User login
Is Vitiligo in Vogue? The Changing Face of Vitiligo
Vitiligo is a disfiguring skin condition that is thought to result from autoimmune destruction of melanocytes in the skin, leading to patchy depigmentation. The prevalence of vitiligo is estimated at 1% worldwide.1 Once seen as merely a cosmetic disorder, it is increasingly recognized for its devastating psychological effects. As skin quality, texture, and color are a few of the first things people notice about others, skin plays a major role in our daily interactions with the world. Vitiligo often affects the face and other visible areas of the body; thus, it is associated with impaired quality of life, and affected individuals often experience psychosocial impairment including anxiety, depression, stigmatization, and self-harm ideation.2 Indeed, vitiligo is a condition with not only a visible skin component but a deeper psychological component that also is important to recognize and address. However, due in large part to recent exposure to vitiligo through mainstream media, general understanding about and attitudes toward this condition are changing. As a result, vitiligo has seen a surge in outreach by those affected by the disease.
Perhaps the most well-known current face of vitiligo is Chantelle Brown-Young, a black fashion model, activist, and vitiligo spokesperson known professionally as Winnie Harlow. Diagnosed with vitiligo in childhood, she revealed she was teased and bullied and at one point contemplated suicide. “The continuous harassment and the despair that [vitiligo] brought on my life was so unbearably dehumanizing that I wanted to kill myself,” she disclosed.3 After competing on America’s Next Top Model in 2014, Winnie Harlow became a household name for redefining global standards of beauty and, in her own words, accepting the differences that make us unique and authentic.4 She went on to speak at the Dove Self-Esteem Project panel at the 2015 Women in the World London Summit and was presented with the Role Model award at the Portuguese GQ Men of the Year event that same year.5
More recently, Amy Deanna, a model with vitiligo, was featured in videos for CoverGirl’s 2018 “I Am What I Make Up” campaign in which she is shown enhancing her various skin tones rather than hiding them by applying both light and dark shades of makeup on her face. In a press release she stated, “Vitiligo awareness is something that is very important to me. Being given a platform to [raise awareness] means so much.”6
Additionally, Brock Elbank, a London-based photographer, recently launched a photograph series of men and women with vitiligo on the digital platform Instagram.7 In a recent interview he stated, “I see beauty in what many see as different. Unique individuals who stand out from the crowd are what inspire me to do what I do.”7
Lee Thomas, a television broadcaster and author of the book Turning White: A Memoir of Change is yet another example of a vitiligo patient who recently stopped hiding his condition. He admitted he has had people refuse to shake his hand due to his condition but has used the experience to educate others. He stated, “Because I’m in this position, I think this is where my next thing is supposed to be. It’s supposed to be about sharing and helping, and hopefully leaving the planet a little better for everybody else who comes along with vitiligo.”8 Thomas is dedicated to inspiring others with the condition and started the Clarity Lee Thomas Foundation to provide emotional and mental support to those with vitiligo.
Critics may say this vitiligo movement is merely another example of exploitation of what is unique or different by mainstream media and the fashion industry, similar to prior movements for plus-sized models, natural hairstyles in black women, and transgender identification. Even if partially true, the ultimate effect has been an increase in attention and representation of individuals with vitiligo in mainstream media. At the time this article was being published (September 2018), an Instagram search for #vitiligo yielded approximately 226,000 posts. For comparison with other much more common dermatologic conditions, #eczema returned approximately 958,000 results, #moles returned approximately 65,000 results, and #skincancer returned approximately 104,000 results. Additionally, the Vitiligo Research Foundation currently has more than 5000 followers on Instagram, which is as many as the Melanoma Research Foundation and almost twice as many as the Skin Cancer Foundation, supporting the idea that mainstream representation of individuals with vitiligo is contributing to raising awareness and backing of organizations aimed at making advancements in this area of dermatology.
As more individuals gain an understanding and curiosity about this disease, perhaps more research and investigation will be done to improve treatment options and outcomes for patients with vitiligo. With this movement, perhaps vitiligo patients will feel more comfortable and confident in their skin.
- Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet. 2015;386:74-84.
- Tomas‐Aragones L, Marron SE. Body image and body dysmorphic concerns. Acta Derm Venereol. 2016;96:47-50.
- Rodney D. From suicide thoughts to finalist in America’s Next Top Model. The Gleaner. February 25, 2014. http://jamaica-gleaner.com/gleaner/20140225/news/news1.html. Accessed September 7, 2018.
- Keyes-Bevan B. Winnie Harlow: her emotional story with vitiligo. Personal Health News website. http://www.personalhealthnews.ca/prevention-and-treatment/her-emotional-story-with-vitiligo. Accessed September 7, 2018.
- Giles K, Davidson R. ‘I think I’m beautiful’: model Winnie Harlow, who suffers from rare vitiligo skin condition, gives empowering talk at Women in the World event. Daily Mail. October 9, 2015. http://www.dailymail.co.uk/tvshowbiz/article-3266579/I-think-m-beautiful-Model-Winnie-Harlow-suffers-rare-Vitiligo-skin-condition-gives-empowering-talk-Women-World-event.html. Updated October 13, 2015. Accessed September 7, 2018.
- Ruffo J. CoverGirl’s first model with vitiligo stars in new campaign: ‘w
e have to be more inclusive.’ People. February 20, 2018. https://people.com/style/covergirl-first-model-with-vitiligo-interview/. Accessed September 25, 2018. - Blair O. This vitiligo photo series is absolutely breathtaking. Cosmopolitan. March 23, 2018. https://www.cosmopolitan.com/uk/beauty-hair/a19494259/vitiligo-photo-series-instagram/. Accessed September 7, 2018.
- Broadcaster opens up about living with vitiligo. People. February 20, 2018. http://people.com/health/lee-thomas-tv-reporter-on-his-vitiligo/. Accessed April 1, 2018.
Vitiligo is a disfiguring skin condition that is thought to result from autoimmune destruction of melanocytes in the skin, leading to patchy depigmentation. The prevalence of vitiligo is estimated at 1% worldwide.1 Once seen as merely a cosmetic disorder, it is increasingly recognized for its devastating psychological effects. As skin quality, texture, and color are a few of the first things people notice about others, skin plays a major role in our daily interactions with the world. Vitiligo often affects the face and other visible areas of the body; thus, it is associated with impaired quality of life, and affected individuals often experience psychosocial impairment including anxiety, depression, stigmatization, and self-harm ideation.2 Indeed, vitiligo is a condition with not only a visible skin component but a deeper psychological component that also is important to recognize and address. However, due in large part to recent exposure to vitiligo through mainstream media, general understanding about and attitudes toward this condition are changing. As a result, vitiligo has seen a surge in outreach by those affected by the disease.
Perhaps the most well-known current face of vitiligo is Chantelle Brown-Young, a black fashion model, activist, and vitiligo spokesperson known professionally as Winnie Harlow. Diagnosed with vitiligo in childhood, she revealed she was teased and bullied and at one point contemplated suicide. “The continuous harassment and the despair that [vitiligo] brought on my life was so unbearably dehumanizing that I wanted to kill myself,” she disclosed.3 After competing on America’s Next Top Model in 2014, Winnie Harlow became a household name for redefining global standards of beauty and, in her own words, accepting the differences that make us unique and authentic.4 She went on to speak at the Dove Self-Esteem Project panel at the 2015 Women in the World London Summit and was presented with the Role Model award at the Portuguese GQ Men of the Year event that same year.5
More recently, Amy Deanna, a model with vitiligo, was featured in videos for CoverGirl’s 2018 “I Am What I Make Up” campaign in which she is shown enhancing her various skin tones rather than hiding them by applying both light and dark shades of makeup on her face. In a press release she stated, “Vitiligo awareness is something that is very important to me. Being given a platform to [raise awareness] means so much.”6
Additionally, Brock Elbank, a London-based photographer, recently launched a photograph series of men and women with vitiligo on the digital platform Instagram.7 In a recent interview he stated, “I see beauty in what many see as different. Unique individuals who stand out from the crowd are what inspire me to do what I do.”7
Lee Thomas, a television broadcaster and author of the book Turning White: A Memoir of Change is yet another example of a vitiligo patient who recently stopped hiding his condition. He admitted he has had people refuse to shake his hand due to his condition but has used the experience to educate others. He stated, “Because I’m in this position, I think this is where my next thing is supposed to be. It’s supposed to be about sharing and helping, and hopefully leaving the planet a little better for everybody else who comes along with vitiligo.”8 Thomas is dedicated to inspiring others with the condition and started the Clarity Lee Thomas Foundation to provide emotional and mental support to those with vitiligo.
Critics may say this vitiligo movement is merely another example of exploitation of what is unique or different by mainstream media and the fashion industry, similar to prior movements for plus-sized models, natural hairstyles in black women, and transgender identification. Even if partially true, the ultimate effect has been an increase in attention and representation of individuals with vitiligo in mainstream media. At the time this article was being published (September 2018), an Instagram search for #vitiligo yielded approximately 226,000 posts. For comparison with other much more common dermatologic conditions, #eczema returned approximately 958,000 results, #moles returned approximately 65,000 results, and #skincancer returned approximately 104,000 results. Additionally, the Vitiligo Research Foundation currently has more than 5000 followers on Instagram, which is as many as the Melanoma Research Foundation and almost twice as many as the Skin Cancer Foundation, supporting the idea that mainstream representation of individuals with vitiligo is contributing to raising awareness and backing of organizations aimed at making advancements in this area of dermatology.
As more individuals gain an understanding and curiosity about this disease, perhaps more research and investigation will be done to improve treatment options and outcomes for patients with vitiligo. With this movement, perhaps vitiligo patients will feel more comfortable and confident in their skin.
Vitiligo is a disfiguring skin condition that is thought to result from autoimmune destruction of melanocytes in the skin, leading to patchy depigmentation. The prevalence of vitiligo is estimated at 1% worldwide.1 Once seen as merely a cosmetic disorder, it is increasingly recognized for its devastating psychological effects. As skin quality, texture, and color are a few of the first things people notice about others, skin plays a major role in our daily interactions with the world. Vitiligo often affects the face and other visible areas of the body; thus, it is associated with impaired quality of life, and affected individuals often experience psychosocial impairment including anxiety, depression, stigmatization, and self-harm ideation.2 Indeed, vitiligo is a condition with not only a visible skin component but a deeper psychological component that also is important to recognize and address. However, due in large part to recent exposure to vitiligo through mainstream media, general understanding about and attitudes toward this condition are changing. As a result, vitiligo has seen a surge in outreach by those affected by the disease.
Perhaps the most well-known current face of vitiligo is Chantelle Brown-Young, a black fashion model, activist, and vitiligo spokesperson known professionally as Winnie Harlow. Diagnosed with vitiligo in childhood, she revealed she was teased and bullied and at one point contemplated suicide. “The continuous harassment and the despair that [vitiligo] brought on my life was so unbearably dehumanizing that I wanted to kill myself,” she disclosed.3 After competing on America’s Next Top Model in 2014, Winnie Harlow became a household name for redefining global standards of beauty and, in her own words, accepting the differences that make us unique and authentic.4 She went on to speak at the Dove Self-Esteem Project panel at the 2015 Women in the World London Summit and was presented with the Role Model award at the Portuguese GQ Men of the Year event that same year.5
More recently, Amy Deanna, a model with vitiligo, was featured in videos for CoverGirl’s 2018 “I Am What I Make Up” campaign in which she is shown enhancing her various skin tones rather than hiding them by applying both light and dark shades of makeup on her face. In a press release she stated, “Vitiligo awareness is something that is very important to me. Being given a platform to [raise awareness] means so much.”6
Additionally, Brock Elbank, a London-based photographer, recently launched a photograph series of men and women with vitiligo on the digital platform Instagram.7 In a recent interview he stated, “I see beauty in what many see as different. Unique individuals who stand out from the crowd are what inspire me to do what I do.”7
Lee Thomas, a television broadcaster and author of the book Turning White: A Memoir of Change is yet another example of a vitiligo patient who recently stopped hiding his condition. He admitted he has had people refuse to shake his hand due to his condition but has used the experience to educate others. He stated, “Because I’m in this position, I think this is where my next thing is supposed to be. It’s supposed to be about sharing and helping, and hopefully leaving the planet a little better for everybody else who comes along with vitiligo.”8 Thomas is dedicated to inspiring others with the condition and started the Clarity Lee Thomas Foundation to provide emotional and mental support to those with vitiligo.
Critics may say this vitiligo movement is merely another example of exploitation of what is unique or different by mainstream media and the fashion industry, similar to prior movements for plus-sized models, natural hairstyles in black women, and transgender identification. Even if partially true, the ultimate effect has been an increase in attention and representation of individuals with vitiligo in mainstream media. At the time this article was being published (September 2018), an Instagram search for #vitiligo yielded approximately 226,000 posts. For comparison with other much more common dermatologic conditions, #eczema returned approximately 958,000 results, #moles returned approximately 65,000 results, and #skincancer returned approximately 104,000 results. Additionally, the Vitiligo Research Foundation currently has more than 5000 followers on Instagram, which is as many as the Melanoma Research Foundation and almost twice as many as the Skin Cancer Foundation, supporting the idea that mainstream representation of individuals with vitiligo is contributing to raising awareness and backing of organizations aimed at making advancements in this area of dermatology.
As more individuals gain an understanding and curiosity about this disease, perhaps more research and investigation will be done to improve treatment options and outcomes for patients with vitiligo. With this movement, perhaps vitiligo patients will feel more comfortable and confident in their skin.
- Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet. 2015;386:74-84.
- Tomas‐Aragones L, Marron SE. Body image and body dysmorphic concerns. Acta Derm Venereol. 2016;96:47-50.
- Rodney D. From suicide thoughts to finalist in America’s Next Top Model. The Gleaner. February 25, 2014. http://jamaica-gleaner.com/gleaner/20140225/news/news1.html. Accessed September 7, 2018.
- Keyes-Bevan B. Winnie Harlow: her emotional story with vitiligo. Personal Health News website. http://www.personalhealthnews.ca/prevention-and-treatment/her-emotional-story-with-vitiligo. Accessed September 7, 2018.
- Giles K, Davidson R. ‘I think I’m beautiful’: model Winnie Harlow, who suffers from rare vitiligo skin condition, gives empowering talk at Women in the World event. Daily Mail. October 9, 2015. http://www.dailymail.co.uk/tvshowbiz/article-3266579/I-think-m-beautiful-Model-Winnie-Harlow-suffers-rare-Vitiligo-skin-condition-gives-empowering-talk-Women-World-event.html. Updated October 13, 2015. Accessed September 7, 2018.
- Ruffo J. CoverGirl’s first model with vitiligo stars in new campaign: ‘w
e have to be more inclusive.’ People. February 20, 2018. https://people.com/style/covergirl-first-model-with-vitiligo-interview/. Accessed September 25, 2018. - Blair O. This vitiligo photo series is absolutely breathtaking. Cosmopolitan. March 23, 2018. https://www.cosmopolitan.com/uk/beauty-hair/a19494259/vitiligo-photo-series-instagram/. Accessed September 7, 2018.
- Broadcaster opens up about living with vitiligo. People. February 20, 2018. http://people.com/health/lee-thomas-tv-reporter-on-his-vitiligo/. Accessed April 1, 2018.
- Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet. 2015;386:74-84.
- Tomas‐Aragones L, Marron SE. Body image and body dysmorphic concerns. Acta Derm Venereol. 2016;96:47-50.
- Rodney D. From suicide thoughts to finalist in America’s Next Top Model. The Gleaner. February 25, 2014. http://jamaica-gleaner.com/gleaner/20140225/news/news1.html. Accessed September 7, 2018.
- Keyes-Bevan B. Winnie Harlow: her emotional story with vitiligo. Personal Health News website. http://www.personalhealthnews.ca/prevention-and-treatment/her-emotional-story-with-vitiligo. Accessed September 7, 2018.
- Giles K, Davidson R. ‘I think I’m beautiful’: model Winnie Harlow, who suffers from rare vitiligo skin condition, gives empowering talk at Women in the World event. Daily Mail. October 9, 2015. http://www.dailymail.co.uk/tvshowbiz/article-3266579/I-think-m-beautiful-Model-Winnie-Harlow-suffers-rare-Vitiligo-skin-condition-gives-empowering-talk-Women-World-event.html. Updated October 13, 2015. Accessed September 7, 2018.
- Ruffo J. CoverGirl’s first model with vitiligo stars in new campaign: ‘w
e have to be more inclusive.’ People. February 20, 2018. https://people.com/style/covergirl-first-model-with-vitiligo-interview/. Accessed September 25, 2018. - Blair O. This vitiligo photo series is absolutely breathtaking. Cosmopolitan. March 23, 2018. https://www.cosmopolitan.com/uk/beauty-hair/a19494259/vitiligo-photo-series-instagram/. Accessed September 7, 2018.
- Broadcaster opens up about living with vitiligo. People. February 20, 2018. http://people.com/health/lee-thomas-tv-reporter-on-his-vitiligo/. Accessed April 1, 2018.
ACOG lends support to bill promoting maternal mortality review committees
The Preventing Maternal Deaths Act of 2018 (H.R. 1318) was the subject of a Sept. 27 hearing of the House Energy and Commerce Health Subcommittee. The bill comes at a time when 700 women a year die as a result of pregnancy or pregnancy-related complications with a rate that is increasing, while 157 of 183 countries around the world are reporting decreasing rates of maternal mortality, according to ACOG.
The bill, authored by Rep. Jamie Herrera Beutler (R-Wash.) and Diana DeGette (D-Colo.) would allocate $58 million for each fiscal year from 2019 through 2023 to support the 33 existing states with maternal mortality review committees (MMRCs) and help the remaining 17 states develop them, as well as to standardize data collection across the nation.
The goal of having these committees in place is to “improve data collection and reporting around maternal mortality, and to develop or support surveillance systems at the local, state, and national level in order to better understand the burden of maternal complications,” a background memo on the hearing noted. “These surveillance efforts include identifying groups of women with disproportionately high rates of maternal mortality and identifying the determinants of disparities in maternal care, health risks, and health outcomes.”
Necessitating this legislation was a data point that was reiterated throughout the course of the hearing – that maternal mortality rates in the United States were on the rise.
“What’s both surprising and devastating is that, despite massive innovation and advances in health care and technology, we’ve experienced recent reports that have indicated that the number of women dying due to pregnancy complications is actually increasing,” Full Committee Chairman Greg Walden (R-Ore.) said in his opening remarks at the hearing. “According to the Centers for Disease Control and Prevention, maternal mortality rates in America have more than doubled since 1987. I think we are asking, how can that be? This is not a statistic any of us wants to hear.”
Chairman Walden acknowledged that there are questions as to whether the increase was a function of better data collection or whether it was an issue with the delivery of health care.
“The bill before us today will help us answer these really important questions and hopefully ensure that expectant newborn mothers receive even better care,” he said.
Lynne M. Coslett-Charlton, MD, ACOG Pennsylvania District legislative chair, offered the organization’s support for the bill.
MMRCs “are multidisciplinary groups of local experts in maternal and public health, as well as patient and community advocates, that closely examine maternal death cases and identify locally relevant ways to prevent future deaths,” she testified before the committee. “While traditional public health surveillance using vital statistics can tell us about trends and disparities, MMRCs are best positioned to comprehensively assess maternal deaths and identify opportunities for prevention.”
Dr. Coslett-Charlton added that to clearly understand why women are dying from preventable maternal complications, which she noted that 60% of maternal deaths are, “every state must have a robust MMRC. The Preventing Maternal Deaths Act will help us reach that goal, and ultimately improve maternal health across this nation,” as these committees review every maternal death and can make a determination as to whether they could have been preventable.
Additionally, the fact that black women face a significantly higher rate of maternal mortality was another data point highlighted during the hearing, further adding to the need for this bill that has bipartisan support and more than 170 cosponsors.
Rep. DeGette called it “one of the most striking aspects” that black women “are nearly four times as likely to experience a pregnancy-related death.”
Stacey D. Stewart, president of the March of Dimes, in her written testimony praised the inclusion in H.R. 1318 of a “demonstration project to determine how best to address disparities in maternal health outcomes.”
The Preventing Maternal Deaths Act of 2018 (H.R. 1318) was the subject of a Sept. 27 hearing of the House Energy and Commerce Health Subcommittee. The bill comes at a time when 700 women a year die as a result of pregnancy or pregnancy-related complications with a rate that is increasing, while 157 of 183 countries around the world are reporting decreasing rates of maternal mortality, according to ACOG.
The bill, authored by Rep. Jamie Herrera Beutler (R-Wash.) and Diana DeGette (D-Colo.) would allocate $58 million for each fiscal year from 2019 through 2023 to support the 33 existing states with maternal mortality review committees (MMRCs) and help the remaining 17 states develop them, as well as to standardize data collection across the nation.
The goal of having these committees in place is to “improve data collection and reporting around maternal mortality, and to develop or support surveillance systems at the local, state, and national level in order to better understand the burden of maternal complications,” a background memo on the hearing noted. “These surveillance efforts include identifying groups of women with disproportionately high rates of maternal mortality and identifying the determinants of disparities in maternal care, health risks, and health outcomes.”
Necessitating this legislation was a data point that was reiterated throughout the course of the hearing – that maternal mortality rates in the United States were on the rise.
“What’s both surprising and devastating is that, despite massive innovation and advances in health care and technology, we’ve experienced recent reports that have indicated that the number of women dying due to pregnancy complications is actually increasing,” Full Committee Chairman Greg Walden (R-Ore.) said in his opening remarks at the hearing. “According to the Centers for Disease Control and Prevention, maternal mortality rates in America have more than doubled since 1987. I think we are asking, how can that be? This is not a statistic any of us wants to hear.”
Chairman Walden acknowledged that there are questions as to whether the increase was a function of better data collection or whether it was an issue with the delivery of health care.
“The bill before us today will help us answer these really important questions and hopefully ensure that expectant newborn mothers receive even better care,” he said.
Lynne M. Coslett-Charlton, MD, ACOG Pennsylvania District legislative chair, offered the organization’s support for the bill.
MMRCs “are multidisciplinary groups of local experts in maternal and public health, as well as patient and community advocates, that closely examine maternal death cases and identify locally relevant ways to prevent future deaths,” she testified before the committee. “While traditional public health surveillance using vital statistics can tell us about trends and disparities, MMRCs are best positioned to comprehensively assess maternal deaths and identify opportunities for prevention.”
Dr. Coslett-Charlton added that to clearly understand why women are dying from preventable maternal complications, which she noted that 60% of maternal deaths are, “every state must have a robust MMRC. The Preventing Maternal Deaths Act will help us reach that goal, and ultimately improve maternal health across this nation,” as these committees review every maternal death and can make a determination as to whether they could have been preventable.
Additionally, the fact that black women face a significantly higher rate of maternal mortality was another data point highlighted during the hearing, further adding to the need for this bill that has bipartisan support and more than 170 cosponsors.
Rep. DeGette called it “one of the most striking aspects” that black women “are nearly four times as likely to experience a pregnancy-related death.”
Stacey D. Stewart, president of the March of Dimes, in her written testimony praised the inclusion in H.R. 1318 of a “demonstration project to determine how best to address disparities in maternal health outcomes.”
The Preventing Maternal Deaths Act of 2018 (H.R. 1318) was the subject of a Sept. 27 hearing of the House Energy and Commerce Health Subcommittee. The bill comes at a time when 700 women a year die as a result of pregnancy or pregnancy-related complications with a rate that is increasing, while 157 of 183 countries around the world are reporting decreasing rates of maternal mortality, according to ACOG.
The bill, authored by Rep. Jamie Herrera Beutler (R-Wash.) and Diana DeGette (D-Colo.) would allocate $58 million for each fiscal year from 2019 through 2023 to support the 33 existing states with maternal mortality review committees (MMRCs) and help the remaining 17 states develop them, as well as to standardize data collection across the nation.
The goal of having these committees in place is to “improve data collection and reporting around maternal mortality, and to develop or support surveillance systems at the local, state, and national level in order to better understand the burden of maternal complications,” a background memo on the hearing noted. “These surveillance efforts include identifying groups of women with disproportionately high rates of maternal mortality and identifying the determinants of disparities in maternal care, health risks, and health outcomes.”
Necessitating this legislation was a data point that was reiterated throughout the course of the hearing – that maternal mortality rates in the United States were on the rise.
“What’s both surprising and devastating is that, despite massive innovation and advances in health care and technology, we’ve experienced recent reports that have indicated that the number of women dying due to pregnancy complications is actually increasing,” Full Committee Chairman Greg Walden (R-Ore.) said in his opening remarks at the hearing. “According to the Centers for Disease Control and Prevention, maternal mortality rates in America have more than doubled since 1987. I think we are asking, how can that be? This is not a statistic any of us wants to hear.”
Chairman Walden acknowledged that there are questions as to whether the increase was a function of better data collection or whether it was an issue with the delivery of health care.
“The bill before us today will help us answer these really important questions and hopefully ensure that expectant newborn mothers receive even better care,” he said.
Lynne M. Coslett-Charlton, MD, ACOG Pennsylvania District legislative chair, offered the organization’s support for the bill.
MMRCs “are multidisciplinary groups of local experts in maternal and public health, as well as patient and community advocates, that closely examine maternal death cases and identify locally relevant ways to prevent future deaths,” she testified before the committee. “While traditional public health surveillance using vital statistics can tell us about trends and disparities, MMRCs are best positioned to comprehensively assess maternal deaths and identify opportunities for prevention.”
Dr. Coslett-Charlton added that to clearly understand why women are dying from preventable maternal complications, which she noted that 60% of maternal deaths are, “every state must have a robust MMRC. The Preventing Maternal Deaths Act will help us reach that goal, and ultimately improve maternal health across this nation,” as these committees review every maternal death and can make a determination as to whether they could have been preventable.
Additionally, the fact that black women face a significantly higher rate of maternal mortality was another data point highlighted during the hearing, further adding to the need for this bill that has bipartisan support and more than 170 cosponsors.
Rep. DeGette called it “one of the most striking aspects” that black women “are nearly four times as likely to experience a pregnancy-related death.”
Stacey D. Stewart, president of the March of Dimes, in her written testimony praised the inclusion in H.R. 1318 of a “demonstration project to determine how best to address disparities in maternal health outcomes.”
REPORTING FROM A HOUSE ENERGY AND COMMERCE HEALTH SUBCOMMITTEE HEARING
Artificial Intelligence in Dermatology: Is Cognitive Computing the Future of Evidence-Based Medicine?
U.S. vs. Europe: Costs of cardiac implant devices compared
Prices that hospitals pay for cardiac implant devices are two to six times higher in the United States than in Europe, according to analysis of a large hospital panel survey.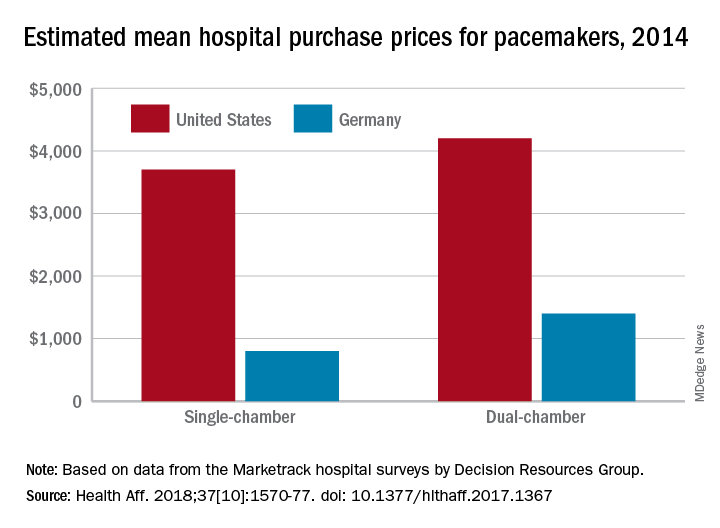
U.S. hospitals had an estimated mean cost of $670 for a bare-metal stent in 2014, compared with $120 in Germany, and the mean costs for dual-chamber pacemakers that year were $4,200 in the United States and $1,400 in Germany, which had lower costs for cardiac devices than the other three European countries – United Kingdom, France, and Italy – included in the study, Martin Wenzl, MSc, and Elias Mossialos, MD, PhD, reported in Health Affairs.
France generally had the highest costs among the European countries, with Italy next and then the United Kingdom. The estimated cost of bare-metal stents was actually higher for French hospitals ($750) than for those in the United States, and Italy had mean prices similar to the United Sates for dual-chamber implantable cardioverter-defibrillators. The prices of implantable cardioverter-defibrillators and cardiac resynchronization devices with defibrillating function were the other exceptions, with the United Kingdom similar to or higher than the United States, said Mr. Wenzl and Dr. Mossialos, both of the London School of Economics and Political Science.
The analysis of data from Decision Resources Group’s Marketrack hospital surveys also showed significant variation between the hospitals in each country, with the exception of France, where payments are based on the specific device rather than the procedure and the system “creates weak incentives for hospitals to negotiate lower prices,” they said. In most of the device categories, “variation between hospitals in each country was similar to variation between countries,” they wrote, adding that prices in general “were only weakly correlated with volumes purchased by hospitals.”
The study was supported by a grant from the Commonwealth Fund. The investigators did not disclose any possible conflicts of interest.
SOURCE: Wenzi M, Mossialos E. Health Aff. 2018;37[10]:1570-77. doi: 10.1377/hlthaff.2017.1367.
Prices that hospitals pay for cardiac implant devices are two to six times higher in the United States than in Europe, according to analysis of a large hospital panel survey.
U.S. hospitals had an estimated mean cost of $670 for a bare-metal stent in 2014, compared with $120 in Germany, and the mean costs for dual-chamber pacemakers that year were $4,200 in the United States and $1,400 in Germany, which had lower costs for cardiac devices than the other three European countries – United Kingdom, France, and Italy – included in the study, Martin Wenzl, MSc, and Elias Mossialos, MD, PhD, reported in Health Affairs.
France generally had the highest costs among the European countries, with Italy next and then the United Kingdom. The estimated cost of bare-metal stents was actually higher for French hospitals ($750) than for those in the United States, and Italy had mean prices similar to the United Sates for dual-chamber implantable cardioverter-defibrillators. The prices of implantable cardioverter-defibrillators and cardiac resynchronization devices with defibrillating function were the other exceptions, with the United Kingdom similar to or higher than the United States, said Mr. Wenzl and Dr. Mossialos, both of the London School of Economics and Political Science.
The analysis of data from Decision Resources Group’s Marketrack hospital surveys also showed significant variation between the hospitals in each country, with the exception of France, where payments are based on the specific device rather than the procedure and the system “creates weak incentives for hospitals to negotiate lower prices,” they said. In most of the device categories, “variation between hospitals in each country was similar to variation between countries,” they wrote, adding that prices in general “were only weakly correlated with volumes purchased by hospitals.”
The study was supported by a grant from the Commonwealth Fund. The investigators did not disclose any possible conflicts of interest.
SOURCE: Wenzi M, Mossialos E. Health Aff. 2018;37[10]:1570-77. doi: 10.1377/hlthaff.2017.1367.
Prices that hospitals pay for cardiac implant devices are two to six times higher in the United States than in Europe, according to analysis of a large hospital panel survey.
U.S. hospitals had an estimated mean cost of $670 for a bare-metal stent in 2014, compared with $120 in Germany, and the mean costs for dual-chamber pacemakers that year were $4,200 in the United States and $1,400 in Germany, which had lower costs for cardiac devices than the other three European countries – United Kingdom, France, and Italy – included in the study, Martin Wenzl, MSc, and Elias Mossialos, MD, PhD, reported in Health Affairs.
France generally had the highest costs among the European countries, with Italy next and then the United Kingdom. The estimated cost of bare-metal stents was actually higher for French hospitals ($750) than for those in the United States, and Italy had mean prices similar to the United Sates for dual-chamber implantable cardioverter-defibrillators. The prices of implantable cardioverter-defibrillators and cardiac resynchronization devices with defibrillating function were the other exceptions, with the United Kingdom similar to or higher than the United States, said Mr. Wenzl and Dr. Mossialos, both of the London School of Economics and Political Science.
The analysis of data from Decision Resources Group’s Marketrack hospital surveys also showed significant variation between the hospitals in each country, with the exception of France, where payments are based on the specific device rather than the procedure and the system “creates weak incentives for hospitals to negotiate lower prices,” they said. In most of the device categories, “variation between hospitals in each country was similar to variation between countries,” they wrote, adding that prices in general “were only weakly correlated with volumes purchased by hospitals.”
The study was supported by a grant from the Commonwealth Fund. The investigators did not disclose any possible conflicts of interest.
SOURCE: Wenzi M, Mossialos E. Health Aff. 2018;37[10]:1570-77. doi: 10.1377/hlthaff.2017.1367.
FROM HEALTH AFFAIRS
PCV13 moderately effective in older adults
ATLANTA – (IPD) caused by PCV13 vaccine serotypes in adults aged 65 years and older, according to a case-control study involving Medicare beneficiaries.
Conversely, the 23-valent pneumococcal polysaccharide vaccine (PPSV23) showed limited effectiveness against serotypes unique to that vaccine in the study, which included 699 cases and more than 10,000 controls, Olivia Almendares, an epidemiologist at the Centers for Disease Control and Prevention, Atlanta, and her colleagues reported in a poster at the International Conference on Emerging Infectious Diseases.
“Vaccine efficacy against PCV13 [plus 6C type, which has cross-reactivity with serotype 6A] was 47% in those who received PCV13 vaccine only,” Ms. Almendares said in an interview, noting that efficacy was 26% against serotype 3 and 67% against other PCV13 serotypes (plus 6C). “Vaccine efficacy against PPSV23-unique types was 36% for those who received only PPSV23.”
Neither vaccine showed effectiveness against serotypes not included in the respective vaccines, she said.
The findings are timely given that the Advisory Committee on Immunization Practices (ACIP) is reevaluating its PCV13 recommendation for adults aged 65 years and older, she added.
“Specifically, ACIP is addressing whether PCV13 should be recommended routinely for all immunocompetent adults aged 65 and older given sustained indirect effects,” she said, explaining that, in 2014 when ACIP recommended routine use of the vaccine in series with PPSV23 for adults aged 65 years and older, the committee recognized that herd immunity effects from PCV13 use in children might eventually limit the utility of this recommendation, and therefore it proposed reevaluation and revision as needed after 4 years.
For the current study, she and her colleagues linked IPD cases in persons aged 65 years and older, which were identified through Active Bacterial Core surveillance during 2015-2016, to records for Centers for Medicare & Medicaid Services (CMS) beneficiaries. Vaccination and medical histories were obtained through medical records, and vaccine effectiveness was estimated as one minus the odds ratio for vaccination with PCV13 only or PPSV23 only versus neither vaccine using conditional logistic regression, with adjustment for sex and underlying medical conditions.
Of 2,246 IPD cases, 1,017 (45%) were matched to Medicare beneficiaries, and 699 were included in the analysis after those with noncontinuous enrollment in Medicare, long-term care residence, and missing census tract data were excluded. The cases were matched based on age, census tract of residence, and length of Medicare enrollment to 10,152 matched controls identified through CMS.
IPD associated with PCV13 (plus type 6C) accounted for 164 (23% of cases), of which 88 (12% of cases) involved serotype 3, and invasive pneumococcal disease associated with PPSV23 accounted for 350 cases (50%), she said.
PCV13 vaccine was given alone in 14% and 18% of cases and controls, respectively; PPSV23 alone was given in 22% and 21% of case patients and controls, respectively; and both vaccines were given in 8% of cases and controls.
Compared with controls, case patients were more likely to be of nonwhite race (16% vs. 11%), to have more than one chronic medical condition (88% vs. 58%), and to have one or more immunocompromising conditions (54% vs. 32%), she and her colleagues reported.
“PCV13 showed moderate overall effectiveness in preventing IPD caused by PCV13 (including 6C), but effectiveness may be lower for serotype 3 than for other PCV13 types,” she said.
“These results are in agreement with those from CAPiTA – a large clinical trial conducted in the Netherlands, which showed PCV13 to be effective against IPD caused by vaccine serotypes among community-dwelling adults aged 65 and older,” she noted. “Additionally, data from CDC surveillance suggest that PCV13-serotype [invasive pneumococcal disease] among children and adults aged 65 and older has declined dramatically following PCV13 introduction for children in 2010, as predicted.”
In fact, among adults aged 65 years and older, PCV13-serotype invasive pneumococcal disease declined by 40% after the vaccine was introduced in children. This corresponds to a change in the annual PCV13-serotype incidence from 14 cases per 100,000 population in 2010 to five cases per 100,000 population in 2014, she said; she added that IPD incidence plateaued in 2014-2016 with vaccine serotypes contributing to a small proportion of overall IPD burden among adults aged 65 years and older.
ACIP’s reevaluation of the PCV13 recommendation is ongoing and will be addressed at upcoming meetings.
“As part of the review process, we look at changes in disease incidence focusing primarily on invasive pneumococcal disease and noninvasive pneumonia, vaccine efficacy and effectiveness, and vaccine safety,” she said. She noted that ACIP currently has no plans to consider revising PCV13 recommendations for adults who have immunocompromising conditions, for whom PCV13 has been recommended since 2012.
Ms. Almendares reported having no disclosures.
SOURCE: Almendares O et al. ICEID 2018, Board 376.
ATLANTA – (IPD) caused by PCV13 vaccine serotypes in adults aged 65 years and older, according to a case-control study involving Medicare beneficiaries.
Conversely, the 23-valent pneumococcal polysaccharide vaccine (PPSV23) showed limited effectiveness against serotypes unique to that vaccine in the study, which included 699 cases and more than 10,000 controls, Olivia Almendares, an epidemiologist at the Centers for Disease Control and Prevention, Atlanta, and her colleagues reported in a poster at the International Conference on Emerging Infectious Diseases.
“Vaccine efficacy against PCV13 [plus 6C type, which has cross-reactivity with serotype 6A] was 47% in those who received PCV13 vaccine only,” Ms. Almendares said in an interview, noting that efficacy was 26% against serotype 3 and 67% against other PCV13 serotypes (plus 6C). “Vaccine efficacy against PPSV23-unique types was 36% for those who received only PPSV23.”
Neither vaccine showed effectiveness against serotypes not included in the respective vaccines, she said.
The findings are timely given that the Advisory Committee on Immunization Practices (ACIP) is reevaluating its PCV13 recommendation for adults aged 65 years and older, she added.
“Specifically, ACIP is addressing whether PCV13 should be recommended routinely for all immunocompetent adults aged 65 and older given sustained indirect effects,” she said, explaining that, in 2014 when ACIP recommended routine use of the vaccine in series with PPSV23 for adults aged 65 years and older, the committee recognized that herd immunity effects from PCV13 use in children might eventually limit the utility of this recommendation, and therefore it proposed reevaluation and revision as needed after 4 years.
For the current study, she and her colleagues linked IPD cases in persons aged 65 years and older, which were identified through Active Bacterial Core surveillance during 2015-2016, to records for Centers for Medicare & Medicaid Services (CMS) beneficiaries. Vaccination and medical histories were obtained through medical records, and vaccine effectiveness was estimated as one minus the odds ratio for vaccination with PCV13 only or PPSV23 only versus neither vaccine using conditional logistic regression, with adjustment for sex and underlying medical conditions.
Of 2,246 IPD cases, 1,017 (45%) were matched to Medicare beneficiaries, and 699 were included in the analysis after those with noncontinuous enrollment in Medicare, long-term care residence, and missing census tract data were excluded. The cases were matched based on age, census tract of residence, and length of Medicare enrollment to 10,152 matched controls identified through CMS.
IPD associated with PCV13 (plus type 6C) accounted for 164 (23% of cases), of which 88 (12% of cases) involved serotype 3, and invasive pneumococcal disease associated with PPSV23 accounted for 350 cases (50%), she said.
PCV13 vaccine was given alone in 14% and 18% of cases and controls, respectively; PPSV23 alone was given in 22% and 21% of case patients and controls, respectively; and both vaccines were given in 8% of cases and controls.
Compared with controls, case patients were more likely to be of nonwhite race (16% vs. 11%), to have more than one chronic medical condition (88% vs. 58%), and to have one or more immunocompromising conditions (54% vs. 32%), she and her colleagues reported.
“PCV13 showed moderate overall effectiveness in preventing IPD caused by PCV13 (including 6C), but effectiveness may be lower for serotype 3 than for other PCV13 types,” she said.
“These results are in agreement with those from CAPiTA – a large clinical trial conducted in the Netherlands, which showed PCV13 to be effective against IPD caused by vaccine serotypes among community-dwelling adults aged 65 and older,” she noted. “Additionally, data from CDC surveillance suggest that PCV13-serotype [invasive pneumococcal disease] among children and adults aged 65 and older has declined dramatically following PCV13 introduction for children in 2010, as predicted.”
In fact, among adults aged 65 years and older, PCV13-serotype invasive pneumococcal disease declined by 40% after the vaccine was introduced in children. This corresponds to a change in the annual PCV13-serotype incidence from 14 cases per 100,000 population in 2010 to five cases per 100,000 population in 2014, she said; she added that IPD incidence plateaued in 2014-2016 with vaccine serotypes contributing to a small proportion of overall IPD burden among adults aged 65 years and older.
ACIP’s reevaluation of the PCV13 recommendation is ongoing and will be addressed at upcoming meetings.
“As part of the review process, we look at changes in disease incidence focusing primarily on invasive pneumococcal disease and noninvasive pneumonia, vaccine efficacy and effectiveness, and vaccine safety,” she said. She noted that ACIP currently has no plans to consider revising PCV13 recommendations for adults who have immunocompromising conditions, for whom PCV13 has been recommended since 2012.
Ms. Almendares reported having no disclosures.
SOURCE: Almendares O et al. ICEID 2018, Board 376.
ATLANTA – (IPD) caused by PCV13 vaccine serotypes in adults aged 65 years and older, according to a case-control study involving Medicare beneficiaries.
Conversely, the 23-valent pneumococcal polysaccharide vaccine (PPSV23) showed limited effectiveness against serotypes unique to that vaccine in the study, which included 699 cases and more than 10,000 controls, Olivia Almendares, an epidemiologist at the Centers for Disease Control and Prevention, Atlanta, and her colleagues reported in a poster at the International Conference on Emerging Infectious Diseases.
“Vaccine efficacy against PCV13 [plus 6C type, which has cross-reactivity with serotype 6A] was 47% in those who received PCV13 vaccine only,” Ms. Almendares said in an interview, noting that efficacy was 26% against serotype 3 and 67% against other PCV13 serotypes (plus 6C). “Vaccine efficacy against PPSV23-unique types was 36% for those who received only PPSV23.”
Neither vaccine showed effectiveness against serotypes not included in the respective vaccines, she said.
The findings are timely given that the Advisory Committee on Immunization Practices (ACIP) is reevaluating its PCV13 recommendation for adults aged 65 years and older, she added.
“Specifically, ACIP is addressing whether PCV13 should be recommended routinely for all immunocompetent adults aged 65 and older given sustained indirect effects,” she said, explaining that, in 2014 when ACIP recommended routine use of the vaccine in series with PPSV23 for adults aged 65 years and older, the committee recognized that herd immunity effects from PCV13 use in children might eventually limit the utility of this recommendation, and therefore it proposed reevaluation and revision as needed after 4 years.
For the current study, she and her colleagues linked IPD cases in persons aged 65 years and older, which were identified through Active Bacterial Core surveillance during 2015-2016, to records for Centers for Medicare & Medicaid Services (CMS) beneficiaries. Vaccination and medical histories were obtained through medical records, and vaccine effectiveness was estimated as one minus the odds ratio for vaccination with PCV13 only or PPSV23 only versus neither vaccine using conditional logistic regression, with adjustment for sex and underlying medical conditions.
Of 2,246 IPD cases, 1,017 (45%) were matched to Medicare beneficiaries, and 699 were included in the analysis after those with noncontinuous enrollment in Medicare, long-term care residence, and missing census tract data were excluded. The cases were matched based on age, census tract of residence, and length of Medicare enrollment to 10,152 matched controls identified through CMS.
IPD associated with PCV13 (plus type 6C) accounted for 164 (23% of cases), of which 88 (12% of cases) involved serotype 3, and invasive pneumococcal disease associated with PPSV23 accounted for 350 cases (50%), she said.
PCV13 vaccine was given alone in 14% and 18% of cases and controls, respectively; PPSV23 alone was given in 22% and 21% of case patients and controls, respectively; and both vaccines were given in 8% of cases and controls.
Compared with controls, case patients were more likely to be of nonwhite race (16% vs. 11%), to have more than one chronic medical condition (88% vs. 58%), and to have one or more immunocompromising conditions (54% vs. 32%), she and her colleagues reported.
“PCV13 showed moderate overall effectiveness in preventing IPD caused by PCV13 (including 6C), but effectiveness may be lower for serotype 3 than for other PCV13 types,” she said.
“These results are in agreement with those from CAPiTA – a large clinical trial conducted in the Netherlands, which showed PCV13 to be effective against IPD caused by vaccine serotypes among community-dwelling adults aged 65 and older,” she noted. “Additionally, data from CDC surveillance suggest that PCV13-serotype [invasive pneumococcal disease] among children and adults aged 65 and older has declined dramatically following PCV13 introduction for children in 2010, as predicted.”
In fact, among adults aged 65 years and older, PCV13-serotype invasive pneumococcal disease declined by 40% after the vaccine was introduced in children. This corresponds to a change in the annual PCV13-serotype incidence from 14 cases per 100,000 population in 2010 to five cases per 100,000 population in 2014, she said; she added that IPD incidence plateaued in 2014-2016 with vaccine serotypes contributing to a small proportion of overall IPD burden among adults aged 65 years and older.
ACIP’s reevaluation of the PCV13 recommendation is ongoing and will be addressed at upcoming meetings.
“As part of the review process, we look at changes in disease incidence focusing primarily on invasive pneumococcal disease and noninvasive pneumonia, vaccine efficacy and effectiveness, and vaccine safety,” she said. She noted that ACIP currently has no plans to consider revising PCV13 recommendations for adults who have immunocompromising conditions, for whom PCV13 has been recommended since 2012.
Ms. Almendares reported having no disclosures.
SOURCE: Almendares O et al. ICEID 2018, Board 376.
REPORTING FROM ICEID 2018
Myomectomy of a large cervical fibroid in a patient desiring future fertility
Uterine fibroids are the most common tumors of the uterus. Clinically significant fibroids that arise from the cervix are less common.1 Removing large cervical fibroids when a patient desires future fertility is a surgical challenge because of the risks of significant blood loss, bladder and ureteral injury, and unplanned hysterectomy. For women who desire future fertility, myomectomy can improve the chances of pregnancy by restoring normal anatomy.2 In this article, we describe a technique for myomectomy with uterine preservation in a patient with a 20-cm cervical fibroid.
CASE Woman with increasing girth and urinary symptoms is unable to conceive
A 33-year-old white woman with a history of 1 prior vaginal delivery presents with symptoms of increasing abdominal girth, intermittent urinary retention and urgency, and inability to become pregnant. She reports normal monthly menstrual periods. On pelvic examination, the ObGyn notes a large fibroid partially protruding through a dilated cervix. Abdominal examination reveals a fundal height at the level of the umbilicus.
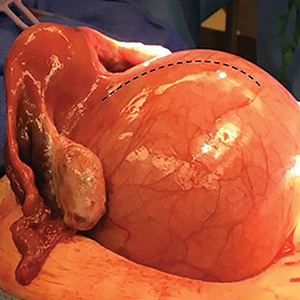
Transvaginal ultrasonography shows a uterus that measures 4.5 x 6.1 x 13.6 cm. Arising from the posterior aspect of the uterine fundus, body, and lower uterine segment is a fibroid that measures 9.7 x 15.5 x 18.9 cm. Magnetic resonance imaging is performed and confirms a fibroid measuring 10 x 16 x 20 cm. The inferior-most aspect of the fibroid appears to be within the endometrial cavity and cervical canal. Most of the fibroid, however, is posterior to the uterus, pressing on and anteriorly displacing the endometrial cavity (FIGURE 1).

What is your surgical approach?
Comprehensive preoperative planning
In this case, the patient should receive extensive preoperative counseling about the significantly increased risk for hysterectomy with an attempted myomectomy. Prior to being scheduled for surgery, she also should have a consultation with a gynecologic oncologist. To optimize visualization during the procedure, we recommend to plan for a midline vertical skin incision. Because of the potential bleeding risks, blood products should be made available in the operating room at the time of surgery.
Techniques for surgery
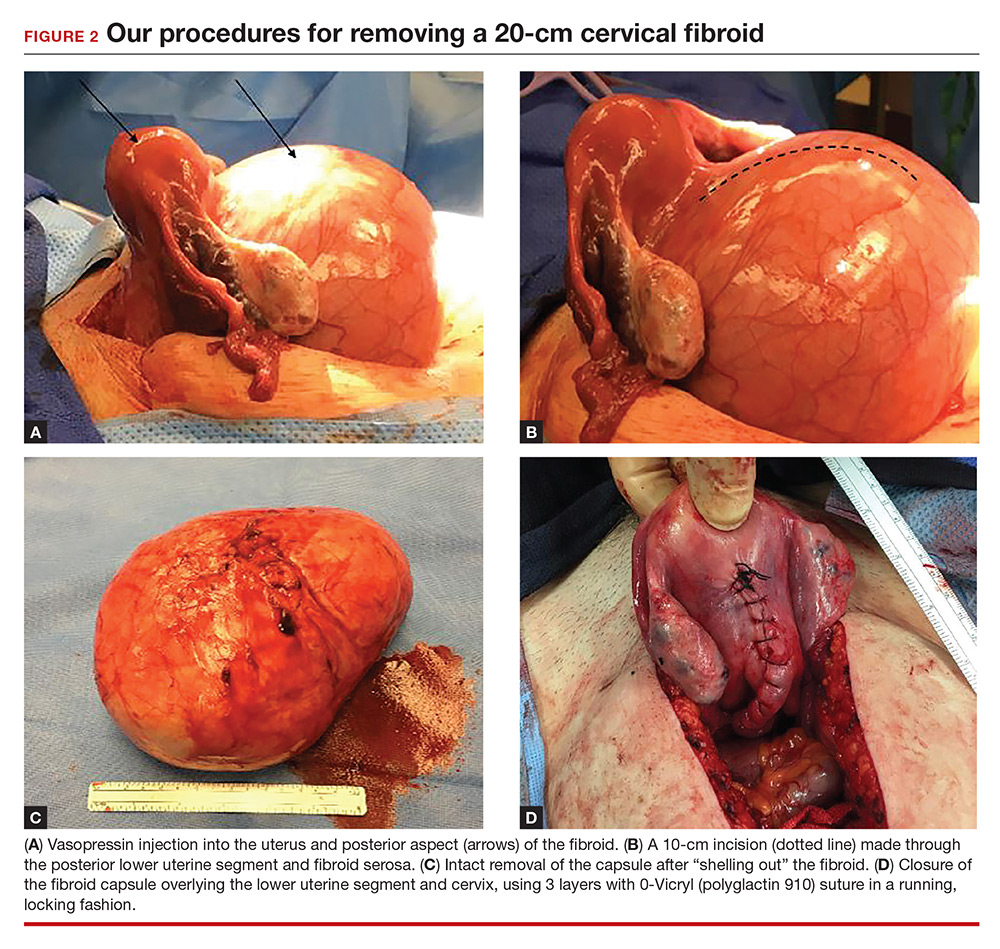
Intraoperatively, a vertical midline incision exteriorizes the uterus from the peritoneal cavity. Opening of the retroperitoneal spaces allows for identification of the ureters. Perform dissection in the midline away from the ureters. Inject vasopressin (5 U) into the uterine fundus. Incise the uterine serosa over the myoma posteriorly in the midline.
Perform a myomectomy, with gentle “shelling out” of the myoma; in this way the specimen can be removed intact. Reapproximate the fibroid cavity in 3 layers with 0-Vicryl (polyglactin 910) suture in a running fashion (FIGURE 2).
Continue to: CASE Resolved
CASE Resolved
The estimated blood loss during surgery was 50 mL. Final pathology reported a 1,660-g intact myoma. The patient’s postoperative course was uncomplicated and she was discharged home on postoperative day 1.
Her postoperative evaluation was 1 month later. Her abdominal incision was well healed. Her fibroid-related symptoms had resolved, and she planned to attempt pregnancy. Cesarean delivery for future pregnancies was recommended.
Increase the chances of a good outcome
Advanced planning for attempted myomectomy of a large cervical fibroid can increase the probability of a successful outcome. We suggest the following:
Counsel the patient on risks. Our preoperative strategy includes extensive counseling on the significantly increased surgical risks and the possibility of unavoidable hysterectomy. Given the anatomic distortion with respect to the ureters, bladder, and major blood vessels, involving gynecologic oncology is beneficial to the surgery planning process.
Prepare for possible transfusion. Ensure blood products are made available in the operating room in case transfusion is needed.
Control bleeding. Randomized studies have shown that intrauterine injection of vasopressin, through its action as a vasoconstrictor, decreases surgical bleeding.3,4 While little data are available on vasopressin’s most effective dosage and dilution, 5 U at a very dilute concentration (0.1–0.2 U/mL) has been recommended.5 A midline cervical incision away from lateral structures and gentle shelling out of the cervical fibroid help to avoid intraoperative damage to the bladder, ureters, and vascular supply.
Close in multiple layers. This approach can prevent a potential space for hematoma accumulation.6 Further, a multiple-layer closure of a myometrial incision may decrease the risk for uterine rupture in subsequent pregnancies.7
Advise abstinence postsurgery. There are no consistent data to guide patient counseling regarding recommendations for the timing of conception following myomectomy. We counseled our patient to abstain from vaginal intercourse for 4 weeks, after which time she soon should attempt to conceive. Although there are no published data regarding when it is best to resume sexual relations following such a surgery, we advise a 1-month period primarily to allow healing of the skin incision. Any further delay in attempting to become pregnant may allow for the growth of additional fibroids.
Plan for future deliveries. When the myomais extensively involved, such as in this case, we recommend cesarean delivery for future pregnancies to avoid the known risk of uterine rupture.8 In general, we recommend cesarean delivery in future pregnancies if an incision larger than 50% of the myometrial thickness is made in the contractile portion of the uterus.
Final takeaway. Despite increased surgical risks, myomectomy of a large cervical fibroid is possible and can alleviate symptoms and improve future fertility.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Ryan GL, Syrop CH, Van Voorhis BJ. Role, epidemiology, and natural history of benign uterine mass lesions. Clin Obstet Gynecol. 2005;48(2):312–324.
- Milazzo GN, Catalano A, Badia V, Mallozzi M, Caserta D. Myoma and myomectomy: poor evidence concern in pregnancy. J Obstet Gynaecol Res. 2017;43(12):1789–1804.
- Okin CR, Guido RS, Meyn LA, Ramanathan S. Vasopressin during abdominal hysterectomy: a randomized controlled trial. Obstet Gynecol. 2001;97(6):867–872.
- Kongnyuy EJ, van den Broek N, Wiysonge CS. A systematic review of randomized controlled trials to reduce hemorrhage during myomectomy for uterine fibroids. Int J Gynaecol Obstet. 2008;100(1):4–9.
- Barbieri RL. Give vasopressin to reduce bleeding in gynecologic surgery. OBG Manage. 2010;22(3):12–15.
- Tian YC, Long TF, Dai YN. Pregnancy outcomes following different surgical approaches of myomectomy. J Obstet Gynaecol Res. 2015;41(3):350–357.
- Bujold E, Bujold C, Hamilton EF, Harel F, Gauthier RJ. The impact of a single-layer or double-layer closure on uterine rupture. Am J Obstet Gynecol. 2002;186(6):1326–1330.
- Claeys J, Hellendoorn I, Hamerlynck T, Bosteels J, Weyers S. The risk of uterine rupture after myomectomy: a systematic review of the literature and meta-analysis. Gynecol Surg. 2014;11(3):197–206.
Uterine fibroids are the most common tumors of the uterus. Clinically significant fibroids that arise from the cervix are less common.1 Removing large cervical fibroids when a patient desires future fertility is a surgical challenge because of the risks of significant blood loss, bladder and ureteral injury, and unplanned hysterectomy. For women who desire future fertility, myomectomy can improve the chances of pregnancy by restoring normal anatomy.2 In this article, we describe a technique for myomectomy with uterine preservation in a patient with a 20-cm cervical fibroid.
CASE Woman with increasing girth and urinary symptoms is unable to conceive
A 33-year-old white woman with a history of 1 prior vaginal delivery presents with symptoms of increasing abdominal girth, intermittent urinary retention and urgency, and inability to become pregnant. She reports normal monthly menstrual periods. On pelvic examination, the ObGyn notes a large fibroid partially protruding through a dilated cervix. Abdominal examination reveals a fundal height at the level of the umbilicus.
Transvaginal ultrasonography shows a uterus that measures 4.5 x 6.1 x 13.6 cm. Arising from the posterior aspect of the uterine fundus, body, and lower uterine segment is a fibroid that measures 9.7 x 15.5 x 18.9 cm. Magnetic resonance imaging is performed and confirms a fibroid measuring 10 x 16 x 20 cm. The inferior-most aspect of the fibroid appears to be within the endometrial cavity and cervical canal. Most of the fibroid, however, is posterior to the uterus, pressing on and anteriorly displacing the endometrial cavity (FIGURE 1).

What is your surgical approach?
Comprehensive preoperative planning
In this case, the patient should receive extensive preoperative counseling about the significantly increased risk for hysterectomy with an attempted myomectomy. Prior to being scheduled for surgery, she also should have a consultation with a gynecologic oncologist. To optimize visualization during the procedure, we recommend to plan for a midline vertical skin incision. Because of the potential bleeding risks, blood products should be made available in the operating room at the time of surgery.
Techniques for surgery
Intraoperatively, a vertical midline incision exteriorizes the uterus from the peritoneal cavity. Opening of the retroperitoneal spaces allows for identification of the ureters. Perform dissection in the midline away from the ureters. Inject vasopressin (5 U) into the uterine fundus. Incise the uterine serosa over the myoma posteriorly in the midline.
Perform a myomectomy, with gentle “shelling out” of the myoma; in this way the specimen can be removed intact. Reapproximate the fibroid cavity in 3 layers with 0-Vicryl (polyglactin 910) suture in a running fashion (FIGURE 2).

Continue to: CASE Resolved
CASE Resolved
The estimated blood loss during surgery was 50 mL. Final pathology reported a 1,660-g intact myoma. The patient’s postoperative course was uncomplicated and she was discharged home on postoperative day 1.
Her postoperative evaluation was 1 month later. Her abdominal incision was well healed. Her fibroid-related symptoms had resolved, and she planned to attempt pregnancy. Cesarean delivery for future pregnancies was recommended.
Increase the chances of a good outcome
Advanced planning for attempted myomectomy of a large cervical fibroid can increase the probability of a successful outcome. We suggest the following:
Counsel the patient on risks. Our preoperative strategy includes extensive counseling on the significantly increased surgical risks and the possibility of unavoidable hysterectomy. Given the anatomic distortion with respect to the ureters, bladder, and major blood vessels, involving gynecologic oncology is beneficial to the surgery planning process.
Prepare for possible transfusion. Ensure blood products are made available in the operating room in case transfusion is needed.
Control bleeding. Randomized studies have shown that intrauterine injection of vasopressin, through its action as a vasoconstrictor, decreases surgical bleeding.3,4 While little data are available on vasopressin’s most effective dosage and dilution, 5 U at a very dilute concentration (0.1–0.2 U/mL) has been recommended.5 A midline cervical incision away from lateral structures and gentle shelling out of the cervical fibroid help to avoid intraoperative damage to the bladder, ureters, and vascular supply.
Close in multiple layers. This approach can prevent a potential space for hematoma accumulation.6 Further, a multiple-layer closure of a myometrial incision may decrease the risk for uterine rupture in subsequent pregnancies.7
Advise abstinence postsurgery. There are no consistent data to guide patient counseling regarding recommendations for the timing of conception following myomectomy. We counseled our patient to abstain from vaginal intercourse for 4 weeks, after which time she soon should attempt to conceive. Although there are no published data regarding when it is best to resume sexual relations following such a surgery, we advise a 1-month period primarily to allow healing of the skin incision. Any further delay in attempting to become pregnant may allow for the growth of additional fibroids.
Plan for future deliveries. When the myomais extensively involved, such as in this case, we recommend cesarean delivery for future pregnancies to avoid the known risk of uterine rupture.8 In general, we recommend cesarean delivery in future pregnancies if an incision larger than 50% of the myometrial thickness is made in the contractile portion of the uterus.
Final takeaway. Despite increased surgical risks, myomectomy of a large cervical fibroid is possible and can alleviate symptoms and improve future fertility.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Uterine fibroids are the most common tumors of the uterus. Clinically significant fibroids that arise from the cervix are less common.1 Removing large cervical fibroids when a patient desires future fertility is a surgical challenge because of the risks of significant blood loss, bladder and ureteral injury, and unplanned hysterectomy. For women who desire future fertility, myomectomy can improve the chances of pregnancy by restoring normal anatomy.2 In this article, we describe a technique for myomectomy with uterine preservation in a patient with a 20-cm cervical fibroid.
CASE Woman with increasing girth and urinary symptoms is unable to conceive
A 33-year-old white woman with a history of 1 prior vaginal delivery presents with symptoms of increasing abdominal girth, intermittent urinary retention and urgency, and inability to become pregnant. She reports normal monthly menstrual periods. On pelvic examination, the ObGyn notes a large fibroid partially protruding through a dilated cervix. Abdominal examination reveals a fundal height at the level of the umbilicus.
Transvaginal ultrasonography shows a uterus that measures 4.5 x 6.1 x 13.6 cm. Arising from the posterior aspect of the uterine fundus, body, and lower uterine segment is a fibroid that measures 9.7 x 15.5 x 18.9 cm. Magnetic resonance imaging is performed and confirms a fibroid measuring 10 x 16 x 20 cm. The inferior-most aspect of the fibroid appears to be within the endometrial cavity and cervical canal. Most of the fibroid, however, is posterior to the uterus, pressing on and anteriorly displacing the endometrial cavity (FIGURE 1).

What is your surgical approach?
Comprehensive preoperative planning
In this case, the patient should receive extensive preoperative counseling about the significantly increased risk for hysterectomy with an attempted myomectomy. Prior to being scheduled for surgery, she also should have a consultation with a gynecologic oncologist. To optimize visualization during the procedure, we recommend to plan for a midline vertical skin incision. Because of the potential bleeding risks, blood products should be made available in the operating room at the time of surgery.
Techniques for surgery
Intraoperatively, a vertical midline incision exteriorizes the uterus from the peritoneal cavity. Opening of the retroperitoneal spaces allows for identification of the ureters. Perform dissection in the midline away from the ureters. Inject vasopressin (5 U) into the uterine fundus. Incise the uterine serosa over the myoma posteriorly in the midline.
Perform a myomectomy, with gentle “shelling out” of the myoma; in this way the specimen can be removed intact. Reapproximate the fibroid cavity in 3 layers with 0-Vicryl (polyglactin 910) suture in a running fashion (FIGURE 2).

Continue to: CASE Resolved
CASE Resolved
The estimated blood loss during surgery was 50 mL. Final pathology reported a 1,660-g intact myoma. The patient’s postoperative course was uncomplicated and she was discharged home on postoperative day 1.
Her postoperative evaluation was 1 month later. Her abdominal incision was well healed. Her fibroid-related symptoms had resolved, and she planned to attempt pregnancy. Cesarean delivery for future pregnancies was recommended.
Increase the chances of a good outcome
Advanced planning for attempted myomectomy of a large cervical fibroid can increase the probability of a successful outcome. We suggest the following:
Counsel the patient on risks. Our preoperative strategy includes extensive counseling on the significantly increased surgical risks and the possibility of unavoidable hysterectomy. Given the anatomic distortion with respect to the ureters, bladder, and major blood vessels, involving gynecologic oncology is beneficial to the surgery planning process.
Prepare for possible transfusion. Ensure blood products are made available in the operating room in case transfusion is needed.
Control bleeding. Randomized studies have shown that intrauterine injection of vasopressin, through its action as a vasoconstrictor, decreases surgical bleeding.3,4 While little data are available on vasopressin’s most effective dosage and dilution, 5 U at a very dilute concentration (0.1–0.2 U/mL) has been recommended.5 A midline cervical incision away from lateral structures and gentle shelling out of the cervical fibroid help to avoid intraoperative damage to the bladder, ureters, and vascular supply.
Close in multiple layers. This approach can prevent a potential space for hematoma accumulation.6 Further, a multiple-layer closure of a myometrial incision may decrease the risk for uterine rupture in subsequent pregnancies.7
Advise abstinence postsurgery. There are no consistent data to guide patient counseling regarding recommendations for the timing of conception following myomectomy. We counseled our patient to abstain from vaginal intercourse for 4 weeks, after which time she soon should attempt to conceive. Although there are no published data regarding when it is best to resume sexual relations following such a surgery, we advise a 1-month period primarily to allow healing of the skin incision. Any further delay in attempting to become pregnant may allow for the growth of additional fibroids.
Plan for future deliveries. When the myomais extensively involved, such as in this case, we recommend cesarean delivery for future pregnancies to avoid the known risk of uterine rupture.8 In general, we recommend cesarean delivery in future pregnancies if an incision larger than 50% of the myometrial thickness is made in the contractile portion of the uterus.
Final takeaway. Despite increased surgical risks, myomectomy of a large cervical fibroid is possible and can alleviate symptoms and improve future fertility.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Ryan GL, Syrop CH, Van Voorhis BJ. Role, epidemiology, and natural history of benign uterine mass lesions. Clin Obstet Gynecol. 2005;48(2):312–324.
- Milazzo GN, Catalano A, Badia V, Mallozzi M, Caserta D. Myoma and myomectomy: poor evidence concern in pregnancy. J Obstet Gynaecol Res. 2017;43(12):1789–1804.
- Okin CR, Guido RS, Meyn LA, Ramanathan S. Vasopressin during abdominal hysterectomy: a randomized controlled trial. Obstet Gynecol. 2001;97(6):867–872.
- Kongnyuy EJ, van den Broek N, Wiysonge CS. A systematic review of randomized controlled trials to reduce hemorrhage during myomectomy for uterine fibroids. Int J Gynaecol Obstet. 2008;100(1):4–9.
- Barbieri RL. Give vasopressin to reduce bleeding in gynecologic surgery. OBG Manage. 2010;22(3):12–15.
- Tian YC, Long TF, Dai YN. Pregnancy outcomes following different surgical approaches of myomectomy. J Obstet Gynaecol Res. 2015;41(3):350–357.
- Bujold E, Bujold C, Hamilton EF, Harel F, Gauthier RJ. The impact of a single-layer or double-layer closure on uterine rupture. Am J Obstet Gynecol. 2002;186(6):1326–1330.
- Claeys J, Hellendoorn I, Hamerlynck T, Bosteels J, Weyers S. The risk of uterine rupture after myomectomy: a systematic review of the literature and meta-analysis. Gynecol Surg. 2014;11(3):197–206.
- Ryan GL, Syrop CH, Van Voorhis BJ. Role, epidemiology, and natural history of benign uterine mass lesions. Clin Obstet Gynecol. 2005;48(2):312–324.
- Milazzo GN, Catalano A, Badia V, Mallozzi M, Caserta D. Myoma and myomectomy: poor evidence concern in pregnancy. J Obstet Gynaecol Res. 2017;43(12):1789–1804.
- Okin CR, Guido RS, Meyn LA, Ramanathan S. Vasopressin during abdominal hysterectomy: a randomized controlled trial. Obstet Gynecol. 2001;97(6):867–872.
- Kongnyuy EJ, van den Broek N, Wiysonge CS. A systematic review of randomized controlled trials to reduce hemorrhage during myomectomy for uterine fibroids. Int J Gynaecol Obstet. 2008;100(1):4–9.
- Barbieri RL. Give vasopressin to reduce bleeding in gynecologic surgery. OBG Manage. 2010;22(3):12–15.
- Tian YC, Long TF, Dai YN. Pregnancy outcomes following different surgical approaches of myomectomy. J Obstet Gynaecol Res. 2015;41(3):350–357.
- Bujold E, Bujold C, Hamilton EF, Harel F, Gauthier RJ. The impact of a single-layer or double-layer closure on uterine rupture. Am J Obstet Gynecol. 2002;186(6):1326–1330.
- Claeys J, Hellendoorn I, Hamerlynck T, Bosteels J, Weyers S. The risk of uterine rupture after myomectomy: a systematic review of the literature and meta-analysis. Gynecol Surg. 2014;11(3):197–206.
SPRINT: Pill burden affects ability to reach systolic BP control
CHICAGO –
“Five or more medications” meant total drug burden, both for hypertension and comorbidities. “When you are treating a patient for hypertension, you care about blood pressure, but you also need to care about” what else they are on, and their total drug burden, “because it affects their ability to get to their blood pressure goal, especially if you’re targeting intensive control,” said lead investigator Catherine Derington, PharmD, a postdoctoral fellow at the University of Colorado, Aurora.
Good old-fashioned exercise and weight loss remain potent non–pill options, she noted at the joint scientific sessions of AHA Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
The take-home message is to use more combination drugs for hypertension “to get patients on fewer pills.” Also, “eliminate drugs [patients] don’t need,” said SPRINT investigator William C. Cushman, MD, professor of medicine and physiology at the University of Tennessee, Memphis, when asked what he thought of the new findings.
SPRINT [Systolic Blood Pressure Intervention Trial] found that targeting systolic blood pressures below 120 mm Hg, as compared with less than 140 mm Hg, led to lower rates of MI, stroke, heart failure, and death from any cause.
Medication burden had no effect on patients in the 140 mm Hg group; they achieved a mean systolic blood pressure (SBP) of 136 mm Hg at 1 year, whether they were on fewer than five drugs a day or more. Hitting that target took an average of 1.8 hypertension medications.
Reaching 120 mm Hg generally took one extra drug (an average of 2.8), and the overall pill burden did matter; 2,463 patients in the intensive arm on fewer than five medications dropped their mean SBP 20 mm Hg at one year, while 1,698 taking more than five had a 15–mm Hg reduction. The group with the lower pill burden had a mean SBP of 120.6 mm Hg and those taking five or more had a mean SBP of 122.5 mm Hg. It’s a small difference but likely important.
Comorbidities in SPRINT included kidney and cardiovascular disease, among others, but the trial excluded patients with diabetes. Many patients were on statins and aspirin.
Pharmacy review is especially a good idea in the elderly.
Patients on five or more medications had higher rates of significant adverse events in the post hoc analysis (about 50% versus about 30%), including hypotension, syncope, electrolyte abnormalities, and acute kidney injury, regardless if they were in the 120–mm Hg or 140–mm Hg group.
About 45% in the intensive arm reported high adherence (Morisky Medication Adherence Scale-8 score greater than 8 at 12 months); medication burden made no difference. Oddly, in the standard-treatment arm, more patients on five or more drugs reported high adherence, 44.5% versus 38.1% among patients taking fewer,
The post hoc analysis was based on pill bottle review at baseline. Medication count did not affect hypertension treatment satisfaction, which was about 84% in the intensive and 77% in the standard arm.
There was no industry funding for the work. Dr. Derington didn’t have any disclosures.
CHICAGO –
“Five or more medications” meant total drug burden, both for hypertension and comorbidities. “When you are treating a patient for hypertension, you care about blood pressure, but you also need to care about” what else they are on, and their total drug burden, “because it affects their ability to get to their blood pressure goal, especially if you’re targeting intensive control,” said lead investigator Catherine Derington, PharmD, a postdoctoral fellow at the University of Colorado, Aurora.
Good old-fashioned exercise and weight loss remain potent non–pill options, she noted at the joint scientific sessions of AHA Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
The take-home message is to use more combination drugs for hypertension “to get patients on fewer pills.” Also, “eliminate drugs [patients] don’t need,” said SPRINT investigator William C. Cushman, MD, professor of medicine and physiology at the University of Tennessee, Memphis, when asked what he thought of the new findings.
SPRINT [Systolic Blood Pressure Intervention Trial] found that targeting systolic blood pressures below 120 mm Hg, as compared with less than 140 mm Hg, led to lower rates of MI, stroke, heart failure, and death from any cause.
Medication burden had no effect on patients in the 140 mm Hg group; they achieved a mean systolic blood pressure (SBP) of 136 mm Hg at 1 year, whether they were on fewer than five drugs a day or more. Hitting that target took an average of 1.8 hypertension medications.
Reaching 120 mm Hg generally took one extra drug (an average of 2.8), and the overall pill burden did matter; 2,463 patients in the intensive arm on fewer than five medications dropped their mean SBP 20 mm Hg at one year, while 1,698 taking more than five had a 15–mm Hg reduction. The group with the lower pill burden had a mean SBP of 120.6 mm Hg and those taking five or more had a mean SBP of 122.5 mm Hg. It’s a small difference but likely important.
Comorbidities in SPRINT included kidney and cardiovascular disease, among others, but the trial excluded patients with diabetes. Many patients were on statins and aspirin.
Pharmacy review is especially a good idea in the elderly.
Patients on five or more medications had higher rates of significant adverse events in the post hoc analysis (about 50% versus about 30%), including hypotension, syncope, electrolyte abnormalities, and acute kidney injury, regardless if they were in the 120–mm Hg or 140–mm Hg group.
About 45% in the intensive arm reported high adherence (Morisky Medication Adherence Scale-8 score greater than 8 at 12 months); medication burden made no difference. Oddly, in the standard-treatment arm, more patients on five or more drugs reported high adherence, 44.5% versus 38.1% among patients taking fewer,
The post hoc analysis was based on pill bottle review at baseline. Medication count did not affect hypertension treatment satisfaction, which was about 84% in the intensive and 77% in the standard arm.
There was no industry funding for the work. Dr. Derington didn’t have any disclosures.
CHICAGO –
“Five or more medications” meant total drug burden, both for hypertension and comorbidities. “When you are treating a patient for hypertension, you care about blood pressure, but you also need to care about” what else they are on, and their total drug burden, “because it affects their ability to get to their blood pressure goal, especially if you’re targeting intensive control,” said lead investigator Catherine Derington, PharmD, a postdoctoral fellow at the University of Colorado, Aurora.
Good old-fashioned exercise and weight loss remain potent non–pill options, she noted at the joint scientific sessions of AHA Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
The take-home message is to use more combination drugs for hypertension “to get patients on fewer pills.” Also, “eliminate drugs [patients] don’t need,” said SPRINT investigator William C. Cushman, MD, professor of medicine and physiology at the University of Tennessee, Memphis, when asked what he thought of the new findings.
SPRINT [Systolic Blood Pressure Intervention Trial] found that targeting systolic blood pressures below 120 mm Hg, as compared with less than 140 mm Hg, led to lower rates of MI, stroke, heart failure, and death from any cause.
Medication burden had no effect on patients in the 140 mm Hg group; they achieved a mean systolic blood pressure (SBP) of 136 mm Hg at 1 year, whether they were on fewer than five drugs a day or more. Hitting that target took an average of 1.8 hypertension medications.
Reaching 120 mm Hg generally took one extra drug (an average of 2.8), and the overall pill burden did matter; 2,463 patients in the intensive arm on fewer than five medications dropped their mean SBP 20 mm Hg at one year, while 1,698 taking more than five had a 15–mm Hg reduction. The group with the lower pill burden had a mean SBP of 120.6 mm Hg and those taking five or more had a mean SBP of 122.5 mm Hg. It’s a small difference but likely important.
Comorbidities in SPRINT included kidney and cardiovascular disease, among others, but the trial excluded patients with diabetes. Many patients were on statins and aspirin.
Pharmacy review is especially a good idea in the elderly.
Patients on five or more medications had higher rates of significant adverse events in the post hoc analysis (about 50% versus about 30%), including hypotension, syncope, electrolyte abnormalities, and acute kidney injury, regardless if they were in the 120–mm Hg or 140–mm Hg group.
About 45% in the intensive arm reported high adherence (Morisky Medication Adherence Scale-8 score greater than 8 at 12 months); medication burden made no difference. Oddly, in the standard-treatment arm, more patients on five or more drugs reported high adherence, 44.5% versus 38.1% among patients taking fewer,
The post hoc analysis was based on pill bottle review at baseline. Medication count did not affect hypertension treatment satisfaction, which was about 84% in the intensive and 77% in the standard arm.
There was no industry funding for the work. Dr. Derington didn’t have any disclosures.
REPORTING FROM JOINT HYPERTENSION 2018
Key clinical point: Consider overall pill burden when managing hypertension.
Major finding: Patients in the SPRINT trial were less likely to meet the intensive treatment goal – systolic blood pressure below 120 mm Hg – if they were taking five or more medications a day.
Study details: Post hoc analysis of the SPRINT trial
Disclosures: There was no industry funding, and the lead investigator didn’t have any disclosures.
Source: Derington C et al. Joint Hypertension 2018, Abstract P208.
Breast cancer risk in type 2 diabetes related to adiposity
ORLANDO – findings from meta-analyses suggest.
In one meta-analysis of data from 21 prospective studies with a total of nearly 15.2 million women, 325,117 breast cancer cases, and a mean follow-up time of 8 years (nearly 33 million person-years), the risk of breast cancer was significantly greater among patients with diabetes than it was among patients without diabetes (summary relative risk, 1.11), Maria Bota reported at the annual scientific sessions of the American Diabetes Association.
However, there was substantial unexplained heterogeneity of results across the individual studies (I2 = 82%), said Ms. Bota, a faculty member at the International Prevention Research Institute, Lyon, France.
“When the analysis was restricted to the 12 studies that adjusted for [body mass index], the summary relative risk decreased to 1.09 and the heterogeneity also decreased to a moderate value of 32%; when the analysis was restricted to the 9 studies that did not adjust for BMI, the summary relative risk increased to 1.14 again, and the heterogeneity increased even more to 91%,” she said.
In an analysis that combined the results of the nine studies that did not adjust for BMI along with crude relative risks from studies that reported both crude and BMI-adjusted relative risks (17 studies in all), the SRR was 1.12, and heterogeneity among the studies was high at 84%.
Additionally, an analysis by menopausal status based on four studies that reported breast cancer in both pre- and postmenopausal women showed SRRs for breast cancer of 0.97 (a 3% decrease in risk) and 1.14 among diabetic vs. nondiabetic premenopausal women and postmenopausal women, respectively, she said, noting that heterogeneity was low (I2 = 0%) among the premenopausal breast cancer study groups and high (I2 = 70%) among the postmenopausal study groups.
The findings provide evidence for a moderately increased risk of breast cancer in women with T2DM, Ms. Bota said.
“However, the effect of the adjustment or lack of adjustment on the heterogeneity suggests that the higher risk of breast cancer in women with diabetes may not be due to diabetes itself, but to adiposity,” she said, adding that “this hypothesis is equally supported by our subgroup analysis according to menopausal status because we saw that the risk of breast cancer was only associated with diabetes in postmenopausal women and this pattern resembles the pattern of the risk of breast cancer associated with adiposity, which is also only increased in postmenopausal women.”
This study was limited by insufficient data for investigating the sources of heterogeneity, she said.
“Therefore we propose ... future pooled analyses based on individual data from good quality prospective studies in order to increase the study power and to do some detailed analysis of the links between adiposity, diabetes, and breast cancer,” she concluded, adding that new studies to examine those relationships only in premenopausal women are also needed
In a separate meta-analysis, she and her colleagues, including Peter Boyle, PhD, president of the International Prevention Research Institute, assessed the association between insulin treatment and breast cancer risk in patients with diabetes.
“The long-acting insulin analogues glargine and detemir have been shown in some studies to be associated with increased risk of breast cancer, and other studies have shown no association between the use of these two compounds and the risk of breast cancer,” Dr. Boyle said in a separate presentation at the ADA meeting. “It was important to sort out a little bit what was going on in the literature.”
Overall, the meta-analysis of data from 12 longitudinal cohort studies – including more than 6,000 cases of breast cancer – showed a slight increase in breast cancer risk in patients taking long-acting insulin (SRR, 1.13) with “a relatively reasonable” level of heterogeneity (I2 = 23%).
“But we see that the story is not that simple,” he said.
For example, some studies included only patients who were prescribed insulin for the first time after the study began (new users), some included only patients who were prescribed insulin before the study began (prevalent users), and some included both (ever users), which may have introduced bias in the results, he explained.
Studies of glargine included 4,168 breast cancer cases over a total of 1,418,743 person-years of observation, and studies of detemir included fewer than 2,047 breast cancer cases (not all studies reported case numbers). Among both new users of glargine and detemir, the SRR was 1.12, suggesting no real association between either glargine or detemir use and breast cancer, he said.
“One important take-home message is that you have to be careful that these pharmaco-epidemiological studies, even when working with the same database, may have conflicting results ... so we still need more robust standards in methods for [such] studies,” he concluded.
Ms. Bota reported having no disclosures. The study presented by Dr. Boyle was funded by Sanofi. Dr. Boyle is president of a charity that has received donations from Pfizer, Roche, Novartis, and Lilly.
SOURCE: Bota M. ADA 2018, Abstract 180-OR; Boyle P. ADA 2018, Abstract 133-OR.
ORLANDO – findings from meta-analyses suggest.
In one meta-analysis of data from 21 prospective studies with a total of nearly 15.2 million women, 325,117 breast cancer cases, and a mean follow-up time of 8 years (nearly 33 million person-years), the risk of breast cancer was significantly greater among patients with diabetes than it was among patients without diabetes (summary relative risk, 1.11), Maria Bota reported at the annual scientific sessions of the American Diabetes Association.
However, there was substantial unexplained heterogeneity of results across the individual studies (I2 = 82%), said Ms. Bota, a faculty member at the International Prevention Research Institute, Lyon, France.
“When the analysis was restricted to the 12 studies that adjusted for [body mass index], the summary relative risk decreased to 1.09 and the heterogeneity also decreased to a moderate value of 32%; when the analysis was restricted to the 9 studies that did not adjust for BMI, the summary relative risk increased to 1.14 again, and the heterogeneity increased even more to 91%,” she said.
In an analysis that combined the results of the nine studies that did not adjust for BMI along with crude relative risks from studies that reported both crude and BMI-adjusted relative risks (17 studies in all), the SRR was 1.12, and heterogeneity among the studies was high at 84%.
Additionally, an analysis by menopausal status based on four studies that reported breast cancer in both pre- and postmenopausal women showed SRRs for breast cancer of 0.97 (a 3% decrease in risk) and 1.14 among diabetic vs. nondiabetic premenopausal women and postmenopausal women, respectively, she said, noting that heterogeneity was low (I2 = 0%) among the premenopausal breast cancer study groups and high (I2 = 70%) among the postmenopausal study groups.
The findings provide evidence for a moderately increased risk of breast cancer in women with T2DM, Ms. Bota said.
“However, the effect of the adjustment or lack of adjustment on the heterogeneity suggests that the higher risk of breast cancer in women with diabetes may not be due to diabetes itself, but to adiposity,” she said, adding that “this hypothesis is equally supported by our subgroup analysis according to menopausal status because we saw that the risk of breast cancer was only associated with diabetes in postmenopausal women and this pattern resembles the pattern of the risk of breast cancer associated with adiposity, which is also only increased in postmenopausal women.”
This study was limited by insufficient data for investigating the sources of heterogeneity, she said.
“Therefore we propose ... future pooled analyses based on individual data from good quality prospective studies in order to increase the study power and to do some detailed analysis of the links between adiposity, diabetes, and breast cancer,” she concluded, adding that new studies to examine those relationships only in premenopausal women are also needed
In a separate meta-analysis, she and her colleagues, including Peter Boyle, PhD, president of the International Prevention Research Institute, assessed the association between insulin treatment and breast cancer risk in patients with diabetes.
“The long-acting insulin analogues glargine and detemir have been shown in some studies to be associated with increased risk of breast cancer, and other studies have shown no association between the use of these two compounds and the risk of breast cancer,” Dr. Boyle said in a separate presentation at the ADA meeting. “It was important to sort out a little bit what was going on in the literature.”
Overall, the meta-analysis of data from 12 longitudinal cohort studies – including more than 6,000 cases of breast cancer – showed a slight increase in breast cancer risk in patients taking long-acting insulin (SRR, 1.13) with “a relatively reasonable” level of heterogeneity (I2 = 23%).
“But we see that the story is not that simple,” he said.
For example, some studies included only patients who were prescribed insulin for the first time after the study began (new users), some included only patients who were prescribed insulin before the study began (prevalent users), and some included both (ever users), which may have introduced bias in the results, he explained.
Studies of glargine included 4,168 breast cancer cases over a total of 1,418,743 person-years of observation, and studies of detemir included fewer than 2,047 breast cancer cases (not all studies reported case numbers). Among both new users of glargine and detemir, the SRR was 1.12, suggesting no real association between either glargine or detemir use and breast cancer, he said.
“One important take-home message is that you have to be careful that these pharmaco-epidemiological studies, even when working with the same database, may have conflicting results ... so we still need more robust standards in methods for [such] studies,” he concluded.
Ms. Bota reported having no disclosures. The study presented by Dr. Boyle was funded by Sanofi. Dr. Boyle is president of a charity that has received donations from Pfizer, Roche, Novartis, and Lilly.
SOURCE: Bota M. ADA 2018, Abstract 180-OR; Boyle P. ADA 2018, Abstract 133-OR.
ORLANDO – findings from meta-analyses suggest.
In one meta-analysis of data from 21 prospective studies with a total of nearly 15.2 million women, 325,117 breast cancer cases, and a mean follow-up time of 8 years (nearly 33 million person-years), the risk of breast cancer was significantly greater among patients with diabetes than it was among patients without diabetes (summary relative risk, 1.11), Maria Bota reported at the annual scientific sessions of the American Diabetes Association.
However, there was substantial unexplained heterogeneity of results across the individual studies (I2 = 82%), said Ms. Bota, a faculty member at the International Prevention Research Institute, Lyon, France.
“When the analysis was restricted to the 12 studies that adjusted for [body mass index], the summary relative risk decreased to 1.09 and the heterogeneity also decreased to a moderate value of 32%; when the analysis was restricted to the 9 studies that did not adjust for BMI, the summary relative risk increased to 1.14 again, and the heterogeneity increased even more to 91%,” she said.
In an analysis that combined the results of the nine studies that did not adjust for BMI along with crude relative risks from studies that reported both crude and BMI-adjusted relative risks (17 studies in all), the SRR was 1.12, and heterogeneity among the studies was high at 84%.
Additionally, an analysis by menopausal status based on four studies that reported breast cancer in both pre- and postmenopausal women showed SRRs for breast cancer of 0.97 (a 3% decrease in risk) and 1.14 among diabetic vs. nondiabetic premenopausal women and postmenopausal women, respectively, she said, noting that heterogeneity was low (I2 = 0%) among the premenopausal breast cancer study groups and high (I2 = 70%) among the postmenopausal study groups.
The findings provide evidence for a moderately increased risk of breast cancer in women with T2DM, Ms. Bota said.
“However, the effect of the adjustment or lack of adjustment on the heterogeneity suggests that the higher risk of breast cancer in women with diabetes may not be due to diabetes itself, but to adiposity,” she said, adding that “this hypothesis is equally supported by our subgroup analysis according to menopausal status because we saw that the risk of breast cancer was only associated with diabetes in postmenopausal women and this pattern resembles the pattern of the risk of breast cancer associated with adiposity, which is also only increased in postmenopausal women.”
This study was limited by insufficient data for investigating the sources of heterogeneity, she said.
“Therefore we propose ... future pooled analyses based on individual data from good quality prospective studies in order to increase the study power and to do some detailed analysis of the links between adiposity, diabetes, and breast cancer,” she concluded, adding that new studies to examine those relationships only in premenopausal women are also needed
In a separate meta-analysis, she and her colleagues, including Peter Boyle, PhD, president of the International Prevention Research Institute, assessed the association between insulin treatment and breast cancer risk in patients with diabetes.
“The long-acting insulin analogues glargine and detemir have been shown in some studies to be associated with increased risk of breast cancer, and other studies have shown no association between the use of these two compounds and the risk of breast cancer,” Dr. Boyle said in a separate presentation at the ADA meeting. “It was important to sort out a little bit what was going on in the literature.”
Overall, the meta-analysis of data from 12 longitudinal cohort studies – including more than 6,000 cases of breast cancer – showed a slight increase in breast cancer risk in patients taking long-acting insulin (SRR, 1.13) with “a relatively reasonable” level of heterogeneity (I2 = 23%).
“But we see that the story is not that simple,” he said.
For example, some studies included only patients who were prescribed insulin for the first time after the study began (new users), some included only patients who were prescribed insulin before the study began (prevalent users), and some included both (ever users), which may have introduced bias in the results, he explained.
Studies of glargine included 4,168 breast cancer cases over a total of 1,418,743 person-years of observation, and studies of detemir included fewer than 2,047 breast cancer cases (not all studies reported case numbers). Among both new users of glargine and detemir, the SRR was 1.12, suggesting no real association between either glargine or detemir use and breast cancer, he said.
“One important take-home message is that you have to be careful that these pharmaco-epidemiological studies, even when working with the same database, may have conflicting results ... so we still need more robust standards in methods for [such] studies,” he concluded.
Ms. Bota reported having no disclosures. The study presented by Dr. Boyle was funded by Sanofi. Dr. Boyle is president of a charity that has received donations from Pfizer, Roche, Novartis, and Lilly.
SOURCE: Bota M. ADA 2018, Abstract 180-OR; Boyle P. ADA 2018, Abstract 133-OR.
REPORTING FROM ADA 2018
Key clinical point: Adiposity accounts for the increased risk of breast cancer among women with diabetes.
Major finding: An analysis of 12 studies that adjusted for BMI showed a summary relative risk for breast cancer of 1.09 in diabetic versus nondiabetic women, with moderate study heterogeneity.
Study details: Meta-analyses including 21 and 12 studies, respectively.
Disclosures: Ms. Bota reported having no disclosures. The study presented by Dr. Boyle was funded by Sanofi. Dr. Boyle is president of a charity that has received donations from Pfizer, Roche, Novartis, and Lilly.
Source: Bota M. ADA 2018, Abstract 180-OR; Boyle P. ADA 2018, Abstract 133-OR.
CKD children need office blood pressures below the 75th percentile
CHICAGO – according to a review of 690 pediatric patients in the Chronic Kidney Disease in Children Cohort Study.
Hypertensive children with CKD were less likely to progress to dialysis or kidney transplant or have a 30% decline in estimated glomerular filtration rate (eGFR) when kept in that range, compared with children above or below it. “Achieved office [blood pressure] between the 50th and 75th percentiles appeared to offer the greatest protection against CKD progression in this cohort,” said investigators led by Joseph Flynn, MD, chief of the nephrology division at Seattle Children’s Hospital.
“We needed to have some [evidence] on what to do based on office blood pressure,” something that had been missing in the literature until now. “I think this is going to be very impactful on the care of children with CKD. Right now, the guidelines say to keep” pressures below the 90th percentile for age and height. “The guidelines [might] need to be changed,” said Dr. Flynn, also the lead author of the American Academy of Pediatrics 2017 blood pressure guidelines for children and adolescents (Pediatrics. 2017 Aug 21. doi: 10.1542/peds.2017-1904).
There was “no evidence to go below the 50th percentile; the 50th-75th seems to be the sweet spot for office blood pressure,” he said at the joint scientific sessions of the AHA Council on Hypertension, the AHA Council on Kidney in Cardiovascular Disease, and the American Society of Hypertension.
The 476 children with nonglomerular CKD actually did worse when their blood pressures were pushed below the 50th percentile, perhaps because of renal hypoperfusion. The 476 children with glomerular CKD did no better or worse below the 50th percentile than they did in the 50th-75th.
Children at or above the 90th percentile had the highest risk of progression, with about 80% needing renal replacement therapy or having a 30% drop in eGFR at 5-8 years of follow-up. Compared with children with glomerular CKD who were in the 90th percentile, the risk hazard (RH) for progression over 3 years was 0.10-0.30 (P less than 0.001) among those children with glomerular CKD who were kept in the 50th-75th percentile. Compared with children with nonglomerular CKD who were in the 90th percentile, the RH over 8 years among those in the 50th-75th percentile was 0.48 (P less than 0.001). Risk of progression in both glomerular and nonglomerular patients in the sweet spot was less than 50% at 5-8 years of follow-up.
When glomerular and nonglomerular patients were considered together, those with pressures below the 50th percentile were less likely to progress than children with pressures between the 75th and 90th, but they were more likely to progress than the 50th-75th percentile group.
Research nurses took three blood pressures by auscultation a minute apart after 5 minutes of rest. Most of the children were treated at first with angiotensin-converting enzyme (ACE) inhibitors/angiotensin receptor blockers, per recommendations. Dihydropyridine calcium channel blockers (for example, amlodipine and nifedipine) were most likely to be used next.
It was a curious finding because dihydropyridines have been show to worsen proteinuria in adults with CKD. The team is investigating to see whether they have the same effect in children. If so, “we’ll be able to tell people to stop using them,” Dr. Flynn said.
The median age in the study was 11.3 years, and almost two-thirds of the subjects were boys. The median duration of disease was 8 years. Some children in the ongoing cohort have been followed for almost 10 years. The analysis is based on children who entered the cohort with hypertension or developed it after enrollment.
The nationwide Chronic Kidney Disease in Children Cohort Study is funded by the National Institutes of Health. Dr. Flynn is an advisor for Ultragenyx and Silvergate Pharmaceuticals.
CHICAGO – according to a review of 690 pediatric patients in the Chronic Kidney Disease in Children Cohort Study.
Hypertensive children with CKD were less likely to progress to dialysis or kidney transplant or have a 30% decline in estimated glomerular filtration rate (eGFR) when kept in that range, compared with children above or below it. “Achieved office [blood pressure] between the 50th and 75th percentiles appeared to offer the greatest protection against CKD progression in this cohort,” said investigators led by Joseph Flynn, MD, chief of the nephrology division at Seattle Children’s Hospital.
“We needed to have some [evidence] on what to do based on office blood pressure,” something that had been missing in the literature until now. “I think this is going to be very impactful on the care of children with CKD. Right now, the guidelines say to keep” pressures below the 90th percentile for age and height. “The guidelines [might] need to be changed,” said Dr. Flynn, also the lead author of the American Academy of Pediatrics 2017 blood pressure guidelines for children and adolescents (Pediatrics. 2017 Aug 21. doi: 10.1542/peds.2017-1904).
There was “no evidence to go below the 50th percentile; the 50th-75th seems to be the sweet spot for office blood pressure,” he said at the joint scientific sessions of the AHA Council on Hypertension, the AHA Council on Kidney in Cardiovascular Disease, and the American Society of Hypertension.
The 476 children with nonglomerular CKD actually did worse when their blood pressures were pushed below the 50th percentile, perhaps because of renal hypoperfusion. The 476 children with glomerular CKD did no better or worse below the 50th percentile than they did in the 50th-75th.
Children at or above the 90th percentile had the highest risk of progression, with about 80% needing renal replacement therapy or having a 30% drop in eGFR at 5-8 years of follow-up. Compared with children with glomerular CKD who were in the 90th percentile, the risk hazard (RH) for progression over 3 years was 0.10-0.30 (P less than 0.001) among those children with glomerular CKD who were kept in the 50th-75th percentile. Compared with children with nonglomerular CKD who were in the 90th percentile, the RH over 8 years among those in the 50th-75th percentile was 0.48 (P less than 0.001). Risk of progression in both glomerular and nonglomerular patients in the sweet spot was less than 50% at 5-8 years of follow-up.
When glomerular and nonglomerular patients were considered together, those with pressures below the 50th percentile were less likely to progress than children with pressures between the 75th and 90th, but they were more likely to progress than the 50th-75th percentile group.
Research nurses took three blood pressures by auscultation a minute apart after 5 minutes of rest. Most of the children were treated at first with angiotensin-converting enzyme (ACE) inhibitors/angiotensin receptor blockers, per recommendations. Dihydropyridine calcium channel blockers (for example, amlodipine and nifedipine) were most likely to be used next.
It was a curious finding because dihydropyridines have been show to worsen proteinuria in adults with CKD. The team is investigating to see whether they have the same effect in children. If so, “we’ll be able to tell people to stop using them,” Dr. Flynn said.
The median age in the study was 11.3 years, and almost two-thirds of the subjects were boys. The median duration of disease was 8 years. Some children in the ongoing cohort have been followed for almost 10 years. The analysis is based on children who entered the cohort with hypertension or developed it after enrollment.
The nationwide Chronic Kidney Disease in Children Cohort Study is funded by the National Institutes of Health. Dr. Flynn is an advisor for Ultragenyx and Silvergate Pharmaceuticals.
CHICAGO – according to a review of 690 pediatric patients in the Chronic Kidney Disease in Children Cohort Study.
Hypertensive children with CKD were less likely to progress to dialysis or kidney transplant or have a 30% decline in estimated glomerular filtration rate (eGFR) when kept in that range, compared with children above or below it. “Achieved office [blood pressure] between the 50th and 75th percentiles appeared to offer the greatest protection against CKD progression in this cohort,” said investigators led by Joseph Flynn, MD, chief of the nephrology division at Seattle Children’s Hospital.
“We needed to have some [evidence] on what to do based on office blood pressure,” something that had been missing in the literature until now. “I think this is going to be very impactful on the care of children with CKD. Right now, the guidelines say to keep” pressures below the 90th percentile for age and height. “The guidelines [might] need to be changed,” said Dr. Flynn, also the lead author of the American Academy of Pediatrics 2017 blood pressure guidelines for children and adolescents (Pediatrics. 2017 Aug 21. doi: 10.1542/peds.2017-1904).
There was “no evidence to go below the 50th percentile; the 50th-75th seems to be the sweet spot for office blood pressure,” he said at the joint scientific sessions of the AHA Council on Hypertension, the AHA Council on Kidney in Cardiovascular Disease, and the American Society of Hypertension.
The 476 children with nonglomerular CKD actually did worse when their blood pressures were pushed below the 50th percentile, perhaps because of renal hypoperfusion. The 476 children with glomerular CKD did no better or worse below the 50th percentile than they did in the 50th-75th.
Children at or above the 90th percentile had the highest risk of progression, with about 80% needing renal replacement therapy or having a 30% drop in eGFR at 5-8 years of follow-up. Compared with children with glomerular CKD who were in the 90th percentile, the risk hazard (RH) for progression over 3 years was 0.10-0.30 (P less than 0.001) among those children with glomerular CKD who were kept in the 50th-75th percentile. Compared with children with nonglomerular CKD who were in the 90th percentile, the RH over 8 years among those in the 50th-75th percentile was 0.48 (P less than 0.001). Risk of progression in both glomerular and nonglomerular patients in the sweet spot was less than 50% at 5-8 years of follow-up.
When glomerular and nonglomerular patients were considered together, those with pressures below the 50th percentile were less likely to progress than children with pressures between the 75th and 90th, but they were more likely to progress than the 50th-75th percentile group.
Research nurses took three blood pressures by auscultation a minute apart after 5 minutes of rest. Most of the children were treated at first with angiotensin-converting enzyme (ACE) inhibitors/angiotensin receptor blockers, per recommendations. Dihydropyridine calcium channel blockers (for example, amlodipine and nifedipine) were most likely to be used next.
It was a curious finding because dihydropyridines have been show to worsen proteinuria in adults with CKD. The team is investigating to see whether they have the same effect in children. If so, “we’ll be able to tell people to stop using them,” Dr. Flynn said.
The median age in the study was 11.3 years, and almost two-thirds of the subjects were boys. The median duration of disease was 8 years. Some children in the ongoing cohort have been followed for almost 10 years. The analysis is based on children who entered the cohort with hypertension or developed it after enrollment.
The nationwide Chronic Kidney Disease in Children Cohort Study is funded by the National Institutes of Health. Dr. Flynn is an advisor for Ultragenyx and Silvergate Pharmaceuticals.
REPORTING FROM JOINT HYPERTENSION 2018
Key clinical point: It’s best to aim for an office blood pressure between the 50th and 75th percentiles in children with CKD.
Major finding: The risk of CKD progression was less than 50% in children kept in that range, which was better than children both above or below it.
Study details: Review of 690 pediatric patients in the Chronic Kidney Disease in Children Cohort Study.
Disclosures: The nationwide Chronic Kidney Disease in Children Cohort Study is funded by the National Institutes of Health. Dr. Joseph Flynn is an advisor for Ultragenyx and Silvergate Pharmaceuticals.
Abortion, the travel ban, and other top Supreme Court rulings affecting your practice
The 2017−2018 term of the Supreme Court of the United States (SCOTUS) was momentous. Justice Anthony Kennedy, who had been the deciding vote in most of the 5 to 4 cases for a generation, announced his retirement as of July 31, 2018. In addition, the Court decided a number of cases of interest to ObGyns. In this article we review some of those cases, as well as consider the future of the Court without Justice Kennedy. In selecting cases, we have given special attention to those in which national medical organizations filed amicus briefs. These “amicus curiae” or “friend of the court” briefs are filed by an entity who is not party to a case but wants to provide information or views to the court.

1. Abortion rulings

The Court decided 2 abortion cases and rejected a request to hear a third.
National Institute of Family and Life Advocates v Becerra
In this case,1 the Court struck down a California law that required pregnancy crisis centers not offering abortions (generally operated by pro-life groups) to provide special notices to clients.2
At stake. These notices would inform clients that California provides free or low-cost services, including abortions, and provide a phone number to call for those services.
There were many amicus briefs filed in this case, including those by the American College of Obstetricians and Gynecologists (ACOG) and other specialty boards,3 as well as the American Association of Pro-Life Obstetricians and Gynecologists and other pro-life organizations.4 ACOG’s brief argued that the California-required notice facilitates the goal of allowing women to receive medical services without harmful delay.
Final ruling. The Court held that the law required clinics to engage in speech with which the clinics disagreed (known as “compelled speech”). It also noted that California disclosure requirements were “wildly underinclusive” because they apply only to some clinics. The majority felt that there was no strong state interest in compelling this speech because there were other alternatives for the state to provide information about the availability of abortion and other services. The Court found that the clinics were likely to succeed on the merits of their claims of a First Amendment (free speech) violation.
Right to abortion for illegal immigrants in custody
A very unusual abortion case involved “Jane Doe,” a minor who was at 8 weeks’ gestation when she illegally crossed the border into the United States.5 She was placed in a federally-funded shelter where she requested an abortion. The facility denied that request.
At stake. Legal argument ensued about releasing her to another facility for an abortion, as the argument was made that pregnant minors who are apprehended crossing into the United States illegally and placed into the custody of federal officials should have abortion access. A lower Court of Appeals ruled against the Trump Administration’s policy of denying abortions to undocumented minors in federal custody. During the process of the federal government taking the case to the Supreme Court, the attorneys for Doe moved appointments around and, without notice, the abortion was performed. Government attorneys said that Doe’s attorneys made “what appear to be material misrepresentations and omissions” designed to “thwart [the Supreme Court’s] review” of the case.5 The government requested that the Court vacate the order of the Court of Appeals so that it could not be used as precedent.
Final ruling. The Court granted the governments request to vacate the lower court’s order because the minor was no longer pregnant and the order was therefore moot. The basic issue in this case (the right of in-custody minors to access abortions) remains unresolved. It is likely to appear before the Court in the future.
Continue to: Access to medical abortions
Access to medical abortions
An Arkansas law requires that a physician administering medical abortions contract with a physician who has admitting privileges at a hospital (a “contracted physician”).
At stake. Planned Parenthood filed suit challenging the requirement as unnecessary and harmful because it would result in the closure of 2 of the 3 abortion providers in Arkansas. ACOG filed an amicus brief urging the Supreme Court to consider the case.6 (Technically this was a petition for a Writ of Certiorari, the procedure by which the Court accepts cases. It accepts only about 1% of applications.) ACOG argued that there was no medical reason for the contracted physician requirement, and noted the harm it would do to women who would not have access to abortions.
Final ruling. On May 29, 2018, the Court declined to hear the case. This case is still active in the lower courts and may eventually return to the Supreme Court.

2. The patent system

The medical profession depends on the patent system to encourage the discovery of new patents efficiently and effectively. In 2012, Congress passed the America Invents Act7 that authorizes a petition by anyone other than the patent holder to the Patent and Trademark Office (PTO) for an “inter partes review” to assess a challenge to the patent’s legitimacy. If the PTO determines that there may be merit to the claim, the Patent Trial and Appeal Board undertakes a trial-like review process that may validate, invalidate, or amend the patent. The Board’s decision is subject to appellate court review.
At stake. This term, the inter partes review was challenged as unconstitutional on technical bases.8
Final ruling. The Court rejected this claim and approved the current administrative inter partes review process. The Court determined that once the Patent Office takes a petition challenging a patent, it must decide all of the claims against the patent, not pick and choose which elements of the challenge to evaluate.9 The Court’s decision upheld patent-review reform, but will require the Patent Office to tweak its procedures.

3. The travel ban

ACOG, the American Medical Association (AMA), the Association of American Medical Colleges, and more than 30 other health care and specialty associations filed an amicus brief regarding one of the most anticipated cases of the term—the “travel ban.”10
At stake. The essential argument of these organizations was that the US health care system depends on professionals from other countries. An efficient and fair immigration program is, therefore, important to advance the nation’s “health security.” During the 2016−2017 term, the Court considered but then removed the issue from its calendar when the Trump Administration issued a revised travel ban.11
In September 2017, President Trump’s proclamation imposed a range of entry restrictions on the citizens of 8 countries, most (but not all) of which are predominantly Muslim. The government indicated that, in a study by Homeland Security and the State Department, these countries were identified as having especially deficient information-sharing practices and presented national security concerns. Trump v Hawaii12 challenged this proclamation.
Final ruling. The majority of the Court upheld the travel ban. For the 5-Justice majority led by Chief Justice Roberts, the case came down to 3 things:
- The Constitution and the laws passed by Congress of necessity give the President great authority to engage in foreign policy, including policies regarding entry into the country.
- The courts are very reluctant to get into the substance of foreign affairs—they are not equipped to know in detail what the facts are, and things change very fast.
- If courts start tinkering with foreign policy and things turn bad, it will appear that the courts are to blame and were interfering in an area about which they are not competent.
Continue to: 4. Did a credit card case add risk to health insurance markets?

It was just a credit card case, but one in which the AMA saw a real risk to regulation of the health insurance markets.
At stake. Technically, Ohio v American Express concerned a claim that American Express (AmEx) violated antitrust laws when it prohibited merchants taking its credit card from “steering” customers to cards with lower fees.13 AmEx maintained that, because credit cards were a special kind of “2-sided” market (connecting merchants on one side and customers on the other), antitrust laws should not be strictly enforced.
The AMA noticed that special rules regarding 2-sided markets might apply to health insurance, and it submitted an amicus brief14 that noted: “dominant health insurance networks … have imposed and could further impose rules or effectively erect barriers that prohibit physicians from referring patients to certain specialists, particularly out-of-network specialists, for innovative and even necessary medical tests.”14 It concluded that the antitrust rule AmEx was suggesting would make it nearly impossible to challenge these unfair provisions in health insurance arrangements.
Final ruling. The Court, however, accepted the AmEx position, making it very difficult to develop an antitrust case against 2-sided markets. It remains to be seen the degree to which the AMA concern about health insurance markets will be realized.

5. Gay wedding and a bakeshop

At stake. In Masterpiece Cakeshop v Colorado, a cakemaker declined to design a cake for a gay wedding and had been disciplined under Colorado law for discriminating against the couple based on sexual orientation.15
Final ruling. The Court, however, found that the Colorado regulators had, ironically, shown such religious animus in the way they treated the baker that the regulators themselves had discriminated on the basis of religion. As a result, the Court reversed the sanctions against the baker.
This decision was fairly narrow. It does not, for example, stand for the proposition that there may be a general religious exception to antidiscrimination laws. The question of broader religious or free-speech objections to antidiscrimination laws remains for another time.
Amicus brief. It was interesting that the American College of Pediatricians, American Association of Pro-Life Obstetricians and Gynecologists, and others, filed an amicus brief to report with concern the “demands that individual medical professionals must perform, assist with, or facilitate abortions, without regard to the teachings of their own faiths, consciences, and convictions.”16 The brief also noted that “issues in the present case implicate the fundamental rights of health care professionals, and to respectfully urge that the Court should by no means permit any weakening or qualification of well-established protections against compelled speech, and of free exercise” of religion.16
Arbitration. The Court upheld, as it has in most recent terms, another arbitration agreement.1 This case concerned an employment agreement in which employees consented to submit to arbitration rather than file lawsuits and not use class action claims.
Search of cell-phone location. Cell phones, whenever turned on, connect with cell towers that record the phone’s location several times a minute. Cell companies store this information, creating a virtual map of where the owner is at all times. The Federal Bureau of Investigation asked a cell company for location information for several people during a 127-day period in which robberies were committed.2 The Court held that the search was illegal in the absence of a warrant.
Public employee unions. The Court held that agency (fair share) fees, in which public employees who are not union members can be required to pay dues for the bargaining and grievance activities (from which they generally benefit), violate the First Amendment. The majority held that forcing public employees to pay fees to unions requires the employees, through those fees, to engage in political activities with which they disagree.3 This is a form of compelled speech, which the Court found violates the First Amendment. Health care professionals who are public employees in positions that have union representation will probably have the opportunity to opt out of agency agreements.
Internet sales tax. The Court permitted states to charge sales tax on out-of-state Internet purchases.4 In doing so, a state may require out-of-state companies to collect taxes on sales to its residents.
References
- Epic Systems Corp. v Lewis, 584 US 16 285 (2018).
- Carpenter v United States, 585 US 16 402 (2018).
- Janus v State, County, and Municipal Employees, 585 US 16 1466 (2018).
- South Dakota v Wayfair, Inc, 585 US 17 494 (2018).
Clues to the future
During the term that ran from October 2, 2017, through June 27, 2018, the Court issued 72 decisions. An unusually high proportion of cases (26%; 19 cases) were decided on a 5 to 4 vote. Last term, the rate of 5 to 4 decisions was 10%; the 6-year average was 18%. The unanimous decision rate was 39% this term, compared with 59% last term, and 50% on average.
The rate of 5 to 4 cases provides a clue about the Court’s general direction. The number of times each Justice was in the majority in those nineteen 5 to 4 decisions included: Chief Justice Roberts, 17; and Justices Kennedy, 16; Gorsuch, 16; Thomas, 15; and Alito, 15; compared with Justices Ginsburg, 5; Breyer, 4; Sotomayor, 4; and Kagan, 3.
The Court convened on October 1, 2018. At this writing, whether the new term starts with 8 or 9 justices remains a question. President Trump nominated Brett Kavanaugh, JD, to take Justice Kennedy’s place on the Court. His professional qualifications and experience appear to make him qualified for a position on the Court, but as we have seen, there are many other elements that go into confirming a Justice’s nomination.
Justice Anthony Kennedy was the deciding vote in the overwhelming majority of the 5 to 4 decisions in 20 of his 30 years on the Court. The areas in which he had an especially important impact include1:
- Gay rights. Justice Kennedy wrote the opinions (usually 5 to 4 decisions) in a number of groundbreaking gay-rights cases, including decriminalizing homosexual conduct, striking down the Defense of Marriage Act, and finding that the Constitution requires states to recognize gay marriage.
- The death penalty. Justice Kennedy wrote decisions that prohibited states from imposing the death penalty for any crime other than murder, for defendants who were under 18 when they committed the crime, and for defendants with serious developmental disabilities. He expressed reservations about long-term solitary confinement, but did not have a case that allowed him to decide its constitutionality.
- The First Amendment. Early in his service on the Court, he held that the First Amendment protected flag burning as a form of speech. He decided many important freespeech and freedom-of-religion cases that have set a standard for protecting those fundamental freedoms.
- Use of health and social science data. Justice Kennedy was more open to mental health information and cited it more often than most other Justices.
- Abortion rights? Many commentators would add protecting the right to choose to have an abortion to the above list. Justice Kennedy was a central figure in one case that declined to back away from Roe v Wade, and joined a more recent decision that struck down a Texas law that created an undue burden on women seeking abortion. Plus, he also voted to uphold abortion restrictions, such as “partial-birth-abortion laws.” So there is a good argument for including abortion rights on the list, although he did not break new ground.
Justice Kennedy as a person
Outside the courtroom, Justice Kennedy is a person of great warmth and compassion. He is a natural teacher and spends a great deal of time with students. When asked how he would like to be remembered, Justice Kennedy once replied, “Somebody who’s decent, and honest, and fair, and who’s absolutely committed to the proposition that freedom is America’s gift to the rest of the world.” I agree with that assessment.
STEVEN R. SMITH, MS, JD
Reference
- South Dakota v Wayfair, Inc, 585 US (2018)
Next term, the Court is scheduled to hear cases regarding pharmaceutical liability, double jeopardy, sex-offender registration, expert witnesses, Social Security disability benefits, and the Age Discrimination in Employment Act. There will be at least 3 arbitration cases. Health care and reproductive rights will continue to be an important part of the Court’s docket.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- National Institute of Family and Life Advocates v Becerra, 585 US 16 1140 (2018).
- California Reproductive Freedom, Accountability, Comprehensive Care, and Transparency Act (FACT Act), Cal. Health & Safety Code Ann. §123470 et seq. (West 2018).
- Brief amici curiae of American Academy of Pediatrics, et al. in National Institute of Family and Life Advocates v Becerra, February 27, 2018.
- Brief amici curiae of American Association of Pro-Life Obstetricians & Gynecologists, et al. in National Institute of Family and Life Advocates v Becerra, January 16, 2018.
- Azar v Garza, 584 US 17 654 (2018).
- Brief amici curiae of American College of Obstetricians and Gynecologists and American Public Health Association in Planned Parenthood of Arkansas and Eastern Oklahoma v Jegley, February 1, 2018.
- Chapter 31, Inter Partes Review. United States Code. Title 35: Patents. Part III, Patents and protection of patents. 2012 Ed. 35 USC 311–319.
- Oil States Energy Services, LLC v Greene’s Energy Group, LLC, 584 US 16 712 (2018).
- SAS Institute Inc. v Iancu, 584 US 16 969 (2018).
- Brief for Association of American Medical Colleges and Others as Amici Curiae Supporting Respondents, Trump v Hawaii. https://www.supremecourt.gov/Docket PDF/17/ 17-965/40128/20180327105855912_17-965%20Amicus%20Br.%20Proclamation.pdf. Accessed September 21, 2018.
- Smith SR, Sanfilippo JS. Supreme Court decisions in 2017 that affected your practice. OBG Manag. 2017;29(12)44–47.
- Trump v Hawaii, 585 US 17 965 (2018).
- Ohio v American Express Co, 585 US 16 1454 (2018).
- Brief amici curiae of American Medical Association and Ohio State Medical Association in Ohio v American Express, December 24, 2017.
- Masterpiece Cakeshop, Ltd. v Colorado Civil Rights Commission, 584 US 16 111 (2018).
- Brief amici curiae of American College of Pediatricians, et al. in Masterpiece Cakeshop v Colorado Civil Rights Commission, September 7, 2017.
The 2017−2018 term of the Supreme Court of the United States (SCOTUS) was momentous. Justice Anthony Kennedy, who had been the deciding vote in most of the 5 to 4 cases for a generation, announced his retirement as of July 31, 2018. In addition, the Court decided a number of cases of interest to ObGyns. In this article we review some of those cases, as well as consider the future of the Court without Justice Kennedy. In selecting cases, we have given special attention to those in which national medical organizations filed amicus briefs. These “amicus curiae” or “friend of the court” briefs are filed by an entity who is not party to a case but wants to provide information or views to the court.

1. Abortion rulings

The Court decided 2 abortion cases and rejected a request to hear a third.
National Institute of Family and Life Advocates v Becerra
In this case,1 the Court struck down a California law that required pregnancy crisis centers not offering abortions (generally operated by pro-life groups) to provide special notices to clients.2
At stake. These notices would inform clients that California provides free or low-cost services, including abortions, and provide a phone number to call for those services.
There were many amicus briefs filed in this case, including those by the American College of Obstetricians and Gynecologists (ACOG) and other specialty boards,3 as well as the American Association of Pro-Life Obstetricians and Gynecologists and other pro-life organizations.4 ACOG’s brief argued that the California-required notice facilitates the goal of allowing women to receive medical services without harmful delay.
Final ruling. The Court held that the law required clinics to engage in speech with which the clinics disagreed (known as “compelled speech”). It also noted that California disclosure requirements were “wildly underinclusive” because they apply only to some clinics. The majority felt that there was no strong state interest in compelling this speech because there were other alternatives for the state to provide information about the availability of abortion and other services. The Court found that the clinics were likely to succeed on the merits of their claims of a First Amendment (free speech) violation.
Right to abortion for illegal immigrants in custody
A very unusual abortion case involved “Jane Doe,” a minor who was at 8 weeks’ gestation when she illegally crossed the border into the United States.5 She was placed in a federally-funded shelter where she requested an abortion. The facility denied that request.
At stake. Legal argument ensued about releasing her to another facility for an abortion, as the argument was made that pregnant minors who are apprehended crossing into the United States illegally and placed into the custody of federal officials should have abortion access. A lower Court of Appeals ruled against the Trump Administration’s policy of denying abortions to undocumented minors in federal custody. During the process of the federal government taking the case to the Supreme Court, the attorneys for Doe moved appointments around and, without notice, the abortion was performed. Government attorneys said that Doe’s attorneys made “what appear to be material misrepresentations and omissions” designed to “thwart [the Supreme Court’s] review” of the case.5 The government requested that the Court vacate the order of the Court of Appeals so that it could not be used as precedent.
Final ruling. The Court granted the governments request to vacate the lower court’s order because the minor was no longer pregnant and the order was therefore moot. The basic issue in this case (the right of in-custody minors to access abortions) remains unresolved. It is likely to appear before the Court in the future.
Continue to: Access to medical abortions
Access to medical abortions
An Arkansas law requires that a physician administering medical abortions contract with a physician who has admitting privileges at a hospital (a “contracted physician”).
At stake. Planned Parenthood filed suit challenging the requirement as unnecessary and harmful because it would result in the closure of 2 of the 3 abortion providers in Arkansas. ACOG filed an amicus brief urging the Supreme Court to consider the case.6 (Technically this was a petition for a Writ of Certiorari, the procedure by which the Court accepts cases. It accepts only about 1% of applications.) ACOG argued that there was no medical reason for the contracted physician requirement, and noted the harm it would do to women who would not have access to abortions.
Final ruling. On May 29, 2018, the Court declined to hear the case. This case is still active in the lower courts and may eventually return to the Supreme Court.

2. The patent system

The medical profession depends on the patent system to encourage the discovery of new patents efficiently and effectively. In 2012, Congress passed the America Invents Act7 that authorizes a petition by anyone other than the patent holder to the Patent and Trademark Office (PTO) for an “inter partes review” to assess a challenge to the patent’s legitimacy. If the PTO determines that there may be merit to the claim, the Patent Trial and Appeal Board undertakes a trial-like review process that may validate, invalidate, or amend the patent. The Board’s decision is subject to appellate court review.
At stake. This term, the inter partes review was challenged as unconstitutional on technical bases.8
Final ruling. The Court rejected this claim and approved the current administrative inter partes review process. The Court determined that once the Patent Office takes a petition challenging a patent, it must decide all of the claims against the patent, not pick and choose which elements of the challenge to evaluate.9 The Court’s decision upheld patent-review reform, but will require the Patent Office to tweak its procedures.

3. The travel ban

ACOG, the American Medical Association (AMA), the Association of American Medical Colleges, and more than 30 other health care and specialty associations filed an amicus brief regarding one of the most anticipated cases of the term—the “travel ban.”10
At stake. The essential argument of these organizations was that the US health care system depends on professionals from other countries. An efficient and fair immigration program is, therefore, important to advance the nation’s “health security.” During the 2016−2017 term, the Court considered but then removed the issue from its calendar when the Trump Administration issued a revised travel ban.11
In September 2017, President Trump’s proclamation imposed a range of entry restrictions on the citizens of 8 countries, most (but not all) of which are predominantly Muslim. The government indicated that, in a study by Homeland Security and the State Department, these countries were identified as having especially deficient information-sharing practices and presented national security concerns. Trump v Hawaii12 challenged this proclamation.
Final ruling. The majority of the Court upheld the travel ban. For the 5-Justice majority led by Chief Justice Roberts, the case came down to 3 things:
- The Constitution and the laws passed by Congress of necessity give the President great authority to engage in foreign policy, including policies regarding entry into the country.
- The courts are very reluctant to get into the substance of foreign affairs—they are not equipped to know in detail what the facts are, and things change very fast.
- If courts start tinkering with foreign policy and things turn bad, it will appear that the courts are to blame and were interfering in an area about which they are not competent.
Continue to: 4. Did a credit card case add risk to health insurance markets?

It was just a credit card case, but one in which the AMA saw a real risk to regulation of the health insurance markets.
At stake. Technically, Ohio v American Express concerned a claim that American Express (AmEx) violated antitrust laws when it prohibited merchants taking its credit card from “steering” customers to cards with lower fees.13 AmEx maintained that, because credit cards were a special kind of “2-sided” market (connecting merchants on one side and customers on the other), antitrust laws should not be strictly enforced.
The AMA noticed that special rules regarding 2-sided markets might apply to health insurance, and it submitted an amicus brief14 that noted: “dominant health insurance networks … have imposed and could further impose rules or effectively erect barriers that prohibit physicians from referring patients to certain specialists, particularly out-of-network specialists, for innovative and even necessary medical tests.”14 It concluded that the antitrust rule AmEx was suggesting would make it nearly impossible to challenge these unfair provisions in health insurance arrangements.
Final ruling. The Court, however, accepted the AmEx position, making it very difficult to develop an antitrust case against 2-sided markets. It remains to be seen the degree to which the AMA concern about health insurance markets will be realized.

5. Gay wedding and a bakeshop

At stake. In Masterpiece Cakeshop v Colorado, a cakemaker declined to design a cake for a gay wedding and had been disciplined under Colorado law for discriminating against the couple based on sexual orientation.15
Final ruling. The Court, however, found that the Colorado regulators had, ironically, shown such religious animus in the way they treated the baker that the regulators themselves had discriminated on the basis of religion. As a result, the Court reversed the sanctions against the baker.
This decision was fairly narrow. It does not, for example, stand for the proposition that there may be a general religious exception to antidiscrimination laws. The question of broader religious or free-speech objections to antidiscrimination laws remains for another time.
Amicus brief. It was interesting that the American College of Pediatricians, American Association of Pro-Life Obstetricians and Gynecologists, and others, filed an amicus brief to report with concern the “demands that individual medical professionals must perform, assist with, or facilitate abortions, without regard to the teachings of their own faiths, consciences, and convictions.”16 The brief also noted that “issues in the present case implicate the fundamental rights of health care professionals, and to respectfully urge that the Court should by no means permit any weakening or qualification of well-established protections against compelled speech, and of free exercise” of religion.16
Arbitration. The Court upheld, as it has in most recent terms, another arbitration agreement.1 This case concerned an employment agreement in which employees consented to submit to arbitration rather than file lawsuits and not use class action claims.
Search of cell-phone location. Cell phones, whenever turned on, connect with cell towers that record the phone’s location several times a minute. Cell companies store this information, creating a virtual map of where the owner is at all times. The Federal Bureau of Investigation asked a cell company for location information for several people during a 127-day period in which robberies were committed.2 The Court held that the search was illegal in the absence of a warrant.
Public employee unions. The Court held that agency (fair share) fees, in which public employees who are not union members can be required to pay dues for the bargaining and grievance activities (from which they generally benefit), violate the First Amendment. The majority held that forcing public employees to pay fees to unions requires the employees, through those fees, to engage in political activities with which they disagree.3 This is a form of compelled speech, which the Court found violates the First Amendment. Health care professionals who are public employees in positions that have union representation will probably have the opportunity to opt out of agency agreements.
Internet sales tax. The Court permitted states to charge sales tax on out-of-state Internet purchases.4 In doing so, a state may require out-of-state companies to collect taxes on sales to its residents.
References
- Epic Systems Corp. v Lewis, 584 US 16 285 (2018).
- Carpenter v United States, 585 US 16 402 (2018).
- Janus v State, County, and Municipal Employees, 585 US 16 1466 (2018).
- South Dakota v Wayfair, Inc, 585 US 17 494 (2018).
Clues to the future
During the term that ran from October 2, 2017, through June 27, 2018, the Court issued 72 decisions. An unusually high proportion of cases (26%; 19 cases) were decided on a 5 to 4 vote. Last term, the rate of 5 to 4 decisions was 10%; the 6-year average was 18%. The unanimous decision rate was 39% this term, compared with 59% last term, and 50% on average.
The rate of 5 to 4 cases provides a clue about the Court’s general direction. The number of times each Justice was in the majority in those nineteen 5 to 4 decisions included: Chief Justice Roberts, 17; and Justices Kennedy, 16; Gorsuch, 16; Thomas, 15; and Alito, 15; compared with Justices Ginsburg, 5; Breyer, 4; Sotomayor, 4; and Kagan, 3.
The Court convened on October 1, 2018. At this writing, whether the new term starts with 8 or 9 justices remains a question. President Trump nominated Brett Kavanaugh, JD, to take Justice Kennedy’s place on the Court. His professional qualifications and experience appear to make him qualified for a position on the Court, but as we have seen, there are many other elements that go into confirming a Justice’s nomination.
Justice Anthony Kennedy was the deciding vote in the overwhelming majority of the 5 to 4 decisions in 20 of his 30 years on the Court. The areas in which he had an especially important impact include1:
- Gay rights. Justice Kennedy wrote the opinions (usually 5 to 4 decisions) in a number of groundbreaking gay-rights cases, including decriminalizing homosexual conduct, striking down the Defense of Marriage Act, and finding that the Constitution requires states to recognize gay marriage.
- The death penalty. Justice Kennedy wrote decisions that prohibited states from imposing the death penalty for any crime other than murder, for defendants who were under 18 when they committed the crime, and for defendants with serious developmental disabilities. He expressed reservations about long-term solitary confinement, but did not have a case that allowed him to decide its constitutionality.
- The First Amendment. Early in his service on the Court, he held that the First Amendment protected flag burning as a form of speech. He decided many important freespeech and freedom-of-religion cases that have set a standard for protecting those fundamental freedoms.
- Use of health and social science data. Justice Kennedy was more open to mental health information and cited it more often than most other Justices.
- Abortion rights? Many commentators would add protecting the right to choose to have an abortion to the above list. Justice Kennedy was a central figure in one case that declined to back away from Roe v Wade, and joined a more recent decision that struck down a Texas law that created an undue burden on women seeking abortion. Plus, he also voted to uphold abortion restrictions, such as “partial-birth-abortion laws.” So there is a good argument for including abortion rights on the list, although he did not break new ground.
Justice Kennedy as a person
Outside the courtroom, Justice Kennedy is a person of great warmth and compassion. He is a natural teacher and spends a great deal of time with students. When asked how he would like to be remembered, Justice Kennedy once replied, “Somebody who’s decent, and honest, and fair, and who’s absolutely committed to the proposition that freedom is America’s gift to the rest of the world.” I agree with that assessment.
STEVEN R. SMITH, MS, JD
Reference
- South Dakota v Wayfair, Inc, 585 US (2018)
Next term, the Court is scheduled to hear cases regarding pharmaceutical liability, double jeopardy, sex-offender registration, expert witnesses, Social Security disability benefits, and the Age Discrimination in Employment Act. There will be at least 3 arbitration cases. Health care and reproductive rights will continue to be an important part of the Court’s docket.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The 2017−2018 term of the Supreme Court of the United States (SCOTUS) was momentous. Justice Anthony Kennedy, who had been the deciding vote in most of the 5 to 4 cases for a generation, announced his retirement as of July 31, 2018. In addition, the Court decided a number of cases of interest to ObGyns. In this article we review some of those cases, as well as consider the future of the Court without Justice Kennedy. In selecting cases, we have given special attention to those in which national medical organizations filed amicus briefs. These “amicus curiae” or “friend of the court” briefs are filed by an entity who is not party to a case but wants to provide information or views to the court.

1. Abortion rulings

The Court decided 2 abortion cases and rejected a request to hear a third.
National Institute of Family and Life Advocates v Becerra
In this case,1 the Court struck down a California law that required pregnancy crisis centers not offering abortions (generally operated by pro-life groups) to provide special notices to clients.2
At stake. These notices would inform clients that California provides free or low-cost services, including abortions, and provide a phone number to call for those services.
There were many amicus briefs filed in this case, including those by the American College of Obstetricians and Gynecologists (ACOG) and other specialty boards,3 as well as the American Association of Pro-Life Obstetricians and Gynecologists and other pro-life organizations.4 ACOG’s brief argued that the California-required notice facilitates the goal of allowing women to receive medical services without harmful delay.
Final ruling. The Court held that the law required clinics to engage in speech with which the clinics disagreed (known as “compelled speech”). It also noted that California disclosure requirements were “wildly underinclusive” because they apply only to some clinics. The majority felt that there was no strong state interest in compelling this speech because there were other alternatives for the state to provide information about the availability of abortion and other services. The Court found that the clinics were likely to succeed on the merits of their claims of a First Amendment (free speech) violation.
Right to abortion for illegal immigrants in custody
A very unusual abortion case involved “Jane Doe,” a minor who was at 8 weeks’ gestation when she illegally crossed the border into the United States.5 She was placed in a federally-funded shelter where she requested an abortion. The facility denied that request.
At stake. Legal argument ensued about releasing her to another facility for an abortion, as the argument was made that pregnant minors who are apprehended crossing into the United States illegally and placed into the custody of federal officials should have abortion access. A lower Court of Appeals ruled against the Trump Administration’s policy of denying abortions to undocumented minors in federal custody. During the process of the federal government taking the case to the Supreme Court, the attorneys for Doe moved appointments around and, without notice, the abortion was performed. Government attorneys said that Doe’s attorneys made “what appear to be material misrepresentations and omissions” designed to “thwart [the Supreme Court’s] review” of the case.5 The government requested that the Court vacate the order of the Court of Appeals so that it could not be used as precedent.
Final ruling. The Court granted the governments request to vacate the lower court’s order because the minor was no longer pregnant and the order was therefore moot. The basic issue in this case (the right of in-custody minors to access abortions) remains unresolved. It is likely to appear before the Court in the future.
Continue to: Access to medical abortions
Access to medical abortions
An Arkansas law requires that a physician administering medical abortions contract with a physician who has admitting privileges at a hospital (a “contracted physician”).
At stake. Planned Parenthood filed suit challenging the requirement as unnecessary and harmful because it would result in the closure of 2 of the 3 abortion providers in Arkansas. ACOG filed an amicus brief urging the Supreme Court to consider the case.6 (Technically this was a petition for a Writ of Certiorari, the procedure by which the Court accepts cases. It accepts only about 1% of applications.) ACOG argued that there was no medical reason for the contracted physician requirement, and noted the harm it would do to women who would not have access to abortions.
Final ruling. On May 29, 2018, the Court declined to hear the case. This case is still active in the lower courts and may eventually return to the Supreme Court.

2. The patent system

The medical profession depends on the patent system to encourage the discovery of new patents efficiently and effectively. In 2012, Congress passed the America Invents Act7 that authorizes a petition by anyone other than the patent holder to the Patent and Trademark Office (PTO) for an “inter partes review” to assess a challenge to the patent’s legitimacy. If the PTO determines that there may be merit to the claim, the Patent Trial and Appeal Board undertakes a trial-like review process that may validate, invalidate, or amend the patent. The Board’s decision is subject to appellate court review.
At stake. This term, the inter partes review was challenged as unconstitutional on technical bases.8
Final ruling. The Court rejected this claim and approved the current administrative inter partes review process. The Court determined that once the Patent Office takes a petition challenging a patent, it must decide all of the claims against the patent, not pick and choose which elements of the challenge to evaluate.9 The Court’s decision upheld patent-review reform, but will require the Patent Office to tweak its procedures.

3. The travel ban

ACOG, the American Medical Association (AMA), the Association of American Medical Colleges, and more than 30 other health care and specialty associations filed an amicus brief regarding one of the most anticipated cases of the term—the “travel ban.”10
At stake. The essential argument of these organizations was that the US health care system depends on professionals from other countries. An efficient and fair immigration program is, therefore, important to advance the nation’s “health security.” During the 2016−2017 term, the Court considered but then removed the issue from its calendar when the Trump Administration issued a revised travel ban.11
In September 2017, President Trump’s proclamation imposed a range of entry restrictions on the citizens of 8 countries, most (but not all) of which are predominantly Muslim. The government indicated that, in a study by Homeland Security and the State Department, these countries were identified as having especially deficient information-sharing practices and presented national security concerns. Trump v Hawaii12 challenged this proclamation.
Final ruling. The majority of the Court upheld the travel ban. For the 5-Justice majority led by Chief Justice Roberts, the case came down to 3 things:
- The Constitution and the laws passed by Congress of necessity give the President great authority to engage in foreign policy, including policies regarding entry into the country.
- The courts are very reluctant to get into the substance of foreign affairs—they are not equipped to know in detail what the facts are, and things change very fast.
- If courts start tinkering with foreign policy and things turn bad, it will appear that the courts are to blame and were interfering in an area about which they are not competent.
Continue to: 4. Did a credit card case add risk to health insurance markets?

It was just a credit card case, but one in which the AMA saw a real risk to regulation of the health insurance markets.
At stake. Technically, Ohio v American Express concerned a claim that American Express (AmEx) violated antitrust laws when it prohibited merchants taking its credit card from “steering” customers to cards with lower fees.13 AmEx maintained that, because credit cards were a special kind of “2-sided” market (connecting merchants on one side and customers on the other), antitrust laws should not be strictly enforced.
The AMA noticed that special rules regarding 2-sided markets might apply to health insurance, and it submitted an amicus brief14 that noted: “dominant health insurance networks … have imposed and could further impose rules or effectively erect barriers that prohibit physicians from referring patients to certain specialists, particularly out-of-network specialists, for innovative and even necessary medical tests.”14 It concluded that the antitrust rule AmEx was suggesting would make it nearly impossible to challenge these unfair provisions in health insurance arrangements.
Final ruling. The Court, however, accepted the AmEx position, making it very difficult to develop an antitrust case against 2-sided markets. It remains to be seen the degree to which the AMA concern about health insurance markets will be realized.

5. Gay wedding and a bakeshop

At stake. In Masterpiece Cakeshop v Colorado, a cakemaker declined to design a cake for a gay wedding and had been disciplined under Colorado law for discriminating against the couple based on sexual orientation.15
Final ruling. The Court, however, found that the Colorado regulators had, ironically, shown such religious animus in the way they treated the baker that the regulators themselves had discriminated on the basis of religion. As a result, the Court reversed the sanctions against the baker.
This decision was fairly narrow. It does not, for example, stand for the proposition that there may be a general religious exception to antidiscrimination laws. The question of broader religious or free-speech objections to antidiscrimination laws remains for another time.
Amicus brief. It was interesting that the American College of Pediatricians, American Association of Pro-Life Obstetricians and Gynecologists, and others, filed an amicus brief to report with concern the “demands that individual medical professionals must perform, assist with, or facilitate abortions, without regard to the teachings of their own faiths, consciences, and convictions.”16 The brief also noted that “issues in the present case implicate the fundamental rights of health care professionals, and to respectfully urge that the Court should by no means permit any weakening or qualification of well-established protections against compelled speech, and of free exercise” of religion.16
Arbitration. The Court upheld, as it has in most recent terms, another arbitration agreement.1 This case concerned an employment agreement in which employees consented to submit to arbitration rather than file lawsuits and not use class action claims.
Search of cell-phone location. Cell phones, whenever turned on, connect with cell towers that record the phone’s location several times a minute. Cell companies store this information, creating a virtual map of where the owner is at all times. The Federal Bureau of Investigation asked a cell company for location information for several people during a 127-day period in which robberies were committed.2 The Court held that the search was illegal in the absence of a warrant.
Public employee unions. The Court held that agency (fair share) fees, in which public employees who are not union members can be required to pay dues for the bargaining and grievance activities (from which they generally benefit), violate the First Amendment. The majority held that forcing public employees to pay fees to unions requires the employees, through those fees, to engage in political activities with which they disagree.3 This is a form of compelled speech, which the Court found violates the First Amendment. Health care professionals who are public employees in positions that have union representation will probably have the opportunity to opt out of agency agreements.
Internet sales tax. The Court permitted states to charge sales tax on out-of-state Internet purchases.4 In doing so, a state may require out-of-state companies to collect taxes on sales to its residents.
References
- Epic Systems Corp. v Lewis, 584 US 16 285 (2018).
- Carpenter v United States, 585 US 16 402 (2018).
- Janus v State, County, and Municipal Employees, 585 US 16 1466 (2018).
- South Dakota v Wayfair, Inc, 585 US 17 494 (2018).
Clues to the future
During the term that ran from October 2, 2017, through June 27, 2018, the Court issued 72 decisions. An unusually high proportion of cases (26%; 19 cases) were decided on a 5 to 4 vote. Last term, the rate of 5 to 4 decisions was 10%; the 6-year average was 18%. The unanimous decision rate was 39% this term, compared with 59% last term, and 50% on average.
The rate of 5 to 4 cases provides a clue about the Court’s general direction. The number of times each Justice was in the majority in those nineteen 5 to 4 decisions included: Chief Justice Roberts, 17; and Justices Kennedy, 16; Gorsuch, 16; Thomas, 15; and Alito, 15; compared with Justices Ginsburg, 5; Breyer, 4; Sotomayor, 4; and Kagan, 3.
The Court convened on October 1, 2018. At this writing, whether the new term starts with 8 or 9 justices remains a question. President Trump nominated Brett Kavanaugh, JD, to take Justice Kennedy’s place on the Court. His professional qualifications and experience appear to make him qualified for a position on the Court, but as we have seen, there are many other elements that go into confirming a Justice’s nomination.
Justice Anthony Kennedy was the deciding vote in the overwhelming majority of the 5 to 4 decisions in 20 of his 30 years on the Court. The areas in which he had an especially important impact include1:
- Gay rights. Justice Kennedy wrote the opinions (usually 5 to 4 decisions) in a number of groundbreaking gay-rights cases, including decriminalizing homosexual conduct, striking down the Defense of Marriage Act, and finding that the Constitution requires states to recognize gay marriage.
- The death penalty. Justice Kennedy wrote decisions that prohibited states from imposing the death penalty for any crime other than murder, for defendants who were under 18 when they committed the crime, and for defendants with serious developmental disabilities. He expressed reservations about long-term solitary confinement, but did not have a case that allowed him to decide its constitutionality.
- The First Amendment. Early in his service on the Court, he held that the First Amendment protected flag burning as a form of speech. He decided many important freespeech and freedom-of-religion cases that have set a standard for protecting those fundamental freedoms.
- Use of health and social science data. Justice Kennedy was more open to mental health information and cited it more often than most other Justices.
- Abortion rights? Many commentators would add protecting the right to choose to have an abortion to the above list. Justice Kennedy was a central figure in one case that declined to back away from Roe v Wade, and joined a more recent decision that struck down a Texas law that created an undue burden on women seeking abortion. Plus, he also voted to uphold abortion restrictions, such as “partial-birth-abortion laws.” So there is a good argument for including abortion rights on the list, although he did not break new ground.
Justice Kennedy as a person
Outside the courtroom, Justice Kennedy is a person of great warmth and compassion. He is a natural teacher and spends a great deal of time with students. When asked how he would like to be remembered, Justice Kennedy once replied, “Somebody who’s decent, and honest, and fair, and who’s absolutely committed to the proposition that freedom is America’s gift to the rest of the world.” I agree with that assessment.
STEVEN R. SMITH, MS, JD
Reference
- South Dakota v Wayfair, Inc, 585 US (2018)
Next term, the Court is scheduled to hear cases regarding pharmaceutical liability, double jeopardy, sex-offender registration, expert witnesses, Social Security disability benefits, and the Age Discrimination in Employment Act. There will be at least 3 arbitration cases. Health care and reproductive rights will continue to be an important part of the Court’s docket.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- National Institute of Family and Life Advocates v Becerra, 585 US 16 1140 (2018).
- California Reproductive Freedom, Accountability, Comprehensive Care, and Transparency Act (FACT Act), Cal. Health & Safety Code Ann. §123470 et seq. (West 2018).
- Brief amici curiae of American Academy of Pediatrics, et al. in National Institute of Family and Life Advocates v Becerra, February 27, 2018.
- Brief amici curiae of American Association of Pro-Life Obstetricians & Gynecologists, et al. in National Institute of Family and Life Advocates v Becerra, January 16, 2018.
- Azar v Garza, 584 US 17 654 (2018).
- Brief amici curiae of American College of Obstetricians and Gynecologists and American Public Health Association in Planned Parenthood of Arkansas and Eastern Oklahoma v Jegley, February 1, 2018.
- Chapter 31, Inter Partes Review. United States Code. Title 35: Patents. Part III, Patents and protection of patents. 2012 Ed. 35 USC 311–319.
- Oil States Energy Services, LLC v Greene’s Energy Group, LLC, 584 US 16 712 (2018).
- SAS Institute Inc. v Iancu, 584 US 16 969 (2018).
- Brief for Association of American Medical Colleges and Others as Amici Curiae Supporting Respondents, Trump v Hawaii. https://www.supremecourt.gov/Docket PDF/17/ 17-965/40128/20180327105855912_17-965%20Amicus%20Br.%20Proclamation.pdf. Accessed September 21, 2018.
- Smith SR, Sanfilippo JS. Supreme Court decisions in 2017 that affected your practice. OBG Manag. 2017;29(12)44–47.
- Trump v Hawaii, 585 US 17 965 (2018).
- Ohio v American Express Co, 585 US 16 1454 (2018).
- Brief amici curiae of American Medical Association and Ohio State Medical Association in Ohio v American Express, December 24, 2017.
- Masterpiece Cakeshop, Ltd. v Colorado Civil Rights Commission, 584 US 16 111 (2018).
- Brief amici curiae of American College of Pediatricians, et al. in Masterpiece Cakeshop v Colorado Civil Rights Commission, September 7, 2017.
- National Institute of Family and Life Advocates v Becerra, 585 US 16 1140 (2018).
- California Reproductive Freedom, Accountability, Comprehensive Care, and Transparency Act (FACT Act), Cal. Health & Safety Code Ann. §123470 et seq. (West 2018).
- Brief amici curiae of American Academy of Pediatrics, et al. in National Institute of Family and Life Advocates v Becerra, February 27, 2018.
- Brief amici curiae of American Association of Pro-Life Obstetricians & Gynecologists, et al. in National Institute of Family and Life Advocates v Becerra, January 16, 2018.
- Azar v Garza, 584 US 17 654 (2018).
- Brief amici curiae of American College of Obstetricians and Gynecologists and American Public Health Association in Planned Parenthood of Arkansas and Eastern Oklahoma v Jegley, February 1, 2018.
- Chapter 31, Inter Partes Review. United States Code. Title 35: Patents. Part III, Patents and protection of patents. 2012 Ed. 35 USC 311–319.
- Oil States Energy Services, LLC v Greene’s Energy Group, LLC, 584 US 16 712 (2018).
- SAS Institute Inc. v Iancu, 584 US 16 969 (2018).
- Brief for Association of American Medical Colleges and Others as Amici Curiae Supporting Respondents, Trump v Hawaii. https://www.supremecourt.gov/Docket PDF/17/ 17-965/40128/20180327105855912_17-965%20Amicus%20Br.%20Proclamation.pdf. Accessed September 21, 2018.
- Smith SR, Sanfilippo JS. Supreme Court decisions in 2017 that affected your practice. OBG Manag. 2017;29(12)44–47.
- Trump v Hawaii, 585 US 17 965 (2018).
- Ohio v American Express Co, 585 US 16 1454 (2018).
- Brief amici curiae of American Medical Association and Ohio State Medical Association in Ohio v American Express, December 24, 2017.
- Masterpiece Cakeshop, Ltd. v Colorado Civil Rights Commission, 584 US 16 111 (2018).
- Brief amici curiae of American College of Pediatricians, et al. in Masterpiece Cakeshop v Colorado Civil Rights Commission, September 7, 2017.