User login
Cardiac Troponins in Low-Risk Pulmonary Embolism Patients: A Systematic Review and Meta-Analysis
Hospital stays for pulmonary embolism (PE) represent a significant cost burden to the United States healthcare system.1 The mean total hospitalization costs for treating a patient with PE ranges widely from $8,764 to $37,006, with an average reported length of stay between 4 and 5 days.2,3 This cost range is attributed to many factors, including type of PE, therapy-induced bleeding risk requiring close monitoring, comorbidities, and social determinants of health. Given that patients with low-risk PE represent the majority of the cases, changes in approaches to care for this population can significantly impact the overall healthcare costs for PE. The European Society of Cardiology (ESC) guidelines incorporate well-validated risk scores, known as the pulmonary embolism severity index (PESI) and the simplified PESI (sPESI) score, and diagnostic test recommendations, including troponin test, echocardiography, and computed tomography, to evaluate patients with PE at varying risk for mortality.4 In these guidelines, the risk stratification algorithm for patients with a low PESI score or a sPESI score of zero does not include checking for the presence of troponin. In reality, practicing hospitalists frequently find that patients receiving a workup in the emergency department for suspected PE undergo troponin test. The ESC guidelines categorize patients with a low-risk score on PESI/sPESI, who subsequently have a positive troponin status, as intermediate low-risk and suggest consideration of hospitalization. The guidelines recommend patients with positive cardiac biomarkers to undergo assessment of right ventricular function through echocardiogram or computed tomography analysis. Moreover, the guidelines support early discharge or ambulatory treatment for low-risk patients who have a negative troponin status.4
The American College of Chest Physicians (ACCP) guidelines on venous thromboembolism (VTE) recommend that cardiac biomarkers should not be measured routinely in all patients with PE and that positive troponin status should discourage physicians from pursuing ambulatory treatment.5 Therefore, ambiguity lies within both guidelines with regard to how hospitalists should interpret a positive troponin status in patients with low risk, which in turn may lead to unnecessary hospitalizations and further imaging. This systematic review and meta-analysis aims to provide clarity, both about gaps in literature and about how practicing hospitalists should interpret troponins in patients with low-risk PE.
METHODS
Data Sources and Searches
This systematic review and meta-analysis was performed in accordance with the established methods and Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines. We searched MEDLINE, SCOPUS, and Cochrane Controlled Trial Registry databases for studies published from inception to December 2016 by using the following key words: pulmonary embolism AND PESI OR “pulmonary embolism severity index.” Only articles written in English language were included. The full articles of potentially eligible studies were reviewed, and articles published only in abstract form were excluded.
Study Selection
Two investigators independently assessed the abstract of each article, and the full article was assessed if it fulfilled the following criteria: (1) the publication must be original; (2) inclusion of objectively diagnosed, hemodynamically stable patients (normotensive patients) with acute PE in the inpatient or outpatient setting; (3) inclusion of patients>19 years old; (4) use of the PESI or sPESI model to stratify patients into a low-risk group irrespective of any evidence of right ventricular dysfunction; and (5) testing of cardiac troponin levels (TnI-troponin I, TnT-troponin T, or hs-TnI/TnT-high sensitivity troponin I/T) in patients. Study design, sample size, duration of follow-up, type of troponin used, definition of hemodynamic stability, and specific type of outcome measured (endpoint) did not affect the study eligibility.
Data Extraction and Risk of Bias Assessment
Statistical Analysis
Data were summarized by using 30-day all-cause mortality only because it is the most consistent endpoint reported by all of the included studies. For each study, 30-day all-cause mortality was analyzed across the 2 troponin groups, and the results were summarized in terms of positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (PLR), negative likelihood ratio (NLR), and odds ratio (OR). To quantify the uncertainty in the LRs and ORs, we calculated 95% confidence intervals (CI).
Overall measures of PPV, NPV, PLR, and NLR were calculated on the pooled collection of data from the studies. LRs are one of the best measures of diagnostic accuracy; therefore, we defined the degree of probability of disease based on simple estimations that were reported by McGee.6 These estimations are independent of pretest probability and include the following: PLR 5.0 increases the probability of the outcome by about 30%, whereas NLR 0.20 decreases the probability of the outcome by 30%. To identify reasonable performance, we defined a PLR > 5 as an increase in moderate to high probability and a NLR < 0.20 as a decrease in moderate to high probability.6
The overall association between 30-day all-cause mortality and troponin classification among patients with low-risk PE was assessed using a mixed effects logistic regression model. The model included a random intercept to account for the correlation among the measurements for patients within a study. The exponentiated regression coefficient for troponin classification is the OR for 30-day all-cause mortality, comparing troponin-positive patients to troponin-negative patients. OR is reported with a 95% CI and a P value. A continuity correction (correction = 0.5) was applied to zero cells. Heterogeneity was measured using Cochran Q statistic and Higgins I2 statistic.
RESULTS
Search Results
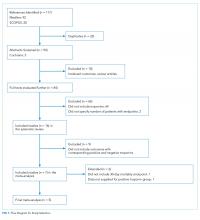
Figure 1 represents the PRISMA flow diagram for literature search and selection process to identify eligible studies for inclusion.
Study Characteristics
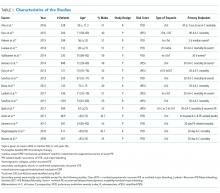
The abstracts of 117 articles were initially identified using the search strategy described above. Of these, 18 articles were deemed appropriate for review based on the criteria outlined in “Study Selection.” The full-text articles of the selected studies were obtained. Upon further evaluation, we identified 16 articles (Figure 1) eligible for the systematic review. Two studies were excluded because they did not provide the number of study participants that met the primary endpoints. The included studies were published from 2009–2016 (Table 1). For patients with low-risk PE, the number of patients with right ventricle dysfunction was either difficult to determine or not reported in all the studies.
Regarding study design, 11 studies were described as prospective cohorts and the remaining 5 studies were identified as retrospective (Table 1). Seven studies stratified participants’ risk of mortality by using sPESI, and 8 studies employed the PESI score. A total of 6952 participants diagnosed with PE were obtained, and 2662 (38%) were recognized as being low-risk based on either the PESI or sPESI. The sample sizes of the individual studies ranged from 121 to 1,291. The studies used either hs-cTnT, hs-cTnI, cTnT, cTnI, or a combination of hs-cTnT and cTnI or cTnT for troponin assay. Most studies used a pre-defined cut-off value to determine positive or negative troponin status.
Thirteen studies reported 30-day event rate as one of the primary endpoints. The 3 other studies included 90-day all-cause mortality, and 2 of them included in-hospital events. Secondary event rates were only reported in 4 studies and consisted of nonfatal PE, nonfatal major bleeding, and PE-related mortality.
Our systematic review revealed that 5 of the 16 studies used either hemodynamic decompensation, cardiopulmonary resuscitation, mechanical ventilation, or a combination of any of these parameters as part of their primary or secondary endpoint. However, none of the studies specified the number of patients that reached any of these endpoints. Furthermore, 10 of the 16 studies did not specify 30-day PE-related mortality outcomes. The most common endpoint was 30-day all-cause mortality, and only 7 studies reported outcomes with positive or negative troponin status.
Outcome Data of All Studies
A total of 2662 participants were categorized as being low risk based on the PESI or sPESI risk score. The pooled rate of PE-related mortality (specified and inferred) was 5 (0.46%) from 6 studies (1,093 patients), in which only 2 studies specified PE-related mortality as the primary endpoint (Vanni [2011]19 and Jimenez [2011]20). The pooled rate of 30-day all-cause mortality was 24 (1.3%) from 12 studies (1882 patients). In 14 studies (2163 patients), the rates of recurrence of PE and major bleeding were 3 (0.14%) and 6 (0.28%), respectively.
Outcomes of Studies with Corresponding Troponin+ and Troponin –
Seven studies used positive or negative troponin status as endpoint to assess low-risk participants (Table 2). However, only 5 studies were included in the final meta-analysis because some data were missing in the Sanchez14 study and the Oszu8 study’s mortality endpoint was more than 30 days. The risk of bias within the studies was evaluated, and for most studies, the quality was of moderate degree (Supplementary Table 1). Table 2 shows the results for the overall pooled data stratified by study. In the pooled data, 463 (67%) patients tested negative for troponin and 228 (33%) tested positive. The overall mortality (from sensitivity analysis) including in-hospital, 30-day, and 90-day mortalities was 1.2%. The NPVs for all individual studies and the overall NPV are 1 or approximately 1. The overall PPVs and by study were low, ranging from 0 to 0.60. The PLRs and NLRs were not estimated for an outcome within an individual study if none of the patients experienced the outcome. When outcomes were only observed among troponin-negative patients, such as in the study of Moores (2009)22 who used 30-day all-cause mortality, the PLR had a value of zero. When outcomes were only observed among troponin-positive patients, as for 30-day all-cause mortality in the Hakemi(2015)9, Lauque (2014)10, and Lankeit(2011)16 studies, the NLR had a value of zero. For zero cells, a continuity correction of 0.5 was applied. The pooled likelihood ratios (LRs) for all-cause mortality were positive LR 2.04 (95% CI, 1.53 to 2.72) and negative LR 0.72 (95% CI, 0.37 to 1.40). The OR for all-cause mortality was 4.79 (95% CI 1.11 to 20.68, P = .0357).
A forest plot was created to visualize the PLR from each study included in the main analysis (Figure 2).
A sensitivity analysis among troponin-positive patients was conducted using 90-day all-cause mortality outcome from the study of Ozsu8 (2015) and the 2 all-cause mortality outcomes from the study of Sanchez14 (2013). The pooled estimates from the 30-day all-cause mortality differed slightly from those previously reported. The PLR increased to 3.40 (95% CI 1.81 to 6.37), and the NLR decreased to 0.59 (95% CI 0.33 to 1.08).
DISCUSSION
In this meta-analysis of 5 studies, which included 691 patients with low-risk PESI or sPESI scores, those tested positive for troponin had nearly a fivefold increased risk of 30-day all-cause mortality compared with patients who tested negative. However, the clinical significance of this association is unclear given that the CI is quite wide and mortality could be associated with PE versus other causes. Similar results were reported by other meta-analyses that consisted of patients with normotensive PE.23-25 To our knowledge, the present meta-analysis is the first to report outcomes in patients with low-risk PE stratified by the presence of cardiac troponin.
A published paper on simplifying the clinical interpretation of LRs state that a positive LR of greater than 5 and a negative LR of less than 0.20 provide dependable evidence regarding reasonable prognostic performance.6 In our analysis, the positive LR was less than 5 and the negative LR’s CI included one. These results suggest a small statistical probability that a patient with a low PESI/sPESI score and a positive troponin status would benefit from inpatient monitoring; simultaneously, a negative troponin does not necessarily translate to safe outpatient therapy, based on our statistical analysis. Previous studies also reported nonextreme positive LRs.23,24 We therefore conclude that low-risk PE patients with positive troponins may be eligible for safe ambulatory treatment or early discharge. However, the number of outcomes of interest (mortality) occurred in only 6 patients among the 228 patients who had positive troponin status. The majority of deaths were reported by Hakemi et al.9 in their retrospective cohort study; as such, drawing conclusions is difficult. Furthermore, the low 30-day all-cause mortality rate of 2.6% in the positive troponin group may have been affected by close monitoring of the patients, who commonly received hemodynamic and oxygen support. Based on these factors, our conclusion is relatively weak, and we cannot recommend a change in practice compared to existing guidelines. In general, additional prospective research is needed to determine whether patients with low-risk PE tested positive for troponin can receive care safely outside the hospital or, rather, require hospitalization similar to patients with intermediate-high risk PE.
We identified a number of other limitations in our analysis. First, aside from the relatively small number of pertinent studies in the literature, most of the studies are of low-moderate quality. Second, the troponin classification in various studies was not conducted using the same assay, and the cut-off value determining positive versus negative results in each case may have differed. These differences may have created some ambiguity or misclassification when the data were pooled together. Third, although the mixed effects logistic regression model controls for some of the variations among patients enrolled in different studies, significant differences exist in terms of patient characteristics or the protocol for follow-up care. This aspect was unaccounted for in this analysis. Lastly, pooled outcome events could not be retrieved from all of the included studies, which would have resulted in a misrepresentation of the true outcomes.
The ESC guidelines suggest avoiding cardiac biomarker testing in patients with low-risk PE because this practice does not have therapeutic implications. Moreover, ESC and ACCP guidelines both state that a positive cardiac biomarker should discourage treatment out of the hospital. The ACCP guidelines further encourage testing of cardiac biomarkers and/or evaluating right ventricular function via echocardiography when uncertainty exists regarding whether patients may require close in-hospital monitoring or not. Although no resounding evidence suggests that troponins have therapeutic implications in patients with low-risk PE, the current guidelines and our meta-analysis cannot offer an overwhelmingly convincing recommendation about whether or not patients with low-risk PE and positive cardiac biomarkers are best treated in the ambulatory or inpatient setting. Such patients may benefit from monitoring in an observation unit (eg, less than 24 or 48 hours), rather than requiring a full admission to the hospital. Nevertheless, our analysis shows that making this determination will require prospective studies that will utilize cardiac troponin status in predicting PE-related events, such as arrhythmia, acute respiratory failure, and hemodynamic decompensation, rather than all-cause mortality.
Until further studies, hospitalists should integrate the use of cardiac troponin and other clinical data, including those available from patient history, physical exam, and other laboratory testing, in determining whether or not to admit, observe, or discharge patients with low-risk PE. As the current guidelines recommend, we support consideration of right ventricular function assessment, via echocardiogram or computed tomography, in patients with positive cardiac troponins even when their PESI/sPESI score is low.
ACKNOWLEDGMENTS
The authors would like to thank Megan Therese Smith, PhD and Lishi Zhang, MS for their contribution in providing a comprehensive statistical analysis of this meta-analysis.
Disclosures
The authors declare no conflicts of interest in the work under consideration for publication. Abdullah Mahayni and Mukti Patel, MD also declared no conflicts of interest with regard to the relevant financial activities outside the submitted work. Omar Darwish, DO and Alpesh Amin, MD also declared no relevant financial activities outside the submitted work; they are speakers for Bristol Myer Squibb and Pfizer regarding the anticoagulant, Apixaban, for treatment of venous thromboembolism and atrial fibrillation.
1. Grosse SD, Nelson RE, Nyarko KA, Richardson LC, Raskob GE. The economic burden of incident venous thromboembolism in the United States: A review of estimated attributable healthcare costs. Thromb Res. 2016;137:3-10 PubMed
2. Fanikos J, Rao A, Seger AC, Carter D, Piazza G, Goldhaber SZ. Hospital Costs of Acute Pulmonary Embolism. Am J Med. 2013;126(2):127-132. PubMed
3. LaMori JC, Shoheiber O, Mody SH, Bookart BK. Inpatient Resource Use and Cost Burden of Deep Vein Thrombosis and Pulmonary Embolism in the United States. Clin Ther. 2015;37(1):62-70. PubMed
4. Konstantinides S, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galié N, et al. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J. 2014;35(43):3033-3080. PubMed
5. Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149(2):315-352. PubMed
6. McGee S. Simplifying Likelihood Ratios. J Gen Intern Med. 2002;17(8):647-650. PubMed
7. Ahn S, Lee Y, Kim WY, Lim KS, Lee J. Prognostic Value of Treatment Setting in Patients With Cancer Having Pulmonary Embolism: Comparison With the Pulmonary Embolism Severity Index. Clin Appl Thromb Hemost. 2016;23(6):615-621. PubMed
8. Ozsu S, Bektas H, Abul Y, Ozlu T, Örem A. Value of Cardiac Troponin and sPESI in Treatment of Pulmonary Thromboembolism at Outpatient Setting. Lung. 2015;193(4):559-565. PubMed
9. Hakemi EU, Alyousef T, Dang G, Hakmei J, Doukky R. The prognostic value of undetectable highly sensitive cardiac troponin I in patients with acute pulmonary embolism. Chest. 2015;147(3):685-694. PubMed
10. Lauque D, Maupas-Schwalm F, Bounes V, et al. Predictive Value of the Heart‐type Fatty Acid–binding Protein and the Pulmonary Embolism Severity Index in Patients With Acute Pulmonary Embolism in the Emergency Department. Acad Emerg Med. 2014;21(10):1143-1150. PubMed
11. Vuilleumier N, Limacher A, Méan M, Choffat J, Lescuyer P, Bounameaux H, et al. Cardiac biomarkers and clinical scores for risk stratification in elderly patients with non‐high‐risk pulmonary embolism. J Intern Med. 2014;277(6):707-716. PubMed
12. Jiménez D, Kopecna D, Tapson V, et al. Derivation and validation of multimarker prognostication for normotensive patients with acute symptomatic pulmonary embolism. Am J Respir Crit Care Med. 2014;189(6):718-726. PubMed
13. Ozsu S, Abul Y, Orem A, et al. Predictive value of troponins and simplified pulmonary embolism severity index in patients with normotensive pulmonary embolism. Multidiscip Respir Med. 2013;8(1):34. PubMed
14. Sanchez O, Trinquart L, Planquette B, et al. Echocardiography and pulmonary embolism severity index have independent prognostic roles in pulmonary embolism. Eur Respir J. 2013;42(3):681-688. PubMed
15. Barra SN, Paiva L, Providéncia R, Fernandes A, Nascimento J, Marques AL. LR–PED Rule: Low Risk Pulmonary Embolism Decision Rule–A new decision score for low risk Pulmonary Embolism. Thromb Res. 2012;130(3):327-333. PubMed
16. Lankeit M, Jiménez D, Kostrubiec M, et al. Predictive Value of the High-Sensitivity Troponin T Assay and the Simplified Pulmonary Embolism Severity Index in Hemodynamically Stable Patients With Acute Pulmonary Embolism A Prospective Validation Study. Circulation. 2011;124(24):2716-2724. PubMed
17. Sánchez D, De Miguel J, Sam A, et al. The effects of cause of death classification on prognostic assessment of patients with pulmonary embolism. J Thromb Haemost. 2011;9(11):2201-2207. PubMed
18. Spirk D, Aujesky D, Husmann M, et al. Cardiac troponin testing and the simplified Pulmonary Embolism Severity Index. J Thromb Haemost. 2011;105(05):978-984. PubMed
19. Vanni S, Nazerian P, Pepe G, et al. Comparison of two prognostic models for acute pulmonary embolism: clinical vs. right ventricular dysfunction‐guided approach. J Thromb Haemos. 2011;9(10):1916-1923. PubMed
20. Jiménez D, Aujesky D, Moores L, et al. Combinations of prognostic tools for identification of high-risk normotensive patients with acute symptomatic pulmonary embolism. Thorax. 2011;66(1):75-81. PubMed
21. Singanayagam A, Scally C, Al-Khairalla MZ, et al. Are biomarkers additive to pulmonary embolism severity index for severity assessment in normotensive patients with acute pulmonary embolism? QJM. 2010;104(2):125-131. PubMed
22. Moores L, Aujesky D, Jimenez D, et al. Pulmonary Embolism Severity Index and troponin testing for the selection of low‐risk patients with acute symptomatic pulmonary embolism. J Thromb Haemost. 2009;8(3):517-522. PubMed
23. Bajaj A, Rathor P, Sehgal V, et al. Prognostic Value of Biomarkers in Acute Non-massive Pulmonary Embolism; A Sysemative Review and Meta-Analysis. Lung. 2015;193(5):639-651. PubMed
24. Jiménez D Uresandi F, Otero R, et al. Troponin-based risk stratification of patients with acute nonmassive pulmonary embolism; a systematic review and metaanalysis. Chest. 2009;136(4):974-982. PubMed
25. Becattini C, Vedovati MC, Agnelli G. Prognostic Value of Troponins in Acute Pulmonary Embolism: A Meta-Analysis. Circulation. 2007;116(4):427-433. PubMed
Hospital stays for pulmonary embolism (PE) represent a significant cost burden to the United States healthcare system.1 The mean total hospitalization costs for treating a patient with PE ranges widely from $8,764 to $37,006, with an average reported length of stay between 4 and 5 days.2,3 This cost range is attributed to many factors, including type of PE, therapy-induced bleeding risk requiring close monitoring, comorbidities, and social determinants of health. Given that patients with low-risk PE represent the majority of the cases, changes in approaches to care for this population can significantly impact the overall healthcare costs for PE. The European Society of Cardiology (ESC) guidelines incorporate well-validated risk scores, known as the pulmonary embolism severity index (PESI) and the simplified PESI (sPESI) score, and diagnostic test recommendations, including troponin test, echocardiography, and computed tomography, to evaluate patients with PE at varying risk for mortality.4 In these guidelines, the risk stratification algorithm for patients with a low PESI score or a sPESI score of zero does not include checking for the presence of troponin. In reality, practicing hospitalists frequently find that patients receiving a workup in the emergency department for suspected PE undergo troponin test. The ESC guidelines categorize patients with a low-risk score on PESI/sPESI, who subsequently have a positive troponin status, as intermediate low-risk and suggest consideration of hospitalization. The guidelines recommend patients with positive cardiac biomarkers to undergo assessment of right ventricular function through echocardiogram or computed tomography analysis. Moreover, the guidelines support early discharge or ambulatory treatment for low-risk patients who have a negative troponin status.4
The American College of Chest Physicians (ACCP) guidelines on venous thromboembolism (VTE) recommend that cardiac biomarkers should not be measured routinely in all patients with PE and that positive troponin status should discourage physicians from pursuing ambulatory treatment.5 Therefore, ambiguity lies within both guidelines with regard to how hospitalists should interpret a positive troponin status in patients with low risk, which in turn may lead to unnecessary hospitalizations and further imaging. This systematic review and meta-analysis aims to provide clarity, both about gaps in literature and about how practicing hospitalists should interpret troponins in patients with low-risk PE.
METHODS
Data Sources and Searches
This systematic review and meta-analysis was performed in accordance with the established methods and Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines. We searched MEDLINE, SCOPUS, and Cochrane Controlled Trial Registry databases for studies published from inception to December 2016 by using the following key words: pulmonary embolism AND PESI OR “pulmonary embolism severity index.” Only articles written in English language were included. The full articles of potentially eligible studies were reviewed, and articles published only in abstract form were excluded.
Study Selection
Two investigators independently assessed the abstract of each article, and the full article was assessed if it fulfilled the following criteria: (1) the publication must be original; (2) inclusion of objectively diagnosed, hemodynamically stable patients (normotensive patients) with acute PE in the inpatient or outpatient setting; (3) inclusion of patients>19 years old; (4) use of the PESI or sPESI model to stratify patients into a low-risk group irrespective of any evidence of right ventricular dysfunction; and (5) testing of cardiac troponin levels (TnI-troponin I, TnT-troponin T, or hs-TnI/TnT-high sensitivity troponin I/T) in patients. Study design, sample size, duration of follow-up, type of troponin used, definition of hemodynamic stability, and specific type of outcome measured (endpoint) did not affect the study eligibility.
Data Extraction and Risk of Bias Assessment
Statistical Analysis
Data were summarized by using 30-day all-cause mortality only because it is the most consistent endpoint reported by all of the included studies. For each study, 30-day all-cause mortality was analyzed across the 2 troponin groups, and the results were summarized in terms of positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (PLR), negative likelihood ratio (NLR), and odds ratio (OR). To quantify the uncertainty in the LRs and ORs, we calculated 95% confidence intervals (CI).
Overall measures of PPV, NPV, PLR, and NLR were calculated on the pooled collection of data from the studies. LRs are one of the best measures of diagnostic accuracy; therefore, we defined the degree of probability of disease based on simple estimations that were reported by McGee.6 These estimations are independent of pretest probability and include the following: PLR 5.0 increases the probability of the outcome by about 30%, whereas NLR 0.20 decreases the probability of the outcome by 30%. To identify reasonable performance, we defined a PLR > 5 as an increase in moderate to high probability and a NLR < 0.20 as a decrease in moderate to high probability.6
The overall association between 30-day all-cause mortality and troponin classification among patients with low-risk PE was assessed using a mixed effects logistic regression model. The model included a random intercept to account for the correlation among the measurements for patients within a study. The exponentiated regression coefficient for troponin classification is the OR for 30-day all-cause mortality, comparing troponin-positive patients to troponin-negative patients. OR is reported with a 95% CI and a P value. A continuity correction (correction = 0.5) was applied to zero cells. Heterogeneity was measured using Cochran Q statistic and Higgins I2 statistic.
RESULTS
Search Results
Figure 1 represents the PRISMA flow diagram for literature search and selection process to identify eligible studies for inclusion.
Study Characteristics
The abstracts of 117 articles were initially identified using the search strategy described above. Of these, 18 articles were deemed appropriate for review based on the criteria outlined in “Study Selection.” The full-text articles of the selected studies were obtained. Upon further evaluation, we identified 16 articles (Figure 1) eligible for the systematic review. Two studies were excluded because they did not provide the number of study participants that met the primary endpoints. The included studies were published from 2009–2016 (Table 1). For patients with low-risk PE, the number of patients with right ventricle dysfunction was either difficult to determine or not reported in all the studies.
Regarding study design, 11 studies were described as prospective cohorts and the remaining 5 studies were identified as retrospective (Table 1). Seven studies stratified participants’ risk of mortality by using sPESI, and 8 studies employed the PESI score. A total of 6952 participants diagnosed with PE were obtained, and 2662 (38%) were recognized as being low-risk based on either the PESI or sPESI. The sample sizes of the individual studies ranged from 121 to 1,291. The studies used either hs-cTnT, hs-cTnI, cTnT, cTnI, or a combination of hs-cTnT and cTnI or cTnT for troponin assay. Most studies used a pre-defined cut-off value to determine positive or negative troponin status.
Thirteen studies reported 30-day event rate as one of the primary endpoints. The 3 other studies included 90-day all-cause mortality, and 2 of them included in-hospital events. Secondary event rates were only reported in 4 studies and consisted of nonfatal PE, nonfatal major bleeding, and PE-related mortality.
Our systematic review revealed that 5 of the 16 studies used either hemodynamic decompensation, cardiopulmonary resuscitation, mechanical ventilation, or a combination of any of these parameters as part of their primary or secondary endpoint. However, none of the studies specified the number of patients that reached any of these endpoints. Furthermore, 10 of the 16 studies did not specify 30-day PE-related mortality outcomes. The most common endpoint was 30-day all-cause mortality, and only 7 studies reported outcomes with positive or negative troponin status.
Outcome Data of All Studies
A total of 2662 participants were categorized as being low risk based on the PESI or sPESI risk score. The pooled rate of PE-related mortality (specified and inferred) was 5 (0.46%) from 6 studies (1,093 patients), in which only 2 studies specified PE-related mortality as the primary endpoint (Vanni [2011]19 and Jimenez [2011]20). The pooled rate of 30-day all-cause mortality was 24 (1.3%) from 12 studies (1882 patients). In 14 studies (2163 patients), the rates of recurrence of PE and major bleeding were 3 (0.14%) and 6 (0.28%), respectively.
Outcomes of Studies with Corresponding Troponin+ and Troponin –
Seven studies used positive or negative troponin status as endpoint to assess low-risk participants (Table 2). However, only 5 studies were included in the final meta-analysis because some data were missing in the Sanchez14 study and the Oszu8 study’s mortality endpoint was more than 30 days. The risk of bias within the studies was evaluated, and for most studies, the quality was of moderate degree (Supplementary Table 1). Table 2 shows the results for the overall pooled data stratified by study. In the pooled data, 463 (67%) patients tested negative for troponin and 228 (33%) tested positive. The overall mortality (from sensitivity analysis) including in-hospital, 30-day, and 90-day mortalities was 1.2%. The NPVs for all individual studies and the overall NPV are 1 or approximately 1. The overall PPVs and by study were low, ranging from 0 to 0.60. The PLRs and NLRs were not estimated for an outcome within an individual study if none of the patients experienced the outcome. When outcomes were only observed among troponin-negative patients, such as in the study of Moores (2009)22 who used 30-day all-cause mortality, the PLR had a value of zero. When outcomes were only observed among troponin-positive patients, as for 30-day all-cause mortality in the Hakemi(2015)9, Lauque (2014)10, and Lankeit(2011)16 studies, the NLR had a value of zero. For zero cells, a continuity correction of 0.5 was applied. The pooled likelihood ratios (LRs) for all-cause mortality were positive LR 2.04 (95% CI, 1.53 to 2.72) and negative LR 0.72 (95% CI, 0.37 to 1.40). The OR for all-cause mortality was 4.79 (95% CI 1.11 to 20.68, P = .0357).
A forest plot was created to visualize the PLR from each study included in the main analysis (Figure 2).
A sensitivity analysis among troponin-positive patients was conducted using 90-day all-cause mortality outcome from the study of Ozsu8 (2015) and the 2 all-cause mortality outcomes from the study of Sanchez14 (2013). The pooled estimates from the 30-day all-cause mortality differed slightly from those previously reported. The PLR increased to 3.40 (95% CI 1.81 to 6.37), and the NLR decreased to 0.59 (95% CI 0.33 to 1.08).
DISCUSSION
In this meta-analysis of 5 studies, which included 691 patients with low-risk PESI or sPESI scores, those tested positive for troponin had nearly a fivefold increased risk of 30-day all-cause mortality compared with patients who tested negative. However, the clinical significance of this association is unclear given that the CI is quite wide and mortality could be associated with PE versus other causes. Similar results were reported by other meta-analyses that consisted of patients with normotensive PE.23-25 To our knowledge, the present meta-analysis is the first to report outcomes in patients with low-risk PE stratified by the presence of cardiac troponin.
A published paper on simplifying the clinical interpretation of LRs state that a positive LR of greater than 5 and a negative LR of less than 0.20 provide dependable evidence regarding reasonable prognostic performance.6 In our analysis, the positive LR was less than 5 and the negative LR’s CI included one. These results suggest a small statistical probability that a patient with a low PESI/sPESI score and a positive troponin status would benefit from inpatient monitoring; simultaneously, a negative troponin does not necessarily translate to safe outpatient therapy, based on our statistical analysis. Previous studies also reported nonextreme positive LRs.23,24 We therefore conclude that low-risk PE patients with positive troponins may be eligible for safe ambulatory treatment or early discharge. However, the number of outcomes of interest (mortality) occurred in only 6 patients among the 228 patients who had positive troponin status. The majority of deaths were reported by Hakemi et al.9 in their retrospective cohort study; as such, drawing conclusions is difficult. Furthermore, the low 30-day all-cause mortality rate of 2.6% in the positive troponin group may have been affected by close monitoring of the patients, who commonly received hemodynamic and oxygen support. Based on these factors, our conclusion is relatively weak, and we cannot recommend a change in practice compared to existing guidelines. In general, additional prospective research is needed to determine whether patients with low-risk PE tested positive for troponin can receive care safely outside the hospital or, rather, require hospitalization similar to patients with intermediate-high risk PE.
We identified a number of other limitations in our analysis. First, aside from the relatively small number of pertinent studies in the literature, most of the studies are of low-moderate quality. Second, the troponin classification in various studies was not conducted using the same assay, and the cut-off value determining positive versus negative results in each case may have differed. These differences may have created some ambiguity or misclassification when the data were pooled together. Third, although the mixed effects logistic regression model controls for some of the variations among patients enrolled in different studies, significant differences exist in terms of patient characteristics or the protocol for follow-up care. This aspect was unaccounted for in this analysis. Lastly, pooled outcome events could not be retrieved from all of the included studies, which would have resulted in a misrepresentation of the true outcomes.
The ESC guidelines suggest avoiding cardiac biomarker testing in patients with low-risk PE because this practice does not have therapeutic implications. Moreover, ESC and ACCP guidelines both state that a positive cardiac biomarker should discourage treatment out of the hospital. The ACCP guidelines further encourage testing of cardiac biomarkers and/or evaluating right ventricular function via echocardiography when uncertainty exists regarding whether patients may require close in-hospital monitoring or not. Although no resounding evidence suggests that troponins have therapeutic implications in patients with low-risk PE, the current guidelines and our meta-analysis cannot offer an overwhelmingly convincing recommendation about whether or not patients with low-risk PE and positive cardiac biomarkers are best treated in the ambulatory or inpatient setting. Such patients may benefit from monitoring in an observation unit (eg, less than 24 or 48 hours), rather than requiring a full admission to the hospital. Nevertheless, our analysis shows that making this determination will require prospective studies that will utilize cardiac troponin status in predicting PE-related events, such as arrhythmia, acute respiratory failure, and hemodynamic decompensation, rather than all-cause mortality.
Until further studies, hospitalists should integrate the use of cardiac troponin and other clinical data, including those available from patient history, physical exam, and other laboratory testing, in determining whether or not to admit, observe, or discharge patients with low-risk PE. As the current guidelines recommend, we support consideration of right ventricular function assessment, via echocardiogram or computed tomography, in patients with positive cardiac troponins even when their PESI/sPESI score is low.
ACKNOWLEDGMENTS
The authors would like to thank Megan Therese Smith, PhD and Lishi Zhang, MS for their contribution in providing a comprehensive statistical analysis of this meta-analysis.
Disclosures
The authors declare no conflicts of interest in the work under consideration for publication. Abdullah Mahayni and Mukti Patel, MD also declared no conflicts of interest with regard to the relevant financial activities outside the submitted work. Omar Darwish, DO and Alpesh Amin, MD also declared no relevant financial activities outside the submitted work; they are speakers for Bristol Myer Squibb and Pfizer regarding the anticoagulant, Apixaban, for treatment of venous thromboembolism and atrial fibrillation.
Hospital stays for pulmonary embolism (PE) represent a significant cost burden to the United States healthcare system.1 The mean total hospitalization costs for treating a patient with PE ranges widely from $8,764 to $37,006, with an average reported length of stay between 4 and 5 days.2,3 This cost range is attributed to many factors, including type of PE, therapy-induced bleeding risk requiring close monitoring, comorbidities, and social determinants of health. Given that patients with low-risk PE represent the majority of the cases, changes in approaches to care for this population can significantly impact the overall healthcare costs for PE. The European Society of Cardiology (ESC) guidelines incorporate well-validated risk scores, known as the pulmonary embolism severity index (PESI) and the simplified PESI (sPESI) score, and diagnostic test recommendations, including troponin test, echocardiography, and computed tomography, to evaluate patients with PE at varying risk for mortality.4 In these guidelines, the risk stratification algorithm for patients with a low PESI score or a sPESI score of zero does not include checking for the presence of troponin. In reality, practicing hospitalists frequently find that patients receiving a workup in the emergency department for suspected PE undergo troponin test. The ESC guidelines categorize patients with a low-risk score on PESI/sPESI, who subsequently have a positive troponin status, as intermediate low-risk and suggest consideration of hospitalization. The guidelines recommend patients with positive cardiac biomarkers to undergo assessment of right ventricular function through echocardiogram or computed tomography analysis. Moreover, the guidelines support early discharge or ambulatory treatment for low-risk patients who have a negative troponin status.4
The American College of Chest Physicians (ACCP) guidelines on venous thromboembolism (VTE) recommend that cardiac biomarkers should not be measured routinely in all patients with PE and that positive troponin status should discourage physicians from pursuing ambulatory treatment.5 Therefore, ambiguity lies within both guidelines with regard to how hospitalists should interpret a positive troponin status in patients with low risk, which in turn may lead to unnecessary hospitalizations and further imaging. This systematic review and meta-analysis aims to provide clarity, both about gaps in literature and about how practicing hospitalists should interpret troponins in patients with low-risk PE.
METHODS
Data Sources and Searches
This systematic review and meta-analysis was performed in accordance with the established methods and Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines. We searched MEDLINE, SCOPUS, and Cochrane Controlled Trial Registry databases for studies published from inception to December 2016 by using the following key words: pulmonary embolism AND PESI OR “pulmonary embolism severity index.” Only articles written in English language were included. The full articles of potentially eligible studies were reviewed, and articles published only in abstract form were excluded.
Study Selection
Two investigators independently assessed the abstract of each article, and the full article was assessed if it fulfilled the following criteria: (1) the publication must be original; (2) inclusion of objectively diagnosed, hemodynamically stable patients (normotensive patients) with acute PE in the inpatient or outpatient setting; (3) inclusion of patients>19 years old; (4) use of the PESI or sPESI model to stratify patients into a low-risk group irrespective of any evidence of right ventricular dysfunction; and (5) testing of cardiac troponin levels (TnI-troponin I, TnT-troponin T, or hs-TnI/TnT-high sensitivity troponin I/T) in patients. Study design, sample size, duration of follow-up, type of troponin used, definition of hemodynamic stability, and specific type of outcome measured (endpoint) did not affect the study eligibility.
Data Extraction and Risk of Bias Assessment
Statistical Analysis
Data were summarized by using 30-day all-cause mortality only because it is the most consistent endpoint reported by all of the included studies. For each study, 30-day all-cause mortality was analyzed across the 2 troponin groups, and the results were summarized in terms of positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (PLR), negative likelihood ratio (NLR), and odds ratio (OR). To quantify the uncertainty in the LRs and ORs, we calculated 95% confidence intervals (CI).
Overall measures of PPV, NPV, PLR, and NLR were calculated on the pooled collection of data from the studies. LRs are one of the best measures of diagnostic accuracy; therefore, we defined the degree of probability of disease based on simple estimations that were reported by McGee.6 These estimations are independent of pretest probability and include the following: PLR 5.0 increases the probability of the outcome by about 30%, whereas NLR 0.20 decreases the probability of the outcome by 30%. To identify reasonable performance, we defined a PLR > 5 as an increase in moderate to high probability and a NLR < 0.20 as a decrease in moderate to high probability.6
The overall association between 30-day all-cause mortality and troponin classification among patients with low-risk PE was assessed using a mixed effects logistic regression model. The model included a random intercept to account for the correlation among the measurements for patients within a study. The exponentiated regression coefficient for troponin classification is the OR for 30-day all-cause mortality, comparing troponin-positive patients to troponin-negative patients. OR is reported with a 95% CI and a P value. A continuity correction (correction = 0.5) was applied to zero cells. Heterogeneity was measured using Cochran Q statistic and Higgins I2 statistic.
RESULTS
Search Results
Figure 1 represents the PRISMA flow diagram for literature search and selection process to identify eligible studies for inclusion.
Study Characteristics
The abstracts of 117 articles were initially identified using the search strategy described above. Of these, 18 articles were deemed appropriate for review based on the criteria outlined in “Study Selection.” The full-text articles of the selected studies were obtained. Upon further evaluation, we identified 16 articles (Figure 1) eligible for the systematic review. Two studies were excluded because they did not provide the number of study participants that met the primary endpoints. The included studies were published from 2009–2016 (Table 1). For patients with low-risk PE, the number of patients with right ventricle dysfunction was either difficult to determine or not reported in all the studies.
Regarding study design, 11 studies were described as prospective cohorts and the remaining 5 studies were identified as retrospective (Table 1). Seven studies stratified participants’ risk of mortality by using sPESI, and 8 studies employed the PESI score. A total of 6952 participants diagnosed with PE were obtained, and 2662 (38%) were recognized as being low-risk based on either the PESI or sPESI. The sample sizes of the individual studies ranged from 121 to 1,291. The studies used either hs-cTnT, hs-cTnI, cTnT, cTnI, or a combination of hs-cTnT and cTnI or cTnT for troponin assay. Most studies used a pre-defined cut-off value to determine positive or negative troponin status.
Thirteen studies reported 30-day event rate as one of the primary endpoints. The 3 other studies included 90-day all-cause mortality, and 2 of them included in-hospital events. Secondary event rates were only reported in 4 studies and consisted of nonfatal PE, nonfatal major bleeding, and PE-related mortality.
Our systematic review revealed that 5 of the 16 studies used either hemodynamic decompensation, cardiopulmonary resuscitation, mechanical ventilation, or a combination of any of these parameters as part of their primary or secondary endpoint. However, none of the studies specified the number of patients that reached any of these endpoints. Furthermore, 10 of the 16 studies did not specify 30-day PE-related mortality outcomes. The most common endpoint was 30-day all-cause mortality, and only 7 studies reported outcomes with positive or negative troponin status.
Outcome Data of All Studies
A total of 2662 participants were categorized as being low risk based on the PESI or sPESI risk score. The pooled rate of PE-related mortality (specified and inferred) was 5 (0.46%) from 6 studies (1,093 patients), in which only 2 studies specified PE-related mortality as the primary endpoint (Vanni [2011]19 and Jimenez [2011]20). The pooled rate of 30-day all-cause mortality was 24 (1.3%) from 12 studies (1882 patients). In 14 studies (2163 patients), the rates of recurrence of PE and major bleeding were 3 (0.14%) and 6 (0.28%), respectively.
Outcomes of Studies with Corresponding Troponin+ and Troponin –
Seven studies used positive or negative troponin status as endpoint to assess low-risk participants (Table 2). However, only 5 studies were included in the final meta-analysis because some data were missing in the Sanchez14 study and the Oszu8 study’s mortality endpoint was more than 30 days. The risk of bias within the studies was evaluated, and for most studies, the quality was of moderate degree (Supplementary Table 1). Table 2 shows the results for the overall pooled data stratified by study. In the pooled data, 463 (67%) patients tested negative for troponin and 228 (33%) tested positive. The overall mortality (from sensitivity analysis) including in-hospital, 30-day, and 90-day mortalities was 1.2%. The NPVs for all individual studies and the overall NPV are 1 or approximately 1. The overall PPVs and by study were low, ranging from 0 to 0.60. The PLRs and NLRs were not estimated for an outcome within an individual study if none of the patients experienced the outcome. When outcomes were only observed among troponin-negative patients, such as in the study of Moores (2009)22 who used 30-day all-cause mortality, the PLR had a value of zero. When outcomes were only observed among troponin-positive patients, as for 30-day all-cause mortality in the Hakemi(2015)9, Lauque (2014)10, and Lankeit(2011)16 studies, the NLR had a value of zero. For zero cells, a continuity correction of 0.5 was applied. The pooled likelihood ratios (LRs) for all-cause mortality were positive LR 2.04 (95% CI, 1.53 to 2.72) and negative LR 0.72 (95% CI, 0.37 to 1.40). The OR for all-cause mortality was 4.79 (95% CI 1.11 to 20.68, P = .0357).
A forest plot was created to visualize the PLR from each study included in the main analysis (Figure 2).
A sensitivity analysis among troponin-positive patients was conducted using 90-day all-cause mortality outcome from the study of Ozsu8 (2015) and the 2 all-cause mortality outcomes from the study of Sanchez14 (2013). The pooled estimates from the 30-day all-cause mortality differed slightly from those previously reported. The PLR increased to 3.40 (95% CI 1.81 to 6.37), and the NLR decreased to 0.59 (95% CI 0.33 to 1.08).
DISCUSSION
In this meta-analysis of 5 studies, which included 691 patients with low-risk PESI or sPESI scores, those tested positive for troponin had nearly a fivefold increased risk of 30-day all-cause mortality compared with patients who tested negative. However, the clinical significance of this association is unclear given that the CI is quite wide and mortality could be associated with PE versus other causes. Similar results were reported by other meta-analyses that consisted of patients with normotensive PE.23-25 To our knowledge, the present meta-analysis is the first to report outcomes in patients with low-risk PE stratified by the presence of cardiac troponin.
A published paper on simplifying the clinical interpretation of LRs state that a positive LR of greater than 5 and a negative LR of less than 0.20 provide dependable evidence regarding reasonable prognostic performance.6 In our analysis, the positive LR was less than 5 and the negative LR’s CI included one. These results suggest a small statistical probability that a patient with a low PESI/sPESI score and a positive troponin status would benefit from inpatient monitoring; simultaneously, a negative troponin does not necessarily translate to safe outpatient therapy, based on our statistical analysis. Previous studies also reported nonextreme positive LRs.23,24 We therefore conclude that low-risk PE patients with positive troponins may be eligible for safe ambulatory treatment or early discharge. However, the number of outcomes of interest (mortality) occurred in only 6 patients among the 228 patients who had positive troponin status. The majority of deaths were reported by Hakemi et al.9 in their retrospective cohort study; as such, drawing conclusions is difficult. Furthermore, the low 30-day all-cause mortality rate of 2.6% in the positive troponin group may have been affected by close monitoring of the patients, who commonly received hemodynamic and oxygen support. Based on these factors, our conclusion is relatively weak, and we cannot recommend a change in practice compared to existing guidelines. In general, additional prospective research is needed to determine whether patients with low-risk PE tested positive for troponin can receive care safely outside the hospital or, rather, require hospitalization similar to patients with intermediate-high risk PE.
We identified a number of other limitations in our analysis. First, aside from the relatively small number of pertinent studies in the literature, most of the studies are of low-moderate quality. Second, the troponin classification in various studies was not conducted using the same assay, and the cut-off value determining positive versus negative results in each case may have differed. These differences may have created some ambiguity or misclassification when the data were pooled together. Third, although the mixed effects logistic regression model controls for some of the variations among patients enrolled in different studies, significant differences exist in terms of patient characteristics or the protocol for follow-up care. This aspect was unaccounted for in this analysis. Lastly, pooled outcome events could not be retrieved from all of the included studies, which would have resulted in a misrepresentation of the true outcomes.
The ESC guidelines suggest avoiding cardiac biomarker testing in patients with low-risk PE because this practice does not have therapeutic implications. Moreover, ESC and ACCP guidelines both state that a positive cardiac biomarker should discourage treatment out of the hospital. The ACCP guidelines further encourage testing of cardiac biomarkers and/or evaluating right ventricular function via echocardiography when uncertainty exists regarding whether patients may require close in-hospital monitoring or not. Although no resounding evidence suggests that troponins have therapeutic implications in patients with low-risk PE, the current guidelines and our meta-analysis cannot offer an overwhelmingly convincing recommendation about whether or not patients with low-risk PE and positive cardiac biomarkers are best treated in the ambulatory or inpatient setting. Such patients may benefit from monitoring in an observation unit (eg, less than 24 or 48 hours), rather than requiring a full admission to the hospital. Nevertheless, our analysis shows that making this determination will require prospective studies that will utilize cardiac troponin status in predicting PE-related events, such as arrhythmia, acute respiratory failure, and hemodynamic decompensation, rather than all-cause mortality.
Until further studies, hospitalists should integrate the use of cardiac troponin and other clinical data, including those available from patient history, physical exam, and other laboratory testing, in determining whether or not to admit, observe, or discharge patients with low-risk PE. As the current guidelines recommend, we support consideration of right ventricular function assessment, via echocardiogram or computed tomography, in patients with positive cardiac troponins even when their PESI/sPESI score is low.
ACKNOWLEDGMENTS
The authors would like to thank Megan Therese Smith, PhD and Lishi Zhang, MS for their contribution in providing a comprehensive statistical analysis of this meta-analysis.
Disclosures
The authors declare no conflicts of interest in the work under consideration for publication. Abdullah Mahayni and Mukti Patel, MD also declared no conflicts of interest with regard to the relevant financial activities outside the submitted work. Omar Darwish, DO and Alpesh Amin, MD also declared no relevant financial activities outside the submitted work; they are speakers for Bristol Myer Squibb and Pfizer regarding the anticoagulant, Apixaban, for treatment of venous thromboembolism and atrial fibrillation.
1. Grosse SD, Nelson RE, Nyarko KA, Richardson LC, Raskob GE. The economic burden of incident venous thromboembolism in the United States: A review of estimated attributable healthcare costs. Thromb Res. 2016;137:3-10 PubMed
2. Fanikos J, Rao A, Seger AC, Carter D, Piazza G, Goldhaber SZ. Hospital Costs of Acute Pulmonary Embolism. Am J Med. 2013;126(2):127-132. PubMed
3. LaMori JC, Shoheiber O, Mody SH, Bookart BK. Inpatient Resource Use and Cost Burden of Deep Vein Thrombosis and Pulmonary Embolism in the United States. Clin Ther. 2015;37(1):62-70. PubMed
4. Konstantinides S, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galié N, et al. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J. 2014;35(43):3033-3080. PubMed
5. Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149(2):315-352. PubMed
6. McGee S. Simplifying Likelihood Ratios. J Gen Intern Med. 2002;17(8):647-650. PubMed
7. Ahn S, Lee Y, Kim WY, Lim KS, Lee J. Prognostic Value of Treatment Setting in Patients With Cancer Having Pulmonary Embolism: Comparison With the Pulmonary Embolism Severity Index. Clin Appl Thromb Hemost. 2016;23(6):615-621. PubMed
8. Ozsu S, Bektas H, Abul Y, Ozlu T, Örem A. Value of Cardiac Troponin and sPESI in Treatment of Pulmonary Thromboembolism at Outpatient Setting. Lung. 2015;193(4):559-565. PubMed
9. Hakemi EU, Alyousef T, Dang G, Hakmei J, Doukky R. The prognostic value of undetectable highly sensitive cardiac troponin I in patients with acute pulmonary embolism. Chest. 2015;147(3):685-694. PubMed
10. Lauque D, Maupas-Schwalm F, Bounes V, et al. Predictive Value of the Heart‐type Fatty Acid–binding Protein and the Pulmonary Embolism Severity Index in Patients With Acute Pulmonary Embolism in the Emergency Department. Acad Emerg Med. 2014;21(10):1143-1150. PubMed
11. Vuilleumier N, Limacher A, Méan M, Choffat J, Lescuyer P, Bounameaux H, et al. Cardiac biomarkers and clinical scores for risk stratification in elderly patients with non‐high‐risk pulmonary embolism. J Intern Med. 2014;277(6):707-716. PubMed
12. Jiménez D, Kopecna D, Tapson V, et al. Derivation and validation of multimarker prognostication for normotensive patients with acute symptomatic pulmonary embolism. Am J Respir Crit Care Med. 2014;189(6):718-726. PubMed
13. Ozsu S, Abul Y, Orem A, et al. Predictive value of troponins and simplified pulmonary embolism severity index in patients with normotensive pulmonary embolism. Multidiscip Respir Med. 2013;8(1):34. PubMed
14. Sanchez O, Trinquart L, Planquette B, et al. Echocardiography and pulmonary embolism severity index have independent prognostic roles in pulmonary embolism. Eur Respir J. 2013;42(3):681-688. PubMed
15. Barra SN, Paiva L, Providéncia R, Fernandes A, Nascimento J, Marques AL. LR–PED Rule: Low Risk Pulmonary Embolism Decision Rule–A new decision score for low risk Pulmonary Embolism. Thromb Res. 2012;130(3):327-333. PubMed
16. Lankeit M, Jiménez D, Kostrubiec M, et al. Predictive Value of the High-Sensitivity Troponin T Assay and the Simplified Pulmonary Embolism Severity Index in Hemodynamically Stable Patients With Acute Pulmonary Embolism A Prospective Validation Study. Circulation. 2011;124(24):2716-2724. PubMed
17. Sánchez D, De Miguel J, Sam A, et al. The effects of cause of death classification on prognostic assessment of patients with pulmonary embolism. J Thromb Haemost. 2011;9(11):2201-2207. PubMed
18. Spirk D, Aujesky D, Husmann M, et al. Cardiac troponin testing and the simplified Pulmonary Embolism Severity Index. J Thromb Haemost. 2011;105(05):978-984. PubMed
19. Vanni S, Nazerian P, Pepe G, et al. Comparison of two prognostic models for acute pulmonary embolism: clinical vs. right ventricular dysfunction‐guided approach. J Thromb Haemos. 2011;9(10):1916-1923. PubMed
20. Jiménez D, Aujesky D, Moores L, et al. Combinations of prognostic tools for identification of high-risk normotensive patients with acute symptomatic pulmonary embolism. Thorax. 2011;66(1):75-81. PubMed
21. Singanayagam A, Scally C, Al-Khairalla MZ, et al. Are biomarkers additive to pulmonary embolism severity index for severity assessment in normotensive patients with acute pulmonary embolism? QJM. 2010;104(2):125-131. PubMed
22. Moores L, Aujesky D, Jimenez D, et al. Pulmonary Embolism Severity Index and troponin testing for the selection of low‐risk patients with acute symptomatic pulmonary embolism. J Thromb Haemost. 2009;8(3):517-522. PubMed
23. Bajaj A, Rathor P, Sehgal V, et al. Prognostic Value of Biomarkers in Acute Non-massive Pulmonary Embolism; A Sysemative Review and Meta-Analysis. Lung. 2015;193(5):639-651. PubMed
24. Jiménez D Uresandi F, Otero R, et al. Troponin-based risk stratification of patients with acute nonmassive pulmonary embolism; a systematic review and metaanalysis. Chest. 2009;136(4):974-982. PubMed
25. Becattini C, Vedovati MC, Agnelli G. Prognostic Value of Troponins in Acute Pulmonary Embolism: A Meta-Analysis. Circulation. 2007;116(4):427-433. PubMed
1. Grosse SD, Nelson RE, Nyarko KA, Richardson LC, Raskob GE. The economic burden of incident venous thromboembolism in the United States: A review of estimated attributable healthcare costs. Thromb Res. 2016;137:3-10 PubMed
2. Fanikos J, Rao A, Seger AC, Carter D, Piazza G, Goldhaber SZ. Hospital Costs of Acute Pulmonary Embolism. Am J Med. 2013;126(2):127-132. PubMed
3. LaMori JC, Shoheiber O, Mody SH, Bookart BK. Inpatient Resource Use and Cost Burden of Deep Vein Thrombosis and Pulmonary Embolism in the United States. Clin Ther. 2015;37(1):62-70. PubMed
4. Konstantinides S, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galié N, et al. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J. 2014;35(43):3033-3080. PubMed
5. Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149(2):315-352. PubMed
6. McGee S. Simplifying Likelihood Ratios. J Gen Intern Med. 2002;17(8):647-650. PubMed
7. Ahn S, Lee Y, Kim WY, Lim KS, Lee J. Prognostic Value of Treatment Setting in Patients With Cancer Having Pulmonary Embolism: Comparison With the Pulmonary Embolism Severity Index. Clin Appl Thromb Hemost. 2016;23(6):615-621. PubMed
8. Ozsu S, Bektas H, Abul Y, Ozlu T, Örem A. Value of Cardiac Troponin and sPESI in Treatment of Pulmonary Thromboembolism at Outpatient Setting. Lung. 2015;193(4):559-565. PubMed
9. Hakemi EU, Alyousef T, Dang G, Hakmei J, Doukky R. The prognostic value of undetectable highly sensitive cardiac troponin I in patients with acute pulmonary embolism. Chest. 2015;147(3):685-694. PubMed
10. Lauque D, Maupas-Schwalm F, Bounes V, et al. Predictive Value of the Heart‐type Fatty Acid–binding Protein and the Pulmonary Embolism Severity Index in Patients With Acute Pulmonary Embolism in the Emergency Department. Acad Emerg Med. 2014;21(10):1143-1150. PubMed
11. Vuilleumier N, Limacher A, Méan M, Choffat J, Lescuyer P, Bounameaux H, et al. Cardiac biomarkers and clinical scores for risk stratification in elderly patients with non‐high‐risk pulmonary embolism. J Intern Med. 2014;277(6):707-716. PubMed
12. Jiménez D, Kopecna D, Tapson V, et al. Derivation and validation of multimarker prognostication for normotensive patients with acute symptomatic pulmonary embolism. Am J Respir Crit Care Med. 2014;189(6):718-726. PubMed
13. Ozsu S, Abul Y, Orem A, et al. Predictive value of troponins and simplified pulmonary embolism severity index in patients with normotensive pulmonary embolism. Multidiscip Respir Med. 2013;8(1):34. PubMed
14. Sanchez O, Trinquart L, Planquette B, et al. Echocardiography and pulmonary embolism severity index have independent prognostic roles in pulmonary embolism. Eur Respir J. 2013;42(3):681-688. PubMed
15. Barra SN, Paiva L, Providéncia R, Fernandes A, Nascimento J, Marques AL. LR–PED Rule: Low Risk Pulmonary Embolism Decision Rule–A new decision score for low risk Pulmonary Embolism. Thromb Res. 2012;130(3):327-333. PubMed
16. Lankeit M, Jiménez D, Kostrubiec M, et al. Predictive Value of the High-Sensitivity Troponin T Assay and the Simplified Pulmonary Embolism Severity Index in Hemodynamically Stable Patients With Acute Pulmonary Embolism A Prospective Validation Study. Circulation. 2011;124(24):2716-2724. PubMed
17. Sánchez D, De Miguel J, Sam A, et al. The effects of cause of death classification on prognostic assessment of patients with pulmonary embolism. J Thromb Haemost. 2011;9(11):2201-2207. PubMed
18. Spirk D, Aujesky D, Husmann M, et al. Cardiac troponin testing and the simplified Pulmonary Embolism Severity Index. J Thromb Haemost. 2011;105(05):978-984. PubMed
19. Vanni S, Nazerian P, Pepe G, et al. Comparison of two prognostic models for acute pulmonary embolism: clinical vs. right ventricular dysfunction‐guided approach. J Thromb Haemos. 2011;9(10):1916-1923. PubMed
20. Jiménez D, Aujesky D, Moores L, et al. Combinations of prognostic tools for identification of high-risk normotensive patients with acute symptomatic pulmonary embolism. Thorax. 2011;66(1):75-81. PubMed
21. Singanayagam A, Scally C, Al-Khairalla MZ, et al. Are biomarkers additive to pulmonary embolism severity index for severity assessment in normotensive patients with acute pulmonary embolism? QJM. 2010;104(2):125-131. PubMed
22. Moores L, Aujesky D, Jimenez D, et al. Pulmonary Embolism Severity Index and troponin testing for the selection of low‐risk patients with acute symptomatic pulmonary embolism. J Thromb Haemost. 2009;8(3):517-522. PubMed
23. Bajaj A, Rathor P, Sehgal V, et al. Prognostic Value of Biomarkers in Acute Non-massive Pulmonary Embolism; A Sysemative Review and Meta-Analysis. Lung. 2015;193(5):639-651. PubMed
24. Jiménez D Uresandi F, Otero R, et al. Troponin-based risk stratification of patients with acute nonmassive pulmonary embolism; a systematic review and metaanalysis. Chest. 2009;136(4):974-982. PubMed
25. Becattini C, Vedovati MC, Agnelli G. Prognostic Value of Troponins in Acute Pulmonary Embolism: A Meta-Analysis. Circulation. 2007;116(4):427-433. PubMed
© 2018 Society of Hospital Medicine
ERRATUM TO: Cardiac Troponins in Low-Risk Pulmonary Embolism Patients: A Systematic Review and Meta-Analysis
The authors would like to make the following corrections to their manuscript, Cardiac Troponins in Low-Risk Pulmonary Embolism Patients: A Systematic Review and Meta-Analysis (doi: 10.12788/jhm.2961), published online first April 25, 2018 (all corrections in bold):
- The last sentence of the results section in the abstract should read: The pooled likelihood ratios (LRs) for all-cause mortality were positive LR 2.04 [95% CI, 1.53 to 2.72] and negative LR 0.72 [95% CI, 0.37 to 1.40].
- In the "All studies pooled" of the last row of Table 2, Tn+ is corrected to 463. See revised table below.
- On page E5, the first paragraph in the "Outcomes of Studies with Corresponding Troponin+ and Troponin-" section beginning with the fifth sentence should read as follows):
"In the pooled data, 463 (67%) patients tested negative for troponin and 228 (33%) tested positive. The overall mortality (from sensitivity analysis) including in-hospital, 30-day, and 90-day mortalities was 1.2%. The NPVs for all individual studies and the overall NPV are 1 or approximately 1. The overall PPVs and by study were low, ranging from 0 to 0.60. The PLRs and NLRs were not estimated for an outcome within an individual study if none of the patients experienced the outcome. When outcomes were only observed among troponin-negative patients, such as in the study of Moore (2009) who used 30-day all-cause mortality, the PLR had a value of zero. When outcomes were only observed among troponin-positive patients, as for 30-day all-cause mortality in the Hakemi9(2015), Lauque10 (2014), and Lankeit16 (2011) studies, the NLR had a value of zero. For zero cells, a continuity correction of 0.5 was applied. The pooled likelihood ratios (LRs) for all-cause mortality were positive LR 2.04 [95% CI, 1.53 to 2.72] and negative LR 0.72 [95% CI, 0.37 to 1.40]. The OR for all-cause mortality was 4.79 [95% CI 1.11 to 20.68, P = .0357].

The authors would like to make the following corrections to their manuscript, Cardiac Troponins in Low-Risk Pulmonary Embolism Patients: A Systematic Review and Meta-Analysis (doi: 10.12788/jhm.2961), published online first April 25, 2018 (all corrections in bold):
- The last sentence of the results section in the abstract should read: The pooled likelihood ratios (LRs) for all-cause mortality were positive LR 2.04 [95% CI, 1.53 to 2.72] and negative LR 0.72 [95% CI, 0.37 to 1.40].
- In the "All studies pooled" of the last row of Table 2, Tn+ is corrected to 463. See revised table below.
- On page E5, the first paragraph in the "Outcomes of Studies with Corresponding Troponin+ and Troponin-" section beginning with the fifth sentence should read as follows):
"In the pooled data, 463 (67%) patients tested negative for troponin and 228 (33%) tested positive. The overall mortality (from sensitivity analysis) including in-hospital, 30-day, and 90-day mortalities was 1.2%. The NPVs for all individual studies and the overall NPV are 1 or approximately 1. The overall PPVs and by study were low, ranging from 0 to 0.60. The PLRs and NLRs were not estimated for an outcome within an individual study if none of the patients experienced the outcome. When outcomes were only observed among troponin-negative patients, such as in the study of Moore (2009) who used 30-day all-cause mortality, the PLR had a value of zero. When outcomes were only observed among troponin-positive patients, as for 30-day all-cause mortality in the Hakemi9(2015), Lauque10 (2014), and Lankeit16 (2011) studies, the NLR had a value of zero. For zero cells, a continuity correction of 0.5 was applied. The pooled likelihood ratios (LRs) for all-cause mortality were positive LR 2.04 [95% CI, 1.53 to 2.72] and negative LR 0.72 [95% CI, 0.37 to 1.40]. The OR for all-cause mortality was 4.79 [95% CI 1.11 to 20.68, P = .0357].

The authors would like to make the following corrections to their manuscript, Cardiac Troponins in Low-Risk Pulmonary Embolism Patients: A Systematic Review and Meta-Analysis (doi: 10.12788/jhm.2961), published online first April 25, 2018 (all corrections in bold):
- The last sentence of the results section in the abstract should read: The pooled likelihood ratios (LRs) for all-cause mortality were positive LR 2.04 [95% CI, 1.53 to 2.72] and negative LR 0.72 [95% CI, 0.37 to 1.40].
- In the "All studies pooled" of the last row of Table 2, Tn+ is corrected to 463. See revised table below.
- On page E5, the first paragraph in the "Outcomes of Studies with Corresponding Troponin+ and Troponin-" section beginning with the fifth sentence should read as follows):
"In the pooled data, 463 (67%) patients tested negative for troponin and 228 (33%) tested positive. The overall mortality (from sensitivity analysis) including in-hospital, 30-day, and 90-day mortalities was 1.2%. The NPVs for all individual studies and the overall NPV are 1 or approximately 1. The overall PPVs and by study were low, ranging from 0 to 0.60. The PLRs and NLRs were not estimated for an outcome within an individual study if none of the patients experienced the outcome. When outcomes were only observed among troponin-negative patients, such as in the study of Moore (2009) who used 30-day all-cause mortality, the PLR had a value of zero. When outcomes were only observed among troponin-positive patients, as for 30-day all-cause mortality in the Hakemi9(2015), Lauque10 (2014), and Lankeit16 (2011) studies, the NLR had a value of zero. For zero cells, a continuity correction of 0.5 was applied. The pooled likelihood ratios (LRs) for all-cause mortality were positive LR 2.04 [95% CI, 1.53 to 2.72] and negative LR 0.72 [95% CI, 0.37 to 1.40]. The OR for all-cause mortality was 4.79 [95% CI 1.11 to 20.68, P = .0357].

© 2018 Society of Hospital Medicine