User login
Predicting failure of nonoperative management of spinal epidural abscess
Clinical question: Can one predict whether nonoperative management of spinal epidural abscesses will fail?
Background: Even though spinal epidural abscesses have a low incidence and nonspecific presentation, a delay in treatment can lead to significant morbidity. Previously, operative management was the preferred treatment; however, improvements in imaging and timing of diagnosis have led to an increased interest in nonoperative management. Few studies have identified possible predictors of failure for nonoperative management, and no algorithm exists for weighing the different possible predictors with the outcome of nonoperative management failure.
Study design: Retrospective cohort study.
Setting: A Massachusetts hospital system with two tertiary academic medical centers and three regional community hospitals.
Synopsis: The study evaluated 1,053 patients admitted with a spinal epidural abscess during 1993-2016. Of these, 432 patients were managed nonoperatively, and 367 were included in the analysis. Failure of nonoperative management occurred in 99 patients (27%). These patients were compared with 266 patients with successful nonoperative management with more than 60 days of follow-up. Six independent factors were associated with failure of nonoperative management including motor deficit at presentation (odds ratio, 7.85), pathological or compression fractures (OR, 6.12), active malignancy (OR, 3.32), diabetes (OR, 2.92), sensory changes at presentation (3.48), and location of the abscess dorsal to the thecal sac (OR, 0.29). Subsequently, a clinical algorithm was created to predict the likelihood of failure of nonoperative management.
Because of its retrospective design, the study was unable to assess the efficacy of surgery versus nonoperative management.
Bottom line: Specific measures of general health, neurologic status at presentation, and anatomical data of a patient with a spinal epidural abscess have led to the development of a clinical algorithm to determine the risk of failure in nonoperative management of spinal epidural abscesses.
Citation: Shah AA et al. Nonoperative management of spinal epidural abscess: Development of a predictive algorithm for failure. J Bone Joint Surg Am. 2018;100(7):546-55.
Dr. Tsien is a hospitalist in the division of hospital medicine in the department of medicine at Loyola University Chicago, Maywood, Ill.
Clinical question: Can one predict whether nonoperative management of spinal epidural abscesses will fail?
Background: Even though spinal epidural abscesses have a low incidence and nonspecific presentation, a delay in treatment can lead to significant morbidity. Previously, operative management was the preferred treatment; however, improvements in imaging and timing of diagnosis have led to an increased interest in nonoperative management. Few studies have identified possible predictors of failure for nonoperative management, and no algorithm exists for weighing the different possible predictors with the outcome of nonoperative management failure.
Study design: Retrospective cohort study.
Setting: A Massachusetts hospital system with two tertiary academic medical centers and three regional community hospitals.
Synopsis: The study evaluated 1,053 patients admitted with a spinal epidural abscess during 1993-2016. Of these, 432 patients were managed nonoperatively, and 367 were included in the analysis. Failure of nonoperative management occurred in 99 patients (27%). These patients were compared with 266 patients with successful nonoperative management with more than 60 days of follow-up. Six independent factors were associated with failure of nonoperative management including motor deficit at presentation (odds ratio, 7.85), pathological or compression fractures (OR, 6.12), active malignancy (OR, 3.32), diabetes (OR, 2.92), sensory changes at presentation (3.48), and location of the abscess dorsal to the thecal sac (OR, 0.29). Subsequently, a clinical algorithm was created to predict the likelihood of failure of nonoperative management.
Because of its retrospective design, the study was unable to assess the efficacy of surgery versus nonoperative management.
Bottom line: Specific measures of general health, neurologic status at presentation, and anatomical data of a patient with a spinal epidural abscess have led to the development of a clinical algorithm to determine the risk of failure in nonoperative management of spinal epidural abscesses.
Citation: Shah AA et al. Nonoperative management of spinal epidural abscess: Development of a predictive algorithm for failure. J Bone Joint Surg Am. 2018;100(7):546-55.
Dr. Tsien is a hospitalist in the division of hospital medicine in the department of medicine at Loyola University Chicago, Maywood, Ill.
Clinical question: Can one predict whether nonoperative management of spinal epidural abscesses will fail?
Background: Even though spinal epidural abscesses have a low incidence and nonspecific presentation, a delay in treatment can lead to significant morbidity. Previously, operative management was the preferred treatment; however, improvements in imaging and timing of diagnosis have led to an increased interest in nonoperative management. Few studies have identified possible predictors of failure for nonoperative management, and no algorithm exists for weighing the different possible predictors with the outcome of nonoperative management failure.
Study design: Retrospective cohort study.
Setting: A Massachusetts hospital system with two tertiary academic medical centers and three regional community hospitals.
Synopsis: The study evaluated 1,053 patients admitted with a spinal epidural abscess during 1993-2016. Of these, 432 patients were managed nonoperatively, and 367 were included in the analysis. Failure of nonoperative management occurred in 99 patients (27%). These patients were compared with 266 patients with successful nonoperative management with more than 60 days of follow-up. Six independent factors were associated with failure of nonoperative management including motor deficit at presentation (odds ratio, 7.85), pathological or compression fractures (OR, 6.12), active malignancy (OR, 3.32), diabetes (OR, 2.92), sensory changes at presentation (3.48), and location of the abscess dorsal to the thecal sac (OR, 0.29). Subsequently, a clinical algorithm was created to predict the likelihood of failure of nonoperative management.
Because of its retrospective design, the study was unable to assess the efficacy of surgery versus nonoperative management.
Bottom line: Specific measures of general health, neurologic status at presentation, and anatomical data of a patient with a spinal epidural abscess have led to the development of a clinical algorithm to determine the risk of failure in nonoperative management of spinal epidural abscesses.
Citation: Shah AA et al. Nonoperative management of spinal epidural abscess: Development of a predictive algorithm for failure. J Bone Joint Surg Am. 2018;100(7):546-55.
Dr. Tsien is a hospitalist in the division of hospital medicine in the department of medicine at Loyola University Chicago, Maywood, Ill.
Research Advances Look Bright for VA
Key leadership in research and oncology addressed attendees of the recent AVAHO annual meeting in Chicago. Carolyn Clancy, MD, deputy undersecretary for health discovery, education and affiliated networks, discussed the important role of cancer in the VA’s research and clinical mission. Neil Spector, MD, director of the National Precision Oncology Program (NPOP) outlined the significant growth in the use of tumor sequencing by NPOP, while Michael Kelley, MD, director of operations for National Oncology discussed the significant strides in opening up access to clinical trials at the VA.
Dr. Clancy addressed AVAHO for the first time and remarked upon the impressive array of research conducted by AVAHO members. “I love the idea of telehealth for genomics,” she remarked about the Genomic Medicine Service Uses Group Telehealth Appointments poster abstract, “it’s brilliant and it’s only the beginning.” The VA’s unique blend of clinical care and research puts it in a unique position to provide cutting edge care to its patients.
Clancy also addressed the larger shift in the VA as it moves from a closed integrated health care system to a high performing network. “We are closer than most systems in this country—public or private—to having a research enterprise that is integral to our mission of providing veterans with great care,” she said. “The magic of bringing [research and clinical] groups together is to enhance the visibility. But frankly it’s also to enhance our capability to take advantage of these assets strategically.” That means providing veterans with “cutting-edge care, and what could be better than that? When we do great things in how we deliver health care that helps your work,” she told attendees.
This shift, as outlined by the VA’s new leadership under Secretary Robert Wilkie and Richard A. Stone, MD, executive in charge of the Veterans Health Administration (VHA), is designed to restore veterans’ trust and confidence in the system, foster an environment of continuous learning to improve quality, and transform the VHA into a “high-reliability organization,” to reduce medical errors. The goal, according to Dr. Clancy, is to develop a culture—like the National Aeronautics and Space Administration or air traffic control systems—where all members of the organization search for and eliminate potential problems. Improving safety, she insisted, has to be a top priority for everyone.
Dr. Clancy also reported that 700,000 veterans enrolled in the VA’s Million Veteran Program (MVP) “We not only have the largest repository of genomic information on people but we also have their clinical data” Later, she told AVAHO members “I am hugely optimistic about the work that is being done in oncology research.” In July, the VA made an arrangement with the National Cancer Institute to allows veterans access to clinical trials. “We need to do more of that, she said, “this is only the beginning of the exciting work we will be doing in cancer research.”
Dr. Spector reported on the progress made by NPOP over the previous year. Currently, NPOP is sequencing solid tumors with a recent biopsy (liquid biopsies are acceptable), but hopes to begin examination of sarcomas and hematologic malignancies. NPOP has grown from about 100 samples analyzed monthly in January 2017 to nearly 350 in June 2018 with a goal of reaching 600 monthly samples across the VA. “You should be sending tumor tissues to be sequenced,” he explained. “It’s free, sequencing tumor tissue is the standard of care, and we need to be sequencing our patients to provide them with an opportunity to get patients onto clinical trials.”
Although the initial analysis can take up to 21 days, the program offers a 72-hour turn-around time for e-consultations. Depending on the quality of trial data, patients may be eligible for treatment even if there is no FDA-approved treatment. According to Spector, the goal of the program is to get patients on the right treatment and avoid costly treatment that will not work for a patient’s cancer type. “We do not want to be giving an expensive drug to someone who will not respond,” he explained.
Multiple efforts are underway to streamline and increase access to oncology care in the VA, according to Dr. Kelley. The development of a national cancer strategy is “long overdue” he admitted, but multiple efforts are underway to including the Fast Track to VA Cancer Care, a single national point of entry for VA cancer care, mechanisms to streamline enrolling patients in non-VA clinical trials, virtual tumor boards, and oncology-specific dashboards. “We have to be transparent and show not only to ourselves, but the whole worlds that we are doing a great job,” he told attendees.
One of the biggest challenges the VA faces will be the roll out of a new electronic health record system. While the new Cerner system has an oncology package, it does not have a cancer registry. According to Kelley, the VA is searching for a commercial system that can interface with Cerner to provide a cancer registry.
Kelley also focused on Annie, a new VA texting platform that allow patients to report on symptoms and get advice The Annie system is automated and allows patients to provide self-care. Already, cancer care providers are experimenting with Annie and Kelley expects the program to develop further.
Key leadership in research and oncology addressed attendees of the recent AVAHO annual meeting in Chicago. Carolyn Clancy, MD, deputy undersecretary for health discovery, education and affiliated networks, discussed the important role of cancer in the VA’s research and clinical mission. Neil Spector, MD, director of the National Precision Oncology Program (NPOP) outlined the significant growth in the use of tumor sequencing by NPOP, while Michael Kelley, MD, director of operations for National Oncology discussed the significant strides in opening up access to clinical trials at the VA.
Dr. Clancy addressed AVAHO for the first time and remarked upon the impressive array of research conducted by AVAHO members. “I love the idea of telehealth for genomics,” she remarked about the Genomic Medicine Service Uses Group Telehealth Appointments poster abstract, “it’s brilliant and it’s only the beginning.” The VA’s unique blend of clinical care and research puts it in a unique position to provide cutting edge care to its patients.
Clancy also addressed the larger shift in the VA as it moves from a closed integrated health care system to a high performing network. “We are closer than most systems in this country—public or private—to having a research enterprise that is integral to our mission of providing veterans with great care,” she said. “The magic of bringing [research and clinical] groups together is to enhance the visibility. But frankly it’s also to enhance our capability to take advantage of these assets strategically.” That means providing veterans with “cutting-edge care, and what could be better than that? When we do great things in how we deliver health care that helps your work,” she told attendees.
This shift, as outlined by the VA’s new leadership under Secretary Robert Wilkie and Richard A. Stone, MD, executive in charge of the Veterans Health Administration (VHA), is designed to restore veterans’ trust and confidence in the system, foster an environment of continuous learning to improve quality, and transform the VHA into a “high-reliability organization,” to reduce medical errors. The goal, according to Dr. Clancy, is to develop a culture—like the National Aeronautics and Space Administration or air traffic control systems—where all members of the organization search for and eliminate potential problems. Improving safety, she insisted, has to be a top priority for everyone.
Dr. Clancy also reported that 700,000 veterans enrolled in the VA’s Million Veteran Program (MVP) “We not only have the largest repository of genomic information on people but we also have their clinical data” Later, she told AVAHO members “I am hugely optimistic about the work that is being done in oncology research.” In July, the VA made an arrangement with the National Cancer Institute to allows veterans access to clinical trials. “We need to do more of that, she said, “this is only the beginning of the exciting work we will be doing in cancer research.”
Dr. Spector reported on the progress made by NPOP over the previous year. Currently, NPOP is sequencing solid tumors with a recent biopsy (liquid biopsies are acceptable), but hopes to begin examination of sarcomas and hematologic malignancies. NPOP has grown from about 100 samples analyzed monthly in January 2017 to nearly 350 in June 2018 with a goal of reaching 600 monthly samples across the VA. “You should be sending tumor tissues to be sequenced,” he explained. “It’s free, sequencing tumor tissue is the standard of care, and we need to be sequencing our patients to provide them with an opportunity to get patients onto clinical trials.”
Although the initial analysis can take up to 21 days, the program offers a 72-hour turn-around time for e-consultations. Depending on the quality of trial data, patients may be eligible for treatment even if there is no FDA-approved treatment. According to Spector, the goal of the program is to get patients on the right treatment and avoid costly treatment that will not work for a patient’s cancer type. “We do not want to be giving an expensive drug to someone who will not respond,” he explained.
Multiple efforts are underway to streamline and increase access to oncology care in the VA, according to Dr. Kelley. The development of a national cancer strategy is “long overdue” he admitted, but multiple efforts are underway to including the Fast Track to VA Cancer Care, a single national point of entry for VA cancer care, mechanisms to streamline enrolling patients in non-VA clinical trials, virtual tumor boards, and oncology-specific dashboards. “We have to be transparent and show not only to ourselves, but the whole worlds that we are doing a great job,” he told attendees.
One of the biggest challenges the VA faces will be the roll out of a new electronic health record system. While the new Cerner system has an oncology package, it does not have a cancer registry. According to Kelley, the VA is searching for a commercial system that can interface with Cerner to provide a cancer registry.
Kelley also focused on Annie, a new VA texting platform that allow patients to report on symptoms and get advice The Annie system is automated and allows patients to provide self-care. Already, cancer care providers are experimenting with Annie and Kelley expects the program to develop further.
Key leadership in research and oncology addressed attendees of the recent AVAHO annual meeting in Chicago. Carolyn Clancy, MD, deputy undersecretary for health discovery, education and affiliated networks, discussed the important role of cancer in the VA’s research and clinical mission. Neil Spector, MD, director of the National Precision Oncology Program (NPOP) outlined the significant growth in the use of tumor sequencing by NPOP, while Michael Kelley, MD, director of operations for National Oncology discussed the significant strides in opening up access to clinical trials at the VA.
Dr. Clancy addressed AVAHO for the first time and remarked upon the impressive array of research conducted by AVAHO members. “I love the idea of telehealth for genomics,” she remarked about the Genomic Medicine Service Uses Group Telehealth Appointments poster abstract, “it’s brilliant and it’s only the beginning.” The VA’s unique blend of clinical care and research puts it in a unique position to provide cutting edge care to its patients.
Clancy also addressed the larger shift in the VA as it moves from a closed integrated health care system to a high performing network. “We are closer than most systems in this country—public or private—to having a research enterprise that is integral to our mission of providing veterans with great care,” she said. “The magic of bringing [research and clinical] groups together is to enhance the visibility. But frankly it’s also to enhance our capability to take advantage of these assets strategically.” That means providing veterans with “cutting-edge care, and what could be better than that? When we do great things in how we deliver health care that helps your work,” she told attendees.
This shift, as outlined by the VA’s new leadership under Secretary Robert Wilkie and Richard A. Stone, MD, executive in charge of the Veterans Health Administration (VHA), is designed to restore veterans’ trust and confidence in the system, foster an environment of continuous learning to improve quality, and transform the VHA into a “high-reliability organization,” to reduce medical errors. The goal, according to Dr. Clancy, is to develop a culture—like the National Aeronautics and Space Administration or air traffic control systems—where all members of the organization search for and eliminate potential problems. Improving safety, she insisted, has to be a top priority for everyone.
Dr. Clancy also reported that 700,000 veterans enrolled in the VA’s Million Veteran Program (MVP) “We not only have the largest repository of genomic information on people but we also have their clinical data” Later, she told AVAHO members “I am hugely optimistic about the work that is being done in oncology research.” In July, the VA made an arrangement with the National Cancer Institute to allows veterans access to clinical trials. “We need to do more of that, she said, “this is only the beginning of the exciting work we will be doing in cancer research.”
Dr. Spector reported on the progress made by NPOP over the previous year. Currently, NPOP is sequencing solid tumors with a recent biopsy (liquid biopsies are acceptable), but hopes to begin examination of sarcomas and hematologic malignancies. NPOP has grown from about 100 samples analyzed monthly in January 2017 to nearly 350 in June 2018 with a goal of reaching 600 monthly samples across the VA. “You should be sending tumor tissues to be sequenced,” he explained. “It’s free, sequencing tumor tissue is the standard of care, and we need to be sequencing our patients to provide them with an opportunity to get patients onto clinical trials.”
Although the initial analysis can take up to 21 days, the program offers a 72-hour turn-around time for e-consultations. Depending on the quality of trial data, patients may be eligible for treatment even if there is no FDA-approved treatment. According to Spector, the goal of the program is to get patients on the right treatment and avoid costly treatment that will not work for a patient’s cancer type. “We do not want to be giving an expensive drug to someone who will not respond,” he explained.
Multiple efforts are underway to streamline and increase access to oncology care in the VA, according to Dr. Kelley. The development of a national cancer strategy is “long overdue” he admitted, but multiple efforts are underway to including the Fast Track to VA Cancer Care, a single national point of entry for VA cancer care, mechanisms to streamline enrolling patients in non-VA clinical trials, virtual tumor boards, and oncology-specific dashboards. “We have to be transparent and show not only to ourselves, but the whole worlds that we are doing a great job,” he told attendees.
One of the biggest challenges the VA faces will be the roll out of a new electronic health record system. While the new Cerner system has an oncology package, it does not have a cancer registry. According to Kelley, the VA is searching for a commercial system that can interface with Cerner to provide a cancer registry.
Kelley also focused on Annie, a new VA texting platform that allow patients to report on symptoms and get advice The Annie system is automated and allows patients to provide self-care. Already, cancer care providers are experimenting with Annie and Kelley expects the program to develop further.
FDA approves Arikayce for MAC lung diseases
that has been caused by members of the Mycobacterium avium complex and is refractory to other treatments.
In a randomized, controlled trial, patients with refractory M. avium complex infections were assigned to receive either Arikayce plus a multidrug antibacterial regimen or just the antibacterial regimen. By 6 months, sputum cultures for 29% of those treated with the combination had shown no mycobacterial growth for 3 consecutive months, whereas this was only true for the cultures for 9% of patients on the multidrug antibacterial regimen alone.
The Arikayce prescribing information includes a boxed warning regarding the increased risk of respiratory conditions, including hypersensitivity pneumonitis, bronchospasm, exacerbation of underlying lung disease, and hemoptysis, some of which have proven serious enough to lead to hospitalization. Other side effects include dysphonia, cough, musculoskeletal pain, nausea, and fatigue.
According to the press announcement from the FDA, this is the first approval under the Limited Population Pathway for Antibacterial and Antifungal Drugs, which was set up by Congress “to advance development and approval of antibacterial and antifungal drugs to treat serious or life-threatening infections in a limited population of patients with unmet need.” It does so by allowing a more streamlined clinical development program that may involve smaller, shorter, or fewer clinical trials.
More information can be found in the full press announcement.
that has been caused by members of the Mycobacterium avium complex and is refractory to other treatments.
In a randomized, controlled trial, patients with refractory M. avium complex infections were assigned to receive either Arikayce plus a multidrug antibacterial regimen or just the antibacterial regimen. By 6 months, sputum cultures for 29% of those treated with the combination had shown no mycobacterial growth for 3 consecutive months, whereas this was only true for the cultures for 9% of patients on the multidrug antibacterial regimen alone.
The Arikayce prescribing information includes a boxed warning regarding the increased risk of respiratory conditions, including hypersensitivity pneumonitis, bronchospasm, exacerbation of underlying lung disease, and hemoptysis, some of which have proven serious enough to lead to hospitalization. Other side effects include dysphonia, cough, musculoskeletal pain, nausea, and fatigue.
According to the press announcement from the FDA, this is the first approval under the Limited Population Pathway for Antibacterial and Antifungal Drugs, which was set up by Congress “to advance development and approval of antibacterial and antifungal drugs to treat serious or life-threatening infections in a limited population of patients with unmet need.” It does so by allowing a more streamlined clinical development program that may involve smaller, shorter, or fewer clinical trials.
More information can be found in the full press announcement.
that has been caused by members of the Mycobacterium avium complex and is refractory to other treatments.
In a randomized, controlled trial, patients with refractory M. avium complex infections were assigned to receive either Arikayce plus a multidrug antibacterial regimen or just the antibacterial regimen. By 6 months, sputum cultures for 29% of those treated with the combination had shown no mycobacterial growth for 3 consecutive months, whereas this was only true for the cultures for 9% of patients on the multidrug antibacterial regimen alone.
The Arikayce prescribing information includes a boxed warning regarding the increased risk of respiratory conditions, including hypersensitivity pneumonitis, bronchospasm, exacerbation of underlying lung disease, and hemoptysis, some of which have proven serious enough to lead to hospitalization. Other side effects include dysphonia, cough, musculoskeletal pain, nausea, and fatigue.
According to the press announcement from the FDA, this is the first approval under the Limited Population Pathway for Antibacterial and Antifungal Drugs, which was set up by Congress “to advance development and approval of antibacterial and antifungal drugs to treat serious or life-threatening infections in a limited population of patients with unmet need.” It does so by allowing a more streamlined clinical development program that may involve smaller, shorter, or fewer clinical trials.
More information can be found in the full press announcement.
Adalimumab safety update finds no new signals
not included in the previous 2009 analysis; their evaluation of data from these 18 trials found no new safety signals, they reported in the British Journal of Dermatology.
Adverse event incidence rates were expressed as events per 100 patient-years of exposure to adalimumab and, among the 3,727 patients who were aged 18 years or older and had moderate to severe plaque psoriasis for at least 6 months, there were 5,430 patient-years of cumulative exposure at the December 2015 cutoff date.
There were 3,798 treatment-related events altogether (70 events/100 patient-years); 269 events (5 events/100 patient-years ) led to discontinuation of treatment. The rates for serious adverse events and serious infections were 8.4 and 1.8 events per 100 patient-years, respectively; the most common types of serious infections were pneumonia and cellulitis.
The rates of the most frequently reported adverse events were comparable with those in the 2009 data set, with the most common being nasopharyngitis, upper respiratory tract infection, and headache. Furthermore, the rates of serious adverse events, serious infections, and malignancies were also stable, even with the increasing adalimumab exposure, and these were mostly consistent with what has been seen in large real-world registries.
The researchers did note that the rates of melanoma and nonmelanoma skin cancer were higher than would be expected in the general population, but they suspected this was at least partly because these psoriasis patients were receiving more frequent skin examinations and more skin cancers were being detected. (Incidence rates for these two cancers were stable during 2009-2015).
The analysis had certain limitations, such as a lack of a long-term comparator group. Also, while some patients continue to receive adalimumab for more than 10 years, the maximum duration of treatment in this analysis was only 5.5 years. Finally, the population in these clinical trials may differ from that seen in general practice settings because of the inclusion/exclusion criteria.
Six authors of the study reported multiple disclosures with pharmaceutical companies, including serving as a consultant, speaker, and/or adviser for, receiving honoraria from, and/or receiving grant/research support from AbbVie, which developed adalimumab and funded/advised this study; two authors are AbbVie employees, one is a former employee.
SOURCE: Leonardi C et al. Br J Dermatol. 2018 Aug 31. doi: 10.1111/bjd.17084.
not included in the previous 2009 analysis; their evaluation of data from these 18 trials found no new safety signals, they reported in the British Journal of Dermatology.
Adverse event incidence rates were expressed as events per 100 patient-years of exposure to adalimumab and, among the 3,727 patients who were aged 18 years or older and had moderate to severe plaque psoriasis for at least 6 months, there were 5,430 patient-years of cumulative exposure at the December 2015 cutoff date.
There were 3,798 treatment-related events altogether (70 events/100 patient-years); 269 events (5 events/100 patient-years ) led to discontinuation of treatment. The rates for serious adverse events and serious infections were 8.4 and 1.8 events per 100 patient-years, respectively; the most common types of serious infections were pneumonia and cellulitis.
The rates of the most frequently reported adverse events were comparable with those in the 2009 data set, with the most common being nasopharyngitis, upper respiratory tract infection, and headache. Furthermore, the rates of serious adverse events, serious infections, and malignancies were also stable, even with the increasing adalimumab exposure, and these were mostly consistent with what has been seen in large real-world registries.
The researchers did note that the rates of melanoma and nonmelanoma skin cancer were higher than would be expected in the general population, but they suspected this was at least partly because these psoriasis patients were receiving more frequent skin examinations and more skin cancers were being detected. (Incidence rates for these two cancers were stable during 2009-2015).
The analysis had certain limitations, such as a lack of a long-term comparator group. Also, while some patients continue to receive adalimumab for more than 10 years, the maximum duration of treatment in this analysis was only 5.5 years. Finally, the population in these clinical trials may differ from that seen in general practice settings because of the inclusion/exclusion criteria.
Six authors of the study reported multiple disclosures with pharmaceutical companies, including serving as a consultant, speaker, and/or adviser for, receiving honoraria from, and/or receiving grant/research support from AbbVie, which developed adalimumab and funded/advised this study; two authors are AbbVie employees, one is a former employee.
SOURCE: Leonardi C et al. Br J Dermatol. 2018 Aug 31. doi: 10.1111/bjd.17084.
not included in the previous 2009 analysis; their evaluation of data from these 18 trials found no new safety signals, they reported in the British Journal of Dermatology.
Adverse event incidence rates were expressed as events per 100 patient-years of exposure to adalimumab and, among the 3,727 patients who were aged 18 years or older and had moderate to severe plaque psoriasis for at least 6 months, there were 5,430 patient-years of cumulative exposure at the December 2015 cutoff date.
There were 3,798 treatment-related events altogether (70 events/100 patient-years); 269 events (5 events/100 patient-years ) led to discontinuation of treatment. The rates for serious adverse events and serious infections were 8.4 and 1.8 events per 100 patient-years, respectively; the most common types of serious infections were pneumonia and cellulitis.
The rates of the most frequently reported adverse events were comparable with those in the 2009 data set, with the most common being nasopharyngitis, upper respiratory tract infection, and headache. Furthermore, the rates of serious adverse events, serious infections, and malignancies were also stable, even with the increasing adalimumab exposure, and these were mostly consistent with what has been seen in large real-world registries.
The researchers did note that the rates of melanoma and nonmelanoma skin cancer were higher than would be expected in the general population, but they suspected this was at least partly because these psoriasis patients were receiving more frequent skin examinations and more skin cancers were being detected. (Incidence rates for these two cancers were stable during 2009-2015).
The analysis had certain limitations, such as a lack of a long-term comparator group. Also, while some patients continue to receive adalimumab for more than 10 years, the maximum duration of treatment in this analysis was only 5.5 years. Finally, the population in these clinical trials may differ from that seen in general practice settings because of the inclusion/exclusion criteria.
Six authors of the study reported multiple disclosures with pharmaceutical companies, including serving as a consultant, speaker, and/or adviser for, receiving honoraria from, and/or receiving grant/research support from AbbVie, which developed adalimumab and funded/advised this study; two authors are AbbVie employees, one is a former employee.
SOURCE: Leonardi C et al. Br J Dermatol. 2018 Aug 31. doi: 10.1111/bjd.17084.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Marcia Morris: College drinking
Dr. Morris is associate professor of psychiatry and associate program director for Student Health Psychiatry at the University of Florida.
Dr. Morris is associate professor of psychiatry and associate program director for Student Health Psychiatry at the University of Florida.
Dr. Morris is associate professor of psychiatry and associate program director for Student Health Psychiatry at the University of Florida.
Neurological disease and aging
Also today, trivalent adjuvant influenza vaccine aIIV3 is safe in elderly adults, pill burden affects the ability to reach systolic BP control, and breast cancer risk in type 2 diabetes related to adiposity.
Amazon Alexa
Apple Podcasts
Spotify
Also today, trivalent adjuvant influenza vaccine aIIV3 is safe in elderly adults, pill burden affects the ability to reach systolic BP control, and breast cancer risk in type 2 diabetes related to adiposity.
Amazon Alexa
Apple Podcasts
Spotify
Also today, trivalent adjuvant influenza vaccine aIIV3 is safe in elderly adults, pill burden affects the ability to reach systolic BP control, and breast cancer risk in type 2 diabetes related to adiposity.
Amazon Alexa
Apple Podcasts
Spotify
Researchers develop genetics-based prognostic tool for MDS
Researchers have developed a new risk model for primary myelodysplastic syndromes (MDS) that integrates genetic and clinical information.
The research team considered the current standard for prognostication—the revised International Prognostic Scoring System (IPSS-R)—to be too complex, limited to newly diagnosed cases, and missing information on mutations and age.
So they devised a “simpler and more contemporary” prognostic system, the Mayo Alliance Prognostic Model for MDS.
The team, from the Mayo Clinic in Rochester, Minnesota, and the National Taiwan University Hospital (NTUH), described the new model in Mayo Clinic Proceedings.
Lead author Ayalew Tefferi, MD, of the Mayo Clinic, said the new model “is not an enhancement of the international prognostic scoring system tool, it's a complete makeover."
The team analyzed mutation information from 357 patients with primary MDS or leukemic transformation treated at the Mayo Clinic from the end of December 1994 through mid-December 2017.
The patients were a median age of 74 and 70% were males.
They compared the Mayo patients to 328 NTUH patients, who were a median age of 66 and 65% were males.
Multivariate analysis of the Mayo cohort identified the following as predictors of inferior overall survival:
- Monosomal karyotype (hazard ratio [HR], 5.2; 95% CI, 3.1-8.6)
- Non-monosomal karyotype abnormalities other than single/double del(5q) (HR, 1.8; 95% CI, 1.3-2.6)
- RUNX1 (HR, 2.0; 95% CI, 1.2-3.1)
- ASXL1 (HR, 1.7; 95% CI, 1.2-2.3) mutations
- Absence of SF3B1 mutations (HR, 1.6; 95% CI, 1.1-2.4)
- Age greater than 70 years (HR, 2.2; 95% CI, 1.6-3.1)
- Hemoglobin level less than 8 g/dL in women or less than 9 g/dL in men (HR, 2.3; 95% CI, 1.7-3.1)
- Platelet count less than 75 x 109/L (HR, 1.5; 95% CI, 1.1-2.1)
- 10% or more bone marrow blasts (HR, 1.7; 95% CI, 1.1-2.8)
They then provided values to reflect the prognostic contribution of each of the above predictors and devised the new 4-tiered Mayo prognostic model.
Median 5-year overall survival rates in the 4 categories in the Mayo model were 73% (low risk), 34% (intermediate-1), 7% (intermediate-2), and 0% (high risk; 9-month median survival).
The team then validated the Mayo alliance model by using the NTUH cohort and compared it to the IPSS-R.
The investigators were able to confirm superior predictive accuracy of their model and a substantial discordance between the the Mayo model and the IPSS-R in terms of the pattern of risk distribution.
Examples of discordance included:
- More than 25% of patients belonging to the high-risk category according to the Mayo alliance model were classified as IPSS-R low or intermediate risk
- Almost 50% of patients with intermediate-2 risk category according to the Mayo alliance model were classified as IPSS-R very low or low risk
- Almost 50% of patients with IPSS-R very low risk were classified as intermediate-2 or intermediate-1 risk according to the Mayo alliance model
The authors wrote that this “suggests a fundamental and not incremental advantage for the new Mayo alliance model.”
Researchers have developed a new risk model for primary myelodysplastic syndromes (MDS) that integrates genetic and clinical information.
The research team considered the current standard for prognostication—the revised International Prognostic Scoring System (IPSS-R)—to be too complex, limited to newly diagnosed cases, and missing information on mutations and age.
So they devised a “simpler and more contemporary” prognostic system, the Mayo Alliance Prognostic Model for MDS.
The team, from the Mayo Clinic in Rochester, Minnesota, and the National Taiwan University Hospital (NTUH), described the new model in Mayo Clinic Proceedings.
Lead author Ayalew Tefferi, MD, of the Mayo Clinic, said the new model “is not an enhancement of the international prognostic scoring system tool, it's a complete makeover."
The team analyzed mutation information from 357 patients with primary MDS or leukemic transformation treated at the Mayo Clinic from the end of December 1994 through mid-December 2017.
The patients were a median age of 74 and 70% were males.
They compared the Mayo patients to 328 NTUH patients, who were a median age of 66 and 65% were males.
Multivariate analysis of the Mayo cohort identified the following as predictors of inferior overall survival:
- Monosomal karyotype (hazard ratio [HR], 5.2; 95% CI, 3.1-8.6)
- Non-monosomal karyotype abnormalities other than single/double del(5q) (HR, 1.8; 95% CI, 1.3-2.6)
- RUNX1 (HR, 2.0; 95% CI, 1.2-3.1)
- ASXL1 (HR, 1.7; 95% CI, 1.2-2.3) mutations
- Absence of SF3B1 mutations (HR, 1.6; 95% CI, 1.1-2.4)
- Age greater than 70 years (HR, 2.2; 95% CI, 1.6-3.1)
- Hemoglobin level less than 8 g/dL in women or less than 9 g/dL in men (HR, 2.3; 95% CI, 1.7-3.1)
- Platelet count less than 75 x 109/L (HR, 1.5; 95% CI, 1.1-2.1)
- 10% or more bone marrow blasts (HR, 1.7; 95% CI, 1.1-2.8)
They then provided values to reflect the prognostic contribution of each of the above predictors and devised the new 4-tiered Mayo prognostic model.
Median 5-year overall survival rates in the 4 categories in the Mayo model were 73% (low risk), 34% (intermediate-1), 7% (intermediate-2), and 0% (high risk; 9-month median survival).
The team then validated the Mayo alliance model by using the NTUH cohort and compared it to the IPSS-R.
The investigators were able to confirm superior predictive accuracy of their model and a substantial discordance between the the Mayo model and the IPSS-R in terms of the pattern of risk distribution.
Examples of discordance included:
- More than 25% of patients belonging to the high-risk category according to the Mayo alliance model were classified as IPSS-R low or intermediate risk
- Almost 50% of patients with intermediate-2 risk category according to the Mayo alliance model were classified as IPSS-R very low or low risk
- Almost 50% of patients with IPSS-R very low risk were classified as intermediate-2 or intermediate-1 risk according to the Mayo alliance model
The authors wrote that this “suggests a fundamental and not incremental advantage for the new Mayo alliance model.”
Researchers have developed a new risk model for primary myelodysplastic syndromes (MDS) that integrates genetic and clinical information.
The research team considered the current standard for prognostication—the revised International Prognostic Scoring System (IPSS-R)—to be too complex, limited to newly diagnosed cases, and missing information on mutations and age.
So they devised a “simpler and more contemporary” prognostic system, the Mayo Alliance Prognostic Model for MDS.
The team, from the Mayo Clinic in Rochester, Minnesota, and the National Taiwan University Hospital (NTUH), described the new model in Mayo Clinic Proceedings.
Lead author Ayalew Tefferi, MD, of the Mayo Clinic, said the new model “is not an enhancement of the international prognostic scoring system tool, it's a complete makeover."
The team analyzed mutation information from 357 patients with primary MDS or leukemic transformation treated at the Mayo Clinic from the end of December 1994 through mid-December 2017.
The patients were a median age of 74 and 70% were males.
They compared the Mayo patients to 328 NTUH patients, who were a median age of 66 and 65% were males.
Multivariate analysis of the Mayo cohort identified the following as predictors of inferior overall survival:
- Monosomal karyotype (hazard ratio [HR], 5.2; 95% CI, 3.1-8.6)
- Non-monosomal karyotype abnormalities other than single/double del(5q) (HR, 1.8; 95% CI, 1.3-2.6)
- RUNX1 (HR, 2.0; 95% CI, 1.2-3.1)
- ASXL1 (HR, 1.7; 95% CI, 1.2-2.3) mutations
- Absence of SF3B1 mutations (HR, 1.6; 95% CI, 1.1-2.4)
- Age greater than 70 years (HR, 2.2; 95% CI, 1.6-3.1)
- Hemoglobin level less than 8 g/dL in women or less than 9 g/dL in men (HR, 2.3; 95% CI, 1.7-3.1)
- Platelet count less than 75 x 109/L (HR, 1.5; 95% CI, 1.1-2.1)
- 10% or more bone marrow blasts (HR, 1.7; 95% CI, 1.1-2.8)
They then provided values to reflect the prognostic contribution of each of the above predictors and devised the new 4-tiered Mayo prognostic model.
Median 5-year overall survival rates in the 4 categories in the Mayo model were 73% (low risk), 34% (intermediate-1), 7% (intermediate-2), and 0% (high risk; 9-month median survival).
The team then validated the Mayo alliance model by using the NTUH cohort and compared it to the IPSS-R.
The investigators were able to confirm superior predictive accuracy of their model and a substantial discordance between the the Mayo model and the IPSS-R in terms of the pattern of risk distribution.
Examples of discordance included:
- More than 25% of patients belonging to the high-risk category according to the Mayo alliance model were classified as IPSS-R low or intermediate risk
- Almost 50% of patients with intermediate-2 risk category according to the Mayo alliance model were classified as IPSS-R very low or low risk
- Almost 50% of patients with IPSS-R very low risk were classified as intermediate-2 or intermediate-1 risk according to the Mayo alliance model
The authors wrote that this “suggests a fundamental and not incremental advantage for the new Mayo alliance model.”
Carfilzomib receives approval for once-weekly dosing
The U.S. Food and Drug Administration (FDA) has approved carfilzomib (Kyprolis) for a once-weekly dosing option in combination with dexamethasone for patients with relapsed or refractory multiple myeloma (MM).
Carfilzomib administered once-weekly at 70 mg/m2 with dexamethasone achieved a superior progression-free survival (PFS) and overall response rates (ORR) compared to twice-weekly carfilzomib at doses of 27 mg/m2.
Carfilzomib is not, however, approved for the twice-weekly 27 mg/m2 dose with dexamethasone alone, but with dexamethasone and lenalidomide.
The FDA based its approval on data from the phase 3 ARROW trial.
The FDA reviewed and approved the supplemental New Drug Application under its Oncology Center of Excellence Real-Time Oncology Review and Assessment Aid pilot program. The program is exploring a more efficient review process to ensure that safe and effective treatments are available to patients as soon as possible.
The FDA approved the carfilzomib application in just over a month.
ARROW
The ARROW study, reported at the 2018 ASCO annual meeting and published in The Lancet, evaluated 478 patients with relapsed or refractory MM who had received at least two but no more than three prior therapies. Prior therapies could include bortezomib and an immunomodulatory drug.
Patients randomized to the investigational arm receive a 30-minute infusion of once-weekly carfilzomib (20 mg/m2 on day 1 of cycle 1; 70 mg/m2 on days 8 and 15 of cycle 1; and 70 mg/m2 on days 1, 8 and 15 of subsequent cycles) with 40 mg of dexamethasone.
Patients randomized to the comparator arm received a 10-minute infusion of twice-weekly carfilzomib (20 mg/m2 on days 1 and 2 of cycle 1; 27 mg/m2 on days 8, 9, 15 and 16 of cycle 1; and 27 mg/m2 on days 1, 2, 8, 9, 15 and 16 of subsequent cycles) with 40 mg of dexamethasone.
Patients in the once-weekly arm achieved a statistically significant 3.7-month improvement in PFS compared to the twice-weekly regimen. Median PFS was 11.2 months for the once-weekly patients and 7.6 months for the twice-weekly group (P=0.0014).
Patients in the once-weekly group had a 62.9% ORR compared to 40.8% for those treated twice weekly (P<0.0001).
More patients (7.1%) in the once-weekly group had complete responses or better than those in the twice-weekly arm (1.7%).
The safety profile of the two arms were comparable, with no new safety risks identified in the once-weekly arm.
Treatment-emergent adverse events occurring in 20% or more patients in either arm included anemia, diarrhea, fatigue, hypertension, insomnia, and pyrexia.
First approved in 2012, carfilzomib has indications for the following in the U.S.:
- Treatment of patients with relapsed or refractory multiple myeloma who have received one to three lines of therapy in combination with dexamethasone or with lenalidomide plus dexamethasone.
- As a single agent for the treatment of patients with relapsed or refractory multiple myeloma who have received one or more lines of therapy.
Amgen manufactures carfilzomib for Onyx Pharmaceuticals, Inc.
Prescribing information for carfilzomib is available online.
The U.S. Food and Drug Administration (FDA) has approved carfilzomib (Kyprolis) for a once-weekly dosing option in combination with dexamethasone for patients with relapsed or refractory multiple myeloma (MM).
Carfilzomib administered once-weekly at 70 mg/m2 with dexamethasone achieved a superior progression-free survival (PFS) and overall response rates (ORR) compared to twice-weekly carfilzomib at doses of 27 mg/m2.
Carfilzomib is not, however, approved for the twice-weekly 27 mg/m2 dose with dexamethasone alone, but with dexamethasone and lenalidomide.
The FDA based its approval on data from the phase 3 ARROW trial.
The FDA reviewed and approved the supplemental New Drug Application under its Oncology Center of Excellence Real-Time Oncology Review and Assessment Aid pilot program. The program is exploring a more efficient review process to ensure that safe and effective treatments are available to patients as soon as possible.
The FDA approved the carfilzomib application in just over a month.
ARROW
The ARROW study, reported at the 2018 ASCO annual meeting and published in The Lancet, evaluated 478 patients with relapsed or refractory MM who had received at least two but no more than three prior therapies. Prior therapies could include bortezomib and an immunomodulatory drug.
Patients randomized to the investigational arm receive a 30-minute infusion of once-weekly carfilzomib (20 mg/m2 on day 1 of cycle 1; 70 mg/m2 on days 8 and 15 of cycle 1; and 70 mg/m2 on days 1, 8 and 15 of subsequent cycles) with 40 mg of dexamethasone.
Patients randomized to the comparator arm received a 10-minute infusion of twice-weekly carfilzomib (20 mg/m2 on days 1 and 2 of cycle 1; 27 mg/m2 on days 8, 9, 15 and 16 of cycle 1; and 27 mg/m2 on days 1, 2, 8, 9, 15 and 16 of subsequent cycles) with 40 mg of dexamethasone.
Patients in the once-weekly arm achieved a statistically significant 3.7-month improvement in PFS compared to the twice-weekly regimen. Median PFS was 11.2 months for the once-weekly patients and 7.6 months for the twice-weekly group (P=0.0014).
Patients in the once-weekly group had a 62.9% ORR compared to 40.8% for those treated twice weekly (P<0.0001).
More patients (7.1%) in the once-weekly group had complete responses or better than those in the twice-weekly arm (1.7%).
The safety profile of the two arms were comparable, with no new safety risks identified in the once-weekly arm.
Treatment-emergent adverse events occurring in 20% or more patients in either arm included anemia, diarrhea, fatigue, hypertension, insomnia, and pyrexia.
First approved in 2012, carfilzomib has indications for the following in the U.S.:
- Treatment of patients with relapsed or refractory multiple myeloma who have received one to three lines of therapy in combination with dexamethasone or with lenalidomide plus dexamethasone.
- As a single agent for the treatment of patients with relapsed or refractory multiple myeloma who have received one or more lines of therapy.
Amgen manufactures carfilzomib for Onyx Pharmaceuticals, Inc.
Prescribing information for carfilzomib is available online.
The U.S. Food and Drug Administration (FDA) has approved carfilzomib (Kyprolis) for a once-weekly dosing option in combination with dexamethasone for patients with relapsed or refractory multiple myeloma (MM).
Carfilzomib administered once-weekly at 70 mg/m2 with dexamethasone achieved a superior progression-free survival (PFS) and overall response rates (ORR) compared to twice-weekly carfilzomib at doses of 27 mg/m2.
Carfilzomib is not, however, approved for the twice-weekly 27 mg/m2 dose with dexamethasone alone, but with dexamethasone and lenalidomide.
The FDA based its approval on data from the phase 3 ARROW trial.
The FDA reviewed and approved the supplemental New Drug Application under its Oncology Center of Excellence Real-Time Oncology Review and Assessment Aid pilot program. The program is exploring a more efficient review process to ensure that safe and effective treatments are available to patients as soon as possible.
The FDA approved the carfilzomib application in just over a month.
ARROW
The ARROW study, reported at the 2018 ASCO annual meeting and published in The Lancet, evaluated 478 patients with relapsed or refractory MM who had received at least two but no more than three prior therapies. Prior therapies could include bortezomib and an immunomodulatory drug.
Patients randomized to the investigational arm receive a 30-minute infusion of once-weekly carfilzomib (20 mg/m2 on day 1 of cycle 1; 70 mg/m2 on days 8 and 15 of cycle 1; and 70 mg/m2 on days 1, 8 and 15 of subsequent cycles) with 40 mg of dexamethasone.
Patients randomized to the comparator arm received a 10-minute infusion of twice-weekly carfilzomib (20 mg/m2 on days 1 and 2 of cycle 1; 27 mg/m2 on days 8, 9, 15 and 16 of cycle 1; and 27 mg/m2 on days 1, 2, 8, 9, 15 and 16 of subsequent cycles) with 40 mg of dexamethasone.
Patients in the once-weekly arm achieved a statistically significant 3.7-month improvement in PFS compared to the twice-weekly regimen. Median PFS was 11.2 months for the once-weekly patients and 7.6 months for the twice-weekly group (P=0.0014).
Patients in the once-weekly group had a 62.9% ORR compared to 40.8% for those treated twice weekly (P<0.0001).
More patients (7.1%) in the once-weekly group had complete responses or better than those in the twice-weekly arm (1.7%).
The safety profile of the two arms were comparable, with no new safety risks identified in the once-weekly arm.
Treatment-emergent adverse events occurring in 20% or more patients in either arm included anemia, diarrhea, fatigue, hypertension, insomnia, and pyrexia.
First approved in 2012, carfilzomib has indications for the following in the U.S.:
- Treatment of patients with relapsed or refractory multiple myeloma who have received one to three lines of therapy in combination with dexamethasone or with lenalidomide plus dexamethasone.
- As a single agent for the treatment of patients with relapsed or refractory multiple myeloma who have received one or more lines of therapy.
Amgen manufactures carfilzomib for Onyx Pharmaceuticals, Inc.
Prescribing information for carfilzomib is available online.
The Rancher, the Roof, and the Rogue Heart
ANSWER
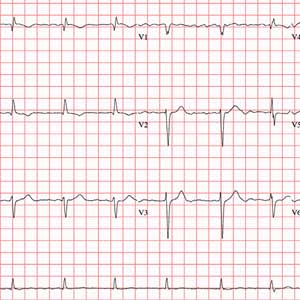
The correct interpretation of this ECG includes atrial fibrillation with variable atrioventricular (AV) block and a left-axis deviation.
Atrial fibrillation is evidenced by the absence of P waves, a consistent PR interval, and an irregularly irregular rhythm. The ventricular rate in atrial fibrillation is due to variable conduction through the AV node, and the 1.6-second pause prior to the last beat on this ECG is due to variable block in the conduction system below the AV node. Pauses such as these may result in clinical symptoms; when seen, the clinician should exercise caution in determining which method (six-second rule, 300/150/100 method) to use to measure the overall heart rate. Note that a pause of undetermined time also exists prior to the first QRS complex seen on the rhythm strip.
Finally, a left-axis deviation is evidenced by an R-wave axis between –30 and –120°.
ANSWER
The correct interpretation of this ECG includes atrial fibrillation with variable atrioventricular (AV) block and a left-axis deviation.
Atrial fibrillation is evidenced by the absence of P waves, a consistent PR interval, and an irregularly irregular rhythm. The ventricular rate in atrial fibrillation is due to variable conduction through the AV node, and the 1.6-second pause prior to the last beat on this ECG is due to variable block in the conduction system below the AV node. Pauses such as these may result in clinical symptoms; when seen, the clinician should exercise caution in determining which method (six-second rule, 300/150/100 method) to use to measure the overall heart rate. Note that a pause of undetermined time also exists prior to the first QRS complex seen on the rhythm strip.
Finally, a left-axis deviation is evidenced by an R-wave axis between –30 and –120°.
ANSWER
The correct interpretation of this ECG includes atrial fibrillation with variable atrioventricular (AV) block and a left-axis deviation.
Atrial fibrillation is evidenced by the absence of P waves, a consistent PR interval, and an irregularly irregular rhythm. The ventricular rate in atrial fibrillation is due to variable conduction through the AV node, and the 1.6-second pause prior to the last beat on this ECG is due to variable block in the conduction system below the AV node. Pauses such as these may result in clinical symptoms; when seen, the clinician should exercise caution in determining which method (six-second rule, 300/150/100 method) to use to measure the overall heart rate. Note that a pause of undetermined time also exists prior to the first QRS complex seen on the rhythm strip.
Finally, a left-axis deviation is evidenced by an R-wave axis between –30 and –120°.
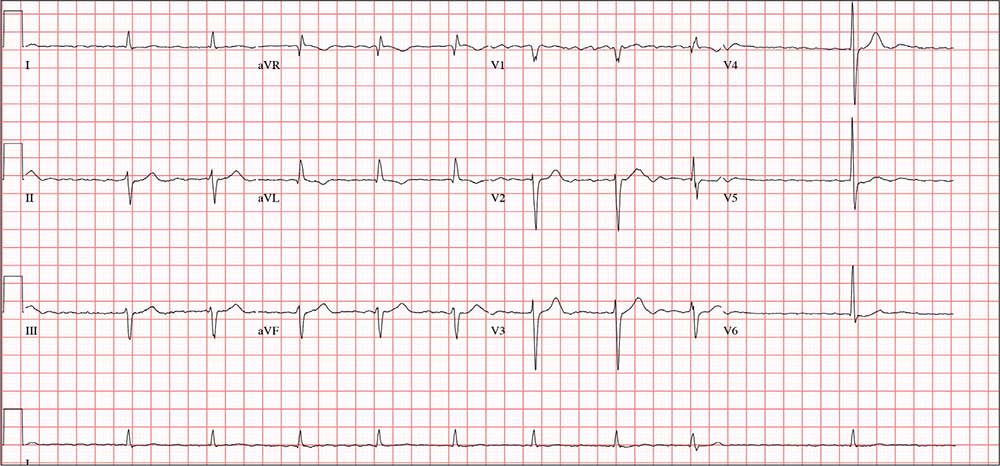
Three days ago, a 74-year-old man fell from his roof, sustaining three fractured ribs and a right-sided hemothorax. He was admitted for treatment: a chest tube to drain the hemothorax and a decortication procedure the following day. His pain has been well controlled postoperatively, and he has been ambulating with his chest tube drainage system on water seal.
This morning, the telemetry technician notices pauses on the patient’s rhythm strips and pages you for interpretation and management. Puzzled as to why this patient was placed on telemetry, you order a 12-lead ECG and review the patient’s electronic medical record (EMR).
Medical history is remarkable for chronic atrial fibrillation, hypertension, hypothyroidism, and a remote history of two transient ischemic attacks (TIAs). His CHA2DS2-VASc score is calculated to be 4, given his age and medical history. When you talk to the patient, he reports no episodes of syncope, near syncope, chest pain, or shortness of breath. He explains that he’s very active and was repairing his roof in preparation for winter when he reached too far and the ladder tipped over.
The patient’s medications include warfarin, hydrochlorothiazide, metoprolol, and propylthiouracil. He has no known drug allergies and denies recreational or homeopathic medication use.
The patient has been a rancher his entire life and doesn’t see himself retiring. He has lived alone on a 200-acre ranch since his wife died of non-Hodgkin lymphoma 12 years ago. He has two adult sons, both in good health, who visit during holidays. The patient was adopted and does not know his family history. He smoked as a young adult but says he hasn’t done so “since cigarettes reached $1.00 per pack.” He has an occasional drink when friends visit but otherwise doesn’t consume alcohol.
The review of systems is remarkable only for a resolving paronychia on his left middle finger.
According to the EMR, his vital signs as of this morning include a blood pressure of 138/94 mm Hg; heart rate, 66 beats/min; respiratory rate, 14 breaths/min; and O2 saturation, 98% on 2L of oxygen via nasal prongs. His height is 6’2” and his weight, 184 lb.
Physical exam reveals an otherwise healthy yet weathered man in no distress. He has multiple old and new areas of ecchymosis on his upper and lower extremities and his right chest. A chest tube is evident exiting the right anterior chest wall. Breath sounds are distant with rhonchi on the right chest and clear and full on the left. The cardiac exam reveals no evidence of jugular venous distention. Heart rhythm is irregularly irregular at a rate of 60 beats/min. There are no murmurs, bruits, or extra heart sounds. The abdomen is soft and scaphoid with no palpable masses. His lower extremities show no evidence of pitting edema, and pulses are strong and full bilaterally. He is alert, oriented, and conversive and does not demonstrate any focal signs.
The ECG you ordered shows an unmeasurable PR interval; QRS duration, 102 ms; QT/QTc interval, 392/397 ms; P axis, unmeasured; R axis, –61°; and T axis, 76°. What is your interpretation?
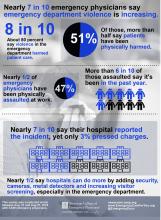
Physical assaults in the emergency department on the rise, survey finds
Those are key findings from an email-based survey administered to ACEP members in August of 2018.
“The results are quite troubling,” Vidor E. Friedman, MD, FACEP, president of ACEP, said during a press briefing at the group’s annual meeting. “Emergency physicians are reporting that violence in emergency departments is increasing and that it’s harming not only physicians and nurses, but also patients and the care that’s being provided to them.”
Dr. Friedman, who practices emergency medicine in Maitland, Fla., recalled one afternoon shift when police brought in a drunk man they had cited for vagrancy after finding him in a ditch by the roadside. It was the man’s first day out of jail in 10 years. “He was a pretty intimidating looking guy,” Dr. Friedman said. “He threatened me and he threatened the staff, so we appropriately restrained him, which only increased his agitation. The fourth time he said he was going to kill me, he told me he was going to put an ice pick in my heart. When his buddies came to pick him up, I had the police escort him to the city limits, and I didn’t sleep at home for a week. This is the kind of thing that health care workers are exposed to on a fairly regular basis.”
The ACEP survey found that emergency physicians across all demographics experience various forms of violence and are increasingly concerned about violence in the ED. “We know that there is gross underreporting of [violence in the ED],” said Terry Kowalenko, MD, FACEP, chair of emergency medicine at Oakland University William Beaumont School of Medicine, Rochester, Mich., and coauthor of a new study that assessed violence against emergency physicians in that state. “Some studies have shown it to be under 50%. The other thing is, it’s very difficult to predict who the perpetrator will be, or who the victim will be. We did find that the time you spend with the patient certainly increases your chances of violence perpetrated against you. Clearly, there are consequences to the victim, the perpetrator, the institution, and potentially, other patients.”
According to Dr. Kowalenko’s own research, 72% of emergency medicine physicians in Michigan reported experiencing violence in the past year. In 2018, an increasing proportion of that state’s emergency physicians reported feeling “constantly fearful” of becoming a victim of violence (8.1% vs. 1.2% in 2005), with 22% reporting feeling frequently fearful (up from 9.4% in 2005).
For the ACEP-sponsored survey, on Aug. 21, 2018, Alexandria, Va.–based Marketing General sent an email to 31,389 ACEP members, inviting them to answer 22 questions in an effort to understand emergency physicians’ views on the level, type, frequency, and impact of violence experienced in the ED. By the time polling closed 6 days later, 3,539 surveys were completed, for a response rate of about 11%. Clinicians in California led the way with 8% of total responses, followed by those in Texas (7%), New York (7%), Florida (5%), Pennsylvania (5%), and Ohio (5%). Nearly two-thirds of respondents (71%) were male, and 25% work for emergency departments with annual patient volumes between 50,001 and 75,000, while another 39% work for departments with even higher volumes.
Nearly half of respondents (47%) reported being physically assaulted while at work in the ED, while 71% have witnessed an assault. Only 10% have experienced neither. The majority of assaults were committed by patients (97%), but 28% involved a patient’s family member or friend. The most common form of assault was a hit or a slap (44%), while other frequent forms of violence included being spit on (30%), punched (28%), or kicked (27%).
Among those who have been physically assaulted, 48% reported that at least half of all assaults were committed by people believed to be seeking drugs or who were under the influence of drugs or alcohol, while 41% said that more than half of assaults were committed by psychiatric patients.
“Our emergency departments are kind of a microcosm of all the challenges that we face today in society: gang violence, gun violence, domestic violence, and psychiatric illnesses [for which] we have a shortage of beds,” said Leigh Vinocur, MD, FACEP, former chair of ACEP’s Emergency Department Violence Committee. “Then there is the opioid crisis.” She added that being in an emergency department can be “an emotionally volatile experience for people from all walks of life. People come in hurt. They’re at their worst; they’re frightened, they’re vulnerable and stressed.”
More than three-quarters of ACEP survey respondents (77%) believe that violence in the ED has harmed patient care, primarily by loss of productivity (81%), emotional trauma (81%), increased wait times (80%), and less focus from emergency staff or physicians (76%). In addition, 49% ranked “increase security” as the most important step hospitals can take to increase ED safety, while the top three contributing factors to violence in the ED were no adequate punitive consequence or response toward the attacker (34%), behavioral health patients (32%), and absence of adequate protective mechanisms for physicians/staff (15%).
When asked how the hospital administration or hospital security responded to the assault, 28% said that the hospital or nursing staff put a behavioral flag in the patient’s medical chart, while 21% said that hospital security arrested the patient for the assault or enlisted law enforcement to arrest the patient.
Finally, nearly half of respondents indicated that hospitals could do more by adding security cameras, metal detectors, and increasing visitor screening. “Obviously this is site-specific,” Dr. Friedman said. “We’re not saying that every emergency department in America needs to have a metal detector out front, but this needs to be something that institutions take seriously, to protect both patients and the providers that work there.”
[email protected]
Those are key findings from an email-based survey administered to ACEP members in August of 2018.
“The results are quite troubling,” Vidor E. Friedman, MD, FACEP, president of ACEP, said during a press briefing at the group’s annual meeting. “Emergency physicians are reporting that violence in emergency departments is increasing and that it’s harming not only physicians and nurses, but also patients and the care that’s being provided to them.”
Dr. Friedman, who practices emergency medicine in Maitland, Fla., recalled one afternoon shift when police brought in a drunk man they had cited for vagrancy after finding him in a ditch by the roadside. It was the man’s first day out of jail in 10 years. “He was a pretty intimidating looking guy,” Dr. Friedman said. “He threatened me and he threatened the staff, so we appropriately restrained him, which only increased his agitation. The fourth time he said he was going to kill me, he told me he was going to put an ice pick in my heart. When his buddies came to pick him up, I had the police escort him to the city limits, and I didn’t sleep at home for a week. This is the kind of thing that health care workers are exposed to on a fairly regular basis.”
The ACEP survey found that emergency physicians across all demographics experience various forms of violence and are increasingly concerned about violence in the ED. “We know that there is gross underreporting of [violence in the ED],” said Terry Kowalenko, MD, FACEP, chair of emergency medicine at Oakland University William Beaumont School of Medicine, Rochester, Mich., and coauthor of a new study that assessed violence against emergency physicians in that state. “Some studies have shown it to be under 50%. The other thing is, it’s very difficult to predict who the perpetrator will be, or who the victim will be. We did find that the time you spend with the patient certainly increases your chances of violence perpetrated against you. Clearly, there are consequences to the victim, the perpetrator, the institution, and potentially, other patients.”
According to Dr. Kowalenko’s own research, 72% of emergency medicine physicians in Michigan reported experiencing violence in the past year. In 2018, an increasing proportion of that state’s emergency physicians reported feeling “constantly fearful” of becoming a victim of violence (8.1% vs. 1.2% in 2005), with 22% reporting feeling frequently fearful (up from 9.4% in 2005).
For the ACEP-sponsored survey, on Aug. 21, 2018, Alexandria, Va.–based Marketing General sent an email to 31,389 ACEP members, inviting them to answer 22 questions in an effort to understand emergency physicians’ views on the level, type, frequency, and impact of violence experienced in the ED. By the time polling closed 6 days later, 3,539 surveys were completed, for a response rate of about 11%. Clinicians in California led the way with 8% of total responses, followed by those in Texas (7%), New York (7%), Florida (5%), Pennsylvania (5%), and Ohio (5%). Nearly two-thirds of respondents (71%) were male, and 25% work for emergency departments with annual patient volumes between 50,001 and 75,000, while another 39% work for departments with even higher volumes.
Nearly half of respondents (47%) reported being physically assaulted while at work in the ED, while 71% have witnessed an assault. Only 10% have experienced neither. The majority of assaults were committed by patients (97%), but 28% involved a patient’s family member or friend. The most common form of assault was a hit or a slap (44%), while other frequent forms of violence included being spit on (30%), punched (28%), or kicked (27%).
Among those who have been physically assaulted, 48% reported that at least half of all assaults were committed by people believed to be seeking drugs or who were under the influence of drugs or alcohol, while 41% said that more than half of assaults were committed by psychiatric patients.
“Our emergency departments are kind of a microcosm of all the challenges that we face today in society: gang violence, gun violence, domestic violence, and psychiatric illnesses [for which] we have a shortage of beds,” said Leigh Vinocur, MD, FACEP, former chair of ACEP’s Emergency Department Violence Committee. “Then there is the opioid crisis.” She added that being in an emergency department can be “an emotionally volatile experience for people from all walks of life. People come in hurt. They’re at their worst; they’re frightened, they’re vulnerable and stressed.”
More than three-quarters of ACEP survey respondents (77%) believe that violence in the ED has harmed patient care, primarily by loss of productivity (81%), emotional trauma (81%), increased wait times (80%), and less focus from emergency staff or physicians (76%). In addition, 49% ranked “increase security” as the most important step hospitals can take to increase ED safety, while the top three contributing factors to violence in the ED were no adequate punitive consequence or response toward the attacker (34%), behavioral health patients (32%), and absence of adequate protective mechanisms for physicians/staff (15%).
When asked how the hospital administration or hospital security responded to the assault, 28% said that the hospital or nursing staff put a behavioral flag in the patient’s medical chart, while 21% said that hospital security arrested the patient for the assault or enlisted law enforcement to arrest the patient.
Finally, nearly half of respondents indicated that hospitals could do more by adding security cameras, metal detectors, and increasing visitor screening. “Obviously this is site-specific,” Dr. Friedman said. “We’re not saying that every emergency department in America needs to have a metal detector out front, but this needs to be something that institutions take seriously, to protect both patients and the providers that work there.”
[email protected]
Those are key findings from an email-based survey administered to ACEP members in August of 2018.
“The results are quite troubling,” Vidor E. Friedman, MD, FACEP, president of ACEP, said during a press briefing at the group’s annual meeting. “Emergency physicians are reporting that violence in emergency departments is increasing and that it’s harming not only physicians and nurses, but also patients and the care that’s being provided to them.”
Dr. Friedman, who practices emergency medicine in Maitland, Fla., recalled one afternoon shift when police brought in a drunk man they had cited for vagrancy after finding him in a ditch by the roadside. It was the man’s first day out of jail in 10 years. “He was a pretty intimidating looking guy,” Dr. Friedman said. “He threatened me and he threatened the staff, so we appropriately restrained him, which only increased his agitation. The fourth time he said he was going to kill me, he told me he was going to put an ice pick in my heart. When his buddies came to pick him up, I had the police escort him to the city limits, and I didn’t sleep at home for a week. This is the kind of thing that health care workers are exposed to on a fairly regular basis.”
The ACEP survey found that emergency physicians across all demographics experience various forms of violence and are increasingly concerned about violence in the ED. “We know that there is gross underreporting of [violence in the ED],” said Terry Kowalenko, MD, FACEP, chair of emergency medicine at Oakland University William Beaumont School of Medicine, Rochester, Mich., and coauthor of a new study that assessed violence against emergency physicians in that state. “Some studies have shown it to be under 50%. The other thing is, it’s very difficult to predict who the perpetrator will be, or who the victim will be. We did find that the time you spend with the patient certainly increases your chances of violence perpetrated against you. Clearly, there are consequences to the victim, the perpetrator, the institution, and potentially, other patients.”
According to Dr. Kowalenko’s own research, 72% of emergency medicine physicians in Michigan reported experiencing violence in the past year. In 2018, an increasing proportion of that state’s emergency physicians reported feeling “constantly fearful” of becoming a victim of violence (8.1% vs. 1.2% in 2005), with 22% reporting feeling frequently fearful (up from 9.4% in 2005).
For the ACEP-sponsored survey, on Aug. 21, 2018, Alexandria, Va.–based Marketing General sent an email to 31,389 ACEP members, inviting them to answer 22 questions in an effort to understand emergency physicians’ views on the level, type, frequency, and impact of violence experienced in the ED. By the time polling closed 6 days later, 3,539 surveys were completed, for a response rate of about 11%. Clinicians in California led the way with 8% of total responses, followed by those in Texas (7%), New York (7%), Florida (5%), Pennsylvania (5%), and Ohio (5%). Nearly two-thirds of respondents (71%) were male, and 25% work for emergency departments with annual patient volumes between 50,001 and 75,000, while another 39% work for departments with even higher volumes.
Nearly half of respondents (47%) reported being physically assaulted while at work in the ED, while 71% have witnessed an assault. Only 10% have experienced neither. The majority of assaults were committed by patients (97%), but 28% involved a patient’s family member or friend. The most common form of assault was a hit or a slap (44%), while other frequent forms of violence included being spit on (30%), punched (28%), or kicked (27%).
Among those who have been physically assaulted, 48% reported that at least half of all assaults were committed by people believed to be seeking drugs or who were under the influence of drugs or alcohol, while 41% said that more than half of assaults were committed by psychiatric patients.
“Our emergency departments are kind of a microcosm of all the challenges that we face today in society: gang violence, gun violence, domestic violence, and psychiatric illnesses [for which] we have a shortage of beds,” said Leigh Vinocur, MD, FACEP, former chair of ACEP’s Emergency Department Violence Committee. “Then there is the opioid crisis.” She added that being in an emergency department can be “an emotionally volatile experience for people from all walks of life. People come in hurt. They’re at their worst; they’re frightened, they’re vulnerable and stressed.”
More than three-quarters of ACEP survey respondents (77%) believe that violence in the ED has harmed patient care, primarily by loss of productivity (81%), emotional trauma (81%), increased wait times (80%), and less focus from emergency staff or physicians (76%). In addition, 49% ranked “increase security” as the most important step hospitals can take to increase ED safety, while the top three contributing factors to violence in the ED were no adequate punitive consequence or response toward the attacker (34%), behavioral health patients (32%), and absence of adequate protective mechanisms for physicians/staff (15%).
When asked how the hospital administration or hospital security responded to the assault, 28% said that the hospital or nursing staff put a behavioral flag in the patient’s medical chart, while 21% said that hospital security arrested the patient for the assault or enlisted law enforcement to arrest the patient.
Finally, nearly half of respondents indicated that hospitals could do more by adding security cameras, metal detectors, and increasing visitor screening. “Obviously this is site-specific,” Dr. Friedman said. “We’re not saying that every emergency department in America needs to have a metal detector out front, but this needs to be something that institutions take seriously, to protect both patients and the providers that work there.”
[email protected]
AT ACEP18