User login
ECMO for ARDS in the modern era
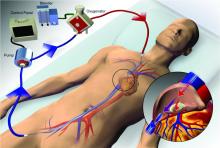
Extracorporeal membrane oxygenation (ECMO) has become increasingly accepted as a rescue therapy for severe respiratory failure from a variety of conditions, though most commonly, the acute respiratory distress syndrome (ARDS) (Thiagarajan R, et al. ASAIO. 2017;63[1]:60). ECMO can provide respiratory or cardiorespiratory support for failing lungs, heart, or both. The most common ECMO configuration used in ARDS is venovenous ECMO, in which blood is withdrawn from a catheter placed in a central vein, pumped through a gas exchange device known as an oxygenator, and returned to the venous system via another catheter. The blood flowing through the oxygenator is separated from a continuous supply of oxygen-rich sweep gas by a semipermeable membrane, across which diffusion-mediated gas exchange occurs, so that the blood exiting it is rich in oxygen and low in carbon dioxide. As venovenous ECMO functions in series with the native circulation, the well-oxygenated blood exiting the ECMO circuit mixes with poorly oxygenated blood flowing through the lungs. Therefore, oxygenation is dependent on native cardiac output to achieve systemic oxygen delivery (Figure 1).
ECMO been used successfully in adults with ARDS since the early 1970s (Hill JD, et al. N Engl J Med. 1972;286[12]:629-34) but, until recently, was limited to small numbers of patients at select global centers and associated with a high-risk profile. In the last decade, however, driven by improvements in ECMO circuit components making the device safer and easier to use, encouraging worldwide experience during the 2009 influenza A (H1N1) pandemic (Davies A, et al. JAMA. 2009;302[17]1888-95), and publication of the Efficacy and Economic Assessment of Conventional Ventilatory Support versus Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure (CESAR) trial (Peek GJ, et al. Lancet. 2009;374[9698]:1351-63), ECMO use has markedly increased.
Despite its rapid growth, however, rigorous evidence supporting the use of ECMO has been lacking. The CESAR trial, while impressive in execution, had methodological issues that limited the strength of its conclusions. CESAR was a pragmatic trial that randomized 180 adults with severe respiratory failure from multiple etiologies to conventional management or transfer to an experienced, ECMO-capable center. CESAR met its primary outcome of improved survival without disability in the ECMO-referred group (63% vs 47%, relative risk [RR] 0.69; 95% confidence interval [CI] 0.05 to 0.97, P=.03), but not all patients in that group ultimately received ECMO. In addition, the use of lung protective ventilation was significantly higher in the ECMO-referred group, making it difficult to separate its benefit from that of ECMO. A conservative interpretation is that CESAR showed the clinical benefit of treatment at an ECMO-capable center, experienced in the management of patients with severe respiratory failure.
Not until the release of the Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome (EOLIA) trial earlier this year (Combes A, et al. N Engl J Med. 2018;378[21]:1965-75), did a modern, randomized controlled trial evaluating the use of ECMO itself exist. The EOLIA trial addressed the limitations of CESAR and randomized adult patients with early, severe ARDS to conventional, standard of care management that included a protocolized lung protective strategy in the control group vs immediate initiation of ECMO combined with an ultra-lung protective strategy (targeting end-inspiratory plateau pressure ≤24 cmH2O) in the intervention group. The primary outcome was all-cause mortality at 60 days. Of note, patients enrolled in EOLIA met entry criteria despite greater than 90% of patients receiving neuromuscular blockade and around 60% treated with prone positioning at the time of randomization (importantly, 90% of control group patients ultimately underwent prone positioning).
EOLIA was powered to detect a 20% decrease in mortality in the ECMO group. Based on trial design and the results of the fourth interim analysis, the trial was stopped for futility to reach that endpoint after enrollment of 249 of a maximum 331 patients. Although a 20% mortality reduction was not achieved, 60-day mortality was notably lower in the ECMO-treated group (35% vs 46%, RR 0.76, 95% CI 0.55 to 1.04, P=.09). The key secondary outcome of risk of treatment failure (defined as death in the ECMO group and death or crossover to ECMO in the control group) favored the ECMO group with a RR for mortality of 0.62 (95% CI, 0.47 to 0.82; P<.001), as did other secondary endpoints such as days free of renal and other organ failure.
A major limitation of the trial was that 35 (28%) of control group patients ultimately crossed over to ECMO, which diluted the effect of ECMO observed in the intention-to-treat analysis. Crossover occurred at clinician discretion an average of 6.5 days after randomization and after stringent criteria for crossover was met. These patients were incredibly ill, with a median oxygen saturation of 77%, rapidly worsening inotropic scores, and lactic acidosis; nine individuals had already suffered cardiac arrest, and six had received ECMO as part of extracorporeal cardiopulmonary resuscitation (ECPR), the initiation of venoarterial ECMO during cardiac arrest in attempt to restore spontaneous circulation. Mortality was considerably worse in the crossover group than in conventionally managed cohort overall, and, notably, 33% of patients crossed over to ECMO still survived.
In order to estimate the effect of ECMO on survival times if crossover had not occurred, the authors performed a post-hoc, rank-preserving structural failure time analysis. Though this relies on some assessment regarding the effect of the treatment itself, it showed a hazard ratio for mortality in the ECMO group of 0.51 (95% CI 0.24 to 1.02, P=.055). Although the EOLIA trial was not positive by traditional interpretation, all three major analyses and all secondary endpoints suggest some degree of benefit in patients with severe ARDS managed with ECMO.
Importantly, ECMO was well tolerated (at least when performed at expert centers, as done in this trial). There were significantly more bleeding events and cases of severe thrombocytopenia in the ECMO-treated group, but massive hemorrhage, ischemic and hemorrhagic stroke, arrhythmias, and other complications were similar.
Where do we go from here? Based on the totality of information, it is reasonable to consider ECMO for cases of severe ARDS not responsive to conventional measures, such as a lung protective ventilator strategy, neuromuscular blockade, and prone positioning. Initiation of ECMO may be reasonable prior to implementation of standard of care therapies, in order to permit safe transfer to an experienced center from a center not able to provide them.
Two take-away points: First, it is important to recognize that much of the clinical benefit derived from ECMO may be beyond its ability to normalize gas exchange and be due, at least in part, to the fact that ECMO allows the enhancement of proven lung protective ventilatory strategies. Initiation of ECMO and the “lung rest” it permits reduce the mechanical power applied to the injured alveoli and may attenuate ventilator-induced lung injury, cytokine release, and multiorgan failure that portend poor clinical outcomes in ARDS. Second, ECMO in EOLIA was conducted at expert centers with relatively low rates of complications.
It is too early to know how the critical care community will view ECMO for ARDS in light of EOLIA as well as a growing body of global ECMO experience, or how its wider application may impact the distribution and organization of ECMO centers. Regardless, of paramount importance in using ECMO as a treatment modality is optimizing patient management both prior to and after its initiation.
Dr. Agerstrand is Assistant Professor of Medicine, Director of the Medical ECMO Program, Columbia University College of Physicians and Surgeons, New York-Presbyterian Hospital.
Extracorporeal membrane oxygenation (ECMO) has become increasingly accepted as a rescue therapy for severe respiratory failure from a variety of conditions, though most commonly, the acute respiratory distress syndrome (ARDS) (Thiagarajan R, et al. ASAIO. 2017;63[1]:60). ECMO can provide respiratory or cardiorespiratory support for failing lungs, heart, or both. The most common ECMO configuration used in ARDS is venovenous ECMO, in which blood is withdrawn from a catheter placed in a central vein, pumped through a gas exchange device known as an oxygenator, and returned to the venous system via another catheter. The blood flowing through the oxygenator is separated from a continuous supply of oxygen-rich sweep gas by a semipermeable membrane, across which diffusion-mediated gas exchange occurs, so that the blood exiting it is rich in oxygen and low in carbon dioxide. As venovenous ECMO functions in series with the native circulation, the well-oxygenated blood exiting the ECMO circuit mixes with poorly oxygenated blood flowing through the lungs. Therefore, oxygenation is dependent on native cardiac output to achieve systemic oxygen delivery (Figure 1).
ECMO been used successfully in adults with ARDS since the early 1970s (Hill JD, et al. N Engl J Med. 1972;286[12]:629-34) but, until recently, was limited to small numbers of patients at select global centers and associated with a high-risk profile. In the last decade, however, driven by improvements in ECMO circuit components making the device safer and easier to use, encouraging worldwide experience during the 2009 influenza A (H1N1) pandemic (Davies A, et al. JAMA. 2009;302[17]1888-95), and publication of the Efficacy and Economic Assessment of Conventional Ventilatory Support versus Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure (CESAR) trial (Peek GJ, et al. Lancet. 2009;374[9698]:1351-63), ECMO use has markedly increased.
Despite its rapid growth, however, rigorous evidence supporting the use of ECMO has been lacking. The CESAR trial, while impressive in execution, had methodological issues that limited the strength of its conclusions. CESAR was a pragmatic trial that randomized 180 adults with severe respiratory failure from multiple etiologies to conventional management or transfer to an experienced, ECMO-capable center. CESAR met its primary outcome of improved survival without disability in the ECMO-referred group (63% vs 47%, relative risk [RR] 0.69; 95% confidence interval [CI] 0.05 to 0.97, P=.03), but not all patients in that group ultimately received ECMO. In addition, the use of lung protective ventilation was significantly higher in the ECMO-referred group, making it difficult to separate its benefit from that of ECMO. A conservative interpretation is that CESAR showed the clinical benefit of treatment at an ECMO-capable center, experienced in the management of patients with severe respiratory failure.
Not until the release of the Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome (EOLIA) trial earlier this year (Combes A, et al. N Engl J Med. 2018;378[21]:1965-75), did a modern, randomized controlled trial evaluating the use of ECMO itself exist. The EOLIA trial addressed the limitations of CESAR and randomized adult patients with early, severe ARDS to conventional, standard of care management that included a protocolized lung protective strategy in the control group vs immediate initiation of ECMO combined with an ultra-lung protective strategy (targeting end-inspiratory plateau pressure ≤24 cmH2O) in the intervention group. The primary outcome was all-cause mortality at 60 days. Of note, patients enrolled in EOLIA met entry criteria despite greater than 90% of patients receiving neuromuscular blockade and around 60% treated with prone positioning at the time of randomization (importantly, 90% of control group patients ultimately underwent prone positioning).
EOLIA was powered to detect a 20% decrease in mortality in the ECMO group. Based on trial design and the results of the fourth interim analysis, the trial was stopped for futility to reach that endpoint after enrollment of 249 of a maximum 331 patients. Although a 20% mortality reduction was not achieved, 60-day mortality was notably lower in the ECMO-treated group (35% vs 46%, RR 0.76, 95% CI 0.55 to 1.04, P=.09). The key secondary outcome of risk of treatment failure (defined as death in the ECMO group and death or crossover to ECMO in the control group) favored the ECMO group with a RR for mortality of 0.62 (95% CI, 0.47 to 0.82; P<.001), as did other secondary endpoints such as days free of renal and other organ failure.
A major limitation of the trial was that 35 (28%) of control group patients ultimately crossed over to ECMO, which diluted the effect of ECMO observed in the intention-to-treat analysis. Crossover occurred at clinician discretion an average of 6.5 days after randomization and after stringent criteria for crossover was met. These patients were incredibly ill, with a median oxygen saturation of 77%, rapidly worsening inotropic scores, and lactic acidosis; nine individuals had already suffered cardiac arrest, and six had received ECMO as part of extracorporeal cardiopulmonary resuscitation (ECPR), the initiation of venoarterial ECMO during cardiac arrest in attempt to restore spontaneous circulation. Mortality was considerably worse in the crossover group than in conventionally managed cohort overall, and, notably, 33% of patients crossed over to ECMO still survived.
In order to estimate the effect of ECMO on survival times if crossover had not occurred, the authors performed a post-hoc, rank-preserving structural failure time analysis. Though this relies on some assessment regarding the effect of the treatment itself, it showed a hazard ratio for mortality in the ECMO group of 0.51 (95% CI 0.24 to 1.02, P=.055). Although the EOLIA trial was not positive by traditional interpretation, all three major analyses and all secondary endpoints suggest some degree of benefit in patients with severe ARDS managed with ECMO.
Importantly, ECMO was well tolerated (at least when performed at expert centers, as done in this trial). There were significantly more bleeding events and cases of severe thrombocytopenia in the ECMO-treated group, but massive hemorrhage, ischemic and hemorrhagic stroke, arrhythmias, and other complications were similar.
Where do we go from here? Based on the totality of information, it is reasonable to consider ECMO for cases of severe ARDS not responsive to conventional measures, such as a lung protective ventilator strategy, neuromuscular blockade, and prone positioning. Initiation of ECMO may be reasonable prior to implementation of standard of care therapies, in order to permit safe transfer to an experienced center from a center not able to provide them.
Two take-away points: First, it is important to recognize that much of the clinical benefit derived from ECMO may be beyond its ability to normalize gas exchange and be due, at least in part, to the fact that ECMO allows the enhancement of proven lung protective ventilatory strategies. Initiation of ECMO and the “lung rest” it permits reduce the mechanical power applied to the injured alveoli and may attenuate ventilator-induced lung injury, cytokine release, and multiorgan failure that portend poor clinical outcomes in ARDS. Second, ECMO in EOLIA was conducted at expert centers with relatively low rates of complications.
It is too early to know how the critical care community will view ECMO for ARDS in light of EOLIA as well as a growing body of global ECMO experience, or how its wider application may impact the distribution and organization of ECMO centers. Regardless, of paramount importance in using ECMO as a treatment modality is optimizing patient management both prior to and after its initiation.
Dr. Agerstrand is Assistant Professor of Medicine, Director of the Medical ECMO Program, Columbia University College of Physicians and Surgeons, New York-Presbyterian Hospital.
Extracorporeal membrane oxygenation (ECMO) has become increasingly accepted as a rescue therapy for severe respiratory failure from a variety of conditions, though most commonly, the acute respiratory distress syndrome (ARDS) (Thiagarajan R, et al. ASAIO. 2017;63[1]:60). ECMO can provide respiratory or cardiorespiratory support for failing lungs, heart, or both. The most common ECMO configuration used in ARDS is venovenous ECMO, in which blood is withdrawn from a catheter placed in a central vein, pumped through a gas exchange device known as an oxygenator, and returned to the venous system via another catheter. The blood flowing through the oxygenator is separated from a continuous supply of oxygen-rich sweep gas by a semipermeable membrane, across which diffusion-mediated gas exchange occurs, so that the blood exiting it is rich in oxygen and low in carbon dioxide. As venovenous ECMO functions in series with the native circulation, the well-oxygenated blood exiting the ECMO circuit mixes with poorly oxygenated blood flowing through the lungs. Therefore, oxygenation is dependent on native cardiac output to achieve systemic oxygen delivery (Figure 1).
ECMO been used successfully in adults with ARDS since the early 1970s (Hill JD, et al. N Engl J Med. 1972;286[12]:629-34) but, until recently, was limited to small numbers of patients at select global centers and associated with a high-risk profile. In the last decade, however, driven by improvements in ECMO circuit components making the device safer and easier to use, encouraging worldwide experience during the 2009 influenza A (H1N1) pandemic (Davies A, et al. JAMA. 2009;302[17]1888-95), and publication of the Efficacy and Economic Assessment of Conventional Ventilatory Support versus Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure (CESAR) trial (Peek GJ, et al. Lancet. 2009;374[9698]:1351-63), ECMO use has markedly increased.
Despite its rapid growth, however, rigorous evidence supporting the use of ECMO has been lacking. The CESAR trial, while impressive in execution, had methodological issues that limited the strength of its conclusions. CESAR was a pragmatic trial that randomized 180 adults with severe respiratory failure from multiple etiologies to conventional management or transfer to an experienced, ECMO-capable center. CESAR met its primary outcome of improved survival without disability in the ECMO-referred group (63% vs 47%, relative risk [RR] 0.69; 95% confidence interval [CI] 0.05 to 0.97, P=.03), but not all patients in that group ultimately received ECMO. In addition, the use of lung protective ventilation was significantly higher in the ECMO-referred group, making it difficult to separate its benefit from that of ECMO. A conservative interpretation is that CESAR showed the clinical benefit of treatment at an ECMO-capable center, experienced in the management of patients with severe respiratory failure.
Not until the release of the Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome (EOLIA) trial earlier this year (Combes A, et al. N Engl J Med. 2018;378[21]:1965-75), did a modern, randomized controlled trial evaluating the use of ECMO itself exist. The EOLIA trial addressed the limitations of CESAR and randomized adult patients with early, severe ARDS to conventional, standard of care management that included a protocolized lung protective strategy in the control group vs immediate initiation of ECMO combined with an ultra-lung protective strategy (targeting end-inspiratory plateau pressure ≤24 cmH2O) in the intervention group. The primary outcome was all-cause mortality at 60 days. Of note, patients enrolled in EOLIA met entry criteria despite greater than 90% of patients receiving neuromuscular blockade and around 60% treated with prone positioning at the time of randomization (importantly, 90% of control group patients ultimately underwent prone positioning).
EOLIA was powered to detect a 20% decrease in mortality in the ECMO group. Based on trial design and the results of the fourth interim analysis, the trial was stopped for futility to reach that endpoint after enrollment of 249 of a maximum 331 patients. Although a 20% mortality reduction was not achieved, 60-day mortality was notably lower in the ECMO-treated group (35% vs 46%, RR 0.76, 95% CI 0.55 to 1.04, P=.09). The key secondary outcome of risk of treatment failure (defined as death in the ECMO group and death or crossover to ECMO in the control group) favored the ECMO group with a RR for mortality of 0.62 (95% CI, 0.47 to 0.82; P<.001), as did other secondary endpoints such as days free of renal and other organ failure.
A major limitation of the trial was that 35 (28%) of control group patients ultimately crossed over to ECMO, which diluted the effect of ECMO observed in the intention-to-treat analysis. Crossover occurred at clinician discretion an average of 6.5 days after randomization and after stringent criteria for crossover was met. These patients were incredibly ill, with a median oxygen saturation of 77%, rapidly worsening inotropic scores, and lactic acidosis; nine individuals had already suffered cardiac arrest, and six had received ECMO as part of extracorporeal cardiopulmonary resuscitation (ECPR), the initiation of venoarterial ECMO during cardiac arrest in attempt to restore spontaneous circulation. Mortality was considerably worse in the crossover group than in conventionally managed cohort overall, and, notably, 33% of patients crossed over to ECMO still survived.
In order to estimate the effect of ECMO on survival times if crossover had not occurred, the authors performed a post-hoc, rank-preserving structural failure time analysis. Though this relies on some assessment regarding the effect of the treatment itself, it showed a hazard ratio for mortality in the ECMO group of 0.51 (95% CI 0.24 to 1.02, P=.055). Although the EOLIA trial was not positive by traditional interpretation, all three major analyses and all secondary endpoints suggest some degree of benefit in patients with severe ARDS managed with ECMO.
Importantly, ECMO was well tolerated (at least when performed at expert centers, as done in this trial). There were significantly more bleeding events and cases of severe thrombocytopenia in the ECMO-treated group, but massive hemorrhage, ischemic and hemorrhagic stroke, arrhythmias, and other complications were similar.
Where do we go from here? Based on the totality of information, it is reasonable to consider ECMO for cases of severe ARDS not responsive to conventional measures, such as a lung protective ventilator strategy, neuromuscular blockade, and prone positioning. Initiation of ECMO may be reasonable prior to implementation of standard of care therapies, in order to permit safe transfer to an experienced center from a center not able to provide them.
Two take-away points: First, it is important to recognize that much of the clinical benefit derived from ECMO may be beyond its ability to normalize gas exchange and be due, at least in part, to the fact that ECMO allows the enhancement of proven lung protective ventilatory strategies. Initiation of ECMO and the “lung rest” it permits reduce the mechanical power applied to the injured alveoli and may attenuate ventilator-induced lung injury, cytokine release, and multiorgan failure that portend poor clinical outcomes in ARDS. Second, ECMO in EOLIA was conducted at expert centers with relatively low rates of complications.
It is too early to know how the critical care community will view ECMO for ARDS in light of EOLIA as well as a growing body of global ECMO experience, or how its wider application may impact the distribution and organization of ECMO centers. Regardless, of paramount importance in using ECMO as a treatment modality is optimizing patient management both prior to and after its initiation.
Dr. Agerstrand is Assistant Professor of Medicine, Director of the Medical ECMO Program, Columbia University College of Physicians and Surgeons, New York-Presbyterian Hospital.
National Board of Echocardiography offering board exam
Due to significant interest in the pulmonary/critical care community, the National Board of Echocardiography (NBE) has opened registration for a board examination as a requirement for national level certification in advanced critical care echocardiography (ACCE). The examination has been developed by the National Board of Medical Examiners; CHEST and the other professional societies are well represented on the writing committee. The first examination is scheduled to be given on January 15, 2019.
The board of the NBE will be the final arbiter for other requirements for certification. We anticipate that these will be available in 2019.
A few essential questions about the certification:
1. Who will be eligible for certification in ACCE?
The policy of the NBE is that any licensed physician may take the examination. Passing the examination confers testamur status, which is only one of several requirements for certification. The board of the NBE will make the final decision as to how to define the clinical background of the candidate that will be required for certification.
2. What will be the requirements for demonstration of competence at image acquisition for ACCE?
Competence at ACCE requires that the intensivist be expert at image acquisition of a comprehensive image set. The board of the NBE will make the final decision as to what constitutes a full ACCE image set, how many studies must be performed by the candidate, and how the studies will be documented. Regarding the latter question, it is likely that there will be a need for identification of qualified mentors to guide the candidate through the process of demonstrating competence in image acquisition.
3. What resources exist to learn more about the examination?
For some suggestions regarding mastery of the cognitive base, Dr. Yonatan Greenstein has set up an independent website that has recommendations about study material and an example of the full ACCE image set (advancedcriticalcareecho.org). The NBE website has a list of subjects that will be covered in the examination. In addition to passing the examination, there will be other elements required for ACCE certification. The NBE has not yet made final decision on the additional requirements. As soon as they are available, they will be posted on the NBE website (echoboards.org).
There is keen interest amongst fellows and junior attendings in the NBE certification who are already competent in whole body ultrasonography. They see ACCE as a natural and necessary extension of their scope of practice, as a means of better helping their critically ill patients, and as a means of acquiring a unique skill that defines them as having a special skill compared with other intensivists. A smaller group of senior attending intensivists are primarily motivated by a well-defined practice-related need of skill at ACCE and/or a strong perception that knowledge of ACCE may directly improve their ability to care for the critically ill patient. Interest in certification extends across the various specialties that provide critical care services. The NBE has indicated that there has been a strong showing of registrations for the examination thus far.
We recommend that candidates for certification consider that passing the examination should be the priority. Collection of the image set may occur in parallel, as the two will complement each other. Preparation for the examination requires intensive study of the cognitive base of ACCE and mastery of image interpretation.
To aid in preparation for the ACCE examination, CHEST is offering a comprehensive review course, Advanced Critical Care Echocardiography Board Review Exam Course, being held at the CHEST Innovation, Simulation, and Training Center, December 7-8, 2018, in Glenview, Illinois.
Due to significant interest in the pulmonary/critical care community, the National Board of Echocardiography (NBE) has opened registration for a board examination as a requirement for national level certification in advanced critical care echocardiography (ACCE). The examination has been developed by the National Board of Medical Examiners; CHEST and the other professional societies are well represented on the writing committee. The first examination is scheduled to be given on January 15, 2019.
The board of the NBE will be the final arbiter for other requirements for certification. We anticipate that these will be available in 2019.
A few essential questions about the certification:
1. Who will be eligible for certification in ACCE?
The policy of the NBE is that any licensed physician may take the examination. Passing the examination confers testamur status, which is only one of several requirements for certification. The board of the NBE will make the final decision as to how to define the clinical background of the candidate that will be required for certification.
2. What will be the requirements for demonstration of competence at image acquisition for ACCE?
Competence at ACCE requires that the intensivist be expert at image acquisition of a comprehensive image set. The board of the NBE will make the final decision as to what constitutes a full ACCE image set, how many studies must be performed by the candidate, and how the studies will be documented. Regarding the latter question, it is likely that there will be a need for identification of qualified mentors to guide the candidate through the process of demonstrating competence in image acquisition.
3. What resources exist to learn more about the examination?
For some suggestions regarding mastery of the cognitive base, Dr. Yonatan Greenstein has set up an independent website that has recommendations about study material and an example of the full ACCE image set (advancedcriticalcareecho.org). The NBE website has a list of subjects that will be covered in the examination. In addition to passing the examination, there will be other elements required for ACCE certification. The NBE has not yet made final decision on the additional requirements. As soon as they are available, they will be posted on the NBE website (echoboards.org).
There is keen interest amongst fellows and junior attendings in the NBE certification who are already competent in whole body ultrasonography. They see ACCE as a natural and necessary extension of their scope of practice, as a means of better helping their critically ill patients, and as a means of acquiring a unique skill that defines them as having a special skill compared with other intensivists. A smaller group of senior attending intensivists are primarily motivated by a well-defined practice-related need of skill at ACCE and/or a strong perception that knowledge of ACCE may directly improve their ability to care for the critically ill patient. Interest in certification extends across the various specialties that provide critical care services. The NBE has indicated that there has been a strong showing of registrations for the examination thus far.
We recommend that candidates for certification consider that passing the examination should be the priority. Collection of the image set may occur in parallel, as the two will complement each other. Preparation for the examination requires intensive study of the cognitive base of ACCE and mastery of image interpretation.
To aid in preparation for the ACCE examination, CHEST is offering a comprehensive review course, Advanced Critical Care Echocardiography Board Review Exam Course, being held at the CHEST Innovation, Simulation, and Training Center, December 7-8, 2018, in Glenview, Illinois.
Due to significant interest in the pulmonary/critical care community, the National Board of Echocardiography (NBE) has opened registration for a board examination as a requirement for national level certification in advanced critical care echocardiography (ACCE). The examination has been developed by the National Board of Medical Examiners; CHEST and the other professional societies are well represented on the writing committee. The first examination is scheduled to be given on January 15, 2019.
The board of the NBE will be the final arbiter for other requirements for certification. We anticipate that these will be available in 2019.
A few essential questions about the certification:
1. Who will be eligible for certification in ACCE?
The policy of the NBE is that any licensed physician may take the examination. Passing the examination confers testamur status, which is only one of several requirements for certification. The board of the NBE will make the final decision as to how to define the clinical background of the candidate that will be required for certification.
2. What will be the requirements for demonstration of competence at image acquisition for ACCE?
Competence at ACCE requires that the intensivist be expert at image acquisition of a comprehensive image set. The board of the NBE will make the final decision as to what constitutes a full ACCE image set, how many studies must be performed by the candidate, and how the studies will be documented. Regarding the latter question, it is likely that there will be a need for identification of qualified mentors to guide the candidate through the process of demonstrating competence in image acquisition.
3. What resources exist to learn more about the examination?
For some suggestions regarding mastery of the cognitive base, Dr. Yonatan Greenstein has set up an independent website that has recommendations about study material and an example of the full ACCE image set (advancedcriticalcareecho.org). The NBE website has a list of subjects that will be covered in the examination. In addition to passing the examination, there will be other elements required for ACCE certification. The NBE has not yet made final decision on the additional requirements. As soon as they are available, they will be posted on the NBE website (echoboards.org).
There is keen interest amongst fellows and junior attendings in the NBE certification who are already competent in whole body ultrasonography. They see ACCE as a natural and necessary extension of their scope of practice, as a means of better helping their critically ill patients, and as a means of acquiring a unique skill that defines them as having a special skill compared with other intensivists. A smaller group of senior attending intensivists are primarily motivated by a well-defined practice-related need of skill at ACCE and/or a strong perception that knowledge of ACCE may directly improve their ability to care for the critically ill patient. Interest in certification extends across the various specialties that provide critical care services. The NBE has indicated that there has been a strong showing of registrations for the examination thus far.
We recommend that candidates for certification consider that passing the examination should be the priority. Collection of the image set may occur in parallel, as the two will complement each other. Preparation for the examination requires intensive study of the cognitive base of ACCE and mastery of image interpretation.
To aid in preparation for the ACCE examination, CHEST is offering a comprehensive review course, Advanced Critical Care Echocardiography Board Review Exam Course, being held at the CHEST Innovation, Simulation, and Training Center, December 7-8, 2018, in Glenview, Illinois.
Interventional Chest/Diagnostic Procedures
Interventional Chest/Diagnostic Procedures
Endobronchial valve therapy receives FDA approval for bronchoscopic LVR
Lung volume reduction surgery (LVRS) is an established approach to improve exercise capacity and lung function in patients with heterogeneous emphysema and may confer survival benefit in patients with apical-predominant disease (Fishman, et al. N Engl J Med. 2003;348[21]:2059). Despite this, LVRS case numbers remain low due to patient and procedural morbidity. Bronchoscopic alternatives for LVRS have advanced considerably over the last decade with endobronchial valve (EBV) therapy emerging as a viable option for select subsets of patients with heterogeneous emphysema. Endobronchial valves are removable devices placed in segmental/subsegmental airways, which allow efflux of air during exhalation but close during inspiration, resulting in distal atelectasis in the absence of collateral ventilation.
The LIBERATE study, a multicenter randomized controlled trial demonstrated improvement in FEV1 ≥15% in 48% of patients after EBV placement compared with 17% of patients receiving standard medical therapy has resulted in FDA approval (Criner G, et al. Am J Respir Crit Care Med. 2018 May 22. doi: 10.1164/rccm.201803-0590OC. [Epub ahead of print]). Patients with EBV had improved subjective dyspnea scores, residual volume, and 6-minute walk distance; however, the pneumothorax rate was 27%.
All study patients with EBV underwent bronchoscopic evaluation for collateral ventilation using a proprietary digital system, which measures expiratory airflow in target airways to establish the presence of collateral ventilation. Previous data have demonstrated improved transplant-free survival when implanted EBVs result in atelectasis of the target lobe, which requires intact interlobar fissures (Garner, et al. Am J Respir Crit Care Med. 2016;194[4]:519). Ongoing clinical trials are attempting to clarify the role of EBV therapy in different phenotypes of COPD, including patients with homogenous emphysema. Long-term follow-up data will be important in determining the broader implementation of bronchoscopic lung volume reduction moving forward.
Vivek Murthy, MD
Jason A. Akulian, MD, FCCP
Steering Committee Members
Pediatric Chest Medicine
CFTR modulators
Cystic fibrosis (CF) is a progressive genetic disorder resulting in multiorgan disease with progressive respiratory decline. CF is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. This codes for the CFTR anion channel and contributes to the movement of salt in and out of the cell. CFTR dysfunction leads to thickened secretions in the lungs and other organs, such as the gut and pancreas. This leads to more lung infections and other organ dysfunction that ultimately leads to premature death.
Established CF treatments include pulmonary and nutritional interventions. CFTR modulators are recent novel therapies that improve the function of CFTR and target the basic defect. Two types of modulator drugs (potentiators and correctors) have been developed with effectiveness depending upon the kind of CF mutation the person has.
CFTR potentiators, such as Kalydeco® (ivacaftor monotherapy), increase the likelihood that the CFTR channel will transport ions through the cell membrane, ie, they increase the channel’s “open probability.” Kalydeco has been approved for patients 12 months or older with mutations that result in partial CFTR protein function in the cell membrane. CFTR correctors, such as lumacaftor and tezacaftor, increase the amount of normal or mutated CFTR protein that gets transported, increasing the amount of CFTR protein on the cell surface. Combination drugs such as Orkambi® (lumacaftor/ivacaftor) for patients 2 years and older, and Symdeko™ (tezacaftor/ivacaftor) for patients 12 years and older, are considered in patients homozygous for the F508del mutation.
Sumit Bhargava, MBBS, FCCP
Steering Committee Member
Pulmonary Physiology, Function, and Rehabilitation
Wildfires, particulate matter, and lung function
In the last 3 decades, human-caused climate change contributed to wildfires in an additional 4.2 million hectares of land across the western US alone. Human impact on climate is responsible for nearly doubling the expected wildfire area (Abatzoglou, et al. PNAS. 2016;113:11770). Year 2017 saw the most destructive wildfires in California recorded to date, and over $2 billion dollars was spent by the US Forest Service, the most-expensive on record. Besides the devastating effects on the forestry and nearby communities, wildfires also generate a large amount of particulate matter (PM). In western US, wildfires contributed to 71.3% of total PM2.5 on days exceeding regulatory PM2.5 standards during 2004-2009 (Liu et al. Clim Change. 2016;138:655). Acute PM exposure is associated with respiratory health effects, such as exacerbation of asthma and COPD, increased ED visits and hospitalization for pneumonia, and increased mortality. Chronic PM2.5 exposure may also affect lung function. Cross-shift and cross-season FEV1 declined by 0.150 L and 0.104 L, respectively in forest firefighters (Betchley, et al. Am. J Ind Med. 1997;31:503). The Children’s Health Study conducted in California found that subjects who were exposed to the highest level of exposure to PM2.5 were five times more likely to have an FEV1 less than 80% of expected FEV1 when they reached 18 years of age than subjects exposed to the lowest level of PM2.5 (Gauderman et al. N Engl J Med. 2004;351:1057). Clinicians should educate patients and the public how to protect our environment and, when wildfires occur, how to protect themselves from exposure to PM.
Thomas W. DeCato, MD
Fellow-in-Training Committee Member
Yuh-Chin T. Huang, MD, FCCP
Steering Committee Member
Pulmonary Vascular Disease
Small increases in pulmonary pressures—big impact
Pulmonary hypertension (PH) is a progressive, life-limiting pulmonary vascular disease that is diagnosed hemodynamically by right-sided heart catheterization (RHC) and defined by a mean pulmonary artery pressure (mPAP) >25 mm Hg (Hoeper MM, et al. JACC. 2013;62(25 Suppl):D42).
The impact of PH on survival both in its “pure” form, pulmonary arterial hypertension, and in the setting of underlying cardiopulmonary disease, is well established. However, the clinical relevance of mildly elevated mPAP, defined as mPAP between 18 and 24 mm Hg, has been unclear until recently. Two large cohort studies have suggested that mild increases in mPAP are clinically relevant. A large retrospective analysis of hemodynamic data from 21,727 US veterans found mildly increased mPAP (19-24 mm Hg) was associated with increased hospitalization and decreased survival (Maron, et al. Circulation. 2016;133:1240).
While this population was skewed toward elderly men, a study from Vanderbilt University that included equal numbers of men and women showed similar results. Patients with mPAP 19-24 mm Hg experienced incrementally increased mortality (HR:1.31, P=.001). Importantly, in the subset of patients who underwent a repeat RHC in follow-up, 61% developed progressive increases of pulmonary pressures (>25 mm Hg) on follow-up RHC suggesting that the disease process may progress in a substantial proportion of patients (Assad, et al. JAMA Cardiol. 2017;2[1]):1361). Combined with prior data from smaller cohorts, these studies highlight the impact of mildly increased pulmonary pressures on outcomes. Given the dearth of available data regarding interventions for these patients, there is an urgent need to study to role of specific therapy for mildly elevated pulmonary pressures.
Vijay Balasubramanian, MD, FCCP
Steering Committee Member
Jean Elwing, MD, FCCP
Steering Committee Vice-Chair
Thoracic Oncology
Multiple tumor nodules in lung cancer diagnosis
Low dose CT (LDCT) scan screening for lung cancer is a recommended preventative modality for adults with a significant smoking history (Mayer et al. Ann Int Med. 2014;160(5):330). The screening approach aims to identify adults at significant risk for lung cancer. The goal is to discover lung cancers at low stage with benign mediastinal nodes for optimal treatment and potential for cure. In a minority, but significant number of cases, the LDCT demonstrates multiple lung nodules or masses confounding the attempt to adequately stage the tumor. Two tumors representing a primary cancer and separate malignant spread, namely, intra-pulmonary metastases, in the same lobe, different ipsilateral lobe, or contralateral lobe would be staged, respectively, as T3, T4, or M1a (Detterbeck et al. Chest. 2013;143(5):e191S). Clearly, if the two tumors are separate unique primary cancers, independent of one another, then at best they would be considered as multiple T1 tumors. The treatment modalities of and clinical survival outcomes for these multiple conditions would be markedly different.
The identification of additional tumors may be synchronous (at the same time of the primary discovery) or metachronous (at a later time than the primary discovery). The approach is basically the same. Two tumors with different histologic types, or having separate in-situ squamous cell carcinoma patterns, or disparate immunohistochemical or molecular expressions, or different genomic profiles or driver mutations may be considered as separate distinct primary malignancies (Detterbeck et al. J Thorac Oncol. 2016;11:639; Nicholson et al. J Thorac Oncol. 2017;13:205). Separate foci of ground-glass opacities with small solid central component indicative of minimally invasive adenocarcinoma may be designated as the highest T-stage. These cited and more challenging cases should be presented to a lung cancer tumor board with multiple specialties represented for analysis and judgment. The approach to diagnostic decision-making and clinical management should involve the expertise of all specialties in the lung cancer patient care team.
Arnold M. Schwartz, MD, PhD, FCCP
Steering Committee Member
Interventional Chest/Diagnostic Procedures
Endobronchial valve therapy receives FDA approval for bronchoscopic LVR
Lung volume reduction surgery (LVRS) is an established approach to improve exercise capacity and lung function in patients with heterogeneous emphysema and may confer survival benefit in patients with apical-predominant disease (Fishman, et al. N Engl J Med. 2003;348[21]:2059). Despite this, LVRS case numbers remain low due to patient and procedural morbidity. Bronchoscopic alternatives for LVRS have advanced considerably over the last decade with endobronchial valve (EBV) therapy emerging as a viable option for select subsets of patients with heterogeneous emphysema. Endobronchial valves are removable devices placed in segmental/subsegmental airways, which allow efflux of air during exhalation but close during inspiration, resulting in distal atelectasis in the absence of collateral ventilation.
The LIBERATE study, a multicenter randomized controlled trial demonstrated improvement in FEV1 ≥15% in 48% of patients after EBV placement compared with 17% of patients receiving standard medical therapy has resulted in FDA approval (Criner G, et al. Am J Respir Crit Care Med. 2018 May 22. doi: 10.1164/rccm.201803-0590OC. [Epub ahead of print]). Patients with EBV had improved subjective dyspnea scores, residual volume, and 6-minute walk distance; however, the pneumothorax rate was 27%.
All study patients with EBV underwent bronchoscopic evaluation for collateral ventilation using a proprietary digital system, which measures expiratory airflow in target airways to establish the presence of collateral ventilation. Previous data have demonstrated improved transplant-free survival when implanted EBVs result in atelectasis of the target lobe, which requires intact interlobar fissures (Garner, et al. Am J Respir Crit Care Med. 2016;194[4]:519). Ongoing clinical trials are attempting to clarify the role of EBV therapy in different phenotypes of COPD, including patients with homogenous emphysema. Long-term follow-up data will be important in determining the broader implementation of bronchoscopic lung volume reduction moving forward.
Vivek Murthy, MD
Jason A. Akulian, MD, FCCP
Steering Committee Members
Pediatric Chest Medicine
CFTR modulators
Cystic fibrosis (CF) is a progressive genetic disorder resulting in multiorgan disease with progressive respiratory decline. CF is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. This codes for the CFTR anion channel and contributes to the movement of salt in and out of the cell. CFTR dysfunction leads to thickened secretions in the lungs and other organs, such as the gut and pancreas. This leads to more lung infections and other organ dysfunction that ultimately leads to premature death.
Established CF treatments include pulmonary and nutritional interventions. CFTR modulators are recent novel therapies that improve the function of CFTR and target the basic defect. Two types of modulator drugs (potentiators and correctors) have been developed with effectiveness depending upon the kind of CF mutation the person has.
CFTR potentiators, such as Kalydeco® (ivacaftor monotherapy), increase the likelihood that the CFTR channel will transport ions through the cell membrane, ie, they increase the channel’s “open probability.” Kalydeco has been approved for patients 12 months or older with mutations that result in partial CFTR protein function in the cell membrane. CFTR correctors, such as lumacaftor and tezacaftor, increase the amount of normal or mutated CFTR protein that gets transported, increasing the amount of CFTR protein on the cell surface. Combination drugs such as Orkambi® (lumacaftor/ivacaftor) for patients 2 years and older, and Symdeko™ (tezacaftor/ivacaftor) for patients 12 years and older, are considered in patients homozygous for the F508del mutation.
Sumit Bhargava, MBBS, FCCP
Steering Committee Member
Pulmonary Physiology, Function, and Rehabilitation
Wildfires, particulate matter, and lung function
In the last 3 decades, human-caused climate change contributed to wildfires in an additional 4.2 million hectares of land across the western US alone. Human impact on climate is responsible for nearly doubling the expected wildfire area (Abatzoglou, et al. PNAS. 2016;113:11770). Year 2017 saw the most destructive wildfires in California recorded to date, and over $2 billion dollars was spent by the US Forest Service, the most-expensive on record. Besides the devastating effects on the forestry and nearby communities, wildfires also generate a large amount of particulate matter (PM). In western US, wildfires contributed to 71.3% of total PM2.5 on days exceeding regulatory PM2.5 standards during 2004-2009 (Liu et al. Clim Change. 2016;138:655). Acute PM exposure is associated with respiratory health effects, such as exacerbation of asthma and COPD, increased ED visits and hospitalization for pneumonia, and increased mortality. Chronic PM2.5 exposure may also affect lung function. Cross-shift and cross-season FEV1 declined by 0.150 L and 0.104 L, respectively in forest firefighters (Betchley, et al. Am. J Ind Med. 1997;31:503). The Children’s Health Study conducted in California found that subjects who were exposed to the highest level of exposure to PM2.5 were five times more likely to have an FEV1 less than 80% of expected FEV1 when they reached 18 years of age than subjects exposed to the lowest level of PM2.5 (Gauderman et al. N Engl J Med. 2004;351:1057). Clinicians should educate patients and the public how to protect our environment and, when wildfires occur, how to protect themselves from exposure to PM.
Thomas W. DeCato, MD
Fellow-in-Training Committee Member
Yuh-Chin T. Huang, MD, FCCP
Steering Committee Member
Pulmonary Vascular Disease
Small increases in pulmonary pressures—big impact
Pulmonary hypertension (PH) is a progressive, life-limiting pulmonary vascular disease that is diagnosed hemodynamically by right-sided heart catheterization (RHC) and defined by a mean pulmonary artery pressure (mPAP) >25 mm Hg (Hoeper MM, et al. JACC. 2013;62(25 Suppl):D42).
The impact of PH on survival both in its “pure” form, pulmonary arterial hypertension, and in the setting of underlying cardiopulmonary disease, is well established. However, the clinical relevance of mildly elevated mPAP, defined as mPAP between 18 and 24 mm Hg, has been unclear until recently. Two large cohort studies have suggested that mild increases in mPAP are clinically relevant. A large retrospective analysis of hemodynamic data from 21,727 US veterans found mildly increased mPAP (19-24 mm Hg) was associated with increased hospitalization and decreased survival (Maron, et al. Circulation. 2016;133:1240).
While this population was skewed toward elderly men, a study from Vanderbilt University that included equal numbers of men and women showed similar results. Patients with mPAP 19-24 mm Hg experienced incrementally increased mortality (HR:1.31, P=.001). Importantly, in the subset of patients who underwent a repeat RHC in follow-up, 61% developed progressive increases of pulmonary pressures (>25 mm Hg) on follow-up RHC suggesting that the disease process may progress in a substantial proportion of patients (Assad, et al. JAMA Cardiol. 2017;2[1]):1361). Combined with prior data from smaller cohorts, these studies highlight the impact of mildly increased pulmonary pressures on outcomes. Given the dearth of available data regarding interventions for these patients, there is an urgent need to study to role of specific therapy for mildly elevated pulmonary pressures.
Vijay Balasubramanian, MD, FCCP
Steering Committee Member
Jean Elwing, MD, FCCP
Steering Committee Vice-Chair
Thoracic Oncology
Multiple tumor nodules in lung cancer diagnosis
Low dose CT (LDCT) scan screening for lung cancer is a recommended preventative modality for adults with a significant smoking history (Mayer et al. Ann Int Med. 2014;160(5):330). The screening approach aims to identify adults at significant risk for lung cancer. The goal is to discover lung cancers at low stage with benign mediastinal nodes for optimal treatment and potential for cure. In a minority, but significant number of cases, the LDCT demonstrates multiple lung nodules or masses confounding the attempt to adequately stage the tumor. Two tumors representing a primary cancer and separate malignant spread, namely, intra-pulmonary metastases, in the same lobe, different ipsilateral lobe, or contralateral lobe would be staged, respectively, as T3, T4, or M1a (Detterbeck et al. Chest. 2013;143(5):e191S). Clearly, if the two tumors are separate unique primary cancers, independent of one another, then at best they would be considered as multiple T1 tumors. The treatment modalities of and clinical survival outcomes for these multiple conditions would be markedly different.
The identification of additional tumors may be synchronous (at the same time of the primary discovery) or metachronous (at a later time than the primary discovery). The approach is basically the same. Two tumors with different histologic types, or having separate in-situ squamous cell carcinoma patterns, or disparate immunohistochemical or molecular expressions, or different genomic profiles or driver mutations may be considered as separate distinct primary malignancies (Detterbeck et al. J Thorac Oncol. 2016;11:639; Nicholson et al. J Thorac Oncol. 2017;13:205). Separate foci of ground-glass opacities with small solid central component indicative of minimally invasive adenocarcinoma may be designated as the highest T-stage. These cited and more challenging cases should be presented to a lung cancer tumor board with multiple specialties represented for analysis and judgment. The approach to diagnostic decision-making and clinical management should involve the expertise of all specialties in the lung cancer patient care team.
Arnold M. Schwartz, MD, PhD, FCCP
Steering Committee Member
Interventional Chest/Diagnostic Procedures
Endobronchial valve therapy receives FDA approval for bronchoscopic LVR
Lung volume reduction surgery (LVRS) is an established approach to improve exercise capacity and lung function in patients with heterogeneous emphysema and may confer survival benefit in patients with apical-predominant disease (Fishman, et al. N Engl J Med. 2003;348[21]:2059). Despite this, LVRS case numbers remain low due to patient and procedural morbidity. Bronchoscopic alternatives for LVRS have advanced considerably over the last decade with endobronchial valve (EBV) therapy emerging as a viable option for select subsets of patients with heterogeneous emphysema. Endobronchial valves are removable devices placed in segmental/subsegmental airways, which allow efflux of air during exhalation but close during inspiration, resulting in distal atelectasis in the absence of collateral ventilation.
The LIBERATE study, a multicenter randomized controlled trial demonstrated improvement in FEV1 ≥15% in 48% of patients after EBV placement compared with 17% of patients receiving standard medical therapy has resulted in FDA approval (Criner G, et al. Am J Respir Crit Care Med. 2018 May 22. doi: 10.1164/rccm.201803-0590OC. [Epub ahead of print]). Patients with EBV had improved subjective dyspnea scores, residual volume, and 6-minute walk distance; however, the pneumothorax rate was 27%.
All study patients with EBV underwent bronchoscopic evaluation for collateral ventilation using a proprietary digital system, which measures expiratory airflow in target airways to establish the presence of collateral ventilation. Previous data have demonstrated improved transplant-free survival when implanted EBVs result in atelectasis of the target lobe, which requires intact interlobar fissures (Garner, et al. Am J Respir Crit Care Med. 2016;194[4]:519). Ongoing clinical trials are attempting to clarify the role of EBV therapy in different phenotypes of COPD, including patients with homogenous emphysema. Long-term follow-up data will be important in determining the broader implementation of bronchoscopic lung volume reduction moving forward.
Vivek Murthy, MD
Jason A. Akulian, MD, FCCP
Steering Committee Members
Pediatric Chest Medicine
CFTR modulators
Cystic fibrosis (CF) is a progressive genetic disorder resulting in multiorgan disease with progressive respiratory decline. CF is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. This codes for the CFTR anion channel and contributes to the movement of salt in and out of the cell. CFTR dysfunction leads to thickened secretions in the lungs and other organs, such as the gut and pancreas. This leads to more lung infections and other organ dysfunction that ultimately leads to premature death.
Established CF treatments include pulmonary and nutritional interventions. CFTR modulators are recent novel therapies that improve the function of CFTR and target the basic defect. Two types of modulator drugs (potentiators and correctors) have been developed with effectiveness depending upon the kind of CF mutation the person has.
CFTR potentiators, such as Kalydeco® (ivacaftor monotherapy), increase the likelihood that the CFTR channel will transport ions through the cell membrane, ie, they increase the channel’s “open probability.” Kalydeco has been approved for patients 12 months or older with mutations that result in partial CFTR protein function in the cell membrane. CFTR correctors, such as lumacaftor and tezacaftor, increase the amount of normal or mutated CFTR protein that gets transported, increasing the amount of CFTR protein on the cell surface. Combination drugs such as Orkambi® (lumacaftor/ivacaftor) for patients 2 years and older, and Symdeko™ (tezacaftor/ivacaftor) for patients 12 years and older, are considered in patients homozygous for the F508del mutation.
Sumit Bhargava, MBBS, FCCP
Steering Committee Member
Pulmonary Physiology, Function, and Rehabilitation
Wildfires, particulate matter, and lung function
In the last 3 decades, human-caused climate change contributed to wildfires in an additional 4.2 million hectares of land across the western US alone. Human impact on climate is responsible for nearly doubling the expected wildfire area (Abatzoglou, et al. PNAS. 2016;113:11770). Year 2017 saw the most destructive wildfires in California recorded to date, and over $2 billion dollars was spent by the US Forest Service, the most-expensive on record. Besides the devastating effects on the forestry and nearby communities, wildfires also generate a large amount of particulate matter (PM). In western US, wildfires contributed to 71.3% of total PM2.5 on days exceeding regulatory PM2.5 standards during 2004-2009 (Liu et al. Clim Change. 2016;138:655). Acute PM exposure is associated with respiratory health effects, such as exacerbation of asthma and COPD, increased ED visits and hospitalization for pneumonia, and increased mortality. Chronic PM2.5 exposure may also affect lung function. Cross-shift and cross-season FEV1 declined by 0.150 L and 0.104 L, respectively in forest firefighters (Betchley, et al. Am. J Ind Med. 1997;31:503). The Children’s Health Study conducted in California found that subjects who were exposed to the highest level of exposure to PM2.5 were five times more likely to have an FEV1 less than 80% of expected FEV1 when they reached 18 years of age than subjects exposed to the lowest level of PM2.5 (Gauderman et al. N Engl J Med. 2004;351:1057). Clinicians should educate patients and the public how to protect our environment and, when wildfires occur, how to protect themselves from exposure to PM.
Thomas W. DeCato, MD
Fellow-in-Training Committee Member
Yuh-Chin T. Huang, MD, FCCP
Steering Committee Member
Pulmonary Vascular Disease
Small increases in pulmonary pressures—big impact
Pulmonary hypertension (PH) is a progressive, life-limiting pulmonary vascular disease that is diagnosed hemodynamically by right-sided heart catheterization (RHC) and defined by a mean pulmonary artery pressure (mPAP) >25 mm Hg (Hoeper MM, et al. JACC. 2013;62(25 Suppl):D42).
The impact of PH on survival both in its “pure” form, pulmonary arterial hypertension, and in the setting of underlying cardiopulmonary disease, is well established. However, the clinical relevance of mildly elevated mPAP, defined as mPAP between 18 and 24 mm Hg, has been unclear until recently. Two large cohort studies have suggested that mild increases in mPAP are clinically relevant. A large retrospective analysis of hemodynamic data from 21,727 US veterans found mildly increased mPAP (19-24 mm Hg) was associated with increased hospitalization and decreased survival (Maron, et al. Circulation. 2016;133:1240).
While this population was skewed toward elderly men, a study from Vanderbilt University that included equal numbers of men and women showed similar results. Patients with mPAP 19-24 mm Hg experienced incrementally increased mortality (HR:1.31, P=.001). Importantly, in the subset of patients who underwent a repeat RHC in follow-up, 61% developed progressive increases of pulmonary pressures (>25 mm Hg) on follow-up RHC suggesting that the disease process may progress in a substantial proportion of patients (Assad, et al. JAMA Cardiol. 2017;2[1]):1361). Combined with prior data from smaller cohorts, these studies highlight the impact of mildly increased pulmonary pressures on outcomes. Given the dearth of available data regarding interventions for these patients, there is an urgent need to study to role of specific therapy for mildly elevated pulmonary pressures.
Vijay Balasubramanian, MD, FCCP
Steering Committee Member
Jean Elwing, MD, FCCP
Steering Committee Vice-Chair
Thoracic Oncology
Multiple tumor nodules in lung cancer diagnosis
Low dose CT (LDCT) scan screening for lung cancer is a recommended preventative modality for adults with a significant smoking history (Mayer et al. Ann Int Med. 2014;160(5):330). The screening approach aims to identify adults at significant risk for lung cancer. The goal is to discover lung cancers at low stage with benign mediastinal nodes for optimal treatment and potential for cure. In a minority, but significant number of cases, the LDCT demonstrates multiple lung nodules or masses confounding the attempt to adequately stage the tumor. Two tumors representing a primary cancer and separate malignant spread, namely, intra-pulmonary metastases, in the same lobe, different ipsilateral lobe, or contralateral lobe would be staged, respectively, as T3, T4, or M1a (Detterbeck et al. Chest. 2013;143(5):e191S). Clearly, if the two tumors are separate unique primary cancers, independent of one another, then at best they would be considered as multiple T1 tumors. The treatment modalities of and clinical survival outcomes for these multiple conditions would be markedly different.
The identification of additional tumors may be synchronous (at the same time of the primary discovery) or metachronous (at a later time than the primary discovery). The approach is basically the same. Two tumors with different histologic types, or having separate in-situ squamous cell carcinoma patterns, or disparate immunohistochemical or molecular expressions, or different genomic profiles or driver mutations may be considered as separate distinct primary malignancies (Detterbeck et al. J Thorac Oncol. 2016;11:639; Nicholson et al. J Thorac Oncol. 2017;13:205). Separate foci of ground-glass opacities with small solid central component indicative of minimally invasive adenocarcinoma may be designated as the highest T-stage. These cited and more challenging cases should be presented to a lung cancer tumor board with multiple specialties represented for analysis and judgment. The approach to diagnostic decision-making and clinical management should involve the expertise of all specialties in the lung cancer patient care team.
Arnold M. Schwartz, MD, PhD, FCCP
Steering Committee Member
This month in the journal CHEST®
Editor’s picks
Original Research
Pilot Feasibility Study in Establishing the Role of Ultrasound-Guided Pleural Biopsies in Pleural Infection (The AUDIO Study). By Dr. I. Psallidas, et al.
Commentary
Sleep Apnea Morbidity: A Consequence of Microbial-Immune Cross-Talk? By Dr. N. Farre, et al.
Evidence-Based Medicine
Treatment of Interstitial Lung Disease-Associated Cough: CHEST guideline and expert panel report. By Dr. S. S. Birring, et al.
Editor’s picks
Original Research
Pilot Feasibility Study in Establishing the Role of Ultrasound-Guided Pleural Biopsies in Pleural Infection (The AUDIO Study). By Dr. I. Psallidas, et al.
Commentary
Sleep Apnea Morbidity: A Consequence of Microbial-Immune Cross-Talk? By Dr. N. Farre, et al.
Evidence-Based Medicine
Treatment of Interstitial Lung Disease-Associated Cough: CHEST guideline and expert panel report. By Dr. S. S. Birring, et al.
Editor’s picks
Original Research
Pilot Feasibility Study in Establishing the Role of Ultrasound-Guided Pleural Biopsies in Pleural Infection (The AUDIO Study). By Dr. I. Psallidas, et al.
Commentary
Sleep Apnea Morbidity: A Consequence of Microbial-Immune Cross-Talk? By Dr. N. Farre, et al.
Evidence-Based Medicine
Treatment of Interstitial Lung Disease-Associated Cough: CHEST guideline and expert panel report. By Dr. S. S. Birring, et al.
Perioperative diabetes and HbA1c in mortality
Clinical question: Do preoperative hemoglobin A1c (HbA1c) and perioperative glucose predict outcomes in patients undergoing noncardiac and cardiac surgeries?
Background: Hyperglycemia in the perioperative period has been associated with infection, delayed wound healing, and postoperative mortality. Studies have investigated the effects of HbA1c or hyperglycemia on postoperative outcomes, but none have been performed to assess the effect of one while controlling for the other.
Study design: Retrospective analysis.
Setting: Single-center, Duke University Health System.
Synopsis: Using a database of electronic health records at Duke University Health System, Durham, N.C., investigators reviewed 13,077 surgeries (6,684 noncardiac and 6,393 cardiac) to determine the association of preoperative HbA1c with perioperative glucose and 30-day mortality. For noncardiac surgery, increased average perioperative glucose was associated with increased mortality (P = .04). In cardiac surgery both low and high average glucose was associated with increased mortality (P = .001). By contrast, HbA1c was not a significant predictor of postoperative mortality in cardiac surgery (P = .08), and in noncardiac surgery, HbA1C was negatively associated with 30-day mortality (P = .01). Overall, perioperative glucose was predictive of 30-day mortality, but HbA1c was not associated with 30-day mortality after researchers controlled for glucose.
Because the study is retrospective, no causal relationship can be established. Hospitalists involved in perioperative care should aim for optimization of glucose control regardless of preoperative HbA1c.
Bottom line: Perioperative glucose is related to surgical outcomes, but HbA1c is a less useful indicator of 30-day postoperative mortality.
Citation: Van den Boom W et al. Effect of A1C and glucose on postoperative mortality in noncardiac and cardiac surgeries. Diabetes Care. 2018 Feb;41:782-8.
Clinical question: Do preoperative hemoglobin A1c (HbA1c) and perioperative glucose predict outcomes in patients undergoing noncardiac and cardiac surgeries?
Background: Hyperglycemia in the perioperative period has been associated with infection, delayed wound healing, and postoperative mortality. Studies have investigated the effects of HbA1c or hyperglycemia on postoperative outcomes, but none have been performed to assess the effect of one while controlling for the other.
Study design: Retrospective analysis.
Setting: Single-center, Duke University Health System.
Synopsis: Using a database of electronic health records at Duke University Health System, Durham, N.C., investigators reviewed 13,077 surgeries (6,684 noncardiac and 6,393 cardiac) to determine the association of preoperative HbA1c with perioperative glucose and 30-day mortality. For noncardiac surgery, increased average perioperative glucose was associated with increased mortality (P = .04). In cardiac surgery both low and high average glucose was associated with increased mortality (P = .001). By contrast, HbA1c was not a significant predictor of postoperative mortality in cardiac surgery (P = .08), and in noncardiac surgery, HbA1C was negatively associated with 30-day mortality (P = .01). Overall, perioperative glucose was predictive of 30-day mortality, but HbA1c was not associated with 30-day mortality after researchers controlled for glucose.
Because the study is retrospective, no causal relationship can be established. Hospitalists involved in perioperative care should aim for optimization of glucose control regardless of preoperative HbA1c.
Bottom line: Perioperative glucose is related to surgical outcomes, but HbA1c is a less useful indicator of 30-day postoperative mortality.
Citation: Van den Boom W et al. Effect of A1C and glucose on postoperative mortality in noncardiac and cardiac surgeries. Diabetes Care. 2018 Feb;41:782-8.
Clinical question: Do preoperative hemoglobin A1c (HbA1c) and perioperative glucose predict outcomes in patients undergoing noncardiac and cardiac surgeries?
Background: Hyperglycemia in the perioperative period has been associated with infection, delayed wound healing, and postoperative mortality. Studies have investigated the effects of HbA1c or hyperglycemia on postoperative outcomes, but none have been performed to assess the effect of one while controlling for the other.
Study design: Retrospective analysis.
Setting: Single-center, Duke University Health System.
Synopsis: Using a database of electronic health records at Duke University Health System, Durham, N.C., investigators reviewed 13,077 surgeries (6,684 noncardiac and 6,393 cardiac) to determine the association of preoperative HbA1c with perioperative glucose and 30-day mortality. For noncardiac surgery, increased average perioperative glucose was associated with increased mortality (P = .04). In cardiac surgery both low and high average glucose was associated with increased mortality (P = .001). By contrast, HbA1c was not a significant predictor of postoperative mortality in cardiac surgery (P = .08), and in noncardiac surgery, HbA1C was negatively associated with 30-day mortality (P = .01). Overall, perioperative glucose was predictive of 30-day mortality, but HbA1c was not associated with 30-day mortality after researchers controlled for glucose.
Because the study is retrospective, no causal relationship can be established. Hospitalists involved in perioperative care should aim for optimization of glucose control regardless of preoperative HbA1c.
Bottom line: Perioperative glucose is related to surgical outcomes, but HbA1c is a less useful indicator of 30-day postoperative mortality.
Citation: Van den Boom W et al. Effect of A1C and glucose on postoperative mortality in noncardiac and cardiac surgeries. Diabetes Care. 2018 Feb;41:782-8.
Syncope alone after age 60 does not require admission
SAN DIEGO – Unless the cause of syncope has been identified after a thorough workup in the emergency department, there is no advantage to admitting patients aged 60 years and older who complain of syncope, an ED-based study has found.
Almost 2,500 patients aged 60 or older with unexplained syncope after a thorough workup had similar 30-day outcomes whether they were admitted to the hospital or sent home from the ED, based on the results of a retrospective study presented at the annual meeting of the American College of Emergency Physicians.
Dr. Marc A. Probst of the Icahn School of Medicine at Mount Sinai, New York, who presented the data, reported that many centers admit older patients with syncope, although the benefit of this practice has not been well established.
In a video interview, Dr. Probst points out how the findings may be useful in guiding clinical decisions or counseling patients when admission is being considered.
SAN DIEGO – Unless the cause of syncope has been identified after a thorough workup in the emergency department, there is no advantage to admitting patients aged 60 years and older who complain of syncope, an ED-based study has found.
Almost 2,500 patients aged 60 or older with unexplained syncope after a thorough workup had similar 30-day outcomes whether they were admitted to the hospital or sent home from the ED, based on the results of a retrospective study presented at the annual meeting of the American College of Emergency Physicians.
Dr. Marc A. Probst of the Icahn School of Medicine at Mount Sinai, New York, who presented the data, reported that many centers admit older patients with syncope, although the benefit of this practice has not been well established.
In a video interview, Dr. Probst points out how the findings may be useful in guiding clinical decisions or counseling patients when admission is being considered.
SAN DIEGO – Unless the cause of syncope has been identified after a thorough workup in the emergency department, there is no advantage to admitting patients aged 60 years and older who complain of syncope, an ED-based study has found.
Almost 2,500 patients aged 60 or older with unexplained syncope after a thorough workup had similar 30-day outcomes whether they were admitted to the hospital or sent home from the ED, based on the results of a retrospective study presented at the annual meeting of the American College of Emergency Physicians.
Dr. Marc A. Probst of the Icahn School of Medicine at Mount Sinai, New York, who presented the data, reported that many centers admit older patients with syncope, although the benefit of this practice has not been well established.
In a video interview, Dr. Probst points out how the findings may be useful in guiding clinical decisions or counseling patients when admission is being considered.
REPORTING FROM ACEP18
Early supportive care cuts costs and admissions in cancer patients undergoing curative treatment
PHOENIX – By starting supportive measures early in the care of cancer patients undergoing curative treatment, a cancer center cut costs, emergency department visits, and admissions, a researcher said at symposium on quality care sponsored by the American Society of Clinical Oncology.
The supportive care pathway resulted in double-digit decreases in admissions and an opportunity cost savings of $1,500 per patient, reported Christopher D. Koprowski, MD, MBA, of Helen F. Graham Cancer Center & Research Institute, Christiana Care Health System, Newark, Del.
Although satisfaction hasn’t been measured yet, anecdotal reports suggest the patient experience has improved because of the multidisciplinary program, which included mandatory supportive care screening and enhancements to computer systems, said Dr. Koprowski, who is director of quality and safety at the cancer center.
“From all outward signs, the patients are extraordinarily grateful in this program,” Dr. Koprowski said in an interview. “I just had one who said that being seen at the same time by all these people just makes things so much easier.”
The Supportive Care of Oncology Patients (SCOOP) clinical pathway, introduced in November 2016, includes palliative and supportive care service screening that occurs during the multidisciplinary visit. The pathway incorporates a checklist integrated into a nurse navigator information system to support care standardization, according to Dr. Koprowski.
Also added were “flags” in the inpatient information system that trigger alerts to navigators, oncologists, and the supportive care service whenever a patient in the SCOOP pathway is admitted, discharged, or seen in the emergency room, he said.
Enrollment in SCOOP was limited to lung, esophageal, head and neck, and colorectal cancer patients receiving concurrent radiation and chemotherapy. Out of approximately 200 eligible patients in the first year, about half entered the clinical pathway, according to Dr. Koprowski.
For that first year, 32% of SCOOP patients had ED visits, compared with 54% of combined modality patients who did not enter the pathway, Dr. Koprowski reported.
Similarly, admissions were 25% for the SCOOP patients and 34% of non-SCOOP patients, and readmissions were seen in 20% versus 32% of those groups, respectively.
These findings are much like what has been seen when early supportive care is introduced in patients with more advanced disease, according to Dr. Koprowski.
He said the SCOOP program was partly inspired by a study in the New England Journal of Medicine showing that patients with advanced non–small cell lung cancer who received early palliative care had longer survival despite less-aggressive care, including reduced use of chemotherapy, at the end of life.
“Early-stage patients aren’t that much different if they are being treated very aggressively with combined modality chemotherapy and radiation,” he said. “The treatment is very, very tough on people.”
Dr. Koprowski and his coinvestigators had no relationships to disclose relevant to the research presented at the ASCO symposium.
SOURCE: Koprowski CD et al. 2018 ASCO Quality Care Symposium, Abstract 142.
PHOENIX – By starting supportive measures early in the care of cancer patients undergoing curative treatment, a cancer center cut costs, emergency department visits, and admissions, a researcher said at symposium on quality care sponsored by the American Society of Clinical Oncology.
The supportive care pathway resulted in double-digit decreases in admissions and an opportunity cost savings of $1,500 per patient, reported Christopher D. Koprowski, MD, MBA, of Helen F. Graham Cancer Center & Research Institute, Christiana Care Health System, Newark, Del.
Although satisfaction hasn’t been measured yet, anecdotal reports suggest the patient experience has improved because of the multidisciplinary program, which included mandatory supportive care screening and enhancements to computer systems, said Dr. Koprowski, who is director of quality and safety at the cancer center.
“From all outward signs, the patients are extraordinarily grateful in this program,” Dr. Koprowski said in an interview. “I just had one who said that being seen at the same time by all these people just makes things so much easier.”
The Supportive Care of Oncology Patients (SCOOP) clinical pathway, introduced in November 2016, includes palliative and supportive care service screening that occurs during the multidisciplinary visit. The pathway incorporates a checklist integrated into a nurse navigator information system to support care standardization, according to Dr. Koprowski.
Also added were “flags” in the inpatient information system that trigger alerts to navigators, oncologists, and the supportive care service whenever a patient in the SCOOP pathway is admitted, discharged, or seen in the emergency room, he said.
Enrollment in SCOOP was limited to lung, esophageal, head and neck, and colorectal cancer patients receiving concurrent radiation and chemotherapy. Out of approximately 200 eligible patients in the first year, about half entered the clinical pathway, according to Dr. Koprowski.
For that first year, 32% of SCOOP patients had ED visits, compared with 54% of combined modality patients who did not enter the pathway, Dr. Koprowski reported.
Similarly, admissions were 25% for the SCOOP patients and 34% of non-SCOOP patients, and readmissions were seen in 20% versus 32% of those groups, respectively.
These findings are much like what has been seen when early supportive care is introduced in patients with more advanced disease, according to Dr. Koprowski.
He said the SCOOP program was partly inspired by a study in the New England Journal of Medicine showing that patients with advanced non–small cell lung cancer who received early palliative care had longer survival despite less-aggressive care, including reduced use of chemotherapy, at the end of life.
“Early-stage patients aren’t that much different if they are being treated very aggressively with combined modality chemotherapy and radiation,” he said. “The treatment is very, very tough on people.”
Dr. Koprowski and his coinvestigators had no relationships to disclose relevant to the research presented at the ASCO symposium.
SOURCE: Koprowski CD et al. 2018 ASCO Quality Care Symposium, Abstract 142.
PHOENIX – By starting supportive measures early in the care of cancer patients undergoing curative treatment, a cancer center cut costs, emergency department visits, and admissions, a researcher said at symposium on quality care sponsored by the American Society of Clinical Oncology.
The supportive care pathway resulted in double-digit decreases in admissions and an opportunity cost savings of $1,500 per patient, reported Christopher D. Koprowski, MD, MBA, of Helen F. Graham Cancer Center & Research Institute, Christiana Care Health System, Newark, Del.
Although satisfaction hasn’t been measured yet, anecdotal reports suggest the patient experience has improved because of the multidisciplinary program, which included mandatory supportive care screening and enhancements to computer systems, said Dr. Koprowski, who is director of quality and safety at the cancer center.
“From all outward signs, the patients are extraordinarily grateful in this program,” Dr. Koprowski said in an interview. “I just had one who said that being seen at the same time by all these people just makes things so much easier.”
The Supportive Care of Oncology Patients (SCOOP) clinical pathway, introduced in November 2016, includes palliative and supportive care service screening that occurs during the multidisciplinary visit. The pathway incorporates a checklist integrated into a nurse navigator information system to support care standardization, according to Dr. Koprowski.
Also added were “flags” in the inpatient information system that trigger alerts to navigators, oncologists, and the supportive care service whenever a patient in the SCOOP pathway is admitted, discharged, or seen in the emergency room, he said.
Enrollment in SCOOP was limited to lung, esophageal, head and neck, and colorectal cancer patients receiving concurrent radiation and chemotherapy. Out of approximately 200 eligible patients in the first year, about half entered the clinical pathway, according to Dr. Koprowski.
For that first year, 32% of SCOOP patients had ED visits, compared with 54% of combined modality patients who did not enter the pathway, Dr. Koprowski reported.
Similarly, admissions were 25% for the SCOOP patients and 34% of non-SCOOP patients, and readmissions were seen in 20% versus 32% of those groups, respectively.
These findings are much like what has been seen when early supportive care is introduced in patients with more advanced disease, according to Dr. Koprowski.
He said the SCOOP program was partly inspired by a study in the New England Journal of Medicine showing that patients with advanced non–small cell lung cancer who received early palliative care had longer survival despite less-aggressive care, including reduced use of chemotherapy, at the end of life.
“Early-stage patients aren’t that much different if they are being treated very aggressively with combined modality chemotherapy and radiation,” he said. “The treatment is very, very tough on people.”
Dr. Koprowski and his coinvestigators had no relationships to disclose relevant to the research presented at the ASCO symposium.
SOURCE: Koprowski CD et al. 2018 ASCO Quality Care Symposium, Abstract 142.
REPORTING FROM THE QUALITY CARE SYMPOSIUM
Key clinical point: Early supportive care measures reduced costs, ED visits, and admissions in cancer patients who underwent combined modality therapy.
Major finding: 32% of patients had ED visits, compared with 54% of patients who did not enter the early supportive care pathway.
Study details: Analysis of cost and health care utilization for approximately 200 patients who underwent concurrent radiation and chemotherapy.
Disclosures: Authors had no relationships to disclose.
Source: Koprowski CD et al. 2018 ASCO Quality Care Symposium, Abstract 142.
PCV13 for older adults
Also today, ED visits that are related to psychiatric complaints are up 20 among elderly, nonobstructive angina should trigger functional testing, and antibiotics trigger proteolytic activity that leads to chronic colitis.
Amazon Alexa
Apple Podcasts
Spotify
Also today, ED visits that are related to psychiatric complaints are up 20 among elderly, nonobstructive angina should trigger functional testing, and antibiotics trigger proteolytic activity that leads to chronic colitis.
Amazon Alexa
Apple Podcasts
Spotify
Also today, ED visits that are related to psychiatric complaints are up 20 among elderly, nonobstructive angina should trigger functional testing, and antibiotics trigger proteolytic activity that leads to chronic colitis.
Amazon Alexa
Apple Podcasts
Spotify
Adding a Cream May Enhance Flu Vaccine Effectiveness
Can a cream help a flu vaccine work better? In a phase 1 clinical trial, researchers from Baylor College of Medicine in Houston, Texas, are testing whether imiquimod cream—commonly used to treat genital warts and some skin cancers—can boost the immune response to an H5N1 flu vaccine. Studies have shown imiquimod generates significantly more robust immune responses.
Participants in the Vaccine and Treatment Evaluation Units trial will be given 2 intradermal doses of an H5N1 vaccine, 21 days apart. In one group, participants will have Aldara (imiquimod cream) applied to their upper arm before each vaccination; in the control group, a placebo cream will be applied.
Participants will return at regular intervals over 7 months to have blood drawn; they also will keep diaries to record symptoms.
The first participant was vaccinated in June. Early results are expected by the end of the year.
Can a cream help a flu vaccine work better? In a phase 1 clinical trial, researchers from Baylor College of Medicine in Houston, Texas, are testing whether imiquimod cream—commonly used to treat genital warts and some skin cancers—can boost the immune response to an H5N1 flu vaccine. Studies have shown imiquimod generates significantly more robust immune responses.
Participants in the Vaccine and Treatment Evaluation Units trial will be given 2 intradermal doses of an H5N1 vaccine, 21 days apart. In one group, participants will have Aldara (imiquimod cream) applied to their upper arm before each vaccination; in the control group, a placebo cream will be applied.
Participants will return at regular intervals over 7 months to have blood drawn; they also will keep diaries to record symptoms.
The first participant was vaccinated in June. Early results are expected by the end of the year.
Can a cream help a flu vaccine work better? In a phase 1 clinical trial, researchers from Baylor College of Medicine in Houston, Texas, are testing whether imiquimod cream—commonly used to treat genital warts and some skin cancers—can boost the immune response to an H5N1 flu vaccine. Studies have shown imiquimod generates significantly more robust immune responses.
Participants in the Vaccine and Treatment Evaluation Units trial will be given 2 intradermal doses of an H5N1 vaccine, 21 days apart. In one group, participants will have Aldara (imiquimod cream) applied to their upper arm before each vaccination; in the control group, a placebo cream will be applied.
Participants will return at regular intervals over 7 months to have blood drawn; they also will keep diaries to record symptoms.
The first participant was vaccinated in June. Early results are expected by the end of the year.
Quadruplet therapy could be the future in MM
New York—Four-drug combinations are holding promise for the treatment of multiple myeloma (MM), although data from additional randomized trials are needed to define their role in clinical practice, according to Natalie S. Callander, MD, of the University of Wisconsin Carbone Cancer Center.
The efficacy of multiple triplet regimens has been well documented, and data are now emerging on the potential of 4-drug combinations.
“Triplet therapy is the standard,” she said during a presentation at the NCCN 13th Annual Congress: Hematologic Malignancies, “and quad therapy may be in the future.”
The study that set the standard for triplets in myeloma, she said, is SWOG 0777, an open label, phase 3 trial that compared bortezomib with lenalidomide and dexamethasone (VRd) to lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma.
Adding bortezomib to lenalidomide and dexamethasone significantly improved both progression-free and overall survival in the 525-patient trial, with a risk-benefit profile that was acceptable. The trial was recently reported in The Lancet.
The median progression free survival (PFS) was 43 months for the triplet, versus 30 months for the two-drug regimen (P=0.0018).
Likewise, median overall survival (OS) was significantly improved, at 75 months versus 64 months for triplet versus doublet therapy (P=0.025).
“Very convincingly, just receiving that short exposure to bortezomib,” she said, “ended up causing a substantial increase of progression-free and overall survival.”
Effective triplet regimens include the combination of carfilzomib, lenalidomide, and dexamethasone (KRd), cyclophosphamide, bortezomib, and dexamethasone (CyBorD), and more recently, ixazomib, lenalidomide, and dexamethasone (IRd).
These regimens have “excellent” response rates and survival data, Dr. Callander noted.
Quadruplet data
The combination of elotuzumab plus VRd produced high response rates that were even higher after transplant, with reasonable toxicity, Dr. Callander said of phase 2 trial data presented at ASCO 2017 (abstract 8002).
Similarly, the combination of daratumumab plus KRd had a 100% rate of partial response or better in phase 2 data also presented at ASCO 2017 (abstract 8000), with rates of very good partial response and complete response that improved with successive cycles of therapy, she said.
Even so, “it remains to be seen whether four drugs will be the new standard,” Dr Callander conjectured.
Four versus three drug strategies are being evaluated in ongoing randomized clinical trials including patients with previously untreated myeloma, she said.
These studies include Cassiopeia (NCT02541383), which is evaluating bortezomib, thalidomide, and dexamethasone with or without daratumumab, and GRIFFIN (NCT02874742), which is looking at VRd with or without daratumumab.
Daratumumab received an additional indication in myeloma, this time as part of a 4-drug regimen, several months ago, Dr. Callander added in a discussion on treatment options for elderly myeloma patients.
The U.S. Food and Drug Administration (FDA) approved the monoclonal antibody in combination with bortezomib, melphalan, and prednisone (VMP) for treatment of newly diagnosed myeloma patients who are transplant ineligible.
That approval was based on results of the multicenter phase 3 ALCYONE (MMY3007) study, published in The New England Journal of Medicine, showing an 18-month PFS of 71.6% for the 4-drug combination versus 50.2% for VMP alone (P<0.001).
New York—Four-drug combinations are holding promise for the treatment of multiple myeloma (MM), although data from additional randomized trials are needed to define their role in clinical practice, according to Natalie S. Callander, MD, of the University of Wisconsin Carbone Cancer Center.
The efficacy of multiple triplet regimens has been well documented, and data are now emerging on the potential of 4-drug combinations.
“Triplet therapy is the standard,” she said during a presentation at the NCCN 13th Annual Congress: Hematologic Malignancies, “and quad therapy may be in the future.”
The study that set the standard for triplets in myeloma, she said, is SWOG 0777, an open label, phase 3 trial that compared bortezomib with lenalidomide and dexamethasone (VRd) to lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma.
Adding bortezomib to lenalidomide and dexamethasone significantly improved both progression-free and overall survival in the 525-patient trial, with a risk-benefit profile that was acceptable. The trial was recently reported in The Lancet.
The median progression free survival (PFS) was 43 months for the triplet, versus 30 months for the two-drug regimen (P=0.0018).
Likewise, median overall survival (OS) was significantly improved, at 75 months versus 64 months for triplet versus doublet therapy (P=0.025).
“Very convincingly, just receiving that short exposure to bortezomib,” she said, “ended up causing a substantial increase of progression-free and overall survival.”
Effective triplet regimens include the combination of carfilzomib, lenalidomide, and dexamethasone (KRd), cyclophosphamide, bortezomib, and dexamethasone (CyBorD), and more recently, ixazomib, lenalidomide, and dexamethasone (IRd).
These regimens have “excellent” response rates and survival data, Dr. Callander noted.
Quadruplet data
The combination of elotuzumab plus VRd produced high response rates that were even higher after transplant, with reasonable toxicity, Dr. Callander said of phase 2 trial data presented at ASCO 2017 (abstract 8002).
Similarly, the combination of daratumumab plus KRd had a 100% rate of partial response or better in phase 2 data also presented at ASCO 2017 (abstract 8000), with rates of very good partial response and complete response that improved with successive cycles of therapy, she said.
Even so, “it remains to be seen whether four drugs will be the new standard,” Dr Callander conjectured.
Four versus three drug strategies are being evaluated in ongoing randomized clinical trials including patients with previously untreated myeloma, she said.
These studies include Cassiopeia (NCT02541383), which is evaluating bortezomib, thalidomide, and dexamethasone with or without daratumumab, and GRIFFIN (NCT02874742), which is looking at VRd with or without daratumumab.
Daratumumab received an additional indication in myeloma, this time as part of a 4-drug regimen, several months ago, Dr. Callander added in a discussion on treatment options for elderly myeloma patients.
The U.S. Food and Drug Administration (FDA) approved the monoclonal antibody in combination with bortezomib, melphalan, and prednisone (VMP) for treatment of newly diagnosed myeloma patients who are transplant ineligible.
That approval was based on results of the multicenter phase 3 ALCYONE (MMY3007) study, published in The New England Journal of Medicine, showing an 18-month PFS of 71.6% for the 4-drug combination versus 50.2% for VMP alone (P<0.001).
New York—Four-drug combinations are holding promise for the treatment of multiple myeloma (MM), although data from additional randomized trials are needed to define their role in clinical practice, according to Natalie S. Callander, MD, of the University of Wisconsin Carbone Cancer Center.
The efficacy of multiple triplet regimens has been well documented, and data are now emerging on the potential of 4-drug combinations.
“Triplet therapy is the standard,” she said during a presentation at the NCCN 13th Annual Congress: Hematologic Malignancies, “and quad therapy may be in the future.”
The study that set the standard for triplets in myeloma, she said, is SWOG 0777, an open label, phase 3 trial that compared bortezomib with lenalidomide and dexamethasone (VRd) to lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma.
Adding bortezomib to lenalidomide and dexamethasone significantly improved both progression-free and overall survival in the 525-patient trial, with a risk-benefit profile that was acceptable. The trial was recently reported in The Lancet.
The median progression free survival (PFS) was 43 months for the triplet, versus 30 months for the two-drug regimen (P=0.0018).
Likewise, median overall survival (OS) was significantly improved, at 75 months versus 64 months for triplet versus doublet therapy (P=0.025).
“Very convincingly, just receiving that short exposure to bortezomib,” she said, “ended up causing a substantial increase of progression-free and overall survival.”
Effective triplet regimens include the combination of carfilzomib, lenalidomide, and dexamethasone (KRd), cyclophosphamide, bortezomib, and dexamethasone (CyBorD), and more recently, ixazomib, lenalidomide, and dexamethasone (IRd).
These regimens have “excellent” response rates and survival data, Dr. Callander noted.
Quadruplet data
The combination of elotuzumab plus VRd produced high response rates that were even higher after transplant, with reasonable toxicity, Dr. Callander said of phase 2 trial data presented at ASCO 2017 (abstract 8002).
Similarly, the combination of daratumumab plus KRd had a 100% rate of partial response or better in phase 2 data also presented at ASCO 2017 (abstract 8000), with rates of very good partial response and complete response that improved with successive cycles of therapy, she said.
Even so, “it remains to be seen whether four drugs will be the new standard,” Dr Callander conjectured.
Four versus three drug strategies are being evaluated in ongoing randomized clinical trials including patients with previously untreated myeloma, she said.
These studies include Cassiopeia (NCT02541383), which is evaluating bortezomib, thalidomide, and dexamethasone with or without daratumumab, and GRIFFIN (NCT02874742), which is looking at VRd with or without daratumumab.
Daratumumab received an additional indication in myeloma, this time as part of a 4-drug regimen, several months ago, Dr. Callander added in a discussion on treatment options for elderly myeloma patients.
The U.S. Food and Drug Administration (FDA) approved the monoclonal antibody in combination with bortezomib, melphalan, and prednisone (VMP) for treatment of newly diagnosed myeloma patients who are transplant ineligible.
That approval was based on results of the multicenter phase 3 ALCYONE (MMY3007) study, published in The New England Journal of Medicine, showing an 18-month PFS of 71.6% for the 4-drug combination versus 50.2% for VMP alone (P<0.001).