User login
Longer withdrawal time in right colon increases adenoma detection rate
PHILADELPHIA – There was a significantly higher adenoma detection rate when the withdrawal rate in the right colon was more than 3 minutes in patients undergoing colonoscopy, according to a recent presentation at the annual meeting of the American College of Gastroenterology.
Although adenomas precede colon cancer in approximately 70% of cases, and detection of adenomas is associated with 5% risk of dying from colorectal cancer, miss rates of adenomas are high in both sides of the colon and ideal withdrawal times are not known, Fahad F. Mir, MD, MSc, from the University of Missouri-Kansas City, said.
“Miss rates are high, especially in the right side of the colon. A colonoscopy offers protection in up to 80% of the left side of the patients but only up to 40%-60% in the right side of patients,” Dr. Mir stated in his presentation. “The quality standard now [for withdrawal time] is 6 minutes, so we hypothesized that adenoma detection rate is not the same if the right colon withdrawal time is equal to or more than 3 minutes, compared to less than 3 minutes.”
The abstract received an ACG Governor’s Award for Excellence in Clinical Research.
Dr. Mir and his colleagues performed a prospective, randomized, case-controlled study of 226 patients undergoing colonoscopy at three endoscopy centers in St. Luke’s Health System, Kansas City, who were aged at least 50 years and had not undergone colonic resections, emergent procedures, or were unable to consent because of mental status or language barrier. Patients were randomized to a control group (113 patients) in whom right colon withdrawal time was under 3 minutes and an intervention group (113 patients) in whom withdrawal time was 3 minutes or more.
There was a 33% adenoma detection rate in the 3 minute or more group, compared with 14% in the less than 3 minutes group (odds ratio, 3.0; 95% confidence interval, 1.62-5.64; P less than .001). Polyp detection rates were 49% in the 3 minutes or more group and 14% in the less than 3 minutes group (OR 5.1; 95% CI, 2.84-9.32; P less than .001). The optimal cut-off point was 3 minutes and 1 second with optimal sensitivity and specificity with an area under the curve of 0.73 (95% CI, 0.65-0.81; P less than .001) for optimal cut-off time for withdrawal from the right colon.
“There was a difference in fellow involvement, where fellows were more likely to be involved when the withdrawal time was more than 3 minutes as opposed to less than 3 minutes; the ADR [adenoma detection rate] was not different based upon fellow involvement,” Dr. Mir said.
The researchers noted similar rates of retroflexion between both groups and said there were no adverse events related to the study in either group. Limitations of the study included its unblinded design, data collection from multiple centers, and a higher rate of previous polyps in patients in the withdrawal in more than 3 minutes group.
Dr. Mir report no relevant conflicts of interest.
SOURCE: Mir FF et al. ACG 2018, Presentation 5.
PHILADELPHIA – There was a significantly higher adenoma detection rate when the withdrawal rate in the right colon was more than 3 minutes in patients undergoing colonoscopy, according to a recent presentation at the annual meeting of the American College of Gastroenterology.
Although adenomas precede colon cancer in approximately 70% of cases, and detection of adenomas is associated with 5% risk of dying from colorectal cancer, miss rates of adenomas are high in both sides of the colon and ideal withdrawal times are not known, Fahad F. Mir, MD, MSc, from the University of Missouri-Kansas City, said.
“Miss rates are high, especially in the right side of the colon. A colonoscopy offers protection in up to 80% of the left side of the patients but only up to 40%-60% in the right side of patients,” Dr. Mir stated in his presentation. “The quality standard now [for withdrawal time] is 6 minutes, so we hypothesized that adenoma detection rate is not the same if the right colon withdrawal time is equal to or more than 3 minutes, compared to less than 3 minutes.”
The abstract received an ACG Governor’s Award for Excellence in Clinical Research.
Dr. Mir and his colleagues performed a prospective, randomized, case-controlled study of 226 patients undergoing colonoscopy at three endoscopy centers in St. Luke’s Health System, Kansas City, who were aged at least 50 years and had not undergone colonic resections, emergent procedures, or were unable to consent because of mental status or language barrier. Patients were randomized to a control group (113 patients) in whom right colon withdrawal time was under 3 minutes and an intervention group (113 patients) in whom withdrawal time was 3 minutes or more.
There was a 33% adenoma detection rate in the 3 minute or more group, compared with 14% in the less than 3 minutes group (odds ratio, 3.0; 95% confidence interval, 1.62-5.64; P less than .001). Polyp detection rates were 49% in the 3 minutes or more group and 14% in the less than 3 minutes group (OR 5.1; 95% CI, 2.84-9.32; P less than .001). The optimal cut-off point was 3 minutes and 1 second with optimal sensitivity and specificity with an area under the curve of 0.73 (95% CI, 0.65-0.81; P less than .001) for optimal cut-off time for withdrawal from the right colon.
“There was a difference in fellow involvement, where fellows were more likely to be involved when the withdrawal time was more than 3 minutes as opposed to less than 3 minutes; the ADR [adenoma detection rate] was not different based upon fellow involvement,” Dr. Mir said.
The researchers noted similar rates of retroflexion between both groups and said there were no adverse events related to the study in either group. Limitations of the study included its unblinded design, data collection from multiple centers, and a higher rate of previous polyps in patients in the withdrawal in more than 3 minutes group.
Dr. Mir report no relevant conflicts of interest.
SOURCE: Mir FF et al. ACG 2018, Presentation 5.
PHILADELPHIA – There was a significantly higher adenoma detection rate when the withdrawal rate in the right colon was more than 3 minutes in patients undergoing colonoscopy, according to a recent presentation at the annual meeting of the American College of Gastroenterology.
Although adenomas precede colon cancer in approximately 70% of cases, and detection of adenomas is associated with 5% risk of dying from colorectal cancer, miss rates of adenomas are high in both sides of the colon and ideal withdrawal times are not known, Fahad F. Mir, MD, MSc, from the University of Missouri-Kansas City, said.
“Miss rates are high, especially in the right side of the colon. A colonoscopy offers protection in up to 80% of the left side of the patients but only up to 40%-60% in the right side of patients,” Dr. Mir stated in his presentation. “The quality standard now [for withdrawal time] is 6 minutes, so we hypothesized that adenoma detection rate is not the same if the right colon withdrawal time is equal to or more than 3 minutes, compared to less than 3 minutes.”
The abstract received an ACG Governor’s Award for Excellence in Clinical Research.
Dr. Mir and his colleagues performed a prospective, randomized, case-controlled study of 226 patients undergoing colonoscopy at three endoscopy centers in St. Luke’s Health System, Kansas City, who were aged at least 50 years and had not undergone colonic resections, emergent procedures, or were unable to consent because of mental status or language barrier. Patients were randomized to a control group (113 patients) in whom right colon withdrawal time was under 3 minutes and an intervention group (113 patients) in whom withdrawal time was 3 minutes or more.
There was a 33% adenoma detection rate in the 3 minute or more group, compared with 14% in the less than 3 minutes group (odds ratio, 3.0; 95% confidence interval, 1.62-5.64; P less than .001). Polyp detection rates were 49% in the 3 minutes or more group and 14% in the less than 3 minutes group (OR 5.1; 95% CI, 2.84-9.32; P less than .001). The optimal cut-off point was 3 minutes and 1 second with optimal sensitivity and specificity with an area under the curve of 0.73 (95% CI, 0.65-0.81; P less than .001) for optimal cut-off time for withdrawal from the right colon.
“There was a difference in fellow involvement, where fellows were more likely to be involved when the withdrawal time was more than 3 minutes as opposed to less than 3 minutes; the ADR [adenoma detection rate] was not different based upon fellow involvement,” Dr. Mir said.
The researchers noted similar rates of retroflexion between both groups and said there were no adverse events related to the study in either group. Limitations of the study included its unblinded design, data collection from multiple centers, and a higher rate of previous polyps in patients in the withdrawal in more than 3 minutes group.
Dr. Mir report no relevant conflicts of interest.
SOURCE: Mir FF et al. ACG 2018, Presentation 5.
REPORTING FROM ACG 2018
Key clinical point: Spending more than 3 minutes in the right colon during withdrawal was associated with a greater adenoma detection rate during colonoscopy.
Major finding: There was a 33% rate of adenoma detection in patients in whom withdrawal time was greater than 3 minutes compared with a 14% detection rate when withdrawal time was under 3 minutes.
Study details: A prospective, randomized, case-controlled study of 226 patients undergoing colonoscopy.
Disclosures: Dr. Mir reports no relevant conflicts of interest.
Source: Mir FF et al. ACG 2018. Presentation 5.
Antimalarial-induced cardiomyopathy in lupus may be underrecognized
Cardiomyopathy induced by antimalarial treatment for systematic lupus erythematosus may not be as rare as previously thought, according to the authors of a case series published Oct. 15 in The Journal of Rheumatology.
The paper describes eight patients attending a lupus clinic, who were diagnosed with definite or possible antimalarial-induced cardiomyopathy over the course of 2 years.
Konstantinos Tselios, MD, PhD, a clinical research fellow at the University of Toronto Lupus Clinic, and his coauthors wrote that antimalarial-induced cardiomyopathy was thought to be relatively rare, with only 47 previous isolated reports, but they suggested the complication may be significantly underrecognized.
“Hypertrophic cardiomyopathy and heart failure, the most common clinical features of AM-induced cardiomyopathy (AMIC), may be falsely attributed to other causes, such as arterial hypertension or ischemic cardiomyopathy,” the authors wrote. “Consequently, nonspecific therapeutic approaches with diuretics and/or antihypertensives will exert minimum or even deleterious effects on such patients.”
All eight patients in this series were female, with a median age of 62.5 years, median disease duration of 35 years, and median antimalarial use of 22 years. They presented with conditions such as heart failure, exertional dyspnea, and pedal edema. Several patients were asymptomatic but had been found to have elevated heart biomarker levels that prompted further investigation.
All patients showed abnormal cardiac troponin I and brain natriuretic peptide levels, and seven of the eight also had chronically elevated creatine phosphokinase.
In three patients, endomyocardial biopsy showed cardiomyocyte vacuolation, intracytoplasmic myelinoid inclusions, and curvilinear bodies.
Four patients were diagnosed based on cardiac MRI, which showed features suggestive of antimalarial-induced cardiomyopathy, including ventricular hypertrophy with or without atrial enlargement and late gadolinium enhancement in a nonvascular pattern.
All patients had left ventricular hypertrophy, and four also had right ventricular hypertrophy. Only one patient showed impaired systolic function, compared with around half of patients in the literature with antimalarial-induced cardiomyopathy, but seven patients showed a restrictive filling pattern of the left ventricle.
“It seems possible that AMIC is a chronic process and systolic dysfunction will become apparent only in late stages,” the authors suggested.
One patient showed complete atrioventricular block, left ventricular and septal hypertrophy, and concomitant ocular toxicity.
After patients stopped antimalarials, the hypertrophy regressed and heart biomarkers decreased in seven patients, but one patient died from refractory heart failure.
Based on their findings, the authors proposed that heart-specific biomarkers be used as a regular screening tool for detecting myocardial injury, followed by more thorough investigations, such as cardiac MRI, in patients with positive biomarker findings.
“However, drug cessation should be prompt and probably upon suspicion of AMIC, because complete investigation may be delayed significantly.”
One author was supported by the Geoff Carr Fellowship from Lupus Ontario. The University of Toronto Lupus Research Program is supported by the University Health Network, Lou and Marissa Rocca, and the Lupus Foundation of Ontario.
SOURCE: Tselios K et al. J Rheumatol, 2018 Oct 15. doi: 10.3899/jrheum.180124.
Cardiomyopathy induced by antimalarial treatment for systematic lupus erythematosus may not be as rare as previously thought, according to the authors of a case series published Oct. 15 in The Journal of Rheumatology.
The paper describes eight patients attending a lupus clinic, who were diagnosed with definite or possible antimalarial-induced cardiomyopathy over the course of 2 years.
Konstantinos Tselios, MD, PhD, a clinical research fellow at the University of Toronto Lupus Clinic, and his coauthors wrote that antimalarial-induced cardiomyopathy was thought to be relatively rare, with only 47 previous isolated reports, but they suggested the complication may be significantly underrecognized.
“Hypertrophic cardiomyopathy and heart failure, the most common clinical features of AM-induced cardiomyopathy (AMIC), may be falsely attributed to other causes, such as arterial hypertension or ischemic cardiomyopathy,” the authors wrote. “Consequently, nonspecific therapeutic approaches with diuretics and/or antihypertensives will exert minimum or even deleterious effects on such patients.”
All eight patients in this series were female, with a median age of 62.5 years, median disease duration of 35 years, and median antimalarial use of 22 years. They presented with conditions such as heart failure, exertional dyspnea, and pedal edema. Several patients were asymptomatic but had been found to have elevated heart biomarker levels that prompted further investigation.
All patients showed abnormal cardiac troponin I and brain natriuretic peptide levels, and seven of the eight also had chronically elevated creatine phosphokinase.
In three patients, endomyocardial biopsy showed cardiomyocyte vacuolation, intracytoplasmic myelinoid inclusions, and curvilinear bodies.
Four patients were diagnosed based on cardiac MRI, which showed features suggestive of antimalarial-induced cardiomyopathy, including ventricular hypertrophy with or without atrial enlargement and late gadolinium enhancement in a nonvascular pattern.
All patients had left ventricular hypertrophy, and four also had right ventricular hypertrophy. Only one patient showed impaired systolic function, compared with around half of patients in the literature with antimalarial-induced cardiomyopathy, but seven patients showed a restrictive filling pattern of the left ventricle.
“It seems possible that AMIC is a chronic process and systolic dysfunction will become apparent only in late stages,” the authors suggested.
One patient showed complete atrioventricular block, left ventricular and septal hypertrophy, and concomitant ocular toxicity.
After patients stopped antimalarials, the hypertrophy regressed and heart biomarkers decreased in seven patients, but one patient died from refractory heart failure.
Based on their findings, the authors proposed that heart-specific biomarkers be used as a regular screening tool for detecting myocardial injury, followed by more thorough investigations, such as cardiac MRI, in patients with positive biomarker findings.
“However, drug cessation should be prompt and probably upon suspicion of AMIC, because complete investigation may be delayed significantly.”
One author was supported by the Geoff Carr Fellowship from Lupus Ontario. The University of Toronto Lupus Research Program is supported by the University Health Network, Lou and Marissa Rocca, and the Lupus Foundation of Ontario.
SOURCE: Tselios K et al. J Rheumatol, 2018 Oct 15. doi: 10.3899/jrheum.180124.
Cardiomyopathy induced by antimalarial treatment for systematic lupus erythematosus may not be as rare as previously thought, according to the authors of a case series published Oct. 15 in The Journal of Rheumatology.
The paper describes eight patients attending a lupus clinic, who were diagnosed with definite or possible antimalarial-induced cardiomyopathy over the course of 2 years.
Konstantinos Tselios, MD, PhD, a clinical research fellow at the University of Toronto Lupus Clinic, and his coauthors wrote that antimalarial-induced cardiomyopathy was thought to be relatively rare, with only 47 previous isolated reports, but they suggested the complication may be significantly underrecognized.
“Hypertrophic cardiomyopathy and heart failure, the most common clinical features of AM-induced cardiomyopathy (AMIC), may be falsely attributed to other causes, such as arterial hypertension or ischemic cardiomyopathy,” the authors wrote. “Consequently, nonspecific therapeutic approaches with diuretics and/or antihypertensives will exert minimum or even deleterious effects on such patients.”
All eight patients in this series were female, with a median age of 62.5 years, median disease duration of 35 years, and median antimalarial use of 22 years. They presented with conditions such as heart failure, exertional dyspnea, and pedal edema. Several patients were asymptomatic but had been found to have elevated heart biomarker levels that prompted further investigation.
All patients showed abnormal cardiac troponin I and brain natriuretic peptide levels, and seven of the eight also had chronically elevated creatine phosphokinase.
In three patients, endomyocardial biopsy showed cardiomyocyte vacuolation, intracytoplasmic myelinoid inclusions, and curvilinear bodies.
Four patients were diagnosed based on cardiac MRI, which showed features suggestive of antimalarial-induced cardiomyopathy, including ventricular hypertrophy with or without atrial enlargement and late gadolinium enhancement in a nonvascular pattern.
All patients had left ventricular hypertrophy, and four also had right ventricular hypertrophy. Only one patient showed impaired systolic function, compared with around half of patients in the literature with antimalarial-induced cardiomyopathy, but seven patients showed a restrictive filling pattern of the left ventricle.
“It seems possible that AMIC is a chronic process and systolic dysfunction will become apparent only in late stages,” the authors suggested.
One patient showed complete atrioventricular block, left ventricular and septal hypertrophy, and concomitant ocular toxicity.
After patients stopped antimalarials, the hypertrophy regressed and heart biomarkers decreased in seven patients, but one patient died from refractory heart failure.
Based on their findings, the authors proposed that heart-specific biomarkers be used as a regular screening tool for detecting myocardial injury, followed by more thorough investigations, such as cardiac MRI, in patients with positive biomarker findings.
“However, drug cessation should be prompt and probably upon suspicion of AMIC, because complete investigation may be delayed significantly.”
One author was supported by the Geoff Carr Fellowship from Lupus Ontario. The University of Toronto Lupus Research Program is supported by the University Health Network, Lou and Marissa Rocca, and the Lupus Foundation of Ontario.
SOURCE: Tselios K et al. J Rheumatol, 2018 Oct 15. doi: 10.3899/jrheum.180124.
FROM THE JOURNAL OF RHEUMATOLOGY
Key clinical point:
Major finding: Elevated cardiac troponin I and brain natriuretic peptide, and abnormal cardiac MRI may indicate antimalarial-induced cardiomyopathy.
Study details: Case series of eight patients with antimalarial-induced cardiomyopathy.
Disclosures: One author was supported by the Geoff Carr Fellowship from Lupus Ontario. The University of Toronto Lupus Research Program is supported by the University Health Network, Lou and Marissa Rocca, and the Lupus Foundation of Ontario.
Source: Tselios K et al. J Rheumatol. 2018 Oct 15. doi: 10.3899/jrheum.180124.
Clinically meaningful change determined for RAPID-3 in active RA
An improvement of nearly 4 points on a 30-point, patient-scored disease severity index appears to be clinically meaningful for adults with active RA, according to a recent clinical trial analysis.
A 3.8-point decrease represented the minimal clinically important improvement in the Routine Assessment of Patient Index Data 3 (RAPID-3) index, investigators reported in The Journal of Rheumatology.
“Clinicians may feel comfortable documenting and monitoring patient status, recognizing an improvement of 3.8 units in patients with active RA to be meaningful in routine patient care,” Michael M. Ward, MD, PhD, of the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), and his coinvestigators wrote.
The RAPID-3 index consists of three patient self-report measures out of the seven-item RA Core Data Set: physical function, pain, and patient’s global assessment. The index was initially designed to be feasible in routine care, such that a patient could fill it out in the waiting area, Dr. Ward and his colleagues noted in their report.
Previous investigations have shown that RAPID-3 is highly correlated with the 28-joint count Disease Activity Score (DAS28) and the Clinical Disease Activity Index, and in one study, it was more likely than erythrocyte sedimentation rate (ESR) to identify incomplete responses to methotrexate.
However, prior to the present study, there were no reported estimates of the minimal clinically important improvement for the RAPID-3 index.
Knowing whether or not a decrease in RAPID-3 is clinically meaningful to patients would help clinicians interpret those changes in response to treatment, Dr. Ward and his coauthors wrote.
In the current study, RAPID-3 was calculated before and after escalation of treatment in a prospective study involving 250 adults with active RA, including 195 women (78%). The mean age of the patients was 51 years, and the median duration of RA was more than 6 years.
At the patients’ baseline visit and evaluation, rheumatologists prescribed prednisone, a new disease-modifying treatment, a new biologic, or increased the dose of a current treatment. The follow-up visit occurred at 4 months for most patients, though follow-up was at 1 month for prednisone-treated patients because of an expected rapid response.
At baseline, the mean RAPID-3 score was 16.3, which improved to 11.1 at the follow-up visit, for a mean change of –5.2 points, according to the investigators. The standardized response mean was –0.79 (95% confidence interval, –0.71 to –0.88), which investigators said demonstrated good sensitivity to change.
They found that the minimal clinically important improvement was –3.8 in a statistical analysis that optimized sensitivity and specificity, and –3.5 and –4.1 by alternate statistical criteria.
However, Dr. Ward and his coauthors cautioned that these estimates should be applied only to patient groups that have high levels of RA activity, similar to this study, in which patients had a baseline mean DAS28-ESR of 6.16 and a mean Simplified Disease Activity Index of 38.6.
Patients with low RA activity are closer to an acceptable symptom state, making the minimal clinically important improvement less relevant. “The margin for symptom improvement becomes smaller and ultimately indiscernible as the level of activity decreases,” they explained in their report.
The study was supported in part through the NIAMS Intramural Research Program and a grant from the U.S. Public Health Service. No conflicts of interest were reported.
SOURCE: Ward MM et al. J Rheumatol. 2018 Oct 15. doi: 10.3899/jrheum.180153.
An improvement of nearly 4 points on a 30-point, patient-scored disease severity index appears to be clinically meaningful for adults with active RA, according to a recent clinical trial analysis.
A 3.8-point decrease represented the minimal clinically important improvement in the Routine Assessment of Patient Index Data 3 (RAPID-3) index, investigators reported in The Journal of Rheumatology.
“Clinicians may feel comfortable documenting and monitoring patient status, recognizing an improvement of 3.8 units in patients with active RA to be meaningful in routine patient care,” Michael M. Ward, MD, PhD, of the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), and his coinvestigators wrote.
The RAPID-3 index consists of three patient self-report measures out of the seven-item RA Core Data Set: physical function, pain, and patient’s global assessment. The index was initially designed to be feasible in routine care, such that a patient could fill it out in the waiting area, Dr. Ward and his colleagues noted in their report.
Previous investigations have shown that RAPID-3 is highly correlated with the 28-joint count Disease Activity Score (DAS28) and the Clinical Disease Activity Index, and in one study, it was more likely than erythrocyte sedimentation rate (ESR) to identify incomplete responses to methotrexate.
However, prior to the present study, there were no reported estimates of the minimal clinically important improvement for the RAPID-3 index.
Knowing whether or not a decrease in RAPID-3 is clinically meaningful to patients would help clinicians interpret those changes in response to treatment, Dr. Ward and his coauthors wrote.
In the current study, RAPID-3 was calculated before and after escalation of treatment in a prospective study involving 250 adults with active RA, including 195 women (78%). The mean age of the patients was 51 years, and the median duration of RA was more than 6 years.
At the patients’ baseline visit and evaluation, rheumatologists prescribed prednisone, a new disease-modifying treatment, a new biologic, or increased the dose of a current treatment. The follow-up visit occurred at 4 months for most patients, though follow-up was at 1 month for prednisone-treated patients because of an expected rapid response.
At baseline, the mean RAPID-3 score was 16.3, which improved to 11.1 at the follow-up visit, for a mean change of –5.2 points, according to the investigators. The standardized response mean was –0.79 (95% confidence interval, –0.71 to –0.88), which investigators said demonstrated good sensitivity to change.
They found that the minimal clinically important improvement was –3.8 in a statistical analysis that optimized sensitivity and specificity, and –3.5 and –4.1 by alternate statistical criteria.
However, Dr. Ward and his coauthors cautioned that these estimates should be applied only to patient groups that have high levels of RA activity, similar to this study, in which patients had a baseline mean DAS28-ESR of 6.16 and a mean Simplified Disease Activity Index of 38.6.
Patients with low RA activity are closer to an acceptable symptom state, making the minimal clinically important improvement less relevant. “The margin for symptom improvement becomes smaller and ultimately indiscernible as the level of activity decreases,” they explained in their report.
The study was supported in part through the NIAMS Intramural Research Program and a grant from the U.S. Public Health Service. No conflicts of interest were reported.
SOURCE: Ward MM et al. J Rheumatol. 2018 Oct 15. doi: 10.3899/jrheum.180153.
An improvement of nearly 4 points on a 30-point, patient-scored disease severity index appears to be clinically meaningful for adults with active RA, according to a recent clinical trial analysis.
A 3.8-point decrease represented the minimal clinically important improvement in the Routine Assessment of Patient Index Data 3 (RAPID-3) index, investigators reported in The Journal of Rheumatology.
“Clinicians may feel comfortable documenting and monitoring patient status, recognizing an improvement of 3.8 units in patients with active RA to be meaningful in routine patient care,” Michael M. Ward, MD, PhD, of the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), and his coinvestigators wrote.
The RAPID-3 index consists of three patient self-report measures out of the seven-item RA Core Data Set: physical function, pain, and patient’s global assessment. The index was initially designed to be feasible in routine care, such that a patient could fill it out in the waiting area, Dr. Ward and his colleagues noted in their report.
Previous investigations have shown that RAPID-3 is highly correlated with the 28-joint count Disease Activity Score (DAS28) and the Clinical Disease Activity Index, and in one study, it was more likely than erythrocyte sedimentation rate (ESR) to identify incomplete responses to methotrexate.
However, prior to the present study, there were no reported estimates of the minimal clinically important improvement for the RAPID-3 index.
Knowing whether or not a decrease in RAPID-3 is clinically meaningful to patients would help clinicians interpret those changes in response to treatment, Dr. Ward and his coauthors wrote.
In the current study, RAPID-3 was calculated before and after escalation of treatment in a prospective study involving 250 adults with active RA, including 195 women (78%). The mean age of the patients was 51 years, and the median duration of RA was more than 6 years.
At the patients’ baseline visit and evaluation, rheumatologists prescribed prednisone, a new disease-modifying treatment, a new biologic, or increased the dose of a current treatment. The follow-up visit occurred at 4 months for most patients, though follow-up was at 1 month for prednisone-treated patients because of an expected rapid response.
At baseline, the mean RAPID-3 score was 16.3, which improved to 11.1 at the follow-up visit, for a mean change of –5.2 points, according to the investigators. The standardized response mean was –0.79 (95% confidence interval, –0.71 to –0.88), which investigators said demonstrated good sensitivity to change.
They found that the minimal clinically important improvement was –3.8 in a statistical analysis that optimized sensitivity and specificity, and –3.5 and –4.1 by alternate statistical criteria.
However, Dr. Ward and his coauthors cautioned that these estimates should be applied only to patient groups that have high levels of RA activity, similar to this study, in which patients had a baseline mean DAS28-ESR of 6.16 and a mean Simplified Disease Activity Index of 38.6.
Patients with low RA activity are closer to an acceptable symptom state, making the minimal clinically important improvement less relevant. “The margin for symptom improvement becomes smaller and ultimately indiscernible as the level of activity decreases,” they explained in their report.
The study was supported in part through the NIAMS Intramural Research Program and a grant from the U.S. Public Health Service. No conflicts of interest were reported.
SOURCE: Ward MM et al. J Rheumatol. 2018 Oct 15. doi: 10.3899/jrheum.180153.
FROM THE JOURNAL OF RHEUMATOLOGY
Key clinical point:
Major finding: Minimal clinically important improvement was –3.8 in a statistical analysis that optimized sensitivity and specificity, and –3.5 and –4.1 by alternate statistical criteria.
Study details: A prospective study of patient-reported outcome measures in 250 adults with active RA.
Disclosures: The study was supported in part through the National Institute of Arthritis and Musculoskeletal and Skin Diseases Intramural Research Program and a grant from the U.S. Public Health Service. No conflicts of interest were reported.
Source: Ward MM et al. J Rheumatol. 2018 Oct 15. doi: 10.3899/jrheum.180153.
Sensory feedback modalities tackle gait, balance problems in PD
NEW YORK – Sending sensory feedback upstream to patients with Parkinson’s disease (PD) may offer a low-risk, nonpharmaceutical method to retain and improve motor function. These interventions may be especially helpful in the subpopulation of patients who are intolerant to exercise, with a growing body of evidence showing sustained benefit for newer sensory stimulation techniques.
“In a healthy person, movement is the seamless integration of sensory and motor systems,” said Ben Weinstock, DPT, speaking at the International Conference on Parkinson’s Disease and Movement Disorders, pointing out that movement stimulates the senses, and sensory stimulation improves movement.
By contrast, patients with PD experience more than just problems with motor function. Patients with PD and sensory or autonomic dysfunction may find these disturbances contributing to motor dysfunction, said Dr. Weinstock, who treats patients with PD and a variety of complex medical conditions in his private practice.
Some of the hallmark features of PD are movement related: the cogwheel rigidity, bradykinesia, and freezing all contribute to poor balance and a fear of falling. Commonly, PD patients also experience fatigue and alterations in cognition and mood.
However, afferent small-fiber neuropathies and centrally mediated mechanisms in PD can also disturb sensory input: Vestibular function, equilibrium, proprioception, and light and deep touch may all be affected, Dr. Weinstock said.
Autonomic dysfunction can be an underappreciated feature of PD, but such manifestations as orthostatic hypotension and poor thermal regulation can have significant negative impact on quality of life for an individual with PD.
Perhaps the gravest variant of autonomic dysregulation, however, is the cardiac denervation that frequently accompanies PD, said Dr. Weinstock. “Although there is a belief that intensive exercise helps people with PD, many individuals are actually exercise intolerant because of loss of cardiac norepinephrine,” he said (J Neurochem. 2014;131[2]:219-228). “A person with PD who is exercise intolerant is at risk” of syncope, falls, and even serious cardiac events during exercise, he noted.
Cardiovascular dysautonomia in PD has been documented in serial 18F-dopamine PET scans, showing progressive reduction in uptake over the course of several years in individual patients (Neurobiol Dis. 2012 June;46[3]:572-80). Similarly, studies have shown lower cardiac radiotracer uptake in patients with PD, compared with normal controls, he said (NPJ Parkinsons Dis. 2017. doi: 10.1038/S41531-017-0017-1).
It’s not easy to determine what level of nonmotor dysfunction a given patient has at a particular point in disease progression, said Dr. Weinstock.
“There is no correlation between motor and nonmotor deterioration,” he said. “Somebody might be newly diagnosed with just a mild tremor and still have significant cardiac denervation.”
Weighing how to help an exercise-intolerant patient with PD means taking into consideration the known risks and side effect profile of PD medications, Dr. Weinstock pointed out. Increasing medications, or beginning a new drug therapy, can mean increased risk for unwanted psychiatric side effects and ototoxicity, among other potential ill effects.
Similarly, the decision to implant deep brain stimulation is not to be taken lightly, since depression can begin or worsen, and any surgical procedure carries risks.
For Dr. Weinstock, using strategies to improve sensory input are “a valid option for people with PD.” Such a strategy is safe, and even brief bouts of stimulation “can have significant, beneficial effects,” he said. “The overall goal is to avoid sedentary behavior,” with its accompanying ills, he said.
Dr. Weinstock noted that he uses different strategies to stimulate the various senses, including bright light therapy, which can help regulate circadian rhythms and promote appropriate melatonin secretion, improving sleep and upping daytime wakefulness.
Another visual strategy when working on gait is to use surface lines, a checkerboard pattern, or other targets that provide a visual goal for step length, which typically shortens with PD progression. Though more high-tech options exist, Dr. Weinstock suggested patients begin with just laying lines of masking tape along the floor to mark the target gait length. “Usually the cheap technique is a good test to see if it’s going to work,” he said.
An auditory strategy to improve the gait cycle is use of a metronome or other rhythmic auditory stimulation; music can be helpful in this regard and as a general cognitive and emotional stimulus, said Dr. Weinstock.
“Loss of smell is an early sign of Parkinson’s,” said Dr. Weinstock, and taste also can be dulled. Though offering tasty meals could help reduce risk of malnutrition in PD patients, “It remains to be seen if aromatherapy can lead to neural plasticity and reverse smell loss in PD.”
Vestibular rehabilitation techniques can help not just with balance, but also with helping to lift mood and improve functional activities, according to one study (Arq Neuropsiquiatr 2009;67[2-A]:219-23).
Other ways to provide proprioceptive feedback include the use of orthotics and textured insoles and the use of a weighted vest. Dr. Weinstock also gives consideration to skin taping, which may give patients useful feedback about their bodies’ position in space, he said.
Intriguing results have been seen with acupuncture, acupressure, and electroacupuncture for PD patients, said Dr. Weinstock. In particular, a technique called automated mechanical pressure stimulation uses a bootlike device to provide mechanical stimulation to points at the head of the great toe and on the ball of the foot at the head of the first metatarsal bone.
One functional magnetic resonance imaging (fMRI) study showed acutely increased resting state functional connectivity after such stimulation, in comparison with a sham procedure that also applied pressure, but over a broader area, he said.
After the stimulation procedure used in the study, the patients who received actual stimulation also saw improved ability to initiate voluntary movements, less tremor and rigidity, and less gait freezing (PLoS One. 2015 Oct 15. doi: 10.1371/journal.pone.0137977).
Other studies of the mechanical stimulation device showed similar results, with some showing that repeated sessions helped maintain these and other benefits, such as improved walking velocity, stride length, and Timed Up and Go results – an assessment of fall risk (Int J Rehabil Res. 2015 Sep;38[3]:238-45). Treatment with the device, dubbed Gondola, is most widely available in Italy, where clinical trials are ongoing.
Stimulation to an acupuncture point located on the proximal lateral leg, near the head of the fibula, showed improvements in gait parameters and in fMRI-assessed brain connectivity as well, noted Dr. Weinstock (CNS Neurosci Ther. 2012 Sep;18[9]:781-90).
“There’s a growing amount of evidence that various types of sensory stimulation can have significant benefits for people with Parkinson’s Disease, especially for those who are exercise intolerant,” said Dr. Weinstock.
Dr. Weinstock reported no relevant disclosures.
[email protected]
NEW YORK – Sending sensory feedback upstream to patients with Parkinson’s disease (PD) may offer a low-risk, nonpharmaceutical method to retain and improve motor function. These interventions may be especially helpful in the subpopulation of patients who are intolerant to exercise, with a growing body of evidence showing sustained benefit for newer sensory stimulation techniques.
“In a healthy person, movement is the seamless integration of sensory and motor systems,” said Ben Weinstock, DPT, speaking at the International Conference on Parkinson’s Disease and Movement Disorders, pointing out that movement stimulates the senses, and sensory stimulation improves movement.
By contrast, patients with PD experience more than just problems with motor function. Patients with PD and sensory or autonomic dysfunction may find these disturbances contributing to motor dysfunction, said Dr. Weinstock, who treats patients with PD and a variety of complex medical conditions in his private practice.
Some of the hallmark features of PD are movement related: the cogwheel rigidity, bradykinesia, and freezing all contribute to poor balance and a fear of falling. Commonly, PD patients also experience fatigue and alterations in cognition and mood.
However, afferent small-fiber neuropathies and centrally mediated mechanisms in PD can also disturb sensory input: Vestibular function, equilibrium, proprioception, and light and deep touch may all be affected, Dr. Weinstock said.
Autonomic dysfunction can be an underappreciated feature of PD, but such manifestations as orthostatic hypotension and poor thermal regulation can have significant negative impact on quality of life for an individual with PD.
Perhaps the gravest variant of autonomic dysregulation, however, is the cardiac denervation that frequently accompanies PD, said Dr. Weinstock. “Although there is a belief that intensive exercise helps people with PD, many individuals are actually exercise intolerant because of loss of cardiac norepinephrine,” he said (J Neurochem. 2014;131[2]:219-228). “A person with PD who is exercise intolerant is at risk” of syncope, falls, and even serious cardiac events during exercise, he noted.
Cardiovascular dysautonomia in PD has been documented in serial 18F-dopamine PET scans, showing progressive reduction in uptake over the course of several years in individual patients (Neurobiol Dis. 2012 June;46[3]:572-80). Similarly, studies have shown lower cardiac radiotracer uptake in patients with PD, compared with normal controls, he said (NPJ Parkinsons Dis. 2017. doi: 10.1038/S41531-017-0017-1).
It’s not easy to determine what level of nonmotor dysfunction a given patient has at a particular point in disease progression, said Dr. Weinstock.
“There is no correlation between motor and nonmotor deterioration,” he said. “Somebody might be newly diagnosed with just a mild tremor and still have significant cardiac denervation.”
Weighing how to help an exercise-intolerant patient with PD means taking into consideration the known risks and side effect profile of PD medications, Dr. Weinstock pointed out. Increasing medications, or beginning a new drug therapy, can mean increased risk for unwanted psychiatric side effects and ototoxicity, among other potential ill effects.
Similarly, the decision to implant deep brain stimulation is not to be taken lightly, since depression can begin or worsen, and any surgical procedure carries risks.
For Dr. Weinstock, using strategies to improve sensory input are “a valid option for people with PD.” Such a strategy is safe, and even brief bouts of stimulation “can have significant, beneficial effects,” he said. “The overall goal is to avoid sedentary behavior,” with its accompanying ills, he said.
Dr. Weinstock noted that he uses different strategies to stimulate the various senses, including bright light therapy, which can help regulate circadian rhythms and promote appropriate melatonin secretion, improving sleep and upping daytime wakefulness.
Another visual strategy when working on gait is to use surface lines, a checkerboard pattern, or other targets that provide a visual goal for step length, which typically shortens with PD progression. Though more high-tech options exist, Dr. Weinstock suggested patients begin with just laying lines of masking tape along the floor to mark the target gait length. “Usually the cheap technique is a good test to see if it’s going to work,” he said.
An auditory strategy to improve the gait cycle is use of a metronome or other rhythmic auditory stimulation; music can be helpful in this regard and as a general cognitive and emotional stimulus, said Dr. Weinstock.
“Loss of smell is an early sign of Parkinson’s,” said Dr. Weinstock, and taste also can be dulled. Though offering tasty meals could help reduce risk of malnutrition in PD patients, “It remains to be seen if aromatherapy can lead to neural plasticity and reverse smell loss in PD.”
Vestibular rehabilitation techniques can help not just with balance, but also with helping to lift mood and improve functional activities, according to one study (Arq Neuropsiquiatr 2009;67[2-A]:219-23).
Other ways to provide proprioceptive feedback include the use of orthotics and textured insoles and the use of a weighted vest. Dr. Weinstock also gives consideration to skin taping, which may give patients useful feedback about their bodies’ position in space, he said.
Intriguing results have been seen with acupuncture, acupressure, and electroacupuncture for PD patients, said Dr. Weinstock. In particular, a technique called automated mechanical pressure stimulation uses a bootlike device to provide mechanical stimulation to points at the head of the great toe and on the ball of the foot at the head of the first metatarsal bone.
One functional magnetic resonance imaging (fMRI) study showed acutely increased resting state functional connectivity after such stimulation, in comparison with a sham procedure that also applied pressure, but over a broader area, he said.
After the stimulation procedure used in the study, the patients who received actual stimulation also saw improved ability to initiate voluntary movements, less tremor and rigidity, and less gait freezing (PLoS One. 2015 Oct 15. doi: 10.1371/journal.pone.0137977).
Other studies of the mechanical stimulation device showed similar results, with some showing that repeated sessions helped maintain these and other benefits, such as improved walking velocity, stride length, and Timed Up and Go results – an assessment of fall risk (Int J Rehabil Res. 2015 Sep;38[3]:238-45). Treatment with the device, dubbed Gondola, is most widely available in Italy, where clinical trials are ongoing.
Stimulation to an acupuncture point located on the proximal lateral leg, near the head of the fibula, showed improvements in gait parameters and in fMRI-assessed brain connectivity as well, noted Dr. Weinstock (CNS Neurosci Ther. 2012 Sep;18[9]:781-90).
“There’s a growing amount of evidence that various types of sensory stimulation can have significant benefits for people with Parkinson’s Disease, especially for those who are exercise intolerant,” said Dr. Weinstock.
Dr. Weinstock reported no relevant disclosures.
[email protected]
NEW YORK – Sending sensory feedback upstream to patients with Parkinson’s disease (PD) may offer a low-risk, nonpharmaceutical method to retain and improve motor function. These interventions may be especially helpful in the subpopulation of patients who are intolerant to exercise, with a growing body of evidence showing sustained benefit for newer sensory stimulation techniques.
“In a healthy person, movement is the seamless integration of sensory and motor systems,” said Ben Weinstock, DPT, speaking at the International Conference on Parkinson’s Disease and Movement Disorders, pointing out that movement stimulates the senses, and sensory stimulation improves movement.
By contrast, patients with PD experience more than just problems with motor function. Patients with PD and sensory or autonomic dysfunction may find these disturbances contributing to motor dysfunction, said Dr. Weinstock, who treats patients with PD and a variety of complex medical conditions in his private practice.
Some of the hallmark features of PD are movement related: the cogwheel rigidity, bradykinesia, and freezing all contribute to poor balance and a fear of falling. Commonly, PD patients also experience fatigue and alterations in cognition and mood.
However, afferent small-fiber neuropathies and centrally mediated mechanisms in PD can also disturb sensory input: Vestibular function, equilibrium, proprioception, and light and deep touch may all be affected, Dr. Weinstock said.
Autonomic dysfunction can be an underappreciated feature of PD, but such manifestations as orthostatic hypotension and poor thermal regulation can have significant negative impact on quality of life for an individual with PD.
Perhaps the gravest variant of autonomic dysregulation, however, is the cardiac denervation that frequently accompanies PD, said Dr. Weinstock. “Although there is a belief that intensive exercise helps people with PD, many individuals are actually exercise intolerant because of loss of cardiac norepinephrine,” he said (J Neurochem. 2014;131[2]:219-228). “A person with PD who is exercise intolerant is at risk” of syncope, falls, and even serious cardiac events during exercise, he noted.
Cardiovascular dysautonomia in PD has been documented in serial 18F-dopamine PET scans, showing progressive reduction in uptake over the course of several years in individual patients (Neurobiol Dis. 2012 June;46[3]:572-80). Similarly, studies have shown lower cardiac radiotracer uptake in patients with PD, compared with normal controls, he said (NPJ Parkinsons Dis. 2017. doi: 10.1038/S41531-017-0017-1).
It’s not easy to determine what level of nonmotor dysfunction a given patient has at a particular point in disease progression, said Dr. Weinstock.
“There is no correlation between motor and nonmotor deterioration,” he said. “Somebody might be newly diagnosed with just a mild tremor and still have significant cardiac denervation.”
Weighing how to help an exercise-intolerant patient with PD means taking into consideration the known risks and side effect profile of PD medications, Dr. Weinstock pointed out. Increasing medications, or beginning a new drug therapy, can mean increased risk for unwanted psychiatric side effects and ototoxicity, among other potential ill effects.
Similarly, the decision to implant deep brain stimulation is not to be taken lightly, since depression can begin or worsen, and any surgical procedure carries risks.
For Dr. Weinstock, using strategies to improve sensory input are “a valid option for people with PD.” Such a strategy is safe, and even brief bouts of stimulation “can have significant, beneficial effects,” he said. “The overall goal is to avoid sedentary behavior,” with its accompanying ills, he said.
Dr. Weinstock noted that he uses different strategies to stimulate the various senses, including bright light therapy, which can help regulate circadian rhythms and promote appropriate melatonin secretion, improving sleep and upping daytime wakefulness.
Another visual strategy when working on gait is to use surface lines, a checkerboard pattern, or other targets that provide a visual goal for step length, which typically shortens with PD progression. Though more high-tech options exist, Dr. Weinstock suggested patients begin with just laying lines of masking tape along the floor to mark the target gait length. “Usually the cheap technique is a good test to see if it’s going to work,” he said.
An auditory strategy to improve the gait cycle is use of a metronome or other rhythmic auditory stimulation; music can be helpful in this regard and as a general cognitive and emotional stimulus, said Dr. Weinstock.
“Loss of smell is an early sign of Parkinson’s,” said Dr. Weinstock, and taste also can be dulled. Though offering tasty meals could help reduce risk of malnutrition in PD patients, “It remains to be seen if aromatherapy can lead to neural plasticity and reverse smell loss in PD.”
Vestibular rehabilitation techniques can help not just with balance, but also with helping to lift mood and improve functional activities, according to one study (Arq Neuropsiquiatr 2009;67[2-A]:219-23).
Other ways to provide proprioceptive feedback include the use of orthotics and textured insoles and the use of a weighted vest. Dr. Weinstock also gives consideration to skin taping, which may give patients useful feedback about their bodies’ position in space, he said.
Intriguing results have been seen with acupuncture, acupressure, and electroacupuncture for PD patients, said Dr. Weinstock. In particular, a technique called automated mechanical pressure stimulation uses a bootlike device to provide mechanical stimulation to points at the head of the great toe and on the ball of the foot at the head of the first metatarsal bone.
One functional magnetic resonance imaging (fMRI) study showed acutely increased resting state functional connectivity after such stimulation, in comparison with a sham procedure that also applied pressure, but over a broader area, he said.
After the stimulation procedure used in the study, the patients who received actual stimulation also saw improved ability to initiate voluntary movements, less tremor and rigidity, and less gait freezing (PLoS One. 2015 Oct 15. doi: 10.1371/journal.pone.0137977).
Other studies of the mechanical stimulation device showed similar results, with some showing that repeated sessions helped maintain these and other benefits, such as improved walking velocity, stride length, and Timed Up and Go results – an assessment of fall risk (Int J Rehabil Res. 2015 Sep;38[3]:238-45). Treatment with the device, dubbed Gondola, is most widely available in Italy, where clinical trials are ongoing.
Stimulation to an acupuncture point located on the proximal lateral leg, near the head of the fibula, showed improvements in gait parameters and in fMRI-assessed brain connectivity as well, noted Dr. Weinstock (CNS Neurosci Ther. 2012 Sep;18[9]:781-90).
“There’s a growing amount of evidence that various types of sensory stimulation can have significant benefits for people with Parkinson’s Disease, especially for those who are exercise intolerant,” said Dr. Weinstock.
Dr. Weinstock reported no relevant disclosures.
[email protected]
EXPERT ANALYSIS FROM ICPDMD 2018
Foot and Ankle Injuries in Soccer
ABSTRACT
The ankle is one of the most commonly injured joints in soccer and represents a significant cost to the healthcare system. The ligaments that stabilize the ankle joint determine its biomechanics—alterations of which result from various soccer-related injuries. Acute sprains are among the most common injury in soccer players and are generally treated conservatively, with emphasis placed on secondary prevention to reduce the risk for future sprains and progression to chronic ankle instability. Repetitive ankle injuries in soccer players may cause chronic ankle instability, which includes both mechanical ligamentous laxity and functional changes. Chronic ankle pathology often requires surgery to repair ligamentous damage and remove soft-tissue or osseous impingement. Proper initial treatment, rehabilitation, and secondary prevention of ankle injuries can limit the amount of time lost from play and avoid negative long-term sequelae (eg, osteochondral lesions, arthritis). On the other hand, high ankle sprains portend a poorer prognosis and a longer recovery. These injuries will typically require surgical stabilization. Impingement-like syndromes of the ankle can undergo an initial trial of conservative treatment; when this fails, however, soccer players respond favorably to arthroscopic debridement of the lesions causing impingement. Finally, other pathologies (eg, stress fractures) are highly encouraged to be treated with surgical stabilization in elite soccer players.
Continue to: EPIDEMIOLOGY
EPIDEMIOLOGY
With roughly 200,000 professional and around 240 million amateur soccer players, soccer has been recognized as the most popular sport worldwide. Nevertheless, given its rising popularity in society, one must also consider the increasing incidence of injuries as a result. Elite soccer players sustain between 10 and 35 injuries per 1000 competitive playing hours.1 Approximately 80% are traumatic, and 20% are overuse injuries.2 Soccer injuries are more frequent with increasing age of the participants, whereas the incidence of injuries in preadolescent players is low. The incidence of injuries has been found to be higher during competition when compared with practice/training sessions, with some studies showing that 59% of injuries occurred during games.2 Amateur or recreational soccer players sustain fewer injuries than professional soccer players, as one would expect, given both the higher intensity of training and match schedule in professionals.
The ankle is one of the most commonly injured joints in soccer, with some studies suggesting it comprises one-fifth of all injuries sustained during soccer, which is only second to those of the knee.2 Ankle sprains specifically are quite a common occurrence in soccer.3-9 A recent study of an English premier league club showed that over a 4-season period, 20% of injuries were of the foot and ankle with a mean return to sport time of 54 days.10 Of all foot and ankle related injuries, ankle sprains are the most common, followed by bruises/contusions, and tendon lesions. Fractures are very rare (1%) in soccer, but when they do occur they impart a much more extended recovery. During the 2010 Fédération Internationale de Football Association (FIFA) World Cup, ankle sprains were among the most common injuries and approximately half lead to players missing training or competitive matches.5
ANATOMY
Knowledge of the biomechanics of both the foot and ankle joints is essential to understand soccer injuries. The ankle joint (talocrural articulation) consists of the distal ends of the tibia and fibula, which form the mortise, and the superior aspect of the talar dome.11 As a hinge joint, the ankle provides 20° of dorsiflexion and 50° of plantar flexion,12 with stability provided by the lateral, medial, and superior ligamentous complexes. The superior articular surface of the talus is narrower posteriorly, which creates a looser fit within the mortise during plantar flexion.11 This decreased stability could help explain why the most common injury in soccer involves a plantar flexion mechanism.13,14 Inferiorly, the talus articulates with the calcaneus to form the subtalar joint. It is at this site that the majority of both foot inversion and eversion occurs. The transverse tarsal joints (Chopart’s joints) separate the hindfoot from the midfoot. Movement of this joint depends on the relative alignment of its 2 articulations: the talonavicular and calcaneocuboid joints. During foot eversion, these 2 joints are parallel to each other allowing supple motion and aiding in shock absorption during the heel strike phase of the gait cycle. With foot inversion, the joints become nonparallel and thus lock the transverse tarsal joints providing a rigid lever needed for push-off.11,12
LATERAL LIGAMENTS
The ankle joint is stabilized laterally by a ligament complex consisting of 3 individual ligaments, all originating from the lateral malleolus: the anterior talofibular ligament (ATFL), the posterior talofibular ligament (PTFL), and the calcaneofibular ligament (CFL) (Figure 1).11,12,15 The ATFL is the primary restraint to inversion in plantar flexion, and it helps resist anterolateral translation of the talus in the mortise. However, it is the weakest and therefore the most frequently injured of the lateral ligaments. The PTFL plays only a supplementary role in ankle stability when the lateral ligament complex is intact. It is under the greatest strain in ankle dorsiflexion and acts to limit posterior talar displacement within the mortise as well as talar external rotation.13,16 The CFL is the primary restraint to inversion in the neutral or dorsiflexed position. It restrains subtalar inversion, thereby limiting talar tilt within the mortise.

DELTOID LIGAMENT
The deltoid ligament complex consists of 6 continuous adjacent superficial and deep ligaments that function synergistically to resist valgus and pronation forces, as well as external rotation of the talus in the mortise.11-13,17 The superficial layer crosses both ankle and subtalar joints. It originates from the anterior colliculus and fans out to insert into the navicular, neck of the talus, sustentaculum tali, and posteromedial talar tubercle. The tibiocalcaneal (sustentaculum tali) portion is the strongest component in the superficial layer and resists calcaneal eversion. The deep layer crosses the ankle joint only. It functions as the primary stabilizer of the medial ankle and prevents both lateral displacement and external rotation of the talus. It originates from the inferior and posterior aspects of the medial malleolus and inserts on the medial and posteromedial aspects of the talus.12,17,18
Continue to: SYNDESMOSIS
SYNDESMOSIS
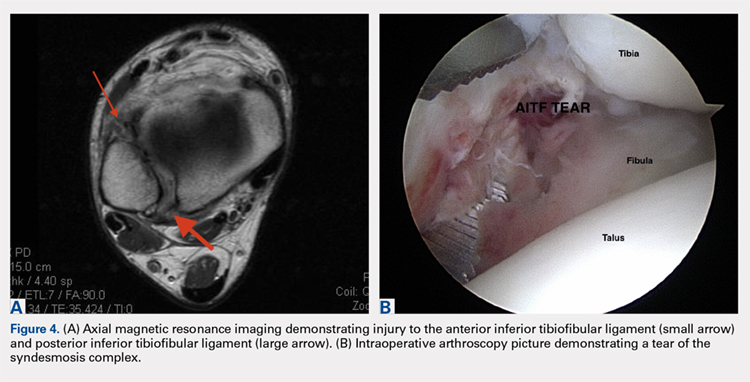
The ankle syndesmosis, or inferior tibiofibular joint, is the distal articulation between the tibia and fibula. The syndesmosis contributes to ankle mortise integrity through its firm fixation of the lateral malleolus against the lateral surface of the talus. Ligaments comprising the ankle syndesmosis include the anterior inferior tibiofibular ligament (AITFL), the posterior inferior tibiofibular ligament (PITFL), the inferior transverse ligament, and the interosseous ligament (IOL).12
ANKLE SPRAINS
Ankle sprains are the most common pathology encountered amongst soccer players, representing from one-half to two-thirds of all ankle related injuries. Most sprains occur outside of player contact.
LATERAL ANKLE SPRAINS AND INSTABILITY
Injury to the lateral ligaments of the ankle represents 77% to 91% of all ankle sprains in soccer.6,19 The greatest risk factor for an ankle sprain in a soccer player is a history of prior sprain.20 Other risk factors include increasing age, player-to-player contact, condition of the pitch, weight-bearing status of the injured limb at the time of injury, and joint instability or laxity.21,22
The evaluation of an ankle sprain to determine its severity is best done after the acute phase, approximately 4 to 7 days after the initial injury when both pain and swelling have subsided.23 The anterior drawer (ATFL instability) and talar tilt (CFL instability) tests are useful in evaluating ankle instability in the delayed or chronic setting; however, both have been shown to have limited sensitivity and significant variability amongst different examiners.24
Clinical examination will direct further diagnostic tests including X-rays, magnetic resonance imaging (MRI), and computed tomography (CT). The Ottawa ankle rules are generally helpful in determining whether plain X-rays are indicated in the acute setting.25,26 (Figure 2) According to these rules, ankle radiographs should be obtained if ankle pain is reported near the malleoli and 1 or more of the following is seen during examination: inability to bear weight immediately after injury and for 4 steps in the emergency department, and bony tenderness at the posterior edge or tip of the malleolus. Stress X-rays are generally not indicated in acute injuries but may be useful in chronic ankle instability cases.23

Continue to: Ankle sprains cover...
Ankle sprains cover a broad spectrum of injuries; therefore, a grading system was devised to aid in guiding treatment. Grade I (mild) sprains are those with minimal swelling and tenderness but have the ligaments still intact. Grade II (moderate) sprains occur when there are partial ligament tears associated with moderate pain, swelling, and tenderness. Finally, Grade III (severe) sprains are complete ligament tears with marked swelling, hemorrhage, tenderness, loss of function, and abnormal joint motion and instability.23, 24
Initial treatment for all ankle sprains is nonoperative and involves the RICE (rest, ice, compression, elevation) protocol with the use of nonsteroidal anti-inflammatory drugs (NSAIDs) during the acute phase (first 4-5 days) with a short period (no >2 weeks) of immobilization.27 Most authors agree that early mobilization followed by phased rehabilitation is warranted to minimize time away from sports.28-31 Prolonged immobilization (>2 weeks) has detrimental effects and may lead to a longer return to play.28-31 The rehabilitation protocol is divided into stages: (1) pain and edema control, (2) range of motion (ROM) and strengthening exercises, (3) soccer specific functional training, and (4) prophylactic intervention with balance and proprioception exercises. Surgical intervention is rarely indicated for acute ankle sprains. There are exceptions, however, such as when ankle sprains are associated with other injuries that require acute intervention (eg, fracture, osteochondral lesion). Surgery is indicated in the setting of chronic, recurrent mechanical instability. Anatomical repairs (modified Brostrom) seem to produce better outcomes than non-anatomical reconstructions (eg, Chrisman-Snook). Surgical outcomes are good, and most athletes are able to return to their pre-injury level of function.32
In athletes, prevention of recurrent sprains is key. Braces may help prevent ankle sprains and bracing has been shown to be superior to taping, as tape loses its restrictive properties within 20 to 30 minutes of initiating activity.33,34 Application of an orthosis (lace-up ankle orthosis) has been shown to reduce the incidence of ankle re-injury in soccer players with previous ankle sprains. Several studies have found minimal, if any, effect of orthoses on athletic performance.20,35,36 Low-profile braces for soccer have been developed which allow for minimal disruption of the player’s boot and space proximally to insert the shin guard. Another essential component of prevention is prophylactic intervention with balance and proprioceptive exercises. A study looking at first division men’s league football (soccer) players in Iran showed a significant decrease in re-injury rates with proprioceptive training.37 In 2003, FIFA introduced a comprehensive warm-up program (FIFA 11+), which has since been shown in several studies to decrease the risk of injury in amateur soccer players.38-40
MEDIAL ANKLE SPRAINS AND INSTABILITY
Soccer places an unusually high demand on both the medial foot and ankle structures when compared with other sports. For instance, striking the ball requires the player to abduct and externally rotate the foot, which preloads medial structures.9 Hintermann18 looked at 54 cases of medial ankle instability and found that injury commonly occurred during landing on an uneven surface, which applies to soccer players when landing after heading the ball or jumping over a tackle. Pronation with eversion and extreme rotational injuries are well known to cause deltoid ligament injury. However, complete rupture of the deltoid ligament is rare and is more often associated with ankle fractures.41 Due to its close proximity and similarly shared function in medial plantar arch stabilization with the tibiospring and spring ligaments, posterior tibialis tendon dysfunction is also frequently seen in medial ankle instability.17 After an acute injury, patients can present with a medial ankle hematoma and pain along the deltoid ligament. Although chronic insufficiency is diagnosed based on the feeling of “giving way,” pain in the medial gutter of the ankle and a valgus and pronation deformity of the foot can be corrected by activating the peroneus tertius muscle. Arthroscopy is the most specific way to confirm clinically suspected instability of the medial ankle; however, MRI can demonstrate loss of organized medial fibers (Figures 3A, 3B).18 Primary surgical repair of deltoid ligament tears yield good to excellent results and should be considered in the soccer player to prevent problems associated with chronic non-repaired tears such as instability, osteoarthritis, and impingement syndromes.18 After surgical repair, players will undergo extensive physical therapy that progresses to sport-specific exercises with the ultimate goal of returning to competitive play around 4-6 months post-operatively.

HIGH ANKLE SPRAINS (SYNDESMOSIS)
High ankle sprains are much less common than low ankle sprains; however, when they do occur they portend a lengthier rehabilitation and a poorer prognosis, especially if undiagnosed. Lubberts and colleagues42 studied the epidemiology of isolated syndesmotic injuries in professional football players. They pooled data from 15 consecutive seasons of European professional football between 2001 and 2016. They examined a total of 3677 players from 61 teams across 17 countries. There were 1320 ankle ligament injuries registered during 15 seasons, of which 94 (7%) were isolated syndesmotic injuries. The incidence of these injuries increased annually between 2001 and 2016. Injuries were 74% contact-related, and isolated syndesmotic injuries were followed by a mean of a 39-day absence.42 Moreover, football players may have an increased risk of syndesmotic sprains due to foot planting and cutting action.41
Continue to: These injuriesa are typically...
These injuries are typically identified with pain over the AITFL and interosseous membrane. Physical examination tests that help identify syndesmotic injuries include the squeeze test, external rotation test, and crossed-leg test.41 The diagnosis can be made on plain X-ray when there is clear diastasis between the distal tibia and fibula. Two critical measurements on plain films are made 1 cm above the tibial plafond and are used to evaluate the integrity of the syndesmosis: tibiofibular clear space >6 mm, and tibiofibular overlap <1 mm, which indicate disruption of the syndesmosis.43 More subtle injuries can be diagnosed with better sensitivity and specificity using MRI, which can also reveal secondary findings such as bone bruises, ATFL injury, osteochondral lesions, and tibiofibular incongruity.44,45 Arthroscopy is an invaluable diagnostic tool for syndesmotic injuries with a characteristic triad finding of PITFL scarring, disrupted interosseous ligament, and posterolateral tibial plafond chondral damage.46
Classification of the ligaments involved can aid in the selection of appropriate treatment. Grade I injuries involve AITFL tears. Grade IIa injuries involve AITFL and IOL tears. Grade IIb injuries include AITFL, PITFL, and IOL tears. Grade III injuries involve injury to all 3 ligaments, as well as a fibular fracture. Conservative treatment is recommended for Grades I and IIa, while surgical intervention is necessary for Grades IIb and III (Figures 4A, 4B). Compared with other ankle sprains, syndesmotic injuries typically require a more prolonged recovery/rehabilitation. Some studies suggest that these injuries require twice as long to heal.47 Hopkinson and colleagues48 reported a mean recovery time of 55 days following syndesmotic injuries in cadets at the United States Military Academy at West Point. Some surgeons advocate surgical intervention in professional athletes with mild sprains to expedite return to play.49
Surgery has been well established as necessary in more severe injuries where there is clear diastasis or instability of the syndesmosis. Traditionally, screws were used for surgical fixation; however, they often required a second surgery for subsequent removal. There is no general consensus on the optimal screw size, level of placement, or timing of removal.50,51 More recently, non-absorbable suture button fixation (eg, TightRope; Arthrex) has become more popular and provides certain advantages over screw fixation, such as avoiding the need for hardware removal. TightRope has been shown to provide more accurate stabilization of the syndesmosis as compared with screw fixation.52 Since malreduction is the most important indicator of poor long-term functional outcome, suture button fixation should be considered in the treatment of the football player.53 Finally, Colcuc and colleagues54 reported a lower complication rate and earlier return to sports in patients treated with knotless suture button devices compared with screw fixation.
OSTEOCHONDRAL LESIONS
Osteochondral lesions (OCLs) are cartilage-bone defects that are usually located in the talus. They can be caused by an acute traumatic event or repetitive microtrauma with no apparent history of trauma (eg, ankle instability). Acute OCLs can occur in soccer secondary to an ankle sprain or ankle fracture. Symptoms of OCLs include pain, swelling, and mechanical symptoms such as catching or locking, and on physical examination, one might see an effusion. The initial imaging modality of choice is radiographing; however, in ankle sprains with continued pain and swelling MRI may be indicated to rule out an underlying OCL. Missed acute lesions have a tendency not to heal and become chronic lesions, which can cause pain and playing disability. It is well established that chronic ankle instability is an important etiologic factor for OCLs. With the normal hydrostatic pressure within the ankle joint, synovial fluid gets pushed into cartilage/bone fissures, which can then lead to cystic degeneration of the subchondral bone.55-57
Surgical repair of an OCL is dependent on both the size and location of the lesion. Acute lesions can be managed by arthroscopic débridement, microfracture, or fixation of the lesion if enough bone remains attached to the chondral lesion. Return to play is based on development and maturation of fibrocartilage over the lesion (debridement/microfracture) or healing and incorporation of the new graft (chondral repair procedures). Meanwhile, chronic lesions can be managed in 1-stage (microfracture, osteochondral autograft transfer or 2-stage (autologous chondrocyte implantation [ACI]) procedures.56-57 Additional biologic healing augmentation with platelet-rich plasma has been described as well.58 Newer techniques in treating chronic talus OCLs, including ones that have failed to respond to bone marrow stimulation techniques, have been developed more recently such as the use of particulated juvenile articular cartilage allograft (DeNovo NT Natural Tissue Graft®; Zimmer Biomet).59 These newer techniques avoid the need for a 2-stage procedure, as is the case with ACI.
Continue to: Further studies are needed...
Further studies are needed to both investigate long-term outcomes and determine the superiority of the arthroscopic juvenile cartilage procedure compared with microfracture and other cartilage resurfacing procedures. When surgically treating OCLs, one must also restore normal ankle joint biomechanics for the lesion to heal. For instance, in the presence of ankle instability, ligament reconstruction must be performed. Also, one should also consider addressing any hindfoot malalignment with an osteotomy (calcaneus, supramalleolar). In a recent retrospective study, van Eekeren and colleagues60 showed that approximately 76% of patients were able to return to sports at long-term follow-up after arthroscopic débridement and bone marrow stimulation of talar OCLs. However, the activity level decreased at long-term follow-up and never attained the pre-injury level.60
ANKLE IMPINGEMENT
ANTERIOR ANKLE IMPINGEMENT (FOOTBALLER'S ANKLE)
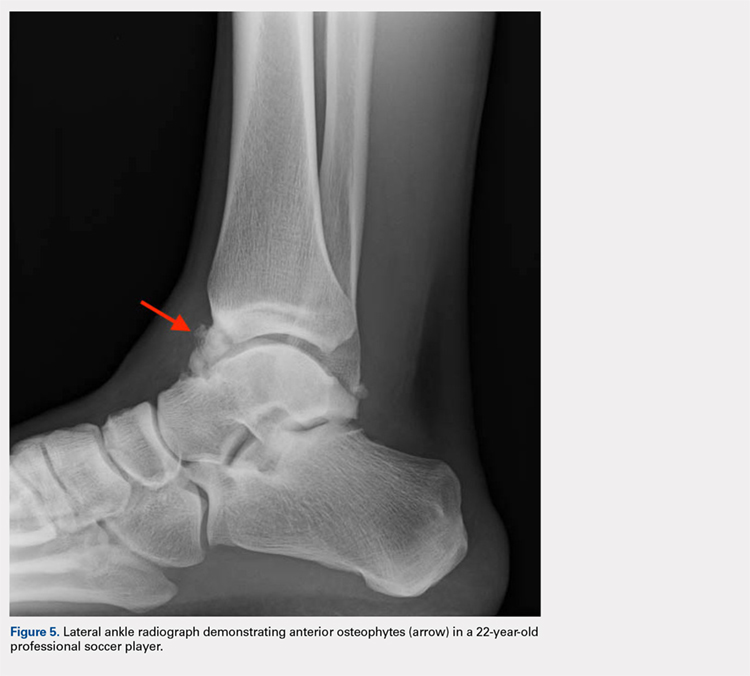
Anterior ankle impingement is caused by anterior osteophytes on both the distal tibia and talar neck. It is thought to be related to repetitive microtrauma to the anteromedial aspect of the ankle from recurrent ball impact.61 It is very common amongst soccer players with some studies suggesting that 60% of soccer players have this syndrome. Ankle impingement is characterized by anterior pain with ankle dorsiflexion, decreased dorsiflexion, and swelling. It is primarily diagnosed with lateral ankle X-rays, which will show the osteophytes. An oblique anteromedial X-ray may increase detection of osteophytes (Figure 5). The early stages of anterior impingement can be treated successfully with injections and heel lifts. Treatment of lesions that fail to respond to conservative management involves arthroscopic or open excision of osteophytes. Most patients with no preexisting osteoarthritis treated arthroscopically will experience pain relief and return to full activity, though recurrent osteophyte formation has been noted at long-term follow-up.62
Anterior ankle impingement is most often caused by acute ankle sprains with an inversion type of mechanism.62 The subsequent reactive inflammation can cause fibrosis leading to distal fascicle enlargement of the AITFL. Impingement in the anterolateral gutter of this enlarged fascicle can also cause both chronic reactive synovitis and chondromalacia of the lateral talar dome.63 MRI can identify abnormal areas of pathology; however, 50% of cases are diagnosed based on clinical examination alone.63 Patients generally present with a history of anterolateral ankle pain and swelling with an occasional popping or snapping sensation.
Soccer players commonly develop anterior bony impingement due to repetitive loading of the anterior ankle from striking the ball. This repetition can lead to osteophyte formation of the anterior distal tibia and talar neck. After the osteophytes form, decreased dorsiflexion can occur due to a mechanical stop and inflammation of the interposed capsule.
The patient will exhibit tenderness to palpation along the anterolateral aspect of the ankle, with pain elicited at extreme passive dorsiflexion.62 Initially, an injection with local anesthetic and corticosteroid can serve both a diagnostic and therapeutic purpose; however, patients who fail conservative treatment can be treated with arthroscopy and resection of the involved scar tissue and osteophytes. The best results are seen in those patients with no concurrent intra-articular lesions or ankle osteoarthritis (Figure 5).62 When treated non-operatively, a player may return to play when pain resolves; however, if treated surgically with arthroscopic debridement/resection, a player must wait until his surgical scars have healed prior to attempting return to play.
Continue to: ANTEROMEDIAL ANKLE IMPINGEMENT
ANTEROMEDIAL ANKLE IMPINGEMENT
Anteromedial ankle impingement is a less common ankle impingement syndrome. It is associated with eversion injuries or following medial malleolar or talar fractures.64,65 Previous injury to the anterior tibiotalar fascicle of the deltoid complex leads to ligament thickening and subsequent impingement in the anteromedial corner of the talus. Adjacent fibrosis and synovitis are common consequences of impingement; however, osteophyte formation and chondral stripping along the anteromedial talus can also be seen. Patients typically complain of pain along the anteromedial joint line that is worse with activity, clicking or popping sensations, and painful, limited dorsiflexion. On examination, impingement can be detected through palpation over the anterior tibiotalar fascicle of the deltoid ligament and eversion or extreme passive dorsiflexion of the foot, all of which will elicit medial ankle tenderness.17,62 Initial treatment consists of rest, physical therapy, and NSAIDs. Refractory cases may be amenable to arthroscopic or open resection of the anterior tibiotalar fascicle with débridement of any adjacent synovitis and scar tissue.62
POSTERIOR ANKLE IMPINGEMENT
Posterior ankle impingement is often referred to as “os trigonum syndrome” since the posterior impingement is frequently associated with a prominent os trigonum. An os trigonum is an accessory ossicle representing the separated posterolateral tubercle of the talus. It is usually asymptomatic. However, in soccer players, pain can occur from impaction between the posterior tibial plafond and the os trigonum, or because of soft tissue compression between the 2 opposing osseous structures. The pain is due to repetitive microtrauma (ankle plantarflexion) or acute forced plantarflexion, which can present as an acute fracture of the os trigonum. Because soccer is a sport requiring both repetitive and extreme plantarflexion, it may predispose players to posterior ankle impingement (Figures 6A, 6B).62,66
Clinically, it can be very difficult to detect and diagnose because the affected structures lie deep and it can coexist with other disease processes (eg, peroneal tendinopathy, Achilles tendinopathy).62,66 Patients will complain of chronic deep posterior ankle pain that is worse with push-off activities (eg, jumping). On examination, patients will exhibit pain with palpation over the posterolateral process and with the crunch test. Lateral radiograph with the foot in plantar flexion will show the os trigonum impinged between the posterior tibial malleolus and the calcaneal tuberosity. An MRI will demonstrate the os trigonum as well as any associated inflammation and edema, while it can also demonstrate coexisting pathologies.
Initial treatment consists of rest, NSAIDs, and taping to prevent plantar flexion. Ultrasound-guided cortisone injection of the capsule and posterior bursa can be both therapeutic and diagnostic. A posterior injection can be used to temporize the symptoms so that the soccer player can make it through the season.
Surgical excision is saved for refractory cases, and this can be done either through an open posterolateral approach or arthroscopic techniques. Recently, Georgiannos and Bisbinas67 showed in an athletic population that endoscopic excision had both a lower complication rate and a quicker return to sports compared with the traditional open approach. Carreira and colleagues68 conducted a retrospective case series of 20 patients (mostly competitive athletes). They found that posterior ankle arthroscopy to address posterior impingement allowed for the maintenance or restoration of anatomic ROM of the ankle and hindfoot, ability to return to at least the previous level of activity, and improvement in objective assessment of pain relief and a higher level of function parameters.68
Continue to: TENDON PATHOLOGY
TENDON PATHOLOGY
SUPERIOR PERONEAL RETINACULUM INJURY
The superior peroneal retinaculum (SPR) forms the roof of the superior peroneal tunnel. The tunnel contains the peroneus brevis and longus tendons and is bordered by the retromalleolar groove of the fibula and the lower aspect of the posterior intramuscular septum of the leg.69,70 The SPR originates from the posterolateral ridge of the fibula and inserts onto the lateral calcaneus, and it is the primary restraint of the peroneal tendons within the retromalleolar sulcus.
Injury to the retinaculum results from both ankle dorsiflexion and inversion, and forceful reflex contraction of the peroneal muscles, which causes subluxation or dislocation of the contained tendons.69 A high level of suspicion is required regarding these injuries since the mechanism of injury is similar to that of a simple lateral ankle sprain. In the setting of retrofibular pain, snapping or popping sensations around the lateral malleolus, or chronic ankle instability that worsens on uneven surfaces, one must consider an injury to the SPR.69 Radiographs are not always diagnostic; however, occasionally on an internal rotation view, one may see a cortical avulsion off the distal tip of the lateral malleolus (“fleck sign”) indicating a rim fracture from an SPR injury (Figure 7). MRI is the best imaging modality to assess the peroneal tendons, as well as an SPR injury. Recently, ultrasound has grown in popularity and may be more useful, since it allows for dynamic evaluation of subluxating/dislocating tendons.69

Conservative management is often associated with poor outcomes, and surgery is indicated for all acute and chronic dislocations in athletes.71 Anatomic reconstruction of the SPR is the preferred surgical method.72 Peroneus brevis debulking and fibular groove deepening may also augment the retinaculum repair.73 van Dijk and colleagues in their systematic review showed that patients treated with both groove deepening and SPR repair have higher rates of return to the sport than patients treated with SPR repair alone.74
STRESS FRACTURES
FIFTH METATARSAL
Fifth metatarsal stress fractures usually occur secondary to lateral overload or avulsion of the peroneus brevis. The fifth metatarsal base can be susceptible to injury in a cavovarus foot. Non-operative treatment typically requires a longer period of immobilization (boot or cast) and necessitates a longer period of non–weight-bearing (anywhere between 6-12 weeks). Therefore, surgery is typically recommended in athletes or in the setting of a recurrent base of the fifth metatarsal fracture to expedite healing and return to play. Return to play is still not recommended until there is evidence of radiographic healing of the fracture. There are certain distinctions with fifth metatarsal stress fractures regarding location and healing rates that need to be taken into account.75,76 In particular, zone 2 injuries (Jones fractures) represent a vascular watershed area, making these fractures prone to nonunion with nonunion rates as high as 15% to 30%. Occasionally, the cavovarus deformity will require correction as well as a reduction in the risk of recurrence or nonunion. Surgical fixation most commonly consists of a single screw placed in an antegrade fashion.77 One must pay attention to screw size since smaller diameter screws (<4.5 mm) are associated with delayed union or nonunion. Moreover, screws that are too long will straighten the curved metatarsal shaft and can lead to fracture distraction or malreduction (Figures 8A, 8B).77
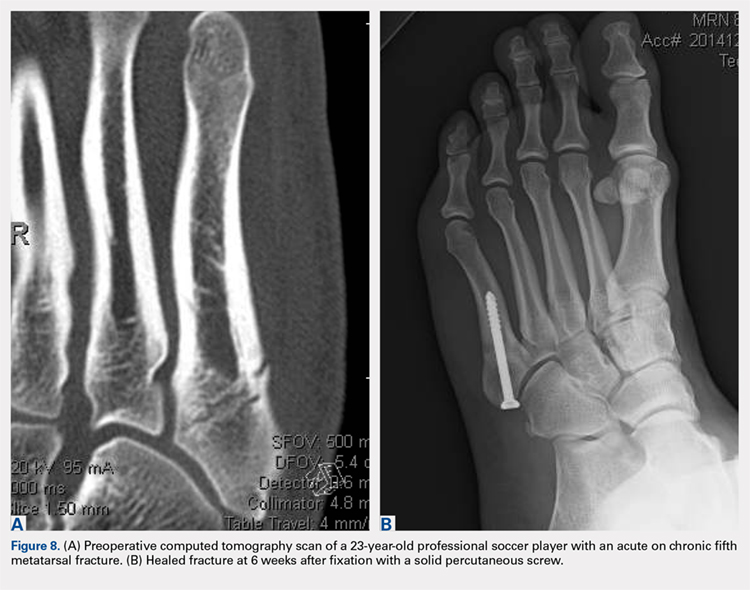
Patients have returned to competitive sports within 6 weeks; however, it should be noted that causes of failure were linked to early return and return to sports before a radiographic union can lead to failure of fixation. Ekstrand and van Dijk78 studied a large group of professional soccer players and found that out of 13,754 injuries, 0.5% (67) were fifth metatarsal fractures. Of note, they found that 45% of players had prodromal symptoms. Furthermore, after surgical treatment the fractures healed faster, compared with conservative treatment (75% vs 33%); however, there was no significant difference in lay-off days between both groups (80 vs 74 days).78 Matsuda and colleagues79 looked at 335 male collegiate soccer players, 29 of whom had a history of a fifth metatarsal stress fracture. They found that playing the midfield position and having an everted rearfoot and inverted forefoot alignment were associated with fifth metatarsal stress fractures.79 Saita and colleagues80 found that restricted hip internal rotation was associated with an increased risk of developing a Jones fracture in 162 professional football players. Finally, Fujitaka and colleagues81 looked at 273 male soccer players between 2005 and 2013. They found an association between weak toe-grip strength and fifth metatarsal fractures, suggesting that weak toe-grip may lead to an increase in the load applied onto the lateral side of the foot, resulting in a stress fracture.81
Continue to: NAVICULAR
NAVICULAR
Another common tarsal bone that sustains stress fractures is the navicular. It is not as common as calcaneal stress fractures in military recruits but can occur in the same type of population, as well as explosive athletics such as sprinters and soccer players. It commonly presents with an indistinct vague achy pain with activity that improves with rest, and pain at the dorsum of the midfoot or along the medial longitudinal arch with activity. It can easily go undiagnosed for quite some time given the difficulty in visualizing the navicular with plain radiographs. Clinically, it is difficult to make the diagnosis, and therefore advanced imaging is necessary when the injury is suspected. Both MRI and CT scans can be used to understand the extent of the injury (Figures 9A-9C). In non-displaced stress fractures, conservative non-operative treatment is the appropriate treatment modality with a brief period of immobilization and non–weight-bearing;82 however, operative treatment is also considered in elite athletes. In either case, return to play is discouraged until there is evidence of radiographic healing. When a displacement is noted, or there is a delay in diagnosis, then operative treatment is recommended.
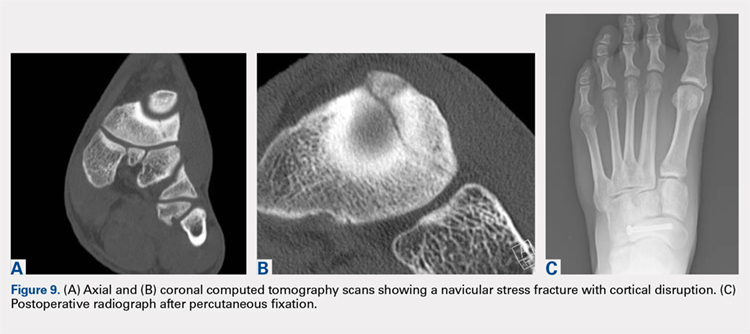
CONCLUSION
Ankle injuries are very common in soccer and can result in decreased performance or significant loss of playing time. Treatment of acute injury generally follows a conservative route, with surgical intervention reserved for severe ruptures or osteochondral fracture of the ankle joint. Chronic ankle pathology resulting in mechanical or functional instability generally requires surgery to repair ligamentous damage and restore normal ankle kinematics. It is critical for the soccer player to receive appropriate rehabilitation prior to returning to play in order to reduce the risk for reinjury and further chronic instability. Prevention and early intervention of ankle injuries is key in preventing the long-term sequelae of ankle injuries, such as arthritis, in former soccer players.
1. Dvorak J, Junge A. Football injuries and physical symptoms. A review of the literature. Am J Sports Med. 2000;28(5 Suppl):S3-S9.
2. Chomiak J, Junge A, Peterson L, Dvorak J. Severe injuries in football players. Am J Sports Med. 2000;28(5 Suppl):S58-S68.
3. Cloke DJ, Ansell P, Avery P, Deehan D. Ankle injuries in football academies: a three-centre prospective study. Br J Sports Med. 2011;45(9):702-708. doi:10.1136/bjsm.2009.067900.
4. Cloke DJ, Spencer S, Hodson A, Deehan D. The epidemiology of ankle injuries occurring in English Football Association academies. Br J Sports Med. 2009;43(14):1119-1125. doi:10.1136/bjsm.2008.052050.
5. Dvorak J, Junge A, Derman W, Schwellnus M. Injuries and illnesses of football players during the 2010 FIFA World Cup. Br J Sports Med. 2011;45(8):626-630. doi:10.1136/bjsm.2010.079905.
6. Ekstrand J, Gillquist J. Soccer injuries and their mechanisms: a prospective study. Med Sci Sports Exerc. 1983;15(3):267-270.
7. Fousekis K, Tsepis E, Vagenas G. Intrinsic risk factors of noncontact ankle sprains in soccer: a prospective study on 100 professional players. Am J Sports Med. 2012;40(8):1842-1850. doi:10.1177/0363546512449602.
8. Gaulrapp H, Becker A, Walther M, Hess H. Injuries in women’s soccer: a 1-year all players prospective field study of the women’s Bundesliga (German premiere league). Clin J Sports Med. 2010;20(4):264-271. doi:10.1097/JSM.0b013e3181e78e33.
9. Morgan BE, Oberlander MA. An examination of injuries in major league soccer. The inaugural season. Am J Sports Med. 2001;29(4):426-430. doi:10.1177/03635465010290040701.
10. Jain N, Murray D, Kemp S, Calder J. Frequency and trends in foot and ankle injuries within an English Premier League Football Club using a new impact factor of injury to identify a focus for injury prevention. Foot Ankle Surg. 2014;20(4):237-240. doi:10.1016/j.fas.2014.05.004.
11. Moore KL, Dalley AF, Agur AMR. Clinically Oriented Anatomy. 6th ed. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins, 2010:xxix, 1134.
12. Thompson JC, Netter FH. Netter’s Concise Orthopaedic Anatomy. 2nd ed. Philadelphia, PA: Saunders Elsevier, 2010:x, 404.
13. Giza E, Mandelbaum B. Chronic footballer’s ankle. In: Football Traumatology. Springer Milan, 2006:333-351.
14. Garrick JG. The frequency of injury, mechanism of injury, and epidemiology of ankle sprains. Am J Sports Med. 1977:5(6):241-242. doi:10.1177/036354657700500606.
15. Agur AMR, Grant JCB. Grant’s Atlas of Anatomy. 13th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins. 2011.
16. Renstrom PA, Konradsen L. Ankle ligament injuries. Br J Sports Med. 1997;31(1):11-20.
17. Chhabra A, Subhawong TK, Carrino JA. MR imaging of deltoid ligament pathologic findings and associated impingement syndromes. Radiographics. 2010;30(3):751-761. doi:10.1148/rg.303095756.
18. Hintermann B. Medial ankle instability. Foot Ankle Clin. 2003;8(4):723-738.
19. Woods C, Hawkins R, Hulse M, Hodson A. The Football Association Medical Research Programme: an audit of injuries in professional football: an analysis of ankle sprains. Br J Sports Med. 2003;37(3):233-238.
20. Thacker SB, Stroup DF, Branche CM, Gilchrist J, Goodman RA, Weitman EA. The prevention of ankle sprains in sports. A systematic review of the literature. Am J Sports Med. 1999;27(6):753-760. doi:10.1177/03635465990270061201.
21. Giza E, Fuller C, Junge A, Dvorak J. Mechanisms of foot and ankle injuries in soccer. Am J Sports Med. 2003;31(4):550-554. doi:10.1177/03635465030310041201.
22. Tucker AM. Common soccer injuries. Diagnosis, treatment and rehabilitation. Sports Med. 1997;23(1):21-32.
23. Lynch SA, Renstrom PA. Treatment of acute lateral ankle ligament rupture in the athlete. Conservative versus surgical treatment. Sports Med. 1999;27(1):61-71.
24. Chan KW, Ding BC, Mroczek KJ. Acute and chronic lateral ankle instability in the athlete. Bull NYU Hosp Jt Dis. 2011;69(1):17-26.
25. Stiell IG, Greenberg GH, McKnight RD, Nair RC, McDowell I, Worthington JR. A study to develop clinical decision rules for the use of radiography in acute ankle injuries. Ann Emerg Med. 1992;21(4):384-390.
26. Bachmann LM, Kolb E, Koller MT, Steurer J, ter Riet G. Accuracy of Ottawa ankle rules to exclude fractures of the ankle and mid-foot: systematic review. BMJ. 2003;326(7386):417. doi:10.1136/bmj.326.7386.417.
27. Balduini FC, Vegso JJ, Torg JS, Torg E. Management and rehabilitation of ligamentous injuries to the ankle. Sports Med. 1987;4(5):364-380.
28. Kerkhoffs GM, Rowe BH, Assendelft WJ, Kelly KD, Struijs PA, van Dijk CN. Immobilisation for acute ankle sprain. A systematic review. Arch Orthop Trauma Surg. 2001;121(8):462-471.
29. Konradsen L, Holmer P, Sondergaard L. Early mobilizing treatment for grade III ankle ligament injuries. Foot Ankle. 1991;12(2):69-73.
30. Eiff MP, Smith AT, Smith GE. Early mobilization versus immobilization in the treatment of lateral ankle sprains. Am J Sports Med. 1994;22(1):83-88. doi:10.1177/036354659402200115.
31. Shrier I. Treatment of lateral collateral ligament sprains of the ankle: a critical appraisal of the literature. Clin J Sport Med. 1995;5(3):187-195.
32. DiGiovanni BF, Partal G, Baumhauer JF. Acute ankle injury and chronic lateral instability in the athlete. Clin Sports Med. 2004;23(1):1-19, v. doi:10.1016/S0278-5919(03)00095-4.
33. Alt W, Lohrer H, Gollhofer A. Functional properties of adhesive ankle taping: neuromuscular and mechanical effects before and after exercise. Foot Ankle Int. 1999;20(4):238-245. doi:10.1177/107110079902000406.
34. Garrick JG, Requa RK. Role of external support in the prevention of ankle sprains. Med Sci Sports. 1973;5(3):200-203.
35. Sharpe SR, Knapik J, Jones B. Ankle braces effectively reduce recurrence of ankle sprains in female soccer players. J Athl Train. 1997;32(1):21-24.
36. Surve I, Schwellnus MP, Noakes T, Lombard C. A fivefold reduction in the incidence of recurrent ankle sprains in soccer players using the Sport-Stirrup orthosis. Am J Sports Med. 1994;22(5):601-606. doi:10.1177/036354659402200506.
37. Mohammadi F. Comparison of 3 preventive methods to reduce the recurrence of ankle inversion sprains in male soccer players. Am J Sports Med. 2007;35(6):922-926. doi:10.1177/0363546507299259.
38. Steffen K, Meeuwisse WH, Romiti M, et al. Evaluation of how different implementation strategies of an injury prevention programme (FIFA 11+) impact team adherence and injury risk in Canadian female youth football players: a cluster-randomised trial. Br J Sports Med. 2013;47(8):480-487. doi:10.1136/bjsports-2012-091887.
39. Steffen K, Emery CA, Romiti M, et al. High adherence to a neuromuscular injury prevention programme (FIFA 11+) improves functional balance and reduces injury risk in Canadian youth female football players: a cluster randomised trial. Br J Sports Med. 2013;47(12):794-802. doi: 10.1136/bjsports-2012-091886.
40. Junge A, Lamprecht M, Stamm H, et al. Countrywide campaign to prevent soccer injuries in Swiss amateur players. Am J Sports Med. 2011;39(1):57-63. doi:10.1177/0363546510377424.
41. Lin CF, Gross ML, Weinhold P. Ankle syndesmosis injuries: anatomy, biomechanics, mechanism of injury, and clinical guidelines for diagnosis and intervention. J Orthop Sports Phys Ther. 2006;36(6):372-384. doi:10.2519/jospt.2006.2195.
42. Lubberts B, D’Hooghe P, Bengtsson H, DiGiovanni CW, Calder J, Ekstrand J. Epidemiology and return to play following isolated syndesmotic injuries of the ankle: a prospective cohort study of 3677 male professional football players in the UEFA Elite Club Injury Study. Br J Sports Med. 2017. doi:10.1136/bjsports-2017-097710.
43. Harper MC, Keller TS. A radiographic evaluation of the tibiofibular syndesmosis. Foot Ankle. 1989;10(3):156-160.
44. Vogl TJ, Hochmuth K, Diebold T, et al. Magnetic resonance imaging in the diagnosis of acute injured distal tibiofibular syndesmosis. Invest Radiol. 1997;32(7):401-409.
45. Brown KW, Morrison WB, Schweitzer ME, Parellada JA, Nothnagel H. MRI findings associated with distal tibiofibular syndesmosis injury. AJR Am J Roentgenol. 2004;182(1):131-136. doi:10.2214/ajr.182.1.1820131.
46. Ogilvie-Harris DJ, Reed SC, Hedman TP. Disruption of the ankle syndesmosis: biomechanical study of the ligamentous restraints. Arthroscopy. 1994;10(5):558-560.
47. Boytim MJ, Fischer DA, Neumann L. Syndesmotic ankle sprains. Am J Sports Med. 1991;19(3):294-298. doi:10.1177/036354659101900315.
48. Hopkinson WJ, St Pierre P, Ryan JB, Wheeler JH. Syndesmosis sprains of the ankle. Foot Ankle. 1990;10(6):325-330. doi:10.1177/107110079001000607.
49. Del Buono A, Florio A, Boccanera MS, Maffulli N. Syndesmosis injuries of the ankle. Curr Rev Musculoskelet Med. 2013;6(4):313-319. doi:10.1007/s12178-013-9183-x.
50. Dattani R, Patnaik S, Kantak A, Srikanth B, Selvan TP. Injuries to the tibiofibular syndesmosis. J Bone Joint Surg Br. 2008;90(4):405-410. doi:10.1302/0301-620X.90B4.19750.
51. Schepers T. To retain or remove the syndesmotic screw: a review of literature. Arch Orthop Trauma Surg. 2011;131(7):879-883. doi:10.1007/s00402-010-1225-x.
52. Naqvi GA, Cunningham P, Lynch B, Galvin R, Awan N. Fixation of ankle syndesmotic injuries: comparison of tightrope fixation and syndesmotic screw fixation for accuracy of syndesmotic reduction. Am J Sports Med. 2012;40(12):2828-2835. doi:10.1177/0363546512461480.
53. Weening B, Bhandari M. Predictors of functional outcome following transsyndesmotic screw fixation of ankle fractures. J Orthop Trauma. 2005;19(2):102-108.
54. Colcuc C, Blank M, Stein T, et al. Lower complication rate and faster return to sports in patients with acute syndesmotic rupture treated with a new knotless suture button device. Knee Surg Sports Traumatol Arthrosc. 2017. doi:10.1007/s00167-017-4820-4823.
55. Savage-Elliott I, Ross KA, Smyth NA, Murawski CD, Kennedy JG. Osteochondral lesions of the talus: a current concepts review and evidence-based treatment paradigm. Foot Ankle Spec. 2014;7(5):414-422. doi:10.1177/1938640014543362.
56. Talusan PG, Milewski MD, Toy JO, Wall EJ. Osteochondritis dissecans of the talus: diagnosis and treatment in athletes. Clin Sports Med. 2014;33(2):267-284. doi:10.1016/j.csm.2014.01.003.
57. Murawski CD, Kennedy JG. Operative treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 2013;95(11):1045-1054. doi:10.2106/JBJS.L.00773.
58. Guney A, Akar M, Karaman I, Oner M, Guney B. Clinical outcomes of platelet rich plasma (PRP) as an adjunct to microfracture surgery in osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2015;23(8):2384-2389. doi:10.1007/s00167-013-2784-5.
59. Hatic SO, Berlet GC. Particulated juvenile articular cartilage graft (DeNovo NT Graft) for treatment of osteochondral lesions of the talus. Foot Ankle Spec. 2010;3(6):361-364. doi:10.1177/1938640010388602.
60. van Eekeren IC, van Bergen CJ, Sierevelt IN, Reilingh ML, van Dijk CN. Return to sports after arthroscopic debridement and bone marrow stimulation of osteochondral talar defects: a 5- to 24-year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1311-1315. doi:10.1007/s00167-016-3992-6.
61. Tol JL, Slim E, van Soest AJ, van Dijk CN. The relationship of the kicking action in soccer and anterior ankle impingement syndrome. A biomechanical analysis. Am J Sports Med. 2002;30(1):45-50. doi:10.1177/03635465020300012101.
62. Sanders TG, Rathur SK. Impingement syndromes of the ankle. Magn Reson Imaging Clin N Am. 2008;16(1):29-38. doi:10.1016/j.mric.2008.02.005.
63. Ogilvie-Harris DJ, Gilbart MK, Chorney K. Chronic pain following ankle sprains in athletes: the role of arthroscopic surgery. Arthroscopy. 1997;13(5):564-574.
64. Robinson P, White LM, Salonen D, Ogilvie-Harris D. Anteromedial impingement of the ankle: using MR arthrography to assess the anteromedial recess. AJR Am J Roentgenol. 2002;178(3):601-604. doi:10.2214/ajr.178.3.1780601.
65. Mosier-La Clair SM, Monroe MT, Manoli A. Medial impingement syndrome of the anterior tibiotalar fascicle of the deltoid ligament on the talus. Foot Ankle Int. 2000;21(5):385-391.
66. Maquirriain J. Posterior ankle impingement syndrome. J Am Acad Orthop Surg. 2005;13(6):365-371.
67. Georgiannos D, Bisbinas I. Endoscopic versus open excision of os trigonum for the treatment of posterior ankle impingement syndrome in an athletic population: a randomized controlled study with 5-year follow-up. Am J Sports Med. 2017;45(6):1388-1394. doi:10.1177/0363546516682498.
68. Carreira DS, Vora AM, Hearne KL, Kozy J. Outcome of arthroscopic treatment of posterior impingement of the ankle. Foot Ankle Int. 2016;37(4):394-400. doi:10.1177/1071100715620857.
69. Roth JA, Taylor WC, Whalen J. Peroneal tendon subluxation: the other lateral ankle injury. Br J Sports Med. 2010;44(14):1047-1053. doi:10.1136/bjsm.2008.057182.
70. Athavale SA, Swathi, Vangara SV. Anatomy of the superior peroneal tunnel. J Bone Joint Surg Am. 2011;93(6):564-571. doi:10.2106/JBJS.17.00836.
71. Porter D, McCarroll J, Knapp E, Torma J. Peroneal tendon subluxation in athletes: fibular groove deepening and retinacular reconstruction. Foot Ankle Int. 2005;26(6):436-441.
72. Ferran NA, Oliva F, Maffulli N. Recurrent subluxation of the peroneal tendons. Sports Med. 2006;36(10):839-846. doi:10.1053/j.jfas.2010.02.007.
73. Saxena A, Ewen B. Peroneal subluxation: surgical results in 31 athletic patients. J Foot Ankle Surg. 2010;49(3):238-241.
74. van Dijk PA, Gianakos AL, Kerkhoffs GM, Kennedy JG. Return to sports and clinical outcomes in patients treated for peroneal tendon dislocation: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1155-1164. doi:10.1007/s00167-015-3833-z.
75. Lee KT, Park YU, Young KW, Kim JS, Kim JB. The plantar gap: another prognostic factor for fifth metatarsal stress fracture. Am J Sports Med. 2011;39(10):2206-2211. doi:10.1177/0363546511414856.
76. Torg JS. Fractures of the base of the fifth metatarsal distal to the tuberosity. Orthopedics. 1990;13:731-737.
77. Smith TO, Clark A, Hing CB. Interventions for treating proximal fifth metatarsal fractures in adults: a meta-analysis of the current evidence-base. Foot Ankle Surg. 2011;17(4):300-307. doi:10.1016/j.fas.2010.12.005.
78. Ekstrand J, van Dijk CN. Fifth metatarsal fractures among male professional footballers: a potential career-ending disease. Br J Sports Med. 2013;47(12):754-758.
79. Matsuda S, Fukubayashi T, Hirose N. Characteristics of the foot static alignment and the plantar pressure associated with fifth metatarsal stress fracture history in male soccer players: a case-control study. Sports Med Open. 2017;3(1):27.
80. Saita Y, Nagao M, Kawasaki T, et al. Range limitation in hip internal rotation and fifth metatarsal stress fractures (Jones fracture) in professional football players. Knee Surg Sports Traumatol Arthrosc. 2018;26(7):1943-1949. doi:10.1007/s00167-017-4552-4.
81. Fujitaka K, Taniguchi A, Isomoto S, et al. Pathogenesis of fifth metatarsal fractures in college soccer players. Orthop J Sports Med. 2015;18;3(9):2325967115603654.
82. Torg J, Moyer J, Gaughan J, Boden B. Management of tarsal navicular stress fractures: conservative versus surgical treatment: a meta-analysis. Am J Sports Med. 2010;38(5):1048-1053.
83. Haytmanek CT, Williams BT, James EW, et al. Radiographic identification of the primary lateral ankle structures. Am J Sports Med. 2015;43(1):79-87. doi:10.1177/0363546514553778.
ABSTRACT
The ankle is one of the most commonly injured joints in soccer and represents a significant cost to the healthcare system. The ligaments that stabilize the ankle joint determine its biomechanics—alterations of which result from various soccer-related injuries. Acute sprains are among the most common injury in soccer players and are generally treated conservatively, with emphasis placed on secondary prevention to reduce the risk for future sprains and progression to chronic ankle instability. Repetitive ankle injuries in soccer players may cause chronic ankle instability, which includes both mechanical ligamentous laxity and functional changes. Chronic ankle pathology often requires surgery to repair ligamentous damage and remove soft-tissue or osseous impingement. Proper initial treatment, rehabilitation, and secondary prevention of ankle injuries can limit the amount of time lost from play and avoid negative long-term sequelae (eg, osteochondral lesions, arthritis). On the other hand, high ankle sprains portend a poorer prognosis and a longer recovery. These injuries will typically require surgical stabilization. Impingement-like syndromes of the ankle can undergo an initial trial of conservative treatment; when this fails, however, soccer players respond favorably to arthroscopic debridement of the lesions causing impingement. Finally, other pathologies (eg, stress fractures) are highly encouraged to be treated with surgical stabilization in elite soccer players.
Continue to: EPIDEMIOLOGY
EPIDEMIOLOGY
With roughly 200,000 professional and around 240 million amateur soccer players, soccer has been recognized as the most popular sport worldwide. Nevertheless, given its rising popularity in society, one must also consider the increasing incidence of injuries as a result. Elite soccer players sustain between 10 and 35 injuries per 1000 competitive playing hours.1 Approximately 80% are traumatic, and 20% are overuse injuries.2 Soccer injuries are more frequent with increasing age of the participants, whereas the incidence of injuries in preadolescent players is low. The incidence of injuries has been found to be higher during competition when compared with practice/training sessions, with some studies showing that 59% of injuries occurred during games.2 Amateur or recreational soccer players sustain fewer injuries than professional soccer players, as one would expect, given both the higher intensity of training and match schedule in professionals.
The ankle is one of the most commonly injured joints in soccer, with some studies suggesting it comprises one-fifth of all injuries sustained during soccer, which is only second to those of the knee.2 Ankle sprains specifically are quite a common occurrence in soccer.3-9 A recent study of an English premier league club showed that over a 4-season period, 20% of injuries were of the foot and ankle with a mean return to sport time of 54 days.10 Of all foot and ankle related injuries, ankle sprains are the most common, followed by bruises/contusions, and tendon lesions. Fractures are very rare (1%) in soccer, but when they do occur they impart a much more extended recovery. During the 2010 Fédération Internationale de Football Association (FIFA) World Cup, ankle sprains were among the most common injuries and approximately half lead to players missing training or competitive matches.5
ANATOMY
Knowledge of the biomechanics of both the foot and ankle joints is essential to understand soccer injuries. The ankle joint (talocrural articulation) consists of the distal ends of the tibia and fibula, which form the mortise, and the superior aspect of the talar dome.11 As a hinge joint, the ankle provides 20° of dorsiflexion and 50° of plantar flexion,12 with stability provided by the lateral, medial, and superior ligamentous complexes. The superior articular surface of the talus is narrower posteriorly, which creates a looser fit within the mortise during plantar flexion.11 This decreased stability could help explain why the most common injury in soccer involves a plantar flexion mechanism.13,14 Inferiorly, the talus articulates with the calcaneus to form the subtalar joint. It is at this site that the majority of both foot inversion and eversion occurs. The transverse tarsal joints (Chopart’s joints) separate the hindfoot from the midfoot. Movement of this joint depends on the relative alignment of its 2 articulations: the talonavicular and calcaneocuboid joints. During foot eversion, these 2 joints are parallel to each other allowing supple motion and aiding in shock absorption during the heel strike phase of the gait cycle. With foot inversion, the joints become nonparallel and thus lock the transverse tarsal joints providing a rigid lever needed for push-off.11,12
LATERAL LIGAMENTS
The ankle joint is stabilized laterally by a ligament complex consisting of 3 individual ligaments, all originating from the lateral malleolus: the anterior talofibular ligament (ATFL), the posterior talofibular ligament (PTFL), and the calcaneofibular ligament (CFL) (Figure 1).11,12,15 The ATFL is the primary restraint to inversion in plantar flexion, and it helps resist anterolateral translation of the talus in the mortise. However, it is the weakest and therefore the most frequently injured of the lateral ligaments. The PTFL plays only a supplementary role in ankle stability when the lateral ligament complex is intact. It is under the greatest strain in ankle dorsiflexion and acts to limit posterior talar displacement within the mortise as well as talar external rotation.13,16 The CFL is the primary restraint to inversion in the neutral or dorsiflexed position. It restrains subtalar inversion, thereby limiting talar tilt within the mortise.

DELTOID LIGAMENT
The deltoid ligament complex consists of 6 continuous adjacent superficial and deep ligaments that function synergistically to resist valgus and pronation forces, as well as external rotation of the talus in the mortise.11-13,17 The superficial layer crosses both ankle and subtalar joints. It originates from the anterior colliculus and fans out to insert into the navicular, neck of the talus, sustentaculum tali, and posteromedial talar tubercle. The tibiocalcaneal (sustentaculum tali) portion is the strongest component in the superficial layer and resists calcaneal eversion. The deep layer crosses the ankle joint only. It functions as the primary stabilizer of the medial ankle and prevents both lateral displacement and external rotation of the talus. It originates from the inferior and posterior aspects of the medial malleolus and inserts on the medial and posteromedial aspects of the talus.12,17,18
Continue to: SYNDESMOSIS
SYNDESMOSIS
The ankle syndesmosis, or inferior tibiofibular joint, is the distal articulation between the tibia and fibula. The syndesmosis contributes to ankle mortise integrity through its firm fixation of the lateral malleolus against the lateral surface of the talus. Ligaments comprising the ankle syndesmosis include the anterior inferior tibiofibular ligament (AITFL), the posterior inferior tibiofibular ligament (PITFL), the inferior transverse ligament, and the interosseous ligament (IOL).12
ANKLE SPRAINS
Ankle sprains are the most common pathology encountered amongst soccer players, representing from one-half to two-thirds of all ankle related injuries. Most sprains occur outside of player contact.
LATERAL ANKLE SPRAINS AND INSTABILITY
Injury to the lateral ligaments of the ankle represents 77% to 91% of all ankle sprains in soccer.6,19 The greatest risk factor for an ankle sprain in a soccer player is a history of prior sprain.20 Other risk factors include increasing age, player-to-player contact, condition of the pitch, weight-bearing status of the injured limb at the time of injury, and joint instability or laxity.21,22
The evaluation of an ankle sprain to determine its severity is best done after the acute phase, approximately 4 to 7 days after the initial injury when both pain and swelling have subsided.23 The anterior drawer (ATFL instability) and talar tilt (CFL instability) tests are useful in evaluating ankle instability in the delayed or chronic setting; however, both have been shown to have limited sensitivity and significant variability amongst different examiners.24
Clinical examination will direct further diagnostic tests including X-rays, magnetic resonance imaging (MRI), and computed tomography (CT). The Ottawa ankle rules are generally helpful in determining whether plain X-rays are indicated in the acute setting.25,26 (Figure 2) According to these rules, ankle radiographs should be obtained if ankle pain is reported near the malleoli and 1 or more of the following is seen during examination: inability to bear weight immediately after injury and for 4 steps in the emergency department, and bony tenderness at the posterior edge or tip of the malleolus. Stress X-rays are generally not indicated in acute injuries but may be useful in chronic ankle instability cases.23

Continue to: Ankle sprains cover...
Ankle sprains cover a broad spectrum of injuries; therefore, a grading system was devised to aid in guiding treatment. Grade I (mild) sprains are those with minimal swelling and tenderness but have the ligaments still intact. Grade II (moderate) sprains occur when there are partial ligament tears associated with moderate pain, swelling, and tenderness. Finally, Grade III (severe) sprains are complete ligament tears with marked swelling, hemorrhage, tenderness, loss of function, and abnormal joint motion and instability.23, 24
Initial treatment for all ankle sprains is nonoperative and involves the RICE (rest, ice, compression, elevation) protocol with the use of nonsteroidal anti-inflammatory drugs (NSAIDs) during the acute phase (first 4-5 days) with a short period (no >2 weeks) of immobilization.27 Most authors agree that early mobilization followed by phased rehabilitation is warranted to minimize time away from sports.28-31 Prolonged immobilization (>2 weeks) has detrimental effects and may lead to a longer return to play.28-31 The rehabilitation protocol is divided into stages: (1) pain and edema control, (2) range of motion (ROM) and strengthening exercises, (3) soccer specific functional training, and (4) prophylactic intervention with balance and proprioception exercises. Surgical intervention is rarely indicated for acute ankle sprains. There are exceptions, however, such as when ankle sprains are associated with other injuries that require acute intervention (eg, fracture, osteochondral lesion). Surgery is indicated in the setting of chronic, recurrent mechanical instability. Anatomical repairs (modified Brostrom) seem to produce better outcomes than non-anatomical reconstructions (eg, Chrisman-Snook). Surgical outcomes are good, and most athletes are able to return to their pre-injury level of function.32
In athletes, prevention of recurrent sprains is key. Braces may help prevent ankle sprains and bracing has been shown to be superior to taping, as tape loses its restrictive properties within 20 to 30 minutes of initiating activity.33,34 Application of an orthosis (lace-up ankle orthosis) has been shown to reduce the incidence of ankle re-injury in soccer players with previous ankle sprains. Several studies have found minimal, if any, effect of orthoses on athletic performance.20,35,36 Low-profile braces for soccer have been developed which allow for minimal disruption of the player’s boot and space proximally to insert the shin guard. Another essential component of prevention is prophylactic intervention with balance and proprioceptive exercises. A study looking at first division men’s league football (soccer) players in Iran showed a significant decrease in re-injury rates with proprioceptive training.37 In 2003, FIFA introduced a comprehensive warm-up program (FIFA 11+), which has since been shown in several studies to decrease the risk of injury in amateur soccer players.38-40
MEDIAL ANKLE SPRAINS AND INSTABILITY
Soccer places an unusually high demand on both the medial foot and ankle structures when compared with other sports. For instance, striking the ball requires the player to abduct and externally rotate the foot, which preloads medial structures.9 Hintermann18 looked at 54 cases of medial ankle instability and found that injury commonly occurred during landing on an uneven surface, which applies to soccer players when landing after heading the ball or jumping over a tackle. Pronation with eversion and extreme rotational injuries are well known to cause deltoid ligament injury. However, complete rupture of the deltoid ligament is rare and is more often associated with ankle fractures.41 Due to its close proximity and similarly shared function in medial plantar arch stabilization with the tibiospring and spring ligaments, posterior tibialis tendon dysfunction is also frequently seen in medial ankle instability.17 After an acute injury, patients can present with a medial ankle hematoma and pain along the deltoid ligament. Although chronic insufficiency is diagnosed based on the feeling of “giving way,” pain in the medial gutter of the ankle and a valgus and pronation deformity of the foot can be corrected by activating the peroneus tertius muscle. Arthroscopy is the most specific way to confirm clinically suspected instability of the medial ankle; however, MRI can demonstrate loss of organized medial fibers (Figures 3A, 3B).18 Primary surgical repair of deltoid ligament tears yield good to excellent results and should be considered in the soccer player to prevent problems associated with chronic non-repaired tears such as instability, osteoarthritis, and impingement syndromes.18 After surgical repair, players will undergo extensive physical therapy that progresses to sport-specific exercises with the ultimate goal of returning to competitive play around 4-6 months post-operatively.

HIGH ANKLE SPRAINS (SYNDESMOSIS)
High ankle sprains are much less common than low ankle sprains; however, when they do occur they portend a lengthier rehabilitation and a poorer prognosis, especially if undiagnosed. Lubberts and colleagues42 studied the epidemiology of isolated syndesmotic injuries in professional football players. They pooled data from 15 consecutive seasons of European professional football between 2001 and 2016. They examined a total of 3677 players from 61 teams across 17 countries. There were 1320 ankle ligament injuries registered during 15 seasons, of which 94 (7%) were isolated syndesmotic injuries. The incidence of these injuries increased annually between 2001 and 2016. Injuries were 74% contact-related, and isolated syndesmotic injuries were followed by a mean of a 39-day absence.42 Moreover, football players may have an increased risk of syndesmotic sprains due to foot planting and cutting action.41
Continue to: These injuriesa are typically...
These injuries are typically identified with pain over the AITFL and interosseous membrane. Physical examination tests that help identify syndesmotic injuries include the squeeze test, external rotation test, and crossed-leg test.41 The diagnosis can be made on plain X-ray when there is clear diastasis between the distal tibia and fibula. Two critical measurements on plain films are made 1 cm above the tibial plafond and are used to evaluate the integrity of the syndesmosis: tibiofibular clear space >6 mm, and tibiofibular overlap <1 mm, which indicate disruption of the syndesmosis.43 More subtle injuries can be diagnosed with better sensitivity and specificity using MRI, which can also reveal secondary findings such as bone bruises, ATFL injury, osteochondral lesions, and tibiofibular incongruity.44,45 Arthroscopy is an invaluable diagnostic tool for syndesmotic injuries with a characteristic triad finding of PITFL scarring, disrupted interosseous ligament, and posterolateral tibial plafond chondral damage.46
Classification of the ligaments involved can aid in the selection of appropriate treatment. Grade I injuries involve AITFL tears. Grade IIa injuries involve AITFL and IOL tears. Grade IIb injuries include AITFL, PITFL, and IOL tears. Grade III injuries involve injury to all 3 ligaments, as well as a fibular fracture. Conservative treatment is recommended for Grades I and IIa, while surgical intervention is necessary for Grades IIb and III (Figures 4A, 4B). Compared with other ankle sprains, syndesmotic injuries typically require a more prolonged recovery/rehabilitation. Some studies suggest that these injuries require twice as long to heal.47 Hopkinson and colleagues48 reported a mean recovery time of 55 days following syndesmotic injuries in cadets at the United States Military Academy at West Point. Some surgeons advocate surgical intervention in professional athletes with mild sprains to expedite return to play.49

Surgery has been well established as necessary in more severe injuries where there is clear diastasis or instability of the syndesmosis. Traditionally, screws were used for surgical fixation; however, they often required a second surgery for subsequent removal. There is no general consensus on the optimal screw size, level of placement, or timing of removal.50,51 More recently, non-absorbable suture button fixation (eg, TightRope; Arthrex) has become more popular and provides certain advantages over screw fixation, such as avoiding the need for hardware removal. TightRope has been shown to provide more accurate stabilization of the syndesmosis as compared with screw fixation.52 Since malreduction is the most important indicator of poor long-term functional outcome, suture button fixation should be considered in the treatment of the football player.53 Finally, Colcuc and colleagues54 reported a lower complication rate and earlier return to sports in patients treated with knotless suture button devices compared with screw fixation.
OSTEOCHONDRAL LESIONS
Osteochondral lesions (OCLs) are cartilage-bone defects that are usually located in the talus. They can be caused by an acute traumatic event or repetitive microtrauma with no apparent history of trauma (eg, ankle instability). Acute OCLs can occur in soccer secondary to an ankle sprain or ankle fracture. Symptoms of OCLs include pain, swelling, and mechanical symptoms such as catching or locking, and on physical examination, one might see an effusion. The initial imaging modality of choice is radiographing; however, in ankle sprains with continued pain and swelling MRI may be indicated to rule out an underlying OCL. Missed acute lesions have a tendency not to heal and become chronic lesions, which can cause pain and playing disability. It is well established that chronic ankle instability is an important etiologic factor for OCLs. With the normal hydrostatic pressure within the ankle joint, synovial fluid gets pushed into cartilage/bone fissures, which can then lead to cystic degeneration of the subchondral bone.55-57
Surgical repair of an OCL is dependent on both the size and location of the lesion. Acute lesions can be managed by arthroscopic débridement, microfracture, or fixation of the lesion if enough bone remains attached to the chondral lesion. Return to play is based on development and maturation of fibrocartilage over the lesion (debridement/microfracture) or healing and incorporation of the new graft (chondral repair procedures). Meanwhile, chronic lesions can be managed in 1-stage (microfracture, osteochondral autograft transfer or 2-stage (autologous chondrocyte implantation [ACI]) procedures.56-57 Additional biologic healing augmentation with platelet-rich plasma has been described as well.58 Newer techniques in treating chronic talus OCLs, including ones that have failed to respond to bone marrow stimulation techniques, have been developed more recently such as the use of particulated juvenile articular cartilage allograft (DeNovo NT Natural Tissue Graft®; Zimmer Biomet).59 These newer techniques avoid the need for a 2-stage procedure, as is the case with ACI.
Continue to: Further studies are needed...
Further studies are needed to both investigate long-term outcomes and determine the superiority of the arthroscopic juvenile cartilage procedure compared with microfracture and other cartilage resurfacing procedures. When surgically treating OCLs, one must also restore normal ankle joint biomechanics for the lesion to heal. For instance, in the presence of ankle instability, ligament reconstruction must be performed. Also, one should also consider addressing any hindfoot malalignment with an osteotomy (calcaneus, supramalleolar). In a recent retrospective study, van Eekeren and colleagues60 showed that approximately 76% of patients were able to return to sports at long-term follow-up after arthroscopic débridement and bone marrow stimulation of talar OCLs. However, the activity level decreased at long-term follow-up and never attained the pre-injury level.60
ANKLE IMPINGEMENT
ANTERIOR ANKLE IMPINGEMENT (FOOTBALLER'S ANKLE)
Anterior ankle impingement is caused by anterior osteophytes on both the distal tibia and talar neck. It is thought to be related to repetitive microtrauma to the anteromedial aspect of the ankle from recurrent ball impact.61 It is very common amongst soccer players with some studies suggesting that 60% of soccer players have this syndrome. Ankle impingement is characterized by anterior pain with ankle dorsiflexion, decreased dorsiflexion, and swelling. It is primarily diagnosed with lateral ankle X-rays, which will show the osteophytes. An oblique anteromedial X-ray may increase detection of osteophytes (Figure 5). The early stages of anterior impingement can be treated successfully with injections and heel lifts. Treatment of lesions that fail to respond to conservative management involves arthroscopic or open excision of osteophytes. Most patients with no preexisting osteoarthritis treated arthroscopically will experience pain relief and return to full activity, though recurrent osteophyte formation has been noted at long-term follow-up.62

Anterior ankle impingement is most often caused by acute ankle sprains with an inversion type of mechanism.62 The subsequent reactive inflammation can cause fibrosis leading to distal fascicle enlargement of the AITFL. Impingement in the anterolateral gutter of this enlarged fascicle can also cause both chronic reactive synovitis and chondromalacia of the lateral talar dome.63 MRI can identify abnormal areas of pathology; however, 50% of cases are diagnosed based on clinical examination alone.63 Patients generally present with a history of anterolateral ankle pain and swelling with an occasional popping or snapping sensation.
Soccer players commonly develop anterior bony impingement due to repetitive loading of the anterior ankle from striking the ball. This repetition can lead to osteophyte formation of the anterior distal tibia and talar neck. After the osteophytes form, decreased dorsiflexion can occur due to a mechanical stop and inflammation of the interposed capsule.
The patient will exhibit tenderness to palpation along the anterolateral aspect of the ankle, with pain elicited at extreme passive dorsiflexion.62 Initially, an injection with local anesthetic and corticosteroid can serve both a diagnostic and therapeutic purpose; however, patients who fail conservative treatment can be treated with arthroscopy and resection of the involved scar tissue and osteophytes. The best results are seen in those patients with no concurrent intra-articular lesions or ankle osteoarthritis (Figure 5).62 When treated non-operatively, a player may return to play when pain resolves; however, if treated surgically with arthroscopic debridement/resection, a player must wait until his surgical scars have healed prior to attempting return to play.
Continue to: ANTEROMEDIAL ANKLE IMPINGEMENT
ANTEROMEDIAL ANKLE IMPINGEMENT
Anteromedial ankle impingement is a less common ankle impingement syndrome. It is associated with eversion injuries or following medial malleolar or talar fractures.64,65 Previous injury to the anterior tibiotalar fascicle of the deltoid complex leads to ligament thickening and subsequent impingement in the anteromedial corner of the talus. Adjacent fibrosis and synovitis are common consequences of impingement; however, osteophyte formation and chondral stripping along the anteromedial talus can also be seen. Patients typically complain of pain along the anteromedial joint line that is worse with activity, clicking or popping sensations, and painful, limited dorsiflexion. On examination, impingement can be detected through palpation over the anterior tibiotalar fascicle of the deltoid ligament and eversion or extreme passive dorsiflexion of the foot, all of which will elicit medial ankle tenderness.17,62 Initial treatment consists of rest, physical therapy, and NSAIDs. Refractory cases may be amenable to arthroscopic or open resection of the anterior tibiotalar fascicle with débridement of any adjacent synovitis and scar tissue.62
POSTERIOR ANKLE IMPINGEMENT
Posterior ankle impingement is often referred to as “os trigonum syndrome” since the posterior impingement is frequently associated with a prominent os trigonum. An os trigonum is an accessory ossicle representing the separated posterolateral tubercle of the talus. It is usually asymptomatic. However, in soccer players, pain can occur from impaction between the posterior tibial plafond and the os trigonum, or because of soft tissue compression between the 2 opposing osseous structures. The pain is due to repetitive microtrauma (ankle plantarflexion) or acute forced plantarflexion, which can present as an acute fracture of the os trigonum. Because soccer is a sport requiring both repetitive and extreme plantarflexion, it may predispose players to posterior ankle impingement (Figures 6A, 6B).62,66

Clinically, it can be very difficult to detect and diagnose because the affected structures lie deep and it can coexist with other disease processes (eg, peroneal tendinopathy, Achilles tendinopathy).62,66 Patients will complain of chronic deep posterior ankle pain that is worse with push-off activities (eg, jumping). On examination, patients will exhibit pain with palpation over the posterolateral process and with the crunch test. Lateral radiograph with the foot in plantar flexion will show the os trigonum impinged between the posterior tibial malleolus and the calcaneal tuberosity. An MRI will demonstrate the os trigonum as well as any associated inflammation and edema, while it can also demonstrate coexisting pathologies.
Initial treatment consists of rest, NSAIDs, and taping to prevent plantar flexion. Ultrasound-guided cortisone injection of the capsule and posterior bursa can be both therapeutic and diagnostic. A posterior injection can be used to temporize the symptoms so that the soccer player can make it through the season.
Surgical excision is saved for refractory cases, and this can be done either through an open posterolateral approach or arthroscopic techniques. Recently, Georgiannos and Bisbinas67 showed in an athletic population that endoscopic excision had both a lower complication rate and a quicker return to sports compared with the traditional open approach. Carreira and colleagues68 conducted a retrospective case series of 20 patients (mostly competitive athletes). They found that posterior ankle arthroscopy to address posterior impingement allowed for the maintenance or restoration of anatomic ROM of the ankle and hindfoot, ability to return to at least the previous level of activity, and improvement in objective assessment of pain relief and a higher level of function parameters.68
Continue to: TENDON PATHOLOGY
TENDON PATHOLOGY
SUPERIOR PERONEAL RETINACULUM INJURY
The superior peroneal retinaculum (SPR) forms the roof of the superior peroneal tunnel. The tunnel contains the peroneus brevis and longus tendons and is bordered by the retromalleolar groove of the fibula and the lower aspect of the posterior intramuscular septum of the leg.69,70 The SPR originates from the posterolateral ridge of the fibula and inserts onto the lateral calcaneus, and it is the primary restraint of the peroneal tendons within the retromalleolar sulcus.
Injury to the retinaculum results from both ankle dorsiflexion and inversion, and forceful reflex contraction of the peroneal muscles, which causes subluxation or dislocation of the contained tendons.69 A high level of suspicion is required regarding these injuries since the mechanism of injury is similar to that of a simple lateral ankle sprain. In the setting of retrofibular pain, snapping or popping sensations around the lateral malleolus, or chronic ankle instability that worsens on uneven surfaces, one must consider an injury to the SPR.69 Radiographs are not always diagnostic; however, occasionally on an internal rotation view, one may see a cortical avulsion off the distal tip of the lateral malleolus (“fleck sign”) indicating a rim fracture from an SPR injury (Figure 7). MRI is the best imaging modality to assess the peroneal tendons, as well as an SPR injury. Recently, ultrasound has grown in popularity and may be more useful, since it allows for dynamic evaluation of subluxating/dislocating tendons.69

Conservative management is often associated with poor outcomes, and surgery is indicated for all acute and chronic dislocations in athletes.71 Anatomic reconstruction of the SPR is the preferred surgical method.72 Peroneus brevis debulking and fibular groove deepening may also augment the retinaculum repair.73 van Dijk and colleagues in their systematic review showed that patients treated with both groove deepening and SPR repair have higher rates of return to the sport than patients treated with SPR repair alone.74
STRESS FRACTURES
FIFTH METATARSAL
Fifth metatarsal stress fractures usually occur secondary to lateral overload or avulsion of the peroneus brevis. The fifth metatarsal base can be susceptible to injury in a cavovarus foot. Non-operative treatment typically requires a longer period of immobilization (boot or cast) and necessitates a longer period of non–weight-bearing (anywhere between 6-12 weeks). Therefore, surgery is typically recommended in athletes or in the setting of a recurrent base of the fifth metatarsal fracture to expedite healing and return to play. Return to play is still not recommended until there is evidence of radiographic healing of the fracture. There are certain distinctions with fifth metatarsal stress fractures regarding location and healing rates that need to be taken into account.75,76 In particular, zone 2 injuries (Jones fractures) represent a vascular watershed area, making these fractures prone to nonunion with nonunion rates as high as 15% to 30%. Occasionally, the cavovarus deformity will require correction as well as a reduction in the risk of recurrence or nonunion. Surgical fixation most commonly consists of a single screw placed in an antegrade fashion.77 One must pay attention to screw size since smaller diameter screws (<4.5 mm) are associated with delayed union or nonunion. Moreover, screws that are too long will straighten the curved metatarsal shaft and can lead to fracture distraction or malreduction (Figures 8A, 8B).77

Patients have returned to competitive sports within 6 weeks; however, it should be noted that causes of failure were linked to early return and return to sports before a radiographic union can lead to failure of fixation. Ekstrand and van Dijk78 studied a large group of professional soccer players and found that out of 13,754 injuries, 0.5% (67) were fifth metatarsal fractures. Of note, they found that 45% of players had prodromal symptoms. Furthermore, after surgical treatment the fractures healed faster, compared with conservative treatment (75% vs 33%); however, there was no significant difference in lay-off days between both groups (80 vs 74 days).78 Matsuda and colleagues79 looked at 335 male collegiate soccer players, 29 of whom had a history of a fifth metatarsal stress fracture. They found that playing the midfield position and having an everted rearfoot and inverted forefoot alignment were associated with fifth metatarsal stress fractures.79 Saita and colleagues80 found that restricted hip internal rotation was associated with an increased risk of developing a Jones fracture in 162 professional football players. Finally, Fujitaka and colleagues81 looked at 273 male soccer players between 2005 and 2013. They found an association between weak toe-grip strength and fifth metatarsal fractures, suggesting that weak toe-grip may lead to an increase in the load applied onto the lateral side of the foot, resulting in a stress fracture.81
Continue to: NAVICULAR
NAVICULAR
Another common tarsal bone that sustains stress fractures is the navicular. It is not as common as calcaneal stress fractures in military recruits but can occur in the same type of population, as well as explosive athletics such as sprinters and soccer players. It commonly presents with an indistinct vague achy pain with activity that improves with rest, and pain at the dorsum of the midfoot or along the medial longitudinal arch with activity. It can easily go undiagnosed for quite some time given the difficulty in visualizing the navicular with plain radiographs. Clinically, it is difficult to make the diagnosis, and therefore advanced imaging is necessary when the injury is suspected. Both MRI and CT scans can be used to understand the extent of the injury (Figures 9A-9C). In non-displaced stress fractures, conservative non-operative treatment is the appropriate treatment modality with a brief period of immobilization and non–weight-bearing;82 however, operative treatment is also considered in elite athletes. In either case, return to play is discouraged until there is evidence of radiographic healing. When a displacement is noted, or there is a delay in diagnosis, then operative treatment is recommended.

CONCLUSION
Ankle injuries are very common in soccer and can result in decreased performance or significant loss of playing time. Treatment of acute injury generally follows a conservative route, with surgical intervention reserved for severe ruptures or osteochondral fracture of the ankle joint. Chronic ankle pathology resulting in mechanical or functional instability generally requires surgery to repair ligamentous damage and restore normal ankle kinematics. It is critical for the soccer player to receive appropriate rehabilitation prior to returning to play in order to reduce the risk for reinjury and further chronic instability. Prevention and early intervention of ankle injuries is key in preventing the long-term sequelae of ankle injuries, such as arthritis, in former soccer players.
ABSTRACT
The ankle is one of the most commonly injured joints in soccer and represents a significant cost to the healthcare system. The ligaments that stabilize the ankle joint determine its biomechanics—alterations of which result from various soccer-related injuries. Acute sprains are among the most common injury in soccer players and are generally treated conservatively, with emphasis placed on secondary prevention to reduce the risk for future sprains and progression to chronic ankle instability. Repetitive ankle injuries in soccer players may cause chronic ankle instability, which includes both mechanical ligamentous laxity and functional changes. Chronic ankle pathology often requires surgery to repair ligamentous damage and remove soft-tissue or osseous impingement. Proper initial treatment, rehabilitation, and secondary prevention of ankle injuries can limit the amount of time lost from play and avoid negative long-term sequelae (eg, osteochondral lesions, arthritis). On the other hand, high ankle sprains portend a poorer prognosis and a longer recovery. These injuries will typically require surgical stabilization. Impingement-like syndromes of the ankle can undergo an initial trial of conservative treatment; when this fails, however, soccer players respond favorably to arthroscopic debridement of the lesions causing impingement. Finally, other pathologies (eg, stress fractures) are highly encouraged to be treated with surgical stabilization in elite soccer players.
Continue to: EPIDEMIOLOGY
EPIDEMIOLOGY
With roughly 200,000 professional and around 240 million amateur soccer players, soccer has been recognized as the most popular sport worldwide. Nevertheless, given its rising popularity in society, one must also consider the increasing incidence of injuries as a result. Elite soccer players sustain between 10 and 35 injuries per 1000 competitive playing hours.1 Approximately 80% are traumatic, and 20% are overuse injuries.2 Soccer injuries are more frequent with increasing age of the participants, whereas the incidence of injuries in preadolescent players is low. The incidence of injuries has been found to be higher during competition when compared with practice/training sessions, with some studies showing that 59% of injuries occurred during games.2 Amateur or recreational soccer players sustain fewer injuries than professional soccer players, as one would expect, given both the higher intensity of training and match schedule in professionals.
The ankle is one of the most commonly injured joints in soccer, with some studies suggesting it comprises one-fifth of all injuries sustained during soccer, which is only second to those of the knee.2 Ankle sprains specifically are quite a common occurrence in soccer.3-9 A recent study of an English premier league club showed that over a 4-season period, 20% of injuries were of the foot and ankle with a mean return to sport time of 54 days.10 Of all foot and ankle related injuries, ankle sprains are the most common, followed by bruises/contusions, and tendon lesions. Fractures are very rare (1%) in soccer, but when they do occur they impart a much more extended recovery. During the 2010 Fédération Internationale de Football Association (FIFA) World Cup, ankle sprains were among the most common injuries and approximately half lead to players missing training or competitive matches.5
ANATOMY
Knowledge of the biomechanics of both the foot and ankle joints is essential to understand soccer injuries. The ankle joint (talocrural articulation) consists of the distal ends of the tibia and fibula, which form the mortise, and the superior aspect of the talar dome.11 As a hinge joint, the ankle provides 20° of dorsiflexion and 50° of plantar flexion,12 with stability provided by the lateral, medial, and superior ligamentous complexes. The superior articular surface of the talus is narrower posteriorly, which creates a looser fit within the mortise during plantar flexion.11 This decreased stability could help explain why the most common injury in soccer involves a plantar flexion mechanism.13,14 Inferiorly, the talus articulates with the calcaneus to form the subtalar joint. It is at this site that the majority of both foot inversion and eversion occurs. The transverse tarsal joints (Chopart’s joints) separate the hindfoot from the midfoot. Movement of this joint depends on the relative alignment of its 2 articulations: the talonavicular and calcaneocuboid joints. During foot eversion, these 2 joints are parallel to each other allowing supple motion and aiding in shock absorption during the heel strike phase of the gait cycle. With foot inversion, the joints become nonparallel and thus lock the transverse tarsal joints providing a rigid lever needed for push-off.11,12
LATERAL LIGAMENTS
The ankle joint is stabilized laterally by a ligament complex consisting of 3 individual ligaments, all originating from the lateral malleolus: the anterior talofibular ligament (ATFL), the posterior talofibular ligament (PTFL), and the calcaneofibular ligament (CFL) (Figure 1).11,12,15 The ATFL is the primary restraint to inversion in plantar flexion, and it helps resist anterolateral translation of the talus in the mortise. However, it is the weakest and therefore the most frequently injured of the lateral ligaments. The PTFL plays only a supplementary role in ankle stability when the lateral ligament complex is intact. It is under the greatest strain in ankle dorsiflexion and acts to limit posterior talar displacement within the mortise as well as talar external rotation.13,16 The CFL is the primary restraint to inversion in the neutral or dorsiflexed position. It restrains subtalar inversion, thereby limiting talar tilt within the mortise.

DELTOID LIGAMENT
The deltoid ligament complex consists of 6 continuous adjacent superficial and deep ligaments that function synergistically to resist valgus and pronation forces, as well as external rotation of the talus in the mortise.11-13,17 The superficial layer crosses both ankle and subtalar joints. It originates from the anterior colliculus and fans out to insert into the navicular, neck of the talus, sustentaculum tali, and posteromedial talar tubercle. The tibiocalcaneal (sustentaculum tali) portion is the strongest component in the superficial layer and resists calcaneal eversion. The deep layer crosses the ankle joint only. It functions as the primary stabilizer of the medial ankle and prevents both lateral displacement and external rotation of the talus. It originates from the inferior and posterior aspects of the medial malleolus and inserts on the medial and posteromedial aspects of the talus.12,17,18
Continue to: SYNDESMOSIS
SYNDESMOSIS
The ankle syndesmosis, or inferior tibiofibular joint, is the distal articulation between the tibia and fibula. The syndesmosis contributes to ankle mortise integrity through its firm fixation of the lateral malleolus against the lateral surface of the talus. Ligaments comprising the ankle syndesmosis include the anterior inferior tibiofibular ligament (AITFL), the posterior inferior tibiofibular ligament (PITFL), the inferior transverse ligament, and the interosseous ligament (IOL).12
ANKLE SPRAINS
Ankle sprains are the most common pathology encountered amongst soccer players, representing from one-half to two-thirds of all ankle related injuries. Most sprains occur outside of player contact.
LATERAL ANKLE SPRAINS AND INSTABILITY
Injury to the lateral ligaments of the ankle represents 77% to 91% of all ankle sprains in soccer.6,19 The greatest risk factor for an ankle sprain in a soccer player is a history of prior sprain.20 Other risk factors include increasing age, player-to-player contact, condition of the pitch, weight-bearing status of the injured limb at the time of injury, and joint instability or laxity.21,22
The evaluation of an ankle sprain to determine its severity is best done after the acute phase, approximately 4 to 7 days after the initial injury when both pain and swelling have subsided.23 The anterior drawer (ATFL instability) and talar tilt (CFL instability) tests are useful in evaluating ankle instability in the delayed or chronic setting; however, both have been shown to have limited sensitivity and significant variability amongst different examiners.24
Clinical examination will direct further diagnostic tests including X-rays, magnetic resonance imaging (MRI), and computed tomography (CT). The Ottawa ankle rules are generally helpful in determining whether plain X-rays are indicated in the acute setting.25,26 (Figure 2) According to these rules, ankle radiographs should be obtained if ankle pain is reported near the malleoli and 1 or more of the following is seen during examination: inability to bear weight immediately after injury and for 4 steps in the emergency department, and bony tenderness at the posterior edge or tip of the malleolus. Stress X-rays are generally not indicated in acute injuries but may be useful in chronic ankle instability cases.23

Continue to: Ankle sprains cover...
Ankle sprains cover a broad spectrum of injuries; therefore, a grading system was devised to aid in guiding treatment. Grade I (mild) sprains are those with minimal swelling and tenderness but have the ligaments still intact. Grade II (moderate) sprains occur when there are partial ligament tears associated with moderate pain, swelling, and tenderness. Finally, Grade III (severe) sprains are complete ligament tears with marked swelling, hemorrhage, tenderness, loss of function, and abnormal joint motion and instability.23, 24
Initial treatment for all ankle sprains is nonoperative and involves the RICE (rest, ice, compression, elevation) protocol with the use of nonsteroidal anti-inflammatory drugs (NSAIDs) during the acute phase (first 4-5 days) with a short period (no >2 weeks) of immobilization.27 Most authors agree that early mobilization followed by phased rehabilitation is warranted to minimize time away from sports.28-31 Prolonged immobilization (>2 weeks) has detrimental effects and may lead to a longer return to play.28-31 The rehabilitation protocol is divided into stages: (1) pain and edema control, (2) range of motion (ROM) and strengthening exercises, (3) soccer specific functional training, and (4) prophylactic intervention with balance and proprioception exercises. Surgical intervention is rarely indicated for acute ankle sprains. There are exceptions, however, such as when ankle sprains are associated with other injuries that require acute intervention (eg, fracture, osteochondral lesion). Surgery is indicated in the setting of chronic, recurrent mechanical instability. Anatomical repairs (modified Brostrom) seem to produce better outcomes than non-anatomical reconstructions (eg, Chrisman-Snook). Surgical outcomes are good, and most athletes are able to return to their pre-injury level of function.32
In athletes, prevention of recurrent sprains is key. Braces may help prevent ankle sprains and bracing has been shown to be superior to taping, as tape loses its restrictive properties within 20 to 30 minutes of initiating activity.33,34 Application of an orthosis (lace-up ankle orthosis) has been shown to reduce the incidence of ankle re-injury in soccer players with previous ankle sprains. Several studies have found minimal, if any, effect of orthoses on athletic performance.20,35,36 Low-profile braces for soccer have been developed which allow for minimal disruption of the player’s boot and space proximally to insert the shin guard. Another essential component of prevention is prophylactic intervention with balance and proprioceptive exercises. A study looking at first division men’s league football (soccer) players in Iran showed a significant decrease in re-injury rates with proprioceptive training.37 In 2003, FIFA introduced a comprehensive warm-up program (FIFA 11+), which has since been shown in several studies to decrease the risk of injury in amateur soccer players.38-40
MEDIAL ANKLE SPRAINS AND INSTABILITY
Soccer places an unusually high demand on both the medial foot and ankle structures when compared with other sports. For instance, striking the ball requires the player to abduct and externally rotate the foot, which preloads medial structures.9 Hintermann18 looked at 54 cases of medial ankle instability and found that injury commonly occurred during landing on an uneven surface, which applies to soccer players when landing after heading the ball or jumping over a tackle. Pronation with eversion and extreme rotational injuries are well known to cause deltoid ligament injury. However, complete rupture of the deltoid ligament is rare and is more often associated with ankle fractures.41 Due to its close proximity and similarly shared function in medial plantar arch stabilization with the tibiospring and spring ligaments, posterior tibialis tendon dysfunction is also frequently seen in medial ankle instability.17 After an acute injury, patients can present with a medial ankle hematoma and pain along the deltoid ligament. Although chronic insufficiency is diagnosed based on the feeling of “giving way,” pain in the medial gutter of the ankle and a valgus and pronation deformity of the foot can be corrected by activating the peroneus tertius muscle. Arthroscopy is the most specific way to confirm clinically suspected instability of the medial ankle; however, MRI can demonstrate loss of organized medial fibers (Figures 3A, 3B).18 Primary surgical repair of deltoid ligament tears yield good to excellent results and should be considered in the soccer player to prevent problems associated with chronic non-repaired tears such as instability, osteoarthritis, and impingement syndromes.18 After surgical repair, players will undergo extensive physical therapy that progresses to sport-specific exercises with the ultimate goal of returning to competitive play around 4-6 months post-operatively.

HIGH ANKLE SPRAINS (SYNDESMOSIS)
High ankle sprains are much less common than low ankle sprains; however, when they do occur they portend a lengthier rehabilitation and a poorer prognosis, especially if undiagnosed. Lubberts and colleagues42 studied the epidemiology of isolated syndesmotic injuries in professional football players. They pooled data from 15 consecutive seasons of European professional football between 2001 and 2016. They examined a total of 3677 players from 61 teams across 17 countries. There were 1320 ankle ligament injuries registered during 15 seasons, of which 94 (7%) were isolated syndesmotic injuries. The incidence of these injuries increased annually between 2001 and 2016. Injuries were 74% contact-related, and isolated syndesmotic injuries were followed by a mean of a 39-day absence.42 Moreover, football players may have an increased risk of syndesmotic sprains due to foot planting and cutting action.41
Continue to: These injuriesa are typically...
These injuries are typically identified with pain over the AITFL and interosseous membrane. Physical examination tests that help identify syndesmotic injuries include the squeeze test, external rotation test, and crossed-leg test.41 The diagnosis can be made on plain X-ray when there is clear diastasis between the distal tibia and fibula. Two critical measurements on plain films are made 1 cm above the tibial plafond and are used to evaluate the integrity of the syndesmosis: tibiofibular clear space >6 mm, and tibiofibular overlap <1 mm, which indicate disruption of the syndesmosis.43 More subtle injuries can be diagnosed with better sensitivity and specificity using MRI, which can also reveal secondary findings such as bone bruises, ATFL injury, osteochondral lesions, and tibiofibular incongruity.44,45 Arthroscopy is an invaluable diagnostic tool for syndesmotic injuries with a characteristic triad finding of PITFL scarring, disrupted interosseous ligament, and posterolateral tibial plafond chondral damage.46
Classification of the ligaments involved can aid in the selection of appropriate treatment. Grade I injuries involve AITFL tears. Grade IIa injuries involve AITFL and IOL tears. Grade IIb injuries include AITFL, PITFL, and IOL tears. Grade III injuries involve injury to all 3 ligaments, as well as a fibular fracture. Conservative treatment is recommended for Grades I and IIa, while surgical intervention is necessary for Grades IIb and III (Figures 4A, 4B). Compared with other ankle sprains, syndesmotic injuries typically require a more prolonged recovery/rehabilitation. Some studies suggest that these injuries require twice as long to heal.47 Hopkinson and colleagues48 reported a mean recovery time of 55 days following syndesmotic injuries in cadets at the United States Military Academy at West Point. Some surgeons advocate surgical intervention in professional athletes with mild sprains to expedite return to play.49

Surgery has been well established as necessary in more severe injuries where there is clear diastasis or instability of the syndesmosis. Traditionally, screws were used for surgical fixation; however, they often required a second surgery for subsequent removal. There is no general consensus on the optimal screw size, level of placement, or timing of removal.50,51 More recently, non-absorbable suture button fixation (eg, TightRope; Arthrex) has become more popular and provides certain advantages over screw fixation, such as avoiding the need for hardware removal. TightRope has been shown to provide more accurate stabilization of the syndesmosis as compared with screw fixation.52 Since malreduction is the most important indicator of poor long-term functional outcome, suture button fixation should be considered in the treatment of the football player.53 Finally, Colcuc and colleagues54 reported a lower complication rate and earlier return to sports in patients treated with knotless suture button devices compared with screw fixation.
OSTEOCHONDRAL LESIONS
Osteochondral lesions (OCLs) are cartilage-bone defects that are usually located in the talus. They can be caused by an acute traumatic event or repetitive microtrauma with no apparent history of trauma (eg, ankle instability). Acute OCLs can occur in soccer secondary to an ankle sprain or ankle fracture. Symptoms of OCLs include pain, swelling, and mechanical symptoms such as catching or locking, and on physical examination, one might see an effusion. The initial imaging modality of choice is radiographing; however, in ankle sprains with continued pain and swelling MRI may be indicated to rule out an underlying OCL. Missed acute lesions have a tendency not to heal and become chronic lesions, which can cause pain and playing disability. It is well established that chronic ankle instability is an important etiologic factor for OCLs. With the normal hydrostatic pressure within the ankle joint, synovial fluid gets pushed into cartilage/bone fissures, which can then lead to cystic degeneration of the subchondral bone.55-57
Surgical repair of an OCL is dependent on both the size and location of the lesion. Acute lesions can be managed by arthroscopic débridement, microfracture, or fixation of the lesion if enough bone remains attached to the chondral lesion. Return to play is based on development and maturation of fibrocartilage over the lesion (debridement/microfracture) or healing and incorporation of the new graft (chondral repair procedures). Meanwhile, chronic lesions can be managed in 1-stage (microfracture, osteochondral autograft transfer or 2-stage (autologous chondrocyte implantation [ACI]) procedures.56-57 Additional biologic healing augmentation with platelet-rich plasma has been described as well.58 Newer techniques in treating chronic talus OCLs, including ones that have failed to respond to bone marrow stimulation techniques, have been developed more recently such as the use of particulated juvenile articular cartilage allograft (DeNovo NT Natural Tissue Graft®; Zimmer Biomet).59 These newer techniques avoid the need for a 2-stage procedure, as is the case with ACI.
Continue to: Further studies are needed...
Further studies are needed to both investigate long-term outcomes and determine the superiority of the arthroscopic juvenile cartilage procedure compared with microfracture and other cartilage resurfacing procedures. When surgically treating OCLs, one must also restore normal ankle joint biomechanics for the lesion to heal. For instance, in the presence of ankle instability, ligament reconstruction must be performed. Also, one should also consider addressing any hindfoot malalignment with an osteotomy (calcaneus, supramalleolar). In a recent retrospective study, van Eekeren and colleagues60 showed that approximately 76% of patients were able to return to sports at long-term follow-up after arthroscopic débridement and bone marrow stimulation of talar OCLs. However, the activity level decreased at long-term follow-up and never attained the pre-injury level.60
ANKLE IMPINGEMENT
ANTERIOR ANKLE IMPINGEMENT (FOOTBALLER'S ANKLE)
Anterior ankle impingement is caused by anterior osteophytes on both the distal tibia and talar neck. It is thought to be related to repetitive microtrauma to the anteromedial aspect of the ankle from recurrent ball impact.61 It is very common amongst soccer players with some studies suggesting that 60% of soccer players have this syndrome. Ankle impingement is characterized by anterior pain with ankle dorsiflexion, decreased dorsiflexion, and swelling. It is primarily diagnosed with lateral ankle X-rays, which will show the osteophytes. An oblique anteromedial X-ray may increase detection of osteophytes (Figure 5). The early stages of anterior impingement can be treated successfully with injections and heel lifts. Treatment of lesions that fail to respond to conservative management involves arthroscopic or open excision of osteophytes. Most patients with no preexisting osteoarthritis treated arthroscopically will experience pain relief and return to full activity, though recurrent osteophyte formation has been noted at long-term follow-up.62

Anterior ankle impingement is most often caused by acute ankle sprains with an inversion type of mechanism.62 The subsequent reactive inflammation can cause fibrosis leading to distal fascicle enlargement of the AITFL. Impingement in the anterolateral gutter of this enlarged fascicle can also cause both chronic reactive synovitis and chondromalacia of the lateral talar dome.63 MRI can identify abnormal areas of pathology; however, 50% of cases are diagnosed based on clinical examination alone.63 Patients generally present with a history of anterolateral ankle pain and swelling with an occasional popping or snapping sensation.
Soccer players commonly develop anterior bony impingement due to repetitive loading of the anterior ankle from striking the ball. This repetition can lead to osteophyte formation of the anterior distal tibia and talar neck. After the osteophytes form, decreased dorsiflexion can occur due to a mechanical stop and inflammation of the interposed capsule.
The patient will exhibit tenderness to palpation along the anterolateral aspect of the ankle, with pain elicited at extreme passive dorsiflexion.62 Initially, an injection with local anesthetic and corticosteroid can serve both a diagnostic and therapeutic purpose; however, patients who fail conservative treatment can be treated with arthroscopy and resection of the involved scar tissue and osteophytes. The best results are seen in those patients with no concurrent intra-articular lesions or ankle osteoarthritis (Figure 5).62 When treated non-operatively, a player may return to play when pain resolves; however, if treated surgically with arthroscopic debridement/resection, a player must wait until his surgical scars have healed prior to attempting return to play.
Continue to: ANTEROMEDIAL ANKLE IMPINGEMENT
ANTEROMEDIAL ANKLE IMPINGEMENT
Anteromedial ankle impingement is a less common ankle impingement syndrome. It is associated with eversion injuries or following medial malleolar or talar fractures.64,65 Previous injury to the anterior tibiotalar fascicle of the deltoid complex leads to ligament thickening and subsequent impingement in the anteromedial corner of the talus. Adjacent fibrosis and synovitis are common consequences of impingement; however, osteophyte formation and chondral stripping along the anteromedial talus can also be seen. Patients typically complain of pain along the anteromedial joint line that is worse with activity, clicking or popping sensations, and painful, limited dorsiflexion. On examination, impingement can be detected through palpation over the anterior tibiotalar fascicle of the deltoid ligament and eversion or extreme passive dorsiflexion of the foot, all of which will elicit medial ankle tenderness.17,62 Initial treatment consists of rest, physical therapy, and NSAIDs. Refractory cases may be amenable to arthroscopic or open resection of the anterior tibiotalar fascicle with débridement of any adjacent synovitis and scar tissue.62
POSTERIOR ANKLE IMPINGEMENT
Posterior ankle impingement is often referred to as “os trigonum syndrome” since the posterior impingement is frequently associated with a prominent os trigonum. An os trigonum is an accessory ossicle representing the separated posterolateral tubercle of the talus. It is usually asymptomatic. However, in soccer players, pain can occur from impaction between the posterior tibial plafond and the os trigonum, or because of soft tissue compression between the 2 opposing osseous structures. The pain is due to repetitive microtrauma (ankle plantarflexion) or acute forced plantarflexion, which can present as an acute fracture of the os trigonum. Because soccer is a sport requiring both repetitive and extreme plantarflexion, it may predispose players to posterior ankle impingement (Figures 6A, 6B).62,66

Clinically, it can be very difficult to detect and diagnose because the affected structures lie deep and it can coexist with other disease processes (eg, peroneal tendinopathy, Achilles tendinopathy).62,66 Patients will complain of chronic deep posterior ankle pain that is worse with push-off activities (eg, jumping). On examination, patients will exhibit pain with palpation over the posterolateral process and with the crunch test. Lateral radiograph with the foot in plantar flexion will show the os trigonum impinged between the posterior tibial malleolus and the calcaneal tuberosity. An MRI will demonstrate the os trigonum as well as any associated inflammation and edema, while it can also demonstrate coexisting pathologies.
Initial treatment consists of rest, NSAIDs, and taping to prevent plantar flexion. Ultrasound-guided cortisone injection of the capsule and posterior bursa can be both therapeutic and diagnostic. A posterior injection can be used to temporize the symptoms so that the soccer player can make it through the season.
Surgical excision is saved for refractory cases, and this can be done either through an open posterolateral approach or arthroscopic techniques. Recently, Georgiannos and Bisbinas67 showed in an athletic population that endoscopic excision had both a lower complication rate and a quicker return to sports compared with the traditional open approach. Carreira and colleagues68 conducted a retrospective case series of 20 patients (mostly competitive athletes). They found that posterior ankle arthroscopy to address posterior impingement allowed for the maintenance or restoration of anatomic ROM of the ankle and hindfoot, ability to return to at least the previous level of activity, and improvement in objective assessment of pain relief and a higher level of function parameters.68
Continue to: TENDON PATHOLOGY
TENDON PATHOLOGY
SUPERIOR PERONEAL RETINACULUM INJURY
The superior peroneal retinaculum (SPR) forms the roof of the superior peroneal tunnel. The tunnel contains the peroneus brevis and longus tendons and is bordered by the retromalleolar groove of the fibula and the lower aspect of the posterior intramuscular septum of the leg.69,70 The SPR originates from the posterolateral ridge of the fibula and inserts onto the lateral calcaneus, and it is the primary restraint of the peroneal tendons within the retromalleolar sulcus.
Injury to the retinaculum results from both ankle dorsiflexion and inversion, and forceful reflex contraction of the peroneal muscles, which causes subluxation or dislocation of the contained tendons.69 A high level of suspicion is required regarding these injuries since the mechanism of injury is similar to that of a simple lateral ankle sprain. In the setting of retrofibular pain, snapping or popping sensations around the lateral malleolus, or chronic ankle instability that worsens on uneven surfaces, one must consider an injury to the SPR.69 Radiographs are not always diagnostic; however, occasionally on an internal rotation view, one may see a cortical avulsion off the distal tip of the lateral malleolus (“fleck sign”) indicating a rim fracture from an SPR injury (Figure 7). MRI is the best imaging modality to assess the peroneal tendons, as well as an SPR injury. Recently, ultrasound has grown in popularity and may be more useful, since it allows for dynamic evaluation of subluxating/dislocating tendons.69

Conservative management is often associated with poor outcomes, and surgery is indicated for all acute and chronic dislocations in athletes.71 Anatomic reconstruction of the SPR is the preferred surgical method.72 Peroneus brevis debulking and fibular groove deepening may also augment the retinaculum repair.73 van Dijk and colleagues in their systematic review showed that patients treated with both groove deepening and SPR repair have higher rates of return to the sport than patients treated with SPR repair alone.74
STRESS FRACTURES
FIFTH METATARSAL
Fifth metatarsal stress fractures usually occur secondary to lateral overload or avulsion of the peroneus brevis. The fifth metatarsal base can be susceptible to injury in a cavovarus foot. Non-operative treatment typically requires a longer period of immobilization (boot or cast) and necessitates a longer period of non–weight-bearing (anywhere between 6-12 weeks). Therefore, surgery is typically recommended in athletes or in the setting of a recurrent base of the fifth metatarsal fracture to expedite healing and return to play. Return to play is still not recommended until there is evidence of radiographic healing of the fracture. There are certain distinctions with fifth metatarsal stress fractures regarding location and healing rates that need to be taken into account.75,76 In particular, zone 2 injuries (Jones fractures) represent a vascular watershed area, making these fractures prone to nonunion with nonunion rates as high as 15% to 30%. Occasionally, the cavovarus deformity will require correction as well as a reduction in the risk of recurrence or nonunion. Surgical fixation most commonly consists of a single screw placed in an antegrade fashion.77 One must pay attention to screw size since smaller diameter screws (<4.5 mm) are associated with delayed union or nonunion. Moreover, screws that are too long will straighten the curved metatarsal shaft and can lead to fracture distraction or malreduction (Figures 8A, 8B).77

Patients have returned to competitive sports within 6 weeks; however, it should be noted that causes of failure were linked to early return and return to sports before a radiographic union can lead to failure of fixation. Ekstrand and van Dijk78 studied a large group of professional soccer players and found that out of 13,754 injuries, 0.5% (67) were fifth metatarsal fractures. Of note, they found that 45% of players had prodromal symptoms. Furthermore, after surgical treatment the fractures healed faster, compared with conservative treatment (75% vs 33%); however, there was no significant difference in lay-off days between both groups (80 vs 74 days).78 Matsuda and colleagues79 looked at 335 male collegiate soccer players, 29 of whom had a history of a fifth metatarsal stress fracture. They found that playing the midfield position and having an everted rearfoot and inverted forefoot alignment were associated with fifth metatarsal stress fractures.79 Saita and colleagues80 found that restricted hip internal rotation was associated with an increased risk of developing a Jones fracture in 162 professional football players. Finally, Fujitaka and colleagues81 looked at 273 male soccer players between 2005 and 2013. They found an association between weak toe-grip strength and fifth metatarsal fractures, suggesting that weak toe-grip may lead to an increase in the load applied onto the lateral side of the foot, resulting in a stress fracture.81
Continue to: NAVICULAR
NAVICULAR
Another common tarsal bone that sustains stress fractures is the navicular. It is not as common as calcaneal stress fractures in military recruits but can occur in the same type of population, as well as explosive athletics such as sprinters and soccer players. It commonly presents with an indistinct vague achy pain with activity that improves with rest, and pain at the dorsum of the midfoot or along the medial longitudinal arch with activity. It can easily go undiagnosed for quite some time given the difficulty in visualizing the navicular with plain radiographs. Clinically, it is difficult to make the diagnosis, and therefore advanced imaging is necessary when the injury is suspected. Both MRI and CT scans can be used to understand the extent of the injury (Figures 9A-9C). In non-displaced stress fractures, conservative non-operative treatment is the appropriate treatment modality with a brief period of immobilization and non–weight-bearing;82 however, operative treatment is also considered in elite athletes. In either case, return to play is discouraged until there is evidence of radiographic healing. When a displacement is noted, or there is a delay in diagnosis, then operative treatment is recommended.

CONCLUSION
Ankle injuries are very common in soccer and can result in decreased performance or significant loss of playing time. Treatment of acute injury generally follows a conservative route, with surgical intervention reserved for severe ruptures or osteochondral fracture of the ankle joint. Chronic ankle pathology resulting in mechanical or functional instability generally requires surgery to repair ligamentous damage and restore normal ankle kinematics. It is critical for the soccer player to receive appropriate rehabilitation prior to returning to play in order to reduce the risk for reinjury and further chronic instability. Prevention and early intervention of ankle injuries is key in preventing the long-term sequelae of ankle injuries, such as arthritis, in former soccer players.
1. Dvorak J, Junge A. Football injuries and physical symptoms. A review of the literature. Am J Sports Med. 2000;28(5 Suppl):S3-S9.
2. Chomiak J, Junge A, Peterson L, Dvorak J. Severe injuries in football players. Am J Sports Med. 2000;28(5 Suppl):S58-S68.
3. Cloke DJ, Ansell P, Avery P, Deehan D. Ankle injuries in football academies: a three-centre prospective study. Br J Sports Med. 2011;45(9):702-708. doi:10.1136/bjsm.2009.067900.
4. Cloke DJ, Spencer S, Hodson A, Deehan D. The epidemiology of ankle injuries occurring in English Football Association academies. Br J Sports Med. 2009;43(14):1119-1125. doi:10.1136/bjsm.2008.052050.
5. Dvorak J, Junge A, Derman W, Schwellnus M. Injuries and illnesses of football players during the 2010 FIFA World Cup. Br J Sports Med. 2011;45(8):626-630. doi:10.1136/bjsm.2010.079905.
6. Ekstrand J, Gillquist J. Soccer injuries and their mechanisms: a prospective study. Med Sci Sports Exerc. 1983;15(3):267-270.
7. Fousekis K, Tsepis E, Vagenas G. Intrinsic risk factors of noncontact ankle sprains in soccer: a prospective study on 100 professional players. Am J Sports Med. 2012;40(8):1842-1850. doi:10.1177/0363546512449602.
8. Gaulrapp H, Becker A, Walther M, Hess H. Injuries in women’s soccer: a 1-year all players prospective field study of the women’s Bundesliga (German premiere league). Clin J Sports Med. 2010;20(4):264-271. doi:10.1097/JSM.0b013e3181e78e33.
9. Morgan BE, Oberlander MA. An examination of injuries in major league soccer. The inaugural season. Am J Sports Med. 2001;29(4):426-430. doi:10.1177/03635465010290040701.
10. Jain N, Murray D, Kemp S, Calder J. Frequency and trends in foot and ankle injuries within an English Premier League Football Club using a new impact factor of injury to identify a focus for injury prevention. Foot Ankle Surg. 2014;20(4):237-240. doi:10.1016/j.fas.2014.05.004.
11. Moore KL, Dalley AF, Agur AMR. Clinically Oriented Anatomy. 6th ed. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins, 2010:xxix, 1134.
12. Thompson JC, Netter FH. Netter’s Concise Orthopaedic Anatomy. 2nd ed. Philadelphia, PA: Saunders Elsevier, 2010:x, 404.
13. Giza E, Mandelbaum B. Chronic footballer’s ankle. In: Football Traumatology. Springer Milan, 2006:333-351.
14. Garrick JG. The frequency of injury, mechanism of injury, and epidemiology of ankle sprains. Am J Sports Med. 1977:5(6):241-242. doi:10.1177/036354657700500606.
15. Agur AMR, Grant JCB. Grant’s Atlas of Anatomy. 13th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins. 2011.
16. Renstrom PA, Konradsen L. Ankle ligament injuries. Br J Sports Med. 1997;31(1):11-20.
17. Chhabra A, Subhawong TK, Carrino JA. MR imaging of deltoid ligament pathologic findings and associated impingement syndromes. Radiographics. 2010;30(3):751-761. doi:10.1148/rg.303095756.
18. Hintermann B. Medial ankle instability. Foot Ankle Clin. 2003;8(4):723-738.
19. Woods C, Hawkins R, Hulse M, Hodson A. The Football Association Medical Research Programme: an audit of injuries in professional football: an analysis of ankle sprains. Br J Sports Med. 2003;37(3):233-238.
20. Thacker SB, Stroup DF, Branche CM, Gilchrist J, Goodman RA, Weitman EA. The prevention of ankle sprains in sports. A systematic review of the literature. Am J Sports Med. 1999;27(6):753-760. doi:10.1177/03635465990270061201.
21. Giza E, Fuller C, Junge A, Dvorak J. Mechanisms of foot and ankle injuries in soccer. Am J Sports Med. 2003;31(4):550-554. doi:10.1177/03635465030310041201.
22. Tucker AM. Common soccer injuries. Diagnosis, treatment and rehabilitation. Sports Med. 1997;23(1):21-32.
23. Lynch SA, Renstrom PA. Treatment of acute lateral ankle ligament rupture in the athlete. Conservative versus surgical treatment. Sports Med. 1999;27(1):61-71.
24. Chan KW, Ding BC, Mroczek KJ. Acute and chronic lateral ankle instability in the athlete. Bull NYU Hosp Jt Dis. 2011;69(1):17-26.
25. Stiell IG, Greenberg GH, McKnight RD, Nair RC, McDowell I, Worthington JR. A study to develop clinical decision rules for the use of radiography in acute ankle injuries. Ann Emerg Med. 1992;21(4):384-390.
26. Bachmann LM, Kolb E, Koller MT, Steurer J, ter Riet G. Accuracy of Ottawa ankle rules to exclude fractures of the ankle and mid-foot: systematic review. BMJ. 2003;326(7386):417. doi:10.1136/bmj.326.7386.417.
27. Balduini FC, Vegso JJ, Torg JS, Torg E. Management and rehabilitation of ligamentous injuries to the ankle. Sports Med. 1987;4(5):364-380.
28. Kerkhoffs GM, Rowe BH, Assendelft WJ, Kelly KD, Struijs PA, van Dijk CN. Immobilisation for acute ankle sprain. A systematic review. Arch Orthop Trauma Surg. 2001;121(8):462-471.
29. Konradsen L, Holmer P, Sondergaard L. Early mobilizing treatment for grade III ankle ligament injuries. Foot Ankle. 1991;12(2):69-73.
30. Eiff MP, Smith AT, Smith GE. Early mobilization versus immobilization in the treatment of lateral ankle sprains. Am J Sports Med. 1994;22(1):83-88. doi:10.1177/036354659402200115.
31. Shrier I. Treatment of lateral collateral ligament sprains of the ankle: a critical appraisal of the literature. Clin J Sport Med. 1995;5(3):187-195.
32. DiGiovanni BF, Partal G, Baumhauer JF. Acute ankle injury and chronic lateral instability in the athlete. Clin Sports Med. 2004;23(1):1-19, v. doi:10.1016/S0278-5919(03)00095-4.
33. Alt W, Lohrer H, Gollhofer A. Functional properties of adhesive ankle taping: neuromuscular and mechanical effects before and after exercise. Foot Ankle Int. 1999;20(4):238-245. doi:10.1177/107110079902000406.
34. Garrick JG, Requa RK. Role of external support in the prevention of ankle sprains. Med Sci Sports. 1973;5(3):200-203.
35. Sharpe SR, Knapik J, Jones B. Ankle braces effectively reduce recurrence of ankle sprains in female soccer players. J Athl Train. 1997;32(1):21-24.
36. Surve I, Schwellnus MP, Noakes T, Lombard C. A fivefold reduction in the incidence of recurrent ankle sprains in soccer players using the Sport-Stirrup orthosis. Am J Sports Med. 1994;22(5):601-606. doi:10.1177/036354659402200506.
37. Mohammadi F. Comparison of 3 preventive methods to reduce the recurrence of ankle inversion sprains in male soccer players. Am J Sports Med. 2007;35(6):922-926. doi:10.1177/0363546507299259.
38. Steffen K, Meeuwisse WH, Romiti M, et al. Evaluation of how different implementation strategies of an injury prevention programme (FIFA 11+) impact team adherence and injury risk in Canadian female youth football players: a cluster-randomised trial. Br J Sports Med. 2013;47(8):480-487. doi:10.1136/bjsports-2012-091887.
39. Steffen K, Emery CA, Romiti M, et al. High adherence to a neuromuscular injury prevention programme (FIFA 11+) improves functional balance and reduces injury risk in Canadian youth female football players: a cluster randomised trial. Br J Sports Med. 2013;47(12):794-802. doi: 10.1136/bjsports-2012-091886.
40. Junge A, Lamprecht M, Stamm H, et al. Countrywide campaign to prevent soccer injuries in Swiss amateur players. Am J Sports Med. 2011;39(1):57-63. doi:10.1177/0363546510377424.
41. Lin CF, Gross ML, Weinhold P. Ankle syndesmosis injuries: anatomy, biomechanics, mechanism of injury, and clinical guidelines for diagnosis and intervention. J Orthop Sports Phys Ther. 2006;36(6):372-384. doi:10.2519/jospt.2006.2195.
42. Lubberts B, D’Hooghe P, Bengtsson H, DiGiovanni CW, Calder J, Ekstrand J. Epidemiology and return to play following isolated syndesmotic injuries of the ankle: a prospective cohort study of 3677 male professional football players in the UEFA Elite Club Injury Study. Br J Sports Med. 2017. doi:10.1136/bjsports-2017-097710.
43. Harper MC, Keller TS. A radiographic evaluation of the tibiofibular syndesmosis. Foot Ankle. 1989;10(3):156-160.
44. Vogl TJ, Hochmuth K, Diebold T, et al. Magnetic resonance imaging in the diagnosis of acute injured distal tibiofibular syndesmosis. Invest Radiol. 1997;32(7):401-409.
45. Brown KW, Morrison WB, Schweitzer ME, Parellada JA, Nothnagel H. MRI findings associated with distal tibiofibular syndesmosis injury. AJR Am J Roentgenol. 2004;182(1):131-136. doi:10.2214/ajr.182.1.1820131.
46. Ogilvie-Harris DJ, Reed SC, Hedman TP. Disruption of the ankle syndesmosis: biomechanical study of the ligamentous restraints. Arthroscopy. 1994;10(5):558-560.
47. Boytim MJ, Fischer DA, Neumann L. Syndesmotic ankle sprains. Am J Sports Med. 1991;19(3):294-298. doi:10.1177/036354659101900315.
48. Hopkinson WJ, St Pierre P, Ryan JB, Wheeler JH. Syndesmosis sprains of the ankle. Foot Ankle. 1990;10(6):325-330. doi:10.1177/107110079001000607.
49. Del Buono A, Florio A, Boccanera MS, Maffulli N. Syndesmosis injuries of the ankle. Curr Rev Musculoskelet Med. 2013;6(4):313-319. doi:10.1007/s12178-013-9183-x.
50. Dattani R, Patnaik S, Kantak A, Srikanth B, Selvan TP. Injuries to the tibiofibular syndesmosis. J Bone Joint Surg Br. 2008;90(4):405-410. doi:10.1302/0301-620X.90B4.19750.
51. Schepers T. To retain or remove the syndesmotic screw: a review of literature. Arch Orthop Trauma Surg. 2011;131(7):879-883. doi:10.1007/s00402-010-1225-x.
52. Naqvi GA, Cunningham P, Lynch B, Galvin R, Awan N. Fixation of ankle syndesmotic injuries: comparison of tightrope fixation and syndesmotic screw fixation for accuracy of syndesmotic reduction. Am J Sports Med. 2012;40(12):2828-2835. doi:10.1177/0363546512461480.
53. Weening B, Bhandari M. Predictors of functional outcome following transsyndesmotic screw fixation of ankle fractures. J Orthop Trauma. 2005;19(2):102-108.
54. Colcuc C, Blank M, Stein T, et al. Lower complication rate and faster return to sports in patients with acute syndesmotic rupture treated with a new knotless suture button device. Knee Surg Sports Traumatol Arthrosc. 2017. doi:10.1007/s00167-017-4820-4823.
55. Savage-Elliott I, Ross KA, Smyth NA, Murawski CD, Kennedy JG. Osteochondral lesions of the talus: a current concepts review and evidence-based treatment paradigm. Foot Ankle Spec. 2014;7(5):414-422. doi:10.1177/1938640014543362.
56. Talusan PG, Milewski MD, Toy JO, Wall EJ. Osteochondritis dissecans of the talus: diagnosis and treatment in athletes. Clin Sports Med. 2014;33(2):267-284. doi:10.1016/j.csm.2014.01.003.
57. Murawski CD, Kennedy JG. Operative treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 2013;95(11):1045-1054. doi:10.2106/JBJS.L.00773.
58. Guney A, Akar M, Karaman I, Oner M, Guney B. Clinical outcomes of platelet rich plasma (PRP) as an adjunct to microfracture surgery in osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2015;23(8):2384-2389. doi:10.1007/s00167-013-2784-5.
59. Hatic SO, Berlet GC. Particulated juvenile articular cartilage graft (DeNovo NT Graft) for treatment of osteochondral lesions of the talus. Foot Ankle Spec. 2010;3(6):361-364. doi:10.1177/1938640010388602.
60. van Eekeren IC, van Bergen CJ, Sierevelt IN, Reilingh ML, van Dijk CN. Return to sports after arthroscopic debridement and bone marrow stimulation of osteochondral talar defects: a 5- to 24-year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1311-1315. doi:10.1007/s00167-016-3992-6.
61. Tol JL, Slim E, van Soest AJ, van Dijk CN. The relationship of the kicking action in soccer and anterior ankle impingement syndrome. A biomechanical analysis. Am J Sports Med. 2002;30(1):45-50. doi:10.1177/03635465020300012101.
62. Sanders TG, Rathur SK. Impingement syndromes of the ankle. Magn Reson Imaging Clin N Am. 2008;16(1):29-38. doi:10.1016/j.mric.2008.02.005.
63. Ogilvie-Harris DJ, Gilbart MK, Chorney K. Chronic pain following ankle sprains in athletes: the role of arthroscopic surgery. Arthroscopy. 1997;13(5):564-574.
64. Robinson P, White LM, Salonen D, Ogilvie-Harris D. Anteromedial impingement of the ankle: using MR arthrography to assess the anteromedial recess. AJR Am J Roentgenol. 2002;178(3):601-604. doi:10.2214/ajr.178.3.1780601.
65. Mosier-La Clair SM, Monroe MT, Manoli A. Medial impingement syndrome of the anterior tibiotalar fascicle of the deltoid ligament on the talus. Foot Ankle Int. 2000;21(5):385-391.
66. Maquirriain J. Posterior ankle impingement syndrome. J Am Acad Orthop Surg. 2005;13(6):365-371.
67. Georgiannos D, Bisbinas I. Endoscopic versus open excision of os trigonum for the treatment of posterior ankle impingement syndrome in an athletic population: a randomized controlled study with 5-year follow-up. Am J Sports Med. 2017;45(6):1388-1394. doi:10.1177/0363546516682498.
68. Carreira DS, Vora AM, Hearne KL, Kozy J. Outcome of arthroscopic treatment of posterior impingement of the ankle. Foot Ankle Int. 2016;37(4):394-400. doi:10.1177/1071100715620857.
69. Roth JA, Taylor WC, Whalen J. Peroneal tendon subluxation: the other lateral ankle injury. Br J Sports Med. 2010;44(14):1047-1053. doi:10.1136/bjsm.2008.057182.
70. Athavale SA, Swathi, Vangara SV. Anatomy of the superior peroneal tunnel. J Bone Joint Surg Am. 2011;93(6):564-571. doi:10.2106/JBJS.17.00836.
71. Porter D, McCarroll J, Knapp E, Torma J. Peroneal tendon subluxation in athletes: fibular groove deepening and retinacular reconstruction. Foot Ankle Int. 2005;26(6):436-441.
72. Ferran NA, Oliva F, Maffulli N. Recurrent subluxation of the peroneal tendons. Sports Med. 2006;36(10):839-846. doi:10.1053/j.jfas.2010.02.007.
73. Saxena A, Ewen B. Peroneal subluxation: surgical results in 31 athletic patients. J Foot Ankle Surg. 2010;49(3):238-241.
74. van Dijk PA, Gianakos AL, Kerkhoffs GM, Kennedy JG. Return to sports and clinical outcomes in patients treated for peroneal tendon dislocation: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1155-1164. doi:10.1007/s00167-015-3833-z.
75. Lee KT, Park YU, Young KW, Kim JS, Kim JB. The plantar gap: another prognostic factor for fifth metatarsal stress fracture. Am J Sports Med. 2011;39(10):2206-2211. doi:10.1177/0363546511414856.
76. Torg JS. Fractures of the base of the fifth metatarsal distal to the tuberosity. Orthopedics. 1990;13:731-737.
77. Smith TO, Clark A, Hing CB. Interventions for treating proximal fifth metatarsal fractures in adults: a meta-analysis of the current evidence-base. Foot Ankle Surg. 2011;17(4):300-307. doi:10.1016/j.fas.2010.12.005.
78. Ekstrand J, van Dijk CN. Fifth metatarsal fractures among male professional footballers: a potential career-ending disease. Br J Sports Med. 2013;47(12):754-758.
79. Matsuda S, Fukubayashi T, Hirose N. Characteristics of the foot static alignment and the plantar pressure associated with fifth metatarsal stress fracture history in male soccer players: a case-control study. Sports Med Open. 2017;3(1):27.
80. Saita Y, Nagao M, Kawasaki T, et al. Range limitation in hip internal rotation and fifth metatarsal stress fractures (Jones fracture) in professional football players. Knee Surg Sports Traumatol Arthrosc. 2018;26(7):1943-1949. doi:10.1007/s00167-017-4552-4.
81. Fujitaka K, Taniguchi A, Isomoto S, et al. Pathogenesis of fifth metatarsal fractures in college soccer players. Orthop J Sports Med. 2015;18;3(9):2325967115603654.
82. Torg J, Moyer J, Gaughan J, Boden B. Management of tarsal navicular stress fractures: conservative versus surgical treatment: a meta-analysis. Am J Sports Med. 2010;38(5):1048-1053.
83. Haytmanek CT, Williams BT, James EW, et al. Radiographic identification of the primary lateral ankle structures. Am J Sports Med. 2015;43(1):79-87. doi:10.1177/0363546514553778.
1. Dvorak J, Junge A. Football injuries and physical symptoms. A review of the literature. Am J Sports Med. 2000;28(5 Suppl):S3-S9.
2. Chomiak J, Junge A, Peterson L, Dvorak J. Severe injuries in football players. Am J Sports Med. 2000;28(5 Suppl):S58-S68.
3. Cloke DJ, Ansell P, Avery P, Deehan D. Ankle injuries in football academies: a three-centre prospective study. Br J Sports Med. 2011;45(9):702-708. doi:10.1136/bjsm.2009.067900.
4. Cloke DJ, Spencer S, Hodson A, Deehan D. The epidemiology of ankle injuries occurring in English Football Association academies. Br J Sports Med. 2009;43(14):1119-1125. doi:10.1136/bjsm.2008.052050.
5. Dvorak J, Junge A, Derman W, Schwellnus M. Injuries and illnesses of football players during the 2010 FIFA World Cup. Br J Sports Med. 2011;45(8):626-630. doi:10.1136/bjsm.2010.079905.
6. Ekstrand J, Gillquist J. Soccer injuries and their mechanisms: a prospective study. Med Sci Sports Exerc. 1983;15(3):267-270.
7. Fousekis K, Tsepis E, Vagenas G. Intrinsic risk factors of noncontact ankle sprains in soccer: a prospective study on 100 professional players. Am J Sports Med. 2012;40(8):1842-1850. doi:10.1177/0363546512449602.
8. Gaulrapp H, Becker A, Walther M, Hess H. Injuries in women’s soccer: a 1-year all players prospective field study of the women’s Bundesliga (German premiere league). Clin J Sports Med. 2010;20(4):264-271. doi:10.1097/JSM.0b013e3181e78e33.
9. Morgan BE, Oberlander MA. An examination of injuries in major league soccer. The inaugural season. Am J Sports Med. 2001;29(4):426-430. doi:10.1177/03635465010290040701.
10. Jain N, Murray D, Kemp S, Calder J. Frequency and trends in foot and ankle injuries within an English Premier League Football Club using a new impact factor of injury to identify a focus for injury prevention. Foot Ankle Surg. 2014;20(4):237-240. doi:10.1016/j.fas.2014.05.004.
11. Moore KL, Dalley AF, Agur AMR. Clinically Oriented Anatomy. 6th ed. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins, 2010:xxix, 1134.
12. Thompson JC, Netter FH. Netter’s Concise Orthopaedic Anatomy. 2nd ed. Philadelphia, PA: Saunders Elsevier, 2010:x, 404.
13. Giza E, Mandelbaum B. Chronic footballer’s ankle. In: Football Traumatology. Springer Milan, 2006:333-351.
14. Garrick JG. The frequency of injury, mechanism of injury, and epidemiology of ankle sprains. Am J Sports Med. 1977:5(6):241-242. doi:10.1177/036354657700500606.
15. Agur AMR, Grant JCB. Grant’s Atlas of Anatomy. 13th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins. 2011.
16. Renstrom PA, Konradsen L. Ankle ligament injuries. Br J Sports Med. 1997;31(1):11-20.
17. Chhabra A, Subhawong TK, Carrino JA. MR imaging of deltoid ligament pathologic findings and associated impingement syndromes. Radiographics. 2010;30(3):751-761. doi:10.1148/rg.303095756.
18. Hintermann B. Medial ankle instability. Foot Ankle Clin. 2003;8(4):723-738.
19. Woods C, Hawkins R, Hulse M, Hodson A. The Football Association Medical Research Programme: an audit of injuries in professional football: an analysis of ankle sprains. Br J Sports Med. 2003;37(3):233-238.
20. Thacker SB, Stroup DF, Branche CM, Gilchrist J, Goodman RA, Weitman EA. The prevention of ankle sprains in sports. A systematic review of the literature. Am J Sports Med. 1999;27(6):753-760. doi:10.1177/03635465990270061201.
21. Giza E, Fuller C, Junge A, Dvorak J. Mechanisms of foot and ankle injuries in soccer. Am J Sports Med. 2003;31(4):550-554. doi:10.1177/03635465030310041201.
22. Tucker AM. Common soccer injuries. Diagnosis, treatment and rehabilitation. Sports Med. 1997;23(1):21-32.
23. Lynch SA, Renstrom PA. Treatment of acute lateral ankle ligament rupture in the athlete. Conservative versus surgical treatment. Sports Med. 1999;27(1):61-71.
24. Chan KW, Ding BC, Mroczek KJ. Acute and chronic lateral ankle instability in the athlete. Bull NYU Hosp Jt Dis. 2011;69(1):17-26.
25. Stiell IG, Greenberg GH, McKnight RD, Nair RC, McDowell I, Worthington JR. A study to develop clinical decision rules for the use of radiography in acute ankle injuries. Ann Emerg Med. 1992;21(4):384-390.
26. Bachmann LM, Kolb E, Koller MT, Steurer J, ter Riet G. Accuracy of Ottawa ankle rules to exclude fractures of the ankle and mid-foot: systematic review. BMJ. 2003;326(7386):417. doi:10.1136/bmj.326.7386.417.
27. Balduini FC, Vegso JJ, Torg JS, Torg E. Management and rehabilitation of ligamentous injuries to the ankle. Sports Med. 1987;4(5):364-380.
28. Kerkhoffs GM, Rowe BH, Assendelft WJ, Kelly KD, Struijs PA, van Dijk CN. Immobilisation for acute ankle sprain. A systematic review. Arch Orthop Trauma Surg. 2001;121(8):462-471.
29. Konradsen L, Holmer P, Sondergaard L. Early mobilizing treatment for grade III ankle ligament injuries. Foot Ankle. 1991;12(2):69-73.
30. Eiff MP, Smith AT, Smith GE. Early mobilization versus immobilization in the treatment of lateral ankle sprains. Am J Sports Med. 1994;22(1):83-88. doi:10.1177/036354659402200115.
31. Shrier I. Treatment of lateral collateral ligament sprains of the ankle: a critical appraisal of the literature. Clin J Sport Med. 1995;5(3):187-195.
32. DiGiovanni BF, Partal G, Baumhauer JF. Acute ankle injury and chronic lateral instability in the athlete. Clin Sports Med. 2004;23(1):1-19, v. doi:10.1016/S0278-5919(03)00095-4.
33. Alt W, Lohrer H, Gollhofer A. Functional properties of adhesive ankle taping: neuromuscular and mechanical effects before and after exercise. Foot Ankle Int. 1999;20(4):238-245. doi:10.1177/107110079902000406.
34. Garrick JG, Requa RK. Role of external support in the prevention of ankle sprains. Med Sci Sports. 1973;5(3):200-203.
35. Sharpe SR, Knapik J, Jones B. Ankle braces effectively reduce recurrence of ankle sprains in female soccer players. J Athl Train. 1997;32(1):21-24.
36. Surve I, Schwellnus MP, Noakes T, Lombard C. A fivefold reduction in the incidence of recurrent ankle sprains in soccer players using the Sport-Stirrup orthosis. Am J Sports Med. 1994;22(5):601-606. doi:10.1177/036354659402200506.
37. Mohammadi F. Comparison of 3 preventive methods to reduce the recurrence of ankle inversion sprains in male soccer players. Am J Sports Med. 2007;35(6):922-926. doi:10.1177/0363546507299259.
38. Steffen K, Meeuwisse WH, Romiti M, et al. Evaluation of how different implementation strategies of an injury prevention programme (FIFA 11+) impact team adherence and injury risk in Canadian female youth football players: a cluster-randomised trial. Br J Sports Med. 2013;47(8):480-487. doi:10.1136/bjsports-2012-091887.
39. Steffen K, Emery CA, Romiti M, et al. High adherence to a neuromuscular injury prevention programme (FIFA 11+) improves functional balance and reduces injury risk in Canadian youth female football players: a cluster randomised trial. Br J Sports Med. 2013;47(12):794-802. doi: 10.1136/bjsports-2012-091886.
40. Junge A, Lamprecht M, Stamm H, et al. Countrywide campaign to prevent soccer injuries in Swiss amateur players. Am J Sports Med. 2011;39(1):57-63. doi:10.1177/0363546510377424.
41. Lin CF, Gross ML, Weinhold P. Ankle syndesmosis injuries: anatomy, biomechanics, mechanism of injury, and clinical guidelines for diagnosis and intervention. J Orthop Sports Phys Ther. 2006;36(6):372-384. doi:10.2519/jospt.2006.2195.
42. Lubberts B, D’Hooghe P, Bengtsson H, DiGiovanni CW, Calder J, Ekstrand J. Epidemiology and return to play following isolated syndesmotic injuries of the ankle: a prospective cohort study of 3677 male professional football players in the UEFA Elite Club Injury Study. Br J Sports Med. 2017. doi:10.1136/bjsports-2017-097710.
43. Harper MC, Keller TS. A radiographic evaluation of the tibiofibular syndesmosis. Foot Ankle. 1989;10(3):156-160.
44. Vogl TJ, Hochmuth K, Diebold T, et al. Magnetic resonance imaging in the diagnosis of acute injured distal tibiofibular syndesmosis. Invest Radiol. 1997;32(7):401-409.
45. Brown KW, Morrison WB, Schweitzer ME, Parellada JA, Nothnagel H. MRI findings associated with distal tibiofibular syndesmosis injury. AJR Am J Roentgenol. 2004;182(1):131-136. doi:10.2214/ajr.182.1.1820131.
46. Ogilvie-Harris DJ, Reed SC, Hedman TP. Disruption of the ankle syndesmosis: biomechanical study of the ligamentous restraints. Arthroscopy. 1994;10(5):558-560.
47. Boytim MJ, Fischer DA, Neumann L. Syndesmotic ankle sprains. Am J Sports Med. 1991;19(3):294-298. doi:10.1177/036354659101900315.
48. Hopkinson WJ, St Pierre P, Ryan JB, Wheeler JH. Syndesmosis sprains of the ankle. Foot Ankle. 1990;10(6):325-330. doi:10.1177/107110079001000607.
49. Del Buono A, Florio A, Boccanera MS, Maffulli N. Syndesmosis injuries of the ankle. Curr Rev Musculoskelet Med. 2013;6(4):313-319. doi:10.1007/s12178-013-9183-x.
50. Dattani R, Patnaik S, Kantak A, Srikanth B, Selvan TP. Injuries to the tibiofibular syndesmosis. J Bone Joint Surg Br. 2008;90(4):405-410. doi:10.1302/0301-620X.90B4.19750.
51. Schepers T. To retain or remove the syndesmotic screw: a review of literature. Arch Orthop Trauma Surg. 2011;131(7):879-883. doi:10.1007/s00402-010-1225-x.
52. Naqvi GA, Cunningham P, Lynch B, Galvin R, Awan N. Fixation of ankle syndesmotic injuries: comparison of tightrope fixation and syndesmotic screw fixation for accuracy of syndesmotic reduction. Am J Sports Med. 2012;40(12):2828-2835. doi:10.1177/0363546512461480.
53. Weening B, Bhandari M. Predictors of functional outcome following transsyndesmotic screw fixation of ankle fractures. J Orthop Trauma. 2005;19(2):102-108.
54. Colcuc C, Blank M, Stein T, et al. Lower complication rate and faster return to sports in patients with acute syndesmotic rupture treated with a new knotless suture button device. Knee Surg Sports Traumatol Arthrosc. 2017. doi:10.1007/s00167-017-4820-4823.
55. Savage-Elliott I, Ross KA, Smyth NA, Murawski CD, Kennedy JG. Osteochondral lesions of the talus: a current concepts review and evidence-based treatment paradigm. Foot Ankle Spec. 2014;7(5):414-422. doi:10.1177/1938640014543362.
56. Talusan PG, Milewski MD, Toy JO, Wall EJ. Osteochondritis dissecans of the talus: diagnosis and treatment in athletes. Clin Sports Med. 2014;33(2):267-284. doi:10.1016/j.csm.2014.01.003.
57. Murawski CD, Kennedy JG. Operative treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 2013;95(11):1045-1054. doi:10.2106/JBJS.L.00773.
58. Guney A, Akar M, Karaman I, Oner M, Guney B. Clinical outcomes of platelet rich plasma (PRP) as an adjunct to microfracture surgery in osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2015;23(8):2384-2389. doi:10.1007/s00167-013-2784-5.
59. Hatic SO, Berlet GC. Particulated juvenile articular cartilage graft (DeNovo NT Graft) for treatment of osteochondral lesions of the talus. Foot Ankle Spec. 2010;3(6):361-364. doi:10.1177/1938640010388602.
60. van Eekeren IC, van Bergen CJ, Sierevelt IN, Reilingh ML, van Dijk CN. Return to sports after arthroscopic debridement and bone marrow stimulation of osteochondral talar defects: a 5- to 24-year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1311-1315. doi:10.1007/s00167-016-3992-6.
61. Tol JL, Slim E, van Soest AJ, van Dijk CN. The relationship of the kicking action in soccer and anterior ankle impingement syndrome. A biomechanical analysis. Am J Sports Med. 2002;30(1):45-50. doi:10.1177/03635465020300012101.
62. Sanders TG, Rathur SK. Impingement syndromes of the ankle. Magn Reson Imaging Clin N Am. 2008;16(1):29-38. doi:10.1016/j.mric.2008.02.005.
63. Ogilvie-Harris DJ, Gilbart MK, Chorney K. Chronic pain following ankle sprains in athletes: the role of arthroscopic surgery. Arthroscopy. 1997;13(5):564-574.
64. Robinson P, White LM, Salonen D, Ogilvie-Harris D. Anteromedial impingement of the ankle: using MR arthrography to assess the anteromedial recess. AJR Am J Roentgenol. 2002;178(3):601-604. doi:10.2214/ajr.178.3.1780601.
65. Mosier-La Clair SM, Monroe MT, Manoli A. Medial impingement syndrome of the anterior tibiotalar fascicle of the deltoid ligament on the talus. Foot Ankle Int. 2000;21(5):385-391.
66. Maquirriain J. Posterior ankle impingement syndrome. J Am Acad Orthop Surg. 2005;13(6):365-371.
67. Georgiannos D, Bisbinas I. Endoscopic versus open excision of os trigonum for the treatment of posterior ankle impingement syndrome in an athletic population: a randomized controlled study with 5-year follow-up. Am J Sports Med. 2017;45(6):1388-1394. doi:10.1177/0363546516682498.
68. Carreira DS, Vora AM, Hearne KL, Kozy J. Outcome of arthroscopic treatment of posterior impingement of the ankle. Foot Ankle Int. 2016;37(4):394-400. doi:10.1177/1071100715620857.
69. Roth JA, Taylor WC, Whalen J. Peroneal tendon subluxation: the other lateral ankle injury. Br J Sports Med. 2010;44(14):1047-1053. doi:10.1136/bjsm.2008.057182.
70. Athavale SA, Swathi, Vangara SV. Anatomy of the superior peroneal tunnel. J Bone Joint Surg Am. 2011;93(6):564-571. doi:10.2106/JBJS.17.00836.
71. Porter D, McCarroll J, Knapp E, Torma J. Peroneal tendon subluxation in athletes: fibular groove deepening and retinacular reconstruction. Foot Ankle Int. 2005;26(6):436-441.
72. Ferran NA, Oliva F, Maffulli N. Recurrent subluxation of the peroneal tendons. Sports Med. 2006;36(10):839-846. doi:10.1053/j.jfas.2010.02.007.
73. Saxena A, Ewen B. Peroneal subluxation: surgical results in 31 athletic patients. J Foot Ankle Surg. 2010;49(3):238-241.
74. van Dijk PA, Gianakos AL, Kerkhoffs GM, Kennedy JG. Return to sports and clinical outcomes in patients treated for peroneal tendon dislocation: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1155-1164. doi:10.1007/s00167-015-3833-z.
75. Lee KT, Park YU, Young KW, Kim JS, Kim JB. The plantar gap: another prognostic factor for fifth metatarsal stress fracture. Am J Sports Med. 2011;39(10):2206-2211. doi:10.1177/0363546511414856.
76. Torg JS. Fractures of the base of the fifth metatarsal distal to the tuberosity. Orthopedics. 1990;13:731-737.
77. Smith TO, Clark A, Hing CB. Interventions for treating proximal fifth metatarsal fractures in adults: a meta-analysis of the current evidence-base. Foot Ankle Surg. 2011;17(4):300-307. doi:10.1016/j.fas.2010.12.005.
78. Ekstrand J, van Dijk CN. Fifth metatarsal fractures among male professional footballers: a potential career-ending disease. Br J Sports Med. 2013;47(12):754-758.
79. Matsuda S, Fukubayashi T, Hirose N. Characteristics of the foot static alignment and the plantar pressure associated with fifth metatarsal stress fracture history in male soccer players: a case-control study. Sports Med Open. 2017;3(1):27.
80. Saita Y, Nagao M, Kawasaki T, et al. Range limitation in hip internal rotation and fifth metatarsal stress fractures (Jones fracture) in professional football players. Knee Surg Sports Traumatol Arthrosc. 2018;26(7):1943-1949. doi:10.1007/s00167-017-4552-4.
81. Fujitaka K, Taniguchi A, Isomoto S, et al. Pathogenesis of fifth metatarsal fractures in college soccer players. Orthop J Sports Med. 2015;18;3(9):2325967115603654.
82. Torg J, Moyer J, Gaughan J, Boden B. Management of tarsal navicular stress fractures: conservative versus surgical treatment: a meta-analysis. Am J Sports Med. 2010;38(5):1048-1053.
83. Haytmanek CT, Williams BT, James EW, et al. Radiographic identification of the primary lateral ankle structures. Am J Sports Med. 2015;43(1):79-87. doi:10.1177/0363546514553778.
TAKE-HOME POINTS
- Soccer injuries of the foot and ankle are becoming more prevalent due to the ever-growing popularity of the sport.
- Low ankle sprains represent the majority of foot and ankle–related injuries due to soccer and most can be treated non-operatively, with an early mobilization protocol followed by a phased rehabilitation.
- High ankle sprains are less common than low ankle sprains; however, they require a lengthier rehabilitation and most of the time are treated surgically.
- Impingement-like syndromes are common among soccer players and can be due to repetitive microtrauma from recurrent ball impact. Most of these syndromes respond favorably to non-operative modalities.
- Stress fractures of the foot, although less common, often require surgical stabilization in soccer players.
DAPT’s benefit after stroke or TIA clusters in first 21 days
MONTREAL – The optimal length for dual antiplatelet therapy in patients who have just had a mild stroke or transient ischemic attack is 21 days, a duration of combined treatment that maximized protection against major ischemic events while minimizing the extra risk for a major hemorrhage, according to a prespecified analysis of data from the POINT trial.
The POINT (Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke) trial randomized 4,881 patients with a very recent mild stroke or transient ischemic attack and without atrial fibrillation to treatment with either clopidogrel plus aspirin or aspirin alone for 90 days. Compared with aspirin alone, dual antiplatelet therapy (DAPT) cut the incidence of a major ischemic event by a relative 25% but also more than doubled the rate of major hemorrhage (New Engl J Med. 2018 Jul 19;377[3]:215-25).
The new, prespecified analysis looked at outcomes on a week-by-week basis over the course of 90 days of treatment, and showed that during the first 21 days the rate of major ischemic events was 5.6% among patients on aspirin only and 3.6% among those on DAPT, a statistically significant 35% relative cut in these adverse outcomes by using DAPT, Jordan J. Elm, PhD, reported at the World Stroke Congress. During the subsequent 69 days on treatment, the incidence of major ischemic events was roughly 1% in both arms of the study, showing that after 3 weeks the incremental benefit from DAPT disappeared, said Dr. Elm, a biostatistician at the Medical University of South Carolina, Charleston.
In contrast, the doubled rate of major hemorrhages (mostly reversible gastrointestinal bleeds) with DAPT, compared with aspirin alone, occurred at a relatively uniform rate throughout the 90 days of treatment, meaning that limiting DAPT to just 21 days could prevent many of the excess hemorrhages.
“These results suggest that limiting clopidogrel plus aspirin use to 21 days may maximize benefit and reduce risk,” Dr. Elm said, especially in light of the findings confirming the efficacy of 21 days of DAPT following a minor stroke or TIA that had been reported several years ago in the CHANCE (Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events) trial (New Engl J Med. 2013 Jul 4;369[1]:11-9).
Although the new finding from the POINT results came in a secondary analysis, it’s statistically legitimate and should be taken into account when writing treatment guidelines, she said, emphasizing that “this is a very important analysis that is not just hypothesis generating.”
Another finding from the new analysis was that a large number of major ischemic events, and hence a large number of the events prevented by DAPT, occurred in the first 2 days following the index event, a finding made possible because the POINT investigators enrolled patients and started treatment within 12 hours of the qualifying events.
“It’s better to start treatment early,” Dr. Elm noted, but she also highlighted that major ischemic events continued to accumulate during days 3-21, suggesting that patients could still benefit from DAPT even if treatment did not start until 24 or 48 hours after their index event.
POINT received no commercial funding aside from study drugs supplied by Sanofi. Dr. Elm reported no disclosures.
SOURCE: Elm JJ et al. World Stroke Congress, Late-breaking session.
The new model using data from the POINT trial confirms what had been previously shown in the CHANCE trial – that 21 days is a sensible cutoff for dual antiplatelet treatment for patients immediately following a mild stroke or transient ischemic attack. Treatment with dual antiplatelet therapy for 21 days provides the same added benefit as 90 days of treatment but with less excess bleeding. The new findings confirm that the CHANCE results were not specific to a Chinese population.

For the time being, clopidogrel is the evidence-based antiplatelet drug to pair with aspirin for this indication. Clopidogrel has the advantages of being generic, cheap, available, and familiar. It’s possible that another P2Y12 inhibitor, such as ticagrelor (Brilinta), might work even better, but that needs to be proven to justify the added expense of a brand-name antiplatelet drug.
Mike Sharma, MD , is a stroke neurologist at McMaster University, Hamilton, Ont. He has been an advisor to Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Janssen Pharmaceuticals, and Pfizer. He made these comments in an interview.
The new model using data from the POINT trial confirms what had been previously shown in the CHANCE trial – that 21 days is a sensible cutoff for dual antiplatelet treatment for patients immediately following a mild stroke or transient ischemic attack. Treatment with dual antiplatelet therapy for 21 days provides the same added benefit as 90 days of treatment but with less excess bleeding. The new findings confirm that the CHANCE results were not specific to a Chinese population.

For the time being, clopidogrel is the evidence-based antiplatelet drug to pair with aspirin for this indication. Clopidogrel has the advantages of being generic, cheap, available, and familiar. It’s possible that another P2Y12 inhibitor, such as ticagrelor (Brilinta), might work even better, but that needs to be proven to justify the added expense of a brand-name antiplatelet drug.
Mike Sharma, MD , is a stroke neurologist at McMaster University, Hamilton, Ont. He has been an advisor to Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Janssen Pharmaceuticals, and Pfizer. He made these comments in an interview.
The new model using data from the POINT trial confirms what had been previously shown in the CHANCE trial – that 21 days is a sensible cutoff for dual antiplatelet treatment for patients immediately following a mild stroke or transient ischemic attack. Treatment with dual antiplatelet therapy for 21 days provides the same added benefit as 90 days of treatment but with less excess bleeding. The new findings confirm that the CHANCE results were not specific to a Chinese population.

For the time being, clopidogrel is the evidence-based antiplatelet drug to pair with aspirin for this indication. Clopidogrel has the advantages of being generic, cheap, available, and familiar. It’s possible that another P2Y12 inhibitor, such as ticagrelor (Brilinta), might work even better, but that needs to be proven to justify the added expense of a brand-name antiplatelet drug.
Mike Sharma, MD , is a stroke neurologist at McMaster University, Hamilton, Ont. He has been an advisor to Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Janssen Pharmaceuticals, and Pfizer. He made these comments in an interview.
MONTREAL – The optimal length for dual antiplatelet therapy in patients who have just had a mild stroke or transient ischemic attack is 21 days, a duration of combined treatment that maximized protection against major ischemic events while minimizing the extra risk for a major hemorrhage, according to a prespecified analysis of data from the POINT trial.
The POINT (Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke) trial randomized 4,881 patients with a very recent mild stroke or transient ischemic attack and without atrial fibrillation to treatment with either clopidogrel plus aspirin or aspirin alone for 90 days. Compared with aspirin alone, dual antiplatelet therapy (DAPT) cut the incidence of a major ischemic event by a relative 25% but also more than doubled the rate of major hemorrhage (New Engl J Med. 2018 Jul 19;377[3]:215-25).
The new, prespecified analysis looked at outcomes on a week-by-week basis over the course of 90 days of treatment, and showed that during the first 21 days the rate of major ischemic events was 5.6% among patients on aspirin only and 3.6% among those on DAPT, a statistically significant 35% relative cut in these adverse outcomes by using DAPT, Jordan J. Elm, PhD, reported at the World Stroke Congress. During the subsequent 69 days on treatment, the incidence of major ischemic events was roughly 1% in both arms of the study, showing that after 3 weeks the incremental benefit from DAPT disappeared, said Dr. Elm, a biostatistician at the Medical University of South Carolina, Charleston.
In contrast, the doubled rate of major hemorrhages (mostly reversible gastrointestinal bleeds) with DAPT, compared with aspirin alone, occurred at a relatively uniform rate throughout the 90 days of treatment, meaning that limiting DAPT to just 21 days could prevent many of the excess hemorrhages.
“These results suggest that limiting clopidogrel plus aspirin use to 21 days may maximize benefit and reduce risk,” Dr. Elm said, especially in light of the findings confirming the efficacy of 21 days of DAPT following a minor stroke or TIA that had been reported several years ago in the CHANCE (Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events) trial (New Engl J Med. 2013 Jul 4;369[1]:11-9).
Although the new finding from the POINT results came in a secondary analysis, it’s statistically legitimate and should be taken into account when writing treatment guidelines, she said, emphasizing that “this is a very important analysis that is not just hypothesis generating.”
Another finding from the new analysis was that a large number of major ischemic events, and hence a large number of the events prevented by DAPT, occurred in the first 2 days following the index event, a finding made possible because the POINT investigators enrolled patients and started treatment within 12 hours of the qualifying events.
“It’s better to start treatment early,” Dr. Elm noted, but she also highlighted that major ischemic events continued to accumulate during days 3-21, suggesting that patients could still benefit from DAPT even if treatment did not start until 24 or 48 hours after their index event.
POINT received no commercial funding aside from study drugs supplied by Sanofi. Dr. Elm reported no disclosures.
SOURCE: Elm JJ et al. World Stroke Congress, Late-breaking session.
MONTREAL – The optimal length for dual antiplatelet therapy in patients who have just had a mild stroke or transient ischemic attack is 21 days, a duration of combined treatment that maximized protection against major ischemic events while minimizing the extra risk for a major hemorrhage, according to a prespecified analysis of data from the POINT trial.
The POINT (Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke) trial randomized 4,881 patients with a very recent mild stroke or transient ischemic attack and without atrial fibrillation to treatment with either clopidogrel plus aspirin or aspirin alone for 90 days. Compared with aspirin alone, dual antiplatelet therapy (DAPT) cut the incidence of a major ischemic event by a relative 25% but also more than doubled the rate of major hemorrhage (New Engl J Med. 2018 Jul 19;377[3]:215-25).
The new, prespecified analysis looked at outcomes on a week-by-week basis over the course of 90 days of treatment, and showed that during the first 21 days the rate of major ischemic events was 5.6% among patients on aspirin only and 3.6% among those on DAPT, a statistically significant 35% relative cut in these adverse outcomes by using DAPT, Jordan J. Elm, PhD, reported at the World Stroke Congress. During the subsequent 69 days on treatment, the incidence of major ischemic events was roughly 1% in both arms of the study, showing that after 3 weeks the incremental benefit from DAPT disappeared, said Dr. Elm, a biostatistician at the Medical University of South Carolina, Charleston.
In contrast, the doubled rate of major hemorrhages (mostly reversible gastrointestinal bleeds) with DAPT, compared with aspirin alone, occurred at a relatively uniform rate throughout the 90 days of treatment, meaning that limiting DAPT to just 21 days could prevent many of the excess hemorrhages.
“These results suggest that limiting clopidogrel plus aspirin use to 21 days may maximize benefit and reduce risk,” Dr. Elm said, especially in light of the findings confirming the efficacy of 21 days of DAPT following a minor stroke or TIA that had been reported several years ago in the CHANCE (Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events) trial (New Engl J Med. 2013 Jul 4;369[1]:11-9).
Although the new finding from the POINT results came in a secondary analysis, it’s statistically legitimate and should be taken into account when writing treatment guidelines, she said, emphasizing that “this is a very important analysis that is not just hypothesis generating.”
Another finding from the new analysis was that a large number of major ischemic events, and hence a large number of the events prevented by DAPT, occurred in the first 2 days following the index event, a finding made possible because the POINT investigators enrolled patients and started treatment within 12 hours of the qualifying events.
“It’s better to start treatment early,” Dr. Elm noted, but she also highlighted that major ischemic events continued to accumulate during days 3-21, suggesting that patients could still benefit from DAPT even if treatment did not start until 24 or 48 hours after their index event.
POINT received no commercial funding aside from study drugs supplied by Sanofi. Dr. Elm reported no disclosures.
SOURCE: Elm JJ et al. World Stroke Congress, Late-breaking session.
REPORTING FROM THE WORLD STROKE CONGRESS
Key clinical point: All of DAPT’s extra benefit over aspirin alone in recent stroke or transient ischemic attack patients happened during the first 21 days.
Major finding: During the first 21 days, DAPT cut major ischemic events by 35%, compared with aspirin only.
Study details: A prespecified, secondary analysis from POINT, a multicenter, randomized trial with 4,881 patients.
Disclosures: POINT received no commercial funding aside from study drugs supplied by Sanofi. Dr. Elm had no disclosures.
Source: Elm JJ et al. World Stroke Congress, Late-breaking session.
Severe chronic malnutrition: What it is and how to diagnose it
My wife and I have traveled a number of times as far east as Kyrgyzstan and as far south as Paraguay to participate in short 1- to 2-week medical clinics. When I participated in a week-long medical clinic in Haiti in early 2017, the CEO of the hosting U.S. organization asked, “I wonder if we are doing any good here?” His organization had been to Onaville, Haiti for the last 4-5 years.
So my wife Stacy, a retired licensed practical nurse, and I, a general pediatrician with an interest in severe acute malnutrition, went on a 3-month medical sabbatical to Onaville. We were self-funded, with the exception of our home church in Senoia, Ga., paying the cost of our lodging during that time.
Prior to the 2010 Haiti earthquake, the government planned Onaville to be a retirement community area, with a population of only about 1,500, I was told. After the devastating temblor, it became one of several areas where the government sent people displaced from Port-au-Prince. The population today is possibly 250,000 or more.
The poverty in this area has “newer” flavor than areas such as Cité Soleil, which has been there for decades. What we found in Onaville – and probably all of Haiti – is an appalling lack of understanding and appreciation about the nature of malnutrition.
Methods and materials for study
The 1981 World Health Organization’s last printed monograph about severe acute nutrition remains essentially today’s cookbook recipe for treatment. Little seems to have changed since then in the literature I’ve reviewed. It didn’t take long after we started seeing the children in Onaville to shift that interest to something much more serious and widespread.
I wanted to start with basic health assessments in the Onaville children around 5 years and under. These children rarely see a physician, and only about half or so get any vaccine. Most parents do not have any immunization records in their possession to even review.
We decided to measure head size, mid-upper arm circumference, height, weight, and hemoglobin levels. Date of birth was recorded, if known or could at best be closely estimated. Vaccination was recorded as a yes or no response. All children also were examined for evidence of things like swelling, marasmic appearance (wasting, loss of body fat and muscle), yellowed hair, eye findings of vitamin A deficiency, etc. I wanted to get some impression about the health of these children in the same way that most mobile medical clinics do in Haiti.
Being a database programmer since I bought my first computer in 1985, and having written and deployed my office’s current EMR system in 2000, I decided before ever arriving in Haiti to write the software needed for this task. Unlike regular office EMRs, there were some special considerations.
Growth charts needed not only to be generated for individuals, but in aggregate. Hemoglobins levels, too, needed charting. While in the United States, I use Centers for Disease Control and Prevention growth chart data, but for Haiti I used WHO growth data. I was able to procure hemoglobin charting data as well. Aggregate data turned out to be key to our conclusions.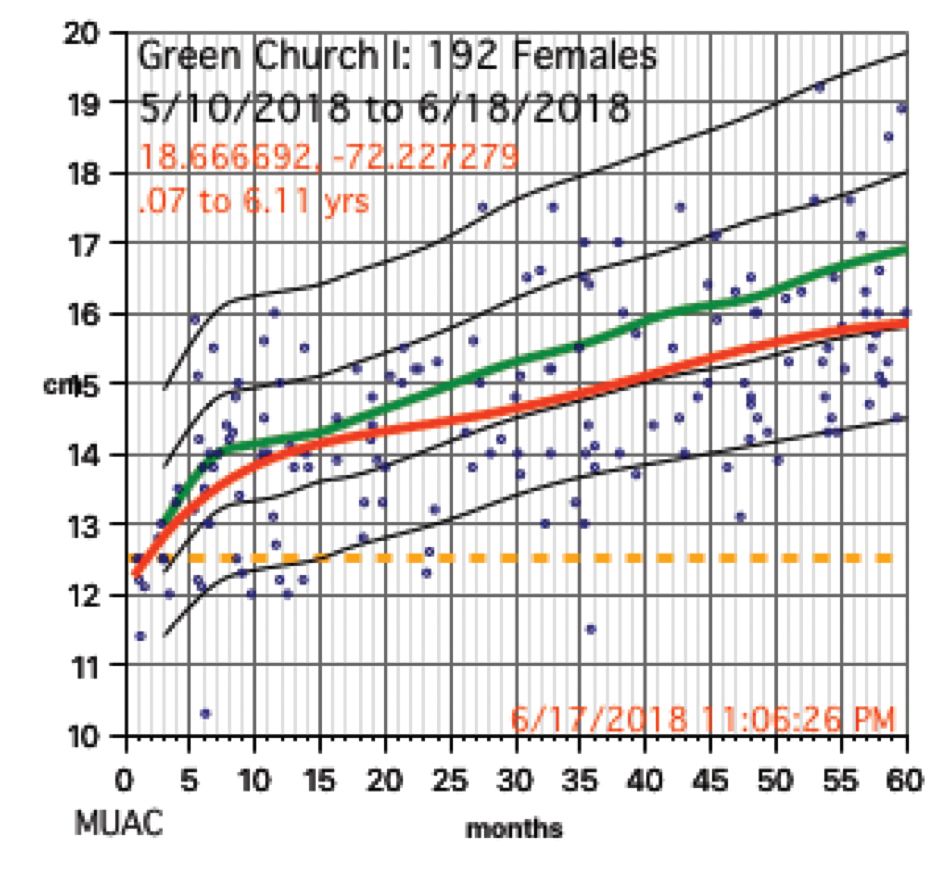
We used a regular consumer quality digital bathroom scale for weights. A sewing tape attached with duct tape to a wall or pillar was used to measure height. Standard head circumference tapes were use to measure heads and arms.
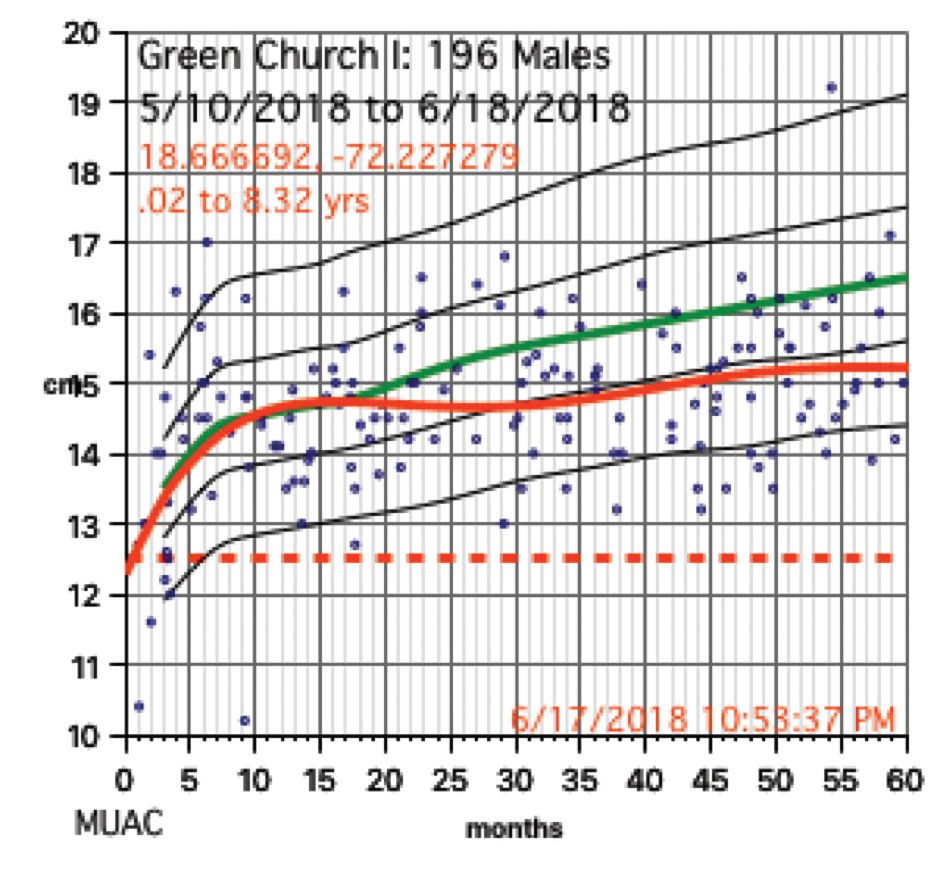
The hemoglobin was measured with a HemoCue Hb 201+ instrument. Size, ruggedness, and cost dictated all our choices because, except for food, we had to carry everything with us. The cost of a new HemoCue was under $400 and each microcuvette test was about $1.50.
Severe anemia
In total, we saw about 386 children, mostly 5 years and under, in Onaville. Toward the end of the 3 months, we were seeing some of those back as follow-ups. One of the first hemoglobins was 4.9 g/dL, with a 5.4 g/dL on repeat. This stunned us. In the first few days, we were seeing what we saw consistently throughout the course of 3 months.
About 19% of these children had hemoglobins from below 9.0 g/dL to below 6 g/dL. More importantly, there was little on physical exam that would trigger one to do a hemoglobin. Low hemoglobins were not associated with yellow-orange hair. No cases of the swelling of kwashiorkor or pencil-like frames of marasmus were seen. 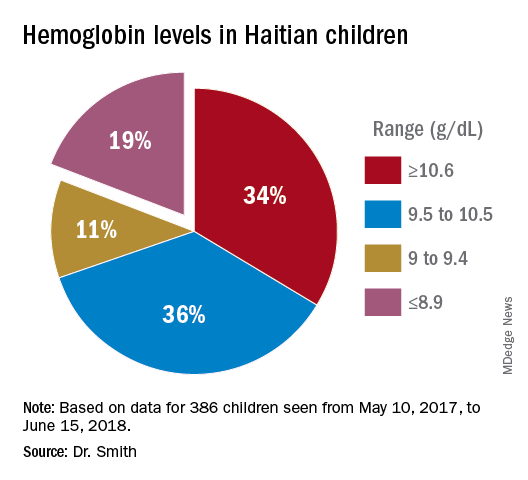
Severe chronic malnutrition
The scatter charts are very telling and the hemoglobin graphs are explosive. What is demonstrated is that this recent population is slowly starving to death. How can the hemoglobins be so very low in comparison to the only slightly lowered mean averages (the solid red line)?
In over 3 decades of pediatric medicine, I rarely have seen children in the United States with hemoglobins below 9.5 g/dL. Often they have other illnesses that clearly point to the cause. Could the 19% of children with severely lowered hemoglobins (below 9.0 g/dL) be caused by sickle cell disease or something else in these Haitian children?
A search for articles where sickle cell was studied revealed a study done at St. Damien Pediatrics Hospital in Port-au-Prince (Blood. 2012;120:4235). The overall incidence of sickle cell disease was this: “Of the 2,258 samples tested, 247 had HbS, fifty-seven had HbC, ten had HbSS, and three had HbSC.” Only 0.57% of these children had sickle or sickle-C disease where one could expect hemoglobins to be as low as in the children of Onaville. Applying that percentage to the 386 children we saw would account for about only 2 children who might have sickling anemia. Yet we had 73 children in our study with severely lowered hemoglobins below 9.0 g/dL. If you estimate that half of the 250,000 people in Onaville are children, that extrapolates to over 47,000 with severe anemia! I think that a study larger than ours needs to be done to better assess that, however.
My best thought is that these children who have little external evidence of abnormality and mildly lowered growth data represent a type of malnutrition that has not been defined, much less addressed. I call this severe chronic malnutrition. The very low hemoglobins indicate to me that this is not simply a lack of iron – although certainly that is a factor – but rather that these children are in a state of chronic protein deprivation. They represent a large pool of children who exist between those with normal nutritional states and those with the kwashiorkor or marasmus of severe acute malnutrition.
A search of the 69,823 ICD-10 codes in my database for “malnutrition” only turns up the ill-defined terms, “Unspecified severe protein-calorie malnutrition,” “Moderate, and Mild protein-calorie malnutrition,” “Unspecified protein-calorie malnutrition,” and “Sequelae of protein-calorie malnutrition.” Whatever each of those means is purely subjective in my opinion.
Without a clear understanding or definition of what is severe chronic malnutrition, we are like the Titanic trying to avoid icebergs on a moonless night. I think we must define severe chronic malnutrition before we really can understand the pathophysiology and treatment of severe acute malnutrition.
The WHO published its last printed monograph, “The treatment and management of severe acute protein-energy malnutrition,” in 1981. This publication is essentially a cookbook approach for what to do, with no clear presentation of the chemical processes and medicine involved. The primary focus for the WHO is mid-upper arm circumference and weight for height. Reading this document might lead one to believe that all malnutrition is acutely severe. It is most certainly not.
Conclusion
The answer to why some children show the swelling of kwashiorkor and some show marasmus probably will not be found in the study of severe acute malnutrition or refeeding syndrome alone. We must go far beyond the WHO’s cookbook recipe.
I think we must start with the study, definition, and treatment of severe chronic malnutrition.
While in Haiti, we shared these data with three organization that are working to provide nutrition in a starving nation. Together, the Baptist Haiti Mission, Mission of Hope Haiti, and Trinity Hope may well be supplying 175,000 meals a day through school lunches and other avenues throughout the country. Their response was telling. Those at Baptist Haiti Mission, an organization with a presence of almost 80 years there, told us that this information was a “big deal.”
The issue for them is the answer to the question, “How can we tell if we are doing any good in our feeding programs?” A lot of money is being thrown into nutrition without tangible ways to assess impact. Clearly parameters such as mid-upper arm circumference and weight for height that WHO advocates is not adequate, as our plots revealed.
We think that a simple, cheap, hemoglobin finger stick can tell us who is falling through the cracks into severe chronic malnutrition and those at risk for severe acute malnutrition. I am an advocate for instituting hemoglobin surveillance as part of all feeding programs. Then we can come up with the cheapest and most effective in-country mechanisms to treat these children.
Indeed that is our next step in working in Haiti.
Dr. Smith is a board certified pediatrician working in McDonough, Ga., with an interest in malnutrition among the children of Haiti. Email him at [email protected].
My wife and I have traveled a number of times as far east as Kyrgyzstan and as far south as Paraguay to participate in short 1- to 2-week medical clinics. When I participated in a week-long medical clinic in Haiti in early 2017, the CEO of the hosting U.S. organization asked, “I wonder if we are doing any good here?” His organization had been to Onaville, Haiti for the last 4-5 years.
So my wife Stacy, a retired licensed practical nurse, and I, a general pediatrician with an interest in severe acute malnutrition, went on a 3-month medical sabbatical to Onaville. We were self-funded, with the exception of our home church in Senoia, Ga., paying the cost of our lodging during that time.
Prior to the 2010 Haiti earthquake, the government planned Onaville to be a retirement community area, with a population of only about 1,500, I was told. After the devastating temblor, it became one of several areas where the government sent people displaced from Port-au-Prince. The population today is possibly 250,000 or more.
The poverty in this area has “newer” flavor than areas such as Cité Soleil, which has been there for decades. What we found in Onaville – and probably all of Haiti – is an appalling lack of understanding and appreciation about the nature of malnutrition.
Methods and materials for study
The 1981 World Health Organization’s last printed monograph about severe acute nutrition remains essentially today’s cookbook recipe for treatment. Little seems to have changed since then in the literature I’ve reviewed. It didn’t take long after we started seeing the children in Onaville to shift that interest to something much more serious and widespread.
I wanted to start with basic health assessments in the Onaville children around 5 years and under. These children rarely see a physician, and only about half or so get any vaccine. Most parents do not have any immunization records in their possession to even review.
We decided to measure head size, mid-upper arm circumference, height, weight, and hemoglobin levels. Date of birth was recorded, if known or could at best be closely estimated. Vaccination was recorded as a yes or no response. All children also were examined for evidence of things like swelling, marasmic appearance (wasting, loss of body fat and muscle), yellowed hair, eye findings of vitamin A deficiency, etc. I wanted to get some impression about the health of these children in the same way that most mobile medical clinics do in Haiti.
Being a database programmer since I bought my first computer in 1985, and having written and deployed my office’s current EMR system in 2000, I decided before ever arriving in Haiti to write the software needed for this task. Unlike regular office EMRs, there were some special considerations.
Growth charts needed not only to be generated for individuals, but in aggregate. Hemoglobins levels, too, needed charting. While in the United States, I use Centers for Disease Control and Prevention growth chart data, but for Haiti I used WHO growth data. I was able to procure hemoglobin charting data as well. Aggregate data turned out to be key to our conclusions.
We used a regular consumer quality digital bathroom scale for weights. A sewing tape attached with duct tape to a wall or pillar was used to measure height. Standard head circumference tapes were use to measure heads and arms.

The hemoglobin was measured with a HemoCue Hb 201+ instrument. Size, ruggedness, and cost dictated all our choices because, except for food, we had to carry everything with us. The cost of a new HemoCue was under $400 and each microcuvette test was about $1.50.
Severe anemia
In total, we saw about 386 children, mostly 5 years and under, in Onaville. Toward the end of the 3 months, we were seeing some of those back as follow-ups. One of the first hemoglobins was 4.9 g/dL, with a 5.4 g/dL on repeat. This stunned us. In the first few days, we were seeing what we saw consistently throughout the course of 3 months.
About 19% of these children had hemoglobins from below 9.0 g/dL to below 6 g/dL. More importantly, there was little on physical exam that would trigger one to do a hemoglobin. Low hemoglobins were not associated with yellow-orange hair. No cases of the swelling of kwashiorkor or pencil-like frames of marasmus were seen. 
Severe chronic malnutrition
The scatter charts are very telling and the hemoglobin graphs are explosive. What is demonstrated is that this recent population is slowly starving to death. How can the hemoglobins be so very low in comparison to the only slightly lowered mean averages (the solid red line)?
In over 3 decades of pediatric medicine, I rarely have seen children in the United States with hemoglobins below 9.5 g/dL. Often they have other illnesses that clearly point to the cause. Could the 19% of children with severely lowered hemoglobins (below 9.0 g/dL) be caused by sickle cell disease or something else in these Haitian children?
A search for articles where sickle cell was studied revealed a study done at St. Damien Pediatrics Hospital in Port-au-Prince (Blood. 2012;120:4235). The overall incidence of sickle cell disease was this: “Of the 2,258 samples tested, 247 had HbS, fifty-seven had HbC, ten had HbSS, and three had HbSC.” Only 0.57% of these children had sickle or sickle-C disease where one could expect hemoglobins to be as low as in the children of Onaville. Applying that percentage to the 386 children we saw would account for about only 2 children who might have sickling anemia. Yet we had 73 children in our study with severely lowered hemoglobins below 9.0 g/dL. If you estimate that half of the 250,000 people in Onaville are children, that extrapolates to over 47,000 with severe anemia! I think that a study larger than ours needs to be done to better assess that, however.
My best thought is that these children who have little external evidence of abnormality and mildly lowered growth data represent a type of malnutrition that has not been defined, much less addressed. I call this severe chronic malnutrition. The very low hemoglobins indicate to me that this is not simply a lack of iron – although certainly that is a factor – but rather that these children are in a state of chronic protein deprivation. They represent a large pool of children who exist between those with normal nutritional states and those with the kwashiorkor or marasmus of severe acute malnutrition.
A search of the 69,823 ICD-10 codes in my database for “malnutrition” only turns up the ill-defined terms, “Unspecified severe protein-calorie malnutrition,” “Moderate, and Mild protein-calorie malnutrition,” “Unspecified protein-calorie malnutrition,” and “Sequelae of protein-calorie malnutrition.” Whatever each of those means is purely subjective in my opinion.
Without a clear understanding or definition of what is severe chronic malnutrition, we are like the Titanic trying to avoid icebergs on a moonless night. I think we must define severe chronic malnutrition before we really can understand the pathophysiology and treatment of severe acute malnutrition.
The WHO published its last printed monograph, “The treatment and management of severe acute protein-energy malnutrition,” in 1981. This publication is essentially a cookbook approach for what to do, with no clear presentation of the chemical processes and medicine involved. The primary focus for the WHO is mid-upper arm circumference and weight for height. Reading this document might lead one to believe that all malnutrition is acutely severe. It is most certainly not.
Conclusion
The answer to why some children show the swelling of kwashiorkor and some show marasmus probably will not be found in the study of severe acute malnutrition or refeeding syndrome alone. We must go far beyond the WHO’s cookbook recipe.
I think we must start with the study, definition, and treatment of severe chronic malnutrition.
While in Haiti, we shared these data with three organization that are working to provide nutrition in a starving nation. Together, the Baptist Haiti Mission, Mission of Hope Haiti, and Trinity Hope may well be supplying 175,000 meals a day through school lunches and other avenues throughout the country. Their response was telling. Those at Baptist Haiti Mission, an organization with a presence of almost 80 years there, told us that this information was a “big deal.”
The issue for them is the answer to the question, “How can we tell if we are doing any good in our feeding programs?” A lot of money is being thrown into nutrition without tangible ways to assess impact. Clearly parameters such as mid-upper arm circumference and weight for height that WHO advocates is not adequate, as our plots revealed.
We think that a simple, cheap, hemoglobin finger stick can tell us who is falling through the cracks into severe chronic malnutrition and those at risk for severe acute malnutrition. I am an advocate for instituting hemoglobin surveillance as part of all feeding programs. Then we can come up with the cheapest and most effective in-country mechanisms to treat these children.
Indeed that is our next step in working in Haiti.
Dr. Smith is a board certified pediatrician working in McDonough, Ga., with an interest in malnutrition among the children of Haiti. Email him at [email protected].
My wife and I have traveled a number of times as far east as Kyrgyzstan and as far south as Paraguay to participate in short 1- to 2-week medical clinics. When I participated in a week-long medical clinic in Haiti in early 2017, the CEO of the hosting U.S. organization asked, “I wonder if we are doing any good here?” His organization had been to Onaville, Haiti for the last 4-5 years.
So my wife Stacy, a retired licensed practical nurse, and I, a general pediatrician with an interest in severe acute malnutrition, went on a 3-month medical sabbatical to Onaville. We were self-funded, with the exception of our home church in Senoia, Ga., paying the cost of our lodging during that time.
Prior to the 2010 Haiti earthquake, the government planned Onaville to be a retirement community area, with a population of only about 1,500, I was told. After the devastating temblor, it became one of several areas where the government sent people displaced from Port-au-Prince. The population today is possibly 250,000 or more.
The poverty in this area has “newer” flavor than areas such as Cité Soleil, which has been there for decades. What we found in Onaville – and probably all of Haiti – is an appalling lack of understanding and appreciation about the nature of malnutrition.
Methods and materials for study
The 1981 World Health Organization’s last printed monograph about severe acute nutrition remains essentially today’s cookbook recipe for treatment. Little seems to have changed since then in the literature I’ve reviewed. It didn’t take long after we started seeing the children in Onaville to shift that interest to something much more serious and widespread.
I wanted to start with basic health assessments in the Onaville children around 5 years and under. These children rarely see a physician, and only about half or so get any vaccine. Most parents do not have any immunization records in their possession to even review.
We decided to measure head size, mid-upper arm circumference, height, weight, and hemoglobin levels. Date of birth was recorded, if known or could at best be closely estimated. Vaccination was recorded as a yes or no response. All children also were examined for evidence of things like swelling, marasmic appearance (wasting, loss of body fat and muscle), yellowed hair, eye findings of vitamin A deficiency, etc. I wanted to get some impression about the health of these children in the same way that most mobile medical clinics do in Haiti.
Being a database programmer since I bought my first computer in 1985, and having written and deployed my office’s current EMR system in 2000, I decided before ever arriving in Haiti to write the software needed for this task. Unlike regular office EMRs, there were some special considerations.
Growth charts needed not only to be generated for individuals, but in aggregate. Hemoglobins levels, too, needed charting. While in the United States, I use Centers for Disease Control and Prevention growth chart data, but for Haiti I used WHO growth data. I was able to procure hemoglobin charting data as well. Aggregate data turned out to be key to our conclusions.
We used a regular consumer quality digital bathroom scale for weights. A sewing tape attached with duct tape to a wall or pillar was used to measure height. Standard head circumference tapes were use to measure heads and arms.

The hemoglobin was measured with a HemoCue Hb 201+ instrument. Size, ruggedness, and cost dictated all our choices because, except for food, we had to carry everything with us. The cost of a new HemoCue was under $400 and each microcuvette test was about $1.50.
Severe anemia
In total, we saw about 386 children, mostly 5 years and under, in Onaville. Toward the end of the 3 months, we were seeing some of those back as follow-ups. One of the first hemoglobins was 4.9 g/dL, with a 5.4 g/dL on repeat. This stunned us. In the first few days, we were seeing what we saw consistently throughout the course of 3 months.
About 19% of these children had hemoglobins from below 9.0 g/dL to below 6 g/dL. More importantly, there was little on physical exam that would trigger one to do a hemoglobin. Low hemoglobins were not associated with yellow-orange hair. No cases of the swelling of kwashiorkor or pencil-like frames of marasmus were seen. 
Severe chronic malnutrition
The scatter charts are very telling and the hemoglobin graphs are explosive. What is demonstrated is that this recent population is slowly starving to death. How can the hemoglobins be so very low in comparison to the only slightly lowered mean averages (the solid red line)?
In over 3 decades of pediatric medicine, I rarely have seen children in the United States with hemoglobins below 9.5 g/dL. Often they have other illnesses that clearly point to the cause. Could the 19% of children with severely lowered hemoglobins (below 9.0 g/dL) be caused by sickle cell disease or something else in these Haitian children?
A search for articles where sickle cell was studied revealed a study done at St. Damien Pediatrics Hospital in Port-au-Prince (Blood. 2012;120:4235). The overall incidence of sickle cell disease was this: “Of the 2,258 samples tested, 247 had HbS, fifty-seven had HbC, ten had HbSS, and three had HbSC.” Only 0.57% of these children had sickle or sickle-C disease where one could expect hemoglobins to be as low as in the children of Onaville. Applying that percentage to the 386 children we saw would account for about only 2 children who might have sickling anemia. Yet we had 73 children in our study with severely lowered hemoglobins below 9.0 g/dL. If you estimate that half of the 250,000 people in Onaville are children, that extrapolates to over 47,000 with severe anemia! I think that a study larger than ours needs to be done to better assess that, however.
My best thought is that these children who have little external evidence of abnormality and mildly lowered growth data represent a type of malnutrition that has not been defined, much less addressed. I call this severe chronic malnutrition. The very low hemoglobins indicate to me that this is not simply a lack of iron – although certainly that is a factor – but rather that these children are in a state of chronic protein deprivation. They represent a large pool of children who exist between those with normal nutritional states and those with the kwashiorkor or marasmus of severe acute malnutrition.
A search of the 69,823 ICD-10 codes in my database for “malnutrition” only turns up the ill-defined terms, “Unspecified severe protein-calorie malnutrition,” “Moderate, and Mild protein-calorie malnutrition,” “Unspecified protein-calorie malnutrition,” and “Sequelae of protein-calorie malnutrition.” Whatever each of those means is purely subjective in my opinion.
Without a clear understanding or definition of what is severe chronic malnutrition, we are like the Titanic trying to avoid icebergs on a moonless night. I think we must define severe chronic malnutrition before we really can understand the pathophysiology and treatment of severe acute malnutrition.
The WHO published its last printed monograph, “The treatment and management of severe acute protein-energy malnutrition,” in 1981. This publication is essentially a cookbook approach for what to do, with no clear presentation of the chemical processes and medicine involved. The primary focus for the WHO is mid-upper arm circumference and weight for height. Reading this document might lead one to believe that all malnutrition is acutely severe. It is most certainly not.
Conclusion
The answer to why some children show the swelling of kwashiorkor and some show marasmus probably will not be found in the study of severe acute malnutrition or refeeding syndrome alone. We must go far beyond the WHO’s cookbook recipe.
I think we must start with the study, definition, and treatment of severe chronic malnutrition.
While in Haiti, we shared these data with three organization that are working to provide nutrition in a starving nation. Together, the Baptist Haiti Mission, Mission of Hope Haiti, and Trinity Hope may well be supplying 175,000 meals a day through school lunches and other avenues throughout the country. Their response was telling. Those at Baptist Haiti Mission, an organization with a presence of almost 80 years there, told us that this information was a “big deal.”
The issue for them is the answer to the question, “How can we tell if we are doing any good in our feeding programs?” A lot of money is being thrown into nutrition without tangible ways to assess impact. Clearly parameters such as mid-upper arm circumference and weight for height that WHO advocates is not adequate, as our plots revealed.
We think that a simple, cheap, hemoglobin finger stick can tell us who is falling through the cracks into severe chronic malnutrition and those at risk for severe acute malnutrition. I am an advocate for instituting hemoglobin surveillance as part of all feeding programs. Then we can come up with the cheapest and most effective in-country mechanisms to treat these children.
Indeed that is our next step in working in Haiti.
Dr. Smith is a board certified pediatrician working in McDonough, Ga., with an interest in malnutrition among the children of Haiti. Email him at [email protected].
Jaya Aysola: Gender inclusivity
Dr. Aysola is an assistant professor of medicine and pediatrics at the Perelman School of Medicine. Her primary appointment in the Divisions of General Internal Medicine.
In early August 2018, Dr. Aysola and her colleagues published a qualitative narrative analysis regarding the perceptions of the factors associated with inclusive workplaces in healthcare.
Dr. Aysola is an assistant professor of medicine and pediatrics at the Perelman School of Medicine. Her primary appointment in the Divisions of General Internal Medicine.
In early August 2018, Dr. Aysola and her colleagues published a qualitative narrative analysis regarding the perceptions of the factors associated with inclusive workplaces in healthcare.
Dr. Aysola is an assistant professor of medicine and pediatrics at the Perelman School of Medicine. Her primary appointment in the Divisions of General Internal Medicine.
In early August 2018, Dr. Aysola and her colleagues published a qualitative narrative analysis regarding the perceptions of the factors associated with inclusive workplaces in healthcare.
Study challenges LVEF assessment before DLBCL treatment
Measuring left ventricular ejection fraction (LVEF) before administering doxorubicin-based chemotherapy doesn’t appear to add clinically meaningful information, according to an analysis of diffuse large B-cell lymphoma (DLBCL) patients.
Current guidelines recommend prescreening with either echocardiography or multiple-gated acquisition (MUGA) scan to identify asymptomatic left ventricular dysfunction before administering doxorubicin-containing chemotherapy, since doxorubicin is known for its cardiotoxicity.
But other studies have challenged the usefulness of routine LVEF screening in DLBCL patients.
In the current study, Deborah L. Enns, PhD, and her colleagues at Virginia Mason Medical Center in Seattle reviewed the medical records of 291 patients diagnosed with DLBCL between 2001 and 2013.
In total, 206 patients with normal LVEF and 8 patients with low LVEF received doxorubicin (P = .006). But while that association appears to support routine prescreening to inform clinical decision making, the link disappeared when the researchers factored out previous cardiac disease (P = .51).
“It is possible that previous [heart failure] may have played a larger role in shaping treatment decisions than did LVEF test results alone,” the researchers wrote. The report is in Mayo Clinic Proceedings: Innovations, Quality & Outcomes.
In addition, for patients who had their LVEF measured, the researchers found no difference in posttreatment incidence of heart failure based on whether patients received doxorubicin (7.0%) or did not (6.8%). The same was true for patients with LVEF values of less than 50% before treatment (13% vs. 14%).
The researchers noted that there are several reasons why LVEF prescreening may not be needed before treatment in DLBCL. For instance, DLBCL patients typically receive low cumulative doses of doxorubicin. Also, doxorubicin’s high efficacy in DLBCL should be balanced with the relatively low risk of death from heart failure due to doxorubicin treatment, at around 4% after 5 years for patients without preexisting cardiac conditions.
“We recommend that the policy of routinely performing prescreening LVEF measurements in all patients with DLBCL before administering anthracycline-based chemotherapy treatments be reevaluated,” the researchers wrote.
They reported having no competing interests.
SOURCE: Enns DL et al. Mayo Clin Proc Innov Qual Outcomes. 2018 Aug 3;2(3):277-85.
Measuring left ventricular ejection fraction (LVEF) before administering doxorubicin-based chemotherapy doesn’t appear to add clinically meaningful information, according to an analysis of diffuse large B-cell lymphoma (DLBCL) patients.
Current guidelines recommend prescreening with either echocardiography or multiple-gated acquisition (MUGA) scan to identify asymptomatic left ventricular dysfunction before administering doxorubicin-containing chemotherapy, since doxorubicin is known for its cardiotoxicity.
But other studies have challenged the usefulness of routine LVEF screening in DLBCL patients.
In the current study, Deborah L. Enns, PhD, and her colleagues at Virginia Mason Medical Center in Seattle reviewed the medical records of 291 patients diagnosed with DLBCL between 2001 and 2013.
In total, 206 patients with normal LVEF and 8 patients with low LVEF received doxorubicin (P = .006). But while that association appears to support routine prescreening to inform clinical decision making, the link disappeared when the researchers factored out previous cardiac disease (P = .51).
“It is possible that previous [heart failure] may have played a larger role in shaping treatment decisions than did LVEF test results alone,” the researchers wrote. The report is in Mayo Clinic Proceedings: Innovations, Quality & Outcomes.
In addition, for patients who had their LVEF measured, the researchers found no difference in posttreatment incidence of heart failure based on whether patients received doxorubicin (7.0%) or did not (6.8%). The same was true for patients with LVEF values of less than 50% before treatment (13% vs. 14%).
The researchers noted that there are several reasons why LVEF prescreening may not be needed before treatment in DLBCL. For instance, DLBCL patients typically receive low cumulative doses of doxorubicin. Also, doxorubicin’s high efficacy in DLBCL should be balanced with the relatively low risk of death from heart failure due to doxorubicin treatment, at around 4% after 5 years for patients without preexisting cardiac conditions.
“We recommend that the policy of routinely performing prescreening LVEF measurements in all patients with DLBCL before administering anthracycline-based chemotherapy treatments be reevaluated,” the researchers wrote.
They reported having no competing interests.
SOURCE: Enns DL et al. Mayo Clin Proc Innov Qual Outcomes. 2018 Aug 3;2(3):277-85.
Measuring left ventricular ejection fraction (LVEF) before administering doxorubicin-based chemotherapy doesn’t appear to add clinically meaningful information, according to an analysis of diffuse large B-cell lymphoma (DLBCL) patients.
Current guidelines recommend prescreening with either echocardiography or multiple-gated acquisition (MUGA) scan to identify asymptomatic left ventricular dysfunction before administering doxorubicin-containing chemotherapy, since doxorubicin is known for its cardiotoxicity.
But other studies have challenged the usefulness of routine LVEF screening in DLBCL patients.
In the current study, Deborah L. Enns, PhD, and her colleagues at Virginia Mason Medical Center in Seattle reviewed the medical records of 291 patients diagnosed with DLBCL between 2001 and 2013.
In total, 206 patients with normal LVEF and 8 patients with low LVEF received doxorubicin (P = .006). But while that association appears to support routine prescreening to inform clinical decision making, the link disappeared when the researchers factored out previous cardiac disease (P = .51).
“It is possible that previous [heart failure] may have played a larger role in shaping treatment decisions than did LVEF test results alone,” the researchers wrote. The report is in Mayo Clinic Proceedings: Innovations, Quality & Outcomes.
In addition, for patients who had their LVEF measured, the researchers found no difference in posttreatment incidence of heart failure based on whether patients received doxorubicin (7.0%) or did not (6.8%). The same was true for patients with LVEF values of less than 50% before treatment (13% vs. 14%).
The researchers noted that there are several reasons why LVEF prescreening may not be needed before treatment in DLBCL. For instance, DLBCL patients typically receive low cumulative doses of doxorubicin. Also, doxorubicin’s high efficacy in DLBCL should be balanced with the relatively low risk of death from heart failure due to doxorubicin treatment, at around 4% after 5 years for patients without preexisting cardiac conditions.
“We recommend that the policy of routinely performing prescreening LVEF measurements in all patients with DLBCL before administering anthracycline-based chemotherapy treatments be reevaluated,” the researchers wrote.
They reported having no competing interests.
SOURCE: Enns DL et al. Mayo Clin Proc Innov Qual Outcomes. 2018 Aug 3;2(3):277-85.
FROM MAYO CLINIC PROCEEDINGS: INNOVATIONS, QUALITY & OUTCOMES
Key clinical point:
Major finding: Among diffuse large B-cell lymphoma (DLBCL) patients who had LVEF measured, the incidence of heart failure post treatment did not differ between patients who received doxorubicin and those who did not (P = 1.0).
Study details: A retrospective analysis of 291 patients diagnosed with DLBCL between 2001 and 2013.
Disclosures: The researchers reported having no competing interests.
Source: Enns DL et al. Mayo Clin Proc Innov Qual Outcomes. 2018 Aug 3;2(3):277-85.
Cross-contamination of Pathology Specimens: A Cautionary Tale
Cross-contamination of pathology specimens is a rare but nonnegligible source of potential morbidity in clinical practice. Contaminant tissue fragments, colloquially referred to as floaters, typically are readily identifiable based on obvious cytomorphologic differences, especially if the tissues arise from different organs; however, one cannot rely on such distinctions in a pathology laboratory dedicated to a single organ system (eg, dermatopathology). The inability to identify quickly and confidently the presence of a contaminant puts the patient at risk for misdiagnosis, which can lead to unnecessary morbidity or even mortality in the case of cancer misdiagnosis. Studies that have been conducted to estimate the incidence of this type of error have suggested an overall incidence rate between approximately 1% and 3%.1,2 Awareness of this phenomenon and careful scrutiny when the histopathologic evidence diverges considerably from the clinical impression is critical for minimizing the negative outcomes that could result from the presence of contaminant tissue. We present a case in which cross-contamination of a pathology specimen led to an initial erroneous diagnosis of an aggressive cutaneous melanoma in a patient with a benign adnexal neoplasm.
Case Report
A 72-year-old man was referred to the Pigmented Lesion and Melanoma Program at Stanford University Medical Center and Cancer Institute (Palo Alto, California) for evaluation and treatment of a presumed stage IIB melanoma on the right preauricular cheek based on a shave biopsy that had been performed (<1 month prior) by his local dermatology provider and subsequently read by an affiliated out-of-state dermatopathology laboratory. Per the clinical history that was gathered at the current presentation, neither the patient nor his wife had noticed the lesion prior to his dermatology provider pointing it out on the day of the biopsy. Additionally, he denied associated pain, bleeding, or ulceration. According to outside medical records, the referring dermatology provider described the lesion as a 4-mm pink pearly papule with telangiectasia favoring a diagnosis of basal cell carcinoma, and a diagnostic shave biopsy was performed. On presentation to our clinic, physical examination of the right preauricular cheek revealed a 4×3-mm depressed erythematous scar with no evidence of residual pigmentation or nodularity (Figure 1). There was no clinically appreciable regional lymphadenopathy.
The original dermatopathology report indicated an invasive melanoma with the following pathologic characteristics: superficial spreading type, Breslow depth of at least 2.16 mm, ulceration, and a mitotic index of 8 mitotic figures/mm2 with transection of the invasive component at the peripheral and deep margins. There was no evidence of regression, perineural invasion, lymphovascular invasion, or microsatellites. Interestingly, the report indicated that there also was a basaloid proliferation with features of cylindroma in the same pathology slide adjacent to the aggressive invasive melanoma that was described. Given the complexity of cases referred to our academic center, the standard of care includes internal dermatopathology review of all outside pathology specimens. This review proved critical to this patient’s care in light of the considerable divergence of the initial pathologic diagnosis and the reported clinical features of the lesion.
Internal review of the single pathology slide received from the referring provider showed a total of 4 sections, 3 of which are shown here (Figure 2A). Three sections, including the one not shown, were all consistent with a diagnosis of cylindroma and showed no evidence of a melanocytic proliferation (Figure 2B). However, the fourth section demonstrated marked morphologic dissimilarity compared to the other 3 sections. This outlier section showed a thick cutaneous melanoma with a Breslow depth of at least 2.1 mm, ulceration, a mitotic rate of 12 mitotic figures/mm2, and broad transection of the invasive component at the peripheral and deep margins (Figures 2C and 2D). Correlation with the gross description of tissue processing on the original pathology report indicating that the specimen had been trisected raised suspicion that the fourth and very dissimilar section could be a contaminant from another source that was incorporated into our patient’s histologic sections during processing. Taken together, these discrepancies made the diagnosis of cylindroma alone far more likely than cutaneous melanoma, but we needed conclusive evidence given the dramatic difference in prognosis and management between a cylindroma and an aggressive cutaneous melanoma.
For further diagnostic clarification, we performed polymorphic short tandem repeat (STR) analysis, a well-described forensic pathology technique, to determine if the melanoma and cylindroma specimens derived from different patients, as we hypothesized. This analysis revealed differences in all but one DNA locus tested between the cylindroma specimen and the melanoma specimen, confirming our hypothesis (Figure 3). Subsequent discussion of the case with staff from the dermatopathology laboratory that processed this specimen provided further support for our suspicion that the invasive melanoma specimen was part of a case processed prior to our patient’s benign lesion. Therefore, the wide local excision for treatment of the suspected melanoma fortunately was canceled, and the patient did not require further treatment of the benign cylindroma. The patient expressed relief and gratitude for this critical clarification and change in management.
Comment
Shah et al3 reported a similar case in which a benign granuloma of the lung masqueraded as a squamous cell carcinoma due to histopathologic contamination. Although few similar cases have been described in the literature, the risk posed by such contamination is remarkable, regardless of whether it occurs during specimen grossing, embedding, sectioning, or staining.1,4,5 This risk is amplified in facilities that process specimens originating predominantly from a single organ system or tissue type, as is often the case in dedicated dermatopathology laboratories. In this scenario, it is unlikely that one could use the presence of tissues from 2 different organ systems on a single slide as a way of easily recognizing the presence of a contaminant and rectifying the error. Additionally, the presence of malignant cells in the contaminant further complicates the problem and requires an investigation that can conclusively distinguish the contaminant from the patient’s actual tissue.
In our case, our dermatology and dermatopathology teams partnered with our molecular pathology team to find a solution. Polymorphic STR analysis via polymerase chain reaction amplification is a sensitive method employed commonly in forensic DNA laboratories for determining whether a sample submitted as evidence belongs to a given suspect.6 Although much more commonly used in forensics, STR analysis does have known roles in clinical medicine, such as chimerism testing after bone marrow or allogeneic stem cell transplantation.7 Given the relatively short period of time it takes along with the convenience of commercially available kits, a high discriminative ability, and well-validated interpretation procedures, STR analysis is an excellent method for determining if a given tissue sample came from a given patient, which is what was needed in our case.
The combined clinical, histopathologic, and molecular data in our case allowed for confident clarification of our patient’s diagnosis, sparing him the morbidity of wide local excision on the face, sentinel lymph node biopsy, and emotional distress associated with a diagnosis of aggressive cutaneous melanoma. Our case highlights the critical importance of internal review of pathology specimens in ensuring proper diagnosis and management and reminds us that, though rare, accidental contamination during processing of pathology specimens is a potential adverse event that must be considered, especially when a pathologic finding diverges considerably from what is anticipated based on the patient’s history and physical examination.
Acknowledgment
The authors express gratitude to the patient described herein who graciously provided permission for us to publish his case and clinical photography.
- Gephardt GN, Zarbo RJ. Extraneous tissue in surgical pathology: a College of American Pathologists Q-Probes study of 275 laboratories. Arch Pathol Lab Med. 1996;120:1009-1014.
- Alam M, Shah AD, Ali S, et al. Floaters in Mohs micrographic surgery [published online June 27, 2013]. Dermatol Surg. 2013;39:1317-1322.
- Shah PA, Prat MP, Hostler DC. Benign granuloma masquerading as squamous cell carcinoma due to a “floater.” Hawaii J Med Public Health. 2017;76(11, suppl 2):19-21.
- Platt E, Sommer P, McDonald L, et al. Tissue floaters and contaminants in the histology laboratory. Arch Pathol Lab Med. 2009;133:973-978.
- Layfield LJ, Witt BL, Metzger KG, et al. Extraneous tissue: a potential source for diagnostic error in surgical pathology. Am J Clin Pathol. 2011;136:767-772.
- Butler JM. Forensic DNA testing. Cold Spring Harb Protoc. 2011;2011:1438-1450.
- Manasatienkij C, Ra-ngabpai C. Clinical application of forensic DNA analysis: a literature review. J Med Assoc Thai. 2012;95:1357-1363.
Cross-contamination of pathology specimens is a rare but nonnegligible source of potential morbidity in clinical practice. Contaminant tissue fragments, colloquially referred to as floaters, typically are readily identifiable based on obvious cytomorphologic differences, especially if the tissues arise from different organs; however, one cannot rely on such distinctions in a pathology laboratory dedicated to a single organ system (eg, dermatopathology). The inability to identify quickly and confidently the presence of a contaminant puts the patient at risk for misdiagnosis, which can lead to unnecessary morbidity or even mortality in the case of cancer misdiagnosis. Studies that have been conducted to estimate the incidence of this type of error have suggested an overall incidence rate between approximately 1% and 3%.1,2 Awareness of this phenomenon and careful scrutiny when the histopathologic evidence diverges considerably from the clinical impression is critical for minimizing the negative outcomes that could result from the presence of contaminant tissue. We present a case in which cross-contamination of a pathology specimen led to an initial erroneous diagnosis of an aggressive cutaneous melanoma in a patient with a benign adnexal neoplasm.
Case Report
A 72-year-old man was referred to the Pigmented Lesion and Melanoma Program at Stanford University Medical Center and Cancer Institute (Palo Alto, California) for evaluation and treatment of a presumed stage IIB melanoma on the right preauricular cheek based on a shave biopsy that had been performed (<1 month prior) by his local dermatology provider and subsequently read by an affiliated out-of-state dermatopathology laboratory. Per the clinical history that was gathered at the current presentation, neither the patient nor his wife had noticed the lesion prior to his dermatology provider pointing it out on the day of the biopsy. Additionally, he denied associated pain, bleeding, or ulceration. According to outside medical records, the referring dermatology provider described the lesion as a 4-mm pink pearly papule with telangiectasia favoring a diagnosis of basal cell carcinoma, and a diagnostic shave biopsy was performed. On presentation to our clinic, physical examination of the right preauricular cheek revealed a 4×3-mm depressed erythematous scar with no evidence of residual pigmentation or nodularity (Figure 1). There was no clinically appreciable regional lymphadenopathy.

The original dermatopathology report indicated an invasive melanoma with the following pathologic characteristics: superficial spreading type, Breslow depth of at least 2.16 mm, ulceration, and a mitotic index of 8 mitotic figures/mm2 with transection of the invasive component at the peripheral and deep margins. There was no evidence of regression, perineural invasion, lymphovascular invasion, or microsatellites. Interestingly, the report indicated that there also was a basaloid proliferation with features of cylindroma in the same pathology slide adjacent to the aggressive invasive melanoma that was described. Given the complexity of cases referred to our academic center, the standard of care includes internal dermatopathology review of all outside pathology specimens. This review proved critical to this patient’s care in light of the considerable divergence of the initial pathologic diagnosis and the reported clinical features of the lesion.
Internal review of the single pathology slide received from the referring provider showed a total of 4 sections, 3 of which are shown here (Figure 2A). Three sections, including the one not shown, were all consistent with a diagnosis of cylindroma and showed no evidence of a melanocytic proliferation (Figure 2B). However, the fourth section demonstrated marked morphologic dissimilarity compared to the other 3 sections. This outlier section showed a thick cutaneous melanoma with a Breslow depth of at least 2.1 mm, ulceration, a mitotic rate of 12 mitotic figures/mm2, and broad transection of the invasive component at the peripheral and deep margins (Figures 2C and 2D). Correlation with the gross description of tissue processing on the original pathology report indicating that the specimen had been trisected raised suspicion that the fourth and very dissimilar section could be a contaminant from another source that was incorporated into our patient’s histologic sections during processing. Taken together, these discrepancies made the diagnosis of cylindroma alone far more likely than cutaneous melanoma, but we needed conclusive evidence given the dramatic difference in prognosis and management between a cylindroma and an aggressive cutaneous melanoma.

For further diagnostic clarification, we performed polymorphic short tandem repeat (STR) analysis, a well-described forensic pathology technique, to determine if the melanoma and cylindroma specimens derived from different patients, as we hypothesized. This analysis revealed differences in all but one DNA locus tested between the cylindroma specimen and the melanoma specimen, confirming our hypothesis (Figure 3). Subsequent discussion of the case with staff from the dermatopathology laboratory that processed this specimen provided further support for our suspicion that the invasive melanoma specimen was part of a case processed prior to our patient’s benign lesion. Therefore, the wide local excision for treatment of the suspected melanoma fortunately was canceled, and the patient did not require further treatment of the benign cylindroma. The patient expressed relief and gratitude for this critical clarification and change in management.

Comment
Shah et al3 reported a similar case in which a benign granuloma of the lung masqueraded as a squamous cell carcinoma due to histopathologic contamination. Although few similar cases have been described in the literature, the risk posed by such contamination is remarkable, regardless of whether it occurs during specimen grossing, embedding, sectioning, or staining.1,4,5 This risk is amplified in facilities that process specimens originating predominantly from a single organ system or tissue type, as is often the case in dedicated dermatopathology laboratories. In this scenario, it is unlikely that one could use the presence of tissues from 2 different organ systems on a single slide as a way of easily recognizing the presence of a contaminant and rectifying the error. Additionally, the presence of malignant cells in the contaminant further complicates the problem and requires an investigation that can conclusively distinguish the contaminant from the patient’s actual tissue.
In our case, our dermatology and dermatopathology teams partnered with our molecular pathology team to find a solution. Polymorphic STR analysis via polymerase chain reaction amplification is a sensitive method employed commonly in forensic DNA laboratories for determining whether a sample submitted as evidence belongs to a given suspect.6 Although much more commonly used in forensics, STR analysis does have known roles in clinical medicine, such as chimerism testing after bone marrow or allogeneic stem cell transplantation.7 Given the relatively short period of time it takes along with the convenience of commercially available kits, a high discriminative ability, and well-validated interpretation procedures, STR analysis is an excellent method for determining if a given tissue sample came from a given patient, which is what was needed in our case.
The combined clinical, histopathologic, and molecular data in our case allowed for confident clarification of our patient’s diagnosis, sparing him the morbidity of wide local excision on the face, sentinel lymph node biopsy, and emotional distress associated with a diagnosis of aggressive cutaneous melanoma. Our case highlights the critical importance of internal review of pathology specimens in ensuring proper diagnosis and management and reminds us that, though rare, accidental contamination during processing of pathology specimens is a potential adverse event that must be considered, especially when a pathologic finding diverges considerably from what is anticipated based on the patient’s history and physical examination.
Acknowledgment
The authors express gratitude to the patient described herein who graciously provided permission for us to publish his case and clinical photography.
Cross-contamination of pathology specimens is a rare but nonnegligible source of potential morbidity in clinical practice. Contaminant tissue fragments, colloquially referred to as floaters, typically are readily identifiable based on obvious cytomorphologic differences, especially if the tissues arise from different organs; however, one cannot rely on such distinctions in a pathology laboratory dedicated to a single organ system (eg, dermatopathology). The inability to identify quickly and confidently the presence of a contaminant puts the patient at risk for misdiagnosis, which can lead to unnecessary morbidity or even mortality in the case of cancer misdiagnosis. Studies that have been conducted to estimate the incidence of this type of error have suggested an overall incidence rate between approximately 1% and 3%.1,2 Awareness of this phenomenon and careful scrutiny when the histopathologic evidence diverges considerably from the clinical impression is critical for minimizing the negative outcomes that could result from the presence of contaminant tissue. We present a case in which cross-contamination of a pathology specimen led to an initial erroneous diagnosis of an aggressive cutaneous melanoma in a patient with a benign adnexal neoplasm.
Case Report
A 72-year-old man was referred to the Pigmented Lesion and Melanoma Program at Stanford University Medical Center and Cancer Institute (Palo Alto, California) for evaluation and treatment of a presumed stage IIB melanoma on the right preauricular cheek based on a shave biopsy that had been performed (<1 month prior) by his local dermatology provider and subsequently read by an affiliated out-of-state dermatopathology laboratory. Per the clinical history that was gathered at the current presentation, neither the patient nor his wife had noticed the lesion prior to his dermatology provider pointing it out on the day of the biopsy. Additionally, he denied associated pain, bleeding, or ulceration. According to outside medical records, the referring dermatology provider described the lesion as a 4-mm pink pearly papule with telangiectasia favoring a diagnosis of basal cell carcinoma, and a diagnostic shave biopsy was performed. On presentation to our clinic, physical examination of the right preauricular cheek revealed a 4×3-mm depressed erythematous scar with no evidence of residual pigmentation or nodularity (Figure 1). There was no clinically appreciable regional lymphadenopathy.

The original dermatopathology report indicated an invasive melanoma with the following pathologic characteristics: superficial spreading type, Breslow depth of at least 2.16 mm, ulceration, and a mitotic index of 8 mitotic figures/mm2 with transection of the invasive component at the peripheral and deep margins. There was no evidence of regression, perineural invasion, lymphovascular invasion, or microsatellites. Interestingly, the report indicated that there also was a basaloid proliferation with features of cylindroma in the same pathology slide adjacent to the aggressive invasive melanoma that was described. Given the complexity of cases referred to our academic center, the standard of care includes internal dermatopathology review of all outside pathology specimens. This review proved critical to this patient’s care in light of the considerable divergence of the initial pathologic diagnosis and the reported clinical features of the lesion.
Internal review of the single pathology slide received from the referring provider showed a total of 4 sections, 3 of which are shown here (Figure 2A). Three sections, including the one not shown, were all consistent with a diagnosis of cylindroma and showed no evidence of a melanocytic proliferation (Figure 2B). However, the fourth section demonstrated marked morphologic dissimilarity compared to the other 3 sections. This outlier section showed a thick cutaneous melanoma with a Breslow depth of at least 2.1 mm, ulceration, a mitotic rate of 12 mitotic figures/mm2, and broad transection of the invasive component at the peripheral and deep margins (Figures 2C and 2D). Correlation with the gross description of tissue processing on the original pathology report indicating that the specimen had been trisected raised suspicion that the fourth and very dissimilar section could be a contaminant from another source that was incorporated into our patient’s histologic sections during processing. Taken together, these discrepancies made the diagnosis of cylindroma alone far more likely than cutaneous melanoma, but we needed conclusive evidence given the dramatic difference in prognosis and management between a cylindroma and an aggressive cutaneous melanoma.

For further diagnostic clarification, we performed polymorphic short tandem repeat (STR) analysis, a well-described forensic pathology technique, to determine if the melanoma and cylindroma specimens derived from different patients, as we hypothesized. This analysis revealed differences in all but one DNA locus tested between the cylindroma specimen and the melanoma specimen, confirming our hypothesis (Figure 3). Subsequent discussion of the case with staff from the dermatopathology laboratory that processed this specimen provided further support for our suspicion that the invasive melanoma specimen was part of a case processed prior to our patient’s benign lesion. Therefore, the wide local excision for treatment of the suspected melanoma fortunately was canceled, and the patient did not require further treatment of the benign cylindroma. The patient expressed relief and gratitude for this critical clarification and change in management.

Comment
Shah et al3 reported a similar case in which a benign granuloma of the lung masqueraded as a squamous cell carcinoma due to histopathologic contamination. Although few similar cases have been described in the literature, the risk posed by such contamination is remarkable, regardless of whether it occurs during specimen grossing, embedding, sectioning, or staining.1,4,5 This risk is amplified in facilities that process specimens originating predominantly from a single organ system or tissue type, as is often the case in dedicated dermatopathology laboratories. In this scenario, it is unlikely that one could use the presence of tissues from 2 different organ systems on a single slide as a way of easily recognizing the presence of a contaminant and rectifying the error. Additionally, the presence of malignant cells in the contaminant further complicates the problem and requires an investigation that can conclusively distinguish the contaminant from the patient’s actual tissue.
In our case, our dermatology and dermatopathology teams partnered with our molecular pathology team to find a solution. Polymorphic STR analysis via polymerase chain reaction amplification is a sensitive method employed commonly in forensic DNA laboratories for determining whether a sample submitted as evidence belongs to a given suspect.6 Although much more commonly used in forensics, STR analysis does have known roles in clinical medicine, such as chimerism testing after bone marrow or allogeneic stem cell transplantation.7 Given the relatively short period of time it takes along with the convenience of commercially available kits, a high discriminative ability, and well-validated interpretation procedures, STR analysis is an excellent method for determining if a given tissue sample came from a given patient, which is what was needed in our case.
The combined clinical, histopathologic, and molecular data in our case allowed for confident clarification of our patient’s diagnosis, sparing him the morbidity of wide local excision on the face, sentinel lymph node biopsy, and emotional distress associated with a diagnosis of aggressive cutaneous melanoma. Our case highlights the critical importance of internal review of pathology specimens in ensuring proper diagnosis and management and reminds us that, though rare, accidental contamination during processing of pathology specimens is a potential adverse event that must be considered, especially when a pathologic finding diverges considerably from what is anticipated based on the patient’s history and physical examination.
Acknowledgment
The authors express gratitude to the patient described herein who graciously provided permission for us to publish his case and clinical photography.
- Gephardt GN, Zarbo RJ. Extraneous tissue in surgical pathology: a College of American Pathologists Q-Probes study of 275 laboratories. Arch Pathol Lab Med. 1996;120:1009-1014.
- Alam M, Shah AD, Ali S, et al. Floaters in Mohs micrographic surgery [published online June 27, 2013]. Dermatol Surg. 2013;39:1317-1322.
- Shah PA, Prat MP, Hostler DC. Benign granuloma masquerading as squamous cell carcinoma due to a “floater.” Hawaii J Med Public Health. 2017;76(11, suppl 2):19-21.
- Platt E, Sommer P, McDonald L, et al. Tissue floaters and contaminants in the histology laboratory. Arch Pathol Lab Med. 2009;133:973-978.
- Layfield LJ, Witt BL, Metzger KG, et al. Extraneous tissue: a potential source for diagnostic error in surgical pathology. Am J Clin Pathol. 2011;136:767-772.
- Butler JM. Forensic DNA testing. Cold Spring Harb Protoc. 2011;2011:1438-1450.
- Manasatienkij C, Ra-ngabpai C. Clinical application of forensic DNA analysis: a literature review. J Med Assoc Thai. 2012;95:1357-1363.
- Gephardt GN, Zarbo RJ. Extraneous tissue in surgical pathology: a College of American Pathologists Q-Probes study of 275 laboratories. Arch Pathol Lab Med. 1996;120:1009-1014.
- Alam M, Shah AD, Ali S, et al. Floaters in Mohs micrographic surgery [published online June 27, 2013]. Dermatol Surg. 2013;39:1317-1322.
- Shah PA, Prat MP, Hostler DC. Benign granuloma masquerading as squamous cell carcinoma due to a “floater.” Hawaii J Med Public Health. 2017;76(11, suppl 2):19-21.
- Platt E, Sommer P, McDonald L, et al. Tissue floaters and contaminants in the histology laboratory. Arch Pathol Lab Med. 2009;133:973-978.
- Layfield LJ, Witt BL, Metzger KG, et al. Extraneous tissue: a potential source for diagnostic error in surgical pathology. Am J Clin Pathol. 2011;136:767-772.
- Butler JM. Forensic DNA testing. Cold Spring Harb Protoc. 2011;2011:1438-1450.
- Manasatienkij C, Ra-ngabpai C. Clinical application of forensic DNA analysis: a literature review. J Med Assoc Thai. 2012;95:1357-1363.
Resident Pearl
- Although cross-contamination of pathology specimens is rare, it does occur and can impact diagnosis and management if detected early.