User login
Pay attention to kidney disease risk in people living with HIV
The prevalence of chronic kidney disease (CKD) in people living with HIV varied widely, depending on population and criteria, according to a systematic literature review of the PubMed and PsycInfo databases for articles published from January 2000 through August 2016.
The review included all studies that involved adults older than 21 years of age, investigated people living with HIV with CKD, reported prevalence of CKD, and were published in a peer-reviewed journal, according to Jungmin Park, PhD, RN, of CHA University, Pocheon-Si, South Korea, and her colleague.
Out of an initial search yielding 1,960 citations in PubMed and 5,356 citations in PsycInfo, the results were pared down to 21 articles, which met all of the inclusion/exclusion criteria and were used for the final analysis.
The risk factors for CKD in people living with HIV cited most often in the studies consisted of medications, hypertension, older age, diabetes mellitus, hepatitis coinfection (with hepatitis C virus more prominent than hepatitis B virus), low CD4+ T-cell count, and race, Dr. Park and her colleague reported.
Of the various risk factors, the only ones unique to HIV were viral load and CD4+ T-cell count. One study reporting on 5,538 treatment-naive patients in mainland China suggested that HIV viral replication in renal cells may be the cause of renal damage in patients with high viral loads, meaning that viral suppression would improve renal function. However, all of these risk factors are intrinsically linked, according to Dr. Park and her colleague. They added that managing viral load alone would be ineffective in preventing CKD: “Therefore [people living with HIV] will need to effectively manage every aspect of their health, including metabolic and cardiovascular systems.”
Of the 43,114 people living with HIV across the 21 studies, 3,218 (7.3%) had CKD. The reported prevalence of CKD ranged from 2.3% to 53.3%, with the African population having the highest prevalence. Some of the wide variation was possibly attributable to differences in the definitions of CKD used across the various studies.
“The risk of under-diagnosis of CKD can lead to long-term health complications. Health care providers must monitor kidney function and treatment for renal damage carefully, especially for people living with HIV with additional diagnoses of diabetes and/or hypertension, and for those who are aging,” Dr. Park and her colleague concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Park, J et al. J Assoc Nurses AIDS Care. 2018;29:655-66.
The prevalence of chronic kidney disease (CKD) in people living with HIV varied widely, depending on population and criteria, according to a systematic literature review of the PubMed and PsycInfo databases for articles published from January 2000 through August 2016.
The review included all studies that involved adults older than 21 years of age, investigated people living with HIV with CKD, reported prevalence of CKD, and were published in a peer-reviewed journal, according to Jungmin Park, PhD, RN, of CHA University, Pocheon-Si, South Korea, and her colleague.
Out of an initial search yielding 1,960 citations in PubMed and 5,356 citations in PsycInfo, the results were pared down to 21 articles, which met all of the inclusion/exclusion criteria and were used for the final analysis.
The risk factors for CKD in people living with HIV cited most often in the studies consisted of medications, hypertension, older age, diabetes mellitus, hepatitis coinfection (with hepatitis C virus more prominent than hepatitis B virus), low CD4+ T-cell count, and race, Dr. Park and her colleague reported.
Of the various risk factors, the only ones unique to HIV were viral load and CD4+ T-cell count. One study reporting on 5,538 treatment-naive patients in mainland China suggested that HIV viral replication in renal cells may be the cause of renal damage in patients with high viral loads, meaning that viral suppression would improve renal function. However, all of these risk factors are intrinsically linked, according to Dr. Park and her colleague. They added that managing viral load alone would be ineffective in preventing CKD: “Therefore [people living with HIV] will need to effectively manage every aspect of their health, including metabolic and cardiovascular systems.”
Of the 43,114 people living with HIV across the 21 studies, 3,218 (7.3%) had CKD. The reported prevalence of CKD ranged from 2.3% to 53.3%, with the African population having the highest prevalence. Some of the wide variation was possibly attributable to differences in the definitions of CKD used across the various studies.
“The risk of under-diagnosis of CKD can lead to long-term health complications. Health care providers must monitor kidney function and treatment for renal damage carefully, especially for people living with HIV with additional diagnoses of diabetes and/or hypertension, and for those who are aging,” Dr. Park and her colleague concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Park, J et al. J Assoc Nurses AIDS Care. 2018;29:655-66.
The prevalence of chronic kidney disease (CKD) in people living with HIV varied widely, depending on population and criteria, according to a systematic literature review of the PubMed and PsycInfo databases for articles published from January 2000 through August 2016.
The review included all studies that involved adults older than 21 years of age, investigated people living with HIV with CKD, reported prevalence of CKD, and were published in a peer-reviewed journal, according to Jungmin Park, PhD, RN, of CHA University, Pocheon-Si, South Korea, and her colleague.
Out of an initial search yielding 1,960 citations in PubMed and 5,356 citations in PsycInfo, the results were pared down to 21 articles, which met all of the inclusion/exclusion criteria and were used for the final analysis.
The risk factors for CKD in people living with HIV cited most often in the studies consisted of medications, hypertension, older age, diabetes mellitus, hepatitis coinfection (with hepatitis C virus more prominent than hepatitis B virus), low CD4+ T-cell count, and race, Dr. Park and her colleague reported.
Of the various risk factors, the only ones unique to HIV were viral load and CD4+ T-cell count. One study reporting on 5,538 treatment-naive patients in mainland China suggested that HIV viral replication in renal cells may be the cause of renal damage in patients with high viral loads, meaning that viral suppression would improve renal function. However, all of these risk factors are intrinsically linked, according to Dr. Park and her colleague. They added that managing viral load alone would be ineffective in preventing CKD: “Therefore [people living with HIV] will need to effectively manage every aspect of their health, including metabolic and cardiovascular systems.”
Of the 43,114 people living with HIV across the 21 studies, 3,218 (7.3%) had CKD. The reported prevalence of CKD ranged from 2.3% to 53.3%, with the African population having the highest prevalence. Some of the wide variation was possibly attributable to differences in the definitions of CKD used across the various studies.
“The risk of under-diagnosis of CKD can lead to long-term health complications. Health care providers must monitor kidney function and treatment for renal damage carefully, especially for people living with HIV with additional diagnoses of diabetes and/or hypertension, and for those who are aging,” Dr. Park and her colleague concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Park, J et al. J Assoc Nurses AIDS Care. 2018;29:655-66.
FROM THE JOURNAL OF THE ASSOCIATION OF NURSES IN AIDS CARE
Key clinical point: Chronic kidney disease in people living with HIV varies widely across geographic regions.
Major finding: The reported prevalence of CKD in PLWH ranged from 2.3% to 53.3%, with the African population having the highest prevalence.
Study details: Systematic literature review of the PubMed and PsycInfo databases for articles published from January 2000 through August 2016.
Disclosures: The authors reported that they had no conflicts of interest.
Source: J Assoc Nurses AIDS Care. 2018;29:655-66).
HIV testing low in U.S. women engaged in risky behavior
HIV testing rates were low among women whose sexual behaviors increased their risk of HIV infection, and they were especially low among women who reported having anal sex, according to a report published in the American Journal of Obstetrics & Gynecology.
Data from the 2011-2015 National Survey of Family Growth were analyzed to estimate the proportion of sexually active, nonpregnant U.S. women aged 15-44 years who had had an HIV test within the past year. The data was stratified by those who reported anal sex and other risk factors, including having more than two sexual partners, condomless sex with a new partner or multiple partners, gonorrhea in the past year, or any history of syphilis, according to Mary Evans, MD, of the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention at the Centers for Disease Control and Prevention and her colleagues.
Among the 42.4 million sexually active women assessed, 9.0 million (20%) reported they had had anal sex in the past year. Of these, 19% reported that their providers asked about their types of intercourse, and 20% reported an HIV test within the past year. Overall, HIV testing was higher among women who reported anal sex and whose providers asked about types of sex engaged in than it was among those women whose provider did not ask (38% vs. 16%, respectively; P less than .001). However, HIV testing in the past year was higher for women with other forms of risky behaviors as compared with anal sex, ranging from 35.8% to 47.2%.
“Women who report sexual behaviors such as anal sex would benefit from an HIV test and an assessment for [prevention with preexposure prophylaxis] eligibility. Women’s health care providers are uniquely poised to provide HIV prevention for women who tend to have frequent encounters with the health care system,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Evans ME et al. Am J Obstet Gynecol. 2018 Oct;219(4):383.e1-7.
HIV testing rates were low among women whose sexual behaviors increased their risk of HIV infection, and they were especially low among women who reported having anal sex, according to a report published in the American Journal of Obstetrics & Gynecology.
Data from the 2011-2015 National Survey of Family Growth were analyzed to estimate the proportion of sexually active, nonpregnant U.S. women aged 15-44 years who had had an HIV test within the past year. The data was stratified by those who reported anal sex and other risk factors, including having more than two sexual partners, condomless sex with a new partner or multiple partners, gonorrhea in the past year, or any history of syphilis, according to Mary Evans, MD, of the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention at the Centers for Disease Control and Prevention and her colleagues.
Among the 42.4 million sexually active women assessed, 9.0 million (20%) reported they had had anal sex in the past year. Of these, 19% reported that their providers asked about their types of intercourse, and 20% reported an HIV test within the past year. Overall, HIV testing was higher among women who reported anal sex and whose providers asked about types of sex engaged in than it was among those women whose provider did not ask (38% vs. 16%, respectively; P less than .001). However, HIV testing in the past year was higher for women with other forms of risky behaviors as compared with anal sex, ranging from 35.8% to 47.2%.
“Women who report sexual behaviors such as anal sex would benefit from an HIV test and an assessment for [prevention with preexposure prophylaxis] eligibility. Women’s health care providers are uniquely poised to provide HIV prevention for women who tend to have frequent encounters with the health care system,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Evans ME et al. Am J Obstet Gynecol. 2018 Oct;219(4):383.e1-7.
HIV testing rates were low among women whose sexual behaviors increased their risk of HIV infection, and they were especially low among women who reported having anal sex, according to a report published in the American Journal of Obstetrics & Gynecology.
Data from the 2011-2015 National Survey of Family Growth were analyzed to estimate the proportion of sexually active, nonpregnant U.S. women aged 15-44 years who had had an HIV test within the past year. The data was stratified by those who reported anal sex and other risk factors, including having more than two sexual partners, condomless sex with a new partner or multiple partners, gonorrhea in the past year, or any history of syphilis, according to Mary Evans, MD, of the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention at the Centers for Disease Control and Prevention and her colleagues.
Among the 42.4 million sexually active women assessed, 9.0 million (20%) reported they had had anal sex in the past year. Of these, 19% reported that their providers asked about their types of intercourse, and 20% reported an HIV test within the past year. Overall, HIV testing was higher among women who reported anal sex and whose providers asked about types of sex engaged in than it was among those women whose provider did not ask (38% vs. 16%, respectively; P less than .001). However, HIV testing in the past year was higher for women with other forms of risky behaviors as compared with anal sex, ranging from 35.8% to 47.2%.
“Women who report sexual behaviors such as anal sex would benefit from an HIV test and an assessment for [prevention with preexposure prophylaxis] eligibility. Women’s health care providers are uniquely poised to provide HIV prevention for women who tend to have frequent encounters with the health care system,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Evans ME et al. Am J Obstet Gynecol. 2018 Oct;219(4):383.e1-7.
FROM THE AMERICAN JOURNAL OF OBSTETRICS & GYNECOLOGY
Key clinical point: Health care providers don’t ask sexually active women about risky behavior that would raise their risk of HIV infection.
Major finding: Of women who reported having anal sex, 19% reported that their providers asked about their types of intercourse.
Study details: Data from the 2011-2015 National Survey of Family Growth.
Disclosures: The authors reported that they had no conflicts of interest.
Source: Evans ME et al. Am J Obstet Gynecol. 2018 Oct;219(4):383.e1-7.
Bipolar patients’ relatives face increased cardiovascular risk
BARCELONA – Young patients recently diagnosed with bipolar disorder are at double the 30-year risk of cardiovascular disease, compared with the general population, and their unaffected first-degree relatives are nearly as high risk, Klara Coello, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
The clinical implication of this finding is that unaffected first-degree relatives of patients with bipolar disorder – an affective disorder typically diagnosed at age 15-24 – should be targeted for intensified primary cardiovascular prevention, with a focus on smoking and dyslipidemia, both of which were more prevalent in these patients and their unaffected relatives than in the general population in her study, noted Dr. Coello, a doctoral candidate with the Copenhagen Affective Disorders Research Center at the University of Copenhagen.
She and her coinvestigators presented a cross-sectional study in which they calculated the 30-year Framingham Risk Scores for 221 patients recently diagnosed bipolar disorder – 95% of whom had been diagnosed within the past 2 years – along with 50 unaffected first-degree relatives and 119 age- and sex-matched controls. The investigators used the Framingham Risk Score because the widely used American Heart Association/American College of Cardiology Atherosclerotic Cardiovascular Disease Risk Estimator applies only to individuals aged 40 and up.
The key findings: The 30-year risk of cardiovascular disease for patients with bipolar was 98.5% greater than that of controls, and the calculated risk of the unaffected first-degree relatives was increased by 85.4%, compared with that of controls.
The Framingham Risk Score is determined on the basis of old-school cardiovascular risk factors, including age, gender, lipids, systolic blood pressure, diabetes, and smoking. 45% of the bipolar patients were smokers, as were 20% of their first-degree relatives and 13% of controls.
The Danish finding of increased cardiovascular risk in young adults with bipolar disorder recapitulates an American Heart Association Scientific Statement, which was published in Circulation (2015 Sep 8;132[10]:965-86). The statement was intended to alert clinicians that these affective disorders constitute “moderate-risk” conditions for arterial dysfunction prior to age 30 and for premature cardiovascular disease (CVD). The statement declared that this risk is likely mediated not only by the classic cardiovascular risk factors but also by disease-related inflammation, oxidative stress, sleep disruption, and the adverse metabolic effects of many psychotropic medications.
“The magnitude of increased risk for CVD in adulthood is substantial,” according to the AHA expert panel’s scientific statement.
Dr. Coello’s study only took into account levels of the traditional cardiovascular risk factors. Where the study broke new ground that hadn’t been explored in the AHA scientific statement, however, was in identifying unaffected first-degree relatives as an additional at-risk group.
She reported having no financial conflicts regarding her study, which constitutes her PhD thesis.
BARCELONA – Young patients recently diagnosed with bipolar disorder are at double the 30-year risk of cardiovascular disease, compared with the general population, and their unaffected first-degree relatives are nearly as high risk, Klara Coello, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
The clinical implication of this finding is that unaffected first-degree relatives of patients with bipolar disorder – an affective disorder typically diagnosed at age 15-24 – should be targeted for intensified primary cardiovascular prevention, with a focus on smoking and dyslipidemia, both of which were more prevalent in these patients and their unaffected relatives than in the general population in her study, noted Dr. Coello, a doctoral candidate with the Copenhagen Affective Disorders Research Center at the University of Copenhagen.
She and her coinvestigators presented a cross-sectional study in which they calculated the 30-year Framingham Risk Scores for 221 patients recently diagnosed bipolar disorder – 95% of whom had been diagnosed within the past 2 years – along with 50 unaffected first-degree relatives and 119 age- and sex-matched controls. The investigators used the Framingham Risk Score because the widely used American Heart Association/American College of Cardiology Atherosclerotic Cardiovascular Disease Risk Estimator applies only to individuals aged 40 and up.
The key findings: The 30-year risk of cardiovascular disease for patients with bipolar was 98.5% greater than that of controls, and the calculated risk of the unaffected first-degree relatives was increased by 85.4%, compared with that of controls.
The Framingham Risk Score is determined on the basis of old-school cardiovascular risk factors, including age, gender, lipids, systolic blood pressure, diabetes, and smoking. 45% of the bipolar patients were smokers, as were 20% of their first-degree relatives and 13% of controls.
The Danish finding of increased cardiovascular risk in young adults with bipolar disorder recapitulates an American Heart Association Scientific Statement, which was published in Circulation (2015 Sep 8;132[10]:965-86). The statement was intended to alert clinicians that these affective disorders constitute “moderate-risk” conditions for arterial dysfunction prior to age 30 and for premature cardiovascular disease (CVD). The statement declared that this risk is likely mediated not only by the classic cardiovascular risk factors but also by disease-related inflammation, oxidative stress, sleep disruption, and the adverse metabolic effects of many psychotropic medications.
“The magnitude of increased risk for CVD in adulthood is substantial,” according to the AHA expert panel’s scientific statement.
Dr. Coello’s study only took into account levels of the traditional cardiovascular risk factors. Where the study broke new ground that hadn’t been explored in the AHA scientific statement, however, was in identifying unaffected first-degree relatives as an additional at-risk group.
She reported having no financial conflicts regarding her study, which constitutes her PhD thesis.
BARCELONA – Young patients recently diagnosed with bipolar disorder are at double the 30-year risk of cardiovascular disease, compared with the general population, and their unaffected first-degree relatives are nearly as high risk, Klara Coello, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
The clinical implication of this finding is that unaffected first-degree relatives of patients with bipolar disorder – an affective disorder typically diagnosed at age 15-24 – should be targeted for intensified primary cardiovascular prevention, with a focus on smoking and dyslipidemia, both of which were more prevalent in these patients and their unaffected relatives than in the general population in her study, noted Dr. Coello, a doctoral candidate with the Copenhagen Affective Disorders Research Center at the University of Copenhagen.
She and her coinvestigators presented a cross-sectional study in which they calculated the 30-year Framingham Risk Scores for 221 patients recently diagnosed bipolar disorder – 95% of whom had been diagnosed within the past 2 years – along with 50 unaffected first-degree relatives and 119 age- and sex-matched controls. The investigators used the Framingham Risk Score because the widely used American Heart Association/American College of Cardiology Atherosclerotic Cardiovascular Disease Risk Estimator applies only to individuals aged 40 and up.
The key findings: The 30-year risk of cardiovascular disease for patients with bipolar was 98.5% greater than that of controls, and the calculated risk of the unaffected first-degree relatives was increased by 85.4%, compared with that of controls.
The Framingham Risk Score is determined on the basis of old-school cardiovascular risk factors, including age, gender, lipids, systolic blood pressure, diabetes, and smoking. 45% of the bipolar patients were smokers, as were 20% of their first-degree relatives and 13% of controls.
The Danish finding of increased cardiovascular risk in young adults with bipolar disorder recapitulates an American Heart Association Scientific Statement, which was published in Circulation (2015 Sep 8;132[10]:965-86). The statement was intended to alert clinicians that these affective disorders constitute “moderate-risk” conditions for arterial dysfunction prior to age 30 and for premature cardiovascular disease (CVD). The statement declared that this risk is likely mediated not only by the classic cardiovascular risk factors but also by disease-related inflammation, oxidative stress, sleep disruption, and the adverse metabolic effects of many psychotropic medications.
“The magnitude of increased risk for CVD in adulthood is substantial,” according to the AHA expert panel’s scientific statement.
Dr. Coello’s study only took into account levels of the traditional cardiovascular risk factors. Where the study broke new ground that hadn’t been explored in the AHA scientific statement, however, was in identifying unaffected first-degree relatives as an additional at-risk group.
She reported having no financial conflicts regarding her study, which constitutes her PhD thesis.
REPORTING FROM THE ECNP CONGRESS
Key clinical point: The first-degree relatives of patients with bipolar disorder should be targeted for intensified primary cardiovascular prevention.
Major finding: Thirty-year cardiovascular risk was increased by 98.5% in recently diagnosed bipolar patients and by 85.4% in their unaffected first-degree relatives, compared with the general population.
Study details: This cross-sectional study involved calculation of 30-year Framingham Risk Scores for 221 patients recently diagnosed with bipolar disorder, 50 unaffected first-degree relatives, and 119 age- and sex-matched controls.
Disclosures: The study presenter reported having no financial conflicts of interest.
Don’t let the mortgage preapproval process give you a stomachache
You are trying to buy your first home. Maybe you have heard stories from family, friends, and colleagues about nightmare scenarios when purchasing a home. There are many facets to the home-financing process, and a little bit of planning can reduce a significant amount of time and stress. Where do you begin? What do lenders look for when preapproving a borrower? What steps do I take to get preapproved for a mortgage loan? This article will help guide you through these initial stages to ultimately guide you to settlement on your new home.
Where to begin?
- Start by drafting a budget. How much of a monthly housing payment can you afford? Planning a budget is an extremely valuable exercise at any point in life, not just when buying a home. Often, borrowers will ask the question “How much can I afford?” The better question to ask is “Can I qualify for a home that meets the maximum monthly payment I have budgeted for?”
- What funds would I use for purchasing a home? Down payments and closing costs can add up quickly. Do you have funds readily available in an account you hold? Will you be obtaining a gift from a family member? Generally, funds for down payment are not allowed to be borrowed, unless the money is coming from an account secured by your own assets (for instance, borrowing from your own retirement account). Don’t think you necessarily need to put 20% down. Some loan programs offer little or no down payment options, while other programs may offer down payment assistance options.
- If you are not aware of your credit standing, run a free credit report to verify accurate information. Federal law allows consumers to access one free credit report annually with each of the three credit bureaus (Equifax, Experian, TransUnion). Knowing your credit history and data on your credit report is very important. If there are known or unknown issues on your credit report, it’s always best to at least be informed. You can access your free report at www.annualcreditreport.com.
- Start planning ahead with some of the documentation you will need for a loan approval. Lenders will request items such as tax returns and W-2s from the past 2 years, your recent pay stubs covering a 30-day period, most recent 2 months asset account statements (bank accounts, investment accounts, retirement accounts, etc.), as well as other documentation, depending on your specific scenario.
What are lenders looking at when preapproving an applicant?
Many people will often start to search for homes without having prepared for the preapproval process. This is not necessarily an issue and it doesn’t mean you will not be preapproved. Planning ahead could help you avoid any unforeseen problems and avoid rushing into the mortgage application process when trying to place an offer on a home.
In addition to supplying information on residence and employment/student history for the past 2 years, there are three primary components to a borrower’s credit portfolio:
1) Debt-to-income ratio: What monthly expenses will show on your credit report (car loans/leases, student loans, credit card payments, personal loans/lines of credit, and mortgages for other properties owned)? Do you own any other real estate? Do you have other required obligations, such as alimony or child support payments? To calculate, first combine these liabilities on a monthly expense basis along with the new proposed monthly housing payment. Take these monthly liabilities and divide by monthly income. Gross income (pretax) for employees of a company they do not own is typically utilized (bonus or commission income can have some alternate rules to be allowed as qualifying income); for self-employed borrowers, tax returns will be required to be reviewed; tax write offs could reduce qualifying income. Self-employed individuals will typically need to show a 2-year income history via personal tax returns (as well as business tax returns if applicable). See Figure 1 for an example of a debt-to-income ratio calculation. Many loan programs will require a debt-to-income ratio of 45% or less. There are various loan programs that will be more or less restrictive than this percentage. A lender will be able to guide you to the proper program for your scenario.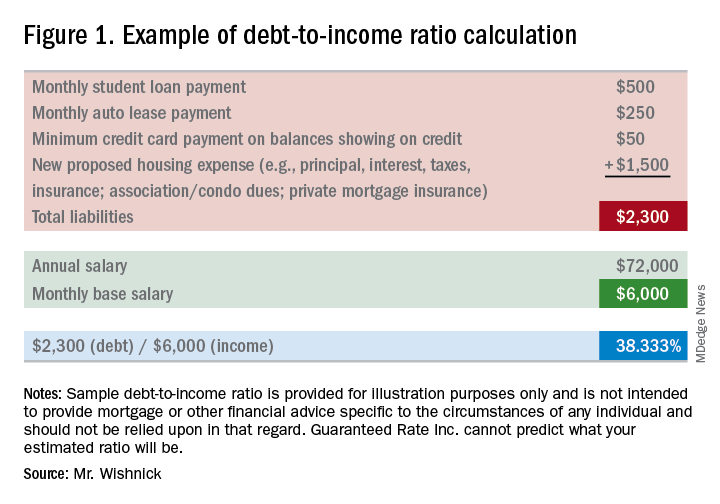
2) Liquid assets: Lenders will review the amount of liquid funds you have available for down payment, closing costs, and any necessary reserves. These may include, but are not limited to, checking/savings/money market accounts, investment accounts (stocks, bonds, mutual funds), and retirement funds. Are there enough allowable funds available for the down payment and closing costs, as well as any required reserves needed for qualification? Large non–payroll deposits can be required to be sourced to make sure the funds are from an allowable source.
3) Credit history/scores: Buying a home will be one of the largest purchases you will make in a lifetime. Credit scores have a major impact on the cost of credit (the interest rate you will obtain). Having higher scores could result in a lower interest rate, as well as open up certain loan programs that may be more advantageous for you. Oftentimes, lenders will take the middle of the three scores as your mortgage score (one score from each of the three credit bureaus). In most cases, if applying jointly, the lowest of the middle scores for all borrowers is the score that is used as the score for the applicants. In general, a 740 middle credit score is considered to be excellent for mortgage financing but is not a requirement for all programs.
**You may have heard about specific mortgage programs for physicians. These programs are intended for use for lesser down payments, and/or not calculating student loan payments when qualifying for home financing. As future income potential is typically not considered when determining debt-to-income ratios, not counting these liabilities potentially increases borrowing power.
You are now ready to be preapproved for mortgage financing. What should you do next?
- Talk to a trusted lender. Ask your real estate agent, family, friends, or colleagues for local lender recommendations. Real estate agents will want to make sure you have spoken with a lender and completed a preapproval application to ensure that you can be preapproved for financing before showing you homes. If you need a loan to purchase a home, a preapproval letter will be required to submit with an offer letter. The application contains questions such as your address and employment history for the past 2 years, income and asset information, as well as a series of other financial information. A hard credit inquiry will need to be performed in order for the lender to issue a preapproval. What should you expect from a lender in addition to competitive rates and an array of programs? Some people prefer more of a hands-on approach. Working with a lender who provides regular status updates and makes him/herself easily accessible for all of your questions can certainly be an attractive feature. Working with a local lender also may be reassuring, as he or she should have plenty of experience with the market in which you are purchasing.
- Search for homes. Upon being given the green light for your preapproval and a price range within your comfort zone, connect with your local real estate professional to search for homes. Plan to spend time with your agent discussing all your needs for your new home.
- Submit an offer. Your lender will be able to provide an estimate of closing costs and monthly payments for homes that you are considering buying before you make an offer. You will want to be sure you are comfortable with the financial obligation prior to making your offer. With your offer, an initial good faith deposit (earnest money deposit) will be required. Your real estate agent will guide you on the proper amount of the deposit.
Conclusion
Once you and the seller have come to terms, you will look to discuss with your lender the rate and program options to secure (locking in an interest rate and program), as well as to complete the formal mortgage application. The lender will request additional documentation, if you have not already provided documents, in order for you to obtain a conditional mortgage commitment. The lender also will order an appraisal to ensure the property value supports the price you have agreed to pay for it. Your real estate agent will guide you through the various deadlines and requirements in the contract for items like home inspections, ordering a title search to obtain title insurance, and other nonfinancing contingencies. Some areas may require attorneys for contract review and closing, which your agent will discuss with you. As you can see, buying a home is not an instant process. Taking the appropriate steps to prepare for your mortgage preapproval could save you a lot of time and stress.
Mr. Wishnick is a 15-year mortgage industry veteran, vice president of mortgage lending with Guaranteed Rate (NMLS #2611) and was ranked as a Top 1% mortgage originator by Mortgage Executive Magazine. He can be reached at [email protected].
All information provided in this publication is for informational and educational purposes only, and in no way is any of the content contained herein to be construed as financial, investment, or legal advice or instruction. Guaranteed Rate does not guarantee the quality, accuracy, completeness or timelines of the information in this publication.
You are trying to buy your first home. Maybe you have heard stories from family, friends, and colleagues about nightmare scenarios when purchasing a home. There are many facets to the home-financing process, and a little bit of planning can reduce a significant amount of time and stress. Where do you begin? What do lenders look for when preapproving a borrower? What steps do I take to get preapproved for a mortgage loan? This article will help guide you through these initial stages to ultimately guide you to settlement on your new home.
Where to begin?
- Start by drafting a budget. How much of a monthly housing payment can you afford? Planning a budget is an extremely valuable exercise at any point in life, not just when buying a home. Often, borrowers will ask the question “How much can I afford?” The better question to ask is “Can I qualify for a home that meets the maximum monthly payment I have budgeted for?”
- What funds would I use for purchasing a home? Down payments and closing costs can add up quickly. Do you have funds readily available in an account you hold? Will you be obtaining a gift from a family member? Generally, funds for down payment are not allowed to be borrowed, unless the money is coming from an account secured by your own assets (for instance, borrowing from your own retirement account). Don’t think you necessarily need to put 20% down. Some loan programs offer little or no down payment options, while other programs may offer down payment assistance options.
- If you are not aware of your credit standing, run a free credit report to verify accurate information. Federal law allows consumers to access one free credit report annually with each of the three credit bureaus (Equifax, Experian, TransUnion). Knowing your credit history and data on your credit report is very important. If there are known or unknown issues on your credit report, it’s always best to at least be informed. You can access your free report at www.annualcreditreport.com.
- Start planning ahead with some of the documentation you will need for a loan approval. Lenders will request items such as tax returns and W-2s from the past 2 years, your recent pay stubs covering a 30-day period, most recent 2 months asset account statements (bank accounts, investment accounts, retirement accounts, etc.), as well as other documentation, depending on your specific scenario.
What are lenders looking at when preapproving an applicant?
Many people will often start to search for homes without having prepared for the preapproval process. This is not necessarily an issue and it doesn’t mean you will not be preapproved. Planning ahead could help you avoid any unforeseen problems and avoid rushing into the mortgage application process when trying to place an offer on a home.
In addition to supplying information on residence and employment/student history for the past 2 years, there are three primary components to a borrower’s credit portfolio:
1) Debt-to-income ratio: What monthly expenses will show on your credit report (car loans/leases, student loans, credit card payments, personal loans/lines of credit, and mortgages for other properties owned)? Do you own any other real estate? Do you have other required obligations, such as alimony or child support payments? To calculate, first combine these liabilities on a monthly expense basis along with the new proposed monthly housing payment. Take these monthly liabilities and divide by monthly income. Gross income (pretax) for employees of a company they do not own is typically utilized (bonus or commission income can have some alternate rules to be allowed as qualifying income); for self-employed borrowers, tax returns will be required to be reviewed; tax write offs could reduce qualifying income. Self-employed individuals will typically need to show a 2-year income history via personal tax returns (as well as business tax returns if applicable). See Figure 1 for an example of a debt-to-income ratio calculation. Many loan programs will require a debt-to-income ratio of 45% or less. There are various loan programs that will be more or less restrictive than this percentage. A lender will be able to guide you to the proper program for your scenario.
2) Liquid assets: Lenders will review the amount of liquid funds you have available for down payment, closing costs, and any necessary reserves. These may include, but are not limited to, checking/savings/money market accounts, investment accounts (stocks, bonds, mutual funds), and retirement funds. Are there enough allowable funds available for the down payment and closing costs, as well as any required reserves needed for qualification? Large non–payroll deposits can be required to be sourced to make sure the funds are from an allowable source.
3) Credit history/scores: Buying a home will be one of the largest purchases you will make in a lifetime. Credit scores have a major impact on the cost of credit (the interest rate you will obtain). Having higher scores could result in a lower interest rate, as well as open up certain loan programs that may be more advantageous for you. Oftentimes, lenders will take the middle of the three scores as your mortgage score (one score from each of the three credit bureaus). In most cases, if applying jointly, the lowest of the middle scores for all borrowers is the score that is used as the score for the applicants. In general, a 740 middle credit score is considered to be excellent for mortgage financing but is not a requirement for all programs.
**You may have heard about specific mortgage programs for physicians. These programs are intended for use for lesser down payments, and/or not calculating student loan payments when qualifying for home financing. As future income potential is typically not considered when determining debt-to-income ratios, not counting these liabilities potentially increases borrowing power.
You are now ready to be preapproved for mortgage financing. What should you do next?
- Talk to a trusted lender. Ask your real estate agent, family, friends, or colleagues for local lender recommendations. Real estate agents will want to make sure you have spoken with a lender and completed a preapproval application to ensure that you can be preapproved for financing before showing you homes. If you need a loan to purchase a home, a preapproval letter will be required to submit with an offer letter. The application contains questions such as your address and employment history for the past 2 years, income and asset information, as well as a series of other financial information. A hard credit inquiry will need to be performed in order for the lender to issue a preapproval. What should you expect from a lender in addition to competitive rates and an array of programs? Some people prefer more of a hands-on approach. Working with a lender who provides regular status updates and makes him/herself easily accessible for all of your questions can certainly be an attractive feature. Working with a local lender also may be reassuring, as he or she should have plenty of experience with the market in which you are purchasing.
- Search for homes. Upon being given the green light for your preapproval and a price range within your comfort zone, connect with your local real estate professional to search for homes. Plan to spend time with your agent discussing all your needs for your new home.
- Submit an offer. Your lender will be able to provide an estimate of closing costs and monthly payments for homes that you are considering buying before you make an offer. You will want to be sure you are comfortable with the financial obligation prior to making your offer. With your offer, an initial good faith deposit (earnest money deposit) will be required. Your real estate agent will guide you on the proper amount of the deposit.
Conclusion
Once you and the seller have come to terms, you will look to discuss with your lender the rate and program options to secure (locking in an interest rate and program), as well as to complete the formal mortgage application. The lender will request additional documentation, if you have not already provided documents, in order for you to obtain a conditional mortgage commitment. The lender also will order an appraisal to ensure the property value supports the price you have agreed to pay for it. Your real estate agent will guide you through the various deadlines and requirements in the contract for items like home inspections, ordering a title search to obtain title insurance, and other nonfinancing contingencies. Some areas may require attorneys for contract review and closing, which your agent will discuss with you. As you can see, buying a home is not an instant process. Taking the appropriate steps to prepare for your mortgage preapproval could save you a lot of time and stress.
Mr. Wishnick is a 15-year mortgage industry veteran, vice president of mortgage lending with Guaranteed Rate (NMLS #2611) and was ranked as a Top 1% mortgage originator by Mortgage Executive Magazine. He can be reached at [email protected].
All information provided in this publication is for informational and educational purposes only, and in no way is any of the content contained herein to be construed as financial, investment, or legal advice or instruction. Guaranteed Rate does not guarantee the quality, accuracy, completeness or timelines of the information in this publication.
You are trying to buy your first home. Maybe you have heard stories from family, friends, and colleagues about nightmare scenarios when purchasing a home. There are many facets to the home-financing process, and a little bit of planning can reduce a significant amount of time and stress. Where do you begin? What do lenders look for when preapproving a borrower? What steps do I take to get preapproved for a mortgage loan? This article will help guide you through these initial stages to ultimately guide you to settlement on your new home.
Where to begin?
- Start by drafting a budget. How much of a monthly housing payment can you afford? Planning a budget is an extremely valuable exercise at any point in life, not just when buying a home. Often, borrowers will ask the question “How much can I afford?” The better question to ask is “Can I qualify for a home that meets the maximum monthly payment I have budgeted for?”
- What funds would I use for purchasing a home? Down payments and closing costs can add up quickly. Do you have funds readily available in an account you hold? Will you be obtaining a gift from a family member? Generally, funds for down payment are not allowed to be borrowed, unless the money is coming from an account secured by your own assets (for instance, borrowing from your own retirement account). Don’t think you necessarily need to put 20% down. Some loan programs offer little or no down payment options, while other programs may offer down payment assistance options.
- If you are not aware of your credit standing, run a free credit report to verify accurate information. Federal law allows consumers to access one free credit report annually with each of the three credit bureaus (Equifax, Experian, TransUnion). Knowing your credit history and data on your credit report is very important. If there are known or unknown issues on your credit report, it’s always best to at least be informed. You can access your free report at www.annualcreditreport.com.
- Start planning ahead with some of the documentation you will need for a loan approval. Lenders will request items such as tax returns and W-2s from the past 2 years, your recent pay stubs covering a 30-day period, most recent 2 months asset account statements (bank accounts, investment accounts, retirement accounts, etc.), as well as other documentation, depending on your specific scenario.
What are lenders looking at when preapproving an applicant?
Many people will often start to search for homes without having prepared for the preapproval process. This is not necessarily an issue and it doesn’t mean you will not be preapproved. Planning ahead could help you avoid any unforeseen problems and avoid rushing into the mortgage application process when trying to place an offer on a home.
In addition to supplying information on residence and employment/student history for the past 2 years, there are three primary components to a borrower’s credit portfolio:
1) Debt-to-income ratio: What monthly expenses will show on your credit report (car loans/leases, student loans, credit card payments, personal loans/lines of credit, and mortgages for other properties owned)? Do you own any other real estate? Do you have other required obligations, such as alimony or child support payments? To calculate, first combine these liabilities on a monthly expense basis along with the new proposed monthly housing payment. Take these monthly liabilities and divide by monthly income. Gross income (pretax) for employees of a company they do not own is typically utilized (bonus or commission income can have some alternate rules to be allowed as qualifying income); for self-employed borrowers, tax returns will be required to be reviewed; tax write offs could reduce qualifying income. Self-employed individuals will typically need to show a 2-year income history via personal tax returns (as well as business tax returns if applicable). See Figure 1 for an example of a debt-to-income ratio calculation. Many loan programs will require a debt-to-income ratio of 45% or less. There are various loan programs that will be more or less restrictive than this percentage. A lender will be able to guide you to the proper program for your scenario.
2) Liquid assets: Lenders will review the amount of liquid funds you have available for down payment, closing costs, and any necessary reserves. These may include, but are not limited to, checking/savings/money market accounts, investment accounts (stocks, bonds, mutual funds), and retirement funds. Are there enough allowable funds available for the down payment and closing costs, as well as any required reserves needed for qualification? Large non–payroll deposits can be required to be sourced to make sure the funds are from an allowable source.
3) Credit history/scores: Buying a home will be one of the largest purchases you will make in a lifetime. Credit scores have a major impact on the cost of credit (the interest rate you will obtain). Having higher scores could result in a lower interest rate, as well as open up certain loan programs that may be more advantageous for you. Oftentimes, lenders will take the middle of the three scores as your mortgage score (one score from each of the three credit bureaus). In most cases, if applying jointly, the lowest of the middle scores for all borrowers is the score that is used as the score for the applicants. In general, a 740 middle credit score is considered to be excellent for mortgage financing but is not a requirement for all programs.
**You may have heard about specific mortgage programs for physicians. These programs are intended for use for lesser down payments, and/or not calculating student loan payments when qualifying for home financing. As future income potential is typically not considered when determining debt-to-income ratios, not counting these liabilities potentially increases borrowing power.
You are now ready to be preapproved for mortgage financing. What should you do next?
- Talk to a trusted lender. Ask your real estate agent, family, friends, or colleagues for local lender recommendations. Real estate agents will want to make sure you have spoken with a lender and completed a preapproval application to ensure that you can be preapproved for financing before showing you homes. If you need a loan to purchase a home, a preapproval letter will be required to submit with an offer letter. The application contains questions such as your address and employment history for the past 2 years, income and asset information, as well as a series of other financial information. A hard credit inquiry will need to be performed in order for the lender to issue a preapproval. What should you expect from a lender in addition to competitive rates and an array of programs? Some people prefer more of a hands-on approach. Working with a lender who provides regular status updates and makes him/herself easily accessible for all of your questions can certainly be an attractive feature. Working with a local lender also may be reassuring, as he or she should have plenty of experience with the market in which you are purchasing.
- Search for homes. Upon being given the green light for your preapproval and a price range within your comfort zone, connect with your local real estate professional to search for homes. Plan to spend time with your agent discussing all your needs for your new home.
- Submit an offer. Your lender will be able to provide an estimate of closing costs and monthly payments for homes that you are considering buying before you make an offer. You will want to be sure you are comfortable with the financial obligation prior to making your offer. With your offer, an initial good faith deposit (earnest money deposit) will be required. Your real estate agent will guide you on the proper amount of the deposit.
Conclusion
Once you and the seller have come to terms, you will look to discuss with your lender the rate and program options to secure (locking in an interest rate and program), as well as to complete the formal mortgage application. The lender will request additional documentation, if you have not already provided documents, in order for you to obtain a conditional mortgage commitment. The lender also will order an appraisal to ensure the property value supports the price you have agreed to pay for it. Your real estate agent will guide you through the various deadlines and requirements in the contract for items like home inspections, ordering a title search to obtain title insurance, and other nonfinancing contingencies. Some areas may require attorneys for contract review and closing, which your agent will discuss with you. As you can see, buying a home is not an instant process. Taking the appropriate steps to prepare for your mortgage preapproval could save you a lot of time and stress.
Mr. Wishnick is a 15-year mortgage industry veteran, vice president of mortgage lending with Guaranteed Rate (NMLS #2611) and was ranked as a Top 1% mortgage originator by Mortgage Executive Magazine. He can be reached at [email protected].
All information provided in this publication is for informational and educational purposes only, and in no way is any of the content contained herein to be construed as financial, investment, or legal advice or instruction. Guaranteed Rate does not guarantee the quality, accuracy, completeness or timelines of the information in this publication.
Advanced training options in inflammatory bowel disease
The global incidence and prevalence of inflammatory bowel disease (IBD) is rising, and it is estimated that by 2025, approximately 2.2 million Americans will be living with this disease. At the same time, there have been several paradigm-changing scientific and medical advances in the understanding and management of IBD. As the diagnostic, therapeutic, and monitoring armamentarium in the management of IBD increases, so is the complexity of the decision making. Advanced concepts and training are often not covered adequately during a general gastroenterology fellowship. In a survey of 160 trainees, more than one-third of fellows did not feel “confident” or “mostly comfortable” with their level of IBD training. Yet, efficient dissemination, effective translation and integration of these advances into clinical practice is paramount to improving quality of care. To facilitate multiple goals as listed in Table 1, advanced training in the field of IBD is increasingly important. In this article, I review different training options available for young gastroenterologists.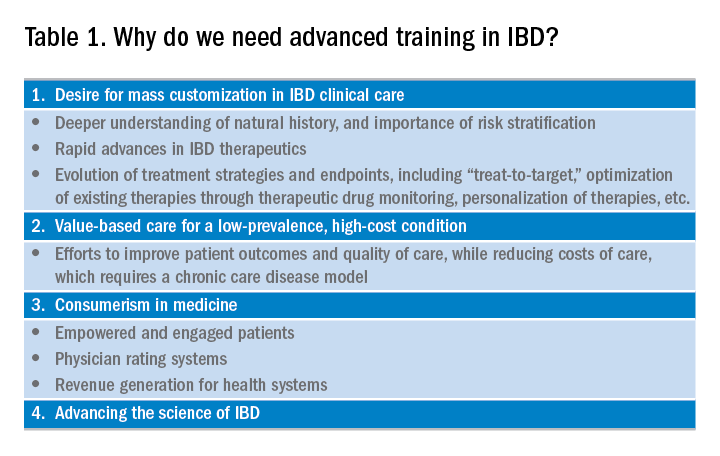
Readers are also directed to an excellent article by David Rubin, MD, published in Gastroenterology in 2015.
Advanced fellowship training in IBD
The most rigorous training in IBD is offered through dedicated advanced fellowships. Currently, there are more than 20 such fellowships in North America, most of them offered at large academic centers with nationally and internationally renowned faculty. These training positions are generally 1 year long, offered after completion of gastroenterology fellowship. The Accreditation Council of the Graduate Medical Education (ACGME) does not accredit these advanced training programs, and there is not a separate American Board of Internal Medicine (ABIM) certification for IBD. Funding of such programs comes from different sources including endowments, private foundations, institutional funds, pharmaceutical company grants, and even limited faculty appointments of the trainees. Though there is currently no official regulatory oversight and requirements, most programs have well-defined curricula covering diverse aspects of IBD care. This core curriculum has been nicely summarized in a recent article by Uma Mahadevan, MD, in Gastroenterology.
Clinical training in these programs is offered through a mix of outpatient IBD clinics (generally three to five clinics/week, with one or more senior IBD-focused faculty member), supervising general gastroenterology fellows for inpatient IBD care, dedicated IBD-focused endoscopy sessions (generally one or two sessions/week) including chromoendoscopy and stricture dilation, as well as formal and informal mentorship by one or more senior faculty members, time and mentorship for scholarly activities, and appropriate evaluation and feedback systems. In addition, most programs offer multidisciplinary training through dedicated clinics with colorectal surgeons (such as pouch clinics, etc.), opportunities for observing and interacting with radiologists, pathologists, psychologists, and dietitians.
There is no centralized application process and prospective applicants should reach out to their program directors and mentors regarding guidance, as well as program directors of specific training programs to learn more about these programs, generally in the second half of their gastroenterology training. The Crohn’s and Colitis Foundation maintains a list of fellowship training programs and appropriate contacts. In choosing a specific program, prospective fellows should consider the rigor and diversity of training, balance between service and scholarship, mentorship opportunities as well as the experience and outcomes of previous fellows in the program. Besides formal interviews at prospective program, fellows should utilize the networking opportunities afforded through the American Gastroenterological Association (both with senior faculty as well as through the Trainee and Early-Career Committee), the Crohn’s and Colitis Foundation as well as other organizations in learning more about programs.
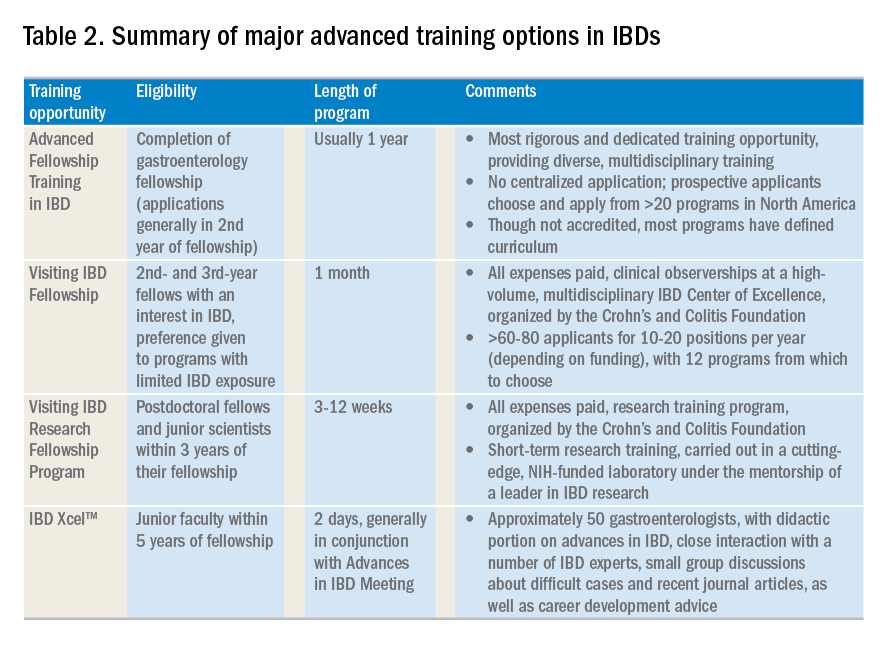
Visiting IBD Fellow Program: Clinical observership, through the Crohn’s and Colitis Foundation
The Visiting IBD Fellow Program – with the support of the Crohn’s and Colitis Foundation – which launched in 2006, arose from the need for immersive training in IBD, especially for fellows for whom IBD exposure may be limited. In this 1-month “observership,” interested 2nd and 3rd year fellows get the opportunity to observe faculty at a high-volume, multidisciplinary IBD Center of Excellence. Besides providing additional knowledge and expertise in the field, this also allows fellows the chance to understand how IBD Centers are set up, so they may seek to replicate similar models as local or regional IBD experts. Currently, 12 centers participate in this program. There is no cost to the fellows who are selected to participate, and all travel expenses and lodging are covered. The program significantly improved the fellows’ knowledge, skills, and attitudes toward IBD and has steadily gained in popularity, with more than 60-80 applicants for 10-20 positions per year (depending on funding). In addition to the clinical exposure, this experience also facilitates networking with faculty and other fellows at participating institutions. Full details of this program can be accessed from the Crohn’s and Colitis Foundation website.
A similar, expenses-paid, abbreviated 3-day program of IBD preceptorship has been launched for advanced practice providers (qualified advanced-practice nurses, nurse practitioners, and physician assistants). This program provides preceptee exposure to medical, surgical, outpatient, and inpatient experiences with patients at a leading academic IBD center.
Visiting IBD Research Fellowship Program, through the Crohn’s and Colitis Foundation
The Crohn’s and Colitis Foundation recently launched a new, short-term, mentored research initiative designed to promote career advancement for talented junior investigators dedicated to IBD research, and to enable knowledge-sharing among leaders in the IBD field. The Foundation encourages outstanding young scientists (postdoctoral studies in the first 3 years of their fellowship), who would like to expand their expertise in IBD research to participate in this short-term research training, carried out in a cutting-edge, NIH-funded laboratory under the mentorship of a leader in IBD research. This all-expense covered 3-12 week rotation provides mentorship and technical training in a state-of-the-art research lab relevant to IBD, with an emphasis on preclinical research most closely relevant to human disease. Details of the program can be found on the Crohn’s and Colitis Foundation website.
IBD Xcel
In 2013, Cornerstones Health, a nonprofit medical education organization, launched a 2-day program dedicated to advances in the field of IBD for junior gastroenterologists within 5 years of completion of their fellowship training. The program includes a didactic component as well as close interaction with a number of IBD experts, small-group discussions about difficult cases, and recent journal articles, as well as career-development advice. The education component is free of cost to selected participants, though travel and housing expenses are not covered.
Besides these dedicated advanced training opportunities, there are major conferences that cover IBD extensively and exclusively. These include the annual Crohn’s and Colitis Congress® conducted jointly by the Crohn’s and Colitis Foundation and the American Gastroenterological Association, the annual Advances in Inflammatory Bowel Diseases through Imedex, the annual European Crohn’s and Colitis Congress, the American College of Gastroenterology’s IBD School, as well as several regional courses conducted throughout the country. In terms of networking opportunities for gastroenterology fellows interested in IBD and junior faculty, REACH-IBD (Rising Educators, Academicians and Clinicians Helping Inflammatory Bowel Disease), founded under the auspices of the Crohn’s and Colitis Foundation in 2013, provides a unique resource. This group is open to all clinical fellows, postdoctoral scientists, and junior faculty (pediatric and adult; medical and surgical specialties, as well as PhDs) less than 7 years out of training with a rank not higher than assistant professor. The mission is to facilitate networking and career development for clinical fellows, postdoctoral scientists, and junior faculty with an interest in IBD; increase active participation of our members in the clinical, educational, scientific, and research programs within the Crohn’s and Colitis Foundation; and foster collaborative research among our members within the Foundation. The group organizes specific breakout events at the Digestive Disease Week® and the annual Crohn’s and Colitis Congress, covering diverse topics such as setting up an IBD practice, funding opportunities, paper and grant writing, career advancement guidance. More information on this can be found on the Crohn’s and Colitis Foundation website.
To summarize, there are numerous opportunities of varying lengths to receive training in inflammatory bowel diseases. This exciting field is expanding at a rapid pace, and instead of limiting management to dedicated IBD Centers of Excellence, there is clear need for effective dissemination of new management approaches and incorporation of quality measures will likely raise the bar for all patients and physicians who care for them.
AGA offers IBD education
Check out AGA’s on-demand IBD education available in AGA University.
Dr. Singh is assistant professor of medicine, division of gastroenterology, University of California, San Diego. He is supported by the American College of Gastroenterology and Crohn’s and Colitis Foundation, has received research grants from Pfizer and AbbVie, and consulting fees from AbbVie, Takeda, and AMAG Pharmaceuticals.
The global incidence and prevalence of inflammatory bowel disease (IBD) is rising, and it is estimated that by 2025, approximately 2.2 million Americans will be living with this disease. At the same time, there have been several paradigm-changing scientific and medical advances in the understanding and management of IBD. As the diagnostic, therapeutic, and monitoring armamentarium in the management of IBD increases, so is the complexity of the decision making. Advanced concepts and training are often not covered adequately during a general gastroenterology fellowship. In a survey of 160 trainees, more than one-third of fellows did not feel “confident” or “mostly comfortable” with their level of IBD training. Yet, efficient dissemination, effective translation and integration of these advances into clinical practice is paramount to improving quality of care. To facilitate multiple goals as listed in Table 1, advanced training in the field of IBD is increasingly important. In this article, I review different training options available for young gastroenterologists.
Readers are also directed to an excellent article by David Rubin, MD, published in Gastroenterology in 2015.
Advanced fellowship training in IBD
The most rigorous training in IBD is offered through dedicated advanced fellowships. Currently, there are more than 20 such fellowships in North America, most of them offered at large academic centers with nationally and internationally renowned faculty. These training positions are generally 1 year long, offered after completion of gastroenterology fellowship. The Accreditation Council of the Graduate Medical Education (ACGME) does not accredit these advanced training programs, and there is not a separate American Board of Internal Medicine (ABIM) certification for IBD. Funding of such programs comes from different sources including endowments, private foundations, institutional funds, pharmaceutical company grants, and even limited faculty appointments of the trainees. Though there is currently no official regulatory oversight and requirements, most programs have well-defined curricula covering diverse aspects of IBD care. This core curriculum has been nicely summarized in a recent article by Uma Mahadevan, MD, in Gastroenterology.
Clinical training in these programs is offered through a mix of outpatient IBD clinics (generally three to five clinics/week, with one or more senior IBD-focused faculty member), supervising general gastroenterology fellows for inpatient IBD care, dedicated IBD-focused endoscopy sessions (generally one or two sessions/week) including chromoendoscopy and stricture dilation, as well as formal and informal mentorship by one or more senior faculty members, time and mentorship for scholarly activities, and appropriate evaluation and feedback systems. In addition, most programs offer multidisciplinary training through dedicated clinics with colorectal surgeons (such as pouch clinics, etc.), opportunities for observing and interacting with radiologists, pathologists, psychologists, and dietitians.
There is no centralized application process and prospective applicants should reach out to their program directors and mentors regarding guidance, as well as program directors of specific training programs to learn more about these programs, generally in the second half of their gastroenterology training. The Crohn’s and Colitis Foundation maintains a list of fellowship training programs and appropriate contacts. In choosing a specific program, prospective fellows should consider the rigor and diversity of training, balance between service and scholarship, mentorship opportunities as well as the experience and outcomes of previous fellows in the program. Besides formal interviews at prospective program, fellows should utilize the networking opportunities afforded through the American Gastroenterological Association (both with senior faculty as well as through the Trainee and Early-Career Committee), the Crohn’s and Colitis Foundation as well as other organizations in learning more about programs.

Visiting IBD Fellow Program: Clinical observership, through the Crohn’s and Colitis Foundation
The Visiting IBD Fellow Program – with the support of the Crohn’s and Colitis Foundation – which launched in 2006, arose from the need for immersive training in IBD, especially for fellows for whom IBD exposure may be limited. In this 1-month “observership,” interested 2nd and 3rd year fellows get the opportunity to observe faculty at a high-volume, multidisciplinary IBD Center of Excellence. Besides providing additional knowledge and expertise in the field, this also allows fellows the chance to understand how IBD Centers are set up, so they may seek to replicate similar models as local or regional IBD experts. Currently, 12 centers participate in this program. There is no cost to the fellows who are selected to participate, and all travel expenses and lodging are covered. The program significantly improved the fellows’ knowledge, skills, and attitudes toward IBD and has steadily gained in popularity, with more than 60-80 applicants for 10-20 positions per year (depending on funding). In addition to the clinical exposure, this experience also facilitates networking with faculty and other fellows at participating institutions. Full details of this program can be accessed from the Crohn’s and Colitis Foundation website.
A similar, expenses-paid, abbreviated 3-day program of IBD preceptorship has been launched for advanced practice providers (qualified advanced-practice nurses, nurse practitioners, and physician assistants). This program provides preceptee exposure to medical, surgical, outpatient, and inpatient experiences with patients at a leading academic IBD center.
Visiting IBD Research Fellowship Program, through the Crohn’s and Colitis Foundation
The Crohn’s and Colitis Foundation recently launched a new, short-term, mentored research initiative designed to promote career advancement for talented junior investigators dedicated to IBD research, and to enable knowledge-sharing among leaders in the IBD field. The Foundation encourages outstanding young scientists (postdoctoral studies in the first 3 years of their fellowship), who would like to expand their expertise in IBD research to participate in this short-term research training, carried out in a cutting-edge, NIH-funded laboratory under the mentorship of a leader in IBD research. This all-expense covered 3-12 week rotation provides mentorship and technical training in a state-of-the-art research lab relevant to IBD, with an emphasis on preclinical research most closely relevant to human disease. Details of the program can be found on the Crohn’s and Colitis Foundation website.
IBD Xcel
In 2013, Cornerstones Health, a nonprofit medical education organization, launched a 2-day program dedicated to advances in the field of IBD for junior gastroenterologists within 5 years of completion of their fellowship training. The program includes a didactic component as well as close interaction with a number of IBD experts, small-group discussions about difficult cases, and recent journal articles, as well as career-development advice. The education component is free of cost to selected participants, though travel and housing expenses are not covered.
Besides these dedicated advanced training opportunities, there are major conferences that cover IBD extensively and exclusively. These include the annual Crohn’s and Colitis Congress® conducted jointly by the Crohn’s and Colitis Foundation and the American Gastroenterological Association, the annual Advances in Inflammatory Bowel Diseases through Imedex, the annual European Crohn’s and Colitis Congress, the American College of Gastroenterology’s IBD School, as well as several regional courses conducted throughout the country. In terms of networking opportunities for gastroenterology fellows interested in IBD and junior faculty, REACH-IBD (Rising Educators, Academicians and Clinicians Helping Inflammatory Bowel Disease), founded under the auspices of the Crohn’s and Colitis Foundation in 2013, provides a unique resource. This group is open to all clinical fellows, postdoctoral scientists, and junior faculty (pediatric and adult; medical and surgical specialties, as well as PhDs) less than 7 years out of training with a rank not higher than assistant professor. The mission is to facilitate networking and career development for clinical fellows, postdoctoral scientists, and junior faculty with an interest in IBD; increase active participation of our members in the clinical, educational, scientific, and research programs within the Crohn’s and Colitis Foundation; and foster collaborative research among our members within the Foundation. The group organizes specific breakout events at the Digestive Disease Week® and the annual Crohn’s and Colitis Congress, covering diverse topics such as setting up an IBD practice, funding opportunities, paper and grant writing, career advancement guidance. More information on this can be found on the Crohn’s and Colitis Foundation website.
To summarize, there are numerous opportunities of varying lengths to receive training in inflammatory bowel diseases. This exciting field is expanding at a rapid pace, and instead of limiting management to dedicated IBD Centers of Excellence, there is clear need for effective dissemination of new management approaches and incorporation of quality measures will likely raise the bar for all patients and physicians who care for them.
AGA offers IBD education
Check out AGA’s on-demand IBD education available in AGA University.
Dr. Singh is assistant professor of medicine, division of gastroenterology, University of California, San Diego. He is supported by the American College of Gastroenterology and Crohn’s and Colitis Foundation, has received research grants from Pfizer and AbbVie, and consulting fees from AbbVie, Takeda, and AMAG Pharmaceuticals.
The global incidence and prevalence of inflammatory bowel disease (IBD) is rising, and it is estimated that by 2025, approximately 2.2 million Americans will be living with this disease. At the same time, there have been several paradigm-changing scientific and medical advances in the understanding and management of IBD. As the diagnostic, therapeutic, and monitoring armamentarium in the management of IBD increases, so is the complexity of the decision making. Advanced concepts and training are often not covered adequately during a general gastroenterology fellowship. In a survey of 160 trainees, more than one-third of fellows did not feel “confident” or “mostly comfortable” with their level of IBD training. Yet, efficient dissemination, effective translation and integration of these advances into clinical practice is paramount to improving quality of care. To facilitate multiple goals as listed in Table 1, advanced training in the field of IBD is increasingly important. In this article, I review different training options available for young gastroenterologists.
Readers are also directed to an excellent article by David Rubin, MD, published in Gastroenterology in 2015.
Advanced fellowship training in IBD
The most rigorous training in IBD is offered through dedicated advanced fellowships. Currently, there are more than 20 such fellowships in North America, most of them offered at large academic centers with nationally and internationally renowned faculty. These training positions are generally 1 year long, offered after completion of gastroenterology fellowship. The Accreditation Council of the Graduate Medical Education (ACGME) does not accredit these advanced training programs, and there is not a separate American Board of Internal Medicine (ABIM) certification for IBD. Funding of such programs comes from different sources including endowments, private foundations, institutional funds, pharmaceutical company grants, and even limited faculty appointments of the trainees. Though there is currently no official regulatory oversight and requirements, most programs have well-defined curricula covering diverse aspects of IBD care. This core curriculum has been nicely summarized in a recent article by Uma Mahadevan, MD, in Gastroenterology.
Clinical training in these programs is offered through a mix of outpatient IBD clinics (generally three to five clinics/week, with one or more senior IBD-focused faculty member), supervising general gastroenterology fellows for inpatient IBD care, dedicated IBD-focused endoscopy sessions (generally one or two sessions/week) including chromoendoscopy and stricture dilation, as well as formal and informal mentorship by one or more senior faculty members, time and mentorship for scholarly activities, and appropriate evaluation and feedback systems. In addition, most programs offer multidisciplinary training through dedicated clinics with colorectal surgeons (such as pouch clinics, etc.), opportunities for observing and interacting with radiologists, pathologists, psychologists, and dietitians.
There is no centralized application process and prospective applicants should reach out to their program directors and mentors regarding guidance, as well as program directors of specific training programs to learn more about these programs, generally in the second half of their gastroenterology training. The Crohn’s and Colitis Foundation maintains a list of fellowship training programs and appropriate contacts. In choosing a specific program, prospective fellows should consider the rigor and diversity of training, balance between service and scholarship, mentorship opportunities as well as the experience and outcomes of previous fellows in the program. Besides formal interviews at prospective program, fellows should utilize the networking opportunities afforded through the American Gastroenterological Association (both with senior faculty as well as through the Trainee and Early-Career Committee), the Crohn’s and Colitis Foundation as well as other organizations in learning more about programs.

Visiting IBD Fellow Program: Clinical observership, through the Crohn’s and Colitis Foundation
The Visiting IBD Fellow Program – with the support of the Crohn’s and Colitis Foundation – which launched in 2006, arose from the need for immersive training in IBD, especially for fellows for whom IBD exposure may be limited. In this 1-month “observership,” interested 2nd and 3rd year fellows get the opportunity to observe faculty at a high-volume, multidisciplinary IBD Center of Excellence. Besides providing additional knowledge and expertise in the field, this also allows fellows the chance to understand how IBD Centers are set up, so they may seek to replicate similar models as local or regional IBD experts. Currently, 12 centers participate in this program. There is no cost to the fellows who are selected to participate, and all travel expenses and lodging are covered. The program significantly improved the fellows’ knowledge, skills, and attitudes toward IBD and has steadily gained in popularity, with more than 60-80 applicants for 10-20 positions per year (depending on funding). In addition to the clinical exposure, this experience also facilitates networking with faculty and other fellows at participating institutions. Full details of this program can be accessed from the Crohn’s and Colitis Foundation website.
A similar, expenses-paid, abbreviated 3-day program of IBD preceptorship has been launched for advanced practice providers (qualified advanced-practice nurses, nurse practitioners, and physician assistants). This program provides preceptee exposure to medical, surgical, outpatient, and inpatient experiences with patients at a leading academic IBD center.
Visiting IBD Research Fellowship Program, through the Crohn’s and Colitis Foundation
The Crohn’s and Colitis Foundation recently launched a new, short-term, mentored research initiative designed to promote career advancement for talented junior investigators dedicated to IBD research, and to enable knowledge-sharing among leaders in the IBD field. The Foundation encourages outstanding young scientists (postdoctoral studies in the first 3 years of their fellowship), who would like to expand their expertise in IBD research to participate in this short-term research training, carried out in a cutting-edge, NIH-funded laboratory under the mentorship of a leader in IBD research. This all-expense covered 3-12 week rotation provides mentorship and technical training in a state-of-the-art research lab relevant to IBD, with an emphasis on preclinical research most closely relevant to human disease. Details of the program can be found on the Crohn’s and Colitis Foundation website.
IBD Xcel
In 2013, Cornerstones Health, a nonprofit medical education organization, launched a 2-day program dedicated to advances in the field of IBD for junior gastroenterologists within 5 years of completion of their fellowship training. The program includes a didactic component as well as close interaction with a number of IBD experts, small-group discussions about difficult cases, and recent journal articles, as well as career-development advice. The education component is free of cost to selected participants, though travel and housing expenses are not covered.
Besides these dedicated advanced training opportunities, there are major conferences that cover IBD extensively and exclusively. These include the annual Crohn’s and Colitis Congress® conducted jointly by the Crohn’s and Colitis Foundation and the American Gastroenterological Association, the annual Advances in Inflammatory Bowel Diseases through Imedex, the annual European Crohn’s and Colitis Congress, the American College of Gastroenterology’s IBD School, as well as several regional courses conducted throughout the country. In terms of networking opportunities for gastroenterology fellows interested in IBD and junior faculty, REACH-IBD (Rising Educators, Academicians and Clinicians Helping Inflammatory Bowel Disease), founded under the auspices of the Crohn’s and Colitis Foundation in 2013, provides a unique resource. This group is open to all clinical fellows, postdoctoral scientists, and junior faculty (pediatric and adult; medical and surgical specialties, as well as PhDs) less than 7 years out of training with a rank not higher than assistant professor. The mission is to facilitate networking and career development for clinical fellows, postdoctoral scientists, and junior faculty with an interest in IBD; increase active participation of our members in the clinical, educational, scientific, and research programs within the Crohn’s and Colitis Foundation; and foster collaborative research among our members within the Foundation. The group organizes specific breakout events at the Digestive Disease Week® and the annual Crohn’s and Colitis Congress, covering diverse topics such as setting up an IBD practice, funding opportunities, paper and grant writing, career advancement guidance. More information on this can be found on the Crohn’s and Colitis Foundation website.
To summarize, there are numerous opportunities of varying lengths to receive training in inflammatory bowel diseases. This exciting field is expanding at a rapid pace, and instead of limiting management to dedicated IBD Centers of Excellence, there is clear need for effective dissemination of new management approaches and incorporation of quality measures will likely raise the bar for all patients and physicians who care for them.
AGA offers IBD education
Check out AGA’s on-demand IBD education available in AGA University.
Dr. Singh is assistant professor of medicine, division of gastroenterology, University of California, San Diego. He is supported by the American College of Gastroenterology and Crohn’s and Colitis Foundation, has received research grants from Pfizer and AbbVie, and consulting fees from AbbVie, Takeda, and AMAG Pharmaceuticals.
How to address reviewer criticism
Authors of manuscripts typically receive one of three responses from journals: 1. Accepted as submitted; 2. Accepted pending revisions (major or minor); and 3. Rejected. Receiving an unconditional acceptance is an unusual fate worth documenting and celebrating. On the other hand, irreversible rejections are so common that authors need to get accustomed to them. Upon receiving an unqualified editorial rejection without a formal review (usually described as priority-related rejection), send the same manuscript out the next day to another journal (with electronic submissions you can do this on the same day).
If your manuscript is rejected after being reviewed, consider seriously the comments given and try to learn from them. Was your study design flawed? Do you need additional data? Were your analyses incomplete or did you employ suboptimal statistical methods? Was your interpretation of the findings far reaching and out of proportion to the actual data? Use this experience and feedback, revise your manuscript, and submit it to a different journal. It is not uncommon to encounter the same reviewer at the next journal; fixing major issues seems responsive and gets you in the door.
To receive a “conditional acceptance” or “rejection with hope” is the most likely “good” editorial response. Avoid a very quick response, because it may be hasty or create an impression of a hasty response. Because most manuscripts with substantial reviews are sent back to the reviewers, the turnaround time in most journals is several weeks and, therefore, there is little to be gained by sending the revised manuscript in 1 day rather than 1 week. The best course of action is to cool down for 1-2 days and then decide and draft responses in 1 week, including planned additional analyses. In the case of seemingly contradictory or numerous requests from reviewers, it is best to carefully examine clues from the editors or associate editors as to the nature and extent of the revision needed. In most instances, we draft the response letter before revising the manuscript. We use the draft letter to obtain specific input from other authors and ‘brainstorm’ about additional analyses that can best address reviewers concerns.
Do the best that you can to fully address all reviewers’ comments. Adequate time should be spent making real changes, including adding additional data or analyses to the manuscript, and taking utmost care in describing and highlighting these changes. If you believe that the reviewers missed a point that was already included in the paper, then point this out as politely as possible as part of the response letter (see below).
In addition to revising your manuscript, you will be asked to prepare a point-by-point response to each of the reviewers’ comments you receive. Thank the editors and reviewers sincerely for their comments and explain how changes based on the comments have made the paper better; they did spend time reviewing your manuscript, and they have not rejected it yet. Reviewers are usually recognized experts, or their apprentices, in the content or method of research employed in your paper. Reviewers are also likely to be authors on papers cited in your manuscript. Avoid unnecessary arguments when possible, especially about noncore issues or about changes that you already conceded. If you are compelled to contest any of the reviewers’ comments, provide substantial evidence that supports your position and be respectful with your responses. Address each comment separately, beginning with the comments raised by the editors followed by those from reviewer one, two, and so on. After each response, clearly point the reviewers and the editors to the revised sections in the manuscript. In case of similar comments, it is acceptable to direct the second (or the third) reviewer to your previous response. Provide new tables, figures, data elements, and references as part of the response letter to make it a stand-alone document. It can be difficult (and annoying) if the reviewer has to flip back and forth between documents to understand the full story.
Appealing editorial decisions consumes a lot of energy, annoys editors and reviewers, and is generally futile. If it is needed, then write a polite, brief appeal letter that summarizes the reasons for the appeal. The most common editorial response to an appeal, which usually follows a several-week delay, is an equally polite affirmation of the original decision. The second and arguably worse outcome is for the manuscript to be sent to two to three new reviewers with another rejection after a several-month delay.
Have colleagues read and comment on your revised paper and use these comments to improve the draft. There is evidence that writing groups are effective in providing suggestions for improving papers: A writing group also keeps the momentum going during the revision process. Setting realistic time lines with the coauthors of the paper is a useful strategy to maintain momentum during revisions.
Writing (and revising) papers can be a highly rewarding activity. Start early, plan carefully, and do not delay the process. Reviewers’ comments are mostly geared toward enhancing the manuscript. Take them seriously, address them fully, and you will have an improved (and we hope, an accepted) manuscript.
Additional reading
El-Serag HB. Writing and publishing scientific papers. Gastroenterology. 2012 Feb;142(2):197-200. doi: 10.1053/j.gastro.2011.12.021. Epub 2011 Dec 16.
Downey SMet al. Manuscript development and publishing: A 5-step approach. Am J Med Sci. 2017 Feb;353(2):132-6. doi: 10.1016/j.amjms.2016.12.005. Epub 2016 Dec 9.
Sullivan GM. What to do when your paper is rejected. J Grad Med Educ. 2015;7:1-3. doi: 10.4300/JGME-D-14-00686.1
Kotz Det al. Effective writing and publishing scientific papers, part XII: responding to reviewers. J Clin Epidemiol. 2014;67:243. doi: 10.1016/j.jclinepi.2013.10.003. Epub 2014 Jan 9.
Dr. El-Serag is chairman of the Margaret M. and Albert B. Alkek department of medicine, Baylor College of Medicine, Houston; incoming president of the American Gastroenterological Association Institute; and past Editor in Chief, Clinical Gastroenterology and Hepatology. Dr. Kanwal is professor of medicine and chief of the section of gastroenterology and hepatology, department of medicine, Baylor College of Medicine and Michael E. DeBakey Veterans Affairs Medical Center, Houston, and Editor in Chief, Clinical Gastroenterology and Hepatology. This material is based on work supported by Cancer Prevention & Research Institute of Texas grant (RP150587). The work is also supported in part by the Center for Gastrointestinal Development, Infection and Injury (NIDDK P30 DK 56338).
Authors of manuscripts typically receive one of three responses from journals: 1. Accepted as submitted; 2. Accepted pending revisions (major or minor); and 3. Rejected. Receiving an unconditional acceptance is an unusual fate worth documenting and celebrating. On the other hand, irreversible rejections are so common that authors need to get accustomed to them. Upon receiving an unqualified editorial rejection without a formal review (usually described as priority-related rejection), send the same manuscript out the next day to another journal (with electronic submissions you can do this on the same day).
If your manuscript is rejected after being reviewed, consider seriously the comments given and try to learn from them. Was your study design flawed? Do you need additional data? Were your analyses incomplete or did you employ suboptimal statistical methods? Was your interpretation of the findings far reaching and out of proportion to the actual data? Use this experience and feedback, revise your manuscript, and submit it to a different journal. It is not uncommon to encounter the same reviewer at the next journal; fixing major issues seems responsive and gets you in the door.
To receive a “conditional acceptance” or “rejection with hope” is the most likely “good” editorial response. Avoid a very quick response, because it may be hasty or create an impression of a hasty response. Because most manuscripts with substantial reviews are sent back to the reviewers, the turnaround time in most journals is several weeks and, therefore, there is little to be gained by sending the revised manuscript in 1 day rather than 1 week. The best course of action is to cool down for 1-2 days and then decide and draft responses in 1 week, including planned additional analyses. In the case of seemingly contradictory or numerous requests from reviewers, it is best to carefully examine clues from the editors or associate editors as to the nature and extent of the revision needed. In most instances, we draft the response letter before revising the manuscript. We use the draft letter to obtain specific input from other authors and ‘brainstorm’ about additional analyses that can best address reviewers concerns.
Do the best that you can to fully address all reviewers’ comments. Adequate time should be spent making real changes, including adding additional data or analyses to the manuscript, and taking utmost care in describing and highlighting these changes. If you believe that the reviewers missed a point that was already included in the paper, then point this out as politely as possible as part of the response letter (see below).
In addition to revising your manuscript, you will be asked to prepare a point-by-point response to each of the reviewers’ comments you receive. Thank the editors and reviewers sincerely for their comments and explain how changes based on the comments have made the paper better; they did spend time reviewing your manuscript, and they have not rejected it yet. Reviewers are usually recognized experts, or their apprentices, in the content or method of research employed in your paper. Reviewers are also likely to be authors on papers cited in your manuscript. Avoid unnecessary arguments when possible, especially about noncore issues or about changes that you already conceded. If you are compelled to contest any of the reviewers’ comments, provide substantial evidence that supports your position and be respectful with your responses. Address each comment separately, beginning with the comments raised by the editors followed by those from reviewer one, two, and so on. After each response, clearly point the reviewers and the editors to the revised sections in the manuscript. In case of similar comments, it is acceptable to direct the second (or the third) reviewer to your previous response. Provide new tables, figures, data elements, and references as part of the response letter to make it a stand-alone document. It can be difficult (and annoying) if the reviewer has to flip back and forth between documents to understand the full story.
Appealing editorial decisions consumes a lot of energy, annoys editors and reviewers, and is generally futile. If it is needed, then write a polite, brief appeal letter that summarizes the reasons for the appeal. The most common editorial response to an appeal, which usually follows a several-week delay, is an equally polite affirmation of the original decision. The second and arguably worse outcome is for the manuscript to be sent to two to three new reviewers with another rejection after a several-month delay.
Have colleagues read and comment on your revised paper and use these comments to improve the draft. There is evidence that writing groups are effective in providing suggestions for improving papers: A writing group also keeps the momentum going during the revision process. Setting realistic time lines with the coauthors of the paper is a useful strategy to maintain momentum during revisions.
Writing (and revising) papers can be a highly rewarding activity. Start early, plan carefully, and do not delay the process. Reviewers’ comments are mostly geared toward enhancing the manuscript. Take them seriously, address them fully, and you will have an improved (and we hope, an accepted) manuscript.
Additional reading
El-Serag HB. Writing and publishing scientific papers. Gastroenterology. 2012 Feb;142(2):197-200. doi: 10.1053/j.gastro.2011.12.021. Epub 2011 Dec 16.
Downey SMet al. Manuscript development and publishing: A 5-step approach. Am J Med Sci. 2017 Feb;353(2):132-6. doi: 10.1016/j.amjms.2016.12.005. Epub 2016 Dec 9.
Sullivan GM. What to do when your paper is rejected. J Grad Med Educ. 2015;7:1-3. doi: 10.4300/JGME-D-14-00686.1
Kotz Det al. Effective writing and publishing scientific papers, part XII: responding to reviewers. J Clin Epidemiol. 2014;67:243. doi: 10.1016/j.jclinepi.2013.10.003. Epub 2014 Jan 9.
Dr. El-Serag is chairman of the Margaret M. and Albert B. Alkek department of medicine, Baylor College of Medicine, Houston; incoming president of the American Gastroenterological Association Institute; and past Editor in Chief, Clinical Gastroenterology and Hepatology. Dr. Kanwal is professor of medicine and chief of the section of gastroenterology and hepatology, department of medicine, Baylor College of Medicine and Michael E. DeBakey Veterans Affairs Medical Center, Houston, and Editor in Chief, Clinical Gastroenterology and Hepatology. This material is based on work supported by Cancer Prevention & Research Institute of Texas grant (RP150587). The work is also supported in part by the Center for Gastrointestinal Development, Infection and Injury (NIDDK P30 DK 56338).
Authors of manuscripts typically receive one of three responses from journals: 1. Accepted as submitted; 2. Accepted pending revisions (major or minor); and 3. Rejected. Receiving an unconditional acceptance is an unusual fate worth documenting and celebrating. On the other hand, irreversible rejections are so common that authors need to get accustomed to them. Upon receiving an unqualified editorial rejection without a formal review (usually described as priority-related rejection), send the same manuscript out the next day to another journal (with electronic submissions you can do this on the same day).
If your manuscript is rejected after being reviewed, consider seriously the comments given and try to learn from them. Was your study design flawed? Do you need additional data? Were your analyses incomplete or did you employ suboptimal statistical methods? Was your interpretation of the findings far reaching and out of proportion to the actual data? Use this experience and feedback, revise your manuscript, and submit it to a different journal. It is not uncommon to encounter the same reviewer at the next journal; fixing major issues seems responsive and gets you in the door.
To receive a “conditional acceptance” or “rejection with hope” is the most likely “good” editorial response. Avoid a very quick response, because it may be hasty or create an impression of a hasty response. Because most manuscripts with substantial reviews are sent back to the reviewers, the turnaround time in most journals is several weeks and, therefore, there is little to be gained by sending the revised manuscript in 1 day rather than 1 week. The best course of action is to cool down for 1-2 days and then decide and draft responses in 1 week, including planned additional analyses. In the case of seemingly contradictory or numerous requests from reviewers, it is best to carefully examine clues from the editors or associate editors as to the nature and extent of the revision needed. In most instances, we draft the response letter before revising the manuscript. We use the draft letter to obtain specific input from other authors and ‘brainstorm’ about additional analyses that can best address reviewers concerns.
Do the best that you can to fully address all reviewers’ comments. Adequate time should be spent making real changes, including adding additional data or analyses to the manuscript, and taking utmost care in describing and highlighting these changes. If you believe that the reviewers missed a point that was already included in the paper, then point this out as politely as possible as part of the response letter (see below).
In addition to revising your manuscript, you will be asked to prepare a point-by-point response to each of the reviewers’ comments you receive. Thank the editors and reviewers sincerely for their comments and explain how changes based on the comments have made the paper better; they did spend time reviewing your manuscript, and they have not rejected it yet. Reviewers are usually recognized experts, or their apprentices, in the content or method of research employed in your paper. Reviewers are also likely to be authors on papers cited in your manuscript. Avoid unnecessary arguments when possible, especially about noncore issues or about changes that you already conceded. If you are compelled to contest any of the reviewers’ comments, provide substantial evidence that supports your position and be respectful with your responses. Address each comment separately, beginning with the comments raised by the editors followed by those from reviewer one, two, and so on. After each response, clearly point the reviewers and the editors to the revised sections in the manuscript. In case of similar comments, it is acceptable to direct the second (or the third) reviewer to your previous response. Provide new tables, figures, data elements, and references as part of the response letter to make it a stand-alone document. It can be difficult (and annoying) if the reviewer has to flip back and forth between documents to understand the full story.
Appealing editorial decisions consumes a lot of energy, annoys editors and reviewers, and is generally futile. If it is needed, then write a polite, brief appeal letter that summarizes the reasons for the appeal. The most common editorial response to an appeal, which usually follows a several-week delay, is an equally polite affirmation of the original decision. The second and arguably worse outcome is for the manuscript to be sent to two to three new reviewers with another rejection after a several-month delay.
Have colleagues read and comment on your revised paper and use these comments to improve the draft. There is evidence that writing groups are effective in providing suggestions for improving papers: A writing group also keeps the momentum going during the revision process. Setting realistic time lines with the coauthors of the paper is a useful strategy to maintain momentum during revisions.
Writing (and revising) papers can be a highly rewarding activity. Start early, plan carefully, and do not delay the process. Reviewers’ comments are mostly geared toward enhancing the manuscript. Take them seriously, address them fully, and you will have an improved (and we hope, an accepted) manuscript.
Additional reading
El-Serag HB. Writing and publishing scientific papers. Gastroenterology. 2012 Feb;142(2):197-200. doi: 10.1053/j.gastro.2011.12.021. Epub 2011 Dec 16.
Downey SMet al. Manuscript development and publishing: A 5-step approach. Am J Med Sci. 2017 Feb;353(2):132-6. doi: 10.1016/j.amjms.2016.12.005. Epub 2016 Dec 9.
Sullivan GM. What to do when your paper is rejected. J Grad Med Educ. 2015;7:1-3. doi: 10.4300/JGME-D-14-00686.1
Kotz Det al. Effective writing and publishing scientific papers, part XII: responding to reviewers. J Clin Epidemiol. 2014;67:243. doi: 10.1016/j.jclinepi.2013.10.003. Epub 2014 Jan 9.
Dr. El-Serag is chairman of the Margaret M. and Albert B. Alkek department of medicine, Baylor College of Medicine, Houston; incoming president of the American Gastroenterological Association Institute; and past Editor in Chief, Clinical Gastroenterology and Hepatology. Dr. Kanwal is professor of medicine and chief of the section of gastroenterology and hepatology, department of medicine, Baylor College of Medicine and Michael E. DeBakey Veterans Affairs Medical Center, Houston, and Editor in Chief, Clinical Gastroenterology and Hepatology. This material is based on work supported by Cancer Prevention & Research Institute of Texas grant (RP150587). The work is also supported in part by the Center for Gastrointestinal Development, Infection and Injury (NIDDK P30 DK 56338).
Glucocorticoids plus tofacitinib may boost herpes zoster risk
Concomitant use of glucocorticoids with tofacitinib in rheumatoid arthritis may double the risk of herpes zoster, but methotrexate does not appear to increase the risk, outcomes from a cohort study of 8,030 rheumatoid arthritis patients – including 222 cases of herpes zoster – suggest.
Using information from Medicare and MarketScan, researchers found that dual therapy with tofacitinib and glucocorticoids was associated with a 96% increase in the risk of herpes zoster, compared with monotherapy with the Janus kinase inhibitor tofacitinib (95% confidence interval, 33%-188%). The crude incidence rate in those taking concomitant tofacitinib and glucocorticoids was 6 cases per 100 patient-years, compared with an incidence rate of 3.4 cases per 100 patient-years with tofacitinib monotherapy.
However, the addition of methotrexate therapy to tofacitinib was not associated with an increased risk of herpes zoster, and the incidence rate in patients on this dual therapy was 3.7 cases per 100 patient-years, Jeffrey R. Curtis, MD, MS, MPH, of the University of Alabama at Birmingham and his coauthors reported in Arthritis Care & Research.
Women – who constituted 80% of the study population – showed a significantly increased risk of herpes zoster, as did older patients.
The study also found that individuals who had received the live herpes zoster vaccine showed a trend toward a decrease in risk.
“We saw a strong trend for decreased risk related to vaccination with the live agent (Zostavax); the concern with this form of vaccination is that any live vaccination is potentially dangerous in patients receiving potent immunosuppression,” Dr. Curtis and his associates wrote.
“The risks for disease flare, and potentially problematic tolerability related to a relatively high incidence of grade 3 (severe) systemic reactogenicity, may limit enthusiasm until specific data in an RA population is available,” they wrote. However, they noted that a randomized, controlled trial of the live virus vaccine was underway in patients with rheumatoid arthritis who were being treated with tumor necrosis factor inhibitors and suggested that vaccination should be considered in at-risk patients who didn’t have contraindications.
The authors noted that the effect of glucocorticoid exposure on herpes zoster risk in patients taking tofacitinib was similar to that seen in rheumatoid arthritis patients receiving conventional synthetic disease-modifying antirheumatic drugs or biologic therapies.
The study was partly funded by the Patient-Centered Outcomes Research Institute. Two authors declared research grants and other funding from the pharmaceutical industry, but no other conflicts of interest were declared.
SOURCE: Curtis J et al. Arthritis Care Res. 2018 Oct 8. doi: 10.1002/acr.23769.
Concomitant use of glucocorticoids with tofacitinib in rheumatoid arthritis may double the risk of herpes zoster, but methotrexate does not appear to increase the risk, outcomes from a cohort study of 8,030 rheumatoid arthritis patients – including 222 cases of herpes zoster – suggest.
Using information from Medicare and MarketScan, researchers found that dual therapy with tofacitinib and glucocorticoids was associated with a 96% increase in the risk of herpes zoster, compared with monotherapy with the Janus kinase inhibitor tofacitinib (95% confidence interval, 33%-188%). The crude incidence rate in those taking concomitant tofacitinib and glucocorticoids was 6 cases per 100 patient-years, compared with an incidence rate of 3.4 cases per 100 patient-years with tofacitinib monotherapy.
However, the addition of methotrexate therapy to tofacitinib was not associated with an increased risk of herpes zoster, and the incidence rate in patients on this dual therapy was 3.7 cases per 100 patient-years, Jeffrey R. Curtis, MD, MS, MPH, of the University of Alabama at Birmingham and his coauthors reported in Arthritis Care & Research.
Women – who constituted 80% of the study population – showed a significantly increased risk of herpes zoster, as did older patients.
The study also found that individuals who had received the live herpes zoster vaccine showed a trend toward a decrease in risk.
“We saw a strong trend for decreased risk related to vaccination with the live agent (Zostavax); the concern with this form of vaccination is that any live vaccination is potentially dangerous in patients receiving potent immunosuppression,” Dr. Curtis and his associates wrote.
“The risks for disease flare, and potentially problematic tolerability related to a relatively high incidence of grade 3 (severe) systemic reactogenicity, may limit enthusiasm until specific data in an RA population is available,” they wrote. However, they noted that a randomized, controlled trial of the live virus vaccine was underway in patients with rheumatoid arthritis who were being treated with tumor necrosis factor inhibitors and suggested that vaccination should be considered in at-risk patients who didn’t have contraindications.
The authors noted that the effect of glucocorticoid exposure on herpes zoster risk in patients taking tofacitinib was similar to that seen in rheumatoid arthritis patients receiving conventional synthetic disease-modifying antirheumatic drugs or biologic therapies.
The study was partly funded by the Patient-Centered Outcomes Research Institute. Two authors declared research grants and other funding from the pharmaceutical industry, but no other conflicts of interest were declared.
SOURCE: Curtis J et al. Arthritis Care Res. 2018 Oct 8. doi: 10.1002/acr.23769.
Concomitant use of glucocorticoids with tofacitinib in rheumatoid arthritis may double the risk of herpes zoster, but methotrexate does not appear to increase the risk, outcomes from a cohort study of 8,030 rheumatoid arthritis patients – including 222 cases of herpes zoster – suggest.
Using information from Medicare and MarketScan, researchers found that dual therapy with tofacitinib and glucocorticoids was associated with a 96% increase in the risk of herpes zoster, compared with monotherapy with the Janus kinase inhibitor tofacitinib (95% confidence interval, 33%-188%). The crude incidence rate in those taking concomitant tofacitinib and glucocorticoids was 6 cases per 100 patient-years, compared with an incidence rate of 3.4 cases per 100 patient-years with tofacitinib monotherapy.
However, the addition of methotrexate therapy to tofacitinib was not associated with an increased risk of herpes zoster, and the incidence rate in patients on this dual therapy was 3.7 cases per 100 patient-years, Jeffrey R. Curtis, MD, MS, MPH, of the University of Alabama at Birmingham and his coauthors reported in Arthritis Care & Research.
Women – who constituted 80% of the study population – showed a significantly increased risk of herpes zoster, as did older patients.
The study also found that individuals who had received the live herpes zoster vaccine showed a trend toward a decrease in risk.
“We saw a strong trend for decreased risk related to vaccination with the live agent (Zostavax); the concern with this form of vaccination is that any live vaccination is potentially dangerous in patients receiving potent immunosuppression,” Dr. Curtis and his associates wrote.
“The risks for disease flare, and potentially problematic tolerability related to a relatively high incidence of grade 3 (severe) systemic reactogenicity, may limit enthusiasm until specific data in an RA population is available,” they wrote. However, they noted that a randomized, controlled trial of the live virus vaccine was underway in patients with rheumatoid arthritis who were being treated with tumor necrosis factor inhibitors and suggested that vaccination should be considered in at-risk patients who didn’t have contraindications.
The authors noted that the effect of glucocorticoid exposure on herpes zoster risk in patients taking tofacitinib was similar to that seen in rheumatoid arthritis patients receiving conventional synthetic disease-modifying antirheumatic drugs or biologic therapies.
The study was partly funded by the Patient-Centered Outcomes Research Institute. Two authors declared research grants and other funding from the pharmaceutical industry, but no other conflicts of interest were declared.
SOURCE: Curtis J et al. Arthritis Care Res. 2018 Oct 8. doi: 10.1002/acr.23769.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: Patients treated with concomitant glucocorticoids and tofacitinib showed increased risk of herpes zoster.
Major finding: Concomitant use of glucocorticoids and tofacitinib is associated with nearly twofold increase in the risk of herpes zoster.
Study details: Cohort study using data from 8,030 rheumatoid arthritis patients.
Disclosures: The study was partly funded by the Patient-Centered Outcomes Research Institute. Two authors declared research grants and other funding from the pharmaceutical industry, but no other conflicts of interest were declared.
Source: Curtis J et al. Arthritis Care Res. 2018 Oct 8. doi: 10.1002/acr.23769.
Solitary Exophytic Plaque on the Left Groin
The Diagnosis: Pemphigus Vegetans
A punch biopsy was taken from the verrucous plaque, and microscopic examination demonstrated prominent epidermal hyperplasia with intraepidermal eosinophilic microabscesses and a superficial dermatitis with abundant eosinophils (Figure 1A). Suprabasal acantholytic cleft formation was noted in a focal area (Figure 1B). Another punch biopsy was performed from the perilesional skin for direct immunofluorescence examination, which revealed intercellular deposits of IgG and C3 throughout the lower half of the epidermis (Figure 1C). Indirect immunofluorescence performed on monkey esophagus substrate showed circulating intercellular IgG antibodies in all the titers of up to 1/160 and an elevated level of IgG antidesmoglein 3 (anti-Dsg3) antibody (enzyme-linked immunosorbent assay index value, >200 RU/mL [reference range, <20 RU/mL]).

Because there was a solitary lesion, the decision was made to perform local treatment. One intralesional triamcinolone acetonide injection (20 mg/mL) resulted in remarkable flattening of the lesion (Figure 2). Subsequently, treatment was continued with clobetasol propionate ointment 3 times weekly for 1 month. During a follow-up period of 2 years, no signs of local relapse or new lesions elsewhere were noted, and the patient continued to be on long-term longitudinal evaluation.

Pemphigus vegetans (PV) is an uncommon variant of pemphigus, typically manifesting with vegetating erosions and plaques localized to the intertriginous areas of the body. Local factors such as semiocclusion, maceration, and/or bacterial or fungal colonization have been hypothesized to account for the distinctive localization and vegetation of the lesions.1,2 Traditionally, 2 clinical subtypes of PV have been described: (1) Hallopeau type presenting with pustules that later evolve into vegetating plaques, and (2) Neumann type that initially manifests as vesicles and bullae with a more disseminated distribution, transforming into hypertrophic masses with erosions.1-5 However, this distinction may not always be clear, and patients with features of both forms have been reported.2,5
At present, our case would best be regarded as a localized form of PV presenting with a solitary lesion. It may progress to more disseminated disease or remain localized during its course; the literature contains reports exemplifying both possibilities. In a large retrospective study from Tunisia encompassing almost 3 decades, the majority of the patients initially presented with unifocal involvement; however, the disease eventually became multifocal in almost all patients during the study period, emphasizing the need for long-term follow-up.2 There also are reports of PV confined to a single anatomic site, such as the scalp, sole, or vulva, that remained localized for years.2,4,6,7 Involvement of the oral mucosa is an important finding of PV and the presenting concern in approximately three-quarters of patients.2 Interestingly, the oral mucosa was not involved in our patient despite the high titer of anti-Dsg3 antibody, which suggests the need for the presence of other factors for clinical expression of the disease.
Although PV is considered a vegetating clinicomorphologic variant of pemphigus vulgaris, PV is histopathologically distinguished from pemphigus vulgaris by the presence of epidermal hyperplasia and intraepidermal eosinophilic microabscesses. Importantly, the epidermis displays signs of exuberant proliferation such as pseudoepitheliomatous hyperplasia and/or papillomatosis of a varying degree.1,2,5 Of note, suprabasal acantholysis is usually overshadowed by the changes in PV and presents only focally, as in our patient. The most common autoantibody profile is IgG targeting Dsg3; however, a spectrum of other autoantibodies has been identified, such as IgG antidesmocollin 3, IgA anti-Dsg3, and IgG anti-Dsg1.8,9
The most important differential diagnosis of PV is pyodermatitis-pyostomatitis vegetans. These 2 entities share many clinical and histopathological features; however, direct immunofluorescence is helpfulfor differentiation because it generally is negative in pyodermatitis-pyostomatitis vegetans.2,10 Furthermore, there is a well-established association between pyodermatitis-pyostomatitis vegetans and inflammatory bowel disorders, whereas PV has anecdotally been linked to malignancy, human immunodeficiency virus infection, and heroin abuse.1,2,10 Our patient was seronegative for human immunodeficiency virus and denied weight loss or loss of appetite. For those cases of PV involving a single anatomic site, the differential diagnosis is broader and encompasses dermatoses such as verrucae, syphilitic chancre, condylomata lata, granuloma inguinale, herpes simplex virus infection, and Kaposi sarcoma.
Treatment of PV is similar to pemphigus vulgaris and consists of a combination of systemic corticosteroids and steroid-sparing agents.1,5 On the other hand, more limited presentations of PV may be suitable for intralesional treatment with triamcinolone acetonide, thus avoiding potential adverse effects of systemic therapy.1,2 In our case with localized involvement, a favorable response was obtained with intralesional triamcinolone acetonide, and we plan to utilize systemic corticosteroids if the disease becomes generalized during follow-up.
- Ruocco V, Ruocco E, Caccavale S, et al. Pemphigus vegetans of the folds (intertriginous areas). Clin Dermatol. 2015;33:471-476.
- Zaraa I, Sellami A, Bouguerra C, et al. Pemphigus vegetans: a clinical, histological, immunopathological and prognostic study. J Eur Acad Dermatol Venereol. 2011;25:1160-1167.
- Madan V, August PJ. Exophytic plaques, blisters, and mouth ulcers. pemphigus vegetans (PV), Neumann type. Arch Dermatol. 2009;145:715-720.
- Mori M, Mariotti G, Grandi V, et al. Pemphigus vegetans of the scalp. J Eur Acad Dermatol Venereol. 2016;30:368-370.
- Monshi B, Marker M, Feichtinger H, et al. Pemphigus vegetans--immunopathological findings in a rare variant of pemphigus vulgaris. J Dtsch Dermatol Ges. 2010;8:179-183.
- Jain VK, Dixit VB, Mohan H. Pemphigus vegetans in an unusual site. Int J Dermatol. 1989;28:352-353.
- Wong KT, Wong KK. A case of acantholytic dermatosis of the vulva with features of pemphigus vegetans. J Cutan Pathol. 1994;21:453-456.
- Morizane S, Yamamoto T, Hisamatsu Y, et al. Pemphigus vegetans with IgG and IgA antidesmoglein 3 antibodies. Br J Dermatol. 2005;153:1236-1237.
- Saruta H, Ishii N, Teye K, et al. Two cases of pemphigus vegetans with IgG anti-desmocollin 3 antibodies. JAMA Dermatol. 2013;149:1209-1213.
- Mehravaran M, Kemény L, Husz S, et al. Pyodermatitis-pyostomatitis vegetans. Br J Dermatol. 1997;137:266-269.
The Diagnosis: Pemphigus Vegetans
A punch biopsy was taken from the verrucous plaque, and microscopic examination demonstrated prominent epidermal hyperplasia with intraepidermal eosinophilic microabscesses and a superficial dermatitis with abundant eosinophils (Figure 1A). Suprabasal acantholytic cleft formation was noted in a focal area (Figure 1B). Another punch biopsy was performed from the perilesional skin for direct immunofluorescence examination, which revealed intercellular deposits of IgG and C3 throughout the lower half of the epidermis (Figure 1C). Indirect immunofluorescence performed on monkey esophagus substrate showed circulating intercellular IgG antibodies in all the titers of up to 1/160 and an elevated level of IgG antidesmoglein 3 (anti-Dsg3) antibody (enzyme-linked immunosorbent assay index value, >200 RU/mL [reference range, <20 RU/mL]).

Because there was a solitary lesion, the decision was made to perform local treatment. One intralesional triamcinolone acetonide injection (20 mg/mL) resulted in remarkable flattening of the lesion (Figure 2). Subsequently, treatment was continued with clobetasol propionate ointment 3 times weekly for 1 month. During a follow-up period of 2 years, no signs of local relapse or new lesions elsewhere were noted, and the patient continued to be on long-term longitudinal evaluation.

Pemphigus vegetans (PV) is an uncommon variant of pemphigus, typically manifesting with vegetating erosions and plaques localized to the intertriginous areas of the body. Local factors such as semiocclusion, maceration, and/or bacterial or fungal colonization have been hypothesized to account for the distinctive localization and vegetation of the lesions.1,2 Traditionally, 2 clinical subtypes of PV have been described: (1) Hallopeau type presenting with pustules that later evolve into vegetating plaques, and (2) Neumann type that initially manifests as vesicles and bullae with a more disseminated distribution, transforming into hypertrophic masses with erosions.1-5 However, this distinction may not always be clear, and patients with features of both forms have been reported.2,5
At present, our case would best be regarded as a localized form of PV presenting with a solitary lesion. It may progress to more disseminated disease or remain localized during its course; the literature contains reports exemplifying both possibilities. In a large retrospective study from Tunisia encompassing almost 3 decades, the majority of the patients initially presented with unifocal involvement; however, the disease eventually became multifocal in almost all patients during the study period, emphasizing the need for long-term follow-up.2 There also are reports of PV confined to a single anatomic site, such as the scalp, sole, or vulva, that remained localized for years.2,4,6,7 Involvement of the oral mucosa is an important finding of PV and the presenting concern in approximately three-quarters of patients.2 Interestingly, the oral mucosa was not involved in our patient despite the high titer of anti-Dsg3 antibody, which suggests the need for the presence of other factors for clinical expression of the disease.
Although PV is considered a vegetating clinicomorphologic variant of pemphigus vulgaris, PV is histopathologically distinguished from pemphigus vulgaris by the presence of epidermal hyperplasia and intraepidermal eosinophilic microabscesses. Importantly, the epidermis displays signs of exuberant proliferation such as pseudoepitheliomatous hyperplasia and/or papillomatosis of a varying degree.1,2,5 Of note, suprabasal acantholysis is usually overshadowed by the changes in PV and presents only focally, as in our patient. The most common autoantibody profile is IgG targeting Dsg3; however, a spectrum of other autoantibodies has been identified, such as IgG antidesmocollin 3, IgA anti-Dsg3, and IgG anti-Dsg1.8,9
The most important differential diagnosis of PV is pyodermatitis-pyostomatitis vegetans. These 2 entities share many clinical and histopathological features; however, direct immunofluorescence is helpfulfor differentiation because it generally is negative in pyodermatitis-pyostomatitis vegetans.2,10 Furthermore, there is a well-established association between pyodermatitis-pyostomatitis vegetans and inflammatory bowel disorders, whereas PV has anecdotally been linked to malignancy, human immunodeficiency virus infection, and heroin abuse.1,2,10 Our patient was seronegative for human immunodeficiency virus and denied weight loss or loss of appetite. For those cases of PV involving a single anatomic site, the differential diagnosis is broader and encompasses dermatoses such as verrucae, syphilitic chancre, condylomata lata, granuloma inguinale, herpes simplex virus infection, and Kaposi sarcoma.
Treatment of PV is similar to pemphigus vulgaris and consists of a combination of systemic corticosteroids and steroid-sparing agents.1,5 On the other hand, more limited presentations of PV may be suitable for intralesional treatment with triamcinolone acetonide, thus avoiding potential adverse effects of systemic therapy.1,2 In our case with localized involvement, a favorable response was obtained with intralesional triamcinolone acetonide, and we plan to utilize systemic corticosteroids if the disease becomes generalized during follow-up.
The Diagnosis: Pemphigus Vegetans
A punch biopsy was taken from the verrucous plaque, and microscopic examination demonstrated prominent epidermal hyperplasia with intraepidermal eosinophilic microabscesses and a superficial dermatitis with abundant eosinophils (Figure 1A). Suprabasal acantholytic cleft formation was noted in a focal area (Figure 1B). Another punch biopsy was performed from the perilesional skin for direct immunofluorescence examination, which revealed intercellular deposits of IgG and C3 throughout the lower half of the epidermis (Figure 1C). Indirect immunofluorescence performed on monkey esophagus substrate showed circulating intercellular IgG antibodies in all the titers of up to 1/160 and an elevated level of IgG antidesmoglein 3 (anti-Dsg3) antibody (enzyme-linked immunosorbent assay index value, >200 RU/mL [reference range, <20 RU/mL]).

Because there was a solitary lesion, the decision was made to perform local treatment. One intralesional triamcinolone acetonide injection (20 mg/mL) resulted in remarkable flattening of the lesion (Figure 2). Subsequently, treatment was continued with clobetasol propionate ointment 3 times weekly for 1 month. During a follow-up period of 2 years, no signs of local relapse or new lesions elsewhere were noted, and the patient continued to be on long-term longitudinal evaluation.

Pemphigus vegetans (PV) is an uncommon variant of pemphigus, typically manifesting with vegetating erosions and plaques localized to the intertriginous areas of the body. Local factors such as semiocclusion, maceration, and/or bacterial or fungal colonization have been hypothesized to account for the distinctive localization and vegetation of the lesions.1,2 Traditionally, 2 clinical subtypes of PV have been described: (1) Hallopeau type presenting with pustules that later evolve into vegetating plaques, and (2) Neumann type that initially manifests as vesicles and bullae with a more disseminated distribution, transforming into hypertrophic masses with erosions.1-5 However, this distinction may not always be clear, and patients with features of both forms have been reported.2,5
At present, our case would best be regarded as a localized form of PV presenting with a solitary lesion. It may progress to more disseminated disease or remain localized during its course; the literature contains reports exemplifying both possibilities. In a large retrospective study from Tunisia encompassing almost 3 decades, the majority of the patients initially presented with unifocal involvement; however, the disease eventually became multifocal in almost all patients during the study period, emphasizing the need for long-term follow-up.2 There also are reports of PV confined to a single anatomic site, such as the scalp, sole, or vulva, that remained localized for years.2,4,6,7 Involvement of the oral mucosa is an important finding of PV and the presenting concern in approximately three-quarters of patients.2 Interestingly, the oral mucosa was not involved in our patient despite the high titer of anti-Dsg3 antibody, which suggests the need for the presence of other factors for clinical expression of the disease.
Although PV is considered a vegetating clinicomorphologic variant of pemphigus vulgaris, PV is histopathologically distinguished from pemphigus vulgaris by the presence of epidermal hyperplasia and intraepidermal eosinophilic microabscesses. Importantly, the epidermis displays signs of exuberant proliferation such as pseudoepitheliomatous hyperplasia and/or papillomatosis of a varying degree.1,2,5 Of note, suprabasal acantholysis is usually overshadowed by the changes in PV and presents only focally, as in our patient. The most common autoantibody profile is IgG targeting Dsg3; however, a spectrum of other autoantibodies has been identified, such as IgG antidesmocollin 3, IgA anti-Dsg3, and IgG anti-Dsg1.8,9
The most important differential diagnosis of PV is pyodermatitis-pyostomatitis vegetans. These 2 entities share many clinical and histopathological features; however, direct immunofluorescence is helpfulfor differentiation because it generally is negative in pyodermatitis-pyostomatitis vegetans.2,10 Furthermore, there is a well-established association between pyodermatitis-pyostomatitis vegetans and inflammatory bowel disorders, whereas PV has anecdotally been linked to malignancy, human immunodeficiency virus infection, and heroin abuse.1,2,10 Our patient was seronegative for human immunodeficiency virus and denied weight loss or loss of appetite. For those cases of PV involving a single anatomic site, the differential diagnosis is broader and encompasses dermatoses such as verrucae, syphilitic chancre, condylomata lata, granuloma inguinale, herpes simplex virus infection, and Kaposi sarcoma.
Treatment of PV is similar to pemphigus vulgaris and consists of a combination of systemic corticosteroids and steroid-sparing agents.1,5 On the other hand, more limited presentations of PV may be suitable for intralesional treatment with triamcinolone acetonide, thus avoiding potential adverse effects of systemic therapy.1,2 In our case with localized involvement, a favorable response was obtained with intralesional triamcinolone acetonide, and we plan to utilize systemic corticosteroids if the disease becomes generalized during follow-up.
- Ruocco V, Ruocco E, Caccavale S, et al. Pemphigus vegetans of the folds (intertriginous areas). Clin Dermatol. 2015;33:471-476.
- Zaraa I, Sellami A, Bouguerra C, et al. Pemphigus vegetans: a clinical, histological, immunopathological and prognostic study. J Eur Acad Dermatol Venereol. 2011;25:1160-1167.
- Madan V, August PJ. Exophytic plaques, blisters, and mouth ulcers. pemphigus vegetans (PV), Neumann type. Arch Dermatol. 2009;145:715-720.
- Mori M, Mariotti G, Grandi V, et al. Pemphigus vegetans of the scalp. J Eur Acad Dermatol Venereol. 2016;30:368-370.
- Monshi B, Marker M, Feichtinger H, et al. Pemphigus vegetans--immunopathological findings in a rare variant of pemphigus vulgaris. J Dtsch Dermatol Ges. 2010;8:179-183.
- Jain VK, Dixit VB, Mohan H. Pemphigus vegetans in an unusual site. Int J Dermatol. 1989;28:352-353.
- Wong KT, Wong KK. A case of acantholytic dermatosis of the vulva with features of pemphigus vegetans. J Cutan Pathol. 1994;21:453-456.
- Morizane S, Yamamoto T, Hisamatsu Y, et al. Pemphigus vegetans with IgG and IgA antidesmoglein 3 antibodies. Br J Dermatol. 2005;153:1236-1237.
- Saruta H, Ishii N, Teye K, et al. Two cases of pemphigus vegetans with IgG anti-desmocollin 3 antibodies. JAMA Dermatol. 2013;149:1209-1213.
- Mehravaran M, Kemény L, Husz S, et al. Pyodermatitis-pyostomatitis vegetans. Br J Dermatol. 1997;137:266-269.
- Ruocco V, Ruocco E, Caccavale S, et al. Pemphigus vegetans of the folds (intertriginous areas). Clin Dermatol. 2015;33:471-476.
- Zaraa I, Sellami A, Bouguerra C, et al. Pemphigus vegetans: a clinical, histological, immunopathological and prognostic study. J Eur Acad Dermatol Venereol. 2011;25:1160-1167.
- Madan V, August PJ. Exophytic plaques, blisters, and mouth ulcers. pemphigus vegetans (PV), Neumann type. Arch Dermatol. 2009;145:715-720.
- Mori M, Mariotti G, Grandi V, et al. Pemphigus vegetans of the scalp. J Eur Acad Dermatol Venereol. 2016;30:368-370.
- Monshi B, Marker M, Feichtinger H, et al. Pemphigus vegetans--immunopathological findings in a rare variant of pemphigus vulgaris. J Dtsch Dermatol Ges. 2010;8:179-183.
- Jain VK, Dixit VB, Mohan H. Pemphigus vegetans in an unusual site. Int J Dermatol. 1989;28:352-353.
- Wong KT, Wong KK. A case of acantholytic dermatosis of the vulva with features of pemphigus vegetans. J Cutan Pathol. 1994;21:453-456.
- Morizane S, Yamamoto T, Hisamatsu Y, et al. Pemphigus vegetans with IgG and IgA antidesmoglein 3 antibodies. Br J Dermatol. 2005;153:1236-1237.
- Saruta H, Ishii N, Teye K, et al. Two cases of pemphigus vegetans with IgG anti-desmocollin 3 antibodies. JAMA Dermatol. 2013;149:1209-1213.
- Mehravaran M, Kemény L, Husz S, et al. Pyodermatitis-pyostomatitis vegetans. Br J Dermatol. 1997;137:266-269.
A 40-year-old otherwise healthy man presented with an exophytic plaque on the left groin of 1 month's duration. The lesion reportedly emerged as pustules that slowly expanded and coalesced. At an outside institution, cryotherapy was planned for a presumed diagnosis of condyloma acuminatum; however, the patient decided to get a second opinion. He denied recent intake of new drugs. Six months prior he had traveled to China and engaged in unprotected sexual intercourse. Physical examination revealed an approximately 4×2-cm exophytic plaque with a partially eroded and exudative surface on the left inguinal fold. Dermatologic examination, including the oral mucosa, was otherwise normal. Complete blood cell count and sexually transmitted disease panel were unremarkable.
CABG vs PCI, new index enhances cardiac CT, and more
, a new index stretches the prognostic power of cardiac CT, a weight-loss drug cuts new-onset diabetes, and a controversial diet score is validated.
Subscribe to Cardiocast wherever you get your podcasts:
Amazon Alexa
Apple Podcasts
, a new index stretches the prognostic power of cardiac CT, a weight-loss drug cuts new-onset diabetes, and a controversial diet score is validated.
Subscribe to Cardiocast wherever you get your podcasts:
Amazon Alexa
Apple Podcasts
, a new index stretches the prognostic power of cardiac CT, a weight-loss drug cuts new-onset diabetes, and a controversial diet score is validated.
Subscribe to Cardiocast wherever you get your podcasts:
Amazon Alexa
Apple Podcasts
Junior faculty guide to preparing a research grant
A wise person once said, “Research is a marathon and not a sprint.” Grant writing is the training for the marathon, and it requires discipline and fortitude to succeed. We are junior faculty members with mentored career development awards who are transitioning to independence. Below, we provide for our junior faculty colleagues some tips that have helped us train for our marathon in research.
Identify great mentors
We all understand that outstanding mentorship is critical to success. With that said, we often struggle to understand what a good mentor is. In regard to grant writing, you need someone who is willing to use red ink. While positive reinforcement may be good for your self-esteem, your mentor needs to be critical so that you can learn how to present the best possible product. In return, you must be an invested mentee who is respectful of the mentor’s time, is prepared for meetings, and responds appropriately to feedback.
Attend workshops
Your home institution and professional societies hold outstanding workshops that provide didactic lessons on both the logistics and mechanics of grant writing and allow you to network with like-minded peers, potential mentors, and staff from the funding organization. One excellent example is the American Gastroenterological Association/American Association for the Study of Liver Diseases (AGA/AASLD) Academic Skills workshop.
Decide on a grant mechanism
There are many different grants available through the government, industry, foundations, and your institution. Each grantor may have a variety of mechanisms from pilot awards to larger multiprovider and institution grants. Deciding which grants to apply for can be more of an art than it is a science. Research the opportunities available to you and develop a long-term plan with your mentors.
Start early and have a plan
An effective grant application is prepared in steps, and every step takes longer than anticipated. About 6 months prior to the deadline, read the instructions and consider using something like a Gantt chart to identify all required sections, special requirements (font, spacing, page limits), and anticipated time of completion. Then, structure a reasonable timeline – and stick to it! Remember to allow ample time for all sections, including the career development plan and research environment. Your institution will probably request the documents early, anywhere from 1 to 2 weeks prior to the deadline so that it can be circulated for institutional signatures. Steady progress wins most races.
Specific aims
There is no grant without a great idea, but not all great ideas are funded. So, the first step is to polish your idea, which must be clearly described on the Specific Aims page, which is a one-page summary that lays the framework for the rest of the grant. For the primary reviewers, it should entice them to read the proposal. For others on the review panel, it may be the only section of your grant that they read. Make sure it is clear and concise. If possible, construct a visually pleasing and easy-to-follow figure that encapsulates your proposal.
Circulate
Ideally, every section of the grant will be circulated but it is critical to have others review the Specific Aims at the very least. Ask not only your mentors and those in the field to critique but also those outside of your area and even your friends and unsuspecting family members; they may not know (or care) about the content but should be able to follow the flow and identify grammatical errors. Remember that everyone is busy, so give ample time for people to review the documents.
Read other proposals
Practice makes perfect. So you can either apply for many grants and make the mistakes yourself or read and review as many proposals as you can to learn from your colleagues’ successes and mistakes. Many institutions, mentors, and colleagues will provide copies of prior applications if you ask. Make sure you know which were successful and try to understand why the others were not successful.
When reading the aims and research strategy, pay close attention to how significance and innovation are detailed. Also, some things like the research environment, which is especially important for career development grants, may be directly applicable to your grant.
Help the reviewer
In general, reviewing grants is a voluntary undertaking. Imagine the reviewer reading your grant at a home filled with screaming children or, alternatively, flying in cramped quarters. Neither situation is stress-free, so put yourself in those positions and decide what you can do to make the reviewer’s job easier.
Use figures and tables to summarize the text, and consider coming back to the figure from your Specific Aims to refer to the specific parts of the proposal. You can decrease reviewer fatigue by using line breaks and fonts to break up sections and highlight important details. This will also be helpful to the reviewers on the panel who were not assigned to your grant and possibly first seeing it during the session.
Learn from rejection
You are either a savant or have not applied for enough grants if you have not received a rejection letter. Often, reviewers provide you with constructive comments, which (after a session of crying in the corner in a fetal position), you can use to improve your grant. Resubmission works!
Apply widely
Identify different possible grants, and work with your mentors on a strategy that allows you to make your idea versatile and package it for various funding mechanisms. Once you have a grant, you can tailor it to other grants as needed. However, remember that quantity does not replace quality, so many poor grants that are not funded will not replace one good one that is funded.
There are multiple approaches to training for the marathon of research, so these tips are not a comprehensive list or mandatory commandments. They have, however, proven invaluable to our mentors and us. Our institutions, societies and government agencies have identified the decline of young scientists and physician-scientists as a major leak in the research pipeline, so there are excellent funding mechanisms that are available to you. Good luck!
We would like to acknowledge Jennifer Weiss, MD, and Sumera Rizvi, MD, for their constructive comments.
Dr. Beyder is with the enteric neuroscience program, a consultant for the department of gastroenterology and hepatology, and an assistant professor of biomedical engineering and physiology at the Mayo Clinic School of Medicine, Rochester, Minn.; Dr. Twyman-Saint Victor is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia.
A wise person once said, “Research is a marathon and not a sprint.” Grant writing is the training for the marathon, and it requires discipline and fortitude to succeed. We are junior faculty members with mentored career development awards who are transitioning to independence. Below, we provide for our junior faculty colleagues some tips that have helped us train for our marathon in research.
Identify great mentors
We all understand that outstanding mentorship is critical to success. With that said, we often struggle to understand what a good mentor is. In regard to grant writing, you need someone who is willing to use red ink. While positive reinforcement may be good for your self-esteem, your mentor needs to be critical so that you can learn how to present the best possible product. In return, you must be an invested mentee who is respectful of the mentor’s time, is prepared for meetings, and responds appropriately to feedback.
Attend workshops
Your home institution and professional societies hold outstanding workshops that provide didactic lessons on both the logistics and mechanics of grant writing and allow you to network with like-minded peers, potential mentors, and staff from the funding organization. One excellent example is the American Gastroenterological Association/American Association for the Study of Liver Diseases (AGA/AASLD) Academic Skills workshop.
Decide on a grant mechanism
There are many different grants available through the government, industry, foundations, and your institution. Each grantor may have a variety of mechanisms from pilot awards to larger multiprovider and institution grants. Deciding which grants to apply for can be more of an art than it is a science. Research the opportunities available to you and develop a long-term plan with your mentors.
Start early and have a plan
An effective grant application is prepared in steps, and every step takes longer than anticipated. About 6 months prior to the deadline, read the instructions and consider using something like a Gantt chart to identify all required sections, special requirements (font, spacing, page limits), and anticipated time of completion. Then, structure a reasonable timeline – and stick to it! Remember to allow ample time for all sections, including the career development plan and research environment. Your institution will probably request the documents early, anywhere from 1 to 2 weeks prior to the deadline so that it can be circulated for institutional signatures. Steady progress wins most races.
Specific aims
There is no grant without a great idea, but not all great ideas are funded. So, the first step is to polish your idea, which must be clearly described on the Specific Aims page, which is a one-page summary that lays the framework for the rest of the grant. For the primary reviewers, it should entice them to read the proposal. For others on the review panel, it may be the only section of your grant that they read. Make sure it is clear and concise. If possible, construct a visually pleasing and easy-to-follow figure that encapsulates your proposal.
Circulate
Ideally, every section of the grant will be circulated but it is critical to have others review the Specific Aims at the very least. Ask not only your mentors and those in the field to critique but also those outside of your area and even your friends and unsuspecting family members; they may not know (or care) about the content but should be able to follow the flow and identify grammatical errors. Remember that everyone is busy, so give ample time for people to review the documents.
Read other proposals
Practice makes perfect. So you can either apply for many grants and make the mistakes yourself or read and review as many proposals as you can to learn from your colleagues’ successes and mistakes. Many institutions, mentors, and colleagues will provide copies of prior applications if you ask. Make sure you know which were successful and try to understand why the others were not successful.
When reading the aims and research strategy, pay close attention to how significance and innovation are detailed. Also, some things like the research environment, which is especially important for career development grants, may be directly applicable to your grant.
Help the reviewer
In general, reviewing grants is a voluntary undertaking. Imagine the reviewer reading your grant at a home filled with screaming children or, alternatively, flying in cramped quarters. Neither situation is stress-free, so put yourself in those positions and decide what you can do to make the reviewer’s job easier.
Use figures and tables to summarize the text, and consider coming back to the figure from your Specific Aims to refer to the specific parts of the proposal. You can decrease reviewer fatigue by using line breaks and fonts to break up sections and highlight important details. This will also be helpful to the reviewers on the panel who were not assigned to your grant and possibly first seeing it during the session.
Learn from rejection
You are either a savant or have not applied for enough grants if you have not received a rejection letter. Often, reviewers provide you with constructive comments, which (after a session of crying in the corner in a fetal position), you can use to improve your grant. Resubmission works!
Apply widely
Identify different possible grants, and work with your mentors on a strategy that allows you to make your idea versatile and package it for various funding mechanisms. Once you have a grant, you can tailor it to other grants as needed. However, remember that quantity does not replace quality, so many poor grants that are not funded will not replace one good one that is funded.
There are multiple approaches to training for the marathon of research, so these tips are not a comprehensive list or mandatory commandments. They have, however, proven invaluable to our mentors and us. Our institutions, societies and government agencies have identified the decline of young scientists and physician-scientists as a major leak in the research pipeline, so there are excellent funding mechanisms that are available to you. Good luck!
We would like to acknowledge Jennifer Weiss, MD, and Sumera Rizvi, MD, for their constructive comments.
Dr. Beyder is with the enteric neuroscience program, a consultant for the department of gastroenterology and hepatology, and an assistant professor of biomedical engineering and physiology at the Mayo Clinic School of Medicine, Rochester, Minn.; Dr. Twyman-Saint Victor is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia.
A wise person once said, “Research is a marathon and not a sprint.” Grant writing is the training for the marathon, and it requires discipline and fortitude to succeed. We are junior faculty members with mentored career development awards who are transitioning to independence. Below, we provide for our junior faculty colleagues some tips that have helped us train for our marathon in research.
Identify great mentors
We all understand that outstanding mentorship is critical to success. With that said, we often struggle to understand what a good mentor is. In regard to grant writing, you need someone who is willing to use red ink. While positive reinforcement may be good for your self-esteem, your mentor needs to be critical so that you can learn how to present the best possible product. In return, you must be an invested mentee who is respectful of the mentor’s time, is prepared for meetings, and responds appropriately to feedback.
Attend workshops
Your home institution and professional societies hold outstanding workshops that provide didactic lessons on both the logistics and mechanics of grant writing and allow you to network with like-minded peers, potential mentors, and staff from the funding organization. One excellent example is the American Gastroenterological Association/American Association for the Study of Liver Diseases (AGA/AASLD) Academic Skills workshop.
Decide on a grant mechanism
There are many different grants available through the government, industry, foundations, and your institution. Each grantor may have a variety of mechanisms from pilot awards to larger multiprovider and institution grants. Deciding which grants to apply for can be more of an art than it is a science. Research the opportunities available to you and develop a long-term plan with your mentors.
Start early and have a plan
An effective grant application is prepared in steps, and every step takes longer than anticipated. About 6 months prior to the deadline, read the instructions and consider using something like a Gantt chart to identify all required sections, special requirements (font, spacing, page limits), and anticipated time of completion. Then, structure a reasonable timeline – and stick to it! Remember to allow ample time for all sections, including the career development plan and research environment. Your institution will probably request the documents early, anywhere from 1 to 2 weeks prior to the deadline so that it can be circulated for institutional signatures. Steady progress wins most races.
Specific aims
There is no grant without a great idea, but not all great ideas are funded. So, the first step is to polish your idea, which must be clearly described on the Specific Aims page, which is a one-page summary that lays the framework for the rest of the grant. For the primary reviewers, it should entice them to read the proposal. For others on the review panel, it may be the only section of your grant that they read. Make sure it is clear and concise. If possible, construct a visually pleasing and easy-to-follow figure that encapsulates your proposal.
Circulate
Ideally, every section of the grant will be circulated but it is critical to have others review the Specific Aims at the very least. Ask not only your mentors and those in the field to critique but also those outside of your area and even your friends and unsuspecting family members; they may not know (or care) about the content but should be able to follow the flow and identify grammatical errors. Remember that everyone is busy, so give ample time for people to review the documents.
Read other proposals
Practice makes perfect. So you can either apply for many grants and make the mistakes yourself or read and review as many proposals as you can to learn from your colleagues’ successes and mistakes. Many institutions, mentors, and colleagues will provide copies of prior applications if you ask. Make sure you know which were successful and try to understand why the others were not successful.
When reading the aims and research strategy, pay close attention to how significance and innovation are detailed. Also, some things like the research environment, which is especially important for career development grants, may be directly applicable to your grant.
Help the reviewer
In general, reviewing grants is a voluntary undertaking. Imagine the reviewer reading your grant at a home filled with screaming children or, alternatively, flying in cramped quarters. Neither situation is stress-free, so put yourself in those positions and decide what you can do to make the reviewer’s job easier.
Use figures and tables to summarize the text, and consider coming back to the figure from your Specific Aims to refer to the specific parts of the proposal. You can decrease reviewer fatigue by using line breaks and fonts to break up sections and highlight important details. This will also be helpful to the reviewers on the panel who were not assigned to your grant and possibly first seeing it during the session.
Learn from rejection
You are either a savant or have not applied for enough grants if you have not received a rejection letter. Often, reviewers provide you with constructive comments, which (after a session of crying in the corner in a fetal position), you can use to improve your grant. Resubmission works!
Apply widely
Identify different possible grants, and work with your mentors on a strategy that allows you to make your idea versatile and package it for various funding mechanisms. Once you have a grant, you can tailor it to other grants as needed. However, remember that quantity does not replace quality, so many poor grants that are not funded will not replace one good one that is funded.
There are multiple approaches to training for the marathon of research, so these tips are not a comprehensive list or mandatory commandments. They have, however, proven invaluable to our mentors and us. Our institutions, societies and government agencies have identified the decline of young scientists and physician-scientists as a major leak in the research pipeline, so there are excellent funding mechanisms that are available to you. Good luck!
We would like to acknowledge Jennifer Weiss, MD, and Sumera Rizvi, MD, for their constructive comments.
Dr. Beyder is with the enteric neuroscience program, a consultant for the department of gastroenterology and hepatology, and an assistant professor of biomedical engineering and physiology at the Mayo Clinic School of Medicine, Rochester, Minn.; Dr. Twyman-Saint Victor is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia.