User login
Stockholm3 Prostate Test Bests PSA for Prostate Cancer Risk in North America
The Stockholm3 (A3P Biomedical) multiparametic blood test has shown accuracy in assessing the risk of prostate cancer, exceeding that of the standard prostate-specific antigen (PSA)-based test, in Swedish patients.
“The Stockholm3 outperformed the PSA test overall and in every subcohort, with an impressive reduction of unnecessary biopsies of 40% to 50%, while maintaining relative sensitivity,” first author Scott E. Eggener, MD, said in presenting the findings at the ASCO Genitourinary Cancers Symposium. The test “has attractive characteristics in a diverse cohort, including within various racial and ethnic subgroups,” added Dr. Eggener, professor of surgery and radiology at the University of Chicago.
While the PSA test, the standard-of-care in prostate cancer risk assessment, reduces mortality, the test is known to have a risk for false positive results, leading to unnecessary prostate biopsies, as well as overdiagnosis of low-risk prostate cancers, Dr. Eggener explained in his talk.
Randomized trials do show “fewer men die from prostate cancer with screening [with PSA testing], however, the likelihood of unnecessarily finding out about a cancer, undergoing treatment, and exposure to potential treatment-related side effects is significantly higher,” Dr. Eggener said in a interview.
The Stockholm3 clinical diagnostic prostate cancer test, which has been used in Sweden and Norway since 2017, was validated in a sample of nearly 60,000 men in the STHLM3 study (doi: 10.1016/S1470-2045[15]00361-7), which was published in The Lancet Oncology in December 2015. That study showed significant improvement over PSA alone detection of prostate cancers with a Gleason score of at least 7 (P < .0001), Dr. Eggener explained.
The test combines five plasma protein markers, including total and free PSA, PSP94, GDF-15 and KLK2, along with 101 genetic markers and clinical patient data, including age, previous biopsy results and family history.
Because the Stockholm3 test was validated in a Swedish population cohort, evidence on the accuracy of the test in other racial and ethnic populations is lacking, the authors noted in the abstract.
Study Methods and Results
To further investigate, Dr. Eggener and his colleagues conducted the prospective SEPTA trial, involving 2,129 men with no known prostate cancer but clinical indications for prostate biopsy, who were referred for prostate biopsy at 17 North American sites between 2019 and 2023.
Among the men, 24% were self-identified as African American/Black; 46% were White/Caucasian; 14% were Hispanic/Latina; and 16% were Asian. The men’s median age was 63; their median PSA value was 6.1 ng/mL, according to the abstract.
Of the patients, 16% received magnetic resonance imaging (MRI)-targeted biopsies and 20% had prior benign biopsies, the abstract notes.
Biopsy results showed that clinically significant prostate cancer, defined as International Society of Urological Pathology (ISUP) Gleason Grade group ≥ 2, was detected in 29% of patients, with 14% having ISUP 1 cancer and 57% of cases having been benign, according to the abstract.
Overall detection rates of grade 2 or higher were 37% for African American/Black, 28% for White/Caucasian, 29% for Hispanic/Latino, and 21% Asian.
In terms of sensitivity of the two tests, the Stockholm3 (cut-point of ≥ 15) was noninferior compared with the traditional PSA cut-point of ≥ 4 ng/mL (relative sensitivity 0.95).
Results were consistent across ethnic subgroups: noninferior sensitivity (0.91-0.98) and superior specificity (2.51-4.70), the abstract authors reported.
Compared with the use of the PSA test’s cut-point of ≥ 4 ng/mL, the use of Stockholm3’s cut-point of ≥ 15 or higher would have reduced unnecessary biopsies by 45% overall, including by 46% among Asian and Black/African American patients, by 53% in Hispanic patients and 42% in White patients, according to the abstract.
Overall, “utilization of Stockholm3 improves the net benefit:harm ratio of PSA screening by identifying nearly all men with Gleason Grade 2 or higher, while minimizing the number of men undergoing biopsy who show no cancer or an indolent cancer (Gleason Grade 1),” Dr. Eggener said in an interview.
Stockholm3 Expected to be Available in U.S. This Year
The test, which has been available in Sweden since 2018, is expected to become commercially available in the United States in early 2024. Dr. Eggener noted that “cost of the test hasn’t been finalized, but will be considerably more expensive than PSA, which is very cheap.”
Commenting on the findings, Bradley McGregor, MD, of the Dana Farber Cancer Institute and an ASCO oncology expert, noted that “ultimately, the goal [of prostate screening] is to be able to better decide when a biopsy is going to yield a clinically relevant prostate cancer, [and] this study gives us some insight of the use of the Stockholm3 tool in a more diverse population.
“How the tool will be utilized in the clinic and in guidelines is something that is a work in progress,” he added. “But I think this provides some reassurances that this will have implications beyond just the homogeneous populations in the original studies.”
Dr. McGregor noted that considerations of the issue of cost should be weighed against the potential costs involved in unnecessary biopsies and a host of other costs that can arise with an inaccurate risk assessment.
“If there is a way to avoid those costs and help us have more confidence in the prostate test results and intervene at an earlier stage, I think that’s exciting,” he said.
Dr. Eggener has consulted for A3P Biomedical but had no financial relationship with the company to disclose.
The Stockholm3 (A3P Biomedical) multiparametic blood test has shown accuracy in assessing the risk of prostate cancer, exceeding that of the standard prostate-specific antigen (PSA)-based test, in Swedish patients.
“The Stockholm3 outperformed the PSA test overall and in every subcohort, with an impressive reduction of unnecessary biopsies of 40% to 50%, while maintaining relative sensitivity,” first author Scott E. Eggener, MD, said in presenting the findings at the ASCO Genitourinary Cancers Symposium. The test “has attractive characteristics in a diverse cohort, including within various racial and ethnic subgroups,” added Dr. Eggener, professor of surgery and radiology at the University of Chicago.
While the PSA test, the standard-of-care in prostate cancer risk assessment, reduces mortality, the test is known to have a risk for false positive results, leading to unnecessary prostate biopsies, as well as overdiagnosis of low-risk prostate cancers, Dr. Eggener explained in his talk.
Randomized trials do show “fewer men die from prostate cancer with screening [with PSA testing], however, the likelihood of unnecessarily finding out about a cancer, undergoing treatment, and exposure to potential treatment-related side effects is significantly higher,” Dr. Eggener said in a interview.
The Stockholm3 clinical diagnostic prostate cancer test, which has been used in Sweden and Norway since 2017, was validated in a sample of nearly 60,000 men in the STHLM3 study (doi: 10.1016/S1470-2045[15]00361-7), which was published in The Lancet Oncology in December 2015. That study showed significant improvement over PSA alone detection of prostate cancers with a Gleason score of at least 7 (P < .0001), Dr. Eggener explained.
The test combines five plasma protein markers, including total and free PSA, PSP94, GDF-15 and KLK2, along with 101 genetic markers and clinical patient data, including age, previous biopsy results and family history.
Because the Stockholm3 test was validated in a Swedish population cohort, evidence on the accuracy of the test in other racial and ethnic populations is lacking, the authors noted in the abstract.
Study Methods and Results
To further investigate, Dr. Eggener and his colleagues conducted the prospective SEPTA trial, involving 2,129 men with no known prostate cancer but clinical indications for prostate biopsy, who were referred for prostate biopsy at 17 North American sites between 2019 and 2023.
Among the men, 24% were self-identified as African American/Black; 46% were White/Caucasian; 14% were Hispanic/Latina; and 16% were Asian. The men’s median age was 63; their median PSA value was 6.1 ng/mL, according to the abstract.
Of the patients, 16% received magnetic resonance imaging (MRI)-targeted biopsies and 20% had prior benign biopsies, the abstract notes.
Biopsy results showed that clinically significant prostate cancer, defined as International Society of Urological Pathology (ISUP) Gleason Grade group ≥ 2, was detected in 29% of patients, with 14% having ISUP 1 cancer and 57% of cases having been benign, according to the abstract.
Overall detection rates of grade 2 or higher were 37% for African American/Black, 28% for White/Caucasian, 29% for Hispanic/Latino, and 21% Asian.
In terms of sensitivity of the two tests, the Stockholm3 (cut-point of ≥ 15) was noninferior compared with the traditional PSA cut-point of ≥ 4 ng/mL (relative sensitivity 0.95).
Results were consistent across ethnic subgroups: noninferior sensitivity (0.91-0.98) and superior specificity (2.51-4.70), the abstract authors reported.
Compared with the use of the PSA test’s cut-point of ≥ 4 ng/mL, the use of Stockholm3’s cut-point of ≥ 15 or higher would have reduced unnecessary biopsies by 45% overall, including by 46% among Asian and Black/African American patients, by 53% in Hispanic patients and 42% in White patients, according to the abstract.
Overall, “utilization of Stockholm3 improves the net benefit:harm ratio of PSA screening by identifying nearly all men with Gleason Grade 2 or higher, while minimizing the number of men undergoing biopsy who show no cancer or an indolent cancer (Gleason Grade 1),” Dr. Eggener said in an interview.
Stockholm3 Expected to be Available in U.S. This Year
The test, which has been available in Sweden since 2018, is expected to become commercially available in the United States in early 2024. Dr. Eggener noted that “cost of the test hasn’t been finalized, but will be considerably more expensive than PSA, which is very cheap.”
Commenting on the findings, Bradley McGregor, MD, of the Dana Farber Cancer Institute and an ASCO oncology expert, noted that “ultimately, the goal [of prostate screening] is to be able to better decide when a biopsy is going to yield a clinically relevant prostate cancer, [and] this study gives us some insight of the use of the Stockholm3 tool in a more diverse population.
“How the tool will be utilized in the clinic and in guidelines is something that is a work in progress,” he added. “But I think this provides some reassurances that this will have implications beyond just the homogeneous populations in the original studies.”
Dr. McGregor noted that considerations of the issue of cost should be weighed against the potential costs involved in unnecessary biopsies and a host of other costs that can arise with an inaccurate risk assessment.
“If there is a way to avoid those costs and help us have more confidence in the prostate test results and intervene at an earlier stage, I think that’s exciting,” he said.
Dr. Eggener has consulted for A3P Biomedical but had no financial relationship with the company to disclose.
The Stockholm3 (A3P Biomedical) multiparametic blood test has shown accuracy in assessing the risk of prostate cancer, exceeding that of the standard prostate-specific antigen (PSA)-based test, in Swedish patients.
“The Stockholm3 outperformed the PSA test overall and in every subcohort, with an impressive reduction of unnecessary biopsies of 40% to 50%, while maintaining relative sensitivity,” first author Scott E. Eggener, MD, said in presenting the findings at the ASCO Genitourinary Cancers Symposium. The test “has attractive characteristics in a diverse cohort, including within various racial and ethnic subgroups,” added Dr. Eggener, professor of surgery and radiology at the University of Chicago.
While the PSA test, the standard-of-care in prostate cancer risk assessment, reduces mortality, the test is known to have a risk for false positive results, leading to unnecessary prostate biopsies, as well as overdiagnosis of low-risk prostate cancers, Dr. Eggener explained in his talk.
Randomized trials do show “fewer men die from prostate cancer with screening [with PSA testing], however, the likelihood of unnecessarily finding out about a cancer, undergoing treatment, and exposure to potential treatment-related side effects is significantly higher,” Dr. Eggener said in a interview.
The Stockholm3 clinical diagnostic prostate cancer test, which has been used in Sweden and Norway since 2017, was validated in a sample of nearly 60,000 men in the STHLM3 study (doi: 10.1016/S1470-2045[15]00361-7), which was published in The Lancet Oncology in December 2015. That study showed significant improvement over PSA alone detection of prostate cancers with a Gleason score of at least 7 (P < .0001), Dr. Eggener explained.
The test combines five plasma protein markers, including total and free PSA, PSP94, GDF-15 and KLK2, along with 101 genetic markers and clinical patient data, including age, previous biopsy results and family history.
Because the Stockholm3 test was validated in a Swedish population cohort, evidence on the accuracy of the test in other racial and ethnic populations is lacking, the authors noted in the abstract.
Study Methods and Results
To further investigate, Dr. Eggener and his colleagues conducted the prospective SEPTA trial, involving 2,129 men with no known prostate cancer but clinical indications for prostate biopsy, who were referred for prostate biopsy at 17 North American sites between 2019 and 2023.
Among the men, 24% were self-identified as African American/Black; 46% were White/Caucasian; 14% were Hispanic/Latina; and 16% were Asian. The men’s median age was 63; their median PSA value was 6.1 ng/mL, according to the abstract.
Of the patients, 16% received magnetic resonance imaging (MRI)-targeted biopsies and 20% had prior benign biopsies, the abstract notes.
Biopsy results showed that clinically significant prostate cancer, defined as International Society of Urological Pathology (ISUP) Gleason Grade group ≥ 2, was detected in 29% of patients, with 14% having ISUP 1 cancer and 57% of cases having been benign, according to the abstract.
Overall detection rates of grade 2 or higher were 37% for African American/Black, 28% for White/Caucasian, 29% for Hispanic/Latino, and 21% Asian.
In terms of sensitivity of the two tests, the Stockholm3 (cut-point of ≥ 15) was noninferior compared with the traditional PSA cut-point of ≥ 4 ng/mL (relative sensitivity 0.95).
Results were consistent across ethnic subgroups: noninferior sensitivity (0.91-0.98) and superior specificity (2.51-4.70), the abstract authors reported.
Compared with the use of the PSA test’s cut-point of ≥ 4 ng/mL, the use of Stockholm3’s cut-point of ≥ 15 or higher would have reduced unnecessary biopsies by 45% overall, including by 46% among Asian and Black/African American patients, by 53% in Hispanic patients and 42% in White patients, according to the abstract.
Overall, “utilization of Stockholm3 improves the net benefit:harm ratio of PSA screening by identifying nearly all men with Gleason Grade 2 or higher, while minimizing the number of men undergoing biopsy who show no cancer or an indolent cancer (Gleason Grade 1),” Dr. Eggener said in an interview.
Stockholm3 Expected to be Available in U.S. This Year
The test, which has been available in Sweden since 2018, is expected to become commercially available in the United States in early 2024. Dr. Eggener noted that “cost of the test hasn’t been finalized, but will be considerably more expensive than PSA, which is very cheap.”
Commenting on the findings, Bradley McGregor, MD, of the Dana Farber Cancer Institute and an ASCO oncology expert, noted that “ultimately, the goal [of prostate screening] is to be able to better decide when a biopsy is going to yield a clinically relevant prostate cancer, [and] this study gives us some insight of the use of the Stockholm3 tool in a more diverse population.
“How the tool will be utilized in the clinic and in guidelines is something that is a work in progress,” he added. “But I think this provides some reassurances that this will have implications beyond just the homogeneous populations in the original studies.”
Dr. McGregor noted that considerations of the issue of cost should be weighed against the potential costs involved in unnecessary biopsies and a host of other costs that can arise with an inaccurate risk assessment.
“If there is a way to avoid those costs and help us have more confidence in the prostate test results and intervene at an earlier stage, I think that’s exciting,” he said.
Dr. Eggener has consulted for A3P Biomedical but had no financial relationship with the company to disclose.
FROM ASCO GU 2024
The Potential Benefits of Dietary Changes in Psoriasis Patients
Psoriasis is a chronic inflammatory skin disease for which several lifestyle factors—smoking, alcohol use, and psychological stress—are associated with higher incidence and more severe disease.1-3 Diet also has been implicated as a factor that can affect psoriasis,4 and many patients have shown interest in possible dietary interventions to help their disease.5
In 2018, the National Psoriasis Foundation (NPF) presented dietary recommendations for patients based on results from a systematic review. From the available literature, only dietary weight reduction with hypocaloric diets in overweight or obese patients could be strongly recommended, and it has been proven that obesity is associated with worse psoriasis severity.6 Other more recent studies have shown that dietary modifications such as intermittent fasting and the ketogenic diet also led to weight loss and improved psoriasis severity in overweight patients; however, it is difficult to discern if the improvement was due to weight loss alone or if the dietary patterns themselves played a role.7,8 The paucity of well-designed studies evaluating the effects of other dietary changes has prevented further guidelines from being written. We propose that dietary patterns such as the Mediterranean diet (MeD) and vegan/vegetarian diets—even without strong data showing benefits in skin disease—may help to decrease systemic inflammation, improve gut dysbiosis, and help decrease the risk for cardiometabolic comorbidities that are associated with psoriasis.
Mediterranean Diet
The MeD is based on the dietary tendencies of inhabitants from the regions surrounding the Mediterranean Sea and is centered around nutrient-rich foods such as vegetables, olive oil, and legumes while limiting meat and dairy.9 The NPF recommended considering a trial of the MeD based on low-quality evidence.6 Observational studies have indicated that psoriasis patients are less likely to adhere to the MeD, but those who do have less severe disease.8 However, a search of PubMed articles indexed for MEDLINE using the terms Mediterranean diet and psoriasis yielded no prospective interventional studies. Given the association of the MeD with less severe disease, it is important to understand which specific foods in the MeD could be beneficial. Intake of omega-3 fatty acids, such as those found in fatty fish, are important for modulation of systemic inflammation.7 High intake of polyphenols—found in fruits and vegetables, extra-virgin olive oil, and wine—also have been implicated in improving inflammatory diseases due to potent antioxidant and anti-inflammatory properties. Individually, fruits, vegetables, whole grains, and sea fish have been associated with lowering C-reactive protein levels, which also is indicative of the benefits of these foods on systemic inflammation.7
Vegan/Vegetarian Diets
Although fruits, vegetables, legumes, and whole grains are a substantial component of the MeD, there are limited data on vegetarian or purely vegan plant-based diets. An observational study from the NPF found that only 48.4% (15/31) of patients on the MeD vs 69.0% (20/29) on a vegan diet reported a favorable skin response.5 Two case reports also have shown beneficial results of a strict vegan diet for psoriasis and psoriatic arthritis, where whole-food plant-based diets also improved joint symptoms.10-12 As with any diet, those who pursue a plant-based diet should strive to consume a variety of foods to avoid nutrient deficiencies. A recent systematic meta-analysis of 141 studies evaluated nutrient status of vegan and vegetarian diets compared to pescovegetarians and those who consume meat. All dietary patterns showed varying degrees of low levels of different nutrients.13 Of note, the researchers found that vitamin B12, vitamin D, iron, zinc, iodine, calcium, and docosahexaenoic acid were lower in plant-based diets. In contrast, folate; vitamins B1, B6, C, and E; polyunsaturated fatty acids; α-linolenic acid; and magnesium intake were higher. Those who consumed meat were at risk for inadequate intake of fiber, polyunsaturated fatty acids, α-linolenic acid, folate, vitamin E, calcium, magnesium, and vitamin D, though vitamin D intake was higher than in vegans/vegetarians.13 The results of this meta-analysis indicated the importance of educating patients on what constitutes a well-rounded, micronutrient-rich diet or appropriate supplementation for any diet.
Effects on Gut Microbiome
Any changes in diet can lead to alterations in the gut microbiome, which may impact skin disease, as evidence indicates a bidirectional relationship between gut and skin health.10 A metagenomic analysis of the gut microbiota in patients with untreated plaque psoriasis revealed a signature dysbiosis for which the researchers developed a psoriasis microbiota index, suggesting the gut microbiota may play a role in psoriasis pathophysiology.14 Research shows that both the MeD and vegan/vegetarian diets, which are relatively rich in fiber and omega-3 fatty acids and low in saturated fat and animal protein compared to many diets, cause increases in dietary fiber–metabolizing bacteria that produce short-chain fatty acids. These short-chain fatty acids improve gut epithelial integrity and alleviate both gut and systemic inflammation.10
The changes to the gut microbiome induced by a high-fat diet also are concerning. In contrast to the MeD or vegan/vegetarian diets, consumption of a high-fat diet induces alterations in the composition of the gut microbiota that in turn increase the release of proinflammatory cytokines and promote higher intestinal permeability.10 Similarly, high sugar consumption promotes increased intestinal permeability and shifts the gut microbiota to organisms that can rapidly utilize simple carbohydrates at the expense of other beneficial organisms, reducing bacterial diversity.15 The Western diet, which is notable for both high fat and high sugar content, is sometimes referred to as a proinflammatory diet and has been shown to worsen psoriasiformlike lesions in mice.16 Importantly, most research indicates that high fat and high sugar consumption appear to be more prevalent in psoriasis patients,8 but the type of fat consumed in the diet matters. The Western diet includes abundant saturated fat found in meat, dairy products, palm and coconut oils, and processed foods, as well as omega-6 fatty acids that are found in meat, poultry, and eggs. Saturated fat has been shown to promote helper T cell (TH17) accumulation in the skin, and omega-6 fatty acids serve as precursors to various inflammatory lipid mediators.4 This distinction of sources of fat between the Western diet and MeD is important in understanding the diets’ different effects on psoriasis and overall health. As previously discussed, the high intake of omega-3 acids in the MeD is one of the ways it may exert its anti-inflammatory benefits.7
Next Steps in Advising Psoriasis Patients
A major limitation of the data for MeD and vegan/vegetarian diets is limited randomized controlled trials evaluating the impact of these diets on psoriasis. Thus, dietary recommendations for psoriasis are not as strong as for other diseases for which more conclusive data exist.8 Although the data on diet and psoriasis are not definitive, perhaps dermatologists should shift the question from “Does this diet definitely improve psoriasis?” to “Does this diet definitely improve my patient’s health as a whole and maybe also their psoriasis?” For instance, the MeD has been shown to reduce the risk for type 2 diabetes mellitus and cardiovascular disease as well as to slow cognitive decline.17 Vegan/vegetarian diets focusing on whole vs processed foods have been shown to be highly effective in combatting obesity, type 2 diabetes mellitus, coronary artery disease including severe atherosclerosis, and hypertension.18 Psoriasis patients are at increased risk for many of the ailments that the MeD and plant-based diets protect against, making these diets potentially even more impactful than for someone without psoriasis.19 Dietary recommendations should still be made in conjunction with continuing traditional therapies for psoriasis and in consultation with the patient’s primary care physician and/or dietitian; however, rather than waiting for more randomized controlled trials before making health-promoting recommendations, what would be the downside of starting now? At worst, the dietary change decreases their risk for several metabolic conditions, and at best they may even see an improvement in their psoriasis.
- Naldi L, Chatenoud L, Linder D, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case–control study. J Invest Dermatol. 2005;125:61-67. doi:10.1111/j.0022-202X.2005.23681.x
- Armstrong AW, Harskamp CT, Dhillon JS, et al. Psoriasis and smoking: a systematic review and meta‐analysis. Br J Dermatol. 2014;170:304-314. doi:10.1111/bjd.12670
- Zhu K, Zhu C, Fan Y. Alcohol consumption and psoriatic risk: a meta‐analysis of case–control studies. J Dermatol. 2012;39:770-773. doi:10.1111/j.1346-8138.2012.01577.x
- Kanda N, Hoashi T, Saeki H. Nutrition and psoriasis. Int J Mol Sci. 2020;21:5405. doi:10.3390/ijms21155405
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther. 2017;7:227-242. doi:10.1007/s13555-017-0183-4
- Ford AR, Siegel M, Bagel J, et al. Dietary recommendations for adults with psoriasis or psoriatic arthritis from the medical board of the National Psoriasis Foundation: a systematic review. JAMA Dermatol. 2018;154:934. doi:10.1001/jamadermatol.2018.1412
- Duchnik E, Kruk J, Tuchowska A, et al. The impact of diet and physical activity on psoriasis: a narrative review of the current evidence. Nutrients. 2023;15:840. doi:10.3390/nu15040840
- Chung M, Bartholomew E, Yeroushalmi S, et al. Dietary intervention and supplements in the management of psoriasis: current perspectives. Psoriasis Targets Ther. 2022;12:151-176. doi:10.2147/PTT.S328581
- Mazza E, Ferro Y, Pujia R, et al. Mediterranean diet in healthy aging. J Nutr Health Aging. 2021;25:1076-1083. doi:10.1007/s12603-021-1675-6
- Flores-Balderas X, Peña-Peña M, Rada KM, et al. Beneficial effects of plant-based diets on skin health and inflammatory skin diseases. Nutrients. 2023;15:2842. doi:10.3390/nu15132842
- Bonjour M, Gabriel S, Valencia A, et al. Challenging case in clinical practice: prolonged water-only fasting followed by an exclusively whole-plant-food diet in the management of severe plaque psoriasis. Integr Complement Ther. 2022;28:85-87. doi:10.1089/ict.2022.29010.mbo
- Lewandowska M, Dunbar K, Kassam S. Managing psoriatic arthritis with a whole food plant-based diet: a case study. Am J Lifestyle Med. 2021;15:402-406. doi:10.1177/1559827621993435
- Neufingerl N, Eilander A. Nutrient intake and status in adults consuming plant-based diets compared to meat-eaters: a systematic review. Nutrients. 2021;14:29. doi:10.3390/nu14010029
- Dei-Cas I, Giliberto F, Luce L, et al. Metagenomic analysis of gut microbiota in non-treated plaque psoriasis patients stratified by disease severity: development of a new psoriasis-microbiome index. Sci Rep. 2020;10:12754. doi:10.1038/s41598-020-69537-3
- Satokari R. High intake of sugar and the balance between pro- and anti-inflammatory gut bacteria. Nutrients. 2020;12:1348. doi:10.3390/nu12051348
- Shi Z, Wu X, Santos Rocha C, et al. Short-term Western diet intake promotes IL-23–mediated skin and joint inflammation accompanied by changes to the gut microbiota in mice. J Invest Dermatol. 2021;141:1780-1791. doi:10.1016/j.jid.2020.11.032
- Romagnolo DF, Selmin OI. Mediterranean diet and prevention of chronic diseases. Nutr Today. 2017;52:208-222. doi:10.1097/NT.0000000000000228
- Tuso PJ, Ismail MH, Ha BP, et al. Nutritional update for physicians: plant-based diets. Perm J. 2013;17:61-66. doi:10.7812/TPP/12-085
- Parisi R, Symmons DPM, Griffiths CEM, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385. doi:10.1038/jid.2012.339
Psoriasis is a chronic inflammatory skin disease for which several lifestyle factors—smoking, alcohol use, and psychological stress—are associated with higher incidence and more severe disease.1-3 Diet also has been implicated as a factor that can affect psoriasis,4 and many patients have shown interest in possible dietary interventions to help their disease.5
In 2018, the National Psoriasis Foundation (NPF) presented dietary recommendations for patients based on results from a systematic review. From the available literature, only dietary weight reduction with hypocaloric diets in overweight or obese patients could be strongly recommended, and it has been proven that obesity is associated with worse psoriasis severity.6 Other more recent studies have shown that dietary modifications such as intermittent fasting and the ketogenic diet also led to weight loss and improved psoriasis severity in overweight patients; however, it is difficult to discern if the improvement was due to weight loss alone or if the dietary patterns themselves played a role.7,8 The paucity of well-designed studies evaluating the effects of other dietary changes has prevented further guidelines from being written. We propose that dietary patterns such as the Mediterranean diet (MeD) and vegan/vegetarian diets—even without strong data showing benefits in skin disease—may help to decrease systemic inflammation, improve gut dysbiosis, and help decrease the risk for cardiometabolic comorbidities that are associated with psoriasis.
Mediterranean Diet
The MeD is based on the dietary tendencies of inhabitants from the regions surrounding the Mediterranean Sea and is centered around nutrient-rich foods such as vegetables, olive oil, and legumes while limiting meat and dairy.9 The NPF recommended considering a trial of the MeD based on low-quality evidence.6 Observational studies have indicated that psoriasis patients are less likely to adhere to the MeD, but those who do have less severe disease.8 However, a search of PubMed articles indexed for MEDLINE using the terms Mediterranean diet and psoriasis yielded no prospective interventional studies. Given the association of the MeD with less severe disease, it is important to understand which specific foods in the MeD could be beneficial. Intake of omega-3 fatty acids, such as those found in fatty fish, are important for modulation of systemic inflammation.7 High intake of polyphenols—found in fruits and vegetables, extra-virgin olive oil, and wine—also have been implicated in improving inflammatory diseases due to potent antioxidant and anti-inflammatory properties. Individually, fruits, vegetables, whole grains, and sea fish have been associated with lowering C-reactive protein levels, which also is indicative of the benefits of these foods on systemic inflammation.7
Vegan/Vegetarian Diets
Although fruits, vegetables, legumes, and whole grains are a substantial component of the MeD, there are limited data on vegetarian or purely vegan plant-based diets. An observational study from the NPF found that only 48.4% (15/31) of patients on the MeD vs 69.0% (20/29) on a vegan diet reported a favorable skin response.5 Two case reports also have shown beneficial results of a strict vegan diet for psoriasis and psoriatic arthritis, where whole-food plant-based diets also improved joint symptoms.10-12 As with any diet, those who pursue a plant-based diet should strive to consume a variety of foods to avoid nutrient deficiencies. A recent systematic meta-analysis of 141 studies evaluated nutrient status of vegan and vegetarian diets compared to pescovegetarians and those who consume meat. All dietary patterns showed varying degrees of low levels of different nutrients.13 Of note, the researchers found that vitamin B12, vitamin D, iron, zinc, iodine, calcium, and docosahexaenoic acid were lower in plant-based diets. In contrast, folate; vitamins B1, B6, C, and E; polyunsaturated fatty acids; α-linolenic acid; and magnesium intake were higher. Those who consumed meat were at risk for inadequate intake of fiber, polyunsaturated fatty acids, α-linolenic acid, folate, vitamin E, calcium, magnesium, and vitamin D, though vitamin D intake was higher than in vegans/vegetarians.13 The results of this meta-analysis indicated the importance of educating patients on what constitutes a well-rounded, micronutrient-rich diet or appropriate supplementation for any diet.
Effects on Gut Microbiome
Any changes in diet can lead to alterations in the gut microbiome, which may impact skin disease, as evidence indicates a bidirectional relationship between gut and skin health.10 A metagenomic analysis of the gut microbiota in patients with untreated plaque psoriasis revealed a signature dysbiosis for which the researchers developed a psoriasis microbiota index, suggesting the gut microbiota may play a role in psoriasis pathophysiology.14 Research shows that both the MeD and vegan/vegetarian diets, which are relatively rich in fiber and omega-3 fatty acids and low in saturated fat and animal protein compared to many diets, cause increases in dietary fiber–metabolizing bacteria that produce short-chain fatty acids. These short-chain fatty acids improve gut epithelial integrity and alleviate both gut and systemic inflammation.10
The changes to the gut microbiome induced by a high-fat diet also are concerning. In contrast to the MeD or vegan/vegetarian diets, consumption of a high-fat diet induces alterations in the composition of the gut microbiota that in turn increase the release of proinflammatory cytokines and promote higher intestinal permeability.10 Similarly, high sugar consumption promotes increased intestinal permeability and shifts the gut microbiota to organisms that can rapidly utilize simple carbohydrates at the expense of other beneficial organisms, reducing bacterial diversity.15 The Western diet, which is notable for both high fat and high sugar content, is sometimes referred to as a proinflammatory diet and has been shown to worsen psoriasiformlike lesions in mice.16 Importantly, most research indicates that high fat and high sugar consumption appear to be more prevalent in psoriasis patients,8 but the type of fat consumed in the diet matters. The Western diet includes abundant saturated fat found in meat, dairy products, palm and coconut oils, and processed foods, as well as omega-6 fatty acids that are found in meat, poultry, and eggs. Saturated fat has been shown to promote helper T cell (TH17) accumulation in the skin, and omega-6 fatty acids serve as precursors to various inflammatory lipid mediators.4 This distinction of sources of fat between the Western diet and MeD is important in understanding the diets’ different effects on psoriasis and overall health. As previously discussed, the high intake of omega-3 acids in the MeD is one of the ways it may exert its anti-inflammatory benefits.7
Next Steps in Advising Psoriasis Patients
A major limitation of the data for MeD and vegan/vegetarian diets is limited randomized controlled trials evaluating the impact of these diets on psoriasis. Thus, dietary recommendations for psoriasis are not as strong as for other diseases for which more conclusive data exist.8 Although the data on diet and psoriasis are not definitive, perhaps dermatologists should shift the question from “Does this diet definitely improve psoriasis?” to “Does this diet definitely improve my patient’s health as a whole and maybe also their psoriasis?” For instance, the MeD has been shown to reduce the risk for type 2 diabetes mellitus and cardiovascular disease as well as to slow cognitive decline.17 Vegan/vegetarian diets focusing on whole vs processed foods have been shown to be highly effective in combatting obesity, type 2 diabetes mellitus, coronary artery disease including severe atherosclerosis, and hypertension.18 Psoriasis patients are at increased risk for many of the ailments that the MeD and plant-based diets protect against, making these diets potentially even more impactful than for someone without psoriasis.19 Dietary recommendations should still be made in conjunction with continuing traditional therapies for psoriasis and in consultation with the patient’s primary care physician and/or dietitian; however, rather than waiting for more randomized controlled trials before making health-promoting recommendations, what would be the downside of starting now? At worst, the dietary change decreases their risk for several metabolic conditions, and at best they may even see an improvement in their psoriasis.
Psoriasis is a chronic inflammatory skin disease for which several lifestyle factors—smoking, alcohol use, and psychological stress—are associated with higher incidence and more severe disease.1-3 Diet also has been implicated as a factor that can affect psoriasis,4 and many patients have shown interest in possible dietary interventions to help their disease.5
In 2018, the National Psoriasis Foundation (NPF) presented dietary recommendations for patients based on results from a systematic review. From the available literature, only dietary weight reduction with hypocaloric diets in overweight or obese patients could be strongly recommended, and it has been proven that obesity is associated with worse psoriasis severity.6 Other more recent studies have shown that dietary modifications such as intermittent fasting and the ketogenic diet also led to weight loss and improved psoriasis severity in overweight patients; however, it is difficult to discern if the improvement was due to weight loss alone or if the dietary patterns themselves played a role.7,8 The paucity of well-designed studies evaluating the effects of other dietary changes has prevented further guidelines from being written. We propose that dietary patterns such as the Mediterranean diet (MeD) and vegan/vegetarian diets—even without strong data showing benefits in skin disease—may help to decrease systemic inflammation, improve gut dysbiosis, and help decrease the risk for cardiometabolic comorbidities that are associated with psoriasis.
Mediterranean Diet
The MeD is based on the dietary tendencies of inhabitants from the regions surrounding the Mediterranean Sea and is centered around nutrient-rich foods such as vegetables, olive oil, and legumes while limiting meat and dairy.9 The NPF recommended considering a trial of the MeD based on low-quality evidence.6 Observational studies have indicated that psoriasis patients are less likely to adhere to the MeD, but those who do have less severe disease.8 However, a search of PubMed articles indexed for MEDLINE using the terms Mediterranean diet and psoriasis yielded no prospective interventional studies. Given the association of the MeD with less severe disease, it is important to understand which specific foods in the MeD could be beneficial. Intake of omega-3 fatty acids, such as those found in fatty fish, are important for modulation of systemic inflammation.7 High intake of polyphenols—found in fruits and vegetables, extra-virgin olive oil, and wine—also have been implicated in improving inflammatory diseases due to potent antioxidant and anti-inflammatory properties. Individually, fruits, vegetables, whole grains, and sea fish have been associated with lowering C-reactive protein levels, which also is indicative of the benefits of these foods on systemic inflammation.7
Vegan/Vegetarian Diets
Although fruits, vegetables, legumes, and whole grains are a substantial component of the MeD, there are limited data on vegetarian or purely vegan plant-based diets. An observational study from the NPF found that only 48.4% (15/31) of patients on the MeD vs 69.0% (20/29) on a vegan diet reported a favorable skin response.5 Two case reports also have shown beneficial results of a strict vegan diet for psoriasis and psoriatic arthritis, where whole-food plant-based diets also improved joint symptoms.10-12 As with any diet, those who pursue a plant-based diet should strive to consume a variety of foods to avoid nutrient deficiencies. A recent systematic meta-analysis of 141 studies evaluated nutrient status of vegan and vegetarian diets compared to pescovegetarians and those who consume meat. All dietary patterns showed varying degrees of low levels of different nutrients.13 Of note, the researchers found that vitamin B12, vitamin D, iron, zinc, iodine, calcium, and docosahexaenoic acid were lower in plant-based diets. In contrast, folate; vitamins B1, B6, C, and E; polyunsaturated fatty acids; α-linolenic acid; and magnesium intake were higher. Those who consumed meat were at risk for inadequate intake of fiber, polyunsaturated fatty acids, α-linolenic acid, folate, vitamin E, calcium, magnesium, and vitamin D, though vitamin D intake was higher than in vegans/vegetarians.13 The results of this meta-analysis indicated the importance of educating patients on what constitutes a well-rounded, micronutrient-rich diet or appropriate supplementation for any diet.
Effects on Gut Microbiome
Any changes in diet can lead to alterations in the gut microbiome, which may impact skin disease, as evidence indicates a bidirectional relationship between gut and skin health.10 A metagenomic analysis of the gut microbiota in patients with untreated plaque psoriasis revealed a signature dysbiosis for which the researchers developed a psoriasis microbiota index, suggesting the gut microbiota may play a role in psoriasis pathophysiology.14 Research shows that both the MeD and vegan/vegetarian diets, which are relatively rich in fiber and omega-3 fatty acids and low in saturated fat and animal protein compared to many diets, cause increases in dietary fiber–metabolizing bacteria that produce short-chain fatty acids. These short-chain fatty acids improve gut epithelial integrity and alleviate both gut and systemic inflammation.10
The changes to the gut microbiome induced by a high-fat diet also are concerning. In contrast to the MeD or vegan/vegetarian diets, consumption of a high-fat diet induces alterations in the composition of the gut microbiota that in turn increase the release of proinflammatory cytokines and promote higher intestinal permeability.10 Similarly, high sugar consumption promotes increased intestinal permeability and shifts the gut microbiota to organisms that can rapidly utilize simple carbohydrates at the expense of other beneficial organisms, reducing bacterial diversity.15 The Western diet, which is notable for both high fat and high sugar content, is sometimes referred to as a proinflammatory diet and has been shown to worsen psoriasiformlike lesions in mice.16 Importantly, most research indicates that high fat and high sugar consumption appear to be more prevalent in psoriasis patients,8 but the type of fat consumed in the diet matters. The Western diet includes abundant saturated fat found in meat, dairy products, palm and coconut oils, and processed foods, as well as omega-6 fatty acids that are found in meat, poultry, and eggs. Saturated fat has been shown to promote helper T cell (TH17) accumulation in the skin, and omega-6 fatty acids serve as precursors to various inflammatory lipid mediators.4 This distinction of sources of fat between the Western diet and MeD is important in understanding the diets’ different effects on psoriasis and overall health. As previously discussed, the high intake of omega-3 acids in the MeD is one of the ways it may exert its anti-inflammatory benefits.7
Next Steps in Advising Psoriasis Patients
A major limitation of the data for MeD and vegan/vegetarian diets is limited randomized controlled trials evaluating the impact of these diets on psoriasis. Thus, dietary recommendations for psoriasis are not as strong as for other diseases for which more conclusive data exist.8 Although the data on diet and psoriasis are not definitive, perhaps dermatologists should shift the question from “Does this diet definitely improve psoriasis?” to “Does this diet definitely improve my patient’s health as a whole and maybe also their psoriasis?” For instance, the MeD has been shown to reduce the risk for type 2 diabetes mellitus and cardiovascular disease as well as to slow cognitive decline.17 Vegan/vegetarian diets focusing on whole vs processed foods have been shown to be highly effective in combatting obesity, type 2 diabetes mellitus, coronary artery disease including severe atherosclerosis, and hypertension.18 Psoriasis patients are at increased risk for many of the ailments that the MeD and plant-based diets protect against, making these diets potentially even more impactful than for someone without psoriasis.19 Dietary recommendations should still be made in conjunction with continuing traditional therapies for psoriasis and in consultation with the patient’s primary care physician and/or dietitian; however, rather than waiting for more randomized controlled trials before making health-promoting recommendations, what would be the downside of starting now? At worst, the dietary change decreases their risk for several metabolic conditions, and at best they may even see an improvement in their psoriasis.
- Naldi L, Chatenoud L, Linder D, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case–control study. J Invest Dermatol. 2005;125:61-67. doi:10.1111/j.0022-202X.2005.23681.x
- Armstrong AW, Harskamp CT, Dhillon JS, et al. Psoriasis and smoking: a systematic review and meta‐analysis. Br J Dermatol. 2014;170:304-314. doi:10.1111/bjd.12670
- Zhu K, Zhu C, Fan Y. Alcohol consumption and psoriatic risk: a meta‐analysis of case–control studies. J Dermatol. 2012;39:770-773. doi:10.1111/j.1346-8138.2012.01577.x
- Kanda N, Hoashi T, Saeki H. Nutrition and psoriasis. Int J Mol Sci. 2020;21:5405. doi:10.3390/ijms21155405
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther. 2017;7:227-242. doi:10.1007/s13555-017-0183-4
- Ford AR, Siegel M, Bagel J, et al. Dietary recommendations for adults with psoriasis or psoriatic arthritis from the medical board of the National Psoriasis Foundation: a systematic review. JAMA Dermatol. 2018;154:934. doi:10.1001/jamadermatol.2018.1412
- Duchnik E, Kruk J, Tuchowska A, et al. The impact of diet and physical activity on psoriasis: a narrative review of the current evidence. Nutrients. 2023;15:840. doi:10.3390/nu15040840
- Chung M, Bartholomew E, Yeroushalmi S, et al. Dietary intervention and supplements in the management of psoriasis: current perspectives. Psoriasis Targets Ther. 2022;12:151-176. doi:10.2147/PTT.S328581
- Mazza E, Ferro Y, Pujia R, et al. Mediterranean diet in healthy aging. J Nutr Health Aging. 2021;25:1076-1083. doi:10.1007/s12603-021-1675-6
- Flores-Balderas X, Peña-Peña M, Rada KM, et al. Beneficial effects of plant-based diets on skin health and inflammatory skin diseases. Nutrients. 2023;15:2842. doi:10.3390/nu15132842
- Bonjour M, Gabriel S, Valencia A, et al. Challenging case in clinical practice: prolonged water-only fasting followed by an exclusively whole-plant-food diet in the management of severe plaque psoriasis. Integr Complement Ther. 2022;28:85-87. doi:10.1089/ict.2022.29010.mbo
- Lewandowska M, Dunbar K, Kassam S. Managing psoriatic arthritis with a whole food plant-based diet: a case study. Am J Lifestyle Med. 2021;15:402-406. doi:10.1177/1559827621993435
- Neufingerl N, Eilander A. Nutrient intake and status in adults consuming plant-based diets compared to meat-eaters: a systematic review. Nutrients. 2021;14:29. doi:10.3390/nu14010029
- Dei-Cas I, Giliberto F, Luce L, et al. Metagenomic analysis of gut microbiota in non-treated plaque psoriasis patients stratified by disease severity: development of a new psoriasis-microbiome index. Sci Rep. 2020;10:12754. doi:10.1038/s41598-020-69537-3
- Satokari R. High intake of sugar and the balance between pro- and anti-inflammatory gut bacteria. Nutrients. 2020;12:1348. doi:10.3390/nu12051348
- Shi Z, Wu X, Santos Rocha C, et al. Short-term Western diet intake promotes IL-23–mediated skin and joint inflammation accompanied by changes to the gut microbiota in mice. J Invest Dermatol. 2021;141:1780-1791. doi:10.1016/j.jid.2020.11.032
- Romagnolo DF, Selmin OI. Mediterranean diet and prevention of chronic diseases. Nutr Today. 2017;52:208-222. doi:10.1097/NT.0000000000000228
- Tuso PJ, Ismail MH, Ha BP, et al. Nutritional update for physicians: plant-based diets. Perm J. 2013;17:61-66. doi:10.7812/TPP/12-085
- Parisi R, Symmons DPM, Griffiths CEM, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385. doi:10.1038/jid.2012.339
- Naldi L, Chatenoud L, Linder D, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case–control study. J Invest Dermatol. 2005;125:61-67. doi:10.1111/j.0022-202X.2005.23681.x
- Armstrong AW, Harskamp CT, Dhillon JS, et al. Psoriasis and smoking: a systematic review and meta‐analysis. Br J Dermatol. 2014;170:304-314. doi:10.1111/bjd.12670
- Zhu K, Zhu C, Fan Y. Alcohol consumption and psoriatic risk: a meta‐analysis of case–control studies. J Dermatol. 2012;39:770-773. doi:10.1111/j.1346-8138.2012.01577.x
- Kanda N, Hoashi T, Saeki H. Nutrition and psoriasis. Int J Mol Sci. 2020;21:5405. doi:10.3390/ijms21155405
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther. 2017;7:227-242. doi:10.1007/s13555-017-0183-4
- Ford AR, Siegel M, Bagel J, et al. Dietary recommendations for adults with psoriasis or psoriatic arthritis from the medical board of the National Psoriasis Foundation: a systematic review. JAMA Dermatol. 2018;154:934. doi:10.1001/jamadermatol.2018.1412
- Duchnik E, Kruk J, Tuchowska A, et al. The impact of diet and physical activity on psoriasis: a narrative review of the current evidence. Nutrients. 2023;15:840. doi:10.3390/nu15040840
- Chung M, Bartholomew E, Yeroushalmi S, et al. Dietary intervention and supplements in the management of psoriasis: current perspectives. Psoriasis Targets Ther. 2022;12:151-176. doi:10.2147/PTT.S328581
- Mazza E, Ferro Y, Pujia R, et al. Mediterranean diet in healthy aging. J Nutr Health Aging. 2021;25:1076-1083. doi:10.1007/s12603-021-1675-6
- Flores-Balderas X, Peña-Peña M, Rada KM, et al. Beneficial effects of plant-based diets on skin health and inflammatory skin diseases. Nutrients. 2023;15:2842. doi:10.3390/nu15132842
- Bonjour M, Gabriel S, Valencia A, et al. Challenging case in clinical practice: prolonged water-only fasting followed by an exclusively whole-plant-food diet in the management of severe plaque psoriasis. Integr Complement Ther. 2022;28:85-87. doi:10.1089/ict.2022.29010.mbo
- Lewandowska M, Dunbar K, Kassam S. Managing psoriatic arthritis with a whole food plant-based diet: a case study. Am J Lifestyle Med. 2021;15:402-406. doi:10.1177/1559827621993435
- Neufingerl N, Eilander A. Nutrient intake and status in adults consuming plant-based diets compared to meat-eaters: a systematic review. Nutrients. 2021;14:29. doi:10.3390/nu14010029
- Dei-Cas I, Giliberto F, Luce L, et al. Metagenomic analysis of gut microbiota in non-treated plaque psoriasis patients stratified by disease severity: development of a new psoriasis-microbiome index. Sci Rep. 2020;10:12754. doi:10.1038/s41598-020-69537-3
- Satokari R. High intake of sugar and the balance between pro- and anti-inflammatory gut bacteria. Nutrients. 2020;12:1348. doi:10.3390/nu12051348
- Shi Z, Wu X, Santos Rocha C, et al. Short-term Western diet intake promotes IL-23–mediated skin and joint inflammation accompanied by changes to the gut microbiota in mice. J Invest Dermatol. 2021;141:1780-1791. doi:10.1016/j.jid.2020.11.032
- Romagnolo DF, Selmin OI. Mediterranean diet and prevention of chronic diseases. Nutr Today. 2017;52:208-222. doi:10.1097/NT.0000000000000228
- Tuso PJ, Ismail MH, Ha BP, et al. Nutritional update for physicians: plant-based diets. Perm J. 2013;17:61-66. doi:10.7812/TPP/12-085
- Parisi R, Symmons DPM, Griffiths CEM, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385. doi:10.1038/jid.2012.339
Practice Points
- Psoriasis is affected by lifestyle factors such as diet, which is an area of interest for many patients.
- Low-calorie diets are strongly recommended for overweight/obese patients with psoriasis to improve their disease.
- Changes in dietary patterns, such as adopting a Mediterranean diet or a plant-based diet, also have shown promise.
The Potential for Artificial Intelligence Tools in Residency Recruitment
According to Electronic Residency Application Service (ERAS) statistics, there were more than 1400 dermatology applicants in 2022, with an average of almost 560 applications received per program.1,2 With the goal to expand the diversity of board-certified dermatologists, there is increasing emphasis on the holistic review of applications, forgoing filtering by discrete metrics such as AOA (American Osteopathic Association) membership and US Medical Licensing Examination (USMLE) scores.3 According to the Association of American Medical Colleges, holistic review focuses on an individual applicant’s experience and unique attributes in addition to their academic achievements.4 Recent strategies to enhance the residency recruitment process have included the introduction of standardized letters of recommendation, preference signaling, and supplemental applications.5,6
Because it has become increasingly important to include applicant factors and achievements that extend beyond academics, the number of data points that are required for holistic review has expanded. If each application required 20 minutes to review, this would result in 166 total hours for complete holistic review of 500 applications. Tools that can facilitate holistic review of candidates and select applicants whose interests and career goals align with individual residency programs have the potential to optimize review. Artificial intelligence (AI) may aid in this process. This column highlights some of the published research on novel AI strategies that have the potential to impact dermatology residency recruitment.
Machine Learning to Screen Applicants
Artificial intelligence involves a machine-based system that can make decisions, predictions, and recommendations when provided a given set of human-defined objectives.7 Autonomous systems, machine learning (ML), and generative AI are examples of AI models.8 Machine learning has been explored to shorten and streamline the application review process and decrease bias. Because ML is a model in which the computer learns patterns based on large amounts of input data,9 it is possible that models could be developed and used in future cycles. Some studies found that applicants were discovered who traditionally would not have made it to the next stage of consideration based primarily on academic metrics.10,11 Burk-Rafel et al10 developed and validated an ML-based decision support tool for residency program directors to use for interview invitation decisions. The tool utilized 61 variables from ERAS data from more than 8000 applications in 3 prior application cycles at a single internal medicine residency program. An interview invitation was designated as the target outcome. Ultimately, the model would output a probability score for an interview invitation. The authors were able to tune the model to a 91% sensitivity and 85% specificity; for a pool of 2000 applicants and an invite rate of 15%, 1475 applicants would be screened out with a negative predictive value of 98% with maintenance of performance, even with removal of USMLE Step 1 examination scores. Their ML model was prospectively validated during an ongoing resident selection cycle, and when compared with human review, the AI model found an additional 20 applicants to invite for interviews. They concluded that this tool could potentially augment the human review process and reveal applicants who may have otherwise been overlooked.10
Rees and Ryder11 utilized another ML screening approach with the target outcome of ranked and matriculated compared with ranked applicants based on ERAS data using 72 unique variables for more than 5000 applicants. Their model was able to identify ranked candidates from the overall applicant pool with high accuracy; identification of ranked applicants that matriculated at the program was more modest but better than random probability.11Both the Burk-Rafel et al10 and Rees and Ryder11 models excluded some unstructured data components of the residency application, such as personal statements, medical student performance evaluation letters, transcripts, and letters of reference, that some may consider strongly in the holistic review process. Drum et al12 explored the value of extraction of this type of data. They created a program to extract “snippets” of text that pertained to values of successful residents for their internal medicine–pediatrics residency program that they previously validated via a modified Delphi method, which then were annotated by expert reviewers. Natural language processing was used to train an ML algorithm (MLA) to classify snippets into 11 value categories. Four values had more than 66% agreement with human annotation: academic strength; leadership; communication; and justice, equity, diversity, and inclusion. Although this MLA has not reached high enough levels of agreement for all the predetermined success values, the authors hope to generate a model that could produce a quantitative score to use as an initial screening tool to select applicants for interview.12 This type of analysis also could be incorporated into other MLAs for further refinement of the mentoring and application process.
Knapke et al13 evaluated the use of a natural language modeling platform to look for semantic patterns in medical school applications that could predict which students would be more likely to pursue family medicine residency, thus beginning the recruitment process even before residency application. This strategy could be particularly valuable for specialties for which there may be greater need in the workforce.
AI for Administrative Purposes
Artificial intelligence also has been used for nonapplication aspects of the residency recruitment process, such as interview scheduling. In the absence of coordinated interview release dates (as was implemented in dermatology starting in the 2020-2021 application cycle), a deluge of responses to schedule an interview comes flooding in as soon as invitations for interviewees are sent out, which can produce anxiety both for applicants and residency program staff as the schedule is sorted out and can create delays at both ends. Stephens et al14 utilized a computerized scheduling program for pediatric surgery fellowship applicants. It was used in 2016 to schedule 26 interviews, and it was found to reduce the average time to schedule an interview from 14.4 hours to 1.7 hours. It also reduced the number of email exchanges needed to finalize scheduling.14
Another aspect of residency recruitment that is amenable to AI is information gathering. Many would-be applicants turn to the internet and social media to learn about residency programs—their unique qualities, assets, and potential alignment of career goals.15 This exchange often is unidirectional, as the applicant clicks through the website searching for information. Yi et al16 explored the use of a chatbot, which mimics human conversation and exchange, on their institution’s pain fellowship website. Fellowship applicants could create specific prompts, such as “Show me faculty that trained at <applicant’s home program>,” and the chatbot would reply with the answer. The researchers sent a survey to all 258 applicants to the pain fellowship program that was completed by 48 applicants. Of these respondents, more than 70% (35/48) utilized the chatbot, and 84% (40/48) stated that they had found the information that was requested. The respondents overall found the chatbot to be a useful and positive experience.16
Specific Tools to Consider
There are some tools that are publicly available for programs and applicants to use that rely on AI.
In collaboration with ERAS and the Association of American Medical Colleges, Cortex powered by Thalamus (SJ MedConnect Inc)(https://thalamusgme.com/cortex-application-screening/) offers technology-assisted holistic review of residency and fellowship applications by utilizing natural language processing and optical character recognition to aggregate data from ERAS.
Tools also are being leveraged by applicants to help them find residency programs that fit their criteria, prepare for interviews, and complete portions of the application. Match A Resident (https://www.matcharesident.com/) is a resource for the international medical graduate community. As part of the service, the “Learn More with MARai” feature uses AI to generate information on residency programs to increase applicants’ confidence going into the interview process.17 Big Interview Medical (https://www.biginterviewmedical.com/ai-feedback), a paid interview preparation system developed by interview experts, utilizes AI to provide feedback to residents practicing for the interview process by measuring the amount of natural eye contact, language used, and pace of speech. A “Power Word” score is provided that incorporates aspects such as using filler words (“umm,” “uhh”). A Pace of Speech Tool provides rate of speaking feedback presuming that there is an ideal pace to decrease the impression that the applicant is nervous. Johnstone et al18 used ChatGPT (https://chat.openai.com/auth/login) to generate 2 personal statements for anesthesia residency applicants. Based on survey responses from 31 program directors, 22 rated the statements as good or excellent.18
Ethnical Concerns and Limitations of AI
The potential use of AI tools by residency applicants inevitably brings forth consideration of biases, ethics, and current limitations. These tools are highly dependent on the quality and quantity of data used for training and validation. Information considered valuable in the holistic review of applications includes unstructured data such as personal statements and letters of recommendation, and incorporating this information can be challenging in ML models, in contrast to discrete structured data such as grades, test scores, and awards. In addition, MLAs depend on large quantities of data to optimize performance.19 Depending on the size of the applicant pool and the amount of data available, this can present a limitation for smaller programs in developing ML tools for residency recruitment. Studies evaluating the use of AI in the residency application process often are from single institutions, and therefore generalizability is uncertain. The risk for latent bias—whereby a historical or pre-existing stereotype gets perpetuated through the system—must be considered, with the development of tools to detect and address this if found. Choosing which data to use to train the model can be tricky as well as choosing the outcome of interest. For these interventions to become more resilient, programs need to self-examine what defines their criteria for a successful match to their program to incorporate this data into their ML studies. The previously described models in this overview focused on outcomes such as whether an applicant was invited to interview, whether the applicant was ranked, and whether the applicant matriculated to their program.10,11 For supervised ML models that rely on outcomes to develop a prediction, continued research as to what outcomes represent resident success (eg, passing board certification examinations, correlation with clinical performance) would be important. There also is the possibility of applicants restructuring their applications to align with goals of an AI-assisted search and using AI to generate part or all of their application. The use of ChatGPT and other AI tools in the preparation of personal statements and curriculum vitae may provide benefits such as improved efficiency and grammar support.20 However, as use becomes more widespread, there is the potential increased similarity of personal statements and likely varied opinions on the use of such tools as writing aids.21,22 Continued efforts to develop guidance on generative AI use cases is ongoing; an example is the launch of VALID AI (https://validai.health/), a collaboration among health systems, health plans, and AI research organizations and nonprofits.23
Final Thoughts
Artificial intelligence tools may be a promising resource for residency and fellowship programs seeking to find meaningful ways to select applicants who are good matches for their training environment. Prioritizing the holistic review of applications has been promoted as a method to evaluate the applicant beyond their test scores and grades. The use of MLAs may streamline this review process, aid in scheduling interviews, and help discover trends in successful matriculants.
- Association of American Medical Colleges. ERAS® Statistics. Accessed January 16, 2024. https://www.aamc.org/data-reports/data/eras-statistics-data
- National Resident Matching Program, Data Release and ResearchCommittee: Results of the 2022 NRMP Program Director Survey. Accessed January 18, 2024. https://www.nrmp.org/wp-content/uploads/2022/09/PD-Survey-Report-2022_FINALrev.pdf
- Isaq NA, Bowers S, Chen ST. Taking a “step” toward diversity in dermatology: de-emphasizing USMLE Step 1 scores in residency applications. Int J Womens Dermatol. 2020;6:209-210. doi:10.1016/j.ijwd.2020.02.008
- Association of American Medical Colleges. Holistic review in medical school admissions. Accessed January 16, 2024. https://students-residents.aamc.org/choosing-medical-career/holistic-review-medical-school-admissions
- Association of American Medical Colleges. The MyERAS® application and program signaling for 2023-24. Accessed January 16, 2024. https://students-residents.aamc.org/applying-residencies-eras/myeras-application-and-program-signaling-2023-24
- Tavarez MM, Baghdassarian A, Bailey J, et al. A call to action for standardizing letters of recommendation. J Grad Med Educ. 2022;14:642-646. doi:10.4300/JGME-D-22-00131.1
- US Department of State. Artificial intelligence (AI). Accessed January 16, 2024. https://www.state.gov/artificial-intelligence/
- Stanford University Human-Centered Artificial Intelligence. Artificial intelligence definitions. Accessed January 16, 2024.https://hai.stanford.edu/sites/default/files/2023-03/AI-Key-Terms-Glossary-Definition.pdf
- Rajkomar A, Dean J, Kohane I. Machine learning in medicine. N Engl J Med. 2019;380:1347-1358. doi:10.1056/NEJMra1814259
- Burk-Rafel J, Reinstein I, Feng J, et al. Development and validation of a machine learning-based decision support tool for residency applicant screening and review. Acad Med. 2021;96(11S):S54-S61. doi:10.1097/ACM.0000000000004317
- Rees CA, Ryder HF. Machine learning for the prediction of ranked applicants and matriculants to an internal medicine residency program. Teach Learn Med. 2023;35:277-286. doi:10.1080/10401334.2022.2059664
- Drum B, Shi J, Peterson B, et al. Using natural language processing and machine learning to identify internal medicine-pediatrics residency values in applications. Acad Med. 2023;98:1278-1282. doi:10.1097/ACM.0000000000005352
- Knapke JM, Mount HR, McCabe E, et al. Early identification of family physicians using qualitative admissions data. Fam Med. 2023;55:245-252. doi:10.22454/FamMed.2023.596964
- Stephens CQ, Hamilton NA, Thompson AE, et al. Use of computerized interview scheduling program for pediatric surgery match applicants. J Pediatr Surg. 2017;52:1056-1059. doi:10.1016/j.jpedsurg.2017.03.033
- Nickles MA, Kulkarni V, Varghese JA, et al. Dermatology residency programs’ websites in the virtual era: a cross-sectional analysis. J Am Acad Dermatol. 2022;86:447-448. doi:10.1016/j.jaad.2021.09.064
- Yi PK, Ray ND, Segall N. A novel use of an artificially intelligent Chatbot and a live, synchronous virtual question-and answer session for fellowship recruitment. BMC Med Educ. 2023;23:152. doi:10.1186/s12909-022-03872-z
- Introducing “Learn More with MARai”—the key to understanding your target residency programs. Match A Resident website. Published September 23, 2023. Accessed January 16, 2024. https://blog.matcharesident.com/ai-powered-residency-insights/
- Johnstone RE, Neely G, Sizemore DC. Artificial intelligence softwarecan generate residency application personal statements that program directors find acceptable and difficult to distinguish from applicant compositions. J Clin Anesth. 2023;89:111185. doi:10.1016/j.jclinane.2023.111185
- Khalid N, Qayyum A, Bilal M, et al. Privacy-preserving artificial intelligence in healthcare: techniques and applications. Comput Biol Med. 2023;158:106848. doi:10.1016/j.compbiomed.2023.106848
- Birt J. How to optimize your resume for AI scanners (with tips). Indeed website. Updated December 30, 2022. Accessed January 16, 2024. https://www.indeed.com/career-advice/resumes-cover-letters/resume-ai
- Patel V, Deleonibus A, Wells MW, et al. Distinguishing authentic voices in the age of ChatGPT: comparing AI-generated and applicant-written personal statements for plastic surgery residency application. Ann Plast Surg. 2023;91:324-325. doi:10.1097/SAP.0000000000003653
- Woodfin MW. The personal statement in the age of artificial intelligence. Acad Med. 2023;98:869. doi:10.1097/ACM.0000000000005266
- Diaz N. UC Davis Health to lead new gen AI collaborative. Beckers Healthcare website. Published October 10, 2023. AccessedJanuary 16, 2024. https://www.beckershospitalreview.com/digital-health/uc-davis-health-to-lead-new-gen-ai-collaborative.html
According to Electronic Residency Application Service (ERAS) statistics, there were more than 1400 dermatology applicants in 2022, with an average of almost 560 applications received per program.1,2 With the goal to expand the diversity of board-certified dermatologists, there is increasing emphasis on the holistic review of applications, forgoing filtering by discrete metrics such as AOA (American Osteopathic Association) membership and US Medical Licensing Examination (USMLE) scores.3 According to the Association of American Medical Colleges, holistic review focuses on an individual applicant’s experience and unique attributes in addition to their academic achievements.4 Recent strategies to enhance the residency recruitment process have included the introduction of standardized letters of recommendation, preference signaling, and supplemental applications.5,6
Because it has become increasingly important to include applicant factors and achievements that extend beyond academics, the number of data points that are required for holistic review has expanded. If each application required 20 minutes to review, this would result in 166 total hours for complete holistic review of 500 applications. Tools that can facilitate holistic review of candidates and select applicants whose interests and career goals align with individual residency programs have the potential to optimize review. Artificial intelligence (AI) may aid in this process. This column highlights some of the published research on novel AI strategies that have the potential to impact dermatology residency recruitment.
Machine Learning to Screen Applicants
Artificial intelligence involves a machine-based system that can make decisions, predictions, and recommendations when provided a given set of human-defined objectives.7 Autonomous systems, machine learning (ML), and generative AI are examples of AI models.8 Machine learning has been explored to shorten and streamline the application review process and decrease bias. Because ML is a model in which the computer learns patterns based on large amounts of input data,9 it is possible that models could be developed and used in future cycles. Some studies found that applicants were discovered who traditionally would not have made it to the next stage of consideration based primarily on academic metrics.10,11 Burk-Rafel et al10 developed and validated an ML-based decision support tool for residency program directors to use for interview invitation decisions. The tool utilized 61 variables from ERAS data from more than 8000 applications in 3 prior application cycles at a single internal medicine residency program. An interview invitation was designated as the target outcome. Ultimately, the model would output a probability score for an interview invitation. The authors were able to tune the model to a 91% sensitivity and 85% specificity; for a pool of 2000 applicants and an invite rate of 15%, 1475 applicants would be screened out with a negative predictive value of 98% with maintenance of performance, even with removal of USMLE Step 1 examination scores. Their ML model was prospectively validated during an ongoing resident selection cycle, and when compared with human review, the AI model found an additional 20 applicants to invite for interviews. They concluded that this tool could potentially augment the human review process and reveal applicants who may have otherwise been overlooked.10
Rees and Ryder11 utilized another ML screening approach with the target outcome of ranked and matriculated compared with ranked applicants based on ERAS data using 72 unique variables for more than 5000 applicants. Their model was able to identify ranked candidates from the overall applicant pool with high accuracy; identification of ranked applicants that matriculated at the program was more modest but better than random probability.11Both the Burk-Rafel et al10 and Rees and Ryder11 models excluded some unstructured data components of the residency application, such as personal statements, medical student performance evaluation letters, transcripts, and letters of reference, that some may consider strongly in the holistic review process. Drum et al12 explored the value of extraction of this type of data. They created a program to extract “snippets” of text that pertained to values of successful residents for their internal medicine–pediatrics residency program that they previously validated via a modified Delphi method, which then were annotated by expert reviewers. Natural language processing was used to train an ML algorithm (MLA) to classify snippets into 11 value categories. Four values had more than 66% agreement with human annotation: academic strength; leadership; communication; and justice, equity, diversity, and inclusion. Although this MLA has not reached high enough levels of agreement for all the predetermined success values, the authors hope to generate a model that could produce a quantitative score to use as an initial screening tool to select applicants for interview.12 This type of analysis also could be incorporated into other MLAs for further refinement of the mentoring and application process.
Knapke et al13 evaluated the use of a natural language modeling platform to look for semantic patterns in medical school applications that could predict which students would be more likely to pursue family medicine residency, thus beginning the recruitment process even before residency application. This strategy could be particularly valuable for specialties for which there may be greater need in the workforce.
AI for Administrative Purposes
Artificial intelligence also has been used for nonapplication aspects of the residency recruitment process, such as interview scheduling. In the absence of coordinated interview release dates (as was implemented in dermatology starting in the 2020-2021 application cycle), a deluge of responses to schedule an interview comes flooding in as soon as invitations for interviewees are sent out, which can produce anxiety both for applicants and residency program staff as the schedule is sorted out and can create delays at both ends. Stephens et al14 utilized a computerized scheduling program for pediatric surgery fellowship applicants. It was used in 2016 to schedule 26 interviews, and it was found to reduce the average time to schedule an interview from 14.4 hours to 1.7 hours. It also reduced the number of email exchanges needed to finalize scheduling.14
Another aspect of residency recruitment that is amenable to AI is information gathering. Many would-be applicants turn to the internet and social media to learn about residency programs—their unique qualities, assets, and potential alignment of career goals.15 This exchange often is unidirectional, as the applicant clicks through the website searching for information. Yi et al16 explored the use of a chatbot, which mimics human conversation and exchange, on their institution’s pain fellowship website. Fellowship applicants could create specific prompts, such as “Show me faculty that trained at <applicant’s home program>,” and the chatbot would reply with the answer. The researchers sent a survey to all 258 applicants to the pain fellowship program that was completed by 48 applicants. Of these respondents, more than 70% (35/48) utilized the chatbot, and 84% (40/48) stated that they had found the information that was requested. The respondents overall found the chatbot to be a useful and positive experience.16
Specific Tools to Consider
There are some tools that are publicly available for programs and applicants to use that rely on AI.
In collaboration with ERAS and the Association of American Medical Colleges, Cortex powered by Thalamus (SJ MedConnect Inc)(https://thalamusgme.com/cortex-application-screening/) offers technology-assisted holistic review of residency and fellowship applications by utilizing natural language processing and optical character recognition to aggregate data from ERAS.
Tools also are being leveraged by applicants to help them find residency programs that fit their criteria, prepare for interviews, and complete portions of the application. Match A Resident (https://www.matcharesident.com/) is a resource for the international medical graduate community. As part of the service, the “Learn More with MARai” feature uses AI to generate information on residency programs to increase applicants’ confidence going into the interview process.17 Big Interview Medical (https://www.biginterviewmedical.com/ai-feedback), a paid interview preparation system developed by interview experts, utilizes AI to provide feedback to residents practicing for the interview process by measuring the amount of natural eye contact, language used, and pace of speech. A “Power Word” score is provided that incorporates aspects such as using filler words (“umm,” “uhh”). A Pace of Speech Tool provides rate of speaking feedback presuming that there is an ideal pace to decrease the impression that the applicant is nervous. Johnstone et al18 used ChatGPT (https://chat.openai.com/auth/login) to generate 2 personal statements for anesthesia residency applicants. Based on survey responses from 31 program directors, 22 rated the statements as good or excellent.18
Ethnical Concerns and Limitations of AI
The potential use of AI tools by residency applicants inevitably brings forth consideration of biases, ethics, and current limitations. These tools are highly dependent on the quality and quantity of data used for training and validation. Information considered valuable in the holistic review of applications includes unstructured data such as personal statements and letters of recommendation, and incorporating this information can be challenging in ML models, in contrast to discrete structured data such as grades, test scores, and awards. In addition, MLAs depend on large quantities of data to optimize performance.19 Depending on the size of the applicant pool and the amount of data available, this can present a limitation for smaller programs in developing ML tools for residency recruitment. Studies evaluating the use of AI in the residency application process often are from single institutions, and therefore generalizability is uncertain. The risk for latent bias—whereby a historical or pre-existing stereotype gets perpetuated through the system—must be considered, with the development of tools to detect and address this if found. Choosing which data to use to train the model can be tricky as well as choosing the outcome of interest. For these interventions to become more resilient, programs need to self-examine what defines their criteria for a successful match to their program to incorporate this data into their ML studies. The previously described models in this overview focused on outcomes such as whether an applicant was invited to interview, whether the applicant was ranked, and whether the applicant matriculated to their program.10,11 For supervised ML models that rely on outcomes to develop a prediction, continued research as to what outcomes represent resident success (eg, passing board certification examinations, correlation with clinical performance) would be important. There also is the possibility of applicants restructuring their applications to align with goals of an AI-assisted search and using AI to generate part or all of their application. The use of ChatGPT and other AI tools in the preparation of personal statements and curriculum vitae may provide benefits such as improved efficiency and grammar support.20 However, as use becomes more widespread, there is the potential increased similarity of personal statements and likely varied opinions on the use of such tools as writing aids.21,22 Continued efforts to develop guidance on generative AI use cases is ongoing; an example is the launch of VALID AI (https://validai.health/), a collaboration among health systems, health plans, and AI research organizations and nonprofits.23
Final Thoughts
Artificial intelligence tools may be a promising resource for residency and fellowship programs seeking to find meaningful ways to select applicants who are good matches for their training environment. Prioritizing the holistic review of applications has been promoted as a method to evaluate the applicant beyond their test scores and grades. The use of MLAs may streamline this review process, aid in scheduling interviews, and help discover trends in successful matriculants.
According to Electronic Residency Application Service (ERAS) statistics, there were more than 1400 dermatology applicants in 2022, with an average of almost 560 applications received per program.1,2 With the goal to expand the diversity of board-certified dermatologists, there is increasing emphasis on the holistic review of applications, forgoing filtering by discrete metrics such as AOA (American Osteopathic Association) membership and US Medical Licensing Examination (USMLE) scores.3 According to the Association of American Medical Colleges, holistic review focuses on an individual applicant’s experience and unique attributes in addition to their academic achievements.4 Recent strategies to enhance the residency recruitment process have included the introduction of standardized letters of recommendation, preference signaling, and supplemental applications.5,6
Because it has become increasingly important to include applicant factors and achievements that extend beyond academics, the number of data points that are required for holistic review has expanded. If each application required 20 minutes to review, this would result in 166 total hours for complete holistic review of 500 applications. Tools that can facilitate holistic review of candidates and select applicants whose interests and career goals align with individual residency programs have the potential to optimize review. Artificial intelligence (AI) may aid in this process. This column highlights some of the published research on novel AI strategies that have the potential to impact dermatology residency recruitment.
Machine Learning to Screen Applicants
Artificial intelligence involves a machine-based system that can make decisions, predictions, and recommendations when provided a given set of human-defined objectives.7 Autonomous systems, machine learning (ML), and generative AI are examples of AI models.8 Machine learning has been explored to shorten and streamline the application review process and decrease bias. Because ML is a model in which the computer learns patterns based on large amounts of input data,9 it is possible that models could be developed and used in future cycles. Some studies found that applicants were discovered who traditionally would not have made it to the next stage of consideration based primarily on academic metrics.10,11 Burk-Rafel et al10 developed and validated an ML-based decision support tool for residency program directors to use for interview invitation decisions. The tool utilized 61 variables from ERAS data from more than 8000 applications in 3 prior application cycles at a single internal medicine residency program. An interview invitation was designated as the target outcome. Ultimately, the model would output a probability score for an interview invitation. The authors were able to tune the model to a 91% sensitivity and 85% specificity; for a pool of 2000 applicants and an invite rate of 15%, 1475 applicants would be screened out with a negative predictive value of 98% with maintenance of performance, even with removal of USMLE Step 1 examination scores. Their ML model was prospectively validated during an ongoing resident selection cycle, and when compared with human review, the AI model found an additional 20 applicants to invite for interviews. They concluded that this tool could potentially augment the human review process and reveal applicants who may have otherwise been overlooked.10
Rees and Ryder11 utilized another ML screening approach with the target outcome of ranked and matriculated compared with ranked applicants based on ERAS data using 72 unique variables for more than 5000 applicants. Their model was able to identify ranked candidates from the overall applicant pool with high accuracy; identification of ranked applicants that matriculated at the program was more modest but better than random probability.11Both the Burk-Rafel et al10 and Rees and Ryder11 models excluded some unstructured data components of the residency application, such as personal statements, medical student performance evaluation letters, transcripts, and letters of reference, that some may consider strongly in the holistic review process. Drum et al12 explored the value of extraction of this type of data. They created a program to extract “snippets” of text that pertained to values of successful residents for their internal medicine–pediatrics residency program that they previously validated via a modified Delphi method, which then were annotated by expert reviewers. Natural language processing was used to train an ML algorithm (MLA) to classify snippets into 11 value categories. Four values had more than 66% agreement with human annotation: academic strength; leadership; communication; and justice, equity, diversity, and inclusion. Although this MLA has not reached high enough levels of agreement for all the predetermined success values, the authors hope to generate a model that could produce a quantitative score to use as an initial screening tool to select applicants for interview.12 This type of analysis also could be incorporated into other MLAs for further refinement of the mentoring and application process.
Knapke et al13 evaluated the use of a natural language modeling platform to look for semantic patterns in medical school applications that could predict which students would be more likely to pursue family medicine residency, thus beginning the recruitment process even before residency application. This strategy could be particularly valuable for specialties for which there may be greater need in the workforce.
AI for Administrative Purposes
Artificial intelligence also has been used for nonapplication aspects of the residency recruitment process, such as interview scheduling. In the absence of coordinated interview release dates (as was implemented in dermatology starting in the 2020-2021 application cycle), a deluge of responses to schedule an interview comes flooding in as soon as invitations for interviewees are sent out, which can produce anxiety both for applicants and residency program staff as the schedule is sorted out and can create delays at both ends. Stephens et al14 utilized a computerized scheduling program for pediatric surgery fellowship applicants. It was used in 2016 to schedule 26 interviews, and it was found to reduce the average time to schedule an interview from 14.4 hours to 1.7 hours. It also reduced the number of email exchanges needed to finalize scheduling.14
Another aspect of residency recruitment that is amenable to AI is information gathering. Many would-be applicants turn to the internet and social media to learn about residency programs—their unique qualities, assets, and potential alignment of career goals.15 This exchange often is unidirectional, as the applicant clicks through the website searching for information. Yi et al16 explored the use of a chatbot, which mimics human conversation and exchange, on their institution’s pain fellowship website. Fellowship applicants could create specific prompts, such as “Show me faculty that trained at <applicant’s home program>,” and the chatbot would reply with the answer. The researchers sent a survey to all 258 applicants to the pain fellowship program that was completed by 48 applicants. Of these respondents, more than 70% (35/48) utilized the chatbot, and 84% (40/48) stated that they had found the information that was requested. The respondents overall found the chatbot to be a useful and positive experience.16
Specific Tools to Consider
There are some tools that are publicly available for programs and applicants to use that rely on AI.
In collaboration with ERAS and the Association of American Medical Colleges, Cortex powered by Thalamus (SJ MedConnect Inc)(https://thalamusgme.com/cortex-application-screening/) offers technology-assisted holistic review of residency and fellowship applications by utilizing natural language processing and optical character recognition to aggregate data from ERAS.
Tools also are being leveraged by applicants to help them find residency programs that fit their criteria, prepare for interviews, and complete portions of the application. Match A Resident (https://www.matcharesident.com/) is a resource for the international medical graduate community. As part of the service, the “Learn More with MARai” feature uses AI to generate information on residency programs to increase applicants’ confidence going into the interview process.17 Big Interview Medical (https://www.biginterviewmedical.com/ai-feedback), a paid interview preparation system developed by interview experts, utilizes AI to provide feedback to residents practicing for the interview process by measuring the amount of natural eye contact, language used, and pace of speech. A “Power Word” score is provided that incorporates aspects such as using filler words (“umm,” “uhh”). A Pace of Speech Tool provides rate of speaking feedback presuming that there is an ideal pace to decrease the impression that the applicant is nervous. Johnstone et al18 used ChatGPT (https://chat.openai.com/auth/login) to generate 2 personal statements for anesthesia residency applicants. Based on survey responses from 31 program directors, 22 rated the statements as good or excellent.18
Ethnical Concerns and Limitations of AI
The potential use of AI tools by residency applicants inevitably brings forth consideration of biases, ethics, and current limitations. These tools are highly dependent on the quality and quantity of data used for training and validation. Information considered valuable in the holistic review of applications includes unstructured data such as personal statements and letters of recommendation, and incorporating this information can be challenging in ML models, in contrast to discrete structured data such as grades, test scores, and awards. In addition, MLAs depend on large quantities of data to optimize performance.19 Depending on the size of the applicant pool and the amount of data available, this can present a limitation for smaller programs in developing ML tools for residency recruitment. Studies evaluating the use of AI in the residency application process often are from single institutions, and therefore generalizability is uncertain. The risk for latent bias—whereby a historical or pre-existing stereotype gets perpetuated through the system—must be considered, with the development of tools to detect and address this if found. Choosing which data to use to train the model can be tricky as well as choosing the outcome of interest. For these interventions to become more resilient, programs need to self-examine what defines their criteria for a successful match to their program to incorporate this data into their ML studies. The previously described models in this overview focused on outcomes such as whether an applicant was invited to interview, whether the applicant was ranked, and whether the applicant matriculated to their program.10,11 For supervised ML models that rely on outcomes to develop a prediction, continued research as to what outcomes represent resident success (eg, passing board certification examinations, correlation with clinical performance) would be important. There also is the possibility of applicants restructuring their applications to align with goals of an AI-assisted search and using AI to generate part or all of their application. The use of ChatGPT and other AI tools in the preparation of personal statements and curriculum vitae may provide benefits such as improved efficiency and grammar support.20 However, as use becomes more widespread, there is the potential increased similarity of personal statements and likely varied opinions on the use of such tools as writing aids.21,22 Continued efforts to develop guidance on generative AI use cases is ongoing; an example is the launch of VALID AI (https://validai.health/), a collaboration among health systems, health plans, and AI research organizations and nonprofits.23
Final Thoughts
Artificial intelligence tools may be a promising resource for residency and fellowship programs seeking to find meaningful ways to select applicants who are good matches for their training environment. Prioritizing the holistic review of applications has been promoted as a method to evaluate the applicant beyond their test scores and grades. The use of MLAs may streamline this review process, aid in scheduling interviews, and help discover trends in successful matriculants.
- Association of American Medical Colleges. ERAS® Statistics. Accessed January 16, 2024. https://www.aamc.org/data-reports/data/eras-statistics-data
- National Resident Matching Program, Data Release and ResearchCommittee: Results of the 2022 NRMP Program Director Survey. Accessed January 18, 2024. https://www.nrmp.org/wp-content/uploads/2022/09/PD-Survey-Report-2022_FINALrev.pdf
- Isaq NA, Bowers S, Chen ST. Taking a “step” toward diversity in dermatology: de-emphasizing USMLE Step 1 scores in residency applications. Int J Womens Dermatol. 2020;6:209-210. doi:10.1016/j.ijwd.2020.02.008
- Association of American Medical Colleges. Holistic review in medical school admissions. Accessed January 16, 2024. https://students-residents.aamc.org/choosing-medical-career/holistic-review-medical-school-admissions
- Association of American Medical Colleges. The MyERAS® application and program signaling for 2023-24. Accessed January 16, 2024. https://students-residents.aamc.org/applying-residencies-eras/myeras-application-and-program-signaling-2023-24
- Tavarez MM, Baghdassarian A, Bailey J, et al. A call to action for standardizing letters of recommendation. J Grad Med Educ. 2022;14:642-646. doi:10.4300/JGME-D-22-00131.1
- US Department of State. Artificial intelligence (AI). Accessed January 16, 2024. https://www.state.gov/artificial-intelligence/
- Stanford University Human-Centered Artificial Intelligence. Artificial intelligence definitions. Accessed January 16, 2024.https://hai.stanford.edu/sites/default/files/2023-03/AI-Key-Terms-Glossary-Definition.pdf
- Rajkomar A, Dean J, Kohane I. Machine learning in medicine. N Engl J Med. 2019;380:1347-1358. doi:10.1056/NEJMra1814259
- Burk-Rafel J, Reinstein I, Feng J, et al. Development and validation of a machine learning-based decision support tool for residency applicant screening and review. Acad Med. 2021;96(11S):S54-S61. doi:10.1097/ACM.0000000000004317
- Rees CA, Ryder HF. Machine learning for the prediction of ranked applicants and matriculants to an internal medicine residency program. Teach Learn Med. 2023;35:277-286. doi:10.1080/10401334.2022.2059664
- Drum B, Shi J, Peterson B, et al. Using natural language processing and machine learning to identify internal medicine-pediatrics residency values in applications. Acad Med. 2023;98:1278-1282. doi:10.1097/ACM.0000000000005352
- Knapke JM, Mount HR, McCabe E, et al. Early identification of family physicians using qualitative admissions data. Fam Med. 2023;55:245-252. doi:10.22454/FamMed.2023.596964
- Stephens CQ, Hamilton NA, Thompson AE, et al. Use of computerized interview scheduling program for pediatric surgery match applicants. J Pediatr Surg. 2017;52:1056-1059. doi:10.1016/j.jpedsurg.2017.03.033
- Nickles MA, Kulkarni V, Varghese JA, et al. Dermatology residency programs’ websites in the virtual era: a cross-sectional analysis. J Am Acad Dermatol. 2022;86:447-448. doi:10.1016/j.jaad.2021.09.064
- Yi PK, Ray ND, Segall N. A novel use of an artificially intelligent Chatbot and a live, synchronous virtual question-and answer session for fellowship recruitment. BMC Med Educ. 2023;23:152. doi:10.1186/s12909-022-03872-z
- Introducing “Learn More with MARai”—the key to understanding your target residency programs. Match A Resident website. Published September 23, 2023. Accessed January 16, 2024. https://blog.matcharesident.com/ai-powered-residency-insights/
- Johnstone RE, Neely G, Sizemore DC. Artificial intelligence softwarecan generate residency application personal statements that program directors find acceptable and difficult to distinguish from applicant compositions. J Clin Anesth. 2023;89:111185. doi:10.1016/j.jclinane.2023.111185
- Khalid N, Qayyum A, Bilal M, et al. Privacy-preserving artificial intelligence in healthcare: techniques and applications. Comput Biol Med. 2023;158:106848. doi:10.1016/j.compbiomed.2023.106848
- Birt J. How to optimize your resume for AI scanners (with tips). Indeed website. Updated December 30, 2022. Accessed January 16, 2024. https://www.indeed.com/career-advice/resumes-cover-letters/resume-ai
- Patel V, Deleonibus A, Wells MW, et al. Distinguishing authentic voices in the age of ChatGPT: comparing AI-generated and applicant-written personal statements for plastic surgery residency application. Ann Plast Surg. 2023;91:324-325. doi:10.1097/SAP.0000000000003653
- Woodfin MW. The personal statement in the age of artificial intelligence. Acad Med. 2023;98:869. doi:10.1097/ACM.0000000000005266
- Diaz N. UC Davis Health to lead new gen AI collaborative. Beckers Healthcare website. Published October 10, 2023. AccessedJanuary 16, 2024. https://www.beckershospitalreview.com/digital-health/uc-davis-health-to-lead-new-gen-ai-collaborative.html
- Association of American Medical Colleges. ERAS® Statistics. Accessed January 16, 2024. https://www.aamc.org/data-reports/data/eras-statistics-data
- National Resident Matching Program, Data Release and ResearchCommittee: Results of the 2022 NRMP Program Director Survey. Accessed January 18, 2024. https://www.nrmp.org/wp-content/uploads/2022/09/PD-Survey-Report-2022_FINALrev.pdf
- Isaq NA, Bowers S, Chen ST. Taking a “step” toward diversity in dermatology: de-emphasizing USMLE Step 1 scores in residency applications. Int J Womens Dermatol. 2020;6:209-210. doi:10.1016/j.ijwd.2020.02.008
- Association of American Medical Colleges. Holistic review in medical school admissions. Accessed January 16, 2024. https://students-residents.aamc.org/choosing-medical-career/holistic-review-medical-school-admissions
- Association of American Medical Colleges. The MyERAS® application and program signaling for 2023-24. Accessed January 16, 2024. https://students-residents.aamc.org/applying-residencies-eras/myeras-application-and-program-signaling-2023-24
- Tavarez MM, Baghdassarian A, Bailey J, et al. A call to action for standardizing letters of recommendation. J Grad Med Educ. 2022;14:642-646. doi:10.4300/JGME-D-22-00131.1
- US Department of State. Artificial intelligence (AI). Accessed January 16, 2024. https://www.state.gov/artificial-intelligence/
- Stanford University Human-Centered Artificial Intelligence. Artificial intelligence definitions. Accessed January 16, 2024.https://hai.stanford.edu/sites/default/files/2023-03/AI-Key-Terms-Glossary-Definition.pdf
- Rajkomar A, Dean J, Kohane I. Machine learning in medicine. N Engl J Med. 2019;380:1347-1358. doi:10.1056/NEJMra1814259
- Burk-Rafel J, Reinstein I, Feng J, et al. Development and validation of a machine learning-based decision support tool for residency applicant screening and review. Acad Med. 2021;96(11S):S54-S61. doi:10.1097/ACM.0000000000004317
- Rees CA, Ryder HF. Machine learning for the prediction of ranked applicants and matriculants to an internal medicine residency program. Teach Learn Med. 2023;35:277-286. doi:10.1080/10401334.2022.2059664
- Drum B, Shi J, Peterson B, et al. Using natural language processing and machine learning to identify internal medicine-pediatrics residency values in applications. Acad Med. 2023;98:1278-1282. doi:10.1097/ACM.0000000000005352
- Knapke JM, Mount HR, McCabe E, et al. Early identification of family physicians using qualitative admissions data. Fam Med. 2023;55:245-252. doi:10.22454/FamMed.2023.596964
- Stephens CQ, Hamilton NA, Thompson AE, et al. Use of computerized interview scheduling program for pediatric surgery match applicants. J Pediatr Surg. 2017;52:1056-1059. doi:10.1016/j.jpedsurg.2017.03.033
- Nickles MA, Kulkarni V, Varghese JA, et al. Dermatology residency programs’ websites in the virtual era: a cross-sectional analysis. J Am Acad Dermatol. 2022;86:447-448. doi:10.1016/j.jaad.2021.09.064
- Yi PK, Ray ND, Segall N. A novel use of an artificially intelligent Chatbot and a live, synchronous virtual question-and answer session for fellowship recruitment. BMC Med Educ. 2023;23:152. doi:10.1186/s12909-022-03872-z
- Introducing “Learn More with MARai”—the key to understanding your target residency programs. Match A Resident website. Published September 23, 2023. Accessed January 16, 2024. https://blog.matcharesident.com/ai-powered-residency-insights/
- Johnstone RE, Neely G, Sizemore DC. Artificial intelligence softwarecan generate residency application personal statements that program directors find acceptable and difficult to distinguish from applicant compositions. J Clin Anesth. 2023;89:111185. doi:10.1016/j.jclinane.2023.111185
- Khalid N, Qayyum A, Bilal M, et al. Privacy-preserving artificial intelligence in healthcare: techniques and applications. Comput Biol Med. 2023;158:106848. doi:10.1016/j.compbiomed.2023.106848
- Birt J. How to optimize your resume for AI scanners (with tips). Indeed website. Updated December 30, 2022. Accessed January 16, 2024. https://www.indeed.com/career-advice/resumes-cover-letters/resume-ai
- Patel V, Deleonibus A, Wells MW, et al. Distinguishing authentic voices in the age of ChatGPT: comparing AI-generated and applicant-written personal statements for plastic surgery residency application. Ann Plast Surg. 2023;91:324-325. doi:10.1097/SAP.0000000000003653
- Woodfin MW. The personal statement in the age of artificial intelligence. Acad Med. 2023;98:869. doi:10.1097/ACM.0000000000005266
- Diaz N. UC Davis Health to lead new gen AI collaborative. Beckers Healthcare website. Published October 10, 2023. AccessedJanuary 16, 2024. https://www.beckershospitalreview.com/digital-health/uc-davis-health-to-lead-new-gen-ai-collaborative.html
Practice Points
- Artificial intelligence solutions may increase the efficiency of the holistic review process and enhance the opportunity to find applicants who may have been overlooked by a traditional review process.
- Artificial intelligence support also may be utilized by applicants to aid in discovering training programs that fit their interests, practice interview strategies, and refine their written application.
Navigating Psoriasis Treatment Innovations
Psoriasis is a chronic autoimmune skin condition that affects approximately 2% to 4% of the US population and notably impacts overall quality of life.1,2 There is no cure for this long-lasting condition. Fortunately, recent developments in research have led to more targeted therapies, paving the way for a more promising transformative landscape of psoriasis management. Herein, we explore the most up-to-date advancements and developments in the realm of psoriasis care.
Emerging Systemic Therapies
Biologics are cutting-edge treatments available for moderate to severe plaque psoriasis, as IL-17A, IL-23, and tumor necrosis factor α (TNF-α) have been recognized as key targets.3
IL-17—Bimekizumab is a unique monoclonal antibody that inhibits the activity of both IL-17A and IL-17F cytokines.3 This treatment was approved by the US Food and Drug Administration (FDA) in October 2023 for patients with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy.4
Bimekizumab outperformed ustekinumab in the BE VIVID phase 3 trial, with 273 of 321 patients (85%) receiving bimekizumab vs 81 of 163 patients (50%) receiving ustekinumab experiencing at least 90% improvement in psoriasis area and severity index (PASI) score at week 16.4 In a 2020 observational study (PSO-BIO-REAL), the efficacy rate of skin clearance after 6 months of treatment with biologics was only 25% (1/4).5 Aside from moderate to severe plaque psoriasis, bimekizumab demonstrated notable improvement in patients with psoriatic arthritis who had inadequate response or intolerance to TNF-α inhibitors compared to a placebo group in the BE COMPLETE phase 3 trial.6
IL-23—Guselkumab, risankizumab, and tildrakizumab are injectable therapies approved by the FDA in 2017 for moderate to severe plaque psoriasis.3 They inhibit IL-23 signaling by targeting the p19 subunit in addition to sparing IL-12.3,7
A novel oral therapeutic peptide, JNJ-2113—the first oral IL-23 receptor antagonist peptide that blocks IL-23 signaling—has been developed, offering a new way to treat moderate to severe plaque psoriasis. Trial results from a phase 2 study (FRONTIER1) have supported JNJ-2113’s advancement into phase 3.7,8 Patients who received JNJ-2113 successfully achieved PASI75 in addition to surpassing PASI90 and PASI100 at greater proportions compared to placebo at week 16.7
The promising early results of JNJ-2113 provide patients with greater flexibility and convenience for treatment options to address the manifestations of psoriasis. Although a considerable number of patients with moderate to severe plaque psoriasis qualify for advanced therapies, a substantial proportion remain untreated. Introducing an oral route of medication administration may help overcome barriers to therapy access due to a greater preference for pills over injections.9
TNF-α Inhibitors—Adalimumab is a TNF-α inhibitor that is used to treat moderate to severe chronic plaque psoriasis in adults who are candidates for systemic phototherapy.1,10 However, one of the main barriers to initiating treatment has been cost. Biosimilars contribute to market competition, thus allowing the possibility of lower drug prices.10
There are 9 FDA-approved biosimilar products for adalimumab, with 2 having interchangeable designation. The first interchangeable biosimilar to enter the US market, adalimumab-adbm, became available in July 2023. In October 2023, adalimumab-afzb was granted interchangeable designation,11 which enables pharmacists to swiftly substitute brand products for lower-cost biosimilars, providing patients with equally safe and effective alternatives without the delay of involving the prescribing clinician.12 Pricing information indicates an initial 5% discount, which may later increase to 60%, from brand name adalimumab. Hopefully, reduced drug costs due to market competition will allow more patients to overcome barriers to therapy access.
IL-12/IL-23—Ustekinumab is a monoclonal antibody that targets IL-12 and IL-23. The FDA recently approved ustekinumab-auub as the first interchangeable ustekinumab biosimilar for the treatment of various inflammatory diseases, including moderate to severe plaque psoriasis and psoriatic arthritis.12,13 The approval of ustekinumab-auub expands therapeutic options for the treatment of diverse inflammatory diseases. As the first interchangeable biosimilar in its category, this development underscores the importance of biosimilars in providing effective and accessible treatment.12,14
Topical Innovations
In October 2023, the FDA approved an expanded indication for roflumilast cream 0.3% to treat children as young as 6 years for plaque psoriasis, even for use in intertriginous areas,15 which is a milestone given the lack of treatment options for the pediatric population because topical steroids, the most common treatment option for plaque psoriasis, can have safety concerns related to long-term use. With the advent of this steroid-free topical agent, pediatric patients have a safe and well-tolerated option for managing plaque psoriasis.16 This promising effort will now expand to trials in children as young as 2 years to test efficacy.16
Engel et al17 proposed a new algorithmic approach to the topical management of psoriasis with roflumilast cream and tapinarof cream as first-line treatments for mild disease due to their novelty in treating intertriginous areas, whereas traditional topical steroids in these areas would be inapt.17 The latest indication for roflumilast cream suggests that this proposed recommendation could be a promising and convenient enhancement to psoriasis management, potentially outperforming traditional topical corticosteroids.15,17
Final Thoughts
Innovative targeted therapies ranging from new biologic agents to broader applications of topical treatments hold the potential to transform conventional psoriasis management with greater efficacy and safety, which can help create a more effective and personalized approach with greater patient satisfaction, ultimately enhancing overall quality of life. The choice of treatment is dependent not only on the severity of the disease but also on accessibility considerations such as cost. Overall, these innovative therapies add substantial value to the treatment armamentarium for psoriasis.
- Li C, Sunhe Y, Zhou H, Dong W. Efficacy and safety evaluations of adalimumab biosimilars in the treatment of psoriasis. J Dermatolog Treat. 2023;34:2249145. doi:10.1080/09546634.2023.2249145
- Liu J, Thatiparthi A, Martin A, et al. Association between psoriasis and thyroid dysfunction among US adults in the 2009-2014 National Health and Nutrition Examination Survey [published online Mary 17, 2021]. J Am Acad Dermatol. 2022;86:897-899. doi:10.1016/j.jaad.2021.03.030
- Lee EB, Amin M, Bhutani T, et al. Emerging therapies in psoriasis: a systematic review. Cutis. 2018;101(3S):5-9.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo-controlled phase 3 trial. Lancet. 2021;397:487-498. doi:10.1016/S0140-6736(21)00125-2
- Seneschal J, Lacour JP, Bewley A, et al. A multinational, prospective, observational study to estimate complete skin clearance in patients with moderate-to-severe plaque PSOriasis treated with BIOlogics in a REAL world setting (PSO-BIO-REAL) [published online June 8, 2020]. J Eur Acad Dermatol Venereol. 2020;34:2566-2573. doi:10.1111/jdv.16568
- Merola JF, Landewé R, McInnes IB, et al. Bimekizumab in patients with active psoriatic arthritis and previous inadequate response or intolerance to tumour necrosis factor-α inhibitors: a randomised, double-blind, placebo-controlled, phase 3 trial (BE COMPLETE)[published online December 6, 2022]. Lancet. 2023;401:38-48. doi:10.1016/S0140-6736(22)02303-0
- Janssen announces positive topline results for JNJ-2113—a novel, first and only oral IL-23 receptor antagonist peptide in development for moderate-to-severe plaque psoriasis. News release. Janssen Pharmaceutical Companies; July 4, 2023.
- Bissonnette R, Pinter A, Ferris L, et al. A Phase 2, randomized, placebo-controlled, dose-ranging study of oral JNJ-77242113 for the treatment of moderate-to-severe plaque psoriasis: FRONTIER 1. Abstract presented at: World Congress of Dermatology, July 3-8, 2023; Singapore.
- Xu Y, Sudharshan L, Hsu MA, et al. Patient preferences associated with therapies for psoriatic arthritis: a conjoint analysis. Am Health Drug Benefits. 2018;11:408-417.
- Maurelli M, Girolomoni G, Gisondi P. Cost per responder of adalimumab biosimilars versus methotrexate in patients with psoriasis: a real-life experience. J Dermatolog Treat. 2023;34:2218504. doi:10.1080/09546634.2023.2218504
- Food and Drug Administration/Center for Drug Evaluation and Research. Expiration of first interchangeable exclusivity (“FIE”) when section 351(l)(6) litigation ends prior to the submission of an application for interchangeability [memorandum]. Published October 3, 2023. Accessed January 18, 2024. https://www.fda.gov/media/173749/download
- US Food & Drug Administration. Biosimilar and interchangeable biologics: more treatment choices. Accessed January 18, 2024. https://www.fda.gov/consumers/consumer-updates/biosimilar-and-interchangeable-biologics-more-treatment-choices
- Chow V, Mytych DT, Das S, et al. Pharmacokinetic similarity of ABP 654, an ustekinumab biosimilar candidate: results from a randomized, double-blind study in healthy subjects [published online July 7, 2023]. Clin Pharmacol Drug Dev. 2023;12:863-873. doi:10.1002/cpdd.1301
- Wezlana (ustekinumab-auub) [prescribing information]. Published October 2023. Accessed January 18, 2024. www.accessdata.fda.gov/drugsatfda_docs/label/2023/761285s000,761331s000lbl.pdf
- ZORYVE (roflumilast) topical cream [prescribing information]. Westlake Village, CA: Arcutis Biotherapeutics. Revised October 2023. Accessed January 18, 2024. https://www.arcutis.com/wp-content/uploads/USPI-roflumilast-cream.pdf
- Lie E, Choi M, Wang SP, et al. Topical management of pediatric psoriasis: a review of new developments and existing therapies. Paediatr Drugs. 2024;26:9-18. doi:10.1007/s40272-023-00592-9
- Engel PV, Smith B, Javadi SS, et al. It is time to consider anew topical algorithm for psoriasis. J Am Acad Dermatol. 2023:S0190-9622(23)02906-7. doi:10.1016/j.jaad.2023.07.1048
Psoriasis is a chronic autoimmune skin condition that affects approximately 2% to 4% of the US population and notably impacts overall quality of life.1,2 There is no cure for this long-lasting condition. Fortunately, recent developments in research have led to more targeted therapies, paving the way for a more promising transformative landscape of psoriasis management. Herein, we explore the most up-to-date advancements and developments in the realm of psoriasis care.
Emerging Systemic Therapies
Biologics are cutting-edge treatments available for moderate to severe plaque psoriasis, as IL-17A, IL-23, and tumor necrosis factor α (TNF-α) have been recognized as key targets.3
IL-17—Bimekizumab is a unique monoclonal antibody that inhibits the activity of both IL-17A and IL-17F cytokines.3 This treatment was approved by the US Food and Drug Administration (FDA) in October 2023 for patients with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy.4
Bimekizumab outperformed ustekinumab in the BE VIVID phase 3 trial, with 273 of 321 patients (85%) receiving bimekizumab vs 81 of 163 patients (50%) receiving ustekinumab experiencing at least 90% improvement in psoriasis area and severity index (PASI) score at week 16.4 In a 2020 observational study (PSO-BIO-REAL), the efficacy rate of skin clearance after 6 months of treatment with biologics was only 25% (1/4).5 Aside from moderate to severe plaque psoriasis, bimekizumab demonstrated notable improvement in patients with psoriatic arthritis who had inadequate response or intolerance to TNF-α inhibitors compared to a placebo group in the BE COMPLETE phase 3 trial.6
IL-23—Guselkumab, risankizumab, and tildrakizumab are injectable therapies approved by the FDA in 2017 for moderate to severe plaque psoriasis.3 They inhibit IL-23 signaling by targeting the p19 subunit in addition to sparing IL-12.3,7
A novel oral therapeutic peptide, JNJ-2113—the first oral IL-23 receptor antagonist peptide that blocks IL-23 signaling—has been developed, offering a new way to treat moderate to severe plaque psoriasis. Trial results from a phase 2 study (FRONTIER1) have supported JNJ-2113’s advancement into phase 3.7,8 Patients who received JNJ-2113 successfully achieved PASI75 in addition to surpassing PASI90 and PASI100 at greater proportions compared to placebo at week 16.7
The promising early results of JNJ-2113 provide patients with greater flexibility and convenience for treatment options to address the manifestations of psoriasis. Although a considerable number of patients with moderate to severe plaque psoriasis qualify for advanced therapies, a substantial proportion remain untreated. Introducing an oral route of medication administration may help overcome barriers to therapy access due to a greater preference for pills over injections.9
TNF-α Inhibitors—Adalimumab is a TNF-α inhibitor that is used to treat moderate to severe chronic plaque psoriasis in adults who are candidates for systemic phototherapy.1,10 However, one of the main barriers to initiating treatment has been cost. Biosimilars contribute to market competition, thus allowing the possibility of lower drug prices.10
There are 9 FDA-approved biosimilar products for adalimumab, with 2 having interchangeable designation. The first interchangeable biosimilar to enter the US market, adalimumab-adbm, became available in July 2023. In October 2023, adalimumab-afzb was granted interchangeable designation,11 which enables pharmacists to swiftly substitute brand products for lower-cost biosimilars, providing patients with equally safe and effective alternatives without the delay of involving the prescribing clinician.12 Pricing information indicates an initial 5% discount, which may later increase to 60%, from brand name adalimumab. Hopefully, reduced drug costs due to market competition will allow more patients to overcome barriers to therapy access.
IL-12/IL-23—Ustekinumab is a monoclonal antibody that targets IL-12 and IL-23. The FDA recently approved ustekinumab-auub as the first interchangeable ustekinumab biosimilar for the treatment of various inflammatory diseases, including moderate to severe plaque psoriasis and psoriatic arthritis.12,13 The approval of ustekinumab-auub expands therapeutic options for the treatment of diverse inflammatory diseases. As the first interchangeable biosimilar in its category, this development underscores the importance of biosimilars in providing effective and accessible treatment.12,14
Topical Innovations
In October 2023, the FDA approved an expanded indication for roflumilast cream 0.3% to treat children as young as 6 years for plaque psoriasis, even for use in intertriginous areas,15 which is a milestone given the lack of treatment options for the pediatric population because topical steroids, the most common treatment option for plaque psoriasis, can have safety concerns related to long-term use. With the advent of this steroid-free topical agent, pediatric patients have a safe and well-tolerated option for managing plaque psoriasis.16 This promising effort will now expand to trials in children as young as 2 years to test efficacy.16
Engel et al17 proposed a new algorithmic approach to the topical management of psoriasis with roflumilast cream and tapinarof cream as first-line treatments for mild disease due to their novelty in treating intertriginous areas, whereas traditional topical steroids in these areas would be inapt.17 The latest indication for roflumilast cream suggests that this proposed recommendation could be a promising and convenient enhancement to psoriasis management, potentially outperforming traditional topical corticosteroids.15,17
Final Thoughts
Innovative targeted therapies ranging from new biologic agents to broader applications of topical treatments hold the potential to transform conventional psoriasis management with greater efficacy and safety, which can help create a more effective and personalized approach with greater patient satisfaction, ultimately enhancing overall quality of life. The choice of treatment is dependent not only on the severity of the disease but also on accessibility considerations such as cost. Overall, these innovative therapies add substantial value to the treatment armamentarium for psoriasis.
Psoriasis is a chronic autoimmune skin condition that affects approximately 2% to 4% of the US population and notably impacts overall quality of life.1,2 There is no cure for this long-lasting condition. Fortunately, recent developments in research have led to more targeted therapies, paving the way for a more promising transformative landscape of psoriasis management. Herein, we explore the most up-to-date advancements and developments in the realm of psoriasis care.
Emerging Systemic Therapies
Biologics are cutting-edge treatments available for moderate to severe plaque psoriasis, as IL-17A, IL-23, and tumor necrosis factor α (TNF-α) have been recognized as key targets.3
IL-17—Bimekizumab is a unique monoclonal antibody that inhibits the activity of both IL-17A and IL-17F cytokines.3 This treatment was approved by the US Food and Drug Administration (FDA) in October 2023 for patients with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy.4
Bimekizumab outperformed ustekinumab in the BE VIVID phase 3 trial, with 273 of 321 patients (85%) receiving bimekizumab vs 81 of 163 patients (50%) receiving ustekinumab experiencing at least 90% improvement in psoriasis area and severity index (PASI) score at week 16.4 In a 2020 observational study (PSO-BIO-REAL), the efficacy rate of skin clearance after 6 months of treatment with biologics was only 25% (1/4).5 Aside from moderate to severe plaque psoriasis, bimekizumab demonstrated notable improvement in patients with psoriatic arthritis who had inadequate response or intolerance to TNF-α inhibitors compared to a placebo group in the BE COMPLETE phase 3 trial.6
IL-23—Guselkumab, risankizumab, and tildrakizumab are injectable therapies approved by the FDA in 2017 for moderate to severe plaque psoriasis.3 They inhibit IL-23 signaling by targeting the p19 subunit in addition to sparing IL-12.3,7
A novel oral therapeutic peptide, JNJ-2113—the first oral IL-23 receptor antagonist peptide that blocks IL-23 signaling—has been developed, offering a new way to treat moderate to severe plaque psoriasis. Trial results from a phase 2 study (FRONTIER1) have supported JNJ-2113’s advancement into phase 3.7,8 Patients who received JNJ-2113 successfully achieved PASI75 in addition to surpassing PASI90 and PASI100 at greater proportions compared to placebo at week 16.7
The promising early results of JNJ-2113 provide patients with greater flexibility and convenience for treatment options to address the manifestations of psoriasis. Although a considerable number of patients with moderate to severe plaque psoriasis qualify for advanced therapies, a substantial proportion remain untreated. Introducing an oral route of medication administration may help overcome barriers to therapy access due to a greater preference for pills over injections.9
TNF-α Inhibitors—Adalimumab is a TNF-α inhibitor that is used to treat moderate to severe chronic plaque psoriasis in adults who are candidates for systemic phototherapy.1,10 However, one of the main barriers to initiating treatment has been cost. Biosimilars contribute to market competition, thus allowing the possibility of lower drug prices.10
There are 9 FDA-approved biosimilar products for adalimumab, with 2 having interchangeable designation. The first interchangeable biosimilar to enter the US market, adalimumab-adbm, became available in July 2023. In October 2023, adalimumab-afzb was granted interchangeable designation,11 which enables pharmacists to swiftly substitute brand products for lower-cost biosimilars, providing patients with equally safe and effective alternatives without the delay of involving the prescribing clinician.12 Pricing information indicates an initial 5% discount, which may later increase to 60%, from brand name adalimumab. Hopefully, reduced drug costs due to market competition will allow more patients to overcome barriers to therapy access.
IL-12/IL-23—Ustekinumab is a monoclonal antibody that targets IL-12 and IL-23. The FDA recently approved ustekinumab-auub as the first interchangeable ustekinumab biosimilar for the treatment of various inflammatory diseases, including moderate to severe plaque psoriasis and psoriatic arthritis.12,13 The approval of ustekinumab-auub expands therapeutic options for the treatment of diverse inflammatory diseases. As the first interchangeable biosimilar in its category, this development underscores the importance of biosimilars in providing effective and accessible treatment.12,14
Topical Innovations
In October 2023, the FDA approved an expanded indication for roflumilast cream 0.3% to treat children as young as 6 years for plaque psoriasis, even for use in intertriginous areas,15 which is a milestone given the lack of treatment options for the pediatric population because topical steroids, the most common treatment option for plaque psoriasis, can have safety concerns related to long-term use. With the advent of this steroid-free topical agent, pediatric patients have a safe and well-tolerated option for managing plaque psoriasis.16 This promising effort will now expand to trials in children as young as 2 years to test efficacy.16
Engel et al17 proposed a new algorithmic approach to the topical management of psoriasis with roflumilast cream and tapinarof cream as first-line treatments for mild disease due to their novelty in treating intertriginous areas, whereas traditional topical steroids in these areas would be inapt.17 The latest indication for roflumilast cream suggests that this proposed recommendation could be a promising and convenient enhancement to psoriasis management, potentially outperforming traditional topical corticosteroids.15,17
Final Thoughts
Innovative targeted therapies ranging from new biologic agents to broader applications of topical treatments hold the potential to transform conventional psoriasis management with greater efficacy and safety, which can help create a more effective and personalized approach with greater patient satisfaction, ultimately enhancing overall quality of life. The choice of treatment is dependent not only on the severity of the disease but also on accessibility considerations such as cost. Overall, these innovative therapies add substantial value to the treatment armamentarium for psoriasis.
- Li C, Sunhe Y, Zhou H, Dong W. Efficacy and safety evaluations of adalimumab biosimilars in the treatment of psoriasis. J Dermatolog Treat. 2023;34:2249145. doi:10.1080/09546634.2023.2249145
- Liu J, Thatiparthi A, Martin A, et al. Association between psoriasis and thyroid dysfunction among US adults in the 2009-2014 National Health and Nutrition Examination Survey [published online Mary 17, 2021]. J Am Acad Dermatol. 2022;86:897-899. doi:10.1016/j.jaad.2021.03.030
- Lee EB, Amin M, Bhutani T, et al. Emerging therapies in psoriasis: a systematic review. Cutis. 2018;101(3S):5-9.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo-controlled phase 3 trial. Lancet. 2021;397:487-498. doi:10.1016/S0140-6736(21)00125-2
- Seneschal J, Lacour JP, Bewley A, et al. A multinational, prospective, observational study to estimate complete skin clearance in patients with moderate-to-severe plaque PSOriasis treated with BIOlogics in a REAL world setting (PSO-BIO-REAL) [published online June 8, 2020]. J Eur Acad Dermatol Venereol. 2020;34:2566-2573. doi:10.1111/jdv.16568
- Merola JF, Landewé R, McInnes IB, et al. Bimekizumab in patients with active psoriatic arthritis and previous inadequate response or intolerance to tumour necrosis factor-α inhibitors: a randomised, double-blind, placebo-controlled, phase 3 trial (BE COMPLETE)[published online December 6, 2022]. Lancet. 2023;401:38-48. doi:10.1016/S0140-6736(22)02303-0
- Janssen announces positive topline results for JNJ-2113—a novel, first and only oral IL-23 receptor antagonist peptide in development for moderate-to-severe plaque psoriasis. News release. Janssen Pharmaceutical Companies; July 4, 2023.
- Bissonnette R, Pinter A, Ferris L, et al. A Phase 2, randomized, placebo-controlled, dose-ranging study of oral JNJ-77242113 for the treatment of moderate-to-severe plaque psoriasis: FRONTIER 1. Abstract presented at: World Congress of Dermatology, July 3-8, 2023; Singapore.
- Xu Y, Sudharshan L, Hsu MA, et al. Patient preferences associated with therapies for psoriatic arthritis: a conjoint analysis. Am Health Drug Benefits. 2018;11:408-417.
- Maurelli M, Girolomoni G, Gisondi P. Cost per responder of adalimumab biosimilars versus methotrexate in patients with psoriasis: a real-life experience. J Dermatolog Treat. 2023;34:2218504. doi:10.1080/09546634.2023.2218504
- Food and Drug Administration/Center for Drug Evaluation and Research. Expiration of first interchangeable exclusivity (“FIE”) when section 351(l)(6) litigation ends prior to the submission of an application for interchangeability [memorandum]. Published October 3, 2023. Accessed January 18, 2024. https://www.fda.gov/media/173749/download
- US Food & Drug Administration. Biosimilar and interchangeable biologics: more treatment choices. Accessed January 18, 2024. https://www.fda.gov/consumers/consumer-updates/biosimilar-and-interchangeable-biologics-more-treatment-choices
- Chow V, Mytych DT, Das S, et al. Pharmacokinetic similarity of ABP 654, an ustekinumab biosimilar candidate: results from a randomized, double-blind study in healthy subjects [published online July 7, 2023]. Clin Pharmacol Drug Dev. 2023;12:863-873. doi:10.1002/cpdd.1301
- Wezlana (ustekinumab-auub) [prescribing information]. Published October 2023. Accessed January 18, 2024. www.accessdata.fda.gov/drugsatfda_docs/label/2023/761285s000,761331s000lbl.pdf
- ZORYVE (roflumilast) topical cream [prescribing information]. Westlake Village, CA: Arcutis Biotherapeutics. Revised October 2023. Accessed January 18, 2024. https://www.arcutis.com/wp-content/uploads/USPI-roflumilast-cream.pdf
- Lie E, Choi M, Wang SP, et al. Topical management of pediatric psoriasis: a review of new developments and existing therapies. Paediatr Drugs. 2024;26:9-18. doi:10.1007/s40272-023-00592-9
- Engel PV, Smith B, Javadi SS, et al. It is time to consider anew topical algorithm for psoriasis. J Am Acad Dermatol. 2023:S0190-9622(23)02906-7. doi:10.1016/j.jaad.2023.07.1048
- Li C, Sunhe Y, Zhou H, Dong W. Efficacy and safety evaluations of adalimumab biosimilars in the treatment of psoriasis. J Dermatolog Treat. 2023;34:2249145. doi:10.1080/09546634.2023.2249145
- Liu J, Thatiparthi A, Martin A, et al. Association between psoriasis and thyroid dysfunction among US adults in the 2009-2014 National Health and Nutrition Examination Survey [published online Mary 17, 2021]. J Am Acad Dermatol. 2022;86:897-899. doi:10.1016/j.jaad.2021.03.030
- Lee EB, Amin M, Bhutani T, et al. Emerging therapies in psoriasis: a systematic review. Cutis. 2018;101(3S):5-9.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo-controlled phase 3 trial. Lancet. 2021;397:487-498. doi:10.1016/S0140-6736(21)00125-2
- Seneschal J, Lacour JP, Bewley A, et al. A multinational, prospective, observational study to estimate complete skin clearance in patients with moderate-to-severe plaque PSOriasis treated with BIOlogics in a REAL world setting (PSO-BIO-REAL) [published online June 8, 2020]. J Eur Acad Dermatol Venereol. 2020;34:2566-2573. doi:10.1111/jdv.16568
- Merola JF, Landewé R, McInnes IB, et al. Bimekizumab in patients with active psoriatic arthritis and previous inadequate response or intolerance to tumour necrosis factor-α inhibitors: a randomised, double-blind, placebo-controlled, phase 3 trial (BE COMPLETE)[published online December 6, 2022]. Lancet. 2023;401:38-48. doi:10.1016/S0140-6736(22)02303-0
- Janssen announces positive topline results for JNJ-2113—a novel, first and only oral IL-23 receptor antagonist peptide in development for moderate-to-severe plaque psoriasis. News release. Janssen Pharmaceutical Companies; July 4, 2023.
- Bissonnette R, Pinter A, Ferris L, et al. A Phase 2, randomized, placebo-controlled, dose-ranging study of oral JNJ-77242113 for the treatment of moderate-to-severe plaque psoriasis: FRONTIER 1. Abstract presented at: World Congress of Dermatology, July 3-8, 2023; Singapore.
- Xu Y, Sudharshan L, Hsu MA, et al. Patient preferences associated with therapies for psoriatic arthritis: a conjoint analysis. Am Health Drug Benefits. 2018;11:408-417.
- Maurelli M, Girolomoni G, Gisondi P. Cost per responder of adalimumab biosimilars versus methotrexate in patients with psoriasis: a real-life experience. J Dermatolog Treat. 2023;34:2218504. doi:10.1080/09546634.2023.2218504
- Food and Drug Administration/Center for Drug Evaluation and Research. Expiration of first interchangeable exclusivity (“FIE”) when section 351(l)(6) litigation ends prior to the submission of an application for interchangeability [memorandum]. Published October 3, 2023. Accessed January 18, 2024. https://www.fda.gov/media/173749/download
- US Food & Drug Administration. Biosimilar and interchangeable biologics: more treatment choices. Accessed January 18, 2024. https://www.fda.gov/consumers/consumer-updates/biosimilar-and-interchangeable-biologics-more-treatment-choices
- Chow V, Mytych DT, Das S, et al. Pharmacokinetic similarity of ABP 654, an ustekinumab biosimilar candidate: results from a randomized, double-blind study in healthy subjects [published online July 7, 2023]. Clin Pharmacol Drug Dev. 2023;12:863-873. doi:10.1002/cpdd.1301
- Wezlana (ustekinumab-auub) [prescribing information]. Published October 2023. Accessed January 18, 2024. www.accessdata.fda.gov/drugsatfda_docs/label/2023/761285s000,761331s000lbl.pdf
- ZORYVE (roflumilast) topical cream [prescribing information]. Westlake Village, CA: Arcutis Biotherapeutics. Revised October 2023. Accessed January 18, 2024. https://www.arcutis.com/wp-content/uploads/USPI-roflumilast-cream.pdf
- Lie E, Choi M, Wang SP, et al. Topical management of pediatric psoriasis: a review of new developments and existing therapies. Paediatr Drugs. 2024;26:9-18. doi:10.1007/s40272-023-00592-9
- Engel PV, Smith B, Javadi SS, et al. It is time to consider anew topical algorithm for psoriasis. J Am Acad Dermatol. 2023:S0190-9622(23)02906-7. doi:10.1016/j.jaad.2023.07.1048
Skin Cancer Screening: The Paradox of Melanoma and Improved All-Cause Mortality
In April 2023, the US Preventive Services Task Force (USPSTF) issued a controversial recommendation that the current evidence is insufficient to assess the benefits vs harms of visual skin examination by clinicians for skin cancer screening in adolescents and adults who do not have signs or symptoms of skin cancer.1,2 This recommendation by the USPSTF has not changed in a quarter century,3 but a recent study described an interesting paradox that should trigger wide evaluation and debate.
Patel et al4 analyzed data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program from January 2000 to December 2018 to identify adults with a diagnosis of first primary melanoma in situ (MIS). Overall mortality was then determined through the National Vital Statistics System, which provides cause-of-death information for all deaths in the United States. The authors found 137,872 patients who had 1—and only 1—MIS discovered over the observation period. These patients predominantly were White (96.7%), and the mean (SD) age at diagnosis was 61.9 (16.5) years. During 910,308 total person-years of follow-up (mean [SD], 6.6 [5.1] years), 893 (0.6%) patients died of melanoma and 17,327 (12.6%) died of any cause. The 15-year melanoma-specific standardized mortality rate (SMR) was 1.89 (95% CI, 1.77-2.02), yet the 15-year overall survival relative to matched population controls was 112.4% (95% CI, 112.0%-112.8%), thus all-cause SMR was significantly lower at 0.68 (95% CI, 0.67-0.7). Although MIS was associated with a small increase in cohort melanoma mortality, overall mortality was actually lower than in the general population.4
Patel et al4 did a further broader search that included an additional 18,379 patients who also experienced a second primary melanoma, of which 6751 (36.7%) were invasive and 11,628 (63.3%) were in situ, with a melanoma-specific survival of 98.2% (95% CI, 97.6%-98.5%). Yet relative all-cause survival was significantly higher at 126.7% (95% CI, 125.5%-128.0%). Even among patients in whom a second primary melanoma was invasive, melanoma-specific survival was reduced to 91.1% (95% CI, 90.0%-92.1%), but relative all-cause survival was 116.7% (95% CI, 115%-118.4%). These data in the overall cohort of 155,251 patients showed a discordance between melanoma mortality, which was 4.27-times higher than in the general population (SMR, 4.27; 95% CI, 4.07-4.48), and a lower risk for death from all causes that was approximately 27% lower than in the general population (SMR, 0.73; 95% CI, 0.72-0.74). The authors showed that their findings were not associated with socioeconomic status.4
The analysis by Patel et al4 is now the second study in the literature to show this discordant melanoma survival pattern. In an earlier Australian study of 2452 melanoma patients, Watts et al5 reported that melanoma detection during routine skin checks was associated with a 25% lower all-cause mortality (hazard ratio, 0.75; 95% CI, 0.63-0.90) but not melanoma-specific mortality after multivariable adjustment for a variety of factors including socioeconomic status.These analyses by 2 different groups of investigators have broad implications. Both groups suggested that the improved life span in melanoma patients may be due to health-seeking behavior, which has been defined as “any action undertaken by individuals who perceive themselves to have a health problem or to be ill for the purpose of finding an appropriate remedy.”6
Once treated for melanoma, it is clear that patients are likely to return at regular intervals for thorough full-body skin examinations, but this activity alone could not be responsible for improved all-cause mortality in the face of increased melanoma-specific mortality. It seems the authors are implying a broader concept of good health behavior, originally defined by MacKian7 as encompassing “activities undertaken to maintain good health, to prevent ill health, as well as dealing with any departure from a good state of health,” such as overt behavioral patterns, actions, and habits with the goal of maintenance, restoration, and improvement of one’s health. A variety of behaviors fall within such a definition including smoking cessation, decreased alcohol use, good diet, more physical activity, safe sexual behavior, scheduling physician visits, medication adherence, vaccination, and yes—screening examinations for health problems.8
The concept that individuals who are diagnosed with melanoma fall into a pattern of good health behavior is an interesting hypothesis that must remain speculative until the multiple aspects of good health behavior are rigorously studied. This concept coexists with the hypothesis of melanoma “overdiagnosis”—the idea that many melanomas are detected that will never lead to death.9 Both concepts deserve further analysis. Unquestionably, a randomized controlled trial could never recruit patients willing to undergo long-term untreated observation of their melanomas to test the hypothesis that their melanoma diagnosis would eventually lead to death. Furthermore, Patel et al4 do suggest that even MIS carries a small but measurable increased risk for death from the disease, which is not particularly supportive of the overdiagnosis hypothesis; however, analysis of the concept that improved individual health behavior is at least in part responsible for the first discovery of melanomas is certainly approachable. Here is the key question: Did the melanoma diagnosis trigger a sudden change in multiple aspects of health behavior that led to significant all-cause mortality benefits? The average age of the population studied by Patel et al4 was approximately 62 years. One wonders whether the consequences of a lifetime of established health behavior patterns can be rapidly modified—certainly possible but again remains to be proven by further studies.
Conversely, the alternative hypothesis is that discovery of MIS was the result of active pursuit of self-examination and screening procedures as part of individually ingrained good health behavior over a lifetime. Goodwin et al10 carried out a study in a sample of the Medicare population aged 69 to 90 years looking at men who had prostate cancer screening via prostate-specific antigen measurement and women who had undergone mammography in older age, compared to the contrast population who had not had these screening procedures. They tracked date of death in Medicare enrollment files. They identified 543,970 women and 362,753 men who were aged 69 to 90 years as of January 1, 2003. Patients were stratified by life expectancy based on age and comorbidity. Within each stratum, the patients with cancer screening had higher actual median survival than those who were not screened, with differences ranging from 1.7 to 2.1 years for women and 0.9 to 1.1 years for men.10 These results were not the result of lower prostate or breast cancer mortality. Rather, one surmises that other health factors yielded lower mortality in the screened cohorts.
A full-body skin examination is a time-consuming process. Patients who come to their physician for a routine annual physical don’t expect a skin examination and very few physicians have the time for a long detailed full-body skin examination. When the patient presents to a dermatologist for an examination, it often is because they have real concerns; for example, they may have had a family member who died of skin cancer, or the patient themself may have noticed a worrisome lesion. Patients, not physicians, are the drivers of skin cancer screening, a fact that often is dismissed by those who are not necessarily supportive of the practice.
In light of the findings of Patel et al,4 it is essential that the USPSTF reviews be reanalyzed to compare skin cancer–specific mortality, all-cause mortality, and lifespan in individuals who pursue skin cancer screening; the reanalysis also should not be exclusively limited to survival. With the advent of the immune checkpoint inhibitors, patients with metastatic melanoma are living much longer.11 The burden of living with metastatic cancer must be characterized and measured to have a complete picture and a valid analysis.
After the release of the USPSTF recommendation, there have been calls for large-scale studies to prove the benefits of skin cancer screening.12 Such studies may be valuable; however, if the hypothesis that overall healthy behavior as the major outcome determinant is substantiated, it may prove quite challenging to perform tests of association with specific interventions. It has been shown that skin cancer screening does lead to discovery of more melanomas,13 yet in light of the paradox described by Patel et al,4 it also is likely that causes of death other than melanoma impact overall mortality. Patients who pursue skin examinations may engage in multiple different health activities that are beneficial in the long term, making it difficult to analyze the specific benefit of skin cancer screening in isolation. It may prove difficult to ask patients to omit selected aspects of healthy behavior to try to prove the point. At this time, there is much more work to be done prior to offering opinions on the importance of skin cancer examination in isolation to improve overall health care. In the meantime, dermatologists owe it to our patients to continue to diligently pursue thorough and detailed skin examinations.
- US Preventive Services Task Force; Mangione CM, Barry MJ, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2023;329:1290-1295.
- Henrikson NB, Ivlev I, Blasi PR, et al. Skin cancer screening: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2023;329:1296-1307.
- US Preventive Services Task Force Guide to Clinical Preventive Services. 2nd ed. Agency for Healthcare Research and Quality; 1996.
- Patel VR, Roberson ML, Pignone MP, et al. Risk of mortality after a diagnosis of melanoma in situ. JAMA Dermatol. 2023;169:703-710.
- Watts CG, McLoughlin K, Goumas C, et al. Association between melanoma detected during routine skin checks and mortality. JAMA Dermatol. 2021;157:1425-1436.
- Chrisman NJ. The health seeking process: an approach to the natural history of illness. Cult Med Psychiatry. 1977;1:351-773.
- MacKian S. A review of health seeking behaviour: problems and prospects. health systems development programme. University of Manchester; 2003. Accessed January 19, 2024. https://assets.publishing.service.gov.uk/media/57a08d1de5274a27b200163d/05-03_health_seeking_behaviour.pdf
- Conner M, Norman P. Health behaviour: current issues and challenges. Psychol Health. 2017;32:895-906.
- Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102:605-613.
- Goodwin JS, Sheffield K, Li S, et al. Receipt of cancer screening is a predictor of life expectancy. J Gen Intern Med. 2016;11:1308-1314.
- Johnson DB, Nebhan CA, Moslehi JJ, et al. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol. 2022;19:254-267.
- Adamson AS. The USPSTF statement on skin cancer screening—not a disappointment but an opportunity. JAMA Dermatol. 2023;159:579-581. doi:10.1001/jamadermatol.2023.0706
- Katalinic A, Eisemann N, Waldmann A. Skin cancer screening in Germany. documenting melanoma incidence and mortality from 2008 to 2013. Dtsch Arztebl Int. 2015;112:629-634.
In April 2023, the US Preventive Services Task Force (USPSTF) issued a controversial recommendation that the current evidence is insufficient to assess the benefits vs harms of visual skin examination by clinicians for skin cancer screening in adolescents and adults who do not have signs or symptoms of skin cancer.1,2 This recommendation by the USPSTF has not changed in a quarter century,3 but a recent study described an interesting paradox that should trigger wide evaluation and debate.
Patel et al4 analyzed data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program from January 2000 to December 2018 to identify adults with a diagnosis of first primary melanoma in situ (MIS). Overall mortality was then determined through the National Vital Statistics System, which provides cause-of-death information for all deaths in the United States. The authors found 137,872 patients who had 1—and only 1—MIS discovered over the observation period. These patients predominantly were White (96.7%), and the mean (SD) age at diagnosis was 61.9 (16.5) years. During 910,308 total person-years of follow-up (mean [SD], 6.6 [5.1] years), 893 (0.6%) patients died of melanoma and 17,327 (12.6%) died of any cause. The 15-year melanoma-specific standardized mortality rate (SMR) was 1.89 (95% CI, 1.77-2.02), yet the 15-year overall survival relative to matched population controls was 112.4% (95% CI, 112.0%-112.8%), thus all-cause SMR was significantly lower at 0.68 (95% CI, 0.67-0.7). Although MIS was associated with a small increase in cohort melanoma mortality, overall mortality was actually lower than in the general population.4
Patel et al4 did a further broader search that included an additional 18,379 patients who also experienced a second primary melanoma, of which 6751 (36.7%) were invasive and 11,628 (63.3%) were in situ, with a melanoma-specific survival of 98.2% (95% CI, 97.6%-98.5%). Yet relative all-cause survival was significantly higher at 126.7% (95% CI, 125.5%-128.0%). Even among patients in whom a second primary melanoma was invasive, melanoma-specific survival was reduced to 91.1% (95% CI, 90.0%-92.1%), but relative all-cause survival was 116.7% (95% CI, 115%-118.4%). These data in the overall cohort of 155,251 patients showed a discordance between melanoma mortality, which was 4.27-times higher than in the general population (SMR, 4.27; 95% CI, 4.07-4.48), and a lower risk for death from all causes that was approximately 27% lower than in the general population (SMR, 0.73; 95% CI, 0.72-0.74). The authors showed that their findings were not associated with socioeconomic status.4
The analysis by Patel et al4 is now the second study in the literature to show this discordant melanoma survival pattern. In an earlier Australian study of 2452 melanoma patients, Watts et al5 reported that melanoma detection during routine skin checks was associated with a 25% lower all-cause mortality (hazard ratio, 0.75; 95% CI, 0.63-0.90) but not melanoma-specific mortality after multivariable adjustment for a variety of factors including socioeconomic status.These analyses by 2 different groups of investigators have broad implications. Both groups suggested that the improved life span in melanoma patients may be due to health-seeking behavior, which has been defined as “any action undertaken by individuals who perceive themselves to have a health problem or to be ill for the purpose of finding an appropriate remedy.”6
Once treated for melanoma, it is clear that patients are likely to return at regular intervals for thorough full-body skin examinations, but this activity alone could not be responsible for improved all-cause mortality in the face of increased melanoma-specific mortality. It seems the authors are implying a broader concept of good health behavior, originally defined by MacKian7 as encompassing “activities undertaken to maintain good health, to prevent ill health, as well as dealing with any departure from a good state of health,” such as overt behavioral patterns, actions, and habits with the goal of maintenance, restoration, and improvement of one’s health. A variety of behaviors fall within such a definition including smoking cessation, decreased alcohol use, good diet, more physical activity, safe sexual behavior, scheduling physician visits, medication adherence, vaccination, and yes—screening examinations for health problems.8
The concept that individuals who are diagnosed with melanoma fall into a pattern of good health behavior is an interesting hypothesis that must remain speculative until the multiple aspects of good health behavior are rigorously studied. This concept coexists with the hypothesis of melanoma “overdiagnosis”—the idea that many melanomas are detected that will never lead to death.9 Both concepts deserve further analysis. Unquestionably, a randomized controlled trial could never recruit patients willing to undergo long-term untreated observation of their melanomas to test the hypothesis that their melanoma diagnosis would eventually lead to death. Furthermore, Patel et al4 do suggest that even MIS carries a small but measurable increased risk for death from the disease, which is not particularly supportive of the overdiagnosis hypothesis; however, analysis of the concept that improved individual health behavior is at least in part responsible for the first discovery of melanomas is certainly approachable. Here is the key question: Did the melanoma diagnosis trigger a sudden change in multiple aspects of health behavior that led to significant all-cause mortality benefits? The average age of the population studied by Patel et al4 was approximately 62 years. One wonders whether the consequences of a lifetime of established health behavior patterns can be rapidly modified—certainly possible but again remains to be proven by further studies.
Conversely, the alternative hypothesis is that discovery of MIS was the result of active pursuit of self-examination and screening procedures as part of individually ingrained good health behavior over a lifetime. Goodwin et al10 carried out a study in a sample of the Medicare population aged 69 to 90 years looking at men who had prostate cancer screening via prostate-specific antigen measurement and women who had undergone mammography in older age, compared to the contrast population who had not had these screening procedures. They tracked date of death in Medicare enrollment files. They identified 543,970 women and 362,753 men who were aged 69 to 90 years as of January 1, 2003. Patients were stratified by life expectancy based on age and comorbidity. Within each stratum, the patients with cancer screening had higher actual median survival than those who were not screened, with differences ranging from 1.7 to 2.1 years for women and 0.9 to 1.1 years for men.10 These results were not the result of lower prostate or breast cancer mortality. Rather, one surmises that other health factors yielded lower mortality in the screened cohorts.
A full-body skin examination is a time-consuming process. Patients who come to their physician for a routine annual physical don’t expect a skin examination and very few physicians have the time for a long detailed full-body skin examination. When the patient presents to a dermatologist for an examination, it often is because they have real concerns; for example, they may have had a family member who died of skin cancer, or the patient themself may have noticed a worrisome lesion. Patients, not physicians, are the drivers of skin cancer screening, a fact that often is dismissed by those who are not necessarily supportive of the practice.
In light of the findings of Patel et al,4 it is essential that the USPSTF reviews be reanalyzed to compare skin cancer–specific mortality, all-cause mortality, and lifespan in individuals who pursue skin cancer screening; the reanalysis also should not be exclusively limited to survival. With the advent of the immune checkpoint inhibitors, patients with metastatic melanoma are living much longer.11 The burden of living with metastatic cancer must be characterized and measured to have a complete picture and a valid analysis.
After the release of the USPSTF recommendation, there have been calls for large-scale studies to prove the benefits of skin cancer screening.12 Such studies may be valuable; however, if the hypothesis that overall healthy behavior as the major outcome determinant is substantiated, it may prove quite challenging to perform tests of association with specific interventions. It has been shown that skin cancer screening does lead to discovery of more melanomas,13 yet in light of the paradox described by Patel et al,4 it also is likely that causes of death other than melanoma impact overall mortality. Patients who pursue skin examinations may engage in multiple different health activities that are beneficial in the long term, making it difficult to analyze the specific benefit of skin cancer screening in isolation. It may prove difficult to ask patients to omit selected aspects of healthy behavior to try to prove the point. At this time, there is much more work to be done prior to offering opinions on the importance of skin cancer examination in isolation to improve overall health care. In the meantime, dermatologists owe it to our patients to continue to diligently pursue thorough and detailed skin examinations.
In April 2023, the US Preventive Services Task Force (USPSTF) issued a controversial recommendation that the current evidence is insufficient to assess the benefits vs harms of visual skin examination by clinicians for skin cancer screening in adolescents and adults who do not have signs or symptoms of skin cancer.1,2 This recommendation by the USPSTF has not changed in a quarter century,3 but a recent study described an interesting paradox that should trigger wide evaluation and debate.
Patel et al4 analyzed data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program from January 2000 to December 2018 to identify adults with a diagnosis of first primary melanoma in situ (MIS). Overall mortality was then determined through the National Vital Statistics System, which provides cause-of-death information for all deaths in the United States. The authors found 137,872 patients who had 1—and only 1—MIS discovered over the observation period. These patients predominantly were White (96.7%), and the mean (SD) age at diagnosis was 61.9 (16.5) years. During 910,308 total person-years of follow-up (mean [SD], 6.6 [5.1] years), 893 (0.6%) patients died of melanoma and 17,327 (12.6%) died of any cause. The 15-year melanoma-specific standardized mortality rate (SMR) was 1.89 (95% CI, 1.77-2.02), yet the 15-year overall survival relative to matched population controls was 112.4% (95% CI, 112.0%-112.8%), thus all-cause SMR was significantly lower at 0.68 (95% CI, 0.67-0.7). Although MIS was associated with a small increase in cohort melanoma mortality, overall mortality was actually lower than in the general population.4
Patel et al4 did a further broader search that included an additional 18,379 patients who also experienced a second primary melanoma, of which 6751 (36.7%) were invasive and 11,628 (63.3%) were in situ, with a melanoma-specific survival of 98.2% (95% CI, 97.6%-98.5%). Yet relative all-cause survival was significantly higher at 126.7% (95% CI, 125.5%-128.0%). Even among patients in whom a second primary melanoma was invasive, melanoma-specific survival was reduced to 91.1% (95% CI, 90.0%-92.1%), but relative all-cause survival was 116.7% (95% CI, 115%-118.4%). These data in the overall cohort of 155,251 patients showed a discordance between melanoma mortality, which was 4.27-times higher than in the general population (SMR, 4.27; 95% CI, 4.07-4.48), and a lower risk for death from all causes that was approximately 27% lower than in the general population (SMR, 0.73; 95% CI, 0.72-0.74). The authors showed that their findings were not associated with socioeconomic status.4
The analysis by Patel et al4 is now the second study in the literature to show this discordant melanoma survival pattern. In an earlier Australian study of 2452 melanoma patients, Watts et al5 reported that melanoma detection during routine skin checks was associated with a 25% lower all-cause mortality (hazard ratio, 0.75; 95% CI, 0.63-0.90) but not melanoma-specific mortality after multivariable adjustment for a variety of factors including socioeconomic status.These analyses by 2 different groups of investigators have broad implications. Both groups suggested that the improved life span in melanoma patients may be due to health-seeking behavior, which has been defined as “any action undertaken by individuals who perceive themselves to have a health problem or to be ill for the purpose of finding an appropriate remedy.”6
Once treated for melanoma, it is clear that patients are likely to return at regular intervals for thorough full-body skin examinations, but this activity alone could not be responsible for improved all-cause mortality in the face of increased melanoma-specific mortality. It seems the authors are implying a broader concept of good health behavior, originally defined by MacKian7 as encompassing “activities undertaken to maintain good health, to prevent ill health, as well as dealing with any departure from a good state of health,” such as overt behavioral patterns, actions, and habits with the goal of maintenance, restoration, and improvement of one’s health. A variety of behaviors fall within such a definition including smoking cessation, decreased alcohol use, good diet, more physical activity, safe sexual behavior, scheduling physician visits, medication adherence, vaccination, and yes—screening examinations for health problems.8
The concept that individuals who are diagnosed with melanoma fall into a pattern of good health behavior is an interesting hypothesis that must remain speculative until the multiple aspects of good health behavior are rigorously studied. This concept coexists with the hypothesis of melanoma “overdiagnosis”—the idea that many melanomas are detected that will never lead to death.9 Both concepts deserve further analysis. Unquestionably, a randomized controlled trial could never recruit patients willing to undergo long-term untreated observation of their melanomas to test the hypothesis that their melanoma diagnosis would eventually lead to death. Furthermore, Patel et al4 do suggest that even MIS carries a small but measurable increased risk for death from the disease, which is not particularly supportive of the overdiagnosis hypothesis; however, analysis of the concept that improved individual health behavior is at least in part responsible for the first discovery of melanomas is certainly approachable. Here is the key question: Did the melanoma diagnosis trigger a sudden change in multiple aspects of health behavior that led to significant all-cause mortality benefits? The average age of the population studied by Patel et al4 was approximately 62 years. One wonders whether the consequences of a lifetime of established health behavior patterns can be rapidly modified—certainly possible but again remains to be proven by further studies.
Conversely, the alternative hypothesis is that discovery of MIS was the result of active pursuit of self-examination and screening procedures as part of individually ingrained good health behavior over a lifetime. Goodwin et al10 carried out a study in a sample of the Medicare population aged 69 to 90 years looking at men who had prostate cancer screening via prostate-specific antigen measurement and women who had undergone mammography in older age, compared to the contrast population who had not had these screening procedures. They tracked date of death in Medicare enrollment files. They identified 543,970 women and 362,753 men who were aged 69 to 90 years as of January 1, 2003. Patients were stratified by life expectancy based on age and comorbidity. Within each stratum, the patients with cancer screening had higher actual median survival than those who were not screened, with differences ranging from 1.7 to 2.1 years for women and 0.9 to 1.1 years for men.10 These results were not the result of lower prostate or breast cancer mortality. Rather, one surmises that other health factors yielded lower mortality in the screened cohorts.
A full-body skin examination is a time-consuming process. Patients who come to their physician for a routine annual physical don’t expect a skin examination and very few physicians have the time for a long detailed full-body skin examination. When the patient presents to a dermatologist for an examination, it often is because they have real concerns; for example, they may have had a family member who died of skin cancer, or the patient themself may have noticed a worrisome lesion. Patients, not physicians, are the drivers of skin cancer screening, a fact that often is dismissed by those who are not necessarily supportive of the practice.
In light of the findings of Patel et al,4 it is essential that the USPSTF reviews be reanalyzed to compare skin cancer–specific mortality, all-cause mortality, and lifespan in individuals who pursue skin cancer screening; the reanalysis also should not be exclusively limited to survival. With the advent of the immune checkpoint inhibitors, patients with metastatic melanoma are living much longer.11 The burden of living with metastatic cancer must be characterized and measured to have a complete picture and a valid analysis.
After the release of the USPSTF recommendation, there have been calls for large-scale studies to prove the benefits of skin cancer screening.12 Such studies may be valuable; however, if the hypothesis that overall healthy behavior as the major outcome determinant is substantiated, it may prove quite challenging to perform tests of association with specific interventions. It has been shown that skin cancer screening does lead to discovery of more melanomas,13 yet in light of the paradox described by Patel et al,4 it also is likely that causes of death other than melanoma impact overall mortality. Patients who pursue skin examinations may engage in multiple different health activities that are beneficial in the long term, making it difficult to analyze the specific benefit of skin cancer screening in isolation. It may prove difficult to ask patients to omit selected aspects of healthy behavior to try to prove the point. At this time, there is much more work to be done prior to offering opinions on the importance of skin cancer examination in isolation to improve overall health care. In the meantime, dermatologists owe it to our patients to continue to diligently pursue thorough and detailed skin examinations.
- US Preventive Services Task Force; Mangione CM, Barry MJ, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2023;329:1290-1295.
- Henrikson NB, Ivlev I, Blasi PR, et al. Skin cancer screening: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2023;329:1296-1307.
- US Preventive Services Task Force Guide to Clinical Preventive Services. 2nd ed. Agency for Healthcare Research and Quality; 1996.
- Patel VR, Roberson ML, Pignone MP, et al. Risk of mortality after a diagnosis of melanoma in situ. JAMA Dermatol. 2023;169:703-710.
- Watts CG, McLoughlin K, Goumas C, et al. Association between melanoma detected during routine skin checks and mortality. JAMA Dermatol. 2021;157:1425-1436.
- Chrisman NJ. The health seeking process: an approach to the natural history of illness. Cult Med Psychiatry. 1977;1:351-773.
- MacKian S. A review of health seeking behaviour: problems and prospects. health systems development programme. University of Manchester; 2003. Accessed January 19, 2024. https://assets.publishing.service.gov.uk/media/57a08d1de5274a27b200163d/05-03_health_seeking_behaviour.pdf
- Conner M, Norman P. Health behaviour: current issues and challenges. Psychol Health. 2017;32:895-906.
- Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102:605-613.
- Goodwin JS, Sheffield K, Li S, et al. Receipt of cancer screening is a predictor of life expectancy. J Gen Intern Med. 2016;11:1308-1314.
- Johnson DB, Nebhan CA, Moslehi JJ, et al. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol. 2022;19:254-267.
- Adamson AS. The USPSTF statement on skin cancer screening—not a disappointment but an opportunity. JAMA Dermatol. 2023;159:579-581. doi:10.1001/jamadermatol.2023.0706
- Katalinic A, Eisemann N, Waldmann A. Skin cancer screening in Germany. documenting melanoma incidence and mortality from 2008 to 2013. Dtsch Arztebl Int. 2015;112:629-634.
- US Preventive Services Task Force; Mangione CM, Barry MJ, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2023;329:1290-1295.
- Henrikson NB, Ivlev I, Blasi PR, et al. Skin cancer screening: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2023;329:1296-1307.
- US Preventive Services Task Force Guide to Clinical Preventive Services. 2nd ed. Agency for Healthcare Research and Quality; 1996.
- Patel VR, Roberson ML, Pignone MP, et al. Risk of mortality after a diagnosis of melanoma in situ. JAMA Dermatol. 2023;169:703-710.
- Watts CG, McLoughlin K, Goumas C, et al. Association between melanoma detected during routine skin checks and mortality. JAMA Dermatol. 2021;157:1425-1436.
- Chrisman NJ. The health seeking process: an approach to the natural history of illness. Cult Med Psychiatry. 1977;1:351-773.
- MacKian S. A review of health seeking behaviour: problems and prospects. health systems development programme. University of Manchester; 2003. Accessed January 19, 2024. https://assets.publishing.service.gov.uk/media/57a08d1de5274a27b200163d/05-03_health_seeking_behaviour.pdf
- Conner M, Norman P. Health behaviour: current issues and challenges. Psychol Health. 2017;32:895-906.
- Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102:605-613.
- Goodwin JS, Sheffield K, Li S, et al. Receipt of cancer screening is a predictor of life expectancy. J Gen Intern Med. 2016;11:1308-1314.
- Johnson DB, Nebhan CA, Moslehi JJ, et al. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol. 2022;19:254-267.
- Adamson AS. The USPSTF statement on skin cancer screening—not a disappointment but an opportunity. JAMA Dermatol. 2023;159:579-581. doi:10.1001/jamadermatol.2023.0706
- Katalinic A, Eisemann N, Waldmann A. Skin cancer screening in Germany. documenting melanoma incidence and mortality from 2008 to 2013. Dtsch Arztebl Int. 2015;112:629-634.
Practice Points
- Screening for skin cancer often is performed at the patient’s request.
- Patients who want full-body skin examinations may exhibit other health-promoting behaviors.
- Studies claiming “overdiagnosis” of skin cancer have not previously evaluated all-cause mortality.
Impact of Ketogenic and Low-Glycemic Diets on Inflammatory Skin Conditions
Inflammatory skin conditions often have a relapsing and remitting course and represent a large proportion of chronic skin diseases. Common inflammatory skin disorders include acne, psoriasis, hidradenitis suppurativa (HS), atopic dermatitis (AD), and seborrheic dermatitis (SD).1 Although each of these conditions has a unique pathogenesis, they all are driven by a background of chronic inflammation. It has been reported that diets with high levels of refined carbohydrates and saturated or trans-fatty acids may exacerbate existing inflammation.2 Consequently, dietary interventions, such as the ketogenic and low-glycemic diets, have potential anti-inflammatory and metabolic effects that are being assessed as stand-alone or adjunctive therapies for dermatologic diseases.
Diet may partially influence systemic inflammation through its effect on weight. Higher body mass index and obesity are linked to a low-grade inflammatory state and higher levels of circulating inflammatory markers. Therefore, weight loss leads to decreases in inflammatory cytokines, including C-reactive protein, tumor necrosis factor α, and IL-6.3 These cytokines and metabolic effects overlap with inflammatory skin condition pathways. It also is posited that decreased insulin release associated with weight loss results in decreased sebaceous lipogenesis and androgens, which drive keratinocyte proliferation and acne development.4,5 For instance, in a 2015 meta-analysis of 5 randomized controlled trials on psoriasis, patients in the weight loss intervention group had more substantial reductions in psoriasis area and severity index (PASI) scores compared with controls receiving usual care (P=.004).6 However, in a systematic review of 35 studies on acne vulgaris, overweight and obese patients (defined by a body mass index of ≥23 kg/m2) had similar odds of having acne compared with normal-weight individuals (P=.671).7
Similar to weight loss, ketogenesis acts as a negative feedback mechanism to reduce insulin release, leading to decreased inflammation and androgens that often exacerbate inflammatory skin diseases.8 Ketogenesis ensues when daily carbohydrate intake is limited to less than 50 g, and long-term adherence to a ketogenic diet results in metabolic reliance on ketone bodies such as acetoacetate, β-hydroxybutyrate, and acetone.9 These metabolites may decrease free radical damage and consequently improve signs and symptoms of acne, psoriasis, and other inflammatory skin diseases.10-12 Similarly, increased ketones also may decrease activation of the NLRP3 (NOD-, LRR-, and Pyrin domain-containing protein 3) inflammasome and therefore reduce inflammatory markers such as IL-1β and IL-1.4,13 Several proposed mechanisms are outlined in the Table.

Collectively, low-glycemic and ketogenic diets have been proposed as potential interventions for reducing inflammatory skin conditions. These dietary approaches are hypothesized to exert their effects by facilitating weight loss, elevating ketone levels, and reducing systemic inflammation. The current review summarizes the existing evidence on ketogenic and low-glycemic diets as treatments for inflammatory skin conditions and evaluates the potential benefits of these dietary interventions in managing and improving outcomes for individuals with inflammatory skin conditions.
Methods
Using PubMed for articles indexed for MEDLINE and Google Scholar, a review of the literature was conducted with a combination of the following search terms: low-glycemic diet, inflammatory, dermatologic, ketogenic diet, inflammation, dermatology, acne, psoriasis, eczema, seborrheic dermatitis, and hidradenitis suppurativa. Reference citations in identified works also were reviewed. Interventional (experimental studies or clinical trials), survey-based, and observational studies that investigated the effects of low-glycemic or ketogenic diets for the treatment of inflammatory skin conditions were included. Inclusion criteria were studies assessing acne, psoriasis, SD, AD, and HS. Exclusion criteria were studies published before 1965; those written in languages other than English; and those analyzing other diets, such as the Mediterranean or low-fat diets. The search yielded a total of 11 observational studies and 4 controlled studies published between 1966 and January 2023. Because this analysis utilized publicly available data and did not qualify as human subject research, institutional review board approval was not required.
Results
Acne Vulgaris—Acne vulgaris is a disease of chronic pilosebaceous inflammation and follicular epithelial proliferation associated with Propionibacterium acnes. The association between acne and low-glycemic diets has been examined in several studies. Diet quality is measured and assessed using the glycemic index (GI), which is the effect of a single food on postprandial blood glucose, and the glycemic load, which is the GI adjusted for carbohydrates per serving.14 High levels of GI and glycemic load are associated with hyperinsulinemia and an increase in insulinlike growth factor 1 concentration that promotes
Six survey-based studies evaluated sugar intake in patients with acne compared to healthy matched controls (eTable). Among these studies, 5 reported higher glycemic loads or daily sugar intake in acne patients compared to individuals without acne.12,19,20,26,28 The remaining study was conducted in 1967 and enrolled 16 acne patients and 32 matched controls. It reported no significant difference in sugar intake between the groups (P>.05).17
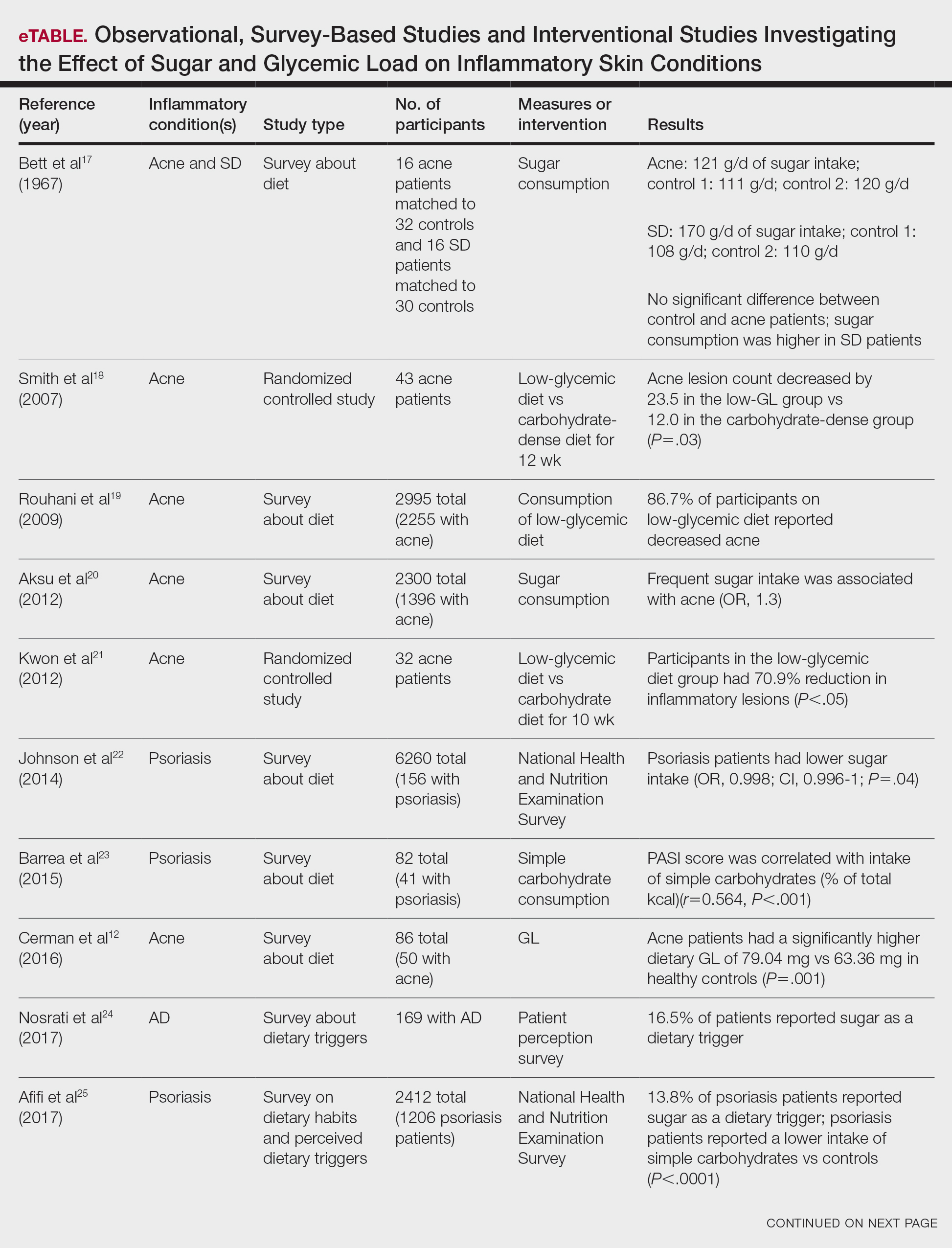

Smith et al18 randomized 43 male patients aged 15 to 25 years with facial acne into 2 cohorts for 12 weeks, each consuming either a low-glycemic diet (25% protein, 45% low-glycemic food [fruits, whole grains], and 30% fat) or a carbohydrate-dense diet of foods with medium to high GI based on prior documentation of the original diet. Patients were instructed to use a noncomedogenic cleanser as their only acne treatment. At 12 weeks, patients consuming the low-glycemic diet had an average of 23.5 fewer inflammatory lesions, while those in the intervention group had 12.0 fewer lesions (P=.03).18
In another controlled study by Kwon et al,21 32 male and female acne patients were randomized to a low-glycemic diet (25% protein, 45% low-glycemic food, and 30% fat) or a standard diet for 10 weeks. Patients on the low-glycemic diet experienced a 70.9% reduction in inflammatory lesions (P<.05). Hematoxylin and eosin staining and image analysis were performed to measure sebaceous gland surface area in the low-glycemic diet group, which decreased from 0.32 to 0.24 mm2 (P=.03). The sebaceous gland surface area in the control group was not reported. Moreover, patients on the low-glycemic diet had reduced IL-8 immunohistochemical staining (decreasing from 2.9 to 1.7 [P=.03]) and sterol regulatory element-binding protein 1 levels (decreasing from 2.6 to 1.3 [P=.03]), suggesting suppression of ongoing inflammation. Patients on the low-glycemic diet had no significant difference in transforming growth factor β1(P=.83). In the control group, there was no difference in IL-8, sterol regulatory element binding protein 1, or transforming growth factor β1 (P>.05) on immunohistochemical staining.21
Psoriasis—Psoriasis is a systemic inflammatory disease characterized by hyperproliferation and aberrant keratinocyte plaque formation. The innate immune response of keratinocytes in response to epidermal damage or infection begins with neutrophil recruitment and dendritic cell activation. Dendritic cell secretion of IL-23 promotes T-cell differentiation into helper T cells (TH1) that subsequently secrete IL-17 and IL-22, thereby stimulating keratinocyte proliferation and eventual plaque formation. The relationship between diet and psoriasis is poorly understood; however, hyperinsulinemia is associated with greater severity of psoriasis.31
Four observational studies examined sugar intake in psoriasis patients. Barrea et al23 conducted a survey-based study of 82 male participants (41 with psoriasis and 41 healthy controls), reporting that PASI score was correlated with intake of simple carbohydrates (percentage of total kilocalorie)(r=0.564, P<.001). Another study by Yamashita et al27 found higher sugar intake in psoriasis patients than controls (P=.003) based on surveys from 70 patients with psoriasis and 70 matched healthy controls.
These findings contrast with 2 survey-based studies by Johnson et al22 and Afifi et al25 of sugar intake in psoriasis patients using the National Health and Nutrition Examination Survey. Johnson et al22 reported reduced sugar intake among 156 psoriasis patients compared with 6104 unmatched controls (odds ratio, 0.998; CI, 0.996-1 [P=.04]) from 2003 to 2006. Similarly, Afifi et al25 reported decreased sugar intake in 1206 psoriasis patients compared with sex- and age-matched controls (P<.0001) in 2009 and 2010. When patients were asked about dietary triggers, 13.8% of psoriasis patients reported sugar as the most common trigger, which was more frequent than alcohol (13.6%), gluten (7.2%), and dairy (6%).25
Castaldo et al29,30 published 2 nonrandomized clinical intervention studies in 2020 and 2021 evaluating the impact of the ketogenic diet on psoriasis. In the first study, 37 psoriasis patients followed a 10-week diet consisting of 4 weeks on a ketogenic diet (500 kcal/d) followed by 6 weeks on a low-caloric Mediterranean diet.29 At the end of the intervention, there was a 17.4% reduction in PASI score, a 33.2-point reduction in itch severity score, and a 13.4-point reduction in the dermatology life quality index score; however, this study did not include a control diet group for comparison.29 The second study included 30 psoriasis patients on a ketogenic diet and 30 control patients without psoriasis on a regular diet.30 The ketogenic diet consisted of 400 to 500 g of vegetables, 20 to 30 g of fat, and a proportion of protein based on body weight with at least 12 g of whey protein and various amino acids. Patients on the ketogenic diet had significant reduction in PASI scores (value relative to clinical features, 1.4916 [P=.007]). Furthermore, concentrations of cytokines IL-2 (P=.04) and IL-1β (P=.006) decreased following the ketogenic diet but were not measured in the control group.30
Seborrheic Dermatitis—Seborrheic dermatitis is associated with overcolonization of Malassezia species near lipid-rich sebaceous glands. Malassezia hydrolyzes free fatty acids, yielding oleic acids and leading to T-cell release of IL-8 and IL-17.32 Literature is sparse regarding how dietary modifications may play a role in disease severity. In a survey study, Bett et al17 compared 16 SD patients to 1:2 matched controls (N=29) to investigate the relationship between sugar consumption and presence of disease. Two control cohorts were selected, 1 from clinic patients diagnosed with verruca and 1 matched by age and sex from a survey-based study at a facility in London, England. Sugar intake was measured both in total grams per day and in “beverage sugar” per day, defined as sugar taken in tea and coffee. There was higher total sugar and higher beverage sugar intake among the SD group compared with both control groups (P<.05).17
Atopic Dermatitis—Atopic dermatitis is a disease of epidermal barrier dysfunction and IgE-mediated allergic sensitization.33 There are several mechanisms by which skin structure may be disrupted. It is well established that filaggrin mutations inhibit stratum corneum maturation and lamellar matrix deposition.34 Upregulation of IL-4–, IL-13–, and IL-17–secreting TH2 cells also is associated with disruption of tight junctions and reduction of filaggrin.35,36 Given that a T cell–mediated inflammatory response is involved in disease pathogenesis, glycemic control is hypothesized to have therapeutic potential.
Nosrati et al24 surveyed 169 AD patients about their perceived dietary triggers through a 61-question survey based on the National Health and Nutrition Examination Survey. Respondents were queried about their perceptions and dietary changes, such as removal or addition of specific food groups and trial of specific diets. Overall, 16.5% of patients reported sugar being a trigger, making it the fourth most common among those surveyed and less common than dairy (24.8%), gluten (18.3%), and alcohol (17.1%).24
Hidradenitis Suppurativa—Hidradenitis suppurativa is driven by hyperkeratosis, dilatation, and occlusion of pilosebaceous follicular ducts, whose eventual rupture evokes a local acute inflammatory response.37 The inciting event for both acne and HS involves mTOR complex–mediated follicular hyperproliferation andinsulinlike growth factor 1 stimulation of androgen receptors in pilosebaceous glands. Given the similarities between the pathogenesis of acne and HS, it is hypothesized that lifestyle changes, including diet modification, may have a beneficial effect on HS.38-40
Comment
Acne—Overall, there is strong evidence supporting the efficacy of a low-glycemic diet in the treatment of acne. Notably, among the 6 observational studies identified, there was 1 conflicting study by Bett et al17 that did not find a statistically significant difference in glucose intake between acne and control patients. However, this study included only 16 acne patients, whereas the other 5 observational studies included 32 to 2255 patients.17 The strongest evidence supporting low-glycemic dietary interventions in acne treatment is from 2 rigorous randomized clinical trials by Kwon et al21 and Smith et al.18 These trials used intention-to-treat models and maintained consistency in gender, age, and acne treatment protocols across both control and treatment groups. To ensure compliance with dietary interventions, daily telephone calls, food logs, and 24-hour urea sampling were utilized. Acne outcomes were assessed by a dermatologist who remained blinded with well-defined outcome measures. An important limitation of these studies is the difficulty in attributing the observed results solely to reduced glucose intake, as low-glycemic diets often lead to other dietary changes, including reduced fat intake and increased nutrient consumption.18,21
A 2022 systematic review of acne by Meixiong et al41 further reinforced the beneficial effects of low-glycemic diets in the management of acne patients. The group reviewed 6 interventional studies and 28 observational studies to investigate the relationship among acne, dairy, and glycemic content and found an association between decreased glucose and dairy on reduction of acne.41
It is likely that the ketogenic diet, which limits glucose, would be beneficial for acne patients. There may be added benefit through elevated ketone bodies and substantially reduced insulin secretion. However, because there are no observational or interventional studies, further research is needed to draw firm conclusions regarding diet for acne treatment. A randomized clinical trial investigating the effects of the ketogenic diet compared to the low-glycemic diet compared to a regular diet would be valuable.
Psoriasis—Among psoriasis studies, there was a lack of consensus regarding glucose intake and correlation with disease. Among the 4 observational studies, 2 reported increased glucose intake among psoriasis patients and 2 reported decreased glucose intake. It is plausible that the variability in studies is due to differences in sample size and diet heterogeneity among study populations. More specifically, Johnson et al22 and Afifi et al25 analyzed large sample sizes of 6260 and 2412 US participants, respectively, and found decreased sugar intake among psoriasis patients compared to controls. In comparison, Barrea et al23 and Yamashita et al27 analyzed substantially smaller and more specific populations consisting of 82 Italian and 140 Japanese participants, respectively; both reported increased glucose intake among psoriasis patients compared to controls. These seemingly antithetical results may be explained by regional dietary differences, with varying proportions of meats, vegetables, antioxidants, and vitamins.
Moreover, the variation among studies may be further explained by the high prevalence of comorbidities among psoriasis patients. In the study by Barrea et al,23 psoriasis patients had higher fasting glucose (P=.004) and insulin (P=.022) levels than healthy patients. After adjusting for body mass index and metabolic syndrome, the correlation coefficient measuring the relationship between the PASI score and intake of simple carbohydrates changed from r=0.564 (P<.001) to r=0.352 (P=.028). The confounding impact of these comorbidities was further highlighted by Yamashita et al,27 who found statistically significant differences in glucose intake between psoriasis and healthy patients (P=.003). However, they reported diminished significance on additional subgroup analysis accounting for potential comorbidities (P=.994).27 Johnson et al22 and Afifi et al25 did not account for comorbidities; therefore, the 4 observational study results must be interpreted cautiously.
The 2 randomized clinical trials by Castaldo et al29,30 weakly suggest that a ketogenic diet may be beneficial for psoriasis patients. The studies have several notable limitations, including insufficient sample sizes and control groups. Thus, the decreased PASI scores reported in psoriasis patients on the ketogenic diets are challenging to interpret. Additionally, both studies placed patients on highly restrictive diets of 500 kcal/d for 4 weeks. The feasibility of recommending such a diet to patients in clinical practice is questionable. Diets of less than 500 kcal/d may be dangerous for patients with underlying comorbidities and are unlikely to serve as long-term solutions.23 To contextualize our findings, a 2022 review by Chung et al42 examined the impact of various diets—low-caloric, gluten-free, Mediterranean, Western, and ketogenic—on psoriasis and reported insufficient evidence to suggest a benefit to the ketogenic diet for psoriasis patients, though the Mediterranean diet may be well suited for psoriasis patients because of improved cardiovascular health and reduced mortality.
Seborrheic Dermatitis—Sanders et al43 found that patients with a high-fruit diet had lower odds of having SD, while those on a Western diet had higher odds of having SD. Although the study did not measure glycemic load, it is conceivable that the high glycemic load characteristic of the Western diet contributed to these findings.43 However, no studies have investigated the direct link between low-glycemic or ketogenic diets and SD, leaving this area open for further study.
Atopic Dermatitis—It has been hypothesized that mitigating T cell–mediated inflammation via glucose control may contribute to the improvement in AD.35,36 However, in one study, 16.5% of AD patients self-identified sugar as a dietary trigger, ranking fourth among other dietary triggers.24 Thus, the connection between glucose levels and AD warrants further exploration.
Hidradenitis Suppurativa—Given the role of metabolic and hormonal influence in HS as well as the overlapping pathophysiology with acne, it is possible that low-glycemic and ketogenic diets may have a role in improving HS.38-40 However, there is a gap in observation and controlled studies investigating the link between low-glycemic or ketogenic diets and HS.
Conclusion
Our analysis focused on interventional and observational research exploring the effects of low-glycemic and ketogenic diets on associations and treatment of inflammatory skin conditions. There is sufficient evidence to counsel acne patients on the benefits of a low-glycemic diet as an adjunctive treatment for acne. Currently, there is insufficient evidence to recommend a low-glycemic or ketogenic diet as a treatment for patients with any other inflammatory skin disease. Prospective and controlled clinical trials are needed to clarify the utility of dietary interventions for treating inflammatory skin conditions.
- Pickett K, Loveman E, Kalita N, et al. Educational interventions to improve quality of life in people with chronic inflammatory skin diseases: systematic reviews of clinical effectiveness and cost-effectiveness. Health Technol Assess. 2015;19:1-176, v-vi.
- Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48:677-685.
- Dowlatshahi EA, van der Voort EA, Arends LR, et al. Markers of systemic inflammation in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2013;169:266-282.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Melnik BC. Acne vulgaris: the metabolic syndrome of the pilosebaceous follicle. Clin Dermatol. 2018;36:29-40.
- Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond). 2015;39:1197-1202.
- Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10:5754.
- Paoli A, Grimaldi K, Toniolo L, et al. Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- Masood W, Annamaraju P, Khan Suheb MZ, et al. Ketogenic diet. StatPearls. StatPearls Publishing; 2023.
- Fomin DA, McDaniel B, Crane J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatolog Treat. 2017;28:484-487.
- Zhang D, Jin W, Wu R, et al. High glucose intake exacerbates autoimmunity through reactive-oxygen-species-mediated TGF-β cytokine activation. Immunity. 2019;51:671-681.e5.
- Cerman AA, Aktas E, Altunay IK, et al. Dietary glycemic factors, insulin resistance, and adiponectin levels in acne vulgaris. J Am Acad Dermatol. 2016;75:155-162.
- Ferrere G, Tidjani Alou M, Liu P, et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight. 2021;6:e145207.
- Burris J, Shikany JM, Rietkerk W, et al. A Low glycemic index and glycemic load diet decreases insulin-like growth factor-1 among adults with moderate and severe acne: a short-duration, 2-week randomized controlled trial. J Acad Nutr Diet. 2018;118:1874-1885.
- Tan JKL, Stein Gold LF, Alexis AF, et al. Current concepts in acne pathogenesis: pathways to inflammation. Semin Cutan Med Surg. 2018;37(3S):S60-S62.
- Kim J, Ochoa MT, Krutzik SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535-1541.
- Bett DG, Morland J, Yudkin J. Sugar consumption in acne vulgaris and seborrhoeic dermatitis. Br Med J. 1967;3:153-155.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Rouhani P, Berman B, Rouhani G. Acne improves with a popular, low glycemic diet from South Beach. J Am Acad Dermatol. 2009;60(Suppl 1):AB14.
- Aksu AE, Metintas S, Saracoglu ZN, et al. Acne: prevalence and relationship with dietary habits in Eskisehir, Turkey. J Eur Acad Dermatol Venereol. 2012;26:1503-1509.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Johnson JA, Ma C, Kanada KN, et al. Diet and nutrition in psoriasis: analysis of the National Health and Nutrition Examination Survey (NHANES) in the United States. J Eur Acad Dermatol Venereol. 2014;28:327-332.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Nosrati A, Afifi L, Danesh MJ, et al. Dietary modifications in atopic dermatitis: patient-reported outcomes. J Dermatolog Treat. 2017;28:523-538.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Burris J, Rietkerk W, Shikany JM, et al. Differences in dietary glycemic load and hormones in New York City adults with no and moderate/severe acne. J Acad Nutr Diet. 2017;117:1375-1383.
- Yamashita H, Morita T, Ito M, et al. Dietary habits in Japanese patients with psoriasis and psoriatic arthritis: low intake of meat in psoriasis and high intake of vitamin A in psoriatic arthritis. J Dermatol. 2019;46:759-769.
- Marson J, Baldwin HE. 12761 Acne, twins, and glycemic index: a sweet pilot study of diet and dietary beliefs. J Am Acad Dermatol. 2020;83(Suppl):AB110.
- Castaldo G, Rastrelli L, Galdo G, et al. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: a proof-of-concept, single-arm, open-label clinical trial. Nutrition. 2020;74:110757.
- Castaldo G, Pagano I, Grimaldi M, et al. Effect of very-low-calorie ketogenic diet on psoriasis patients: a nuclear magnetic resonance-based metabolomic study. J Proteome Res. 2021;20:1509-1521.
- Ip W, Kirchhof MG. Glycemic control in the treatment of psoriasis. Dermatology. 2017;233:23-29.
- Vijaya Chandra SH, Srinivas R, Dawson TL Jr, et al. Cutaneous Malassezia: commensal, pathogen, or protector? Front Cell Infect Microbiol. 2020;10:614446.
- David Boothe W, Tarbox JA, Tarbox MB. Atopic dermatitis: pathophysiology. Adv Exp Med Biol. 2017;1027:21-37.
- Guttman-Yassky E, Hanifin JM, Boguniewicz M, et al. The role of phosphodiesterase 4 in the pathophysiology of atopic dermatitis and the perspective for its inhibition. Exp Dermatol. 2019;28:3-10.
- Furue K, Ito T, Tsuji G, et al. The IL-13–OVOL1–FLG axis in atopic dermatitis. Immunology. 2019;158:281-286.
- Renert-Yuval Y, Guttman-Yassky E. New treatments for atopic dermatitis targeting beyond IL-4/IL-13 cytokines. Ann Allergy Asthma Immunol. 2020;124:28-35.
- Sellheyer K, Krahl D. “Hidradenitis suppurativa” is acne inversa! An appeal to (finally) abandon a misnomer. Int J Dermatol. 2005;44:535-540.
- Danby FW, Margesson LJ. Hidradenitis suppurativa. Dermatol Clin. 2010;28:779-793.
- Fernandez JM, Marr KD, Hendricks AJ, et al. Alleviating and exacerbating foods in hidradenitis suppurativa. Dermatol Ther. 2020;33:E14246.
- Yamanaka-Takaichi M, Revankar R, Shih T, et al. Expert consensus on priority research gaps in dietary and lifestyle factors in hidradenitis suppurativa: a Delphi consensus study. Arch Dermatol Res. 2023;315:2129-2136.
- Meixiong J, Ricco C, Vasavda C, et al. Diet and acne: a systematic review. JAAD Int. 2022;7:95-112.
- Chung M, Bartholomew E, Yeroushalmi S, et al. Dietary intervention and supplements in the management of psoriasis: current perspectives. Psoriasis (Auckland). 2022;12:151-176. doi:10.2147/PTT.S328581
- Sanders MGH, Pardo LM, Ginger RS, et al. Association between diet and seborrheic dermatitis: a cross-sectional study. J Invest Dermatol. 2019;139:108-114.
Inflammatory skin conditions often have a relapsing and remitting course and represent a large proportion of chronic skin diseases. Common inflammatory skin disorders include acne, psoriasis, hidradenitis suppurativa (HS), atopic dermatitis (AD), and seborrheic dermatitis (SD).1 Although each of these conditions has a unique pathogenesis, they all are driven by a background of chronic inflammation. It has been reported that diets with high levels of refined carbohydrates and saturated or trans-fatty acids may exacerbate existing inflammation.2 Consequently, dietary interventions, such as the ketogenic and low-glycemic diets, have potential anti-inflammatory and metabolic effects that are being assessed as stand-alone or adjunctive therapies for dermatologic diseases.
Diet may partially influence systemic inflammation through its effect on weight. Higher body mass index and obesity are linked to a low-grade inflammatory state and higher levels of circulating inflammatory markers. Therefore, weight loss leads to decreases in inflammatory cytokines, including C-reactive protein, tumor necrosis factor α, and IL-6.3 These cytokines and metabolic effects overlap with inflammatory skin condition pathways. It also is posited that decreased insulin release associated with weight loss results in decreased sebaceous lipogenesis and androgens, which drive keratinocyte proliferation and acne development.4,5 For instance, in a 2015 meta-analysis of 5 randomized controlled trials on psoriasis, patients in the weight loss intervention group had more substantial reductions in psoriasis area and severity index (PASI) scores compared with controls receiving usual care (P=.004).6 However, in a systematic review of 35 studies on acne vulgaris, overweight and obese patients (defined by a body mass index of ≥23 kg/m2) had similar odds of having acne compared with normal-weight individuals (P=.671).7
Similar to weight loss, ketogenesis acts as a negative feedback mechanism to reduce insulin release, leading to decreased inflammation and androgens that often exacerbate inflammatory skin diseases.8 Ketogenesis ensues when daily carbohydrate intake is limited to less than 50 g, and long-term adherence to a ketogenic diet results in metabolic reliance on ketone bodies such as acetoacetate, β-hydroxybutyrate, and acetone.9 These metabolites may decrease free radical damage and consequently improve signs and symptoms of acne, psoriasis, and other inflammatory skin diseases.10-12 Similarly, increased ketones also may decrease activation of the NLRP3 (NOD-, LRR-, and Pyrin domain-containing protein 3) inflammasome and therefore reduce inflammatory markers such as IL-1β and IL-1.4,13 Several proposed mechanisms are outlined in the Table.

Collectively, low-glycemic and ketogenic diets have been proposed as potential interventions for reducing inflammatory skin conditions. These dietary approaches are hypothesized to exert their effects by facilitating weight loss, elevating ketone levels, and reducing systemic inflammation. The current review summarizes the existing evidence on ketogenic and low-glycemic diets as treatments for inflammatory skin conditions and evaluates the potential benefits of these dietary interventions in managing and improving outcomes for individuals with inflammatory skin conditions.
Methods
Using PubMed for articles indexed for MEDLINE and Google Scholar, a review of the literature was conducted with a combination of the following search terms: low-glycemic diet, inflammatory, dermatologic, ketogenic diet, inflammation, dermatology, acne, psoriasis, eczema, seborrheic dermatitis, and hidradenitis suppurativa. Reference citations in identified works also were reviewed. Interventional (experimental studies or clinical trials), survey-based, and observational studies that investigated the effects of low-glycemic or ketogenic diets for the treatment of inflammatory skin conditions were included. Inclusion criteria were studies assessing acne, psoriasis, SD, AD, and HS. Exclusion criteria were studies published before 1965; those written in languages other than English; and those analyzing other diets, such as the Mediterranean or low-fat diets. The search yielded a total of 11 observational studies and 4 controlled studies published between 1966 and January 2023. Because this analysis utilized publicly available data and did not qualify as human subject research, institutional review board approval was not required.
Results
Acne Vulgaris—Acne vulgaris is a disease of chronic pilosebaceous inflammation and follicular epithelial proliferation associated with Propionibacterium acnes. The association between acne and low-glycemic diets has been examined in several studies. Diet quality is measured and assessed using the glycemic index (GI), which is the effect of a single food on postprandial blood glucose, and the glycemic load, which is the GI adjusted for carbohydrates per serving.14 High levels of GI and glycemic load are associated with hyperinsulinemia and an increase in insulinlike growth factor 1 concentration that promotes
Six survey-based studies evaluated sugar intake in patients with acne compared to healthy matched controls (eTable). Among these studies, 5 reported higher glycemic loads or daily sugar intake in acne patients compared to individuals without acne.12,19,20,26,28 The remaining study was conducted in 1967 and enrolled 16 acne patients and 32 matched controls. It reported no significant difference in sugar intake between the groups (P>.05).17


Smith et al18 randomized 43 male patients aged 15 to 25 years with facial acne into 2 cohorts for 12 weeks, each consuming either a low-glycemic diet (25% protein, 45% low-glycemic food [fruits, whole grains], and 30% fat) or a carbohydrate-dense diet of foods with medium to high GI based on prior documentation of the original diet. Patients were instructed to use a noncomedogenic cleanser as their only acne treatment. At 12 weeks, patients consuming the low-glycemic diet had an average of 23.5 fewer inflammatory lesions, while those in the intervention group had 12.0 fewer lesions (P=.03).18
In another controlled study by Kwon et al,21 32 male and female acne patients were randomized to a low-glycemic diet (25% protein, 45% low-glycemic food, and 30% fat) or a standard diet for 10 weeks. Patients on the low-glycemic diet experienced a 70.9% reduction in inflammatory lesions (P<.05). Hematoxylin and eosin staining and image analysis were performed to measure sebaceous gland surface area in the low-glycemic diet group, which decreased from 0.32 to 0.24 mm2 (P=.03). The sebaceous gland surface area in the control group was not reported. Moreover, patients on the low-glycemic diet had reduced IL-8 immunohistochemical staining (decreasing from 2.9 to 1.7 [P=.03]) and sterol regulatory element-binding protein 1 levels (decreasing from 2.6 to 1.3 [P=.03]), suggesting suppression of ongoing inflammation. Patients on the low-glycemic diet had no significant difference in transforming growth factor β1(P=.83). In the control group, there was no difference in IL-8, sterol regulatory element binding protein 1, or transforming growth factor β1 (P>.05) on immunohistochemical staining.21
Psoriasis—Psoriasis is a systemic inflammatory disease characterized by hyperproliferation and aberrant keratinocyte plaque formation. The innate immune response of keratinocytes in response to epidermal damage or infection begins with neutrophil recruitment and dendritic cell activation. Dendritic cell secretion of IL-23 promotes T-cell differentiation into helper T cells (TH1) that subsequently secrete IL-17 and IL-22, thereby stimulating keratinocyte proliferation and eventual plaque formation. The relationship between diet and psoriasis is poorly understood; however, hyperinsulinemia is associated with greater severity of psoriasis.31
Four observational studies examined sugar intake in psoriasis patients. Barrea et al23 conducted a survey-based study of 82 male participants (41 with psoriasis and 41 healthy controls), reporting that PASI score was correlated with intake of simple carbohydrates (percentage of total kilocalorie)(r=0.564, P<.001). Another study by Yamashita et al27 found higher sugar intake in psoriasis patients than controls (P=.003) based on surveys from 70 patients with psoriasis and 70 matched healthy controls.
These findings contrast with 2 survey-based studies by Johnson et al22 and Afifi et al25 of sugar intake in psoriasis patients using the National Health and Nutrition Examination Survey. Johnson et al22 reported reduced sugar intake among 156 psoriasis patients compared with 6104 unmatched controls (odds ratio, 0.998; CI, 0.996-1 [P=.04]) from 2003 to 2006. Similarly, Afifi et al25 reported decreased sugar intake in 1206 psoriasis patients compared with sex- and age-matched controls (P<.0001) in 2009 and 2010. When patients were asked about dietary triggers, 13.8% of psoriasis patients reported sugar as the most common trigger, which was more frequent than alcohol (13.6%), gluten (7.2%), and dairy (6%).25
Castaldo et al29,30 published 2 nonrandomized clinical intervention studies in 2020 and 2021 evaluating the impact of the ketogenic diet on psoriasis. In the first study, 37 psoriasis patients followed a 10-week diet consisting of 4 weeks on a ketogenic diet (500 kcal/d) followed by 6 weeks on a low-caloric Mediterranean diet.29 At the end of the intervention, there was a 17.4% reduction in PASI score, a 33.2-point reduction in itch severity score, and a 13.4-point reduction in the dermatology life quality index score; however, this study did not include a control diet group for comparison.29 The second study included 30 psoriasis patients on a ketogenic diet and 30 control patients without psoriasis on a regular diet.30 The ketogenic diet consisted of 400 to 500 g of vegetables, 20 to 30 g of fat, and a proportion of protein based on body weight with at least 12 g of whey protein and various amino acids. Patients on the ketogenic diet had significant reduction in PASI scores (value relative to clinical features, 1.4916 [P=.007]). Furthermore, concentrations of cytokines IL-2 (P=.04) and IL-1β (P=.006) decreased following the ketogenic diet but were not measured in the control group.30
Seborrheic Dermatitis—Seborrheic dermatitis is associated with overcolonization of Malassezia species near lipid-rich sebaceous glands. Malassezia hydrolyzes free fatty acids, yielding oleic acids and leading to T-cell release of IL-8 and IL-17.32 Literature is sparse regarding how dietary modifications may play a role in disease severity. In a survey study, Bett et al17 compared 16 SD patients to 1:2 matched controls (N=29) to investigate the relationship between sugar consumption and presence of disease. Two control cohorts were selected, 1 from clinic patients diagnosed with verruca and 1 matched by age and sex from a survey-based study at a facility in London, England. Sugar intake was measured both in total grams per day and in “beverage sugar” per day, defined as sugar taken in tea and coffee. There was higher total sugar and higher beverage sugar intake among the SD group compared with both control groups (P<.05).17
Atopic Dermatitis—Atopic dermatitis is a disease of epidermal barrier dysfunction and IgE-mediated allergic sensitization.33 There are several mechanisms by which skin structure may be disrupted. It is well established that filaggrin mutations inhibit stratum corneum maturation and lamellar matrix deposition.34 Upregulation of IL-4–, IL-13–, and IL-17–secreting TH2 cells also is associated with disruption of tight junctions and reduction of filaggrin.35,36 Given that a T cell–mediated inflammatory response is involved in disease pathogenesis, glycemic control is hypothesized to have therapeutic potential.
Nosrati et al24 surveyed 169 AD patients about their perceived dietary triggers through a 61-question survey based on the National Health and Nutrition Examination Survey. Respondents were queried about their perceptions and dietary changes, such as removal or addition of specific food groups and trial of specific diets. Overall, 16.5% of patients reported sugar being a trigger, making it the fourth most common among those surveyed and less common than dairy (24.8%), gluten (18.3%), and alcohol (17.1%).24
Hidradenitis Suppurativa—Hidradenitis suppurativa is driven by hyperkeratosis, dilatation, and occlusion of pilosebaceous follicular ducts, whose eventual rupture evokes a local acute inflammatory response.37 The inciting event for both acne and HS involves mTOR complex–mediated follicular hyperproliferation andinsulinlike growth factor 1 stimulation of androgen receptors in pilosebaceous glands. Given the similarities between the pathogenesis of acne and HS, it is hypothesized that lifestyle changes, including diet modification, may have a beneficial effect on HS.38-40
Comment
Acne—Overall, there is strong evidence supporting the efficacy of a low-glycemic diet in the treatment of acne. Notably, among the 6 observational studies identified, there was 1 conflicting study by Bett et al17 that did not find a statistically significant difference in glucose intake between acne and control patients. However, this study included only 16 acne patients, whereas the other 5 observational studies included 32 to 2255 patients.17 The strongest evidence supporting low-glycemic dietary interventions in acne treatment is from 2 rigorous randomized clinical trials by Kwon et al21 and Smith et al.18 These trials used intention-to-treat models and maintained consistency in gender, age, and acne treatment protocols across both control and treatment groups. To ensure compliance with dietary interventions, daily telephone calls, food logs, and 24-hour urea sampling were utilized. Acne outcomes were assessed by a dermatologist who remained blinded with well-defined outcome measures. An important limitation of these studies is the difficulty in attributing the observed results solely to reduced glucose intake, as low-glycemic diets often lead to other dietary changes, including reduced fat intake and increased nutrient consumption.18,21
A 2022 systematic review of acne by Meixiong et al41 further reinforced the beneficial effects of low-glycemic diets in the management of acne patients. The group reviewed 6 interventional studies and 28 observational studies to investigate the relationship among acne, dairy, and glycemic content and found an association between decreased glucose and dairy on reduction of acne.41
It is likely that the ketogenic diet, which limits glucose, would be beneficial for acne patients. There may be added benefit through elevated ketone bodies and substantially reduced insulin secretion. However, because there are no observational or interventional studies, further research is needed to draw firm conclusions regarding diet for acne treatment. A randomized clinical trial investigating the effects of the ketogenic diet compared to the low-glycemic diet compared to a regular diet would be valuable.
Psoriasis—Among psoriasis studies, there was a lack of consensus regarding glucose intake and correlation with disease. Among the 4 observational studies, 2 reported increased glucose intake among psoriasis patients and 2 reported decreased glucose intake. It is plausible that the variability in studies is due to differences in sample size and diet heterogeneity among study populations. More specifically, Johnson et al22 and Afifi et al25 analyzed large sample sizes of 6260 and 2412 US participants, respectively, and found decreased sugar intake among psoriasis patients compared to controls. In comparison, Barrea et al23 and Yamashita et al27 analyzed substantially smaller and more specific populations consisting of 82 Italian and 140 Japanese participants, respectively; both reported increased glucose intake among psoriasis patients compared to controls. These seemingly antithetical results may be explained by regional dietary differences, with varying proportions of meats, vegetables, antioxidants, and vitamins.
Moreover, the variation among studies may be further explained by the high prevalence of comorbidities among psoriasis patients. In the study by Barrea et al,23 psoriasis patients had higher fasting glucose (P=.004) and insulin (P=.022) levels than healthy patients. After adjusting for body mass index and metabolic syndrome, the correlation coefficient measuring the relationship between the PASI score and intake of simple carbohydrates changed from r=0.564 (P<.001) to r=0.352 (P=.028). The confounding impact of these comorbidities was further highlighted by Yamashita et al,27 who found statistically significant differences in glucose intake between psoriasis and healthy patients (P=.003). However, they reported diminished significance on additional subgroup analysis accounting for potential comorbidities (P=.994).27 Johnson et al22 and Afifi et al25 did not account for comorbidities; therefore, the 4 observational study results must be interpreted cautiously.
The 2 randomized clinical trials by Castaldo et al29,30 weakly suggest that a ketogenic diet may be beneficial for psoriasis patients. The studies have several notable limitations, including insufficient sample sizes and control groups. Thus, the decreased PASI scores reported in psoriasis patients on the ketogenic diets are challenging to interpret. Additionally, both studies placed patients on highly restrictive diets of 500 kcal/d for 4 weeks. The feasibility of recommending such a diet to patients in clinical practice is questionable. Diets of less than 500 kcal/d may be dangerous for patients with underlying comorbidities and are unlikely to serve as long-term solutions.23 To contextualize our findings, a 2022 review by Chung et al42 examined the impact of various diets—low-caloric, gluten-free, Mediterranean, Western, and ketogenic—on psoriasis and reported insufficient evidence to suggest a benefit to the ketogenic diet for psoriasis patients, though the Mediterranean diet may be well suited for psoriasis patients because of improved cardiovascular health and reduced mortality.
Seborrheic Dermatitis—Sanders et al43 found that patients with a high-fruit diet had lower odds of having SD, while those on a Western diet had higher odds of having SD. Although the study did not measure glycemic load, it is conceivable that the high glycemic load characteristic of the Western diet contributed to these findings.43 However, no studies have investigated the direct link between low-glycemic or ketogenic diets and SD, leaving this area open for further study.
Atopic Dermatitis—It has been hypothesized that mitigating T cell–mediated inflammation via glucose control may contribute to the improvement in AD.35,36 However, in one study, 16.5% of AD patients self-identified sugar as a dietary trigger, ranking fourth among other dietary triggers.24 Thus, the connection between glucose levels and AD warrants further exploration.
Hidradenitis Suppurativa—Given the role of metabolic and hormonal influence in HS as well as the overlapping pathophysiology with acne, it is possible that low-glycemic and ketogenic diets may have a role in improving HS.38-40 However, there is a gap in observation and controlled studies investigating the link between low-glycemic or ketogenic diets and HS.
Conclusion
Our analysis focused on interventional and observational research exploring the effects of low-glycemic and ketogenic diets on associations and treatment of inflammatory skin conditions. There is sufficient evidence to counsel acne patients on the benefits of a low-glycemic diet as an adjunctive treatment for acne. Currently, there is insufficient evidence to recommend a low-glycemic or ketogenic diet as a treatment for patients with any other inflammatory skin disease. Prospective and controlled clinical trials are needed to clarify the utility of dietary interventions for treating inflammatory skin conditions.
Inflammatory skin conditions often have a relapsing and remitting course and represent a large proportion of chronic skin diseases. Common inflammatory skin disorders include acne, psoriasis, hidradenitis suppurativa (HS), atopic dermatitis (AD), and seborrheic dermatitis (SD).1 Although each of these conditions has a unique pathogenesis, they all are driven by a background of chronic inflammation. It has been reported that diets with high levels of refined carbohydrates and saturated or trans-fatty acids may exacerbate existing inflammation.2 Consequently, dietary interventions, such as the ketogenic and low-glycemic diets, have potential anti-inflammatory and metabolic effects that are being assessed as stand-alone or adjunctive therapies for dermatologic diseases.
Diet may partially influence systemic inflammation through its effect on weight. Higher body mass index and obesity are linked to a low-grade inflammatory state and higher levels of circulating inflammatory markers. Therefore, weight loss leads to decreases in inflammatory cytokines, including C-reactive protein, tumor necrosis factor α, and IL-6.3 These cytokines and metabolic effects overlap with inflammatory skin condition pathways. It also is posited that decreased insulin release associated with weight loss results in decreased sebaceous lipogenesis and androgens, which drive keratinocyte proliferation and acne development.4,5 For instance, in a 2015 meta-analysis of 5 randomized controlled trials on psoriasis, patients in the weight loss intervention group had more substantial reductions in psoriasis area and severity index (PASI) scores compared with controls receiving usual care (P=.004).6 However, in a systematic review of 35 studies on acne vulgaris, overweight and obese patients (defined by a body mass index of ≥23 kg/m2) had similar odds of having acne compared with normal-weight individuals (P=.671).7
Similar to weight loss, ketogenesis acts as a negative feedback mechanism to reduce insulin release, leading to decreased inflammation and androgens that often exacerbate inflammatory skin diseases.8 Ketogenesis ensues when daily carbohydrate intake is limited to less than 50 g, and long-term adherence to a ketogenic diet results in metabolic reliance on ketone bodies such as acetoacetate, β-hydroxybutyrate, and acetone.9 These metabolites may decrease free radical damage and consequently improve signs and symptoms of acne, psoriasis, and other inflammatory skin diseases.10-12 Similarly, increased ketones also may decrease activation of the NLRP3 (NOD-, LRR-, and Pyrin domain-containing protein 3) inflammasome and therefore reduce inflammatory markers such as IL-1β and IL-1.4,13 Several proposed mechanisms are outlined in the Table.

Collectively, low-glycemic and ketogenic diets have been proposed as potential interventions for reducing inflammatory skin conditions. These dietary approaches are hypothesized to exert their effects by facilitating weight loss, elevating ketone levels, and reducing systemic inflammation. The current review summarizes the existing evidence on ketogenic and low-glycemic diets as treatments for inflammatory skin conditions and evaluates the potential benefits of these dietary interventions in managing and improving outcomes for individuals with inflammatory skin conditions.
Methods
Using PubMed for articles indexed for MEDLINE and Google Scholar, a review of the literature was conducted with a combination of the following search terms: low-glycemic diet, inflammatory, dermatologic, ketogenic diet, inflammation, dermatology, acne, psoriasis, eczema, seborrheic dermatitis, and hidradenitis suppurativa. Reference citations in identified works also were reviewed. Interventional (experimental studies or clinical trials), survey-based, and observational studies that investigated the effects of low-glycemic or ketogenic diets for the treatment of inflammatory skin conditions were included. Inclusion criteria were studies assessing acne, psoriasis, SD, AD, and HS. Exclusion criteria were studies published before 1965; those written in languages other than English; and those analyzing other diets, such as the Mediterranean or low-fat diets. The search yielded a total of 11 observational studies and 4 controlled studies published between 1966 and January 2023. Because this analysis utilized publicly available data and did not qualify as human subject research, institutional review board approval was not required.
Results
Acne Vulgaris—Acne vulgaris is a disease of chronic pilosebaceous inflammation and follicular epithelial proliferation associated with Propionibacterium acnes. The association between acne and low-glycemic diets has been examined in several studies. Diet quality is measured and assessed using the glycemic index (GI), which is the effect of a single food on postprandial blood glucose, and the glycemic load, which is the GI adjusted for carbohydrates per serving.14 High levels of GI and glycemic load are associated with hyperinsulinemia and an increase in insulinlike growth factor 1 concentration that promotes
Six survey-based studies evaluated sugar intake in patients with acne compared to healthy matched controls (eTable). Among these studies, 5 reported higher glycemic loads or daily sugar intake in acne patients compared to individuals without acne.12,19,20,26,28 The remaining study was conducted in 1967 and enrolled 16 acne patients and 32 matched controls. It reported no significant difference in sugar intake between the groups (P>.05).17


Smith et al18 randomized 43 male patients aged 15 to 25 years with facial acne into 2 cohorts for 12 weeks, each consuming either a low-glycemic diet (25% protein, 45% low-glycemic food [fruits, whole grains], and 30% fat) or a carbohydrate-dense diet of foods with medium to high GI based on prior documentation of the original diet. Patients were instructed to use a noncomedogenic cleanser as their only acne treatment. At 12 weeks, patients consuming the low-glycemic diet had an average of 23.5 fewer inflammatory lesions, while those in the intervention group had 12.0 fewer lesions (P=.03).18
In another controlled study by Kwon et al,21 32 male and female acne patients were randomized to a low-glycemic diet (25% protein, 45% low-glycemic food, and 30% fat) or a standard diet for 10 weeks. Patients on the low-glycemic diet experienced a 70.9% reduction in inflammatory lesions (P<.05). Hematoxylin and eosin staining and image analysis were performed to measure sebaceous gland surface area in the low-glycemic diet group, which decreased from 0.32 to 0.24 mm2 (P=.03). The sebaceous gland surface area in the control group was not reported. Moreover, patients on the low-glycemic diet had reduced IL-8 immunohistochemical staining (decreasing from 2.9 to 1.7 [P=.03]) and sterol regulatory element-binding protein 1 levels (decreasing from 2.6 to 1.3 [P=.03]), suggesting suppression of ongoing inflammation. Patients on the low-glycemic diet had no significant difference in transforming growth factor β1(P=.83). In the control group, there was no difference in IL-8, sterol regulatory element binding protein 1, or transforming growth factor β1 (P>.05) on immunohistochemical staining.21
Psoriasis—Psoriasis is a systemic inflammatory disease characterized by hyperproliferation and aberrant keratinocyte plaque formation. The innate immune response of keratinocytes in response to epidermal damage or infection begins with neutrophil recruitment and dendritic cell activation. Dendritic cell secretion of IL-23 promotes T-cell differentiation into helper T cells (TH1) that subsequently secrete IL-17 and IL-22, thereby stimulating keratinocyte proliferation and eventual plaque formation. The relationship between diet and psoriasis is poorly understood; however, hyperinsulinemia is associated with greater severity of psoriasis.31
Four observational studies examined sugar intake in psoriasis patients. Barrea et al23 conducted a survey-based study of 82 male participants (41 with psoriasis and 41 healthy controls), reporting that PASI score was correlated with intake of simple carbohydrates (percentage of total kilocalorie)(r=0.564, P<.001). Another study by Yamashita et al27 found higher sugar intake in psoriasis patients than controls (P=.003) based on surveys from 70 patients with psoriasis and 70 matched healthy controls.
These findings contrast with 2 survey-based studies by Johnson et al22 and Afifi et al25 of sugar intake in psoriasis patients using the National Health and Nutrition Examination Survey. Johnson et al22 reported reduced sugar intake among 156 psoriasis patients compared with 6104 unmatched controls (odds ratio, 0.998; CI, 0.996-1 [P=.04]) from 2003 to 2006. Similarly, Afifi et al25 reported decreased sugar intake in 1206 psoriasis patients compared with sex- and age-matched controls (P<.0001) in 2009 and 2010. When patients were asked about dietary triggers, 13.8% of psoriasis patients reported sugar as the most common trigger, which was more frequent than alcohol (13.6%), gluten (7.2%), and dairy (6%).25
Castaldo et al29,30 published 2 nonrandomized clinical intervention studies in 2020 and 2021 evaluating the impact of the ketogenic diet on psoriasis. In the first study, 37 psoriasis patients followed a 10-week diet consisting of 4 weeks on a ketogenic diet (500 kcal/d) followed by 6 weeks on a low-caloric Mediterranean diet.29 At the end of the intervention, there was a 17.4% reduction in PASI score, a 33.2-point reduction in itch severity score, and a 13.4-point reduction in the dermatology life quality index score; however, this study did not include a control diet group for comparison.29 The second study included 30 psoriasis patients on a ketogenic diet and 30 control patients without psoriasis on a regular diet.30 The ketogenic diet consisted of 400 to 500 g of vegetables, 20 to 30 g of fat, and a proportion of protein based on body weight with at least 12 g of whey protein and various amino acids. Patients on the ketogenic diet had significant reduction in PASI scores (value relative to clinical features, 1.4916 [P=.007]). Furthermore, concentrations of cytokines IL-2 (P=.04) and IL-1β (P=.006) decreased following the ketogenic diet but were not measured in the control group.30
Seborrheic Dermatitis—Seborrheic dermatitis is associated with overcolonization of Malassezia species near lipid-rich sebaceous glands. Malassezia hydrolyzes free fatty acids, yielding oleic acids and leading to T-cell release of IL-8 and IL-17.32 Literature is sparse regarding how dietary modifications may play a role in disease severity. In a survey study, Bett et al17 compared 16 SD patients to 1:2 matched controls (N=29) to investigate the relationship between sugar consumption and presence of disease. Two control cohorts were selected, 1 from clinic patients diagnosed with verruca and 1 matched by age and sex from a survey-based study at a facility in London, England. Sugar intake was measured both in total grams per day and in “beverage sugar” per day, defined as sugar taken in tea and coffee. There was higher total sugar and higher beverage sugar intake among the SD group compared with both control groups (P<.05).17
Atopic Dermatitis—Atopic dermatitis is a disease of epidermal barrier dysfunction and IgE-mediated allergic sensitization.33 There are several mechanisms by which skin structure may be disrupted. It is well established that filaggrin mutations inhibit stratum corneum maturation and lamellar matrix deposition.34 Upregulation of IL-4–, IL-13–, and IL-17–secreting TH2 cells also is associated with disruption of tight junctions and reduction of filaggrin.35,36 Given that a T cell–mediated inflammatory response is involved in disease pathogenesis, glycemic control is hypothesized to have therapeutic potential.
Nosrati et al24 surveyed 169 AD patients about their perceived dietary triggers through a 61-question survey based on the National Health and Nutrition Examination Survey. Respondents were queried about their perceptions and dietary changes, such as removal or addition of specific food groups and trial of specific diets. Overall, 16.5% of patients reported sugar being a trigger, making it the fourth most common among those surveyed and less common than dairy (24.8%), gluten (18.3%), and alcohol (17.1%).24
Hidradenitis Suppurativa—Hidradenitis suppurativa is driven by hyperkeratosis, dilatation, and occlusion of pilosebaceous follicular ducts, whose eventual rupture evokes a local acute inflammatory response.37 The inciting event for both acne and HS involves mTOR complex–mediated follicular hyperproliferation andinsulinlike growth factor 1 stimulation of androgen receptors in pilosebaceous glands. Given the similarities between the pathogenesis of acne and HS, it is hypothesized that lifestyle changes, including diet modification, may have a beneficial effect on HS.38-40
Comment
Acne—Overall, there is strong evidence supporting the efficacy of a low-glycemic diet in the treatment of acne. Notably, among the 6 observational studies identified, there was 1 conflicting study by Bett et al17 that did not find a statistically significant difference in glucose intake between acne and control patients. However, this study included only 16 acne patients, whereas the other 5 observational studies included 32 to 2255 patients.17 The strongest evidence supporting low-glycemic dietary interventions in acne treatment is from 2 rigorous randomized clinical trials by Kwon et al21 and Smith et al.18 These trials used intention-to-treat models and maintained consistency in gender, age, and acne treatment protocols across both control and treatment groups. To ensure compliance with dietary interventions, daily telephone calls, food logs, and 24-hour urea sampling were utilized. Acne outcomes were assessed by a dermatologist who remained blinded with well-defined outcome measures. An important limitation of these studies is the difficulty in attributing the observed results solely to reduced glucose intake, as low-glycemic diets often lead to other dietary changes, including reduced fat intake and increased nutrient consumption.18,21
A 2022 systematic review of acne by Meixiong et al41 further reinforced the beneficial effects of low-glycemic diets in the management of acne patients. The group reviewed 6 interventional studies and 28 observational studies to investigate the relationship among acne, dairy, and glycemic content and found an association between decreased glucose and dairy on reduction of acne.41
It is likely that the ketogenic diet, which limits glucose, would be beneficial for acne patients. There may be added benefit through elevated ketone bodies and substantially reduced insulin secretion. However, because there are no observational or interventional studies, further research is needed to draw firm conclusions regarding diet for acne treatment. A randomized clinical trial investigating the effects of the ketogenic diet compared to the low-glycemic diet compared to a regular diet would be valuable.
Psoriasis—Among psoriasis studies, there was a lack of consensus regarding glucose intake and correlation with disease. Among the 4 observational studies, 2 reported increased glucose intake among psoriasis patients and 2 reported decreased glucose intake. It is plausible that the variability in studies is due to differences in sample size and diet heterogeneity among study populations. More specifically, Johnson et al22 and Afifi et al25 analyzed large sample sizes of 6260 and 2412 US participants, respectively, and found decreased sugar intake among psoriasis patients compared to controls. In comparison, Barrea et al23 and Yamashita et al27 analyzed substantially smaller and more specific populations consisting of 82 Italian and 140 Japanese participants, respectively; both reported increased glucose intake among psoriasis patients compared to controls. These seemingly antithetical results may be explained by regional dietary differences, with varying proportions of meats, vegetables, antioxidants, and vitamins.
Moreover, the variation among studies may be further explained by the high prevalence of comorbidities among psoriasis patients. In the study by Barrea et al,23 psoriasis patients had higher fasting glucose (P=.004) and insulin (P=.022) levels than healthy patients. After adjusting for body mass index and metabolic syndrome, the correlation coefficient measuring the relationship between the PASI score and intake of simple carbohydrates changed from r=0.564 (P<.001) to r=0.352 (P=.028). The confounding impact of these comorbidities was further highlighted by Yamashita et al,27 who found statistically significant differences in glucose intake between psoriasis and healthy patients (P=.003). However, they reported diminished significance on additional subgroup analysis accounting for potential comorbidities (P=.994).27 Johnson et al22 and Afifi et al25 did not account for comorbidities; therefore, the 4 observational study results must be interpreted cautiously.
The 2 randomized clinical trials by Castaldo et al29,30 weakly suggest that a ketogenic diet may be beneficial for psoriasis patients. The studies have several notable limitations, including insufficient sample sizes and control groups. Thus, the decreased PASI scores reported in psoriasis patients on the ketogenic diets are challenging to interpret. Additionally, both studies placed patients on highly restrictive diets of 500 kcal/d for 4 weeks. The feasibility of recommending such a diet to patients in clinical practice is questionable. Diets of less than 500 kcal/d may be dangerous for patients with underlying comorbidities and are unlikely to serve as long-term solutions.23 To contextualize our findings, a 2022 review by Chung et al42 examined the impact of various diets—low-caloric, gluten-free, Mediterranean, Western, and ketogenic—on psoriasis and reported insufficient evidence to suggest a benefit to the ketogenic diet for psoriasis patients, though the Mediterranean diet may be well suited for psoriasis patients because of improved cardiovascular health and reduced mortality.
Seborrheic Dermatitis—Sanders et al43 found that patients with a high-fruit diet had lower odds of having SD, while those on a Western diet had higher odds of having SD. Although the study did not measure glycemic load, it is conceivable that the high glycemic load characteristic of the Western diet contributed to these findings.43 However, no studies have investigated the direct link between low-glycemic or ketogenic diets and SD, leaving this area open for further study.
Atopic Dermatitis—It has been hypothesized that mitigating T cell–mediated inflammation via glucose control may contribute to the improvement in AD.35,36 However, in one study, 16.5% of AD patients self-identified sugar as a dietary trigger, ranking fourth among other dietary triggers.24 Thus, the connection between glucose levels and AD warrants further exploration.
Hidradenitis Suppurativa—Given the role of metabolic and hormonal influence in HS as well as the overlapping pathophysiology with acne, it is possible that low-glycemic and ketogenic diets may have a role in improving HS.38-40 However, there is a gap in observation and controlled studies investigating the link between low-glycemic or ketogenic diets and HS.
Conclusion
Our analysis focused on interventional and observational research exploring the effects of low-glycemic and ketogenic diets on associations and treatment of inflammatory skin conditions. There is sufficient evidence to counsel acne patients on the benefits of a low-glycemic diet as an adjunctive treatment for acne. Currently, there is insufficient evidence to recommend a low-glycemic or ketogenic diet as a treatment for patients with any other inflammatory skin disease. Prospective and controlled clinical trials are needed to clarify the utility of dietary interventions for treating inflammatory skin conditions.
- Pickett K, Loveman E, Kalita N, et al. Educational interventions to improve quality of life in people with chronic inflammatory skin diseases: systematic reviews of clinical effectiveness and cost-effectiveness. Health Technol Assess. 2015;19:1-176, v-vi.
- Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48:677-685.
- Dowlatshahi EA, van der Voort EA, Arends LR, et al. Markers of systemic inflammation in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2013;169:266-282.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Melnik BC. Acne vulgaris: the metabolic syndrome of the pilosebaceous follicle. Clin Dermatol. 2018;36:29-40.
- Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond). 2015;39:1197-1202.
- Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10:5754.
- Paoli A, Grimaldi K, Toniolo L, et al. Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- Masood W, Annamaraju P, Khan Suheb MZ, et al. Ketogenic diet. StatPearls. StatPearls Publishing; 2023.
- Fomin DA, McDaniel B, Crane J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatolog Treat. 2017;28:484-487.
- Zhang D, Jin W, Wu R, et al. High glucose intake exacerbates autoimmunity through reactive-oxygen-species-mediated TGF-β cytokine activation. Immunity. 2019;51:671-681.e5.
- Cerman AA, Aktas E, Altunay IK, et al. Dietary glycemic factors, insulin resistance, and adiponectin levels in acne vulgaris. J Am Acad Dermatol. 2016;75:155-162.
- Ferrere G, Tidjani Alou M, Liu P, et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight. 2021;6:e145207.
- Burris J, Shikany JM, Rietkerk W, et al. A Low glycemic index and glycemic load diet decreases insulin-like growth factor-1 among adults with moderate and severe acne: a short-duration, 2-week randomized controlled trial. J Acad Nutr Diet. 2018;118:1874-1885.
- Tan JKL, Stein Gold LF, Alexis AF, et al. Current concepts in acne pathogenesis: pathways to inflammation. Semin Cutan Med Surg. 2018;37(3S):S60-S62.
- Kim J, Ochoa MT, Krutzik SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535-1541.
- Bett DG, Morland J, Yudkin J. Sugar consumption in acne vulgaris and seborrhoeic dermatitis. Br Med J. 1967;3:153-155.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Rouhani P, Berman B, Rouhani G. Acne improves with a popular, low glycemic diet from South Beach. J Am Acad Dermatol. 2009;60(Suppl 1):AB14.
- Aksu AE, Metintas S, Saracoglu ZN, et al. Acne: prevalence and relationship with dietary habits in Eskisehir, Turkey. J Eur Acad Dermatol Venereol. 2012;26:1503-1509.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Johnson JA, Ma C, Kanada KN, et al. Diet and nutrition in psoriasis: analysis of the National Health and Nutrition Examination Survey (NHANES) in the United States. J Eur Acad Dermatol Venereol. 2014;28:327-332.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Nosrati A, Afifi L, Danesh MJ, et al. Dietary modifications in atopic dermatitis: patient-reported outcomes. J Dermatolog Treat. 2017;28:523-538.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Burris J, Rietkerk W, Shikany JM, et al. Differences in dietary glycemic load and hormones in New York City adults with no and moderate/severe acne. J Acad Nutr Diet. 2017;117:1375-1383.
- Yamashita H, Morita T, Ito M, et al. Dietary habits in Japanese patients with psoriasis and psoriatic arthritis: low intake of meat in psoriasis and high intake of vitamin A in psoriatic arthritis. J Dermatol. 2019;46:759-769.
- Marson J, Baldwin HE. 12761 Acne, twins, and glycemic index: a sweet pilot study of diet and dietary beliefs. J Am Acad Dermatol. 2020;83(Suppl):AB110.
- Castaldo G, Rastrelli L, Galdo G, et al. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: a proof-of-concept, single-arm, open-label clinical trial. Nutrition. 2020;74:110757.
- Castaldo G, Pagano I, Grimaldi M, et al. Effect of very-low-calorie ketogenic diet on psoriasis patients: a nuclear magnetic resonance-based metabolomic study. J Proteome Res. 2021;20:1509-1521.
- Ip W, Kirchhof MG. Glycemic control in the treatment of psoriasis. Dermatology. 2017;233:23-29.
- Vijaya Chandra SH, Srinivas R, Dawson TL Jr, et al. Cutaneous Malassezia: commensal, pathogen, or protector? Front Cell Infect Microbiol. 2020;10:614446.
- David Boothe W, Tarbox JA, Tarbox MB. Atopic dermatitis: pathophysiology. Adv Exp Med Biol. 2017;1027:21-37.
- Guttman-Yassky E, Hanifin JM, Boguniewicz M, et al. The role of phosphodiesterase 4 in the pathophysiology of atopic dermatitis and the perspective for its inhibition. Exp Dermatol. 2019;28:3-10.
- Furue K, Ito T, Tsuji G, et al. The IL-13–OVOL1–FLG axis in atopic dermatitis. Immunology. 2019;158:281-286.
- Renert-Yuval Y, Guttman-Yassky E. New treatments for atopic dermatitis targeting beyond IL-4/IL-13 cytokines. Ann Allergy Asthma Immunol. 2020;124:28-35.
- Sellheyer K, Krahl D. “Hidradenitis suppurativa” is acne inversa! An appeal to (finally) abandon a misnomer. Int J Dermatol. 2005;44:535-540.
- Danby FW, Margesson LJ. Hidradenitis suppurativa. Dermatol Clin. 2010;28:779-793.
- Fernandez JM, Marr KD, Hendricks AJ, et al. Alleviating and exacerbating foods in hidradenitis suppurativa. Dermatol Ther. 2020;33:E14246.
- Yamanaka-Takaichi M, Revankar R, Shih T, et al. Expert consensus on priority research gaps in dietary and lifestyle factors in hidradenitis suppurativa: a Delphi consensus study. Arch Dermatol Res. 2023;315:2129-2136.
- Meixiong J, Ricco C, Vasavda C, et al. Diet and acne: a systematic review. JAAD Int. 2022;7:95-112.
- Chung M, Bartholomew E, Yeroushalmi S, et al. Dietary intervention and supplements in the management of psoriasis: current perspectives. Psoriasis (Auckland). 2022;12:151-176. doi:10.2147/PTT.S328581
- Sanders MGH, Pardo LM, Ginger RS, et al. Association between diet and seborrheic dermatitis: a cross-sectional study. J Invest Dermatol. 2019;139:108-114.
- Pickett K, Loveman E, Kalita N, et al. Educational interventions to improve quality of life in people with chronic inflammatory skin diseases: systematic reviews of clinical effectiveness and cost-effectiveness. Health Technol Assess. 2015;19:1-176, v-vi.
- Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48:677-685.
- Dowlatshahi EA, van der Voort EA, Arends LR, et al. Markers of systemic inflammation in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2013;169:266-282.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Melnik BC. Acne vulgaris: the metabolic syndrome of the pilosebaceous follicle. Clin Dermatol. 2018;36:29-40.
- Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond). 2015;39:1197-1202.
- Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10:5754.
- Paoli A, Grimaldi K, Toniolo L, et al. Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- Masood W, Annamaraju P, Khan Suheb MZ, et al. Ketogenic diet. StatPearls. StatPearls Publishing; 2023.
- Fomin DA, McDaniel B, Crane J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatolog Treat. 2017;28:484-487.
- Zhang D, Jin W, Wu R, et al. High glucose intake exacerbates autoimmunity through reactive-oxygen-species-mediated TGF-β cytokine activation. Immunity. 2019;51:671-681.e5.
- Cerman AA, Aktas E, Altunay IK, et al. Dietary glycemic factors, insulin resistance, and adiponectin levels in acne vulgaris. J Am Acad Dermatol. 2016;75:155-162.
- Ferrere G, Tidjani Alou M, Liu P, et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight. 2021;6:e145207.
- Burris J, Shikany JM, Rietkerk W, et al. A Low glycemic index and glycemic load diet decreases insulin-like growth factor-1 among adults with moderate and severe acne: a short-duration, 2-week randomized controlled trial. J Acad Nutr Diet. 2018;118:1874-1885.
- Tan JKL, Stein Gold LF, Alexis AF, et al. Current concepts in acne pathogenesis: pathways to inflammation. Semin Cutan Med Surg. 2018;37(3S):S60-S62.
- Kim J, Ochoa MT, Krutzik SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535-1541.
- Bett DG, Morland J, Yudkin J. Sugar consumption in acne vulgaris and seborrhoeic dermatitis. Br Med J. 1967;3:153-155.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Rouhani P, Berman B, Rouhani G. Acne improves with a popular, low glycemic diet from South Beach. J Am Acad Dermatol. 2009;60(Suppl 1):AB14.
- Aksu AE, Metintas S, Saracoglu ZN, et al. Acne: prevalence and relationship with dietary habits in Eskisehir, Turkey. J Eur Acad Dermatol Venereol. 2012;26:1503-1509.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Johnson JA, Ma C, Kanada KN, et al. Diet and nutrition in psoriasis: analysis of the National Health and Nutrition Examination Survey (NHANES) in the United States. J Eur Acad Dermatol Venereol. 2014;28:327-332.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Nosrati A, Afifi L, Danesh MJ, et al. Dietary modifications in atopic dermatitis: patient-reported outcomes. J Dermatolog Treat. 2017;28:523-538.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Burris J, Rietkerk W, Shikany JM, et al. Differences in dietary glycemic load and hormones in New York City adults with no and moderate/severe acne. J Acad Nutr Diet. 2017;117:1375-1383.
- Yamashita H, Morita T, Ito M, et al. Dietary habits in Japanese patients with psoriasis and psoriatic arthritis: low intake of meat in psoriasis and high intake of vitamin A in psoriatic arthritis. J Dermatol. 2019;46:759-769.
- Marson J, Baldwin HE. 12761 Acne, twins, and glycemic index: a sweet pilot study of diet and dietary beliefs. J Am Acad Dermatol. 2020;83(Suppl):AB110.
- Castaldo G, Rastrelli L, Galdo G, et al. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: a proof-of-concept, single-arm, open-label clinical trial. Nutrition. 2020;74:110757.
- Castaldo G, Pagano I, Grimaldi M, et al. Effect of very-low-calorie ketogenic diet on psoriasis patients: a nuclear magnetic resonance-based metabolomic study. J Proteome Res. 2021;20:1509-1521.
- Ip W, Kirchhof MG. Glycemic control in the treatment of psoriasis. Dermatology. 2017;233:23-29.
- Vijaya Chandra SH, Srinivas R, Dawson TL Jr, et al. Cutaneous Malassezia: commensal, pathogen, or protector? Front Cell Infect Microbiol. 2020;10:614446.
- David Boothe W, Tarbox JA, Tarbox MB. Atopic dermatitis: pathophysiology. Adv Exp Med Biol. 2017;1027:21-37.
- Guttman-Yassky E, Hanifin JM, Boguniewicz M, et al. The role of phosphodiesterase 4 in the pathophysiology of atopic dermatitis and the perspective for its inhibition. Exp Dermatol. 2019;28:3-10.
- Furue K, Ito T, Tsuji G, et al. The IL-13–OVOL1–FLG axis in atopic dermatitis. Immunology. 2019;158:281-286.
- Renert-Yuval Y, Guttman-Yassky E. New treatments for atopic dermatitis targeting beyond IL-4/IL-13 cytokines. Ann Allergy Asthma Immunol. 2020;124:28-35.
- Sellheyer K, Krahl D. “Hidradenitis suppurativa” is acne inversa! An appeal to (finally) abandon a misnomer. Int J Dermatol. 2005;44:535-540.
- Danby FW, Margesson LJ. Hidradenitis suppurativa. Dermatol Clin. 2010;28:779-793.
- Fernandez JM, Marr KD, Hendricks AJ, et al. Alleviating and exacerbating foods in hidradenitis suppurativa. Dermatol Ther. 2020;33:E14246.
- Yamanaka-Takaichi M, Revankar R, Shih T, et al. Expert consensus on priority research gaps in dietary and lifestyle factors in hidradenitis suppurativa: a Delphi consensus study. Arch Dermatol Res. 2023;315:2129-2136.
- Meixiong J, Ricco C, Vasavda C, et al. Diet and acne: a systematic review. JAAD Int. 2022;7:95-112.
- Chung M, Bartholomew E, Yeroushalmi S, et al. Dietary intervention and supplements in the management of psoriasis: current perspectives. Psoriasis (Auckland). 2022;12:151-176. doi:10.2147/PTT.S328581
- Sanders MGH, Pardo LM, Ginger RS, et al. Association between diet and seborrheic dermatitis: a cross-sectional study. J Invest Dermatol. 2019;139:108-114.
Practice Points
- As the ketogenic diet gains in popularity, dermatologists may inform patients that there is emerging evidence supporting the idea that low-glycemic diets may contribute to improvement in inflammatory skin conditions.
- Dermatologists may educate patients about the potential benefits of a low-glycemic diet as a supplementary treatment for acne based on existing evidence.
- Current evidence is insufficient to endorse a ketogenic diet as superior to other dietary approaches in treating inflammatory skin conditions.
Punch Biopsy Extraction With Fingers
Practice Gap
Punch biopsies are utilized frequently by dermatologists to aid in the diagnosis of various skin diseases.1 When performing a punch biopsy, dermatologists are taught to use either forceps or skin hooks in addition to scissors to extract the tissue from the skin.2 However, the use of these sterile instruments for a simple biopsy adds extra costs to the procedure. Herein, a cheaper and often faster method of obtaining the specimen from the patient is described.
The Technique
A 3- or 4-mm disposable punch biopsy tool is employed for this method. After locally anesthetizing the skin, the skin is punched to a subcutaneous depth utilizing the full length of the blade and a little extra pressure is applied downward while stretching the skin around (Figure, A). This may be helpful to dislodge the punch specimen from the surrounding skin. The specimen now can be easily removed by gently grasping it with the thumb and index finger (Figure, B and C). It then can be transferred immediately to the formalin container.
Practice Implications
This technique saves time as well as financial and environmental costs associated with the use of sterile instruments. An additional advantage to this simple method is avoiding specimen crush injuries, which are common when using forceps. This solution works in most cases but may not be suitable for certain special anatomic locations such as the scalp, nose, and ears.
- Gronbeck C, Feng H. Volume and distribution of skin biopsies performed by dermatologists and other health care providers in the Medicare population in 2019. J Am Acad Dermatol. 2022;87:675-678.
- Bolognia JL, Schaffer JV, Cerroni L. Dermatology. Elsevier; 2018.
Practice Gap
Punch biopsies are utilized frequently by dermatologists to aid in the diagnosis of various skin diseases.1 When performing a punch biopsy, dermatologists are taught to use either forceps or skin hooks in addition to scissors to extract the tissue from the skin.2 However, the use of these sterile instruments for a simple biopsy adds extra costs to the procedure. Herein, a cheaper and often faster method of obtaining the specimen from the patient is described.
The Technique
A 3- or 4-mm disposable punch biopsy tool is employed for this method. After locally anesthetizing the skin, the skin is punched to a subcutaneous depth utilizing the full length of the blade and a little extra pressure is applied downward while stretching the skin around (Figure, A). This may be helpful to dislodge the punch specimen from the surrounding skin. The specimen now can be easily removed by gently grasping it with the thumb and index finger (Figure, B and C). It then can be transferred immediately to the formalin container.

Practice Implications
This technique saves time as well as financial and environmental costs associated with the use of sterile instruments. An additional advantage to this simple method is avoiding specimen crush injuries, which are common when using forceps. This solution works in most cases but may not be suitable for certain special anatomic locations such as the scalp, nose, and ears.
Practice Gap
Punch biopsies are utilized frequently by dermatologists to aid in the diagnosis of various skin diseases.1 When performing a punch biopsy, dermatologists are taught to use either forceps or skin hooks in addition to scissors to extract the tissue from the skin.2 However, the use of these sterile instruments for a simple biopsy adds extra costs to the procedure. Herein, a cheaper and often faster method of obtaining the specimen from the patient is described.
The Technique
A 3- or 4-mm disposable punch biopsy tool is employed for this method. After locally anesthetizing the skin, the skin is punched to a subcutaneous depth utilizing the full length of the blade and a little extra pressure is applied downward while stretching the skin around (Figure, A). This may be helpful to dislodge the punch specimen from the surrounding skin. The specimen now can be easily removed by gently grasping it with the thumb and index finger (Figure, B and C). It then can be transferred immediately to the formalin container.

Practice Implications
This technique saves time as well as financial and environmental costs associated with the use of sterile instruments. An additional advantage to this simple method is avoiding specimen crush injuries, which are common when using forceps. This solution works in most cases but may not be suitable for certain special anatomic locations such as the scalp, nose, and ears.
- Gronbeck C, Feng H. Volume and distribution of skin biopsies performed by dermatologists and other health care providers in the Medicare population in 2019. J Am Acad Dermatol. 2022;87:675-678.
- Bolognia JL, Schaffer JV, Cerroni L. Dermatology. Elsevier; 2018.
- Gronbeck C, Feng H. Volume and distribution of skin biopsies performed by dermatologists and other health care providers in the Medicare population in 2019. J Am Acad Dermatol. 2022;87:675-678.
- Bolognia JL, Schaffer JV, Cerroni L. Dermatology. Elsevier; 2018.
PCPs Increasingly Chained to EHRs
If you feel like the day doesn’t hold enough hours for you to get your work done, you’re right: (EHRs).
Investigators followed 141 academic PCPs between May 2019 and March 2023 and found they spent considerably more time engaging in EHR tasks during the final year of the study than in the prepandemic period. EHR time increased by over 8% on days with scheduled appointments and almost 20% on days without scheduled appointments.
“Physicians spend an unsustainable amount of time on EHR-based work, and that amount has increased steadily from 2019 to 2023,” Christine Sinsky, MD, vice president of professional satisfaction at the American Medical Association (AMA) and the senior author of the study, told this news organization. “It is imperative for healthcare systems to develop strategies to change the overall EHR workload trajectory to minimize PCPs’ occupational stress, including improved workflows, where the work is more appropriately distributed amongst the team.”
The study was published online on January 22, 2024, in the Annals of Family Medicine.
‘Pajama Time’
Dr. Sinsky said the motivation for conducting the current study was that PCPs have reported an increase in their workload, especially EHR tasks outside of work (“pajama time”) since the onset of the pandemic.
The research followed up on a 2017 analysis from the same group and other findings showing an increase in the time physicians spend in EHR tasks and the number of Inbox messages they receive from patients seeking medical advice increased during the months following the start of the pandemic.
“As a busy practicing PCP with a large panel of patients, my sense was that the workload was increasing even more, which is what our study confirmed,” said Brian G. Arndt, MD, of the Department of Family Medicine and Community Heath at the University of Wisconsin School of Medicine and Public Health, in Madison, Wisconsin, who led the new study.
The researchers analyzed EHR usage of 141 academic PCPs practicing family medicine, internal medicine, and general pediatrics, two thirds (66.7%) of whom were female. They compared the amount of time spent on EHR tasks during four timespans:
- May 2019 to February 2020
- June 2020 to March 2021
- May 2021 to March 2022
- April 2022 to March 2023
Each PCP’s time and Inbox message volume were calculated and then normalized over 8 hours of scheduled clinic appointments.
Increased Time, Increased Burnout
The study found evidence PCPs have reduced their clinical hours in response to their growing digital workload.
“We have a serious shortage of primary care physicians,” Dr. Sinsky said. “When PCPs cut back their clinical [work] as a coping mechanism for an unmanageable workload, this further exacerbates the primary care shortage, reducing access to care for patients.”
The researchers found increases from the first prepandemic period to the final period of their study in average time that PCPs spent at the EHR per 8 hours of scheduled clinic appointments (Table).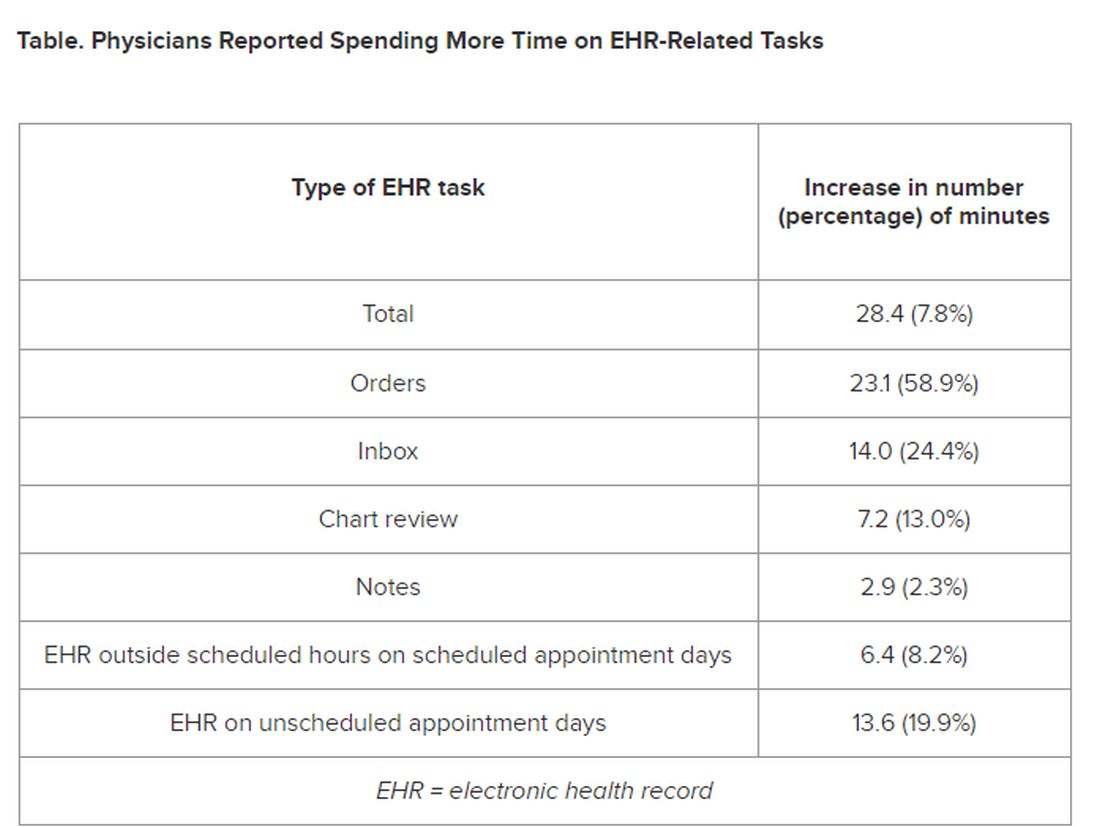
PCPs were inundated with several types of EHR-related responsibilities, including more medical advice requests (+55.5%) and more prescription messages (+19.5%) per 8 hours of scheduled clinic appointments. On the other hand, they had slightly fewer patient calls (−10.5%) and messages concerning test results (−2.7%).
A recent study of 307 PCPs across 31 primary care practices paralleled these findings. It found that physicians spent 36.2 minutes on the EHR per visit (interquartile range, 28.9-45.7 minutes). Included were 6.2 minutes of “pajama time” per visit and 7.8 minutes on the EHR per visit.
The amount of EHR time exceeded the amount of time allotted to a primary care visit (30 minutes). The authors commented that the EHR time burden “and the burnout associated with this burden represent a serious threat to the primary care physician workforce.”
“As more health systems across the country transition from fee-for-service to value-based payment arrangements, they need to balance the time PCPs and their care teams need for face-to-face care — in-person or video visits — with the increasing asynchronous care patients are seeking from us through the portal, for example, MyChart,” Dr. Arndt said.
Sinsky noted that when patients receive care from a PCP, quality is higher and costs are lower. “When access to primary care is further limited by virtue of physicians being overwhelmed by administrative work implemented via the EHR, so that they are reducing their hours, then we can expect negative consequences for patient care and costs of care.”
Tips for Reducing EHR Time
Arndt noted that some “brief investments” of time with patients “lead to high rates of return on decreased MyChart messaging.” For example, he has said to patients: “In the future, there’s no need to respond in MyChart with a ‘Thank you.’” Or “In the future, if you have questions from preappointment labs, no need to send me a separate message in MyChart prior to your visit since they’re typically just a few days out. I look closely at your labs and would always pick up the phone and call you if there was anything more urgent or pressing that needs more immediate action.”
Sinsky recommended two “high-yield opportunities” to reduce EHR-associated workload. The AMA offers a brief Inbox reduction checklist as well as a detailed toolkit to guide physicians and operational leaders in reducing the volume of unnecessary Inbox messages, she said.
Distribution of work among the team also can reduce the time physicians spent on order entry. “It doesn’t take a medical school education to enter orders for flu shots, lipid profiles, mammograms, and other tests, and yet we have primary care physicians around the country spending an hour or more per 8 hours of patient visits on this task,” she said.
‘Growing Mountain’
Sally Baxter, MD, assistant professor of ophthalmology and division chief for Ophthalmology Informatics and Data Sciences at University of California San Diego, said, “Studies like this ... are important for continuing to quantify the burden of EHR work and to evaluate potential interventions to reduce this burden and subsequent burnout.”
Baxter’s health system allows physicians to bill for asynchronous messaging when certain eligibility criteria are met. “This can deter frivolous messaging and also provide some compensation for the work involved,” she said.
“In addition, we’ve recently piloted using AI tools to help draft replies to patient messages in the EHR as another approach to tackling this important issue,” said Baxter, who wasn’t involved with the current study.
Eve Rittenberg, MD, an assistant professor at Harvard Medical School and a PCP at Brigham and Women’s Hospital Fish Center for Women’s Health, in Boston, recommended that healthcare systems “monitor EHR workload across gender, specialty, and other variables to develop equitable support and compensation models.”
Dr. Rittenberg, who wasn’t involved with the current study, said healthcare systems should consider supporting physicians by blocking out time during clinic sessions to manage their EHR work. “Cross-coverage systems are vital so that on their days off, physicians can unplug from the computer and know that their patients’ needs are being met,” she added.
This work was supported in part by the AMA Practice Transformation Initiative: EHR-Use Metrics Research which provided grant funding to several of the authors. Sinsky is employed by the AMA. Dr. Arndt and coauthors disclosed no relevant financial information. Dr. Baxter received nonfinancial support from Optonmed and Topcon for research studies and collaborated with some of the study authors on other research but not this particular study. Dr. Rittenberg received internal funding from the Brigham Care Redesign Incubator and Startup Program, Brigham and Women’s Hospital, for a previous pilot project of inbasket cross-coverage. She had no relevant current disclosures.
A version of this article appeared on Medscape.com.
If you feel like the day doesn’t hold enough hours for you to get your work done, you’re right: (EHRs).
Investigators followed 141 academic PCPs between May 2019 and March 2023 and found they spent considerably more time engaging in EHR tasks during the final year of the study than in the prepandemic period. EHR time increased by over 8% on days with scheduled appointments and almost 20% on days without scheduled appointments.
“Physicians spend an unsustainable amount of time on EHR-based work, and that amount has increased steadily from 2019 to 2023,” Christine Sinsky, MD, vice president of professional satisfaction at the American Medical Association (AMA) and the senior author of the study, told this news organization. “It is imperative for healthcare systems to develop strategies to change the overall EHR workload trajectory to minimize PCPs’ occupational stress, including improved workflows, where the work is more appropriately distributed amongst the team.”
The study was published online on January 22, 2024, in the Annals of Family Medicine.
‘Pajama Time’
Dr. Sinsky said the motivation for conducting the current study was that PCPs have reported an increase in their workload, especially EHR tasks outside of work (“pajama time”) since the onset of the pandemic.
The research followed up on a 2017 analysis from the same group and other findings showing an increase in the time physicians spend in EHR tasks and the number of Inbox messages they receive from patients seeking medical advice increased during the months following the start of the pandemic.
“As a busy practicing PCP with a large panel of patients, my sense was that the workload was increasing even more, which is what our study confirmed,” said Brian G. Arndt, MD, of the Department of Family Medicine and Community Heath at the University of Wisconsin School of Medicine and Public Health, in Madison, Wisconsin, who led the new study.
The researchers analyzed EHR usage of 141 academic PCPs practicing family medicine, internal medicine, and general pediatrics, two thirds (66.7%) of whom were female. They compared the amount of time spent on EHR tasks during four timespans:
- May 2019 to February 2020
- June 2020 to March 2021
- May 2021 to March 2022
- April 2022 to March 2023
Each PCP’s time and Inbox message volume were calculated and then normalized over 8 hours of scheduled clinic appointments.
Increased Time, Increased Burnout
The study found evidence PCPs have reduced their clinical hours in response to their growing digital workload.
“We have a serious shortage of primary care physicians,” Dr. Sinsky said. “When PCPs cut back their clinical [work] as a coping mechanism for an unmanageable workload, this further exacerbates the primary care shortage, reducing access to care for patients.”
The researchers found increases from the first prepandemic period to the final period of their study in average time that PCPs spent at the EHR per 8 hours of scheduled clinic appointments (Table).
PCPs were inundated with several types of EHR-related responsibilities, including more medical advice requests (+55.5%) and more prescription messages (+19.5%) per 8 hours of scheduled clinic appointments. On the other hand, they had slightly fewer patient calls (−10.5%) and messages concerning test results (−2.7%).
A recent study of 307 PCPs across 31 primary care practices paralleled these findings. It found that physicians spent 36.2 minutes on the EHR per visit (interquartile range, 28.9-45.7 minutes). Included were 6.2 minutes of “pajama time” per visit and 7.8 minutes on the EHR per visit.
The amount of EHR time exceeded the amount of time allotted to a primary care visit (30 minutes). The authors commented that the EHR time burden “and the burnout associated with this burden represent a serious threat to the primary care physician workforce.”
“As more health systems across the country transition from fee-for-service to value-based payment arrangements, they need to balance the time PCPs and their care teams need for face-to-face care — in-person or video visits — with the increasing asynchronous care patients are seeking from us through the portal, for example, MyChart,” Dr. Arndt said.
Sinsky noted that when patients receive care from a PCP, quality is higher and costs are lower. “When access to primary care is further limited by virtue of physicians being overwhelmed by administrative work implemented via the EHR, so that they are reducing their hours, then we can expect negative consequences for patient care and costs of care.”
Tips for Reducing EHR Time
Arndt noted that some “brief investments” of time with patients “lead to high rates of return on decreased MyChart messaging.” For example, he has said to patients: “In the future, there’s no need to respond in MyChart with a ‘Thank you.’” Or “In the future, if you have questions from preappointment labs, no need to send me a separate message in MyChart prior to your visit since they’re typically just a few days out. I look closely at your labs and would always pick up the phone and call you if there was anything more urgent or pressing that needs more immediate action.”
Sinsky recommended two “high-yield opportunities” to reduce EHR-associated workload. The AMA offers a brief Inbox reduction checklist as well as a detailed toolkit to guide physicians and operational leaders in reducing the volume of unnecessary Inbox messages, she said.
Distribution of work among the team also can reduce the time physicians spent on order entry. “It doesn’t take a medical school education to enter orders for flu shots, lipid profiles, mammograms, and other tests, and yet we have primary care physicians around the country spending an hour or more per 8 hours of patient visits on this task,” she said.
‘Growing Mountain’
Sally Baxter, MD, assistant professor of ophthalmology and division chief for Ophthalmology Informatics and Data Sciences at University of California San Diego, said, “Studies like this ... are important for continuing to quantify the burden of EHR work and to evaluate potential interventions to reduce this burden and subsequent burnout.”
Baxter’s health system allows physicians to bill for asynchronous messaging when certain eligibility criteria are met. “This can deter frivolous messaging and also provide some compensation for the work involved,” she said.
“In addition, we’ve recently piloted using AI tools to help draft replies to patient messages in the EHR as another approach to tackling this important issue,” said Baxter, who wasn’t involved with the current study.
Eve Rittenberg, MD, an assistant professor at Harvard Medical School and a PCP at Brigham and Women’s Hospital Fish Center for Women’s Health, in Boston, recommended that healthcare systems “monitor EHR workload across gender, specialty, and other variables to develop equitable support and compensation models.”
Dr. Rittenberg, who wasn’t involved with the current study, said healthcare systems should consider supporting physicians by blocking out time during clinic sessions to manage their EHR work. “Cross-coverage systems are vital so that on their days off, physicians can unplug from the computer and know that their patients’ needs are being met,” she added.
This work was supported in part by the AMA Practice Transformation Initiative: EHR-Use Metrics Research which provided grant funding to several of the authors. Sinsky is employed by the AMA. Dr. Arndt and coauthors disclosed no relevant financial information. Dr. Baxter received nonfinancial support from Optonmed and Topcon for research studies and collaborated with some of the study authors on other research but not this particular study. Dr. Rittenberg received internal funding from the Brigham Care Redesign Incubator and Startup Program, Brigham and Women’s Hospital, for a previous pilot project of inbasket cross-coverage. She had no relevant current disclosures.
A version of this article appeared on Medscape.com.
If you feel like the day doesn’t hold enough hours for you to get your work done, you’re right: (EHRs).
Investigators followed 141 academic PCPs between May 2019 and March 2023 and found they spent considerably more time engaging in EHR tasks during the final year of the study than in the prepandemic period. EHR time increased by over 8% on days with scheduled appointments and almost 20% on days without scheduled appointments.
“Physicians spend an unsustainable amount of time on EHR-based work, and that amount has increased steadily from 2019 to 2023,” Christine Sinsky, MD, vice president of professional satisfaction at the American Medical Association (AMA) and the senior author of the study, told this news organization. “It is imperative for healthcare systems to develop strategies to change the overall EHR workload trajectory to minimize PCPs’ occupational stress, including improved workflows, where the work is more appropriately distributed amongst the team.”
The study was published online on January 22, 2024, in the Annals of Family Medicine.
‘Pajama Time’
Dr. Sinsky said the motivation for conducting the current study was that PCPs have reported an increase in their workload, especially EHR tasks outside of work (“pajama time”) since the onset of the pandemic.
The research followed up on a 2017 analysis from the same group and other findings showing an increase in the time physicians spend in EHR tasks and the number of Inbox messages they receive from patients seeking medical advice increased during the months following the start of the pandemic.
“As a busy practicing PCP with a large panel of patients, my sense was that the workload was increasing even more, which is what our study confirmed,” said Brian G. Arndt, MD, of the Department of Family Medicine and Community Heath at the University of Wisconsin School of Medicine and Public Health, in Madison, Wisconsin, who led the new study.
The researchers analyzed EHR usage of 141 academic PCPs practicing family medicine, internal medicine, and general pediatrics, two thirds (66.7%) of whom were female. They compared the amount of time spent on EHR tasks during four timespans:
- May 2019 to February 2020
- June 2020 to March 2021
- May 2021 to March 2022
- April 2022 to March 2023
Each PCP’s time and Inbox message volume were calculated and then normalized over 8 hours of scheduled clinic appointments.
Increased Time, Increased Burnout
The study found evidence PCPs have reduced their clinical hours in response to their growing digital workload.
“We have a serious shortage of primary care physicians,” Dr. Sinsky said. “When PCPs cut back their clinical [work] as a coping mechanism for an unmanageable workload, this further exacerbates the primary care shortage, reducing access to care for patients.”
The researchers found increases from the first prepandemic period to the final period of their study in average time that PCPs spent at the EHR per 8 hours of scheduled clinic appointments (Table).
PCPs were inundated with several types of EHR-related responsibilities, including more medical advice requests (+55.5%) and more prescription messages (+19.5%) per 8 hours of scheduled clinic appointments. On the other hand, they had slightly fewer patient calls (−10.5%) and messages concerning test results (−2.7%).
A recent study of 307 PCPs across 31 primary care practices paralleled these findings. It found that physicians spent 36.2 minutes on the EHR per visit (interquartile range, 28.9-45.7 minutes). Included were 6.2 minutes of “pajama time” per visit and 7.8 minutes on the EHR per visit.
The amount of EHR time exceeded the amount of time allotted to a primary care visit (30 minutes). The authors commented that the EHR time burden “and the burnout associated with this burden represent a serious threat to the primary care physician workforce.”
“As more health systems across the country transition from fee-for-service to value-based payment arrangements, they need to balance the time PCPs and their care teams need for face-to-face care — in-person or video visits — with the increasing asynchronous care patients are seeking from us through the portal, for example, MyChart,” Dr. Arndt said.
Sinsky noted that when patients receive care from a PCP, quality is higher and costs are lower. “When access to primary care is further limited by virtue of physicians being overwhelmed by administrative work implemented via the EHR, so that they are reducing their hours, then we can expect negative consequences for patient care and costs of care.”
Tips for Reducing EHR Time
Arndt noted that some “brief investments” of time with patients “lead to high rates of return on decreased MyChart messaging.” For example, he has said to patients: “In the future, there’s no need to respond in MyChart with a ‘Thank you.’” Or “In the future, if you have questions from preappointment labs, no need to send me a separate message in MyChart prior to your visit since they’re typically just a few days out. I look closely at your labs and would always pick up the phone and call you if there was anything more urgent or pressing that needs more immediate action.”
Sinsky recommended two “high-yield opportunities” to reduce EHR-associated workload. The AMA offers a brief Inbox reduction checklist as well as a detailed toolkit to guide physicians and operational leaders in reducing the volume of unnecessary Inbox messages, she said.
Distribution of work among the team also can reduce the time physicians spent on order entry. “It doesn’t take a medical school education to enter orders for flu shots, lipid profiles, mammograms, and other tests, and yet we have primary care physicians around the country spending an hour or more per 8 hours of patient visits on this task,” she said.
‘Growing Mountain’
Sally Baxter, MD, assistant professor of ophthalmology and division chief for Ophthalmology Informatics and Data Sciences at University of California San Diego, said, “Studies like this ... are important for continuing to quantify the burden of EHR work and to evaluate potential interventions to reduce this burden and subsequent burnout.”
Baxter’s health system allows physicians to bill for asynchronous messaging when certain eligibility criteria are met. “This can deter frivolous messaging and also provide some compensation for the work involved,” she said.
“In addition, we’ve recently piloted using AI tools to help draft replies to patient messages in the EHR as another approach to tackling this important issue,” said Baxter, who wasn’t involved with the current study.
Eve Rittenberg, MD, an assistant professor at Harvard Medical School and a PCP at Brigham and Women’s Hospital Fish Center for Women’s Health, in Boston, recommended that healthcare systems “monitor EHR workload across gender, specialty, and other variables to develop equitable support and compensation models.”
Dr. Rittenberg, who wasn’t involved with the current study, said healthcare systems should consider supporting physicians by blocking out time during clinic sessions to manage their EHR work. “Cross-coverage systems are vital so that on their days off, physicians can unplug from the computer and know that their patients’ needs are being met,” she added.
This work was supported in part by the AMA Practice Transformation Initiative: EHR-Use Metrics Research which provided grant funding to several of the authors. Sinsky is employed by the AMA. Dr. Arndt and coauthors disclosed no relevant financial information. Dr. Baxter received nonfinancial support from Optonmed and Topcon for research studies and collaborated with some of the study authors on other research but not this particular study. Dr. Rittenberg received internal funding from the Brigham Care Redesign Incubator and Startup Program, Brigham and Women’s Hospital, for a previous pilot project of inbasket cross-coverage. She had no relevant current disclosures.
A version of this article appeared on Medscape.com.
Sodium vs Potassium for Lowering Blood Pressure?
A pair of dueling editorials in the journal Hypertension debate whether our focus should be on sodium or its often neglected partner, potassium.
A meta-analysis of 85 trials showed a consistent and linear. It may also depend on where you live and whether your concern is treating individuals or implementing effective food policy.
The Case for Sodium Restriction
Stephen Juraschek, MD, PhD, of the Beth Israel Deaconess Medical Center, Boston, Massachusetts, co-author of one editorial, told me in a zoom interview that he believes his side of the debate clearly has the stronger argument. Of the two cations in question, there has been infinitely more ink spilled about sodium.
Studies such as INTERSALT, the DASH diet, and TOHP may be the most well-known, but there are many, many intervention studies of sodium restriction’s effect on blood pressure. A meta-analysis of 85 trials of showed a consistent and linear relationship between sodium reduction and blood pressure. In contrast, the evidence base for potassium is more limited and less consistent. There are half as many trials with potassium, and its ability to lower blood pressure may depend on how much sodium is present in the diet.
An outlier in the sodium restriction evidence base is the PURE study, which suggested that extreme sodium restriction could increase cardiovascular mortality, but the trial suffered from two potential issues. First, it used a single spot urine specimen to measure sodium rather than the generally accepted more accurate 24-hour urine collection. A reanalysis of the TOHP study using a spot urine rather than a 24-hour urine collection changed the relationship between sodium intake and mortality and possibly explained the U-shaped association observed in PURE. Second, PURE was an observational cohort and was prone to confounding, or in this case, reverse causation. Why did people who consumed very little salt have an increased risk for cardiovascular disease? It is very possible that people with a high risk for cardiovascular disease were told to consume less salt to begin with. Hence B led to A rather than A leading to B.
The debate on sodium restriction has been bitter at times. Opposing camps formed, and people took sides in the “salt wars.” A group of researchers, termed the Jackson 6, met and decided to end the controversy by running a randomized trial in US prisons (having discounted the options of long-term care homes and military bases). They detailed their plan in an editorial in Hypertension. The study never came to fruition for two reasons: the obvious ethical problems of experimenting on prisoners and the revelation of undisclosed salt industry funding.
More recent studies have mercifully been more conventional. The SSaSS study, a randomized controlled trial of a salt substitute, provided the cardiovascular outcomes data that many were waiting for. And CARDIA-SSBP, a cross-over randomized trial recently presented at the American Heart Association meeting, showed that reducing dietary sodium was on par with medication when it came to lowering blood pressure.
For Dr. Juraschek, the evidence is clear: “If you were going to choose one, I would say the weight of the evidence is still really heavily on the sodium side.”
The Case for Potassium Supplementation
The evidence for salt restriction notwithstanding, Swapnil Hiremath, MD, MPH, from the University of Ottawa, Ontario, Canada, argued in his editorial that potassium supplementation has gotten short shrift. Though he admits the studies for potassium supplementation have been smaller and sometimes rely on observational evidence, the evidence is there. In the distal convoluted tubule, the sodium chloride cotransporter (NCC), aka the potassium switch, is turned on by low potassium levels and leads to sodium reabsorption by the kidney even in settings of high sodium intake (Figure). To nonnephrologists, renal physiology may be a black box. But if you quickly brush up on the mechanism of action of thiazide diuretics, the preceding descriptor will make more sense.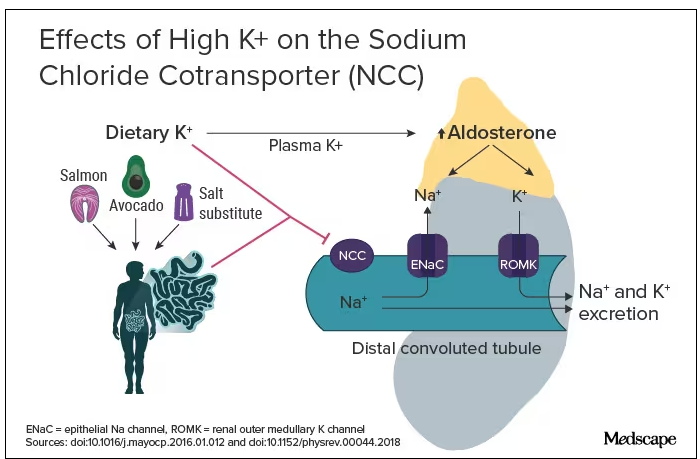
Dr. Hiremath points out that the DASH diet study also got patients to increase their potassium intake by eating more fruits and vegetables. Furthermore, the SSaSS study tested a salt substitute that was 25% potassium (and 75% sodium).
How much blood pressure lowering is due to sodium restriction vs potassium supplementation is a complex question because lowering sodium intake will invariably lead to more potassium intake. “It’s very hard to untangle the relationship,” Dr. Hiremath said in an interview. “It’s sort of synergistic but it’s not completely additive. It’s not as if you add four and four and get eight.” But he maintains there is more evidence regarding the benefit of potassium supplementation than many realize.
Realistic Diets and Taste Issues
“We know that increasing potassium, decreasing sodium is useful. The question is how do we do that?” says Dr. Hiremath. Should we encourage fruit and vegetable consumption in a healthy diet, give potassium supplements, or encourage the use of low-sodium salt substitutes?
Recommending a healthier diet with more fruits and vegetables is a no-brainer. But getting people to do it is hard. In a world where fruit is more expensive than junk food is, economic realities may drive food choice regardless of our best efforts. The 4700 mg of potassium in the DASH eating plan is the equivalent of eleven bananas daily; although not impossible, it would require a substantive shift in eating patterns for most people.
Given that we prescribe iron, vitamin B12, calcium, and vitamin D to patients who need them, why not potassium tablets to help with blood pressure? Granted, there are concerns about inducing hyperkalemia. Also, why not just prescribe a proven anti-hypertensive, such as ramipril, which has the added benefit of helping with renal protection or cardiac remodeling? Dr. Hiremath points out that patients are far less reluctant to take dietary supplements. Medication is something you take when sick. A supplement is seen as “natural” and “healthy” and might be more attractive to people resistant to prescription meds.
Another drawback of oral potassium supplementation is taste. In a Consumer Reports taste test, potassium chloride fared poorly. It was bitter and had a metallic aftertaste. At least one tester wouldn’t ever consume it again. Potassium citrate is slightly more palpable.
Salt substitutes, like the 75:25 ratio of sodium to potassium used in SSaSS, may be as high as you can go for potassium in any low-sodium salt alternative. If you go any higher than that, the taste will just turn people off, suggests Dr. Hiremath.
But SsaSS, which was done in China, may not be relevant to North America. In China, most sodium is added during cooking at home, and the consumption of processed foods is low. For the typical North American, roughly three quarters of the sodium eaten is added to their food by someone else; only about 15% is added during cooking at home or at the dinner table. If you aren’t someone who cooks, buying a salt substitute is probably not going to have much impact.
Given that reality, Dr. Juraschek thinks we need to target the sodium in processed foods. “There’s just so much sodium in so many products,” he says. “When you think about public policy, it’s most expeditious for there to be more regulation about how much is added to our food supply vs trying to get people to consume eight to 12 servings of fruit.”
No Salt War Here
Despite their different editorial takes, Dr. Hiremath and Dr. Juraschek largely agree on the broad strokes of the problem. This isn’t X (or Twitter) after all. Potassium supplementation may be useful in some parts of the world but may not address the underlying problem in countries where processed foods are the source of most dietary sodium.
The CARDIA-SSBP trial showed that a very low–sodium diet had the same blood pressure–lowering effect as a first-line antihypertensive, but most people will not be able to limit themselves to 500 mg of dietary sodium per day. In CARDIA-SSBP, just as in DASH, participants were provided with meals from study kitchens. They were not just told to eat less salt, which would almost certainly have failed.
“We should aim for stuff that is practical and doable rather than aim for stuff that cannot be done,” according to Dr. Hiremath. Whether that should be salt substitutes or policy change may depend on which part of the planet you live on.
One recent positive change may herald the beginning of a policy change, at least in the United States. In March 2023, the US Food and Drug Administration proposed a rule change to allow salt substitutes to be labeled as salt. This would make it easier for food manufacturers to swap out sodium chloride for a low-sodium alternative and reduce the amount of sodium in the US diet without having a large impact on taste and consumer uptake. Both Dr. Hiremath and Dr. Juraschek agree that it may not be enough on its own but that it’s a start.
Christopher Labos is a cardiologist with a degree in epidemiology. He spends most of his time doing things that he doesn’t get paid for, like research, teaching, and podcasting. Occasionally, he finds time to practice cardiology to pay the rent. He realizes that half of his research findings will be disproved in 5 years; he just doesn’t know which half. He is a regular contributor to the Montreal Gazette, CJAD radio, and CTV television in Montreal, and is host of the award-winning podcast The Body of Evidence.
A version of this article appeared on Medscape.com.
A pair of dueling editorials in the journal Hypertension debate whether our focus should be on sodium or its often neglected partner, potassium.
A meta-analysis of 85 trials showed a consistent and linear. It may also depend on where you live and whether your concern is treating individuals or implementing effective food policy.
The Case for Sodium Restriction
Stephen Juraschek, MD, PhD, of the Beth Israel Deaconess Medical Center, Boston, Massachusetts, co-author of one editorial, told me in a zoom interview that he believes his side of the debate clearly has the stronger argument. Of the two cations in question, there has been infinitely more ink spilled about sodium.
Studies such as INTERSALT, the DASH diet, and TOHP may be the most well-known, but there are many, many intervention studies of sodium restriction’s effect on blood pressure. A meta-analysis of 85 trials of showed a consistent and linear relationship between sodium reduction and blood pressure. In contrast, the evidence base for potassium is more limited and less consistent. There are half as many trials with potassium, and its ability to lower blood pressure may depend on how much sodium is present in the diet.
An outlier in the sodium restriction evidence base is the PURE study, which suggested that extreme sodium restriction could increase cardiovascular mortality, but the trial suffered from two potential issues. First, it used a single spot urine specimen to measure sodium rather than the generally accepted more accurate 24-hour urine collection. A reanalysis of the TOHP study using a spot urine rather than a 24-hour urine collection changed the relationship between sodium intake and mortality and possibly explained the U-shaped association observed in PURE. Second, PURE was an observational cohort and was prone to confounding, or in this case, reverse causation. Why did people who consumed very little salt have an increased risk for cardiovascular disease? It is very possible that people with a high risk for cardiovascular disease were told to consume less salt to begin with. Hence B led to A rather than A leading to B.
The debate on sodium restriction has been bitter at times. Opposing camps formed, and people took sides in the “salt wars.” A group of researchers, termed the Jackson 6, met and decided to end the controversy by running a randomized trial in US prisons (having discounted the options of long-term care homes and military bases). They detailed their plan in an editorial in Hypertension. The study never came to fruition for two reasons: the obvious ethical problems of experimenting on prisoners and the revelation of undisclosed salt industry funding.
More recent studies have mercifully been more conventional. The SSaSS study, a randomized controlled trial of a salt substitute, provided the cardiovascular outcomes data that many were waiting for. And CARDIA-SSBP, a cross-over randomized trial recently presented at the American Heart Association meeting, showed that reducing dietary sodium was on par with medication when it came to lowering blood pressure.
For Dr. Juraschek, the evidence is clear: “If you were going to choose one, I would say the weight of the evidence is still really heavily on the sodium side.”
The Case for Potassium Supplementation
The evidence for salt restriction notwithstanding, Swapnil Hiremath, MD, MPH, from the University of Ottawa, Ontario, Canada, argued in his editorial that potassium supplementation has gotten short shrift. Though he admits the studies for potassium supplementation have been smaller and sometimes rely on observational evidence, the evidence is there. In the distal convoluted tubule, the sodium chloride cotransporter (NCC), aka the potassium switch, is turned on by low potassium levels and leads to sodium reabsorption by the kidney even in settings of high sodium intake (Figure). To nonnephrologists, renal physiology may be a black box. But if you quickly brush up on the mechanism of action of thiazide diuretics, the preceding descriptor will make more sense.
Dr. Hiremath points out that the DASH diet study also got patients to increase their potassium intake by eating more fruits and vegetables. Furthermore, the SSaSS study tested a salt substitute that was 25% potassium (and 75% sodium).
How much blood pressure lowering is due to sodium restriction vs potassium supplementation is a complex question because lowering sodium intake will invariably lead to more potassium intake. “It’s very hard to untangle the relationship,” Dr. Hiremath said in an interview. “It’s sort of synergistic but it’s not completely additive. It’s not as if you add four and four and get eight.” But he maintains there is more evidence regarding the benefit of potassium supplementation than many realize.
Realistic Diets and Taste Issues
“We know that increasing potassium, decreasing sodium is useful. The question is how do we do that?” says Dr. Hiremath. Should we encourage fruit and vegetable consumption in a healthy diet, give potassium supplements, or encourage the use of low-sodium salt substitutes?
Recommending a healthier diet with more fruits and vegetables is a no-brainer. But getting people to do it is hard. In a world where fruit is more expensive than junk food is, economic realities may drive food choice regardless of our best efforts. The 4700 mg of potassium in the DASH eating plan is the equivalent of eleven bananas daily; although not impossible, it would require a substantive shift in eating patterns for most people.
Given that we prescribe iron, vitamin B12, calcium, and vitamin D to patients who need them, why not potassium tablets to help with blood pressure? Granted, there are concerns about inducing hyperkalemia. Also, why not just prescribe a proven anti-hypertensive, such as ramipril, which has the added benefit of helping with renal protection or cardiac remodeling? Dr. Hiremath points out that patients are far less reluctant to take dietary supplements. Medication is something you take when sick. A supplement is seen as “natural” and “healthy” and might be more attractive to people resistant to prescription meds.
Another drawback of oral potassium supplementation is taste. In a Consumer Reports taste test, potassium chloride fared poorly. It was bitter and had a metallic aftertaste. At least one tester wouldn’t ever consume it again. Potassium citrate is slightly more palpable.
Salt substitutes, like the 75:25 ratio of sodium to potassium used in SSaSS, may be as high as you can go for potassium in any low-sodium salt alternative. If you go any higher than that, the taste will just turn people off, suggests Dr. Hiremath.
But SsaSS, which was done in China, may not be relevant to North America. In China, most sodium is added during cooking at home, and the consumption of processed foods is low. For the typical North American, roughly three quarters of the sodium eaten is added to their food by someone else; only about 15% is added during cooking at home or at the dinner table. If you aren’t someone who cooks, buying a salt substitute is probably not going to have much impact.
Given that reality, Dr. Juraschek thinks we need to target the sodium in processed foods. “There’s just so much sodium in so many products,” he says. “When you think about public policy, it’s most expeditious for there to be more regulation about how much is added to our food supply vs trying to get people to consume eight to 12 servings of fruit.”
No Salt War Here
Despite their different editorial takes, Dr. Hiremath and Dr. Juraschek largely agree on the broad strokes of the problem. This isn’t X (or Twitter) after all. Potassium supplementation may be useful in some parts of the world but may not address the underlying problem in countries where processed foods are the source of most dietary sodium.
The CARDIA-SSBP trial showed that a very low–sodium diet had the same blood pressure–lowering effect as a first-line antihypertensive, but most people will not be able to limit themselves to 500 mg of dietary sodium per day. In CARDIA-SSBP, just as in DASH, participants were provided with meals from study kitchens. They were not just told to eat less salt, which would almost certainly have failed.
“We should aim for stuff that is practical and doable rather than aim for stuff that cannot be done,” according to Dr. Hiremath. Whether that should be salt substitutes or policy change may depend on which part of the planet you live on.
One recent positive change may herald the beginning of a policy change, at least in the United States. In March 2023, the US Food and Drug Administration proposed a rule change to allow salt substitutes to be labeled as salt. This would make it easier for food manufacturers to swap out sodium chloride for a low-sodium alternative and reduce the amount of sodium in the US diet without having a large impact on taste and consumer uptake. Both Dr. Hiremath and Dr. Juraschek agree that it may not be enough on its own but that it’s a start.
Christopher Labos is a cardiologist with a degree in epidemiology. He spends most of his time doing things that he doesn’t get paid for, like research, teaching, and podcasting. Occasionally, he finds time to practice cardiology to pay the rent. He realizes that half of his research findings will be disproved in 5 years; he just doesn’t know which half. He is a regular contributor to the Montreal Gazette, CJAD radio, and CTV television in Montreal, and is host of the award-winning podcast The Body of Evidence.
A version of this article appeared on Medscape.com.
A pair of dueling editorials in the journal Hypertension debate whether our focus should be on sodium or its often neglected partner, potassium.
A meta-analysis of 85 trials showed a consistent and linear. It may also depend on where you live and whether your concern is treating individuals or implementing effective food policy.
The Case for Sodium Restriction
Stephen Juraschek, MD, PhD, of the Beth Israel Deaconess Medical Center, Boston, Massachusetts, co-author of one editorial, told me in a zoom interview that he believes his side of the debate clearly has the stronger argument. Of the two cations in question, there has been infinitely more ink spilled about sodium.
Studies such as INTERSALT, the DASH diet, and TOHP may be the most well-known, but there are many, many intervention studies of sodium restriction’s effect on blood pressure. A meta-analysis of 85 trials of showed a consistent and linear relationship between sodium reduction and blood pressure. In contrast, the evidence base for potassium is more limited and less consistent. There are half as many trials with potassium, and its ability to lower blood pressure may depend on how much sodium is present in the diet.
An outlier in the sodium restriction evidence base is the PURE study, which suggested that extreme sodium restriction could increase cardiovascular mortality, but the trial suffered from two potential issues. First, it used a single spot urine specimen to measure sodium rather than the generally accepted more accurate 24-hour urine collection. A reanalysis of the TOHP study using a spot urine rather than a 24-hour urine collection changed the relationship between sodium intake and mortality and possibly explained the U-shaped association observed in PURE. Second, PURE was an observational cohort and was prone to confounding, or in this case, reverse causation. Why did people who consumed very little salt have an increased risk for cardiovascular disease? It is very possible that people with a high risk for cardiovascular disease were told to consume less salt to begin with. Hence B led to A rather than A leading to B.
The debate on sodium restriction has been bitter at times. Opposing camps formed, and people took sides in the “salt wars.” A group of researchers, termed the Jackson 6, met and decided to end the controversy by running a randomized trial in US prisons (having discounted the options of long-term care homes and military bases). They detailed their plan in an editorial in Hypertension. The study never came to fruition for two reasons: the obvious ethical problems of experimenting on prisoners and the revelation of undisclosed salt industry funding.
More recent studies have mercifully been more conventional. The SSaSS study, a randomized controlled trial of a salt substitute, provided the cardiovascular outcomes data that many were waiting for. And CARDIA-SSBP, a cross-over randomized trial recently presented at the American Heart Association meeting, showed that reducing dietary sodium was on par with medication when it came to lowering blood pressure.
For Dr. Juraschek, the evidence is clear: “If you were going to choose one, I would say the weight of the evidence is still really heavily on the sodium side.”
The Case for Potassium Supplementation
The evidence for salt restriction notwithstanding, Swapnil Hiremath, MD, MPH, from the University of Ottawa, Ontario, Canada, argued in his editorial that potassium supplementation has gotten short shrift. Though he admits the studies for potassium supplementation have been smaller and sometimes rely on observational evidence, the evidence is there. In the distal convoluted tubule, the sodium chloride cotransporter (NCC), aka the potassium switch, is turned on by low potassium levels and leads to sodium reabsorption by the kidney even in settings of high sodium intake (Figure). To nonnephrologists, renal physiology may be a black box. But if you quickly brush up on the mechanism of action of thiazide diuretics, the preceding descriptor will make more sense.
Dr. Hiremath points out that the DASH diet study also got patients to increase their potassium intake by eating more fruits and vegetables. Furthermore, the SSaSS study tested a salt substitute that was 25% potassium (and 75% sodium).
How much blood pressure lowering is due to sodium restriction vs potassium supplementation is a complex question because lowering sodium intake will invariably lead to more potassium intake. “It’s very hard to untangle the relationship,” Dr. Hiremath said in an interview. “It’s sort of synergistic but it’s not completely additive. It’s not as if you add four and four and get eight.” But he maintains there is more evidence regarding the benefit of potassium supplementation than many realize.
Realistic Diets and Taste Issues
“We know that increasing potassium, decreasing sodium is useful. The question is how do we do that?” says Dr. Hiremath. Should we encourage fruit and vegetable consumption in a healthy diet, give potassium supplements, or encourage the use of low-sodium salt substitutes?
Recommending a healthier diet with more fruits and vegetables is a no-brainer. But getting people to do it is hard. In a world where fruit is more expensive than junk food is, economic realities may drive food choice regardless of our best efforts. The 4700 mg of potassium in the DASH eating plan is the equivalent of eleven bananas daily; although not impossible, it would require a substantive shift in eating patterns for most people.
Given that we prescribe iron, vitamin B12, calcium, and vitamin D to patients who need them, why not potassium tablets to help with blood pressure? Granted, there are concerns about inducing hyperkalemia. Also, why not just prescribe a proven anti-hypertensive, such as ramipril, which has the added benefit of helping with renal protection or cardiac remodeling? Dr. Hiremath points out that patients are far less reluctant to take dietary supplements. Medication is something you take when sick. A supplement is seen as “natural” and “healthy” and might be more attractive to people resistant to prescription meds.
Another drawback of oral potassium supplementation is taste. In a Consumer Reports taste test, potassium chloride fared poorly. It was bitter and had a metallic aftertaste. At least one tester wouldn’t ever consume it again. Potassium citrate is slightly more palpable.
Salt substitutes, like the 75:25 ratio of sodium to potassium used in SSaSS, may be as high as you can go for potassium in any low-sodium salt alternative. If you go any higher than that, the taste will just turn people off, suggests Dr. Hiremath.
But SsaSS, which was done in China, may not be relevant to North America. In China, most sodium is added during cooking at home, and the consumption of processed foods is low. For the typical North American, roughly three quarters of the sodium eaten is added to their food by someone else; only about 15% is added during cooking at home or at the dinner table. If you aren’t someone who cooks, buying a salt substitute is probably not going to have much impact.
Given that reality, Dr. Juraschek thinks we need to target the sodium in processed foods. “There’s just so much sodium in so many products,” he says. “When you think about public policy, it’s most expeditious for there to be more regulation about how much is added to our food supply vs trying to get people to consume eight to 12 servings of fruit.”
No Salt War Here
Despite their different editorial takes, Dr. Hiremath and Dr. Juraschek largely agree on the broad strokes of the problem. This isn’t X (or Twitter) after all. Potassium supplementation may be useful in some parts of the world but may not address the underlying problem in countries where processed foods are the source of most dietary sodium.
The CARDIA-SSBP trial showed that a very low–sodium diet had the same blood pressure–lowering effect as a first-line antihypertensive, but most people will not be able to limit themselves to 500 mg of dietary sodium per day. In CARDIA-SSBP, just as in DASH, participants were provided with meals from study kitchens. They were not just told to eat less salt, which would almost certainly have failed.
“We should aim for stuff that is practical and doable rather than aim for stuff that cannot be done,” according to Dr. Hiremath. Whether that should be salt substitutes or policy change may depend on which part of the planet you live on.
One recent positive change may herald the beginning of a policy change, at least in the United States. In March 2023, the US Food and Drug Administration proposed a rule change to allow salt substitutes to be labeled as salt. This would make it easier for food manufacturers to swap out sodium chloride for a low-sodium alternative and reduce the amount of sodium in the US diet without having a large impact on taste and consumer uptake. Both Dr. Hiremath and Dr. Juraschek agree that it may not be enough on its own but that it’s a start.
Christopher Labos is a cardiologist with a degree in epidemiology. He spends most of his time doing things that he doesn’t get paid for, like research, teaching, and podcasting. Occasionally, he finds time to practice cardiology to pay the rent. He realizes that half of his research findings will be disproved in 5 years; he just doesn’t know which half. He is a regular contributor to the Montreal Gazette, CJAD radio, and CTV television in Montreal, and is host of the award-winning podcast The Body of Evidence.
A version of this article appeared on Medscape.com.
More Young Women Being Diagnosed With Breast Cancer Than Ever Before
This transcript has been edited for clarity.
From the year 2000 until around 2016, the incidence of breast cancer among young women — those under age 50 — rose steadily, if slowly.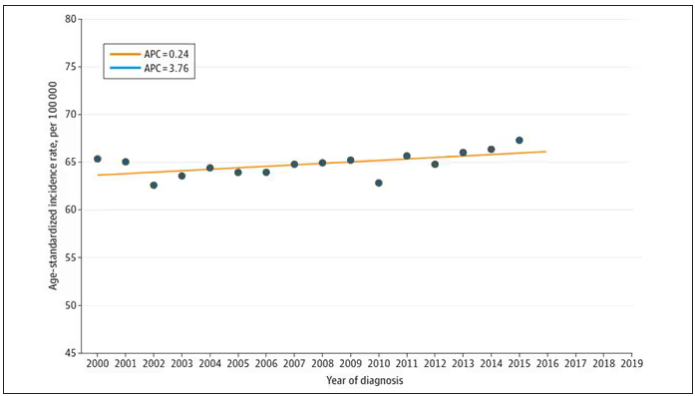
And then this happened: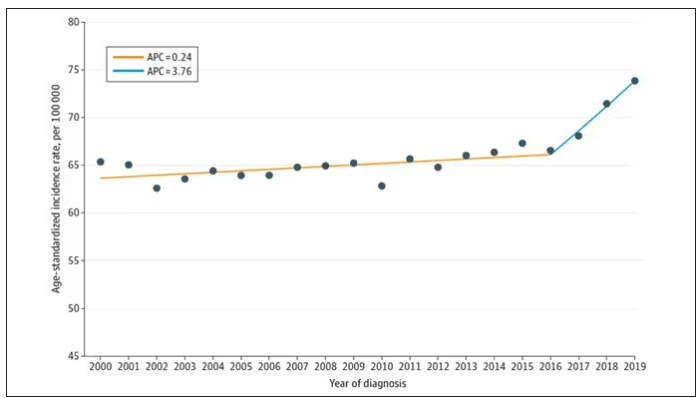
I look at a lot of graphs in my line of work, and it’s not too often that one actually makes me say “What the hell?” out loud. But this one did. Why are young women all of a sudden more likely to get breast cancer?
The graph comes from this paper, Breast cancer incidence among us women aged 20 to 49 years by race, stage, and hormone receptor status, appearing in JAMA Network Open
Researchers from Washington University in St. Louis utilized SEER registries to conduct their analyses. SEER is a public database from the National Cancer Institute with coverage of 27% of the US population and a long track record of statistical backbone to translate the data from SEER to numbers that are representative of the population at large.
From 2000 to 2019, more than 200,000 women were diagnosed with primary invasive breast cancer in the dataset, and I’ve already given you the top-line results. Of course, when you see a graph like this, the next question really needs to be why?
Fortunately, the SEER dataset contains a lot more information than simply whether someone was diagnosed with cancer. In the case of breast cancer, there is information about the patient’s demographics, the hormone status of the cancer, the stage, and so on. Using those additional data points can help the authors, and us, start to formulate some hypotheses as to what is happening here.
Let’s start with something a bit tricky about this kind of data. We see an uptick in new breast cancer diagnoses among young women in recent years. We need to tease that uptick apart a bit. It could be that it is the year that is the key factor here. In other words, it is simply that more women are getting breast cancer since 2016 and so more young women are getting breast cancer since 2016. These are known as period effects.
Or is there something unique to young women — something about their environmental exposures that put them at higher risk than they would have been had they been born at some other time? These are known as cohort effects.
The researchers teased these two effects apart, as you can see here, and concluded that, well, it’s both.
Stage of cancer at diagnosis can give us some more insight into what is happening. These results are pretty interesting. These higher cancer rates are due primarily to stage I and stage IV cancers, not stage II and stage III cancers.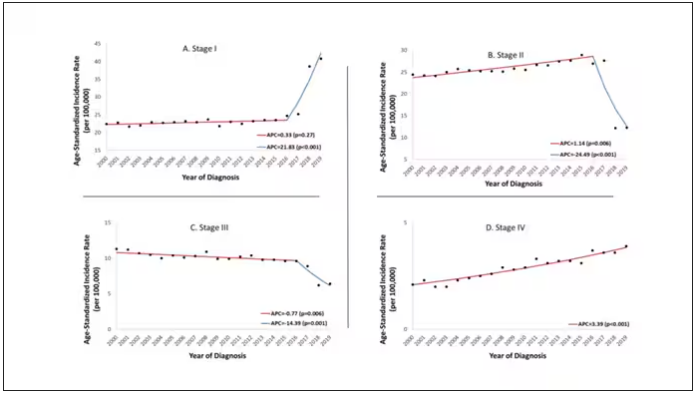
The rising incidence of stage I cancers could reflect better detection, though many of the women in this cohort would not have been old enough to quality for screening mammograms. That said, increased awareness about genetic risk and family history might be leading younger women to get screened, picking up more early cancers. Additionally, much of the increased incidence was with estrogen receptor–positive tumors, which might reflect the fact that women in this cohort are tending to have fewer children, and children later in life.
So why the rise in stage IV breast cancer? Well, precisely because younger women are not recommended to get screening mammograms; those who detect a lump on their own are likely to be at a more advanced stage. But I’m not sure why that would be changing recently. The authors argue that an increase in overweight and obesity in the country might be to blame here. Prior studies have shown that higher BMI is associated with higher stage at breast cancer diagnosis.
Of course, we can speculate as to multiple other causes as well: environmental toxins, pollution, hormone exposures, and so on. Figuring this out will be the work of multiple other studies. In the meantime, we should remember that the landscape of cancer is continuously changing. And that means we need to adapt to it. If these trends continue, national agencies may need to reconsider their guidelines for when screening mammography should begin — at least in some groups of young women.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
From the year 2000 until around 2016, the incidence of breast cancer among young women — those under age 50 — rose steadily, if slowly.
And then this happened:
I look at a lot of graphs in my line of work, and it’s not too often that one actually makes me say “What the hell?” out loud. But this one did. Why are young women all of a sudden more likely to get breast cancer?
The graph comes from this paper, Breast cancer incidence among us women aged 20 to 49 years by race, stage, and hormone receptor status, appearing in JAMA Network Open
Researchers from Washington University in St. Louis utilized SEER registries to conduct their analyses. SEER is a public database from the National Cancer Institute with coverage of 27% of the US population and a long track record of statistical backbone to translate the data from SEER to numbers that are representative of the population at large.
From 2000 to 2019, more than 200,000 women were diagnosed with primary invasive breast cancer in the dataset, and I’ve already given you the top-line results. Of course, when you see a graph like this, the next question really needs to be why?
Fortunately, the SEER dataset contains a lot more information than simply whether someone was diagnosed with cancer. In the case of breast cancer, there is information about the patient’s demographics, the hormone status of the cancer, the stage, and so on. Using those additional data points can help the authors, and us, start to formulate some hypotheses as to what is happening here.
Let’s start with something a bit tricky about this kind of data. We see an uptick in new breast cancer diagnoses among young women in recent years. We need to tease that uptick apart a bit. It could be that it is the year that is the key factor here. In other words, it is simply that more women are getting breast cancer since 2016 and so more young women are getting breast cancer since 2016. These are known as period effects.
Or is there something unique to young women — something about their environmental exposures that put them at higher risk than they would have been had they been born at some other time? These are known as cohort effects.
The researchers teased these two effects apart, as you can see here, and concluded that, well, it’s both.
Stage of cancer at diagnosis can give us some more insight into what is happening. These results are pretty interesting. These higher cancer rates are due primarily to stage I and stage IV cancers, not stage II and stage III cancers.
The rising incidence of stage I cancers could reflect better detection, though many of the women in this cohort would not have been old enough to quality for screening mammograms. That said, increased awareness about genetic risk and family history might be leading younger women to get screened, picking up more early cancers. Additionally, much of the increased incidence was with estrogen receptor–positive tumors, which might reflect the fact that women in this cohort are tending to have fewer children, and children later in life.
So why the rise in stage IV breast cancer? Well, precisely because younger women are not recommended to get screening mammograms; those who detect a lump on their own are likely to be at a more advanced stage. But I’m not sure why that would be changing recently. The authors argue that an increase in overweight and obesity in the country might be to blame here. Prior studies have shown that higher BMI is associated with higher stage at breast cancer diagnosis.
Of course, we can speculate as to multiple other causes as well: environmental toxins, pollution, hormone exposures, and so on. Figuring this out will be the work of multiple other studies. In the meantime, we should remember that the landscape of cancer is continuously changing. And that means we need to adapt to it. If these trends continue, national agencies may need to reconsider their guidelines for when screening mammography should begin — at least in some groups of young women.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
From the year 2000 until around 2016, the incidence of breast cancer among young women — those under age 50 — rose steadily, if slowly.
And then this happened:
I look at a lot of graphs in my line of work, and it’s not too often that one actually makes me say “What the hell?” out loud. But this one did. Why are young women all of a sudden more likely to get breast cancer?
The graph comes from this paper, Breast cancer incidence among us women aged 20 to 49 years by race, stage, and hormone receptor status, appearing in JAMA Network Open
Researchers from Washington University in St. Louis utilized SEER registries to conduct their analyses. SEER is a public database from the National Cancer Institute with coverage of 27% of the US population and a long track record of statistical backbone to translate the data from SEER to numbers that are representative of the population at large.
From 2000 to 2019, more than 200,000 women were diagnosed with primary invasive breast cancer in the dataset, and I’ve already given you the top-line results. Of course, when you see a graph like this, the next question really needs to be why?
Fortunately, the SEER dataset contains a lot more information than simply whether someone was diagnosed with cancer. In the case of breast cancer, there is information about the patient’s demographics, the hormone status of the cancer, the stage, and so on. Using those additional data points can help the authors, and us, start to formulate some hypotheses as to what is happening here.
Let’s start with something a bit tricky about this kind of data. We see an uptick in new breast cancer diagnoses among young women in recent years. We need to tease that uptick apart a bit. It could be that it is the year that is the key factor here. In other words, it is simply that more women are getting breast cancer since 2016 and so more young women are getting breast cancer since 2016. These are known as period effects.
Or is there something unique to young women — something about their environmental exposures that put them at higher risk than they would have been had they been born at some other time? These are known as cohort effects.
The researchers teased these two effects apart, as you can see here, and concluded that, well, it’s both.
Stage of cancer at diagnosis can give us some more insight into what is happening. These results are pretty interesting. These higher cancer rates are due primarily to stage I and stage IV cancers, not stage II and stage III cancers.
The rising incidence of stage I cancers could reflect better detection, though many of the women in this cohort would not have been old enough to quality for screening mammograms. That said, increased awareness about genetic risk and family history might be leading younger women to get screened, picking up more early cancers. Additionally, much of the increased incidence was with estrogen receptor–positive tumors, which might reflect the fact that women in this cohort are tending to have fewer children, and children later in life.
So why the rise in stage IV breast cancer? Well, precisely because younger women are not recommended to get screening mammograms; those who detect a lump on their own are likely to be at a more advanced stage. But I’m not sure why that would be changing recently. The authors argue that an increase in overweight and obesity in the country might be to blame here. Prior studies have shown that higher BMI is associated with higher stage at breast cancer diagnosis.
Of course, we can speculate as to multiple other causes as well: environmental toxins, pollution, hormone exposures, and so on. Figuring this out will be the work of multiple other studies. In the meantime, we should remember that the landscape of cancer is continuously changing. And that means we need to adapt to it. If these trends continue, national agencies may need to reconsider their guidelines for when screening mammography should begin — at least in some groups of young women.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.