User login
Do Your Patients Hate Exercise? Suggest They Do This Instead
Have patients who want to lose weight? Tell them to put on their dancing shoes.
Dancing can be an effective fat-loss tool for people who are overweight or have obesity, according to a recent meta-analysis in PLOS One.
Participants who danced three times a week for at least 3 months reaped maximum benefits. And the more they let loose, the better — more creative dance forms led to more pronounced improvements in body composition.
The study builds on previous research that suggests dance can be beneficial for weight loss and overall health. A 2017 meta-analysis found that dance significantly improved body composition, blood biomarkers, and musculoskeletal function. Other research has linked dance with improvements in cognitive function, mental health, and quality of life.
What makes dance special? It’s a full-body workout that might be easier to stick with than other exercises. “Enjoyment” is key for sustainability, the researchers wrote: “As a form of physical activity that integrates exercise, entertainment, and sociality, dance possesses innate advantages in fostering motivation for exercise.”
“The best exercise is the one you’ll do every day, and something that you like to do,” said Nicholas Pennings, DO, chair and associate professor of family medicine at Campbell University, Buies Creek, NC. (Dr. Pennings was not involved in the study.) For patients who enjoy dancing, dance could be that thing — or at least one workout to add to the mix.
Help your patients get started with these tips.
Frame it as a hobby, not exercise. Ask what hobbies they used to enjoy in high school, suggests Deirdre Mattina, MD, a cardiologist at the Cleveland Clinic and a former professional dancer. “ This can sometimes evoke happy memories of younger years and perhaps hobbies that they’d given up because they thought they were too old,” she said. If they used to play sports or dance, that’s your in. “I usually talk about hot yoga as a transition to get back their flexibility and then something like a dance aerobics or Zumba class to start.”
Recommend a group class. “Any intervention promoting social relationships is expected to increase adherence,” said Giulio Marchesini Reggiani, MD, a recently retired professor of internal medicine and dietetics at the University of Bologna in Italy. “You are motivated by the group, and you create a relationship among participants, and this means that you are no longer alone.” Try local gyms, health clubs, or even dance studios (yes, where kids go — they offer adult classes, too).
Help patients find their unique groove. Dr. Mattina has some patients who take cardio dance classes, some who line dance, and others who pole dance or heels dance. “Those are the things that keep it fun,” she said. “It doesn’t seem like exercise. It seems more like going out and hanging out.”
Encourage those who “don’t know how to dance.” You don’t need fancy choreography or the grace of a prima ballerina.”Simply move aided by the music,” said Dr. Reggiani. “As long as you start engaging in physical activity, you improve your health, and you improve your movement.” Suggest patients start with beginner Zumba or a step class to get the hang of moving to a beat. Or try a home dance video, like Barre Blend by BODi (which offers a 14-day free trial). “You can try taking a couple classes in the privacy of your own home first, so you feel comfortable getting out there and doing it with a group,” said Dr. Mattina.
Modify as needed. If a patient has mobility limitations or lower-body pain, they can still dance — just do the upper-body portion of the moves. “Dance involves both upper and lower body movement, and so many dance activities could easily be performed in a chair,” said Dr. Pennings. A good joint-friendly option: Some health clubs offer dance classes that take place in a swimming pool.
Involve the whole family. Support from a partner can help patients stick with exercise, said Dr. Reggiani, and dance can also help a couple strengthen their bond. Invite kids and grandparents to join, too. “Dancing is something that can be done at any age,” said Dr. Reggiani. “For kids, it is important to make it fun,” said Dr. Pennings. “Start when they are young with music they are familiar with and enjoy.” For skeptical partners? “Keep it simple and nonjudgmental,” he said.
Remind patients to warm up. We lose flexibility with age, so ease into it, said Dr. Mattina. Many classes include warmups, but if you’re at home, do a few minutes of light, low-impact cardio — jumping jacks, mountain climbers, jogging, or brisk walking — before stretching. Or just put on a slow song and start lightly bouncing to the beat or stepping your feet to one side, together, then to the other side and together.
Tell them to take dance breaks. No time to join a class? Break up the workday with a few 10-minute dance parties. (That’s about three songs.) “Short bursts of exercise throughout the day, like if you do 10 minutes of exercise six times a day, actually has a greater health benefit than doing 60 minutes of continuous exercise,” said Dr. Pennings. It helps counter the negative effects of prolonged sitting “by increasing blood flow and increasing utilization of your muscles.”
Manage expectations about weight loss. Patients often have outsized expectations about how much weight they’ll lose when starting a new exercise regimen, Dr. Pennings said. Dancing burns about 300 calories per hour, so it takes roughly 12 hours to lose one pound. Consistency over time is the key. “My goal is to both emphasize the health benefits of exercise while maintaining realistic expectations about weight loss,” said Dr. Pennings. Focus less on the weight part and highlight other benefits: Dancing builds strength, balance, and coordination, said Dr. Pennings. It can help improve blood pressure and other heart health markers and boost cognition in older adults. And it’s fun.
A version of this article appeared on Medscape.com.
Have patients who want to lose weight? Tell them to put on their dancing shoes.
Dancing can be an effective fat-loss tool for people who are overweight or have obesity, according to a recent meta-analysis in PLOS One.
Participants who danced three times a week for at least 3 months reaped maximum benefits. And the more they let loose, the better — more creative dance forms led to more pronounced improvements in body composition.
The study builds on previous research that suggests dance can be beneficial for weight loss and overall health. A 2017 meta-analysis found that dance significantly improved body composition, blood biomarkers, and musculoskeletal function. Other research has linked dance with improvements in cognitive function, mental health, and quality of life.
What makes dance special? It’s a full-body workout that might be easier to stick with than other exercises. “Enjoyment” is key for sustainability, the researchers wrote: “As a form of physical activity that integrates exercise, entertainment, and sociality, dance possesses innate advantages in fostering motivation for exercise.”
“The best exercise is the one you’ll do every day, and something that you like to do,” said Nicholas Pennings, DO, chair and associate professor of family medicine at Campbell University, Buies Creek, NC. (Dr. Pennings was not involved in the study.) For patients who enjoy dancing, dance could be that thing — or at least one workout to add to the mix.
Help your patients get started with these tips.
Frame it as a hobby, not exercise. Ask what hobbies they used to enjoy in high school, suggests Deirdre Mattina, MD, a cardiologist at the Cleveland Clinic and a former professional dancer. “ This can sometimes evoke happy memories of younger years and perhaps hobbies that they’d given up because they thought they were too old,” she said. If they used to play sports or dance, that’s your in. “I usually talk about hot yoga as a transition to get back their flexibility and then something like a dance aerobics or Zumba class to start.”
Recommend a group class. “Any intervention promoting social relationships is expected to increase adherence,” said Giulio Marchesini Reggiani, MD, a recently retired professor of internal medicine and dietetics at the University of Bologna in Italy. “You are motivated by the group, and you create a relationship among participants, and this means that you are no longer alone.” Try local gyms, health clubs, or even dance studios (yes, where kids go — they offer adult classes, too).
Help patients find their unique groove. Dr. Mattina has some patients who take cardio dance classes, some who line dance, and others who pole dance or heels dance. “Those are the things that keep it fun,” she said. “It doesn’t seem like exercise. It seems more like going out and hanging out.”
Encourage those who “don’t know how to dance.” You don’t need fancy choreography or the grace of a prima ballerina.”Simply move aided by the music,” said Dr. Reggiani. “As long as you start engaging in physical activity, you improve your health, and you improve your movement.” Suggest patients start with beginner Zumba or a step class to get the hang of moving to a beat. Or try a home dance video, like Barre Blend by BODi (which offers a 14-day free trial). “You can try taking a couple classes in the privacy of your own home first, so you feel comfortable getting out there and doing it with a group,” said Dr. Mattina.
Modify as needed. If a patient has mobility limitations or lower-body pain, they can still dance — just do the upper-body portion of the moves. “Dance involves both upper and lower body movement, and so many dance activities could easily be performed in a chair,” said Dr. Pennings. A good joint-friendly option: Some health clubs offer dance classes that take place in a swimming pool.
Involve the whole family. Support from a partner can help patients stick with exercise, said Dr. Reggiani, and dance can also help a couple strengthen their bond. Invite kids and grandparents to join, too. “Dancing is something that can be done at any age,” said Dr. Reggiani. “For kids, it is important to make it fun,” said Dr. Pennings. “Start when they are young with music they are familiar with and enjoy.” For skeptical partners? “Keep it simple and nonjudgmental,” he said.
Remind patients to warm up. We lose flexibility with age, so ease into it, said Dr. Mattina. Many classes include warmups, but if you’re at home, do a few minutes of light, low-impact cardio — jumping jacks, mountain climbers, jogging, or brisk walking — before stretching. Or just put on a slow song and start lightly bouncing to the beat or stepping your feet to one side, together, then to the other side and together.
Tell them to take dance breaks. No time to join a class? Break up the workday with a few 10-minute dance parties. (That’s about three songs.) “Short bursts of exercise throughout the day, like if you do 10 minutes of exercise six times a day, actually has a greater health benefit than doing 60 minutes of continuous exercise,” said Dr. Pennings. It helps counter the negative effects of prolonged sitting “by increasing blood flow and increasing utilization of your muscles.”
Manage expectations about weight loss. Patients often have outsized expectations about how much weight they’ll lose when starting a new exercise regimen, Dr. Pennings said. Dancing burns about 300 calories per hour, so it takes roughly 12 hours to lose one pound. Consistency over time is the key. “My goal is to both emphasize the health benefits of exercise while maintaining realistic expectations about weight loss,” said Dr. Pennings. Focus less on the weight part and highlight other benefits: Dancing builds strength, balance, and coordination, said Dr. Pennings. It can help improve blood pressure and other heart health markers and boost cognition in older adults. And it’s fun.
A version of this article appeared on Medscape.com.
Have patients who want to lose weight? Tell them to put on their dancing shoes.
Dancing can be an effective fat-loss tool for people who are overweight or have obesity, according to a recent meta-analysis in PLOS One.
Participants who danced three times a week for at least 3 months reaped maximum benefits. And the more they let loose, the better — more creative dance forms led to more pronounced improvements in body composition.
The study builds on previous research that suggests dance can be beneficial for weight loss and overall health. A 2017 meta-analysis found that dance significantly improved body composition, blood biomarkers, and musculoskeletal function. Other research has linked dance with improvements in cognitive function, mental health, and quality of life.
What makes dance special? It’s a full-body workout that might be easier to stick with than other exercises. “Enjoyment” is key for sustainability, the researchers wrote: “As a form of physical activity that integrates exercise, entertainment, and sociality, dance possesses innate advantages in fostering motivation for exercise.”
“The best exercise is the one you’ll do every day, and something that you like to do,” said Nicholas Pennings, DO, chair and associate professor of family medicine at Campbell University, Buies Creek, NC. (Dr. Pennings was not involved in the study.) For patients who enjoy dancing, dance could be that thing — or at least one workout to add to the mix.
Help your patients get started with these tips.
Frame it as a hobby, not exercise. Ask what hobbies they used to enjoy in high school, suggests Deirdre Mattina, MD, a cardiologist at the Cleveland Clinic and a former professional dancer. “ This can sometimes evoke happy memories of younger years and perhaps hobbies that they’d given up because they thought they were too old,” she said. If they used to play sports or dance, that’s your in. “I usually talk about hot yoga as a transition to get back their flexibility and then something like a dance aerobics or Zumba class to start.”
Recommend a group class. “Any intervention promoting social relationships is expected to increase adherence,” said Giulio Marchesini Reggiani, MD, a recently retired professor of internal medicine and dietetics at the University of Bologna in Italy. “You are motivated by the group, and you create a relationship among participants, and this means that you are no longer alone.” Try local gyms, health clubs, or even dance studios (yes, where kids go — they offer adult classes, too).
Help patients find their unique groove. Dr. Mattina has some patients who take cardio dance classes, some who line dance, and others who pole dance or heels dance. “Those are the things that keep it fun,” she said. “It doesn’t seem like exercise. It seems more like going out and hanging out.”
Encourage those who “don’t know how to dance.” You don’t need fancy choreography or the grace of a prima ballerina.”Simply move aided by the music,” said Dr. Reggiani. “As long as you start engaging in physical activity, you improve your health, and you improve your movement.” Suggest patients start with beginner Zumba or a step class to get the hang of moving to a beat. Or try a home dance video, like Barre Blend by BODi (which offers a 14-day free trial). “You can try taking a couple classes in the privacy of your own home first, so you feel comfortable getting out there and doing it with a group,” said Dr. Mattina.
Modify as needed. If a patient has mobility limitations or lower-body pain, they can still dance — just do the upper-body portion of the moves. “Dance involves both upper and lower body movement, and so many dance activities could easily be performed in a chair,” said Dr. Pennings. A good joint-friendly option: Some health clubs offer dance classes that take place in a swimming pool.
Involve the whole family. Support from a partner can help patients stick with exercise, said Dr. Reggiani, and dance can also help a couple strengthen their bond. Invite kids and grandparents to join, too. “Dancing is something that can be done at any age,” said Dr. Reggiani. “For kids, it is important to make it fun,” said Dr. Pennings. “Start when they are young with music they are familiar with and enjoy.” For skeptical partners? “Keep it simple and nonjudgmental,” he said.
Remind patients to warm up. We lose flexibility with age, so ease into it, said Dr. Mattina. Many classes include warmups, but if you’re at home, do a few minutes of light, low-impact cardio — jumping jacks, mountain climbers, jogging, or brisk walking — before stretching. Or just put on a slow song and start lightly bouncing to the beat or stepping your feet to one side, together, then to the other side and together.
Tell them to take dance breaks. No time to join a class? Break up the workday with a few 10-minute dance parties. (That’s about three songs.) “Short bursts of exercise throughout the day, like if you do 10 minutes of exercise six times a day, actually has a greater health benefit than doing 60 minutes of continuous exercise,” said Dr. Pennings. It helps counter the negative effects of prolonged sitting “by increasing blood flow and increasing utilization of your muscles.”
Manage expectations about weight loss. Patients often have outsized expectations about how much weight they’ll lose when starting a new exercise regimen, Dr. Pennings said. Dancing burns about 300 calories per hour, so it takes roughly 12 hours to lose one pound. Consistency over time is the key. “My goal is to both emphasize the health benefits of exercise while maintaining realistic expectations about weight loss,” said Dr. Pennings. Focus less on the weight part and highlight other benefits: Dancing builds strength, balance, and coordination, said Dr. Pennings. It can help improve blood pressure and other heart health markers and boost cognition in older adults. And it’s fun.
A version of this article appeared on Medscape.com.
New Tech Could Record Deep-Brain Activity From Surface
Modern technology for recording deep-brain activity involves sharp metal electrodes that penetrate the tissue, causing damage that can compromise the signal and limiting how often they can be used.
A rapidly growing area in materials science and engineering aims to fix the problem by designing electrodes that are softer, smaller, and flexible — safer for use inside the delicate tissues of the brain. On January 17, researchers from the University of California, San Diego, reported the development of a thin, flexible electrode that can be inserted deep within the brain and communicate with sensors on the surface.
But what if you could record detailed deep-brain activity without piercing the brain?
A team of researchers (as it happens, also from UC San Diego) have developed a thin, flexible implant that “resides on the brain’s surface” and “can infer neural activity from deeper layers,” said Duygu Kuzum, PhD, a professor of electrical and computer engineering, who led the research.
By combining electrical and optical imaging methods, and artificial intelligence, the researchers used the device — a polymer strip packed with graphene electrodes — to predict deep calcium activity from surface signals, according to a proof-of-concept study published this month in Nature Nanotechnology.
“Almost everything we know about how neurons behave in living brains comes from data collected with either electrophysiology or two-photon imaging,” said neuroscientist Joshua H. Siegle, PhD, of the Allen Institute for Neural Dynamics in Seattle , who not involved in the study. “ Until now, these two methods have rarely been used simultaneously.”
The technology, which has been tested in mice, could help advance our knowledge of how the brain works and may lead to new minimally invasive treatments for neurologic disorders.
Multimodal Neurotech: The Power of 2-in-1
Electrical and optical methods for recording brain activity have been crucial in advancing neurophysiologic science, but each technique has its limits. Electrical recordings provide high “temporal resolution”; they reveal when activation is happening, but not really where. Optical imaging, on the other hand, offers high “spatial resolution,” showing which area of the brain is lighting up, but its measurements may not correspond with the activity’s timing.
Research over the past decade has explored how to combine and harness the strengths of both methods. One potential solution is to use electrodes made of transparent materials such as graphene, allowing a clear field of view for a microscope during imaging. Recently, University of Pennsylvania scientists used graphene electrodes to illuminate the neural dynamics of seizures.
But there are challenges. If graphene electrodes are very small — in this case, 20 µm in diameter — they become more resistant to the flow of electricity. Dr. Kuzum and colleagues addressed this by adding tiny platinum particles to improve electrical conductivity. Long graphene wires connect electrodes to the circuit board, but defects in graphene can interrupt the signal, so they made each wire with two layers; any defects in one wire could be hidden by the other.
By combining the two methods (microelectrode arrays and two-photon imaging), the researchers could see both when brain activity was happening and where, including in deeper layers. They discovered a correlation between electrical responses on the surface and cellular calcium activity deeper down. The team used these data to create a neural network (a type of artificial intelligence that learns to recognize patterns) that predicts deep calcium activity from surface-level readings.
The tech could help scientists study brain activity “in a way not possible with current single-function tools,” said Luyao Lu, PhD, professor of biomedical engineering at George Washington University in Washington, DC, who was not involved in the study. It could shed light on interactions between vascular and electrical activity, or explain how place cells (neurons in the hippocampus) are so efficient at creating spatial memory.
It could also pave the way for minimally invasive neural prosthetics or targeted treatments for neurologic disorders, the researchers say. Implanting the device would be a “straightforward process” similar to placing electrocorticography grids in patients with epilepsy, said Dr. Kuzum.
But first, the team plans to do more studies in animal models before testing the tech in clinical settings, Dr. Kuzum added.
A version of this article appeared on Medscape.com.
Modern technology for recording deep-brain activity involves sharp metal electrodes that penetrate the tissue, causing damage that can compromise the signal and limiting how often they can be used.
A rapidly growing area in materials science and engineering aims to fix the problem by designing electrodes that are softer, smaller, and flexible — safer for use inside the delicate tissues of the brain. On January 17, researchers from the University of California, San Diego, reported the development of a thin, flexible electrode that can be inserted deep within the brain and communicate with sensors on the surface.
But what if you could record detailed deep-brain activity without piercing the brain?
A team of researchers (as it happens, also from UC San Diego) have developed a thin, flexible implant that “resides on the brain’s surface” and “can infer neural activity from deeper layers,” said Duygu Kuzum, PhD, a professor of electrical and computer engineering, who led the research.
By combining electrical and optical imaging methods, and artificial intelligence, the researchers used the device — a polymer strip packed with graphene electrodes — to predict deep calcium activity from surface signals, according to a proof-of-concept study published this month in Nature Nanotechnology.
“Almost everything we know about how neurons behave in living brains comes from data collected with either electrophysiology or two-photon imaging,” said neuroscientist Joshua H. Siegle, PhD, of the Allen Institute for Neural Dynamics in Seattle , who not involved in the study. “ Until now, these two methods have rarely been used simultaneously.”
The technology, which has been tested in mice, could help advance our knowledge of how the brain works and may lead to new minimally invasive treatments for neurologic disorders.
Multimodal Neurotech: The Power of 2-in-1
Electrical and optical methods for recording brain activity have been crucial in advancing neurophysiologic science, but each technique has its limits. Electrical recordings provide high “temporal resolution”; they reveal when activation is happening, but not really where. Optical imaging, on the other hand, offers high “spatial resolution,” showing which area of the brain is lighting up, but its measurements may not correspond with the activity’s timing.
Research over the past decade has explored how to combine and harness the strengths of both methods. One potential solution is to use electrodes made of transparent materials such as graphene, allowing a clear field of view for a microscope during imaging. Recently, University of Pennsylvania scientists used graphene electrodes to illuminate the neural dynamics of seizures.
But there are challenges. If graphene electrodes are very small — in this case, 20 µm in diameter — they become more resistant to the flow of electricity. Dr. Kuzum and colleagues addressed this by adding tiny platinum particles to improve electrical conductivity. Long graphene wires connect electrodes to the circuit board, but defects in graphene can interrupt the signal, so they made each wire with two layers; any defects in one wire could be hidden by the other.
By combining the two methods (microelectrode arrays and two-photon imaging), the researchers could see both when brain activity was happening and where, including in deeper layers. They discovered a correlation between electrical responses on the surface and cellular calcium activity deeper down. The team used these data to create a neural network (a type of artificial intelligence that learns to recognize patterns) that predicts deep calcium activity from surface-level readings.
The tech could help scientists study brain activity “in a way not possible with current single-function tools,” said Luyao Lu, PhD, professor of biomedical engineering at George Washington University in Washington, DC, who was not involved in the study. It could shed light on interactions between vascular and electrical activity, or explain how place cells (neurons in the hippocampus) are so efficient at creating spatial memory.
It could also pave the way for minimally invasive neural prosthetics or targeted treatments for neurologic disorders, the researchers say. Implanting the device would be a “straightforward process” similar to placing electrocorticography grids in patients with epilepsy, said Dr. Kuzum.
But first, the team plans to do more studies in animal models before testing the tech in clinical settings, Dr. Kuzum added.
A version of this article appeared on Medscape.com.
Modern technology for recording deep-brain activity involves sharp metal electrodes that penetrate the tissue, causing damage that can compromise the signal and limiting how often they can be used.
A rapidly growing area in materials science and engineering aims to fix the problem by designing electrodes that are softer, smaller, and flexible — safer for use inside the delicate tissues of the brain. On January 17, researchers from the University of California, San Diego, reported the development of a thin, flexible electrode that can be inserted deep within the brain and communicate with sensors on the surface.
But what if you could record detailed deep-brain activity without piercing the brain?
A team of researchers (as it happens, also from UC San Diego) have developed a thin, flexible implant that “resides on the brain’s surface” and “can infer neural activity from deeper layers,” said Duygu Kuzum, PhD, a professor of electrical and computer engineering, who led the research.
By combining electrical and optical imaging methods, and artificial intelligence, the researchers used the device — a polymer strip packed with graphene electrodes — to predict deep calcium activity from surface signals, according to a proof-of-concept study published this month in Nature Nanotechnology.
“Almost everything we know about how neurons behave in living brains comes from data collected with either electrophysiology or two-photon imaging,” said neuroscientist Joshua H. Siegle, PhD, of the Allen Institute for Neural Dynamics in Seattle , who not involved in the study. “ Until now, these two methods have rarely been used simultaneously.”
The technology, which has been tested in mice, could help advance our knowledge of how the brain works and may lead to new minimally invasive treatments for neurologic disorders.
Multimodal Neurotech: The Power of 2-in-1
Electrical and optical methods for recording brain activity have been crucial in advancing neurophysiologic science, but each technique has its limits. Electrical recordings provide high “temporal resolution”; they reveal when activation is happening, but not really where. Optical imaging, on the other hand, offers high “spatial resolution,” showing which area of the brain is lighting up, but its measurements may not correspond with the activity’s timing.
Research over the past decade has explored how to combine and harness the strengths of both methods. One potential solution is to use electrodes made of transparent materials such as graphene, allowing a clear field of view for a microscope during imaging. Recently, University of Pennsylvania scientists used graphene electrodes to illuminate the neural dynamics of seizures.
But there are challenges. If graphene electrodes are very small — in this case, 20 µm in diameter — they become more resistant to the flow of electricity. Dr. Kuzum and colleagues addressed this by adding tiny platinum particles to improve electrical conductivity. Long graphene wires connect electrodes to the circuit board, but defects in graphene can interrupt the signal, so they made each wire with two layers; any defects in one wire could be hidden by the other.
By combining the two methods (microelectrode arrays and two-photon imaging), the researchers could see both when brain activity was happening and where, including in deeper layers. They discovered a correlation between electrical responses on the surface and cellular calcium activity deeper down. The team used these data to create a neural network (a type of artificial intelligence that learns to recognize patterns) that predicts deep calcium activity from surface-level readings.
The tech could help scientists study brain activity “in a way not possible with current single-function tools,” said Luyao Lu, PhD, professor of biomedical engineering at George Washington University in Washington, DC, who was not involved in the study. It could shed light on interactions between vascular and electrical activity, or explain how place cells (neurons in the hippocampus) are so efficient at creating spatial memory.
It could also pave the way for minimally invasive neural prosthetics or targeted treatments for neurologic disorders, the researchers say. Implanting the device would be a “straightforward process” similar to placing electrocorticography grids in patients with epilepsy, said Dr. Kuzum.
But first, the team plans to do more studies in animal models before testing the tech in clinical settings, Dr. Kuzum added.
A version of this article appeared on Medscape.com.
FROM NATURE NANOTECHNOLOGY
Do ‘Forever Chemicals’ Affect Bone Health in Youth?
Bone health begins in childhood, particularly during the rapid bone accrual phase of puberty, which is essential for attaining optimal peak bone mass. Peak bone mass is achieved in early adult life and affects both immediate and future fracture risk. Genetic, nutritional, exercise-related, and hormonal factors, and certain diseases and medications, have deleterious effects on bone health.
In addition, emerging data suggest that certain manmade chemicals known as per- and polyfluoroalkyl substances (PFAS) may affect bone accrual during this important period and potentially increase the risk for osteoporosis in adulthood. Osteoporosis refers to increased fracture risk because of low bone density and affects a large proportion of postmenopausal women and older men.
New evidence comes from a recent study conducted by investigators from the Keck School of Medicine, who examined the impact of exposure to PFAS on skeletal outcomes in youth. Of note, participants were primarily Hispanic; this population has a higher risk for osteoporosis in adulthood. PFAS are manmade chemicals with water- and grease-resistant properties. They are used in a variety of products, such as nonstick cookware, food packaging, water-repellent clothing, stain-resistant fabrics, carpets, and in certain industrial processes. They are pervasive in the environment, in wildlife, and in humans.
Use and production of certain PFAS, such as perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA), have decreased over the past two decades, with a significant reduction in blood concentrations of these chemicals. However, they can be resistant to degradation and have very long half-lives. As a consequence, these «forever chemicals» continue to linger in the environment. Also, the risk for exposure to other PFAS persists, and almost every individual has detectable levels of PFAS in blood.
Scientists are still learning about the impact of environmental chemicals on bone health. In contrast, other factors that may jeopardize pubertal bone accrual and peak bone mass acquisition have been studied extensively, with guidelines for management of the consequent poor skeletal health.
For PFAS, studies have reported deleterious effects on various body systems, such as the liver, immune system, thyroid, and the developing brain. The limited data related to bone suggest negative associations between certain, but not all, PFAS and bone density — ie, the higher the exposure, the worse the impact on bone health.
PFAS may affect health through alterations in the endocrine system. They have been associated with lower levels of testosterone and downregulation of its receptor (and testosterone is known to modulate bone formation and bone loss). On the other hand, some PFAS are estrogenic, which should be beneficial to bone. A direct impact of PFAS on pathways regulating activity of osteoblasts (bone-forming cells) and osteoclasts (bone-resorbing cells) has also been postulated, with conflicting results.
Previous research on PFAS and human bone health has found mixed results. In adolescents, Xiong and colleagues reported negative associations of PFOS, PFOA, and perfluorononanoic acid (PFNA), but not perfluorohexane sulfonic acid (PFHxS), levels with bone density at various sites, mostly in females. Carwile and associates reported similar negative associations of blood concentrations of PFOA and PFOS and urinary concentrations of phthalates with bone density in adolescents, but only in males. Lin and coworkers also reported negative associations of PFOA and bone density in adult premenopausal women, but found no associations of PFOA and PFOS concentrations with self-reported fractures, suggesting questionable biological significance of these findings. These were all cross-sectional studies and did not report on the impact of these chemicals on longitudinal bone accrual.
In the recent study, Beglarian and colleagues examined the impact of PFAS on longitudinal changes in bone density in adolescents, drawn from the Study of Latino Adolescents at Risk of Type 2 Diabetes (SOLAR) cohort and young adults from the Southern California Children’s Health Study (CHS) cohort. They found that in adolescents, higher baseline concentrations of PFOS predicted lower bone accrual over time. In young adults, there was a similar negative association of PFOS concentrations and bone density at baseline, but not with longitudinal bone accrual. In this study, other PFAS were not associated with bone outcomes.
Overall, research appears to suggest that PFOA, PFOS, and PFNA may have deleterious effects on bone density and bone accrual over time. However, data are not consistent across studies and across sexes, and more research is necessary to conclusively define the impact of these chemicals on skeletal health, particularly during the critical pubertal years of maximal bone accrual. In the meantime, continued efforts are necessary to reduce to concentrations of these PFAS in the environment.
Dr. Misra disclosed ties with AbbVie, Sanofi, and Ipsen.
A version of this article appeared on Medscape.com.
Bone health begins in childhood, particularly during the rapid bone accrual phase of puberty, which is essential for attaining optimal peak bone mass. Peak bone mass is achieved in early adult life and affects both immediate and future fracture risk. Genetic, nutritional, exercise-related, and hormonal factors, and certain diseases and medications, have deleterious effects on bone health.
In addition, emerging data suggest that certain manmade chemicals known as per- and polyfluoroalkyl substances (PFAS) may affect bone accrual during this important period and potentially increase the risk for osteoporosis in adulthood. Osteoporosis refers to increased fracture risk because of low bone density and affects a large proportion of postmenopausal women and older men.
New evidence comes from a recent study conducted by investigators from the Keck School of Medicine, who examined the impact of exposure to PFAS on skeletal outcomes in youth. Of note, participants were primarily Hispanic; this population has a higher risk for osteoporosis in adulthood. PFAS are manmade chemicals with water- and grease-resistant properties. They are used in a variety of products, such as nonstick cookware, food packaging, water-repellent clothing, stain-resistant fabrics, carpets, and in certain industrial processes. They are pervasive in the environment, in wildlife, and in humans.
Use and production of certain PFAS, such as perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA), have decreased over the past two decades, with a significant reduction in blood concentrations of these chemicals. However, they can be resistant to degradation and have very long half-lives. As a consequence, these «forever chemicals» continue to linger in the environment. Also, the risk for exposure to other PFAS persists, and almost every individual has detectable levels of PFAS in blood.
Scientists are still learning about the impact of environmental chemicals on bone health. In contrast, other factors that may jeopardize pubertal bone accrual and peak bone mass acquisition have been studied extensively, with guidelines for management of the consequent poor skeletal health.
For PFAS, studies have reported deleterious effects on various body systems, such as the liver, immune system, thyroid, and the developing brain. The limited data related to bone suggest negative associations between certain, but not all, PFAS and bone density — ie, the higher the exposure, the worse the impact on bone health.
PFAS may affect health through alterations in the endocrine system. They have been associated with lower levels of testosterone and downregulation of its receptor (and testosterone is known to modulate bone formation and bone loss). On the other hand, some PFAS are estrogenic, which should be beneficial to bone. A direct impact of PFAS on pathways regulating activity of osteoblasts (bone-forming cells) and osteoclasts (bone-resorbing cells) has also been postulated, with conflicting results.
Previous research on PFAS and human bone health has found mixed results. In adolescents, Xiong and colleagues reported negative associations of PFOS, PFOA, and perfluorononanoic acid (PFNA), but not perfluorohexane sulfonic acid (PFHxS), levels with bone density at various sites, mostly in females. Carwile and associates reported similar negative associations of blood concentrations of PFOA and PFOS and urinary concentrations of phthalates with bone density in adolescents, but only in males. Lin and coworkers also reported negative associations of PFOA and bone density in adult premenopausal women, but found no associations of PFOA and PFOS concentrations with self-reported fractures, suggesting questionable biological significance of these findings. These were all cross-sectional studies and did not report on the impact of these chemicals on longitudinal bone accrual.
In the recent study, Beglarian and colleagues examined the impact of PFAS on longitudinal changes in bone density in adolescents, drawn from the Study of Latino Adolescents at Risk of Type 2 Diabetes (SOLAR) cohort and young adults from the Southern California Children’s Health Study (CHS) cohort. They found that in adolescents, higher baseline concentrations of PFOS predicted lower bone accrual over time. In young adults, there was a similar negative association of PFOS concentrations and bone density at baseline, but not with longitudinal bone accrual. In this study, other PFAS were not associated with bone outcomes.
Overall, research appears to suggest that PFOA, PFOS, and PFNA may have deleterious effects on bone density and bone accrual over time. However, data are not consistent across studies and across sexes, and more research is necessary to conclusively define the impact of these chemicals on skeletal health, particularly during the critical pubertal years of maximal bone accrual. In the meantime, continued efforts are necessary to reduce to concentrations of these PFAS in the environment.
Dr. Misra disclosed ties with AbbVie, Sanofi, and Ipsen.
A version of this article appeared on Medscape.com.
Bone health begins in childhood, particularly during the rapid bone accrual phase of puberty, which is essential for attaining optimal peak bone mass. Peak bone mass is achieved in early adult life and affects both immediate and future fracture risk. Genetic, nutritional, exercise-related, and hormonal factors, and certain diseases and medications, have deleterious effects on bone health.
In addition, emerging data suggest that certain manmade chemicals known as per- and polyfluoroalkyl substances (PFAS) may affect bone accrual during this important period and potentially increase the risk for osteoporosis in adulthood. Osteoporosis refers to increased fracture risk because of low bone density and affects a large proportion of postmenopausal women and older men.
New evidence comes from a recent study conducted by investigators from the Keck School of Medicine, who examined the impact of exposure to PFAS on skeletal outcomes in youth. Of note, participants were primarily Hispanic; this population has a higher risk for osteoporosis in adulthood. PFAS are manmade chemicals with water- and grease-resistant properties. They are used in a variety of products, such as nonstick cookware, food packaging, water-repellent clothing, stain-resistant fabrics, carpets, and in certain industrial processes. They are pervasive in the environment, in wildlife, and in humans.
Use and production of certain PFAS, such as perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA), have decreased over the past two decades, with a significant reduction in blood concentrations of these chemicals. However, they can be resistant to degradation and have very long half-lives. As a consequence, these «forever chemicals» continue to linger in the environment. Also, the risk for exposure to other PFAS persists, and almost every individual has detectable levels of PFAS in blood.
Scientists are still learning about the impact of environmental chemicals on bone health. In contrast, other factors that may jeopardize pubertal bone accrual and peak bone mass acquisition have been studied extensively, with guidelines for management of the consequent poor skeletal health.
For PFAS, studies have reported deleterious effects on various body systems, such as the liver, immune system, thyroid, and the developing brain. The limited data related to bone suggest negative associations between certain, but not all, PFAS and bone density — ie, the higher the exposure, the worse the impact on bone health.
PFAS may affect health through alterations in the endocrine system. They have been associated with lower levels of testosterone and downregulation of its receptor (and testosterone is known to modulate bone formation and bone loss). On the other hand, some PFAS are estrogenic, which should be beneficial to bone. A direct impact of PFAS on pathways regulating activity of osteoblasts (bone-forming cells) and osteoclasts (bone-resorbing cells) has also been postulated, with conflicting results.
Previous research on PFAS and human bone health has found mixed results. In adolescents, Xiong and colleagues reported negative associations of PFOS, PFOA, and perfluorononanoic acid (PFNA), but not perfluorohexane sulfonic acid (PFHxS), levels with bone density at various sites, mostly in females. Carwile and associates reported similar negative associations of blood concentrations of PFOA and PFOS and urinary concentrations of phthalates with bone density in adolescents, but only in males. Lin and coworkers also reported negative associations of PFOA and bone density in adult premenopausal women, but found no associations of PFOA and PFOS concentrations with self-reported fractures, suggesting questionable biological significance of these findings. These were all cross-sectional studies and did not report on the impact of these chemicals on longitudinal bone accrual.
In the recent study, Beglarian and colleagues examined the impact of PFAS on longitudinal changes in bone density in adolescents, drawn from the Study of Latino Adolescents at Risk of Type 2 Diabetes (SOLAR) cohort and young adults from the Southern California Children’s Health Study (CHS) cohort. They found that in adolescents, higher baseline concentrations of PFOS predicted lower bone accrual over time. In young adults, there was a similar negative association of PFOS concentrations and bone density at baseline, but not with longitudinal bone accrual. In this study, other PFAS were not associated with bone outcomes.
Overall, research appears to suggest that PFOA, PFOS, and PFNA may have deleterious effects on bone density and bone accrual over time. However, data are not consistent across studies and across sexes, and more research is necessary to conclusively define the impact of these chemicals on skeletal health, particularly during the critical pubertal years of maximal bone accrual. In the meantime, continued efforts are necessary to reduce to concentrations of these PFAS in the environment.
Dr. Misra disclosed ties with AbbVie, Sanofi, and Ipsen.
A version of this article appeared on Medscape.com.
Expanding the Psoriasis Framework: Immunopathogenesis and Treatment Updates
Psoriasis is a chronic inflammatory disease that affects approximately 3% of the US population.1 Plaque psoriasis comprises 80% to 90% of cases, while pustular, erythrodermic, guttate, inverse, and palmoplantar disease are less common variants (Figure 1). Psoriatic skin manifestations range from localized to widespread or generalized disease with recurrent flares. Body surface area or psoriasis area and severity index (PASI) measurements primarily focus on skin manifestations and are important for evaluating disease activity and response to treatment, but they have inherent limitations: they do not capture extracutaneous disease activity, systemic inflammation, comorbid conditions, quality of life impact, or the economic burden of psoriasis.
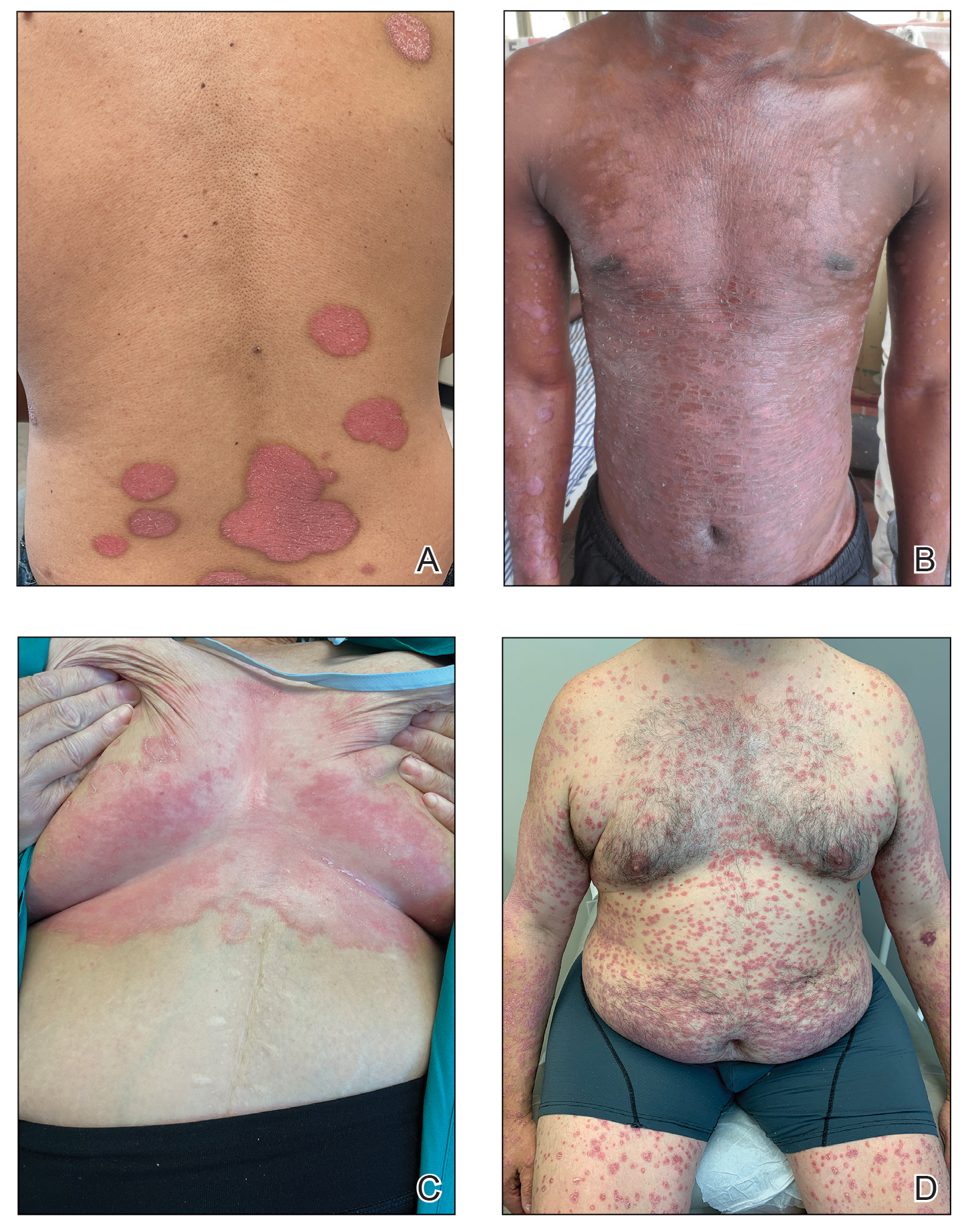
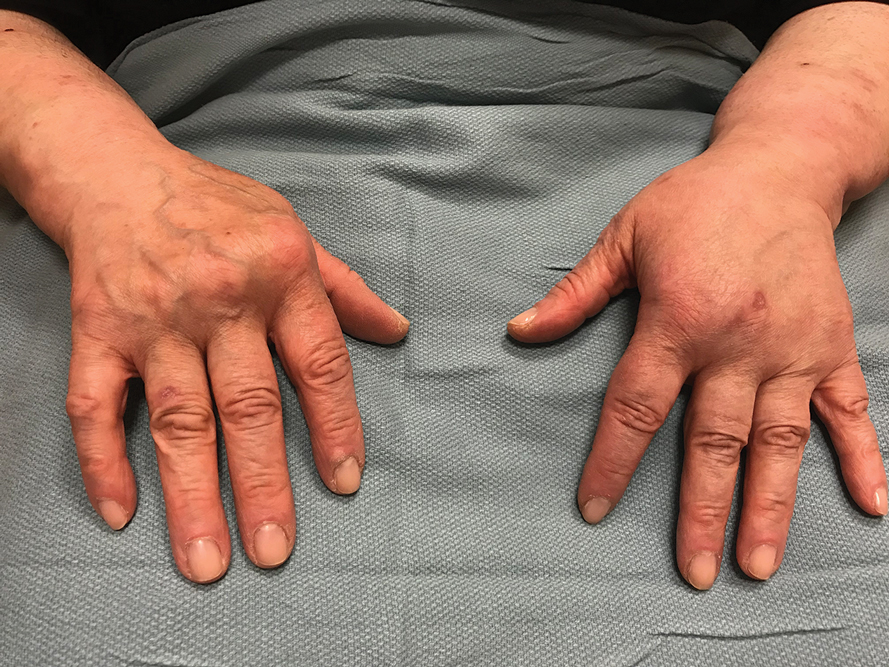
A common manifestation of psoriasis is psoriatic arthritis (PsA), which can involve the nails, joints, ligaments, or tendons in 30% to 41% of affected individuals (Figure 2).2,3 A growing number of psoriasis-associated comorbidities also have been reported including metabolic syndrome4; hyperlipidemia5; cardiovascular disease6; stroke7; hypertension8; obesity9; sleep disorders10; malignancy11; infections12; inflammatory bowel disease13; and mental health disorders such as depression,14 anxiety,15 and suicidal ideation.15 Psoriatic disease also interferes with daily life activities and a patient’s overall quality of life, including interpersonal relationships, intimacy, employment, and work productivity.16 Finally, the total estimated cost of psoriasis-related health care is more than $35 billion annually,17 representing a substantial economic burden to our health care system and individual patients.
The overall burden of psoriatic disease has declined markedly in the last 2 decades due to revolutionary advances in our understanding of the immunopathogenesis of psoriasis and the subsequent development of improved therapies that predominantly interrupt IL-23/IL-17 cytokine signaling; however, critical knowledge and treatment gaps persist, underscoring the importance of ongoing clinical and research efforts in psoriatic disease. We review the working immune model of psoriasis, summarize related immune discoveries, and highlight recent therapeutic innovations that are shaping psoriatic disease management.
Current Immune Model of Psoriatic Disease
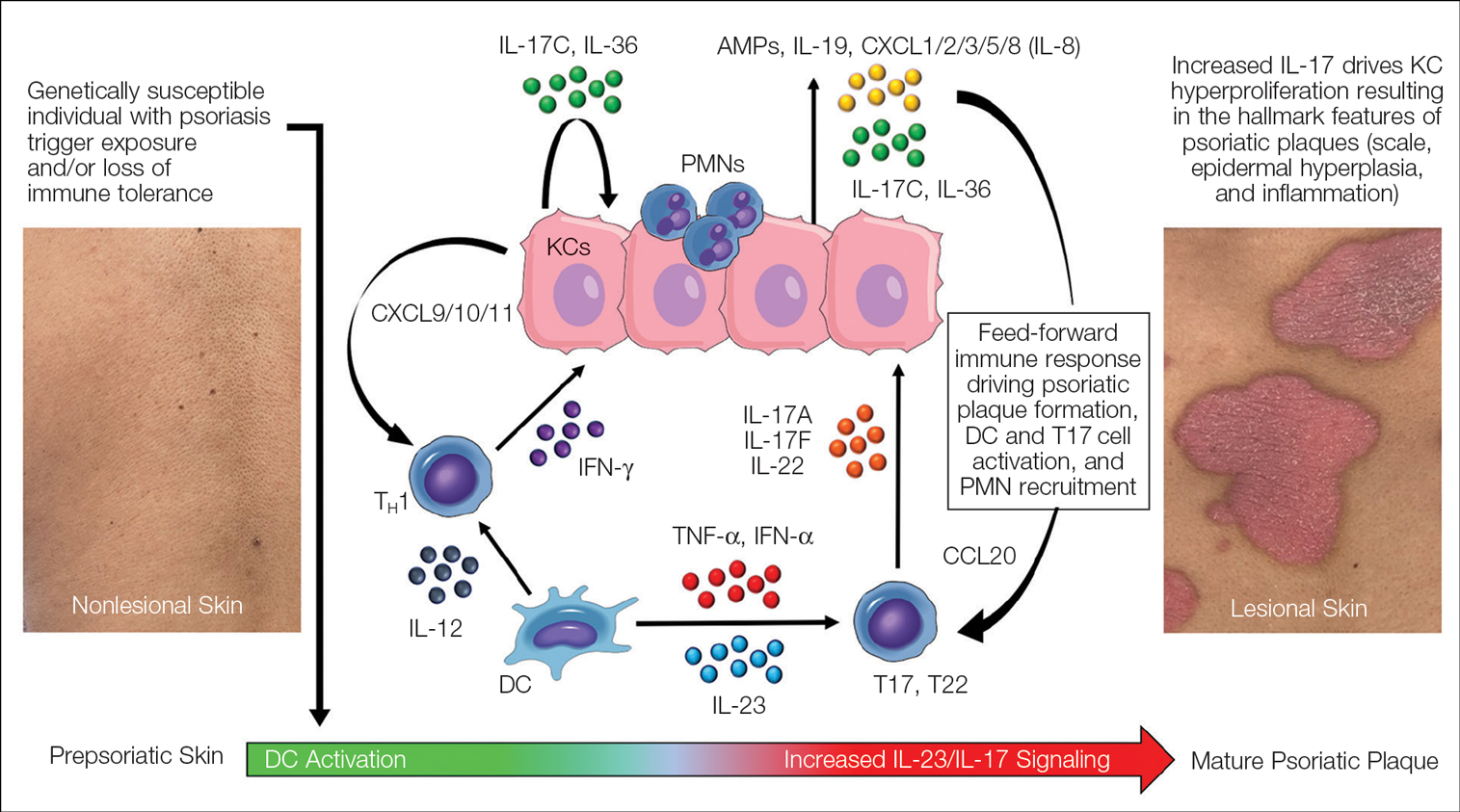
Psoriasis is an autoinflammatory T cell–mediated disease with negligible contributions from the humoral immune response. Early clinical observations reported increased inflammatory infiltrates in psoriatic skin lesions primarily consisting of both CD4+ and CD8+ T-cell populations.18,19 Additionally, patients treated with broad-acting, systemic immunosuppressive medications (eg, cyclosporine, oral corticosteroids) experienced improvement of psoriatic lesions and normalization of the immune infiltrates observed in skin biopsy specimens.20,21 These early clinical findings led to more sophisticated experimentation in xenotransplant models of psoriasis,22,23 which explored the clinical efficacy of several less immunosuppressive (eg, methotrexate, anti–tumor necrosis factor [TNF] biologics)24 or T cell–specific agents (eg, alefacept, abatacept, efalizumab).25-27 The results of these translational studies provided indisputable evidence for the role of the dysregulated immune response as the primary pathogenic process driving plaque formation; they also led to a paradigm shift in how the immunopathogenesis of psoriatic disease was viewed and paved the way for the identification and targeting of other specific proinflammatory signals produced by activated dendritic cell (DC) and T-lymphocyte populations. Among the psoriasis-associated cytokines subsequently identified and studied, elevated IL-23 and IL-17 cytokine levels in psoriatic skin were most closely associated with disease activity, and rapid normalization of IL-23/IL-17 signaling in response to effective oral or injectable antipsoriatic treatments was the hallmark of skin clearance.28 The predominant role of IL-23/IL-17 signaling in the development and maintenance of psoriatic disease is the central feature of all working immune models for this disease (Figure 3).
Psoriasis-Associated Genetic and Environmental Risk Factors
The exact sequence of events that lead to the initiation and formation of plaque psoriasis in susceptible individuals is still poorly understood; however, several important risk factors and key immune events have been identified. First, decades of genetic research have reported more than 80 known psoriasis-associated susceptibility loci,29 which explains approximately 50% of psoriasis heritability. The major genetic determinant of psoriasis, HLA-C*06:02 (formerly HLA-Cw6), resides in the major histocompatibility complex class I region on chromosome 6p21.3 (psoriasis susceptibility gene 1, PSORS1) and is most strongly associated with psoriatic disease.30 Less common psoriasis-associated susceptibility genes also are known to directly or indirectly impact innate and adaptive immune functions that contribute to the pathogenesis of psoriasis.
Second, several nongenetic environmental risk factors for psoriasis have been reported across diverse patient populations, including skin trauma/injury, infections, alcohol/tobacco use, obesity, medication exposure (eg, lithium, antimalarials, beta-blockers), and stress.31 These genetic and/or environmental risk factors can trigger the onset of psoriatic disease at any stage of life, though most patients develop disease in early adulthood or later (age range, 50–60 years). Some patients never develop psoriasis despite exposure to environmental risk factors and/or a genetic makeup that is similar to affected first-degree relatives, which requires further study.
Prepsoriatic Skin and Initiation of Plaque Development
In response to environmental stimuli and/or other triggers of the immune system, DC and resident IL-17–producing T-cell (T17) populations become activated in predisposed individuals. Dendritic cell activation leads to the upregulation and increase of several proinflammatory cytokines, including TNF, interferon (IFN) α, IFN-γ, IL-12, and IL-23. Tumor necrosis factor and IL-23 play a vital role in psoriasis by helping to regulate the polarization and expansion of T22 and T17 cells in the skin, whereas IL-12 promotes a corresponding type 1 inflammatory response.32 Increased IL-17 and IL-22 result in alteration of the terminal differentiation and proliferative potential of epidermal keratinocytes, leading to the early clinical hallmarks of psoriatic plaques. The potential contribution of overexpressed psoriasis-related autoantigens, such as LL-37/cathelicidin, ADAMTSL5, and PLA2G4D,33 in the initiation of psoriatic plaques has been suggested but is poorly characterized.34 Whether these specific autoantigens or others presented by HLA-C variants found on antigen-presenting cells are required for the breakdown of immune tolerance and psoriatic disease initiation is highly relevant but requires further investigation and validation.
Feed-Forward Inflammation, Mature Psoriatic Plaques, and Resident Memory T Cells
In response to the upstream production of IL-23 by dermal DCs, high levels of IL-17 cytokines can be found in mature psoriatic plaques. The IL-17 family consists of 6 dimeric cytokines (IL-17A through IL-17F) that provide innate cutaneous protection against bacterial, viral, and fungal infectious agents, such as Candida albicans. Unlike other IL-17 isoforms, IL-17A and IL-17F share the same receptor complex and have the highest structural homology of any pair (approximately 50% similar).35 The relative expression of IL-17F is higher than IL-17A in psoriasis,36 though IL-17A has been considered as the predominant IL-17 cytokine found in psoriatic skin lesions due to its higher potency.
Binding of IL-17A/F with the IL-17 receptor (IL-17R) on keratinocytes contributes to the development of psoriatic plaques by inducing epidermal hyperplasia via activation of CCAAT/enhancer-binding proteins β and δ, nuclear factor κB, and signal transducer and activator of transcription 1 gene (STAT1).37,38 This also increases the expression of other keratinocyte-derived proteins (eg, human β-defensins, S-100 proteins, LL-37, other antimicrobial peptides, IL-19, IL-36, IL-17C) that act as reinforcing proinflammatory signals or chemotactic factors (eg, chemokine [C-C motif] ligand 20 [CCL20], chemokine [C-C motif] ligand 1/2/3/5 [CXCL1/2/3/5], CXCL8, IL-8) that facilitate the recruitment of additional immune cells to the skin including polymorphonuclear neutrophils (PMNs), macrophages, and DCs.39-41 Routine immunohistochemical staining for these keratinocyte-derived proteins reveals a striking epidermal gene expression gradient wherein levels of IL-17–induced proteins are most highly expressed in the uppermost layers of keratinocytes and facilitate the recruitment of immune cells into the epidermis. Activated T17 cells also stimulate the production of keratinocyte-derived chemokines (eg, CXCL9/10/11), which recruit type 1 inflammatory T-cell populations into developing psoriatic plaques.42,43 Finally, TNF, IL-36, and IL-17C cytokines act synergistically with IL-17A/F to amplify the proinflammatory effects of IL-17 signaling and further stimulate their production from T17 cell populations.40 This inflammatory circuit in the skin creates and supports a self-amplifying or positive feedback loop between the skin and immune system that commonly is referred to as feed-forward inflammation (Figure 3).34 The feed-forward inflammatory loop in psoriasis—predominantly driven by increased IL-23/IL-17 signaling—best characterizes the mature psoriatic plaque.
Several findings suggest that the influx of persistent, long-lived resident memory T cells (Trms) may contribute to the mature psoriatic plaque. It is believed that CD8+CD103+CD49a− Trm cell populations may be responsible for the sharply demarcated borders of untreated psoriasis plaques or their recurrence at specific body sites such as the scalp, buttocks, extremity extensor surfaces, umbilicus, or acral skin following specific stimuli or trauma (Koebner phenomenon or isomorphic response).44,45 It is not known if repeated stimuli or trauma induce disease formation via the activation of Trm cell populations; further study in large patient cohorts is needed, but this remains an intriguing area of study for durable treatment responses and potential cures for psoriasis.
Recent Discoveries in Psoriatic Disease
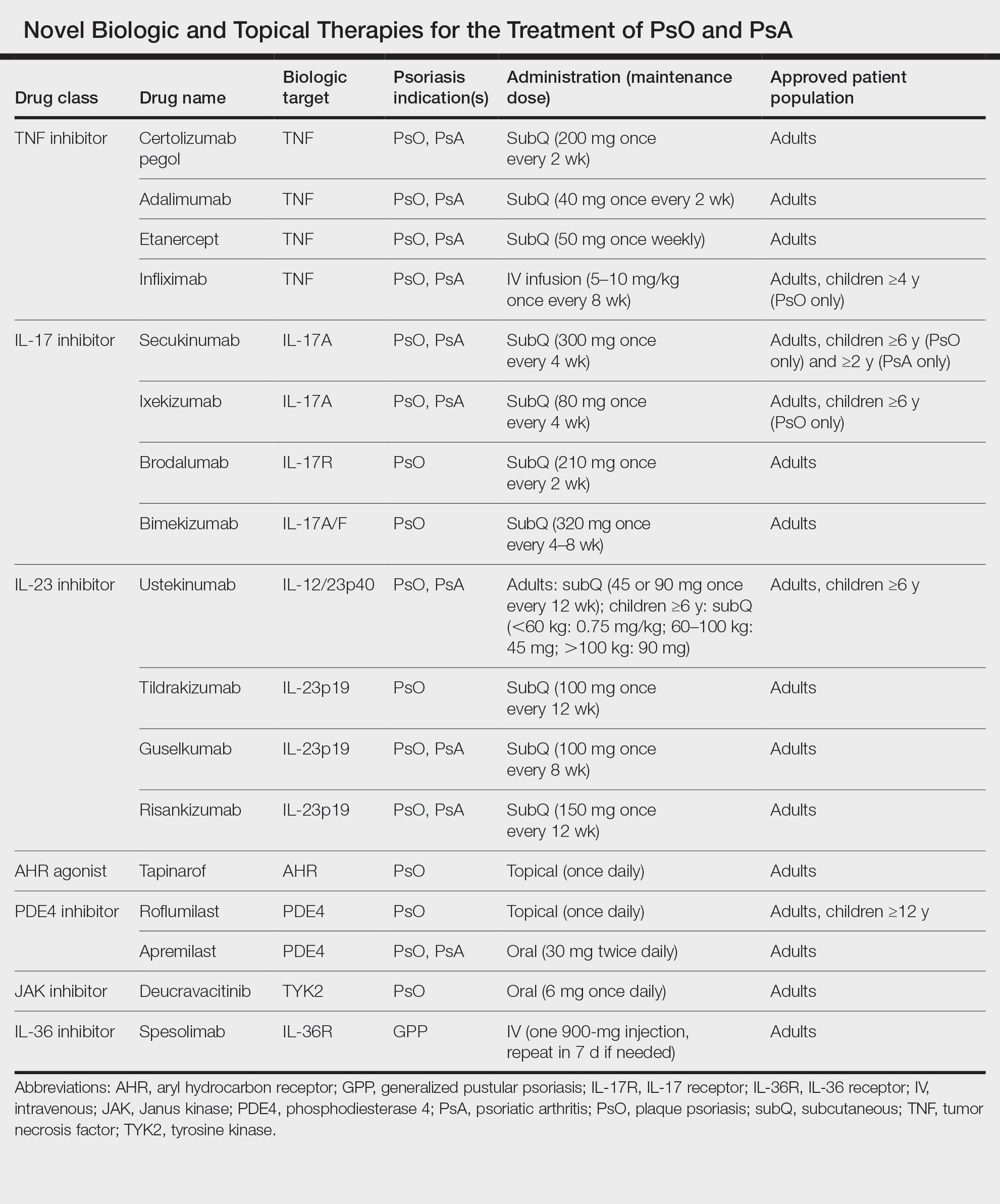
Remarkable treatment outcomes for psoriasis have been achieved with multiple selective IL-17 and IL-23 inhibitors (eTable). As demonstrated in several pivotal phase 3 clinical trials for members of these classes of medications, the majority of treated psoriasis patients achieved PASI90 clearance.46 Due to their more favorable dosing schedule (ie, fewer injections) and ability to induce a durable remissionlike treatment response, IL-23 inhibitors have become the preferred treatment class for cutaneous disease, while IL-17 inhibitors may be preferred when treating patients with both plaque psoriasis and PsA.47,48 Nevertheless, the complexity of this disease is punctuated by treated patients who do not adequately respond to selective IL-23/IL-17 blockade.49 Recent and emerging treatments may shed light on these recalcitrant cases and will add to the rapidly growing arsenal of available psoriasis therapies.
The Role of IL-17F in Psoriasis and Other Inflammatory Skin Diseases
Dysregulation of IL-17A and IL-17F is associated with several chronic inflammatory conditions, such as psoriasis and PsA.35,50 Both cytokines, either as homodimers or heterodimers, can selectively bind to the heterodimeric IL-17R formed by the IL-17RA and IL-17RC subunits.35 IL-17F and IL-17C also can synergize with TNF and other cytokines to promote and support the self-sustaining inflammatory circuits in mature psoriatic plaques, though their inflammatory effects in the skin are more limited than IL-17A.51,52 Therefore, incomplete blockade of IL-17 signaling (ie, unopposed IL-17F and IL-17C) represents a potential mechanism to explain the persistence of psoriasis in patients treated with selective IL-17A inhibitors. This hypothesis is supported by reports of psoriasis patients who have inadequate clinical responses to selective IL-17A inhibition but subsequently improve with IL-17R blockade, which results in disruption of IL-17A as well as IL-17C/E/F cytokine signaling. This formed the basis for further study into the specific role of IL-17F in psoriatic disease and any potential therapeutic benefits associated with its inhibition.
Recently approved in the European Union, Canada, Australia, Japan, the United Kingdom, and the United States for moderate to severe psoriasis, bimekizumab is a novel humanized IgG antibody that selectively inhibits both IL-17A and IL-17F cytokines.53 Specifically, bimekizumab simultaneously prevents binding of IL-17A/A, IL-17A/F, and IL-17F/F dimers with the IL-17R. Compared to other IL-17 and IL-23 biologic therapies, bimekizumab (320 mg) achieved relatively higher response rates for PASI75, PASI90, and PASI100.49 Neutralization of IL-17A and IL-17F by bimekizumab also resulted in more complete suppression of cytokine responses and PMN chemotaxis than either cytokine alone in treated PsA patients,54 which is notable because of the incremental benefits of recent IL-23 and IL-17 inhibitors on inflammatory arthritis symptoms in contrast to the substantial improvements observed for cutaneous disease with those same agents.
The primary disadvantage of bimekizumab and its more complete blockade of the IL-17 signaling pathway is that treated patients have a substantially increased risk for oral candidiasis (>10%).55 However, the precise link between candidiasis and IL-17 blockade is not yet fully understood because other targeted agents that also broadly suppress IL-17 signaling (ie, IL-17R, IL-23 inhibitors) are associated with much lower rates of candidiasis.56-58 Bimekizumab also is being investigated as a novel therapy for hidradenitis suppurativa and will provide important reference information regarding the role for bispecific biologic agents in the treatment of chronic inflammatory skin diseases.59
IL-36 Signaling and Generalized Pustular Psoriasis
Recent genetic and clinical studies have expanded our understanding of the role of IL-36 signaling in the immunopathogenesis of pustular psoriasis variants. Generalized pustular psoriasis (GPP) is a rare distinct psoriasis subtype characterized by the recurrent development of widespread erythema, superficial sterile pustules, and desquamation. Systemic symptoms such as fever, malaise, itching, and skin pain accompany acute GPP flares.60 Generalized pustular psoriasis is more common in female patients (in contrast with plaque psoriasis), and acute flares may be caused by multiple stimuli including infections, hypocalcemia, initiation or discontinuation of medications (eg, oral corticosteroids), pregnancy, or stress.61,62 Flares of GPP often require emergency or in-patient care, as untreated symptoms increase the risk for severe health complications such as secondary infections, sepsis, or multisystem organ failure.63 The prevalence of GPP is estimated to be approximately 1 in 10,000 individuals in the United States,64-67 with mortality rates ranging from 0 to 3.3 deaths per 100 patient-years.67
In contrast to plaque psoriasis, aberrant IL-36 signaling is the predominant driver of GPP. IL-36 is a member of the IL-1 cytokine family that includes three IL-36 agonists (IL-36α, IL-36β, IL-36γ) and 1 endogenous antagonist (IL-36Ra, encoded by IL36RN).68 The immunopathogenesis of GPP involves dysregulation of the IL-36–chemokine–PMN axis, resulting in unopposed IL-36 signaling and the subsequent recruitment and influx of PMNs into the epidermis. IL36RN mutations are strongly associated with GPP and result in impaired function of the IL-36Ra protein, leading to unopposed IL-36 signaling.69 However, approximately two-thirds of GPP patients lack identifiable gene mutations, suggesting other immune mechanisms or triggers causing upregulated IL-36 signaling.70 In response to these triggers, increased IL-36 cytokines released by keratinocytes bind to the IL-36R, resulting in substantial keratinocyte hyperproliferation, increased IL-36 levels, and the expression of hundreds of additional inflammatory signals (eg, IL-17C, antimicrobial peptides, TNF, IL-6).71 Increased IL-36 levels also drive the production of PMN chemotactic proteins (eg, CXCL1/2/3/5/6/8 and CXCR1/2) and act synergistically with IL-17 cytokines to create an autoamplifying circuit that is analogous to the feed-forward inflammatory loop in plaque psoriasis.72 Biopsies of involved GPP skin reveal increased expression of IL-36 in the uppermost layers of the epidermis, which creates a gene expression gradient that acts as a strong attractant for PMNs and forms the basis for the hallmark pustular lesions observed in GPP patients.
Until recently, treatment strategies for GPP involved the off-label use of topical, oral, or biologic therapies approved for plaque psoriasis, which often was associated with variable or incomplete disease control. In September 2022, the US Food and Drug Administration (FDA) approved intravenous spesolimab as a first-in-class humanized monoclonal IgG1 antibody for the treatment of GPP flares in adults. Spesolimab binds to IL-36R and prevents its activation by its endogenous agonists. A phase 2, randomized, 12-week clinical trial (Effisayil-1) evaluated the efficacy and safety of a single 900-mg intravenous dose of spesolimab followed by an optional second dose 1 week later for inadequate treatment responses in 53 enrolled GPP patients (2:1 treatment to placebo randomization).73 Remarkably, more than half (19/35 [54%]) of GPP patients experienced complete resolution of pustules (GPP physician global assessment subscore of 0 [range, 0–4]) and showed sustained efficacy out to week 12 after just 1 or 2 doses of spesolimab. Overall, the safety profile of spesolimab was good; asthenia, fatigue, nausea, vomiting, headache, pruritus, infusion-related reaction and symptoms, and mild infections (eg, urinary tract infection) were the most common adverse events reported.73
Imsidolimab, a high-affinity humanized IgG4 monoclonal antibody that binds and blocks activation of IL-36R, also has completed phase 2 testing,74 with phase 3 study results expected in early 2024. The rapid onset of action and overall safety of imsidolimab was in line with and similar to spesolimab. Future approval of imsidolimab would add to the limited treatment options available for GPP and has the additional convenience of being administered to patients subcutaneously. Overall, the development of selective IL-36R inhibitors offers a much-needed therapeutic option for GPP and illustrates the importance of translational research.
Role of Tyrosine Kinase in Psoriatic Disease
The Janus kinase (JAK) enzyme family consists of 4 enzymes—tyrosine kinase 2 (TYK2), JAK1, JAK2, and JAK3—that function as intracellular transduction signals that mediate the biologic response of most extracellular cytokines and growth factors.75 Critical psoriasis-related cytokines are dependent on intact JAK-STAT signaling, including IL-23, IL-12, and type I IFNs. In 2010, a genome-wide association identified TYK2 as a psoriasis susceptibility locus,76 and loss-of-function TYK2 mutations confer a reduced risk for psoriasis.77 Unlike other JAK isoforms, TYK2 mediates biologic functions that are highly restricted to the immune responses associated with IL-23, IL-12, and type I IFN signaling.78,79 For these reasons, blockade of TYK2 signaling is an attractive therapeutic target for the potential treatment of psoriatic disease.
In September 2022, the FDA approved deucravacitinib as a first-in-class, oral, selective TYK2 inhibitor for the treatment of adult patients with moderate to severe plaque psoriasis. It was the first FDA approval of an oral small-molecule treatment for plaque psoriasis in nearly a decade. Deucravacitinib inhibits TYK2 signaling via selective binding of its unique regulatory domain, resulting in a conformational (allosteric) change that interferes with its active domain.80 This novel mechanism of action limits the unwanted blockade of other broad biologic processes mediated by JAK1/2/3. Of note, the FDA did not issue any boxed warnings for deucravacitinib as it did for other FDA-approved JAK inhibitors.
In a head-to-head, 52-week, double-blind, prospective, randomized, phase 3 study, deucravacitinib showed clear superiority over apremilast for PASI75 at week 16 (53.0% [271/511] vs 39.8% [101/254]) and week 24 (58.7% [296/504] vs 37.8% [96/254]).81 Clinical responses were sustained through week 52 and showed efficacy for difficult-to-treat areas such as the scalp, acral sites, and nails. Other advantages of deucravacitinib include once-daily dosing with no need for dose titration or adjustments for renal insufficiency as well as the absence of statistically significant differences in gastrointestinal tract symptoms compared to placebo. The most common adverse effects included nasopharyngitis, upper respiratory tract infections, headache, diarrhea, and herpes infections.81 The potential benefit of deucravacitinib for PsA and psoriasis comorbidities remains to be seen, but it is promising due to its simultaneous disruption of multiple psoriasis-related cytokine networks. Several other TYK2 inhibitors are being developed for psoriatic disease and related inflammatory conditions, underscoring the promise of targeting this intracellular pathway.
Aryl Hydrocarbon Receptor Agonism
Topical steroids are the mainstay treatment option for localized or limited plaque psoriasis due to their potent immunosuppressive effect on the skin and relatively low cost. Combined with vitamin D analogs, topical steroids result in marked improvements in disease severity and improved tolerability.82 However, chronic use of topical steroids is limited by the need for twice-daily application, resulting in poor treatment compliance; loss of efficacy over time; risk for steroid-induced skin atrophy on special body sites; and patient concerns of potential systemic effects. The discovery of novel drug targets amenable to topical inhibition is needed.
Dysregulated aryl hydrocarbon receptor (AHR) levels have been reported in atopic dermatitis and psoriasis.83 Aryl hydrocarbon receptors are ubiquitously expressed in many cell types and play an integral role in immune homeostasis within the skin, skin barrier function, protection against oxidative stressors, and regulation of proliferating melanocytes and keratinocytes.84,85 They are widely expressed in multiple immune cell types (eg, antigen-presenting cells, T lymphocytes, fibroblasts) and modulate the differentiation of T17 and T22 cells as well as their balance with regulatory T-cell populations.86 In keratinocytes, AHR helps to regulate terminal differentiation, enhance skin barrier integrity via AHR-dependent filaggrin (FLG) expression, and prevent transepidermal water loss.87,88 The mechanisms by which AHR ligands lead to the upregulation or downregulation of specific genes is intricate and highly context dependent, such as the specific ligand and cell type involved. In preclinical studies, AHR-deficient mice develop psoriasiform skin inflammation, increased IL-17 and IL-22 expression, and abnormal skin barrier function.89 Keratinocytes treated with AHR ligands in vitro modulated psoriasis-associated inflammatory cytokines, such as IL-6, IL-8, and type I and II IFNs.89,90 The use of coal tar, one of the earliest historical treatments for psoriasis, is thought to activate AHRs in the skin via organic compound mixtures containing polyaromatic hydrocarbons that help normalize the proinflammatory environment in psoriatic skin.91
In June 2022, the FDA approved tapinarof as a first-in-class, topical, nonsteroidal AHR agonist for the treatment of plaque psoriasis in adults. Although the exact mechanism of action for tapinarof has not been fully elucidated, early studies suggest that its primary function is the activation of AHR, leading to reduced T-cell expansion and T17 cell differentiation. In the imiquimod mouse model, cytokine expression of IL-17A, IL-17F, IL-19, IL-22, IL-23A, and IL-lβ in psoriasiform skin lesions were downregulated following tapinarof treatment.92 In humans, tapinarof treatment is associated with a remittive effect, in which the average time for tapinarof-treated psoriasis lesions to remain clear was approximately 4 months.93 Preliminary research investigating the mechanism by which tapinarof induces this remittive effect is ongoing and may involve the reduced activation and influx of T17 and Trm populations into the skin.94 However, these preclinical studies were performed on healthy dermatome-derived skin tissue cultured in T17-skewing conditions and needs to be replicated in larger samples sizes using human-derived psoriatic tissue. Alternatively, a strong inhibitory effect on IL-23 cytokine signaling may, in part, explain the remittive effect of tapinarof, as an analogous response is observed in patients who start and discontinue treatment with selective IL-23 antagonists. Regardless, the once-daily dosing of tapinarof and sustained treatment response is appealing to psoriasis patients. Tapinarof generally is well tolerated with mild folliculitis (>20% of patients) and contact dermatitis (5% of patients) reported as the most common skin-related adverse events.
New Roles for Phosphodiesterase 4 Inhibition
Phosphodiesterases (PDEs) are enzymes that hydrolyze cyclic nucleotides (eg, cyclic adenosine monophosphate) to regulate intracellular secondary messengers involved in the inflammatory response. One of several enzymes in the PDE family, PDE4, has been shown to have greater activity in psoriatic skin compared to healthy skin.95 Phosphodiesterase inhibitors decrease the degradation of cyclic adenosine monophosphate, which triggers protein kinase A to downregulate proinflammatory (eg, TNF-α, IL-6, IL-17, IL-12, IL-23) cytokines and increased expression of anti-inflammatory signals such as IL-10.96,97 Apremilast, the first oral PDE4 inhibitor approved by the FDA for psoriasis, offered a safe alternative to traditional oral immunosuppressive agents that had extensive risks and potential end-organ adverse effects. Unfortunately, apremilast demonstrated modest efficacy for psoriatic disease (better efficacy in the skin vs joint manifestations) and was supplanted easily by next-generation targeted biologic agents that were more efficacious and lacked the troublesome gastrointestinal tract adverse effects of PDE4 inhibition.98
Crisaborole became the first topical PDE4 inhibitor approved in the United States in December 2016 for twice-daily treatment of atopic dermatitis. Although phase 2 trial results were reported in psoriasis, this indication was never pursued, presumably due to similar improvements in primary outcome measures at week 12, compared to placebo (ClinicalTrials.gov Identifier NCT01300052).
In July 2022, the first topical PDE4 inhibitor indicated for plaque psoriasis was approved by the FDA—roflumilast cream 0.3% for once-daily use in individuals 12 years and older. Roflumilast was found to be clinically efficacious as early as 2 weeks after its use in an early-phase clinical trial.99 In 2 phase 3 clinical trials (DERMIS-1 and DERMIS-2), roflumilast significantly increased the proportion of patients achieving PASI75 at week 8 compared to vehicle (39%–41.6% vs 5.3%–7.6%, respectively)(P<.001).100 Overall, this nonsteroidal topical therapy was found to be well tolerated, with infrequent reports of application site pain or irritation as adverse events. Similar to tapinarof, patients can apply roflumilast on all body surface areas including the face, external genitalia, and other intertriginous areas.100 Importantly, the broad immune impact of PDE4 inhibition suggests that topical roflumilast likely will be an effective treatment for several additional inflammatory conditions, including seborrheic dermatitis and atopic dermatitis, which would expand the clinical utility of this specific medication.
Conclusion
In the last 2 decades, we have witnessed a translational revolution in our understanding of the underlying genetics and immunology of psoriatic disease. Psoriasis is widely considered one of the best-managed inflammatory conditions in all of medicine due to the development and availability of highly targeted, effective topical and systemic therapies that predominantly disrupt IL-23/IL-17 cytokine signaling in affected tissues. However, future clinical studies and laboratory research are necessary to elucidate the precise cause of psoriasis as well as the underlying genetic and immune signaling pathways driving less common clinical variants and recalcitrant disease.
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Ogdie A, Langan S, Love T, et al. Prevalence and treatment patterns of psoriatic arthritis in the UK. Rheumatology (Oxford). 2013;52:568-575. doi:10.1093/rheumatology/kes324
- Mease PJ, Gladman DD, Papp KA, et al. Prevalence of rheumatologistdiagnosed psoriatic arthritis in patients with psoriasis in European/ North American dermatology clinics. J Am Acad Dermatol. 2013;69:729- 735. doi:10.1016/j.jaad.2013.07.023
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132(3 pt 1):556-562. doi:10.1038/jid.2011.365
- Wu S, Li WQ, Han J, et al. Hypercholesterolemia and risk of incident psoriasis and psoriatic arthritis in US women. Arthritis Rheumatol. 2014;66:304-310. doi:10.1002/art.38227
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and hypertension: a systematic review and meta-analysis of observational studies. J Hypertens. 2013;31:433-442; discussion 442-443. doi:10.1097/HJH.0b013e32835bcce1
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418. doi:10.1038 /jid.2009.112
- Armstrong AW, Lin SW, Chambers CJ, et al. Psoriasis and hypertension severity: results from a case-control study. PLoS One. 2011;6:E18227. doi:10.1371/journal.pone.0018227
- Naldi L, Chatenoud L, Linder D, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case-control study. J Invest Dermatol. 2005;125:61-67. doi:10.1111/j.0022-202X.2005.23681.x
- Yang YW, Kang JH, Lin HC. Increased risk of psoriasis following obstructive sleep apnea: a longitudinal population-based study. Sleep Med. 2012;13:285-289. doi:10.1016/j.sleep.2011.07.018
- Vaengebjerg S, Skov L, Egeberg A, et al. Prevalence, incidence, and risk of cancer in patients with psoriasis and psoriatic arthritis: a systematic review and meta-analysis. JAMA Dermatol. 2020;156:421-429. doi:10.1001/jamadermatol.2020.0024
- Loft N, Skov L, Richardson C, et al. A nationwide population-based cohort study of the incidence of severe and rare infections among adults with psoriasis in Denmark. Br J Dermatol. 2022;187:353-363. doi:10.1111/bjd.21595
- Fu Y, Lee CH, Chi CC. Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol. 2018;154:1417-1423. doi:10.1001/jamadermatol.2018.3631
- Dowlatshahi EA, Wakkee M, Arends LR, et al. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134:1542-1551. doi:10.1038/jid.2013.508
- Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety, and suicidality in patients with psoriasis: a populationbased cohort study. Arch Dermatol. 2010;146:891-895. doi:10.1001 /archdermatol.2010.186
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113. doi:10.1016/j.jaad.2018.11.058
- Vanderpuye-Orgle J, Zhao Y, Lu J, et al. Evaluating the economic burden of psoriasis in the United States. J Am Acad Dermatol. 2015;72:961-967. doi:10.1016/j.jaad.2015.02.1099
- Bos JD, Hagenaars C, Das PK, et al. Predominance of “memory” T cells (CD4+, CDw29+) over “naive” T cells (CD4+, CD45R+) in both normal and diseased human skin. Arch Dermatol Res. 1989;281:24-30. doi:10.1007/BF00424268
- Bos JD, Hulsebosch HJ, Krieg SR, et al. Immunocompetent cells in psoriasis. in situ immunophenotyping by monoclonal antibodies. Arch Dermatol Res. 1983;275:181-189. doi:10.1007/BF00510050
- Griffiths CE, Powles AV, Leonard JN, et al. Clearance of psoriasis with low dose cyclosporin. Br Med J (Clin Res Ed). 1986;293:731-732. doi:10.1136/bmj.293.6549.731
- Ellis CN, Gorsulowsky DC, Hamilton TA, et al. Cyclosporine improves psoriasis in a double-blind study. JAMA. 1986;256:3110-3116.
- Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233-240. doi:10.1084/jem.20041257
- Ferber IA, Brocke S, Taylor-Edwards C, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J Immunol. 1996;156:5-7.
- Bharadwaj R, Lusi CF, Mashayekh S, et al. Methotrexate suppresses psoriatic skin inflammation by inhibiting muropeptide transporter SLC46A2 activity. Immunity. 2023;56:998-1012. doi:10.1016/j. immuni.2023.04.001
- Jenneck C, Novak N. The safety and efficacy of alefacept in the treatment of chronic plaque psoriasis. Ther Clin Risk Manag. 2007;3:411-420.
- Pariser DM, Gordon KB, Papp KA, et al. Clinical efficacy of efalizumab in patients with chronic plaque psoriasis: results from three randomized placebo-controlled phase III trials: part I. J Cutan Med Surg. 2005; 9:303-312. doi:10.1007/s10227-005-0116-1
- Mease PJ, Gottlieb AB, van der Heijde D, et al. Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis. Ann Rheum Dis. 2017;76:1550-1558. doi:10.1136/annrheumdis-2016-210724
- Hawkes JE, Yan BY, Chan TC, et al. Discovery of the IL-23/IL-17 signaling pathway and the treatment of psoriasis. J Immunol. 2018;201:1605-1613. doi:10.4049/jimmunol.1800013
- Ogawa K, Okada Y. The current landscape of psoriasis genetics in 2020. J Dermatol Sci. 2020;99:2-8. doi:10.1016/j.jdermsci.2020.05.008
- Arakawa A, Siewert K, Stohr J, et al. Melanocyte antigen triggers autoimmunity in human psoriasis. J Exp Med. 2015;212:2203-2212. doi:10.1084/jem.20151093
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263-271. doi:10.1016/S0140-6736(07)61128-3
- Wang CQF, Akalu YT, Suarez-Farinas M, et al. IL-17 and TNF synergistically modulate cytokine expression while suppressing melanogenesis: potential relevance to psoriasis. J Invest Dermatol. 2013;133:2741-2752. doi:10.1038/jid.2013.237
- Cheung KL, Jarrett R, Subramaniam S, et la. Psoriatic T cells recognize neolipid antigens generated by mast cell phospholipase delivered by exosomes and presented by CD1a. J Exp Med. 2016;213:2399-2412. doi:10.1084/jem.20160258
- Hawkes JE, Gonzalez JA, Krueger JG. Autoimmunity in psoriasis: evidence for specific autoantigens. Curr Dermatol Rep. 2017;6:104-112. doi:10.1007/s13671-017-0177-6
- Johansen C, Usher PA, Kjellerup RB, et al. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br J Dermatol. 2009;160:319-324. doi:10.1111/j.1365-2133 .2008.08902.x
- Kolbinger F, Loesche C, Valentin MA, et al. beta-Defensin 2 is a responsive biomarker of IL-17A-driven skin pathology in patients with psoriasis. J Allergy Clin Immunol. 2017;139:923-932. doi:10.1016/j .jaci.2016.06.038
- Ruddy MJ, Wong GC, Liu XK, et al. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J Biol Chem. 2004;279:2559-2567. doi:10.1074/jbc.M308809200
- Shen F, Hu Z, Goswami J, et al. Identification of common transcriptional regulatory elements in interleukin-17 target genes. J Biol Chem. 2006;281:24138-24148. doi:10.1074/jbc.M604597200
- Harper EG, Guo C, Rizzo H, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol. 2009;129:2175-2183. doi:10.1038/jid.2009.65
- Chiricozzi A, Guttman-Yassky E, Suarez-Farinas M, et al. Integrative responses to IL-17 and TNF-alpha in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J Invest Dermatol. 2011;131:677-687. doi:10.1038/jid.2010.340
- Homey B, Dieu-Nosjean MC, Wiesenborn A, et al. Up-regulation of macrophage inflammatory protein-3 alpha/CCL20 and CC chemokine receptor 6 in psoriasis. J Immunol. 2000;164:6621-6632. doi:10.4049 /jimmunol.164.12.6621
- Stephen-Victor E, Fickenscher H, Bayry J. IL-26: an emerging proinflammatory member of the IL-10 Cytokine family with multifaceted actions in antiviral, antimicrobial, and autoimmune responses. PLoS Pathog. 2016;12:E1005624. doi:10.1371/journal.ppat.1005624
- Wolk K, Witte K, Witte E, et al. IL-29 is produced by T(H)17 cells and mediates the cutaneous antiviral competence in psoriasis [published online September 25, 2013]. Sci Transl Med. doi:10.1126 /scitranslmed.3006245
- Kasprowicz-Furmanczyk M, Czerwinska J, Placek W, et al. Assessment of the tissue resident memory cells in lesional skin of patients with psoriasis and in healthy skin of healthy volunteers. Int J Environ Res Public Health. 2021;18:11251. doi:10.3390/ijerph182111251
- Cheuk S, Schlums H, Gallais Serezal I, et al. CD49a expression defines tissue-resident CD8(+) T cells poised for cytotoxic function in human skin. Immunity. 2017;46:287-300. doi:10.1016/j.immuni.2017.01.009
- Sawyer LM, Malottki K, Sabry-Grant C, et al. Assessing the relative efficacy of interleukin-17 and interleukin-23 targeted treatments for moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis of PASI response. PLoS One. 2019;14:E0220868. doi:10.1371/journal.pone.0220868
- Coates LC, Kavanaugh A, Mease PJ, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68:1060-1071. doi:10.1002/art.39573
- Wang EA, Suzuki E, Maverakis E, et al. Targeting IL-17 in psoriatic arthritis. Eur J Rheumatol. 2017;4:272-277. doi:10.5152/eurjrheum.2017.17037
- Armstrong A, Fahrbach K, Leonardi C, et al. Efficacy of bimekizumab and other biologics in moderate to severe plaque psoriasis: a systematic literature review and a network meta-analysis. Dermatol Ther (Heidelb). 2022;12:1777-1792. doi:10.1007/s13555-022-00760-8
- van Baarsen LG, Lebre MC, van der Coelen D, et al. Heterogeneous expression pattern of interleukin 17A (IL-17A), IL-17F and their receptors in synovium of rheumatoid arthritis, psoriatic arthritis and osteoarthritis: possible explanation for nonresponse to anti-IL-17 therapy? Arthritis Res Ther. 2014;16:426. doi:10.1186/s13075-014-0426-z
- Hot A, Zrioual S, Toh ML, et al. IL-17A- versus IL-17F-induced intracellular signal transduction pathways and modulation by IL-17RA and IL-17RC RNA interference in rheumatoid synoviocytes. Ann Rheum Dis. 2011;70:341-348. doi:10.1136/ard.2010.132233
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894. doi:10.3389/fimmu.2020.01894
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475-486. doi:10.1016/S0140-6736(21)00126-4
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532. doi:10.1136 /annrheumdis-2017-212127
- Gordon KB, Langley RG, Warren RB, et al. Bimekizumab safety in patients with moderate to severe plaque psoriasis: pooled results from phase 2 and phase 3 randomized clinical trials. JAMA Dermatol. 2022;158:735-744. doi:10.1001/jamadermatol.2022.1185
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152. doi:10.1056/NEJMoa2102383
- Reich K, Iversen L, Puig L, et al. Long-term efficacy and safety of brodalumab in moderate-to-severe plaque psoriasis: a post hoc pooled analysis of AMAGINE-2 and -3. J Eur Acad Dermatol Venereol. 2022;36:1275-1283. doi:10.1111/jdv.18068
- Papp KA, Blauvelt A, Puig L, et al. Long-term safety and efficacy of risankizumab for the treatment of moderate-to-severe plaque psoriasis: interim analysis of the LIMMitless open-label extension trial up to 5 years of follow-up. J Am Acad Dermatol. 2023;89:1149-1158. doi: 10.1016/j.jaad.2023.07.1024
- Glatt S, Jemec GBE, Forman S, et al. Efficacy and safety of bimekizumab in moderate to severe hidradenitis suppurativa: a phase 2, doubleblind, placebo-controlled randomized clinical trial. JAMA Dermatol. 2021;157:1279-1288. doi:10.1001/jamadermatol.2021.2905
- Choon SE, Lai NM, Mohammad NA, et al. Clinical profile, morbidity, and outcome of adult-onset generalized pustular psoriasis: analysis of 102 cases seen in a tertiary hospital in Johor, Malaysia. Int J Dermatol. 2014;53:676-684. doi:10.1111/ijd.12070
- Zheng M, Jullien D, Eyerich K. The prevalence and disease characteristics of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23 (suppl 1):5-12. doi:10.1007/s40257-021-00664-x
- Fujita H, Gooderham M, Romiti R. Diagnosis of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23(suppl 1):31-38. doi:10.1007/s40257-021-00652-1
- Choon SE, Navarini AA, Pinter A. Clinical course and characteristics of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23 (suppl 1):21-29. doi:10.1007/s40257-021-00654-z
- Augey F, Renaudier P, Nicolas JF. Generalized pustular psoriasis (Zumbusch): a French epidemiological survey. Eur J Dermatol. 2006;16:669-673.
- Ohkawara A, Yasuda H, Kobayashi H, et al. Generalized pustular psoriasis in Japan: two distinct groups formed by differences in symptoms and genetic background. Acta Derm Venereol. 1996;76:68-71. doi:10.2340/00015555766871
- Lee JY, Kang S, Park JS, et al. Prevalence of psoriasis in Korea: A population-based epidemiological study using the Korean National Health Insurance database. Ann Dermatol. 2017;29:761-767. doi:10.5021 /ad.2017.29.6.761
- Prinz JC, Choon SE, Griffiths CEM, et al. Prevalence, comorbidities and mortality of generalized pustular psoriasis: a literature review. J Eur Acad Dermatol Venereol. 2023;37:256-273. doi:10.1111/jdv.18720
- Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109-120. doi:10.1016/j.jaci.2016.08.056
- Rajan N, Sinclair N, Nakai H, et al. A tale of two sisters: identical IL36RN mutations and discordant phenotypes. Br J Dermatol. 2016;174:417-420. doi:10.1111/bjd.14003
- Ly K, Beck KM, Smith MP, et al. Diagnosis and screening of patients with generalized pustular psoriasis. Psoriasis (Auckl). 2019;9:37-42. doi:10.2147/PTT.S181808
- Sugiura K. Role of interleukin 36 in generalised pustular psoriasis and beyond. Dermatol Ther (Heidelb). 2022;12:315-328. doi:10.1007 /s13555-021-00677-8
- Akiyama M, Takeichi T, McGrath JA, et al. Autoinflammatory keratinization diseases: an emerging concept encompassing various inflammatory keratinization disorders of the skin. J Dermatol Sci. 2018;90:105-111. doi:10.1016/j.jdermsci.2018.01.012
- Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440. doi:10.1056/NEJMoa2111563
- Warren RB, Reich A, Kaszuba A, et al. Imsidolimab, an anti-IL-36 receptor monoclonal antibody for the treatment of generalised pustular psoriasis: results from the phase 2 GALLOP trial. Br J Dermatol. 2023;189:161-169. doi:10.1093/bjd/ljad083
- Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017; 18:374-384. doi:10.1038/ni.3691
- Genetic Analysis of Psoriasis Consortium & the Wellcome Trust Case Control Consortium 2; Strange A, Capon F, et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat Genet. 2010;42:985-990. doi:10.1038/ng.694
- Enerback C, Sandin C, Lambert S, et al. The psoriasis-protective TYK2 I684S variant impairs IL-12 stimulated pSTAT4 response in skin-homing CD4+ and CD8+ memory T-cells. Sci Rep. 2018;8:7043. doi:10.1038/s41598-018-25282-2
- Shimoda K, Kato K, Aoki K, et al. Tyk2 plays a restricted role in IFN alpha signaling, although it is required for IL-12-mediated T cell function. Immunity. 2000;13:561-571. doi:10.1016/s1074-7613(00)00055-8
- Karaghiosoff M, Neubauer H, Lassnig C, et al. Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity. 2000;13:549-560. doi:10.1016/s1074-7613(00)00054-6
- Burke JR, Cheng L, Gillooly KM, et al. Autoimmune pathways in mice and humans are blocked by pharmacological stabilization of the TYK2 pseudokinase domain [published online July 24, 2019]. Sci Transl Med. doi:10.1126/scitranslmed.aaw1736
- Strober B, Thaci D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 program for evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51. doi:10.1016/j.jaad.2022.08.061
- Stein Gold L, Lebwohl M, Menter A, et al. Aerosol foam formulation of fixed combination calcipotriene plus betamethasone dipropionate is highly efficacious in patients with psoriasis vulgaris: pooled data from three randomized controlled studies. J Drugs Dermatol. 2016;15:951-957.
- Beranek M, Fiala Z, Kremlacek J, et al. Serum levels of aryl hydrocarbon receptor, cytochromes p450 1a1 and 1b1 in patients with exacerbated psoriasis vulgaris. Folia Biol (Praha). 2018;64:97-102.
- Esser C, Rannug A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol Rev. 2015;67:259- 279. doi:10.1124/pr.114.009001
- Furue M, Uchi H, Mitoma C, et al. Antioxidants for healthy skin: the emerging role of aryl hydrocarbon receptors and nuclear factorerythroid 2-related factor-2. Nutrients. 2017;9:223. doi:10.3390/nu9030223
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684. doi:10.1016/S0140-6736(08)60726-6
- Sutter CH, Olesen KM, Bhuju J, et al. AHR regulates metabolic reprogramming to promote SIRT1-dependent keratinocyte differentiation. J Invest Dermatol. 2019;139:818-826. doi:10.1016/j.jid.2018.10.019
- Haas K, Weighardt H, Deenen R, et al. Aryl hydrocarbon receptor in keratinocytes is essential for murine skin barrier integrity. J Invest Dermatol. 2016;136:2260-2269. doi:10.1016/j.jid.2016.06.627
- Di Meglio P, Duarte JH, Ahlfors H, et al. Activation of the aryl hydrocarbon receptor dampens the severity of inflammatory skin conditions. Immunity. 2014;40:989-1001. doi:10.1016/j.immuni.2014.04.019
- Kim HO, Kim JH, Chung BY, et al. Increased expression of the aryl hydrocarbon receptor in patients with chronic inflammatory skin diseases. Exp Dermatol. 2014;23:278-281. doi:10.1111/exd.12350
- van den Bogaard EH, Bergboer JG, Vonk-Bergers M, et al. Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J Clin Invest. 2013;123:917-927. doi:10.1172/JCI65642
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AHR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137:2110-2119. doi:10.1016/j.jid.2017.05.004
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806. doi:10.1016/j.jaad.2022.06.1171
- Mooney N, Teague JE, Gehad AE, et al. Tapinarof inhibits the formation, cytokine production, and persistence of resident memory T cells in vitro. SKIN J Cutan Med. 2023;7:S194. doi:10.25251/skin.7.supp.194
- Schafer PH, Truzzi F, Parton A, et al. Phosphodiesterase 4 in inflammatory diseases: effects of apremilast in psoriatic blood and in dermal myofibroblasts through the PDE4/CD271 complex. Cell Signal. 2016;28:753-763. doi:10.1016/j.cellsig.2016.01.007
- Li H, Zuo J, Tang W. Phosphodiesterase-4 inhibitors for the treatment of inflammatory diseases. Front Pharmacol. 2018;9:1048. doi:10.3389/ fphar.2018.01048
- Schafer PH, Parton A, Gandhi AK, et al. Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br J Pharmacol. 2010;159:842-855. doi:10.1111/j.1476-5381.2009.00559.x
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49. doi:10.1016/j .jaad.2015.03.049
- Papp KA, Gooderham M, Droege M, et al. Roflumilast cream improves signs and symptoms of plaque psoriasis: results from a phase 1/2a randomized, controlled study. J Drugs Dermatol. 2020;19:734-740. doi:10.36849/JDD.2020.5370
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084. doi:10.1001/jama.2022.15632
Psoriasis is a chronic inflammatory disease that affects approximately 3% of the US population.1 Plaque psoriasis comprises 80% to 90% of cases, while pustular, erythrodermic, guttate, inverse, and palmoplantar disease are less common variants (Figure 1). Psoriatic skin manifestations range from localized to widespread or generalized disease with recurrent flares. Body surface area or psoriasis area and severity index (PASI) measurements primarily focus on skin manifestations and are important for evaluating disease activity and response to treatment, but they have inherent limitations: they do not capture extracutaneous disease activity, systemic inflammation, comorbid conditions, quality of life impact, or the economic burden of psoriasis.

A common manifestation of psoriasis is psoriatic arthritis (PsA), which can involve the nails, joints, ligaments, or tendons in 30% to 41% of affected individuals (Figure 2).2,3 A growing number of psoriasis-associated comorbidities also have been reported including metabolic syndrome4; hyperlipidemia5; cardiovascular disease6; stroke7; hypertension8; obesity9; sleep disorders10; malignancy11; infections12; inflammatory bowel disease13; and mental health disorders such as depression,14 anxiety,15 and suicidal ideation.15 Psoriatic disease also interferes with daily life activities and a patient’s overall quality of life, including interpersonal relationships, intimacy, employment, and work productivity.16 Finally, the total estimated cost of psoriasis-related health care is more than $35 billion annually,17 representing a substantial economic burden to our health care system and individual patients.

The overall burden of psoriatic disease has declined markedly in the last 2 decades due to revolutionary advances in our understanding of the immunopathogenesis of psoriasis and the subsequent development of improved therapies that predominantly interrupt IL-23/IL-17 cytokine signaling; however, critical knowledge and treatment gaps persist, underscoring the importance of ongoing clinical and research efforts in psoriatic disease. We review the working immune model of psoriasis, summarize related immune discoveries, and highlight recent therapeutic innovations that are shaping psoriatic disease management.
Current Immune Model of Psoriatic Disease
Psoriasis is an autoinflammatory T cell–mediated disease with negligible contributions from the humoral immune response. Early clinical observations reported increased inflammatory infiltrates in psoriatic skin lesions primarily consisting of both CD4+ and CD8+ T-cell populations.18,19 Additionally, patients treated with broad-acting, systemic immunosuppressive medications (eg, cyclosporine, oral corticosteroids) experienced improvement of psoriatic lesions and normalization of the immune infiltrates observed in skin biopsy specimens.20,21 These early clinical findings led to more sophisticated experimentation in xenotransplant models of psoriasis,22,23 which explored the clinical efficacy of several less immunosuppressive (eg, methotrexate, anti–tumor necrosis factor [TNF] biologics)24 or T cell–specific agents (eg, alefacept, abatacept, efalizumab).25-27 The results of these translational studies provided indisputable evidence for the role of the dysregulated immune response as the primary pathogenic process driving plaque formation; they also led to a paradigm shift in how the immunopathogenesis of psoriatic disease was viewed and paved the way for the identification and targeting of other specific proinflammatory signals produced by activated dendritic cell (DC) and T-lymphocyte populations. Among the psoriasis-associated cytokines subsequently identified and studied, elevated IL-23 and IL-17 cytokine levels in psoriatic skin were most closely associated with disease activity, and rapid normalization of IL-23/IL-17 signaling in response to effective oral or injectable antipsoriatic treatments was the hallmark of skin clearance.28 The predominant role of IL-23/IL-17 signaling in the development and maintenance of psoriatic disease is the central feature of all working immune models for this disease (Figure 3).

Psoriasis-Associated Genetic and Environmental Risk Factors
The exact sequence of events that lead to the initiation and formation of plaque psoriasis in susceptible individuals is still poorly understood; however, several important risk factors and key immune events have been identified. First, decades of genetic research have reported more than 80 known psoriasis-associated susceptibility loci,29 which explains approximately 50% of psoriasis heritability. The major genetic determinant of psoriasis, HLA-C*06:02 (formerly HLA-Cw6), resides in the major histocompatibility complex class I region on chromosome 6p21.3 (psoriasis susceptibility gene 1, PSORS1) and is most strongly associated with psoriatic disease.30 Less common psoriasis-associated susceptibility genes also are known to directly or indirectly impact innate and adaptive immune functions that contribute to the pathogenesis of psoriasis.
Second, several nongenetic environmental risk factors for psoriasis have been reported across diverse patient populations, including skin trauma/injury, infections, alcohol/tobacco use, obesity, medication exposure (eg, lithium, antimalarials, beta-blockers), and stress.31 These genetic and/or environmental risk factors can trigger the onset of psoriatic disease at any stage of life, though most patients develop disease in early adulthood or later (age range, 50–60 years). Some patients never develop psoriasis despite exposure to environmental risk factors and/or a genetic makeup that is similar to affected first-degree relatives, which requires further study.
Prepsoriatic Skin and Initiation of Plaque Development
In response to environmental stimuli and/or other triggers of the immune system, DC and resident IL-17–producing T-cell (T17) populations become activated in predisposed individuals. Dendritic cell activation leads to the upregulation and increase of several proinflammatory cytokines, including TNF, interferon (IFN) α, IFN-γ, IL-12, and IL-23. Tumor necrosis factor and IL-23 play a vital role in psoriasis by helping to regulate the polarization and expansion of T22 and T17 cells in the skin, whereas IL-12 promotes a corresponding type 1 inflammatory response.32 Increased IL-17 and IL-22 result in alteration of the terminal differentiation and proliferative potential of epidermal keratinocytes, leading to the early clinical hallmarks of psoriatic plaques. The potential contribution of overexpressed psoriasis-related autoantigens, such as LL-37/cathelicidin, ADAMTSL5, and PLA2G4D,33 in the initiation of psoriatic plaques has been suggested but is poorly characterized.34 Whether these specific autoantigens or others presented by HLA-C variants found on antigen-presenting cells are required for the breakdown of immune tolerance and psoriatic disease initiation is highly relevant but requires further investigation and validation.
Feed-Forward Inflammation, Mature Psoriatic Plaques, and Resident Memory T Cells
In response to the upstream production of IL-23 by dermal DCs, high levels of IL-17 cytokines can be found in mature psoriatic plaques. The IL-17 family consists of 6 dimeric cytokines (IL-17A through IL-17F) that provide innate cutaneous protection against bacterial, viral, and fungal infectious agents, such as Candida albicans. Unlike other IL-17 isoforms, IL-17A and IL-17F share the same receptor complex and have the highest structural homology of any pair (approximately 50% similar).35 The relative expression of IL-17F is higher than IL-17A in psoriasis,36 though IL-17A has been considered as the predominant IL-17 cytokine found in psoriatic skin lesions due to its higher potency.
Binding of IL-17A/F with the IL-17 receptor (IL-17R) on keratinocytes contributes to the development of psoriatic plaques by inducing epidermal hyperplasia via activation of CCAAT/enhancer-binding proteins β and δ, nuclear factor κB, and signal transducer and activator of transcription 1 gene (STAT1).37,38 This also increases the expression of other keratinocyte-derived proteins (eg, human β-defensins, S-100 proteins, LL-37, other antimicrobial peptides, IL-19, IL-36, IL-17C) that act as reinforcing proinflammatory signals or chemotactic factors (eg, chemokine [C-C motif] ligand 20 [CCL20], chemokine [C-C motif] ligand 1/2/3/5 [CXCL1/2/3/5], CXCL8, IL-8) that facilitate the recruitment of additional immune cells to the skin including polymorphonuclear neutrophils (PMNs), macrophages, and DCs.39-41 Routine immunohistochemical staining for these keratinocyte-derived proteins reveals a striking epidermal gene expression gradient wherein levels of IL-17–induced proteins are most highly expressed in the uppermost layers of keratinocytes and facilitate the recruitment of immune cells into the epidermis. Activated T17 cells also stimulate the production of keratinocyte-derived chemokines (eg, CXCL9/10/11), which recruit type 1 inflammatory T-cell populations into developing psoriatic plaques.42,43 Finally, TNF, IL-36, and IL-17C cytokines act synergistically with IL-17A/F to amplify the proinflammatory effects of IL-17 signaling and further stimulate their production from T17 cell populations.40 This inflammatory circuit in the skin creates and supports a self-amplifying or positive feedback loop between the skin and immune system that commonly is referred to as feed-forward inflammation (Figure 3).34 The feed-forward inflammatory loop in psoriasis—predominantly driven by increased IL-23/IL-17 signaling—best characterizes the mature psoriatic plaque.
Several findings suggest that the influx of persistent, long-lived resident memory T cells (Trms) may contribute to the mature psoriatic plaque. It is believed that CD8+CD103+CD49a− Trm cell populations may be responsible for the sharply demarcated borders of untreated psoriasis plaques or their recurrence at specific body sites such as the scalp, buttocks, extremity extensor surfaces, umbilicus, or acral skin following specific stimuli or trauma (Koebner phenomenon or isomorphic response).44,45 It is not known if repeated stimuli or trauma induce disease formation via the activation of Trm cell populations; further study in large patient cohorts is needed, but this remains an intriguing area of study for durable treatment responses and potential cures for psoriasis.
Recent Discoveries in Psoriatic Disease
Remarkable treatment outcomes for psoriasis have been achieved with multiple selective IL-17 and IL-23 inhibitors (eTable). As demonstrated in several pivotal phase 3 clinical trials for members of these classes of medications, the majority of treated psoriasis patients achieved PASI90 clearance.46 Due to their more favorable dosing schedule (ie, fewer injections) and ability to induce a durable remissionlike treatment response, IL-23 inhibitors have become the preferred treatment class for cutaneous disease, while IL-17 inhibitors may be preferred when treating patients with both plaque psoriasis and PsA.47,48 Nevertheless, the complexity of this disease is punctuated by treated patients who do not adequately respond to selective IL-23/IL-17 blockade.49 Recent and emerging treatments may shed light on these recalcitrant cases and will add to the rapidly growing arsenal of available psoriasis therapies.
The Role of IL-17F in Psoriasis and Other Inflammatory Skin Diseases
Dysregulation of IL-17A and IL-17F is associated with several chronic inflammatory conditions, such as psoriasis and PsA.35,50 Both cytokines, either as homodimers or heterodimers, can selectively bind to the heterodimeric IL-17R formed by the IL-17RA and IL-17RC subunits.35 IL-17F and IL-17C also can synergize with TNF and other cytokines to promote and support the self-sustaining inflammatory circuits in mature psoriatic plaques, though their inflammatory effects in the skin are more limited than IL-17A.51,52 Therefore, incomplete blockade of IL-17 signaling (ie, unopposed IL-17F and IL-17C) represents a potential mechanism to explain the persistence of psoriasis in patients treated with selective IL-17A inhibitors. This hypothesis is supported by reports of psoriasis patients who have inadequate clinical responses to selective IL-17A inhibition but subsequently improve with IL-17R blockade, which results in disruption of IL-17A as well as IL-17C/E/F cytokine signaling. This formed the basis for further study into the specific role of IL-17F in psoriatic disease and any potential therapeutic benefits associated with its inhibition.
Recently approved in the European Union, Canada, Australia, Japan, the United Kingdom, and the United States for moderate to severe psoriasis, bimekizumab is a novel humanized IgG antibody that selectively inhibits both IL-17A and IL-17F cytokines.53 Specifically, bimekizumab simultaneously prevents binding of IL-17A/A, IL-17A/F, and IL-17F/F dimers with the IL-17R. Compared to other IL-17 and IL-23 biologic therapies, bimekizumab (320 mg) achieved relatively higher response rates for PASI75, PASI90, and PASI100.49 Neutralization of IL-17A and IL-17F by bimekizumab also resulted in more complete suppression of cytokine responses and PMN chemotaxis than either cytokine alone in treated PsA patients,54 which is notable because of the incremental benefits of recent IL-23 and IL-17 inhibitors on inflammatory arthritis symptoms in contrast to the substantial improvements observed for cutaneous disease with those same agents.
The primary disadvantage of bimekizumab and its more complete blockade of the IL-17 signaling pathway is that treated patients have a substantially increased risk for oral candidiasis (>10%).55 However, the precise link between candidiasis and IL-17 blockade is not yet fully understood because other targeted agents that also broadly suppress IL-17 signaling (ie, IL-17R, IL-23 inhibitors) are associated with much lower rates of candidiasis.56-58 Bimekizumab also is being investigated as a novel therapy for hidradenitis suppurativa and will provide important reference information regarding the role for bispecific biologic agents in the treatment of chronic inflammatory skin diseases.59
IL-36 Signaling and Generalized Pustular Psoriasis
Recent genetic and clinical studies have expanded our understanding of the role of IL-36 signaling in the immunopathogenesis of pustular psoriasis variants. Generalized pustular psoriasis (GPP) is a rare distinct psoriasis subtype characterized by the recurrent development of widespread erythema, superficial sterile pustules, and desquamation. Systemic symptoms such as fever, malaise, itching, and skin pain accompany acute GPP flares.60 Generalized pustular psoriasis is more common in female patients (in contrast with plaque psoriasis), and acute flares may be caused by multiple stimuli including infections, hypocalcemia, initiation or discontinuation of medications (eg, oral corticosteroids), pregnancy, or stress.61,62 Flares of GPP often require emergency or in-patient care, as untreated symptoms increase the risk for severe health complications such as secondary infections, sepsis, or multisystem organ failure.63 The prevalence of GPP is estimated to be approximately 1 in 10,000 individuals in the United States,64-67 with mortality rates ranging from 0 to 3.3 deaths per 100 patient-years.67
In contrast to plaque psoriasis, aberrant IL-36 signaling is the predominant driver of GPP. IL-36 is a member of the IL-1 cytokine family that includes three IL-36 agonists (IL-36α, IL-36β, IL-36γ) and 1 endogenous antagonist (IL-36Ra, encoded by IL36RN).68 The immunopathogenesis of GPP involves dysregulation of the IL-36–chemokine–PMN axis, resulting in unopposed IL-36 signaling and the subsequent recruitment and influx of PMNs into the epidermis. IL36RN mutations are strongly associated with GPP and result in impaired function of the IL-36Ra protein, leading to unopposed IL-36 signaling.69 However, approximately two-thirds of GPP patients lack identifiable gene mutations, suggesting other immune mechanisms or triggers causing upregulated IL-36 signaling.70 In response to these triggers, increased IL-36 cytokines released by keratinocytes bind to the IL-36R, resulting in substantial keratinocyte hyperproliferation, increased IL-36 levels, and the expression of hundreds of additional inflammatory signals (eg, IL-17C, antimicrobial peptides, TNF, IL-6).71 Increased IL-36 levels also drive the production of PMN chemotactic proteins (eg, CXCL1/2/3/5/6/8 and CXCR1/2) and act synergistically with IL-17 cytokines to create an autoamplifying circuit that is analogous to the feed-forward inflammatory loop in plaque psoriasis.72 Biopsies of involved GPP skin reveal increased expression of IL-36 in the uppermost layers of the epidermis, which creates a gene expression gradient that acts as a strong attractant for PMNs and forms the basis for the hallmark pustular lesions observed in GPP patients.
Until recently, treatment strategies for GPP involved the off-label use of topical, oral, or biologic therapies approved for plaque psoriasis, which often was associated with variable or incomplete disease control. In September 2022, the US Food and Drug Administration (FDA) approved intravenous spesolimab as a first-in-class humanized monoclonal IgG1 antibody for the treatment of GPP flares in adults. Spesolimab binds to IL-36R and prevents its activation by its endogenous agonists. A phase 2, randomized, 12-week clinical trial (Effisayil-1) evaluated the efficacy and safety of a single 900-mg intravenous dose of spesolimab followed by an optional second dose 1 week later for inadequate treatment responses in 53 enrolled GPP patients (2:1 treatment to placebo randomization).73 Remarkably, more than half (19/35 [54%]) of GPP patients experienced complete resolution of pustules (GPP physician global assessment subscore of 0 [range, 0–4]) and showed sustained efficacy out to week 12 after just 1 or 2 doses of spesolimab. Overall, the safety profile of spesolimab was good; asthenia, fatigue, nausea, vomiting, headache, pruritus, infusion-related reaction and symptoms, and mild infections (eg, urinary tract infection) were the most common adverse events reported.73
Imsidolimab, a high-affinity humanized IgG4 monoclonal antibody that binds and blocks activation of IL-36R, also has completed phase 2 testing,74 with phase 3 study results expected in early 2024. The rapid onset of action and overall safety of imsidolimab was in line with and similar to spesolimab. Future approval of imsidolimab would add to the limited treatment options available for GPP and has the additional convenience of being administered to patients subcutaneously. Overall, the development of selective IL-36R inhibitors offers a much-needed therapeutic option for GPP and illustrates the importance of translational research.
Role of Tyrosine Kinase in Psoriatic Disease
The Janus kinase (JAK) enzyme family consists of 4 enzymes—tyrosine kinase 2 (TYK2), JAK1, JAK2, and JAK3—that function as intracellular transduction signals that mediate the biologic response of most extracellular cytokines and growth factors.75 Critical psoriasis-related cytokines are dependent on intact JAK-STAT signaling, including IL-23, IL-12, and type I IFNs. In 2010, a genome-wide association identified TYK2 as a psoriasis susceptibility locus,76 and loss-of-function TYK2 mutations confer a reduced risk for psoriasis.77 Unlike other JAK isoforms, TYK2 mediates biologic functions that are highly restricted to the immune responses associated with IL-23, IL-12, and type I IFN signaling.78,79 For these reasons, blockade of TYK2 signaling is an attractive therapeutic target for the potential treatment of psoriatic disease.
In September 2022, the FDA approved deucravacitinib as a first-in-class, oral, selective TYK2 inhibitor for the treatment of adult patients with moderate to severe plaque psoriasis. It was the first FDA approval of an oral small-molecule treatment for plaque psoriasis in nearly a decade. Deucravacitinib inhibits TYK2 signaling via selective binding of its unique regulatory domain, resulting in a conformational (allosteric) change that interferes with its active domain.80 This novel mechanism of action limits the unwanted blockade of other broad biologic processes mediated by JAK1/2/3. Of note, the FDA did not issue any boxed warnings for deucravacitinib as it did for other FDA-approved JAK inhibitors.
In a head-to-head, 52-week, double-blind, prospective, randomized, phase 3 study, deucravacitinib showed clear superiority over apremilast for PASI75 at week 16 (53.0% [271/511] vs 39.8% [101/254]) and week 24 (58.7% [296/504] vs 37.8% [96/254]).81 Clinical responses were sustained through week 52 and showed efficacy for difficult-to-treat areas such as the scalp, acral sites, and nails. Other advantages of deucravacitinib include once-daily dosing with no need for dose titration or adjustments for renal insufficiency as well as the absence of statistically significant differences in gastrointestinal tract symptoms compared to placebo. The most common adverse effects included nasopharyngitis, upper respiratory tract infections, headache, diarrhea, and herpes infections.81 The potential benefit of deucravacitinib for PsA and psoriasis comorbidities remains to be seen, but it is promising due to its simultaneous disruption of multiple psoriasis-related cytokine networks. Several other TYK2 inhibitors are being developed for psoriatic disease and related inflammatory conditions, underscoring the promise of targeting this intracellular pathway.
Aryl Hydrocarbon Receptor Agonism
Topical steroids are the mainstay treatment option for localized or limited plaque psoriasis due to their potent immunosuppressive effect on the skin and relatively low cost. Combined with vitamin D analogs, topical steroids result in marked improvements in disease severity and improved tolerability.82 However, chronic use of topical steroids is limited by the need for twice-daily application, resulting in poor treatment compliance; loss of efficacy over time; risk for steroid-induced skin atrophy on special body sites; and patient concerns of potential systemic effects. The discovery of novel drug targets amenable to topical inhibition is needed.
Dysregulated aryl hydrocarbon receptor (AHR) levels have been reported in atopic dermatitis and psoriasis.83 Aryl hydrocarbon receptors are ubiquitously expressed in many cell types and play an integral role in immune homeostasis within the skin, skin barrier function, protection against oxidative stressors, and regulation of proliferating melanocytes and keratinocytes.84,85 They are widely expressed in multiple immune cell types (eg, antigen-presenting cells, T lymphocytes, fibroblasts) and modulate the differentiation of T17 and T22 cells as well as their balance with regulatory T-cell populations.86 In keratinocytes, AHR helps to regulate terminal differentiation, enhance skin barrier integrity via AHR-dependent filaggrin (FLG) expression, and prevent transepidermal water loss.87,88 The mechanisms by which AHR ligands lead to the upregulation or downregulation of specific genes is intricate and highly context dependent, such as the specific ligand and cell type involved. In preclinical studies, AHR-deficient mice develop psoriasiform skin inflammation, increased IL-17 and IL-22 expression, and abnormal skin barrier function.89 Keratinocytes treated with AHR ligands in vitro modulated psoriasis-associated inflammatory cytokines, such as IL-6, IL-8, and type I and II IFNs.89,90 The use of coal tar, one of the earliest historical treatments for psoriasis, is thought to activate AHRs in the skin via organic compound mixtures containing polyaromatic hydrocarbons that help normalize the proinflammatory environment in psoriatic skin.91
In June 2022, the FDA approved tapinarof as a first-in-class, topical, nonsteroidal AHR agonist for the treatment of plaque psoriasis in adults. Although the exact mechanism of action for tapinarof has not been fully elucidated, early studies suggest that its primary function is the activation of AHR, leading to reduced T-cell expansion and T17 cell differentiation. In the imiquimod mouse model, cytokine expression of IL-17A, IL-17F, IL-19, IL-22, IL-23A, and IL-lβ in psoriasiform skin lesions were downregulated following tapinarof treatment.92 In humans, tapinarof treatment is associated with a remittive effect, in which the average time for tapinarof-treated psoriasis lesions to remain clear was approximately 4 months.93 Preliminary research investigating the mechanism by which tapinarof induces this remittive effect is ongoing and may involve the reduced activation and influx of T17 and Trm populations into the skin.94 However, these preclinical studies were performed on healthy dermatome-derived skin tissue cultured in T17-skewing conditions and needs to be replicated in larger samples sizes using human-derived psoriatic tissue. Alternatively, a strong inhibitory effect on IL-23 cytokine signaling may, in part, explain the remittive effect of tapinarof, as an analogous response is observed in patients who start and discontinue treatment with selective IL-23 antagonists. Regardless, the once-daily dosing of tapinarof and sustained treatment response is appealing to psoriasis patients. Tapinarof generally is well tolerated with mild folliculitis (>20% of patients) and contact dermatitis (5% of patients) reported as the most common skin-related adverse events.
New Roles for Phosphodiesterase 4 Inhibition
Phosphodiesterases (PDEs) are enzymes that hydrolyze cyclic nucleotides (eg, cyclic adenosine monophosphate) to regulate intracellular secondary messengers involved in the inflammatory response. One of several enzymes in the PDE family, PDE4, has been shown to have greater activity in psoriatic skin compared to healthy skin.95 Phosphodiesterase inhibitors decrease the degradation of cyclic adenosine monophosphate, which triggers protein kinase A to downregulate proinflammatory (eg, TNF-α, IL-6, IL-17, IL-12, IL-23) cytokines and increased expression of anti-inflammatory signals such as IL-10.96,97 Apremilast, the first oral PDE4 inhibitor approved by the FDA for psoriasis, offered a safe alternative to traditional oral immunosuppressive agents that had extensive risks and potential end-organ adverse effects. Unfortunately, apremilast demonstrated modest efficacy for psoriatic disease (better efficacy in the skin vs joint manifestations) and was supplanted easily by next-generation targeted biologic agents that were more efficacious and lacked the troublesome gastrointestinal tract adverse effects of PDE4 inhibition.98
Crisaborole became the first topical PDE4 inhibitor approved in the United States in December 2016 for twice-daily treatment of atopic dermatitis. Although phase 2 trial results were reported in psoriasis, this indication was never pursued, presumably due to similar improvements in primary outcome measures at week 12, compared to placebo (ClinicalTrials.gov Identifier NCT01300052).
In July 2022, the first topical PDE4 inhibitor indicated for plaque psoriasis was approved by the FDA—roflumilast cream 0.3% for once-daily use in individuals 12 years and older. Roflumilast was found to be clinically efficacious as early as 2 weeks after its use in an early-phase clinical trial.99 In 2 phase 3 clinical trials (DERMIS-1 and DERMIS-2), roflumilast significantly increased the proportion of patients achieving PASI75 at week 8 compared to vehicle (39%–41.6% vs 5.3%–7.6%, respectively)(P<.001).100 Overall, this nonsteroidal topical therapy was found to be well tolerated, with infrequent reports of application site pain or irritation as adverse events. Similar to tapinarof, patients can apply roflumilast on all body surface areas including the face, external genitalia, and other intertriginous areas.100 Importantly, the broad immune impact of PDE4 inhibition suggests that topical roflumilast likely will be an effective treatment for several additional inflammatory conditions, including seborrheic dermatitis and atopic dermatitis, which would expand the clinical utility of this specific medication.
Conclusion
In the last 2 decades, we have witnessed a translational revolution in our understanding of the underlying genetics and immunology of psoriatic disease. Psoriasis is widely considered one of the best-managed inflammatory conditions in all of medicine due to the development and availability of highly targeted, effective topical and systemic therapies that predominantly disrupt IL-23/IL-17 cytokine signaling in affected tissues. However, future clinical studies and laboratory research are necessary to elucidate the precise cause of psoriasis as well as the underlying genetic and immune signaling pathways driving less common clinical variants and recalcitrant disease.

Psoriasis is a chronic inflammatory disease that affects approximately 3% of the US population.1 Plaque psoriasis comprises 80% to 90% of cases, while pustular, erythrodermic, guttate, inverse, and palmoplantar disease are less common variants (Figure 1). Psoriatic skin manifestations range from localized to widespread or generalized disease with recurrent flares. Body surface area or psoriasis area and severity index (PASI) measurements primarily focus on skin manifestations and are important for evaluating disease activity and response to treatment, but they have inherent limitations: they do not capture extracutaneous disease activity, systemic inflammation, comorbid conditions, quality of life impact, or the economic burden of psoriasis.

A common manifestation of psoriasis is psoriatic arthritis (PsA), which can involve the nails, joints, ligaments, or tendons in 30% to 41% of affected individuals (Figure 2).2,3 A growing number of psoriasis-associated comorbidities also have been reported including metabolic syndrome4; hyperlipidemia5; cardiovascular disease6; stroke7; hypertension8; obesity9; sleep disorders10; malignancy11; infections12; inflammatory bowel disease13; and mental health disorders such as depression,14 anxiety,15 and suicidal ideation.15 Psoriatic disease also interferes with daily life activities and a patient’s overall quality of life, including interpersonal relationships, intimacy, employment, and work productivity.16 Finally, the total estimated cost of psoriasis-related health care is more than $35 billion annually,17 representing a substantial economic burden to our health care system and individual patients.

The overall burden of psoriatic disease has declined markedly in the last 2 decades due to revolutionary advances in our understanding of the immunopathogenesis of psoriasis and the subsequent development of improved therapies that predominantly interrupt IL-23/IL-17 cytokine signaling; however, critical knowledge and treatment gaps persist, underscoring the importance of ongoing clinical and research efforts in psoriatic disease. We review the working immune model of psoriasis, summarize related immune discoveries, and highlight recent therapeutic innovations that are shaping psoriatic disease management.
Current Immune Model of Psoriatic Disease
Psoriasis is an autoinflammatory T cell–mediated disease with negligible contributions from the humoral immune response. Early clinical observations reported increased inflammatory infiltrates in psoriatic skin lesions primarily consisting of both CD4+ and CD8+ T-cell populations.18,19 Additionally, patients treated with broad-acting, systemic immunosuppressive medications (eg, cyclosporine, oral corticosteroids) experienced improvement of psoriatic lesions and normalization of the immune infiltrates observed in skin biopsy specimens.20,21 These early clinical findings led to more sophisticated experimentation in xenotransplant models of psoriasis,22,23 which explored the clinical efficacy of several less immunosuppressive (eg, methotrexate, anti–tumor necrosis factor [TNF] biologics)24 or T cell–specific agents (eg, alefacept, abatacept, efalizumab).25-27 The results of these translational studies provided indisputable evidence for the role of the dysregulated immune response as the primary pathogenic process driving plaque formation; they also led to a paradigm shift in how the immunopathogenesis of psoriatic disease was viewed and paved the way for the identification and targeting of other specific proinflammatory signals produced by activated dendritic cell (DC) and T-lymphocyte populations. Among the psoriasis-associated cytokines subsequently identified and studied, elevated IL-23 and IL-17 cytokine levels in psoriatic skin were most closely associated with disease activity, and rapid normalization of IL-23/IL-17 signaling in response to effective oral or injectable antipsoriatic treatments was the hallmark of skin clearance.28 The predominant role of IL-23/IL-17 signaling in the development and maintenance of psoriatic disease is the central feature of all working immune models for this disease (Figure 3).

Psoriasis-Associated Genetic and Environmental Risk Factors
The exact sequence of events that lead to the initiation and formation of plaque psoriasis in susceptible individuals is still poorly understood; however, several important risk factors and key immune events have been identified. First, decades of genetic research have reported more than 80 known psoriasis-associated susceptibility loci,29 which explains approximately 50% of psoriasis heritability. The major genetic determinant of psoriasis, HLA-C*06:02 (formerly HLA-Cw6), resides in the major histocompatibility complex class I region on chromosome 6p21.3 (psoriasis susceptibility gene 1, PSORS1) and is most strongly associated with psoriatic disease.30 Less common psoriasis-associated susceptibility genes also are known to directly or indirectly impact innate and adaptive immune functions that contribute to the pathogenesis of psoriasis.
Second, several nongenetic environmental risk factors for psoriasis have been reported across diverse patient populations, including skin trauma/injury, infections, alcohol/tobacco use, obesity, medication exposure (eg, lithium, antimalarials, beta-blockers), and stress.31 These genetic and/or environmental risk factors can trigger the onset of psoriatic disease at any stage of life, though most patients develop disease in early adulthood or later (age range, 50–60 years). Some patients never develop psoriasis despite exposure to environmental risk factors and/or a genetic makeup that is similar to affected first-degree relatives, which requires further study.
Prepsoriatic Skin and Initiation of Plaque Development
In response to environmental stimuli and/or other triggers of the immune system, DC and resident IL-17–producing T-cell (T17) populations become activated in predisposed individuals. Dendritic cell activation leads to the upregulation and increase of several proinflammatory cytokines, including TNF, interferon (IFN) α, IFN-γ, IL-12, and IL-23. Tumor necrosis factor and IL-23 play a vital role in psoriasis by helping to regulate the polarization and expansion of T22 and T17 cells in the skin, whereas IL-12 promotes a corresponding type 1 inflammatory response.32 Increased IL-17 and IL-22 result in alteration of the terminal differentiation and proliferative potential of epidermal keratinocytes, leading to the early clinical hallmarks of psoriatic plaques. The potential contribution of overexpressed psoriasis-related autoantigens, such as LL-37/cathelicidin, ADAMTSL5, and PLA2G4D,33 in the initiation of psoriatic plaques has been suggested but is poorly characterized.34 Whether these specific autoantigens or others presented by HLA-C variants found on antigen-presenting cells are required for the breakdown of immune tolerance and psoriatic disease initiation is highly relevant but requires further investigation and validation.
Feed-Forward Inflammation, Mature Psoriatic Plaques, and Resident Memory T Cells
In response to the upstream production of IL-23 by dermal DCs, high levels of IL-17 cytokines can be found in mature psoriatic plaques. The IL-17 family consists of 6 dimeric cytokines (IL-17A through IL-17F) that provide innate cutaneous protection against bacterial, viral, and fungal infectious agents, such as Candida albicans. Unlike other IL-17 isoforms, IL-17A and IL-17F share the same receptor complex and have the highest structural homology of any pair (approximately 50% similar).35 The relative expression of IL-17F is higher than IL-17A in psoriasis,36 though IL-17A has been considered as the predominant IL-17 cytokine found in psoriatic skin lesions due to its higher potency.
Binding of IL-17A/F with the IL-17 receptor (IL-17R) on keratinocytes contributes to the development of psoriatic plaques by inducing epidermal hyperplasia via activation of CCAAT/enhancer-binding proteins β and δ, nuclear factor κB, and signal transducer and activator of transcription 1 gene (STAT1).37,38 This also increases the expression of other keratinocyte-derived proteins (eg, human β-defensins, S-100 proteins, LL-37, other antimicrobial peptides, IL-19, IL-36, IL-17C) that act as reinforcing proinflammatory signals or chemotactic factors (eg, chemokine [C-C motif] ligand 20 [CCL20], chemokine [C-C motif] ligand 1/2/3/5 [CXCL1/2/3/5], CXCL8, IL-8) that facilitate the recruitment of additional immune cells to the skin including polymorphonuclear neutrophils (PMNs), macrophages, and DCs.39-41 Routine immunohistochemical staining for these keratinocyte-derived proteins reveals a striking epidermal gene expression gradient wherein levels of IL-17–induced proteins are most highly expressed in the uppermost layers of keratinocytes and facilitate the recruitment of immune cells into the epidermis. Activated T17 cells also stimulate the production of keratinocyte-derived chemokines (eg, CXCL9/10/11), which recruit type 1 inflammatory T-cell populations into developing psoriatic plaques.42,43 Finally, TNF, IL-36, and IL-17C cytokines act synergistically with IL-17A/F to amplify the proinflammatory effects of IL-17 signaling and further stimulate their production from T17 cell populations.40 This inflammatory circuit in the skin creates and supports a self-amplifying or positive feedback loop between the skin and immune system that commonly is referred to as feed-forward inflammation (Figure 3).34 The feed-forward inflammatory loop in psoriasis—predominantly driven by increased IL-23/IL-17 signaling—best characterizes the mature psoriatic plaque.
Several findings suggest that the influx of persistent, long-lived resident memory T cells (Trms) may contribute to the mature psoriatic plaque. It is believed that CD8+CD103+CD49a− Trm cell populations may be responsible for the sharply demarcated borders of untreated psoriasis plaques or their recurrence at specific body sites such as the scalp, buttocks, extremity extensor surfaces, umbilicus, or acral skin following specific stimuli or trauma (Koebner phenomenon or isomorphic response).44,45 It is not known if repeated stimuli or trauma induce disease formation via the activation of Trm cell populations; further study in large patient cohorts is needed, but this remains an intriguing area of study for durable treatment responses and potential cures for psoriasis.
Recent Discoveries in Psoriatic Disease
Remarkable treatment outcomes for psoriasis have been achieved with multiple selective IL-17 and IL-23 inhibitors (eTable). As demonstrated in several pivotal phase 3 clinical trials for members of these classes of medications, the majority of treated psoriasis patients achieved PASI90 clearance.46 Due to their more favorable dosing schedule (ie, fewer injections) and ability to induce a durable remissionlike treatment response, IL-23 inhibitors have become the preferred treatment class for cutaneous disease, while IL-17 inhibitors may be preferred when treating patients with both plaque psoriasis and PsA.47,48 Nevertheless, the complexity of this disease is punctuated by treated patients who do not adequately respond to selective IL-23/IL-17 blockade.49 Recent and emerging treatments may shed light on these recalcitrant cases and will add to the rapidly growing arsenal of available psoriasis therapies.
The Role of IL-17F in Psoriasis and Other Inflammatory Skin Diseases
Dysregulation of IL-17A and IL-17F is associated with several chronic inflammatory conditions, such as psoriasis and PsA.35,50 Both cytokines, either as homodimers or heterodimers, can selectively bind to the heterodimeric IL-17R formed by the IL-17RA and IL-17RC subunits.35 IL-17F and IL-17C also can synergize with TNF and other cytokines to promote and support the self-sustaining inflammatory circuits in mature psoriatic plaques, though their inflammatory effects in the skin are more limited than IL-17A.51,52 Therefore, incomplete blockade of IL-17 signaling (ie, unopposed IL-17F and IL-17C) represents a potential mechanism to explain the persistence of psoriasis in patients treated with selective IL-17A inhibitors. This hypothesis is supported by reports of psoriasis patients who have inadequate clinical responses to selective IL-17A inhibition but subsequently improve with IL-17R blockade, which results in disruption of IL-17A as well as IL-17C/E/F cytokine signaling. This formed the basis for further study into the specific role of IL-17F in psoriatic disease and any potential therapeutic benefits associated with its inhibition.
Recently approved in the European Union, Canada, Australia, Japan, the United Kingdom, and the United States for moderate to severe psoriasis, bimekizumab is a novel humanized IgG antibody that selectively inhibits both IL-17A and IL-17F cytokines.53 Specifically, bimekizumab simultaneously prevents binding of IL-17A/A, IL-17A/F, and IL-17F/F dimers with the IL-17R. Compared to other IL-17 and IL-23 biologic therapies, bimekizumab (320 mg) achieved relatively higher response rates for PASI75, PASI90, and PASI100.49 Neutralization of IL-17A and IL-17F by bimekizumab also resulted in more complete suppression of cytokine responses and PMN chemotaxis than either cytokine alone in treated PsA patients,54 which is notable because of the incremental benefits of recent IL-23 and IL-17 inhibitors on inflammatory arthritis symptoms in contrast to the substantial improvements observed for cutaneous disease with those same agents.
The primary disadvantage of bimekizumab and its more complete blockade of the IL-17 signaling pathway is that treated patients have a substantially increased risk for oral candidiasis (>10%).55 However, the precise link between candidiasis and IL-17 blockade is not yet fully understood because other targeted agents that also broadly suppress IL-17 signaling (ie, IL-17R, IL-23 inhibitors) are associated with much lower rates of candidiasis.56-58 Bimekizumab also is being investigated as a novel therapy for hidradenitis suppurativa and will provide important reference information regarding the role for bispecific biologic agents in the treatment of chronic inflammatory skin diseases.59
IL-36 Signaling and Generalized Pustular Psoriasis
Recent genetic and clinical studies have expanded our understanding of the role of IL-36 signaling in the immunopathogenesis of pustular psoriasis variants. Generalized pustular psoriasis (GPP) is a rare distinct psoriasis subtype characterized by the recurrent development of widespread erythema, superficial sterile pustules, and desquamation. Systemic symptoms such as fever, malaise, itching, and skin pain accompany acute GPP flares.60 Generalized pustular psoriasis is more common in female patients (in contrast with plaque psoriasis), and acute flares may be caused by multiple stimuli including infections, hypocalcemia, initiation or discontinuation of medications (eg, oral corticosteroids), pregnancy, or stress.61,62 Flares of GPP often require emergency or in-patient care, as untreated symptoms increase the risk for severe health complications such as secondary infections, sepsis, or multisystem organ failure.63 The prevalence of GPP is estimated to be approximately 1 in 10,000 individuals in the United States,64-67 with mortality rates ranging from 0 to 3.3 deaths per 100 patient-years.67
In contrast to plaque psoriasis, aberrant IL-36 signaling is the predominant driver of GPP. IL-36 is a member of the IL-1 cytokine family that includes three IL-36 agonists (IL-36α, IL-36β, IL-36γ) and 1 endogenous antagonist (IL-36Ra, encoded by IL36RN).68 The immunopathogenesis of GPP involves dysregulation of the IL-36–chemokine–PMN axis, resulting in unopposed IL-36 signaling and the subsequent recruitment and influx of PMNs into the epidermis. IL36RN mutations are strongly associated with GPP and result in impaired function of the IL-36Ra protein, leading to unopposed IL-36 signaling.69 However, approximately two-thirds of GPP patients lack identifiable gene mutations, suggesting other immune mechanisms or triggers causing upregulated IL-36 signaling.70 In response to these triggers, increased IL-36 cytokines released by keratinocytes bind to the IL-36R, resulting in substantial keratinocyte hyperproliferation, increased IL-36 levels, and the expression of hundreds of additional inflammatory signals (eg, IL-17C, antimicrobial peptides, TNF, IL-6).71 Increased IL-36 levels also drive the production of PMN chemotactic proteins (eg, CXCL1/2/3/5/6/8 and CXCR1/2) and act synergistically with IL-17 cytokines to create an autoamplifying circuit that is analogous to the feed-forward inflammatory loop in plaque psoriasis.72 Biopsies of involved GPP skin reveal increased expression of IL-36 in the uppermost layers of the epidermis, which creates a gene expression gradient that acts as a strong attractant for PMNs and forms the basis for the hallmark pustular lesions observed in GPP patients.
Until recently, treatment strategies for GPP involved the off-label use of topical, oral, or biologic therapies approved for plaque psoriasis, which often was associated with variable or incomplete disease control. In September 2022, the US Food and Drug Administration (FDA) approved intravenous spesolimab as a first-in-class humanized monoclonal IgG1 antibody for the treatment of GPP flares in adults. Spesolimab binds to IL-36R and prevents its activation by its endogenous agonists. A phase 2, randomized, 12-week clinical trial (Effisayil-1) evaluated the efficacy and safety of a single 900-mg intravenous dose of spesolimab followed by an optional second dose 1 week later for inadequate treatment responses in 53 enrolled GPP patients (2:1 treatment to placebo randomization).73 Remarkably, more than half (19/35 [54%]) of GPP patients experienced complete resolution of pustules (GPP physician global assessment subscore of 0 [range, 0–4]) and showed sustained efficacy out to week 12 after just 1 or 2 doses of spesolimab. Overall, the safety profile of spesolimab was good; asthenia, fatigue, nausea, vomiting, headache, pruritus, infusion-related reaction and symptoms, and mild infections (eg, urinary tract infection) were the most common adverse events reported.73
Imsidolimab, a high-affinity humanized IgG4 monoclonal antibody that binds and blocks activation of IL-36R, also has completed phase 2 testing,74 with phase 3 study results expected in early 2024. The rapid onset of action and overall safety of imsidolimab was in line with and similar to spesolimab. Future approval of imsidolimab would add to the limited treatment options available for GPP and has the additional convenience of being administered to patients subcutaneously. Overall, the development of selective IL-36R inhibitors offers a much-needed therapeutic option for GPP and illustrates the importance of translational research.
Role of Tyrosine Kinase in Psoriatic Disease
The Janus kinase (JAK) enzyme family consists of 4 enzymes—tyrosine kinase 2 (TYK2), JAK1, JAK2, and JAK3—that function as intracellular transduction signals that mediate the biologic response of most extracellular cytokines and growth factors.75 Critical psoriasis-related cytokines are dependent on intact JAK-STAT signaling, including IL-23, IL-12, and type I IFNs. In 2010, a genome-wide association identified TYK2 as a psoriasis susceptibility locus,76 and loss-of-function TYK2 mutations confer a reduced risk for psoriasis.77 Unlike other JAK isoforms, TYK2 mediates biologic functions that are highly restricted to the immune responses associated with IL-23, IL-12, and type I IFN signaling.78,79 For these reasons, blockade of TYK2 signaling is an attractive therapeutic target for the potential treatment of psoriatic disease.
In September 2022, the FDA approved deucravacitinib as a first-in-class, oral, selective TYK2 inhibitor for the treatment of adult patients with moderate to severe plaque psoriasis. It was the first FDA approval of an oral small-molecule treatment for plaque psoriasis in nearly a decade. Deucravacitinib inhibits TYK2 signaling via selective binding of its unique regulatory domain, resulting in a conformational (allosteric) change that interferes with its active domain.80 This novel mechanism of action limits the unwanted blockade of other broad biologic processes mediated by JAK1/2/3. Of note, the FDA did not issue any boxed warnings for deucravacitinib as it did for other FDA-approved JAK inhibitors.
In a head-to-head, 52-week, double-blind, prospective, randomized, phase 3 study, deucravacitinib showed clear superiority over apremilast for PASI75 at week 16 (53.0% [271/511] vs 39.8% [101/254]) and week 24 (58.7% [296/504] vs 37.8% [96/254]).81 Clinical responses were sustained through week 52 and showed efficacy for difficult-to-treat areas such as the scalp, acral sites, and nails. Other advantages of deucravacitinib include once-daily dosing with no need for dose titration or adjustments for renal insufficiency as well as the absence of statistically significant differences in gastrointestinal tract symptoms compared to placebo. The most common adverse effects included nasopharyngitis, upper respiratory tract infections, headache, diarrhea, and herpes infections.81 The potential benefit of deucravacitinib for PsA and psoriasis comorbidities remains to be seen, but it is promising due to its simultaneous disruption of multiple psoriasis-related cytokine networks. Several other TYK2 inhibitors are being developed for psoriatic disease and related inflammatory conditions, underscoring the promise of targeting this intracellular pathway.
Aryl Hydrocarbon Receptor Agonism
Topical steroids are the mainstay treatment option for localized or limited plaque psoriasis due to their potent immunosuppressive effect on the skin and relatively low cost. Combined with vitamin D analogs, topical steroids result in marked improvements in disease severity and improved tolerability.82 However, chronic use of topical steroids is limited by the need for twice-daily application, resulting in poor treatment compliance; loss of efficacy over time; risk for steroid-induced skin atrophy on special body sites; and patient concerns of potential systemic effects. The discovery of novel drug targets amenable to topical inhibition is needed.
Dysregulated aryl hydrocarbon receptor (AHR) levels have been reported in atopic dermatitis and psoriasis.83 Aryl hydrocarbon receptors are ubiquitously expressed in many cell types and play an integral role in immune homeostasis within the skin, skin barrier function, protection against oxidative stressors, and regulation of proliferating melanocytes and keratinocytes.84,85 They are widely expressed in multiple immune cell types (eg, antigen-presenting cells, T lymphocytes, fibroblasts) and modulate the differentiation of T17 and T22 cells as well as their balance with regulatory T-cell populations.86 In keratinocytes, AHR helps to regulate terminal differentiation, enhance skin barrier integrity via AHR-dependent filaggrin (FLG) expression, and prevent transepidermal water loss.87,88 The mechanisms by which AHR ligands lead to the upregulation or downregulation of specific genes is intricate and highly context dependent, such as the specific ligand and cell type involved. In preclinical studies, AHR-deficient mice develop psoriasiform skin inflammation, increased IL-17 and IL-22 expression, and abnormal skin barrier function.89 Keratinocytes treated with AHR ligands in vitro modulated psoriasis-associated inflammatory cytokines, such as IL-6, IL-8, and type I and II IFNs.89,90 The use of coal tar, one of the earliest historical treatments for psoriasis, is thought to activate AHRs in the skin via organic compound mixtures containing polyaromatic hydrocarbons that help normalize the proinflammatory environment in psoriatic skin.91
In June 2022, the FDA approved tapinarof as a first-in-class, topical, nonsteroidal AHR agonist for the treatment of plaque psoriasis in adults. Although the exact mechanism of action for tapinarof has not been fully elucidated, early studies suggest that its primary function is the activation of AHR, leading to reduced T-cell expansion and T17 cell differentiation. In the imiquimod mouse model, cytokine expression of IL-17A, IL-17F, IL-19, IL-22, IL-23A, and IL-lβ in psoriasiform skin lesions were downregulated following tapinarof treatment.92 In humans, tapinarof treatment is associated with a remittive effect, in which the average time for tapinarof-treated psoriasis lesions to remain clear was approximately 4 months.93 Preliminary research investigating the mechanism by which tapinarof induces this remittive effect is ongoing and may involve the reduced activation and influx of T17 and Trm populations into the skin.94 However, these preclinical studies were performed on healthy dermatome-derived skin tissue cultured in T17-skewing conditions and needs to be replicated in larger samples sizes using human-derived psoriatic tissue. Alternatively, a strong inhibitory effect on IL-23 cytokine signaling may, in part, explain the remittive effect of tapinarof, as an analogous response is observed in patients who start and discontinue treatment with selective IL-23 antagonists. Regardless, the once-daily dosing of tapinarof and sustained treatment response is appealing to psoriasis patients. Tapinarof generally is well tolerated with mild folliculitis (>20% of patients) and contact dermatitis (5% of patients) reported as the most common skin-related adverse events.
New Roles for Phosphodiesterase 4 Inhibition
Phosphodiesterases (PDEs) are enzymes that hydrolyze cyclic nucleotides (eg, cyclic adenosine monophosphate) to regulate intracellular secondary messengers involved in the inflammatory response. One of several enzymes in the PDE family, PDE4, has been shown to have greater activity in psoriatic skin compared to healthy skin.95 Phosphodiesterase inhibitors decrease the degradation of cyclic adenosine monophosphate, which triggers protein kinase A to downregulate proinflammatory (eg, TNF-α, IL-6, IL-17, IL-12, IL-23) cytokines and increased expression of anti-inflammatory signals such as IL-10.96,97 Apremilast, the first oral PDE4 inhibitor approved by the FDA for psoriasis, offered a safe alternative to traditional oral immunosuppressive agents that had extensive risks and potential end-organ adverse effects. Unfortunately, apremilast demonstrated modest efficacy for psoriatic disease (better efficacy in the skin vs joint manifestations) and was supplanted easily by next-generation targeted biologic agents that were more efficacious and lacked the troublesome gastrointestinal tract adverse effects of PDE4 inhibition.98
Crisaborole became the first topical PDE4 inhibitor approved in the United States in December 2016 for twice-daily treatment of atopic dermatitis. Although phase 2 trial results were reported in psoriasis, this indication was never pursued, presumably due to similar improvements in primary outcome measures at week 12, compared to placebo (ClinicalTrials.gov Identifier NCT01300052).
In July 2022, the first topical PDE4 inhibitor indicated for plaque psoriasis was approved by the FDA—roflumilast cream 0.3% for once-daily use in individuals 12 years and older. Roflumilast was found to be clinically efficacious as early as 2 weeks after its use in an early-phase clinical trial.99 In 2 phase 3 clinical trials (DERMIS-1 and DERMIS-2), roflumilast significantly increased the proportion of patients achieving PASI75 at week 8 compared to vehicle (39%–41.6% vs 5.3%–7.6%, respectively)(P<.001).100 Overall, this nonsteroidal topical therapy was found to be well tolerated, with infrequent reports of application site pain or irritation as adverse events. Similar to tapinarof, patients can apply roflumilast on all body surface areas including the face, external genitalia, and other intertriginous areas.100 Importantly, the broad immune impact of PDE4 inhibition suggests that topical roflumilast likely will be an effective treatment for several additional inflammatory conditions, including seborrheic dermatitis and atopic dermatitis, which would expand the clinical utility of this specific medication.
Conclusion
In the last 2 decades, we have witnessed a translational revolution in our understanding of the underlying genetics and immunology of psoriatic disease. Psoriasis is widely considered one of the best-managed inflammatory conditions in all of medicine due to the development and availability of highly targeted, effective topical and systemic therapies that predominantly disrupt IL-23/IL-17 cytokine signaling in affected tissues. However, future clinical studies and laboratory research are necessary to elucidate the precise cause of psoriasis as well as the underlying genetic and immune signaling pathways driving less common clinical variants and recalcitrant disease.

- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Ogdie A, Langan S, Love T, et al. Prevalence and treatment patterns of psoriatic arthritis in the UK. Rheumatology (Oxford). 2013;52:568-575. doi:10.1093/rheumatology/kes324
- Mease PJ, Gladman DD, Papp KA, et al. Prevalence of rheumatologistdiagnosed psoriatic arthritis in patients with psoriasis in European/ North American dermatology clinics. J Am Acad Dermatol. 2013;69:729- 735. doi:10.1016/j.jaad.2013.07.023
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132(3 pt 1):556-562. doi:10.1038/jid.2011.365
- Wu S, Li WQ, Han J, et al. Hypercholesterolemia and risk of incident psoriasis and psoriatic arthritis in US women. Arthritis Rheumatol. 2014;66:304-310. doi:10.1002/art.38227
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and hypertension: a systematic review and meta-analysis of observational studies. J Hypertens. 2013;31:433-442; discussion 442-443. doi:10.1097/HJH.0b013e32835bcce1
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418. doi:10.1038 /jid.2009.112
- Armstrong AW, Lin SW, Chambers CJ, et al. Psoriasis and hypertension severity: results from a case-control study. PLoS One. 2011;6:E18227. doi:10.1371/journal.pone.0018227
- Naldi L, Chatenoud L, Linder D, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case-control study. J Invest Dermatol. 2005;125:61-67. doi:10.1111/j.0022-202X.2005.23681.x
- Yang YW, Kang JH, Lin HC. Increased risk of psoriasis following obstructive sleep apnea: a longitudinal population-based study. Sleep Med. 2012;13:285-289. doi:10.1016/j.sleep.2011.07.018
- Vaengebjerg S, Skov L, Egeberg A, et al. Prevalence, incidence, and risk of cancer in patients with psoriasis and psoriatic arthritis: a systematic review and meta-analysis. JAMA Dermatol. 2020;156:421-429. doi:10.1001/jamadermatol.2020.0024
- Loft N, Skov L, Richardson C, et al. A nationwide population-based cohort study of the incidence of severe and rare infections among adults with psoriasis in Denmark. Br J Dermatol. 2022;187:353-363. doi:10.1111/bjd.21595
- Fu Y, Lee CH, Chi CC. Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol. 2018;154:1417-1423. doi:10.1001/jamadermatol.2018.3631
- Dowlatshahi EA, Wakkee M, Arends LR, et al. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134:1542-1551. doi:10.1038/jid.2013.508
- Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety, and suicidality in patients with psoriasis: a populationbased cohort study. Arch Dermatol. 2010;146:891-895. doi:10.1001 /archdermatol.2010.186
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113. doi:10.1016/j.jaad.2018.11.058
- Vanderpuye-Orgle J, Zhao Y, Lu J, et al. Evaluating the economic burden of psoriasis in the United States. J Am Acad Dermatol. 2015;72:961-967. doi:10.1016/j.jaad.2015.02.1099
- Bos JD, Hagenaars C, Das PK, et al. Predominance of “memory” T cells (CD4+, CDw29+) over “naive” T cells (CD4+, CD45R+) in both normal and diseased human skin. Arch Dermatol Res. 1989;281:24-30. doi:10.1007/BF00424268
- Bos JD, Hulsebosch HJ, Krieg SR, et al. Immunocompetent cells in psoriasis. in situ immunophenotyping by monoclonal antibodies. Arch Dermatol Res. 1983;275:181-189. doi:10.1007/BF00510050
- Griffiths CE, Powles AV, Leonard JN, et al. Clearance of psoriasis with low dose cyclosporin. Br Med J (Clin Res Ed). 1986;293:731-732. doi:10.1136/bmj.293.6549.731
- Ellis CN, Gorsulowsky DC, Hamilton TA, et al. Cyclosporine improves psoriasis in a double-blind study. JAMA. 1986;256:3110-3116.
- Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233-240. doi:10.1084/jem.20041257
- Ferber IA, Brocke S, Taylor-Edwards C, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J Immunol. 1996;156:5-7.
- Bharadwaj R, Lusi CF, Mashayekh S, et al. Methotrexate suppresses psoriatic skin inflammation by inhibiting muropeptide transporter SLC46A2 activity. Immunity. 2023;56:998-1012. doi:10.1016/j. immuni.2023.04.001
- Jenneck C, Novak N. The safety and efficacy of alefacept in the treatment of chronic plaque psoriasis. Ther Clin Risk Manag. 2007;3:411-420.
- Pariser DM, Gordon KB, Papp KA, et al. Clinical efficacy of efalizumab in patients with chronic plaque psoriasis: results from three randomized placebo-controlled phase III trials: part I. J Cutan Med Surg. 2005; 9:303-312. doi:10.1007/s10227-005-0116-1
- Mease PJ, Gottlieb AB, van der Heijde D, et al. Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis. Ann Rheum Dis. 2017;76:1550-1558. doi:10.1136/annrheumdis-2016-210724
- Hawkes JE, Yan BY, Chan TC, et al. Discovery of the IL-23/IL-17 signaling pathway and the treatment of psoriasis. J Immunol. 2018;201:1605-1613. doi:10.4049/jimmunol.1800013
- Ogawa K, Okada Y. The current landscape of psoriasis genetics in 2020. J Dermatol Sci. 2020;99:2-8. doi:10.1016/j.jdermsci.2020.05.008
- Arakawa A, Siewert K, Stohr J, et al. Melanocyte antigen triggers autoimmunity in human psoriasis. J Exp Med. 2015;212:2203-2212. doi:10.1084/jem.20151093
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263-271. doi:10.1016/S0140-6736(07)61128-3
- Wang CQF, Akalu YT, Suarez-Farinas M, et al. IL-17 and TNF synergistically modulate cytokine expression while suppressing melanogenesis: potential relevance to psoriasis. J Invest Dermatol. 2013;133:2741-2752. doi:10.1038/jid.2013.237
- Cheung KL, Jarrett R, Subramaniam S, et la. Psoriatic T cells recognize neolipid antigens generated by mast cell phospholipase delivered by exosomes and presented by CD1a. J Exp Med. 2016;213:2399-2412. doi:10.1084/jem.20160258
- Hawkes JE, Gonzalez JA, Krueger JG. Autoimmunity in psoriasis: evidence for specific autoantigens. Curr Dermatol Rep. 2017;6:104-112. doi:10.1007/s13671-017-0177-6
- Johansen C, Usher PA, Kjellerup RB, et al. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br J Dermatol. 2009;160:319-324. doi:10.1111/j.1365-2133 .2008.08902.x
- Kolbinger F, Loesche C, Valentin MA, et al. beta-Defensin 2 is a responsive biomarker of IL-17A-driven skin pathology in patients with psoriasis. J Allergy Clin Immunol. 2017;139:923-932. doi:10.1016/j .jaci.2016.06.038
- Ruddy MJ, Wong GC, Liu XK, et al. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J Biol Chem. 2004;279:2559-2567. doi:10.1074/jbc.M308809200
- Shen F, Hu Z, Goswami J, et al. Identification of common transcriptional regulatory elements in interleukin-17 target genes. J Biol Chem. 2006;281:24138-24148. doi:10.1074/jbc.M604597200
- Harper EG, Guo C, Rizzo H, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol. 2009;129:2175-2183. doi:10.1038/jid.2009.65
- Chiricozzi A, Guttman-Yassky E, Suarez-Farinas M, et al. Integrative responses to IL-17 and TNF-alpha in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J Invest Dermatol. 2011;131:677-687. doi:10.1038/jid.2010.340
- Homey B, Dieu-Nosjean MC, Wiesenborn A, et al. Up-regulation of macrophage inflammatory protein-3 alpha/CCL20 and CC chemokine receptor 6 in psoriasis. J Immunol. 2000;164:6621-6632. doi:10.4049 /jimmunol.164.12.6621
- Stephen-Victor E, Fickenscher H, Bayry J. IL-26: an emerging proinflammatory member of the IL-10 Cytokine family with multifaceted actions in antiviral, antimicrobial, and autoimmune responses. PLoS Pathog. 2016;12:E1005624. doi:10.1371/journal.ppat.1005624
- Wolk K, Witte K, Witte E, et al. IL-29 is produced by T(H)17 cells and mediates the cutaneous antiviral competence in psoriasis [published online September 25, 2013]. Sci Transl Med. doi:10.1126 /scitranslmed.3006245
- Kasprowicz-Furmanczyk M, Czerwinska J, Placek W, et al. Assessment of the tissue resident memory cells in lesional skin of patients with psoriasis and in healthy skin of healthy volunteers. Int J Environ Res Public Health. 2021;18:11251. doi:10.3390/ijerph182111251
- Cheuk S, Schlums H, Gallais Serezal I, et al. CD49a expression defines tissue-resident CD8(+) T cells poised for cytotoxic function in human skin. Immunity. 2017;46:287-300. doi:10.1016/j.immuni.2017.01.009
- Sawyer LM, Malottki K, Sabry-Grant C, et al. Assessing the relative efficacy of interleukin-17 and interleukin-23 targeted treatments for moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis of PASI response. PLoS One. 2019;14:E0220868. doi:10.1371/journal.pone.0220868
- Coates LC, Kavanaugh A, Mease PJ, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68:1060-1071. doi:10.1002/art.39573
- Wang EA, Suzuki E, Maverakis E, et al. Targeting IL-17 in psoriatic arthritis. Eur J Rheumatol. 2017;4:272-277. doi:10.5152/eurjrheum.2017.17037
- Armstrong A, Fahrbach K, Leonardi C, et al. Efficacy of bimekizumab and other biologics in moderate to severe plaque psoriasis: a systematic literature review and a network meta-analysis. Dermatol Ther (Heidelb). 2022;12:1777-1792. doi:10.1007/s13555-022-00760-8
- van Baarsen LG, Lebre MC, van der Coelen D, et al. Heterogeneous expression pattern of interleukin 17A (IL-17A), IL-17F and their receptors in synovium of rheumatoid arthritis, psoriatic arthritis and osteoarthritis: possible explanation for nonresponse to anti-IL-17 therapy? Arthritis Res Ther. 2014;16:426. doi:10.1186/s13075-014-0426-z
- Hot A, Zrioual S, Toh ML, et al. IL-17A- versus IL-17F-induced intracellular signal transduction pathways and modulation by IL-17RA and IL-17RC RNA interference in rheumatoid synoviocytes. Ann Rheum Dis. 2011;70:341-348. doi:10.1136/ard.2010.132233
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894. doi:10.3389/fimmu.2020.01894
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475-486. doi:10.1016/S0140-6736(21)00126-4
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532. doi:10.1136 /annrheumdis-2017-212127
- Gordon KB, Langley RG, Warren RB, et al. Bimekizumab safety in patients with moderate to severe plaque psoriasis: pooled results from phase 2 and phase 3 randomized clinical trials. JAMA Dermatol. 2022;158:735-744. doi:10.1001/jamadermatol.2022.1185
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152. doi:10.1056/NEJMoa2102383
- Reich K, Iversen L, Puig L, et al. Long-term efficacy and safety of brodalumab in moderate-to-severe plaque psoriasis: a post hoc pooled analysis of AMAGINE-2 and -3. J Eur Acad Dermatol Venereol. 2022;36:1275-1283. doi:10.1111/jdv.18068
- Papp KA, Blauvelt A, Puig L, et al. Long-term safety and efficacy of risankizumab for the treatment of moderate-to-severe plaque psoriasis: interim analysis of the LIMMitless open-label extension trial up to 5 years of follow-up. J Am Acad Dermatol. 2023;89:1149-1158. doi: 10.1016/j.jaad.2023.07.1024
- Glatt S, Jemec GBE, Forman S, et al. Efficacy and safety of bimekizumab in moderate to severe hidradenitis suppurativa: a phase 2, doubleblind, placebo-controlled randomized clinical trial. JAMA Dermatol. 2021;157:1279-1288. doi:10.1001/jamadermatol.2021.2905
- Choon SE, Lai NM, Mohammad NA, et al. Clinical profile, morbidity, and outcome of adult-onset generalized pustular psoriasis: analysis of 102 cases seen in a tertiary hospital in Johor, Malaysia. Int J Dermatol. 2014;53:676-684. doi:10.1111/ijd.12070
- Zheng M, Jullien D, Eyerich K. The prevalence and disease characteristics of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23 (suppl 1):5-12. doi:10.1007/s40257-021-00664-x
- Fujita H, Gooderham M, Romiti R. Diagnosis of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23(suppl 1):31-38. doi:10.1007/s40257-021-00652-1
- Choon SE, Navarini AA, Pinter A. Clinical course and characteristics of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23 (suppl 1):21-29. doi:10.1007/s40257-021-00654-z
- Augey F, Renaudier P, Nicolas JF. Generalized pustular psoriasis (Zumbusch): a French epidemiological survey. Eur J Dermatol. 2006;16:669-673.
- Ohkawara A, Yasuda H, Kobayashi H, et al. Generalized pustular psoriasis in Japan: two distinct groups formed by differences in symptoms and genetic background. Acta Derm Venereol. 1996;76:68-71. doi:10.2340/00015555766871
- Lee JY, Kang S, Park JS, et al. Prevalence of psoriasis in Korea: A population-based epidemiological study using the Korean National Health Insurance database. Ann Dermatol. 2017;29:761-767. doi:10.5021 /ad.2017.29.6.761
- Prinz JC, Choon SE, Griffiths CEM, et al. Prevalence, comorbidities and mortality of generalized pustular psoriasis: a literature review. J Eur Acad Dermatol Venereol. 2023;37:256-273. doi:10.1111/jdv.18720
- Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109-120. doi:10.1016/j.jaci.2016.08.056
- Rajan N, Sinclair N, Nakai H, et al. A tale of two sisters: identical IL36RN mutations and discordant phenotypes. Br J Dermatol. 2016;174:417-420. doi:10.1111/bjd.14003
- Ly K, Beck KM, Smith MP, et al. Diagnosis and screening of patients with generalized pustular psoriasis. Psoriasis (Auckl). 2019;9:37-42. doi:10.2147/PTT.S181808
- Sugiura K. Role of interleukin 36 in generalised pustular psoriasis and beyond. Dermatol Ther (Heidelb). 2022;12:315-328. doi:10.1007 /s13555-021-00677-8
- Akiyama M, Takeichi T, McGrath JA, et al. Autoinflammatory keratinization diseases: an emerging concept encompassing various inflammatory keratinization disorders of the skin. J Dermatol Sci. 2018;90:105-111. doi:10.1016/j.jdermsci.2018.01.012
- Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440. doi:10.1056/NEJMoa2111563
- Warren RB, Reich A, Kaszuba A, et al. Imsidolimab, an anti-IL-36 receptor monoclonal antibody for the treatment of generalised pustular psoriasis: results from the phase 2 GALLOP trial. Br J Dermatol. 2023;189:161-169. doi:10.1093/bjd/ljad083
- Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017; 18:374-384. doi:10.1038/ni.3691
- Genetic Analysis of Psoriasis Consortium & the Wellcome Trust Case Control Consortium 2; Strange A, Capon F, et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat Genet. 2010;42:985-990. doi:10.1038/ng.694
- Enerback C, Sandin C, Lambert S, et al. The psoriasis-protective TYK2 I684S variant impairs IL-12 stimulated pSTAT4 response in skin-homing CD4+ and CD8+ memory T-cells. Sci Rep. 2018;8:7043. doi:10.1038/s41598-018-25282-2
- Shimoda K, Kato K, Aoki K, et al. Tyk2 plays a restricted role in IFN alpha signaling, although it is required for IL-12-mediated T cell function. Immunity. 2000;13:561-571. doi:10.1016/s1074-7613(00)00055-8
- Karaghiosoff M, Neubauer H, Lassnig C, et al. Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity. 2000;13:549-560. doi:10.1016/s1074-7613(00)00054-6
- Burke JR, Cheng L, Gillooly KM, et al. Autoimmune pathways in mice and humans are blocked by pharmacological stabilization of the TYK2 pseudokinase domain [published online July 24, 2019]. Sci Transl Med. doi:10.1126/scitranslmed.aaw1736
- Strober B, Thaci D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 program for evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51. doi:10.1016/j.jaad.2022.08.061
- Stein Gold L, Lebwohl M, Menter A, et al. Aerosol foam formulation of fixed combination calcipotriene plus betamethasone dipropionate is highly efficacious in patients with psoriasis vulgaris: pooled data from three randomized controlled studies. J Drugs Dermatol. 2016;15:951-957.
- Beranek M, Fiala Z, Kremlacek J, et al. Serum levels of aryl hydrocarbon receptor, cytochromes p450 1a1 and 1b1 in patients with exacerbated psoriasis vulgaris. Folia Biol (Praha). 2018;64:97-102.
- Esser C, Rannug A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol Rev. 2015;67:259- 279. doi:10.1124/pr.114.009001
- Furue M, Uchi H, Mitoma C, et al. Antioxidants for healthy skin: the emerging role of aryl hydrocarbon receptors and nuclear factorerythroid 2-related factor-2. Nutrients. 2017;9:223. doi:10.3390/nu9030223
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684. doi:10.1016/S0140-6736(08)60726-6
- Sutter CH, Olesen KM, Bhuju J, et al. AHR regulates metabolic reprogramming to promote SIRT1-dependent keratinocyte differentiation. J Invest Dermatol. 2019;139:818-826. doi:10.1016/j.jid.2018.10.019
- Haas K, Weighardt H, Deenen R, et al. Aryl hydrocarbon receptor in keratinocytes is essential for murine skin barrier integrity. J Invest Dermatol. 2016;136:2260-2269. doi:10.1016/j.jid.2016.06.627
- Di Meglio P, Duarte JH, Ahlfors H, et al. Activation of the aryl hydrocarbon receptor dampens the severity of inflammatory skin conditions. Immunity. 2014;40:989-1001. doi:10.1016/j.immuni.2014.04.019
- Kim HO, Kim JH, Chung BY, et al. Increased expression of the aryl hydrocarbon receptor in patients with chronic inflammatory skin diseases. Exp Dermatol. 2014;23:278-281. doi:10.1111/exd.12350
- van den Bogaard EH, Bergboer JG, Vonk-Bergers M, et al. Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J Clin Invest. 2013;123:917-927. doi:10.1172/JCI65642
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AHR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137:2110-2119. doi:10.1016/j.jid.2017.05.004
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806. doi:10.1016/j.jaad.2022.06.1171
- Mooney N, Teague JE, Gehad AE, et al. Tapinarof inhibits the formation, cytokine production, and persistence of resident memory T cells in vitro. SKIN J Cutan Med. 2023;7:S194. doi:10.25251/skin.7.supp.194
- Schafer PH, Truzzi F, Parton A, et al. Phosphodiesterase 4 in inflammatory diseases: effects of apremilast in psoriatic blood and in dermal myofibroblasts through the PDE4/CD271 complex. Cell Signal. 2016;28:753-763. doi:10.1016/j.cellsig.2016.01.007
- Li H, Zuo J, Tang W. Phosphodiesterase-4 inhibitors for the treatment of inflammatory diseases. Front Pharmacol. 2018;9:1048. doi:10.3389/ fphar.2018.01048
- Schafer PH, Parton A, Gandhi AK, et al. Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br J Pharmacol. 2010;159:842-855. doi:10.1111/j.1476-5381.2009.00559.x
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49. doi:10.1016/j .jaad.2015.03.049
- Papp KA, Gooderham M, Droege M, et al. Roflumilast cream improves signs and symptoms of plaque psoriasis: results from a phase 1/2a randomized, controlled study. J Drugs Dermatol. 2020;19:734-740. doi:10.36849/JDD.2020.5370
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084. doi:10.1001/jama.2022.15632
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Ogdie A, Langan S, Love T, et al. Prevalence and treatment patterns of psoriatic arthritis in the UK. Rheumatology (Oxford). 2013;52:568-575. doi:10.1093/rheumatology/kes324
- Mease PJ, Gladman DD, Papp KA, et al. Prevalence of rheumatologistdiagnosed psoriatic arthritis in patients with psoriasis in European/ North American dermatology clinics. J Am Acad Dermatol. 2013;69:729- 735. doi:10.1016/j.jaad.2013.07.023
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132(3 pt 1):556-562. doi:10.1038/jid.2011.365
- Wu S, Li WQ, Han J, et al. Hypercholesterolemia and risk of incident psoriasis and psoriatic arthritis in US women. Arthritis Rheumatol. 2014;66:304-310. doi:10.1002/art.38227
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and hypertension: a systematic review and meta-analysis of observational studies. J Hypertens. 2013;31:433-442; discussion 442-443. doi:10.1097/HJH.0b013e32835bcce1
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418. doi:10.1038 /jid.2009.112
- Armstrong AW, Lin SW, Chambers CJ, et al. Psoriasis and hypertension severity: results from a case-control study. PLoS One. 2011;6:E18227. doi:10.1371/journal.pone.0018227
- Naldi L, Chatenoud L, Linder D, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case-control study. J Invest Dermatol. 2005;125:61-67. doi:10.1111/j.0022-202X.2005.23681.x
- Yang YW, Kang JH, Lin HC. Increased risk of psoriasis following obstructive sleep apnea: a longitudinal population-based study. Sleep Med. 2012;13:285-289. doi:10.1016/j.sleep.2011.07.018
- Vaengebjerg S, Skov L, Egeberg A, et al. Prevalence, incidence, and risk of cancer in patients with psoriasis and psoriatic arthritis: a systematic review and meta-analysis. JAMA Dermatol. 2020;156:421-429. doi:10.1001/jamadermatol.2020.0024
- Loft N, Skov L, Richardson C, et al. A nationwide population-based cohort study of the incidence of severe and rare infections among adults with psoriasis in Denmark. Br J Dermatol. 2022;187:353-363. doi:10.1111/bjd.21595
- Fu Y, Lee CH, Chi CC. Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol. 2018;154:1417-1423. doi:10.1001/jamadermatol.2018.3631
- Dowlatshahi EA, Wakkee M, Arends LR, et al. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134:1542-1551. doi:10.1038/jid.2013.508
- Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety, and suicidality in patients with psoriasis: a populationbased cohort study. Arch Dermatol. 2010;146:891-895. doi:10.1001 /archdermatol.2010.186
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113. doi:10.1016/j.jaad.2018.11.058
- Vanderpuye-Orgle J, Zhao Y, Lu J, et al. Evaluating the economic burden of psoriasis in the United States. J Am Acad Dermatol. 2015;72:961-967. doi:10.1016/j.jaad.2015.02.1099
- Bos JD, Hagenaars C, Das PK, et al. Predominance of “memory” T cells (CD4+, CDw29+) over “naive” T cells (CD4+, CD45R+) in both normal and diseased human skin. Arch Dermatol Res. 1989;281:24-30. doi:10.1007/BF00424268
- Bos JD, Hulsebosch HJ, Krieg SR, et al. Immunocompetent cells in psoriasis. in situ immunophenotyping by monoclonal antibodies. Arch Dermatol Res. 1983;275:181-189. doi:10.1007/BF00510050
- Griffiths CE, Powles AV, Leonard JN, et al. Clearance of psoriasis with low dose cyclosporin. Br Med J (Clin Res Ed). 1986;293:731-732. doi:10.1136/bmj.293.6549.731
- Ellis CN, Gorsulowsky DC, Hamilton TA, et al. Cyclosporine improves psoriasis in a double-blind study. JAMA. 1986;256:3110-3116.
- Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233-240. doi:10.1084/jem.20041257
- Ferber IA, Brocke S, Taylor-Edwards C, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J Immunol. 1996;156:5-7.
- Bharadwaj R, Lusi CF, Mashayekh S, et al. Methotrexate suppresses psoriatic skin inflammation by inhibiting muropeptide transporter SLC46A2 activity. Immunity. 2023;56:998-1012. doi:10.1016/j. immuni.2023.04.001
- Jenneck C, Novak N. The safety and efficacy of alefacept in the treatment of chronic plaque psoriasis. Ther Clin Risk Manag. 2007;3:411-420.
- Pariser DM, Gordon KB, Papp KA, et al. Clinical efficacy of efalizumab in patients with chronic plaque psoriasis: results from three randomized placebo-controlled phase III trials: part I. J Cutan Med Surg. 2005; 9:303-312. doi:10.1007/s10227-005-0116-1
- Mease PJ, Gottlieb AB, van der Heijde D, et al. Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis. Ann Rheum Dis. 2017;76:1550-1558. doi:10.1136/annrheumdis-2016-210724
- Hawkes JE, Yan BY, Chan TC, et al. Discovery of the IL-23/IL-17 signaling pathway and the treatment of psoriasis. J Immunol. 2018;201:1605-1613. doi:10.4049/jimmunol.1800013
- Ogawa K, Okada Y. The current landscape of psoriasis genetics in 2020. J Dermatol Sci. 2020;99:2-8. doi:10.1016/j.jdermsci.2020.05.008
- Arakawa A, Siewert K, Stohr J, et al. Melanocyte antigen triggers autoimmunity in human psoriasis. J Exp Med. 2015;212:2203-2212. doi:10.1084/jem.20151093
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263-271. doi:10.1016/S0140-6736(07)61128-3
- Wang CQF, Akalu YT, Suarez-Farinas M, et al. IL-17 and TNF synergistically modulate cytokine expression while suppressing melanogenesis: potential relevance to psoriasis. J Invest Dermatol. 2013;133:2741-2752. doi:10.1038/jid.2013.237
- Cheung KL, Jarrett R, Subramaniam S, et la. Psoriatic T cells recognize neolipid antigens generated by mast cell phospholipase delivered by exosomes and presented by CD1a. J Exp Med. 2016;213:2399-2412. doi:10.1084/jem.20160258
- Hawkes JE, Gonzalez JA, Krueger JG. Autoimmunity in psoriasis: evidence for specific autoantigens. Curr Dermatol Rep. 2017;6:104-112. doi:10.1007/s13671-017-0177-6
- Johansen C, Usher PA, Kjellerup RB, et al. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br J Dermatol. 2009;160:319-324. doi:10.1111/j.1365-2133 .2008.08902.x
- Kolbinger F, Loesche C, Valentin MA, et al. beta-Defensin 2 is a responsive biomarker of IL-17A-driven skin pathology in patients with psoriasis. J Allergy Clin Immunol. 2017;139:923-932. doi:10.1016/j .jaci.2016.06.038
- Ruddy MJ, Wong GC, Liu XK, et al. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J Biol Chem. 2004;279:2559-2567. doi:10.1074/jbc.M308809200
- Shen F, Hu Z, Goswami J, et al. Identification of common transcriptional regulatory elements in interleukin-17 target genes. J Biol Chem. 2006;281:24138-24148. doi:10.1074/jbc.M604597200
- Harper EG, Guo C, Rizzo H, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol. 2009;129:2175-2183. doi:10.1038/jid.2009.65
- Chiricozzi A, Guttman-Yassky E, Suarez-Farinas M, et al. Integrative responses to IL-17 and TNF-alpha in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J Invest Dermatol. 2011;131:677-687. doi:10.1038/jid.2010.340
- Homey B, Dieu-Nosjean MC, Wiesenborn A, et al. Up-regulation of macrophage inflammatory protein-3 alpha/CCL20 and CC chemokine receptor 6 in psoriasis. J Immunol. 2000;164:6621-6632. doi:10.4049 /jimmunol.164.12.6621
- Stephen-Victor E, Fickenscher H, Bayry J. IL-26: an emerging proinflammatory member of the IL-10 Cytokine family with multifaceted actions in antiviral, antimicrobial, and autoimmune responses. PLoS Pathog. 2016;12:E1005624. doi:10.1371/journal.ppat.1005624
- Wolk K, Witte K, Witte E, et al. IL-29 is produced by T(H)17 cells and mediates the cutaneous antiviral competence in psoriasis [published online September 25, 2013]. Sci Transl Med. doi:10.1126 /scitranslmed.3006245
- Kasprowicz-Furmanczyk M, Czerwinska J, Placek W, et al. Assessment of the tissue resident memory cells in lesional skin of patients with psoriasis and in healthy skin of healthy volunteers. Int J Environ Res Public Health. 2021;18:11251. doi:10.3390/ijerph182111251
- Cheuk S, Schlums H, Gallais Serezal I, et al. CD49a expression defines tissue-resident CD8(+) T cells poised for cytotoxic function in human skin. Immunity. 2017;46:287-300. doi:10.1016/j.immuni.2017.01.009
- Sawyer LM, Malottki K, Sabry-Grant C, et al. Assessing the relative efficacy of interleukin-17 and interleukin-23 targeted treatments for moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis of PASI response. PLoS One. 2019;14:E0220868. doi:10.1371/journal.pone.0220868
- Coates LC, Kavanaugh A, Mease PJ, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68:1060-1071. doi:10.1002/art.39573
- Wang EA, Suzuki E, Maverakis E, et al. Targeting IL-17 in psoriatic arthritis. Eur J Rheumatol. 2017;4:272-277. doi:10.5152/eurjrheum.2017.17037
- Armstrong A, Fahrbach K, Leonardi C, et al. Efficacy of bimekizumab and other biologics in moderate to severe plaque psoriasis: a systematic literature review and a network meta-analysis. Dermatol Ther (Heidelb). 2022;12:1777-1792. doi:10.1007/s13555-022-00760-8
- van Baarsen LG, Lebre MC, van der Coelen D, et al. Heterogeneous expression pattern of interleukin 17A (IL-17A), IL-17F and their receptors in synovium of rheumatoid arthritis, psoriatic arthritis and osteoarthritis: possible explanation for nonresponse to anti-IL-17 therapy? Arthritis Res Ther. 2014;16:426. doi:10.1186/s13075-014-0426-z
- Hot A, Zrioual S, Toh ML, et al. IL-17A- versus IL-17F-induced intracellular signal transduction pathways and modulation by IL-17RA and IL-17RC RNA interference in rheumatoid synoviocytes. Ann Rheum Dis. 2011;70:341-348. doi:10.1136/ard.2010.132233
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894. doi:10.3389/fimmu.2020.01894
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475-486. doi:10.1016/S0140-6736(21)00126-4
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532. doi:10.1136 /annrheumdis-2017-212127
- Gordon KB, Langley RG, Warren RB, et al. Bimekizumab safety in patients with moderate to severe plaque psoriasis: pooled results from phase 2 and phase 3 randomized clinical trials. JAMA Dermatol. 2022;158:735-744. doi:10.1001/jamadermatol.2022.1185
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152. doi:10.1056/NEJMoa2102383
- Reich K, Iversen L, Puig L, et al. Long-term efficacy and safety of brodalumab in moderate-to-severe plaque psoriasis: a post hoc pooled analysis of AMAGINE-2 and -3. J Eur Acad Dermatol Venereol. 2022;36:1275-1283. doi:10.1111/jdv.18068
- Papp KA, Blauvelt A, Puig L, et al. Long-term safety and efficacy of risankizumab for the treatment of moderate-to-severe plaque psoriasis: interim analysis of the LIMMitless open-label extension trial up to 5 years of follow-up. J Am Acad Dermatol. 2023;89:1149-1158. doi: 10.1016/j.jaad.2023.07.1024
- Glatt S, Jemec GBE, Forman S, et al. Efficacy and safety of bimekizumab in moderate to severe hidradenitis suppurativa: a phase 2, doubleblind, placebo-controlled randomized clinical trial. JAMA Dermatol. 2021;157:1279-1288. doi:10.1001/jamadermatol.2021.2905
- Choon SE, Lai NM, Mohammad NA, et al. Clinical profile, morbidity, and outcome of adult-onset generalized pustular psoriasis: analysis of 102 cases seen in a tertiary hospital in Johor, Malaysia. Int J Dermatol. 2014;53:676-684. doi:10.1111/ijd.12070
- Zheng M, Jullien D, Eyerich K. The prevalence and disease characteristics of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23 (suppl 1):5-12. doi:10.1007/s40257-021-00664-x
- Fujita H, Gooderham M, Romiti R. Diagnosis of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23(suppl 1):31-38. doi:10.1007/s40257-021-00652-1
- Choon SE, Navarini AA, Pinter A. Clinical course and characteristics of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23 (suppl 1):21-29. doi:10.1007/s40257-021-00654-z
- Augey F, Renaudier P, Nicolas JF. Generalized pustular psoriasis (Zumbusch): a French epidemiological survey. Eur J Dermatol. 2006;16:669-673.
- Ohkawara A, Yasuda H, Kobayashi H, et al. Generalized pustular psoriasis in Japan: two distinct groups formed by differences in symptoms and genetic background. Acta Derm Venereol. 1996;76:68-71. doi:10.2340/00015555766871
- Lee JY, Kang S, Park JS, et al. Prevalence of psoriasis in Korea: A population-based epidemiological study using the Korean National Health Insurance database. Ann Dermatol. 2017;29:761-767. doi:10.5021 /ad.2017.29.6.761
- Prinz JC, Choon SE, Griffiths CEM, et al. Prevalence, comorbidities and mortality of generalized pustular psoriasis: a literature review. J Eur Acad Dermatol Venereol. 2023;37:256-273. doi:10.1111/jdv.18720
- Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109-120. doi:10.1016/j.jaci.2016.08.056
- Rajan N, Sinclair N, Nakai H, et al. A tale of two sisters: identical IL36RN mutations and discordant phenotypes. Br J Dermatol. 2016;174:417-420. doi:10.1111/bjd.14003
- Ly K, Beck KM, Smith MP, et al. Diagnosis and screening of patients with generalized pustular psoriasis. Psoriasis (Auckl). 2019;9:37-42. doi:10.2147/PTT.S181808
- Sugiura K. Role of interleukin 36 in generalised pustular psoriasis and beyond. Dermatol Ther (Heidelb). 2022;12:315-328. doi:10.1007 /s13555-021-00677-8
- Akiyama M, Takeichi T, McGrath JA, et al. Autoinflammatory keratinization diseases: an emerging concept encompassing various inflammatory keratinization disorders of the skin. J Dermatol Sci. 2018;90:105-111. doi:10.1016/j.jdermsci.2018.01.012
- Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440. doi:10.1056/NEJMoa2111563
- Warren RB, Reich A, Kaszuba A, et al. Imsidolimab, an anti-IL-36 receptor monoclonal antibody for the treatment of generalised pustular psoriasis: results from the phase 2 GALLOP trial. Br J Dermatol. 2023;189:161-169. doi:10.1093/bjd/ljad083
- Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017; 18:374-384. doi:10.1038/ni.3691
- Genetic Analysis of Psoriasis Consortium & the Wellcome Trust Case Control Consortium 2; Strange A, Capon F, et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat Genet. 2010;42:985-990. doi:10.1038/ng.694
- Enerback C, Sandin C, Lambert S, et al. The psoriasis-protective TYK2 I684S variant impairs IL-12 stimulated pSTAT4 response in skin-homing CD4+ and CD8+ memory T-cells. Sci Rep. 2018;8:7043. doi:10.1038/s41598-018-25282-2
- Shimoda K, Kato K, Aoki K, et al. Tyk2 plays a restricted role in IFN alpha signaling, although it is required for IL-12-mediated T cell function. Immunity. 2000;13:561-571. doi:10.1016/s1074-7613(00)00055-8
- Karaghiosoff M, Neubauer H, Lassnig C, et al. Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity. 2000;13:549-560. doi:10.1016/s1074-7613(00)00054-6
- Burke JR, Cheng L, Gillooly KM, et al. Autoimmune pathways in mice and humans are blocked by pharmacological stabilization of the TYK2 pseudokinase domain [published online July 24, 2019]. Sci Transl Med. doi:10.1126/scitranslmed.aaw1736
- Strober B, Thaci D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 program for evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51. doi:10.1016/j.jaad.2022.08.061
- Stein Gold L, Lebwohl M, Menter A, et al. Aerosol foam formulation of fixed combination calcipotriene plus betamethasone dipropionate is highly efficacious in patients with psoriasis vulgaris: pooled data from three randomized controlled studies. J Drugs Dermatol. 2016;15:951-957.
- Beranek M, Fiala Z, Kremlacek J, et al. Serum levels of aryl hydrocarbon receptor, cytochromes p450 1a1 and 1b1 in patients with exacerbated psoriasis vulgaris. Folia Biol (Praha). 2018;64:97-102.
- Esser C, Rannug A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol Rev. 2015;67:259- 279. doi:10.1124/pr.114.009001
- Furue M, Uchi H, Mitoma C, et al. Antioxidants for healthy skin: the emerging role of aryl hydrocarbon receptors and nuclear factorerythroid 2-related factor-2. Nutrients. 2017;9:223. doi:10.3390/nu9030223
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684. doi:10.1016/S0140-6736(08)60726-6
- Sutter CH, Olesen KM, Bhuju J, et al. AHR regulates metabolic reprogramming to promote SIRT1-dependent keratinocyte differentiation. J Invest Dermatol. 2019;139:818-826. doi:10.1016/j.jid.2018.10.019
- Haas K, Weighardt H, Deenen R, et al. Aryl hydrocarbon receptor in keratinocytes is essential for murine skin barrier integrity. J Invest Dermatol. 2016;136:2260-2269. doi:10.1016/j.jid.2016.06.627
- Di Meglio P, Duarte JH, Ahlfors H, et al. Activation of the aryl hydrocarbon receptor dampens the severity of inflammatory skin conditions. Immunity. 2014;40:989-1001. doi:10.1016/j.immuni.2014.04.019
- Kim HO, Kim JH, Chung BY, et al. Increased expression of the aryl hydrocarbon receptor in patients with chronic inflammatory skin diseases. Exp Dermatol. 2014;23:278-281. doi:10.1111/exd.12350
- van den Bogaard EH, Bergboer JG, Vonk-Bergers M, et al. Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J Clin Invest. 2013;123:917-927. doi:10.1172/JCI65642
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AHR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137:2110-2119. doi:10.1016/j.jid.2017.05.004
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806. doi:10.1016/j.jaad.2022.06.1171
- Mooney N, Teague JE, Gehad AE, et al. Tapinarof inhibits the formation, cytokine production, and persistence of resident memory T cells in vitro. SKIN J Cutan Med. 2023;7:S194. doi:10.25251/skin.7.supp.194
- Schafer PH, Truzzi F, Parton A, et al. Phosphodiesterase 4 in inflammatory diseases: effects of apremilast in psoriatic blood and in dermal myofibroblasts through the PDE4/CD271 complex. Cell Signal. 2016;28:753-763. doi:10.1016/j.cellsig.2016.01.007
- Li H, Zuo J, Tang W. Phosphodiesterase-4 inhibitors for the treatment of inflammatory diseases. Front Pharmacol. 2018;9:1048. doi:10.3389/ fphar.2018.01048
- Schafer PH, Parton A, Gandhi AK, et al. Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br J Pharmacol. 2010;159:842-855. doi:10.1111/j.1476-5381.2009.00559.x
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49. doi:10.1016/j .jaad.2015.03.049
- Papp KA, Gooderham M, Droege M, et al. Roflumilast cream improves signs and symptoms of plaque psoriasis: results from a phase 1/2a randomized, controlled study. J Drugs Dermatol. 2020;19:734-740. doi:10.36849/JDD.2020.5370
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084. doi:10.1001/jama.2022.15632
Practice Points
- Psoriasis is a chronic inflammatory condition characterized by systemic inflammation and dysregulated IL-23/IL-17 signaling.
- Modern discoveries highlight the role of additional immune signals in psoriatic disease such as IL-17C, IL-17F, IL-36, and tyrosine kinase 2, which also contribute to disease development.
- Novel systemic, oral, and topical therapies have become available and add to the rapidly growing armamentarium of safe and effective treatments for psoriatic disease.
Colchicine May Benefit Patients With Diabetes and Recent MI
TOPLINE:
A daily low dose of colchicine significantly reduces ischemic cardiovascular events in patients with type 2 diabetes (T2D) and a recent myocardial infarction (MI).
METHODOLOGY:
- After an MI, patients with vs without T2D have a higher risk for another cardiovascular event.
- The Colchicine Cardiovascular Outcomes Trial (COLCOT), a randomized, double-blinded trial, found a lower risk for ischemic cardiovascular events with 0.5 mg colchicine taken daily vs placebo, initiated within 30 days of an MI.
- Researchers conducted a prespecified subgroup analysis of 959 adult patients with T2D (mean age, 62.4 years; 22.2% women) in COLCOT (462 patients in colchicine and 497 patients in placebo groups).
- The primary efficacy endpoint was a composite of cardiovascular death, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina requiring coronary revascularization within a median 23 months.
- The patients were taking a variety of appropriate medications, including aspirin and another antiplatelet agent and a statin (98%-99%) and metformin (75%-76%).
TAKEAWAY:
- The risk for the primary endpoint was reduced by 35% in patients with T2D who received colchicine than in those who received placebo (hazard ratio, 0.65; P = .03).
- The primary endpoint event rate per 100 patient-months was significantly lower in the colchicine group than in the placebo group (rate ratio, 0.53; P = .01).
- The frequencies of adverse events were similar in both the treatment and placebo groups (14.6% and 12.8%, respectively; P = .41), with gastrointestinal adverse events being the most common.
- In COLCOT, patients with T2D had a 1.86-fold higher risk for a primary endpoint cardiovascular event, but there was no significant difference in the primary endpoint between those with and without T2D on colchicine.
IN PRACTICE:
“Patients with both T2D and a recent MI derive a large benefit from inflammation-reducing therapy with colchicine,” the authors noted.
SOURCE:
This study, led by François Roubille, University Hospital of Montpellier, France, was published online on January 5, 2024, in Diabetes Care.
LIMITATIONS:
Patients were not stratified at inclusion for the presence of diabetes. Also, the study did not evaluate the role of glycated hemoglobin and low-density lipoprotein cholesterol, as well as the effects of different glucose-lowering medications or possible hypoglycemic episodes.
DISCLOSURES:
The COLCOT study was funded by the Government of Quebec, the Canadian Institutes of Health Research, and philanthropic foundations. Coauthors Jean-Claude Tardif and Wolfgang Koenig declared receiving research grants, honoraria, advisory board fees, and lecture fees from pharmaceutical companies, as well as having other ties with various sources.
A version of this article appeared on Medscape.com.
TOPLINE:
A daily low dose of colchicine significantly reduces ischemic cardiovascular events in patients with type 2 diabetes (T2D) and a recent myocardial infarction (MI).
METHODOLOGY:
- After an MI, patients with vs without T2D have a higher risk for another cardiovascular event.
- The Colchicine Cardiovascular Outcomes Trial (COLCOT), a randomized, double-blinded trial, found a lower risk for ischemic cardiovascular events with 0.5 mg colchicine taken daily vs placebo, initiated within 30 days of an MI.
- Researchers conducted a prespecified subgroup analysis of 959 adult patients with T2D (mean age, 62.4 years; 22.2% women) in COLCOT (462 patients in colchicine and 497 patients in placebo groups).
- The primary efficacy endpoint was a composite of cardiovascular death, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina requiring coronary revascularization within a median 23 months.
- The patients were taking a variety of appropriate medications, including aspirin and another antiplatelet agent and a statin (98%-99%) and metformin (75%-76%).
TAKEAWAY:
- The risk for the primary endpoint was reduced by 35% in patients with T2D who received colchicine than in those who received placebo (hazard ratio, 0.65; P = .03).
- The primary endpoint event rate per 100 patient-months was significantly lower in the colchicine group than in the placebo group (rate ratio, 0.53; P = .01).
- The frequencies of adverse events were similar in both the treatment and placebo groups (14.6% and 12.8%, respectively; P = .41), with gastrointestinal adverse events being the most common.
- In COLCOT, patients with T2D had a 1.86-fold higher risk for a primary endpoint cardiovascular event, but there was no significant difference in the primary endpoint between those with and without T2D on colchicine.
IN PRACTICE:
“Patients with both T2D and a recent MI derive a large benefit from inflammation-reducing therapy with colchicine,” the authors noted.
SOURCE:
This study, led by François Roubille, University Hospital of Montpellier, France, was published online on January 5, 2024, in Diabetes Care.
LIMITATIONS:
Patients were not stratified at inclusion for the presence of diabetes. Also, the study did not evaluate the role of glycated hemoglobin and low-density lipoprotein cholesterol, as well as the effects of different glucose-lowering medications or possible hypoglycemic episodes.
DISCLOSURES:
The COLCOT study was funded by the Government of Quebec, the Canadian Institutes of Health Research, and philanthropic foundations. Coauthors Jean-Claude Tardif and Wolfgang Koenig declared receiving research grants, honoraria, advisory board fees, and lecture fees from pharmaceutical companies, as well as having other ties with various sources.
A version of this article appeared on Medscape.com.
TOPLINE:
A daily low dose of colchicine significantly reduces ischemic cardiovascular events in patients with type 2 diabetes (T2D) and a recent myocardial infarction (MI).
METHODOLOGY:
- After an MI, patients with vs without T2D have a higher risk for another cardiovascular event.
- The Colchicine Cardiovascular Outcomes Trial (COLCOT), a randomized, double-blinded trial, found a lower risk for ischemic cardiovascular events with 0.5 mg colchicine taken daily vs placebo, initiated within 30 days of an MI.
- Researchers conducted a prespecified subgroup analysis of 959 adult patients with T2D (mean age, 62.4 years; 22.2% women) in COLCOT (462 patients in colchicine and 497 patients in placebo groups).
- The primary efficacy endpoint was a composite of cardiovascular death, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina requiring coronary revascularization within a median 23 months.
- The patients were taking a variety of appropriate medications, including aspirin and another antiplatelet agent and a statin (98%-99%) and metformin (75%-76%).
TAKEAWAY:
- The risk for the primary endpoint was reduced by 35% in patients with T2D who received colchicine than in those who received placebo (hazard ratio, 0.65; P = .03).
- The primary endpoint event rate per 100 patient-months was significantly lower in the colchicine group than in the placebo group (rate ratio, 0.53; P = .01).
- The frequencies of adverse events were similar in both the treatment and placebo groups (14.6% and 12.8%, respectively; P = .41), with gastrointestinal adverse events being the most common.
- In COLCOT, patients with T2D had a 1.86-fold higher risk for a primary endpoint cardiovascular event, but there was no significant difference in the primary endpoint between those with and without T2D on colchicine.
IN PRACTICE:
“Patients with both T2D and a recent MI derive a large benefit from inflammation-reducing therapy with colchicine,” the authors noted.
SOURCE:
This study, led by François Roubille, University Hospital of Montpellier, France, was published online on January 5, 2024, in Diabetes Care.
LIMITATIONS:
Patients were not stratified at inclusion for the presence of diabetes. Also, the study did not evaluate the role of glycated hemoglobin and low-density lipoprotein cholesterol, as well as the effects of different glucose-lowering medications or possible hypoglycemic episodes.
DISCLOSURES:
The COLCOT study was funded by the Government of Quebec, the Canadian Institutes of Health Research, and philanthropic foundations. Coauthors Jean-Claude Tardif and Wolfgang Koenig declared receiving research grants, honoraria, advisory board fees, and lecture fees from pharmaceutical companies, as well as having other ties with various sources.
A version of this article appeared on Medscape.com.
FDA Expands Dupilumab for EoE to Younger Children
The US Food and Drug Administration (FDA) has approved dupilumab (Dupixent, Regeneron/Sanofi) for the treatment of eosinophilic esophagitis (EoE) in children aged 1-11 years and weighing ≥ 15 kg. It is the first and only medicine approved to treat these patients.
, as reported by this news organization.
EoE is a chronic inflammatory disorder driven by type 2 inflammation that damages the esophagus and causes difficulty swallowing and eating.
Dupilumab is a monoclonal antibody that acts to inhibit part of the inflammatory pathway.
EoE KIDS Trial
The FDA approval of dupilumab for younger children is based on results from the phase 3 randomized, double-blind, placebo-controlled EoE KIDS trial, which had two parts.
Part A was a 16-week double-blind treatment period that evaluated the safety and efficacy of dupilumab in a tiered weight-based dosing schema.
At 16 weeks, 66% of children who received higher dose dupilumab at tiered dosing regimens based on weight achieved histologic disease remission (six or fewer eosinophils/high power field), which was the primary endpoint, compared with only 3% of children who received placebo.
In addition, a greater decrease in the proportion of days with one or more signs of EoE according to the Pediatric EoE Sign/Symptom Questionnaire caregiver version (PESQ-C) was observed in children treated with dupilumab at 16 weeks compared placebo.
Part B was a 36-week extended active treatment period in which eligible children from Part A in the dupilumab group continued to receive their dose level and those in the placebo group in Part A switched to active treatment.
Histologic remission was sustained at week 52 in 53% of children treated with dupilumab in Parts A and B. Histologic remission was also achieved at week 52 in 53% of children who switched to dupilumab from placebo in Part B.
The safety profile of dupilumab observed through 16 weeks in these children was generally in line to that seen through 24 weeks in persons aged 12 years or older with EoE.
The most common adverse events (≥ 2%) more frequently observed with dupilumab than with placebo were injection site reactions, upper respiratory tract infections, arthralgia, and herpes viral infections. In EoE KIDS Part B, one case of helminth infection was reported in the dupilumab arm.
Full prescribing information is available online.
A version of this article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved dupilumab (Dupixent, Regeneron/Sanofi) for the treatment of eosinophilic esophagitis (EoE) in children aged 1-11 years and weighing ≥ 15 kg. It is the first and only medicine approved to treat these patients.
, as reported by this news organization.
EoE is a chronic inflammatory disorder driven by type 2 inflammation that damages the esophagus and causes difficulty swallowing and eating.
Dupilumab is a monoclonal antibody that acts to inhibit part of the inflammatory pathway.
EoE KIDS Trial
The FDA approval of dupilumab for younger children is based on results from the phase 3 randomized, double-blind, placebo-controlled EoE KIDS trial, which had two parts.
Part A was a 16-week double-blind treatment period that evaluated the safety and efficacy of dupilumab in a tiered weight-based dosing schema.
At 16 weeks, 66% of children who received higher dose dupilumab at tiered dosing regimens based on weight achieved histologic disease remission (six or fewer eosinophils/high power field), which was the primary endpoint, compared with only 3% of children who received placebo.
In addition, a greater decrease in the proportion of days with one or more signs of EoE according to the Pediatric EoE Sign/Symptom Questionnaire caregiver version (PESQ-C) was observed in children treated with dupilumab at 16 weeks compared placebo.
Part B was a 36-week extended active treatment period in which eligible children from Part A in the dupilumab group continued to receive their dose level and those in the placebo group in Part A switched to active treatment.
Histologic remission was sustained at week 52 in 53% of children treated with dupilumab in Parts A and B. Histologic remission was also achieved at week 52 in 53% of children who switched to dupilumab from placebo in Part B.
The safety profile of dupilumab observed through 16 weeks in these children was generally in line to that seen through 24 weeks in persons aged 12 years or older with EoE.
The most common adverse events (≥ 2%) more frequently observed with dupilumab than with placebo were injection site reactions, upper respiratory tract infections, arthralgia, and herpes viral infections. In EoE KIDS Part B, one case of helminth infection was reported in the dupilumab arm.
Full prescribing information is available online.
A version of this article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved dupilumab (Dupixent, Regeneron/Sanofi) for the treatment of eosinophilic esophagitis (EoE) in children aged 1-11 years and weighing ≥ 15 kg. It is the first and only medicine approved to treat these patients.
, as reported by this news organization.
EoE is a chronic inflammatory disorder driven by type 2 inflammation that damages the esophagus and causes difficulty swallowing and eating.
Dupilumab is a monoclonal antibody that acts to inhibit part of the inflammatory pathway.
EoE KIDS Trial
The FDA approval of dupilumab for younger children is based on results from the phase 3 randomized, double-blind, placebo-controlled EoE KIDS trial, which had two parts.
Part A was a 16-week double-blind treatment period that evaluated the safety and efficacy of dupilumab in a tiered weight-based dosing schema.
At 16 weeks, 66% of children who received higher dose dupilumab at tiered dosing regimens based on weight achieved histologic disease remission (six or fewer eosinophils/high power field), which was the primary endpoint, compared with only 3% of children who received placebo.
In addition, a greater decrease in the proportion of days with one or more signs of EoE according to the Pediatric EoE Sign/Symptom Questionnaire caregiver version (PESQ-C) was observed in children treated with dupilumab at 16 weeks compared placebo.
Part B was a 36-week extended active treatment period in which eligible children from Part A in the dupilumab group continued to receive their dose level and those in the placebo group in Part A switched to active treatment.
Histologic remission was sustained at week 52 in 53% of children treated with dupilumab in Parts A and B. Histologic remission was also achieved at week 52 in 53% of children who switched to dupilumab from placebo in Part B.
The safety profile of dupilumab observed through 16 weeks in these children was generally in line to that seen through 24 weeks in persons aged 12 years or older with EoE.
The most common adverse events (≥ 2%) more frequently observed with dupilumab than with placebo were injection site reactions, upper respiratory tract infections, arthralgia, and herpes viral infections. In EoE KIDS Part B, one case of helminth infection was reported in the dupilumab arm.
Full prescribing information is available online.
A version of this article first appeared on Medscape.com.
How to Motivate Pain Patients to Try Nondrug Options
This transcript has been edited for clarity.
Neha Pathak, MD: Hello. Today, we’re talking to Dr. Daniel Clauw, a professor at the University of Michigan in Ann Arbor, who is running a major trial on treatments for chronic back pain. We’re talking today about managing back pain in the post-opioid world. Thank you so much, Dr. Clauw, for taking the time to be our resident pain consultant today. Managing chronic pain can lead to a large amount of burnout and helplessness in the clinic setting. That’s the reality with some of the modalities that patients are requesting; there is still confusion about what is optimal for a particular type of patient, this feeling that we’re not really helping people get better, and whenever patients come in, that’s always still their chief complaint.
How would you advise providers to think about that and to settle into their role as communicators about better strategies without the burnout?
Daniel Clauw, MD: The first thing is to broaden the number of other providers that you get involved in these individuals’ care as the evidence base for all of these nonpharmacologic therapies being effective in chronic pain increases and increases. As third-party payers begin to reimburse for more and more of these therapies, it’s really difficult to manage chronic pain patients if you’re trying to do it alone on an island.
If you can, identify the good physical therapists in your community that are going to really work with people to give them an exercise program that they can use at home; find a pain psychologist that can offer some cognitive-behavioral therapy (CBT) for insomnia and some CBT for pain; and in the subset of patients with trauma, give them the emotional awareness of the neural reprocessing therapy for that specific subset.
As you start to identify more and more of these nonpharmacologic therapies that you want your patients to try, each of those has a set of providers and they can be incredibly helpful so that you, as the primary care provider (PCP), don’t really feel overwhelmed that you’re it, that you’re the only one.
Many of these individuals have more time to spend, and they have more one-on-one in-person time than you do as a primary care physician in the current healthcare system. Many of those providers have become really good at doing amateur CBT, goal-setting, and some of the other things that you need to do when you manage chronic pain patients. Try to find that other group of people that you can send your patients to that are going to be offering some of these nonpharmacologic therapies, and they’ll really help you manage these individuals.
Dr. Pathak: I think a couple of things come up for me. One is that we have to maybe broaden thinking about pain management, not only as multimodal strategies but also as multidisciplinary strategies. To your point, I think that’s really important. I also worry and wonder about health equity concerns, because just as overburdened as many PCPs are, we’re seeing it’s very difficult to get into physical therapy or to get into a setting where you’d be able to receive CBT for your pain. Any thoughts on those types of considerations?
Dr. Clauw: That’s a huge problem. Our group and many other groups in the pain space are developing websites, smartphone apps, and things like that to try to get some of these things directly to individuals with pain, not only for the reasons that you stated but also so that persons with pain don’t have to become patients. Our healthcare systems often make pain worse rather than better.
There were some great articles in The Lancet about 5 years ago talking about low back pain and that in different countries, the healthcare systems, for different reasons, have a tendency to actually make low back pain worse because they do too much surgery, immobilize people, or things like that rather than just not make them better. I think we’ve overmedicalized chronic pain in some settings, and much of what we’re trying to lead people to are things that are parts of wellness programs. The NIH National Center for Complementary and Integrative Health director talks about whole person health often.
I think that these interdisciplinary, integrative approaches are what we have to be using for chronic pain patients. I tell pain patients that, among acupuncture, acupressure, mindfulness, five different forms of CBT, yoga, and tai chi, I don’t know which of those is going to work, but I know that about 1 in 3 individuals that tries each of those therapies gets a benefit. What I really should be doing most is incentivizing people and motivating people to keep trying some of those nonpharmacologic approaches that they haven’t yet tried, because when they find one that works for them, then they will integrate it into their day-to-day life.
The other trick I would use for primary care physicians or anyone managing chronic pain patients is, don’t try to incentivize a pain patient to go try a new nonpharmacologic therapy or start an exercise program because you want their pain score to go from a 6 to a 3. Incentivize them by asking them, what are two or three things that you’re not able to do now because you have chronic pain that you’d really like to be able to do?
You’d like to play nine holes of golf; you’d like to be able to hug your grandchild; or you’d like to be able to do something else. Use those functional goals that are patient0driven to motivate your patients to do these things, because that will work much better. Again, any of us are inherently more likely to take the time and the effort to do some of these nonpharmacologic therapies if it’s for a reason that internally motivates us.
Dr. Pathak: I think that’s great. I’m very privileged to work within the Veterans Affairs (VA) healthcare system. I think that there’s been a huge shift within VA healthcare to provide these ancillary services, whether it’s yoga, tai chi, or acupuncture, as an adjunct to the pain management strategy.
Also, what comes up for me, as you’re saying, is grounding the point that instead of relying on a pain score — which can be objective and different from patient to patient and even within a patient — we should choose a smart goal that is almost more objective when it’s functional. Your goal is to walk two blocks to the mailbox. Can we achieve that as part of your pain control strategy?
I so appreciate your taking the time to be our pain consultant today. I really appreciate our discussion, and I’d like to hand it over to you for any final thoughts.
Dr. Clauw: I’d add that when you’re seeing chronic pain patients, many of them are going to have comorbid sleep problems. They’re going to have comorbid problems with fatigue and memory problems, especially the central nervous system–driven forms of pain that we now call nociplastic pain. Look at those as therapeutic targets.
If you’re befuddled because you’ve tried many different things for pain in this individual you’ve been seeing for a while, focus on their sleep and focus on getting them more active. Don’t use the word exercise — because that scares chronic pain patients — but focus on getting them more active.
There are many different tactics and strategies that you can use to motivate the patients to try some of these new nonpharmacologic approaches as the evidence base continues to increase.
Dr. Pathak: Thank you so much, again, to Dr. Clauw for joining us and being our pain consultant, really helping us to think about managing back pain in the postopioid world.
Dr. Pathak is Chief Physician Editor, Health and Lifestyle Medicine, WebMD. She has disclosed no relevant financial relationships. Dr. Clauw is Director, Chronic Pain and Fatigue Research Center, Department of Anesthesia, University of Michigan, Ann Arbor. He disclosed ties with Tonix and Viatris.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Neha Pathak, MD: Hello. Today, we’re talking to Dr. Daniel Clauw, a professor at the University of Michigan in Ann Arbor, who is running a major trial on treatments for chronic back pain. We’re talking today about managing back pain in the post-opioid world. Thank you so much, Dr. Clauw, for taking the time to be our resident pain consultant today. Managing chronic pain can lead to a large amount of burnout and helplessness in the clinic setting. That’s the reality with some of the modalities that patients are requesting; there is still confusion about what is optimal for a particular type of patient, this feeling that we’re not really helping people get better, and whenever patients come in, that’s always still their chief complaint.
How would you advise providers to think about that and to settle into their role as communicators about better strategies without the burnout?
Daniel Clauw, MD: The first thing is to broaden the number of other providers that you get involved in these individuals’ care as the evidence base for all of these nonpharmacologic therapies being effective in chronic pain increases and increases. As third-party payers begin to reimburse for more and more of these therapies, it’s really difficult to manage chronic pain patients if you’re trying to do it alone on an island.
If you can, identify the good physical therapists in your community that are going to really work with people to give them an exercise program that they can use at home; find a pain psychologist that can offer some cognitive-behavioral therapy (CBT) for insomnia and some CBT for pain; and in the subset of patients with trauma, give them the emotional awareness of the neural reprocessing therapy for that specific subset.
As you start to identify more and more of these nonpharmacologic therapies that you want your patients to try, each of those has a set of providers and they can be incredibly helpful so that you, as the primary care provider (PCP), don’t really feel overwhelmed that you’re it, that you’re the only one.
Many of these individuals have more time to spend, and they have more one-on-one in-person time than you do as a primary care physician in the current healthcare system. Many of those providers have become really good at doing amateur CBT, goal-setting, and some of the other things that you need to do when you manage chronic pain patients. Try to find that other group of people that you can send your patients to that are going to be offering some of these nonpharmacologic therapies, and they’ll really help you manage these individuals.
Dr. Pathak: I think a couple of things come up for me. One is that we have to maybe broaden thinking about pain management, not only as multimodal strategies but also as multidisciplinary strategies. To your point, I think that’s really important. I also worry and wonder about health equity concerns, because just as overburdened as many PCPs are, we’re seeing it’s very difficult to get into physical therapy or to get into a setting where you’d be able to receive CBT for your pain. Any thoughts on those types of considerations?
Dr. Clauw: That’s a huge problem. Our group and many other groups in the pain space are developing websites, smartphone apps, and things like that to try to get some of these things directly to individuals with pain, not only for the reasons that you stated but also so that persons with pain don’t have to become patients. Our healthcare systems often make pain worse rather than better.
There were some great articles in The Lancet about 5 years ago talking about low back pain and that in different countries, the healthcare systems, for different reasons, have a tendency to actually make low back pain worse because they do too much surgery, immobilize people, or things like that rather than just not make them better. I think we’ve overmedicalized chronic pain in some settings, and much of what we’re trying to lead people to are things that are parts of wellness programs. The NIH National Center for Complementary and Integrative Health director talks about whole person health often.
I think that these interdisciplinary, integrative approaches are what we have to be using for chronic pain patients. I tell pain patients that, among acupuncture, acupressure, mindfulness, five different forms of CBT, yoga, and tai chi, I don’t know which of those is going to work, but I know that about 1 in 3 individuals that tries each of those therapies gets a benefit. What I really should be doing most is incentivizing people and motivating people to keep trying some of those nonpharmacologic approaches that they haven’t yet tried, because when they find one that works for them, then they will integrate it into their day-to-day life.
The other trick I would use for primary care physicians or anyone managing chronic pain patients is, don’t try to incentivize a pain patient to go try a new nonpharmacologic therapy or start an exercise program because you want their pain score to go from a 6 to a 3. Incentivize them by asking them, what are two or three things that you’re not able to do now because you have chronic pain that you’d really like to be able to do?
You’d like to play nine holes of golf; you’d like to be able to hug your grandchild; or you’d like to be able to do something else. Use those functional goals that are patient0driven to motivate your patients to do these things, because that will work much better. Again, any of us are inherently more likely to take the time and the effort to do some of these nonpharmacologic therapies if it’s for a reason that internally motivates us.
Dr. Pathak: I think that’s great. I’m very privileged to work within the Veterans Affairs (VA) healthcare system. I think that there’s been a huge shift within VA healthcare to provide these ancillary services, whether it’s yoga, tai chi, or acupuncture, as an adjunct to the pain management strategy.
Also, what comes up for me, as you’re saying, is grounding the point that instead of relying on a pain score — which can be objective and different from patient to patient and even within a patient — we should choose a smart goal that is almost more objective when it’s functional. Your goal is to walk two blocks to the mailbox. Can we achieve that as part of your pain control strategy?
I so appreciate your taking the time to be our pain consultant today. I really appreciate our discussion, and I’d like to hand it over to you for any final thoughts.
Dr. Clauw: I’d add that when you’re seeing chronic pain patients, many of them are going to have comorbid sleep problems. They’re going to have comorbid problems with fatigue and memory problems, especially the central nervous system–driven forms of pain that we now call nociplastic pain. Look at those as therapeutic targets.
If you’re befuddled because you’ve tried many different things for pain in this individual you’ve been seeing for a while, focus on their sleep and focus on getting them more active. Don’t use the word exercise — because that scares chronic pain patients — but focus on getting them more active.
There are many different tactics and strategies that you can use to motivate the patients to try some of these new nonpharmacologic approaches as the evidence base continues to increase.
Dr. Pathak: Thank you so much, again, to Dr. Clauw for joining us and being our pain consultant, really helping us to think about managing back pain in the postopioid world.
Dr. Pathak is Chief Physician Editor, Health and Lifestyle Medicine, WebMD. She has disclosed no relevant financial relationships. Dr. Clauw is Director, Chronic Pain and Fatigue Research Center, Department of Anesthesia, University of Michigan, Ann Arbor. He disclosed ties with Tonix and Viatris.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Neha Pathak, MD: Hello. Today, we’re talking to Dr. Daniel Clauw, a professor at the University of Michigan in Ann Arbor, who is running a major trial on treatments for chronic back pain. We’re talking today about managing back pain in the post-opioid world. Thank you so much, Dr. Clauw, for taking the time to be our resident pain consultant today. Managing chronic pain can lead to a large amount of burnout and helplessness in the clinic setting. That’s the reality with some of the modalities that patients are requesting; there is still confusion about what is optimal for a particular type of patient, this feeling that we’re not really helping people get better, and whenever patients come in, that’s always still their chief complaint.
How would you advise providers to think about that and to settle into their role as communicators about better strategies without the burnout?
Daniel Clauw, MD: The first thing is to broaden the number of other providers that you get involved in these individuals’ care as the evidence base for all of these nonpharmacologic therapies being effective in chronic pain increases and increases. As third-party payers begin to reimburse for more and more of these therapies, it’s really difficult to manage chronic pain patients if you’re trying to do it alone on an island.
If you can, identify the good physical therapists in your community that are going to really work with people to give them an exercise program that they can use at home; find a pain psychologist that can offer some cognitive-behavioral therapy (CBT) for insomnia and some CBT for pain; and in the subset of patients with trauma, give them the emotional awareness of the neural reprocessing therapy for that specific subset.
As you start to identify more and more of these nonpharmacologic therapies that you want your patients to try, each of those has a set of providers and they can be incredibly helpful so that you, as the primary care provider (PCP), don’t really feel overwhelmed that you’re it, that you’re the only one.
Many of these individuals have more time to spend, and they have more one-on-one in-person time than you do as a primary care physician in the current healthcare system. Many of those providers have become really good at doing amateur CBT, goal-setting, and some of the other things that you need to do when you manage chronic pain patients. Try to find that other group of people that you can send your patients to that are going to be offering some of these nonpharmacologic therapies, and they’ll really help you manage these individuals.
Dr. Pathak: I think a couple of things come up for me. One is that we have to maybe broaden thinking about pain management, not only as multimodal strategies but also as multidisciplinary strategies. To your point, I think that’s really important. I also worry and wonder about health equity concerns, because just as overburdened as many PCPs are, we’re seeing it’s very difficult to get into physical therapy or to get into a setting where you’d be able to receive CBT for your pain. Any thoughts on those types of considerations?
Dr. Clauw: That’s a huge problem. Our group and many other groups in the pain space are developing websites, smartphone apps, and things like that to try to get some of these things directly to individuals with pain, not only for the reasons that you stated but also so that persons with pain don’t have to become patients. Our healthcare systems often make pain worse rather than better.
There were some great articles in The Lancet about 5 years ago talking about low back pain and that in different countries, the healthcare systems, for different reasons, have a tendency to actually make low back pain worse because they do too much surgery, immobilize people, or things like that rather than just not make them better. I think we’ve overmedicalized chronic pain in some settings, and much of what we’re trying to lead people to are things that are parts of wellness programs. The NIH National Center for Complementary and Integrative Health director talks about whole person health often.
I think that these interdisciplinary, integrative approaches are what we have to be using for chronic pain patients. I tell pain patients that, among acupuncture, acupressure, mindfulness, five different forms of CBT, yoga, and tai chi, I don’t know which of those is going to work, but I know that about 1 in 3 individuals that tries each of those therapies gets a benefit. What I really should be doing most is incentivizing people and motivating people to keep trying some of those nonpharmacologic approaches that they haven’t yet tried, because when they find one that works for them, then they will integrate it into their day-to-day life.
The other trick I would use for primary care physicians or anyone managing chronic pain patients is, don’t try to incentivize a pain patient to go try a new nonpharmacologic therapy or start an exercise program because you want their pain score to go from a 6 to a 3. Incentivize them by asking them, what are two or three things that you’re not able to do now because you have chronic pain that you’d really like to be able to do?
You’d like to play nine holes of golf; you’d like to be able to hug your grandchild; or you’d like to be able to do something else. Use those functional goals that are patient0driven to motivate your patients to do these things, because that will work much better. Again, any of us are inherently more likely to take the time and the effort to do some of these nonpharmacologic therapies if it’s for a reason that internally motivates us.
Dr. Pathak: I think that’s great. I’m very privileged to work within the Veterans Affairs (VA) healthcare system. I think that there’s been a huge shift within VA healthcare to provide these ancillary services, whether it’s yoga, tai chi, or acupuncture, as an adjunct to the pain management strategy.
Also, what comes up for me, as you’re saying, is grounding the point that instead of relying on a pain score — which can be objective and different from patient to patient and even within a patient — we should choose a smart goal that is almost more objective when it’s functional. Your goal is to walk two blocks to the mailbox. Can we achieve that as part of your pain control strategy?
I so appreciate your taking the time to be our pain consultant today. I really appreciate our discussion, and I’d like to hand it over to you for any final thoughts.
Dr. Clauw: I’d add that when you’re seeing chronic pain patients, many of them are going to have comorbid sleep problems. They’re going to have comorbid problems with fatigue and memory problems, especially the central nervous system–driven forms of pain that we now call nociplastic pain. Look at those as therapeutic targets.
If you’re befuddled because you’ve tried many different things for pain in this individual you’ve been seeing for a while, focus on their sleep and focus on getting them more active. Don’t use the word exercise — because that scares chronic pain patients — but focus on getting them more active.
There are many different tactics and strategies that you can use to motivate the patients to try some of these new nonpharmacologic approaches as the evidence base continues to increase.
Dr. Pathak: Thank you so much, again, to Dr. Clauw for joining us and being our pain consultant, really helping us to think about managing back pain in the postopioid world.
Dr. Pathak is Chief Physician Editor, Health and Lifestyle Medicine, WebMD. She has disclosed no relevant financial relationships. Dr. Clauw is Director, Chronic Pain and Fatigue Research Center, Department of Anesthesia, University of Michigan, Ann Arbor. He disclosed ties with Tonix and Viatris.
A version of this article appeared on Medscape.com.
e-Cigarettes Best Nicotine Gum for Smoking Cessation
UPDATE: On March 29, 2024, the authors of this study published in JAMA Internal Medicine issued a formal retraction of their article. "Unfortunately, we have found significant coding errors that are difficult to rectify," the author wrote. "We also discovered discrepancies in the calculation process that cast doubt on the accuracy and reliability of the reported findings." The CHEST Physician® Editorial Board apologizes for any confusion this may have caused.
TOPLINE:
and as effective as varenicline in achieving sustained abstinence at 6 months, a randomized trial found. Questions about the long-term safety of e-cigarettes remain, however, according to the researchers.
METHODOLOGY:
- The study included 1068 participants in China who were smoking at least 10 cigarettes per day.
- They were randomly assigned to undergo 12 weeks of treatment with a cartridge-based e-cigarette, varenicline, or nicotine chewing gum.
TAKEAWAY:
- At 6 months, the biochemically validated rate of quitting was 15.7% for those who received e-cigarettes, 14.2% for those who received varenicline, and 8.8% for those who chewed nicotine gum.
- At 6 months, 62.8% of participants in the e-cigarette arm were still using the devices, whereas those in the other study arms had not continued their treatments.
- Adverse reactions with e-cigarettes and nicotine chewing gum included irritation of the throat and mouth, which occurred in 7%-8% of participants.
- In the varenicline group, 8.8% experienced nausea.
- No serious adverse events were reported.
IN PRACTICE:
“A moderate approach would be to recommend approved medications as the first step and, if that fails, then inform the patient of the evidence regarding the use of electronic cigarettes as a possible approach, acknowledging all its caveats,” Dorothy K. Hatsukami, PhD, with the University of Minnesota in Minneapolis, and Judith J. Prochaska, PhD, MPH, with Stanford (California) University, wrote in an invited commentary.
SOURCE:
Zhao Liu, PhD, with the China-Japan Friendship Hospital in Beijing, was the corresponding author for the study. The study was published online on January 29, 2024, in JAMA Internal Medicine.
LIMITATIONS:
The trial had an open-label design, so participants’ expectations about their assigned treatment may have influenced the results.
The study did not include participants older than 45 years, so it is unclear how the results apply to older populations.
More studies are needed to see whether continued use of e-cigarettes is beneficial or harmful, the researchers wrote.
Combining forms of nicotine replacement therapy, such as gum plus a patch, may be more effective than a single form, but the trial did not assess a combined approach, the commentary authors noted. The dose of nicotine gum for some participants may have been suboptimal, they added.
DISCLOSURES:
The study was supported by the Scientific Research Project Fund of China-Japan Friendship Hospital. The researchers had no conflict of interest disclosures. Dr. Prochaska disclosed receiving fees from Achieve Life Sciences, OneLeaf, and attorneys who are involved in litigation against tobacco companies.
A version of this article appeared on Medscape.com.
UPDATE: On March 29, 2024, the authors of this study published in JAMA Internal Medicine issued a formal retraction of their article. "Unfortunately, we have found significant coding errors that are difficult to rectify," the author wrote. "We also discovered discrepancies in the calculation process that cast doubt on the accuracy and reliability of the reported findings." The CHEST Physician® Editorial Board apologizes for any confusion this may have caused.
TOPLINE:
and as effective as varenicline in achieving sustained abstinence at 6 months, a randomized trial found. Questions about the long-term safety of e-cigarettes remain, however, according to the researchers.
METHODOLOGY:
- The study included 1068 participants in China who were smoking at least 10 cigarettes per day.
- They were randomly assigned to undergo 12 weeks of treatment with a cartridge-based e-cigarette, varenicline, or nicotine chewing gum.
TAKEAWAY:
- At 6 months, the biochemically validated rate of quitting was 15.7% for those who received e-cigarettes, 14.2% for those who received varenicline, and 8.8% for those who chewed nicotine gum.
- At 6 months, 62.8% of participants in the e-cigarette arm were still using the devices, whereas those in the other study arms had not continued their treatments.
- Adverse reactions with e-cigarettes and nicotine chewing gum included irritation of the throat and mouth, which occurred in 7%-8% of participants.
- In the varenicline group, 8.8% experienced nausea.
- No serious adverse events were reported.
IN PRACTICE:
“A moderate approach would be to recommend approved medications as the first step and, if that fails, then inform the patient of the evidence regarding the use of electronic cigarettes as a possible approach, acknowledging all its caveats,” Dorothy K. Hatsukami, PhD, with the University of Minnesota in Minneapolis, and Judith J. Prochaska, PhD, MPH, with Stanford (California) University, wrote in an invited commentary.
SOURCE:
Zhao Liu, PhD, with the China-Japan Friendship Hospital in Beijing, was the corresponding author for the study. The study was published online on January 29, 2024, in JAMA Internal Medicine.
LIMITATIONS:
The trial had an open-label design, so participants’ expectations about their assigned treatment may have influenced the results.
The study did not include participants older than 45 years, so it is unclear how the results apply to older populations.
More studies are needed to see whether continued use of e-cigarettes is beneficial or harmful, the researchers wrote.
Combining forms of nicotine replacement therapy, such as gum plus a patch, may be more effective than a single form, but the trial did not assess a combined approach, the commentary authors noted. The dose of nicotine gum for some participants may have been suboptimal, they added.
DISCLOSURES:
The study was supported by the Scientific Research Project Fund of China-Japan Friendship Hospital. The researchers had no conflict of interest disclosures. Dr. Prochaska disclosed receiving fees from Achieve Life Sciences, OneLeaf, and attorneys who are involved in litigation against tobacco companies.
A version of this article appeared on Medscape.com.
UPDATE: On March 29, 2024, the authors of this study published in JAMA Internal Medicine issued a formal retraction of their article. "Unfortunately, we have found significant coding errors that are difficult to rectify," the author wrote. "We also discovered discrepancies in the calculation process that cast doubt on the accuracy and reliability of the reported findings." The CHEST Physician® Editorial Board apologizes for any confusion this may have caused.
TOPLINE:
and as effective as varenicline in achieving sustained abstinence at 6 months, a randomized trial found. Questions about the long-term safety of e-cigarettes remain, however, according to the researchers.
METHODOLOGY:
- The study included 1068 participants in China who were smoking at least 10 cigarettes per day.
- They were randomly assigned to undergo 12 weeks of treatment with a cartridge-based e-cigarette, varenicline, or nicotine chewing gum.
TAKEAWAY:
- At 6 months, the biochemically validated rate of quitting was 15.7% for those who received e-cigarettes, 14.2% for those who received varenicline, and 8.8% for those who chewed nicotine gum.
- At 6 months, 62.8% of participants in the e-cigarette arm were still using the devices, whereas those in the other study arms had not continued their treatments.
- Adverse reactions with e-cigarettes and nicotine chewing gum included irritation of the throat and mouth, which occurred in 7%-8% of participants.
- In the varenicline group, 8.8% experienced nausea.
- No serious adverse events were reported.
IN PRACTICE:
“A moderate approach would be to recommend approved medications as the first step and, if that fails, then inform the patient of the evidence regarding the use of electronic cigarettes as a possible approach, acknowledging all its caveats,” Dorothy K. Hatsukami, PhD, with the University of Minnesota in Minneapolis, and Judith J. Prochaska, PhD, MPH, with Stanford (California) University, wrote in an invited commentary.
SOURCE:
Zhao Liu, PhD, with the China-Japan Friendship Hospital in Beijing, was the corresponding author for the study. The study was published online on January 29, 2024, in JAMA Internal Medicine.
LIMITATIONS:
The trial had an open-label design, so participants’ expectations about their assigned treatment may have influenced the results.
The study did not include participants older than 45 years, so it is unclear how the results apply to older populations.
More studies are needed to see whether continued use of e-cigarettes is beneficial or harmful, the researchers wrote.
Combining forms of nicotine replacement therapy, such as gum plus a patch, may be more effective than a single form, but the trial did not assess a combined approach, the commentary authors noted. The dose of nicotine gum for some participants may have been suboptimal, they added.
DISCLOSURES:
The study was supported by the Scientific Research Project Fund of China-Japan Friendship Hospital. The researchers had no conflict of interest disclosures. Dr. Prochaska disclosed receiving fees from Achieve Life Sciences, OneLeaf, and attorneys who are involved in litigation against tobacco companies.
A version of this article appeared on Medscape.com.
HPV Vaccine Shown to Be Highly Effective in Girls Years Later
TOPLINE:
METHODOLOGY:
- Cervical cancer is the fourth most common cancer among women worldwide.
- Programs to provide Cervarix, a bivalent vaccine, began in the United Kingdom in 2007.
- After the initiation of the programs, administering the vaccine became part of routine care for girls starting at age 12 years.
- Researchers collected data in 2020 from 447,845 women born between 1988 and 1996 from the Scottish cervical cancer screening system to assess the efficacy of Cervarix in lowering rates of cervical cancer.
- They correlated the rate of cervical cancer per 100,000 person-years with data on women regarding vaccination status, age when vaccinated, and deprivation in areas like income, housing, and health.
TAKEAWAY:
- No cases of cervical cancer were found among women who were immunized at ages 12 or 13 years, no matter how many doses they received.
- Women who were immunized between ages 14 and 18 years and received three doses had fewer instances of cervical cancer compared with unvaccinated women regardless of deprivation status (3.2 cases per 100,00 women vs 8.4 cases per 100,000).
IN PRACTICE:
“Continued participation in screening and monitoring of outcomes is required, however, to assess the effects of changes in vaccines used and dosage schedules since the start of vaccination in Scotland in 2008 and the longevity of protection the vaccines offer.”
SOURCE:
The study was led by Timothy J. Palmer, PhD, Scottish Clinical Lead for Cervical Screening at Public Health Scotland.
LIMITATIONS:
Only 14,645 women had received just one or two doses, which may have affected the statistical analysis.
DISCLOSURES:
The study was funded by Public Health Scotland. A coauthor reports attending an advisory board meeting for HOLOGIC and Vaccitech. Her institution received research funding or gratis support funding from Cepheid, Euroimmun, GeneFirst, SelfScreen, Hiantis, Seegene, Roche, Hologic, and Vaccitech in the past 3 years.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Cervical cancer is the fourth most common cancer among women worldwide.
- Programs to provide Cervarix, a bivalent vaccine, began in the United Kingdom in 2007.
- After the initiation of the programs, administering the vaccine became part of routine care for girls starting at age 12 years.
- Researchers collected data in 2020 from 447,845 women born between 1988 and 1996 from the Scottish cervical cancer screening system to assess the efficacy of Cervarix in lowering rates of cervical cancer.
- They correlated the rate of cervical cancer per 100,000 person-years with data on women regarding vaccination status, age when vaccinated, and deprivation in areas like income, housing, and health.
TAKEAWAY:
- No cases of cervical cancer were found among women who were immunized at ages 12 or 13 years, no matter how many doses they received.
- Women who were immunized between ages 14 and 18 years and received three doses had fewer instances of cervical cancer compared with unvaccinated women regardless of deprivation status (3.2 cases per 100,00 women vs 8.4 cases per 100,000).
IN PRACTICE:
“Continued participation in screening and monitoring of outcomes is required, however, to assess the effects of changes in vaccines used and dosage schedules since the start of vaccination in Scotland in 2008 and the longevity of protection the vaccines offer.”
SOURCE:
The study was led by Timothy J. Palmer, PhD, Scottish Clinical Lead for Cervical Screening at Public Health Scotland.
LIMITATIONS:
Only 14,645 women had received just one or two doses, which may have affected the statistical analysis.
DISCLOSURES:
The study was funded by Public Health Scotland. A coauthor reports attending an advisory board meeting for HOLOGIC and Vaccitech. Her institution received research funding or gratis support funding from Cepheid, Euroimmun, GeneFirst, SelfScreen, Hiantis, Seegene, Roche, Hologic, and Vaccitech in the past 3 years.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Cervical cancer is the fourth most common cancer among women worldwide.
- Programs to provide Cervarix, a bivalent vaccine, began in the United Kingdom in 2007.
- After the initiation of the programs, administering the vaccine became part of routine care for girls starting at age 12 years.
- Researchers collected data in 2020 from 447,845 women born between 1988 and 1996 from the Scottish cervical cancer screening system to assess the efficacy of Cervarix in lowering rates of cervical cancer.
- They correlated the rate of cervical cancer per 100,000 person-years with data on women regarding vaccination status, age when vaccinated, and deprivation in areas like income, housing, and health.
TAKEAWAY:
- No cases of cervical cancer were found among women who were immunized at ages 12 or 13 years, no matter how many doses they received.
- Women who were immunized between ages 14 and 18 years and received three doses had fewer instances of cervical cancer compared with unvaccinated women regardless of deprivation status (3.2 cases per 100,00 women vs 8.4 cases per 100,000).
IN PRACTICE:
“Continued participation in screening and monitoring of outcomes is required, however, to assess the effects of changes in vaccines used and dosage schedules since the start of vaccination in Scotland in 2008 and the longevity of protection the vaccines offer.”
SOURCE:
The study was led by Timothy J. Palmer, PhD, Scottish Clinical Lead for Cervical Screening at Public Health Scotland.
LIMITATIONS:
Only 14,645 women had received just one or two doses, which may have affected the statistical analysis.
DISCLOSURES:
The study was funded by Public Health Scotland. A coauthor reports attending an advisory board meeting for HOLOGIC and Vaccitech. Her institution received research funding or gratis support funding from Cepheid, Euroimmun, GeneFirst, SelfScreen, Hiantis, Seegene, Roche, Hologic, and Vaccitech in the past 3 years.
A version of this article appeared on Medscape.com.
Chemo-Free Maintenance Strategies May Boost Survival in TNBC
TOPLINE:
METHODOLOGY:
- First-line standard therapy for advanced TNBC generally includes taxane- or platinum-based chemotherapy which poses challenging toxicities. Exploring chemotherapy-free maintenance strategies may provide adequate disease control and improve patient quality of life.
- The researchers evaluated 45 patients, at five sites in the Republic of Korea, the United States, and Singapore, with TNBC who had ongoing stable disease or complete/partial response from first- or second-line platinum-based chemotherapy.
- The patients were randomized 1:1 to receive olaparib 300 mg twice daily with or without durvalumab 1500 mg on day 1 every 4 weeks.
- The authors compared PFS with a historical control of continued platinum-based therapy. An improvement to 4 months with maintenance therapy was considered clinically significant.
TAKEAWAY:
- After a follow-up of 9.8 months, patients who received olaparib alone demonstrated median PFS of 4.0 months, and those who received the combination therapy had median PFS of 6.1 months.
- Clinical benefit rates, defined as stable disease for at least 24 weeks or complete/partial response, were reported in 44% of the monotherapy group and 36% of the combination therapy group.
- Sustained clinical benefit was evident irrespective of germline BRCA mutation or programmed death-ligand 1 status, although it tended to be associated with complete or partial response to prior platinum.
- Grade 3-4 adverse events were reported in nine patients (39%) in the olaparib arm and eight patients (36%) in the combination arm. No treatment-related deaths or new safety signals were observed.
IN PRACTICE:
“Maintenance regimens are rarely used in [triple-negative breast cancer] but offer the possibility of more tolerable long-term treatment avoiding some of the chemotherapy-related side effects of more aggressive regimens, as is standard in the first-line treatment of HER2-positive advanced breast cancer,” the researchers concluded.
SOURCE:
This study, led by Tira J. Tan from Duke-NUS Medical School, Singapore, was published online on January 18, 2024, in Clinical Cancer Research.
LIMITATIONS:
The main limitations were the small sample size and lack of a standard control arm. Most patients (76%) were Asian, limiting generalizability. The trial was not designed to compare olaparib monotherapy and olaparib plus durvalumab regimens.
DISCLOSURES:
AstraZeneca Pharmaceuticals LP supported this study. Several authors reported financial support from various sources.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- First-line standard therapy for advanced TNBC generally includes taxane- or platinum-based chemotherapy which poses challenging toxicities. Exploring chemotherapy-free maintenance strategies may provide adequate disease control and improve patient quality of life.
- The researchers evaluated 45 patients, at five sites in the Republic of Korea, the United States, and Singapore, with TNBC who had ongoing stable disease or complete/partial response from first- or second-line platinum-based chemotherapy.
- The patients were randomized 1:1 to receive olaparib 300 mg twice daily with or without durvalumab 1500 mg on day 1 every 4 weeks.
- The authors compared PFS with a historical control of continued platinum-based therapy. An improvement to 4 months with maintenance therapy was considered clinically significant.
TAKEAWAY:
- After a follow-up of 9.8 months, patients who received olaparib alone demonstrated median PFS of 4.0 months, and those who received the combination therapy had median PFS of 6.1 months.
- Clinical benefit rates, defined as stable disease for at least 24 weeks or complete/partial response, were reported in 44% of the monotherapy group and 36% of the combination therapy group.
- Sustained clinical benefit was evident irrespective of germline BRCA mutation or programmed death-ligand 1 status, although it tended to be associated with complete or partial response to prior platinum.
- Grade 3-4 adverse events were reported in nine patients (39%) in the olaparib arm and eight patients (36%) in the combination arm. No treatment-related deaths or new safety signals were observed.
IN PRACTICE:
“Maintenance regimens are rarely used in [triple-negative breast cancer] but offer the possibility of more tolerable long-term treatment avoiding some of the chemotherapy-related side effects of more aggressive regimens, as is standard in the first-line treatment of HER2-positive advanced breast cancer,” the researchers concluded.
SOURCE:
This study, led by Tira J. Tan from Duke-NUS Medical School, Singapore, was published online on January 18, 2024, in Clinical Cancer Research.
LIMITATIONS:
The main limitations were the small sample size and lack of a standard control arm. Most patients (76%) were Asian, limiting generalizability. The trial was not designed to compare olaparib monotherapy and olaparib plus durvalumab regimens.
DISCLOSURES:
AstraZeneca Pharmaceuticals LP supported this study. Several authors reported financial support from various sources.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- First-line standard therapy for advanced TNBC generally includes taxane- or platinum-based chemotherapy which poses challenging toxicities. Exploring chemotherapy-free maintenance strategies may provide adequate disease control and improve patient quality of life.
- The researchers evaluated 45 patients, at five sites in the Republic of Korea, the United States, and Singapore, with TNBC who had ongoing stable disease or complete/partial response from first- or second-line platinum-based chemotherapy.
- The patients were randomized 1:1 to receive olaparib 300 mg twice daily with or without durvalumab 1500 mg on day 1 every 4 weeks.
- The authors compared PFS with a historical control of continued platinum-based therapy. An improvement to 4 months with maintenance therapy was considered clinically significant.
TAKEAWAY:
- After a follow-up of 9.8 months, patients who received olaparib alone demonstrated median PFS of 4.0 months, and those who received the combination therapy had median PFS of 6.1 months.
- Clinical benefit rates, defined as stable disease for at least 24 weeks or complete/partial response, were reported in 44% of the monotherapy group and 36% of the combination therapy group.
- Sustained clinical benefit was evident irrespective of germline BRCA mutation or programmed death-ligand 1 status, although it tended to be associated with complete or partial response to prior platinum.
- Grade 3-4 adverse events were reported in nine patients (39%) in the olaparib arm and eight patients (36%) in the combination arm. No treatment-related deaths or new safety signals were observed.
IN PRACTICE:
“Maintenance regimens are rarely used in [triple-negative breast cancer] but offer the possibility of more tolerable long-term treatment avoiding some of the chemotherapy-related side effects of more aggressive regimens, as is standard in the first-line treatment of HER2-positive advanced breast cancer,” the researchers concluded.
SOURCE:
This study, led by Tira J. Tan from Duke-NUS Medical School, Singapore, was published online on January 18, 2024, in Clinical Cancer Research.
LIMITATIONS:
The main limitations were the small sample size and lack of a standard control arm. Most patients (76%) were Asian, limiting generalizability. The trial was not designed to compare olaparib monotherapy and olaparib plus durvalumab regimens.
DISCLOSURES:
AstraZeneca Pharmaceuticals LP supported this study. Several authors reported financial support from various sources.
A version of this article appeared on Medscape.com.