User login
Implementing Trustworthy AI in VA High Reliability Health Care Organizations
Artificial intelligence (AI) has lagged in health care but has considerable potential to improve quality, safety, clinician experience, and access to care. It is being tested in areas like billing, hospital operations, and preventing adverse events (eg, sepsis mortality) with some early success. However, there are still many barriers preventing the widespread use of AI, such as data problems, mismatched rewards, and workplace obstacles. Innovative projects, partnerships, better rewards, and more investment could remove barriers. Implemented reliably and safely, AI can add to what clinicians know, help them work faster, cut costs, and, most importantly, improve patient care.1
AI can potentially bring several clinical benefits, such as reducing the administrative strain on clinicians and granting them more time for direct patient care. It can also improve diagnostic accuracy by analyzing patient data and diagnostic images, providing differential diagnoses, and increasing access to care by providing medical information and essential online services to patients.2
High Reliability Organizations
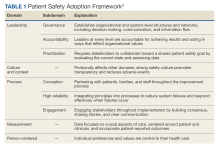
High reliability health care organizations have considerable experience safely launching new programs. For example, the Patient Safety Adoption Framework gives practical tips for smoothly rolling out safety initiatives (Table 1). Developed with experts and diverse views, this framework has 5 key areas: leadership, culture and context, process, measurement, and person-centeredness. These address adoption problems, guide leaders step-by-step, and focus on leadership buy-in, safety culture, cooperation, and local customization. Checklists and tools make it systematic to go from ideas to action on patient safety.3
Leadership involves establishing organizational commitment behind new safety programs. This visible commitment signals importance and priorities to others. Leaders model desired behaviors and language around safety, allocate resources, remove obstacles, and keep initiatives energized over time through consistent messaging.4 Culture and context recognizes that safety culture differs across units and facilities. Local input tailors programs to fit and examines strengths to build on, like psychological safety. Surveys gauge the existing culture and its need for change. Process details how to plan, design, test, implement, and improve new safety practices and provides a phased roadmap from idea to results. Measurement collects data to drive improvement and show impact. Metrics track progress and allow benchmarking. Person-centeredness puts patients first in safety efforts through participation, education, and transparency.
The Veterans Health Administration piloted a comprehensive high reliability hospital (HRH) model. Over 3 years, the Veterans Health Administration focused on leadership, culture, and process improvement at a hospital. After initiating the model, the pilot hospital improved its safety culture, reported more minor safety issues, and reduced deaths and complications better than other hospitals. The high-reliability approach successfully instilled principles and improved culture and outcomes. The HRH model is set to be expanded to 18 more US Department of Veterans Affairs (VA) sites for further evaluation across diverse settings.5
Trustworthy AI Framework
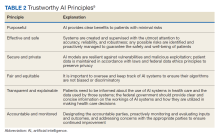
AI systems are growing more powerful and widespread, including in health care. Unfortunately, irresponsible AI can introduce new harm. ChatGPT and other large language models, for example, sometimes are known to provide erroneous information in a compelling way. Clinicians and patients who use such programs can act on such information, which would lead to unforeseen negative consequences. Several frameworks on ethical AI have come from governmental groups.6-9 In 2023, the VA National AI Institute suggested a Trustworthy AI Framework based on core principles tailored for federal health care. The framework has 6 key principles: purposeful, effective and safe, secure and private, fair and equitable, transparent and explainable, and accountable and monitored (Table 2).10
First, AI must clearly help veterans while minimizing risks. To ensure purpose, the VA will assess patient and clinician needs and design AI that targets meaningful problems to avoid scope creep or feature bloat. For example, adding new features to the AI software after release can clutter and complicate the interface, making it difficult to use. Rigorous testing will confirm that AI meets intent prior to deployment. Second, AI is designed and checked for effectiveness, safety, and reliability. The VA pledges to monitor AI’s impact to ensure it performs as expected without unintended consequences. Algorithms will be stress tested across representative datasets and approval processes will screen for safety issues. Third, AI models are secured from vulnerabilities and misuse. Technical controls will prevent unauthorized access or changes to AI systems. Audits will check for appropriate internal usage per policies. Continual patches and upgrades will maintain security. Fourth, the VA manages AI for fairness, avoiding bias. They will proactively assess datasets and algorithms for potential biases based on protected attributes like race, gender, or age. Biased outputs will be addressed through techniques such as data augmentation, reweighting, and algorithm tweaks. Fifth, transparency explains AI’s role in care. Documentation will detail an AI system’s data sources, methodology, testing, limitations, and integration with clinical workflows. Clinicians and patients will receive education on interpreting AI outputs. Finally, the VA pledges to closely monitor AI systems to sustain trust. The VA will establish oversight processes to quickly identify any declines in reliability or unfair impacts on subgroups. AI models will be retrained as needed based on incoming data patterns.
Each Trustworthy AI Framework principle connects to others in existing frameworks. The purpose principle aligns with human-centric AI focused on benefits. Effectiveness and safety link to technical robustness and risk management principles. Security maps to privacy protection principles. Fairness connects to principles of avoiding bias and discrimination. Transparency corresponds with accountable and explainable AI. Monitoring and accountability tie back to governance principles. Overall, the VA framework aims to guide ethical AI based on context. It offers a model for managing risks and building trust in health care AI.
Combining VA principles with high-reliability safety principles can ensure that AI benefits veterans. The leadership and culture aspects will drive commitment to trustworthy AI practices. Leaders will communicate the importance of responsible AI through words and actions. Culture surveys can assess baseline awareness of AI ethics issues to target education. AI security and fairness will be emphasized as safety critical. The process aspect will institute policies and procedures to uphold AI principles through the project lifecycle. For example, structured testing processes will validate safety. Measurement will collect data on principles like transparency and fairness. Dashboards can track metrics like explainability and biases. A patient-centered approach will incorporate veteran perspectives on AI through participatory design and advisory councils. They can give input on AI explainability and potential biases based on their diverse backgrounds.
Conclusions
Joint principles will lead to successful AI that improves care while proactively managing risks. Involve leaders to stress the necessity of eliminating biases. Build security into the AI development process. Co-design AI transparency features with end users. Closely monitor the impact of AI across safety, fairness, and other principles. Adhering to both Trustworthy AI and high reliability organizations principles will earn veterans’ confidence. Health care organizations like the VA can integrate ethical AI safely via established frameworks. With responsible design and implementation, AI’s potential to enhance care quality, safety, and access can be realized.
Acknowledgments
We would like to acknowledge Joshua Mueller, Theo Tiffney, John Zachary, and Gil Alterovitz for their excellent work creating the VA Trustworthy Principles. This material is the result of work supported by resources and the use of facilities at the James A. Haley Veterans’ Hospital.
1. Sahni NR, Carrus B. Artificial intelligence in U.S. health care delivery. N Engl J Med. 2023;389(4):348-358. doi:10.1056/NEJMra2204673
2. Borkowski AA, Jakey CE, Mastorides SM, et al. Applications of ChatGPT and large language models in medicine and health care: benefits and pitfalls. Fed Pract. 2023;40(6):170-173. doi:10.12788/fp.0386
3. Moyal-Smith R, Margo J, Maloney FL, et al. The patient safety adoption framework: a practical framework to bridge the know-do gap. J Patient Saf. 2023;19(4):243-248. doi:10.1097/PTS.0000000000001118
4. Isaacks DB, Anderson TM, Moore SC, Patterson W, Govindan S. High reliability organization principles improve VA workplace burnout: the Truman THRIVE2 model. Am J Med Qual. 2021;36(6):422-428. doi:10.1097/01.JMQ.0000735516.35323.97
5. Sculli GL, Pendley-Louis R, Neily J, et al. A high-reliability organization framework for health care: a multiyear implementation strategy and associated outcomes. J Patient Saf. 2022;18(1):64-70. doi:10.1097/PTS.0000000000000788
6. National Institute of Standards and Technology. AI risk management framework. Accessed January 2, 2024. https://www.nist.gov/itl/ai-risk-management-framework
7. Executive Office of the President, Office of Science and Technology Policy. Blueprint for an AI Bill of Rights. Accessed January 11, 2024. https://www.whitehouse.gov/ostp/ai-bill-of-rights
8. Executive Office of the President. Executive Order 13960: promoting the use of trustworthy artificial intelligence in the federal government. Fed Regist. 2020;89(236):78939-78943.
9. Biden JR. Executive Order on the safe, secure, and trustworthy development and use of artificial intelligence. Published October 30, 2023. Accessed January 11, 2024. https://www.whitehouse.gov/briefing-room/presidential-actions/2023/10/30/executive-order-on-the-safe-secure-and-trustworthy-development-and-use-of-artificial-intelligence/
10. US Department of Veterans Affairs. Trustworthy AI. Accessed January 11, 2024. https://department.va.gov/ai/trustworthy/
Artificial intelligence (AI) has lagged in health care but has considerable potential to improve quality, safety, clinician experience, and access to care. It is being tested in areas like billing, hospital operations, and preventing adverse events (eg, sepsis mortality) with some early success. However, there are still many barriers preventing the widespread use of AI, such as data problems, mismatched rewards, and workplace obstacles. Innovative projects, partnerships, better rewards, and more investment could remove barriers. Implemented reliably and safely, AI can add to what clinicians know, help them work faster, cut costs, and, most importantly, improve patient care.1
AI can potentially bring several clinical benefits, such as reducing the administrative strain on clinicians and granting them more time for direct patient care. It can also improve diagnostic accuracy by analyzing patient data and diagnostic images, providing differential diagnoses, and increasing access to care by providing medical information and essential online services to patients.2
High Reliability Organizations
High reliability health care organizations have considerable experience safely launching new programs. For example, the Patient Safety Adoption Framework gives practical tips for smoothly rolling out safety initiatives (Table 1). Developed with experts and diverse views, this framework has 5 key areas: leadership, culture and context, process, measurement, and person-centeredness. These address adoption problems, guide leaders step-by-step, and focus on leadership buy-in, safety culture, cooperation, and local customization. Checklists and tools make it systematic to go from ideas to action on patient safety.3
Leadership involves establishing organizational commitment behind new safety programs. This visible commitment signals importance and priorities to others. Leaders model desired behaviors and language around safety, allocate resources, remove obstacles, and keep initiatives energized over time through consistent messaging.4 Culture and context recognizes that safety culture differs across units and facilities. Local input tailors programs to fit and examines strengths to build on, like psychological safety. Surveys gauge the existing culture and its need for change. Process details how to plan, design, test, implement, and improve new safety practices and provides a phased roadmap from idea to results. Measurement collects data to drive improvement and show impact. Metrics track progress and allow benchmarking. Person-centeredness puts patients first in safety efforts through participation, education, and transparency.
The Veterans Health Administration piloted a comprehensive high reliability hospital (HRH) model. Over 3 years, the Veterans Health Administration focused on leadership, culture, and process improvement at a hospital. After initiating the model, the pilot hospital improved its safety culture, reported more minor safety issues, and reduced deaths and complications better than other hospitals. The high-reliability approach successfully instilled principles and improved culture and outcomes. The HRH model is set to be expanded to 18 more US Department of Veterans Affairs (VA) sites for further evaluation across diverse settings.5
Trustworthy AI Framework
AI systems are growing more powerful and widespread, including in health care. Unfortunately, irresponsible AI can introduce new harm. ChatGPT and other large language models, for example, sometimes are known to provide erroneous information in a compelling way. Clinicians and patients who use such programs can act on such information, which would lead to unforeseen negative consequences. Several frameworks on ethical AI have come from governmental groups.6-9 In 2023, the VA National AI Institute suggested a Trustworthy AI Framework based on core principles tailored for federal health care. The framework has 6 key principles: purposeful, effective and safe, secure and private, fair and equitable, transparent and explainable, and accountable and monitored (Table 2).10
First, AI must clearly help veterans while minimizing risks. To ensure purpose, the VA will assess patient and clinician needs and design AI that targets meaningful problems to avoid scope creep or feature bloat. For example, adding new features to the AI software after release can clutter and complicate the interface, making it difficult to use. Rigorous testing will confirm that AI meets intent prior to deployment. Second, AI is designed and checked for effectiveness, safety, and reliability. The VA pledges to monitor AI’s impact to ensure it performs as expected without unintended consequences. Algorithms will be stress tested across representative datasets and approval processes will screen for safety issues. Third, AI models are secured from vulnerabilities and misuse. Technical controls will prevent unauthorized access or changes to AI systems. Audits will check for appropriate internal usage per policies. Continual patches and upgrades will maintain security. Fourth, the VA manages AI for fairness, avoiding bias. They will proactively assess datasets and algorithms for potential biases based on protected attributes like race, gender, or age. Biased outputs will be addressed through techniques such as data augmentation, reweighting, and algorithm tweaks. Fifth, transparency explains AI’s role in care. Documentation will detail an AI system’s data sources, methodology, testing, limitations, and integration with clinical workflows. Clinicians and patients will receive education on interpreting AI outputs. Finally, the VA pledges to closely monitor AI systems to sustain trust. The VA will establish oversight processes to quickly identify any declines in reliability or unfair impacts on subgroups. AI models will be retrained as needed based on incoming data patterns.
Each Trustworthy AI Framework principle connects to others in existing frameworks. The purpose principle aligns with human-centric AI focused on benefits. Effectiveness and safety link to technical robustness and risk management principles. Security maps to privacy protection principles. Fairness connects to principles of avoiding bias and discrimination. Transparency corresponds with accountable and explainable AI. Monitoring and accountability tie back to governance principles. Overall, the VA framework aims to guide ethical AI based on context. It offers a model for managing risks and building trust in health care AI.
Combining VA principles with high-reliability safety principles can ensure that AI benefits veterans. The leadership and culture aspects will drive commitment to trustworthy AI practices. Leaders will communicate the importance of responsible AI through words and actions. Culture surveys can assess baseline awareness of AI ethics issues to target education. AI security and fairness will be emphasized as safety critical. The process aspect will institute policies and procedures to uphold AI principles through the project lifecycle. For example, structured testing processes will validate safety. Measurement will collect data on principles like transparency and fairness. Dashboards can track metrics like explainability and biases. A patient-centered approach will incorporate veteran perspectives on AI through participatory design and advisory councils. They can give input on AI explainability and potential biases based on their diverse backgrounds.
Conclusions
Joint principles will lead to successful AI that improves care while proactively managing risks. Involve leaders to stress the necessity of eliminating biases. Build security into the AI development process. Co-design AI transparency features with end users. Closely monitor the impact of AI across safety, fairness, and other principles. Adhering to both Trustworthy AI and high reliability organizations principles will earn veterans’ confidence. Health care organizations like the VA can integrate ethical AI safely via established frameworks. With responsible design and implementation, AI’s potential to enhance care quality, safety, and access can be realized.
Acknowledgments
We would like to acknowledge Joshua Mueller, Theo Tiffney, John Zachary, and Gil Alterovitz for their excellent work creating the VA Trustworthy Principles. This material is the result of work supported by resources and the use of facilities at the James A. Haley Veterans’ Hospital.
Artificial intelligence (AI) has lagged in health care but has considerable potential to improve quality, safety, clinician experience, and access to care. It is being tested in areas like billing, hospital operations, and preventing adverse events (eg, sepsis mortality) with some early success. However, there are still many barriers preventing the widespread use of AI, such as data problems, mismatched rewards, and workplace obstacles. Innovative projects, partnerships, better rewards, and more investment could remove barriers. Implemented reliably and safely, AI can add to what clinicians know, help them work faster, cut costs, and, most importantly, improve patient care.1
AI can potentially bring several clinical benefits, such as reducing the administrative strain on clinicians and granting them more time for direct patient care. It can also improve diagnostic accuracy by analyzing patient data and diagnostic images, providing differential diagnoses, and increasing access to care by providing medical information and essential online services to patients.2
High Reliability Organizations
High reliability health care organizations have considerable experience safely launching new programs. For example, the Patient Safety Adoption Framework gives practical tips for smoothly rolling out safety initiatives (Table 1). Developed with experts and diverse views, this framework has 5 key areas: leadership, culture and context, process, measurement, and person-centeredness. These address adoption problems, guide leaders step-by-step, and focus on leadership buy-in, safety culture, cooperation, and local customization. Checklists and tools make it systematic to go from ideas to action on patient safety.3
Leadership involves establishing organizational commitment behind new safety programs. This visible commitment signals importance and priorities to others. Leaders model desired behaviors and language around safety, allocate resources, remove obstacles, and keep initiatives energized over time through consistent messaging.4 Culture and context recognizes that safety culture differs across units and facilities. Local input tailors programs to fit and examines strengths to build on, like psychological safety. Surveys gauge the existing culture and its need for change. Process details how to plan, design, test, implement, and improve new safety practices and provides a phased roadmap from idea to results. Measurement collects data to drive improvement and show impact. Metrics track progress and allow benchmarking. Person-centeredness puts patients first in safety efforts through participation, education, and transparency.
The Veterans Health Administration piloted a comprehensive high reliability hospital (HRH) model. Over 3 years, the Veterans Health Administration focused on leadership, culture, and process improvement at a hospital. After initiating the model, the pilot hospital improved its safety culture, reported more minor safety issues, and reduced deaths and complications better than other hospitals. The high-reliability approach successfully instilled principles and improved culture and outcomes. The HRH model is set to be expanded to 18 more US Department of Veterans Affairs (VA) sites for further evaluation across diverse settings.5
Trustworthy AI Framework
AI systems are growing more powerful and widespread, including in health care. Unfortunately, irresponsible AI can introduce new harm. ChatGPT and other large language models, for example, sometimes are known to provide erroneous information in a compelling way. Clinicians and patients who use such programs can act on such information, which would lead to unforeseen negative consequences. Several frameworks on ethical AI have come from governmental groups.6-9 In 2023, the VA National AI Institute suggested a Trustworthy AI Framework based on core principles tailored for federal health care. The framework has 6 key principles: purposeful, effective and safe, secure and private, fair and equitable, transparent and explainable, and accountable and monitored (Table 2).10
First, AI must clearly help veterans while minimizing risks. To ensure purpose, the VA will assess patient and clinician needs and design AI that targets meaningful problems to avoid scope creep or feature bloat. For example, adding new features to the AI software after release can clutter and complicate the interface, making it difficult to use. Rigorous testing will confirm that AI meets intent prior to deployment. Second, AI is designed and checked for effectiveness, safety, and reliability. The VA pledges to monitor AI’s impact to ensure it performs as expected without unintended consequences. Algorithms will be stress tested across representative datasets and approval processes will screen for safety issues. Third, AI models are secured from vulnerabilities and misuse. Technical controls will prevent unauthorized access or changes to AI systems. Audits will check for appropriate internal usage per policies. Continual patches and upgrades will maintain security. Fourth, the VA manages AI for fairness, avoiding bias. They will proactively assess datasets and algorithms for potential biases based on protected attributes like race, gender, or age. Biased outputs will be addressed through techniques such as data augmentation, reweighting, and algorithm tweaks. Fifth, transparency explains AI’s role in care. Documentation will detail an AI system’s data sources, methodology, testing, limitations, and integration with clinical workflows. Clinicians and patients will receive education on interpreting AI outputs. Finally, the VA pledges to closely monitor AI systems to sustain trust. The VA will establish oversight processes to quickly identify any declines in reliability or unfair impacts on subgroups. AI models will be retrained as needed based on incoming data patterns.
Each Trustworthy AI Framework principle connects to others in existing frameworks. The purpose principle aligns with human-centric AI focused on benefits. Effectiveness and safety link to technical robustness and risk management principles. Security maps to privacy protection principles. Fairness connects to principles of avoiding bias and discrimination. Transparency corresponds with accountable and explainable AI. Monitoring and accountability tie back to governance principles. Overall, the VA framework aims to guide ethical AI based on context. It offers a model for managing risks and building trust in health care AI.
Combining VA principles with high-reliability safety principles can ensure that AI benefits veterans. The leadership and culture aspects will drive commitment to trustworthy AI practices. Leaders will communicate the importance of responsible AI through words and actions. Culture surveys can assess baseline awareness of AI ethics issues to target education. AI security and fairness will be emphasized as safety critical. The process aspect will institute policies and procedures to uphold AI principles through the project lifecycle. For example, structured testing processes will validate safety. Measurement will collect data on principles like transparency and fairness. Dashboards can track metrics like explainability and biases. A patient-centered approach will incorporate veteran perspectives on AI through participatory design and advisory councils. They can give input on AI explainability and potential biases based on their diverse backgrounds.
Conclusions
Joint principles will lead to successful AI that improves care while proactively managing risks. Involve leaders to stress the necessity of eliminating biases. Build security into the AI development process. Co-design AI transparency features with end users. Closely monitor the impact of AI across safety, fairness, and other principles. Adhering to both Trustworthy AI and high reliability organizations principles will earn veterans’ confidence. Health care organizations like the VA can integrate ethical AI safely via established frameworks. With responsible design and implementation, AI’s potential to enhance care quality, safety, and access can be realized.
Acknowledgments
We would like to acknowledge Joshua Mueller, Theo Tiffney, John Zachary, and Gil Alterovitz for their excellent work creating the VA Trustworthy Principles. This material is the result of work supported by resources and the use of facilities at the James A. Haley Veterans’ Hospital.
1. Sahni NR, Carrus B. Artificial intelligence in U.S. health care delivery. N Engl J Med. 2023;389(4):348-358. doi:10.1056/NEJMra2204673
2. Borkowski AA, Jakey CE, Mastorides SM, et al. Applications of ChatGPT and large language models in medicine and health care: benefits and pitfalls. Fed Pract. 2023;40(6):170-173. doi:10.12788/fp.0386
3. Moyal-Smith R, Margo J, Maloney FL, et al. The patient safety adoption framework: a practical framework to bridge the know-do gap. J Patient Saf. 2023;19(4):243-248. doi:10.1097/PTS.0000000000001118
4. Isaacks DB, Anderson TM, Moore SC, Patterson W, Govindan S. High reliability organization principles improve VA workplace burnout: the Truman THRIVE2 model. Am J Med Qual. 2021;36(6):422-428. doi:10.1097/01.JMQ.0000735516.35323.97
5. Sculli GL, Pendley-Louis R, Neily J, et al. A high-reliability organization framework for health care: a multiyear implementation strategy and associated outcomes. J Patient Saf. 2022;18(1):64-70. doi:10.1097/PTS.0000000000000788
6. National Institute of Standards and Technology. AI risk management framework. Accessed January 2, 2024. https://www.nist.gov/itl/ai-risk-management-framework
7. Executive Office of the President, Office of Science and Technology Policy. Blueprint for an AI Bill of Rights. Accessed January 11, 2024. https://www.whitehouse.gov/ostp/ai-bill-of-rights
8. Executive Office of the President. Executive Order 13960: promoting the use of trustworthy artificial intelligence in the federal government. Fed Regist. 2020;89(236):78939-78943.
9. Biden JR. Executive Order on the safe, secure, and trustworthy development and use of artificial intelligence. Published October 30, 2023. Accessed January 11, 2024. https://www.whitehouse.gov/briefing-room/presidential-actions/2023/10/30/executive-order-on-the-safe-secure-and-trustworthy-development-and-use-of-artificial-intelligence/
10. US Department of Veterans Affairs. Trustworthy AI. Accessed January 11, 2024. https://department.va.gov/ai/trustworthy/
1. Sahni NR, Carrus B. Artificial intelligence in U.S. health care delivery. N Engl J Med. 2023;389(4):348-358. doi:10.1056/NEJMra2204673
2. Borkowski AA, Jakey CE, Mastorides SM, et al. Applications of ChatGPT and large language models in medicine and health care: benefits and pitfalls. Fed Pract. 2023;40(6):170-173. doi:10.12788/fp.0386
3. Moyal-Smith R, Margo J, Maloney FL, et al. The patient safety adoption framework: a practical framework to bridge the know-do gap. J Patient Saf. 2023;19(4):243-248. doi:10.1097/PTS.0000000000001118
4. Isaacks DB, Anderson TM, Moore SC, Patterson W, Govindan S. High reliability organization principles improve VA workplace burnout: the Truman THRIVE2 model. Am J Med Qual. 2021;36(6):422-428. doi:10.1097/01.JMQ.0000735516.35323.97
5. Sculli GL, Pendley-Louis R, Neily J, et al. A high-reliability organization framework for health care: a multiyear implementation strategy and associated outcomes. J Patient Saf. 2022;18(1):64-70. doi:10.1097/PTS.0000000000000788
6. National Institute of Standards and Technology. AI risk management framework. Accessed January 2, 2024. https://www.nist.gov/itl/ai-risk-management-framework
7. Executive Office of the President, Office of Science and Technology Policy. Blueprint for an AI Bill of Rights. Accessed January 11, 2024. https://www.whitehouse.gov/ostp/ai-bill-of-rights
8. Executive Office of the President. Executive Order 13960: promoting the use of trustworthy artificial intelligence in the federal government. Fed Regist. 2020;89(236):78939-78943.
9. Biden JR. Executive Order on the safe, secure, and trustworthy development and use of artificial intelligence. Published October 30, 2023. Accessed January 11, 2024. https://www.whitehouse.gov/briefing-room/presidential-actions/2023/10/30/executive-order-on-the-safe-secure-and-trustworthy-development-and-use-of-artificial-intelligence/
10. US Department of Veterans Affairs. Trustworthy AI. Accessed January 11, 2024. https://department.va.gov/ai/trustworthy/
Most Americans Believe Bariatric Surgery Is Shortcut, Should Be ‘Last Resort’: Survey
Most Americans’ views about obesity and bariatric surgery are colored by stigmas, according to a new survey from the healthcare system at Orlando Health.
For example, most Americans believe that weight loss surgery should be pursued only as a last resort and that bariatric surgery is a shortcut to shedding pounds, the survey found.
Common stigmas could be deterring people who qualify for bariatric surgery from pursuing it, according to Orlando Health, located in Florida.
“Bariatric surgery is by no means an easy way out. If you have the courage to ask for help and commit to doing the hard work of changing your diet and improving your life, you’re a champion in my book,” said Andre Teixeira, MD, medical director and bariatric surgeon at Orlando Health Weight Loss and Bariatric Surgery Institute, Orlando, Florida.
“Surgery is simply a tool to jumpstart that change,” he said. “After surgery, it is up to the patient to learn how to eat well, implement exercise into their routine, and shift their mindset to maintain their health for the rest of their lives.”
The survey results were published in January by Orlando Health.
Surveying Americans
The national survey, conducted for Orlando Health by the market research firm Ipsos in early November 2023, asked 1017 US adults whether they agreed or disagreed with several statements about weight loss and bariatric surgery. The statements and responses are as follows:
- “Weight loss surgery is a shortcut to shedding pounds” — 60% strongly or somewhat agreed, 38% strongly or somewhat disagreed, and the remainder declined to answer.
- “Weight loss surgery is cosmetic and mainly impacts appearance” — 37% strongly or somewhat agreed, 61% strongly or somewhat disagreed, and the remainder declined to respond.
- “Exercise and diet should be enough for weight loss” — 61% strongly or somewhat agreed, 37% strongly or somewhat disagreed, and the remainder declined to respond.
- “Weight loss surgery should only be pursued as a last resort” — 79% strongly or somewhat agreed, 19% strongly or somewhat disagreed, and the remainder declined to answer.
- “Surgery should be more socially accepted as a way to lose weight” — 46% strongly or somewhat agreed, 52% strongly or somewhat disagreed, and the remainder declined to respond.
Men’s responses indicated that they are more likely to have negative views toward weight loss surgery than women. For example, 66% of men vs 54% of women respondents see weight loss surgery as a shortcut to losing weight. Conversely, 42% of men vs 50% of women said that surgery should be a more socially accepted weight loss method.
Opinions that might interfere with the willingness to have weight loss surgery were apparent among people with obesity. The survey found that 65% of respondents with obesity and 59% with extreme obesity view surgery as a shortcut. Eighty-two percent of respondents with obesity and 68% with extreme obesity see surgery as a last resort.
At the end of 2022, the American Society of Metabolic and Bariatric Surgery and the International Federation for the Surgery of Obesity and Metabolic Disorders updated their guidelines for metabolic and bariatric surgery for the first time since 1991, with the aim of expanding access to surgery, Orlando Health noted. However, only 1% of those who are clinically eligible end up undergoing weight loss surgery, even with advancements in laparoscopic and robotic techniques that have made it safer and less invasive, the health system added.
“Because of the stigma around obesity and bariatric surgery, so many of my patients feel defeated if they can’t lose weight on their own,” said Muhammad Ghanem, MD, a bariatric surgeon at Orlando Health.
“But when I tell them obesity is a disease and that many of its causes are outside of their control, you can see their relief,” he said. “They often even shed a tear because they’ve struggled with their weight all their lives and finally have some validation.”
Individualizing Treatment
Obesity treatment plans should be tailored to patients on the basis of individual factors such as body mass index, existing medical conditions, and family history, Dr. Teixeira said.
Besides bariatric surgery, patients also may consider options such as counseling, lifestyle changes, and medications including the latest weight loss drugs, he added.
The clinical approach to obesity treatment has evolved, said Miguel Burch, MD, director of general surgery and chief of minimally invasive and gastrointestinal surgery at Cedars-Sinai, Los Angeles, California, who was not involved in the survey.
“At one point in my career, I could say the only proven durable treatment for obesity is weight loss surgery. This was in the context of patients who were morbidly obese requiring risk reduction, not for a year or two but for decades, and not for 10-20 pounds but for 40-60 pounds of weight loss,” said Dr. Burch, who also directs the bariatric surgery program at Torrance Memorial Medical Center, Torrance, California.
“That was a previous era. We are now in a new one with the weight loss drugs,” Dr. Burch said. “In fact, it’s wonderful to have the opportunity to serve so many patients with an option other than just surgery.”
Still, Dr. Burch added, “we have to change the way we look at obesity management as being either surgery or medicine and start thinking about it more as a multidisciplinary approach to a chronic and potentially relapsing disease.”
A version of this article appeared on Medscape.com.
Most Americans’ views about obesity and bariatric surgery are colored by stigmas, according to a new survey from the healthcare system at Orlando Health.
For example, most Americans believe that weight loss surgery should be pursued only as a last resort and that bariatric surgery is a shortcut to shedding pounds, the survey found.
Common stigmas could be deterring people who qualify for bariatric surgery from pursuing it, according to Orlando Health, located in Florida.
“Bariatric surgery is by no means an easy way out. If you have the courage to ask for help and commit to doing the hard work of changing your diet and improving your life, you’re a champion in my book,” said Andre Teixeira, MD, medical director and bariatric surgeon at Orlando Health Weight Loss and Bariatric Surgery Institute, Orlando, Florida.
“Surgery is simply a tool to jumpstart that change,” he said. “After surgery, it is up to the patient to learn how to eat well, implement exercise into their routine, and shift their mindset to maintain their health for the rest of their lives.”
The survey results were published in January by Orlando Health.
Surveying Americans
The national survey, conducted for Orlando Health by the market research firm Ipsos in early November 2023, asked 1017 US adults whether they agreed or disagreed with several statements about weight loss and bariatric surgery. The statements and responses are as follows:
- “Weight loss surgery is a shortcut to shedding pounds” — 60% strongly or somewhat agreed, 38% strongly or somewhat disagreed, and the remainder declined to answer.
- “Weight loss surgery is cosmetic and mainly impacts appearance” — 37% strongly or somewhat agreed, 61% strongly or somewhat disagreed, and the remainder declined to respond.
- “Exercise and diet should be enough for weight loss” — 61% strongly or somewhat agreed, 37% strongly or somewhat disagreed, and the remainder declined to respond.
- “Weight loss surgery should only be pursued as a last resort” — 79% strongly or somewhat agreed, 19% strongly or somewhat disagreed, and the remainder declined to answer.
- “Surgery should be more socially accepted as a way to lose weight” — 46% strongly or somewhat agreed, 52% strongly or somewhat disagreed, and the remainder declined to respond.
Men’s responses indicated that they are more likely to have negative views toward weight loss surgery than women. For example, 66% of men vs 54% of women respondents see weight loss surgery as a shortcut to losing weight. Conversely, 42% of men vs 50% of women said that surgery should be a more socially accepted weight loss method.
Opinions that might interfere with the willingness to have weight loss surgery were apparent among people with obesity. The survey found that 65% of respondents with obesity and 59% with extreme obesity view surgery as a shortcut. Eighty-two percent of respondents with obesity and 68% with extreme obesity see surgery as a last resort.
At the end of 2022, the American Society of Metabolic and Bariatric Surgery and the International Federation for the Surgery of Obesity and Metabolic Disorders updated their guidelines for metabolic and bariatric surgery for the first time since 1991, with the aim of expanding access to surgery, Orlando Health noted. However, only 1% of those who are clinically eligible end up undergoing weight loss surgery, even with advancements in laparoscopic and robotic techniques that have made it safer and less invasive, the health system added.
“Because of the stigma around obesity and bariatric surgery, so many of my patients feel defeated if they can’t lose weight on their own,” said Muhammad Ghanem, MD, a bariatric surgeon at Orlando Health.
“But when I tell them obesity is a disease and that many of its causes are outside of their control, you can see their relief,” he said. “They often even shed a tear because they’ve struggled with their weight all their lives and finally have some validation.”
Individualizing Treatment
Obesity treatment plans should be tailored to patients on the basis of individual factors such as body mass index, existing medical conditions, and family history, Dr. Teixeira said.
Besides bariatric surgery, patients also may consider options such as counseling, lifestyle changes, and medications including the latest weight loss drugs, he added.
The clinical approach to obesity treatment has evolved, said Miguel Burch, MD, director of general surgery and chief of minimally invasive and gastrointestinal surgery at Cedars-Sinai, Los Angeles, California, who was not involved in the survey.
“At one point in my career, I could say the only proven durable treatment for obesity is weight loss surgery. This was in the context of patients who were morbidly obese requiring risk reduction, not for a year or two but for decades, and not for 10-20 pounds but for 40-60 pounds of weight loss,” said Dr. Burch, who also directs the bariatric surgery program at Torrance Memorial Medical Center, Torrance, California.
“That was a previous era. We are now in a new one with the weight loss drugs,” Dr. Burch said. “In fact, it’s wonderful to have the opportunity to serve so many patients with an option other than just surgery.”
Still, Dr. Burch added, “we have to change the way we look at obesity management as being either surgery or medicine and start thinking about it more as a multidisciplinary approach to a chronic and potentially relapsing disease.”
A version of this article appeared on Medscape.com.
Most Americans’ views about obesity and bariatric surgery are colored by stigmas, according to a new survey from the healthcare system at Orlando Health.
For example, most Americans believe that weight loss surgery should be pursued only as a last resort and that bariatric surgery is a shortcut to shedding pounds, the survey found.
Common stigmas could be deterring people who qualify for bariatric surgery from pursuing it, according to Orlando Health, located in Florida.
“Bariatric surgery is by no means an easy way out. If you have the courage to ask for help and commit to doing the hard work of changing your diet and improving your life, you’re a champion in my book,” said Andre Teixeira, MD, medical director and bariatric surgeon at Orlando Health Weight Loss and Bariatric Surgery Institute, Orlando, Florida.
“Surgery is simply a tool to jumpstart that change,” he said. “After surgery, it is up to the patient to learn how to eat well, implement exercise into their routine, and shift their mindset to maintain their health for the rest of their lives.”
The survey results were published in January by Orlando Health.
Surveying Americans
The national survey, conducted for Orlando Health by the market research firm Ipsos in early November 2023, asked 1017 US adults whether they agreed or disagreed with several statements about weight loss and bariatric surgery. The statements and responses are as follows:
- “Weight loss surgery is a shortcut to shedding pounds” — 60% strongly or somewhat agreed, 38% strongly or somewhat disagreed, and the remainder declined to answer.
- “Weight loss surgery is cosmetic and mainly impacts appearance” — 37% strongly or somewhat agreed, 61% strongly or somewhat disagreed, and the remainder declined to respond.
- “Exercise and diet should be enough for weight loss” — 61% strongly or somewhat agreed, 37% strongly or somewhat disagreed, and the remainder declined to respond.
- “Weight loss surgery should only be pursued as a last resort” — 79% strongly or somewhat agreed, 19% strongly or somewhat disagreed, and the remainder declined to answer.
- “Surgery should be more socially accepted as a way to lose weight” — 46% strongly or somewhat agreed, 52% strongly or somewhat disagreed, and the remainder declined to respond.
Men’s responses indicated that they are more likely to have negative views toward weight loss surgery than women. For example, 66% of men vs 54% of women respondents see weight loss surgery as a shortcut to losing weight. Conversely, 42% of men vs 50% of women said that surgery should be a more socially accepted weight loss method.
Opinions that might interfere with the willingness to have weight loss surgery were apparent among people with obesity. The survey found that 65% of respondents with obesity and 59% with extreme obesity view surgery as a shortcut. Eighty-two percent of respondents with obesity and 68% with extreme obesity see surgery as a last resort.
At the end of 2022, the American Society of Metabolic and Bariatric Surgery and the International Federation for the Surgery of Obesity and Metabolic Disorders updated their guidelines for metabolic and bariatric surgery for the first time since 1991, with the aim of expanding access to surgery, Orlando Health noted. However, only 1% of those who are clinically eligible end up undergoing weight loss surgery, even with advancements in laparoscopic and robotic techniques that have made it safer and less invasive, the health system added.
“Because of the stigma around obesity and bariatric surgery, so many of my patients feel defeated if they can’t lose weight on their own,” said Muhammad Ghanem, MD, a bariatric surgeon at Orlando Health.
“But when I tell them obesity is a disease and that many of its causes are outside of their control, you can see their relief,” he said. “They often even shed a tear because they’ve struggled with their weight all their lives and finally have some validation.”
Individualizing Treatment
Obesity treatment plans should be tailored to patients on the basis of individual factors such as body mass index, existing medical conditions, and family history, Dr. Teixeira said.
Besides bariatric surgery, patients also may consider options such as counseling, lifestyle changes, and medications including the latest weight loss drugs, he added.
The clinical approach to obesity treatment has evolved, said Miguel Burch, MD, director of general surgery and chief of minimally invasive and gastrointestinal surgery at Cedars-Sinai, Los Angeles, California, who was not involved in the survey.
“At one point in my career, I could say the only proven durable treatment for obesity is weight loss surgery. This was in the context of patients who were morbidly obese requiring risk reduction, not for a year or two but for decades, and not for 10-20 pounds but for 40-60 pounds of weight loss,” said Dr. Burch, who also directs the bariatric surgery program at Torrance Memorial Medical Center, Torrance, California.
“That was a previous era. We are now in a new one with the weight loss drugs,” Dr. Burch said. “In fact, it’s wonderful to have the opportunity to serve so many patients with an option other than just surgery.”
Still, Dr. Burch added, “we have to change the way we look at obesity management as being either surgery or medicine and start thinking about it more as a multidisciplinary approach to a chronic and potentially relapsing disease.”
A version of this article appeared on Medscape.com.
Working together
Dear Friends,
After 6 months in my first faculty position, I have come to appreciate the term “multidisciplinary approach” more than ever. Not only does this facilitate optimal patient care, but I have personally learned so much from experts in other fields. This theme resonates across this issue of The New Gastroenterologist, from treating complex gallbladder disease, to caring for sexual and gender minorities, and collaborating with the tech industry to advance patient care.
Our “In Focus” feature, written by Dr. Andrew Gilman and Dr. Todd Baron, is on endoscopic management of gallbladder disease. They review endoscopic treatment options in patients with benign gallbladder disease, with emphasis on working with surgical and interventional radiology colleagues, as well as relaying endoscopic tips and techniques to achieve success in these complicated procedures.
In the “Short Clinical Reviews” section, Dr. David Chiang and Dr. Victor Chedid highlight the gaps in research and clinical care and competency for sexual and gender minorities, particularly in patients with inflammatory bowel disease. They describe the creation of the Pride in IBD clinic at Mayo Clinic in Rochester, Minn., that creates a culturally sensitive space to care for this community.
As trainees transition to early faculty, becoming a mentor is a new role that can be very rewarding and daunting at the same time. Dr. Anna Lok, recipient of the AGA’s Distinguished Mentor Award, and Dr. Vincent Chen share invaluable experiences and advice on being a mentor from senior and early-career perspectives, respectively. Similarly in the transition to early faculty, Erin Anderson, CPA, answers five common financial questions that arise to better understand and manage a significant increase in salary.
Lastly, Dr. Shifa Umar describes her unique experience as part of the AGA’s annual Tech Summit Fellows Program, a cross-section of medicine, technology, and innovation.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Jillian Schweitzer ([email protected]), managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: The concept of the clinicopathologic conference (CPC) was introduced by Dr. Walter B. Cannon as a medical student at Harvard Medical School.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Dear Friends,
After 6 months in my first faculty position, I have come to appreciate the term “multidisciplinary approach” more than ever. Not only does this facilitate optimal patient care, but I have personally learned so much from experts in other fields. This theme resonates across this issue of The New Gastroenterologist, from treating complex gallbladder disease, to caring for sexual and gender minorities, and collaborating with the tech industry to advance patient care.
Our “In Focus” feature, written by Dr. Andrew Gilman and Dr. Todd Baron, is on endoscopic management of gallbladder disease. They review endoscopic treatment options in patients with benign gallbladder disease, with emphasis on working with surgical and interventional radiology colleagues, as well as relaying endoscopic tips and techniques to achieve success in these complicated procedures.
In the “Short Clinical Reviews” section, Dr. David Chiang and Dr. Victor Chedid highlight the gaps in research and clinical care and competency for sexual and gender minorities, particularly in patients with inflammatory bowel disease. They describe the creation of the Pride in IBD clinic at Mayo Clinic in Rochester, Minn., that creates a culturally sensitive space to care for this community.
As trainees transition to early faculty, becoming a mentor is a new role that can be very rewarding and daunting at the same time. Dr. Anna Lok, recipient of the AGA’s Distinguished Mentor Award, and Dr. Vincent Chen share invaluable experiences and advice on being a mentor from senior and early-career perspectives, respectively. Similarly in the transition to early faculty, Erin Anderson, CPA, answers five common financial questions that arise to better understand and manage a significant increase in salary.
Lastly, Dr. Shifa Umar describes her unique experience as part of the AGA’s annual Tech Summit Fellows Program, a cross-section of medicine, technology, and innovation.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Jillian Schweitzer ([email protected]), managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: The concept of the clinicopathologic conference (CPC) was introduced by Dr. Walter B. Cannon as a medical student at Harvard Medical School.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Dear Friends,
After 6 months in my first faculty position, I have come to appreciate the term “multidisciplinary approach” more than ever. Not only does this facilitate optimal patient care, but I have personally learned so much from experts in other fields. This theme resonates across this issue of The New Gastroenterologist, from treating complex gallbladder disease, to caring for sexual and gender minorities, and collaborating with the tech industry to advance patient care.
Our “In Focus” feature, written by Dr. Andrew Gilman and Dr. Todd Baron, is on endoscopic management of gallbladder disease. They review endoscopic treatment options in patients with benign gallbladder disease, with emphasis on working with surgical and interventional radiology colleagues, as well as relaying endoscopic tips and techniques to achieve success in these complicated procedures.
In the “Short Clinical Reviews” section, Dr. David Chiang and Dr. Victor Chedid highlight the gaps in research and clinical care and competency for sexual and gender minorities, particularly in patients with inflammatory bowel disease. They describe the creation of the Pride in IBD clinic at Mayo Clinic in Rochester, Minn., that creates a culturally sensitive space to care for this community.
As trainees transition to early faculty, becoming a mentor is a new role that can be very rewarding and daunting at the same time. Dr. Anna Lok, recipient of the AGA’s Distinguished Mentor Award, and Dr. Vincent Chen share invaluable experiences and advice on being a mentor from senior and early-career perspectives, respectively. Similarly in the transition to early faculty, Erin Anderson, CPA, answers five common financial questions that arise to better understand and manage a significant increase in salary.
Lastly, Dr. Shifa Umar describes her unique experience as part of the AGA’s annual Tech Summit Fellows Program, a cross-section of medicine, technology, and innovation.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Jillian Schweitzer ([email protected]), managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: The concept of the clinicopathologic conference (CPC) was introduced by Dr. Walter B. Cannon as a medical student at Harvard Medical School.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Moving the Field FORWARD
As an organization, AGA has invested heavily in programs and initiatives to support the professional development of its members across career stages. This includes programs such as the AGA-AASLD Academic Skills Workshop (in which I was fortunate to participate in 2016), Women’s Leadership and Executive Leadership Conferences (with the Midwest Women in GI Regional Workshop taking place later this month), and the AGA Research Foundation Awards Program, which distributes over $2 million in funding annually to support promising early career and senior investigators.
AGA’s Fostering Opportunities Resulting in Workforce and Research Diversity (FORWARD) Program, which was first funded by the National Institutes of Health in 2018 and is focused on improving the diversity of the GI research workforce, is another shining example. Led by Dr. Byron Cryer and Dr. Sandra Quezada, the program recently welcomed its 3rd cohort of participants, including 14 mentees and 28 senior and near-peer mentors.
We are pleased to frequently highlight these programs in the pages of GI & Hepatology News, and hope you enjoy learning more about each of these initiatives in future issues.
In this month’s issue of GIHN, we highlight AGA’s newest Clinical Practice Guideline focused on management of pouchitis. We also report on the results of a recent RCT published in the New England Journal of Medicine demonstrating the efficacy of thalidomide as a treatment for recurrent bleeding due to small-intestinal angiodysplasia and summarize other key journal content impacting your clinical practice. In our February Member Spotlight, we feature Dr. Rajeev Jain of Texas Digestive Disease Consultants, a former AGA Governing Board member, and learn about his advocacy work to improve patient care and reduce physician burnout through insurance coverage and MOC reform. We hope you enjoy this, and all the exciting content included in our February issue!
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
As an organization, AGA has invested heavily in programs and initiatives to support the professional development of its members across career stages. This includes programs such as the AGA-AASLD Academic Skills Workshop (in which I was fortunate to participate in 2016), Women’s Leadership and Executive Leadership Conferences (with the Midwest Women in GI Regional Workshop taking place later this month), and the AGA Research Foundation Awards Program, which distributes over $2 million in funding annually to support promising early career and senior investigators.
AGA’s Fostering Opportunities Resulting in Workforce and Research Diversity (FORWARD) Program, which was first funded by the National Institutes of Health in 2018 and is focused on improving the diversity of the GI research workforce, is another shining example. Led by Dr. Byron Cryer and Dr. Sandra Quezada, the program recently welcomed its 3rd cohort of participants, including 14 mentees and 28 senior and near-peer mentors.
We are pleased to frequently highlight these programs in the pages of GI & Hepatology News, and hope you enjoy learning more about each of these initiatives in future issues.
In this month’s issue of GIHN, we highlight AGA’s newest Clinical Practice Guideline focused on management of pouchitis. We also report on the results of a recent RCT published in the New England Journal of Medicine demonstrating the efficacy of thalidomide as a treatment for recurrent bleeding due to small-intestinal angiodysplasia and summarize other key journal content impacting your clinical practice. In our February Member Spotlight, we feature Dr. Rajeev Jain of Texas Digestive Disease Consultants, a former AGA Governing Board member, and learn about his advocacy work to improve patient care and reduce physician burnout through insurance coverage and MOC reform. We hope you enjoy this, and all the exciting content included in our February issue!
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
As an organization, AGA has invested heavily in programs and initiatives to support the professional development of its members across career stages. This includes programs such as the AGA-AASLD Academic Skills Workshop (in which I was fortunate to participate in 2016), Women’s Leadership and Executive Leadership Conferences (with the Midwest Women in GI Regional Workshop taking place later this month), and the AGA Research Foundation Awards Program, which distributes over $2 million in funding annually to support promising early career and senior investigators.
AGA’s Fostering Opportunities Resulting in Workforce and Research Diversity (FORWARD) Program, which was first funded by the National Institutes of Health in 2018 and is focused on improving the diversity of the GI research workforce, is another shining example. Led by Dr. Byron Cryer and Dr. Sandra Quezada, the program recently welcomed its 3rd cohort of participants, including 14 mentees and 28 senior and near-peer mentors.
We are pleased to frequently highlight these programs in the pages of GI & Hepatology News, and hope you enjoy learning more about each of these initiatives in future issues.
In this month’s issue of GIHN, we highlight AGA’s newest Clinical Practice Guideline focused on management of pouchitis. We also report on the results of a recent RCT published in the New England Journal of Medicine demonstrating the efficacy of thalidomide as a treatment for recurrent bleeding due to small-intestinal angiodysplasia and summarize other key journal content impacting your clinical practice. In our February Member Spotlight, we feature Dr. Rajeev Jain of Texas Digestive Disease Consultants, a former AGA Governing Board member, and learn about his advocacy work to improve patient care and reduce physician burnout through insurance coverage and MOC reform. We hope you enjoy this, and all the exciting content included in our February issue!
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Psychogenic Purpura
To the Editor:
A 14-year-old Black adolescent girl presented with episodic, painful, edematous plaques that occurred symmetrically on the arms and legs of 5 years’ duration. The plaques evolved into hyperpigmented patches within 24 to 48 hours before eventually resolving. Fatigue, headache, arthralgias of the arms and legs, chest pain, abdominal pain, nausea, and vomiting variably accompanied these episodes.
Prior to visiting our clinic, the patient had been seen by numerous specialists. A review of her medical records revealed an initial diagnosis of Henoch-Schönlein purpura (HSP), then urticarial vasculitis. She had been treated with antihistamines, topical and systemic steroids, hydroxychloroquine, mycophenolate mofetil, dapsone, azathioprine, and gabapentin. All treatments were ineffectual. She underwent extensive diagnostic testing and imaging, which were normal or noncontributory, including type I allergy testing; multiple exhaustive batteries of hematologic testing; and computed tomography/magnetic resonance imaging/magnetic resonance angiography of the brain, chest, abdomen, and pelvic region. Biopsies from symptomatic segments of the gastrointestinal tract were normal.
Chronic treatment with systemic steroids over 9 months resulted in gastritis and an episode of hematemesis requiring emergent hospitalization. A lengthy multidisciplinary evaluation was conducted at the patient’s local community hospital; the team concluded that she had an urticarial-type rash with accompanying symptoms that did not have an autoimmune, rheumatologic, or inflammatory basis.
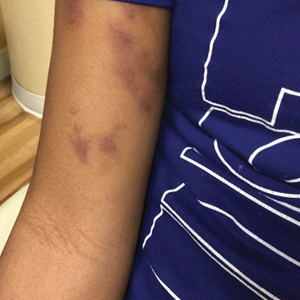
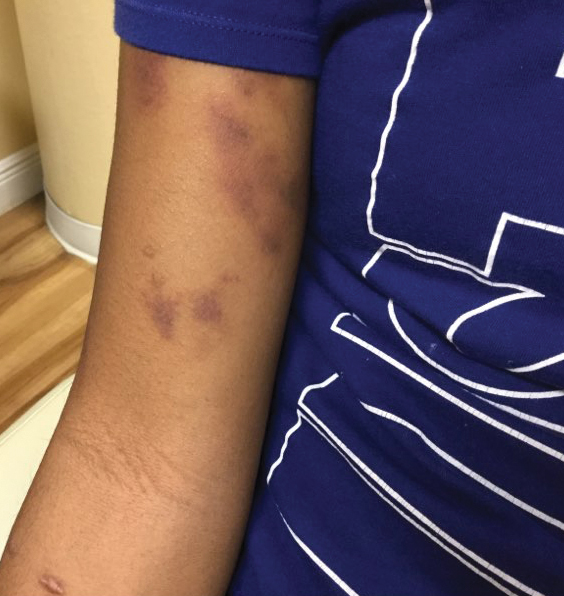
The patient’s medical history was remarkable for recent-onset panic attacks. Her family medical history was noncontributory. Physical examination revealed multiple violaceous hyperpigmented patches diffusely located on the proximal upper arms (Figure 1). There were no additional findings on physical examination.
Punch biopsies were performed on lesional areas of the arm. Histopathology indicated a mild superficial perivascular dermal mixed infiltrate and extravasated erythrocytes (Figure 2). Direct immunofluorescence (DIF) testing was negative for vasculitis. Immunohistochemical stains for CD117 and tryptase demonstrated a slight increase in the number of dermal mast cells; however, the increase was not sufficient to diagnose cutaneous mastocytosis, which was in the differential. We proposed a diagnosis of psychogenic purpura (PP)(also known as Gardner-Diamond syndrome). She was treated with gabapentin, a selective serotonin reuptake inhibitor, and cognitive therapy. Unfortunately, after starting therapy the patient was lost to follow-up.
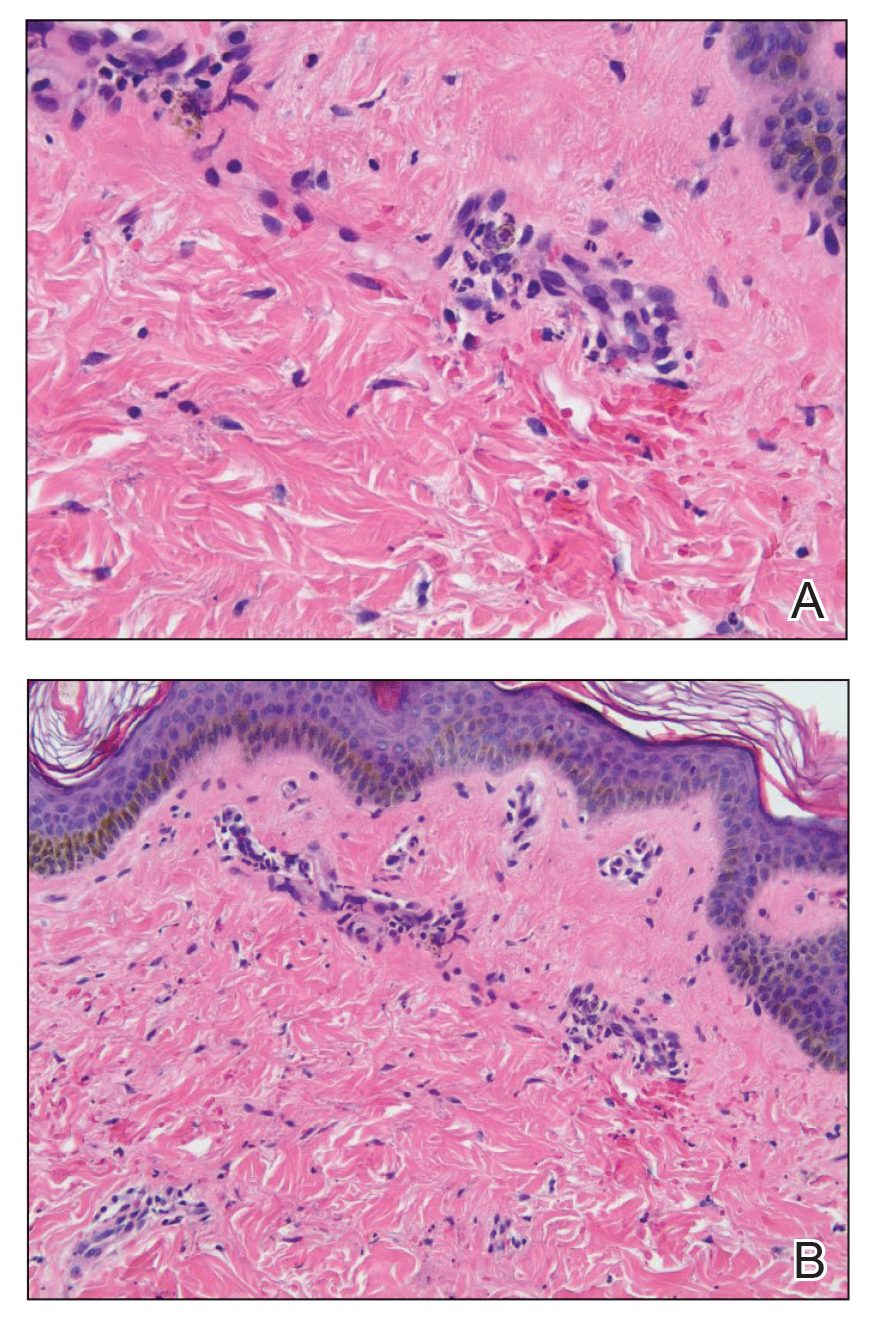
Psychogenic purpura is a rare vasculopathy of unknown etiology that may be a special form of factitious disorder.1,2 In one study, PP occurred predominantly in females aged 15 to 66 years, with a median onset age of 33 years.3 A prodrome of localized itching, burning, and/or pain precedes the development of edematous plaques. The plaques evolve into painful ecchymoses within 1 to 2 days and resolve in 10 days or fewer without treatment. Lesions most commonly occur on the extremities but may occur anywhere on the body. The most common associated finding is an underlying depressive disorder. Episodes may be accompanied by headache, dizziness, fatigue, fever, arthralgia, nausea, vomiting, abdominal pain, menstrual irregularities, myalgia, and urologic conditions.
In 1955, Gardner and Diamond4 described the first cases of PP in 4 female patients at Peter Bent Brigham Hospital in Boston, Massachusetts. The investigators were able to replicate the painful ecchymoses with intradermal injection of the patient’s own erythrocytes into the skin. They proposed that the underlying pathogenesis involved autosensitization to erythrocyte stroma.4 Since then, others have suggested that the pathogenesis may include autosensitization to erythrocyte phosphatidylserine, tonus dysregulation of venous capillaries, abnormal endothelial fibrin synthesis, and capillary wall instability.5-7
Histopathology typically reveals superficial and deep perivascular inflammation with extravasated erythrocytes. Direct immunofluorescence is negative for vasculitis.8 Diagnostics and laboratory findings for underlying systemic illness are negative or noncontributory. Cutaneous injection of 1 mL of the patient’s own washed erythrocytes may result in the formation of the characteristic painful plaques within 24 hours; however, this test is limited by lack of standardization and low sensitivity.3
Psychogenic purpura may share clinical features with cutaneous small vessel vasculitis, such as HSP or urticarial vasculitis. Some of the findings that our patient was experiencing, including purpura, arthralgia, and abdominal pain, are associated with HSP. However, HSP typically is self-limiting and classically features palpable purpura distributed across the lower extremities and buttocks. Histopathology demonstrates the classic findings of leukocytoclastic vasculitis; DIF typically is positive for perivascular IgA and C3 deposition. Increased serum IgA may be present.9 Urticarial vasculitis appears as erythematous indurated wheals that favor a proximal extremity and truncal distribution. They characteristically last longer than 24 hours, are frequently associated with nonprodromal pain or burning, and resolve with hyperpigmentation. Arthralgia and gastrointestinal, renal, pulmonary, cardiac, and neurologic symptoms may be present, especially in patients with low complement levels.10 Skin biopsy demonstrates leukocytoclasia that must be accompanied by vessel wall necrosis. Fibrinoid deposition, erythrocyte extravasation, or perivascular inflammation may be present. In 70% of cases revealing perivascular immunoglobulin, C3, and fibrinogen deposition, DIF is positive. Serum C1q autoantibody may be associated with the hypocomplementemic form.10
The classic histopathologic findings in leukocytoclastic vasculitis include transmural neutrophilic infiltration of the walls of small vessels, fibrinoid necrosis of vessel walls, leukocytoclasia, extravasated erythrocytes, and signs of endothelial cell damage.9 A prior punch biopsy in this patient demonstrated rare neutrophilic nuclear debris within the vessel walls without fibrin deposition. Although the presence of nuclear debris and extravasated erythrocytes could be compatible with a manifestation of urticarial vasculitis, the lack of direct evidence of vessel wall necrosis combined with subsequent biopsies unequivocally ruled out cutaneous small vessel vasculitis in our patient.
Psychogenic purpura has been reported to occur frequently in the background of psycho-emotional distress. In 1989, Ratnoff11 noted that many of the patients he was treating at the University Hospitals of Cleveland, Ohio, had a depressive syndrome. A review of patients treated at the Mayo Clinic in Rochester, Minnesota, illustrated concomitant psychiatric illnesses in 41 of 76 (54%) patients treated for PP, most commonly depressive, personality, and anxiety disorders.3
There is no consensus on therapy for PP. Treatment is based on providing symptomatic relief and relieving underlying psychiatric distress. Block et al12 found the use of selective serotonin reuptake inhibitors, tricyclic antidepressants, and psychotherapy to be successful in improving symptoms and reducing lesions at follow-up visits.
- Piette WW. Purpura: mechanisms and differential diagnosis. In: Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier; 2018:376-389.
- Harth W, Taube KM, Gieler U. Factitious disorders in dermatology. J Dtsch Dermatol Ges. 2010;8:361-372.
- Sridharan M, Ali U, Hook CC, et al. The Mayo Clinic experience with psychogenic purpura (Gardner-Diamond syndrome). Am J Med Sci. 2019;357:411‐420.
- Gardner FH, Diamond LK. Autoerythrocyte sensitization; a form of purpura producing painful bruising following autosensitization to red blood cells in certain women. Blood. 1955;10:675-690.
- Groch GS, Finch SC, Rogoway W, et al. Studies in the pathogenesis of autoerythrocyte sensitization syndrome. Blood. 1966;28:19-33.
- Strunecká A, Krpejsová L, Palecek J, et al. Transbilayer redistribution of phosphatidylserine in erythrocytes of a patient with autoerythrocyte sensitization syndrome (psychogenic purpura). Folia Haematol Int Mag Klin Morphol Blutforsch. 1990;117:829-841.
- Merlen JF. Ecchymotic patches of the fingers and Gardner-Diamond vascular purpura. Phlebologie. 1987;40:473-487.
- Ivanov OL, Lvov AN, Michenko AV, et al. Autoerythrocyte sensitization syndrome (Gardner-Diamond syndrome): review of the literature. J Eur Acad Dermatol Venereol. 2009;23:499-504.
- Wetter DA, Dutz JP, Shinkai K, et al. Cutaneous vasculitis. In: Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier; 2018:409-439.
- Hamad A, Jithpratuck W, Krishnaswamy G. Urticarial vasculitis and associated disorders. Ann Allergy Asthma Immunol. 2017;118:394-398.
- Ratnoff OD. Psychogenic purpura (autoerythrocyte sensitization): an unsolved dilemma. Am J Med. 1989;87:16N-21N.
- Block ME, Sitenga JL, Lehrer M, et al. Gardner‐Diamond syndrome: a systematic review of treatment options for a rare psychodermatological disorder. Int J Dermatol. 2019;58:782-787.
To the Editor:
A 14-year-old Black adolescent girl presented with episodic, painful, edematous plaques that occurred symmetrically on the arms and legs of 5 years’ duration. The plaques evolved into hyperpigmented patches within 24 to 48 hours before eventually resolving. Fatigue, headache, arthralgias of the arms and legs, chest pain, abdominal pain, nausea, and vomiting variably accompanied these episodes.
Prior to visiting our clinic, the patient had been seen by numerous specialists. A review of her medical records revealed an initial diagnosis of Henoch-Schönlein purpura (HSP), then urticarial vasculitis. She had been treated with antihistamines, topical and systemic steroids, hydroxychloroquine, mycophenolate mofetil, dapsone, azathioprine, and gabapentin. All treatments were ineffectual. She underwent extensive diagnostic testing and imaging, which were normal or noncontributory, including type I allergy testing; multiple exhaustive batteries of hematologic testing; and computed tomography/magnetic resonance imaging/magnetic resonance angiography of the brain, chest, abdomen, and pelvic region. Biopsies from symptomatic segments of the gastrointestinal tract were normal.
Chronic treatment with systemic steroids over 9 months resulted in gastritis and an episode of hematemesis requiring emergent hospitalization. A lengthy multidisciplinary evaluation was conducted at the patient’s local community hospital; the team concluded that she had an urticarial-type rash with accompanying symptoms that did not have an autoimmune, rheumatologic, or inflammatory basis.
The patient’s medical history was remarkable for recent-onset panic attacks. Her family medical history was noncontributory. Physical examination revealed multiple violaceous hyperpigmented patches diffusely located on the proximal upper arms (Figure 1). There were no additional findings on physical examination.

Punch biopsies were performed on lesional areas of the arm. Histopathology indicated a mild superficial perivascular dermal mixed infiltrate and extravasated erythrocytes (Figure 2). Direct immunofluorescence (DIF) testing was negative for vasculitis. Immunohistochemical stains for CD117 and tryptase demonstrated a slight increase in the number of dermal mast cells; however, the increase was not sufficient to diagnose cutaneous mastocytosis, which was in the differential. We proposed a diagnosis of psychogenic purpura (PP)(also known as Gardner-Diamond syndrome). She was treated with gabapentin, a selective serotonin reuptake inhibitor, and cognitive therapy. Unfortunately, after starting therapy the patient was lost to follow-up.

Psychogenic purpura is a rare vasculopathy of unknown etiology that may be a special form of factitious disorder.1,2 In one study, PP occurred predominantly in females aged 15 to 66 years, with a median onset age of 33 years.3 A prodrome of localized itching, burning, and/or pain precedes the development of edematous plaques. The plaques evolve into painful ecchymoses within 1 to 2 days and resolve in 10 days or fewer without treatment. Lesions most commonly occur on the extremities but may occur anywhere on the body. The most common associated finding is an underlying depressive disorder. Episodes may be accompanied by headache, dizziness, fatigue, fever, arthralgia, nausea, vomiting, abdominal pain, menstrual irregularities, myalgia, and urologic conditions.
In 1955, Gardner and Diamond4 described the first cases of PP in 4 female patients at Peter Bent Brigham Hospital in Boston, Massachusetts. The investigators were able to replicate the painful ecchymoses with intradermal injection of the patient’s own erythrocytes into the skin. They proposed that the underlying pathogenesis involved autosensitization to erythrocyte stroma.4 Since then, others have suggested that the pathogenesis may include autosensitization to erythrocyte phosphatidylserine, tonus dysregulation of venous capillaries, abnormal endothelial fibrin synthesis, and capillary wall instability.5-7
Histopathology typically reveals superficial and deep perivascular inflammation with extravasated erythrocytes. Direct immunofluorescence is negative for vasculitis.8 Diagnostics and laboratory findings for underlying systemic illness are negative or noncontributory. Cutaneous injection of 1 mL of the patient’s own washed erythrocytes may result in the formation of the characteristic painful plaques within 24 hours; however, this test is limited by lack of standardization and low sensitivity.3
Psychogenic purpura may share clinical features with cutaneous small vessel vasculitis, such as HSP or urticarial vasculitis. Some of the findings that our patient was experiencing, including purpura, arthralgia, and abdominal pain, are associated with HSP. However, HSP typically is self-limiting and classically features palpable purpura distributed across the lower extremities and buttocks. Histopathology demonstrates the classic findings of leukocytoclastic vasculitis; DIF typically is positive for perivascular IgA and C3 deposition. Increased serum IgA may be present.9 Urticarial vasculitis appears as erythematous indurated wheals that favor a proximal extremity and truncal distribution. They characteristically last longer than 24 hours, are frequently associated with nonprodromal pain or burning, and resolve with hyperpigmentation. Arthralgia and gastrointestinal, renal, pulmonary, cardiac, and neurologic symptoms may be present, especially in patients with low complement levels.10 Skin biopsy demonstrates leukocytoclasia that must be accompanied by vessel wall necrosis. Fibrinoid deposition, erythrocyte extravasation, or perivascular inflammation may be present. In 70% of cases revealing perivascular immunoglobulin, C3, and fibrinogen deposition, DIF is positive. Serum C1q autoantibody may be associated with the hypocomplementemic form.10
The classic histopathologic findings in leukocytoclastic vasculitis include transmural neutrophilic infiltration of the walls of small vessels, fibrinoid necrosis of vessel walls, leukocytoclasia, extravasated erythrocytes, and signs of endothelial cell damage.9 A prior punch biopsy in this patient demonstrated rare neutrophilic nuclear debris within the vessel walls without fibrin deposition. Although the presence of nuclear debris and extravasated erythrocytes could be compatible with a manifestation of urticarial vasculitis, the lack of direct evidence of vessel wall necrosis combined with subsequent biopsies unequivocally ruled out cutaneous small vessel vasculitis in our patient.
Psychogenic purpura has been reported to occur frequently in the background of psycho-emotional distress. In 1989, Ratnoff11 noted that many of the patients he was treating at the University Hospitals of Cleveland, Ohio, had a depressive syndrome. A review of patients treated at the Mayo Clinic in Rochester, Minnesota, illustrated concomitant psychiatric illnesses in 41 of 76 (54%) patients treated for PP, most commonly depressive, personality, and anxiety disorders.3
There is no consensus on therapy for PP. Treatment is based on providing symptomatic relief and relieving underlying psychiatric distress. Block et al12 found the use of selective serotonin reuptake inhibitors, tricyclic antidepressants, and psychotherapy to be successful in improving symptoms and reducing lesions at follow-up visits.
To the Editor:
A 14-year-old Black adolescent girl presented with episodic, painful, edematous plaques that occurred symmetrically on the arms and legs of 5 years’ duration. The plaques evolved into hyperpigmented patches within 24 to 48 hours before eventually resolving. Fatigue, headache, arthralgias of the arms and legs, chest pain, abdominal pain, nausea, and vomiting variably accompanied these episodes.
Prior to visiting our clinic, the patient had been seen by numerous specialists. A review of her medical records revealed an initial diagnosis of Henoch-Schönlein purpura (HSP), then urticarial vasculitis. She had been treated with antihistamines, topical and systemic steroids, hydroxychloroquine, mycophenolate mofetil, dapsone, azathioprine, and gabapentin. All treatments were ineffectual. She underwent extensive diagnostic testing and imaging, which were normal or noncontributory, including type I allergy testing; multiple exhaustive batteries of hematologic testing; and computed tomography/magnetic resonance imaging/magnetic resonance angiography of the brain, chest, abdomen, and pelvic region. Biopsies from symptomatic segments of the gastrointestinal tract were normal.
Chronic treatment with systemic steroids over 9 months resulted in gastritis and an episode of hematemesis requiring emergent hospitalization. A lengthy multidisciplinary evaluation was conducted at the patient’s local community hospital; the team concluded that she had an urticarial-type rash with accompanying symptoms that did not have an autoimmune, rheumatologic, or inflammatory basis.
The patient’s medical history was remarkable for recent-onset panic attacks. Her family medical history was noncontributory. Physical examination revealed multiple violaceous hyperpigmented patches diffusely located on the proximal upper arms (Figure 1). There were no additional findings on physical examination.

Punch biopsies were performed on lesional areas of the arm. Histopathology indicated a mild superficial perivascular dermal mixed infiltrate and extravasated erythrocytes (Figure 2). Direct immunofluorescence (DIF) testing was negative for vasculitis. Immunohistochemical stains for CD117 and tryptase demonstrated a slight increase in the number of dermal mast cells; however, the increase was not sufficient to diagnose cutaneous mastocytosis, which was in the differential. We proposed a diagnosis of psychogenic purpura (PP)(also known as Gardner-Diamond syndrome). She was treated with gabapentin, a selective serotonin reuptake inhibitor, and cognitive therapy. Unfortunately, after starting therapy the patient was lost to follow-up.

Psychogenic purpura is a rare vasculopathy of unknown etiology that may be a special form of factitious disorder.1,2 In one study, PP occurred predominantly in females aged 15 to 66 years, with a median onset age of 33 years.3 A prodrome of localized itching, burning, and/or pain precedes the development of edematous plaques. The plaques evolve into painful ecchymoses within 1 to 2 days and resolve in 10 days or fewer without treatment. Lesions most commonly occur on the extremities but may occur anywhere on the body. The most common associated finding is an underlying depressive disorder. Episodes may be accompanied by headache, dizziness, fatigue, fever, arthralgia, nausea, vomiting, abdominal pain, menstrual irregularities, myalgia, and urologic conditions.
In 1955, Gardner and Diamond4 described the first cases of PP in 4 female patients at Peter Bent Brigham Hospital in Boston, Massachusetts. The investigators were able to replicate the painful ecchymoses with intradermal injection of the patient’s own erythrocytes into the skin. They proposed that the underlying pathogenesis involved autosensitization to erythrocyte stroma.4 Since then, others have suggested that the pathogenesis may include autosensitization to erythrocyte phosphatidylserine, tonus dysregulation of venous capillaries, abnormal endothelial fibrin synthesis, and capillary wall instability.5-7
Histopathology typically reveals superficial and deep perivascular inflammation with extravasated erythrocytes. Direct immunofluorescence is negative for vasculitis.8 Diagnostics and laboratory findings for underlying systemic illness are negative or noncontributory. Cutaneous injection of 1 mL of the patient’s own washed erythrocytes may result in the formation of the characteristic painful plaques within 24 hours; however, this test is limited by lack of standardization and low sensitivity.3
Psychogenic purpura may share clinical features with cutaneous small vessel vasculitis, such as HSP or urticarial vasculitis. Some of the findings that our patient was experiencing, including purpura, arthralgia, and abdominal pain, are associated with HSP. However, HSP typically is self-limiting and classically features palpable purpura distributed across the lower extremities and buttocks. Histopathology demonstrates the classic findings of leukocytoclastic vasculitis; DIF typically is positive for perivascular IgA and C3 deposition. Increased serum IgA may be present.9 Urticarial vasculitis appears as erythematous indurated wheals that favor a proximal extremity and truncal distribution. They characteristically last longer than 24 hours, are frequently associated with nonprodromal pain or burning, and resolve with hyperpigmentation. Arthralgia and gastrointestinal, renal, pulmonary, cardiac, and neurologic symptoms may be present, especially in patients with low complement levels.10 Skin biopsy demonstrates leukocytoclasia that must be accompanied by vessel wall necrosis. Fibrinoid deposition, erythrocyte extravasation, or perivascular inflammation may be present. In 70% of cases revealing perivascular immunoglobulin, C3, and fibrinogen deposition, DIF is positive. Serum C1q autoantibody may be associated with the hypocomplementemic form.10
The classic histopathologic findings in leukocytoclastic vasculitis include transmural neutrophilic infiltration of the walls of small vessels, fibrinoid necrosis of vessel walls, leukocytoclasia, extravasated erythrocytes, and signs of endothelial cell damage.9 A prior punch biopsy in this patient demonstrated rare neutrophilic nuclear debris within the vessel walls without fibrin deposition. Although the presence of nuclear debris and extravasated erythrocytes could be compatible with a manifestation of urticarial vasculitis, the lack of direct evidence of vessel wall necrosis combined with subsequent biopsies unequivocally ruled out cutaneous small vessel vasculitis in our patient.
Psychogenic purpura has been reported to occur frequently in the background of psycho-emotional distress. In 1989, Ratnoff11 noted that many of the patients he was treating at the University Hospitals of Cleveland, Ohio, had a depressive syndrome. A review of patients treated at the Mayo Clinic in Rochester, Minnesota, illustrated concomitant psychiatric illnesses in 41 of 76 (54%) patients treated for PP, most commonly depressive, personality, and anxiety disorders.3
There is no consensus on therapy for PP. Treatment is based on providing symptomatic relief and relieving underlying psychiatric distress. Block et al12 found the use of selective serotonin reuptake inhibitors, tricyclic antidepressants, and psychotherapy to be successful in improving symptoms and reducing lesions at follow-up visits.
- Piette WW. Purpura: mechanisms and differential diagnosis. In: Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier; 2018:376-389.
- Harth W, Taube KM, Gieler U. Factitious disorders in dermatology. J Dtsch Dermatol Ges. 2010;8:361-372.
- Sridharan M, Ali U, Hook CC, et al. The Mayo Clinic experience with psychogenic purpura (Gardner-Diamond syndrome). Am J Med Sci. 2019;357:411‐420.
- Gardner FH, Diamond LK. Autoerythrocyte sensitization; a form of purpura producing painful bruising following autosensitization to red blood cells in certain women. Blood. 1955;10:675-690.
- Groch GS, Finch SC, Rogoway W, et al. Studies in the pathogenesis of autoerythrocyte sensitization syndrome. Blood. 1966;28:19-33.
- Strunecká A, Krpejsová L, Palecek J, et al. Transbilayer redistribution of phosphatidylserine in erythrocytes of a patient with autoerythrocyte sensitization syndrome (psychogenic purpura). Folia Haematol Int Mag Klin Morphol Blutforsch. 1990;117:829-841.
- Merlen JF. Ecchymotic patches of the fingers and Gardner-Diamond vascular purpura. Phlebologie. 1987;40:473-487.
- Ivanov OL, Lvov AN, Michenko AV, et al. Autoerythrocyte sensitization syndrome (Gardner-Diamond syndrome): review of the literature. J Eur Acad Dermatol Venereol. 2009;23:499-504.
- Wetter DA, Dutz JP, Shinkai K, et al. Cutaneous vasculitis. In: Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier; 2018:409-439.
- Hamad A, Jithpratuck W, Krishnaswamy G. Urticarial vasculitis and associated disorders. Ann Allergy Asthma Immunol. 2017;118:394-398.
- Ratnoff OD. Psychogenic purpura (autoerythrocyte sensitization): an unsolved dilemma. Am J Med. 1989;87:16N-21N.
- Block ME, Sitenga JL, Lehrer M, et al. Gardner‐Diamond syndrome: a systematic review of treatment options for a rare psychodermatological disorder. Int J Dermatol. 2019;58:782-787.
- Piette WW. Purpura: mechanisms and differential diagnosis. In: Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier; 2018:376-389.
- Harth W, Taube KM, Gieler U. Factitious disorders in dermatology. J Dtsch Dermatol Ges. 2010;8:361-372.
- Sridharan M, Ali U, Hook CC, et al. The Mayo Clinic experience with psychogenic purpura (Gardner-Diamond syndrome). Am J Med Sci. 2019;357:411‐420.
- Gardner FH, Diamond LK. Autoerythrocyte sensitization; a form of purpura producing painful bruising following autosensitization to red blood cells in certain women. Blood. 1955;10:675-690.
- Groch GS, Finch SC, Rogoway W, et al. Studies in the pathogenesis of autoerythrocyte sensitization syndrome. Blood. 1966;28:19-33.
- Strunecká A, Krpejsová L, Palecek J, et al. Transbilayer redistribution of phosphatidylserine in erythrocytes of a patient with autoerythrocyte sensitization syndrome (psychogenic purpura). Folia Haematol Int Mag Klin Morphol Blutforsch. 1990;117:829-841.
- Merlen JF. Ecchymotic patches of the fingers and Gardner-Diamond vascular purpura. Phlebologie. 1987;40:473-487.
- Ivanov OL, Lvov AN, Michenko AV, et al. Autoerythrocyte sensitization syndrome (Gardner-Diamond syndrome): review of the literature. J Eur Acad Dermatol Venereol. 2009;23:499-504.
- Wetter DA, Dutz JP, Shinkai K, et al. Cutaneous vasculitis. In: Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier; 2018:409-439.
- Hamad A, Jithpratuck W, Krishnaswamy G. Urticarial vasculitis and associated disorders. Ann Allergy Asthma Immunol. 2017;118:394-398.
- Ratnoff OD. Psychogenic purpura (autoerythrocyte sensitization): an unsolved dilemma. Am J Med. 1989;87:16N-21N.
- Block ME, Sitenga JL, Lehrer M, et al. Gardner‐Diamond syndrome: a systematic review of treatment options for a rare psychodermatological disorder. Int J Dermatol. 2019;58:782-787.
PRACTICE POINTS
- Psychogenic purpura is a rare vasculopathy characterized by painful recurrent episodes of purpura. It is a diagnosis of exclusion that may manifest with signs similar to cutaneous small vessel vasculitis.
- Awareness of this condition could help prevent unnecessary diagnostics, medications, and adverse events.
Endoscopic Management of Benign Gallbladder Disease
Introduction
The treatment of benign gallbladder disease has changed substantially in the past decade, but this represents only a snapshot in the evolutionary history of the management of this organ. What began as a problem managed exclusively by open cholecystectomy (CCY) transitioned into a race toward minimally invasive approaches in the 1980s, with advances from gastroenterology, surgery, and radiology.
The opening strides were made in 1980 with the first description of percutaneous cholecystostomy (PC) by Dr. R.W. Radder.1 Shortly thereafter, in 1984, Dr. Richard Kozarek first reported the feasibility of selective cystic duct cannulation during endoscopic retrograde cholangiopancreatography (ERCP).2 Subsequent stenting for the treatment of acute cholecystitis (endoscopic transpapillary gallbladder drainage, ET-GBD) was then reported by Tamada et. al. in 1991.3 Not to be outdone, the first laparoscopic cholecystectomy (LC) was completed by Dr. Med Erich Mühe of Germany in 1985.4 More recently, with the expansion of interventional endoscopic ultrasound (EUS), the first transmural EUS-guided gallbladder drainage (EUS-GBD) was described by Dr. Baron and Dr. Topazian in 2007.5
The subsequent advent of lumen apposing metal stents (LAMS) has cemented EUS-GBD in the toolbox of treatment for benign gallbladder disease. Results of a recent prospective multicenter trial, with a Food and Drug Administration–approved protocol and investigational device exemption, have been published, opening the door for the expansion of FDA approved indications for this device.6
Benign gallbladder disease encompasses both polyps (benign and premalignant) and cholecystitis (acute/chronic, calculous/acalculous), in addition to others. The four management techniques (LC, PC, ET-GBD, and EUS-GBD) have filled integral niches in the management of these patients. Even gallbladder polyps have not been able to escape the reach of endoscopic approaches with the recent description of LAMS-assisted polypectomy as part of a gallbladder preserving strategy.7,8 While EUS-GBD also has been used for biliary decompression in the presence of a patent cystic duct and absence of cholecystitis, .9 Both of these techniques have gained wide recognition and/or guideline support for their use from the American Society for Gastrointestinal Endoscopy (ASGE) and the European Society of Gastrointestinal Endoscopy (ESGE).10,11 In addition, there is now one FDA-approved stent device for treatment of acute cholecystitis in patients unfit for surgery.
Techniques & Tips
ET-GBD
- During ERCP, after successful cannulation of the bile duct, attempted wire cannulation of the cystic duct is performed.
A cholangiogram, which clearly delineates the insertion of the cystic duct into the main bile duct, can enhance cannulation success. Rotatable fluoroscopy can facilitate identification.
- After anatomy is clear, wire access is often best achieved using a sphincterotome or stone retrieval (occlusion) balloon.
The balloon, once inflated, can be pulled downward to establish traction on the main bile duct, which can straighten the approach.
- After superficial wire engagement into the cystic duct, the accessory used can be slowly advanced into the cystic duct to stabilize the catheter and then navigate the valves of Heister to reach the gallbladder lumen.
Use of a sphincterotome, which directs toward the patient’s right (most often direction of cystic duct takeoff), is helpful. Angled guidewires are preferable. We often use a 0.035-inch, 260-cm angled hydrophilic wire (GLIDEWIRE; Terumo, Somerset, NJ) to overcome this challenging portion of ET-GBD.
If despite the above maneuvers the guidewire has failed to enter the cystic duct, cholangioscopy can be used to identify the orifice and/or stabilize deep wire cannulation. This is often cumbersome, time consuming, does not always produce success, and requires additional expertise.
- If a stone is encountered that cannot be extracted or traversed by a guidewire, cholangioscopy with electrohydraulic lithotripsy can be pursued.
- After the guidewire has entered the gallbladder, a 5 French or 7 French plastic double pigtail stent is placed. Typical lengths are 9-15 cm.
Some authors prefer to use two side-by-side plastic stents.12 This has been shown retrospectively to enhance the long term clinical success of ET-GBD but with additional technical difficulty.
- This stent can remain in place indefinitely and need not be exchanged, though it should be removed just prior to CCY if pursued. Alternatively, the surgeon can be alerted to its presence and, if comfortable, it can be removed intraoperatively.
EUS-GBD
- Use of fluoroscopy is optional but can enhance technical success in selected situations.
- Conversion, or internalization, of PC is reasonable and can enhance patient quality of life.13
- If the gallbladder wall is not in close apposition to the duodenal (or gastric) wall, consider measuring the distance.
We preferentially use 10-mm diameter by 10-mm saddle length LAMS for EUS-GBD, unless the above distance warrants use of a 15-mm by 15-mm LAMS (AXIOS, Boston Scientific, Marlborough, MA). If the distance is greater than 15 mm, consider searching for an alternative site, using a traditional biliary fully covered self-expandable metal stent (FCSEMS) for longer length, or converting to ET-GBD. Smaller diameter (8 mm) with an 8-mm saddle length can be used as well. The optimal diameter is unknown and also dependent on whether transluminal endoscopic diagnosis or therapy is a consideration.
- If there is difficulty locating the gallbladder, it may be decompressed or small (particularly if PC or a partial CCY has already been performed).
If a cholecystostomy tube is in place, instillation of sterile water via the tube can sometimes improve the target for LAMS placement, though caution should be made to not over-distend the gallbladder. ERCP with placement of a nasobiliary tube into the gallbladder can also serve this purpose and has been previously described.14
The gallbladder can be punctured with a 19-gauge FNA needle to instill sterile water and distend the gallbladder with the added benefit of being able to pass a guidewire, which may enhance procedural safety in difficult cases. However, success of this technique is contingent on fluid remaining within the gallbladder and not transiting out via the cystic duct. Expedient exchange of the FNA needle for the LAMS device may be necessary.
- Attempt to confirm location within the duodenum prior to puncture, as gastric origins can pose unique ramifications (i.e. potential for partial gastric outlet obstruction, obstruction of LAMS with food debris, etc.).
It can be easy to mistake an unintentional pre-pyloric position for a position within the duodenum since the working channel is behind (proximal to) the echoprobe.
- Turning off Doppler flow prior to advancement of the cautery enhanced LAMS can reduce obscurement of views on entry into the gallbladder. Lack of certainty about entry or misdeployment after presumed entry herald the most challenging aspect of EUS-GBD.
Utilization of a previously placed guidewire or advancement of one preloaded into the LAMS can aid in both enhancing confidence in location and assist with salvage maneuvers, if needed.
- After successful deployment of the LAMS we routinely place a double pigtail plastic stent through it (typically 7 French by 4 cm) to maintain patency. This may also prevent bleeding from the LAMS flange abrading the wall of either lumen.
- We routinely exchange the LAMS for two double pigtail plastic stents (typically 7 French by 4 cm) 4 weeks after initial placement especially when there is a more than modest residual stone burden (data in press). These plastic stents can remain in place indefinitely.
This exchange can be deferred if the patient is not expected to survive until the one-year anniversary of LAMS deployment. After one year the LAMS plastic covering may degrade and pose additional problems.15
LAMS Misdeployment Salvage Tips
- Salvage techniques can vary from simple to complex.
- If a wire is in place, it can be used to balloon or catheter dilate the tract and place a FCSEMS traversing the gallbladder and duodenal/gastric lumens. A similar approach can be used if the LAMS deployed on only one side (gallbladder or duodenum/stomach) and the other flange is within the peritoneum.
- The most challenging scenario to salvage is if the LAMS is misdeployed or becomes dislodged and no wire is present. This is why the use of a guidewire, even if preloaded into the LAMS and placement is freehand, is essential for EUS-GBD. A potential technique is to balloon dilate the duodenal/gastric defect and drive the endoscope into the peritoneum to reconnect that lumen to the gallbladder defect or LAMS, depending on the site of misdeployment. Doing so requires a high degree of commitment and skill and should not be done casually.
- If uncertainty remains or if misdeployment has occurred and salvage attempts have failed, consider closure of the duodenal/gastric defect and conversion to ET-GBD.
This may both treat the initial procedural indication and assist with what is essentially a large bile leak, which might also require percutaneous therapy for non-surgical management.
- For endoscopists with limited experience at salvage techniques, it is reasonable for the threshold for conversion to be low, assuming experience with and confidence in ET-GBD is high.
- If salvage is successful but ambiguity remains, consider obtaining a cholangiogram via the LAMS to confirm positioning and absence of leak.
Adverse Events
Both ET-GBD and EUS-GBD should be performed by an endoscopist comfortable with their techniques and the management of their adverse events (AEs). Rates for EUS-GBD AEs in patients at high risk for LC were reported in one international multicenter registry to be 15.3% with a 30-day mortality of 9.2%, with a significant predictor of AE being endoscopist experience less than 25 procedures.16 A meta-analysis also found an overall AE rate of 18.31%, with rates for perforation and stent related AEs (i.e. migration, occlusion, pneumoperitoneum) being 6.71% and 8.16%, respectively.17 For this reason, we recommend that patients with cholecystitis who are deemed to be poor surgical candidates be transferred to a tertiary referral center with expertise in these approaches. Rates of AEs for ET-GBD are similar to that for standard ERCP, with reported ranges of 5%-10.3%.10
Comparisons Between Techniques
The decision on which technique to utilize for endoscopic management of cholecystitis or symptomatic cholelithiasis depends first and foremost on the expertise and comfort level of the endoscopist. Given the additional training that an advanced endoscopist needs to perform EUS-GBD, combined with the perhaps slightly higher AE rate and permanency of endoscopic cholecystostomy, it is reasonable to proceed with a trial of ET-GBD if confidence is insufficient. However, ET-GBD can certainly be more technically challenging and less effective than EUS-GBD, with lower reported technical and clinical success rates (technical 85.3% vs 93.0%, clinical 95.2% vs 97.3%).18 Despite this, the rate of recurrence of cholecystitis is similar between ET-GBD and EUS-GBD (4.6% vs 4.2%).19 As stated above in the Techniques & Tips section, some authors utilize two plastic stents for ET-GBD for this purpose, though with increased technical difficulty. It is important to remember that these numbers, when paired with AE rates, represent the achievements of expert endoscopists.
Discussion with your surgery team is important when deciding modality. If the patient is felt to be a potential candidate for CCY, and EUS-GBD is not being used as a destination therapy, the surgeon may prefer ET-GBD. EUS-GBD may enhance the difficulty of CCY, though at least one study demonstrated that this was no different than PC with similar rates of conversion from LC to open CCY.20 This conversation is most critical for patients who are potential liver transplant candidates. For patients where this is not a consideration there is some evidence to suggest equivalency between LC and EUS-GBD, though certainly EUS-GBD has not yet supplanted LC as the treatment of choice.21
While there may eventually be a shift towards EUS-GBD instead of LC in certain patient groups, what is clearer are the advantages of EUS-GBD over PC. One recent meta-analysis revealed that EUS-GBD has significantly favorable odds of overall adverse events (OR 0.43, 95% CI 0.18-1.00), shorter hospital stay (2.76 less days, 95% CI 0.31-5.20 less days), reinterventions (OR 0.15, 95% CI 0.02-0.98), and unplanned readmissions (OR 0.14, 95% CI 0.03-0.70) compared to PC.22 Beyond the data, though, are the emotional and psychological impacts an external drain can have on a patient.
Conclusion
When expertise is available, endoscopic treatment of benign gallbladder disease has a definite role but should be undertaken only by those with the experience and skill to safely do so. Decision to proceed, especially with EUS-GBD, should be accompanied by conversation and collaboration with surgical teams. If a patient is under consideration for PC instead of LC, it may be worthwhile to seek consultation with a local center with expertise in EUS-GBD or ET-GBD. The adoption of these techniques is part of the paradigm shift, seen broadly throughout medicine, towards minimally invasive interventions, particularly in advanced endoscopy.
Dr. Gilman (X @a_gilman) and Dr. Baron (X @EndoTx) are with the University of North Carolina, Chapel Hill, Division of Gastroenterology & Hepatology. Dr. Gilman has no relevant financial disclosures. Dr. Baron is a consultant and speaker for Ambu, Boston Scientific, Cook Endoscopy, Medtronic, Olympus America, and W.L. Gore.
References
1. Radder RW. Ultrasonically guided percutaneous catheter drainage for gallbladder empyema. Diagn Imaging. 1980;49:330-333.
2. Kozarek RA. Selective cannulation of the cystic duct at time of ERCP. J Clin Gastroenterol. 1984;6:37-40.
3. Tamada K et al. Efficacy of endoscopic retrograde cholecystoendoprosthesis (ERCCE) for cholecystitis. Endoscopy. 1991;23:2-3.
4. Reynolds W. The first laparoscopic cholecystectomy. JSLS. 2001;5:89-94.
5. Baron TH, Topazian MD. Endoscopic transduodenal drainage of the gallbladder: Implications for endoluminal treatment of gallbladder disease. Gastrointest Endosc. 2007 Apr;65(4):735-7. doi: 10.1016/j.gie.2006.07.041.
6. Irani SS et al. Endoscopic ultrasound-guided transluminal gallbladder drainage in patients with acute cholecystitis: A prospective multicenter trial. Ann Surg. 2023 Sep 1;278(3):e556-e562. doi: 10.1097/SLA.0000000000005784.
7. Shen Y et al. Endoscopic ultrasound-guided cholecystostomy for resection of gallbladder polyps with lumen-apposing metal stent. Medicine (Baltimore). 2020 Oct 23;99(43):e22903. doi: 10.1097/MD.0000000000022903.
8. Pang H et al. Endoscopic ultrasound-guided gallbladder endoscopic mucosal resection: A pilot porcine study. Minim Invasive Ther Allied Technol. 2023 Feb;32(1):24-32. doi: 10.1080/13645706.2022.2153228.
9. Imai H et al. EUS-guided gallbladder drainage for rescue treatment of malignant distal biliary obstruction after unsuccessful ERCP. Gastrointest Endosc. 2016 Jul;84(1):147-51. doi: 10.1016/j.gie.2015.12.024.
10. Saumoy M et al. Endoscopic therapies for gallbladder drainage. Gastrointest Endosc. 2021 Oct;94(4):671-84. doi: 10.1016/j.gie.2021.05.031.
11. Van der Merwe SW et al. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022 Feb;54(2):185-205. doi: 10.1055/a-1717-1391.
12. Storm AC et al. Transpapillary gallbladder stent placement for long-term therapy of acute cholecystitis. Gastrointest Endosc. 2021 Oct;94(4):742-8 e1. doi: 10.1016/j.gie.2021.03.025.
13. James TW, Baron TH. Converting percutaneous gallbladder drainage to internal drainage using EUS-guided therapy: A review of current practices and procedures. Endosc Ultrasound. 2018 Mar-Apr;7(2):93-6. doi: 10.4103/eus.eus_110_17.
14. James TW, Baron TH. Transpapillary nasocystic tube placement to allow gallbladder distention for EUS-guided cholecystoduodenostomy. VideoGIE. 2019 Dec;4(12):561-2. doi: 10.1016/j.vgie.2019.08.009.
15. Gilman AJ, Baron TH. Delamination of a lumen-apposing metal stent with tissue ingrowth and stent-in-stent removal. Gastrointest Endosc. 2023 Sep;98(3):451-3. doi: 10.1016/j.gie.2023.04.2087.
16. Teoh AY et al. Outcomes of an international multicenter registry on EUS-guided gallbladder drainage in patients at high risk for cholecystectomy. Endosc Int Open. 2019 Aug;7(8):E964-E973. doi: 10.1055/a-0915-2098.
17. Kalva NR et al. Efficacy and safety of lumen apposing self-expandable metal stents for EUS guided cholecystostomy: A meta-analysis and systematic review. Can J Gastroenterol Hepatol. 2018;2018:7070961. doi: 10.1155/2018/7070961.
18. Khan MA et al. Efficacy and safety of endoscopic gallbladder drainage in acute cholecystitis: Is it better than percutaneous gallbladder drainage? Gastrointest Endosc. 2017 Jan;85(1):76-87 e3. doi: 10.1016/j.gie.2016.06.032.
19. Mohan BP et al. Endoscopic ultrasound-guided gallbladder drainage, transpapillary drainage, or percutaneous drainage in high risk acute cholecystitis patients: a systematic review and comparative meta-analysis. Endoscopy. 2020 Feb;52(2):96-106. doi: 10.1055/a-1020-3932.
20. Jang JW et al. Endoscopic ultrasound-guided transmural and percutaneous transhepatic gallbladder drainage are comparable for acute cholecystitis. Gastroenterology. 2012 Apr;142(4):805-11. doi: 10.1053/j.gastro.2011.12.051.
21. Teoh AYB et al. EUS-guided gallbladder drainage versus laparoscopic cholecystectomy for acute cholecystitis: a propensity score analysis with 1-year follow-up data. Gastrointest Endosc. 2021 Mar;93(3):577-83. doi: 10.1016/j.gie.2020.06.066.
22. Luk SW et al. Endoscopic ultrasound-guided gallbladder drainage versus percutaneous cholecystostomy for high risk surgical patients with acute cholecystitis: a systematic review and meta-analysis. Endoscopy. 2019 Aug;51(8):722-32. doi: 10.1055/a-0929-6603.
Introduction
The treatment of benign gallbladder disease has changed substantially in the past decade, but this represents only a snapshot in the evolutionary history of the management of this organ. What began as a problem managed exclusively by open cholecystectomy (CCY) transitioned into a race toward minimally invasive approaches in the 1980s, with advances from gastroenterology, surgery, and radiology.
The opening strides were made in 1980 with the first description of percutaneous cholecystostomy (PC) by Dr. R.W. Radder.1 Shortly thereafter, in 1984, Dr. Richard Kozarek first reported the feasibility of selective cystic duct cannulation during endoscopic retrograde cholangiopancreatography (ERCP).2 Subsequent stenting for the treatment of acute cholecystitis (endoscopic transpapillary gallbladder drainage, ET-GBD) was then reported by Tamada et. al. in 1991.3 Not to be outdone, the first laparoscopic cholecystectomy (LC) was completed by Dr. Med Erich Mühe of Germany in 1985.4 More recently, with the expansion of interventional endoscopic ultrasound (EUS), the first transmural EUS-guided gallbladder drainage (EUS-GBD) was described by Dr. Baron and Dr. Topazian in 2007.5
The subsequent advent of lumen apposing metal stents (LAMS) has cemented EUS-GBD in the toolbox of treatment for benign gallbladder disease. Results of a recent prospective multicenter trial, with a Food and Drug Administration–approved protocol and investigational device exemption, have been published, opening the door for the expansion of FDA approved indications for this device.6
Benign gallbladder disease encompasses both polyps (benign and premalignant) and cholecystitis (acute/chronic, calculous/acalculous), in addition to others. The four management techniques (LC, PC, ET-GBD, and EUS-GBD) have filled integral niches in the management of these patients. Even gallbladder polyps have not been able to escape the reach of endoscopic approaches with the recent description of LAMS-assisted polypectomy as part of a gallbladder preserving strategy.7,8 While EUS-GBD also has been used for biliary decompression in the presence of a patent cystic duct and absence of cholecystitis, .9 Both of these techniques have gained wide recognition and/or guideline support for their use from the American Society for Gastrointestinal Endoscopy (ASGE) and the European Society of Gastrointestinal Endoscopy (ESGE).10,11 In addition, there is now one FDA-approved stent device for treatment of acute cholecystitis in patients unfit for surgery.
Techniques & Tips
ET-GBD
- During ERCP, after successful cannulation of the bile duct, attempted wire cannulation of the cystic duct is performed.
A cholangiogram, which clearly delineates the insertion of the cystic duct into the main bile duct, can enhance cannulation success. Rotatable fluoroscopy can facilitate identification.
- After anatomy is clear, wire access is often best achieved using a sphincterotome or stone retrieval (occlusion) balloon.
The balloon, once inflated, can be pulled downward to establish traction on the main bile duct, which can straighten the approach.
- After superficial wire engagement into the cystic duct, the accessory used can be slowly advanced into the cystic duct to stabilize the catheter and then navigate the valves of Heister to reach the gallbladder lumen.
Use of a sphincterotome, which directs toward the patient’s right (most often direction of cystic duct takeoff), is helpful. Angled guidewires are preferable. We often use a 0.035-inch, 260-cm angled hydrophilic wire (GLIDEWIRE; Terumo, Somerset, NJ) to overcome this challenging portion of ET-GBD.
If despite the above maneuvers the guidewire has failed to enter the cystic duct, cholangioscopy can be used to identify the orifice and/or stabilize deep wire cannulation. This is often cumbersome, time consuming, does not always produce success, and requires additional expertise.
- If a stone is encountered that cannot be extracted or traversed by a guidewire, cholangioscopy with electrohydraulic lithotripsy can be pursued.
- After the guidewire has entered the gallbladder, a 5 French or 7 French plastic double pigtail stent is placed. Typical lengths are 9-15 cm.
Some authors prefer to use two side-by-side plastic stents.12 This has been shown retrospectively to enhance the long term clinical success of ET-GBD but with additional technical difficulty.
- This stent can remain in place indefinitely and need not be exchanged, though it should be removed just prior to CCY if pursued. Alternatively, the surgeon can be alerted to its presence and, if comfortable, it can be removed intraoperatively.
EUS-GBD
- Use of fluoroscopy is optional but can enhance technical success in selected situations.
- Conversion, or internalization, of PC is reasonable and can enhance patient quality of life.13
- If the gallbladder wall is not in close apposition to the duodenal (or gastric) wall, consider measuring the distance.
We preferentially use 10-mm diameter by 10-mm saddle length LAMS for EUS-GBD, unless the above distance warrants use of a 15-mm by 15-mm LAMS (AXIOS, Boston Scientific, Marlborough, MA). If the distance is greater than 15 mm, consider searching for an alternative site, using a traditional biliary fully covered self-expandable metal stent (FCSEMS) for longer length, or converting to ET-GBD. Smaller diameter (8 mm) with an 8-mm saddle length can be used as well. The optimal diameter is unknown and also dependent on whether transluminal endoscopic diagnosis or therapy is a consideration.
- If there is difficulty locating the gallbladder, it may be decompressed or small (particularly if PC or a partial CCY has already been performed).
If a cholecystostomy tube is in place, instillation of sterile water via the tube can sometimes improve the target for LAMS placement, though caution should be made to not over-distend the gallbladder. ERCP with placement of a nasobiliary tube into the gallbladder can also serve this purpose and has been previously described.14
The gallbladder can be punctured with a 19-gauge FNA needle to instill sterile water and distend the gallbladder with the added benefit of being able to pass a guidewire, which may enhance procedural safety in difficult cases. However, success of this technique is contingent on fluid remaining within the gallbladder and not transiting out via the cystic duct. Expedient exchange of the FNA needle for the LAMS device may be necessary.
- Attempt to confirm location within the duodenum prior to puncture, as gastric origins can pose unique ramifications (i.e. potential for partial gastric outlet obstruction, obstruction of LAMS with food debris, etc.).
It can be easy to mistake an unintentional pre-pyloric position for a position within the duodenum since the working channel is behind (proximal to) the echoprobe.
- Turning off Doppler flow prior to advancement of the cautery enhanced LAMS can reduce obscurement of views on entry into the gallbladder. Lack of certainty about entry or misdeployment after presumed entry herald the most challenging aspect of EUS-GBD.
Utilization of a previously placed guidewire or advancement of one preloaded into the LAMS can aid in both enhancing confidence in location and assist with salvage maneuvers, if needed.
- After successful deployment of the LAMS we routinely place a double pigtail plastic stent through it (typically 7 French by 4 cm) to maintain patency. This may also prevent bleeding from the LAMS flange abrading the wall of either lumen.
- We routinely exchange the LAMS for two double pigtail plastic stents (typically 7 French by 4 cm) 4 weeks after initial placement especially when there is a more than modest residual stone burden (data in press). These plastic stents can remain in place indefinitely.
This exchange can be deferred if the patient is not expected to survive until the one-year anniversary of LAMS deployment. After one year the LAMS plastic covering may degrade and pose additional problems.15
LAMS Misdeployment Salvage Tips
- Salvage techniques can vary from simple to complex.
- If a wire is in place, it can be used to balloon or catheter dilate the tract and place a FCSEMS traversing the gallbladder and duodenal/gastric lumens. A similar approach can be used if the LAMS deployed on only one side (gallbladder or duodenum/stomach) and the other flange is within the peritoneum.
- The most challenging scenario to salvage is if the LAMS is misdeployed or becomes dislodged and no wire is present. This is why the use of a guidewire, even if preloaded into the LAMS and placement is freehand, is essential for EUS-GBD. A potential technique is to balloon dilate the duodenal/gastric defect and drive the endoscope into the peritoneum to reconnect that lumen to the gallbladder defect or LAMS, depending on the site of misdeployment. Doing so requires a high degree of commitment and skill and should not be done casually.
- If uncertainty remains or if misdeployment has occurred and salvage attempts have failed, consider closure of the duodenal/gastric defect and conversion to ET-GBD.
This may both treat the initial procedural indication and assist with what is essentially a large bile leak, which might also require percutaneous therapy for non-surgical management.
- For endoscopists with limited experience at salvage techniques, it is reasonable for the threshold for conversion to be low, assuming experience with and confidence in ET-GBD is high.
- If salvage is successful but ambiguity remains, consider obtaining a cholangiogram via the LAMS to confirm positioning and absence of leak.
Adverse Events
Both ET-GBD and EUS-GBD should be performed by an endoscopist comfortable with their techniques and the management of their adverse events (AEs). Rates for EUS-GBD AEs in patients at high risk for LC were reported in one international multicenter registry to be 15.3% with a 30-day mortality of 9.2%, with a significant predictor of AE being endoscopist experience less than 25 procedures.16 A meta-analysis also found an overall AE rate of 18.31%, with rates for perforation and stent related AEs (i.e. migration, occlusion, pneumoperitoneum) being 6.71% and 8.16%, respectively.17 For this reason, we recommend that patients with cholecystitis who are deemed to be poor surgical candidates be transferred to a tertiary referral center with expertise in these approaches. Rates of AEs for ET-GBD are similar to that for standard ERCP, with reported ranges of 5%-10.3%.10
Comparisons Between Techniques
The decision on which technique to utilize for endoscopic management of cholecystitis or symptomatic cholelithiasis depends first and foremost on the expertise and comfort level of the endoscopist. Given the additional training that an advanced endoscopist needs to perform EUS-GBD, combined with the perhaps slightly higher AE rate and permanency of endoscopic cholecystostomy, it is reasonable to proceed with a trial of ET-GBD if confidence is insufficient. However, ET-GBD can certainly be more technically challenging and less effective than EUS-GBD, with lower reported technical and clinical success rates (technical 85.3% vs 93.0%, clinical 95.2% vs 97.3%).18 Despite this, the rate of recurrence of cholecystitis is similar between ET-GBD and EUS-GBD (4.6% vs 4.2%).19 As stated above in the Techniques & Tips section, some authors utilize two plastic stents for ET-GBD for this purpose, though with increased technical difficulty. It is important to remember that these numbers, when paired with AE rates, represent the achievements of expert endoscopists.
Discussion with your surgery team is important when deciding modality. If the patient is felt to be a potential candidate for CCY, and EUS-GBD is not being used as a destination therapy, the surgeon may prefer ET-GBD. EUS-GBD may enhance the difficulty of CCY, though at least one study demonstrated that this was no different than PC with similar rates of conversion from LC to open CCY.20 This conversation is most critical for patients who are potential liver transplant candidates. For patients where this is not a consideration there is some evidence to suggest equivalency between LC and EUS-GBD, though certainly EUS-GBD has not yet supplanted LC as the treatment of choice.21
While there may eventually be a shift towards EUS-GBD instead of LC in certain patient groups, what is clearer are the advantages of EUS-GBD over PC. One recent meta-analysis revealed that EUS-GBD has significantly favorable odds of overall adverse events (OR 0.43, 95% CI 0.18-1.00), shorter hospital stay (2.76 less days, 95% CI 0.31-5.20 less days), reinterventions (OR 0.15, 95% CI 0.02-0.98), and unplanned readmissions (OR 0.14, 95% CI 0.03-0.70) compared to PC.22 Beyond the data, though, are the emotional and psychological impacts an external drain can have on a patient.
Conclusion
When expertise is available, endoscopic treatment of benign gallbladder disease has a definite role but should be undertaken only by those with the experience and skill to safely do so. Decision to proceed, especially with EUS-GBD, should be accompanied by conversation and collaboration with surgical teams. If a patient is under consideration for PC instead of LC, it may be worthwhile to seek consultation with a local center with expertise in EUS-GBD or ET-GBD. The adoption of these techniques is part of the paradigm shift, seen broadly throughout medicine, towards minimally invasive interventions, particularly in advanced endoscopy.
Dr. Gilman (X @a_gilman) and Dr. Baron (X @EndoTx) are with the University of North Carolina, Chapel Hill, Division of Gastroenterology & Hepatology. Dr. Gilman has no relevant financial disclosures. Dr. Baron is a consultant and speaker for Ambu, Boston Scientific, Cook Endoscopy, Medtronic, Olympus America, and W.L. Gore.
References
1. Radder RW. Ultrasonically guided percutaneous catheter drainage for gallbladder empyema. Diagn Imaging. 1980;49:330-333.
2. Kozarek RA. Selective cannulation of the cystic duct at time of ERCP. J Clin Gastroenterol. 1984;6:37-40.
3. Tamada K et al. Efficacy of endoscopic retrograde cholecystoendoprosthesis (ERCCE) for cholecystitis. Endoscopy. 1991;23:2-3.
4. Reynolds W. The first laparoscopic cholecystectomy. JSLS. 2001;5:89-94.
5. Baron TH, Topazian MD. Endoscopic transduodenal drainage of the gallbladder: Implications for endoluminal treatment of gallbladder disease. Gastrointest Endosc. 2007 Apr;65(4):735-7. doi: 10.1016/j.gie.2006.07.041.
6. Irani SS et al. Endoscopic ultrasound-guided transluminal gallbladder drainage in patients with acute cholecystitis: A prospective multicenter trial. Ann Surg. 2023 Sep 1;278(3):e556-e562. doi: 10.1097/SLA.0000000000005784.
7. Shen Y et al. Endoscopic ultrasound-guided cholecystostomy for resection of gallbladder polyps with lumen-apposing metal stent. Medicine (Baltimore). 2020 Oct 23;99(43):e22903. doi: 10.1097/MD.0000000000022903.
8. Pang H et al. Endoscopic ultrasound-guided gallbladder endoscopic mucosal resection: A pilot porcine study. Minim Invasive Ther Allied Technol. 2023 Feb;32(1):24-32. doi: 10.1080/13645706.2022.2153228.
9. Imai H et al. EUS-guided gallbladder drainage for rescue treatment of malignant distal biliary obstruction after unsuccessful ERCP. Gastrointest Endosc. 2016 Jul;84(1):147-51. doi: 10.1016/j.gie.2015.12.024.
10. Saumoy M et al. Endoscopic therapies for gallbladder drainage. Gastrointest Endosc. 2021 Oct;94(4):671-84. doi: 10.1016/j.gie.2021.05.031.
11. Van der Merwe SW et al. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022 Feb;54(2):185-205. doi: 10.1055/a-1717-1391.
12. Storm AC et al. Transpapillary gallbladder stent placement for long-term therapy of acute cholecystitis. Gastrointest Endosc. 2021 Oct;94(4):742-8 e1. doi: 10.1016/j.gie.2021.03.025.
13. James TW, Baron TH. Converting percutaneous gallbladder drainage to internal drainage using EUS-guided therapy: A review of current practices and procedures. Endosc Ultrasound. 2018 Mar-Apr;7(2):93-6. doi: 10.4103/eus.eus_110_17.
14. James TW, Baron TH. Transpapillary nasocystic tube placement to allow gallbladder distention for EUS-guided cholecystoduodenostomy. VideoGIE. 2019 Dec;4(12):561-2. doi: 10.1016/j.vgie.2019.08.009.
15. Gilman AJ, Baron TH. Delamination of a lumen-apposing metal stent with tissue ingrowth and stent-in-stent removal. Gastrointest Endosc. 2023 Sep;98(3):451-3. doi: 10.1016/j.gie.2023.04.2087.
16. Teoh AY et al. Outcomes of an international multicenter registry on EUS-guided gallbladder drainage in patients at high risk for cholecystectomy. Endosc Int Open. 2019 Aug;7(8):E964-E973. doi: 10.1055/a-0915-2098.
17. Kalva NR et al. Efficacy and safety of lumen apposing self-expandable metal stents for EUS guided cholecystostomy: A meta-analysis and systematic review. Can J Gastroenterol Hepatol. 2018;2018:7070961. doi: 10.1155/2018/7070961.
18. Khan MA et al. Efficacy and safety of endoscopic gallbladder drainage in acute cholecystitis: Is it better than percutaneous gallbladder drainage? Gastrointest Endosc. 2017 Jan;85(1):76-87 e3. doi: 10.1016/j.gie.2016.06.032.
19. Mohan BP et al. Endoscopic ultrasound-guided gallbladder drainage, transpapillary drainage, or percutaneous drainage in high risk acute cholecystitis patients: a systematic review and comparative meta-analysis. Endoscopy. 2020 Feb;52(2):96-106. doi: 10.1055/a-1020-3932.
20. Jang JW et al. Endoscopic ultrasound-guided transmural and percutaneous transhepatic gallbladder drainage are comparable for acute cholecystitis. Gastroenterology. 2012 Apr;142(4):805-11. doi: 10.1053/j.gastro.2011.12.051.
21. Teoh AYB et al. EUS-guided gallbladder drainage versus laparoscopic cholecystectomy for acute cholecystitis: a propensity score analysis with 1-year follow-up data. Gastrointest Endosc. 2021 Mar;93(3):577-83. doi: 10.1016/j.gie.2020.06.066.
22. Luk SW et al. Endoscopic ultrasound-guided gallbladder drainage versus percutaneous cholecystostomy for high risk surgical patients with acute cholecystitis: a systematic review and meta-analysis. Endoscopy. 2019 Aug;51(8):722-32. doi: 10.1055/a-0929-6603.
Introduction
The treatment of benign gallbladder disease has changed substantially in the past decade, but this represents only a snapshot in the evolutionary history of the management of this organ. What began as a problem managed exclusively by open cholecystectomy (CCY) transitioned into a race toward minimally invasive approaches in the 1980s, with advances from gastroenterology, surgery, and radiology.
The opening strides were made in 1980 with the first description of percutaneous cholecystostomy (PC) by Dr. R.W. Radder.1 Shortly thereafter, in 1984, Dr. Richard Kozarek first reported the feasibility of selective cystic duct cannulation during endoscopic retrograde cholangiopancreatography (ERCP).2 Subsequent stenting for the treatment of acute cholecystitis (endoscopic transpapillary gallbladder drainage, ET-GBD) was then reported by Tamada et. al. in 1991.3 Not to be outdone, the first laparoscopic cholecystectomy (LC) was completed by Dr. Med Erich Mühe of Germany in 1985.4 More recently, with the expansion of interventional endoscopic ultrasound (EUS), the first transmural EUS-guided gallbladder drainage (EUS-GBD) was described by Dr. Baron and Dr. Topazian in 2007.5
The subsequent advent of lumen apposing metal stents (LAMS) has cemented EUS-GBD in the toolbox of treatment for benign gallbladder disease. Results of a recent prospective multicenter trial, with a Food and Drug Administration–approved protocol and investigational device exemption, have been published, opening the door for the expansion of FDA approved indications for this device.6
Benign gallbladder disease encompasses both polyps (benign and premalignant) and cholecystitis (acute/chronic, calculous/acalculous), in addition to others. The four management techniques (LC, PC, ET-GBD, and EUS-GBD) have filled integral niches in the management of these patients. Even gallbladder polyps have not been able to escape the reach of endoscopic approaches with the recent description of LAMS-assisted polypectomy as part of a gallbladder preserving strategy.7,8 While EUS-GBD also has been used for biliary decompression in the presence of a patent cystic duct and absence of cholecystitis, .9 Both of these techniques have gained wide recognition and/or guideline support for their use from the American Society for Gastrointestinal Endoscopy (ASGE) and the European Society of Gastrointestinal Endoscopy (ESGE).10,11 In addition, there is now one FDA-approved stent device for treatment of acute cholecystitis in patients unfit for surgery.
Techniques & Tips
ET-GBD
- During ERCP, after successful cannulation of the bile duct, attempted wire cannulation of the cystic duct is performed.
A cholangiogram, which clearly delineates the insertion of the cystic duct into the main bile duct, can enhance cannulation success. Rotatable fluoroscopy can facilitate identification.
- After anatomy is clear, wire access is often best achieved using a sphincterotome or stone retrieval (occlusion) balloon.
The balloon, once inflated, can be pulled downward to establish traction on the main bile duct, which can straighten the approach.
- After superficial wire engagement into the cystic duct, the accessory used can be slowly advanced into the cystic duct to stabilize the catheter and then navigate the valves of Heister to reach the gallbladder lumen.
Use of a sphincterotome, which directs toward the patient’s right (most often direction of cystic duct takeoff), is helpful. Angled guidewires are preferable. We often use a 0.035-inch, 260-cm angled hydrophilic wire (GLIDEWIRE; Terumo, Somerset, NJ) to overcome this challenging portion of ET-GBD.
If despite the above maneuvers the guidewire has failed to enter the cystic duct, cholangioscopy can be used to identify the orifice and/or stabilize deep wire cannulation. This is often cumbersome, time consuming, does not always produce success, and requires additional expertise.
- If a stone is encountered that cannot be extracted or traversed by a guidewire, cholangioscopy with electrohydraulic lithotripsy can be pursued.
- After the guidewire has entered the gallbladder, a 5 French or 7 French plastic double pigtail stent is placed. Typical lengths are 9-15 cm.
Some authors prefer to use two side-by-side plastic stents.12 This has been shown retrospectively to enhance the long term clinical success of ET-GBD but with additional technical difficulty.
- This stent can remain in place indefinitely and need not be exchanged, though it should be removed just prior to CCY if pursued. Alternatively, the surgeon can be alerted to its presence and, if comfortable, it can be removed intraoperatively.
EUS-GBD
- Use of fluoroscopy is optional but can enhance technical success in selected situations.
- Conversion, or internalization, of PC is reasonable and can enhance patient quality of life.13
- If the gallbladder wall is not in close apposition to the duodenal (or gastric) wall, consider measuring the distance.
We preferentially use 10-mm diameter by 10-mm saddle length LAMS for EUS-GBD, unless the above distance warrants use of a 15-mm by 15-mm LAMS (AXIOS, Boston Scientific, Marlborough, MA). If the distance is greater than 15 mm, consider searching for an alternative site, using a traditional biliary fully covered self-expandable metal stent (FCSEMS) for longer length, or converting to ET-GBD. Smaller diameter (8 mm) with an 8-mm saddle length can be used as well. The optimal diameter is unknown and also dependent on whether transluminal endoscopic diagnosis or therapy is a consideration.
- If there is difficulty locating the gallbladder, it may be decompressed or small (particularly if PC or a partial CCY has already been performed).
If a cholecystostomy tube is in place, instillation of sterile water via the tube can sometimes improve the target for LAMS placement, though caution should be made to not over-distend the gallbladder. ERCP with placement of a nasobiliary tube into the gallbladder can also serve this purpose and has been previously described.14
The gallbladder can be punctured with a 19-gauge FNA needle to instill sterile water and distend the gallbladder with the added benefit of being able to pass a guidewire, which may enhance procedural safety in difficult cases. However, success of this technique is contingent on fluid remaining within the gallbladder and not transiting out via the cystic duct. Expedient exchange of the FNA needle for the LAMS device may be necessary.
- Attempt to confirm location within the duodenum prior to puncture, as gastric origins can pose unique ramifications (i.e. potential for partial gastric outlet obstruction, obstruction of LAMS with food debris, etc.).
It can be easy to mistake an unintentional pre-pyloric position for a position within the duodenum since the working channel is behind (proximal to) the echoprobe.
- Turning off Doppler flow prior to advancement of the cautery enhanced LAMS can reduce obscurement of views on entry into the gallbladder. Lack of certainty about entry or misdeployment after presumed entry herald the most challenging aspect of EUS-GBD.
Utilization of a previously placed guidewire or advancement of one preloaded into the LAMS can aid in both enhancing confidence in location and assist with salvage maneuvers, if needed.
- After successful deployment of the LAMS we routinely place a double pigtail plastic stent through it (typically 7 French by 4 cm) to maintain patency. This may also prevent bleeding from the LAMS flange abrading the wall of either lumen.
- We routinely exchange the LAMS for two double pigtail plastic stents (typically 7 French by 4 cm) 4 weeks after initial placement especially when there is a more than modest residual stone burden (data in press). These plastic stents can remain in place indefinitely.
This exchange can be deferred if the patient is not expected to survive until the one-year anniversary of LAMS deployment. After one year the LAMS plastic covering may degrade and pose additional problems.15
LAMS Misdeployment Salvage Tips
- Salvage techniques can vary from simple to complex.
- If a wire is in place, it can be used to balloon or catheter dilate the tract and place a FCSEMS traversing the gallbladder and duodenal/gastric lumens. A similar approach can be used if the LAMS deployed on only one side (gallbladder or duodenum/stomach) and the other flange is within the peritoneum.
- The most challenging scenario to salvage is if the LAMS is misdeployed or becomes dislodged and no wire is present. This is why the use of a guidewire, even if preloaded into the LAMS and placement is freehand, is essential for EUS-GBD. A potential technique is to balloon dilate the duodenal/gastric defect and drive the endoscope into the peritoneum to reconnect that lumen to the gallbladder defect or LAMS, depending on the site of misdeployment. Doing so requires a high degree of commitment and skill and should not be done casually.
- If uncertainty remains or if misdeployment has occurred and salvage attempts have failed, consider closure of the duodenal/gastric defect and conversion to ET-GBD.
This may both treat the initial procedural indication and assist with what is essentially a large bile leak, which might also require percutaneous therapy for non-surgical management.
- For endoscopists with limited experience at salvage techniques, it is reasonable for the threshold for conversion to be low, assuming experience with and confidence in ET-GBD is high.
- If salvage is successful but ambiguity remains, consider obtaining a cholangiogram via the LAMS to confirm positioning and absence of leak.
Adverse Events
Both ET-GBD and EUS-GBD should be performed by an endoscopist comfortable with their techniques and the management of their adverse events (AEs). Rates for EUS-GBD AEs in patients at high risk for LC were reported in one international multicenter registry to be 15.3% with a 30-day mortality of 9.2%, with a significant predictor of AE being endoscopist experience less than 25 procedures.16 A meta-analysis also found an overall AE rate of 18.31%, with rates for perforation and stent related AEs (i.e. migration, occlusion, pneumoperitoneum) being 6.71% and 8.16%, respectively.17 For this reason, we recommend that patients with cholecystitis who are deemed to be poor surgical candidates be transferred to a tertiary referral center with expertise in these approaches. Rates of AEs for ET-GBD are similar to that for standard ERCP, with reported ranges of 5%-10.3%.10
Comparisons Between Techniques
The decision on which technique to utilize for endoscopic management of cholecystitis or symptomatic cholelithiasis depends first and foremost on the expertise and comfort level of the endoscopist. Given the additional training that an advanced endoscopist needs to perform EUS-GBD, combined with the perhaps slightly higher AE rate and permanency of endoscopic cholecystostomy, it is reasonable to proceed with a trial of ET-GBD if confidence is insufficient. However, ET-GBD can certainly be more technically challenging and less effective than EUS-GBD, with lower reported technical and clinical success rates (technical 85.3% vs 93.0%, clinical 95.2% vs 97.3%).18 Despite this, the rate of recurrence of cholecystitis is similar between ET-GBD and EUS-GBD (4.6% vs 4.2%).19 As stated above in the Techniques & Tips section, some authors utilize two plastic stents for ET-GBD for this purpose, though with increased technical difficulty. It is important to remember that these numbers, when paired with AE rates, represent the achievements of expert endoscopists.
Discussion with your surgery team is important when deciding modality. If the patient is felt to be a potential candidate for CCY, and EUS-GBD is not being used as a destination therapy, the surgeon may prefer ET-GBD. EUS-GBD may enhance the difficulty of CCY, though at least one study demonstrated that this was no different than PC with similar rates of conversion from LC to open CCY.20 This conversation is most critical for patients who are potential liver transplant candidates. For patients where this is not a consideration there is some evidence to suggest equivalency between LC and EUS-GBD, though certainly EUS-GBD has not yet supplanted LC as the treatment of choice.21
While there may eventually be a shift towards EUS-GBD instead of LC in certain patient groups, what is clearer are the advantages of EUS-GBD over PC. One recent meta-analysis revealed that EUS-GBD has significantly favorable odds of overall adverse events (OR 0.43, 95% CI 0.18-1.00), shorter hospital stay (2.76 less days, 95% CI 0.31-5.20 less days), reinterventions (OR 0.15, 95% CI 0.02-0.98), and unplanned readmissions (OR 0.14, 95% CI 0.03-0.70) compared to PC.22 Beyond the data, though, are the emotional and psychological impacts an external drain can have on a patient.
Conclusion
When expertise is available, endoscopic treatment of benign gallbladder disease has a definite role but should be undertaken only by those with the experience and skill to safely do so. Decision to proceed, especially with EUS-GBD, should be accompanied by conversation and collaboration with surgical teams. If a patient is under consideration for PC instead of LC, it may be worthwhile to seek consultation with a local center with expertise in EUS-GBD or ET-GBD. The adoption of these techniques is part of the paradigm shift, seen broadly throughout medicine, towards minimally invasive interventions, particularly in advanced endoscopy.
Dr. Gilman (X @a_gilman) and Dr. Baron (X @EndoTx) are with the University of North Carolina, Chapel Hill, Division of Gastroenterology & Hepatology. Dr. Gilman has no relevant financial disclosures. Dr. Baron is a consultant and speaker for Ambu, Boston Scientific, Cook Endoscopy, Medtronic, Olympus America, and W.L. Gore.
References
1. Radder RW. Ultrasonically guided percutaneous catheter drainage for gallbladder empyema. Diagn Imaging. 1980;49:330-333.
2. Kozarek RA. Selective cannulation of the cystic duct at time of ERCP. J Clin Gastroenterol. 1984;6:37-40.
3. Tamada K et al. Efficacy of endoscopic retrograde cholecystoendoprosthesis (ERCCE) for cholecystitis. Endoscopy. 1991;23:2-3.
4. Reynolds W. The first laparoscopic cholecystectomy. JSLS. 2001;5:89-94.
5. Baron TH, Topazian MD. Endoscopic transduodenal drainage of the gallbladder: Implications for endoluminal treatment of gallbladder disease. Gastrointest Endosc. 2007 Apr;65(4):735-7. doi: 10.1016/j.gie.2006.07.041.
6. Irani SS et al. Endoscopic ultrasound-guided transluminal gallbladder drainage in patients with acute cholecystitis: A prospective multicenter trial. Ann Surg. 2023 Sep 1;278(3):e556-e562. doi: 10.1097/SLA.0000000000005784.
7. Shen Y et al. Endoscopic ultrasound-guided cholecystostomy for resection of gallbladder polyps with lumen-apposing metal stent. Medicine (Baltimore). 2020 Oct 23;99(43):e22903. doi: 10.1097/MD.0000000000022903.
8. Pang H et al. Endoscopic ultrasound-guided gallbladder endoscopic mucosal resection: A pilot porcine study. Minim Invasive Ther Allied Technol. 2023 Feb;32(1):24-32. doi: 10.1080/13645706.2022.2153228.
9. Imai H et al. EUS-guided gallbladder drainage for rescue treatment of malignant distal biliary obstruction after unsuccessful ERCP. Gastrointest Endosc. 2016 Jul;84(1):147-51. doi: 10.1016/j.gie.2015.12.024.
10. Saumoy M et al. Endoscopic therapies for gallbladder drainage. Gastrointest Endosc. 2021 Oct;94(4):671-84. doi: 10.1016/j.gie.2021.05.031.
11. Van der Merwe SW et al. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022 Feb;54(2):185-205. doi: 10.1055/a-1717-1391.
12. Storm AC et al. Transpapillary gallbladder stent placement for long-term therapy of acute cholecystitis. Gastrointest Endosc. 2021 Oct;94(4):742-8 e1. doi: 10.1016/j.gie.2021.03.025.
13. James TW, Baron TH. Converting percutaneous gallbladder drainage to internal drainage using EUS-guided therapy: A review of current practices and procedures. Endosc Ultrasound. 2018 Mar-Apr;7(2):93-6. doi: 10.4103/eus.eus_110_17.
14. James TW, Baron TH. Transpapillary nasocystic tube placement to allow gallbladder distention for EUS-guided cholecystoduodenostomy. VideoGIE. 2019 Dec;4(12):561-2. doi: 10.1016/j.vgie.2019.08.009.
15. Gilman AJ, Baron TH. Delamination of a lumen-apposing metal stent with tissue ingrowth and stent-in-stent removal. Gastrointest Endosc. 2023 Sep;98(3):451-3. doi: 10.1016/j.gie.2023.04.2087.
16. Teoh AY et al. Outcomes of an international multicenter registry on EUS-guided gallbladder drainage in patients at high risk for cholecystectomy. Endosc Int Open. 2019 Aug;7(8):E964-E973. doi: 10.1055/a-0915-2098.
17. Kalva NR et al. Efficacy and safety of lumen apposing self-expandable metal stents for EUS guided cholecystostomy: A meta-analysis and systematic review. Can J Gastroenterol Hepatol. 2018;2018:7070961. doi: 10.1155/2018/7070961.
18. Khan MA et al. Efficacy and safety of endoscopic gallbladder drainage in acute cholecystitis: Is it better than percutaneous gallbladder drainage? Gastrointest Endosc. 2017 Jan;85(1):76-87 e3. doi: 10.1016/j.gie.2016.06.032.
19. Mohan BP et al. Endoscopic ultrasound-guided gallbladder drainage, transpapillary drainage, or percutaneous drainage in high risk acute cholecystitis patients: a systematic review and comparative meta-analysis. Endoscopy. 2020 Feb;52(2):96-106. doi: 10.1055/a-1020-3932.
20. Jang JW et al. Endoscopic ultrasound-guided transmural and percutaneous transhepatic gallbladder drainage are comparable for acute cholecystitis. Gastroenterology. 2012 Apr;142(4):805-11. doi: 10.1053/j.gastro.2011.12.051.
21. Teoh AYB et al. EUS-guided gallbladder drainage versus laparoscopic cholecystectomy for acute cholecystitis: a propensity score analysis with 1-year follow-up data. Gastrointest Endosc. 2021 Mar;93(3):577-83. doi: 10.1016/j.gie.2020.06.066.
22. Luk SW et al. Endoscopic ultrasound-guided gallbladder drainage versus percutaneous cholecystostomy for high risk surgical patients with acute cholecystitis: a systematic review and meta-analysis. Endoscopy. 2019 Aug;51(8):722-32. doi: 10.1055/a-0929-6603.
Gastroenterologist advocates for fair coverage, reduced physician burden
Faced with an opportunity to advocate for patients, Rajeev Jain, MD, AGAF, is never afraid to speak up. He recently spoke out publicly against a major payer’s new advance notification process for colonoscopy and endoscopy procedures, cautioning it was a glidepath toward far-reaching prior authorization requirements.
UnitedHealthcare plans to collect a larger scope of data for this new policy, “which I fear will disrupt and deny patients’ access to lifesaving care,” Dr. Jain, a gastroenterologist with Texas Digestive Disease Consultants and a member of the American Gastroenterological Association’s (AGA) Prior Authorization Reform Task Force, wrote in an opinion piece in the Dallas Morning News.
Insurance coverage should be fair to the end goal of taking good care of patients, said Dr. Jain. “And if they’re putting processes in place, which are solely to be an impediment to excellent care, then that’s not right.”
Through his extensive participation in AGA panels and other influential groups, Dr. Jain has sought to improve clinical practice and reduce physician burnout. As Director and now Chair of the Board of Directors” of the American Board of Internal Medicine, Dr. Jain participated in conversations to make the maintenance of certification (MOC) process more accessible and less burdensome for doctors.
People spent a lot of time studying for ABIM’s 10-year MOC exam, sometimes even taking a course to help them pass. Now, there’s an option in all specialties to take a 30-question exam every quarter.
On average, it takes someone roughly 2 minutes to answer each question on this short exam. “Per quarter, you’re roughly spending an hour to do that instead of taking a big 10-year exam, where people were spending money and missing work,” said Dr. Jain. This modality enables physicians to meet credentialing requirements “in a way that it meets many of the desires of our practitioners,” he added.
Dr. Jain expounded on his work to advocate for patients and physicians in an interview.
Q: I’d like to discuss your opinion piece on UnitedHealthcare’s advanced notification process. Where does that policy stand now? I’m wondering if your opinion piece led to any changes.
Dr. Jain: There’s not a metric I can use to measure its success. But I will tell you this: I’ve had numerous patients mention to me, “Hey, I saw your article in the Dallas Morning News. That was great.” And that would lead to a conversation.
Q: Why do you think UHC’s policy was a tool for prior authorization?
Dr. Jain: Imagine you go to see a gastroenterologist in clinic, and the GI believes you need a procedure for certain symptoms or abnormal laboratory tests or imaging. It’s not a screening procedure. It’s a diagnostic procedure. Now, the insurance company is going to say, “Well, we can’t schedule that until you do a preauthorization.”
That could take a day. It could take a week. It could take longer. And now, the patient has lost that moment where they can get this settled. It’s not just the schedule for the patient. They’re going to get anesthesia, be it conscious sedation or deeper sedation, and they’re going to need a ride home. They have to coordinate things with family members or friends. Those little logistics add up to a lot of times why patients cancel or don’t show up or don’t follow through, because we couldn’t get it scheduled at that moment.
I feel like we are trying to attack this problem from many different angles, and my opinion piece was one of those tactics. The patients and the rank-and-file gastroenterologists appreciate the AGA being at the forefront of this issue.
Q: Your interests range from colon cancer to Barrett’s esophagus and inflammatory bowel disease (IBD). Is there an area of focus you feel passionate about?
Dr. Jain: Through AGA, I was the cochair of the IBD Parenthood Project, which convened subject-matter experts outside of GI, including maternal-fetal medicine, lactation experts, and patients. We came up with a care pathway for women in their reproductive years who have inflammatory bowel disease, including how they should think about family planning and what they should do during pregnancy and then the postpartum. Those kinds of things have really kept me energized. It’s sort of an antidote to burnout.
Q: Who are your mentors?
Dr. Jain: I would say the late Dan Foster, MD, who was the chair of medicine at UT Southwestern, and Mark Feldman, MD, AGAF, who held leadership roles at the Dallas VA Medical Center and then Texas Health Dallas. He retired a few years ago. They both expected physicians to understand the knowledge of how we were taking care of the patient and our professionalism. There’s also my senior partner, Peter Loeb, MD, AGAF, who’s now retired. He had an insatiable appetite for knowledge. Every time I’d come back from a meeting, he’d say, “Rajeev, tell me three things you learned.” He always kept patients as the primary North Star; that whatever we did, we were thinking, “Is it best for the patient?”
Lightning Round:
Favorite type of music?
1980s alternative
Favorite movie genre?
Comedy
Cat person or dog person?
Dog
Favorite sport:
College football
What song do you have to sing along with when you hear it?
“I Ran,” by a Flock of Seagulls
Faced with an opportunity to advocate for patients, Rajeev Jain, MD, AGAF, is never afraid to speak up. He recently spoke out publicly against a major payer’s new advance notification process for colonoscopy and endoscopy procedures, cautioning it was a glidepath toward far-reaching prior authorization requirements.
UnitedHealthcare plans to collect a larger scope of data for this new policy, “which I fear will disrupt and deny patients’ access to lifesaving care,” Dr. Jain, a gastroenterologist with Texas Digestive Disease Consultants and a member of the American Gastroenterological Association’s (AGA) Prior Authorization Reform Task Force, wrote in an opinion piece in the Dallas Morning News.
Insurance coverage should be fair to the end goal of taking good care of patients, said Dr. Jain. “And if they’re putting processes in place, which are solely to be an impediment to excellent care, then that’s not right.”
Through his extensive participation in AGA panels and other influential groups, Dr. Jain has sought to improve clinical practice and reduce physician burnout. As Director and now Chair of the Board of Directors” of the American Board of Internal Medicine, Dr. Jain participated in conversations to make the maintenance of certification (MOC) process more accessible and less burdensome for doctors.
People spent a lot of time studying for ABIM’s 10-year MOC exam, sometimes even taking a course to help them pass. Now, there’s an option in all specialties to take a 30-question exam every quarter.
On average, it takes someone roughly 2 minutes to answer each question on this short exam. “Per quarter, you’re roughly spending an hour to do that instead of taking a big 10-year exam, where people were spending money and missing work,” said Dr. Jain. This modality enables physicians to meet credentialing requirements “in a way that it meets many of the desires of our practitioners,” he added.
Dr. Jain expounded on his work to advocate for patients and physicians in an interview.
Q: I’d like to discuss your opinion piece on UnitedHealthcare’s advanced notification process. Where does that policy stand now? I’m wondering if your opinion piece led to any changes.
Dr. Jain: There’s not a metric I can use to measure its success. But I will tell you this: I’ve had numerous patients mention to me, “Hey, I saw your article in the Dallas Morning News. That was great.” And that would lead to a conversation.
Q: Why do you think UHC’s policy was a tool for prior authorization?
Dr. Jain: Imagine you go to see a gastroenterologist in clinic, and the GI believes you need a procedure for certain symptoms or abnormal laboratory tests or imaging. It’s not a screening procedure. It’s a diagnostic procedure. Now, the insurance company is going to say, “Well, we can’t schedule that until you do a preauthorization.”
That could take a day. It could take a week. It could take longer. And now, the patient has lost that moment where they can get this settled. It’s not just the schedule for the patient. They’re going to get anesthesia, be it conscious sedation or deeper sedation, and they’re going to need a ride home. They have to coordinate things with family members or friends. Those little logistics add up to a lot of times why patients cancel or don’t show up or don’t follow through, because we couldn’t get it scheduled at that moment.
I feel like we are trying to attack this problem from many different angles, and my opinion piece was one of those tactics. The patients and the rank-and-file gastroenterologists appreciate the AGA being at the forefront of this issue.
Q: Your interests range from colon cancer to Barrett’s esophagus and inflammatory bowel disease (IBD). Is there an area of focus you feel passionate about?
Dr. Jain: Through AGA, I was the cochair of the IBD Parenthood Project, which convened subject-matter experts outside of GI, including maternal-fetal medicine, lactation experts, and patients. We came up with a care pathway for women in their reproductive years who have inflammatory bowel disease, including how they should think about family planning and what they should do during pregnancy and then the postpartum. Those kinds of things have really kept me energized. It’s sort of an antidote to burnout.
Q: Who are your mentors?
Dr. Jain: I would say the late Dan Foster, MD, who was the chair of medicine at UT Southwestern, and Mark Feldman, MD, AGAF, who held leadership roles at the Dallas VA Medical Center and then Texas Health Dallas. He retired a few years ago. They both expected physicians to understand the knowledge of how we were taking care of the patient and our professionalism. There’s also my senior partner, Peter Loeb, MD, AGAF, who’s now retired. He had an insatiable appetite for knowledge. Every time I’d come back from a meeting, he’d say, “Rajeev, tell me three things you learned.” He always kept patients as the primary North Star; that whatever we did, we were thinking, “Is it best for the patient?”
Lightning Round:
Favorite type of music?
1980s alternative
Favorite movie genre?
Comedy
Cat person or dog person?
Dog
Favorite sport:
College football
What song do you have to sing along with when you hear it?
“I Ran,” by a Flock of Seagulls
Faced with an opportunity to advocate for patients, Rajeev Jain, MD, AGAF, is never afraid to speak up. He recently spoke out publicly against a major payer’s new advance notification process for colonoscopy and endoscopy procedures, cautioning it was a glidepath toward far-reaching prior authorization requirements.
UnitedHealthcare plans to collect a larger scope of data for this new policy, “which I fear will disrupt and deny patients’ access to lifesaving care,” Dr. Jain, a gastroenterologist with Texas Digestive Disease Consultants and a member of the American Gastroenterological Association’s (AGA) Prior Authorization Reform Task Force, wrote in an opinion piece in the Dallas Morning News.
Insurance coverage should be fair to the end goal of taking good care of patients, said Dr. Jain. “And if they’re putting processes in place, which are solely to be an impediment to excellent care, then that’s not right.”
Through his extensive participation in AGA panels and other influential groups, Dr. Jain has sought to improve clinical practice and reduce physician burnout. As Director and now Chair of the Board of Directors” of the American Board of Internal Medicine, Dr. Jain participated in conversations to make the maintenance of certification (MOC) process more accessible and less burdensome for doctors.
People spent a lot of time studying for ABIM’s 10-year MOC exam, sometimes even taking a course to help them pass. Now, there’s an option in all specialties to take a 30-question exam every quarter.
On average, it takes someone roughly 2 minutes to answer each question on this short exam. “Per quarter, you’re roughly spending an hour to do that instead of taking a big 10-year exam, where people were spending money and missing work,” said Dr. Jain. This modality enables physicians to meet credentialing requirements “in a way that it meets many of the desires of our practitioners,” he added.
Dr. Jain expounded on his work to advocate for patients and physicians in an interview.
Q: I’d like to discuss your opinion piece on UnitedHealthcare’s advanced notification process. Where does that policy stand now? I’m wondering if your opinion piece led to any changes.
Dr. Jain: There’s not a metric I can use to measure its success. But I will tell you this: I’ve had numerous patients mention to me, “Hey, I saw your article in the Dallas Morning News. That was great.” And that would lead to a conversation.
Q: Why do you think UHC’s policy was a tool for prior authorization?
Dr. Jain: Imagine you go to see a gastroenterologist in clinic, and the GI believes you need a procedure for certain symptoms or abnormal laboratory tests or imaging. It’s not a screening procedure. It’s a diagnostic procedure. Now, the insurance company is going to say, “Well, we can’t schedule that until you do a preauthorization.”
That could take a day. It could take a week. It could take longer. And now, the patient has lost that moment where they can get this settled. It’s not just the schedule for the patient. They’re going to get anesthesia, be it conscious sedation or deeper sedation, and they’re going to need a ride home. They have to coordinate things with family members or friends. Those little logistics add up to a lot of times why patients cancel or don’t show up or don’t follow through, because we couldn’t get it scheduled at that moment.
I feel like we are trying to attack this problem from many different angles, and my opinion piece was one of those tactics. The patients and the rank-and-file gastroenterologists appreciate the AGA being at the forefront of this issue.
Q: Your interests range from colon cancer to Barrett’s esophagus and inflammatory bowel disease (IBD). Is there an area of focus you feel passionate about?
Dr. Jain: Through AGA, I was the cochair of the IBD Parenthood Project, which convened subject-matter experts outside of GI, including maternal-fetal medicine, lactation experts, and patients. We came up with a care pathway for women in their reproductive years who have inflammatory bowel disease, including how they should think about family planning and what they should do during pregnancy and then the postpartum. Those kinds of things have really kept me energized. It’s sort of an antidote to burnout.
Q: Who are your mentors?
Dr. Jain: I would say the late Dan Foster, MD, who was the chair of medicine at UT Southwestern, and Mark Feldman, MD, AGAF, who held leadership roles at the Dallas VA Medical Center and then Texas Health Dallas. He retired a few years ago. They both expected physicians to understand the knowledge of how we were taking care of the patient and our professionalism. There’s also my senior partner, Peter Loeb, MD, AGAF, who’s now retired. He had an insatiable appetite for knowledge. Every time I’d come back from a meeting, he’d say, “Rajeev, tell me three things you learned.” He always kept patients as the primary North Star; that whatever we did, we were thinking, “Is it best for the patient?”
Lightning Round:
Favorite type of music?
1980s alternative
Favorite movie genre?
Comedy
Cat person or dog person?
Dog
Favorite sport:
College football
What song do you have to sing along with when you hear it?
“I Ran,” by a Flock of Seagulls
Struggling to Stay Awake While Driving a Sign of OSA?
TOPLINE:
Individuals who frequently used one or more coping strategies to stay awake while driving are significantly more likely to be diagnosed with obstructive sleep apnea (OSA) than are those who don’t use such coping strategies, new research showed.
METHODOLOGY:
- Investigators analyzed data on 119 participants with an Epworth Sleepiness Score (ESS) of > 10 who were being considered for a continuous positive airway pressure (CPAP) trial or who drove regularly.
- A total of 105 healthy volunteers with an ESS score of < 10 with no symptoms of OSA were recruited as controls.
- All participants completed questionnaires about how sleepiness affected their driving.
TAKEAWAY:
- Participants with OSA were more likely to feel sleepy while driving than controls (P = .0002).
- Participants with OSA were significantly more likely than were controls to use at least one coping strategy “frequently” vs control participants (43.7% vs 10.5%; P ≤ .0001).
- Strategies included rolling down the window, drinking tea or coffee, or listening to music at a high volume.
- Participants with OSA were significantly more likely to have either reported an accident or have been involved in an accident irrespective of any insurance claims in the last year than controls (16.8% vs 2.85%; P ≤ .0013).
IN PRACTICE:
“Our research suggests that untreated OSA patients often use coping strategies that could be surrogate markers of sleepiness,” lead author Akshay Dwarakanath, MD, said in a press release. “Asking about these strategies in the clinic may help doctors identifying patients who are at risk of driving incidents and to advise appropriately.”
SOURCE:
Akshay Dwarakanath, MD, of St. James University Hospital in Leeds, England, led the study, which was published online on January 17, 2024, in ERJ Open Research.
LIMITATIONS:
Investigators only evaluated patients with OSA with symptoms severe enough to warrant a CPAP trial and who needed to be assessed to determine if they should be allowed to continue to drive. Participant reporting and recall bias was another potential limitation.
DISCLOSURES:
There was no information available about study funding, and study authors had no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Individuals who frequently used one or more coping strategies to stay awake while driving are significantly more likely to be diagnosed with obstructive sleep apnea (OSA) than are those who don’t use such coping strategies, new research showed.
METHODOLOGY:
- Investigators analyzed data on 119 participants with an Epworth Sleepiness Score (ESS) of > 10 who were being considered for a continuous positive airway pressure (CPAP) trial or who drove regularly.
- A total of 105 healthy volunteers with an ESS score of < 10 with no symptoms of OSA were recruited as controls.
- All participants completed questionnaires about how sleepiness affected their driving.
TAKEAWAY:
- Participants with OSA were more likely to feel sleepy while driving than controls (P = .0002).
- Participants with OSA were significantly more likely than were controls to use at least one coping strategy “frequently” vs control participants (43.7% vs 10.5%; P ≤ .0001).
- Strategies included rolling down the window, drinking tea or coffee, or listening to music at a high volume.
- Participants with OSA were significantly more likely to have either reported an accident or have been involved in an accident irrespective of any insurance claims in the last year than controls (16.8% vs 2.85%; P ≤ .0013).
IN PRACTICE:
“Our research suggests that untreated OSA patients often use coping strategies that could be surrogate markers of sleepiness,” lead author Akshay Dwarakanath, MD, said in a press release. “Asking about these strategies in the clinic may help doctors identifying patients who are at risk of driving incidents and to advise appropriately.”
SOURCE:
Akshay Dwarakanath, MD, of St. James University Hospital in Leeds, England, led the study, which was published online on January 17, 2024, in ERJ Open Research.
LIMITATIONS:
Investigators only evaluated patients with OSA with symptoms severe enough to warrant a CPAP trial and who needed to be assessed to determine if they should be allowed to continue to drive. Participant reporting and recall bias was another potential limitation.
DISCLOSURES:
There was no information available about study funding, and study authors had no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Individuals who frequently used one or more coping strategies to stay awake while driving are significantly more likely to be diagnosed with obstructive sleep apnea (OSA) than are those who don’t use such coping strategies, new research showed.
METHODOLOGY:
- Investigators analyzed data on 119 participants with an Epworth Sleepiness Score (ESS) of > 10 who were being considered for a continuous positive airway pressure (CPAP) trial or who drove regularly.
- A total of 105 healthy volunteers with an ESS score of < 10 with no symptoms of OSA were recruited as controls.
- All participants completed questionnaires about how sleepiness affected their driving.
TAKEAWAY:
- Participants with OSA were more likely to feel sleepy while driving than controls (P = .0002).
- Participants with OSA were significantly more likely than were controls to use at least one coping strategy “frequently” vs control participants (43.7% vs 10.5%; P ≤ .0001).
- Strategies included rolling down the window, drinking tea or coffee, or listening to music at a high volume.
- Participants with OSA were significantly more likely to have either reported an accident or have been involved in an accident irrespective of any insurance claims in the last year than controls (16.8% vs 2.85%; P ≤ .0013).
IN PRACTICE:
“Our research suggests that untreated OSA patients often use coping strategies that could be surrogate markers of sleepiness,” lead author Akshay Dwarakanath, MD, said in a press release. “Asking about these strategies in the clinic may help doctors identifying patients who are at risk of driving incidents and to advise appropriately.”
SOURCE:
Akshay Dwarakanath, MD, of St. James University Hospital in Leeds, England, led the study, which was published online on January 17, 2024, in ERJ Open Research.
LIMITATIONS:
Investigators only evaluated patients with OSA with symptoms severe enough to warrant a CPAP trial and who needed to be assessed to determine if they should be allowed to continue to drive. Participant reporting and recall bias was another potential limitation.
DISCLOSURES:
There was no information available about study funding, and study authors had no disclosures.
A version of this article appeared on Medscape.com.
No Impact of Legalized Cannabis on Opioid Prescriptions, Mortality
TOPLINE:
Legalization of recreational and medical cannabis is not associated with a reduction in opioid prescriptions or overall opioid overdose mortality, a new study suggested. However, investigators did find that recreational cannabis laws may be tied to a potential reduction in synthetic opioid deaths.
METHODOLOGY:
- Investigators analyzed state-level data from the US Centers for Disease Control and Prevention and other databases (2006-2020) on the number of opioid prescriptions (per 100,000 persons).
- Prescription opioids included buprenorphine (except products to treat opioid use disorder), codeine, fentanyl, hydrocodone, hydromorphone, methadone, morphine, oxycodone, oxymorphone, propoxyphene, tapentadol, and tramadol.
- Researchers used regression analyses to account for poverty rates and real gross domestic product and a generalized difference-in-differences method that accounted for staggered implementation of cannabis laws.
TAKEAWAY:
- During the full study period, 13 states legalized recreational cannabis and 23 legalized medical cannabis.
- No statistically significant association was found between recreational cannabis laws and opioid prescriptions (3.08 fewer prescriptions per 100 persons; P = .17) or overall opioid overdose mortality (3.05 fewer deaths per 100,000; P = .24).
- The changes in outcomes associated with medical cannabis laws were larger in magnitude than those for recreational cannabis laws but also not statistically significant (3.54 additional prescriptions per 100 persons; P = .17 and 3.09 additional deaths per 100,000; P = .07).
- A potential reduction was found in synthetic opioid deaths associated specifically with states that had recreational cannabis laws (4.9 fewer deaths per 100,000; P = .04), but there were no differences in overdose deaths with other opioids.
IN PRACTICE:
“These results contrast with recent studies that suggested that recreational and medical cannabis legalization are associated with reductions in opioid prescriptions and medical cannabis legalization is associated with an increase in opioid mortality,” the authors wrote.
SOURCE:
Hai V. Nguyen, PhD, of the School of Pharmacy, Memorial University of Newfoundland, St. John’s, Canada, was the lead and corresponding author of the study. It was published online on January 19, 2024, in JAMA Health Forum.
A version of this article appeared on Medscape.com.
TOPLINE:
Legalization of recreational and medical cannabis is not associated with a reduction in opioid prescriptions or overall opioid overdose mortality, a new study suggested. However, investigators did find that recreational cannabis laws may be tied to a potential reduction in synthetic opioid deaths.
METHODOLOGY:
- Investigators analyzed state-level data from the US Centers for Disease Control and Prevention and other databases (2006-2020) on the number of opioid prescriptions (per 100,000 persons).
- Prescription opioids included buprenorphine (except products to treat opioid use disorder), codeine, fentanyl, hydrocodone, hydromorphone, methadone, morphine, oxycodone, oxymorphone, propoxyphene, tapentadol, and tramadol.
- Researchers used regression analyses to account for poverty rates and real gross domestic product and a generalized difference-in-differences method that accounted for staggered implementation of cannabis laws.
TAKEAWAY:
- During the full study period, 13 states legalized recreational cannabis and 23 legalized medical cannabis.
- No statistically significant association was found between recreational cannabis laws and opioid prescriptions (3.08 fewer prescriptions per 100 persons; P = .17) or overall opioid overdose mortality (3.05 fewer deaths per 100,000; P = .24).
- The changes in outcomes associated with medical cannabis laws were larger in magnitude than those for recreational cannabis laws but also not statistically significant (3.54 additional prescriptions per 100 persons; P = .17 and 3.09 additional deaths per 100,000; P = .07).
- A potential reduction was found in synthetic opioid deaths associated specifically with states that had recreational cannabis laws (4.9 fewer deaths per 100,000; P = .04), but there were no differences in overdose deaths with other opioids.
IN PRACTICE:
“These results contrast with recent studies that suggested that recreational and medical cannabis legalization are associated with reductions in opioid prescriptions and medical cannabis legalization is associated with an increase in opioid mortality,” the authors wrote.
SOURCE:
Hai V. Nguyen, PhD, of the School of Pharmacy, Memorial University of Newfoundland, St. John’s, Canada, was the lead and corresponding author of the study. It was published online on January 19, 2024, in JAMA Health Forum.
A version of this article appeared on Medscape.com.
TOPLINE:
Legalization of recreational and medical cannabis is not associated with a reduction in opioid prescriptions or overall opioid overdose mortality, a new study suggested. However, investigators did find that recreational cannabis laws may be tied to a potential reduction in synthetic opioid deaths.
METHODOLOGY:
- Investigators analyzed state-level data from the US Centers for Disease Control and Prevention and other databases (2006-2020) on the number of opioid prescriptions (per 100,000 persons).
- Prescription opioids included buprenorphine (except products to treat opioid use disorder), codeine, fentanyl, hydrocodone, hydromorphone, methadone, morphine, oxycodone, oxymorphone, propoxyphene, tapentadol, and tramadol.
- Researchers used regression analyses to account for poverty rates and real gross domestic product and a generalized difference-in-differences method that accounted for staggered implementation of cannabis laws.
TAKEAWAY:
- During the full study period, 13 states legalized recreational cannabis and 23 legalized medical cannabis.
- No statistically significant association was found between recreational cannabis laws and opioid prescriptions (3.08 fewer prescriptions per 100 persons; P = .17) or overall opioid overdose mortality (3.05 fewer deaths per 100,000; P = .24).
- The changes in outcomes associated with medical cannabis laws were larger in magnitude than those for recreational cannabis laws but also not statistically significant (3.54 additional prescriptions per 100 persons; P = .17 and 3.09 additional deaths per 100,000; P = .07).
- A potential reduction was found in synthetic opioid deaths associated specifically with states that had recreational cannabis laws (4.9 fewer deaths per 100,000; P = .04), but there were no differences in overdose deaths with other opioids.
IN PRACTICE:
“These results contrast with recent studies that suggested that recreational and medical cannabis legalization are associated with reductions in opioid prescriptions and medical cannabis legalization is associated with an increase in opioid mortality,” the authors wrote.
SOURCE:
Hai V. Nguyen, PhD, of the School of Pharmacy, Memorial University of Newfoundland, St. John’s, Canada, was the lead and corresponding author of the study. It was published online on January 19, 2024, in JAMA Health Forum.
A version of this article appeared on Medscape.com.
Small PFS gain in metastatic prostate cancer with TKI and ICI
The combination of the tyrosine kinase inhibitor (TKI), cabozantinib (Cabometyx), and the immune checkpoint inhibitor (ICI), atezolizumab (Tecentriq), was associated with a median PFS of 6.3 months vs 4.2 months for patients assigned to second hormonal therapy with either abiraterone (Zytiga) and prednisone, or enzalutamide (Xtandi) in the CONTACT-02 trial, Neeraj Agarwal, MD, reported at the ASCO Genitourinary Cancers Symposium.
“CONTACT 2 is the first phase 3 trial of the TKI/ICI combination to show statistically significant improvement in PFS in patients with mCRPC,” said Dr. Agarwal, of the Huntsman Cancer Institute at the University of Utah in Salt Lake City.
The data support the combination of cabozantinib and atezolizumab as a potential new treatment option for patients with mCRPC that has progressed on novel hormonal therapy, he said.
Study Design Questioned
That opinion, however, was not shared by Kim N. Chi, MD, of the University of British Columbia in Vancouver, BC, Canada, the invited discussant.
Dr. Chi acknowledged that the study results as presented were positive, but also pointed to several limitations, including the small difference between the treatment groups in radiographic progression-free survival (rPFS).
“I would say the rPFS benefit is modest, and in the absence of other improvements the difference in the median rPFS is equivalent from one scan to the next in the scanning cycle. I would argue about the clinical significance of that,” he said.
He also noted that there was no improvement in the investigational arm in patient-reported outcomes, and that pain progression and quality-of-life deterioration occurred within 2 to 4 months, which is “quite quick.”
Additionally, he questioned the choice of an androgen receptor pathway inhibitor (ARPI) switch as the control arm of the study.
“I’d also argue that ARPI switch is not the best standard of care for this patient population with measurable disease and 40% visceral metastases; there are better options,” he said.
For example, in phase 3 trials, docetaxel and cabazitaxel (Jevtana) have consistently demonstrated radiographic PFS of 8 to 9 months. In addition, lutetium-177–PSMA-617, a radioligand therapy that delivers beta-particle radiation to PSMA-expressing cells and the tumor microenvironment, has also been shown to have PFS and overall survival benefits, he said.
“Irrespective of regulatory decisions, I personally could not recommend this at this time, given the data that we’ve seen and the better options that are available for this patient population,” Dr. Chi said.
Real-World Practice
“Kim Chi offered a pretty fair critique and summary of the control arm, but in real world practice, ARPI switch, from abi [abiraterone] to enza [enzalutamide] or enza to abi continues to be used in routine clinical practice for various reasons,” Xin Gao, MD, a genitourinary oncologist at Mass General Cancer Center in Boston, said in an interview.
“There are patients who can’t tolerate chemotherapy or don’t want chemotherapy, and we do know also that there are patients who can benefit from an ARPI switch, especially some patients with more indolent disease,” said Dr. Gao, who attended the presentation but was not involved in the study.
He noted that some patients being switched from abiraterone to enzalutamide have clinical responses, and that the ARPIs are generally more tolerable than chemotherapy.
In addition, CONTACT-02 is one of a series of trials in which ARPI switch was used as the control arm, and many of these trials were initiated before there were data confirming the superior efficacy of some newer therapeutic options, Dr. Gao noted.
He agreed, however that there is growing evidence to show that ARPI switch may not be the optimal choice for patients with more measurable disease, especially visceral metastases, and other more aggressive forms of mCRPC.
CONTACT-02 Details
Investigators in the phase 3 study screened 866 men with mCRPC and after stratification by liver metastases, prior docetaxel use for castration-sensitive prostate cancer, and disease stage for which the first novel hormonal therapy was given. About 500 patients (507) were randomized to receive either oral cabozantinib 40 mg daily plus intravenous atezolizumab 1200 mg every 3 weeks or second hormonal therapy with either abiraterone 1000 mg with oral prednisone 5 mg twice daily, or oral enzalutamide 160 mg daily.
After a median follow-up of 14.3 months in the PFS intention-to-treat population, the median PFS by blinded central review was 6.3 months with cabozantinib/atezolizumab and 4.2 months with second hormonal therapy. This translated into a hazard ratio of 0.64 (P = .0002). The results were similar for a PFS analysis according to Prostate Cancer Working Group 3 criteria.
The combination was also associated with modest improvements in PFS in prespecified subgroups, including patients who had liver or bone metastases and those who had previously received docetaxel.
There were no significant differences in overall survival at the time of data cutoff. Overall survival data were not mature and will be reported at a later date.
Disease control rates, a composite of complete and partial responses and stable disease, were 73% with the combination and 55% with second hormonal therapy (P value not shown).
Safety Data
The safety analysis indicated that patients found the ARPI switch easier to tolerate than the combination.
Adverse events leading to dose reductions occurred in 40% of patients on the combination, vs 3% of patients on second hormonal therapy, and treatment-related adverse events leading to discontinuation occurred in 13% and 2%, respectively.
Grade 3 or 4 adverse events occurred in 48% of patients assigned to the combination vs. 23% of patients assigned to the ARPI switch.
In all, 8% of patients on the combination and 12% on second hormonal therapy died on study, but none of the deaths were deemed to be treatment related.
CONTACT-02 was sponsored by Exelixis in partnerships with Ipsen and Takeda.
Dr. Agarwal disclosed institutional research funding from Exelixis, Roche, Takeda, and others, and travel expenses from Pfizer. Dr. Chi disclosed honoraria, a consulting/advisory role and institutional research funding with Roche and others. Dr. Gao has served as a consultant or advisor to several companies, not including the sponsors of the study, and has served as principal investigator at his institution, which has received research funding from Exelixis, Takeda, and others.
The combination of the tyrosine kinase inhibitor (TKI), cabozantinib (Cabometyx), and the immune checkpoint inhibitor (ICI), atezolizumab (Tecentriq), was associated with a median PFS of 6.3 months vs 4.2 months for patients assigned to second hormonal therapy with either abiraterone (Zytiga) and prednisone, or enzalutamide (Xtandi) in the CONTACT-02 trial, Neeraj Agarwal, MD, reported at the ASCO Genitourinary Cancers Symposium.
“CONTACT 2 is the first phase 3 trial of the TKI/ICI combination to show statistically significant improvement in PFS in patients with mCRPC,” said Dr. Agarwal, of the Huntsman Cancer Institute at the University of Utah in Salt Lake City.
The data support the combination of cabozantinib and atezolizumab as a potential new treatment option for patients with mCRPC that has progressed on novel hormonal therapy, he said.
Study Design Questioned
That opinion, however, was not shared by Kim N. Chi, MD, of the University of British Columbia in Vancouver, BC, Canada, the invited discussant.
Dr. Chi acknowledged that the study results as presented were positive, but also pointed to several limitations, including the small difference between the treatment groups in radiographic progression-free survival (rPFS).
“I would say the rPFS benefit is modest, and in the absence of other improvements the difference in the median rPFS is equivalent from one scan to the next in the scanning cycle. I would argue about the clinical significance of that,” he said.
He also noted that there was no improvement in the investigational arm in patient-reported outcomes, and that pain progression and quality-of-life deterioration occurred within 2 to 4 months, which is “quite quick.”
Additionally, he questioned the choice of an androgen receptor pathway inhibitor (ARPI) switch as the control arm of the study.
“I’d also argue that ARPI switch is not the best standard of care for this patient population with measurable disease and 40% visceral metastases; there are better options,” he said.
For example, in phase 3 trials, docetaxel and cabazitaxel (Jevtana) have consistently demonstrated radiographic PFS of 8 to 9 months. In addition, lutetium-177–PSMA-617, a radioligand therapy that delivers beta-particle radiation to PSMA-expressing cells and the tumor microenvironment, has also been shown to have PFS and overall survival benefits, he said.
“Irrespective of regulatory decisions, I personally could not recommend this at this time, given the data that we’ve seen and the better options that are available for this patient population,” Dr. Chi said.
Real-World Practice
“Kim Chi offered a pretty fair critique and summary of the control arm, but in real world practice, ARPI switch, from abi [abiraterone] to enza [enzalutamide] or enza to abi continues to be used in routine clinical practice for various reasons,” Xin Gao, MD, a genitourinary oncologist at Mass General Cancer Center in Boston, said in an interview.
“There are patients who can’t tolerate chemotherapy or don’t want chemotherapy, and we do know also that there are patients who can benefit from an ARPI switch, especially some patients with more indolent disease,” said Dr. Gao, who attended the presentation but was not involved in the study.
He noted that some patients being switched from abiraterone to enzalutamide have clinical responses, and that the ARPIs are generally more tolerable than chemotherapy.
In addition, CONTACT-02 is one of a series of trials in which ARPI switch was used as the control arm, and many of these trials were initiated before there were data confirming the superior efficacy of some newer therapeutic options, Dr. Gao noted.
He agreed, however that there is growing evidence to show that ARPI switch may not be the optimal choice for patients with more measurable disease, especially visceral metastases, and other more aggressive forms of mCRPC.
CONTACT-02 Details
Investigators in the phase 3 study screened 866 men with mCRPC and after stratification by liver metastases, prior docetaxel use for castration-sensitive prostate cancer, and disease stage for which the first novel hormonal therapy was given. About 500 patients (507) were randomized to receive either oral cabozantinib 40 mg daily plus intravenous atezolizumab 1200 mg every 3 weeks or second hormonal therapy with either abiraterone 1000 mg with oral prednisone 5 mg twice daily, or oral enzalutamide 160 mg daily.
After a median follow-up of 14.3 months in the PFS intention-to-treat population, the median PFS by blinded central review was 6.3 months with cabozantinib/atezolizumab and 4.2 months with second hormonal therapy. This translated into a hazard ratio of 0.64 (P = .0002). The results were similar for a PFS analysis according to Prostate Cancer Working Group 3 criteria.
The combination was also associated with modest improvements in PFS in prespecified subgroups, including patients who had liver or bone metastases and those who had previously received docetaxel.
There were no significant differences in overall survival at the time of data cutoff. Overall survival data were not mature and will be reported at a later date.
Disease control rates, a composite of complete and partial responses and stable disease, were 73% with the combination and 55% with second hormonal therapy (P value not shown).
Safety Data
The safety analysis indicated that patients found the ARPI switch easier to tolerate than the combination.
Adverse events leading to dose reductions occurred in 40% of patients on the combination, vs 3% of patients on second hormonal therapy, and treatment-related adverse events leading to discontinuation occurred in 13% and 2%, respectively.
Grade 3 or 4 adverse events occurred in 48% of patients assigned to the combination vs. 23% of patients assigned to the ARPI switch.
In all, 8% of patients on the combination and 12% on second hormonal therapy died on study, but none of the deaths were deemed to be treatment related.
CONTACT-02 was sponsored by Exelixis in partnerships with Ipsen and Takeda.
Dr. Agarwal disclosed institutional research funding from Exelixis, Roche, Takeda, and others, and travel expenses from Pfizer. Dr. Chi disclosed honoraria, a consulting/advisory role and institutional research funding with Roche and others. Dr. Gao has served as a consultant or advisor to several companies, not including the sponsors of the study, and has served as principal investigator at his institution, which has received research funding from Exelixis, Takeda, and others.
The combination of the tyrosine kinase inhibitor (TKI), cabozantinib (Cabometyx), and the immune checkpoint inhibitor (ICI), atezolizumab (Tecentriq), was associated with a median PFS of 6.3 months vs 4.2 months for patients assigned to second hormonal therapy with either abiraterone (Zytiga) and prednisone, or enzalutamide (Xtandi) in the CONTACT-02 trial, Neeraj Agarwal, MD, reported at the ASCO Genitourinary Cancers Symposium.
“CONTACT 2 is the first phase 3 trial of the TKI/ICI combination to show statistically significant improvement in PFS in patients with mCRPC,” said Dr. Agarwal, of the Huntsman Cancer Institute at the University of Utah in Salt Lake City.
The data support the combination of cabozantinib and atezolizumab as a potential new treatment option for patients with mCRPC that has progressed on novel hormonal therapy, he said.
Study Design Questioned
That opinion, however, was not shared by Kim N. Chi, MD, of the University of British Columbia in Vancouver, BC, Canada, the invited discussant.
Dr. Chi acknowledged that the study results as presented were positive, but also pointed to several limitations, including the small difference between the treatment groups in radiographic progression-free survival (rPFS).
“I would say the rPFS benefit is modest, and in the absence of other improvements the difference in the median rPFS is equivalent from one scan to the next in the scanning cycle. I would argue about the clinical significance of that,” he said.
He also noted that there was no improvement in the investigational arm in patient-reported outcomes, and that pain progression and quality-of-life deterioration occurred within 2 to 4 months, which is “quite quick.”
Additionally, he questioned the choice of an androgen receptor pathway inhibitor (ARPI) switch as the control arm of the study.
“I’d also argue that ARPI switch is not the best standard of care for this patient population with measurable disease and 40% visceral metastases; there are better options,” he said.
For example, in phase 3 trials, docetaxel and cabazitaxel (Jevtana) have consistently demonstrated radiographic PFS of 8 to 9 months. In addition, lutetium-177–PSMA-617, a radioligand therapy that delivers beta-particle radiation to PSMA-expressing cells and the tumor microenvironment, has also been shown to have PFS and overall survival benefits, he said.
“Irrespective of regulatory decisions, I personally could not recommend this at this time, given the data that we’ve seen and the better options that are available for this patient population,” Dr. Chi said.
Real-World Practice
“Kim Chi offered a pretty fair critique and summary of the control arm, but in real world practice, ARPI switch, from abi [abiraterone] to enza [enzalutamide] or enza to abi continues to be used in routine clinical practice for various reasons,” Xin Gao, MD, a genitourinary oncologist at Mass General Cancer Center in Boston, said in an interview.
“There are patients who can’t tolerate chemotherapy or don’t want chemotherapy, and we do know also that there are patients who can benefit from an ARPI switch, especially some patients with more indolent disease,” said Dr. Gao, who attended the presentation but was not involved in the study.
He noted that some patients being switched from abiraterone to enzalutamide have clinical responses, and that the ARPIs are generally more tolerable than chemotherapy.
In addition, CONTACT-02 is one of a series of trials in which ARPI switch was used as the control arm, and many of these trials were initiated before there were data confirming the superior efficacy of some newer therapeutic options, Dr. Gao noted.
He agreed, however that there is growing evidence to show that ARPI switch may not be the optimal choice for patients with more measurable disease, especially visceral metastases, and other more aggressive forms of mCRPC.
CONTACT-02 Details
Investigators in the phase 3 study screened 866 men with mCRPC and after stratification by liver metastases, prior docetaxel use for castration-sensitive prostate cancer, and disease stage for which the first novel hormonal therapy was given. About 500 patients (507) were randomized to receive either oral cabozantinib 40 mg daily plus intravenous atezolizumab 1200 mg every 3 weeks or second hormonal therapy with either abiraterone 1000 mg with oral prednisone 5 mg twice daily, or oral enzalutamide 160 mg daily.
After a median follow-up of 14.3 months in the PFS intention-to-treat population, the median PFS by blinded central review was 6.3 months with cabozantinib/atezolizumab and 4.2 months with second hormonal therapy. This translated into a hazard ratio of 0.64 (P = .0002). The results were similar for a PFS analysis according to Prostate Cancer Working Group 3 criteria.
The combination was also associated with modest improvements in PFS in prespecified subgroups, including patients who had liver or bone metastases and those who had previously received docetaxel.
There were no significant differences in overall survival at the time of data cutoff. Overall survival data were not mature and will be reported at a later date.
Disease control rates, a composite of complete and partial responses and stable disease, were 73% with the combination and 55% with second hormonal therapy (P value not shown).
Safety Data
The safety analysis indicated that patients found the ARPI switch easier to tolerate than the combination.
Adverse events leading to dose reductions occurred in 40% of patients on the combination, vs 3% of patients on second hormonal therapy, and treatment-related adverse events leading to discontinuation occurred in 13% and 2%, respectively.
Grade 3 or 4 adverse events occurred in 48% of patients assigned to the combination vs. 23% of patients assigned to the ARPI switch.
In all, 8% of patients on the combination and 12% on second hormonal therapy died on study, but none of the deaths were deemed to be treatment related.
CONTACT-02 was sponsored by Exelixis in partnerships with Ipsen and Takeda.
Dr. Agarwal disclosed institutional research funding from Exelixis, Roche, Takeda, and others, and travel expenses from Pfizer. Dr. Chi disclosed honoraria, a consulting/advisory role and institutional research funding with Roche and others. Dr. Gao has served as a consultant or advisor to several companies, not including the sponsors of the study, and has served as principal investigator at his institution, which has received research funding from Exelixis, Takeda, and others.
FROM ASCO GU 2024