User login
My Kidney Is Fine, Can’t You Cystatin C?
Clinicians usually measure renal function by using surrogate markers because directly measuring glomerular filtration rate (GFR) is not routinely feasible in a clinical setting.1,2 Creatinine (Cr) and cystatin C (CysC) are the 2 main surrogate molecules used to estimate GFR.3
Creatine is a molecule nonenzymatically converted into Cr, weighing only 113 Da in skeletal muscles.4 It is then filtered at the glomeruli and secreted at the proximal tubules of the kidneys. However, serum Cr (sCr) levels are affected by several factors, including age, biological sex, liver function, diet, and muscle mass.5 Historically, sCr levels also are affected by race.5 In an early study of factors affecting accurate GFR, researchers reported that self-identified African American patients had a 16% higher GFR than those who did not when using Cr.6 Despite this, the inclusion of Cr on a basic metabolic panel has allowed automatic reporting of an estimated GFR using sCr (eGFRCr) to be readily available.7
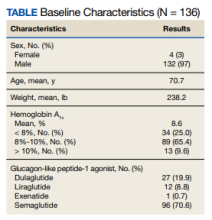
In comparison to Cr, CysC is an endogenous protein weighing 13 kDa produced by all nucleated cells.8,9 CysC is filtered by the kidney at the glomeruli and completely reabsorbed and catabolized by epithelial cells at the proximal tubule.9 Since production is not dependent on skeletal muscle, there are fewer physiological impacts on serum concentration of CysC. Levels of CysC may be elevated by factors shown in the Table.
Estimating Glomerular Filtration Rates
Multiple equations were developed to mitigate the impact of extraneous factors on the accuracy of an eGFRCr. The first widely used equation that included a variable adjustment for race was the Modification of Diet in Renal Disease study, presented in 2006.10 The equation increased the accuracy of eGFRCr further by adjusting for sex and age. It was followed by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation in 2009, which was more accurate at higher GFR levels.11
CysC was simultaneously studied as an alternative to Cr with multiple equation iterations shown to be viable in various populations as early as 2003.12-15 However, it was not until 2012 that an equation for the use of CysC was offered for widespread use as an alternative to Cr alongside further refinement of the CKD-EPI equation for Cr.16 A new formula was presented in 2021 to use both sCr and serum CysC levels to obtain a more accurate estimation of GFR.17 Research continues its effort to accurately estimate GFR for diagnosing kidney disease and assessing comorbidities relating to decreased kidney function.3
All historical equations attempted to mitigate the potential impact of race on sCr level when calculating eGFRCr by assigning a separate variable for African American patients. As an unintended adverse effect, these equations may have led to discrimination by having a different equation for African American patients.18 Moreover, these Cr-based equations remain less accurate in patients with varied muscle mass, such as older patients, bodybuilders, athletes, and individuals with varied extremes of daily protein intake.1,8,9,19 Several medications can also directly affect Cr clearance, reducing its ability to act as a surrogate for kidney function.1 In this case report, we discuss an African American patient with high muscle mass and protein intake who was initially diagnosed with kidney disease based on an elevated Cr and found to be misdiagnosed based on the use of CysC for a more accurate GFR estimation.
Case Presentation
A 35-year-old African American man serving in the military and recently diagnosed with HIV was referred to a nephrology clinic for further evaluation of an acute elevation in sCr. Before treatment for HIV, a brief record review showed a baseline Cr of about 1.3 mg/dL, with an eGFRCr of 75 mL/min/1.73 m2.20 In the same month, the patient was prescribed bictegravir/emtricitabine/tenofovir alafenamide, an HIV drug with nephrotoxic potential.21 The patient's total viral load remained low, and CD4 count remained > 500 after initiation of the HIV treatment. He was in his normal state of health and had no known contributory history before his HIV diagnosis. Cr readings peaked at 1.83 mg/dL after starting the HIV treatment and remained elevated to 1.73 mg/dL over the next few months, corresponding to CKD stage 3A. Because bictegravir/emtricitabine/tenofovir alafenamide is cleared by the kidneys and has a nephrotoxic profile, the clinical care team considered dosage adjustment or a medication switch given his observed elevated eGFRCr based on the CKD-EPI 2021 equation for Cr alone. It was also noted that the patient had a similar Cr spike to 1.83 mg/dL in 2018 without any identifiable renal insult or symptoms (Figure).
Diagnostic Evaluation
The primary care team ordered a renal ultrasound and referred the patient to the nephrology clinic. The nephrologist ordered the following laboratory studies: urine microalbumin to Cr ratio, basic metabolic panel (BMP), comprehensive metabolic panel (CMP), urinalysis, urine protein, urine Cr, parathyroid hormone level, hemoglobin A1c, complement component 3/4 panels, antinuclear and antineutrophil cytoplasmic antibodies titers, glomerular basement membrane antibody titer, urine light chains, serum protein electrophoresis, κ/λ ratio, viral hepatitis panel, and rapid plasma reagin testing. Much of this laboratory evaluation served to rule out any secondary causes of kidney disease, including autoimmune disease, monoclonal or polyclonal gammopathies, diabetic nephropathy or glomerulosclerosis, and nephrotic or nephritic syndromes.
All laboratory studies returned within normal limits; no proteinuria was discovered on urinalysis, and no abnormalities were visualized on renal ultrasound. Bictegravir/emtricitabine/tenofovir alafenamide nephrotoxicity was highest among the differential diagnoses due to the timing of Cr elevation coinciding with the initiation of the medications. The patient's CysC level was 0.85 mg/dL with a calculated eGFRCys of 125 mL/min/1.73 m2. The calculated sCR and serum cystatin C (eGFRCr-Cys) using the new 2021 equation and when adjusting for body surface area placed his eGFR at 92 mL/min/1.73 m2.20
The patient’s eGFRCys reassured the care team that the patient’s renal function was not acutely or chronically impacted by bictegravir/emtricitabine/tenofovir alafenamide, resulting in avoidance of unnecessary dosage adjustment or discontinuation of the HIV treatment. The patient reported a chronic habit of protein and creatine supplementation and bodybuilding, which likely further compounded the discrepancy between eGFRCr and eGFRCys and explained his previous elevation in Cr in 2018.
Follow-up
The patient underwent serial monitoring that revealed a stable Cr and unremarkable eGFR, ruling out CKD. There has been no evidence of worsening kidney disease to date, and the patient remained on his initial HIV regimen.
Discussion
This case shows the importance of using CysC as an alternative or confirmatory marker compared with sCr to estimate GFR in patients with high muscle mass and/or high creatine intake, such as many in the US Department of Defense (DoD) and US Department of Veterans Affairs (VA) patient populations. In the presented case, recorded Cr levels climbed from baseline Cr with the initiation of bictegravir/emtricitabine/tenofovir alafenamide. This raised the concern that HIV treatment was leading to the development of kidney damage.22
Diagnosis of kidney disease as opposed to the normal decline of eGFR with age in individuals without intrinsic CKD requires GFR ≥ 60 mL/min/1.73 m2 with kidney damage (proteinuria or radiological abnormalities, etc) or GFR < 135 to 140 mL/min/1.73 m2 minus the patient’s age in years.23 The patient’s Cr peak at 1.83 mg/dL in 2018 led to an inappropriate diagnosis of kidney disease stage 3a based on an eGFRCr (2021 equation) of 52 mL/min/1.73 m2 when not corrected for body surface area.20 However, using the new 2021 equation using both Cr and CysC, the patient’s eGFRCr-Cys was 92 mL/min/1.73 m2 after a correction for body surface area.
The 2009 CKD-EPI recommended the calculation of eGFR based on SCr concentration using age, sex, and race while the 2021 CKD-EPI recommended the exclusion of race.3 Both equations are less accurate in African American patients, individuals taking medications that interfere with Cr secretion and assay, and patients taking creatine supplements, high daily protein intake, or with high muscle mass.7 These settings result in a decreased eGFRCr without corresponding eGFRCys changes. Using SCr and CysC together, the eGFRCr-Cys yields improved concordance to measured GFR across race groups compared to GFR estimation based on Cr alone, which can avoid unnecessary expensive diagnostic workup, inappropriate kidney disease diagnosis, incorrect dosing of drugs, and accurately represent the military readiness of patients. Interestingly, in African American patients with recently diagnosed HIV, CKD-EPI using both Cr and CysC without race inclusion led to only a 2.9% overestimation of GFR and was the only equation with no statistically significant bias compared with measured GFR.24
A March 2023 case involving an otherwise healthy 26-year-old male active-duty US Navy member with a history of excessive protein supplement intake and intense exercise < 24 hours before laboratory work was diagnosed with CKD after a measured Cr of 16 mg/dL and an eGFRCr of 4 mL/min/1.73 m2 without any other evidence of kidney disease. His CysC remained within normal limits, resulting in a normal eGFRCys of 121 mL/min/1.73 m2, indicating no CKD. His Cr and eGFR recovered 10 days after his clinic visit and cessation of his supplement intake. These findings may not be uncommon given that 65% of active-duty military use protein supplements and 38% use other performance-enhancing supplements, such as creatine, according to a study.25
Unfortunately, the BMP/CMP traditionally used at VA centers use the eGFRCr equation, and it is unknown how many primary care practitioners recognize the limitations of these metabolic panels on accurate estimation of kidney function. However, in 2022 an expert panel including VA physicians recommended the immediate use of eGFRCr-Cys or eGFRCys for confirmatory testing and potentially screening of CKD.26 A small number of VAs have since adopted this recommendation, which should lead to fewer misdiagnoses among US military members as clinicians should now have access to more accurate measurements of GFR.
The VA spends about $18 billion (excluding dialysis) for care for 1.1 to 2.5 million VA patients with CKD.27 The majority of these diagnoses were undoubtedly made using the eGFRCr equation, raising the question of how many may be misdiagnosed. Assessment with CysC is currently relatively expensive, but it will likely become more affordable as the use of CysC as a confirmatory test increases.5 The cost of a sCr test is about $2.50, while CysC costs about $10.60, with variation from laboratory to laboratory.28 By comparison, a renal ultrasound costs $99 to $140 for uninsured patients.29 Furthermore, the cost of CysC testing is likely to trend downward as more facilities adopt the use of CysC measurements, which can be run on the same analytical equipment currently used for Cr measurements. Currently, most laboratories do not have established assays to use in-house and thus require CysC to be sent out to a laboratory, which increases result time and makes Cr a more attractive option. As more laboratories adopt assays for CysC, the cost of reagents will further decrease.
Given such considerations, confirmation testing of kidney function with CysC in specific patient populations with decreased eGFRCr without other features of CKD can offer great medical and financial benefits. A 2023 KDIGO report noted that many individuals may be mistakenly diagnosed with CKD when using eGFRCr.3 KDIGO noted that a 2013 meta-analysis of 90,000 individuals found that with a Cr-based eGFR of 45 to 59 mL/min/1.73 m2 (42%) had a CysC-based eGFR of ≥ 60 mL/min/1.73 m2. An eGFRCr of 45 to 59 represents 54% of all patients with CKD, amounting to millions of people (including current and former military personnel).3,29-31 Correcting a misdiagnosis of CKD would bring significant relief to patients and save millions in health care spending.
Conclusions
In patients who meet CKD criteria using eGFRCr but without other features of CKD, we recommend using confirmatory CysC levels and the eGFRCr-Cys equation. This will align care with the KDIGO guidelines and could be a cost-effective step toward improving military patient care. Further work in this area should focus on determining the knowledge gaps in primary care practitioners’ understanding of the limits of eGFRCr, the potential mitigation of concomitant CysC testing in equivocal CKD cases, and the cost-effectiveness and increased utilization of CysC.
1. Gabriel R. Time to scrap creatinine clearance? Br Med J (Clin Res Ed). 1986;293(6555):1119-1120. doi:10.1136/bmj.293.6555.1119
2. Swan SK. The search continues—an ideal marker of GFR. Clin Chem. 1997;43(6):913-914.doi:10.1093/clinchem/43.6.913 3. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1).
4. Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80(3):1107-1213. doi:10.1152/physrev.2000.80.3.1107
5. Ferguson TW, Komenda P, Tangri N. Cystatin C as a biomarker for estimating glomerular filtration rate. Curr Opin Nephrol Hypertens. 2015;24(3):295-300. doi:10.1097/mnh.0000000000000115
6. Levey AS, Titan SM, Powe NR, Coresh J, Inker LA. Kidney disease, race, and GFR estimation. Clin J Am Soc Nephrol. 2020;15(8):1203-1212. doi:10.2215/cjn.12791019
7. Shlipak MG, Tummalapalli SL, Boulware LE, et al; Conference Participants. The case for early identification and intervention of chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int. 2021;99(1):34-47. doi:10.1016/j.kint.2020.10.012
8. O’Riordan SE, Webb MC, Stowe HJ, et al. Cystatin C improves the detection of mild renal dysfunction in older patients. Ann Clin Biochem. 2003;40(pt 6):648-655. doi:10.1258/000456303770367243
9. Stevens LA, Schmid CH, Greene T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75(6):652-660. doi:10.1038/ki.2008.638
10. Levey AS, Coresh J, Greene T, et al; Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247-254. doi:10.7326/0003-4819-145-4-200608150-00004
11. Levey AS, Stevens LA, Schmid CH, et al; Chronic Kidney Disease Epidemiology Collaboration. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi:10.7326/0003-4819-150-9-200905050-00006
12. Pöge U, Gerhardt T, Stoffel-Wagner B, Klehr HU, Sauerbruch T, Woitas RP. Calculation of glomerular filtration rate based on cystatin C in cirrhotic patients. Nephrol Dial Transplant. 2006;21(3):660-664. doi:10.1093/ndt/gfi305
13. Larsson A, Malm J, Grubb A, Hansson LO. Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scand J Clin Lab Invest. 2004;64(1):25-30. doi:10.1080/00365510410003723.
14. Macisaac RJ, Tsalamandris C, Thomas MC, et al. Estimating glomerular filtration rate in diabetes: a comparison of cystatin-C- and creatinine-based methods. Diabetologia. 2006;49(7):1686-1689. doi:10.1007/s00125-006-0275-7
15. Rule AD, Bergstralh EJ, Slezak JM, Bergert J, Larson TS. Glomerular filtration rate estimated by cystatin C among different clinical presentations. Kidney Int. 2006;69(2):399-405. doi:10.1038/sj.ki.5000073
16. Inker LA, Schmid CH, Tighiouart H, et al; Chronic Kidney Disease Epidemiology Collaboration Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20-29. doi:10.1056/NEJMoa1114248
17. Shlipak MG, Matsushita K, Ärnlöv J, et al; CKD Prognosis Consortium. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932-943. doi:10.1056/NEJMoa1214234
18. Inker LA, Eneanya ND, Coresh J, et al; Chronic Kidney Disease Epidemiology Collaboration. New creatinine- and cystatin C–Based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737-1749. doi:10.1056/NEJMoa2102953
19. Oterdoom LH, Gansevoort RT, Schouten JP, de Jong PE, Gans ROB, Bakker SJL. Urinary creatinine excretion, an indirect measure of muscle mass, is an independent predictor of cardiovascular disease and mortality in the general population. Atherosclerosis. 2009;207(2):534-540. doi.10.1016/j.atherosclerosis.2009.05.010
20. National Kidney Foundation Inc. eGFR calculator. Accessed October 20, 2023. https://www.kidney.org/professionals/kdoqi/gfr_calculator
21. Ueaphongsukkit T, Gatechompol S, Avihingsanon A, et al. Tenofovir alafenamide nephrotoxicity: a case report and literature review. AIDS Res Ther. 2021;18(1):53. doi:10.1186/s12981-021-00380-w
22. D’Agati V, Appel GB. Renal pathology of human immunodeficiency virus infection. Semin Nephrol. 1998;18(4):406-421.
23. Glassock RJ, Winearls C. Ageing and the glomerular filtration rate: truths and consequences. Trans Am Clin Climatol Assoc. 2009;120:419-428.
24. Seape T, Gounden V, van Deventer HE, Candy GP, George JA. Cystatin C- and creatinine-based equations in the assessment of renal function in HIV-positive patients prior to commencing highly active antiretroviral therapy. Ann Clin Biochem. 2016;53(pt 1):58-66. doi:10.1177/0004563215579695
25. Tobin TW, Thurlow JS, Yuan CM. A healthy active duty soldier with an elevated serum creatinine. Mil Med. 2023;188(3-4):e866-e869. doi:10.1093/milmed/usab163
26. Delgado C, Baweja M, Crews DC, et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. Am J Kidney Dis. 2022;79(2):268-288.e1. doi:10.1053/j.ajkd.2021.08.003
27. Saran R, Pearson A, Tilea A, et al; VA-REINS Steering Committee; VA Advisory Board. Burden and cost of caring for us veterans with CKD: initial findings from the VA Renal Information System (VA-REINS). Am J Kidney Dis. 2021;77(3):397-405. doi:10.1053/j.ajkd.2020.07.013
28. Zoler ML. Nephrologists make the case for cystatin C-based eGFR. Accessed October 20, 2023. https://www.medscape.com/viewarticle/951335#vp_2
29. Versaw N. How much does an ultrasound cost? Updated February 2022. Accessed October 20, 2023. https://www.compare.com/health/healthcare-resources/how-much-does-an-ultrasound-cost
30. Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379(9811):165-180. doi:10.1016/S0140-6736(11)60178-5
31. Shlipak MG, Matsushita K, Ärnlöv J, et al; CKD Prognosis Consortium. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932-943. doi:10.1056/NEJMoa1214234
Clinicians usually measure renal function by using surrogate markers because directly measuring glomerular filtration rate (GFR) is not routinely feasible in a clinical setting.1,2 Creatinine (Cr) and cystatin C (CysC) are the 2 main surrogate molecules used to estimate GFR.3
Creatine is a molecule nonenzymatically converted into Cr, weighing only 113 Da in skeletal muscles.4 It is then filtered at the glomeruli and secreted at the proximal tubules of the kidneys. However, serum Cr (sCr) levels are affected by several factors, including age, biological sex, liver function, diet, and muscle mass.5 Historically, sCr levels also are affected by race.5 In an early study of factors affecting accurate GFR, researchers reported that self-identified African American patients had a 16% higher GFR than those who did not when using Cr.6 Despite this, the inclusion of Cr on a basic metabolic panel has allowed automatic reporting of an estimated GFR using sCr (eGFRCr) to be readily available.7
In comparison to Cr, CysC is an endogenous protein weighing 13 kDa produced by all nucleated cells.8,9 CysC is filtered by the kidney at the glomeruli and completely reabsorbed and catabolized by epithelial cells at the proximal tubule.9 Since production is not dependent on skeletal muscle, there are fewer physiological impacts on serum concentration of CysC. Levels of CysC may be elevated by factors shown in the Table.
Estimating Glomerular Filtration Rates
Multiple equations were developed to mitigate the impact of extraneous factors on the accuracy of an eGFRCr. The first widely used equation that included a variable adjustment for race was the Modification of Diet in Renal Disease study, presented in 2006.10 The equation increased the accuracy of eGFRCr further by adjusting for sex and age. It was followed by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation in 2009, which was more accurate at higher GFR levels.11
CysC was simultaneously studied as an alternative to Cr with multiple equation iterations shown to be viable in various populations as early as 2003.12-15 However, it was not until 2012 that an equation for the use of CysC was offered for widespread use as an alternative to Cr alongside further refinement of the CKD-EPI equation for Cr.16 A new formula was presented in 2021 to use both sCr and serum CysC levels to obtain a more accurate estimation of GFR.17 Research continues its effort to accurately estimate GFR for diagnosing kidney disease and assessing comorbidities relating to decreased kidney function.3
All historical equations attempted to mitigate the potential impact of race on sCr level when calculating eGFRCr by assigning a separate variable for African American patients. As an unintended adverse effect, these equations may have led to discrimination by having a different equation for African American patients.18 Moreover, these Cr-based equations remain less accurate in patients with varied muscle mass, such as older patients, bodybuilders, athletes, and individuals with varied extremes of daily protein intake.1,8,9,19 Several medications can also directly affect Cr clearance, reducing its ability to act as a surrogate for kidney function.1 In this case report, we discuss an African American patient with high muscle mass and protein intake who was initially diagnosed with kidney disease based on an elevated Cr and found to be misdiagnosed based on the use of CysC for a more accurate GFR estimation.
Case Presentation
A 35-year-old African American man serving in the military and recently diagnosed with HIV was referred to a nephrology clinic for further evaluation of an acute elevation in sCr. Before treatment for HIV, a brief record review showed a baseline Cr of about 1.3 mg/dL, with an eGFRCr of 75 mL/min/1.73 m2.20 In the same month, the patient was prescribed bictegravir/emtricitabine/tenofovir alafenamide, an HIV drug with nephrotoxic potential.21 The patient's total viral load remained low, and CD4 count remained > 500 after initiation of the HIV treatment. He was in his normal state of health and had no known contributory history before his HIV diagnosis. Cr readings peaked at 1.83 mg/dL after starting the HIV treatment and remained elevated to 1.73 mg/dL over the next few months, corresponding to CKD stage 3A. Because bictegravir/emtricitabine/tenofovir alafenamide is cleared by the kidneys and has a nephrotoxic profile, the clinical care team considered dosage adjustment or a medication switch given his observed elevated eGFRCr based on the CKD-EPI 2021 equation for Cr alone. It was also noted that the patient had a similar Cr spike to 1.83 mg/dL in 2018 without any identifiable renal insult or symptoms (Figure).
Diagnostic Evaluation
The primary care team ordered a renal ultrasound and referred the patient to the nephrology clinic. The nephrologist ordered the following laboratory studies: urine microalbumin to Cr ratio, basic metabolic panel (BMP), comprehensive metabolic panel (CMP), urinalysis, urine protein, urine Cr, parathyroid hormone level, hemoglobin A1c, complement component 3/4 panels, antinuclear and antineutrophil cytoplasmic antibodies titers, glomerular basement membrane antibody titer, urine light chains, serum protein electrophoresis, κ/λ ratio, viral hepatitis panel, and rapid plasma reagin testing. Much of this laboratory evaluation served to rule out any secondary causes of kidney disease, including autoimmune disease, monoclonal or polyclonal gammopathies, diabetic nephropathy or glomerulosclerosis, and nephrotic or nephritic syndromes.
All laboratory studies returned within normal limits; no proteinuria was discovered on urinalysis, and no abnormalities were visualized on renal ultrasound. Bictegravir/emtricitabine/tenofovir alafenamide nephrotoxicity was highest among the differential diagnoses due to the timing of Cr elevation coinciding with the initiation of the medications. The patient's CysC level was 0.85 mg/dL with a calculated eGFRCys of 125 mL/min/1.73 m2. The calculated sCR and serum cystatin C (eGFRCr-Cys) using the new 2021 equation and when adjusting for body surface area placed his eGFR at 92 mL/min/1.73 m2.20
The patient’s eGFRCys reassured the care team that the patient’s renal function was not acutely or chronically impacted by bictegravir/emtricitabine/tenofovir alafenamide, resulting in avoidance of unnecessary dosage adjustment or discontinuation of the HIV treatment. The patient reported a chronic habit of protein and creatine supplementation and bodybuilding, which likely further compounded the discrepancy between eGFRCr and eGFRCys and explained his previous elevation in Cr in 2018.
Follow-up
The patient underwent serial monitoring that revealed a stable Cr and unremarkable eGFR, ruling out CKD. There has been no evidence of worsening kidney disease to date, and the patient remained on his initial HIV regimen.
Discussion
This case shows the importance of using CysC as an alternative or confirmatory marker compared with sCr to estimate GFR in patients with high muscle mass and/or high creatine intake, such as many in the US Department of Defense (DoD) and US Department of Veterans Affairs (VA) patient populations. In the presented case, recorded Cr levels climbed from baseline Cr with the initiation of bictegravir/emtricitabine/tenofovir alafenamide. This raised the concern that HIV treatment was leading to the development of kidney damage.22
Diagnosis of kidney disease as opposed to the normal decline of eGFR with age in individuals without intrinsic CKD requires GFR ≥ 60 mL/min/1.73 m2 with kidney damage (proteinuria or radiological abnormalities, etc) or GFR < 135 to 140 mL/min/1.73 m2 minus the patient’s age in years.23 The patient’s Cr peak at 1.83 mg/dL in 2018 led to an inappropriate diagnosis of kidney disease stage 3a based on an eGFRCr (2021 equation) of 52 mL/min/1.73 m2 when not corrected for body surface area.20 However, using the new 2021 equation using both Cr and CysC, the patient’s eGFRCr-Cys was 92 mL/min/1.73 m2 after a correction for body surface area.
The 2009 CKD-EPI recommended the calculation of eGFR based on SCr concentration using age, sex, and race while the 2021 CKD-EPI recommended the exclusion of race.3 Both equations are less accurate in African American patients, individuals taking medications that interfere with Cr secretion and assay, and patients taking creatine supplements, high daily protein intake, or with high muscle mass.7 These settings result in a decreased eGFRCr without corresponding eGFRCys changes. Using SCr and CysC together, the eGFRCr-Cys yields improved concordance to measured GFR across race groups compared to GFR estimation based on Cr alone, which can avoid unnecessary expensive diagnostic workup, inappropriate kidney disease diagnosis, incorrect dosing of drugs, and accurately represent the military readiness of patients. Interestingly, in African American patients with recently diagnosed HIV, CKD-EPI using both Cr and CysC without race inclusion led to only a 2.9% overestimation of GFR and was the only equation with no statistically significant bias compared with measured GFR.24
A March 2023 case involving an otherwise healthy 26-year-old male active-duty US Navy member with a history of excessive protein supplement intake and intense exercise < 24 hours before laboratory work was diagnosed with CKD after a measured Cr of 16 mg/dL and an eGFRCr of 4 mL/min/1.73 m2 without any other evidence of kidney disease. His CysC remained within normal limits, resulting in a normal eGFRCys of 121 mL/min/1.73 m2, indicating no CKD. His Cr and eGFR recovered 10 days after his clinic visit and cessation of his supplement intake. These findings may not be uncommon given that 65% of active-duty military use protein supplements and 38% use other performance-enhancing supplements, such as creatine, according to a study.25
Unfortunately, the BMP/CMP traditionally used at VA centers use the eGFRCr equation, and it is unknown how many primary care practitioners recognize the limitations of these metabolic panels on accurate estimation of kidney function. However, in 2022 an expert panel including VA physicians recommended the immediate use of eGFRCr-Cys or eGFRCys for confirmatory testing and potentially screening of CKD.26 A small number of VAs have since adopted this recommendation, which should lead to fewer misdiagnoses among US military members as clinicians should now have access to more accurate measurements of GFR.
The VA spends about $18 billion (excluding dialysis) for care for 1.1 to 2.5 million VA patients with CKD.27 The majority of these diagnoses were undoubtedly made using the eGFRCr equation, raising the question of how many may be misdiagnosed. Assessment with CysC is currently relatively expensive, but it will likely become more affordable as the use of CysC as a confirmatory test increases.5 The cost of a sCr test is about $2.50, while CysC costs about $10.60, with variation from laboratory to laboratory.28 By comparison, a renal ultrasound costs $99 to $140 for uninsured patients.29 Furthermore, the cost of CysC testing is likely to trend downward as more facilities adopt the use of CysC measurements, which can be run on the same analytical equipment currently used for Cr measurements. Currently, most laboratories do not have established assays to use in-house and thus require CysC to be sent out to a laboratory, which increases result time and makes Cr a more attractive option. As more laboratories adopt assays for CysC, the cost of reagents will further decrease.
Given such considerations, confirmation testing of kidney function with CysC in specific patient populations with decreased eGFRCr without other features of CKD can offer great medical and financial benefits. A 2023 KDIGO report noted that many individuals may be mistakenly diagnosed with CKD when using eGFRCr.3 KDIGO noted that a 2013 meta-analysis of 90,000 individuals found that with a Cr-based eGFR of 45 to 59 mL/min/1.73 m2 (42%) had a CysC-based eGFR of ≥ 60 mL/min/1.73 m2. An eGFRCr of 45 to 59 represents 54% of all patients with CKD, amounting to millions of people (including current and former military personnel).3,29-31 Correcting a misdiagnosis of CKD would bring significant relief to patients and save millions in health care spending.
Conclusions
In patients who meet CKD criteria using eGFRCr but without other features of CKD, we recommend using confirmatory CysC levels and the eGFRCr-Cys equation. This will align care with the KDIGO guidelines and could be a cost-effective step toward improving military patient care. Further work in this area should focus on determining the knowledge gaps in primary care practitioners’ understanding of the limits of eGFRCr, the potential mitigation of concomitant CysC testing in equivocal CKD cases, and the cost-effectiveness and increased utilization of CysC.
Clinicians usually measure renal function by using surrogate markers because directly measuring glomerular filtration rate (GFR) is not routinely feasible in a clinical setting.1,2 Creatinine (Cr) and cystatin C (CysC) are the 2 main surrogate molecules used to estimate GFR.3
Creatine is a molecule nonenzymatically converted into Cr, weighing only 113 Da in skeletal muscles.4 It is then filtered at the glomeruli and secreted at the proximal tubules of the kidneys. However, serum Cr (sCr) levels are affected by several factors, including age, biological sex, liver function, diet, and muscle mass.5 Historically, sCr levels also are affected by race.5 In an early study of factors affecting accurate GFR, researchers reported that self-identified African American patients had a 16% higher GFR than those who did not when using Cr.6 Despite this, the inclusion of Cr on a basic metabolic panel has allowed automatic reporting of an estimated GFR using sCr (eGFRCr) to be readily available.7
In comparison to Cr, CysC is an endogenous protein weighing 13 kDa produced by all nucleated cells.8,9 CysC is filtered by the kidney at the glomeruli and completely reabsorbed and catabolized by epithelial cells at the proximal tubule.9 Since production is not dependent on skeletal muscle, there are fewer physiological impacts on serum concentration of CysC. Levels of CysC may be elevated by factors shown in the Table.
Estimating Glomerular Filtration Rates
Multiple equations were developed to mitigate the impact of extraneous factors on the accuracy of an eGFRCr. The first widely used equation that included a variable adjustment for race was the Modification of Diet in Renal Disease study, presented in 2006.10 The equation increased the accuracy of eGFRCr further by adjusting for sex and age. It was followed by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation in 2009, which was more accurate at higher GFR levels.11
CysC was simultaneously studied as an alternative to Cr with multiple equation iterations shown to be viable in various populations as early as 2003.12-15 However, it was not until 2012 that an equation for the use of CysC was offered for widespread use as an alternative to Cr alongside further refinement of the CKD-EPI equation for Cr.16 A new formula was presented in 2021 to use both sCr and serum CysC levels to obtain a more accurate estimation of GFR.17 Research continues its effort to accurately estimate GFR for diagnosing kidney disease and assessing comorbidities relating to decreased kidney function.3
All historical equations attempted to mitigate the potential impact of race on sCr level when calculating eGFRCr by assigning a separate variable for African American patients. As an unintended adverse effect, these equations may have led to discrimination by having a different equation for African American patients.18 Moreover, these Cr-based equations remain less accurate in patients with varied muscle mass, such as older patients, bodybuilders, athletes, and individuals with varied extremes of daily protein intake.1,8,9,19 Several medications can also directly affect Cr clearance, reducing its ability to act as a surrogate for kidney function.1 In this case report, we discuss an African American patient with high muscle mass and protein intake who was initially diagnosed with kidney disease based on an elevated Cr and found to be misdiagnosed based on the use of CysC for a more accurate GFR estimation.
Case Presentation
A 35-year-old African American man serving in the military and recently diagnosed with HIV was referred to a nephrology clinic for further evaluation of an acute elevation in sCr. Before treatment for HIV, a brief record review showed a baseline Cr of about 1.3 mg/dL, with an eGFRCr of 75 mL/min/1.73 m2.20 In the same month, the patient was prescribed bictegravir/emtricitabine/tenofovir alafenamide, an HIV drug with nephrotoxic potential.21 The patient's total viral load remained low, and CD4 count remained > 500 after initiation of the HIV treatment. He was in his normal state of health and had no known contributory history before his HIV diagnosis. Cr readings peaked at 1.83 mg/dL after starting the HIV treatment and remained elevated to 1.73 mg/dL over the next few months, corresponding to CKD stage 3A. Because bictegravir/emtricitabine/tenofovir alafenamide is cleared by the kidneys and has a nephrotoxic profile, the clinical care team considered dosage adjustment or a medication switch given his observed elevated eGFRCr based on the CKD-EPI 2021 equation for Cr alone. It was also noted that the patient had a similar Cr spike to 1.83 mg/dL in 2018 without any identifiable renal insult or symptoms (Figure).
Diagnostic Evaluation
The primary care team ordered a renal ultrasound and referred the patient to the nephrology clinic. The nephrologist ordered the following laboratory studies: urine microalbumin to Cr ratio, basic metabolic panel (BMP), comprehensive metabolic panel (CMP), urinalysis, urine protein, urine Cr, parathyroid hormone level, hemoglobin A1c, complement component 3/4 panels, antinuclear and antineutrophil cytoplasmic antibodies titers, glomerular basement membrane antibody titer, urine light chains, serum protein electrophoresis, κ/λ ratio, viral hepatitis panel, and rapid plasma reagin testing. Much of this laboratory evaluation served to rule out any secondary causes of kidney disease, including autoimmune disease, monoclonal or polyclonal gammopathies, diabetic nephropathy or glomerulosclerosis, and nephrotic or nephritic syndromes.
All laboratory studies returned within normal limits; no proteinuria was discovered on urinalysis, and no abnormalities were visualized on renal ultrasound. Bictegravir/emtricitabine/tenofovir alafenamide nephrotoxicity was highest among the differential diagnoses due to the timing of Cr elevation coinciding with the initiation of the medications. The patient's CysC level was 0.85 mg/dL with a calculated eGFRCys of 125 mL/min/1.73 m2. The calculated sCR and serum cystatin C (eGFRCr-Cys) using the new 2021 equation and when adjusting for body surface area placed his eGFR at 92 mL/min/1.73 m2.20
The patient’s eGFRCys reassured the care team that the patient’s renal function was not acutely or chronically impacted by bictegravir/emtricitabine/tenofovir alafenamide, resulting in avoidance of unnecessary dosage adjustment or discontinuation of the HIV treatment. The patient reported a chronic habit of protein and creatine supplementation and bodybuilding, which likely further compounded the discrepancy between eGFRCr and eGFRCys and explained his previous elevation in Cr in 2018.
Follow-up
The patient underwent serial monitoring that revealed a stable Cr and unremarkable eGFR, ruling out CKD. There has been no evidence of worsening kidney disease to date, and the patient remained on his initial HIV regimen.
Discussion
This case shows the importance of using CysC as an alternative or confirmatory marker compared with sCr to estimate GFR in patients with high muscle mass and/or high creatine intake, such as many in the US Department of Defense (DoD) and US Department of Veterans Affairs (VA) patient populations. In the presented case, recorded Cr levels climbed from baseline Cr with the initiation of bictegravir/emtricitabine/tenofovir alafenamide. This raised the concern that HIV treatment was leading to the development of kidney damage.22
Diagnosis of kidney disease as opposed to the normal decline of eGFR with age in individuals without intrinsic CKD requires GFR ≥ 60 mL/min/1.73 m2 with kidney damage (proteinuria or radiological abnormalities, etc) or GFR < 135 to 140 mL/min/1.73 m2 minus the patient’s age in years.23 The patient’s Cr peak at 1.83 mg/dL in 2018 led to an inappropriate diagnosis of kidney disease stage 3a based on an eGFRCr (2021 equation) of 52 mL/min/1.73 m2 when not corrected for body surface area.20 However, using the new 2021 equation using both Cr and CysC, the patient’s eGFRCr-Cys was 92 mL/min/1.73 m2 after a correction for body surface area.
The 2009 CKD-EPI recommended the calculation of eGFR based on SCr concentration using age, sex, and race while the 2021 CKD-EPI recommended the exclusion of race.3 Both equations are less accurate in African American patients, individuals taking medications that interfere with Cr secretion and assay, and patients taking creatine supplements, high daily protein intake, or with high muscle mass.7 These settings result in a decreased eGFRCr without corresponding eGFRCys changes. Using SCr and CysC together, the eGFRCr-Cys yields improved concordance to measured GFR across race groups compared to GFR estimation based on Cr alone, which can avoid unnecessary expensive diagnostic workup, inappropriate kidney disease diagnosis, incorrect dosing of drugs, and accurately represent the military readiness of patients. Interestingly, in African American patients with recently diagnosed HIV, CKD-EPI using both Cr and CysC without race inclusion led to only a 2.9% overestimation of GFR and was the only equation with no statistically significant bias compared with measured GFR.24
A March 2023 case involving an otherwise healthy 26-year-old male active-duty US Navy member with a history of excessive protein supplement intake and intense exercise < 24 hours before laboratory work was diagnosed with CKD after a measured Cr of 16 mg/dL and an eGFRCr of 4 mL/min/1.73 m2 without any other evidence of kidney disease. His CysC remained within normal limits, resulting in a normal eGFRCys of 121 mL/min/1.73 m2, indicating no CKD. His Cr and eGFR recovered 10 days after his clinic visit and cessation of his supplement intake. These findings may not be uncommon given that 65% of active-duty military use protein supplements and 38% use other performance-enhancing supplements, such as creatine, according to a study.25
Unfortunately, the BMP/CMP traditionally used at VA centers use the eGFRCr equation, and it is unknown how many primary care practitioners recognize the limitations of these metabolic panels on accurate estimation of kidney function. However, in 2022 an expert panel including VA physicians recommended the immediate use of eGFRCr-Cys or eGFRCys for confirmatory testing and potentially screening of CKD.26 A small number of VAs have since adopted this recommendation, which should lead to fewer misdiagnoses among US military members as clinicians should now have access to more accurate measurements of GFR.
The VA spends about $18 billion (excluding dialysis) for care for 1.1 to 2.5 million VA patients with CKD.27 The majority of these diagnoses were undoubtedly made using the eGFRCr equation, raising the question of how many may be misdiagnosed. Assessment with CysC is currently relatively expensive, but it will likely become more affordable as the use of CysC as a confirmatory test increases.5 The cost of a sCr test is about $2.50, while CysC costs about $10.60, with variation from laboratory to laboratory.28 By comparison, a renal ultrasound costs $99 to $140 for uninsured patients.29 Furthermore, the cost of CysC testing is likely to trend downward as more facilities adopt the use of CysC measurements, which can be run on the same analytical equipment currently used for Cr measurements. Currently, most laboratories do not have established assays to use in-house and thus require CysC to be sent out to a laboratory, which increases result time and makes Cr a more attractive option. As more laboratories adopt assays for CysC, the cost of reagents will further decrease.
Given such considerations, confirmation testing of kidney function with CysC in specific patient populations with decreased eGFRCr without other features of CKD can offer great medical and financial benefits. A 2023 KDIGO report noted that many individuals may be mistakenly diagnosed with CKD when using eGFRCr.3 KDIGO noted that a 2013 meta-analysis of 90,000 individuals found that with a Cr-based eGFR of 45 to 59 mL/min/1.73 m2 (42%) had a CysC-based eGFR of ≥ 60 mL/min/1.73 m2. An eGFRCr of 45 to 59 represents 54% of all patients with CKD, amounting to millions of people (including current and former military personnel).3,29-31 Correcting a misdiagnosis of CKD would bring significant relief to patients and save millions in health care spending.
Conclusions
In patients who meet CKD criteria using eGFRCr but without other features of CKD, we recommend using confirmatory CysC levels and the eGFRCr-Cys equation. This will align care with the KDIGO guidelines and could be a cost-effective step toward improving military patient care. Further work in this area should focus on determining the knowledge gaps in primary care practitioners’ understanding of the limits of eGFRCr, the potential mitigation of concomitant CysC testing in equivocal CKD cases, and the cost-effectiveness and increased utilization of CysC.
1. Gabriel R. Time to scrap creatinine clearance? Br Med J (Clin Res Ed). 1986;293(6555):1119-1120. doi:10.1136/bmj.293.6555.1119
2. Swan SK. The search continues—an ideal marker of GFR. Clin Chem. 1997;43(6):913-914.doi:10.1093/clinchem/43.6.913 3. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1).
4. Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80(3):1107-1213. doi:10.1152/physrev.2000.80.3.1107
5. Ferguson TW, Komenda P, Tangri N. Cystatin C as a biomarker for estimating glomerular filtration rate. Curr Opin Nephrol Hypertens. 2015;24(3):295-300. doi:10.1097/mnh.0000000000000115
6. Levey AS, Titan SM, Powe NR, Coresh J, Inker LA. Kidney disease, race, and GFR estimation. Clin J Am Soc Nephrol. 2020;15(8):1203-1212. doi:10.2215/cjn.12791019
7. Shlipak MG, Tummalapalli SL, Boulware LE, et al; Conference Participants. The case for early identification and intervention of chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int. 2021;99(1):34-47. doi:10.1016/j.kint.2020.10.012
8. O’Riordan SE, Webb MC, Stowe HJ, et al. Cystatin C improves the detection of mild renal dysfunction in older patients. Ann Clin Biochem. 2003;40(pt 6):648-655. doi:10.1258/000456303770367243
9. Stevens LA, Schmid CH, Greene T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75(6):652-660. doi:10.1038/ki.2008.638
10. Levey AS, Coresh J, Greene T, et al; Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247-254. doi:10.7326/0003-4819-145-4-200608150-00004
11. Levey AS, Stevens LA, Schmid CH, et al; Chronic Kidney Disease Epidemiology Collaboration. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi:10.7326/0003-4819-150-9-200905050-00006
12. Pöge U, Gerhardt T, Stoffel-Wagner B, Klehr HU, Sauerbruch T, Woitas RP. Calculation of glomerular filtration rate based on cystatin C in cirrhotic patients. Nephrol Dial Transplant. 2006;21(3):660-664. doi:10.1093/ndt/gfi305
13. Larsson A, Malm J, Grubb A, Hansson LO. Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scand J Clin Lab Invest. 2004;64(1):25-30. doi:10.1080/00365510410003723.
14. Macisaac RJ, Tsalamandris C, Thomas MC, et al. Estimating glomerular filtration rate in diabetes: a comparison of cystatin-C- and creatinine-based methods. Diabetologia. 2006;49(7):1686-1689. doi:10.1007/s00125-006-0275-7
15. Rule AD, Bergstralh EJ, Slezak JM, Bergert J, Larson TS. Glomerular filtration rate estimated by cystatin C among different clinical presentations. Kidney Int. 2006;69(2):399-405. doi:10.1038/sj.ki.5000073
16. Inker LA, Schmid CH, Tighiouart H, et al; Chronic Kidney Disease Epidemiology Collaboration Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20-29. doi:10.1056/NEJMoa1114248
17. Shlipak MG, Matsushita K, Ärnlöv J, et al; CKD Prognosis Consortium. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932-943. doi:10.1056/NEJMoa1214234
18. Inker LA, Eneanya ND, Coresh J, et al; Chronic Kidney Disease Epidemiology Collaboration. New creatinine- and cystatin C–Based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737-1749. doi:10.1056/NEJMoa2102953
19. Oterdoom LH, Gansevoort RT, Schouten JP, de Jong PE, Gans ROB, Bakker SJL. Urinary creatinine excretion, an indirect measure of muscle mass, is an independent predictor of cardiovascular disease and mortality in the general population. Atherosclerosis. 2009;207(2):534-540. doi.10.1016/j.atherosclerosis.2009.05.010
20. National Kidney Foundation Inc. eGFR calculator. Accessed October 20, 2023. https://www.kidney.org/professionals/kdoqi/gfr_calculator
21. Ueaphongsukkit T, Gatechompol S, Avihingsanon A, et al. Tenofovir alafenamide nephrotoxicity: a case report and literature review. AIDS Res Ther. 2021;18(1):53. doi:10.1186/s12981-021-00380-w
22. D’Agati V, Appel GB. Renal pathology of human immunodeficiency virus infection. Semin Nephrol. 1998;18(4):406-421.
23. Glassock RJ, Winearls C. Ageing and the glomerular filtration rate: truths and consequences. Trans Am Clin Climatol Assoc. 2009;120:419-428.
24. Seape T, Gounden V, van Deventer HE, Candy GP, George JA. Cystatin C- and creatinine-based equations in the assessment of renal function in HIV-positive patients prior to commencing highly active antiretroviral therapy. Ann Clin Biochem. 2016;53(pt 1):58-66. doi:10.1177/0004563215579695
25. Tobin TW, Thurlow JS, Yuan CM. A healthy active duty soldier with an elevated serum creatinine. Mil Med. 2023;188(3-4):e866-e869. doi:10.1093/milmed/usab163
26. Delgado C, Baweja M, Crews DC, et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. Am J Kidney Dis. 2022;79(2):268-288.e1. doi:10.1053/j.ajkd.2021.08.003
27. Saran R, Pearson A, Tilea A, et al; VA-REINS Steering Committee; VA Advisory Board. Burden and cost of caring for us veterans with CKD: initial findings from the VA Renal Information System (VA-REINS). Am J Kidney Dis. 2021;77(3):397-405. doi:10.1053/j.ajkd.2020.07.013
28. Zoler ML. Nephrologists make the case for cystatin C-based eGFR. Accessed October 20, 2023. https://www.medscape.com/viewarticle/951335#vp_2
29. Versaw N. How much does an ultrasound cost? Updated February 2022. Accessed October 20, 2023. https://www.compare.com/health/healthcare-resources/how-much-does-an-ultrasound-cost
30. Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379(9811):165-180. doi:10.1016/S0140-6736(11)60178-5
31. Shlipak MG, Matsushita K, Ärnlöv J, et al; CKD Prognosis Consortium. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932-943. doi:10.1056/NEJMoa1214234
1. Gabriel R. Time to scrap creatinine clearance? Br Med J (Clin Res Ed). 1986;293(6555):1119-1120. doi:10.1136/bmj.293.6555.1119
2. Swan SK. The search continues—an ideal marker of GFR. Clin Chem. 1997;43(6):913-914.doi:10.1093/clinchem/43.6.913 3. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1).
4. Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80(3):1107-1213. doi:10.1152/physrev.2000.80.3.1107
5. Ferguson TW, Komenda P, Tangri N. Cystatin C as a biomarker for estimating glomerular filtration rate. Curr Opin Nephrol Hypertens. 2015;24(3):295-300. doi:10.1097/mnh.0000000000000115
6. Levey AS, Titan SM, Powe NR, Coresh J, Inker LA. Kidney disease, race, and GFR estimation. Clin J Am Soc Nephrol. 2020;15(8):1203-1212. doi:10.2215/cjn.12791019
7. Shlipak MG, Tummalapalli SL, Boulware LE, et al; Conference Participants. The case for early identification and intervention of chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int. 2021;99(1):34-47. doi:10.1016/j.kint.2020.10.012
8. O’Riordan SE, Webb MC, Stowe HJ, et al. Cystatin C improves the detection of mild renal dysfunction in older patients. Ann Clin Biochem. 2003;40(pt 6):648-655. doi:10.1258/000456303770367243
9. Stevens LA, Schmid CH, Greene T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75(6):652-660. doi:10.1038/ki.2008.638
10. Levey AS, Coresh J, Greene T, et al; Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247-254. doi:10.7326/0003-4819-145-4-200608150-00004
11. Levey AS, Stevens LA, Schmid CH, et al; Chronic Kidney Disease Epidemiology Collaboration. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi:10.7326/0003-4819-150-9-200905050-00006
12. Pöge U, Gerhardt T, Stoffel-Wagner B, Klehr HU, Sauerbruch T, Woitas RP. Calculation of glomerular filtration rate based on cystatin C in cirrhotic patients. Nephrol Dial Transplant. 2006;21(3):660-664. doi:10.1093/ndt/gfi305
13. Larsson A, Malm J, Grubb A, Hansson LO. Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scand J Clin Lab Invest. 2004;64(1):25-30. doi:10.1080/00365510410003723.
14. Macisaac RJ, Tsalamandris C, Thomas MC, et al. Estimating glomerular filtration rate in diabetes: a comparison of cystatin-C- and creatinine-based methods. Diabetologia. 2006;49(7):1686-1689. doi:10.1007/s00125-006-0275-7
15. Rule AD, Bergstralh EJ, Slezak JM, Bergert J, Larson TS. Glomerular filtration rate estimated by cystatin C among different clinical presentations. Kidney Int. 2006;69(2):399-405. doi:10.1038/sj.ki.5000073
16. Inker LA, Schmid CH, Tighiouart H, et al; Chronic Kidney Disease Epidemiology Collaboration Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20-29. doi:10.1056/NEJMoa1114248
17. Shlipak MG, Matsushita K, Ärnlöv J, et al; CKD Prognosis Consortium. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932-943. doi:10.1056/NEJMoa1214234
18. Inker LA, Eneanya ND, Coresh J, et al; Chronic Kidney Disease Epidemiology Collaboration. New creatinine- and cystatin C–Based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737-1749. doi:10.1056/NEJMoa2102953
19. Oterdoom LH, Gansevoort RT, Schouten JP, de Jong PE, Gans ROB, Bakker SJL. Urinary creatinine excretion, an indirect measure of muscle mass, is an independent predictor of cardiovascular disease and mortality in the general population. Atherosclerosis. 2009;207(2):534-540. doi.10.1016/j.atherosclerosis.2009.05.010
20. National Kidney Foundation Inc. eGFR calculator. Accessed October 20, 2023. https://www.kidney.org/professionals/kdoqi/gfr_calculator
21. Ueaphongsukkit T, Gatechompol S, Avihingsanon A, et al. Tenofovir alafenamide nephrotoxicity: a case report and literature review. AIDS Res Ther. 2021;18(1):53. doi:10.1186/s12981-021-00380-w
22. D’Agati V, Appel GB. Renal pathology of human immunodeficiency virus infection. Semin Nephrol. 1998;18(4):406-421.
23. Glassock RJ, Winearls C. Ageing and the glomerular filtration rate: truths and consequences. Trans Am Clin Climatol Assoc. 2009;120:419-428.
24. Seape T, Gounden V, van Deventer HE, Candy GP, George JA. Cystatin C- and creatinine-based equations in the assessment of renal function in HIV-positive patients prior to commencing highly active antiretroviral therapy. Ann Clin Biochem. 2016;53(pt 1):58-66. doi:10.1177/0004563215579695
25. Tobin TW, Thurlow JS, Yuan CM. A healthy active duty soldier with an elevated serum creatinine. Mil Med. 2023;188(3-4):e866-e869. doi:10.1093/milmed/usab163
26. Delgado C, Baweja M, Crews DC, et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. Am J Kidney Dis. 2022;79(2):268-288.e1. doi:10.1053/j.ajkd.2021.08.003
27. Saran R, Pearson A, Tilea A, et al; VA-REINS Steering Committee; VA Advisory Board. Burden and cost of caring for us veterans with CKD: initial findings from the VA Renal Information System (VA-REINS). Am J Kidney Dis. 2021;77(3):397-405. doi:10.1053/j.ajkd.2020.07.013
28. Zoler ML. Nephrologists make the case for cystatin C-based eGFR. Accessed October 20, 2023. https://www.medscape.com/viewarticle/951335#vp_2
29. Versaw N. How much does an ultrasound cost? Updated February 2022. Accessed October 20, 2023. https://www.compare.com/health/healthcare-resources/how-much-does-an-ultrasound-cost
30. Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379(9811):165-180. doi:10.1016/S0140-6736(11)60178-5
31. Shlipak MG, Matsushita K, Ärnlöv J, et al; CKD Prognosis Consortium. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932-943. doi:10.1056/NEJMoa1214234
Why Everyone Needs Their Own Emergency Medicine Doctor
How emerging models come close to making this reality
As emergency medicine doctors, we regularly give medical advice to family and close friends when they get sick or are injured and don’t know what to do. In a matter of moments, we triage, diagnose, and assemble a logical plan, whatever the issue may be. This skill comes from our training and years of experience in treating emergencies and also routine medical matters. The value proposition is clear.
Frankly, it’s a service everyone should have. Think about the potential time and money saved if this option for medical care and triage was broadly available. Overtriage would plummet. That’s when people run to the emergency department (ED) and wait endless hours, only to be reassured or receive limited treatment. Undertriage would also decline. That’s when people should go to the ED but, unwisely, wait. For example, this may occur when symptoms of dizziness end up being a stroke.
Why doesn’t everyone have an ED doctor they can call? The primary reason is that the current system mostly doesn’t support it. The most common scenario is that insurance companies pay us to see patients in an expensive box called the ED. Most EDs are situated within an even more expensive box, called a hospital.
Here’s the good news: Better access to emergency care and people who are formally trained in emergency medicine and routine matters of urgent care is increasing.
One example is telemedicine, where a remote doctor — either your own or a doctor through an app — conducts a visit. Telemedicine is more common since the pandemic, now that insurance pays for it. In emergency situations, it’s rare that your own doctor can see you immediately by telemedicine. By contrast, direct-to-consumer telemedicine (eg, Teladoc, Doctor On Demand, and others) connects you with a random doctor.
In many apps, it’s unclear not only who the doctor is, but more importantly, what their specific medical specialty or training is. It may be an ED doctor evaluating your child’s fever, or it may be a retired general surgeon or an adult rheumatology specialist in the midst of their fellowship, making an extra buck, who may have no pediatric training.
Training Matters
Clinical training and whether the doctor knows you matters. A recent JAMA study from Ontario, Canada, found that patients with virtual visits who saw outside family physicians (whom they had never met) compared with their own family physicians were 66% more likely to visit an ED within 7 days after the visit. This illustrates the importance of understanding your personal history in assessing acute symptoms.
Some healthcare systems do use ED physicians for on-demand telehealth services, such as Thomas Jefferson’s JeffConnect. Amazon Clinic recently entered this space, providing condition-specific acute or chronic care to adults aged 18-64 years for a fee that is, notably, not covered by insurance.
A second innovative approach, albeit not specifically in the realm of a personal emergency medicine doctor, is artificial intelligence (AI)–powered kiosks. A concierge medicine company known as Forward recently unveiled an innovative concept known as CarePods that are now available in Sacramento, California; Chandler, Arizona; and Chicago. For a membership fee, you swipe into what looks like an oversize, space-age porta-potty. You sit in a chair and run through a series of health apps, which includes a biometric body scan along with mental health screenings. It even takes your blood (without a needle) and sequences your DNA. Results are reviewed by a doctor (not yours) who talks to you by video. They advertise that AI helps make the diagnosis. Although diagnostic AI is emerging and exciting, its benefit is not clear in emergency conditions. Yet, one clear value in a kiosk over telemedicine is the ability to obtain vital signs and lab results, which are useful for diagnosis.
Another approach is the telehealth offerings used in integrated systems of care, such as Kaiser Permanente. Kaiser is both an insurance company and a deliverer of healthcare services. Kaiser maintains a nurse call center and can handle urgent e-visits. Integrated systems not only help triage patients’ acute issues but also have access to their personal health histories. They can also provide a definitive plan for in-person treatment or a specific referral. A downside of integrated care is that it often limits your choice of provider.
Insurance companies also maintain call-in lines such as HumanaFirst, which is also staffed by nurses. We have not seen data on the calls such services receive, but we doubt people that want to call their insurance company when sick or injured, knowing that the insurer benefits when you receive less care. Additionally, studies have found that nurse-only triage is not as effective as physician triage and results in higher ED referral rates.
The Concierge Option
Probably the closest thing to having your own personal emergency medicine doctor is concierge medicine, which combines personalized care and accessibility. Concierge doctors come in many forms, but they usually charge a fixed fee for 24/7 availability and same-day appointments. A downside of concierge medicine is its expense ($2000–$3500 per year), and that many don’t take insurance. Concierge medicine is also criticized because, as doctors gravitate toward it, people in the community often lose their physician if they can’t afford the fees.
Ultimately, remote medical advice for emergency care is clearly evolving in new ways. The inability of traditional care models to achieve this goal will lead to innovation to improve the available options that have led us to think outside of the proverbial “box” we refer to as the ED-in-the-case.
At this time, will any option come close to having a personal emergency medicine physician willing to answer your questions, real-time, as with family and close friends? We think not.
But the future certainly holds promise for alternatives that will hopefully make payers and the Centers for Medicare & Medicaid Services take notice. Innovations in personalized care that reduce costs will be critical in our current healthcare landscape.
Dr. Pines is clinical professor of emergency medicine at George Washington University in Washington, DC, and chief of clinical innovation at US Acute Care Solutions in Canton, Ohio. He disclosed ties with CSL Behring and Abbott Point-of-Care. Dr. Glatter is assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is a medical advisor for Medscape and hosts the Hot Topics in EM series. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
How emerging models come close to making this reality
How emerging models come close to making this reality
As emergency medicine doctors, we regularly give medical advice to family and close friends when they get sick or are injured and don’t know what to do. In a matter of moments, we triage, diagnose, and assemble a logical plan, whatever the issue may be. This skill comes from our training and years of experience in treating emergencies and also routine medical matters. The value proposition is clear.
Frankly, it’s a service everyone should have. Think about the potential time and money saved if this option for medical care and triage was broadly available. Overtriage would plummet. That’s when people run to the emergency department (ED) and wait endless hours, only to be reassured or receive limited treatment. Undertriage would also decline. That’s when people should go to the ED but, unwisely, wait. For example, this may occur when symptoms of dizziness end up being a stroke.
Why doesn’t everyone have an ED doctor they can call? The primary reason is that the current system mostly doesn’t support it. The most common scenario is that insurance companies pay us to see patients in an expensive box called the ED. Most EDs are situated within an even more expensive box, called a hospital.
Here’s the good news: Better access to emergency care and people who are formally trained in emergency medicine and routine matters of urgent care is increasing.
One example is telemedicine, where a remote doctor — either your own or a doctor through an app — conducts a visit. Telemedicine is more common since the pandemic, now that insurance pays for it. In emergency situations, it’s rare that your own doctor can see you immediately by telemedicine. By contrast, direct-to-consumer telemedicine (eg, Teladoc, Doctor On Demand, and others) connects you with a random doctor.
In many apps, it’s unclear not only who the doctor is, but more importantly, what their specific medical specialty or training is. It may be an ED doctor evaluating your child’s fever, or it may be a retired general surgeon or an adult rheumatology specialist in the midst of their fellowship, making an extra buck, who may have no pediatric training.
Training Matters
Clinical training and whether the doctor knows you matters. A recent JAMA study from Ontario, Canada, found that patients with virtual visits who saw outside family physicians (whom they had never met) compared with their own family physicians were 66% more likely to visit an ED within 7 days after the visit. This illustrates the importance of understanding your personal history in assessing acute symptoms.
Some healthcare systems do use ED physicians for on-demand telehealth services, such as Thomas Jefferson’s JeffConnect. Amazon Clinic recently entered this space, providing condition-specific acute or chronic care to adults aged 18-64 years for a fee that is, notably, not covered by insurance.
A second innovative approach, albeit not specifically in the realm of a personal emergency medicine doctor, is artificial intelligence (AI)–powered kiosks. A concierge medicine company known as Forward recently unveiled an innovative concept known as CarePods that are now available in Sacramento, California; Chandler, Arizona; and Chicago. For a membership fee, you swipe into what looks like an oversize, space-age porta-potty. You sit in a chair and run through a series of health apps, which includes a biometric body scan along with mental health screenings. It even takes your blood (without a needle) and sequences your DNA. Results are reviewed by a doctor (not yours) who talks to you by video. They advertise that AI helps make the diagnosis. Although diagnostic AI is emerging and exciting, its benefit is not clear in emergency conditions. Yet, one clear value in a kiosk over telemedicine is the ability to obtain vital signs and lab results, which are useful for diagnosis.
Another approach is the telehealth offerings used in integrated systems of care, such as Kaiser Permanente. Kaiser is both an insurance company and a deliverer of healthcare services. Kaiser maintains a nurse call center and can handle urgent e-visits. Integrated systems not only help triage patients’ acute issues but also have access to their personal health histories. They can also provide a definitive plan for in-person treatment or a specific referral. A downside of integrated care is that it often limits your choice of provider.
Insurance companies also maintain call-in lines such as HumanaFirst, which is also staffed by nurses. We have not seen data on the calls such services receive, but we doubt people that want to call their insurance company when sick or injured, knowing that the insurer benefits when you receive less care. Additionally, studies have found that nurse-only triage is not as effective as physician triage and results in higher ED referral rates.
The Concierge Option
Probably the closest thing to having your own personal emergency medicine doctor is concierge medicine, which combines personalized care and accessibility. Concierge doctors come in many forms, but they usually charge a fixed fee for 24/7 availability and same-day appointments. A downside of concierge medicine is its expense ($2000–$3500 per year), and that many don’t take insurance. Concierge medicine is also criticized because, as doctors gravitate toward it, people in the community often lose their physician if they can’t afford the fees.
Ultimately, remote medical advice for emergency care is clearly evolving in new ways. The inability of traditional care models to achieve this goal will lead to innovation to improve the available options that have led us to think outside of the proverbial “box” we refer to as the ED-in-the-case.
At this time, will any option come close to having a personal emergency medicine physician willing to answer your questions, real-time, as with family and close friends? We think not.
But the future certainly holds promise for alternatives that will hopefully make payers and the Centers for Medicare & Medicaid Services take notice. Innovations in personalized care that reduce costs will be critical in our current healthcare landscape.
Dr. Pines is clinical professor of emergency medicine at George Washington University in Washington, DC, and chief of clinical innovation at US Acute Care Solutions in Canton, Ohio. He disclosed ties with CSL Behring and Abbott Point-of-Care. Dr. Glatter is assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is a medical advisor for Medscape and hosts the Hot Topics in EM series. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
As emergency medicine doctors, we regularly give medical advice to family and close friends when they get sick or are injured and don’t know what to do. In a matter of moments, we triage, diagnose, and assemble a logical plan, whatever the issue may be. This skill comes from our training and years of experience in treating emergencies and also routine medical matters. The value proposition is clear.
Frankly, it’s a service everyone should have. Think about the potential time and money saved if this option for medical care and triage was broadly available. Overtriage would plummet. That’s when people run to the emergency department (ED) and wait endless hours, only to be reassured or receive limited treatment. Undertriage would also decline. That’s when people should go to the ED but, unwisely, wait. For example, this may occur when symptoms of dizziness end up being a stroke.
Why doesn’t everyone have an ED doctor they can call? The primary reason is that the current system mostly doesn’t support it. The most common scenario is that insurance companies pay us to see patients in an expensive box called the ED. Most EDs are situated within an even more expensive box, called a hospital.
Here’s the good news: Better access to emergency care and people who are formally trained in emergency medicine and routine matters of urgent care is increasing.
One example is telemedicine, where a remote doctor — either your own or a doctor through an app — conducts a visit. Telemedicine is more common since the pandemic, now that insurance pays for it. In emergency situations, it’s rare that your own doctor can see you immediately by telemedicine. By contrast, direct-to-consumer telemedicine (eg, Teladoc, Doctor On Demand, and others) connects you with a random doctor.
In many apps, it’s unclear not only who the doctor is, but more importantly, what their specific medical specialty or training is. It may be an ED doctor evaluating your child’s fever, or it may be a retired general surgeon or an adult rheumatology specialist in the midst of their fellowship, making an extra buck, who may have no pediatric training.
Training Matters
Clinical training and whether the doctor knows you matters. A recent JAMA study from Ontario, Canada, found that patients with virtual visits who saw outside family physicians (whom they had never met) compared with their own family physicians were 66% more likely to visit an ED within 7 days after the visit. This illustrates the importance of understanding your personal history in assessing acute symptoms.
Some healthcare systems do use ED physicians for on-demand telehealth services, such as Thomas Jefferson’s JeffConnect. Amazon Clinic recently entered this space, providing condition-specific acute or chronic care to adults aged 18-64 years for a fee that is, notably, not covered by insurance.
A second innovative approach, albeit not specifically in the realm of a personal emergency medicine doctor, is artificial intelligence (AI)–powered kiosks. A concierge medicine company known as Forward recently unveiled an innovative concept known as CarePods that are now available in Sacramento, California; Chandler, Arizona; and Chicago. For a membership fee, you swipe into what looks like an oversize, space-age porta-potty. You sit in a chair and run through a series of health apps, which includes a biometric body scan along with mental health screenings. It even takes your blood (without a needle) and sequences your DNA. Results are reviewed by a doctor (not yours) who talks to you by video. They advertise that AI helps make the diagnosis. Although diagnostic AI is emerging and exciting, its benefit is not clear in emergency conditions. Yet, one clear value in a kiosk over telemedicine is the ability to obtain vital signs and lab results, which are useful for diagnosis.
Another approach is the telehealth offerings used in integrated systems of care, such as Kaiser Permanente. Kaiser is both an insurance company and a deliverer of healthcare services. Kaiser maintains a nurse call center and can handle urgent e-visits. Integrated systems not only help triage patients’ acute issues but also have access to their personal health histories. They can also provide a definitive plan for in-person treatment or a specific referral. A downside of integrated care is that it often limits your choice of provider.
Insurance companies also maintain call-in lines such as HumanaFirst, which is also staffed by nurses. We have not seen data on the calls such services receive, but we doubt people that want to call their insurance company when sick or injured, knowing that the insurer benefits when you receive less care. Additionally, studies have found that nurse-only triage is not as effective as physician triage and results in higher ED referral rates.
The Concierge Option
Probably the closest thing to having your own personal emergency medicine doctor is concierge medicine, which combines personalized care and accessibility. Concierge doctors come in many forms, but they usually charge a fixed fee for 24/7 availability and same-day appointments. A downside of concierge medicine is its expense ($2000–$3500 per year), and that many don’t take insurance. Concierge medicine is also criticized because, as doctors gravitate toward it, people in the community often lose their physician if they can’t afford the fees.
Ultimately, remote medical advice for emergency care is clearly evolving in new ways. The inability of traditional care models to achieve this goal will lead to innovation to improve the available options that have led us to think outside of the proverbial “box” we refer to as the ED-in-the-case.
At this time, will any option come close to having a personal emergency medicine physician willing to answer your questions, real-time, as with family and close friends? We think not.
But the future certainly holds promise for alternatives that will hopefully make payers and the Centers for Medicare & Medicaid Services take notice. Innovations in personalized care that reduce costs will be critical in our current healthcare landscape.
Dr. Pines is clinical professor of emergency medicine at George Washington University in Washington, DC, and chief of clinical innovation at US Acute Care Solutions in Canton, Ohio. He disclosed ties with CSL Behring and Abbott Point-of-Care. Dr. Glatter is assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is a medical advisor for Medscape and hosts the Hot Topics in EM series. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
A Cross-sectional Analysis of Regional Trends in Medicare Reimbursement for Phototherapy Services From 2010 to 2023
To the Editor:
Phototherapy regularly is utilized in the outpatient setting to address various skin pathologies, including atopic dermatitis, psoriasis, pruritus, vitiligo, and mycosis fungoides.1,2 Phototherapy is broadly defined by the measured administration of nonionizing radiation within the UV range including wavelengths within the UVA (eg, psoralen sensitizer plus UVA-1) and UVB (eg, broadband UVB, narrowband UVB) spectrums.1,3 Generally, the mechanism of action is derived from effects on inflammatory components of cutaneous disorders and the induction of apoptosis, both precipitating numerous downstream events.4
From 2015 to 2018, there were more than 1.3 million outpatient phototherapy visits in the United States, with the most common procedural indications being dermatitis not otherwise specified, atopic dermatitis, and pruritus.5 From 2000 to 2015, the quantity of phototherapy services billed to Medicare trended upwards by an average of 5% per year, increasing from 334,670 in the year 2000 to 692,093 in 2015.6 Therefore, an illustration of associated costs would be beneficial. Additionally, because total cost and physician reimbursement fluctuate from year to year, studies demonstrating overall trends can inform both US policymakers and physicians. There is a paucity of research on geographical trends for procedural reimbursements in dermatology for phototherapy. Understanding geographic trends of reimbursement could duly serve to optimize dermatologist practice patterns involving access to viable and quality care for patients seeking treatment as well as draw health policymakers’ attention to striking adjustments in physician fees. Therefore, in this study we aimed to illustrate the most recent regional payment trends in phototherapy procedures for Medicare B patients.
We queried the Centers for Medicare & Medicaid Services Medicare Physician Fee Schedule (MPFS) database (https://www.cms.gov/medicare/payment/fee-schedules/physician/lookup-tool) for the years 2010 to 2023 for Current Procedural Terminology (CPT) codes common to phototherapy procedures: actinotherapy (96900); photochemotherapy by Goeckerman treatment or using petrolatum and UVB (96910); photochemotherapy using psoralen plus UVA (96912); and photochemotherapy of severe dermatoses requiring a minimum of 4 hours of care under direct physician supervision (96913). Nonfacility prices for these procedures were analyzed. For 2010, due to midyear alterations to Medicare reimbursement (owed to bills HR 3962 and HR 4872), the mean price data of MPFS files 2010A and 2010B were used. All dollar values were converted to January 2023 US dollars using corresponding consumer price index inflation data. The Medicare Administrative Contractors were used to group state pricing information by region in accordance with established US Census Bureau subdivisions (https://www.census.gov/programs-surveys/economic-census/guidance-geographies/levels.html). Weighted percentage change in reimbursement rate was calculated using physician (MD or DO) utilization (procedure volume) data available in the 2020 Physician and Other Practitioners Public Use File (https://data.cms.gov/provider-summary-by-type-of-service/medicare-physician-other-practitioners/medicare-physician-other-practitioners-by-provider-and-service). All descriptive statistics and visualization were generated using R software (v4.2.2)(R Development Core Team).
Table 1 provides physician utilization data and the corresponding number of Part B beneficiaries for phototherapy procedures in 2020. There were 65,045 services of actinotherapy provided to a total of 6855 unique Part B beneficiaries, 173,979 services of photochemotherapy by Goeckerman treatment or using petrolatum and UVB provided to 13,122 unique Part B beneficiaries, 2524 services of photochemotherapy using psoralen plus UVA provided to a total of 357 unique Part B beneficiaries, and 37 services of photochemotherapy of severe dermatoses requiring a minimum of 4 hours of care under direct physician supervision provided to a total of 27 unique Part B beneficiaries.
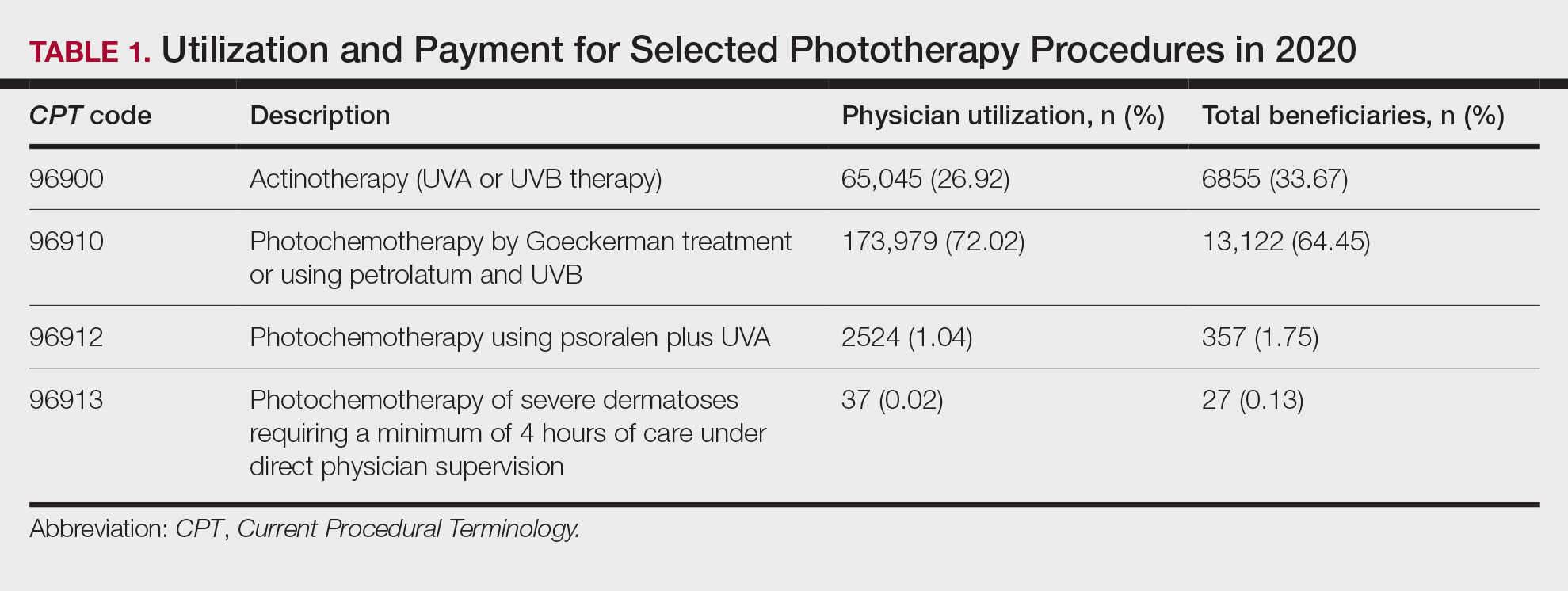
On average (unweighted), phototherapy reimbursement rates in the North increased by 0.68% between 2010 and 2023 (Table 2). After weighting for 2020 physician utilization, the average change in reimbursement rate was +19.37%. During this time period, CPT code 96910 reported the greatest adjusted increase in reimbursement (+31.45%)($98.12 to $128.98; compound annual growth rate [CAGR], +0.0213), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−12.76%)($126.09 to $109.97; CAGR, −0.0105). For CPT code 96900, the reported adjusted decrease in reimbursement was −11.68% ($30.21 to $26.68; CAGR, −0.0095), and for CPT code 96913, the reported adjusted decrease in reimbursement was −4.27% ($174.03 to $166.60; CAGR, −0.0034).
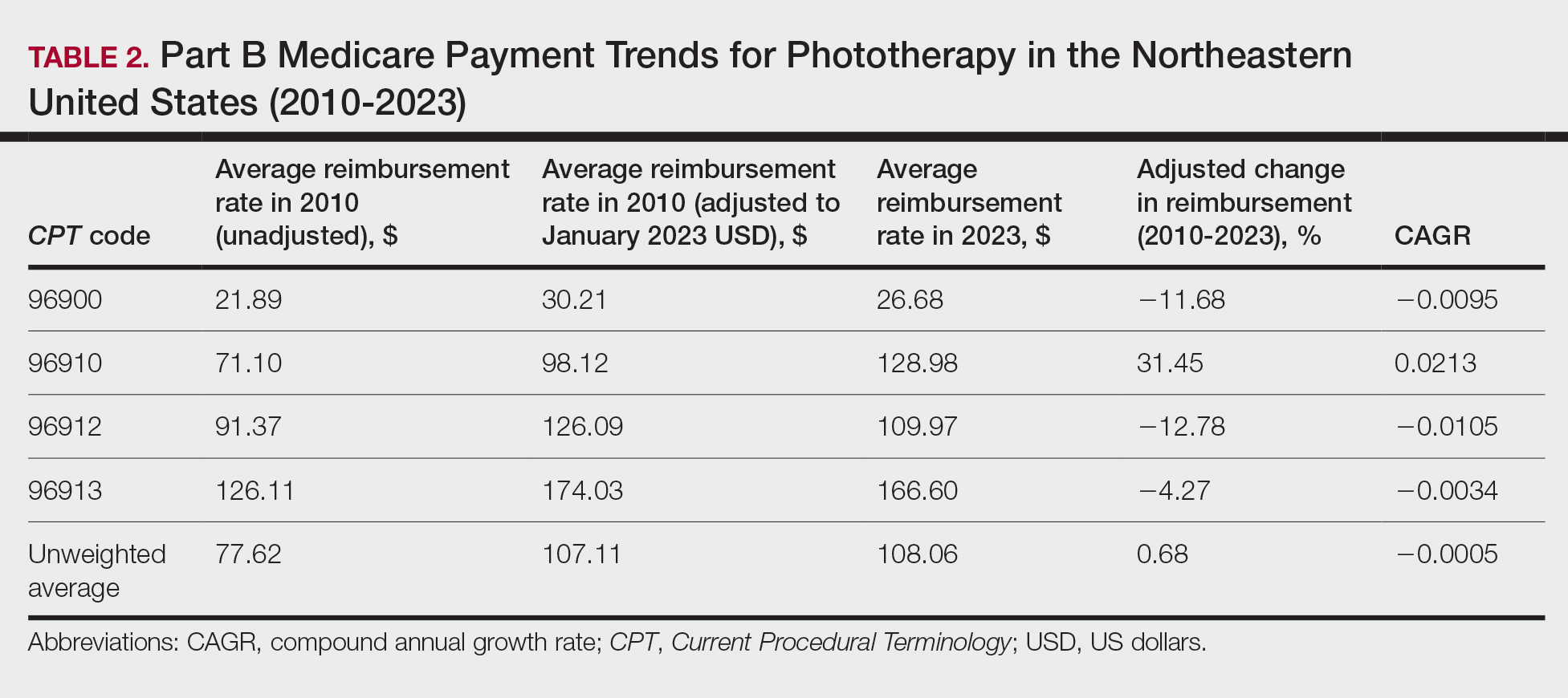
On average (unweighted), phototherapy reimbursement rates in the Midwest increased by 8.40% between 2010 and 2023 (Table 3). After weighting for 2020 physician utilization, the average change in reimbursement rate was +28.53%. During this time period, CPT code 96910 reported the greatest adjusted change in reimbursement (+41.48%)($80.42 to $113.78; CAGR, +0.0270), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−6.14%)($103.28 to $97.03; CAGR, −0.0049). For CPT code 96900, the reported adjusted decrease in reimbursement was −4.73% ($24.69 to $23.52; CAGR, −0.0037), and for CPT code 96913, the reported adjusted increase in reimbursement was +2.99% ($142.72 to $146.99; CAGR, +0.0023).
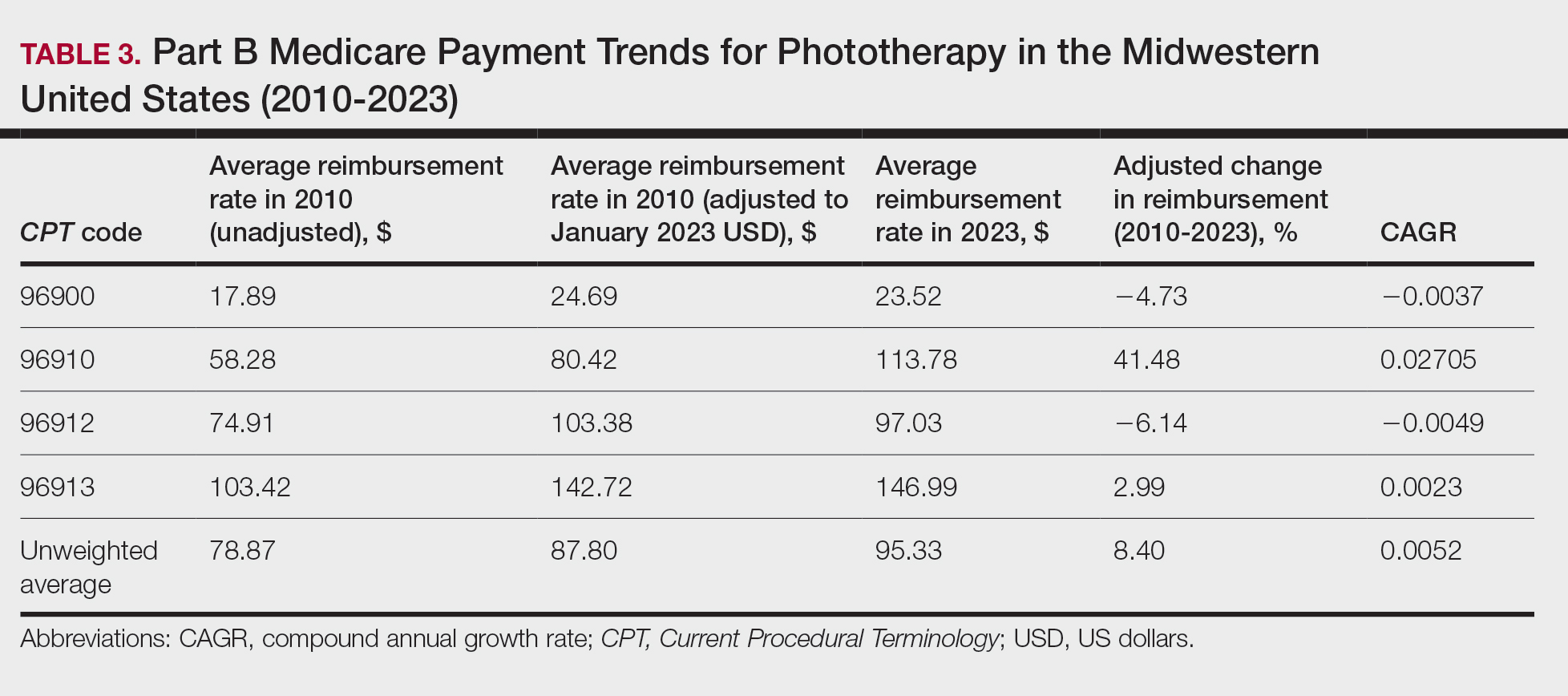
On average (unweighted), phototherapy reimbursement rates in the South decreased by 2.62% between 2010 and 2023 (Table 4). After weighting for 2020 physician utilization, the average change in reimbursement rate was +15.41%. During this time period, CPT code 96910 reported the greatest adjusted change in reimbursement (+27.26%)($90.40 to $115.04 USD; CAGR, +0.0187), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−15.50%)($116.08 to $98.09; CAGR, −0.0129). For CPT code 96900, the reported adjusted decrease in reimbursement was −15.06% ($28.02 to $23.80; CAGR, −0.0125), and for CPT code 96913, the reported adjusted decrease in reimbursement was −7.19% ($160.11 to $148.61; CAGR, −0.0057).
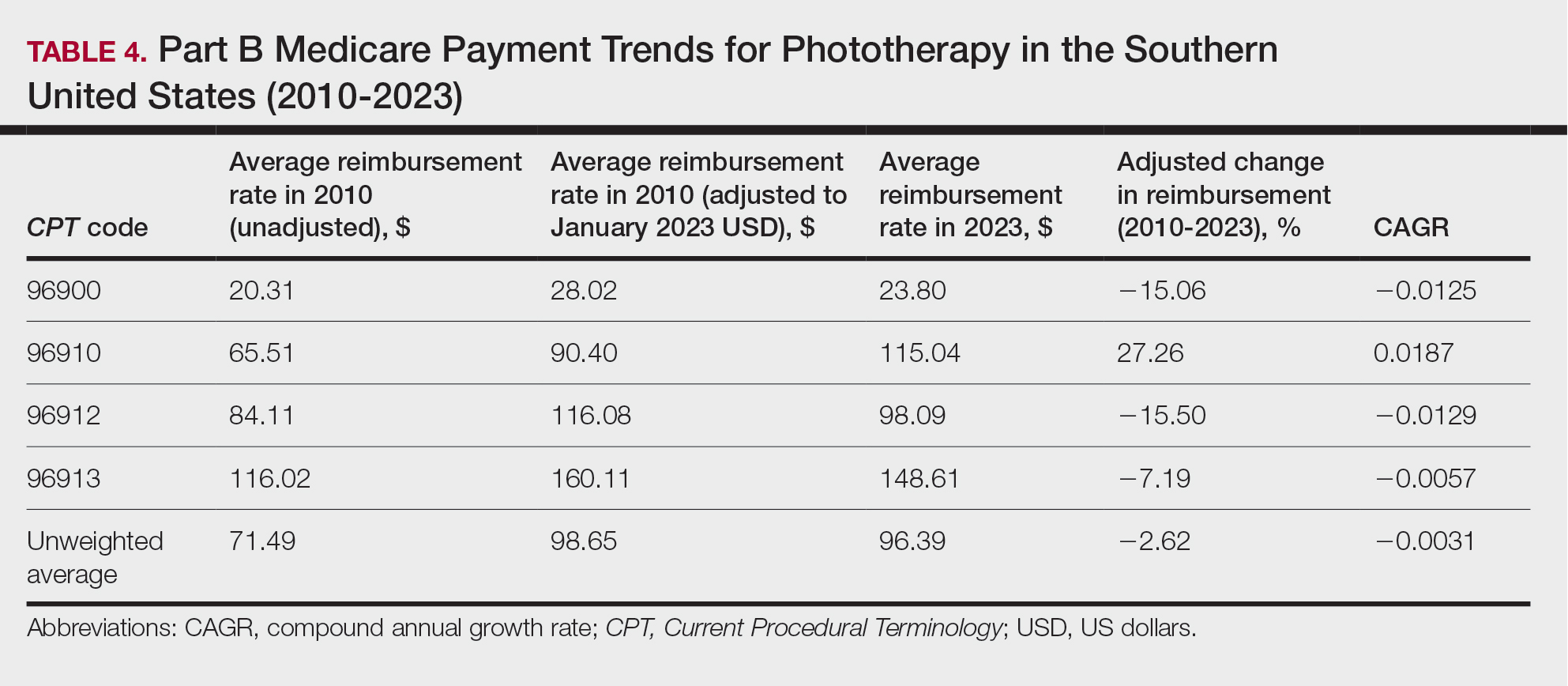
On average (unweighted), phototherapy reimbursement rates in the West increased by 27.53% between 2010 and 2023 (Table 5). After weighting for 2020 physician utilization, the average change in reimbursement rate was +51.16%. Reimbursement for all analyzed procedures increased in the western United States. During this time period, CPT code 96910 reported the greatest adjusted increase in reimbursement (+66.56%)($80.84 to $134.65; CAGR, +0.0400), and CPT code 96912 reported the lowest adjusted increase in reimbursement (+10.64%)($103.88 to $114.93; CAGR, +0.0078). For CPT code 96900, the reported adjusted increase in reimbursement was 11.54% ($24.88 to $27.75; CAGR, +0.0084), and for CPT code 96913, the reported adjusted increase in reimbursement was 21.38% ($143.39 to $174.04; CAGR, +0.0150).
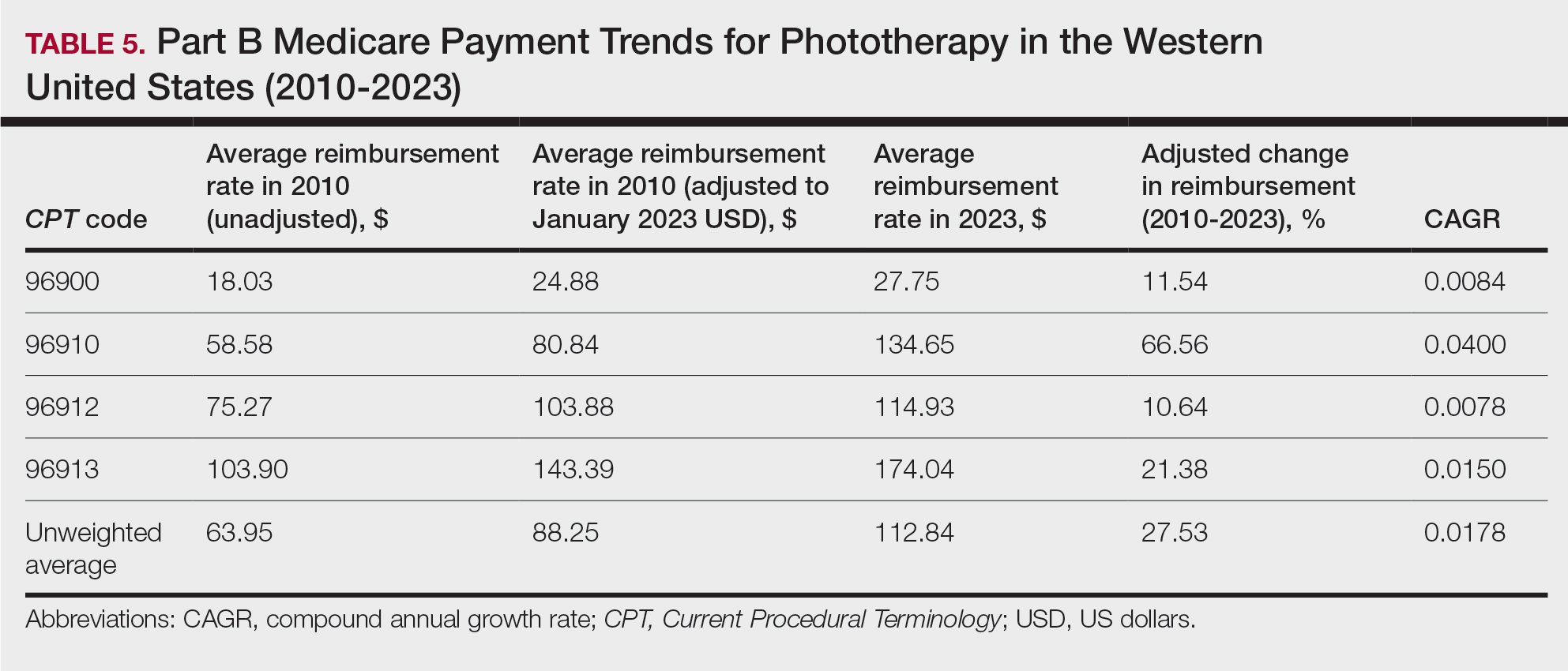
In this study evaluating geographical payment trends for phototherapy from 2010 to 2023, we demonstrated regional inconsistency in mean inflation-adjusted Medicare reimbursement rates. We found that all phototherapy procedures had increased reimbursement in the western United States, whereas all other regions reported cuts in reimbursement rates for at least half of the analyzed procedures. After adjusting for procedure utilization by physicians, weighted mean reimbursement for phototherapy increased in all US regions.
In a cross-sectional study that explored trends in the geographic distribution of dermatologists from 2012 to 2017, dermatologists in the northeastern and western United States were more likely to be located in higher-income zip codes, whereas dermatologists in the southern United States were more likely to be located in lower-income zip codes,7 suggesting that payment rate changes are not concordant with cost of living. Additionally, Lauck and colleagues8 observed that 75% of the top 20 most common procedures performed by dermatologists had decreased reimbursement (mean change, −10.8%) from 2011 to 2021. Other studies on Medicare reimbursement trends over the last 2 decades have reported major decreases within other specialties, suggesting that declining Medicare reimbursements are not unique to dermatology.9,10 It is critical to monitor these developments, as the Centers for Medicare & Medicaid Services emphasized health care policy changes aimed at increasing reimbursements for evaluation and management services with compensatory payment cuts in billing for procedural services.11
Mazmudar et al12 previously reported a mean reimbursement decrease of −6.6% for laser/phototherapy procedures between 2007 and 2021, but these data did not include the heavily utilized Goeckerman treatment. Changes in reimbursement pose major ramifications for dermatologists—for practice size, scope, and longevity—as rates influence changes in commercial insurance reimbursements.13 Medicare plays a major role in the US health care system as the second largest expenditure14; indeed, between 2000 and 2015, Part B billing volume for phototherapy procedures increased 5% annually. However, phototherapy remains inaccessible in many locations due to unequal regional distribution of phototherapy clinics.6 Moreover, home phototherapy units are not yet widely utilized because of safety and efficacy concerns, lack of physician oversight, and difficulty obtaining insurance coverage.15 Acknowledgment and consideration of these geographical trends may persuasively allow policymakers, hospitals, and physicians to facilitate cost-effective phototherapy reimbursements that ensure continued access to quality and sustainable dermatologic care in the United States that tailor to regional needs.
In sum, this analysis reveals regional trends in Part B physician reimbursement for phototherapy procedures, with all US regions reporting a mean increase in phototherapy reimbursement after adjusting for utilization, albeit to varying degrees. Mean reimbursement for photochemotherapy by Goeckerman treatment or using petrolatum and UVB increased most among phototherapy procedures. Mean reimbursement for both actinotherapy and photochemotherapy using psoralen plus UVA decreased in all regions except the western United States.
Limitations include the restriction to Part B MPFS and the reliance on single-year (2020) physician utilization data to compute weighted changes in average reimbursement across a multiyear range, effectively restricting sweeping conclusions. Still, this study puts forth actionable insights for dermatologists and policymakers alike to appreciate and consider.
- Rathod DG, Muneer H, Masood S. Phototherapy. StatPearls. StatPearls Publishing; 2002.
- Branisteanu DE, Dirzu DS, Toader MP, et al. Phototherapy in dermatological maladies (Review). Exp Ther Med. 2022;23:259. doi:10.3892/etm.2022.11184
- Barros NM, Sbroglio LL, Buffara MO, et al. Phototherapy. An Bras Dermatol. 2021;96:397-407. doi:10.1016/j.abd.2021.03.001
- Vieyra-Garcia PA, Wolf P. A deep dive into UV-based phototherapy: mechanisms of action and emerging molecular targets in inflammation and cancer. Pharmacol Ther. 2021;222:107784. doi:10.1016/j.pharmthera.2020.107784
- Oulee A, Javadi SS, Martin A, et al. Phototherapy trends in dermatology 2015-2018. J Dermatolog Treat. 2022;33:2545-2546. doi:10.1080/09546634.2021.2019660
- Tan SY, Buzney E, Mostaghimi A. Trends in phototherapy utilization among Medicare beneficiaries in the United States, 2000 to 2015. J Am Acad Dermatol. 2018;79:672-679. doi:10.1016/j.jaad.2018.03.018
- Benlagha I, Nguyen BM. Changes in dermatology practice characteristics in the United States from 2012 to 2017. JAAD Int. 2021;3:92-101. doi:10.1016/j.jdin.2021.03.005
- Lauck K, Nguyen QB, Hebert A. Trends in Medicare reimbursement within dermatology: 2011-2021. Skin. 2022;6:122-131. doi:10.25251/skin.6.2.5
- Smith JF, Moore ML, Pollock JR, et al. National and geographic trends in Medicare reimbursement rates for orthopedic shoulder and upper extremity surgery from 2000 to 2020. J Shoulder Elbow Surg. 2022;31:860-867. doi:10.1016/j.jse.2021.09.001
- Haglin JM, Eltorai AEM, Richter KR, et al. Medicare reimbursement for general surgery procedures: 2000 to 2018. Ann Surg. 2020;271:17-22. doi:10.1097/SLA.0000000000003289
- Fleishon HB. Evaluation and management coding initiative. J Am Coll Radiol. 2020;17:1539-1540. doi:10.1016/j.jacr.2020.09.057
- Mazmudar RS, Sheth A, Tripathi R, et al. Inflation-adjusted trends in Medicare reimbursement for common dermatologic procedures, 2007-2021. JAMA Dermatol. 2021;157:1355-1358. doi:10.1001/jamadermatol.2021.3453
- Clemens J, Gottlieb JD. In the shadow of a giant: Medicare’s influence on private physician payments. J Polit Econ. 2017;125:1-39. doi:10.1086/689772
- Ya J, Ezaldein HH, Scott JF. Trends in Medicare utilization by dermatologists, 2012-2015. JAMA Dermatol. 2019;155:471-474. doi:10.1001/jamadermatol.2018.4212
- Rajpara AN, O’Neill JL, Nolan BV, et al. Review of home phototherapy. Dermatol Online J. 2010;16:2.
To the Editor:
Phototherapy regularly is utilized in the outpatient setting to address various skin pathologies, including atopic dermatitis, psoriasis, pruritus, vitiligo, and mycosis fungoides.1,2 Phototherapy is broadly defined by the measured administration of nonionizing radiation within the UV range including wavelengths within the UVA (eg, psoralen sensitizer plus UVA-1) and UVB (eg, broadband UVB, narrowband UVB) spectrums.1,3 Generally, the mechanism of action is derived from effects on inflammatory components of cutaneous disorders and the induction of apoptosis, both precipitating numerous downstream events.4
From 2015 to 2018, there were more than 1.3 million outpatient phototherapy visits in the United States, with the most common procedural indications being dermatitis not otherwise specified, atopic dermatitis, and pruritus.5 From 2000 to 2015, the quantity of phototherapy services billed to Medicare trended upwards by an average of 5% per year, increasing from 334,670 in the year 2000 to 692,093 in 2015.6 Therefore, an illustration of associated costs would be beneficial. Additionally, because total cost and physician reimbursement fluctuate from year to year, studies demonstrating overall trends can inform both US policymakers and physicians. There is a paucity of research on geographical trends for procedural reimbursements in dermatology for phototherapy. Understanding geographic trends of reimbursement could duly serve to optimize dermatologist practice patterns involving access to viable and quality care for patients seeking treatment as well as draw health policymakers’ attention to striking adjustments in physician fees. Therefore, in this study we aimed to illustrate the most recent regional payment trends in phototherapy procedures for Medicare B patients.
We queried the Centers for Medicare & Medicaid Services Medicare Physician Fee Schedule (MPFS) database (https://www.cms.gov/medicare/payment/fee-schedules/physician/lookup-tool) for the years 2010 to 2023 for Current Procedural Terminology (CPT) codes common to phototherapy procedures: actinotherapy (96900); photochemotherapy by Goeckerman treatment or using petrolatum and UVB (96910); photochemotherapy using psoralen plus UVA (96912); and photochemotherapy of severe dermatoses requiring a minimum of 4 hours of care under direct physician supervision (96913). Nonfacility prices for these procedures were analyzed. For 2010, due to midyear alterations to Medicare reimbursement (owed to bills HR 3962 and HR 4872), the mean price data of MPFS files 2010A and 2010B were used. All dollar values were converted to January 2023 US dollars using corresponding consumer price index inflation data. The Medicare Administrative Contractors were used to group state pricing information by region in accordance with established US Census Bureau subdivisions (https://www.census.gov/programs-surveys/economic-census/guidance-geographies/levels.html). Weighted percentage change in reimbursement rate was calculated using physician (MD or DO) utilization (procedure volume) data available in the 2020 Physician and Other Practitioners Public Use File (https://data.cms.gov/provider-summary-by-type-of-service/medicare-physician-other-practitioners/medicare-physician-other-practitioners-by-provider-and-service). All descriptive statistics and visualization were generated using R software (v4.2.2)(R Development Core Team).
Table 1 provides physician utilization data and the corresponding number of Part B beneficiaries for phototherapy procedures in 2020. There were 65,045 services of actinotherapy provided to a total of 6855 unique Part B beneficiaries, 173,979 services of photochemotherapy by Goeckerman treatment or using petrolatum and UVB provided to 13,122 unique Part B beneficiaries, 2524 services of photochemotherapy using psoralen plus UVA provided to a total of 357 unique Part B beneficiaries, and 37 services of photochemotherapy of severe dermatoses requiring a minimum of 4 hours of care under direct physician supervision provided to a total of 27 unique Part B beneficiaries.

On average (unweighted), phototherapy reimbursement rates in the North increased by 0.68% between 2010 and 2023 (Table 2). After weighting for 2020 physician utilization, the average change in reimbursement rate was +19.37%. During this time period, CPT code 96910 reported the greatest adjusted increase in reimbursement (+31.45%)($98.12 to $128.98; compound annual growth rate [CAGR], +0.0213), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−12.76%)($126.09 to $109.97; CAGR, −0.0105). For CPT code 96900, the reported adjusted decrease in reimbursement was −11.68% ($30.21 to $26.68; CAGR, −0.0095), and for CPT code 96913, the reported adjusted decrease in reimbursement was −4.27% ($174.03 to $166.60; CAGR, −0.0034).

On average (unweighted), phototherapy reimbursement rates in the Midwest increased by 8.40% between 2010 and 2023 (Table 3). After weighting for 2020 physician utilization, the average change in reimbursement rate was +28.53%. During this time period, CPT code 96910 reported the greatest adjusted change in reimbursement (+41.48%)($80.42 to $113.78; CAGR, +0.0270), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−6.14%)($103.28 to $97.03; CAGR, −0.0049). For CPT code 96900, the reported adjusted decrease in reimbursement was −4.73% ($24.69 to $23.52; CAGR, −0.0037), and for CPT code 96913, the reported adjusted increase in reimbursement was +2.99% ($142.72 to $146.99; CAGR, +0.0023).

On average (unweighted), phototherapy reimbursement rates in the South decreased by 2.62% between 2010 and 2023 (Table 4). After weighting for 2020 physician utilization, the average change in reimbursement rate was +15.41%. During this time period, CPT code 96910 reported the greatest adjusted change in reimbursement (+27.26%)($90.40 to $115.04 USD; CAGR, +0.0187), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−15.50%)($116.08 to $98.09; CAGR, −0.0129). For CPT code 96900, the reported adjusted decrease in reimbursement was −15.06% ($28.02 to $23.80; CAGR, −0.0125), and for CPT code 96913, the reported adjusted decrease in reimbursement was −7.19% ($160.11 to $148.61; CAGR, −0.0057).

On average (unweighted), phototherapy reimbursement rates in the West increased by 27.53% between 2010 and 2023 (Table 5). After weighting for 2020 physician utilization, the average change in reimbursement rate was +51.16%. Reimbursement for all analyzed procedures increased in the western United States. During this time period, CPT code 96910 reported the greatest adjusted increase in reimbursement (+66.56%)($80.84 to $134.65; CAGR, +0.0400), and CPT code 96912 reported the lowest adjusted increase in reimbursement (+10.64%)($103.88 to $114.93; CAGR, +0.0078). For CPT code 96900, the reported adjusted increase in reimbursement was 11.54% ($24.88 to $27.75; CAGR, +0.0084), and for CPT code 96913, the reported adjusted increase in reimbursement was 21.38% ($143.39 to $174.04; CAGR, +0.0150).

In this study evaluating geographical payment trends for phototherapy from 2010 to 2023, we demonstrated regional inconsistency in mean inflation-adjusted Medicare reimbursement rates. We found that all phototherapy procedures had increased reimbursement in the western United States, whereas all other regions reported cuts in reimbursement rates for at least half of the analyzed procedures. After adjusting for procedure utilization by physicians, weighted mean reimbursement for phototherapy increased in all US regions.
In a cross-sectional study that explored trends in the geographic distribution of dermatologists from 2012 to 2017, dermatologists in the northeastern and western United States were more likely to be located in higher-income zip codes, whereas dermatologists in the southern United States were more likely to be located in lower-income zip codes,7 suggesting that payment rate changes are not concordant with cost of living. Additionally, Lauck and colleagues8 observed that 75% of the top 20 most common procedures performed by dermatologists had decreased reimbursement (mean change, −10.8%) from 2011 to 2021. Other studies on Medicare reimbursement trends over the last 2 decades have reported major decreases within other specialties, suggesting that declining Medicare reimbursements are not unique to dermatology.9,10 It is critical to monitor these developments, as the Centers for Medicare & Medicaid Services emphasized health care policy changes aimed at increasing reimbursements for evaluation and management services with compensatory payment cuts in billing for procedural services.11
Mazmudar et al12 previously reported a mean reimbursement decrease of −6.6% for laser/phototherapy procedures between 2007 and 2021, but these data did not include the heavily utilized Goeckerman treatment. Changes in reimbursement pose major ramifications for dermatologists—for practice size, scope, and longevity—as rates influence changes in commercial insurance reimbursements.13 Medicare plays a major role in the US health care system as the second largest expenditure14; indeed, between 2000 and 2015, Part B billing volume for phototherapy procedures increased 5% annually. However, phototherapy remains inaccessible in many locations due to unequal regional distribution of phototherapy clinics.6 Moreover, home phototherapy units are not yet widely utilized because of safety and efficacy concerns, lack of physician oversight, and difficulty obtaining insurance coverage.15 Acknowledgment and consideration of these geographical trends may persuasively allow policymakers, hospitals, and physicians to facilitate cost-effective phototherapy reimbursements that ensure continued access to quality and sustainable dermatologic care in the United States that tailor to regional needs.
In sum, this analysis reveals regional trends in Part B physician reimbursement for phototherapy procedures, with all US regions reporting a mean increase in phototherapy reimbursement after adjusting for utilization, albeit to varying degrees. Mean reimbursement for photochemotherapy by Goeckerman treatment or using petrolatum and UVB increased most among phototherapy procedures. Mean reimbursement for both actinotherapy and photochemotherapy using psoralen plus UVA decreased in all regions except the western United States.
Limitations include the restriction to Part B MPFS and the reliance on single-year (2020) physician utilization data to compute weighted changes in average reimbursement across a multiyear range, effectively restricting sweeping conclusions. Still, this study puts forth actionable insights for dermatologists and policymakers alike to appreciate and consider.
To the Editor:
Phototherapy regularly is utilized in the outpatient setting to address various skin pathologies, including atopic dermatitis, psoriasis, pruritus, vitiligo, and mycosis fungoides.1,2 Phototherapy is broadly defined by the measured administration of nonionizing radiation within the UV range including wavelengths within the UVA (eg, psoralen sensitizer plus UVA-1) and UVB (eg, broadband UVB, narrowband UVB) spectrums.1,3 Generally, the mechanism of action is derived from effects on inflammatory components of cutaneous disorders and the induction of apoptosis, both precipitating numerous downstream events.4
From 2015 to 2018, there were more than 1.3 million outpatient phototherapy visits in the United States, with the most common procedural indications being dermatitis not otherwise specified, atopic dermatitis, and pruritus.5 From 2000 to 2015, the quantity of phototherapy services billed to Medicare trended upwards by an average of 5% per year, increasing from 334,670 in the year 2000 to 692,093 in 2015.6 Therefore, an illustration of associated costs would be beneficial. Additionally, because total cost and physician reimbursement fluctuate from year to year, studies demonstrating overall trends can inform both US policymakers and physicians. There is a paucity of research on geographical trends for procedural reimbursements in dermatology for phototherapy. Understanding geographic trends of reimbursement could duly serve to optimize dermatologist practice patterns involving access to viable and quality care for patients seeking treatment as well as draw health policymakers’ attention to striking adjustments in physician fees. Therefore, in this study we aimed to illustrate the most recent regional payment trends in phototherapy procedures for Medicare B patients.
We queried the Centers for Medicare & Medicaid Services Medicare Physician Fee Schedule (MPFS) database (https://www.cms.gov/medicare/payment/fee-schedules/physician/lookup-tool) for the years 2010 to 2023 for Current Procedural Terminology (CPT) codes common to phototherapy procedures: actinotherapy (96900); photochemotherapy by Goeckerman treatment or using petrolatum and UVB (96910); photochemotherapy using psoralen plus UVA (96912); and photochemotherapy of severe dermatoses requiring a minimum of 4 hours of care under direct physician supervision (96913). Nonfacility prices for these procedures were analyzed. For 2010, due to midyear alterations to Medicare reimbursement (owed to bills HR 3962 and HR 4872), the mean price data of MPFS files 2010A and 2010B were used. All dollar values were converted to January 2023 US dollars using corresponding consumer price index inflation data. The Medicare Administrative Contractors were used to group state pricing information by region in accordance with established US Census Bureau subdivisions (https://www.census.gov/programs-surveys/economic-census/guidance-geographies/levels.html). Weighted percentage change in reimbursement rate was calculated using physician (MD or DO) utilization (procedure volume) data available in the 2020 Physician and Other Practitioners Public Use File (https://data.cms.gov/provider-summary-by-type-of-service/medicare-physician-other-practitioners/medicare-physician-other-practitioners-by-provider-and-service). All descriptive statistics and visualization were generated using R software (v4.2.2)(R Development Core Team).
Table 1 provides physician utilization data and the corresponding number of Part B beneficiaries for phototherapy procedures in 2020. There were 65,045 services of actinotherapy provided to a total of 6855 unique Part B beneficiaries, 173,979 services of photochemotherapy by Goeckerman treatment or using petrolatum and UVB provided to 13,122 unique Part B beneficiaries, 2524 services of photochemotherapy using psoralen plus UVA provided to a total of 357 unique Part B beneficiaries, and 37 services of photochemotherapy of severe dermatoses requiring a minimum of 4 hours of care under direct physician supervision provided to a total of 27 unique Part B beneficiaries.

On average (unweighted), phototherapy reimbursement rates in the North increased by 0.68% between 2010 and 2023 (Table 2). After weighting for 2020 physician utilization, the average change in reimbursement rate was +19.37%. During this time period, CPT code 96910 reported the greatest adjusted increase in reimbursement (+31.45%)($98.12 to $128.98; compound annual growth rate [CAGR], +0.0213), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−12.76%)($126.09 to $109.97; CAGR, −0.0105). For CPT code 96900, the reported adjusted decrease in reimbursement was −11.68% ($30.21 to $26.68; CAGR, −0.0095), and for CPT code 96913, the reported adjusted decrease in reimbursement was −4.27% ($174.03 to $166.60; CAGR, −0.0034).

On average (unweighted), phototherapy reimbursement rates in the Midwest increased by 8.40% between 2010 and 2023 (Table 3). After weighting for 2020 physician utilization, the average change in reimbursement rate was +28.53%. During this time period, CPT code 96910 reported the greatest adjusted change in reimbursement (+41.48%)($80.42 to $113.78; CAGR, +0.0270), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−6.14%)($103.28 to $97.03; CAGR, −0.0049). For CPT code 96900, the reported adjusted decrease in reimbursement was −4.73% ($24.69 to $23.52; CAGR, −0.0037), and for CPT code 96913, the reported adjusted increase in reimbursement was +2.99% ($142.72 to $146.99; CAGR, +0.0023).

On average (unweighted), phototherapy reimbursement rates in the South decreased by 2.62% between 2010 and 2023 (Table 4). After weighting for 2020 physician utilization, the average change in reimbursement rate was +15.41%. During this time period, CPT code 96910 reported the greatest adjusted change in reimbursement (+27.26%)($90.40 to $115.04 USD; CAGR, +0.0187), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−15.50%)($116.08 to $98.09; CAGR, −0.0129). For CPT code 96900, the reported adjusted decrease in reimbursement was −15.06% ($28.02 to $23.80; CAGR, −0.0125), and for CPT code 96913, the reported adjusted decrease in reimbursement was −7.19% ($160.11 to $148.61; CAGR, −0.0057).

On average (unweighted), phototherapy reimbursement rates in the West increased by 27.53% between 2010 and 2023 (Table 5). After weighting for 2020 physician utilization, the average change in reimbursement rate was +51.16%. Reimbursement for all analyzed procedures increased in the western United States. During this time period, CPT code 96910 reported the greatest adjusted increase in reimbursement (+66.56%)($80.84 to $134.65; CAGR, +0.0400), and CPT code 96912 reported the lowest adjusted increase in reimbursement (+10.64%)($103.88 to $114.93; CAGR, +0.0078). For CPT code 96900, the reported adjusted increase in reimbursement was 11.54% ($24.88 to $27.75; CAGR, +0.0084), and for CPT code 96913, the reported adjusted increase in reimbursement was 21.38% ($143.39 to $174.04; CAGR, +0.0150).

In this study evaluating geographical payment trends for phototherapy from 2010 to 2023, we demonstrated regional inconsistency in mean inflation-adjusted Medicare reimbursement rates. We found that all phototherapy procedures had increased reimbursement in the western United States, whereas all other regions reported cuts in reimbursement rates for at least half of the analyzed procedures. After adjusting for procedure utilization by physicians, weighted mean reimbursement for phototherapy increased in all US regions.
In a cross-sectional study that explored trends in the geographic distribution of dermatologists from 2012 to 2017, dermatologists in the northeastern and western United States were more likely to be located in higher-income zip codes, whereas dermatologists in the southern United States were more likely to be located in lower-income zip codes,7 suggesting that payment rate changes are not concordant with cost of living. Additionally, Lauck and colleagues8 observed that 75% of the top 20 most common procedures performed by dermatologists had decreased reimbursement (mean change, −10.8%) from 2011 to 2021. Other studies on Medicare reimbursement trends over the last 2 decades have reported major decreases within other specialties, suggesting that declining Medicare reimbursements are not unique to dermatology.9,10 It is critical to monitor these developments, as the Centers for Medicare & Medicaid Services emphasized health care policy changes aimed at increasing reimbursements for evaluation and management services with compensatory payment cuts in billing for procedural services.11
Mazmudar et al12 previously reported a mean reimbursement decrease of −6.6% for laser/phototherapy procedures between 2007 and 2021, but these data did not include the heavily utilized Goeckerman treatment. Changes in reimbursement pose major ramifications for dermatologists—for practice size, scope, and longevity—as rates influence changes in commercial insurance reimbursements.13 Medicare plays a major role in the US health care system as the second largest expenditure14; indeed, between 2000 and 2015, Part B billing volume for phototherapy procedures increased 5% annually. However, phototherapy remains inaccessible in many locations due to unequal regional distribution of phototherapy clinics.6 Moreover, home phototherapy units are not yet widely utilized because of safety and efficacy concerns, lack of physician oversight, and difficulty obtaining insurance coverage.15 Acknowledgment and consideration of these geographical trends may persuasively allow policymakers, hospitals, and physicians to facilitate cost-effective phototherapy reimbursements that ensure continued access to quality and sustainable dermatologic care in the United States that tailor to regional needs.
In sum, this analysis reveals regional trends in Part B physician reimbursement for phototherapy procedures, with all US regions reporting a mean increase in phototherapy reimbursement after adjusting for utilization, albeit to varying degrees. Mean reimbursement for photochemotherapy by Goeckerman treatment or using petrolatum and UVB increased most among phototherapy procedures. Mean reimbursement for both actinotherapy and photochemotherapy using psoralen plus UVA decreased in all regions except the western United States.
Limitations include the restriction to Part B MPFS and the reliance on single-year (2020) physician utilization data to compute weighted changes in average reimbursement across a multiyear range, effectively restricting sweeping conclusions. Still, this study puts forth actionable insights for dermatologists and policymakers alike to appreciate and consider.
- Rathod DG, Muneer H, Masood S. Phototherapy. StatPearls. StatPearls Publishing; 2002.
- Branisteanu DE, Dirzu DS, Toader MP, et al. Phototherapy in dermatological maladies (Review). Exp Ther Med. 2022;23:259. doi:10.3892/etm.2022.11184
- Barros NM, Sbroglio LL, Buffara MO, et al. Phototherapy. An Bras Dermatol. 2021;96:397-407. doi:10.1016/j.abd.2021.03.001
- Vieyra-Garcia PA, Wolf P. A deep dive into UV-based phototherapy: mechanisms of action and emerging molecular targets in inflammation and cancer. Pharmacol Ther. 2021;222:107784. doi:10.1016/j.pharmthera.2020.107784
- Oulee A, Javadi SS, Martin A, et al. Phototherapy trends in dermatology 2015-2018. J Dermatolog Treat. 2022;33:2545-2546. doi:10.1080/09546634.2021.2019660
- Tan SY, Buzney E, Mostaghimi A. Trends in phototherapy utilization among Medicare beneficiaries in the United States, 2000 to 2015. J Am Acad Dermatol. 2018;79:672-679. doi:10.1016/j.jaad.2018.03.018
- Benlagha I, Nguyen BM. Changes in dermatology practice characteristics in the United States from 2012 to 2017. JAAD Int. 2021;3:92-101. doi:10.1016/j.jdin.2021.03.005
- Lauck K, Nguyen QB, Hebert A. Trends in Medicare reimbursement within dermatology: 2011-2021. Skin. 2022;6:122-131. doi:10.25251/skin.6.2.5
- Smith JF, Moore ML, Pollock JR, et al. National and geographic trends in Medicare reimbursement rates for orthopedic shoulder and upper extremity surgery from 2000 to 2020. J Shoulder Elbow Surg. 2022;31:860-867. doi:10.1016/j.jse.2021.09.001
- Haglin JM, Eltorai AEM, Richter KR, et al. Medicare reimbursement for general surgery procedures: 2000 to 2018. Ann Surg. 2020;271:17-22. doi:10.1097/SLA.0000000000003289
- Fleishon HB. Evaluation and management coding initiative. J Am Coll Radiol. 2020;17:1539-1540. doi:10.1016/j.jacr.2020.09.057
- Mazmudar RS, Sheth A, Tripathi R, et al. Inflation-adjusted trends in Medicare reimbursement for common dermatologic procedures, 2007-2021. JAMA Dermatol. 2021;157:1355-1358. doi:10.1001/jamadermatol.2021.3453
- Clemens J, Gottlieb JD. In the shadow of a giant: Medicare’s influence on private physician payments. J Polit Econ. 2017;125:1-39. doi:10.1086/689772
- Ya J, Ezaldein HH, Scott JF. Trends in Medicare utilization by dermatologists, 2012-2015. JAMA Dermatol. 2019;155:471-474. doi:10.1001/jamadermatol.2018.4212
- Rajpara AN, O’Neill JL, Nolan BV, et al. Review of home phototherapy. Dermatol Online J. 2010;16:2.
- Rathod DG, Muneer H, Masood S. Phototherapy. StatPearls. StatPearls Publishing; 2002.
- Branisteanu DE, Dirzu DS, Toader MP, et al. Phototherapy in dermatological maladies (Review). Exp Ther Med. 2022;23:259. doi:10.3892/etm.2022.11184
- Barros NM, Sbroglio LL, Buffara MO, et al. Phototherapy. An Bras Dermatol. 2021;96:397-407. doi:10.1016/j.abd.2021.03.001
- Vieyra-Garcia PA, Wolf P. A deep dive into UV-based phototherapy: mechanisms of action and emerging molecular targets in inflammation and cancer. Pharmacol Ther. 2021;222:107784. doi:10.1016/j.pharmthera.2020.107784
- Oulee A, Javadi SS, Martin A, et al. Phototherapy trends in dermatology 2015-2018. J Dermatolog Treat. 2022;33:2545-2546. doi:10.1080/09546634.2021.2019660
- Tan SY, Buzney E, Mostaghimi A. Trends in phototherapy utilization among Medicare beneficiaries in the United States, 2000 to 2015. J Am Acad Dermatol. 2018;79:672-679. doi:10.1016/j.jaad.2018.03.018
- Benlagha I, Nguyen BM. Changes in dermatology practice characteristics in the United States from 2012 to 2017. JAAD Int. 2021;3:92-101. doi:10.1016/j.jdin.2021.03.005
- Lauck K, Nguyen QB, Hebert A. Trends in Medicare reimbursement within dermatology: 2011-2021. Skin. 2022;6:122-131. doi:10.25251/skin.6.2.5
- Smith JF, Moore ML, Pollock JR, et al. National and geographic trends in Medicare reimbursement rates for orthopedic shoulder and upper extremity surgery from 2000 to 2020. J Shoulder Elbow Surg. 2022;31:860-867. doi:10.1016/j.jse.2021.09.001
- Haglin JM, Eltorai AEM, Richter KR, et al. Medicare reimbursement for general surgery procedures: 2000 to 2018. Ann Surg. 2020;271:17-22. doi:10.1097/SLA.0000000000003289
- Fleishon HB. Evaluation and management coding initiative. J Am Coll Radiol. 2020;17:1539-1540. doi:10.1016/j.jacr.2020.09.057
- Mazmudar RS, Sheth A, Tripathi R, et al. Inflation-adjusted trends in Medicare reimbursement for common dermatologic procedures, 2007-2021. JAMA Dermatol. 2021;157:1355-1358. doi:10.1001/jamadermatol.2021.3453
- Clemens J, Gottlieb JD. In the shadow of a giant: Medicare’s influence on private physician payments. J Polit Econ. 2017;125:1-39. doi:10.1086/689772
- Ya J, Ezaldein HH, Scott JF. Trends in Medicare utilization by dermatologists, 2012-2015. JAMA Dermatol. 2019;155:471-474. doi:10.1001/jamadermatol.2018.4212
- Rajpara AN, O’Neill JL, Nolan BV, et al. Review of home phototherapy. Dermatol Online J. 2010;16:2.
Practice Points
- After weighting for procedure utilization, mean reimbursement for phototherapy increased across all US regions from 2010 to 2023 (mean change, +28.62%), yet with marked regional diversity.
- The southern United States reported the least growth in weighted mean reimbursement (+15.41%), and the western United States reported the greatest growth in weighted mean reimbursement (+51.16%).
- Region- and procedure-specific payment changes are especially valuable to dermatologists and policymakers alike, potentially reinvigorating payment reform discussions.
Thiazide-Induced Hyponatremia Presenting as a Fall in an Older Adult
Hypertension is a major risk factor for heart disease, stroke, and kidney disease. The prevalence of hypertension increases with age, primarily due to age-related changes in arterial physiology.1 For older adults, current guidelines regarding blood pressure (BP) treatment goals vary. The American Heart Association/American College of Cardiology 2017 clinical practice guidelines recommend a systolic BP (SBP) treatment goal of < 130 mm Hg for community-dwelling, ambulatory, noninstitutionalized adults aged ≥ 65 years; whereas the American College of Physicians/American Academy of Family Physicians recommend a goal of < 150 mm Hg for those aged ≥ 60 years without comorbidities and < 140 mm Hg for those with increased cardiovascular risk.1-3 Regardless of the specific threshold, agreement that some degree of BP control even in those with advanced age improves outcomes.2
First-line therapy for uncomplicated hypertension includes thiazide diuretics, long-acting calcium channel blockers, and renin-angiotensin system inhibitors. When choosing between these options, it is recommended to engage in shared decision making and to consider the patient’s comorbidities. Among patients who are likely to require a second agent (eg, if initial BP is > 20/10 mm Hg above goal), it is recommended to begin both drugs at the same time, preferably benazepril plus amlodipine due to the reduction in cardiovascular events reported in the ACCOMPLISH trial.4 If BP remains elevated despite 2 agents at moderate to maximum doses, it is important to investigate for secondary hypertension causes and to explore medication adherence as possible etiologies of treatment failure. Older adults are often at higher risk of adverse drug events due to age-related changes in pharmacodynamics. Despite this, there are no guidelines for choosing between different classes of antihypertensives in this population. We present a case of thiazide-induced hyponatremia in an older adult and review the risks of thiazide use in this population.
Case Presentation
A man aged > 90 years was admitted to the hospital after a syncopal episode. His history was significant for hypertension, hyperlipidemia, and vitamin D deficiency. At the time, his home medications were amlodipine 5 mg daily, atorvastatin 40 mg daily, ergocalciferol 50,000 IU weekly, and polyethylene glycol 17 g daily as needed. His syncope workup was unremarkable and included negative orthostatic vital signs, normal serial troponins, an electrocardiogram without ischemic changes, normal serum creatinine, sodium, and glucose, and a head computed tomography without any acute abnormality. Throughout the patient’s hospital stay, he had multiple elevated SBP readings, including many > 200 mm Hg. On discharge, in addition to continuing his home medications, he was started on valsartan 20 mg daily and enrolled in a remote BP monitoring program.
Three weeks later, the patient was seen by their primary care practitioner for follow-up. He reported adherence to his antihypertensive regimen. However, his remote BP monitoring revealed persistently elevated BPs, with an average of 179/79 mm Hg, a high of 205/85 mm Hg, and a low of 150/67 mm Hg over the previous 7 days. Laboratory tests obtained at the visit were notable for serum sodium of 138 mmol/L and potassium of 4.1 mmol/L. His weight was 87 kg. Given persistently elevated BP readings, in addition to continuing his amlodipine 5 mg daily and valsartan 20 mg daily, he was started on hydrochlorothiazide 25 mg daily, with plans to repeat a basic metabolic panel in 2 weeks.
Two weeks later, he fell after getting out of his bed. On examination, he was noted to have dry mucous membranes, and although no formal delirium screening was performed, he was able to repeat the months of the year backward. Vital signs were notable for positive postural hypertension, and his laboratory tests revealed a normal serum creatinine, serum sodium of 117 mmol/L
Discussion
Although thiazide diuretics are recommended as first-line therapy for uncomplicated hypertension, they are known to cause electrolyte abnormalities, including hypomagnesemia, hypokalemia, and hyponatremia.4 These metabolic derangements are more likely to occur in older adults. One study of adults aged ≥ 65 years found that at 9 months of follow-up, 14.3% of new thiazide users had developed a thiazide-related metabolic adverse event (hyponatremia < 135 mmol/L, hypokalemia < 3.5 mmol/L, and decrease in estimated glomerular filtration rate by > 25%) compared with 6.0% of nonusers (P < .001; number needed to harm [NNH] = 12).5 In addition, 3.8% of new thiazide users had an emergency department visit or were hospitalized for complications related to thiazides compared with only 2.0% of nonusers (P = .02; NNH = 56).5 Independent risk factors for thiazide-induced hyponatremia include high-comorbidity burden, low body weight, low-normal or unmeasured serum sodium, low potassium, and aged > 70 years.5-7 Each 10-year increment in age is associated with a 2-fold increase in risk, suggesting that older adults are at a much higher risk for hyponatremia than their younger peers.6
Despite their designation as a first-line option for uncomplicated hypertension, thiazide diuretics may cause more harm than good in some older adults, especially those with additional risk factors for thiazide-induced hyponatremia. In this population, these adverse effects should be discussed before starting thiazides for the treatment of hypertension. If thiazides are initiated, they should be started at the lowest possible dose, and plans made to monitor bloodwork within 1 to 2 weeks of initiation or dose change and periodically thereafter while the patient remains on the therapy.
Medication Management in Older Adults
Due to the risks of medication use in older adults, the phrase “start low, go slow” is commonly used in geriatric medicine to describe the optimal method for initiation and up-titration of new medication with the hope of mitigating adverse drug events. In our case, we started valsartan at 20 mg daily—one-fourth the recommended initial dose. Although this strategy is reasonable to “start low,” we were not surprised to find that the patient’s BP did not markedly improve on such a low dose. The team could have increased the valsartan dose to a therapeutically efficacious dose before choosing to add another hypertensive agent. In alignment with geriatric prescribing principles, starting at the lowest possible dose of hydrochlorothiazide is recommended.5 However, the clinician started hydrochlorothiazide at 25 mg daily, potentially increasing this patient’s risk of electrolyte abnormalities and eventual fall.
Managing hypertension also invites a discussion of polypharmacy and medication adherence. Older adults are at risk of polypharmacy, defined as the prescription of 5 or more medications.8 Polypharmacy is associated with increased hospitalizations, higher costs of care for individuals and health care systems, increased risks of adverse drug events, medication nonadherence, and lower quality of life for patients.9 In some situations, the risks of polypharmacy may outweigh the benefits of using multiple antihypertensives with different mechanisms of action if patients can reach their BP goal on the maximum dose of a single agent. For patients taking multiple antihypertensives, it is important to routinely monitor BP and assess whether deprescribing is indicated. Cognitive impairment and decreased social support may affect medication adherence for older adults.6 Clinicians should be aware of strategies, such as medication reminders and pillboxes, to increase antihypertensive medication adherence. Polypills that contain 2 antihypertensives can be another tool used to manage older adults to increase adherence and decrease health care costs.10
A current strategy that encompasses discussing many, if not all, of these noted elements is the Institute for Healthcare Improvement’s Age-Friendly Health System. This framework uses evidence-based tools to provide care for older adults across all clinical settings and highlights the 4Ms: what matters, medication, mentation, and mobility.11 Medication considers whether a medication is necessary, whether its use has benefits that outweigh the risks, and how it interacts with what matters, mentation, and mobility. In particular, what matters plays an important role in hypertension management in older adults given the recommended target BP differs, depending on which specialty organization guideline is followed. By better understanding what matters to patients, including their goals and priorities, clinicians can engage patients in shared decision making and provide individualized recommendations based on geriatric principles (eg, start low, go slow, principles of medication adherence) and patient comorbidities (eg, medical history and risk factors for hyponatremia) to help patients make a more informed choice about their antihypertensive treatment regimen (Figure).
Conclusions
This case illustrates the need for a specialized approach to hypertension management in older adults and the risks of thiazide diuretics in this population. Clinicians should consider BP goals, patient-specific factors, and principles of medication management in older adults. If initiating thiazide therapy, discuss the risks associated with use, start at the lowest possible dose, and monitor bloodwork within 1 to 2 weeks of initiation/dose change and periodically thereafter while the patient remains on the therapy to decrease the risk of adverse events. Finally, the Institute for Healthcare Improvement’s Age-Friendly Health System framework can be a useful when considering the addition of a new medication in an older adult’s treatment plan.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the New England Geriatrics Research, Education, and Clinical Center, Veterans Affairs Boston Healthcare System, and the Cincinnati VeteransAffairs Medical Center.
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127-e248. doi:10.1016/j.jacc.2017.11.006
2. Davis LL. Hypertension: how low to go when treating older adults. J Nurse Pract. 2019;15(1):1-6. doi:10.1016/j.nurpra.2018.10.010
3. Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166(6):430-437. doi:10.7326/M16-1785
4. Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011;57(20):2037-2114. doi:10.1016/j.jacc.2011.01.008
5. Makam AN, Boscardin WJ, Miao Y, Steinman MA. Risk of thiazide-induced metabolic adverse events in older adults. J Am Geriatr Soc. 2014;62(6):1039-1045. doi:10.1111/jgs.12839
6. Chow KM, Szeto CC, Wong TY, Leung CB, Li PK. Risk factors for thiazide-induced hyponatraemia. QJM. 2003;96(12):911-917. doi:10.1093/qjmed/hcg157
7. Clayton JA, Rodgers S, Blakey J, Avery A, Hall IP. Thiazide diuretic prescription and electrolyte abnormalities in primary care. Br J Clin Pharmacol. 2006;61(1):87-95. doi:10.1111/j.1365-2125.2005.02531.x
8. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28(2):173-186. doi:10.1016/j.cger.2012.01.002
9. Benetos A, Petrovic M, Strandberg T. Hypertension management in older and frail older patients. Circ Res. 2019;124(7):1045-1060. doi:10.1161/CIRCRESAHA.118.313236
10. Sherrill B, Halpern M, Khan S, Zhang J, Panjabi S. Single-pill vs free-equivalent combination therapies for hypertension: a meta-analysis of health care costs and adherence. J Clin Hypertens (Greenwich). 2011;13(12):898-909. doi:10.1111/j.1751-7176.2011.00550.x
11. Mate K, Fulmer T, Pelton L, et al. Evidence for the 4Ms: interactions and outcomes across the care continuum. J Aging Health. 2021;33(7-8):469-481. doi:10.1177/0898264321991658
Hypertension is a major risk factor for heart disease, stroke, and kidney disease. The prevalence of hypertension increases with age, primarily due to age-related changes in arterial physiology.1 For older adults, current guidelines regarding blood pressure (BP) treatment goals vary. The American Heart Association/American College of Cardiology 2017 clinical practice guidelines recommend a systolic BP (SBP) treatment goal of < 130 mm Hg for community-dwelling, ambulatory, noninstitutionalized adults aged ≥ 65 years; whereas the American College of Physicians/American Academy of Family Physicians recommend a goal of < 150 mm Hg for those aged ≥ 60 years without comorbidities and < 140 mm Hg for those with increased cardiovascular risk.1-3 Regardless of the specific threshold, agreement that some degree of BP control even in those with advanced age improves outcomes.2
First-line therapy for uncomplicated hypertension includes thiazide diuretics, long-acting calcium channel blockers, and renin-angiotensin system inhibitors. When choosing between these options, it is recommended to engage in shared decision making and to consider the patient’s comorbidities. Among patients who are likely to require a second agent (eg, if initial BP is > 20/10 mm Hg above goal), it is recommended to begin both drugs at the same time, preferably benazepril plus amlodipine due to the reduction in cardiovascular events reported in the ACCOMPLISH trial.4 If BP remains elevated despite 2 agents at moderate to maximum doses, it is important to investigate for secondary hypertension causes and to explore medication adherence as possible etiologies of treatment failure. Older adults are often at higher risk of adverse drug events due to age-related changes in pharmacodynamics. Despite this, there are no guidelines for choosing between different classes of antihypertensives in this population. We present a case of thiazide-induced hyponatremia in an older adult and review the risks of thiazide use in this population.
Case Presentation
A man aged > 90 years was admitted to the hospital after a syncopal episode. His history was significant for hypertension, hyperlipidemia, and vitamin D deficiency. At the time, his home medications were amlodipine 5 mg daily, atorvastatin 40 mg daily, ergocalciferol 50,000 IU weekly, and polyethylene glycol 17 g daily as needed. His syncope workup was unremarkable and included negative orthostatic vital signs, normal serial troponins, an electrocardiogram without ischemic changes, normal serum creatinine, sodium, and glucose, and a head computed tomography without any acute abnormality. Throughout the patient’s hospital stay, he had multiple elevated SBP readings, including many > 200 mm Hg. On discharge, in addition to continuing his home medications, he was started on valsartan 20 mg daily and enrolled in a remote BP monitoring program.
Three weeks later, the patient was seen by their primary care practitioner for follow-up. He reported adherence to his antihypertensive regimen. However, his remote BP monitoring revealed persistently elevated BPs, with an average of 179/79 mm Hg, a high of 205/85 mm Hg, and a low of 150/67 mm Hg over the previous 7 days. Laboratory tests obtained at the visit were notable for serum sodium of 138 mmol/L and potassium of 4.1 mmol/L. His weight was 87 kg. Given persistently elevated BP readings, in addition to continuing his amlodipine 5 mg daily and valsartan 20 mg daily, he was started on hydrochlorothiazide 25 mg daily, with plans to repeat a basic metabolic panel in 2 weeks.
Two weeks later, he fell after getting out of his bed. On examination, he was noted to have dry mucous membranes, and although no formal delirium screening was performed, he was able to repeat the months of the year backward. Vital signs were notable for positive postural hypertension, and his laboratory tests revealed a normal serum creatinine, serum sodium of 117 mmol/L
Discussion
Although thiazide diuretics are recommended as first-line therapy for uncomplicated hypertension, they are known to cause electrolyte abnormalities, including hypomagnesemia, hypokalemia, and hyponatremia.4 These metabolic derangements are more likely to occur in older adults. One study of adults aged ≥ 65 years found that at 9 months of follow-up, 14.3% of new thiazide users had developed a thiazide-related metabolic adverse event (hyponatremia < 135 mmol/L, hypokalemia < 3.5 mmol/L, and decrease in estimated glomerular filtration rate by > 25%) compared with 6.0% of nonusers (P < .001; number needed to harm [NNH] = 12).5 In addition, 3.8% of new thiazide users had an emergency department visit or were hospitalized for complications related to thiazides compared with only 2.0% of nonusers (P = .02; NNH = 56).5 Independent risk factors for thiazide-induced hyponatremia include high-comorbidity burden, low body weight, low-normal or unmeasured serum sodium, low potassium, and aged > 70 years.5-7 Each 10-year increment in age is associated with a 2-fold increase in risk, suggesting that older adults are at a much higher risk for hyponatremia than their younger peers.6
Despite their designation as a first-line option for uncomplicated hypertension, thiazide diuretics may cause more harm than good in some older adults, especially those with additional risk factors for thiazide-induced hyponatremia. In this population, these adverse effects should be discussed before starting thiazides for the treatment of hypertension. If thiazides are initiated, they should be started at the lowest possible dose, and plans made to monitor bloodwork within 1 to 2 weeks of initiation or dose change and periodically thereafter while the patient remains on the therapy.
Medication Management in Older Adults
Due to the risks of medication use in older adults, the phrase “start low, go slow” is commonly used in geriatric medicine to describe the optimal method for initiation and up-titration of new medication with the hope of mitigating adverse drug events. In our case, we started valsartan at 20 mg daily—one-fourth the recommended initial dose. Although this strategy is reasonable to “start low,” we were not surprised to find that the patient’s BP did not markedly improve on such a low dose. The team could have increased the valsartan dose to a therapeutically efficacious dose before choosing to add another hypertensive agent. In alignment with geriatric prescribing principles, starting at the lowest possible dose of hydrochlorothiazide is recommended.5 However, the clinician started hydrochlorothiazide at 25 mg daily, potentially increasing this patient’s risk of electrolyte abnormalities and eventual fall.
Managing hypertension also invites a discussion of polypharmacy and medication adherence. Older adults are at risk of polypharmacy, defined as the prescription of 5 or more medications.8 Polypharmacy is associated with increased hospitalizations, higher costs of care for individuals and health care systems, increased risks of adverse drug events, medication nonadherence, and lower quality of life for patients.9 In some situations, the risks of polypharmacy may outweigh the benefits of using multiple antihypertensives with different mechanisms of action if patients can reach their BP goal on the maximum dose of a single agent. For patients taking multiple antihypertensives, it is important to routinely monitor BP and assess whether deprescribing is indicated. Cognitive impairment and decreased social support may affect medication adherence for older adults.6 Clinicians should be aware of strategies, such as medication reminders and pillboxes, to increase antihypertensive medication adherence. Polypills that contain 2 antihypertensives can be another tool used to manage older adults to increase adherence and decrease health care costs.10
A current strategy that encompasses discussing many, if not all, of these noted elements is the Institute for Healthcare Improvement’s Age-Friendly Health System. This framework uses evidence-based tools to provide care for older adults across all clinical settings and highlights the 4Ms: what matters, medication, mentation, and mobility.11 Medication considers whether a medication is necessary, whether its use has benefits that outweigh the risks, and how it interacts with what matters, mentation, and mobility. In particular, what matters plays an important role in hypertension management in older adults given the recommended target BP differs, depending on which specialty organization guideline is followed. By better understanding what matters to patients, including their goals and priorities, clinicians can engage patients in shared decision making and provide individualized recommendations based on geriatric principles (eg, start low, go slow, principles of medication adherence) and patient comorbidities (eg, medical history and risk factors for hyponatremia) to help patients make a more informed choice about their antihypertensive treatment regimen (Figure).
Conclusions
This case illustrates the need for a specialized approach to hypertension management in older adults and the risks of thiazide diuretics in this population. Clinicians should consider BP goals, patient-specific factors, and principles of medication management in older adults. If initiating thiazide therapy, discuss the risks associated with use, start at the lowest possible dose, and monitor bloodwork within 1 to 2 weeks of initiation/dose change and periodically thereafter while the patient remains on the therapy to decrease the risk of adverse events. Finally, the Institute for Healthcare Improvement’s Age-Friendly Health System framework can be a useful when considering the addition of a new medication in an older adult’s treatment plan.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the New England Geriatrics Research, Education, and Clinical Center, Veterans Affairs Boston Healthcare System, and the Cincinnati VeteransAffairs Medical Center.
Hypertension is a major risk factor for heart disease, stroke, and kidney disease. The prevalence of hypertension increases with age, primarily due to age-related changes in arterial physiology.1 For older adults, current guidelines regarding blood pressure (BP) treatment goals vary. The American Heart Association/American College of Cardiology 2017 clinical practice guidelines recommend a systolic BP (SBP) treatment goal of < 130 mm Hg for community-dwelling, ambulatory, noninstitutionalized adults aged ≥ 65 years; whereas the American College of Physicians/American Academy of Family Physicians recommend a goal of < 150 mm Hg for those aged ≥ 60 years without comorbidities and < 140 mm Hg for those with increased cardiovascular risk.1-3 Regardless of the specific threshold, agreement that some degree of BP control even in those with advanced age improves outcomes.2
First-line therapy for uncomplicated hypertension includes thiazide diuretics, long-acting calcium channel blockers, and renin-angiotensin system inhibitors. When choosing between these options, it is recommended to engage in shared decision making and to consider the patient’s comorbidities. Among patients who are likely to require a second agent (eg, if initial BP is > 20/10 mm Hg above goal), it is recommended to begin both drugs at the same time, preferably benazepril plus amlodipine due to the reduction in cardiovascular events reported in the ACCOMPLISH trial.4 If BP remains elevated despite 2 agents at moderate to maximum doses, it is important to investigate for secondary hypertension causes and to explore medication adherence as possible etiologies of treatment failure. Older adults are often at higher risk of adverse drug events due to age-related changes in pharmacodynamics. Despite this, there are no guidelines for choosing between different classes of antihypertensives in this population. We present a case of thiazide-induced hyponatremia in an older adult and review the risks of thiazide use in this population.
Case Presentation
A man aged > 90 years was admitted to the hospital after a syncopal episode. His history was significant for hypertension, hyperlipidemia, and vitamin D deficiency. At the time, his home medications were amlodipine 5 mg daily, atorvastatin 40 mg daily, ergocalciferol 50,000 IU weekly, and polyethylene glycol 17 g daily as needed. His syncope workup was unremarkable and included negative orthostatic vital signs, normal serial troponins, an electrocardiogram without ischemic changes, normal serum creatinine, sodium, and glucose, and a head computed tomography without any acute abnormality. Throughout the patient’s hospital stay, he had multiple elevated SBP readings, including many > 200 mm Hg. On discharge, in addition to continuing his home medications, he was started on valsartan 20 mg daily and enrolled in a remote BP monitoring program.
Three weeks later, the patient was seen by their primary care practitioner for follow-up. He reported adherence to his antihypertensive regimen. However, his remote BP monitoring revealed persistently elevated BPs, with an average of 179/79 mm Hg, a high of 205/85 mm Hg, and a low of 150/67 mm Hg over the previous 7 days. Laboratory tests obtained at the visit were notable for serum sodium of 138 mmol/L and potassium of 4.1 mmol/L. His weight was 87 kg. Given persistently elevated BP readings, in addition to continuing his amlodipine 5 mg daily and valsartan 20 mg daily, he was started on hydrochlorothiazide 25 mg daily, with plans to repeat a basic metabolic panel in 2 weeks.
Two weeks later, he fell after getting out of his bed. On examination, he was noted to have dry mucous membranes, and although no formal delirium screening was performed, he was able to repeat the months of the year backward. Vital signs were notable for positive postural hypertension, and his laboratory tests revealed a normal serum creatinine, serum sodium of 117 mmol/L
Discussion
Although thiazide diuretics are recommended as first-line therapy for uncomplicated hypertension, they are known to cause electrolyte abnormalities, including hypomagnesemia, hypokalemia, and hyponatremia.4 These metabolic derangements are more likely to occur in older adults. One study of adults aged ≥ 65 years found that at 9 months of follow-up, 14.3% of new thiazide users had developed a thiazide-related metabolic adverse event (hyponatremia < 135 mmol/L, hypokalemia < 3.5 mmol/L, and decrease in estimated glomerular filtration rate by > 25%) compared with 6.0% of nonusers (P < .001; number needed to harm [NNH] = 12).5 In addition, 3.8% of new thiazide users had an emergency department visit or were hospitalized for complications related to thiazides compared with only 2.0% of nonusers (P = .02; NNH = 56).5 Independent risk factors for thiazide-induced hyponatremia include high-comorbidity burden, low body weight, low-normal or unmeasured serum sodium, low potassium, and aged > 70 years.5-7 Each 10-year increment in age is associated with a 2-fold increase in risk, suggesting that older adults are at a much higher risk for hyponatremia than their younger peers.6
Despite their designation as a first-line option for uncomplicated hypertension, thiazide diuretics may cause more harm than good in some older adults, especially those with additional risk factors for thiazide-induced hyponatremia. In this population, these adverse effects should be discussed before starting thiazides for the treatment of hypertension. If thiazides are initiated, they should be started at the lowest possible dose, and plans made to monitor bloodwork within 1 to 2 weeks of initiation or dose change and periodically thereafter while the patient remains on the therapy.
Medication Management in Older Adults
Due to the risks of medication use in older adults, the phrase “start low, go slow” is commonly used in geriatric medicine to describe the optimal method for initiation and up-titration of new medication with the hope of mitigating adverse drug events. In our case, we started valsartan at 20 mg daily—one-fourth the recommended initial dose. Although this strategy is reasonable to “start low,” we were not surprised to find that the patient’s BP did not markedly improve on such a low dose. The team could have increased the valsartan dose to a therapeutically efficacious dose before choosing to add another hypertensive agent. In alignment with geriatric prescribing principles, starting at the lowest possible dose of hydrochlorothiazide is recommended.5 However, the clinician started hydrochlorothiazide at 25 mg daily, potentially increasing this patient’s risk of electrolyte abnormalities and eventual fall.
Managing hypertension also invites a discussion of polypharmacy and medication adherence. Older adults are at risk of polypharmacy, defined as the prescription of 5 or more medications.8 Polypharmacy is associated with increased hospitalizations, higher costs of care for individuals and health care systems, increased risks of adverse drug events, medication nonadherence, and lower quality of life for patients.9 In some situations, the risks of polypharmacy may outweigh the benefits of using multiple antihypertensives with different mechanisms of action if patients can reach their BP goal on the maximum dose of a single agent. For patients taking multiple antihypertensives, it is important to routinely monitor BP and assess whether deprescribing is indicated. Cognitive impairment and decreased social support may affect medication adherence for older adults.6 Clinicians should be aware of strategies, such as medication reminders and pillboxes, to increase antihypertensive medication adherence. Polypills that contain 2 antihypertensives can be another tool used to manage older adults to increase adherence and decrease health care costs.10
A current strategy that encompasses discussing many, if not all, of these noted elements is the Institute for Healthcare Improvement’s Age-Friendly Health System. This framework uses evidence-based tools to provide care for older adults across all clinical settings and highlights the 4Ms: what matters, medication, mentation, and mobility.11 Medication considers whether a medication is necessary, whether its use has benefits that outweigh the risks, and how it interacts with what matters, mentation, and mobility. In particular, what matters plays an important role in hypertension management in older adults given the recommended target BP differs, depending on which specialty organization guideline is followed. By better understanding what matters to patients, including their goals and priorities, clinicians can engage patients in shared decision making and provide individualized recommendations based on geriatric principles (eg, start low, go slow, principles of medication adherence) and patient comorbidities (eg, medical history and risk factors for hyponatremia) to help patients make a more informed choice about their antihypertensive treatment regimen (Figure).
Conclusions
This case illustrates the need for a specialized approach to hypertension management in older adults and the risks of thiazide diuretics in this population. Clinicians should consider BP goals, patient-specific factors, and principles of medication management in older adults. If initiating thiazide therapy, discuss the risks associated with use, start at the lowest possible dose, and monitor bloodwork within 1 to 2 weeks of initiation/dose change and periodically thereafter while the patient remains on the therapy to decrease the risk of adverse events. Finally, the Institute for Healthcare Improvement’s Age-Friendly Health System framework can be a useful when considering the addition of a new medication in an older adult’s treatment plan.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the New England Geriatrics Research, Education, and Clinical Center, Veterans Affairs Boston Healthcare System, and the Cincinnati VeteransAffairs Medical Center.
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127-e248. doi:10.1016/j.jacc.2017.11.006
2. Davis LL. Hypertension: how low to go when treating older adults. J Nurse Pract. 2019;15(1):1-6. doi:10.1016/j.nurpra.2018.10.010
3. Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166(6):430-437. doi:10.7326/M16-1785
4. Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011;57(20):2037-2114. doi:10.1016/j.jacc.2011.01.008
5. Makam AN, Boscardin WJ, Miao Y, Steinman MA. Risk of thiazide-induced metabolic adverse events in older adults. J Am Geriatr Soc. 2014;62(6):1039-1045. doi:10.1111/jgs.12839
6. Chow KM, Szeto CC, Wong TY, Leung CB, Li PK. Risk factors for thiazide-induced hyponatraemia. QJM. 2003;96(12):911-917. doi:10.1093/qjmed/hcg157
7. Clayton JA, Rodgers S, Blakey J, Avery A, Hall IP. Thiazide diuretic prescription and electrolyte abnormalities in primary care. Br J Clin Pharmacol. 2006;61(1):87-95. doi:10.1111/j.1365-2125.2005.02531.x
8. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28(2):173-186. doi:10.1016/j.cger.2012.01.002
9. Benetos A, Petrovic M, Strandberg T. Hypertension management in older and frail older patients. Circ Res. 2019;124(7):1045-1060. doi:10.1161/CIRCRESAHA.118.313236
10. Sherrill B, Halpern M, Khan S, Zhang J, Panjabi S. Single-pill vs free-equivalent combination therapies for hypertension: a meta-analysis of health care costs and adherence. J Clin Hypertens (Greenwich). 2011;13(12):898-909. doi:10.1111/j.1751-7176.2011.00550.x
11. Mate K, Fulmer T, Pelton L, et al. Evidence for the 4Ms: interactions and outcomes across the care continuum. J Aging Health. 2021;33(7-8):469-481. doi:10.1177/0898264321991658
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127-e248. doi:10.1016/j.jacc.2017.11.006
2. Davis LL. Hypertension: how low to go when treating older adults. J Nurse Pract. 2019;15(1):1-6. doi:10.1016/j.nurpra.2018.10.010
3. Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166(6):430-437. doi:10.7326/M16-1785
4. Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011;57(20):2037-2114. doi:10.1016/j.jacc.2011.01.008
5. Makam AN, Boscardin WJ, Miao Y, Steinman MA. Risk of thiazide-induced metabolic adverse events in older adults. J Am Geriatr Soc. 2014;62(6):1039-1045. doi:10.1111/jgs.12839
6. Chow KM, Szeto CC, Wong TY, Leung CB, Li PK. Risk factors for thiazide-induced hyponatraemia. QJM. 2003;96(12):911-917. doi:10.1093/qjmed/hcg157
7. Clayton JA, Rodgers S, Blakey J, Avery A, Hall IP. Thiazide diuretic prescription and electrolyte abnormalities in primary care. Br J Clin Pharmacol. 2006;61(1):87-95. doi:10.1111/j.1365-2125.2005.02531.x
8. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28(2):173-186. doi:10.1016/j.cger.2012.01.002
9. Benetos A, Petrovic M, Strandberg T. Hypertension management in older and frail older patients. Circ Res. 2019;124(7):1045-1060. doi:10.1161/CIRCRESAHA.118.313236
10. Sherrill B, Halpern M, Khan S, Zhang J, Panjabi S. Single-pill vs free-equivalent combination therapies for hypertension: a meta-analysis of health care costs and adherence. J Clin Hypertens (Greenwich). 2011;13(12):898-909. doi:10.1111/j.1751-7176.2011.00550.x
11. Mate K, Fulmer T, Pelton L, et al. Evidence for the 4Ms: interactions and outcomes across the care continuum. J Aging Health. 2021;33(7-8):469-481. doi:10.1177/0898264321991658
Reducing or Discontinuing Insulin or Sulfonylurea When Initiating a Glucagon-like Peptide-1 Agonist
Hypoglycemia and weight gain are well-known adverse effects that can result from insulin and sulfonylureas in patients with type 2 diabetes mellitus (T2DM).1,2 Insulin and sulfonylurea medications can cause additional weight gain in patients who are overweight or obese, which can increase the burden of diabetes therapy with added medications, raise the risk of hypoglycemia complications, and raise atherosclerotic cardiovascular disease risk factors.3 Although increasing the insulin or sulfonylurea dose is an option health care practitioners or pharmacists have, this approach can increase the risk of hypoglycemia, especially in older adults, such as the veteran population, which could lead to complications, such as falls.2
Previous studies focusing on hypoglycemic events in patients with T2DM showed that glucagon-like peptide-1 (GLP-1) agonist monotherapy has a low incidence of a hypoglycemic events. However, when a GLP-1 agonist is combined with insulin or sulfonylureas, patients have an increased chance of a hypoglycemic event.3-8 According to the prescribing information for semaglutide, 1.6% to 3.8% of patients on a GLP-1 agonist monotherapy reported a documented symptomatic hypoglycemic event (blood glucose ≤ 70 mg/dL), based on semaglutide dosing. 9 Patients on combination therapy of a GLP-1 agonist and basal insulin and a GLP-1 agonist and a sulfonylurea reported a documented symptomatic hypoglycemic event ranging from 16.7% to 29.8% and 17.3% to 24.4%, respectively.9 The incidences of hypoglycemia thus dramatically increase with combination therapy of a GLP-1 agonist plus insulin or a sulfonylurea.
When adding a GLP-1 agonist to insulin or a sulfonylurea, clinicians must be mindful of the increased risk of hypoglycemia. Per the warnings and precautions in the prescribing information of GLP-1 agonists, concomitant use with insulin or a sulfonylurea may increase the risk of hypoglycemia, and reducing the dose of insulin or a sulfonylurea may be necessary.9-11 According to the American College of Cardiology guidelines, when starting a GLP-1 agonist, the insulin dose should be decreased by about 20% in patients with a well-controlled hemoglobin A1c (HbA1c).12
This study aimed to determine the percentage of patients who required dose reductions or discontinuations of insulin and sulfonylureas with the addition of a GLP-1 agonist. Understanding necessary dose reductions or discontinuations of these concomitant diabetes agents can assist pharmacists in preventing hypoglycemia and minimizing weight gain.
Methods
This clinical review was a single-center, retrospective chart review of patients prescribed a GLP-1 agonist while on insulin or a sulfonylurea between January 1, 2019, and September 30, 2022, at the Wilkes-Barre Veterans Affairs Medical Center (WBVAMC) in Pennsylvania and managed in a pharmacist-led patient aligned care team (PACT) clinic. It was determined by the US Department of Veterans Affairs Office of Research and Development that an institutional review board or other review committee approval was not needed for this nonresearch Veterans Health Administration quality assurance and improvement project. Patients aged ≥ 18 years were included in this study. Patients were excluded if they were not on insulin or a sulfonylurea when starting a GLP-1 agonist, started a GLP-1 agonist outside of the retrospective chart review dates, or were prescribed a GLP-1 agonist by anyone other than a pharmacist in their PACT clinic. This included if a GLP-1 agonist was prescribed by a primary care physician, endocrinologist, or someone outside the VA system.
The primary study outcomes were to determine the percentage of patients with a dose reduction of insulin or sulfonylurea and discontinuation of insulin or a sulfonylurea at intervals of 0 (baseline), 3, 6, and 12 months. Secondary outcomes included changes in HbA1c and body weight measured at the same intervals of 0 (baseline), 3, 6, and 12 months.
Data were collected using the VA Computerized Patient Record System (CPRS) and stored in a locked spreadsheet. Descriptive statistics were used to analyze the data. Patient data included the number of patients on insulin or a sulfonylurea when initiating a GLP-1 agonist, the percentage of patients started on a certain GLP-1 agonist (dulaglutide, liraglutide, exenatide, and semaglutide), and the percentage of patients with a baseline HbA1c of < 8%, 8% to 10%, and > 10%. The GLP-1 agonist formulary was adjusted during the time of this retrospective chart review. Patients who were not on semaglutide were switched over if they were on another GLP-1 agonist as semaglutide became the preferred GLP-1 agonist.
Patients were considered to have a dose reduction or discontinuation of insulin or a sulfonylurea if the dose or medication they were on decreased or was discontinued permanently within 12 months of starting a GLP-1 agonist. For example, if a patient who was administering 10 units of insulin daily was decreased to 8 but later increased back to 10, this was not counted as a dose reduction. If a patient discontinued insulin or a sulfonylurea and then restarted it within 12 months of initiating a GLP-1 agonist, this was not counted as a discontinuation.
Results
This retrospective review included 136 patients; 96 patients taking insulin and 54 taking a sulfonylurea when they started a GLP-1 agonist. Fourteen patients were on both. Criteria for use, which are clinical criteria to determine if a patient is eligible for the use of a given medication, are used within the VA. The inclusion criteria for a patient initiating a GLP-1 agonist is that the patient must have atherosclerotic cardiovascular disease or chronic kidney disease with the patient receiving metformin (unless unable to use metformin) and empagliflozin (unless unable to use empagliflozin).
The baseline mean age and weight for the patient population in this retrospective chart review was 70.7 years and 238.2 lb, respectively. Ninety-six patients (70.6%) were started on semaglutide, 27 (19.9%) on dulaglutide, 12 (8.8%) on liraglutide, and 1 (0.7%) on exenatide. The mean HbA1c when patients initiated a GLP-1 agonist was 8.6%. When starting a GLP-1 agonist, 34 patients (25.0%) had an HbA1c < 8%, 89 (65.4%) had an HbA1c between 8% to 10%, and 13 (9.6%) had an HbA1c > 10% (Table).
For the primary results, 25 patients (26.0%) had a dose reduction of insulin when they started a GLP-1 agonist, and 55 patients (57.3%) had at least 1 insulin dose reduction within the year follow-up. Seven patients (13.0%) had a dose reduction of a sulfonylurea when they started a GLP-1 agonist, and 16 patients (29.6%) had at least 1 dose reduction of a sulfonylurea within the year follow-up. Six patients (6.3%) discontinued insulin use when they initially started a GLP-1 agonist, and 14 patients (14.6%) discontinued insulin use within the year follow-up. Eleven patients (20.4%) discontinued sulfonylurea use when they initially started a GLP-1 agonist, and 21 patients (38.9%) discontinued sulfonylurea use within the year follow-up (Figure).
Fourteen patients were on both insulin and a sulfonylurea. Two patients (14.3%) had a dose reduction of insulin when they started a GLP-1 agonist, and 5 (35.7%) had ≥ 1 insulin dose reduction within the year follow-up. Three patients (21.4%) had a dose reduction of a sulfonylurea when they started a GLP-1 agonist, and 6 (42.9%) had ≥ 1 dose reduction of a sulfonylurea within the year follow-up. Seven patients (50.0%) discontinued sulfonylurea and 3 (21.4%) discontinued insulin at any time throughout the year. The majority of the discontinuations were at the initial start of GLP-1 agonist therapy.
The mean HbA1c for patients on GLP-1 agonist was 8.6% at baseline, 8.0% at 0 to 3 months, 7.6% at 3 to 6 months, and 7.5% at 12 months. Patients experienced a mean HbA1c reduction of 1.1%. The mean weight when a GLP-1 agonist was started was 238.2 lb, 236.0 lb at 0 to 3 months, 223.8 lb at 3 to 6 months, and 224.3 lb after 12 months. Study participants lost a mean weight of 13.9 lb while on a GLP-1 agonist.
Discussion
While this study did not examine why there were dose reductions or discontinuations, we can hypothesize that insulin or sulfonylureas were reduced or discontinued due to a myriad of reasons, such as prophylactic dosing per guidelines, patients having a hypoglycemic event, or pharmacists anticipating potential low blood glucose trends. Also, there could have been numerous reasons GLP-1 agonists were started in patients on insulin or a sulfonylurea, such as HbA1c not being within goal range, cardiovascular benefits (reduce risk of stroke, heart attack, and death), weight loss, and renal protection, such as preventing albuminuria.13,14
This retrospective chart review found a large proportion of patients had a dose reduction of insulin (57.3%) or sulfonylurea (29.6%). The percentage of patients with a dose reduction was potentially underestimated as patients were not counted if they discontinued insulin or sulfonylurea. Concomitant use of GLP-1 agonists with insulin or a sulfonylurea may increase the risk of hypoglycemia and reducing the dose of insulin or a sulfonylurea may be necessary.9-11 The dose reductions in this study show that pharmacists within pharmacy-led PACT clinics monitor for or attempt to prevent hypoglycemia, which aligns with the prescribing information of GLP-1 agonists. While increasing the insulin or sulfonylurea dose is an option for patients, this approach can increase the risk of hypoglycemia, especially in an older population, like this one with a mean age > 70 years. The large proportions of patients with dose reductions or insulin and sulfonylurea discontinuations suggest that pharmacists may need to take a more cautious approach when initiating a GLP-1 agonist to prevent adverse health outcomes related to low blood sugar for older adults, such as falls and fractures.
Insulin was discontinued in 20.4% of patients and sulfonylurea was discontinued in 38.9% of patients within 12 months after starting a GLP-1 agonist. When a patient was on both insulin and a sulfonylurea, the percentage of patients who discontinued insulin (21.4%) or a sulfonylurea (50.0%) was higher compared with patients just on insulin (14.6%) or a sulfonylurea (38.9%) alone. Patients on both insulin and a sulfonylurea may need closer monitoring due to a higher incidence of discontinuations when these diabetes agents are administered in combination.
Within 12 months of patients receiving a GLP-1 agonist, the mean HbA1c reduction was 1.1%, which is comparable to other GLP-1 agonist clinical trials. For semaglutide 0.5 mg and 1.0 mg dosages, the mean HbA1c reduction was 1.4% and 1.6%, respectively.9 For dulaglutide 0.75 mg and 1.5 mg dosages, the mean HbA1c reduction ranged from 0.7% to 1.6% and 0.8% to 1.6%, respectively.10 For liraglutide 1.8 mg dosage, the mean HbA1c reduction ranged from 1.0% to 1.5%.11 The mean weight loss in this study was 13.9 lb. Along with HbA1c, weight loss in this review was comparable to other GLP-1 agonist clinical trials. Patients administering semaglutide lost up to 14 lb, patients taking dulaglutide lost up to 10.1 lb, and patients on liraglutide lost on average 6.2 lb.9-11 Even with medications such as insulin and sulfonylurea that have the side effects of hypoglycemia and weight gain, adding a GLP-1 agonist showed a reduction in HbA1c and weight loss relatively similar to previous clinical trials.
A study on the effects of adding semaglutide to insulin regimens in March 2023 by Meyer and colleagues displayed similar results to this retrospective chart review. That study concluded that there was blood glucose improvement (HbA1c reduction of 1.3%) in patients after 6 months despite a decrease in the insulin dose. Also, patients lost a mean weight of 11 lb during the 6-month trial.3 This retrospective chart review at the WBVAMC adds to the body of research that supports potential reductions or discontinuations of insulin and/or sulfonylureas with the addition of a GLP-1 agonist.
Limitations
Several limitations of this study should be considered when evaluating the results. This review was comprised of a mostly older, male population, which results in a low generalizability to organizations other than VA medical centers. In addition, this study only evaluated patients on a GLP-1 agonist followed in a pharmacist-led PACT clinic. This study excluded patients who were prescribed a GLP-1 agonist by an endocrinologist or a pharmacist at one of the community-based outpatient clinics affiliated with WBVAMC, or a pharmacist or clinician outside the VA. The sole focus of this study was patients in a pharmacist-led VAMC clinic. Not all patient data may have been included in the study. If a patient did not have an appointment at baseline, 3, 6, and 12 months or did not obtain laboratory tests, HbA1c and weights were not recorded. Data were collected during the COVID-19 pandemic and in-person appointments were potentially switched to phone or video appointments. There were many instances during this chart review where a weight was not recorded at each time interval. Also, this study did not consider any other diabetes medications the patient was taking. There were many instances where the patient was taking metformin and/or sodium-glucose cotransporter-2 (SGLT-2) inhibitors. These medications along with diet could have affected the weight results as metformin is weight neutral and SGLT-2 inhibitors promote weight loss.15 Lastly, this study did not evaluate the amount of insulin reduced, only if there was a dose reduction or discontinuation of insulin and/or a sulfonylurea.
Conclusions
Dose reductions and a discontinuation of insulin or a sulfonylurea with the addition of a GLP-1 agonist may be needed. Patients on both insulin and a sulfonylurea may need closer monitoring due to the higher incidences of discontinuations compared with patients on just 1 of these agents. Dose reductions or discontinuations of these diabetic agents can promote positive patient outcomes, such as preventing hypoglycemia, minimizing weight gain, increasing weight loss, and reducing HbA1c levels.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Wilkes-Barre Veterans Affairs Medical Center in Pennsylvania.
1. ElSayed NA, Aleppo G, Aroda VR, et al. 8. Obesity and weight management for the prevention and treatment of type 2 diabetes: standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S128-S139. doi:10.2337/dc23-S008
2. ElSayed NA, Aleppo G, Aroda VE, et al. Older adults: standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S216-S229. doi:10.2337/dc23-S013
3. Meyer J, Dreischmeier E, Lehmann M, Phelan J. The effects of adding semaglutide to high daily dose insulin regimens in patients with type 2 diabetes. Ann Pharmacother. 2023;57(3):241-250. doi:10.1177/10600280221107381
4. Rodbard HW, Lingvay I, Reed J, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab. 2018;103(6):2291-2301. doi:10.1210/jc.2018-00070
5. Anderson SL, Trujillo JM. Basal insulin use with GLP-1 receptor agonists. Diabetes Spectr. 2016;29(3):152-160. doi:10.2337/diaspect.29.3.152
6. Castek SL, Healey LC, Kania DS, Vernon VP, Dawson AJ. Assessment of glucagon-like peptide-1 receptor agonists in veterans taking basal/bolus insulin regimens. Fed Pract. 2022;39(suppl 5):S18-S23. doi:10.12788/fp.0317
7. Chen M, Vider E, Plakogiannis R. Insulin dosage adjustments after initiation of GLP-1 receptor agonists in patients with type 2 diabetes. J Pharm Pract. 2022;35(4):511-517. doi:10.1177/0897190021993625
8. Seino Y, Min KW, Niemoeller E, Takami A; EFC10887 GETGOAL-L Asia Study Investigators. Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia). Diabetes Obes Metab. 2012;14(10):910-917. doi:10.1111/j.1463-1326.2012.01618.x.
9. Ozempic (semaglutide) injection. Package insert. Novo Nordisk Inc; 2022. https://www.ozempic.com/prescribing-information.html
10. Trulicity (dulaglutide) injection. Prescribing information. Lilly and Company; 2022. Accessed December 20, 2023. https://pi.lilly.com/us/trulicity-uspi.pdf
11. Victoza (liraglutide) injection. Prescribing information. Novo Nordisk Inc; 2022. Accessed December 20, 2023. https://www.novo-pi.com/victoza.pdf
12. Das SR, Everett BM, Birtcher KK, et al. 2020 expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76(9):1117-1145. doi:10.1016/j.jacc.2020.05.037
13. Granata A, Maccarrone R, Anzaldi M, et al. GLP-1 receptor agonists and renal outcomes in patients with diabetes mellitus type 2 and diabetic kidney disease: state of the art. Clin Kidney J. 2022;15(9):1657-1665. Published 2022 Mar 12. doi:10.1093/ckj/sfac069
14. Marx N, Husain M, Lehrke M, Verma S, Sattar N. GLP-1 receptor agonists for the reduction of atherosclerotic cardiovascular risk in patients with type 2 diabetes. Circulation. 2022;146(24):1882-1894. doi:10.1161/CIRCULATIONAHA.122.059595
15. Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2022;65(12):1925-1966. doi:10.1007/s00125-022-05787-2
Hypoglycemia and weight gain are well-known adverse effects that can result from insulin and sulfonylureas in patients with type 2 diabetes mellitus (T2DM).1,2 Insulin and sulfonylurea medications can cause additional weight gain in patients who are overweight or obese, which can increase the burden of diabetes therapy with added medications, raise the risk of hypoglycemia complications, and raise atherosclerotic cardiovascular disease risk factors.3 Although increasing the insulin or sulfonylurea dose is an option health care practitioners or pharmacists have, this approach can increase the risk of hypoglycemia, especially in older adults, such as the veteran population, which could lead to complications, such as falls.2
Previous studies focusing on hypoglycemic events in patients with T2DM showed that glucagon-like peptide-1 (GLP-1) agonist monotherapy has a low incidence of a hypoglycemic events. However, when a GLP-1 agonist is combined with insulin or sulfonylureas, patients have an increased chance of a hypoglycemic event.3-8 According to the prescribing information for semaglutide, 1.6% to 3.8% of patients on a GLP-1 agonist monotherapy reported a documented symptomatic hypoglycemic event (blood glucose ≤ 70 mg/dL), based on semaglutide dosing. 9 Patients on combination therapy of a GLP-1 agonist and basal insulin and a GLP-1 agonist and a sulfonylurea reported a documented symptomatic hypoglycemic event ranging from 16.7% to 29.8% and 17.3% to 24.4%, respectively.9 The incidences of hypoglycemia thus dramatically increase with combination therapy of a GLP-1 agonist plus insulin or a sulfonylurea.
When adding a GLP-1 agonist to insulin or a sulfonylurea, clinicians must be mindful of the increased risk of hypoglycemia. Per the warnings and precautions in the prescribing information of GLP-1 agonists, concomitant use with insulin or a sulfonylurea may increase the risk of hypoglycemia, and reducing the dose of insulin or a sulfonylurea may be necessary.9-11 According to the American College of Cardiology guidelines, when starting a GLP-1 agonist, the insulin dose should be decreased by about 20% in patients with a well-controlled hemoglobin A1c (HbA1c).12
This study aimed to determine the percentage of patients who required dose reductions or discontinuations of insulin and sulfonylureas with the addition of a GLP-1 agonist. Understanding necessary dose reductions or discontinuations of these concomitant diabetes agents can assist pharmacists in preventing hypoglycemia and minimizing weight gain.
Methods
This clinical review was a single-center, retrospective chart review of patients prescribed a GLP-1 agonist while on insulin or a sulfonylurea between January 1, 2019, and September 30, 2022, at the Wilkes-Barre Veterans Affairs Medical Center (WBVAMC) in Pennsylvania and managed in a pharmacist-led patient aligned care team (PACT) clinic. It was determined by the US Department of Veterans Affairs Office of Research and Development that an institutional review board or other review committee approval was not needed for this nonresearch Veterans Health Administration quality assurance and improvement project. Patients aged ≥ 18 years were included in this study. Patients were excluded if they were not on insulin or a sulfonylurea when starting a GLP-1 agonist, started a GLP-1 agonist outside of the retrospective chart review dates, or were prescribed a GLP-1 agonist by anyone other than a pharmacist in their PACT clinic. This included if a GLP-1 agonist was prescribed by a primary care physician, endocrinologist, or someone outside the VA system.
The primary study outcomes were to determine the percentage of patients with a dose reduction of insulin or sulfonylurea and discontinuation of insulin or a sulfonylurea at intervals of 0 (baseline), 3, 6, and 12 months. Secondary outcomes included changes in HbA1c and body weight measured at the same intervals of 0 (baseline), 3, 6, and 12 months.
Data were collected using the VA Computerized Patient Record System (CPRS) and stored in a locked spreadsheet. Descriptive statistics were used to analyze the data. Patient data included the number of patients on insulin or a sulfonylurea when initiating a GLP-1 agonist, the percentage of patients started on a certain GLP-1 agonist (dulaglutide, liraglutide, exenatide, and semaglutide), and the percentage of patients with a baseline HbA1c of < 8%, 8% to 10%, and > 10%. The GLP-1 agonist formulary was adjusted during the time of this retrospective chart review. Patients who were not on semaglutide were switched over if they were on another GLP-1 agonist as semaglutide became the preferred GLP-1 agonist.
Patients were considered to have a dose reduction or discontinuation of insulin or a sulfonylurea if the dose or medication they were on decreased or was discontinued permanently within 12 months of starting a GLP-1 agonist. For example, if a patient who was administering 10 units of insulin daily was decreased to 8 but later increased back to 10, this was not counted as a dose reduction. If a patient discontinued insulin or a sulfonylurea and then restarted it within 12 months of initiating a GLP-1 agonist, this was not counted as a discontinuation.
Results
This retrospective review included 136 patients; 96 patients taking insulin and 54 taking a sulfonylurea when they started a GLP-1 agonist. Fourteen patients were on both. Criteria for use, which are clinical criteria to determine if a patient is eligible for the use of a given medication, are used within the VA. The inclusion criteria for a patient initiating a GLP-1 agonist is that the patient must have atherosclerotic cardiovascular disease or chronic kidney disease with the patient receiving metformin (unless unable to use metformin) and empagliflozin (unless unable to use empagliflozin).
The baseline mean age and weight for the patient population in this retrospective chart review was 70.7 years and 238.2 lb, respectively. Ninety-six patients (70.6%) were started on semaglutide, 27 (19.9%) on dulaglutide, 12 (8.8%) on liraglutide, and 1 (0.7%) on exenatide. The mean HbA1c when patients initiated a GLP-1 agonist was 8.6%. When starting a GLP-1 agonist, 34 patients (25.0%) had an HbA1c < 8%, 89 (65.4%) had an HbA1c between 8% to 10%, and 13 (9.6%) had an HbA1c > 10% (Table).
For the primary results, 25 patients (26.0%) had a dose reduction of insulin when they started a GLP-1 agonist, and 55 patients (57.3%) had at least 1 insulin dose reduction within the year follow-up. Seven patients (13.0%) had a dose reduction of a sulfonylurea when they started a GLP-1 agonist, and 16 patients (29.6%) had at least 1 dose reduction of a sulfonylurea within the year follow-up. Six patients (6.3%) discontinued insulin use when they initially started a GLP-1 agonist, and 14 patients (14.6%) discontinued insulin use within the year follow-up. Eleven patients (20.4%) discontinued sulfonylurea use when they initially started a GLP-1 agonist, and 21 patients (38.9%) discontinued sulfonylurea use within the year follow-up (Figure).
Fourteen patients were on both insulin and a sulfonylurea. Two patients (14.3%) had a dose reduction of insulin when they started a GLP-1 agonist, and 5 (35.7%) had ≥ 1 insulin dose reduction within the year follow-up. Three patients (21.4%) had a dose reduction of a sulfonylurea when they started a GLP-1 agonist, and 6 (42.9%) had ≥ 1 dose reduction of a sulfonylurea within the year follow-up. Seven patients (50.0%) discontinued sulfonylurea and 3 (21.4%) discontinued insulin at any time throughout the year. The majority of the discontinuations were at the initial start of GLP-1 agonist therapy.
The mean HbA1c for patients on GLP-1 agonist was 8.6% at baseline, 8.0% at 0 to 3 months, 7.6% at 3 to 6 months, and 7.5% at 12 months. Patients experienced a mean HbA1c reduction of 1.1%. The mean weight when a GLP-1 agonist was started was 238.2 lb, 236.0 lb at 0 to 3 months, 223.8 lb at 3 to 6 months, and 224.3 lb after 12 months. Study participants lost a mean weight of 13.9 lb while on a GLP-1 agonist.
Discussion
While this study did not examine why there were dose reductions or discontinuations, we can hypothesize that insulin or sulfonylureas were reduced or discontinued due to a myriad of reasons, such as prophylactic dosing per guidelines, patients having a hypoglycemic event, or pharmacists anticipating potential low blood glucose trends. Also, there could have been numerous reasons GLP-1 agonists were started in patients on insulin or a sulfonylurea, such as HbA1c not being within goal range, cardiovascular benefits (reduce risk of stroke, heart attack, and death), weight loss, and renal protection, such as preventing albuminuria.13,14
This retrospective chart review found a large proportion of patients had a dose reduction of insulin (57.3%) or sulfonylurea (29.6%). The percentage of patients with a dose reduction was potentially underestimated as patients were not counted if they discontinued insulin or sulfonylurea. Concomitant use of GLP-1 agonists with insulin or a sulfonylurea may increase the risk of hypoglycemia and reducing the dose of insulin or a sulfonylurea may be necessary.9-11 The dose reductions in this study show that pharmacists within pharmacy-led PACT clinics monitor for or attempt to prevent hypoglycemia, which aligns with the prescribing information of GLP-1 agonists. While increasing the insulin or sulfonylurea dose is an option for patients, this approach can increase the risk of hypoglycemia, especially in an older population, like this one with a mean age > 70 years. The large proportions of patients with dose reductions or insulin and sulfonylurea discontinuations suggest that pharmacists may need to take a more cautious approach when initiating a GLP-1 agonist to prevent adverse health outcomes related to low blood sugar for older adults, such as falls and fractures.
Insulin was discontinued in 20.4% of patients and sulfonylurea was discontinued in 38.9% of patients within 12 months after starting a GLP-1 agonist. When a patient was on both insulin and a sulfonylurea, the percentage of patients who discontinued insulin (21.4%) or a sulfonylurea (50.0%) was higher compared with patients just on insulin (14.6%) or a sulfonylurea (38.9%) alone. Patients on both insulin and a sulfonylurea may need closer monitoring due to a higher incidence of discontinuations when these diabetes agents are administered in combination.
Within 12 months of patients receiving a GLP-1 agonist, the mean HbA1c reduction was 1.1%, which is comparable to other GLP-1 agonist clinical trials. For semaglutide 0.5 mg and 1.0 mg dosages, the mean HbA1c reduction was 1.4% and 1.6%, respectively.9 For dulaglutide 0.75 mg and 1.5 mg dosages, the mean HbA1c reduction ranged from 0.7% to 1.6% and 0.8% to 1.6%, respectively.10 For liraglutide 1.8 mg dosage, the mean HbA1c reduction ranged from 1.0% to 1.5%.11 The mean weight loss in this study was 13.9 lb. Along with HbA1c, weight loss in this review was comparable to other GLP-1 agonist clinical trials. Patients administering semaglutide lost up to 14 lb, patients taking dulaglutide lost up to 10.1 lb, and patients on liraglutide lost on average 6.2 lb.9-11 Even with medications such as insulin and sulfonylurea that have the side effects of hypoglycemia and weight gain, adding a GLP-1 agonist showed a reduction in HbA1c and weight loss relatively similar to previous clinical trials.
A study on the effects of adding semaglutide to insulin regimens in March 2023 by Meyer and colleagues displayed similar results to this retrospective chart review. That study concluded that there was blood glucose improvement (HbA1c reduction of 1.3%) in patients after 6 months despite a decrease in the insulin dose. Also, patients lost a mean weight of 11 lb during the 6-month trial.3 This retrospective chart review at the WBVAMC adds to the body of research that supports potential reductions or discontinuations of insulin and/or sulfonylureas with the addition of a GLP-1 agonist.
Limitations
Several limitations of this study should be considered when evaluating the results. This review was comprised of a mostly older, male population, which results in a low generalizability to organizations other than VA medical centers. In addition, this study only evaluated patients on a GLP-1 agonist followed in a pharmacist-led PACT clinic. This study excluded patients who were prescribed a GLP-1 agonist by an endocrinologist or a pharmacist at one of the community-based outpatient clinics affiliated with WBVAMC, or a pharmacist or clinician outside the VA. The sole focus of this study was patients in a pharmacist-led VAMC clinic. Not all patient data may have been included in the study. If a patient did not have an appointment at baseline, 3, 6, and 12 months or did not obtain laboratory tests, HbA1c and weights were not recorded. Data were collected during the COVID-19 pandemic and in-person appointments were potentially switched to phone or video appointments. There were many instances during this chart review where a weight was not recorded at each time interval. Also, this study did not consider any other diabetes medications the patient was taking. There were many instances where the patient was taking metformin and/or sodium-glucose cotransporter-2 (SGLT-2) inhibitors. These medications along with diet could have affected the weight results as metformin is weight neutral and SGLT-2 inhibitors promote weight loss.15 Lastly, this study did not evaluate the amount of insulin reduced, only if there was a dose reduction or discontinuation of insulin and/or a sulfonylurea.
Conclusions
Dose reductions and a discontinuation of insulin or a sulfonylurea with the addition of a GLP-1 agonist may be needed. Patients on both insulin and a sulfonylurea may need closer monitoring due to the higher incidences of discontinuations compared with patients on just 1 of these agents. Dose reductions or discontinuations of these diabetic agents can promote positive patient outcomes, such as preventing hypoglycemia, minimizing weight gain, increasing weight loss, and reducing HbA1c levels.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Wilkes-Barre Veterans Affairs Medical Center in Pennsylvania.
Hypoglycemia and weight gain are well-known adverse effects that can result from insulin and sulfonylureas in patients with type 2 diabetes mellitus (T2DM).1,2 Insulin and sulfonylurea medications can cause additional weight gain in patients who are overweight or obese, which can increase the burden of diabetes therapy with added medications, raise the risk of hypoglycemia complications, and raise atherosclerotic cardiovascular disease risk factors.3 Although increasing the insulin or sulfonylurea dose is an option health care practitioners or pharmacists have, this approach can increase the risk of hypoglycemia, especially in older adults, such as the veteran population, which could lead to complications, such as falls.2
Previous studies focusing on hypoglycemic events in patients with T2DM showed that glucagon-like peptide-1 (GLP-1) agonist monotherapy has a low incidence of a hypoglycemic events. However, when a GLP-1 agonist is combined with insulin or sulfonylureas, patients have an increased chance of a hypoglycemic event.3-8 According to the prescribing information for semaglutide, 1.6% to 3.8% of patients on a GLP-1 agonist monotherapy reported a documented symptomatic hypoglycemic event (blood glucose ≤ 70 mg/dL), based on semaglutide dosing. 9 Patients on combination therapy of a GLP-1 agonist and basal insulin and a GLP-1 agonist and a sulfonylurea reported a documented symptomatic hypoglycemic event ranging from 16.7% to 29.8% and 17.3% to 24.4%, respectively.9 The incidences of hypoglycemia thus dramatically increase with combination therapy of a GLP-1 agonist plus insulin or a sulfonylurea.
When adding a GLP-1 agonist to insulin or a sulfonylurea, clinicians must be mindful of the increased risk of hypoglycemia. Per the warnings and precautions in the prescribing information of GLP-1 agonists, concomitant use with insulin or a sulfonylurea may increase the risk of hypoglycemia, and reducing the dose of insulin or a sulfonylurea may be necessary.9-11 According to the American College of Cardiology guidelines, when starting a GLP-1 agonist, the insulin dose should be decreased by about 20% in patients with a well-controlled hemoglobin A1c (HbA1c).12
This study aimed to determine the percentage of patients who required dose reductions or discontinuations of insulin and sulfonylureas with the addition of a GLP-1 agonist. Understanding necessary dose reductions or discontinuations of these concomitant diabetes agents can assist pharmacists in preventing hypoglycemia and minimizing weight gain.
Methods
This clinical review was a single-center, retrospective chart review of patients prescribed a GLP-1 agonist while on insulin or a sulfonylurea between January 1, 2019, and September 30, 2022, at the Wilkes-Barre Veterans Affairs Medical Center (WBVAMC) in Pennsylvania and managed in a pharmacist-led patient aligned care team (PACT) clinic. It was determined by the US Department of Veterans Affairs Office of Research and Development that an institutional review board or other review committee approval was not needed for this nonresearch Veterans Health Administration quality assurance and improvement project. Patients aged ≥ 18 years were included in this study. Patients were excluded if they were not on insulin or a sulfonylurea when starting a GLP-1 agonist, started a GLP-1 agonist outside of the retrospective chart review dates, or were prescribed a GLP-1 agonist by anyone other than a pharmacist in their PACT clinic. This included if a GLP-1 agonist was prescribed by a primary care physician, endocrinologist, or someone outside the VA system.
The primary study outcomes were to determine the percentage of patients with a dose reduction of insulin or sulfonylurea and discontinuation of insulin or a sulfonylurea at intervals of 0 (baseline), 3, 6, and 12 months. Secondary outcomes included changes in HbA1c and body weight measured at the same intervals of 0 (baseline), 3, 6, and 12 months.
Data were collected using the VA Computerized Patient Record System (CPRS) and stored in a locked spreadsheet. Descriptive statistics were used to analyze the data. Patient data included the number of patients on insulin or a sulfonylurea when initiating a GLP-1 agonist, the percentage of patients started on a certain GLP-1 agonist (dulaglutide, liraglutide, exenatide, and semaglutide), and the percentage of patients with a baseline HbA1c of < 8%, 8% to 10%, and > 10%. The GLP-1 agonist formulary was adjusted during the time of this retrospective chart review. Patients who were not on semaglutide were switched over if they were on another GLP-1 agonist as semaglutide became the preferred GLP-1 agonist.
Patients were considered to have a dose reduction or discontinuation of insulin or a sulfonylurea if the dose or medication they were on decreased or was discontinued permanently within 12 months of starting a GLP-1 agonist. For example, if a patient who was administering 10 units of insulin daily was decreased to 8 but later increased back to 10, this was not counted as a dose reduction. If a patient discontinued insulin or a sulfonylurea and then restarted it within 12 months of initiating a GLP-1 agonist, this was not counted as a discontinuation.
Results
This retrospective review included 136 patients; 96 patients taking insulin and 54 taking a sulfonylurea when they started a GLP-1 agonist. Fourteen patients were on both. Criteria for use, which are clinical criteria to determine if a patient is eligible for the use of a given medication, are used within the VA. The inclusion criteria for a patient initiating a GLP-1 agonist is that the patient must have atherosclerotic cardiovascular disease or chronic kidney disease with the patient receiving metformin (unless unable to use metformin) and empagliflozin (unless unable to use empagliflozin).
The baseline mean age and weight for the patient population in this retrospective chart review was 70.7 years and 238.2 lb, respectively. Ninety-six patients (70.6%) were started on semaglutide, 27 (19.9%) on dulaglutide, 12 (8.8%) on liraglutide, and 1 (0.7%) on exenatide. The mean HbA1c when patients initiated a GLP-1 agonist was 8.6%. When starting a GLP-1 agonist, 34 patients (25.0%) had an HbA1c < 8%, 89 (65.4%) had an HbA1c between 8% to 10%, and 13 (9.6%) had an HbA1c > 10% (Table).
For the primary results, 25 patients (26.0%) had a dose reduction of insulin when they started a GLP-1 agonist, and 55 patients (57.3%) had at least 1 insulin dose reduction within the year follow-up. Seven patients (13.0%) had a dose reduction of a sulfonylurea when they started a GLP-1 agonist, and 16 patients (29.6%) had at least 1 dose reduction of a sulfonylurea within the year follow-up. Six patients (6.3%) discontinued insulin use when they initially started a GLP-1 agonist, and 14 patients (14.6%) discontinued insulin use within the year follow-up. Eleven patients (20.4%) discontinued sulfonylurea use when they initially started a GLP-1 agonist, and 21 patients (38.9%) discontinued sulfonylurea use within the year follow-up (Figure).
Fourteen patients were on both insulin and a sulfonylurea. Two patients (14.3%) had a dose reduction of insulin when they started a GLP-1 agonist, and 5 (35.7%) had ≥ 1 insulin dose reduction within the year follow-up. Three patients (21.4%) had a dose reduction of a sulfonylurea when they started a GLP-1 agonist, and 6 (42.9%) had ≥ 1 dose reduction of a sulfonylurea within the year follow-up. Seven patients (50.0%) discontinued sulfonylurea and 3 (21.4%) discontinued insulin at any time throughout the year. The majority of the discontinuations were at the initial start of GLP-1 agonist therapy.
The mean HbA1c for patients on GLP-1 agonist was 8.6% at baseline, 8.0% at 0 to 3 months, 7.6% at 3 to 6 months, and 7.5% at 12 months. Patients experienced a mean HbA1c reduction of 1.1%. The mean weight when a GLP-1 agonist was started was 238.2 lb, 236.0 lb at 0 to 3 months, 223.8 lb at 3 to 6 months, and 224.3 lb after 12 months. Study participants lost a mean weight of 13.9 lb while on a GLP-1 agonist.
Discussion
While this study did not examine why there were dose reductions or discontinuations, we can hypothesize that insulin or sulfonylureas were reduced or discontinued due to a myriad of reasons, such as prophylactic dosing per guidelines, patients having a hypoglycemic event, or pharmacists anticipating potential low blood glucose trends. Also, there could have been numerous reasons GLP-1 agonists were started in patients on insulin or a sulfonylurea, such as HbA1c not being within goal range, cardiovascular benefits (reduce risk of stroke, heart attack, and death), weight loss, and renal protection, such as preventing albuminuria.13,14
This retrospective chart review found a large proportion of patients had a dose reduction of insulin (57.3%) or sulfonylurea (29.6%). The percentage of patients with a dose reduction was potentially underestimated as patients were not counted if they discontinued insulin or sulfonylurea. Concomitant use of GLP-1 agonists with insulin or a sulfonylurea may increase the risk of hypoglycemia and reducing the dose of insulin or a sulfonylurea may be necessary.9-11 The dose reductions in this study show that pharmacists within pharmacy-led PACT clinics monitor for or attempt to prevent hypoglycemia, which aligns with the prescribing information of GLP-1 agonists. While increasing the insulin or sulfonylurea dose is an option for patients, this approach can increase the risk of hypoglycemia, especially in an older population, like this one with a mean age > 70 years. The large proportions of patients with dose reductions or insulin and sulfonylurea discontinuations suggest that pharmacists may need to take a more cautious approach when initiating a GLP-1 agonist to prevent adverse health outcomes related to low blood sugar for older adults, such as falls and fractures.
Insulin was discontinued in 20.4% of patients and sulfonylurea was discontinued in 38.9% of patients within 12 months after starting a GLP-1 agonist. When a patient was on both insulin and a sulfonylurea, the percentage of patients who discontinued insulin (21.4%) or a sulfonylurea (50.0%) was higher compared with patients just on insulin (14.6%) or a sulfonylurea (38.9%) alone. Patients on both insulin and a sulfonylurea may need closer monitoring due to a higher incidence of discontinuations when these diabetes agents are administered in combination.
Within 12 months of patients receiving a GLP-1 agonist, the mean HbA1c reduction was 1.1%, which is comparable to other GLP-1 agonist clinical trials. For semaglutide 0.5 mg and 1.0 mg dosages, the mean HbA1c reduction was 1.4% and 1.6%, respectively.9 For dulaglutide 0.75 mg and 1.5 mg dosages, the mean HbA1c reduction ranged from 0.7% to 1.6% and 0.8% to 1.6%, respectively.10 For liraglutide 1.8 mg dosage, the mean HbA1c reduction ranged from 1.0% to 1.5%.11 The mean weight loss in this study was 13.9 lb. Along with HbA1c, weight loss in this review was comparable to other GLP-1 agonist clinical trials. Patients administering semaglutide lost up to 14 lb, patients taking dulaglutide lost up to 10.1 lb, and patients on liraglutide lost on average 6.2 lb.9-11 Even with medications such as insulin and sulfonylurea that have the side effects of hypoglycemia and weight gain, adding a GLP-1 agonist showed a reduction in HbA1c and weight loss relatively similar to previous clinical trials.
A study on the effects of adding semaglutide to insulin regimens in March 2023 by Meyer and colleagues displayed similar results to this retrospective chart review. That study concluded that there was blood glucose improvement (HbA1c reduction of 1.3%) in patients after 6 months despite a decrease in the insulin dose. Also, patients lost a mean weight of 11 lb during the 6-month trial.3 This retrospective chart review at the WBVAMC adds to the body of research that supports potential reductions or discontinuations of insulin and/or sulfonylureas with the addition of a GLP-1 agonist.
Limitations
Several limitations of this study should be considered when evaluating the results. This review was comprised of a mostly older, male population, which results in a low generalizability to organizations other than VA medical centers. In addition, this study only evaluated patients on a GLP-1 agonist followed in a pharmacist-led PACT clinic. This study excluded patients who were prescribed a GLP-1 agonist by an endocrinologist or a pharmacist at one of the community-based outpatient clinics affiliated with WBVAMC, or a pharmacist or clinician outside the VA. The sole focus of this study was patients in a pharmacist-led VAMC clinic. Not all patient data may have been included in the study. If a patient did not have an appointment at baseline, 3, 6, and 12 months or did not obtain laboratory tests, HbA1c and weights were not recorded. Data were collected during the COVID-19 pandemic and in-person appointments were potentially switched to phone or video appointments. There were many instances during this chart review where a weight was not recorded at each time interval. Also, this study did not consider any other diabetes medications the patient was taking. There were many instances where the patient was taking metformin and/or sodium-glucose cotransporter-2 (SGLT-2) inhibitors. These medications along with diet could have affected the weight results as metformin is weight neutral and SGLT-2 inhibitors promote weight loss.15 Lastly, this study did not evaluate the amount of insulin reduced, only if there was a dose reduction or discontinuation of insulin and/or a sulfonylurea.
Conclusions
Dose reductions and a discontinuation of insulin or a sulfonylurea with the addition of a GLP-1 agonist may be needed. Patients on both insulin and a sulfonylurea may need closer monitoring due to the higher incidences of discontinuations compared with patients on just 1 of these agents. Dose reductions or discontinuations of these diabetic agents can promote positive patient outcomes, such as preventing hypoglycemia, minimizing weight gain, increasing weight loss, and reducing HbA1c levels.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Wilkes-Barre Veterans Affairs Medical Center in Pennsylvania.
1. ElSayed NA, Aleppo G, Aroda VR, et al. 8. Obesity and weight management for the prevention and treatment of type 2 diabetes: standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S128-S139. doi:10.2337/dc23-S008
2. ElSayed NA, Aleppo G, Aroda VE, et al. Older adults: standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S216-S229. doi:10.2337/dc23-S013
3. Meyer J, Dreischmeier E, Lehmann M, Phelan J. The effects of adding semaglutide to high daily dose insulin regimens in patients with type 2 diabetes. Ann Pharmacother. 2023;57(3):241-250. doi:10.1177/10600280221107381
4. Rodbard HW, Lingvay I, Reed J, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab. 2018;103(6):2291-2301. doi:10.1210/jc.2018-00070
5. Anderson SL, Trujillo JM. Basal insulin use with GLP-1 receptor agonists. Diabetes Spectr. 2016;29(3):152-160. doi:10.2337/diaspect.29.3.152
6. Castek SL, Healey LC, Kania DS, Vernon VP, Dawson AJ. Assessment of glucagon-like peptide-1 receptor agonists in veterans taking basal/bolus insulin regimens. Fed Pract. 2022;39(suppl 5):S18-S23. doi:10.12788/fp.0317
7. Chen M, Vider E, Plakogiannis R. Insulin dosage adjustments after initiation of GLP-1 receptor agonists in patients with type 2 diabetes. J Pharm Pract. 2022;35(4):511-517. doi:10.1177/0897190021993625
8. Seino Y, Min KW, Niemoeller E, Takami A; EFC10887 GETGOAL-L Asia Study Investigators. Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia). Diabetes Obes Metab. 2012;14(10):910-917. doi:10.1111/j.1463-1326.2012.01618.x.
9. Ozempic (semaglutide) injection. Package insert. Novo Nordisk Inc; 2022. https://www.ozempic.com/prescribing-information.html
10. Trulicity (dulaglutide) injection. Prescribing information. Lilly and Company; 2022. Accessed December 20, 2023. https://pi.lilly.com/us/trulicity-uspi.pdf
11. Victoza (liraglutide) injection. Prescribing information. Novo Nordisk Inc; 2022. Accessed December 20, 2023. https://www.novo-pi.com/victoza.pdf
12. Das SR, Everett BM, Birtcher KK, et al. 2020 expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76(9):1117-1145. doi:10.1016/j.jacc.2020.05.037
13. Granata A, Maccarrone R, Anzaldi M, et al. GLP-1 receptor agonists and renal outcomes in patients with diabetes mellitus type 2 and diabetic kidney disease: state of the art. Clin Kidney J. 2022;15(9):1657-1665. Published 2022 Mar 12. doi:10.1093/ckj/sfac069
14. Marx N, Husain M, Lehrke M, Verma S, Sattar N. GLP-1 receptor agonists for the reduction of atherosclerotic cardiovascular risk in patients with type 2 diabetes. Circulation. 2022;146(24):1882-1894. doi:10.1161/CIRCULATIONAHA.122.059595
15. Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2022;65(12):1925-1966. doi:10.1007/s00125-022-05787-2
1. ElSayed NA, Aleppo G, Aroda VR, et al. 8. Obesity and weight management for the prevention and treatment of type 2 diabetes: standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S128-S139. doi:10.2337/dc23-S008
2. ElSayed NA, Aleppo G, Aroda VE, et al. Older adults: standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S216-S229. doi:10.2337/dc23-S013
3. Meyer J, Dreischmeier E, Lehmann M, Phelan J. The effects of adding semaglutide to high daily dose insulin regimens in patients with type 2 diabetes. Ann Pharmacother. 2023;57(3):241-250. doi:10.1177/10600280221107381
4. Rodbard HW, Lingvay I, Reed J, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab. 2018;103(6):2291-2301. doi:10.1210/jc.2018-00070
5. Anderson SL, Trujillo JM. Basal insulin use with GLP-1 receptor agonists. Diabetes Spectr. 2016;29(3):152-160. doi:10.2337/diaspect.29.3.152
6. Castek SL, Healey LC, Kania DS, Vernon VP, Dawson AJ. Assessment of glucagon-like peptide-1 receptor agonists in veterans taking basal/bolus insulin regimens. Fed Pract. 2022;39(suppl 5):S18-S23. doi:10.12788/fp.0317
7. Chen M, Vider E, Plakogiannis R. Insulin dosage adjustments after initiation of GLP-1 receptor agonists in patients with type 2 diabetes. J Pharm Pract. 2022;35(4):511-517. doi:10.1177/0897190021993625
8. Seino Y, Min KW, Niemoeller E, Takami A; EFC10887 GETGOAL-L Asia Study Investigators. Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia). Diabetes Obes Metab. 2012;14(10):910-917. doi:10.1111/j.1463-1326.2012.01618.x.
9. Ozempic (semaglutide) injection. Package insert. Novo Nordisk Inc; 2022. https://www.ozempic.com/prescribing-information.html
10. Trulicity (dulaglutide) injection. Prescribing information. Lilly and Company; 2022. Accessed December 20, 2023. https://pi.lilly.com/us/trulicity-uspi.pdf
11. Victoza (liraglutide) injection. Prescribing information. Novo Nordisk Inc; 2022. Accessed December 20, 2023. https://www.novo-pi.com/victoza.pdf
12. Das SR, Everett BM, Birtcher KK, et al. 2020 expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76(9):1117-1145. doi:10.1016/j.jacc.2020.05.037
13. Granata A, Maccarrone R, Anzaldi M, et al. GLP-1 receptor agonists and renal outcomes in patients with diabetes mellitus type 2 and diabetic kidney disease: state of the art. Clin Kidney J. 2022;15(9):1657-1665. Published 2022 Mar 12. doi:10.1093/ckj/sfac069
14. Marx N, Husain M, Lehrke M, Verma S, Sattar N. GLP-1 receptor agonists for the reduction of atherosclerotic cardiovascular risk in patients with type 2 diabetes. Circulation. 2022;146(24):1882-1894. doi:10.1161/CIRCULATIONAHA.122.059595
15. Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2022;65(12):1925-1966. doi:10.1007/s00125-022-05787-2
The Impact of a Paracentesis Clinic on Internal Medicine Resident Procedural Competency
Competency in paracentesis is an important procedural skill for medical practitioners caring for patients with decompensated liver cirrhosis. Paracentesis is performed to drain ascitic fluid for both diagnosis and/or therapeutic purposes.1 While this procedure can be performed without the use of ultrasound, it is preferable to use ultrasound to identify an area of fluid that is away from dangerous anatomy including bowel loops, the liver, and spleen. After prepping the area, lidocaine is administered locally. A catheter is then inserted until fluid begins flowing freely. The catheter is connected to a suction canister or collection kit, and the patient is monitored until the flow ceases. Samples can be sent for analysis to determine the etiology of ascites, identify concerns for infection, and more.
Paracentesis is a very common procedure. Barsuk and colleagues noted that between 2010 and 2012, 97,577 procedures were performed across 120 academic medical centers and 290 affiliated hospitals.2 Patients undergo paracentesis in a variety of settings including the emergency department, inpatient hospitalizations, and clinics. Some patients may require only 1 paracentesis procedure while others may require it regularly.
Due to the rising need for paracentesis in the Central Texas Veterans Affairs Hospital (CTVAH) in Temple, a paracentesis clinic was started in February 2018. The goal of the paracentesis clinic was multifocal—to reduce hospital admissions, improve access to regularly scheduled procedures, decrease wait times, and increase patient satisfaction.3 Through the CTVAH affiliation with the Texas A&M internal medicine residency program, the paracentesis clinic started involving and training residents on this procedure. Up to 3 residents are on weekly rotation and can perform up to 6 paracentesis procedures in a week. The purpose of this article was to evaluate resident competency in paracentesis after completion of the paracentesis clinic.
Methods
The paracentesis clinic schedules up to 3 patients on Tuesdays and Thursdays between 8
A survey was sent via email to all categorical internal medicine residents across all 3 program years at the time of data collection. Competency for paracentesis sign-off was defined as completing and logging 5 procedures supervised by a competent physician who confirmed that all portions of the procedure were performed correctly. Residents were also asked to answer questions on a scale from 1 to 10, with 1 representing no confidence and 10 representing strong confidence to practice independently (Table).
We also evaluated the number of procedures performed by internal medicine residents 3 years before the clinic was started in 2015 up to the completion of 2022. The numbers were obtained by examining procedural log data for each year for all internal medicine residents.
Results
Thirty-three residents completed the survey: 10 first-year internal medicine residents (PGY1), 12 second-year residents (PGY2), and 11 third-year residents (PGY3). The mean participation was 4.8 paracentesis sessions per person for the duration of the study. The range of paracentesis procedures performed varied based on PGY year: PGY1s performed 1 to > 10 procedures, PGY2s performed 2 to > 10 procedures, and PGY3s performed 5 to > 10 procedures. Thirty-six percent of residents completed > 10 procedures in the paracentesis clinic; 82% of PGY3s had completed > 10 procedures by December of their third year. Twenty-six residents (79%) were credentialed to perform paracentesis procedures independently after performing > 5 procedures, and 7 residents were not yet cleared for procedural independence.
In the survey, residents rated their comfort with performing paracentesis procedures independently at a mean of 7.9. The mean comfort reported by PGY1s was 7.2, PGY2s was 7.3, and PGY3s was 9.3. Residents also rated their opinion on whether or not the paracentesis clinic adequately prepared them for paracentesis procedural independence; the mean was 8.9 across all residents.
The total number of procedures performed by residents at CTVAH also increased. Starting in 2015, 3 years before the clinic was started, 38 procedures were performed by internal medicine residents, followed by 72 procedures in 2016; 76 in 2017; 58 in 2018; 94 in 2019; 88 in 2020; 136 in 2021; and 188 in 2022.
Discussion
Paracentesis is a simple but invasive procedure to relieve ascites, often relieving patients’ symptoms, preventing hospital admission, and increasing patient satisfaction.4 The CTVAH does not have the capacity to perform outpatient paracentesis effectively in its emergency or radiology departments. Furthermore, the use of the emergency or radiology departments for routine paracentesis may not be feasible due to the acuity of care being provided, as these procedures can be time consuming and can draw away critical resources and time from patients that need emergent care. The paracentesis clinic was then formed to provide veterans access to the procedural care they need, while also preparing residents to ably and confidently perform the procedure independently.
Based on our study, most residents were cleared to independently perform paracentesis procedures across all 3 years, with 79% of residents having completed the required 5 supervised procedures to independently practice. A study assessing unsupervised practice standards showed that paracentesis skill declines as soon as 3 months after training. However, retraining was shown to potentially interrupt this skill decline.5 Studies have shown that procedure-driven electives or services significantly improved paracentesis certification rates and total logged procedures, with minimal funding or scheduling changes required.6 Our clinic showed a significant increase in the number of procedures logged starting with the minimum of 38 procedures in 2015 and ending with 188 procedures logged at the end of 2022.
By allowing residents to routinely return to the paracentesis clinic across all 3 years, residents were more likely to feel comfortable independently performing the procedure, with residents reporting a mean comfort score of 7.9. The spaced repetition and ability to work with the clinic during elective time allows regular opportunities to undergo supervised training in a controlled environment and created scheduled retraining opportunities. Future studies should evaluate residents prior to each paracentesis clinic to ascertain if skill decline is occurring at a slower rate.
The inpatient effect of the clinic is also multifocal. Pham and colleagues showed that integrating paracentesis into timely training can reduce paracentesis delay and delays in care.7 By increasing the volume of procedures each resident performs and creating a sense of confidence amongst residents, the clinic increases the number of residents able and willing to perform inpatient procedures, thus reducing the number of unnecessary consultations and hospital resources. One of the reasons the paracentesis clinic was started was to allow patients to have scheduled times to remove fluid from their abdomen, thus cutting down on emergency department procedures and unnecessary admissions. Additionally, the benefits of early paracentesis procedural performance by residents and internal medicine physicians have been demonstrated in the literature. A study by Gaetano and colleagues noted that patients undergoing early paracentesis had reduced mortality of 5.5% vs 7.5% in those undergoing late paracentesis.8 This study also showed the in-hospital mortality rate was decreased with paracentesis (6.3%) vs without paracentesis (8.9%).8 By offering residents a chance to participate in the clinic, we have shown that regular opportunities to perform paracentesis may increase the number of physicians capable of independently practicing, improve procedural competency, and improve patient access to this procedure.
Limitations
Our study was not free of bias and has potential weaknesses. The survey was sent to all current residents who have participated in the paracentesis clinic, but not every resident filled out the survey (55% of all residents across 3 years completed the survey, 68.7% who had done clinic that year completed the survey). There is a possibility that those not signed off avoided doing the survey, but we are unable to confirm this. The survey also depended on resident recall of the number of paracenteses completed or looking at their procedure log. It is possible that some procedures were not documented, changing the true number. Additionally, rating comfortability with procedures is subjective, which may also create a source of potential weakness. Future projects should include a baseline survey for residents, followed by a repeat survey a year later to show changes from baseline competency.
Conclusions
A dedicated paracentesis clinic with internal medicine resident involvement may increase resident paracentesis procedural independence, the number of procedures available and performed, and procedural comfort level.
1. Aponte EM, O’Rourke MC, Katta S. Paracentesis. StatPearls [internet]. September 5, 2022. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK435998
2. Barsuk JH, Feinglass J, Kozmic SE, Hohmann SF, Ganger D, Wayne DB. Specialties performing paracentesis procedures at university hospitals: implications for training and certification. J Hosp Med. 2014;9(3):162-168. doi:10.1002/jhm.2153
3. Cheng Y-W, Sandrasegaran K, Cheng K, et al. A dedicated paracentesis clinic decreases healthcare utilization for serial paracenteses in decompensated cirrhosis. Abdominal Radiology. 2017;43(8):2190-2197. doi:10.1007/s00261-017-1406-y
4. Wang J, Khan S, Wyer P, et al. The role of ultrasound-guided therapeutic paracentesis in an outpatient transitional care program: A case series. Am J Hospice Palliat Med. 2018;35(9):1256-1260. doi:10.1177/1049909118755378
5. Sall D, Warm EJ, Kinnear B, Kelleher M, Jandarov R, O’Toole J. See one, do one, forget one: early skill decay after paracentesis training. J Gen Int Med. 2020;36(5):1346-1351. doi:10.1007/s11606-020-06242-x
6. Berger M, Divilov V, Paredes H, Kesar V, Sun E. Improving resident paracentesis certification rates by using an innovative resident driven procedure service. Am J Gastroenterol. 2018;113(suppl). doi:10.14309/00000434-201810001-00980
7. Pham C, Xu A, Suaez MG. S1250 a pilot study to improve resident paracentesis training and reduce paracentesis delay in admitted patients with cirrhosis. Am J Gastroenterol. 2022;117(10S). doi:10.14309/01.ajg.0000861640.53682.93
8. Gaetano JN, Micic D, Aronsohn A, et al. The benefit of paracentesis on hospitalized adults with cirrhosis and ascites. J Gastroenterol Hepatol. 2016;31(5):1025-1030. doi:10.1111/jgh.13255
Competency in paracentesis is an important procedural skill for medical practitioners caring for patients with decompensated liver cirrhosis. Paracentesis is performed to drain ascitic fluid for both diagnosis and/or therapeutic purposes.1 While this procedure can be performed without the use of ultrasound, it is preferable to use ultrasound to identify an area of fluid that is away from dangerous anatomy including bowel loops, the liver, and spleen. After prepping the area, lidocaine is administered locally. A catheter is then inserted until fluid begins flowing freely. The catheter is connected to a suction canister or collection kit, and the patient is monitored until the flow ceases. Samples can be sent for analysis to determine the etiology of ascites, identify concerns for infection, and more.
Paracentesis is a very common procedure. Barsuk and colleagues noted that between 2010 and 2012, 97,577 procedures were performed across 120 academic medical centers and 290 affiliated hospitals.2 Patients undergo paracentesis in a variety of settings including the emergency department, inpatient hospitalizations, and clinics. Some patients may require only 1 paracentesis procedure while others may require it regularly.
Due to the rising need for paracentesis in the Central Texas Veterans Affairs Hospital (CTVAH) in Temple, a paracentesis clinic was started in February 2018. The goal of the paracentesis clinic was multifocal—to reduce hospital admissions, improve access to regularly scheduled procedures, decrease wait times, and increase patient satisfaction.3 Through the CTVAH affiliation with the Texas A&M internal medicine residency program, the paracentesis clinic started involving and training residents on this procedure. Up to 3 residents are on weekly rotation and can perform up to 6 paracentesis procedures in a week. The purpose of this article was to evaluate resident competency in paracentesis after completion of the paracentesis clinic.
Methods
The paracentesis clinic schedules up to 3 patients on Tuesdays and Thursdays between 8
A survey was sent via email to all categorical internal medicine residents across all 3 program years at the time of data collection. Competency for paracentesis sign-off was defined as completing and logging 5 procedures supervised by a competent physician who confirmed that all portions of the procedure were performed correctly. Residents were also asked to answer questions on a scale from 1 to 10, with 1 representing no confidence and 10 representing strong confidence to practice independently (Table).
We also evaluated the number of procedures performed by internal medicine residents 3 years before the clinic was started in 2015 up to the completion of 2022. The numbers were obtained by examining procedural log data for each year for all internal medicine residents.
Results
Thirty-three residents completed the survey: 10 first-year internal medicine residents (PGY1), 12 second-year residents (PGY2), and 11 third-year residents (PGY3). The mean participation was 4.8 paracentesis sessions per person for the duration of the study. The range of paracentesis procedures performed varied based on PGY year: PGY1s performed 1 to > 10 procedures, PGY2s performed 2 to > 10 procedures, and PGY3s performed 5 to > 10 procedures. Thirty-six percent of residents completed > 10 procedures in the paracentesis clinic; 82% of PGY3s had completed > 10 procedures by December of their third year. Twenty-six residents (79%) were credentialed to perform paracentesis procedures independently after performing > 5 procedures, and 7 residents were not yet cleared for procedural independence.
In the survey, residents rated their comfort with performing paracentesis procedures independently at a mean of 7.9. The mean comfort reported by PGY1s was 7.2, PGY2s was 7.3, and PGY3s was 9.3. Residents also rated their opinion on whether or not the paracentesis clinic adequately prepared them for paracentesis procedural independence; the mean was 8.9 across all residents.
The total number of procedures performed by residents at CTVAH also increased. Starting in 2015, 3 years before the clinic was started, 38 procedures were performed by internal medicine residents, followed by 72 procedures in 2016; 76 in 2017; 58 in 2018; 94 in 2019; 88 in 2020; 136 in 2021; and 188 in 2022.
Discussion
Paracentesis is a simple but invasive procedure to relieve ascites, often relieving patients’ symptoms, preventing hospital admission, and increasing patient satisfaction.4 The CTVAH does not have the capacity to perform outpatient paracentesis effectively in its emergency or radiology departments. Furthermore, the use of the emergency or radiology departments for routine paracentesis may not be feasible due to the acuity of care being provided, as these procedures can be time consuming and can draw away critical resources and time from patients that need emergent care. The paracentesis clinic was then formed to provide veterans access to the procedural care they need, while also preparing residents to ably and confidently perform the procedure independently.
Based on our study, most residents were cleared to independently perform paracentesis procedures across all 3 years, with 79% of residents having completed the required 5 supervised procedures to independently practice. A study assessing unsupervised practice standards showed that paracentesis skill declines as soon as 3 months after training. However, retraining was shown to potentially interrupt this skill decline.5 Studies have shown that procedure-driven electives or services significantly improved paracentesis certification rates and total logged procedures, with minimal funding or scheduling changes required.6 Our clinic showed a significant increase in the number of procedures logged starting with the minimum of 38 procedures in 2015 and ending with 188 procedures logged at the end of 2022.
By allowing residents to routinely return to the paracentesis clinic across all 3 years, residents were more likely to feel comfortable independently performing the procedure, with residents reporting a mean comfort score of 7.9. The spaced repetition and ability to work with the clinic during elective time allows regular opportunities to undergo supervised training in a controlled environment and created scheduled retraining opportunities. Future studies should evaluate residents prior to each paracentesis clinic to ascertain if skill decline is occurring at a slower rate.
The inpatient effect of the clinic is also multifocal. Pham and colleagues showed that integrating paracentesis into timely training can reduce paracentesis delay and delays in care.7 By increasing the volume of procedures each resident performs and creating a sense of confidence amongst residents, the clinic increases the number of residents able and willing to perform inpatient procedures, thus reducing the number of unnecessary consultations and hospital resources. One of the reasons the paracentesis clinic was started was to allow patients to have scheduled times to remove fluid from their abdomen, thus cutting down on emergency department procedures and unnecessary admissions. Additionally, the benefits of early paracentesis procedural performance by residents and internal medicine physicians have been demonstrated in the literature. A study by Gaetano and colleagues noted that patients undergoing early paracentesis had reduced mortality of 5.5% vs 7.5% in those undergoing late paracentesis.8 This study also showed the in-hospital mortality rate was decreased with paracentesis (6.3%) vs without paracentesis (8.9%).8 By offering residents a chance to participate in the clinic, we have shown that regular opportunities to perform paracentesis may increase the number of physicians capable of independently practicing, improve procedural competency, and improve patient access to this procedure.
Limitations
Our study was not free of bias and has potential weaknesses. The survey was sent to all current residents who have participated in the paracentesis clinic, but not every resident filled out the survey (55% of all residents across 3 years completed the survey, 68.7% who had done clinic that year completed the survey). There is a possibility that those not signed off avoided doing the survey, but we are unable to confirm this. The survey also depended on resident recall of the number of paracenteses completed or looking at their procedure log. It is possible that some procedures were not documented, changing the true number. Additionally, rating comfortability with procedures is subjective, which may also create a source of potential weakness. Future projects should include a baseline survey for residents, followed by a repeat survey a year later to show changes from baseline competency.
Conclusions
A dedicated paracentesis clinic with internal medicine resident involvement may increase resident paracentesis procedural independence, the number of procedures available and performed, and procedural comfort level.
Competency in paracentesis is an important procedural skill for medical practitioners caring for patients with decompensated liver cirrhosis. Paracentesis is performed to drain ascitic fluid for both diagnosis and/or therapeutic purposes.1 While this procedure can be performed without the use of ultrasound, it is preferable to use ultrasound to identify an area of fluid that is away from dangerous anatomy including bowel loops, the liver, and spleen. After prepping the area, lidocaine is administered locally. A catheter is then inserted until fluid begins flowing freely. The catheter is connected to a suction canister or collection kit, and the patient is monitored until the flow ceases. Samples can be sent for analysis to determine the etiology of ascites, identify concerns for infection, and more.
Paracentesis is a very common procedure. Barsuk and colleagues noted that between 2010 and 2012, 97,577 procedures were performed across 120 academic medical centers and 290 affiliated hospitals.2 Patients undergo paracentesis in a variety of settings including the emergency department, inpatient hospitalizations, and clinics. Some patients may require only 1 paracentesis procedure while others may require it regularly.
Due to the rising need for paracentesis in the Central Texas Veterans Affairs Hospital (CTVAH) in Temple, a paracentesis clinic was started in February 2018. The goal of the paracentesis clinic was multifocal—to reduce hospital admissions, improve access to regularly scheduled procedures, decrease wait times, and increase patient satisfaction.3 Through the CTVAH affiliation with the Texas A&M internal medicine residency program, the paracentesis clinic started involving and training residents on this procedure. Up to 3 residents are on weekly rotation and can perform up to 6 paracentesis procedures in a week. The purpose of this article was to evaluate resident competency in paracentesis after completion of the paracentesis clinic.
Methods
The paracentesis clinic schedules up to 3 patients on Tuesdays and Thursdays between 8
A survey was sent via email to all categorical internal medicine residents across all 3 program years at the time of data collection. Competency for paracentesis sign-off was defined as completing and logging 5 procedures supervised by a competent physician who confirmed that all portions of the procedure were performed correctly. Residents were also asked to answer questions on a scale from 1 to 10, with 1 representing no confidence and 10 representing strong confidence to practice independently (Table).
We also evaluated the number of procedures performed by internal medicine residents 3 years before the clinic was started in 2015 up to the completion of 2022. The numbers were obtained by examining procedural log data for each year for all internal medicine residents.
Results
Thirty-three residents completed the survey: 10 first-year internal medicine residents (PGY1), 12 second-year residents (PGY2), and 11 third-year residents (PGY3). The mean participation was 4.8 paracentesis sessions per person for the duration of the study. The range of paracentesis procedures performed varied based on PGY year: PGY1s performed 1 to > 10 procedures, PGY2s performed 2 to > 10 procedures, and PGY3s performed 5 to > 10 procedures. Thirty-six percent of residents completed > 10 procedures in the paracentesis clinic; 82% of PGY3s had completed > 10 procedures by December of their third year. Twenty-six residents (79%) were credentialed to perform paracentesis procedures independently after performing > 5 procedures, and 7 residents were not yet cleared for procedural independence.
In the survey, residents rated their comfort with performing paracentesis procedures independently at a mean of 7.9. The mean comfort reported by PGY1s was 7.2, PGY2s was 7.3, and PGY3s was 9.3. Residents also rated their opinion on whether or not the paracentesis clinic adequately prepared them for paracentesis procedural independence; the mean was 8.9 across all residents.
The total number of procedures performed by residents at CTVAH also increased. Starting in 2015, 3 years before the clinic was started, 38 procedures were performed by internal medicine residents, followed by 72 procedures in 2016; 76 in 2017; 58 in 2018; 94 in 2019; 88 in 2020; 136 in 2021; and 188 in 2022.
Discussion
Paracentesis is a simple but invasive procedure to relieve ascites, often relieving patients’ symptoms, preventing hospital admission, and increasing patient satisfaction.4 The CTVAH does not have the capacity to perform outpatient paracentesis effectively in its emergency or radiology departments. Furthermore, the use of the emergency or radiology departments for routine paracentesis may not be feasible due to the acuity of care being provided, as these procedures can be time consuming and can draw away critical resources and time from patients that need emergent care. The paracentesis clinic was then formed to provide veterans access to the procedural care they need, while also preparing residents to ably and confidently perform the procedure independently.
Based on our study, most residents were cleared to independently perform paracentesis procedures across all 3 years, with 79% of residents having completed the required 5 supervised procedures to independently practice. A study assessing unsupervised practice standards showed that paracentesis skill declines as soon as 3 months after training. However, retraining was shown to potentially interrupt this skill decline.5 Studies have shown that procedure-driven electives or services significantly improved paracentesis certification rates and total logged procedures, with minimal funding or scheduling changes required.6 Our clinic showed a significant increase in the number of procedures logged starting with the minimum of 38 procedures in 2015 and ending with 188 procedures logged at the end of 2022.
By allowing residents to routinely return to the paracentesis clinic across all 3 years, residents were more likely to feel comfortable independently performing the procedure, with residents reporting a mean comfort score of 7.9. The spaced repetition and ability to work with the clinic during elective time allows regular opportunities to undergo supervised training in a controlled environment and created scheduled retraining opportunities. Future studies should evaluate residents prior to each paracentesis clinic to ascertain if skill decline is occurring at a slower rate.
The inpatient effect of the clinic is also multifocal. Pham and colleagues showed that integrating paracentesis into timely training can reduce paracentesis delay and delays in care.7 By increasing the volume of procedures each resident performs and creating a sense of confidence amongst residents, the clinic increases the number of residents able and willing to perform inpatient procedures, thus reducing the number of unnecessary consultations and hospital resources. One of the reasons the paracentesis clinic was started was to allow patients to have scheduled times to remove fluid from their abdomen, thus cutting down on emergency department procedures and unnecessary admissions. Additionally, the benefits of early paracentesis procedural performance by residents and internal medicine physicians have been demonstrated in the literature. A study by Gaetano and colleagues noted that patients undergoing early paracentesis had reduced mortality of 5.5% vs 7.5% in those undergoing late paracentesis.8 This study also showed the in-hospital mortality rate was decreased with paracentesis (6.3%) vs without paracentesis (8.9%).8 By offering residents a chance to participate in the clinic, we have shown that regular opportunities to perform paracentesis may increase the number of physicians capable of independently practicing, improve procedural competency, and improve patient access to this procedure.
Limitations
Our study was not free of bias and has potential weaknesses. The survey was sent to all current residents who have participated in the paracentesis clinic, but not every resident filled out the survey (55% of all residents across 3 years completed the survey, 68.7% who had done clinic that year completed the survey). There is a possibility that those not signed off avoided doing the survey, but we are unable to confirm this. The survey also depended on resident recall of the number of paracenteses completed or looking at their procedure log. It is possible that some procedures were not documented, changing the true number. Additionally, rating comfortability with procedures is subjective, which may also create a source of potential weakness. Future projects should include a baseline survey for residents, followed by a repeat survey a year later to show changes from baseline competency.
Conclusions
A dedicated paracentesis clinic with internal medicine resident involvement may increase resident paracentesis procedural independence, the number of procedures available and performed, and procedural comfort level.
1. Aponte EM, O’Rourke MC, Katta S. Paracentesis. StatPearls [internet]. September 5, 2022. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK435998
2. Barsuk JH, Feinglass J, Kozmic SE, Hohmann SF, Ganger D, Wayne DB. Specialties performing paracentesis procedures at university hospitals: implications for training and certification. J Hosp Med. 2014;9(3):162-168. doi:10.1002/jhm.2153
3. Cheng Y-W, Sandrasegaran K, Cheng K, et al. A dedicated paracentesis clinic decreases healthcare utilization for serial paracenteses in decompensated cirrhosis. Abdominal Radiology. 2017;43(8):2190-2197. doi:10.1007/s00261-017-1406-y
4. Wang J, Khan S, Wyer P, et al. The role of ultrasound-guided therapeutic paracentesis in an outpatient transitional care program: A case series. Am J Hospice Palliat Med. 2018;35(9):1256-1260. doi:10.1177/1049909118755378
5. Sall D, Warm EJ, Kinnear B, Kelleher M, Jandarov R, O’Toole J. See one, do one, forget one: early skill decay after paracentesis training. J Gen Int Med. 2020;36(5):1346-1351. doi:10.1007/s11606-020-06242-x
6. Berger M, Divilov V, Paredes H, Kesar V, Sun E. Improving resident paracentesis certification rates by using an innovative resident driven procedure service. Am J Gastroenterol. 2018;113(suppl). doi:10.14309/00000434-201810001-00980
7. Pham C, Xu A, Suaez MG. S1250 a pilot study to improve resident paracentesis training and reduce paracentesis delay in admitted patients with cirrhosis. Am J Gastroenterol. 2022;117(10S). doi:10.14309/01.ajg.0000861640.53682.93
8. Gaetano JN, Micic D, Aronsohn A, et al. The benefit of paracentesis on hospitalized adults with cirrhosis and ascites. J Gastroenterol Hepatol. 2016;31(5):1025-1030. doi:10.1111/jgh.13255
1. Aponte EM, O’Rourke MC, Katta S. Paracentesis. StatPearls [internet]. September 5, 2022. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK435998
2. Barsuk JH, Feinglass J, Kozmic SE, Hohmann SF, Ganger D, Wayne DB. Specialties performing paracentesis procedures at university hospitals: implications for training and certification. J Hosp Med. 2014;9(3):162-168. doi:10.1002/jhm.2153
3. Cheng Y-W, Sandrasegaran K, Cheng K, et al. A dedicated paracentesis clinic decreases healthcare utilization for serial paracenteses in decompensated cirrhosis. Abdominal Radiology. 2017;43(8):2190-2197. doi:10.1007/s00261-017-1406-y
4. Wang J, Khan S, Wyer P, et al. The role of ultrasound-guided therapeutic paracentesis in an outpatient transitional care program: A case series. Am J Hospice Palliat Med. 2018;35(9):1256-1260. doi:10.1177/1049909118755378
5. Sall D, Warm EJ, Kinnear B, Kelleher M, Jandarov R, O’Toole J. See one, do one, forget one: early skill decay after paracentesis training. J Gen Int Med. 2020;36(5):1346-1351. doi:10.1007/s11606-020-06242-x
6. Berger M, Divilov V, Paredes H, Kesar V, Sun E. Improving resident paracentesis certification rates by using an innovative resident driven procedure service. Am J Gastroenterol. 2018;113(suppl). doi:10.14309/00000434-201810001-00980
7. Pham C, Xu A, Suaez MG. S1250 a pilot study to improve resident paracentesis training and reduce paracentesis delay in admitted patients with cirrhosis. Am J Gastroenterol. 2022;117(10S). doi:10.14309/01.ajg.0000861640.53682.93
8. Gaetano JN, Micic D, Aronsohn A, et al. The benefit of paracentesis on hospitalized adults with cirrhosis and ascites. J Gastroenterol Hepatol. 2016;31(5):1025-1030. doi:10.1111/jgh.13255
Piperacillin/Tazobactam Use vs Cefepime May Be Associated With Acute Decompensated Heart Failure
Piperacillin/tazobactam (PTZ) is a combination IV antibiotic comprised of the semisynthetic antipseudomonal β-lactam, piperacillin sodium, and the β-lactamase inhibitor, tazobactam sodium.1 PTZ is extensively prescribed in the hospital setting for a multitude of infections including but not limited to the US Food and Drug Administration–approved indications: intra-abdominal infection, skin and skin structure infection (SSTI), urinary tract infection (UTI), and pneumonia. Given its broad spectrum of activity and relative safety profile, PTZ is a mainstay of many empiric IV antibiotic regimens. The primary elimination pathway for PTZ is renal excretion, and dosage adjustments are recommended with reduced creatinine clearance. Additionally, PTZ use has been associated with acute renal injury and delayed renal recovery.1-3
There are various mechanisms through which medications can contribute to acute decomopensated heart failure (ADHF).4 These mechanisms include direct cardiotoxicity; negative inotropic, lusitropic, or chronotropic effects; exacerbating hypertension; sodium loading; and drug-drug interactions that limit the benefits of heart failure (HF) medications. One potentially overlooked constituent of PTZ is the sodium content, with the standard formulation containing 65 mg of sodium per gram of piperacillin.1-3 Furthermore, PTZ must be diluted in 50 to 150 mL of diluent, commonly 0.9% sodium chloride, which can contribute an additional 177 to 531 mg of sodium per dose. PTZ prescribing information advises caution for use in patients with decreased renal, hepatic, and/or cardiac function and notes that geriatric patients, particularly with HF, may be at risk of impaired natriuresis in the setting of large sodium doses.
It is estimated that roughly 6.2 million adults in the United States have HF and prevalence continues to rise.5,6 Mortality rates after hospitalization due to HF are 20% to 25% at 1 year. Health care expenditures for the management of HF surpass $30 billion per year in the US, with most of this cost attributed to hospitalizations. Consequently, it is important to continue to identify and practice preventative strategies when managing patients with HF.
Methods
This single-center, retrospective, cohort study was conducted at James H. Quillen Veterans Affairs Medical Center (JHQVAMC) in Mountain Home, Tennessee, a 174-bed tertiary medical center. The purpose of this study was to compare the incidence of ADHF in patients who received PTZ vs cefepime (CFP). This project was reviewed by the JHQVAMC Institutional Review Board and deemed exempt as a clinical process improvement operations activity.
The antimicrobial stewardship team at JHQVAMC reviewed the use of PTZ in veterans between January 1, 2018, to December 31, 2019, and compared baseline demographics, history of HF, and outcomes in patients receiving analogous broad-spectrum empiric antibiotic therapy with CFP.
Statistical Analysis
Analysis was conducted with R Software. Pearson χ2 and t tests were used to compare baseline demographics, length of stay, readmission, and mortality. Significance used was α = .05.
Results
A retrospective chart review was performed on 389 veterans. Of the 389, 204 patients received at least 24 hours of PTZ, and 185 patients received CFP. The mean age in both groups was 75 years. Patients in the PTZ group were more likely to have been admitted with the diagnosis of pneumonia (105 vs 49, P < .001). However, 29 patients (15.7%) in the CFP group were admitted with a UTI diagnosis compared with 6 patients (2.9%) in the PTZ group (P < .001) and 62 patients (33.5%) in the CFP group were admitted with a SSTI diagnosis compared with 48 patients (23.5%) in the PTZ group (P = .03). Otherwise, there were no differences between other admitting diagnoses. Additionally, there was no difference in prior history of HF between groups (Table 1).
Twenty-five patients (12.3%) in the PTZ group and 4 patients (2.2%) in the CFP group were subsequently diagnosed with ADHF (P < .001). Hospital readmissions due to HF were higher in the PTZ group compared with the CFP group (11 vs 2, P = .02). Hospital readmission due to other causes was not significantly different between groups. Hospital readmission due to infection occurred in 18 patients who received PTZ and 25 who received CFP (8.8% vs 13.5%, P = .14). Hospital readmission due to any other indication occurred in 24 patients who received PTZ and 24 who received CFP (11.8% vs 13.0%, P = .72). There was no statistically significant difference in all-cause mortality during the associated admission or within 6 months of discharge between groups, with 59 total deaths in the PTZ group and 50 in the CFP group (28.9% vs 27.0%, P = .63).
There was no difference in length of stay outcomes between patients receiving PTZ compared with CFP. Twenty-eight patients in the PTZ group and 20 in the CFP group had a length of stay duration of < 3 days (13.7% vs 10.8%, P = .46). Seventy-three patients in the PTZ group and 76 in the CFP group had a length of stay duration of 4 to 6 days (36.3% vs 41.1%, P = .28). One hundred three patients in the PTZ group and 89 in the CFP group had a length of stay duration ≥ 7 days (50.5% vs 48.1%, P = .78). Table 2 includes a complete overview of primary and secondary endpoint results.
Discussion
The American Heart Association (AHA) lists PTZ as a medication that may cause or exacerbate HF, though no studies have identified a clear association between PTZ use and ADHF.4 Sodium restriction is consistently recommended as an important strategy for the prevention of ADHF. Accordingly, PTZ prescribing information and the AHA advise careful consideration with PTZ use in this patient population.1,4
The specific mechanism responsible for the association of PTZ with cardiac-related adverse outcomes is unclear. It is easy to presume that the sodium content of PTZ is solely responsible; however, other antibiotic regimens not included as agents of concern by the AHA, such as meropenem, can approach similar overall daily sodium amounts.4,7 Additionally, total sodium and volume can also be contributed by various IV medications and fluids. This study did not evaluate total sodium intake from all sources, but it is notable that this study identified a possible trend toward the risk of ADHF with PTZ use in a routine practice environment. It is reasonable to postulate additional intrinsic properties of PTZ may be contributing to the development of ADHF, such as its association with renal injury possibly resulting in increased fluid retainment and subsequent fluid volume overload.1,2,4 Other hypothesized mechanisms may include those previously described, such as direct myocardial toxicity; negative inotropic, lusitropic, or chronotropic effects; exacerbating hypertension; and drug-drug interactions that limit the benefits of HF medications, although these have not been overtly associated with PTZ in the literature to date.4,8
ADHF can present similarly to other acute pulmonary conditions, including pneumonia.9,10 It is important to acknowledge the challenge this creates for diagnosticians to differentiate between these conditions rapidly and precisely. As a result, this patient population is likely at increased risk of IV antibiotic exposure. Other studies have identified worse outcomes in patients who receive potentially unwarranted IV antibiotics in patients with ADHF.9,10 The results of this study further emphasize the importance of careful considerate antibiotic selection and overall avoidance of unnecessary antibiotic exposure to limit potential adverse outcomes.
Limitations
There are various limitations to this study. Firstly, no women were included due to the predominantly male population within the Veterans Health Administration system. Secondly, this study was retrospective in design and was therefore limited to the completeness and accuracy of the available data collected. Additionally, this study evaluated any ADHF episode during the associated hospitalization as the primary endpoint. The time to diagnosis of ADHF in relation to PTZ initiation was not evaluated, which may have helped better elucidate this possible association. Furthermore, while a significant statistical difference was identified, the smaller sample size may have limited the ability to accurately identify differences in lower event rate outcomes.
Conclusions
This study identifies an association between PTZ use and significant cardiac-related adverse outcomes, including increased incidence of ADHF and readmission due to HF exacerbation. While more research is needed to define the exact mechanisms by which PTZ may precipitate acute decompensation in patients with HF, it is judicious to consider close monitoring or the avoidance of PTZ when appropriate antibiotic alternatives are available in patients with a known history of HF.
1. Zosyn. Package insert. Wyeth Pharmaceuticals; 2020.
2. Jensen JU, Hein L, Lundgren B, et al. Kidney failure related to broad-spectrum antibiotics in critically ill patients: secondary end point results from a 1200 patient randomised trial. BMJ Open. 2012;2(2):e000635. Published 2012 Mar 11. doi:10.1136/bmjopen-2011-000635
3. Kadomura S, Takekuma Y, Sato Y, et al. Higher incidence of acute kidney injury in patients treated with piperacillin/tazobactam than in patients treated with cefepime: a single-center retrospective cohort study. J Pharm Health Care Sci. 2019;5:13. Published 2019 Jun 12. doi:10.1186/s40780-019-0142-6
4. Page RL 2nd, O’Bryant CL, Cheng D, et al. Drugs that may cause or exacerbate heart failure: a scientific statement from the American Heart Association. Circulation. 2016;134(6):e32-e69. doi:10.1161/CIR.0000000000000426
5. Bozkurt B, Hershberger RE, Butler J, et al. 2021 ACC/AHA key data elements and definitions for heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical data standards. J Am Coll Cardiol. 2021;77(16):2053-2150.
6. Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254-e743. doi:10.1161/CIR.0000000000000950
7. Merrem. Package insert. Pfizer Labs; 2021.
8. Keller GA, Alvarez PA, Ponte ML, et al. Drug-induced QTc interval prolongation: a multicenter study to detect drugs and clinical factors involved in every day practice. Curr Drug Saf. 2016;11(1):86-98. doi:10.2174/1574886311207040262
9. Wu S, Alikhil M, Forsyth R, Allen B. Impact of potentially unwarranted intravenous antibiotics targeting pulmonary infections in acute decompensated heart failure. J Pharm Technol. 2021;37(6):298-303. doi:10.1177/87551225211038020
10. Frisbee J, Heidel RH, Rasnake MS. Adverse outcomes associated with potentially inappropriate antibiotic use in heart failure admissions. Open Forum Infect Dis. 2019;6(6):ofz220. doi:10.1093/ofid/ofz220
Piperacillin/tazobactam (PTZ) is a combination IV antibiotic comprised of the semisynthetic antipseudomonal β-lactam, piperacillin sodium, and the β-lactamase inhibitor, tazobactam sodium.1 PTZ is extensively prescribed in the hospital setting for a multitude of infections including but not limited to the US Food and Drug Administration–approved indications: intra-abdominal infection, skin and skin structure infection (SSTI), urinary tract infection (UTI), and pneumonia. Given its broad spectrum of activity and relative safety profile, PTZ is a mainstay of many empiric IV antibiotic regimens. The primary elimination pathway for PTZ is renal excretion, and dosage adjustments are recommended with reduced creatinine clearance. Additionally, PTZ use has been associated with acute renal injury and delayed renal recovery.1-3
There are various mechanisms through which medications can contribute to acute decomopensated heart failure (ADHF).4 These mechanisms include direct cardiotoxicity; negative inotropic, lusitropic, or chronotropic effects; exacerbating hypertension; sodium loading; and drug-drug interactions that limit the benefits of heart failure (HF) medications. One potentially overlooked constituent of PTZ is the sodium content, with the standard formulation containing 65 mg of sodium per gram of piperacillin.1-3 Furthermore, PTZ must be diluted in 50 to 150 mL of diluent, commonly 0.9% sodium chloride, which can contribute an additional 177 to 531 mg of sodium per dose. PTZ prescribing information advises caution for use in patients with decreased renal, hepatic, and/or cardiac function and notes that geriatric patients, particularly with HF, may be at risk of impaired natriuresis in the setting of large sodium doses.
It is estimated that roughly 6.2 million adults in the United States have HF and prevalence continues to rise.5,6 Mortality rates after hospitalization due to HF are 20% to 25% at 1 year. Health care expenditures for the management of HF surpass $30 billion per year in the US, with most of this cost attributed to hospitalizations. Consequently, it is important to continue to identify and practice preventative strategies when managing patients with HF.
Methods
This single-center, retrospective, cohort study was conducted at James H. Quillen Veterans Affairs Medical Center (JHQVAMC) in Mountain Home, Tennessee, a 174-bed tertiary medical center. The purpose of this study was to compare the incidence of ADHF in patients who received PTZ vs cefepime (CFP). This project was reviewed by the JHQVAMC Institutional Review Board and deemed exempt as a clinical process improvement operations activity.
The antimicrobial stewardship team at JHQVAMC reviewed the use of PTZ in veterans between January 1, 2018, to December 31, 2019, and compared baseline demographics, history of HF, and outcomes in patients receiving analogous broad-spectrum empiric antibiotic therapy with CFP.
Statistical Analysis
Analysis was conducted with R Software. Pearson χ2 and t tests were used to compare baseline demographics, length of stay, readmission, and mortality. Significance used was α = .05.
Results
A retrospective chart review was performed on 389 veterans. Of the 389, 204 patients received at least 24 hours of PTZ, and 185 patients received CFP. The mean age in both groups was 75 years. Patients in the PTZ group were more likely to have been admitted with the diagnosis of pneumonia (105 vs 49, P < .001). However, 29 patients (15.7%) in the CFP group were admitted with a UTI diagnosis compared with 6 patients (2.9%) in the PTZ group (P < .001) and 62 patients (33.5%) in the CFP group were admitted with a SSTI diagnosis compared with 48 patients (23.5%) in the PTZ group (P = .03). Otherwise, there were no differences between other admitting diagnoses. Additionally, there was no difference in prior history of HF between groups (Table 1).
Twenty-five patients (12.3%) in the PTZ group and 4 patients (2.2%) in the CFP group were subsequently diagnosed with ADHF (P < .001). Hospital readmissions due to HF were higher in the PTZ group compared with the CFP group (11 vs 2, P = .02). Hospital readmission due to other causes was not significantly different between groups. Hospital readmission due to infection occurred in 18 patients who received PTZ and 25 who received CFP (8.8% vs 13.5%, P = .14). Hospital readmission due to any other indication occurred in 24 patients who received PTZ and 24 who received CFP (11.8% vs 13.0%, P = .72). There was no statistically significant difference in all-cause mortality during the associated admission or within 6 months of discharge between groups, with 59 total deaths in the PTZ group and 50 in the CFP group (28.9% vs 27.0%, P = .63).
There was no difference in length of stay outcomes between patients receiving PTZ compared with CFP. Twenty-eight patients in the PTZ group and 20 in the CFP group had a length of stay duration of < 3 days (13.7% vs 10.8%, P = .46). Seventy-three patients in the PTZ group and 76 in the CFP group had a length of stay duration of 4 to 6 days (36.3% vs 41.1%, P = .28). One hundred three patients in the PTZ group and 89 in the CFP group had a length of stay duration ≥ 7 days (50.5% vs 48.1%, P = .78). Table 2 includes a complete overview of primary and secondary endpoint results.
Discussion
The American Heart Association (AHA) lists PTZ as a medication that may cause or exacerbate HF, though no studies have identified a clear association between PTZ use and ADHF.4 Sodium restriction is consistently recommended as an important strategy for the prevention of ADHF. Accordingly, PTZ prescribing information and the AHA advise careful consideration with PTZ use in this patient population.1,4
The specific mechanism responsible for the association of PTZ with cardiac-related adverse outcomes is unclear. It is easy to presume that the sodium content of PTZ is solely responsible; however, other antibiotic regimens not included as agents of concern by the AHA, such as meropenem, can approach similar overall daily sodium amounts.4,7 Additionally, total sodium and volume can also be contributed by various IV medications and fluids. This study did not evaluate total sodium intake from all sources, but it is notable that this study identified a possible trend toward the risk of ADHF with PTZ use in a routine practice environment. It is reasonable to postulate additional intrinsic properties of PTZ may be contributing to the development of ADHF, such as its association with renal injury possibly resulting in increased fluid retainment and subsequent fluid volume overload.1,2,4 Other hypothesized mechanisms may include those previously described, such as direct myocardial toxicity; negative inotropic, lusitropic, or chronotropic effects; exacerbating hypertension; and drug-drug interactions that limit the benefits of HF medications, although these have not been overtly associated with PTZ in the literature to date.4,8
ADHF can present similarly to other acute pulmonary conditions, including pneumonia.9,10 It is important to acknowledge the challenge this creates for diagnosticians to differentiate between these conditions rapidly and precisely. As a result, this patient population is likely at increased risk of IV antibiotic exposure. Other studies have identified worse outcomes in patients who receive potentially unwarranted IV antibiotics in patients with ADHF.9,10 The results of this study further emphasize the importance of careful considerate antibiotic selection and overall avoidance of unnecessary antibiotic exposure to limit potential adverse outcomes.
Limitations
There are various limitations to this study. Firstly, no women were included due to the predominantly male population within the Veterans Health Administration system. Secondly, this study was retrospective in design and was therefore limited to the completeness and accuracy of the available data collected. Additionally, this study evaluated any ADHF episode during the associated hospitalization as the primary endpoint. The time to diagnosis of ADHF in relation to PTZ initiation was not evaluated, which may have helped better elucidate this possible association. Furthermore, while a significant statistical difference was identified, the smaller sample size may have limited the ability to accurately identify differences in lower event rate outcomes.
Conclusions
This study identifies an association between PTZ use and significant cardiac-related adverse outcomes, including increased incidence of ADHF and readmission due to HF exacerbation. While more research is needed to define the exact mechanisms by which PTZ may precipitate acute decompensation in patients with HF, it is judicious to consider close monitoring or the avoidance of PTZ when appropriate antibiotic alternatives are available in patients with a known history of HF.
Piperacillin/tazobactam (PTZ) is a combination IV antibiotic comprised of the semisynthetic antipseudomonal β-lactam, piperacillin sodium, and the β-lactamase inhibitor, tazobactam sodium.1 PTZ is extensively prescribed in the hospital setting for a multitude of infections including but not limited to the US Food and Drug Administration–approved indications: intra-abdominal infection, skin and skin structure infection (SSTI), urinary tract infection (UTI), and pneumonia. Given its broad spectrum of activity and relative safety profile, PTZ is a mainstay of many empiric IV antibiotic regimens. The primary elimination pathway for PTZ is renal excretion, and dosage adjustments are recommended with reduced creatinine clearance. Additionally, PTZ use has been associated with acute renal injury and delayed renal recovery.1-3
There are various mechanisms through which medications can contribute to acute decomopensated heart failure (ADHF).4 These mechanisms include direct cardiotoxicity; negative inotropic, lusitropic, or chronotropic effects; exacerbating hypertension; sodium loading; and drug-drug interactions that limit the benefits of heart failure (HF) medications. One potentially overlooked constituent of PTZ is the sodium content, with the standard formulation containing 65 mg of sodium per gram of piperacillin.1-3 Furthermore, PTZ must be diluted in 50 to 150 mL of diluent, commonly 0.9% sodium chloride, which can contribute an additional 177 to 531 mg of sodium per dose. PTZ prescribing information advises caution for use in patients with decreased renal, hepatic, and/or cardiac function and notes that geriatric patients, particularly with HF, may be at risk of impaired natriuresis in the setting of large sodium doses.
It is estimated that roughly 6.2 million adults in the United States have HF and prevalence continues to rise.5,6 Mortality rates after hospitalization due to HF are 20% to 25% at 1 year. Health care expenditures for the management of HF surpass $30 billion per year in the US, with most of this cost attributed to hospitalizations. Consequently, it is important to continue to identify and practice preventative strategies when managing patients with HF.
Methods
This single-center, retrospective, cohort study was conducted at James H. Quillen Veterans Affairs Medical Center (JHQVAMC) in Mountain Home, Tennessee, a 174-bed tertiary medical center. The purpose of this study was to compare the incidence of ADHF in patients who received PTZ vs cefepime (CFP). This project was reviewed by the JHQVAMC Institutional Review Board and deemed exempt as a clinical process improvement operations activity.
The antimicrobial stewardship team at JHQVAMC reviewed the use of PTZ in veterans between January 1, 2018, to December 31, 2019, and compared baseline demographics, history of HF, and outcomes in patients receiving analogous broad-spectrum empiric antibiotic therapy with CFP.
Statistical Analysis
Analysis was conducted with R Software. Pearson χ2 and t tests were used to compare baseline demographics, length of stay, readmission, and mortality. Significance used was α = .05.
Results
A retrospective chart review was performed on 389 veterans. Of the 389, 204 patients received at least 24 hours of PTZ, and 185 patients received CFP. The mean age in both groups was 75 years. Patients in the PTZ group were more likely to have been admitted with the diagnosis of pneumonia (105 vs 49, P < .001). However, 29 patients (15.7%) in the CFP group were admitted with a UTI diagnosis compared with 6 patients (2.9%) in the PTZ group (P < .001) and 62 patients (33.5%) in the CFP group were admitted with a SSTI diagnosis compared with 48 patients (23.5%) in the PTZ group (P = .03). Otherwise, there were no differences between other admitting diagnoses. Additionally, there was no difference in prior history of HF between groups (Table 1).
Twenty-five patients (12.3%) in the PTZ group and 4 patients (2.2%) in the CFP group were subsequently diagnosed with ADHF (P < .001). Hospital readmissions due to HF were higher in the PTZ group compared with the CFP group (11 vs 2, P = .02). Hospital readmission due to other causes was not significantly different between groups. Hospital readmission due to infection occurred in 18 patients who received PTZ and 25 who received CFP (8.8% vs 13.5%, P = .14). Hospital readmission due to any other indication occurred in 24 patients who received PTZ and 24 who received CFP (11.8% vs 13.0%, P = .72). There was no statistically significant difference in all-cause mortality during the associated admission or within 6 months of discharge between groups, with 59 total deaths in the PTZ group and 50 in the CFP group (28.9% vs 27.0%, P = .63).
There was no difference in length of stay outcomes between patients receiving PTZ compared with CFP. Twenty-eight patients in the PTZ group and 20 in the CFP group had a length of stay duration of < 3 days (13.7% vs 10.8%, P = .46). Seventy-three patients in the PTZ group and 76 in the CFP group had a length of stay duration of 4 to 6 days (36.3% vs 41.1%, P = .28). One hundred three patients in the PTZ group and 89 in the CFP group had a length of stay duration ≥ 7 days (50.5% vs 48.1%, P = .78). Table 2 includes a complete overview of primary and secondary endpoint results.
Discussion
The American Heart Association (AHA) lists PTZ as a medication that may cause or exacerbate HF, though no studies have identified a clear association between PTZ use and ADHF.4 Sodium restriction is consistently recommended as an important strategy for the prevention of ADHF. Accordingly, PTZ prescribing information and the AHA advise careful consideration with PTZ use in this patient population.1,4
The specific mechanism responsible for the association of PTZ with cardiac-related adverse outcomes is unclear. It is easy to presume that the sodium content of PTZ is solely responsible; however, other antibiotic regimens not included as agents of concern by the AHA, such as meropenem, can approach similar overall daily sodium amounts.4,7 Additionally, total sodium and volume can also be contributed by various IV medications and fluids. This study did not evaluate total sodium intake from all sources, but it is notable that this study identified a possible trend toward the risk of ADHF with PTZ use in a routine practice environment. It is reasonable to postulate additional intrinsic properties of PTZ may be contributing to the development of ADHF, such as its association with renal injury possibly resulting in increased fluid retainment and subsequent fluid volume overload.1,2,4 Other hypothesized mechanisms may include those previously described, such as direct myocardial toxicity; negative inotropic, lusitropic, or chronotropic effects; exacerbating hypertension; and drug-drug interactions that limit the benefits of HF medications, although these have not been overtly associated with PTZ in the literature to date.4,8
ADHF can present similarly to other acute pulmonary conditions, including pneumonia.9,10 It is important to acknowledge the challenge this creates for diagnosticians to differentiate between these conditions rapidly and precisely. As a result, this patient population is likely at increased risk of IV antibiotic exposure. Other studies have identified worse outcomes in patients who receive potentially unwarranted IV antibiotics in patients with ADHF.9,10 The results of this study further emphasize the importance of careful considerate antibiotic selection and overall avoidance of unnecessary antibiotic exposure to limit potential adverse outcomes.
Limitations
There are various limitations to this study. Firstly, no women were included due to the predominantly male population within the Veterans Health Administration system. Secondly, this study was retrospective in design and was therefore limited to the completeness and accuracy of the available data collected. Additionally, this study evaluated any ADHF episode during the associated hospitalization as the primary endpoint. The time to diagnosis of ADHF in relation to PTZ initiation was not evaluated, which may have helped better elucidate this possible association. Furthermore, while a significant statistical difference was identified, the smaller sample size may have limited the ability to accurately identify differences in lower event rate outcomes.
Conclusions
This study identifies an association between PTZ use and significant cardiac-related adverse outcomes, including increased incidence of ADHF and readmission due to HF exacerbation. While more research is needed to define the exact mechanisms by which PTZ may precipitate acute decompensation in patients with HF, it is judicious to consider close monitoring or the avoidance of PTZ when appropriate antibiotic alternatives are available in patients with a known history of HF.
1. Zosyn. Package insert. Wyeth Pharmaceuticals; 2020.
2. Jensen JU, Hein L, Lundgren B, et al. Kidney failure related to broad-spectrum antibiotics in critically ill patients: secondary end point results from a 1200 patient randomised trial. BMJ Open. 2012;2(2):e000635. Published 2012 Mar 11. doi:10.1136/bmjopen-2011-000635
3. Kadomura S, Takekuma Y, Sato Y, et al. Higher incidence of acute kidney injury in patients treated with piperacillin/tazobactam than in patients treated with cefepime: a single-center retrospective cohort study. J Pharm Health Care Sci. 2019;5:13. Published 2019 Jun 12. doi:10.1186/s40780-019-0142-6
4. Page RL 2nd, O’Bryant CL, Cheng D, et al. Drugs that may cause or exacerbate heart failure: a scientific statement from the American Heart Association. Circulation. 2016;134(6):e32-e69. doi:10.1161/CIR.0000000000000426
5. Bozkurt B, Hershberger RE, Butler J, et al. 2021 ACC/AHA key data elements and definitions for heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical data standards. J Am Coll Cardiol. 2021;77(16):2053-2150.
6. Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254-e743. doi:10.1161/CIR.0000000000000950
7. Merrem. Package insert. Pfizer Labs; 2021.
8. Keller GA, Alvarez PA, Ponte ML, et al. Drug-induced QTc interval prolongation: a multicenter study to detect drugs and clinical factors involved in every day practice. Curr Drug Saf. 2016;11(1):86-98. doi:10.2174/1574886311207040262
9. Wu S, Alikhil M, Forsyth R, Allen B. Impact of potentially unwarranted intravenous antibiotics targeting pulmonary infections in acute decompensated heart failure. J Pharm Technol. 2021;37(6):298-303. doi:10.1177/87551225211038020
10. Frisbee J, Heidel RH, Rasnake MS. Adverse outcomes associated with potentially inappropriate antibiotic use in heart failure admissions. Open Forum Infect Dis. 2019;6(6):ofz220. doi:10.1093/ofid/ofz220
1. Zosyn. Package insert. Wyeth Pharmaceuticals; 2020.
2. Jensen JU, Hein L, Lundgren B, et al. Kidney failure related to broad-spectrum antibiotics in critically ill patients: secondary end point results from a 1200 patient randomised trial. BMJ Open. 2012;2(2):e000635. Published 2012 Mar 11. doi:10.1136/bmjopen-2011-000635
3. Kadomura S, Takekuma Y, Sato Y, et al. Higher incidence of acute kidney injury in patients treated with piperacillin/tazobactam than in patients treated with cefepime: a single-center retrospective cohort study. J Pharm Health Care Sci. 2019;5:13. Published 2019 Jun 12. doi:10.1186/s40780-019-0142-6
4. Page RL 2nd, O’Bryant CL, Cheng D, et al. Drugs that may cause or exacerbate heart failure: a scientific statement from the American Heart Association. Circulation. 2016;134(6):e32-e69. doi:10.1161/CIR.0000000000000426
5. Bozkurt B, Hershberger RE, Butler J, et al. 2021 ACC/AHA key data elements and definitions for heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical data standards. J Am Coll Cardiol. 2021;77(16):2053-2150.
6. Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254-e743. doi:10.1161/CIR.0000000000000950
7. Merrem. Package insert. Pfizer Labs; 2021.
8. Keller GA, Alvarez PA, Ponte ML, et al. Drug-induced QTc interval prolongation: a multicenter study to detect drugs and clinical factors involved in every day practice. Curr Drug Saf. 2016;11(1):86-98. doi:10.2174/1574886311207040262
9. Wu S, Alikhil M, Forsyth R, Allen B. Impact of potentially unwarranted intravenous antibiotics targeting pulmonary infections in acute decompensated heart failure. J Pharm Technol. 2021;37(6):298-303. doi:10.1177/87551225211038020
10. Frisbee J, Heidel RH, Rasnake MS. Adverse outcomes associated with potentially inappropriate antibiotic use in heart failure admissions. Open Forum Infect Dis. 2019;6(6):ofz220. doi:10.1093/ofid/ofz220
Unlikely Breakthrough of the Year: Chemo for Lung Cancer
This transcript has been edited for clarity.
I’ve been spending time recently reflecting on the biggest developments from last year. I have to say that the breakthrough of the year, based on the amount of data presented and the importance of the data, is chemotherapy. I never thought I would say that. Many folks have tried to relegate chemotherapy to the museum, but last year it came to the forefront.
Let’s start with neoadjuvant therapy. We now have multiple drug approvals for giving a checkpoint inhibitor and neoadjuvant therapy in what I would say is a new standard of care for patients with locally advanced lung cancers who are candidates for surgery. In all those trials, there was a clear improvement in progression-free survival by adding a checkpoint inhibitor to chemotherapy. The cornerstone of this regimen is chemotherapy.
What about adjuvant therapy? I think one of the most astounding pieces of data last year was in the adjuvant realm. In the trial comparing adjuvant osimertinib with placebo in patients with EGFR-mutant disease, patients who received chemotherapy in addition to osimertinib had a 7% improvement in 5-year survival. Patients who had placebo, who got chemotherapy vs didn’t, had a 9% improvement in 5-year survival. Those are huge numbers for that kind of metric, and it happened with chemotherapy.
What about targeted therapies? Again, I think people were astounded that, by adding chemotherapy to osimertinib compared with osimertinib alone, there was a 9-month improvement overall in progression-free survival. I think in the presentation of the data that has been made, the most remarkable piece of data is that, in patients with brain metastases, chemotherapy on top of osimertinib improved progression-free survival. Not only did it improve progression-free survival, but it did it with brain metastases, where people think it just doesn’t help at all.
What about other, newer agents with chemotherapy? Amivantamab, I would say, has hitched itself to chemotherapy. A trial in EGFR exon 20 compared chemo to amivantamab plus chemotherapy. There again, chemo is the common denominator. Amivantamab added approximately 5 months of improved progression-free survival. Again, chemo was used. In adjuvant, neoadjuvant, and targeted therapies, chemotherapy adds.
What about the second line? I think everybody was very disappointed when second-line sotorasib gave a very tiny amount of progression-free survival improvement over docetaxel. I think we all want more for our patients than we can deliver with docetaxel. The roughly 5-week improvement seen with sotorasib was one that raised a question about the place of sotorasib in this setting.
Clearly, we’ve all seen patients have an excellent result with sotorasib as an additional option for treating patients with long progression-free survival, high rates of response, and good tolerability even at the 960 mg dose. But in the randomized trial, it wasn’t better than docetaxel. Again, I think we were disappointed with tusamitamab ravtansine in that it could not beat docetaxel either. I think the idea here is that chemo still has a huge place and still remains the treatment that we have to beat.
We’re all very excited about the antibody-drug conjugates and I think everybody sees them as another advance. Many folks have said that they are just a more precise way of delivering chemotherapy, and when you look at the side effects, it supports that — they’re largely side effects of chemotherapy with these drugs across the board. Also, when you look at the patterns of resistance, the resistance really isn’t a resistance to the targeted therapy; it’s a resistance to chemotherapy more than anything else.
So we’re happy that the antibody-drug conjugates are available and we were disappointed with tusamitamab ravtansine because we thought that it could beat docetaxel. But in truth, it didn’t, and unfortunately, that pivotal trial led to the end of the entire development program for that agent, as stated in a press release.
The molecule or treatment of the year is chemotherapy — added to targeted therapies, used with immunotherapy, and now attached to antibodies as part of antibody-drug conjugates. I think it remains, more than any one treatment, a very effective treatment for patients and deserves to be used.
There are a lot of choices here. I think you have to be very careful to choose wisely, and you also have to be careful because chemotherapy has side effects. The nice thing is that many of those side effects can be ameliorated. We have to make sure that we use all the supportive medications we can.
Who would have thought that chemotherapy would be the treatment of the year in 2023 for lung cancers?
Dr. Kris is chief of the thoracic oncology service and the William and Joy Ruane Chair in Thoracic Oncology at Memorial Sloan Kettering Cancer Center in New York City. He disclosed ties with AstraZeneca, Roche/Genentech, Ariad Pharmaceuticals, Pfizer Inc, and PUMA.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I’ve been spending time recently reflecting on the biggest developments from last year. I have to say that the breakthrough of the year, based on the amount of data presented and the importance of the data, is chemotherapy. I never thought I would say that. Many folks have tried to relegate chemotherapy to the museum, but last year it came to the forefront.
Let’s start with neoadjuvant therapy. We now have multiple drug approvals for giving a checkpoint inhibitor and neoadjuvant therapy in what I would say is a new standard of care for patients with locally advanced lung cancers who are candidates for surgery. In all those trials, there was a clear improvement in progression-free survival by adding a checkpoint inhibitor to chemotherapy. The cornerstone of this regimen is chemotherapy.
What about adjuvant therapy? I think one of the most astounding pieces of data last year was in the adjuvant realm. In the trial comparing adjuvant osimertinib with placebo in patients with EGFR-mutant disease, patients who received chemotherapy in addition to osimertinib had a 7% improvement in 5-year survival. Patients who had placebo, who got chemotherapy vs didn’t, had a 9% improvement in 5-year survival. Those are huge numbers for that kind of metric, and it happened with chemotherapy.
What about targeted therapies? Again, I think people were astounded that, by adding chemotherapy to osimertinib compared with osimertinib alone, there was a 9-month improvement overall in progression-free survival. I think in the presentation of the data that has been made, the most remarkable piece of data is that, in patients with brain metastases, chemotherapy on top of osimertinib improved progression-free survival. Not only did it improve progression-free survival, but it did it with brain metastases, where people think it just doesn’t help at all.
What about other, newer agents with chemotherapy? Amivantamab, I would say, has hitched itself to chemotherapy. A trial in EGFR exon 20 compared chemo to amivantamab plus chemotherapy. There again, chemo is the common denominator. Amivantamab added approximately 5 months of improved progression-free survival. Again, chemo was used. In adjuvant, neoadjuvant, and targeted therapies, chemotherapy adds.
What about the second line? I think everybody was very disappointed when second-line sotorasib gave a very tiny amount of progression-free survival improvement over docetaxel. I think we all want more for our patients than we can deliver with docetaxel. The roughly 5-week improvement seen with sotorasib was one that raised a question about the place of sotorasib in this setting.
Clearly, we’ve all seen patients have an excellent result with sotorasib as an additional option for treating patients with long progression-free survival, high rates of response, and good tolerability even at the 960 mg dose. But in the randomized trial, it wasn’t better than docetaxel. Again, I think we were disappointed with tusamitamab ravtansine in that it could not beat docetaxel either. I think the idea here is that chemo still has a huge place and still remains the treatment that we have to beat.
We’re all very excited about the antibody-drug conjugates and I think everybody sees them as another advance. Many folks have said that they are just a more precise way of delivering chemotherapy, and when you look at the side effects, it supports that — they’re largely side effects of chemotherapy with these drugs across the board. Also, when you look at the patterns of resistance, the resistance really isn’t a resistance to the targeted therapy; it’s a resistance to chemotherapy more than anything else.
So we’re happy that the antibody-drug conjugates are available and we were disappointed with tusamitamab ravtansine because we thought that it could beat docetaxel. But in truth, it didn’t, and unfortunately, that pivotal trial led to the end of the entire development program for that agent, as stated in a press release.
The molecule or treatment of the year is chemotherapy — added to targeted therapies, used with immunotherapy, and now attached to antibodies as part of antibody-drug conjugates. I think it remains, more than any one treatment, a very effective treatment for patients and deserves to be used.
There are a lot of choices here. I think you have to be very careful to choose wisely, and you also have to be careful because chemotherapy has side effects. The nice thing is that many of those side effects can be ameliorated. We have to make sure that we use all the supportive medications we can.
Who would have thought that chemotherapy would be the treatment of the year in 2023 for lung cancers?
Dr. Kris is chief of the thoracic oncology service and the William and Joy Ruane Chair in Thoracic Oncology at Memorial Sloan Kettering Cancer Center in New York City. He disclosed ties with AstraZeneca, Roche/Genentech, Ariad Pharmaceuticals, Pfizer Inc, and PUMA.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I’ve been spending time recently reflecting on the biggest developments from last year. I have to say that the breakthrough of the year, based on the amount of data presented and the importance of the data, is chemotherapy. I never thought I would say that. Many folks have tried to relegate chemotherapy to the museum, but last year it came to the forefront.
Let’s start with neoadjuvant therapy. We now have multiple drug approvals for giving a checkpoint inhibitor and neoadjuvant therapy in what I would say is a new standard of care for patients with locally advanced lung cancers who are candidates for surgery. In all those trials, there was a clear improvement in progression-free survival by adding a checkpoint inhibitor to chemotherapy. The cornerstone of this regimen is chemotherapy.
What about adjuvant therapy? I think one of the most astounding pieces of data last year was in the adjuvant realm. In the trial comparing adjuvant osimertinib with placebo in patients with EGFR-mutant disease, patients who received chemotherapy in addition to osimertinib had a 7% improvement in 5-year survival. Patients who had placebo, who got chemotherapy vs didn’t, had a 9% improvement in 5-year survival. Those are huge numbers for that kind of metric, and it happened with chemotherapy.
What about targeted therapies? Again, I think people were astounded that, by adding chemotherapy to osimertinib compared with osimertinib alone, there was a 9-month improvement overall in progression-free survival. I think in the presentation of the data that has been made, the most remarkable piece of data is that, in patients with brain metastases, chemotherapy on top of osimertinib improved progression-free survival. Not only did it improve progression-free survival, but it did it with brain metastases, where people think it just doesn’t help at all.
What about other, newer agents with chemotherapy? Amivantamab, I would say, has hitched itself to chemotherapy. A trial in EGFR exon 20 compared chemo to amivantamab plus chemotherapy. There again, chemo is the common denominator. Amivantamab added approximately 5 months of improved progression-free survival. Again, chemo was used. In adjuvant, neoadjuvant, and targeted therapies, chemotherapy adds.
What about the second line? I think everybody was very disappointed when second-line sotorasib gave a very tiny amount of progression-free survival improvement over docetaxel. I think we all want more for our patients than we can deliver with docetaxel. The roughly 5-week improvement seen with sotorasib was one that raised a question about the place of sotorasib in this setting.
Clearly, we’ve all seen patients have an excellent result with sotorasib as an additional option for treating patients with long progression-free survival, high rates of response, and good tolerability even at the 960 mg dose. But in the randomized trial, it wasn’t better than docetaxel. Again, I think we were disappointed with tusamitamab ravtansine in that it could not beat docetaxel either. I think the idea here is that chemo still has a huge place and still remains the treatment that we have to beat.
We’re all very excited about the antibody-drug conjugates and I think everybody sees them as another advance. Many folks have said that they are just a more precise way of delivering chemotherapy, and when you look at the side effects, it supports that — they’re largely side effects of chemotherapy with these drugs across the board. Also, when you look at the patterns of resistance, the resistance really isn’t a resistance to the targeted therapy; it’s a resistance to chemotherapy more than anything else.
So we’re happy that the antibody-drug conjugates are available and we were disappointed with tusamitamab ravtansine because we thought that it could beat docetaxel. But in truth, it didn’t, and unfortunately, that pivotal trial led to the end of the entire development program for that agent, as stated in a press release.
The molecule or treatment of the year is chemotherapy — added to targeted therapies, used with immunotherapy, and now attached to antibodies as part of antibody-drug conjugates. I think it remains, more than any one treatment, a very effective treatment for patients and deserves to be used.
There are a lot of choices here. I think you have to be very careful to choose wisely, and you also have to be careful because chemotherapy has side effects. The nice thing is that many of those side effects can be ameliorated. We have to make sure that we use all the supportive medications we can.
Who would have thought that chemotherapy would be the treatment of the year in 2023 for lung cancers?
Dr. Kris is chief of the thoracic oncology service and the William and Joy Ruane Chair in Thoracic Oncology at Memorial Sloan Kettering Cancer Center in New York City. He disclosed ties with AstraZeneca, Roche/Genentech, Ariad Pharmaceuticals, Pfizer Inc, and PUMA.
A version of this article appeared on Medscape.com.
Treating Acne Scars Can Improve Aesthetics, Quality of Life
ORLANDO, FLORIDA — For some people, acne carries a one-two punch. First, they experience acne that is significant enough to decrease their quality of life, followed by scarring that can last a lifetime. For those patients, dermatologists have several options: Subcision to lift the depression of the scar, laser treatment to lower the height of scar tissue, and injections to fill scars.
“In my practice, I find that ,” Robyn Siperstein, MD, said at the annual ODAC Dermatology, Aesthetic & Surgical Conference.
Dr. Siperstein starts by identifying the type of acne scar — rolling scars, boxcar scars, or ice pick scars. Rolling scars tend to be shallower with no sharp edges; boxcar scars are deeper, more defined round or oval depressions; and ice pick scars, as the name suggests, look like someone stuck tiny ice picks into the skin, leaving a sunken or pitted appearance.
“It’s really important to categorize so that we know which ones are going to be effectively treated with different modalities and which ones aren’t, so that we can give our patients realistic expectations,” said Dr. Siperstein, a cosmetic dermatologist in private practice in Boca Raton, Florida, and a clinical affiliate associate professor of dermatology at Florida Atlantic University, Boca Raton.
“There’s not going to be one treatment that’s right for everything,” she said. Different approaches may be required even for the same patient because some people present with all three types of acne scars, she added.
Combining Treatments
When it comes to injecting dermal fillers into acne scars to lift the depressed areas, the US Food and Drug Administration approved a filler with polymethyl methacrylate filler and bovine collagen (Bellafill) for this indication (moderate to severe, atrophic, distensible facial acne scars on the cheek in patients over age 21) in 2015. “And off-label, I use hyaluronic acid in my practice,” Dr. Siperstein said. Each filler “probably works a little bit better or differently on different types of scars.”
For rolling scars, she recommends hyaluronic acid (HA) dermal filler for everyone. “Of course, this is my opinion.” She was also a lead investigator in a randomized, placebo-controlled split-face study comparing HA filler with saline for correcting atrophic facial scars in 15 patients. The HA filler emerged superior, although there were some improvements with saline.
In her clinical experience, patients are happy with the results and ask, “Why didn’t the last four doctors do this?”
Boxcar scars are more challenging to fill with HA. In some cases, Dr. Siperstein is able to raise the depressed portion of the scar, but some of the vertical edges remain. In this scenario, she might combine treatments. Laser resurfacing, for example, might help convert boxcar scars into rolling scars, which then can be filled more successfully.
“Ice pick scars are tough,” Dr. Siperstein said. A punch removal technique can work in some cases, or she might try the “cross technique.” This involves placing acetic acid inside the scar using a Q-tip. “You have to be really careful,” she added, “because if you get it around the edges, it’s actually going to make the scar bigger.”
Choosing the Right Candidates
Selecting the right candidate for HA treatment of acne scars is essential. Dr. Siperstein shared the example of a lifeguard who had prominent acne scarring down the center of his chest. “He was embarrassed to go to the beach and take off his shirt. He said he felt like he had bullet holes in his chest.”
One month following treatment, “he had a really nice improvement, and now he feels really comfortable,” she said.
Some dermatologists might be reluctant to consider HA fillers for acne scarring because there is a misconception that HA is short-acting, lasting 6 months to 1 year before the effect wears off. That impression can persist from company-sponsored studies that limit follow-up to 6 months or 1 year “to get their drug to market,” she noted.
Also adding to this impression is that HA fillers in wrinkles may not last as long. Dr. Siperstein explained that wrinkles on the face are dynamic and constantly moving. In contrast, acne scars experience less movement, which helps the HA last longer. There is MRI evidence that shows HA fillers last over 2 years in the face, she added.
One tip to predict how well an acne scar might respond to filler injections is to squeeze it and look for the “dimple sign.” If the floor of the scar lifts up when squeezed, “we know that they’ll be a good candidate for hyaluronic acid filler.” Another tip is to inject HA in a retrograde technique high up in the skin. Inject tiny amounts — microdroplets — of the HA filler high on the dermis, she advised.
Deeper injections run the risk of raising the entire scar instead of filling it, she added.
Like many dermatologic procedures, before and after photos are essential to demonstrate improvements, Dr. Siperstein pointed out. Patients are often skeptical. “This happens a lot with acne scar patients. They’ve been to a million places that have promised results, they have not gotten them, and they are frustrated.”
Acne scars can result from picking, inflammation, or treatment. “This is what we see all day in clinic,” Dr. Siperstein said. “Somebody who had to undergo Accutane treatment but unfortunately is left with holes. This is a huge psychological burden on our patients,” she said, describing a younger patient who had scarring, which “led to depression — it was ruining his life.”
“His mom was willing to do whatever it took. And I said, You know what, I think filler will be enough,” Dr. Siperstein said. She counseled them that treatment would not make the scars disappear completely. But patients used to 10% improvements are very happy when their acne scars look 80% or 90% better, she added.
Dr. Siperstein received grant or research support and is a member of the speakers bureau for Allergan and Galderma. She is also a consultant/advisory board member for Allergan.
A version of this article appeared on Medscape.com.
ORLANDO, FLORIDA — For some people, acne carries a one-two punch. First, they experience acne that is significant enough to decrease their quality of life, followed by scarring that can last a lifetime. For those patients, dermatologists have several options: Subcision to lift the depression of the scar, laser treatment to lower the height of scar tissue, and injections to fill scars.
“In my practice, I find that ,” Robyn Siperstein, MD, said at the annual ODAC Dermatology, Aesthetic & Surgical Conference.
Dr. Siperstein starts by identifying the type of acne scar — rolling scars, boxcar scars, or ice pick scars. Rolling scars tend to be shallower with no sharp edges; boxcar scars are deeper, more defined round or oval depressions; and ice pick scars, as the name suggests, look like someone stuck tiny ice picks into the skin, leaving a sunken or pitted appearance.
“It’s really important to categorize so that we know which ones are going to be effectively treated with different modalities and which ones aren’t, so that we can give our patients realistic expectations,” said Dr. Siperstein, a cosmetic dermatologist in private practice in Boca Raton, Florida, and a clinical affiliate associate professor of dermatology at Florida Atlantic University, Boca Raton.
“There’s not going to be one treatment that’s right for everything,” she said. Different approaches may be required even for the same patient because some people present with all three types of acne scars, she added.
Combining Treatments
When it comes to injecting dermal fillers into acne scars to lift the depressed areas, the US Food and Drug Administration approved a filler with polymethyl methacrylate filler and bovine collagen (Bellafill) for this indication (moderate to severe, atrophic, distensible facial acne scars on the cheek in patients over age 21) in 2015. “And off-label, I use hyaluronic acid in my practice,” Dr. Siperstein said. Each filler “probably works a little bit better or differently on different types of scars.”
For rolling scars, she recommends hyaluronic acid (HA) dermal filler for everyone. “Of course, this is my opinion.” She was also a lead investigator in a randomized, placebo-controlled split-face study comparing HA filler with saline for correcting atrophic facial scars in 15 patients. The HA filler emerged superior, although there were some improvements with saline.
In her clinical experience, patients are happy with the results and ask, “Why didn’t the last four doctors do this?”
Boxcar scars are more challenging to fill with HA. In some cases, Dr. Siperstein is able to raise the depressed portion of the scar, but some of the vertical edges remain. In this scenario, she might combine treatments. Laser resurfacing, for example, might help convert boxcar scars into rolling scars, which then can be filled more successfully.
“Ice pick scars are tough,” Dr. Siperstein said. A punch removal technique can work in some cases, or she might try the “cross technique.” This involves placing acetic acid inside the scar using a Q-tip. “You have to be really careful,” she added, “because if you get it around the edges, it’s actually going to make the scar bigger.”
Choosing the Right Candidates
Selecting the right candidate for HA treatment of acne scars is essential. Dr. Siperstein shared the example of a lifeguard who had prominent acne scarring down the center of his chest. “He was embarrassed to go to the beach and take off his shirt. He said he felt like he had bullet holes in his chest.”
One month following treatment, “he had a really nice improvement, and now he feels really comfortable,” she said.
Some dermatologists might be reluctant to consider HA fillers for acne scarring because there is a misconception that HA is short-acting, lasting 6 months to 1 year before the effect wears off. That impression can persist from company-sponsored studies that limit follow-up to 6 months or 1 year “to get their drug to market,” she noted.
Also adding to this impression is that HA fillers in wrinkles may not last as long. Dr. Siperstein explained that wrinkles on the face are dynamic and constantly moving. In contrast, acne scars experience less movement, which helps the HA last longer. There is MRI evidence that shows HA fillers last over 2 years in the face, she added.
One tip to predict how well an acne scar might respond to filler injections is to squeeze it and look for the “dimple sign.” If the floor of the scar lifts up when squeezed, “we know that they’ll be a good candidate for hyaluronic acid filler.” Another tip is to inject HA in a retrograde technique high up in the skin. Inject tiny amounts — microdroplets — of the HA filler high on the dermis, she advised.
Deeper injections run the risk of raising the entire scar instead of filling it, she added.
Like many dermatologic procedures, before and after photos are essential to demonstrate improvements, Dr. Siperstein pointed out. Patients are often skeptical. “This happens a lot with acne scar patients. They’ve been to a million places that have promised results, they have not gotten them, and they are frustrated.”
Acne scars can result from picking, inflammation, or treatment. “This is what we see all day in clinic,” Dr. Siperstein said. “Somebody who had to undergo Accutane treatment but unfortunately is left with holes. This is a huge psychological burden on our patients,” she said, describing a younger patient who had scarring, which “led to depression — it was ruining his life.”
“His mom was willing to do whatever it took. And I said, You know what, I think filler will be enough,” Dr. Siperstein said. She counseled them that treatment would not make the scars disappear completely. But patients used to 10% improvements are very happy when their acne scars look 80% or 90% better, she added.
Dr. Siperstein received grant or research support and is a member of the speakers bureau for Allergan and Galderma. She is also a consultant/advisory board member for Allergan.
A version of this article appeared on Medscape.com.
ORLANDO, FLORIDA — For some people, acne carries a one-two punch. First, they experience acne that is significant enough to decrease their quality of life, followed by scarring that can last a lifetime. For those patients, dermatologists have several options: Subcision to lift the depression of the scar, laser treatment to lower the height of scar tissue, and injections to fill scars.
“In my practice, I find that ,” Robyn Siperstein, MD, said at the annual ODAC Dermatology, Aesthetic & Surgical Conference.
Dr. Siperstein starts by identifying the type of acne scar — rolling scars, boxcar scars, or ice pick scars. Rolling scars tend to be shallower with no sharp edges; boxcar scars are deeper, more defined round or oval depressions; and ice pick scars, as the name suggests, look like someone stuck tiny ice picks into the skin, leaving a sunken or pitted appearance.
“It’s really important to categorize so that we know which ones are going to be effectively treated with different modalities and which ones aren’t, so that we can give our patients realistic expectations,” said Dr. Siperstein, a cosmetic dermatologist in private practice in Boca Raton, Florida, and a clinical affiliate associate professor of dermatology at Florida Atlantic University, Boca Raton.
“There’s not going to be one treatment that’s right for everything,” she said. Different approaches may be required even for the same patient because some people present with all three types of acne scars, she added.
Combining Treatments
When it comes to injecting dermal fillers into acne scars to lift the depressed areas, the US Food and Drug Administration approved a filler with polymethyl methacrylate filler and bovine collagen (Bellafill) for this indication (moderate to severe, atrophic, distensible facial acne scars on the cheek in patients over age 21) in 2015. “And off-label, I use hyaluronic acid in my practice,” Dr. Siperstein said. Each filler “probably works a little bit better or differently on different types of scars.”
For rolling scars, she recommends hyaluronic acid (HA) dermal filler for everyone. “Of course, this is my opinion.” She was also a lead investigator in a randomized, placebo-controlled split-face study comparing HA filler with saline for correcting atrophic facial scars in 15 patients. The HA filler emerged superior, although there were some improvements with saline.
In her clinical experience, patients are happy with the results and ask, “Why didn’t the last four doctors do this?”
Boxcar scars are more challenging to fill with HA. In some cases, Dr. Siperstein is able to raise the depressed portion of the scar, but some of the vertical edges remain. In this scenario, she might combine treatments. Laser resurfacing, for example, might help convert boxcar scars into rolling scars, which then can be filled more successfully.
“Ice pick scars are tough,” Dr. Siperstein said. A punch removal technique can work in some cases, or she might try the “cross technique.” This involves placing acetic acid inside the scar using a Q-tip. “You have to be really careful,” she added, “because if you get it around the edges, it’s actually going to make the scar bigger.”
Choosing the Right Candidates
Selecting the right candidate for HA treatment of acne scars is essential. Dr. Siperstein shared the example of a lifeguard who had prominent acne scarring down the center of his chest. “He was embarrassed to go to the beach and take off his shirt. He said he felt like he had bullet holes in his chest.”
One month following treatment, “he had a really nice improvement, and now he feels really comfortable,” she said.
Some dermatologists might be reluctant to consider HA fillers for acne scarring because there is a misconception that HA is short-acting, lasting 6 months to 1 year before the effect wears off. That impression can persist from company-sponsored studies that limit follow-up to 6 months or 1 year “to get their drug to market,” she noted.
Also adding to this impression is that HA fillers in wrinkles may not last as long. Dr. Siperstein explained that wrinkles on the face are dynamic and constantly moving. In contrast, acne scars experience less movement, which helps the HA last longer. There is MRI evidence that shows HA fillers last over 2 years in the face, she added.
One tip to predict how well an acne scar might respond to filler injections is to squeeze it and look for the “dimple sign.” If the floor of the scar lifts up when squeezed, “we know that they’ll be a good candidate for hyaluronic acid filler.” Another tip is to inject HA in a retrograde technique high up in the skin. Inject tiny amounts — microdroplets — of the HA filler high on the dermis, she advised.
Deeper injections run the risk of raising the entire scar instead of filling it, she added.
Like many dermatologic procedures, before and after photos are essential to demonstrate improvements, Dr. Siperstein pointed out. Patients are often skeptical. “This happens a lot with acne scar patients. They’ve been to a million places that have promised results, they have not gotten them, and they are frustrated.”
Acne scars can result from picking, inflammation, or treatment. “This is what we see all day in clinic,” Dr. Siperstein said. “Somebody who had to undergo Accutane treatment but unfortunately is left with holes. This is a huge psychological burden on our patients,” she said, describing a younger patient who had scarring, which “led to depression — it was ruining his life.”
“His mom was willing to do whatever it took. And I said, You know what, I think filler will be enough,” Dr. Siperstein said. She counseled them that treatment would not make the scars disappear completely. But patients used to 10% improvements are very happy when their acne scars look 80% or 90% better, she added.
Dr. Siperstein received grant or research support and is a member of the speakers bureau for Allergan and Galderma. She is also a consultant/advisory board member for Allergan.
A version of this article appeared on Medscape.com.
FROM ODAC 2024
In Search of the Nation’s Primary Care Providers
Clinicians at Valley-Wide Health Systems never know who will appear at their clinic in San Luis, a town of about 600 people in southern Colorado.
“If someone’s in labor, they’ll show up. If someone has a laceration, they’ll show up,” said nurse practitioner Emelin Martinez, the chief medical officer for the healthcare system serving 13 rural Colorado counties.
But she struggled to find a full-time medical provider for that clinic, the only one in Costilla County. Born and raised in the area, Martinez filled some of the gap by driving about 45 minutes from Alamosa, the nearest city, once a week for months. A physician assistant from another town chipped in, too.
As one of the nation’s more than 1000 federally designated primary care shortage areas, Costilla County has many carrots to dangle in front of medical providers willing to practice there, including federal student loan repayments, bonus Medicare payments, and expedited visas for foreign clinicians. Still, Ms. Martinez said, its latest opening remained unfilled for more than a year. Not a single physician applied.
Policymakers have long tried to lure more primary care providers to the areas of the nation that have fewer than one physician for every 3500 residents. Recent examples include the Biden administration boosting funding in 2022 to address shortages and Sen. Bernie Sanders (I-Vt.) pushing sweeping primary care legislation in 2023.
But researchers steeped in the issue have a persistent frustration: It’s hard to know if any policy is working given that the data the federal government collects on primary care shortage areas has been flawed for a long time. One of the biggest gaps is that the system counts only physicians, not the myriad other healthcare professionals who now provide much of our nation›s primary care.
Additionally, a Health Affairs study shows the federal designations, which help allocate an estimated $1 billion in annual funding through at least 20 federal programs aimed at boosting primary care capacity, haven›t helped much.
In fact, Costilla County is among more than 180 federally designated areas that have remained stuck on the primary care shortage list for at least 40 years, according to a KFF Health News analysis. That›s even as the overall number of licensed US physicians more than doubled from 1990 to 2022 to over 1 million, according to the Federation of State Medical Boards, outpacing overall population growth.
No one disputes that much of the nation is starved for primary care clinicians, with patients having to wait weeks to get appointments or travel long distances for basic preventive care. Many doctors decide against primary care career paths, let alone practicing in isolated communities, because those jobs entail heavy workloads and earn less money and respect than specialists. But how does the nation solve the problem without knowing exactly where it is? And what tools must be used? Does a physician need to be the one providing the care?
Whitney Zahnd, president of the board of the Iowa Rural Health Association, said the fact that some rural areas have had such federal shortage designations for decades doesn’t prove they are ineffective. “Had the program not been there, would it have been even worse?” she said.
Federal funding supports 18,000 primary care doctors, nurse practitioners, and physician assistants to provide care to more than 18 million patients in the highest-need urban and rural communities across the country, said David Bowman, a spokesperson for the Health Resources and Services Administration, which manages the shortage designations. He said more than 80% of clinicians who get such scholarships or loan repayments continue to practice in shortage areas beyond their obligation of several years.
But that doesn’t mean they stick around forever.
Justin Markowski, a Yale School of Public Health doctoral student, coauthored the Health Affairs study that found the federal shortage designation makes no difference in upping physician density long-term. He is skeptical of policy ideas that promise big primary care fixes. That includes the Biden administration’s investment in more scholarships and loan repayments through the National Health Service Corps.
“You’re just throwing more money at a set of programs that don’t really seem to work,” he said. “We’ll see in a few years, but I’ll be shocked if it actually moved any physicians or any other advanced practice providers.”
One possible explanation for the persistence of shortage areas is that such incentives are too small or too fleeting.
But another issue is how shortages are measured. The government considers geographic shortage areas, now numbering just over 1000, but also population groups such as migrant farmworkers and individual facilities such as prisons that lack enough providers. Yet it’s up to state offices to identify populations and locations that might qualify as shortage areas and submit them to HRSA, which then scores the extent of any shortages. The funding and staffing for those state offices vary, creating an uneven foundation from which to map actual shortages.
“Some states became very adept at the equivalent of gerrymandering, where they were piecing together census blocks or census tracts in odd shapes in order to maximize the areas that are eligible,” said Stephen Petterson, a senior scholar at the Robert Graham Center, a policy think tank in Washington, DC, that focuses on primary care.
The federal Government Accountability Office has highlighted such issues since at least 1995, when it released a report identifying widespread data problems with the shortage area system and concluding it had “little assurance that federal funds are used where most needed.” The report noted one of the persistent shortcomings is that the system counts only physicians, not other key primary care providers.
Since 1998, federal officials have made three attempts to update the 1970s-era rules that define what counts as a shortage area. The authors of the Affordable Care Act tried most recently, tasking a committee of experts to decide on an update.
Among other things, the committee concluded in its 2011 report that nurse practitioners, physician assistants, and certified nurse midwives should be counted as primary care providers. But the recommendations fell short by just a handful of votes.
“We failed and the committee as a whole failed and HRSA failed by not moving the process forward,” said Petterson, who presented to the committee on how to comprehensively measure primary care needs.
Steve Holloway, who directs the Colorado health department’s Primary Care Office, served on the committee. Without action at the federal level, he then led a team to create Colorado’s own health professional shortage area designations that factor in nurse practitioners and physician assistants, not just doctors.
He said it’s taken about 6 years to create a tool and map of Colorado to answer a deceptively simple question: “How many actual flesh-and-blood, live clinicians are seeing patients?”
Ed Salsberg, who was the lead federal government representative on that committee and who headed HRSA’s National Center for Health Workforce Analysis, said the rest of the nation needs more precise data, too.
“It’s so important for the nation to target its resources to the highest-need communities,” he said. “It’s time again to try one more time to develop an improved methodology.”
In the past few years, more readily available data from insurance claims has allowed researchers to distinguish the medical providers who are practicing primary care from those who have specialized or retired.
Candice Chen, an associate professor of health policy and management at George Washington University’s Fitzhugh Mullan Institute for Health Workforce Equity, used claims data that reflects one large slice of the American population — about 66 million Medicaid beneficiaries — to map the primary care workforce.
Meanwhile, Monica O’Reilly-Jacob, a nurse-scientist who recently moved from Boston College to Columbia University’s School of Nursing, studied Medicare claims to conclude that fewer than 70% of physicians typically considered primary care providers were actually providing primary care. The rest, she said, often find more lucrative positions, such as subspecializing or working in hospitals. By contrast, nurse practitioners are likely undercounted. Her study found that close to half are providing primary care.
But such publicly available data leaves out much of the country, given that fewer than 40% of Americans are insured through Medicaid or Medicare.
“There’s no government organization that’s tracking: Who trained in what, where, and where are they now, and what are they practicing,” said Alison Huffstetler, medical director of the Robert Graham Center. “And if we don’t know who is doing what kind of care — and where — then there is no way for us to equitably manage the patient-to-clinician ratio across every state.”
In Costilla County, Ms. Martinez finally found someone to provide primary care: An experienced physician assistant who moved from Texas in December.
The physician assistant’s presence should bump the county out of its dire shortage, according to Colorado’s measure. But since he isn’t a physician, he’ll remain invisible in the national data and Costilla County will likely remain on the books as a federal shortage area.
Data reporter Hannah Recht, data editor Holly K. Hacker, and rural editor/correspondent Tony Leys contributed to this report.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF — an independent source of health policy research, polling, and journalism.
Clinicians at Valley-Wide Health Systems never know who will appear at their clinic in San Luis, a town of about 600 people in southern Colorado.
“If someone’s in labor, they’ll show up. If someone has a laceration, they’ll show up,” said nurse practitioner Emelin Martinez, the chief medical officer for the healthcare system serving 13 rural Colorado counties.
But she struggled to find a full-time medical provider for that clinic, the only one in Costilla County. Born and raised in the area, Martinez filled some of the gap by driving about 45 minutes from Alamosa, the nearest city, once a week for months. A physician assistant from another town chipped in, too.
As one of the nation’s more than 1000 federally designated primary care shortage areas, Costilla County has many carrots to dangle in front of medical providers willing to practice there, including federal student loan repayments, bonus Medicare payments, and expedited visas for foreign clinicians. Still, Ms. Martinez said, its latest opening remained unfilled for more than a year. Not a single physician applied.
Policymakers have long tried to lure more primary care providers to the areas of the nation that have fewer than one physician for every 3500 residents. Recent examples include the Biden administration boosting funding in 2022 to address shortages and Sen. Bernie Sanders (I-Vt.) pushing sweeping primary care legislation in 2023.
But researchers steeped in the issue have a persistent frustration: It’s hard to know if any policy is working given that the data the federal government collects on primary care shortage areas has been flawed for a long time. One of the biggest gaps is that the system counts only physicians, not the myriad other healthcare professionals who now provide much of our nation›s primary care.
Additionally, a Health Affairs study shows the federal designations, which help allocate an estimated $1 billion in annual funding through at least 20 federal programs aimed at boosting primary care capacity, haven›t helped much.
In fact, Costilla County is among more than 180 federally designated areas that have remained stuck on the primary care shortage list for at least 40 years, according to a KFF Health News analysis. That›s even as the overall number of licensed US physicians more than doubled from 1990 to 2022 to over 1 million, according to the Federation of State Medical Boards, outpacing overall population growth.
No one disputes that much of the nation is starved for primary care clinicians, with patients having to wait weeks to get appointments or travel long distances for basic preventive care. Many doctors decide against primary care career paths, let alone practicing in isolated communities, because those jobs entail heavy workloads and earn less money and respect than specialists. But how does the nation solve the problem without knowing exactly where it is? And what tools must be used? Does a physician need to be the one providing the care?
Whitney Zahnd, president of the board of the Iowa Rural Health Association, said the fact that some rural areas have had such federal shortage designations for decades doesn’t prove they are ineffective. “Had the program not been there, would it have been even worse?” she said.
Federal funding supports 18,000 primary care doctors, nurse practitioners, and physician assistants to provide care to more than 18 million patients in the highest-need urban and rural communities across the country, said David Bowman, a spokesperson for the Health Resources and Services Administration, which manages the shortage designations. He said more than 80% of clinicians who get such scholarships or loan repayments continue to practice in shortage areas beyond their obligation of several years.
But that doesn’t mean they stick around forever.
Justin Markowski, a Yale School of Public Health doctoral student, coauthored the Health Affairs study that found the federal shortage designation makes no difference in upping physician density long-term. He is skeptical of policy ideas that promise big primary care fixes. That includes the Biden administration’s investment in more scholarships and loan repayments through the National Health Service Corps.
“You’re just throwing more money at a set of programs that don’t really seem to work,” he said. “We’ll see in a few years, but I’ll be shocked if it actually moved any physicians or any other advanced practice providers.”
One possible explanation for the persistence of shortage areas is that such incentives are too small or too fleeting.
But another issue is how shortages are measured. The government considers geographic shortage areas, now numbering just over 1000, but also population groups such as migrant farmworkers and individual facilities such as prisons that lack enough providers. Yet it’s up to state offices to identify populations and locations that might qualify as shortage areas and submit them to HRSA, which then scores the extent of any shortages. The funding and staffing for those state offices vary, creating an uneven foundation from which to map actual shortages.
“Some states became very adept at the equivalent of gerrymandering, where they were piecing together census blocks or census tracts in odd shapes in order to maximize the areas that are eligible,” said Stephen Petterson, a senior scholar at the Robert Graham Center, a policy think tank in Washington, DC, that focuses on primary care.
The federal Government Accountability Office has highlighted such issues since at least 1995, when it released a report identifying widespread data problems with the shortage area system and concluding it had “little assurance that federal funds are used where most needed.” The report noted one of the persistent shortcomings is that the system counts only physicians, not other key primary care providers.
Since 1998, federal officials have made three attempts to update the 1970s-era rules that define what counts as a shortage area. The authors of the Affordable Care Act tried most recently, tasking a committee of experts to decide on an update.
Among other things, the committee concluded in its 2011 report that nurse practitioners, physician assistants, and certified nurse midwives should be counted as primary care providers. But the recommendations fell short by just a handful of votes.
“We failed and the committee as a whole failed and HRSA failed by not moving the process forward,” said Petterson, who presented to the committee on how to comprehensively measure primary care needs.
Steve Holloway, who directs the Colorado health department’s Primary Care Office, served on the committee. Without action at the federal level, he then led a team to create Colorado’s own health professional shortage area designations that factor in nurse practitioners and physician assistants, not just doctors.
He said it’s taken about 6 years to create a tool and map of Colorado to answer a deceptively simple question: “How many actual flesh-and-blood, live clinicians are seeing patients?”
Ed Salsberg, who was the lead federal government representative on that committee and who headed HRSA’s National Center for Health Workforce Analysis, said the rest of the nation needs more precise data, too.
“It’s so important for the nation to target its resources to the highest-need communities,” he said. “It’s time again to try one more time to develop an improved methodology.”
In the past few years, more readily available data from insurance claims has allowed researchers to distinguish the medical providers who are practicing primary care from those who have specialized or retired.
Candice Chen, an associate professor of health policy and management at George Washington University’s Fitzhugh Mullan Institute for Health Workforce Equity, used claims data that reflects one large slice of the American population — about 66 million Medicaid beneficiaries — to map the primary care workforce.
Meanwhile, Monica O’Reilly-Jacob, a nurse-scientist who recently moved from Boston College to Columbia University’s School of Nursing, studied Medicare claims to conclude that fewer than 70% of physicians typically considered primary care providers were actually providing primary care. The rest, she said, often find more lucrative positions, such as subspecializing or working in hospitals. By contrast, nurse practitioners are likely undercounted. Her study found that close to half are providing primary care.
But such publicly available data leaves out much of the country, given that fewer than 40% of Americans are insured through Medicaid or Medicare.
“There’s no government organization that’s tracking: Who trained in what, where, and where are they now, and what are they practicing,” said Alison Huffstetler, medical director of the Robert Graham Center. “And if we don’t know who is doing what kind of care — and where — then there is no way for us to equitably manage the patient-to-clinician ratio across every state.”
In Costilla County, Ms. Martinez finally found someone to provide primary care: An experienced physician assistant who moved from Texas in December.
The physician assistant’s presence should bump the county out of its dire shortage, according to Colorado’s measure. But since he isn’t a physician, he’ll remain invisible in the national data and Costilla County will likely remain on the books as a federal shortage area.
Data reporter Hannah Recht, data editor Holly K. Hacker, and rural editor/correspondent Tony Leys contributed to this report.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF — an independent source of health policy research, polling, and journalism.
Clinicians at Valley-Wide Health Systems never know who will appear at their clinic in San Luis, a town of about 600 people in southern Colorado.
“If someone’s in labor, they’ll show up. If someone has a laceration, they’ll show up,” said nurse practitioner Emelin Martinez, the chief medical officer for the healthcare system serving 13 rural Colorado counties.
But she struggled to find a full-time medical provider for that clinic, the only one in Costilla County. Born and raised in the area, Martinez filled some of the gap by driving about 45 minutes from Alamosa, the nearest city, once a week for months. A physician assistant from another town chipped in, too.
As one of the nation’s more than 1000 federally designated primary care shortage areas, Costilla County has many carrots to dangle in front of medical providers willing to practice there, including federal student loan repayments, bonus Medicare payments, and expedited visas for foreign clinicians. Still, Ms. Martinez said, its latest opening remained unfilled for more than a year. Not a single physician applied.
Policymakers have long tried to lure more primary care providers to the areas of the nation that have fewer than one physician for every 3500 residents. Recent examples include the Biden administration boosting funding in 2022 to address shortages and Sen. Bernie Sanders (I-Vt.) pushing sweeping primary care legislation in 2023.
But researchers steeped in the issue have a persistent frustration: It’s hard to know if any policy is working given that the data the federal government collects on primary care shortage areas has been flawed for a long time. One of the biggest gaps is that the system counts only physicians, not the myriad other healthcare professionals who now provide much of our nation›s primary care.
Additionally, a Health Affairs study shows the federal designations, which help allocate an estimated $1 billion in annual funding through at least 20 federal programs aimed at boosting primary care capacity, haven›t helped much.
In fact, Costilla County is among more than 180 federally designated areas that have remained stuck on the primary care shortage list for at least 40 years, according to a KFF Health News analysis. That›s even as the overall number of licensed US physicians more than doubled from 1990 to 2022 to over 1 million, according to the Federation of State Medical Boards, outpacing overall population growth.
No one disputes that much of the nation is starved for primary care clinicians, with patients having to wait weeks to get appointments or travel long distances for basic preventive care. Many doctors decide against primary care career paths, let alone practicing in isolated communities, because those jobs entail heavy workloads and earn less money and respect than specialists. But how does the nation solve the problem without knowing exactly where it is? And what tools must be used? Does a physician need to be the one providing the care?
Whitney Zahnd, president of the board of the Iowa Rural Health Association, said the fact that some rural areas have had such federal shortage designations for decades doesn’t prove they are ineffective. “Had the program not been there, would it have been even worse?” she said.
Federal funding supports 18,000 primary care doctors, nurse practitioners, and physician assistants to provide care to more than 18 million patients in the highest-need urban and rural communities across the country, said David Bowman, a spokesperson for the Health Resources and Services Administration, which manages the shortage designations. He said more than 80% of clinicians who get such scholarships or loan repayments continue to practice in shortage areas beyond their obligation of several years.
But that doesn’t mean they stick around forever.
Justin Markowski, a Yale School of Public Health doctoral student, coauthored the Health Affairs study that found the federal shortage designation makes no difference in upping physician density long-term. He is skeptical of policy ideas that promise big primary care fixes. That includes the Biden administration’s investment in more scholarships and loan repayments through the National Health Service Corps.
“You’re just throwing more money at a set of programs that don’t really seem to work,” he said. “We’ll see in a few years, but I’ll be shocked if it actually moved any physicians or any other advanced practice providers.”
One possible explanation for the persistence of shortage areas is that such incentives are too small or too fleeting.
But another issue is how shortages are measured. The government considers geographic shortage areas, now numbering just over 1000, but also population groups such as migrant farmworkers and individual facilities such as prisons that lack enough providers. Yet it’s up to state offices to identify populations and locations that might qualify as shortage areas and submit them to HRSA, which then scores the extent of any shortages. The funding and staffing for those state offices vary, creating an uneven foundation from which to map actual shortages.
“Some states became very adept at the equivalent of gerrymandering, where they were piecing together census blocks or census tracts in odd shapes in order to maximize the areas that are eligible,” said Stephen Petterson, a senior scholar at the Robert Graham Center, a policy think tank in Washington, DC, that focuses on primary care.
The federal Government Accountability Office has highlighted such issues since at least 1995, when it released a report identifying widespread data problems with the shortage area system and concluding it had “little assurance that federal funds are used where most needed.” The report noted one of the persistent shortcomings is that the system counts only physicians, not other key primary care providers.
Since 1998, federal officials have made three attempts to update the 1970s-era rules that define what counts as a shortage area. The authors of the Affordable Care Act tried most recently, tasking a committee of experts to decide on an update.
Among other things, the committee concluded in its 2011 report that nurse practitioners, physician assistants, and certified nurse midwives should be counted as primary care providers. But the recommendations fell short by just a handful of votes.
“We failed and the committee as a whole failed and HRSA failed by not moving the process forward,” said Petterson, who presented to the committee on how to comprehensively measure primary care needs.
Steve Holloway, who directs the Colorado health department’s Primary Care Office, served on the committee. Without action at the federal level, he then led a team to create Colorado’s own health professional shortage area designations that factor in nurse practitioners and physician assistants, not just doctors.
He said it’s taken about 6 years to create a tool and map of Colorado to answer a deceptively simple question: “How many actual flesh-and-blood, live clinicians are seeing patients?”
Ed Salsberg, who was the lead federal government representative on that committee and who headed HRSA’s National Center for Health Workforce Analysis, said the rest of the nation needs more precise data, too.
“It’s so important for the nation to target its resources to the highest-need communities,” he said. “It’s time again to try one more time to develop an improved methodology.”
In the past few years, more readily available data from insurance claims has allowed researchers to distinguish the medical providers who are practicing primary care from those who have specialized or retired.
Candice Chen, an associate professor of health policy and management at George Washington University’s Fitzhugh Mullan Institute for Health Workforce Equity, used claims data that reflects one large slice of the American population — about 66 million Medicaid beneficiaries — to map the primary care workforce.
Meanwhile, Monica O’Reilly-Jacob, a nurse-scientist who recently moved from Boston College to Columbia University’s School of Nursing, studied Medicare claims to conclude that fewer than 70% of physicians typically considered primary care providers were actually providing primary care. The rest, she said, often find more lucrative positions, such as subspecializing or working in hospitals. By contrast, nurse practitioners are likely undercounted. Her study found that close to half are providing primary care.
But such publicly available data leaves out much of the country, given that fewer than 40% of Americans are insured through Medicaid or Medicare.
“There’s no government organization that’s tracking: Who trained in what, where, and where are they now, and what are they practicing,” said Alison Huffstetler, medical director of the Robert Graham Center. “And if we don’t know who is doing what kind of care — and where — then there is no way for us to equitably manage the patient-to-clinician ratio across every state.”
In Costilla County, Ms. Martinez finally found someone to provide primary care: An experienced physician assistant who moved from Texas in December.
The physician assistant’s presence should bump the county out of its dire shortage, according to Colorado’s measure. But since he isn’t a physician, he’ll remain invisible in the national data and Costilla County will likely remain on the books as a federal shortage area.
Data reporter Hannah Recht, data editor Holly K. Hacker, and rural editor/correspondent Tony Leys contributed to this report.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF — an independent source of health policy research, polling, and journalism.