User login
In memory of Dr. Carl Compton Bell
It was a simple message in the body of an email: “A strong voice in and for psychiatry is now silent.”
That is how I shared the news of the passing of Carl Compton Bell, MD, with the leadership of the American Psychiatric Association and what I think Carl would have approved be shared. Although he was a member of APA, he was never interested in the trappings of leadership there or any other organizations of which he was a longtime member, really. He preferred to “do the work” and was known to not suffer fools who were in it to promote themselves. He was always ready, willing, and able to offer guidance or assistance in your work and never failed to have an opinion on what else you needed to do. Some of my favorite memories of Carl are the talks he initiated at the drop of a hat where he “dropped some knowledge” about what he was doing or what you should be doing.
Upon hearing of his death, I described him to someone as fearless, unapologetic, smart, and ready to advocate for black people at the drop of one of the many hats he wore over the years. In fact, his decades of wardrobes is one the other things many of us will remember – the CMHC baseball cap with the “Stop Black on Black” crime T-shirt, the Obama cap paired with an assortment of message T-shirts (depending on what issue he was focused on at the time), and, most recently, the longer hair sticking out from under the wide brim leather cowboy hat with the highway patrol polarized sunglasses.
And whether it was the Surgeon General or an audience at the Carter Center, the message was consistent and powerful. An international researcher, clinician, teacher, and author of more than 500 books, chapters, and articles, he spent most of his career directly addressing issues of violence and HIV prevention, misdiagnosis of psychiatric disorders in African Americans, and the psychological effects on children exposed to violence.
Honoring the legacy of Carl Bell is about more than how we can all follow in his footsteps and more about being like him – unapologetically fearless and focused on improving the health, mental health, and overall well-being of black people. He was very clear that his talents, his skills, his focus – whether it was clinical care, training, or research – would be on black people, and he was often amused at the response, mostly from white people, when he stated clearly that this was his focus. He was often challenged by them, and his response as I frequently heard him say was: “I care about black people; I want to help black people.” I think he basically felt that, if it was good for black people, it would also benefit everyone else.
So, there’s a lesson for us as we heap on the well-deserved accolades on him and his life’s work, and reminisce about our personal encounters and experiences with him over the decades. As we reflect on what he meant to each of us as a friend, a colleague, and a history maker, I think the lesson is that if Carl were here today, he’d say: “OK, that’s all good, thank you for the nice words but what are you doing for black people today? What are you doing to improve their health and life condition today?” I think if we really want to honor his legacy and continue his work, we must be as fearless and focused as he was as we follow his lead and carry on with the work that promotes mental health in the black community. And when we are challenged for wanting to do this work, we must be just as unapologetic and thoughtful as he was, even channeling our own “Carl Bell” moment if needed. As a lifelong martial arts practitioner, I will end with this: “The bamboo which bends in the wind is stronger than the mighty oak which breaks in a storm.” Carl was the bamboo, and he’s with the Ancestors now, encouraging us to do the work and bend not break. Rest, my brother; job well done!
Dr. Stewart is immediate past president of the American Psychiatric Association.
It was a simple message in the body of an email: “A strong voice in and for psychiatry is now silent.”
That is how I shared the news of the passing of Carl Compton Bell, MD, with the leadership of the American Psychiatric Association and what I think Carl would have approved be shared. Although he was a member of APA, he was never interested in the trappings of leadership there or any other organizations of which he was a longtime member, really. He preferred to “do the work” and was known to not suffer fools who were in it to promote themselves. He was always ready, willing, and able to offer guidance or assistance in your work and never failed to have an opinion on what else you needed to do. Some of my favorite memories of Carl are the talks he initiated at the drop of a hat where he “dropped some knowledge” about what he was doing or what you should be doing.
Upon hearing of his death, I described him to someone as fearless, unapologetic, smart, and ready to advocate for black people at the drop of one of the many hats he wore over the years. In fact, his decades of wardrobes is one the other things many of us will remember – the CMHC baseball cap with the “Stop Black on Black” crime T-shirt, the Obama cap paired with an assortment of message T-shirts (depending on what issue he was focused on at the time), and, most recently, the longer hair sticking out from under the wide brim leather cowboy hat with the highway patrol polarized sunglasses.
And whether it was the Surgeon General or an audience at the Carter Center, the message was consistent and powerful. An international researcher, clinician, teacher, and author of more than 500 books, chapters, and articles, he spent most of his career directly addressing issues of violence and HIV prevention, misdiagnosis of psychiatric disorders in African Americans, and the psychological effects on children exposed to violence.
Honoring the legacy of Carl Bell is about more than how we can all follow in his footsteps and more about being like him – unapologetically fearless and focused on improving the health, mental health, and overall well-being of black people. He was very clear that his talents, his skills, his focus – whether it was clinical care, training, or research – would be on black people, and he was often amused at the response, mostly from white people, when he stated clearly that this was his focus. He was often challenged by them, and his response as I frequently heard him say was: “I care about black people; I want to help black people.” I think he basically felt that, if it was good for black people, it would also benefit everyone else.
So, there’s a lesson for us as we heap on the well-deserved accolades on him and his life’s work, and reminisce about our personal encounters and experiences with him over the decades. As we reflect on what he meant to each of us as a friend, a colleague, and a history maker, I think the lesson is that if Carl were here today, he’d say: “OK, that’s all good, thank you for the nice words but what are you doing for black people today? What are you doing to improve their health and life condition today?” I think if we really want to honor his legacy and continue his work, we must be as fearless and focused as he was as we follow his lead and carry on with the work that promotes mental health in the black community. And when we are challenged for wanting to do this work, we must be just as unapologetic and thoughtful as he was, even channeling our own “Carl Bell” moment if needed. As a lifelong martial arts practitioner, I will end with this: “The bamboo which bends in the wind is stronger than the mighty oak which breaks in a storm.” Carl was the bamboo, and he’s with the Ancestors now, encouraging us to do the work and bend not break. Rest, my brother; job well done!
Dr. Stewart is immediate past president of the American Psychiatric Association.
It was a simple message in the body of an email: “A strong voice in and for psychiatry is now silent.”
That is how I shared the news of the passing of Carl Compton Bell, MD, with the leadership of the American Psychiatric Association and what I think Carl would have approved be shared. Although he was a member of APA, he was never interested in the trappings of leadership there or any other organizations of which he was a longtime member, really. He preferred to “do the work” and was known to not suffer fools who were in it to promote themselves. He was always ready, willing, and able to offer guidance or assistance in your work and never failed to have an opinion on what else you needed to do. Some of my favorite memories of Carl are the talks he initiated at the drop of a hat where he “dropped some knowledge” about what he was doing or what you should be doing.
Upon hearing of his death, I described him to someone as fearless, unapologetic, smart, and ready to advocate for black people at the drop of one of the many hats he wore over the years. In fact, his decades of wardrobes is one the other things many of us will remember – the CMHC baseball cap with the “Stop Black on Black” crime T-shirt, the Obama cap paired with an assortment of message T-shirts (depending on what issue he was focused on at the time), and, most recently, the longer hair sticking out from under the wide brim leather cowboy hat with the highway patrol polarized sunglasses.
And whether it was the Surgeon General or an audience at the Carter Center, the message was consistent and powerful. An international researcher, clinician, teacher, and author of more than 500 books, chapters, and articles, he spent most of his career directly addressing issues of violence and HIV prevention, misdiagnosis of psychiatric disorders in African Americans, and the psychological effects on children exposed to violence.
Honoring the legacy of Carl Bell is about more than how we can all follow in his footsteps and more about being like him – unapologetically fearless and focused on improving the health, mental health, and overall well-being of black people. He was very clear that his talents, his skills, his focus – whether it was clinical care, training, or research – would be on black people, and he was often amused at the response, mostly from white people, when he stated clearly that this was his focus. He was often challenged by them, and his response as I frequently heard him say was: “I care about black people; I want to help black people.” I think he basically felt that, if it was good for black people, it would also benefit everyone else.
So, there’s a lesson for us as we heap on the well-deserved accolades on him and his life’s work, and reminisce about our personal encounters and experiences with him over the decades. As we reflect on what he meant to each of us as a friend, a colleague, and a history maker, I think the lesson is that if Carl were here today, he’d say: “OK, that’s all good, thank you for the nice words but what are you doing for black people today? What are you doing to improve their health and life condition today?” I think if we really want to honor his legacy and continue his work, we must be as fearless and focused as he was as we follow his lead and carry on with the work that promotes mental health in the black community. And when we are challenged for wanting to do this work, we must be just as unapologetic and thoughtful as he was, even channeling our own “Carl Bell” moment if needed. As a lifelong martial arts practitioner, I will end with this: “The bamboo which bends in the wind is stronger than the mighty oak which breaks in a storm.” Carl was the bamboo, and he’s with the Ancestors now, encouraging us to do the work and bend not break. Rest, my brother; job well done!
Dr. Stewart is immediate past president of the American Psychiatric Association.
Hormone therapy and cognition: What is best for the midlife brain?
CASE HT for vasomotor symptoms in perimenopausal woman with cognitive concerns
Jackie is a 49-year-old woman. Her body mass index is 33 kg/m2, and she has mild hypertension that is effectively controlled with antihypertensive medications. Otherwise, she is in good health.During her annual gynecologic exam, she reports that for the past 9 months her menstrual cycles have not been as regular as they used to be and that 3 months ago she skipped a cycle. She is having bothersome vasomotor symptoms (VMS) and is concerned about her memory. She says she is forgetful at work and in social situations. During a recent presentation, she could not remember the name of one of her former clients. At a work happy hour, she forgot the name of her coworker’s husband, although she did remember it later after returning home.
Her mother has Alzheimer disease (AD), and Jackie worries about whether she, too, might be developing dementia and whether her memory will fail her in social situations.
She is concerned about using hormone therapy (HT) for her vasomotor symptoms because she has heard that it can lead to breast cancer and/or AD.
How would you advise her?
HT remains the most effective treatment for bothersome VMS, but concerns about its cognitive safety persist. Such concerns, and indeed a black-box warning about the risk of dementia with HT use, initially arose following the 2003 publication of the Women’s Health Initiative Memory Study (WHIMS), a randomized, placebo-controlled trial of HT for the primary prevention of dementia in women aged 65 years and older at baseline.1 The study found that combination estrogen/progestin therapy was associated with a 2-fold increase in dementia when compared with placebo.
One of the critical questions arising even before WHIMS was whether the cognitive risks associated with HT that were seen in WHIMS apply to younger women. Attempting to answer the question and adding fuel to the fire are the results of a recent case-control study from Finland.2 This study compared HT use in Finnish women with and without AD and found that HT use was higher among Finnish women with AD compared with those without AD, regardless of age. The authors concluded, “Our data must be implemented into information for the present and future users of HT, even though the absolute risk increase is small.”
However, given the limitations inherent to observational and registry studies, and the contrasting findings of 3 high-quality, randomized controlled trials (RCTs; more details below), providers actually can reassure younger peri- and postmenopausal women about the cognitive safety of HT.3 They also can explain to patients that cognitive symptoms like the ones described in the case example are normal and provide general guidance to midlife women on how to optimize brain health.

Continue to: Closer look at WHI and RCT research pinpoints cognitively neutral HT...
Closer look at WHI and RCT research pinpoints cognitively neutral HT
In WHIMS, the combination of conjugated equine estrogen (CEE; 0.625 mg/d) plus medroxyprogesterone acetate (MPA; 2.5 mg/d) led to a doubling of the risk of all-cause dementia compared with placebo in a sample of 4,532 women aged 65 years and older at baseline.1 CEE alone (0.625 mg) did not lead to an increased risk of all-cause dementia.4
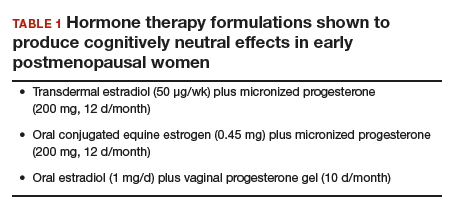
Whether those formulations led to cognitive impairment in younger postmenopausal women was the focus of WHIMS-Younger (WHIMS-Y), which involved WHI participants aged 50 to 55 years at baseline.5 Results revealed neutral cognitive effects (ie, no differences in cognitive performance in women randomly assigned to HT or placebo) in women tested 7.2 years after the end of the WHI trial. WHIMS-Y findings indicated that there were no sustained cognitive risks of CEE or CEE/MPA therapy. Two randomized, placebo-controlled trials involving younger postmenopausal women yielded similar findings.6,7 HT shown to produce cognitively neutral effects during active treatment included transdermal estradiol plus micronized progesterone,6 CEE plus progesterone,6 and oral estradiol plus vaginal progesterone gel.7 The findings of these randomized trials are critical for guiding decisions regarding the cognitive risks of HT in early postmenopausal women (TABLE 1).
What about women with VMS?
A key gap in knowledge about the cognitive effects of HT is whether HT confers cognitive advantages to women with bothersome VMS. This is a striking absence given that the key indication for HT is the treatment of VMS. While some symptomatic women were included in the trials of HT in younger postmenopausal women described above, no large trial to date has selectively enrolled women with moderate-to-severe VMS to determine if HT is cognitively neutral, beneficial, or detrimental in that group. Some studies involving midlife women have found associations between VMS (as measured with ambulatory skin conductance monitors) and multiple measures of brain health, including memory performance,8 small ischemic lesions on structural brain scans,9 and altered brain function.10 In a small trial of a nonhormonal intervention for VMS, improvement in VMS following the intervention was directly related to improvement in memory performance.11 The reliability of these findings continues to be evaluated but raises the hypothesis that VMS treatments might improve memory in midlife women.
Memory complaints common among midlife women
About 60% of women report an undesirable change in memory performance at midlife as compared with earlier in their lives.12,13 Complaints of forgetfulness are higher in perimenopausal and postmenopausal women compared with premenopausal women, even when those women are similar in age.14 Two large prospective studies found that memory performance decreases during the perimenopause and then rebounds, suggesting a transient decrease in memory.15,16 Although cognitive complaints are common among women in their 40s and 50s, AD is rare in that age group. The risk is largely limited to those women with a parent who developed dementia before age 65, as such cases suggest a familial form of AD.
Continue to: What causes cognitive difficulties during midlife?
What causes cognitive difficulties during midlife?
First, some cognitive decline is expected at midlife based on increasing age. Second, above and beyond the role of chronologic aging (ie, getting one year older each year), ovarian aging plays a role. A role of estrogen was verified in clinical trials showing that memory decreased following oophorectomy in premenopausal women in their 40s but returned to presurgical levels following treatment with estrogen therapy (ET).17 Cohort studies indicate that women who undergo oophorectomy before the typical age of menopause are at increased risk for cognitive impairment or dementia, but those who take ET after oophorectomy until the typical age of menopause do not show that risk.18
Third, cognitive problems are linked not only to VMS but also to sleep disturbance, depressed mood, and increased anxiety—all of which are common in midlife women.15,19 Lastly, health factors play a role. Hypertension, obesity, insulin resistance, diabetes, and smoking are associated with adverse brain changes at midlife.20
Giving advice to your patients
First, normalize the cognitive complaints, noting that some cognitive changes are an expected part of aging for all people regardless of whether they are male or female. Advise that while the best studies indicate that these cognitive lapses are especially common in perimenopausal women, they appear to be temporary; women are likely to resume normal cognitive function once the hormonal changes associated with menopause subside.15,16 Note that the one unknown is the role that VMS play in memory problems and that some studies indicate a link between VMS and cognitive problems. Women may experience some cognitive improvement if VMS are effectively treated.
Advise patients that the Endocrine Society, the North American Menopause Society (NAMS), and the International Menopause Society all have published guidelines saying that the benefits of HT outweigh the risks for most women aged 50 to 60 years.21 For concerns about the cognitive adverse effects of HT, discuss the best quality evidence—that which comes from randomized trials—which shows no harmful effects of HT in midlife women.5-7 Especially reassuring is that one of these high-quality studies was conducted by the same researchers who found that HT can be risky in older women (ie, the WHI Investigators).5
Going one step further: Protecting brain health
As primary care providers to midlife women, ObGyns can go one step further and advise patients on how to proactively nurture their brain health. Great evidence-based resources for information on maintaining brain health include the Alzheimer’s Association (https://www.alz.org) and the Women’s Brain Health Initiative (https://womensbrainhealth.org). Primary prevention of AD begins decades before the typical age of an AD diagnosis, and many risk factors for AD are modifiable.22 Patients can keep their brains healthy through myriad approaches including treating hypertension, reducing body mass index, engaging in regular aerobic exercise (brisk walking is fine), eating a Mediterranean diet, maintaining an active social life, and engaging in novel challenging activities like learning a new language or a new skill like dancing.20
Also important is the overlap between cognitive issues, mood, and alcohol use. In the opening case, Jackie mentions alcohol use and social withdrawal. According to the National Institute on Alcohol Abuse and Alcoholism (NIAAA), low-risk drinking for women is defined as no more than 3 drinks on any single day and no more than 7 drinks per week.23 Heavy alcohol use not only affects brain function but also mood, and depressed mood can lead women to drink excessively.24
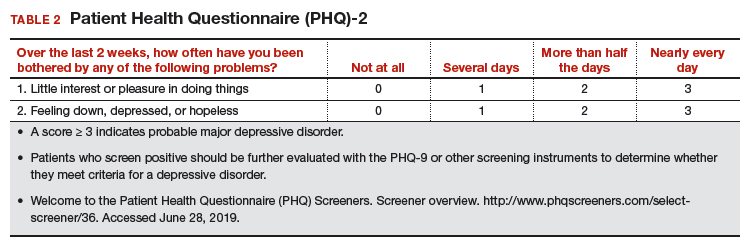
In addition, Jackie’s mother has AD, and that stressor can contribute to depressed feelings, especially if Jackie is involved in caregiving. A quick screen for depression with an instrument like the Patient Health Questionnaire-2 (PHQ-2; TABLE 2)25 can rule out a more serious mood disorder—an approach that is particularly important for patients with a history of major depression, as 58% of those patients experience a major depressive episode during the menopausal transition.26 For this reason, it is important to ask patients like Jackie if they have a history of depression; if they do and were treated medically, consider prescribing the antidepressant that worked in the past. For information on menopause and mood-related issues, providers can access new guidelines from NAMS and the National Network of Depression Centers (NNDC).27 There is also a handy patient information sheet to accompany those guidelines on the NAMS website (https://www.menopause.org/).
Continue to: CASE Resolved...
CASE Resolved
When approaching Jackie, most importantly, I would normalize her experience and tell her that memory problems are common in the menopausal transition, especially for women with bothersome VMS. Research suggests that the memory problems she is experiencing are related to hormonal changes and not to AD, and that her memory will likely improve once she has transitioned through the menopause. I would tell her that AD is rare at midlife unless there is a family history of early onset of AD (before age 65), and I would verify the age at which her mother was diagnosed to confirm that it was late-onset AD.
For now, I would recommend that she be prescribed HT for her bothersome hot flashes using one of the “safe” formulations in the Table on page 24. I also would tell her that there is much she can do to lower her risk of AD and that it is best to start now as she enters her 50s because that is when AD changes typically start in the brain, and she can start to prevent those changes now.
I would tell her that experts in the field of AD agree that these lifestyle interventions are currently the best way to prevent AD and that the more of them she engages in, the more her brain will benefit. I would advise her to continue to manage her hypertension and to consider ways of lowering her BMI to enhance her brain health. Engaging in regular brisk walking or other aerobic exercise, as well as incorporating more of the Mediterranean diet into her daily food intake would also benefit her brain. As a working woman, she is exercising her brain, and she should consider other cognitively challenging activities to keep her brain in good shape.
I would follow up with her in a few months to see if her memory functioning is better. If it is not, and if her VMS continue to be bothersome, I would increase her dose of HT. Only if her VMS are treated but her memory problems are getting worse would I screen her with a Mini-Mental State Exam and refer her to a neurologist for an evaluation.
- Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651-2662.
- Savolainen-Peltonen H, Rahkola-Soisalo P, Hoti F, et al. Use of postmenopausal hormone therapy and risk of Alzheimer’s disease in Finland: nationwide case-control study. BMJ. 2019;364:1665.
- Maki PM, Girard LM, Manson JE. Menopausal hormone therapy and cognition. BMJ. 2019;364:1877.
- Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947-2958.
- Espeland MA, Shumaker SA, Leng I, et al. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med. 2013;173:1429-1436.
- Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-cognitive and affective study. PLoS Med. 2015;12:e1001833.
- Henderson VW, St. John JA, Hodis HN, et al. Cognitive effects of estradiol after menopause: a randomized trial of the timing hypothesis. Neurology. 2016;87:699-708.
- Maki PM, Drogos LL, Rubin LH, et al. Objective hot flashes are negatively related to verbal memory performance in midlife women. Menopause. 2008;15:848-856.
- Thurston RC, Aizenstein HJ, Derby CA, et al. Menopausal hot flashes and white matter hyperintensities. Menopause. 2016;23:27-32.
- Thurston RC, Maki PM, Derby CA, et al. Menopausal hot flashes and the default mode network. Fertil Steril. 2015;103:1572-1578.e1.
- Maki PM, Rubin LH, Savarese A, et al. Stellate ganglion blockade and verbal memory in midlife women: evidence from a randomized trial. Maturitas. 2016;92:123-129.
- Woods NF, Mitchell ES, Adams C. Memory functioning among midlife women: observations from the Seattle Midlife Women’s Health Study. Menopause. 2000;7:257-265.
- Sullivan Mitchell E, Fugate Woods N. Midlife women’s attributions about perceived memory changes: observations from the Seattle Midlife Women’s Health Study. J Womens Health Gend Based Med. 2001;10:351-362.
- Gold EB, Sternfeld B, Kelsey JL, et al. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40-55 years of age. Am J Epidemiol. 2000;152:463-473.
CASE HT for vasomotor symptoms in perimenopausal woman with cognitive concerns
Jackie is a 49-year-old woman. Her body mass index is 33 kg/m2, and she has mild hypertension that is effectively controlled with antihypertensive medications. Otherwise, she is in good health.During her annual gynecologic exam, she reports that for the past 9 months her menstrual cycles have not been as regular as they used to be and that 3 months ago she skipped a cycle. She is having bothersome vasomotor symptoms (VMS) and is concerned about her memory. She says she is forgetful at work and in social situations. During a recent presentation, she could not remember the name of one of her former clients. At a work happy hour, she forgot the name of her coworker’s husband, although she did remember it later after returning home.
Her mother has Alzheimer disease (AD), and Jackie worries about whether she, too, might be developing dementia and whether her memory will fail her in social situations.
She is concerned about using hormone therapy (HT) for her vasomotor symptoms because she has heard that it can lead to breast cancer and/or AD.
How would you advise her?
HT remains the most effective treatment for bothersome VMS, but concerns about its cognitive safety persist. Such concerns, and indeed a black-box warning about the risk of dementia with HT use, initially arose following the 2003 publication of the Women’s Health Initiative Memory Study (WHIMS), a randomized, placebo-controlled trial of HT for the primary prevention of dementia in women aged 65 years and older at baseline.1 The study found that combination estrogen/progestin therapy was associated with a 2-fold increase in dementia when compared with placebo.
One of the critical questions arising even before WHIMS was whether the cognitive risks associated with HT that were seen in WHIMS apply to younger women. Attempting to answer the question and adding fuel to the fire are the results of a recent case-control study from Finland.2 This study compared HT use in Finnish women with and without AD and found that HT use was higher among Finnish women with AD compared with those without AD, regardless of age. The authors concluded, “Our data must be implemented into information for the present and future users of HT, even though the absolute risk increase is small.”
However, given the limitations inherent to observational and registry studies, and the contrasting findings of 3 high-quality, randomized controlled trials (RCTs; more details below), providers actually can reassure younger peri- and postmenopausal women about the cognitive safety of HT.3 They also can explain to patients that cognitive symptoms like the ones described in the case example are normal and provide general guidance to midlife women on how to optimize brain health.

Continue to: Closer look at WHI and RCT research pinpoints cognitively neutral HT...
Closer look at WHI and RCT research pinpoints cognitively neutral HT
In WHIMS, the combination of conjugated equine estrogen (CEE; 0.625 mg/d) plus medroxyprogesterone acetate (MPA; 2.5 mg/d) led to a doubling of the risk of all-cause dementia compared with placebo in a sample of 4,532 women aged 65 years and older at baseline.1 CEE alone (0.625 mg) did not lead to an increased risk of all-cause dementia.4
Whether those formulations led to cognitive impairment in younger postmenopausal women was the focus of WHIMS-Younger (WHIMS-Y), which involved WHI participants aged 50 to 55 years at baseline.5 Results revealed neutral cognitive effects (ie, no differences in cognitive performance in women randomly assigned to HT or placebo) in women tested 7.2 years after the end of the WHI trial. WHIMS-Y findings indicated that there were no sustained cognitive risks of CEE or CEE/MPA therapy. Two randomized, placebo-controlled trials involving younger postmenopausal women yielded similar findings.6,7 HT shown to produce cognitively neutral effects during active treatment included transdermal estradiol plus micronized progesterone,6 CEE plus progesterone,6 and oral estradiol plus vaginal progesterone gel.7 The findings of these randomized trials are critical for guiding decisions regarding the cognitive risks of HT in early postmenopausal women (TABLE 1).

What about women with VMS?
A key gap in knowledge about the cognitive effects of HT is whether HT confers cognitive advantages to women with bothersome VMS. This is a striking absence given that the key indication for HT is the treatment of VMS. While some symptomatic women were included in the trials of HT in younger postmenopausal women described above, no large trial to date has selectively enrolled women with moderate-to-severe VMS to determine if HT is cognitively neutral, beneficial, or detrimental in that group. Some studies involving midlife women have found associations between VMS (as measured with ambulatory skin conductance monitors) and multiple measures of brain health, including memory performance,8 small ischemic lesions on structural brain scans,9 and altered brain function.10 In a small trial of a nonhormonal intervention for VMS, improvement in VMS following the intervention was directly related to improvement in memory performance.11 The reliability of these findings continues to be evaluated but raises the hypothesis that VMS treatments might improve memory in midlife women.
Memory complaints common among midlife women
About 60% of women report an undesirable change in memory performance at midlife as compared with earlier in their lives.12,13 Complaints of forgetfulness are higher in perimenopausal and postmenopausal women compared with premenopausal women, even when those women are similar in age.14 Two large prospective studies found that memory performance decreases during the perimenopause and then rebounds, suggesting a transient decrease in memory.15,16 Although cognitive complaints are common among women in their 40s and 50s, AD is rare in that age group. The risk is largely limited to those women with a parent who developed dementia before age 65, as such cases suggest a familial form of AD.
Continue to: What causes cognitive difficulties during midlife?
What causes cognitive difficulties during midlife?
First, some cognitive decline is expected at midlife based on increasing age. Second, above and beyond the role of chronologic aging (ie, getting one year older each year), ovarian aging plays a role. A role of estrogen was verified in clinical trials showing that memory decreased following oophorectomy in premenopausal women in their 40s but returned to presurgical levels following treatment with estrogen therapy (ET).17 Cohort studies indicate that women who undergo oophorectomy before the typical age of menopause are at increased risk for cognitive impairment or dementia, but those who take ET after oophorectomy until the typical age of menopause do not show that risk.18
Third, cognitive problems are linked not only to VMS but also to sleep disturbance, depressed mood, and increased anxiety—all of which are common in midlife women.15,19 Lastly, health factors play a role. Hypertension, obesity, insulin resistance, diabetes, and smoking are associated with adverse brain changes at midlife.20
Giving advice to your patients
First, normalize the cognitive complaints, noting that some cognitive changes are an expected part of aging for all people regardless of whether they are male or female. Advise that while the best studies indicate that these cognitive lapses are especially common in perimenopausal women, they appear to be temporary; women are likely to resume normal cognitive function once the hormonal changes associated with menopause subside.15,16 Note that the one unknown is the role that VMS play in memory problems and that some studies indicate a link between VMS and cognitive problems. Women may experience some cognitive improvement if VMS are effectively treated.
Advise patients that the Endocrine Society, the North American Menopause Society (NAMS), and the International Menopause Society all have published guidelines saying that the benefits of HT outweigh the risks for most women aged 50 to 60 years.21 For concerns about the cognitive adverse effects of HT, discuss the best quality evidence—that which comes from randomized trials—which shows no harmful effects of HT in midlife women.5-7 Especially reassuring is that one of these high-quality studies was conducted by the same researchers who found that HT can be risky in older women (ie, the WHI Investigators).5
Going one step further: Protecting brain health
As primary care providers to midlife women, ObGyns can go one step further and advise patients on how to proactively nurture their brain health. Great evidence-based resources for information on maintaining brain health include the Alzheimer’s Association (https://www.alz.org) and the Women’s Brain Health Initiative (https://womensbrainhealth.org). Primary prevention of AD begins decades before the typical age of an AD diagnosis, and many risk factors for AD are modifiable.22 Patients can keep their brains healthy through myriad approaches including treating hypertension, reducing body mass index, engaging in regular aerobic exercise (brisk walking is fine), eating a Mediterranean diet, maintaining an active social life, and engaging in novel challenging activities like learning a new language or a new skill like dancing.20
Also important is the overlap between cognitive issues, mood, and alcohol use. In the opening case, Jackie mentions alcohol use and social withdrawal. According to the National Institute on Alcohol Abuse and Alcoholism (NIAAA), low-risk drinking for women is defined as no more than 3 drinks on any single day and no more than 7 drinks per week.23 Heavy alcohol use not only affects brain function but also mood, and depressed mood can lead women to drink excessively.24
In addition, Jackie’s mother has AD, and that stressor can contribute to depressed feelings, especially if Jackie is involved in caregiving. A quick screen for depression with an instrument like the Patient Health Questionnaire-2 (PHQ-2; TABLE 2)25 can rule out a more serious mood disorder—an approach that is particularly important for patients with a history of major depression, as 58% of those patients experience a major depressive episode during the menopausal transition.26 For this reason, it is important to ask patients like Jackie if they have a history of depression; if they do and were treated medically, consider prescribing the antidepressant that worked in the past. For information on menopause and mood-related issues, providers can access new guidelines from NAMS and the National Network of Depression Centers (NNDC).27 There is also a handy patient information sheet to accompany those guidelines on the NAMS website (https://www.menopause.org/).

Continue to: CASE Resolved...
CASE Resolved
When approaching Jackie, most importantly, I would normalize her experience and tell her that memory problems are common in the menopausal transition, especially for women with bothersome VMS. Research suggests that the memory problems she is experiencing are related to hormonal changes and not to AD, and that her memory will likely improve once she has transitioned through the menopause. I would tell her that AD is rare at midlife unless there is a family history of early onset of AD (before age 65), and I would verify the age at which her mother was diagnosed to confirm that it was late-onset AD.
For now, I would recommend that she be prescribed HT for her bothersome hot flashes using one of the “safe” formulations in the Table on page 24. I also would tell her that there is much she can do to lower her risk of AD and that it is best to start now as she enters her 50s because that is when AD changes typically start in the brain, and she can start to prevent those changes now.
I would tell her that experts in the field of AD agree that these lifestyle interventions are currently the best way to prevent AD and that the more of them she engages in, the more her brain will benefit. I would advise her to continue to manage her hypertension and to consider ways of lowering her BMI to enhance her brain health. Engaging in regular brisk walking or other aerobic exercise, as well as incorporating more of the Mediterranean diet into her daily food intake would also benefit her brain. As a working woman, she is exercising her brain, and she should consider other cognitively challenging activities to keep her brain in good shape.
I would follow up with her in a few months to see if her memory functioning is better. If it is not, and if her VMS continue to be bothersome, I would increase her dose of HT. Only if her VMS are treated but her memory problems are getting worse would I screen her with a Mini-Mental State Exam and refer her to a neurologist for an evaluation.
CASE HT for vasomotor symptoms in perimenopausal woman with cognitive concerns
Jackie is a 49-year-old woman. Her body mass index is 33 kg/m2, and she has mild hypertension that is effectively controlled with antihypertensive medications. Otherwise, she is in good health.During her annual gynecologic exam, she reports that for the past 9 months her menstrual cycles have not been as regular as they used to be and that 3 months ago she skipped a cycle. She is having bothersome vasomotor symptoms (VMS) and is concerned about her memory. She says she is forgetful at work and in social situations. During a recent presentation, she could not remember the name of one of her former clients. At a work happy hour, she forgot the name of her coworker’s husband, although she did remember it later after returning home.
Her mother has Alzheimer disease (AD), and Jackie worries about whether she, too, might be developing dementia and whether her memory will fail her in social situations.
She is concerned about using hormone therapy (HT) for her vasomotor symptoms because she has heard that it can lead to breast cancer and/or AD.
How would you advise her?
HT remains the most effective treatment for bothersome VMS, but concerns about its cognitive safety persist. Such concerns, and indeed a black-box warning about the risk of dementia with HT use, initially arose following the 2003 publication of the Women’s Health Initiative Memory Study (WHIMS), a randomized, placebo-controlled trial of HT for the primary prevention of dementia in women aged 65 years and older at baseline.1 The study found that combination estrogen/progestin therapy was associated with a 2-fold increase in dementia when compared with placebo.
One of the critical questions arising even before WHIMS was whether the cognitive risks associated with HT that were seen in WHIMS apply to younger women. Attempting to answer the question and adding fuel to the fire are the results of a recent case-control study from Finland.2 This study compared HT use in Finnish women with and without AD and found that HT use was higher among Finnish women with AD compared with those without AD, regardless of age. The authors concluded, “Our data must be implemented into information for the present and future users of HT, even though the absolute risk increase is small.”
However, given the limitations inherent to observational and registry studies, and the contrasting findings of 3 high-quality, randomized controlled trials (RCTs; more details below), providers actually can reassure younger peri- and postmenopausal women about the cognitive safety of HT.3 They also can explain to patients that cognitive symptoms like the ones described in the case example are normal and provide general guidance to midlife women on how to optimize brain health.

Continue to: Closer look at WHI and RCT research pinpoints cognitively neutral HT...
Closer look at WHI and RCT research pinpoints cognitively neutral HT
In WHIMS, the combination of conjugated equine estrogen (CEE; 0.625 mg/d) plus medroxyprogesterone acetate (MPA; 2.5 mg/d) led to a doubling of the risk of all-cause dementia compared with placebo in a sample of 4,532 women aged 65 years and older at baseline.1 CEE alone (0.625 mg) did not lead to an increased risk of all-cause dementia.4
Whether those formulations led to cognitive impairment in younger postmenopausal women was the focus of WHIMS-Younger (WHIMS-Y), which involved WHI participants aged 50 to 55 years at baseline.5 Results revealed neutral cognitive effects (ie, no differences in cognitive performance in women randomly assigned to HT or placebo) in women tested 7.2 years after the end of the WHI trial. WHIMS-Y findings indicated that there were no sustained cognitive risks of CEE or CEE/MPA therapy. Two randomized, placebo-controlled trials involving younger postmenopausal women yielded similar findings.6,7 HT shown to produce cognitively neutral effects during active treatment included transdermal estradiol plus micronized progesterone,6 CEE plus progesterone,6 and oral estradiol plus vaginal progesterone gel.7 The findings of these randomized trials are critical for guiding decisions regarding the cognitive risks of HT in early postmenopausal women (TABLE 1).

What about women with VMS?
A key gap in knowledge about the cognitive effects of HT is whether HT confers cognitive advantages to women with bothersome VMS. This is a striking absence given that the key indication for HT is the treatment of VMS. While some symptomatic women were included in the trials of HT in younger postmenopausal women described above, no large trial to date has selectively enrolled women with moderate-to-severe VMS to determine if HT is cognitively neutral, beneficial, or detrimental in that group. Some studies involving midlife women have found associations between VMS (as measured with ambulatory skin conductance monitors) and multiple measures of brain health, including memory performance,8 small ischemic lesions on structural brain scans,9 and altered brain function.10 In a small trial of a nonhormonal intervention for VMS, improvement in VMS following the intervention was directly related to improvement in memory performance.11 The reliability of these findings continues to be evaluated but raises the hypothesis that VMS treatments might improve memory in midlife women.
Memory complaints common among midlife women
About 60% of women report an undesirable change in memory performance at midlife as compared with earlier in their lives.12,13 Complaints of forgetfulness are higher in perimenopausal and postmenopausal women compared with premenopausal women, even when those women are similar in age.14 Two large prospective studies found that memory performance decreases during the perimenopause and then rebounds, suggesting a transient decrease in memory.15,16 Although cognitive complaints are common among women in their 40s and 50s, AD is rare in that age group. The risk is largely limited to those women with a parent who developed dementia before age 65, as such cases suggest a familial form of AD.
Continue to: What causes cognitive difficulties during midlife?
What causes cognitive difficulties during midlife?
First, some cognitive decline is expected at midlife based on increasing age. Second, above and beyond the role of chronologic aging (ie, getting one year older each year), ovarian aging plays a role. A role of estrogen was verified in clinical trials showing that memory decreased following oophorectomy in premenopausal women in their 40s but returned to presurgical levels following treatment with estrogen therapy (ET).17 Cohort studies indicate that women who undergo oophorectomy before the typical age of menopause are at increased risk for cognitive impairment or dementia, but those who take ET after oophorectomy until the typical age of menopause do not show that risk.18
Third, cognitive problems are linked not only to VMS but also to sleep disturbance, depressed mood, and increased anxiety—all of which are common in midlife women.15,19 Lastly, health factors play a role. Hypertension, obesity, insulin resistance, diabetes, and smoking are associated with adverse brain changes at midlife.20
Giving advice to your patients
First, normalize the cognitive complaints, noting that some cognitive changes are an expected part of aging for all people regardless of whether they are male or female. Advise that while the best studies indicate that these cognitive lapses are especially common in perimenopausal women, they appear to be temporary; women are likely to resume normal cognitive function once the hormonal changes associated with menopause subside.15,16 Note that the one unknown is the role that VMS play in memory problems and that some studies indicate a link between VMS and cognitive problems. Women may experience some cognitive improvement if VMS are effectively treated.
Advise patients that the Endocrine Society, the North American Menopause Society (NAMS), and the International Menopause Society all have published guidelines saying that the benefits of HT outweigh the risks for most women aged 50 to 60 years.21 For concerns about the cognitive adverse effects of HT, discuss the best quality evidence—that which comes from randomized trials—which shows no harmful effects of HT in midlife women.5-7 Especially reassuring is that one of these high-quality studies was conducted by the same researchers who found that HT can be risky in older women (ie, the WHI Investigators).5
Going one step further: Protecting brain health
As primary care providers to midlife women, ObGyns can go one step further and advise patients on how to proactively nurture their brain health. Great evidence-based resources for information on maintaining brain health include the Alzheimer’s Association (https://www.alz.org) and the Women’s Brain Health Initiative (https://womensbrainhealth.org). Primary prevention of AD begins decades before the typical age of an AD diagnosis, and many risk factors for AD are modifiable.22 Patients can keep their brains healthy through myriad approaches including treating hypertension, reducing body mass index, engaging in regular aerobic exercise (brisk walking is fine), eating a Mediterranean diet, maintaining an active social life, and engaging in novel challenging activities like learning a new language or a new skill like dancing.20
Also important is the overlap between cognitive issues, mood, and alcohol use. In the opening case, Jackie mentions alcohol use and social withdrawal. According to the National Institute on Alcohol Abuse and Alcoholism (NIAAA), low-risk drinking for women is defined as no more than 3 drinks on any single day and no more than 7 drinks per week.23 Heavy alcohol use not only affects brain function but also mood, and depressed mood can lead women to drink excessively.24
In addition, Jackie’s mother has AD, and that stressor can contribute to depressed feelings, especially if Jackie is involved in caregiving. A quick screen for depression with an instrument like the Patient Health Questionnaire-2 (PHQ-2; TABLE 2)25 can rule out a more serious mood disorder—an approach that is particularly important for patients with a history of major depression, as 58% of those patients experience a major depressive episode during the menopausal transition.26 For this reason, it is important to ask patients like Jackie if they have a history of depression; if they do and were treated medically, consider prescribing the antidepressant that worked in the past. For information on menopause and mood-related issues, providers can access new guidelines from NAMS and the National Network of Depression Centers (NNDC).27 There is also a handy patient information sheet to accompany those guidelines on the NAMS website (https://www.menopause.org/).

Continue to: CASE Resolved...
CASE Resolved
When approaching Jackie, most importantly, I would normalize her experience and tell her that memory problems are common in the menopausal transition, especially for women with bothersome VMS. Research suggests that the memory problems she is experiencing are related to hormonal changes and not to AD, and that her memory will likely improve once she has transitioned through the menopause. I would tell her that AD is rare at midlife unless there is a family history of early onset of AD (before age 65), and I would verify the age at which her mother was diagnosed to confirm that it was late-onset AD.
For now, I would recommend that she be prescribed HT for her bothersome hot flashes using one of the “safe” formulations in the Table on page 24. I also would tell her that there is much she can do to lower her risk of AD and that it is best to start now as she enters her 50s because that is when AD changes typically start in the brain, and she can start to prevent those changes now.
I would tell her that experts in the field of AD agree that these lifestyle interventions are currently the best way to prevent AD and that the more of them she engages in, the more her brain will benefit. I would advise her to continue to manage her hypertension and to consider ways of lowering her BMI to enhance her brain health. Engaging in regular brisk walking or other aerobic exercise, as well as incorporating more of the Mediterranean diet into her daily food intake would also benefit her brain. As a working woman, she is exercising her brain, and she should consider other cognitively challenging activities to keep her brain in good shape.
I would follow up with her in a few months to see if her memory functioning is better. If it is not, and if her VMS continue to be bothersome, I would increase her dose of HT. Only if her VMS are treated but her memory problems are getting worse would I screen her with a Mini-Mental State Exam and refer her to a neurologist for an evaluation.
- Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651-2662.
- Savolainen-Peltonen H, Rahkola-Soisalo P, Hoti F, et al. Use of postmenopausal hormone therapy and risk of Alzheimer’s disease in Finland: nationwide case-control study. BMJ. 2019;364:1665.
- Maki PM, Girard LM, Manson JE. Menopausal hormone therapy and cognition. BMJ. 2019;364:1877.
- Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947-2958.
- Espeland MA, Shumaker SA, Leng I, et al. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med. 2013;173:1429-1436.
- Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-cognitive and affective study. PLoS Med. 2015;12:e1001833.
- Henderson VW, St. John JA, Hodis HN, et al. Cognitive effects of estradiol after menopause: a randomized trial of the timing hypothesis. Neurology. 2016;87:699-708.
- Maki PM, Drogos LL, Rubin LH, et al. Objective hot flashes are negatively related to verbal memory performance in midlife women. Menopause. 2008;15:848-856.
- Thurston RC, Aizenstein HJ, Derby CA, et al. Menopausal hot flashes and white matter hyperintensities. Menopause. 2016;23:27-32.
- Thurston RC, Maki PM, Derby CA, et al. Menopausal hot flashes and the default mode network. Fertil Steril. 2015;103:1572-1578.e1.
- Maki PM, Rubin LH, Savarese A, et al. Stellate ganglion blockade and verbal memory in midlife women: evidence from a randomized trial. Maturitas. 2016;92:123-129.
- Woods NF, Mitchell ES, Adams C. Memory functioning among midlife women: observations from the Seattle Midlife Women’s Health Study. Menopause. 2000;7:257-265.
- Sullivan Mitchell E, Fugate Woods N. Midlife women’s attributions about perceived memory changes: observations from the Seattle Midlife Women’s Health Study. J Womens Health Gend Based Med. 2001;10:351-362.
- Gold EB, Sternfeld B, Kelsey JL, et al. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40-55 years of age. Am J Epidemiol. 2000;152:463-473.
- Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651-2662.
- Savolainen-Peltonen H, Rahkola-Soisalo P, Hoti F, et al. Use of postmenopausal hormone therapy and risk of Alzheimer’s disease in Finland: nationwide case-control study. BMJ. 2019;364:1665.
- Maki PM, Girard LM, Manson JE. Menopausal hormone therapy and cognition. BMJ. 2019;364:1877.
- Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947-2958.
- Espeland MA, Shumaker SA, Leng I, et al. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med. 2013;173:1429-1436.
- Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-cognitive and affective study. PLoS Med. 2015;12:e1001833.
- Henderson VW, St. John JA, Hodis HN, et al. Cognitive effects of estradiol after menopause: a randomized trial of the timing hypothesis. Neurology. 2016;87:699-708.
- Maki PM, Drogos LL, Rubin LH, et al. Objective hot flashes are negatively related to verbal memory performance in midlife women. Menopause. 2008;15:848-856.
- Thurston RC, Aizenstein HJ, Derby CA, et al. Menopausal hot flashes and white matter hyperintensities. Menopause. 2016;23:27-32.
- Thurston RC, Maki PM, Derby CA, et al. Menopausal hot flashes and the default mode network. Fertil Steril. 2015;103:1572-1578.e1.
- Maki PM, Rubin LH, Savarese A, et al. Stellate ganglion blockade and verbal memory in midlife women: evidence from a randomized trial. Maturitas. 2016;92:123-129.
- Woods NF, Mitchell ES, Adams C. Memory functioning among midlife women: observations from the Seattle Midlife Women’s Health Study. Menopause. 2000;7:257-265.
- Sullivan Mitchell E, Fugate Woods N. Midlife women’s attributions about perceived memory changes: observations from the Seattle Midlife Women’s Health Study. J Womens Health Gend Based Med. 2001;10:351-362.
- Gold EB, Sternfeld B, Kelsey JL, et al. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40-55 years of age. Am J Epidemiol. 2000;152:463-473.
Office hysteroscopic evaluation of postmenopausal bleeding
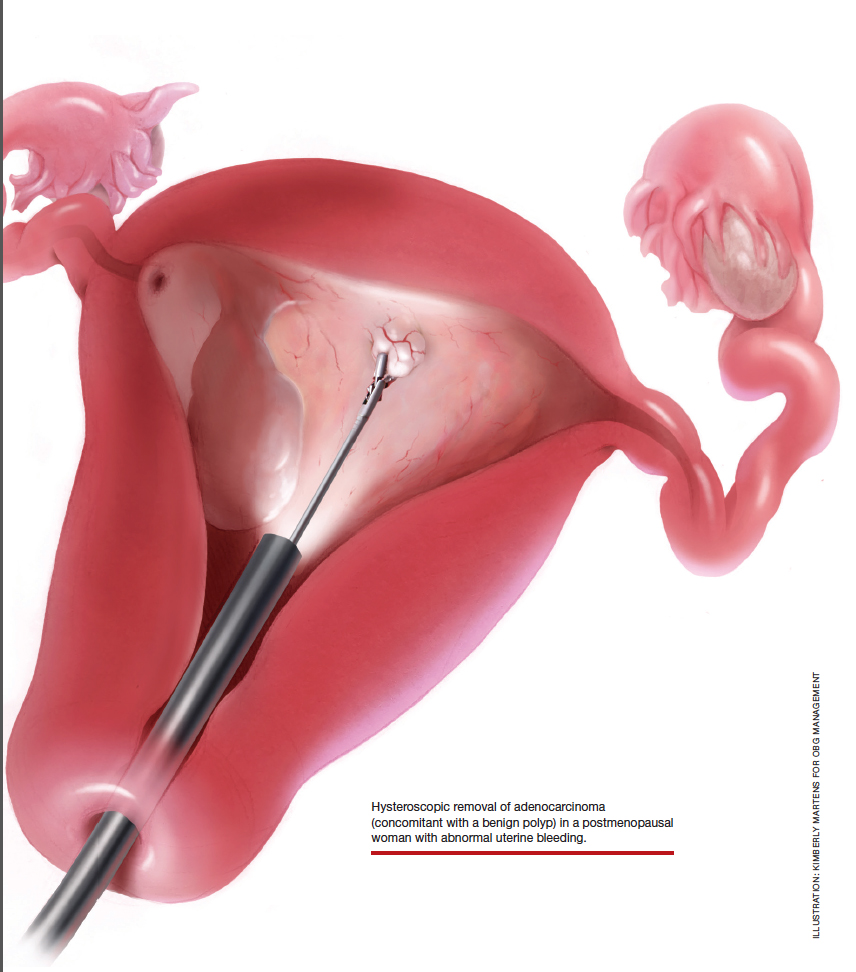
Postmenopausal bleeding (PMB) is the presenting sign in most cases of endometrial carcinoma. Prompt evaluation of PMB can exclude, or diagnose, endometrial carcinoma.1 Although no general consensus exists for PMB evaluation, it involves endometrial assessment with transvaginal ultrasonography (TVUS) and subsequent endometrial biopsy when a thickened endometrium is found. When biopsy results reveal insufficient or scant tissue, further investigation into the etiology of PMB should include office hysteroscopy with possible directed biopsy. In this article I discuss the prevalence of PMB and steps for evaluation, providing clinical takeaways.
Postmenopausal bleeding: Its risk for cancer
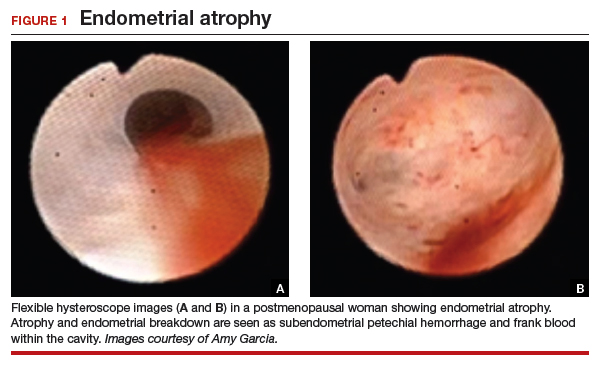
Abnormal uterine bleeding (AUB) in a postmenopausal woman is of particular concern to the gynecologist and the patient because of the increased possibility of endometrial carcinoma in this age group. AUB is present in more than 90% of postmenopausal women with endometrial carcinoma, which leads to diagnosis in the early stages of the disease. Approximately 3% to 7% of postmenopausal women with PMB will have endometrial carcinoma.2 Most women with PMB, however, experience bleeding secondary to atrophic changes of the vagina or endometrium and not to endometrial carcinoma. (FIGURE 1, VIDEO 1) In addition, women who take gonadal steroids for hormone replacement therapy (HRT) may experience breakthrough bleeding that leads to initial investigation with TVUS.
Video 1

The risk of malignancy in polyps in postmenopausal women over the age of 59 who present with PMB is approximately 12%, and hysteroscopic resection should routinely be performed. For asymptomatic patients, the risk of a malignant lesion is low—approximately 3%—and for these women intervention should be assessed individually for the risks of carcinoma and benefits of hysteroscopic removal.3
Clinical takeaway. The high possibility of endometrial carcinoma in postmenopausal women warrants that any patient who is symptomatic with PMB should be presumed to have endometrial cancer until the diagnostic evaluation process proves she does not.
Evaluation of postmenopausal bleeding
Transvaginal ultrasound
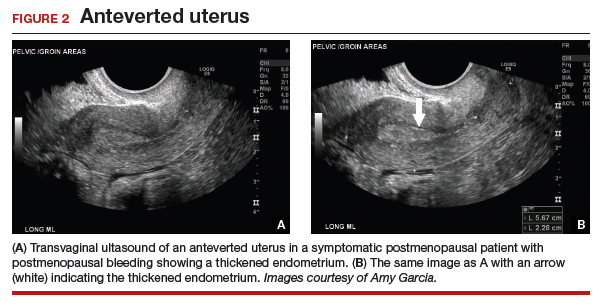
As mentioned, no general consensus exists for the evaluation of PMB; however, initial evaluation by TVUS is recommended. The American College of Obstetricians and Gynecologists (ACOG) concluded that when the endometrium measures ≤4 mm with TVUS, the likelihood that bleeding is secondary to endometrial carcinoma is less than 1% (negative predictive value 99%), and endometrial biopsy is not recommended.3 Endometrial sampling in this clinical scenario likely will result in insufficient tissue for evaluation, and it is reasonable to consider initial management for atrophy. A thickened endometrium on TVUS (>4 mm in a postmenopausal woman with PMB) warrants additional evaluation with endometrial sampling (FIGURE 2).
Clinical takeaway. A thickened endometrium on TVUS ≥4 mm in a postmenopausal woman with PMB warrants additional evaluation with endometrial sampling.
Endometrial biopsy
An endometrial biopsy is performed to determine whether endometrial cancer or precancer is present in women with AUB. ACOG recommends that endometrial biopsy be performed for women older than age 45. It is also appropriate in women younger than 45 years if they have risk factors for developing endometrial cancer, including unopposed estrogen exposure (obesity, ovulatory dysfunction), failed medical management of AUB, or persistence of AUB.4
Continue to: Endometrial biopsy has some...
Endometrial biopsy has some diagnostic shortcomings, however. In 2016 a systematic review and meta-analysis found that, in women with PMB, the specificity of endometrial biopsy was 98% to 100% (accurate diagnosis with a positive result). The sensitivity (ability to make an accurate diagnosis) of endometrial biopsy to identify endometrial pathology (carcinoma, atypical hyperplasia, and polyps) is lower than typically thought. These investigators found an endometrial biopsy failure rate of 11% (range, 1% to 53%) and rate of insufficient samples of 31% (range, 7% to 76%). In women with insufficient or failed samples, endometrial cancer or precancer was found in 7% (range, 0% to 18%).5 Therefore, a negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative. The results of endometrial biopsy are only an endpoint to the evaluation of PMB when atypical hyperplasia or endometrial cancer is identified.
Clinical takeaway. A negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative.
Hysteroscopy
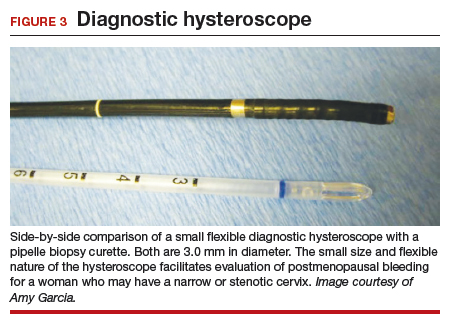
Hysteroscopy is the gold standard for evaluating the uterine cavity, diagnosing intrauterine pathology, and operative intervention for some causes of AUB. It also is easily performed in the office. This makes the hysteroscope an essential instrument for the gynecologist. Dr. Linda Bradley, a preeminent leader in hysteroscopic surgical education, has coined the phrase, “My hysteroscope is my stethoscope.”6 As gynecologists, we should be as adept at using a hysteroscope in the office as the cardiologist is at using a stethoscope.
It has been known for some time that hysteroscopy improves our diagnostic capabilities over blinded procedures such as endometrial biopsy and dilation and curettage (D&C). As far back as 1989, Dr. Frank Loffer reported the increased sensitivity (ability to make an accurate diagnosis) of hysteroscopy with directed biopsy over blinded D&C (98% vs 65%) in the evaluation of AUB.7 Evaluation of the endometrium with D&C is no longer recommended; yet today, few gynecologists perform hysteroscopic-directed biopsy for AUB evaluation instead of blinded tissue sampling despite the clinical superiority and in-office capabilities (FIGURE 3).
Continue to: Hysteroscopy and endometrial carcinoma...
Hysteroscopy and endometrial carcinoma
The most common type of gynecologic cancer in the United States is endometrial adenocarcinoma (type 1 endometrial cancer). There is some concern about the effect of hysteroscopy on endometrial cancer prognosis and the spread of cells to the peritoneum at the time of hysteroscopy. A large meta-analysis found that hysteroscopy performed in the presence of type 1 endometrial cancer statistically significantly increased the likelihood of positive intraperitoneal cytology; however, it did not alter the clinical outcome. It was recommended that hysteroscopy not be avoided for this reason and is helpful in the diagnosis of endometrial cancer, especially in the early stages of disease.8
For endometrial cancer type 2 (serous carcinoma, clear cell carcinoma, and carcinosarcoma), Chen and colleagues reported a statistically significant increase in positive peritoneal cytology for cancers evaluated by hysteroscopy versus D&C. The disease-specific survival for the hysteroscopy group was 60 months, compared with 71 months for the D&C group. While this finding was not statistically significant, it was clinically relevant, and the effect of hysteroscopy on prognosis with type 2 endometrial cancer is unclear.9
A common occurrence in the evaluation of postmenopausal bleeding (PMB) is an initial TVUS finding of an enlarged endometrium and an endometrial biopsy that is negative or reveals scant or insufficient tissue. Unfortunately, the diagnostic evaluation process often stops here, and a diagnosis for the PMB is never actually identified. Here are several clinical scenarios that highlight the need for hysteroscopy in the initial evaluation of PMB, especially when there is a discordance between transvaginal ultrasonography (TVUS) and endometrial biopsy findings.
Patient 1: Discordant TVUS and biopsy, with benign findings
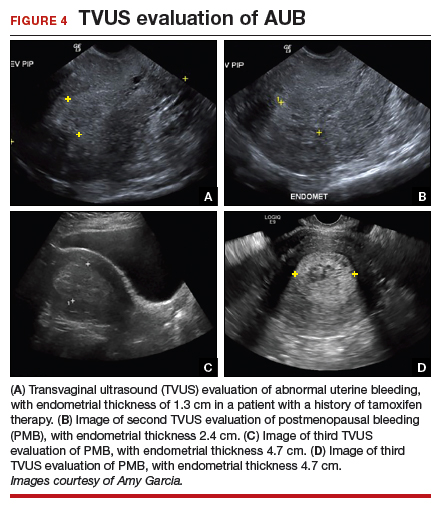
The patient is a 52-year-old woman who presented to her gynecologist reporting abnormal uterine bleeding (AUB). She has a history of breast cancer, and she completed tamoxifen treatment. Pelvic ultrasonography was performed; an enlarged endometrial stripe of 1.3 cm was found (FIGURE 4A). Endometrial biopsy was performed, showing adequate tissue but with a negative result. The patient is told that she is likely perimenopausal, which is the reason for her bleeding.
At the time of referral, the patient is evaluated with in-office hysteroscopy. Diagnosis of a 5 cm x 7 cm benign endometrial polyp is made. An uneventful hysteroscopic polypectomy is performed (VIDEO 2).
Video 2

This scenario illustrates the shortcoming of initial evaluation by not performing a hysteroscopy, especially in a woman with a thickened endometrium with previous tamoxifen therapy. Subsequent visits failed to correlate bleeding etiology with discordant TVUS and endometrial biopsy results with hysteroscopy, and no hysteroscopy was performed in the operating room at the time of D&C.
Patient 2: Discordant TVUS and biopsy, with premalignant findings
The patient is a 62-year-old woman who had incidental findings of a thickened endometrium on computed tomography scan of the pelvis. TVUS confirmed a thickened endometrium measuring 17 mm, and an endometrial biopsy showed scant tissue.
At the time of referral, a diagnostic hysteroscopy was performed in the office. Endometrial atrophy, a large benign appearing polyp, and focal abnormal appearing tissue were seen (FIGURE 5). A decision for polypectomy and directed biopsy was made. Histology findings confirmed benign polyp and atypical hyperplasia (VIDEO 3).
Video 3

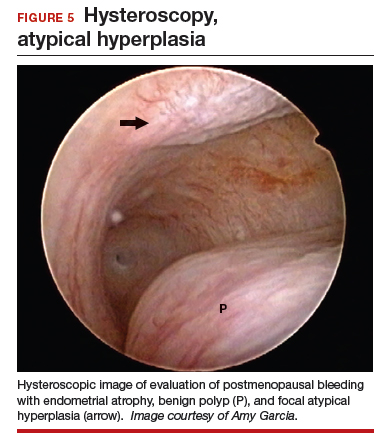
This scenario illustrates that while the patient was asymptomatic, there was discordance between the TVUS and endometrial biopsy. Hysteroscopy identified a benign endometrial polyp, which is common in asymptomatic postmenopausal patients with a thickened endometrium and endometrial biopsy showing scant tissue. However, addition of the diagnostic hysteroscopy identified focal precancerous tissue, removed under directed biopsy.
Patient 3: Discordant TVUS and biopsy, with malignant findings
The patient is a 68-year-old woman with PMB. TVUS showed a thickened endometrium measuring 14 mm. An endometrial biopsy was negative, showing scant tissue. No additional diagnostic evaluation or management was offered.
Video 4A

At the time of referral, the patient was evaluated with in-office diagnostic hysteroscopy, and the patient was found to have endometrial atrophy, benign appearing polyps, and focal abnormal tissue (FIGURE 6). A decision for polypectomy and directed biopsy was made. Histology confirmed benign polyps and grade 1 adenocarcinoma (VIDEOS 4A, 4B, 4C).
Video 4B

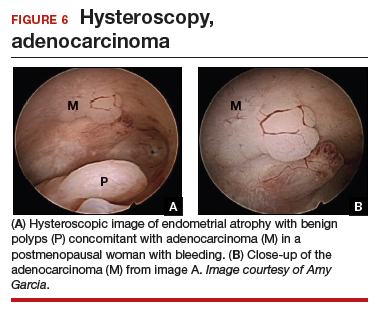
This scenario illustrates the possibility of having multiple endometrial pathologies present at the time of discordant TVUS and endometrial biopsy. Hysteroscopy plays a critical role in additional evaluation and diagnosis of endometrial carcinoma with directed biopsy, especially in a symptomatic woman with PMB.
Video 4C

Conclusion
Evaluation of PMB begins with a screening TVUS. Findings of an endometrium of ≤4 mm indicate a very low likelihood of the presence of endometrial cancer, and treatment for atrophy or changes to hormone replacement therapy regimen is reasonable first-line management; endometrial biopsy is not recommended. For patients with persistent PMB or thickened endometrium ≥4 mm on TVUS, biopsy sampling of the endometrium should be performed. If the endometrial biopsy does not explain the etiology of the PMB with atypical hyperplasia or endometrial cancer, then hysteroscopy should be performed to evaluate for focal endometrial disease and possible directed biopsy.
- ACOG Committee Opinion no. 734: the role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018;131:e124-e129.
- Goldstein SR. Appropriate evaluation of postmenopausal bleeding. Menopause. 2018;25:1476-1478.
- Bel S, Billard C, Godet J, et al. Risk of malignancy on suspicion of polyps in menopausal women. Eur J Obstet Gynecol Reprod Biol. 2017;216:138-142.
- Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- van Hanegem N, Prins MM, Bongers MY. The accuracy of endometrial sampling in women with postmenopausal bleeding: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2016;197:147-155.
- Embracing hysteroscopy. September 6, 2017. https://consultqd.clevelandclinic.org/embracing-hysteroscopy/. Accessed July 22, 2019.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73:16-20.
- Chang YN, Zhang Y, Wang LP, et al. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96:957-961.
- Chen J, Clark LH, Kong WM, et al. Does hysteroscopy worsen prognosis in women with type II endometrial carcinoma? PLoS One. 2017;12:e0174226.
Postmenopausal bleeding (PMB) is the presenting sign in most cases of endometrial carcinoma. Prompt evaluation of PMB can exclude, or diagnose, endometrial carcinoma.1 Although no general consensus exists for PMB evaluation, it involves endometrial assessment with transvaginal ultrasonography (TVUS) and subsequent endometrial biopsy when a thickened endometrium is found. When biopsy results reveal insufficient or scant tissue, further investigation into the etiology of PMB should include office hysteroscopy with possible directed biopsy. In this article I discuss the prevalence of PMB and steps for evaluation, providing clinical takeaways.

Postmenopausal bleeding: Its risk for cancer
Abnormal uterine bleeding (AUB) in a postmenopausal woman is of particular concern to the gynecologist and the patient because of the increased possibility of endometrial carcinoma in this age group. AUB is present in more than 90% of postmenopausal women with endometrial carcinoma, which leads to diagnosis in the early stages of the disease. Approximately 3% to 7% of postmenopausal women with PMB will have endometrial carcinoma.2 Most women with PMB, however, experience bleeding secondary to atrophic changes of the vagina or endometrium and not to endometrial carcinoma. (FIGURE 1, VIDEO 1) In addition, women who take gonadal steroids for hormone replacement therapy (HRT) may experience breakthrough bleeding that leads to initial investigation with TVUS.
Video 1

The risk of malignancy in polyps in postmenopausal women over the age of 59 who present with PMB is approximately 12%, and hysteroscopic resection should routinely be performed. For asymptomatic patients, the risk of a malignant lesion is low—approximately 3%—and for these women intervention should be assessed individually for the risks of carcinoma and benefits of hysteroscopic removal.3
Clinical takeaway. The high possibility of endometrial carcinoma in postmenopausal women warrants that any patient who is symptomatic with PMB should be presumed to have endometrial cancer until the diagnostic evaluation process proves she does not.

Evaluation of postmenopausal bleeding
Transvaginal ultrasound
As mentioned, no general consensus exists for the evaluation of PMB; however, initial evaluation by TVUS is recommended. The American College of Obstetricians and Gynecologists (ACOG) concluded that when the endometrium measures ≤4 mm with TVUS, the likelihood that bleeding is secondary to endometrial carcinoma is less than 1% (negative predictive value 99%), and endometrial biopsy is not recommended.3 Endometrial sampling in this clinical scenario likely will result in insufficient tissue for evaluation, and it is reasonable to consider initial management for atrophy. A thickened endometrium on TVUS (>4 mm in a postmenopausal woman with PMB) warrants additional evaluation with endometrial sampling (FIGURE 2).
Clinical takeaway. A thickened endometrium on TVUS ≥4 mm in a postmenopausal woman with PMB warrants additional evaluation with endometrial sampling.

Endometrial biopsy
An endometrial biopsy is performed to determine whether endometrial cancer or precancer is present in women with AUB. ACOG recommends that endometrial biopsy be performed for women older than age 45. It is also appropriate in women younger than 45 years if they have risk factors for developing endometrial cancer, including unopposed estrogen exposure (obesity, ovulatory dysfunction), failed medical management of AUB, or persistence of AUB.4
Continue to: Endometrial biopsy has some...
Endometrial biopsy has some diagnostic shortcomings, however. In 2016 a systematic review and meta-analysis found that, in women with PMB, the specificity of endometrial biopsy was 98% to 100% (accurate diagnosis with a positive result). The sensitivity (ability to make an accurate diagnosis) of endometrial biopsy to identify endometrial pathology (carcinoma, atypical hyperplasia, and polyps) is lower than typically thought. These investigators found an endometrial biopsy failure rate of 11% (range, 1% to 53%) and rate of insufficient samples of 31% (range, 7% to 76%). In women with insufficient or failed samples, endometrial cancer or precancer was found in 7% (range, 0% to 18%).5 Therefore, a negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative. The results of endometrial biopsy are only an endpoint to the evaluation of PMB when atypical hyperplasia or endometrial cancer is identified.
Clinical takeaway. A negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative.
Hysteroscopy
Hysteroscopy is the gold standard for evaluating the uterine cavity, diagnosing intrauterine pathology, and operative intervention for some causes of AUB. It also is easily performed in the office. This makes the hysteroscope an essential instrument for the gynecologist. Dr. Linda Bradley, a preeminent leader in hysteroscopic surgical education, has coined the phrase, “My hysteroscope is my stethoscope.”6 As gynecologists, we should be as adept at using a hysteroscope in the office as the cardiologist is at using a stethoscope.
It has been known for some time that hysteroscopy improves our diagnostic capabilities over blinded procedures such as endometrial biopsy and dilation and curettage (D&C). As far back as 1989, Dr. Frank Loffer reported the increased sensitivity (ability to make an accurate diagnosis) of hysteroscopy with directed biopsy over blinded D&C (98% vs 65%) in the evaluation of AUB.7 Evaluation of the endometrium with D&C is no longer recommended; yet today, few gynecologists perform hysteroscopic-directed biopsy for AUB evaluation instead of blinded tissue sampling despite the clinical superiority and in-office capabilities (FIGURE 3).

Continue to: Hysteroscopy and endometrial carcinoma...
Hysteroscopy and endometrial carcinoma
The most common type of gynecologic cancer in the United States is endometrial adenocarcinoma (type 1 endometrial cancer). There is some concern about the effect of hysteroscopy on endometrial cancer prognosis and the spread of cells to the peritoneum at the time of hysteroscopy. A large meta-analysis found that hysteroscopy performed in the presence of type 1 endometrial cancer statistically significantly increased the likelihood of positive intraperitoneal cytology; however, it did not alter the clinical outcome. It was recommended that hysteroscopy not be avoided for this reason and is helpful in the diagnosis of endometrial cancer, especially in the early stages of disease.8
For endometrial cancer type 2 (serous carcinoma, clear cell carcinoma, and carcinosarcoma), Chen and colleagues reported a statistically significant increase in positive peritoneal cytology for cancers evaluated by hysteroscopy versus D&C. The disease-specific survival for the hysteroscopy group was 60 months, compared with 71 months for the D&C group. While this finding was not statistically significant, it was clinically relevant, and the effect of hysteroscopy on prognosis with type 2 endometrial cancer is unclear.9
A common occurrence in the evaluation of postmenopausal bleeding (PMB) is an initial TVUS finding of an enlarged endometrium and an endometrial biopsy that is negative or reveals scant or insufficient tissue. Unfortunately, the diagnostic evaluation process often stops here, and a diagnosis for the PMB is never actually identified. Here are several clinical scenarios that highlight the need for hysteroscopy in the initial evaluation of PMB, especially when there is a discordance between transvaginal ultrasonography (TVUS) and endometrial biopsy findings.
Patient 1: Discordant TVUS and biopsy, with benign findings
The patient is a 52-year-old woman who presented to her gynecologist reporting abnormal uterine bleeding (AUB). She has a history of breast cancer, and she completed tamoxifen treatment. Pelvic ultrasonography was performed; an enlarged endometrial stripe of 1.3 cm was found (FIGURE 4A). Endometrial biopsy was performed, showing adequate tissue but with a negative result. The patient is told that she is likely perimenopausal, which is the reason for her bleeding.

At the time of referral, the patient is evaluated with in-office hysteroscopy. Diagnosis of a 5 cm x 7 cm benign endometrial polyp is made. An uneventful hysteroscopic polypectomy is performed (VIDEO 2).
Video 2

This scenario illustrates the shortcoming of initial evaluation by not performing a hysteroscopy, especially in a woman with a thickened endometrium with previous tamoxifen therapy. Subsequent visits failed to correlate bleeding etiology with discordant TVUS and endometrial biopsy results with hysteroscopy, and no hysteroscopy was performed in the operating room at the time of D&C.
Patient 2: Discordant TVUS and biopsy, with premalignant findings
The patient is a 62-year-old woman who had incidental findings of a thickened endometrium on computed tomography scan of the pelvis. TVUS confirmed a thickened endometrium measuring 17 mm, and an endometrial biopsy showed scant tissue.
At the time of referral, a diagnostic hysteroscopy was performed in the office. Endometrial atrophy, a large benign appearing polyp, and focal abnormal appearing tissue were seen (FIGURE 5). A decision for polypectomy and directed biopsy was made. Histology findings confirmed benign polyp and atypical hyperplasia (VIDEO 3).
Video 3


This scenario illustrates that while the patient was asymptomatic, there was discordance between the TVUS and endometrial biopsy. Hysteroscopy identified a benign endometrial polyp, which is common in asymptomatic postmenopausal patients with a thickened endometrium and endometrial biopsy showing scant tissue. However, addition of the diagnostic hysteroscopy identified focal precancerous tissue, removed under directed biopsy.
Patient 3: Discordant TVUS and biopsy, with malignant findings
The patient is a 68-year-old woman with PMB. TVUS showed a thickened endometrium measuring 14 mm. An endometrial biopsy was negative, showing scant tissue. No additional diagnostic evaluation or management was offered.
Video 4A

At the time of referral, the patient was evaluated with in-office diagnostic hysteroscopy, and the patient was found to have endometrial atrophy, benign appearing polyps, and focal abnormal tissue (FIGURE 6). A decision for polypectomy and directed biopsy was made. Histology confirmed benign polyps and grade 1 adenocarcinoma (VIDEOS 4A, 4B, 4C).
Video 4B


This scenario illustrates the possibility of having multiple endometrial pathologies present at the time of discordant TVUS and endometrial biopsy. Hysteroscopy plays a critical role in additional evaluation and diagnosis of endometrial carcinoma with directed biopsy, especially in a symptomatic woman with PMB.
Video 4C

Conclusion
Evaluation of PMB begins with a screening TVUS. Findings of an endometrium of ≤4 mm indicate a very low likelihood of the presence of endometrial cancer, and treatment for atrophy or changes to hormone replacement therapy regimen is reasonable first-line management; endometrial biopsy is not recommended. For patients with persistent PMB or thickened endometrium ≥4 mm on TVUS, biopsy sampling of the endometrium should be performed. If the endometrial biopsy does not explain the etiology of the PMB with atypical hyperplasia or endometrial cancer, then hysteroscopy should be performed to evaluate for focal endometrial disease and possible directed biopsy.
Postmenopausal bleeding (PMB) is the presenting sign in most cases of endometrial carcinoma. Prompt evaluation of PMB can exclude, or diagnose, endometrial carcinoma.1 Although no general consensus exists for PMB evaluation, it involves endometrial assessment with transvaginal ultrasonography (TVUS) and subsequent endometrial biopsy when a thickened endometrium is found. When biopsy results reveal insufficient or scant tissue, further investigation into the etiology of PMB should include office hysteroscopy with possible directed biopsy. In this article I discuss the prevalence of PMB and steps for evaluation, providing clinical takeaways.

Postmenopausal bleeding: Its risk for cancer
Abnormal uterine bleeding (AUB) in a postmenopausal woman is of particular concern to the gynecologist and the patient because of the increased possibility of endometrial carcinoma in this age group. AUB is present in more than 90% of postmenopausal women with endometrial carcinoma, which leads to diagnosis in the early stages of the disease. Approximately 3% to 7% of postmenopausal women with PMB will have endometrial carcinoma.2 Most women with PMB, however, experience bleeding secondary to atrophic changes of the vagina or endometrium and not to endometrial carcinoma. (FIGURE 1, VIDEO 1) In addition, women who take gonadal steroids for hormone replacement therapy (HRT) may experience breakthrough bleeding that leads to initial investigation with TVUS.
Video 1

The risk of malignancy in polyps in postmenopausal women over the age of 59 who present with PMB is approximately 12%, and hysteroscopic resection should routinely be performed. For asymptomatic patients, the risk of a malignant lesion is low—approximately 3%—and for these women intervention should be assessed individually for the risks of carcinoma and benefits of hysteroscopic removal.3
Clinical takeaway. The high possibility of endometrial carcinoma in postmenopausal women warrants that any patient who is symptomatic with PMB should be presumed to have endometrial cancer until the diagnostic evaluation process proves she does not.

Evaluation of postmenopausal bleeding
Transvaginal ultrasound
As mentioned, no general consensus exists for the evaluation of PMB; however, initial evaluation by TVUS is recommended. The American College of Obstetricians and Gynecologists (ACOG) concluded that when the endometrium measures ≤4 mm with TVUS, the likelihood that bleeding is secondary to endometrial carcinoma is less than 1% (negative predictive value 99%), and endometrial biopsy is not recommended.3 Endometrial sampling in this clinical scenario likely will result in insufficient tissue for evaluation, and it is reasonable to consider initial management for atrophy. A thickened endometrium on TVUS (>4 mm in a postmenopausal woman with PMB) warrants additional evaluation with endometrial sampling (FIGURE 2).
Clinical takeaway. A thickened endometrium on TVUS ≥4 mm in a postmenopausal woman with PMB warrants additional evaluation with endometrial sampling.

Endometrial biopsy
An endometrial biopsy is performed to determine whether endometrial cancer or precancer is present in women with AUB. ACOG recommends that endometrial biopsy be performed for women older than age 45. It is also appropriate in women younger than 45 years if they have risk factors for developing endometrial cancer, including unopposed estrogen exposure (obesity, ovulatory dysfunction), failed medical management of AUB, or persistence of AUB.4
Continue to: Endometrial biopsy has some...
Endometrial biopsy has some diagnostic shortcomings, however. In 2016 a systematic review and meta-analysis found that, in women with PMB, the specificity of endometrial biopsy was 98% to 100% (accurate diagnosis with a positive result). The sensitivity (ability to make an accurate diagnosis) of endometrial biopsy to identify endometrial pathology (carcinoma, atypical hyperplasia, and polyps) is lower than typically thought. These investigators found an endometrial biopsy failure rate of 11% (range, 1% to 53%) and rate of insufficient samples of 31% (range, 7% to 76%). In women with insufficient or failed samples, endometrial cancer or precancer was found in 7% (range, 0% to 18%).5 Therefore, a negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative. The results of endometrial biopsy are only an endpoint to the evaluation of PMB when atypical hyperplasia or endometrial cancer is identified.
Clinical takeaway. A negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative.
Hysteroscopy
Hysteroscopy is the gold standard for evaluating the uterine cavity, diagnosing intrauterine pathology, and operative intervention for some causes of AUB. It also is easily performed in the office. This makes the hysteroscope an essential instrument for the gynecologist. Dr. Linda Bradley, a preeminent leader in hysteroscopic surgical education, has coined the phrase, “My hysteroscope is my stethoscope.”6 As gynecologists, we should be as adept at using a hysteroscope in the office as the cardiologist is at using a stethoscope.
It has been known for some time that hysteroscopy improves our diagnostic capabilities over blinded procedures such as endometrial biopsy and dilation and curettage (D&C). As far back as 1989, Dr. Frank Loffer reported the increased sensitivity (ability to make an accurate diagnosis) of hysteroscopy with directed biopsy over blinded D&C (98% vs 65%) in the evaluation of AUB.7 Evaluation of the endometrium with D&C is no longer recommended; yet today, few gynecologists perform hysteroscopic-directed biopsy for AUB evaluation instead of blinded tissue sampling despite the clinical superiority and in-office capabilities (FIGURE 3).

Continue to: Hysteroscopy and endometrial carcinoma...
Hysteroscopy and endometrial carcinoma
The most common type of gynecologic cancer in the United States is endometrial adenocarcinoma (type 1 endometrial cancer). There is some concern about the effect of hysteroscopy on endometrial cancer prognosis and the spread of cells to the peritoneum at the time of hysteroscopy. A large meta-analysis found that hysteroscopy performed in the presence of type 1 endometrial cancer statistically significantly increased the likelihood of positive intraperitoneal cytology; however, it did not alter the clinical outcome. It was recommended that hysteroscopy not be avoided for this reason and is helpful in the diagnosis of endometrial cancer, especially in the early stages of disease.8
For endometrial cancer type 2 (serous carcinoma, clear cell carcinoma, and carcinosarcoma), Chen and colleagues reported a statistically significant increase in positive peritoneal cytology for cancers evaluated by hysteroscopy versus D&C. The disease-specific survival for the hysteroscopy group was 60 months, compared with 71 months for the D&C group. While this finding was not statistically significant, it was clinically relevant, and the effect of hysteroscopy on prognosis with type 2 endometrial cancer is unclear.9
A common occurrence in the evaluation of postmenopausal bleeding (PMB) is an initial TVUS finding of an enlarged endometrium and an endometrial biopsy that is negative or reveals scant or insufficient tissue. Unfortunately, the diagnostic evaluation process often stops here, and a diagnosis for the PMB is never actually identified. Here are several clinical scenarios that highlight the need for hysteroscopy in the initial evaluation of PMB, especially when there is a discordance between transvaginal ultrasonography (TVUS) and endometrial biopsy findings.
Patient 1: Discordant TVUS and biopsy, with benign findings
The patient is a 52-year-old woman who presented to her gynecologist reporting abnormal uterine bleeding (AUB). She has a history of breast cancer, and she completed tamoxifen treatment. Pelvic ultrasonography was performed; an enlarged endometrial stripe of 1.3 cm was found (FIGURE 4A). Endometrial biopsy was performed, showing adequate tissue but with a negative result. The patient is told that she is likely perimenopausal, which is the reason for her bleeding.

At the time of referral, the patient is evaluated with in-office hysteroscopy. Diagnosis of a 5 cm x 7 cm benign endometrial polyp is made. An uneventful hysteroscopic polypectomy is performed (VIDEO 2).
Video 2

This scenario illustrates the shortcoming of initial evaluation by not performing a hysteroscopy, especially in a woman with a thickened endometrium with previous tamoxifen therapy. Subsequent visits failed to correlate bleeding etiology with discordant TVUS and endometrial biopsy results with hysteroscopy, and no hysteroscopy was performed in the operating room at the time of D&C.
Patient 2: Discordant TVUS and biopsy, with premalignant findings
The patient is a 62-year-old woman who had incidental findings of a thickened endometrium on computed tomography scan of the pelvis. TVUS confirmed a thickened endometrium measuring 17 mm, and an endometrial biopsy showed scant tissue.
At the time of referral, a diagnostic hysteroscopy was performed in the office. Endometrial atrophy, a large benign appearing polyp, and focal abnormal appearing tissue were seen (FIGURE 5). A decision for polypectomy and directed biopsy was made. Histology findings confirmed benign polyp and atypical hyperplasia (VIDEO 3).
Video 3


This scenario illustrates that while the patient was asymptomatic, there was discordance between the TVUS and endometrial biopsy. Hysteroscopy identified a benign endometrial polyp, which is common in asymptomatic postmenopausal patients with a thickened endometrium and endometrial biopsy showing scant tissue. However, addition of the diagnostic hysteroscopy identified focal precancerous tissue, removed under directed biopsy.
Patient 3: Discordant TVUS and biopsy, with malignant findings
The patient is a 68-year-old woman with PMB. TVUS showed a thickened endometrium measuring 14 mm. An endometrial biopsy was negative, showing scant tissue. No additional diagnostic evaluation or management was offered.
Video 4A

At the time of referral, the patient was evaluated with in-office diagnostic hysteroscopy, and the patient was found to have endometrial atrophy, benign appearing polyps, and focal abnormal tissue (FIGURE 6). A decision for polypectomy and directed biopsy was made. Histology confirmed benign polyps and grade 1 adenocarcinoma (VIDEOS 4A, 4B, 4C).
Video 4B


This scenario illustrates the possibility of having multiple endometrial pathologies present at the time of discordant TVUS and endometrial biopsy. Hysteroscopy plays a critical role in additional evaluation and diagnosis of endometrial carcinoma with directed biopsy, especially in a symptomatic woman with PMB.
Video 4C

Conclusion
Evaluation of PMB begins with a screening TVUS. Findings of an endometrium of ≤4 mm indicate a very low likelihood of the presence of endometrial cancer, and treatment for atrophy or changes to hormone replacement therapy regimen is reasonable first-line management; endometrial biopsy is not recommended. For patients with persistent PMB or thickened endometrium ≥4 mm on TVUS, biopsy sampling of the endometrium should be performed. If the endometrial biopsy does not explain the etiology of the PMB with atypical hyperplasia or endometrial cancer, then hysteroscopy should be performed to evaluate for focal endometrial disease and possible directed biopsy.
- ACOG Committee Opinion no. 734: the role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018;131:e124-e129.
- Goldstein SR. Appropriate evaluation of postmenopausal bleeding. Menopause. 2018;25:1476-1478.
- Bel S, Billard C, Godet J, et al. Risk of malignancy on suspicion of polyps in menopausal women. Eur J Obstet Gynecol Reprod Biol. 2017;216:138-142.
- Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- van Hanegem N, Prins MM, Bongers MY. The accuracy of endometrial sampling in women with postmenopausal bleeding: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2016;197:147-155.
- Embracing hysteroscopy. September 6, 2017. https://consultqd.clevelandclinic.org/embracing-hysteroscopy/. Accessed July 22, 2019.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73:16-20.
- Chang YN, Zhang Y, Wang LP, et al. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96:957-961.
- Chen J, Clark LH, Kong WM, et al. Does hysteroscopy worsen prognosis in women with type II endometrial carcinoma? PLoS One. 2017;12:e0174226.
- ACOG Committee Opinion no. 734: the role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018;131:e124-e129.
- Goldstein SR. Appropriate evaluation of postmenopausal bleeding. Menopause. 2018;25:1476-1478.
- Bel S, Billard C, Godet J, et al. Risk of malignancy on suspicion of polyps in menopausal women. Eur J Obstet Gynecol Reprod Biol. 2017;216:138-142.
- Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- van Hanegem N, Prins MM, Bongers MY. The accuracy of endometrial sampling in women with postmenopausal bleeding: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2016;197:147-155.
- Embracing hysteroscopy. September 6, 2017. https://consultqd.clevelandclinic.org/embracing-hysteroscopy/. Accessed July 22, 2019.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73:16-20.
- Chang YN, Zhang Y, Wang LP, et al. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96:957-961.
- Chen J, Clark LH, Kong WM, et al. Does hysteroscopy worsen prognosis in women with type II endometrial carcinoma? PLoS One. 2017;12:e0174226.
The states of health care: Ranking the best and worst
and the last frontier is also last in the nation in health care.
In the 2019 edition of its annual ranking of state health care systems, personal finance website WalletHub named Minnesota the best less than a week after U.S. News & World Report called the Mayo Clinic the top hospital in the country.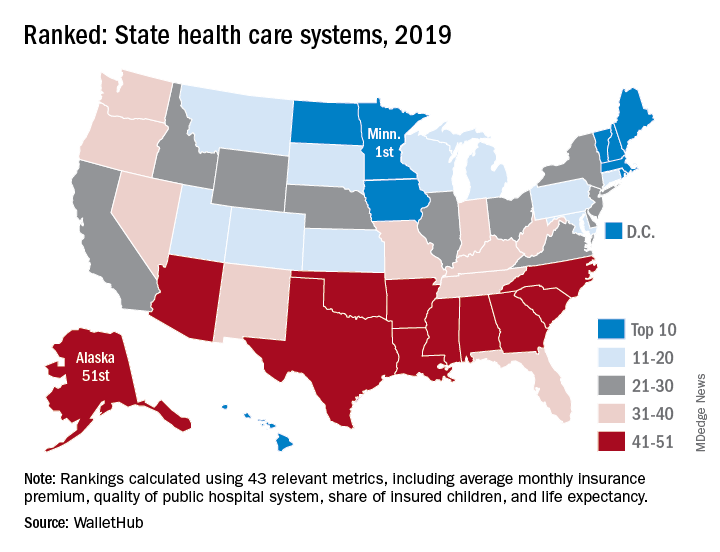
The WalletHub ranking, which included Washington, D.C., also put Alaska’s health care system at No. 51. Just one step above in the 50th spot was North Carolina, with Mississippi (49), South Carolina (48), and Arkansas (47) occupying the rest of the bottom five. Louisiana, which jumped from 51st in 2018 to 44th this year, was the only state to move out of the bottom five, WalletHub reported.
Joining Minnesota in the top five were Massachusetts (2), Rhode Island (3), D.C. (4), and Vermont (5), which was the home of last year’s best health care. North Dakota, which moved up 12 spots this year all the way to 9th, had the second-largest climb of any state: Montana catapulted 15 spots this year to finish 17th overall, WalletHub said.
For 2019, the company compared the states and D.C. using 43 measures of cost, accessibility, and outcome. The cost dimension’s six metrics included cost of a medical visit and share of adults with no doctor visits because of cost. The accessibility dimension consisted of 23 metrics, including hospital beds per capita, geriatricians per population aged 65 years and older, and Medicaid acceptance rate among physicians. The outcomes dimension included 14 metrics, among them share of patients readmitted to hospitals and share of nonimmunized children.
Minnesota was the only state to finish in the top 10 of all three dimensions. D.C. was ranked first in cost and Maine was the leader in access – as each was last year – and Massachusetts was ranked first in outcomes, the WalletHub data show.
The lowest-ranked states for each category in 2019 were the same as in 2018: Alaska (cost), Texas (access), and Mississippi (outcomes), according to the WalletHub analysis, which was based on data from 21 sources, including the Bureau of Labor Statistics, the Centers for Medicare & Medicaid Services, and the Kaiser Family Foundation.
and the last frontier is also last in the nation in health care.
In the 2019 edition of its annual ranking of state health care systems, personal finance website WalletHub named Minnesota the best less than a week after U.S. News & World Report called the Mayo Clinic the top hospital in the country.
The WalletHub ranking, which included Washington, D.C., also put Alaska’s health care system at No. 51. Just one step above in the 50th spot was North Carolina, with Mississippi (49), South Carolina (48), and Arkansas (47) occupying the rest of the bottom five. Louisiana, which jumped from 51st in 2018 to 44th this year, was the only state to move out of the bottom five, WalletHub reported.
Joining Minnesota in the top five were Massachusetts (2), Rhode Island (3), D.C. (4), and Vermont (5), which was the home of last year’s best health care. North Dakota, which moved up 12 spots this year all the way to 9th, had the second-largest climb of any state: Montana catapulted 15 spots this year to finish 17th overall, WalletHub said.
For 2019, the company compared the states and D.C. using 43 measures of cost, accessibility, and outcome. The cost dimension’s six metrics included cost of a medical visit and share of adults with no doctor visits because of cost. The accessibility dimension consisted of 23 metrics, including hospital beds per capita, geriatricians per population aged 65 years and older, and Medicaid acceptance rate among physicians. The outcomes dimension included 14 metrics, among them share of patients readmitted to hospitals and share of nonimmunized children.
Minnesota was the only state to finish in the top 10 of all three dimensions. D.C. was ranked first in cost and Maine was the leader in access – as each was last year – and Massachusetts was ranked first in outcomes, the WalletHub data show.
The lowest-ranked states for each category in 2019 were the same as in 2018: Alaska (cost), Texas (access), and Mississippi (outcomes), according to the WalletHub analysis, which was based on data from 21 sources, including the Bureau of Labor Statistics, the Centers for Medicare & Medicaid Services, and the Kaiser Family Foundation.
and the last frontier is also last in the nation in health care.
In the 2019 edition of its annual ranking of state health care systems, personal finance website WalletHub named Minnesota the best less than a week after U.S. News & World Report called the Mayo Clinic the top hospital in the country.
The WalletHub ranking, which included Washington, D.C., also put Alaska’s health care system at No. 51. Just one step above in the 50th spot was North Carolina, with Mississippi (49), South Carolina (48), and Arkansas (47) occupying the rest of the bottom five. Louisiana, which jumped from 51st in 2018 to 44th this year, was the only state to move out of the bottom five, WalletHub reported.
Joining Minnesota in the top five were Massachusetts (2), Rhode Island (3), D.C. (4), and Vermont (5), which was the home of last year’s best health care. North Dakota, which moved up 12 spots this year all the way to 9th, had the second-largest climb of any state: Montana catapulted 15 spots this year to finish 17th overall, WalletHub said.
For 2019, the company compared the states and D.C. using 43 measures of cost, accessibility, and outcome. The cost dimension’s six metrics included cost of a medical visit and share of adults with no doctor visits because of cost. The accessibility dimension consisted of 23 metrics, including hospital beds per capita, geriatricians per population aged 65 years and older, and Medicaid acceptance rate among physicians. The outcomes dimension included 14 metrics, among them share of patients readmitted to hospitals and share of nonimmunized children.
Minnesota was the only state to finish in the top 10 of all three dimensions. D.C. was ranked first in cost and Maine was the leader in access – as each was last year – and Massachusetts was ranked first in outcomes, the WalletHub data show.
The lowest-ranked states for each category in 2019 were the same as in 2018: Alaska (cost), Texas (access), and Mississippi (outcomes), according to the WalletHub analysis, which was based on data from 21 sources, including the Bureau of Labor Statistics, the Centers for Medicare & Medicaid Services, and the Kaiser Family Foundation.
Burnout gets personal for 68% of physicians
by real-time market insights technology firm InCrowd.
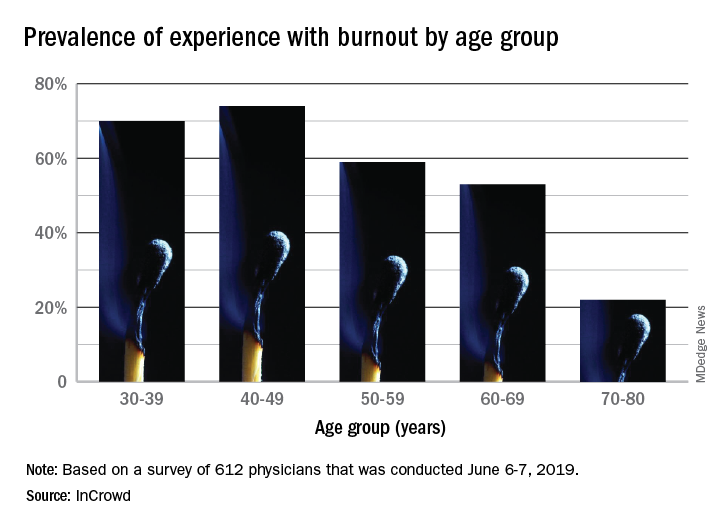
The overall prevalence of personal burnout experience was 68% among respondents, and another 28% said that they had not felt burned out but knew other physicians who had, InCrowd reported Aug. 6.
Specialty appeared to play a part given that 79% of primary care physicians reported experiencing burnout versus 57% of specialists. In response to an open-ended question about ability to manage burnout, the most common answer (23%) was that specialty played a large role, with “no role/all specialties affected equally” next at 13%. Equal proportions of respondents, however, said that specialists (24%) and primary care physicians (24%) were the group most affected, InCrowd said.
There was also a disconnect regarding age. When answering another open-ended question about the effects of age, 23% of those surveyed said that older physicians are more affected, compared with 9% who put the greater burden on younger physicians. The self-reporting of burnout, however, showed that younger physicians were much more likely to experience its effects than their older counterparts: 70% of those aged 30-39 years and 74% of those 40-49 versus 22% of those aged 70-80, InCrowd reported.
InCrowd noted that its results fall within the range of other recent surveys involving burnout in physicians that have shown levels that were lower, at 44% (MedScape, 2019) or 43.9% (American Academy of Family Physicians, 2019), and those that were higher, at 77.8% (The Physicians Foundation/Merritt Hawkins, 2018).
“The alarming persistence of physician burnout over the years and across multiple studies unfortunately demonstrates that we have not yet turned the tide on this problematic issue,” Diane Hayes, PhD, president of InCrowd, said in a statement accompanying the survey results. “Since we last looked at this in 2016, there really haven’t been any notable improvements. The healthcare industry would benefit from refining and expanding current initiatives to assure adequate staffing levels needed to deliver the quality care patients deserve.”
The survey was conducted June 6-7, 2019, and involved responses from 612 physicians (51% primary care providers, 49% specialists).
by real-time market insights technology firm InCrowd.

The overall prevalence of personal burnout experience was 68% among respondents, and another 28% said that they had not felt burned out but knew other physicians who had, InCrowd reported Aug. 6.
Specialty appeared to play a part given that 79% of primary care physicians reported experiencing burnout versus 57% of specialists. In response to an open-ended question about ability to manage burnout, the most common answer (23%) was that specialty played a large role, with “no role/all specialties affected equally” next at 13%. Equal proportions of respondents, however, said that specialists (24%) and primary care physicians (24%) were the group most affected, InCrowd said.
There was also a disconnect regarding age. When answering another open-ended question about the effects of age, 23% of those surveyed said that older physicians are more affected, compared with 9% who put the greater burden on younger physicians. The self-reporting of burnout, however, showed that younger physicians were much more likely to experience its effects than their older counterparts: 70% of those aged 30-39 years and 74% of those 40-49 versus 22% of those aged 70-80, InCrowd reported.
InCrowd noted that its results fall within the range of other recent surveys involving burnout in physicians that have shown levels that were lower, at 44% (MedScape, 2019) or 43.9% (American Academy of Family Physicians, 2019), and those that were higher, at 77.8% (The Physicians Foundation/Merritt Hawkins, 2018).
“The alarming persistence of physician burnout over the years and across multiple studies unfortunately demonstrates that we have not yet turned the tide on this problematic issue,” Diane Hayes, PhD, president of InCrowd, said in a statement accompanying the survey results. “Since we last looked at this in 2016, there really haven’t been any notable improvements. The healthcare industry would benefit from refining and expanding current initiatives to assure adequate staffing levels needed to deliver the quality care patients deserve.”
The survey was conducted June 6-7, 2019, and involved responses from 612 physicians (51% primary care providers, 49% specialists).
by real-time market insights technology firm InCrowd.

The overall prevalence of personal burnout experience was 68% among respondents, and another 28% said that they had not felt burned out but knew other physicians who had, InCrowd reported Aug. 6.
Specialty appeared to play a part given that 79% of primary care physicians reported experiencing burnout versus 57% of specialists. In response to an open-ended question about ability to manage burnout, the most common answer (23%) was that specialty played a large role, with “no role/all specialties affected equally” next at 13%. Equal proportions of respondents, however, said that specialists (24%) and primary care physicians (24%) were the group most affected, InCrowd said.
There was also a disconnect regarding age. When answering another open-ended question about the effects of age, 23% of those surveyed said that older physicians are more affected, compared with 9% who put the greater burden on younger physicians. The self-reporting of burnout, however, showed that younger physicians were much more likely to experience its effects than their older counterparts: 70% of those aged 30-39 years and 74% of those 40-49 versus 22% of those aged 70-80, InCrowd reported.
InCrowd noted that its results fall within the range of other recent surveys involving burnout in physicians that have shown levels that were lower, at 44% (MedScape, 2019) or 43.9% (American Academy of Family Physicians, 2019), and those that were higher, at 77.8% (The Physicians Foundation/Merritt Hawkins, 2018).
“The alarming persistence of physician burnout over the years and across multiple studies unfortunately demonstrates that we have not yet turned the tide on this problematic issue,” Diane Hayes, PhD, president of InCrowd, said in a statement accompanying the survey results. “Since we last looked at this in 2016, there really haven’t been any notable improvements. The healthcare industry would benefit from refining and expanding current initiatives to assure adequate staffing levels needed to deliver the quality care patients deserve.”
The survey was conducted June 6-7, 2019, and involved responses from 612 physicians (51% primary care providers, 49% specialists).
“Hidden” HIV in Cerebrospinal Fluid Cells May Lead to Cognitive Problems
Even when a standard HIV RNA test is negative, some patients may still have viral DNA in their cerebrospinal fluid (CSF)—and that could be a predictor of later memory and concentration problems, say researchers who conducted a National Institute of Health (NIH)-funded study of patients on long-term antiretroviral therapy (ART).
The 69 participants, enrolled in the AIDS Clinical Trials Group HIV Reservoirs Cohort Study, had infections controlled with ART for a median of 9 years. Calling it a “striking observation,” the researchers found nearly half of the patients had viral DNA in CSF cells, although the standard viral load tests of the cell-free CSF fluid were positive in only 4% of the patients.
Of the 30 patients with persistent HIV, 9 (30%) experienced neurocognitive difficulties in a 7-domain neuropsychological test battery. Among 35 participants with no detectable HIV DNA in CSF, 4 (11%) were clinically impaired.
The low rates of detectable HIV RNA in the cell-free CSF fraction and within CSF cell pellets suggest low levels of HIV transcription within cells and infrequent release into the extracellular space during systemically suppressive ART, the researchers say.
The brain is one of the first targets of the virus, and CNS manifestations are common. Neurocognitive impairment in HIV-positive patients is probably related to multiple factors, including HIV infection, age, neuroinflammation, and comorbid conditions, including substance abuse, the researchers say. There also may be a “legacy effect,” in which processes associated with long-term exposure to HIV before ART leads to irreversible neurologic injury and more extensive infection of CSF cells. Lack of an association with inflammatory biomarkers in this study suggested that current inflammation does not lead to present neurocognitive dysfunction, the researchers say, but does not rule out prior inflammation as the underlying cause of neuronal injury.
Still, given that brain tissue in living individuals is “inaccessible” (as the researchers put it), CSF offers a window into neuropathogenesis of HIV. Studies have found a range between 15% and 55% of participants develop an HIV-associated neurocognitive disorder (HAND). The new findings may support a role of persistent HIV-infected cells, but the researchers emphasize that the association does not confirm that HIV DNA causes HAND. However, they add, persistent HIV in “sanctuary sites” despite ART presents a barrier to curing the infection. Their study, they say, indicates that examination of CSF cells is important in assessing residual HIV in compartments during ART.
Even when a standard HIV RNA test is negative, some patients may still have viral DNA in their cerebrospinal fluid (CSF)—and that could be a predictor of later memory and concentration problems, say researchers who conducted a National Institute of Health (NIH)-funded study of patients on long-term antiretroviral therapy (ART).
The 69 participants, enrolled in the AIDS Clinical Trials Group HIV Reservoirs Cohort Study, had infections controlled with ART for a median of 9 years. Calling it a “striking observation,” the researchers found nearly half of the patients had viral DNA in CSF cells, although the standard viral load tests of the cell-free CSF fluid were positive in only 4% of the patients.
Of the 30 patients with persistent HIV, 9 (30%) experienced neurocognitive difficulties in a 7-domain neuropsychological test battery. Among 35 participants with no detectable HIV DNA in CSF, 4 (11%) were clinically impaired.
The low rates of detectable HIV RNA in the cell-free CSF fraction and within CSF cell pellets suggest low levels of HIV transcription within cells and infrequent release into the extracellular space during systemically suppressive ART, the researchers say.
The brain is one of the first targets of the virus, and CNS manifestations are common. Neurocognitive impairment in HIV-positive patients is probably related to multiple factors, including HIV infection, age, neuroinflammation, and comorbid conditions, including substance abuse, the researchers say. There also may be a “legacy effect,” in which processes associated with long-term exposure to HIV before ART leads to irreversible neurologic injury and more extensive infection of CSF cells. Lack of an association with inflammatory biomarkers in this study suggested that current inflammation does not lead to present neurocognitive dysfunction, the researchers say, but does not rule out prior inflammation as the underlying cause of neuronal injury.
Still, given that brain tissue in living individuals is “inaccessible” (as the researchers put it), CSF offers a window into neuropathogenesis of HIV. Studies have found a range between 15% and 55% of participants develop an HIV-associated neurocognitive disorder (HAND). The new findings may support a role of persistent HIV-infected cells, but the researchers emphasize that the association does not confirm that HIV DNA causes HAND. However, they add, persistent HIV in “sanctuary sites” despite ART presents a barrier to curing the infection. Their study, they say, indicates that examination of CSF cells is important in assessing residual HIV in compartments during ART.
Even when a standard HIV RNA test is negative, some patients may still have viral DNA in their cerebrospinal fluid (CSF)—and that could be a predictor of later memory and concentration problems, say researchers who conducted a National Institute of Health (NIH)-funded study of patients on long-term antiretroviral therapy (ART).
The 69 participants, enrolled in the AIDS Clinical Trials Group HIV Reservoirs Cohort Study, had infections controlled with ART for a median of 9 years. Calling it a “striking observation,” the researchers found nearly half of the patients had viral DNA in CSF cells, although the standard viral load tests of the cell-free CSF fluid were positive in only 4% of the patients.
Of the 30 patients with persistent HIV, 9 (30%) experienced neurocognitive difficulties in a 7-domain neuropsychological test battery. Among 35 participants with no detectable HIV DNA in CSF, 4 (11%) were clinically impaired.
The low rates of detectable HIV RNA in the cell-free CSF fraction and within CSF cell pellets suggest low levels of HIV transcription within cells and infrequent release into the extracellular space during systemically suppressive ART, the researchers say.
The brain is one of the first targets of the virus, and CNS manifestations are common. Neurocognitive impairment in HIV-positive patients is probably related to multiple factors, including HIV infection, age, neuroinflammation, and comorbid conditions, including substance abuse, the researchers say. There also may be a “legacy effect,” in which processes associated with long-term exposure to HIV before ART leads to irreversible neurologic injury and more extensive infection of CSF cells. Lack of an association with inflammatory biomarkers in this study suggested that current inflammation does not lead to present neurocognitive dysfunction, the researchers say, but does not rule out prior inflammation as the underlying cause of neuronal injury.
Still, given that brain tissue in living individuals is “inaccessible” (as the researchers put it), CSF offers a window into neuropathogenesis of HIV. Studies have found a range between 15% and 55% of participants develop an HIV-associated neurocognitive disorder (HAND). The new findings may support a role of persistent HIV-infected cells, but the researchers emphasize that the association does not confirm that HIV DNA causes HAND. However, they add, persistent HIV in “sanctuary sites” despite ART presents a barrier to curing the infection. Their study, they say, indicates that examination of CSF cells is important in assessing residual HIV in compartments during ART.
Half of psychiatry, psychology trial abstracts contain spin
About half of papers in a sample of psychiatry and psychology journals have evidence of “spin” in the abstract, according to an analysis published in BMJ Evidence-Based Medicine.
Samuel Jellison, a third-year medical student at Oklahoma State University, Tulsa, and coauthors wrote that the results of randomized, controlled trials should be reported objectively because of their importance for psychiatry clinical practice. However, given that researchers were allowed more latitude in the abstract of a paper to highlight certain results or conclusions, the abstract might not accurately represent the findings of the study.
To evaluate this, the authors investigated the use of spin in abstracts, which they defined as “‘use of specific reporting strategies, from whatever motive, to highlight that the experimental treatment is beneficial, despite a statistically nonsignificant difference for the primary outcome, or to distract the reader from statistically nonsignificant results.”
They analyzed 116 randomized, controlled trials of interventions where there was a nonsignificant primary endpoint and found that 56% of those contained spin in the abstract. Spin was evident in 2% of publication titles, 21% of abstract results sections, and 49.1% of abstract conclusions sections.
Spin was more common in trials of pharmacologic treatments, compared with nonpharmacologic treatments. However, the study did not find a higher level of spin in industry-funded studies, and in fact, spin was more common in publicly funded research.
The most common form of spin was focusing on a statistically significant primary or secondary endpoint while omitting one or more nonsignificant primary endpoints. Other spin strategies included claiming noninferiority or equivalence for a statistically nonsignificant endpoint; using phrases such as “trend towards significance”; or focusing on statistically significant subgroup analyses, such as per protocol instead of intention to treat.
The authors observed that most physicians only read article abstracts, and up to one-quarter of editorial decisions are based on the abstract alone.
“Adding spin to the abstract of an article may mislead physicians who are attempting to draw conclusions about a treatment for patients,” they wrote, while calling for efforts to discourage spin in abstracts.
In an interview, Paul S. Nestadt, MD, said the findings were not surprising.
“The proportion [56%] of psychiatry and psychology abstracts which Jellison et al. found to contain spin is similar to that found in broader studies of all biomedical literature in previous reviews,” said Dr. Nestadt, assistant professor in the department of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore. “It is disheartening that attempts to mislead have become ‘standard of care’ in medical literature, but it is a predictable outcome of increasing competition for shrinking grant funding, awarded partly on the basis of publication history in leading journals that maintain clear publication biases toward positive results.
“As the authors point out, we all share a responsibility to call out spin when we see it, whether in our role as reviewer, editor, coauthor, or as the writers themselves.”
Neither Mr. Jellison nor his coauthors reported funding or conflicts of interest.
SOURCE: Jellison S et al. BMJ Evid Based Med. 2019 Aug 5. doi: 10.1136/bmjebm-2019-111176.
About half of papers in a sample of psychiatry and psychology journals have evidence of “spin” in the abstract, according to an analysis published in BMJ Evidence-Based Medicine.
Samuel Jellison, a third-year medical student at Oklahoma State University, Tulsa, and coauthors wrote that the results of randomized, controlled trials should be reported objectively because of their importance for psychiatry clinical practice. However, given that researchers were allowed more latitude in the abstract of a paper to highlight certain results or conclusions, the abstract might not accurately represent the findings of the study.
To evaluate this, the authors investigated the use of spin in abstracts, which they defined as “‘use of specific reporting strategies, from whatever motive, to highlight that the experimental treatment is beneficial, despite a statistically nonsignificant difference for the primary outcome, or to distract the reader from statistically nonsignificant results.”
They analyzed 116 randomized, controlled trials of interventions where there was a nonsignificant primary endpoint and found that 56% of those contained spin in the abstract. Spin was evident in 2% of publication titles, 21% of abstract results sections, and 49.1% of abstract conclusions sections.
Spin was more common in trials of pharmacologic treatments, compared with nonpharmacologic treatments. However, the study did not find a higher level of spin in industry-funded studies, and in fact, spin was more common in publicly funded research.
The most common form of spin was focusing on a statistically significant primary or secondary endpoint while omitting one or more nonsignificant primary endpoints. Other spin strategies included claiming noninferiority or equivalence for a statistically nonsignificant endpoint; using phrases such as “trend towards significance”; or focusing on statistically significant subgroup analyses, such as per protocol instead of intention to treat.
The authors observed that most physicians only read article abstracts, and up to one-quarter of editorial decisions are based on the abstract alone.
“Adding spin to the abstract of an article may mislead physicians who are attempting to draw conclusions about a treatment for patients,” they wrote, while calling for efforts to discourage spin in abstracts.
In an interview, Paul S. Nestadt, MD, said the findings were not surprising.
“The proportion [56%] of psychiatry and psychology abstracts which Jellison et al. found to contain spin is similar to that found in broader studies of all biomedical literature in previous reviews,” said Dr. Nestadt, assistant professor in the department of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore. “It is disheartening that attempts to mislead have become ‘standard of care’ in medical literature, but it is a predictable outcome of increasing competition for shrinking grant funding, awarded partly on the basis of publication history in leading journals that maintain clear publication biases toward positive results.
“As the authors point out, we all share a responsibility to call out spin when we see it, whether in our role as reviewer, editor, coauthor, or as the writers themselves.”
Neither Mr. Jellison nor his coauthors reported funding or conflicts of interest.
SOURCE: Jellison S et al. BMJ Evid Based Med. 2019 Aug 5. doi: 10.1136/bmjebm-2019-111176.
About half of papers in a sample of psychiatry and psychology journals have evidence of “spin” in the abstract, according to an analysis published in BMJ Evidence-Based Medicine.
Samuel Jellison, a third-year medical student at Oklahoma State University, Tulsa, and coauthors wrote that the results of randomized, controlled trials should be reported objectively because of their importance for psychiatry clinical practice. However, given that researchers were allowed more latitude in the abstract of a paper to highlight certain results or conclusions, the abstract might not accurately represent the findings of the study.
To evaluate this, the authors investigated the use of spin in abstracts, which they defined as “‘use of specific reporting strategies, from whatever motive, to highlight that the experimental treatment is beneficial, despite a statistically nonsignificant difference for the primary outcome, or to distract the reader from statistically nonsignificant results.”
They analyzed 116 randomized, controlled trials of interventions where there was a nonsignificant primary endpoint and found that 56% of those contained spin in the abstract. Spin was evident in 2% of publication titles, 21% of abstract results sections, and 49.1% of abstract conclusions sections.
Spin was more common in trials of pharmacologic treatments, compared with nonpharmacologic treatments. However, the study did not find a higher level of spin in industry-funded studies, and in fact, spin was more common in publicly funded research.
The most common form of spin was focusing on a statistically significant primary or secondary endpoint while omitting one or more nonsignificant primary endpoints. Other spin strategies included claiming noninferiority or equivalence for a statistically nonsignificant endpoint; using phrases such as “trend towards significance”; or focusing on statistically significant subgroup analyses, such as per protocol instead of intention to treat.
The authors observed that most physicians only read article abstracts, and up to one-quarter of editorial decisions are based on the abstract alone.
“Adding spin to the abstract of an article may mislead physicians who are attempting to draw conclusions about a treatment for patients,” they wrote, while calling for efforts to discourage spin in abstracts.
In an interview, Paul S. Nestadt, MD, said the findings were not surprising.
“The proportion [56%] of psychiatry and psychology abstracts which Jellison et al. found to contain spin is similar to that found in broader studies of all biomedical literature in previous reviews,” said Dr. Nestadt, assistant professor in the department of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore. “It is disheartening that attempts to mislead have become ‘standard of care’ in medical literature, but it is a predictable outcome of increasing competition for shrinking grant funding, awarded partly on the basis of publication history in leading journals that maintain clear publication biases toward positive results.
“As the authors point out, we all share a responsibility to call out spin when we see it, whether in our role as reviewer, editor, coauthor, or as the writers themselves.”
Neither Mr. Jellison nor his coauthors reported funding or conflicts of interest.
SOURCE: Jellison S et al. BMJ Evid Based Med. 2019 Aug 5. doi: 10.1136/bmjebm-2019-111176.
FROM BMJ EVIDENCE-BASED MEDICINE
Key clinical point. Spin is common in abstracts of psychiatry and psychology clinical trial papers.
Major finding: Spin was found in 56% of abstracts for psychiatry and psychology trials with a nonsignificant primary outcome.
Study details: An analysis of 116 randomized, controlled trials of interventions where there was a nonsignificant primary outcome.
Disclosures: No funding or conflicts of interest were reported.
Source: Jellison S et al. BMJ Evid Based Med. 2019 Aug 5. doi: 10.1136/bmjebm-2019-111176.
Lynch syndrome screening shows low efficiency in elderly
The need for universal screening for Lynch syndrome in elderly patients with newly diagnosed colorectal cancer (CRC) has been questioned, according to results from a retrospective cohort study.
In addition, discontinuing reflex CRC screening for Lynch syndrome in patients over age 80 years could be feasible because of very low efficiency.
“The universal strategy advocates screening all patients with newly diagnosed CRC for Lynch syndrome and has been shown to be the most sensitive method,” wrote Dan Li, MD, of Kaiser Permanente Northern California, Santa Clara, and colleagues. The findings were published in Annals of Internal Medicine.
The researchers studied 3,891 patients with newly diagnosed CRC who were screened for Lynch syndrome from 2011 to 2016. Data were collected from a population-based screening program at Kaiser Permanente Northern California.
“The system provides comprehensive medical care for more than 4 million members across 21 medical centers covering urban, suburban, and semirural areas,” Dr. Li and his colleagues wrote.
To compare universal and age-restricted screening, the team obtained surgical samples of all newly diagnosed CRC tumors and tested them for reflex mismatch repair protein expression using immunohistochemistry.
Subsequently, the age-restricted screening groups were divided into several age categories, ranging from age 50 to 85 years.
The diagnostic yield, defined as the “percentage of patients with pathogenic reflex mismatch repair gene variants among all patients with CRC screened with immunohistochemistry,” was measured and compared with the universal screening technique.
“We calculated the number of patients with CRC who needed to be screened in each age group to identify one case of Lynch syndrome by dividing the number of patients screened in each age group by the number of Lynch syndrome cases diagnosed in that group,” they explained.
After analysis, the researchers detected a total of 63 cases of Lynch syndrome (diagnostic yield, 1.62%) with universal screening, among which 5 (7.9%) were over age 70 years and 1 (1.6%) was over age 80 years.
When patients with CRC who were universally screened were used as the denominator, 58 cases (diagnostic yield, 1.49%) were detected in those with CRC diagnosed at or prior to age 70 years.
In addition, in patients diagnosed at or before age 75 and 80 years, 60 and 62 cases of Lynch syndrome (diagnostic yield, 1.54% and 1.59%) were detected, respectively.
“The incremental diagnostic yield decreased substantially after age 70 to 75 years,” they wrote.
With these findings, Dr. Li and his colleagues suggested that cessation of screening for Lynch syndrome post age 80 years may be acceptable, especially in resource-limited environments.
“Using age as the primary criterion is a simple method of selecting patients for Lynch syndrome screening in clinical practice,” they added.
In accordance with previous studies, a major reduction in Lynch syndrome incidence has been noted among elderly populations.
There remains a need for additional studies exploring the effects of diagnosing Lynch syndrome in elderly patients on family members.
The researchers acknowledged a key limitation of the study was that patients who did not finish germline analysis but were eligible for it were excluded from certain measurements. To reduce potential bias, the team conducted a sensitivity analysis, and the findings were negligible with respect to main results.
“Given the geographic variation in the reported prevalence of Lynch syndrome, the diagnostic efficiency of Lynch syndrome screening among elderly populations should be further investigated in other populations,” they concluded.
The study was funded by Kaiser Permanente Northern California. The authors reported financial affiliations with Bayer, Clinical Genomics, Covidien, Exact Sciences, Motus GI, Quorum, Universal DX, and the National Cancer Institute.
SOURCE: Li D et al. Ann Intern Med. 2019 Jun 11. doi: 10.7326/M18-3316.
The need for universal screening for Lynch syndrome in elderly patients with newly diagnosed colorectal cancer (CRC) has been questioned, according to results from a retrospective cohort study.
In addition, discontinuing reflex CRC screening for Lynch syndrome in patients over age 80 years could be feasible because of very low efficiency.
“The universal strategy advocates screening all patients with newly diagnosed CRC for Lynch syndrome and has been shown to be the most sensitive method,” wrote Dan Li, MD, of Kaiser Permanente Northern California, Santa Clara, and colleagues. The findings were published in Annals of Internal Medicine.
The researchers studied 3,891 patients with newly diagnosed CRC who were screened for Lynch syndrome from 2011 to 2016. Data were collected from a population-based screening program at Kaiser Permanente Northern California.
“The system provides comprehensive medical care for more than 4 million members across 21 medical centers covering urban, suburban, and semirural areas,” Dr. Li and his colleagues wrote.
To compare universal and age-restricted screening, the team obtained surgical samples of all newly diagnosed CRC tumors and tested them for reflex mismatch repair protein expression using immunohistochemistry.
Subsequently, the age-restricted screening groups were divided into several age categories, ranging from age 50 to 85 years.
The diagnostic yield, defined as the “percentage of patients with pathogenic reflex mismatch repair gene variants among all patients with CRC screened with immunohistochemistry,” was measured and compared with the universal screening technique.
“We calculated the number of patients with CRC who needed to be screened in each age group to identify one case of Lynch syndrome by dividing the number of patients screened in each age group by the number of Lynch syndrome cases diagnosed in that group,” they explained.
After analysis, the researchers detected a total of 63 cases of Lynch syndrome (diagnostic yield, 1.62%) with universal screening, among which 5 (7.9%) were over age 70 years and 1 (1.6%) was over age 80 years.
When patients with CRC who were universally screened were used as the denominator, 58 cases (diagnostic yield, 1.49%) were detected in those with CRC diagnosed at or prior to age 70 years.
In addition, in patients diagnosed at or before age 75 and 80 years, 60 and 62 cases of Lynch syndrome (diagnostic yield, 1.54% and 1.59%) were detected, respectively.
“The incremental diagnostic yield decreased substantially after age 70 to 75 years,” they wrote.
With these findings, Dr. Li and his colleagues suggested that cessation of screening for Lynch syndrome post age 80 years may be acceptable, especially in resource-limited environments.
“Using age as the primary criterion is a simple method of selecting patients for Lynch syndrome screening in clinical practice,” they added.
In accordance with previous studies, a major reduction in Lynch syndrome incidence has been noted among elderly populations.
There remains a need for additional studies exploring the effects of diagnosing Lynch syndrome in elderly patients on family members.
The researchers acknowledged a key limitation of the study was that patients who did not finish germline analysis but were eligible for it were excluded from certain measurements. To reduce potential bias, the team conducted a sensitivity analysis, and the findings were negligible with respect to main results.
“Given the geographic variation in the reported prevalence of Lynch syndrome, the diagnostic efficiency of Lynch syndrome screening among elderly populations should be further investigated in other populations,” they concluded.
The study was funded by Kaiser Permanente Northern California. The authors reported financial affiliations with Bayer, Clinical Genomics, Covidien, Exact Sciences, Motus GI, Quorum, Universal DX, and the National Cancer Institute.
SOURCE: Li D et al. Ann Intern Med. 2019 Jun 11. doi: 10.7326/M18-3316.
The need for universal screening for Lynch syndrome in elderly patients with newly diagnosed colorectal cancer (CRC) has been questioned, according to results from a retrospective cohort study.
In addition, discontinuing reflex CRC screening for Lynch syndrome in patients over age 80 years could be feasible because of very low efficiency.
“The universal strategy advocates screening all patients with newly diagnosed CRC for Lynch syndrome and has been shown to be the most sensitive method,” wrote Dan Li, MD, of Kaiser Permanente Northern California, Santa Clara, and colleagues. The findings were published in Annals of Internal Medicine.
The researchers studied 3,891 patients with newly diagnosed CRC who were screened for Lynch syndrome from 2011 to 2016. Data were collected from a population-based screening program at Kaiser Permanente Northern California.
“The system provides comprehensive medical care for more than 4 million members across 21 medical centers covering urban, suburban, and semirural areas,” Dr. Li and his colleagues wrote.
To compare universal and age-restricted screening, the team obtained surgical samples of all newly diagnosed CRC tumors and tested them for reflex mismatch repair protein expression using immunohistochemistry.
Subsequently, the age-restricted screening groups were divided into several age categories, ranging from age 50 to 85 years.
The diagnostic yield, defined as the “percentage of patients with pathogenic reflex mismatch repair gene variants among all patients with CRC screened with immunohistochemistry,” was measured and compared with the universal screening technique.
“We calculated the number of patients with CRC who needed to be screened in each age group to identify one case of Lynch syndrome by dividing the number of patients screened in each age group by the number of Lynch syndrome cases diagnosed in that group,” they explained.
After analysis, the researchers detected a total of 63 cases of Lynch syndrome (diagnostic yield, 1.62%) with universal screening, among which 5 (7.9%) were over age 70 years and 1 (1.6%) was over age 80 years.
When patients with CRC who were universally screened were used as the denominator, 58 cases (diagnostic yield, 1.49%) were detected in those with CRC diagnosed at or prior to age 70 years.
In addition, in patients diagnosed at or before age 75 and 80 years, 60 and 62 cases of Lynch syndrome (diagnostic yield, 1.54% and 1.59%) were detected, respectively.
“The incremental diagnostic yield decreased substantially after age 70 to 75 years,” they wrote.
With these findings, Dr. Li and his colleagues suggested that cessation of screening for Lynch syndrome post age 80 years may be acceptable, especially in resource-limited environments.
“Using age as the primary criterion is a simple method of selecting patients for Lynch syndrome screening in clinical practice,” they added.
In accordance with previous studies, a major reduction in Lynch syndrome incidence has been noted among elderly populations.
There remains a need for additional studies exploring the effects of diagnosing Lynch syndrome in elderly patients on family members.
The researchers acknowledged a key limitation of the study was that patients who did not finish germline analysis but were eligible for it were excluded from certain measurements. To reduce potential bias, the team conducted a sensitivity analysis, and the findings were negligible with respect to main results.
“Given the geographic variation in the reported prevalence of Lynch syndrome, the diagnostic efficiency of Lynch syndrome screening among elderly populations should be further investigated in other populations,” they concluded.
The study was funded by Kaiser Permanente Northern California. The authors reported financial affiliations with Bayer, Clinical Genomics, Covidien, Exact Sciences, Motus GI, Quorum, Universal DX, and the National Cancer Institute.
SOURCE: Li D et al. Ann Intern Med. 2019 Jun 11. doi: 10.7326/M18-3316.
FROM ANNALS OF INTERNAL MEDICINE
CVD risk after breast cancer: Adipose distribution trumps BMI
When it comes to cardiovascular disease (CVD) risk after a breast cancer diagnosis, it’s not so much a matter of the amount of body fat carried but rather where it is located, results of a retrospective cohort study of nearly 3,000 survivors suggest.
“It is well known that higher body mass index (BMI) is associated with CVD mortality in the general population. However, BMI is not always an accurate proxy for individual-level adiposity and does not describe adipose tissue distribution,” note lead investigator Elizabeth M. Cespedes Feliciano, ScD, of Kaiser Permanente Northern California, Oakland, Calif., and coinvestigators.
The investigators studied 2,943 survivors of nonmetastatic breast cancer having a mean age of 56 years who were initially CVD free, using clinically acquired CT scans obtained near diagnosis to measure adiposity in three compartments: visceral, subcutaneous, and intramuscular.
The cohort experienced 328 CVD events (nonfatal stroke, myocardial infarction, heart failure, or CVD death) during a median follow-up of 6 years, the investigators reported in Journal of Clinical Oncology. The 10-year cumulative incidence was 15%.
In analyses that were adjusted for potential confounders and took into account competing risks, survivors’ CVD risk increased significantly with each standard deviation (SD) increase in visceral adiposity (hazard ratio, 1.15; 95% confidence interval, 1.03-1.29) and each SD increase in intramuscular adiposity (HR, 1.21; 95% CI, 1.06-1.37). The association for subcutaneous adiposity was not significant.
Findings were similar across all BMI categories. Of particular note, among survivors having a normal BMI, risk of CVD events increased by 70% with each SD greater visceral adiposity (HR, 1.70; 95% CI, 1.10-2.62).
Risk also rose with BMI exceeding the normal range, but the association became significant only in survivors with a BMI placing them in obesity class II (35 kg/m2 or greater) (HR, 1.70; 95% CI, 1.20-2.42).
“Although it has been assumed that excess adiposity increases the risk of CVD after breast cancer, this first-of-its-kind study demonstrates that adipose tissue distribution best identifies patients with breast cancer with higher CVD risk after diagnosis, including those with normal BMI,” Dr. Cespedes Feliciano and coinvestigators note.
“Software is now available that automatically measures body composition from clinically acquired CT scans, facilitating clinical integration,” they note. “Measures of adipose tissue distribution from CT or anthropometry (e.g., waist circumference) may help identify individuals with high CVD risk and tailor prevention efforts to patients’ body composition.”
Dr. Cespedes Feliciano disclosed no relevant conflicts of interest. The study did not receive any specific funding.
SOURCE: Cespedes Feliciano EM et al. J Clin Oncol. 2019 Aug 1. doi: 10.1200/JCO.19.00286.
When it comes to cardiovascular disease (CVD) risk after a breast cancer diagnosis, it’s not so much a matter of the amount of body fat carried but rather where it is located, results of a retrospective cohort study of nearly 3,000 survivors suggest.
“It is well known that higher body mass index (BMI) is associated with CVD mortality in the general population. However, BMI is not always an accurate proxy for individual-level adiposity and does not describe adipose tissue distribution,” note lead investigator Elizabeth M. Cespedes Feliciano, ScD, of Kaiser Permanente Northern California, Oakland, Calif., and coinvestigators.
The investigators studied 2,943 survivors of nonmetastatic breast cancer having a mean age of 56 years who were initially CVD free, using clinically acquired CT scans obtained near diagnosis to measure adiposity in three compartments: visceral, subcutaneous, and intramuscular.
The cohort experienced 328 CVD events (nonfatal stroke, myocardial infarction, heart failure, or CVD death) during a median follow-up of 6 years, the investigators reported in Journal of Clinical Oncology. The 10-year cumulative incidence was 15%.
In analyses that were adjusted for potential confounders and took into account competing risks, survivors’ CVD risk increased significantly with each standard deviation (SD) increase in visceral adiposity (hazard ratio, 1.15; 95% confidence interval, 1.03-1.29) and each SD increase in intramuscular adiposity (HR, 1.21; 95% CI, 1.06-1.37). The association for subcutaneous adiposity was not significant.
Findings were similar across all BMI categories. Of particular note, among survivors having a normal BMI, risk of CVD events increased by 70% with each SD greater visceral adiposity (HR, 1.70; 95% CI, 1.10-2.62).
Risk also rose with BMI exceeding the normal range, but the association became significant only in survivors with a BMI placing them in obesity class II (35 kg/m2 or greater) (HR, 1.70; 95% CI, 1.20-2.42).
“Although it has been assumed that excess adiposity increases the risk of CVD after breast cancer, this first-of-its-kind study demonstrates that adipose tissue distribution best identifies patients with breast cancer with higher CVD risk after diagnosis, including those with normal BMI,” Dr. Cespedes Feliciano and coinvestigators note.
“Software is now available that automatically measures body composition from clinically acquired CT scans, facilitating clinical integration,” they note. “Measures of adipose tissue distribution from CT or anthropometry (e.g., waist circumference) may help identify individuals with high CVD risk and tailor prevention efforts to patients’ body composition.”
Dr. Cespedes Feliciano disclosed no relevant conflicts of interest. The study did not receive any specific funding.
SOURCE: Cespedes Feliciano EM et al. J Clin Oncol. 2019 Aug 1. doi: 10.1200/JCO.19.00286.
When it comes to cardiovascular disease (CVD) risk after a breast cancer diagnosis, it’s not so much a matter of the amount of body fat carried but rather where it is located, results of a retrospective cohort study of nearly 3,000 survivors suggest.
“It is well known that higher body mass index (BMI) is associated with CVD mortality in the general population. However, BMI is not always an accurate proxy for individual-level adiposity and does not describe adipose tissue distribution,” note lead investigator Elizabeth M. Cespedes Feliciano, ScD, of Kaiser Permanente Northern California, Oakland, Calif., and coinvestigators.
The investigators studied 2,943 survivors of nonmetastatic breast cancer having a mean age of 56 years who were initially CVD free, using clinically acquired CT scans obtained near diagnosis to measure adiposity in three compartments: visceral, subcutaneous, and intramuscular.
The cohort experienced 328 CVD events (nonfatal stroke, myocardial infarction, heart failure, or CVD death) during a median follow-up of 6 years, the investigators reported in Journal of Clinical Oncology. The 10-year cumulative incidence was 15%.
In analyses that were adjusted for potential confounders and took into account competing risks, survivors’ CVD risk increased significantly with each standard deviation (SD) increase in visceral adiposity (hazard ratio, 1.15; 95% confidence interval, 1.03-1.29) and each SD increase in intramuscular adiposity (HR, 1.21; 95% CI, 1.06-1.37). The association for subcutaneous adiposity was not significant.
Findings were similar across all BMI categories. Of particular note, among survivors having a normal BMI, risk of CVD events increased by 70% with each SD greater visceral adiposity (HR, 1.70; 95% CI, 1.10-2.62).
Risk also rose with BMI exceeding the normal range, but the association became significant only in survivors with a BMI placing them in obesity class II (35 kg/m2 or greater) (HR, 1.70; 95% CI, 1.20-2.42).
“Although it has been assumed that excess adiposity increases the risk of CVD after breast cancer, this first-of-its-kind study demonstrates that adipose tissue distribution best identifies patients with breast cancer with higher CVD risk after diagnosis, including those with normal BMI,” Dr. Cespedes Feliciano and coinvestigators note.
“Software is now available that automatically measures body composition from clinically acquired CT scans, facilitating clinical integration,” they note. “Measures of adipose tissue distribution from CT or anthropometry (e.g., waist circumference) may help identify individuals with high CVD risk and tailor prevention efforts to patients’ body composition.”
Dr. Cespedes Feliciano disclosed no relevant conflicts of interest. The study did not receive any specific funding.
SOURCE: Cespedes Feliciano EM et al. J Clin Oncol. 2019 Aug 1. doi: 10.1200/JCO.19.00286.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
International lupus community sets out top barriers to improving lupus outcomes
The heterogeneity of lupus and the subsequent lack of a clear disease definition have been identified by an international group of experts as the primary barriers hindering timely diagnosis, improved treatment options, and appropriate access to care.
A report published in Lupus Science & Medicine titled “Global Consensus Building and Prioritization of Fundamental Lupus Challenges: The ALPHA Project” describes the results of a first-ever global consensus on key barriers to advances in lupus care, including a lack of validated biomarkers and flawed clinical trial design.
A lack of access to medical professionals familiar with lupus, challenges in managing lupus because of social determinants, and lack of treatment adherence were also considered to be barriers to improving the outcomes of people living with lupus.
First author Susan Manzi, MD, codirector of the Lupus Center of Excellence at Allegheny Health Network, Pittsburgh, and her colleagues said that, in contrast to other autoimmune diseases such as rheumatoid arthritis and psoriasis, the field of lupus has struggled with establishing a clear pathway for lupus drug development because of “persistent challenges in understanding the biology of the disease, defining clinical trial entry criteria and end points, developing instruments to measure changes in clinical activity, and controlling background medications.”
The authors noted that the intention of the Addressing Lupus Pillars for Health Advancement (ALPHA) Project was to build on the work of other initiatives, including some that were international in scope or were still ongoing.
“The ALPHA project was founded as the first step in an ongoing commitment to identify, prioritize, and implement strategies to address the most pressing challenges that limit progress in lupus across the continuum,” they wrote. In a joint initiative, the Lupus Foundation of America (LFA) and the Tufts Center for the Study of Drug Development (Tufts CSDD) set up a Global Advisory Committee (GAC) that included 13 lupus experts from the United States, Australia, United Kingdom, Germany, and South Korea to guide and oversee the study. Members had extensive knowledge of the disease, with specific expertise in rheumatology, dermatology, immunology, nephrology, and pediatrics.
Next, in-depth interviews were conducted with 17 experts who were well respected in the lupus scientific and care communities and represented all stakeholders. Using information garnered from these interviews, the LFA, Tufts CSDD, and GAC collaborated to develop a survey that included 23 questions addressing attitudes and perceptions about lupus as well as the prioritization of the most pressing challenges to improving diagnosis, care, treatment, and research.
The online survey was sent to 366 candidates, from whom the researchers received 127 completed responses. Of these, 82 (65%) were clinician-researcher-scientists and 14 (11%) worked in industry/biotechnology, 13 (10%) were researcher-scientists, and 12 (9%) were clinicians; 5% marked “other.”
The research team used a weighting system to prioritize barriers ranked by respondents, whereby higher ratings represented the challenges of highest impact (a score of 9 was highest rating, with 1 the lowest).
Survey respondents ranked the following as the top barriers to improving outcomes in lupus:
- A lack of diagnostic, predictive, and prognostic biomarkers for lupus (weighted prioritization score of 7.294) and lack of biomarkers to predict drug response in clinical trials (weighted prioritization score of 6.614).
- Flawed clinical trial design (weighted prioritization score of 6.370).
- Lack of access to clinicians familiar with lupus (weighted prioritization score of 6.873), and limited awareness of lupus among nonexpert medical professionals (weighted prioritization score of 5.800).
- Barriers to effective management of lupus because of social determinants of care in predominantly lower socioeconomic status areas (weighted prioritization score of 6.937).
- A lack of treatment adherence (weighted prioritization score of 6.717).
“A strong consensus built throughout the study, as themes and insights gathered from the in-depth interviews were highly consistent with those collected in the survey,” the researchers noted.
They said it was not surprising that the development of biomarkers had received a high ranking, as advances in this area would help accelerate drug development and precision medicine as well as more practical aspects of clinical care.
The research team acknowledged that substantial funds would be needed to address the top priorities identified in the study, and some of the issues may be more easily addressed than others.
“In the past decade, the overall funding landscape for lupus has been on a decline, particularly through the National Institutes of Health – the largest public funder of lupus research in the world – during a time in which arguably, lupus research has been prolific,” they wrote.
They concluded that comprehensive measures were needed to transform the lupus research and health care landscape.
“Lupus experts must convene to determine feasible and coordinated approaches for addressing long-standing barriers across the global lupus community,” they stressed.
The next part of the project will involve an international stakeholder meeting to develop a global road map of specific recommendations to address identified barriers, which “may include multipronged strategies using regulatory and advocacy approaches, scientific consensus building, communication efforts, among other possible tactics,” they added.
The ALPHA Project was launched in partnership with founding partner EMD Serono Research & Development (a business of Merck KGaA) and through additional support by GlaxoSmithKline. Many authors of the report had financial connections to the pharmaceutical industry.
SOURCE: Manzi S et al. Lupus Sci Med. 2019;6:e000342. doi: 10.1136/lupus-2019-000342.
The heterogeneity of lupus and the subsequent lack of a clear disease definition have been identified by an international group of experts as the primary barriers hindering timely diagnosis, improved treatment options, and appropriate access to care.
A report published in Lupus Science & Medicine titled “Global Consensus Building and Prioritization of Fundamental Lupus Challenges: The ALPHA Project” describes the results of a first-ever global consensus on key barriers to advances in lupus care, including a lack of validated biomarkers and flawed clinical trial design.
A lack of access to medical professionals familiar with lupus, challenges in managing lupus because of social determinants, and lack of treatment adherence were also considered to be barriers to improving the outcomes of people living with lupus.
First author Susan Manzi, MD, codirector of the Lupus Center of Excellence at Allegheny Health Network, Pittsburgh, and her colleagues said that, in contrast to other autoimmune diseases such as rheumatoid arthritis and psoriasis, the field of lupus has struggled with establishing a clear pathway for lupus drug development because of “persistent challenges in understanding the biology of the disease, defining clinical trial entry criteria and end points, developing instruments to measure changes in clinical activity, and controlling background medications.”
The authors noted that the intention of the Addressing Lupus Pillars for Health Advancement (ALPHA) Project was to build on the work of other initiatives, including some that were international in scope or were still ongoing.
“The ALPHA project was founded as the first step in an ongoing commitment to identify, prioritize, and implement strategies to address the most pressing challenges that limit progress in lupus across the continuum,” they wrote. In a joint initiative, the Lupus Foundation of America (LFA) and the Tufts Center for the Study of Drug Development (Tufts CSDD) set up a Global Advisory Committee (GAC) that included 13 lupus experts from the United States, Australia, United Kingdom, Germany, and South Korea to guide and oversee the study. Members had extensive knowledge of the disease, with specific expertise in rheumatology, dermatology, immunology, nephrology, and pediatrics.
Next, in-depth interviews were conducted with 17 experts who were well respected in the lupus scientific and care communities and represented all stakeholders. Using information garnered from these interviews, the LFA, Tufts CSDD, and GAC collaborated to develop a survey that included 23 questions addressing attitudes and perceptions about lupus as well as the prioritization of the most pressing challenges to improving diagnosis, care, treatment, and research.
The online survey was sent to 366 candidates, from whom the researchers received 127 completed responses. Of these, 82 (65%) were clinician-researcher-scientists and 14 (11%) worked in industry/biotechnology, 13 (10%) were researcher-scientists, and 12 (9%) were clinicians; 5% marked “other.”
The research team used a weighting system to prioritize barriers ranked by respondents, whereby higher ratings represented the challenges of highest impact (a score of 9 was highest rating, with 1 the lowest).
Survey respondents ranked the following as the top barriers to improving outcomes in lupus:
- A lack of diagnostic, predictive, and prognostic biomarkers for lupus (weighted prioritization score of 7.294) and lack of biomarkers to predict drug response in clinical trials (weighted prioritization score of 6.614).
- Flawed clinical trial design (weighted prioritization score of 6.370).
- Lack of access to clinicians familiar with lupus (weighted prioritization score of 6.873), and limited awareness of lupus among nonexpert medical professionals (weighted prioritization score of 5.800).
- Barriers to effective management of lupus because of social determinants of care in predominantly lower socioeconomic status areas (weighted prioritization score of 6.937).
- A lack of treatment adherence (weighted prioritization score of 6.717).
“A strong consensus built throughout the study, as themes and insights gathered from the in-depth interviews were highly consistent with those collected in the survey,” the researchers noted.
They said it was not surprising that the development of biomarkers had received a high ranking, as advances in this area would help accelerate drug development and precision medicine as well as more practical aspects of clinical care.
The research team acknowledged that substantial funds would be needed to address the top priorities identified in the study, and some of the issues may be more easily addressed than others.
“In the past decade, the overall funding landscape for lupus has been on a decline, particularly through the National Institutes of Health – the largest public funder of lupus research in the world – during a time in which arguably, lupus research has been prolific,” they wrote.
They concluded that comprehensive measures were needed to transform the lupus research and health care landscape.
“Lupus experts must convene to determine feasible and coordinated approaches for addressing long-standing barriers across the global lupus community,” they stressed.
The next part of the project will involve an international stakeholder meeting to develop a global road map of specific recommendations to address identified barriers, which “may include multipronged strategies using regulatory and advocacy approaches, scientific consensus building, communication efforts, among other possible tactics,” they added.
The ALPHA Project was launched in partnership with founding partner EMD Serono Research & Development (a business of Merck KGaA) and through additional support by GlaxoSmithKline. Many authors of the report had financial connections to the pharmaceutical industry.
SOURCE: Manzi S et al. Lupus Sci Med. 2019;6:e000342. doi: 10.1136/lupus-2019-000342.
The heterogeneity of lupus and the subsequent lack of a clear disease definition have been identified by an international group of experts as the primary barriers hindering timely diagnosis, improved treatment options, and appropriate access to care.
A report published in Lupus Science & Medicine titled “Global Consensus Building and Prioritization of Fundamental Lupus Challenges: The ALPHA Project” describes the results of a first-ever global consensus on key barriers to advances in lupus care, including a lack of validated biomarkers and flawed clinical trial design.
A lack of access to medical professionals familiar with lupus, challenges in managing lupus because of social determinants, and lack of treatment adherence were also considered to be barriers to improving the outcomes of people living with lupus.
First author Susan Manzi, MD, codirector of the Lupus Center of Excellence at Allegheny Health Network, Pittsburgh, and her colleagues said that, in contrast to other autoimmune diseases such as rheumatoid arthritis and psoriasis, the field of lupus has struggled with establishing a clear pathway for lupus drug development because of “persistent challenges in understanding the biology of the disease, defining clinical trial entry criteria and end points, developing instruments to measure changes in clinical activity, and controlling background medications.”
The authors noted that the intention of the Addressing Lupus Pillars for Health Advancement (ALPHA) Project was to build on the work of other initiatives, including some that were international in scope or were still ongoing.
“The ALPHA project was founded as the first step in an ongoing commitment to identify, prioritize, and implement strategies to address the most pressing challenges that limit progress in lupus across the continuum,” they wrote. In a joint initiative, the Lupus Foundation of America (LFA) and the Tufts Center for the Study of Drug Development (Tufts CSDD) set up a Global Advisory Committee (GAC) that included 13 lupus experts from the United States, Australia, United Kingdom, Germany, and South Korea to guide and oversee the study. Members had extensive knowledge of the disease, with specific expertise in rheumatology, dermatology, immunology, nephrology, and pediatrics.
Next, in-depth interviews were conducted with 17 experts who were well respected in the lupus scientific and care communities and represented all stakeholders. Using information garnered from these interviews, the LFA, Tufts CSDD, and GAC collaborated to develop a survey that included 23 questions addressing attitudes and perceptions about lupus as well as the prioritization of the most pressing challenges to improving diagnosis, care, treatment, and research.
The online survey was sent to 366 candidates, from whom the researchers received 127 completed responses. Of these, 82 (65%) were clinician-researcher-scientists and 14 (11%) worked in industry/biotechnology, 13 (10%) were researcher-scientists, and 12 (9%) were clinicians; 5% marked “other.”
The research team used a weighting system to prioritize barriers ranked by respondents, whereby higher ratings represented the challenges of highest impact (a score of 9 was highest rating, with 1 the lowest).
Survey respondents ranked the following as the top barriers to improving outcomes in lupus:
- A lack of diagnostic, predictive, and prognostic biomarkers for lupus (weighted prioritization score of 7.294) and lack of biomarkers to predict drug response in clinical trials (weighted prioritization score of 6.614).
- Flawed clinical trial design (weighted prioritization score of 6.370).
- Lack of access to clinicians familiar with lupus (weighted prioritization score of 6.873), and limited awareness of lupus among nonexpert medical professionals (weighted prioritization score of 5.800).
- Barriers to effective management of lupus because of social determinants of care in predominantly lower socioeconomic status areas (weighted prioritization score of 6.937).
- A lack of treatment adherence (weighted prioritization score of 6.717).
“A strong consensus built throughout the study, as themes and insights gathered from the in-depth interviews were highly consistent with those collected in the survey,” the researchers noted.
They said it was not surprising that the development of biomarkers had received a high ranking, as advances in this area would help accelerate drug development and precision medicine as well as more practical aspects of clinical care.
The research team acknowledged that substantial funds would be needed to address the top priorities identified in the study, and some of the issues may be more easily addressed than others.
“In the past decade, the overall funding landscape for lupus has been on a decline, particularly through the National Institutes of Health – the largest public funder of lupus research in the world – during a time in which arguably, lupus research has been prolific,” they wrote.
They concluded that comprehensive measures were needed to transform the lupus research and health care landscape.
“Lupus experts must convene to determine feasible and coordinated approaches for addressing long-standing barriers across the global lupus community,” they stressed.
The next part of the project will involve an international stakeholder meeting to develop a global road map of specific recommendations to address identified barriers, which “may include multipronged strategies using regulatory and advocacy approaches, scientific consensus building, communication efforts, among other possible tactics,” they added.
The ALPHA Project was launched in partnership with founding partner EMD Serono Research & Development (a business of Merck KGaA) and through additional support by GlaxoSmithKline. Many authors of the report had financial connections to the pharmaceutical industry.
SOURCE: Manzi S et al. Lupus Sci Med. 2019;6:e000342. doi: 10.1136/lupus-2019-000342.
REPORTING FROM LUPUS SCIENCE & MEDICINE







