User login
Preeclampsia doubles risk of postpartum transfusion reactions
Women with preeclampsia were found to be at the highest risk for transfusion reactions when receiving a blood transfusion post partum, according to results from a retrospective study.
Additionally, all women who received a transfusion postpartum were twice as likely to experience a procedure-related complication, compared with nonpregnant controls who received identical care.
“The objective of our study was to assess the incidence and risk factors for postpartum [transfusion reactions] in women transfused with red blood cells, plasma, or platelets post partum,” wrote Lars Thurn, PhD, of the Karolinska Institute in Stockholm and colleagues. The findings were reported in Blood Advances.
The researchers conducted a population-based cohort study that included a total of 517,854 women who gave birth in Stockholm County over a period of 21 years. Of those included, 12,183 (2.4%) received a blood transfusion postpartum.
Data was obtained from the Swedish National Birth Registry and was linked to the Stockholm Transfusion Database in order to evaluate the risk of transfusion reactions in pregnant women versus nonpregnant controls.
The researchers identified a total of 96 transfusion reactions postpartum for a prevalence of 79 per 10,000, compared with 40 per 10,000 among nonpregnant controls (odds ratio, 2.0; 95% confidence interval, 1.6-2.5).
The risk of transfusion-related reactions was more than double in pregnant women with preeclampsia versus pregnant women without the condition (OR, 2.1; 95% CI, 1.7-2.6).
“Preeclampsia, induced labor, and preterm delivery were significant risk factors for [transfusion reactions], but we found no differences due to parity, donor gender, or blood group,” the researchers wrote.
The large sample size was a major strength of the study, while a key limitation was the retrospective design.
“Our findings suggest heightened attention be paid when patients with preeclampsia are being evaluated for blood transfusions post partum,” the researchers concluded.
The study was partially funded by Södra Sjukvårdsregionen. The researchers reported having no conflicts of interest.
SOURCE: Thurn L et al. Blood Adv. 2019 Jul 31. doi: 10.1182/bloodadvances.2019000074.
Women with preeclampsia were found to be at the highest risk for transfusion reactions when receiving a blood transfusion post partum, according to results from a retrospective study.
Additionally, all women who received a transfusion postpartum were twice as likely to experience a procedure-related complication, compared with nonpregnant controls who received identical care.
“The objective of our study was to assess the incidence and risk factors for postpartum [transfusion reactions] in women transfused with red blood cells, plasma, or platelets post partum,” wrote Lars Thurn, PhD, of the Karolinska Institute in Stockholm and colleagues. The findings were reported in Blood Advances.
The researchers conducted a population-based cohort study that included a total of 517,854 women who gave birth in Stockholm County over a period of 21 years. Of those included, 12,183 (2.4%) received a blood transfusion postpartum.
Data was obtained from the Swedish National Birth Registry and was linked to the Stockholm Transfusion Database in order to evaluate the risk of transfusion reactions in pregnant women versus nonpregnant controls.
The researchers identified a total of 96 transfusion reactions postpartum for a prevalence of 79 per 10,000, compared with 40 per 10,000 among nonpregnant controls (odds ratio, 2.0; 95% confidence interval, 1.6-2.5).
The risk of transfusion-related reactions was more than double in pregnant women with preeclampsia versus pregnant women without the condition (OR, 2.1; 95% CI, 1.7-2.6).
“Preeclampsia, induced labor, and preterm delivery were significant risk factors for [transfusion reactions], but we found no differences due to parity, donor gender, or blood group,” the researchers wrote.
The large sample size was a major strength of the study, while a key limitation was the retrospective design.
“Our findings suggest heightened attention be paid when patients with preeclampsia are being evaluated for blood transfusions post partum,” the researchers concluded.
The study was partially funded by Södra Sjukvårdsregionen. The researchers reported having no conflicts of interest.
SOURCE: Thurn L et al. Blood Adv. 2019 Jul 31. doi: 10.1182/bloodadvances.2019000074.
Women with preeclampsia were found to be at the highest risk for transfusion reactions when receiving a blood transfusion post partum, according to results from a retrospective study.
Additionally, all women who received a transfusion postpartum were twice as likely to experience a procedure-related complication, compared with nonpregnant controls who received identical care.
“The objective of our study was to assess the incidence and risk factors for postpartum [transfusion reactions] in women transfused with red blood cells, plasma, or platelets post partum,” wrote Lars Thurn, PhD, of the Karolinska Institute in Stockholm and colleagues. The findings were reported in Blood Advances.
The researchers conducted a population-based cohort study that included a total of 517,854 women who gave birth in Stockholm County over a period of 21 years. Of those included, 12,183 (2.4%) received a blood transfusion postpartum.
Data was obtained from the Swedish National Birth Registry and was linked to the Stockholm Transfusion Database in order to evaluate the risk of transfusion reactions in pregnant women versus nonpregnant controls.
The researchers identified a total of 96 transfusion reactions postpartum for a prevalence of 79 per 10,000, compared with 40 per 10,000 among nonpregnant controls (odds ratio, 2.0; 95% confidence interval, 1.6-2.5).
The risk of transfusion-related reactions was more than double in pregnant women with preeclampsia versus pregnant women without the condition (OR, 2.1; 95% CI, 1.7-2.6).
“Preeclampsia, induced labor, and preterm delivery were significant risk factors for [transfusion reactions], but we found no differences due to parity, donor gender, or blood group,” the researchers wrote.
The large sample size was a major strength of the study, while a key limitation was the retrospective design.
“Our findings suggest heightened attention be paid when patients with preeclampsia are being evaluated for blood transfusions post partum,” the researchers concluded.
The study was partially funded by Södra Sjukvårdsregionen. The researchers reported having no conflicts of interest.
SOURCE: Thurn L et al. Blood Adv. 2019 Jul 31. doi: 10.1182/bloodadvances.2019000074.
FROM BLOOD ADVANCES
Enteral feeding is safe during bronchiolitis HFNC
SEATTLE – There were no cases of aspiration with enteric feeds of 60 children aged up to 2 years on high flow nasal cannula (HFNC) for bronchiolitis at the University of Oklahoma Children’s Hospital, Oklahoma City, according to research presented at the 2019 Pediatric Hospital Medicine Conference.
HFNC has become common for bronchiolitis management; it often saves infants from intubation. However, many providers opt for total parenteral nutrition during therapy instead of enteral feeding because of concerns about aspiration pneumonia.
Pediatricians at the children’s hospital began to wonder if the concern was really necessary. There have been reports of safe feeding during HFNC, and “clinical care literature has shown that feeding the gut throughout illness improves outcomes,” said lead investigator, Sarah Walter, MD, a third-year pediatrics resident at the hospital.
So her team took a leap of faith. They consulted the HFNC literature, asked their fellow providers what they would be comfortable with, and instituted a pediatric HFNC enteral feeding protocol at the children’s hospital for use on inpatient floors, pediatric ICUs, and elsewhere.
Feedings – formula or breast milk – are triggered by stable respiratory Tal scores over 8 hours, meaning that respiratory rates, breath sounds, and accessory muscle use were stable or improving. Children on a flow of 6 L/min or less, with a respiratory rate below 60 breaths per minute, are started on oral feeds, and those on higher flows on nasogastric (NG) tube feeds.
Feeds are started at 1 mL/kg per hour and advanced by the same amount every 3 hours until volume goals are reached; IV fluids are tapered accordingly. It’s a standing order, so nurses are able to initiate and advance feeding as indicated, any time of day.
Feeding was temporarily suspended in only 17 children: 6 for emesis, 6 for worsening respiratory scores, and the rest for dislodged NG tubes, procedures, or other issues. Enteric feeds were restarted with two stable scores below 7 points, at half the rate at which they were stopped.
NG tubes were used in over half of the 478 nursing shifts during which the 60 children – the majority aged 4-24 months – were fed; oral feeds in more than a third; and gastric tubes and other options in the rest. IV nutrition was used during just 1.8% of the shifts.
Enteric feeds were given up to a flow rate of 3.5 L/kg. There were no aspirations, even when children vomited. “We have seen good results so far that feeding is safe in these children,” Dr. Walters said.
“Our hospitalist team has been very receptive; they have been using the order set pretty continuously.” Parents also feel better when they know their children were “getting food in their belly,” even if by NG tube. “It’s important for family satisfaction,” she said.
The next step is to assess impact on length of stay, and education efforts to encourage broader use of the order set.
There was no external funding, and Dr. Walter had no disclosures. The meeting was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
SEATTLE – There were no cases of aspiration with enteric feeds of 60 children aged up to 2 years on high flow nasal cannula (HFNC) for bronchiolitis at the University of Oklahoma Children’s Hospital, Oklahoma City, according to research presented at the 2019 Pediatric Hospital Medicine Conference.
HFNC has become common for bronchiolitis management; it often saves infants from intubation. However, many providers opt for total parenteral nutrition during therapy instead of enteral feeding because of concerns about aspiration pneumonia.
Pediatricians at the children’s hospital began to wonder if the concern was really necessary. There have been reports of safe feeding during HFNC, and “clinical care literature has shown that feeding the gut throughout illness improves outcomes,” said lead investigator, Sarah Walter, MD, a third-year pediatrics resident at the hospital.
So her team took a leap of faith. They consulted the HFNC literature, asked their fellow providers what they would be comfortable with, and instituted a pediatric HFNC enteral feeding protocol at the children’s hospital for use on inpatient floors, pediatric ICUs, and elsewhere.
Feedings – formula or breast milk – are triggered by stable respiratory Tal scores over 8 hours, meaning that respiratory rates, breath sounds, and accessory muscle use were stable or improving. Children on a flow of 6 L/min or less, with a respiratory rate below 60 breaths per minute, are started on oral feeds, and those on higher flows on nasogastric (NG) tube feeds.
Feeds are started at 1 mL/kg per hour and advanced by the same amount every 3 hours until volume goals are reached; IV fluids are tapered accordingly. It’s a standing order, so nurses are able to initiate and advance feeding as indicated, any time of day.
Feeding was temporarily suspended in only 17 children: 6 for emesis, 6 for worsening respiratory scores, and the rest for dislodged NG tubes, procedures, or other issues. Enteric feeds were restarted with two stable scores below 7 points, at half the rate at which they were stopped.
NG tubes were used in over half of the 478 nursing shifts during which the 60 children – the majority aged 4-24 months – were fed; oral feeds in more than a third; and gastric tubes and other options in the rest. IV nutrition was used during just 1.8% of the shifts.
Enteric feeds were given up to a flow rate of 3.5 L/kg. There were no aspirations, even when children vomited. “We have seen good results so far that feeding is safe in these children,” Dr. Walters said.
“Our hospitalist team has been very receptive; they have been using the order set pretty continuously.” Parents also feel better when they know their children were “getting food in their belly,” even if by NG tube. “It’s important for family satisfaction,” she said.
The next step is to assess impact on length of stay, and education efforts to encourage broader use of the order set.
There was no external funding, and Dr. Walter had no disclosures. The meeting was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
SEATTLE – There were no cases of aspiration with enteric feeds of 60 children aged up to 2 years on high flow nasal cannula (HFNC) for bronchiolitis at the University of Oklahoma Children’s Hospital, Oklahoma City, according to research presented at the 2019 Pediatric Hospital Medicine Conference.
HFNC has become common for bronchiolitis management; it often saves infants from intubation. However, many providers opt for total parenteral nutrition during therapy instead of enteral feeding because of concerns about aspiration pneumonia.
Pediatricians at the children’s hospital began to wonder if the concern was really necessary. There have been reports of safe feeding during HFNC, and “clinical care literature has shown that feeding the gut throughout illness improves outcomes,” said lead investigator, Sarah Walter, MD, a third-year pediatrics resident at the hospital.
So her team took a leap of faith. They consulted the HFNC literature, asked their fellow providers what they would be comfortable with, and instituted a pediatric HFNC enteral feeding protocol at the children’s hospital for use on inpatient floors, pediatric ICUs, and elsewhere.
Feedings – formula or breast milk – are triggered by stable respiratory Tal scores over 8 hours, meaning that respiratory rates, breath sounds, and accessory muscle use were stable or improving. Children on a flow of 6 L/min or less, with a respiratory rate below 60 breaths per minute, are started on oral feeds, and those on higher flows on nasogastric (NG) tube feeds.
Feeds are started at 1 mL/kg per hour and advanced by the same amount every 3 hours until volume goals are reached; IV fluids are tapered accordingly. It’s a standing order, so nurses are able to initiate and advance feeding as indicated, any time of day.
Feeding was temporarily suspended in only 17 children: 6 for emesis, 6 for worsening respiratory scores, and the rest for dislodged NG tubes, procedures, or other issues. Enteric feeds were restarted with two stable scores below 7 points, at half the rate at which they were stopped.
NG tubes were used in over half of the 478 nursing shifts during which the 60 children – the majority aged 4-24 months – were fed; oral feeds in more than a third; and gastric tubes and other options in the rest. IV nutrition was used during just 1.8% of the shifts.
Enteric feeds were given up to a flow rate of 3.5 L/kg. There were no aspirations, even when children vomited. “We have seen good results so far that feeding is safe in these children,” Dr. Walters said.
“Our hospitalist team has been very receptive; they have been using the order set pretty continuously.” Parents also feel better when they know their children were “getting food in their belly,” even if by NG tube. “It’s important for family satisfaction,” she said.
The next step is to assess impact on length of stay, and education efforts to encourage broader use of the order set.
There was no external funding, and Dr. Walter had no disclosures. The meeting was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
REPORTING FROM PHM 2019
Large prospective trial offers reassurance for long-term PPI use
Aside from a possible increased risk of enteric infections, long-term use of the proton pump inhibitor (PPI) pantoprazole appears safe in patients with stable atherosclerotic vascular disease, according to a prospective trial involving more than 17,000 participants.
In contrast with published observational studies, the present trial found no associations between long-term PPI use and previously reported risks such as pneumonia, fracture, or cerebrovascular events, according to lead author Paul Moayyedi, MB ChB, PhD, of McMaster University in Hamilton, Ont., and colleagues.
“To our knowledge, this is the largest PPI trial for any indication and the first prospective randomized trial to evaluate the many long-term safety concerns related to PPI therapy,” the investigators wrote in Gastroenterology. “It is reassuring that there was no evidence for harm for most of these events other than an excess of enteric infections.”
“Given how commonly acid suppressive medications are used, it is important to ensure that this class of drugs is safe,” the investigators wrote. They noted that patients are often alarmed by “sensational headlines” about PPI safety. “There are balancing articles that more carefully discuss the risks and benefits of taking PPI therapy but these receive less media attention,” the investigators added.
The present, prospective trial, COMPASS, involved 17,598 participants from 33 countries with stable peripheral artery disease and cardiovascular disease. “We use the term participants, rather than patients, as not all of those taking part in this research would have been patients throughout the trial but all participated in the randomized controlled trial,” the investigators wrote.
In addition to evaluating the safety of pantoprazole, the study was initially designed to measure the efficacy of pantoprazole for preventing upper gastrointestinal events in participants taking rivaroxaban and/or aspirin, which, in combination, were recently shown to reduce cardiovascular outcomes among patients with stable cardiovascular conditions. As such, participants in the trial were randomized to one of three groups: 100-mg aspirin once daily, 5-mg rivaroxaban twice daily, or 2.5-mg rivaroxaban twice daily combined with 100-mg aspirin once daily. The primary efficacy outcomes for these three groups were stroke, myocardial infarction, and cardiovascular death. This portion of the trial was discontinued early because of evidence that showed the superiority of combination therapy over aspirin alone; however, the pantoprazole component of the trial continued, as planned, for 3 years.
At baseline, about two-thirds of participants (64%) were not taking a PPI, requiring randomization to either 40-mg pantoprazole once daily or matching placebo. Pantoprazole safety outcomes centered on those previously reported by observational studies, including dementia, chronic kidney disease, gastric atrophy, fracture, cancer, pneumonia, diabetes mellitus, chronic obstructive lung disease, Clostrididoides difficile infection, and other enteric infections. Hospitalization rates for noncardiovascular and cardiovascular events were also reported. Data were gathered via questionnaires, which were conducted every 6 months.
Most patients in the trial (78%) were male, and 23% were current smokers. Smaller proportions of the population were taking an NSAID (5%) and/or had a history of peptic ulcer disease (2.6%). The median follow-up was 3.01 years, ranging from 2.49 to 3.59 years. Permanent discontinuations occurred at approximately equal rates in the pantoprazole (21%) and placebo (22%) group after a median of 11 months (338 days). In both groups, more than 96% of participants who continued treatment took their medications as prescribed at least 80% of the time.
Analysis of cardiovascular outcomes revealed no significant differences between placebo and pantoprazole groups. Of all the evaluated safety measures, only enteric infections differed significantly between groups, occurring at a higher rate in the pantoprazole group than in the placebo group (1.4% vs. 1.0%; odds ratio, 1.33; 95% confidence interval, 1.01-1.75). Although C. difficile infection was more common among pantoprazole users, only 13 such events occurred, precluding statistical significance.
According to the investigators, these findings should offer reassurance to PPI prescribers and users; they noted that previous findings from observational studies warrant skepticism. “A significant proportion of patients are prescribed PPI therapy inappropriately, and in these cases, it is reasonable to advocate strategies to discontinue acid suppression. However, when there is a clinical need for PPI therapy, these data suggest that the benefits are likely to outweigh any putative risks.”
In regard to the possible increased risk of enteric infection, the investigators again urged a conservative interpretation, as the increased rate of enteric infection among PPI users was still lower than rates reported by systematic reviews. “The data in the current randomized trial were not adjusted for multiple testing so this result should be interpreted with caution,” the investigators wrote. Although acid suppression may allow for increased ingestion of pathogenic organisms, which could theoretically increase the risk of enteric infection, the investigators stated that the benefits of PPIs likely outweigh their risks.
The COMPASS trial was funded by Bayer AG. The investigators disclosed additional relationships with Bayer, Allergan, Takeda, Janssen, and others.
SOURCE: Moayyedi P et al. Gastro. 2019 May 29. doi: 10.1053/j.gastro.2019.05.056.
AGA patient education on GERD can help your patients better understand and manage the disorder. Post this education or your practice website or share you’re your patients at https://www.gastro.org/practice-guidance/gi-patient-center/topic/gastroesophageal-reflux-disease-gerd.
Aside from a possible increased risk of enteric infections, long-term use of the proton pump inhibitor (PPI) pantoprazole appears safe in patients with stable atherosclerotic vascular disease, according to a prospective trial involving more than 17,000 participants.
In contrast with published observational studies, the present trial found no associations between long-term PPI use and previously reported risks such as pneumonia, fracture, or cerebrovascular events, according to lead author Paul Moayyedi, MB ChB, PhD, of McMaster University in Hamilton, Ont., and colleagues.
“To our knowledge, this is the largest PPI trial for any indication and the first prospective randomized trial to evaluate the many long-term safety concerns related to PPI therapy,” the investigators wrote in Gastroenterology. “It is reassuring that there was no evidence for harm for most of these events other than an excess of enteric infections.”
“Given how commonly acid suppressive medications are used, it is important to ensure that this class of drugs is safe,” the investigators wrote. They noted that patients are often alarmed by “sensational headlines” about PPI safety. “There are balancing articles that more carefully discuss the risks and benefits of taking PPI therapy but these receive less media attention,” the investigators added.
The present, prospective trial, COMPASS, involved 17,598 participants from 33 countries with stable peripheral artery disease and cardiovascular disease. “We use the term participants, rather than patients, as not all of those taking part in this research would have been patients throughout the trial but all participated in the randomized controlled trial,” the investigators wrote.
In addition to evaluating the safety of pantoprazole, the study was initially designed to measure the efficacy of pantoprazole for preventing upper gastrointestinal events in participants taking rivaroxaban and/or aspirin, which, in combination, were recently shown to reduce cardiovascular outcomes among patients with stable cardiovascular conditions. As such, participants in the trial were randomized to one of three groups: 100-mg aspirin once daily, 5-mg rivaroxaban twice daily, or 2.5-mg rivaroxaban twice daily combined with 100-mg aspirin once daily. The primary efficacy outcomes for these three groups were stroke, myocardial infarction, and cardiovascular death. This portion of the trial was discontinued early because of evidence that showed the superiority of combination therapy over aspirin alone; however, the pantoprazole component of the trial continued, as planned, for 3 years.
At baseline, about two-thirds of participants (64%) were not taking a PPI, requiring randomization to either 40-mg pantoprazole once daily or matching placebo. Pantoprazole safety outcomes centered on those previously reported by observational studies, including dementia, chronic kidney disease, gastric atrophy, fracture, cancer, pneumonia, diabetes mellitus, chronic obstructive lung disease, Clostrididoides difficile infection, and other enteric infections. Hospitalization rates for noncardiovascular and cardiovascular events were also reported. Data were gathered via questionnaires, which were conducted every 6 months.
Most patients in the trial (78%) were male, and 23% were current smokers. Smaller proportions of the population were taking an NSAID (5%) and/or had a history of peptic ulcer disease (2.6%). The median follow-up was 3.01 years, ranging from 2.49 to 3.59 years. Permanent discontinuations occurred at approximately equal rates in the pantoprazole (21%) and placebo (22%) group after a median of 11 months (338 days). In both groups, more than 96% of participants who continued treatment took their medications as prescribed at least 80% of the time.
Analysis of cardiovascular outcomes revealed no significant differences between placebo and pantoprazole groups. Of all the evaluated safety measures, only enteric infections differed significantly between groups, occurring at a higher rate in the pantoprazole group than in the placebo group (1.4% vs. 1.0%; odds ratio, 1.33; 95% confidence interval, 1.01-1.75). Although C. difficile infection was more common among pantoprazole users, only 13 such events occurred, precluding statistical significance.
According to the investigators, these findings should offer reassurance to PPI prescribers and users; they noted that previous findings from observational studies warrant skepticism. “A significant proportion of patients are prescribed PPI therapy inappropriately, and in these cases, it is reasonable to advocate strategies to discontinue acid suppression. However, when there is a clinical need for PPI therapy, these data suggest that the benefits are likely to outweigh any putative risks.”
In regard to the possible increased risk of enteric infection, the investigators again urged a conservative interpretation, as the increased rate of enteric infection among PPI users was still lower than rates reported by systematic reviews. “The data in the current randomized trial were not adjusted for multiple testing so this result should be interpreted with caution,” the investigators wrote. Although acid suppression may allow for increased ingestion of pathogenic organisms, which could theoretically increase the risk of enteric infection, the investigators stated that the benefits of PPIs likely outweigh their risks.
The COMPASS trial was funded by Bayer AG. The investigators disclosed additional relationships with Bayer, Allergan, Takeda, Janssen, and others.
SOURCE: Moayyedi P et al. Gastro. 2019 May 29. doi: 10.1053/j.gastro.2019.05.056.
AGA patient education on GERD can help your patients better understand and manage the disorder. Post this education or your practice website or share you’re your patients at https://www.gastro.org/practice-guidance/gi-patient-center/topic/gastroesophageal-reflux-disease-gerd.
Aside from a possible increased risk of enteric infections, long-term use of the proton pump inhibitor (PPI) pantoprazole appears safe in patients with stable atherosclerotic vascular disease, according to a prospective trial involving more than 17,000 participants.
In contrast with published observational studies, the present trial found no associations between long-term PPI use and previously reported risks such as pneumonia, fracture, or cerebrovascular events, according to lead author Paul Moayyedi, MB ChB, PhD, of McMaster University in Hamilton, Ont., and colleagues.
“To our knowledge, this is the largest PPI trial for any indication and the first prospective randomized trial to evaluate the many long-term safety concerns related to PPI therapy,” the investigators wrote in Gastroenterology. “It is reassuring that there was no evidence for harm for most of these events other than an excess of enteric infections.”
“Given how commonly acid suppressive medications are used, it is important to ensure that this class of drugs is safe,” the investigators wrote. They noted that patients are often alarmed by “sensational headlines” about PPI safety. “There are balancing articles that more carefully discuss the risks and benefits of taking PPI therapy but these receive less media attention,” the investigators added.
The present, prospective trial, COMPASS, involved 17,598 participants from 33 countries with stable peripheral artery disease and cardiovascular disease. “We use the term participants, rather than patients, as not all of those taking part in this research would have been patients throughout the trial but all participated in the randomized controlled trial,” the investigators wrote.
In addition to evaluating the safety of pantoprazole, the study was initially designed to measure the efficacy of pantoprazole for preventing upper gastrointestinal events in participants taking rivaroxaban and/or aspirin, which, in combination, were recently shown to reduce cardiovascular outcomes among patients with stable cardiovascular conditions. As such, participants in the trial were randomized to one of three groups: 100-mg aspirin once daily, 5-mg rivaroxaban twice daily, or 2.5-mg rivaroxaban twice daily combined with 100-mg aspirin once daily. The primary efficacy outcomes for these three groups were stroke, myocardial infarction, and cardiovascular death. This portion of the trial was discontinued early because of evidence that showed the superiority of combination therapy over aspirin alone; however, the pantoprazole component of the trial continued, as planned, for 3 years.
At baseline, about two-thirds of participants (64%) were not taking a PPI, requiring randomization to either 40-mg pantoprazole once daily or matching placebo. Pantoprazole safety outcomes centered on those previously reported by observational studies, including dementia, chronic kidney disease, gastric atrophy, fracture, cancer, pneumonia, diabetes mellitus, chronic obstructive lung disease, Clostrididoides difficile infection, and other enteric infections. Hospitalization rates for noncardiovascular and cardiovascular events were also reported. Data were gathered via questionnaires, which were conducted every 6 months.
Most patients in the trial (78%) were male, and 23% were current smokers. Smaller proportions of the population were taking an NSAID (5%) and/or had a history of peptic ulcer disease (2.6%). The median follow-up was 3.01 years, ranging from 2.49 to 3.59 years. Permanent discontinuations occurred at approximately equal rates in the pantoprazole (21%) and placebo (22%) group after a median of 11 months (338 days). In both groups, more than 96% of participants who continued treatment took their medications as prescribed at least 80% of the time.
Analysis of cardiovascular outcomes revealed no significant differences between placebo and pantoprazole groups. Of all the evaluated safety measures, only enteric infections differed significantly between groups, occurring at a higher rate in the pantoprazole group than in the placebo group (1.4% vs. 1.0%; odds ratio, 1.33; 95% confidence interval, 1.01-1.75). Although C. difficile infection was more common among pantoprazole users, only 13 such events occurred, precluding statistical significance.
According to the investigators, these findings should offer reassurance to PPI prescribers and users; they noted that previous findings from observational studies warrant skepticism. “A significant proportion of patients are prescribed PPI therapy inappropriately, and in these cases, it is reasonable to advocate strategies to discontinue acid suppression. However, when there is a clinical need for PPI therapy, these data suggest that the benefits are likely to outweigh any putative risks.”
In regard to the possible increased risk of enteric infection, the investigators again urged a conservative interpretation, as the increased rate of enteric infection among PPI users was still lower than rates reported by systematic reviews. “The data in the current randomized trial were not adjusted for multiple testing so this result should be interpreted with caution,” the investigators wrote. Although acid suppression may allow for increased ingestion of pathogenic organisms, which could theoretically increase the risk of enteric infection, the investigators stated that the benefits of PPIs likely outweigh their risks.
The COMPASS trial was funded by Bayer AG. The investigators disclosed additional relationships with Bayer, Allergan, Takeda, Janssen, and others.
SOURCE: Moayyedi P et al. Gastro. 2019 May 29. doi: 10.1053/j.gastro.2019.05.056.
AGA patient education on GERD can help your patients better understand and manage the disorder. Post this education or your practice website or share you’re your patients at https://www.gastro.org/practice-guidance/gi-patient-center/topic/gastroesophageal-reflux-disease-gerd.
FROM GASTROENTEROLOGY
Key clinical point: Aside from a possible increased risk of enteric infections, long-term use of pantoprazole is safe in patients with stable peripheral artery and cardiovascular disease.
Major finding: Enteric infections were 33% more common in the pantoprazole group than in the placebo group.
Study details: A placebo-controlled, double-blind, randomized trial involving 17,598 patients with stable peripheral artery disease and cardiovascular disease.
Disclosures: The COMPASS trial was funded by Bayer AG. The investigators disclosed relationships with Bayer, Allergan, Takeda, Janssen, and others.
Source: Moayyedi P et al. Gastroenterology. 2019 May 29. doi: 10.1053/j.gastro.2019.05.056.
Cost a factor in breast cancer treatment decisions
Treatment costs are a significant factor in women’s decision making around breast cancer surgery, investigators reported.
With the health care costs of breast cancer estimated to reach $20 billion by 2020 in the United States, many of those costs are being shifted onto patients themselves, wrote Rachel A. Greenup, MD, from Duke University, Durham, N.C., and coauthors in the Journal of Oncology Practice.
“This financial hardship is now recognized as a major adverse effect of cancer care and has been associated with reduced quality of life, nonadherence, and an increased risk of early mortality,” they wrote.
Researchers surveyed 607 women with a history of breast cancer to examine the impact that cost had on their decisions about surgery and what financial harm they had experienced after breast cancer surgery.
Overall, 43% of women said they considered costs when making decisions about breast cancer treatment, 28% said cost influenced their decision making around breast cancer surgery, and 14% said costs were extremely important in that decision.
Women in the lowest income bracket – earning at or below $45,000 per year – identified cost as the most influential factor in their decision about breast cancer surgery, above loss of sensation, breast preservation or appearance, the need for long-term surveillance, or avoiding radiation.
However, more than three-quarters of women said they never discussed costs with their medical team.
Bilateral mastectomy, with and without reconstruction, was associated with higher patient-reported out-of-pocket costs, higher debt, higher rates of cancer-induced financial hardship, and higher rates of altered or reduced employment, compared with breast-conserving surgery.
More than one-third of participants reported significant to catastrophic financial burden because of their breast cancer care.
Even in the highest income brackets, two-thirds of women were financially unprepared for the cost of treatment, and 26% said their treatment costs were higher than expected.
The authors commented that “cost transparency” was uncommon between oncologically equivalent surgical treatments, “thus, patients with breast cancer may unknowingly be guiding therapeutic decisions that increase the risk of financial harm.”
“To date, patient out-of-pocket costs and subsequent risk of financial harm have not been routinely incorporated into shared decisions for breast cancer surgery, a process that has otherwise highly revered patient values,” they wrote.
The investigators suggested that revealing the greater risk for financial burden associated with treatments like bilateral mastectomy could help inform surgical treatment decisions.
The study was supported by the National Institutes of Health and the Duke Cancer Institute. Six authors reported honoraria, research funding, prior employment, and other support from the pharmaceutical sector.
SOURCE: Greenup RA et al. J Oncol Pract. 2019 Jul 29. doi: 10.1200/JOP.18.00796.
Treatment costs are a significant factor in women’s decision making around breast cancer surgery, investigators reported.
With the health care costs of breast cancer estimated to reach $20 billion by 2020 in the United States, many of those costs are being shifted onto patients themselves, wrote Rachel A. Greenup, MD, from Duke University, Durham, N.C., and coauthors in the Journal of Oncology Practice.
“This financial hardship is now recognized as a major adverse effect of cancer care and has been associated with reduced quality of life, nonadherence, and an increased risk of early mortality,” they wrote.
Researchers surveyed 607 women with a history of breast cancer to examine the impact that cost had on their decisions about surgery and what financial harm they had experienced after breast cancer surgery.
Overall, 43% of women said they considered costs when making decisions about breast cancer treatment, 28% said cost influenced their decision making around breast cancer surgery, and 14% said costs were extremely important in that decision.
Women in the lowest income bracket – earning at or below $45,000 per year – identified cost as the most influential factor in their decision about breast cancer surgery, above loss of sensation, breast preservation or appearance, the need for long-term surveillance, or avoiding radiation.
However, more than three-quarters of women said they never discussed costs with their medical team.
Bilateral mastectomy, with and without reconstruction, was associated with higher patient-reported out-of-pocket costs, higher debt, higher rates of cancer-induced financial hardship, and higher rates of altered or reduced employment, compared with breast-conserving surgery.
More than one-third of participants reported significant to catastrophic financial burden because of their breast cancer care.
Even in the highest income brackets, two-thirds of women were financially unprepared for the cost of treatment, and 26% said their treatment costs were higher than expected.
The authors commented that “cost transparency” was uncommon between oncologically equivalent surgical treatments, “thus, patients with breast cancer may unknowingly be guiding therapeutic decisions that increase the risk of financial harm.”
“To date, patient out-of-pocket costs and subsequent risk of financial harm have not been routinely incorporated into shared decisions for breast cancer surgery, a process that has otherwise highly revered patient values,” they wrote.
The investigators suggested that revealing the greater risk for financial burden associated with treatments like bilateral mastectomy could help inform surgical treatment decisions.
The study was supported by the National Institutes of Health and the Duke Cancer Institute. Six authors reported honoraria, research funding, prior employment, and other support from the pharmaceutical sector.
SOURCE: Greenup RA et al. J Oncol Pract. 2019 Jul 29. doi: 10.1200/JOP.18.00796.
Treatment costs are a significant factor in women’s decision making around breast cancer surgery, investigators reported.
With the health care costs of breast cancer estimated to reach $20 billion by 2020 in the United States, many of those costs are being shifted onto patients themselves, wrote Rachel A. Greenup, MD, from Duke University, Durham, N.C., and coauthors in the Journal of Oncology Practice.
“This financial hardship is now recognized as a major adverse effect of cancer care and has been associated with reduced quality of life, nonadherence, and an increased risk of early mortality,” they wrote.
Researchers surveyed 607 women with a history of breast cancer to examine the impact that cost had on their decisions about surgery and what financial harm they had experienced after breast cancer surgery.
Overall, 43% of women said they considered costs when making decisions about breast cancer treatment, 28% said cost influenced their decision making around breast cancer surgery, and 14% said costs were extremely important in that decision.
Women in the lowest income bracket – earning at or below $45,000 per year – identified cost as the most influential factor in their decision about breast cancer surgery, above loss of sensation, breast preservation or appearance, the need for long-term surveillance, or avoiding radiation.
However, more than three-quarters of women said they never discussed costs with their medical team.
Bilateral mastectomy, with and without reconstruction, was associated with higher patient-reported out-of-pocket costs, higher debt, higher rates of cancer-induced financial hardship, and higher rates of altered or reduced employment, compared with breast-conserving surgery.
More than one-third of participants reported significant to catastrophic financial burden because of their breast cancer care.
Even in the highest income brackets, two-thirds of women were financially unprepared for the cost of treatment, and 26% said their treatment costs were higher than expected.
The authors commented that “cost transparency” was uncommon between oncologically equivalent surgical treatments, “thus, patients with breast cancer may unknowingly be guiding therapeutic decisions that increase the risk of financial harm.”
“To date, patient out-of-pocket costs and subsequent risk of financial harm have not been routinely incorporated into shared decisions for breast cancer surgery, a process that has otherwise highly revered patient values,” they wrote.
The investigators suggested that revealing the greater risk for financial burden associated with treatments like bilateral mastectomy could help inform surgical treatment decisions.
The study was supported by the National Institutes of Health and the Duke Cancer Institute. Six authors reported honoraria, research funding, prior employment, and other support from the pharmaceutical sector.
SOURCE: Greenup RA et al. J Oncol Pract. 2019 Jul 29. doi: 10.1200/JOP.18.00796.
FROM THE JOURNAL OF ONCOLOGY PRACTICE
DRC Ebola epidemic continues unabated despite international response
, “currently the outbreak continues at the same pace, so we don’t see evidence of slowing,” according to Henry Walke, MD, director of the Division of Preparedness and Emerging Infections and Incident Manager, 2018 CDC Ebola Response, Centers for Disease Control and Prevention.
He added that new cases of Ebola have been seen in Goma, which is outside the initial outbreak area. Goma is the largest city in the eastern part of the DRC and a major trading port.
Dr. Walke made his remarks in a telephone media briefing Aug. 1 by the U. S. Department of Health and Human Services outlining the current state of the U.S. response to the outbreak.
He described the efforts of the CDC to provide support to the DRC both from Atlanta and in the field. These efforts included support for vaccination activities in DRC’s North Kivu and Ituri provinces for the population and for at-risk health-care workers in the DRC and neighboring countries. In addition, the United States is involved in the testing of experimental therapeutics and vaccines in the DRC in an effort to aid in this and future outbreaks.
“There are no cases of Ebola in the United States,” said Dr. Walke, and the CDC believes the risk to the United States from the outbreak is low. He cited the limited number of travelers from DRC. “There [are] about 16,000 from the DRC to the U.S. on an annual basis, and only about 100 from Goma itself. There aren’t direct flights and we have at the Goma airport both entry and exit screening.”
According to a World Health Organization report, this Ebola outbreak is the second deadliest on record and has killed 1,750 people out of around 2,518 confirmed cases as of July 23.
Efforts to control the epidemic are severely hampered by civil unrest in the area, public mistrust of the government and health care workers, and a comparative lack of international aid compared to previous Ebola outbreaks.
, “currently the outbreak continues at the same pace, so we don’t see evidence of slowing,” according to Henry Walke, MD, director of the Division of Preparedness and Emerging Infections and Incident Manager, 2018 CDC Ebola Response, Centers for Disease Control and Prevention.
He added that new cases of Ebola have been seen in Goma, which is outside the initial outbreak area. Goma is the largest city in the eastern part of the DRC and a major trading port.
Dr. Walke made his remarks in a telephone media briefing Aug. 1 by the U. S. Department of Health and Human Services outlining the current state of the U.S. response to the outbreak.
He described the efforts of the CDC to provide support to the DRC both from Atlanta and in the field. These efforts included support for vaccination activities in DRC’s North Kivu and Ituri provinces for the population and for at-risk health-care workers in the DRC and neighboring countries. In addition, the United States is involved in the testing of experimental therapeutics and vaccines in the DRC in an effort to aid in this and future outbreaks.
“There are no cases of Ebola in the United States,” said Dr. Walke, and the CDC believes the risk to the United States from the outbreak is low. He cited the limited number of travelers from DRC. “There [are] about 16,000 from the DRC to the U.S. on an annual basis, and only about 100 from Goma itself. There aren’t direct flights and we have at the Goma airport both entry and exit screening.”
According to a World Health Organization report, this Ebola outbreak is the second deadliest on record and has killed 1,750 people out of around 2,518 confirmed cases as of July 23.
Efforts to control the epidemic are severely hampered by civil unrest in the area, public mistrust of the government and health care workers, and a comparative lack of international aid compared to previous Ebola outbreaks.
, “currently the outbreak continues at the same pace, so we don’t see evidence of slowing,” according to Henry Walke, MD, director of the Division of Preparedness and Emerging Infections and Incident Manager, 2018 CDC Ebola Response, Centers for Disease Control and Prevention.
He added that new cases of Ebola have been seen in Goma, which is outside the initial outbreak area. Goma is the largest city in the eastern part of the DRC and a major trading port.
Dr. Walke made his remarks in a telephone media briefing Aug. 1 by the U. S. Department of Health and Human Services outlining the current state of the U.S. response to the outbreak.
He described the efforts of the CDC to provide support to the DRC both from Atlanta and in the field. These efforts included support for vaccination activities in DRC’s North Kivu and Ituri provinces for the population and for at-risk health-care workers in the DRC and neighboring countries. In addition, the United States is involved in the testing of experimental therapeutics and vaccines in the DRC in an effort to aid in this and future outbreaks.
“There are no cases of Ebola in the United States,” said Dr. Walke, and the CDC believes the risk to the United States from the outbreak is low. He cited the limited number of travelers from DRC. “There [are] about 16,000 from the DRC to the U.S. on an annual basis, and only about 100 from Goma itself. There aren’t direct flights and we have at the Goma airport both entry and exit screening.”
According to a World Health Organization report, this Ebola outbreak is the second deadliest on record and has killed 1,750 people out of around 2,518 confirmed cases as of July 23.
Efforts to control the epidemic are severely hampered by civil unrest in the area, public mistrust of the government and health care workers, and a comparative lack of international aid compared to previous Ebola outbreaks.
REPORTING FROM A MEDIA BRIEFING BY HHS
Conflicts of interest common among authors of ASCO guidelines
A significant number of physicians who author practice guidelines are not reporting financial conflicts of interest, a study finds.
Lead author Ramy R. Saleh, MD, of the University of Toronto, and colleagues searched The American Society of Clinical Oncology (ASCO) website to identify all clinical practice guidelines (CPGs) for systemic therapy published between August 2013 and June 2018. Investigators analyzed self-reported author financial conflicts of interest and funding sources and also reviewed The Open Payments database to identify compensation to guideline authors. Researchers categorized conflicts of interest into two groups: research funding (which could include departmental and/or hospital funding) and nonresearch payments (including travel expenses, honoraria, employment, and stock ownership to the individual author).
The initial search identified 121 CPGs published by ASCO between August 2013 and August 2018 of which 26 guidelines were selected because of their focus on systemic treatment. Findings showed that 239 guideline authors who were not exempt from reporting received industry payments, but only 184 (77%) disclosed these payments, according to the study in Cancer. The mean total of all undisclosed payments from 2013 to 2017 received by CPG authors was $187,503 and the median was $30,500. Of the 55 authors with undisclosed conflicts of interest, 34 authors (62%) received more than $1,000 of nonresearch funding, and 19 authors (35%) received more than $5,000 per calendar year.
The majority of the authors with undisclosed conflicts were medical oncologists, the investigators found. Radiation oncologists and surgeons had similar proportions of undisclosed financial conflicts.
The researchers concluded that financial conflicts of interest among authors of ASCO guidelines are common and are not disclosed in a substantial number of cases. The findings indicate that current self-disclosure practices are not adequate for accurately reporting conflicts, they noted.
“Improved transparency of [financial conflicts of interest should become standard practice among CPG authors,” the investigators wrote. “Professional societies and journal editors need to create a mechanism to verify self-reported [financial conflicts of interest].”
Source: Saleh et. al. 2019 July 29 doi: 10.1002/cncr.32408.
The study by Saleh et al. illustrates the need for a better disclosure system that is more consistent and allows for potential conflicts of interest to be more easily identified and managed, says Clifford A. Hudis, MD, of The American Society of Clinical Oncology.
In an editorial accompanying Dr. Saleh’s study in the July 29 issue of Cancer, Dr. Hudis and coauthor Robert W. Carlson, MD, of the National Comprehensive Cancer Network, write that while disclosure compliance is important, they do not believe the lack of disclosures reported in the analysis “represent malintent or malfeasance on the part of authors or a lack of diligence by the involved institutions.
“Instead, this represents one more in a potentially endless number of illustrative specific examples of all that is wrong — and must be fixed—with disclosure as currently practiced in the United States,” the authors wrote.
Dr. Hudis and Dr. Carlson outlined several possible solutions for a better disclosure system, including making the definitions of research funding, consultancy, honoraria, and travel support standardized and applied consistently. In addition, one source of universal disclosure should be developed within the house of medicine that provides a simple, easy-to-use, easily vetted, shared, and accessible resource that allows for the easy documentation, confirmation, and sharing of potential conflicts, according to the authors. Finally, companies that are subject to sunshine reporting should be required to notify covered individuals, in nearly real time, “when and what they are reporting so that there is no disconnect or time lag,” the doctors wrote.
Clifford A. Hudis is CEO for the American Society of Clinical Oncology and Robert W. Carlson is CEO for the National Comprehensive Cancer Network. Dr. Carlson reports being issued US patent D848,448S for Evidence Blocks (part of National Comprehensive Cancer Network guidelines).
The study by Saleh et al. illustrates the need for a better disclosure system that is more consistent and allows for potential conflicts of interest to be more easily identified and managed, says Clifford A. Hudis, MD, of The American Society of Clinical Oncology.
In an editorial accompanying Dr. Saleh’s study in the July 29 issue of Cancer, Dr. Hudis and coauthor Robert W. Carlson, MD, of the National Comprehensive Cancer Network, write that while disclosure compliance is important, they do not believe the lack of disclosures reported in the analysis “represent malintent or malfeasance on the part of authors or a lack of diligence by the involved institutions.
“Instead, this represents one more in a potentially endless number of illustrative specific examples of all that is wrong — and must be fixed—with disclosure as currently practiced in the United States,” the authors wrote.
Dr. Hudis and Dr. Carlson outlined several possible solutions for a better disclosure system, including making the definitions of research funding, consultancy, honoraria, and travel support standardized and applied consistently. In addition, one source of universal disclosure should be developed within the house of medicine that provides a simple, easy-to-use, easily vetted, shared, and accessible resource that allows for the easy documentation, confirmation, and sharing of potential conflicts, according to the authors. Finally, companies that are subject to sunshine reporting should be required to notify covered individuals, in nearly real time, “when and what they are reporting so that there is no disconnect or time lag,” the doctors wrote.
Clifford A. Hudis is CEO for the American Society of Clinical Oncology and Robert W. Carlson is CEO for the National Comprehensive Cancer Network. Dr. Carlson reports being issued US patent D848,448S for Evidence Blocks (part of National Comprehensive Cancer Network guidelines).
The study by Saleh et al. illustrates the need for a better disclosure system that is more consistent and allows for potential conflicts of interest to be more easily identified and managed, says Clifford A. Hudis, MD, of The American Society of Clinical Oncology.
In an editorial accompanying Dr. Saleh’s study in the July 29 issue of Cancer, Dr. Hudis and coauthor Robert W. Carlson, MD, of the National Comprehensive Cancer Network, write that while disclosure compliance is important, they do not believe the lack of disclosures reported in the analysis “represent malintent or malfeasance on the part of authors or a lack of diligence by the involved institutions.
“Instead, this represents one more in a potentially endless number of illustrative specific examples of all that is wrong — and must be fixed—with disclosure as currently practiced in the United States,” the authors wrote.
Dr. Hudis and Dr. Carlson outlined several possible solutions for a better disclosure system, including making the definitions of research funding, consultancy, honoraria, and travel support standardized and applied consistently. In addition, one source of universal disclosure should be developed within the house of medicine that provides a simple, easy-to-use, easily vetted, shared, and accessible resource that allows for the easy documentation, confirmation, and sharing of potential conflicts, according to the authors. Finally, companies that are subject to sunshine reporting should be required to notify covered individuals, in nearly real time, “when and what they are reporting so that there is no disconnect or time lag,” the doctors wrote.
Clifford A. Hudis is CEO for the American Society of Clinical Oncology and Robert W. Carlson is CEO for the National Comprehensive Cancer Network. Dr. Carlson reports being issued US patent D848,448S for Evidence Blocks (part of National Comprehensive Cancer Network guidelines).
A significant number of physicians who author practice guidelines are not reporting financial conflicts of interest, a study finds.
Lead author Ramy R. Saleh, MD, of the University of Toronto, and colleagues searched The American Society of Clinical Oncology (ASCO) website to identify all clinical practice guidelines (CPGs) for systemic therapy published between August 2013 and June 2018. Investigators analyzed self-reported author financial conflicts of interest and funding sources and also reviewed The Open Payments database to identify compensation to guideline authors. Researchers categorized conflicts of interest into two groups: research funding (which could include departmental and/or hospital funding) and nonresearch payments (including travel expenses, honoraria, employment, and stock ownership to the individual author).
The initial search identified 121 CPGs published by ASCO between August 2013 and August 2018 of which 26 guidelines were selected because of their focus on systemic treatment. Findings showed that 239 guideline authors who were not exempt from reporting received industry payments, but only 184 (77%) disclosed these payments, according to the study in Cancer. The mean total of all undisclosed payments from 2013 to 2017 received by CPG authors was $187,503 and the median was $30,500. Of the 55 authors with undisclosed conflicts of interest, 34 authors (62%) received more than $1,000 of nonresearch funding, and 19 authors (35%) received more than $5,000 per calendar year.
The majority of the authors with undisclosed conflicts were medical oncologists, the investigators found. Radiation oncologists and surgeons had similar proportions of undisclosed financial conflicts.
The researchers concluded that financial conflicts of interest among authors of ASCO guidelines are common and are not disclosed in a substantial number of cases. The findings indicate that current self-disclosure practices are not adequate for accurately reporting conflicts, they noted.
“Improved transparency of [financial conflicts of interest should become standard practice among CPG authors,” the investigators wrote. “Professional societies and journal editors need to create a mechanism to verify self-reported [financial conflicts of interest].”
Source: Saleh et. al. 2019 July 29 doi: 10.1002/cncr.32408.
A significant number of physicians who author practice guidelines are not reporting financial conflicts of interest, a study finds.
Lead author Ramy R. Saleh, MD, of the University of Toronto, and colleagues searched The American Society of Clinical Oncology (ASCO) website to identify all clinical practice guidelines (CPGs) for systemic therapy published between August 2013 and June 2018. Investigators analyzed self-reported author financial conflicts of interest and funding sources and also reviewed The Open Payments database to identify compensation to guideline authors. Researchers categorized conflicts of interest into two groups: research funding (which could include departmental and/or hospital funding) and nonresearch payments (including travel expenses, honoraria, employment, and stock ownership to the individual author).
The initial search identified 121 CPGs published by ASCO between August 2013 and August 2018 of which 26 guidelines were selected because of their focus on systemic treatment. Findings showed that 239 guideline authors who were not exempt from reporting received industry payments, but only 184 (77%) disclosed these payments, according to the study in Cancer. The mean total of all undisclosed payments from 2013 to 2017 received by CPG authors was $187,503 and the median was $30,500. Of the 55 authors with undisclosed conflicts of interest, 34 authors (62%) received more than $1,000 of nonresearch funding, and 19 authors (35%) received more than $5,000 per calendar year.
The majority of the authors with undisclosed conflicts were medical oncologists, the investigators found. Radiation oncologists and surgeons had similar proportions of undisclosed financial conflicts.
The researchers concluded that financial conflicts of interest among authors of ASCO guidelines are common and are not disclosed in a substantial number of cases. The findings indicate that current self-disclosure practices are not adequate for accurately reporting conflicts, they noted.
“Improved transparency of [financial conflicts of interest should become standard practice among CPG authors,” the investigators wrote. “Professional societies and journal editors need to create a mechanism to verify self-reported [financial conflicts of interest].”
Source: Saleh et. al. 2019 July 29 doi: 10.1002/cncr.32408.
Immune checkpoint inhibitors and locally ablative therapy in NSCLC
In this edition of “How I will treat my next patient,” I take a look at two phase 2 trials in stage IV non–small cell lung cancer (NSCLC) patients that appeared recently in JAMA Oncology. One summarizes a trial in stage IV NSCLC with four or fewer sites of metastasis (oligometastatic disease or OM), in which pembrolizumab is added to locally ablative therapy (LAT). The other examines whether LAT potentiates the response to immuno-oncology (I/O) in distant sites that were unexposed to LAT.
I/O added to LAT in OM-NSCLC
Joshua M. Bauml, MD, of the University of Pennsylvania, Philadelphia, and colleagues, published findings from a nonrandomized phase 2 trial in OM-NSCLC in which patients could receive LAT by any technique (JAMA Oncol. 2019 Jul 11. doi: 10.1001/jamaoncol.2019.1449). Patients could have synchronous or metachronous OM-NSCLC, any histology, and any PD-L1 tumor proportion score. Patients with more than four sites of metastatic disease that regressed to OM-NSCLC after prior therapy (i.e., “oligoremnant NSCLC”) were excluded.
They reported on 51 patients who received conventional-dose pembrolizumab for eight cycles after LAT. Patients without toxicity or progression were allowed to receive up to eight additional cycles of pembrolizumab. The median progression-free survival (PFS) was 19.1 months (95% confidence interval, 9.4-28.7 months), significantly longer than the historical comparison group (median PFS, 6.6 months; P = .005). Additionally, the 24-month overall survival (OS) was 77.5%. With respect to safety, no quality of life decrement or new safety signals were seen.
What this means in practice
As Dr. Bauml and colleagues suggest, there is strong theoretical rationale for believing that OM-NSCLC represents a special, potentially curable, population of stage IV NSCLC patients. Like the recently published work of Daniel R. Gomez, MD, of the University of Texas MD Anderson Cancer Center, Houston, and colleagues (J Clin Oncol. 2019 Jun 20;37[18]:1558-65), who studied LAT in comparison with consolidative/maintenance chemotherapy in a slightly different population of OM-NSCLC patients, the current trial moves clinical research forward.
Practically, this study has limitations that should temper a clinician’s enthusiasm for adopting the strategy of LAT, followed by I/O, as standard practice: small patient numbers, most with only one site of OM-NSCLC; comparison with historical controls; and no meaningful information about patient subsets who benefit from I/O and who do not. As the authors suggest, this study provides a strong rationale for a phase 3 trial with stratification for variables that could influence outcome. It does not inform clinical practice at the present time.
LAT added to I/O in stage IV NSCLC
We have limited ability to identify (the majority of) patients with metastatic NSCLC who will not benefit from I/O and no proven interventions to augment benefit in (the majority of) patients with low PD-L1 tumor proportion scores and/or low tumor mutation burden. However, the PEMBRO-RT study was designed to investigate whether LAT with stereotactic body radiation therapy (SBRT) could exploit the hypothesized increase in tumor antigen release and antigen presentation that could lead to better responses to I/O in untreated sites of disease among all patients with stage IV NSCLC.
As reported by Willemijn S.M.E. Theelen, MD, of the Netherlands Cancer Institute in Amsterdam and colleagues, the PEMBRO-RT study randomized 76 patients with stage IV NSCLC to pembro following SBRT to a single metastatic site (the experimental arm of the trial) or pembrolizumab alone. Pembrolizumab was given in a conventional dose and schedule in both arms of the trial and was administered within 7 days after SBRT on the experimental arm (JAMA Oncol. 2019 Jul 11. doi: 10.1001/jamaoncol.2019.1478).
The primary outcome was the overall response rate (ORR) at 12 weeks. Among patients on the experimental versus control arms, the ORR was 36% and 18%, respectively (P = .07). This did not meet the prespecified endpoint of improving ORR from 20% to 50% at 12 weeks. Additionally, although improved on the pembro plus SBRT arm of the trial, the median PFS and OS did not meet statistical criteria for improvement over the control arm, except among the 47 patients in the PD-L1 negative subset.
What this means in practice
There are a lot of potentially relevant variables in this small, randomized phase 2 study. As the authors discuss, if there is a dose and schedule of RT that facilitates antigen release and presentation and or an ideal latent period after radiotherapy that promotes an “abscopal effect” from I/O, it is unclear whether the ideal schema was used in the PEMBRO-RT trial.
At present, if a patient with stage IV NSCLC requires LAT for clinical reasons during I/O treatment, the patient can receive it safely, but without the expectation that the LAT will augment overall benefit from I/O. Additional preclinical work will need to help guide us about a rational way to design the next trial to test the concept of supra-additive benefit from these modalities. Not only is this combination “not ready for prime time” in clinical care, but it’s not ready for the large numbers of patients in a phase 3 clinical trial.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I will treat my next patient,” I take a look at two phase 2 trials in stage IV non–small cell lung cancer (NSCLC) patients that appeared recently in JAMA Oncology. One summarizes a trial in stage IV NSCLC with four or fewer sites of metastasis (oligometastatic disease or OM), in which pembrolizumab is added to locally ablative therapy (LAT). The other examines whether LAT potentiates the response to immuno-oncology (I/O) in distant sites that were unexposed to LAT.
I/O added to LAT in OM-NSCLC
Joshua M. Bauml, MD, of the University of Pennsylvania, Philadelphia, and colleagues, published findings from a nonrandomized phase 2 trial in OM-NSCLC in which patients could receive LAT by any technique (JAMA Oncol. 2019 Jul 11. doi: 10.1001/jamaoncol.2019.1449). Patients could have synchronous or metachronous OM-NSCLC, any histology, and any PD-L1 tumor proportion score. Patients with more than four sites of metastatic disease that regressed to OM-NSCLC after prior therapy (i.e., “oligoremnant NSCLC”) were excluded.
They reported on 51 patients who received conventional-dose pembrolizumab for eight cycles after LAT. Patients without toxicity or progression were allowed to receive up to eight additional cycles of pembrolizumab. The median progression-free survival (PFS) was 19.1 months (95% confidence interval, 9.4-28.7 months), significantly longer than the historical comparison group (median PFS, 6.6 months; P = .005). Additionally, the 24-month overall survival (OS) was 77.5%. With respect to safety, no quality of life decrement or new safety signals were seen.
What this means in practice
As Dr. Bauml and colleagues suggest, there is strong theoretical rationale for believing that OM-NSCLC represents a special, potentially curable, population of stage IV NSCLC patients. Like the recently published work of Daniel R. Gomez, MD, of the University of Texas MD Anderson Cancer Center, Houston, and colleagues (J Clin Oncol. 2019 Jun 20;37[18]:1558-65), who studied LAT in comparison with consolidative/maintenance chemotherapy in a slightly different population of OM-NSCLC patients, the current trial moves clinical research forward.
Practically, this study has limitations that should temper a clinician’s enthusiasm for adopting the strategy of LAT, followed by I/O, as standard practice: small patient numbers, most with only one site of OM-NSCLC; comparison with historical controls; and no meaningful information about patient subsets who benefit from I/O and who do not. As the authors suggest, this study provides a strong rationale for a phase 3 trial with stratification for variables that could influence outcome. It does not inform clinical practice at the present time.
LAT added to I/O in stage IV NSCLC
We have limited ability to identify (the majority of) patients with metastatic NSCLC who will not benefit from I/O and no proven interventions to augment benefit in (the majority of) patients with low PD-L1 tumor proportion scores and/or low tumor mutation burden. However, the PEMBRO-RT study was designed to investigate whether LAT with stereotactic body radiation therapy (SBRT) could exploit the hypothesized increase in tumor antigen release and antigen presentation that could lead to better responses to I/O in untreated sites of disease among all patients with stage IV NSCLC.
As reported by Willemijn S.M.E. Theelen, MD, of the Netherlands Cancer Institute in Amsterdam and colleagues, the PEMBRO-RT study randomized 76 patients with stage IV NSCLC to pembro following SBRT to a single metastatic site (the experimental arm of the trial) or pembrolizumab alone. Pembrolizumab was given in a conventional dose and schedule in both arms of the trial and was administered within 7 days after SBRT on the experimental arm (JAMA Oncol. 2019 Jul 11. doi: 10.1001/jamaoncol.2019.1478).
The primary outcome was the overall response rate (ORR) at 12 weeks. Among patients on the experimental versus control arms, the ORR was 36% and 18%, respectively (P = .07). This did not meet the prespecified endpoint of improving ORR from 20% to 50% at 12 weeks. Additionally, although improved on the pembro plus SBRT arm of the trial, the median PFS and OS did not meet statistical criteria for improvement over the control arm, except among the 47 patients in the PD-L1 negative subset.
What this means in practice
There are a lot of potentially relevant variables in this small, randomized phase 2 study. As the authors discuss, if there is a dose and schedule of RT that facilitates antigen release and presentation and or an ideal latent period after radiotherapy that promotes an “abscopal effect” from I/O, it is unclear whether the ideal schema was used in the PEMBRO-RT trial.
At present, if a patient with stage IV NSCLC requires LAT for clinical reasons during I/O treatment, the patient can receive it safely, but without the expectation that the LAT will augment overall benefit from I/O. Additional preclinical work will need to help guide us about a rational way to design the next trial to test the concept of supra-additive benefit from these modalities. Not only is this combination “not ready for prime time” in clinical care, but it’s not ready for the large numbers of patients in a phase 3 clinical trial.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I will treat my next patient,” I take a look at two phase 2 trials in stage IV non–small cell lung cancer (NSCLC) patients that appeared recently in JAMA Oncology. One summarizes a trial in stage IV NSCLC with four or fewer sites of metastasis (oligometastatic disease or OM), in which pembrolizumab is added to locally ablative therapy (LAT). The other examines whether LAT potentiates the response to immuno-oncology (I/O) in distant sites that were unexposed to LAT.
I/O added to LAT in OM-NSCLC
Joshua M. Bauml, MD, of the University of Pennsylvania, Philadelphia, and colleagues, published findings from a nonrandomized phase 2 trial in OM-NSCLC in which patients could receive LAT by any technique (JAMA Oncol. 2019 Jul 11. doi: 10.1001/jamaoncol.2019.1449). Patients could have synchronous or metachronous OM-NSCLC, any histology, and any PD-L1 tumor proportion score. Patients with more than four sites of metastatic disease that regressed to OM-NSCLC after prior therapy (i.e., “oligoremnant NSCLC”) were excluded.
They reported on 51 patients who received conventional-dose pembrolizumab for eight cycles after LAT. Patients without toxicity or progression were allowed to receive up to eight additional cycles of pembrolizumab. The median progression-free survival (PFS) was 19.1 months (95% confidence interval, 9.4-28.7 months), significantly longer than the historical comparison group (median PFS, 6.6 months; P = .005). Additionally, the 24-month overall survival (OS) was 77.5%. With respect to safety, no quality of life decrement or new safety signals were seen.
What this means in practice
As Dr. Bauml and colleagues suggest, there is strong theoretical rationale for believing that OM-NSCLC represents a special, potentially curable, population of stage IV NSCLC patients. Like the recently published work of Daniel R. Gomez, MD, of the University of Texas MD Anderson Cancer Center, Houston, and colleagues (J Clin Oncol. 2019 Jun 20;37[18]:1558-65), who studied LAT in comparison with consolidative/maintenance chemotherapy in a slightly different population of OM-NSCLC patients, the current trial moves clinical research forward.
Practically, this study has limitations that should temper a clinician’s enthusiasm for adopting the strategy of LAT, followed by I/O, as standard practice: small patient numbers, most with only one site of OM-NSCLC; comparison with historical controls; and no meaningful information about patient subsets who benefit from I/O and who do not. As the authors suggest, this study provides a strong rationale for a phase 3 trial with stratification for variables that could influence outcome. It does not inform clinical practice at the present time.
LAT added to I/O in stage IV NSCLC
We have limited ability to identify (the majority of) patients with metastatic NSCLC who will not benefit from I/O and no proven interventions to augment benefit in (the majority of) patients with low PD-L1 tumor proportion scores and/or low tumor mutation burden. However, the PEMBRO-RT study was designed to investigate whether LAT with stereotactic body radiation therapy (SBRT) could exploit the hypothesized increase in tumor antigen release and antigen presentation that could lead to better responses to I/O in untreated sites of disease among all patients with stage IV NSCLC.
As reported by Willemijn S.M.E. Theelen, MD, of the Netherlands Cancer Institute in Amsterdam and colleagues, the PEMBRO-RT study randomized 76 patients with stage IV NSCLC to pembro following SBRT to a single metastatic site (the experimental arm of the trial) or pembrolizumab alone. Pembrolizumab was given in a conventional dose and schedule in both arms of the trial and was administered within 7 days after SBRT on the experimental arm (JAMA Oncol. 2019 Jul 11. doi: 10.1001/jamaoncol.2019.1478).
The primary outcome was the overall response rate (ORR) at 12 weeks. Among patients on the experimental versus control arms, the ORR was 36% and 18%, respectively (P = .07). This did not meet the prespecified endpoint of improving ORR from 20% to 50% at 12 weeks. Additionally, although improved on the pembro plus SBRT arm of the trial, the median PFS and OS did not meet statistical criteria for improvement over the control arm, except among the 47 patients in the PD-L1 negative subset.
What this means in practice
There are a lot of potentially relevant variables in this small, randomized phase 2 study. As the authors discuss, if there is a dose and schedule of RT that facilitates antigen release and presentation and or an ideal latent period after radiotherapy that promotes an “abscopal effect” from I/O, it is unclear whether the ideal schema was used in the PEMBRO-RT trial.
At present, if a patient with stage IV NSCLC requires LAT for clinical reasons during I/O treatment, the patient can receive it safely, but without the expectation that the LAT will augment overall benefit from I/O. Additional preclinical work will need to help guide us about a rational way to design the next trial to test the concept of supra-additive benefit from these modalities. Not only is this combination “not ready for prime time” in clinical care, but it’s not ready for the large numbers of patients in a phase 3 clinical trial.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
Illusion of options
Mr. M wanted a second opinion. He was almost 80 years old and had been healthy his entire life. But recent abdominal discomfort prompted a CT scan, which prompted a biopsy. It appeared the tumor had started in his pancreas and then spread to the lymph nodes and the wall of his abdomen.
He asked his doctor to “give it to him straight,” and she did. She told him that it was incurable, but that chemotherapy might slow it down. He asked how long he had, and she said less than a year.
He wanted a straight answer, but that wasn’t the answer he wanted. Who would? So he did some reading and decided to come to a large academic hospital an hour away for a second opinion.
I interviewed him and then scrolled through his CT scans outside the room. There were a few things we could do, the attending and I discussed. We would send his tumor for genetic testing to see if there were any cancer mutations that could be targeted with drugs more specific than standard chemotherapy. We would also refer him to our cancer genetics clinic to get his blood tested for inherited mutations.
But mostly, all of that would likely turn up negative. Mostly, we agreed with his local oncologist.
We went back in the room. Explaining the genetic testing took the length of the visit because this is not a straightforward concept. We explained the difference between tumor mutations and inherited mutations. We wrote down a list of genetic variations we could discover. We discussed treatment options that could go along with each.
Do you have any questions?
He broke down. He reached for the tissue box sitting on the exam room table. “I feel so much better,” he said. “This is why I came here.” He felt safe, reassured, and hopeful.
I was happy to be helpful, but later, as I wrote my clinic note about him, I felt uneasy about the visit.
Everything we said was true. But somehow, it still felt as though we left him with an overly optimistic view of his illness. Did our emphasis on what could be done overshadow that it was unlikely to change the big picture? Did our in-depth discussion of slim possibilities mask that his prognosis was, in fact, still grim?
Working at a large academic medical center, I see many patients who come for a second opinion. I’m incredibly fortunate to learn at a place that is not just up to date in the most cutting-edge treatments but often leading in innovation.
And so we offer patients these options. They sound novel and exciting. They fill patients with hope because they fill the field with hope. I, too, get enraptured with the possibilities – circulating tumor DNA and clinical trials and targeted therapies.
At big cancer meetings every year, oncologists come together and speak about cancer therapies with enthusiasm and hope. Advances have exploded; it’s an exciting time to be learning and practicing.
And yet, the reality for many patients is very different. We are still discussing hospice after one line of chemotherapy has failed. We are still gently holding hands and saying that we have no more options to treat their aggressive cancers.
How can both of these worlds coexist? How can both be true?
A few years ago, a friend was diagnosed with a devastating neurologic condition. I went to a clinical trials website and typed in her disease. Immediately, hundreds of options popped up. I felt hopeful. The field is moving forward, I thought. There are options.
But in the exam room, there were none. When I asked about what I had read, the neurologist explained how many of these possibilities were being investigated. But in the end, my friend really had no good options.
After my visit with Mr. M, I thought about how commonly this story plays out in my field of hematology and oncology. Yes, there are instances in which we find a mutation that drastically changes management. It’s wonderful to witness: patients handed an ominous diagnosis and then living their normal lives, in remission or with stable disease, years later.
We all hope for that. But we rarely get it. The challenge comes when we spend 95% of a visit talking about something with a 1% chance of working. The numbers don’t add up – it’s an equation that easily results in false understanding. Cancer can be glossed with a veneer of innovative options, obscuring the reality that none are likely to work.
Weaving both truths into the conversation is a difficult skill, but one I decided to be more cognizant of after my encounter with Mr. M.
At our next visit, we were still waiting on the test results. But I decided to speak with him candidly. It’s important to have a plan B, I said, and asked what would be important to him if his time were limited. He nodded, thinking about this. “I’ve just been holding out hope for the mutation,” he admitted.
The next week his genetic testing came back negative, and he decided to get palliative chemotherapy closer to home. He had no reason to come to a large academic hospital anymore. With nothing special to offer him, I never saw him again.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz and listen to her each week on the Blood & Cancer podcast.
Mr. M wanted a second opinion. He was almost 80 years old and had been healthy his entire life. But recent abdominal discomfort prompted a CT scan, which prompted a biopsy. It appeared the tumor had started in his pancreas and then spread to the lymph nodes and the wall of his abdomen.
He asked his doctor to “give it to him straight,” and she did. She told him that it was incurable, but that chemotherapy might slow it down. He asked how long he had, and she said less than a year.
He wanted a straight answer, but that wasn’t the answer he wanted. Who would? So he did some reading and decided to come to a large academic hospital an hour away for a second opinion.
I interviewed him and then scrolled through his CT scans outside the room. There were a few things we could do, the attending and I discussed. We would send his tumor for genetic testing to see if there were any cancer mutations that could be targeted with drugs more specific than standard chemotherapy. We would also refer him to our cancer genetics clinic to get his blood tested for inherited mutations.
But mostly, all of that would likely turn up negative. Mostly, we agreed with his local oncologist.
We went back in the room. Explaining the genetic testing took the length of the visit because this is not a straightforward concept. We explained the difference between tumor mutations and inherited mutations. We wrote down a list of genetic variations we could discover. We discussed treatment options that could go along with each.
Do you have any questions?
He broke down. He reached for the tissue box sitting on the exam room table. “I feel so much better,” he said. “This is why I came here.” He felt safe, reassured, and hopeful.
I was happy to be helpful, but later, as I wrote my clinic note about him, I felt uneasy about the visit.
Everything we said was true. But somehow, it still felt as though we left him with an overly optimistic view of his illness. Did our emphasis on what could be done overshadow that it was unlikely to change the big picture? Did our in-depth discussion of slim possibilities mask that his prognosis was, in fact, still grim?
Working at a large academic medical center, I see many patients who come for a second opinion. I’m incredibly fortunate to learn at a place that is not just up to date in the most cutting-edge treatments but often leading in innovation.
And so we offer patients these options. They sound novel and exciting. They fill patients with hope because they fill the field with hope. I, too, get enraptured with the possibilities – circulating tumor DNA and clinical trials and targeted therapies.
At big cancer meetings every year, oncologists come together and speak about cancer therapies with enthusiasm and hope. Advances have exploded; it’s an exciting time to be learning and practicing.
And yet, the reality for many patients is very different. We are still discussing hospice after one line of chemotherapy has failed. We are still gently holding hands and saying that we have no more options to treat their aggressive cancers.
How can both of these worlds coexist? How can both be true?
A few years ago, a friend was diagnosed with a devastating neurologic condition. I went to a clinical trials website and typed in her disease. Immediately, hundreds of options popped up. I felt hopeful. The field is moving forward, I thought. There are options.
But in the exam room, there were none. When I asked about what I had read, the neurologist explained how many of these possibilities were being investigated. But in the end, my friend really had no good options.
After my visit with Mr. M, I thought about how commonly this story plays out in my field of hematology and oncology. Yes, there are instances in which we find a mutation that drastically changes management. It’s wonderful to witness: patients handed an ominous diagnosis and then living their normal lives, in remission or with stable disease, years later.
We all hope for that. But we rarely get it. The challenge comes when we spend 95% of a visit talking about something with a 1% chance of working. The numbers don’t add up – it’s an equation that easily results in false understanding. Cancer can be glossed with a veneer of innovative options, obscuring the reality that none are likely to work.
Weaving both truths into the conversation is a difficult skill, but one I decided to be more cognizant of after my encounter with Mr. M.
At our next visit, we were still waiting on the test results. But I decided to speak with him candidly. It’s important to have a plan B, I said, and asked what would be important to him if his time were limited. He nodded, thinking about this. “I’ve just been holding out hope for the mutation,” he admitted.
The next week his genetic testing came back negative, and he decided to get palliative chemotherapy closer to home. He had no reason to come to a large academic hospital anymore. With nothing special to offer him, I never saw him again.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz and listen to her each week on the Blood & Cancer podcast.
Mr. M wanted a second opinion. He was almost 80 years old and had been healthy his entire life. But recent abdominal discomfort prompted a CT scan, which prompted a biopsy. It appeared the tumor had started in his pancreas and then spread to the lymph nodes and the wall of his abdomen.
He asked his doctor to “give it to him straight,” and she did. She told him that it was incurable, but that chemotherapy might slow it down. He asked how long he had, and she said less than a year.
He wanted a straight answer, but that wasn’t the answer he wanted. Who would? So he did some reading and decided to come to a large academic hospital an hour away for a second opinion.
I interviewed him and then scrolled through his CT scans outside the room. There were a few things we could do, the attending and I discussed. We would send his tumor for genetic testing to see if there were any cancer mutations that could be targeted with drugs more specific than standard chemotherapy. We would also refer him to our cancer genetics clinic to get his blood tested for inherited mutations.
But mostly, all of that would likely turn up negative. Mostly, we agreed with his local oncologist.
We went back in the room. Explaining the genetic testing took the length of the visit because this is not a straightforward concept. We explained the difference between tumor mutations and inherited mutations. We wrote down a list of genetic variations we could discover. We discussed treatment options that could go along with each.
Do you have any questions?
He broke down. He reached for the tissue box sitting on the exam room table. “I feel so much better,” he said. “This is why I came here.” He felt safe, reassured, and hopeful.
I was happy to be helpful, but later, as I wrote my clinic note about him, I felt uneasy about the visit.
Everything we said was true. But somehow, it still felt as though we left him with an overly optimistic view of his illness. Did our emphasis on what could be done overshadow that it was unlikely to change the big picture? Did our in-depth discussion of slim possibilities mask that his prognosis was, in fact, still grim?
Working at a large academic medical center, I see many patients who come for a second opinion. I’m incredibly fortunate to learn at a place that is not just up to date in the most cutting-edge treatments but often leading in innovation.
And so we offer patients these options. They sound novel and exciting. They fill patients with hope because they fill the field with hope. I, too, get enraptured with the possibilities – circulating tumor DNA and clinical trials and targeted therapies.
At big cancer meetings every year, oncologists come together and speak about cancer therapies with enthusiasm and hope. Advances have exploded; it’s an exciting time to be learning and practicing.
And yet, the reality for many patients is very different. We are still discussing hospice after one line of chemotherapy has failed. We are still gently holding hands and saying that we have no more options to treat their aggressive cancers.
How can both of these worlds coexist? How can both be true?
A few years ago, a friend was diagnosed with a devastating neurologic condition. I went to a clinical trials website and typed in her disease. Immediately, hundreds of options popped up. I felt hopeful. The field is moving forward, I thought. There are options.
But in the exam room, there were none. When I asked about what I had read, the neurologist explained how many of these possibilities were being investigated. But in the end, my friend really had no good options.
After my visit with Mr. M, I thought about how commonly this story plays out in my field of hematology and oncology. Yes, there are instances in which we find a mutation that drastically changes management. It’s wonderful to witness: patients handed an ominous diagnosis and then living their normal lives, in remission or with stable disease, years later.
We all hope for that. But we rarely get it. The challenge comes when we spend 95% of a visit talking about something with a 1% chance of working. The numbers don’t add up – it’s an equation that easily results in false understanding. Cancer can be glossed with a veneer of innovative options, obscuring the reality that none are likely to work.
Weaving both truths into the conversation is a difficult skill, but one I decided to be more cognizant of after my encounter with Mr. M.
At our next visit, we were still waiting on the test results. But I decided to speak with him candidly. It’s important to have a plan B, I said, and asked what would be important to him if his time were limited. He nodded, thinking about this. “I’ve just been holding out hope for the mutation,” he admitted.
The next week his genetic testing came back negative, and he decided to get palliative chemotherapy closer to home. He had no reason to come to a large academic hospital anymore. With nothing special to offer him, I never saw him again.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz and listen to her each week on the Blood & Cancer podcast.
VHA Practice Guideline Recommendations for Diffuse Gliomas (FULL)
Over the past few decades, our understanding of the molecular underpinning of primary neoplasms of the central nervous system (CNS) has progressed substantially. Thanks in large part to this expansion in our knowledge base, the World Health Organization (WHO) has recently updated its classification of tumors of the CNS.1 One of the key elements of this update was the inclusion of molecular diagnostic criteria for the classification of infiltrating gliomas. While the previous classification system was based upon histologic subtypes of the tumor (astrocytoma, oligodendroglioma, and oligoastrocytoma), the revised classification system incorporates molecular testing to establish the genetic characteristics of the tumor to reach a final integrated diagnosis.
In this article, we present 3 cases to highlight some of these recent changes in the WHO diagnostic categories of primary CNS tumors and to illustrate the role of specific molecular tests in reaching a final integrated diagnosis. We then propose a clinical practice guideline for the Veterans Health Administration (VHA) that recommends use of molecular testing for veterans as part of the diagnostic workup of primary CNS neoplasms.
Purpose
In 2013 the VHA National Director of Pathology & Laboratory Medicine Services (P&LMS) chartered a national molecular genetics pathology workgroup (MGPW) that was charged with 4 specific tasks: (1) Provide recommendations about the effective use of molecular genetic testing for veterans; (2) Promote increased quality and availability of molecular testing within the VHA; (3) Encourage internal referral testing; and (4) Create an organizational structure and policies for molecular genetic testing and laboratory developed tests. The workgroup is currently composed of 4 subcommittees: genetic medicine, hematopathology, pharmacogenomics, and molecular oncology. The molecular oncology subcommittee is focused upon molecular genetic testing for solid tumors.
This article is intended to be the first of several publications from the molecular oncology subcommittee of the MGPW that address some of the aforementioned tasks. Similar to the recent publication from the hematopathology subcommittee of the MGPW, this article focuses on CNS neoplasms.2
Scope of Problem
The incidence of tumors of the CNS in the US population varies among age groups. It is the most common solid tumor in children aged < 14 years and represents a significant cause of mortality across all age groups.3 Of CNS tumors, diffuse gliomas comprise about 20% of the tumors and more than 70% of the primary malignant CNS tumors.3 Analysis of the VA Central Cancer Registry data from 2010 to 2014 identified 1,186 veterans (about 237 veterans per year) who were diagnosed with diffuse gliomas. (Lynch, Kulich, Colman, unpublished data, February 2018). While the majority (nearly 80%) of these cases were glioblastomas (GBMs), unfortunately a majority of these cases did not undergo molecular testing (Lynch, Kulich, Colman, unpublished data, February 2018).
Although this low rate of testing may be in part reflective of the period from which these data were gleaned (ie, prior to the WHO release of their updated the classification of tumors of the CNS), it is important to raise VA practitioners’ awareness of these recent changes to ensure that veterans receive the proper diagnosis and treatment for their disease. Thus, while the number of veterans diagnosed with diffuse gliomas within the VHA is relatively small in comparison to other malignancies, such as prostatic adenocarcinomas and lung carcinomas, the majority of diffuse gliomas do not seem to be receiving the molecular testing that would be necessary for (1) appropriate classification under the recently revised WHO recommendations; and (2) making important treatment decisions.
Case Presentations
Case 1. A veteran of the Gulf War presented with a 3-month history of possible narcoleptic events associated with a motor vehicle accident. Magnetic resonance imaging (MRI) revealed a large left frontal mass lesion with minimal surrounding edema without appreciable contrast enhancement (Figures 1A, 1B, and 1C).
Neither mitotic figures nor endothelial proliferation were identified. Immunohistochemical stains revealed a lack of R132H mutant IDH1 protein expression, a loss of nuclear staining for ATRX protein within a substantial number of cells, and a clonal pattern of p53 protein overexpression (Figures 1E, 1F, and 1G). The lesion demonstrated diffuse glial fibrillary acidic protein (GFAP) immunoreactivity and a low proliferation index (as determined by Ki-67 staining; estimated at less than 5%) (Figures 1H and 1I).
Based upon these results, an initial morphologic diagnosis of diffuse glioma was issued, and tissue was subjected to a variety of nucleic acid-based tests. While fluorescence in situ hybridization (FISH) studies were negative for 1p/19q codeletion, pyrosequencing analysis revealed the presence of a c.394C>T (R132C) mutation of the IDH1 gene (Figure 1J). The University of Pittsburgh Medical Center’s GlioSeq targeted next-generation sequence (NGS) analysis confirmed the presence of the c.394C > T mutation in IDH1 gene.4 Based upon this additional information, a final integrated morphologic and molecular diagnosis of diffuse astrocytoma, IDH-mutant was rendered.
Case 2. A Vietnam War veteran presented with a 6-week history of new onset falls with associated left lower extremity weakness. A MRI revealed a right frontoparietal mass lesion with surrounding edema without appreciable contrast enhancement (Figures 2A, 2B, and 2C).
Immunohistochemical stains revealed R132H mutant IDH1 protein expression, retention of nuclear staining for ATRX protein, the lack of a clonal pattern of p53 protein overexpression, diffuse GFAP immunoreactivity, and a proliferation index (as determined by Ki-67 staining) focally approaching 20% (Figures 2E, 2F, 2G, 2H and 2I).
Based upon these results, an initial morphologic diagnosis of diffuse (high grade) glioma was issued, and tissue was subjected to a variety of nucleic acid-based tests. The FISH studies were positive for 1p/19q codeletion, and pyrosequencing analysis confirmed the immunohistochemical findings of a c.395G>A (R132H) mutation of the IDH1 gene (Figure 2J). GlioSeq targeted NGS analysis confirmed the presence of the c.395G>A mutation in the IDH1 gene, a mutation in the telomerase reverse transcriptase (TERT) promoter, and possible decreased copy number of the CIC (chromosome 1p) and FUBP1 (chromosome 19q) genes.
A final integrated morphologic and molecular diagnosis of anaplastic oligodendroglioma, IDH-mutant and 1p/19q-codeleted was rendered based on the additional information. With this final diagnosis, methylation analysis of the MGMT gene promoter, which was performed for prognostic and predictive purposes, was identified in this case.5,6
Case 3. A veteran of the Vietnam War presented with a new onset seizure. A MRI revealed a focally contrast-enhancing mass with surrounding edema within the left frontal lobe (Figures 3A, 3B, and 3C).
Hematoxylin and eosin (H&E) stained sections following formalin fixation and paraffin embedding demonstrated similar findings (Figure 3D), and while mitotic figures were readily identified, areas of necrosis were not identified and endothelial proliferation was not a prominent feature. Immunohistochemical stains revealed no evidence of R132H mutant IDH1 protein expression, retention of nuclear staining for ATRX protein, a clonal pattern of p53 protein overexpression, patchy GFAP immunoreactivity, and a proliferation index (as determined by Ki-67 staining) focally approaching 50% (Figures 3E, 3F, 3G, 3H, and 3I).
Based upon these results, an initial morphologic diagnosis of diffuse (high grade) glioma was issued, and the tissue was subjected to a variety of nucleic acid-based tests. The FISH studies were negative for EGFR gene amplification and 1p/19q codeletion, although a gain of the long arm of chromosome 1 was detected. Pyrosequencing analysis for mutations in codon 132 of the IDH1 gene revealed no mutations (Figure 3J). GlioSeq targeted NGS analysis identified mutations within the NF1, TP53, and PIK3CA genes without evidence of mutations in the IDH1, IDH2, ATRX, H3F3A, or EGFR genes or the TERT promoter. Based upon this additional information, a final integrated morphologic and molecular diagnosis of GBM, IDH wild-type was issued. The MGMT gene promoter was negative for methylation, a finding that has prognostic and predictive impact with regard to treatment with temazolamide.7-9
New Diffuse Glioma Classification
Since the issuance of the previous edition of the WHO classification of CNS tumors in 2007, several sentinel discoveries have been made that have advanced our understanding of the underlying biology of primary CNS neoplasms. Since a detailed review of these findings is beyond the scope and purpose of this manuscript and salient reviews on the topic can be found elsewhere, we will focus on the molecular findings that have been incorporated into the recently revised WHO classification.10 The importance of providing such information for proper patient management is illustrated by the recent acknowledgement by the American Academy of Neurology that molecular testing of brain tumors is a specific area in which there is a need for quality improvement.11 Therefore, it is critical that these underlying molecular abnormalities are identified to allow for proper classification and treatment of diffuse gliomas in the veteran population.
As noted previously, based on VA cancer registry data, diffuse gliomas are the most commonly encountered primary CNS cancers in the veteran population. Several of the aforementioned seminal discoveries have been incorporated into the updated classification of diffuse gliomas. While the recently updated WHO classification allows for the assignment of “not otherwise specified (NOS)” diagnostic designation, this category must be limited to cases where there is insufficient data to allow for a more precise classification due to sample limitations and not simply due to a failure of VA pathology laboratories to pursue the appropriate diagnostic testing.
Figure 4 presents the recommended diagnostic workflow for the workup of diffuse gliomas. As illustrated in the above cases, a variety of different methodologies, including immunohistochemical, FISH, loss of heterozygosity analysis, traditional and NGS may be applied when elucidating the status of molecular events at critical diagnostic branch points.
Diagnostic Uses of Molecular Testing
While the case studies in this article demonstrate the use of ancillary testing and provide a suggested strategy for properly subclassifying diffuse gliomas, inherent in this strategy is the assumption that, based upon the initial clinical and pathologic information available, one can accurately categorize the lesion as a diffuse glioma. In reality, such a distinction is not always a straightforward endeavor. It is well recognized that a proportion of low-grade, typically radiologically circumscribed, CNS neoplasms, such as pilocytic astrocytomas and glioneuronal tumors, may infiltrate the surrounding brain parenchyma. In addition, many of these low-grade CNS neoplasms also may have growth patterns that are shared with diffuse gliomas, a diagnostic challenge that often can be further hampered by the inherent limitations involved in obtaining adequate samples for diagnosis from the CNS.
Although there are limitations and caveats, molecular diagnostic testing may be invaluable in properly classifying CNS tumors in such situations. The finding of mutations in the IDH1 or IDH2 genes has been shown to be very valuable in distinguishing low-grade diffuse glioma from both nonneoplastic and low-grade circumscribed neuroepithelial neoplasms that may exhibit growth patterns that can mimic those of diffuse gliomas.15-17 Conversely, finding abnormalities in the BRAF gene in a brain neoplasm that has a low-grade morphology suggests that the lesion may represent one of these low-grade lesions such as a pleomorphic xanthoastrocytoma, pilocytic astrocytoma, or mixed neuronal-glial tumor as opposed to a diffuse glioma.18,19
Depending upon the environment in which one practices, small biopsy specimens may be prevalent, and unfortunately, it is not uncommon to obtain a biopsy that exhibits a histologic growth pattern that is discordant from what one would predict based on the clinical context and imaging findings. Molecular testing may be useful in resolving discordances in such situations. If a biopsy of a ring-enhancing lesion demonstrates a diffuse glioma that doesn’t meet WHO grade IV criteria, applying methodologies that look for genetic features commonly encountered in high-grade astrocytomas may identify genetic abnormalities that suggest a more aggressive lesion than is indicated by the histologic findings. The presence of genetic abnormalities such as homozygous deletion of the CDKN2A gene, TERT promoter mutation, loss of heterozygosity of chromosome 10q and/or phosphatase and tensin homolog (PTEN) mutations, EGFR gene amplification or the presence of the EGFR variant III are a few findings that would suggest the aforementioned sample may represent an undersampling of a higher grade diffuse astrocytoma, which would be important information to convey to the treating clinicians.20-26
Testing In the VA
The goals of the MPWG include promoting increased quality and availability of genetic testing within the VHA as well as encouraging internal referral testing. An informal survey of the chiefs of VA Pathology and Laboratory Medicine Services was conducted in November of 2017 in an attempt to identify internal VA pathology laboratories currently conducting testing that may be of use in the workup of diffuse gliomas (Table 1).
The VA currently offers NGS panels for patients with advanced-stage malignancies under the auspices of the Precision Oncology Program, whose reports provide both (1) mutational analyses for genes such as TP53, ATRX, NF1, BRAF, PTEN, TERT IDH1, and IDH2 that may be useful in the proper classifying of high-grade diffuse gliomas; and (2) information regarding clinical trials for which the veteran may be eligible for based on their glioma’s mutational profile. Interested VA providers should visit tinyurl.com/precisiononcology/ for more information about this program. Finally, although internal testing within VA laboratories is recommended to allow for the development of more cost-effective testing, testing may be performed through many nationally contracted reference laboratories.
Conclusion
In light of the recent progress made in our understanding of the molecular events of gliomagenesis, the way we diagnose diffuse gliomas within the CNS has undergone a major paradigm shift. While histology still plays a critical role in the process, we believe that additional ancillary testing is a requirement for all diffuse gliomas diagnosed within VA pathology laboratories. In the context of recently encountered cases, we have provided a recommended workflow highlighting the testing that can be performed to allow for the proper diagnosis of our veterans with diffuse gliomas (Figure 4).
Unless limited by the amount of tissue available for such tests, ancillary testing must be performed on all diffuse gliomas diagnosed within the VA system to ensure proper diagnosis and treatment of our veterans with diffuse gliomas.
Acknowledgments
The authors thank Dr. Craig M. Horbinski (Feinberg School of Medicine, Northwestern University) and Dr. Geoffrey H. Murdoch (University of Pittsburgh) for their constructive criticism of the manuscript. We also thank the following individuals for past service as members of the molecular oncology subcommittee of the MGPW: Dr. George Ansstas (Washington University School of Medicine), Dr. Osssama Hemadeh (Bay Pines VA Health Care System), Dr. James Herman (VA Pittsburgh Healthcare System), and Dr. Ryan Phan (formerly of the VA Greater Los Angeles Healthcare System) as well as the members of the Veterans Administration pathology and laboratory medicine service molecular genetics pathology workgroup.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies.
Dr. Kulich is the Acting Chief of Pathology and Laboratory Medicine Service at VA Pittsburgh Healthcare System and member of the Division of Neuropathology at University of Pittsburgh Department of Pathology, Dr. Duvvuri is an Otolaryngologist at VA Pittsburgh Healthcare System, and Dr. Passero is the Section Chief of Hematology\Oncology at VA Pittsburgh Healthcare System in Pennsylvania. Dr. Becker is an Oncologist at VA-New York Harbor Healthcare System. Dr. Dacic is a Pathologist at University of Pittsburgh Department of Pathology in Pennsylvania. Dr. Ehsan is Chief of Pathology and Laboratory Medicine Services at the South Texas Veterans Healthcare System in San Antonio. Dr. Gutkin is the former Chief of Pathology and Laboratory Medicine Service at VA Pittsburgh Healthcare System. Dr. Hou is a Pathologist at St. Louis VA Medical Center in Missouri. Dr. Icardi is the VA National Director of Pathology and Laboratory Medicine Services. Dr. Lyle is a Pathologist at Bay Pine Health Care System in Florida. Dr. Lynch is an Investigator at VA Salt Lake Health Care System Informatics and Computing Infrastructure. Dr. Montgomery is an Oncologist at VA Puget Sound Health Care System, in Seattle, Washington. Dr. Przygodzki is the Director of Genomic Medicine Implementation and Associate Director of Genomic Medicine for the VA. Dr. Colman is a Neuro-Oncologist at George E. Wahlen VA Medical Center and the Director of Medical Neuro-Oncology at the Huntsman Cancer Institute, Salt Lake City, Utah.
Correspondence: Dr. Kulich ([email protected])
1. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803-820.
2. Wang-Rodriguez J, Yunes A, Phan R, et al. The challenges of precision medicine and new advances in molecular diagnostic testing in hematolymphoid malignancies: impact on the VHA. Fed Pract. 2017;34(suppl 5):S38-S49.
3. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017;19(suppl 5):v1-v88.
4. Nikiforova MN, Wald AI, Melan MA, et al. Targeted next-generation sequencing panel (GlioSeq) provides comprehensive genetic profiling of central nervous system tumors. Neuro Oncol. 2016;18(3)379-387.
5. Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90(19):1473-1479.
6. van den Bent MJ, Erdem-Eraslan L, Idbaih A, et al. MGMT-STP27 methylation status as predictive marker for response to PCV in anaplastic oligodendrogliomas and oligoastrocytomas. A report from EORTC study 26951. Clin Cancer Res. 2013;19(19):5513-5522.
7. Stupp R, Hegi ME, Mason WP, et al; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459-466.
8. Malmstrom A, Gronberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916-926.
9. van den Bent MJ, Kros JM. Predictive and prognostic markers in neuro-oncology. J Neuropathol Exp Neurol. 2007;66(12):1074-1081.
10. Chen R, Smith-Cohn M, Cohen AL, Colman H. Glioma subclassifications and their clinical significance. Neurotherapeutics. 2017;14(2):284-297.
11. Jordan JT, Sanders AE, Armstrong T, et al. Quality improvement in neurology: neuro-oncology quality measurement set. Neurology. 2018;90(14):652-658.
12. Chen L, Voronovich Z, Clark K, et al. Predicting the likelihood of an isocitrate dehydrogenase 1 or 2 mutation in diagnoses of infiltrative glioma. Neuro Oncol. 2014;16(11):1478-1483.
13. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997-1003.
14. Wick W, Platten M, Meisner C, et al; NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707-715.
15. Horbinski C, Kofler J, Kelly LM, Murdoch GH, Nikiforova MN. Diagnostic use of IDH1/2 mutation analysis in routine clinical testing of formalin-fixed, paraffin-embedded glioma tissues. J Neuropathol Exp Neurol. 2009;68(12):1319-1325.
16. Camelo-Piragua S, Jansen M, Ganguly A, Kim JC, Louis DN, Nutt CL. Mutant IDH1-specific immunohistochemistry distinguishes diffuse astrocytoma from astrocytosis. Acta Neuropathol. 2010;119(4):509-511.
17. Horbinski C, Kofler J, Yeaney G, et al. Isocitrate dehydrogenase 1 analysis differentiates gangliogliomas from infiltrative gliomas. Brain Pathol. 2011;21(5):564-574.
18. Berghoff AS, Preusser M. BRAF alterations in brain tumours: molecular pathology and therapeutic opportunities. Curr Opin Neurol. 2014;27(6):689-696.
19. Korshunov A, Meyer J, Capper D, et al. Combined molecular analysis of BRAF and IDH1 distinguishes pilocytic astrocytoma from diffuse astrocytoma. Acta Neuropathol. 2009;118(3):401-405.
20. Fuller CE, Schmidt RE, Roth KA, et al. Clinical utility of fluorescence in situ hybridization (FISH) in morphologically ambiguous gliomas with hybrid oligodendroglial/astrocytic features. J Neuropathol Exp Neurol. 2003;62(11):1118-1128.
21. Horbinski C. Practical molecular diagnostics in neuropathology: making a tough job a little easier. Semin Diagn Pathol. 2010;27(2):105-113.
22. Fuller GN, Bigner SH. Amplified cellular oncogenes in neoplasms of the human central nervous system. Mutat Res. 1992;276(3):299-306.
23. Brennan CW, Verhaak RG, McKenna A, et al; TCGA Research Network. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462-477.
24. Aldape K, Zadeh G, Mansouri S, Reifenberger G, von Deimling A. Glioblastoma: pathology, molecular mechanisms and markers. Acta Neuropathol. 2015;129(6):829-848.
25. Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110(15):6021-6026.
26. Nikiforova MN, Hamilton RL. Molecular diagnostics of gliomas. Arch Pathol Lab Med. 2011;135(5):558-568.
Over the past few decades, our understanding of the molecular underpinning of primary neoplasms of the central nervous system (CNS) has progressed substantially. Thanks in large part to this expansion in our knowledge base, the World Health Organization (WHO) has recently updated its classification of tumors of the CNS.1 One of the key elements of this update was the inclusion of molecular diagnostic criteria for the classification of infiltrating gliomas. While the previous classification system was based upon histologic subtypes of the tumor (astrocytoma, oligodendroglioma, and oligoastrocytoma), the revised classification system incorporates molecular testing to establish the genetic characteristics of the tumor to reach a final integrated diagnosis.
In this article, we present 3 cases to highlight some of these recent changes in the WHO diagnostic categories of primary CNS tumors and to illustrate the role of specific molecular tests in reaching a final integrated diagnosis. We then propose a clinical practice guideline for the Veterans Health Administration (VHA) that recommends use of molecular testing for veterans as part of the diagnostic workup of primary CNS neoplasms.
Purpose
In 2013 the VHA National Director of Pathology & Laboratory Medicine Services (P&LMS) chartered a national molecular genetics pathology workgroup (MGPW) that was charged with 4 specific tasks: (1) Provide recommendations about the effective use of molecular genetic testing for veterans; (2) Promote increased quality and availability of molecular testing within the VHA; (3) Encourage internal referral testing; and (4) Create an organizational structure and policies for molecular genetic testing and laboratory developed tests. The workgroup is currently composed of 4 subcommittees: genetic medicine, hematopathology, pharmacogenomics, and molecular oncology. The molecular oncology subcommittee is focused upon molecular genetic testing for solid tumors.
This article is intended to be the first of several publications from the molecular oncology subcommittee of the MGPW that address some of the aforementioned tasks. Similar to the recent publication from the hematopathology subcommittee of the MGPW, this article focuses on CNS neoplasms.2
Scope of Problem
The incidence of tumors of the CNS in the US population varies among age groups. It is the most common solid tumor in children aged < 14 years and represents a significant cause of mortality across all age groups.3 Of CNS tumors, diffuse gliomas comprise about 20% of the tumors and more than 70% of the primary malignant CNS tumors.3 Analysis of the VA Central Cancer Registry data from 2010 to 2014 identified 1,186 veterans (about 237 veterans per year) who were diagnosed with diffuse gliomas. (Lynch, Kulich, Colman, unpublished data, February 2018). While the majority (nearly 80%) of these cases were glioblastomas (GBMs), unfortunately a majority of these cases did not undergo molecular testing (Lynch, Kulich, Colman, unpublished data, February 2018).
Although this low rate of testing may be in part reflective of the period from which these data were gleaned (ie, prior to the WHO release of their updated the classification of tumors of the CNS), it is important to raise VA practitioners’ awareness of these recent changes to ensure that veterans receive the proper diagnosis and treatment for their disease. Thus, while the number of veterans diagnosed with diffuse gliomas within the VHA is relatively small in comparison to other malignancies, such as prostatic adenocarcinomas and lung carcinomas, the majority of diffuse gliomas do not seem to be receiving the molecular testing that would be necessary for (1) appropriate classification under the recently revised WHO recommendations; and (2) making important treatment decisions.
Case Presentations
Case 1. A veteran of the Gulf War presented with a 3-month history of possible narcoleptic events associated with a motor vehicle accident. Magnetic resonance imaging (MRI) revealed a large left frontal mass lesion with minimal surrounding edema without appreciable contrast enhancement (Figures 1A, 1B, and 1C).
Neither mitotic figures nor endothelial proliferation were identified. Immunohistochemical stains revealed a lack of R132H mutant IDH1 protein expression, a loss of nuclear staining for ATRX protein within a substantial number of cells, and a clonal pattern of p53 protein overexpression (Figures 1E, 1F, and 1G). The lesion demonstrated diffuse glial fibrillary acidic protein (GFAP) immunoreactivity and a low proliferation index (as determined by Ki-67 staining; estimated at less than 5%) (Figures 1H and 1I).
Based upon these results, an initial morphologic diagnosis of diffuse glioma was issued, and tissue was subjected to a variety of nucleic acid-based tests. While fluorescence in situ hybridization (FISH) studies were negative for 1p/19q codeletion, pyrosequencing analysis revealed the presence of a c.394C>T (R132C) mutation of the IDH1 gene (Figure 1J). The University of Pittsburgh Medical Center’s GlioSeq targeted next-generation sequence (NGS) analysis confirmed the presence of the c.394C > T mutation in IDH1 gene.4 Based upon this additional information, a final integrated morphologic and molecular diagnosis of diffuse astrocytoma, IDH-mutant was rendered.
Case 2. A Vietnam War veteran presented with a 6-week history of new onset falls with associated left lower extremity weakness. A MRI revealed a right frontoparietal mass lesion with surrounding edema without appreciable contrast enhancement (Figures 2A, 2B, and 2C).
Immunohistochemical stains revealed R132H mutant IDH1 protein expression, retention of nuclear staining for ATRX protein, the lack of a clonal pattern of p53 protein overexpression, diffuse GFAP immunoreactivity, and a proliferation index (as determined by Ki-67 staining) focally approaching 20% (Figures 2E, 2F, 2G, 2H and 2I).
Based upon these results, an initial morphologic diagnosis of diffuse (high grade) glioma was issued, and tissue was subjected to a variety of nucleic acid-based tests. The FISH studies were positive for 1p/19q codeletion, and pyrosequencing analysis confirmed the immunohistochemical findings of a c.395G>A (R132H) mutation of the IDH1 gene (Figure 2J). GlioSeq targeted NGS analysis confirmed the presence of the c.395G>A mutation in the IDH1 gene, a mutation in the telomerase reverse transcriptase (TERT) promoter, and possible decreased copy number of the CIC (chromosome 1p) and FUBP1 (chromosome 19q) genes.
A final integrated morphologic and molecular diagnosis of anaplastic oligodendroglioma, IDH-mutant and 1p/19q-codeleted was rendered based on the additional information. With this final diagnosis, methylation analysis of the MGMT gene promoter, which was performed for prognostic and predictive purposes, was identified in this case.5,6
Case 3. A veteran of the Vietnam War presented with a new onset seizure. A MRI revealed a focally contrast-enhancing mass with surrounding edema within the left frontal lobe (Figures 3A, 3B, and 3C).
Hematoxylin and eosin (H&E) stained sections following formalin fixation and paraffin embedding demonstrated similar findings (Figure 3D), and while mitotic figures were readily identified, areas of necrosis were not identified and endothelial proliferation was not a prominent feature. Immunohistochemical stains revealed no evidence of R132H mutant IDH1 protein expression, retention of nuclear staining for ATRX protein, a clonal pattern of p53 protein overexpression, patchy GFAP immunoreactivity, and a proliferation index (as determined by Ki-67 staining) focally approaching 50% (Figures 3E, 3F, 3G, 3H, and 3I).
Based upon these results, an initial morphologic diagnosis of diffuse (high grade) glioma was issued, and the tissue was subjected to a variety of nucleic acid-based tests. The FISH studies were negative for EGFR gene amplification and 1p/19q codeletion, although a gain of the long arm of chromosome 1 was detected. Pyrosequencing analysis for mutations in codon 132 of the IDH1 gene revealed no mutations (Figure 3J). GlioSeq targeted NGS analysis identified mutations within the NF1, TP53, and PIK3CA genes without evidence of mutations in the IDH1, IDH2, ATRX, H3F3A, or EGFR genes or the TERT promoter. Based upon this additional information, a final integrated morphologic and molecular diagnosis of GBM, IDH wild-type was issued. The MGMT gene promoter was negative for methylation, a finding that has prognostic and predictive impact with regard to treatment with temazolamide.7-9
New Diffuse Glioma Classification
Since the issuance of the previous edition of the WHO classification of CNS tumors in 2007, several sentinel discoveries have been made that have advanced our understanding of the underlying biology of primary CNS neoplasms. Since a detailed review of these findings is beyond the scope and purpose of this manuscript and salient reviews on the topic can be found elsewhere, we will focus on the molecular findings that have been incorporated into the recently revised WHO classification.10 The importance of providing such information for proper patient management is illustrated by the recent acknowledgement by the American Academy of Neurology that molecular testing of brain tumors is a specific area in which there is a need for quality improvement.11 Therefore, it is critical that these underlying molecular abnormalities are identified to allow for proper classification and treatment of diffuse gliomas in the veteran population.
As noted previously, based on VA cancer registry data, diffuse gliomas are the most commonly encountered primary CNS cancers in the veteran population. Several of the aforementioned seminal discoveries have been incorporated into the updated classification of diffuse gliomas. While the recently updated WHO classification allows for the assignment of “not otherwise specified (NOS)” diagnostic designation, this category must be limited to cases where there is insufficient data to allow for a more precise classification due to sample limitations and not simply due to a failure of VA pathology laboratories to pursue the appropriate diagnostic testing.
Figure 4 presents the recommended diagnostic workflow for the workup of diffuse gliomas. As illustrated in the above cases, a variety of different methodologies, including immunohistochemical, FISH, loss of heterozygosity analysis, traditional and NGS may be applied when elucidating the status of molecular events at critical diagnostic branch points.
Diagnostic Uses of Molecular Testing
While the case studies in this article demonstrate the use of ancillary testing and provide a suggested strategy for properly subclassifying diffuse gliomas, inherent in this strategy is the assumption that, based upon the initial clinical and pathologic information available, one can accurately categorize the lesion as a diffuse glioma. In reality, such a distinction is not always a straightforward endeavor. It is well recognized that a proportion of low-grade, typically radiologically circumscribed, CNS neoplasms, such as pilocytic astrocytomas and glioneuronal tumors, may infiltrate the surrounding brain parenchyma. In addition, many of these low-grade CNS neoplasms also may have growth patterns that are shared with diffuse gliomas, a diagnostic challenge that often can be further hampered by the inherent limitations involved in obtaining adequate samples for diagnosis from the CNS.
Although there are limitations and caveats, molecular diagnostic testing may be invaluable in properly classifying CNS tumors in such situations. The finding of mutations in the IDH1 or IDH2 genes has been shown to be very valuable in distinguishing low-grade diffuse glioma from both nonneoplastic and low-grade circumscribed neuroepithelial neoplasms that may exhibit growth patterns that can mimic those of diffuse gliomas.15-17 Conversely, finding abnormalities in the BRAF gene in a brain neoplasm that has a low-grade morphology suggests that the lesion may represent one of these low-grade lesions such as a pleomorphic xanthoastrocytoma, pilocytic astrocytoma, or mixed neuronal-glial tumor as opposed to a diffuse glioma.18,19
Depending upon the environment in which one practices, small biopsy specimens may be prevalent, and unfortunately, it is not uncommon to obtain a biopsy that exhibits a histologic growth pattern that is discordant from what one would predict based on the clinical context and imaging findings. Molecular testing may be useful in resolving discordances in such situations. If a biopsy of a ring-enhancing lesion demonstrates a diffuse glioma that doesn’t meet WHO grade IV criteria, applying methodologies that look for genetic features commonly encountered in high-grade astrocytomas may identify genetic abnormalities that suggest a more aggressive lesion than is indicated by the histologic findings. The presence of genetic abnormalities such as homozygous deletion of the CDKN2A gene, TERT promoter mutation, loss of heterozygosity of chromosome 10q and/or phosphatase and tensin homolog (PTEN) mutations, EGFR gene amplification or the presence of the EGFR variant III are a few findings that would suggest the aforementioned sample may represent an undersampling of a higher grade diffuse astrocytoma, which would be important information to convey to the treating clinicians.20-26
Testing In the VA
The goals of the MPWG include promoting increased quality and availability of genetic testing within the VHA as well as encouraging internal referral testing. An informal survey of the chiefs of VA Pathology and Laboratory Medicine Services was conducted in November of 2017 in an attempt to identify internal VA pathology laboratories currently conducting testing that may be of use in the workup of diffuse gliomas (Table 1).
The VA currently offers NGS panels for patients with advanced-stage malignancies under the auspices of the Precision Oncology Program, whose reports provide both (1) mutational analyses for genes such as TP53, ATRX, NF1, BRAF, PTEN, TERT IDH1, and IDH2 that may be useful in the proper classifying of high-grade diffuse gliomas; and (2) information regarding clinical trials for which the veteran may be eligible for based on their glioma’s mutational profile. Interested VA providers should visit tinyurl.com/precisiononcology/ for more information about this program. Finally, although internal testing within VA laboratories is recommended to allow for the development of more cost-effective testing, testing may be performed through many nationally contracted reference laboratories.
Conclusion
In light of the recent progress made in our understanding of the molecular events of gliomagenesis, the way we diagnose diffuse gliomas within the CNS has undergone a major paradigm shift. While histology still plays a critical role in the process, we believe that additional ancillary testing is a requirement for all diffuse gliomas diagnosed within VA pathology laboratories. In the context of recently encountered cases, we have provided a recommended workflow highlighting the testing that can be performed to allow for the proper diagnosis of our veterans with diffuse gliomas (Figure 4).
Unless limited by the amount of tissue available for such tests, ancillary testing must be performed on all diffuse gliomas diagnosed within the VA system to ensure proper diagnosis and treatment of our veterans with diffuse gliomas.

Acknowledgments
The authors thank Dr. Craig M. Horbinski (Feinberg School of Medicine, Northwestern University) and Dr. Geoffrey H. Murdoch (University of Pittsburgh) for their constructive criticism of the manuscript. We also thank the following individuals for past service as members of the molecular oncology subcommittee of the MGPW: Dr. George Ansstas (Washington University School of Medicine), Dr. Osssama Hemadeh (Bay Pines VA Health Care System), Dr. James Herman (VA Pittsburgh Healthcare System), and Dr. Ryan Phan (formerly of the VA Greater Los Angeles Healthcare System) as well as the members of the Veterans Administration pathology and laboratory medicine service molecular genetics pathology workgroup.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies.
Dr. Kulich is the Acting Chief of Pathology and Laboratory Medicine Service at VA Pittsburgh Healthcare System and member of the Division of Neuropathology at University of Pittsburgh Department of Pathology, Dr. Duvvuri is an Otolaryngologist at VA Pittsburgh Healthcare System, and Dr. Passero is the Section Chief of Hematology\Oncology at VA Pittsburgh Healthcare System in Pennsylvania. Dr. Becker is an Oncologist at VA-New York Harbor Healthcare System. Dr. Dacic is a Pathologist at University of Pittsburgh Department of Pathology in Pennsylvania. Dr. Ehsan is Chief of Pathology and Laboratory Medicine Services at the South Texas Veterans Healthcare System in San Antonio. Dr. Gutkin is the former Chief of Pathology and Laboratory Medicine Service at VA Pittsburgh Healthcare System. Dr. Hou is a Pathologist at St. Louis VA Medical Center in Missouri. Dr. Icardi is the VA National Director of Pathology and Laboratory Medicine Services. Dr. Lyle is a Pathologist at Bay Pine Health Care System in Florida. Dr. Lynch is an Investigator at VA Salt Lake Health Care System Informatics and Computing Infrastructure. Dr. Montgomery is an Oncologist at VA Puget Sound Health Care System, in Seattle, Washington. Dr. Przygodzki is the Director of Genomic Medicine Implementation and Associate Director of Genomic Medicine for the VA. Dr. Colman is a Neuro-Oncologist at George E. Wahlen VA Medical Center and the Director of Medical Neuro-Oncology at the Huntsman Cancer Institute, Salt Lake City, Utah.
Correspondence: Dr. Kulich ([email protected])
Over the past few decades, our understanding of the molecular underpinning of primary neoplasms of the central nervous system (CNS) has progressed substantially. Thanks in large part to this expansion in our knowledge base, the World Health Organization (WHO) has recently updated its classification of tumors of the CNS.1 One of the key elements of this update was the inclusion of molecular diagnostic criteria for the classification of infiltrating gliomas. While the previous classification system was based upon histologic subtypes of the tumor (astrocytoma, oligodendroglioma, and oligoastrocytoma), the revised classification system incorporates molecular testing to establish the genetic characteristics of the tumor to reach a final integrated diagnosis.
In this article, we present 3 cases to highlight some of these recent changes in the WHO diagnostic categories of primary CNS tumors and to illustrate the role of specific molecular tests in reaching a final integrated diagnosis. We then propose a clinical practice guideline for the Veterans Health Administration (VHA) that recommends use of molecular testing for veterans as part of the diagnostic workup of primary CNS neoplasms.
Purpose
In 2013 the VHA National Director of Pathology & Laboratory Medicine Services (P&LMS) chartered a national molecular genetics pathology workgroup (MGPW) that was charged with 4 specific tasks: (1) Provide recommendations about the effective use of molecular genetic testing for veterans; (2) Promote increased quality and availability of molecular testing within the VHA; (3) Encourage internal referral testing; and (4) Create an organizational structure and policies for molecular genetic testing and laboratory developed tests. The workgroup is currently composed of 4 subcommittees: genetic medicine, hematopathology, pharmacogenomics, and molecular oncology. The molecular oncology subcommittee is focused upon molecular genetic testing for solid tumors.
This article is intended to be the first of several publications from the molecular oncology subcommittee of the MGPW that address some of the aforementioned tasks. Similar to the recent publication from the hematopathology subcommittee of the MGPW, this article focuses on CNS neoplasms.2
Scope of Problem
The incidence of tumors of the CNS in the US population varies among age groups. It is the most common solid tumor in children aged < 14 years and represents a significant cause of mortality across all age groups.3 Of CNS tumors, diffuse gliomas comprise about 20% of the tumors and more than 70% of the primary malignant CNS tumors.3 Analysis of the VA Central Cancer Registry data from 2010 to 2014 identified 1,186 veterans (about 237 veterans per year) who were diagnosed with diffuse gliomas. (Lynch, Kulich, Colman, unpublished data, February 2018). While the majority (nearly 80%) of these cases were glioblastomas (GBMs), unfortunately a majority of these cases did not undergo molecular testing (Lynch, Kulich, Colman, unpublished data, February 2018).
Although this low rate of testing may be in part reflective of the period from which these data were gleaned (ie, prior to the WHO release of their updated the classification of tumors of the CNS), it is important to raise VA practitioners’ awareness of these recent changes to ensure that veterans receive the proper diagnosis and treatment for their disease. Thus, while the number of veterans diagnosed with diffuse gliomas within the VHA is relatively small in comparison to other malignancies, such as prostatic adenocarcinomas and lung carcinomas, the majority of diffuse gliomas do not seem to be receiving the molecular testing that would be necessary for (1) appropriate classification under the recently revised WHO recommendations; and (2) making important treatment decisions.
Case Presentations
Case 1. A veteran of the Gulf War presented with a 3-month history of possible narcoleptic events associated with a motor vehicle accident. Magnetic resonance imaging (MRI) revealed a large left frontal mass lesion with minimal surrounding edema without appreciable contrast enhancement (Figures 1A, 1B, and 1C).
Neither mitotic figures nor endothelial proliferation were identified. Immunohistochemical stains revealed a lack of R132H mutant IDH1 protein expression, a loss of nuclear staining for ATRX protein within a substantial number of cells, and a clonal pattern of p53 protein overexpression (Figures 1E, 1F, and 1G). The lesion demonstrated diffuse glial fibrillary acidic protein (GFAP) immunoreactivity and a low proliferation index (as determined by Ki-67 staining; estimated at less than 5%) (Figures 1H and 1I).
Based upon these results, an initial morphologic diagnosis of diffuse glioma was issued, and tissue was subjected to a variety of nucleic acid-based tests. While fluorescence in situ hybridization (FISH) studies were negative for 1p/19q codeletion, pyrosequencing analysis revealed the presence of a c.394C>T (R132C) mutation of the IDH1 gene (Figure 1J). The University of Pittsburgh Medical Center’s GlioSeq targeted next-generation sequence (NGS) analysis confirmed the presence of the c.394C > T mutation in IDH1 gene.4 Based upon this additional information, a final integrated morphologic and molecular diagnosis of diffuse astrocytoma, IDH-mutant was rendered.
Case 2. A Vietnam War veteran presented with a 6-week history of new onset falls with associated left lower extremity weakness. A MRI revealed a right frontoparietal mass lesion with surrounding edema without appreciable contrast enhancement (Figures 2A, 2B, and 2C).
Immunohistochemical stains revealed R132H mutant IDH1 protein expression, retention of nuclear staining for ATRX protein, the lack of a clonal pattern of p53 protein overexpression, diffuse GFAP immunoreactivity, and a proliferation index (as determined by Ki-67 staining) focally approaching 20% (Figures 2E, 2F, 2G, 2H and 2I).
Based upon these results, an initial morphologic diagnosis of diffuse (high grade) glioma was issued, and tissue was subjected to a variety of nucleic acid-based tests. The FISH studies were positive for 1p/19q codeletion, and pyrosequencing analysis confirmed the immunohistochemical findings of a c.395G>A (R132H) mutation of the IDH1 gene (Figure 2J). GlioSeq targeted NGS analysis confirmed the presence of the c.395G>A mutation in the IDH1 gene, a mutation in the telomerase reverse transcriptase (TERT) promoter, and possible decreased copy number of the CIC (chromosome 1p) and FUBP1 (chromosome 19q) genes.
A final integrated morphologic and molecular diagnosis of anaplastic oligodendroglioma, IDH-mutant and 1p/19q-codeleted was rendered based on the additional information. With this final diagnosis, methylation analysis of the MGMT gene promoter, which was performed for prognostic and predictive purposes, was identified in this case.5,6
Case 3. A veteran of the Vietnam War presented with a new onset seizure. A MRI revealed a focally contrast-enhancing mass with surrounding edema within the left frontal lobe (Figures 3A, 3B, and 3C).
Hematoxylin and eosin (H&E) stained sections following formalin fixation and paraffin embedding demonstrated similar findings (Figure 3D), and while mitotic figures were readily identified, areas of necrosis were not identified and endothelial proliferation was not a prominent feature. Immunohistochemical stains revealed no evidence of R132H mutant IDH1 protein expression, retention of nuclear staining for ATRX protein, a clonal pattern of p53 protein overexpression, patchy GFAP immunoreactivity, and a proliferation index (as determined by Ki-67 staining) focally approaching 50% (Figures 3E, 3F, 3G, 3H, and 3I).
Based upon these results, an initial morphologic diagnosis of diffuse (high grade) glioma was issued, and the tissue was subjected to a variety of nucleic acid-based tests. The FISH studies were negative for EGFR gene amplification and 1p/19q codeletion, although a gain of the long arm of chromosome 1 was detected. Pyrosequencing analysis for mutations in codon 132 of the IDH1 gene revealed no mutations (Figure 3J). GlioSeq targeted NGS analysis identified mutations within the NF1, TP53, and PIK3CA genes without evidence of mutations in the IDH1, IDH2, ATRX, H3F3A, or EGFR genes or the TERT promoter. Based upon this additional information, a final integrated morphologic and molecular diagnosis of GBM, IDH wild-type was issued. The MGMT gene promoter was negative for methylation, a finding that has prognostic and predictive impact with regard to treatment with temazolamide.7-9
New Diffuse Glioma Classification
Since the issuance of the previous edition of the WHO classification of CNS tumors in 2007, several sentinel discoveries have been made that have advanced our understanding of the underlying biology of primary CNS neoplasms. Since a detailed review of these findings is beyond the scope and purpose of this manuscript and salient reviews on the topic can be found elsewhere, we will focus on the molecular findings that have been incorporated into the recently revised WHO classification.10 The importance of providing such information for proper patient management is illustrated by the recent acknowledgement by the American Academy of Neurology that molecular testing of brain tumors is a specific area in which there is a need for quality improvement.11 Therefore, it is critical that these underlying molecular abnormalities are identified to allow for proper classification and treatment of diffuse gliomas in the veteran population.
As noted previously, based on VA cancer registry data, diffuse gliomas are the most commonly encountered primary CNS cancers in the veteran population. Several of the aforementioned seminal discoveries have been incorporated into the updated classification of diffuse gliomas. While the recently updated WHO classification allows for the assignment of “not otherwise specified (NOS)” diagnostic designation, this category must be limited to cases where there is insufficient data to allow for a more precise classification due to sample limitations and not simply due to a failure of VA pathology laboratories to pursue the appropriate diagnostic testing.
Figure 4 presents the recommended diagnostic workflow for the workup of diffuse gliomas. As illustrated in the above cases, a variety of different methodologies, including immunohistochemical, FISH, loss of heterozygosity analysis, traditional and NGS may be applied when elucidating the status of molecular events at critical diagnostic branch points.
Diagnostic Uses of Molecular Testing
While the case studies in this article demonstrate the use of ancillary testing and provide a suggested strategy for properly subclassifying diffuse gliomas, inherent in this strategy is the assumption that, based upon the initial clinical and pathologic information available, one can accurately categorize the lesion as a diffuse glioma. In reality, such a distinction is not always a straightforward endeavor. It is well recognized that a proportion of low-grade, typically radiologically circumscribed, CNS neoplasms, such as pilocytic astrocytomas and glioneuronal tumors, may infiltrate the surrounding brain parenchyma. In addition, many of these low-grade CNS neoplasms also may have growth patterns that are shared with diffuse gliomas, a diagnostic challenge that often can be further hampered by the inherent limitations involved in obtaining adequate samples for diagnosis from the CNS.
Although there are limitations and caveats, molecular diagnostic testing may be invaluable in properly classifying CNS tumors in such situations. The finding of mutations in the IDH1 or IDH2 genes has been shown to be very valuable in distinguishing low-grade diffuse glioma from both nonneoplastic and low-grade circumscribed neuroepithelial neoplasms that may exhibit growth patterns that can mimic those of diffuse gliomas.15-17 Conversely, finding abnormalities in the BRAF gene in a brain neoplasm that has a low-grade morphology suggests that the lesion may represent one of these low-grade lesions such as a pleomorphic xanthoastrocytoma, pilocytic astrocytoma, or mixed neuronal-glial tumor as opposed to a diffuse glioma.18,19
Depending upon the environment in which one practices, small biopsy specimens may be prevalent, and unfortunately, it is not uncommon to obtain a biopsy that exhibits a histologic growth pattern that is discordant from what one would predict based on the clinical context and imaging findings. Molecular testing may be useful in resolving discordances in such situations. If a biopsy of a ring-enhancing lesion demonstrates a diffuse glioma that doesn’t meet WHO grade IV criteria, applying methodologies that look for genetic features commonly encountered in high-grade astrocytomas may identify genetic abnormalities that suggest a more aggressive lesion than is indicated by the histologic findings. The presence of genetic abnormalities such as homozygous deletion of the CDKN2A gene, TERT promoter mutation, loss of heterozygosity of chromosome 10q and/or phosphatase and tensin homolog (PTEN) mutations, EGFR gene amplification or the presence of the EGFR variant III are a few findings that would suggest the aforementioned sample may represent an undersampling of a higher grade diffuse astrocytoma, which would be important information to convey to the treating clinicians.20-26
Testing In the VA
The goals of the MPWG include promoting increased quality and availability of genetic testing within the VHA as well as encouraging internal referral testing. An informal survey of the chiefs of VA Pathology and Laboratory Medicine Services was conducted in November of 2017 in an attempt to identify internal VA pathology laboratories currently conducting testing that may be of use in the workup of diffuse gliomas (Table 1).
The VA currently offers NGS panels for patients with advanced-stage malignancies under the auspices of the Precision Oncology Program, whose reports provide both (1) mutational analyses for genes such as TP53, ATRX, NF1, BRAF, PTEN, TERT IDH1, and IDH2 that may be useful in the proper classifying of high-grade diffuse gliomas; and (2) information regarding clinical trials for which the veteran may be eligible for based on their glioma’s mutational profile. Interested VA providers should visit tinyurl.com/precisiononcology/ for more information about this program. Finally, although internal testing within VA laboratories is recommended to allow for the development of more cost-effective testing, testing may be performed through many nationally contracted reference laboratories.
Conclusion
In light of the recent progress made in our understanding of the molecular events of gliomagenesis, the way we diagnose diffuse gliomas within the CNS has undergone a major paradigm shift. While histology still plays a critical role in the process, we believe that additional ancillary testing is a requirement for all diffuse gliomas diagnosed within VA pathology laboratories. In the context of recently encountered cases, we have provided a recommended workflow highlighting the testing that can be performed to allow for the proper diagnosis of our veterans with diffuse gliomas (Figure 4).
Unless limited by the amount of tissue available for such tests, ancillary testing must be performed on all diffuse gliomas diagnosed within the VA system to ensure proper diagnosis and treatment of our veterans with diffuse gliomas.

Acknowledgments
The authors thank Dr. Craig M. Horbinski (Feinberg School of Medicine, Northwestern University) and Dr. Geoffrey H. Murdoch (University of Pittsburgh) for their constructive criticism of the manuscript. We also thank the following individuals for past service as members of the molecular oncology subcommittee of the MGPW: Dr. George Ansstas (Washington University School of Medicine), Dr. Osssama Hemadeh (Bay Pines VA Health Care System), Dr. James Herman (VA Pittsburgh Healthcare System), and Dr. Ryan Phan (formerly of the VA Greater Los Angeles Healthcare System) as well as the members of the Veterans Administration pathology and laboratory medicine service molecular genetics pathology workgroup.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies.
Dr. Kulich is the Acting Chief of Pathology and Laboratory Medicine Service at VA Pittsburgh Healthcare System and member of the Division of Neuropathology at University of Pittsburgh Department of Pathology, Dr. Duvvuri is an Otolaryngologist at VA Pittsburgh Healthcare System, and Dr. Passero is the Section Chief of Hematology\Oncology at VA Pittsburgh Healthcare System in Pennsylvania. Dr. Becker is an Oncologist at VA-New York Harbor Healthcare System. Dr. Dacic is a Pathologist at University of Pittsburgh Department of Pathology in Pennsylvania. Dr. Ehsan is Chief of Pathology and Laboratory Medicine Services at the South Texas Veterans Healthcare System in San Antonio. Dr. Gutkin is the former Chief of Pathology and Laboratory Medicine Service at VA Pittsburgh Healthcare System. Dr. Hou is a Pathologist at St. Louis VA Medical Center in Missouri. Dr. Icardi is the VA National Director of Pathology and Laboratory Medicine Services. Dr. Lyle is a Pathologist at Bay Pine Health Care System in Florida. Dr. Lynch is an Investigator at VA Salt Lake Health Care System Informatics and Computing Infrastructure. Dr. Montgomery is an Oncologist at VA Puget Sound Health Care System, in Seattle, Washington. Dr. Przygodzki is the Director of Genomic Medicine Implementation and Associate Director of Genomic Medicine for the VA. Dr. Colman is a Neuro-Oncologist at George E. Wahlen VA Medical Center and the Director of Medical Neuro-Oncology at the Huntsman Cancer Institute, Salt Lake City, Utah.
Correspondence: Dr. Kulich ([email protected])
1. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803-820.
2. Wang-Rodriguez J, Yunes A, Phan R, et al. The challenges of precision medicine and new advances in molecular diagnostic testing in hematolymphoid malignancies: impact on the VHA. Fed Pract. 2017;34(suppl 5):S38-S49.
3. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017;19(suppl 5):v1-v88.
4. Nikiforova MN, Wald AI, Melan MA, et al. Targeted next-generation sequencing panel (GlioSeq) provides comprehensive genetic profiling of central nervous system tumors. Neuro Oncol. 2016;18(3)379-387.
5. Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90(19):1473-1479.
6. van den Bent MJ, Erdem-Eraslan L, Idbaih A, et al. MGMT-STP27 methylation status as predictive marker for response to PCV in anaplastic oligodendrogliomas and oligoastrocytomas. A report from EORTC study 26951. Clin Cancer Res. 2013;19(19):5513-5522.
7. Stupp R, Hegi ME, Mason WP, et al; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459-466.
8. Malmstrom A, Gronberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916-926.
9. van den Bent MJ, Kros JM. Predictive and prognostic markers in neuro-oncology. J Neuropathol Exp Neurol. 2007;66(12):1074-1081.
10. Chen R, Smith-Cohn M, Cohen AL, Colman H. Glioma subclassifications and their clinical significance. Neurotherapeutics. 2017;14(2):284-297.
11. Jordan JT, Sanders AE, Armstrong T, et al. Quality improvement in neurology: neuro-oncology quality measurement set. Neurology. 2018;90(14):652-658.
12. Chen L, Voronovich Z, Clark K, et al. Predicting the likelihood of an isocitrate dehydrogenase 1 or 2 mutation in diagnoses of infiltrative glioma. Neuro Oncol. 2014;16(11):1478-1483.
13. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997-1003.
14. Wick W, Platten M, Meisner C, et al; NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707-715.
15. Horbinski C, Kofler J, Kelly LM, Murdoch GH, Nikiforova MN. Diagnostic use of IDH1/2 mutation analysis in routine clinical testing of formalin-fixed, paraffin-embedded glioma tissues. J Neuropathol Exp Neurol. 2009;68(12):1319-1325.
16. Camelo-Piragua S, Jansen M, Ganguly A, Kim JC, Louis DN, Nutt CL. Mutant IDH1-specific immunohistochemistry distinguishes diffuse astrocytoma from astrocytosis. Acta Neuropathol. 2010;119(4):509-511.
17. Horbinski C, Kofler J, Yeaney G, et al. Isocitrate dehydrogenase 1 analysis differentiates gangliogliomas from infiltrative gliomas. Brain Pathol. 2011;21(5):564-574.
18. Berghoff AS, Preusser M. BRAF alterations in brain tumours: molecular pathology and therapeutic opportunities. Curr Opin Neurol. 2014;27(6):689-696.
19. Korshunov A, Meyer J, Capper D, et al. Combined molecular analysis of BRAF and IDH1 distinguishes pilocytic astrocytoma from diffuse astrocytoma. Acta Neuropathol. 2009;118(3):401-405.
20. Fuller CE, Schmidt RE, Roth KA, et al. Clinical utility of fluorescence in situ hybridization (FISH) in morphologically ambiguous gliomas with hybrid oligodendroglial/astrocytic features. J Neuropathol Exp Neurol. 2003;62(11):1118-1128.
21. Horbinski C. Practical molecular diagnostics in neuropathology: making a tough job a little easier. Semin Diagn Pathol. 2010;27(2):105-113.
22. Fuller GN, Bigner SH. Amplified cellular oncogenes in neoplasms of the human central nervous system. Mutat Res. 1992;276(3):299-306.
23. Brennan CW, Verhaak RG, McKenna A, et al; TCGA Research Network. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462-477.
24. Aldape K, Zadeh G, Mansouri S, Reifenberger G, von Deimling A. Glioblastoma: pathology, molecular mechanisms and markers. Acta Neuropathol. 2015;129(6):829-848.
25. Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110(15):6021-6026.
26. Nikiforova MN, Hamilton RL. Molecular diagnostics of gliomas. Arch Pathol Lab Med. 2011;135(5):558-568.
1. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803-820.
2. Wang-Rodriguez J, Yunes A, Phan R, et al. The challenges of precision medicine and new advances in molecular diagnostic testing in hematolymphoid malignancies: impact on the VHA. Fed Pract. 2017;34(suppl 5):S38-S49.
3. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017;19(suppl 5):v1-v88.
4. Nikiforova MN, Wald AI, Melan MA, et al. Targeted next-generation sequencing panel (GlioSeq) provides comprehensive genetic profiling of central nervous system tumors. Neuro Oncol. 2016;18(3)379-387.
5. Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90(19):1473-1479.
6. van den Bent MJ, Erdem-Eraslan L, Idbaih A, et al. MGMT-STP27 methylation status as predictive marker for response to PCV in anaplastic oligodendrogliomas and oligoastrocytomas. A report from EORTC study 26951. Clin Cancer Res. 2013;19(19):5513-5522.
7. Stupp R, Hegi ME, Mason WP, et al; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459-466.
8. Malmstrom A, Gronberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916-926.
9. van den Bent MJ, Kros JM. Predictive and prognostic markers in neuro-oncology. J Neuropathol Exp Neurol. 2007;66(12):1074-1081.
10. Chen R, Smith-Cohn M, Cohen AL, Colman H. Glioma subclassifications and their clinical significance. Neurotherapeutics. 2017;14(2):284-297.
11. Jordan JT, Sanders AE, Armstrong T, et al. Quality improvement in neurology: neuro-oncology quality measurement set. Neurology. 2018;90(14):652-658.
12. Chen L, Voronovich Z, Clark K, et al. Predicting the likelihood of an isocitrate dehydrogenase 1 or 2 mutation in diagnoses of infiltrative glioma. Neuro Oncol. 2014;16(11):1478-1483.
13. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997-1003.
14. Wick W, Platten M, Meisner C, et al; NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707-715.
15. Horbinski C, Kofler J, Kelly LM, Murdoch GH, Nikiforova MN. Diagnostic use of IDH1/2 mutation analysis in routine clinical testing of formalin-fixed, paraffin-embedded glioma tissues. J Neuropathol Exp Neurol. 2009;68(12):1319-1325.
16. Camelo-Piragua S, Jansen M, Ganguly A, Kim JC, Louis DN, Nutt CL. Mutant IDH1-specific immunohistochemistry distinguishes diffuse astrocytoma from astrocytosis. Acta Neuropathol. 2010;119(4):509-511.
17. Horbinski C, Kofler J, Yeaney G, et al. Isocitrate dehydrogenase 1 analysis differentiates gangliogliomas from infiltrative gliomas. Brain Pathol. 2011;21(5):564-574.
18. Berghoff AS, Preusser M. BRAF alterations in brain tumours: molecular pathology and therapeutic opportunities. Curr Opin Neurol. 2014;27(6):689-696.
19. Korshunov A, Meyer J, Capper D, et al. Combined molecular analysis of BRAF and IDH1 distinguishes pilocytic astrocytoma from diffuse astrocytoma. Acta Neuropathol. 2009;118(3):401-405.
20. Fuller CE, Schmidt RE, Roth KA, et al. Clinical utility of fluorescence in situ hybridization (FISH) in morphologically ambiguous gliomas with hybrid oligodendroglial/astrocytic features. J Neuropathol Exp Neurol. 2003;62(11):1118-1128.
21. Horbinski C. Practical molecular diagnostics in neuropathology: making a tough job a little easier. Semin Diagn Pathol. 2010;27(2):105-113.
22. Fuller GN, Bigner SH. Amplified cellular oncogenes in neoplasms of the human central nervous system. Mutat Res. 1992;276(3):299-306.
23. Brennan CW, Verhaak RG, McKenna A, et al; TCGA Research Network. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462-477.
24. Aldape K, Zadeh G, Mansouri S, Reifenberger G, von Deimling A. Glioblastoma: pathology, molecular mechanisms and markers. Acta Neuropathol. 2015;129(6):829-848.
25. Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110(15):6021-6026.
26. Nikiforova MN, Hamilton RL. Molecular diagnostics of gliomas. Arch Pathol Lab Med. 2011;135(5):558-568.
Rheumatoid Arthritis: Therapeutic Strategies After Inadequate Response to Initial TNF Inhibitor Therapy
From the University of Iowa Hospitals and Clinics, Iowa City, IA.
Abstract
- Objective: To discuss the variability in response to tumor necrosis factor inhibitors (TNFis) observed in patients with rheumatoid arthritis (RA) and discuss therapeutic options for patients who do not respond to initial TNFi therapy.
- Methods: Review of the literature.
- Results: Optimal treatment of RA aims at achieving and then maintaining remission or low disease activity. In a patient with an inadequate response to initial biologic therapy, several therapeutic options exist. Current evidence supports TNFi dose escalation for only infliximab; optimization of concurrent conventional synthetic disease-modifying antirheumatic drug (csDMARD) or switching to a different csDMARD are other options. Cycling (switching to an alternative TNFi) and swapping (switching to a therapy with a different mode of action) strategies are other alternate approaches supported by many observational studies. While no head-to-head trials exist directly comparing the 2 strategies, data suggest superiority of the swapping strategy over the cycling approach. Also, several studies have shown that switching to a drug with a different mechanism of action is associated with higher treatment persistence and lower health care costs than TNFi cycling.
- Conclusion: Physicians have a growing list of treatment options to help their patients with RA achieve disease remission. The choice of best treatment for a given patient needs to be individualized, keeping in mind other factors, including comorbidities.
Keywords: biologics; rheumatoid arthritis; swapping strategy; cycling strategy; TNF inhibitors.
Following the discovery of tumor necrosis factor (TNF) as a proinflammatory cytokine 30 years ago, the use of TNF antagonists has revolutionized the treatment of rheumatoid arthritis (RA). Although TNF inhibitors (TNFIs) are frequently used as a first-line biologic disease-modifying antirheumatic drug (bDMARD), they are not uniformly efficacious in achieving remission in all patients with RA. This article highlights the reasons for such variability in observed response and discusses therapeutic options for patients who do not respond to TNFi therapy.
Case Presentation
A 60-year-old woman is evaluated in the clinic for complaints of pain in her hands, morning stiffness lasting 2 hours, and swelling in her wrists, all of which have been ongoing for 3 months. Physical exam reveals evidence of active inflammation, with synovitis in her second, third, and fourth metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints bilaterally, swelling over both wrists, and a weak grip. Inflammatory markers are elevated, and rheumatoid factor and anti-cyclic citrullinated peptide (anti-CCP) are both positive at high titer. Radiographs reveal evidence of small erosions at the third and fourth MCPs and PIPs bilaterally and periarticular osteopenia. The patient is diagnosed with seropositive, erosive RA based on history, physical exam, laboratory studies, and imaging. She is started on 20 mg of prednisone for acute treatment of her symptoms along with methotrexate, and, initially, her symptoms are well controlled. A few months after starting treatment, she develops voluminous diarrhea that necessitates cessation of methotrexate. Leflunomide also causes similar symptoms. The combination of sulfasalazine and hydroxychloroquine does not adequately control her symptoms, and ongoing use of low-dose glucocorticoids is required to improve functionality in all joints. Using the treat-to-target (T2T) strategy, adalimumab is initiated. However, she continues to report persistent swelling and pain and still requests oral glucocorticoids to help decrease inflammation. The 28-joint Disease Activity Score (DAS28) is 4.8, suggestive of moderate disease activity.
Why are TNFi agents sometimes ineffective?
The introduction of monoclonal antibodies and fusion proteins to block TNF and other cytokines was a remarkable development in the treatment of RA that revolutionized patient care. Despite the efficacy of TNFis, clinical response to these agents is not universal and only some patients achieve complete remission. In targeting the eventual goal of remission or low disease activity in patients with RA, the concept of “TNF failure” becomes extremely relevant. These inadequate responses to anti-TNF therapy may be due to primary failures, or complete lack of clinical response after initiation of the bDMARD, and secondary failures, or the loss of initially achieved clinical response to therapy. Other reasons for discontinuation of a given TNFi include partial disease control and intolerance to the medication (possible injection-site or infusion reactions). Keystone and Kavanaugh1 divided causes of failure of TNF agents into 2 broad categories: perceptual (related to natural variations in disease course like hormonal variation and physical and emotional stress) and pathophysiological failures (genetic variations, high body mass index, concomitant cigarette use).
Another important consideration in patients treated with a TNFi is the consequent formation of anti-drug antibodies (ADAs). TNFi agents are immunogenic and normally elicit an immune response. The appearance of such ADAs may reduce the bioavailability of free drug, resulting in a decreased clinical response,2 or may lead to serious adverse effects.
How common is discontinuation of the first TNFi?
Several studies have reported that the prevalence of primary failure, secondary failure, and intolerance to TNFis ranges from 30% to 40%.3-6 Female sex,7 concurrent prednisone use,8 high disease activity scores,6,8,9 and the absence of treatment with low-dose methotrexate7,8 have all been shown to be negative predictors of bDMARD retention and response.10
Are there any factors that predict TNFi failure?
There are no specific parameters to accurately predict responses to TNFI therapy.11 Several clinical and molecular biomarkers in synovium (initial TNF levels, macrophages, T cells)12 and peripheral blood (serum myeloid-related protein 8 and 14 complex levels,13 prealbumin, platelet factor 4, and S100A12)14 have been described as predictors of clinical response to TNFis, but their utility in clinical practice has not been established and the use of these markers has not yet been incorporated into clinical guidelines.
How is disease activity measured in patients with RA?
In 2010 an international expert consensus panel published treatment recommendations for RA that emphasized a T2T strategy of individualizing and escalating treatment to achieve the lowest disease activity or remission. In clinical practice, numerous tools are available to measure RA disease activity. Herein, we mention several that are most commonly used in clinical practice.
DAS28 combines single activity measures into an overall continuous measure of disease activity and has been endorsed by both the American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR). It includes a 28-swollen joint count (SJC), 28-tender joint count (TJC), erythrocyte sedimentation rate (ESR; can also be calculated using C-reactive protein [CRP]), and a patient global assessment (PtGA). The cut-offs used for DAS28 interpretation are as follows: remission (< 2.6), low (≥ 2.6 but ≤ 3.2), moderate (> 3.2 but ≤ 5.1), or high (> 5.1).15 Some of the difficulties in using DAS28 in daily clinical practice include the need for a lab value and the time needed to perform the joint counts. Note also that due to the inclusion of ESR, which is influenced by age and other factors, DAS28 may underestimate remission in the elderly.
Another measure of RA disease activity is the Simplified Disease Activity Index (SDAI), which includes 28 SJC, 28 TJC, PtGA, provider global assessment (PrGA), and CRP in mg/dL. The level of disease activity using the SDAI is interpreted as: remission (SDAI ≤ 3.3), low (≥ 3.4 but ≤ 11), moderate (> 11 but ≤ 26), or high (> 26). The advantage of the SDAI is that a calculator or computer is not required for calculations. Another measure, the Clinical Disease Activity Index (CDAI), includes a 28 SJC, 28 TJC, PtGA, and PrGA. Because a laboratory value is not needed to calculate the CDAI, it is well-suited for use in clinical practice. When using the CDAI, the level of disease activity can be defined as remission (CDAI ≤ 2.8), low (> 2.8 but ≤ 10), moderate (> 10 but ≤ 22), or high (> 22). Again, as with the SDAI, a calculator or computer is not needed for calculations.
What are the alternative treatment options after first biologic failure?
In patients who have failed treatment with an initial biologic, usually a TNFi, the treating rheumatologist has the following options (Figure), with the best treatment strategy being driven by individualized patient and disease-related factors (Table 1 and Table 2):
- TNFi dose escalation
- Trial of an alternate TNFi agent (the “cycling” strategy)
- Optimization of therapy conjoined with a conventional synthetic DMARD (csDMARD)
- Use of a non-TNF biologic or targeted synthetic DMARD (the “swapping” strategy)
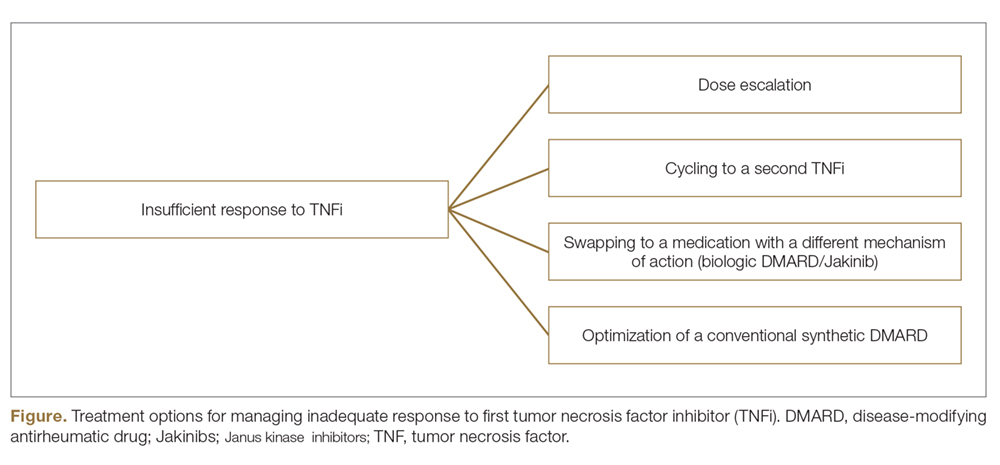
If all the listed strategies fail, the next step can be the addition of short-term, low-dose glucocorticoid therapy.
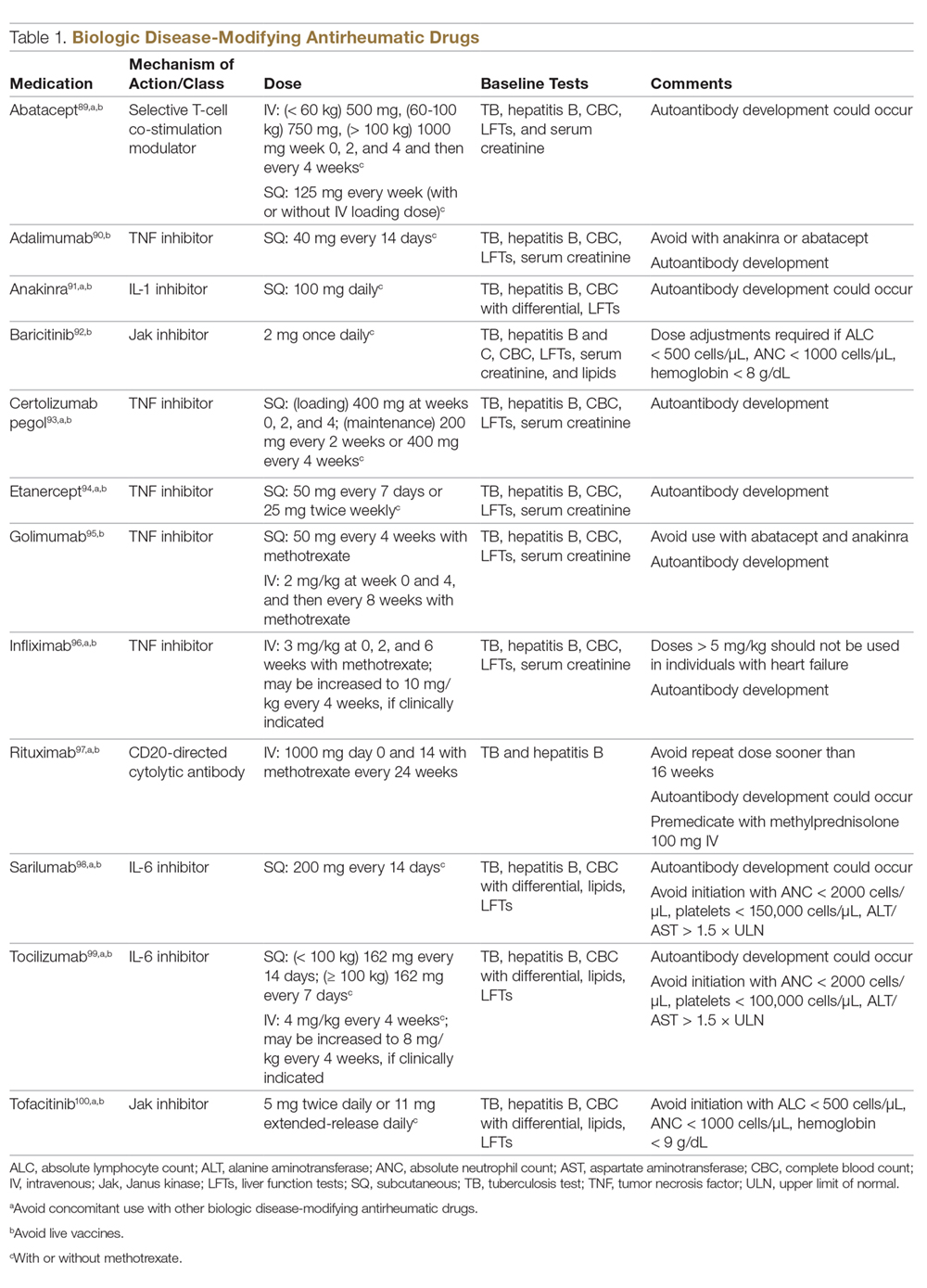
TNFi Dose Escalation
The available data have demonstrated the safety, efficacy, and cost-effectiveness of dose escalation in patients with RA receiving infliximab.16-18 The ATTRACT trial first demonstrated this, with greater clinical and radiographic improvements in those with higher trough serum concentrations, suggesting that doses higher than 3 mg/kg or more frequent than every 8 weeks may be needed for full response in some patients.19
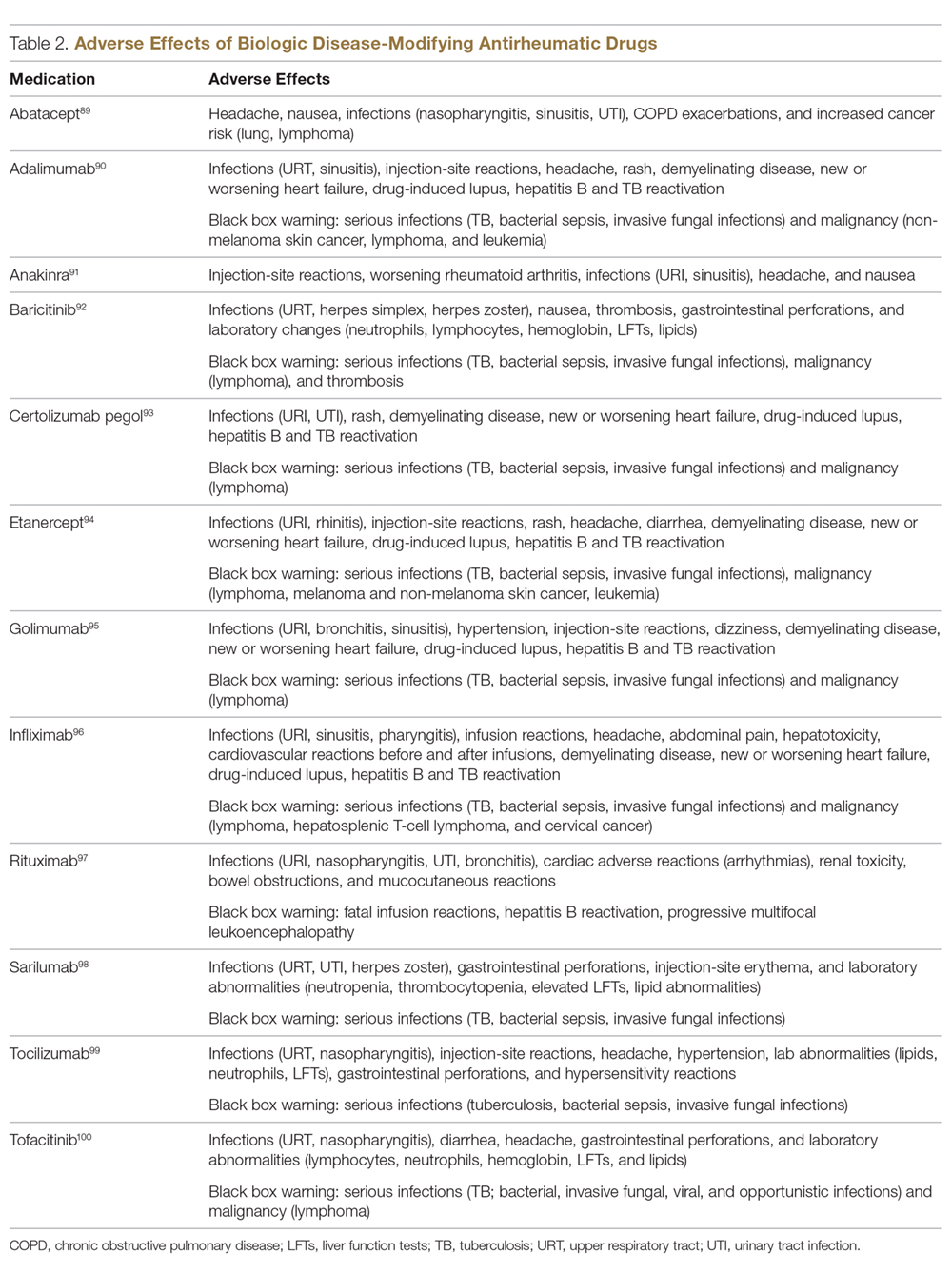
There is a lack of studies in RA patients to determine the most effective dose escalation strategy. A study in patients with Crohn disease showed that intensification to 10 mg/kg every 8 weeks (dose doubling) was at least as effective as 5 mg/kg every 4 weeks (halving interval) at 12 months.16 Due to greater patient and administration convenience of dose-doubling, this strategy may be preferred.17 A starting dose of 10 mg/kg every 8 weeks is not routinely recommended due to an increased risk of serious infection; these adverse events were not found when the dose was gradually increased, as clinically indicated, starting at 3 mg/kg.19,20 Further studies are needed to explore this approach in RA patients.
These results, however, have not been replicated with other TNFi agents. No significant clinical improvements were identified with etanercept 50 mg twice weekly,21 adalimumab 40 mg every week in the PREMIER trial,18 or certolizumab 400 mg every other week in an open-label extension phase of the RAPID 1 study.22 A Japanese study found significantly worse clinical outcomes with dose escalation of golimumab.23 Conversely, 2 studies found clinical benefits after escalating the tocilizumab dose, the first a real-world review from the Consortium of Rheumatology Researchers of North America (CORRONA) registry using the intravenous formulation,24 and the other the BREVACTA study utilizing subcutaneous tocilizumab.25 No studies to date have been published on dose escalation of abatacept in patients with RA who respond poorly. Overall, previous studies support dose escalation in individuals being treated with infliximab to improve clinical outcomes, but additional studies are needed for other bDMARDs.
Trial of an Alternate TNF Agent: The “Cycling” Strategy
Per the ACR/EULAR26,27 guidelines, all approved bDMARDs may be used without hierarchical positioning. However, after the failure of a TNFi agent, these guidelines do not provide specific advice about a preference between the “cycling” strategy (switching to an alternative TNFi) and “swapping” strategy (switching to a therapy with a different mode of action). Cycling might work for several reasons, including differences in the agents’ molecular structure, immunological mechanism of action, immunogenicity, and pharmacokinetics.28-30 The cycling strategy is a well-established approach adopted by more than 94% of practicing rheumatologists, according to a national survey,31 and its efficacy is supported by trials and additional observational studies.32-35
The greater clinical effectiveness of switching to infliximab compared with continuing with etanercept in patients with inadequate response to etanercept (n = 28) was suggested in the open-label OPPOSITE trial.36 Data from the GO-AFTER trial37 suggests that a greater proportion of patients with RA refractory to adalimumab, etanercept, or infliximab who were treated with golimumab achieved an ACR20 and ACR50 response compared with patients who received placebo, and this response persisted through 5 years.38 More recently, certolizumab pegol and adalimumab were compared head-to-head in the EXXELERATE trial.39 The results of this trial revealed the adequate efficacy of cycling to another TNFi after primary insufficient response to the first.
In studies from Finland and Sweden,35,40 it has been observed that a better response is achieved in patients in whom TNF failure was initially due to secondary failure or intolerance rather than primary failure. A post-hoc analysis of the results of the GO-AFTER trial41 and from a few observational studies35,40,42 revealed that switching from one TNFi to another, especially from a monoclonal antibody to a soluble receptor, was often more beneficial for RA patients than switching from a soluble receptor to a monoclonal antibody.
Optimization of Therapy Conjoined with csDMARDs
Methotrexate is one of the oldest and most effective csDMARDs available for the treatment of RA.43 The 2016 EULAR guidelines recommend the addition of methotrexate and/or other csDMARDs to potentiate the effect of bDMARDs.26 In the case of TNFi therapy, the observed synergistic effect between the monoclonal antibody and methotrexate may be explained by sustained suppression of ADA formation.44 In the TEMPO,45 PREMIER,18 and GO-BEFORE46 trials, the addition of methotrexate led to improved clinical and radiological outcomes in patients treated with etanercept, adalimumab, and golimumab,47 respectively. These findings were also demonstrated in several registries, where significant improvement in clinical response and retention rate of the TNFi agents was noted. Results have been replicated with non-TNFi bDMARDs, including abatacept48,49 and rituximab.50 Patients treated with interleukin (IL)-6 inhibitors in combination with methotrexate have shown significantly less radiographic progression compared to those treated with tocilizumab alone and those treated with monotherapy tocilizumab versus monotherapy methotrexate.51,52 Results possibly favor the use of IL-6 inhibitors alone in those who cannot tolerate or have contraindications to methotrexate.
An open prospective study by Cohen et al added methotrexate to the treatment regimens of individuals on bDMARD monotherapy with a primary failure and found favorable changes in ACR20 and DAS28 scores at 3 and 12 months and therapeutic biological response (ESR, CRP) at 3 months.53 Unlike monotherapy, in these situations methotrexate is known to be efficacious even at a lower dose, possibly at 7.5 mg to 10 mg per week. Some studies have shown that methotrexate administered parenterally may be more efficacious than when given orally.54-58
In clinical trials and observational studies, leflunomide, sulfasalazine, and hydroxychloroquine have been used as alternate csDMARDs added to the treatment regimen.59-62 There are, however, only 2 trials comparing the efficacy of methotrexate with that of other csDMARDs as concomitant treatment in patients with inadequate response to TNFi therapy. The RABBIT trial found a slight decrease in effectiveness with concomitant TNFi and leflunomide compared to TNFi/methotrexate, but overall each group had similar EULAR responses at 24 months.63 A study by De Stefano et al found comparable ACR20 and DAS28 responses among individuals receiving TNFis with methotrexate or leflunomide.61
The “Swapping” Strategy
The efficacy of the swapping strategy has been shown in 3 randomized clinical trials demonstrating the superiority of abatacept, tocilizumab, and rituximab in the treatment of individuals with RA refractory to TNFis. Tocilizumab was studied in the RADIATE64 trial, which involved 499 patients with inadequate response to 1 or more TNFi agents. The primary endpoint (24-week ACR20) was achieved by 50.0%, 30.4%, and 10.1% of patients in the 8 mg/kg, 4 mg/kg, and control groups, respectively (P < 0.001 for both tocilizumab groups versus placebo). The utility of abatacept as second-line therapy after initial TNF failure was evaluated in the ATTAIN65 study. Participants with an inadequate response to etanercept or infliximab were randomly assigned to receive either abatacept or placebo. ACR50 response rates after 6 months of treatment were 20.3% with abatacept and 3.8% with placebo (P < 0.001). The SWITCH-RA study,66 an observational study, compared rituximab to TNFis in 1112 participants with inadequate response to initial anti-TNF therapy. At 6 months, mean change in DAS28 was small but significantly greater for the rituximab group (–1.5 vs –1.1; P = 0.007). The difference in response rates was greatest among seropositive patients. These data suggest that rituximab has efficacy following TNFi failure, particularly for seropositive patients. Additionally, REFLEX67 is the sole randomized controlled trial in patients with insufficient response to TNFis that showed significant prevention of radiographic progression at week 56 in patients on rituximab compared to placebo (mean change from baseline in total Genant-modified Sharp score, 1.00 vs 2.31, respectively; P = 0.005).
One study randomly assigned 399 patients with active RA who had inadequate response to prior TNFi therapy to tofacitinib68 (5 mg twice daily or 10 mg twice daily) or placebo, both with methotrexate.6 After 3 months of treatment, ACR20 response rates (41.7% for 5 mg, 28.1% for 10 mg, 24.4% for placebo) and DAS28 remission rates (6.7% for 5 mg, 8.8% for 10 mg, 1.7% for placebo) were significantly greater among patients treated with tofacitinib compared to those treated with placebo. More recently, the RA-BEACON trial69 demonstrated a consistent, beneficial treatment effect of baricitinib in patients with insufficient response to 1 or more TNFis. In this trial, 527 patients with an inadequate response to bDMARDs were randomly assigned to receive baricitinib 2 mg or 4 mg daily or placebo for 24 weeks. A higher proportion of patients receiving baricitinib 4 mg had an ACR20 response at week 12 compared with those treated with placebo (55% vs 27%, P < 0.001), and patients receiving the 4-mg dose had significant improvements from baseline in DAS28 and Health Assessment Questionnaire–Disability Index scores (P < 0.001 for both comparisons).
To Cycle or to Swap?
Several observational studies (SCQM-RA,70 STURE,71 BSRBR,72 Favalli,43 MIRAR,73 SWITCH-RA,74 ROC72) have clearly demonstrated that the swapping strategy is favored over the cycling strategy. In the ROC study,72 patients were randomly assigned (based on physician discretion) to receive a non-TNF biologic or a TNFi. More patients in the non-TNF group than in the TNFi group showed low disease activity at week 24 (45% vs 28%; odds ratio [OR], 2.09; 95% confidence interval [CI], 1.27-3.43; P = 0.004) and at week 52 (41% vs 23%; OR, 2.26; 95% CI, 1.33-3.86; P = 0.003). The authors concluded that in patients having an insufficient response to TNFi therapy, a non-TNF biologic agent may be more effective than a second TNFi drug. Only a few studies75-77 have demonstrated similar results between the 2 strategies. Overall, the available evidence seems to suggest the superiority of the swapping over the cycling strategy.
An important clinical pearl to keep in mind is that both swapping and cycling strategies might theoretically increase the risk of infection; however, limited evidence is reported in the literature. In a large retrospective analysis78 of data on 4332 RA patients from a large US claims database, patients who had cycled between TNFi agents had a 30% to 40% increased risk of infection compared to patients treated with rituximab. Patients on infliximab had a 62% higher hazard of severe infections, and this has also been reported in an observational study.79 In another study,70 41% of 201 patients with RA followed between 1999 and 2013 who swapped to abatacept/rituximab or tocilizumab developed adverse events, as compared to 59% of those who switched to a second TNFi.
What are recent trends in the use of bDMARDs?
Currently, there are no specific guidelines or biomarkers available to facilitate selection of specific treatment from among the classes of biologics. With the development of several new drugs and regulatory approval of baricitinib, physicians now have several biologic options to treat patients. A recent large time-trend study80 deriving data from more than 200,000 patients with RA showed that etanercept remains the most frequently used agent for the treatment of RA; it also showed that the use of adalimumab and infliximab is decreasing, and that the use of newer agents, especially abatacept, golimumab, and certolizumab, has considerably risen in recent years. In this study, abatacept, rituximab, certolizumab, golimumab, tocilizumab, and tofacitinib accounted for 13.2%, 13.8%, 6.9%, 11.9%, and 7.5% switches from first TNFi therapy.
Jin et al81 studied factors associated with the choice of bDMARD for initial and subsequent use. They found that patients with commercial insurance had an 87% higher likelihood of initiating a bDMARD. In the Medicaid subgroup, African Americans had lower odds of initiating and switching bDMARDs than non-Hispanic whites. Prior use of steroids and nonbiologic DMARDs predicted both bDMARD initiation and subsequent switching. Etanercept, adalimumab, and infliximab were the most commonly used first- and second-line bDMARDS; patients on anakinra and golimumab were most likely to be switched to other bDMARDs.
Which treatment strategy is the most cost-effective?
Several studies have reported better treatment persistence rates among patients who are treated with the swapping strategy compared to the cycling strategy. In a retrospective analysis of claims data,82 the authors examined treatment persistence and health care costs in patients switching to biologics with a different mechanism of action or cycling to another TNFi. The mean cost was significantly lower among patients treated using the swapping strategy than among the TNFi cyclers, both for the total cost of care for RA and for the total cost of the targeted DMARDs in the first year after the change in therapy. The authors concluded that switching to a drug with a different mechanism of action is associated with higher treatment persistence and lower health care costs than TNFi cycling.
What about biosimilars?
Biosimilars are copies of already licensed biologics that are very similar to the biologics, but are made by different sponsors using independently derived cell lines and separately developed manufacturing processes.83 Regarding biosimilar use, EULAR26 states that biosimilar bDMARDs approved by the European Medicines Agency or US Food and Drug Administration have similar efficacy and safety as the originator bDMARDs, and recommends them as preferred agents if they are indeed appreciably cheaper than originator or other bDMARDs.
What are the novel treatment targets in RA?
New therapeutics for RA continue to be developed. One of the new agents is peficitinib (ASP015K), an oral, once-daily Janus kinase (Jak) inhibitor targeting Jak-1, Jak-2, and tyrosine kinase-2, with moderate selectivity for Jak-3. In a phase 2b trial, 100-mg and 150-mg doses of peficitinib achieved a statistically significant ACR20 response (48.3% and 56.3%) compared to placebo (29.4%) at 12 weeks.84
Given the benefit of targeting TNF-α and IL-17 in RA, a novel molecule (ABT-122) that targets both human TNF and IL-17 has been developed. Two phase 1 studies85 showed that dual neutralization of TNF and IL-17 with ABT-122 has characteristics acceptable for further exploration of therapeutic potential of this agent in TNF- and IL-17A–driven immune-mediated inflammatory diseases. Another novel drug is mavrilimumab, a human monoclonal antibody that targets granulocyte–macrophage colony-stimulating factor receptor α. A recent studyshowed that long-term treatment with mavrilimumab maintained response and was well-tolerated, with no increased incidence of treatment-emergent adverse events.86
Namilumab (AMG203) is an immunoglobulin G1 monoclonal antibody that binds with high affinity to the GM-CSF ligand. In a phase 1b, randomized, double-blind study (PRIORA)87 to assess namilumab in treating active, mild-to-moderate RA, significant improvement was seen in the DAS28-CRP score with namilumab (150 and 300 mg groups combined) compared with placebo at day 43 (P = 0.0117) and also 8 weeks after last dosing at day 99 (P = 0.0154). Adverse events were similar across different doses of namilumab and placebo, and included nasopharyngitis and exacerbation/worsening of RA. Another drug showing promise in RA is fosdagrocorat (PF-04171327), a potential dissociated agonist of the glucocorticoid receptor. A multicenter, double-blind, parallel-group, active- and placebo-controlled phase 2 study randomly assigned 86 patients to receive fosdagrocorat 10 mg, fosdagrocorat 25 mg, prednisone 5 mg, or placebo, all with stable background methotrexate therapy.88 Both fosdagrocorat doses demonstrated efficacy in improving signs and symptoms in RA patients, with manageable adverse events.
Case Conclusion
There are several available treatment options for the case patient. Based on the PREMIER trial, solely increasing the dose of adalimumab is unlikely to provide a therapeutic benefit. Adding low-dose methotrexate (possibly via a parenteral route because of patient-reported gastrointestinal discomfort) might provide some synergistic and therapeutic effect. However, because of primary failure with TNFi therapy, she may benefit from the initiation of a biologic with a different mechanism of action (ie, swapping strategy). Therapeutic options include tocilizumab, abatacept, rituximab, and the Jak inhibitors (tofacitinib and baricitinib).
Summary
The optimal treatment of RA aims at achieving, and then maintaining, remission or a low disease activity. The choice of best treatment must be individualized to the patient, keeping in mind other factors, including comorbidities like fibromyalgia, history of diverticulitis (prior to use of tocilizumab), history of chronic obstructive pulmonary disease (prior to the use of abatacept), malignancy, and the presence of risk factors for infections (age, diabetes, chronic bronchitis). In a patient with inadequate response to initial biologic therapy, several options exist for the rheumatologist. Current evidence supports TNFi dose escalation for only infliximab; optimization of concurrent csDMARD or switching to a different csDMARD are other options. Cycling and swapping are other alternate approaches supported by many observational studies. While no head-to-head trials exist comparing the 2 strategies, data suggest superiority of the swapping strategy over the cycling approach. With the continuing development of novel therapeutics in RA, physicians have a growing list of treatment options to help their patients achieve disease remission.
Corresponding author: Namrata Singh, MD, 200 Hawkins Drive, Iowa City, IA 52242.
Financial disclosures: None.
1. Keystone ED, Kavanaugh KA. What to do with TNF failures. Expert Opin Drug Saf. 2005;4:149-155.
2. Kalden JR, Schulze-Koops H. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol. 2017;13:707-718.
3. Weinblatt ME, Kremer JM, Bankhurst AD, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999;340:253-259.
4. Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50(5):1400-1411.
5. Lipsky PE, van der Heijde DM, St Clair EW, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343:1594-1602.
6. Finckh A, Simard JF, Gabay C, et al. Evidence for differential acquired drug resistance to anti-tumour necrosis factor agents in rheumatoid arthritis. Ann Rheum Dis. 2006;65:746-752.
7. Souto A, Maneiro JR, Gomez-Reino JJ. Rate of discontinuation and drug survival of biologic therapies in rheumatoid arthritis: a systematic review and meta-analysis of drug registries and health care databases. Rheumatology. 2016;55:523-534.
8. Hetland ML, Christensen IJ, Tarp U, et al. Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum. 2010;62:22-32.
9. Gabay C, Riek M, Scherer A, et al. Effectiveness of biologic DMARDs in monotherapy versus in combination with synthetic DMARDs in rheumatoid arthritis: data from the Swiss Clinical Quality Management Registry. Rheumatology. 2015;54(9):1664-1672.
10. Ebina K, Hashimoto M, Yamamoto W, et al. Drug retention and discontinuation reasons between seven biologics in patients with rheumatoid arthritis-The ANSWER cohort study. PloS One. 2018;13:e0194130.
11. Wijbrandts CA, Tak PP. Prediction of response to targeted treatment in rheumatoid arthritis. Mayo Clin Proc. 2017;92:1129-1143.
12. Ulfgren AK, Andersson U, Engstrom M, et al. Systemic anti-tumor necrosis factor alpha therapy in rheumatoid arthritis down-regulates synovial tumor necrosis factor alpha synthesis. Arthritis Rheum. 2000;43:2391-2396.
13. Choi IY, Gerlag DM, Herenius MJ, et al. MRP8/14 serum levels as a strong predictor of response to biological treatments in patients with rheumatoid arthritis. Ann Rheum Dis. 2015;74:499-505.
14. Nguyen MVC, Baillet A, Romand X, et al. Prealbumin, platelet factor 4 and S100A12 combination at baseline predicts good response to TNF alpha inhibitors in rheumatoid arthritis. Joint Bone Spine. 2019;86:195-201.
15. Anderson JK, Zimmerman L, Caplan L, Michaud K. Measures of rheumatoid arthritis disease activity: Patient (PtGA) and Provider (PrGA) Global Assessment of Disease Activity, Disease Activity Score (DAS) and Disease Activity Score with 28-Joint Counts (DAS28), Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI), Patient Activity Score (PAS) and Patient Activity Score-II (PASII), Routine Assessment of Patient Index Data (RAPID), Rheumatoid Arthritis Disease Activity Index (RADAI) and Rheumatoid Arthritis Disease Activity Index-5 (RADAI-5), Chronic Arthritis Systemic Index (CASI), Patient-Based Disease Activity Score With ESR (PDAS1) and Patient-Based Disease Activity Score without ESR (PDAS2), and Mean Overall Index for Rheumatoid Arthritis (MOI-RA). Arthritis Care Res. 2011;63(suppl 11):S14-S36.
16. Katz L, Gisbert JP, Manoogian B, et al. Doubling the infliximab dose versus halving the infusion intervals in Crohn’s disease patients with loss of response. Inflamm Bowel Dis. 2012;18:2026-2033.
17. Durez P, Van den Bosch F, Corluy L, et al. A dose adjustment in patients with rheumatoid arthritis not optimally responding to a standard dose of infliximab of 3 mg/kg every 8 weeks can be effective: a Belgian prospective study. Rheumatology. 2005;44:465-468.
18. Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26-37.
19. St Clair EW, Wagner CL, Fasanmade AA, et al. The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46:1451-1459.
20. Rahman MU, Strusberg I, Geusens P, et al. Double-blinded infliximab dose escalation in patients with rheumatoid arthritis. Ann Rheum Dis. 2007;66:1233-1238.
21. Weinblatt ME, Schiff MH, Ruderman EM, et al. Efficacy and safety of etanercept 50 mg twice a week in patients with rheumatoid arthritis who had a suboptimal response to etanercept 50 mg once a week: results of a multicenter, randomized, double-blind, active drug-controlled study. Arthritis Rheum. 2008;58:1921-1930.
22. Curtis JR, Chen L, Luijtens K, et al. Dose escalation of certolizumab pegol from 200 mg to 400 mg every other week provides no additional efficacy in rheumatoid arthritis: an analysis of individual patient-level data. Arthritis Rheum. 2011;63:2203-2208.
23. Okazaki M, Kobayashi H, Ishii Y, et al. Real-world treatment patterns for golimumab and concomitant medications in Japanese rheumatoid arthritis patients. Rheumatol Ther. 2018;5:185-201.
24. Pappas DA, John A, Curtis JR, et al. Dosing of intravenous tocilizumab in a real-world setting of rheumatoid arthritis: analyses from the Corrona Registry. Rheumatol Ther. 2016;3:103-115.
25. Kivitz A, Olech E, Borofsky MA, et al. Two-year efficacy and safety of subcutaneous tocilizumab in combination with disease-modifying antirheumatic drugs including escalation to weekly dosing in rheumatoid arthritis. J Rheumatol. 2018;45:456-464.
26. Smolen JS, Landewe R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76:960-977.
27. Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. 2016;68:1-25.
28. Tabrizi MA, Tseng CM, Roskos LK. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today. 2006;11:81-88.
29. Keizer RJ, Huitema AD, Schellens JH, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:493-507.
30. Navarro Coy NC, Brown S, Bosworth A, et al. The ‘Switch’ study protocol: a randomised-controlled trial of switching to an alternative tumour-necrosis factor (TNF)-inhibitor drug or abatacept or rituximab in patients with rheumatoid arthritis who have failed an initial TNF-inhibitor drug. BMC Musculoskelet Disord. 2014;15:452.
31. Kamal KM, Madhavan SS, Hornsby JA, et al. Use of tumor necrosis factor inhibitors in rheumatoid arthritis: a national survey of practicing United States rheumatologists. Joint Bone Spine. 2006;73:718-724.
32. Gomez-Reino JJ, Carmona L, BIOBADASER Group. Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients over a four-year period. Arthritis Res Ther. 2006;8:R29.
33. Caporali R, Sarzi-Puttini P, Atzeni F, et al. Switching TNF-alpha antagonists in rheumatoid arthritis: The experience of the LORHEN registry. Autoimmun Rev. 2010;9:465-469.
34. Iannone F, Trotta F, Monteccuco C, et al. Etanercept maintains the clinical benefit achieved by infliximab in patients with rheumatoid arthritis who discontinued infliximab because of side effects. Ann Rheum Dis. 2007;66:249-252.
35. Virkki LM, Valleala H, Takakubo Y, et al. Outcomes of switching anti-TNF drugs in rheumatoid arthritis—a study based on observational data from the Finnish Register of Biological Treatment (ROB-FIN). Clin Rheumatol. 2011;30:1447-1454.
36. Furst DE, Gaylis N, Bray V, et al. Open-label, pilot protocol of patients with rheumatoid arthritis who switch to infliximab after an incomplete response to etanercept: the opposite study. Ann Rheum Dis. 2007;66:893-899.
37. Smolen JS, Kay J, Doyle MK, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet. 2009;374:210-221.
38. Smolen JS, Kay J, Doyle M, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumor necrosis factor α inhibitors: findings with up to five years of treatment in the multicenter, randomized, double-blind, placebo-controlled, phase 3 GO-AFTER study. Arthritis Res Ther. 2015;17:14.
39. Smolen JS, Burmester G-R, Combe B, et al. Head-to-head comparison of certolizumab pegol versus adalimumab in rheumatoid arthritis: 2-year efficacy and safety results from the randomised EXXELERATE study. Lancet. 2016;388:2763-2774.
40. Chatzidionysiou K, Askling J, Eriksson J, et al. Effectiveness of TNF inhibitor switch in RA: results from the national Swedish register. Ann Rheum Dis. 2015;74:890.
41. Smolen JS, Kay J, Doyle M, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumor necrosis factor alpha inhibitors: findings with up to five years of treatment in the multicenter, randomized, double-blind, placebo-controlled, phase 3 GO-AFTER study. Arthritis Res Ther. 2015;17:14.
42. Lequerré T, Farran É, Ménard J-F, et al. Switching from an anti-TNF monoclonal antibody to soluble TNF-receptor yields better results than vice versa: An observational retrospective study of 72 rheumatoid arthritis switchers. Joint Bone Spine. 2015;82:330-337.
43. Favalli EG, Biggioggero M, Meroni PL. Methotrexate for the treatment of rheumatoid arthritis in the biologic era: Still an “anchor” drug? Autoimmun Rev. 2014;13:1102-1108.
44. Kalden JR, Schulze-Koops H. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol. 2017;13:707-718.
45. Klareskog L, van der Heijde D, de Jager JP, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363:675-681.
46. Emery P, Fleischmann RM, Strusberg I, et al. Efficacy and safety of subcutaneous golimumab in methotrexate-naive patients with rheumatoid arthritis: five-year results of a randomized clinical trial. Arthritis Care Res. 2016;68:744-752.
47. Emery P, Fleischmann RM, Moreland LW, et al. Golimumab, a human anti-tumor necrosis factor alpha monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: twenty-four-week results of a phase III, multicenter, randomized, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum. 2009;60:2272-2283.
48. Emery P, Burmester GR, Bykerk VP, et al. Evaluating drug-free remission with abatacept in early rheumatoid arthritis: results from the phase 3b, multicentre, randomised, active-controlled AVERT study of 24 months, with a 12-month, double-blind treatment period. Ann Rheum Dis. 2015;74:19-26.
49. Westhovens R, Robles M, Ximenes AC, et al. Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Ann Rheum Dis. 2009;68:1870-1877.
50. Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793-2806.
51. Burmester GR, Rigby WF, van Vollenhoven RF, et al. Tocilizumab in early progressive rheumatoid arthritis: FUNCTION, a randomised controlled trial. Ann Rheum Dis. 2016;75:1081-1091.
52. Bijlsma JWJ, Welsing PMJ, Woodworth TG, et al. Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (U-Act-Early): a multicentre, randomised, double-blind, double-dummy, strategy trial. Lancet. 2016;388:343-355.
53. Cohen JD, Zaltni S, Kaiser MJ, et al. Secondary addition of methotrexate to partial responders to etanercept alone is effective in severe rheumatoid arthritis. Ann Rheum Dis. 2004;63:209-210.
54. Hamilton RA, Kremer JM. Why intramuscular methotrexate may be more efficacious than oral dosing in patients with rheumatoid arthritis. Br J Rheumatol. 1997;36:86-90.
55. Hoekstra M, Haagsma C, Neef C, et al. Bioavailability of higher dose methotrexate comparing oral and subcutaneous administration in patients with rheumatoid arthritis. J Rheumatol. 2004;31:645-648.
56. Herman RA, Veng-Pedersen P, Hoffman J, et al. Pharmacokinetics of low-dose methotrexate in rheumatoid arthritis patients. J Pharm Sci. 1989;78:165-171.
57. Schiff MH, Jaffe JS, Freundlich B. Head-to-head, randomised, crossover study of oral versus subcutaneous methotrexate in patients with rheumatoid arthritis: drug-exposure limitations of oral methotrexate at doses ± 15 mg may be overcome with subcutaneous administration. Ann Rheum Dis. 2014;73:1549-1551.
58. Hazlewood GS, Thorne JC, Pope JE, et al. The comparative effectiveness of oral versus subcutaneous methotrexate for the treatment of early rheumatoid arthritis. Ann Rheum Dis. 2016;75:1003-1008.
59. O’Dell JR, Petersen K, Leff R, et al. Etanercept in combination with sulfasalazine, hydroxychloroquine, or gold in the treatment of rheumatoid arthritis. J Rheumatol. 2006;33:213-218.
60. Finckh A, Dehler S, Gabay C. The effectiveness of leflunomide as a co-therapy of tumour necrosis factor inhibitors in rheumatoid arthritis: a population-based study. Ann Rheum Dis. 2009;68:33-39.
61. De Stefano R, Frati E, Nargi F, et al. Comparison of combination therapies in the treatment of rheumatoid arthritis: leflunomide-anti-TNF-alpha versus methotrexate-anti-TNF-alpha. Clin Rheumatol. 2010;29:517-524.
62. Combe B, Codreanu C, Fiocco U, et al. Etanercept and sulfasalazine, alone and combined, in patients with active rheumatoid arthritis despite receiving sulfasalazine: a double-blind comparison. Ann Rheum Dis. 2006;65:1357-1362.
63. Strangfeld A, Hierse F, Kekow J, et al. Comparative effectiveness of tumour necrosis factor α inhibitors in combination with either methotrexate or leflunomide. Ann Rheum Dis. 2009;68:1856.
64. Emery P, Keystone E, Tony HP, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis. 2008;67:1516.
65. Genovese MC, Becker J-C, Schiff M, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor α inhibition. N Engl J Med. 2005;353:1114-1123.
66. Emery P, Gottenberg JE, Rubbert-Roth A, et al. Rituximab versus an alternative TNF inhibitor in patients with rheumatoid arthritis who failed to respond to a single previous TNF inhibitor: SWITCH-RA, a global, observational, comparative effectiveness study. Ann Rheum Dis. 2015;74:979-984.
67. Keystone E, Emery P, Peterfy CG, et al. Rituximab inhibits structural joint damage in patients with rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitor therapies. Ann Rheum Dis. 2009;68:216.
68. Burmester GR, Blanco R, Charles-Schoeman C, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013;381:451-460.
69. Genovese MC, Kremer J, Zamani O, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med. 2016;374:1243-1252.
70. Favalli EG, Biggioggero M, Marchesoni A, Meroni PL. Survival on treatment with second-line biologic therapy: a cohort study comparing cycling and swap strategies. Rheumatology. 2014;53:1664-1668.
71. Harrold LR, Reed GW, Solomon DH, et al. Comparative effectiveness of abatacept versus tocilizumab in rheumatoid arthritis patients with prior TNFi exposure in the US Corrona registry. Arthritis Res Ther. 2016;18:280.
72. Gottenberg J, Brocq O, Perdriger A, et al. Non–TNF-targeted biologic vs a second anti-TNF drug to treat rheumatoid arthritis in patients with insufficient response to a first anti-TNF drug: A randomized clinical trial. JAMA. 2016;316:1172-1180.
73. Pascart T, Philippe P, Drumez E, et al. Comparative efficacy of tocilizumab, abatacept and rituximab after non-TNF inhibitor failure: results from a multicentre study. Int J Rheum Dis. 2016;19:1093-1102.
74. Akiyama M, Kaneko Y, Kondo H, Takeuchi T. Comparison of the clinical effectiveness of tumour necrosis factor inhibitors and abatacept after insufficient response to tocilizumab in patients with rheumatoid arthritis. Clin Rheumatol. 2016;35:2829-2834.
75. Schoels M, Aletaha D, Smolen JS, Wong JB. Comparative effectiveness and safety of biological treatment options after tumour necrosis factor α inhibitor failure in rheumatoid arthritis: systematic review and indirect pairwise meta-analysis. Ann Rheum Dis. 2012;71:1303.
76. Soliman MM, Hyrich KL, Lunt M, et al. Rituximab or a second anti-tumor necrosis factor therapy for rheumatoid arthritis patients who have failed their first anti-tumor necrosis factor therapy? Comparative analysis from the British Society for Rheumatology Biologics Register. Arthritis Care Res. 2012;64:1108-1115.
77. Chatzidionysiou K, Vollenhoven RF. Rituximab versus anti-TNF in patients who previously failed one TNF inhibitor in an observational cohort. Scand J Rheumatol. 2013;42:190-195.
78. Johnston SS, Turpcu A, Shi N, et al. Risk of infections in rheumatoid arthritis patients switching from anti-TNF agents to rituximab, abatacept, or another anti-TNF agent, a retrospective administrative claims analysis. Semim Arthritis Rheum. 2013;43:39-47.
79. Curtis JR, Xie F, Chen L, et al. The comparative risk of serious infections among rheumatoid arthritis patients starting or switching biological agents. Ann Rheum Dis. 2011;70:1401.
80. Desai RJ, Solomon DH, Jin Y, et al. Temporal trends in use of biologic DMARDs for rheumatoid arthritis in the United States: a cohort study of publicly and privately insured patients. J Manag Care Spec Pharm. 2017;23:809-814.
81. Jin Y, Desai RJ, Liu J, et al. Factors associated with initial or subsequent choice of biologic disease-modifying antirheumatic drugs for treatment of rheumatoid arthritis. Arthritis Res Ther. 2017;19:159.
82. Bonafede MMK, McMorrow D, Proudfoot C, et al. Treatment persistence and healthcare costs among patients with rheumatoid arthritis after a change in targeted therapy. Am Health Drug Benefits. 2018;11:192-202.
83. US Food and Drug Administration. Biosimilars are safe, effective treatment options. www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/therapeuticbiologicapplications/biosimilars/. Accessed November 9, 2018.
84. Genovese MC, Greenwald M, Codding C, et al. Peficitinib, a JAK inhibitor, in combination with limited conventional synthetic disease-modifying antirheumatic drugs in the treatment of moderate-to-severe rheumatoid arthritis. Arthritis Rheumatol. 2017;69:932-942.
85. Fleischmann RM, Wagner F, Kivitz AJ, et al. Safety, tolerability, and pharmacodynamics of ABT-122, a tumor necrosis factor- and interleukin-17-targeted dual variable domain immunoglobulin, in patients with rheumatoid arthritis. Arthritis Rheumatol. 2017;69:2283-2291.
86. Burmester GR, McInnes IB, Kremer JM, et al. Mavrilimumab, a fully human granulocyte-macrophage colony-stimulating factor receptor alpha monoclonal antibody: long-term safety and efficacy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2018;70:679-689.
87. Huizinga TW, Batalov A, Stoilov R, et al. Phase 1b randomized, double-blind study of namilumab, an anti-granulocyte macrophage colony-stimulating factor monoclonal antibody, in mild-to-moderate rheumatoid arthritis. Arthritis Res Ther. 2017;19:53.
88. Stock T, Fleishaker D, Wang X, et al. Improved disease activity with fosdagrocorat (PF-04171327), a partial agonist of the glucocorticoid receptor, in patients with rheumatoid arthritis: a Phase 2 randomized study. Int J Rheum Dis. 2017;20:960-970.
89. Orencia [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2013.
90. Humira[package insert]. North Chicago, IL: AbbVie; 2012.
91. Kineret [package insert]. Stockholm, Sweden: Sobi; 2012.
92. Olumiant [package insert]. Indianapolis, IN: Lilly USA, LLC; 2018.
93. Cimzia [package insert]. Smyrna, GA: UCB, Inc; 2008.
94. Enbrel [package insert]. Thousand Oaks, CA: Immunex Corporation; 1998.
95. Simponi [package insert]. Horsham, PA: Janssen Biotech, Inc; 2009.
96. Remicade [package insert]. Horsham, PA: Janssen Biotech, Inc; 1998.
97. Rituxan [package insert]. South San Francisco, CA: Genetech, Inc; 1997.
98. Kevzara [package insert]. Bridgewater, NJ: Sanofi-Aventis US LLC; 2018.
99. Actemra [package insert]. South San Francisco, CA: Genentech, Inc; 2013.
100. Xeljanz [package insert]. New York, NY: Pfizer Inc; 2016.
From the University of Iowa Hospitals and Clinics, Iowa City, IA.
Abstract
- Objective: To discuss the variability in response to tumor necrosis factor inhibitors (TNFis) observed in patients with rheumatoid arthritis (RA) and discuss therapeutic options for patients who do not respond to initial TNFi therapy.
- Methods: Review of the literature.
- Results: Optimal treatment of RA aims at achieving and then maintaining remission or low disease activity. In a patient with an inadequate response to initial biologic therapy, several therapeutic options exist. Current evidence supports TNFi dose escalation for only infliximab; optimization of concurrent conventional synthetic disease-modifying antirheumatic drug (csDMARD) or switching to a different csDMARD are other options. Cycling (switching to an alternative TNFi) and swapping (switching to a therapy with a different mode of action) strategies are other alternate approaches supported by many observational studies. While no head-to-head trials exist directly comparing the 2 strategies, data suggest superiority of the swapping strategy over the cycling approach. Also, several studies have shown that switching to a drug with a different mechanism of action is associated with higher treatment persistence and lower health care costs than TNFi cycling.
- Conclusion: Physicians have a growing list of treatment options to help their patients with RA achieve disease remission. The choice of best treatment for a given patient needs to be individualized, keeping in mind other factors, including comorbidities.
Keywords: biologics; rheumatoid arthritis; swapping strategy; cycling strategy; TNF inhibitors.
Following the discovery of tumor necrosis factor (TNF) as a proinflammatory cytokine 30 years ago, the use of TNF antagonists has revolutionized the treatment of rheumatoid arthritis (RA). Although TNF inhibitors (TNFIs) are frequently used as a first-line biologic disease-modifying antirheumatic drug (bDMARD), they are not uniformly efficacious in achieving remission in all patients with RA. This article highlights the reasons for such variability in observed response and discusses therapeutic options for patients who do not respond to TNFi therapy.
Case Presentation
A 60-year-old woman is evaluated in the clinic for complaints of pain in her hands, morning stiffness lasting 2 hours, and swelling in her wrists, all of which have been ongoing for 3 months. Physical exam reveals evidence of active inflammation, with synovitis in her second, third, and fourth metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints bilaterally, swelling over both wrists, and a weak grip. Inflammatory markers are elevated, and rheumatoid factor and anti-cyclic citrullinated peptide (anti-CCP) are both positive at high titer. Radiographs reveal evidence of small erosions at the third and fourth MCPs and PIPs bilaterally and periarticular osteopenia. The patient is diagnosed with seropositive, erosive RA based on history, physical exam, laboratory studies, and imaging. She is started on 20 mg of prednisone for acute treatment of her symptoms along with methotrexate, and, initially, her symptoms are well controlled. A few months after starting treatment, she develops voluminous diarrhea that necessitates cessation of methotrexate. Leflunomide also causes similar symptoms. The combination of sulfasalazine and hydroxychloroquine does not adequately control her symptoms, and ongoing use of low-dose glucocorticoids is required to improve functionality in all joints. Using the treat-to-target (T2T) strategy, adalimumab is initiated. However, she continues to report persistent swelling and pain and still requests oral glucocorticoids to help decrease inflammation. The 28-joint Disease Activity Score (DAS28) is 4.8, suggestive of moderate disease activity.
Why are TNFi agents sometimes ineffective?
The introduction of monoclonal antibodies and fusion proteins to block TNF and other cytokines was a remarkable development in the treatment of RA that revolutionized patient care. Despite the efficacy of TNFis, clinical response to these agents is not universal and only some patients achieve complete remission. In targeting the eventual goal of remission or low disease activity in patients with RA, the concept of “TNF failure” becomes extremely relevant. These inadequate responses to anti-TNF therapy may be due to primary failures, or complete lack of clinical response after initiation of the bDMARD, and secondary failures, or the loss of initially achieved clinical response to therapy. Other reasons for discontinuation of a given TNFi include partial disease control and intolerance to the medication (possible injection-site or infusion reactions). Keystone and Kavanaugh1 divided causes of failure of TNF agents into 2 broad categories: perceptual (related to natural variations in disease course like hormonal variation and physical and emotional stress) and pathophysiological failures (genetic variations, high body mass index, concomitant cigarette use).
Another important consideration in patients treated with a TNFi is the consequent formation of anti-drug antibodies (ADAs). TNFi agents are immunogenic and normally elicit an immune response. The appearance of such ADAs may reduce the bioavailability of free drug, resulting in a decreased clinical response,2 or may lead to serious adverse effects.
How common is discontinuation of the first TNFi?
Several studies have reported that the prevalence of primary failure, secondary failure, and intolerance to TNFis ranges from 30% to 40%.3-6 Female sex,7 concurrent prednisone use,8 high disease activity scores,6,8,9 and the absence of treatment with low-dose methotrexate7,8 have all been shown to be negative predictors of bDMARD retention and response.10
Are there any factors that predict TNFi failure?
There are no specific parameters to accurately predict responses to TNFI therapy.11 Several clinical and molecular biomarkers in synovium (initial TNF levels, macrophages, T cells)12 and peripheral blood (serum myeloid-related protein 8 and 14 complex levels,13 prealbumin, platelet factor 4, and S100A12)14 have been described as predictors of clinical response to TNFis, but their utility in clinical practice has not been established and the use of these markers has not yet been incorporated into clinical guidelines.
How is disease activity measured in patients with RA?
In 2010 an international expert consensus panel published treatment recommendations for RA that emphasized a T2T strategy of individualizing and escalating treatment to achieve the lowest disease activity or remission. In clinical practice, numerous tools are available to measure RA disease activity. Herein, we mention several that are most commonly used in clinical practice.
DAS28 combines single activity measures into an overall continuous measure of disease activity and has been endorsed by both the American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR). It includes a 28-swollen joint count (SJC), 28-tender joint count (TJC), erythrocyte sedimentation rate (ESR; can also be calculated using C-reactive protein [CRP]), and a patient global assessment (PtGA). The cut-offs used for DAS28 interpretation are as follows: remission (< 2.6), low (≥ 2.6 but ≤ 3.2), moderate (> 3.2 but ≤ 5.1), or high (> 5.1).15 Some of the difficulties in using DAS28 in daily clinical practice include the need for a lab value and the time needed to perform the joint counts. Note also that due to the inclusion of ESR, which is influenced by age and other factors, DAS28 may underestimate remission in the elderly.
Another measure of RA disease activity is the Simplified Disease Activity Index (SDAI), which includes 28 SJC, 28 TJC, PtGA, provider global assessment (PrGA), and CRP in mg/dL. The level of disease activity using the SDAI is interpreted as: remission (SDAI ≤ 3.3), low (≥ 3.4 but ≤ 11), moderate (> 11 but ≤ 26), or high (> 26). The advantage of the SDAI is that a calculator or computer is not required for calculations. Another measure, the Clinical Disease Activity Index (CDAI), includes a 28 SJC, 28 TJC, PtGA, and PrGA. Because a laboratory value is not needed to calculate the CDAI, it is well-suited for use in clinical practice. When using the CDAI, the level of disease activity can be defined as remission (CDAI ≤ 2.8), low (> 2.8 but ≤ 10), moderate (> 10 but ≤ 22), or high (> 22). Again, as with the SDAI, a calculator or computer is not needed for calculations.
What are the alternative treatment options after first biologic failure?
In patients who have failed treatment with an initial biologic, usually a TNFi, the treating rheumatologist has the following options (Figure), with the best treatment strategy being driven by individualized patient and disease-related factors (Table 1 and Table 2):
- TNFi dose escalation
- Trial of an alternate TNFi agent (the “cycling” strategy)
- Optimization of therapy conjoined with a conventional synthetic DMARD (csDMARD)
- Use of a non-TNF biologic or targeted synthetic DMARD (the “swapping” strategy)

If all the listed strategies fail, the next step can be the addition of short-term, low-dose glucocorticoid therapy.

TNFi Dose Escalation
The available data have demonstrated the safety, efficacy, and cost-effectiveness of dose escalation in patients with RA receiving infliximab.16-18 The ATTRACT trial first demonstrated this, with greater clinical and radiographic improvements in those with higher trough serum concentrations, suggesting that doses higher than 3 mg/kg or more frequent than every 8 weeks may be needed for full response in some patients.19

There is a lack of studies in RA patients to determine the most effective dose escalation strategy. A study in patients with Crohn disease showed that intensification to 10 mg/kg every 8 weeks (dose doubling) was at least as effective as 5 mg/kg every 4 weeks (halving interval) at 12 months.16 Due to greater patient and administration convenience of dose-doubling, this strategy may be preferred.17 A starting dose of 10 mg/kg every 8 weeks is not routinely recommended due to an increased risk of serious infection; these adverse events were not found when the dose was gradually increased, as clinically indicated, starting at 3 mg/kg.19,20 Further studies are needed to explore this approach in RA patients.
These results, however, have not been replicated with other TNFi agents. No significant clinical improvements were identified with etanercept 50 mg twice weekly,21 adalimumab 40 mg every week in the PREMIER trial,18 or certolizumab 400 mg every other week in an open-label extension phase of the RAPID 1 study.22 A Japanese study found significantly worse clinical outcomes with dose escalation of golimumab.23 Conversely, 2 studies found clinical benefits after escalating the tocilizumab dose, the first a real-world review from the Consortium of Rheumatology Researchers of North America (CORRONA) registry using the intravenous formulation,24 and the other the BREVACTA study utilizing subcutaneous tocilizumab.25 No studies to date have been published on dose escalation of abatacept in patients with RA who respond poorly. Overall, previous studies support dose escalation in individuals being treated with infliximab to improve clinical outcomes, but additional studies are needed for other bDMARDs.
Trial of an Alternate TNF Agent: The “Cycling” Strategy
Per the ACR/EULAR26,27 guidelines, all approved bDMARDs may be used without hierarchical positioning. However, after the failure of a TNFi agent, these guidelines do not provide specific advice about a preference between the “cycling” strategy (switching to an alternative TNFi) and “swapping” strategy (switching to a therapy with a different mode of action). Cycling might work for several reasons, including differences in the agents’ molecular structure, immunological mechanism of action, immunogenicity, and pharmacokinetics.28-30 The cycling strategy is a well-established approach adopted by more than 94% of practicing rheumatologists, according to a national survey,31 and its efficacy is supported by trials and additional observational studies.32-35
The greater clinical effectiveness of switching to infliximab compared with continuing with etanercept in patients with inadequate response to etanercept (n = 28) was suggested in the open-label OPPOSITE trial.36 Data from the GO-AFTER trial37 suggests that a greater proportion of patients with RA refractory to adalimumab, etanercept, or infliximab who were treated with golimumab achieved an ACR20 and ACR50 response compared with patients who received placebo, and this response persisted through 5 years.38 More recently, certolizumab pegol and adalimumab were compared head-to-head in the EXXELERATE trial.39 The results of this trial revealed the adequate efficacy of cycling to another TNFi after primary insufficient response to the first.
In studies from Finland and Sweden,35,40 it has been observed that a better response is achieved in patients in whom TNF failure was initially due to secondary failure or intolerance rather than primary failure. A post-hoc analysis of the results of the GO-AFTER trial41 and from a few observational studies35,40,42 revealed that switching from one TNFi to another, especially from a monoclonal antibody to a soluble receptor, was often more beneficial for RA patients than switching from a soluble receptor to a monoclonal antibody.
Optimization of Therapy Conjoined with csDMARDs
Methotrexate is one of the oldest and most effective csDMARDs available for the treatment of RA.43 The 2016 EULAR guidelines recommend the addition of methotrexate and/or other csDMARDs to potentiate the effect of bDMARDs.26 In the case of TNFi therapy, the observed synergistic effect between the monoclonal antibody and methotrexate may be explained by sustained suppression of ADA formation.44 In the TEMPO,45 PREMIER,18 and GO-BEFORE46 trials, the addition of methotrexate led to improved clinical and radiological outcomes in patients treated with etanercept, adalimumab, and golimumab,47 respectively. These findings were also demonstrated in several registries, where significant improvement in clinical response and retention rate of the TNFi agents was noted. Results have been replicated with non-TNFi bDMARDs, including abatacept48,49 and rituximab.50 Patients treated with interleukin (IL)-6 inhibitors in combination with methotrexate have shown significantly less radiographic progression compared to those treated with tocilizumab alone and those treated with monotherapy tocilizumab versus monotherapy methotrexate.51,52 Results possibly favor the use of IL-6 inhibitors alone in those who cannot tolerate or have contraindications to methotrexate.
An open prospective study by Cohen et al added methotrexate to the treatment regimens of individuals on bDMARD monotherapy with a primary failure and found favorable changes in ACR20 and DAS28 scores at 3 and 12 months and therapeutic biological response (ESR, CRP) at 3 months.53 Unlike monotherapy, in these situations methotrexate is known to be efficacious even at a lower dose, possibly at 7.5 mg to 10 mg per week. Some studies have shown that methotrexate administered parenterally may be more efficacious than when given orally.54-58
In clinical trials and observational studies, leflunomide, sulfasalazine, and hydroxychloroquine have been used as alternate csDMARDs added to the treatment regimen.59-62 There are, however, only 2 trials comparing the efficacy of methotrexate with that of other csDMARDs as concomitant treatment in patients with inadequate response to TNFi therapy. The RABBIT trial found a slight decrease in effectiveness with concomitant TNFi and leflunomide compared to TNFi/methotrexate, but overall each group had similar EULAR responses at 24 months.63 A study by De Stefano et al found comparable ACR20 and DAS28 responses among individuals receiving TNFis with methotrexate or leflunomide.61
The “Swapping” Strategy
The efficacy of the swapping strategy has been shown in 3 randomized clinical trials demonstrating the superiority of abatacept, tocilizumab, and rituximab in the treatment of individuals with RA refractory to TNFis. Tocilizumab was studied in the RADIATE64 trial, which involved 499 patients with inadequate response to 1 or more TNFi agents. The primary endpoint (24-week ACR20) was achieved by 50.0%, 30.4%, and 10.1% of patients in the 8 mg/kg, 4 mg/kg, and control groups, respectively (P < 0.001 for both tocilizumab groups versus placebo). The utility of abatacept as second-line therapy after initial TNF failure was evaluated in the ATTAIN65 study. Participants with an inadequate response to etanercept or infliximab were randomly assigned to receive either abatacept or placebo. ACR50 response rates after 6 months of treatment were 20.3% with abatacept and 3.8% with placebo (P < 0.001). The SWITCH-RA study,66 an observational study, compared rituximab to TNFis in 1112 participants with inadequate response to initial anti-TNF therapy. At 6 months, mean change in DAS28 was small but significantly greater for the rituximab group (–1.5 vs –1.1; P = 0.007). The difference in response rates was greatest among seropositive patients. These data suggest that rituximab has efficacy following TNFi failure, particularly for seropositive patients. Additionally, REFLEX67 is the sole randomized controlled trial in patients with insufficient response to TNFis that showed significant prevention of radiographic progression at week 56 in patients on rituximab compared to placebo (mean change from baseline in total Genant-modified Sharp score, 1.00 vs 2.31, respectively; P = 0.005).
One study randomly assigned 399 patients with active RA who had inadequate response to prior TNFi therapy to tofacitinib68 (5 mg twice daily or 10 mg twice daily) or placebo, both with methotrexate.6 After 3 months of treatment, ACR20 response rates (41.7% for 5 mg, 28.1% for 10 mg, 24.4% for placebo) and DAS28 remission rates (6.7% for 5 mg, 8.8% for 10 mg, 1.7% for placebo) were significantly greater among patients treated with tofacitinib compared to those treated with placebo. More recently, the RA-BEACON trial69 demonstrated a consistent, beneficial treatment effect of baricitinib in patients with insufficient response to 1 or more TNFis. In this trial, 527 patients with an inadequate response to bDMARDs were randomly assigned to receive baricitinib 2 mg or 4 mg daily or placebo for 24 weeks. A higher proportion of patients receiving baricitinib 4 mg had an ACR20 response at week 12 compared with those treated with placebo (55% vs 27%, P < 0.001), and patients receiving the 4-mg dose had significant improvements from baseline in DAS28 and Health Assessment Questionnaire–Disability Index scores (P < 0.001 for both comparisons).
To Cycle or to Swap?
Several observational studies (SCQM-RA,70 STURE,71 BSRBR,72 Favalli,43 MIRAR,73 SWITCH-RA,74 ROC72) have clearly demonstrated that the swapping strategy is favored over the cycling strategy. In the ROC study,72 patients were randomly assigned (based on physician discretion) to receive a non-TNF biologic or a TNFi. More patients in the non-TNF group than in the TNFi group showed low disease activity at week 24 (45% vs 28%; odds ratio [OR], 2.09; 95% confidence interval [CI], 1.27-3.43; P = 0.004) and at week 52 (41% vs 23%; OR, 2.26; 95% CI, 1.33-3.86; P = 0.003). The authors concluded that in patients having an insufficient response to TNFi therapy, a non-TNF biologic agent may be more effective than a second TNFi drug. Only a few studies75-77 have demonstrated similar results between the 2 strategies. Overall, the available evidence seems to suggest the superiority of the swapping over the cycling strategy.
An important clinical pearl to keep in mind is that both swapping and cycling strategies might theoretically increase the risk of infection; however, limited evidence is reported in the literature. In a large retrospective analysis78 of data on 4332 RA patients from a large US claims database, patients who had cycled between TNFi agents had a 30% to 40% increased risk of infection compared to patients treated with rituximab. Patients on infliximab had a 62% higher hazard of severe infections, and this has also been reported in an observational study.79 In another study,70 41% of 201 patients with RA followed between 1999 and 2013 who swapped to abatacept/rituximab or tocilizumab developed adverse events, as compared to 59% of those who switched to a second TNFi.
What are recent trends in the use of bDMARDs?
Currently, there are no specific guidelines or biomarkers available to facilitate selection of specific treatment from among the classes of biologics. With the development of several new drugs and regulatory approval of baricitinib, physicians now have several biologic options to treat patients. A recent large time-trend study80 deriving data from more than 200,000 patients with RA showed that etanercept remains the most frequently used agent for the treatment of RA; it also showed that the use of adalimumab and infliximab is decreasing, and that the use of newer agents, especially abatacept, golimumab, and certolizumab, has considerably risen in recent years. In this study, abatacept, rituximab, certolizumab, golimumab, tocilizumab, and tofacitinib accounted for 13.2%, 13.8%, 6.9%, 11.9%, and 7.5% switches from first TNFi therapy.
Jin et al81 studied factors associated with the choice of bDMARD for initial and subsequent use. They found that patients with commercial insurance had an 87% higher likelihood of initiating a bDMARD. In the Medicaid subgroup, African Americans had lower odds of initiating and switching bDMARDs than non-Hispanic whites. Prior use of steroids and nonbiologic DMARDs predicted both bDMARD initiation and subsequent switching. Etanercept, adalimumab, and infliximab were the most commonly used first- and second-line bDMARDS; patients on anakinra and golimumab were most likely to be switched to other bDMARDs.
Which treatment strategy is the most cost-effective?
Several studies have reported better treatment persistence rates among patients who are treated with the swapping strategy compared to the cycling strategy. In a retrospective analysis of claims data,82 the authors examined treatment persistence and health care costs in patients switching to biologics with a different mechanism of action or cycling to another TNFi. The mean cost was significantly lower among patients treated using the swapping strategy than among the TNFi cyclers, both for the total cost of care for RA and for the total cost of the targeted DMARDs in the first year after the change in therapy. The authors concluded that switching to a drug with a different mechanism of action is associated with higher treatment persistence and lower health care costs than TNFi cycling.
What about biosimilars?
Biosimilars are copies of already licensed biologics that are very similar to the biologics, but are made by different sponsors using independently derived cell lines and separately developed manufacturing processes.83 Regarding biosimilar use, EULAR26 states that biosimilar bDMARDs approved by the European Medicines Agency or US Food and Drug Administration have similar efficacy and safety as the originator bDMARDs, and recommends them as preferred agents if they are indeed appreciably cheaper than originator or other bDMARDs.
What are the novel treatment targets in RA?
New therapeutics for RA continue to be developed. One of the new agents is peficitinib (ASP015K), an oral, once-daily Janus kinase (Jak) inhibitor targeting Jak-1, Jak-2, and tyrosine kinase-2, with moderate selectivity for Jak-3. In a phase 2b trial, 100-mg and 150-mg doses of peficitinib achieved a statistically significant ACR20 response (48.3% and 56.3%) compared to placebo (29.4%) at 12 weeks.84
Given the benefit of targeting TNF-α and IL-17 in RA, a novel molecule (ABT-122) that targets both human TNF and IL-17 has been developed. Two phase 1 studies85 showed that dual neutralization of TNF and IL-17 with ABT-122 has characteristics acceptable for further exploration of therapeutic potential of this agent in TNF- and IL-17A–driven immune-mediated inflammatory diseases. Another novel drug is mavrilimumab, a human monoclonal antibody that targets granulocyte–macrophage colony-stimulating factor receptor α. A recent studyshowed that long-term treatment with mavrilimumab maintained response and was well-tolerated, with no increased incidence of treatment-emergent adverse events.86
Namilumab (AMG203) is an immunoglobulin G1 monoclonal antibody that binds with high affinity to the GM-CSF ligand. In a phase 1b, randomized, double-blind study (PRIORA)87 to assess namilumab in treating active, mild-to-moderate RA, significant improvement was seen in the DAS28-CRP score with namilumab (150 and 300 mg groups combined) compared with placebo at day 43 (P = 0.0117) and also 8 weeks after last dosing at day 99 (P = 0.0154). Adverse events were similar across different doses of namilumab and placebo, and included nasopharyngitis and exacerbation/worsening of RA. Another drug showing promise in RA is fosdagrocorat (PF-04171327), a potential dissociated agonist of the glucocorticoid receptor. A multicenter, double-blind, parallel-group, active- and placebo-controlled phase 2 study randomly assigned 86 patients to receive fosdagrocorat 10 mg, fosdagrocorat 25 mg, prednisone 5 mg, or placebo, all with stable background methotrexate therapy.88 Both fosdagrocorat doses demonstrated efficacy in improving signs and symptoms in RA patients, with manageable adverse events.
Case Conclusion
There are several available treatment options for the case patient. Based on the PREMIER trial, solely increasing the dose of adalimumab is unlikely to provide a therapeutic benefit. Adding low-dose methotrexate (possibly via a parenteral route because of patient-reported gastrointestinal discomfort) might provide some synergistic and therapeutic effect. However, because of primary failure with TNFi therapy, she may benefit from the initiation of a biologic with a different mechanism of action (ie, swapping strategy). Therapeutic options include tocilizumab, abatacept, rituximab, and the Jak inhibitors (tofacitinib and baricitinib).
Summary
The optimal treatment of RA aims at achieving, and then maintaining, remission or a low disease activity. The choice of best treatment must be individualized to the patient, keeping in mind other factors, including comorbidities like fibromyalgia, history of diverticulitis (prior to use of tocilizumab), history of chronic obstructive pulmonary disease (prior to the use of abatacept), malignancy, and the presence of risk factors for infections (age, diabetes, chronic bronchitis). In a patient with inadequate response to initial biologic therapy, several options exist for the rheumatologist. Current evidence supports TNFi dose escalation for only infliximab; optimization of concurrent csDMARD or switching to a different csDMARD are other options. Cycling and swapping are other alternate approaches supported by many observational studies. While no head-to-head trials exist comparing the 2 strategies, data suggest superiority of the swapping strategy over the cycling approach. With the continuing development of novel therapeutics in RA, physicians have a growing list of treatment options to help their patients achieve disease remission.
Corresponding author: Namrata Singh, MD, 200 Hawkins Drive, Iowa City, IA 52242.
Financial disclosures: None.
From the University of Iowa Hospitals and Clinics, Iowa City, IA.
Abstract
- Objective: To discuss the variability in response to tumor necrosis factor inhibitors (TNFis) observed in patients with rheumatoid arthritis (RA) and discuss therapeutic options for patients who do not respond to initial TNFi therapy.
- Methods: Review of the literature.
- Results: Optimal treatment of RA aims at achieving and then maintaining remission or low disease activity. In a patient with an inadequate response to initial biologic therapy, several therapeutic options exist. Current evidence supports TNFi dose escalation for only infliximab; optimization of concurrent conventional synthetic disease-modifying antirheumatic drug (csDMARD) or switching to a different csDMARD are other options. Cycling (switching to an alternative TNFi) and swapping (switching to a therapy with a different mode of action) strategies are other alternate approaches supported by many observational studies. While no head-to-head trials exist directly comparing the 2 strategies, data suggest superiority of the swapping strategy over the cycling approach. Also, several studies have shown that switching to a drug with a different mechanism of action is associated with higher treatment persistence and lower health care costs than TNFi cycling.
- Conclusion: Physicians have a growing list of treatment options to help their patients with RA achieve disease remission. The choice of best treatment for a given patient needs to be individualized, keeping in mind other factors, including comorbidities.
Keywords: biologics; rheumatoid arthritis; swapping strategy; cycling strategy; TNF inhibitors.
Following the discovery of tumor necrosis factor (TNF) as a proinflammatory cytokine 30 years ago, the use of TNF antagonists has revolutionized the treatment of rheumatoid arthritis (RA). Although TNF inhibitors (TNFIs) are frequently used as a first-line biologic disease-modifying antirheumatic drug (bDMARD), they are not uniformly efficacious in achieving remission in all patients with RA. This article highlights the reasons for such variability in observed response and discusses therapeutic options for patients who do not respond to TNFi therapy.
Case Presentation
A 60-year-old woman is evaluated in the clinic for complaints of pain in her hands, morning stiffness lasting 2 hours, and swelling in her wrists, all of which have been ongoing for 3 months. Physical exam reveals evidence of active inflammation, with synovitis in her second, third, and fourth metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints bilaterally, swelling over both wrists, and a weak grip. Inflammatory markers are elevated, and rheumatoid factor and anti-cyclic citrullinated peptide (anti-CCP) are both positive at high titer. Radiographs reveal evidence of small erosions at the third and fourth MCPs and PIPs bilaterally and periarticular osteopenia. The patient is diagnosed with seropositive, erosive RA based on history, physical exam, laboratory studies, and imaging. She is started on 20 mg of prednisone for acute treatment of her symptoms along with methotrexate, and, initially, her symptoms are well controlled. A few months after starting treatment, she develops voluminous diarrhea that necessitates cessation of methotrexate. Leflunomide also causes similar symptoms. The combination of sulfasalazine and hydroxychloroquine does not adequately control her symptoms, and ongoing use of low-dose glucocorticoids is required to improve functionality in all joints. Using the treat-to-target (T2T) strategy, adalimumab is initiated. However, she continues to report persistent swelling and pain and still requests oral glucocorticoids to help decrease inflammation. The 28-joint Disease Activity Score (DAS28) is 4.8, suggestive of moderate disease activity.
Why are TNFi agents sometimes ineffective?
The introduction of monoclonal antibodies and fusion proteins to block TNF and other cytokines was a remarkable development in the treatment of RA that revolutionized patient care. Despite the efficacy of TNFis, clinical response to these agents is not universal and only some patients achieve complete remission. In targeting the eventual goal of remission or low disease activity in patients with RA, the concept of “TNF failure” becomes extremely relevant. These inadequate responses to anti-TNF therapy may be due to primary failures, or complete lack of clinical response after initiation of the bDMARD, and secondary failures, or the loss of initially achieved clinical response to therapy. Other reasons for discontinuation of a given TNFi include partial disease control and intolerance to the medication (possible injection-site or infusion reactions). Keystone and Kavanaugh1 divided causes of failure of TNF agents into 2 broad categories: perceptual (related to natural variations in disease course like hormonal variation and physical and emotional stress) and pathophysiological failures (genetic variations, high body mass index, concomitant cigarette use).
Another important consideration in patients treated with a TNFi is the consequent formation of anti-drug antibodies (ADAs). TNFi agents are immunogenic and normally elicit an immune response. The appearance of such ADAs may reduce the bioavailability of free drug, resulting in a decreased clinical response,2 or may lead to serious adverse effects.
How common is discontinuation of the first TNFi?
Several studies have reported that the prevalence of primary failure, secondary failure, and intolerance to TNFis ranges from 30% to 40%.3-6 Female sex,7 concurrent prednisone use,8 high disease activity scores,6,8,9 and the absence of treatment with low-dose methotrexate7,8 have all been shown to be negative predictors of bDMARD retention and response.10
Are there any factors that predict TNFi failure?
There are no specific parameters to accurately predict responses to TNFI therapy.11 Several clinical and molecular biomarkers in synovium (initial TNF levels, macrophages, T cells)12 and peripheral blood (serum myeloid-related protein 8 and 14 complex levels,13 prealbumin, platelet factor 4, and S100A12)14 have been described as predictors of clinical response to TNFis, but their utility in clinical practice has not been established and the use of these markers has not yet been incorporated into clinical guidelines.
How is disease activity measured in patients with RA?
In 2010 an international expert consensus panel published treatment recommendations for RA that emphasized a T2T strategy of individualizing and escalating treatment to achieve the lowest disease activity or remission. In clinical practice, numerous tools are available to measure RA disease activity. Herein, we mention several that are most commonly used in clinical practice.
DAS28 combines single activity measures into an overall continuous measure of disease activity and has been endorsed by both the American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR). It includes a 28-swollen joint count (SJC), 28-tender joint count (TJC), erythrocyte sedimentation rate (ESR; can also be calculated using C-reactive protein [CRP]), and a patient global assessment (PtGA). The cut-offs used for DAS28 interpretation are as follows: remission (< 2.6), low (≥ 2.6 but ≤ 3.2), moderate (> 3.2 but ≤ 5.1), or high (> 5.1).15 Some of the difficulties in using DAS28 in daily clinical practice include the need for a lab value and the time needed to perform the joint counts. Note also that due to the inclusion of ESR, which is influenced by age and other factors, DAS28 may underestimate remission in the elderly.
Another measure of RA disease activity is the Simplified Disease Activity Index (SDAI), which includes 28 SJC, 28 TJC, PtGA, provider global assessment (PrGA), and CRP in mg/dL. The level of disease activity using the SDAI is interpreted as: remission (SDAI ≤ 3.3), low (≥ 3.4 but ≤ 11), moderate (> 11 but ≤ 26), or high (> 26). The advantage of the SDAI is that a calculator or computer is not required for calculations. Another measure, the Clinical Disease Activity Index (CDAI), includes a 28 SJC, 28 TJC, PtGA, and PrGA. Because a laboratory value is not needed to calculate the CDAI, it is well-suited for use in clinical practice. When using the CDAI, the level of disease activity can be defined as remission (CDAI ≤ 2.8), low (> 2.8 but ≤ 10), moderate (> 10 but ≤ 22), or high (> 22). Again, as with the SDAI, a calculator or computer is not needed for calculations.
What are the alternative treatment options after first biologic failure?
In patients who have failed treatment with an initial biologic, usually a TNFi, the treating rheumatologist has the following options (Figure), with the best treatment strategy being driven by individualized patient and disease-related factors (Table 1 and Table 2):
- TNFi dose escalation
- Trial of an alternate TNFi agent (the “cycling” strategy)
- Optimization of therapy conjoined with a conventional synthetic DMARD (csDMARD)
- Use of a non-TNF biologic or targeted synthetic DMARD (the “swapping” strategy)

If all the listed strategies fail, the next step can be the addition of short-term, low-dose glucocorticoid therapy.

TNFi Dose Escalation
The available data have demonstrated the safety, efficacy, and cost-effectiveness of dose escalation in patients with RA receiving infliximab.16-18 The ATTRACT trial first demonstrated this, with greater clinical and radiographic improvements in those with higher trough serum concentrations, suggesting that doses higher than 3 mg/kg or more frequent than every 8 weeks may be needed for full response in some patients.19

There is a lack of studies in RA patients to determine the most effective dose escalation strategy. A study in patients with Crohn disease showed that intensification to 10 mg/kg every 8 weeks (dose doubling) was at least as effective as 5 mg/kg every 4 weeks (halving interval) at 12 months.16 Due to greater patient and administration convenience of dose-doubling, this strategy may be preferred.17 A starting dose of 10 mg/kg every 8 weeks is not routinely recommended due to an increased risk of serious infection; these adverse events were not found when the dose was gradually increased, as clinically indicated, starting at 3 mg/kg.19,20 Further studies are needed to explore this approach in RA patients.
These results, however, have not been replicated with other TNFi agents. No significant clinical improvements were identified with etanercept 50 mg twice weekly,21 adalimumab 40 mg every week in the PREMIER trial,18 or certolizumab 400 mg every other week in an open-label extension phase of the RAPID 1 study.22 A Japanese study found significantly worse clinical outcomes with dose escalation of golimumab.23 Conversely, 2 studies found clinical benefits after escalating the tocilizumab dose, the first a real-world review from the Consortium of Rheumatology Researchers of North America (CORRONA) registry using the intravenous formulation,24 and the other the BREVACTA study utilizing subcutaneous tocilizumab.25 No studies to date have been published on dose escalation of abatacept in patients with RA who respond poorly. Overall, previous studies support dose escalation in individuals being treated with infliximab to improve clinical outcomes, but additional studies are needed for other bDMARDs.
Trial of an Alternate TNF Agent: The “Cycling” Strategy
Per the ACR/EULAR26,27 guidelines, all approved bDMARDs may be used without hierarchical positioning. However, after the failure of a TNFi agent, these guidelines do not provide specific advice about a preference between the “cycling” strategy (switching to an alternative TNFi) and “swapping” strategy (switching to a therapy with a different mode of action). Cycling might work for several reasons, including differences in the agents’ molecular structure, immunological mechanism of action, immunogenicity, and pharmacokinetics.28-30 The cycling strategy is a well-established approach adopted by more than 94% of practicing rheumatologists, according to a national survey,31 and its efficacy is supported by trials and additional observational studies.32-35
The greater clinical effectiveness of switching to infliximab compared with continuing with etanercept in patients with inadequate response to etanercept (n = 28) was suggested in the open-label OPPOSITE trial.36 Data from the GO-AFTER trial37 suggests that a greater proportion of patients with RA refractory to adalimumab, etanercept, or infliximab who were treated with golimumab achieved an ACR20 and ACR50 response compared with patients who received placebo, and this response persisted through 5 years.38 More recently, certolizumab pegol and adalimumab were compared head-to-head in the EXXELERATE trial.39 The results of this trial revealed the adequate efficacy of cycling to another TNFi after primary insufficient response to the first.
In studies from Finland and Sweden,35,40 it has been observed that a better response is achieved in patients in whom TNF failure was initially due to secondary failure or intolerance rather than primary failure. A post-hoc analysis of the results of the GO-AFTER trial41 and from a few observational studies35,40,42 revealed that switching from one TNFi to another, especially from a monoclonal antibody to a soluble receptor, was often more beneficial for RA patients than switching from a soluble receptor to a monoclonal antibody.
Optimization of Therapy Conjoined with csDMARDs
Methotrexate is one of the oldest and most effective csDMARDs available for the treatment of RA.43 The 2016 EULAR guidelines recommend the addition of methotrexate and/or other csDMARDs to potentiate the effect of bDMARDs.26 In the case of TNFi therapy, the observed synergistic effect between the monoclonal antibody and methotrexate may be explained by sustained suppression of ADA formation.44 In the TEMPO,45 PREMIER,18 and GO-BEFORE46 trials, the addition of methotrexate led to improved clinical and radiological outcomes in patients treated with etanercept, adalimumab, and golimumab,47 respectively. These findings were also demonstrated in several registries, where significant improvement in clinical response and retention rate of the TNFi agents was noted. Results have been replicated with non-TNFi bDMARDs, including abatacept48,49 and rituximab.50 Patients treated with interleukin (IL)-6 inhibitors in combination with methotrexate have shown significantly less radiographic progression compared to those treated with tocilizumab alone and those treated with monotherapy tocilizumab versus monotherapy methotrexate.51,52 Results possibly favor the use of IL-6 inhibitors alone in those who cannot tolerate or have contraindications to methotrexate.
An open prospective study by Cohen et al added methotrexate to the treatment regimens of individuals on bDMARD monotherapy with a primary failure and found favorable changes in ACR20 and DAS28 scores at 3 and 12 months and therapeutic biological response (ESR, CRP) at 3 months.53 Unlike monotherapy, in these situations methotrexate is known to be efficacious even at a lower dose, possibly at 7.5 mg to 10 mg per week. Some studies have shown that methotrexate administered parenterally may be more efficacious than when given orally.54-58
In clinical trials and observational studies, leflunomide, sulfasalazine, and hydroxychloroquine have been used as alternate csDMARDs added to the treatment regimen.59-62 There are, however, only 2 trials comparing the efficacy of methotrexate with that of other csDMARDs as concomitant treatment in patients with inadequate response to TNFi therapy. The RABBIT trial found a slight decrease in effectiveness with concomitant TNFi and leflunomide compared to TNFi/methotrexate, but overall each group had similar EULAR responses at 24 months.63 A study by De Stefano et al found comparable ACR20 and DAS28 responses among individuals receiving TNFis with methotrexate or leflunomide.61
The “Swapping” Strategy
The efficacy of the swapping strategy has been shown in 3 randomized clinical trials demonstrating the superiority of abatacept, tocilizumab, and rituximab in the treatment of individuals with RA refractory to TNFis. Tocilizumab was studied in the RADIATE64 trial, which involved 499 patients with inadequate response to 1 or more TNFi agents. The primary endpoint (24-week ACR20) was achieved by 50.0%, 30.4%, and 10.1% of patients in the 8 mg/kg, 4 mg/kg, and control groups, respectively (P < 0.001 for both tocilizumab groups versus placebo). The utility of abatacept as second-line therapy after initial TNF failure was evaluated in the ATTAIN65 study. Participants with an inadequate response to etanercept or infliximab were randomly assigned to receive either abatacept or placebo. ACR50 response rates after 6 months of treatment were 20.3% with abatacept and 3.8% with placebo (P < 0.001). The SWITCH-RA study,66 an observational study, compared rituximab to TNFis in 1112 participants with inadequate response to initial anti-TNF therapy. At 6 months, mean change in DAS28 was small but significantly greater for the rituximab group (–1.5 vs –1.1; P = 0.007). The difference in response rates was greatest among seropositive patients. These data suggest that rituximab has efficacy following TNFi failure, particularly for seropositive patients. Additionally, REFLEX67 is the sole randomized controlled trial in patients with insufficient response to TNFis that showed significant prevention of radiographic progression at week 56 in patients on rituximab compared to placebo (mean change from baseline in total Genant-modified Sharp score, 1.00 vs 2.31, respectively; P = 0.005).
One study randomly assigned 399 patients with active RA who had inadequate response to prior TNFi therapy to tofacitinib68 (5 mg twice daily or 10 mg twice daily) or placebo, both with methotrexate.6 After 3 months of treatment, ACR20 response rates (41.7% for 5 mg, 28.1% for 10 mg, 24.4% for placebo) and DAS28 remission rates (6.7% for 5 mg, 8.8% for 10 mg, 1.7% for placebo) were significantly greater among patients treated with tofacitinib compared to those treated with placebo. More recently, the RA-BEACON trial69 demonstrated a consistent, beneficial treatment effect of baricitinib in patients with insufficient response to 1 or more TNFis. In this trial, 527 patients with an inadequate response to bDMARDs were randomly assigned to receive baricitinib 2 mg or 4 mg daily or placebo for 24 weeks. A higher proportion of patients receiving baricitinib 4 mg had an ACR20 response at week 12 compared with those treated with placebo (55% vs 27%, P < 0.001), and patients receiving the 4-mg dose had significant improvements from baseline in DAS28 and Health Assessment Questionnaire–Disability Index scores (P < 0.001 for both comparisons).
To Cycle or to Swap?
Several observational studies (SCQM-RA,70 STURE,71 BSRBR,72 Favalli,43 MIRAR,73 SWITCH-RA,74 ROC72) have clearly demonstrated that the swapping strategy is favored over the cycling strategy. In the ROC study,72 patients were randomly assigned (based on physician discretion) to receive a non-TNF biologic or a TNFi. More patients in the non-TNF group than in the TNFi group showed low disease activity at week 24 (45% vs 28%; odds ratio [OR], 2.09; 95% confidence interval [CI], 1.27-3.43; P = 0.004) and at week 52 (41% vs 23%; OR, 2.26; 95% CI, 1.33-3.86; P = 0.003). The authors concluded that in patients having an insufficient response to TNFi therapy, a non-TNF biologic agent may be more effective than a second TNFi drug. Only a few studies75-77 have demonstrated similar results between the 2 strategies. Overall, the available evidence seems to suggest the superiority of the swapping over the cycling strategy.
An important clinical pearl to keep in mind is that both swapping and cycling strategies might theoretically increase the risk of infection; however, limited evidence is reported in the literature. In a large retrospective analysis78 of data on 4332 RA patients from a large US claims database, patients who had cycled between TNFi agents had a 30% to 40% increased risk of infection compared to patients treated with rituximab. Patients on infliximab had a 62% higher hazard of severe infections, and this has also been reported in an observational study.79 In another study,70 41% of 201 patients with RA followed between 1999 and 2013 who swapped to abatacept/rituximab or tocilizumab developed adverse events, as compared to 59% of those who switched to a second TNFi.
What are recent trends in the use of bDMARDs?
Currently, there are no specific guidelines or biomarkers available to facilitate selection of specific treatment from among the classes of biologics. With the development of several new drugs and regulatory approval of baricitinib, physicians now have several biologic options to treat patients. A recent large time-trend study80 deriving data from more than 200,000 patients with RA showed that etanercept remains the most frequently used agent for the treatment of RA; it also showed that the use of adalimumab and infliximab is decreasing, and that the use of newer agents, especially abatacept, golimumab, and certolizumab, has considerably risen in recent years. In this study, abatacept, rituximab, certolizumab, golimumab, tocilizumab, and tofacitinib accounted for 13.2%, 13.8%, 6.9%, 11.9%, and 7.5% switches from first TNFi therapy.
Jin et al81 studied factors associated with the choice of bDMARD for initial and subsequent use. They found that patients with commercial insurance had an 87% higher likelihood of initiating a bDMARD. In the Medicaid subgroup, African Americans had lower odds of initiating and switching bDMARDs than non-Hispanic whites. Prior use of steroids and nonbiologic DMARDs predicted both bDMARD initiation and subsequent switching. Etanercept, adalimumab, and infliximab were the most commonly used first- and second-line bDMARDS; patients on anakinra and golimumab were most likely to be switched to other bDMARDs.
Which treatment strategy is the most cost-effective?
Several studies have reported better treatment persistence rates among patients who are treated with the swapping strategy compared to the cycling strategy. In a retrospective analysis of claims data,82 the authors examined treatment persistence and health care costs in patients switching to biologics with a different mechanism of action or cycling to another TNFi. The mean cost was significantly lower among patients treated using the swapping strategy than among the TNFi cyclers, both for the total cost of care for RA and for the total cost of the targeted DMARDs in the first year after the change in therapy. The authors concluded that switching to a drug with a different mechanism of action is associated with higher treatment persistence and lower health care costs than TNFi cycling.
What about biosimilars?
Biosimilars are copies of already licensed biologics that are very similar to the biologics, but are made by different sponsors using independently derived cell lines and separately developed manufacturing processes.83 Regarding biosimilar use, EULAR26 states that biosimilar bDMARDs approved by the European Medicines Agency or US Food and Drug Administration have similar efficacy and safety as the originator bDMARDs, and recommends them as preferred agents if they are indeed appreciably cheaper than originator or other bDMARDs.
What are the novel treatment targets in RA?
New therapeutics for RA continue to be developed. One of the new agents is peficitinib (ASP015K), an oral, once-daily Janus kinase (Jak) inhibitor targeting Jak-1, Jak-2, and tyrosine kinase-2, with moderate selectivity for Jak-3. In a phase 2b trial, 100-mg and 150-mg doses of peficitinib achieved a statistically significant ACR20 response (48.3% and 56.3%) compared to placebo (29.4%) at 12 weeks.84
Given the benefit of targeting TNF-α and IL-17 in RA, a novel molecule (ABT-122) that targets both human TNF and IL-17 has been developed. Two phase 1 studies85 showed that dual neutralization of TNF and IL-17 with ABT-122 has characteristics acceptable for further exploration of therapeutic potential of this agent in TNF- and IL-17A–driven immune-mediated inflammatory diseases. Another novel drug is mavrilimumab, a human monoclonal antibody that targets granulocyte–macrophage colony-stimulating factor receptor α. A recent studyshowed that long-term treatment with mavrilimumab maintained response and was well-tolerated, with no increased incidence of treatment-emergent adverse events.86
Namilumab (AMG203) is an immunoglobulin G1 monoclonal antibody that binds with high affinity to the GM-CSF ligand. In a phase 1b, randomized, double-blind study (PRIORA)87 to assess namilumab in treating active, mild-to-moderate RA, significant improvement was seen in the DAS28-CRP score with namilumab (150 and 300 mg groups combined) compared with placebo at day 43 (P = 0.0117) and also 8 weeks after last dosing at day 99 (P = 0.0154). Adverse events were similar across different doses of namilumab and placebo, and included nasopharyngitis and exacerbation/worsening of RA. Another drug showing promise in RA is fosdagrocorat (PF-04171327), a potential dissociated agonist of the glucocorticoid receptor. A multicenter, double-blind, parallel-group, active- and placebo-controlled phase 2 study randomly assigned 86 patients to receive fosdagrocorat 10 mg, fosdagrocorat 25 mg, prednisone 5 mg, or placebo, all with stable background methotrexate therapy.88 Both fosdagrocorat doses demonstrated efficacy in improving signs and symptoms in RA patients, with manageable adverse events.
Case Conclusion
There are several available treatment options for the case patient. Based on the PREMIER trial, solely increasing the dose of adalimumab is unlikely to provide a therapeutic benefit. Adding low-dose methotrexate (possibly via a parenteral route because of patient-reported gastrointestinal discomfort) might provide some synergistic and therapeutic effect. However, because of primary failure with TNFi therapy, she may benefit from the initiation of a biologic with a different mechanism of action (ie, swapping strategy). Therapeutic options include tocilizumab, abatacept, rituximab, and the Jak inhibitors (tofacitinib and baricitinib).
Summary
The optimal treatment of RA aims at achieving, and then maintaining, remission or a low disease activity. The choice of best treatment must be individualized to the patient, keeping in mind other factors, including comorbidities like fibromyalgia, history of diverticulitis (prior to use of tocilizumab), history of chronic obstructive pulmonary disease (prior to the use of abatacept), malignancy, and the presence of risk factors for infections (age, diabetes, chronic bronchitis). In a patient with inadequate response to initial biologic therapy, several options exist for the rheumatologist. Current evidence supports TNFi dose escalation for only infliximab; optimization of concurrent csDMARD or switching to a different csDMARD are other options. Cycling and swapping are other alternate approaches supported by many observational studies. While no head-to-head trials exist comparing the 2 strategies, data suggest superiority of the swapping strategy over the cycling approach. With the continuing development of novel therapeutics in RA, physicians have a growing list of treatment options to help their patients achieve disease remission.
Corresponding author: Namrata Singh, MD, 200 Hawkins Drive, Iowa City, IA 52242.
Financial disclosures: None.
1. Keystone ED, Kavanaugh KA. What to do with TNF failures. Expert Opin Drug Saf. 2005;4:149-155.
2. Kalden JR, Schulze-Koops H. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol. 2017;13:707-718.
3. Weinblatt ME, Kremer JM, Bankhurst AD, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999;340:253-259.
4. Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50(5):1400-1411.
5. Lipsky PE, van der Heijde DM, St Clair EW, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343:1594-1602.
6. Finckh A, Simard JF, Gabay C, et al. Evidence for differential acquired drug resistance to anti-tumour necrosis factor agents in rheumatoid arthritis. Ann Rheum Dis. 2006;65:746-752.
7. Souto A, Maneiro JR, Gomez-Reino JJ. Rate of discontinuation and drug survival of biologic therapies in rheumatoid arthritis: a systematic review and meta-analysis of drug registries and health care databases. Rheumatology. 2016;55:523-534.
8. Hetland ML, Christensen IJ, Tarp U, et al. Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum. 2010;62:22-32.
9. Gabay C, Riek M, Scherer A, et al. Effectiveness of biologic DMARDs in monotherapy versus in combination with synthetic DMARDs in rheumatoid arthritis: data from the Swiss Clinical Quality Management Registry. Rheumatology. 2015;54(9):1664-1672.
10. Ebina K, Hashimoto M, Yamamoto W, et al. Drug retention and discontinuation reasons between seven biologics in patients with rheumatoid arthritis-The ANSWER cohort study. PloS One. 2018;13:e0194130.
11. Wijbrandts CA, Tak PP. Prediction of response to targeted treatment in rheumatoid arthritis. Mayo Clin Proc. 2017;92:1129-1143.
12. Ulfgren AK, Andersson U, Engstrom M, et al. Systemic anti-tumor necrosis factor alpha therapy in rheumatoid arthritis down-regulates synovial tumor necrosis factor alpha synthesis. Arthritis Rheum. 2000;43:2391-2396.
13. Choi IY, Gerlag DM, Herenius MJ, et al. MRP8/14 serum levels as a strong predictor of response to biological treatments in patients with rheumatoid arthritis. Ann Rheum Dis. 2015;74:499-505.
14. Nguyen MVC, Baillet A, Romand X, et al. Prealbumin, platelet factor 4 and S100A12 combination at baseline predicts good response to TNF alpha inhibitors in rheumatoid arthritis. Joint Bone Spine. 2019;86:195-201.
15. Anderson JK, Zimmerman L, Caplan L, Michaud K. Measures of rheumatoid arthritis disease activity: Patient (PtGA) and Provider (PrGA) Global Assessment of Disease Activity, Disease Activity Score (DAS) and Disease Activity Score with 28-Joint Counts (DAS28), Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI), Patient Activity Score (PAS) and Patient Activity Score-II (PASII), Routine Assessment of Patient Index Data (RAPID), Rheumatoid Arthritis Disease Activity Index (RADAI) and Rheumatoid Arthritis Disease Activity Index-5 (RADAI-5), Chronic Arthritis Systemic Index (CASI), Patient-Based Disease Activity Score With ESR (PDAS1) and Patient-Based Disease Activity Score without ESR (PDAS2), and Mean Overall Index for Rheumatoid Arthritis (MOI-RA). Arthritis Care Res. 2011;63(suppl 11):S14-S36.
16. Katz L, Gisbert JP, Manoogian B, et al. Doubling the infliximab dose versus halving the infusion intervals in Crohn’s disease patients with loss of response. Inflamm Bowel Dis. 2012;18:2026-2033.
17. Durez P, Van den Bosch F, Corluy L, et al. A dose adjustment in patients with rheumatoid arthritis not optimally responding to a standard dose of infliximab of 3 mg/kg every 8 weeks can be effective: a Belgian prospective study. Rheumatology. 2005;44:465-468.
18. Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26-37.
19. St Clair EW, Wagner CL, Fasanmade AA, et al. The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46:1451-1459.
20. Rahman MU, Strusberg I, Geusens P, et al. Double-blinded infliximab dose escalation in patients with rheumatoid arthritis. Ann Rheum Dis. 2007;66:1233-1238.
21. Weinblatt ME, Schiff MH, Ruderman EM, et al. Efficacy and safety of etanercept 50 mg twice a week in patients with rheumatoid arthritis who had a suboptimal response to etanercept 50 mg once a week: results of a multicenter, randomized, double-blind, active drug-controlled study. Arthritis Rheum. 2008;58:1921-1930.
22. Curtis JR, Chen L, Luijtens K, et al. Dose escalation of certolizumab pegol from 200 mg to 400 mg every other week provides no additional efficacy in rheumatoid arthritis: an analysis of individual patient-level data. Arthritis Rheum. 2011;63:2203-2208.
23. Okazaki M, Kobayashi H, Ishii Y, et al. Real-world treatment patterns for golimumab and concomitant medications in Japanese rheumatoid arthritis patients. Rheumatol Ther. 2018;5:185-201.
24. Pappas DA, John A, Curtis JR, et al. Dosing of intravenous tocilizumab in a real-world setting of rheumatoid arthritis: analyses from the Corrona Registry. Rheumatol Ther. 2016;3:103-115.
25. Kivitz A, Olech E, Borofsky MA, et al. Two-year efficacy and safety of subcutaneous tocilizumab in combination with disease-modifying antirheumatic drugs including escalation to weekly dosing in rheumatoid arthritis. J Rheumatol. 2018;45:456-464.
26. Smolen JS, Landewe R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76:960-977.
27. Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. 2016;68:1-25.
28. Tabrizi MA, Tseng CM, Roskos LK. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today. 2006;11:81-88.
29. Keizer RJ, Huitema AD, Schellens JH, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:493-507.
30. Navarro Coy NC, Brown S, Bosworth A, et al. The ‘Switch’ study protocol: a randomised-controlled trial of switching to an alternative tumour-necrosis factor (TNF)-inhibitor drug or abatacept or rituximab in patients with rheumatoid arthritis who have failed an initial TNF-inhibitor drug. BMC Musculoskelet Disord. 2014;15:452.
31. Kamal KM, Madhavan SS, Hornsby JA, et al. Use of tumor necrosis factor inhibitors in rheumatoid arthritis: a national survey of practicing United States rheumatologists. Joint Bone Spine. 2006;73:718-724.
32. Gomez-Reino JJ, Carmona L, BIOBADASER Group. Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients over a four-year period. Arthritis Res Ther. 2006;8:R29.
33. Caporali R, Sarzi-Puttini P, Atzeni F, et al. Switching TNF-alpha antagonists in rheumatoid arthritis: The experience of the LORHEN registry. Autoimmun Rev. 2010;9:465-469.
34. Iannone F, Trotta F, Monteccuco C, et al. Etanercept maintains the clinical benefit achieved by infliximab in patients with rheumatoid arthritis who discontinued infliximab because of side effects. Ann Rheum Dis. 2007;66:249-252.
35. Virkki LM, Valleala H, Takakubo Y, et al. Outcomes of switching anti-TNF drugs in rheumatoid arthritis—a study based on observational data from the Finnish Register of Biological Treatment (ROB-FIN). Clin Rheumatol. 2011;30:1447-1454.
36. Furst DE, Gaylis N, Bray V, et al. Open-label, pilot protocol of patients with rheumatoid arthritis who switch to infliximab after an incomplete response to etanercept: the opposite study. Ann Rheum Dis. 2007;66:893-899.
37. Smolen JS, Kay J, Doyle MK, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet. 2009;374:210-221.
38. Smolen JS, Kay J, Doyle M, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumor necrosis factor α inhibitors: findings with up to five years of treatment in the multicenter, randomized, double-blind, placebo-controlled, phase 3 GO-AFTER study. Arthritis Res Ther. 2015;17:14.
39. Smolen JS, Burmester G-R, Combe B, et al. Head-to-head comparison of certolizumab pegol versus adalimumab in rheumatoid arthritis: 2-year efficacy and safety results from the randomised EXXELERATE study. Lancet. 2016;388:2763-2774.
40. Chatzidionysiou K, Askling J, Eriksson J, et al. Effectiveness of TNF inhibitor switch in RA: results from the national Swedish register. Ann Rheum Dis. 2015;74:890.
41. Smolen JS, Kay J, Doyle M, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumor necrosis factor alpha inhibitors: findings with up to five years of treatment in the multicenter, randomized, double-blind, placebo-controlled, phase 3 GO-AFTER study. Arthritis Res Ther. 2015;17:14.
42. Lequerré T, Farran É, Ménard J-F, et al. Switching from an anti-TNF monoclonal antibody to soluble TNF-receptor yields better results than vice versa: An observational retrospective study of 72 rheumatoid arthritis switchers. Joint Bone Spine. 2015;82:330-337.
43. Favalli EG, Biggioggero M, Meroni PL. Methotrexate for the treatment of rheumatoid arthritis in the biologic era: Still an “anchor” drug? Autoimmun Rev. 2014;13:1102-1108.
44. Kalden JR, Schulze-Koops H. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol. 2017;13:707-718.
45. Klareskog L, van der Heijde D, de Jager JP, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363:675-681.
46. Emery P, Fleischmann RM, Strusberg I, et al. Efficacy and safety of subcutaneous golimumab in methotrexate-naive patients with rheumatoid arthritis: five-year results of a randomized clinical trial. Arthritis Care Res. 2016;68:744-752.
47. Emery P, Fleischmann RM, Moreland LW, et al. Golimumab, a human anti-tumor necrosis factor alpha monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: twenty-four-week results of a phase III, multicenter, randomized, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum. 2009;60:2272-2283.
48. Emery P, Burmester GR, Bykerk VP, et al. Evaluating drug-free remission with abatacept in early rheumatoid arthritis: results from the phase 3b, multicentre, randomised, active-controlled AVERT study of 24 months, with a 12-month, double-blind treatment period. Ann Rheum Dis. 2015;74:19-26.
49. Westhovens R, Robles M, Ximenes AC, et al. Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Ann Rheum Dis. 2009;68:1870-1877.
50. Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793-2806.
51. Burmester GR, Rigby WF, van Vollenhoven RF, et al. Tocilizumab in early progressive rheumatoid arthritis: FUNCTION, a randomised controlled trial. Ann Rheum Dis. 2016;75:1081-1091.
52. Bijlsma JWJ, Welsing PMJ, Woodworth TG, et al. Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (U-Act-Early): a multicentre, randomised, double-blind, double-dummy, strategy trial. Lancet. 2016;388:343-355.
53. Cohen JD, Zaltni S, Kaiser MJ, et al. Secondary addition of methotrexate to partial responders to etanercept alone is effective in severe rheumatoid arthritis. Ann Rheum Dis. 2004;63:209-210.
54. Hamilton RA, Kremer JM. Why intramuscular methotrexate may be more efficacious than oral dosing in patients with rheumatoid arthritis. Br J Rheumatol. 1997;36:86-90.
55. Hoekstra M, Haagsma C, Neef C, et al. Bioavailability of higher dose methotrexate comparing oral and subcutaneous administration in patients with rheumatoid arthritis. J Rheumatol. 2004;31:645-648.
56. Herman RA, Veng-Pedersen P, Hoffman J, et al. Pharmacokinetics of low-dose methotrexate in rheumatoid arthritis patients. J Pharm Sci. 1989;78:165-171.
57. Schiff MH, Jaffe JS, Freundlich B. Head-to-head, randomised, crossover study of oral versus subcutaneous methotrexate in patients with rheumatoid arthritis: drug-exposure limitations of oral methotrexate at doses ± 15 mg may be overcome with subcutaneous administration. Ann Rheum Dis. 2014;73:1549-1551.
58. Hazlewood GS, Thorne JC, Pope JE, et al. The comparative effectiveness of oral versus subcutaneous methotrexate for the treatment of early rheumatoid arthritis. Ann Rheum Dis. 2016;75:1003-1008.
59. O’Dell JR, Petersen K, Leff R, et al. Etanercept in combination with sulfasalazine, hydroxychloroquine, or gold in the treatment of rheumatoid arthritis. J Rheumatol. 2006;33:213-218.
60. Finckh A, Dehler S, Gabay C. The effectiveness of leflunomide as a co-therapy of tumour necrosis factor inhibitors in rheumatoid arthritis: a population-based study. Ann Rheum Dis. 2009;68:33-39.
61. De Stefano R, Frati E, Nargi F, et al. Comparison of combination therapies in the treatment of rheumatoid arthritis: leflunomide-anti-TNF-alpha versus methotrexate-anti-TNF-alpha. Clin Rheumatol. 2010;29:517-524.
62. Combe B, Codreanu C, Fiocco U, et al. Etanercept and sulfasalazine, alone and combined, in patients with active rheumatoid arthritis despite receiving sulfasalazine: a double-blind comparison. Ann Rheum Dis. 2006;65:1357-1362.
63. Strangfeld A, Hierse F, Kekow J, et al. Comparative effectiveness of tumour necrosis factor α inhibitors in combination with either methotrexate or leflunomide. Ann Rheum Dis. 2009;68:1856.
64. Emery P, Keystone E, Tony HP, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis. 2008;67:1516.
65. Genovese MC, Becker J-C, Schiff M, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor α inhibition. N Engl J Med. 2005;353:1114-1123.
66. Emery P, Gottenberg JE, Rubbert-Roth A, et al. Rituximab versus an alternative TNF inhibitor in patients with rheumatoid arthritis who failed to respond to a single previous TNF inhibitor: SWITCH-RA, a global, observational, comparative effectiveness study. Ann Rheum Dis. 2015;74:979-984.
67. Keystone E, Emery P, Peterfy CG, et al. Rituximab inhibits structural joint damage in patients with rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitor therapies. Ann Rheum Dis. 2009;68:216.
68. Burmester GR, Blanco R, Charles-Schoeman C, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013;381:451-460.
69. Genovese MC, Kremer J, Zamani O, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med. 2016;374:1243-1252.
70. Favalli EG, Biggioggero M, Marchesoni A, Meroni PL. Survival on treatment with second-line biologic therapy: a cohort study comparing cycling and swap strategies. Rheumatology. 2014;53:1664-1668.
71. Harrold LR, Reed GW, Solomon DH, et al. Comparative effectiveness of abatacept versus tocilizumab in rheumatoid arthritis patients with prior TNFi exposure in the US Corrona registry. Arthritis Res Ther. 2016;18:280.
72. Gottenberg J, Brocq O, Perdriger A, et al. Non–TNF-targeted biologic vs a second anti-TNF drug to treat rheumatoid arthritis in patients with insufficient response to a first anti-TNF drug: A randomized clinical trial. JAMA. 2016;316:1172-1180.
73. Pascart T, Philippe P, Drumez E, et al. Comparative efficacy of tocilizumab, abatacept and rituximab after non-TNF inhibitor failure: results from a multicentre study. Int J Rheum Dis. 2016;19:1093-1102.
74. Akiyama M, Kaneko Y, Kondo H, Takeuchi T. Comparison of the clinical effectiveness of tumour necrosis factor inhibitors and abatacept after insufficient response to tocilizumab in patients with rheumatoid arthritis. Clin Rheumatol. 2016;35:2829-2834.
75. Schoels M, Aletaha D, Smolen JS, Wong JB. Comparative effectiveness and safety of biological treatment options after tumour necrosis factor α inhibitor failure in rheumatoid arthritis: systematic review and indirect pairwise meta-analysis. Ann Rheum Dis. 2012;71:1303.
76. Soliman MM, Hyrich KL, Lunt M, et al. Rituximab or a second anti-tumor necrosis factor therapy for rheumatoid arthritis patients who have failed their first anti-tumor necrosis factor therapy? Comparative analysis from the British Society for Rheumatology Biologics Register. Arthritis Care Res. 2012;64:1108-1115.
77. Chatzidionysiou K, Vollenhoven RF. Rituximab versus anti-TNF in patients who previously failed one TNF inhibitor in an observational cohort. Scand J Rheumatol. 2013;42:190-195.
78. Johnston SS, Turpcu A, Shi N, et al. Risk of infections in rheumatoid arthritis patients switching from anti-TNF agents to rituximab, abatacept, or another anti-TNF agent, a retrospective administrative claims analysis. Semim Arthritis Rheum. 2013;43:39-47.
79. Curtis JR, Xie F, Chen L, et al. The comparative risk of serious infections among rheumatoid arthritis patients starting or switching biological agents. Ann Rheum Dis. 2011;70:1401.
80. Desai RJ, Solomon DH, Jin Y, et al. Temporal trends in use of biologic DMARDs for rheumatoid arthritis in the United States: a cohort study of publicly and privately insured patients. J Manag Care Spec Pharm. 2017;23:809-814.
81. Jin Y, Desai RJ, Liu J, et al. Factors associated with initial or subsequent choice of biologic disease-modifying antirheumatic drugs for treatment of rheumatoid arthritis. Arthritis Res Ther. 2017;19:159.
82. Bonafede MMK, McMorrow D, Proudfoot C, et al. Treatment persistence and healthcare costs among patients with rheumatoid arthritis after a change in targeted therapy. Am Health Drug Benefits. 2018;11:192-202.
83. US Food and Drug Administration. Biosimilars are safe, effective treatment options. www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/therapeuticbiologicapplications/biosimilars/. Accessed November 9, 2018.
84. Genovese MC, Greenwald M, Codding C, et al. Peficitinib, a JAK inhibitor, in combination with limited conventional synthetic disease-modifying antirheumatic drugs in the treatment of moderate-to-severe rheumatoid arthritis. Arthritis Rheumatol. 2017;69:932-942.
85. Fleischmann RM, Wagner F, Kivitz AJ, et al. Safety, tolerability, and pharmacodynamics of ABT-122, a tumor necrosis factor- and interleukin-17-targeted dual variable domain immunoglobulin, in patients with rheumatoid arthritis. Arthritis Rheumatol. 2017;69:2283-2291.
86. Burmester GR, McInnes IB, Kremer JM, et al. Mavrilimumab, a fully human granulocyte-macrophage colony-stimulating factor receptor alpha monoclonal antibody: long-term safety and efficacy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2018;70:679-689.
87. Huizinga TW, Batalov A, Stoilov R, et al. Phase 1b randomized, double-blind study of namilumab, an anti-granulocyte macrophage colony-stimulating factor monoclonal antibody, in mild-to-moderate rheumatoid arthritis. Arthritis Res Ther. 2017;19:53.
88. Stock T, Fleishaker D, Wang X, et al. Improved disease activity with fosdagrocorat (PF-04171327), a partial agonist of the glucocorticoid receptor, in patients with rheumatoid arthritis: a Phase 2 randomized study. Int J Rheum Dis. 2017;20:960-970.
89. Orencia [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2013.
90. Humira[package insert]. North Chicago, IL: AbbVie; 2012.
91. Kineret [package insert]. Stockholm, Sweden: Sobi; 2012.
92. Olumiant [package insert]. Indianapolis, IN: Lilly USA, LLC; 2018.
93. Cimzia [package insert]. Smyrna, GA: UCB, Inc; 2008.
94. Enbrel [package insert]. Thousand Oaks, CA: Immunex Corporation; 1998.
95. Simponi [package insert]. Horsham, PA: Janssen Biotech, Inc; 2009.
96. Remicade [package insert]. Horsham, PA: Janssen Biotech, Inc; 1998.
97. Rituxan [package insert]. South San Francisco, CA: Genetech, Inc; 1997.
98. Kevzara [package insert]. Bridgewater, NJ: Sanofi-Aventis US LLC; 2018.
99. Actemra [package insert]. South San Francisco, CA: Genentech, Inc; 2013.
100. Xeljanz [package insert]. New York, NY: Pfizer Inc; 2016.
1. Keystone ED, Kavanaugh KA. What to do with TNF failures. Expert Opin Drug Saf. 2005;4:149-155.
2. Kalden JR, Schulze-Koops H. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol. 2017;13:707-718.
3. Weinblatt ME, Kremer JM, Bankhurst AD, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999;340:253-259.
4. Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50(5):1400-1411.
5. Lipsky PE, van der Heijde DM, St Clair EW, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343:1594-1602.
6. Finckh A, Simard JF, Gabay C, et al. Evidence for differential acquired drug resistance to anti-tumour necrosis factor agents in rheumatoid arthritis. Ann Rheum Dis. 2006;65:746-752.
7. Souto A, Maneiro JR, Gomez-Reino JJ. Rate of discontinuation and drug survival of biologic therapies in rheumatoid arthritis: a systematic review and meta-analysis of drug registries and health care databases. Rheumatology. 2016;55:523-534.
8. Hetland ML, Christensen IJ, Tarp U, et al. Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum. 2010;62:22-32.
9. Gabay C, Riek M, Scherer A, et al. Effectiveness of biologic DMARDs in monotherapy versus in combination with synthetic DMARDs in rheumatoid arthritis: data from the Swiss Clinical Quality Management Registry. Rheumatology. 2015;54(9):1664-1672.
10. Ebina K, Hashimoto M, Yamamoto W, et al. Drug retention and discontinuation reasons between seven biologics in patients with rheumatoid arthritis-The ANSWER cohort study. PloS One. 2018;13:e0194130.
11. Wijbrandts CA, Tak PP. Prediction of response to targeted treatment in rheumatoid arthritis. Mayo Clin Proc. 2017;92:1129-1143.
12. Ulfgren AK, Andersson U, Engstrom M, et al. Systemic anti-tumor necrosis factor alpha therapy in rheumatoid arthritis down-regulates synovial tumor necrosis factor alpha synthesis. Arthritis Rheum. 2000;43:2391-2396.
13. Choi IY, Gerlag DM, Herenius MJ, et al. MRP8/14 serum levels as a strong predictor of response to biological treatments in patients with rheumatoid arthritis. Ann Rheum Dis. 2015;74:499-505.
14. Nguyen MVC, Baillet A, Romand X, et al. Prealbumin, platelet factor 4 and S100A12 combination at baseline predicts good response to TNF alpha inhibitors in rheumatoid arthritis. Joint Bone Spine. 2019;86:195-201.
15. Anderson JK, Zimmerman L, Caplan L, Michaud K. Measures of rheumatoid arthritis disease activity: Patient (PtGA) and Provider (PrGA) Global Assessment of Disease Activity, Disease Activity Score (DAS) and Disease Activity Score with 28-Joint Counts (DAS28), Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI), Patient Activity Score (PAS) and Patient Activity Score-II (PASII), Routine Assessment of Patient Index Data (RAPID), Rheumatoid Arthritis Disease Activity Index (RADAI) and Rheumatoid Arthritis Disease Activity Index-5 (RADAI-5), Chronic Arthritis Systemic Index (CASI), Patient-Based Disease Activity Score With ESR (PDAS1) and Patient-Based Disease Activity Score without ESR (PDAS2), and Mean Overall Index for Rheumatoid Arthritis (MOI-RA). Arthritis Care Res. 2011;63(suppl 11):S14-S36.
16. Katz L, Gisbert JP, Manoogian B, et al. Doubling the infliximab dose versus halving the infusion intervals in Crohn’s disease patients with loss of response. Inflamm Bowel Dis. 2012;18:2026-2033.
17. Durez P, Van den Bosch F, Corluy L, et al. A dose adjustment in patients with rheumatoid arthritis not optimally responding to a standard dose of infliximab of 3 mg/kg every 8 weeks can be effective: a Belgian prospective study. Rheumatology. 2005;44:465-468.
18. Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26-37.
19. St Clair EW, Wagner CL, Fasanmade AA, et al. The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46:1451-1459.
20. Rahman MU, Strusberg I, Geusens P, et al. Double-blinded infliximab dose escalation in patients with rheumatoid arthritis. Ann Rheum Dis. 2007;66:1233-1238.
21. Weinblatt ME, Schiff MH, Ruderman EM, et al. Efficacy and safety of etanercept 50 mg twice a week in patients with rheumatoid arthritis who had a suboptimal response to etanercept 50 mg once a week: results of a multicenter, randomized, double-blind, active drug-controlled study. Arthritis Rheum. 2008;58:1921-1930.
22. Curtis JR, Chen L, Luijtens K, et al. Dose escalation of certolizumab pegol from 200 mg to 400 mg every other week provides no additional efficacy in rheumatoid arthritis: an analysis of individual patient-level data. Arthritis Rheum. 2011;63:2203-2208.
23. Okazaki M, Kobayashi H, Ishii Y, et al. Real-world treatment patterns for golimumab and concomitant medications in Japanese rheumatoid arthritis patients. Rheumatol Ther. 2018;5:185-201.
24. Pappas DA, John A, Curtis JR, et al. Dosing of intravenous tocilizumab in a real-world setting of rheumatoid arthritis: analyses from the Corrona Registry. Rheumatol Ther. 2016;3:103-115.
25. Kivitz A, Olech E, Borofsky MA, et al. Two-year efficacy and safety of subcutaneous tocilizumab in combination with disease-modifying antirheumatic drugs including escalation to weekly dosing in rheumatoid arthritis. J Rheumatol. 2018;45:456-464.
26. Smolen JS, Landewe R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76:960-977.
27. Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. 2016;68:1-25.
28. Tabrizi MA, Tseng CM, Roskos LK. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today. 2006;11:81-88.
29. Keizer RJ, Huitema AD, Schellens JH, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:493-507.
30. Navarro Coy NC, Brown S, Bosworth A, et al. The ‘Switch’ study protocol: a randomised-controlled trial of switching to an alternative tumour-necrosis factor (TNF)-inhibitor drug or abatacept or rituximab in patients with rheumatoid arthritis who have failed an initial TNF-inhibitor drug. BMC Musculoskelet Disord. 2014;15:452.
31. Kamal KM, Madhavan SS, Hornsby JA, et al. Use of tumor necrosis factor inhibitors in rheumatoid arthritis: a national survey of practicing United States rheumatologists. Joint Bone Spine. 2006;73:718-724.
32. Gomez-Reino JJ, Carmona L, BIOBADASER Group. Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients over a four-year period. Arthritis Res Ther. 2006;8:R29.
33. Caporali R, Sarzi-Puttini P, Atzeni F, et al. Switching TNF-alpha antagonists in rheumatoid arthritis: The experience of the LORHEN registry. Autoimmun Rev. 2010;9:465-469.
34. Iannone F, Trotta F, Monteccuco C, et al. Etanercept maintains the clinical benefit achieved by infliximab in patients with rheumatoid arthritis who discontinued infliximab because of side effects. Ann Rheum Dis. 2007;66:249-252.
35. Virkki LM, Valleala H, Takakubo Y, et al. Outcomes of switching anti-TNF drugs in rheumatoid arthritis—a study based on observational data from the Finnish Register of Biological Treatment (ROB-FIN). Clin Rheumatol. 2011;30:1447-1454.
36. Furst DE, Gaylis N, Bray V, et al. Open-label, pilot protocol of patients with rheumatoid arthritis who switch to infliximab after an incomplete response to etanercept: the opposite study. Ann Rheum Dis. 2007;66:893-899.
37. Smolen JS, Kay J, Doyle MK, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet. 2009;374:210-221.
38. Smolen JS, Kay J, Doyle M, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumor necrosis factor α inhibitors: findings with up to five years of treatment in the multicenter, randomized, double-blind, placebo-controlled, phase 3 GO-AFTER study. Arthritis Res Ther. 2015;17:14.
39. Smolen JS, Burmester G-R, Combe B, et al. Head-to-head comparison of certolizumab pegol versus adalimumab in rheumatoid arthritis: 2-year efficacy and safety results from the randomised EXXELERATE study. Lancet. 2016;388:2763-2774.
40. Chatzidionysiou K, Askling J, Eriksson J, et al. Effectiveness of TNF inhibitor switch in RA: results from the national Swedish register. Ann Rheum Dis. 2015;74:890.
41. Smolen JS, Kay J, Doyle M, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumor necrosis factor alpha inhibitors: findings with up to five years of treatment in the multicenter, randomized, double-blind, placebo-controlled, phase 3 GO-AFTER study. Arthritis Res Ther. 2015;17:14.
42. Lequerré T, Farran É, Ménard J-F, et al. Switching from an anti-TNF monoclonal antibody to soluble TNF-receptor yields better results than vice versa: An observational retrospective study of 72 rheumatoid arthritis switchers. Joint Bone Spine. 2015;82:330-337.
43. Favalli EG, Biggioggero M, Meroni PL. Methotrexate for the treatment of rheumatoid arthritis in the biologic era: Still an “anchor” drug? Autoimmun Rev. 2014;13:1102-1108.
44. Kalden JR, Schulze-Koops H. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol. 2017;13:707-718.
45. Klareskog L, van der Heijde D, de Jager JP, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363:675-681.
46. Emery P, Fleischmann RM, Strusberg I, et al. Efficacy and safety of subcutaneous golimumab in methotrexate-naive patients with rheumatoid arthritis: five-year results of a randomized clinical trial. Arthritis Care Res. 2016;68:744-752.
47. Emery P, Fleischmann RM, Moreland LW, et al. Golimumab, a human anti-tumor necrosis factor alpha monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: twenty-four-week results of a phase III, multicenter, randomized, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum. 2009;60:2272-2283.
48. Emery P, Burmester GR, Bykerk VP, et al. Evaluating drug-free remission with abatacept in early rheumatoid arthritis: results from the phase 3b, multicentre, randomised, active-controlled AVERT study of 24 months, with a 12-month, double-blind treatment period. Ann Rheum Dis. 2015;74:19-26.
49. Westhovens R, Robles M, Ximenes AC, et al. Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Ann Rheum Dis. 2009;68:1870-1877.
50. Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793-2806.
51. Burmester GR, Rigby WF, van Vollenhoven RF, et al. Tocilizumab in early progressive rheumatoid arthritis: FUNCTION, a randomised controlled trial. Ann Rheum Dis. 2016;75:1081-1091.
52. Bijlsma JWJ, Welsing PMJ, Woodworth TG, et al. Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (U-Act-Early): a multicentre, randomised, double-blind, double-dummy, strategy trial. Lancet. 2016;388:343-355.
53. Cohen JD, Zaltni S, Kaiser MJ, et al. Secondary addition of methotrexate to partial responders to etanercept alone is effective in severe rheumatoid arthritis. Ann Rheum Dis. 2004;63:209-210.
54. Hamilton RA, Kremer JM. Why intramuscular methotrexate may be more efficacious than oral dosing in patients with rheumatoid arthritis. Br J Rheumatol. 1997;36:86-90.
55. Hoekstra M, Haagsma C, Neef C, et al. Bioavailability of higher dose methotrexate comparing oral and subcutaneous administration in patients with rheumatoid arthritis. J Rheumatol. 2004;31:645-648.
56. Herman RA, Veng-Pedersen P, Hoffman J, et al. Pharmacokinetics of low-dose methotrexate in rheumatoid arthritis patients. J Pharm Sci. 1989;78:165-171.
57. Schiff MH, Jaffe JS, Freundlich B. Head-to-head, randomised, crossover study of oral versus subcutaneous methotrexate in patients with rheumatoid arthritis: drug-exposure limitations of oral methotrexate at doses ± 15 mg may be overcome with subcutaneous administration. Ann Rheum Dis. 2014;73:1549-1551.
58. Hazlewood GS, Thorne JC, Pope JE, et al. The comparative effectiveness of oral versus subcutaneous methotrexate for the treatment of early rheumatoid arthritis. Ann Rheum Dis. 2016;75:1003-1008.
59. O’Dell JR, Petersen K, Leff R, et al. Etanercept in combination with sulfasalazine, hydroxychloroquine, or gold in the treatment of rheumatoid arthritis. J Rheumatol. 2006;33:213-218.
60. Finckh A, Dehler S, Gabay C. The effectiveness of leflunomide as a co-therapy of tumour necrosis factor inhibitors in rheumatoid arthritis: a population-based study. Ann Rheum Dis. 2009;68:33-39.
61. De Stefano R, Frati E, Nargi F, et al. Comparison of combination therapies in the treatment of rheumatoid arthritis: leflunomide-anti-TNF-alpha versus methotrexate-anti-TNF-alpha. Clin Rheumatol. 2010;29:517-524.
62. Combe B, Codreanu C, Fiocco U, et al. Etanercept and sulfasalazine, alone and combined, in patients with active rheumatoid arthritis despite receiving sulfasalazine: a double-blind comparison. Ann Rheum Dis. 2006;65:1357-1362.
63. Strangfeld A, Hierse F, Kekow J, et al. Comparative effectiveness of tumour necrosis factor α inhibitors in combination with either methotrexate or leflunomide. Ann Rheum Dis. 2009;68:1856.
64. Emery P, Keystone E, Tony HP, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis. 2008;67:1516.
65. Genovese MC, Becker J-C, Schiff M, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor α inhibition. N Engl J Med. 2005;353:1114-1123.
66. Emery P, Gottenberg JE, Rubbert-Roth A, et al. Rituximab versus an alternative TNF inhibitor in patients with rheumatoid arthritis who failed to respond to a single previous TNF inhibitor: SWITCH-RA, a global, observational, comparative effectiveness study. Ann Rheum Dis. 2015;74:979-984.
67. Keystone E, Emery P, Peterfy CG, et al. Rituximab inhibits structural joint damage in patients with rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitor therapies. Ann Rheum Dis. 2009;68:216.
68. Burmester GR, Blanco R, Charles-Schoeman C, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013;381:451-460.
69. Genovese MC, Kremer J, Zamani O, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med. 2016;374:1243-1252.
70. Favalli EG, Biggioggero M, Marchesoni A, Meroni PL. Survival on treatment with second-line biologic therapy: a cohort study comparing cycling and swap strategies. Rheumatology. 2014;53:1664-1668.
71. Harrold LR, Reed GW, Solomon DH, et al. Comparative effectiveness of abatacept versus tocilizumab in rheumatoid arthritis patients with prior TNFi exposure in the US Corrona registry. Arthritis Res Ther. 2016;18:280.
72. Gottenberg J, Brocq O, Perdriger A, et al. Non–TNF-targeted biologic vs a second anti-TNF drug to treat rheumatoid arthritis in patients with insufficient response to a first anti-TNF drug: A randomized clinical trial. JAMA. 2016;316:1172-1180.
73. Pascart T, Philippe P, Drumez E, et al. Comparative efficacy of tocilizumab, abatacept and rituximab after non-TNF inhibitor failure: results from a multicentre study. Int J Rheum Dis. 2016;19:1093-1102.
74. Akiyama M, Kaneko Y, Kondo H, Takeuchi T. Comparison of the clinical effectiveness of tumour necrosis factor inhibitors and abatacept after insufficient response to tocilizumab in patients with rheumatoid arthritis. Clin Rheumatol. 2016;35:2829-2834.
75. Schoels M, Aletaha D, Smolen JS, Wong JB. Comparative effectiveness and safety of biological treatment options after tumour necrosis factor α inhibitor failure in rheumatoid arthritis: systematic review and indirect pairwise meta-analysis. Ann Rheum Dis. 2012;71:1303.
76. Soliman MM, Hyrich KL, Lunt M, et al. Rituximab or a second anti-tumor necrosis factor therapy for rheumatoid arthritis patients who have failed their first anti-tumor necrosis factor therapy? Comparative analysis from the British Society for Rheumatology Biologics Register. Arthritis Care Res. 2012;64:1108-1115.
77. Chatzidionysiou K, Vollenhoven RF. Rituximab versus anti-TNF in patients who previously failed one TNF inhibitor in an observational cohort. Scand J Rheumatol. 2013;42:190-195.
78. Johnston SS, Turpcu A, Shi N, et al. Risk of infections in rheumatoid arthritis patients switching from anti-TNF agents to rituximab, abatacept, or another anti-TNF agent, a retrospective administrative claims analysis. Semim Arthritis Rheum. 2013;43:39-47.
79. Curtis JR, Xie F, Chen L, et al. The comparative risk of serious infections among rheumatoid arthritis patients starting or switching biological agents. Ann Rheum Dis. 2011;70:1401.
80. Desai RJ, Solomon DH, Jin Y, et al. Temporal trends in use of biologic DMARDs for rheumatoid arthritis in the United States: a cohort study of publicly and privately insured patients. J Manag Care Spec Pharm. 2017;23:809-814.
81. Jin Y, Desai RJ, Liu J, et al. Factors associated with initial or subsequent choice of biologic disease-modifying antirheumatic drugs for treatment of rheumatoid arthritis. Arthritis Res Ther. 2017;19:159.
82. Bonafede MMK, McMorrow D, Proudfoot C, et al. Treatment persistence and healthcare costs among patients with rheumatoid arthritis after a change in targeted therapy. Am Health Drug Benefits. 2018;11:192-202.
83. US Food and Drug Administration. Biosimilars are safe, effective treatment options. www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/therapeuticbiologicapplications/biosimilars/. Accessed November 9, 2018.
84. Genovese MC, Greenwald M, Codding C, et al. Peficitinib, a JAK inhibitor, in combination with limited conventional synthetic disease-modifying antirheumatic drugs in the treatment of moderate-to-severe rheumatoid arthritis. Arthritis Rheumatol. 2017;69:932-942.
85. Fleischmann RM, Wagner F, Kivitz AJ, et al. Safety, tolerability, and pharmacodynamics of ABT-122, a tumor necrosis factor- and interleukin-17-targeted dual variable domain immunoglobulin, in patients with rheumatoid arthritis. Arthritis Rheumatol. 2017;69:2283-2291.
86. Burmester GR, McInnes IB, Kremer JM, et al. Mavrilimumab, a fully human granulocyte-macrophage colony-stimulating factor receptor alpha monoclonal antibody: long-term safety and efficacy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2018;70:679-689.
87. Huizinga TW, Batalov A, Stoilov R, et al. Phase 1b randomized, double-blind study of namilumab, an anti-granulocyte macrophage colony-stimulating factor monoclonal antibody, in mild-to-moderate rheumatoid arthritis. Arthritis Res Ther. 2017;19:53.
88. Stock T, Fleishaker D, Wang X, et al. Improved disease activity with fosdagrocorat (PF-04171327), a partial agonist of the glucocorticoid receptor, in patients with rheumatoid arthritis: a Phase 2 randomized study. Int J Rheum Dis. 2017;20:960-970.
89. Orencia [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2013.
90. Humira[package insert]. North Chicago, IL: AbbVie; 2012.
91. Kineret [package insert]. Stockholm, Sweden: Sobi; 2012.
92. Olumiant [package insert]. Indianapolis, IN: Lilly USA, LLC; 2018.
93. Cimzia [package insert]. Smyrna, GA: UCB, Inc; 2008.
94. Enbrel [package insert]. Thousand Oaks, CA: Immunex Corporation; 1998.
95. Simponi [package insert]. Horsham, PA: Janssen Biotech, Inc; 2009.
96. Remicade [package insert]. Horsham, PA: Janssen Biotech, Inc; 1998.
97. Rituxan [package insert]. South San Francisco, CA: Genetech, Inc; 1997.
98. Kevzara [package insert]. Bridgewater, NJ: Sanofi-Aventis US LLC; 2018.
99. Actemra [package insert]. South San Francisco, CA: Genentech, Inc; 2013.
100. Xeljanz [package insert]. New York, NY: Pfizer Inc; 2016.