User login
Association of Nausea and Length of Stay with Carbohydrate Loading Prior to Total Joint Arthroplasty
From Stony Brook Medical Center, Stony Brook, NY (Dr. Blum), and NYU Winthrop Medical Center,
Abstract
- Background: Enhanced Recovery After Surgery (ERAS) is a multimodal, standardized approach to the surgical patient that incorporates evidenced-based interventions designed to achieve rapid recovery after surgery by minimizing the patient’s stress response. One aspect of ERAS, carbohydrate loading, has been shown in multiple randomized controlled trials to result in postoperative benefits in patients undergoing colorectal surgery, but there appears to be insufficient data to make definitive recommendations for or against carbohydrate loading in joint replacement patients.
- Objective: To evaluate postoperative nausea and length of stay (LOS) after a preoperative carbohydrate loading protocol was initiated for patients undergoing total joint replacement.
- Design: Retrospective chart review.
- Setting and participants: 100 patients who underwent either total knee or hip arthroplasty at Winthrop University Hospital, Mineola, NY, in the past 4 years and either had (n = 50) or had not received preoperative carbohydrate supplements (n = 50).
- Methods: Using the total joint database, the medical record was reviewed for the patient’s demographics, LOS, documentation of postoperative nausea, and number of doses of antiemetic medication given to the patient.
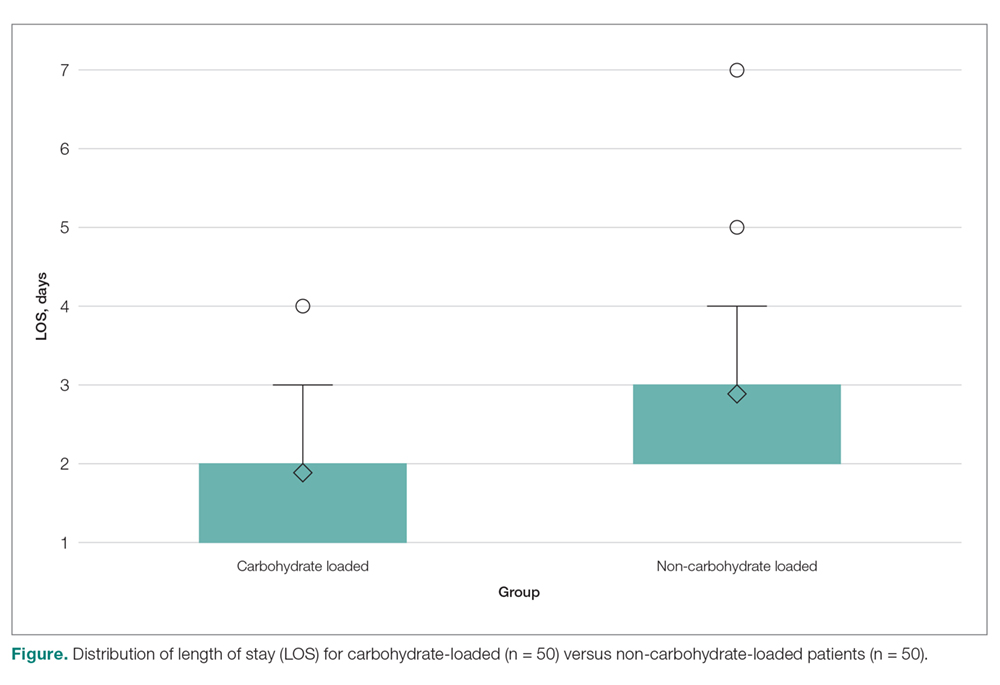
- Results: The mean LOS for the carbohydrate-loading group and non-carbohydrate group was 1.9 days and 2.6 days. respectively, a difference of 0.70 days (P < 0.0001). The carbohydrate-loaded group received a total of 13 doses of antiemetic medications and the non-carbohydrate group received 21 doses. The average number of antiemetic doses given to a patient postoperatively was 0.26 for the carbohydrate-loaded group and 0.42 for the non-carbohydrate-loaded group. The difference was 0.16 doses (P < 0.7815).
- Conclusion: The implementation of carbohydrate loading decreased LOS for joint replacement patients by approximately 1 day. Additionally, there was a trend towards decreased antiemetic use and fewer documented cases of postoperative nausea after carbohydrate loading.
Keywords: carbohydrate loading, ERAS, joint arthroplasty, length of stay, nausea.
Enhanced Recovery After Surgery (ERAS) is a multimodal, standardized approach to the surgical patient that incorporates evidenced-based interventions designed to achieve rapid recovery after surgery by minimizing the patient’s stress response.1-4 The ERAS protocols have been shown to reduce complications, decrease length of stay (LOS), and improve patient outcomes.3-7 The program was originally designed to facilitate recovery after colorectal operative procedures by maintaining preoperative organ function and reducing the postoperative stress response. This was done through a coordinated program of preoperative counseling, optimizing nutritional status, standardizing analgesic regimens, and early mobilization.3
The principles of an ERAS program with standardized pre- and postoperative protocols appear ideally suited for the total joint arthroplasty patient.1,3-5 Prior studies have demonstrated ERAS to be effective in facilitating decreased LOS, with no apparent increase in readmission rates or complications for both colorectal and joint arthroplasty patients.1-7 The protocols have also been shown to be cost-effective, with decreased incidence of postoperative complications, including thromboembolic disease and infections.3,4,6
An important tenet of ERAS protocols is optimizing the nutritional status of the patient prior to surgery.6 This includes avoidance of preoperative fasting in conjunction with carbohydrate loading. ERAS protocols instruct the patient to ingest a carbohydrate-rich beverage 2 hours prior to surgery. The concept of allowing a patient to eat prior to surgery is based on the preference for the patient to present for surgery in an anabolic rather than a catabolic state.2,3,11 Patients in an anabolic state undergo less postoperative protein and nitrogen losses, which appears to facilitate wound healing.2,6,11
There have been multiple randomized controlled trials demonstrating the postoperative benefits of carbohydrate loading prior to colorectal surgery.2,6
Another potential benefit of preoperative carbohydrate loading is a decrease in postoperative nausea.1,5,12-14 A decrease in nausea in theory would allow for earlier mobilization with physical therapy and potentially a shorter LOS. Hence, the goal of this study was to examine the impact of preoperative carbohydrate loading on postoperative nausea directly, as well as on LOS, at a single institution in the setting of an ERAS protocol.
Methods
We retrospectively reviewed the records of 100 patients who underwent total hip or total knee replacement between 2014 and 2018 at NYU Winthrop University Hospital, Mineola, NY. Fifty patients had received preoperative carbohydrate supplements and 50 patients had not. The remainder of the total joint protocol was identical for the 2 groups.
Protocol
All patients attended preoperative educational classes. For patients receiving carbohydrate loading, written and oral instructions were given for the patient to drink Ensure Clear followed by 8 ounces of water before going to bed the night before surgery. They were also instructed to drink the Ensure Pre-Surgery Drink 2 hours prior to their operative procedure. Patients with diabetes were instructed to drink the Ensure Glucerna Clear drink the night before surgery. No carbohydrate drink was given on the day of surgery until a finger-stick glucose level was performed upon arrival at the hospital. Spinal anesthesia was utilized in all patients, with adductor canal block supplementation for patients undergoing total knee replacement. Orders were written to have physical therapy evaluate the patients in the PACU to facilitate ambulation. Pre- and postoperative pain protocols were identical for the 2 groups.
Data Collection
A chart review was performed using the patients’ medical record numbers from the joint replacement database at our institution. Exemption was obtained for the project from our institution’s Institutional Review Board (IRB).
Analysis
Descriptive statistics (mean, standard deviation, and median for continuous variables; frequencies and percentages for categorical variables) were calculated separately by group. The 2 groups were compared using the chi-square test or Fisher’s exact test, as deemed appropriate, for categorical variables, the 2-sample t-test for age, and the Mann-Whitney test for LOS and number of antiemetic doses given. A result was considered statistically significant at the P < 0.05 level of significance. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
The carbohydrate-loading group (n = 50) and the non-carbohydrate-loading group (n = 50) were comparable for age, gender, type of arthroplasty, episodes of vomiting, diabetes, and nerve block (Table). 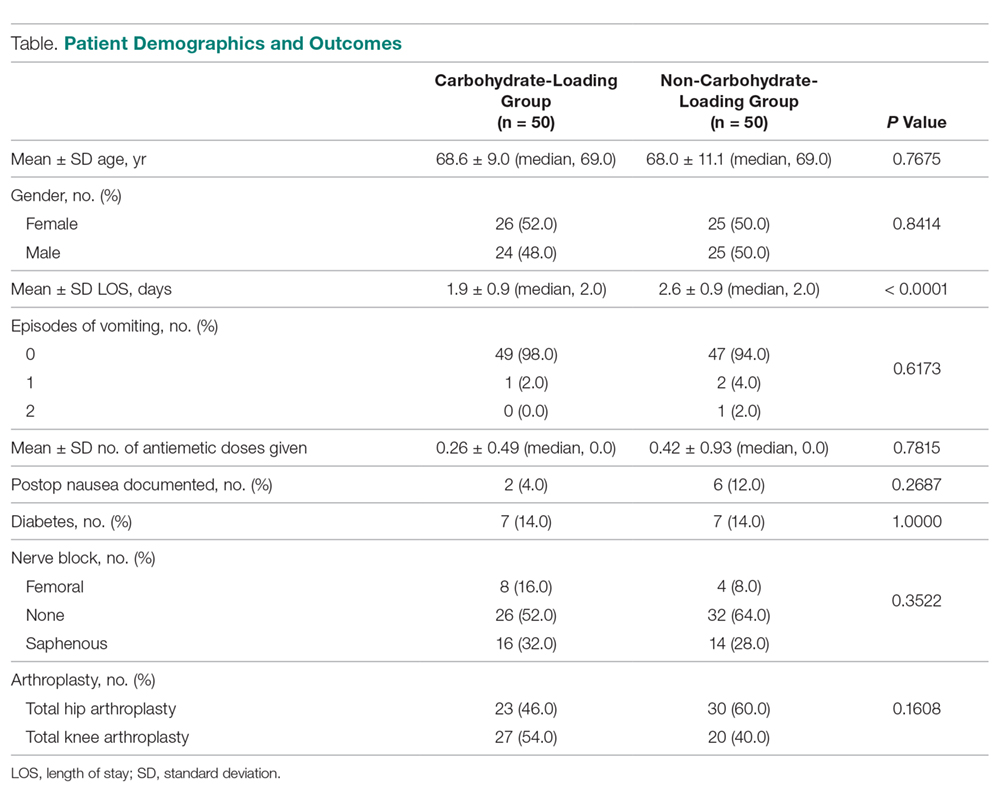
Discussion
In this study we explored whether carbohydrate loading prior to total joint replacement influenced postoperative nausea and LOS in a single institution. The 2 groups appeared similar in terms of demographics as well as the types of surgical procedures performed. After initiation of the carbohydrate-loading protocol, LOS decreased by approximately 1 day. There was also a trend toward decreased usage of antiemetics in the carbohydrate-loaded group, although the final values were not statistically significant. There were also fewer documented cases of postoperative nausea in the carbohydrate-loaded group.
The failure to find a statistical difference in postoperative antiemetic usage between carbohydrate-loaded and non-carbohydrate-loaded patients may be due to incomplete documentation (ie, not all patients who were nauseous having their symptoms documented in the chart). Due to the small number of antiemetic doses given to each patient, we may have lacked the necessary numbers to visualize the difference between the groups. We were unable to perform a post-hoc power calculation with our current data. Additionally, the decrease seen in LOS may not have been due solely to carbohydrate loading, since the data were collected over multiple years during implementation of the ERAS protocol. There is a possibility that the ERAS protocol, which is multimodal, was better implemented as time progressed, adding a confounding variable to our data. Despite these limitations, however, we were able to demonstrate a decreased LOS for patients who underwent total joint replacement with the initiation of a preoperative carbohydrate-loading ERAS protocol. Furthermore, there was a trend toward decreased documented postoperative nausea and decreased antiemetic use in the group that avoided fasting and received carbohydrate supplements.
This decrease in LOS by almost 1 day is consistent with multiple prior studies that demonstrated a similar decrease when implementing an ERAS protocol.3-5,7 The trend towards lower antiemetic use and less postoperative nausea in the carbohydrate-loading ERAS protocol gives merit to further research on this topic, with the goal of finding an optimal preoperative practice that allows patients to experience rapid mobilization, minimal postoperative nausea, and faster recovery overall.
Conclusion
Corresponding author: Christopher L. Blum, MD, Stony Brook Medical Center, Stony Brook, NY; [email protected].
Financial disclosures: None.
1. Proudfoot S, Bennett B, Duff S, Palmer J. Implementation and effects of Enhanced Recovery After Surgery for hip and knee replacements and fractured neck of femur in New Zealand orthopaedic services. N Z Med J. 2017;130:77-90.
2. Geltzeiler CB, Rotramel A, Wilson C, et al. Prospective study of colorectal enhanced recovery after surgery in a community hospital. JAMA Surg. 2014;149:955-961.
3. Soffin EM, YaDeau JT. Enhanced recovery after surgery for primary hip and knee arthroplasty: a review of the evidence. Br J Anaesth. 2016;117(suppl 3):iii62-iii72.
4. Stowers MD, Manuopangai L, Hill AG, et al. Enhanced Recovery After Surgery in elective hip and knee arthroplasty reduces length of hospital stay. ANZ J Surg. 2016;86:475-479.
5. Gwynne-Jones DP, Martin G, Crane C. Enhanced Recovery After Surgery for hip and knee replacements. Orthop Nurs. 2017;36:203-210.
6. Semerjian A, Milbar N, Kates M, et al. Hospital charges and length of stay following radical cystectomy in the enhanced recovery after surgery era. Urology. 2018;111:86-91.
7. Stambough JB, Nunley RM, Curry MC, et al. Rapid recovery protocols for primary total hip arthroplasty can safely reduce length of stay without increasing readmissions. J Arthroplasty. 2015;30:521-526.
8. Ljungqvist O, Soreide E. Preoperative fasting. Br J Surg. 2003;90:400-406.
9. Riis J, Lomholt B, Haxholdt O, et al. Immediate and long-term mental recovery from general versus epidural anesthesia in elderly patients. Acta Anaesthesiol Scand. 1983;27:44-49.
10. Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183:630-641.
11. Svanfeldt M, Thorell A, Hausel J, Soop M, et al. Randomized clinical trial of the effect of preoperative oral carbohydrate treatment on postoperative whole-body protein and glucose kinetics. Br J Surg. 2007;94:1342-1350.
12. Halaszynski TM, Juda R, Silverman DG. Optimizing postoperative outcomes with efficient preoperative assessment and management. Crit Care Med. 2004;32(4 suppl):S76-S86.
13. Aronsson A, Al-Ani NA, Brismar K, Hedstrom M. A carbohydrate-rich drink shortly before surgery affected IGF-I bioavailability after a total hip replacement. A double-blind placebo controlled study on 29 patients. Aging Clin Exp Res. 2009;21:97-101.
14. Bilku DK, Dennison AR, Hall TC, Metcalfe MS, Garcea G. Role of preoperative carbohydrate loading: a systematic review. Ann R Coll Surg Engl. 2014;96:15-22.
From Stony Brook Medical Center, Stony Brook, NY (Dr. Blum), and NYU Winthrop Medical Center,
Abstract
- Background: Enhanced Recovery After Surgery (ERAS) is a multimodal, standardized approach to the surgical patient that incorporates evidenced-based interventions designed to achieve rapid recovery after surgery by minimizing the patient’s stress response. One aspect of ERAS, carbohydrate loading, has been shown in multiple randomized controlled trials to result in postoperative benefits in patients undergoing colorectal surgery, but there appears to be insufficient data to make definitive recommendations for or against carbohydrate loading in joint replacement patients.
- Objective: To evaluate postoperative nausea and length of stay (LOS) after a preoperative carbohydrate loading protocol was initiated for patients undergoing total joint replacement.
- Design: Retrospective chart review.
- Setting and participants: 100 patients who underwent either total knee or hip arthroplasty at Winthrop University Hospital, Mineola, NY, in the past 4 years and either had (n = 50) or had not received preoperative carbohydrate supplements (n = 50).
- Methods: Using the total joint database, the medical record was reviewed for the patient’s demographics, LOS, documentation of postoperative nausea, and number of doses of antiemetic medication given to the patient.
- Results: The mean LOS for the carbohydrate-loading group and non-carbohydrate group was 1.9 days and 2.6 days. respectively, a difference of 0.70 days (P < 0.0001). The carbohydrate-loaded group received a total of 13 doses of antiemetic medications and the non-carbohydrate group received 21 doses. The average number of antiemetic doses given to a patient postoperatively was 0.26 for the carbohydrate-loaded group and 0.42 for the non-carbohydrate-loaded group. The difference was 0.16 doses (P < 0.7815).
- Conclusion: The implementation of carbohydrate loading decreased LOS for joint replacement patients by approximately 1 day. Additionally, there was a trend towards decreased antiemetic use and fewer documented cases of postoperative nausea after carbohydrate loading.
Keywords: carbohydrate loading, ERAS, joint arthroplasty, length of stay, nausea.
Enhanced Recovery After Surgery (ERAS) is a multimodal, standardized approach to the surgical patient that incorporates evidenced-based interventions designed to achieve rapid recovery after surgery by minimizing the patient’s stress response.1-4 The ERAS protocols have been shown to reduce complications, decrease length of stay (LOS), and improve patient outcomes.3-7 The program was originally designed to facilitate recovery after colorectal operative procedures by maintaining preoperative organ function and reducing the postoperative stress response. This was done through a coordinated program of preoperative counseling, optimizing nutritional status, standardizing analgesic regimens, and early mobilization.3
The principles of an ERAS program with standardized pre- and postoperative protocols appear ideally suited for the total joint arthroplasty patient.1,3-5 Prior studies have demonstrated ERAS to be effective in facilitating decreased LOS, with no apparent increase in readmission rates or complications for both colorectal and joint arthroplasty patients.1-7 The protocols have also been shown to be cost-effective, with decreased incidence of postoperative complications, including thromboembolic disease and infections.3,4,6
An important tenet of ERAS protocols is optimizing the nutritional status of the patient prior to surgery.6 This includes avoidance of preoperative fasting in conjunction with carbohydrate loading. ERAS protocols instruct the patient to ingest a carbohydrate-rich beverage 2 hours prior to surgery. The concept of allowing a patient to eat prior to surgery is based on the preference for the patient to present for surgery in an anabolic rather than a catabolic state.2,3,11 Patients in an anabolic state undergo less postoperative protein and nitrogen losses, which appears to facilitate wound healing.2,6,11
There have been multiple randomized controlled trials demonstrating the postoperative benefits of carbohydrate loading prior to colorectal surgery.2,6
Another potential benefit of preoperative carbohydrate loading is a decrease in postoperative nausea.1,5,12-14 A decrease in nausea in theory would allow for earlier mobilization with physical therapy and potentially a shorter LOS. Hence, the goal of this study was to examine the impact of preoperative carbohydrate loading on postoperative nausea directly, as well as on LOS, at a single institution in the setting of an ERAS protocol.
Methods
We retrospectively reviewed the records of 100 patients who underwent total hip or total knee replacement between 2014 and 2018 at NYU Winthrop University Hospital, Mineola, NY. Fifty patients had received preoperative carbohydrate supplements and 50 patients had not. The remainder of the total joint protocol was identical for the 2 groups.
Protocol
All patients attended preoperative educational classes. For patients receiving carbohydrate loading, written and oral instructions were given for the patient to drink Ensure Clear followed by 8 ounces of water before going to bed the night before surgery. They were also instructed to drink the Ensure Pre-Surgery Drink 2 hours prior to their operative procedure. Patients with diabetes were instructed to drink the Ensure Glucerna Clear drink the night before surgery. No carbohydrate drink was given on the day of surgery until a finger-stick glucose level was performed upon arrival at the hospital. Spinal anesthesia was utilized in all patients, with adductor canal block supplementation for patients undergoing total knee replacement. Orders were written to have physical therapy evaluate the patients in the PACU to facilitate ambulation. Pre- and postoperative pain protocols were identical for the 2 groups.
Data Collection
A chart review was performed using the patients’ medical record numbers from the joint replacement database at our institution. Exemption was obtained for the project from our institution’s Institutional Review Board (IRB).
Analysis
Descriptive statistics (mean, standard deviation, and median for continuous variables; frequencies and percentages for categorical variables) were calculated separately by group. The 2 groups were compared using the chi-square test or Fisher’s exact test, as deemed appropriate, for categorical variables, the 2-sample t-test for age, and the Mann-Whitney test for LOS and number of antiemetic doses given. A result was considered statistically significant at the P < 0.05 level of significance. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
The carbohydrate-loading group (n = 50) and the non-carbohydrate-loading group (n = 50) were comparable for age, gender, type of arthroplasty, episodes of vomiting, diabetes, and nerve block (Table). 

Discussion
In this study we explored whether carbohydrate loading prior to total joint replacement influenced postoperative nausea and LOS in a single institution. The 2 groups appeared similar in terms of demographics as well as the types of surgical procedures performed. After initiation of the carbohydrate-loading protocol, LOS decreased by approximately 1 day. There was also a trend toward decreased usage of antiemetics in the carbohydrate-loaded group, although the final values were not statistically significant. There were also fewer documented cases of postoperative nausea in the carbohydrate-loaded group.
The failure to find a statistical difference in postoperative antiemetic usage between carbohydrate-loaded and non-carbohydrate-loaded patients may be due to incomplete documentation (ie, not all patients who were nauseous having their symptoms documented in the chart). Due to the small number of antiemetic doses given to each patient, we may have lacked the necessary numbers to visualize the difference between the groups. We were unable to perform a post-hoc power calculation with our current data. Additionally, the decrease seen in LOS may not have been due solely to carbohydrate loading, since the data were collected over multiple years during implementation of the ERAS protocol. There is a possibility that the ERAS protocol, which is multimodal, was better implemented as time progressed, adding a confounding variable to our data. Despite these limitations, however, we were able to demonstrate a decreased LOS for patients who underwent total joint replacement with the initiation of a preoperative carbohydrate-loading ERAS protocol. Furthermore, there was a trend toward decreased documented postoperative nausea and decreased antiemetic use in the group that avoided fasting and received carbohydrate supplements.
This decrease in LOS by almost 1 day is consistent with multiple prior studies that demonstrated a similar decrease when implementing an ERAS protocol.3-5,7 The trend towards lower antiemetic use and less postoperative nausea in the carbohydrate-loading ERAS protocol gives merit to further research on this topic, with the goal of finding an optimal preoperative practice that allows patients to experience rapid mobilization, minimal postoperative nausea, and faster recovery overall.
Conclusion
Corresponding author: Christopher L. Blum, MD, Stony Brook Medical Center, Stony Brook, NY; [email protected].
Financial disclosures: None.
From Stony Brook Medical Center, Stony Brook, NY (Dr. Blum), and NYU Winthrop Medical Center,
Abstract
- Background: Enhanced Recovery After Surgery (ERAS) is a multimodal, standardized approach to the surgical patient that incorporates evidenced-based interventions designed to achieve rapid recovery after surgery by minimizing the patient’s stress response. One aspect of ERAS, carbohydrate loading, has been shown in multiple randomized controlled trials to result in postoperative benefits in patients undergoing colorectal surgery, but there appears to be insufficient data to make definitive recommendations for or against carbohydrate loading in joint replacement patients.
- Objective: To evaluate postoperative nausea and length of stay (LOS) after a preoperative carbohydrate loading protocol was initiated for patients undergoing total joint replacement.
- Design: Retrospective chart review.
- Setting and participants: 100 patients who underwent either total knee or hip arthroplasty at Winthrop University Hospital, Mineola, NY, in the past 4 years and either had (n = 50) or had not received preoperative carbohydrate supplements (n = 50).
- Methods: Using the total joint database, the medical record was reviewed for the patient’s demographics, LOS, documentation of postoperative nausea, and number of doses of antiemetic medication given to the patient.
- Results: The mean LOS for the carbohydrate-loading group and non-carbohydrate group was 1.9 days and 2.6 days. respectively, a difference of 0.70 days (P < 0.0001). The carbohydrate-loaded group received a total of 13 doses of antiemetic medications and the non-carbohydrate group received 21 doses. The average number of antiemetic doses given to a patient postoperatively was 0.26 for the carbohydrate-loaded group and 0.42 for the non-carbohydrate-loaded group. The difference was 0.16 doses (P < 0.7815).
- Conclusion: The implementation of carbohydrate loading decreased LOS for joint replacement patients by approximately 1 day. Additionally, there was a trend towards decreased antiemetic use and fewer documented cases of postoperative nausea after carbohydrate loading.
Keywords: carbohydrate loading, ERAS, joint arthroplasty, length of stay, nausea.
Enhanced Recovery After Surgery (ERAS) is a multimodal, standardized approach to the surgical patient that incorporates evidenced-based interventions designed to achieve rapid recovery after surgery by minimizing the patient’s stress response.1-4 The ERAS protocols have been shown to reduce complications, decrease length of stay (LOS), and improve patient outcomes.3-7 The program was originally designed to facilitate recovery after colorectal operative procedures by maintaining preoperative organ function and reducing the postoperative stress response. This was done through a coordinated program of preoperative counseling, optimizing nutritional status, standardizing analgesic regimens, and early mobilization.3
The principles of an ERAS program with standardized pre- and postoperative protocols appear ideally suited for the total joint arthroplasty patient.1,3-5 Prior studies have demonstrated ERAS to be effective in facilitating decreased LOS, with no apparent increase in readmission rates or complications for both colorectal and joint arthroplasty patients.1-7 The protocols have also been shown to be cost-effective, with decreased incidence of postoperative complications, including thromboembolic disease and infections.3,4,6
An important tenet of ERAS protocols is optimizing the nutritional status of the patient prior to surgery.6 This includes avoidance of preoperative fasting in conjunction with carbohydrate loading. ERAS protocols instruct the patient to ingest a carbohydrate-rich beverage 2 hours prior to surgery. The concept of allowing a patient to eat prior to surgery is based on the preference for the patient to present for surgery in an anabolic rather than a catabolic state.2,3,11 Patients in an anabolic state undergo less postoperative protein and nitrogen losses, which appears to facilitate wound healing.2,6,11
There have been multiple randomized controlled trials demonstrating the postoperative benefits of carbohydrate loading prior to colorectal surgery.2,6
Another potential benefit of preoperative carbohydrate loading is a decrease in postoperative nausea.1,5,12-14 A decrease in nausea in theory would allow for earlier mobilization with physical therapy and potentially a shorter LOS. Hence, the goal of this study was to examine the impact of preoperative carbohydrate loading on postoperative nausea directly, as well as on LOS, at a single institution in the setting of an ERAS protocol.
Methods
We retrospectively reviewed the records of 100 patients who underwent total hip or total knee replacement between 2014 and 2018 at NYU Winthrop University Hospital, Mineola, NY. Fifty patients had received preoperative carbohydrate supplements and 50 patients had not. The remainder of the total joint protocol was identical for the 2 groups.
Protocol
All patients attended preoperative educational classes. For patients receiving carbohydrate loading, written and oral instructions were given for the patient to drink Ensure Clear followed by 8 ounces of water before going to bed the night before surgery. They were also instructed to drink the Ensure Pre-Surgery Drink 2 hours prior to their operative procedure. Patients with diabetes were instructed to drink the Ensure Glucerna Clear drink the night before surgery. No carbohydrate drink was given on the day of surgery until a finger-stick glucose level was performed upon arrival at the hospital. Spinal anesthesia was utilized in all patients, with adductor canal block supplementation for patients undergoing total knee replacement. Orders were written to have physical therapy evaluate the patients in the PACU to facilitate ambulation. Pre- and postoperative pain protocols were identical for the 2 groups.
Data Collection
A chart review was performed using the patients’ medical record numbers from the joint replacement database at our institution. Exemption was obtained for the project from our institution’s Institutional Review Board (IRB).
Analysis
Descriptive statistics (mean, standard deviation, and median for continuous variables; frequencies and percentages for categorical variables) were calculated separately by group. The 2 groups were compared using the chi-square test or Fisher’s exact test, as deemed appropriate, for categorical variables, the 2-sample t-test for age, and the Mann-Whitney test for LOS and number of antiemetic doses given. A result was considered statistically significant at the P < 0.05 level of significance. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
The carbohydrate-loading group (n = 50) and the non-carbohydrate-loading group (n = 50) were comparable for age, gender, type of arthroplasty, episodes of vomiting, diabetes, and nerve block (Table). 

Discussion
In this study we explored whether carbohydrate loading prior to total joint replacement influenced postoperative nausea and LOS in a single institution. The 2 groups appeared similar in terms of demographics as well as the types of surgical procedures performed. After initiation of the carbohydrate-loading protocol, LOS decreased by approximately 1 day. There was also a trend toward decreased usage of antiemetics in the carbohydrate-loaded group, although the final values were not statistically significant. There were also fewer documented cases of postoperative nausea in the carbohydrate-loaded group.
The failure to find a statistical difference in postoperative antiemetic usage between carbohydrate-loaded and non-carbohydrate-loaded patients may be due to incomplete documentation (ie, not all patients who were nauseous having their symptoms documented in the chart). Due to the small number of antiemetic doses given to each patient, we may have lacked the necessary numbers to visualize the difference between the groups. We were unable to perform a post-hoc power calculation with our current data. Additionally, the decrease seen in LOS may not have been due solely to carbohydrate loading, since the data were collected over multiple years during implementation of the ERAS protocol. There is a possibility that the ERAS protocol, which is multimodal, was better implemented as time progressed, adding a confounding variable to our data. Despite these limitations, however, we were able to demonstrate a decreased LOS for patients who underwent total joint replacement with the initiation of a preoperative carbohydrate-loading ERAS protocol. Furthermore, there was a trend toward decreased documented postoperative nausea and decreased antiemetic use in the group that avoided fasting and received carbohydrate supplements.
This decrease in LOS by almost 1 day is consistent with multiple prior studies that demonstrated a similar decrease when implementing an ERAS protocol.3-5,7 The trend towards lower antiemetic use and less postoperative nausea in the carbohydrate-loading ERAS protocol gives merit to further research on this topic, with the goal of finding an optimal preoperative practice that allows patients to experience rapid mobilization, minimal postoperative nausea, and faster recovery overall.
Conclusion
Corresponding author: Christopher L. Blum, MD, Stony Brook Medical Center, Stony Brook, NY; [email protected].
Financial disclosures: None.
1. Proudfoot S, Bennett B, Duff S, Palmer J. Implementation and effects of Enhanced Recovery After Surgery for hip and knee replacements and fractured neck of femur in New Zealand orthopaedic services. N Z Med J. 2017;130:77-90.
2. Geltzeiler CB, Rotramel A, Wilson C, et al. Prospective study of colorectal enhanced recovery after surgery in a community hospital. JAMA Surg. 2014;149:955-961.
3. Soffin EM, YaDeau JT. Enhanced recovery after surgery for primary hip and knee arthroplasty: a review of the evidence. Br J Anaesth. 2016;117(suppl 3):iii62-iii72.
4. Stowers MD, Manuopangai L, Hill AG, et al. Enhanced Recovery After Surgery in elective hip and knee arthroplasty reduces length of hospital stay. ANZ J Surg. 2016;86:475-479.
5. Gwynne-Jones DP, Martin G, Crane C. Enhanced Recovery After Surgery for hip and knee replacements. Orthop Nurs. 2017;36:203-210.
6. Semerjian A, Milbar N, Kates M, et al. Hospital charges and length of stay following radical cystectomy in the enhanced recovery after surgery era. Urology. 2018;111:86-91.
7. Stambough JB, Nunley RM, Curry MC, et al. Rapid recovery protocols for primary total hip arthroplasty can safely reduce length of stay without increasing readmissions. J Arthroplasty. 2015;30:521-526.
8. Ljungqvist O, Soreide E. Preoperative fasting. Br J Surg. 2003;90:400-406.
9. Riis J, Lomholt B, Haxholdt O, et al. Immediate and long-term mental recovery from general versus epidural anesthesia in elderly patients. Acta Anaesthesiol Scand. 1983;27:44-49.
10. Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183:630-641.
11. Svanfeldt M, Thorell A, Hausel J, Soop M, et al. Randomized clinical trial of the effect of preoperative oral carbohydrate treatment on postoperative whole-body protein and glucose kinetics. Br J Surg. 2007;94:1342-1350.
12. Halaszynski TM, Juda R, Silverman DG. Optimizing postoperative outcomes with efficient preoperative assessment and management. Crit Care Med. 2004;32(4 suppl):S76-S86.
13. Aronsson A, Al-Ani NA, Brismar K, Hedstrom M. A carbohydrate-rich drink shortly before surgery affected IGF-I bioavailability after a total hip replacement. A double-blind placebo controlled study on 29 patients. Aging Clin Exp Res. 2009;21:97-101.
14. Bilku DK, Dennison AR, Hall TC, Metcalfe MS, Garcea G. Role of preoperative carbohydrate loading: a systematic review. Ann R Coll Surg Engl. 2014;96:15-22.
1. Proudfoot S, Bennett B, Duff S, Palmer J. Implementation and effects of Enhanced Recovery After Surgery for hip and knee replacements and fractured neck of femur in New Zealand orthopaedic services. N Z Med J. 2017;130:77-90.
2. Geltzeiler CB, Rotramel A, Wilson C, et al. Prospective study of colorectal enhanced recovery after surgery in a community hospital. JAMA Surg. 2014;149:955-961.
3. Soffin EM, YaDeau JT. Enhanced recovery after surgery for primary hip and knee arthroplasty: a review of the evidence. Br J Anaesth. 2016;117(suppl 3):iii62-iii72.
4. Stowers MD, Manuopangai L, Hill AG, et al. Enhanced Recovery After Surgery in elective hip and knee arthroplasty reduces length of hospital stay. ANZ J Surg. 2016;86:475-479.
5. Gwynne-Jones DP, Martin G, Crane C. Enhanced Recovery After Surgery for hip and knee replacements. Orthop Nurs. 2017;36:203-210.
6. Semerjian A, Milbar N, Kates M, et al. Hospital charges and length of stay following radical cystectomy in the enhanced recovery after surgery era. Urology. 2018;111:86-91.
7. Stambough JB, Nunley RM, Curry MC, et al. Rapid recovery protocols for primary total hip arthroplasty can safely reduce length of stay without increasing readmissions. J Arthroplasty. 2015;30:521-526.
8. Ljungqvist O, Soreide E. Preoperative fasting. Br J Surg. 2003;90:400-406.
9. Riis J, Lomholt B, Haxholdt O, et al. Immediate and long-term mental recovery from general versus epidural anesthesia in elderly patients. Acta Anaesthesiol Scand. 1983;27:44-49.
10. Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183:630-641.
11. Svanfeldt M, Thorell A, Hausel J, Soop M, et al. Randomized clinical trial of the effect of preoperative oral carbohydrate treatment on postoperative whole-body protein and glucose kinetics. Br J Surg. 2007;94:1342-1350.
12. Halaszynski TM, Juda R, Silverman DG. Optimizing postoperative outcomes with efficient preoperative assessment and management. Crit Care Med. 2004;32(4 suppl):S76-S86.
13. Aronsson A, Al-Ani NA, Brismar K, Hedstrom M. A carbohydrate-rich drink shortly before surgery affected IGF-I bioavailability after a total hip replacement. A double-blind placebo controlled study on 29 patients. Aging Clin Exp Res. 2009;21:97-101.
14. Bilku DK, Dennison AR, Hall TC, Metcalfe MS, Garcea G. Role of preoperative carbohydrate loading: a systematic review. Ann R Coll Surg Engl. 2014;96:15-22.
Decreasing Treatment of Asymptomatic Bacteriuria: An Interprofessional Approach to Antibiotic Stewardship
From the Mayo Clinic, Rochester, MN.
Abstract
- Objective: Asymptomatic bacteriuria (ASB) denotes asymptomatic carriage of bacteria within the urinary tract and does not require treatment in most patient populations. Unnecessary antimicrobial treatment has several consequences, including promotion of antimicrobial resistance, potential for medication adverse effects, and risk for Clostridiodes difficile infection. The aim of this quality improvement effort was to decrease both the unnecessary ordering of urine culture studies and unnecessary treatment of ASB.
- Methods: This is a single-center study of patients who received care on 3 internal medicine units at a large, academic medical center. We sought to determine the impact of information technology and educational interventions to decrease both inappropriate urine culture ordering and treatment of ASB. Data from included patients were collected over 3 1-month time periods: baseline, post-information technology intervention, and post-educational intervention.
- Results: There was a reduction in the percentage of patients who received antibiotics for ASB in the post-education intervention period as compared to baseline (35% vs 42%). The proportion of total urine cultures ordered by internal medicine clinicians did not change after an information technology intervention to redesign the computerized physician order entry screen for urine cultures.
- Conclusion: Educational interventions are effective ways to reduce rates of inappropriate treatment of ASB in patients admitted to internal medicine services.
Keywords: asymptomatic bacteriuria, UTI, information technology, education, quality.
Asymptomatic bacteriuria (ASB) is a common condition in which bacteria are recovered from a urine culture (UC) in patients without symptoms suggestive of urinary tract infection (UTI), with no pathologic consequences to most patients who are not treated.1,2 Patients with ASB do not exhibit symptoms of a UTI such as dysuria, increased frequency of urination, increased urgency, suprapubic tenderness, or costovertebral pain. Treatment with antibiotics is not indicated for most patients with ASB.1,3 According to the Infectious Diseases Society of America (IDSA), screening for bacteriuria and treatment for positive results is only indicated during pregnancy and prior to urologic procedures with anticipated breach of the mucosal lining.1
An estimated 20% to 52% of patients in hospital settings receive inappropriate treatment with antibiotics for ASB.4 Unnecessary prescribing of antibiotics has several negative consequences, including increased rates of antibiotic resistance, Clostridioides difficile infection, and medication adverse events, as well as increased health care costs.2,5 Antimicrobial stewardship programs to improve judicious use of antimicrobials are paramount to reducing these consequences, and their importance is heightened with recent requirements for antimicrobial stewardship put forth by The Joint Commission and the Centers for Medicare & Medicaid Services.6,7
A previous review of UC and antimicrobial use in patients for purposes of quality improvement at our institution over a 2-month period showed that of 59 patients with positive UCs, 47 patients (80%) did not have documented symptoms of a UTI. Of these 47 patients with ASB, 29 (61.7%) received antimicrobial treatment unnecessarily (unpublished data). We convened a group of clinicians and nonclinicians representing the areas of infectious disease, pharmacy, microbiology, statistics, and hospital internal medicine (IM) to examine the unnecessary treatment of ASB in our institution. Our objective was to address 2 antimicrobial stewardship issues: inappropriate UC ordering and unnecessary use of antibiotics to treat ASB. Our aim was to reduce the inappropriate ordering of UCs and to reduce treatment of ASB.
Methods
Setting
The study was conducted on 3 IM nursing units with a total of 83 beds at a large tertiary care academic medical center in the midwestern United States, and was approved by the organization’s Institutional Review Board.
Participants
We included all non-pregnant patients aged 18 years or older who received care from an IM primary service. These patients were admitted directly to an IM team through the emergency department (ED) or transferred to an IM team after an initial stay in the intensive care unit.
Data Source
Microbiology laboratory reports generated from the electronic health record were used to identify all patients with a collected UC sample who received care from an IM service prior to discharge. Urine samples were collected by midstream catch or catheterization. Data on urine Gram stain and urine dipstick were not included. Henceforth, the phrase “urine culture order” indicates that a UC was both ordered and performed. Data reports were generated for the month of August 2016 to determine the baseline number of UCs ordered. Charts of patients with positive UCs were reviewed to determine if antibiotics were started for the positive UC and whether the patient had signs or symptoms consistent with a UTI. If antibiotics were started in the absence of signs or symptoms to support a UTI, the patient was determined to have been unnecessarily treated for ASB. Reports were then generated for the month after each intervention was implemented, with the same chart review undertaken for positive UCs. Bacteriuria was defined in our study as the presence of microbial growth greater than 10,000 CFU/mL in UC.
Interventions
Initial analysis by our study group determined that lack of electronic clinical decision support (CDS) at the point of care and provider knowledge gaps in interpreting positive UCs were the 2 main contributors to unnecessary UC orders and unnecessary treatment of positive UCs, respectively. We reviewed the work of other groups who reported interventions to decrease treatment of ASB, ranging from educational presentations to pocket cards and treatment algorithms.8-13 We hypothesized that there would be a decrease in UC orders with CDS embedded in the computerized order entry screen, and that we would decrease unnecessary treatment of positive UCs by educating clinicians on indications for appropriate antibiotic prescribing in the setting of a positive UC.
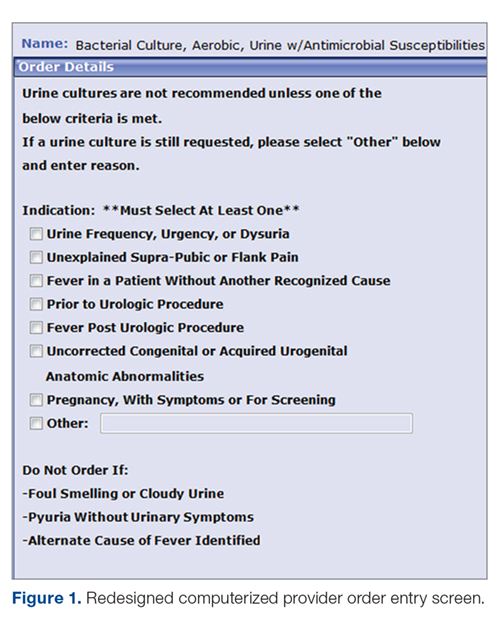
Information technology intervention. The first intervention implemented involved redesign of the UC ordering screen in the computerized physician order entry (CPOE) system. This intervention went live hospital-wide, including the IM floors, intensive care units, and all other areas except the ED, on February 1, 2017 (Figure 1). The ordering screen required the prescriber to select from a list of appropriate indications for ordering a UC, including urine frequency, urgency, or dysuria; unexplained suprapubic or flank pain; fever in patients without another recognized cause; screening obtained prior to urologic procedure; or screening during pregnancy. An additional message advised prescribers to avoid ordering the culture if the patient had malodorous or cloudy urine, pyuria without urinary symptoms, or had an alternative cause of fever. Before we implemented the information technology (IT) intervention, there had been no specific point-of-care guidance on UC ordering.
Educational intervention. The second intervention, driven by clinical pharmacists, involved active and passive education of prescribers specifically designed to address unnecessary treatment of ASB. The IT intervention with CDS for UC ordering remained live. Presentations designed by the study group summarizing the appropriate indications for ordering a UC, distinguishing ASB from UTI, and discouraging treatment of ASB were delivered via a variety of routes by clinical pharmacists to nurses, nurse practitioners, physician assistants, pharmacists, medical residents, and staff physicians providing care to patients on the 3 IM units over a 1-month period in March 2017. The presentations contained the same basic content, but the information was delivered to target each specific audience group.
Medical residents received a 10-minute live presentation during a conference. Nurse practitioners, physician assistants, and staff physicians received a presentation via email, and highlights of the presentation were delivered by clinical pharmacists at their respective monthly group meetings. A handout was presented to nursing staff at nursing huddles, and presentation slides were distributed by email. Educational posters were posted in the medical resident workrooms, nursing breakrooms, and staff bathrooms on the units.
Outcome Measurements
The endpoints of interest were the percentage of patients with positive UCs unnecessarily treated for ASB before and after each intervention and the number of UCs ordered at baseline and after implementation of each intervention. Counterbalance measures assessed included the incidence of UTI, pyelonephritis, or urosepsis within 7 days of positive UC for patients who did not receive antibiotic treatment for ASB.
Results
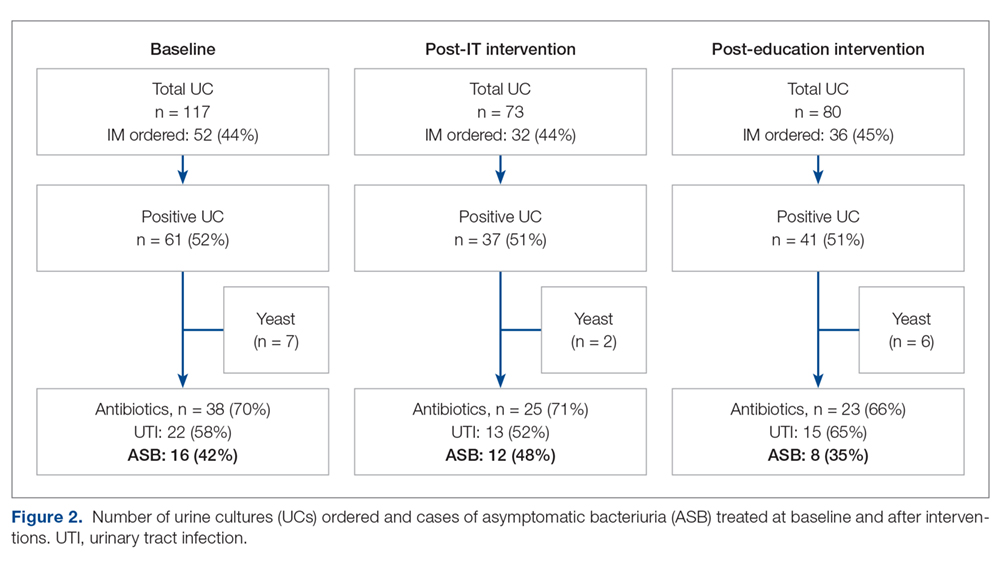
Data from a total of 270 cultures were examined from IM nursing units. A total of 117 UCs were ordered during the baseline period before interventions were implemented. For a period of 1 month following activation of the IT intervention, 73 UCs were ordered. For a period of 1 month following the educational interventions, 80 UCs were ordered. Of these, 61 (52%) UCs were positive at baseline, 37 (51%) after the IT intervention, and 41 (51%) after the educational intervention. Patient characteristics were similar between the 3 groups (Table); 64.7% of patients were female in their early to mid-seventies. The majority of UCs were ordered by providers in the ED in all 3 periods examined (51%-70%). The percentage of patients who received antibiotics prior to UC for another indication (including bacteriuria) in the baseline, post-IT intervention, and post-education intervention groups were 30%, 27%, and 45%, respectively.
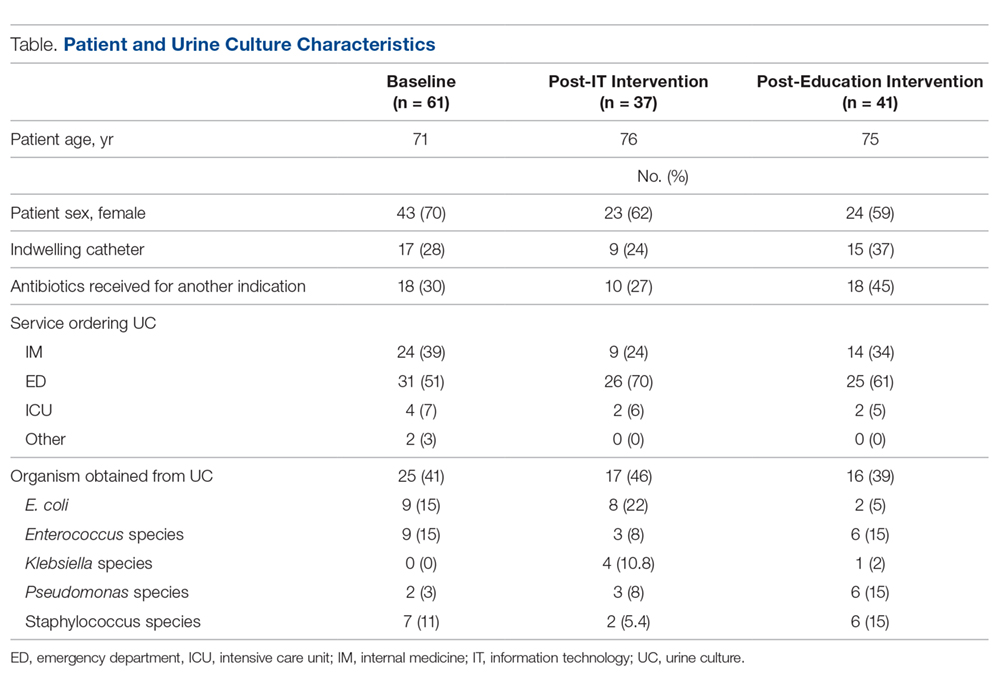
The study outcomes are summarized in Figure 2. Among patients with positive cultures, there was not a reduction in inappropriate treatment of ASB compared to baseline after the IT intervention (48% vs 42%). Following the education intervention, there was a reduction in unnecessary ASB treatment as compared both to baseline (35% vs 42%) and to post-IT intervention (35% vs 48%). There was no difference between the 3 study periods in the percentage of total UCs ordered by IM clinicians. The counterbalance measure showed that 1 patient who did not receive antibiotics within 7 days of a positive UC developed pyelonephritis, UTI, or sepsis due to a UTI in each intervention group.
Discussion
The results of this study demonstrate the role of multimodal interventions in antimicrobial stewardship and add to the growing body of evidence supporting the work of antimicrobial stewardship programs. Our multidisciplinary study group and multipronged intervention follow recent guideline recommendations for antimicrobial stewardship program interventions against unnecessary treatment of ASB.14 Initial analysis by our study group determined lack of CDS at the point of care and provider knowledge gaps in interpreting positive UCs as the 2 main contributors to unnecessary UC orders and unnecessary treatment of positive UCs in our local practice culture. The IT component of our intervention was intended to provide CDS for ordering UCs, and the education component focused on informing clinicians’ treatment decisions for positive UCs.
It has been suggested that the type of stewardship intervention that is most effective fits the specific needs and resources of an institution.14,15 And although the IDSA does not recommend education as a stand-alone intervention,16 we found it to be an effective intervention for our clinicians in our work environment. However, since the CPOE guidance was in place during the educational study periods, it is possible that the effect was due to a combination of these 2 approaches. Our pre-intervention ASB treatment rates were consistent with a recent meta-analysis in which the rate of inappropriate treatment of ASB was 45%.17 This meta-analysis found educational and organizational interventions led to a mean absolute risk reduction of 33%. After the education intervention, we saw a 7% decrease in unnecessary treatment of ASB compared to baseline, and a 13% decrease compared to the month just prior to the educational intervention.
Lessons learned from our work included how clear review of local processes can inform quality improvement interventions. For instance, we initially hypothesized that IM clinicians would benefit from point-of-care CDS guidance, but such guidance used alone without educational interventions was not supported by the results. We also determined that the majority of UCs from patients on general medicine units were ordered by ED providers. This revealed an opportunity to implement similar interventions in the ED, as this was the initial point of contact for many of these patients.
As with any clinical intervention, the anticipated benefits should be weighed against potential harm. Using counterbalance measures, we found there was minimal risk in the occurrence of UTI, pyelonephritis, or sepsis if clinicians avoided treating ASB. This finding is consistent with IDSA guideline recommendations and other studies that suggest that withholding treatment for asymptomatic bacteriuria does not lead to worse outcomes.1
This study has several limitations. Data were obtained through review of the electronic health record and therefore documentation may be incomplete. Also, antimicrobials for empiric coverage or treatment for other infections (eg, pneumonia, sepsis) may have confounded our results, as empirical antimicrobials were given to 27% to 45% of patients prior to UC. This was a quality improvement project carried out over defined time intervals, and thus our sample size was limited and not adequately powered to show statistical significance. Additionally, given the bundling of interventions, it is difficult to determine the impact of each intervention independently. Although CDS for UC ordering may not have influenced ordering, it is possible that the IT intervention raised awareness of ASB and influenced treatment practices.
Conclusion
Our work supports the principles of antibiotic stewardship as brought forth by IDSA.16 This work was the effort of a multidisciplinary team, which aligns with recommendations by Daniel and colleagues, published after our study had ended, for reducing overtreatment of ASB.14 Additionally, our study results provided valuable information for our institution. Although improvements in management of ASB were modest, the success of provider education and identification of other work areas and clinicians to target for future intervention were helpful in consideration of further studies. This work will also aid us in developing an expected effect size for future studies. We plan to provide ongoing education for IM providers as well as education in the ED to target providers who make first contact with patients admitted to inpatient services. In addition, the CPOE UC ordering screen message will continue to be used hospital-wide and will be expanded to the ED ordering system. Our interventions, experiences, and challenges may be used by other institutions to design effective antimicrobial stewardship interventions directed towards reducing rates of inappropriate ASB treatment.
Corresponding author: Prasanna P. Narayanan, PharmD, 200 First Street SW, Rochester, MN 55905; [email protected].
Financial disclosures: None.
1. Nicolle LE, Gupta K, Bradley SF, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68:e83–75.
2. Trautner BW, Grigoryan L, Petersen NJ, et al. Effectiveness of an antimicrobial stewardship approach for urinary catheter-associated asymptomatic bacteriuria. JAMA Intern Med. 2015;175:1120-1127.
3. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309-332.
4. Trautner BW. Asymptomatic bacteriuria: when the treatment is worse than the disease. Nat Rev Urol. 2011;9:85-93.
5. Costelloe C, Metcalfe C, Lovering A, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096.
6. The Joint Commission. Prepublication Requirements: New antimicrobial stewardship standard. Jun 22, 2016. www.jointcommission.org/assets/1/6/HAP-CAH_Antimicrobial_Prepub.pdf. Accessed January 24, 2019.
7. Federal Register. Medicare and Medicaid Programs; Hospital and Critical Access Hospital (CAH) Changes to Promote Innovation, Flexibility, and Improvement in Patient Care.Centers for Medicare & Medicaid Services. June 16, 2016. CMS-3295-P
8. Hartley SE, Kuhn L, Valley S, et al. Evaluating a hospitalist-based intervention to decrease unnecessary antimicrobial use in patients with asymptomatic bacteriuria. Infect Control Hosp Epidemiol. 2016;37:1044-1051.
9. Pavese P, Saurel N, Labarere J, et al. Does an educational session with an infectious diseases physician reduce the use of inappropriate antibiotic therapy for inpatients with positive urine culture results? A controlled before-and-after study. Infect Control Hosp Epidemiol. 2009;30:596-599.
10. Kelley D, Aaronson P, Poon E, et al. Evaluation of an antimicrobial stewardship approach to minimize overuse of antibiotics in patients with asymptomatic bacteriuria. Infect Control Hosp Epidemiol. 2014;35:193-195.
11. Chowdhury F, Sarkar K, Branche A, et al. Preventing the inappropriate treatment of asymptomatic bacteriuria at a community teaching hospital. J Community Hosp Intern Med Perspect. 2012;2.
12. Bonnal C, Baune B, Mion M, et al. Bacteriuria in a geriatric hospital: impact of an antibiotic improvement program. J Am Med Dir Assoc. 2008;9:605-609.
13. Linares LA, Thornton DJ, Strymish J, et al. Electronic memorandum decreases unnecessary antimicrobial use for asymptomatic bacteriuria and culture-negative pyuria. Infect Control Hosp Epidemiol. 2011;32:644-648.
14. Daniel M, Keller S, Mozafarihashjin M, et al. An implementation guide to reducing overtreatment of asymptomatic bacteriuria. JAMA Intern Med. 2018;178:271-276.
15. Redwood R, Knobloch MJ, Pellegrini DC, et al. Reducing unnecessary culturing: a systems approach to evaluating urine culture ordering and collection practices among nurses in two acute care settings. Antimicrob Resist Infect Control. 2018;7:4.
16. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62:e51–e7.
17. Flokas ME, Andreatos N, Alevizakos M, et al. Inappropriate management of asymptomatic patients with positive urine cultures: a systematic review and meta-analysis. Open Forum Infect Dis. 2017;4:1-10.
From the Mayo Clinic, Rochester, MN.
Abstract
- Objective: Asymptomatic bacteriuria (ASB) denotes asymptomatic carriage of bacteria within the urinary tract and does not require treatment in most patient populations. Unnecessary antimicrobial treatment has several consequences, including promotion of antimicrobial resistance, potential for medication adverse effects, and risk for Clostridiodes difficile infection. The aim of this quality improvement effort was to decrease both the unnecessary ordering of urine culture studies and unnecessary treatment of ASB.
- Methods: This is a single-center study of patients who received care on 3 internal medicine units at a large, academic medical center. We sought to determine the impact of information technology and educational interventions to decrease both inappropriate urine culture ordering and treatment of ASB. Data from included patients were collected over 3 1-month time periods: baseline, post-information technology intervention, and post-educational intervention.
- Results: There was a reduction in the percentage of patients who received antibiotics for ASB in the post-education intervention period as compared to baseline (35% vs 42%). The proportion of total urine cultures ordered by internal medicine clinicians did not change after an information technology intervention to redesign the computerized physician order entry screen for urine cultures.
- Conclusion: Educational interventions are effective ways to reduce rates of inappropriate treatment of ASB in patients admitted to internal medicine services.
Keywords: asymptomatic bacteriuria, UTI, information technology, education, quality.
Asymptomatic bacteriuria (ASB) is a common condition in which bacteria are recovered from a urine culture (UC) in patients without symptoms suggestive of urinary tract infection (UTI), with no pathologic consequences to most patients who are not treated.1,2 Patients with ASB do not exhibit symptoms of a UTI such as dysuria, increased frequency of urination, increased urgency, suprapubic tenderness, or costovertebral pain. Treatment with antibiotics is not indicated for most patients with ASB.1,3 According to the Infectious Diseases Society of America (IDSA), screening for bacteriuria and treatment for positive results is only indicated during pregnancy and prior to urologic procedures with anticipated breach of the mucosal lining.1
An estimated 20% to 52% of patients in hospital settings receive inappropriate treatment with antibiotics for ASB.4 Unnecessary prescribing of antibiotics has several negative consequences, including increased rates of antibiotic resistance, Clostridioides difficile infection, and medication adverse events, as well as increased health care costs.2,5 Antimicrobial stewardship programs to improve judicious use of antimicrobials are paramount to reducing these consequences, and their importance is heightened with recent requirements for antimicrobial stewardship put forth by The Joint Commission and the Centers for Medicare & Medicaid Services.6,7
A previous review of UC and antimicrobial use in patients for purposes of quality improvement at our institution over a 2-month period showed that of 59 patients with positive UCs, 47 patients (80%) did not have documented symptoms of a UTI. Of these 47 patients with ASB, 29 (61.7%) received antimicrobial treatment unnecessarily (unpublished data). We convened a group of clinicians and nonclinicians representing the areas of infectious disease, pharmacy, microbiology, statistics, and hospital internal medicine (IM) to examine the unnecessary treatment of ASB in our institution. Our objective was to address 2 antimicrobial stewardship issues: inappropriate UC ordering and unnecessary use of antibiotics to treat ASB. Our aim was to reduce the inappropriate ordering of UCs and to reduce treatment of ASB.
Methods
Setting
The study was conducted on 3 IM nursing units with a total of 83 beds at a large tertiary care academic medical center in the midwestern United States, and was approved by the organization’s Institutional Review Board.
Participants
We included all non-pregnant patients aged 18 years or older who received care from an IM primary service. These patients were admitted directly to an IM team through the emergency department (ED) or transferred to an IM team after an initial stay in the intensive care unit.
Data Source
Microbiology laboratory reports generated from the electronic health record were used to identify all patients with a collected UC sample who received care from an IM service prior to discharge. Urine samples were collected by midstream catch or catheterization. Data on urine Gram stain and urine dipstick were not included. Henceforth, the phrase “urine culture order” indicates that a UC was both ordered and performed. Data reports were generated for the month of August 2016 to determine the baseline number of UCs ordered. Charts of patients with positive UCs were reviewed to determine if antibiotics were started for the positive UC and whether the patient had signs or symptoms consistent with a UTI. If antibiotics were started in the absence of signs or symptoms to support a UTI, the patient was determined to have been unnecessarily treated for ASB. Reports were then generated for the month after each intervention was implemented, with the same chart review undertaken for positive UCs. Bacteriuria was defined in our study as the presence of microbial growth greater than 10,000 CFU/mL in UC.
Interventions
Initial analysis by our study group determined that lack of electronic clinical decision support (CDS) at the point of care and provider knowledge gaps in interpreting positive UCs were the 2 main contributors to unnecessary UC orders and unnecessary treatment of positive UCs, respectively. We reviewed the work of other groups who reported interventions to decrease treatment of ASB, ranging from educational presentations to pocket cards and treatment algorithms.8-13 We hypothesized that there would be a decrease in UC orders with CDS embedded in the computerized order entry screen, and that we would decrease unnecessary treatment of positive UCs by educating clinicians on indications for appropriate antibiotic prescribing in the setting of a positive UC.
Information technology intervention. The first intervention implemented involved redesign of the UC ordering screen in the computerized physician order entry (CPOE) system. This intervention went live hospital-wide, including the IM floors, intensive care units, and all other areas except the ED, on February 1, 2017 (Figure 1). The ordering screen required the prescriber to select from a list of appropriate indications for ordering a UC, including urine frequency, urgency, or dysuria; unexplained suprapubic or flank pain; fever in patients without another recognized cause; screening obtained prior to urologic procedure; or screening during pregnancy. An additional message advised prescribers to avoid ordering the culture if the patient had malodorous or cloudy urine, pyuria without urinary symptoms, or had an alternative cause of fever. Before we implemented the information technology (IT) intervention, there had been no specific point-of-care guidance on UC ordering.

Educational intervention. The second intervention, driven by clinical pharmacists, involved active and passive education of prescribers specifically designed to address unnecessary treatment of ASB. The IT intervention with CDS for UC ordering remained live. Presentations designed by the study group summarizing the appropriate indications for ordering a UC, distinguishing ASB from UTI, and discouraging treatment of ASB were delivered via a variety of routes by clinical pharmacists to nurses, nurse practitioners, physician assistants, pharmacists, medical residents, and staff physicians providing care to patients on the 3 IM units over a 1-month period in March 2017. The presentations contained the same basic content, but the information was delivered to target each specific audience group.
Medical residents received a 10-minute live presentation during a conference. Nurse practitioners, physician assistants, and staff physicians received a presentation via email, and highlights of the presentation were delivered by clinical pharmacists at their respective monthly group meetings. A handout was presented to nursing staff at nursing huddles, and presentation slides were distributed by email. Educational posters were posted in the medical resident workrooms, nursing breakrooms, and staff bathrooms on the units.
Outcome Measurements
The endpoints of interest were the percentage of patients with positive UCs unnecessarily treated for ASB before and after each intervention and the number of UCs ordered at baseline and after implementation of each intervention. Counterbalance measures assessed included the incidence of UTI, pyelonephritis, or urosepsis within 7 days of positive UC for patients who did not receive antibiotic treatment for ASB.
Results
Data from a total of 270 cultures were examined from IM nursing units. A total of 117 UCs were ordered during the baseline period before interventions were implemented. For a period of 1 month following activation of the IT intervention, 73 UCs were ordered. For a period of 1 month following the educational interventions, 80 UCs were ordered. Of these, 61 (52%) UCs were positive at baseline, 37 (51%) after the IT intervention, and 41 (51%) after the educational intervention. Patient characteristics were similar between the 3 groups (Table); 64.7% of patients were female in their early to mid-seventies. The majority of UCs were ordered by providers in the ED in all 3 periods examined (51%-70%). The percentage of patients who received antibiotics prior to UC for another indication (including bacteriuria) in the baseline, post-IT intervention, and post-education intervention groups were 30%, 27%, and 45%, respectively.

The study outcomes are summarized in Figure 2. Among patients with positive cultures, there was not a reduction in inappropriate treatment of ASB compared to baseline after the IT intervention (48% vs 42%). Following the education intervention, there was a reduction in unnecessary ASB treatment as compared both to baseline (35% vs 42%) and to post-IT intervention (35% vs 48%). There was no difference between the 3 study periods in the percentage of total UCs ordered by IM clinicians. The counterbalance measure showed that 1 patient who did not receive antibiotics within 7 days of a positive UC developed pyelonephritis, UTI, or sepsis due to a UTI in each intervention group.

Discussion
The results of this study demonstrate the role of multimodal interventions in antimicrobial stewardship and add to the growing body of evidence supporting the work of antimicrobial stewardship programs. Our multidisciplinary study group and multipronged intervention follow recent guideline recommendations for antimicrobial stewardship program interventions against unnecessary treatment of ASB.14 Initial analysis by our study group determined lack of CDS at the point of care and provider knowledge gaps in interpreting positive UCs as the 2 main contributors to unnecessary UC orders and unnecessary treatment of positive UCs in our local practice culture. The IT component of our intervention was intended to provide CDS for ordering UCs, and the education component focused on informing clinicians’ treatment decisions for positive UCs.
It has been suggested that the type of stewardship intervention that is most effective fits the specific needs and resources of an institution.14,15 And although the IDSA does not recommend education as a stand-alone intervention,16 we found it to be an effective intervention for our clinicians in our work environment. However, since the CPOE guidance was in place during the educational study periods, it is possible that the effect was due to a combination of these 2 approaches. Our pre-intervention ASB treatment rates were consistent with a recent meta-analysis in which the rate of inappropriate treatment of ASB was 45%.17 This meta-analysis found educational and organizational interventions led to a mean absolute risk reduction of 33%. After the education intervention, we saw a 7% decrease in unnecessary treatment of ASB compared to baseline, and a 13% decrease compared to the month just prior to the educational intervention.
Lessons learned from our work included how clear review of local processes can inform quality improvement interventions. For instance, we initially hypothesized that IM clinicians would benefit from point-of-care CDS guidance, but such guidance used alone without educational interventions was not supported by the results. We also determined that the majority of UCs from patients on general medicine units were ordered by ED providers. This revealed an opportunity to implement similar interventions in the ED, as this was the initial point of contact for many of these patients.
As with any clinical intervention, the anticipated benefits should be weighed against potential harm. Using counterbalance measures, we found there was minimal risk in the occurrence of UTI, pyelonephritis, or sepsis if clinicians avoided treating ASB. This finding is consistent with IDSA guideline recommendations and other studies that suggest that withholding treatment for asymptomatic bacteriuria does not lead to worse outcomes.1
This study has several limitations. Data were obtained through review of the electronic health record and therefore documentation may be incomplete. Also, antimicrobials for empiric coverage or treatment for other infections (eg, pneumonia, sepsis) may have confounded our results, as empirical antimicrobials were given to 27% to 45% of patients prior to UC. This was a quality improvement project carried out over defined time intervals, and thus our sample size was limited and not adequately powered to show statistical significance. Additionally, given the bundling of interventions, it is difficult to determine the impact of each intervention independently. Although CDS for UC ordering may not have influenced ordering, it is possible that the IT intervention raised awareness of ASB and influenced treatment practices.
Conclusion
Our work supports the principles of antibiotic stewardship as brought forth by IDSA.16 This work was the effort of a multidisciplinary team, which aligns with recommendations by Daniel and colleagues, published after our study had ended, for reducing overtreatment of ASB.14 Additionally, our study results provided valuable information for our institution. Although improvements in management of ASB were modest, the success of provider education and identification of other work areas and clinicians to target for future intervention were helpful in consideration of further studies. This work will also aid us in developing an expected effect size for future studies. We plan to provide ongoing education for IM providers as well as education in the ED to target providers who make first contact with patients admitted to inpatient services. In addition, the CPOE UC ordering screen message will continue to be used hospital-wide and will be expanded to the ED ordering system. Our interventions, experiences, and challenges may be used by other institutions to design effective antimicrobial stewardship interventions directed towards reducing rates of inappropriate ASB treatment.
Corresponding author: Prasanna P. Narayanan, PharmD, 200 First Street SW, Rochester, MN 55905; [email protected].
Financial disclosures: None.
From the Mayo Clinic, Rochester, MN.
Abstract
- Objective: Asymptomatic bacteriuria (ASB) denotes asymptomatic carriage of bacteria within the urinary tract and does not require treatment in most patient populations. Unnecessary antimicrobial treatment has several consequences, including promotion of antimicrobial resistance, potential for medication adverse effects, and risk for Clostridiodes difficile infection. The aim of this quality improvement effort was to decrease both the unnecessary ordering of urine culture studies and unnecessary treatment of ASB.
- Methods: This is a single-center study of patients who received care on 3 internal medicine units at a large, academic medical center. We sought to determine the impact of information technology and educational interventions to decrease both inappropriate urine culture ordering and treatment of ASB. Data from included patients were collected over 3 1-month time periods: baseline, post-information technology intervention, and post-educational intervention.
- Results: There was a reduction in the percentage of patients who received antibiotics for ASB in the post-education intervention period as compared to baseline (35% vs 42%). The proportion of total urine cultures ordered by internal medicine clinicians did not change after an information technology intervention to redesign the computerized physician order entry screen for urine cultures.
- Conclusion: Educational interventions are effective ways to reduce rates of inappropriate treatment of ASB in patients admitted to internal medicine services.
Keywords: asymptomatic bacteriuria, UTI, information technology, education, quality.
Asymptomatic bacteriuria (ASB) is a common condition in which bacteria are recovered from a urine culture (UC) in patients without symptoms suggestive of urinary tract infection (UTI), with no pathologic consequences to most patients who are not treated.1,2 Patients with ASB do not exhibit symptoms of a UTI such as dysuria, increased frequency of urination, increased urgency, suprapubic tenderness, or costovertebral pain. Treatment with antibiotics is not indicated for most patients with ASB.1,3 According to the Infectious Diseases Society of America (IDSA), screening for bacteriuria and treatment for positive results is only indicated during pregnancy and prior to urologic procedures with anticipated breach of the mucosal lining.1
An estimated 20% to 52% of patients in hospital settings receive inappropriate treatment with antibiotics for ASB.4 Unnecessary prescribing of antibiotics has several negative consequences, including increased rates of antibiotic resistance, Clostridioides difficile infection, and medication adverse events, as well as increased health care costs.2,5 Antimicrobial stewardship programs to improve judicious use of antimicrobials are paramount to reducing these consequences, and their importance is heightened with recent requirements for antimicrobial stewardship put forth by The Joint Commission and the Centers for Medicare & Medicaid Services.6,7
A previous review of UC and antimicrobial use in patients for purposes of quality improvement at our institution over a 2-month period showed that of 59 patients with positive UCs, 47 patients (80%) did not have documented symptoms of a UTI. Of these 47 patients with ASB, 29 (61.7%) received antimicrobial treatment unnecessarily (unpublished data). We convened a group of clinicians and nonclinicians representing the areas of infectious disease, pharmacy, microbiology, statistics, and hospital internal medicine (IM) to examine the unnecessary treatment of ASB in our institution. Our objective was to address 2 antimicrobial stewardship issues: inappropriate UC ordering and unnecessary use of antibiotics to treat ASB. Our aim was to reduce the inappropriate ordering of UCs and to reduce treatment of ASB.
Methods
Setting
The study was conducted on 3 IM nursing units with a total of 83 beds at a large tertiary care academic medical center in the midwestern United States, and was approved by the organization’s Institutional Review Board.
Participants
We included all non-pregnant patients aged 18 years or older who received care from an IM primary service. These patients were admitted directly to an IM team through the emergency department (ED) or transferred to an IM team after an initial stay in the intensive care unit.
Data Source
Microbiology laboratory reports generated from the electronic health record were used to identify all patients with a collected UC sample who received care from an IM service prior to discharge. Urine samples were collected by midstream catch or catheterization. Data on urine Gram stain and urine dipstick were not included. Henceforth, the phrase “urine culture order” indicates that a UC was both ordered and performed. Data reports were generated for the month of August 2016 to determine the baseline number of UCs ordered. Charts of patients with positive UCs were reviewed to determine if antibiotics were started for the positive UC and whether the patient had signs or symptoms consistent with a UTI. If antibiotics were started in the absence of signs or symptoms to support a UTI, the patient was determined to have been unnecessarily treated for ASB. Reports were then generated for the month after each intervention was implemented, with the same chart review undertaken for positive UCs. Bacteriuria was defined in our study as the presence of microbial growth greater than 10,000 CFU/mL in UC.
Interventions
Initial analysis by our study group determined that lack of electronic clinical decision support (CDS) at the point of care and provider knowledge gaps in interpreting positive UCs were the 2 main contributors to unnecessary UC orders and unnecessary treatment of positive UCs, respectively. We reviewed the work of other groups who reported interventions to decrease treatment of ASB, ranging from educational presentations to pocket cards and treatment algorithms.8-13 We hypothesized that there would be a decrease in UC orders with CDS embedded in the computerized order entry screen, and that we would decrease unnecessary treatment of positive UCs by educating clinicians on indications for appropriate antibiotic prescribing in the setting of a positive UC.
Information technology intervention. The first intervention implemented involved redesign of the UC ordering screen in the computerized physician order entry (CPOE) system. This intervention went live hospital-wide, including the IM floors, intensive care units, and all other areas except the ED, on February 1, 2017 (Figure 1). The ordering screen required the prescriber to select from a list of appropriate indications for ordering a UC, including urine frequency, urgency, or dysuria; unexplained suprapubic or flank pain; fever in patients without another recognized cause; screening obtained prior to urologic procedure; or screening during pregnancy. An additional message advised prescribers to avoid ordering the culture if the patient had malodorous or cloudy urine, pyuria without urinary symptoms, or had an alternative cause of fever. Before we implemented the information technology (IT) intervention, there had been no specific point-of-care guidance on UC ordering.

Educational intervention. The second intervention, driven by clinical pharmacists, involved active and passive education of prescribers specifically designed to address unnecessary treatment of ASB. The IT intervention with CDS for UC ordering remained live. Presentations designed by the study group summarizing the appropriate indications for ordering a UC, distinguishing ASB from UTI, and discouraging treatment of ASB were delivered via a variety of routes by clinical pharmacists to nurses, nurse practitioners, physician assistants, pharmacists, medical residents, and staff physicians providing care to patients on the 3 IM units over a 1-month period in March 2017. The presentations contained the same basic content, but the information was delivered to target each specific audience group.
Medical residents received a 10-minute live presentation during a conference. Nurse practitioners, physician assistants, and staff physicians received a presentation via email, and highlights of the presentation were delivered by clinical pharmacists at their respective monthly group meetings. A handout was presented to nursing staff at nursing huddles, and presentation slides were distributed by email. Educational posters were posted in the medical resident workrooms, nursing breakrooms, and staff bathrooms on the units.
Outcome Measurements
The endpoints of interest were the percentage of patients with positive UCs unnecessarily treated for ASB before and after each intervention and the number of UCs ordered at baseline and after implementation of each intervention. Counterbalance measures assessed included the incidence of UTI, pyelonephritis, or urosepsis within 7 days of positive UC for patients who did not receive antibiotic treatment for ASB.
Results
Data from a total of 270 cultures were examined from IM nursing units. A total of 117 UCs were ordered during the baseline period before interventions were implemented. For a period of 1 month following activation of the IT intervention, 73 UCs were ordered. For a period of 1 month following the educational interventions, 80 UCs were ordered. Of these, 61 (52%) UCs were positive at baseline, 37 (51%) after the IT intervention, and 41 (51%) after the educational intervention. Patient characteristics were similar between the 3 groups (Table); 64.7% of patients were female in their early to mid-seventies. The majority of UCs were ordered by providers in the ED in all 3 periods examined (51%-70%). The percentage of patients who received antibiotics prior to UC for another indication (including bacteriuria) in the baseline, post-IT intervention, and post-education intervention groups were 30%, 27%, and 45%, respectively.

The study outcomes are summarized in Figure 2. Among patients with positive cultures, there was not a reduction in inappropriate treatment of ASB compared to baseline after the IT intervention (48% vs 42%). Following the education intervention, there was a reduction in unnecessary ASB treatment as compared both to baseline (35% vs 42%) and to post-IT intervention (35% vs 48%). There was no difference between the 3 study periods in the percentage of total UCs ordered by IM clinicians. The counterbalance measure showed that 1 patient who did not receive antibiotics within 7 days of a positive UC developed pyelonephritis, UTI, or sepsis due to a UTI in each intervention group.

Discussion
The results of this study demonstrate the role of multimodal interventions in antimicrobial stewardship and add to the growing body of evidence supporting the work of antimicrobial stewardship programs. Our multidisciplinary study group and multipronged intervention follow recent guideline recommendations for antimicrobial stewardship program interventions against unnecessary treatment of ASB.14 Initial analysis by our study group determined lack of CDS at the point of care and provider knowledge gaps in interpreting positive UCs as the 2 main contributors to unnecessary UC orders and unnecessary treatment of positive UCs in our local practice culture. The IT component of our intervention was intended to provide CDS for ordering UCs, and the education component focused on informing clinicians’ treatment decisions for positive UCs.
It has been suggested that the type of stewardship intervention that is most effective fits the specific needs and resources of an institution.14,15 And although the IDSA does not recommend education as a stand-alone intervention,16 we found it to be an effective intervention for our clinicians in our work environment. However, since the CPOE guidance was in place during the educational study periods, it is possible that the effect was due to a combination of these 2 approaches. Our pre-intervention ASB treatment rates were consistent with a recent meta-analysis in which the rate of inappropriate treatment of ASB was 45%.17 This meta-analysis found educational and organizational interventions led to a mean absolute risk reduction of 33%. After the education intervention, we saw a 7% decrease in unnecessary treatment of ASB compared to baseline, and a 13% decrease compared to the month just prior to the educational intervention.
Lessons learned from our work included how clear review of local processes can inform quality improvement interventions. For instance, we initially hypothesized that IM clinicians would benefit from point-of-care CDS guidance, but such guidance used alone without educational interventions was not supported by the results. We also determined that the majority of UCs from patients on general medicine units were ordered by ED providers. This revealed an opportunity to implement similar interventions in the ED, as this was the initial point of contact for many of these patients.
As with any clinical intervention, the anticipated benefits should be weighed against potential harm. Using counterbalance measures, we found there was minimal risk in the occurrence of UTI, pyelonephritis, or sepsis if clinicians avoided treating ASB. This finding is consistent with IDSA guideline recommendations and other studies that suggest that withholding treatment for asymptomatic bacteriuria does not lead to worse outcomes.1
This study has several limitations. Data were obtained through review of the electronic health record and therefore documentation may be incomplete. Also, antimicrobials for empiric coverage or treatment for other infections (eg, pneumonia, sepsis) may have confounded our results, as empirical antimicrobials were given to 27% to 45% of patients prior to UC. This was a quality improvement project carried out over defined time intervals, and thus our sample size was limited and not adequately powered to show statistical significance. Additionally, given the bundling of interventions, it is difficult to determine the impact of each intervention independently. Although CDS for UC ordering may not have influenced ordering, it is possible that the IT intervention raised awareness of ASB and influenced treatment practices.
Conclusion
Our work supports the principles of antibiotic stewardship as brought forth by IDSA.16 This work was the effort of a multidisciplinary team, which aligns with recommendations by Daniel and colleagues, published after our study had ended, for reducing overtreatment of ASB.14 Additionally, our study results provided valuable information for our institution. Although improvements in management of ASB were modest, the success of provider education and identification of other work areas and clinicians to target for future intervention were helpful in consideration of further studies. This work will also aid us in developing an expected effect size for future studies. We plan to provide ongoing education for IM providers as well as education in the ED to target providers who make first contact with patients admitted to inpatient services. In addition, the CPOE UC ordering screen message will continue to be used hospital-wide and will be expanded to the ED ordering system. Our interventions, experiences, and challenges may be used by other institutions to design effective antimicrobial stewardship interventions directed towards reducing rates of inappropriate ASB treatment.
Corresponding author: Prasanna P. Narayanan, PharmD, 200 First Street SW, Rochester, MN 55905; [email protected].
Financial disclosures: None.
1. Nicolle LE, Gupta K, Bradley SF, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68:e83–75.
2. Trautner BW, Grigoryan L, Petersen NJ, et al. Effectiveness of an antimicrobial stewardship approach for urinary catheter-associated asymptomatic bacteriuria. JAMA Intern Med. 2015;175:1120-1127.
3. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309-332.
4. Trautner BW. Asymptomatic bacteriuria: when the treatment is worse than the disease. Nat Rev Urol. 2011;9:85-93.
5. Costelloe C, Metcalfe C, Lovering A, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096.
6. The Joint Commission. Prepublication Requirements: New antimicrobial stewardship standard. Jun 22, 2016. www.jointcommission.org/assets/1/6/HAP-CAH_Antimicrobial_Prepub.pdf. Accessed January 24, 2019.
7. Federal Register. Medicare and Medicaid Programs; Hospital and Critical Access Hospital (CAH) Changes to Promote Innovation, Flexibility, and Improvement in Patient Care.Centers for Medicare & Medicaid Services. June 16, 2016. CMS-3295-P
8. Hartley SE, Kuhn L, Valley S, et al. Evaluating a hospitalist-based intervention to decrease unnecessary antimicrobial use in patients with asymptomatic bacteriuria. Infect Control Hosp Epidemiol. 2016;37:1044-1051.
9. Pavese P, Saurel N, Labarere J, et al. Does an educational session with an infectious diseases physician reduce the use of inappropriate antibiotic therapy for inpatients with positive urine culture results? A controlled before-and-after study. Infect Control Hosp Epidemiol. 2009;30:596-599.
10. Kelley D, Aaronson P, Poon E, et al. Evaluation of an antimicrobial stewardship approach to minimize overuse of antibiotics in patients with asymptomatic bacteriuria. Infect Control Hosp Epidemiol. 2014;35:193-195.
11. Chowdhury F, Sarkar K, Branche A, et al. Preventing the inappropriate treatment of asymptomatic bacteriuria at a community teaching hospital. J Community Hosp Intern Med Perspect. 2012;2.
12. Bonnal C, Baune B, Mion M, et al. Bacteriuria in a geriatric hospital: impact of an antibiotic improvement program. J Am Med Dir Assoc. 2008;9:605-609.
13. Linares LA, Thornton DJ, Strymish J, et al. Electronic memorandum decreases unnecessary antimicrobial use for asymptomatic bacteriuria and culture-negative pyuria. Infect Control Hosp Epidemiol. 2011;32:644-648.
14. Daniel M, Keller S, Mozafarihashjin M, et al. An implementation guide to reducing overtreatment of asymptomatic bacteriuria. JAMA Intern Med. 2018;178:271-276.
15. Redwood R, Knobloch MJ, Pellegrini DC, et al. Reducing unnecessary culturing: a systems approach to evaluating urine culture ordering and collection practices among nurses in two acute care settings. Antimicrob Resist Infect Control. 2018;7:4.
16. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62:e51–e7.
17. Flokas ME, Andreatos N, Alevizakos M, et al. Inappropriate management of asymptomatic patients with positive urine cultures: a systematic review and meta-analysis. Open Forum Infect Dis. 2017;4:1-10.
1. Nicolle LE, Gupta K, Bradley SF, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68:e83–75.
2. Trautner BW, Grigoryan L, Petersen NJ, et al. Effectiveness of an antimicrobial stewardship approach for urinary catheter-associated asymptomatic bacteriuria. JAMA Intern Med. 2015;175:1120-1127.
3. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309-332.
4. Trautner BW. Asymptomatic bacteriuria: when the treatment is worse than the disease. Nat Rev Urol. 2011;9:85-93.
5. Costelloe C, Metcalfe C, Lovering A, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096.
6. The Joint Commission. Prepublication Requirements: New antimicrobial stewardship standard. Jun 22, 2016. www.jointcommission.org/assets/1/6/HAP-CAH_Antimicrobial_Prepub.pdf. Accessed January 24, 2019.
7. Federal Register. Medicare and Medicaid Programs; Hospital and Critical Access Hospital (CAH) Changes to Promote Innovation, Flexibility, and Improvement in Patient Care.Centers for Medicare & Medicaid Services. June 16, 2016. CMS-3295-P
8. Hartley SE, Kuhn L, Valley S, et al. Evaluating a hospitalist-based intervention to decrease unnecessary antimicrobial use in patients with asymptomatic bacteriuria. Infect Control Hosp Epidemiol. 2016;37:1044-1051.
9. Pavese P, Saurel N, Labarere J, et al. Does an educational session with an infectious diseases physician reduce the use of inappropriate antibiotic therapy for inpatients with positive urine culture results? A controlled before-and-after study. Infect Control Hosp Epidemiol. 2009;30:596-599.
10. Kelley D, Aaronson P, Poon E, et al. Evaluation of an antimicrobial stewardship approach to minimize overuse of antibiotics in patients with asymptomatic bacteriuria. Infect Control Hosp Epidemiol. 2014;35:193-195.
11. Chowdhury F, Sarkar K, Branche A, et al. Preventing the inappropriate treatment of asymptomatic bacteriuria at a community teaching hospital. J Community Hosp Intern Med Perspect. 2012;2.
12. Bonnal C, Baune B, Mion M, et al. Bacteriuria in a geriatric hospital: impact of an antibiotic improvement program. J Am Med Dir Assoc. 2008;9:605-609.
13. Linares LA, Thornton DJ, Strymish J, et al. Electronic memorandum decreases unnecessary antimicrobial use for asymptomatic bacteriuria and culture-negative pyuria. Infect Control Hosp Epidemiol. 2011;32:644-648.
14. Daniel M, Keller S, Mozafarihashjin M, et al. An implementation guide to reducing overtreatment of asymptomatic bacteriuria. JAMA Intern Med. 2018;178:271-276.
15. Redwood R, Knobloch MJ, Pellegrini DC, et al. Reducing unnecessary culturing: a systems approach to evaluating urine culture ordering and collection practices among nurses in two acute care settings. Antimicrob Resist Infect Control. 2018;7:4.
16. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62:e51–e7.
17. Flokas ME, Andreatos N, Alevizakos M, et al. Inappropriate management of asymptomatic patients with positive urine cultures: a systematic review and meta-analysis. Open Forum Infect Dis. 2017;4:1-10.
Elevating Critical Care Pharmacy Services in a Resource-Limited Environment Through Establishment of a Pharmacist Team
From Robert Wood Johnson University Hospital Hamilton, Hamilton, NJ.
Abstract
- Background: Critical care pharmacy services are often provided by clinical specialists during limited hours and, otherwise, by general practice pharmacists, leading to varied level, expertise, and multidisciplinary expectations of these services.
- Objective: Since no published descriptions of successful models sustaining routine, high-quality critical care pharmacy services in a community-based, resource-limited environment exist, a critical care pharmacist team (CCPT) was created to meet this goal. After successful launch, the initiative’s primary goal was to assess whether team formation indeed standardized and increased the level of pharmacy services routinely provided. The secondary goal was to demonstrate cultural acceptance, and thus sustainability, of the model.
- Methods: A CCPT was formed from existing pharmacist resources. A longitudinal educational plan, including classroom, bedside, and practice modeling, assured consistent skills, knowledge, and confidence. Interventions performed by pharmacists before and after implementation were assessed to determine whether the model standardized type and level of service. Surveys of the CCPT and multidisciplinary teams assessed perceptions of expertise, confidence, and value as surrogates for model success and sustainability.
- Results: Interventions after CCPT formation reflected elevated and standardized critical care pharmacy services that advanced the multidisciplinary team’s perception of the pharmacist as an integral, essential team member. CCPT members felt empowered, as reflected by self-directed enrollment in PharmD programs and/or obtaining board certification. This success subsequently served to improve the culture of cooperation and spark similar evolution of other disciplines.
- Conclusion: The standardization and optimization of pharmacy services through a dedicated CCPT improved continuity of care and standardized multidisciplinary team expectations.
Keywords: critical care; clinical pharmacist; pharmaceutical care; standards of practice.
There has been significant evolution in the role, training, and overall understanding of the impact of critical care pharmacists over the past 2 decades. The specialized knowledge and role of pharmacists make them essential links in the provision of quality critical care services.1 The Society of Critical Care Medicine (SCCM) and the American College of Clinical Pharmacy (ACCP) have defined the level of clinical practice and specialized skills that characterize the critical care pharmacist and have made recommendations regarding both the personnel requirements for the provision of pharmaceutical care to critically ill patients and the fundamental, desirable, and optimal pharmacy services that should be provided to these patients (Table 1).2 Despite this, only two-thirds of US intensive care units (ICUs) have clinical pharmacists/specialists (defined as spending at least 50% of their time providing clinical services), resulting in fundamental activities dominating routine pharmacist services.3 The clinical nature of most desirable and optimal activities, such as code response and pharmacist-driven protocol management, is limited, but these activities correlate with decreases in mortality across hospitalized populations.4
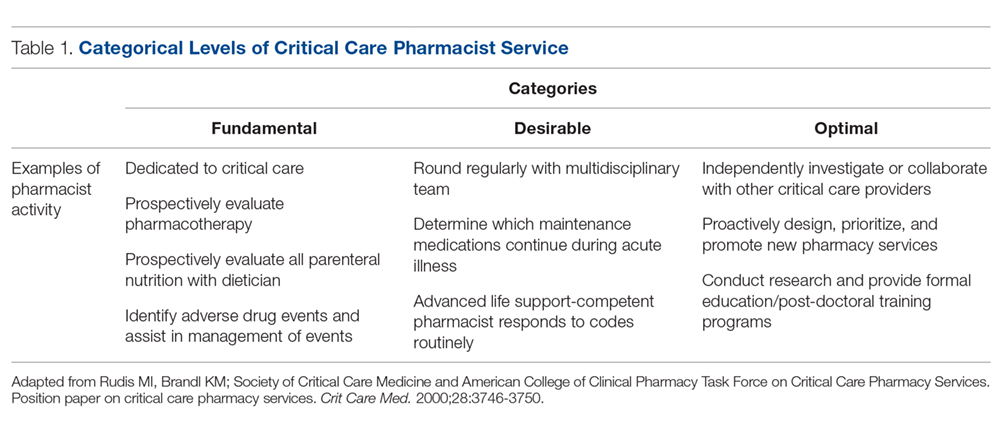
Despite their demonstrated benefit and recognized role, critical care pharmacists remain a limited resource with limited physical presence in ICUs.5 This presents hospital pharmacies with a real dilemma: given that clinical pharmacy specialists are often a limited resource, what services (fundamental, desirable, or optimal) should be provided by which pharmacists over what hours and on which days? For many hospitals, personnel resources allow for a clinical pharmacy specialist (either trained or with significant experience in critical care) to participate in multidisciplinary rounds, but do not allow a specialist to be present 7 days per week across all times of the day. As a result, routine services may be inconsistent and limited to activities that are fundamental-to-desirable, due to the varied educational and training backgrounds of pharmacists providing nonrounding services. Where gaps have been identified, remote (tele-health) provision of targeted ICU pharmacist services are beneficial.5
In our organization, we recognized the significant variation created by this resource-defined model and sought to develop a process to move closer to published best practice standards for quality services2 through the creation of a formalized critical care pharmacist team (CCPT). This change was spurred by the transition of our organization’s clinical pharmacist to a board-certified, faculty-based specialist, which in turn spurred new focus on standardizing both the type and quality of services provided by the entire pharmacy team, targeting a higher, more consistent level of pharmacist care which better aligned with SCCM/ACCP-defined activities associated with quality services. The specialist proposed the formation of a CCPT, a process that involved targeted, intensive education and clinical skills development of a narrow pharmacist audience; administration approved this plan, provided that the CCPT arose from existing resources. This realignment focused on ensuring continuity of services across pharmacist roles (ie, rounding vs satellite) as well as across times (both days of the week and shifts). This report describes the methods used to recruit, train, and sustain a CCPT; the resulting changes observed in levels of pharmacy services after CCPT implementation; and the impressions of the CCPT members and the multidisciplinary team (physicians, nurses, dieticians, respiratory therapists, chaplains, and social workers in addition to the pharmacist), as cultural integration and perceived value are essential for sustainability and growth of the model.
Methods
Setting
Robert Wood Johnson University Hospital Hamilton is a 248-bed suburban community hospital in New Jersey with a 20-bed ICU that provides level II6 critical care services as part of an 11-hospital system. Critical care pharmacy services spanned from fundamental (eg, order review) to optimal (eg, independent pharmacotherapy evaluation) activities, with tremendous variability associated with who was engaged in care. In this original model, weekday ICU pharmacy services were provided by satellite-based general practice staff pharmacists (satellite pharmacy located in the ICU provides services for ICU, telemetry, and the emergency department) across 2 shifts (0700-2300; 9 pharmacists during the day shift and 2 on the evening shift). Satellite pharmacists largely focused on traditional/fundamental pharmacy practice, including order review, drug therapy evaluation, and adverse drug event identification. Additionally, a hospital-based, residency-trained clinical pharmacist rounded 3 days per week. General practice staff pharmacists provided weekend and overnight services. Very limited, prospective, independent clinical evaluation or individualized pharmacotherapy optimization occurred routinely. No established clinical assessment priorities or strategies existed, and thus expectations of pharmacy services were associated with the individual pharmacist present.
Team Structure and Recruitment
The staff pharmacists were well-established, with each having 25 to 41 years of practice experience. All 11 full-time staff pharmacists graduated with Bachelor of Science degrees in pharmacy, and 5 of them had returned to acquire Doctor of Pharmacy degrees prior to the initiative. None had completed post-doctoral training residencies, as residencies were not the standard when these pharmacists entered practice. The staffing model necessitated that pharmacists maintain Basic Life Support (BLS) and Advanced Cardiac Life Support (ACLS) competency as members of inpatient emergency response teams.
Three volunteers were recruited to the initial transformational process. These volunteer pharmacists were preferentially assigned to the ICU, with a clinically focused weekend rotation, to provide 7-day/week rounding continuity, but maintained general competencies and cross-functionality. Weekend responsibilities included critical care assessments and multidisciplinary rounding, inpatient emergency response, patient education/medication histories, and inpatient warfarin management consultations.
Team Training and Development
Longitudinal education of the CCPT included classroom, bedside, and practice-modeling training strategies to complement routine exposure and integration into the pharmacist’s practice in providing direct patient care. Concentrated learning occurred over a 3-month period, with extended bedside and patient-case-based learning continuing for another 3 months. Expectations of the critical care pharmacist as an independent consultant to the interdisciplinary team targeting holistic pharmacotherapy optimization were established, instilling independence and accountability within the role. Next, lecture and bedside training targeted the development of crucial assessment skills, including an understanding of device and equipment implications on pharmacotherapy decisions, pharmacokinetic and pharmacodynamic variations in critically ill patients, and supportive care. A minimum of 5 hours of group lectures were included for all members of the CCPT, with additional instruction provided based on individual needs. Lectures explored the evidence and practice associated with common diagnoses, including review of related literature, core guidelines, and institutional order sets. Fundamental topics included pain, agitation, and delirium (PAD) during mechanical ventilation, infectious diseases, and hemodynamic management.
To reinforce knowledge, build bedside assessment skills, and increase confidence, pharmacists routinely partnered with the specialist during independent morning bedside evaluations and rounds. Over time, the specialist role became increasingly supportive as the critical care pharmacist grew into the primary role. On weekends the specialist was not present but remained on call to discuss cases with the rounding critical care pharmacist. This served to reinforce clinical decision-making and expand knowledge; these patient-specific lessons were communicated with the team to support continued development and standardization.
In addition to these internal efforts, the specialist simultaneously recalibrated expectations among key ICU stakeholders, establishing uniform quality and scope of service from the CCPT. Historically, physicians and nurses sought input from specific pharmacists, and thus a cultural change regarding the perceived value of the team was required. To reinforce this, those demanding a specific pharmacist were referred to the CCPT member present.
The initial training process involved a significant proportion of the specialist’s time. Initially focused on classroom lecture and core skills development, time increasingly focused on individual learner’s needs and learning styles. Mentoring and partnering were key during this period. In the first 6 months, weekend calls were routine, but these quickly tapered as the team gained experience and confidence in their knowledge and skills.
Tools and Team Support
Beyond standardizing knowledge and skills, team effectiveness depended on establishing routine assessment criteria (Table 2), communication tools, and references. Rounding and sign-out processes were standardized to support continuity of care. A patient census report generated by the clinical computer system was used as the daily worksheet and was stored on a sign-out clipboard to readily communicate clinically pertinent history, assessments, recommendations, and pending follow-up. The report included patient demographics, admitting diagnosis, and a list of consulting physicians. The pharmacist routinely recorded daily bedside observations, his/her independent assessments (topics outlined in Table 2), pertinent history, events, and goals established on rounds. Verbal sign-out occurred twice daily (during weekdays)—from the rounding to satellite pharmacist after rounds (unless 1 person fulfilled both roles) and between day and evening shifts. Additionally, a resource binder provided rapid accessibility to key information (eg, published evidence, tools, institutional protocols), with select references residing on the sign-out clipboard for immediate access during rounding.
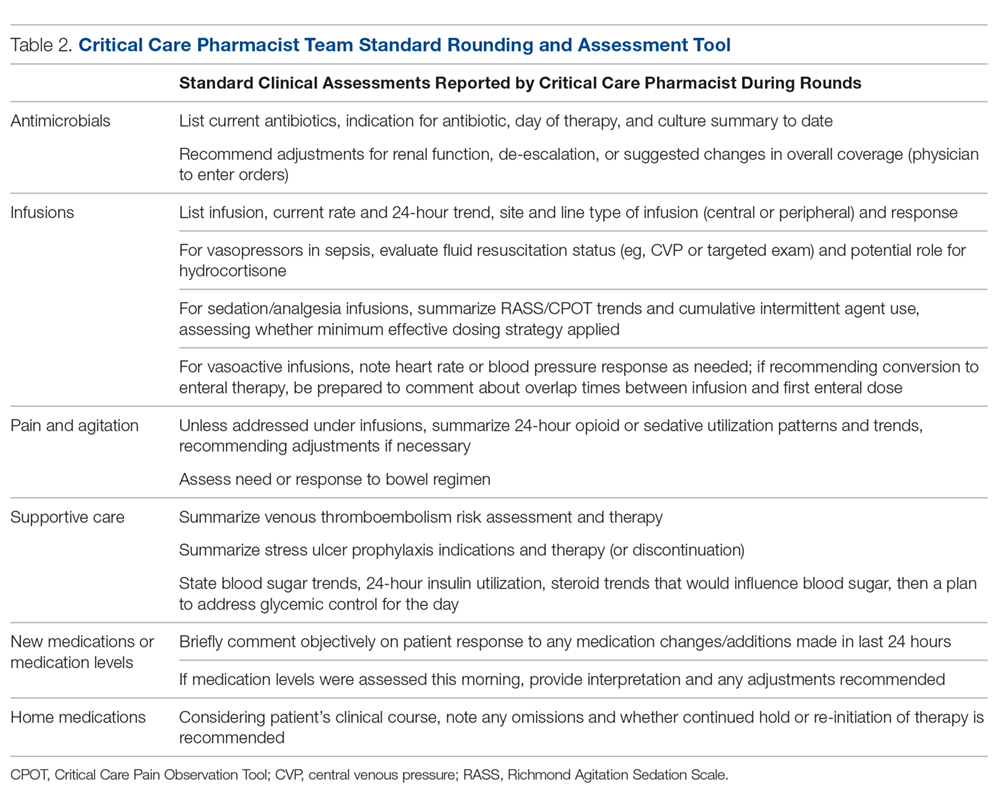
Monthly meetings were established to promote full engagement of the team, demonstrate ownership, and provide opportunity for discussion and information sharing. Meetings covered operational updates, strategic development of the service, educational topics, and discussions of difficult cases.
Assessment
While not directly studied, existing evidence suggests that appropriately trained critical care pharmacists should be able to perform a broad range of services, from fundamental to optimal.7 To evaluate if CCPT training elevated and standardized the type of interventions routinely made, services provided prior to the team’s formation were compared to those provided after formation through interrogation of the institution’s surveillance system. As a baseline, a comparison of the types of ICU interventions documented by the specialist during a 2-month period prior to the team’s formation were compared to the interventions documented by the staff pharmacists who became part of the CCPT. Since standardization of skills and practice were goals of the CCPT formation, the same comparison was conducted after team formation to assess whether the intervention types normalized across roles, reflecting a consistent level of service.
As assignment to the CCPT is voluntary, with no additional compensation or tangible benefits, the success of the CCPT relies on active pharmacist engagement and ongoing commitment. Thus, a personal belief that their commitment was valuable and increased professional satisfaction was key to sustain change. An online, voluntary, anonymous survey was conducted to assess the CCPT member’s perceptions of their preparedness, development of skills and comfort level, and acceptance by the multidisciplinary team, as these elements would influence members’ beliefs regarding the impact and value of the team and their justification for commitment to continuous, uncompensated learning and training. Their thoughts on professional satisfaction and development were collected as a surrogate for the model’s sustainability.
Success and sustainability also depend on the multidisciplinary team’s acceptance and perceived value of the CCPT, especially given its evolution from a model in which clinical feedback was sought and accepted exclusively from the specialist. To evaluate these components, an online, voluntary, anonymous survey of the multidisciplinary members was conducted.
Results
CCPT Interventions and Level of Service
Prior to CCPT formation, intervention categories documented by the specialist differed from those of the staff (Figure 1). The staff’s baseline interventions represented those arising from the established, routine assessments performed by all pharmacists for all inpatients, such as renal dose assessments. The specialist’s interventions largely focused on independent pharmacotherapy assessments and optimization strategies. After team formation, intervention type became increasingly consistent across the CCPT, with all members aligning with the specialist’s interventions. Intervention categories reflected the clinically focused, independent assessments targeted during training (eg, supportive care and pain/sedation assessment), expanding beyond the routine assessments performed across the general hospitalized population.
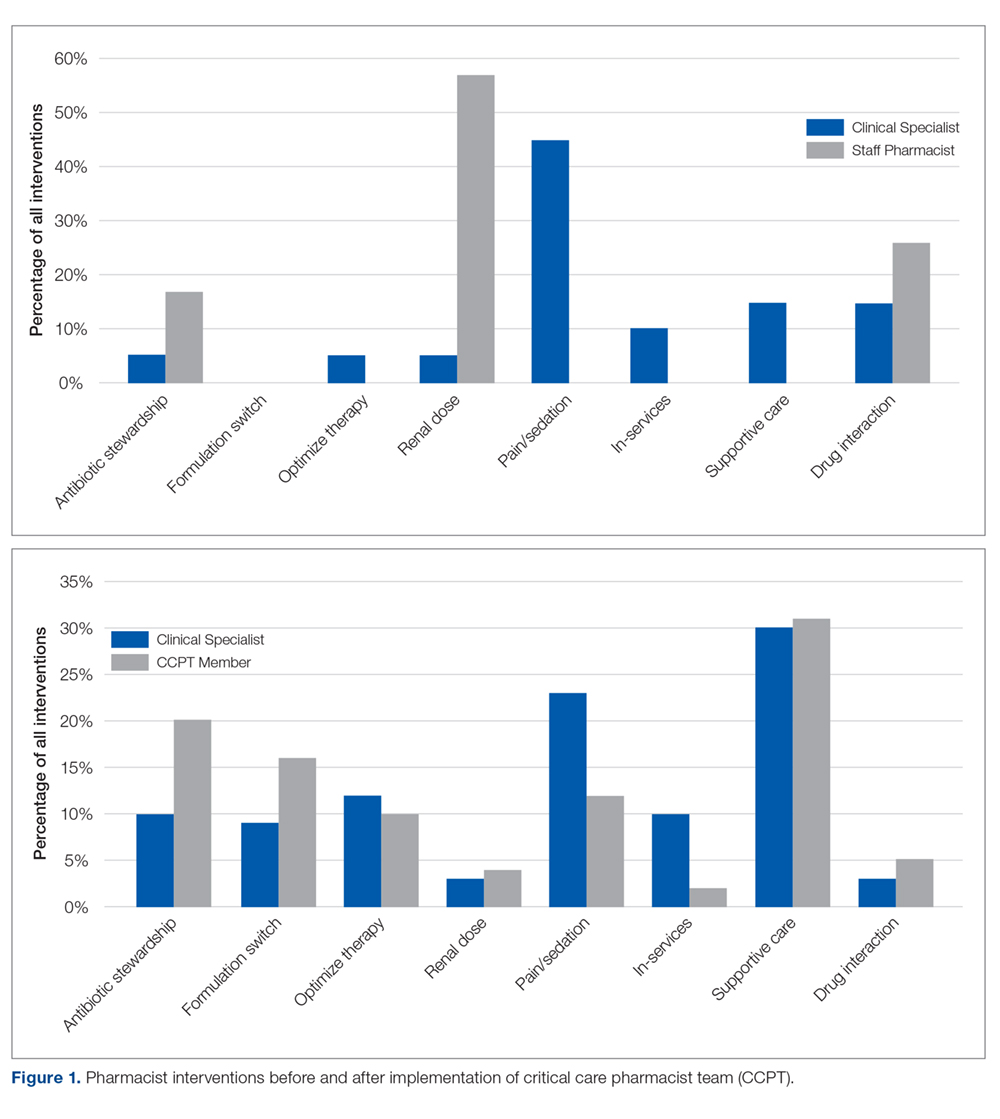
When compared to SCCM/ACCP ideals, these interventions corresponded with an expansion from routinely fundamental to routinely broad (ie, fundamental, desirable, and optimal) critical care pharmacist activities, thus elevating the overall quality of services provided by the team while assuring continuity. Desirable activities adopted by the CCPT included multidisciplinary rounding on all ICU patients; drug history review for appropriate management during acute illness; and training of students and providing educational in-services. Optimal activities routinely integrated included independent and/or collaborative investigation of ICU guidelines/protocol impact and scholarship in peer-reviewed publications. Prior to CCPT formation, staff involvement of desirable activities was limited to resuscitation event response and clarification of effective dosage regimens, with no involvement in optimal activities.
CCPT Impressions
The online, voluntary, anonymous survey was completed by 5 of the 6 staff members (the 3 original members plus 3 staff members who were added several months into the program to enhance continuity and cross-shift coverage) comprising the team. Using a 5-point Likert scale, members ranked their comfort level with their critical care knowledge, bedside skills, ability to actively participate in rounds, and ability to address controversial clinical issues in their staffing role prior to team formation (ie, baseline) compared to their current CCPT practice. Overall, self-assessments reflected perceived increases across all categories. Prior to CCPT training and implementation, all team members were “not at all,” “slightly comfortable,” or “somewhat comfortable” with these points, while after training and implementation all reported being “comfortable” or “very comfortable” with the same points. All members reported feeling better prepared and confident in caring for critically ill patients and felt that the team and its standardized approach enhanced medication safety. When asked about their impressions of the perceived value of the CCPT by interdisciplinary peers, pharmacists felt it was perceived as bringing “a lot” or “a great deal” of value. Additionally, all members uniformly felt that the team supported their professional growth and enhanced their professional satisfaction.
Multidisciplinary Impressions of Service and Value
A total of 29 (90%) multidisciplinary team members completed the online, voluntary, anonymous survey of their impressions of the CCPT’s service and impact. Surveys represented the impressions of critical care physicians, the unit’s nursing leadership (administrative and clinical), nursing education, staff nurses, social work, and pastoral care. Using a 5-point Likert scale, all respondents reported that they “agreed” or “entirely agreed” that the CCPT enhanced care. Specifically, they reported that pharmacists were more visible and engaged, and provided more consistent and reliable care regardless of which member was present. Services were seen as more robust and seamless, meeting interdisciplinary needs. The CCPT was viewed as a cohesive, efficient group. Respondents felt that the CCPT’s presence and engagement on weekends enhanced continuity of pharmaceutical care. As a result, the CCPT was seen as enhancing interdisciplinary understanding of the pharmacist’s value in critical care.
Discussion
Realignment and development of existing personnel resources allowed our organization to assure greater continuity, consistency, and quality of pharmacy care in the critical care setting (Figure 2). By standardizing expectations and broadening multidisciplinary understanding of the CCPT’s unique value, the pharmacist’s role was solidified and became an integral, active part of routine patient bedside care.
Prior to forming the CCPT, the physical presence of the pharmacist, as well as the services provided, were inconsistent. While a general practice pharmacist was in the satellite pharmacy within the ICU for up to 2 shifts on weekdays, pharmacists largely focused on traditional functions associated with order review and drug dispensing or established hospital-wide programs such as renal dosing or intravenous-to-oral formulation switches. The pharmacist remained in the satellite, not visible on rounds or at the bedside. In fact, there was a clear lack of comfort, frequently articulated by the pharmacists, with clinical questions that required bedside assessment, leading to routine escalation to the clinical specialist, who was not always readily available. This dynamic set an expectation for the multidisciplinary team that there were segregated pharmacy services—the satellite provided order review and product and the clinical specialist, in the limited hours present, provided clinical consultation and education. The formation of the CCPT abolished this tiered level of expectations, establishing a physical and clinical presence of a critical care pharmacist with equal capability and comfort. Both the pharmacist and multidisciplinary members perceived enhancements and value associated with the standardization and consistency provided by implementing the CCPT. Intervention data from before and after team formation support that routine interventions in critical care normalized the care provided and increased the robustness of critical care pharmacy services, with a strong shift to both clinical and academic activities considered desirable to optimal by SCCM/ACCP standards.
The benefit of pharmacist presence in the ICU is well described, with studies showing that the presence of a pharmacist is associated with medication error prevention and adverse drug event identification.8-10 However, this body of evidence applies no standardized definition regarding critical care pharmacist qualifications, with many studies pre-dating the wider availability of post-doctoral training programs and national board certification for critical care pharmacists.11 Training and certification structures have evolved with increased recognition of the specialization required to optimize the pharmacist’s role in providing quality care, albeit at a slower pace than published standards.1,2 In 2018, 136 organizations offered America Society of Health-System Pharmacists–accredited critical care pharmacy residencies.12 National recognition of expertise as a critical care pharmacist was established by the Board of Pharmacy Specialists in 2015, with more than 1600 pharmacists currently recognized.12 Our project is the only known description of a pharmacist practice model that increases critical care pharmacist availability through the application of standardized criteria incorporating these updated qualifications, thus ensuring expertise and experience that correlates with practice quality and consistency.
Despite the advancements achieved through this project, several limitations exist. First, while this model largely normalized services over the day and evening shifts, our night shift continues to be covered by 1 general practice pharmacist. More recently, resource reallocation mandated reduction in satellite hours, although that CCPT member remains available from the main pharmacy. The specialist remains on call to support the general practice pharmacists, but in-house expertise cannot be made available in the absence of additional resources. To optimize existing staffing, the specialist begins clinical evaluations during the early morning, overlapping with the night-shift prior to the satellite pharmacist’s arrival. This both provides some pharmacist presence at the bedside for night shift nurses and extends the hours during which a critical care pharmacist is physically available. Second, while all efforts are made to stagger time off, unavoidable gaps in critical care pharmacist coverage occur; expansion of the original team from 3 to 6 members has greatly reduced the likelihood of such gaps. Last, the program was designed to achieve routine integration of activities shown in the literature as being associated with quality, and those activities were assessed as a surrogate for quality.
Informal input, confirmed through survey data, from various disciplines on our team has consistently supported that the establishment of the CCPT has met a need by both standardizing critical care pharmacy practice and optimizing the pharmacist role within the team. While we recognize the limitations associated with the size of these surveys, they represent large proportions of our team and reflect key elements known to be important in sustaining long-term cultural change—a belief that what one is doing is both justified and valuable. This success has been a catalyst for several ongoing projects, fostering the development and adoption of critical care pharmacist protocols to allow more autonomous practice within our scope. Team development and movement toward robust protocol management has sparked a cultural evolution across disciplines as we strive to achieve the SCCM description of a highly effective team2,13 that emphasizes each discipline practicing fully within its scope in a horizontal team structure. Thus, the ICU medical director has used the success of the CCPT structure as an example to support optimization and development of the practice by other disciplines within the team. This has led to a significant revision in our rounding structure and interdisciplinary care model.14
The survey of CCPT members revealed that the model both engaged and stimulated the pharmacists involved, reflective of the autonomy and accountability required for sustainable, transformational cultural change. Within a year of entering the CCPT, 2 of the 3 pharmacists initially engaged had earned their board certification in pharmacotherapy (ie, BCPS) and the other, who had not acquired her Doctor of Pharmacy degree prior to the CCPT initiative, enrolled in a program to do so. The pharmacists expressed that they obtained BCPS over the newly available critical care certification because of the expectation that they maintain expertise across patient populations. This level of self-driven motivation in the absence of compensation reflects the value and professional satisfaction gained from being voluntary members of the CCPT.
Conclusion
Critical care pharmacy practice has continued to evolve to include increasingly specialized training for newer graduates and, more recently, the availability of critical care pharmacist board certification. While it is optimal to apply these standards when filling open critical care pharmacist positions, many hospitals require existing staff to fulfill multiple roles across various patient populations, leading to a variation in educational, training, and practice backgrounds for pharmacists currently practicing in the ICU. To minimize the variation associated with this resource-limited structure in a manner that standardized and elevated the type and level of service provided, we created a CCPT with existing pharmacists who were willing to accept intensive training and demonstrate an ongoing commitment to maintain defined competencies and skills. Our goal was to solidify the essential role of the critical care pharmacist in providing quality critical care services as described in the literature. The CCPT was well-received by the multidisciplinary team and served as an example for other disciplines that had similar struggles. The team’s success expanded into several other ongoing initiatives, including critical care pharmacist–driven protocols.
Acknowledgment: The authors thank Nina Roberts, MSN, RN, CCRN, NEA-BC, and Carol Ash, DO, MBA, MHCDS, the ICU Nursing and Medical Directors, respectively, at the time of this program’s initiation, for supporting the development of the critical care pharmacist team initiative and review of this manuscript.
Corresponding author: Liza Barbarello Andrews, PharmD, BCCCP, BCPS, Ernest Mario School of Pharmacy, Rutgers, The State University of New Jersey, 160 Frelinghuysen Road, Piscataway, NJ 08854; [email protected].
Financial disclosures: None.
1. Brilli RJ, Spevetz A, Branson RD, et al. American College of Critical Care Medicine Task Force on Models of Critical Care Delivery. Critical care delivery in the intensive care unit: defining clinical roles and the best practice model. Crit Care Med. 2001;29:2007-2019.
2. Rudis MI, Brandl KM; Society of Critical Care Medicine and American College of Clinical Pharmacy Task Force on Critical Care Pharmacy Services. Position paper on critical care pharmacy services. Crit Care Med. 2000;28:3746-3750.
3. MacLaren R, Devlin JW, Martin SJ, et al. Critical care pharmacy services in United States hospitals. Ann Pharmacother. 2006;40:612-618.
4. Bond CA, Raehl CL. Clinical pharmacy services, pharmacy staffing, and hospital mortality rates. Pharmacotherapy. 2007;27:481-493.
5. Forni A, Skahan N, Hartman CA, et al. Evaluation of the impact of a tele-ICU pharmacist on the management of sedation in critically ill mechanically ventilated patients. Ann Pharmacother. 2010;44:432-438.
6. Haupt MT, Bekes CE, Brilli RJ, et al. Guidelines on critical care services and personnel: recommendations based on a system of categorization on three levels of care. Crit Care Med. 2003;31:2677-2683.
7. Board of Pharmacy Specialties. Critical Care Pharmacy. www.bpsweb.org/bps-specialties/critical-care-pharmacy/.
8. Montazeri M, Cook DJ. Impact of a clinical pharmacist in a multidisciplinary intensive care unit. Crit Care Med. 1994;22:1044-1048.
9. Leape L, Cullen D, Clapp M, et al. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit. JAMA. 1999;282:267-270.
10. Horn E, Jacobi J. The critical care pharmacist: evolution of an essential team member. Crit Care Med. 2006;34(suppl):S46-S51.
11. Jacobi J. Measuring the impact of a pharmacist in the intensive care unit—are all pharmacists created equal? J Crit Care. 2015;30:1127-1128.
12. American Society of HealthSystem Pharmacists. Online residency directory. https://accred.ashp.org/aps/pages/directory/residencyProgramSearch.aspx. Accessed June 26, 2019.
13. Weled BJ, Adzhigirey LA, Hodgman TM, et al. Critical care delivery: the importance of process of care and ICU structure to improved outcomes: an update from the American College of Critical Care Medicine Task Force on Models of Critical Care. Crit Care Med. 2015;43:1520-1525.
14. Andrews LB, Roberts N, Ash C, et al. The LOTUS: a journey to value-based, patient-centered care. Creat Nurs. 2019;25:17-24.
From Robert Wood Johnson University Hospital Hamilton, Hamilton, NJ.
Abstract
- Background: Critical care pharmacy services are often provided by clinical specialists during limited hours and, otherwise, by general practice pharmacists, leading to varied level, expertise, and multidisciplinary expectations of these services.
- Objective: Since no published descriptions of successful models sustaining routine, high-quality critical care pharmacy services in a community-based, resource-limited environment exist, a critical care pharmacist team (CCPT) was created to meet this goal. After successful launch, the initiative’s primary goal was to assess whether team formation indeed standardized and increased the level of pharmacy services routinely provided. The secondary goal was to demonstrate cultural acceptance, and thus sustainability, of the model.
- Methods: A CCPT was formed from existing pharmacist resources. A longitudinal educational plan, including classroom, bedside, and practice modeling, assured consistent skills, knowledge, and confidence. Interventions performed by pharmacists before and after implementation were assessed to determine whether the model standardized type and level of service. Surveys of the CCPT and multidisciplinary teams assessed perceptions of expertise, confidence, and value as surrogates for model success and sustainability.
- Results: Interventions after CCPT formation reflected elevated and standardized critical care pharmacy services that advanced the multidisciplinary team’s perception of the pharmacist as an integral, essential team member. CCPT members felt empowered, as reflected by self-directed enrollment in PharmD programs and/or obtaining board certification. This success subsequently served to improve the culture of cooperation and spark similar evolution of other disciplines.
- Conclusion: The standardization and optimization of pharmacy services through a dedicated CCPT improved continuity of care and standardized multidisciplinary team expectations.
Keywords: critical care; clinical pharmacist; pharmaceutical care; standards of practice.
There has been significant evolution in the role, training, and overall understanding of the impact of critical care pharmacists over the past 2 decades. The specialized knowledge and role of pharmacists make them essential links in the provision of quality critical care services.1 The Society of Critical Care Medicine (SCCM) and the American College of Clinical Pharmacy (ACCP) have defined the level of clinical practice and specialized skills that characterize the critical care pharmacist and have made recommendations regarding both the personnel requirements for the provision of pharmaceutical care to critically ill patients and the fundamental, desirable, and optimal pharmacy services that should be provided to these patients (Table 1).2 Despite this, only two-thirds of US intensive care units (ICUs) have clinical pharmacists/specialists (defined as spending at least 50% of their time providing clinical services), resulting in fundamental activities dominating routine pharmacist services.3 The clinical nature of most desirable and optimal activities, such as code response and pharmacist-driven protocol management, is limited, but these activities correlate with decreases in mortality across hospitalized populations.4

Despite their demonstrated benefit and recognized role, critical care pharmacists remain a limited resource with limited physical presence in ICUs.5 This presents hospital pharmacies with a real dilemma: given that clinical pharmacy specialists are often a limited resource, what services (fundamental, desirable, or optimal) should be provided by which pharmacists over what hours and on which days? For many hospitals, personnel resources allow for a clinical pharmacy specialist (either trained or with significant experience in critical care) to participate in multidisciplinary rounds, but do not allow a specialist to be present 7 days per week across all times of the day. As a result, routine services may be inconsistent and limited to activities that are fundamental-to-desirable, due to the varied educational and training backgrounds of pharmacists providing nonrounding services. Where gaps have been identified, remote (tele-health) provision of targeted ICU pharmacist services are beneficial.5
In our organization, we recognized the significant variation created by this resource-defined model and sought to develop a process to move closer to published best practice standards for quality services2 through the creation of a formalized critical care pharmacist team (CCPT). This change was spurred by the transition of our organization’s clinical pharmacist to a board-certified, faculty-based specialist, which in turn spurred new focus on standardizing both the type and quality of services provided by the entire pharmacy team, targeting a higher, more consistent level of pharmacist care which better aligned with SCCM/ACCP-defined activities associated with quality services. The specialist proposed the formation of a CCPT, a process that involved targeted, intensive education and clinical skills development of a narrow pharmacist audience; administration approved this plan, provided that the CCPT arose from existing resources. This realignment focused on ensuring continuity of services across pharmacist roles (ie, rounding vs satellite) as well as across times (both days of the week and shifts). This report describes the methods used to recruit, train, and sustain a CCPT; the resulting changes observed in levels of pharmacy services after CCPT implementation; and the impressions of the CCPT members and the multidisciplinary team (physicians, nurses, dieticians, respiratory therapists, chaplains, and social workers in addition to the pharmacist), as cultural integration and perceived value are essential for sustainability and growth of the model.
Methods
Setting
Robert Wood Johnson University Hospital Hamilton is a 248-bed suburban community hospital in New Jersey with a 20-bed ICU that provides level II6 critical care services as part of an 11-hospital system. Critical care pharmacy services spanned from fundamental (eg, order review) to optimal (eg, independent pharmacotherapy evaluation) activities, with tremendous variability associated with who was engaged in care. In this original model, weekday ICU pharmacy services were provided by satellite-based general practice staff pharmacists (satellite pharmacy located in the ICU provides services for ICU, telemetry, and the emergency department) across 2 shifts (0700-2300; 9 pharmacists during the day shift and 2 on the evening shift). Satellite pharmacists largely focused on traditional/fundamental pharmacy practice, including order review, drug therapy evaluation, and adverse drug event identification. Additionally, a hospital-based, residency-trained clinical pharmacist rounded 3 days per week. General practice staff pharmacists provided weekend and overnight services. Very limited, prospective, independent clinical evaluation or individualized pharmacotherapy optimization occurred routinely. No established clinical assessment priorities or strategies existed, and thus expectations of pharmacy services were associated with the individual pharmacist present.
Team Structure and Recruitment
The staff pharmacists were well-established, with each having 25 to 41 years of practice experience. All 11 full-time staff pharmacists graduated with Bachelor of Science degrees in pharmacy, and 5 of them had returned to acquire Doctor of Pharmacy degrees prior to the initiative. None had completed post-doctoral training residencies, as residencies were not the standard when these pharmacists entered practice. The staffing model necessitated that pharmacists maintain Basic Life Support (BLS) and Advanced Cardiac Life Support (ACLS) competency as members of inpatient emergency response teams.
Three volunteers were recruited to the initial transformational process. These volunteer pharmacists were preferentially assigned to the ICU, with a clinically focused weekend rotation, to provide 7-day/week rounding continuity, but maintained general competencies and cross-functionality. Weekend responsibilities included critical care assessments and multidisciplinary rounding, inpatient emergency response, patient education/medication histories, and inpatient warfarin management consultations.
Team Training and Development
Longitudinal education of the CCPT included classroom, bedside, and practice-modeling training strategies to complement routine exposure and integration into the pharmacist’s practice in providing direct patient care. Concentrated learning occurred over a 3-month period, with extended bedside and patient-case-based learning continuing for another 3 months. Expectations of the critical care pharmacist as an independent consultant to the interdisciplinary team targeting holistic pharmacotherapy optimization were established, instilling independence and accountability within the role. Next, lecture and bedside training targeted the development of crucial assessment skills, including an understanding of device and equipment implications on pharmacotherapy decisions, pharmacokinetic and pharmacodynamic variations in critically ill patients, and supportive care. A minimum of 5 hours of group lectures were included for all members of the CCPT, with additional instruction provided based on individual needs. Lectures explored the evidence and practice associated with common diagnoses, including review of related literature, core guidelines, and institutional order sets. Fundamental topics included pain, agitation, and delirium (PAD) during mechanical ventilation, infectious diseases, and hemodynamic management.
To reinforce knowledge, build bedside assessment skills, and increase confidence, pharmacists routinely partnered with the specialist during independent morning bedside evaluations and rounds. Over time, the specialist role became increasingly supportive as the critical care pharmacist grew into the primary role. On weekends the specialist was not present but remained on call to discuss cases with the rounding critical care pharmacist. This served to reinforce clinical decision-making and expand knowledge; these patient-specific lessons were communicated with the team to support continued development and standardization.
In addition to these internal efforts, the specialist simultaneously recalibrated expectations among key ICU stakeholders, establishing uniform quality and scope of service from the CCPT. Historically, physicians and nurses sought input from specific pharmacists, and thus a cultural change regarding the perceived value of the team was required. To reinforce this, those demanding a specific pharmacist were referred to the CCPT member present.
The initial training process involved a significant proportion of the specialist’s time. Initially focused on classroom lecture and core skills development, time increasingly focused on individual learner’s needs and learning styles. Mentoring and partnering were key during this period. In the first 6 months, weekend calls were routine, but these quickly tapered as the team gained experience and confidence in their knowledge and skills.
Tools and Team Support
Beyond standardizing knowledge and skills, team effectiveness depended on establishing routine assessment criteria (Table 2), communication tools, and references. Rounding and sign-out processes were standardized to support continuity of care. A patient census report generated by the clinical computer system was used as the daily worksheet and was stored on a sign-out clipboard to readily communicate clinically pertinent history, assessments, recommendations, and pending follow-up. The report included patient demographics, admitting diagnosis, and a list of consulting physicians. The pharmacist routinely recorded daily bedside observations, his/her independent assessments (topics outlined in Table 2), pertinent history, events, and goals established on rounds. Verbal sign-out occurred twice daily (during weekdays)—from the rounding to satellite pharmacist after rounds (unless 1 person fulfilled both roles) and between day and evening shifts. Additionally, a resource binder provided rapid accessibility to key information (eg, published evidence, tools, institutional protocols), with select references residing on the sign-out clipboard for immediate access during rounding.

Monthly meetings were established to promote full engagement of the team, demonstrate ownership, and provide opportunity for discussion and information sharing. Meetings covered operational updates, strategic development of the service, educational topics, and discussions of difficult cases.
Assessment
While not directly studied, existing evidence suggests that appropriately trained critical care pharmacists should be able to perform a broad range of services, from fundamental to optimal.7 To evaluate if CCPT training elevated and standardized the type of interventions routinely made, services provided prior to the team’s formation were compared to those provided after formation through interrogation of the institution’s surveillance system. As a baseline, a comparison of the types of ICU interventions documented by the specialist during a 2-month period prior to the team’s formation were compared to the interventions documented by the staff pharmacists who became part of the CCPT. Since standardization of skills and practice were goals of the CCPT formation, the same comparison was conducted after team formation to assess whether the intervention types normalized across roles, reflecting a consistent level of service.
As assignment to the CCPT is voluntary, with no additional compensation or tangible benefits, the success of the CCPT relies on active pharmacist engagement and ongoing commitment. Thus, a personal belief that their commitment was valuable and increased professional satisfaction was key to sustain change. An online, voluntary, anonymous survey was conducted to assess the CCPT member’s perceptions of their preparedness, development of skills and comfort level, and acceptance by the multidisciplinary team, as these elements would influence members’ beliefs regarding the impact and value of the team and their justification for commitment to continuous, uncompensated learning and training. Their thoughts on professional satisfaction and development were collected as a surrogate for the model’s sustainability.
Success and sustainability also depend on the multidisciplinary team’s acceptance and perceived value of the CCPT, especially given its evolution from a model in which clinical feedback was sought and accepted exclusively from the specialist. To evaluate these components, an online, voluntary, anonymous survey of the multidisciplinary members was conducted.
Results
CCPT Interventions and Level of Service
Prior to CCPT formation, intervention categories documented by the specialist differed from those of the staff (Figure 1). The staff’s baseline interventions represented those arising from the established, routine assessments performed by all pharmacists for all inpatients, such as renal dose assessments. The specialist’s interventions largely focused on independent pharmacotherapy assessments and optimization strategies. After team formation, intervention type became increasingly consistent across the CCPT, with all members aligning with the specialist’s interventions. Intervention categories reflected the clinically focused, independent assessments targeted during training (eg, supportive care and pain/sedation assessment), expanding beyond the routine assessments performed across the general hospitalized population.

When compared to SCCM/ACCP ideals, these interventions corresponded with an expansion from routinely fundamental to routinely broad (ie, fundamental, desirable, and optimal) critical care pharmacist activities, thus elevating the overall quality of services provided by the team while assuring continuity. Desirable activities adopted by the CCPT included multidisciplinary rounding on all ICU patients; drug history review for appropriate management during acute illness; and training of students and providing educational in-services. Optimal activities routinely integrated included independent and/or collaborative investigation of ICU guidelines/protocol impact and scholarship in peer-reviewed publications. Prior to CCPT formation, staff involvement of desirable activities was limited to resuscitation event response and clarification of effective dosage regimens, with no involvement in optimal activities.
CCPT Impressions
The online, voluntary, anonymous survey was completed by 5 of the 6 staff members (the 3 original members plus 3 staff members who were added several months into the program to enhance continuity and cross-shift coverage) comprising the team. Using a 5-point Likert scale, members ranked their comfort level with their critical care knowledge, bedside skills, ability to actively participate in rounds, and ability to address controversial clinical issues in their staffing role prior to team formation (ie, baseline) compared to their current CCPT practice. Overall, self-assessments reflected perceived increases across all categories. Prior to CCPT training and implementation, all team members were “not at all,” “slightly comfortable,” or “somewhat comfortable” with these points, while after training and implementation all reported being “comfortable” or “very comfortable” with the same points. All members reported feeling better prepared and confident in caring for critically ill patients and felt that the team and its standardized approach enhanced medication safety. When asked about their impressions of the perceived value of the CCPT by interdisciplinary peers, pharmacists felt it was perceived as bringing “a lot” or “a great deal” of value. Additionally, all members uniformly felt that the team supported their professional growth and enhanced their professional satisfaction.
Multidisciplinary Impressions of Service and Value
A total of 29 (90%) multidisciplinary team members completed the online, voluntary, anonymous survey of their impressions of the CCPT’s service and impact. Surveys represented the impressions of critical care physicians, the unit’s nursing leadership (administrative and clinical), nursing education, staff nurses, social work, and pastoral care. Using a 5-point Likert scale, all respondents reported that they “agreed” or “entirely agreed” that the CCPT enhanced care. Specifically, they reported that pharmacists were more visible and engaged, and provided more consistent and reliable care regardless of which member was present. Services were seen as more robust and seamless, meeting interdisciplinary needs. The CCPT was viewed as a cohesive, efficient group. Respondents felt that the CCPT’s presence and engagement on weekends enhanced continuity of pharmaceutical care. As a result, the CCPT was seen as enhancing interdisciplinary understanding of the pharmacist’s value in critical care.
Discussion
Realignment and development of existing personnel resources allowed our organization to assure greater continuity, consistency, and quality of pharmacy care in the critical care setting (Figure 2). By standardizing expectations and broadening multidisciplinary understanding of the CCPT’s unique value, the pharmacist’s role was solidified and became an integral, active part of routine patient bedside care.

Prior to forming the CCPT, the physical presence of the pharmacist, as well as the services provided, were inconsistent. While a general practice pharmacist was in the satellite pharmacy within the ICU for up to 2 shifts on weekdays, pharmacists largely focused on traditional functions associated with order review and drug dispensing or established hospital-wide programs such as renal dosing or intravenous-to-oral formulation switches. The pharmacist remained in the satellite, not visible on rounds or at the bedside. In fact, there was a clear lack of comfort, frequently articulated by the pharmacists, with clinical questions that required bedside assessment, leading to routine escalation to the clinical specialist, who was not always readily available. This dynamic set an expectation for the multidisciplinary team that there were segregated pharmacy services—the satellite provided order review and product and the clinical specialist, in the limited hours present, provided clinical consultation and education. The formation of the CCPT abolished this tiered level of expectations, establishing a physical and clinical presence of a critical care pharmacist with equal capability and comfort. Both the pharmacist and multidisciplinary members perceived enhancements and value associated with the standardization and consistency provided by implementing the CCPT. Intervention data from before and after team formation support that routine interventions in critical care normalized the care provided and increased the robustness of critical care pharmacy services, with a strong shift to both clinical and academic activities considered desirable to optimal by SCCM/ACCP standards.
The benefit of pharmacist presence in the ICU is well described, with studies showing that the presence of a pharmacist is associated with medication error prevention and adverse drug event identification.8-10 However, this body of evidence applies no standardized definition regarding critical care pharmacist qualifications, with many studies pre-dating the wider availability of post-doctoral training programs and national board certification for critical care pharmacists.11 Training and certification structures have evolved with increased recognition of the specialization required to optimize the pharmacist’s role in providing quality care, albeit at a slower pace than published standards.1,2 In 2018, 136 organizations offered America Society of Health-System Pharmacists–accredited critical care pharmacy residencies.12 National recognition of expertise as a critical care pharmacist was established by the Board of Pharmacy Specialists in 2015, with more than 1600 pharmacists currently recognized.12 Our project is the only known description of a pharmacist practice model that increases critical care pharmacist availability through the application of standardized criteria incorporating these updated qualifications, thus ensuring expertise and experience that correlates with practice quality and consistency.
Despite the advancements achieved through this project, several limitations exist. First, while this model largely normalized services over the day and evening shifts, our night shift continues to be covered by 1 general practice pharmacist. More recently, resource reallocation mandated reduction in satellite hours, although that CCPT member remains available from the main pharmacy. The specialist remains on call to support the general practice pharmacists, but in-house expertise cannot be made available in the absence of additional resources. To optimize existing staffing, the specialist begins clinical evaluations during the early morning, overlapping with the night-shift prior to the satellite pharmacist’s arrival. This both provides some pharmacist presence at the bedside for night shift nurses and extends the hours during which a critical care pharmacist is physically available. Second, while all efforts are made to stagger time off, unavoidable gaps in critical care pharmacist coverage occur; expansion of the original team from 3 to 6 members has greatly reduced the likelihood of such gaps. Last, the program was designed to achieve routine integration of activities shown in the literature as being associated with quality, and those activities were assessed as a surrogate for quality.
Informal input, confirmed through survey data, from various disciplines on our team has consistently supported that the establishment of the CCPT has met a need by both standardizing critical care pharmacy practice and optimizing the pharmacist role within the team. While we recognize the limitations associated with the size of these surveys, they represent large proportions of our team and reflect key elements known to be important in sustaining long-term cultural change—a belief that what one is doing is both justified and valuable. This success has been a catalyst for several ongoing projects, fostering the development and adoption of critical care pharmacist protocols to allow more autonomous practice within our scope. Team development and movement toward robust protocol management has sparked a cultural evolution across disciplines as we strive to achieve the SCCM description of a highly effective team2,13 that emphasizes each discipline practicing fully within its scope in a horizontal team structure. Thus, the ICU medical director has used the success of the CCPT structure as an example to support optimization and development of the practice by other disciplines within the team. This has led to a significant revision in our rounding structure and interdisciplinary care model.14
The survey of CCPT members revealed that the model both engaged and stimulated the pharmacists involved, reflective of the autonomy and accountability required for sustainable, transformational cultural change. Within a year of entering the CCPT, 2 of the 3 pharmacists initially engaged had earned their board certification in pharmacotherapy (ie, BCPS) and the other, who had not acquired her Doctor of Pharmacy degree prior to the CCPT initiative, enrolled in a program to do so. The pharmacists expressed that they obtained BCPS over the newly available critical care certification because of the expectation that they maintain expertise across patient populations. This level of self-driven motivation in the absence of compensation reflects the value and professional satisfaction gained from being voluntary members of the CCPT.
Conclusion
Critical care pharmacy practice has continued to evolve to include increasingly specialized training for newer graduates and, more recently, the availability of critical care pharmacist board certification. While it is optimal to apply these standards when filling open critical care pharmacist positions, many hospitals require existing staff to fulfill multiple roles across various patient populations, leading to a variation in educational, training, and practice backgrounds for pharmacists currently practicing in the ICU. To minimize the variation associated with this resource-limited structure in a manner that standardized and elevated the type and level of service provided, we created a CCPT with existing pharmacists who were willing to accept intensive training and demonstrate an ongoing commitment to maintain defined competencies and skills. Our goal was to solidify the essential role of the critical care pharmacist in providing quality critical care services as described in the literature. The CCPT was well-received by the multidisciplinary team and served as an example for other disciplines that had similar struggles. The team’s success expanded into several other ongoing initiatives, including critical care pharmacist–driven protocols.
Acknowledgment: The authors thank Nina Roberts, MSN, RN, CCRN, NEA-BC, and Carol Ash, DO, MBA, MHCDS, the ICU Nursing and Medical Directors, respectively, at the time of this program’s initiation, for supporting the development of the critical care pharmacist team initiative and review of this manuscript.
Corresponding author: Liza Barbarello Andrews, PharmD, BCCCP, BCPS, Ernest Mario School of Pharmacy, Rutgers, The State University of New Jersey, 160 Frelinghuysen Road, Piscataway, NJ 08854; [email protected].
Financial disclosures: None.
From Robert Wood Johnson University Hospital Hamilton, Hamilton, NJ.
Abstract
- Background: Critical care pharmacy services are often provided by clinical specialists during limited hours and, otherwise, by general practice pharmacists, leading to varied level, expertise, and multidisciplinary expectations of these services.
- Objective: Since no published descriptions of successful models sustaining routine, high-quality critical care pharmacy services in a community-based, resource-limited environment exist, a critical care pharmacist team (CCPT) was created to meet this goal. After successful launch, the initiative’s primary goal was to assess whether team formation indeed standardized and increased the level of pharmacy services routinely provided. The secondary goal was to demonstrate cultural acceptance, and thus sustainability, of the model.
- Methods: A CCPT was formed from existing pharmacist resources. A longitudinal educational plan, including classroom, bedside, and practice modeling, assured consistent skills, knowledge, and confidence. Interventions performed by pharmacists before and after implementation were assessed to determine whether the model standardized type and level of service. Surveys of the CCPT and multidisciplinary teams assessed perceptions of expertise, confidence, and value as surrogates for model success and sustainability.
- Results: Interventions after CCPT formation reflected elevated and standardized critical care pharmacy services that advanced the multidisciplinary team’s perception of the pharmacist as an integral, essential team member. CCPT members felt empowered, as reflected by self-directed enrollment in PharmD programs and/or obtaining board certification. This success subsequently served to improve the culture of cooperation and spark similar evolution of other disciplines.
- Conclusion: The standardization and optimization of pharmacy services through a dedicated CCPT improved continuity of care and standardized multidisciplinary team expectations.
Keywords: critical care; clinical pharmacist; pharmaceutical care; standards of practice.
There has been significant evolution in the role, training, and overall understanding of the impact of critical care pharmacists over the past 2 decades. The specialized knowledge and role of pharmacists make them essential links in the provision of quality critical care services.1 The Society of Critical Care Medicine (SCCM) and the American College of Clinical Pharmacy (ACCP) have defined the level of clinical practice and specialized skills that characterize the critical care pharmacist and have made recommendations regarding both the personnel requirements for the provision of pharmaceutical care to critically ill patients and the fundamental, desirable, and optimal pharmacy services that should be provided to these patients (Table 1).2 Despite this, only two-thirds of US intensive care units (ICUs) have clinical pharmacists/specialists (defined as spending at least 50% of their time providing clinical services), resulting in fundamental activities dominating routine pharmacist services.3 The clinical nature of most desirable and optimal activities, such as code response and pharmacist-driven protocol management, is limited, but these activities correlate with decreases in mortality across hospitalized populations.4

Despite their demonstrated benefit and recognized role, critical care pharmacists remain a limited resource with limited physical presence in ICUs.5 This presents hospital pharmacies with a real dilemma: given that clinical pharmacy specialists are often a limited resource, what services (fundamental, desirable, or optimal) should be provided by which pharmacists over what hours and on which days? For many hospitals, personnel resources allow for a clinical pharmacy specialist (either trained or with significant experience in critical care) to participate in multidisciplinary rounds, but do not allow a specialist to be present 7 days per week across all times of the day. As a result, routine services may be inconsistent and limited to activities that are fundamental-to-desirable, due to the varied educational and training backgrounds of pharmacists providing nonrounding services. Where gaps have been identified, remote (tele-health) provision of targeted ICU pharmacist services are beneficial.5
In our organization, we recognized the significant variation created by this resource-defined model and sought to develop a process to move closer to published best practice standards for quality services2 through the creation of a formalized critical care pharmacist team (CCPT). This change was spurred by the transition of our organization’s clinical pharmacist to a board-certified, faculty-based specialist, which in turn spurred new focus on standardizing both the type and quality of services provided by the entire pharmacy team, targeting a higher, more consistent level of pharmacist care which better aligned with SCCM/ACCP-defined activities associated with quality services. The specialist proposed the formation of a CCPT, a process that involved targeted, intensive education and clinical skills development of a narrow pharmacist audience; administration approved this plan, provided that the CCPT arose from existing resources. This realignment focused on ensuring continuity of services across pharmacist roles (ie, rounding vs satellite) as well as across times (both days of the week and shifts). This report describes the methods used to recruit, train, and sustain a CCPT; the resulting changes observed in levels of pharmacy services after CCPT implementation; and the impressions of the CCPT members and the multidisciplinary team (physicians, nurses, dieticians, respiratory therapists, chaplains, and social workers in addition to the pharmacist), as cultural integration and perceived value are essential for sustainability and growth of the model.
Methods
Setting
Robert Wood Johnson University Hospital Hamilton is a 248-bed suburban community hospital in New Jersey with a 20-bed ICU that provides level II6 critical care services as part of an 11-hospital system. Critical care pharmacy services spanned from fundamental (eg, order review) to optimal (eg, independent pharmacotherapy evaluation) activities, with tremendous variability associated with who was engaged in care. In this original model, weekday ICU pharmacy services were provided by satellite-based general practice staff pharmacists (satellite pharmacy located in the ICU provides services for ICU, telemetry, and the emergency department) across 2 shifts (0700-2300; 9 pharmacists during the day shift and 2 on the evening shift). Satellite pharmacists largely focused on traditional/fundamental pharmacy practice, including order review, drug therapy evaluation, and adverse drug event identification. Additionally, a hospital-based, residency-trained clinical pharmacist rounded 3 days per week. General practice staff pharmacists provided weekend and overnight services. Very limited, prospective, independent clinical evaluation or individualized pharmacotherapy optimization occurred routinely. No established clinical assessment priorities or strategies existed, and thus expectations of pharmacy services were associated with the individual pharmacist present.
Team Structure and Recruitment
The staff pharmacists were well-established, with each having 25 to 41 years of practice experience. All 11 full-time staff pharmacists graduated with Bachelor of Science degrees in pharmacy, and 5 of them had returned to acquire Doctor of Pharmacy degrees prior to the initiative. None had completed post-doctoral training residencies, as residencies were not the standard when these pharmacists entered practice. The staffing model necessitated that pharmacists maintain Basic Life Support (BLS) and Advanced Cardiac Life Support (ACLS) competency as members of inpatient emergency response teams.
Three volunteers were recruited to the initial transformational process. These volunteer pharmacists were preferentially assigned to the ICU, with a clinically focused weekend rotation, to provide 7-day/week rounding continuity, but maintained general competencies and cross-functionality. Weekend responsibilities included critical care assessments and multidisciplinary rounding, inpatient emergency response, patient education/medication histories, and inpatient warfarin management consultations.
Team Training and Development
Longitudinal education of the CCPT included classroom, bedside, and practice-modeling training strategies to complement routine exposure and integration into the pharmacist’s practice in providing direct patient care. Concentrated learning occurred over a 3-month period, with extended bedside and patient-case-based learning continuing for another 3 months. Expectations of the critical care pharmacist as an independent consultant to the interdisciplinary team targeting holistic pharmacotherapy optimization were established, instilling independence and accountability within the role. Next, lecture and bedside training targeted the development of crucial assessment skills, including an understanding of device and equipment implications on pharmacotherapy decisions, pharmacokinetic and pharmacodynamic variations in critically ill patients, and supportive care. A minimum of 5 hours of group lectures were included for all members of the CCPT, with additional instruction provided based on individual needs. Lectures explored the evidence and practice associated with common diagnoses, including review of related literature, core guidelines, and institutional order sets. Fundamental topics included pain, agitation, and delirium (PAD) during mechanical ventilation, infectious diseases, and hemodynamic management.
To reinforce knowledge, build bedside assessment skills, and increase confidence, pharmacists routinely partnered with the specialist during independent morning bedside evaluations and rounds. Over time, the specialist role became increasingly supportive as the critical care pharmacist grew into the primary role. On weekends the specialist was not present but remained on call to discuss cases with the rounding critical care pharmacist. This served to reinforce clinical decision-making and expand knowledge; these patient-specific lessons were communicated with the team to support continued development and standardization.
In addition to these internal efforts, the specialist simultaneously recalibrated expectations among key ICU stakeholders, establishing uniform quality and scope of service from the CCPT. Historically, physicians and nurses sought input from specific pharmacists, and thus a cultural change regarding the perceived value of the team was required. To reinforce this, those demanding a specific pharmacist were referred to the CCPT member present.
The initial training process involved a significant proportion of the specialist’s time. Initially focused on classroom lecture and core skills development, time increasingly focused on individual learner’s needs and learning styles. Mentoring and partnering were key during this period. In the first 6 months, weekend calls were routine, but these quickly tapered as the team gained experience and confidence in their knowledge and skills.
Tools and Team Support
Beyond standardizing knowledge and skills, team effectiveness depended on establishing routine assessment criteria (Table 2), communication tools, and references. Rounding and sign-out processes were standardized to support continuity of care. A patient census report generated by the clinical computer system was used as the daily worksheet and was stored on a sign-out clipboard to readily communicate clinically pertinent history, assessments, recommendations, and pending follow-up. The report included patient demographics, admitting diagnosis, and a list of consulting physicians. The pharmacist routinely recorded daily bedside observations, his/her independent assessments (topics outlined in Table 2), pertinent history, events, and goals established on rounds. Verbal sign-out occurred twice daily (during weekdays)—from the rounding to satellite pharmacist after rounds (unless 1 person fulfilled both roles) and between day and evening shifts. Additionally, a resource binder provided rapid accessibility to key information (eg, published evidence, tools, institutional protocols), with select references residing on the sign-out clipboard for immediate access during rounding.

Monthly meetings were established to promote full engagement of the team, demonstrate ownership, and provide opportunity for discussion and information sharing. Meetings covered operational updates, strategic development of the service, educational topics, and discussions of difficult cases.
Assessment
While not directly studied, existing evidence suggests that appropriately trained critical care pharmacists should be able to perform a broad range of services, from fundamental to optimal.7 To evaluate if CCPT training elevated and standardized the type of interventions routinely made, services provided prior to the team’s formation were compared to those provided after formation through interrogation of the institution’s surveillance system. As a baseline, a comparison of the types of ICU interventions documented by the specialist during a 2-month period prior to the team’s formation were compared to the interventions documented by the staff pharmacists who became part of the CCPT. Since standardization of skills and practice were goals of the CCPT formation, the same comparison was conducted after team formation to assess whether the intervention types normalized across roles, reflecting a consistent level of service.
As assignment to the CCPT is voluntary, with no additional compensation or tangible benefits, the success of the CCPT relies on active pharmacist engagement and ongoing commitment. Thus, a personal belief that their commitment was valuable and increased professional satisfaction was key to sustain change. An online, voluntary, anonymous survey was conducted to assess the CCPT member’s perceptions of their preparedness, development of skills and comfort level, and acceptance by the multidisciplinary team, as these elements would influence members’ beliefs regarding the impact and value of the team and their justification for commitment to continuous, uncompensated learning and training. Their thoughts on professional satisfaction and development were collected as a surrogate for the model’s sustainability.
Success and sustainability also depend on the multidisciplinary team’s acceptance and perceived value of the CCPT, especially given its evolution from a model in which clinical feedback was sought and accepted exclusively from the specialist. To evaluate these components, an online, voluntary, anonymous survey of the multidisciplinary members was conducted.
Results
CCPT Interventions and Level of Service
Prior to CCPT formation, intervention categories documented by the specialist differed from those of the staff (Figure 1). The staff’s baseline interventions represented those arising from the established, routine assessments performed by all pharmacists for all inpatients, such as renal dose assessments. The specialist’s interventions largely focused on independent pharmacotherapy assessments and optimization strategies. After team formation, intervention type became increasingly consistent across the CCPT, with all members aligning with the specialist’s interventions. Intervention categories reflected the clinically focused, independent assessments targeted during training (eg, supportive care and pain/sedation assessment), expanding beyond the routine assessments performed across the general hospitalized population.

When compared to SCCM/ACCP ideals, these interventions corresponded with an expansion from routinely fundamental to routinely broad (ie, fundamental, desirable, and optimal) critical care pharmacist activities, thus elevating the overall quality of services provided by the team while assuring continuity. Desirable activities adopted by the CCPT included multidisciplinary rounding on all ICU patients; drug history review for appropriate management during acute illness; and training of students and providing educational in-services. Optimal activities routinely integrated included independent and/or collaborative investigation of ICU guidelines/protocol impact and scholarship in peer-reviewed publications. Prior to CCPT formation, staff involvement of desirable activities was limited to resuscitation event response and clarification of effective dosage regimens, with no involvement in optimal activities.
CCPT Impressions
The online, voluntary, anonymous survey was completed by 5 of the 6 staff members (the 3 original members plus 3 staff members who were added several months into the program to enhance continuity and cross-shift coverage) comprising the team. Using a 5-point Likert scale, members ranked their comfort level with their critical care knowledge, bedside skills, ability to actively participate in rounds, and ability to address controversial clinical issues in their staffing role prior to team formation (ie, baseline) compared to their current CCPT practice. Overall, self-assessments reflected perceived increases across all categories. Prior to CCPT training and implementation, all team members were “not at all,” “slightly comfortable,” or “somewhat comfortable” with these points, while after training and implementation all reported being “comfortable” or “very comfortable” with the same points. All members reported feeling better prepared and confident in caring for critically ill patients and felt that the team and its standardized approach enhanced medication safety. When asked about their impressions of the perceived value of the CCPT by interdisciplinary peers, pharmacists felt it was perceived as bringing “a lot” or “a great deal” of value. Additionally, all members uniformly felt that the team supported their professional growth and enhanced their professional satisfaction.
Multidisciplinary Impressions of Service and Value
A total of 29 (90%) multidisciplinary team members completed the online, voluntary, anonymous survey of their impressions of the CCPT’s service and impact. Surveys represented the impressions of critical care physicians, the unit’s nursing leadership (administrative and clinical), nursing education, staff nurses, social work, and pastoral care. Using a 5-point Likert scale, all respondents reported that they “agreed” or “entirely agreed” that the CCPT enhanced care. Specifically, they reported that pharmacists were more visible and engaged, and provided more consistent and reliable care regardless of which member was present. Services were seen as more robust and seamless, meeting interdisciplinary needs. The CCPT was viewed as a cohesive, efficient group. Respondents felt that the CCPT’s presence and engagement on weekends enhanced continuity of pharmaceutical care. As a result, the CCPT was seen as enhancing interdisciplinary understanding of the pharmacist’s value in critical care.
Discussion
Realignment and development of existing personnel resources allowed our organization to assure greater continuity, consistency, and quality of pharmacy care in the critical care setting (Figure 2). By standardizing expectations and broadening multidisciplinary understanding of the CCPT’s unique value, the pharmacist’s role was solidified and became an integral, active part of routine patient bedside care.

Prior to forming the CCPT, the physical presence of the pharmacist, as well as the services provided, were inconsistent. While a general practice pharmacist was in the satellite pharmacy within the ICU for up to 2 shifts on weekdays, pharmacists largely focused on traditional functions associated with order review and drug dispensing or established hospital-wide programs such as renal dosing or intravenous-to-oral formulation switches. The pharmacist remained in the satellite, not visible on rounds or at the bedside. In fact, there was a clear lack of comfort, frequently articulated by the pharmacists, with clinical questions that required bedside assessment, leading to routine escalation to the clinical specialist, who was not always readily available. This dynamic set an expectation for the multidisciplinary team that there were segregated pharmacy services—the satellite provided order review and product and the clinical specialist, in the limited hours present, provided clinical consultation and education. The formation of the CCPT abolished this tiered level of expectations, establishing a physical and clinical presence of a critical care pharmacist with equal capability and comfort. Both the pharmacist and multidisciplinary members perceived enhancements and value associated with the standardization and consistency provided by implementing the CCPT. Intervention data from before and after team formation support that routine interventions in critical care normalized the care provided and increased the robustness of critical care pharmacy services, with a strong shift to both clinical and academic activities considered desirable to optimal by SCCM/ACCP standards.
The benefit of pharmacist presence in the ICU is well described, with studies showing that the presence of a pharmacist is associated with medication error prevention and adverse drug event identification.8-10 However, this body of evidence applies no standardized definition regarding critical care pharmacist qualifications, with many studies pre-dating the wider availability of post-doctoral training programs and national board certification for critical care pharmacists.11 Training and certification structures have evolved with increased recognition of the specialization required to optimize the pharmacist’s role in providing quality care, albeit at a slower pace than published standards.1,2 In 2018, 136 organizations offered America Society of Health-System Pharmacists–accredited critical care pharmacy residencies.12 National recognition of expertise as a critical care pharmacist was established by the Board of Pharmacy Specialists in 2015, with more than 1600 pharmacists currently recognized.12 Our project is the only known description of a pharmacist practice model that increases critical care pharmacist availability through the application of standardized criteria incorporating these updated qualifications, thus ensuring expertise and experience that correlates with practice quality and consistency.
Despite the advancements achieved through this project, several limitations exist. First, while this model largely normalized services over the day and evening shifts, our night shift continues to be covered by 1 general practice pharmacist. More recently, resource reallocation mandated reduction in satellite hours, although that CCPT member remains available from the main pharmacy. The specialist remains on call to support the general practice pharmacists, but in-house expertise cannot be made available in the absence of additional resources. To optimize existing staffing, the specialist begins clinical evaluations during the early morning, overlapping with the night-shift prior to the satellite pharmacist’s arrival. This both provides some pharmacist presence at the bedside for night shift nurses and extends the hours during which a critical care pharmacist is physically available. Second, while all efforts are made to stagger time off, unavoidable gaps in critical care pharmacist coverage occur; expansion of the original team from 3 to 6 members has greatly reduced the likelihood of such gaps. Last, the program was designed to achieve routine integration of activities shown in the literature as being associated with quality, and those activities were assessed as a surrogate for quality.
Informal input, confirmed through survey data, from various disciplines on our team has consistently supported that the establishment of the CCPT has met a need by both standardizing critical care pharmacy practice and optimizing the pharmacist role within the team. While we recognize the limitations associated with the size of these surveys, they represent large proportions of our team and reflect key elements known to be important in sustaining long-term cultural change—a belief that what one is doing is both justified and valuable. This success has been a catalyst for several ongoing projects, fostering the development and adoption of critical care pharmacist protocols to allow more autonomous practice within our scope. Team development and movement toward robust protocol management has sparked a cultural evolution across disciplines as we strive to achieve the SCCM description of a highly effective team2,13 that emphasizes each discipline practicing fully within its scope in a horizontal team structure. Thus, the ICU medical director has used the success of the CCPT structure as an example to support optimization and development of the practice by other disciplines within the team. This has led to a significant revision in our rounding structure and interdisciplinary care model.14
The survey of CCPT members revealed that the model both engaged and stimulated the pharmacists involved, reflective of the autonomy and accountability required for sustainable, transformational cultural change. Within a year of entering the CCPT, 2 of the 3 pharmacists initially engaged had earned their board certification in pharmacotherapy (ie, BCPS) and the other, who had not acquired her Doctor of Pharmacy degree prior to the CCPT initiative, enrolled in a program to do so. The pharmacists expressed that they obtained BCPS over the newly available critical care certification because of the expectation that they maintain expertise across patient populations. This level of self-driven motivation in the absence of compensation reflects the value and professional satisfaction gained from being voluntary members of the CCPT.
Conclusion
Critical care pharmacy practice has continued to evolve to include increasingly specialized training for newer graduates and, more recently, the availability of critical care pharmacist board certification. While it is optimal to apply these standards when filling open critical care pharmacist positions, many hospitals require existing staff to fulfill multiple roles across various patient populations, leading to a variation in educational, training, and practice backgrounds for pharmacists currently practicing in the ICU. To minimize the variation associated with this resource-limited structure in a manner that standardized and elevated the type and level of service provided, we created a CCPT with existing pharmacists who were willing to accept intensive training and demonstrate an ongoing commitment to maintain defined competencies and skills. Our goal was to solidify the essential role of the critical care pharmacist in providing quality critical care services as described in the literature. The CCPT was well-received by the multidisciplinary team and served as an example for other disciplines that had similar struggles. The team’s success expanded into several other ongoing initiatives, including critical care pharmacist–driven protocols.
Acknowledgment: The authors thank Nina Roberts, MSN, RN, CCRN, NEA-BC, and Carol Ash, DO, MBA, MHCDS, the ICU Nursing and Medical Directors, respectively, at the time of this program’s initiation, for supporting the development of the critical care pharmacist team initiative and review of this manuscript.
Corresponding author: Liza Barbarello Andrews, PharmD, BCCCP, BCPS, Ernest Mario School of Pharmacy, Rutgers, The State University of New Jersey, 160 Frelinghuysen Road, Piscataway, NJ 08854; [email protected].
Financial disclosures: None.
1. Brilli RJ, Spevetz A, Branson RD, et al. American College of Critical Care Medicine Task Force on Models of Critical Care Delivery. Critical care delivery in the intensive care unit: defining clinical roles and the best practice model. Crit Care Med. 2001;29:2007-2019.
2. Rudis MI, Brandl KM; Society of Critical Care Medicine and American College of Clinical Pharmacy Task Force on Critical Care Pharmacy Services. Position paper on critical care pharmacy services. Crit Care Med. 2000;28:3746-3750.
3. MacLaren R, Devlin JW, Martin SJ, et al. Critical care pharmacy services in United States hospitals. Ann Pharmacother. 2006;40:612-618.
4. Bond CA, Raehl CL. Clinical pharmacy services, pharmacy staffing, and hospital mortality rates. Pharmacotherapy. 2007;27:481-493.
5. Forni A, Skahan N, Hartman CA, et al. Evaluation of the impact of a tele-ICU pharmacist on the management of sedation in critically ill mechanically ventilated patients. Ann Pharmacother. 2010;44:432-438.
6. Haupt MT, Bekes CE, Brilli RJ, et al. Guidelines on critical care services and personnel: recommendations based on a system of categorization on three levels of care. Crit Care Med. 2003;31:2677-2683.
7. Board of Pharmacy Specialties. Critical Care Pharmacy. www.bpsweb.org/bps-specialties/critical-care-pharmacy/.
8. Montazeri M, Cook DJ. Impact of a clinical pharmacist in a multidisciplinary intensive care unit. Crit Care Med. 1994;22:1044-1048.
9. Leape L, Cullen D, Clapp M, et al. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit. JAMA. 1999;282:267-270.
10. Horn E, Jacobi J. The critical care pharmacist: evolution of an essential team member. Crit Care Med. 2006;34(suppl):S46-S51.
11. Jacobi J. Measuring the impact of a pharmacist in the intensive care unit—are all pharmacists created equal? J Crit Care. 2015;30:1127-1128.
12. American Society of HealthSystem Pharmacists. Online residency directory. https://accred.ashp.org/aps/pages/directory/residencyProgramSearch.aspx. Accessed June 26, 2019.
13. Weled BJ, Adzhigirey LA, Hodgman TM, et al. Critical care delivery: the importance of process of care and ICU structure to improved outcomes: an update from the American College of Critical Care Medicine Task Force on Models of Critical Care. Crit Care Med. 2015;43:1520-1525.
14. Andrews LB, Roberts N, Ash C, et al. The LOTUS: a journey to value-based, patient-centered care. Creat Nurs. 2019;25:17-24.
1. Brilli RJ, Spevetz A, Branson RD, et al. American College of Critical Care Medicine Task Force on Models of Critical Care Delivery. Critical care delivery in the intensive care unit: defining clinical roles and the best practice model. Crit Care Med. 2001;29:2007-2019.
2. Rudis MI, Brandl KM; Society of Critical Care Medicine and American College of Clinical Pharmacy Task Force on Critical Care Pharmacy Services. Position paper on critical care pharmacy services. Crit Care Med. 2000;28:3746-3750.
3. MacLaren R, Devlin JW, Martin SJ, et al. Critical care pharmacy services in United States hospitals. Ann Pharmacother. 2006;40:612-618.
4. Bond CA, Raehl CL. Clinical pharmacy services, pharmacy staffing, and hospital mortality rates. Pharmacotherapy. 2007;27:481-493.
5. Forni A, Skahan N, Hartman CA, et al. Evaluation of the impact of a tele-ICU pharmacist on the management of sedation in critically ill mechanically ventilated patients. Ann Pharmacother. 2010;44:432-438.
6. Haupt MT, Bekes CE, Brilli RJ, et al. Guidelines on critical care services and personnel: recommendations based on a system of categorization on three levels of care. Crit Care Med. 2003;31:2677-2683.
7. Board of Pharmacy Specialties. Critical Care Pharmacy. www.bpsweb.org/bps-specialties/critical-care-pharmacy/.
8. Montazeri M, Cook DJ. Impact of a clinical pharmacist in a multidisciplinary intensive care unit. Crit Care Med. 1994;22:1044-1048.
9. Leape L, Cullen D, Clapp M, et al. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit. JAMA. 1999;282:267-270.
10. Horn E, Jacobi J. The critical care pharmacist: evolution of an essential team member. Crit Care Med. 2006;34(suppl):S46-S51.
11. Jacobi J. Measuring the impact of a pharmacist in the intensive care unit—are all pharmacists created equal? J Crit Care. 2015;30:1127-1128.
12. American Society of HealthSystem Pharmacists. Online residency directory. https://accred.ashp.org/aps/pages/directory/residencyProgramSearch.aspx. Accessed June 26, 2019.
13. Weled BJ, Adzhigirey LA, Hodgman TM, et al. Critical care delivery: the importance of process of care and ICU structure to improved outcomes: an update from the American College of Critical Care Medicine Task Force on Models of Critical Care. Crit Care Med. 2015;43:1520-1525.
14. Andrews LB, Roberts N, Ash C, et al. The LOTUS: a journey to value-based, patient-centered care. Creat Nurs. 2019;25:17-24.
Long-Term Exercise Training in Older Adults Is Associated with Reduced Injurious Falls and Fractures
Study Overview
Objective. To evaluate the association between long-term exercise interventions (duration ≥ 1 year) and risks of falls, injurious falls, multiple falls, fractures, hospitalization, and mortality in older adults.
Design. A systematic review of randomized controlled trials (RCTs) with preplanned meta-analysis was conducted to investigate the association between long-term exercise interventions and falls and fall-related adverse outcomes in adults older than 60 years. A literature search using electronic databases, including PubMed, Cochrane Central Register of Controlled Trials, SportDiscus, PsychInfo, and Ageline, was performed between February 20 and March 5, 2018. Studies selected were RCTs with exercise duration of 1 year or longer, where effects of exercise intervention were compared with a comparator group of participants aged 60 years or older. Articles were independently screened, abstracted, and assessed for risk of bias by 2 raters, who resolved divergences in data extraction and synthesis via in-person meetings.
Setting and participants. A total of 46 studies (22,709 participants; median of 203 participants per study) were included in the review and 40 studies (21,868 participants) were included in the meta-analysis. The participants’ mean age was 73.1 ± 7.1 years, and 66.3% (15,054 participants) were women. Studies were mostly conducted in Europe (n = 15), North America (n = 13), and Oceania (n = 10). Multicomponent training involving multiple exercises (eg, aerobic, strength and balance; 29 RCTs) was the most common intervention modality, followed by aerobic (8 RCTs) and strength (5 RCTs) training. Exercise interventions had a mean frequency of 3 times/week, with each session lasting approximately 50 minutes, and were administered at a moderate intensity. The average compliance rate with exercise training was 65%. Comparator groups were often active controls that ranged from attention controls to more intensive interventions.
Main outcome measures. The 6 binary outcomes investigated were fallers who fell at least once, multiple times, or at least twice; fractures; hospitalization; and mortality. Estimates of outcomes were combined using risk ratios (RRs) using DerSimonian and Laird’s random-effects model (Mantel-Haenszel method). Heterogeneity was evaluated using I2 statistics, and trials with low rates of compliance (< 30%) with exercise intervention or high attrition (> 40%) were excluded in primary analyses.
Main results. Exercise training significantly reduced the risk of falls by 12% (n = 20 RCTs; 4420 participants; RR, 0.88; 95% confidence interval [CI], 0.79-0.98) and injurious falls by 26% (9 RCTs; 4481 participants; RR, 0.74; 95% CI, 0.62-0.88), and reduced the risk of fractures by 16% (19 RCTs; 8410 participants; RR, 0.84; 95% CI, 0.71-1.00; P = 0.05). Exercise training did not decrease the risk of multiple falls (13 RCTs; 3060 participants; RR, 0.86; 95% CI, 0.68-1.08), hospitalization (12 RCTs; 5639 participants; RR 0.94; 95% CI, 0.80-1.12), or mortality (29 RCTs; 11,441 participants; RR 0.96; 95% CI, 0.85-1.09). Sensitivity analyses yielded similar results, with the exception of the fixed-effect meta-analysis for the risk of fracture that showed a significant effect of long-term exercise training (RR, 0.84; 95% CI, 0.70-1.00; P = 0.047). Meta-regression analysis on mortality and falls suggested that exercise frequency between 2 and 3 times per week was optimal and beneficial.
Conclusion. Long-term exercise training of 1 year or longer in duration is associated with a reduction in falls, injurious falls, and fractures in older adults. Moreover, moderate intensity, multicomponent exercise training performed 2 to 3 times weekly is likely safe and effective in this vulnerable population.
Commentary
Falls are exceedingly common (1 in 3 older Americans fall each year) and are the leading cause of fatal and nonfatal injuries in persons over the age of 65 years.1,2 While fall prevention is a public health priority and a topic of interest in many research studies, there are important gaps in knowledge regarding optimal strategies to prevent falls and fall-related injuries in this high-risk population. The study reported by de Souto Barreto and colleagues provides new insights to address several of these gaps and may have a significant impact on the clinical practice of fall prevention in geriatric medicine.
Studies show that a single exercise intervention of short- to medium-term duration can prevent falls in community-dwelling older adults.3 However, the effects of long-term exercise training (ie, intervention lasting longer than a year) on fall prevention in this population is less well characterized. This study is the first meta-analysis that aimed to evaluate the potential beneficial impact of long-term exercise training on falls and adverse fall-related outcomes in adults ≥ 60 years of age who are prone to falls. The study’s findings indicate that long-term exercise training reduces the risk of falling by 12%, injurious falls by 26%, and factures by 16%. These results are important in that they add compelling evidence that exercise training of any duration can reduce falls and some fall-related adverse outcomes. Furthermore, the positive effects of long-term exercise training appear to mitigate some of the fatal and nonfatal injuries attributable to falls—the leading cause of such injuries in older adults.
The modality (type) and dose (frequency) of exercise training are important components of “exercise prescription” for older adults. However, there is a lack of research evidence to help clearly define these exercise parameters to better guide development of consensus exercise recommendations for older patients. This gap in knowledge limits the clinicians’ ability to recommend evidence-based treatment regimens to older adults who are at higher risk for falls. Moreover, although exercise programs are rarely associated with serious adverse events, recent findings from the Lifestyle Interventions and Independence for Elders (LIFE) study found a modest and nonstatistically significant association between long-term, moderate-intensity physical activity programs and an increase in hospitalizations and mortality in older adults.4,5 Taken together, these gaps in knowledge highlight the urgent need to better understand the optimal methods for administering exercise programs in older adults as well as the need for critical appraisals of the benefits and harms associated with long-term exercise training in this vulnerable population.
The results reported by de Souto Barreto and colleagues helped to address these questions. In this study, the authors found that long-term multicomponent training, particularly moderate intensity with balance exercises performed 2 to 3 times a week, appears to be a safe and effective intervention for reducing falls and injurious falls in older adults. Importantly, this type of long-term exercise regimen does not increase hospitalization and mortality, and thus supports the notion that exercise therapy is safe in older adults. Therefore, information gained from this meta-analysis should help to guide clinicians to devise a patient-centered exercise prescription for fall prevention.
The current study was well designed and has a number of strengths. The design of the systematic review and meta-analysis allowed aggregation of data from multiple trials, resulting in a more robust point estimate to evaluate the effects of long-term exercise training on falls and fall-related outcomes that otherwise cannot be achieved with individual trials. In addition, the emphasis on long-term exercise training in older adults in the setting of falls and adverse fall-related outcomes addresses a key area of research that currently lacks a sufficient evidence base. There are also several limitations in this study, primarily due to the nature of its meta-analysis design. For instance, the study populations included in the analysis are highly heterogeneous and range from those with dementia to healthy participants. In addition, long-term exercise training, defined as a duration ≥ 1 year, was arbitrarily established as the minimum period of intervention. Thus, potential important studies that include interventions of significant duration, but less than 1 year, may not have been captured in this analysis.
Applications for Clinical Practice
Falls in older adults are common and may lead to devastating health consequences. The implementation of a long-term, multicomponent, moderate-intensity exercise regimen performed 2 to 3 times weekly can reduce falls and injurious falls in older adults.
—Fred Ko, MD, MS
1. Schiller JS, Kramarow EA, Dey AN. Fall injury episodes among noninstitutionalized older adults: United States, 2001-2003. Adv Data. 2007(392);1-16.
2. Sterling DA, O’Connor JA, Bonadies J. Geriatric falls: injury severity is high and disproportionate to mechanism. J Trauma. 2001;50:116-119.
3. Sherrington C, Michaleff ZA, Fairhall N, et al. Exercise to prevent falls in older adults: an updated systematic review and meta-analysis. Br J Sports Med. 2017;51:1750-1758.
4. Liu CJ, Latham, NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev. 2009;CD002759.
5. Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311:2387-2396.
Study Overview
Objective. To evaluate the association between long-term exercise interventions (duration ≥ 1 year) and risks of falls, injurious falls, multiple falls, fractures, hospitalization, and mortality in older adults.
Design. A systematic review of randomized controlled trials (RCTs) with preplanned meta-analysis was conducted to investigate the association between long-term exercise interventions and falls and fall-related adverse outcomes in adults older than 60 years. A literature search using electronic databases, including PubMed, Cochrane Central Register of Controlled Trials, SportDiscus, PsychInfo, and Ageline, was performed between February 20 and March 5, 2018. Studies selected were RCTs with exercise duration of 1 year or longer, where effects of exercise intervention were compared with a comparator group of participants aged 60 years or older. Articles were independently screened, abstracted, and assessed for risk of bias by 2 raters, who resolved divergences in data extraction and synthesis via in-person meetings.
Setting and participants. A total of 46 studies (22,709 participants; median of 203 participants per study) were included in the review and 40 studies (21,868 participants) were included in the meta-analysis. The participants’ mean age was 73.1 ± 7.1 years, and 66.3% (15,054 participants) were women. Studies were mostly conducted in Europe (n = 15), North America (n = 13), and Oceania (n = 10). Multicomponent training involving multiple exercises (eg, aerobic, strength and balance; 29 RCTs) was the most common intervention modality, followed by aerobic (8 RCTs) and strength (5 RCTs) training. Exercise interventions had a mean frequency of 3 times/week, with each session lasting approximately 50 minutes, and were administered at a moderate intensity. The average compliance rate with exercise training was 65%. Comparator groups were often active controls that ranged from attention controls to more intensive interventions.
Main outcome measures. The 6 binary outcomes investigated were fallers who fell at least once, multiple times, or at least twice; fractures; hospitalization; and mortality. Estimates of outcomes were combined using risk ratios (RRs) using DerSimonian and Laird’s random-effects model (Mantel-Haenszel method). Heterogeneity was evaluated using I2 statistics, and trials with low rates of compliance (< 30%) with exercise intervention or high attrition (> 40%) were excluded in primary analyses.
Main results. Exercise training significantly reduced the risk of falls by 12% (n = 20 RCTs; 4420 participants; RR, 0.88; 95% confidence interval [CI], 0.79-0.98) and injurious falls by 26% (9 RCTs; 4481 participants; RR, 0.74; 95% CI, 0.62-0.88), and reduced the risk of fractures by 16% (19 RCTs; 8410 participants; RR, 0.84; 95% CI, 0.71-1.00; P = 0.05). Exercise training did not decrease the risk of multiple falls (13 RCTs; 3060 participants; RR, 0.86; 95% CI, 0.68-1.08), hospitalization (12 RCTs; 5639 participants; RR 0.94; 95% CI, 0.80-1.12), or mortality (29 RCTs; 11,441 participants; RR 0.96; 95% CI, 0.85-1.09). Sensitivity analyses yielded similar results, with the exception of the fixed-effect meta-analysis for the risk of fracture that showed a significant effect of long-term exercise training (RR, 0.84; 95% CI, 0.70-1.00; P = 0.047). Meta-regression analysis on mortality and falls suggested that exercise frequency between 2 and 3 times per week was optimal and beneficial.
Conclusion. Long-term exercise training of 1 year or longer in duration is associated with a reduction in falls, injurious falls, and fractures in older adults. Moreover, moderate intensity, multicomponent exercise training performed 2 to 3 times weekly is likely safe and effective in this vulnerable population.
Commentary
Falls are exceedingly common (1 in 3 older Americans fall each year) and are the leading cause of fatal and nonfatal injuries in persons over the age of 65 years.1,2 While fall prevention is a public health priority and a topic of interest in many research studies, there are important gaps in knowledge regarding optimal strategies to prevent falls and fall-related injuries in this high-risk population. The study reported by de Souto Barreto and colleagues provides new insights to address several of these gaps and may have a significant impact on the clinical practice of fall prevention in geriatric medicine.
Studies show that a single exercise intervention of short- to medium-term duration can prevent falls in community-dwelling older adults.3 However, the effects of long-term exercise training (ie, intervention lasting longer than a year) on fall prevention in this population is less well characterized. This study is the first meta-analysis that aimed to evaluate the potential beneficial impact of long-term exercise training on falls and adverse fall-related outcomes in adults ≥ 60 years of age who are prone to falls. The study’s findings indicate that long-term exercise training reduces the risk of falling by 12%, injurious falls by 26%, and factures by 16%. These results are important in that they add compelling evidence that exercise training of any duration can reduce falls and some fall-related adverse outcomes. Furthermore, the positive effects of long-term exercise training appear to mitigate some of the fatal and nonfatal injuries attributable to falls—the leading cause of such injuries in older adults.
The modality (type) and dose (frequency) of exercise training are important components of “exercise prescription” for older adults. However, there is a lack of research evidence to help clearly define these exercise parameters to better guide development of consensus exercise recommendations for older patients. This gap in knowledge limits the clinicians’ ability to recommend evidence-based treatment regimens to older adults who are at higher risk for falls. Moreover, although exercise programs are rarely associated with serious adverse events, recent findings from the Lifestyle Interventions and Independence for Elders (LIFE) study found a modest and nonstatistically significant association between long-term, moderate-intensity physical activity programs and an increase in hospitalizations and mortality in older adults.4,5 Taken together, these gaps in knowledge highlight the urgent need to better understand the optimal methods for administering exercise programs in older adults as well as the need for critical appraisals of the benefits and harms associated with long-term exercise training in this vulnerable population.
The results reported by de Souto Barreto and colleagues helped to address these questions. In this study, the authors found that long-term multicomponent training, particularly moderate intensity with balance exercises performed 2 to 3 times a week, appears to be a safe and effective intervention for reducing falls and injurious falls in older adults. Importantly, this type of long-term exercise regimen does not increase hospitalization and mortality, and thus supports the notion that exercise therapy is safe in older adults. Therefore, information gained from this meta-analysis should help to guide clinicians to devise a patient-centered exercise prescription for fall prevention.
The current study was well designed and has a number of strengths. The design of the systematic review and meta-analysis allowed aggregation of data from multiple trials, resulting in a more robust point estimate to evaluate the effects of long-term exercise training on falls and fall-related outcomes that otherwise cannot be achieved with individual trials. In addition, the emphasis on long-term exercise training in older adults in the setting of falls and adverse fall-related outcomes addresses a key area of research that currently lacks a sufficient evidence base. There are also several limitations in this study, primarily due to the nature of its meta-analysis design. For instance, the study populations included in the analysis are highly heterogeneous and range from those with dementia to healthy participants. In addition, long-term exercise training, defined as a duration ≥ 1 year, was arbitrarily established as the minimum period of intervention. Thus, potential important studies that include interventions of significant duration, but less than 1 year, may not have been captured in this analysis.
Applications for Clinical Practice
Falls in older adults are common and may lead to devastating health consequences. The implementation of a long-term, multicomponent, moderate-intensity exercise regimen performed 2 to 3 times weekly can reduce falls and injurious falls in older adults.
—Fred Ko, MD, MS
Study Overview
Objective. To evaluate the association between long-term exercise interventions (duration ≥ 1 year) and risks of falls, injurious falls, multiple falls, fractures, hospitalization, and mortality in older adults.
Design. A systematic review of randomized controlled trials (RCTs) with preplanned meta-analysis was conducted to investigate the association between long-term exercise interventions and falls and fall-related adverse outcomes in adults older than 60 years. A literature search using electronic databases, including PubMed, Cochrane Central Register of Controlled Trials, SportDiscus, PsychInfo, and Ageline, was performed between February 20 and March 5, 2018. Studies selected were RCTs with exercise duration of 1 year or longer, where effects of exercise intervention were compared with a comparator group of participants aged 60 years or older. Articles were independently screened, abstracted, and assessed for risk of bias by 2 raters, who resolved divergences in data extraction and synthesis via in-person meetings.
Setting and participants. A total of 46 studies (22,709 participants; median of 203 participants per study) were included in the review and 40 studies (21,868 participants) were included in the meta-analysis. The participants’ mean age was 73.1 ± 7.1 years, and 66.3% (15,054 participants) were women. Studies were mostly conducted in Europe (n = 15), North America (n = 13), and Oceania (n = 10). Multicomponent training involving multiple exercises (eg, aerobic, strength and balance; 29 RCTs) was the most common intervention modality, followed by aerobic (8 RCTs) and strength (5 RCTs) training. Exercise interventions had a mean frequency of 3 times/week, with each session lasting approximately 50 minutes, and were administered at a moderate intensity. The average compliance rate with exercise training was 65%. Comparator groups were often active controls that ranged from attention controls to more intensive interventions.
Main outcome measures. The 6 binary outcomes investigated were fallers who fell at least once, multiple times, or at least twice; fractures; hospitalization; and mortality. Estimates of outcomes were combined using risk ratios (RRs) using DerSimonian and Laird’s random-effects model (Mantel-Haenszel method). Heterogeneity was evaluated using I2 statistics, and trials with low rates of compliance (< 30%) with exercise intervention or high attrition (> 40%) were excluded in primary analyses.
Main results. Exercise training significantly reduced the risk of falls by 12% (n = 20 RCTs; 4420 participants; RR, 0.88; 95% confidence interval [CI], 0.79-0.98) and injurious falls by 26% (9 RCTs; 4481 participants; RR, 0.74; 95% CI, 0.62-0.88), and reduced the risk of fractures by 16% (19 RCTs; 8410 participants; RR, 0.84; 95% CI, 0.71-1.00; P = 0.05). Exercise training did not decrease the risk of multiple falls (13 RCTs; 3060 participants; RR, 0.86; 95% CI, 0.68-1.08), hospitalization (12 RCTs; 5639 participants; RR 0.94; 95% CI, 0.80-1.12), or mortality (29 RCTs; 11,441 participants; RR 0.96; 95% CI, 0.85-1.09). Sensitivity analyses yielded similar results, with the exception of the fixed-effect meta-analysis for the risk of fracture that showed a significant effect of long-term exercise training (RR, 0.84; 95% CI, 0.70-1.00; P = 0.047). Meta-regression analysis on mortality and falls suggested that exercise frequency between 2 and 3 times per week was optimal and beneficial.
Conclusion. Long-term exercise training of 1 year or longer in duration is associated with a reduction in falls, injurious falls, and fractures in older adults. Moreover, moderate intensity, multicomponent exercise training performed 2 to 3 times weekly is likely safe and effective in this vulnerable population.
Commentary
Falls are exceedingly common (1 in 3 older Americans fall each year) and are the leading cause of fatal and nonfatal injuries in persons over the age of 65 years.1,2 While fall prevention is a public health priority and a topic of interest in many research studies, there are important gaps in knowledge regarding optimal strategies to prevent falls and fall-related injuries in this high-risk population. The study reported by de Souto Barreto and colleagues provides new insights to address several of these gaps and may have a significant impact on the clinical practice of fall prevention in geriatric medicine.
Studies show that a single exercise intervention of short- to medium-term duration can prevent falls in community-dwelling older adults.3 However, the effects of long-term exercise training (ie, intervention lasting longer than a year) on fall prevention in this population is less well characterized. This study is the first meta-analysis that aimed to evaluate the potential beneficial impact of long-term exercise training on falls and adverse fall-related outcomes in adults ≥ 60 years of age who are prone to falls. The study’s findings indicate that long-term exercise training reduces the risk of falling by 12%, injurious falls by 26%, and factures by 16%. These results are important in that they add compelling evidence that exercise training of any duration can reduce falls and some fall-related adverse outcomes. Furthermore, the positive effects of long-term exercise training appear to mitigate some of the fatal and nonfatal injuries attributable to falls—the leading cause of such injuries in older adults.
The modality (type) and dose (frequency) of exercise training are important components of “exercise prescription” for older adults. However, there is a lack of research evidence to help clearly define these exercise parameters to better guide development of consensus exercise recommendations for older patients. This gap in knowledge limits the clinicians’ ability to recommend evidence-based treatment regimens to older adults who are at higher risk for falls. Moreover, although exercise programs are rarely associated with serious adverse events, recent findings from the Lifestyle Interventions and Independence for Elders (LIFE) study found a modest and nonstatistically significant association between long-term, moderate-intensity physical activity programs and an increase in hospitalizations and mortality in older adults.4,5 Taken together, these gaps in knowledge highlight the urgent need to better understand the optimal methods for administering exercise programs in older adults as well as the need for critical appraisals of the benefits and harms associated with long-term exercise training in this vulnerable population.
The results reported by de Souto Barreto and colleagues helped to address these questions. In this study, the authors found that long-term multicomponent training, particularly moderate intensity with balance exercises performed 2 to 3 times a week, appears to be a safe and effective intervention for reducing falls and injurious falls in older adults. Importantly, this type of long-term exercise regimen does not increase hospitalization and mortality, and thus supports the notion that exercise therapy is safe in older adults. Therefore, information gained from this meta-analysis should help to guide clinicians to devise a patient-centered exercise prescription for fall prevention.
The current study was well designed and has a number of strengths. The design of the systematic review and meta-analysis allowed aggregation of data from multiple trials, resulting in a more robust point estimate to evaluate the effects of long-term exercise training on falls and fall-related outcomes that otherwise cannot be achieved with individual trials. In addition, the emphasis on long-term exercise training in older adults in the setting of falls and adverse fall-related outcomes addresses a key area of research that currently lacks a sufficient evidence base. There are also several limitations in this study, primarily due to the nature of its meta-analysis design. For instance, the study populations included in the analysis are highly heterogeneous and range from those with dementia to healthy participants. In addition, long-term exercise training, defined as a duration ≥ 1 year, was arbitrarily established as the minimum period of intervention. Thus, potential important studies that include interventions of significant duration, but less than 1 year, may not have been captured in this analysis.
Applications for Clinical Practice
Falls in older adults are common and may lead to devastating health consequences. The implementation of a long-term, multicomponent, moderate-intensity exercise regimen performed 2 to 3 times weekly can reduce falls and injurious falls in older adults.
—Fred Ko, MD, MS
1. Schiller JS, Kramarow EA, Dey AN. Fall injury episodes among noninstitutionalized older adults: United States, 2001-2003. Adv Data. 2007(392);1-16.
2. Sterling DA, O’Connor JA, Bonadies J. Geriatric falls: injury severity is high and disproportionate to mechanism. J Trauma. 2001;50:116-119.
3. Sherrington C, Michaleff ZA, Fairhall N, et al. Exercise to prevent falls in older adults: an updated systematic review and meta-analysis. Br J Sports Med. 2017;51:1750-1758.
4. Liu CJ, Latham, NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev. 2009;CD002759.
5. Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311:2387-2396.
1. Schiller JS, Kramarow EA, Dey AN. Fall injury episodes among noninstitutionalized older adults: United States, 2001-2003. Adv Data. 2007(392);1-16.
2. Sterling DA, O’Connor JA, Bonadies J. Geriatric falls: injury severity is high and disproportionate to mechanism. J Trauma. 2001;50:116-119.
3. Sherrington C, Michaleff ZA, Fairhall N, et al. Exercise to prevent falls in older adults: an updated systematic review and meta-analysis. Br J Sports Med. 2017;51:1750-1758.
4. Liu CJ, Latham, NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev. 2009;CD002759.
5. Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311:2387-2396.
Receipt of Primary Care Linked to High-Value Care, Better Health Care Experience
Study Overview
Objective. To examine whether receiving primary care is associated with receipt of high-value services and low-value services and quality of patient experience.
Design. Secondary data analysis of the Medical Expenditure
To define whether a respondent received primary care, respondents were asked if they have a “usual source of care” and to provide the name of a physician they usually visit if they “are sick or need advice” about their health. Four additional questions asked respondents if they would visit their usual source of care for (1) “new health problems,” (2) “preventive health care such as general checkups, examinations, and immunizations,” (3) “ongoing health problems,” and (4) “referrals to other health professionals when needed.” These questions were intended to reflect the essential functions of primary care: providing first contact care that is comprehensive, continuous, and coordinated. Any respondents who indicated that they did not have a usual source of care or answered no to any of the 4 questions were considered to not have primary care. Among respondents who identified a usual source of care, 95% met criteria for having primary care.
Setting and participants. The study included 49,286 US adults with primary care and 21,133 US adults without primary care. The average age was 50 years (95% confidence interval [CI], 50-51) among those with primary care and 38 years (95% CI, 38-39) among those without primary care. Among those who had primary care, 55% were female, 50% were non-Hispanic white, 32% Hispanic, and 13% black; among those without primary care, 43% were female, 43% were non-Hispanic white, 35% Hispanic, and 13% black. Among respondents with primary care, 58% considered their health status to be excellent or very good, as compared with 66% of respondents without primary care. Lack of insurance was reported by 7% of respondents with primary care and 34% of respondents without primary care. Chronic disease was reported in 78% of respondents without primary care, as compared with 42% of respondents with primary care. The study uses propensity score matching methods to produce a matched cohort, taking into account potential confounders. The matching procedure resulted in a final sample of 43,766 respondents with primary care matched to 17,964 respondents without primary care.
Main outcome measures. Main study outcome measures included 39 quality measures aggregated into quality composites (6 high-value services and 4 low-value services), and 7 patient care experience measures aggregated into an overall patient experience rating and 2 experience composites. High-value services are defined as delivery of services that are likely of benefit, and include the use of recommended cancer screening such as colorectal cancer screening in appropriate age groups; recommended diagnostic and preventive testing such as cholesterol measurement and influenza vaccination; recommended diabetes care such as hemoglobin A1c measurement; recommended medical treatment for medical conditions such as heart failure, coronary artery disease, and chronic obstructive pulmonary disease; and recommended counseling such as smoking cessation. Low-value services are defined as delivery of services that are considered either inappropriate or of little to no benefit, and include cancer screening in older adults; inappropriate use of antibiotics such as for bronchitis; inappropriate medical treatment such as anxiolytic, sedative, or hypnotic prescriptions for older adults; and inappropriate imaging tests for certain conditions.
Composites of underuse (high-value care) and overuse (low-value care) were constructed from each measure of high- or low-value services by identifying respondents who were eligible for the measure and determining the proportion in which recommended care was delivered (for high-value measures) or avoided (for low-value measures). Patient care experience was measured by standardized CAHPS (Consumer Assessment of Healthcare Providers and Systems) measurement for global rating of health care, doctor communication, and access to care. The patient care experience measures were dichotomized into positive responses as a rating of 8, 9, or 10 on items scored from 0 to 10, and 4 for items scored from 1 to 4. The experience composite was constructed by computing the mean for each respondent and then the mean for all respondents.
Main results. The study found that respondents with primary care were more likely to receive high-value care in 4 of 5 composite measures—cancer screening, diagnostic and preventive testing, diabetes care, and recommended counseling such as smoking cessation—but not in the composite recommended treatment for specific medical conditions such as heart failure. Respondents with primary care were more likely to receive recommended cancer screening, as compared to those without primary care (78% vs 67%, respectively, with a difference of 10.8%; 95% CI, 8.5%-13.0%). Respondents with primary care were also more likely to receive recommended diagnostic and preventive testing (with a difference of 9.9%; 95% CI, 8.7%-11.2%), to receive high-value diabetes care (with a difference of 7.8%; 95% CI, 1.2%-14.4%), and to receive counselling (with a difference of 6.9%; 95% CI, 4.1%-9.7%) when compared to respondents without primary care. However the rates of receipt of high-value medical treatments were similar among respondents with or without primary care (with a difference of –4.6% (95% CI, –14.3% to 5.0%). In contrast, rates of low-value care were similar for those with or without primary care in 3 of 4 composites, including low-value cancer screening, medical treatment, and imaging, while those with primary care had higher rates of low-value antibiotic use (with a difference of 11.0%; 95% CI, 2.8%-19.3%). Respondents with primary care reported better patient care experience, including global rating of their health care, physician communication, and access to care, when compared to those without primary care.
Conclusion. Receipt of primary care is associated with a better patient care experience, more high-value care, and slightly more low-value care.
Commentary
Primary care has long been considered the bedrock of modern health care, and the delivery of comprehensive, continuous, high-quality primary care yields benefits to patients and the health care system.1 Primary care is associated with better outcomes, such as lower mortality and reduced rates of potentially avoidable hospitalizations, and people living in areas with higher concentrations of primary care are more likely to report better health.2 Primary care is also associated with reductions in health care cost and utilization while maintaining quality.2 The current study adds to what is known about the potential benefits of primary care by directly examining the association of the use of primary care versus no primary care with outcomes of high-value care, low-value care, and patient care experience. Because this study used nationally representative data, it was able to examine adults in all age groups, not only older adults in Medicare, which prior studies have relied on.3 The study’s findings—that adults seen in primary care receive more high-value care and report better care experiences—are not surprising. The study also found that slightly more low-value care is being delivered in primary care. These findings are consistent with prior studies. Also, although primary care overall may be associated with health care benefits, there is substantial variation in the rates of overuse (of low-value care) and underuse (of high-value care) in primary care, and this may represent opportunities for improvement.4
This study has several limitations. Because the study defined primary care using questions that identify essential elements of primary care—first contact, comprehensiveness, continuity, and coordinated care—the findings may not apply to all individuals who have identified a primary care provider, but only to those who experience comprehensive, continuous, and coordinated care. Inclusion of all individuals who identify a usual source of primary care as the sole criteria may attenuate the association of primary care with the outcome measures. It is, however, reassuring that among those who identified a usual source of care (primary care), 95% indicated that they have care that is consistent with the principles of first contact care, comprehensiveness, continuity, and coordinated care. Another limitation is that the use of the criteria to indicate high- or low-value care may not capture the nuances of patient-centered care, preferences, or individualized decision-making that occurs in clinical care. Nonetheless, definitions used in the study for high- and low-value care are consistent with prior literature, and offer a standardized measure to indicate quality of care.
Applications for Clinical Practice
A recent trend in health care is the shift of continuity of care from primary care providers or practices to facility-based care or no continuity of care at all, and this shift disproportionately affects patients with low income and is associated with more emergency room visits.5 The current study makes a strong case for the potential benefits of receiving primary care that is comprehensive, continuous, and coordinated, as patients in primary care are more likely to receive high-value over low-value care, and to have a better care experience. The ongoing debate on changes to the health care system and insurance options must take into account the impact of any changes on the population receiving primary care coverage, with the goal that more, rather than fewer, individuals realize the potential benefits of comprehensive primary care.
— William W. Hung, MD MPH
1. Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83:457-502.
2. American College of Physicians. How is a shortage of primary care physicians affecting the quality and cost of medical care? www.acponline.org/acp_policy/policies/primary_care_shortage_affecting_hc_2008.pdf. Published 2008. Accessed June 11, 2019.
3. Bazemore A, Petterson S, Peterson LE, et al. Higher primary care physician continuity is associated with lower costs and hospitalizations. Ann Fam Med. 2018;16:492-497.
4. O’Sullivan JW, Albasri A, Nicholson BD, et al. Overtesting and undertesting in primary care: a systematic review and meta-analysis. BMJ Open. 2018;8:e018557.
5. Liaw W, Jetty A, Petterson S, et al. Trends in the types of usual sources of care: a shift from people to places or nothing at all. Health Serv Res. 2018;53:2346-2367.
Study Overview
Objective. To examine whether receiving primary care is associated with receipt of high-value services and low-value services and quality of patient experience.
Design. Secondary data analysis of the Medical Expenditure
To define whether a respondent received primary care, respondents were asked if they have a “usual source of care” and to provide the name of a physician they usually visit if they “are sick or need advice” about their health. Four additional questions asked respondents if they would visit their usual source of care for (1) “new health problems,” (2) “preventive health care such as general checkups, examinations, and immunizations,” (3) “ongoing health problems,” and (4) “referrals to other health professionals when needed.” These questions were intended to reflect the essential functions of primary care: providing first contact care that is comprehensive, continuous, and coordinated. Any respondents who indicated that they did not have a usual source of care or answered no to any of the 4 questions were considered to not have primary care. Among respondents who identified a usual source of care, 95% met criteria for having primary care.
Setting and participants. The study included 49,286 US adults with primary care and 21,133 US adults without primary care. The average age was 50 years (95% confidence interval [CI], 50-51) among those with primary care and 38 years (95% CI, 38-39) among those without primary care. Among those who had primary care, 55% were female, 50% were non-Hispanic white, 32% Hispanic, and 13% black; among those without primary care, 43% were female, 43% were non-Hispanic white, 35% Hispanic, and 13% black. Among respondents with primary care, 58% considered their health status to be excellent or very good, as compared with 66% of respondents without primary care. Lack of insurance was reported by 7% of respondents with primary care and 34% of respondents without primary care. Chronic disease was reported in 78% of respondents without primary care, as compared with 42% of respondents with primary care. The study uses propensity score matching methods to produce a matched cohort, taking into account potential confounders. The matching procedure resulted in a final sample of 43,766 respondents with primary care matched to 17,964 respondents without primary care.
Main outcome measures. Main study outcome measures included 39 quality measures aggregated into quality composites (6 high-value services and 4 low-value services), and 7 patient care experience measures aggregated into an overall patient experience rating and 2 experience composites. High-value services are defined as delivery of services that are likely of benefit, and include the use of recommended cancer screening such as colorectal cancer screening in appropriate age groups; recommended diagnostic and preventive testing such as cholesterol measurement and influenza vaccination; recommended diabetes care such as hemoglobin A1c measurement; recommended medical treatment for medical conditions such as heart failure, coronary artery disease, and chronic obstructive pulmonary disease; and recommended counseling such as smoking cessation. Low-value services are defined as delivery of services that are considered either inappropriate or of little to no benefit, and include cancer screening in older adults; inappropriate use of antibiotics such as for bronchitis; inappropriate medical treatment such as anxiolytic, sedative, or hypnotic prescriptions for older adults; and inappropriate imaging tests for certain conditions.
Composites of underuse (high-value care) and overuse (low-value care) were constructed from each measure of high- or low-value services by identifying respondents who were eligible for the measure and determining the proportion in which recommended care was delivered (for high-value measures) or avoided (for low-value measures). Patient care experience was measured by standardized CAHPS (Consumer Assessment of Healthcare Providers and Systems) measurement for global rating of health care, doctor communication, and access to care. The patient care experience measures were dichotomized into positive responses as a rating of 8, 9, or 10 on items scored from 0 to 10, and 4 for items scored from 1 to 4. The experience composite was constructed by computing the mean for each respondent and then the mean for all respondents.
Main results. The study found that respondents with primary care were more likely to receive high-value care in 4 of 5 composite measures—cancer screening, diagnostic and preventive testing, diabetes care, and recommended counseling such as smoking cessation—but not in the composite recommended treatment for specific medical conditions such as heart failure. Respondents with primary care were more likely to receive recommended cancer screening, as compared to those without primary care (78% vs 67%, respectively, with a difference of 10.8%; 95% CI, 8.5%-13.0%). Respondents with primary care were also more likely to receive recommended diagnostic and preventive testing (with a difference of 9.9%; 95% CI, 8.7%-11.2%), to receive high-value diabetes care (with a difference of 7.8%; 95% CI, 1.2%-14.4%), and to receive counselling (with a difference of 6.9%; 95% CI, 4.1%-9.7%) when compared to respondents without primary care. However the rates of receipt of high-value medical treatments were similar among respondents with or without primary care (with a difference of –4.6% (95% CI, –14.3% to 5.0%). In contrast, rates of low-value care were similar for those with or without primary care in 3 of 4 composites, including low-value cancer screening, medical treatment, and imaging, while those with primary care had higher rates of low-value antibiotic use (with a difference of 11.0%; 95% CI, 2.8%-19.3%). Respondents with primary care reported better patient care experience, including global rating of their health care, physician communication, and access to care, when compared to those without primary care.
Conclusion. Receipt of primary care is associated with a better patient care experience, more high-value care, and slightly more low-value care.
Commentary
Primary care has long been considered the bedrock of modern health care, and the delivery of comprehensive, continuous, high-quality primary care yields benefits to patients and the health care system.1 Primary care is associated with better outcomes, such as lower mortality and reduced rates of potentially avoidable hospitalizations, and people living in areas with higher concentrations of primary care are more likely to report better health.2 Primary care is also associated with reductions in health care cost and utilization while maintaining quality.2 The current study adds to what is known about the potential benefits of primary care by directly examining the association of the use of primary care versus no primary care with outcomes of high-value care, low-value care, and patient care experience. Because this study used nationally representative data, it was able to examine adults in all age groups, not only older adults in Medicare, which prior studies have relied on.3 The study’s findings—that adults seen in primary care receive more high-value care and report better care experiences—are not surprising. The study also found that slightly more low-value care is being delivered in primary care. These findings are consistent with prior studies. Also, although primary care overall may be associated with health care benefits, there is substantial variation in the rates of overuse (of low-value care) and underuse (of high-value care) in primary care, and this may represent opportunities for improvement.4
This study has several limitations. Because the study defined primary care using questions that identify essential elements of primary care—first contact, comprehensiveness, continuity, and coordinated care—the findings may not apply to all individuals who have identified a primary care provider, but only to those who experience comprehensive, continuous, and coordinated care. Inclusion of all individuals who identify a usual source of primary care as the sole criteria may attenuate the association of primary care with the outcome measures. It is, however, reassuring that among those who identified a usual source of care (primary care), 95% indicated that they have care that is consistent with the principles of first contact care, comprehensiveness, continuity, and coordinated care. Another limitation is that the use of the criteria to indicate high- or low-value care may not capture the nuances of patient-centered care, preferences, or individualized decision-making that occurs in clinical care. Nonetheless, definitions used in the study for high- and low-value care are consistent with prior literature, and offer a standardized measure to indicate quality of care.
Applications for Clinical Practice
A recent trend in health care is the shift of continuity of care from primary care providers or practices to facility-based care or no continuity of care at all, and this shift disproportionately affects patients with low income and is associated with more emergency room visits.5 The current study makes a strong case for the potential benefits of receiving primary care that is comprehensive, continuous, and coordinated, as patients in primary care are more likely to receive high-value over low-value care, and to have a better care experience. The ongoing debate on changes to the health care system and insurance options must take into account the impact of any changes on the population receiving primary care coverage, with the goal that more, rather than fewer, individuals realize the potential benefits of comprehensive primary care.
— William W. Hung, MD MPH
Study Overview
Objective. To examine whether receiving primary care is associated with receipt of high-value services and low-value services and quality of patient experience.
Design. Secondary data analysis of the Medical Expenditure
To define whether a respondent received primary care, respondents were asked if they have a “usual source of care” and to provide the name of a physician they usually visit if they “are sick or need advice” about their health. Four additional questions asked respondents if they would visit their usual source of care for (1) “new health problems,” (2) “preventive health care such as general checkups, examinations, and immunizations,” (3) “ongoing health problems,” and (4) “referrals to other health professionals when needed.” These questions were intended to reflect the essential functions of primary care: providing first contact care that is comprehensive, continuous, and coordinated. Any respondents who indicated that they did not have a usual source of care or answered no to any of the 4 questions were considered to not have primary care. Among respondents who identified a usual source of care, 95% met criteria for having primary care.
Setting and participants. The study included 49,286 US adults with primary care and 21,133 US adults without primary care. The average age was 50 years (95% confidence interval [CI], 50-51) among those with primary care and 38 years (95% CI, 38-39) among those without primary care. Among those who had primary care, 55% were female, 50% were non-Hispanic white, 32% Hispanic, and 13% black; among those without primary care, 43% were female, 43% were non-Hispanic white, 35% Hispanic, and 13% black. Among respondents with primary care, 58% considered their health status to be excellent or very good, as compared with 66% of respondents without primary care. Lack of insurance was reported by 7% of respondents with primary care and 34% of respondents without primary care. Chronic disease was reported in 78% of respondents without primary care, as compared with 42% of respondents with primary care. The study uses propensity score matching methods to produce a matched cohort, taking into account potential confounders. The matching procedure resulted in a final sample of 43,766 respondents with primary care matched to 17,964 respondents without primary care.
Main outcome measures. Main study outcome measures included 39 quality measures aggregated into quality composites (6 high-value services and 4 low-value services), and 7 patient care experience measures aggregated into an overall patient experience rating and 2 experience composites. High-value services are defined as delivery of services that are likely of benefit, and include the use of recommended cancer screening such as colorectal cancer screening in appropriate age groups; recommended diagnostic and preventive testing such as cholesterol measurement and influenza vaccination; recommended diabetes care such as hemoglobin A1c measurement; recommended medical treatment for medical conditions such as heart failure, coronary artery disease, and chronic obstructive pulmonary disease; and recommended counseling such as smoking cessation. Low-value services are defined as delivery of services that are considered either inappropriate or of little to no benefit, and include cancer screening in older adults; inappropriate use of antibiotics such as for bronchitis; inappropriate medical treatment such as anxiolytic, sedative, or hypnotic prescriptions for older adults; and inappropriate imaging tests for certain conditions.
Composites of underuse (high-value care) and overuse (low-value care) were constructed from each measure of high- or low-value services by identifying respondents who were eligible for the measure and determining the proportion in which recommended care was delivered (for high-value measures) or avoided (for low-value measures). Patient care experience was measured by standardized CAHPS (Consumer Assessment of Healthcare Providers and Systems) measurement for global rating of health care, doctor communication, and access to care. The patient care experience measures were dichotomized into positive responses as a rating of 8, 9, or 10 on items scored from 0 to 10, and 4 for items scored from 1 to 4. The experience composite was constructed by computing the mean for each respondent and then the mean for all respondents.
Main results. The study found that respondents with primary care were more likely to receive high-value care in 4 of 5 composite measures—cancer screening, diagnostic and preventive testing, diabetes care, and recommended counseling such as smoking cessation—but not in the composite recommended treatment for specific medical conditions such as heart failure. Respondents with primary care were more likely to receive recommended cancer screening, as compared to those without primary care (78% vs 67%, respectively, with a difference of 10.8%; 95% CI, 8.5%-13.0%). Respondents with primary care were also more likely to receive recommended diagnostic and preventive testing (with a difference of 9.9%; 95% CI, 8.7%-11.2%), to receive high-value diabetes care (with a difference of 7.8%; 95% CI, 1.2%-14.4%), and to receive counselling (with a difference of 6.9%; 95% CI, 4.1%-9.7%) when compared to respondents without primary care. However the rates of receipt of high-value medical treatments were similar among respondents with or without primary care (with a difference of –4.6% (95% CI, –14.3% to 5.0%). In contrast, rates of low-value care were similar for those with or without primary care in 3 of 4 composites, including low-value cancer screening, medical treatment, and imaging, while those with primary care had higher rates of low-value antibiotic use (with a difference of 11.0%; 95% CI, 2.8%-19.3%). Respondents with primary care reported better patient care experience, including global rating of their health care, physician communication, and access to care, when compared to those without primary care.
Conclusion. Receipt of primary care is associated with a better patient care experience, more high-value care, and slightly more low-value care.
Commentary
Primary care has long been considered the bedrock of modern health care, and the delivery of comprehensive, continuous, high-quality primary care yields benefits to patients and the health care system.1 Primary care is associated with better outcomes, such as lower mortality and reduced rates of potentially avoidable hospitalizations, and people living in areas with higher concentrations of primary care are more likely to report better health.2 Primary care is also associated with reductions in health care cost and utilization while maintaining quality.2 The current study adds to what is known about the potential benefits of primary care by directly examining the association of the use of primary care versus no primary care with outcomes of high-value care, low-value care, and patient care experience. Because this study used nationally representative data, it was able to examine adults in all age groups, not only older adults in Medicare, which prior studies have relied on.3 The study’s findings—that adults seen in primary care receive more high-value care and report better care experiences—are not surprising. The study also found that slightly more low-value care is being delivered in primary care. These findings are consistent with prior studies. Also, although primary care overall may be associated with health care benefits, there is substantial variation in the rates of overuse (of low-value care) and underuse (of high-value care) in primary care, and this may represent opportunities for improvement.4
This study has several limitations. Because the study defined primary care using questions that identify essential elements of primary care—first contact, comprehensiveness, continuity, and coordinated care—the findings may not apply to all individuals who have identified a primary care provider, but only to those who experience comprehensive, continuous, and coordinated care. Inclusion of all individuals who identify a usual source of primary care as the sole criteria may attenuate the association of primary care with the outcome measures. It is, however, reassuring that among those who identified a usual source of care (primary care), 95% indicated that they have care that is consistent with the principles of first contact care, comprehensiveness, continuity, and coordinated care. Another limitation is that the use of the criteria to indicate high- or low-value care may not capture the nuances of patient-centered care, preferences, or individualized decision-making that occurs in clinical care. Nonetheless, definitions used in the study for high- and low-value care are consistent with prior literature, and offer a standardized measure to indicate quality of care.
Applications for Clinical Practice
A recent trend in health care is the shift of continuity of care from primary care providers or practices to facility-based care or no continuity of care at all, and this shift disproportionately affects patients with low income and is associated with more emergency room visits.5 The current study makes a strong case for the potential benefits of receiving primary care that is comprehensive, continuous, and coordinated, as patients in primary care are more likely to receive high-value over low-value care, and to have a better care experience. The ongoing debate on changes to the health care system and insurance options must take into account the impact of any changes on the population receiving primary care coverage, with the goal that more, rather than fewer, individuals realize the potential benefits of comprehensive primary care.
— William W. Hung, MD MPH
1. Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83:457-502.
2. American College of Physicians. How is a shortage of primary care physicians affecting the quality and cost of medical care? www.acponline.org/acp_policy/policies/primary_care_shortage_affecting_hc_2008.pdf. Published 2008. Accessed June 11, 2019.
3. Bazemore A, Petterson S, Peterson LE, et al. Higher primary care physician continuity is associated with lower costs and hospitalizations. Ann Fam Med. 2018;16:492-497.
4. O’Sullivan JW, Albasri A, Nicholson BD, et al. Overtesting and undertesting in primary care: a systematic review and meta-analysis. BMJ Open. 2018;8:e018557.
5. Liaw W, Jetty A, Petterson S, et al. Trends in the types of usual sources of care: a shift from people to places or nothing at all. Health Serv Res. 2018;53:2346-2367.
1. Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83:457-502.
2. American College of Physicians. How is a shortage of primary care physicians affecting the quality and cost of medical care? www.acponline.org/acp_policy/policies/primary_care_shortage_affecting_hc_2008.pdf. Published 2008. Accessed June 11, 2019.
3. Bazemore A, Petterson S, Peterson LE, et al. Higher primary care physician continuity is associated with lower costs and hospitalizations. Ann Fam Med. 2018;16:492-497.
4. O’Sullivan JW, Albasri A, Nicholson BD, et al. Overtesting and undertesting in primary care: a systematic review and meta-analysis. BMJ Open. 2018;8:e018557.
5. Liaw W, Jetty A, Petterson S, et al. Trends in the types of usual sources of care: a shift from people to places or nothing at all. Health Serv Res. 2018;53:2346-2367.
Enzalutamide Improves Progression-Free and Overall Survival in Metastatic Hormone-Sensitive Prostate Cancer
Study Overview
Objective. To evaluate the efficacy of enzalutamide compared with standard first-line testosterone suppression in men with newly diagnosed metastatic, castrate-sensitive prostate cancer.
Design. Multinational, open-label, randomized phase 3 trial.
Setting and participants. 1125 men were randomly assigned to receive enzalutamide (563 patients) or standard care (562 patients) from March 2014 through March 2017. Eligible patients had a histologic diagnosis of prostate adenocarcinoma with metastases documented by conventional imaging with computed tomography (CT) and/or technetium-99 bone scan. Prior use of adjuvant testosterone suppression was allowed for up to 2 years, provided this had been completed at least 12 months prior to enrollment.
Intervention. Patients were randomized in a 1:1 fashion to receive enzalutamide 160 mg daily or nonsteroidal antiandrogen therapy with bicalutamide, nilutamide, or flutamide. All patients received testosterone suppression with goserelin, leuprolide, or degarelix. Therapy was continued until disease progression or intolerable adverse effects occurred. In November 2014 the protocol was amended to allow for early administration of docetaxel 75 mg/m2 every 3 weeks for 6 cycles and androgen suppression. Patients were stratified according to having received docetaxel prior to randomization. This amendment was based on evidence of improved survival noted with this combination, and the decision to add docetaxel was up to the treating physician. The randomization was further stratified by disease volume, the use of bone-modifying agents, and comorbidity scores. High-volume disease was defined as the presence of visceral metastases or at least 4 bone lesions, with at least 1 being in the appendicular skeleton.
Main outcome measures. The primary endpoint was overall survival (OS). The secondary endpoints were prostate-specific antigen (PSA) progression-free survival (PFS), clinical PFS, death from any cause, or the last known follow-up PSA. PSA progression was defined as an increase in PSA level from the nadir value by ≥ 25% and by ≥ 2 ng/mL.
Main results. The baseline characteristics were well balanced between the treatment arms. High-volume disease was present in 52% of patients. Early docetaxel was planned in 45% of patients; however, 22 patients in whom docetaxel treatment was planned did not receive it. All 6 cycles of docetaxel were given to 159 patients in the enzalutamide group and 181 patients in the standard-care group. After a median follow-up of 34 months, there were 102 deaths in the enzalutamide group and 143 deaths in the standard-care group, with a hazard ratio (HR) for death of 0.67 (95% confidence interval [CI], 0.52-0.86; P = 0.002). Early docetaxel treatment, volume of disease, and use of bone-modifying agents did not affect this outcome. At 3 years, the OS was 80% in the enzalutamide group and 72% in the standard-care group. The rate of PSA-determined PFS was higher in the enzalutamide group compared with the standard group (3-year event-free survival, 67% and 37%, respectively), with a HR of 0.39 (95% CI, 0.33-0.47; P < 0.001). There were fewer clinical PFS events in the enzalutamide group (167 events vs 320 events), with a HR of 0.40 (95% CI, 0.33-0.49; P < 0.001). Analysis of the stratified subgroups showed the effect on OS was diminished in those with use of bone-modifying agents, those with high-volume disease, and those who received early docetaxel. The clinical PFS benefit was maintained across all subgroups, albeit with a smaller effect in those with high-volume disease and in those with early docetaxel treatment.
Treatment discontinuation for reasons other than progressive disease occurred in 12% of those in the enzalutamide group and 19% of those in the standard-care group. Overall, the adverse events were consistent with the known safety profiles of the treatment regimen. Seizures occurred in 7 patients on enzalutamide and no patients in the standard-care group. Fatigue was more common with enzalutamide.
Conclusion. Enzalutamide treatment was associated with significantly longer PFS and OS compared with standard care in men with metastatic, hormone-sensitive prostate cancer receiving testosterone suppression.
Commentary
The current study shows that the addition of enzalutamide to standard androgen deprivation therapy (ADT) improves OS and PFS in men with newly diagnosed metastatic, hormone-sensitive prostate cancer. Until recently, antiandrogen therapy had been the standard of care for these men; however, with the advent of novel antiandrogen agents, outcomes in men with metastatic prostate cancer in both the androgen-sensitive and castrate-resistant settings have steadily improved.1-5 In the castrate-resistant setting, enzalutamide has previously been shown to improve survival in chemotherapy-naïve patients and those previously exposed to docetaxel chemotherapy.5-7 Similarly, in the hormone-sensitive setting the combination of ADT with either abiraterone or chemotherapy has been shown to improve outcomes. In the phase 3 LATITUDE and STAMPEDE trials, the combination of abiraterone plus prednisone and ADT resulted in a 30% and 37% improvement in OS, respectively.1,2 Six cycles of docetaxel in combination with ADT also resulted in a 37% increase in OS in those with high-volume metastatic disease.3
The current study adds to the growing body of literature suggesting that combination therapy in the upfront, hormone-sensitive setting improves outcomes. In the CHAARTED trial, the combination of docetaxel and ADT improved survival in men with high-volume disease, but it did not seem to benefit those with lower-volume disease.3 However, the current data suggests a survival advantage with enzalutamide with low-volume disease as well. The use of docetaxel was similar between the 2 groups, and this suggests that the benefits of enzalutamide cannot be attributed to early integration of docetaxel. It is important to note that the subgroup analysis of those who received early docetaxel showed that these patients did not experience the same survival benefit as those who did not receive docetaxel. However, this trial was not powered for this analysis, and thus it should be interpreted with caution. PFS benefit was maintained across those who received and did not receive early docetaxel. Also worth noting is the increased docetaxel-related toxicity in the combination docetaxel and enzalutamide arm of this study. The neurological toxicity of enzalutamide was again noted, with 7 seizure events documented in this study.
Because this report on the ENZAMET study is an interim analysis, it will be important to follow these outcomes as the data set matures to ensure these effects are maintained over time. Additionally, it will be important to see what implications the addition of enzalutamide have on quality of life measures, as these data have not yet been published.
Applications for Clinical Practice
The ENZAMET study provides evidence that in men with metastatic, hormone-sensitive prostate cancer receiving ADT, the addition of enzalutamide improves PFS and OS. In men who received early docetaxel, enzalutamide was associated with increased toxicity. Additionally, while PFS was improved in men who received enzalutamide and docetaxel, OS was not improved. The neurologic toxicities of enzalutamide should be considered, particularly in those with a prior history of seizure disorders. Based on these data, enzalutamide in combination with ADT represents a reasonable treatment option in men with metastatic, hormone-sensitive prostate cancer.
—Daniel Isaac, DO, MS
1. Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352-360.
2. James ND, de Bono JS, Spears MR, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338-351.
3. Kytriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36:1080-1087.
4. Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naïve men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomized, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152-160.
5. Beer TM, Armstrong AJ, Rathkopf D, et al. Enzalutamide in men with chemotherapy-naïve metastatic castration-resistant prostate cancer: extended analysis of the phase 3 PREVAIL study. Eur Urol. 2017;71:151-154.
6. Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187-1197.
7. Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with non-metastatic castration resistant prostate cancer. N Engl J Med. 2018;378:2465-2474.
Study Overview
Objective. To evaluate the efficacy of enzalutamide compared with standard first-line testosterone suppression in men with newly diagnosed metastatic, castrate-sensitive prostate cancer.
Design. Multinational, open-label, randomized phase 3 trial.
Setting and participants. 1125 men were randomly assigned to receive enzalutamide (563 patients) or standard care (562 patients) from March 2014 through March 2017. Eligible patients had a histologic diagnosis of prostate adenocarcinoma with metastases documented by conventional imaging with computed tomography (CT) and/or technetium-99 bone scan. Prior use of adjuvant testosterone suppression was allowed for up to 2 years, provided this had been completed at least 12 months prior to enrollment.
Intervention. Patients were randomized in a 1:1 fashion to receive enzalutamide 160 mg daily or nonsteroidal antiandrogen therapy with bicalutamide, nilutamide, or flutamide. All patients received testosterone suppression with goserelin, leuprolide, or degarelix. Therapy was continued until disease progression or intolerable adverse effects occurred. In November 2014 the protocol was amended to allow for early administration of docetaxel 75 mg/m2 every 3 weeks for 6 cycles and androgen suppression. Patients were stratified according to having received docetaxel prior to randomization. This amendment was based on evidence of improved survival noted with this combination, and the decision to add docetaxel was up to the treating physician. The randomization was further stratified by disease volume, the use of bone-modifying agents, and comorbidity scores. High-volume disease was defined as the presence of visceral metastases or at least 4 bone lesions, with at least 1 being in the appendicular skeleton.
Main outcome measures. The primary endpoint was overall survival (OS). The secondary endpoints were prostate-specific antigen (PSA) progression-free survival (PFS), clinical PFS, death from any cause, or the last known follow-up PSA. PSA progression was defined as an increase in PSA level from the nadir value by ≥ 25% and by ≥ 2 ng/mL.
Main results. The baseline characteristics were well balanced between the treatment arms. High-volume disease was present in 52% of patients. Early docetaxel was planned in 45% of patients; however, 22 patients in whom docetaxel treatment was planned did not receive it. All 6 cycles of docetaxel were given to 159 patients in the enzalutamide group and 181 patients in the standard-care group. After a median follow-up of 34 months, there were 102 deaths in the enzalutamide group and 143 deaths in the standard-care group, with a hazard ratio (HR) for death of 0.67 (95% confidence interval [CI], 0.52-0.86; P = 0.002). Early docetaxel treatment, volume of disease, and use of bone-modifying agents did not affect this outcome. At 3 years, the OS was 80% in the enzalutamide group and 72% in the standard-care group. The rate of PSA-determined PFS was higher in the enzalutamide group compared with the standard group (3-year event-free survival, 67% and 37%, respectively), with a HR of 0.39 (95% CI, 0.33-0.47; P < 0.001). There were fewer clinical PFS events in the enzalutamide group (167 events vs 320 events), with a HR of 0.40 (95% CI, 0.33-0.49; P < 0.001). Analysis of the stratified subgroups showed the effect on OS was diminished in those with use of bone-modifying agents, those with high-volume disease, and those who received early docetaxel. The clinical PFS benefit was maintained across all subgroups, albeit with a smaller effect in those with high-volume disease and in those with early docetaxel treatment.
Treatment discontinuation for reasons other than progressive disease occurred in 12% of those in the enzalutamide group and 19% of those in the standard-care group. Overall, the adverse events were consistent with the known safety profiles of the treatment regimen. Seizures occurred in 7 patients on enzalutamide and no patients in the standard-care group. Fatigue was more common with enzalutamide.
Conclusion. Enzalutamide treatment was associated with significantly longer PFS and OS compared with standard care in men with metastatic, hormone-sensitive prostate cancer receiving testosterone suppression.
Commentary
The current study shows that the addition of enzalutamide to standard androgen deprivation therapy (ADT) improves OS and PFS in men with newly diagnosed metastatic, hormone-sensitive prostate cancer. Until recently, antiandrogen therapy had been the standard of care for these men; however, with the advent of novel antiandrogen agents, outcomes in men with metastatic prostate cancer in both the androgen-sensitive and castrate-resistant settings have steadily improved.1-5 In the castrate-resistant setting, enzalutamide has previously been shown to improve survival in chemotherapy-naïve patients and those previously exposed to docetaxel chemotherapy.5-7 Similarly, in the hormone-sensitive setting the combination of ADT with either abiraterone or chemotherapy has been shown to improve outcomes. In the phase 3 LATITUDE and STAMPEDE trials, the combination of abiraterone plus prednisone and ADT resulted in a 30% and 37% improvement in OS, respectively.1,2 Six cycles of docetaxel in combination with ADT also resulted in a 37% increase in OS in those with high-volume metastatic disease.3
The current study adds to the growing body of literature suggesting that combination therapy in the upfront, hormone-sensitive setting improves outcomes. In the CHAARTED trial, the combination of docetaxel and ADT improved survival in men with high-volume disease, but it did not seem to benefit those with lower-volume disease.3 However, the current data suggests a survival advantage with enzalutamide with low-volume disease as well. The use of docetaxel was similar between the 2 groups, and this suggests that the benefits of enzalutamide cannot be attributed to early integration of docetaxel. It is important to note that the subgroup analysis of those who received early docetaxel showed that these patients did not experience the same survival benefit as those who did not receive docetaxel. However, this trial was not powered for this analysis, and thus it should be interpreted with caution. PFS benefit was maintained across those who received and did not receive early docetaxel. Also worth noting is the increased docetaxel-related toxicity in the combination docetaxel and enzalutamide arm of this study. The neurological toxicity of enzalutamide was again noted, with 7 seizure events documented in this study.
Because this report on the ENZAMET study is an interim analysis, it will be important to follow these outcomes as the data set matures to ensure these effects are maintained over time. Additionally, it will be important to see what implications the addition of enzalutamide have on quality of life measures, as these data have not yet been published.
Applications for Clinical Practice
The ENZAMET study provides evidence that in men with metastatic, hormone-sensitive prostate cancer receiving ADT, the addition of enzalutamide improves PFS and OS. In men who received early docetaxel, enzalutamide was associated with increased toxicity. Additionally, while PFS was improved in men who received enzalutamide and docetaxel, OS was not improved. The neurologic toxicities of enzalutamide should be considered, particularly in those with a prior history of seizure disorders. Based on these data, enzalutamide in combination with ADT represents a reasonable treatment option in men with metastatic, hormone-sensitive prostate cancer.
—Daniel Isaac, DO, MS
Study Overview
Objective. To evaluate the efficacy of enzalutamide compared with standard first-line testosterone suppression in men with newly diagnosed metastatic, castrate-sensitive prostate cancer.
Design. Multinational, open-label, randomized phase 3 trial.
Setting and participants. 1125 men were randomly assigned to receive enzalutamide (563 patients) or standard care (562 patients) from March 2014 through March 2017. Eligible patients had a histologic diagnosis of prostate adenocarcinoma with metastases documented by conventional imaging with computed tomography (CT) and/or technetium-99 bone scan. Prior use of adjuvant testosterone suppression was allowed for up to 2 years, provided this had been completed at least 12 months prior to enrollment.
Intervention. Patients were randomized in a 1:1 fashion to receive enzalutamide 160 mg daily or nonsteroidal antiandrogen therapy with bicalutamide, nilutamide, or flutamide. All patients received testosterone suppression with goserelin, leuprolide, or degarelix. Therapy was continued until disease progression or intolerable adverse effects occurred. In November 2014 the protocol was amended to allow for early administration of docetaxel 75 mg/m2 every 3 weeks for 6 cycles and androgen suppression. Patients were stratified according to having received docetaxel prior to randomization. This amendment was based on evidence of improved survival noted with this combination, and the decision to add docetaxel was up to the treating physician. The randomization was further stratified by disease volume, the use of bone-modifying agents, and comorbidity scores. High-volume disease was defined as the presence of visceral metastases or at least 4 bone lesions, with at least 1 being in the appendicular skeleton.
Main outcome measures. The primary endpoint was overall survival (OS). The secondary endpoints were prostate-specific antigen (PSA) progression-free survival (PFS), clinical PFS, death from any cause, or the last known follow-up PSA. PSA progression was defined as an increase in PSA level from the nadir value by ≥ 25% and by ≥ 2 ng/mL.
Main results. The baseline characteristics were well balanced between the treatment arms. High-volume disease was present in 52% of patients. Early docetaxel was planned in 45% of patients; however, 22 patients in whom docetaxel treatment was planned did not receive it. All 6 cycles of docetaxel were given to 159 patients in the enzalutamide group and 181 patients in the standard-care group. After a median follow-up of 34 months, there were 102 deaths in the enzalutamide group and 143 deaths in the standard-care group, with a hazard ratio (HR) for death of 0.67 (95% confidence interval [CI], 0.52-0.86; P = 0.002). Early docetaxel treatment, volume of disease, and use of bone-modifying agents did not affect this outcome. At 3 years, the OS was 80% in the enzalutamide group and 72% in the standard-care group. The rate of PSA-determined PFS was higher in the enzalutamide group compared with the standard group (3-year event-free survival, 67% and 37%, respectively), with a HR of 0.39 (95% CI, 0.33-0.47; P < 0.001). There were fewer clinical PFS events in the enzalutamide group (167 events vs 320 events), with a HR of 0.40 (95% CI, 0.33-0.49; P < 0.001). Analysis of the stratified subgroups showed the effect on OS was diminished in those with use of bone-modifying agents, those with high-volume disease, and those who received early docetaxel. The clinical PFS benefit was maintained across all subgroups, albeit with a smaller effect in those with high-volume disease and in those with early docetaxel treatment.
Treatment discontinuation for reasons other than progressive disease occurred in 12% of those in the enzalutamide group and 19% of those in the standard-care group. Overall, the adverse events were consistent with the known safety profiles of the treatment regimen. Seizures occurred in 7 patients on enzalutamide and no patients in the standard-care group. Fatigue was more common with enzalutamide.
Conclusion. Enzalutamide treatment was associated with significantly longer PFS and OS compared with standard care in men with metastatic, hormone-sensitive prostate cancer receiving testosterone suppression.
Commentary
The current study shows that the addition of enzalutamide to standard androgen deprivation therapy (ADT) improves OS and PFS in men with newly diagnosed metastatic, hormone-sensitive prostate cancer. Until recently, antiandrogen therapy had been the standard of care for these men; however, with the advent of novel antiandrogen agents, outcomes in men with metastatic prostate cancer in both the androgen-sensitive and castrate-resistant settings have steadily improved.1-5 In the castrate-resistant setting, enzalutamide has previously been shown to improve survival in chemotherapy-naïve patients and those previously exposed to docetaxel chemotherapy.5-7 Similarly, in the hormone-sensitive setting the combination of ADT with either abiraterone or chemotherapy has been shown to improve outcomes. In the phase 3 LATITUDE and STAMPEDE trials, the combination of abiraterone plus prednisone and ADT resulted in a 30% and 37% improvement in OS, respectively.1,2 Six cycles of docetaxel in combination with ADT also resulted in a 37% increase in OS in those with high-volume metastatic disease.3
The current study adds to the growing body of literature suggesting that combination therapy in the upfront, hormone-sensitive setting improves outcomes. In the CHAARTED trial, the combination of docetaxel and ADT improved survival in men with high-volume disease, but it did not seem to benefit those with lower-volume disease.3 However, the current data suggests a survival advantage with enzalutamide with low-volume disease as well. The use of docetaxel was similar between the 2 groups, and this suggests that the benefits of enzalutamide cannot be attributed to early integration of docetaxel. It is important to note that the subgroup analysis of those who received early docetaxel showed that these patients did not experience the same survival benefit as those who did not receive docetaxel. However, this trial was not powered for this analysis, and thus it should be interpreted with caution. PFS benefit was maintained across those who received and did not receive early docetaxel. Also worth noting is the increased docetaxel-related toxicity in the combination docetaxel and enzalutamide arm of this study. The neurological toxicity of enzalutamide was again noted, with 7 seizure events documented in this study.
Because this report on the ENZAMET study is an interim analysis, it will be important to follow these outcomes as the data set matures to ensure these effects are maintained over time. Additionally, it will be important to see what implications the addition of enzalutamide have on quality of life measures, as these data have not yet been published.
Applications for Clinical Practice
The ENZAMET study provides evidence that in men with metastatic, hormone-sensitive prostate cancer receiving ADT, the addition of enzalutamide improves PFS and OS. In men who received early docetaxel, enzalutamide was associated with increased toxicity. Additionally, while PFS was improved in men who received enzalutamide and docetaxel, OS was not improved. The neurologic toxicities of enzalutamide should be considered, particularly in those with a prior history of seizure disorders. Based on these data, enzalutamide in combination with ADT represents a reasonable treatment option in men with metastatic, hormone-sensitive prostate cancer.
—Daniel Isaac, DO, MS
1. Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352-360.
2. James ND, de Bono JS, Spears MR, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338-351.
3. Kytriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36:1080-1087.
4. Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naïve men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomized, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152-160.
5. Beer TM, Armstrong AJ, Rathkopf D, et al. Enzalutamide in men with chemotherapy-naïve metastatic castration-resistant prostate cancer: extended analysis of the phase 3 PREVAIL study. Eur Urol. 2017;71:151-154.
6. Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187-1197.
7. Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with non-metastatic castration resistant prostate cancer. N Engl J Med. 2018;378:2465-2474.
1. Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352-360.
2. James ND, de Bono JS, Spears MR, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338-351.
3. Kytriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36:1080-1087.
4. Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naïve men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomized, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152-160.
5. Beer TM, Armstrong AJ, Rathkopf D, et al. Enzalutamide in men with chemotherapy-naïve metastatic castration-resistant prostate cancer: extended analysis of the phase 3 PREVAIL study. Eur Urol. 2017;71:151-154.
6. Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187-1197.
7. Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with non-metastatic castration resistant prostate cancer. N Engl J Med. 2018;378:2465-2474.
Rescue fantasies
In Walter Mitty moments, many of us daydream of glory: We’ll make that big discovery, score that disruptive app, homer in the bottom of the ninth to win the series. Then we wake up.
Those of us in the helping professions have fantasies, too, though fewer as times goes by. But the temptation to dream, day or night, never quite goes away. ...
Curtis is 45. He’s had eczema forever. It covers half his body. Topical steroids and courses of prednisone have failed him for decades. Maybe he’ll respond to dupilumab. Maybe his insurer will let him try.
The insurer rejects my Prior Authorization request; guidelines won’t authorize dupilumab unless the patient has failed on pimecrolimus.
Pimecrolimus?!!!
I figure – what the heck – I’ll dash off a stem-winder of a letter to the insurer’s medical director.
Esteemed Director,
Like every doctor, I spend my days filling out Prior Authorization forms. These are tedious but at least make some sense on their own terms. But your rejection of dupilumab is so silly that I must object.
My patient is 6-feet tall. Half his body has been covered with eczema for a long time. No expert could possibly have told you that someone who failed oral and topical steroids would respond to pimecrolimus. Besides, how many gallons of pimecrolimus would it take to smear all over a man this size in a useless effort to show it doesn’t work?
Cordially,
Two days later they approved dupilumab. Triumph! Excited, I call Curtis to tell him the news.
Curtis does not respond.
My staff calls three times. He doesn’t call back.
I write Curtis a letter. Nothing.
Maybe the Prior Authorization form chased away his eczema.
Not long after Curtis, Warren comes by. In his mid-50s, Warren is miserable. “I had a responsible job,” he says. “Now I feel as though my brain is disintegrating. For the last month, I’ve had worms crawling out of my pores. ...”
I don’t know about you, dear colleagues, but nothing stirs within me a deeper sense of futility than a patient with parasitical delusions.
“Here,” says Warren, on cue, “I brought some worms in,” handing me the requisite rumpled tissue filled with squiggles of mucus.
“Look, Warren,” I say, “you’re not going to like hearing this, but there are no worms coming out of you.”
“There aren’t?”
“You think you have them, but you need help realizing you don’t. You should see a psychiatrist.”
“Really?” says Warren. “If you think it would help, that would be wonderful. Could you help me find one?”
In all my years, no patient with parasitic delusions has ever responded positively to my suggesting a psychiatric referral. Maybe I can actually help this man!
A shrink I know refers me to a colleague at TweedleDum Medical Empire, who is most cordial. “Yes,” he says, “we work closely with dermatology and handle such patients all the time. Recent symptom onset does suggest an organic cause. Have him call my appointment coordinator.”
Which I do, with great excitement. Warren is enthused too. His emails express optimism and deep gratitude, catnip to a rescue fantasist.
What follows is – not much. Warren calls me. His insurer has balked, because his primary care is at TweedleDee Medical Empire. Courtney at TweedleDum should straighten it out, but she is away. For a very long time. And so forth.
Days go by. Weeks. Emails fly back and forth. Warren wavers between hope and despair. He is waiting for Courtney. I am waiting for Godot.
I put my staff on it. Three hours later they find Courtney. In person. It’s all set!
I let Warren know. And then ...
Nothing.
Warren stops answering my emails. I write the cordial psychiatrist at TweedleDum.
No response.
My batting average with delusional parasitosis remains an immaculate 0.000.
Rescuing people is tricky. You need to know a bit. You need to persevere. You need contacts. You need luck.
And the patient needs to want to be rescued.
Not for nothing do they call them Rescue Fantasies.
Now I can go back to work on that disruptive app. Just wait, my friends – it’s going to disrupt the world and change everything!
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. He had no disclosures relevant to this column. Write to him at [email protected].
In Walter Mitty moments, many of us daydream of glory: We’ll make that big discovery, score that disruptive app, homer in the bottom of the ninth to win the series. Then we wake up.
Those of us in the helping professions have fantasies, too, though fewer as times goes by. But the temptation to dream, day or night, never quite goes away. ...
Curtis is 45. He’s had eczema forever. It covers half his body. Topical steroids and courses of prednisone have failed him for decades. Maybe he’ll respond to dupilumab. Maybe his insurer will let him try.
The insurer rejects my Prior Authorization request; guidelines won’t authorize dupilumab unless the patient has failed on pimecrolimus.
Pimecrolimus?!!!
I figure – what the heck – I’ll dash off a stem-winder of a letter to the insurer’s medical director.
Esteemed Director,
Like every doctor, I spend my days filling out Prior Authorization forms. These are tedious but at least make some sense on their own terms. But your rejection of dupilumab is so silly that I must object.
My patient is 6-feet tall. Half his body has been covered with eczema for a long time. No expert could possibly have told you that someone who failed oral and topical steroids would respond to pimecrolimus. Besides, how many gallons of pimecrolimus would it take to smear all over a man this size in a useless effort to show it doesn’t work?
Cordially,
Two days later they approved dupilumab. Triumph! Excited, I call Curtis to tell him the news.
Curtis does not respond.
My staff calls three times. He doesn’t call back.
I write Curtis a letter. Nothing.
Maybe the Prior Authorization form chased away his eczema.
Not long after Curtis, Warren comes by. In his mid-50s, Warren is miserable. “I had a responsible job,” he says. “Now I feel as though my brain is disintegrating. For the last month, I’ve had worms crawling out of my pores. ...”
I don’t know about you, dear colleagues, but nothing stirs within me a deeper sense of futility than a patient with parasitical delusions.
“Here,” says Warren, on cue, “I brought some worms in,” handing me the requisite rumpled tissue filled with squiggles of mucus.
“Look, Warren,” I say, “you’re not going to like hearing this, but there are no worms coming out of you.”
“There aren’t?”
“You think you have them, but you need help realizing you don’t. You should see a psychiatrist.”
“Really?” says Warren. “If you think it would help, that would be wonderful. Could you help me find one?”
In all my years, no patient with parasitic delusions has ever responded positively to my suggesting a psychiatric referral. Maybe I can actually help this man!
A shrink I know refers me to a colleague at TweedleDum Medical Empire, who is most cordial. “Yes,” he says, “we work closely with dermatology and handle such patients all the time. Recent symptom onset does suggest an organic cause. Have him call my appointment coordinator.”
Which I do, with great excitement. Warren is enthused too. His emails express optimism and deep gratitude, catnip to a rescue fantasist.
What follows is – not much. Warren calls me. His insurer has balked, because his primary care is at TweedleDee Medical Empire. Courtney at TweedleDum should straighten it out, but she is away. For a very long time. And so forth.
Days go by. Weeks. Emails fly back and forth. Warren wavers between hope and despair. He is waiting for Courtney. I am waiting for Godot.
I put my staff on it. Three hours later they find Courtney. In person. It’s all set!
I let Warren know. And then ...
Nothing.
Warren stops answering my emails. I write the cordial psychiatrist at TweedleDum.
No response.
My batting average with delusional parasitosis remains an immaculate 0.000.
Rescuing people is tricky. You need to know a bit. You need to persevere. You need contacts. You need luck.
And the patient needs to want to be rescued.
Not for nothing do they call them Rescue Fantasies.
Now I can go back to work on that disruptive app. Just wait, my friends – it’s going to disrupt the world and change everything!
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. He had no disclosures relevant to this column. Write to him at [email protected].
In Walter Mitty moments, many of us daydream of glory: We’ll make that big discovery, score that disruptive app, homer in the bottom of the ninth to win the series. Then we wake up.
Those of us in the helping professions have fantasies, too, though fewer as times goes by. But the temptation to dream, day or night, never quite goes away. ...
Curtis is 45. He’s had eczema forever. It covers half his body. Topical steroids and courses of prednisone have failed him for decades. Maybe he’ll respond to dupilumab. Maybe his insurer will let him try.
The insurer rejects my Prior Authorization request; guidelines won’t authorize dupilumab unless the patient has failed on pimecrolimus.
Pimecrolimus?!!!
I figure – what the heck – I’ll dash off a stem-winder of a letter to the insurer’s medical director.
Esteemed Director,
Like every doctor, I spend my days filling out Prior Authorization forms. These are tedious but at least make some sense on their own terms. But your rejection of dupilumab is so silly that I must object.
My patient is 6-feet tall. Half his body has been covered with eczema for a long time. No expert could possibly have told you that someone who failed oral and topical steroids would respond to pimecrolimus. Besides, how many gallons of pimecrolimus would it take to smear all over a man this size in a useless effort to show it doesn’t work?
Cordially,
Two days later they approved dupilumab. Triumph! Excited, I call Curtis to tell him the news.
Curtis does not respond.
My staff calls three times. He doesn’t call back.
I write Curtis a letter. Nothing.
Maybe the Prior Authorization form chased away his eczema.
Not long after Curtis, Warren comes by. In his mid-50s, Warren is miserable. “I had a responsible job,” he says. “Now I feel as though my brain is disintegrating. For the last month, I’ve had worms crawling out of my pores. ...”
I don’t know about you, dear colleagues, but nothing stirs within me a deeper sense of futility than a patient with parasitical delusions.
“Here,” says Warren, on cue, “I brought some worms in,” handing me the requisite rumpled tissue filled with squiggles of mucus.
“Look, Warren,” I say, “you’re not going to like hearing this, but there are no worms coming out of you.”
“There aren’t?”
“You think you have them, but you need help realizing you don’t. You should see a psychiatrist.”
“Really?” says Warren. “If you think it would help, that would be wonderful. Could you help me find one?”
In all my years, no patient with parasitic delusions has ever responded positively to my suggesting a psychiatric referral. Maybe I can actually help this man!
A shrink I know refers me to a colleague at TweedleDum Medical Empire, who is most cordial. “Yes,” he says, “we work closely with dermatology and handle such patients all the time. Recent symptom onset does suggest an organic cause. Have him call my appointment coordinator.”
Which I do, with great excitement. Warren is enthused too. His emails express optimism and deep gratitude, catnip to a rescue fantasist.
What follows is – not much. Warren calls me. His insurer has balked, because his primary care is at TweedleDee Medical Empire. Courtney at TweedleDum should straighten it out, but she is away. For a very long time. And so forth.
Days go by. Weeks. Emails fly back and forth. Warren wavers between hope and despair. He is waiting for Courtney. I am waiting for Godot.
I put my staff on it. Three hours later they find Courtney. In person. It’s all set!
I let Warren know. And then ...
Nothing.
Warren stops answering my emails. I write the cordial psychiatrist at TweedleDum.
No response.
My batting average with delusional parasitosis remains an immaculate 0.000.
Rescuing people is tricky. You need to know a bit. You need to persevere. You need contacts. You need luck.
And the patient needs to want to be rescued.
Not for nothing do they call them Rescue Fantasies.
Now I can go back to work on that disruptive app. Just wait, my friends – it’s going to disrupt the world and change everything!
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. He had no disclosures relevant to this column. Write to him at [email protected].
Posttraumatic headache may be associated with reduced pain thresholds
PHILADELPHIA – , according to results of a pilot study presented at the annual meeting of the American Headache Society. The findings suggest that patients with posttraumatic headache have abnormal, multimodal sensory processing, said Amaal J. Starling, MD, a neurologist at Mayo Clinic in Phoenix.
Mild traumatic brain injury (TBI) is a growing public health problem. Headache is the most common symptom after mild TBI, and often the most debilitating symptom for these patients. No Food and Drug Administration–approved treatments are available for patients with posttraumatic headache, and about three-quarters of these patients report that current treatments bring them no relief.
Identifying novel targets and developing new treatment options will require a deeper understanding of the pathophysiology of posttraumatic headache, said Dr. Starling. She and her colleagues conducted a pilot study to characterize allodynia, cutaneous heat pain thresholds, photophobia, and light-induced pain thresholds objectively in patients with posttraumatic headache, compared with healthy controls.
Participants were exposed to a bright-light stressor
The researchers enrolled 20 patients between ages 18 years and 65 years with posttraumatic headache attributed to mild TBI in their study. They matched these patients by age with 20 healthy controls. Dr. Starling and colleagues evaluated all participants prospectively using the Allodynia Symptom Checklist (ASC-12), Photosensitivity Assessment Questionnaire (PAQ), State-Trait Anxiety Inventory (STAI), and Beck Depression Inventory (BDI).
The investigators performed quantitative sensory testing to measure each participant’s cutaneous forearm heat pain threshold. Using a progressive light stimulation device, they quantified each participant’s light-induced pain threshold. Finally, Dr. Starling and colleagues obtained participants’ cutaneous heat pain thresholds immediately after, 10 minutes after, and 40 minutes after exposing them to a bright-light stressor.
The researchers found no significant differences between groups in age, gender, or race. The population’s average age was 41 years. Approximately 70% of the sample was female. Among participants with posttraumatic headache, the average time since the onset of posttraumatic headache was 46 months. The average number of headache days per month in that group was 17.2, which represented “a significantly high headache burden,” said Dr. Starling. Approximately 80% of patients with posttraumatic headache had headaches with a migraine phenotype.
Patients’ pain thresholds were lower
STAI and BDI scores were significantly higher among patients with posttraumatic headache, compared with controls. Mean PAQ score was 0.62 among patients and 0.24 among controls, representing significantly greater photophobia symptom severity among patients, said Dr. Starling.
Light-induced pain thresholds were significantly lower in patients with posttraumatic headache (median, 90.5 lux), compared with healthy controls (median, 863.5 lux), independent of depression and anxiety. Allodynia symptom severity was significantly higher in patients with posttraumatic headache (mean ASC-12 score, 5.7), compared with controls (mean ASC-12 score, 0.98).
In addition, the mean baseline cutaneous heat pain threshold was 40.8° C in patients with posttraumatic headache and 44.4° C in healthy controls. When participants were subjected to the bright-light stressor, the immediate change in heat pain threshold was significant in patients with posttraumatic headache (−1.9° C), compared with healthy controls. The difference between groups was not significant at 10 and 40 minutes after exposure to the stressor, however. The light intensity inducing moderate pain was 688 lux in patients with posttraumatic headache, compared with 6,000 lux in healthy controls.
“Our next steps are going to be replicating this [study] in a larger population, as well as determining whether any type of intervention would change these different types of sensory sensitivities and thresholds,” said Dr. Starling. She and her colleagues will use this human research model to examine whether posttraumatic headache differs from other headache disorders such as migraine and to examine potential differences between acute and persistent posttraumatic headache.
The study was funded through an intramural Mayo Clinic early career research award.
SOURCE: Starling AJ et al. AHS 2019. Abstract OR14.
PHILADELPHIA – , according to results of a pilot study presented at the annual meeting of the American Headache Society. The findings suggest that patients with posttraumatic headache have abnormal, multimodal sensory processing, said Amaal J. Starling, MD, a neurologist at Mayo Clinic in Phoenix.
Mild traumatic brain injury (TBI) is a growing public health problem. Headache is the most common symptom after mild TBI, and often the most debilitating symptom for these patients. No Food and Drug Administration–approved treatments are available for patients with posttraumatic headache, and about three-quarters of these patients report that current treatments bring them no relief.
Identifying novel targets and developing new treatment options will require a deeper understanding of the pathophysiology of posttraumatic headache, said Dr. Starling. She and her colleagues conducted a pilot study to characterize allodynia, cutaneous heat pain thresholds, photophobia, and light-induced pain thresholds objectively in patients with posttraumatic headache, compared with healthy controls.
Participants were exposed to a bright-light stressor
The researchers enrolled 20 patients between ages 18 years and 65 years with posttraumatic headache attributed to mild TBI in their study. They matched these patients by age with 20 healthy controls. Dr. Starling and colleagues evaluated all participants prospectively using the Allodynia Symptom Checklist (ASC-12), Photosensitivity Assessment Questionnaire (PAQ), State-Trait Anxiety Inventory (STAI), and Beck Depression Inventory (BDI).
The investigators performed quantitative sensory testing to measure each participant’s cutaneous forearm heat pain threshold. Using a progressive light stimulation device, they quantified each participant’s light-induced pain threshold. Finally, Dr. Starling and colleagues obtained participants’ cutaneous heat pain thresholds immediately after, 10 minutes after, and 40 minutes after exposing them to a bright-light stressor.
The researchers found no significant differences between groups in age, gender, or race. The population’s average age was 41 years. Approximately 70% of the sample was female. Among participants with posttraumatic headache, the average time since the onset of posttraumatic headache was 46 months. The average number of headache days per month in that group was 17.2, which represented “a significantly high headache burden,” said Dr. Starling. Approximately 80% of patients with posttraumatic headache had headaches with a migraine phenotype.
Patients’ pain thresholds were lower
STAI and BDI scores were significantly higher among patients with posttraumatic headache, compared with controls. Mean PAQ score was 0.62 among patients and 0.24 among controls, representing significantly greater photophobia symptom severity among patients, said Dr. Starling.
Light-induced pain thresholds were significantly lower in patients with posttraumatic headache (median, 90.5 lux), compared with healthy controls (median, 863.5 lux), independent of depression and anxiety. Allodynia symptom severity was significantly higher in patients with posttraumatic headache (mean ASC-12 score, 5.7), compared with controls (mean ASC-12 score, 0.98).
In addition, the mean baseline cutaneous heat pain threshold was 40.8° C in patients with posttraumatic headache and 44.4° C in healthy controls. When participants were subjected to the bright-light stressor, the immediate change in heat pain threshold was significant in patients with posttraumatic headache (−1.9° C), compared with healthy controls. The difference between groups was not significant at 10 and 40 minutes after exposure to the stressor, however. The light intensity inducing moderate pain was 688 lux in patients with posttraumatic headache, compared with 6,000 lux in healthy controls.
“Our next steps are going to be replicating this [study] in a larger population, as well as determining whether any type of intervention would change these different types of sensory sensitivities and thresholds,” said Dr. Starling. She and her colleagues will use this human research model to examine whether posttraumatic headache differs from other headache disorders such as migraine and to examine potential differences between acute and persistent posttraumatic headache.
The study was funded through an intramural Mayo Clinic early career research award.
SOURCE: Starling AJ et al. AHS 2019. Abstract OR14.
PHILADELPHIA – , according to results of a pilot study presented at the annual meeting of the American Headache Society. The findings suggest that patients with posttraumatic headache have abnormal, multimodal sensory processing, said Amaal J. Starling, MD, a neurologist at Mayo Clinic in Phoenix.
Mild traumatic brain injury (TBI) is a growing public health problem. Headache is the most common symptom after mild TBI, and often the most debilitating symptom for these patients. No Food and Drug Administration–approved treatments are available for patients with posttraumatic headache, and about three-quarters of these patients report that current treatments bring them no relief.
Identifying novel targets and developing new treatment options will require a deeper understanding of the pathophysiology of posttraumatic headache, said Dr. Starling. She and her colleagues conducted a pilot study to characterize allodynia, cutaneous heat pain thresholds, photophobia, and light-induced pain thresholds objectively in patients with posttraumatic headache, compared with healthy controls.
Participants were exposed to a bright-light stressor
The researchers enrolled 20 patients between ages 18 years and 65 years with posttraumatic headache attributed to mild TBI in their study. They matched these patients by age with 20 healthy controls. Dr. Starling and colleagues evaluated all participants prospectively using the Allodynia Symptom Checklist (ASC-12), Photosensitivity Assessment Questionnaire (PAQ), State-Trait Anxiety Inventory (STAI), and Beck Depression Inventory (BDI).
The investigators performed quantitative sensory testing to measure each participant’s cutaneous forearm heat pain threshold. Using a progressive light stimulation device, they quantified each participant’s light-induced pain threshold. Finally, Dr. Starling and colleagues obtained participants’ cutaneous heat pain thresholds immediately after, 10 minutes after, and 40 minutes after exposing them to a bright-light stressor.
The researchers found no significant differences between groups in age, gender, or race. The population’s average age was 41 years. Approximately 70% of the sample was female. Among participants with posttraumatic headache, the average time since the onset of posttraumatic headache was 46 months. The average number of headache days per month in that group was 17.2, which represented “a significantly high headache burden,” said Dr. Starling. Approximately 80% of patients with posttraumatic headache had headaches with a migraine phenotype.
Patients’ pain thresholds were lower
STAI and BDI scores were significantly higher among patients with posttraumatic headache, compared with controls. Mean PAQ score was 0.62 among patients and 0.24 among controls, representing significantly greater photophobia symptom severity among patients, said Dr. Starling.
Light-induced pain thresholds were significantly lower in patients with posttraumatic headache (median, 90.5 lux), compared with healthy controls (median, 863.5 lux), independent of depression and anxiety. Allodynia symptom severity was significantly higher in patients with posttraumatic headache (mean ASC-12 score, 5.7), compared with controls (mean ASC-12 score, 0.98).
In addition, the mean baseline cutaneous heat pain threshold was 40.8° C in patients with posttraumatic headache and 44.4° C in healthy controls. When participants were subjected to the bright-light stressor, the immediate change in heat pain threshold was significant in patients with posttraumatic headache (−1.9° C), compared with healthy controls. The difference between groups was not significant at 10 and 40 minutes after exposure to the stressor, however. The light intensity inducing moderate pain was 688 lux in patients with posttraumatic headache, compared with 6,000 lux in healthy controls.
“Our next steps are going to be replicating this [study] in a larger population, as well as determining whether any type of intervention would change these different types of sensory sensitivities and thresholds,” said Dr. Starling. She and her colleagues will use this human research model to examine whether posttraumatic headache differs from other headache disorders such as migraine and to examine potential differences between acute and persistent posttraumatic headache.
The study was funded through an intramural Mayo Clinic early career research award.
SOURCE: Starling AJ et al. AHS 2019. Abstract OR14.
REPORTING FROM AHS 2019
Pediatric Dermatology: Summer 2019
Click here to read the supplement.
The 2019 Pediatric Dermatology supplement features a selection of articles published in Dermatology News and Pediatric News, with commentary by Lawrence F. Eichenfield, MD, and Robert Sidbury, MD.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital in San Diego. He is vice chair of dermatology and professor of dermatology and pediatrics at the University of California, San Diego.
Dr. Sidbury is chief of dermatology at Seattle Children’s Hospital and professor, department of pediatrics, University of Washington, Seattle.
Highlights include:
- Atopic dermatitis update: The field continues to evolve
- When treating impetigo, be aware of antibiotic resistance patterns
- Don’t sweat axillary hyperhidrosis
- Premature children’s skin is different
Click here to read the supplement.
Click here to read the supplement.
The 2019 Pediatric Dermatology supplement features a selection of articles published in Dermatology News and Pediatric News, with commentary by Lawrence F. Eichenfield, MD, and Robert Sidbury, MD.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital in San Diego. He is vice chair of dermatology and professor of dermatology and pediatrics at the University of California, San Diego.
Dr. Sidbury is chief of dermatology at Seattle Children’s Hospital and professor, department of pediatrics, University of Washington, Seattle.
Highlights include:
- Atopic dermatitis update: The field continues to evolve
- When treating impetigo, be aware of antibiotic resistance patterns
- Don’t sweat axillary hyperhidrosis
- Premature children’s skin is different
Click here to read the supplement.
Click here to read the supplement.
The 2019 Pediatric Dermatology supplement features a selection of articles published in Dermatology News and Pediatric News, with commentary by Lawrence F. Eichenfield, MD, and Robert Sidbury, MD.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital in San Diego. He is vice chair of dermatology and professor of dermatology and pediatrics at the University of California, San Diego.
Dr. Sidbury is chief of dermatology at Seattle Children’s Hospital and professor, department of pediatrics, University of Washington, Seattle.
Highlights include:
- Atopic dermatitis update: The field continues to evolve
- When treating impetigo, be aware of antibiotic resistance patterns
- Don’t sweat axillary hyperhidrosis
- Premature children’s skin is different
Click here to read the supplement.
Adding clinical research to your practice ‘not something you take lightly’
AUSTIN, TEX. – The way , because not everyone is cut out for it.
“This is not something you take lightly,” Dr. Hebert, chief of pediatric dermatology at the University of Texas Health Science Center, Houston, said at the annual meeting of the Society for Pediatric Dermatology. “There will be days when you feel like you are going into combat. If you are not prepared for that, maybe stick to clinical practice. That plan might be a bit simpler.”
For more than 30 years, Dr. Hebert has been engaged in dermatology clinical research, with a focus on atopic dermatitis, psoriasis, and hyperhidrosis. Her team includes a full-time research fellow, a full-time nurse practitioner, and a part-time clinical trials coordinator. “We work very hard, and I am responsible for their salaries,” she said. “There is considerable pressure from year to year, but they work hard and are very loyal. They represent a great portion of the success that we have in clinical research. You need a team, and you have to be a team leader in order to undertake this.”
Dr. Hebert offered the following tips for incorporating clinical research into your practice:
• Set aside allocated time. “This is not a side job within your current occupation,” she emphasized. “You really need allocated time to do research, and you need protected academic time if you work in that environment. You also need protected academic time for your staff to conduct research.”
• Get to know the players involved. This includes forming professional relationships with internal and external review boards, and understanding contract and grants. “This collaboration is a lot like getting into a sport,” she explained. “You have to know the rules, you have to know how to play with strategy, and you have to be very effective.”
Review boards that work with academic centers include the Western Institutional Review Board (WIRB) and Advarra. “You also need to understand time lines, what it takes to go from submission to implementation,” Dr. Hebert said. “That time cannot be too long, or you will be off the list of desired investigators because you cannot get up and running in a timely fashion. Your team has to be behind you in order to make this happen.” [She recommended the National Association of Healthcare Revenue Integrity publication, “The Practical Guide to Clinical Trials Billing.”]
• Consider space and equipment needs. This includes space for patient charts, cameras, scales, electronic devices for data capture, computer equipment, and other instruments you may need to carry out your work. Dr. Hebert’s clinic houses about a half-dozen EKG machines dedicated to research projects. “You also need a lot of storage,” she added. “Our university requires us to save every piece of paper, every communication for 15 years. Our university does allocate that space.”
Sponsored research opportunities include working with the National Institutes of Health, Department of Defense, pharmaceutical companies, and over-the-counter product companies. “You can also initiate your own research,” she said. “One way to get started is to partner with an established researcher. That could be someone in your department, in a different department, or in a clinical research unit within your institution. They can help you with infrastructure and they can sometimes do your research submissions for you.”
Other ways to explore research opportunities include reaching out to medical science liaisons and clinical trial research organizations, and hiring fellows with research experience. “The bottom line is, talk to a lot of people you do not know,” Dr. Hebert said. “That is the real secret to getting research studies.”
There is another player in the field to become familiar with: contract research organizations (CROs), which provide support to pharmaceutical industries in the form of research services outsourced on a contract basis. They receive payment from the sponsor to conduct clinical research operations. “Many times the sponsor does not entirely know what the CRO is doing with regard to the interface with you as an investigator,” said Dr. Hebert, who has conducted more than 160 trials, including 65 in atopic dermatitis alone. “Sometimes you have to go over the head of the CRO to address issues that come up. The reality is, if the sponsor does not know you are not getting paid or having obstacles put in front of you that may not be in your contract, that constitutes a problem. You have to learn to be a bit shrewd in this game of research.”
She added that clinicians who choose to conduct clinical research come to learn “a whole new language,” such as what a confidential disclosure agreement is. “You also have to undergo CITI [Collaborative Institutional Training Initiative] training to meet the principles of good clinical practice,” she said. Other specifics include infectious substance shipping training per International Air Transport Association shipping guidelines, protocol training in arenas such as Eczema Area and Severity Index scoring and Psoriasis Area Severity Index scoring, and electronic case report form/electronic data–capture report training. “We also have a lot of equipment training because we are constantly getting new tablets for recording patient data and so forth,” she said. “This is something that has helped me become more technically equipped to handle all of this data capture.”
Dr. Hebert closed her remarks by noting that conducting clinical research can be a rewarding endeavor. “You really do address unmet needs, and you give a lot of education to residents, students, and fellows,” she said. “Clinical research provides research opportunities for those interested in dermatology. You have those moments when you see patients get better, and this represents the best patient encounter you could ever hope for.”
She reported having no relevant financial disclosures.
AUSTIN, TEX. – The way , because not everyone is cut out for it.
“This is not something you take lightly,” Dr. Hebert, chief of pediatric dermatology at the University of Texas Health Science Center, Houston, said at the annual meeting of the Society for Pediatric Dermatology. “There will be days when you feel like you are going into combat. If you are not prepared for that, maybe stick to clinical practice. That plan might be a bit simpler.”
For more than 30 years, Dr. Hebert has been engaged in dermatology clinical research, with a focus on atopic dermatitis, psoriasis, and hyperhidrosis. Her team includes a full-time research fellow, a full-time nurse practitioner, and a part-time clinical trials coordinator. “We work very hard, and I am responsible for their salaries,” she said. “There is considerable pressure from year to year, but they work hard and are very loyal. They represent a great portion of the success that we have in clinical research. You need a team, and you have to be a team leader in order to undertake this.”
Dr. Hebert offered the following tips for incorporating clinical research into your practice:
• Set aside allocated time. “This is not a side job within your current occupation,” she emphasized. “You really need allocated time to do research, and you need protected academic time if you work in that environment. You also need protected academic time for your staff to conduct research.”
• Get to know the players involved. This includes forming professional relationships with internal and external review boards, and understanding contract and grants. “This collaboration is a lot like getting into a sport,” she explained. “You have to know the rules, you have to know how to play with strategy, and you have to be very effective.”
Review boards that work with academic centers include the Western Institutional Review Board (WIRB) and Advarra. “You also need to understand time lines, what it takes to go from submission to implementation,” Dr. Hebert said. “That time cannot be too long, or you will be off the list of desired investigators because you cannot get up and running in a timely fashion. Your team has to be behind you in order to make this happen.” [She recommended the National Association of Healthcare Revenue Integrity publication, “The Practical Guide to Clinical Trials Billing.”]
• Consider space and equipment needs. This includes space for patient charts, cameras, scales, electronic devices for data capture, computer equipment, and other instruments you may need to carry out your work. Dr. Hebert’s clinic houses about a half-dozen EKG machines dedicated to research projects. “You also need a lot of storage,” she added. “Our university requires us to save every piece of paper, every communication for 15 years. Our university does allocate that space.”
Sponsored research opportunities include working with the National Institutes of Health, Department of Defense, pharmaceutical companies, and over-the-counter product companies. “You can also initiate your own research,” she said. “One way to get started is to partner with an established researcher. That could be someone in your department, in a different department, or in a clinical research unit within your institution. They can help you with infrastructure and they can sometimes do your research submissions for you.”
Other ways to explore research opportunities include reaching out to medical science liaisons and clinical trial research organizations, and hiring fellows with research experience. “The bottom line is, talk to a lot of people you do not know,” Dr. Hebert said. “That is the real secret to getting research studies.”
There is another player in the field to become familiar with: contract research organizations (CROs), which provide support to pharmaceutical industries in the form of research services outsourced on a contract basis. They receive payment from the sponsor to conduct clinical research operations. “Many times the sponsor does not entirely know what the CRO is doing with regard to the interface with you as an investigator,” said Dr. Hebert, who has conducted more than 160 trials, including 65 in atopic dermatitis alone. “Sometimes you have to go over the head of the CRO to address issues that come up. The reality is, if the sponsor does not know you are not getting paid or having obstacles put in front of you that may not be in your contract, that constitutes a problem. You have to learn to be a bit shrewd in this game of research.”
She added that clinicians who choose to conduct clinical research come to learn “a whole new language,” such as what a confidential disclosure agreement is. “You also have to undergo CITI [Collaborative Institutional Training Initiative] training to meet the principles of good clinical practice,” she said. Other specifics include infectious substance shipping training per International Air Transport Association shipping guidelines, protocol training in arenas such as Eczema Area and Severity Index scoring and Psoriasis Area Severity Index scoring, and electronic case report form/electronic data–capture report training. “We also have a lot of equipment training because we are constantly getting new tablets for recording patient data and so forth,” she said. “This is something that has helped me become more technically equipped to handle all of this data capture.”
Dr. Hebert closed her remarks by noting that conducting clinical research can be a rewarding endeavor. “You really do address unmet needs, and you give a lot of education to residents, students, and fellows,” she said. “Clinical research provides research opportunities for those interested in dermatology. You have those moments when you see patients get better, and this represents the best patient encounter you could ever hope for.”
She reported having no relevant financial disclosures.
AUSTIN, TEX. – The way , because not everyone is cut out for it.
“This is not something you take lightly,” Dr. Hebert, chief of pediatric dermatology at the University of Texas Health Science Center, Houston, said at the annual meeting of the Society for Pediatric Dermatology. “There will be days when you feel like you are going into combat. If you are not prepared for that, maybe stick to clinical practice. That plan might be a bit simpler.”
For more than 30 years, Dr. Hebert has been engaged in dermatology clinical research, with a focus on atopic dermatitis, psoriasis, and hyperhidrosis. Her team includes a full-time research fellow, a full-time nurse practitioner, and a part-time clinical trials coordinator. “We work very hard, and I am responsible for their salaries,” she said. “There is considerable pressure from year to year, but they work hard and are very loyal. They represent a great portion of the success that we have in clinical research. You need a team, and you have to be a team leader in order to undertake this.”
Dr. Hebert offered the following tips for incorporating clinical research into your practice:
• Set aside allocated time. “This is not a side job within your current occupation,” she emphasized. “You really need allocated time to do research, and you need protected academic time if you work in that environment. You also need protected academic time for your staff to conduct research.”
• Get to know the players involved. This includes forming professional relationships with internal and external review boards, and understanding contract and grants. “This collaboration is a lot like getting into a sport,” she explained. “You have to know the rules, you have to know how to play with strategy, and you have to be very effective.”
Review boards that work with academic centers include the Western Institutional Review Board (WIRB) and Advarra. “You also need to understand time lines, what it takes to go from submission to implementation,” Dr. Hebert said. “That time cannot be too long, or you will be off the list of desired investigators because you cannot get up and running in a timely fashion. Your team has to be behind you in order to make this happen.” [She recommended the National Association of Healthcare Revenue Integrity publication, “The Practical Guide to Clinical Trials Billing.”]
• Consider space and equipment needs. This includes space for patient charts, cameras, scales, electronic devices for data capture, computer equipment, and other instruments you may need to carry out your work. Dr. Hebert’s clinic houses about a half-dozen EKG machines dedicated to research projects. “You also need a lot of storage,” she added. “Our university requires us to save every piece of paper, every communication for 15 years. Our university does allocate that space.”
Sponsored research opportunities include working with the National Institutes of Health, Department of Defense, pharmaceutical companies, and over-the-counter product companies. “You can also initiate your own research,” she said. “One way to get started is to partner with an established researcher. That could be someone in your department, in a different department, or in a clinical research unit within your institution. They can help you with infrastructure and they can sometimes do your research submissions for you.”
Other ways to explore research opportunities include reaching out to medical science liaisons and clinical trial research organizations, and hiring fellows with research experience. “The bottom line is, talk to a lot of people you do not know,” Dr. Hebert said. “That is the real secret to getting research studies.”
There is another player in the field to become familiar with: contract research organizations (CROs), which provide support to pharmaceutical industries in the form of research services outsourced on a contract basis. They receive payment from the sponsor to conduct clinical research operations. “Many times the sponsor does not entirely know what the CRO is doing with regard to the interface with you as an investigator,” said Dr. Hebert, who has conducted more than 160 trials, including 65 in atopic dermatitis alone. “Sometimes you have to go over the head of the CRO to address issues that come up. The reality is, if the sponsor does not know you are not getting paid or having obstacles put in front of you that may not be in your contract, that constitutes a problem. You have to learn to be a bit shrewd in this game of research.”
She added that clinicians who choose to conduct clinical research come to learn “a whole new language,” such as what a confidential disclosure agreement is. “You also have to undergo CITI [Collaborative Institutional Training Initiative] training to meet the principles of good clinical practice,” she said. Other specifics include infectious substance shipping training per International Air Transport Association shipping guidelines, protocol training in arenas such as Eczema Area and Severity Index scoring and Psoriasis Area Severity Index scoring, and electronic case report form/electronic data–capture report training. “We also have a lot of equipment training because we are constantly getting new tablets for recording patient data and so forth,” she said. “This is something that has helped me become more technically equipped to handle all of this data capture.”
Dr. Hebert closed her remarks by noting that conducting clinical research can be a rewarding endeavor. “You really do address unmet needs, and you give a lot of education to residents, students, and fellows,” she said. “Clinical research provides research opportunities for those interested in dermatology. You have those moments when you see patients get better, and this represents the best patient encounter you could ever hope for.”
She reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM SPD 2019




