User login
Professional coaching keeps doctors in the game
Physicians who receive professional coaching are less emotionally exhausted and less vulnerable to burnout, according to the results of a pilot study.
“This intervention adds to the growing literature of evidence-based approaches to promote physician well-being and should be considered a complementary strategy to be deployed in combination with other organizational approaches to improve system-level drivers of work-related stressors,” wrote Liselotte N. Dyrbye, MD, of the Mayo Clinic in Rochester, Minn., and coauthors in JAMA Internal Medicine.
Dr. Dyrbye and colleagues conducted a randomized pilot study of 88 Mayo Clinic physicians in the departments of medicine, family medicine, and pediatrics. Half (n = 44) received 3.5 hours of sessions facilitated by a professional coach. The other half (n = 44) served as controls. Participants’ well-being – in regard to burnout, quality of life, resilience, job satisfaction, engagement, and meaning at work – was surveyed at baseline and the study’s completion.
Physicians in the coaching group participated in a 1-hour initial telephone session, designed to establish a relationship between the physician and coach, as well as to assess needs, set goals, identify values, and create an action plan. During follow-up sessions, coaches would check in, help plan and set goals, and suggest strategies/changes to incorporate into daily life. Physicians were permitted to ask for support on any issue, but also were expected to see as many patients as their colleagues outside of the study.
After 6 months, physicians in the coaching group saw a significant decrease in emotional exhaustion by a mean of 5.2 points, compared with an increase of 1.5 points in the control group. At 5 months, absolute rates of high emotional exhaustion decreased by 19.5% in the coaching group and increased by 9.8% in the control group and absolute rates of overall burnout decreased by 17.1% in the coaching group and increased by 4.9% in the control group. Quality of life and resilience scores also improved, though there were no notable differences between groups in measures of job satisfaction, engagement, and meaning at work.
The authors noted their study’s limitations, which included a modest sample size and a volunteer group of participants.
In addition, the lower percentage of men in the study – 48 of 88 participants were women – may be a result of factors that deserve further investigation. Finally, burnout rates among volunteers were higher than those among other physicians, suggesting that “the study appealed to those in greatest need of the intervention.”
The study was funded by the Mayo Clinic department of medicine’s Program on Physician Well-Being and the Physician Foundation. Two of the authors – Dr. Dyrbye and Tait D. Shanafelt, MD, of Stanford (Calif.) University – reported being the coinventors of, and receiving royalties for, the Physician Well-Being Index, Medical Student Well-Being Index, Nurse Well-Being Index, and the Well-Being Index.
SOURCE: Dyrbye LN et al. JAMA Intern Med. 2019 Aug 5. doi: 10.1001/jamainternmed.2019.2425.
Physicians who receive professional coaching are less emotionally exhausted and less vulnerable to burnout, according to the results of a pilot study.
“This intervention adds to the growing literature of evidence-based approaches to promote physician well-being and should be considered a complementary strategy to be deployed in combination with other organizational approaches to improve system-level drivers of work-related stressors,” wrote Liselotte N. Dyrbye, MD, of the Mayo Clinic in Rochester, Minn., and coauthors in JAMA Internal Medicine.
Dr. Dyrbye and colleagues conducted a randomized pilot study of 88 Mayo Clinic physicians in the departments of medicine, family medicine, and pediatrics. Half (n = 44) received 3.5 hours of sessions facilitated by a professional coach. The other half (n = 44) served as controls. Participants’ well-being – in regard to burnout, quality of life, resilience, job satisfaction, engagement, and meaning at work – was surveyed at baseline and the study’s completion.
Physicians in the coaching group participated in a 1-hour initial telephone session, designed to establish a relationship between the physician and coach, as well as to assess needs, set goals, identify values, and create an action plan. During follow-up sessions, coaches would check in, help plan and set goals, and suggest strategies/changes to incorporate into daily life. Physicians were permitted to ask for support on any issue, but also were expected to see as many patients as their colleagues outside of the study.
After 6 months, physicians in the coaching group saw a significant decrease in emotional exhaustion by a mean of 5.2 points, compared with an increase of 1.5 points in the control group. At 5 months, absolute rates of high emotional exhaustion decreased by 19.5% in the coaching group and increased by 9.8% in the control group and absolute rates of overall burnout decreased by 17.1% in the coaching group and increased by 4.9% in the control group. Quality of life and resilience scores also improved, though there were no notable differences between groups in measures of job satisfaction, engagement, and meaning at work.
The authors noted their study’s limitations, which included a modest sample size and a volunteer group of participants.
In addition, the lower percentage of men in the study – 48 of 88 participants were women – may be a result of factors that deserve further investigation. Finally, burnout rates among volunteers were higher than those among other physicians, suggesting that “the study appealed to those in greatest need of the intervention.”
The study was funded by the Mayo Clinic department of medicine’s Program on Physician Well-Being and the Physician Foundation. Two of the authors – Dr. Dyrbye and Tait D. Shanafelt, MD, of Stanford (Calif.) University – reported being the coinventors of, and receiving royalties for, the Physician Well-Being Index, Medical Student Well-Being Index, Nurse Well-Being Index, and the Well-Being Index.
SOURCE: Dyrbye LN et al. JAMA Intern Med. 2019 Aug 5. doi: 10.1001/jamainternmed.2019.2425.
Physicians who receive professional coaching are less emotionally exhausted and less vulnerable to burnout, according to the results of a pilot study.
“This intervention adds to the growing literature of evidence-based approaches to promote physician well-being and should be considered a complementary strategy to be deployed in combination with other organizational approaches to improve system-level drivers of work-related stressors,” wrote Liselotte N. Dyrbye, MD, of the Mayo Clinic in Rochester, Minn., and coauthors in JAMA Internal Medicine.
Dr. Dyrbye and colleagues conducted a randomized pilot study of 88 Mayo Clinic physicians in the departments of medicine, family medicine, and pediatrics. Half (n = 44) received 3.5 hours of sessions facilitated by a professional coach. The other half (n = 44) served as controls. Participants’ well-being – in regard to burnout, quality of life, resilience, job satisfaction, engagement, and meaning at work – was surveyed at baseline and the study’s completion.
Physicians in the coaching group participated in a 1-hour initial telephone session, designed to establish a relationship between the physician and coach, as well as to assess needs, set goals, identify values, and create an action plan. During follow-up sessions, coaches would check in, help plan and set goals, and suggest strategies/changes to incorporate into daily life. Physicians were permitted to ask for support on any issue, but also were expected to see as many patients as their colleagues outside of the study.
After 6 months, physicians in the coaching group saw a significant decrease in emotional exhaustion by a mean of 5.2 points, compared with an increase of 1.5 points in the control group. At 5 months, absolute rates of high emotional exhaustion decreased by 19.5% in the coaching group and increased by 9.8% in the control group and absolute rates of overall burnout decreased by 17.1% in the coaching group and increased by 4.9% in the control group. Quality of life and resilience scores also improved, though there were no notable differences between groups in measures of job satisfaction, engagement, and meaning at work.
The authors noted their study’s limitations, which included a modest sample size and a volunteer group of participants.
In addition, the lower percentage of men in the study – 48 of 88 participants were women – may be a result of factors that deserve further investigation. Finally, burnout rates among volunteers were higher than those among other physicians, suggesting that “the study appealed to those in greatest need of the intervention.”
The study was funded by the Mayo Clinic department of medicine’s Program on Physician Well-Being and the Physician Foundation. Two of the authors – Dr. Dyrbye and Tait D. Shanafelt, MD, of Stanford (Calif.) University – reported being the coinventors of, and receiving royalties for, the Physician Well-Being Index, Medical Student Well-Being Index, Nurse Well-Being Index, and the Well-Being Index.
SOURCE: Dyrbye LN et al. JAMA Intern Med. 2019 Aug 5. doi: 10.1001/jamainternmed.2019.2425.
FROM JAMA INTERNAL MEDICINE
Antiepileptic drug outcomes have remained flat for 3 decades
BANGKOK – Since founding the Epilepsy Unit at Glasgow’s Western Infirmary 37 years ago, Martin J. Brodie, MD, has seen many changes in the field, including the introduction of more than a dozen new antiepileptic drugs (AEDs) in the past 2 decades.
And based upon this vast clinical experience coupled with his leadership of landmark studies, he has a message for his physician colleagues and their epilepsy patients. And it’s not pretty.
“Has the probability of achieving seizure freedom increased significantly in the last 3 decades? Regrettably, the answer is no,” he declared at the International Epilepsy Congress.
“Over all these years, in terms of seizure freedom there has been no real difference in outcome. There’s really quite a long way to go before we can say that we are doing all that well for people,” he said at the congress sponsored by the International League Against Epilepsy.
In the year 2000, he and his coinvestigators published a prospective, longitudinal, observational cohort study of 470 newly diagnosed patients with epilepsy treated at the Western Infirmary during 1982-1997, all with a minimum of 2 years’ follow-up. Sixty-one percent achieved complete freedom from seizures for at least 1 year on monotherapy, and another 3% did so on polytherapy, for a total rate of 64% (N Engl J Med. 2000 Feb 3;342[5]:314-19).
But these were patients who by and large were treated with older AEDs such as carbamazepine, which has since fallen by the wayside because of toxicities. Scottish neurologists now generally turn to lamotrigine (Lamictal), levetiracetam (Spritam), and other, newer AEDs. So Dr. Brodie and his coworkers recently published a follow-up study, this one featuring 30 years of longitudinal follow-up of 1,795 patients newly treated for epilepsy with AEDs, new and old, during 1982-2012. The investigators demonstrated that the seizure-free survival curves over time were virtually superimposable. In the larger, more recent study, remission was achieved in 55% of patients with AED monotherapy and in another 9% with polytherapy, for a total rate of 64%, identical to the rate in the 2000 study, and as was the case in the earlier study, 36% of patients remained uncontrolled (JAMA Neurol. 2018 Mar 1;75[3]:279-86).
“Overall, the way this population behaves, there’s no difference in efficacy and no difference in tolerability whether you’re using old drugs used properly or new drugs used properly,” said Dr. Brodie, professor of neurology at the University of Glasgow (Scotland).
It’s noteworthy that Sir William R. Gowers, the Londoner who has been called the greatest neurologist of all time, reported a 70% seizure-free rate in 1881, while Dr. Brodie and workers achieved a 64% rate in their 30-year study. “It’s interesting that the numbers are so bad, really, I suppose,” Dr. Brodie commented.
How about outcomes in pediatric epilepsy?
Dr. Brodie and coworkers recently published a 30-year prospective cohort study of 332 adolescent epilepsy patients newly diagnosed and treated at the Western Infirmary during 1982-2012. At the end of the study, 67% were seizure-free for at least the past year, a feat accomplished via monotherapy in 83% of cases. The seizure-free rate was 72% in those with generalized epilepsy, significantly better than the 60% figure in those with focal epilepsy. The efficacy rate was 74% with newer AED monotherapy and similar at 77% with monotherapy older drugs. Adverse event rates ranged from a low of 12% with lamotrigine to 56% with topiramate (Topamax), according to the findings published in Epilepsia (2019 Jun;60[6]:1083-90).
Roughly similar outcomes have been reported from Norway in a study of 600 children with epilepsy, median age 7 years, with a median follow-up of 5.8 years that is considerably shorter than that in the Glasgow pediatric study. Overall, 59% of the Norwegian children remained seizure free for at least 1 year, 30% developed drug-resistant epilepsy, and 11% followed an intermediate remitting/relapsing course (Pediatrics. 2018 Jun. doi: 10.1542/peds.2017-4016).
Why the decades of flat pharmacologic outcomes?
The consistently suboptimal seizure-free outcomes obtained over the past 30 years shouldn’t really be surprising, according to Dr. Brodie.
“Although we think we have lots of mechanisms of action and lots of differences between the drugs, they’re arguably all antiseizure drugs and not antiepilepsy drugs. We don’t treat the whale; we treat the spout. We don’t treat what we cannot see; we treat what we can see, which is the seizures, but we’re not influencing the long-term outcome,” the neurologist explained.
The compelling case for early epilepsy surgery
Epilepsy surgery remains underutilized, according to Dr. Brodie and other experts.
The International League Against Epilepsy defines drug-resistant epilepsy as failure to achieve sustained seizure freedom after adequate trials of two tolerated and appropriately chosen and used AED schedules. Dr. Brodie’s work was influential in creating that definition because his data demonstrated the sharply diminishing returns of additional drug trials.
“When do we consider epilepsy surgery? Arguably, the earlier, the better. After two drugs have failed appropriately, I don’t think anybody in this room would argue about that, although people in some of the other rooms might,” he said at the congress.
Influential in his thinking on this score were the impressive results of an early study, the first-ever randomized trial of surgery for epilepsy. In 80 patients with a 21-year history of drug-refractory temporal lobe epilepsy who were randomized to surgery or 1 year of AED therapy, at 1 year of follow-up blinded epileptologists rated 58% of surgically treated patients as free from seizures that impair awareness of self and surroundings, compared with just 8% in the AED group (N Engl J Med. 2001 Aug 2;345[5]:311-8).
“That’s a big outcome, and I’m very keen to ensure that my data continue to drive the push for early surgery,” according to the neurologist.
A Cochrane review of 177 studies totaling more than 16,000 patients concluded that 65% of epilepsy patients had good outcomes following surgery. Prognostic factors associated with better surgical outcomes included complete surgical resection of the epileptogenic focus, the presence of mesial temporal sclerosis, concordance of MRI and EEG findings, and an absence of cortical dysplasia (Cochrane Database Syst Rev. 2019;6:CD010541. doi: 10.1002/14651858.CD010541.pub3).
In addition, a systematic review and meta-analysis by Canadian investigators found that 72% of adults with lesional epilepsy identified by MRI or histopathology were seizure-free after surgery, compared with 36% of those with nonlesional epilepsy. The disparity in outcomes was similar in pediatric epilepsy patients, with seizure freedom after surgery in 74% of those with lesional disease versus 45% with nonlesional epilepsy (Epilepsy Res. 2010 May;89[2-3]:310-8).
Whither are neurostimulatory device therapies headed?
Dr. Brodie was quick to admit that as a pharmacologic researcher, device modalities including vagus nerve stimulation, responsive neurostimulation, and deep brain stimulation are outside his area of expertise. But he’s been following developments in the field with interest.
“These device therapies have shown efficacy in short-term randomized trials, but very few patients attain long-term seizure freedom. I think these are largely palliative techniques. I gave up on these techniques a long time ago because I felt it was a very costly way of reducing seizures by a relatively small margin, and really we need to go a little bit further than that. But I know there’s a lot of work going on at the moment,” he said.
Dr. Brodie reported serving on the scientific advisory boards of more than a half dozen pharmaceutical companies.
BANGKOK – Since founding the Epilepsy Unit at Glasgow’s Western Infirmary 37 years ago, Martin J. Brodie, MD, has seen many changes in the field, including the introduction of more than a dozen new antiepileptic drugs (AEDs) in the past 2 decades.
And based upon this vast clinical experience coupled with his leadership of landmark studies, he has a message for his physician colleagues and their epilepsy patients. And it’s not pretty.
“Has the probability of achieving seizure freedom increased significantly in the last 3 decades? Regrettably, the answer is no,” he declared at the International Epilepsy Congress.
“Over all these years, in terms of seizure freedom there has been no real difference in outcome. There’s really quite a long way to go before we can say that we are doing all that well for people,” he said at the congress sponsored by the International League Against Epilepsy.
In the year 2000, he and his coinvestigators published a prospective, longitudinal, observational cohort study of 470 newly diagnosed patients with epilepsy treated at the Western Infirmary during 1982-1997, all with a minimum of 2 years’ follow-up. Sixty-one percent achieved complete freedom from seizures for at least 1 year on monotherapy, and another 3% did so on polytherapy, for a total rate of 64% (N Engl J Med. 2000 Feb 3;342[5]:314-19).
But these were patients who by and large were treated with older AEDs such as carbamazepine, which has since fallen by the wayside because of toxicities. Scottish neurologists now generally turn to lamotrigine (Lamictal), levetiracetam (Spritam), and other, newer AEDs. So Dr. Brodie and his coworkers recently published a follow-up study, this one featuring 30 years of longitudinal follow-up of 1,795 patients newly treated for epilepsy with AEDs, new and old, during 1982-2012. The investigators demonstrated that the seizure-free survival curves over time were virtually superimposable. In the larger, more recent study, remission was achieved in 55% of patients with AED monotherapy and in another 9% with polytherapy, for a total rate of 64%, identical to the rate in the 2000 study, and as was the case in the earlier study, 36% of patients remained uncontrolled (JAMA Neurol. 2018 Mar 1;75[3]:279-86).
“Overall, the way this population behaves, there’s no difference in efficacy and no difference in tolerability whether you’re using old drugs used properly or new drugs used properly,” said Dr. Brodie, professor of neurology at the University of Glasgow (Scotland).
It’s noteworthy that Sir William R. Gowers, the Londoner who has been called the greatest neurologist of all time, reported a 70% seizure-free rate in 1881, while Dr. Brodie and workers achieved a 64% rate in their 30-year study. “It’s interesting that the numbers are so bad, really, I suppose,” Dr. Brodie commented.
How about outcomes in pediatric epilepsy?
Dr. Brodie and coworkers recently published a 30-year prospective cohort study of 332 adolescent epilepsy patients newly diagnosed and treated at the Western Infirmary during 1982-2012. At the end of the study, 67% were seizure-free for at least the past year, a feat accomplished via monotherapy in 83% of cases. The seizure-free rate was 72% in those with generalized epilepsy, significantly better than the 60% figure in those with focal epilepsy. The efficacy rate was 74% with newer AED monotherapy and similar at 77% with monotherapy older drugs. Adverse event rates ranged from a low of 12% with lamotrigine to 56% with topiramate (Topamax), according to the findings published in Epilepsia (2019 Jun;60[6]:1083-90).
Roughly similar outcomes have been reported from Norway in a study of 600 children with epilepsy, median age 7 years, with a median follow-up of 5.8 years that is considerably shorter than that in the Glasgow pediatric study. Overall, 59% of the Norwegian children remained seizure free for at least 1 year, 30% developed drug-resistant epilepsy, and 11% followed an intermediate remitting/relapsing course (Pediatrics. 2018 Jun. doi: 10.1542/peds.2017-4016).
Why the decades of flat pharmacologic outcomes?
The consistently suboptimal seizure-free outcomes obtained over the past 30 years shouldn’t really be surprising, according to Dr. Brodie.
“Although we think we have lots of mechanisms of action and lots of differences between the drugs, they’re arguably all antiseizure drugs and not antiepilepsy drugs. We don’t treat the whale; we treat the spout. We don’t treat what we cannot see; we treat what we can see, which is the seizures, but we’re not influencing the long-term outcome,” the neurologist explained.
The compelling case for early epilepsy surgery
Epilepsy surgery remains underutilized, according to Dr. Brodie and other experts.
The International League Against Epilepsy defines drug-resistant epilepsy as failure to achieve sustained seizure freedom after adequate trials of two tolerated and appropriately chosen and used AED schedules. Dr. Brodie’s work was influential in creating that definition because his data demonstrated the sharply diminishing returns of additional drug trials.
“When do we consider epilepsy surgery? Arguably, the earlier, the better. After two drugs have failed appropriately, I don’t think anybody in this room would argue about that, although people in some of the other rooms might,” he said at the congress.
Influential in his thinking on this score were the impressive results of an early study, the first-ever randomized trial of surgery for epilepsy. In 80 patients with a 21-year history of drug-refractory temporal lobe epilepsy who were randomized to surgery or 1 year of AED therapy, at 1 year of follow-up blinded epileptologists rated 58% of surgically treated patients as free from seizures that impair awareness of self and surroundings, compared with just 8% in the AED group (N Engl J Med. 2001 Aug 2;345[5]:311-8).
“That’s a big outcome, and I’m very keen to ensure that my data continue to drive the push for early surgery,” according to the neurologist.
A Cochrane review of 177 studies totaling more than 16,000 patients concluded that 65% of epilepsy patients had good outcomes following surgery. Prognostic factors associated with better surgical outcomes included complete surgical resection of the epileptogenic focus, the presence of mesial temporal sclerosis, concordance of MRI and EEG findings, and an absence of cortical dysplasia (Cochrane Database Syst Rev. 2019;6:CD010541. doi: 10.1002/14651858.CD010541.pub3).
In addition, a systematic review and meta-analysis by Canadian investigators found that 72% of adults with lesional epilepsy identified by MRI or histopathology were seizure-free after surgery, compared with 36% of those with nonlesional epilepsy. The disparity in outcomes was similar in pediatric epilepsy patients, with seizure freedom after surgery in 74% of those with lesional disease versus 45% with nonlesional epilepsy (Epilepsy Res. 2010 May;89[2-3]:310-8).
Whither are neurostimulatory device therapies headed?
Dr. Brodie was quick to admit that as a pharmacologic researcher, device modalities including vagus nerve stimulation, responsive neurostimulation, and deep brain stimulation are outside his area of expertise. But he’s been following developments in the field with interest.
“These device therapies have shown efficacy in short-term randomized trials, but very few patients attain long-term seizure freedom. I think these are largely palliative techniques. I gave up on these techniques a long time ago because I felt it was a very costly way of reducing seizures by a relatively small margin, and really we need to go a little bit further than that. But I know there’s a lot of work going on at the moment,” he said.
Dr. Brodie reported serving on the scientific advisory boards of more than a half dozen pharmaceutical companies.
BANGKOK – Since founding the Epilepsy Unit at Glasgow’s Western Infirmary 37 years ago, Martin J. Brodie, MD, has seen many changes in the field, including the introduction of more than a dozen new antiepileptic drugs (AEDs) in the past 2 decades.
And based upon this vast clinical experience coupled with his leadership of landmark studies, he has a message for his physician colleagues and their epilepsy patients. And it’s not pretty.
“Has the probability of achieving seizure freedom increased significantly in the last 3 decades? Regrettably, the answer is no,” he declared at the International Epilepsy Congress.
“Over all these years, in terms of seizure freedom there has been no real difference in outcome. There’s really quite a long way to go before we can say that we are doing all that well for people,” he said at the congress sponsored by the International League Against Epilepsy.
In the year 2000, he and his coinvestigators published a prospective, longitudinal, observational cohort study of 470 newly diagnosed patients with epilepsy treated at the Western Infirmary during 1982-1997, all with a minimum of 2 years’ follow-up. Sixty-one percent achieved complete freedom from seizures for at least 1 year on monotherapy, and another 3% did so on polytherapy, for a total rate of 64% (N Engl J Med. 2000 Feb 3;342[5]:314-19).
But these were patients who by and large were treated with older AEDs such as carbamazepine, which has since fallen by the wayside because of toxicities. Scottish neurologists now generally turn to lamotrigine (Lamictal), levetiracetam (Spritam), and other, newer AEDs. So Dr. Brodie and his coworkers recently published a follow-up study, this one featuring 30 years of longitudinal follow-up of 1,795 patients newly treated for epilepsy with AEDs, new and old, during 1982-2012. The investigators demonstrated that the seizure-free survival curves over time were virtually superimposable. In the larger, more recent study, remission was achieved in 55% of patients with AED monotherapy and in another 9% with polytherapy, for a total rate of 64%, identical to the rate in the 2000 study, and as was the case in the earlier study, 36% of patients remained uncontrolled (JAMA Neurol. 2018 Mar 1;75[3]:279-86).
“Overall, the way this population behaves, there’s no difference in efficacy and no difference in tolerability whether you’re using old drugs used properly or new drugs used properly,” said Dr. Brodie, professor of neurology at the University of Glasgow (Scotland).
It’s noteworthy that Sir William R. Gowers, the Londoner who has been called the greatest neurologist of all time, reported a 70% seizure-free rate in 1881, while Dr. Brodie and workers achieved a 64% rate in their 30-year study. “It’s interesting that the numbers are so bad, really, I suppose,” Dr. Brodie commented.
How about outcomes in pediatric epilepsy?
Dr. Brodie and coworkers recently published a 30-year prospective cohort study of 332 adolescent epilepsy patients newly diagnosed and treated at the Western Infirmary during 1982-2012. At the end of the study, 67% were seizure-free for at least the past year, a feat accomplished via monotherapy in 83% of cases. The seizure-free rate was 72% in those with generalized epilepsy, significantly better than the 60% figure in those with focal epilepsy. The efficacy rate was 74% with newer AED monotherapy and similar at 77% with monotherapy older drugs. Adverse event rates ranged from a low of 12% with lamotrigine to 56% with topiramate (Topamax), according to the findings published in Epilepsia (2019 Jun;60[6]:1083-90).
Roughly similar outcomes have been reported from Norway in a study of 600 children with epilepsy, median age 7 years, with a median follow-up of 5.8 years that is considerably shorter than that in the Glasgow pediatric study. Overall, 59% of the Norwegian children remained seizure free for at least 1 year, 30% developed drug-resistant epilepsy, and 11% followed an intermediate remitting/relapsing course (Pediatrics. 2018 Jun. doi: 10.1542/peds.2017-4016).
Why the decades of flat pharmacologic outcomes?
The consistently suboptimal seizure-free outcomes obtained over the past 30 years shouldn’t really be surprising, according to Dr. Brodie.
“Although we think we have lots of mechanisms of action and lots of differences between the drugs, they’re arguably all antiseizure drugs and not antiepilepsy drugs. We don’t treat the whale; we treat the spout. We don’t treat what we cannot see; we treat what we can see, which is the seizures, but we’re not influencing the long-term outcome,” the neurologist explained.
The compelling case for early epilepsy surgery
Epilepsy surgery remains underutilized, according to Dr. Brodie and other experts.
The International League Against Epilepsy defines drug-resistant epilepsy as failure to achieve sustained seizure freedom after adequate trials of two tolerated and appropriately chosen and used AED schedules. Dr. Brodie’s work was influential in creating that definition because his data demonstrated the sharply diminishing returns of additional drug trials.
“When do we consider epilepsy surgery? Arguably, the earlier, the better. After two drugs have failed appropriately, I don’t think anybody in this room would argue about that, although people in some of the other rooms might,” he said at the congress.
Influential in his thinking on this score were the impressive results of an early study, the first-ever randomized trial of surgery for epilepsy. In 80 patients with a 21-year history of drug-refractory temporal lobe epilepsy who were randomized to surgery or 1 year of AED therapy, at 1 year of follow-up blinded epileptologists rated 58% of surgically treated patients as free from seizures that impair awareness of self and surroundings, compared with just 8% in the AED group (N Engl J Med. 2001 Aug 2;345[5]:311-8).
“That’s a big outcome, and I’m very keen to ensure that my data continue to drive the push for early surgery,” according to the neurologist.
A Cochrane review of 177 studies totaling more than 16,000 patients concluded that 65% of epilepsy patients had good outcomes following surgery. Prognostic factors associated with better surgical outcomes included complete surgical resection of the epileptogenic focus, the presence of mesial temporal sclerosis, concordance of MRI and EEG findings, and an absence of cortical dysplasia (Cochrane Database Syst Rev. 2019;6:CD010541. doi: 10.1002/14651858.CD010541.pub3).
In addition, a systematic review and meta-analysis by Canadian investigators found that 72% of adults with lesional epilepsy identified by MRI or histopathology were seizure-free after surgery, compared with 36% of those with nonlesional epilepsy. The disparity in outcomes was similar in pediatric epilepsy patients, with seizure freedom after surgery in 74% of those with lesional disease versus 45% with nonlesional epilepsy (Epilepsy Res. 2010 May;89[2-3]:310-8).
Whither are neurostimulatory device therapies headed?
Dr. Brodie was quick to admit that as a pharmacologic researcher, device modalities including vagus nerve stimulation, responsive neurostimulation, and deep brain stimulation are outside his area of expertise. But he’s been following developments in the field with interest.
“These device therapies have shown efficacy in short-term randomized trials, but very few patients attain long-term seizure freedom. I think these are largely palliative techniques. I gave up on these techniques a long time ago because I felt it was a very costly way of reducing seizures by a relatively small margin, and really we need to go a little bit further than that. But I know there’s a lot of work going on at the moment,” he said.
Dr. Brodie reported serving on the scientific advisory boards of more than a half dozen pharmaceutical companies.
EXPERT ANALYSIS FROM IEC 2019
Should You Switch the DAPT Agent a Month After ACS?
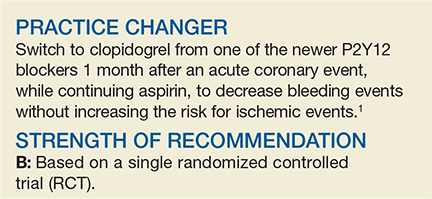
A 60-year-old man visits your clinic 30 days after he was hospitalized for acute coronary syndrome (ACS) due to ST-elevation myocardial infarction (STEMI). The patient underwent percutaneous coronary intervention (PCI) with placement of a stent and received aspirin and a loading dose of ticagrelor for antiplatelet therapy. He was discharged on dual antiplatelet therapy (DAPT) consisting of daily aspirin and ticagrelor. He asks about the risk for bleeding associated with these medications. Should you recommend any changes?
Platelet inhibition during and after ACS to prevent recurrent ischemic events is a cornerstone of treatment for patients after a myocardial infarction (MI).2 Current American College of Cardiology/American Heart Association and European Society of Cardiology guidelines recommend that patients with coronary artery disease who recently had an MI continue DAPT with aspirin and a P2Y12 blocker (clopidogrel, ticlopidine, ticagrelor, prasugrel, or cangrelor) for 12 months following ACS to reduce recurrent ischemia.2-4
Studies have shown that using the newer P2Y12 inhibitors (prasugrel and ticagrelor) after PCI leads to a significant reduction in recurrent ischemic events, compared with clopidogrel.5-7 These data prompted a guideline change recommending the use of the newer agents over clopidogrel for 12 months following PCI.2 Follow-up studies show strong evidence for the use of the newer P2Y12 agents in the first month following PCI, but they also demonstrate an increased bleeding risk in the maintenance phase (from 30 days to 12 months post-PCI).6,7 This increased risk is the basis for the study by Cuisset et al, which examined switching from a newer P2Y12 agent to clopidogrel after the initial 30-day period following PCI.
STUDY SUMMARY
Switched DAPT is superior
This open-label RCT (N = 646) evaluated changing DAPT from aspirin plus a newer P2Y12 blocker (prasugrel or ticagrelor) to a combination of aspirin and clopidogrel after the first month of DAPT post-ACS.1 Prior to PCI, patients received a loading dose of ticagrelor (180 mg) or prasugrel (60 mg). Subsequently, all patients took aspirin (75 mg/d) and either prasugrel (10 mg/d) or ticagrelor (90 mg bid) for 1 month. After 30 days, participants who had no adverse events were randomly assigned in a 1:1 ratio to continue the aspirin and newer P2Y12 blocker regimen or switch to aspirin and clopidogrel (75 mg/d). In the following year, researchers examined the composite outcome of cardiovascular death, urgent revascularization, stroke, and major bleeding (defined by a Bleeding Academic Research Consortium [BARC] classification ≥ Type 2 at 1-year post-ACS).
Of the participants (average age, 60), 40% had a STEMI and 60% had a non-STEMI. Overall, 43% of patients were prescribed ticagrelor and 57% prasugrel. At 1 year, 86% of the switched-DAPT group and 75% of the unchanged-DAPT group were still taking their medication. The composite outcome at 1-year follow-up was lower in the switched group compared with the unchanged group (13.4% vs 26.3%; hazard ratio [HR], 0.48; 95% confidence interval [CI], 0.34-0.68; number needed to treat [NNT], 8).
Bleeding events (ranging from minimal to fatal) were lower in the switched group (9.3% vs 23.5%; HR, 0.39; 95% CI, 0.27-0.57; NNT, 7) and events identified as BARC ≥ Type 2 (defined as needing medical treatment) were also lower in this group (4% vs 14.9%; HR, 0.30, 95% CI, 0.18-0.50; NNT, 9). There were no significant differences in reported recurrent cardiovascular ischemic events (9.3% vs 11.5%; HR, 0.80, 95% CI, 0.50-1.29).
WHAT’S NEW
Less bleeding, no increase in ischemic events
Cardiology guidelines recommend the newer P2Y12 blockers as part of DAPT after ACS, but this trial showed switching to clopidogrel for DAPT after 30 days of treatment lowers bleeding events with no difference in recurrent ischemic events.2-4
Continue to: CAVEATS
CAVEATS
Less-than-ideal study methods
In this open-label and unblinded study, the investigators adjudicating critical events were blinded to the treatment allocation. However, patients could self-report minor bleeding and medication discontinuation for which no consultation was sought. In addition, the investigators used opaque envelopes—a less-than-ideal method—to conceal allocation at enrollment.
CHALLENGES TO IMPLEMENTATION
PCP may not change cardiologist’s prescription
Implementing this practice is facilitated by the comparatively lower cost of clopidogrel versus the newer P2Y12 blockers. However, after ACS and PCI treatment, cardiologists usually initiate antiplatelet therapy and may continue to manage patients after discharge. The primary care provider (PCP) may not be responsible for the DAPT switch initially; furthermore, ordering a switch may require coordination if the PCP is hesitant to change the cardiologist’s prescription. Lastly, guidelines currently recommend using the newer P2Y12 blockers for 12 months.2 CR
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[3]:162,164).
1. Cuisset T, Deharo P, Quilici J, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. 2017;38(41):3070-3078.
2. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68(10):1082-1115.
3. Steg PG, James SK, Atar D, et al; Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33(20):2569-2619.
4. Roffi M, Patrono C, Collet J-P, et al; ESC Scientific Document Group. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2015;37(3):267-315.
5. Antman EM, Wiviott SD, Murphy SA, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a TRITON-TIMI 38 (TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel-Thrombolysis In Myocardial Infarction) analysis. J Am Coll Cardiol. 2008;51(21): 2028-2033.
6. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045-1057.
7. Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001-2015.

A 60-year-old man visits your clinic 30 days after he was hospitalized for acute coronary syndrome (ACS) due to ST-elevation myocardial infarction (STEMI). The patient underwent percutaneous coronary intervention (PCI) with placement of a stent and received aspirin and a loading dose of ticagrelor for antiplatelet therapy. He was discharged on dual antiplatelet therapy (DAPT) consisting of daily aspirin and ticagrelor. He asks about the risk for bleeding associated with these medications. Should you recommend any changes?
Platelet inhibition during and after ACS to prevent recurrent ischemic events is a cornerstone of treatment for patients after a myocardial infarction (MI).2 Current American College of Cardiology/American Heart Association and European Society of Cardiology guidelines recommend that patients with coronary artery disease who recently had an MI continue DAPT with aspirin and a P2Y12 blocker (clopidogrel, ticlopidine, ticagrelor, prasugrel, or cangrelor) for 12 months following ACS to reduce recurrent ischemia.2-4
Studies have shown that using the newer P2Y12 inhibitors (prasugrel and ticagrelor) after PCI leads to a significant reduction in recurrent ischemic events, compared with clopidogrel.5-7 These data prompted a guideline change recommending the use of the newer agents over clopidogrel for 12 months following PCI.2 Follow-up studies show strong evidence for the use of the newer P2Y12 agents in the first month following PCI, but they also demonstrate an increased bleeding risk in the maintenance phase (from 30 days to 12 months post-PCI).6,7 This increased risk is the basis for the study by Cuisset et al, which examined switching from a newer P2Y12 agent to clopidogrel after the initial 30-day period following PCI.
STUDY SUMMARY
Switched DAPT is superior
This open-label RCT (N = 646) evaluated changing DAPT from aspirin plus a newer P2Y12 blocker (prasugrel or ticagrelor) to a combination of aspirin and clopidogrel after the first month of DAPT post-ACS.1 Prior to PCI, patients received a loading dose of ticagrelor (180 mg) or prasugrel (60 mg). Subsequently, all patients took aspirin (75 mg/d) and either prasugrel (10 mg/d) or ticagrelor (90 mg bid) for 1 month. After 30 days, participants who had no adverse events were randomly assigned in a 1:1 ratio to continue the aspirin and newer P2Y12 blocker regimen or switch to aspirin and clopidogrel (75 mg/d). In the following year, researchers examined the composite outcome of cardiovascular death, urgent revascularization, stroke, and major bleeding (defined by a Bleeding Academic Research Consortium [BARC] classification ≥ Type 2 at 1-year post-ACS).
Of the participants (average age, 60), 40% had a STEMI and 60% had a non-STEMI. Overall, 43% of patients were prescribed ticagrelor and 57% prasugrel. At 1 year, 86% of the switched-DAPT group and 75% of the unchanged-DAPT group were still taking their medication. The composite outcome at 1-year follow-up was lower in the switched group compared with the unchanged group (13.4% vs 26.3%; hazard ratio [HR], 0.48; 95% confidence interval [CI], 0.34-0.68; number needed to treat [NNT], 8).
Bleeding events (ranging from minimal to fatal) were lower in the switched group (9.3% vs 23.5%; HR, 0.39; 95% CI, 0.27-0.57; NNT, 7) and events identified as BARC ≥ Type 2 (defined as needing medical treatment) were also lower in this group (4% vs 14.9%; HR, 0.30, 95% CI, 0.18-0.50; NNT, 9). There were no significant differences in reported recurrent cardiovascular ischemic events (9.3% vs 11.5%; HR, 0.80, 95% CI, 0.50-1.29).
WHAT’S NEW
Less bleeding, no increase in ischemic events
Cardiology guidelines recommend the newer P2Y12 blockers as part of DAPT after ACS, but this trial showed switching to clopidogrel for DAPT after 30 days of treatment lowers bleeding events with no difference in recurrent ischemic events.2-4
Continue to: CAVEATS
CAVEATS
Less-than-ideal study methods
In this open-label and unblinded study, the investigators adjudicating critical events were blinded to the treatment allocation. However, patients could self-report minor bleeding and medication discontinuation for which no consultation was sought. In addition, the investigators used opaque envelopes—a less-than-ideal method—to conceal allocation at enrollment.
CHALLENGES TO IMPLEMENTATION
PCP may not change cardiologist’s prescription
Implementing this practice is facilitated by the comparatively lower cost of clopidogrel versus the newer P2Y12 blockers. However, after ACS and PCI treatment, cardiologists usually initiate antiplatelet therapy and may continue to manage patients after discharge. The primary care provider (PCP) may not be responsible for the DAPT switch initially; furthermore, ordering a switch may require coordination if the PCP is hesitant to change the cardiologist’s prescription. Lastly, guidelines currently recommend using the newer P2Y12 blockers for 12 months.2 CR
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[3]:162,164).

A 60-year-old man visits your clinic 30 days after he was hospitalized for acute coronary syndrome (ACS) due to ST-elevation myocardial infarction (STEMI). The patient underwent percutaneous coronary intervention (PCI) with placement of a stent and received aspirin and a loading dose of ticagrelor for antiplatelet therapy. He was discharged on dual antiplatelet therapy (DAPT) consisting of daily aspirin and ticagrelor. He asks about the risk for bleeding associated with these medications. Should you recommend any changes?
Platelet inhibition during and after ACS to prevent recurrent ischemic events is a cornerstone of treatment for patients after a myocardial infarction (MI).2 Current American College of Cardiology/American Heart Association and European Society of Cardiology guidelines recommend that patients with coronary artery disease who recently had an MI continue DAPT with aspirin and a P2Y12 blocker (clopidogrel, ticlopidine, ticagrelor, prasugrel, or cangrelor) for 12 months following ACS to reduce recurrent ischemia.2-4
Studies have shown that using the newer P2Y12 inhibitors (prasugrel and ticagrelor) after PCI leads to a significant reduction in recurrent ischemic events, compared with clopidogrel.5-7 These data prompted a guideline change recommending the use of the newer agents over clopidogrel for 12 months following PCI.2 Follow-up studies show strong evidence for the use of the newer P2Y12 agents in the first month following PCI, but they also demonstrate an increased bleeding risk in the maintenance phase (from 30 days to 12 months post-PCI).6,7 This increased risk is the basis for the study by Cuisset et al, which examined switching from a newer P2Y12 agent to clopidogrel after the initial 30-day period following PCI.
STUDY SUMMARY
Switched DAPT is superior
This open-label RCT (N = 646) evaluated changing DAPT from aspirin plus a newer P2Y12 blocker (prasugrel or ticagrelor) to a combination of aspirin and clopidogrel after the first month of DAPT post-ACS.1 Prior to PCI, patients received a loading dose of ticagrelor (180 mg) or prasugrel (60 mg). Subsequently, all patients took aspirin (75 mg/d) and either prasugrel (10 mg/d) or ticagrelor (90 mg bid) for 1 month. After 30 days, participants who had no adverse events were randomly assigned in a 1:1 ratio to continue the aspirin and newer P2Y12 blocker regimen or switch to aspirin and clopidogrel (75 mg/d). In the following year, researchers examined the composite outcome of cardiovascular death, urgent revascularization, stroke, and major bleeding (defined by a Bleeding Academic Research Consortium [BARC] classification ≥ Type 2 at 1-year post-ACS).
Of the participants (average age, 60), 40% had a STEMI and 60% had a non-STEMI. Overall, 43% of patients were prescribed ticagrelor and 57% prasugrel. At 1 year, 86% of the switched-DAPT group and 75% of the unchanged-DAPT group were still taking their medication. The composite outcome at 1-year follow-up was lower in the switched group compared with the unchanged group (13.4% vs 26.3%; hazard ratio [HR], 0.48; 95% confidence interval [CI], 0.34-0.68; number needed to treat [NNT], 8).
Bleeding events (ranging from minimal to fatal) were lower in the switched group (9.3% vs 23.5%; HR, 0.39; 95% CI, 0.27-0.57; NNT, 7) and events identified as BARC ≥ Type 2 (defined as needing medical treatment) were also lower in this group (4% vs 14.9%; HR, 0.30, 95% CI, 0.18-0.50; NNT, 9). There were no significant differences in reported recurrent cardiovascular ischemic events (9.3% vs 11.5%; HR, 0.80, 95% CI, 0.50-1.29).
WHAT’S NEW
Less bleeding, no increase in ischemic events
Cardiology guidelines recommend the newer P2Y12 blockers as part of DAPT after ACS, but this trial showed switching to clopidogrel for DAPT after 30 days of treatment lowers bleeding events with no difference in recurrent ischemic events.2-4
Continue to: CAVEATS
CAVEATS
Less-than-ideal study methods
In this open-label and unblinded study, the investigators adjudicating critical events were blinded to the treatment allocation. However, patients could self-report minor bleeding and medication discontinuation for which no consultation was sought. In addition, the investigators used opaque envelopes—a less-than-ideal method—to conceal allocation at enrollment.
CHALLENGES TO IMPLEMENTATION
PCP may not change cardiologist’s prescription
Implementing this practice is facilitated by the comparatively lower cost of clopidogrel versus the newer P2Y12 blockers. However, after ACS and PCI treatment, cardiologists usually initiate antiplatelet therapy and may continue to manage patients after discharge. The primary care provider (PCP) may not be responsible for the DAPT switch initially; furthermore, ordering a switch may require coordination if the PCP is hesitant to change the cardiologist’s prescription. Lastly, guidelines currently recommend using the newer P2Y12 blockers for 12 months.2 CR
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[3]:162,164).
1. Cuisset T, Deharo P, Quilici J, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. 2017;38(41):3070-3078.
2. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68(10):1082-1115.
3. Steg PG, James SK, Atar D, et al; Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33(20):2569-2619.
4. Roffi M, Patrono C, Collet J-P, et al; ESC Scientific Document Group. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2015;37(3):267-315.
5. Antman EM, Wiviott SD, Murphy SA, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a TRITON-TIMI 38 (TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel-Thrombolysis In Myocardial Infarction) analysis. J Am Coll Cardiol. 2008;51(21): 2028-2033.
6. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045-1057.
7. Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001-2015.
1. Cuisset T, Deharo P, Quilici J, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. 2017;38(41):3070-3078.
2. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68(10):1082-1115.
3. Steg PG, James SK, Atar D, et al; Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33(20):2569-2619.
4. Roffi M, Patrono C, Collet J-P, et al; ESC Scientific Document Group. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2015;37(3):267-315.
5. Antman EM, Wiviott SD, Murphy SA, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a TRITON-TIMI 38 (TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel-Thrombolysis In Myocardial Infarction) analysis. J Am Coll Cardiol. 2008;51(21): 2028-2033.
6. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045-1057.
7. Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001-2015.
Benefit of chemo + RT for high-risk endometrial cancer increases with time
Benefits of adding chemotherapy to pelvic radiotherapy in localized high-risk endometrial cancer increase with follow-up, finds an updated analysis of the randomized PORTEC-3 trial.
The relative risk-benefit profile of chemoradiotherapy is uncertain, with some evidence suggesting it varies according to disease histology and stage, noted lead investigator Stephanie de Boer, MD, department of radiation oncology, Leiden (the Netherlands) University Medical Center, and coinvestigators.
PORTEC-3, a multicenter phase 3 trial, enrolled women who had undergone surgery for high-risk endometrial cancer: FIGO 2009 stage I, endometrioid grade 3 cancer with deep myometrial invasion, lymphovascular space invasion, or both; stage II or III disease; or stage I to III disease with serous or clear cell histology. In all, 660 women were evenly assigned to external-beam radiotherapy alone (48.6 Gy in 1.8-Gy fractions, on 5 days per week) or radiotherapy and chemotherapy (two cycles of cisplatin given during radiotherapy, followed by four cycles of carboplatin and paclitaxel).
A previous analysis, at a median follow-up of 60.2 months (5.0 years), showed a significant 5-year failure-free survival benefit of chemoradiotherapy over radiotherapy (hazard ratio [HR], 0.71; P = .022) but only a trend toward an overall survival benefit (Lancet Oncol. 2018;19[3]:295-309).
The updated analysis, now at a median follow-up of 72.6 months (6.1 years), was reported in Lancet Oncology and showed that chemoradiotherapy was still significantly superior to radiotherapy alone in terms of 5-year failure-free survival (76.5% vs. 69.1%; adjusted HR, 0.70; P = .016) but was now also significantly superior in terms of 5-year overall survival (81.4% vs. 76.1%; adjusted HR, 0.70; P = .034)
The 5-year probability of distant metastasis was lower with chemoradiotherapy than with radiotherapy alone (21.4% vs. 29.1%; HR, 0.74; P = .047). The two groups did not differ significantly with respect to isolated vaginal recurrence as first site (0.3% in each group) or isolated pelvic recurrence as first site (0.9% in each group).
Only a single patient, in the chemoradiotherapy group, experienced a grade 4 adverse event (ileus or obstruction). The chemoradiotherapy and radiotherapy-only groups were similar on the rate of grade 3 adverse events (8% vs. 5%; P = .24), the most common of which was hypertension. However, the former had a higher rate of grade 2 or worse adverse events (38% vs. 23%; P = .002), such as persistent sensory neuropathy (6% vs. 0%). None of the patients died from treatment-related causes.
“Combined adjuvant chemotherapy and radiotherapy should be discussed and recommended as a new standard of care, especially for women with stage III endometrial cancer or serous cancers, or both,” Dr. de Boer and coinvestigators maintained. “Shared decision making between doctors and their patients remains essential to weigh the costs and benefits for individual patients.
“Molecular analysis has the potential to improve risk stratification and should be used to identify subgroups that can derive the greatest benefit from chemotherapy and to select patients for targeted therapies; molecular studies on tissue samples donated by PORTEC-3 trial participants are ongoing,” they noted.
Dr. de Boer disclosed no competing interests in relation to the study. The study was supported in part by the Dutch Cancer Society, Cancer Research UK, National Health and Medical Research Council, Cancer Australia, the Italian Medicines Agency, and the Canadian Cancer Society Research Institute.
SOURCE: de Boer SM et al. Lancet Oncol. 2019 July 22. doi: 10.1016/S1470-2045(19)30395-X.
Results of PORTEC-3 are potentially practice changing but generate several questions relevant to optimizing adjuvant therapy for high-risk endometrial cancer, Marcus Randall, MD, contends in a commentary (Lancet Oncol. 2019 Jul 22. doi: 10.1016/S1470-2045[19]30416-4).
One question is applicability of the findings across trial subgroups, which is still uncertain. “However, taking into account the statistical limitations of subgroup analyses, the therapeutic benefit of combined chemotherapy and radiotherapy (vs. radiotherapy alone) appeared to remain confined to patients with stage III disease and those with serous carcinomas of all stages,” he noted.
Another question is whether chemotherapy alone is sufficient. Here, results from trials conducted by the Gynecologic Oncology Group (now NRG Oncology) suggest that omitting pelvic radiotherapy increases the risk of locoregional failure, according to Dr. Randall.
A final question is whether there is a preferred approach for combining chemotherapy with radiotherapy. “Increasing evidence supports the use of upfront systemic therapy, when combined with radiotherapy, as a strategy to maximise both systemic and local control. ... Many clinicians often use this regimen as a preferred adjuvant approach in locally advanced endometrial cancer,” he noted.
“Based on outstanding work done by the PORTEC Study Group and others, we have made good progress in improving outcomes for women with high-risk and locally advanced endometrial cancers. However, we are not there yet,” Dr. Randall concludes.
Marcus Randall, MD, is with the department of radiation medicine, University of Kentucky, Lexington. He has no disclosures related to the commentary.
Results of PORTEC-3 are potentially practice changing but generate several questions relevant to optimizing adjuvant therapy for high-risk endometrial cancer, Marcus Randall, MD, contends in a commentary (Lancet Oncol. 2019 Jul 22. doi: 10.1016/S1470-2045[19]30416-4).
One question is applicability of the findings across trial subgroups, which is still uncertain. “However, taking into account the statistical limitations of subgroup analyses, the therapeutic benefit of combined chemotherapy and radiotherapy (vs. radiotherapy alone) appeared to remain confined to patients with stage III disease and those with serous carcinomas of all stages,” he noted.
Another question is whether chemotherapy alone is sufficient. Here, results from trials conducted by the Gynecologic Oncology Group (now NRG Oncology) suggest that omitting pelvic radiotherapy increases the risk of locoregional failure, according to Dr. Randall.
A final question is whether there is a preferred approach for combining chemotherapy with radiotherapy. “Increasing evidence supports the use of upfront systemic therapy, when combined with radiotherapy, as a strategy to maximise both systemic and local control. ... Many clinicians often use this regimen as a preferred adjuvant approach in locally advanced endometrial cancer,” he noted.
“Based on outstanding work done by the PORTEC Study Group and others, we have made good progress in improving outcomes for women with high-risk and locally advanced endometrial cancers. However, we are not there yet,” Dr. Randall concludes.
Marcus Randall, MD, is with the department of radiation medicine, University of Kentucky, Lexington. He has no disclosures related to the commentary.
Results of PORTEC-3 are potentially practice changing but generate several questions relevant to optimizing adjuvant therapy for high-risk endometrial cancer, Marcus Randall, MD, contends in a commentary (Lancet Oncol. 2019 Jul 22. doi: 10.1016/S1470-2045[19]30416-4).
One question is applicability of the findings across trial subgroups, which is still uncertain. “However, taking into account the statistical limitations of subgroup analyses, the therapeutic benefit of combined chemotherapy and radiotherapy (vs. radiotherapy alone) appeared to remain confined to patients with stage III disease and those with serous carcinomas of all stages,” he noted.
Another question is whether chemotherapy alone is sufficient. Here, results from trials conducted by the Gynecologic Oncology Group (now NRG Oncology) suggest that omitting pelvic radiotherapy increases the risk of locoregional failure, according to Dr. Randall.
A final question is whether there is a preferred approach for combining chemotherapy with radiotherapy. “Increasing evidence supports the use of upfront systemic therapy, when combined with radiotherapy, as a strategy to maximise both systemic and local control. ... Many clinicians often use this regimen as a preferred adjuvant approach in locally advanced endometrial cancer,” he noted.
“Based on outstanding work done by the PORTEC Study Group and others, we have made good progress in improving outcomes for women with high-risk and locally advanced endometrial cancers. However, we are not there yet,” Dr. Randall concludes.
Marcus Randall, MD, is with the department of radiation medicine, University of Kentucky, Lexington. He has no disclosures related to the commentary.
Benefits of adding chemotherapy to pelvic radiotherapy in localized high-risk endometrial cancer increase with follow-up, finds an updated analysis of the randomized PORTEC-3 trial.
The relative risk-benefit profile of chemoradiotherapy is uncertain, with some evidence suggesting it varies according to disease histology and stage, noted lead investigator Stephanie de Boer, MD, department of radiation oncology, Leiden (the Netherlands) University Medical Center, and coinvestigators.
PORTEC-3, a multicenter phase 3 trial, enrolled women who had undergone surgery for high-risk endometrial cancer: FIGO 2009 stage I, endometrioid grade 3 cancer with deep myometrial invasion, lymphovascular space invasion, or both; stage II or III disease; or stage I to III disease with serous or clear cell histology. In all, 660 women were evenly assigned to external-beam radiotherapy alone (48.6 Gy in 1.8-Gy fractions, on 5 days per week) or radiotherapy and chemotherapy (two cycles of cisplatin given during radiotherapy, followed by four cycles of carboplatin and paclitaxel).
A previous analysis, at a median follow-up of 60.2 months (5.0 years), showed a significant 5-year failure-free survival benefit of chemoradiotherapy over radiotherapy (hazard ratio [HR], 0.71; P = .022) but only a trend toward an overall survival benefit (Lancet Oncol. 2018;19[3]:295-309).
The updated analysis, now at a median follow-up of 72.6 months (6.1 years), was reported in Lancet Oncology and showed that chemoradiotherapy was still significantly superior to radiotherapy alone in terms of 5-year failure-free survival (76.5% vs. 69.1%; adjusted HR, 0.70; P = .016) but was now also significantly superior in terms of 5-year overall survival (81.4% vs. 76.1%; adjusted HR, 0.70; P = .034)
The 5-year probability of distant metastasis was lower with chemoradiotherapy than with radiotherapy alone (21.4% vs. 29.1%; HR, 0.74; P = .047). The two groups did not differ significantly with respect to isolated vaginal recurrence as first site (0.3% in each group) or isolated pelvic recurrence as first site (0.9% in each group).
Only a single patient, in the chemoradiotherapy group, experienced a grade 4 adverse event (ileus or obstruction). The chemoradiotherapy and radiotherapy-only groups were similar on the rate of grade 3 adverse events (8% vs. 5%; P = .24), the most common of which was hypertension. However, the former had a higher rate of grade 2 or worse adverse events (38% vs. 23%; P = .002), such as persistent sensory neuropathy (6% vs. 0%). None of the patients died from treatment-related causes.
“Combined adjuvant chemotherapy and radiotherapy should be discussed and recommended as a new standard of care, especially for women with stage III endometrial cancer or serous cancers, or both,” Dr. de Boer and coinvestigators maintained. “Shared decision making between doctors and their patients remains essential to weigh the costs and benefits for individual patients.
“Molecular analysis has the potential to improve risk stratification and should be used to identify subgroups that can derive the greatest benefit from chemotherapy and to select patients for targeted therapies; molecular studies on tissue samples donated by PORTEC-3 trial participants are ongoing,” they noted.
Dr. de Boer disclosed no competing interests in relation to the study. The study was supported in part by the Dutch Cancer Society, Cancer Research UK, National Health and Medical Research Council, Cancer Australia, the Italian Medicines Agency, and the Canadian Cancer Society Research Institute.
SOURCE: de Boer SM et al. Lancet Oncol. 2019 July 22. doi: 10.1016/S1470-2045(19)30395-X.
Benefits of adding chemotherapy to pelvic radiotherapy in localized high-risk endometrial cancer increase with follow-up, finds an updated analysis of the randomized PORTEC-3 trial.
The relative risk-benefit profile of chemoradiotherapy is uncertain, with some evidence suggesting it varies according to disease histology and stage, noted lead investigator Stephanie de Boer, MD, department of radiation oncology, Leiden (the Netherlands) University Medical Center, and coinvestigators.
PORTEC-3, a multicenter phase 3 trial, enrolled women who had undergone surgery for high-risk endometrial cancer: FIGO 2009 stage I, endometrioid grade 3 cancer with deep myometrial invasion, lymphovascular space invasion, or both; stage II or III disease; or stage I to III disease with serous or clear cell histology. In all, 660 women were evenly assigned to external-beam radiotherapy alone (48.6 Gy in 1.8-Gy fractions, on 5 days per week) or radiotherapy and chemotherapy (two cycles of cisplatin given during radiotherapy, followed by four cycles of carboplatin and paclitaxel).
A previous analysis, at a median follow-up of 60.2 months (5.0 years), showed a significant 5-year failure-free survival benefit of chemoradiotherapy over radiotherapy (hazard ratio [HR], 0.71; P = .022) but only a trend toward an overall survival benefit (Lancet Oncol. 2018;19[3]:295-309).
The updated analysis, now at a median follow-up of 72.6 months (6.1 years), was reported in Lancet Oncology and showed that chemoradiotherapy was still significantly superior to radiotherapy alone in terms of 5-year failure-free survival (76.5% vs. 69.1%; adjusted HR, 0.70; P = .016) but was now also significantly superior in terms of 5-year overall survival (81.4% vs. 76.1%; adjusted HR, 0.70; P = .034)
The 5-year probability of distant metastasis was lower with chemoradiotherapy than with radiotherapy alone (21.4% vs. 29.1%; HR, 0.74; P = .047). The two groups did not differ significantly with respect to isolated vaginal recurrence as first site (0.3% in each group) or isolated pelvic recurrence as first site (0.9% in each group).
Only a single patient, in the chemoradiotherapy group, experienced a grade 4 adverse event (ileus or obstruction). The chemoradiotherapy and radiotherapy-only groups were similar on the rate of grade 3 adverse events (8% vs. 5%; P = .24), the most common of which was hypertension. However, the former had a higher rate of grade 2 or worse adverse events (38% vs. 23%; P = .002), such as persistent sensory neuropathy (6% vs. 0%). None of the patients died from treatment-related causes.
“Combined adjuvant chemotherapy and radiotherapy should be discussed and recommended as a new standard of care, especially for women with stage III endometrial cancer or serous cancers, or both,” Dr. de Boer and coinvestigators maintained. “Shared decision making between doctors and their patients remains essential to weigh the costs and benefits for individual patients.
“Molecular analysis has the potential to improve risk stratification and should be used to identify subgroups that can derive the greatest benefit from chemotherapy and to select patients for targeted therapies; molecular studies on tissue samples donated by PORTEC-3 trial participants are ongoing,” they noted.
Dr. de Boer disclosed no competing interests in relation to the study. The study was supported in part by the Dutch Cancer Society, Cancer Research UK, National Health and Medical Research Council, Cancer Australia, the Italian Medicines Agency, and the Canadian Cancer Society Research Institute.
SOURCE: de Boer SM et al. Lancet Oncol. 2019 July 22. doi: 10.1016/S1470-2045(19)30395-X.
FROM LANCET ONCOLOGY
Commentary: Medical educators must do more to prevent physician suicides
while describing two exemplars at providing physicians with mental health support, in a recently published commentary.
“I want to call attention to the gap between what educators think and say is available, in terms of mental health support, and what trainees experience,” Dr. Poorman said in a statement on her piece in the Journal of Patient Safety and Risk Management. She shared the results of an anonymous depression survey of interns, which showed that 41.8% of participants screened positive for depression. Dr. Poorman also provided statistics on physician suicide, including that at least 66 residents killed themselves between 2000 and 2014, according to ACGME, and that another source estimated that 300-400 physician suicide deaths occur annually.
“When it comes to mental illness and suicide, we are all at risk, but we too often lacked compassion in the way we approach our colleagues. We have lacked the courage to fight the stigma that is killing us. We have not asked whether our unwillingness to reform medical training has eroded the empathy of generations of doctors. We have not done enough to fight medical boards that ask doctors about mental health diagnoses in the same way they ask if we have domestic violence charges,” wrote Dr. Poorman, who practices at a University of Washington neighborhood clinic in Kent.
“A focus on the occupational risks we face would shift us away from ‘wellness’ and ‘resilience,’ and place the onus on schools, training programs, and hospitals to do better for providers and patients,” continued Dr. Poorman, who serves on the editorial advisory board of Internal Medicine News.
She commended Oregon Health and Science University, Portland, and Stanford (Calif.) University’s divisions of general surgery for providing “rigorously confidential mental health support” through a wellness and suicide prevention program for residents and faculty, and a wellness program for residents “that emphasizes relationships, structural support, and psychological safety,” respectively. Dr. Poorman also applauded both for speaking openly about physician suicides.
SOURCE: Poorman E. J Patient Saf Risk Manag. 2019 Aug 5. doi: 10.1177/2516043519866993.
This article was updated 8/5/19.
while describing two exemplars at providing physicians with mental health support, in a recently published commentary.
“I want to call attention to the gap between what educators think and say is available, in terms of mental health support, and what trainees experience,” Dr. Poorman said in a statement on her piece in the Journal of Patient Safety and Risk Management. She shared the results of an anonymous depression survey of interns, which showed that 41.8% of participants screened positive for depression. Dr. Poorman also provided statistics on physician suicide, including that at least 66 residents killed themselves between 2000 and 2014, according to ACGME, and that another source estimated that 300-400 physician suicide deaths occur annually.
“When it comes to mental illness and suicide, we are all at risk, but we too often lacked compassion in the way we approach our colleagues. We have lacked the courage to fight the stigma that is killing us. We have not asked whether our unwillingness to reform medical training has eroded the empathy of generations of doctors. We have not done enough to fight medical boards that ask doctors about mental health diagnoses in the same way they ask if we have domestic violence charges,” wrote Dr. Poorman, who practices at a University of Washington neighborhood clinic in Kent.
“A focus on the occupational risks we face would shift us away from ‘wellness’ and ‘resilience,’ and place the onus on schools, training programs, and hospitals to do better for providers and patients,” continued Dr. Poorman, who serves on the editorial advisory board of Internal Medicine News.
She commended Oregon Health and Science University, Portland, and Stanford (Calif.) University’s divisions of general surgery for providing “rigorously confidential mental health support” through a wellness and suicide prevention program for residents and faculty, and a wellness program for residents “that emphasizes relationships, structural support, and psychological safety,” respectively. Dr. Poorman also applauded both for speaking openly about physician suicides.
SOURCE: Poorman E. J Patient Saf Risk Manag. 2019 Aug 5. doi: 10.1177/2516043519866993.
This article was updated 8/5/19.
while describing two exemplars at providing physicians with mental health support, in a recently published commentary.
“I want to call attention to the gap between what educators think and say is available, in terms of mental health support, and what trainees experience,” Dr. Poorman said in a statement on her piece in the Journal of Patient Safety and Risk Management. She shared the results of an anonymous depression survey of interns, which showed that 41.8% of participants screened positive for depression. Dr. Poorman also provided statistics on physician suicide, including that at least 66 residents killed themselves between 2000 and 2014, according to ACGME, and that another source estimated that 300-400 physician suicide deaths occur annually.
“When it comes to mental illness and suicide, we are all at risk, but we too often lacked compassion in the way we approach our colleagues. We have lacked the courage to fight the stigma that is killing us. We have not asked whether our unwillingness to reform medical training has eroded the empathy of generations of doctors. We have not done enough to fight medical boards that ask doctors about mental health diagnoses in the same way they ask if we have domestic violence charges,” wrote Dr. Poorman, who practices at a University of Washington neighborhood clinic in Kent.
“A focus on the occupational risks we face would shift us away from ‘wellness’ and ‘resilience,’ and place the onus on schools, training programs, and hospitals to do better for providers and patients,” continued Dr. Poorman, who serves on the editorial advisory board of Internal Medicine News.
She commended Oregon Health and Science University, Portland, and Stanford (Calif.) University’s divisions of general surgery for providing “rigorously confidential mental health support” through a wellness and suicide prevention program for residents and faculty, and a wellness program for residents “that emphasizes relationships, structural support, and psychological safety,” respectively. Dr. Poorman also applauded both for speaking openly about physician suicides.
SOURCE: Poorman E. J Patient Saf Risk Manag. 2019 Aug 5. doi: 10.1177/2516043519866993.
This article was updated 8/5/19.
FROM THE JOURNAL OF PATIENT SAFETY AND RISK MANAGEMENT
MS Highlights From the AAN & CMSC Annual Meetings
This supplement to Neurology Reviews compiles MS-related news briefs from the 2019 annual meetings of the American Academy of Neurology, held in Philadelphia in early May, and the Consortium of Multiple Sclerosis Centers, held in Seattle in late May.
This supplement to Neurology Reviews compiles MS-related news briefs from the 2019 annual meetings of the American Academy of Neurology, held in Philadelphia in early May, and the Consortium of Multiple Sclerosis Centers, held in Seattle in late May.
This supplement to Neurology Reviews compiles MS-related news briefs from the 2019 annual meetings of the American Academy of Neurology, held in Philadelphia in early May, and the Consortium of Multiple Sclerosis Centers, held in Seattle in late May.
Generalist knowledge is an asset
Hospitalists trained in family medicine
Lori J. Heim, MD, FAAFP, a hospitalist in practice at Scotland Memorial Hospital in Laurinburg, N.C., for the past 10 years, recalls when she first decided to pursue hospital medicine as a career. As a family physician in private practice who admitted patients to the local hospital in Pinehurst, N.C., and even followed them into the ICU, she needed a more flexible schedule when she became president-elect of the American Academy of Family Physicians (AAFP).
“My local hospital told me they had a policy against hiring family physicians as hospitalists. They didn’t consider us qualified,” Dr. Heim said. “I was incredulous when I first heard that because I already had full admitting privileges at the hospital. It made no sense, since they allowed me to manage my patients in the ICU.”
Then an opportunity opened at Scotland Memorial, located an hour away. “That has been a fabulous experience for me,” she said. The transition was relatively easy, following more than 2 decades of office practice. Dr. Heim’s hospitalist group now includes eight full-time clinicians who have a mix of family medicine and internal medicine backgrounds.
“I’ve never felt anything other than collegial support here. We go to the ER to evaluate patients and decide whether to admit them, and we do a lot of medical procedures. I’m not practicing pediatrics currently, but I have no problem conducting a gynecological exam. I think my experience in family medicine and primary care has been an asset,” Dr. Heim said. “I’m not sure I would be a hospitalist today if I had not been elected president of AAFP, but it was fortuitous.”
Respect for HTFMs is growing
Hospitalists trained in family medicine (HTFM) are a small but important segment of this field and of the membership of the Society of Hospital Medicine. The board specialties of physicians who work in the hospital are not always broken out in existing databases, but HTFMs are believed to represent about 8% of SHM members, and somewhere around 10%-15% of the total hospitalist workforce. According to SHM’s 2018 State of Hospital Medicine Report, 65% of hospital medicine groups employed at least one family medicine–trained provider in their group.1
SHM’s Special Interest Group (SIG) for HTFMs reports to the society’s Board of Directors. The American Academy of Family Medicine, with 131,400 members, also has a Member Interest Group (MIG) for HTFMs. When AAFP recently surveyed its members to identify their primary patient care practice location, only 4% named the hospital (not including the emergency department), while 3% said the hospital emergency department.2
Among 32,450 adult primary care-trained hospitalists surveyed for the June 2016 AAMC In Brief of the American Association of Medical Colleges, 81.9% of the hospitalists identified internal medicine as their specialty, while 5.2% identified themselves as family physicians.3 A 2014 Medical Group Management Association survey, which reported data for 4,200 hospitalists working in community hospitals, found that 82% were internal medicine trained, versus 10% in family medicine and 7% in pediatrics.
Family medicine hospitalists may be more common in rural areas or in small hospitals – where a clinician is often expected to wear more hats, said hospitalist David Goldstein, MD, FHM, assistant director of the family medicine residency program at Natividad Medical Center, Salinas, Calif., and cochair of SHM’s family medicine SIG. “In a smaller hospital, if there’s not sufficient volume to support full-time pediatric and adult hospital medicine services, a family medicine hospitalist might do both – and even help staff the ICU.”
A decade or so ago, much of the professional literature about the role of HTFMs suggested that some had experienced a lack of respect or of equal job opportunities, while others faced pay differentials.3-5 Since then, the field of hospital medicine has come a long way toward recognizing their contributions, although there are still hurdles to overcome, mainly involving issues of credentialing, to allow HTFMs to play equal roles in the hospital, the ICU, or in residency training. The SHM 2018 State of Hospital Medicine Report reveals that HTFMs actually made slightly higher salaries on average than their internist colleagues, $301,833 versus $300,030.
Prior to the advent of hospital medicine, both family medicine and internal medicine physicians practiced in much the same way in their medical offices, and visited their patients in the hospital, said Claudia Geyer, MD, SFHM, system chief of hospital medicine at Central Maine Healthcare in Lewiston. She is trained and boarded in both family and internal medicine. “When hospital medicine launched, its heavy academic emphasis on internists led to underrecognition of the continued contributions of family medicine. Family physicians never left the hospital setting and – in certain locales – were the predominant hospitalists. We just waited for the recognition to catch up with the reality,” Dr. Geyer said.
“I don’t feel family medicine for hospitalists is nearly the stepchild of internal medicine that it was when I first started,” Dr. Heim said. “In my multihospital hospitalist group, I haven’t seen anything to suggest that they treat family medicine hospitalists as second class.” The demand for hospitalists is greater than internists can fill, while clearly the public is not concerned about these distinctions, she said.
Whether clinicians are board certified in family medicine or internal medicine may be less important to their skills for practicing in the hospital than which residency program they completed, what emphasis it placed on working in the hospital or ICU, electives completed, and other past experience. “Some family medicine residencies offer more or less hospital experience,” Dr. Heim said.
Jasen Gundersen, MD, MBA, CPE, SFHM, president of acute and post-acute services for the national hospital services company TeamHealth, agreed that there has been dramatic improvement in the status of HTFMs. He is one, and still practices as a hospitalist at Boca Raton (Fla.) Regional Hospital when administrative responsibilities permit.
TeamHealth has long been open to family medicine doctors, Dr. Gundersen added, although some of the medical staff at hospitals that contract with TeamHealth have issues with it. “We will talk to them about it,” he said. “We hire hospitalists who can do the work, and we evaluate them based on their background and skill set, where they’ve practiced and for how long. We want people who are experienced and good at managing hospitalized patients. For new residency grads, we look at their electives and the focus of their training.”
What is home for HTFMs?
Where are HTFMs most likely to find their professional home? “That’s hard to answer,” said Patricia Seymour, MD, FHM, FAAFP, an academic hospitalist at the University of Massachusetts-Worcester. “In the last 4-5 years, SHM has worked very hard to create a space for HTFMs. AAFP has a hospital medicine track at their annual meeting, and that’s a good thing. But they also need to protect family physicians’ right to practice in any setting they choose. For those pursuing hospital medicine, there’s a different career trajectory, different CME needs, and different recertification needs.”
Dr. Seymour is the executive cochair of SHM’s family medicine SIG and serves as interim chief of a family medicine hospitalist group that provides inpatient training for a family practice residency, where up to a third of the 12 residents each year go on to pursue hospital medicine as a career. “We have the second-oldest family medicine–specific hospitalist group in the country, so our residency training has an emphasis on hospital medicine,” she explained.
“Because I’m a practicing hospitalist, the residents come to me seeking advice. I appreciate the training I received as a family physician in communication science, palliative care, geriatrics, family systems theory, and public health. I wouldn’t have done it any other way, and that’s how I counsel our students and residents,” she said. Others suggest that the generalist training and diverse experiences of family medicine can be a gift for a doctor who later chooses hospital medicine.
AAFP is a large umbrella organization and the majority of its members practice primary care, Dr. Heim said. “I don’t know the percentage of HTFMs who are members of AAFP. Some no doubt belong to both AAFP and SHM.” Even though both groups have recognized this important subset of their members who chose the field of hospital medicine and its status as a career track, it can be a stretch for family medicine to embrace hospitalists.
“It inherently goes against our training, which is to work in outpatient, inpatient, obstetric, pediatric, and adult settings,” Dr. Heim said. “It’s difficult to reconcile giving up a big part of what defined your training – that range of settings. I remember feeling like I should apologize to other family medicine doctors for choosing this path.”
Credentialing opportunities and barriers
For the diverse group of practicing HTFMs, credentialing and scope of practice represent their biggest current issues. A designation of Focused Practice in Hospital Medicine (FPHM) has been offered jointly since 2010 by the American Board of Family Medicine (ABFM) and the American Board of Internal Medicine (ABIM), although their specific requirements vary.
Eligible hospitalist candidates for the focused practice exam must have an unrestricted medical license, maintenance of current primary certification, and verification of three years of unsupervised hospital medicine practice experience. ABIM views FPHM not as a subspecialty, but as a variation of internal medicine certification, identifying diplomates who are board-certified in internal medicine with a hospital medicine specialization. They do not have to take the general internal medicine recertification exam if they qualify for FPHM.
ABFM-certified family physicians who work primarily in a hospital setting can take the same test for FPHM, with the same eligibility requirements. But ABFM does not consider focused practice a subspecialty, or the Certificate of Added Qualifications in Family Medicine as sufficient for board certification. That means family physicians also need to take its general board exam in order to maintain their ABFM board certification.
ABFM’s decision not to accept the focused practice designation as sufficient for boarding was disappointing to a lot of hospitalists, said Laura “Nell” Hodo, MD, FAAFP, chair of AAFP’s hospital medicine MIG, and a pediatric academic hospitalist at Icahn School of Medicine at Mount Sinai, New York. “Many family physicians practice hospital medicine exclusively and would prefer to take one boarding exam instead of two, and not have to do CME and board review in areas where we don’t practice anymore,” Dr. Hodo said, adding that she hopes that this decision could be revisited in the future.
A number of 1-year hospital medicine fellowships across the country provide additional training opportunities for both family practice and internal medicine residency graduates. These fellowships do not offer board certification or designated specialty credentialing for hospitalists and are not recognized by the American College of Graduate Medical Education (ACGME), which sets standards for residency and fellowship training. “But they reflect a need and an interest in optimizing the knowledge of hospital medicine and developing the specific skills needed to practice it well,” Dr. Geyer noted.
She directs a program for one to three fellows per year out of the Central Maine Family Medicine Residency program and Central Maine Medical Center in Lewiston, and is now recruiting her tenth class. At least 13 other hospital medicine fellowships, out of about 40 nationwide, are family medicine based. “We rely heavily on the Core Competencies in Hospital Medicine developed by SHM, which emphasize clinical conditions, medical procedures, and health care systems. Gaining fluency in the latter is really what makes hospital medicine unique,” Dr. Geyer said.
Often residency graduates seeking work in hospital medicine are insufficiently prepared for hospital billing and coding, enacting safe transitions of care, providing palliative care, and understanding how to impact their health care systems for quality improvement, patient safety and the like, she added.
Dr. Geyer said her fellowship does not mean just being a poorly paid hospitalist for a year. “The fellows are clearly trainees, getting the full benefit of our supervision and supplemental training focused on enhanced clinical and procedural exposure, but also on academics, quality improvement, leadership, and efficiency,” she said. “All of our fellows join SHM, go to the Annual Conference, propose case studies, do longitudinal quality or safety projects, and learn the other aspects of hospital medicine not well-taught in residency. We train them to be highly functional hospitalists right out of the gate.”
Until recently, another barrier for HTFMs was their ability to be on the faculty of internal medicine residency programs. Previous language from ACGME indicated that family medicine-trained physicians could not serve as faculty for these programs, Dr. Goldstein said. SHM has lobbied ACGME to change that rule, which could enable family medicine hospitalists who had achieved FPHM designation to be attendings and to teach internal medicine residents.
Needed in critical care – but not credentialed
One of the biggest frustrations for family medicine hospitalists is clarifying their role in the ICU. SHM’s Education Committee recently surveyed hospitalist members who practice in the ICU, finding that at least half felt obliged to practice beyond their scope, 90 percent occasionally perceived insufficient support from intensivists, and two-thirds reported moderate difficulty transferring patients to higher levels of intensive care.7 The respondents overwhelmingly indicated that they wanted more training and education in critical care medicine.
“I want to highlight the fact that in some settings family physicians are the sole providers of critical care,” Dr. Goldstein said. Meanwhile, the standards of the Leapfrog Group, a coalition of health care purchasers, call for ICUs to be staffed by physicians certified in critical care, even though there is a growing shortage of credentialed intensivists to treat an increasing number of older, sicker, critically ill patients.
Some internal medicine physicians don’t want to have anything to do with the ICU because of the medical and legal risks, said David Aymond, MD, a family physician and hospitalist at Byrd Regional Hospital in Leesville, La. “There’s a bunch of sick people in the ICU, and when some doctors like me started doing critical care, we realized we liked it. Depending on your locale, if you are doing hospital medicine, critically ill patients are going to fall in your lap,” he said. “But if you don’t have the skills, that could lead to poor outcomes and unnecessary transfers.”
Dr. Aymond started his career in family medicine. “When I got into residency, I saw how much critical care was needed in rural communities. I decided I would learn everything I could about it. I did a hospital medicine fellowship at the University of Alabama, which included considerable involvement in the ICU. When I went to Byrd Regional, a 60-bed facility with eight ICU beds, we did all of the critical care, and word started to spread in the community. My hospitalist partner and I are now on call 24/7 alternating weeks, doing the majority of the critical care and taking care of anything that goes on in an ICU at a larger center, although we often lack access to consultation services,” he explained.
“We needed to get the attention of the Society of Critical Care Medicine (SCCM) to communicate the scope of this problem. These doctors are doing critical care but there is no official medical training or recognition for them. So they’re legally out on a limb, even though often they are literally the only person available to do it,” Dr. Aymond said. “Certainly there’s a skills gap between HTFMs and board-certified intensivists, but some of that gap has to do with the volume of patients they have seen in the ICU and their comfort level,” he said.
SHM is pursuing initiatives to help address this gap, including collaborating with SCCM on developing a rigorous critical care training curriculum for internal medicine and family medicine hospitalists, with coursework drawn from existing sources, said Eric Siegal, MD, SFHM, a critical care physician in Milwaukee. “It doesn’t replace a 2-year critical care fellowship, but it will be a lot more than what’s currently out there for the nonintensivist who practices in the ICU.” SCCM has approved moving forward with the advanced training curriculum, he said.
Another priority is to try to create a pathway that could permit family medicine–trained hospitalists to apply for existing critical care fellowships, as internal medicine doctors are now able to do. SHM has lobbied ABFM to create a pathway to subspecialty certification in critical care medicine, similar to those that exist for internists and emergency physicians, Dr. Goldstein said, adding that ACGME, which controls access to fellowships, will be the next step. Dr. Aymond expects that there will be a lot of hoops to jump through.
“David Aymond is an exceptional hospitalist,” Dr. Siegal added. “He thinks and talks like an intensivist, but it took concerted and self-directed effort for him to get there. Family practitioners are a significant part of the rural critical care workforce, but their training generally does not adequately prepare them for this role – unless they have made a conscious effort to pursue additional training,” he said.
“My message to family practitioners is not that they’re not good enough to do this, but rather that they are being asked to do something they weren’t trained for. How can we help them do it well?”
References
1. Society of Hospital Medicine (SHM) Practice Analysis Committee. 2018 State of Hospital Medicine Report; Oct 2018.
2. American Academy of Family Physicians Member Census, Dec 31, 2017.
3. Jones KC et al. Hospitalists: A growing part of the primary care workforce. AAMC Analysis in Brief; June 2016; 16(5):1.
4. Berczuk C. Uniquely positioned. The Hospitalist; July 2009.
5. Iqbal Y. Family medicine hospitalists: Separate and unequal? Today’s Hospitalist; May 2007.
6. Kinnan JP. The family way. The Hospitalist; Nov 2007.
7. Sweigart JR et al. Characterizing hospitalist practice and perceptions of critical care delivery. J Hosp Med. 2018 Jan 1;13(1):6-12.
Hospitalists trained in family medicine
Hospitalists trained in family medicine
Lori J. Heim, MD, FAAFP, a hospitalist in practice at Scotland Memorial Hospital in Laurinburg, N.C., for the past 10 years, recalls when she first decided to pursue hospital medicine as a career. As a family physician in private practice who admitted patients to the local hospital in Pinehurst, N.C., and even followed them into the ICU, she needed a more flexible schedule when she became president-elect of the American Academy of Family Physicians (AAFP).
“My local hospital told me they had a policy against hiring family physicians as hospitalists. They didn’t consider us qualified,” Dr. Heim said. “I was incredulous when I first heard that because I already had full admitting privileges at the hospital. It made no sense, since they allowed me to manage my patients in the ICU.”
Then an opportunity opened at Scotland Memorial, located an hour away. “That has been a fabulous experience for me,” she said. The transition was relatively easy, following more than 2 decades of office practice. Dr. Heim’s hospitalist group now includes eight full-time clinicians who have a mix of family medicine and internal medicine backgrounds.
“I’ve never felt anything other than collegial support here. We go to the ER to evaluate patients and decide whether to admit them, and we do a lot of medical procedures. I’m not practicing pediatrics currently, but I have no problem conducting a gynecological exam. I think my experience in family medicine and primary care has been an asset,” Dr. Heim said. “I’m not sure I would be a hospitalist today if I had not been elected president of AAFP, but it was fortuitous.”
Respect for HTFMs is growing
Hospitalists trained in family medicine (HTFM) are a small but important segment of this field and of the membership of the Society of Hospital Medicine. The board specialties of physicians who work in the hospital are not always broken out in existing databases, but HTFMs are believed to represent about 8% of SHM members, and somewhere around 10%-15% of the total hospitalist workforce. According to SHM’s 2018 State of Hospital Medicine Report, 65% of hospital medicine groups employed at least one family medicine–trained provider in their group.1
SHM’s Special Interest Group (SIG) for HTFMs reports to the society’s Board of Directors. The American Academy of Family Medicine, with 131,400 members, also has a Member Interest Group (MIG) for HTFMs. When AAFP recently surveyed its members to identify their primary patient care practice location, only 4% named the hospital (not including the emergency department), while 3% said the hospital emergency department.2
Among 32,450 adult primary care-trained hospitalists surveyed for the June 2016 AAMC In Brief of the American Association of Medical Colleges, 81.9% of the hospitalists identified internal medicine as their specialty, while 5.2% identified themselves as family physicians.3 A 2014 Medical Group Management Association survey, which reported data for 4,200 hospitalists working in community hospitals, found that 82% were internal medicine trained, versus 10% in family medicine and 7% in pediatrics.
Family medicine hospitalists may be more common in rural areas or in small hospitals – where a clinician is often expected to wear more hats, said hospitalist David Goldstein, MD, FHM, assistant director of the family medicine residency program at Natividad Medical Center, Salinas, Calif., and cochair of SHM’s family medicine SIG. “In a smaller hospital, if there’s not sufficient volume to support full-time pediatric and adult hospital medicine services, a family medicine hospitalist might do both – and even help staff the ICU.”
A decade or so ago, much of the professional literature about the role of HTFMs suggested that some had experienced a lack of respect or of equal job opportunities, while others faced pay differentials.3-5 Since then, the field of hospital medicine has come a long way toward recognizing their contributions, although there are still hurdles to overcome, mainly involving issues of credentialing, to allow HTFMs to play equal roles in the hospital, the ICU, or in residency training. The SHM 2018 State of Hospital Medicine Report reveals that HTFMs actually made slightly higher salaries on average than their internist colleagues, $301,833 versus $300,030.
Prior to the advent of hospital medicine, both family medicine and internal medicine physicians practiced in much the same way in their medical offices, and visited their patients in the hospital, said Claudia Geyer, MD, SFHM, system chief of hospital medicine at Central Maine Healthcare in Lewiston. She is trained and boarded in both family and internal medicine. “When hospital medicine launched, its heavy academic emphasis on internists led to underrecognition of the continued contributions of family medicine. Family physicians never left the hospital setting and – in certain locales – were the predominant hospitalists. We just waited for the recognition to catch up with the reality,” Dr. Geyer said.
“I don’t feel family medicine for hospitalists is nearly the stepchild of internal medicine that it was when I first started,” Dr. Heim said. “In my multihospital hospitalist group, I haven’t seen anything to suggest that they treat family medicine hospitalists as second class.” The demand for hospitalists is greater than internists can fill, while clearly the public is not concerned about these distinctions, she said.
Whether clinicians are board certified in family medicine or internal medicine may be less important to their skills for practicing in the hospital than which residency program they completed, what emphasis it placed on working in the hospital or ICU, electives completed, and other past experience. “Some family medicine residencies offer more or less hospital experience,” Dr. Heim said.
Jasen Gundersen, MD, MBA, CPE, SFHM, president of acute and post-acute services for the national hospital services company TeamHealth, agreed that there has been dramatic improvement in the status of HTFMs. He is one, and still practices as a hospitalist at Boca Raton (Fla.) Regional Hospital when administrative responsibilities permit.
TeamHealth has long been open to family medicine doctors, Dr. Gundersen added, although some of the medical staff at hospitals that contract with TeamHealth have issues with it. “We will talk to them about it,” he said. “We hire hospitalists who can do the work, and we evaluate them based on their background and skill set, where they’ve practiced and for how long. We want people who are experienced and good at managing hospitalized patients. For new residency grads, we look at their electives and the focus of their training.”
What is home for HTFMs?
Where are HTFMs most likely to find their professional home? “That’s hard to answer,” said Patricia Seymour, MD, FHM, FAAFP, an academic hospitalist at the University of Massachusetts-Worcester. “In the last 4-5 years, SHM has worked very hard to create a space for HTFMs. AAFP has a hospital medicine track at their annual meeting, and that’s a good thing. But they also need to protect family physicians’ right to practice in any setting they choose. For those pursuing hospital medicine, there’s a different career trajectory, different CME needs, and different recertification needs.”
Dr. Seymour is the executive cochair of SHM’s family medicine SIG and serves as interim chief of a family medicine hospitalist group that provides inpatient training for a family practice residency, where up to a third of the 12 residents each year go on to pursue hospital medicine as a career. “We have the second-oldest family medicine–specific hospitalist group in the country, so our residency training has an emphasis on hospital medicine,” she explained.
“Because I’m a practicing hospitalist, the residents come to me seeking advice. I appreciate the training I received as a family physician in communication science, palliative care, geriatrics, family systems theory, and public health. I wouldn’t have done it any other way, and that’s how I counsel our students and residents,” she said. Others suggest that the generalist training and diverse experiences of family medicine can be a gift for a doctor who later chooses hospital medicine.
AAFP is a large umbrella organization and the majority of its members practice primary care, Dr. Heim said. “I don’t know the percentage of HTFMs who are members of AAFP. Some no doubt belong to both AAFP and SHM.” Even though both groups have recognized this important subset of their members who chose the field of hospital medicine and its status as a career track, it can be a stretch for family medicine to embrace hospitalists.
“It inherently goes against our training, which is to work in outpatient, inpatient, obstetric, pediatric, and adult settings,” Dr. Heim said. “It’s difficult to reconcile giving up a big part of what defined your training – that range of settings. I remember feeling like I should apologize to other family medicine doctors for choosing this path.”
Credentialing opportunities and barriers
For the diverse group of practicing HTFMs, credentialing and scope of practice represent their biggest current issues. A designation of Focused Practice in Hospital Medicine (FPHM) has been offered jointly since 2010 by the American Board of Family Medicine (ABFM) and the American Board of Internal Medicine (ABIM), although their specific requirements vary.
Eligible hospitalist candidates for the focused practice exam must have an unrestricted medical license, maintenance of current primary certification, and verification of three years of unsupervised hospital medicine practice experience. ABIM views FPHM not as a subspecialty, but as a variation of internal medicine certification, identifying diplomates who are board-certified in internal medicine with a hospital medicine specialization. They do not have to take the general internal medicine recertification exam if they qualify for FPHM.
ABFM-certified family physicians who work primarily in a hospital setting can take the same test for FPHM, with the same eligibility requirements. But ABFM does not consider focused practice a subspecialty, or the Certificate of Added Qualifications in Family Medicine as sufficient for board certification. That means family physicians also need to take its general board exam in order to maintain their ABFM board certification.
ABFM’s decision not to accept the focused practice designation as sufficient for boarding was disappointing to a lot of hospitalists, said Laura “Nell” Hodo, MD, FAAFP, chair of AAFP’s hospital medicine MIG, and a pediatric academic hospitalist at Icahn School of Medicine at Mount Sinai, New York. “Many family physicians practice hospital medicine exclusively and would prefer to take one boarding exam instead of two, and not have to do CME and board review in areas where we don’t practice anymore,” Dr. Hodo said, adding that she hopes that this decision could be revisited in the future.
A number of 1-year hospital medicine fellowships across the country provide additional training opportunities for both family practice and internal medicine residency graduates. These fellowships do not offer board certification or designated specialty credentialing for hospitalists and are not recognized by the American College of Graduate Medical Education (ACGME), which sets standards for residency and fellowship training. “But they reflect a need and an interest in optimizing the knowledge of hospital medicine and developing the specific skills needed to practice it well,” Dr. Geyer noted.
She directs a program for one to three fellows per year out of the Central Maine Family Medicine Residency program and Central Maine Medical Center in Lewiston, and is now recruiting her tenth class. At least 13 other hospital medicine fellowships, out of about 40 nationwide, are family medicine based. “We rely heavily on the Core Competencies in Hospital Medicine developed by SHM, which emphasize clinical conditions, medical procedures, and health care systems. Gaining fluency in the latter is really what makes hospital medicine unique,” Dr. Geyer said.
Often residency graduates seeking work in hospital medicine are insufficiently prepared for hospital billing and coding, enacting safe transitions of care, providing palliative care, and understanding how to impact their health care systems for quality improvement, patient safety and the like, she added.
Dr. Geyer said her fellowship does not mean just being a poorly paid hospitalist for a year. “The fellows are clearly trainees, getting the full benefit of our supervision and supplemental training focused on enhanced clinical and procedural exposure, but also on academics, quality improvement, leadership, and efficiency,” she said. “All of our fellows join SHM, go to the Annual Conference, propose case studies, do longitudinal quality or safety projects, and learn the other aspects of hospital medicine not well-taught in residency. We train them to be highly functional hospitalists right out of the gate.”
Until recently, another barrier for HTFMs was their ability to be on the faculty of internal medicine residency programs. Previous language from ACGME indicated that family medicine-trained physicians could not serve as faculty for these programs, Dr. Goldstein said. SHM has lobbied ACGME to change that rule, which could enable family medicine hospitalists who had achieved FPHM designation to be attendings and to teach internal medicine residents.
Needed in critical care – but not credentialed
One of the biggest frustrations for family medicine hospitalists is clarifying their role in the ICU. SHM’s Education Committee recently surveyed hospitalist members who practice in the ICU, finding that at least half felt obliged to practice beyond their scope, 90 percent occasionally perceived insufficient support from intensivists, and two-thirds reported moderate difficulty transferring patients to higher levels of intensive care.7 The respondents overwhelmingly indicated that they wanted more training and education in critical care medicine.
“I want to highlight the fact that in some settings family physicians are the sole providers of critical care,” Dr. Goldstein said. Meanwhile, the standards of the Leapfrog Group, a coalition of health care purchasers, call for ICUs to be staffed by physicians certified in critical care, even though there is a growing shortage of credentialed intensivists to treat an increasing number of older, sicker, critically ill patients.
Some internal medicine physicians don’t want to have anything to do with the ICU because of the medical and legal risks, said David Aymond, MD, a family physician and hospitalist at Byrd Regional Hospital in Leesville, La. “There’s a bunch of sick people in the ICU, and when some doctors like me started doing critical care, we realized we liked it. Depending on your locale, if you are doing hospital medicine, critically ill patients are going to fall in your lap,” he said. “But if you don’t have the skills, that could lead to poor outcomes and unnecessary transfers.”
Dr. Aymond started his career in family medicine. “When I got into residency, I saw how much critical care was needed in rural communities. I decided I would learn everything I could about it. I did a hospital medicine fellowship at the University of Alabama, which included considerable involvement in the ICU. When I went to Byrd Regional, a 60-bed facility with eight ICU beds, we did all of the critical care, and word started to spread in the community. My hospitalist partner and I are now on call 24/7 alternating weeks, doing the majority of the critical care and taking care of anything that goes on in an ICU at a larger center, although we often lack access to consultation services,” he explained.
“We needed to get the attention of the Society of Critical Care Medicine (SCCM) to communicate the scope of this problem. These doctors are doing critical care but there is no official medical training or recognition for them. So they’re legally out on a limb, even though often they are literally the only person available to do it,” Dr. Aymond said. “Certainly there’s a skills gap between HTFMs and board-certified intensivists, but some of that gap has to do with the volume of patients they have seen in the ICU and their comfort level,” he said.
SHM is pursuing initiatives to help address this gap, including collaborating with SCCM on developing a rigorous critical care training curriculum for internal medicine and family medicine hospitalists, with coursework drawn from existing sources, said Eric Siegal, MD, SFHM, a critical care physician in Milwaukee. “It doesn’t replace a 2-year critical care fellowship, but it will be a lot more than what’s currently out there for the nonintensivist who practices in the ICU.” SCCM has approved moving forward with the advanced training curriculum, he said.
Another priority is to try to create a pathway that could permit family medicine–trained hospitalists to apply for existing critical care fellowships, as internal medicine doctors are now able to do. SHM has lobbied ABFM to create a pathway to subspecialty certification in critical care medicine, similar to those that exist for internists and emergency physicians, Dr. Goldstein said, adding that ACGME, which controls access to fellowships, will be the next step. Dr. Aymond expects that there will be a lot of hoops to jump through.
“David Aymond is an exceptional hospitalist,” Dr. Siegal added. “He thinks and talks like an intensivist, but it took concerted and self-directed effort for him to get there. Family practitioners are a significant part of the rural critical care workforce, but their training generally does not adequately prepare them for this role – unless they have made a conscious effort to pursue additional training,” he said.
“My message to family practitioners is not that they’re not good enough to do this, but rather that they are being asked to do something they weren’t trained for. How can we help them do it well?”
References
1. Society of Hospital Medicine (SHM) Practice Analysis Committee. 2018 State of Hospital Medicine Report; Oct 2018.
2. American Academy of Family Physicians Member Census, Dec 31, 2017.
3. Jones KC et al. Hospitalists: A growing part of the primary care workforce. AAMC Analysis in Brief; June 2016; 16(5):1.
4. Berczuk C. Uniquely positioned. The Hospitalist; July 2009.
5. Iqbal Y. Family medicine hospitalists: Separate and unequal? Today’s Hospitalist; May 2007.
6. Kinnan JP. The family way. The Hospitalist; Nov 2007.
7. Sweigart JR et al. Characterizing hospitalist practice and perceptions of critical care delivery. J Hosp Med. 2018 Jan 1;13(1):6-12.
Lori J. Heim, MD, FAAFP, a hospitalist in practice at Scotland Memorial Hospital in Laurinburg, N.C., for the past 10 years, recalls when she first decided to pursue hospital medicine as a career. As a family physician in private practice who admitted patients to the local hospital in Pinehurst, N.C., and even followed them into the ICU, she needed a more flexible schedule when she became president-elect of the American Academy of Family Physicians (AAFP).
“My local hospital told me they had a policy against hiring family physicians as hospitalists. They didn’t consider us qualified,” Dr. Heim said. “I was incredulous when I first heard that because I already had full admitting privileges at the hospital. It made no sense, since they allowed me to manage my patients in the ICU.”
Then an opportunity opened at Scotland Memorial, located an hour away. “That has been a fabulous experience for me,” she said. The transition was relatively easy, following more than 2 decades of office practice. Dr. Heim’s hospitalist group now includes eight full-time clinicians who have a mix of family medicine and internal medicine backgrounds.
“I’ve never felt anything other than collegial support here. We go to the ER to evaluate patients and decide whether to admit them, and we do a lot of medical procedures. I’m not practicing pediatrics currently, but I have no problem conducting a gynecological exam. I think my experience in family medicine and primary care has been an asset,” Dr. Heim said. “I’m not sure I would be a hospitalist today if I had not been elected president of AAFP, but it was fortuitous.”
Respect for HTFMs is growing
Hospitalists trained in family medicine (HTFM) are a small but important segment of this field and of the membership of the Society of Hospital Medicine. The board specialties of physicians who work in the hospital are not always broken out in existing databases, but HTFMs are believed to represent about 8% of SHM members, and somewhere around 10%-15% of the total hospitalist workforce. According to SHM’s 2018 State of Hospital Medicine Report, 65% of hospital medicine groups employed at least one family medicine–trained provider in their group.1
SHM’s Special Interest Group (SIG) for HTFMs reports to the society’s Board of Directors. The American Academy of Family Medicine, with 131,400 members, also has a Member Interest Group (MIG) for HTFMs. When AAFP recently surveyed its members to identify their primary patient care practice location, only 4% named the hospital (not including the emergency department), while 3% said the hospital emergency department.2
Among 32,450 adult primary care-trained hospitalists surveyed for the June 2016 AAMC In Brief of the American Association of Medical Colleges, 81.9% of the hospitalists identified internal medicine as their specialty, while 5.2% identified themselves as family physicians.3 A 2014 Medical Group Management Association survey, which reported data for 4,200 hospitalists working in community hospitals, found that 82% were internal medicine trained, versus 10% in family medicine and 7% in pediatrics.
Family medicine hospitalists may be more common in rural areas or in small hospitals – where a clinician is often expected to wear more hats, said hospitalist David Goldstein, MD, FHM, assistant director of the family medicine residency program at Natividad Medical Center, Salinas, Calif., and cochair of SHM’s family medicine SIG. “In a smaller hospital, if there’s not sufficient volume to support full-time pediatric and adult hospital medicine services, a family medicine hospitalist might do both – and even help staff the ICU.”
A decade or so ago, much of the professional literature about the role of HTFMs suggested that some had experienced a lack of respect or of equal job opportunities, while others faced pay differentials.3-5 Since then, the field of hospital medicine has come a long way toward recognizing their contributions, although there are still hurdles to overcome, mainly involving issues of credentialing, to allow HTFMs to play equal roles in the hospital, the ICU, or in residency training. The SHM 2018 State of Hospital Medicine Report reveals that HTFMs actually made slightly higher salaries on average than their internist colleagues, $301,833 versus $300,030.
Prior to the advent of hospital medicine, both family medicine and internal medicine physicians practiced in much the same way in their medical offices, and visited their patients in the hospital, said Claudia Geyer, MD, SFHM, system chief of hospital medicine at Central Maine Healthcare in Lewiston. She is trained and boarded in both family and internal medicine. “When hospital medicine launched, its heavy academic emphasis on internists led to underrecognition of the continued contributions of family medicine. Family physicians never left the hospital setting and – in certain locales – were the predominant hospitalists. We just waited for the recognition to catch up with the reality,” Dr. Geyer said.
“I don’t feel family medicine for hospitalists is nearly the stepchild of internal medicine that it was when I first started,” Dr. Heim said. “In my multihospital hospitalist group, I haven’t seen anything to suggest that they treat family medicine hospitalists as second class.” The demand for hospitalists is greater than internists can fill, while clearly the public is not concerned about these distinctions, she said.
Whether clinicians are board certified in family medicine or internal medicine may be less important to their skills for practicing in the hospital than which residency program they completed, what emphasis it placed on working in the hospital or ICU, electives completed, and other past experience. “Some family medicine residencies offer more or less hospital experience,” Dr. Heim said.
Jasen Gundersen, MD, MBA, CPE, SFHM, president of acute and post-acute services for the national hospital services company TeamHealth, agreed that there has been dramatic improvement in the status of HTFMs. He is one, and still practices as a hospitalist at Boca Raton (Fla.) Regional Hospital when administrative responsibilities permit.
TeamHealth has long been open to family medicine doctors, Dr. Gundersen added, although some of the medical staff at hospitals that contract with TeamHealth have issues with it. “We will talk to them about it,” he said. “We hire hospitalists who can do the work, and we evaluate them based on their background and skill set, where they’ve practiced and for how long. We want people who are experienced and good at managing hospitalized patients. For new residency grads, we look at their electives and the focus of their training.”
What is home for HTFMs?
Where are HTFMs most likely to find their professional home? “That’s hard to answer,” said Patricia Seymour, MD, FHM, FAAFP, an academic hospitalist at the University of Massachusetts-Worcester. “In the last 4-5 years, SHM has worked very hard to create a space for HTFMs. AAFP has a hospital medicine track at their annual meeting, and that’s a good thing. But they also need to protect family physicians’ right to practice in any setting they choose. For those pursuing hospital medicine, there’s a different career trajectory, different CME needs, and different recertification needs.”
Dr. Seymour is the executive cochair of SHM’s family medicine SIG and serves as interim chief of a family medicine hospitalist group that provides inpatient training for a family practice residency, where up to a third of the 12 residents each year go on to pursue hospital medicine as a career. “We have the second-oldest family medicine–specific hospitalist group in the country, so our residency training has an emphasis on hospital medicine,” she explained.
“Because I’m a practicing hospitalist, the residents come to me seeking advice. I appreciate the training I received as a family physician in communication science, palliative care, geriatrics, family systems theory, and public health. I wouldn’t have done it any other way, and that’s how I counsel our students and residents,” she said. Others suggest that the generalist training and diverse experiences of family medicine can be a gift for a doctor who later chooses hospital medicine.
AAFP is a large umbrella organization and the majority of its members practice primary care, Dr. Heim said. “I don’t know the percentage of HTFMs who are members of AAFP. Some no doubt belong to both AAFP and SHM.” Even though both groups have recognized this important subset of their members who chose the field of hospital medicine and its status as a career track, it can be a stretch for family medicine to embrace hospitalists.
“It inherently goes against our training, which is to work in outpatient, inpatient, obstetric, pediatric, and adult settings,” Dr. Heim said. “It’s difficult to reconcile giving up a big part of what defined your training – that range of settings. I remember feeling like I should apologize to other family medicine doctors for choosing this path.”
Credentialing opportunities and barriers
For the diverse group of practicing HTFMs, credentialing and scope of practice represent their biggest current issues. A designation of Focused Practice in Hospital Medicine (FPHM) has been offered jointly since 2010 by the American Board of Family Medicine (ABFM) and the American Board of Internal Medicine (ABIM), although their specific requirements vary.
Eligible hospitalist candidates for the focused practice exam must have an unrestricted medical license, maintenance of current primary certification, and verification of three years of unsupervised hospital medicine practice experience. ABIM views FPHM not as a subspecialty, but as a variation of internal medicine certification, identifying diplomates who are board-certified in internal medicine with a hospital medicine specialization. They do not have to take the general internal medicine recertification exam if they qualify for FPHM.
ABFM-certified family physicians who work primarily in a hospital setting can take the same test for FPHM, with the same eligibility requirements. But ABFM does not consider focused practice a subspecialty, or the Certificate of Added Qualifications in Family Medicine as sufficient for board certification. That means family physicians also need to take its general board exam in order to maintain their ABFM board certification.
ABFM’s decision not to accept the focused practice designation as sufficient for boarding was disappointing to a lot of hospitalists, said Laura “Nell” Hodo, MD, FAAFP, chair of AAFP’s hospital medicine MIG, and a pediatric academic hospitalist at Icahn School of Medicine at Mount Sinai, New York. “Many family physicians practice hospital medicine exclusively and would prefer to take one boarding exam instead of two, and not have to do CME and board review in areas where we don’t practice anymore,” Dr. Hodo said, adding that she hopes that this decision could be revisited in the future.
A number of 1-year hospital medicine fellowships across the country provide additional training opportunities for both family practice and internal medicine residency graduates. These fellowships do not offer board certification or designated specialty credentialing for hospitalists and are not recognized by the American College of Graduate Medical Education (ACGME), which sets standards for residency and fellowship training. “But they reflect a need and an interest in optimizing the knowledge of hospital medicine and developing the specific skills needed to practice it well,” Dr. Geyer noted.
She directs a program for one to three fellows per year out of the Central Maine Family Medicine Residency program and Central Maine Medical Center in Lewiston, and is now recruiting her tenth class. At least 13 other hospital medicine fellowships, out of about 40 nationwide, are family medicine based. “We rely heavily on the Core Competencies in Hospital Medicine developed by SHM, which emphasize clinical conditions, medical procedures, and health care systems. Gaining fluency in the latter is really what makes hospital medicine unique,” Dr. Geyer said.
Often residency graduates seeking work in hospital medicine are insufficiently prepared for hospital billing and coding, enacting safe transitions of care, providing palliative care, and understanding how to impact their health care systems for quality improvement, patient safety and the like, she added.
Dr. Geyer said her fellowship does not mean just being a poorly paid hospitalist for a year. “The fellows are clearly trainees, getting the full benefit of our supervision and supplemental training focused on enhanced clinical and procedural exposure, but also on academics, quality improvement, leadership, and efficiency,” she said. “All of our fellows join SHM, go to the Annual Conference, propose case studies, do longitudinal quality or safety projects, and learn the other aspects of hospital medicine not well-taught in residency. We train them to be highly functional hospitalists right out of the gate.”
Until recently, another barrier for HTFMs was their ability to be on the faculty of internal medicine residency programs. Previous language from ACGME indicated that family medicine-trained physicians could not serve as faculty for these programs, Dr. Goldstein said. SHM has lobbied ACGME to change that rule, which could enable family medicine hospitalists who had achieved FPHM designation to be attendings and to teach internal medicine residents.
Needed in critical care – but not credentialed
One of the biggest frustrations for family medicine hospitalists is clarifying their role in the ICU. SHM’s Education Committee recently surveyed hospitalist members who practice in the ICU, finding that at least half felt obliged to practice beyond their scope, 90 percent occasionally perceived insufficient support from intensivists, and two-thirds reported moderate difficulty transferring patients to higher levels of intensive care.7 The respondents overwhelmingly indicated that they wanted more training and education in critical care medicine.
“I want to highlight the fact that in some settings family physicians are the sole providers of critical care,” Dr. Goldstein said. Meanwhile, the standards of the Leapfrog Group, a coalition of health care purchasers, call for ICUs to be staffed by physicians certified in critical care, even though there is a growing shortage of credentialed intensivists to treat an increasing number of older, sicker, critically ill patients.
Some internal medicine physicians don’t want to have anything to do with the ICU because of the medical and legal risks, said David Aymond, MD, a family physician and hospitalist at Byrd Regional Hospital in Leesville, La. “There’s a bunch of sick people in the ICU, and when some doctors like me started doing critical care, we realized we liked it. Depending on your locale, if you are doing hospital medicine, critically ill patients are going to fall in your lap,” he said. “But if you don’t have the skills, that could lead to poor outcomes and unnecessary transfers.”
Dr. Aymond started his career in family medicine. “When I got into residency, I saw how much critical care was needed in rural communities. I decided I would learn everything I could about it. I did a hospital medicine fellowship at the University of Alabama, which included considerable involvement in the ICU. When I went to Byrd Regional, a 60-bed facility with eight ICU beds, we did all of the critical care, and word started to spread in the community. My hospitalist partner and I are now on call 24/7 alternating weeks, doing the majority of the critical care and taking care of anything that goes on in an ICU at a larger center, although we often lack access to consultation services,” he explained.
“We needed to get the attention of the Society of Critical Care Medicine (SCCM) to communicate the scope of this problem. These doctors are doing critical care but there is no official medical training or recognition for them. So they’re legally out on a limb, even though often they are literally the only person available to do it,” Dr. Aymond said. “Certainly there’s a skills gap between HTFMs and board-certified intensivists, but some of that gap has to do with the volume of patients they have seen in the ICU and their comfort level,” he said.
SHM is pursuing initiatives to help address this gap, including collaborating with SCCM on developing a rigorous critical care training curriculum for internal medicine and family medicine hospitalists, with coursework drawn from existing sources, said Eric Siegal, MD, SFHM, a critical care physician in Milwaukee. “It doesn’t replace a 2-year critical care fellowship, but it will be a lot more than what’s currently out there for the nonintensivist who practices in the ICU.” SCCM has approved moving forward with the advanced training curriculum, he said.
Another priority is to try to create a pathway that could permit family medicine–trained hospitalists to apply for existing critical care fellowships, as internal medicine doctors are now able to do. SHM has lobbied ABFM to create a pathway to subspecialty certification in critical care medicine, similar to those that exist for internists and emergency physicians, Dr. Goldstein said, adding that ACGME, which controls access to fellowships, will be the next step. Dr. Aymond expects that there will be a lot of hoops to jump through.
“David Aymond is an exceptional hospitalist,” Dr. Siegal added. “He thinks and talks like an intensivist, but it took concerted and self-directed effort for him to get there. Family practitioners are a significant part of the rural critical care workforce, but their training generally does not adequately prepare them for this role – unless they have made a conscious effort to pursue additional training,” he said.
“My message to family practitioners is not that they’re not good enough to do this, but rather that they are being asked to do something they weren’t trained for. How can we help them do it well?”
References
1. Society of Hospital Medicine (SHM) Practice Analysis Committee. 2018 State of Hospital Medicine Report; Oct 2018.
2. American Academy of Family Physicians Member Census, Dec 31, 2017.
3. Jones KC et al. Hospitalists: A growing part of the primary care workforce. AAMC Analysis in Brief; June 2016; 16(5):1.
4. Berczuk C. Uniquely positioned. The Hospitalist; July 2009.
5. Iqbal Y. Family medicine hospitalists: Separate and unequal? Today’s Hospitalist; May 2007.
6. Kinnan JP. The family way. The Hospitalist; Nov 2007.
7. Sweigart JR et al. Characterizing hospitalist practice and perceptions of critical care delivery. J Hosp Med. 2018 Jan 1;13(1):6-12.
About half of NCI cancer center directors received industry payments in 2017
Slightly less than half of directors at National Cancer Institute–designated cancer centers received payments from industry in 2017, according to recent analysis of the Centers for Medicare & Medicaid Services Open Payments database.
That number is lower than in 2014, but approximately one-fourth of National Cancer Institute (NCI) directors received payments of $5,000 or more that were not related to research, David Carr, MD, of the University of California in San Diego, and H. Gilbert Welch, MD, MPH, of Thetford, Vt., reported in a research letter in JAMA Internal Medicine.
Although cancer care is shaped by public funding through centers under NCI, “[c]ancer care is also shaped by industry, because developing new cancer therapeutics represents a major market opportunity,” Dr. Carr and Dr. Welch said. “Industry payments to academic physicians risk blurring the line between innovation and the evaluation of new therapies.”
In their research letter, Dr. Carr and Dr. Welch examined research- and non–research-based payments made to 53 physician cancer center directors between 2015 and 2017. In 2016, 44 of 53 directors held their current positions, while 41 of 53 directors held their position in 2015.
Analysis from 2017 showed $4.42 million in total industry payments were distributed to the directors, consisting of $1.89 million in research-based payments and $2.53 million in nonresearch payments across 22 directors. There were 27 directors (51%) who did not receive any payments from industry.
The authors found 19 directors (36%) received payments of $5,000 or more, while 12 of 53 directors (23%) received nonresearch payments of $5,000 or more. The largest payment received in 2017 for research was $863,000. Two directors received industry payments at $50,000 or higher that significantly exceeded payments other directors received, and one director received $2.27 million for “compensation for services other than consulting, including serving as faculty or a speaker at a venue other than a continuing education program.”
The NCI currently defines any remuneration above $5,000 to be of “significant financial interest.”
“Our findings raise the question of whether industry payments to the directors of publicly supported institutions, such as NCI-designated cancer centers, serve the public interest,” Dr. Carr and Dr. Welch noted. “Policy makers—and the public—should consider whether such payments should be allowed, limited (e.g., to less than $5,000 a year), or eliminated.”
The authors reported no conflicts of interest.
SOURCE: Carr D, et al. JAMA Intern Med. 2019 Aug 5. doi: 10.1001/jamainternmed.2019.3098.
Slightly less than half of directors at National Cancer Institute–designated cancer centers received payments from industry in 2017, according to recent analysis of the Centers for Medicare & Medicaid Services Open Payments database.
That number is lower than in 2014, but approximately one-fourth of National Cancer Institute (NCI) directors received payments of $5,000 or more that were not related to research, David Carr, MD, of the University of California in San Diego, and H. Gilbert Welch, MD, MPH, of Thetford, Vt., reported in a research letter in JAMA Internal Medicine.
Although cancer care is shaped by public funding through centers under NCI, “[c]ancer care is also shaped by industry, because developing new cancer therapeutics represents a major market opportunity,” Dr. Carr and Dr. Welch said. “Industry payments to academic physicians risk blurring the line between innovation and the evaluation of new therapies.”
In their research letter, Dr. Carr and Dr. Welch examined research- and non–research-based payments made to 53 physician cancer center directors between 2015 and 2017. In 2016, 44 of 53 directors held their current positions, while 41 of 53 directors held their position in 2015.
Analysis from 2017 showed $4.42 million in total industry payments were distributed to the directors, consisting of $1.89 million in research-based payments and $2.53 million in nonresearch payments across 22 directors. There were 27 directors (51%) who did not receive any payments from industry.
The authors found 19 directors (36%) received payments of $5,000 or more, while 12 of 53 directors (23%) received nonresearch payments of $5,000 or more. The largest payment received in 2017 for research was $863,000. Two directors received industry payments at $50,000 or higher that significantly exceeded payments other directors received, and one director received $2.27 million for “compensation for services other than consulting, including serving as faculty or a speaker at a venue other than a continuing education program.”
The NCI currently defines any remuneration above $5,000 to be of “significant financial interest.”
“Our findings raise the question of whether industry payments to the directors of publicly supported institutions, such as NCI-designated cancer centers, serve the public interest,” Dr. Carr and Dr. Welch noted. “Policy makers—and the public—should consider whether such payments should be allowed, limited (e.g., to less than $5,000 a year), or eliminated.”
The authors reported no conflicts of interest.
SOURCE: Carr D, et al. JAMA Intern Med. 2019 Aug 5. doi: 10.1001/jamainternmed.2019.3098.
Slightly less than half of directors at National Cancer Institute–designated cancer centers received payments from industry in 2017, according to recent analysis of the Centers for Medicare & Medicaid Services Open Payments database.
That number is lower than in 2014, but approximately one-fourth of National Cancer Institute (NCI) directors received payments of $5,000 or more that were not related to research, David Carr, MD, of the University of California in San Diego, and H. Gilbert Welch, MD, MPH, of Thetford, Vt., reported in a research letter in JAMA Internal Medicine.
Although cancer care is shaped by public funding through centers under NCI, “[c]ancer care is also shaped by industry, because developing new cancer therapeutics represents a major market opportunity,” Dr. Carr and Dr. Welch said. “Industry payments to academic physicians risk blurring the line between innovation and the evaluation of new therapies.”
In their research letter, Dr. Carr and Dr. Welch examined research- and non–research-based payments made to 53 physician cancer center directors between 2015 and 2017. In 2016, 44 of 53 directors held their current positions, while 41 of 53 directors held their position in 2015.
Analysis from 2017 showed $4.42 million in total industry payments were distributed to the directors, consisting of $1.89 million in research-based payments and $2.53 million in nonresearch payments across 22 directors. There were 27 directors (51%) who did not receive any payments from industry.
The authors found 19 directors (36%) received payments of $5,000 or more, while 12 of 53 directors (23%) received nonresearch payments of $5,000 or more. The largest payment received in 2017 for research was $863,000. Two directors received industry payments at $50,000 or higher that significantly exceeded payments other directors received, and one director received $2.27 million for “compensation for services other than consulting, including serving as faculty or a speaker at a venue other than a continuing education program.”
The NCI currently defines any remuneration above $5,000 to be of “significant financial interest.”
“Our findings raise the question of whether industry payments to the directors of publicly supported institutions, such as NCI-designated cancer centers, serve the public interest,” Dr. Carr and Dr. Welch noted. “Policy makers—and the public—should consider whether such payments should be allowed, limited (e.g., to less than $5,000 a year), or eliminated.”
The authors reported no conflicts of interest.
SOURCE: Carr D, et al. JAMA Intern Med. 2019 Aug 5. doi: 10.1001/jamainternmed.2019.3098.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Slightly less than half of directors at National Cancer Institute (NCI)–designated cancer centers received industry payments in 2017.
Major finding: Directors were more likely to receive non–research-based industry payments (23%), and the largest payment was to one director for $2.27 million.
Study details: An analysis of industry payments to 53 NCI-designated cancer center directors in 2017 using the Centers for Medicare & Medicaid Services Open Payments database.
Disclosures: The authors reported no conflicts of interest.
Source: Carr D et al. JAMA Intern Med. 2019 Aug 5. doi: 10.1001/jamainternmed.2019.3098.
Propose Ideas for Sessions for VAM 2020
The Society for Vascular Surgery seeks proposals for invited sessions for the 2020 Vascular Annual Meeting, to be held June 17 to 20 in Toronto, Ontario, Canada. Scientific sessions will be June 18 to 20 and exhibits will be open June 18 to 19. Proposals should include the session's educational benefit, a short outline of the program topic, session goals and target audience, among other information. Obtain the information/submission form here. Email completed forms to [email protected].
The Society for Vascular Surgery seeks proposals for invited sessions for the 2020 Vascular Annual Meeting, to be held June 17 to 20 in Toronto, Ontario, Canada. Scientific sessions will be June 18 to 20 and exhibits will be open June 18 to 19. Proposals should include the session's educational benefit, a short outline of the program topic, session goals and target audience, among other information. Obtain the information/submission form here. Email completed forms to [email protected].
The Society for Vascular Surgery seeks proposals for invited sessions for the 2020 Vascular Annual Meeting, to be held June 17 to 20 in Toronto, Ontario, Canada. Scientific sessions will be June 18 to 20 and exhibits will be open June 18 to 19. Proposals should include the session's educational benefit, a short outline of the program topic, session goals and target audience, among other information. Obtain the information/submission form here. Email completed forms to [email protected].
VAM on Demand Now Available
All who attended the 2019 Vascular Annual Meeting can now review sessions they attended, as well as “attend” those that they missed. Those who weren’t at VAM can now experience all they missed. Slides and audio presentations of nearly every session are included in the now available VAM on Demand. The cost for one year of access is $199 for VAM attendees and $499 for non-attendees. Those who purchased VAM on Demand before the meeting ended can visit this site to gain access by logging in with their SVS credentials.
All who attended the 2019 Vascular Annual Meeting can now review sessions they attended, as well as “attend” those that they missed. Those who weren’t at VAM can now experience all they missed. Slides and audio presentations of nearly every session are included in the now available VAM on Demand. The cost for one year of access is $199 for VAM attendees and $499 for non-attendees. Those who purchased VAM on Demand before the meeting ended can visit this site to gain access by logging in with their SVS credentials.
All who attended the 2019 Vascular Annual Meeting can now review sessions they attended, as well as “attend” those that they missed. Those who weren’t at VAM can now experience all they missed. Slides and audio presentations of nearly every session are included in the now available VAM on Demand. The cost for one year of access is $199 for VAM attendees and $499 for non-attendees. Those who purchased VAM on Demand before the meeting ended can visit this site to gain access by logging in with their SVS credentials.














