User login
IV fluid weaning unnecessary after gastroenteritis rehydration
SEATTLE – Intravenous fluids can simply be stopped after children with acute viral gastroenteritis are rehydrated in the hospital; there’s no need for a slow wean, according to a review at the Connecticut Children’s Medical Center, Hartford.
Researchers found that children leave the hospital hours sooner, with no ill effects. “This study suggests that slowly weaning IV fluids may not be necessary,” said lead investigator Danielle Klima, DO, a University of Connecticut pediatrics resident.
The team at Connecticut Children’s noticed that weaning practices after gastroenteritis rehydration varied widely on the pediatric floors, and appeared to be largely provider dependent, with “much subjective decision making.” The team wanted to see if it made a difference one way or the other, Dr. Klima said at Pediatric Hospital Medicine.
During respiratory season, “our pediatric floors are surging. Saving even a couple hours to get these kids out” quicker matters, she said, noting that it’s likely the first time the issue has been studied.
The team reviewed 153 children aged 2 months to 18 years, 95 of whom had IV fluids stopped once physicians deemed they were fluid resuscitated and ready for an oral feeding trial; the other 58 were weaned, with at least two reductions by half before final discontinuation.
There were no significant differences in age, gender, race, or insurance type between the two groups. The mean age was 2.6 years, and there were slightly more boys. The ED triage level was a mean of 3.2 points in both groups on a scale of 1-5, with 1 being the most urgent. Children with serious comorbidities, chronic diarrhea, feeding tubes, severe electrolyte abnormalities, or feeding problems were among those excluded.
Overall length of stay was 36 hours in the stop group versus 40.5 hours in the weaning group (P = .004). Children left the hospital about 6 hours after IV fluids were discontinued, versus 26 hours after weaning was started (P less than .001).
Electrolyte abnormalities on admission were more common in the weaning group (65% versus 57%), but not significantly so (P = .541). Electrolyte abnormalities were also more common at the end of fluid resuscitation in the weaning arm, but again not significantly (65% 42%, P = .077).
Fluid resuscitation needed to be restarted in 15 children in the stop group (16%), versus 11 (19%) in the wean arm (P = .459). One child in the stop group (1%) versus four (7%) who were weaned were readmitted to the hospital within a week for acute viral gastroenteritis (P = .067).
“I expected we were taking a more conservative weaning approach in younger infants,” but age didn’t seem to affect whether patients were weaned or not, Dr. Klima said.
With the results in hand, “our group is taking a closer look at exactly what we are doing,” perhaps with an eye toward standardization or even a randomized trial, she said.
She noted that weaning still makes sense for a fussy toddler who refuses to take anything by mouth.
There was no external funding, and Dr. Klima had no disclosures. The conference was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
SEATTLE – Intravenous fluids can simply be stopped after children with acute viral gastroenteritis are rehydrated in the hospital; there’s no need for a slow wean, according to a review at the Connecticut Children’s Medical Center, Hartford.
Researchers found that children leave the hospital hours sooner, with no ill effects. “This study suggests that slowly weaning IV fluids may not be necessary,” said lead investigator Danielle Klima, DO, a University of Connecticut pediatrics resident.
The team at Connecticut Children’s noticed that weaning practices after gastroenteritis rehydration varied widely on the pediatric floors, and appeared to be largely provider dependent, with “much subjective decision making.” The team wanted to see if it made a difference one way or the other, Dr. Klima said at Pediatric Hospital Medicine.
During respiratory season, “our pediatric floors are surging. Saving even a couple hours to get these kids out” quicker matters, she said, noting that it’s likely the first time the issue has been studied.
The team reviewed 153 children aged 2 months to 18 years, 95 of whom had IV fluids stopped once physicians deemed they were fluid resuscitated and ready for an oral feeding trial; the other 58 were weaned, with at least two reductions by half before final discontinuation.
There were no significant differences in age, gender, race, or insurance type between the two groups. The mean age was 2.6 years, and there were slightly more boys. The ED triage level was a mean of 3.2 points in both groups on a scale of 1-5, with 1 being the most urgent. Children with serious comorbidities, chronic diarrhea, feeding tubes, severe electrolyte abnormalities, or feeding problems were among those excluded.
Overall length of stay was 36 hours in the stop group versus 40.5 hours in the weaning group (P = .004). Children left the hospital about 6 hours after IV fluids were discontinued, versus 26 hours after weaning was started (P less than .001).
Electrolyte abnormalities on admission were more common in the weaning group (65% versus 57%), but not significantly so (P = .541). Electrolyte abnormalities were also more common at the end of fluid resuscitation in the weaning arm, but again not significantly (65% 42%, P = .077).
Fluid resuscitation needed to be restarted in 15 children in the stop group (16%), versus 11 (19%) in the wean arm (P = .459). One child in the stop group (1%) versus four (7%) who were weaned were readmitted to the hospital within a week for acute viral gastroenteritis (P = .067).
“I expected we were taking a more conservative weaning approach in younger infants,” but age didn’t seem to affect whether patients were weaned or not, Dr. Klima said.
With the results in hand, “our group is taking a closer look at exactly what we are doing,” perhaps with an eye toward standardization or even a randomized trial, she said.
She noted that weaning still makes sense for a fussy toddler who refuses to take anything by mouth.
There was no external funding, and Dr. Klima had no disclosures. The conference was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
SEATTLE – Intravenous fluids can simply be stopped after children with acute viral gastroenteritis are rehydrated in the hospital; there’s no need for a slow wean, according to a review at the Connecticut Children’s Medical Center, Hartford.
Researchers found that children leave the hospital hours sooner, with no ill effects. “This study suggests that slowly weaning IV fluids may not be necessary,” said lead investigator Danielle Klima, DO, a University of Connecticut pediatrics resident.
The team at Connecticut Children’s noticed that weaning practices after gastroenteritis rehydration varied widely on the pediatric floors, and appeared to be largely provider dependent, with “much subjective decision making.” The team wanted to see if it made a difference one way or the other, Dr. Klima said at Pediatric Hospital Medicine.
During respiratory season, “our pediatric floors are surging. Saving even a couple hours to get these kids out” quicker matters, she said, noting that it’s likely the first time the issue has been studied.
The team reviewed 153 children aged 2 months to 18 years, 95 of whom had IV fluids stopped once physicians deemed they were fluid resuscitated and ready for an oral feeding trial; the other 58 were weaned, with at least two reductions by half before final discontinuation.
There were no significant differences in age, gender, race, or insurance type between the two groups. The mean age was 2.6 years, and there were slightly more boys. The ED triage level was a mean of 3.2 points in both groups on a scale of 1-5, with 1 being the most urgent. Children with serious comorbidities, chronic diarrhea, feeding tubes, severe electrolyte abnormalities, or feeding problems were among those excluded.
Overall length of stay was 36 hours in the stop group versus 40.5 hours in the weaning group (P = .004). Children left the hospital about 6 hours after IV fluids were discontinued, versus 26 hours after weaning was started (P less than .001).
Electrolyte abnormalities on admission were more common in the weaning group (65% versus 57%), but not significantly so (P = .541). Electrolyte abnormalities were also more common at the end of fluid resuscitation in the weaning arm, but again not significantly (65% 42%, P = .077).
Fluid resuscitation needed to be restarted in 15 children in the stop group (16%), versus 11 (19%) in the wean arm (P = .459). One child in the stop group (1%) versus four (7%) who were weaned were readmitted to the hospital within a week for acute viral gastroenteritis (P = .067).
“I expected we were taking a more conservative weaning approach in younger infants,” but age didn’t seem to affect whether patients were weaned or not, Dr. Klima said.
With the results in hand, “our group is taking a closer look at exactly what we are doing,” perhaps with an eye toward standardization or even a randomized trial, she said.
She noted that weaning still makes sense for a fussy toddler who refuses to take anything by mouth.
There was no external funding, and Dr. Klima had no disclosures. The conference was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
REPORTING FROM PHM 2019
Are your patients ready for the transition to adult care?
AUSTIN, TEX. – All too often, children and adolescents stumble on their way to adult health care – if they make it at all.
In fact, data from an ongoing survey by the Department of Health & Human Services and the Health Resources and Services Administration indicate that only 40% of children with special health care needs aged 12-17 years receive the services necessary to make transitions to adult health care.
“Most fall off the proverbial cliff and do not engage with adult providers,” Cynthia Peacock, MD, said at the annual meeting of the Society for Pediatric Dermatology. She recalled meeting with one individual who, after being a patient at the children’s hospital for 13 years, was told over the phone to transition to an adult provider with not so much as a promised letter of introduction being sent on. “She did not know about the adult health care culture, which is fractured in care, relies on the patient to be their own advocate, and wants patients to be able to do their visit in 10-15 minutes.”
Dr. Peacock, medical director of the Transition Medicine Clinic at Baylor College of Medicine, Houston, described “Starting at age 12 is not too early,” she said. “It gives you time so that you can keep introducing the concept when that individual keeps coming back to see you, especially if it’s a chronic condition.”
She recommended that clinicians ask several questions to assess transition readiness of pediatric patients to adult care, including, Do you know your medications? Do you know how to take them? Do you know how to refill them? Do you know how to discuss them? Can you discuss your medical condition with the adult doctor? Can you call for a doctor’s appointment or get a prescription filled? “Adolescents are notorious for calling [the doctor’s office], and if they’re told they can’t make an appointment, that’s it; they stop right there,” Dr. Peacock said. “They don’t tend to problem solve. They don’t engage.”
Studies have suggested that the transfer of care is more likely to be successful if a formal transition program is in place to prepare the patient and to facilitate the change in health care providers. “There is a growing evidence base in the literature that skills training for young people with chronic illnesses can be associated with positive outcomes,” she said. “This can be as easy as telling the individual, ‘Do a book report about your condition. Talk to a friend. Tell a friend what your condition is. Or, do school science fair project and talk to your class about what you have.’ Get them past that uncomfortable feeling of having to talk about it.”
The earlier this happens, the better. “We know from research that if you get them to be their own [health care] advocate, that’s one less thing they have to do in the adult health care system,” she said. “They will move on to other things, such as getting a job or going to college.”
Dr. Peacock, who is board certified in pediatrics and internal medicine, added that providing adolescents with the option of being seen by professionals without their parents is considered best practice. “You could start by introducing the concept at age 12, but say at age 13, ‘I want to spend a minute with you alone without your parent. I want you to bring in questions that you want to ask me that you may not want to ask in front of your parents,’” she said. “I guarantee you that on that third visit the adolescent will ask you a question.”
Optimistic messaging is another component of effective planning. “You may see someone in your office with a disease that you know has high mortality and high morbidity, and you’re trying to help the family cope,” Dr. Peacock said. “That young person needs to be asked, ‘What are your plans for your future?’ Think about it: In 10 or 20 years when you’re transitioning that individual out of your health care system, what medical miracles have happened?” She recalled visiting with the mother of a patient with Down syndrome who had significant congenital heart disease. He was in his 30s and struggled to keep his behavior in check. “We were trying to develop a behavior plan, but his past care team had never put one in place for him,” Dr. Peacock said. “The mother looked at me and said, accusingly, ‘It’s your fault. It’s all of your doctors’ faults because they never told me that this would happen. They told me to take him home and spoil him because he would not be around at this age.’”
Effective transition handoffs are collaborative, she continued, with care plans built around what is likely to happen with the patient over time. “At Baylor College of Medicine, pediatric dermatologists follow patients for life, but I take over everything else adult care related,” Dr. Peacock said. “The pediatric dermatologist has me come over to the hospital when we’re talking about quality-of-life issues – about advance care, advanced directives, those types of things.”
She recommends not transferring care to an adult provider during pregnancy, hospitalization, during active disease, or during changes to a patient’s medical therapy. “When pediatricians call me from the hospital and they want an urgent transfer, I tell them, ‘Your emergency is not my urgency. Get everything ready; get them discharged. Get them followed up and back on their chronic care management, and I’ll be happy to do that transition for you,’ ” she said. “It’s also good to leave that door open for the adult provider to call you. Give them your cell phone number because it may be just one question, like, ‘Can you tell me why his liver enzymes are elevated? We can’t figure it out.’ ”
Dr. Peacock advises pediatric providers to develop processes within their own practice that facilitates transfer, “even if it just means sharing information with the adult provider at the end of the time you’re seeing that young adult. Know your systems and your resources. Get that medical summary done, even if it’s making the patient do the medical summary.” More information for clinicians and for patients and their families can be found at www.gottransition.org.
She reported having no relevant financial disclosures.
AUSTIN, TEX. – All too often, children and adolescents stumble on their way to adult health care – if they make it at all.
In fact, data from an ongoing survey by the Department of Health & Human Services and the Health Resources and Services Administration indicate that only 40% of children with special health care needs aged 12-17 years receive the services necessary to make transitions to adult health care.
“Most fall off the proverbial cliff and do not engage with adult providers,” Cynthia Peacock, MD, said at the annual meeting of the Society for Pediatric Dermatology. She recalled meeting with one individual who, after being a patient at the children’s hospital for 13 years, was told over the phone to transition to an adult provider with not so much as a promised letter of introduction being sent on. “She did not know about the adult health care culture, which is fractured in care, relies on the patient to be their own advocate, and wants patients to be able to do their visit in 10-15 minutes.”
Dr. Peacock, medical director of the Transition Medicine Clinic at Baylor College of Medicine, Houston, described “Starting at age 12 is not too early,” she said. “It gives you time so that you can keep introducing the concept when that individual keeps coming back to see you, especially if it’s a chronic condition.”
She recommended that clinicians ask several questions to assess transition readiness of pediatric patients to adult care, including, Do you know your medications? Do you know how to take them? Do you know how to refill them? Do you know how to discuss them? Can you discuss your medical condition with the adult doctor? Can you call for a doctor’s appointment or get a prescription filled? “Adolescents are notorious for calling [the doctor’s office], and if they’re told they can’t make an appointment, that’s it; they stop right there,” Dr. Peacock said. “They don’t tend to problem solve. They don’t engage.”
Studies have suggested that the transfer of care is more likely to be successful if a formal transition program is in place to prepare the patient and to facilitate the change in health care providers. “There is a growing evidence base in the literature that skills training for young people with chronic illnesses can be associated with positive outcomes,” she said. “This can be as easy as telling the individual, ‘Do a book report about your condition. Talk to a friend. Tell a friend what your condition is. Or, do school science fair project and talk to your class about what you have.’ Get them past that uncomfortable feeling of having to talk about it.”
The earlier this happens, the better. “We know from research that if you get them to be their own [health care] advocate, that’s one less thing they have to do in the adult health care system,” she said. “They will move on to other things, such as getting a job or going to college.”
Dr. Peacock, who is board certified in pediatrics and internal medicine, added that providing adolescents with the option of being seen by professionals without their parents is considered best practice. “You could start by introducing the concept at age 12, but say at age 13, ‘I want to spend a minute with you alone without your parent. I want you to bring in questions that you want to ask me that you may not want to ask in front of your parents,’” she said. “I guarantee you that on that third visit the adolescent will ask you a question.”
Optimistic messaging is another component of effective planning. “You may see someone in your office with a disease that you know has high mortality and high morbidity, and you’re trying to help the family cope,” Dr. Peacock said. “That young person needs to be asked, ‘What are your plans for your future?’ Think about it: In 10 or 20 years when you’re transitioning that individual out of your health care system, what medical miracles have happened?” She recalled visiting with the mother of a patient with Down syndrome who had significant congenital heart disease. He was in his 30s and struggled to keep his behavior in check. “We were trying to develop a behavior plan, but his past care team had never put one in place for him,” Dr. Peacock said. “The mother looked at me and said, accusingly, ‘It’s your fault. It’s all of your doctors’ faults because they never told me that this would happen. They told me to take him home and spoil him because he would not be around at this age.’”
Effective transition handoffs are collaborative, she continued, with care plans built around what is likely to happen with the patient over time. “At Baylor College of Medicine, pediatric dermatologists follow patients for life, but I take over everything else adult care related,” Dr. Peacock said. “The pediatric dermatologist has me come over to the hospital when we’re talking about quality-of-life issues – about advance care, advanced directives, those types of things.”
She recommends not transferring care to an adult provider during pregnancy, hospitalization, during active disease, or during changes to a patient’s medical therapy. “When pediatricians call me from the hospital and they want an urgent transfer, I tell them, ‘Your emergency is not my urgency. Get everything ready; get them discharged. Get them followed up and back on their chronic care management, and I’ll be happy to do that transition for you,’ ” she said. “It’s also good to leave that door open for the adult provider to call you. Give them your cell phone number because it may be just one question, like, ‘Can you tell me why his liver enzymes are elevated? We can’t figure it out.’ ”
Dr. Peacock advises pediatric providers to develop processes within their own practice that facilitates transfer, “even if it just means sharing information with the adult provider at the end of the time you’re seeing that young adult. Know your systems and your resources. Get that medical summary done, even if it’s making the patient do the medical summary.” More information for clinicians and for patients and their families can be found at www.gottransition.org.
She reported having no relevant financial disclosures.
AUSTIN, TEX. – All too often, children and adolescents stumble on their way to adult health care – if they make it at all.
In fact, data from an ongoing survey by the Department of Health & Human Services and the Health Resources and Services Administration indicate that only 40% of children with special health care needs aged 12-17 years receive the services necessary to make transitions to adult health care.
“Most fall off the proverbial cliff and do not engage with adult providers,” Cynthia Peacock, MD, said at the annual meeting of the Society for Pediatric Dermatology. She recalled meeting with one individual who, after being a patient at the children’s hospital for 13 years, was told over the phone to transition to an adult provider with not so much as a promised letter of introduction being sent on. “She did not know about the adult health care culture, which is fractured in care, relies on the patient to be their own advocate, and wants patients to be able to do their visit in 10-15 minutes.”
Dr. Peacock, medical director of the Transition Medicine Clinic at Baylor College of Medicine, Houston, described “Starting at age 12 is not too early,” she said. “It gives you time so that you can keep introducing the concept when that individual keeps coming back to see you, especially if it’s a chronic condition.”
She recommended that clinicians ask several questions to assess transition readiness of pediatric patients to adult care, including, Do you know your medications? Do you know how to take them? Do you know how to refill them? Do you know how to discuss them? Can you discuss your medical condition with the adult doctor? Can you call for a doctor’s appointment or get a prescription filled? “Adolescents are notorious for calling [the doctor’s office], and if they’re told they can’t make an appointment, that’s it; they stop right there,” Dr. Peacock said. “They don’t tend to problem solve. They don’t engage.”
Studies have suggested that the transfer of care is more likely to be successful if a formal transition program is in place to prepare the patient and to facilitate the change in health care providers. “There is a growing evidence base in the literature that skills training for young people with chronic illnesses can be associated with positive outcomes,” she said. “This can be as easy as telling the individual, ‘Do a book report about your condition. Talk to a friend. Tell a friend what your condition is. Or, do school science fair project and talk to your class about what you have.’ Get them past that uncomfortable feeling of having to talk about it.”
The earlier this happens, the better. “We know from research that if you get them to be their own [health care] advocate, that’s one less thing they have to do in the adult health care system,” she said. “They will move on to other things, such as getting a job or going to college.”
Dr. Peacock, who is board certified in pediatrics and internal medicine, added that providing adolescents with the option of being seen by professionals without their parents is considered best practice. “You could start by introducing the concept at age 12, but say at age 13, ‘I want to spend a minute with you alone without your parent. I want you to bring in questions that you want to ask me that you may not want to ask in front of your parents,’” she said. “I guarantee you that on that third visit the adolescent will ask you a question.”
Optimistic messaging is another component of effective planning. “You may see someone in your office with a disease that you know has high mortality and high morbidity, and you’re trying to help the family cope,” Dr. Peacock said. “That young person needs to be asked, ‘What are your plans for your future?’ Think about it: In 10 or 20 years when you’re transitioning that individual out of your health care system, what medical miracles have happened?” She recalled visiting with the mother of a patient with Down syndrome who had significant congenital heart disease. He was in his 30s and struggled to keep his behavior in check. “We were trying to develop a behavior plan, but his past care team had never put one in place for him,” Dr. Peacock said. “The mother looked at me and said, accusingly, ‘It’s your fault. It’s all of your doctors’ faults because they never told me that this would happen. They told me to take him home and spoil him because he would not be around at this age.’”
Effective transition handoffs are collaborative, she continued, with care plans built around what is likely to happen with the patient over time. “At Baylor College of Medicine, pediatric dermatologists follow patients for life, but I take over everything else adult care related,” Dr. Peacock said. “The pediatric dermatologist has me come over to the hospital when we’re talking about quality-of-life issues – about advance care, advanced directives, those types of things.”
She recommends not transferring care to an adult provider during pregnancy, hospitalization, during active disease, or during changes to a patient’s medical therapy. “When pediatricians call me from the hospital and they want an urgent transfer, I tell them, ‘Your emergency is not my urgency. Get everything ready; get them discharged. Get them followed up and back on their chronic care management, and I’ll be happy to do that transition for you,’ ” she said. “It’s also good to leave that door open for the adult provider to call you. Give them your cell phone number because it may be just one question, like, ‘Can you tell me why his liver enzymes are elevated? We can’t figure it out.’ ”
Dr. Peacock advises pediatric providers to develop processes within their own practice that facilitates transfer, “even if it just means sharing information with the adult provider at the end of the time you’re seeing that young adult. Know your systems and your resources. Get that medical summary done, even if it’s making the patient do the medical summary.” More information for clinicians and for patients and their families can be found at www.gottransition.org.
She reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM SPD 2019
Vaccination is not associated with increased risk of MS
(MS), according to an analysis published July 30 in Neurology. Although the results suggest that vaccination is associated with a lower likelihood of incident MS within the following 5 years, “these data alone do not allow for any conclusion regarding a possible protective effect of vaccinations regarding the development of MS,” wrote Alexander Hapfelmeier, PhD, of the Technical University of Munich and colleagues.
In recent years, researchers have proposed and investigated various potential environmental risk factors for the development of MS. Vaccination is one proposed environmental risk factor, but case reports and small studies have yielded conflicting results about its association with incident MS.
To examine this question more closely, Dr. Hapfelmeier and colleagues performed a systematic retrospective analysis of ambulatory claims data held by the Bavarian Association of Statutory Health Insurance Physicians. They reviewed the data to identify patients with new-onset MS and at least two ICD-10 diagnoses of the disorder. They next identified two control cohorts of participants diagnosed with other autoimmune diseases: Crohn’s disease and psoriasis. Finally, they randomly selected a third control cohort of patients without any of these diagnoses and matched them by age, sex, and district to patients with MS in a 5:1 ratio. Eligible participants were younger than 70 years.
Dr. Hapfelmeier and colleagues reviewed the incidence and frequency of vaccinations (such as those targeting tick-borne encephalitis, human papillomavirus, and influenza virus) in all cohorts. They created unconditional logistic regression models to assess the association between vaccination and MS. They also created separate models to contrast the MS cohort with each of the control cohorts.
The researchers included 12,262 patients with MS, 19,296 patients with Crohn’s disease, 112,292 patients with psoriasis, and 79,185 participants without these autoimmune diseases in their analysis. They found 456 participants with Crohn’s disease and psoriasis, 216 participants with MS and psoriasis, 48 participants with Crohn’s disease and MS, and 2 participants with Crohn’s disease, psoriasis, and MS. Dr. Hapfelmeier and colleagues allocated these participants to each of the respective cohorts and did not analyze them differently because of the comparatively small sample sizes.
The investigators analyzed the occurrence of vaccination in all participants during the 5 years before first diagnosis. Among patients who received vaccination, the odds ratio of MS was 0.870 in participants without autoimmune disease, 0.919 in participants with Crohn’s disease, and 0.973 in participants with psoriasis. Decreased risk of MS was most notable for vaccinations against influenza and tick-borne encephalitis. The results were consistent regardless of time frame, control cohort, and definition of MS.
The subjective definition of the MS cohort was a limitation of the study, but the authors addressed it by also using several strict definitions of that cohort. Another limitation is that the source data may reflect entry errors and incorrect coding.
A grant from the German Federal Ministry of Education and Research Competence Network MS supported the study. The authors had no conflicts that were relevant to the topic of the study.
SOURCE: Hapfelmeier A et al. Neurology. 2019 Jul 30. doi: 10.1212/WNL.0000000000008012.
The analysis by Hapfelmeier et al. provides important evidence that vaccinations are not associated with multiple sclerosis (MS), said E. Ann Yeh, MD, a neurologist at the Hospital for Sick Children in Toronto, and Jennifer Graves, MD, PhD, a neurologist at the University of California, San Diego, in an accompanying editorial. On the contrary, the evidence supports a potential protective effect of vaccines on the risk of developing MS, they said.
“The reasons for this [finding] cannot be gleaned from this study and may range from biological to sociocultural/demographic reasons,” the authors added. “Infection, rather than vaccination, may be an MS trigger, or individuals obtaining vaccinations may be practicing other healthy behaviors protective for MS. These possibilities should be the subject of future studies.”
Until future studies are completed and their results published, the findings of Hapfelmeier et al. offer “strong evidence to share with worried patients and families when faced with the question of whether a vaccine in the recent or relatively distant past triggered the individual’s MS,” said Dr. Yeh and Dr. Graves.
The authors had various relationships with industry, including serving on advisory boards for and receiving funding from pharmaceutical companies.
The analysis by Hapfelmeier et al. provides important evidence that vaccinations are not associated with multiple sclerosis (MS), said E. Ann Yeh, MD, a neurologist at the Hospital for Sick Children in Toronto, and Jennifer Graves, MD, PhD, a neurologist at the University of California, San Diego, in an accompanying editorial. On the contrary, the evidence supports a potential protective effect of vaccines on the risk of developing MS, they said.
“The reasons for this [finding] cannot be gleaned from this study and may range from biological to sociocultural/demographic reasons,” the authors added. “Infection, rather than vaccination, may be an MS trigger, or individuals obtaining vaccinations may be practicing other healthy behaviors protective for MS. These possibilities should be the subject of future studies.”
Until future studies are completed and their results published, the findings of Hapfelmeier et al. offer “strong evidence to share with worried patients and families when faced with the question of whether a vaccine in the recent or relatively distant past triggered the individual’s MS,” said Dr. Yeh and Dr. Graves.
The authors had various relationships with industry, including serving on advisory boards for and receiving funding from pharmaceutical companies.
The analysis by Hapfelmeier et al. provides important evidence that vaccinations are not associated with multiple sclerosis (MS), said E. Ann Yeh, MD, a neurologist at the Hospital for Sick Children in Toronto, and Jennifer Graves, MD, PhD, a neurologist at the University of California, San Diego, in an accompanying editorial. On the contrary, the evidence supports a potential protective effect of vaccines on the risk of developing MS, they said.
“The reasons for this [finding] cannot be gleaned from this study and may range from biological to sociocultural/demographic reasons,” the authors added. “Infection, rather than vaccination, may be an MS trigger, or individuals obtaining vaccinations may be practicing other healthy behaviors protective for MS. These possibilities should be the subject of future studies.”
Until future studies are completed and their results published, the findings of Hapfelmeier et al. offer “strong evidence to share with worried patients and families when faced with the question of whether a vaccine in the recent or relatively distant past triggered the individual’s MS,” said Dr. Yeh and Dr. Graves.
The authors had various relationships with industry, including serving on advisory boards for and receiving funding from pharmaceutical companies.
(MS), according to an analysis published July 30 in Neurology. Although the results suggest that vaccination is associated with a lower likelihood of incident MS within the following 5 years, “these data alone do not allow for any conclusion regarding a possible protective effect of vaccinations regarding the development of MS,” wrote Alexander Hapfelmeier, PhD, of the Technical University of Munich and colleagues.
In recent years, researchers have proposed and investigated various potential environmental risk factors for the development of MS. Vaccination is one proposed environmental risk factor, but case reports and small studies have yielded conflicting results about its association with incident MS.
To examine this question more closely, Dr. Hapfelmeier and colleagues performed a systematic retrospective analysis of ambulatory claims data held by the Bavarian Association of Statutory Health Insurance Physicians. They reviewed the data to identify patients with new-onset MS and at least two ICD-10 diagnoses of the disorder. They next identified two control cohorts of participants diagnosed with other autoimmune diseases: Crohn’s disease and psoriasis. Finally, they randomly selected a third control cohort of patients without any of these diagnoses and matched them by age, sex, and district to patients with MS in a 5:1 ratio. Eligible participants were younger than 70 years.
Dr. Hapfelmeier and colleagues reviewed the incidence and frequency of vaccinations (such as those targeting tick-borne encephalitis, human papillomavirus, and influenza virus) in all cohorts. They created unconditional logistic regression models to assess the association between vaccination and MS. They also created separate models to contrast the MS cohort with each of the control cohorts.
The researchers included 12,262 patients with MS, 19,296 patients with Crohn’s disease, 112,292 patients with psoriasis, and 79,185 participants without these autoimmune diseases in their analysis. They found 456 participants with Crohn’s disease and psoriasis, 216 participants with MS and psoriasis, 48 participants with Crohn’s disease and MS, and 2 participants with Crohn’s disease, psoriasis, and MS. Dr. Hapfelmeier and colleagues allocated these participants to each of the respective cohorts and did not analyze them differently because of the comparatively small sample sizes.
The investigators analyzed the occurrence of vaccination in all participants during the 5 years before first diagnosis. Among patients who received vaccination, the odds ratio of MS was 0.870 in participants without autoimmune disease, 0.919 in participants with Crohn’s disease, and 0.973 in participants with psoriasis. Decreased risk of MS was most notable for vaccinations against influenza and tick-borne encephalitis. The results were consistent regardless of time frame, control cohort, and definition of MS.
The subjective definition of the MS cohort was a limitation of the study, but the authors addressed it by also using several strict definitions of that cohort. Another limitation is that the source data may reflect entry errors and incorrect coding.
A grant from the German Federal Ministry of Education and Research Competence Network MS supported the study. The authors had no conflicts that were relevant to the topic of the study.
SOURCE: Hapfelmeier A et al. Neurology. 2019 Jul 30. doi: 10.1212/WNL.0000000000008012.
(MS), according to an analysis published July 30 in Neurology. Although the results suggest that vaccination is associated with a lower likelihood of incident MS within the following 5 years, “these data alone do not allow for any conclusion regarding a possible protective effect of vaccinations regarding the development of MS,” wrote Alexander Hapfelmeier, PhD, of the Technical University of Munich and colleagues.
In recent years, researchers have proposed and investigated various potential environmental risk factors for the development of MS. Vaccination is one proposed environmental risk factor, but case reports and small studies have yielded conflicting results about its association with incident MS.
To examine this question more closely, Dr. Hapfelmeier and colleagues performed a systematic retrospective analysis of ambulatory claims data held by the Bavarian Association of Statutory Health Insurance Physicians. They reviewed the data to identify patients with new-onset MS and at least two ICD-10 diagnoses of the disorder. They next identified two control cohorts of participants diagnosed with other autoimmune diseases: Crohn’s disease and psoriasis. Finally, they randomly selected a third control cohort of patients without any of these diagnoses and matched them by age, sex, and district to patients with MS in a 5:1 ratio. Eligible participants were younger than 70 years.
Dr. Hapfelmeier and colleagues reviewed the incidence and frequency of vaccinations (such as those targeting tick-borne encephalitis, human papillomavirus, and influenza virus) in all cohorts. They created unconditional logistic regression models to assess the association between vaccination and MS. They also created separate models to contrast the MS cohort with each of the control cohorts.
The researchers included 12,262 patients with MS, 19,296 patients with Crohn’s disease, 112,292 patients with psoriasis, and 79,185 participants without these autoimmune diseases in their analysis. They found 456 participants with Crohn’s disease and psoriasis, 216 participants with MS and psoriasis, 48 participants with Crohn’s disease and MS, and 2 participants with Crohn’s disease, psoriasis, and MS. Dr. Hapfelmeier and colleagues allocated these participants to each of the respective cohorts and did not analyze them differently because of the comparatively small sample sizes.
The investigators analyzed the occurrence of vaccination in all participants during the 5 years before first diagnosis. Among patients who received vaccination, the odds ratio of MS was 0.870 in participants without autoimmune disease, 0.919 in participants with Crohn’s disease, and 0.973 in participants with psoriasis. Decreased risk of MS was most notable for vaccinations against influenza and tick-borne encephalitis. The results were consistent regardless of time frame, control cohort, and definition of MS.
The subjective definition of the MS cohort was a limitation of the study, but the authors addressed it by also using several strict definitions of that cohort. Another limitation is that the source data may reflect entry errors and incorrect coding.
A grant from the German Federal Ministry of Education and Research Competence Network MS supported the study. The authors had no conflicts that were relevant to the topic of the study.
SOURCE: Hapfelmeier A et al. Neurology. 2019 Jul 30. doi: 10.1212/WNL.0000000000008012.
FROM NEUROLOGY
Benzodiazepines, hypnotics don’t increase Alzheimer’s pathology
LOS ANGELES – Benzodiazepines and hypnotics, including the so-called “Z drugs,” don’t significantly increase the pathological features typical of Alzheimer’s disease but long-term users may experience some neuronal loss in the nucleus basalis, Chris Fox, MD, reported at the Alzheimer’s Association International Conference.
The nucleus basalis is rich in cholinergic neurons and associated with arousing stimuli, including positive and aversive appetite, sustained attention, and the interplay of reality and visual perception.
“Neuronal loss in the nucleus basalis offers mechanisms for the impact of benzodiazepine and anticholinergic drug use on the aging brain and highlights important areas for future research,” said Dr. Fox, professor of clinical psychiatry at the University of East Anglia, Norwich, England.
“The risk [for taking a Z drug] in the United Kingdom is high, with about 7.5 million older adults using potentially inappropriately prescribed anticholinergic and/or Z-drug medications. Despite well-documented cognitive impairment associated with these medicines, hypnotics are still used for long durations and exceed the recommended limits,” Dr. Fox said. “There’s no association with better cognition, quality of life, or improved behavior when they are given to people with dementia. In fact, we’ve seen a 60% increased risk of hip fractures – an increase from a 3% to a 15% yearly risk.”
Dr. Fox and colleagues studied the brains of 337 subjects who were included in the U.K. Medical Research Council’s Cognitive Function and Ageing Studies (CFAS). The study was intended to explore the incidence of dementia in the United Kingdom, examine incidence variation among regions, and explore factors increasing dementia risk and rate of progression.
The first study, which began in 1989 and lasted until 2015, followed subjects older than 65 years for up to 12 years. Each subject was regularly interviewed and underwent cognitive testing about every 1.5 years. Benzodiazepine use was considered an especially important aspect, because the medications are frequently used in the elderly and seem linked to injuries and cognitive status at last follow-up.
In CFAS, 21% of subjects reported at least one incidence of anticholinergic use, and 12% reported recurrent use. Another 17% reported any hypnotic use, and 11% reported recurrent use. The main indications were as an antidepressant (13%), for urological issues (4%), as antiparkinsonism drugs (1%), as antipsychotics (3%), and as antihistamines (3%). Overall, 18% reported concurrent use of benzodiazepines and hypnotics. At time of death, 46% had a diagnosis of dementia.
“Those reporting benzodiazepine use were more likely to be women and to have depression or sleep problems,” Dr. Fox noted, although he didn’t give specific hazard ratios. After adjustment for numerous factors, including age, sex, stroke, hypertension, depression, anxiety, asthma, Parkinson’s disease, duration of sleep problems, education, and smoking, he found no statistically increased risk of amyloid brain plaques or tau tangles, the pathologic hallmarks of Alzheimer’s disease.
Anticholinergic use was associated with a significant 60% reduction in cortical atrophy (odds ratio, 0.40) and recurrent use with a 61% reduction in amyloid angiopathy (OR, 0.39).
However, both medication classes were associated with greater neuronal loss in the nucleus basalis. Recurrent use of anticholinergic drugs increased neuronal loss by 300% (OR, 4.12), while any use nearly tripled it (OR, 2.87). Recurrent use of benzodiazepines was associated with increased neuronal loss in the region (OR, 3.76) as well. However, these associations did not reach statistical significance. But there was a statistically significant association with any use of benzodiazepines and neuronal loss in the nucleus basalis (OR, 6.84).
“We did find greater neuronal loss in the nucleus basalis associated with benzodiazepine and anticholinergic drugs use,” Dr. Fox said. “The nucleus basalis is rich in neurons that stimulate the cholinergic system of the neocortex. Neuronal loss in this region is thought to occur in the early stages of Alzheimer’s. Other studies have suggested that volume loss in the basal forebrain cholinergic site leads to widespread cortical atrophy in patients with mild cognitive impairment. We did not observe the widespread cortical atrophy, however.
“Given that the strongest associations were observed for benzodiazepines and neuronal loss in the nucleus basalis, it may be that the drugs were prescribed to treat the symptoms of ‘cholinergic deficiency syndrome,’ Our findings suggest that the symptoms of dementia lead to an increase of benzodiazepines as opposed to the medications actually causing Alzheimer’s disease,” he said.
Dr. Fox reported no financial disclosures.
SOURCE: Fox C et al. AAIC 2019, Abstract 34017.
LOS ANGELES – Benzodiazepines and hypnotics, including the so-called “Z drugs,” don’t significantly increase the pathological features typical of Alzheimer’s disease but long-term users may experience some neuronal loss in the nucleus basalis, Chris Fox, MD, reported at the Alzheimer’s Association International Conference.
The nucleus basalis is rich in cholinergic neurons and associated with arousing stimuli, including positive and aversive appetite, sustained attention, and the interplay of reality and visual perception.
“Neuronal loss in the nucleus basalis offers mechanisms for the impact of benzodiazepine and anticholinergic drug use on the aging brain and highlights important areas for future research,” said Dr. Fox, professor of clinical psychiatry at the University of East Anglia, Norwich, England.
“The risk [for taking a Z drug] in the United Kingdom is high, with about 7.5 million older adults using potentially inappropriately prescribed anticholinergic and/or Z-drug medications. Despite well-documented cognitive impairment associated with these medicines, hypnotics are still used for long durations and exceed the recommended limits,” Dr. Fox said. “There’s no association with better cognition, quality of life, or improved behavior when they are given to people with dementia. In fact, we’ve seen a 60% increased risk of hip fractures – an increase from a 3% to a 15% yearly risk.”
Dr. Fox and colleagues studied the brains of 337 subjects who were included in the U.K. Medical Research Council’s Cognitive Function and Ageing Studies (CFAS). The study was intended to explore the incidence of dementia in the United Kingdom, examine incidence variation among regions, and explore factors increasing dementia risk and rate of progression.
The first study, which began in 1989 and lasted until 2015, followed subjects older than 65 years for up to 12 years. Each subject was regularly interviewed and underwent cognitive testing about every 1.5 years. Benzodiazepine use was considered an especially important aspect, because the medications are frequently used in the elderly and seem linked to injuries and cognitive status at last follow-up.
In CFAS, 21% of subjects reported at least one incidence of anticholinergic use, and 12% reported recurrent use. Another 17% reported any hypnotic use, and 11% reported recurrent use. The main indications were as an antidepressant (13%), for urological issues (4%), as antiparkinsonism drugs (1%), as antipsychotics (3%), and as antihistamines (3%). Overall, 18% reported concurrent use of benzodiazepines and hypnotics. At time of death, 46% had a diagnosis of dementia.
“Those reporting benzodiazepine use were more likely to be women and to have depression or sleep problems,” Dr. Fox noted, although he didn’t give specific hazard ratios. After adjustment for numerous factors, including age, sex, stroke, hypertension, depression, anxiety, asthma, Parkinson’s disease, duration of sleep problems, education, and smoking, he found no statistically increased risk of amyloid brain plaques or tau tangles, the pathologic hallmarks of Alzheimer’s disease.
Anticholinergic use was associated with a significant 60% reduction in cortical atrophy (odds ratio, 0.40) and recurrent use with a 61% reduction in amyloid angiopathy (OR, 0.39).
However, both medication classes were associated with greater neuronal loss in the nucleus basalis. Recurrent use of anticholinergic drugs increased neuronal loss by 300% (OR, 4.12), while any use nearly tripled it (OR, 2.87). Recurrent use of benzodiazepines was associated with increased neuronal loss in the region (OR, 3.76) as well. However, these associations did not reach statistical significance. But there was a statistically significant association with any use of benzodiazepines and neuronal loss in the nucleus basalis (OR, 6.84).
“We did find greater neuronal loss in the nucleus basalis associated with benzodiazepine and anticholinergic drugs use,” Dr. Fox said. “The nucleus basalis is rich in neurons that stimulate the cholinergic system of the neocortex. Neuronal loss in this region is thought to occur in the early stages of Alzheimer’s. Other studies have suggested that volume loss in the basal forebrain cholinergic site leads to widespread cortical atrophy in patients with mild cognitive impairment. We did not observe the widespread cortical atrophy, however.
“Given that the strongest associations were observed for benzodiazepines and neuronal loss in the nucleus basalis, it may be that the drugs were prescribed to treat the symptoms of ‘cholinergic deficiency syndrome,’ Our findings suggest that the symptoms of dementia lead to an increase of benzodiazepines as opposed to the medications actually causing Alzheimer’s disease,” he said.
Dr. Fox reported no financial disclosures.
SOURCE: Fox C et al. AAIC 2019, Abstract 34017.
LOS ANGELES – Benzodiazepines and hypnotics, including the so-called “Z drugs,” don’t significantly increase the pathological features typical of Alzheimer’s disease but long-term users may experience some neuronal loss in the nucleus basalis, Chris Fox, MD, reported at the Alzheimer’s Association International Conference.
The nucleus basalis is rich in cholinergic neurons and associated with arousing stimuli, including positive and aversive appetite, sustained attention, and the interplay of reality and visual perception.
“Neuronal loss in the nucleus basalis offers mechanisms for the impact of benzodiazepine and anticholinergic drug use on the aging brain and highlights important areas for future research,” said Dr. Fox, professor of clinical psychiatry at the University of East Anglia, Norwich, England.
“The risk [for taking a Z drug] in the United Kingdom is high, with about 7.5 million older adults using potentially inappropriately prescribed anticholinergic and/or Z-drug medications. Despite well-documented cognitive impairment associated with these medicines, hypnotics are still used for long durations and exceed the recommended limits,” Dr. Fox said. “There’s no association with better cognition, quality of life, or improved behavior when they are given to people with dementia. In fact, we’ve seen a 60% increased risk of hip fractures – an increase from a 3% to a 15% yearly risk.”
Dr. Fox and colleagues studied the brains of 337 subjects who were included in the U.K. Medical Research Council’s Cognitive Function and Ageing Studies (CFAS). The study was intended to explore the incidence of dementia in the United Kingdom, examine incidence variation among regions, and explore factors increasing dementia risk and rate of progression.
The first study, which began in 1989 and lasted until 2015, followed subjects older than 65 years for up to 12 years. Each subject was regularly interviewed and underwent cognitive testing about every 1.5 years. Benzodiazepine use was considered an especially important aspect, because the medications are frequently used in the elderly and seem linked to injuries and cognitive status at last follow-up.
In CFAS, 21% of subjects reported at least one incidence of anticholinergic use, and 12% reported recurrent use. Another 17% reported any hypnotic use, and 11% reported recurrent use. The main indications were as an antidepressant (13%), for urological issues (4%), as antiparkinsonism drugs (1%), as antipsychotics (3%), and as antihistamines (3%). Overall, 18% reported concurrent use of benzodiazepines and hypnotics. At time of death, 46% had a diagnosis of dementia.
“Those reporting benzodiazepine use were more likely to be women and to have depression or sleep problems,” Dr. Fox noted, although he didn’t give specific hazard ratios. After adjustment for numerous factors, including age, sex, stroke, hypertension, depression, anxiety, asthma, Parkinson’s disease, duration of sleep problems, education, and smoking, he found no statistically increased risk of amyloid brain plaques or tau tangles, the pathologic hallmarks of Alzheimer’s disease.
Anticholinergic use was associated with a significant 60% reduction in cortical atrophy (odds ratio, 0.40) and recurrent use with a 61% reduction in amyloid angiopathy (OR, 0.39).
However, both medication classes were associated with greater neuronal loss in the nucleus basalis. Recurrent use of anticholinergic drugs increased neuronal loss by 300% (OR, 4.12), while any use nearly tripled it (OR, 2.87). Recurrent use of benzodiazepines was associated with increased neuronal loss in the region (OR, 3.76) as well. However, these associations did not reach statistical significance. But there was a statistically significant association with any use of benzodiazepines and neuronal loss in the nucleus basalis (OR, 6.84).
“We did find greater neuronal loss in the nucleus basalis associated with benzodiazepine and anticholinergic drugs use,” Dr. Fox said. “The nucleus basalis is rich in neurons that stimulate the cholinergic system of the neocortex. Neuronal loss in this region is thought to occur in the early stages of Alzheimer’s. Other studies have suggested that volume loss in the basal forebrain cholinergic site leads to widespread cortical atrophy in patients with mild cognitive impairment. We did not observe the widespread cortical atrophy, however.
“Given that the strongest associations were observed for benzodiazepines and neuronal loss in the nucleus basalis, it may be that the drugs were prescribed to treat the symptoms of ‘cholinergic deficiency syndrome,’ Our findings suggest that the symptoms of dementia lead to an increase of benzodiazepines as opposed to the medications actually causing Alzheimer’s disease,” he said.
Dr. Fox reported no financial disclosures.
SOURCE: Fox C et al. AAIC 2019, Abstract 34017.
REPORTING FROM AAIC 2019
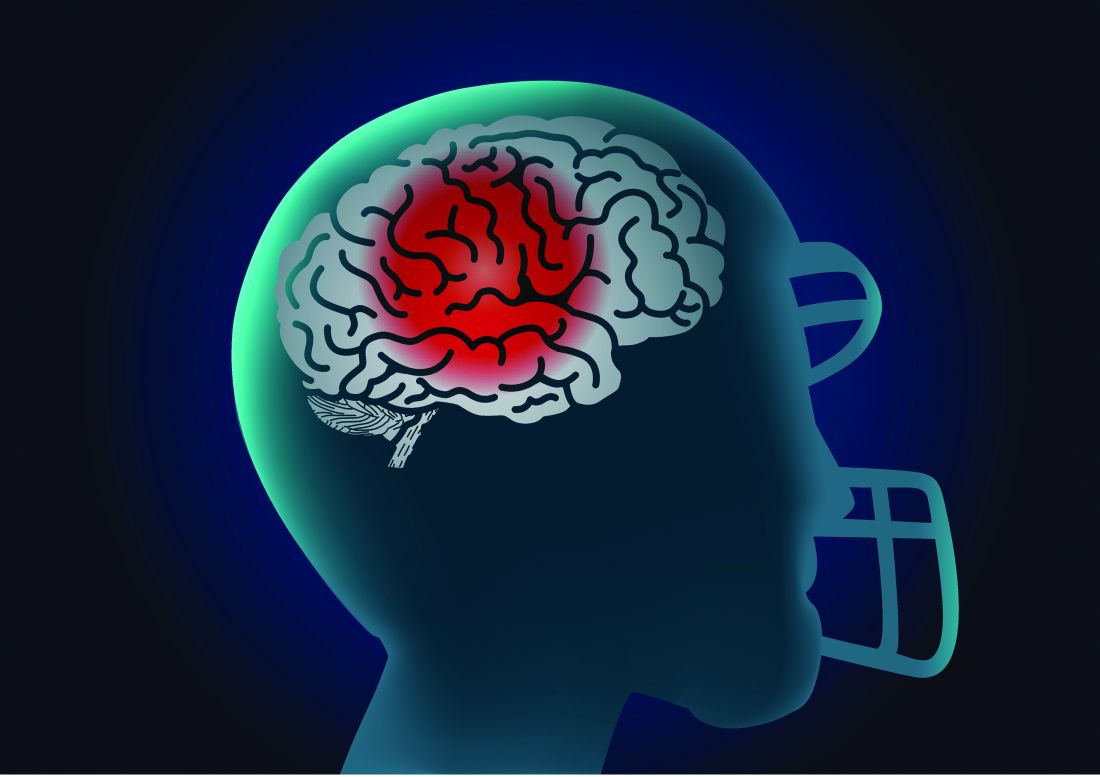
Researchers examine potential causes of dementia in CTE
, according to a cross-sectional study published online Aug. 5 in JAMA Neurology.
The study of older, deceased former American football players with CTE showed that more years of play were associated with more severe white matter rarefaction and greater burden of neurofibrillary tau tangles in the dorsolateral frontal cortex, wrote Michael L. Alosco, PhD, assistant professor of neurology at Boston University’s CTE Center, and colleagues.
An analysis of donated brains
Repetitive head impacts are associated with CTE. The clinical presentation of CTE includes cognitive, behavioral, and mood changes that can progress to dementia. The contributions of pathologic changes in phosphorylated tau, white matter degeneration, and cerebrovascular disease to dementia in the context of CTE are poorly understood. Dr. Alosco and colleagues examined arteriosclerosis, infarcts, microinfarcts, microbleeds, and white matter rarefaction in donated brains to illuminate these contributions.
The researchers examined data from the Understanding Neurologic Injury and Traumatic Encephalopathy (UNITE) Study and Veterans Affairs–Boston University–Concussion Legacy Foundation brain bank. The population included deceased men who had played football and had received a neuropathologic diagnosis of CTE. Eligible participants had a history of repetitive head impacts. Brains that had been donated after a prolonged time postmortem and those with poor tissue quality were excluded.
Neuropathologists blinded to clinical data analyzed patients’ CTE stage and severity of neurofibrillary tangle burden in the dorsolateral frontal cortex as semiquantitative scales of phosphorylated tau severity. Neurofibrillary tangle burden was dichotomized as none or mild versus moderate or severe. The neuropathologists also rated white matter rarefaction and arteriolosclerosis severity using a scale of 0 points (i.e., none) to 3 points (i.e., severe changes). The investigators obtained clinical data through online surveys and retrospective telephone interviews with informants. They adjudicated consensus diagnoses of dementia based on modified criteria from DSM-IV.
White matter rarefaction was common
Dr. Alosco and colleagues included 180 individuals in their analysis, excluding those aged younger than 40 years because of low pathologic burden and minimal presence of dementia. Mean age at death was nearly 68 years. Fifty patients had no or mild neurofibrillary tangle burden, and 130 had moderate to severe burden. Thirty-five patients had CTE at stage I or II, and 145 had CTE at stage III or IV. In all, 120 patients were determined to have had dementia. About 47% of the sample had moderate to severe white matter rarefaction, and about 47% had arteriolosclerosis. Infarcts, microinfarcts, and microbleeds were uncommon.
When the investigators created a simultaneous equations regression model and controlled for age and race, they found that more years of play was associated with more severe white matter rarefaction, greater phosphorylated tau accumulation, and high CTE stage. Furthermore, white matter rarefaction and dorsolateral frontal cortex neurofibrillary tangles were associated with dementia. The association of years of play with dementia was mediated by white matter rarefaction and neurofibrillary tangle burden. Arteriolosclerosis was not associated with years of play, but arteriolosclerosis was independently associated with dementia.
The odds ratio for dementia was 1.69 among participants with more severe white matter rarefaction and 1.81 among patients with arteriolosclerosis. After the researchers controlled for age and race, the odds ratio of dementia was 2.65 among participants with a high neurofibrillary tangle burden, compared with participants with a low burden.
“Studies that include direct cardiovascular disease and repetitive head impacts metrics and refined measures of white matter integrity are needed to improve understanding of the pathogenesis of white matter rarefaction and cerebral small vessel changes in CTE,” Dr. Alosco and colleagues wrote.
The study was funded by grants from the National Institute on Aging, National Institute of Neurological Disorders and Stroke, the Department of Veterans Affairs, the Nick and Lynn Buoniconti Foundation, and the National Center for Advancing Translational Sciences. Some of the authors reported financial ties to the pharmaceutical industry and serving on professional sports committees.
SOURCE: Alosco ML et al. JAMA Neurol. 2019 Aug 5. doi: 10.1001/jamaneurol.2019.2244.
The study by Alosco et al. provides new insights into the pathogenesis of dementia in deceased former football players with chronic traumatic encephalopathy (CTE), Julie A. Schneider, MD, professor of neuropathology at Rush University, Chicago, wrote in an accompanying editorial (JAMA Neurol. 2019 Aug 5. doi: 10.1001/jamaneurol.2019.1089).
Significant and widespread white matter injury is an established result of head trauma resulting from acceleration-deceleration injuries. In addition, studies of single and repetitive traumatic brain injury have shown disruption of axons and white matter. The findings of Alosco et al. “underscore the importance of studying the risk factors and mechanisms for the white matter rarefaction, in addition to the tauopathy, in individuals who have played U.S. football and have CTE,” Dr. Schneider wrote.
The comprehensive neuropathologic examinations, advanced statistical techniques, and multiple sensitivity analyses that the investigators performed are among the study’s strengths. An important limitation, however, is selection bias. “The frequency of pathologic characteristics in this group should not be generalized to estimate the prevalence of neuropathologic conditions in living individuals who have played or are playing U.S. football,” Dr. Schneider wrote. “Moreover, individuals who played football who were selected for autopsy and found to have CTE may differ in other important ways from those who did not undergo autopsy or did not have CTE.” Recall bias could alter associations between years of play and dementia diagnosis, and the study’s semiquantitative assessments could result in decreased power to observe relevant associations, she said.
“In spite of these limitations, the authors should be applauded for elegant work and compelling support for multiple pathologic pathways to dementia in football players with CTE,” Dr. Schneider concluded.
Dr. Schneider is with the Rush Alzheimer’s Disease Center at Rush University, Chicago. She has been an expert consultant for the National Football League and the National Hockey League.
The study by Alosco et al. provides new insights into the pathogenesis of dementia in deceased former football players with chronic traumatic encephalopathy (CTE), Julie A. Schneider, MD, professor of neuropathology at Rush University, Chicago, wrote in an accompanying editorial (JAMA Neurol. 2019 Aug 5. doi: 10.1001/jamaneurol.2019.1089).
Significant and widespread white matter injury is an established result of head trauma resulting from acceleration-deceleration injuries. In addition, studies of single and repetitive traumatic brain injury have shown disruption of axons and white matter. The findings of Alosco et al. “underscore the importance of studying the risk factors and mechanisms for the white matter rarefaction, in addition to the tauopathy, in individuals who have played U.S. football and have CTE,” Dr. Schneider wrote.
The comprehensive neuropathologic examinations, advanced statistical techniques, and multiple sensitivity analyses that the investigators performed are among the study’s strengths. An important limitation, however, is selection bias. “The frequency of pathologic characteristics in this group should not be generalized to estimate the prevalence of neuropathologic conditions in living individuals who have played or are playing U.S. football,” Dr. Schneider wrote. “Moreover, individuals who played football who were selected for autopsy and found to have CTE may differ in other important ways from those who did not undergo autopsy or did not have CTE.” Recall bias could alter associations between years of play and dementia diagnosis, and the study’s semiquantitative assessments could result in decreased power to observe relevant associations, she said.
“In spite of these limitations, the authors should be applauded for elegant work and compelling support for multiple pathologic pathways to dementia in football players with CTE,” Dr. Schneider concluded.
Dr. Schneider is with the Rush Alzheimer’s Disease Center at Rush University, Chicago. She has been an expert consultant for the National Football League and the National Hockey League.
The study by Alosco et al. provides new insights into the pathogenesis of dementia in deceased former football players with chronic traumatic encephalopathy (CTE), Julie A. Schneider, MD, professor of neuropathology at Rush University, Chicago, wrote in an accompanying editorial (JAMA Neurol. 2019 Aug 5. doi: 10.1001/jamaneurol.2019.1089).
Significant and widespread white matter injury is an established result of head trauma resulting from acceleration-deceleration injuries. In addition, studies of single and repetitive traumatic brain injury have shown disruption of axons and white matter. The findings of Alosco et al. “underscore the importance of studying the risk factors and mechanisms for the white matter rarefaction, in addition to the tauopathy, in individuals who have played U.S. football and have CTE,” Dr. Schneider wrote.
The comprehensive neuropathologic examinations, advanced statistical techniques, and multiple sensitivity analyses that the investigators performed are among the study’s strengths. An important limitation, however, is selection bias. “The frequency of pathologic characteristics in this group should not be generalized to estimate the prevalence of neuropathologic conditions in living individuals who have played or are playing U.S. football,” Dr. Schneider wrote. “Moreover, individuals who played football who were selected for autopsy and found to have CTE may differ in other important ways from those who did not undergo autopsy or did not have CTE.” Recall bias could alter associations between years of play and dementia diagnosis, and the study’s semiquantitative assessments could result in decreased power to observe relevant associations, she said.
“In spite of these limitations, the authors should be applauded for elegant work and compelling support for multiple pathologic pathways to dementia in football players with CTE,” Dr. Schneider concluded.
Dr. Schneider is with the Rush Alzheimer’s Disease Center at Rush University, Chicago. She has been an expert consultant for the National Football League and the National Hockey League.
, according to a cross-sectional study published online Aug. 5 in JAMA Neurology.
The study of older, deceased former American football players with CTE showed that more years of play were associated with more severe white matter rarefaction and greater burden of neurofibrillary tau tangles in the dorsolateral frontal cortex, wrote Michael L. Alosco, PhD, assistant professor of neurology at Boston University’s CTE Center, and colleagues.
An analysis of donated brains
Repetitive head impacts are associated with CTE. The clinical presentation of CTE includes cognitive, behavioral, and mood changes that can progress to dementia. The contributions of pathologic changes in phosphorylated tau, white matter degeneration, and cerebrovascular disease to dementia in the context of CTE are poorly understood. Dr. Alosco and colleagues examined arteriosclerosis, infarcts, microinfarcts, microbleeds, and white matter rarefaction in donated brains to illuminate these contributions.
The researchers examined data from the Understanding Neurologic Injury and Traumatic Encephalopathy (UNITE) Study and Veterans Affairs–Boston University–Concussion Legacy Foundation brain bank. The population included deceased men who had played football and had received a neuropathologic diagnosis of CTE. Eligible participants had a history of repetitive head impacts. Brains that had been donated after a prolonged time postmortem and those with poor tissue quality were excluded.
Neuropathologists blinded to clinical data analyzed patients’ CTE stage and severity of neurofibrillary tangle burden in the dorsolateral frontal cortex as semiquantitative scales of phosphorylated tau severity. Neurofibrillary tangle burden was dichotomized as none or mild versus moderate or severe. The neuropathologists also rated white matter rarefaction and arteriolosclerosis severity using a scale of 0 points (i.e., none) to 3 points (i.e., severe changes). The investigators obtained clinical data through online surveys and retrospective telephone interviews with informants. They adjudicated consensus diagnoses of dementia based on modified criteria from DSM-IV.
White matter rarefaction was common
Dr. Alosco and colleagues included 180 individuals in their analysis, excluding those aged younger than 40 years because of low pathologic burden and minimal presence of dementia. Mean age at death was nearly 68 years. Fifty patients had no or mild neurofibrillary tangle burden, and 130 had moderate to severe burden. Thirty-five patients had CTE at stage I or II, and 145 had CTE at stage III or IV. In all, 120 patients were determined to have had dementia. About 47% of the sample had moderate to severe white matter rarefaction, and about 47% had arteriolosclerosis. Infarcts, microinfarcts, and microbleeds were uncommon.
When the investigators created a simultaneous equations regression model and controlled for age and race, they found that more years of play was associated with more severe white matter rarefaction, greater phosphorylated tau accumulation, and high CTE stage. Furthermore, white matter rarefaction and dorsolateral frontal cortex neurofibrillary tangles were associated with dementia. The association of years of play with dementia was mediated by white matter rarefaction and neurofibrillary tangle burden. Arteriolosclerosis was not associated with years of play, but arteriolosclerosis was independently associated with dementia.
The odds ratio for dementia was 1.69 among participants with more severe white matter rarefaction and 1.81 among patients with arteriolosclerosis. After the researchers controlled for age and race, the odds ratio of dementia was 2.65 among participants with a high neurofibrillary tangle burden, compared with participants with a low burden.
“Studies that include direct cardiovascular disease and repetitive head impacts metrics and refined measures of white matter integrity are needed to improve understanding of the pathogenesis of white matter rarefaction and cerebral small vessel changes in CTE,” Dr. Alosco and colleagues wrote.
The study was funded by grants from the National Institute on Aging, National Institute of Neurological Disorders and Stroke, the Department of Veterans Affairs, the Nick and Lynn Buoniconti Foundation, and the National Center for Advancing Translational Sciences. Some of the authors reported financial ties to the pharmaceutical industry and serving on professional sports committees.
SOURCE: Alosco ML et al. JAMA Neurol. 2019 Aug 5. doi: 10.1001/jamaneurol.2019.2244.
, according to a cross-sectional study published online Aug. 5 in JAMA Neurology.
The study of older, deceased former American football players with CTE showed that more years of play were associated with more severe white matter rarefaction and greater burden of neurofibrillary tau tangles in the dorsolateral frontal cortex, wrote Michael L. Alosco, PhD, assistant professor of neurology at Boston University’s CTE Center, and colleagues.
An analysis of donated brains
Repetitive head impacts are associated with CTE. The clinical presentation of CTE includes cognitive, behavioral, and mood changes that can progress to dementia. The contributions of pathologic changes in phosphorylated tau, white matter degeneration, and cerebrovascular disease to dementia in the context of CTE are poorly understood. Dr. Alosco and colleagues examined arteriosclerosis, infarcts, microinfarcts, microbleeds, and white matter rarefaction in donated brains to illuminate these contributions.
The researchers examined data from the Understanding Neurologic Injury and Traumatic Encephalopathy (UNITE) Study and Veterans Affairs–Boston University–Concussion Legacy Foundation brain bank. The population included deceased men who had played football and had received a neuropathologic diagnosis of CTE. Eligible participants had a history of repetitive head impacts. Brains that had been donated after a prolonged time postmortem and those with poor tissue quality were excluded.
Neuropathologists blinded to clinical data analyzed patients’ CTE stage and severity of neurofibrillary tangle burden in the dorsolateral frontal cortex as semiquantitative scales of phosphorylated tau severity. Neurofibrillary tangle burden was dichotomized as none or mild versus moderate or severe. The neuropathologists also rated white matter rarefaction and arteriolosclerosis severity using a scale of 0 points (i.e., none) to 3 points (i.e., severe changes). The investigators obtained clinical data through online surveys and retrospective telephone interviews with informants. They adjudicated consensus diagnoses of dementia based on modified criteria from DSM-IV.
White matter rarefaction was common
Dr. Alosco and colleagues included 180 individuals in their analysis, excluding those aged younger than 40 years because of low pathologic burden and minimal presence of dementia. Mean age at death was nearly 68 years. Fifty patients had no or mild neurofibrillary tangle burden, and 130 had moderate to severe burden. Thirty-five patients had CTE at stage I or II, and 145 had CTE at stage III or IV. In all, 120 patients were determined to have had dementia. About 47% of the sample had moderate to severe white matter rarefaction, and about 47% had arteriolosclerosis. Infarcts, microinfarcts, and microbleeds were uncommon.
When the investigators created a simultaneous equations regression model and controlled for age and race, they found that more years of play was associated with more severe white matter rarefaction, greater phosphorylated tau accumulation, and high CTE stage. Furthermore, white matter rarefaction and dorsolateral frontal cortex neurofibrillary tangles were associated with dementia. The association of years of play with dementia was mediated by white matter rarefaction and neurofibrillary tangle burden. Arteriolosclerosis was not associated with years of play, but arteriolosclerosis was independently associated with dementia.
The odds ratio for dementia was 1.69 among participants with more severe white matter rarefaction and 1.81 among patients with arteriolosclerosis. After the researchers controlled for age and race, the odds ratio of dementia was 2.65 among participants with a high neurofibrillary tangle burden, compared with participants with a low burden.
“Studies that include direct cardiovascular disease and repetitive head impacts metrics and refined measures of white matter integrity are needed to improve understanding of the pathogenesis of white matter rarefaction and cerebral small vessel changes in CTE,” Dr. Alosco and colleagues wrote.
The study was funded by grants from the National Institute on Aging, National Institute of Neurological Disorders and Stroke, the Department of Veterans Affairs, the Nick and Lynn Buoniconti Foundation, and the National Center for Advancing Translational Sciences. Some of the authors reported financial ties to the pharmaceutical industry and serving on professional sports committees.
SOURCE: Alosco ML et al. JAMA Neurol. 2019 Aug 5. doi: 10.1001/jamaneurol.2019.2244.
FROM JAMA NEUROLOGY
Tales From VA Anesthesiology
The patient grabbed my attention as I glanced through our clinic schedule. It was his age: He was 99 years old and scheduled for eye surgery. The plastic surgery resident’s note read: “Patient understands that this would involve surgery under general anesthesia and is agreeable to moving forward...Extremely high risk of anesthesia emphasized.”
I reviewed the patient’s history. At baseline, he had severe pulmonary hypertension, severe aortic stenosis (AS), diastolic heart failure, chronic atrial fibrillation, chronic kidney disease (estimated glomerular filtration rate of 26 mL/min [normal is > 60 mL/min]), anemia (hematocrit 26%), and a standing do not resuscitate (DNR) order. His maximal daily exercise was walking slowly across a room, primarily limited by joint pain. Recent geropsychiatry notes indicated mild cognitive impairment. The anesthesia record from an urgent hip fracture repair 7 months before under general anesthesia was unremarkable.
I phoned the attending plastic surgeon. Our conversation was as follows:
“Hi, I’m about to see a 99-year-old patient with a DNR who is scheduled for resection of an eyelid tumor. His medical history makes me nervous. Are you sure this is a good idea?”“Hmmm, 99-year-old…okay, that’s right,” he responded. “He has an invasive squamous that could become a big problem. The actual procedure is under 10 minutes. Waiting for the pathology report will be the longest part of the procedure.”
“Can it be done under local?” I asked.
“Yes,” he replied.
“Okay, I’ll talk to him and call you back.”
I found the patient in the waiting room, flanked by his 2 daughters and invited them into the clinic room. After introductions, I began asking whether they had any questions about the anesthesia. By midsentence a daughter was prompting him to discuss what happened “last time.” He described a history of posttraumatic stress disorder (PTSD) stemming from his hip surgery, which he blamed squarely on the anesthesia. His emotion was evident in the gathering pauses. “I hate that I am so emotional since they kept me awake during my surgery.”
Through the fog of multiple accounts, it became clear that he was traumatized by the loss of control during the administration of and emergence from the anesthesia.
“They told me it was only oxygen,” he said. “They lied. There was a taste to it…I was awake and skinned alive…They said I was a monster when I woke up thrashing.” He went on, explaining that in the recovery room “there were 2 people bothering me, man-handling me, asking me questions.”
One of his daughters showed me pictures of bruises on his face from ripping off the mask and pulling out the breathing tube. They were visibly upset by the memory of his postoperative combativeness and paranoia. The note written by the orthopedic surgery resident on the day after surgery stated succinctly, “Doing well, had some delirium from anesthesia overnight.” Subsequent geropsychiatry home visits attested to intrusive thoughts, flashbacks, and nightmares from his time as a combat soldier in World War II, 65 years in the past.
“It took me months…months to recover,” he said.
He was in the mood to reminisce, however, perhaps a willful distraction. He had the floor for at least 30 minutes, during which I spoke about 5 sentences. With every sad story he told there was a happy, humorous one, such as meeting his future wife while on leave in New Zealand during the war, recalled down to exact dates. And another story:
There we were in New Caledonia. All our supplies went out to replace what sank on [USS] Coolidge, including a lot of food. Well, there were deer on the island. So we took out a truck and a rifle and wouldn’t you know we came upon a roadblock in the form of a big steer. We figured it looked enough like a deer. My buddy shot it dead with one shot. We dressed it and loaded it into the jeep. Hardly before we even got back to the mess hall, the officers’ cook came sniffing around. He and our captain agreed it was easily the biggest deer they’d ever seen and appropriated it to the officers’ mess. Next day the CO [commanding officer] of the whole outfit came by and announced it was the best tasting ‘venison’ he’d ever had. I heard the farmer got paid a pretty penny for that steer. I didn’t get a damn bite.
He delivered this last bit with relish.
When the conversation returned to anesthesia, I read them the record of his hip fracture repair. I explained that on the face of it, the report seemed uneventful. One daughter asked astute questions about his awareness. I explained that although awareness during general anesthesia is possible, it seemed from the record, he’d had plenty of anesthesia during the case and that there is always less at the beginning and end, the periods that apparently had caused him distress. I also explained that most studies report the incidence of true awareness as at most 1 out of thousands of events and that he had none of the established risk factors for it, such as female gender, young age, chronic substance abuse, cardiac and obstetric surgery, and history of awareness.1
The other daughter wondered why he was so agitated afterward. I recited data on the frequency of postoperative delirium in elderly patients but explained that the range is wide, depending on the study and population, from about 1% in elderly patients undergoing ambulatory surgery to 65% for open aortic surgery.2,3 I added that their father had 2 of the strongest risk factors for delirium, advanced age and cognitive impairment.3 Only after airing each question about the hip surgery in detail were they ready to discuss the eye surgery.
He started that conversation with the right question: “Do I really need it?”
I quoted my surgical colleague’s concern. I told him that, should he opt to undergo the surgery, I was confident that this time around his experience would be different from the last.
“If you’re okay with it, all you need is some numbing medicine from the surgeon; you won’t need any anesthesia from me.”
I walked step-by-step through what they could expect on the day of surgery. Maintaining control was of obvious importance to him. He felt comfortable going forward. His daughters intuited that less would be more for a quick recovery.
We then addressed the DNR directive. I acknowledged his absolute right to self-determination and explained that the need for resuscitation is, at times, a consequence of the surgery and anesthesia. I reassured them that our plan made resuscitation and intubation highly unlikely. They also asked to use any interventions necessary to restart his heart if it should stop beating. I documented their decision in my notes and communicated it to the surgical team. We had talked for 90 minutes.
I met the patient and his daughters on the day of surgery in the preoperative holding area. I inserted an IV, applied electrocardiography leads, and affixed a pulse oximeter and a noninvasive blood pressure cuff. In the operating room (OR) we took time to place his 99-year-old joints into, as he said, the “least worst” position. He tolerated the injection of the local by the surgeon perfectly well. We were in the OR for 3 hours, during which he taught me a fair amount about boating and outboard engines among other things. Pathology reported clean margins. He was discharged home soon after and had an uneventful recovery.
Patient-First Approach
A core competency of the Accreditation Council for Graduate Medical Education for an anesthesia residency is the Interpersonal and Communication Skills program. A comprehensive discussion of communication is far beyond the scope here. But not surprisingly, deficient communication between physicians and patients can cause emotional distress, significant dissatisfaction among family members, and negative patient judgment of how well we communicate.4-6 These observations are particularly true in our increasingly elderly surgical population, in which both surgeons and anesthesiologists often feel unequal to the task of discussing concepts such as code status.7,8
In our practice and in residency training, the preoperative clinic often is the location where patient/provider communication occurs. Here we consider the latest American College of Cardiology/American Heart Association guidelines, examine airways, review electrocardiograms, and formulate plans agreeable to and understood by our anxious patients and their families. The potent anxiolytic effect of a preoperative visit by an anesthesiologist is well established.9 Anxiety about surgery is a risk factor for impaired decision making before surgery.10 And surgery is traumatic—as many as 7.6% of postoperative patients experience symptoms consistent with PTSD attributable to the surgery, placing it on a par with being mugged (8.0%).11,12
The patient in this case presented several communication challenges even absent his revelation of prior traumatic experience with anesthesia. He was elderly, anxious, and had multiple comorbidities. He had mild cognitive impairment and required a code status discussion. There also were the clinical challenges—navigating a 99-year-old with severe aortic stenosis and a right ventricular systolic pressure > 90 mm Hg through a general anesthetic gave me a sinking feeling.
He was fortunate that the procedure could be done with local anesthesia, mitigating his risk of cognitive dysfunction, including delirium. He also was fortunate in that his anesthesiologist and surgeon had created a collaborative, patient-first approach and that his US Department of Veterans Affairs (VA) clinic had the time, space, and staffing to accommodate an unexpected 90-minute visit. A big investment in communication, mainly my keeping quiet, made the intraoperative management simple. Such is life in an integrated health care system without financial incentives for high-volume care—and another reminder that VA physicians are blessed to guide patients through some of the most vulnerable and distressing moments of their lives.
Postscript
During the preparation of this manuscript, the patient passed away at the age of 100. His obituary was consistent with what I had learned about him and his family during our 2 encounters: a long successful career in local industry; extensive involvement in his community; an avid sportsman; and nearly 30 grandchildren, great-grandchildren, and great-great grandchildren. But there was one more detail that never came up during my extensive discussion with him and his daughters: He was awarded the Purple Heart for his service in World War II.
1. Ghoneim MM, Block RI, Haffarnan M, Mathews MJ. Awareness during anesthesia: risk factors, causes and sequelae: a review of reported cases in the literature. Anesth Analg. 2009;108(2):527-535.
2. Aya AGM, Pouchain PH, Thomas H, Ripart J, Cuvillon P. Incidence of postoperative delirium in elderly ambulatory patients: a prospective evaluation using the FAM-CAM instrument. J Clin Anesth. 2019;53:35-38.
3. Raats JW, Steunenberg SL, de Lange DC, van der Laan L. Risk factors of post-operative delirium after elective vascular surgery in the elderly: a systematic review. Int J Surg. 2016;35:1-6.
4. Roter DL, Hall JA, Kern DE, Barker LR, Cole KA, Roca RP. Improving physicians’ interviewing skills and reducing patients’ emotional distress: a randomized clinical trial. Arch Intern Med. 1995;155(17):1877-1884.
5. Wright AA, Keating NL, Ayanian JZ, et al. Family perspectives on aggressive cancer care near the end of life. JAMA. 2016;315(3):284-292.
6. Hall JA, Roter DL, Rand CS. Communication of affect between patient and physician. J Health Soc Behav. 1981;22(1):18-30.
7. Cooper Z, Meyers M, Keating NL, Gu X, Lipsitz SR, Rogers SO. Resident education and management of end-of-life care: the resident’s perspective. J Surg Educ. 2010;67(2):79-84.
8. Hickey TR, Cooper Z, Urman RD, Hepner DL, Bader AM. An agenda for improving perioperative code status discussion. A A Case Rep. 2016;6(12):411-415.
9. Egbert LD, Battit GE, Turndorf H, Beecher HK. The value of the preoperative visit by an anesthetist. JAMA. 1963;185(7):553-555.
10. Ankuda CK, Block SD, Cooper Z, et al. Measuring critical deficits in shared decision making before elective surgery. Patient Educ Couns. 2014;94(3):328-333.
11. Whitlock EL, Rodebaugh TL, Hassett AL, et al. Psychological sequelae of surgery in a prospective cohort of patients from three intraoperative awareness prevention trials. Anesth Analg. 2015;120(1):87-95.
12. Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatry. 1998;55(7):626-632.
The patient grabbed my attention as I glanced through our clinic schedule. It was his age: He was 99 years old and scheduled for eye surgery. The plastic surgery resident’s note read: “Patient understands that this would involve surgery under general anesthesia and is agreeable to moving forward...Extremely high risk of anesthesia emphasized.”
I reviewed the patient’s history. At baseline, he had severe pulmonary hypertension, severe aortic stenosis (AS), diastolic heart failure, chronic atrial fibrillation, chronic kidney disease (estimated glomerular filtration rate of 26 mL/min [normal is > 60 mL/min]), anemia (hematocrit 26%), and a standing do not resuscitate (DNR) order. His maximal daily exercise was walking slowly across a room, primarily limited by joint pain. Recent geropsychiatry notes indicated mild cognitive impairment. The anesthesia record from an urgent hip fracture repair 7 months before under general anesthesia was unremarkable.
I phoned the attending plastic surgeon. Our conversation was as follows:
“Hi, I’m about to see a 99-year-old patient with a DNR who is scheduled for resection of an eyelid tumor. His medical history makes me nervous. Are you sure this is a good idea?”“Hmmm, 99-year-old…okay, that’s right,” he responded. “He has an invasive squamous that could become a big problem. The actual procedure is under 10 minutes. Waiting for the pathology report will be the longest part of the procedure.”
“Can it be done under local?” I asked.
“Yes,” he replied.
“Okay, I’ll talk to him and call you back.”
I found the patient in the waiting room, flanked by his 2 daughters and invited them into the clinic room. After introductions, I began asking whether they had any questions about the anesthesia. By midsentence a daughter was prompting him to discuss what happened “last time.” He described a history of posttraumatic stress disorder (PTSD) stemming from his hip surgery, which he blamed squarely on the anesthesia. His emotion was evident in the gathering pauses. “I hate that I am so emotional since they kept me awake during my surgery.”
Through the fog of multiple accounts, it became clear that he was traumatized by the loss of control during the administration of and emergence from the anesthesia.
“They told me it was only oxygen,” he said. “They lied. There was a taste to it…I was awake and skinned alive…They said I was a monster when I woke up thrashing.” He went on, explaining that in the recovery room “there were 2 people bothering me, man-handling me, asking me questions.”
One of his daughters showed me pictures of bruises on his face from ripping off the mask and pulling out the breathing tube. They were visibly upset by the memory of his postoperative combativeness and paranoia. The note written by the orthopedic surgery resident on the day after surgery stated succinctly, “Doing well, had some delirium from anesthesia overnight.” Subsequent geropsychiatry home visits attested to intrusive thoughts, flashbacks, and nightmares from his time as a combat soldier in World War II, 65 years in the past.
“It took me months…months to recover,” he said.
He was in the mood to reminisce, however, perhaps a willful distraction. He had the floor for at least 30 minutes, during which I spoke about 5 sentences. With every sad story he told there was a happy, humorous one, such as meeting his future wife while on leave in New Zealand during the war, recalled down to exact dates. And another story:
There we were in New Caledonia. All our supplies went out to replace what sank on [USS] Coolidge, including a lot of food. Well, there were deer on the island. So we took out a truck and a rifle and wouldn’t you know we came upon a roadblock in the form of a big steer. We figured it looked enough like a deer. My buddy shot it dead with one shot. We dressed it and loaded it into the jeep. Hardly before we even got back to the mess hall, the officers’ cook came sniffing around. He and our captain agreed it was easily the biggest deer they’d ever seen and appropriated it to the officers’ mess. Next day the CO [commanding officer] of the whole outfit came by and announced it was the best tasting ‘venison’ he’d ever had. I heard the farmer got paid a pretty penny for that steer. I didn’t get a damn bite.
He delivered this last bit with relish.
When the conversation returned to anesthesia, I read them the record of his hip fracture repair. I explained that on the face of it, the report seemed uneventful. One daughter asked astute questions about his awareness. I explained that although awareness during general anesthesia is possible, it seemed from the record, he’d had plenty of anesthesia during the case and that there is always less at the beginning and end, the periods that apparently had caused him distress. I also explained that most studies report the incidence of true awareness as at most 1 out of thousands of events and that he had none of the established risk factors for it, such as female gender, young age, chronic substance abuse, cardiac and obstetric surgery, and history of awareness.1
The other daughter wondered why he was so agitated afterward. I recited data on the frequency of postoperative delirium in elderly patients but explained that the range is wide, depending on the study and population, from about 1% in elderly patients undergoing ambulatory surgery to 65% for open aortic surgery.2,3 I added that their father had 2 of the strongest risk factors for delirium, advanced age and cognitive impairment.3 Only after airing each question about the hip surgery in detail were they ready to discuss the eye surgery.
He started that conversation with the right question: “Do I really need it?”
I quoted my surgical colleague’s concern. I told him that, should he opt to undergo the surgery, I was confident that this time around his experience would be different from the last.
“If you’re okay with it, all you need is some numbing medicine from the surgeon; you won’t need any anesthesia from me.”
I walked step-by-step through what they could expect on the day of surgery. Maintaining control was of obvious importance to him. He felt comfortable going forward. His daughters intuited that less would be more for a quick recovery.
We then addressed the DNR directive. I acknowledged his absolute right to self-determination and explained that the need for resuscitation is, at times, a consequence of the surgery and anesthesia. I reassured them that our plan made resuscitation and intubation highly unlikely. They also asked to use any interventions necessary to restart his heart if it should stop beating. I documented their decision in my notes and communicated it to the surgical team. We had talked for 90 minutes.
I met the patient and his daughters on the day of surgery in the preoperative holding area. I inserted an IV, applied electrocardiography leads, and affixed a pulse oximeter and a noninvasive blood pressure cuff. In the operating room (OR) we took time to place his 99-year-old joints into, as he said, the “least worst” position. He tolerated the injection of the local by the surgeon perfectly well. We were in the OR for 3 hours, during which he taught me a fair amount about boating and outboard engines among other things. Pathology reported clean margins. He was discharged home soon after and had an uneventful recovery.
Patient-First Approach
A core competency of the Accreditation Council for Graduate Medical Education for an anesthesia residency is the Interpersonal and Communication Skills program. A comprehensive discussion of communication is far beyond the scope here. But not surprisingly, deficient communication between physicians and patients can cause emotional distress, significant dissatisfaction among family members, and negative patient judgment of how well we communicate.4-6 These observations are particularly true in our increasingly elderly surgical population, in which both surgeons and anesthesiologists often feel unequal to the task of discussing concepts such as code status.7,8
In our practice and in residency training, the preoperative clinic often is the location where patient/provider communication occurs. Here we consider the latest American College of Cardiology/American Heart Association guidelines, examine airways, review electrocardiograms, and formulate plans agreeable to and understood by our anxious patients and their families. The potent anxiolytic effect of a preoperative visit by an anesthesiologist is well established.9 Anxiety about surgery is a risk factor for impaired decision making before surgery.10 And surgery is traumatic—as many as 7.6% of postoperative patients experience symptoms consistent with PTSD attributable to the surgery, placing it on a par with being mugged (8.0%).11,12
The patient in this case presented several communication challenges even absent his revelation of prior traumatic experience with anesthesia. He was elderly, anxious, and had multiple comorbidities. He had mild cognitive impairment and required a code status discussion. There also were the clinical challenges—navigating a 99-year-old with severe aortic stenosis and a right ventricular systolic pressure > 90 mm Hg through a general anesthetic gave me a sinking feeling.
He was fortunate that the procedure could be done with local anesthesia, mitigating his risk of cognitive dysfunction, including delirium. He also was fortunate in that his anesthesiologist and surgeon had created a collaborative, patient-first approach and that his US Department of Veterans Affairs (VA) clinic had the time, space, and staffing to accommodate an unexpected 90-minute visit. A big investment in communication, mainly my keeping quiet, made the intraoperative management simple. Such is life in an integrated health care system without financial incentives for high-volume care—and another reminder that VA physicians are blessed to guide patients through some of the most vulnerable and distressing moments of their lives.
Postscript
During the preparation of this manuscript, the patient passed away at the age of 100. His obituary was consistent with what I had learned about him and his family during our 2 encounters: a long successful career in local industry; extensive involvement in his community; an avid sportsman; and nearly 30 grandchildren, great-grandchildren, and great-great grandchildren. But there was one more detail that never came up during my extensive discussion with him and his daughters: He was awarded the Purple Heart for his service in World War II.
The patient grabbed my attention as I glanced through our clinic schedule. It was his age: He was 99 years old and scheduled for eye surgery. The plastic surgery resident’s note read: “Patient understands that this would involve surgery under general anesthesia and is agreeable to moving forward...Extremely high risk of anesthesia emphasized.”
I reviewed the patient’s history. At baseline, he had severe pulmonary hypertension, severe aortic stenosis (AS), diastolic heart failure, chronic atrial fibrillation, chronic kidney disease (estimated glomerular filtration rate of 26 mL/min [normal is > 60 mL/min]), anemia (hematocrit 26%), and a standing do not resuscitate (DNR) order. His maximal daily exercise was walking slowly across a room, primarily limited by joint pain. Recent geropsychiatry notes indicated mild cognitive impairment. The anesthesia record from an urgent hip fracture repair 7 months before under general anesthesia was unremarkable.
I phoned the attending plastic surgeon. Our conversation was as follows:
“Hi, I’m about to see a 99-year-old patient with a DNR who is scheduled for resection of an eyelid tumor. His medical history makes me nervous. Are you sure this is a good idea?”“Hmmm, 99-year-old…okay, that’s right,” he responded. “He has an invasive squamous that could become a big problem. The actual procedure is under 10 minutes. Waiting for the pathology report will be the longest part of the procedure.”
“Can it be done under local?” I asked.
“Yes,” he replied.
“Okay, I’ll talk to him and call you back.”
I found the patient in the waiting room, flanked by his 2 daughters and invited them into the clinic room. After introductions, I began asking whether they had any questions about the anesthesia. By midsentence a daughter was prompting him to discuss what happened “last time.” He described a history of posttraumatic stress disorder (PTSD) stemming from his hip surgery, which he blamed squarely on the anesthesia. His emotion was evident in the gathering pauses. “I hate that I am so emotional since they kept me awake during my surgery.”
Through the fog of multiple accounts, it became clear that he was traumatized by the loss of control during the administration of and emergence from the anesthesia.
“They told me it was only oxygen,” he said. “They lied. There was a taste to it…I was awake and skinned alive…They said I was a monster when I woke up thrashing.” He went on, explaining that in the recovery room “there were 2 people bothering me, man-handling me, asking me questions.”
One of his daughters showed me pictures of bruises on his face from ripping off the mask and pulling out the breathing tube. They were visibly upset by the memory of his postoperative combativeness and paranoia. The note written by the orthopedic surgery resident on the day after surgery stated succinctly, “Doing well, had some delirium from anesthesia overnight.” Subsequent geropsychiatry home visits attested to intrusive thoughts, flashbacks, and nightmares from his time as a combat soldier in World War II, 65 years in the past.
“It took me months…months to recover,” he said.
He was in the mood to reminisce, however, perhaps a willful distraction. He had the floor for at least 30 minutes, during which I spoke about 5 sentences. With every sad story he told there was a happy, humorous one, such as meeting his future wife while on leave in New Zealand during the war, recalled down to exact dates. And another story:
There we were in New Caledonia. All our supplies went out to replace what sank on [USS] Coolidge, including a lot of food. Well, there were deer on the island. So we took out a truck and a rifle and wouldn’t you know we came upon a roadblock in the form of a big steer. We figured it looked enough like a deer. My buddy shot it dead with one shot. We dressed it and loaded it into the jeep. Hardly before we even got back to the mess hall, the officers’ cook came sniffing around. He and our captain agreed it was easily the biggest deer they’d ever seen and appropriated it to the officers’ mess. Next day the CO [commanding officer] of the whole outfit came by and announced it was the best tasting ‘venison’ he’d ever had. I heard the farmer got paid a pretty penny for that steer. I didn’t get a damn bite.
He delivered this last bit with relish.
When the conversation returned to anesthesia, I read them the record of his hip fracture repair. I explained that on the face of it, the report seemed uneventful. One daughter asked astute questions about his awareness. I explained that although awareness during general anesthesia is possible, it seemed from the record, he’d had plenty of anesthesia during the case and that there is always less at the beginning and end, the periods that apparently had caused him distress. I also explained that most studies report the incidence of true awareness as at most 1 out of thousands of events and that he had none of the established risk factors for it, such as female gender, young age, chronic substance abuse, cardiac and obstetric surgery, and history of awareness.1
The other daughter wondered why he was so agitated afterward. I recited data on the frequency of postoperative delirium in elderly patients but explained that the range is wide, depending on the study and population, from about 1% in elderly patients undergoing ambulatory surgery to 65% for open aortic surgery.2,3 I added that their father had 2 of the strongest risk factors for delirium, advanced age and cognitive impairment.3 Only after airing each question about the hip surgery in detail were they ready to discuss the eye surgery.
He started that conversation with the right question: “Do I really need it?”
I quoted my surgical colleague’s concern. I told him that, should he opt to undergo the surgery, I was confident that this time around his experience would be different from the last.
“If you’re okay with it, all you need is some numbing medicine from the surgeon; you won’t need any anesthesia from me.”
I walked step-by-step through what they could expect on the day of surgery. Maintaining control was of obvious importance to him. He felt comfortable going forward. His daughters intuited that less would be more for a quick recovery.
We then addressed the DNR directive. I acknowledged his absolute right to self-determination and explained that the need for resuscitation is, at times, a consequence of the surgery and anesthesia. I reassured them that our plan made resuscitation and intubation highly unlikely. They also asked to use any interventions necessary to restart his heart if it should stop beating. I documented their decision in my notes and communicated it to the surgical team. We had talked for 90 minutes.
I met the patient and his daughters on the day of surgery in the preoperative holding area. I inserted an IV, applied electrocardiography leads, and affixed a pulse oximeter and a noninvasive blood pressure cuff. In the operating room (OR) we took time to place his 99-year-old joints into, as he said, the “least worst” position. He tolerated the injection of the local by the surgeon perfectly well. We were in the OR for 3 hours, during which he taught me a fair amount about boating and outboard engines among other things. Pathology reported clean margins. He was discharged home soon after and had an uneventful recovery.
Patient-First Approach
A core competency of the Accreditation Council for Graduate Medical Education for an anesthesia residency is the Interpersonal and Communication Skills program. A comprehensive discussion of communication is far beyond the scope here. But not surprisingly, deficient communication between physicians and patients can cause emotional distress, significant dissatisfaction among family members, and negative patient judgment of how well we communicate.4-6 These observations are particularly true in our increasingly elderly surgical population, in which both surgeons and anesthesiologists often feel unequal to the task of discussing concepts such as code status.7,8
In our practice and in residency training, the preoperative clinic often is the location where patient/provider communication occurs. Here we consider the latest American College of Cardiology/American Heart Association guidelines, examine airways, review electrocardiograms, and formulate plans agreeable to and understood by our anxious patients and their families. The potent anxiolytic effect of a preoperative visit by an anesthesiologist is well established.9 Anxiety about surgery is a risk factor for impaired decision making before surgery.10 And surgery is traumatic—as many as 7.6% of postoperative patients experience symptoms consistent with PTSD attributable to the surgery, placing it on a par with being mugged (8.0%).11,12
The patient in this case presented several communication challenges even absent his revelation of prior traumatic experience with anesthesia. He was elderly, anxious, and had multiple comorbidities. He had mild cognitive impairment and required a code status discussion. There also were the clinical challenges—navigating a 99-year-old with severe aortic stenosis and a right ventricular systolic pressure > 90 mm Hg through a general anesthetic gave me a sinking feeling.
He was fortunate that the procedure could be done with local anesthesia, mitigating his risk of cognitive dysfunction, including delirium. He also was fortunate in that his anesthesiologist and surgeon had created a collaborative, patient-first approach and that his US Department of Veterans Affairs (VA) clinic had the time, space, and staffing to accommodate an unexpected 90-minute visit. A big investment in communication, mainly my keeping quiet, made the intraoperative management simple. Such is life in an integrated health care system without financial incentives for high-volume care—and another reminder that VA physicians are blessed to guide patients through some of the most vulnerable and distressing moments of their lives.
Postscript
During the preparation of this manuscript, the patient passed away at the age of 100. His obituary was consistent with what I had learned about him and his family during our 2 encounters: a long successful career in local industry; extensive involvement in his community; an avid sportsman; and nearly 30 grandchildren, great-grandchildren, and great-great grandchildren. But there was one more detail that never came up during my extensive discussion with him and his daughters: He was awarded the Purple Heart for his service in World War II.
1. Ghoneim MM, Block RI, Haffarnan M, Mathews MJ. Awareness during anesthesia: risk factors, causes and sequelae: a review of reported cases in the literature. Anesth Analg. 2009;108(2):527-535.
2. Aya AGM, Pouchain PH, Thomas H, Ripart J, Cuvillon P. Incidence of postoperative delirium in elderly ambulatory patients: a prospective evaluation using the FAM-CAM instrument. J Clin Anesth. 2019;53:35-38.
3. Raats JW, Steunenberg SL, de Lange DC, van der Laan L. Risk factors of post-operative delirium after elective vascular surgery in the elderly: a systematic review. Int J Surg. 2016;35:1-6.
4. Roter DL, Hall JA, Kern DE, Barker LR, Cole KA, Roca RP. Improving physicians’ interviewing skills and reducing patients’ emotional distress: a randomized clinical trial. Arch Intern Med. 1995;155(17):1877-1884.
5. Wright AA, Keating NL, Ayanian JZ, et al. Family perspectives on aggressive cancer care near the end of life. JAMA. 2016;315(3):284-292.
6. Hall JA, Roter DL, Rand CS. Communication of affect between patient and physician. J Health Soc Behav. 1981;22(1):18-30.
7. Cooper Z, Meyers M, Keating NL, Gu X, Lipsitz SR, Rogers SO. Resident education and management of end-of-life care: the resident’s perspective. J Surg Educ. 2010;67(2):79-84.
8. Hickey TR, Cooper Z, Urman RD, Hepner DL, Bader AM. An agenda for improving perioperative code status discussion. A A Case Rep. 2016;6(12):411-415.
9. Egbert LD, Battit GE, Turndorf H, Beecher HK. The value of the preoperative visit by an anesthetist. JAMA. 1963;185(7):553-555.
10. Ankuda CK, Block SD, Cooper Z, et al. Measuring critical deficits in shared decision making before elective surgery. Patient Educ Couns. 2014;94(3):328-333.
11. Whitlock EL, Rodebaugh TL, Hassett AL, et al. Psychological sequelae of surgery in a prospective cohort of patients from three intraoperative awareness prevention trials. Anesth Analg. 2015;120(1):87-95.
12. Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatry. 1998;55(7):626-632.
1. Ghoneim MM, Block RI, Haffarnan M, Mathews MJ. Awareness during anesthesia: risk factors, causes and sequelae: a review of reported cases in the literature. Anesth Analg. 2009;108(2):527-535.
2. Aya AGM, Pouchain PH, Thomas H, Ripart J, Cuvillon P. Incidence of postoperative delirium in elderly ambulatory patients: a prospective evaluation using the FAM-CAM instrument. J Clin Anesth. 2019;53:35-38.
3. Raats JW, Steunenberg SL, de Lange DC, van der Laan L. Risk factors of post-operative delirium after elective vascular surgery in the elderly: a systematic review. Int J Surg. 2016;35:1-6.
4. Roter DL, Hall JA, Kern DE, Barker LR, Cole KA, Roca RP. Improving physicians’ interviewing skills and reducing patients’ emotional distress: a randomized clinical trial. Arch Intern Med. 1995;155(17):1877-1884.
5. Wright AA, Keating NL, Ayanian JZ, et al. Family perspectives on aggressive cancer care near the end of life. JAMA. 2016;315(3):284-292.
6. Hall JA, Roter DL, Rand CS. Communication of affect between patient and physician. J Health Soc Behav. 1981;22(1):18-30.
7. Cooper Z, Meyers M, Keating NL, Gu X, Lipsitz SR, Rogers SO. Resident education and management of end-of-life care: the resident’s perspective. J Surg Educ. 2010;67(2):79-84.
8. Hickey TR, Cooper Z, Urman RD, Hepner DL, Bader AM. An agenda for improving perioperative code status discussion. A A Case Rep. 2016;6(12):411-415.
9. Egbert LD, Battit GE, Turndorf H, Beecher HK. The value of the preoperative visit by an anesthetist. JAMA. 1963;185(7):553-555.
10. Ankuda CK, Block SD, Cooper Z, et al. Measuring critical deficits in shared decision making before elective surgery. Patient Educ Couns. 2014;94(3):328-333.
11. Whitlock EL, Rodebaugh TL, Hassett AL, et al. Psychological sequelae of surgery in a prospective cohort of patients from three intraoperative awareness prevention trials. Anesth Analg. 2015;120(1):87-95.
12. Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatry. 1998;55(7):626-632.
Portrayal of Federal Endoscopy Technology
To the Editor: I was excited to see that in the latest issue of Federal Practitioner there is an article titled “Unrelated Death After Colorectal Cancer Screening: Implications for Improving Colonoscopy Referrals.”1 In fact, it made the cover! But your cover image showed what appears to be an ancient (an ancient artifact, perhaps)—did I mention ancient?—fiber-optic endoscope. Fiber-optic endoscopes haven’t been used in maybe 20 years. High-definition endoscopy is the standard of care. Before that it was standard definition. The cover image suggests that federal endoscopists may be using museum-quality colonoscopes, which I know is not the case. I just wanted to point out what I found to be humorous.
Thank you for opportunity to share my opinion.
CDR R. Daniel Lawson, MD, MC, USN
Head, Endoscopy
Naval Medical Center San Diego
Owner, Lawson GI LLC
Gastroenterologist
Response: Dr. Lawson, thank you for your concern. The image in question was selected by myself and the art director and not the authors of the article in question, purely for its recognizable and iconic nature. The image was in no way meant to portray the current state of the technology used at federal facilities. We regret that it may have confused or misled any readers about the current standard of endoscopy care. In the future we will retire such images to the museums where they belong.
Reid A. Paul, MA
Editor
1. Gawron A, Bielefeldt K. Unrelated death after colorectal cancer screening: implications for improving colonoscopy referrals. Fed Pract. 2019;36(6):262-270.
To the Editor: I was excited to see that in the latest issue of Federal Practitioner there is an article titled “Unrelated Death After Colorectal Cancer Screening: Implications for Improving Colonoscopy Referrals.”1 In fact, it made the cover! But your cover image showed what appears to be an ancient (an ancient artifact, perhaps)—did I mention ancient?—fiber-optic endoscope. Fiber-optic endoscopes haven’t been used in maybe 20 years. High-definition endoscopy is the standard of care. Before that it was standard definition. The cover image suggests that federal endoscopists may be using museum-quality colonoscopes, which I know is not the case. I just wanted to point out what I found to be humorous.
Thank you for opportunity to share my opinion.
CDR R. Daniel Lawson, MD, MC, USN
Head, Endoscopy
Naval Medical Center San Diego
Owner, Lawson GI LLC
Gastroenterologist
Response: Dr. Lawson, thank you for your concern. The image in question was selected by myself and the art director and not the authors of the article in question, purely for its recognizable and iconic nature. The image was in no way meant to portray the current state of the technology used at federal facilities. We regret that it may have confused or misled any readers about the current standard of endoscopy care. In the future we will retire such images to the museums where they belong.
Reid A. Paul, MA
Editor
To the Editor: I was excited to see that in the latest issue of Federal Practitioner there is an article titled “Unrelated Death After Colorectal Cancer Screening: Implications for Improving Colonoscopy Referrals.”1 In fact, it made the cover! But your cover image showed what appears to be an ancient (an ancient artifact, perhaps)—did I mention ancient?—fiber-optic endoscope. Fiber-optic endoscopes haven’t been used in maybe 20 years. High-definition endoscopy is the standard of care. Before that it was standard definition. The cover image suggests that federal endoscopists may be using museum-quality colonoscopes, which I know is not the case. I just wanted to point out what I found to be humorous.
Thank you for opportunity to share my opinion.
CDR R. Daniel Lawson, MD, MC, USN
Head, Endoscopy
Naval Medical Center San Diego
Owner, Lawson GI LLC
Gastroenterologist
Response: Dr. Lawson, thank you for your concern. The image in question was selected by myself and the art director and not the authors of the article in question, purely for its recognizable and iconic nature. The image was in no way meant to portray the current state of the technology used at federal facilities. We regret that it may have confused or misled any readers about the current standard of endoscopy care. In the future we will retire such images to the museums where they belong.
Reid A. Paul, MA
Editor
1. Gawron A, Bielefeldt K. Unrelated death after colorectal cancer screening: implications for improving colonoscopy referrals. Fed Pract. 2019;36(6):262-270.
1. Gawron A, Bielefeldt K. Unrelated death after colorectal cancer screening: implications for improving colonoscopy referrals. Fed Pract. 2019;36(6):262-270.
Of God and Country
Whoever seeks to set one religion against another seeks to destroy all religion.1
President Franklin D. Roosevelt
Recently, a US Department of Veterans Affairs (VA) colleague knowing of my background in religious studies asked me what I thought of the recent change in VA religious policy. VA Secretary Robert Wilke had announced on July 3 that VA was revising its policies on religious symbols at all VA facilities and religious and pastoral care in the Veterans Health Administration, respectively.2,3 A news release from the VA Office of Public and Intergovernmental Affairs designated the changes as an “overhaul.”4
The revisions in these VA directives are designed to address confusion and inconsistency regarding displays of religious matters, not just between different VA medical centers (VAMCs) but even within a single facility. From my decades as a federal practitioner and ethicist, I can attest to the confusion. I have heard or read from staff and leaders of VAMCs everything from “VA prohibits all religious symbols so take that Christmas tree down” to “it is fine to host holiday parties complete with decorations.” There certainly was a need for clarity, transparency, and fairness in VA policy regarding religious and spiritual symbolism. This editorial will discuss how, why, and whether the policy accomplishes this organizational ethics purpose.
The new policies have 3 aims: (1) to permit VA facilities to publicly display religious content in appropriate circumstances; (2) to allow patients and their guests to request and receive religious literature, sacred texts, and spiritual symbols during visits to VA chapels or episodes of treatment; and (3) to permit VA facilities to receive and dispense donations of religious literature, cards, and symbols to VA patrons under appropriate circumstances or when they ask for them.
Secretary Wilke announced the aim of the revised directives: “These important changes will bring simplicity and clarity to our policies governing religious and spiritual symbols, helping ensure we are consistently complying with the First Amendment to the US Constitution at thousands of facilities across the department.”4 As with most US Department of Defense (DoD) and VA decisions about potentially controversial issues, this one has a backstory involving 2 high-profile court cases that provide a deeper understanding of the subtext of the policy change.
In February 2019, the US Supreme Court heard oral arguments for The American Legion v American Humanist Association, the most recent of a long line of important cases about the First Amendment and its freedom of religion guarantee.5 This case involved veterans—although not the VA or DoD—and is of prima facie interest for those invested or interested in the VA’s position on religion. A 40-foot cross had stood in a veteran memorial park in Bladensburg, Maryland, for decades. In the 1960s the park became the property of the Maryland National Capital Park and Planning Commission (MNCPPC), which assumed the responsibility for upkeep for the cross at considerable expense. The American Humanist Association, an organization advocating for church-state separation, sued the MNCPPC on the grounds it violated the establishment clause of the First Amendment by promoting Christianity as a federally supported religion.
The US District Court found in favor of MNCPPC, but an appeals court reversed that decision. The American Legion, a major force in VA politics, joined MNCPPC to appeal the case to the Supreme Court. The Court issued a 7 to 2 decision, which ruled that the cross did not violate the establishment clause. Even though the cross began as religious symbol, with the passage of time the High Court opined that the cross had become a historic memorial honoring those who fought in the First World War, which rose above its purely Christian meaning.5
The American Legion website explicitly credited their success before the Supreme Court as the impetus for VA policy changes.6 Hence, from the perspective of VA leadership, this wider latitude for religious expression, which the revised policy now allows, renderings VA practice consonant with the authoritative interpreters of constitutional law—the highest court in the land.
Of course, on a question that has been so divisive for the nation since its founding, there are many who protest this extension of religious liberty in the federal health care system. Veterans stand tall on both sides of this divide. In May 2019 a US Air Force veteran filed a federal lawsuit against the Manchester VAMC director asking the court to remove a Christian Bible from a public display.
Air Force Times compared the resulting melee to actual combat!7 As with the first case, such legal battles are ripe territory for advocacy and lobbying organizations of all political stripes to weigh in while promoting their own ideologic agendas. The Military Religious Freedom Foundation assumed the mantle on behalf of the Air Force veteran in the Manchester suit. The news media reported that the plaintiff in the case identified himself as a committed Christian. According to the news reports, what worried this veteran was the same thing that troubled President Roosevelt in 1940: By featuring the Christian Bible, the VA excluded other faith groups.1 Other veterans and some veteran religious organizations objected just as strenuously to its removal, likely done to reduce potential for violence. Veterans opposing the inclusion of the Bible in the display also grounded their arguments in the First Amendment clause that prohibits the federal government from establishing or favoring any religion.8
Presumptively, displays of such religious symbols may well be supported in VA policy as a protected expression of religion, which Secretary Wilke stated was the other primary aim of the revisions. “We want to make sure that all of our veterans and their families feel welcome at VA, no matter their religious beliefs. Protecting religious liberty is a key part of how we accomplish that goal.”4
In the middle of this sensitive controversy are the many veterans and their families that third parties—for profit, for politics, for publicity—have far too often manipulated for their own purposes. If you want to get an idea of the scope of these diverse stakeholders, just peruse the amicus briefs submitted to the Supreme Court on both sides of the issues in The American Legion v American Humanist Association.8
VA data show that veterans while being more religious than the general public are religiously diverse: 2015 data on the religion of veterans in every state listed 13 different faith communities.9 My response to the colleague who asked me about my opinion of the VA policies changes was based on the background narrative recounted here. My rsponse, in light of Roosevelt’s concern and this snippet of a much larger swath of legal machinations, is the change in the VA policy is reasonable as long as it “has room for the expression of those whose trust is in God, in country, in neither, and in both.” We know from research that religion is a strength and a support to many veterans and that spirituality as an aspect of psychological therapies and pastoral counseling has shown healing power for the wounds of war.10 Yet we also know that religiously based hatred and discrimination are among the most divisive and destructive forces that threaten our democracy. Let’s all hope—and those who pray do so—that these policy changes deter the latter and promote the former.
1. Roosevelt FD. The Public Papers and Addresses of Franklin D. Roosevelt. 1940 volume, War-and Aid to Democracies: With a Special Introduction and Explanatory Notes by President Roosevelt [Book 1]. New York: Macmillan; 1941:537.
2. US Department of Veterans Affairs, Veterans Health Administration. VA Directive 0022: Religious symbols in VA facilities. https://www.va.gov/vapubs/viewPublication.asp?Pub_ID=849. Published July 3, 2019. Accessed July 18, 2019.
3. US Department of Veterans Affairs, Veterans Health Administration. SVA Directive 1111(1): Spiritual and pastoral care in the Veterans Health Administration. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=4299. Published November 22, 2016. Amended July 3, 2019. Accessed July 22, 2019.
4. VA Office of Public and Intergovernmental Affairs. VA overhauls religious and spiritual symbol policies to protect religious liberty. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5279. Updated July 3, 2019. Accessed July 22, 2019.
5. Oyez. The American Legion v American Humanist Association. www.oyez.org/cases/2018/17-1717. Accessed July 16, 2019.
6. The American Legion. Legion salutes VA policy change for religious freedom. https://www.legion.org/honor/246151/legion-salutes-va-policy-change-victory-religious-freedom. Published July 03, 2019. Accessed July 22, 2019.
7. Miller K. Lawsuit filed over Bible display at New Hampshire VA Hospital; uproar ensues. https://www.airforcetimes.com/news/your-military/2019/05/07/lawsuit-filed-over-bible-display-at-new-hampshire-va-hospital-uproar-ensues. Published May 7, 2019. Accessed July 22, 2019.
8. Scotusblog. The American Legion v American Humanist Association. https://www.scotusblog.com/case-files/cases/the-american-legion-v-american-humanist-association. Accessed July 22, 2019.
9. US Department of Veterans Affairs. Veterans religions by state 2015. https://www.va.gov/vetdata/docs/SpecialReports/Veterans_Religion_by_State.xlsx. Accessed July 22, 2019.
10. Smothers ZPW. Koenig HG. Spiritual interventions in veterans with PTSD: a systematic review. J Relig Health. 2018;57(5):2033-2048.
Whoever seeks to set one religion against another seeks to destroy all religion.1
President Franklin D. Roosevelt
Recently, a US Department of Veterans Affairs (VA) colleague knowing of my background in religious studies asked me what I thought of the recent change in VA religious policy. VA Secretary Robert Wilke had announced on July 3 that VA was revising its policies on religious symbols at all VA facilities and religious and pastoral care in the Veterans Health Administration, respectively.2,3 A news release from the VA Office of Public and Intergovernmental Affairs designated the changes as an “overhaul.”4
The revisions in these VA directives are designed to address confusion and inconsistency regarding displays of religious matters, not just between different VA medical centers (VAMCs) but even within a single facility. From my decades as a federal practitioner and ethicist, I can attest to the confusion. I have heard or read from staff and leaders of VAMCs everything from “VA prohibits all religious symbols so take that Christmas tree down” to “it is fine to host holiday parties complete with decorations.” There certainly was a need for clarity, transparency, and fairness in VA policy regarding religious and spiritual symbolism. This editorial will discuss how, why, and whether the policy accomplishes this organizational ethics purpose.
The new policies have 3 aims: (1) to permit VA facilities to publicly display religious content in appropriate circumstances; (2) to allow patients and their guests to request and receive religious literature, sacred texts, and spiritual symbols during visits to VA chapels or episodes of treatment; and (3) to permit VA facilities to receive and dispense donations of religious literature, cards, and symbols to VA patrons under appropriate circumstances or when they ask for them.
Secretary Wilke announced the aim of the revised directives: “These important changes will bring simplicity and clarity to our policies governing religious and spiritual symbols, helping ensure we are consistently complying with the First Amendment to the US Constitution at thousands of facilities across the department.”4 As with most US Department of Defense (DoD) and VA decisions about potentially controversial issues, this one has a backstory involving 2 high-profile court cases that provide a deeper understanding of the subtext of the policy change.
In February 2019, the US Supreme Court heard oral arguments for The American Legion v American Humanist Association, the most recent of a long line of important cases about the First Amendment and its freedom of religion guarantee.5 This case involved veterans—although not the VA or DoD—and is of prima facie interest for those invested or interested in the VA’s position on religion. A 40-foot cross had stood in a veteran memorial park in Bladensburg, Maryland, for decades. In the 1960s the park became the property of the Maryland National Capital Park and Planning Commission (MNCPPC), which assumed the responsibility for upkeep for the cross at considerable expense. The American Humanist Association, an organization advocating for church-state separation, sued the MNCPPC on the grounds it violated the establishment clause of the First Amendment by promoting Christianity as a federally supported religion.
The US District Court found in favor of MNCPPC, but an appeals court reversed that decision. The American Legion, a major force in VA politics, joined MNCPPC to appeal the case to the Supreme Court. The Court issued a 7 to 2 decision, which ruled that the cross did not violate the establishment clause. Even though the cross began as religious symbol, with the passage of time the High Court opined that the cross had become a historic memorial honoring those who fought in the First World War, which rose above its purely Christian meaning.5
The American Legion website explicitly credited their success before the Supreme Court as the impetus for VA policy changes.6 Hence, from the perspective of VA leadership, this wider latitude for religious expression, which the revised policy now allows, renderings VA practice consonant with the authoritative interpreters of constitutional law—the highest court in the land.
Of course, on a question that has been so divisive for the nation since its founding, there are many who protest this extension of religious liberty in the federal health care system. Veterans stand tall on both sides of this divide. In May 2019 a US Air Force veteran filed a federal lawsuit against the Manchester VAMC director asking the court to remove a Christian Bible from a public display.
Air Force Times compared the resulting melee to actual combat!7 As with the first case, such legal battles are ripe territory for advocacy and lobbying organizations of all political stripes to weigh in while promoting their own ideologic agendas. The Military Religious Freedom Foundation assumed the mantle on behalf of the Air Force veteran in the Manchester suit. The news media reported that the plaintiff in the case identified himself as a committed Christian. According to the news reports, what worried this veteran was the same thing that troubled President Roosevelt in 1940: By featuring the Christian Bible, the VA excluded other faith groups.1 Other veterans and some veteran religious organizations objected just as strenuously to its removal, likely done to reduce potential for violence. Veterans opposing the inclusion of the Bible in the display also grounded their arguments in the First Amendment clause that prohibits the federal government from establishing or favoring any religion.8
Presumptively, displays of such religious symbols may well be supported in VA policy as a protected expression of religion, which Secretary Wilke stated was the other primary aim of the revisions. “We want to make sure that all of our veterans and their families feel welcome at VA, no matter their religious beliefs. Protecting religious liberty is a key part of how we accomplish that goal.”4
In the middle of this sensitive controversy are the many veterans and their families that third parties—for profit, for politics, for publicity—have far too often manipulated for their own purposes. If you want to get an idea of the scope of these diverse stakeholders, just peruse the amicus briefs submitted to the Supreme Court on both sides of the issues in The American Legion v American Humanist Association.8
VA data show that veterans while being more religious than the general public are religiously diverse: 2015 data on the religion of veterans in every state listed 13 different faith communities.9 My response to the colleague who asked me about my opinion of the VA policies changes was based on the background narrative recounted here. My rsponse, in light of Roosevelt’s concern and this snippet of a much larger swath of legal machinations, is the change in the VA policy is reasonable as long as it “has room for the expression of those whose trust is in God, in country, in neither, and in both.” We know from research that religion is a strength and a support to many veterans and that spirituality as an aspect of psychological therapies and pastoral counseling has shown healing power for the wounds of war.10 Yet we also know that religiously based hatred and discrimination are among the most divisive and destructive forces that threaten our democracy. Let’s all hope—and those who pray do so—that these policy changes deter the latter and promote the former.
Whoever seeks to set one religion against another seeks to destroy all religion.1
President Franklin D. Roosevelt
Recently, a US Department of Veterans Affairs (VA) colleague knowing of my background in religious studies asked me what I thought of the recent change in VA religious policy. VA Secretary Robert Wilke had announced on July 3 that VA was revising its policies on religious symbols at all VA facilities and religious and pastoral care in the Veterans Health Administration, respectively.2,3 A news release from the VA Office of Public and Intergovernmental Affairs designated the changes as an “overhaul.”4
The revisions in these VA directives are designed to address confusion and inconsistency regarding displays of religious matters, not just between different VA medical centers (VAMCs) but even within a single facility. From my decades as a federal practitioner and ethicist, I can attest to the confusion. I have heard or read from staff and leaders of VAMCs everything from “VA prohibits all religious symbols so take that Christmas tree down” to “it is fine to host holiday parties complete with decorations.” There certainly was a need for clarity, transparency, and fairness in VA policy regarding religious and spiritual symbolism. This editorial will discuss how, why, and whether the policy accomplishes this organizational ethics purpose.
The new policies have 3 aims: (1) to permit VA facilities to publicly display religious content in appropriate circumstances; (2) to allow patients and their guests to request and receive religious literature, sacred texts, and spiritual symbols during visits to VA chapels or episodes of treatment; and (3) to permit VA facilities to receive and dispense donations of religious literature, cards, and symbols to VA patrons under appropriate circumstances or when they ask for them.
Secretary Wilke announced the aim of the revised directives: “These important changes will bring simplicity and clarity to our policies governing religious and spiritual symbols, helping ensure we are consistently complying with the First Amendment to the US Constitution at thousands of facilities across the department.”4 As with most US Department of Defense (DoD) and VA decisions about potentially controversial issues, this one has a backstory involving 2 high-profile court cases that provide a deeper understanding of the subtext of the policy change.
In February 2019, the US Supreme Court heard oral arguments for The American Legion v American Humanist Association, the most recent of a long line of important cases about the First Amendment and its freedom of religion guarantee.5 This case involved veterans—although not the VA or DoD—and is of prima facie interest for those invested or interested in the VA’s position on religion. A 40-foot cross had stood in a veteran memorial park in Bladensburg, Maryland, for decades. In the 1960s the park became the property of the Maryland National Capital Park and Planning Commission (MNCPPC), which assumed the responsibility for upkeep for the cross at considerable expense. The American Humanist Association, an organization advocating for church-state separation, sued the MNCPPC on the grounds it violated the establishment clause of the First Amendment by promoting Christianity as a federally supported religion.
The US District Court found in favor of MNCPPC, but an appeals court reversed that decision. The American Legion, a major force in VA politics, joined MNCPPC to appeal the case to the Supreme Court. The Court issued a 7 to 2 decision, which ruled that the cross did not violate the establishment clause. Even though the cross began as religious symbol, with the passage of time the High Court opined that the cross had become a historic memorial honoring those who fought in the First World War, which rose above its purely Christian meaning.5
The American Legion website explicitly credited their success before the Supreme Court as the impetus for VA policy changes.6 Hence, from the perspective of VA leadership, this wider latitude for religious expression, which the revised policy now allows, renderings VA practice consonant with the authoritative interpreters of constitutional law—the highest court in the land.
Of course, on a question that has been so divisive for the nation since its founding, there are many who protest this extension of religious liberty in the federal health care system. Veterans stand tall on both sides of this divide. In May 2019 a US Air Force veteran filed a federal lawsuit against the Manchester VAMC director asking the court to remove a Christian Bible from a public display.
Air Force Times compared the resulting melee to actual combat!7 As with the first case, such legal battles are ripe territory for advocacy and lobbying organizations of all political stripes to weigh in while promoting their own ideologic agendas. The Military Religious Freedom Foundation assumed the mantle on behalf of the Air Force veteran in the Manchester suit. The news media reported that the plaintiff in the case identified himself as a committed Christian. According to the news reports, what worried this veteran was the same thing that troubled President Roosevelt in 1940: By featuring the Christian Bible, the VA excluded other faith groups.1 Other veterans and some veteran religious organizations objected just as strenuously to its removal, likely done to reduce potential for violence. Veterans opposing the inclusion of the Bible in the display also grounded their arguments in the First Amendment clause that prohibits the federal government from establishing or favoring any religion.8
Presumptively, displays of such religious symbols may well be supported in VA policy as a protected expression of religion, which Secretary Wilke stated was the other primary aim of the revisions. “We want to make sure that all of our veterans and their families feel welcome at VA, no matter their religious beliefs. Protecting religious liberty is a key part of how we accomplish that goal.”4
In the middle of this sensitive controversy are the many veterans and their families that third parties—for profit, for politics, for publicity—have far too often manipulated for their own purposes. If you want to get an idea of the scope of these diverse stakeholders, just peruse the amicus briefs submitted to the Supreme Court on both sides of the issues in The American Legion v American Humanist Association.8
VA data show that veterans while being more religious than the general public are religiously diverse: 2015 data on the religion of veterans in every state listed 13 different faith communities.9 My response to the colleague who asked me about my opinion of the VA policies changes was based on the background narrative recounted here. My rsponse, in light of Roosevelt’s concern and this snippet of a much larger swath of legal machinations, is the change in the VA policy is reasonable as long as it “has room for the expression of those whose trust is in God, in country, in neither, and in both.” We know from research that religion is a strength and a support to many veterans and that spirituality as an aspect of psychological therapies and pastoral counseling has shown healing power for the wounds of war.10 Yet we also know that religiously based hatred and discrimination are among the most divisive and destructive forces that threaten our democracy. Let’s all hope—and those who pray do so—that these policy changes deter the latter and promote the former.
1. Roosevelt FD. The Public Papers and Addresses of Franklin D. Roosevelt. 1940 volume, War-and Aid to Democracies: With a Special Introduction and Explanatory Notes by President Roosevelt [Book 1]. New York: Macmillan; 1941:537.
2. US Department of Veterans Affairs, Veterans Health Administration. VA Directive 0022: Religious symbols in VA facilities. https://www.va.gov/vapubs/viewPublication.asp?Pub_ID=849. Published July 3, 2019. Accessed July 18, 2019.
3. US Department of Veterans Affairs, Veterans Health Administration. SVA Directive 1111(1): Spiritual and pastoral care in the Veterans Health Administration. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=4299. Published November 22, 2016. Amended July 3, 2019. Accessed July 22, 2019.
4. VA Office of Public and Intergovernmental Affairs. VA overhauls religious and spiritual symbol policies to protect religious liberty. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5279. Updated July 3, 2019. Accessed July 22, 2019.
5. Oyez. The American Legion v American Humanist Association. www.oyez.org/cases/2018/17-1717. Accessed July 16, 2019.
6. The American Legion. Legion salutes VA policy change for religious freedom. https://www.legion.org/honor/246151/legion-salutes-va-policy-change-victory-religious-freedom. Published July 03, 2019. Accessed July 22, 2019.
7. Miller K. Lawsuit filed over Bible display at New Hampshire VA Hospital; uproar ensues. https://www.airforcetimes.com/news/your-military/2019/05/07/lawsuit-filed-over-bible-display-at-new-hampshire-va-hospital-uproar-ensues. Published May 7, 2019. Accessed July 22, 2019.
8. Scotusblog. The American Legion v American Humanist Association. https://www.scotusblog.com/case-files/cases/the-american-legion-v-american-humanist-association. Accessed July 22, 2019.
9. US Department of Veterans Affairs. Veterans religions by state 2015. https://www.va.gov/vetdata/docs/SpecialReports/Veterans_Religion_by_State.xlsx. Accessed July 22, 2019.
10. Smothers ZPW. Koenig HG. Spiritual interventions in veterans with PTSD: a systematic review. J Relig Health. 2018;57(5):2033-2048.
1. Roosevelt FD. The Public Papers and Addresses of Franklin D. Roosevelt. 1940 volume, War-and Aid to Democracies: With a Special Introduction and Explanatory Notes by President Roosevelt [Book 1]. New York: Macmillan; 1941:537.
2. US Department of Veterans Affairs, Veterans Health Administration. VA Directive 0022: Religious symbols in VA facilities. https://www.va.gov/vapubs/viewPublication.asp?Pub_ID=849. Published July 3, 2019. Accessed July 18, 2019.
3. US Department of Veterans Affairs, Veterans Health Administration. SVA Directive 1111(1): Spiritual and pastoral care in the Veterans Health Administration. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=4299. Published November 22, 2016. Amended July 3, 2019. Accessed July 22, 2019.
4. VA Office of Public and Intergovernmental Affairs. VA overhauls religious and spiritual symbol policies to protect religious liberty. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5279. Updated July 3, 2019. Accessed July 22, 2019.
5. Oyez. The American Legion v American Humanist Association. www.oyez.org/cases/2018/17-1717. Accessed July 16, 2019.
6. The American Legion. Legion salutes VA policy change for religious freedom. https://www.legion.org/honor/246151/legion-salutes-va-policy-change-victory-religious-freedom. Published July 03, 2019. Accessed July 22, 2019.
7. Miller K. Lawsuit filed over Bible display at New Hampshire VA Hospital; uproar ensues. https://www.airforcetimes.com/news/your-military/2019/05/07/lawsuit-filed-over-bible-display-at-new-hampshire-va-hospital-uproar-ensues. Published May 7, 2019. Accessed July 22, 2019.
8. Scotusblog. The American Legion v American Humanist Association. https://www.scotusblog.com/case-files/cases/the-american-legion-v-american-humanist-association. Accessed July 22, 2019.
9. US Department of Veterans Affairs. Veterans religions by state 2015. https://www.va.gov/vetdata/docs/SpecialReports/Veterans_Religion_by_State.xlsx. Accessed July 22, 2019.
10. Smothers ZPW. Koenig HG. Spiritual interventions in veterans with PTSD: a systematic review. J Relig Health. 2018;57(5):2033-2048.
A Reticular Rash on the Leg
A 73-year-old male veteran with a history of ischemic stroke with left-sided deficits and edema, falls, poorly controlled hypertension, active tobacco use, obesity, and prediabetes was assessed on a routine visit by our home-based primary care team and found to have a new, unilateral, asymptomatic rash. He reported feeling no pain in the affected area or any significant increase in the baseline left lower extremity edema and weakness resulting from his stroke 2 years prior.
On the left lateral leg from mid-thigh to mid-calf, there was a nontender, flat, reticulated rash with pigmentary alteration ranging from light brown to dark brown (Figure).
On further questioning, the patient reported regular use of a space heater because his gas furnace had been destroyed in an earthquake more than 20 years before. He would place this heater close to his left leg when using the computer or while sleeping in his wheelchair.
- What is your diagnosis?
- How would you treat this patient?
Our Diagnosis
Erythema ab igne, also called hot water bottle rash, is a clinical diagnosis based on characteristic cutaneous findings and a clear history of chronic, moderate heat or infrared exposure.1 Although exposure to space heaters, open fire, radiators, hot water bottles, and heating pads are the classic causes, recently there have been reports of laptop computers, cell phones, infrared food lamps, automobile seat heaters, and heated recliners causing the same type of skin reaction.2
With chronic moderate heat or infrared exposure, the rash usually progresses over days to months. It begins as a mild, transient, reticulated, erythematous rash, which follows the pattern of the cutaneous venous plexus and resolves minutes to hours after removal of the offending source as vasodilation resolves. After months of continued exposure, the dermis around the affected vasculature eventually becomes hyperpigmented due to the deposition of melanin and sometimes hemosiderin.
The rash is usually asymptomatic but has been associated with pain, pruritis, and/or tingling. Once the diagnosis is made, treatment involves removal of the offending source. The discoloration may resolve over months to years, but permanent hyperpigmentation is not uncommon. There are a few case reports on treatment using Nd-Yag laser therapy, topical hydroquinone and tretinoin, 5-fluorouracil, and systemic mesoglycan with topical bioflavonoids.2-4
While the prognosis of erythema ab igne is excellent if detected early, failure to recognize this condition and remove the offending source can lead to sequalae, such as squamous cell carcinoma, poorly differentiated carcinoma, cutaneous marginal zone lymphoma, and Merkel cell carcinoma.5-8 Development of malignancy typically has a latency period of > 30 years. Patients should have periodic surveillance of their skin and any suspicious lesion in the involved area should be considered for biopsy.
Rashes may represent systemic or more localized pathology (Table). In contrast to erythema ab igne, the rash associated with a vasculitic process (autoimmune, drug-induced, or infectious) tends to be more generalized and bilateral but still follows the pattern of the cutaneous venous plexus. An example of this would be livedo reticularis. Although this rash is reticular, it is not hyperpigmented.9 A variant of livedo reticularis is cutis marmorata, which develops in response to cold exposure, particularly in infants or in the setting of hypothyroidism.Cutis marmorata is erythematous, blanchable, and reversible with rewarming. Unlike erythema ab igne, there is no hyperpigmentation and tends to be more diffuse.10
When evaluating a reticular rash, consider local and systemic etiologies. If more localized and hyperpigmented, ask about heat or infrared exposure. This may point to a diagnosis of erythema ab igne.
1. Page EH, Shear NH. Temperature-dependent skin disorders. J Am Acad Dermatol. 1988;18(5, pt 1):1003-1019.
2. Tan S, Bertucci V. Erythema ab igne: an old condition new again. CMAJ. 2000;162(1):77-78.
3. Kim HW, Kim EJ, Park HC, Ko JY, Ro YS, Kim JE. Erythema ab igne successfully treated with low fluenced 1,064-nm Q-switched Neodymium-Doped Yttrium Aluminum Garnet laser. J Cosmet Laser Ther. 2014;16(3):147-148.
4. Gianfaldoni S, Gianfaldoni R, Tchernev G, Lotti J, Wollina U, Lotti T. Erythema ab igne successfully treated with mesoglycan and bioflavonoids: a case-report. Open Access Maced J Med Sci. 2017;5(4):432-435.
5. Arrington JH 3rd, Lockman DS. Thermal keratoses and squamous cell carcinoma in situ associated with erythema ab igne. AMA Arch Derm. 1979;115(10):1226-1228.
6. Sigmon JR, Cantrell J, Teague D, Sangueza O, Sheehan DJ. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35(6):676-678
7. Wharton J, Roffwarg D, Miller J, Sheehan DJ. Cutaneous marginal zone lymphoma arising in the setting of erythema ab igne. J Am Acad Dermatol. 2010;62(6):1080-1081.
8. Jones CS. Development of neuroendocrine (Merkel cell) carcinoma mixed with squamous cell carcinoma in erythema ab igne. Arch Dermatol. 1988;124(1):110-113.
9. Sajjan VV, Lunge S, Swamy MB, Pandit AM. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6(5):315-321.
10. O’Connor NR, McLaughlin MR, Ham P. Newborn skin: part I. Common rashes. Am Fam Physician. 2008;77(1):47-52.
A 73-year-old male veteran with a history of ischemic stroke with left-sided deficits and edema, falls, poorly controlled hypertension, active tobacco use, obesity, and prediabetes was assessed on a routine visit by our home-based primary care team and found to have a new, unilateral, asymptomatic rash. He reported feeling no pain in the affected area or any significant increase in the baseline left lower extremity edema and weakness resulting from his stroke 2 years prior.
On the left lateral leg from mid-thigh to mid-calf, there was a nontender, flat, reticulated rash with pigmentary alteration ranging from light brown to dark brown (Figure).
On further questioning, the patient reported regular use of a space heater because his gas furnace had been destroyed in an earthquake more than 20 years before. He would place this heater close to his left leg when using the computer or while sleeping in his wheelchair.
- What is your diagnosis?
- How would you treat this patient?
Our Diagnosis
Erythema ab igne, also called hot water bottle rash, is a clinical diagnosis based on characteristic cutaneous findings and a clear history of chronic, moderate heat or infrared exposure.1 Although exposure to space heaters, open fire, radiators, hot water bottles, and heating pads are the classic causes, recently there have been reports of laptop computers, cell phones, infrared food lamps, automobile seat heaters, and heated recliners causing the same type of skin reaction.2
With chronic moderate heat or infrared exposure, the rash usually progresses over days to months. It begins as a mild, transient, reticulated, erythematous rash, which follows the pattern of the cutaneous venous plexus and resolves minutes to hours after removal of the offending source as vasodilation resolves. After months of continued exposure, the dermis around the affected vasculature eventually becomes hyperpigmented due to the deposition of melanin and sometimes hemosiderin.
The rash is usually asymptomatic but has been associated with pain, pruritis, and/or tingling. Once the diagnosis is made, treatment involves removal of the offending source. The discoloration may resolve over months to years, but permanent hyperpigmentation is not uncommon. There are a few case reports on treatment using Nd-Yag laser therapy, topical hydroquinone and tretinoin, 5-fluorouracil, and systemic mesoglycan with topical bioflavonoids.2-4
While the prognosis of erythema ab igne is excellent if detected early, failure to recognize this condition and remove the offending source can lead to sequalae, such as squamous cell carcinoma, poorly differentiated carcinoma, cutaneous marginal zone lymphoma, and Merkel cell carcinoma.5-8 Development of malignancy typically has a latency period of > 30 years. Patients should have periodic surveillance of their skin and any suspicious lesion in the involved area should be considered for biopsy.
Rashes may represent systemic or more localized pathology (Table). In contrast to erythema ab igne, the rash associated with a vasculitic process (autoimmune, drug-induced, or infectious) tends to be more generalized and bilateral but still follows the pattern of the cutaneous venous plexus. An example of this would be livedo reticularis. Although this rash is reticular, it is not hyperpigmented.9 A variant of livedo reticularis is cutis marmorata, which develops in response to cold exposure, particularly in infants or in the setting of hypothyroidism.Cutis marmorata is erythematous, blanchable, and reversible with rewarming. Unlike erythema ab igne, there is no hyperpigmentation and tends to be more diffuse.10
When evaluating a reticular rash, consider local and systemic etiologies. If more localized and hyperpigmented, ask about heat or infrared exposure. This may point to a diagnosis of erythema ab igne.
A 73-year-old male veteran with a history of ischemic stroke with left-sided deficits and edema, falls, poorly controlled hypertension, active tobacco use, obesity, and prediabetes was assessed on a routine visit by our home-based primary care team and found to have a new, unilateral, asymptomatic rash. He reported feeling no pain in the affected area or any significant increase in the baseline left lower extremity edema and weakness resulting from his stroke 2 years prior.
On the left lateral leg from mid-thigh to mid-calf, there was a nontender, flat, reticulated rash with pigmentary alteration ranging from light brown to dark brown (Figure).
On further questioning, the patient reported regular use of a space heater because his gas furnace had been destroyed in an earthquake more than 20 years before. He would place this heater close to his left leg when using the computer or while sleeping in his wheelchair.
- What is your diagnosis?
- How would you treat this patient?
Our Diagnosis
Erythema ab igne, also called hot water bottle rash, is a clinical diagnosis based on characteristic cutaneous findings and a clear history of chronic, moderate heat or infrared exposure.1 Although exposure to space heaters, open fire, radiators, hot water bottles, and heating pads are the classic causes, recently there have been reports of laptop computers, cell phones, infrared food lamps, automobile seat heaters, and heated recliners causing the same type of skin reaction.2
With chronic moderate heat or infrared exposure, the rash usually progresses over days to months. It begins as a mild, transient, reticulated, erythematous rash, which follows the pattern of the cutaneous venous plexus and resolves minutes to hours after removal of the offending source as vasodilation resolves. After months of continued exposure, the dermis around the affected vasculature eventually becomes hyperpigmented due to the deposition of melanin and sometimes hemosiderin.
The rash is usually asymptomatic but has been associated with pain, pruritis, and/or tingling. Once the diagnosis is made, treatment involves removal of the offending source. The discoloration may resolve over months to years, but permanent hyperpigmentation is not uncommon. There are a few case reports on treatment using Nd-Yag laser therapy, topical hydroquinone and tretinoin, 5-fluorouracil, and systemic mesoglycan with topical bioflavonoids.2-4
While the prognosis of erythema ab igne is excellent if detected early, failure to recognize this condition and remove the offending source can lead to sequalae, such as squamous cell carcinoma, poorly differentiated carcinoma, cutaneous marginal zone lymphoma, and Merkel cell carcinoma.5-8 Development of malignancy typically has a latency period of > 30 years. Patients should have periodic surveillance of their skin and any suspicious lesion in the involved area should be considered for biopsy.
Rashes may represent systemic or more localized pathology (Table). In contrast to erythema ab igne, the rash associated with a vasculitic process (autoimmune, drug-induced, or infectious) tends to be more generalized and bilateral but still follows the pattern of the cutaneous venous plexus. An example of this would be livedo reticularis. Although this rash is reticular, it is not hyperpigmented.9 A variant of livedo reticularis is cutis marmorata, which develops in response to cold exposure, particularly in infants or in the setting of hypothyroidism.Cutis marmorata is erythematous, blanchable, and reversible with rewarming. Unlike erythema ab igne, there is no hyperpigmentation and tends to be more diffuse.10
When evaluating a reticular rash, consider local and systemic etiologies. If more localized and hyperpigmented, ask about heat or infrared exposure. This may point to a diagnosis of erythema ab igne.
1. Page EH, Shear NH. Temperature-dependent skin disorders. J Am Acad Dermatol. 1988;18(5, pt 1):1003-1019.
2. Tan S, Bertucci V. Erythema ab igne: an old condition new again. CMAJ. 2000;162(1):77-78.
3. Kim HW, Kim EJ, Park HC, Ko JY, Ro YS, Kim JE. Erythema ab igne successfully treated with low fluenced 1,064-nm Q-switched Neodymium-Doped Yttrium Aluminum Garnet laser. J Cosmet Laser Ther. 2014;16(3):147-148.
4. Gianfaldoni S, Gianfaldoni R, Tchernev G, Lotti J, Wollina U, Lotti T. Erythema ab igne successfully treated with mesoglycan and bioflavonoids: a case-report. Open Access Maced J Med Sci. 2017;5(4):432-435.
5. Arrington JH 3rd, Lockman DS. Thermal keratoses and squamous cell carcinoma in situ associated with erythema ab igne. AMA Arch Derm. 1979;115(10):1226-1228.
6. Sigmon JR, Cantrell J, Teague D, Sangueza O, Sheehan DJ. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35(6):676-678
7. Wharton J, Roffwarg D, Miller J, Sheehan DJ. Cutaneous marginal zone lymphoma arising in the setting of erythema ab igne. J Am Acad Dermatol. 2010;62(6):1080-1081.
8. Jones CS. Development of neuroendocrine (Merkel cell) carcinoma mixed with squamous cell carcinoma in erythema ab igne. Arch Dermatol. 1988;124(1):110-113.
9. Sajjan VV, Lunge S, Swamy MB, Pandit AM. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6(5):315-321.
10. O’Connor NR, McLaughlin MR, Ham P. Newborn skin: part I. Common rashes. Am Fam Physician. 2008;77(1):47-52.
1. Page EH, Shear NH. Temperature-dependent skin disorders. J Am Acad Dermatol. 1988;18(5, pt 1):1003-1019.
2. Tan S, Bertucci V. Erythema ab igne: an old condition new again. CMAJ. 2000;162(1):77-78.
3. Kim HW, Kim EJ, Park HC, Ko JY, Ro YS, Kim JE. Erythema ab igne successfully treated with low fluenced 1,064-nm Q-switched Neodymium-Doped Yttrium Aluminum Garnet laser. J Cosmet Laser Ther. 2014;16(3):147-148.
4. Gianfaldoni S, Gianfaldoni R, Tchernev G, Lotti J, Wollina U, Lotti T. Erythema ab igne successfully treated with mesoglycan and bioflavonoids: a case-report. Open Access Maced J Med Sci. 2017;5(4):432-435.
5. Arrington JH 3rd, Lockman DS. Thermal keratoses and squamous cell carcinoma in situ associated with erythema ab igne. AMA Arch Derm. 1979;115(10):1226-1228.
6. Sigmon JR, Cantrell J, Teague D, Sangueza O, Sheehan DJ. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35(6):676-678
7. Wharton J, Roffwarg D, Miller J, Sheehan DJ. Cutaneous marginal zone lymphoma arising in the setting of erythema ab igne. J Am Acad Dermatol. 2010;62(6):1080-1081.
8. Jones CS. Development of neuroendocrine (Merkel cell) carcinoma mixed with squamous cell carcinoma in erythema ab igne. Arch Dermatol. 1988;124(1):110-113.
9. Sajjan VV, Lunge S, Swamy MB, Pandit AM. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6(5):315-321.
10. O’Connor NR, McLaughlin MR, Ham P. Newborn skin: part I. Common rashes. Am Fam Physician. 2008;77(1):47-52.
Shoulder Injury Related to Vaccine Administration: A Rare Reaction
Localized reactions and transient pain at the site of vaccine administration are frequent and well-described occurrences that are typically short-lived and mild in nature. The most common findings at the injection site are soreness, erythema, and edema.1 Although less common, generalized shoulder dysfunction after vaccine administration also has been reported. Bodor and colleagues described a peri-articular inflammatory response that led to shoulder pain and weakness.2 A single case report by Kuether and colleagues described atraumatic osteonecrosis of the humeral head after H1N1 vaccine administration in the deltoid.3 In 2010, shoulder injury related to vaccine administration (SIRVA) was described by Atanasoff and colleagues as the rapid onset of shoulder pain and dysfunction persisting as a complication of deltoid muscle vaccination in a case series of 13 patients.4 In our report, we present a case of an active-duty male eventually diagnosed with SIRVA after influenza vaccination and discuss factors that may prevent vaccine-related shoulder injuries.
Case Presentation
A 31-year-old active-duty male presented to the Allergy clinic for evaluation of persistent left shoulder pain and decreased range of motion (ROM) following influenza vaccination 4 months prior. He reported a history of chronic low back and right shoulder pain. Although the patient had a traumatic injury to his right shoulder, which was corrected with surgery, he had no surgeries on the left shoulder. He reported no prior pain or known trauma to his left shoulder. He had no personal or family history of atopy or vaccine reactions.
The patient weighed 91 kg and received an intramuscular (IM) quadrivalent influenza vaccine with a 25-gauge, 1-inch needle during a mass influenza immunization. He recalled that the site of vaccination was slightly more than 3 cm below the top of the shoulder in a region correlating to the left deltoid. The vaccine was administered while he was standing with his arm extended, adducted, and internally rotated. The patient experienced intense pain immediately after the vaccination and noted decreased ROM. Initially, he dismissed the pain and decreased ROM as routine but sought medical attention when there was no improvement after 3 weeks.
Six weeks after the onset of symptoms, a magnetic resonance image (MRI) revealed tendinopathy of the left distal subscapularis, infraspinatus, supraspinatus, and teres minor tendon. These findings were suggestive of a small partial thickness tear of the supraspinatus (Figure 1), possible calcific tendinopathy of the distal teres minor (Figure 2), and underlying humeral head edema (Figure 3). The patient was evaluated by Orthopedics and experienced no relief from ibuprofen, celecoxib, and a steroid/lidocaine intra-articular injection. Laboratory studies included an unremarkable complete blood count and erythrocyte sedimentation rate. He was diagnosed with SIRVA and continued in physical therapy with incomplete resolution of symptoms 6 months postvaccination.
Discussion
According to a 2018 report issued by the Centers for Disease Control and Prevention, local reactions following immunizations are seen in up to 80% of administered vaccine doses.1 While most of these reactions are mild, transient, cutaneous reactions, rarely these also may persist and impact quality of life significantly. SIRVA is one such process that can lead to persistent musculoskeletal dysfunction. SIRVA presents as shoulder pain and limited ROM that occurs after the administration of an injectable vaccine. In 2011, the Institute of Medicine determined that evidence supported a causal relationship between vaccine administration and deltoid bursitis.5
In 2017, SIRVA was included in the Vaccine Injury Compensation Program (VICP), a federal program that can provide compensation to individuals injured by certain vaccines.6 A diagnosis of SIRVA can be considered in patients who experience pain within 48 hours of vaccination, have no prior history of pain or dysfunction of the affected shoulder prior to vaccine administration, and have symptoms limited to the shoulder in which the vaccine was administered where no other abnormality is present to explain these symptoms (eg, brachial neuritis, other neuropathy). Currently, patients with back pain or musculoskeletal complaints that do not include the shoulder following deltoid vaccination do not meet the reporting criteria for SIRVA in the VICP.6
The exact prevalence or incidence of SIRVA is unknown. In a 2017 systematic review of the literature and the Spanish Pharmacovigilance System database, Martín Arias and colleagues found 45 cases of new onset, unilateral shoulder dysfunction without associated neuropathy or autoimmune conditions following vaccine administration. They noted a female to male predominance (71.1% vs 28.9%) with a mean age of 53.6 years (range 22-89 y). Most of the cases occurred following influenza vaccine (62%); pneumococcal vaccine was the next most common (13%).7 Shoulder injury also has been reported after tetanus-diphtheria toxoids, human papilloma virus, and hepatitis A virus vaccines.4,7 The review noted that all patients had onset of pain within the first week following vaccination with the majority (81%) having pain in the first 24 hours. Two cases found in the Spanish database had pain onset 2 months postvaccination.7 Atanasoff and colleagues found that 93% of patients had pain onset within 24 hours of vaccination with 54% reporting immediate pain.4
The Vaccine Adverse Event Reporting System (VAERS) tracks reports of shoulder dysfunction following certain vaccinations, but the system is unable to establish causality. According to VAERS reporting, between 2010 and 2016, there were 1006 possible reports of shoulder dysfunction following inactivated influenza vaccination (IIV) compared with an estimated 130 million doses of IIV given each influenza season in the US.8
Bodor and Montalvo postulated that vaccine antigen was being over penetrated into the synovial space of the shoulder, as the subdeltoid/subacromial bursa is located a mere 0.8 to 1.6 cm below the skin surface in patients with healthy body mass index.2 Atanasoff and colleagues expounded that antibodies from previous vaccination or natural infection may then form antigen-antibody complexes, creating prolonged local immune and inflammatory responses leading to bursitis or tendonitis.4 Martín Arias and colleagues hypothesized that improper injection technique, including wrong insertion angle, incorrect needle type/size, and failure to account for the patient’s physical characteristics were the most likely causes of SIRVA.7
Proper vaccine administration ensures that vaccinations are delivered in a safe and efficacious manner. Safe vaccination practices include the use of trained personnel who receive comprehensive, competency-based training regarding vaccine administration.1 Aspiration prior to an injection is a practice that has not been evaluated fully. Given that the 2 routinely recommended locations for IM vaccines (deltoid muscle in adults or vastus lateralis muscle in infants) lack large blood vessels, the practice of aspiration prior to an IM vaccine is not currently deemed necessary.1 Additional safe vaccine practices include the selection of appropriate needle length for muscle penetration and that anatomic landmarks determine the location of vaccination.1 Despite this, in a survey of 100 medical professionals, half could not name any structure at risk from improper deltoid vaccination technique.9
Cook and colleagues used anthropomorphic data to evaluate the potential for injury to the subdeltoid/subacromial bursa and/or the axillary nerve.10 Based on these data, they recommended safe IM vaccine administration can be assured by using the midpoint of the deltoid muscle located midway between the acromion and deltoid tuberosity with the arm abducted to 60°.10,11 In 46% of SIRVA cases described by Atanasoff and colleagues, patients reported that the vaccine was administered “too high.”4 The study also recommended that the clinician and the patient be in the seated position to ensure proper needle angle and location of administration.4 For most adults, a 1-inch needle is appropriate for vaccine administration in the deltoid; however, in females weighing < 70 kg and males < 75 kg, a 5/8-inch needle is recommended to avoid injury.7
Our 91-kg patient was appropriately administered his vaccine with a 1-inch needle. As he experienced immediate pain, it is unlikely that his symptoms were due to an immune-mediated process, as this would not be expected to occur immediately. Improper location of vaccine administration is a proposed mechanism of injury for our patient, though this cannot be confirmed by history alone. His prior history of traumatic injury to the opposite shoulder could represent a confounding factor as no prior imaging was available for the vaccine-affected shoulder. A preexisting shoulder abnormality or injury cannot be completely excluded, and it is possible that an underlying prior shoulder injury was aggravated postvaccination.
Evaluation and Treatment
There is no standardized approach for the evaluation of SIRVA to date. Awareness of SIRVA and a high index of suspicion are necessary to evaluate patients with shoulder concerns postvaccination. Laboratory evaluation should be considered to evaluate for other potential diagnoses (eg, infection, rheumatologic concerns). Routine X-rays are not helpful in cases of SIRVA. Ultrasound may be considered as it can show bursa abnormalities consistent with bursitis.2 MRI of the affected shoulder may provide improved diagnostic capability if SIRVA is suspected. MRI findings vary but include intraosseous edema, bursitis, tendonitis, and rotator cuff tears.4,12 Complete rotator cuff tears were found in 15% of cases reviewed by Atanasoff and colleagues.4 While there is no recommended timing for MRI, 63% of MRIs were performed within 3 months of symptom onset.4 As SIRVA is not a neurologic injury, nerve conduction, electromyographic studies, and neurologic evaluation or testing are expected to be normal.
Treatment of SIRVA and other vaccine-related shoulder injuries typically have involved pain management (eg, nonsteroidal anti-inflammatory agents), intra-articular steroid injections, and physical therapy, though some patients never experience complete resolution of symptoms.2,4,7 Both patients with vaccination-related shoulder dysfunction described by Bodor and colleagues improved after intra-articular triamcinolone injections, with up to 3 injections before complete resolution of pain in one patient.2 Orthopedics evaluation may need to be considered for persistent symptoms. According to Atanasoff and colleagues, most patients were symptomatic for at least 6 months, and complete recovery was seen in less than one-third of patients.4 Although the development of SIRVA is not a contraindication to future doses of the presumed causative vaccine, subsequent vaccination should include careful consideration of other administration sites if possible (eg, vastus lateralis may be used for IM injections in adults) (Figure 4).
Reporting
A diagnosis or concern for SIRVA also should be reported to the VAERS, the national database established in order to detect possible safety problems with US-licensed vaccines. VAERS reports can be submitted by anyone with concerns for vaccine adverse reactions, including patients, caregivers, and health care professionals at vaers.hhs.gov/reportevent.html. Additional information regarding VICP can be obtained at www.hrsa.gov/vaccine-compensation/index.html.
Military-Specific Issues
The military values readiness, which includes ensuring that active-duty members remain up-to-date on life-saving vaccinations. Immunization is of critical importance to mobility and success of the overall mission. Mobility processing lines where immunizations can be provided to multiple active-duty members can be a successful strategy for mass immunizations. Although the quick administration of immunizations maintains readiness and provides a medically necessary service, it also may increase the chances of incorrect vaccine placement in the deltoid, causing long-term shoulder immobility that may impact a service member’s retainability. The benefits of mobility processing lines can continue to outweigh the risks of immunization administration by ensuring proper staff training, seating both the administrator and recipient of vaccination, and selecting a proper needle length and site of administration specific to each recipient.
Conclusion
Correct administration of vaccines is of utmost importance in preventing SIRVA and other vaccine-related shoulder dysfunctions. Proper staff training and refresher training can help prevent vaccine-related shoulder injuries. Additionally, clinicians should be aware of this potential complication and maintain a high index of suspicion when evaluating patients with postvaccination shoulder complaints.
1. Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases. https://www.cdc.gov/vaccines/pubs/pinkbook/vac-admin.html. Published 2015. Accessed June 3, 2019.
2. Bodor M, Montalvo E. Vaccination-related shoulder dysfunction. Vaccine. 2007;25(4):585-587.
3. Kuether G, Dietrich B, Smith T, Peter C, Gruessner S. Atraumatic osteonecrosis of the humeral head after influenza A-(H1N1) v-2009 vaccination. Vaccine. 2011;29(40):6830-6833.
4. Atanasoff S, Ryan T, Lightfoot R, Johann-Liang R. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010;28(51):8049-8052.
5. Institute of Medicine. Adverse effects of vaccines: evidence and causality. http://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2011/Adverse-Effects-of-Vaccines-Evidence-and-Causality/Vaccine-report-brief-FINAL.pdf. Published August 2011. Accessed June 3, 2019.
6. Health Resources and Services Administration, Health and Human Services Administration. National vaccine injury compensation program: revisions to the vaccine injury table. https://www.federalregister.gov/documents/2017/01/19/2017-00701/national-vaccine-injury-compensation-program-revisions-to-the-vaccine-injury-table. Published January 19, 2017. Accessed June 3, 2019.
7. Martín Arias LH, Sanz Fadrique R, Sáinz Gil M, Salgueiro-Vazquez ME. Risk of bursitis and other injuries and dysfunctions of the shoulder following vaccinations. Vaccine. 2017;35(37):4870-4876.
8. Centers for Disease Control and Prevention. Reports of shoulder dysfunction following inactivated influenza vaccine in the Vaccine Adverse Event Reporting System (VAERS), 2010-2016. https://stacks.cdc.gov/view/cdc/57624. Published January 4, 2018. Accessed June 3, 2019.
9. McGarvey MA, Hooper AC. The deltoid intramuscular injection site in the adult. Current practice among general practitioners and practice nurses. Ir Med J. 2005;98(4):105-107.
10. Cook IF. An evidence based protocol for the prevention of upper arm injury related to vaccine administration (UAIRVA). Hum Vaccin. 2011;7(8):845-848.
11. Cook IF. Best vaccination practice and medically attended injection site events following deltoid intramuscular injection. Hum Vaccin Immunother. 2015;11(5):1184-1191.
12. Okur G, Chaney KA, Lomasney LM. Magnetic resonance imaging of abnormal shoulder pain following influenza vaccination. Skeletal Radiol. 2014;43(9):1325-1331.
Localized reactions and transient pain at the site of vaccine administration are frequent and well-described occurrences that are typically short-lived and mild in nature. The most common findings at the injection site are soreness, erythema, and edema.1 Although less common, generalized shoulder dysfunction after vaccine administration also has been reported. Bodor and colleagues described a peri-articular inflammatory response that led to shoulder pain and weakness.2 A single case report by Kuether and colleagues described atraumatic osteonecrosis of the humeral head after H1N1 vaccine administration in the deltoid.3 In 2010, shoulder injury related to vaccine administration (SIRVA) was described by Atanasoff and colleagues as the rapid onset of shoulder pain and dysfunction persisting as a complication of deltoid muscle vaccination in a case series of 13 patients.4 In our report, we present a case of an active-duty male eventually diagnosed with SIRVA after influenza vaccination and discuss factors that may prevent vaccine-related shoulder injuries.
Case Presentation
A 31-year-old active-duty male presented to the Allergy clinic for evaluation of persistent left shoulder pain and decreased range of motion (ROM) following influenza vaccination 4 months prior. He reported a history of chronic low back and right shoulder pain. Although the patient had a traumatic injury to his right shoulder, which was corrected with surgery, he had no surgeries on the left shoulder. He reported no prior pain or known trauma to his left shoulder. He had no personal or family history of atopy or vaccine reactions.
The patient weighed 91 kg and received an intramuscular (IM) quadrivalent influenza vaccine with a 25-gauge, 1-inch needle during a mass influenza immunization. He recalled that the site of vaccination was slightly more than 3 cm below the top of the shoulder in a region correlating to the left deltoid. The vaccine was administered while he was standing with his arm extended, adducted, and internally rotated. The patient experienced intense pain immediately after the vaccination and noted decreased ROM. Initially, he dismissed the pain and decreased ROM as routine but sought medical attention when there was no improvement after 3 weeks.
Six weeks after the onset of symptoms, a magnetic resonance image (MRI) revealed tendinopathy of the left distal subscapularis, infraspinatus, supraspinatus, and teres minor tendon. These findings were suggestive of a small partial thickness tear of the supraspinatus (Figure 1), possible calcific tendinopathy of the distal teres minor (Figure 2), and underlying humeral head edema (Figure 3). The patient was evaluated by Orthopedics and experienced no relief from ibuprofen, celecoxib, and a steroid/lidocaine intra-articular injection. Laboratory studies included an unremarkable complete blood count and erythrocyte sedimentation rate. He was diagnosed with SIRVA and continued in physical therapy with incomplete resolution of symptoms 6 months postvaccination.
Discussion
According to a 2018 report issued by the Centers for Disease Control and Prevention, local reactions following immunizations are seen in up to 80% of administered vaccine doses.1 While most of these reactions are mild, transient, cutaneous reactions, rarely these also may persist and impact quality of life significantly. SIRVA is one such process that can lead to persistent musculoskeletal dysfunction. SIRVA presents as shoulder pain and limited ROM that occurs after the administration of an injectable vaccine. In 2011, the Institute of Medicine determined that evidence supported a causal relationship between vaccine administration and deltoid bursitis.5
In 2017, SIRVA was included in the Vaccine Injury Compensation Program (VICP), a federal program that can provide compensation to individuals injured by certain vaccines.6 A diagnosis of SIRVA can be considered in patients who experience pain within 48 hours of vaccination, have no prior history of pain or dysfunction of the affected shoulder prior to vaccine administration, and have symptoms limited to the shoulder in which the vaccine was administered where no other abnormality is present to explain these symptoms (eg, brachial neuritis, other neuropathy). Currently, patients with back pain or musculoskeletal complaints that do not include the shoulder following deltoid vaccination do not meet the reporting criteria for SIRVA in the VICP.6
The exact prevalence or incidence of SIRVA is unknown. In a 2017 systematic review of the literature and the Spanish Pharmacovigilance System database, Martín Arias and colleagues found 45 cases of new onset, unilateral shoulder dysfunction without associated neuropathy or autoimmune conditions following vaccine administration. They noted a female to male predominance (71.1% vs 28.9%) with a mean age of 53.6 years (range 22-89 y). Most of the cases occurred following influenza vaccine (62%); pneumococcal vaccine was the next most common (13%).7 Shoulder injury also has been reported after tetanus-diphtheria toxoids, human papilloma virus, and hepatitis A virus vaccines.4,7 The review noted that all patients had onset of pain within the first week following vaccination with the majority (81%) having pain in the first 24 hours. Two cases found in the Spanish database had pain onset 2 months postvaccination.7 Atanasoff and colleagues found that 93% of patients had pain onset within 24 hours of vaccination with 54% reporting immediate pain.4
The Vaccine Adverse Event Reporting System (VAERS) tracks reports of shoulder dysfunction following certain vaccinations, but the system is unable to establish causality. According to VAERS reporting, between 2010 and 2016, there were 1006 possible reports of shoulder dysfunction following inactivated influenza vaccination (IIV) compared with an estimated 130 million doses of IIV given each influenza season in the US.8
Bodor and Montalvo postulated that vaccine antigen was being over penetrated into the synovial space of the shoulder, as the subdeltoid/subacromial bursa is located a mere 0.8 to 1.6 cm below the skin surface in patients with healthy body mass index.2 Atanasoff and colleagues expounded that antibodies from previous vaccination or natural infection may then form antigen-antibody complexes, creating prolonged local immune and inflammatory responses leading to bursitis or tendonitis.4 Martín Arias and colleagues hypothesized that improper injection technique, including wrong insertion angle, incorrect needle type/size, and failure to account for the patient’s physical characteristics were the most likely causes of SIRVA.7
Proper vaccine administration ensures that vaccinations are delivered in a safe and efficacious manner. Safe vaccination practices include the use of trained personnel who receive comprehensive, competency-based training regarding vaccine administration.1 Aspiration prior to an injection is a practice that has not been evaluated fully. Given that the 2 routinely recommended locations for IM vaccines (deltoid muscle in adults or vastus lateralis muscle in infants) lack large blood vessels, the practice of aspiration prior to an IM vaccine is not currently deemed necessary.1 Additional safe vaccine practices include the selection of appropriate needle length for muscle penetration and that anatomic landmarks determine the location of vaccination.1 Despite this, in a survey of 100 medical professionals, half could not name any structure at risk from improper deltoid vaccination technique.9
Cook and colleagues used anthropomorphic data to evaluate the potential for injury to the subdeltoid/subacromial bursa and/or the axillary nerve.10 Based on these data, they recommended safe IM vaccine administration can be assured by using the midpoint of the deltoid muscle located midway between the acromion and deltoid tuberosity with the arm abducted to 60°.10,11 In 46% of SIRVA cases described by Atanasoff and colleagues, patients reported that the vaccine was administered “too high.”4 The study also recommended that the clinician and the patient be in the seated position to ensure proper needle angle and location of administration.4 For most adults, a 1-inch needle is appropriate for vaccine administration in the deltoid; however, in females weighing < 70 kg and males < 75 kg, a 5/8-inch needle is recommended to avoid injury.7
Our 91-kg patient was appropriately administered his vaccine with a 1-inch needle. As he experienced immediate pain, it is unlikely that his symptoms were due to an immune-mediated process, as this would not be expected to occur immediately. Improper location of vaccine administration is a proposed mechanism of injury for our patient, though this cannot be confirmed by history alone. His prior history of traumatic injury to the opposite shoulder could represent a confounding factor as no prior imaging was available for the vaccine-affected shoulder. A preexisting shoulder abnormality or injury cannot be completely excluded, and it is possible that an underlying prior shoulder injury was aggravated postvaccination.
Evaluation and Treatment
There is no standardized approach for the evaluation of SIRVA to date. Awareness of SIRVA and a high index of suspicion are necessary to evaluate patients with shoulder concerns postvaccination. Laboratory evaluation should be considered to evaluate for other potential diagnoses (eg, infection, rheumatologic concerns). Routine X-rays are not helpful in cases of SIRVA. Ultrasound may be considered as it can show bursa abnormalities consistent with bursitis.2 MRI of the affected shoulder may provide improved diagnostic capability if SIRVA is suspected. MRI findings vary but include intraosseous edema, bursitis, tendonitis, and rotator cuff tears.4,12 Complete rotator cuff tears were found in 15% of cases reviewed by Atanasoff and colleagues.4 While there is no recommended timing for MRI, 63% of MRIs were performed within 3 months of symptom onset.4 As SIRVA is not a neurologic injury, nerve conduction, electromyographic studies, and neurologic evaluation or testing are expected to be normal.
Treatment of SIRVA and other vaccine-related shoulder injuries typically have involved pain management (eg, nonsteroidal anti-inflammatory agents), intra-articular steroid injections, and physical therapy, though some patients never experience complete resolution of symptoms.2,4,7 Both patients with vaccination-related shoulder dysfunction described by Bodor and colleagues improved after intra-articular triamcinolone injections, with up to 3 injections before complete resolution of pain in one patient.2 Orthopedics evaluation may need to be considered for persistent symptoms. According to Atanasoff and colleagues, most patients were symptomatic for at least 6 months, and complete recovery was seen in less than one-third of patients.4 Although the development of SIRVA is not a contraindication to future doses of the presumed causative vaccine, subsequent vaccination should include careful consideration of other administration sites if possible (eg, vastus lateralis may be used for IM injections in adults) (Figure 4).
Reporting
A diagnosis or concern for SIRVA also should be reported to the VAERS, the national database established in order to detect possible safety problems with US-licensed vaccines. VAERS reports can be submitted by anyone with concerns for vaccine adverse reactions, including patients, caregivers, and health care professionals at vaers.hhs.gov/reportevent.html. Additional information regarding VICP can be obtained at www.hrsa.gov/vaccine-compensation/index.html.
Military-Specific Issues
The military values readiness, which includes ensuring that active-duty members remain up-to-date on life-saving vaccinations. Immunization is of critical importance to mobility and success of the overall mission. Mobility processing lines where immunizations can be provided to multiple active-duty members can be a successful strategy for mass immunizations. Although the quick administration of immunizations maintains readiness and provides a medically necessary service, it also may increase the chances of incorrect vaccine placement in the deltoid, causing long-term shoulder immobility that may impact a service member’s retainability. The benefits of mobility processing lines can continue to outweigh the risks of immunization administration by ensuring proper staff training, seating both the administrator and recipient of vaccination, and selecting a proper needle length and site of administration specific to each recipient.
Conclusion
Correct administration of vaccines is of utmost importance in preventing SIRVA and other vaccine-related shoulder dysfunctions. Proper staff training and refresher training can help prevent vaccine-related shoulder injuries. Additionally, clinicians should be aware of this potential complication and maintain a high index of suspicion when evaluating patients with postvaccination shoulder complaints.
Localized reactions and transient pain at the site of vaccine administration are frequent and well-described occurrences that are typically short-lived and mild in nature. The most common findings at the injection site are soreness, erythema, and edema.1 Although less common, generalized shoulder dysfunction after vaccine administration also has been reported. Bodor and colleagues described a peri-articular inflammatory response that led to shoulder pain and weakness.2 A single case report by Kuether and colleagues described atraumatic osteonecrosis of the humeral head after H1N1 vaccine administration in the deltoid.3 In 2010, shoulder injury related to vaccine administration (SIRVA) was described by Atanasoff and colleagues as the rapid onset of shoulder pain and dysfunction persisting as a complication of deltoid muscle vaccination in a case series of 13 patients.4 In our report, we present a case of an active-duty male eventually diagnosed with SIRVA after influenza vaccination and discuss factors that may prevent vaccine-related shoulder injuries.
Case Presentation
A 31-year-old active-duty male presented to the Allergy clinic for evaluation of persistent left shoulder pain and decreased range of motion (ROM) following influenza vaccination 4 months prior. He reported a history of chronic low back and right shoulder pain. Although the patient had a traumatic injury to his right shoulder, which was corrected with surgery, he had no surgeries on the left shoulder. He reported no prior pain or known trauma to his left shoulder. He had no personal or family history of atopy or vaccine reactions.
The patient weighed 91 kg and received an intramuscular (IM) quadrivalent influenza vaccine with a 25-gauge, 1-inch needle during a mass influenza immunization. He recalled that the site of vaccination was slightly more than 3 cm below the top of the shoulder in a region correlating to the left deltoid. The vaccine was administered while he was standing with his arm extended, adducted, and internally rotated. The patient experienced intense pain immediately after the vaccination and noted decreased ROM. Initially, he dismissed the pain and decreased ROM as routine but sought medical attention when there was no improvement after 3 weeks.
Six weeks after the onset of symptoms, a magnetic resonance image (MRI) revealed tendinopathy of the left distal subscapularis, infraspinatus, supraspinatus, and teres minor tendon. These findings were suggestive of a small partial thickness tear of the supraspinatus (Figure 1), possible calcific tendinopathy of the distal teres minor (Figure 2), and underlying humeral head edema (Figure 3). The patient was evaluated by Orthopedics and experienced no relief from ibuprofen, celecoxib, and a steroid/lidocaine intra-articular injection. Laboratory studies included an unremarkable complete blood count and erythrocyte sedimentation rate. He was diagnosed with SIRVA and continued in physical therapy with incomplete resolution of symptoms 6 months postvaccination.
Discussion
According to a 2018 report issued by the Centers for Disease Control and Prevention, local reactions following immunizations are seen in up to 80% of administered vaccine doses.1 While most of these reactions are mild, transient, cutaneous reactions, rarely these also may persist and impact quality of life significantly. SIRVA is one such process that can lead to persistent musculoskeletal dysfunction. SIRVA presents as shoulder pain and limited ROM that occurs after the administration of an injectable vaccine. In 2011, the Institute of Medicine determined that evidence supported a causal relationship between vaccine administration and deltoid bursitis.5
In 2017, SIRVA was included in the Vaccine Injury Compensation Program (VICP), a federal program that can provide compensation to individuals injured by certain vaccines.6 A diagnosis of SIRVA can be considered in patients who experience pain within 48 hours of vaccination, have no prior history of pain or dysfunction of the affected shoulder prior to vaccine administration, and have symptoms limited to the shoulder in which the vaccine was administered where no other abnormality is present to explain these symptoms (eg, brachial neuritis, other neuropathy). Currently, patients with back pain or musculoskeletal complaints that do not include the shoulder following deltoid vaccination do not meet the reporting criteria for SIRVA in the VICP.6
The exact prevalence or incidence of SIRVA is unknown. In a 2017 systematic review of the literature and the Spanish Pharmacovigilance System database, Martín Arias and colleagues found 45 cases of new onset, unilateral shoulder dysfunction without associated neuropathy or autoimmune conditions following vaccine administration. They noted a female to male predominance (71.1% vs 28.9%) with a mean age of 53.6 years (range 22-89 y). Most of the cases occurred following influenza vaccine (62%); pneumococcal vaccine was the next most common (13%).7 Shoulder injury also has been reported after tetanus-diphtheria toxoids, human papilloma virus, and hepatitis A virus vaccines.4,7 The review noted that all patients had onset of pain within the first week following vaccination with the majority (81%) having pain in the first 24 hours. Two cases found in the Spanish database had pain onset 2 months postvaccination.7 Atanasoff and colleagues found that 93% of patients had pain onset within 24 hours of vaccination with 54% reporting immediate pain.4
The Vaccine Adverse Event Reporting System (VAERS) tracks reports of shoulder dysfunction following certain vaccinations, but the system is unable to establish causality. According to VAERS reporting, between 2010 and 2016, there were 1006 possible reports of shoulder dysfunction following inactivated influenza vaccination (IIV) compared with an estimated 130 million doses of IIV given each influenza season in the US.8
Bodor and Montalvo postulated that vaccine antigen was being over penetrated into the synovial space of the shoulder, as the subdeltoid/subacromial bursa is located a mere 0.8 to 1.6 cm below the skin surface in patients with healthy body mass index.2 Atanasoff and colleagues expounded that antibodies from previous vaccination or natural infection may then form antigen-antibody complexes, creating prolonged local immune and inflammatory responses leading to bursitis or tendonitis.4 Martín Arias and colleagues hypothesized that improper injection technique, including wrong insertion angle, incorrect needle type/size, and failure to account for the patient’s physical characteristics were the most likely causes of SIRVA.7
Proper vaccine administration ensures that vaccinations are delivered in a safe and efficacious manner. Safe vaccination practices include the use of trained personnel who receive comprehensive, competency-based training regarding vaccine administration.1 Aspiration prior to an injection is a practice that has not been evaluated fully. Given that the 2 routinely recommended locations for IM vaccines (deltoid muscle in adults or vastus lateralis muscle in infants) lack large blood vessels, the practice of aspiration prior to an IM vaccine is not currently deemed necessary.1 Additional safe vaccine practices include the selection of appropriate needle length for muscle penetration and that anatomic landmarks determine the location of vaccination.1 Despite this, in a survey of 100 medical professionals, half could not name any structure at risk from improper deltoid vaccination technique.9
Cook and colleagues used anthropomorphic data to evaluate the potential for injury to the subdeltoid/subacromial bursa and/or the axillary nerve.10 Based on these data, they recommended safe IM vaccine administration can be assured by using the midpoint of the deltoid muscle located midway between the acromion and deltoid tuberosity with the arm abducted to 60°.10,11 In 46% of SIRVA cases described by Atanasoff and colleagues, patients reported that the vaccine was administered “too high.”4 The study also recommended that the clinician and the patient be in the seated position to ensure proper needle angle and location of administration.4 For most adults, a 1-inch needle is appropriate for vaccine administration in the deltoid; however, in females weighing < 70 kg and males < 75 kg, a 5/8-inch needle is recommended to avoid injury.7
Our 91-kg patient was appropriately administered his vaccine with a 1-inch needle. As he experienced immediate pain, it is unlikely that his symptoms were due to an immune-mediated process, as this would not be expected to occur immediately. Improper location of vaccine administration is a proposed mechanism of injury for our patient, though this cannot be confirmed by history alone. His prior history of traumatic injury to the opposite shoulder could represent a confounding factor as no prior imaging was available for the vaccine-affected shoulder. A preexisting shoulder abnormality or injury cannot be completely excluded, and it is possible that an underlying prior shoulder injury was aggravated postvaccination.
Evaluation and Treatment
There is no standardized approach for the evaluation of SIRVA to date. Awareness of SIRVA and a high index of suspicion are necessary to evaluate patients with shoulder concerns postvaccination. Laboratory evaluation should be considered to evaluate for other potential diagnoses (eg, infection, rheumatologic concerns). Routine X-rays are not helpful in cases of SIRVA. Ultrasound may be considered as it can show bursa abnormalities consistent with bursitis.2 MRI of the affected shoulder may provide improved diagnostic capability if SIRVA is suspected. MRI findings vary but include intraosseous edema, bursitis, tendonitis, and rotator cuff tears.4,12 Complete rotator cuff tears were found in 15% of cases reviewed by Atanasoff and colleagues.4 While there is no recommended timing for MRI, 63% of MRIs were performed within 3 months of symptom onset.4 As SIRVA is not a neurologic injury, nerve conduction, electromyographic studies, and neurologic evaluation or testing are expected to be normal.
Treatment of SIRVA and other vaccine-related shoulder injuries typically have involved pain management (eg, nonsteroidal anti-inflammatory agents), intra-articular steroid injections, and physical therapy, though some patients never experience complete resolution of symptoms.2,4,7 Both patients with vaccination-related shoulder dysfunction described by Bodor and colleagues improved after intra-articular triamcinolone injections, with up to 3 injections before complete resolution of pain in one patient.2 Orthopedics evaluation may need to be considered for persistent symptoms. According to Atanasoff and colleagues, most patients were symptomatic for at least 6 months, and complete recovery was seen in less than one-third of patients.4 Although the development of SIRVA is not a contraindication to future doses of the presumed causative vaccine, subsequent vaccination should include careful consideration of other administration sites if possible (eg, vastus lateralis may be used for IM injections in adults) (Figure 4).
Reporting
A diagnosis or concern for SIRVA also should be reported to the VAERS, the national database established in order to detect possible safety problems with US-licensed vaccines. VAERS reports can be submitted by anyone with concerns for vaccine adverse reactions, including patients, caregivers, and health care professionals at vaers.hhs.gov/reportevent.html. Additional information regarding VICP can be obtained at www.hrsa.gov/vaccine-compensation/index.html.
Military-Specific Issues
The military values readiness, which includes ensuring that active-duty members remain up-to-date on life-saving vaccinations. Immunization is of critical importance to mobility and success of the overall mission. Mobility processing lines where immunizations can be provided to multiple active-duty members can be a successful strategy for mass immunizations. Although the quick administration of immunizations maintains readiness and provides a medically necessary service, it also may increase the chances of incorrect vaccine placement in the deltoid, causing long-term shoulder immobility that may impact a service member’s retainability. The benefits of mobility processing lines can continue to outweigh the risks of immunization administration by ensuring proper staff training, seating both the administrator and recipient of vaccination, and selecting a proper needle length and site of administration specific to each recipient.
Conclusion
Correct administration of vaccines is of utmost importance in preventing SIRVA and other vaccine-related shoulder dysfunctions. Proper staff training and refresher training can help prevent vaccine-related shoulder injuries. Additionally, clinicians should be aware of this potential complication and maintain a high index of suspicion when evaluating patients with postvaccination shoulder complaints.
1. Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases. https://www.cdc.gov/vaccines/pubs/pinkbook/vac-admin.html. Published 2015. Accessed June 3, 2019.
2. Bodor M, Montalvo E. Vaccination-related shoulder dysfunction. Vaccine. 2007;25(4):585-587.
3. Kuether G, Dietrich B, Smith T, Peter C, Gruessner S. Atraumatic osteonecrosis of the humeral head after influenza A-(H1N1) v-2009 vaccination. Vaccine. 2011;29(40):6830-6833.
4. Atanasoff S, Ryan T, Lightfoot R, Johann-Liang R. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010;28(51):8049-8052.
5. Institute of Medicine. Adverse effects of vaccines: evidence and causality. http://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2011/Adverse-Effects-of-Vaccines-Evidence-and-Causality/Vaccine-report-brief-FINAL.pdf. Published August 2011. Accessed June 3, 2019.
6. Health Resources and Services Administration, Health and Human Services Administration. National vaccine injury compensation program: revisions to the vaccine injury table. https://www.federalregister.gov/documents/2017/01/19/2017-00701/national-vaccine-injury-compensation-program-revisions-to-the-vaccine-injury-table. Published January 19, 2017. Accessed June 3, 2019.
7. Martín Arias LH, Sanz Fadrique R, Sáinz Gil M, Salgueiro-Vazquez ME. Risk of bursitis and other injuries and dysfunctions of the shoulder following vaccinations. Vaccine. 2017;35(37):4870-4876.
8. Centers for Disease Control and Prevention. Reports of shoulder dysfunction following inactivated influenza vaccine in the Vaccine Adverse Event Reporting System (VAERS), 2010-2016. https://stacks.cdc.gov/view/cdc/57624. Published January 4, 2018. Accessed June 3, 2019.
9. McGarvey MA, Hooper AC. The deltoid intramuscular injection site in the adult. Current practice among general practitioners and practice nurses. Ir Med J. 2005;98(4):105-107.
10. Cook IF. An evidence based protocol for the prevention of upper arm injury related to vaccine administration (UAIRVA). Hum Vaccin. 2011;7(8):845-848.
11. Cook IF. Best vaccination practice and medically attended injection site events following deltoid intramuscular injection. Hum Vaccin Immunother. 2015;11(5):1184-1191.
12. Okur G, Chaney KA, Lomasney LM. Magnetic resonance imaging of abnormal shoulder pain following influenza vaccination. Skeletal Radiol. 2014;43(9):1325-1331.
1. Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases. https://www.cdc.gov/vaccines/pubs/pinkbook/vac-admin.html. Published 2015. Accessed June 3, 2019.
2. Bodor M, Montalvo E. Vaccination-related shoulder dysfunction. Vaccine. 2007;25(4):585-587.
3. Kuether G, Dietrich B, Smith T, Peter C, Gruessner S. Atraumatic osteonecrosis of the humeral head after influenza A-(H1N1) v-2009 vaccination. Vaccine. 2011;29(40):6830-6833.
4. Atanasoff S, Ryan T, Lightfoot R, Johann-Liang R. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010;28(51):8049-8052.
5. Institute of Medicine. Adverse effects of vaccines: evidence and causality. http://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2011/Adverse-Effects-of-Vaccines-Evidence-and-Causality/Vaccine-report-brief-FINAL.pdf. Published August 2011. Accessed June 3, 2019.
6. Health Resources and Services Administration, Health and Human Services Administration. National vaccine injury compensation program: revisions to the vaccine injury table. https://www.federalregister.gov/documents/2017/01/19/2017-00701/national-vaccine-injury-compensation-program-revisions-to-the-vaccine-injury-table. Published January 19, 2017. Accessed June 3, 2019.
7. Martín Arias LH, Sanz Fadrique R, Sáinz Gil M, Salgueiro-Vazquez ME. Risk of bursitis and other injuries and dysfunctions of the shoulder following vaccinations. Vaccine. 2017;35(37):4870-4876.
8. Centers for Disease Control and Prevention. Reports of shoulder dysfunction following inactivated influenza vaccine in the Vaccine Adverse Event Reporting System (VAERS), 2010-2016. https://stacks.cdc.gov/view/cdc/57624. Published January 4, 2018. Accessed June 3, 2019.
9. McGarvey MA, Hooper AC. The deltoid intramuscular injection site in the adult. Current practice among general practitioners and practice nurses. Ir Med J. 2005;98(4):105-107.
10. Cook IF. An evidence based protocol for the prevention of upper arm injury related to vaccine administration (UAIRVA). Hum Vaccin. 2011;7(8):845-848.
11. Cook IF. Best vaccination practice and medically attended injection site events following deltoid intramuscular injection. Hum Vaccin Immunother. 2015;11(5):1184-1191.
12. Okur G, Chaney KA, Lomasney LM. Magnetic resonance imaging of abnormal shoulder pain following influenza vaccination. Skeletal Radiol. 2014;43(9):1325-1331.