User login
Maximize your leadership in academic hospital medicine
AHA Level 2 course now available
Over the past 2 decades, hospital medicine has grown from a nascent collection of hospitalists to one of the fastest growing specialties, with more than 60,000 active practitioners today.
Ten years ago, the need for mentoring and growth of a new generation of young academic faculty led to the development of the first Academic Hospitalist Academy (AHA) through the coordinated efforts of the Society of Hospital Medicine, the Society of General Internal Medicine, and the Association of Clinical Leaders of General Internal Medicine.
As modern medicine moves at an increasing pace, the intersection of patient care, research, and education has opened further opportunities for fostering the expertise of hospital medicine practitioners. The next level of training is now available with the advent of AHA’s Level 2 course.
Ever wonder why the new clinical service you’ve designed to improve physician and patient efficiency isn’t functioning like it did in the beginning? Patients are staying longer in the hospital, and physicians are working harder. The principles of change management, personal leadership styles, and adult learning will be covered in the AHA Level 2 course. How do I get my project funded and then what do I do with the results? Keys to negotiating for time and resources as well as the skills to write and disseminate your work are integrated into the curriculum.
Participants will be engaged in an interactive course designed around the challenges of practicing and leading in an academic environment. AHA Level 2 aims to help attendees – regardless of their areas of interest – identify and acquire the skills necessary to advance their career, describe the business and cultural landscape of academic health systems, and learn how to leverage that knowledge; to list resources and techniques to continue to further build their skills, and identify and pursue their unique scholarly niche.
Based on the success of AHA’s Level 1 course and the feedback from the almost 1,000 participants who have attended, AHA Level 2 is a 2.5-day course that will allow for the exchange of ideas and skills from nationally regarded faculty and fellow attendees. Through plenary sessions, workshops, small groups, and networking opportunities, attendees will be immersed in the realm of modern academic hospital medicine. The new course is offered in parallel with AHA Level 1 at the Inverness Resort, outside of Denver, on Sept. 10-12, 2019.
The course will leave attendees with an individualized career plan and enhance their area of expertise. The lessons learned and shared will allow participants to return to their institutions and continue to lead in the areas of patient care, financial resourcefulness, and the education of current and future generations of hospital medicine specialists.
Dr. O’Dorisio is a Med-Peds hospitalist at the Ohio State University, Columbus.
AHA Level 2 course now available
AHA Level 2 course now available
Over the past 2 decades, hospital medicine has grown from a nascent collection of hospitalists to one of the fastest growing specialties, with more than 60,000 active practitioners today.
Ten years ago, the need for mentoring and growth of a new generation of young academic faculty led to the development of the first Academic Hospitalist Academy (AHA) through the coordinated efforts of the Society of Hospital Medicine, the Society of General Internal Medicine, and the Association of Clinical Leaders of General Internal Medicine.
As modern medicine moves at an increasing pace, the intersection of patient care, research, and education has opened further opportunities for fostering the expertise of hospital medicine practitioners. The next level of training is now available with the advent of AHA’s Level 2 course.
Ever wonder why the new clinical service you’ve designed to improve physician and patient efficiency isn’t functioning like it did in the beginning? Patients are staying longer in the hospital, and physicians are working harder. The principles of change management, personal leadership styles, and adult learning will be covered in the AHA Level 2 course. How do I get my project funded and then what do I do with the results? Keys to negotiating for time and resources as well as the skills to write and disseminate your work are integrated into the curriculum.
Participants will be engaged in an interactive course designed around the challenges of practicing and leading in an academic environment. AHA Level 2 aims to help attendees – regardless of their areas of interest – identify and acquire the skills necessary to advance their career, describe the business and cultural landscape of academic health systems, and learn how to leverage that knowledge; to list resources and techniques to continue to further build their skills, and identify and pursue their unique scholarly niche.
Based on the success of AHA’s Level 1 course and the feedback from the almost 1,000 participants who have attended, AHA Level 2 is a 2.5-day course that will allow for the exchange of ideas and skills from nationally regarded faculty and fellow attendees. Through plenary sessions, workshops, small groups, and networking opportunities, attendees will be immersed in the realm of modern academic hospital medicine. The new course is offered in parallel with AHA Level 1 at the Inverness Resort, outside of Denver, on Sept. 10-12, 2019.
The course will leave attendees with an individualized career plan and enhance their area of expertise. The lessons learned and shared will allow participants to return to their institutions and continue to lead in the areas of patient care, financial resourcefulness, and the education of current and future generations of hospital medicine specialists.
Dr. O’Dorisio is a Med-Peds hospitalist at the Ohio State University, Columbus.
Over the past 2 decades, hospital medicine has grown from a nascent collection of hospitalists to one of the fastest growing specialties, with more than 60,000 active practitioners today.
Ten years ago, the need for mentoring and growth of a new generation of young academic faculty led to the development of the first Academic Hospitalist Academy (AHA) through the coordinated efforts of the Society of Hospital Medicine, the Society of General Internal Medicine, and the Association of Clinical Leaders of General Internal Medicine.
As modern medicine moves at an increasing pace, the intersection of patient care, research, and education has opened further opportunities for fostering the expertise of hospital medicine practitioners. The next level of training is now available with the advent of AHA’s Level 2 course.
Ever wonder why the new clinical service you’ve designed to improve physician and patient efficiency isn’t functioning like it did in the beginning? Patients are staying longer in the hospital, and physicians are working harder. The principles of change management, personal leadership styles, and adult learning will be covered in the AHA Level 2 course. How do I get my project funded and then what do I do with the results? Keys to negotiating for time and resources as well as the skills to write and disseminate your work are integrated into the curriculum.
Participants will be engaged in an interactive course designed around the challenges of practicing and leading in an academic environment. AHA Level 2 aims to help attendees – regardless of their areas of interest – identify and acquire the skills necessary to advance their career, describe the business and cultural landscape of academic health systems, and learn how to leverage that knowledge; to list resources and techniques to continue to further build their skills, and identify and pursue their unique scholarly niche.
Based on the success of AHA’s Level 1 course and the feedback from the almost 1,000 participants who have attended, AHA Level 2 is a 2.5-day course that will allow for the exchange of ideas and skills from nationally regarded faculty and fellow attendees. Through plenary sessions, workshops, small groups, and networking opportunities, attendees will be immersed in the realm of modern academic hospital medicine. The new course is offered in parallel with AHA Level 1 at the Inverness Resort, outside of Denver, on Sept. 10-12, 2019.
The course will leave attendees with an individualized career plan and enhance their area of expertise. The lessons learned and shared will allow participants to return to their institutions and continue to lead in the areas of patient care, financial resourcefulness, and the education of current and future generations of hospital medicine specialists.
Dr. O’Dorisio is a Med-Peds hospitalist at the Ohio State University, Columbus.
Psoriasis patients on biologics show improved heart health
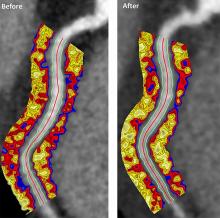
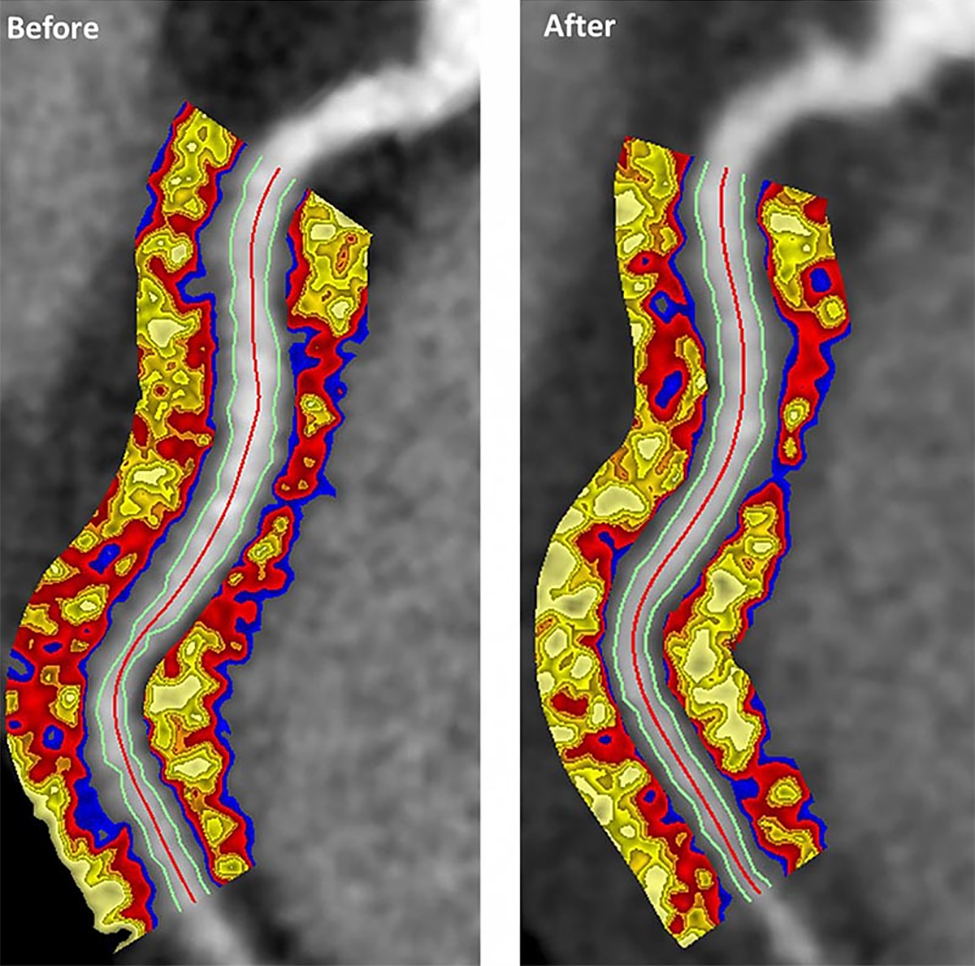
Biologics improved coronary inflammation as well as psoriasis symptoms, according to data from the perivascular fat attenuation index in 134 adults identified using coronary CT angiography.
“The perivascular fat attenuation index [FAI] is a [CT]-based, novel, noninvasive imaging technique that allows for direct visualization and quantification of coronary inflammation using differential mapping of attenuation gradients in pericoronary fat,” wrote Youssef A. Elnabawi, MD, of the National Heart, Lung, and Blood Institute and colleagues. Biologics have been associated with reduced noncalcified coronary plaques in psoriasis patients, which suggests possible reduction in coronary inflammation as well.
In a study published in JAMA Cardiology, the researchers analyzed data from 134 adults with moderate to severe psoriasis who received no biologic therapy for at least 3 months before starting the study. Of these, 52 chose not to receive biologics, and served as controls while being treated with topical or light therapies. The participants are part of the Psoriasis Atherosclerosis Cardiometabolic Initiative, an ongoing, prospective cohort study. The average age of the patients was 51 years, and 63% were male.
The 82 patients given biologics received anti–tumor necrosis factor–alpha, anti–interleukin-12/23, or anti-IL-17 for 1 year. Overall, patients on biologics showed a significant decrease in FAI from a median of –71.22 Hounsfield units (HU) at baseline to a median of –76.06 at 1 year. These patients also showed significant improvement in Psoriasis Area and Severity Index scores, from a median of 7.7 at baseline to a median of 3.2 at 1 year. The control patients not on biologics showed no significant changes in FAI, with a median of –71.98 HU at baseline and –72.66 HU at 1 year.
The changes were consistent among the various biologics used, and The median FAI for patients on anti–tumor necrosis factor–alpha changed from –71.25 at baseline to –75.49 at 1 year; median FAI for both IL-12/23 and anti-IL-17 treatment groups changed from –71.18 HU at baseline to –76.92 at 1 year.
In addition, only patients treated with biologics showed a significant reduction in median C-reactive protein levels from baseline (2.2 mg/L vs. 1.3 mg/L). The changes in FAI were not associated with the presence of coronary plaques, the researchers noted.
The study findings were limited by several factors, including the observational design, small size, and lack of data on cardiovascular endpoints. “Future studies will be needed to explore whether the residual CV risk detected by perivascular FAI can be attenuated using targeted anti-inflammatory interventions,” they wrote.
However, the results suggest that biologics impact coronary vasculature at the microenvironmental level, and that FAI can be a noninvasive, cost-effective way to stratify patients at increased risk for cardiovascular disease, the researchers noted.
“We believe that the strength of perivascular FAI in risk stratifying patients with increased coronary inflammation will allow for better identification of patients at increased risk of future myocardial events that are not captured by traditional CV risk factors,” they wrote.
The study was funded by the National Institutes of Health, several research foundations, Elsevier, Colgate-Palmolive, and Genentech. Dr. Elnabawi had no financial conflicts to disclose; several coauthors reported relationships with multiple companies. One coauthor disclosed a pending and licensed patent to a novel tool for cardiovascular risk stratification based on the CT attenuation of perivascular tissue (OxScore) and a pending and licensed patent to perivascular texture index.
SOURCE: Elnabawi YA et al. JAMA Cardiol. 2019 Jul 31. doi: 10.1001/jamacardio.2019.2589.
Biologics improved coronary inflammation as well as psoriasis symptoms, according to data from the perivascular fat attenuation index in 134 adults identified using coronary CT angiography.
“The perivascular fat attenuation index [FAI] is a [CT]-based, novel, noninvasive imaging technique that allows for direct visualization and quantification of coronary inflammation using differential mapping of attenuation gradients in pericoronary fat,” wrote Youssef A. Elnabawi, MD, of the National Heart, Lung, and Blood Institute and colleagues. Biologics have been associated with reduced noncalcified coronary plaques in psoriasis patients, which suggests possible reduction in coronary inflammation as well.
In a study published in JAMA Cardiology, the researchers analyzed data from 134 adults with moderate to severe psoriasis who received no biologic therapy for at least 3 months before starting the study. Of these, 52 chose not to receive biologics, and served as controls while being treated with topical or light therapies. The participants are part of the Psoriasis Atherosclerosis Cardiometabolic Initiative, an ongoing, prospective cohort study. The average age of the patients was 51 years, and 63% were male.
The 82 patients given biologics received anti–tumor necrosis factor–alpha, anti–interleukin-12/23, or anti-IL-17 for 1 year. Overall, patients on biologics showed a significant decrease in FAI from a median of –71.22 Hounsfield units (HU) at baseline to a median of –76.06 at 1 year. These patients also showed significant improvement in Psoriasis Area and Severity Index scores, from a median of 7.7 at baseline to a median of 3.2 at 1 year. The control patients not on biologics showed no significant changes in FAI, with a median of –71.98 HU at baseline and –72.66 HU at 1 year.
The changes were consistent among the various biologics used, and The median FAI for patients on anti–tumor necrosis factor–alpha changed from –71.25 at baseline to –75.49 at 1 year; median FAI for both IL-12/23 and anti-IL-17 treatment groups changed from –71.18 HU at baseline to –76.92 at 1 year.
In addition, only patients treated with biologics showed a significant reduction in median C-reactive protein levels from baseline (2.2 mg/L vs. 1.3 mg/L). The changes in FAI were not associated with the presence of coronary plaques, the researchers noted.
The study findings were limited by several factors, including the observational design, small size, and lack of data on cardiovascular endpoints. “Future studies will be needed to explore whether the residual CV risk detected by perivascular FAI can be attenuated using targeted anti-inflammatory interventions,” they wrote.
However, the results suggest that biologics impact coronary vasculature at the microenvironmental level, and that FAI can be a noninvasive, cost-effective way to stratify patients at increased risk for cardiovascular disease, the researchers noted.
“We believe that the strength of perivascular FAI in risk stratifying patients with increased coronary inflammation will allow for better identification of patients at increased risk of future myocardial events that are not captured by traditional CV risk factors,” they wrote.
The study was funded by the National Institutes of Health, several research foundations, Elsevier, Colgate-Palmolive, and Genentech. Dr. Elnabawi had no financial conflicts to disclose; several coauthors reported relationships with multiple companies. One coauthor disclosed a pending and licensed patent to a novel tool for cardiovascular risk stratification based on the CT attenuation of perivascular tissue (OxScore) and a pending and licensed patent to perivascular texture index.
SOURCE: Elnabawi YA et al. JAMA Cardiol. 2019 Jul 31. doi: 10.1001/jamacardio.2019.2589.
Biologics improved coronary inflammation as well as psoriasis symptoms, according to data from the perivascular fat attenuation index in 134 adults identified using coronary CT angiography.
“The perivascular fat attenuation index [FAI] is a [CT]-based, novel, noninvasive imaging technique that allows for direct visualization and quantification of coronary inflammation using differential mapping of attenuation gradients in pericoronary fat,” wrote Youssef A. Elnabawi, MD, of the National Heart, Lung, and Blood Institute and colleagues. Biologics have been associated with reduced noncalcified coronary plaques in psoriasis patients, which suggests possible reduction in coronary inflammation as well.
In a study published in JAMA Cardiology, the researchers analyzed data from 134 adults with moderate to severe psoriasis who received no biologic therapy for at least 3 months before starting the study. Of these, 52 chose not to receive biologics, and served as controls while being treated with topical or light therapies. The participants are part of the Psoriasis Atherosclerosis Cardiometabolic Initiative, an ongoing, prospective cohort study. The average age of the patients was 51 years, and 63% were male.
The 82 patients given biologics received anti–tumor necrosis factor–alpha, anti–interleukin-12/23, or anti-IL-17 for 1 year. Overall, patients on biologics showed a significant decrease in FAI from a median of –71.22 Hounsfield units (HU) at baseline to a median of –76.06 at 1 year. These patients also showed significant improvement in Psoriasis Area and Severity Index scores, from a median of 7.7 at baseline to a median of 3.2 at 1 year. The control patients not on biologics showed no significant changes in FAI, with a median of –71.98 HU at baseline and –72.66 HU at 1 year.
The changes were consistent among the various biologics used, and The median FAI for patients on anti–tumor necrosis factor–alpha changed from –71.25 at baseline to –75.49 at 1 year; median FAI for both IL-12/23 and anti-IL-17 treatment groups changed from –71.18 HU at baseline to –76.92 at 1 year.
In addition, only patients treated with biologics showed a significant reduction in median C-reactive protein levels from baseline (2.2 mg/L vs. 1.3 mg/L). The changes in FAI were not associated with the presence of coronary plaques, the researchers noted.
The study findings were limited by several factors, including the observational design, small size, and lack of data on cardiovascular endpoints. “Future studies will be needed to explore whether the residual CV risk detected by perivascular FAI can be attenuated using targeted anti-inflammatory interventions,” they wrote.
However, the results suggest that biologics impact coronary vasculature at the microenvironmental level, and that FAI can be a noninvasive, cost-effective way to stratify patients at increased risk for cardiovascular disease, the researchers noted.
“We believe that the strength of perivascular FAI in risk stratifying patients with increased coronary inflammation will allow for better identification of patients at increased risk of future myocardial events that are not captured by traditional CV risk factors,” they wrote.
The study was funded by the National Institutes of Health, several research foundations, Elsevier, Colgate-Palmolive, and Genentech. Dr. Elnabawi had no financial conflicts to disclose; several coauthors reported relationships with multiple companies. One coauthor disclosed a pending and licensed patent to a novel tool for cardiovascular risk stratification based on the CT attenuation of perivascular tissue (OxScore) and a pending and licensed patent to perivascular texture index.
SOURCE: Elnabawi YA et al. JAMA Cardiol. 2019 Jul 31. doi: 10.1001/jamacardio.2019.2589.
FROM JAMA CARDIOLOGY
Sleep aids and dementia: Studies find both risks and benefits
LOS ANGELES – While a large number of older adults take prescription and nonprescription medications to help them sleep, the effect of these medications on dementia risk is unclear, with most researchers advocating a cautious and conservative approach to prescribing.
Research is increasingly revealing a bidirectional relationship between sleep and dementia. Poor sleep – especially from insomnia, sleep deprivation, or obstructive sleep apnea – is known to increase dementia risk. Dementias, meanwhile, are associated with serious circadian rhythm disturbances, leading to nighttime sleep loss and increasing the likelihood of institutionalization.
At the Alzheimer’s Association International Conference, researchers presented findings assessing the links between sleep medication use and dementia and also what agents or approaches might safely improve sleep in people with sleep disorders who are at risk for dementia or who have been diagnosed with dementia.
Sex- and race-based differences in risk
Yue Leng, PhD, of the University of California, San Francisco, reported a link between frequent sleep medication use and later dementia – but only in white adults. Dr. Leng presented findings from the National Institutes of Health–funded Health, Aging, and Body Composition Study, which recruited 3,068 subjects aged 70-79 and followed them for 15 years. At baseline, 2.7% of African Americans and 7.7% of whites in the study reported taking sleep medications “often” or “almost always.”
Dr. Leng and her colleagues found that white subjects who reported taking sleep aids five or more times a month at baseline had a nearly 80% higher risk of developing dementia during the course of the study (hazard ratio, 1.79; 95% confidence interval, 1.21-2.66), compared with people who reported never taking sleep aids or taking them less frequently.
The researchers saw no between-sex differences for this finding, and adjusted for a variety of genetic and lifestyle confounders. Importantly, no significant increase in dementia risk was seen for black subjects, who made up more than one-third of the cohort.
Dr. Leng told the conference that the researchers could not explain why black participants did not see similarly increased dementia risk. Also, she noted, researchers did not have information on the specific sleep medications people used: benzodiazepines, antihistamines, antidepressants, or other types of drugs. Nonetheless, she told the conference, the findings ratified the cautious approach many dementia experts are already stressing.
“Do we really need to prescribe so many sleep meds to older adults who are already at risk for cognitive impairment?” Dr. Leng said, adding: “I am a big advocate of behavioral sleep interventions.” People with clinical sleep problems “should be referred to sleep centers” for a fuller assessment before medication is prescribed, she said.
Findings from another cohort study, meanwhile, suggest that there could be sex-related differences in how sleep aids affect dementia risk. Investigators at Utah State University in Logan used data from some 3,656 older adults in the Cache County Study on Memory and Aging, an NIH-backed cohort study of white adults in Utah without dementia at baseline who were followed for 12 years.
The investigators, led by doctoral student Elizabeth Vernon, found that men reporting use of sleep medication saw more than threefold higher risk of developing Alzheimer’s disease than did men who did not use sleep aids (HR, 3.604; P = .0001).
Women who did not report having sleep disturbance but used sleep-inducing medications were at nearly fourfold greater risk for developing Alzheimer’s disease (HR, 3.916; P = .0001). Women who self-reported sleep disturbances at baseline, meanwhile, saw a reduction in Alzheimer’s risk of about one-third associated with the use of sleep medications.
Ms. Vernon told the conference that, despite the finding of risk reduction for this particular group of women, caution in prescribing sleep aids was warranted.
Common sleep drugs linked to cognitive aging
Chris Fox, MD, a researcher at the University of East Anglia in Norwich, England, and his colleagues demonstrated in 2018 that long-term exposure to anticholinergic drugs, a class that includes some antidepressants and antihistamines used to promote sleep, was associated with a higher risk of dementia, while use of benzodiazepines, a class of sedatives used commonly in older people as sleep aids, was not. (Whether benzodiazepine exposure relates to dementia remains controversial.)
At AAIC 2019, Dr. Fox presented findings from a study of 337 brains in a U.K. brain bank, of which 17% and 21% came from users of benzodiazepines and anticholinergic drugs, whose usage history was well documented. Dr. Fox and his colleagues found that, while neither anticholinergic nor benzodiazepine exposure was associated with brain pathology specific to that seen in Alzheimer’s disease, both classes of drugs were associated with “slight signals in neuronal loss” in one brain region, the nucleus basalis of Meynert. Dr. Fox described the drugs as causing “an increase in cognitive aging” which could bear on Alzheimer’s risk without being directly causative.
Newer sleep drugs may help Alzheimer’s patients
Scientists working for drug manufacturers presented findings on agents to counter the circadian rhythm disturbances seen in people with Alzheimer’s disease. Margaret Moline, PhD, of Eisai in Woodcliff Lake, N.J., showed some results from a phase 2, dose-ranging, placebo-controlled study of the experimental agent lemborexant in 62 subjects aged 60-90 with mild to moderate Alzheimer’s disease and sleep disturbances. (Lemborexant, an orexin receptor agonist that acts to regulate wakefulness, is being investigated in a broad range of sleep disorders.) Patients were randomized to one of four doses of lemborexant or placebo and wore a device for sleep monitoring. Nighttime activity indicating arousal was significantly lower for people in two dosage arms, 5 mg and 10 mg, compared with placebo, and treatment groups saw trends toward less sleep fragmentation and higher total sleep time, Dr. Moline told the conference.
Suvorexant (Belsomra), the only orexin receptor antagonist currently licensed as a sleep aid, is also being tested in people with Alzheimer’s disease. At AAIC 2019, Joseph Herring, MD, PhD, of Merck in Kenilworth, N.J., presented results from a placebo-controlled trial of 277 patients with Alzheimer’s disease and insomnia, and reported that treatment with 10 or 20 mg of suvorexant over 4 weeks was associated with about an extra half hour of total nightly sleep, with a 73-minute mean increase from baseline, compared with 45 minutes for patients receiving placebo (95% CI, 11-45; P less than .005).
Trazodone linked to slower cognitive decline
An inexpensive antidepressant used in low doses as a sleep aid, including in people with Alzheimer’s disease, was associated with a delay in cognitive decline in older adults, according to results from a retrospective study. Elissaios Karageorgiou, MD, PhD, of the University of California, San Francisco, and the Neurological Institute of Athens presented results derived from two cohorts: patients enrolled at the UCSF Memory and Aging Center and women enrolled in the Study for Osteoporotic Fractures (SOF) in Women. The investigators were able to identify trazodone users in the studies (with two or more contiguous study visits reporting trazodone use) and match them with control patients from the same cohorts who did not use trazodone.
Trazodone was studied because previous research suggests it increases total sleep time in patients with Alzheimer’s disease without affecting next-day cognitive performance.
Trazodone-using patients in the UCSF cohort (n = 25) saw significantly less decline in Mini Mental State Exam (MMSE) scores over 4 years, compared with nonusers (0.27 vs. 0.70 points per year; P = .023), an effect that remained statistically significant even after adjusting for sedative and stimulant use and the expected progression of Alzheimer’s disease pathology. Importantly, the slower decline was seen only among subjects with sleep complaints at baseline and especially those whose sleep improved over time, suggesting that the cognitive benefit was mediated by improved sleep.
In the SOF cohort of 46 trazodone users matched with 148 nonusers, no significant protective or negative effect related to long-term trazodone use was found using the MMSE or the Trails Making Test. In this analysis, however, baseline and longitudinal sleep quality was not captured in the group-matching process, neither was the use of other medications. The patient group was slightly older, and all patients were women.
Dr. Karageorgiou said in an interview that the link between improved sleep, trazodone, and cognition needs to be validated in prospective intervention studies. Trazodone, he said, appears to work best in people with a specific type of insomnia characterized by cortical and behavioral hyperarousal, and its cognitive effect appears limited to people whose sleep improves with treatment. “You’re not going to see long-term cognitive benefits if it’s not improving your sleep,” Dr. Karageorgiou said. “So, whether trazodone improves sleep or not in a patient after a few months can be an early indicator for the clinician to continue using it or suspend it, because it is unlikely to help their cognition otherwise.”
He stressed that physicians need to be broadly focused on improving sleep to help patients with, or at risk for, dementia by consolidating their sleep rhythms.
“Trazodone is not the magic bullet, and I don’t think we will ever have a magic bullet,” Dr. Karageorgiou said. “Because when our brain degenerates, it’s not just one chemical, or one system, it’s many. And our body changes as well. The important thing is to help the patient consolidate their rhythms, whether through light therapy, daily exercise, cognitive behavioral therapy for insomnia, or other evidence-based interventions and their combination. The same applies for a person with dementia as for the rest of us.”
None of the investigators outside of the industry-sponsored studies had relevant disclosures.
LOS ANGELES – While a large number of older adults take prescription and nonprescription medications to help them sleep, the effect of these medications on dementia risk is unclear, with most researchers advocating a cautious and conservative approach to prescribing.
Research is increasingly revealing a bidirectional relationship between sleep and dementia. Poor sleep – especially from insomnia, sleep deprivation, or obstructive sleep apnea – is known to increase dementia risk. Dementias, meanwhile, are associated with serious circadian rhythm disturbances, leading to nighttime sleep loss and increasing the likelihood of institutionalization.
At the Alzheimer’s Association International Conference, researchers presented findings assessing the links between sleep medication use and dementia and also what agents or approaches might safely improve sleep in people with sleep disorders who are at risk for dementia or who have been diagnosed with dementia.
Sex- and race-based differences in risk
Yue Leng, PhD, of the University of California, San Francisco, reported a link between frequent sleep medication use and later dementia – but only in white adults. Dr. Leng presented findings from the National Institutes of Health–funded Health, Aging, and Body Composition Study, which recruited 3,068 subjects aged 70-79 and followed them for 15 years. At baseline, 2.7% of African Americans and 7.7% of whites in the study reported taking sleep medications “often” or “almost always.”
Dr. Leng and her colleagues found that white subjects who reported taking sleep aids five or more times a month at baseline had a nearly 80% higher risk of developing dementia during the course of the study (hazard ratio, 1.79; 95% confidence interval, 1.21-2.66), compared with people who reported never taking sleep aids or taking them less frequently.
The researchers saw no between-sex differences for this finding, and adjusted for a variety of genetic and lifestyle confounders. Importantly, no significant increase in dementia risk was seen for black subjects, who made up more than one-third of the cohort.
Dr. Leng told the conference that the researchers could not explain why black participants did not see similarly increased dementia risk. Also, she noted, researchers did not have information on the specific sleep medications people used: benzodiazepines, antihistamines, antidepressants, or other types of drugs. Nonetheless, she told the conference, the findings ratified the cautious approach many dementia experts are already stressing.
“Do we really need to prescribe so many sleep meds to older adults who are already at risk for cognitive impairment?” Dr. Leng said, adding: “I am a big advocate of behavioral sleep interventions.” People with clinical sleep problems “should be referred to sleep centers” for a fuller assessment before medication is prescribed, she said.
Findings from another cohort study, meanwhile, suggest that there could be sex-related differences in how sleep aids affect dementia risk. Investigators at Utah State University in Logan used data from some 3,656 older adults in the Cache County Study on Memory and Aging, an NIH-backed cohort study of white adults in Utah without dementia at baseline who were followed for 12 years.
The investigators, led by doctoral student Elizabeth Vernon, found that men reporting use of sleep medication saw more than threefold higher risk of developing Alzheimer’s disease than did men who did not use sleep aids (HR, 3.604; P = .0001).
Women who did not report having sleep disturbance but used sleep-inducing medications were at nearly fourfold greater risk for developing Alzheimer’s disease (HR, 3.916; P = .0001). Women who self-reported sleep disturbances at baseline, meanwhile, saw a reduction in Alzheimer’s risk of about one-third associated with the use of sleep medications.
Ms. Vernon told the conference that, despite the finding of risk reduction for this particular group of women, caution in prescribing sleep aids was warranted.
Common sleep drugs linked to cognitive aging
Chris Fox, MD, a researcher at the University of East Anglia in Norwich, England, and his colleagues demonstrated in 2018 that long-term exposure to anticholinergic drugs, a class that includes some antidepressants and antihistamines used to promote sleep, was associated with a higher risk of dementia, while use of benzodiazepines, a class of sedatives used commonly in older people as sleep aids, was not. (Whether benzodiazepine exposure relates to dementia remains controversial.)
At AAIC 2019, Dr. Fox presented findings from a study of 337 brains in a U.K. brain bank, of which 17% and 21% came from users of benzodiazepines and anticholinergic drugs, whose usage history was well documented. Dr. Fox and his colleagues found that, while neither anticholinergic nor benzodiazepine exposure was associated with brain pathology specific to that seen in Alzheimer’s disease, both classes of drugs were associated with “slight signals in neuronal loss” in one brain region, the nucleus basalis of Meynert. Dr. Fox described the drugs as causing “an increase in cognitive aging” which could bear on Alzheimer’s risk without being directly causative.
Newer sleep drugs may help Alzheimer’s patients
Scientists working for drug manufacturers presented findings on agents to counter the circadian rhythm disturbances seen in people with Alzheimer’s disease. Margaret Moline, PhD, of Eisai in Woodcliff Lake, N.J., showed some results from a phase 2, dose-ranging, placebo-controlled study of the experimental agent lemborexant in 62 subjects aged 60-90 with mild to moderate Alzheimer’s disease and sleep disturbances. (Lemborexant, an orexin receptor agonist that acts to regulate wakefulness, is being investigated in a broad range of sleep disorders.) Patients were randomized to one of four doses of lemborexant or placebo and wore a device for sleep monitoring. Nighttime activity indicating arousal was significantly lower for people in two dosage arms, 5 mg and 10 mg, compared with placebo, and treatment groups saw trends toward less sleep fragmentation and higher total sleep time, Dr. Moline told the conference.
Suvorexant (Belsomra), the only orexin receptor antagonist currently licensed as a sleep aid, is also being tested in people with Alzheimer’s disease. At AAIC 2019, Joseph Herring, MD, PhD, of Merck in Kenilworth, N.J., presented results from a placebo-controlled trial of 277 patients with Alzheimer’s disease and insomnia, and reported that treatment with 10 or 20 mg of suvorexant over 4 weeks was associated with about an extra half hour of total nightly sleep, with a 73-minute mean increase from baseline, compared with 45 minutes for patients receiving placebo (95% CI, 11-45; P less than .005).
Trazodone linked to slower cognitive decline
An inexpensive antidepressant used in low doses as a sleep aid, including in people with Alzheimer’s disease, was associated with a delay in cognitive decline in older adults, according to results from a retrospective study. Elissaios Karageorgiou, MD, PhD, of the University of California, San Francisco, and the Neurological Institute of Athens presented results derived from two cohorts: patients enrolled at the UCSF Memory and Aging Center and women enrolled in the Study for Osteoporotic Fractures (SOF) in Women. The investigators were able to identify trazodone users in the studies (with two or more contiguous study visits reporting trazodone use) and match them with control patients from the same cohorts who did not use trazodone.
Trazodone was studied because previous research suggests it increases total sleep time in patients with Alzheimer’s disease without affecting next-day cognitive performance.
Trazodone-using patients in the UCSF cohort (n = 25) saw significantly less decline in Mini Mental State Exam (MMSE) scores over 4 years, compared with nonusers (0.27 vs. 0.70 points per year; P = .023), an effect that remained statistically significant even after adjusting for sedative and stimulant use and the expected progression of Alzheimer’s disease pathology. Importantly, the slower decline was seen only among subjects with sleep complaints at baseline and especially those whose sleep improved over time, suggesting that the cognitive benefit was mediated by improved sleep.
In the SOF cohort of 46 trazodone users matched with 148 nonusers, no significant protective or negative effect related to long-term trazodone use was found using the MMSE or the Trails Making Test. In this analysis, however, baseline and longitudinal sleep quality was not captured in the group-matching process, neither was the use of other medications. The patient group was slightly older, and all patients were women.
Dr. Karageorgiou said in an interview that the link between improved sleep, trazodone, and cognition needs to be validated in prospective intervention studies. Trazodone, he said, appears to work best in people with a specific type of insomnia characterized by cortical and behavioral hyperarousal, and its cognitive effect appears limited to people whose sleep improves with treatment. “You’re not going to see long-term cognitive benefits if it’s not improving your sleep,” Dr. Karageorgiou said. “So, whether trazodone improves sleep or not in a patient after a few months can be an early indicator for the clinician to continue using it or suspend it, because it is unlikely to help their cognition otherwise.”
He stressed that physicians need to be broadly focused on improving sleep to help patients with, or at risk for, dementia by consolidating their sleep rhythms.
“Trazodone is not the magic bullet, and I don’t think we will ever have a magic bullet,” Dr. Karageorgiou said. “Because when our brain degenerates, it’s not just one chemical, or one system, it’s many. And our body changes as well. The important thing is to help the patient consolidate their rhythms, whether through light therapy, daily exercise, cognitive behavioral therapy for insomnia, or other evidence-based interventions and their combination. The same applies for a person with dementia as for the rest of us.”
None of the investigators outside of the industry-sponsored studies had relevant disclosures.
LOS ANGELES – While a large number of older adults take prescription and nonprescription medications to help them sleep, the effect of these medications on dementia risk is unclear, with most researchers advocating a cautious and conservative approach to prescribing.
Research is increasingly revealing a bidirectional relationship between sleep and dementia. Poor sleep – especially from insomnia, sleep deprivation, or obstructive sleep apnea – is known to increase dementia risk. Dementias, meanwhile, are associated with serious circadian rhythm disturbances, leading to nighttime sleep loss and increasing the likelihood of institutionalization.
At the Alzheimer’s Association International Conference, researchers presented findings assessing the links between sleep medication use and dementia and also what agents or approaches might safely improve sleep in people with sleep disorders who are at risk for dementia or who have been diagnosed with dementia.
Sex- and race-based differences in risk
Yue Leng, PhD, of the University of California, San Francisco, reported a link between frequent sleep medication use and later dementia – but only in white adults. Dr. Leng presented findings from the National Institutes of Health–funded Health, Aging, and Body Composition Study, which recruited 3,068 subjects aged 70-79 and followed them for 15 years. At baseline, 2.7% of African Americans and 7.7% of whites in the study reported taking sleep medications “often” or “almost always.”
Dr. Leng and her colleagues found that white subjects who reported taking sleep aids five or more times a month at baseline had a nearly 80% higher risk of developing dementia during the course of the study (hazard ratio, 1.79; 95% confidence interval, 1.21-2.66), compared with people who reported never taking sleep aids or taking them less frequently.
The researchers saw no between-sex differences for this finding, and adjusted for a variety of genetic and lifestyle confounders. Importantly, no significant increase in dementia risk was seen for black subjects, who made up more than one-third of the cohort.
Dr. Leng told the conference that the researchers could not explain why black participants did not see similarly increased dementia risk. Also, she noted, researchers did not have information on the specific sleep medications people used: benzodiazepines, antihistamines, antidepressants, or other types of drugs. Nonetheless, she told the conference, the findings ratified the cautious approach many dementia experts are already stressing.
“Do we really need to prescribe so many sleep meds to older adults who are already at risk for cognitive impairment?” Dr. Leng said, adding: “I am a big advocate of behavioral sleep interventions.” People with clinical sleep problems “should be referred to sleep centers” for a fuller assessment before medication is prescribed, she said.
Findings from another cohort study, meanwhile, suggest that there could be sex-related differences in how sleep aids affect dementia risk. Investigators at Utah State University in Logan used data from some 3,656 older adults in the Cache County Study on Memory and Aging, an NIH-backed cohort study of white adults in Utah without dementia at baseline who were followed for 12 years.
The investigators, led by doctoral student Elizabeth Vernon, found that men reporting use of sleep medication saw more than threefold higher risk of developing Alzheimer’s disease than did men who did not use sleep aids (HR, 3.604; P = .0001).
Women who did not report having sleep disturbance but used sleep-inducing medications were at nearly fourfold greater risk for developing Alzheimer’s disease (HR, 3.916; P = .0001). Women who self-reported sleep disturbances at baseline, meanwhile, saw a reduction in Alzheimer’s risk of about one-third associated with the use of sleep medications.
Ms. Vernon told the conference that, despite the finding of risk reduction for this particular group of women, caution in prescribing sleep aids was warranted.
Common sleep drugs linked to cognitive aging
Chris Fox, MD, a researcher at the University of East Anglia in Norwich, England, and his colleagues demonstrated in 2018 that long-term exposure to anticholinergic drugs, a class that includes some antidepressants and antihistamines used to promote sleep, was associated with a higher risk of dementia, while use of benzodiazepines, a class of sedatives used commonly in older people as sleep aids, was not. (Whether benzodiazepine exposure relates to dementia remains controversial.)
At AAIC 2019, Dr. Fox presented findings from a study of 337 brains in a U.K. brain bank, of which 17% and 21% came from users of benzodiazepines and anticholinergic drugs, whose usage history was well documented. Dr. Fox and his colleagues found that, while neither anticholinergic nor benzodiazepine exposure was associated with brain pathology specific to that seen in Alzheimer’s disease, both classes of drugs were associated with “slight signals in neuronal loss” in one brain region, the nucleus basalis of Meynert. Dr. Fox described the drugs as causing “an increase in cognitive aging” which could bear on Alzheimer’s risk without being directly causative.
Newer sleep drugs may help Alzheimer’s patients
Scientists working for drug manufacturers presented findings on agents to counter the circadian rhythm disturbances seen in people with Alzheimer’s disease. Margaret Moline, PhD, of Eisai in Woodcliff Lake, N.J., showed some results from a phase 2, dose-ranging, placebo-controlled study of the experimental agent lemborexant in 62 subjects aged 60-90 with mild to moderate Alzheimer’s disease and sleep disturbances. (Lemborexant, an orexin receptor agonist that acts to regulate wakefulness, is being investigated in a broad range of sleep disorders.) Patients were randomized to one of four doses of lemborexant or placebo and wore a device for sleep monitoring. Nighttime activity indicating arousal was significantly lower for people in two dosage arms, 5 mg and 10 mg, compared with placebo, and treatment groups saw trends toward less sleep fragmentation and higher total sleep time, Dr. Moline told the conference.
Suvorexant (Belsomra), the only orexin receptor antagonist currently licensed as a sleep aid, is also being tested in people with Alzheimer’s disease. At AAIC 2019, Joseph Herring, MD, PhD, of Merck in Kenilworth, N.J., presented results from a placebo-controlled trial of 277 patients with Alzheimer’s disease and insomnia, and reported that treatment with 10 or 20 mg of suvorexant over 4 weeks was associated with about an extra half hour of total nightly sleep, with a 73-minute mean increase from baseline, compared with 45 minutes for patients receiving placebo (95% CI, 11-45; P less than .005).
Trazodone linked to slower cognitive decline
An inexpensive antidepressant used in low doses as a sleep aid, including in people with Alzheimer’s disease, was associated with a delay in cognitive decline in older adults, according to results from a retrospective study. Elissaios Karageorgiou, MD, PhD, of the University of California, San Francisco, and the Neurological Institute of Athens presented results derived from two cohorts: patients enrolled at the UCSF Memory and Aging Center and women enrolled in the Study for Osteoporotic Fractures (SOF) in Women. The investigators were able to identify trazodone users in the studies (with two or more contiguous study visits reporting trazodone use) and match them with control patients from the same cohorts who did not use trazodone.
Trazodone was studied because previous research suggests it increases total sleep time in patients with Alzheimer’s disease without affecting next-day cognitive performance.
Trazodone-using patients in the UCSF cohort (n = 25) saw significantly less decline in Mini Mental State Exam (MMSE) scores over 4 years, compared with nonusers (0.27 vs. 0.70 points per year; P = .023), an effect that remained statistically significant even after adjusting for sedative and stimulant use and the expected progression of Alzheimer’s disease pathology. Importantly, the slower decline was seen only among subjects with sleep complaints at baseline and especially those whose sleep improved over time, suggesting that the cognitive benefit was mediated by improved sleep.
In the SOF cohort of 46 trazodone users matched with 148 nonusers, no significant protective or negative effect related to long-term trazodone use was found using the MMSE or the Trails Making Test. In this analysis, however, baseline and longitudinal sleep quality was not captured in the group-matching process, neither was the use of other medications. The patient group was slightly older, and all patients were women.
Dr. Karageorgiou said in an interview that the link between improved sleep, trazodone, and cognition needs to be validated in prospective intervention studies. Trazodone, he said, appears to work best in people with a specific type of insomnia characterized by cortical and behavioral hyperarousal, and its cognitive effect appears limited to people whose sleep improves with treatment. “You’re not going to see long-term cognitive benefits if it’s not improving your sleep,” Dr. Karageorgiou said. “So, whether trazodone improves sleep or not in a patient after a few months can be an early indicator for the clinician to continue using it or suspend it, because it is unlikely to help their cognition otherwise.”
He stressed that physicians need to be broadly focused on improving sleep to help patients with, or at risk for, dementia by consolidating their sleep rhythms.
“Trazodone is not the magic bullet, and I don’t think we will ever have a magic bullet,” Dr. Karageorgiou said. “Because when our brain degenerates, it’s not just one chemical, or one system, it’s many. And our body changes as well. The important thing is to help the patient consolidate their rhythms, whether through light therapy, daily exercise, cognitive behavioral therapy for insomnia, or other evidence-based interventions and their combination. The same applies for a person with dementia as for the rest of us.”
None of the investigators outside of the industry-sponsored studies had relevant disclosures.
REPORTING FROM AAIC 2019
mRNA technology for respiratory vaccines impresses
LJUBLJANA, SLOVENIA – Encouraging safety and immunogenicity results reported from phase 1 studies of the first mRNA vaccines against the potentially pandemic H10N8 avian influenza and H7N9 influenza viruses suggest a bright future for what appears to be a breakthrough technology in vaccine development.
“We have developed an mRNA platform that has the potential to be quite applicable to the vaccine space. It’s an agile platform with the potential for relatively rapid development of vaccine antigen without the use of dedicated facilities, or growth in eggs, or insects, or mammalian cells,” Lori Panther, MD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
“We now have a platform that is relatively plug and play. If one has the mRNA sequence that you’re after to produce the protein that you’re after, it is a relatively repetitive process somewhat irrespective of the goal of the protein that you’re going to manufacture. We’re introducing an mRNA into our cellular machinery – the destination is the cellular ribosome – where it hopefully is able to be translated with fidelity into the target protein. Essentially it’s like the biological equivalent of a software hack for our own cells,” explained Dr. Panther, who is director of clinical development for infectious diseases at Moderna, in Cambridge, Mass.
Indeed, Moderna has numerous ongoing or recently completed phase 1 clinical trials of mRNA vaccines developed to protect against a raft of viral infections: respiratory syncytial virus, cytomegalovirus (NCT03382405), zika, chikungunya (NCT03829384), human metapneumovirus, and parainfluenza virus 3, as well as the aforementioned H10N8 and H7N9 influenza viruses. And an mRNA varicella zoster virus vaccine is in preclinical studies.
The mRNA vaccines closely mimic native viral infections, eliciting both B- and T-cell responses.
Moreover, the company also has ongoing phase 1 studies of mRNA-based cancer vaccines – therapies targeting solid tumors and lymphomas – as well as mRNA-directed increased production of relaxin as a treatment for heart failure and of vascular endothelial growth factor to treat myocardial ischemia.
“For the purposes of my company, the desired protein at this juncture could be an antibody, it could be a tumor antigen, it could be an enzyme that will replace an enzyme that’s lacking in somebody with an inborn error of metabolism. Or it could be a vaccine antigen target,” Dr. Panther said.
In addition to highlighting the results of the two phase 1 proof-of-concept studies of mRNA vaccines targeting the feared H10N8 and H7N9 influenza viruses, she presented interim results of an ongoing 1-year study of an mRNA vaccine that contains two antigens simultaneously targeting human metapneumovirus (hMPV) and parainfluenza virus 3 (PIV3).
“The rationale behind this study is that, taken together, these are two viruses that are responsible for a fair bit of disease burden in terms of lower respiratory tract infections and hospitalizations in children [younger] than 12 months of age, which will be the target population,” the infectious disease specialist noted.
The early positive results of the mRNA influenza vaccine studies were of particular interest to her audience of pediatric infectious disease specialists. Since the first human H7N9 infections were reported in China in 2013, five outbreaks have occurred involving more than 1,500 documented infections, resulting in more than 600 deaths. And ever since the virulent H10N8 avian influenza virus popped up on the radar in 2013, infectious disease physicians the world over have been waiting for the other shoe to drop.
There is obvious appeal to a novel, precise, and rapidly scalable technology such as that promised by intracellular delivery of mRNA in order to ramp up high-volume production of effective vaccines in the face of a looming pandemic threat. Elsewhere at the meeting, it was noted that, during the H1N1 pandemic of 2009, it took 6 months for the first vaccine doses to become available using current antiquated egg-based production methods. Another 2 months elapsed before the necessary millions of doses were produced.
The details of the two phase 1 studies of the mRNA vaccines against H7N9 and H10N8 influenza have recently been published (Vaccine. 2019 May 31;37[25]:3326-34). The vaccines, delivered in the conventional manner via injection into the deltoid muscle, were well tolerated, with the most common adverse events being the familiar ones: injection site pain, erythema, headache, fatigue, and myalgia. The immune response was robust and durable.
In response to an audience question, Dr. Panther said the mRNA vaccines are amenable to development as intranasal formulations.
The ongoing 12-month, phase 1, dose-ranging study of the mRNA hMPV/PIV3 virus vaccine includes 124 healthy adults at three U.S. sites who received two vaccinations on days 1 and 28. One month after a single vaccination, hMPV neutralizing antibody titers were 6.2-6.4 times those in the placebo arm; PIV3 neutralization titers were increased 3.3-fold. The second injection didn’t further boost antibody titers, suggesting that, at least in this study population of preexposed adults, a single vaccination is sufficient.
The use of mRNA technology has been a long time in coming. Dr. Panther explained why: “It’s a big trick to take an mRNA that by its own nature is a pretty fragile molecule and to get it past the degrading enzymes, like RNAses, that are out to chew it up immediately, and then to sneak it across the cellular membrane and into the cytoplasm, all the while avoiding the innate immune responses that exist solely to recognize RNA that looks foreign and chew it up.”
Moderna has accomplished this using a proprietary lipid nanoparticle delivery system.
“Essentially it’s a lipid shield that surrounds the mRNAs and ushers them past those enzymes and past the innate immune response that would otherwise destroy them,” according to Dr. Panther.
She and her colleagues believe they may eventually be able to change the nucleotide sequence of their manufactured mRNAs in order to expand the immunogenicity epitope and achieve a stronger immune response than would result from natural infection.
LJUBLJANA, SLOVENIA – Encouraging safety and immunogenicity results reported from phase 1 studies of the first mRNA vaccines against the potentially pandemic H10N8 avian influenza and H7N9 influenza viruses suggest a bright future for what appears to be a breakthrough technology in vaccine development.
“We have developed an mRNA platform that has the potential to be quite applicable to the vaccine space. It’s an agile platform with the potential for relatively rapid development of vaccine antigen without the use of dedicated facilities, or growth in eggs, or insects, or mammalian cells,” Lori Panther, MD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
“We now have a platform that is relatively plug and play. If one has the mRNA sequence that you’re after to produce the protein that you’re after, it is a relatively repetitive process somewhat irrespective of the goal of the protein that you’re going to manufacture. We’re introducing an mRNA into our cellular machinery – the destination is the cellular ribosome – where it hopefully is able to be translated with fidelity into the target protein. Essentially it’s like the biological equivalent of a software hack for our own cells,” explained Dr. Panther, who is director of clinical development for infectious diseases at Moderna, in Cambridge, Mass.
Indeed, Moderna has numerous ongoing or recently completed phase 1 clinical trials of mRNA vaccines developed to protect against a raft of viral infections: respiratory syncytial virus, cytomegalovirus (NCT03382405), zika, chikungunya (NCT03829384), human metapneumovirus, and parainfluenza virus 3, as well as the aforementioned H10N8 and H7N9 influenza viruses. And an mRNA varicella zoster virus vaccine is in preclinical studies.
The mRNA vaccines closely mimic native viral infections, eliciting both B- and T-cell responses.
Moreover, the company also has ongoing phase 1 studies of mRNA-based cancer vaccines – therapies targeting solid tumors and lymphomas – as well as mRNA-directed increased production of relaxin as a treatment for heart failure and of vascular endothelial growth factor to treat myocardial ischemia.
“For the purposes of my company, the desired protein at this juncture could be an antibody, it could be a tumor antigen, it could be an enzyme that will replace an enzyme that’s lacking in somebody with an inborn error of metabolism. Or it could be a vaccine antigen target,” Dr. Panther said.
In addition to highlighting the results of the two phase 1 proof-of-concept studies of mRNA vaccines targeting the feared H10N8 and H7N9 influenza viruses, she presented interim results of an ongoing 1-year study of an mRNA vaccine that contains two antigens simultaneously targeting human metapneumovirus (hMPV) and parainfluenza virus 3 (PIV3).
“The rationale behind this study is that, taken together, these are two viruses that are responsible for a fair bit of disease burden in terms of lower respiratory tract infections and hospitalizations in children [younger] than 12 months of age, which will be the target population,” the infectious disease specialist noted.
The early positive results of the mRNA influenza vaccine studies were of particular interest to her audience of pediatric infectious disease specialists. Since the first human H7N9 infections were reported in China in 2013, five outbreaks have occurred involving more than 1,500 documented infections, resulting in more than 600 deaths. And ever since the virulent H10N8 avian influenza virus popped up on the radar in 2013, infectious disease physicians the world over have been waiting for the other shoe to drop.
There is obvious appeal to a novel, precise, and rapidly scalable technology such as that promised by intracellular delivery of mRNA in order to ramp up high-volume production of effective vaccines in the face of a looming pandemic threat. Elsewhere at the meeting, it was noted that, during the H1N1 pandemic of 2009, it took 6 months for the first vaccine doses to become available using current antiquated egg-based production methods. Another 2 months elapsed before the necessary millions of doses were produced.
The details of the two phase 1 studies of the mRNA vaccines against H7N9 and H10N8 influenza have recently been published (Vaccine. 2019 May 31;37[25]:3326-34). The vaccines, delivered in the conventional manner via injection into the deltoid muscle, were well tolerated, with the most common adverse events being the familiar ones: injection site pain, erythema, headache, fatigue, and myalgia. The immune response was robust and durable.
In response to an audience question, Dr. Panther said the mRNA vaccines are amenable to development as intranasal formulations.
The ongoing 12-month, phase 1, dose-ranging study of the mRNA hMPV/PIV3 virus vaccine includes 124 healthy adults at three U.S. sites who received two vaccinations on days 1 and 28. One month after a single vaccination, hMPV neutralizing antibody titers were 6.2-6.4 times those in the placebo arm; PIV3 neutralization titers were increased 3.3-fold. The second injection didn’t further boost antibody titers, suggesting that, at least in this study population of preexposed adults, a single vaccination is sufficient.
The use of mRNA technology has been a long time in coming. Dr. Panther explained why: “It’s a big trick to take an mRNA that by its own nature is a pretty fragile molecule and to get it past the degrading enzymes, like RNAses, that are out to chew it up immediately, and then to sneak it across the cellular membrane and into the cytoplasm, all the while avoiding the innate immune responses that exist solely to recognize RNA that looks foreign and chew it up.”
Moderna has accomplished this using a proprietary lipid nanoparticle delivery system.
“Essentially it’s a lipid shield that surrounds the mRNAs and ushers them past those enzymes and past the innate immune response that would otherwise destroy them,” according to Dr. Panther.
She and her colleagues believe they may eventually be able to change the nucleotide sequence of their manufactured mRNAs in order to expand the immunogenicity epitope and achieve a stronger immune response than would result from natural infection.
LJUBLJANA, SLOVENIA – Encouraging safety and immunogenicity results reported from phase 1 studies of the first mRNA vaccines against the potentially pandemic H10N8 avian influenza and H7N9 influenza viruses suggest a bright future for what appears to be a breakthrough technology in vaccine development.
“We have developed an mRNA platform that has the potential to be quite applicable to the vaccine space. It’s an agile platform with the potential for relatively rapid development of vaccine antigen without the use of dedicated facilities, or growth in eggs, or insects, or mammalian cells,” Lori Panther, MD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
“We now have a platform that is relatively plug and play. If one has the mRNA sequence that you’re after to produce the protein that you’re after, it is a relatively repetitive process somewhat irrespective of the goal of the protein that you’re going to manufacture. We’re introducing an mRNA into our cellular machinery – the destination is the cellular ribosome – where it hopefully is able to be translated with fidelity into the target protein. Essentially it’s like the biological equivalent of a software hack for our own cells,” explained Dr. Panther, who is director of clinical development for infectious diseases at Moderna, in Cambridge, Mass.
Indeed, Moderna has numerous ongoing or recently completed phase 1 clinical trials of mRNA vaccines developed to protect against a raft of viral infections: respiratory syncytial virus, cytomegalovirus (NCT03382405), zika, chikungunya (NCT03829384), human metapneumovirus, and parainfluenza virus 3, as well as the aforementioned H10N8 and H7N9 influenza viruses. And an mRNA varicella zoster virus vaccine is in preclinical studies.
The mRNA vaccines closely mimic native viral infections, eliciting both B- and T-cell responses.
Moreover, the company also has ongoing phase 1 studies of mRNA-based cancer vaccines – therapies targeting solid tumors and lymphomas – as well as mRNA-directed increased production of relaxin as a treatment for heart failure and of vascular endothelial growth factor to treat myocardial ischemia.
“For the purposes of my company, the desired protein at this juncture could be an antibody, it could be a tumor antigen, it could be an enzyme that will replace an enzyme that’s lacking in somebody with an inborn error of metabolism. Or it could be a vaccine antigen target,” Dr. Panther said.
In addition to highlighting the results of the two phase 1 proof-of-concept studies of mRNA vaccines targeting the feared H10N8 and H7N9 influenza viruses, she presented interim results of an ongoing 1-year study of an mRNA vaccine that contains two antigens simultaneously targeting human metapneumovirus (hMPV) and parainfluenza virus 3 (PIV3).
“The rationale behind this study is that, taken together, these are two viruses that are responsible for a fair bit of disease burden in terms of lower respiratory tract infections and hospitalizations in children [younger] than 12 months of age, which will be the target population,” the infectious disease specialist noted.
The early positive results of the mRNA influenza vaccine studies were of particular interest to her audience of pediatric infectious disease specialists. Since the first human H7N9 infections were reported in China in 2013, five outbreaks have occurred involving more than 1,500 documented infections, resulting in more than 600 deaths. And ever since the virulent H10N8 avian influenza virus popped up on the radar in 2013, infectious disease physicians the world over have been waiting for the other shoe to drop.
There is obvious appeal to a novel, precise, and rapidly scalable technology such as that promised by intracellular delivery of mRNA in order to ramp up high-volume production of effective vaccines in the face of a looming pandemic threat. Elsewhere at the meeting, it was noted that, during the H1N1 pandemic of 2009, it took 6 months for the first vaccine doses to become available using current antiquated egg-based production methods. Another 2 months elapsed before the necessary millions of doses were produced.
The details of the two phase 1 studies of the mRNA vaccines against H7N9 and H10N8 influenza have recently been published (Vaccine. 2019 May 31;37[25]:3326-34). The vaccines, delivered in the conventional manner via injection into the deltoid muscle, were well tolerated, with the most common adverse events being the familiar ones: injection site pain, erythema, headache, fatigue, and myalgia. The immune response was robust and durable.
In response to an audience question, Dr. Panther said the mRNA vaccines are amenable to development as intranasal formulations.
The ongoing 12-month, phase 1, dose-ranging study of the mRNA hMPV/PIV3 virus vaccine includes 124 healthy adults at three U.S. sites who received two vaccinations on days 1 and 28. One month after a single vaccination, hMPV neutralizing antibody titers were 6.2-6.4 times those in the placebo arm; PIV3 neutralization titers were increased 3.3-fold. The second injection didn’t further boost antibody titers, suggesting that, at least in this study population of preexposed adults, a single vaccination is sufficient.
The use of mRNA technology has been a long time in coming. Dr. Panther explained why: “It’s a big trick to take an mRNA that by its own nature is a pretty fragile molecule and to get it past the degrading enzymes, like RNAses, that are out to chew it up immediately, and then to sneak it across the cellular membrane and into the cytoplasm, all the while avoiding the innate immune responses that exist solely to recognize RNA that looks foreign and chew it up.”
Moderna has accomplished this using a proprietary lipid nanoparticle delivery system.
“Essentially it’s a lipid shield that surrounds the mRNAs and ushers them past those enzymes and past the innate immune response that would otherwise destroy them,” according to Dr. Panther.
She and her colleagues believe they may eventually be able to change the nucleotide sequence of their manufactured mRNAs in order to expand the immunogenicity epitope and achieve a stronger immune response than would result from natural infection.
REPORTING FROM ESPID 2019
High EPclin score equals chemo benefit in ER+/HER2– breast cancer
A study modeling outcomes for women with estrogen receptor–positive, HER2-negative breast cancer indicates that those with the highest score on a clinicomolecular test would be most likely to benefit from chemotherapy to reduce the risk of distant recurrence.
The model, created by William Gradishar, MD, from the Robert H. Lurie Comprehensive Cancer Center in Chicago, and colleagues, uses validated 10-year risk of distant breast recurrences as a function of the EndoPredict (EPclin) 12-gene clinicomolecular assay score.
The model suggests that for patients with a low EPclin score, chemotherapy would offer no additional benefit over endocrine therapy, whereas those with high scores would have an increase in recurrence-free survival with chemotherapy.
“Overall, this demonstrates that EndoPredict provides guidance on the expected absolute benefit from adjuvant chemotherapy in addition to prognostic information for patients with ER-positive, HER2-negative early-stage breast cancer. Therefore, EndoPredict can identify patients likely to benefit sufficiently from adjuvant chemotherapy to justify associated toxicities,” Dr. Gradishar and associates wrote in Precision Oncology.
The assay has previously been shown to accurately predict the risk of distant metastases in patients with estrogen receptor–positive, human epidermal growth factor receptor–2 negative (ER+/HER2–) breast cancer, but the ability to predict absolute benefit of chemotherapy is less clear, and it would be unethical to conduct a randomized trial with a no-chemotherapy arm, the investigators noted.
Instead, the investigators created a mathematical model to try to answer the question. They determined the average relative benefit of chemotherapy for reducing distant recurrence using a published meta-analysis from the Early Breast Cancer Trialists’ Collaborative Group, and they estimated absolute chemotherapy benefit differences across a range of interaction strengths between relative chemotherapy benefit and the EPclin score. Finally, they calculated average absolute benefit for patients with high and low EPclin scores using the distribution of scores in 2,185 samples tested by Myriad Genetics, maker of the assay.
They found that the average expected absolute benefit of chemotherapy treatment for patients with a low EPclin score was 1.8% assuming no interaction between prognostic factors and chemotherapy benefit, and 1.5% for maximal interaction, suggesting no significant added benefit from chemotherapy.
In contrast, for patients with a high EPclin score, the absolute benefit assuming no interaction was 5.3% and the benefit assuming maximal interaction was 7.3%.
“Overall, these data demonstrate that high EPclin scores are associated with maximal predicted chemotherapy benefit, and low EPclin scores are associated with no clinically meaningful benefit. This association is irrespective of interaction strength between EPclin and predicted chemotherapy benefit, and the impact of any interaction on absolute benefit is much smaller than the ability to accurately estimate absolute risk,” they wrote.
The study was supported by Myriad Genetics. Dr. Gradishar disclosed consulting or advisory roles with Genentech and Roche. Five of the coauthors are employees of Myriad Genetics or Myriad International.
SOURCE: Gradishar W et al. Precision Oncology. 2016 Aug. 6. doi: 10.1200/PO.18.00361.
A study modeling outcomes for women with estrogen receptor–positive, HER2-negative breast cancer indicates that those with the highest score on a clinicomolecular test would be most likely to benefit from chemotherapy to reduce the risk of distant recurrence.
The model, created by William Gradishar, MD, from the Robert H. Lurie Comprehensive Cancer Center in Chicago, and colleagues, uses validated 10-year risk of distant breast recurrences as a function of the EndoPredict (EPclin) 12-gene clinicomolecular assay score.
The model suggests that for patients with a low EPclin score, chemotherapy would offer no additional benefit over endocrine therapy, whereas those with high scores would have an increase in recurrence-free survival with chemotherapy.
“Overall, this demonstrates that EndoPredict provides guidance on the expected absolute benefit from adjuvant chemotherapy in addition to prognostic information for patients with ER-positive, HER2-negative early-stage breast cancer. Therefore, EndoPredict can identify patients likely to benefit sufficiently from adjuvant chemotherapy to justify associated toxicities,” Dr. Gradishar and associates wrote in Precision Oncology.
The assay has previously been shown to accurately predict the risk of distant metastases in patients with estrogen receptor–positive, human epidermal growth factor receptor–2 negative (ER+/HER2–) breast cancer, but the ability to predict absolute benefit of chemotherapy is less clear, and it would be unethical to conduct a randomized trial with a no-chemotherapy arm, the investigators noted.
Instead, the investigators created a mathematical model to try to answer the question. They determined the average relative benefit of chemotherapy for reducing distant recurrence using a published meta-analysis from the Early Breast Cancer Trialists’ Collaborative Group, and they estimated absolute chemotherapy benefit differences across a range of interaction strengths between relative chemotherapy benefit and the EPclin score. Finally, they calculated average absolute benefit for patients with high and low EPclin scores using the distribution of scores in 2,185 samples tested by Myriad Genetics, maker of the assay.
They found that the average expected absolute benefit of chemotherapy treatment for patients with a low EPclin score was 1.8% assuming no interaction between prognostic factors and chemotherapy benefit, and 1.5% for maximal interaction, suggesting no significant added benefit from chemotherapy.
In contrast, for patients with a high EPclin score, the absolute benefit assuming no interaction was 5.3% and the benefit assuming maximal interaction was 7.3%.
“Overall, these data demonstrate that high EPclin scores are associated with maximal predicted chemotherapy benefit, and low EPclin scores are associated with no clinically meaningful benefit. This association is irrespective of interaction strength between EPclin and predicted chemotherapy benefit, and the impact of any interaction on absolute benefit is much smaller than the ability to accurately estimate absolute risk,” they wrote.
The study was supported by Myriad Genetics. Dr. Gradishar disclosed consulting or advisory roles with Genentech and Roche. Five of the coauthors are employees of Myriad Genetics or Myriad International.
SOURCE: Gradishar W et al. Precision Oncology. 2016 Aug. 6. doi: 10.1200/PO.18.00361.
A study modeling outcomes for women with estrogen receptor–positive, HER2-negative breast cancer indicates that those with the highest score on a clinicomolecular test would be most likely to benefit from chemotherapy to reduce the risk of distant recurrence.
The model, created by William Gradishar, MD, from the Robert H. Lurie Comprehensive Cancer Center in Chicago, and colleagues, uses validated 10-year risk of distant breast recurrences as a function of the EndoPredict (EPclin) 12-gene clinicomolecular assay score.
The model suggests that for patients with a low EPclin score, chemotherapy would offer no additional benefit over endocrine therapy, whereas those with high scores would have an increase in recurrence-free survival with chemotherapy.
“Overall, this demonstrates that EndoPredict provides guidance on the expected absolute benefit from adjuvant chemotherapy in addition to prognostic information for patients with ER-positive, HER2-negative early-stage breast cancer. Therefore, EndoPredict can identify patients likely to benefit sufficiently from adjuvant chemotherapy to justify associated toxicities,” Dr. Gradishar and associates wrote in Precision Oncology.
The assay has previously been shown to accurately predict the risk of distant metastases in patients with estrogen receptor–positive, human epidermal growth factor receptor–2 negative (ER+/HER2–) breast cancer, but the ability to predict absolute benefit of chemotherapy is less clear, and it would be unethical to conduct a randomized trial with a no-chemotherapy arm, the investigators noted.
Instead, the investigators created a mathematical model to try to answer the question. They determined the average relative benefit of chemotherapy for reducing distant recurrence using a published meta-analysis from the Early Breast Cancer Trialists’ Collaborative Group, and they estimated absolute chemotherapy benefit differences across a range of interaction strengths between relative chemotherapy benefit and the EPclin score. Finally, they calculated average absolute benefit for patients with high and low EPclin scores using the distribution of scores in 2,185 samples tested by Myriad Genetics, maker of the assay.
They found that the average expected absolute benefit of chemotherapy treatment for patients with a low EPclin score was 1.8% assuming no interaction between prognostic factors and chemotherapy benefit, and 1.5% for maximal interaction, suggesting no significant added benefit from chemotherapy.
In contrast, for patients with a high EPclin score, the absolute benefit assuming no interaction was 5.3% and the benefit assuming maximal interaction was 7.3%.
“Overall, these data demonstrate that high EPclin scores are associated with maximal predicted chemotherapy benefit, and low EPclin scores are associated with no clinically meaningful benefit. This association is irrespective of interaction strength between EPclin and predicted chemotherapy benefit, and the impact of any interaction on absolute benefit is much smaller than the ability to accurately estimate absolute risk,” they wrote.
The study was supported by Myriad Genetics. Dr. Gradishar disclosed consulting or advisory roles with Genentech and Roche. Five of the coauthors are employees of Myriad Genetics or Myriad International.
SOURCE: Gradishar W et al. Precision Oncology. 2016 Aug. 6. doi: 10.1200/PO.18.00361.
FROM PRECISION ONCOLOGY
Patient and family education of asthma management is critical
ORLANDO – according to a speaker at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
“Every contact with them is a teachable moment,” Mary Lou Hayden, RN, MS, FNP-BC, AE-C, a board-certified nurse practitioner and asthma educator, said in her presentation. “You want to make sure you’re involving the important people in their lives to help them.”
Education for asthma includes teaching patients and their families the difference between long-term control and reliever medications; the proper timing and technique with the medications, as well as the importance of adherence; how to recognize and avoid triggers for asthma; how to self-monitor their asthma and control the disease; and when to seek medication care, she said.
“We review their inhaler technique every time they come in,” she added.
According to the American Lung Association, patients learn in visual, auditory, and kinesthetic styles. Teaching patients in a kinesthetic style by actually showing the patient how to take the medication through example will help the patient learn through feeling, or muscle memory. This also method works even if patients do not have the medication with them at the time, said Ms. Hayden.
“Let’s say, you don’t have [the medication], but you prescribe it,” she said. “When they come back, tell them to bring their bag of medications and make sure you go back through because if they can kinesthetically use it correctly, they’ve already mastered the visual and the auditory piece.
Written action plans are also important to successful asthma management. The plan should be tailored to the patient’s disease severity, loss of control, and include information like the peak expiratory flow and medication types, dosages, and frequencies. The action plan should also be available at home, daycare, and school. “You want them to know how to recognize their symptoms, what to do about their symptoms, and when to contact you or go to urgent care or [the emergency room],” said Ms. Hayden.
To simplify the plan, Ms. Hayden recommended zoning actions based on color, like the asthma action plan provided by the American Academy of Allergy, Asthma & Immunology. The AAAAI plan uses traffic colors to signify how well controlled a patient’s asthma is, with green indicating well-controlled disease, yellow denoting worsening asthma, and red indicating that the asthma needs to be treated right away.
Action plans should also address a patient’s health literacy level and culture. “Think about who’s going to be using it,” said Ms. Hayden.
The goal of asthma therapy is to prevent chronic or problematic symptoms, lower use of short-acting beta-agonists, maintain good pulmonary function, normalize activity levels at school and work, prevent exacerbations and hospitalizations, and meet the patient’s expectations, as well as those of their family. “If you’re thinking only severe patients have exacerbations that are near fatal or fatal, that’s not true,” she said. It’s “very common for somebody with a very mild and intermittent asthma to go to severe in a very short period of time.”
When properly implemented, patient education is performed at the time of diagnosis, is done according to a plan, is integrated into care, reinforces important information, improves adherence, is individualized to the patient and addresses their needs, and builds a partnership between provider and patient.
“We really are thinking of the team concept: us, the patient and the important people the patient’s lives, and other clinicians that might be involved with other diseases to care for the patient,” said Ms. Hayden.
Ms. Hayden reports no relevant conflicts of interest. Global Academy for Medical Education and this news organization are owned by the same parent company.
ORLANDO – according to a speaker at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
“Every contact with them is a teachable moment,” Mary Lou Hayden, RN, MS, FNP-BC, AE-C, a board-certified nurse practitioner and asthma educator, said in her presentation. “You want to make sure you’re involving the important people in their lives to help them.”
Education for asthma includes teaching patients and their families the difference between long-term control and reliever medications; the proper timing and technique with the medications, as well as the importance of adherence; how to recognize and avoid triggers for asthma; how to self-monitor their asthma and control the disease; and when to seek medication care, she said.
“We review their inhaler technique every time they come in,” she added.
According to the American Lung Association, patients learn in visual, auditory, and kinesthetic styles. Teaching patients in a kinesthetic style by actually showing the patient how to take the medication through example will help the patient learn through feeling, or muscle memory. This also method works even if patients do not have the medication with them at the time, said Ms. Hayden.
“Let’s say, you don’t have [the medication], but you prescribe it,” she said. “When they come back, tell them to bring their bag of medications and make sure you go back through because if they can kinesthetically use it correctly, they’ve already mastered the visual and the auditory piece.
Written action plans are also important to successful asthma management. The plan should be tailored to the patient’s disease severity, loss of control, and include information like the peak expiratory flow and medication types, dosages, and frequencies. The action plan should also be available at home, daycare, and school. “You want them to know how to recognize their symptoms, what to do about their symptoms, and when to contact you or go to urgent care or [the emergency room],” said Ms. Hayden.
To simplify the plan, Ms. Hayden recommended zoning actions based on color, like the asthma action plan provided by the American Academy of Allergy, Asthma & Immunology. The AAAAI plan uses traffic colors to signify how well controlled a patient’s asthma is, with green indicating well-controlled disease, yellow denoting worsening asthma, and red indicating that the asthma needs to be treated right away.
Action plans should also address a patient’s health literacy level and culture. “Think about who’s going to be using it,” said Ms. Hayden.
The goal of asthma therapy is to prevent chronic or problematic symptoms, lower use of short-acting beta-agonists, maintain good pulmonary function, normalize activity levels at school and work, prevent exacerbations and hospitalizations, and meet the patient’s expectations, as well as those of their family. “If you’re thinking only severe patients have exacerbations that are near fatal or fatal, that’s not true,” she said. It’s “very common for somebody with a very mild and intermittent asthma to go to severe in a very short period of time.”
When properly implemented, patient education is performed at the time of diagnosis, is done according to a plan, is integrated into care, reinforces important information, improves adherence, is individualized to the patient and addresses their needs, and builds a partnership between provider and patient.
“We really are thinking of the team concept: us, the patient and the important people the patient’s lives, and other clinicians that might be involved with other diseases to care for the patient,” said Ms. Hayden.
Ms. Hayden reports no relevant conflicts of interest. Global Academy for Medical Education and this news organization are owned by the same parent company.
ORLANDO – according to a speaker at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
“Every contact with them is a teachable moment,” Mary Lou Hayden, RN, MS, FNP-BC, AE-C, a board-certified nurse practitioner and asthma educator, said in her presentation. “You want to make sure you’re involving the important people in their lives to help them.”
Education for asthma includes teaching patients and their families the difference between long-term control and reliever medications; the proper timing and technique with the medications, as well as the importance of adherence; how to recognize and avoid triggers for asthma; how to self-monitor their asthma and control the disease; and when to seek medication care, she said.
“We review their inhaler technique every time they come in,” she added.
According to the American Lung Association, patients learn in visual, auditory, and kinesthetic styles. Teaching patients in a kinesthetic style by actually showing the patient how to take the medication through example will help the patient learn through feeling, or muscle memory. This also method works even if patients do not have the medication with them at the time, said Ms. Hayden.
“Let’s say, you don’t have [the medication], but you prescribe it,” she said. “When they come back, tell them to bring their bag of medications and make sure you go back through because if they can kinesthetically use it correctly, they’ve already mastered the visual and the auditory piece.
Written action plans are also important to successful asthma management. The plan should be tailored to the patient’s disease severity, loss of control, and include information like the peak expiratory flow and medication types, dosages, and frequencies. The action plan should also be available at home, daycare, and school. “You want them to know how to recognize their symptoms, what to do about their symptoms, and when to contact you or go to urgent care or [the emergency room],” said Ms. Hayden.
To simplify the plan, Ms. Hayden recommended zoning actions based on color, like the asthma action plan provided by the American Academy of Allergy, Asthma & Immunology. The AAAAI plan uses traffic colors to signify how well controlled a patient’s asthma is, with green indicating well-controlled disease, yellow denoting worsening asthma, and red indicating that the asthma needs to be treated right away.
Action plans should also address a patient’s health literacy level and culture. “Think about who’s going to be using it,” said Ms. Hayden.
The goal of asthma therapy is to prevent chronic or problematic symptoms, lower use of short-acting beta-agonists, maintain good pulmonary function, normalize activity levels at school and work, prevent exacerbations and hospitalizations, and meet the patient’s expectations, as well as those of their family. “If you’re thinking only severe patients have exacerbations that are near fatal or fatal, that’s not true,” she said. It’s “very common for somebody with a very mild and intermittent asthma to go to severe in a very short period of time.”
When properly implemented, patient education is performed at the time of diagnosis, is done according to a plan, is integrated into care, reinforces important information, improves adherence, is individualized to the patient and addresses their needs, and builds a partnership between provider and patient.
“We really are thinking of the team concept: us, the patient and the important people the patient’s lives, and other clinicians that might be involved with other diseases to care for the patient,” said Ms. Hayden.
Ms. Hayden reports no relevant conflicts of interest. Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM CARPS 2019
CDC finds that too little naloxone is dispensed
Although the CDC recommends that clinicians consider prescribing naloxone, which can reverse the effects of an opioid overdose, to patients who receive high-dose opioid prescriptions, one naloxone prescription was dispensed in 2018 for every 69 such patients, according to a Vital Signs investigation published Aug. 6 in the Morbidity and Mortality Weekly Report.
Approximately 9 million more naloxone prescriptions could have been dispensed in 2018 if every patient with a high-dose opioid prescription were offered the drug, according to the agency. In addition, the rate at which naloxone is dispensed varies significantly according to region.
“Thousands of Americans are alive today thanks to the use of naloxone,” said Alex M. Azar, secretary of Health and Human Services, in a press release. “Giving people a chance to survive an opioid overdose and safely enter recovery is one of the five key pillars of our HHS strategy for ending the overdose epidemic. With help from Congress, the private sector, state, and local governments and communities, targeted access to naloxone has expanded dramatically over the last several years, but today’s CDC report is a reminder that there is much more all of us need to do to save lives.”
Investigators examined retail pharmacy data
In 2017, 47,600 (67.8%) drug overdose deaths in the United States involved opioids. For decades, emergency medical service providers have administered naloxone to patients with suspected drug overdose. A major focus of public health initiatives intended to address the opioid overdose crisis has been to increase access to naloxone through clinician prescribing and pharmacy dispensing. The CDC recommends considering prescribing naloxone to patients with a history of overdose or substance use disorder, those receiving opioid dosages of 50 morphine milligram equivalents per day or greater (that is, high-dose prescriptions), and those who are using benzodiazepines concurrently.
Investigators at the CDC examined retail pharmacy data from IQVIA, a company that maintains information on prescriptions from approximately 50,400 retail pharmacies. They extracted data from 2012 through 2018 to analyze naloxone dispensing by region, urban versus rural status, prescriber specialty, and recipient characteristics (for example, age group, sex, out-of-pocket costs, and method of payment).
Dispensations doubled from 2017 to 2018
Naloxone dispensing from retail pharmacies increased from 0.4 prescriptions per 100,000 in 2012 to 170.2 prescriptions per 100,000 in 2018. From 2017 to 2018 alone, the number of prescriptions dispensed increased by 106%.
Despite consistency among state laws, naloxone dispensation varied by region. The average rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 0.2 in the lowest quartile to 2.9 in the highest quartile. In 2018, the rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 1.5 in metropolitan counties and 1.6 in the Northeast to 1.2 in rural counties and 1.3 in the Midwest. Rural counties were nearly three times more likely to be low-dispensing counties, compared with metropolitan counties.
The rate of naloxone prescriptions per 100 high-dose opioid prescriptions also varied by provider specialty. This rate was lowest among surgeons (0.2) and highest among psychiatrists (12.9).
Most naloxone prescriptions entailed out-of-pocket costs. About 71% of prescriptions paid for by Medicare entailed out-of-pocket costs, compared with 43.8% of prescriptions paid for by Medicaid, and 41.5% of prescriptions paid for by commercial insurance.
 Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
More can be done

“It is clear from the data that there is still much needed education around the important role naloxone plays in reducing overdose deaths,” said Robert R. Redfield, MD, director of the CDC, in a press release. “The time is now to ensure all individuals who are prescribed high-dose opioids also receive naloxone as a potential life-saving intervention. As we aggressively confront what is the public health crisis of our time, CDC will continue to stress with health care providers the benefit of making this overdose-reversing medicine available to patients.”
“While we’ve seen these important increases [in naloxone prescriptions], we are not as far along as we’d like to be,” said Anne Schuchat, MD, principal deputy director of the CDC, during a press conference. “Cost is one of the issues, but I think awareness is another.” These data should prompt pharmacies to make sure that they stock naloxone and remind clinicians to consider naloxone when they prescribe opioids, she added. Patients and their family members should be aware of naloxone and ask their health care providers about it. “We’d really like to see the increase [in naloxone prescriptions] move much more rapidly,” she concluded.
The investigators disclosed no potential conflicts of interest.
SOURCE: Guy GP et al. MMWR Morb Mortal Wkly Rep. 2019 Aug 6.
Although the CDC recommends that clinicians consider prescribing naloxone, which can reverse the effects of an opioid overdose, to patients who receive high-dose opioid prescriptions, one naloxone prescription was dispensed in 2018 for every 69 such patients, according to a Vital Signs investigation published Aug. 6 in the Morbidity and Mortality Weekly Report.
Approximately 9 million more naloxone prescriptions could have been dispensed in 2018 if every patient with a high-dose opioid prescription were offered the drug, according to the agency. In addition, the rate at which naloxone is dispensed varies significantly according to region.
“Thousands of Americans are alive today thanks to the use of naloxone,” said Alex M. Azar, secretary of Health and Human Services, in a press release. “Giving people a chance to survive an opioid overdose and safely enter recovery is one of the five key pillars of our HHS strategy for ending the overdose epidemic. With help from Congress, the private sector, state, and local governments and communities, targeted access to naloxone has expanded dramatically over the last several years, but today’s CDC report is a reminder that there is much more all of us need to do to save lives.”
Investigators examined retail pharmacy data
In 2017, 47,600 (67.8%) drug overdose deaths in the United States involved opioids. For decades, emergency medical service providers have administered naloxone to patients with suspected drug overdose. A major focus of public health initiatives intended to address the opioid overdose crisis has been to increase access to naloxone through clinician prescribing and pharmacy dispensing. The CDC recommends considering prescribing naloxone to patients with a history of overdose or substance use disorder, those receiving opioid dosages of 50 morphine milligram equivalents per day or greater (that is, high-dose prescriptions), and those who are using benzodiazepines concurrently.
Investigators at the CDC examined retail pharmacy data from IQVIA, a company that maintains information on prescriptions from approximately 50,400 retail pharmacies. They extracted data from 2012 through 2018 to analyze naloxone dispensing by region, urban versus rural status, prescriber specialty, and recipient characteristics (for example, age group, sex, out-of-pocket costs, and method of payment).
Dispensations doubled from 2017 to 2018
Naloxone dispensing from retail pharmacies increased from 0.4 prescriptions per 100,000 in 2012 to 170.2 prescriptions per 100,000 in 2018. From 2017 to 2018 alone, the number of prescriptions dispensed increased by 106%.
Despite consistency among state laws, naloxone dispensation varied by region. The average rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 0.2 in the lowest quartile to 2.9 in the highest quartile. In 2018, the rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 1.5 in metropolitan counties and 1.6 in the Northeast to 1.2 in rural counties and 1.3 in the Midwest. Rural counties were nearly three times more likely to be low-dispensing counties, compared with metropolitan counties.
The rate of naloxone prescriptions per 100 high-dose opioid prescriptions also varied by provider specialty. This rate was lowest among surgeons (0.2) and highest among psychiatrists (12.9).
Most naloxone prescriptions entailed out-of-pocket costs. About 71% of prescriptions paid for by Medicare entailed out-of-pocket costs, compared with 43.8% of prescriptions paid for by Medicaid, and 41.5% of prescriptions paid for by commercial insurance.
 Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
More can be done

“It is clear from the data that there is still much needed education around the important role naloxone plays in reducing overdose deaths,” said Robert R. Redfield, MD, director of the CDC, in a press release. “The time is now to ensure all individuals who are prescribed high-dose opioids also receive naloxone as a potential life-saving intervention. As we aggressively confront what is the public health crisis of our time, CDC will continue to stress with health care providers the benefit of making this overdose-reversing medicine available to patients.”
“While we’ve seen these important increases [in naloxone prescriptions], we are not as far along as we’d like to be,” said Anne Schuchat, MD, principal deputy director of the CDC, during a press conference. “Cost is one of the issues, but I think awareness is another.” These data should prompt pharmacies to make sure that they stock naloxone and remind clinicians to consider naloxone when they prescribe opioids, she added. Patients and their family members should be aware of naloxone and ask their health care providers about it. “We’d really like to see the increase [in naloxone prescriptions] move much more rapidly,” she concluded.
The investigators disclosed no potential conflicts of interest.
SOURCE: Guy GP et al. MMWR Morb Mortal Wkly Rep. 2019 Aug 6.
Although the CDC recommends that clinicians consider prescribing naloxone, which can reverse the effects of an opioid overdose, to patients who receive high-dose opioid prescriptions, one naloxone prescription was dispensed in 2018 for every 69 such patients, according to a Vital Signs investigation published Aug. 6 in the Morbidity and Mortality Weekly Report.
Approximately 9 million more naloxone prescriptions could have been dispensed in 2018 if every patient with a high-dose opioid prescription were offered the drug, according to the agency. In addition, the rate at which naloxone is dispensed varies significantly according to region.
“Thousands of Americans are alive today thanks to the use of naloxone,” said Alex M. Azar, secretary of Health and Human Services, in a press release. “Giving people a chance to survive an opioid overdose and safely enter recovery is one of the five key pillars of our HHS strategy for ending the overdose epidemic. With help from Congress, the private sector, state, and local governments and communities, targeted access to naloxone has expanded dramatically over the last several years, but today’s CDC report is a reminder that there is much more all of us need to do to save lives.”
Investigators examined retail pharmacy data
In 2017, 47,600 (67.8%) drug overdose deaths in the United States involved opioids. For decades, emergency medical service providers have administered naloxone to patients with suspected drug overdose. A major focus of public health initiatives intended to address the opioid overdose crisis has been to increase access to naloxone through clinician prescribing and pharmacy dispensing. The CDC recommends considering prescribing naloxone to patients with a history of overdose or substance use disorder, those receiving opioid dosages of 50 morphine milligram equivalents per day or greater (that is, high-dose prescriptions), and those who are using benzodiazepines concurrently.
Investigators at the CDC examined retail pharmacy data from IQVIA, a company that maintains information on prescriptions from approximately 50,400 retail pharmacies. They extracted data from 2012 through 2018 to analyze naloxone dispensing by region, urban versus rural status, prescriber specialty, and recipient characteristics (for example, age group, sex, out-of-pocket costs, and method of payment).
Dispensations doubled from 2017 to 2018
Naloxone dispensing from retail pharmacies increased from 0.4 prescriptions per 100,000 in 2012 to 170.2 prescriptions per 100,000 in 2018. From 2017 to 2018 alone, the number of prescriptions dispensed increased by 106%.
Despite consistency among state laws, naloxone dispensation varied by region. The average rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 0.2 in the lowest quartile to 2.9 in the highest quartile. In 2018, the rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 1.5 in metropolitan counties and 1.6 in the Northeast to 1.2 in rural counties and 1.3 in the Midwest. Rural counties were nearly three times more likely to be low-dispensing counties, compared with metropolitan counties.
The rate of naloxone prescriptions per 100 high-dose opioid prescriptions also varied by provider specialty. This rate was lowest among surgeons (0.2) and highest among psychiatrists (12.9).
Most naloxone prescriptions entailed out-of-pocket costs. About 71% of prescriptions paid for by Medicare entailed out-of-pocket costs, compared with 43.8% of prescriptions paid for by Medicaid, and 41.5% of prescriptions paid for by commercial insurance.
 Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
More can be done

“It is clear from the data that there is still much needed education around the important role naloxone plays in reducing overdose deaths,” said Robert R. Redfield, MD, director of the CDC, in a press release. “The time is now to ensure all individuals who are prescribed high-dose opioids also receive naloxone as a potential life-saving intervention. As we aggressively confront what is the public health crisis of our time, CDC will continue to stress with health care providers the benefit of making this overdose-reversing medicine available to patients.”
“While we’ve seen these important increases [in naloxone prescriptions], we are not as far along as we’d like to be,” said Anne Schuchat, MD, principal deputy director of the CDC, during a press conference. “Cost is one of the issues, but I think awareness is another.” These data should prompt pharmacies to make sure that they stock naloxone and remind clinicians to consider naloxone when they prescribe opioids, she added. Patients and their family members should be aware of naloxone and ask their health care providers about it. “We’d really like to see the increase [in naloxone prescriptions] move much more rapidly,” she concluded.
The investigators disclosed no potential conflicts of interest.
SOURCE: Guy GP et al. MMWR Morb Mortal Wkly Rep. 2019 Aug 6.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Preoperative tramadol fails to improve function after knee surgery
according to findings of a study based on pre- and postsurgery data.
Tramadol has become a popular choice for nonoperative knee pain relief because of its low potential for abuse and favorable safety profile, but its impact on postoperative outcomes when given before knee surgery has not been well studied, wrote Adam Driesman, MD, of the New York University Langone Orthopedic Hospital and colleagues.
In a study published in the Journal of Arthroplasty, the researchers compared patient-reported outcomes (PRO) after total knee arthroplasty among 136 patients who received no opiates, 21 who received tramadol, and 42 who received other opiates. All patients who did not have preoperative and postoperative PRO scores were excluded
All patients received the same multimodal perioperative pain protocol, and all were placed on oxycodone postoperatively for maintenance and breakthrough pain as needed, with discharge prescriptions for acetaminophen/oxycodone combination (Percocet) for breakthrough pain.
Patients preoperative assessment using the Knee Disability and Osteoarthritis Outcome Score Jr. (KOOS, JR.) were similar among the groups prior to surgery; baseline scores for the groups receiving either tramadol, no opiates, or other opiates were 49.95, 50.4, and 48.0, respectively. Demographics also were not significantly different among the groups.
At 3 months, the average KOOS, JR., score for the tramadol group (62.4) was significantly lower, compared with the other-opiate group (67.1) and treatment-naive group (70.1). In addition, patients in the tramadol group had the least change in scores on KOOS, JR., with an average of 12.5 points, compared with 19.1-point and 20.1-point improvements, respectively, in the alternate-opiate group and opiate-naive group.
The data expand on previous findings that patients given preoperative opioids had proportionally less postoperative pain relief than those not on opioids, the researchers said, but noted that they were surprised by the worse outcomes in the tramadol group given its demonstrated side-effect profile.
The study findings were limited by several factors including the retrospective design and relatively short follow-up period, as well as the inability to accurately determine outpatient medication use, not only of opioids, but of nonopioid postoperative pain medications that could have affected the results, the researchers said.
“However, given the conflicting evidence presented in this study and despite the 2013 American Academy of Orthopedic Surgeons Clinical Practice Guidelines, it is recommended providers remain very conservative in their administration of outpatient narcotics including tramadol prior to surgery,” they concluded.
SOURCE: Driesman A et al. J Arthroplasty. 2019;34(8):1662-66.
according to findings of a study based on pre- and postsurgery data.
Tramadol has become a popular choice for nonoperative knee pain relief because of its low potential for abuse and favorable safety profile, but its impact on postoperative outcomes when given before knee surgery has not been well studied, wrote Adam Driesman, MD, of the New York University Langone Orthopedic Hospital and colleagues.
In a study published in the Journal of Arthroplasty, the researchers compared patient-reported outcomes (PRO) after total knee arthroplasty among 136 patients who received no opiates, 21 who received tramadol, and 42 who received other opiates. All patients who did not have preoperative and postoperative PRO scores were excluded
All patients received the same multimodal perioperative pain protocol, and all were placed on oxycodone postoperatively for maintenance and breakthrough pain as needed, with discharge prescriptions for acetaminophen/oxycodone combination (Percocet) for breakthrough pain.
Patients preoperative assessment using the Knee Disability and Osteoarthritis Outcome Score Jr. (KOOS, JR.) were similar among the groups prior to surgery; baseline scores for the groups receiving either tramadol, no opiates, or other opiates were 49.95, 50.4, and 48.0, respectively. Demographics also were not significantly different among the groups.
At 3 months, the average KOOS, JR., score for the tramadol group (62.4) was significantly lower, compared with the other-opiate group (67.1) and treatment-naive group (70.1). In addition, patients in the tramadol group had the least change in scores on KOOS, JR., with an average of 12.5 points, compared with 19.1-point and 20.1-point improvements, respectively, in the alternate-opiate group and opiate-naive group.
The data expand on previous findings that patients given preoperative opioids had proportionally less postoperative pain relief than those not on opioids, the researchers said, but noted that they were surprised by the worse outcomes in the tramadol group given its demonstrated side-effect profile.
The study findings were limited by several factors including the retrospective design and relatively short follow-up period, as well as the inability to accurately determine outpatient medication use, not only of opioids, but of nonopioid postoperative pain medications that could have affected the results, the researchers said.
“However, given the conflicting evidence presented in this study and despite the 2013 American Academy of Orthopedic Surgeons Clinical Practice Guidelines, it is recommended providers remain very conservative in their administration of outpatient narcotics including tramadol prior to surgery,” they concluded.
SOURCE: Driesman A et al. J Arthroplasty. 2019;34(8):1662-66.
according to findings of a study based on pre- and postsurgery data.
Tramadol has become a popular choice for nonoperative knee pain relief because of its low potential for abuse and favorable safety profile, but its impact on postoperative outcomes when given before knee surgery has not been well studied, wrote Adam Driesman, MD, of the New York University Langone Orthopedic Hospital and colleagues.
In a study published in the Journal of Arthroplasty, the researchers compared patient-reported outcomes (PRO) after total knee arthroplasty among 136 patients who received no opiates, 21 who received tramadol, and 42 who received other opiates. All patients who did not have preoperative and postoperative PRO scores were excluded
All patients received the same multimodal perioperative pain protocol, and all were placed on oxycodone postoperatively for maintenance and breakthrough pain as needed, with discharge prescriptions for acetaminophen/oxycodone combination (Percocet) for breakthrough pain.
Patients preoperative assessment using the Knee Disability and Osteoarthritis Outcome Score Jr. (KOOS, JR.) were similar among the groups prior to surgery; baseline scores for the groups receiving either tramadol, no opiates, or other opiates were 49.95, 50.4, and 48.0, respectively. Demographics also were not significantly different among the groups.
At 3 months, the average KOOS, JR., score for the tramadol group (62.4) was significantly lower, compared with the other-opiate group (67.1) and treatment-naive group (70.1). In addition, patients in the tramadol group had the least change in scores on KOOS, JR., with an average of 12.5 points, compared with 19.1-point and 20.1-point improvements, respectively, in the alternate-opiate group and opiate-naive group.
The data expand on previous findings that patients given preoperative opioids had proportionally less postoperative pain relief than those not on opioids, the researchers said, but noted that they were surprised by the worse outcomes in the tramadol group given its demonstrated side-effect profile.
The study findings were limited by several factors including the retrospective design and relatively short follow-up period, as well as the inability to accurately determine outpatient medication use, not only of opioids, but of nonopioid postoperative pain medications that could have affected the results, the researchers said.
“However, given the conflicting evidence presented in this study and despite the 2013 American Academy of Orthopedic Surgeons Clinical Practice Guidelines, it is recommended providers remain very conservative in their administration of outpatient narcotics including tramadol prior to surgery,” they concluded.
SOURCE: Driesman A et al. J Arthroplasty. 2019;34(8):1662-66.
FROM THE JOURNAL OF ARTHROPLASTY
Endoscopic duodenal mucosal resection found effective for some patients with T2D
Among patients with suboptimally controlled type 2 diabetes who use oral glucose-lowering medication, endoscopic duodenal mucosal resection (DMR) can be implemented safely and effectively, results from a multicenter, international, phase 2 study demonstrated.
“DMR elicited a substantial improvement in parameters of glycemia as well as a decrease in liver transaminase levels at 24 weeks, which was sustained at 12 months post procedure,” researchers led by Annieke C.G. van Baar, MD, wrote in a study published online in Gut. “These findings were also associated with an improvement in patients’ diabetes treatment satisfaction.”
For the study, Dr. van Baar, of the department of gastroenterology and hepatology at Amsterdam University Medical Center, and colleagues at seven clinical sites enrolled 46 patients with type 2 diabetes who were on stable glucose-lowering medication to undergo DMR. The procedure “involves circumferential hydrothermal ablation of the duodenal mucosa resulting in subsequent regeneration of the mucosa,” they wrote. “Before ablation, the mucosa is lifted with saline to protect the outer layers of the duodenum.” DMR was performed under either general anesthesia or deep sedation with propofol by a single endoscopist at each site with extensive experience in therapeutic upper GI endoscopy and guidewire management.
The mean age of the study participants was 55 years and 63% were male. Of the 46 patients, 37 (80%) underwent complete DMR and results were reported for 36 of them. A total of 24 patients had at least one adverse event related to DMR (52%), mostly GI symptoms such as diarrhea, abdominal pain, nausea, and oropharyngeal pain. Of these, 81% were mild. One serious adverse event was considered to be related to the procedure. “This concerned a patient with general malaise, mild fever, and increased C-reactive protein level on the first day after DMR,” the researchers wrote. “The mild fever resolved within 24 hours and [C-reactive protein] level normalized within 3 days.” No unanticipated adverse events were reported.
During follow-up measures taken 24 weeks after their DMR, hemoglobin A1c fell by a mean of 10 mmol/mol (P less than .001), fasting plasma glucose by 1.7 mmol/L (P less than .001), and the Homeostatic Model Assessment of Insulin Resistance improved significantly (P less than .001). In addition, the procedure conferred a moderate reduction in weight (a mean loss of 2.5 kg) and a decrease in hepatic transaminase levels. The effects were sustained at 12 months.
“While the majority of patients showed a durable glycemic response over 12 months, a minority exhibited less benefit from DMR and required additional glucose-lowering medication at 24 weeks,” the researchers wrote. “Of note, approximately two-thirds of the patients who required addition of antidiabetic medication in the latter phase of study had undergone insulin secretagogue medication withdrawal at screening. For future study, it may not be necessary to discontinue these medications before DMR, and this will allow an even more precise measure of DMR effect.”
Dr. van Baar and colleagues acknowledged certain limitations of the phase 2 study, including its open-label, uncontrolled design. “The results of this multicenter study need to be confirmed in a proper controlled study. Nevertheless, this study forms the requisite solid foundation for further research, and controlled studies are currently underway.”
The study was funded by Fractyl Laboratories. Dr. van Baar reported having no financial disclosures. Four of the study authors reported having financial relationships with numerous pharmaceutical and device companies.
SOURCE: van Baar ACG et al. Gut. 2019 Jul 22. doi: 10.1136/gutjnl-2019-318349.
Among patients with suboptimally controlled type 2 diabetes who use oral glucose-lowering medication, endoscopic duodenal mucosal resection (DMR) can be implemented safely and effectively, results from a multicenter, international, phase 2 study demonstrated.
“DMR elicited a substantial improvement in parameters of glycemia as well as a decrease in liver transaminase levels at 24 weeks, which was sustained at 12 months post procedure,” researchers led by Annieke C.G. van Baar, MD, wrote in a study published online in Gut. “These findings were also associated with an improvement in patients’ diabetes treatment satisfaction.”
For the study, Dr. van Baar, of the department of gastroenterology and hepatology at Amsterdam University Medical Center, and colleagues at seven clinical sites enrolled 46 patients with type 2 diabetes who were on stable glucose-lowering medication to undergo DMR. The procedure “involves circumferential hydrothermal ablation of the duodenal mucosa resulting in subsequent regeneration of the mucosa,” they wrote. “Before ablation, the mucosa is lifted with saline to protect the outer layers of the duodenum.” DMR was performed under either general anesthesia or deep sedation with propofol by a single endoscopist at each site with extensive experience in therapeutic upper GI endoscopy and guidewire management.
The mean age of the study participants was 55 years and 63% were male. Of the 46 patients, 37 (80%) underwent complete DMR and results were reported for 36 of them. A total of 24 patients had at least one adverse event related to DMR (52%), mostly GI symptoms such as diarrhea, abdominal pain, nausea, and oropharyngeal pain. Of these, 81% were mild. One serious adverse event was considered to be related to the procedure. “This concerned a patient with general malaise, mild fever, and increased C-reactive protein level on the first day after DMR,” the researchers wrote. “The mild fever resolved within 24 hours and [C-reactive protein] level normalized within 3 days.” No unanticipated adverse events were reported.
During follow-up measures taken 24 weeks after their DMR, hemoglobin A1c fell by a mean of 10 mmol/mol (P less than .001), fasting plasma glucose by 1.7 mmol/L (P less than .001), and the Homeostatic Model Assessment of Insulin Resistance improved significantly (P less than .001). In addition, the procedure conferred a moderate reduction in weight (a mean loss of 2.5 kg) and a decrease in hepatic transaminase levels. The effects were sustained at 12 months.
“While the majority of patients showed a durable glycemic response over 12 months, a minority exhibited less benefit from DMR and required additional glucose-lowering medication at 24 weeks,” the researchers wrote. “Of note, approximately two-thirds of the patients who required addition of antidiabetic medication in the latter phase of study had undergone insulin secretagogue medication withdrawal at screening. For future study, it may not be necessary to discontinue these medications before DMR, and this will allow an even more precise measure of DMR effect.”
Dr. van Baar and colleagues acknowledged certain limitations of the phase 2 study, including its open-label, uncontrolled design. “The results of this multicenter study need to be confirmed in a proper controlled study. Nevertheless, this study forms the requisite solid foundation for further research, and controlled studies are currently underway.”
The study was funded by Fractyl Laboratories. Dr. van Baar reported having no financial disclosures. Four of the study authors reported having financial relationships with numerous pharmaceutical and device companies.
SOURCE: van Baar ACG et al. Gut. 2019 Jul 22. doi: 10.1136/gutjnl-2019-318349.
Among patients with suboptimally controlled type 2 diabetes who use oral glucose-lowering medication, endoscopic duodenal mucosal resection (DMR) can be implemented safely and effectively, results from a multicenter, international, phase 2 study demonstrated.
“DMR elicited a substantial improvement in parameters of glycemia as well as a decrease in liver transaminase levels at 24 weeks, which was sustained at 12 months post procedure,” researchers led by Annieke C.G. van Baar, MD, wrote in a study published online in Gut. “These findings were also associated with an improvement in patients’ diabetes treatment satisfaction.”
For the study, Dr. van Baar, of the department of gastroenterology and hepatology at Amsterdam University Medical Center, and colleagues at seven clinical sites enrolled 46 patients with type 2 diabetes who were on stable glucose-lowering medication to undergo DMR. The procedure “involves circumferential hydrothermal ablation of the duodenal mucosa resulting in subsequent regeneration of the mucosa,” they wrote. “Before ablation, the mucosa is lifted with saline to protect the outer layers of the duodenum.” DMR was performed under either general anesthesia or deep sedation with propofol by a single endoscopist at each site with extensive experience in therapeutic upper GI endoscopy and guidewire management.
The mean age of the study participants was 55 years and 63% were male. Of the 46 patients, 37 (80%) underwent complete DMR and results were reported for 36 of them. A total of 24 patients had at least one adverse event related to DMR (52%), mostly GI symptoms such as diarrhea, abdominal pain, nausea, and oropharyngeal pain. Of these, 81% were mild. One serious adverse event was considered to be related to the procedure. “This concerned a patient with general malaise, mild fever, and increased C-reactive protein level on the first day after DMR,” the researchers wrote. “The mild fever resolved within 24 hours and [C-reactive protein] level normalized within 3 days.” No unanticipated adverse events were reported.
During follow-up measures taken 24 weeks after their DMR, hemoglobin A1c fell by a mean of 10 mmol/mol (P less than .001), fasting plasma glucose by 1.7 mmol/L (P less than .001), and the Homeostatic Model Assessment of Insulin Resistance improved significantly (P less than .001). In addition, the procedure conferred a moderate reduction in weight (a mean loss of 2.5 kg) and a decrease in hepatic transaminase levels. The effects were sustained at 12 months.
“While the majority of patients showed a durable glycemic response over 12 months, a minority exhibited less benefit from DMR and required additional glucose-lowering medication at 24 weeks,” the researchers wrote. “Of note, approximately two-thirds of the patients who required addition of antidiabetic medication in the latter phase of study had undergone insulin secretagogue medication withdrawal at screening. For future study, it may not be necessary to discontinue these medications before DMR, and this will allow an even more precise measure of DMR effect.”
Dr. van Baar and colleagues acknowledged certain limitations of the phase 2 study, including its open-label, uncontrolled design. “The results of this multicenter study need to be confirmed in a proper controlled study. Nevertheless, this study forms the requisite solid foundation for further research, and controlled studies are currently underway.”
The study was funded by Fractyl Laboratories. Dr. van Baar reported having no financial disclosures. Four of the study authors reported having financial relationships with numerous pharmaceutical and device companies.
SOURCE: van Baar ACG et al. Gut. 2019 Jul 22. doi: 10.1136/gutjnl-2019-318349.
FROM GUT
Rubbery Nodule on the Face of an Infant
The Diagnosis: Juvenile Xanthogranuloma
Juvenile xanthogranuloma (JXG) was first described in 1905 by Adamson1 as solitary or multiple plaquiform or nodular lesions that are yellow to yellowish brown. In 1954, Helwig and Hackney2 coined the term juvenile xanthogranuloma to define this histiocytic cutaneous granulomatous tumor.
Juvenile xanthogranuloma is a rare dermatologic disorder that may be present at birth and primarily affects infants and young children. The benign lesions generally occur in the first 4 years of life, with a median age of onset of 2 years.3 Lesions range in size from millimeters to several centimeters in diameter.4 The skin of the head and neck is the most commonly involved site in JXG. The most frequent noncutaneous site of JXG involvement is the eye, particularly the iris, accounting for 0.4% of cases.5,6 Extracutaneous sites such as the heart, liver, lungs, spleen, oral cavity, and brain also may be involved.4
Most children with JXG are asymptomatic. Skin lesions present as well-demarcated, rubbery, tan-orange papules or nodules. They usually are solitary, and multiple nodules can increase the risk for extracutaneous involvement.4 A case series of patients with neurofibromatosis type 1 showed 14 of 77 (18%) patients examined in the first year of life presented with JXG or other non–Langerhans cell histiocytosis.7 The adult form of cutaneous xanthogranuloma often presents with severe bronchial asthma.8
Histopathologic examination of a biopsy of the lesion typically demonstrates well-circumscribed nodules with dense infiltrates of polyhedral histiocytes with vaculoles.3-7 In 85% of cases, Touton giant cells are present.4,9 A prominent vascular network often is present, which was observed in our patient’s biopsy (Figure 1). Immunohistochemistry typically is positive for CD14, CD68, CD163, fascin, and factor XIIIa.4,10 Classically, the cells are negative for S-100 and CD1a, which differentiates these lesions from Langerhans cell histiocytosis.4-7,10 Our patient demonstrated scattered S-100–positive cells representing background dendritic cells and macrophages (Figure 2). The remainder of the clinical, morphologic, and immunophenotypic findings were consistent with non–Langerhans cell histiocytosis, specifically JXG. 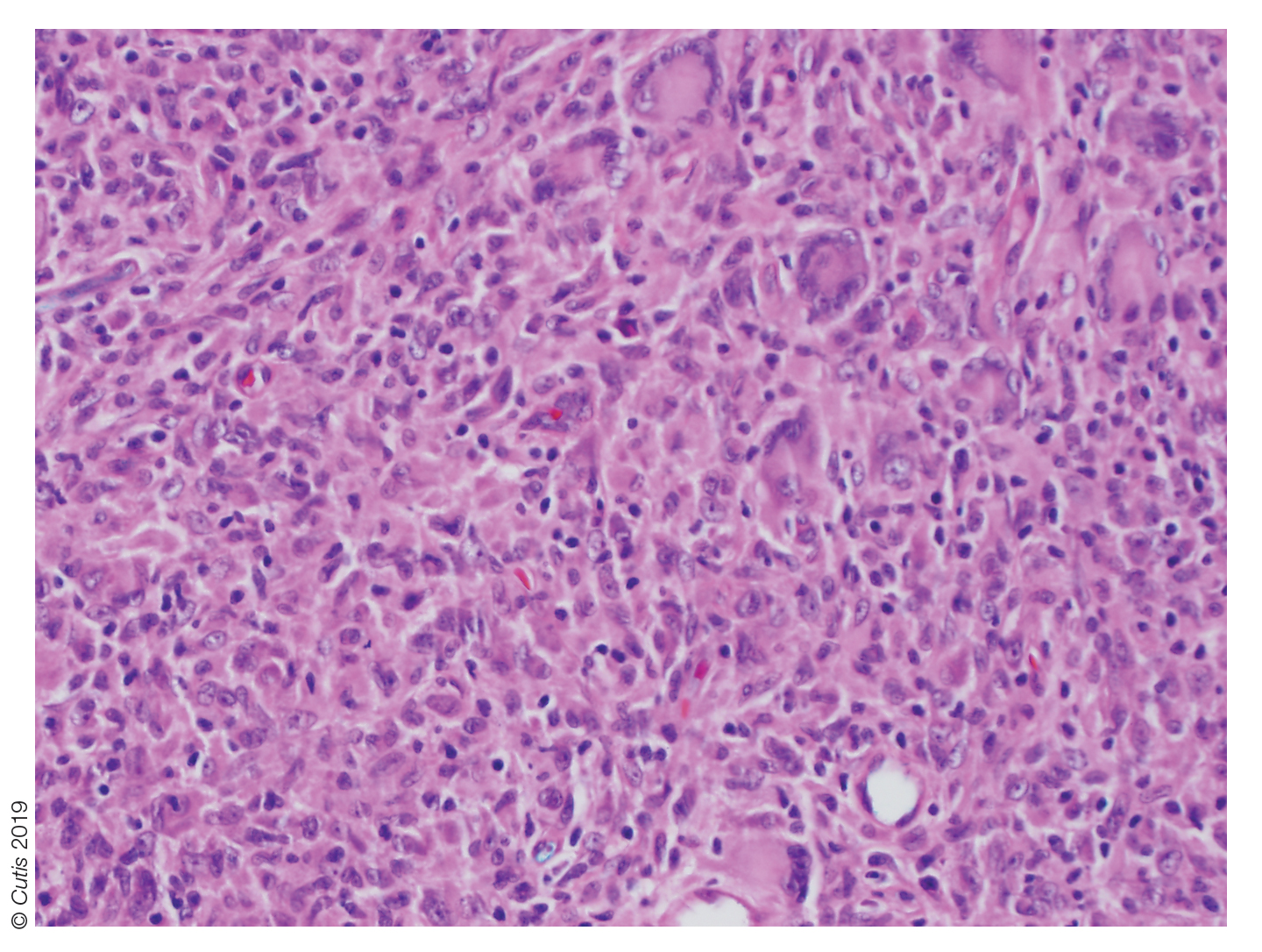
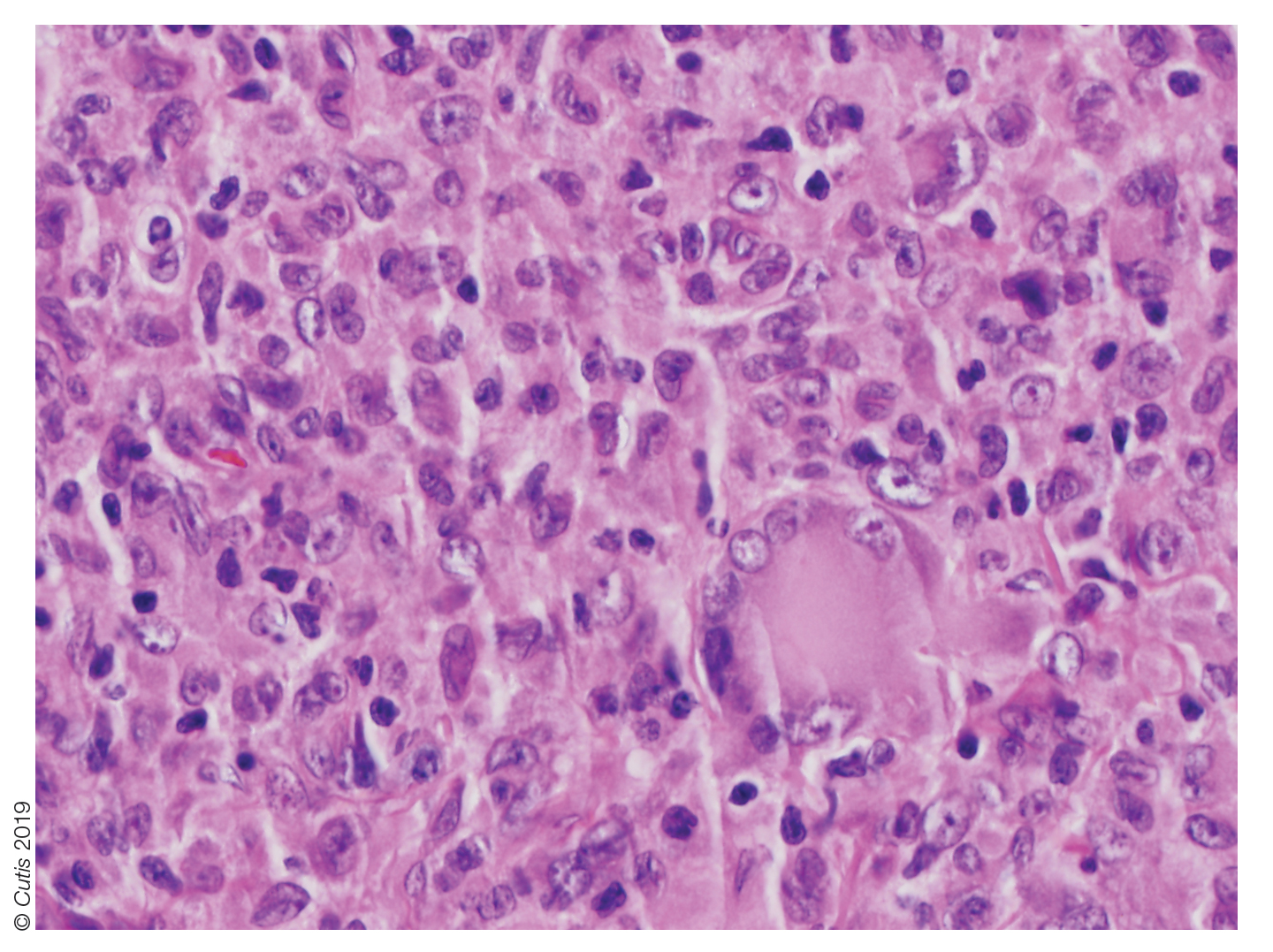
Biopsy should be performed in all cases, and basic laboratory test results such as a complete blood cell count and basic metabolic panel also are appropriate. Routine referral of all patients with cutaneous JXG for ophthalmologic evaluation is not recommended.11 Most patients with ocular involvement present with acute ocular concerns; asymptomatic eye involvement is rare. It is reasonable to consider referral to ophthalmology for patients younger than 2 years who present with multiple lesions, as they may have a higher risk for ocular involvement.11
Juvenile xanthogranuloma usually is a benign disorder with management involving observation, as the lesions typically involute spontaneously.3-7,9,10,12 Systemic or intralesional corticosteroids may be used for treatment in lesions that do not resolve. Ocular JXG refractory to steroid treatment has been managed in several cases with intravitreal bevacizumab.13 Additionally, surgical excision can be considered if malignancy is suspected, the lesion does not resolve with observation or steroid treatment, or the lesion is located near vital structures.4-7,9-13
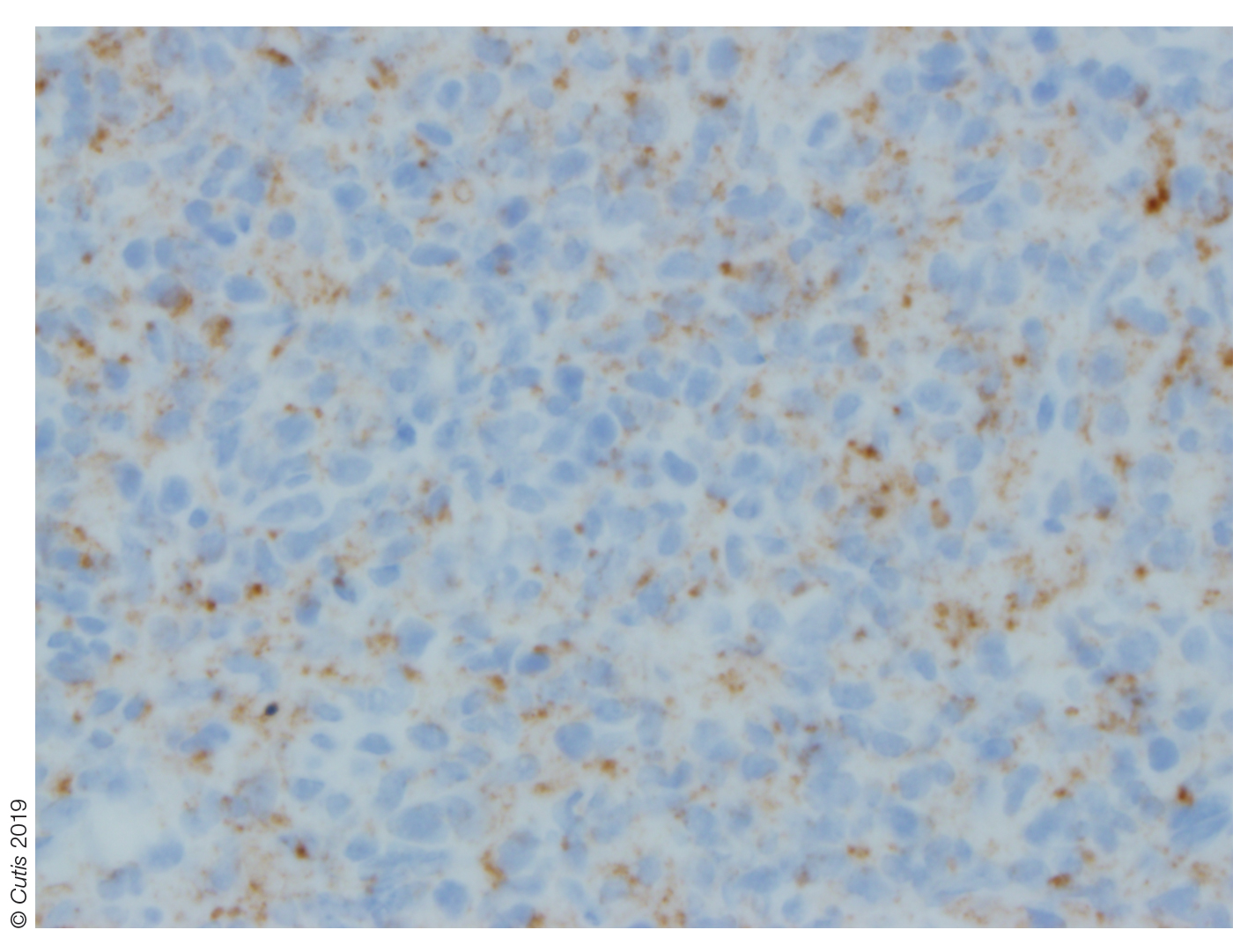
Spitz nevus presents as a single dome-shaped papule, but histology shows a symmetrical proliferation of spindle and epithelioid cells. Trachoma can present in and around the eye as a follicular hypertrophy but most commonly is seen in the conjunctiva. Dermoid cysts present as solitary subcutaneous cystic lesions; histology demonstrates a lining of keratinizing squamous epithelium with the presence of pilosebaceous structures. Dermatofibroma appears as a tan to reddish-brown papule in an area of prior minor trauma; pathology demonstrates an acanthotic epidermis with an underlying zone of normal papillary dermis and unencapsulated lesions with spindle cells overlapping in fascicles and whorls at the periphery.
1. Adamson HG. Society intelligence: the Dermatological Society of
London. Br J Dermatol. 1905;17:222-223.
2. Helwig E, Hackney VC. Juvenile xanthogranuloma (nevoxanthoendothelioma).
Am J Pathol. 1954;30:625-626.
3. Farrugia EJ, Stephen AP, Raza SA. Juvenile xanthogranuloma of
temporal bone—a case report. J Laryngol Otol. 1997;111:63-65.
4. Cypel TK, Zuker RM. Juvenile xanthogranuloma: case report and review
of the literature. Can J Plast Surg. 2008;16:175-177.
5. Chang MW, Frieden IJ, Good W. The risk of intraocular juvenile
xanthogranuloma: survey of current practices and assessment of risk.
J Am Acad Dermatol. 1996;34:445-449.
6. Zimmerman LE. Ocular lesions of juvenile xanthogranuloma.
nevoxanthoendothelioma. Am J Ophthalmol. 1965;60:1011-1035.
7. Cambiaghi S, Restano L, Caputo R. Juvenile xanthogranuloma associated
with neurofibromatosis 1: 14 patients without evidence of hematologic
malignancies. Pediatr Dermatol. 2004;21:97-101.
8. Stover DG, Alapati S, Regueira O, et al. Treatment of juvenile xanthogranuloma.
Pediatr Blood Cancer. 2008;51:130-133.
9. Dehner LP. Juvenile xanthogranulomas in the first two decades of life. a
clinicopathologic study of 174 cases with cutaneous and extracutaneous
manifestations. Am J Surg Pathol. 2003;27:579-593.
10. Weitzman S, Jaffe R. Uncommon histiocytic disorders: the non-
Langerhans cell histiocytoses. Pediatr Blood Cancer. 2005;45:256-264.
11. Chang MW, Frieden IJ, Good W. The risk of intraocular juvenile
xanthogranuloma: survey of current practices and assessment of risk.
J Am Acad Dermatol. 1996;34:445.
12. Eggli KD, Caro P, Quioque T, et al. Juvenile xanthogranuloma: non-X
histiocytosis with systemic involvement. Pediatr Radiol. 1992;22:374-376.
13. Ashkenazy N, Henry CR, Abbey AM, et al. Successful treatment of juvenile
xanthogranuloma using bevacizumab. J AAPOS. 2014;18:295-297.
The Diagnosis: Juvenile Xanthogranuloma
Juvenile xanthogranuloma (JXG) was first described in 1905 by Adamson1 as solitary or multiple plaquiform or nodular lesions that are yellow to yellowish brown. In 1954, Helwig and Hackney2 coined the term juvenile xanthogranuloma to define this histiocytic cutaneous granulomatous tumor.
Juvenile xanthogranuloma is a rare dermatologic disorder that may be present at birth and primarily affects infants and young children. The benign lesions generally occur in the first 4 years of life, with a median age of onset of 2 years.3 Lesions range in size from millimeters to several centimeters in diameter.4 The skin of the head and neck is the most commonly involved site in JXG. The most frequent noncutaneous site of JXG involvement is the eye, particularly the iris, accounting for 0.4% of cases.5,6 Extracutaneous sites such as the heart, liver, lungs, spleen, oral cavity, and brain also may be involved.4
Most children with JXG are asymptomatic. Skin lesions present as well-demarcated, rubbery, tan-orange papules or nodules. They usually are solitary, and multiple nodules can increase the risk for extracutaneous involvement.4 A case series of patients with neurofibromatosis type 1 showed 14 of 77 (18%) patients examined in the first year of life presented with JXG or other non–Langerhans cell histiocytosis.7 The adult form of cutaneous xanthogranuloma often presents with severe bronchial asthma.8
Histopathologic examination of a biopsy of the lesion typically demonstrates well-circumscribed nodules with dense infiltrates of polyhedral histiocytes with vaculoles.3-7 In 85% of cases, Touton giant cells are present.4,9 A prominent vascular network often is present, which was observed in our patient’s biopsy (Figure 1). Immunohistochemistry typically is positive for CD14, CD68, CD163, fascin, and factor XIIIa.4,10 Classically, the cells are negative for S-100 and CD1a, which differentiates these lesions from Langerhans cell histiocytosis.4-7,10 Our patient demonstrated scattered S-100–positive cells representing background dendritic cells and macrophages (Figure 2). The remainder of the clinical, morphologic, and immunophenotypic findings were consistent with non–Langerhans cell histiocytosis, specifically JXG. 

Biopsy should be performed in all cases, and basic laboratory test results such as a complete blood cell count and basic metabolic panel also are appropriate. Routine referral of all patients with cutaneous JXG for ophthalmologic evaluation is not recommended.11 Most patients with ocular involvement present with acute ocular concerns; asymptomatic eye involvement is rare. It is reasonable to consider referral to ophthalmology for patients younger than 2 years who present with multiple lesions, as they may have a higher risk for ocular involvement.11
Juvenile xanthogranuloma usually is a benign disorder with management involving observation, as the lesions typically involute spontaneously.3-7,9,10,12 Systemic or intralesional corticosteroids may be used for treatment in lesions that do not resolve. Ocular JXG refractory to steroid treatment has been managed in several cases with intravitreal bevacizumab.13 Additionally, surgical excision can be considered if malignancy is suspected, the lesion does not resolve with observation or steroid treatment, or the lesion is located near vital structures.4-7,9-13

Spitz nevus presents as a single dome-shaped papule, but histology shows a symmetrical proliferation of spindle and epithelioid cells. Trachoma can present in and around the eye as a follicular hypertrophy but most commonly is seen in the conjunctiva. Dermoid cysts present as solitary subcutaneous cystic lesions; histology demonstrates a lining of keratinizing squamous epithelium with the presence of pilosebaceous structures. Dermatofibroma appears as a tan to reddish-brown papule in an area of prior minor trauma; pathology demonstrates an acanthotic epidermis with an underlying zone of normal papillary dermis and unencapsulated lesions with spindle cells overlapping in fascicles and whorls at the periphery.
The Diagnosis: Juvenile Xanthogranuloma
Juvenile xanthogranuloma (JXG) was first described in 1905 by Adamson1 as solitary or multiple plaquiform or nodular lesions that are yellow to yellowish brown. In 1954, Helwig and Hackney2 coined the term juvenile xanthogranuloma to define this histiocytic cutaneous granulomatous tumor.
Juvenile xanthogranuloma is a rare dermatologic disorder that may be present at birth and primarily affects infants and young children. The benign lesions generally occur in the first 4 years of life, with a median age of onset of 2 years.3 Lesions range in size from millimeters to several centimeters in diameter.4 The skin of the head and neck is the most commonly involved site in JXG. The most frequent noncutaneous site of JXG involvement is the eye, particularly the iris, accounting for 0.4% of cases.5,6 Extracutaneous sites such as the heart, liver, lungs, spleen, oral cavity, and brain also may be involved.4
Most children with JXG are asymptomatic. Skin lesions present as well-demarcated, rubbery, tan-orange papules or nodules. They usually are solitary, and multiple nodules can increase the risk for extracutaneous involvement.4 A case series of patients with neurofibromatosis type 1 showed 14 of 77 (18%) patients examined in the first year of life presented with JXG or other non–Langerhans cell histiocytosis.7 The adult form of cutaneous xanthogranuloma often presents with severe bronchial asthma.8
Histopathologic examination of a biopsy of the lesion typically demonstrates well-circumscribed nodules with dense infiltrates of polyhedral histiocytes with vaculoles.3-7 In 85% of cases, Touton giant cells are present.4,9 A prominent vascular network often is present, which was observed in our patient’s biopsy (Figure 1). Immunohistochemistry typically is positive for CD14, CD68, CD163, fascin, and factor XIIIa.4,10 Classically, the cells are negative for S-100 and CD1a, which differentiates these lesions from Langerhans cell histiocytosis.4-7,10 Our patient demonstrated scattered S-100–positive cells representing background dendritic cells and macrophages (Figure 2). The remainder of the clinical, morphologic, and immunophenotypic findings were consistent with non–Langerhans cell histiocytosis, specifically JXG. 

Biopsy should be performed in all cases, and basic laboratory test results such as a complete blood cell count and basic metabolic panel also are appropriate. Routine referral of all patients with cutaneous JXG for ophthalmologic evaluation is not recommended.11 Most patients with ocular involvement present with acute ocular concerns; asymptomatic eye involvement is rare. It is reasonable to consider referral to ophthalmology for patients younger than 2 years who present with multiple lesions, as they may have a higher risk for ocular involvement.11
Juvenile xanthogranuloma usually is a benign disorder with management involving observation, as the lesions typically involute spontaneously.3-7,9,10,12 Systemic or intralesional corticosteroids may be used for treatment in lesions that do not resolve. Ocular JXG refractory to steroid treatment has been managed in several cases with intravitreal bevacizumab.13 Additionally, surgical excision can be considered if malignancy is suspected, the lesion does not resolve with observation or steroid treatment, or the lesion is located near vital structures.4-7,9-13

Spitz nevus presents as a single dome-shaped papule, but histology shows a symmetrical proliferation of spindle and epithelioid cells. Trachoma can present in and around the eye as a follicular hypertrophy but most commonly is seen in the conjunctiva. Dermoid cysts present as solitary subcutaneous cystic lesions; histology demonstrates a lining of keratinizing squamous epithelium with the presence of pilosebaceous structures. Dermatofibroma appears as a tan to reddish-brown papule in an area of prior minor trauma; pathology demonstrates an acanthotic epidermis with an underlying zone of normal papillary dermis and unencapsulated lesions with spindle cells overlapping in fascicles and whorls at the periphery.
1. Adamson HG. Society intelligence: the Dermatological Society of
London. Br J Dermatol. 1905;17:222-223.
2. Helwig E, Hackney VC. Juvenile xanthogranuloma (nevoxanthoendothelioma).
Am J Pathol. 1954;30:625-626.
3. Farrugia EJ, Stephen AP, Raza SA. Juvenile xanthogranuloma of
temporal bone—a case report. J Laryngol Otol. 1997;111:63-65.
4. Cypel TK, Zuker RM. Juvenile xanthogranuloma: case report and review
of the literature. Can J Plast Surg. 2008;16:175-177.
5. Chang MW, Frieden IJ, Good W. The risk of intraocular juvenile
xanthogranuloma: survey of current practices and assessment of risk.
J Am Acad Dermatol. 1996;34:445-449.
6. Zimmerman LE. Ocular lesions of juvenile xanthogranuloma.
nevoxanthoendothelioma. Am J Ophthalmol. 1965;60:1011-1035.
7. Cambiaghi S, Restano L, Caputo R. Juvenile xanthogranuloma associated
with neurofibromatosis 1: 14 patients without evidence of hematologic
malignancies. Pediatr Dermatol. 2004;21:97-101.
8. Stover DG, Alapati S, Regueira O, et al. Treatment of juvenile xanthogranuloma.
Pediatr Blood Cancer. 2008;51:130-133.
9. Dehner LP. Juvenile xanthogranulomas in the first two decades of life. a
clinicopathologic study of 174 cases with cutaneous and extracutaneous
manifestations. Am J Surg Pathol. 2003;27:579-593.
10. Weitzman S, Jaffe R. Uncommon histiocytic disorders: the non-
Langerhans cell histiocytoses. Pediatr Blood Cancer. 2005;45:256-264.
11. Chang MW, Frieden IJ, Good W. The risk of intraocular juvenile
xanthogranuloma: survey of current practices and assessment of risk.
J Am Acad Dermatol. 1996;34:445.
12. Eggli KD, Caro P, Quioque T, et al. Juvenile xanthogranuloma: non-X
histiocytosis with systemic involvement. Pediatr Radiol. 1992;22:374-376.
13. Ashkenazy N, Henry CR, Abbey AM, et al. Successful treatment of juvenile
xanthogranuloma using bevacizumab. J AAPOS. 2014;18:295-297.
1. Adamson HG. Society intelligence: the Dermatological Society of
London. Br J Dermatol. 1905;17:222-223.
2. Helwig E, Hackney VC. Juvenile xanthogranuloma (nevoxanthoendothelioma).
Am J Pathol. 1954;30:625-626.
3. Farrugia EJ, Stephen AP, Raza SA. Juvenile xanthogranuloma of
temporal bone—a case report. J Laryngol Otol. 1997;111:63-65.
4. Cypel TK, Zuker RM. Juvenile xanthogranuloma: case report and review
of the literature. Can J Plast Surg. 2008;16:175-177.
5. Chang MW, Frieden IJ, Good W. The risk of intraocular juvenile
xanthogranuloma: survey of current practices and assessment of risk.
J Am Acad Dermatol. 1996;34:445-449.
6. Zimmerman LE. Ocular lesions of juvenile xanthogranuloma.
nevoxanthoendothelioma. Am J Ophthalmol. 1965;60:1011-1035.
7. Cambiaghi S, Restano L, Caputo R. Juvenile xanthogranuloma associated
with neurofibromatosis 1: 14 patients without evidence of hematologic
malignancies. Pediatr Dermatol. 2004;21:97-101.
8. Stover DG, Alapati S, Regueira O, et al. Treatment of juvenile xanthogranuloma.
Pediatr Blood Cancer. 2008;51:130-133.
9. Dehner LP. Juvenile xanthogranulomas in the first two decades of life. a
clinicopathologic study of 174 cases with cutaneous and extracutaneous
manifestations. Am J Surg Pathol. 2003;27:579-593.
10. Weitzman S, Jaffe R. Uncommon histiocytic disorders: the non-
Langerhans cell histiocytoses. Pediatr Blood Cancer. 2005;45:256-264.
11. Chang MW, Frieden IJ, Good W. The risk of intraocular juvenile
xanthogranuloma: survey of current practices and assessment of risk.
J Am Acad Dermatol. 1996;34:445.
12. Eggli KD, Caro P, Quioque T, et al. Juvenile xanthogranuloma: non-X
histiocytosis with systemic involvement. Pediatr Radiol. 1992;22:374-376.
13. Ashkenazy N, Henry CR, Abbey AM, et al. Successful treatment of juvenile
xanthogranuloma using bevacizumab. J AAPOS. 2014;18:295-297.
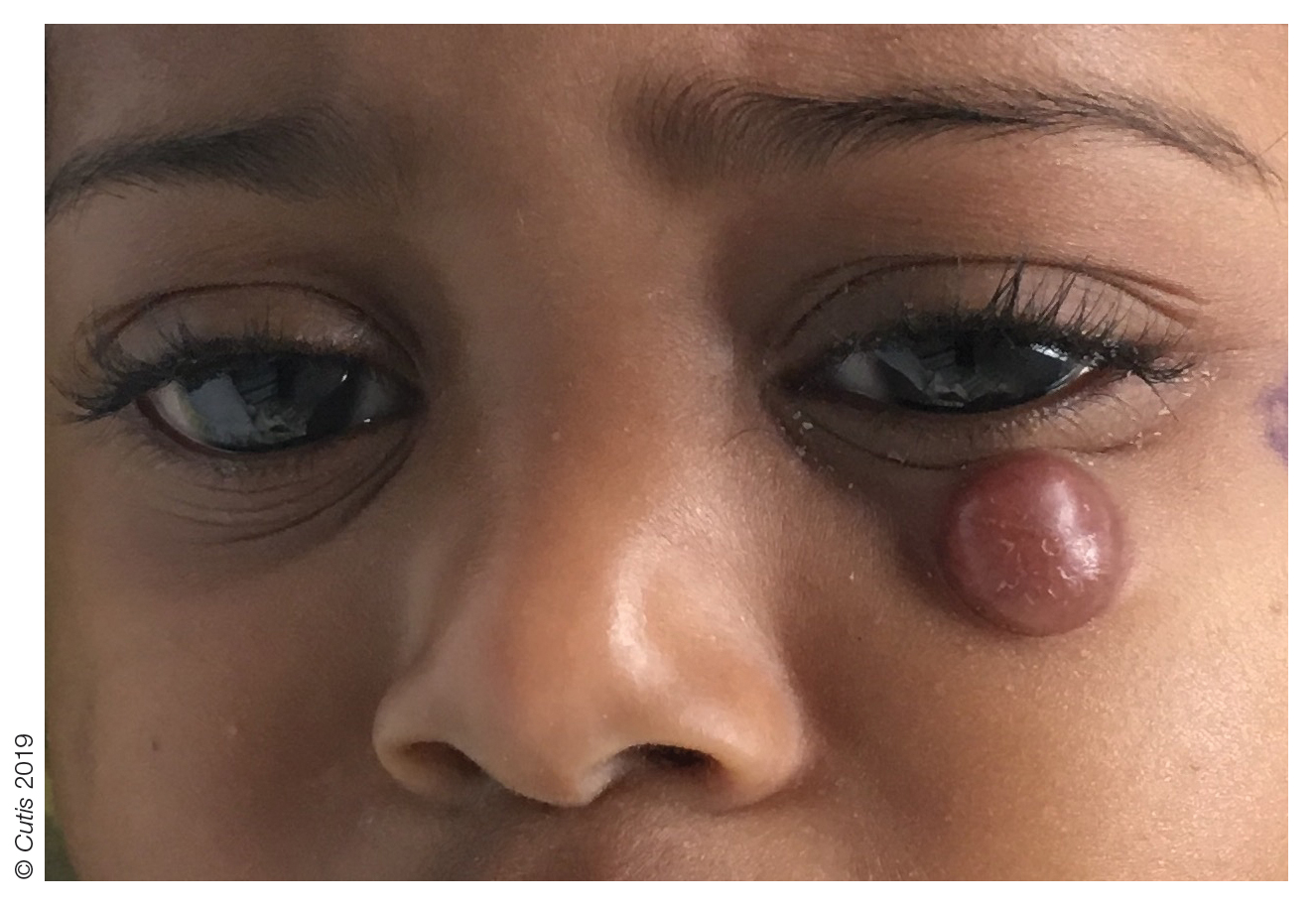
A 10-month-old girl presented with a facial nodule of 7 months’ duration that started as a small lesion. On physical examination, a single 10×10-mm, nontender, well-circumscribed, firm, freely mobile nodule was observed in the left infraorbital area. The patient was born full term at 37 weeks’ gestation via spontaneous vaginal delivery and had no other notable findings on physical examination. Excision was performed by an oculoplastic surgeon. Pathology revealed a relatively well-circumscribed, diffuse, dermal infiltrate of cells arranged in short fascicles and a storiform pattern. The cells had abundant clear to amphophilic cytoplasm, ovoid to reniform nuclei with vesicular chromatin and focal grooves, and diffuse CD68+ immunoreactivity, as well as scattered S-100–positive cells. The patient did well with the excision and no new lesions have developed.