User login
What’s new for CHEST 2019?
Head to New Orleans this October for CHEST Annual Meeting 2019 for the latest original research, postgraduate courses, interactive case-based discussions, simulation sessions, CHEST Games, and more! CHEST 2019 allows clinician members of the entire health-care team to stay up to date on pulmonary, critical care, and sleep medicine. There are many new and exciting things happening at CHEST 2019, and we are excited to give you a sneak peek.
The simulation sessions are better than ever and include a full day of cadaver-based courses and brand new hands-on sessions in bronchoscopy, advanced critical care echocardiography, and airway management, that will put your skills to the test. You don’t want to miss these simulation sessions that allow you to learn from our expert faculty to advance and develop valuable skills and apply your knowledge.
Visit CHEST in the exhibit hall to see the new additions we have added to amplify your experience. The new FISH Bowl innovation competition will allow you to learn about new solutions and ideas that were submitted in education and clinical disease for pulmonary, critical care, and sleep medicine. The finalists will be presenting live in Experience CHEST and competing for prizes in each category. CHEST games will be back again in a new space in the exhibit hall. Be sure to bring your team to play the popular Nodal Nemesis and the other games that test your skills in new and creative ways.
CHEST 2019 plans to make your life easier by providing you with the latest updates in patient care at the annual meeting, but we are also planning on making it easier in other ways. New this year, you can update your professional headshot in our new complimentary headshot booth. Plan on a visit to the new CHEST Wellness Zone. This area is designed to help you relax and recharge while at CHEST and includes meditation, posture consultants, aromatherapy, foot massage, and yoga. Attend CHEST 2019 with some peace of mind knowing that your children can be cared for at the Kiddie Corp childcare program for kids ages 6 months to 12 years.
According to William Kelly, MD, FCCP, CHEST 2019 Program Chair, “We are excited about these new opportunities that will help you improve your patient care. We’re taking concrete steps to make your learning, your practice, and your life a little easier.”
We look forward to seeing you at CHEST 2019 in New Orleans, Louisiana, October 19-23!
Head to New Orleans this October for CHEST Annual Meeting 2019 for the latest original research, postgraduate courses, interactive case-based discussions, simulation sessions, CHEST Games, and more! CHEST 2019 allows clinician members of the entire health-care team to stay up to date on pulmonary, critical care, and sleep medicine. There are many new and exciting things happening at CHEST 2019, and we are excited to give you a sneak peek.
The simulation sessions are better than ever and include a full day of cadaver-based courses and brand new hands-on sessions in bronchoscopy, advanced critical care echocardiography, and airway management, that will put your skills to the test. You don’t want to miss these simulation sessions that allow you to learn from our expert faculty to advance and develop valuable skills and apply your knowledge.
Visit CHEST in the exhibit hall to see the new additions we have added to amplify your experience. The new FISH Bowl innovation competition will allow you to learn about new solutions and ideas that were submitted in education and clinical disease for pulmonary, critical care, and sleep medicine. The finalists will be presenting live in Experience CHEST and competing for prizes in each category. CHEST games will be back again in a new space in the exhibit hall. Be sure to bring your team to play the popular Nodal Nemesis and the other games that test your skills in new and creative ways.
CHEST 2019 plans to make your life easier by providing you with the latest updates in patient care at the annual meeting, but we are also planning on making it easier in other ways. New this year, you can update your professional headshot in our new complimentary headshot booth. Plan on a visit to the new CHEST Wellness Zone. This area is designed to help you relax and recharge while at CHEST and includes meditation, posture consultants, aromatherapy, foot massage, and yoga. Attend CHEST 2019 with some peace of mind knowing that your children can be cared for at the Kiddie Corp childcare program for kids ages 6 months to 12 years.
According to William Kelly, MD, FCCP, CHEST 2019 Program Chair, “We are excited about these new opportunities that will help you improve your patient care. We’re taking concrete steps to make your learning, your practice, and your life a little easier.”
We look forward to seeing you at CHEST 2019 in New Orleans, Louisiana, October 19-23!
Head to New Orleans this October for CHEST Annual Meeting 2019 for the latest original research, postgraduate courses, interactive case-based discussions, simulation sessions, CHEST Games, and more! CHEST 2019 allows clinician members of the entire health-care team to stay up to date on pulmonary, critical care, and sleep medicine. There are many new and exciting things happening at CHEST 2019, and we are excited to give you a sneak peek.
The simulation sessions are better than ever and include a full day of cadaver-based courses and brand new hands-on sessions in bronchoscopy, advanced critical care echocardiography, and airway management, that will put your skills to the test. You don’t want to miss these simulation sessions that allow you to learn from our expert faculty to advance and develop valuable skills and apply your knowledge.
Visit CHEST in the exhibit hall to see the new additions we have added to amplify your experience. The new FISH Bowl innovation competition will allow you to learn about new solutions and ideas that were submitted in education and clinical disease for pulmonary, critical care, and sleep medicine. The finalists will be presenting live in Experience CHEST and competing for prizes in each category. CHEST games will be back again in a new space in the exhibit hall. Be sure to bring your team to play the popular Nodal Nemesis and the other games that test your skills in new and creative ways.
CHEST 2019 plans to make your life easier by providing you with the latest updates in patient care at the annual meeting, but we are also planning on making it easier in other ways. New this year, you can update your professional headshot in our new complimentary headshot booth. Plan on a visit to the new CHEST Wellness Zone. This area is designed to help you relax and recharge while at CHEST and includes meditation, posture consultants, aromatherapy, foot massage, and yoga. Attend CHEST 2019 with some peace of mind knowing that your children can be cared for at the Kiddie Corp childcare program for kids ages 6 months to 12 years.
According to William Kelly, MD, FCCP, CHEST 2019 Program Chair, “We are excited about these new opportunities that will help you improve your patient care. We’re taking concrete steps to make your learning, your practice, and your life a little easier.”
We look forward to seeing you at CHEST 2019 in New Orleans, Louisiana, October 19-23!
Vaping in 2019: Risk vs. reward
The prevalence and popularity of electronic cigarettes or “vaping” have grown dramatically over the last several years in the United States. Although new studies targeting these products are being done at increasing frequency, there remains a relative paucity of data regarding the long-term risks. Proponents argue that they can be used as a cessation tool for smokers, or failing that, a safer replacement for traditional cigarettes. Opponents make the case that the perception of safety could contribute to increased use in people who may have otherwise never smoked, leading to an overall increase in nicotine use and addiction. This is most readily seen in the adolescent population, where use has skyrocketed, leading to concerns about how electronic cigarettes are marketed to youth, as well as the ease of access.
Basics of vaping (devices)
In its most basic form, an electronic cigarette consists of a battery that powers a heating coil. This heating coil applies heat to a wick, which is soaked in liquid, “vape juice,” converting it into a vapor that is then directly inhaled. However, there can be many variations on this simple theme. Early generation products resembled traditional cigarettes in size and shape and were marketed as smoking cessation aids. Newer devices have abandoned this look and strategy. Preloaded cartridges have been replaced by large tanks that the user can fill with the liquid of their choosing. Multiple tanks can be purchased for a single device, enabling the user to have multiple flavors or various levels of nicotine dosing on hand for quick changing, depending on user preference or mood. Additionally, there are variable voltage settings, resulting in different styles of vapor and/or “throat hit” (the description of the desired burning vs smooth effect of the vapor on the oropharynx). This type of device invites experimentation. Multiple flavors can be used in isolation or mixed together at various temperatures. It no longer resembles classic cigarettes, and the flavor and experience are more prominently promoted. One can see that this device has more appeal to a “never smoker” than the original products, and there is concern that it is being marketed as such with some success (Dinakar C, et al. N Engl J Med. 2016;375[14]:1372).
E-liquid
Perhaps more important than the devices themselves is an understanding of the components of the liquid used to generate the inhaled aerosol.
Typically, four components are present:
• Propylene glycol
• Vegetable glycerin
• Flavoring
• Nicotine
The first two components are generally considered nontoxic, based on their use as food additives. However, inhalation is a novel route of entry and the long-term effects on the respiratory tract are unclear.
The third component, “flavorings,” is a catch-all term for the hundreds of different flavors and styles of e-liquids available today, ranging from menthol to fruit or candy and everything in between. It is difficult to account for all the potential effects of the numerous flavorings being used, especially when some are combined by the end user to various degrees.
Nicotine is present, specified in varying doses. However, vaping style, experience, and type of device used can dramatically affect how much is absorbed, making dosages difficult to predict. Additionally, labeled doses are prone to wide ranges of error (Schraufnagel DE, et al. Am J Respir Crit Care Med. 2014;190[6]:611).
What are the risks?
Cancer
A handful of known carcinogens can be found in inhaled vapor, including formaldehyde, acetaldehyde, acrolein, toluene, and nitrosamines. However, they are present in far lower concentrations than in traditional cigarettes (Goniewicz ML, et al. JAMA Netw Open. 2018;1[8]e185937). This leads to the natural assumption that vaping, while not benign, poses a much lower cancer risk when compared with smoking. Whether that is borne out in the long term remains to be seen.
Pulmonary function
The long-term effect on pulmonary function is not known. Small studies have shown no significant changes to spirometry after acute exposure to vapor. More data are needed in this area (Palazzolo DL. Frontiers Public Health. 2013;1[56]1-20).
Wound healing
An animal study has shown evidence of poor wound healing extrapolated from skin flap necrosis in rats. Exposure to vapor vs smoke yielded similar results, and both were worse than the sham arm (Troiano C, et al. JAMA Facial Plast Surg. 2019;21[1]:5). While it is difficult to know how to apply this clinically, it may be prudent to advise patients to abstain while in preparation for elective surgery.
Cardiovascular/stroke
Much of the cardiovascular toxicity from cigarette use is tied to the myriad of complex toxic particles produced in inhaled smoke, the vast majority of which are not present in e-cigarette vapor. While nicotine itself has known acute cardiovascular effects, including tachycardia and vasoconstriction, a tolerance to these effects occurs over time. Previous evaluations of nicotine replacement therapies and smokeless tobacco for their cardiovascular effects have had mixed results. But, there appears to be a trend toward minimal cardiovascular risk when using “cleaner” products, such as nicotine replacement therapy compared with smokeless tobacco (Benowitz NL, et al. Nature Rev Cardiol. 2017;14[8]:447). Whether this can be extrapolated to electronic cigarette use is unknown but is encouraging.
Alternative toxicity
In addition to the above risks that are in comparison to traditional smoking, vaping also introduces novel toxicities. There are case reports of lipoid pneumonia, ARDS, hypersensitivity pneumonitis, eosinophilic pneumonia, and diffuse alveola hemorrhage. Burns from malfunctioning devices must also be considered, as there is a wide array of products available, at differing levels of build quality.
Toxic oral ingestion of nicotine, especially by children, has led to increased calls to poison centers. For a small child, this can be fatal. Regulation of labels and containers could curtail this issue. But, public education regarding the toxicity of these substances when ingested in large quantities is also important. If there is a lack of understanding about this danger, then typical safeguards are easily overlooked by individual users.
Are there benefits?
Smoking cessation
Compared with other products, such as nicotine patches, gum, and pharmaceutical methods, e-cigarettes most closely mimic the actual experience of smoking. For some, the habit and ritual of smoking is as much a part of the addiction as nicotine. Vaping has the potential to help alleviate this difficult aspect of cessation. Data involving early generation products failed to show a significant advantage. Newer devices that are more pleasurable to use and offer more efficient nicotine delivery may be more effective. Indeed, a recent study in the New England Journal of Medicine from this year demonstrated improved smoking cessation compared with traditional methods, using second generation vape devices (Hajek P, et al. N Engl J Med. 2019;380[7]629). It will be interesting to see if this can be repeatable going forward and if protocols can be established to maximize effectiveness.
As outlined above, it is difficult to make definitive conclusions or recommendations regarding electronic cigarette use at the present time. The risk of cancer and cardiopulmonary disease is likely to be significantly lower but not eliminated. Use as a smoking cessation aid is starting to show promise. Even without cessation, ongoing vaping is likely to be safer than ongoing smoking. Two caveats to this remain: some patients, in an effort to quit smoking, may take up vaping but eventually become “dual users.” This scenario has been associated with higher toxic exposure and possibly worse outcomes. The second caveat is that while there is promise to using this as a cessation tool, it should not yet replace other more well-studied, first-line agents in this regard. It should, perhaps, target patients who are motivated to quit but have failed more traditional methods. Finally, there continues to be concern that vaping could appeal to never smokers, given its perceived safety profile and ease of use in public places. This could lead to an overall increase in nicotine addiction, which could be a significant step backwards.
Dr. Clark is Assistant Professor, Pulmonary and Critical Care Medicine, UT Southwestern Medical Center, Dallas, Texas.
The prevalence and popularity of electronic cigarettes or “vaping” have grown dramatically over the last several years in the United States. Although new studies targeting these products are being done at increasing frequency, there remains a relative paucity of data regarding the long-term risks. Proponents argue that they can be used as a cessation tool for smokers, or failing that, a safer replacement for traditional cigarettes. Opponents make the case that the perception of safety could contribute to increased use in people who may have otherwise never smoked, leading to an overall increase in nicotine use and addiction. This is most readily seen in the adolescent population, where use has skyrocketed, leading to concerns about how electronic cigarettes are marketed to youth, as well as the ease of access.
Basics of vaping (devices)
In its most basic form, an electronic cigarette consists of a battery that powers a heating coil. This heating coil applies heat to a wick, which is soaked in liquid, “vape juice,” converting it into a vapor that is then directly inhaled. However, there can be many variations on this simple theme. Early generation products resembled traditional cigarettes in size and shape and were marketed as smoking cessation aids. Newer devices have abandoned this look and strategy. Preloaded cartridges have been replaced by large tanks that the user can fill with the liquid of their choosing. Multiple tanks can be purchased for a single device, enabling the user to have multiple flavors or various levels of nicotine dosing on hand for quick changing, depending on user preference or mood. Additionally, there are variable voltage settings, resulting in different styles of vapor and/or “throat hit” (the description of the desired burning vs smooth effect of the vapor on the oropharynx). This type of device invites experimentation. Multiple flavors can be used in isolation or mixed together at various temperatures. It no longer resembles classic cigarettes, and the flavor and experience are more prominently promoted. One can see that this device has more appeal to a “never smoker” than the original products, and there is concern that it is being marketed as such with some success (Dinakar C, et al. N Engl J Med. 2016;375[14]:1372).
E-liquid
Perhaps more important than the devices themselves is an understanding of the components of the liquid used to generate the inhaled aerosol.
Typically, four components are present:
• Propylene glycol
• Vegetable glycerin
• Flavoring
• Nicotine
The first two components are generally considered nontoxic, based on their use as food additives. However, inhalation is a novel route of entry and the long-term effects on the respiratory tract are unclear.
The third component, “flavorings,” is a catch-all term for the hundreds of different flavors and styles of e-liquids available today, ranging from menthol to fruit or candy and everything in between. It is difficult to account for all the potential effects of the numerous flavorings being used, especially when some are combined by the end user to various degrees.
Nicotine is present, specified in varying doses. However, vaping style, experience, and type of device used can dramatically affect how much is absorbed, making dosages difficult to predict. Additionally, labeled doses are prone to wide ranges of error (Schraufnagel DE, et al. Am J Respir Crit Care Med. 2014;190[6]:611).
What are the risks?
Cancer
A handful of known carcinogens can be found in inhaled vapor, including formaldehyde, acetaldehyde, acrolein, toluene, and nitrosamines. However, they are present in far lower concentrations than in traditional cigarettes (Goniewicz ML, et al. JAMA Netw Open. 2018;1[8]e185937). This leads to the natural assumption that vaping, while not benign, poses a much lower cancer risk when compared with smoking. Whether that is borne out in the long term remains to be seen.
Pulmonary function
The long-term effect on pulmonary function is not known. Small studies have shown no significant changes to spirometry after acute exposure to vapor. More data are needed in this area (Palazzolo DL. Frontiers Public Health. 2013;1[56]1-20).
Wound healing
An animal study has shown evidence of poor wound healing extrapolated from skin flap necrosis in rats. Exposure to vapor vs smoke yielded similar results, and both were worse than the sham arm (Troiano C, et al. JAMA Facial Plast Surg. 2019;21[1]:5). While it is difficult to know how to apply this clinically, it may be prudent to advise patients to abstain while in preparation for elective surgery.
Cardiovascular/stroke
Much of the cardiovascular toxicity from cigarette use is tied to the myriad of complex toxic particles produced in inhaled smoke, the vast majority of which are not present in e-cigarette vapor. While nicotine itself has known acute cardiovascular effects, including tachycardia and vasoconstriction, a tolerance to these effects occurs over time. Previous evaluations of nicotine replacement therapies and smokeless tobacco for their cardiovascular effects have had mixed results. But, there appears to be a trend toward minimal cardiovascular risk when using “cleaner” products, such as nicotine replacement therapy compared with smokeless tobacco (Benowitz NL, et al. Nature Rev Cardiol. 2017;14[8]:447). Whether this can be extrapolated to electronic cigarette use is unknown but is encouraging.
Alternative toxicity
In addition to the above risks that are in comparison to traditional smoking, vaping also introduces novel toxicities. There are case reports of lipoid pneumonia, ARDS, hypersensitivity pneumonitis, eosinophilic pneumonia, and diffuse alveola hemorrhage. Burns from malfunctioning devices must also be considered, as there is a wide array of products available, at differing levels of build quality.
Toxic oral ingestion of nicotine, especially by children, has led to increased calls to poison centers. For a small child, this can be fatal. Regulation of labels and containers could curtail this issue. But, public education regarding the toxicity of these substances when ingested in large quantities is also important. If there is a lack of understanding about this danger, then typical safeguards are easily overlooked by individual users.
Are there benefits?
Smoking cessation
Compared with other products, such as nicotine patches, gum, and pharmaceutical methods, e-cigarettes most closely mimic the actual experience of smoking. For some, the habit and ritual of smoking is as much a part of the addiction as nicotine. Vaping has the potential to help alleviate this difficult aspect of cessation. Data involving early generation products failed to show a significant advantage. Newer devices that are more pleasurable to use and offer more efficient nicotine delivery may be more effective. Indeed, a recent study in the New England Journal of Medicine from this year demonstrated improved smoking cessation compared with traditional methods, using second generation vape devices (Hajek P, et al. N Engl J Med. 2019;380[7]629). It will be interesting to see if this can be repeatable going forward and if protocols can be established to maximize effectiveness.
As outlined above, it is difficult to make definitive conclusions or recommendations regarding electronic cigarette use at the present time. The risk of cancer and cardiopulmonary disease is likely to be significantly lower but not eliminated. Use as a smoking cessation aid is starting to show promise. Even without cessation, ongoing vaping is likely to be safer than ongoing smoking. Two caveats to this remain: some patients, in an effort to quit smoking, may take up vaping but eventually become “dual users.” This scenario has been associated with higher toxic exposure and possibly worse outcomes. The second caveat is that while there is promise to using this as a cessation tool, it should not yet replace other more well-studied, first-line agents in this regard. It should, perhaps, target patients who are motivated to quit but have failed more traditional methods. Finally, there continues to be concern that vaping could appeal to never smokers, given its perceived safety profile and ease of use in public places. This could lead to an overall increase in nicotine addiction, which could be a significant step backwards.
Dr. Clark is Assistant Professor, Pulmonary and Critical Care Medicine, UT Southwestern Medical Center, Dallas, Texas.
The prevalence and popularity of electronic cigarettes or “vaping” have grown dramatically over the last several years in the United States. Although new studies targeting these products are being done at increasing frequency, there remains a relative paucity of data regarding the long-term risks. Proponents argue that they can be used as a cessation tool for smokers, or failing that, a safer replacement for traditional cigarettes. Opponents make the case that the perception of safety could contribute to increased use in people who may have otherwise never smoked, leading to an overall increase in nicotine use and addiction. This is most readily seen in the adolescent population, where use has skyrocketed, leading to concerns about how electronic cigarettes are marketed to youth, as well as the ease of access.
Basics of vaping (devices)
In its most basic form, an electronic cigarette consists of a battery that powers a heating coil. This heating coil applies heat to a wick, which is soaked in liquid, “vape juice,” converting it into a vapor that is then directly inhaled. However, there can be many variations on this simple theme. Early generation products resembled traditional cigarettes in size and shape and were marketed as smoking cessation aids. Newer devices have abandoned this look and strategy. Preloaded cartridges have been replaced by large tanks that the user can fill with the liquid of their choosing. Multiple tanks can be purchased for a single device, enabling the user to have multiple flavors or various levels of nicotine dosing on hand for quick changing, depending on user preference or mood. Additionally, there are variable voltage settings, resulting in different styles of vapor and/or “throat hit” (the description of the desired burning vs smooth effect of the vapor on the oropharynx). This type of device invites experimentation. Multiple flavors can be used in isolation or mixed together at various temperatures. It no longer resembles classic cigarettes, and the flavor and experience are more prominently promoted. One can see that this device has more appeal to a “never smoker” than the original products, and there is concern that it is being marketed as such with some success (Dinakar C, et al. N Engl J Med. 2016;375[14]:1372).
E-liquid
Perhaps more important than the devices themselves is an understanding of the components of the liquid used to generate the inhaled aerosol.
Typically, four components are present:
• Propylene glycol
• Vegetable glycerin
• Flavoring
• Nicotine
The first two components are generally considered nontoxic, based on their use as food additives. However, inhalation is a novel route of entry and the long-term effects on the respiratory tract are unclear.
The third component, “flavorings,” is a catch-all term for the hundreds of different flavors and styles of e-liquids available today, ranging from menthol to fruit or candy and everything in between. It is difficult to account for all the potential effects of the numerous flavorings being used, especially when some are combined by the end user to various degrees.
Nicotine is present, specified in varying doses. However, vaping style, experience, and type of device used can dramatically affect how much is absorbed, making dosages difficult to predict. Additionally, labeled doses are prone to wide ranges of error (Schraufnagel DE, et al. Am J Respir Crit Care Med. 2014;190[6]:611).
What are the risks?
Cancer
A handful of known carcinogens can be found in inhaled vapor, including formaldehyde, acetaldehyde, acrolein, toluene, and nitrosamines. However, they are present in far lower concentrations than in traditional cigarettes (Goniewicz ML, et al. JAMA Netw Open. 2018;1[8]e185937). This leads to the natural assumption that vaping, while not benign, poses a much lower cancer risk when compared with smoking. Whether that is borne out in the long term remains to be seen.
Pulmonary function
The long-term effect on pulmonary function is not known. Small studies have shown no significant changes to spirometry after acute exposure to vapor. More data are needed in this area (Palazzolo DL. Frontiers Public Health. 2013;1[56]1-20).
Wound healing
An animal study has shown evidence of poor wound healing extrapolated from skin flap necrosis in rats. Exposure to vapor vs smoke yielded similar results, and both were worse than the sham arm (Troiano C, et al. JAMA Facial Plast Surg. 2019;21[1]:5). While it is difficult to know how to apply this clinically, it may be prudent to advise patients to abstain while in preparation for elective surgery.
Cardiovascular/stroke
Much of the cardiovascular toxicity from cigarette use is tied to the myriad of complex toxic particles produced in inhaled smoke, the vast majority of which are not present in e-cigarette vapor. While nicotine itself has known acute cardiovascular effects, including tachycardia and vasoconstriction, a tolerance to these effects occurs over time. Previous evaluations of nicotine replacement therapies and smokeless tobacco for their cardiovascular effects have had mixed results. But, there appears to be a trend toward minimal cardiovascular risk when using “cleaner” products, such as nicotine replacement therapy compared with smokeless tobacco (Benowitz NL, et al. Nature Rev Cardiol. 2017;14[8]:447). Whether this can be extrapolated to electronic cigarette use is unknown but is encouraging.
Alternative toxicity
In addition to the above risks that are in comparison to traditional smoking, vaping also introduces novel toxicities. There are case reports of lipoid pneumonia, ARDS, hypersensitivity pneumonitis, eosinophilic pneumonia, and diffuse alveola hemorrhage. Burns from malfunctioning devices must also be considered, as there is a wide array of products available, at differing levels of build quality.
Toxic oral ingestion of nicotine, especially by children, has led to increased calls to poison centers. For a small child, this can be fatal. Regulation of labels and containers could curtail this issue. But, public education regarding the toxicity of these substances when ingested in large quantities is also important. If there is a lack of understanding about this danger, then typical safeguards are easily overlooked by individual users.
Are there benefits?
Smoking cessation
Compared with other products, such as nicotine patches, gum, and pharmaceutical methods, e-cigarettes most closely mimic the actual experience of smoking. For some, the habit and ritual of smoking is as much a part of the addiction as nicotine. Vaping has the potential to help alleviate this difficult aspect of cessation. Data involving early generation products failed to show a significant advantage. Newer devices that are more pleasurable to use and offer more efficient nicotine delivery may be more effective. Indeed, a recent study in the New England Journal of Medicine from this year demonstrated improved smoking cessation compared with traditional methods, using second generation vape devices (Hajek P, et al. N Engl J Med. 2019;380[7]629). It will be interesting to see if this can be repeatable going forward and if protocols can be established to maximize effectiveness.
As outlined above, it is difficult to make definitive conclusions or recommendations regarding electronic cigarette use at the present time. The risk of cancer and cardiopulmonary disease is likely to be significantly lower but not eliminated. Use as a smoking cessation aid is starting to show promise. Even without cessation, ongoing vaping is likely to be safer than ongoing smoking. Two caveats to this remain: some patients, in an effort to quit smoking, may take up vaping but eventually become “dual users.” This scenario has been associated with higher toxic exposure and possibly worse outcomes. The second caveat is that while there is promise to using this as a cessation tool, it should not yet replace other more well-studied, first-line agents in this regard. It should, perhaps, target patients who are motivated to quit but have failed more traditional methods. Finally, there continues to be concern that vaping could appeal to never smokers, given its perceived safety profile and ease of use in public places. This could lead to an overall increase in nicotine addiction, which could be a significant step backwards.
Dr. Clark is Assistant Professor, Pulmonary and Critical Care Medicine, UT Southwestern Medical Center, Dallas, Texas.
CHEST Foundation at Board Review
The CHEST Foundation is excited to be a part of this year’s CHEST Board Review in Phoenix, and we can’t wait to see you! We are hosting two receptions and invite you to attend and learn more about how the CHEST Foundation supports you, your colleagues, your patients, and the greater community while also taking the time to relax with your peers and board review faculty. The receptions are scheduled for Saturday, August 17 (for Sleep and Critical Care Board Review), and Wednesday, August 21 (for Pulmonary Board Review) immediately following your scheduled sessions. Please join us for hors d’oeuvres and beverages. This year, we are featuring surprise, guest speakers from CHEST leadership who will share why they are passionate about the Foundation’s mission and offer simple ways you can become further involved with the CHEST Foundation. You won’t want to miss this networking opportunity and the chance to learn more about what the Foundation has been doing!
This summer, we are focused on supporting young and early-career clinicians and are raising money at this year’s at Board Review to support travel grants to CHEST 2019. These travel grants provide early-career clinicians the funds needed to attend CHEST 2019. This program further develops the future leaders of CHEST and allows clinicians to take full advantage of career-development and networking opportunities that the annual meeting offers. If you’re interested in how you can make a difference in someone’s life, visit our website (foundation.chestnet.org), or find us at Board Review! We would love to share more with you about all the great work the Foundation is doing.
We can’t wait to see you in Phoenix to celebrate all your hard work!
The CHEST Foundation is excited to be a part of this year’s CHEST Board Review in Phoenix, and we can’t wait to see you! We are hosting two receptions and invite you to attend and learn more about how the CHEST Foundation supports you, your colleagues, your patients, and the greater community while also taking the time to relax with your peers and board review faculty. The receptions are scheduled for Saturday, August 17 (for Sleep and Critical Care Board Review), and Wednesday, August 21 (for Pulmonary Board Review) immediately following your scheduled sessions. Please join us for hors d’oeuvres and beverages. This year, we are featuring surprise, guest speakers from CHEST leadership who will share why they are passionate about the Foundation’s mission and offer simple ways you can become further involved with the CHEST Foundation. You won’t want to miss this networking opportunity and the chance to learn more about what the Foundation has been doing!
This summer, we are focused on supporting young and early-career clinicians and are raising money at this year’s at Board Review to support travel grants to CHEST 2019. These travel grants provide early-career clinicians the funds needed to attend CHEST 2019. This program further develops the future leaders of CHEST and allows clinicians to take full advantage of career-development and networking opportunities that the annual meeting offers. If you’re interested in how you can make a difference in someone’s life, visit our website (foundation.chestnet.org), or find us at Board Review! We would love to share more with you about all the great work the Foundation is doing.
We can’t wait to see you in Phoenix to celebrate all your hard work!
The CHEST Foundation is excited to be a part of this year’s CHEST Board Review in Phoenix, and we can’t wait to see you! We are hosting two receptions and invite you to attend and learn more about how the CHEST Foundation supports you, your colleagues, your patients, and the greater community while also taking the time to relax with your peers and board review faculty. The receptions are scheduled for Saturday, August 17 (for Sleep and Critical Care Board Review), and Wednesday, August 21 (for Pulmonary Board Review) immediately following your scheduled sessions. Please join us for hors d’oeuvres and beverages. This year, we are featuring surprise, guest speakers from CHEST leadership who will share why they are passionate about the Foundation’s mission and offer simple ways you can become further involved with the CHEST Foundation. You won’t want to miss this networking opportunity and the chance to learn more about what the Foundation has been doing!
This summer, we are focused on supporting young and early-career clinicians and are raising money at this year’s at Board Review to support travel grants to CHEST 2019. These travel grants provide early-career clinicians the funds needed to attend CHEST 2019. This program further develops the future leaders of CHEST and allows clinicians to take full advantage of career-development and networking opportunities that the annual meeting offers. If you’re interested in how you can make a difference in someone’s life, visit our website (foundation.chestnet.org), or find us at Board Review! We would love to share more with you about all the great work the Foundation is doing.
We can’t wait to see you in Phoenix to celebrate all your hard work!
In Memoriam: Mark J. Rosen, MD, Master FCCP
Past President (2006-2007) of the American College of Chest Physicians, leader, educator, mentor, and friend, Dr. Mark Rosen, Master FCCP, died on July 3, 2019. Dr. Rosen’s distinguished career in pulmonary and critical care medicine spanned more than 4 decades, marked by his deep commitments to medical education and patient care. His research and administrative accomplishments at New York City and Long Island hospitals were many, but clinical medicine and teaching were always at the top of his list. Mark’s unmistakable way of incorporating both clarity and humor into his roles of clinician, teacher, colleague, and friend provided us all with respect and adoration for this unforgettable individual.
Mark’s distinguished leadership involvement with CHEST began well before his term as President. Two years after completing his fellowships in pulmonary and critical care medicine, he became an FCCP in 1982, and his engagement with the American College of Chest Physicians began. During the 1990s and into the 2000s, Mark provided CHEST with his teaching expertise serving as faculty and director for the Pulmonary Board Review Courses. In 1998, he was Chair of the CHEST Annual Meeting, and from 1999 to 2005, he served on the ACCP-SEEK Editorial Boards for Pulmonary Disease and Critical Care Medicine. Mark served on the CHEST Board of Regents for many years, on the CHEST Foundation Board of Trustees, and as a Chair or member on numerous CHEST committees, some of which included Education, Nominations, Membership, Marketing, and Finance. He was the CHEST Governor for the City of New York and Chair of the Council of Governors. His leadership in all of these capacities was exemplary, as was his guidance as CHEST President from 2006 to 2007. Most recently, Mark served as CHEST Director of Global Education and Strategic Development (2011-2014) followed by CHEST Medical Director (2014-2016). Mark strived to uphold and strengthen the quality of the education that CHEST provided to all health-care professionals. His imprint on the educational and clinical foundations of CHEST, along with the many friendships he made along the way, will be remembered always.
CHEST extends heartfelt condolences to Mark’s wife of 37 years, Ilene, and the Rosen family and many friends and colleagues.
Past President (2006-2007) of the American College of Chest Physicians, leader, educator, mentor, and friend, Dr. Mark Rosen, Master FCCP, died on July 3, 2019. Dr. Rosen’s distinguished career in pulmonary and critical care medicine spanned more than 4 decades, marked by his deep commitments to medical education and patient care. His research and administrative accomplishments at New York City and Long Island hospitals were many, but clinical medicine and teaching were always at the top of his list. Mark’s unmistakable way of incorporating both clarity and humor into his roles of clinician, teacher, colleague, and friend provided us all with respect and adoration for this unforgettable individual.
Mark’s distinguished leadership involvement with CHEST began well before his term as President. Two years after completing his fellowships in pulmonary and critical care medicine, he became an FCCP in 1982, and his engagement with the American College of Chest Physicians began. During the 1990s and into the 2000s, Mark provided CHEST with his teaching expertise serving as faculty and director for the Pulmonary Board Review Courses. In 1998, he was Chair of the CHEST Annual Meeting, and from 1999 to 2005, he served on the ACCP-SEEK Editorial Boards for Pulmonary Disease and Critical Care Medicine. Mark served on the CHEST Board of Regents for many years, on the CHEST Foundation Board of Trustees, and as a Chair or member on numerous CHEST committees, some of which included Education, Nominations, Membership, Marketing, and Finance. He was the CHEST Governor for the City of New York and Chair of the Council of Governors. His leadership in all of these capacities was exemplary, as was his guidance as CHEST President from 2006 to 2007. Most recently, Mark served as CHEST Director of Global Education and Strategic Development (2011-2014) followed by CHEST Medical Director (2014-2016). Mark strived to uphold and strengthen the quality of the education that CHEST provided to all health-care professionals. His imprint on the educational and clinical foundations of CHEST, along with the many friendships he made along the way, will be remembered always.
CHEST extends heartfelt condolences to Mark’s wife of 37 years, Ilene, and the Rosen family and many friends and colleagues.
Past President (2006-2007) of the American College of Chest Physicians, leader, educator, mentor, and friend, Dr. Mark Rosen, Master FCCP, died on July 3, 2019. Dr. Rosen’s distinguished career in pulmonary and critical care medicine spanned more than 4 decades, marked by his deep commitments to medical education and patient care. His research and administrative accomplishments at New York City and Long Island hospitals were many, but clinical medicine and teaching were always at the top of his list. Mark’s unmistakable way of incorporating both clarity and humor into his roles of clinician, teacher, colleague, and friend provided us all with respect and adoration for this unforgettable individual.
Mark’s distinguished leadership involvement with CHEST began well before his term as President. Two years after completing his fellowships in pulmonary and critical care medicine, he became an FCCP in 1982, and his engagement with the American College of Chest Physicians began. During the 1990s and into the 2000s, Mark provided CHEST with his teaching expertise serving as faculty and director for the Pulmonary Board Review Courses. In 1998, he was Chair of the CHEST Annual Meeting, and from 1999 to 2005, he served on the ACCP-SEEK Editorial Boards for Pulmonary Disease and Critical Care Medicine. Mark served on the CHEST Board of Regents for many years, on the CHEST Foundation Board of Trustees, and as a Chair or member on numerous CHEST committees, some of which included Education, Nominations, Membership, Marketing, and Finance. He was the CHEST Governor for the City of New York and Chair of the Council of Governors. His leadership in all of these capacities was exemplary, as was his guidance as CHEST President from 2006 to 2007. Most recently, Mark served as CHEST Director of Global Education and Strategic Development (2011-2014) followed by CHEST Medical Director (2014-2016). Mark strived to uphold and strengthen the quality of the education that CHEST provided to all health-care professionals. His imprint on the educational and clinical foundations of CHEST, along with the many friendships he made along the way, will be remembered always.
CHEST extends heartfelt condolences to Mark’s wife of 37 years, Ilene, and the Rosen family and many friends and colleagues.
New Editor in Chief takes the reins
CHEST welcomed Peter J. Mazzone, MD, MPH, FCCP, in July, as the new Editor in Chief of the journal CHEST®. Dr. Mazzone is the Director of the Lung Cancer Program and Lung Cancer Screening Program for the Respiratory Institute at the Cleveland Clinic in Ohio.
His clinical interests include nodule management and the prevention, screening, diagnosis, staging, and characterization of lung cancer; his research has focused on the development of molecular biomarkers for lung cancer detection. Dr. Mazzone has been a member of CHEST since 1999 and an FCCP since 2004. He has served in several CHEST leadership positions, including member of the CHEST Lung Cancer Living Guidelines Steering Committee and program chair for the CHEST 2017 annual meeting, among others. Dr. Mazzone has provided some insights into the structure and strategies of the journal going forward, so don’t miss his editorial in the July issue of CHEST®.
CHEST welcomed Peter J. Mazzone, MD, MPH, FCCP, in July, as the new Editor in Chief of the journal CHEST®. Dr. Mazzone is the Director of the Lung Cancer Program and Lung Cancer Screening Program for the Respiratory Institute at the Cleveland Clinic in Ohio.
His clinical interests include nodule management and the prevention, screening, diagnosis, staging, and characterization of lung cancer; his research has focused on the development of molecular biomarkers for lung cancer detection. Dr. Mazzone has been a member of CHEST since 1999 and an FCCP since 2004. He has served in several CHEST leadership positions, including member of the CHEST Lung Cancer Living Guidelines Steering Committee and program chair for the CHEST 2017 annual meeting, among others. Dr. Mazzone has provided some insights into the structure and strategies of the journal going forward, so don’t miss his editorial in the July issue of CHEST®.
CHEST welcomed Peter J. Mazzone, MD, MPH, FCCP, in July, as the new Editor in Chief of the journal CHEST®. Dr. Mazzone is the Director of the Lung Cancer Program and Lung Cancer Screening Program for the Respiratory Institute at the Cleveland Clinic in Ohio.
His clinical interests include nodule management and the prevention, screening, diagnosis, staging, and characterization of lung cancer; his research has focused on the development of molecular biomarkers for lung cancer detection. Dr. Mazzone has been a member of CHEST since 1999 and an FCCP since 2004. He has served in several CHEST leadership positions, including member of the CHEST Lung Cancer Living Guidelines Steering Committee and program chair for the CHEST 2017 annual meeting, among others. Dr. Mazzone has provided some insights into the structure and strategies of the journal going forward, so don’t miss his editorial in the July issue of CHEST®.
Environmental Scan: Economy and workforce
The health care workforce is being transformed by profound demographic changes and the steady growth of the U.S. health sector. In addition, the movement of physicians out of private practice to employment by medical centers has accelerated. And a new generation of health care professionals is demanding a sustainable work/life balance. These trends will combine to change the work environment of chest physicians.
Spending
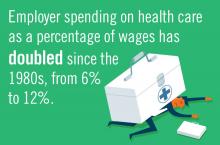
The United States spends about twice as much on health care as any other industrialized nation and this fact is driving an increasingly urgent public discussion about options and means of reducing costs.1 Medicare and Medicaid already account for about a quarter of federal government spending and those numbers are expected to rise as baby boomers age.1 Employer spending on health care as a percentage of wages has doubled since the 1980s.2
Workforce supply
An expanding health care sector means a growing demand for health care labor. Health care occupations are projected to grow 18% from 2016 to 2026, faster than the average for all occupations and adding 2.4 million new jobs to the economy.3 Expert testimony before the U.S. Senate Committee on Health, Education, Labor, and Pensions in May 2018 projected shortages of physicians in the coming years. According to estimates of the Health Resources and Services Administration (HRSA), there is a need for 13,800 additional primary care physicians in areas – especially rural – that are designated as health professional shortage areas. Signs of a worsening situation include projected shortages of 20,000 primary care physicians by 2025, according to HRSA, and 42,600-121,300 physicians by 2030, according to the Association of American Medical Colleges. The demand for physicians will exceed supply by 46,000-90,000 by 2025. An update to that research increased the projected shortage range to 61,700-94,700 by 2025.4 These shortages will result in recruiting challenges for many medical centers, especially those in rural areas.
Employment
Private practice is becoming the less common structure of employment for physicians. According to American Medical Association data, physician ownership of practices dropped below 50% for the first time in 2016.5 The trend toward employed versus private practice physicians is expected to continue. The size of practices is growing, with about one-third of physicians working in a hospital-owned practice or employed directly by a hospital and around 40% in practices of 10 physicians or more.5 Of every 10 physician practices, 3 were hospital owned in 2016.6 Physicians are being called upon to do more data entry and administrative work; 21% of physicians’ time is now spent on nonclinical paperwork.7 The ripple-out effects of what amounts to a seismic shift in the work structure and work environment for physicians are only beginning to be studied in terms of overall personal satisfaction and impact on patient care.
Stephanie M. Levine, MD, FCCP, the designate president of the American College of Chest Physicians and professor of medicine in the division of pulmonary diseases and critical care medicine at the University of Texas, San Antonio, recognizes the significance of the move from private practice to employment and suggests that advantages could be offset by some potential negatives practicing chest physicians. She noted, “Pros include potentially more job security, more predictable work hours, perhaps a reduction in some of the traditional administrative ‘hassles’ with running a private practice, and possibly a better and healthier work/life balance. Some think that physician input and leadership in the employed model may have more influence on a health care system than in an individual private practice. Nonclinical work may be decreased, but it is not clear that this is true.
“The negatives include a loss of autonomy, a potential loss of personal ownership of our patients’ health, and the loss of a unique personal culture of private practice. Physicians may be subject to metrics imposed by the employer. In addition, we may see more job turnover since physicians could be less invested emotionally and financially; fewer patients seen since the structure is often salary based and not based on productivity; and increased shift work, set work hours, and schedules. Thus, the employer-based model may actually contribute to the ongoing physician shortage.”
Dr. Levine stressed the role of training programs to prepare physicians for what may lie ahead. “Training programs must prepare physicians for what to expect as employees.”
Changing expectations
An evolution of expectations about a healthy work/life balance has occurred in many professions, including the health care profession. While younger practitioners may be more likely to embrace the changes occurring within health care, they are often more vocal about their desire for a healthy work/life balance and may be less likely to spend time away from family and friends rather than completing administrative tasks. Parenting is increasingly regarded by women and men as compatible with a full and rewarding career as a physician. So these changing expectations about work/life balances mean health care institutions will have to adjust their own expectations in order to recruit and maintain top-quality staff.
Stress and burnout
Workforce shortages, overwhelming administrative tasks, and a variety of forces that come with employment in a large medical system are causing stress and burnout in many physicians. In a 2018 Medscape study of more than 15,000 physicians, 42% reported burnout, and 15% admitted to experiencing either clinical or colloquial forms of depression.8 Dr. Levine acknowledges that many chest physicians are at risk for burnout. “In our field of medicine, particularly with those that practice in an intensive care setting, we are faced with the high stress and emotional experiences we encounter in the life and death nature of our jobs. We care for the sickest patient population, and are often facing life and death clinical needs as well as end-of-life discussions and care. Burnout is a potential threat to both patient safety and the quality of healthcare that we practice.”
Dr. Levine strongly urges colleagues to remain vigilant to this potentially devastating condition in their fellow physicians and in themselves. She said, “If you suspect you are feeling the symptoms of burnout, or have been told so by a colleague, then talk to a peer or colleague, take personal time to do something you enjoy, and/or join a support group. But better than that, try to preempt burnout by developing a strong emotional peer support group in or out of work, practicing mindfulness training, and paying attention to wellness and self-care.”
Burnout is finally being recognized by medical institutions as a significant factor in physician health and performance, and in the recruitment and attrition of staff. Dr. Levine sees progress in how health care institutions deal with burnout, wellness, and work/life balance among staff and trainees. In a hopeful note, Dr. Levine suggested that institutional responses to burnout and the workplace factors that fuel burnout may improve work conditions for physicians in the future.
These trends in the U.S. economy and workforce will mean a steady growth of the health care sector for the foreseeable future, continued political and social pressure to control costs, fewer physicians in private practice, and a potential move away from unhealthy work/life ratios currently so common among physicians.
Dr. Levine concluded that it is up to training programs to prepare trainees for these sea changes to the practice of medicine.
References
1. https://www.healthleadersmedia.com/finance/healthcare-spending-20-gdp-thats-economy-wide-problem
2. PwC Health Research Institute
3. https://www.bls.gov/ooh/healthcare/home.htm
4. https://www.hfma.org/Content.aspx?id=60811
5. https://www.ama-assn.org/about-ama/research/physician-practice-benchmark-survey
6. http://www.physiciansadvocacyinstitute.org/
7. https://omahamedical.com/wp-content/uploads/2016/12/2016-Survey-of-Americas-Physicians-Practice-Patterns-and-Perspectives.pdf
8. https://www.medscape.com/slideshow/2018-lifestyle-burnout-depression-6009235
The health care workforce is being transformed by profound demographic changes and the steady growth of the U.S. health sector. In addition, the movement of physicians out of private practice to employment by medical centers has accelerated. And a new generation of health care professionals is demanding a sustainable work/life balance. These trends will combine to change the work environment of chest physicians.
Spending
The United States spends about twice as much on health care as any other industrialized nation and this fact is driving an increasingly urgent public discussion about options and means of reducing costs.1 Medicare and Medicaid already account for about a quarter of federal government spending and those numbers are expected to rise as baby boomers age.1 Employer spending on health care as a percentage of wages has doubled since the 1980s.2
Workforce supply
An expanding health care sector means a growing demand for health care labor. Health care occupations are projected to grow 18% from 2016 to 2026, faster than the average for all occupations and adding 2.4 million new jobs to the economy.3 Expert testimony before the U.S. Senate Committee on Health, Education, Labor, and Pensions in May 2018 projected shortages of physicians in the coming years. According to estimates of the Health Resources and Services Administration (HRSA), there is a need for 13,800 additional primary care physicians in areas – especially rural – that are designated as health professional shortage areas. Signs of a worsening situation include projected shortages of 20,000 primary care physicians by 2025, according to HRSA, and 42,600-121,300 physicians by 2030, according to the Association of American Medical Colleges. The demand for physicians will exceed supply by 46,000-90,000 by 2025. An update to that research increased the projected shortage range to 61,700-94,700 by 2025.4 These shortages will result in recruiting challenges for many medical centers, especially those in rural areas.
Employment
Private practice is becoming the less common structure of employment for physicians. According to American Medical Association data, physician ownership of practices dropped below 50% for the first time in 2016.5 The trend toward employed versus private practice physicians is expected to continue. The size of practices is growing, with about one-third of physicians working in a hospital-owned practice or employed directly by a hospital and around 40% in practices of 10 physicians or more.5 Of every 10 physician practices, 3 were hospital owned in 2016.6 Physicians are being called upon to do more data entry and administrative work; 21% of physicians’ time is now spent on nonclinical paperwork.7 The ripple-out effects of what amounts to a seismic shift in the work structure and work environment for physicians are only beginning to be studied in terms of overall personal satisfaction and impact on patient care.
Stephanie M. Levine, MD, FCCP, the designate president of the American College of Chest Physicians and professor of medicine in the division of pulmonary diseases and critical care medicine at the University of Texas, San Antonio, recognizes the significance of the move from private practice to employment and suggests that advantages could be offset by some potential negatives practicing chest physicians. She noted, “Pros include potentially more job security, more predictable work hours, perhaps a reduction in some of the traditional administrative ‘hassles’ with running a private practice, and possibly a better and healthier work/life balance. Some think that physician input and leadership in the employed model may have more influence on a health care system than in an individual private practice. Nonclinical work may be decreased, but it is not clear that this is true.
“The negatives include a loss of autonomy, a potential loss of personal ownership of our patients’ health, and the loss of a unique personal culture of private practice. Physicians may be subject to metrics imposed by the employer. In addition, we may see more job turnover since physicians could be less invested emotionally and financially; fewer patients seen since the structure is often salary based and not based on productivity; and increased shift work, set work hours, and schedules. Thus, the employer-based model may actually contribute to the ongoing physician shortage.”
Dr. Levine stressed the role of training programs to prepare physicians for what may lie ahead. “Training programs must prepare physicians for what to expect as employees.”
Changing expectations
An evolution of expectations about a healthy work/life balance has occurred in many professions, including the health care profession. While younger practitioners may be more likely to embrace the changes occurring within health care, they are often more vocal about their desire for a healthy work/life balance and may be less likely to spend time away from family and friends rather than completing administrative tasks. Parenting is increasingly regarded by women and men as compatible with a full and rewarding career as a physician. So these changing expectations about work/life balances mean health care institutions will have to adjust their own expectations in order to recruit and maintain top-quality staff.
Stress and burnout
Workforce shortages, overwhelming administrative tasks, and a variety of forces that come with employment in a large medical system are causing stress and burnout in many physicians. In a 2018 Medscape study of more than 15,000 physicians, 42% reported burnout, and 15% admitted to experiencing either clinical or colloquial forms of depression.8 Dr. Levine acknowledges that many chest physicians are at risk for burnout. “In our field of medicine, particularly with those that practice in an intensive care setting, we are faced with the high stress and emotional experiences we encounter in the life and death nature of our jobs. We care for the sickest patient population, and are often facing life and death clinical needs as well as end-of-life discussions and care. Burnout is a potential threat to both patient safety and the quality of healthcare that we practice.”
Dr. Levine strongly urges colleagues to remain vigilant to this potentially devastating condition in their fellow physicians and in themselves. She said, “If you suspect you are feeling the symptoms of burnout, or have been told so by a colleague, then talk to a peer or colleague, take personal time to do something you enjoy, and/or join a support group. But better than that, try to preempt burnout by developing a strong emotional peer support group in or out of work, practicing mindfulness training, and paying attention to wellness and self-care.”
Burnout is finally being recognized by medical institutions as a significant factor in physician health and performance, and in the recruitment and attrition of staff. Dr. Levine sees progress in how health care institutions deal with burnout, wellness, and work/life balance among staff and trainees. In a hopeful note, Dr. Levine suggested that institutional responses to burnout and the workplace factors that fuel burnout may improve work conditions for physicians in the future.
These trends in the U.S. economy and workforce will mean a steady growth of the health care sector for the foreseeable future, continued political and social pressure to control costs, fewer physicians in private practice, and a potential move away from unhealthy work/life ratios currently so common among physicians.
Dr. Levine concluded that it is up to training programs to prepare trainees for these sea changes to the practice of medicine.
References
1. https://www.healthleadersmedia.com/finance/healthcare-spending-20-gdp-thats-economy-wide-problem
2. PwC Health Research Institute
3. https://www.bls.gov/ooh/healthcare/home.htm
4. https://www.hfma.org/Content.aspx?id=60811
5. https://www.ama-assn.org/about-ama/research/physician-practice-benchmark-survey
6. http://www.physiciansadvocacyinstitute.org/
7. https://omahamedical.com/wp-content/uploads/2016/12/2016-Survey-of-Americas-Physicians-Practice-Patterns-and-Perspectives.pdf
8. https://www.medscape.com/slideshow/2018-lifestyle-burnout-depression-6009235
The health care workforce is being transformed by profound demographic changes and the steady growth of the U.S. health sector. In addition, the movement of physicians out of private practice to employment by medical centers has accelerated. And a new generation of health care professionals is demanding a sustainable work/life balance. These trends will combine to change the work environment of chest physicians.
Spending
The United States spends about twice as much on health care as any other industrialized nation and this fact is driving an increasingly urgent public discussion about options and means of reducing costs.1 Medicare and Medicaid already account for about a quarter of federal government spending and those numbers are expected to rise as baby boomers age.1 Employer spending on health care as a percentage of wages has doubled since the 1980s.2
Workforce supply
An expanding health care sector means a growing demand for health care labor. Health care occupations are projected to grow 18% from 2016 to 2026, faster than the average for all occupations and adding 2.4 million new jobs to the economy.3 Expert testimony before the U.S. Senate Committee on Health, Education, Labor, and Pensions in May 2018 projected shortages of physicians in the coming years. According to estimates of the Health Resources and Services Administration (HRSA), there is a need for 13,800 additional primary care physicians in areas – especially rural – that are designated as health professional shortage areas. Signs of a worsening situation include projected shortages of 20,000 primary care physicians by 2025, according to HRSA, and 42,600-121,300 physicians by 2030, according to the Association of American Medical Colleges. The demand for physicians will exceed supply by 46,000-90,000 by 2025. An update to that research increased the projected shortage range to 61,700-94,700 by 2025.4 These shortages will result in recruiting challenges for many medical centers, especially those in rural areas.
Employment
Private practice is becoming the less common structure of employment for physicians. According to American Medical Association data, physician ownership of practices dropped below 50% for the first time in 2016.5 The trend toward employed versus private practice physicians is expected to continue. The size of practices is growing, with about one-third of physicians working in a hospital-owned practice or employed directly by a hospital and around 40% in practices of 10 physicians or more.5 Of every 10 physician practices, 3 were hospital owned in 2016.6 Physicians are being called upon to do more data entry and administrative work; 21% of physicians’ time is now spent on nonclinical paperwork.7 The ripple-out effects of what amounts to a seismic shift in the work structure and work environment for physicians are only beginning to be studied in terms of overall personal satisfaction and impact on patient care.
Stephanie M. Levine, MD, FCCP, the designate president of the American College of Chest Physicians and professor of medicine in the division of pulmonary diseases and critical care medicine at the University of Texas, San Antonio, recognizes the significance of the move from private practice to employment and suggests that advantages could be offset by some potential negatives practicing chest physicians. She noted, “Pros include potentially more job security, more predictable work hours, perhaps a reduction in some of the traditional administrative ‘hassles’ with running a private practice, and possibly a better and healthier work/life balance. Some think that physician input and leadership in the employed model may have more influence on a health care system than in an individual private practice. Nonclinical work may be decreased, but it is not clear that this is true.
“The negatives include a loss of autonomy, a potential loss of personal ownership of our patients’ health, and the loss of a unique personal culture of private practice. Physicians may be subject to metrics imposed by the employer. In addition, we may see more job turnover since physicians could be less invested emotionally and financially; fewer patients seen since the structure is often salary based and not based on productivity; and increased shift work, set work hours, and schedules. Thus, the employer-based model may actually contribute to the ongoing physician shortage.”
Dr. Levine stressed the role of training programs to prepare physicians for what may lie ahead. “Training programs must prepare physicians for what to expect as employees.”
Changing expectations
An evolution of expectations about a healthy work/life balance has occurred in many professions, including the health care profession. While younger practitioners may be more likely to embrace the changes occurring within health care, they are often more vocal about their desire for a healthy work/life balance and may be less likely to spend time away from family and friends rather than completing administrative tasks. Parenting is increasingly regarded by women and men as compatible with a full and rewarding career as a physician. So these changing expectations about work/life balances mean health care institutions will have to adjust their own expectations in order to recruit and maintain top-quality staff.
Stress and burnout
Workforce shortages, overwhelming administrative tasks, and a variety of forces that come with employment in a large medical system are causing stress and burnout in many physicians. In a 2018 Medscape study of more than 15,000 physicians, 42% reported burnout, and 15% admitted to experiencing either clinical or colloquial forms of depression.8 Dr. Levine acknowledges that many chest physicians are at risk for burnout. “In our field of medicine, particularly with those that practice in an intensive care setting, we are faced with the high stress and emotional experiences we encounter in the life and death nature of our jobs. We care for the sickest patient population, and are often facing life and death clinical needs as well as end-of-life discussions and care. Burnout is a potential threat to both patient safety and the quality of healthcare that we practice.”
Dr. Levine strongly urges colleagues to remain vigilant to this potentially devastating condition in their fellow physicians and in themselves. She said, “If you suspect you are feeling the symptoms of burnout, or have been told so by a colleague, then talk to a peer or colleague, take personal time to do something you enjoy, and/or join a support group. But better than that, try to preempt burnout by developing a strong emotional peer support group in or out of work, practicing mindfulness training, and paying attention to wellness and self-care.”
Burnout is finally being recognized by medical institutions as a significant factor in physician health and performance, and in the recruitment and attrition of staff. Dr. Levine sees progress in how health care institutions deal with burnout, wellness, and work/life balance among staff and trainees. In a hopeful note, Dr. Levine suggested that institutional responses to burnout and the workplace factors that fuel burnout may improve work conditions for physicians in the future.
These trends in the U.S. economy and workforce will mean a steady growth of the health care sector for the foreseeable future, continued political and social pressure to control costs, fewer physicians in private practice, and a potential move away from unhealthy work/life ratios currently so common among physicians.
Dr. Levine concluded that it is up to training programs to prepare trainees for these sea changes to the practice of medicine.
References
1. https://www.healthleadersmedia.com/finance/healthcare-spending-20-gdp-thats-economy-wide-problem
2. PwC Health Research Institute
3. https://www.bls.gov/ooh/healthcare/home.htm
4. https://www.hfma.org/Content.aspx?id=60811
5. https://www.ama-assn.org/about-ama/research/physician-practice-benchmark-survey
6. http://www.physiciansadvocacyinstitute.org/
7. https://omahamedical.com/wp-content/uploads/2016/12/2016-Survey-of-Americas-Physicians-Practice-Patterns-and-Perspectives.pdf
8. https://www.medscape.com/slideshow/2018-lifestyle-burnout-depression-6009235
Lancet joins movement to reject ‘manels’
The Lancet Group’s 18 medical journals have committed to ensuring that their editorial advisory boards include at least 50% female members by the end of 2019 as just one component of the diversity and gender parity initiative unveiled Aug. 8.
“The case for gender equity and diversity is clear: Teams that are diverse in terms of gender, ethnicity, and social background produce better health science, are more highly cited, generate a broader range of ideas and innovations, and better represent society,” group editors wrote in their comment (Lancet. 2019 Aug 10;394:452-3). They emphasized the importance of increasing inclusion in science “across gender, ethnicity, geography, and other social categories.”
The Diversity Pledge states the group’s commitment “to increasing diversity and inclusion in research and publishing, and in particular to increasing the representation of women and colleagues from low-income and middle-income countries among our editorial advisers, peer reviewers, and authors.”
The No All-Male Panel Policy echoes a call from the National Institutes of Health for ending the “Manel Tradition,” as NIH Director Francis S. Collins, MD, PhD, wrote in early June. Recognizing the need “to combat cultural forces that tolerate gender harassment and limit the advancement of women,” Dr. Collins pledged to decline speaking invitations if “attention to inclusiveness” is not clear in the event’s agenda.
Discussion of “manels” – all-male panels – and the decision to boycott them has been picking up speed in scientific, medical and even business circles over the past several years. The BBC highlighted a popular blog that shamed events with all-male panels in 2015, and a 2018 study more formally concluded that male scientists had considerably more opportunities to speak and present at the world’s largest geophysical conference.
One business and development leader even included space on his website to allow other leaders to pledge not to “serve on a panel of two people or more unless there is at least one woman on the panel, not including the chair.” More than 2,000 leaders from across the globe already have signed.
Six months ago, the Lancet published a special theme issue on women in science, medicine, and global health. The editors noted in the issue that women comprise fewer than a third of authors and reviewers in high-impact medical journals – just one example of the underrepresentation of women and people in color in medical publishing. The group is now revamping their systems to address the disparities.
“An upcoming update of our online submission system will have a field for self-selected gender, so we can better track representation across genders among authors, reviewers, editors, and editorial advisers, along with country of origin,” the editors wrote.
But they acknowledged that their efforts are just one piece of the academic ecosystem and called on others’ participation. “We encourage other publishers, journals, and members of the science community to contribute to these pledges.”
SOURCE: Lancet. 2019 Aug 10;394:452-3.
The Lancet Group’s 18 medical journals have committed to ensuring that their editorial advisory boards include at least 50% female members by the end of 2019 as just one component of the diversity and gender parity initiative unveiled Aug. 8.
“The case for gender equity and diversity is clear: Teams that are diverse in terms of gender, ethnicity, and social background produce better health science, are more highly cited, generate a broader range of ideas and innovations, and better represent society,” group editors wrote in their comment (Lancet. 2019 Aug 10;394:452-3). They emphasized the importance of increasing inclusion in science “across gender, ethnicity, geography, and other social categories.”
The Diversity Pledge states the group’s commitment “to increasing diversity and inclusion in research and publishing, and in particular to increasing the representation of women and colleagues from low-income and middle-income countries among our editorial advisers, peer reviewers, and authors.”
The No All-Male Panel Policy echoes a call from the National Institutes of Health for ending the “Manel Tradition,” as NIH Director Francis S. Collins, MD, PhD, wrote in early June. Recognizing the need “to combat cultural forces that tolerate gender harassment and limit the advancement of women,” Dr. Collins pledged to decline speaking invitations if “attention to inclusiveness” is not clear in the event’s agenda.
Discussion of “manels” – all-male panels – and the decision to boycott them has been picking up speed in scientific, medical and even business circles over the past several years. The BBC highlighted a popular blog that shamed events with all-male panels in 2015, and a 2018 study more formally concluded that male scientists had considerably more opportunities to speak and present at the world’s largest geophysical conference.
One business and development leader even included space on his website to allow other leaders to pledge not to “serve on a panel of two people or more unless there is at least one woman on the panel, not including the chair.” More than 2,000 leaders from across the globe already have signed.
Six months ago, the Lancet published a special theme issue on women in science, medicine, and global health. The editors noted in the issue that women comprise fewer than a third of authors and reviewers in high-impact medical journals – just one example of the underrepresentation of women and people in color in medical publishing. The group is now revamping their systems to address the disparities.
“An upcoming update of our online submission system will have a field for self-selected gender, so we can better track representation across genders among authors, reviewers, editors, and editorial advisers, along with country of origin,” the editors wrote.
But they acknowledged that their efforts are just one piece of the academic ecosystem and called on others’ participation. “We encourage other publishers, journals, and members of the science community to contribute to these pledges.”
SOURCE: Lancet. 2019 Aug 10;394:452-3.
The Lancet Group’s 18 medical journals have committed to ensuring that their editorial advisory boards include at least 50% female members by the end of 2019 as just one component of the diversity and gender parity initiative unveiled Aug. 8.
“The case for gender equity and diversity is clear: Teams that are diverse in terms of gender, ethnicity, and social background produce better health science, are more highly cited, generate a broader range of ideas and innovations, and better represent society,” group editors wrote in their comment (Lancet. 2019 Aug 10;394:452-3). They emphasized the importance of increasing inclusion in science “across gender, ethnicity, geography, and other social categories.”
The Diversity Pledge states the group’s commitment “to increasing diversity and inclusion in research and publishing, and in particular to increasing the representation of women and colleagues from low-income and middle-income countries among our editorial advisers, peer reviewers, and authors.”
The No All-Male Panel Policy echoes a call from the National Institutes of Health for ending the “Manel Tradition,” as NIH Director Francis S. Collins, MD, PhD, wrote in early June. Recognizing the need “to combat cultural forces that tolerate gender harassment and limit the advancement of women,” Dr. Collins pledged to decline speaking invitations if “attention to inclusiveness” is not clear in the event’s agenda.
Discussion of “manels” – all-male panels – and the decision to boycott them has been picking up speed in scientific, medical and even business circles over the past several years. The BBC highlighted a popular blog that shamed events with all-male panels in 2015, and a 2018 study more formally concluded that male scientists had considerably more opportunities to speak and present at the world’s largest geophysical conference.
One business and development leader even included space on his website to allow other leaders to pledge not to “serve on a panel of two people or more unless there is at least one woman on the panel, not including the chair.” More than 2,000 leaders from across the globe already have signed.
Six months ago, the Lancet published a special theme issue on women in science, medicine, and global health. The editors noted in the issue that women comprise fewer than a third of authors and reviewers in high-impact medical journals – just one example of the underrepresentation of women and people in color in medical publishing. The group is now revamping their systems to address the disparities.
“An upcoming update of our online submission system will have a field for self-selected gender, so we can better track representation across genders among authors, reviewers, editors, and editorial advisers, along with country of origin,” the editors wrote.
But they acknowledged that their efforts are just one piece of the academic ecosystem and called on others’ participation. “We encourage other publishers, journals, and members of the science community to contribute to these pledges.”
SOURCE: Lancet. 2019 Aug 10;394:452-3.
FROM THE LANCET
Dr. Carl Bell’s research broke new ground
Psychiatrist educated the field with his work on gun violence, prenatal alcohol exposure
With the heart of a child and the spirit of a warrior, Carl Bell always spoke his truth. And, he did so in his own inimitable way. Sporting his signature dark brown wide-brim leather cowboy hat or NMA (National Medical Association) baseball cap, aviator sunglasses, and accompanying Superman belt buckle, Carl Compton Bell, MD, – psychiatrist, researcher, mental health advocate, father, grandfather, friend, colleague, pioneer, and servant – was driven by a deep commitment to serve others.
As those who truly knew him can attest, it is not hyperbole to say that Carl Compton Bell was one of the most genuine, brilliant, and humble physicians of our professional community and time.
My collaboration and friendship began with Dr. Bell began during the summer of 2016 as I was preparing for the 2017 Washington Psychiatric Society’s (WPS) Presidential Symposium at Saint Elizabeths Hospital. As president of WPS, I had chosen gun violence as my topic and sought out Dr. Bell because of his work on the South Side of Chicago, where he had devoted himself, becoming an internationally known clinician, researcher, and mental health advocate for those personally affected by violence and trauma. He immediately accepted.
In his presentation, “Gun Violence, Urban Youth and Mental Illness,” he reviewed his research on the neurocognitive behavioral effects of prenatal exposure to alcohol and its relation to the neurodevelopmental dynamics of youth violence, intimate partner violence, and mass shootings. Dr. Bell suggested that the relationship between prenatal exposure to alcohol and the diagnosis of numerous psychiatric conditions had been underestimated in the medical community. He eventually summarized his work in Fetal Alcohol Exposure in the African-American Community, published by Third World Press (2018). This vital resource not only summarizes in plain language the scope of the problem of prenatal alcohol exposure but is a narrative of Carl Bell’s life journey.
After the symposium, he would send articles, while warning, “I can bombard you with stuff.” Sometimes we would not speak for weeks at a time while I digested the resources he had shared. However, whenever I picked up the phone to call and respond to what he had provided, he would answer the phone, “Yessssss?” – as if he were anticipating my call and was ready to address any queries or comments I might have. Even when he were about to board a plane or charting – after making rounds on his patients while listening to the music of James Brown – he would answer the phone, even if only to coordinate a more mutually convenient time to connect.
During the process of digesting the plethora of articles and resources he provided on prenatal fetal alcohol exposure, including the 1996 Institute of Medicine’s report and the American Medical Association’s 2017 resolution supporting the addition of adequate amounts of choline to prenatal vitamins, I found myself immersed in neuroscience topics, such as the role of neuronal acetylcholine receptor subunit alpha-7 in the formation of neurotransmitters, the strengthening of cell membranes, and the promotion of proper brain and spinal cord development. Dr. Bell spoke authoritatively about the neuroscience and the public health implications. One of his mantras was “Where is the data? You’ve got to have data.”
The information that he shared became the foundation of the action paper calling for the American Psychiatric Association (APA) to endorse the AMA’s resolution supporting the addition of adequate amounts (450 mg/d for pregnant women) of phosphatidylcholine to prenatal vitamins. The APA Assembly passed this action paper in May 2018.
He was also responsible for a second action paper, “Psychiatric Management of the Impact of Racism on Social and Clinical Events,” which passed at the same May 2018 assembly. Dr. Bell agreed to coauthor this paper, which was only fitting since the paper was a further elaboration of his efforts with the APA Caucus of Black Psychiatrists to implore the APA to acknowledge the deleterious effects of racism on both the victim and perpetrator.
While researching this topic, I had come across his 1980 article, Racism: A Symptom of the Narcissistic Personality Disorder (J Nat Med Assoc. 1980 Jul;72[7]:661-5), in which Dr. Bell applied psychoanalytic theory to posit that racism is one psychic derivative through which narcissism may manifest itself.
Although he was not formally trained as a psychoanalyst, he had benefited from strong psychoanalytic supervision at the Illinois State Psychiatric Institute, a training program of the University of Illinois at Chicago. He wrote confidently and clearly, applying self-psychology principles. He had the gravitas to write and speak about a range of topics, from neuroscience, psychotherapy, medical management of illness, and mental health advocacy. His 387-page curriculum vitae of 500+ articles, chapters, and books on mental health issues is a catalog of evidence that he had given thought to just about any topic along the spectrum of psychiatry and beyond.
In July 2018, after leaving a performance of “Hamilton” at the Kennedy Center, a lyric from the song “Non-Stop” stayed with me:
Why do you write like you’re running out of time?
(Why do you write like you’re running out of time?)
Write day and night like you’re running out of time?
The pace at which he read, wrote, lectured, researched, collaborated, and served on committees reminded me of the prodigious work of the former Secretary of the Treasury. When I shared this with Dr. Bell, he volunteered that he wrote to clear his mind. I suggested that, like other true writers, it seemed that he had to write. He did not disagree.
But, what was most meaningful about his productivity was his generosity of spirit. Any conversation was an opportunity for him to thoughtfully and respectfully share his knowledge. For example, once, we were discussing a clinical case that included the differential diagnosis of a patient, who happened to be African American, who was having auditory hallucinations. Dr. Bell might have been the first psychiatrist to alert the medical community about the misdiagnosis of schizophrenia among African Americans with bipolar disorder (J Nat Med Assoc. 1980 Feb 72[2]:141-5).
Contrary to my expectation that he was going to remind me of the tendency to misdiagnose, he instead offered, “You know, there are 40 reasons for auditory hallucinations.” Not what I had expected, yet, a response that reflected his continually giving nature and sharing of his abundance of gems. He was always teaching.
I later learned that his workday at Jackson Park Hospital usually ended at 2 p.m. He had treated patients, and supervised medical students and residents there for more than 40 years. The afternoons afforded him time to read, write, listen to music (Ella Fitzgerald), watch movies, and spend time with his adult children, to whom he was quite devoted. Dr. Bell was an avid martial artist and enjoyed sharing this practice with his son, William.
He was a longtime active member of the National Medical Association, recently receiving its prestigious Distinguished Service Award in Hawaii for his “exceptional work in medical service, medical research, and academic medicine.” It would be his last professional talk, though his delivery would belie his numbered days.
He was a former vice president of the Black Psychiatrists of America (BPA) and for 10 years had been the editor of the BPA Newsletter. Conversations were often peppered with anecdotes from time spent with other pioneering ancestors, such as Chester “Chet” Pierce, MD, Jeanne Spurlock, MD, Robert Phillips, MD, PhD, Charles Prudhomme, MD, Frances Cress Welsing, MD, and others. Dr. Bell was at the tail end of a generation of African American psychiatrists who had experienced firsthand the transition from segregation to federally mandated integration of our society.
Dr. Bell and his peers applied their education and training to improve clinical care for all, to decrease health inequities, and to eliminate disparities. It is evident that he loved his people and committed his life to addressing the needs of marginalized communities, those without the benefit of abundant resources, and those disproportionately affected by violence and trauma. As he stated in his last book, Fetal Alcohol Exposure in the African-American Community:
“I should add, my main concern is African American people living within the United States of America where in one community the rate of Fetal Alcohol Exposure is 388/1,000 people. ... However, this problem extends much further. Fetal Alcohol Exposure (FAE) is increasingly being found to (be) problematic in people of color around the world: Native Americans in Canada ... Aboriginal people in Australia ... and various tribes of people on the continent of Africa. ... Lastly, while the problem of Fetal Alcohol Exposure seems to be disproportionately affecting people of color, it also affects people who lack pigment in their skin. For example, FAE is a problem in Russia. From a public health perspective, so often people of color are like the proverbial “canary in a coal mine,” i.e., if there is poisonous gas in the coal mine, the canary will die first, warning the miners that they need to do something about it.”
However, Dr. Bell was a Distinguished Lifetime Fellow of the American Psychiatric Association and a Lifetime Fellow of the American College of Psychiatrists. He was a founding member of the board of directors of the National Commission on Correctional Health, and a member of prominent work groups of the National Institutes of Mental Health and the National Academy of Medicine (formerly the Institute of Medicine). And Dr. Bell’s mentees and students transcend generations, race, religions, professional disciplines, and national boundaries.
Because Dr. Bell was grounded and never forgot his roots, it was in these professional society circles that he ensured that clinicians with more privilege and limited or no exposure to communities of color were educated about the needs of those he treated. Without exposure to Carl Bell, it is likely that many of our psychiatric colleagues would remain unaware of both the brilliant dynamic resources and enormous challenges that are found in the black community and communities of color. By sharing his work with the house of medicine, he obviated the excuse of doing nothing because of ignorance.
I last saw Dr. Bell in San Francisco toward the end of the 2019 annual APA meeting. He had received the APA’s Adolf* Meyer Award for lifetime achievement. Afterward, I joined him for a dim sum lunch in Chinatown with two of his colleagues and Joseph Calhoun, his mentee in the APA’s Black Men in Psychiatry Early Pipeline Program. As we walked back to our respective hotels, we paused at what is now the Chinese Affirmative Action Center. We learned that this site had been the home of one of Dr. Bell’s former martial arts instructors. As Dr. Bell recounted his martial arts training, the reverence for his sensei was evident in his eyes.
When I reflect on how much I learned from and about Carl Bell in such a short period of time, I realize that he was one of those people who was so present and so astute that he allowed you to know him while he was giving.
So, how do we honor someone who gave so much of himself? When I now think of the lyric from “Hamilton” – “Why do you write like you’re running out of time?” – I realize that we get it twisted when we associate running out of time with our elders and their phase of life. It was not Carl Bell who was running out of time. He had been extraordinarily respectful of the space, time, and energy allotted to him in his lifetime. He would say, “People squander their personal resources.” He certainly had not squandered his.
As we reflect and mourn his passing, we will hear about his candor, authenticity, integrity, discipline, reliability, dedication, and serving spirit. This is called character.
Dr. Bell was beyond generous with his life, and it is going to take decades, if not more, for us to digest the compendium of knowledge that he left behind. I ask you: How will you use that knowledge to advance the causes he so diligently devoted his life to solving?
To Carl Compton Bell, I say, Well done. Thank you. And, rest now my dear brother.
Dr. Dunlap, a psychiatrist and psychoanalyst who practices in Washington, is a Washington Psychiatric Society representative to the APA Assembly, a past president of the Washington Psychiatric Society, and clinical professor of psychiatry and behavioral sciences at George Washington University, Washington. She is interested in the role “difference” – race, culture, and ethnicity – plays in interpersonal relationships and group dynamics.
*This column was updated 9/3/2019.
Psychiatrist educated the field with his work on gun violence, prenatal alcohol exposure
Psychiatrist educated the field with his work on gun violence, prenatal alcohol exposure
With the heart of a child and the spirit of a warrior, Carl Bell always spoke his truth. And, he did so in his own inimitable way. Sporting his signature dark brown wide-brim leather cowboy hat or NMA (National Medical Association) baseball cap, aviator sunglasses, and accompanying Superman belt buckle, Carl Compton Bell, MD, – psychiatrist, researcher, mental health advocate, father, grandfather, friend, colleague, pioneer, and servant – was driven by a deep commitment to serve others.
As those who truly knew him can attest, it is not hyperbole to say that Carl Compton Bell was one of the most genuine, brilliant, and humble physicians of our professional community and time.
My collaboration and friendship began with Dr. Bell began during the summer of 2016 as I was preparing for the 2017 Washington Psychiatric Society’s (WPS) Presidential Symposium at Saint Elizabeths Hospital. As president of WPS, I had chosen gun violence as my topic and sought out Dr. Bell because of his work on the South Side of Chicago, where he had devoted himself, becoming an internationally known clinician, researcher, and mental health advocate for those personally affected by violence and trauma. He immediately accepted.
In his presentation, “Gun Violence, Urban Youth and Mental Illness,” he reviewed his research on the neurocognitive behavioral effects of prenatal exposure to alcohol and its relation to the neurodevelopmental dynamics of youth violence, intimate partner violence, and mass shootings. Dr. Bell suggested that the relationship between prenatal exposure to alcohol and the diagnosis of numerous psychiatric conditions had been underestimated in the medical community. He eventually summarized his work in Fetal Alcohol Exposure in the African-American Community, published by Third World Press (2018). This vital resource not only summarizes in plain language the scope of the problem of prenatal alcohol exposure but is a narrative of Carl Bell’s life journey.
After the symposium, he would send articles, while warning, “I can bombard you with stuff.” Sometimes we would not speak for weeks at a time while I digested the resources he had shared. However, whenever I picked up the phone to call and respond to what he had provided, he would answer the phone, “Yessssss?” – as if he were anticipating my call and was ready to address any queries or comments I might have. Even when he were about to board a plane or charting – after making rounds on his patients while listening to the music of James Brown – he would answer the phone, even if only to coordinate a more mutually convenient time to connect.
During the process of digesting the plethora of articles and resources he provided on prenatal fetal alcohol exposure, including the 1996 Institute of Medicine’s report and the American Medical Association’s 2017 resolution supporting the addition of adequate amounts of choline to prenatal vitamins, I found myself immersed in neuroscience topics, such as the role of neuronal acetylcholine receptor subunit alpha-7 in the formation of neurotransmitters, the strengthening of cell membranes, and the promotion of proper brain and spinal cord development. Dr. Bell spoke authoritatively about the neuroscience and the public health implications. One of his mantras was “Where is the data? You’ve got to have data.”
The information that he shared became the foundation of the action paper calling for the American Psychiatric Association (APA) to endorse the AMA’s resolution supporting the addition of adequate amounts (450 mg/d for pregnant women) of phosphatidylcholine to prenatal vitamins. The APA Assembly passed this action paper in May 2018.
He was also responsible for a second action paper, “Psychiatric Management of the Impact of Racism on Social and Clinical Events,” which passed at the same May 2018 assembly. Dr. Bell agreed to coauthor this paper, which was only fitting since the paper was a further elaboration of his efforts with the APA Caucus of Black Psychiatrists to implore the APA to acknowledge the deleterious effects of racism on both the victim and perpetrator.
While researching this topic, I had come across his 1980 article, Racism: A Symptom of the Narcissistic Personality Disorder (J Nat Med Assoc. 1980 Jul;72[7]:661-5), in which Dr. Bell applied psychoanalytic theory to posit that racism is one psychic derivative through which narcissism may manifest itself.
Although he was not formally trained as a psychoanalyst, he had benefited from strong psychoanalytic supervision at the Illinois State Psychiatric Institute, a training program of the University of Illinois at Chicago. He wrote confidently and clearly, applying self-psychology principles. He had the gravitas to write and speak about a range of topics, from neuroscience, psychotherapy, medical management of illness, and mental health advocacy. His 387-page curriculum vitae of 500+ articles, chapters, and books on mental health issues is a catalog of evidence that he had given thought to just about any topic along the spectrum of psychiatry and beyond.
In July 2018, after leaving a performance of “Hamilton” at the Kennedy Center, a lyric from the song “Non-Stop” stayed with me:
Why do you write like you’re running out of time?
(Why do you write like you’re running out of time?)
Write day and night like you’re running out of time?
The pace at which he read, wrote, lectured, researched, collaborated, and served on committees reminded me of the prodigious work of the former Secretary of the Treasury. When I shared this with Dr. Bell, he volunteered that he wrote to clear his mind. I suggested that, like other true writers, it seemed that he had to write. He did not disagree.
But, what was most meaningful about his productivity was his generosity of spirit. Any conversation was an opportunity for him to thoughtfully and respectfully share his knowledge. For example, once, we were discussing a clinical case that included the differential diagnosis of a patient, who happened to be African American, who was having auditory hallucinations. Dr. Bell might have been the first psychiatrist to alert the medical community about the misdiagnosis of schizophrenia among African Americans with bipolar disorder (J Nat Med Assoc. 1980 Feb 72[2]:141-5).
Contrary to my expectation that he was going to remind me of the tendency to misdiagnose, he instead offered, “You know, there are 40 reasons for auditory hallucinations.” Not what I had expected, yet, a response that reflected his continually giving nature and sharing of his abundance of gems. He was always teaching.
I later learned that his workday at Jackson Park Hospital usually ended at 2 p.m. He had treated patients, and supervised medical students and residents there for more than 40 years. The afternoons afforded him time to read, write, listen to music (Ella Fitzgerald), watch movies, and spend time with his adult children, to whom he was quite devoted. Dr. Bell was an avid martial artist and enjoyed sharing this practice with his son, William.
He was a longtime active member of the National Medical Association, recently receiving its prestigious Distinguished Service Award in Hawaii for his “exceptional work in medical service, medical research, and academic medicine.” It would be his last professional talk, though his delivery would belie his numbered days.
He was a former vice president of the Black Psychiatrists of America (BPA) and for 10 years had been the editor of the BPA Newsletter. Conversations were often peppered with anecdotes from time spent with other pioneering ancestors, such as Chester “Chet” Pierce, MD, Jeanne Spurlock, MD, Robert Phillips, MD, PhD, Charles Prudhomme, MD, Frances Cress Welsing, MD, and others. Dr. Bell was at the tail end of a generation of African American psychiatrists who had experienced firsthand the transition from segregation to federally mandated integration of our society.
Dr. Bell and his peers applied their education and training to improve clinical care for all, to decrease health inequities, and to eliminate disparities. It is evident that he loved his people and committed his life to addressing the needs of marginalized communities, those without the benefit of abundant resources, and those disproportionately affected by violence and trauma. As he stated in his last book, Fetal Alcohol Exposure in the African-American Community:
“I should add, my main concern is African American people living within the United States of America where in one community the rate of Fetal Alcohol Exposure is 388/1,000 people. ... However, this problem extends much further. Fetal Alcohol Exposure (FAE) is increasingly being found to (be) problematic in people of color around the world: Native Americans in Canada ... Aboriginal people in Australia ... and various tribes of people on the continent of Africa. ... Lastly, while the problem of Fetal Alcohol Exposure seems to be disproportionately affecting people of color, it also affects people who lack pigment in their skin. For example, FAE is a problem in Russia. From a public health perspective, so often people of color are like the proverbial “canary in a coal mine,” i.e., if there is poisonous gas in the coal mine, the canary will die first, warning the miners that they need to do something about it.”
However, Dr. Bell was a Distinguished Lifetime Fellow of the American Psychiatric Association and a Lifetime Fellow of the American College of Psychiatrists. He was a founding member of the board of directors of the National Commission on Correctional Health, and a member of prominent work groups of the National Institutes of Mental Health and the National Academy of Medicine (formerly the Institute of Medicine). And Dr. Bell’s mentees and students transcend generations, race, religions, professional disciplines, and national boundaries.
Because Dr. Bell was grounded and never forgot his roots, it was in these professional society circles that he ensured that clinicians with more privilege and limited or no exposure to communities of color were educated about the needs of those he treated. Without exposure to Carl Bell, it is likely that many of our psychiatric colleagues would remain unaware of both the brilliant dynamic resources and enormous challenges that are found in the black community and communities of color. By sharing his work with the house of medicine, he obviated the excuse of doing nothing because of ignorance.
I last saw Dr. Bell in San Francisco toward the end of the 2019 annual APA meeting. He had received the APA’s Adolf* Meyer Award for lifetime achievement. Afterward, I joined him for a dim sum lunch in Chinatown with two of his colleagues and Joseph Calhoun, his mentee in the APA’s Black Men in Psychiatry Early Pipeline Program. As we walked back to our respective hotels, we paused at what is now the Chinese Affirmative Action Center. We learned that this site had been the home of one of Dr. Bell’s former martial arts instructors. As Dr. Bell recounted his martial arts training, the reverence for his sensei was evident in his eyes.
When I reflect on how much I learned from and about Carl Bell in such a short period of time, I realize that he was one of those people who was so present and so astute that he allowed you to know him while he was giving.
So, how do we honor someone who gave so much of himself? When I now think of the lyric from “Hamilton” – “Why do you write like you’re running out of time?” – I realize that we get it twisted when we associate running out of time with our elders and their phase of life. It was not Carl Bell who was running out of time. He had been extraordinarily respectful of the space, time, and energy allotted to him in his lifetime. He would say, “People squander their personal resources.” He certainly had not squandered his.
As we reflect and mourn his passing, we will hear about his candor, authenticity, integrity, discipline, reliability, dedication, and serving spirit. This is called character.
Dr. Bell was beyond generous with his life, and it is going to take decades, if not more, for us to digest the compendium of knowledge that he left behind. I ask you: How will you use that knowledge to advance the causes he so diligently devoted his life to solving?
To Carl Compton Bell, I say, Well done. Thank you. And, rest now my dear brother.
Dr. Dunlap, a psychiatrist and psychoanalyst who practices in Washington, is a Washington Psychiatric Society representative to the APA Assembly, a past president of the Washington Psychiatric Society, and clinical professor of psychiatry and behavioral sciences at George Washington University, Washington. She is interested in the role “difference” – race, culture, and ethnicity – plays in interpersonal relationships and group dynamics.
*This column was updated 9/3/2019.
With the heart of a child and the spirit of a warrior, Carl Bell always spoke his truth. And, he did so in his own inimitable way. Sporting his signature dark brown wide-brim leather cowboy hat or NMA (National Medical Association) baseball cap, aviator sunglasses, and accompanying Superman belt buckle, Carl Compton Bell, MD, – psychiatrist, researcher, mental health advocate, father, grandfather, friend, colleague, pioneer, and servant – was driven by a deep commitment to serve others.
As those who truly knew him can attest, it is not hyperbole to say that Carl Compton Bell was one of the most genuine, brilliant, and humble physicians of our professional community and time.
My collaboration and friendship began with Dr. Bell began during the summer of 2016 as I was preparing for the 2017 Washington Psychiatric Society’s (WPS) Presidential Symposium at Saint Elizabeths Hospital. As president of WPS, I had chosen gun violence as my topic and sought out Dr. Bell because of his work on the South Side of Chicago, where he had devoted himself, becoming an internationally known clinician, researcher, and mental health advocate for those personally affected by violence and trauma. He immediately accepted.
In his presentation, “Gun Violence, Urban Youth and Mental Illness,” he reviewed his research on the neurocognitive behavioral effects of prenatal exposure to alcohol and its relation to the neurodevelopmental dynamics of youth violence, intimate partner violence, and mass shootings. Dr. Bell suggested that the relationship between prenatal exposure to alcohol and the diagnosis of numerous psychiatric conditions had been underestimated in the medical community. He eventually summarized his work in Fetal Alcohol Exposure in the African-American Community, published by Third World Press (2018). This vital resource not only summarizes in plain language the scope of the problem of prenatal alcohol exposure but is a narrative of Carl Bell’s life journey.
After the symposium, he would send articles, while warning, “I can bombard you with stuff.” Sometimes we would not speak for weeks at a time while I digested the resources he had shared. However, whenever I picked up the phone to call and respond to what he had provided, he would answer the phone, “Yessssss?” – as if he were anticipating my call and was ready to address any queries or comments I might have. Even when he were about to board a plane or charting – after making rounds on his patients while listening to the music of James Brown – he would answer the phone, even if only to coordinate a more mutually convenient time to connect.
During the process of digesting the plethora of articles and resources he provided on prenatal fetal alcohol exposure, including the 1996 Institute of Medicine’s report and the American Medical Association’s 2017 resolution supporting the addition of adequate amounts of choline to prenatal vitamins, I found myself immersed in neuroscience topics, such as the role of neuronal acetylcholine receptor subunit alpha-7 in the formation of neurotransmitters, the strengthening of cell membranes, and the promotion of proper brain and spinal cord development. Dr. Bell spoke authoritatively about the neuroscience and the public health implications. One of his mantras was “Where is the data? You’ve got to have data.”
The information that he shared became the foundation of the action paper calling for the American Psychiatric Association (APA) to endorse the AMA’s resolution supporting the addition of adequate amounts (450 mg/d for pregnant women) of phosphatidylcholine to prenatal vitamins. The APA Assembly passed this action paper in May 2018.
He was also responsible for a second action paper, “Psychiatric Management of the Impact of Racism on Social and Clinical Events,” which passed at the same May 2018 assembly. Dr. Bell agreed to coauthor this paper, which was only fitting since the paper was a further elaboration of his efforts with the APA Caucus of Black Psychiatrists to implore the APA to acknowledge the deleterious effects of racism on both the victim and perpetrator.
While researching this topic, I had come across his 1980 article, Racism: A Symptom of the Narcissistic Personality Disorder (J Nat Med Assoc. 1980 Jul;72[7]:661-5), in which Dr. Bell applied psychoanalytic theory to posit that racism is one psychic derivative through which narcissism may manifest itself.
Although he was not formally trained as a psychoanalyst, he had benefited from strong psychoanalytic supervision at the Illinois State Psychiatric Institute, a training program of the University of Illinois at Chicago. He wrote confidently and clearly, applying self-psychology principles. He had the gravitas to write and speak about a range of topics, from neuroscience, psychotherapy, medical management of illness, and mental health advocacy. His 387-page curriculum vitae of 500+ articles, chapters, and books on mental health issues is a catalog of evidence that he had given thought to just about any topic along the spectrum of psychiatry and beyond.
In July 2018, after leaving a performance of “Hamilton” at the Kennedy Center, a lyric from the song “Non-Stop” stayed with me:
Why do you write like you’re running out of time?
(Why do you write like you’re running out of time?)
Write day and night like you’re running out of time?
The pace at which he read, wrote, lectured, researched, collaborated, and served on committees reminded me of the prodigious work of the former Secretary of the Treasury. When I shared this with Dr. Bell, he volunteered that he wrote to clear his mind. I suggested that, like other true writers, it seemed that he had to write. He did not disagree.
But, what was most meaningful about his productivity was his generosity of spirit. Any conversation was an opportunity for him to thoughtfully and respectfully share his knowledge. For example, once, we were discussing a clinical case that included the differential diagnosis of a patient, who happened to be African American, who was having auditory hallucinations. Dr. Bell might have been the first psychiatrist to alert the medical community about the misdiagnosis of schizophrenia among African Americans with bipolar disorder (J Nat Med Assoc. 1980 Feb 72[2]:141-5).
Contrary to my expectation that he was going to remind me of the tendency to misdiagnose, he instead offered, “You know, there are 40 reasons for auditory hallucinations.” Not what I had expected, yet, a response that reflected his continually giving nature and sharing of his abundance of gems. He was always teaching.
I later learned that his workday at Jackson Park Hospital usually ended at 2 p.m. He had treated patients, and supervised medical students and residents there for more than 40 years. The afternoons afforded him time to read, write, listen to music (Ella Fitzgerald), watch movies, and spend time with his adult children, to whom he was quite devoted. Dr. Bell was an avid martial artist and enjoyed sharing this practice with his son, William.
He was a longtime active member of the National Medical Association, recently receiving its prestigious Distinguished Service Award in Hawaii for his “exceptional work in medical service, medical research, and academic medicine.” It would be his last professional talk, though his delivery would belie his numbered days.
He was a former vice president of the Black Psychiatrists of America (BPA) and for 10 years had been the editor of the BPA Newsletter. Conversations were often peppered with anecdotes from time spent with other pioneering ancestors, such as Chester “Chet” Pierce, MD, Jeanne Spurlock, MD, Robert Phillips, MD, PhD, Charles Prudhomme, MD, Frances Cress Welsing, MD, and others. Dr. Bell was at the tail end of a generation of African American psychiatrists who had experienced firsthand the transition from segregation to federally mandated integration of our society.
Dr. Bell and his peers applied their education and training to improve clinical care for all, to decrease health inequities, and to eliminate disparities. It is evident that he loved his people and committed his life to addressing the needs of marginalized communities, those without the benefit of abundant resources, and those disproportionately affected by violence and trauma. As he stated in his last book, Fetal Alcohol Exposure in the African-American Community:
“I should add, my main concern is African American people living within the United States of America where in one community the rate of Fetal Alcohol Exposure is 388/1,000 people. ... However, this problem extends much further. Fetal Alcohol Exposure (FAE) is increasingly being found to (be) problematic in people of color around the world: Native Americans in Canada ... Aboriginal people in Australia ... and various tribes of people on the continent of Africa. ... Lastly, while the problem of Fetal Alcohol Exposure seems to be disproportionately affecting people of color, it also affects people who lack pigment in their skin. For example, FAE is a problem in Russia. From a public health perspective, so often people of color are like the proverbial “canary in a coal mine,” i.e., if there is poisonous gas in the coal mine, the canary will die first, warning the miners that they need to do something about it.”
However, Dr. Bell was a Distinguished Lifetime Fellow of the American Psychiatric Association and a Lifetime Fellow of the American College of Psychiatrists. He was a founding member of the board of directors of the National Commission on Correctional Health, and a member of prominent work groups of the National Institutes of Mental Health and the National Academy of Medicine (formerly the Institute of Medicine). And Dr. Bell’s mentees and students transcend generations, race, religions, professional disciplines, and national boundaries.
Because Dr. Bell was grounded and never forgot his roots, it was in these professional society circles that he ensured that clinicians with more privilege and limited or no exposure to communities of color were educated about the needs of those he treated. Without exposure to Carl Bell, it is likely that many of our psychiatric colleagues would remain unaware of both the brilliant dynamic resources and enormous challenges that are found in the black community and communities of color. By sharing his work with the house of medicine, he obviated the excuse of doing nothing because of ignorance.
I last saw Dr. Bell in San Francisco toward the end of the 2019 annual APA meeting. He had received the APA’s Adolf* Meyer Award for lifetime achievement. Afterward, I joined him for a dim sum lunch in Chinatown with two of his colleagues and Joseph Calhoun, his mentee in the APA’s Black Men in Psychiatry Early Pipeline Program. As we walked back to our respective hotels, we paused at what is now the Chinese Affirmative Action Center. We learned that this site had been the home of one of Dr. Bell’s former martial arts instructors. As Dr. Bell recounted his martial arts training, the reverence for his sensei was evident in his eyes.
When I reflect on how much I learned from and about Carl Bell in such a short period of time, I realize that he was one of those people who was so present and so astute that he allowed you to know him while he was giving.
So, how do we honor someone who gave so much of himself? When I now think of the lyric from “Hamilton” – “Why do you write like you’re running out of time?” – I realize that we get it twisted when we associate running out of time with our elders and their phase of life. It was not Carl Bell who was running out of time. He had been extraordinarily respectful of the space, time, and energy allotted to him in his lifetime. He would say, “People squander their personal resources.” He certainly had not squandered his.
As we reflect and mourn his passing, we will hear about his candor, authenticity, integrity, discipline, reliability, dedication, and serving spirit. This is called character.
Dr. Bell was beyond generous with his life, and it is going to take decades, if not more, for us to digest the compendium of knowledge that he left behind. I ask you: How will you use that knowledge to advance the causes he so diligently devoted his life to solving?
To Carl Compton Bell, I say, Well done. Thank you. And, rest now my dear brother.
Dr. Dunlap, a psychiatrist and psychoanalyst who practices in Washington, is a Washington Psychiatric Society representative to the APA Assembly, a past president of the Washington Psychiatric Society, and clinical professor of psychiatry and behavioral sciences at George Washington University, Washington. She is interested in the role “difference” – race, culture, and ethnicity – plays in interpersonal relationships and group dynamics.
*This column was updated 9/3/2019.
Sleep disorder treatment tied to lower suicide attempt risk in veterans
in a large case-control matched study of patients in the Veterans Health Administration database.
However, treatment for sleep disorders was correlated to a reduced risk for suicide attempts.
Todd M. Bishop, PhD, of the Center of Excellence for Suicide Prevention, Canandaigua (N.Y.) VA Medical Center, and the department of psychiatry, University of Rochester (N.Y.) Medical Center, and his colleagues wrote that suicide is the 10th most frequent cause of death in the United States, and “nowhere is the suicide rate more alarming than among military veterans, who after adjusting for age and gender, have an approximately 1.5 times greater risk for suicide as compared to the civilian population.”
Previous research has explored the link between sleep disturbances and suicide attempts. But less has been done to look at specific sleep problems, and little research has examined the role of sleep medicine interventions and suicide attempt risk.
The investigators conducted a study to establish the association between suicide attempts and specific sleep disorders, and to examine the correlation between sleep medicine treatment and suicide attempts. Their sample consisted of 60,102 veterans who had received care within the VHA between Oct. 1, 2012, and Sept. 20, 2014. Half of the sample had a documented suicide attempt in the medical record (n = 30,051) and half did not (n = 30,051). The overall sample was predominately male (87.1%) with a mean age of 48.6 years. More than half the sample identified as white (67.4%).
Suicide attempts, sleep disturbance, and medical and mental health comorbidities were identified via ICD codes and prescription records. The predominant sleep disorders studied were insomnia, sleep-related breathing disorder (SRBD), and nightmares. The first suicide attempt in the study period was determined to be the index date for the case-control matching.
Overall, sleep disturbances were much more prevalent among cases than controls (insomnia, 46.2% vs. 12.6%), sleep-related breathing disorder (8.6% vs. 4.8%), and nightmares (7.1% vs. 1.6%). A logistic regression analysis was undertaken to examine the relationship between specific sleep disorders and suicide attempts. Insomnia, nightmares, and SRBD were each associated with increased odds of a suicide attempt with the following odds ratios: insomnia (odds ratio, 5.62; 95% confidence interval, 5.39-5.86), nightmares (OR, 2.49; 95% CI, 2.23-2.77), and sleep-related breathing disorder (OR, 1.37; 95% CI, 1.27-1.48).
A second model included known drivers of suicide attempts (PTSD, depression, anxiety disorders, schizophrenia, bipolar disorder, substance use disorder, medical comorbidity, and obesity). But after controlling for these factors, neither nightmares (OR, 0.96; 95% CI, 0.85-1.09) nor sleep-related breathing disorders (OR, 0.87, 95% CI, 0.79-0.94) remained positively associated with suicide attempt, but the association of insomnia with suicide attempt was maintained (OR, 1.51; 95% CI, 1.43-1.59).
The question of the impact of sleep medicine interventions on suicide attempts was studied with a third regression model adding the number of sleep medicine clinic visits in the 180 days prior to the suicide attempt index date as an independent variable. The variables in this model were limited to insomnia, SRBD, and nightmares. The investigators found that “for each sleep medicine clinic visit within the 6 months prior to index date the likelihood of suicide attempt is 11% less (OR, 0.89; 95% CI, 0.82-0.97).”
The limitations of the study include the lack of information on sleep treatment modalities or medications provided during the clinic visits, and the overlapping of sleep disturbance with other mental health conditions, such as alcohol dependence and PTSD. In addition, “some insomnia medications are labeled for risk of suicidal ideation and behavior, so there is some chance that the medications rather than insomnia itself were associated with the increased suicidal behavior,” the investigators wrote.
In addition to an analysis of specific types of sleep disorders associated with suicide attempts, the study showed that treatment of sleep disorders may have an important role in suicide prevention. The investigators concluded: “Identifying populations at risk for suicide prior to a first attempt is an important, but difficult task of suicide prevention. Prevention efforts can be aimed at modifiable risk factors that arise early on a patient’s trajectory toward a suicide attempt.”
The study was supported by the VISN 2 Center of Excellence for Suicide Prevention, Canandaigua VAMC. The authors had no disclosures.
SOURCE: Bishop TM et al. Sleep Med. 2019 Jul 25. doi: 10.1016/j.sleep.2019.07.016.
in a large case-control matched study of patients in the Veterans Health Administration database.
However, treatment for sleep disorders was correlated to a reduced risk for suicide attempts.
Todd M. Bishop, PhD, of the Center of Excellence for Suicide Prevention, Canandaigua (N.Y.) VA Medical Center, and the department of psychiatry, University of Rochester (N.Y.) Medical Center, and his colleagues wrote that suicide is the 10th most frequent cause of death in the United States, and “nowhere is the suicide rate more alarming than among military veterans, who after adjusting for age and gender, have an approximately 1.5 times greater risk for suicide as compared to the civilian population.”
Previous research has explored the link between sleep disturbances and suicide attempts. But less has been done to look at specific sleep problems, and little research has examined the role of sleep medicine interventions and suicide attempt risk.
The investigators conducted a study to establish the association between suicide attempts and specific sleep disorders, and to examine the correlation between sleep medicine treatment and suicide attempts. Their sample consisted of 60,102 veterans who had received care within the VHA between Oct. 1, 2012, and Sept. 20, 2014. Half of the sample had a documented suicide attempt in the medical record (n = 30,051) and half did not (n = 30,051). The overall sample was predominately male (87.1%) with a mean age of 48.6 years. More than half the sample identified as white (67.4%).
Suicide attempts, sleep disturbance, and medical and mental health comorbidities were identified via ICD codes and prescription records. The predominant sleep disorders studied were insomnia, sleep-related breathing disorder (SRBD), and nightmares. The first suicide attempt in the study period was determined to be the index date for the case-control matching.
Overall, sleep disturbances were much more prevalent among cases than controls (insomnia, 46.2% vs. 12.6%), sleep-related breathing disorder (8.6% vs. 4.8%), and nightmares (7.1% vs. 1.6%). A logistic regression analysis was undertaken to examine the relationship between specific sleep disorders and suicide attempts. Insomnia, nightmares, and SRBD were each associated with increased odds of a suicide attempt with the following odds ratios: insomnia (odds ratio, 5.62; 95% confidence interval, 5.39-5.86), nightmares (OR, 2.49; 95% CI, 2.23-2.77), and sleep-related breathing disorder (OR, 1.37; 95% CI, 1.27-1.48).
A second model included known drivers of suicide attempts (PTSD, depression, anxiety disorders, schizophrenia, bipolar disorder, substance use disorder, medical comorbidity, and obesity). But after controlling for these factors, neither nightmares (OR, 0.96; 95% CI, 0.85-1.09) nor sleep-related breathing disorders (OR, 0.87, 95% CI, 0.79-0.94) remained positively associated with suicide attempt, but the association of insomnia with suicide attempt was maintained (OR, 1.51; 95% CI, 1.43-1.59).
The question of the impact of sleep medicine interventions on suicide attempts was studied with a third regression model adding the number of sleep medicine clinic visits in the 180 days prior to the suicide attempt index date as an independent variable. The variables in this model were limited to insomnia, SRBD, and nightmares. The investigators found that “for each sleep medicine clinic visit within the 6 months prior to index date the likelihood of suicide attempt is 11% less (OR, 0.89; 95% CI, 0.82-0.97).”
The limitations of the study include the lack of information on sleep treatment modalities or medications provided during the clinic visits, and the overlapping of sleep disturbance with other mental health conditions, such as alcohol dependence and PTSD. In addition, “some insomnia medications are labeled for risk of suicidal ideation and behavior, so there is some chance that the medications rather than insomnia itself were associated with the increased suicidal behavior,” the investigators wrote.
In addition to an analysis of specific types of sleep disorders associated with suicide attempts, the study showed that treatment of sleep disorders may have an important role in suicide prevention. The investigators concluded: “Identifying populations at risk for suicide prior to a first attempt is an important, but difficult task of suicide prevention. Prevention efforts can be aimed at modifiable risk factors that arise early on a patient’s trajectory toward a suicide attempt.”
The study was supported by the VISN 2 Center of Excellence for Suicide Prevention, Canandaigua VAMC. The authors had no disclosures.
SOURCE: Bishop TM et al. Sleep Med. 2019 Jul 25. doi: 10.1016/j.sleep.2019.07.016.
in a large case-control matched study of patients in the Veterans Health Administration database.
However, treatment for sleep disorders was correlated to a reduced risk for suicide attempts.
Todd M. Bishop, PhD, of the Center of Excellence for Suicide Prevention, Canandaigua (N.Y.) VA Medical Center, and the department of psychiatry, University of Rochester (N.Y.) Medical Center, and his colleagues wrote that suicide is the 10th most frequent cause of death in the United States, and “nowhere is the suicide rate more alarming than among military veterans, who after adjusting for age and gender, have an approximately 1.5 times greater risk for suicide as compared to the civilian population.”
Previous research has explored the link between sleep disturbances and suicide attempts. But less has been done to look at specific sleep problems, and little research has examined the role of sleep medicine interventions and suicide attempt risk.
The investigators conducted a study to establish the association between suicide attempts and specific sleep disorders, and to examine the correlation between sleep medicine treatment and suicide attempts. Their sample consisted of 60,102 veterans who had received care within the VHA between Oct. 1, 2012, and Sept. 20, 2014. Half of the sample had a documented suicide attempt in the medical record (n = 30,051) and half did not (n = 30,051). The overall sample was predominately male (87.1%) with a mean age of 48.6 years. More than half the sample identified as white (67.4%).
Suicide attempts, sleep disturbance, and medical and mental health comorbidities were identified via ICD codes and prescription records. The predominant sleep disorders studied were insomnia, sleep-related breathing disorder (SRBD), and nightmares. The first suicide attempt in the study period was determined to be the index date for the case-control matching.
Overall, sleep disturbances were much more prevalent among cases than controls (insomnia, 46.2% vs. 12.6%), sleep-related breathing disorder (8.6% vs. 4.8%), and nightmares (7.1% vs. 1.6%). A logistic regression analysis was undertaken to examine the relationship between specific sleep disorders and suicide attempts. Insomnia, nightmares, and SRBD were each associated with increased odds of a suicide attempt with the following odds ratios: insomnia (odds ratio, 5.62; 95% confidence interval, 5.39-5.86), nightmares (OR, 2.49; 95% CI, 2.23-2.77), and sleep-related breathing disorder (OR, 1.37; 95% CI, 1.27-1.48).
A second model included known drivers of suicide attempts (PTSD, depression, anxiety disorders, schizophrenia, bipolar disorder, substance use disorder, medical comorbidity, and obesity). But after controlling for these factors, neither nightmares (OR, 0.96; 95% CI, 0.85-1.09) nor sleep-related breathing disorders (OR, 0.87, 95% CI, 0.79-0.94) remained positively associated with suicide attempt, but the association of insomnia with suicide attempt was maintained (OR, 1.51; 95% CI, 1.43-1.59).
The question of the impact of sleep medicine interventions on suicide attempts was studied with a third regression model adding the number of sleep medicine clinic visits in the 180 days prior to the suicide attempt index date as an independent variable. The variables in this model were limited to insomnia, SRBD, and nightmares. The investigators found that “for each sleep medicine clinic visit within the 6 months prior to index date the likelihood of suicide attempt is 11% less (OR, 0.89; 95% CI, 0.82-0.97).”
The limitations of the study include the lack of information on sleep treatment modalities or medications provided during the clinic visits, and the overlapping of sleep disturbance with other mental health conditions, such as alcohol dependence and PTSD. In addition, “some insomnia medications are labeled for risk of suicidal ideation and behavior, so there is some chance that the medications rather than insomnia itself were associated with the increased suicidal behavior,” the investigators wrote.
In addition to an analysis of specific types of sleep disorders associated with suicide attempts, the study showed that treatment of sleep disorders may have an important role in suicide prevention. The investigators concluded: “Identifying populations at risk for suicide prior to a first attempt is an important, but difficult task of suicide prevention. Prevention efforts can be aimed at modifiable risk factors that arise early on a patient’s trajectory toward a suicide attempt.”
The study was supported by the VISN 2 Center of Excellence for Suicide Prevention, Canandaigua VAMC. The authors had no disclosures.
SOURCE: Bishop TM et al. Sleep Med. 2019 Jul 25. doi: 10.1016/j.sleep.2019.07.016.
REPORTING FROM SLEEP MEDICINE
Transient opioid use linked to COPD exacerbation
according to a study of Medicaid claims data.
The data showed that opioid exposure in the prior 7 days was significantly associated with acute respiratory exacerbation. The odds of exacerbation increased as the morphine-equivalent daily dose increased and as the exposure window decreased.
These results “underline the immediacy of the risk of opioid use,” according to Yiran Rong of the department of pharmacy administration at the University of Mississippi in University and colleagues. Ms. Rong and colleagues reported their findings in the American Journal of Epidemiology.
The researchers analyzed Mississippi Medicaid administrative claims data from 2013-2017, which included 1,354 beneficiaries with 1,972 exacerbation events. The beneficiaries had a mean age of 53.11 years, 69.9% were female, and 59.7% were white. The patients had an average of 1.46 exacerbation events, and 62.27% of these events occurred in patients who had an opioid prescription filled in the previous 7 days.
The researchers compared the frequency and dose of opioid exposure in the 7 days before an exacerbation to the opioid exposure in 10 control periods, each 7 days long.
Opioid exposure in the prior 7 days was associated with an 80.8% increase in the odds of exacerbation. The odds ratio, adjusted for exposure to bronchodilators, corticosteroids, benzodiazepines, and beta-blockers, was 1.81 (95% confidence interval, 1.60-2.05).
Opioid exposure was associated with exacerbation in patients with a single exacerbation event (OR, 1.91; 95% CI, 1.61, 2.27), multiple events (OR, 1.71; 95% CI, 1.45-2.01), events recorded in the emergency department (OR, 2.01; 95% CI, 1.71-2.35), and events recorded in the hospital (OR, 1.47; 95% CI, 1.21-1.79).
The odds of exacerbation increased as the morphine-equivalent daily dose increased. Each 25-mg increase in morphine-equivalent daily dose was associated with an 11.2% increase in the odds of exacerbation (OR, 1.11; 95% CI, 1.04-1.20).
“This dose-response relationship is consistent with previously established evidence … and is indicative of the need for caution in prescribing high doses of opioids to COPD patients,” the researchers wrote.
They also found the odds of exacerbation increased as the exposure window decreased. The OR was 1.74 (95% CI, 1.54-1.97) for opioid exposure in an 8-day window before exacerbation and 2.00 (95% CI, 1.73-2.30) for opioid exposure in a 5-day window before exacerbation.
This suggests that “opioid-induced respiratory depression has a very short-term onset,” according to the researchers.
The team noted that this study has limitations, including its retrospective, observational nature, but the results suggest transient opioid use is associated with acute respiratory exacerbation of COPD.
There was no funding for this study, and none of the researchers declared conflicts of interest.
SOURCE: Rong Y et al. Am J Epidemiol. 2019 Jul 30. doi: 10.1093/aje/kwz169.
according to a study of Medicaid claims data.
The data showed that opioid exposure in the prior 7 days was significantly associated with acute respiratory exacerbation. The odds of exacerbation increased as the morphine-equivalent daily dose increased and as the exposure window decreased.
These results “underline the immediacy of the risk of opioid use,” according to Yiran Rong of the department of pharmacy administration at the University of Mississippi in University and colleagues. Ms. Rong and colleagues reported their findings in the American Journal of Epidemiology.
The researchers analyzed Mississippi Medicaid administrative claims data from 2013-2017, which included 1,354 beneficiaries with 1,972 exacerbation events. The beneficiaries had a mean age of 53.11 years, 69.9% were female, and 59.7% were white. The patients had an average of 1.46 exacerbation events, and 62.27% of these events occurred in patients who had an opioid prescription filled in the previous 7 days.
The researchers compared the frequency and dose of opioid exposure in the 7 days before an exacerbation to the opioid exposure in 10 control periods, each 7 days long.
Opioid exposure in the prior 7 days was associated with an 80.8% increase in the odds of exacerbation. The odds ratio, adjusted for exposure to bronchodilators, corticosteroids, benzodiazepines, and beta-blockers, was 1.81 (95% confidence interval, 1.60-2.05).
Opioid exposure was associated with exacerbation in patients with a single exacerbation event (OR, 1.91; 95% CI, 1.61, 2.27), multiple events (OR, 1.71; 95% CI, 1.45-2.01), events recorded in the emergency department (OR, 2.01; 95% CI, 1.71-2.35), and events recorded in the hospital (OR, 1.47; 95% CI, 1.21-1.79).
The odds of exacerbation increased as the morphine-equivalent daily dose increased. Each 25-mg increase in morphine-equivalent daily dose was associated with an 11.2% increase in the odds of exacerbation (OR, 1.11; 95% CI, 1.04-1.20).
“This dose-response relationship is consistent with previously established evidence … and is indicative of the need for caution in prescribing high doses of opioids to COPD patients,” the researchers wrote.
They also found the odds of exacerbation increased as the exposure window decreased. The OR was 1.74 (95% CI, 1.54-1.97) for opioid exposure in an 8-day window before exacerbation and 2.00 (95% CI, 1.73-2.30) for opioid exposure in a 5-day window before exacerbation.
This suggests that “opioid-induced respiratory depression has a very short-term onset,” according to the researchers.
The team noted that this study has limitations, including its retrospective, observational nature, but the results suggest transient opioid use is associated with acute respiratory exacerbation of COPD.
There was no funding for this study, and none of the researchers declared conflicts of interest.
SOURCE: Rong Y et al. Am J Epidemiol. 2019 Jul 30. doi: 10.1093/aje/kwz169.
according to a study of Medicaid claims data.
The data showed that opioid exposure in the prior 7 days was significantly associated with acute respiratory exacerbation. The odds of exacerbation increased as the morphine-equivalent daily dose increased and as the exposure window decreased.
These results “underline the immediacy of the risk of opioid use,” according to Yiran Rong of the department of pharmacy administration at the University of Mississippi in University and colleagues. Ms. Rong and colleagues reported their findings in the American Journal of Epidemiology.
The researchers analyzed Mississippi Medicaid administrative claims data from 2013-2017, which included 1,354 beneficiaries with 1,972 exacerbation events. The beneficiaries had a mean age of 53.11 years, 69.9% were female, and 59.7% were white. The patients had an average of 1.46 exacerbation events, and 62.27% of these events occurred in patients who had an opioid prescription filled in the previous 7 days.
The researchers compared the frequency and dose of opioid exposure in the 7 days before an exacerbation to the opioid exposure in 10 control periods, each 7 days long.
Opioid exposure in the prior 7 days was associated with an 80.8% increase in the odds of exacerbation. The odds ratio, adjusted for exposure to bronchodilators, corticosteroids, benzodiazepines, and beta-blockers, was 1.81 (95% confidence interval, 1.60-2.05).
Opioid exposure was associated with exacerbation in patients with a single exacerbation event (OR, 1.91; 95% CI, 1.61, 2.27), multiple events (OR, 1.71; 95% CI, 1.45-2.01), events recorded in the emergency department (OR, 2.01; 95% CI, 1.71-2.35), and events recorded in the hospital (OR, 1.47; 95% CI, 1.21-1.79).
The odds of exacerbation increased as the morphine-equivalent daily dose increased. Each 25-mg increase in morphine-equivalent daily dose was associated with an 11.2% increase in the odds of exacerbation (OR, 1.11; 95% CI, 1.04-1.20).
“This dose-response relationship is consistent with previously established evidence … and is indicative of the need for caution in prescribing high doses of opioids to COPD patients,” the researchers wrote.
They also found the odds of exacerbation increased as the exposure window decreased. The OR was 1.74 (95% CI, 1.54-1.97) for opioid exposure in an 8-day window before exacerbation and 2.00 (95% CI, 1.73-2.30) for opioid exposure in a 5-day window before exacerbation.
This suggests that “opioid-induced respiratory depression has a very short-term onset,” according to the researchers.
The team noted that this study has limitations, including its retrospective, observational nature, but the results suggest transient opioid use is associated with acute respiratory exacerbation of COPD.
There was no funding for this study, and none of the researchers declared conflicts of interest.
SOURCE: Rong Y et al. Am J Epidemiol. 2019 Jul 30. doi: 10.1093/aje/kwz169.
FROM THE AMERICAN JOURNAL OF EPIDEMIOLOGY