User login
How Do Drug Shortages Affect Dermatologists?
The frequency of drug shortages in the United States has considerably increased over the last decade, affecting different areas of health care practice.1,2 Basic products needed to care for patients in hospitals and clinics are many of the same drugs that are in short supply.3 This issue has become an ongoing public health concern that directly affects health care providers and their patients.4 In dermatology, similar to other specialties, success often is influenced by the efficacy of medications used to treat patients, and lack of appropriate medications has the potential to diminish health outcomes. Therefore, it is imperative for dermatology providers to recognize the factors that contribute to this issue, understand the effects of drug shortages on patients, and learn how they can improve stewardship of scarce resources and contribute to the solution.
Causes of Drug Shortages
Drug shortages can occur due to discontinuations, delays, or manufacturing and quality problems.5 Shortages of the most basic hospital products represent market failure.1 In such cases, a small number of manufacturers supply these products, and if a manufacturer discontinues a particular product—as in the case of lidocaine with epinephrine—a shortage results, as the current system does not have the capacity to deal with such as issue.1,6
An important playmaker affecting the market for medical supplies and drugs are group purchasing organizations (GPOs). The 4 largest GPOs in the United States account for 90% of the medical supply market.7 Although they have simplified the process for hospitals to purchase supplies by taking on the work and expense of dealing with hundreds of manufacturers, GPOs have considerable power to affect the supply chain. By allowing certain manufacturers to become the sole suppliers of products in return for premium fees, GPOs have narrowed the supply chain of key products to sometimes only 1 or 2 manufacturers.7 This practice may lead to decreased capacity of regional and national supply chains, setting up the system to eventual product shortage in scenarios of production problems or a decrease in the already limited number of manufacturers.
The US Food and Drug Administration (FDA) works closely with manufacturers to prevent or reduce the impact of drug shortages. Although the FDA recently has taken more action to address the issue, solutions such as allowing imported products and underlying or approving new suppliers are only temporary fixes.1 The root of the problem needs to be dealt with by ensuring there is a broad competitive supply chain.
Impact on Dermatologists
The nationwide shortage of lidocaine with epinephrine that occurred in 2017 is a specific example of how drug shortages affect dermatologists.6 This product is used in the typical dermatology clinic on a daily basis for biopsies. Possible solutions to decrease usage include drawing up 1.5 mL lidocaine with epinephrine instead of 3 mL and mixing readily available normal saline with lidocaine to produce a 1:200,000 mixture to yield a 0.5% concentration that still maintains good vasoconstrictor effects. Options for dermatologists who run out of lidocaine with epinephrine are to either use lidocaine without epinephrine, which disrupts optimal patient care, or to purchase 1% lidocaine with epinephrine at a much higher cost.6 A study that analyzed changes in drug pricing following shortages in the United States indicated that prices of drugs facing a shortage increased more than twice as quickly as expected between 2015 and 2016 vs those that were not in shortage, which may reflect opportunistic behaviors of drug manufacturers during shortages.8
The American Academy of Dermatology Association has created a letter and encouraged patients to notify their lawmakers about the severity of the drug shortage issue. Given the shortage of local anesthetics and their importance to the practice of dermatology, the American Academy of Dermatology Association also has created guidelines discussing local anesthetics that could be an alternative to lidocaine for office-based dermatologic surgery.9
Final Thoughts
Dermatology practitioners should be aware of current shortages impacting their practice and address the potential shortage proactively. We propose that dermatology clinics should keep an emergency reservoir of products routinely used in practice that currently are on the FDA drug shortage list, particularly lidocaine hydrochloride (with and without epinephrine) and sodium bicarbonate,10 which may diminish the negative impact a shortage may have on the high quality of health care we strive to provide. On a bigger scale, providers should be more proactive to have their voices heard and get involved with policymaking given the potential for patient harm and suboptimal care associated with drug shortages.
- Mazer-Amirshahi M, Fox ER, Zocchi MS, et al. Longitudinal trends in US shortages of sterile solutions, 2001-17. Am J Health Syst Pharm. 2018;75:1903-1908.
- Fox ER, Sweet BV, Jensen V. Drug shortages: a complex health care crisis. Mayo Clin Proc. 2014;89:361-373.
- Drug shortages roundtable: minimizing impact on patient care [published online March 15, 2018]. Am J Health Syst Pharm. 2018;75:816-820.
- Fox ER, McLaughlin MM. ASHP guidelines on managing drug product shortages. Am J Health Syst Pharm. 2018;75:1742-1750.
- Bowles SK. Drug shortages: more than just a background noise [published online February 28, 2018]. Can J Hosp Pharm. 2019;72:3-4.
- Bodie B, Brodell RT, Helms SE. Shortage of lidocaine with epinephrine: causes and solutions. J Am Acad Dermatol. 2018;79:392-393.
- Bruhn WE, Fracica EA, Makary MA. Group purchasing organizations, health care costs, and drug shortages. JAMA. 2018;320:1859-1860.
- Hernandez I, Sampathkumar S, Good CB, et al. Changes in drug pricing after drug shortages in the United States. Ann Intern Med. 2018;170:74-76.
- AADA, other specialties continue pressing FDA on drug shortages American Academy of Dermatology Association website.
https://www.aad.org/advocacy/news/news/2018/02/aada-other-specialties-continue-pressing-fda-on-drug-shortages. Published February 23, 2018. Accessed July 24, 2019. - FDA drug shortages. US Food & Drug Administration website. https://www.aad.org/advocacy/drug-pricing-and-availability/dermatologic-drug-shortages. Accessed July 24, 2019.
The frequency of drug shortages in the United States has considerably increased over the last decade, affecting different areas of health care practice.1,2 Basic products needed to care for patients in hospitals and clinics are many of the same drugs that are in short supply.3 This issue has become an ongoing public health concern that directly affects health care providers and their patients.4 In dermatology, similar to other specialties, success often is influenced by the efficacy of medications used to treat patients, and lack of appropriate medications has the potential to diminish health outcomes. Therefore, it is imperative for dermatology providers to recognize the factors that contribute to this issue, understand the effects of drug shortages on patients, and learn how they can improve stewardship of scarce resources and contribute to the solution.
Causes of Drug Shortages
Drug shortages can occur due to discontinuations, delays, or manufacturing and quality problems.5 Shortages of the most basic hospital products represent market failure.1 In such cases, a small number of manufacturers supply these products, and if a manufacturer discontinues a particular product—as in the case of lidocaine with epinephrine—a shortage results, as the current system does not have the capacity to deal with such as issue.1,6
An important playmaker affecting the market for medical supplies and drugs are group purchasing organizations (GPOs). The 4 largest GPOs in the United States account for 90% of the medical supply market.7 Although they have simplified the process for hospitals to purchase supplies by taking on the work and expense of dealing with hundreds of manufacturers, GPOs have considerable power to affect the supply chain. By allowing certain manufacturers to become the sole suppliers of products in return for premium fees, GPOs have narrowed the supply chain of key products to sometimes only 1 or 2 manufacturers.7 This practice may lead to decreased capacity of regional and national supply chains, setting up the system to eventual product shortage in scenarios of production problems or a decrease in the already limited number of manufacturers.
The US Food and Drug Administration (FDA) works closely with manufacturers to prevent or reduce the impact of drug shortages. Although the FDA recently has taken more action to address the issue, solutions such as allowing imported products and underlying or approving new suppliers are only temporary fixes.1 The root of the problem needs to be dealt with by ensuring there is a broad competitive supply chain.
Impact on Dermatologists
The nationwide shortage of lidocaine with epinephrine that occurred in 2017 is a specific example of how drug shortages affect dermatologists.6 This product is used in the typical dermatology clinic on a daily basis for biopsies. Possible solutions to decrease usage include drawing up 1.5 mL lidocaine with epinephrine instead of 3 mL and mixing readily available normal saline with lidocaine to produce a 1:200,000 mixture to yield a 0.5% concentration that still maintains good vasoconstrictor effects. Options for dermatologists who run out of lidocaine with epinephrine are to either use lidocaine without epinephrine, which disrupts optimal patient care, or to purchase 1% lidocaine with epinephrine at a much higher cost.6 A study that analyzed changes in drug pricing following shortages in the United States indicated that prices of drugs facing a shortage increased more than twice as quickly as expected between 2015 and 2016 vs those that were not in shortage, which may reflect opportunistic behaviors of drug manufacturers during shortages.8
The American Academy of Dermatology Association has created a letter and encouraged patients to notify their lawmakers about the severity of the drug shortage issue. Given the shortage of local anesthetics and their importance to the practice of dermatology, the American Academy of Dermatology Association also has created guidelines discussing local anesthetics that could be an alternative to lidocaine for office-based dermatologic surgery.9
Final Thoughts
Dermatology practitioners should be aware of current shortages impacting their practice and address the potential shortage proactively. We propose that dermatology clinics should keep an emergency reservoir of products routinely used in practice that currently are on the FDA drug shortage list, particularly lidocaine hydrochloride (with and without epinephrine) and sodium bicarbonate,10 which may diminish the negative impact a shortage may have on the high quality of health care we strive to provide. On a bigger scale, providers should be more proactive to have their voices heard and get involved with policymaking given the potential for patient harm and suboptimal care associated with drug shortages.
The frequency of drug shortages in the United States has considerably increased over the last decade, affecting different areas of health care practice.1,2 Basic products needed to care for patients in hospitals and clinics are many of the same drugs that are in short supply.3 This issue has become an ongoing public health concern that directly affects health care providers and their patients.4 In dermatology, similar to other specialties, success often is influenced by the efficacy of medications used to treat patients, and lack of appropriate medications has the potential to diminish health outcomes. Therefore, it is imperative for dermatology providers to recognize the factors that contribute to this issue, understand the effects of drug shortages on patients, and learn how they can improve stewardship of scarce resources and contribute to the solution.
Causes of Drug Shortages
Drug shortages can occur due to discontinuations, delays, or manufacturing and quality problems.5 Shortages of the most basic hospital products represent market failure.1 In such cases, a small number of manufacturers supply these products, and if a manufacturer discontinues a particular product—as in the case of lidocaine with epinephrine—a shortage results, as the current system does not have the capacity to deal with such as issue.1,6
An important playmaker affecting the market for medical supplies and drugs are group purchasing organizations (GPOs). The 4 largest GPOs in the United States account for 90% of the medical supply market.7 Although they have simplified the process for hospitals to purchase supplies by taking on the work and expense of dealing with hundreds of manufacturers, GPOs have considerable power to affect the supply chain. By allowing certain manufacturers to become the sole suppliers of products in return for premium fees, GPOs have narrowed the supply chain of key products to sometimes only 1 or 2 manufacturers.7 This practice may lead to decreased capacity of regional and national supply chains, setting up the system to eventual product shortage in scenarios of production problems or a decrease in the already limited number of manufacturers.
The US Food and Drug Administration (FDA) works closely with manufacturers to prevent or reduce the impact of drug shortages. Although the FDA recently has taken more action to address the issue, solutions such as allowing imported products and underlying or approving new suppliers are only temporary fixes.1 The root of the problem needs to be dealt with by ensuring there is a broad competitive supply chain.
Impact on Dermatologists
The nationwide shortage of lidocaine with epinephrine that occurred in 2017 is a specific example of how drug shortages affect dermatologists.6 This product is used in the typical dermatology clinic on a daily basis for biopsies. Possible solutions to decrease usage include drawing up 1.5 mL lidocaine with epinephrine instead of 3 mL and mixing readily available normal saline with lidocaine to produce a 1:200,000 mixture to yield a 0.5% concentration that still maintains good vasoconstrictor effects. Options for dermatologists who run out of lidocaine with epinephrine are to either use lidocaine without epinephrine, which disrupts optimal patient care, or to purchase 1% lidocaine with epinephrine at a much higher cost.6 A study that analyzed changes in drug pricing following shortages in the United States indicated that prices of drugs facing a shortage increased more than twice as quickly as expected between 2015 and 2016 vs those that were not in shortage, which may reflect opportunistic behaviors of drug manufacturers during shortages.8
The American Academy of Dermatology Association has created a letter and encouraged patients to notify their lawmakers about the severity of the drug shortage issue. Given the shortage of local anesthetics and their importance to the practice of dermatology, the American Academy of Dermatology Association also has created guidelines discussing local anesthetics that could be an alternative to lidocaine for office-based dermatologic surgery.9
Final Thoughts
Dermatology practitioners should be aware of current shortages impacting their practice and address the potential shortage proactively. We propose that dermatology clinics should keep an emergency reservoir of products routinely used in practice that currently are on the FDA drug shortage list, particularly lidocaine hydrochloride (with and without epinephrine) and sodium bicarbonate,10 which may diminish the negative impact a shortage may have on the high quality of health care we strive to provide. On a bigger scale, providers should be more proactive to have their voices heard and get involved with policymaking given the potential for patient harm and suboptimal care associated with drug shortages.
- Mazer-Amirshahi M, Fox ER, Zocchi MS, et al. Longitudinal trends in US shortages of sterile solutions, 2001-17. Am J Health Syst Pharm. 2018;75:1903-1908.
- Fox ER, Sweet BV, Jensen V. Drug shortages: a complex health care crisis. Mayo Clin Proc. 2014;89:361-373.
- Drug shortages roundtable: minimizing impact on patient care [published online March 15, 2018]. Am J Health Syst Pharm. 2018;75:816-820.
- Fox ER, McLaughlin MM. ASHP guidelines on managing drug product shortages. Am J Health Syst Pharm. 2018;75:1742-1750.
- Bowles SK. Drug shortages: more than just a background noise [published online February 28, 2018]. Can J Hosp Pharm. 2019;72:3-4.
- Bodie B, Brodell RT, Helms SE. Shortage of lidocaine with epinephrine: causes and solutions. J Am Acad Dermatol. 2018;79:392-393.
- Bruhn WE, Fracica EA, Makary MA. Group purchasing organizations, health care costs, and drug shortages. JAMA. 2018;320:1859-1860.
- Hernandez I, Sampathkumar S, Good CB, et al. Changes in drug pricing after drug shortages in the United States. Ann Intern Med. 2018;170:74-76.
- AADA, other specialties continue pressing FDA on drug shortages American Academy of Dermatology Association website.
https://www.aad.org/advocacy/news/news/2018/02/aada-other-specialties-continue-pressing-fda-on-drug-shortages. Published February 23, 2018. Accessed July 24, 2019. - FDA drug shortages. US Food & Drug Administration website. https://www.aad.org/advocacy/drug-pricing-and-availability/dermatologic-drug-shortages. Accessed July 24, 2019.
- Mazer-Amirshahi M, Fox ER, Zocchi MS, et al. Longitudinal trends in US shortages of sterile solutions, 2001-17. Am J Health Syst Pharm. 2018;75:1903-1908.
- Fox ER, Sweet BV, Jensen V. Drug shortages: a complex health care crisis. Mayo Clin Proc. 2014;89:361-373.
- Drug shortages roundtable: minimizing impact on patient care [published online March 15, 2018]. Am J Health Syst Pharm. 2018;75:816-820.
- Fox ER, McLaughlin MM. ASHP guidelines on managing drug product shortages. Am J Health Syst Pharm. 2018;75:1742-1750.
- Bowles SK. Drug shortages: more than just a background noise [published online February 28, 2018]. Can J Hosp Pharm. 2019;72:3-4.
- Bodie B, Brodell RT, Helms SE. Shortage of lidocaine with epinephrine: causes and solutions. J Am Acad Dermatol. 2018;79:392-393.
- Bruhn WE, Fracica EA, Makary MA. Group purchasing organizations, health care costs, and drug shortages. JAMA. 2018;320:1859-1860.
- Hernandez I, Sampathkumar S, Good CB, et al. Changes in drug pricing after drug shortages in the United States. Ann Intern Med. 2018;170:74-76.
- AADA, other specialties continue pressing FDA on drug shortages American Academy of Dermatology Association website.
https://www.aad.org/advocacy/news/news/2018/02/aada-other-specialties-continue-pressing-fda-on-drug-shortages. Published February 23, 2018. Accessed July 24, 2019. - FDA drug shortages. US Food & Drug Administration website. https://www.aad.org/advocacy/drug-pricing-and-availability/dermatologic-drug-shortages. Accessed July 24, 2019.
Intraoperative Electrosurgical Smoke During Outpatient Surgery: A Survey of Dermatologic Surgeon and Staff Preferences
A growing body of evidence shows that electrosurgical smoke contains both harmful chemicals as well as live material, including blood particles, bacteria, and viruses.1 Both human immunodeficiency virus and human papillomavirus have been identified in surgical smoke plumes, and bacterial colony growth has been demonstrated from electrosurgical smoke specimens, specifically Staphylococcus, Corynebacterium, and Neisseria species.2-8 Treating 1 g of tissue with electrocoagulation produces chemical by-products equivalent to burning 6 unfiltered cigarettes,9 which is twice the amount of chemical by-products produced by CO2 laser vaporization of the same quantity of tissue. It is a common misconception that electrosurgical smoke is less hazardous than smoke produced by ablative CO2 procedures.9 Many chemicals are present in electrosurgical smoke plumes, including nitriles, benzenes, carbon monoxide, hydrogen cyanide, indoles, phenols, pyridine, pyrrole, styrene, toluene, and xylene.10-12 In animal model studies of rat lungs exposed to surgical smoke, pathologic changes, including interstitial pneumonia, bronchiolitis, and emphysema, have been shown in a dose-dependent manner.1,13-16 Diseases and symptoms linked to inhalation of electrosurgical smoke in humans include anemia, eye irritation, hypoxia, dizziness, nasopharyngeal lesions, vomiting, sneezing, throat irritation, and weakness.1,8,17-19 A study of 153 dermatology residents found that more than 70% reported receiving no formal education on the hazards of electrosurgical smoke.20 Approximately 45% were unaware if they had access to smoke evacuation in rooms where electrosurgery was performed. More than 76% were concerned with the infectious risk of electrosurgical smoke, and more than 71% were concerned with its potential carcinogenic risk.20
We surveyed dermatologists who perform skin surgery as well as staff members with respect to their experiences with electrosurgical smoke and to observe any difference that information on the potential hazards of electrosurgical smoke may have on their attitudes and preferences.
Materials and Methods
Survey Instrument
We developed a REDCap survey consisting of 17 questions that was approved by the executive committees of the American College of Mohs Surgery and the American Society for Dermatologic Surgery for distribution to their dermatologist memberships. It was emailed to eligible participants using their mailing lists. Although the survey was sent directly to member physicians, it was recommended that they forward the survey to their clinical staff to complete.
After responding to an initial set of survey questions, respondents were informed that there is growing evidence of potential harms of inhalation of surgical smoke. They then were asked the same series of survey questions in light of this information.
Statistical Analysis
Statistical analysis of the survey responses was then completed, and free-text responses as a final question of the survey were assessed for themes. Preintervention responses of staff and clinicians noticing smoke and being bothered by smoke were assessed using proportions and 95% confidence interval (CI) estimates of the proportions. On most questions, respondents could answer on a scale of 1 to 10. Responses of 5 to 10 on noticing smoke and 5 to 10 on being bothered or troubled by the smoke smell were grouped for analyses. A cross-tabulation using the Bhapkar test for marginal homogeneity was used to assess if information presented on potential smoke hazards changed responses. A Cochran-Mantel-Haenszel test for ordinal responses was used to determine differences between surgeons and staff. A McNemar test was used to determine statistical significance of change in responses to cost. Statistical analysis was performed using SAS version 9.
Results
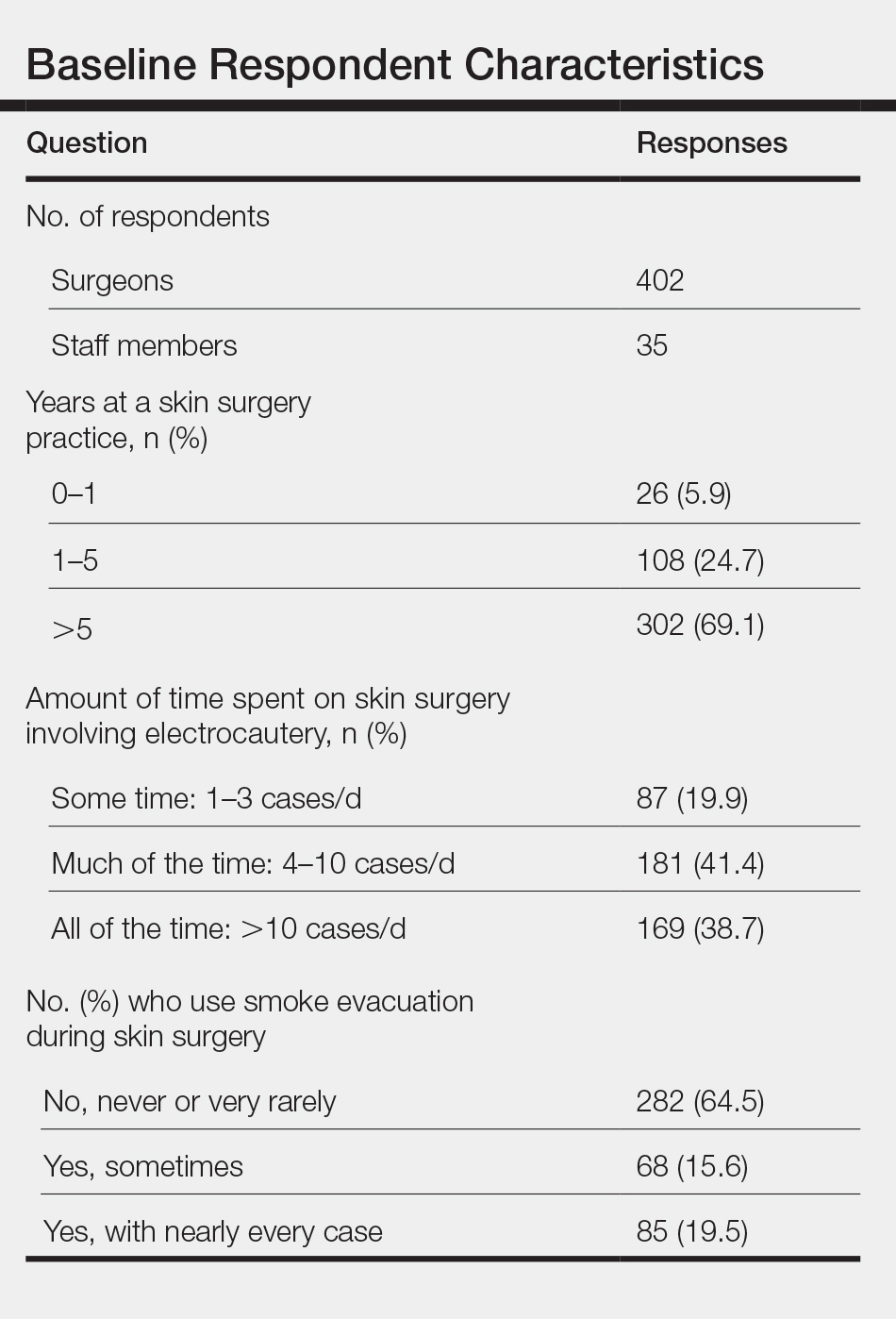
There was a total of 443 responses to our questionnaire. Two respondents answered that they did not work in an office where skin surgery was performed, and 4 respondents did not answer any questions and were therefore excluded, leaving a total of 437 responses (402 physicians and 35 staff members). A summary of the characteristics of the respondents is shown in the Table. Some respondents did not answer each question, leading to fewer than 437 answers for some questions.
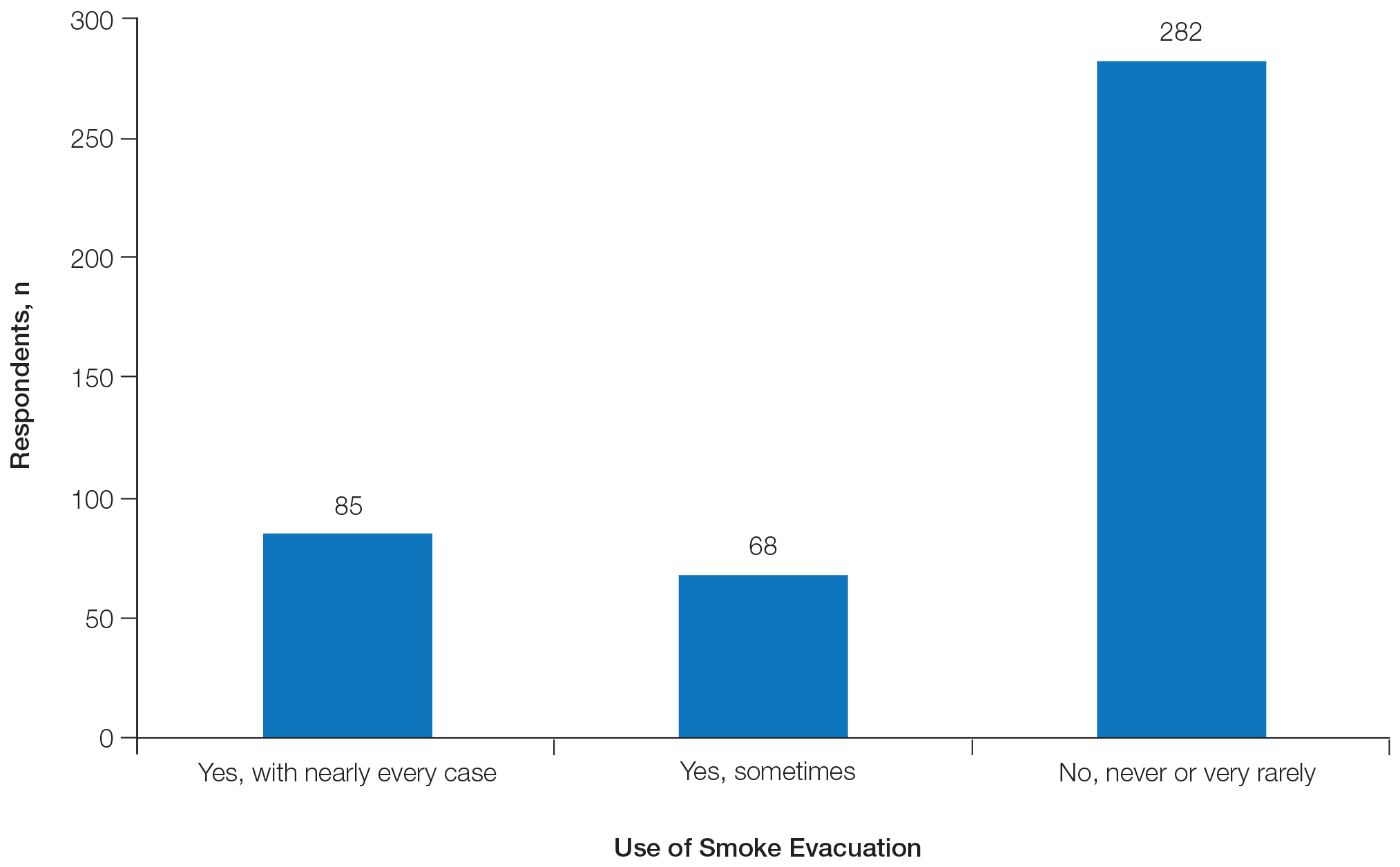
Two hundred eighty-two respondents (64.5%) never or very rarely used smoke evacuation during skin surgical procedures, and only 85 (19.5%) used smoke evacuation with nearly every case. The remaining respondents sometimes used smoke evacuation (Figure 1).
Prior to being presented with the potential dangers of electrosurgical smoke and using a value of 5 to 10 to determine if respondents noticed smoke, 54.4% (95% CI, 49.5%-59.1%) did notice intraoperative smoke during procedures. Using a value of 5 to 10 to indicate if respondents were bothered or troubled by the smoke smell, 35.5% (95% CI, 31.0%-40.2%) were bothered or troubled by intraoperative smoke prior to potential hazards being presented.
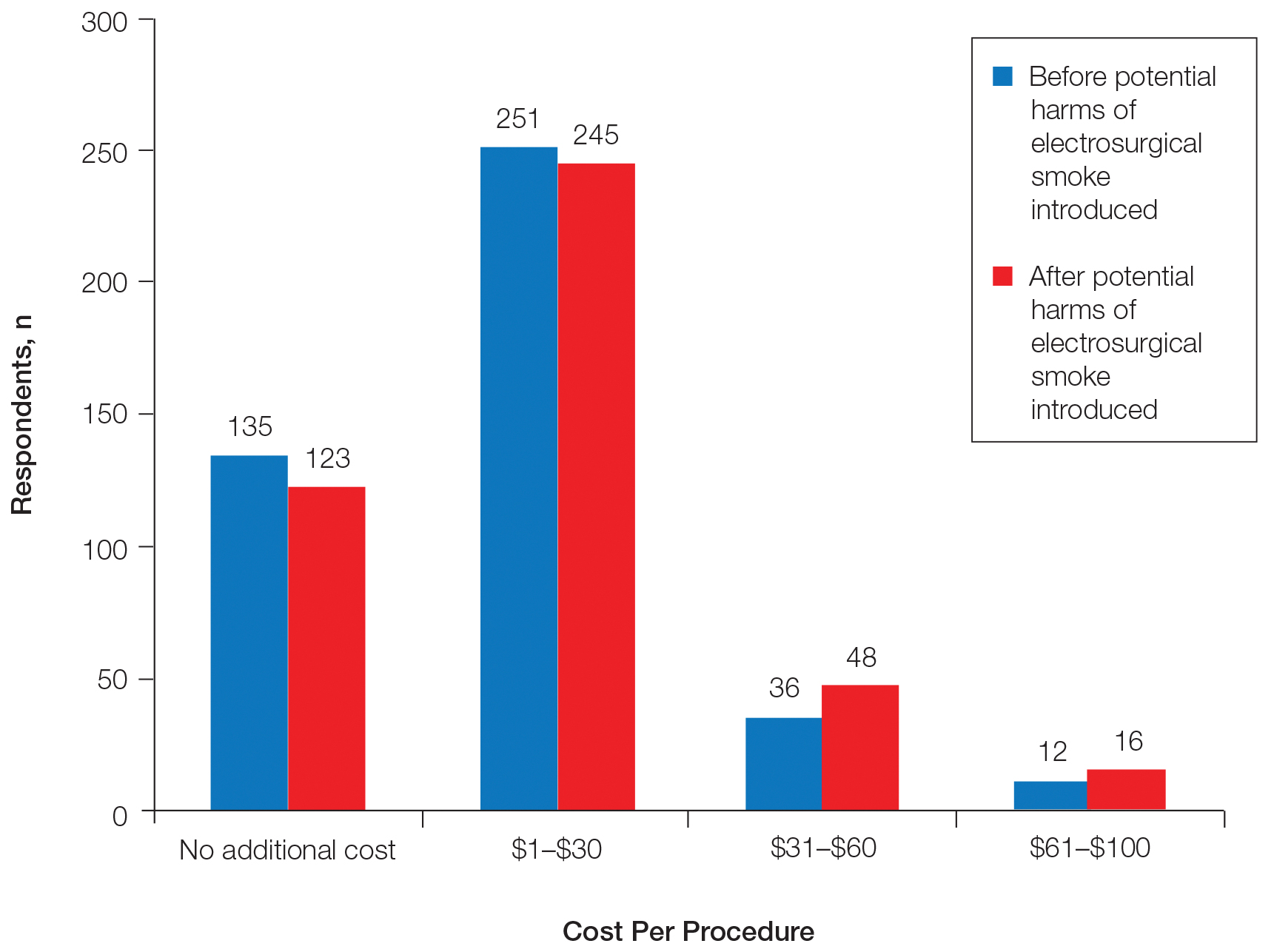
Regarding acceptable increase in cost per procedure for smoke evacuation at baseline, 68.9% of respondents favored additional cost; 57.8% of respondents chose the lowest cost grouping of $1 to $30. After being presented with information about the potential harm of intraoperative smoke, the respondents in favor of additional cost increased to 71.5%, which was a small but statistically significant change (P=.0075)(Figure 2).
Respondents were sorted into groups consisting of those who never used smoke evacuation, those who used it occasionally, and those who used it with all smoke-producing procedures. The degree to which respondents noticed intraoperative smoke was strongly correlated with their use of smoke evacuation; those who never used smoke evacuation noticed the presence of smoke more, and those who always used smoke evacuation noticed it less (P=.0002). Similar trends were noted regarding if the smoke smell bothered or troubled respondents (P=.0014).
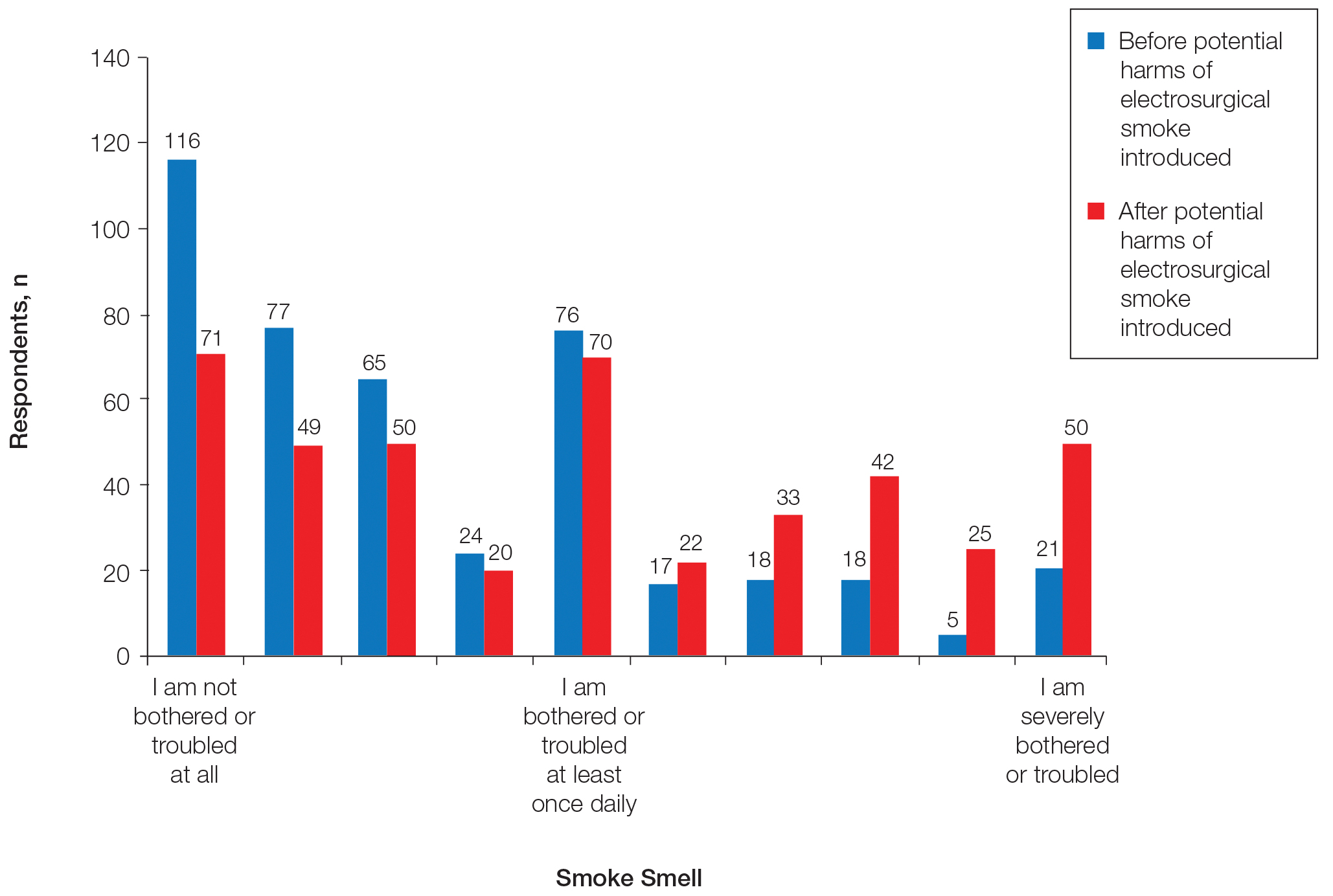
After being presented with the potential risks of electrosurgical smoke, 29 more respondents answered that they were severely bothered by electrosurgical smoke, whereas 45 fewer respondents selected that they were not bothered or troubled at all by electrosurgical smoke (Figure 3). This difference was statistically significant (P<.0001). Fifteen more respondents answered that they would be much more likely to choose to work at a practice with smoke evacuation once the potential harm of electrosurgical smoke was introduced, and 11 were somewhat more likely to choose a practice with smoke evacuation (P<.0001).
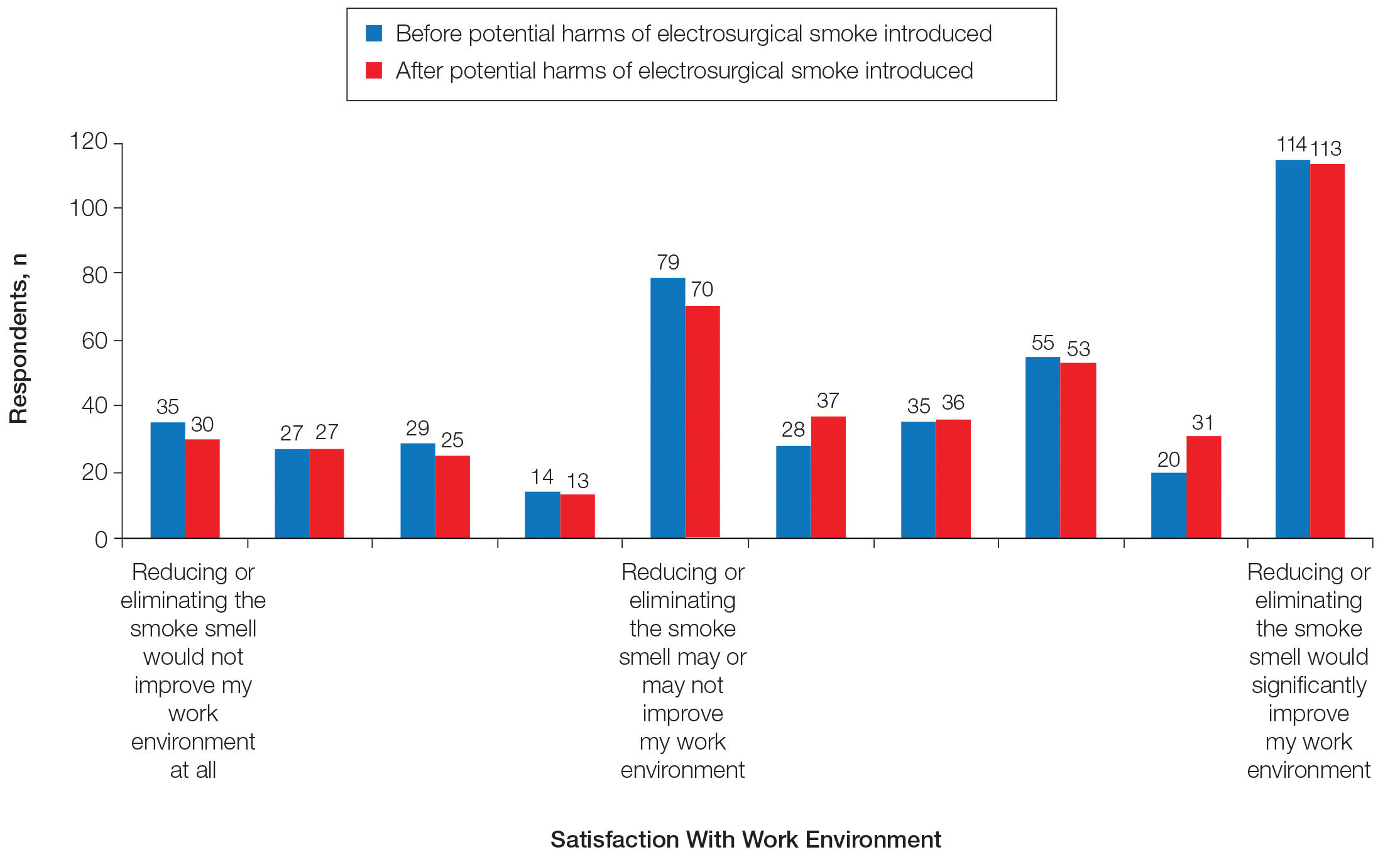
Information about the potential harm of electrosurgical smoke did not statistically significantly affect satisfaction with work environment (P=.3139)(Figure 4).
There were no statistically significant differences between surgeon and staff responses on any questions.
Comment
Developing evidence of health risks associated with electrosurgical smoke plumes has led to an increasing interest in the use of smoke protection or remediation tools during surgical procedures. High-filtration face masks and smoke-evacuation devices protect physicians, staff members, and patients, as well as improve the patient’s clinical experience.
Our study was designed to query dermatologists who perform skin surgery as well as staff members with respect to their experiences with electrosurgical smoke and to observe any difference that information on the potential hazards of electrosurgical smoke may have on their attitudes and preferences. We received 437 responses to our survey (Table). At baseline, 54.4% of respondents noticed and 35.5% were bothered or troubled by the smoke smell produced during skin electrosurgery. These data were intuitively associated in a statistically significant manner with the use of smoke evacuation for respondents; those respondents who more commonly used smoke evacuation were bothered less by electrosurgical smoke, and those respondents who used smoke evacuation less often were more likely to notice and be bothered by surgical smoke.
Once our respondents were presented with the potentially harmful effects of electrosurgical smoke, they became significantly more likely to be bothered by electrosurgical smoke and to want to work in a practice where smoke evacuation was available. This information, however, did not change respondents’ satisfaction with their work environment, and no statistically significant differences were noted between physicians and staff.
At baseline, 68.9% of respondents favored additional cost for smoke evacuation, with approximately 58% favoring the lowest cost category we presented ($1–$30). After being presented with information about the potential dangers of electrosurgical smoke, 71.5% were in favor of increased cost for smoke evacuation, which was a small but statistically significant increase.
The open-comment section of the survey provided interesting insight into the opinions of our respondents on smoke remediation. It is important to note that statistical analysis cannot be performed with these data, and firm generalizable conclusions cannot be drawn from them; however, they reveal topics that may guide further research and policy and certainly merit mention. Of 437 respondents, 108 left free-text comments. Twenty-six percent were categorized as unqualified proponents (in favor of smoke remediation) and 45% as qualified proponents (defined as an individual who verbalized a desire for smoke remediation but also cited a factor limiting their ability to use it, such as cost or staff availability). Only 12% were firmly against smoke remediation, while the remaining 17% did not comment discernibly for or against smoke remediation, indicating that a majority (71% of our comment section respondents) were in favor of some type of smoke remediation, especially if obstacles such as cost could be addressed. Only a small minority was firmly against smoke remediation.
The comments section of our survey highlighted some of the concerns that dermatologic surgeons and their staff have with electrosurgical smoke evacuation. Thirty percent cited cost as an obstacle to use of these devices, and several comments raised concern about increasing overhead and regulatory demands placed on practices. Many indicated that, without sufficient evidence of the harm caused by electrosurgical smoke, regulation that forces use of smoke remediation devices would represent a costly unfunded mandate. Others referenced the logistical challenges of smoke evacuation and the need for staff assistance. Newer smoke-evacuation wands built into cautery pens address much of this concern regarding logistical and staff challenges and further allow the evacuator tip to be located where it is most effective: 1 cm to 2 in from the point of cautery.21,22
Additionally, 12% of commenters noted that their patients were bothered by the smell of electrosurgical smoke, which is a point that requires further research and is the focus of a current randomized trial at our institution (ClinicalTrials.gov Identifier NCT02958826).
Our current study is limited in that it is a survey and therefore is subject to response bias. Further, some may assert that the hazards of electrosurgical smoke are not settled science, and although we agree with this point on some level, the study aim was not to prove risk but rather to assess current attitudes and see if awareness of a potential risk influenced those attitudes. Additionally, most responses were from physicians—only 35 responses were from nonphysician staff—so it may be difficult to generalize the findings of this study to staff. The large number of physician respondents, however, can be seen as a strength, and the findings are likely much more generalizable to providers who routinely perform clinic-based surgical procedures involving electrosurgery.
Conclusion
Our study shows that most dermatologists who perform skin surgery notice and are bothered by the smoke produced by electrosurgery to at least some extent. When presented with the possibility that inhaling electrosurgical smoke may be harmful, dermatologists were more likely to be bothered by electrosurgical smoke, more likely to prefer a practice environment where smoke evacuation was available, and more likely to be willing to bear additional cost for smoke evacuation. The free-text comments on our survey highlighted that many dermatologic surgeons are proponents of smoke evacuation but have concerns about cost and potential regulatory challenges associated with smoke evacuation, especially if the potential risks are not settled science. Many logistical concerns for smoke evacuation are addressed with the use of integrated devices. More research is needed to determine the health effects of the surgical smoke we are exposed to daily and the optimal way to limit any risk.
Acknowledgment
The authors would like to thank Richard W. Madsen, PhD (Columbia, Missouri), biostatistician, for his valuable guidance in the statistical analysis of data, interpretation of results, and editorial support in finalizing the manuscript.
- Lewin J, Brauer J, Ostad A. Surgical smoke and the dermatologist. J Am Acad Dermatol. 2011;65:636-641.
- Garden JM, O’Banion MK, Shelnitz LS, et al. Papillomavirus in the vapor of carbon dioxide laser-treated verrucae. JAMA. 1988;259:1199-1202.
- Sawchuk WS, Weber PJ, Lowy DR, et al. Infectious papillomavirus in the vapor of warts treated with carbon dioxide laser or electrocoagulation: detection and protection. J Am Acad Dermatol. 1989;21:41-49.
- Baggish MS, Poiesz BJ, Joret D, et al. Presence of human immunodeficiency virus DNA in laser smoke. Lasers Surg Med. 1991;11:197-203.
- Capizzi PJ, Clay RP, Battey MJ. Microbiologic activity in laser resurfacing plume and debris. Lasers Surg Med. 1998;23:172-174.
- Sebben JE. The hazards of electrosurgery. J Am Acad Dermatol. 1987;16:869-872.
- Bigony L. Risks associated with exposure to surgical smoke plume: a review of the literature. AORN J. 2007;86:1013-1020.
- Barrett WL, Garber SM. Surgical smoke: a review of the literature. Surg Endosc. 2003;17:979-987.
- Tomita Y, Mihashi S, Nagata K, et al. Mutagenicity of smoke condensates induced by CO2-laser irradiation and electrocauterization. Mutat Res. 1981;89:145-149.
- Hollmann R, Hort CE, Kammer E, et al. Smoke in the operating theater: an unregarded source of danger. Plast Reconstr Surg. 2004;114:458-463.
- Hensman C, Baty D, Willis RG, et al. Chemical composition of smoke produced by high-frequency electrosurgery in a closed gaseous environment. An in vitro study. Surg Endosc. 1998;12:1017-1019.
- Ulmer B. The hazards of surgical smoke. AORN J. 2008;87:721-734; quiz 735-738.
- Baggish MS, Baltoyannis P, Sze E. Protection of the rat lung from the harmful effects of laser smoke. Lasers Surg Med. 1988;8:248-253.
- Baggish MS, Elbakry M. The effects of laser smoke on the lungs of rats. Am J Obstet Gynecol. 1987;156:1260-1265.
- Freitag L, Chapman GA, Sielczak M, et al. Laser smoke effect on the bronchial system. Lasers Surg Med. 1987;7:283-288.
- Gracie KW. Hazards of vaporized tissue plume. Surgical Technologist. 2001;33:20-26.
- Giordano BP. Don’t be a victim of surgical smoke. AORN J. 1996;63:520, 522.
- Dikes CN. Is it safe to allow smoke in our operating room? Todays Surg Nurse. 1999;21:15-21; quiz 38-39.
- Wu MP, Ou CS, Chen SL, et al. Complications and recommended practices for electrosurgery in laparoscopy. Am J Surg. 2000;179:67-73.
- Chapman LW, Korta DZ, Lee PK, et al. Awareness of surgical smoke risks and assessment of safety practices during electrosurgery among US dermatology residents. JAMA Dermatol. 2017;153:467-468.
- Trevor M. Presence of virus in CO2 laser plumes raises infection concern. Hosp Infect Control. 1987;14:166-167.
- Smith JP, Moss CE, Bryant CJ, et al. Evaluation of a smoke evacuator used for laser surgery. Lasers Surg Med. 1989;9:276-281.
A growing body of evidence shows that electrosurgical smoke contains both harmful chemicals as well as live material, including blood particles, bacteria, and viruses.1 Both human immunodeficiency virus and human papillomavirus have been identified in surgical smoke plumes, and bacterial colony growth has been demonstrated from electrosurgical smoke specimens, specifically Staphylococcus, Corynebacterium, and Neisseria species.2-8 Treating 1 g of tissue with electrocoagulation produces chemical by-products equivalent to burning 6 unfiltered cigarettes,9 which is twice the amount of chemical by-products produced by CO2 laser vaporization of the same quantity of tissue. It is a common misconception that electrosurgical smoke is less hazardous than smoke produced by ablative CO2 procedures.9 Many chemicals are present in electrosurgical smoke plumes, including nitriles, benzenes, carbon monoxide, hydrogen cyanide, indoles, phenols, pyridine, pyrrole, styrene, toluene, and xylene.10-12 In animal model studies of rat lungs exposed to surgical smoke, pathologic changes, including interstitial pneumonia, bronchiolitis, and emphysema, have been shown in a dose-dependent manner.1,13-16 Diseases and symptoms linked to inhalation of electrosurgical smoke in humans include anemia, eye irritation, hypoxia, dizziness, nasopharyngeal lesions, vomiting, sneezing, throat irritation, and weakness.1,8,17-19 A study of 153 dermatology residents found that more than 70% reported receiving no formal education on the hazards of electrosurgical smoke.20 Approximately 45% were unaware if they had access to smoke evacuation in rooms where electrosurgery was performed. More than 76% were concerned with the infectious risk of electrosurgical smoke, and more than 71% were concerned with its potential carcinogenic risk.20
We surveyed dermatologists who perform skin surgery as well as staff members with respect to their experiences with electrosurgical smoke and to observe any difference that information on the potential hazards of electrosurgical smoke may have on their attitudes and preferences.
Materials and Methods
Survey Instrument
We developed a REDCap survey consisting of 17 questions that was approved by the executive committees of the American College of Mohs Surgery and the American Society for Dermatologic Surgery for distribution to their dermatologist memberships. It was emailed to eligible participants using their mailing lists. Although the survey was sent directly to member physicians, it was recommended that they forward the survey to their clinical staff to complete.
After responding to an initial set of survey questions, respondents were informed that there is growing evidence of potential harms of inhalation of surgical smoke. They then were asked the same series of survey questions in light of this information.
Statistical Analysis
Statistical analysis of the survey responses was then completed, and free-text responses as a final question of the survey were assessed for themes. Preintervention responses of staff and clinicians noticing smoke and being bothered by smoke were assessed using proportions and 95% confidence interval (CI) estimates of the proportions. On most questions, respondents could answer on a scale of 1 to 10. Responses of 5 to 10 on noticing smoke and 5 to 10 on being bothered or troubled by the smoke smell were grouped for analyses. A cross-tabulation using the Bhapkar test for marginal homogeneity was used to assess if information presented on potential smoke hazards changed responses. A Cochran-Mantel-Haenszel test for ordinal responses was used to determine differences between surgeons and staff. A McNemar test was used to determine statistical significance of change in responses to cost. Statistical analysis was performed using SAS version 9.
Results
There was a total of 443 responses to our questionnaire. Two respondents answered that they did not work in an office where skin surgery was performed, and 4 respondents did not answer any questions and were therefore excluded, leaving a total of 437 responses (402 physicians and 35 staff members). A summary of the characteristics of the respondents is shown in the Table. Some respondents did not answer each question, leading to fewer than 437 answers for some questions.

Two hundred eighty-two respondents (64.5%) never or very rarely used smoke evacuation during skin surgical procedures, and only 85 (19.5%) used smoke evacuation with nearly every case. The remaining respondents sometimes used smoke evacuation (Figure 1).

Prior to being presented with the potential dangers of electrosurgical smoke and using a value of 5 to 10 to determine if respondents noticed smoke, 54.4% (95% CI, 49.5%-59.1%) did notice intraoperative smoke during procedures. Using a value of 5 to 10 to indicate if respondents were bothered or troubled by the smoke smell, 35.5% (95% CI, 31.0%-40.2%) were bothered or troubled by intraoperative smoke prior to potential hazards being presented.
Regarding acceptable increase in cost per procedure for smoke evacuation at baseline, 68.9% of respondents favored additional cost; 57.8% of respondents chose the lowest cost grouping of $1 to $30. After being presented with information about the potential harm of intraoperative smoke, the respondents in favor of additional cost increased to 71.5%, which was a small but statistically significant change (P=.0075)(Figure 2).

Respondents were sorted into groups consisting of those who never used smoke evacuation, those who used it occasionally, and those who used it with all smoke-producing procedures. The degree to which respondents noticed intraoperative smoke was strongly correlated with their use of smoke evacuation; those who never used smoke evacuation noticed the presence of smoke more, and those who always used smoke evacuation noticed it less (P=.0002). Similar trends were noted regarding if the smoke smell bothered or troubled respondents (P=.0014).
After being presented with the potential risks of electrosurgical smoke, 29 more respondents answered that they were severely bothered by electrosurgical smoke, whereas 45 fewer respondents selected that they were not bothered or troubled at all by electrosurgical smoke (Figure 3). This difference was statistically significant (P<.0001). Fifteen more respondents answered that they would be much more likely to choose to work at a practice with smoke evacuation once the potential harm of electrosurgical smoke was introduced, and 11 were somewhat more likely to choose a practice with smoke evacuation (P<.0001).

Information about the potential harm of electrosurgical smoke did not statistically significantly affect satisfaction with work environment (P=.3139)(Figure 4).

There were no statistically significant differences between surgeon and staff responses on any questions.
Comment
Developing evidence of health risks associated with electrosurgical smoke plumes has led to an increasing interest in the use of smoke protection or remediation tools during surgical procedures. High-filtration face masks and smoke-evacuation devices protect physicians, staff members, and patients, as well as improve the patient’s clinical experience.
Our study was designed to query dermatologists who perform skin surgery as well as staff members with respect to their experiences with electrosurgical smoke and to observe any difference that information on the potential hazards of electrosurgical smoke may have on their attitudes and preferences. We received 437 responses to our survey (Table). At baseline, 54.4% of respondents noticed and 35.5% were bothered or troubled by the smoke smell produced during skin electrosurgery. These data were intuitively associated in a statistically significant manner with the use of smoke evacuation for respondents; those respondents who more commonly used smoke evacuation were bothered less by electrosurgical smoke, and those respondents who used smoke evacuation less often were more likely to notice and be bothered by surgical smoke.
Once our respondents were presented with the potentially harmful effects of electrosurgical smoke, they became significantly more likely to be bothered by electrosurgical smoke and to want to work in a practice where smoke evacuation was available. This information, however, did not change respondents’ satisfaction with their work environment, and no statistically significant differences were noted between physicians and staff.
At baseline, 68.9% of respondents favored additional cost for smoke evacuation, with approximately 58% favoring the lowest cost category we presented ($1–$30). After being presented with information about the potential dangers of electrosurgical smoke, 71.5% were in favor of increased cost for smoke evacuation, which was a small but statistically significant increase.
The open-comment section of the survey provided interesting insight into the opinions of our respondents on smoke remediation. It is important to note that statistical analysis cannot be performed with these data, and firm generalizable conclusions cannot be drawn from them; however, they reveal topics that may guide further research and policy and certainly merit mention. Of 437 respondents, 108 left free-text comments. Twenty-six percent were categorized as unqualified proponents (in favor of smoke remediation) and 45% as qualified proponents (defined as an individual who verbalized a desire for smoke remediation but also cited a factor limiting their ability to use it, such as cost or staff availability). Only 12% were firmly against smoke remediation, while the remaining 17% did not comment discernibly for or against smoke remediation, indicating that a majority (71% of our comment section respondents) were in favor of some type of smoke remediation, especially if obstacles such as cost could be addressed. Only a small minority was firmly against smoke remediation.
The comments section of our survey highlighted some of the concerns that dermatologic surgeons and their staff have with electrosurgical smoke evacuation. Thirty percent cited cost as an obstacle to use of these devices, and several comments raised concern about increasing overhead and regulatory demands placed on practices. Many indicated that, without sufficient evidence of the harm caused by electrosurgical smoke, regulation that forces use of smoke remediation devices would represent a costly unfunded mandate. Others referenced the logistical challenges of smoke evacuation and the need for staff assistance. Newer smoke-evacuation wands built into cautery pens address much of this concern regarding logistical and staff challenges and further allow the evacuator tip to be located where it is most effective: 1 cm to 2 in from the point of cautery.21,22
Additionally, 12% of commenters noted that their patients were bothered by the smell of electrosurgical smoke, which is a point that requires further research and is the focus of a current randomized trial at our institution (ClinicalTrials.gov Identifier NCT02958826).
Our current study is limited in that it is a survey and therefore is subject to response bias. Further, some may assert that the hazards of electrosurgical smoke are not settled science, and although we agree with this point on some level, the study aim was not to prove risk but rather to assess current attitudes and see if awareness of a potential risk influenced those attitudes. Additionally, most responses were from physicians—only 35 responses were from nonphysician staff—so it may be difficult to generalize the findings of this study to staff. The large number of physician respondents, however, can be seen as a strength, and the findings are likely much more generalizable to providers who routinely perform clinic-based surgical procedures involving electrosurgery.
Conclusion
Our study shows that most dermatologists who perform skin surgery notice and are bothered by the smoke produced by electrosurgery to at least some extent. When presented with the possibility that inhaling electrosurgical smoke may be harmful, dermatologists were more likely to be bothered by electrosurgical smoke, more likely to prefer a practice environment where smoke evacuation was available, and more likely to be willing to bear additional cost for smoke evacuation. The free-text comments on our survey highlighted that many dermatologic surgeons are proponents of smoke evacuation but have concerns about cost and potential regulatory challenges associated with smoke evacuation, especially if the potential risks are not settled science. Many logistical concerns for smoke evacuation are addressed with the use of integrated devices. More research is needed to determine the health effects of the surgical smoke we are exposed to daily and the optimal way to limit any risk.
Acknowledgment
The authors would like to thank Richard W. Madsen, PhD (Columbia, Missouri), biostatistician, for his valuable guidance in the statistical analysis of data, interpretation of results, and editorial support in finalizing the manuscript.
A growing body of evidence shows that electrosurgical smoke contains both harmful chemicals as well as live material, including blood particles, bacteria, and viruses.1 Both human immunodeficiency virus and human papillomavirus have been identified in surgical smoke plumes, and bacterial colony growth has been demonstrated from electrosurgical smoke specimens, specifically Staphylococcus, Corynebacterium, and Neisseria species.2-8 Treating 1 g of tissue with electrocoagulation produces chemical by-products equivalent to burning 6 unfiltered cigarettes,9 which is twice the amount of chemical by-products produced by CO2 laser vaporization of the same quantity of tissue. It is a common misconception that electrosurgical smoke is less hazardous than smoke produced by ablative CO2 procedures.9 Many chemicals are present in electrosurgical smoke plumes, including nitriles, benzenes, carbon monoxide, hydrogen cyanide, indoles, phenols, pyridine, pyrrole, styrene, toluene, and xylene.10-12 In animal model studies of rat lungs exposed to surgical smoke, pathologic changes, including interstitial pneumonia, bronchiolitis, and emphysema, have been shown in a dose-dependent manner.1,13-16 Diseases and symptoms linked to inhalation of electrosurgical smoke in humans include anemia, eye irritation, hypoxia, dizziness, nasopharyngeal lesions, vomiting, sneezing, throat irritation, and weakness.1,8,17-19 A study of 153 dermatology residents found that more than 70% reported receiving no formal education on the hazards of electrosurgical smoke.20 Approximately 45% were unaware if they had access to smoke evacuation in rooms where electrosurgery was performed. More than 76% were concerned with the infectious risk of electrosurgical smoke, and more than 71% were concerned with its potential carcinogenic risk.20
We surveyed dermatologists who perform skin surgery as well as staff members with respect to their experiences with electrosurgical smoke and to observe any difference that information on the potential hazards of electrosurgical smoke may have on their attitudes and preferences.
Materials and Methods
Survey Instrument
We developed a REDCap survey consisting of 17 questions that was approved by the executive committees of the American College of Mohs Surgery and the American Society for Dermatologic Surgery for distribution to their dermatologist memberships. It was emailed to eligible participants using their mailing lists. Although the survey was sent directly to member physicians, it was recommended that they forward the survey to their clinical staff to complete.
After responding to an initial set of survey questions, respondents were informed that there is growing evidence of potential harms of inhalation of surgical smoke. They then were asked the same series of survey questions in light of this information.
Statistical Analysis
Statistical analysis of the survey responses was then completed, and free-text responses as a final question of the survey were assessed for themes. Preintervention responses of staff and clinicians noticing smoke and being bothered by smoke were assessed using proportions and 95% confidence interval (CI) estimates of the proportions. On most questions, respondents could answer on a scale of 1 to 10. Responses of 5 to 10 on noticing smoke and 5 to 10 on being bothered or troubled by the smoke smell were grouped for analyses. A cross-tabulation using the Bhapkar test for marginal homogeneity was used to assess if information presented on potential smoke hazards changed responses. A Cochran-Mantel-Haenszel test for ordinal responses was used to determine differences between surgeons and staff. A McNemar test was used to determine statistical significance of change in responses to cost. Statistical analysis was performed using SAS version 9.
Results
There was a total of 443 responses to our questionnaire. Two respondents answered that they did not work in an office where skin surgery was performed, and 4 respondents did not answer any questions and were therefore excluded, leaving a total of 437 responses (402 physicians and 35 staff members). A summary of the characteristics of the respondents is shown in the Table. Some respondents did not answer each question, leading to fewer than 437 answers for some questions.

Two hundred eighty-two respondents (64.5%) never or very rarely used smoke evacuation during skin surgical procedures, and only 85 (19.5%) used smoke evacuation with nearly every case. The remaining respondents sometimes used smoke evacuation (Figure 1).

Prior to being presented with the potential dangers of electrosurgical smoke and using a value of 5 to 10 to determine if respondents noticed smoke, 54.4% (95% CI, 49.5%-59.1%) did notice intraoperative smoke during procedures. Using a value of 5 to 10 to indicate if respondents were bothered or troubled by the smoke smell, 35.5% (95% CI, 31.0%-40.2%) were bothered or troubled by intraoperative smoke prior to potential hazards being presented.
Regarding acceptable increase in cost per procedure for smoke evacuation at baseline, 68.9% of respondents favored additional cost; 57.8% of respondents chose the lowest cost grouping of $1 to $30. After being presented with information about the potential harm of intraoperative smoke, the respondents in favor of additional cost increased to 71.5%, which was a small but statistically significant change (P=.0075)(Figure 2).

Respondents were sorted into groups consisting of those who never used smoke evacuation, those who used it occasionally, and those who used it with all smoke-producing procedures. The degree to which respondents noticed intraoperative smoke was strongly correlated with their use of smoke evacuation; those who never used smoke evacuation noticed the presence of smoke more, and those who always used smoke evacuation noticed it less (P=.0002). Similar trends were noted regarding if the smoke smell bothered or troubled respondents (P=.0014).
After being presented with the potential risks of electrosurgical smoke, 29 more respondents answered that they were severely bothered by electrosurgical smoke, whereas 45 fewer respondents selected that they were not bothered or troubled at all by electrosurgical smoke (Figure 3). This difference was statistically significant (P<.0001). Fifteen more respondents answered that they would be much more likely to choose to work at a practice with smoke evacuation once the potential harm of electrosurgical smoke was introduced, and 11 were somewhat more likely to choose a practice with smoke evacuation (P<.0001).

Information about the potential harm of electrosurgical smoke did not statistically significantly affect satisfaction with work environment (P=.3139)(Figure 4).

There were no statistically significant differences between surgeon and staff responses on any questions.
Comment
Developing evidence of health risks associated with electrosurgical smoke plumes has led to an increasing interest in the use of smoke protection or remediation tools during surgical procedures. High-filtration face masks and smoke-evacuation devices protect physicians, staff members, and patients, as well as improve the patient’s clinical experience.
Our study was designed to query dermatologists who perform skin surgery as well as staff members with respect to their experiences with electrosurgical smoke and to observe any difference that information on the potential hazards of electrosurgical smoke may have on their attitudes and preferences. We received 437 responses to our survey (Table). At baseline, 54.4% of respondents noticed and 35.5% were bothered or troubled by the smoke smell produced during skin electrosurgery. These data were intuitively associated in a statistically significant manner with the use of smoke evacuation for respondents; those respondents who more commonly used smoke evacuation were bothered less by electrosurgical smoke, and those respondents who used smoke evacuation less often were more likely to notice and be bothered by surgical smoke.
Once our respondents were presented with the potentially harmful effects of electrosurgical smoke, they became significantly more likely to be bothered by electrosurgical smoke and to want to work in a practice where smoke evacuation was available. This information, however, did not change respondents’ satisfaction with their work environment, and no statistically significant differences were noted between physicians and staff.
At baseline, 68.9% of respondents favored additional cost for smoke evacuation, with approximately 58% favoring the lowest cost category we presented ($1–$30). After being presented with information about the potential dangers of electrosurgical smoke, 71.5% were in favor of increased cost for smoke evacuation, which was a small but statistically significant increase.
The open-comment section of the survey provided interesting insight into the opinions of our respondents on smoke remediation. It is important to note that statistical analysis cannot be performed with these data, and firm generalizable conclusions cannot be drawn from them; however, they reveal topics that may guide further research and policy and certainly merit mention. Of 437 respondents, 108 left free-text comments. Twenty-six percent were categorized as unqualified proponents (in favor of smoke remediation) and 45% as qualified proponents (defined as an individual who verbalized a desire for smoke remediation but also cited a factor limiting their ability to use it, such as cost or staff availability). Only 12% were firmly against smoke remediation, while the remaining 17% did not comment discernibly for or against smoke remediation, indicating that a majority (71% of our comment section respondents) were in favor of some type of smoke remediation, especially if obstacles such as cost could be addressed. Only a small minority was firmly against smoke remediation.
The comments section of our survey highlighted some of the concerns that dermatologic surgeons and their staff have with electrosurgical smoke evacuation. Thirty percent cited cost as an obstacle to use of these devices, and several comments raised concern about increasing overhead and regulatory demands placed on practices. Many indicated that, without sufficient evidence of the harm caused by electrosurgical smoke, regulation that forces use of smoke remediation devices would represent a costly unfunded mandate. Others referenced the logistical challenges of smoke evacuation and the need for staff assistance. Newer smoke-evacuation wands built into cautery pens address much of this concern regarding logistical and staff challenges and further allow the evacuator tip to be located where it is most effective: 1 cm to 2 in from the point of cautery.21,22
Additionally, 12% of commenters noted that their patients were bothered by the smell of electrosurgical smoke, which is a point that requires further research and is the focus of a current randomized trial at our institution (ClinicalTrials.gov Identifier NCT02958826).
Our current study is limited in that it is a survey and therefore is subject to response bias. Further, some may assert that the hazards of electrosurgical smoke are not settled science, and although we agree with this point on some level, the study aim was not to prove risk but rather to assess current attitudes and see if awareness of a potential risk influenced those attitudes. Additionally, most responses were from physicians—only 35 responses were from nonphysician staff—so it may be difficult to generalize the findings of this study to staff. The large number of physician respondents, however, can be seen as a strength, and the findings are likely much more generalizable to providers who routinely perform clinic-based surgical procedures involving electrosurgery.
Conclusion
Our study shows that most dermatologists who perform skin surgery notice and are bothered by the smoke produced by electrosurgery to at least some extent. When presented with the possibility that inhaling electrosurgical smoke may be harmful, dermatologists were more likely to be bothered by electrosurgical smoke, more likely to prefer a practice environment where smoke evacuation was available, and more likely to be willing to bear additional cost for smoke evacuation. The free-text comments on our survey highlighted that many dermatologic surgeons are proponents of smoke evacuation but have concerns about cost and potential regulatory challenges associated with smoke evacuation, especially if the potential risks are not settled science. Many logistical concerns for smoke evacuation are addressed with the use of integrated devices. More research is needed to determine the health effects of the surgical smoke we are exposed to daily and the optimal way to limit any risk.
Acknowledgment
The authors would like to thank Richard W. Madsen, PhD (Columbia, Missouri), biostatistician, for his valuable guidance in the statistical analysis of data, interpretation of results, and editorial support in finalizing the manuscript.
- Lewin J, Brauer J, Ostad A. Surgical smoke and the dermatologist. J Am Acad Dermatol. 2011;65:636-641.
- Garden JM, O’Banion MK, Shelnitz LS, et al. Papillomavirus in the vapor of carbon dioxide laser-treated verrucae. JAMA. 1988;259:1199-1202.
- Sawchuk WS, Weber PJ, Lowy DR, et al. Infectious papillomavirus in the vapor of warts treated with carbon dioxide laser or electrocoagulation: detection and protection. J Am Acad Dermatol. 1989;21:41-49.
- Baggish MS, Poiesz BJ, Joret D, et al. Presence of human immunodeficiency virus DNA in laser smoke. Lasers Surg Med. 1991;11:197-203.
- Capizzi PJ, Clay RP, Battey MJ. Microbiologic activity in laser resurfacing plume and debris. Lasers Surg Med. 1998;23:172-174.
- Sebben JE. The hazards of electrosurgery. J Am Acad Dermatol. 1987;16:869-872.
- Bigony L. Risks associated with exposure to surgical smoke plume: a review of the literature. AORN J. 2007;86:1013-1020.
- Barrett WL, Garber SM. Surgical smoke: a review of the literature. Surg Endosc. 2003;17:979-987.
- Tomita Y, Mihashi S, Nagata K, et al. Mutagenicity of smoke condensates induced by CO2-laser irradiation and electrocauterization. Mutat Res. 1981;89:145-149.
- Hollmann R, Hort CE, Kammer E, et al. Smoke in the operating theater: an unregarded source of danger. Plast Reconstr Surg. 2004;114:458-463.
- Hensman C, Baty D, Willis RG, et al. Chemical composition of smoke produced by high-frequency electrosurgery in a closed gaseous environment. An in vitro study. Surg Endosc. 1998;12:1017-1019.
- Ulmer B. The hazards of surgical smoke. AORN J. 2008;87:721-734; quiz 735-738.
- Baggish MS, Baltoyannis P, Sze E. Protection of the rat lung from the harmful effects of laser smoke. Lasers Surg Med. 1988;8:248-253.
- Baggish MS, Elbakry M. The effects of laser smoke on the lungs of rats. Am J Obstet Gynecol. 1987;156:1260-1265.
- Freitag L, Chapman GA, Sielczak M, et al. Laser smoke effect on the bronchial system. Lasers Surg Med. 1987;7:283-288.
- Gracie KW. Hazards of vaporized tissue plume. Surgical Technologist. 2001;33:20-26.
- Giordano BP. Don’t be a victim of surgical smoke. AORN J. 1996;63:520, 522.
- Dikes CN. Is it safe to allow smoke in our operating room? Todays Surg Nurse. 1999;21:15-21; quiz 38-39.
- Wu MP, Ou CS, Chen SL, et al. Complications and recommended practices for electrosurgery in laparoscopy. Am J Surg. 2000;179:67-73.
- Chapman LW, Korta DZ, Lee PK, et al. Awareness of surgical smoke risks and assessment of safety practices during electrosurgery among US dermatology residents. JAMA Dermatol. 2017;153:467-468.
- Trevor M. Presence of virus in CO2 laser plumes raises infection concern. Hosp Infect Control. 1987;14:166-167.
- Smith JP, Moss CE, Bryant CJ, et al. Evaluation of a smoke evacuator used for laser surgery. Lasers Surg Med. 1989;9:276-281.
- Lewin J, Brauer J, Ostad A. Surgical smoke and the dermatologist. J Am Acad Dermatol. 2011;65:636-641.
- Garden JM, O’Banion MK, Shelnitz LS, et al. Papillomavirus in the vapor of carbon dioxide laser-treated verrucae. JAMA. 1988;259:1199-1202.
- Sawchuk WS, Weber PJ, Lowy DR, et al. Infectious papillomavirus in the vapor of warts treated with carbon dioxide laser or electrocoagulation: detection and protection. J Am Acad Dermatol. 1989;21:41-49.
- Baggish MS, Poiesz BJ, Joret D, et al. Presence of human immunodeficiency virus DNA in laser smoke. Lasers Surg Med. 1991;11:197-203.
- Capizzi PJ, Clay RP, Battey MJ. Microbiologic activity in laser resurfacing plume and debris. Lasers Surg Med. 1998;23:172-174.
- Sebben JE. The hazards of electrosurgery. J Am Acad Dermatol. 1987;16:869-872.
- Bigony L. Risks associated with exposure to surgical smoke plume: a review of the literature. AORN J. 2007;86:1013-1020.
- Barrett WL, Garber SM. Surgical smoke: a review of the literature. Surg Endosc. 2003;17:979-987.
- Tomita Y, Mihashi S, Nagata K, et al. Mutagenicity of smoke condensates induced by CO2-laser irradiation and electrocauterization. Mutat Res. 1981;89:145-149.
- Hollmann R, Hort CE, Kammer E, et al. Smoke in the operating theater: an unregarded source of danger. Plast Reconstr Surg. 2004;114:458-463.
- Hensman C, Baty D, Willis RG, et al. Chemical composition of smoke produced by high-frequency electrosurgery in a closed gaseous environment. An in vitro study. Surg Endosc. 1998;12:1017-1019.
- Ulmer B. The hazards of surgical smoke. AORN J. 2008;87:721-734; quiz 735-738.
- Baggish MS, Baltoyannis P, Sze E. Protection of the rat lung from the harmful effects of laser smoke. Lasers Surg Med. 1988;8:248-253.
- Baggish MS, Elbakry M. The effects of laser smoke on the lungs of rats. Am J Obstet Gynecol. 1987;156:1260-1265.
- Freitag L, Chapman GA, Sielczak M, et al. Laser smoke effect on the bronchial system. Lasers Surg Med. 1987;7:283-288.
- Gracie KW. Hazards of vaporized tissue plume. Surgical Technologist. 2001;33:20-26.
- Giordano BP. Don’t be a victim of surgical smoke. AORN J. 1996;63:520, 522.
- Dikes CN. Is it safe to allow smoke in our operating room? Todays Surg Nurse. 1999;21:15-21; quiz 38-39.
- Wu MP, Ou CS, Chen SL, et al. Complications and recommended practices for electrosurgery in laparoscopy. Am J Surg. 2000;179:67-73.
- Chapman LW, Korta DZ, Lee PK, et al. Awareness of surgical smoke risks and assessment of safety practices during electrosurgery among US dermatology residents. JAMA Dermatol. 2017;153:467-468.
- Trevor M. Presence of virus in CO2 laser plumes raises infection concern. Hosp Infect Control. 1987;14:166-167.
- Smith JP, Moss CE, Bryant CJ, et al. Evaluation of a smoke evacuator used for laser surgery. Lasers Surg Med. 1989;9:276-281.
Practice Points
- Growing evidence suggests that the surgical smoke plume generated during electrosurgery may be harmful if inhaled.
- Our survey indicates that this information may affect clinician and staff perceptions about exposure to electrosurgical smoke and its remediation.
Diversity and Inclusivity Are Essential to the Future of Dermatology
Over the last 5 years, there has been an important dialogue among dermatologists about diversity in our specialty that has shifted the mind-set of the dermatology community and highlighted an intent to build a diverse workforce. It is important to reflect on this effort and acknowledge the progress that has been made. Additionally, it also is important to envision what our ideal specialty will look like 10 years from now and to discuss specific ways that we can achieve that vision for the future of dermatology.
At the 2015 Annual Meeting of the American Academy of Dermatology (AAD), Bruce E. Wintroub, MD, highlighted the importance of diversity in dermatology when he presented the Clarence S. Livingood lecture.1 His discussion was followed by a call to action from Pandya et al2 in 2016, which described the lack of diversity in our specialty (the second least diverse specialty in medicine) and proposed specific steps that can be taken by individuals and organizations to address the issue. In line with this effort, the AAD’s Diversity Task Force, Diversity Mentorship Program,3 and Diversity Champion Initiative were created. The latter program enlisted dermatology residency programs across the country to select a diversity champion who would lead efforts to increase diversity in each participating department, including mentorship of underrepresented-in-medicine college and medical students. The AAD’s 2019 Diversity Champion Workshop4 (September 12–13, 2019) will be held for the first time prior to the Association of Professors of Dermatology Annual Meeting (September 13–14, 2019) in an attempt to scale up the Diversity Champion Initiative. This workshop has galvanized widespread support and will be collaboratively hosted by the AAD, Association of Professors of Dermatology, Skin of Color Society, Society for Investigative Dermatology, and Women’s Dermatologic Society.
Current diversity efforts have largely focused on increasing representation in the dermatology workforce. A publication in 2017 challenged the tenets of dermatology resident selection and advocated for holistic review of residency program applicants as one way to address the lack of diversity in dermatology.5 This viewpoint highlighted that dermatology’s traditional focus on US Medical Licensing Examination scores and Alpha Omega Alpha Honor Medical Society membership leads to bias6-8; the viewpoint proposed several ways to change the resident selection process to enhance diversity.5 A recent proposal to eliminate numerical scores on the US Medical Licensing Examination Step 1 and move to a pass/fail grading system aligns well with this viewpoint.9 Defining best practices to perform holistic reviews is an ongoing effort and challenge for many programs, one that will be discussed at the AAD’s 2019 Diversity Champion Workshop. Implementing best practices will require individual residency programs to develop review processes tailored to departmental resources and strengths. Achieving increased representation must be an active process starting with an explicit commitment to improving diversity.
Through these efforts, we are poised to improve our specialty; however, it is critical to recognize that simply increasing the number of underrepresented dermatologists is not enough to improve diversity in dermatology. What does meaningful change look like? In 10 years, we hope that, in addition to a more inclusive workforce, we will see expanded diversity efforts beyond race and ethnicity; improved cultural competence within dermatology departments and organizations that creates more inclusive places to work, learn, and practice medicine; intentional broader representation in dermatology leadership; high-quality, evidence-based, inclusive, and culturally competent education, patient care, and research; and equal and improved outcomes for all of our patients, particularly those who traditionally experience health care disparities. To this end, ensuring diversity in research and publications is paramount. Academic journals should be actively working to include articles in the literature that help us better understand health care differences, including research that examines the presentations of skin disease in a broad spectrum of study populations, as well as to spotlight and solicit content from diverse voices. Inclusion of a diverse range of participants in research based on human subjects should be a requirement for publication, which would ensure more generalizable data. Diversity in clinical trials is improving,10 but more effort should be devoted to further increasing diversity in medical research. In particular, we need to broaden the inclusivity of dermatology research efforts and outcomes data to include more patients with skin of color as well as other underrepresented groups, thus helping to improve our understanding of the differential effects of certain interventions.
We also must educate trainees and practicing dermatologists to better understand the diagnosis and management of skin diseases in all populations; to this end, it is essential to develop a culturally competent curriculum and continuing medical education on diseases of the skin and hair that affect patients with skin of color as well as cutaneous conditions that present in groups such as sexual and gender minorities.11,12 All dermatologists—not just the experts in academic skin of color and other specialty clinics—should have expertise in the dermatologic care of diverse patients.
We have made notable and important strides with regard to diversity in dermatology by beginning this conversation, identifying problems, coming up with solutions, and implementing them.13 This progress has been made relatively quickly and is commendable; however, we have more work to do before our specialty is inclusive of underrepresented-in-medicine physicians and provides excellent care to all patients.
- Wintroub BE. Dermatology: insuring the future for the patients we serve. Presented at: 73rd Annual Meeting of the American Academy of Dermatology; March 20-24, 2015; San Francisco, California.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Diversity Mentorship Program: current mentors. American Academy of Dermatology website. https://www.aad.org/members/leadership-institute/mentoring/diversity-mentorship-program-current-mentors. Accessed July 17, 2019.
- Diversity Champion Workshop. American Academy of Dermatology website. https://www.aad.org/meetings/diversity-champion-workshop. Accessed July 17, 2019.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260.
- McGaghie WC, Cohen ER, Wayne DB. Are United States Medical Licensing Exam Step 1 and 2 scores valid measures for postgraduate medical residency selection decisions? Acad Med. 2011;86:48-52.
- Edmond MB, Deschenes JL, Eckler M, et al. Racial bias in using USMLE step 1 scores to grant internal medicine residency interviews. Acad Med. 2001;76:1253-1256.
- Boatright D, Ross D, O’Connor P, et al. Racial disparities in medical student membership in the Alpha Omega Alpha Honor Society. JAMA Intern Med. 2017;177:659-665.
- The conversation continues: exploring possible changes to USMLE score reporting. US Medical Licensing Examination website. https://www.usmle.org/usmlescoring/. Accessed July 17, 2019.
- Charrow A, Xia FD, Joyce C, et al. Diversity in dermatology clinical trials: a systematic review. JAMA Dermatol. 2017;153:193-198.
- Vashi NA, Patzelt N, Wirya S, et al. Dermatoses caused by cultural practices: therapeutic cultural practices. J Am Acad Dermatol. 2018;79:1-16.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
Over the last 5 years, there has been an important dialogue among dermatologists about diversity in our specialty that has shifted the mind-set of the dermatology community and highlighted an intent to build a diverse workforce. It is important to reflect on this effort and acknowledge the progress that has been made. Additionally, it also is important to envision what our ideal specialty will look like 10 years from now and to discuss specific ways that we can achieve that vision for the future of dermatology.
At the 2015 Annual Meeting of the American Academy of Dermatology (AAD), Bruce E. Wintroub, MD, highlighted the importance of diversity in dermatology when he presented the Clarence S. Livingood lecture.1 His discussion was followed by a call to action from Pandya et al2 in 2016, which described the lack of diversity in our specialty (the second least diverse specialty in medicine) and proposed specific steps that can be taken by individuals and organizations to address the issue. In line with this effort, the AAD’s Diversity Task Force, Diversity Mentorship Program,3 and Diversity Champion Initiative were created. The latter program enlisted dermatology residency programs across the country to select a diversity champion who would lead efforts to increase diversity in each participating department, including mentorship of underrepresented-in-medicine college and medical students. The AAD’s 2019 Diversity Champion Workshop4 (September 12–13, 2019) will be held for the first time prior to the Association of Professors of Dermatology Annual Meeting (September 13–14, 2019) in an attempt to scale up the Diversity Champion Initiative. This workshop has galvanized widespread support and will be collaboratively hosted by the AAD, Association of Professors of Dermatology, Skin of Color Society, Society for Investigative Dermatology, and Women’s Dermatologic Society.
Current diversity efforts have largely focused on increasing representation in the dermatology workforce. A publication in 2017 challenged the tenets of dermatology resident selection and advocated for holistic review of residency program applicants as one way to address the lack of diversity in dermatology.5 This viewpoint highlighted that dermatology’s traditional focus on US Medical Licensing Examination scores and Alpha Omega Alpha Honor Medical Society membership leads to bias6-8; the viewpoint proposed several ways to change the resident selection process to enhance diversity.5 A recent proposal to eliminate numerical scores on the US Medical Licensing Examination Step 1 and move to a pass/fail grading system aligns well with this viewpoint.9 Defining best practices to perform holistic reviews is an ongoing effort and challenge for many programs, one that will be discussed at the AAD’s 2019 Diversity Champion Workshop. Implementing best practices will require individual residency programs to develop review processes tailored to departmental resources and strengths. Achieving increased representation must be an active process starting with an explicit commitment to improving diversity.
Through these efforts, we are poised to improve our specialty; however, it is critical to recognize that simply increasing the number of underrepresented dermatologists is not enough to improve diversity in dermatology. What does meaningful change look like? In 10 years, we hope that, in addition to a more inclusive workforce, we will see expanded diversity efforts beyond race and ethnicity; improved cultural competence within dermatology departments and organizations that creates more inclusive places to work, learn, and practice medicine; intentional broader representation in dermatology leadership; high-quality, evidence-based, inclusive, and culturally competent education, patient care, and research; and equal and improved outcomes for all of our patients, particularly those who traditionally experience health care disparities. To this end, ensuring diversity in research and publications is paramount. Academic journals should be actively working to include articles in the literature that help us better understand health care differences, including research that examines the presentations of skin disease in a broad spectrum of study populations, as well as to spotlight and solicit content from diverse voices. Inclusion of a diverse range of participants in research based on human subjects should be a requirement for publication, which would ensure more generalizable data. Diversity in clinical trials is improving,10 but more effort should be devoted to further increasing diversity in medical research. In particular, we need to broaden the inclusivity of dermatology research efforts and outcomes data to include more patients with skin of color as well as other underrepresented groups, thus helping to improve our understanding of the differential effects of certain interventions.
We also must educate trainees and practicing dermatologists to better understand the diagnosis and management of skin diseases in all populations; to this end, it is essential to develop a culturally competent curriculum and continuing medical education on diseases of the skin and hair that affect patients with skin of color as well as cutaneous conditions that present in groups such as sexual and gender minorities.11,12 All dermatologists—not just the experts in academic skin of color and other specialty clinics—should have expertise in the dermatologic care of diverse patients.
We have made notable and important strides with regard to diversity in dermatology by beginning this conversation, identifying problems, coming up with solutions, and implementing them.13 This progress has been made relatively quickly and is commendable; however, we have more work to do before our specialty is inclusive of underrepresented-in-medicine physicians and provides excellent care to all patients.
Over the last 5 years, there has been an important dialogue among dermatologists about diversity in our specialty that has shifted the mind-set of the dermatology community and highlighted an intent to build a diverse workforce. It is important to reflect on this effort and acknowledge the progress that has been made. Additionally, it also is important to envision what our ideal specialty will look like 10 years from now and to discuss specific ways that we can achieve that vision for the future of dermatology.
At the 2015 Annual Meeting of the American Academy of Dermatology (AAD), Bruce E. Wintroub, MD, highlighted the importance of diversity in dermatology when he presented the Clarence S. Livingood lecture.1 His discussion was followed by a call to action from Pandya et al2 in 2016, which described the lack of diversity in our specialty (the second least diverse specialty in medicine) and proposed specific steps that can be taken by individuals and organizations to address the issue. In line with this effort, the AAD’s Diversity Task Force, Diversity Mentorship Program,3 and Diversity Champion Initiative were created. The latter program enlisted dermatology residency programs across the country to select a diversity champion who would lead efforts to increase diversity in each participating department, including mentorship of underrepresented-in-medicine college and medical students. The AAD’s 2019 Diversity Champion Workshop4 (September 12–13, 2019) will be held for the first time prior to the Association of Professors of Dermatology Annual Meeting (September 13–14, 2019) in an attempt to scale up the Diversity Champion Initiative. This workshop has galvanized widespread support and will be collaboratively hosted by the AAD, Association of Professors of Dermatology, Skin of Color Society, Society for Investigative Dermatology, and Women’s Dermatologic Society.
Current diversity efforts have largely focused on increasing representation in the dermatology workforce. A publication in 2017 challenged the tenets of dermatology resident selection and advocated for holistic review of residency program applicants as one way to address the lack of diversity in dermatology.5 This viewpoint highlighted that dermatology’s traditional focus on US Medical Licensing Examination scores and Alpha Omega Alpha Honor Medical Society membership leads to bias6-8; the viewpoint proposed several ways to change the resident selection process to enhance diversity.5 A recent proposal to eliminate numerical scores on the US Medical Licensing Examination Step 1 and move to a pass/fail grading system aligns well with this viewpoint.9 Defining best practices to perform holistic reviews is an ongoing effort and challenge for many programs, one that will be discussed at the AAD’s 2019 Diversity Champion Workshop. Implementing best practices will require individual residency programs to develop review processes tailored to departmental resources and strengths. Achieving increased representation must be an active process starting with an explicit commitment to improving diversity.
Through these efforts, we are poised to improve our specialty; however, it is critical to recognize that simply increasing the number of underrepresented dermatologists is not enough to improve diversity in dermatology. What does meaningful change look like? In 10 years, we hope that, in addition to a more inclusive workforce, we will see expanded diversity efforts beyond race and ethnicity; improved cultural competence within dermatology departments and organizations that creates more inclusive places to work, learn, and practice medicine; intentional broader representation in dermatology leadership; high-quality, evidence-based, inclusive, and culturally competent education, patient care, and research; and equal and improved outcomes for all of our patients, particularly those who traditionally experience health care disparities. To this end, ensuring diversity in research and publications is paramount. Academic journals should be actively working to include articles in the literature that help us better understand health care differences, including research that examines the presentations of skin disease in a broad spectrum of study populations, as well as to spotlight and solicit content from diverse voices. Inclusion of a diverse range of participants in research based on human subjects should be a requirement for publication, which would ensure more generalizable data. Diversity in clinical trials is improving,10 but more effort should be devoted to further increasing diversity in medical research. In particular, we need to broaden the inclusivity of dermatology research efforts and outcomes data to include more patients with skin of color as well as other underrepresented groups, thus helping to improve our understanding of the differential effects of certain interventions.
We also must educate trainees and practicing dermatologists to better understand the diagnosis and management of skin diseases in all populations; to this end, it is essential to develop a culturally competent curriculum and continuing medical education on diseases of the skin and hair that affect patients with skin of color as well as cutaneous conditions that present in groups such as sexual and gender minorities.11,12 All dermatologists—not just the experts in academic skin of color and other specialty clinics—should have expertise in the dermatologic care of diverse patients.
We have made notable and important strides with regard to diversity in dermatology by beginning this conversation, identifying problems, coming up with solutions, and implementing them.13 This progress has been made relatively quickly and is commendable; however, we have more work to do before our specialty is inclusive of underrepresented-in-medicine physicians and provides excellent care to all patients.
- Wintroub BE. Dermatology: insuring the future for the patients we serve. Presented at: 73rd Annual Meeting of the American Academy of Dermatology; March 20-24, 2015; San Francisco, California.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Diversity Mentorship Program: current mentors. American Academy of Dermatology website. https://www.aad.org/members/leadership-institute/mentoring/diversity-mentorship-program-current-mentors. Accessed July 17, 2019.
- Diversity Champion Workshop. American Academy of Dermatology website. https://www.aad.org/meetings/diversity-champion-workshop. Accessed July 17, 2019.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260.
- McGaghie WC, Cohen ER, Wayne DB. Are United States Medical Licensing Exam Step 1 and 2 scores valid measures for postgraduate medical residency selection decisions? Acad Med. 2011;86:48-52.
- Edmond MB, Deschenes JL, Eckler M, et al. Racial bias in using USMLE step 1 scores to grant internal medicine residency interviews. Acad Med. 2001;76:1253-1256.
- Boatright D, Ross D, O’Connor P, et al. Racial disparities in medical student membership in the Alpha Omega Alpha Honor Society. JAMA Intern Med. 2017;177:659-665.
- The conversation continues: exploring possible changes to USMLE score reporting. US Medical Licensing Examination website. https://www.usmle.org/usmlescoring/. Accessed July 17, 2019.
- Charrow A, Xia FD, Joyce C, et al. Diversity in dermatology clinical trials: a systematic review. JAMA Dermatol. 2017;153:193-198.
- Vashi NA, Patzelt N, Wirya S, et al. Dermatoses caused by cultural practices: therapeutic cultural practices. J Am Acad Dermatol. 2018;79:1-16.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Wintroub BE. Dermatology: insuring the future for the patients we serve. Presented at: 73rd Annual Meeting of the American Academy of Dermatology; March 20-24, 2015; San Francisco, California.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Diversity Mentorship Program: current mentors. American Academy of Dermatology website. https://www.aad.org/members/leadership-institute/mentoring/diversity-mentorship-program-current-mentors. Accessed July 17, 2019.
- Diversity Champion Workshop. American Academy of Dermatology website. https://www.aad.org/meetings/diversity-champion-workshop. Accessed July 17, 2019.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260.
- McGaghie WC, Cohen ER, Wayne DB. Are United States Medical Licensing Exam Step 1 and 2 scores valid measures for postgraduate medical residency selection decisions? Acad Med. 2011;86:48-52.
- Edmond MB, Deschenes JL, Eckler M, et al. Racial bias in using USMLE step 1 scores to grant internal medicine residency interviews. Acad Med. 2001;76:1253-1256.
- Boatright D, Ross D, O’Connor P, et al. Racial disparities in medical student membership in the Alpha Omega Alpha Honor Society. JAMA Intern Med. 2017;177:659-665.
- The conversation continues: exploring possible changes to USMLE score reporting. US Medical Licensing Examination website. https://www.usmle.org/usmlescoring/. Accessed July 17, 2019.
- Charrow A, Xia FD, Joyce C, et al. Diversity in dermatology clinical trials: a systematic review. JAMA Dermatol. 2017;153:193-198.
- Vashi NA, Patzelt N, Wirya S, et al. Dermatoses caused by cultural practices: therapeutic cultural practices. J Am Acad Dermatol. 2018;79:1-16.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
Methylisothiazolinone and Isothiazolinone Allergy
Unless you have been living under a rock, you probably already know that the preservative methylisothiazolinone (MI) has caused an epidemic of allergic contact dermatitis (ACD) and was named the 2013 American Contact Dermatitis Society Allergen of the Year.1 Methylisothiazolinone is not new on the market, but its solo use as a preservative is relatively new. In this article, we review the emergence of MI as a common allergen, discuss North American MI patch test results, and describe common and uncommon sources of MI exposure. We also explore the related isothiazolinones, benzisothiazolinone (BIT) and octylisothiazolinone (OIT).
Background
Methylchloroisothiazolinone (MCI) and MI have been utilized as a preservative in a 3:1 ratio since the 1980s. In 2005, MI was first used alone as a preservative in personal care products in concentrations of up to 100 ppm, which represented a 25-fold increase in exposure to MI in personal care products and thus unleashed an epidemic of ACD.1 In the 2015 to 2016 cycle of the North American Contact Dermatitis Group (NACDG) patch testing results, MI was found to be positive in 13.4% of patch tested patients (N=5597) and also had the highest significance-prevalence index number, a calculation that represents the relevance of positive reactions in relationship to prevalence.2 In Europe, MI is banned in leave-on products and is allowed in rinse-off products in concentrations of up to 15 ppm. In the United States, the Cosmetic Ingredient Review panel concluded that MI is safe at a maximum concentration up to 100 ppm in rinse-off products and safe in leave-on products when formulated to be nonsensitizing, which may be determined based on a quantitative risk assessment.3
It is recommended that MI be patch tested at a concentration of 2000 ppm (0.2% aqueous).4 Testing at lower concentrations may result in missed positives. In addition, it should be noted that MCI/MI is present in the T.R.U.E. Test (SmartPractice), but MI alone is not.
Sources of MI Exposure
The first few case reports of MI contact allergy were associated with occupational exposures. In 2004, Isaksson et al5 reported 2 cases of MI allergy following exposure to wallpaper glue and a chemical burn from a biocide, respectively. Soon after, Thyssen et al6 reported 4 occupational cases of MI allergy at a paint manufacturing plant.
An early case series of MI contact allergy associated with personal care products was published in 2010 in which the authors described adults with ACD from wet wipes and a makeup remover that contained MI.7 A more recent report indicated that MI is now an infrequent ingredient in wet wipes but is still found in a wide variety of household and personal care products.8 A 2017 query of the American Contact Dermatitis Society’s Contact Allergy Management Program (CAMP) database revealed that 12.9% of all products contained MI. Furthermore, CAMP data revealed that MI was the most commonly found preservative in both hair care and household products.9 An additional CAMP database study revealed that 53% of shampoos and 45% of conditioners contained MI, and it also was commonly found in hair dyes, soaps and cleansers, hand cleaners and sanitizers, vaginal hygiene products, sunscreens, and moisturizers.10
Household products represent an important source of MI exposure. A chemical analysis of water-based paints identified the presence of isothiazolinones. Contact allergy from isothiazolinones in paint can present as either direct or airborne-pattern contact dermatitis.11 Sodium bisulfite has been used to inactivate MCI/MI in wall paint and could be utilized in severe cases of airborne contact dermatitis.12 Off-gassing may take up to 5.5 weeks before the paint cures and the isothiazolinone level decreases.13 A 2016 analysis of household products in the CAMP database revealed that MI commonly was found in dishwashing soap (64%), followed by household cleaners (47%), laundry softeners/additives (30%), surface disinfectants (27%), and laundry detergents (13%).10 Because certain chemical ingredients are not always listed on household product labels, patients with MI contact allergy may be at higher risk for unanticipated exposure to this allergen.
Dear reader, we know that you know all of this. We know that you have been watching the MI epidemic and have followed its every turn. But something that may be new to you are the unique MI exposures identified over the last several years.
In 2017, MI was identified in the glue used to affix 3 layers of the upper portion of a shoe.14 In addition, a recent chemical analysis of US consumer adhesives confirmed the presence of isothiazolinones in 50% (19/38) of products; 44.7% (17/38) specifically contained MI.15 Slime, the sticky play substance that children concoct out of household materials, has caused ACD, and not surprisingly, MI has been identified as a culprit allergen. In one case report, contact allergy was caused by MI present in a slime mixture made up of laundry detergent, dish soap, shampoo, and hand cream.16 In another case series, 3 children with MI contact allergy had played with slime that included dishwashing liquid, which contained MI, along with polyvinyl acetate glue and liquid soap components.17 Another case report documented slime made from MI-containing school glue as the source of ACD.18 Isothiazolinones also have been identified as causative allergens in “noise putty,” another homemade play item.19
Additionally, there has been a report of contact allergy to MI in a designer eyeglass frame.20 There also have been several documented cases of ACD to MCI/MI aerosolized from water used during ironing.21,22
There also have been several reports of photoaggravated ACD and possible photoallergic contact dermatitis from MI.23,24 In such cases, patients also may have transient photosensitivity even when MI exposure is discontinued; therefore, MI should be considered for inclusion in photopatch test panels when relevant.
Methylisothiazolinone contact allergy also should be considered for products that do not list MI on the label, which presents another potential exposure. In products that do not list MI as an ingredient on the label, its presence may be due to inclusion of the preservative in raw materials used in production. For example, a patient who reacted to a facial mask gel had a positive patch test reaction to MI, the facial mask gel, and sodium hyaluronate, the raw ingredient in the gel. Further analysis revealed that MI was unexpectedly present in the sodium hyaluronate.25 Similar scenarios have been reported in association with facial wet wipes,26 an exfoliating facial sponge,27 and a polyurethane sponge from a wound vacuum pump,28 among others.
Other Isothiazolinones
Other isothiazolinones also are known to cause ACD, albeit less commonly than MI. Benzisothiazolinone has been identified in glues, cleaning agents, paints, and industrial chemicals; unlike MI, the presence of BIT is infrequent in personal care products.15,29 This chemical is not commonly included in patch test screening series in the United States but is currently present in the NACDG screening series as BIT 0.1% in petrolatum.
Octylisothiazolinone (OIT) has been reported in leather furniture, belts, shoes, and watchbands, as well as industrial chemicals.30,31 Similar to BIT, OIT is not commonly tested in screening series in the United States; the NACDG tests this chemical as OIT 0.025% in petrolatum.
The cross-reaction patterns between the isothiazolinones remain uncertain. A study in mice supported cross-reactivity between MI, OIT, and BIT32; however, several clinical epidemiologic studies suggested that although there is evidence that there may be cross-reactivity between OIT and MI, concomitant positive BIT and MI reactions more likely represent cosensitization.33-35
Final Interpretation
Methylisothiazolinone continues to have high positive patch test rates in North American patch test populations and should be tested at a concentration of 2000 ppm (0.2% aqueous). Methylisothiazolinone may now be rare in wet wipes, but it is still present in numerous personal care products including hair care products, liquid soaps, and cleaning products. Novel exposures to MI include paint, slime, and glues. It also is important to remember that MI can cause photoaggravated or photoallergic contact dermatitis and might be a worthy addition to photopatch test trays. Finally, keep a look out for BIT and OIT, which may be present in industrial chemicals, glues, paints, cleaning products, and leather items
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis. 2013;24:2-6. 2. DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Cosmetic Ingredient Review. Amended safety assessment of methylisothiazolinone as used in cosmetics. https://www.cir-safety.org/sites/default/files/mthiaz092014FR_final.pdf. Released October 8, 2014. Accessed July 9, 2019.
- Isaksson M, Ale I, Andersen KE, et al. Multicenter patch testing with methylisothiazolinone and methylchloroisothiazolinone/methylisothiazolinone within the International Contact Dermatitis Research Group. Dermatitis. 2017;28:210-214.
- Isaksson M, Gruvberger B, Bruze M. Occupational contact allergy and dermatitis from methylisothiazolinone after contact with wallcovering glue and after a chemical burn from a biocide. Dermatitis. 2004;15:201-205.
- Thyssen JP, Sederberg-Olsen N, Thomsen JF, et al. Contact dermatitis from methylisothiazolinone in a paint factory. Contact Dermatitis. 2006;54:322-324.
- García-Gavín J, Vansina S, Kerre S, et al. Methylisothiazolinone, an emerging allergen in cosmetics? Contact Dermatitis. 2010;63:96-101.
- Hamann CR, Sahni S, Zug KA. Methylisothiazolinone: still on leave-on products, but no longer on baby wipes. Dermatitis. 2019;30:173-174.
- Beene KM, Scheman A, Severson D, et al. Prevalence of preservatives across all product types in the Contact Allergen Management Program. Dermatitis. 2017;28:81-87.
- Scheman A, Severson D. American Contact Dermatitis Society Contact Allergy Management Program: an epidemiologic tool to quantify ingredient usage. Dermatitis. 2016;27:11-13.
- Goodier MC, Siegel PD, Zang LY, et al. Isothiazolinone in residential interior wall paint: a high-performance liquid chromatographic-mass spectrometry analysis. Dermatitis. 2018;29:332-338.
- Bohn S, Niederer M, Brehm K, et al. Airborne contact dermatitis from methylchloroisothiazolinone in wall paint. abolition of symptoms by chemical allergen inactivation. Contact Dermatitis. 2000;42:196-201.
- Amsler E, Aerts O, Raison-Peyron N, et al; Dermatology Allergy Group (DAG) of the French Society of Dermatology. Airborne allergic contact dermatitis caused by isothiazolinones in water-based paints: a retrospective study of 44 cases. Contact Dermatitis. 2017;77:163-170.
- Silva CA, El-Houri RB, Christensen LP, et al. Contact allergy caused by methylisothiazolinone in shoe glue. Contact Dermatitis. 2017;77:175-176.
- Goodier MC, Zang LY, Siegel PD, et al. Isothiazolinone content of US consumer adhesives: ultrahigh-performance liquid chromatographic mass spectrometry analysis. Dermatitis. 2019;30:129-134.
- Anderson LE, Treat JR, Brod BA, et al. “Slime” contact dermatitis: case report and review of relevant allergens. Pediatr Dermatol. 2019;36:335-337.
- Salman A, Demir G, Apti O. “Slime”: a trending cause of isothiazolinone contact allergy in children. Contact Dermatitis. 2019;80:409-411.
- Zhang AJ, Boyd AH, Asch S, et al. Allergic contact dermatitis to slime: the epidemic of isothiazolinone allergy encompasses school glue. Pediatr Dermatol. 2019;36:e37-e38.
- Ducharme O, Labadie M, Briand SM, et al. Allergic contact dermatitis in a child caused by isothiazolinones in a “noise putty.” Contact Dermatitis. 2018;79:393-394.
- El-Houri RB, Christensen LP, Persson C, et al. Methylisothiazolinone in a designer spectacle frame—a surprising finding. Contact Dermatitis. 2016;75:310-312.
- Atkar R, Todd P. Four cases of allergic contact dermatitis caused by methylchloroisothiazolinone/methylisothiazolinone in ironing water. Contact Dermatitis. 2016;75:316-317.
- Hunter KJ, Shelley JC, Haworth AE. Airborne allergic contact dermatitis to methylchloroisothiazolinone/methylisothiazolinone in ironing water. Contact Dermatitis. 2008;58:183-184.
- Aerts O, Goossens A, Marguery MC, et al. Photoaggravated allergic contact dermatitis and transient photosensitivity caused by methylisothiazolinone. Contact Dermatitis. 2018;78:241-245.
- Trokoudes D, Banerjee P, Fityan A, et al. Photoaggravated contact dermatitis caused by methylisothiazolinone. Contact Dermatitis. 2017;76:303-304.
- Kerre S, Naessens T, Theunis M, et al. Facial dermatitis caused by undeclared methylisothiazolinone in a gel mask: is the preservation of raw materials in cosmetics a cause of concern? Contact Dermatitis. 2018;78:421-424.
- Isaksson M, Persson L. ‘Mislabelled’ make-up remover wet wipes as a cause of severe, recalcitrant facial eczema [published online March 27, 2015]. Contact Dermatitis. 2015;73:56-59.
- Madsen JT, Andersen KE, Nielsen DT, et al. Undisclosed presence of methylisothiazolinone in ‘100% natural’ Konjac® sponge. Contact Dermatitis. 2016;75:308-309.
- Schliemann S, Isaksson M, Persson C, et al. Allergic contact dermatitis caused by methylchloroisothiazolinone/methylisothiazolinone in a medical device. Contact Dermatitis. 2016;75:312-314.
- Kaur-Knudsen D, Menné T, Christina Carlsen B. Systemic allergic dermatitis following airborne exposure to 1,2-benzisothiazolin-3-one. Contact Dermatitis. 2012;67:310-312.
- Aerts O, Meert H, Romaen E, et al. Octylisothiazolinone, an additional cause of allergic contact dermatitis caused by leather: case series and potential implications for the study of cross-reactivity with methylisothiazolinone. Contact Dermatitis. 2016;75:276-284.
- Alipour Tehrany Y, Quenan S, Bugey A, et al. Allergic contact dermatitis caused by octylisothiazolinone in a leather sofa. Contact Dermatitis. 2018;79:188-189.
- Schwensen JF, Menné Bonefeld C, Zachariae C, et al. Cross-reactivity between methylisothiazolinone, octylisothiazolinone and benzisothiazolinone using a modified local lymph node assay. Br J Dermatol. 2017;176:176-183.
- Aalto-Korte K, Suuronen K. Patterns of concomitant allergic reactions in patients suggest cross-sensitization between octylisothiazolinone and methylisothiazolinone. Contact Dermatitis. 2017;77:385-389.
- Craig S, Urwin R, Latheef F, et al. Patch test clinic experience of potential cross-reactivity of isothiazolinones. Contact Dermatitis. 2017;76:299-300.
- Geier J, Lessmann H, Schnuch A, et al. Concomitant reactivity to methylisothiazolinone, benzisothiazolinone, and octylisothiazolinone. International Network of Departments of Dermatology data, 2009-2013. Contact Dermatitis. 2015;72:337-339.
Unless you have been living under a rock, you probably already know that the preservative methylisothiazolinone (MI) has caused an epidemic of allergic contact dermatitis (ACD) and was named the 2013 American Contact Dermatitis Society Allergen of the Year.1 Methylisothiazolinone is not new on the market, but its solo use as a preservative is relatively new. In this article, we review the emergence of MI as a common allergen, discuss North American MI patch test results, and describe common and uncommon sources of MI exposure. We also explore the related isothiazolinones, benzisothiazolinone (BIT) and octylisothiazolinone (OIT).
Background
Methylchloroisothiazolinone (MCI) and MI have been utilized as a preservative in a 3:1 ratio since the 1980s. In 2005, MI was first used alone as a preservative in personal care products in concentrations of up to 100 ppm, which represented a 25-fold increase in exposure to MI in personal care products and thus unleashed an epidemic of ACD.1 In the 2015 to 2016 cycle of the North American Contact Dermatitis Group (NACDG) patch testing results, MI was found to be positive in 13.4% of patch tested patients (N=5597) and also had the highest significance-prevalence index number, a calculation that represents the relevance of positive reactions in relationship to prevalence.2 In Europe, MI is banned in leave-on products and is allowed in rinse-off products in concentrations of up to 15 ppm. In the United States, the Cosmetic Ingredient Review panel concluded that MI is safe at a maximum concentration up to 100 ppm in rinse-off products and safe in leave-on products when formulated to be nonsensitizing, which may be determined based on a quantitative risk assessment.3
It is recommended that MI be patch tested at a concentration of 2000 ppm (0.2% aqueous).4 Testing at lower concentrations may result in missed positives. In addition, it should be noted that MCI/MI is present in the T.R.U.E. Test (SmartPractice), but MI alone is not.
Sources of MI Exposure
The first few case reports of MI contact allergy were associated with occupational exposures. In 2004, Isaksson et al5 reported 2 cases of MI allergy following exposure to wallpaper glue and a chemical burn from a biocide, respectively. Soon after, Thyssen et al6 reported 4 occupational cases of MI allergy at a paint manufacturing plant.
An early case series of MI contact allergy associated with personal care products was published in 2010 in which the authors described adults with ACD from wet wipes and a makeup remover that contained MI.7 A more recent report indicated that MI is now an infrequent ingredient in wet wipes but is still found in a wide variety of household and personal care products.8 A 2017 query of the American Contact Dermatitis Society’s Contact Allergy Management Program (CAMP) database revealed that 12.9% of all products contained MI. Furthermore, CAMP data revealed that MI was the most commonly found preservative in both hair care and household products.9 An additional CAMP database study revealed that 53% of shampoos and 45% of conditioners contained MI, and it also was commonly found in hair dyes, soaps and cleansers, hand cleaners and sanitizers, vaginal hygiene products, sunscreens, and moisturizers.10
Household products represent an important source of MI exposure. A chemical analysis of water-based paints identified the presence of isothiazolinones. Contact allergy from isothiazolinones in paint can present as either direct or airborne-pattern contact dermatitis.11 Sodium bisulfite has been used to inactivate MCI/MI in wall paint and could be utilized in severe cases of airborne contact dermatitis.12 Off-gassing may take up to 5.5 weeks before the paint cures and the isothiazolinone level decreases.13 A 2016 analysis of household products in the CAMP database revealed that MI commonly was found in dishwashing soap (64%), followed by household cleaners (47%), laundry softeners/additives (30%), surface disinfectants (27%), and laundry detergents (13%).10 Because certain chemical ingredients are not always listed on household product labels, patients with MI contact allergy may be at higher risk for unanticipated exposure to this allergen.
Dear reader, we know that you know all of this. We know that you have been watching the MI epidemic and have followed its every turn. But something that may be new to you are the unique MI exposures identified over the last several years.
In 2017, MI was identified in the glue used to affix 3 layers of the upper portion of a shoe.14 In addition, a recent chemical analysis of US consumer adhesives confirmed the presence of isothiazolinones in 50% (19/38) of products; 44.7% (17/38) specifically contained MI.15 Slime, the sticky play substance that children concoct out of household materials, has caused ACD, and not surprisingly, MI has been identified as a culprit allergen. In one case report, contact allergy was caused by MI present in a slime mixture made up of laundry detergent, dish soap, shampoo, and hand cream.16 In another case series, 3 children with MI contact allergy had played with slime that included dishwashing liquid, which contained MI, along with polyvinyl acetate glue and liquid soap components.17 Another case report documented slime made from MI-containing school glue as the source of ACD.18 Isothiazolinones also have been identified as causative allergens in “noise putty,” another homemade play item.19
Additionally, there has been a report of contact allergy to MI in a designer eyeglass frame.20 There also have been several documented cases of ACD to MCI/MI aerosolized from water used during ironing.21,22
There also have been several reports of photoaggravated ACD and possible photoallergic contact dermatitis from MI.23,24 In such cases, patients also may have transient photosensitivity even when MI exposure is discontinued; therefore, MI should be considered for inclusion in photopatch test panels when relevant.
Methylisothiazolinone contact allergy also should be considered for products that do not list MI on the label, which presents another potential exposure. In products that do not list MI as an ingredient on the label, its presence may be due to inclusion of the preservative in raw materials used in production. For example, a patient who reacted to a facial mask gel had a positive patch test reaction to MI, the facial mask gel, and sodium hyaluronate, the raw ingredient in the gel. Further analysis revealed that MI was unexpectedly present in the sodium hyaluronate.25 Similar scenarios have been reported in association with facial wet wipes,26 an exfoliating facial sponge,27 and a polyurethane sponge from a wound vacuum pump,28 among others.
Other Isothiazolinones
Other isothiazolinones also are known to cause ACD, albeit less commonly than MI. Benzisothiazolinone has been identified in glues, cleaning agents, paints, and industrial chemicals; unlike MI, the presence of BIT is infrequent in personal care products.15,29 This chemical is not commonly included in patch test screening series in the United States but is currently present in the NACDG screening series as BIT 0.1% in petrolatum.
Octylisothiazolinone (OIT) has been reported in leather furniture, belts, shoes, and watchbands, as well as industrial chemicals.30,31 Similar to BIT, OIT is not commonly tested in screening series in the United States; the NACDG tests this chemical as OIT 0.025% in petrolatum.
The cross-reaction patterns between the isothiazolinones remain uncertain. A study in mice supported cross-reactivity between MI, OIT, and BIT32; however, several clinical epidemiologic studies suggested that although there is evidence that there may be cross-reactivity between OIT and MI, concomitant positive BIT and MI reactions more likely represent cosensitization.33-35
Final Interpretation
Methylisothiazolinone continues to have high positive patch test rates in North American patch test populations and should be tested at a concentration of 2000 ppm (0.2% aqueous). Methylisothiazolinone may now be rare in wet wipes, but it is still present in numerous personal care products including hair care products, liquid soaps, and cleaning products. Novel exposures to MI include paint, slime, and glues. It also is important to remember that MI can cause photoaggravated or photoallergic contact dermatitis and might be a worthy addition to photopatch test trays. Finally, keep a look out for BIT and OIT, which may be present in industrial chemicals, glues, paints, cleaning products, and leather items
Unless you have been living under a rock, you probably already know that the preservative methylisothiazolinone (MI) has caused an epidemic of allergic contact dermatitis (ACD) and was named the 2013 American Contact Dermatitis Society Allergen of the Year.1 Methylisothiazolinone is not new on the market, but its solo use as a preservative is relatively new. In this article, we review the emergence of MI as a common allergen, discuss North American MI patch test results, and describe common and uncommon sources of MI exposure. We also explore the related isothiazolinones, benzisothiazolinone (BIT) and octylisothiazolinone (OIT).
Background
Methylchloroisothiazolinone (MCI) and MI have been utilized as a preservative in a 3:1 ratio since the 1980s. In 2005, MI was first used alone as a preservative in personal care products in concentrations of up to 100 ppm, which represented a 25-fold increase in exposure to MI in personal care products and thus unleashed an epidemic of ACD.1 In the 2015 to 2016 cycle of the North American Contact Dermatitis Group (NACDG) patch testing results, MI was found to be positive in 13.4% of patch tested patients (N=5597) and also had the highest significance-prevalence index number, a calculation that represents the relevance of positive reactions in relationship to prevalence.2 In Europe, MI is banned in leave-on products and is allowed in rinse-off products in concentrations of up to 15 ppm. In the United States, the Cosmetic Ingredient Review panel concluded that MI is safe at a maximum concentration up to 100 ppm in rinse-off products and safe in leave-on products when formulated to be nonsensitizing, which may be determined based on a quantitative risk assessment.3
It is recommended that MI be patch tested at a concentration of 2000 ppm (0.2% aqueous).4 Testing at lower concentrations may result in missed positives. In addition, it should be noted that MCI/MI is present in the T.R.U.E. Test (SmartPractice), but MI alone is not.
Sources of MI Exposure
The first few case reports of MI contact allergy were associated with occupational exposures. In 2004, Isaksson et al5 reported 2 cases of MI allergy following exposure to wallpaper glue and a chemical burn from a biocide, respectively. Soon after, Thyssen et al6 reported 4 occupational cases of MI allergy at a paint manufacturing plant.
An early case series of MI contact allergy associated with personal care products was published in 2010 in which the authors described adults with ACD from wet wipes and a makeup remover that contained MI.7 A more recent report indicated that MI is now an infrequent ingredient in wet wipes but is still found in a wide variety of household and personal care products.8 A 2017 query of the American Contact Dermatitis Society’s Contact Allergy Management Program (CAMP) database revealed that 12.9% of all products contained MI. Furthermore, CAMP data revealed that MI was the most commonly found preservative in both hair care and household products.9 An additional CAMP database study revealed that 53% of shampoos and 45% of conditioners contained MI, and it also was commonly found in hair dyes, soaps and cleansers, hand cleaners and sanitizers, vaginal hygiene products, sunscreens, and moisturizers.10
Household products represent an important source of MI exposure. A chemical analysis of water-based paints identified the presence of isothiazolinones. Contact allergy from isothiazolinones in paint can present as either direct or airborne-pattern contact dermatitis.11 Sodium bisulfite has been used to inactivate MCI/MI in wall paint and could be utilized in severe cases of airborne contact dermatitis.12 Off-gassing may take up to 5.5 weeks before the paint cures and the isothiazolinone level decreases.13 A 2016 analysis of household products in the CAMP database revealed that MI commonly was found in dishwashing soap (64%), followed by household cleaners (47%), laundry softeners/additives (30%), surface disinfectants (27%), and laundry detergents (13%).10 Because certain chemical ingredients are not always listed on household product labels, patients with MI contact allergy may be at higher risk for unanticipated exposure to this allergen.
Dear reader, we know that you know all of this. We know that you have been watching the MI epidemic and have followed its every turn. But something that may be new to you are the unique MI exposures identified over the last several years.
In 2017, MI was identified in the glue used to affix 3 layers of the upper portion of a shoe.14 In addition, a recent chemical analysis of US consumer adhesives confirmed the presence of isothiazolinones in 50% (19/38) of products; 44.7% (17/38) specifically contained MI.15 Slime, the sticky play substance that children concoct out of household materials, has caused ACD, and not surprisingly, MI has been identified as a culprit allergen. In one case report, contact allergy was caused by MI present in a slime mixture made up of laundry detergent, dish soap, shampoo, and hand cream.16 In another case series, 3 children with MI contact allergy had played with slime that included dishwashing liquid, which contained MI, along with polyvinyl acetate glue and liquid soap components.17 Another case report documented slime made from MI-containing school glue as the source of ACD.18 Isothiazolinones also have been identified as causative allergens in “noise putty,” another homemade play item.19
Additionally, there has been a report of contact allergy to MI in a designer eyeglass frame.20 There also have been several documented cases of ACD to MCI/MI aerosolized from water used during ironing.21,22
There also have been several reports of photoaggravated ACD and possible photoallergic contact dermatitis from MI.23,24 In such cases, patients also may have transient photosensitivity even when MI exposure is discontinued; therefore, MI should be considered for inclusion in photopatch test panels when relevant.
Methylisothiazolinone contact allergy also should be considered for products that do not list MI on the label, which presents another potential exposure. In products that do not list MI as an ingredient on the label, its presence may be due to inclusion of the preservative in raw materials used in production. For example, a patient who reacted to a facial mask gel had a positive patch test reaction to MI, the facial mask gel, and sodium hyaluronate, the raw ingredient in the gel. Further analysis revealed that MI was unexpectedly present in the sodium hyaluronate.25 Similar scenarios have been reported in association with facial wet wipes,26 an exfoliating facial sponge,27 and a polyurethane sponge from a wound vacuum pump,28 among others.
Other Isothiazolinones
Other isothiazolinones also are known to cause ACD, albeit less commonly than MI. Benzisothiazolinone has been identified in glues, cleaning agents, paints, and industrial chemicals; unlike MI, the presence of BIT is infrequent in personal care products.15,29 This chemical is not commonly included in patch test screening series in the United States but is currently present in the NACDG screening series as BIT 0.1% in petrolatum.
Octylisothiazolinone (OIT) has been reported in leather furniture, belts, shoes, and watchbands, as well as industrial chemicals.30,31 Similar to BIT, OIT is not commonly tested in screening series in the United States; the NACDG tests this chemical as OIT 0.025% in petrolatum.
The cross-reaction patterns between the isothiazolinones remain uncertain. A study in mice supported cross-reactivity between MI, OIT, and BIT32; however, several clinical epidemiologic studies suggested that although there is evidence that there may be cross-reactivity between OIT and MI, concomitant positive BIT and MI reactions more likely represent cosensitization.33-35
Final Interpretation
Methylisothiazolinone continues to have high positive patch test rates in North American patch test populations and should be tested at a concentration of 2000 ppm (0.2% aqueous). Methylisothiazolinone may now be rare in wet wipes, but it is still present in numerous personal care products including hair care products, liquid soaps, and cleaning products. Novel exposures to MI include paint, slime, and glues. It also is important to remember that MI can cause photoaggravated or photoallergic contact dermatitis and might be a worthy addition to photopatch test trays. Finally, keep a look out for BIT and OIT, which may be present in industrial chemicals, glues, paints, cleaning products, and leather items
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis. 2013;24:2-6. 2. DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Cosmetic Ingredient Review. Amended safety assessment of methylisothiazolinone as used in cosmetics. https://www.cir-safety.org/sites/default/files/mthiaz092014FR_final.pdf. Released October 8, 2014. Accessed July 9, 2019.
- Isaksson M, Ale I, Andersen KE, et al. Multicenter patch testing with methylisothiazolinone and methylchloroisothiazolinone/methylisothiazolinone within the International Contact Dermatitis Research Group. Dermatitis. 2017;28:210-214.
- Isaksson M, Gruvberger B, Bruze M. Occupational contact allergy and dermatitis from methylisothiazolinone after contact with wallcovering glue and after a chemical burn from a biocide. Dermatitis. 2004;15:201-205.
- Thyssen JP, Sederberg-Olsen N, Thomsen JF, et al. Contact dermatitis from methylisothiazolinone in a paint factory. Contact Dermatitis. 2006;54:322-324.
- García-Gavín J, Vansina S, Kerre S, et al. Methylisothiazolinone, an emerging allergen in cosmetics? Contact Dermatitis. 2010;63:96-101.
- Hamann CR, Sahni S, Zug KA. Methylisothiazolinone: still on leave-on products, but no longer on baby wipes. Dermatitis. 2019;30:173-174.
- Beene KM, Scheman A, Severson D, et al. Prevalence of preservatives across all product types in the Contact Allergen Management Program. Dermatitis. 2017;28:81-87.
- Scheman A, Severson D. American Contact Dermatitis Society Contact Allergy Management Program: an epidemiologic tool to quantify ingredient usage. Dermatitis. 2016;27:11-13.
- Goodier MC, Siegel PD, Zang LY, et al. Isothiazolinone in residential interior wall paint: a high-performance liquid chromatographic-mass spectrometry analysis. Dermatitis. 2018;29:332-338.
- Bohn S, Niederer M, Brehm K, et al. Airborne contact dermatitis from methylchloroisothiazolinone in wall paint. abolition of symptoms by chemical allergen inactivation. Contact Dermatitis. 2000;42:196-201.
- Amsler E, Aerts O, Raison-Peyron N, et al; Dermatology Allergy Group (DAG) of the French Society of Dermatology. Airborne allergic contact dermatitis caused by isothiazolinones in water-based paints: a retrospective study of 44 cases. Contact Dermatitis. 2017;77:163-170.
- Silva CA, El-Houri RB, Christensen LP, et al. Contact allergy caused by methylisothiazolinone in shoe glue. Contact Dermatitis. 2017;77:175-176.
- Goodier MC, Zang LY, Siegel PD, et al. Isothiazolinone content of US consumer adhesives: ultrahigh-performance liquid chromatographic mass spectrometry analysis. Dermatitis. 2019;30:129-134.
- Anderson LE, Treat JR, Brod BA, et al. “Slime” contact dermatitis: case report and review of relevant allergens. Pediatr Dermatol. 2019;36:335-337.
- Salman A, Demir G, Apti O. “Slime”: a trending cause of isothiazolinone contact allergy in children. Contact Dermatitis. 2019;80:409-411.
- Zhang AJ, Boyd AH, Asch S, et al. Allergic contact dermatitis to slime: the epidemic of isothiazolinone allergy encompasses school glue. Pediatr Dermatol. 2019;36:e37-e38.
- Ducharme O, Labadie M, Briand SM, et al. Allergic contact dermatitis in a child caused by isothiazolinones in a “noise putty.” Contact Dermatitis. 2018;79:393-394.
- El-Houri RB, Christensen LP, Persson C, et al. Methylisothiazolinone in a designer spectacle frame—a surprising finding. Contact Dermatitis. 2016;75:310-312.
- Atkar R, Todd P. Four cases of allergic contact dermatitis caused by methylchloroisothiazolinone/methylisothiazolinone in ironing water. Contact Dermatitis. 2016;75:316-317.
- Hunter KJ, Shelley JC, Haworth AE. Airborne allergic contact dermatitis to methylchloroisothiazolinone/methylisothiazolinone in ironing water. Contact Dermatitis. 2008;58:183-184.
- Aerts O, Goossens A, Marguery MC, et al. Photoaggravated allergic contact dermatitis and transient photosensitivity caused by methylisothiazolinone. Contact Dermatitis. 2018;78:241-245.
- Trokoudes D, Banerjee P, Fityan A, et al. Photoaggravated contact dermatitis caused by methylisothiazolinone. Contact Dermatitis. 2017;76:303-304.
- Kerre S, Naessens T, Theunis M, et al. Facial dermatitis caused by undeclared methylisothiazolinone in a gel mask: is the preservation of raw materials in cosmetics a cause of concern? Contact Dermatitis. 2018;78:421-424.
- Isaksson M, Persson L. ‘Mislabelled’ make-up remover wet wipes as a cause of severe, recalcitrant facial eczema [published online March 27, 2015]. Contact Dermatitis. 2015;73:56-59.
- Madsen JT, Andersen KE, Nielsen DT, et al. Undisclosed presence of methylisothiazolinone in ‘100% natural’ Konjac® sponge. Contact Dermatitis. 2016;75:308-309.
- Schliemann S, Isaksson M, Persson C, et al. Allergic contact dermatitis caused by methylchloroisothiazolinone/methylisothiazolinone in a medical device. Contact Dermatitis. 2016;75:312-314.
- Kaur-Knudsen D, Menné T, Christina Carlsen B. Systemic allergic dermatitis following airborne exposure to 1,2-benzisothiazolin-3-one. Contact Dermatitis. 2012;67:310-312.
- Aerts O, Meert H, Romaen E, et al. Octylisothiazolinone, an additional cause of allergic contact dermatitis caused by leather: case series and potential implications for the study of cross-reactivity with methylisothiazolinone. Contact Dermatitis. 2016;75:276-284.
- Alipour Tehrany Y, Quenan S, Bugey A, et al. Allergic contact dermatitis caused by octylisothiazolinone in a leather sofa. Contact Dermatitis. 2018;79:188-189.
- Schwensen JF, Menné Bonefeld C, Zachariae C, et al. Cross-reactivity between methylisothiazolinone, octylisothiazolinone and benzisothiazolinone using a modified local lymph node assay. Br J Dermatol. 2017;176:176-183.
- Aalto-Korte K, Suuronen K. Patterns of concomitant allergic reactions in patients suggest cross-sensitization between octylisothiazolinone and methylisothiazolinone. Contact Dermatitis. 2017;77:385-389.
- Craig S, Urwin R, Latheef F, et al. Patch test clinic experience of potential cross-reactivity of isothiazolinones. Contact Dermatitis. 2017;76:299-300.
- Geier J, Lessmann H, Schnuch A, et al. Concomitant reactivity to methylisothiazolinone, benzisothiazolinone, and octylisothiazolinone. International Network of Departments of Dermatology data, 2009-2013. Contact Dermatitis. 2015;72:337-339.
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis. 2013;24:2-6. 2. DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Cosmetic Ingredient Review. Amended safety assessment of methylisothiazolinone as used in cosmetics. https://www.cir-safety.org/sites/default/files/mthiaz092014FR_final.pdf. Released October 8, 2014. Accessed July 9, 2019.
- Isaksson M, Ale I, Andersen KE, et al. Multicenter patch testing with methylisothiazolinone and methylchloroisothiazolinone/methylisothiazolinone within the International Contact Dermatitis Research Group. Dermatitis. 2017;28:210-214.
- Isaksson M, Gruvberger B, Bruze M. Occupational contact allergy and dermatitis from methylisothiazolinone after contact with wallcovering glue and after a chemical burn from a biocide. Dermatitis. 2004;15:201-205.
- Thyssen JP, Sederberg-Olsen N, Thomsen JF, et al. Contact dermatitis from methylisothiazolinone in a paint factory. Contact Dermatitis. 2006;54:322-324.
- García-Gavín J, Vansina S, Kerre S, et al. Methylisothiazolinone, an emerging allergen in cosmetics? Contact Dermatitis. 2010;63:96-101.
- Hamann CR, Sahni S, Zug KA. Methylisothiazolinone: still on leave-on products, but no longer on baby wipes. Dermatitis. 2019;30:173-174.
- Beene KM, Scheman A, Severson D, et al. Prevalence of preservatives across all product types in the Contact Allergen Management Program. Dermatitis. 2017;28:81-87.
- Scheman A, Severson D. American Contact Dermatitis Society Contact Allergy Management Program: an epidemiologic tool to quantify ingredient usage. Dermatitis. 2016;27:11-13.
- Goodier MC, Siegel PD, Zang LY, et al. Isothiazolinone in residential interior wall paint: a high-performance liquid chromatographic-mass spectrometry analysis. Dermatitis. 2018;29:332-338.
- Bohn S, Niederer M, Brehm K, et al. Airborne contact dermatitis from methylchloroisothiazolinone in wall paint. abolition of symptoms by chemical allergen inactivation. Contact Dermatitis. 2000;42:196-201.
- Amsler E, Aerts O, Raison-Peyron N, et al; Dermatology Allergy Group (DAG) of the French Society of Dermatology. Airborne allergic contact dermatitis caused by isothiazolinones in water-based paints: a retrospective study of 44 cases. Contact Dermatitis. 2017;77:163-170.
- Silva CA, El-Houri RB, Christensen LP, et al. Contact allergy caused by methylisothiazolinone in shoe glue. Contact Dermatitis. 2017;77:175-176.
- Goodier MC, Zang LY, Siegel PD, et al. Isothiazolinone content of US consumer adhesives: ultrahigh-performance liquid chromatographic mass spectrometry analysis. Dermatitis. 2019;30:129-134.
- Anderson LE, Treat JR, Brod BA, et al. “Slime” contact dermatitis: case report and review of relevant allergens. Pediatr Dermatol. 2019;36:335-337.
- Salman A, Demir G, Apti O. “Slime”: a trending cause of isothiazolinone contact allergy in children. Contact Dermatitis. 2019;80:409-411.
- Zhang AJ, Boyd AH, Asch S, et al. Allergic contact dermatitis to slime: the epidemic of isothiazolinone allergy encompasses school glue. Pediatr Dermatol. 2019;36:e37-e38.
- Ducharme O, Labadie M, Briand SM, et al. Allergic contact dermatitis in a child caused by isothiazolinones in a “noise putty.” Contact Dermatitis. 2018;79:393-394.
- El-Houri RB, Christensen LP, Persson C, et al. Methylisothiazolinone in a designer spectacle frame—a surprising finding. Contact Dermatitis. 2016;75:310-312.
- Atkar R, Todd P. Four cases of allergic contact dermatitis caused by methylchloroisothiazolinone/methylisothiazolinone in ironing water. Contact Dermatitis. 2016;75:316-317.
- Hunter KJ, Shelley JC, Haworth AE. Airborne allergic contact dermatitis to methylchloroisothiazolinone/methylisothiazolinone in ironing water. Contact Dermatitis. 2008;58:183-184.
- Aerts O, Goossens A, Marguery MC, et al. Photoaggravated allergic contact dermatitis and transient photosensitivity caused by methylisothiazolinone. Contact Dermatitis. 2018;78:241-245.
- Trokoudes D, Banerjee P, Fityan A, et al. Photoaggravated contact dermatitis caused by methylisothiazolinone. Contact Dermatitis. 2017;76:303-304.
- Kerre S, Naessens T, Theunis M, et al. Facial dermatitis caused by undeclared methylisothiazolinone in a gel mask: is the preservation of raw materials in cosmetics a cause of concern? Contact Dermatitis. 2018;78:421-424.
- Isaksson M, Persson L. ‘Mislabelled’ make-up remover wet wipes as a cause of severe, recalcitrant facial eczema [published online March 27, 2015]. Contact Dermatitis. 2015;73:56-59.
- Madsen JT, Andersen KE, Nielsen DT, et al. Undisclosed presence of methylisothiazolinone in ‘100% natural’ Konjac® sponge. Contact Dermatitis. 2016;75:308-309.
- Schliemann S, Isaksson M, Persson C, et al. Allergic contact dermatitis caused by methylchloroisothiazolinone/methylisothiazolinone in a medical device. Contact Dermatitis. 2016;75:312-314.
- Kaur-Knudsen D, Menné T, Christina Carlsen B. Systemic allergic dermatitis following airborne exposure to 1,2-benzisothiazolin-3-one. Contact Dermatitis. 2012;67:310-312.
- Aerts O, Meert H, Romaen E, et al. Octylisothiazolinone, an additional cause of allergic contact dermatitis caused by leather: case series and potential implications for the study of cross-reactivity with methylisothiazolinone. Contact Dermatitis. 2016;75:276-284.
- Alipour Tehrany Y, Quenan S, Bugey A, et al. Allergic contact dermatitis caused by octylisothiazolinone in a leather sofa. Contact Dermatitis. 2018;79:188-189.
- Schwensen JF, Menné Bonefeld C, Zachariae C, et al. Cross-reactivity between methylisothiazolinone, octylisothiazolinone and benzisothiazolinone using a modified local lymph node assay. Br J Dermatol. 2017;176:176-183.
- Aalto-Korte K, Suuronen K. Patterns of concomitant allergic reactions in patients suggest cross-sensitization between octylisothiazolinone and methylisothiazolinone. Contact Dermatitis. 2017;77:385-389.
- Craig S, Urwin R, Latheef F, et al. Patch test clinic experience of potential cross-reactivity of isothiazolinones. Contact Dermatitis. 2017;76:299-300.
- Geier J, Lessmann H, Schnuch A, et al. Concomitant reactivity to methylisothiazolinone, benzisothiazolinone, and octylisothiazolinone. International Network of Departments of Dermatology data, 2009-2013. Contact Dermatitis. 2015;72:337-339.
Practice Points
- Methylisothiazolinone (MI) is a preservative found in water-based personal care products and is a common allergen in patch-tested populations.
- Methylisothiazolinone also has been identified in household products, industrial chemicals, paint, adhesives, and other unique sources.
- Benzisothiazolinone and octylisothiazolinone are structurally similar to MI, and a subset of MI-allergic patients may need to avoid them.
The changing landscape of medical education
A brave new world
It’s Monday morning, and your intern is presenting an overnight admission. Lost in the details of his disorganized introduction, your mind wanders. “Why doesn’t this intern know how to present? When I trained, all those admissions during long sleepless nights really taught me to do this right.” But can we equate hours worked with competency achieved? And if not, what is the alternative? This article introduces some major changes in medical education and their implications for hospitalists.
Most hospitalists trained in an educational system influenced by Sir William Osler. In the early 1900s, he introduced the natural method of teaching, positing that student exposure to patients and experience over time ensured that physicians in training would become competent doctors.1 His influence led to the current structure of medical education, which includes conventional third-year clerkships and time-limited rotations (such as a 2-week nephrology block).
While familiarity may be comforting, there are signs our current model of medical education is inefficient, inadequate, and obsolete.
For one, the traditional system is failing to adequately prepare physicians to provide safe and complex care. Reports, such as the Institute of Medicine’s (IOM) “To Err is Human,”2 describe a high rate of preventable errors, highlighting considerable room for improvement in training the next generation of physicians.3,4
Meanwhile, trainees are still largely being deemed ready for the workforce by length of training completed (for example, completion of four-year medical school) rather than a skill set distinctly achieved. Our system leaves little flexibility to individualize learner goals, which is significant given some students and residents take shorter or longer periods of time to achieve proficiency. In addition, learner outcomes can be quite variable, as we have all experienced.
Even our methods of assessment may not adequately evaluate trainees’ skill sets. For example, most clerkships still rely heavily on the shelf exam5 as a surrogate for medical knowledge. As such, learners may conclude that testing performance trumps development of other professional skills.6 Efforts are being made to revamp evaluation systems to reflect mastery (such as Entrustable Professional Activities, or EPAs) toward competencies.7 Still, many institutions continue to rely on faculty evaluations that often reflect interpersonal dynamics rather than true critical thinking skills.6
Recognizing the above limitations, many educators have called for changing to outcome-based, or competency-based, training (CBME). CBME targets attainment of skills in performing concrete critical clinical activities,8 such as identifying unstable patients, providing initial management, and obtaining help. To be successful, supervisors must directly observe trainees, assess demonstrated skills, and provide feedback about progress.
Unfortunately, this considerable investment of time and effort is often poorly compensated. Additionally, unanswered questions remain. For example, how will residency programs continue to challenge physicians deemed “competent” in a required skill? What happens when a trainee is deficient and not appropriately progressing in a required skill? Is flexible training time part of the future of medical education? While CBME appears to be a more effective method of education, questions like these must be addressed during implementation.
Beyond the fact that hours worked cannot be used as a surrogate for competency, excessive unregulated work hours can be detrimental to learners, their supervisors, and patients. In 2003, the Accreditation Council for Graduate Medical Education (ACGME) implemented a major change in medical education: duty hour limitations. The premise that sleep-deprived providers are more prone to error is well established. However, controversy remains as to whether these regulations translate into improved patient care and provider well-being. Studies published following the ACGME change demonstrate increasing burnout among physicians,9-11 which has led some educators to explore the potential relationship between burnout and duty hour restrictions.
The recent “iCOMPARE” trial, which compared internal medicine (IM) residencies with “standard duty-hour” policies to those with “flexible” policies (that is, they did not specify limits on shift length or mandatory time off between shifts), supported a lack of correlation between hours worked and burnout.12 Researchers administered the Maslach Burnout Inventory to all participants.13 While those in the “flexible hours” arm reported greater dissatisfaction with the effect of the program on their personal lives, both groups reported significant burnout, with interns recording high scores in emotional exhaustion (79% in flexible programs vs. 72% in standard), depersonalization (75% vs. 72%), and lack of personal accomplishment (71% vs. 69%).
Disturbingly, these scores were not restricted to interns but were present in all residents. The good news? Limiting duty hours does not cause burnout. On the other hand, it does not protect from burnout. Trainee burnout appears to transcend the issue of hours worked. Clearly, we need to address the systemic flaws in our work environments that contribute to this epidemic. Nationwide, educators and organizations are continuing to define causes of burnout and test interventions to improve wellness.
A final front of change in medical education worth mentioning is the use of the electronic medical record (EMR). While the EMR has improved many aspects of patient care, its implementation is associated with decreased time spent with patients and parallels the rise in burnout. Another unforeseen consequence has been its disruptive impact on medical student documentation. A national survey of clerkship directors found that, while 64% of programs allowed students to use the EMR, only two-thirds of those programs permitted students to document electronically.14
Many institutions limit student access because of either liability concerns or the fact that student notes cannot be used to support medical billing. Concerning workarounds among preceptors, such as logging in students under their own credentials to write notes, have been identified.15 Yet medical students need to learn how to document a clinical encounter and maintain medical records.7,16 Authoring notes engages students, promotes a sense of patient ownership, and empowers them to feel like essential team members. Participating in the EMR also allows for critical feedback and skill development.
In 2016, the Society of Hospital Medicine joined several major internal medicine organizations in asking the federal government to reconsider guidelines prohibiting attendings from referring to medical student notes. In February 2018, the Centers for Medicare & Medicaid Services (CMS) revised its student documentation guidelines (see Box A), allowing teaching physicians to use all student documentation (not just Review of Systems, Family History, and Social History) for billable services.
While the guidelines officially went into effect in March 2018, many institutions are still fine-tuning their implementation, in part because of nonspecific policy language. For instance, if a student composes a note and a resident edits and signs it, can the attending physician simply cosign the resident note? Also, once a student has presented a case, can the attending see the patient and verify findings without the student present?
Despite the above challenges, the revision to CMS guidelines is a significant “win” and can potentially reduce the documentation burden on teaching physicians. With more oversight of their notes, the next generation of students will be encouraged to produce accurate, high-quality documentation.
In summary, these changes in the way we define competency, in duty hours, and in the use of the EMR demonstrate that medical education is continuously improving via robust critique and educator engagement in outcomes. We are fortunate to train in a system that respects the scientific method and utilizes data and critical events to drive important changes in practice. Understanding these changes might help hospitalists relate to the backgrounds and needs of learners. And who knows – maybe next time that intern will do a better job presenting!
Dr. Kwan is a hospitalist at the Veterans Affairs San Diego Healthcare System (VASDHS) and an associate professor at the University of California, San Diego, in the division of hospital medicine. He is the chair of the SHM Physicians in Training committee. Dr. Sebasky is an associate clinical professor at UCSD in the division of hospital medicine. Dr. Muchmore is a hematologist/oncologist and professor of clinical medicine in the department of medicine at UCSD and associate chief of staff for education at VASDHS.
References
1. Osler W. “The Hospital as a College.” In Aequanimitas. Osler W, Ed. (Philadelphia: P. Blakiston’s Son & Co., 1932).
2. Kohn LT, Corrigan JM, Donaldson MS, eds. To Err Is Human: Building a Safer Health Care System. (Washington: National Academies Press, 1999).
3. Ten Cate O. Competency-based postgraduate medical education: Past, present and future. GMS J Med Educ. 2017 Nov 15. doi: 10.3205/zma001146.
4. Carraccio C, Englander R, Van Melle E, et al. Advancing competency-based medical education: A charter for clinician–educators. Acad Med. 2016;91(5):645-9.
5. 2016 NBME Clinical Clerkship Subject Examination Survey.
6. Mehta NB, Hull AL, Young JB, et al. Just imagine: New paradigms for medical education. Acad Med. 2013;88(10):1418-23.
7. Fazio SB, Ledford CH, Aronowitz PB, et al. Competency-based medical education in the internal medicine clerkship: A report from the Alliance for Academic Internal Medicine Undergraduate Medical Education Task Force. Acad Med. 2018;93(3):421-7.
8. Ten Cate O, Scheele F. Competency-based postgraduate training: Can we bridge the gap between theory and clinical practice? Acad Med. 2007 Jun;82(6):542-7.
9. Dewa CS, Loong D, Bonato S, et al. The relationship between physician burnout and quality of healthcare in terms of safety and acceptability: A systematic review. BMJ Open. 2017. doi: 10.1136/bmjopen-2016-015141.
10. Hall LH, Johnson J, Watt I, et al. Healthcare Staff wellbeing, burnout, and patient safety: A systematic review. PLoS ONE. 2016. doi: 10.1371/journal.pone.0159015.
11. Salyers MP, Bonfils KA, Luther L, et al. The relationship between professional burnout and quality and safety in healthcare: A meta-analysis. Gen Intern Med. 2017 Apr; 32(4):475-82.
12. Desai SV, Asch DA, Bellini LM, et al. Education outcomes in a duty hour flexibility trial in internal medicine. N Engl J Med. 2018 378:1494-508.
13. Maslach C, Jackson SE, Leiter MP. Maslach burnout inventory manual. 3rd ed. (Palo Alto, CA: Consulting Psychologists Press, 1996).
14. Hammoud MM, Margo K, Christner JG, et al. Opportunities and challenges in integrating electronic health records into undergraduate medical education: A national survey of clerkship directors. Teach Learn Med. 2012;24(3):219-24.
15. White J, Anthony D, WinklerPrins V, et al. Electronic medical records, medical students, and ambulatory family physicians: A multi-institution study. Acad Med. 2017;92(10):1485-90.
16. Pageler NM, Friedman CP, Longhurst CA. Refocusing medical education in the EMR era. JAMA 2013;310(21):2249-50.
Box A
“Students may document services in the medical record. However, the teaching physician must verify in the medical record all student documentation or findings, including history, physical exam, and/or medical decision making. The teaching physician must personally perform (or re-perform) the physical exam and medical decision making activities of the E/M service being billed, but may verify any student documentation of them in the medical record, rather than re-documenting this work.”
A brave new world
A brave new world
It’s Monday morning, and your intern is presenting an overnight admission. Lost in the details of his disorganized introduction, your mind wanders. “Why doesn’t this intern know how to present? When I trained, all those admissions during long sleepless nights really taught me to do this right.” But can we equate hours worked with competency achieved? And if not, what is the alternative? This article introduces some major changes in medical education and their implications for hospitalists.
Most hospitalists trained in an educational system influenced by Sir William Osler. In the early 1900s, he introduced the natural method of teaching, positing that student exposure to patients and experience over time ensured that physicians in training would become competent doctors.1 His influence led to the current structure of medical education, which includes conventional third-year clerkships and time-limited rotations (such as a 2-week nephrology block).
While familiarity may be comforting, there are signs our current model of medical education is inefficient, inadequate, and obsolete.
For one, the traditional system is failing to adequately prepare physicians to provide safe and complex care. Reports, such as the Institute of Medicine’s (IOM) “To Err is Human,”2 describe a high rate of preventable errors, highlighting considerable room for improvement in training the next generation of physicians.3,4
Meanwhile, trainees are still largely being deemed ready for the workforce by length of training completed (for example, completion of four-year medical school) rather than a skill set distinctly achieved. Our system leaves little flexibility to individualize learner goals, which is significant given some students and residents take shorter or longer periods of time to achieve proficiency. In addition, learner outcomes can be quite variable, as we have all experienced.
Even our methods of assessment may not adequately evaluate trainees’ skill sets. For example, most clerkships still rely heavily on the shelf exam5 as a surrogate for medical knowledge. As such, learners may conclude that testing performance trumps development of other professional skills.6 Efforts are being made to revamp evaluation systems to reflect mastery (such as Entrustable Professional Activities, or EPAs) toward competencies.7 Still, many institutions continue to rely on faculty evaluations that often reflect interpersonal dynamics rather than true critical thinking skills.6
Recognizing the above limitations, many educators have called for changing to outcome-based, or competency-based, training (CBME). CBME targets attainment of skills in performing concrete critical clinical activities,8 such as identifying unstable patients, providing initial management, and obtaining help. To be successful, supervisors must directly observe trainees, assess demonstrated skills, and provide feedback about progress.
Unfortunately, this considerable investment of time and effort is often poorly compensated. Additionally, unanswered questions remain. For example, how will residency programs continue to challenge physicians deemed “competent” in a required skill? What happens when a trainee is deficient and not appropriately progressing in a required skill? Is flexible training time part of the future of medical education? While CBME appears to be a more effective method of education, questions like these must be addressed during implementation.
Beyond the fact that hours worked cannot be used as a surrogate for competency, excessive unregulated work hours can be detrimental to learners, their supervisors, and patients. In 2003, the Accreditation Council for Graduate Medical Education (ACGME) implemented a major change in medical education: duty hour limitations. The premise that sleep-deprived providers are more prone to error is well established. However, controversy remains as to whether these regulations translate into improved patient care and provider well-being. Studies published following the ACGME change demonstrate increasing burnout among physicians,9-11 which has led some educators to explore the potential relationship between burnout and duty hour restrictions.
The recent “iCOMPARE” trial, which compared internal medicine (IM) residencies with “standard duty-hour” policies to those with “flexible” policies (that is, they did not specify limits on shift length or mandatory time off between shifts), supported a lack of correlation between hours worked and burnout.12 Researchers administered the Maslach Burnout Inventory to all participants.13 While those in the “flexible hours” arm reported greater dissatisfaction with the effect of the program on their personal lives, both groups reported significant burnout, with interns recording high scores in emotional exhaustion (79% in flexible programs vs. 72% in standard), depersonalization (75% vs. 72%), and lack of personal accomplishment (71% vs. 69%).
Disturbingly, these scores were not restricted to interns but were present in all residents. The good news? Limiting duty hours does not cause burnout. On the other hand, it does not protect from burnout. Trainee burnout appears to transcend the issue of hours worked. Clearly, we need to address the systemic flaws in our work environments that contribute to this epidemic. Nationwide, educators and organizations are continuing to define causes of burnout and test interventions to improve wellness.
A final front of change in medical education worth mentioning is the use of the electronic medical record (EMR). While the EMR has improved many aspects of patient care, its implementation is associated with decreased time spent with patients and parallels the rise in burnout. Another unforeseen consequence has been its disruptive impact on medical student documentation. A national survey of clerkship directors found that, while 64% of programs allowed students to use the EMR, only two-thirds of those programs permitted students to document electronically.14
Many institutions limit student access because of either liability concerns or the fact that student notes cannot be used to support medical billing. Concerning workarounds among preceptors, such as logging in students under their own credentials to write notes, have been identified.15 Yet medical students need to learn how to document a clinical encounter and maintain medical records.7,16 Authoring notes engages students, promotes a sense of patient ownership, and empowers them to feel like essential team members. Participating in the EMR also allows for critical feedback and skill development.
In 2016, the Society of Hospital Medicine joined several major internal medicine organizations in asking the federal government to reconsider guidelines prohibiting attendings from referring to medical student notes. In February 2018, the Centers for Medicare & Medicaid Services (CMS) revised its student documentation guidelines (see Box A), allowing teaching physicians to use all student documentation (not just Review of Systems, Family History, and Social History) for billable services.
While the guidelines officially went into effect in March 2018, many institutions are still fine-tuning their implementation, in part because of nonspecific policy language. For instance, if a student composes a note and a resident edits and signs it, can the attending physician simply cosign the resident note? Also, once a student has presented a case, can the attending see the patient and verify findings without the student present?
Despite the above challenges, the revision to CMS guidelines is a significant “win” and can potentially reduce the documentation burden on teaching physicians. With more oversight of their notes, the next generation of students will be encouraged to produce accurate, high-quality documentation.
In summary, these changes in the way we define competency, in duty hours, and in the use of the EMR demonstrate that medical education is continuously improving via robust critique and educator engagement in outcomes. We are fortunate to train in a system that respects the scientific method and utilizes data and critical events to drive important changes in practice. Understanding these changes might help hospitalists relate to the backgrounds and needs of learners. And who knows – maybe next time that intern will do a better job presenting!
Dr. Kwan is a hospitalist at the Veterans Affairs San Diego Healthcare System (VASDHS) and an associate professor at the University of California, San Diego, in the division of hospital medicine. He is the chair of the SHM Physicians in Training committee. Dr. Sebasky is an associate clinical professor at UCSD in the division of hospital medicine. Dr. Muchmore is a hematologist/oncologist and professor of clinical medicine in the department of medicine at UCSD and associate chief of staff for education at VASDHS.
References
1. Osler W. “The Hospital as a College.” In Aequanimitas. Osler W, Ed. (Philadelphia: P. Blakiston’s Son & Co., 1932).
2. Kohn LT, Corrigan JM, Donaldson MS, eds. To Err Is Human: Building a Safer Health Care System. (Washington: National Academies Press, 1999).
3. Ten Cate O. Competency-based postgraduate medical education: Past, present and future. GMS J Med Educ. 2017 Nov 15. doi: 10.3205/zma001146.
4. Carraccio C, Englander R, Van Melle E, et al. Advancing competency-based medical education: A charter for clinician–educators. Acad Med. 2016;91(5):645-9.
5. 2016 NBME Clinical Clerkship Subject Examination Survey.
6. Mehta NB, Hull AL, Young JB, et al. Just imagine: New paradigms for medical education. Acad Med. 2013;88(10):1418-23.
7. Fazio SB, Ledford CH, Aronowitz PB, et al. Competency-based medical education in the internal medicine clerkship: A report from the Alliance for Academic Internal Medicine Undergraduate Medical Education Task Force. Acad Med. 2018;93(3):421-7.
8. Ten Cate O, Scheele F. Competency-based postgraduate training: Can we bridge the gap between theory and clinical practice? Acad Med. 2007 Jun;82(6):542-7.
9. Dewa CS, Loong D, Bonato S, et al. The relationship between physician burnout and quality of healthcare in terms of safety and acceptability: A systematic review. BMJ Open. 2017. doi: 10.1136/bmjopen-2016-015141.
10. Hall LH, Johnson J, Watt I, et al. Healthcare Staff wellbeing, burnout, and patient safety: A systematic review. PLoS ONE. 2016. doi: 10.1371/journal.pone.0159015.
11. Salyers MP, Bonfils KA, Luther L, et al. The relationship between professional burnout and quality and safety in healthcare: A meta-analysis. Gen Intern Med. 2017 Apr; 32(4):475-82.
12. Desai SV, Asch DA, Bellini LM, et al. Education outcomes in a duty hour flexibility trial in internal medicine. N Engl J Med. 2018 378:1494-508.
13. Maslach C, Jackson SE, Leiter MP. Maslach burnout inventory manual. 3rd ed. (Palo Alto, CA: Consulting Psychologists Press, 1996).
14. Hammoud MM, Margo K, Christner JG, et al. Opportunities and challenges in integrating electronic health records into undergraduate medical education: A national survey of clerkship directors. Teach Learn Med. 2012;24(3):219-24.
15. White J, Anthony D, WinklerPrins V, et al. Electronic medical records, medical students, and ambulatory family physicians: A multi-institution study. Acad Med. 2017;92(10):1485-90.
16. Pageler NM, Friedman CP, Longhurst CA. Refocusing medical education in the EMR era. JAMA 2013;310(21):2249-50.
Box A
“Students may document services in the medical record. However, the teaching physician must verify in the medical record all student documentation or findings, including history, physical exam, and/or medical decision making. The teaching physician must personally perform (or re-perform) the physical exam and medical decision making activities of the E/M service being billed, but may verify any student documentation of them in the medical record, rather than re-documenting this work.”
It’s Monday morning, and your intern is presenting an overnight admission. Lost in the details of his disorganized introduction, your mind wanders. “Why doesn’t this intern know how to present? When I trained, all those admissions during long sleepless nights really taught me to do this right.” But can we equate hours worked with competency achieved? And if not, what is the alternative? This article introduces some major changes in medical education and their implications for hospitalists.
Most hospitalists trained in an educational system influenced by Sir William Osler. In the early 1900s, he introduced the natural method of teaching, positing that student exposure to patients and experience over time ensured that physicians in training would become competent doctors.1 His influence led to the current structure of medical education, which includes conventional third-year clerkships and time-limited rotations (such as a 2-week nephrology block).
While familiarity may be comforting, there are signs our current model of medical education is inefficient, inadequate, and obsolete.
For one, the traditional system is failing to adequately prepare physicians to provide safe and complex care. Reports, such as the Institute of Medicine’s (IOM) “To Err is Human,”2 describe a high rate of preventable errors, highlighting considerable room for improvement in training the next generation of physicians.3,4
Meanwhile, trainees are still largely being deemed ready for the workforce by length of training completed (for example, completion of four-year medical school) rather than a skill set distinctly achieved. Our system leaves little flexibility to individualize learner goals, which is significant given some students and residents take shorter or longer periods of time to achieve proficiency. In addition, learner outcomes can be quite variable, as we have all experienced.
Even our methods of assessment may not adequately evaluate trainees’ skill sets. For example, most clerkships still rely heavily on the shelf exam5 as a surrogate for medical knowledge. As such, learners may conclude that testing performance trumps development of other professional skills.6 Efforts are being made to revamp evaluation systems to reflect mastery (such as Entrustable Professional Activities, or EPAs) toward competencies.7 Still, many institutions continue to rely on faculty evaluations that often reflect interpersonal dynamics rather than true critical thinking skills.6
Recognizing the above limitations, many educators have called for changing to outcome-based, or competency-based, training (CBME). CBME targets attainment of skills in performing concrete critical clinical activities,8 such as identifying unstable patients, providing initial management, and obtaining help. To be successful, supervisors must directly observe trainees, assess demonstrated skills, and provide feedback about progress.
Unfortunately, this considerable investment of time and effort is often poorly compensated. Additionally, unanswered questions remain. For example, how will residency programs continue to challenge physicians deemed “competent” in a required skill? What happens when a trainee is deficient and not appropriately progressing in a required skill? Is flexible training time part of the future of medical education? While CBME appears to be a more effective method of education, questions like these must be addressed during implementation.
Beyond the fact that hours worked cannot be used as a surrogate for competency, excessive unregulated work hours can be detrimental to learners, their supervisors, and patients. In 2003, the Accreditation Council for Graduate Medical Education (ACGME) implemented a major change in medical education: duty hour limitations. The premise that sleep-deprived providers are more prone to error is well established. However, controversy remains as to whether these regulations translate into improved patient care and provider well-being. Studies published following the ACGME change demonstrate increasing burnout among physicians,9-11 which has led some educators to explore the potential relationship between burnout and duty hour restrictions.
The recent “iCOMPARE” trial, which compared internal medicine (IM) residencies with “standard duty-hour” policies to those with “flexible” policies (that is, they did not specify limits on shift length or mandatory time off between shifts), supported a lack of correlation between hours worked and burnout.12 Researchers administered the Maslach Burnout Inventory to all participants.13 While those in the “flexible hours” arm reported greater dissatisfaction with the effect of the program on their personal lives, both groups reported significant burnout, with interns recording high scores in emotional exhaustion (79% in flexible programs vs. 72% in standard), depersonalization (75% vs. 72%), and lack of personal accomplishment (71% vs. 69%).
Disturbingly, these scores were not restricted to interns but were present in all residents. The good news? Limiting duty hours does not cause burnout. On the other hand, it does not protect from burnout. Trainee burnout appears to transcend the issue of hours worked. Clearly, we need to address the systemic flaws in our work environments that contribute to this epidemic. Nationwide, educators and organizations are continuing to define causes of burnout and test interventions to improve wellness.
A final front of change in medical education worth mentioning is the use of the electronic medical record (EMR). While the EMR has improved many aspects of patient care, its implementation is associated with decreased time spent with patients and parallels the rise in burnout. Another unforeseen consequence has been its disruptive impact on medical student documentation. A national survey of clerkship directors found that, while 64% of programs allowed students to use the EMR, only two-thirds of those programs permitted students to document electronically.14
Many institutions limit student access because of either liability concerns or the fact that student notes cannot be used to support medical billing. Concerning workarounds among preceptors, such as logging in students under their own credentials to write notes, have been identified.15 Yet medical students need to learn how to document a clinical encounter and maintain medical records.7,16 Authoring notes engages students, promotes a sense of patient ownership, and empowers them to feel like essential team members. Participating in the EMR also allows for critical feedback and skill development.
In 2016, the Society of Hospital Medicine joined several major internal medicine organizations in asking the federal government to reconsider guidelines prohibiting attendings from referring to medical student notes. In February 2018, the Centers for Medicare & Medicaid Services (CMS) revised its student documentation guidelines (see Box A), allowing teaching physicians to use all student documentation (not just Review of Systems, Family History, and Social History) for billable services.
While the guidelines officially went into effect in March 2018, many institutions are still fine-tuning their implementation, in part because of nonspecific policy language. For instance, if a student composes a note and a resident edits and signs it, can the attending physician simply cosign the resident note? Also, once a student has presented a case, can the attending see the patient and verify findings without the student present?
Despite the above challenges, the revision to CMS guidelines is a significant “win” and can potentially reduce the documentation burden on teaching physicians. With more oversight of their notes, the next generation of students will be encouraged to produce accurate, high-quality documentation.
In summary, these changes in the way we define competency, in duty hours, and in the use of the EMR demonstrate that medical education is continuously improving via robust critique and educator engagement in outcomes. We are fortunate to train in a system that respects the scientific method and utilizes data and critical events to drive important changes in practice. Understanding these changes might help hospitalists relate to the backgrounds and needs of learners. And who knows – maybe next time that intern will do a better job presenting!
Dr. Kwan is a hospitalist at the Veterans Affairs San Diego Healthcare System (VASDHS) and an associate professor at the University of California, San Diego, in the division of hospital medicine. He is the chair of the SHM Physicians in Training committee. Dr. Sebasky is an associate clinical professor at UCSD in the division of hospital medicine. Dr. Muchmore is a hematologist/oncologist and professor of clinical medicine in the department of medicine at UCSD and associate chief of staff for education at VASDHS.
References
1. Osler W. “The Hospital as a College.” In Aequanimitas. Osler W, Ed. (Philadelphia: P. Blakiston’s Son & Co., 1932).
2. Kohn LT, Corrigan JM, Donaldson MS, eds. To Err Is Human: Building a Safer Health Care System. (Washington: National Academies Press, 1999).
3. Ten Cate O. Competency-based postgraduate medical education: Past, present and future. GMS J Med Educ. 2017 Nov 15. doi: 10.3205/zma001146.
4. Carraccio C, Englander R, Van Melle E, et al. Advancing competency-based medical education: A charter for clinician–educators. Acad Med. 2016;91(5):645-9.
5. 2016 NBME Clinical Clerkship Subject Examination Survey.
6. Mehta NB, Hull AL, Young JB, et al. Just imagine: New paradigms for medical education. Acad Med. 2013;88(10):1418-23.
7. Fazio SB, Ledford CH, Aronowitz PB, et al. Competency-based medical education in the internal medicine clerkship: A report from the Alliance for Academic Internal Medicine Undergraduate Medical Education Task Force. Acad Med. 2018;93(3):421-7.
8. Ten Cate O, Scheele F. Competency-based postgraduate training: Can we bridge the gap between theory and clinical practice? Acad Med. 2007 Jun;82(6):542-7.
9. Dewa CS, Loong D, Bonato S, et al. The relationship between physician burnout and quality of healthcare in terms of safety and acceptability: A systematic review. BMJ Open. 2017. doi: 10.1136/bmjopen-2016-015141.
10. Hall LH, Johnson J, Watt I, et al. Healthcare Staff wellbeing, burnout, and patient safety: A systematic review. PLoS ONE. 2016. doi: 10.1371/journal.pone.0159015.
11. Salyers MP, Bonfils KA, Luther L, et al. The relationship between professional burnout and quality and safety in healthcare: A meta-analysis. Gen Intern Med. 2017 Apr; 32(4):475-82.
12. Desai SV, Asch DA, Bellini LM, et al. Education outcomes in a duty hour flexibility trial in internal medicine. N Engl J Med. 2018 378:1494-508.
13. Maslach C, Jackson SE, Leiter MP. Maslach burnout inventory manual. 3rd ed. (Palo Alto, CA: Consulting Psychologists Press, 1996).
14. Hammoud MM, Margo K, Christner JG, et al. Opportunities and challenges in integrating electronic health records into undergraduate medical education: A national survey of clerkship directors. Teach Learn Med. 2012;24(3):219-24.
15. White J, Anthony D, WinklerPrins V, et al. Electronic medical records, medical students, and ambulatory family physicians: A multi-institution study. Acad Med. 2017;92(10):1485-90.
16. Pageler NM, Friedman CP, Longhurst CA. Refocusing medical education in the EMR era. JAMA 2013;310(21):2249-50.
Box A
“Students may document services in the medical record. However, the teaching physician must verify in the medical record all student documentation or findings, including history, physical exam, and/or medical decision making. The teaching physician must personally perform (or re-perform) the physical exam and medical decision making activities of the E/M service being billed, but may verify any student documentation of them in the medical record, rather than re-documenting this work.”
Clinical Pearl: Topical Timolol for Refractory Hypergranulation
Practice Gap
Hypergranulation is a frequent complication of dermatologic surgery, especially when surgical defects are left to heal by secondary intention (eg, after electrodesiccation and curettage). Although management of postoperative hypergranulation with routine wound care, superpotent topical corticosteroids, and/or topical silver nitrate often is effective, refractory cases pose a difficult challenge given the paucity of treatment options. Effective management of these cases is important because hypergranulation can delay wound healing, cause patient discomfort, and lead to poor wound cosmesis.
The Technique
If refractory hypergranulation fails to respond to treatment with routine wound care and topical silver nitrate, we prescribe twice-daily application of timolol maleate ophthalmic gel forming solution 0.5% for up to 14 days or until complete resolution of the hypergranulation is achieved. We counsel patients to continue routine wound care with daily dressing changes in conjunction with topical timolol application.
We initiated treatment with topical timolol in a patient who developed hypergranulation at 2 separate electrodesiccation and curettage sites that was refractory to 6 weeks of routine wound care with white petrolatum under nonadherent sterile gauze dressings and 2 subsequent topical silver nitrate applications (Figure 1). After 2 weeks of treatment with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 2). Another patient presented with hypergranulation that developed following a traumatic injury on the left upper arm and had been treated unsuccessfully for several months at a wound care clinic with daily nonadherent sterile gauze dressings and both topical and oral antibiotics (Figure 3A). After treatment for 9 days with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 3B).
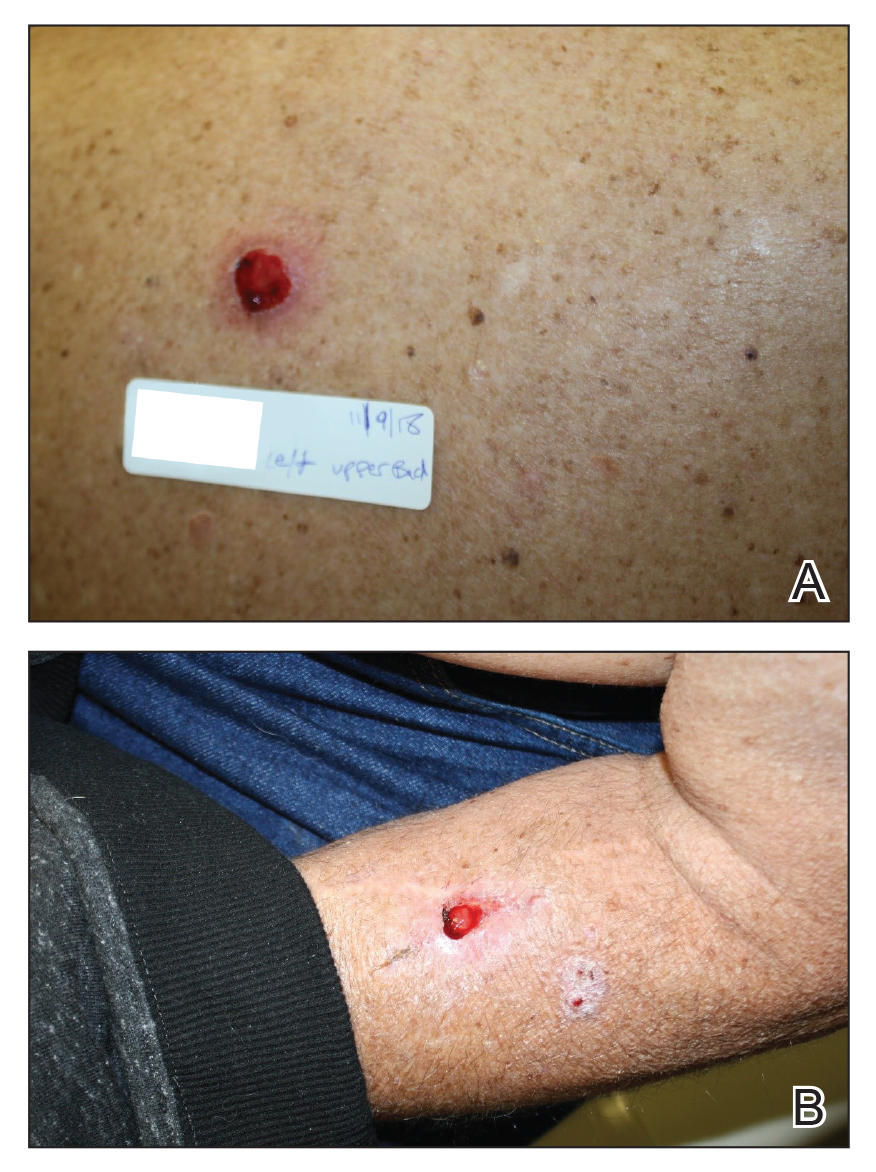
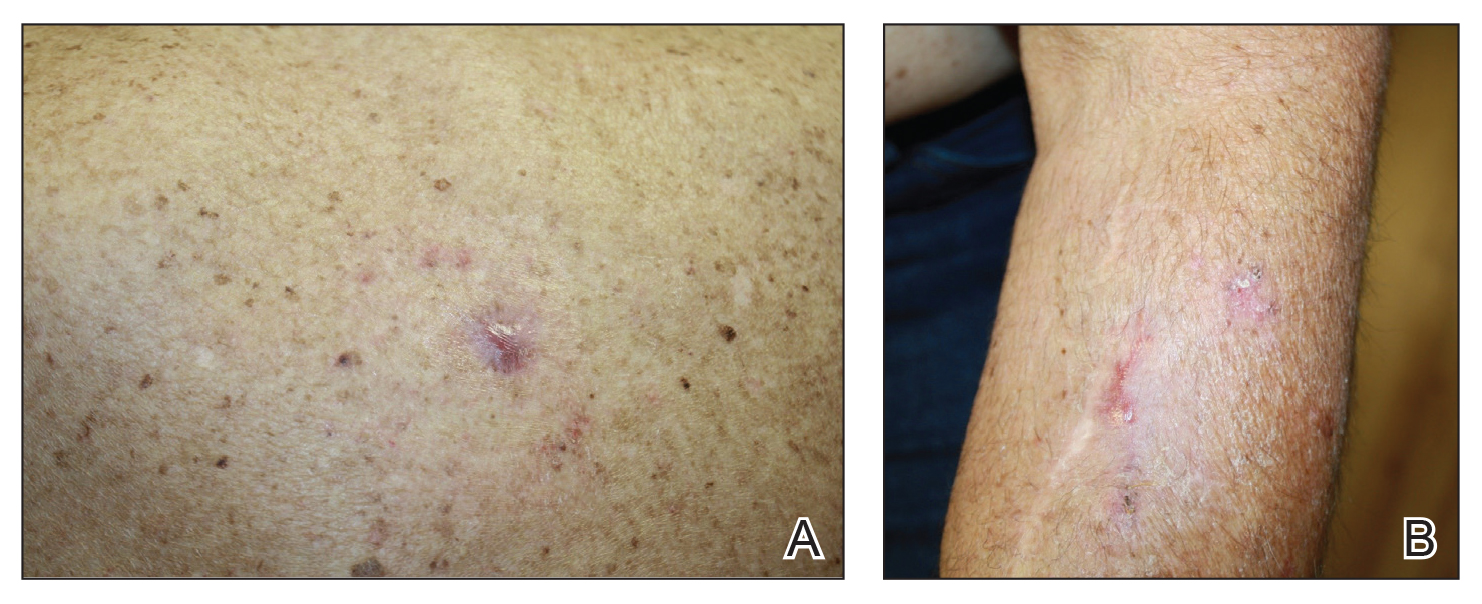
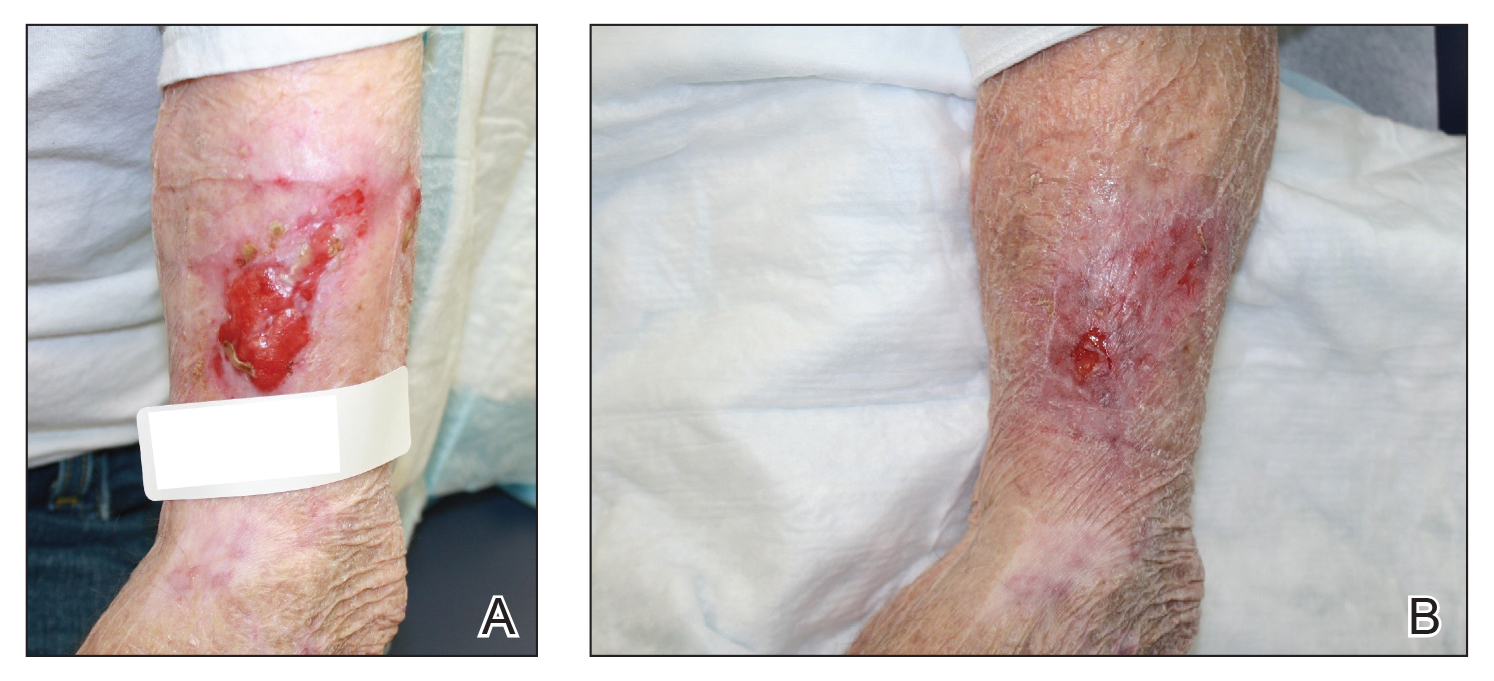
Practice Implications
Beta-blockers are increasingly being used for management of chronic nonhealing wounds since the 1990s when oral administration of propranolol initially was reported to be an effective adjuvant therapy for managing severe burns.1 Since then, topical beta-blockers have been reported to be effective for management of ulcerated hemangiomas, venous stasis ulcers, chronic diabetic ulcers, and chronic nonhealing surgical wounds; however, there are no known reports of using topical beta-blockers for management of hypergranulation.2-5 We found timolol ophthalmic gel to be an excellent second-line therapy for management of postoperative hypergranulation if prior treatment with routine wound care and superpotent topical corticosteroids has failed. To date, we have found no reported adverse effects from the use of topical timolol for this indication that have required discontinuation of the medication. Use of this simple and safe intervention can be effective as a solution to a common postoperative condition.
- Herndon DN, Hart DW, Wolf SE, et al. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223-1229.
- Pope E, Chakkittakandiyil A. Topical timolol gel for infantile hemangiomas: a pilot study. Arch Dermatol. 2010;146:564-565.
- Braun L, Lamel S, Richmond N, et al. Topical timolol for recalcitrant wounds. JAMA Dermatol. 2013;149:1400-1402.
- Thomas B, Kurien J, Jose T, et al. Topical timolol promotes healing of chronic leg ulcer. J Vasc Surg. 2017;5:844-850.
- Tang J, Dosal J, Kirsner RS. Topical timolol for a refractory wound. Dermatol Surg. 2012;38:135-138.
Practice Gap
Hypergranulation is a frequent complication of dermatologic surgery, especially when surgical defects are left to heal by secondary intention (eg, after electrodesiccation and curettage). Although management of postoperative hypergranulation with routine wound care, superpotent topical corticosteroids, and/or topical silver nitrate often is effective, refractory cases pose a difficult challenge given the paucity of treatment options. Effective management of these cases is important because hypergranulation can delay wound healing, cause patient discomfort, and lead to poor wound cosmesis.
The Technique
If refractory hypergranulation fails to respond to treatment with routine wound care and topical silver nitrate, we prescribe twice-daily application of timolol maleate ophthalmic gel forming solution 0.5% for up to 14 days or until complete resolution of the hypergranulation is achieved. We counsel patients to continue routine wound care with daily dressing changes in conjunction with topical timolol application.
We initiated treatment with topical timolol in a patient who developed hypergranulation at 2 separate electrodesiccation and curettage sites that was refractory to 6 weeks of routine wound care with white petrolatum under nonadherent sterile gauze dressings and 2 subsequent topical silver nitrate applications (Figure 1). After 2 weeks of treatment with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 2). Another patient presented with hypergranulation that developed following a traumatic injury on the left upper arm and had been treated unsuccessfully for several months at a wound care clinic with daily nonadherent sterile gauze dressings and both topical and oral antibiotics (Figure 3A). After treatment for 9 days with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 3B).



Practice Implications
Beta-blockers are increasingly being used for management of chronic nonhealing wounds since the 1990s when oral administration of propranolol initially was reported to be an effective adjuvant therapy for managing severe burns.1 Since then, topical beta-blockers have been reported to be effective for management of ulcerated hemangiomas, venous stasis ulcers, chronic diabetic ulcers, and chronic nonhealing surgical wounds; however, there are no known reports of using topical beta-blockers for management of hypergranulation.2-5 We found timolol ophthalmic gel to be an excellent second-line therapy for management of postoperative hypergranulation if prior treatment with routine wound care and superpotent topical corticosteroids has failed. To date, we have found no reported adverse effects from the use of topical timolol for this indication that have required discontinuation of the medication. Use of this simple and safe intervention can be effective as a solution to a common postoperative condition.
Practice Gap
Hypergranulation is a frequent complication of dermatologic surgery, especially when surgical defects are left to heal by secondary intention (eg, after electrodesiccation and curettage). Although management of postoperative hypergranulation with routine wound care, superpotent topical corticosteroids, and/or topical silver nitrate often is effective, refractory cases pose a difficult challenge given the paucity of treatment options. Effective management of these cases is important because hypergranulation can delay wound healing, cause patient discomfort, and lead to poor wound cosmesis.
The Technique
If refractory hypergranulation fails to respond to treatment with routine wound care and topical silver nitrate, we prescribe twice-daily application of timolol maleate ophthalmic gel forming solution 0.5% for up to 14 days or until complete resolution of the hypergranulation is achieved. We counsel patients to continue routine wound care with daily dressing changes in conjunction with topical timolol application.
We initiated treatment with topical timolol in a patient who developed hypergranulation at 2 separate electrodesiccation and curettage sites that was refractory to 6 weeks of routine wound care with white petrolatum under nonadherent sterile gauze dressings and 2 subsequent topical silver nitrate applications (Figure 1). After 2 weeks of treatment with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 2). Another patient presented with hypergranulation that developed following a traumatic injury on the left upper arm and had been treated unsuccessfully for several months at a wound care clinic with daily nonadherent sterile gauze dressings and both topical and oral antibiotics (Figure 3A). After treatment for 9 days with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 3B).



Practice Implications
Beta-blockers are increasingly being used for management of chronic nonhealing wounds since the 1990s when oral administration of propranolol initially was reported to be an effective adjuvant therapy for managing severe burns.1 Since then, topical beta-blockers have been reported to be effective for management of ulcerated hemangiomas, venous stasis ulcers, chronic diabetic ulcers, and chronic nonhealing surgical wounds; however, there are no known reports of using topical beta-blockers for management of hypergranulation.2-5 We found timolol ophthalmic gel to be an excellent second-line therapy for management of postoperative hypergranulation if prior treatment with routine wound care and superpotent topical corticosteroids has failed. To date, we have found no reported adverse effects from the use of topical timolol for this indication that have required discontinuation of the medication. Use of this simple and safe intervention can be effective as a solution to a common postoperative condition.
- Herndon DN, Hart DW, Wolf SE, et al. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223-1229.
- Pope E, Chakkittakandiyil A. Topical timolol gel for infantile hemangiomas: a pilot study. Arch Dermatol. 2010;146:564-565.
- Braun L, Lamel S, Richmond N, et al. Topical timolol for recalcitrant wounds. JAMA Dermatol. 2013;149:1400-1402.
- Thomas B, Kurien J, Jose T, et al. Topical timolol promotes healing of chronic leg ulcer. J Vasc Surg. 2017;5:844-850.
- Tang J, Dosal J, Kirsner RS. Topical timolol for a refractory wound. Dermatol Surg. 2012;38:135-138.
- Herndon DN, Hart DW, Wolf SE, et al. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223-1229.
- Pope E, Chakkittakandiyil A. Topical timolol gel for infantile hemangiomas: a pilot study. Arch Dermatol. 2010;146:564-565.
- Braun L, Lamel S, Richmond N, et al. Topical timolol for recalcitrant wounds. JAMA Dermatol. 2013;149:1400-1402.
- Thomas B, Kurien J, Jose T, et al. Topical timolol promotes healing of chronic leg ulcer. J Vasc Surg. 2017;5:844-850.
- Tang J, Dosal J, Kirsner RS. Topical timolol for a refractory wound. Dermatol Surg. 2012;38:135-138.
Recurrence of Linear Basal Cell Carcinoma
Case Report
A 63-year-old man was evaluated in the Mohs clinic for a lesion on the right supraclavicular neck, which he described as a linear asymptomatic “birthmark” that had been present since childhood and stable for many years. It began to enlarge approximately 5 years prior, became increasingly red, and had occasional crusting. The lesion also gradually became more irritated with repeated mild trauma when he carried a backpack while hiking. On physical examination, a 10×2-cm, linear, pink plaque with an irregular border, translucent rolled edges, and central smooth atrophic skin was seen on the right supraclavicular neck (Figure). There was no visible epidermal nevus or nevus sebaceous in the area. A shave biopsy of the lesion confirmed the pathologic diagnosis of basal cell carcinoma, nodular type, along with the morphologic diagnosis of linear basal cell carcinoma (LBCC). The tumor was completely removed with standard excision using 5-mm margins.

Approximately 10 months after the original excision, the patient developed an irritated erosion that occasionally bled when his backpack rubbed against it. He returned to the clinic after the erosion failed to heal. Physical examination revealed a 1.4×0.7-cm, eroded, pink papule with large telangiectases at the superior pole of the excision scar. A shave biopsy confirmed the diagnosis of a recurrent infiltrative basal cell carcinoma. The tumor was then completely excised using Mohs micrographic surgery.
Comment
Linear basal cell carcinoma, first described by Lewis1 in 1985, is a rare morphologic variant of basal cell carcinoma. In 2011, Al-Niaimi and Lyon2 performed a comprehensive literature search on LBCC (1985-2008) and found only 39 cases (including 2 of their own) had been published since the pioneer case in 1985. It was determined that the most common sites affected were the periorbital area and neck (n=13 each [67%]), and the majority were histologically nodular (n=27 [69%]). Mohs micrographic surgery was the most common treatment method (n=23 [59%]), followed by primary excision (n=17 [44%]). A history of trauma, radiotherapy, or prior operation in association with the site of the LBCC was discovered in only 7 cases (18%).2 Although Peschen et al3 proposed that trauma—both physical and surgical—and radiotherapy may play a role in the development of LBCCs, the low incidence reported suggests that other factors may be involved. To determine if genetic factors were contributing to the development of LBCCs, Yamaguchi et al4 investigated the expression of p27 and PCTAIRE1, both known to contribute to tumorigenesis when mutated, as well as somatic gene mutations using deep sequencing in a case of LBCC; they found no associated genetic mutation.
Reported Cases of LBCC
According to a PubMed search of articles indexed for MEDLINE using the terms linear and basal cell carcinoma, 67 cases (including the current case) of LBCC have been published since 1985. The patient demographics, anatomic location, histologic subtype, treatment methods, and frequency of recurrence for all reported cases of LBCC are summarized in the Table.1-24 There were 36 women and 31 men, with an average age of 70 years (range, 40–92 years). The most commonly affected sites were the periocular region (n=27) and neck (n=18). Histologically, most LBCCs were nodular (n=35), with the next most common histologic subtype being infiltrative (n=20), which included the morphoeic, metatypical, and micronodular subtypes under the overarching infiltrative subtype. The most frequently chosen treatment option was primary excision (n=38 [57%]), followed by Mohs micrographic surgery (n=28 [42%]). Risk factors previously identified by Al-Niaimi and Lyon,2 including trauma, radiotherapy, or prior operation, were reported in 12 of 67 cases. Recurrence was reported in only 2 of 67 cases, 1 being the current case; however, an accurate recurrence rate could not be calculated due to lack of follow-up or short length of follow-up in most of the reported cases.
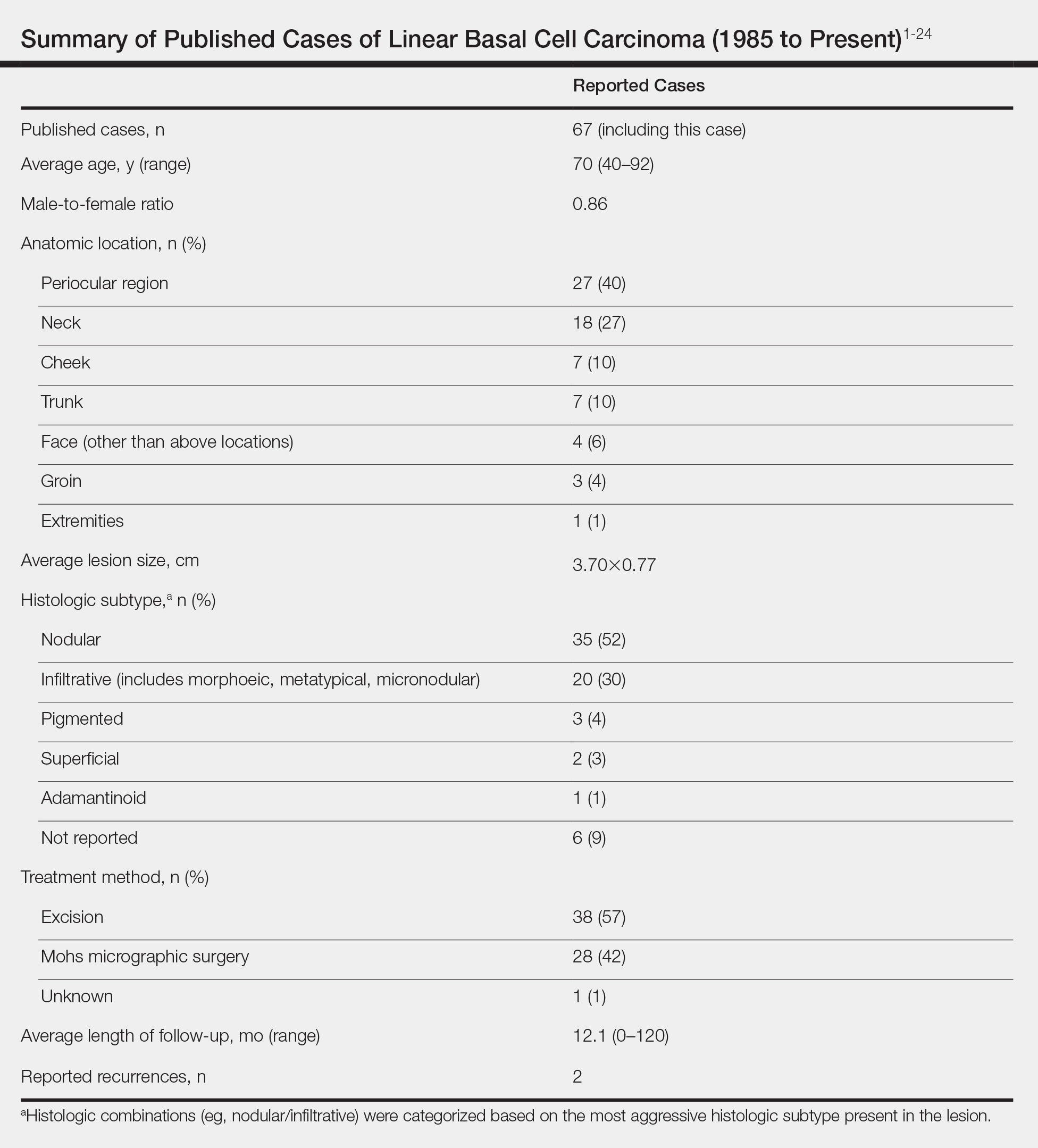
Presentation and Treatment
Currently, there are no set criteria for the diagnosis of LBCC, but it has been shown to follow a characteristic morphologic pattern, favoring extension in one direction leading to a length-to-width ratio that typically is at least 3 to 1.5 With most lesions presenting in the periocular region along relaxed skin tension lines, it has been speculated that these tumors expand along wrinkles.2 Pierard and Lapiere25 proposed that the preferential parallel orientation and a straightening of thin collagen bundles and elastic fibers within the reticular dermis combined with relaxed skin tension lines and muscle contraction perpendicular to these stromal parts may influence the growth of tumors preferentially in one direction, contributing to linearity of the lesion. In addition, the clinical appearance is not a reliable indicator of subclinical extension.2 Therefore, Lim et al6 recommended Mohs micrographic surgery as the best initial treatment of LBCCs.
Conclusion
Linear basal cell carcinoma should be considered a distinct morphologic variant of basal cell carcinoma. Although likely underreported, this variant is uncommon. It presents most often in the periocular and neck regions. The most common histologic subtypes are nodular and infiltrative. Because of the likelihood of subclinical spread, LBCC should be regarded as a high-risk subtype. As such, Mohs micrographic surgery or excision with complete circumferential peripheral and deep margin assessment is recommended as first-line treatment of LBCC.6
- Lewis JE. Linear basal cell epithelioma. Int J Dermatol. 1985;24:124-125.
- Al-Niaimi F, Lyon CC. Linear basal cell carcinoma: a distinct condition? Clin Exp Dermatol. 2011;36:231-234.
- Peschen M, Lo JS, Snow SN, et al. Linear basal cell carcinoma. Cutis. 1993;51:287-289.
- Yamaguchi Y, Yanagi T, Imafuku K, et al. A case of linear basal cell carcinoma: evaluation of proliferative activity by immunohistochemical staining of PCTAIRE1 and p27. J Eur Acad Dermatol Venereol. 2017;31:E359-E362.
- Mavirakis I, Malhotra R, Selva D, et al. Linear basal cell carcinoma: a distinct clinical entity. J Plast Reconstr Aesthet Surg. 2006;59:419-423.
- Lim KK, Randle HW, Roenigk RK, et al. Linear basal cell carcinoma: report of seventeen cases and review of the presentation and treatment. Dermatol Surg. 1999;25:63-67.
- Pardavila R, Rosón E, De la torre C, et al. Linear basal cell carcinoma. report of two cases [in Spanish]. Actas Dermosifiliogr. 2007;98:291.
- Shinsuke K, Hirohiko K, Yasuhiro T, et al. Linear basal cell carcinoma in an Asian patient. Open Ophthalmol J. 2007;1:20-22.
- Ning C, Chao S. Linear basal cell carcinoma of the scrotum. Dermatol Sinica. 2002;20:57-62.
- Chopra KF, Cohen PR. Linear basal cell carcinomas: report of multiple sequential tumors localized to a radiotherapy port and review of the literature. Tex Med. 1997;93:57-59.
- da Silva MO, Dadalt P, Santos OL, et al. Linear basal cell carcinoma. Int J Dermatol. 1995;34:488.
- Warthan TL, Lewis JE. Giant linear basal cell epithelioma. Int J Dermatol. 1994;33:284.
- Lewis JE. Linear basal cell epithelioma. Int J Dermatol. 1989;28:682-684.
- Alcántara-Reifs CM, Salido-Vallejo R, González-Menchen A, et al. Linear basal cell carcinoma: report of three cases with dermoscopic findings. Indian J Dermatol Venereol Leprol. 2016;82:708-711.
- Lee MS, Cho E, Lee JH, et al. Linearly curved, blackish macule on the wrist. Cutis. 2016;97:384, 406-407.
- Bajaj S, Sharma PK, Kar HK. Linear adamantinoid basal cell carcinoma in the axilla. Dermatol Online J. 2015;21. pii:13030/qt8k0713nb.
- Iga N, Sakurai K, Fujii H, et al. Linear basal cell carcinoma at the external genitalia. J Dermatol. 2014;41:275-276.
- Ichinokawa Y, Ohtuki A, Hattori M, et al. Linear basal cell carcinoma: a case report. Case Rep Dermatol. 2011;3:142-146.
- Becher GL, Affleck A, Fleming C, et al. Linear basal cell carcinoma occurs most commonly on the lower eyelid. Clin Exp Dermatol. 2011;36:311-312.
- Jellouli A, Triki S, Zghal M, et al. Linear basal cell carcinoma. Actas Dermosifiliogr. 2010;101:648-650.
- Takiyoshi N, Nakano H, Kaneko T, et al. A linear basal cell carcinoma undergoing spontaneous regression. Clin Exp Dermatol. 2009;34:E411-E413.
- Yoleri L, Ozden S, Kandiloglu A. A 46-year-old male with an ulcerated linear lesion on his neck. Ann Saudi Med. 2008;28:57-58.
- Palleschi GM, Corradini D, Bruscino N, et al. Linear basal cell carcinoma: clinical significance and better surgical approach. G Ital Dermatol Venereol. 2016;151:119-121.
- Rodriguez-Garijo N, Redondo P. Linear basal cell carcinoma of the lower eyelid: reconstruction with a musculocutaneous transposition flap. JAAD Case Rep. 2018;4:633-635.
- Pierard GE, Lapiere CM. Microanatomy of the dermis in relation to relaxed skin tension lines and Langer’s lines. Am J Dermatopathol. 1987;9:219-224.
Case Report
A 63-year-old man was evaluated in the Mohs clinic for a lesion on the right supraclavicular neck, which he described as a linear asymptomatic “birthmark” that had been present since childhood and stable for many years. It began to enlarge approximately 5 years prior, became increasingly red, and had occasional crusting. The lesion also gradually became more irritated with repeated mild trauma when he carried a backpack while hiking. On physical examination, a 10×2-cm, linear, pink plaque with an irregular border, translucent rolled edges, and central smooth atrophic skin was seen on the right supraclavicular neck (Figure). There was no visible epidermal nevus or nevus sebaceous in the area. A shave biopsy of the lesion confirmed the pathologic diagnosis of basal cell carcinoma, nodular type, along with the morphologic diagnosis of linear basal cell carcinoma (LBCC). The tumor was completely removed with standard excision using 5-mm margins.

Approximately 10 months after the original excision, the patient developed an irritated erosion that occasionally bled when his backpack rubbed against it. He returned to the clinic after the erosion failed to heal. Physical examination revealed a 1.4×0.7-cm, eroded, pink papule with large telangiectases at the superior pole of the excision scar. A shave biopsy confirmed the diagnosis of a recurrent infiltrative basal cell carcinoma. The tumor was then completely excised using Mohs micrographic surgery.
Comment
Linear basal cell carcinoma, first described by Lewis1 in 1985, is a rare morphologic variant of basal cell carcinoma. In 2011, Al-Niaimi and Lyon2 performed a comprehensive literature search on LBCC (1985-2008) and found only 39 cases (including 2 of their own) had been published since the pioneer case in 1985. It was determined that the most common sites affected were the periorbital area and neck (n=13 each [67%]), and the majority were histologically nodular (n=27 [69%]). Mohs micrographic surgery was the most common treatment method (n=23 [59%]), followed by primary excision (n=17 [44%]). A history of trauma, radiotherapy, or prior operation in association with the site of the LBCC was discovered in only 7 cases (18%).2 Although Peschen et al3 proposed that trauma—both physical and surgical—and radiotherapy may play a role in the development of LBCCs, the low incidence reported suggests that other factors may be involved. To determine if genetic factors were contributing to the development of LBCCs, Yamaguchi et al4 investigated the expression of p27 and PCTAIRE1, both known to contribute to tumorigenesis when mutated, as well as somatic gene mutations using deep sequencing in a case of LBCC; they found no associated genetic mutation.
Reported Cases of LBCC
According to a PubMed search of articles indexed for MEDLINE using the terms linear and basal cell carcinoma, 67 cases (including the current case) of LBCC have been published since 1985. The patient demographics, anatomic location, histologic subtype, treatment methods, and frequency of recurrence for all reported cases of LBCC are summarized in the Table.1-24 There were 36 women and 31 men, with an average age of 70 years (range, 40–92 years). The most commonly affected sites were the periocular region (n=27) and neck (n=18). Histologically, most LBCCs were nodular (n=35), with the next most common histologic subtype being infiltrative (n=20), which included the morphoeic, metatypical, and micronodular subtypes under the overarching infiltrative subtype. The most frequently chosen treatment option was primary excision (n=38 [57%]), followed by Mohs micrographic surgery (n=28 [42%]). Risk factors previously identified by Al-Niaimi and Lyon,2 including trauma, radiotherapy, or prior operation, were reported in 12 of 67 cases. Recurrence was reported in only 2 of 67 cases, 1 being the current case; however, an accurate recurrence rate could not be calculated due to lack of follow-up or short length of follow-up in most of the reported cases.

Presentation and Treatment
Currently, there are no set criteria for the diagnosis of LBCC, but it has been shown to follow a characteristic morphologic pattern, favoring extension in one direction leading to a length-to-width ratio that typically is at least 3 to 1.5 With most lesions presenting in the periocular region along relaxed skin tension lines, it has been speculated that these tumors expand along wrinkles.2 Pierard and Lapiere25 proposed that the preferential parallel orientation and a straightening of thin collagen bundles and elastic fibers within the reticular dermis combined with relaxed skin tension lines and muscle contraction perpendicular to these stromal parts may influence the growth of tumors preferentially in one direction, contributing to linearity of the lesion. In addition, the clinical appearance is not a reliable indicator of subclinical extension.2 Therefore, Lim et al6 recommended Mohs micrographic surgery as the best initial treatment of LBCCs.
Conclusion
Linear basal cell carcinoma should be considered a distinct morphologic variant of basal cell carcinoma. Although likely underreported, this variant is uncommon. It presents most often in the periocular and neck regions. The most common histologic subtypes are nodular and infiltrative. Because of the likelihood of subclinical spread, LBCC should be regarded as a high-risk subtype. As such, Mohs micrographic surgery or excision with complete circumferential peripheral and deep margin assessment is recommended as first-line treatment of LBCC.6
Case Report
A 63-year-old man was evaluated in the Mohs clinic for a lesion on the right supraclavicular neck, which he described as a linear asymptomatic “birthmark” that had been present since childhood and stable for many years. It began to enlarge approximately 5 years prior, became increasingly red, and had occasional crusting. The lesion also gradually became more irritated with repeated mild trauma when he carried a backpack while hiking. On physical examination, a 10×2-cm, linear, pink plaque with an irregular border, translucent rolled edges, and central smooth atrophic skin was seen on the right supraclavicular neck (Figure). There was no visible epidermal nevus or nevus sebaceous in the area. A shave biopsy of the lesion confirmed the pathologic diagnosis of basal cell carcinoma, nodular type, along with the morphologic diagnosis of linear basal cell carcinoma (LBCC). The tumor was completely removed with standard excision using 5-mm margins.

Approximately 10 months after the original excision, the patient developed an irritated erosion that occasionally bled when his backpack rubbed against it. He returned to the clinic after the erosion failed to heal. Physical examination revealed a 1.4×0.7-cm, eroded, pink papule with large telangiectases at the superior pole of the excision scar. A shave biopsy confirmed the diagnosis of a recurrent infiltrative basal cell carcinoma. The tumor was then completely excised using Mohs micrographic surgery.
Comment
Linear basal cell carcinoma, first described by Lewis1 in 1985, is a rare morphologic variant of basal cell carcinoma. In 2011, Al-Niaimi and Lyon2 performed a comprehensive literature search on LBCC (1985-2008) and found only 39 cases (including 2 of their own) had been published since the pioneer case in 1985. It was determined that the most common sites affected were the periorbital area and neck (n=13 each [67%]), and the majority were histologically nodular (n=27 [69%]). Mohs micrographic surgery was the most common treatment method (n=23 [59%]), followed by primary excision (n=17 [44%]). A history of trauma, radiotherapy, or prior operation in association with the site of the LBCC was discovered in only 7 cases (18%).2 Although Peschen et al3 proposed that trauma—both physical and surgical—and radiotherapy may play a role in the development of LBCCs, the low incidence reported suggests that other factors may be involved. To determine if genetic factors were contributing to the development of LBCCs, Yamaguchi et al4 investigated the expression of p27 and PCTAIRE1, both known to contribute to tumorigenesis when mutated, as well as somatic gene mutations using deep sequencing in a case of LBCC; they found no associated genetic mutation.
Reported Cases of LBCC
According to a PubMed search of articles indexed for MEDLINE using the terms linear and basal cell carcinoma, 67 cases (including the current case) of LBCC have been published since 1985. The patient demographics, anatomic location, histologic subtype, treatment methods, and frequency of recurrence for all reported cases of LBCC are summarized in the Table.1-24 There were 36 women and 31 men, with an average age of 70 years (range, 40–92 years). The most commonly affected sites were the periocular region (n=27) and neck (n=18). Histologically, most LBCCs were nodular (n=35), with the next most common histologic subtype being infiltrative (n=20), which included the morphoeic, metatypical, and micronodular subtypes under the overarching infiltrative subtype. The most frequently chosen treatment option was primary excision (n=38 [57%]), followed by Mohs micrographic surgery (n=28 [42%]). Risk factors previously identified by Al-Niaimi and Lyon,2 including trauma, radiotherapy, or prior operation, were reported in 12 of 67 cases. Recurrence was reported in only 2 of 67 cases, 1 being the current case; however, an accurate recurrence rate could not be calculated due to lack of follow-up or short length of follow-up in most of the reported cases.

Presentation and Treatment
Currently, there are no set criteria for the diagnosis of LBCC, but it has been shown to follow a characteristic morphologic pattern, favoring extension in one direction leading to a length-to-width ratio that typically is at least 3 to 1.5 With most lesions presenting in the periocular region along relaxed skin tension lines, it has been speculated that these tumors expand along wrinkles.2 Pierard and Lapiere25 proposed that the preferential parallel orientation and a straightening of thin collagen bundles and elastic fibers within the reticular dermis combined with relaxed skin tension lines and muscle contraction perpendicular to these stromal parts may influence the growth of tumors preferentially in one direction, contributing to linearity of the lesion. In addition, the clinical appearance is not a reliable indicator of subclinical extension.2 Therefore, Lim et al6 recommended Mohs micrographic surgery as the best initial treatment of LBCCs.
Conclusion
Linear basal cell carcinoma should be considered a distinct morphologic variant of basal cell carcinoma. Although likely underreported, this variant is uncommon. It presents most often in the periocular and neck regions. The most common histologic subtypes are nodular and infiltrative. Because of the likelihood of subclinical spread, LBCC should be regarded as a high-risk subtype. As such, Mohs micrographic surgery or excision with complete circumferential peripheral and deep margin assessment is recommended as first-line treatment of LBCC.6
- Lewis JE. Linear basal cell epithelioma. Int J Dermatol. 1985;24:124-125.
- Al-Niaimi F, Lyon CC. Linear basal cell carcinoma: a distinct condition? Clin Exp Dermatol. 2011;36:231-234.
- Peschen M, Lo JS, Snow SN, et al. Linear basal cell carcinoma. Cutis. 1993;51:287-289.
- Yamaguchi Y, Yanagi T, Imafuku K, et al. A case of linear basal cell carcinoma: evaluation of proliferative activity by immunohistochemical staining of PCTAIRE1 and p27. J Eur Acad Dermatol Venereol. 2017;31:E359-E362.
- Mavirakis I, Malhotra R, Selva D, et al. Linear basal cell carcinoma: a distinct clinical entity. J Plast Reconstr Aesthet Surg. 2006;59:419-423.
- Lim KK, Randle HW, Roenigk RK, et al. Linear basal cell carcinoma: report of seventeen cases and review of the presentation and treatment. Dermatol Surg. 1999;25:63-67.
- Pardavila R, Rosón E, De la torre C, et al. Linear basal cell carcinoma. report of two cases [in Spanish]. Actas Dermosifiliogr. 2007;98:291.
- Shinsuke K, Hirohiko K, Yasuhiro T, et al. Linear basal cell carcinoma in an Asian patient. Open Ophthalmol J. 2007;1:20-22.
- Ning C, Chao S. Linear basal cell carcinoma of the scrotum. Dermatol Sinica. 2002;20:57-62.
- Chopra KF, Cohen PR. Linear basal cell carcinomas: report of multiple sequential tumors localized to a radiotherapy port and review of the literature. Tex Med. 1997;93:57-59.
- da Silva MO, Dadalt P, Santos OL, et al. Linear basal cell carcinoma. Int J Dermatol. 1995;34:488.
- Warthan TL, Lewis JE. Giant linear basal cell epithelioma. Int J Dermatol. 1994;33:284.
- Lewis JE. Linear basal cell epithelioma. Int J Dermatol. 1989;28:682-684.
- Alcántara-Reifs CM, Salido-Vallejo R, González-Menchen A, et al. Linear basal cell carcinoma: report of three cases with dermoscopic findings. Indian J Dermatol Venereol Leprol. 2016;82:708-711.
- Lee MS, Cho E, Lee JH, et al. Linearly curved, blackish macule on the wrist. Cutis. 2016;97:384, 406-407.
- Bajaj S, Sharma PK, Kar HK. Linear adamantinoid basal cell carcinoma in the axilla. Dermatol Online J. 2015;21. pii:13030/qt8k0713nb.
- Iga N, Sakurai K, Fujii H, et al. Linear basal cell carcinoma at the external genitalia. J Dermatol. 2014;41:275-276.
- Ichinokawa Y, Ohtuki A, Hattori M, et al. Linear basal cell carcinoma: a case report. Case Rep Dermatol. 2011;3:142-146.
- Becher GL, Affleck A, Fleming C, et al. Linear basal cell carcinoma occurs most commonly on the lower eyelid. Clin Exp Dermatol. 2011;36:311-312.
- Jellouli A, Triki S, Zghal M, et al. Linear basal cell carcinoma. Actas Dermosifiliogr. 2010;101:648-650.
- Takiyoshi N, Nakano H, Kaneko T, et al. A linear basal cell carcinoma undergoing spontaneous regression. Clin Exp Dermatol. 2009;34:E411-E413.
- Yoleri L, Ozden S, Kandiloglu A. A 46-year-old male with an ulcerated linear lesion on his neck. Ann Saudi Med. 2008;28:57-58.
- Palleschi GM, Corradini D, Bruscino N, et al. Linear basal cell carcinoma: clinical significance and better surgical approach. G Ital Dermatol Venereol. 2016;151:119-121.
- Rodriguez-Garijo N, Redondo P. Linear basal cell carcinoma of the lower eyelid: reconstruction with a musculocutaneous transposition flap. JAAD Case Rep. 2018;4:633-635.
- Pierard GE, Lapiere CM. Microanatomy of the dermis in relation to relaxed skin tension lines and Langer’s lines. Am J Dermatopathol. 1987;9:219-224.
- Lewis JE. Linear basal cell epithelioma. Int J Dermatol. 1985;24:124-125.
- Al-Niaimi F, Lyon CC. Linear basal cell carcinoma: a distinct condition? Clin Exp Dermatol. 2011;36:231-234.
- Peschen M, Lo JS, Snow SN, et al. Linear basal cell carcinoma. Cutis. 1993;51:287-289.
- Yamaguchi Y, Yanagi T, Imafuku K, et al. A case of linear basal cell carcinoma: evaluation of proliferative activity by immunohistochemical staining of PCTAIRE1 and p27. J Eur Acad Dermatol Venereol. 2017;31:E359-E362.
- Mavirakis I, Malhotra R, Selva D, et al. Linear basal cell carcinoma: a distinct clinical entity. J Plast Reconstr Aesthet Surg. 2006;59:419-423.
- Lim KK, Randle HW, Roenigk RK, et al. Linear basal cell carcinoma: report of seventeen cases and review of the presentation and treatment. Dermatol Surg. 1999;25:63-67.
- Pardavila R, Rosón E, De la torre C, et al. Linear basal cell carcinoma. report of two cases [in Spanish]. Actas Dermosifiliogr. 2007;98:291.
- Shinsuke K, Hirohiko K, Yasuhiro T, et al. Linear basal cell carcinoma in an Asian patient. Open Ophthalmol J. 2007;1:20-22.
- Ning C, Chao S. Linear basal cell carcinoma of the scrotum. Dermatol Sinica. 2002;20:57-62.
- Chopra KF, Cohen PR. Linear basal cell carcinomas: report of multiple sequential tumors localized to a radiotherapy port and review of the literature. Tex Med. 1997;93:57-59.
- da Silva MO, Dadalt P, Santos OL, et al. Linear basal cell carcinoma. Int J Dermatol. 1995;34:488.
- Warthan TL, Lewis JE. Giant linear basal cell epithelioma. Int J Dermatol. 1994;33:284.
- Lewis JE. Linear basal cell epithelioma. Int J Dermatol. 1989;28:682-684.
- Alcántara-Reifs CM, Salido-Vallejo R, González-Menchen A, et al. Linear basal cell carcinoma: report of three cases with dermoscopic findings. Indian J Dermatol Venereol Leprol. 2016;82:708-711.
- Lee MS, Cho E, Lee JH, et al. Linearly curved, blackish macule on the wrist. Cutis. 2016;97:384, 406-407.
- Bajaj S, Sharma PK, Kar HK. Linear adamantinoid basal cell carcinoma in the axilla. Dermatol Online J. 2015;21. pii:13030/qt8k0713nb.
- Iga N, Sakurai K, Fujii H, et al. Linear basal cell carcinoma at the external genitalia. J Dermatol. 2014;41:275-276.
- Ichinokawa Y, Ohtuki A, Hattori M, et al. Linear basal cell carcinoma: a case report. Case Rep Dermatol. 2011;3:142-146.
- Becher GL, Affleck A, Fleming C, et al. Linear basal cell carcinoma occurs most commonly on the lower eyelid. Clin Exp Dermatol. 2011;36:311-312.
- Jellouli A, Triki S, Zghal M, et al. Linear basal cell carcinoma. Actas Dermosifiliogr. 2010;101:648-650.
- Takiyoshi N, Nakano H, Kaneko T, et al. A linear basal cell carcinoma undergoing spontaneous regression. Clin Exp Dermatol. 2009;34:E411-E413.
- Yoleri L, Ozden S, Kandiloglu A. A 46-year-old male with an ulcerated linear lesion on his neck. Ann Saudi Med. 2008;28:57-58.
- Palleschi GM, Corradini D, Bruscino N, et al. Linear basal cell carcinoma: clinical significance and better surgical approach. G Ital Dermatol Venereol. 2016;151:119-121.
- Rodriguez-Garijo N, Redondo P. Linear basal cell carcinoma of the lower eyelid: reconstruction with a musculocutaneous transposition flap. JAAD Case Rep. 2018;4:633-635.
- Pierard GE, Lapiere CM. Microanatomy of the dermis in relation to relaxed skin tension lines and Langer’s lines. Am J Dermatopathol. 1987;9:219-224.
Practice Points
- Linear basal cell carcinoma (LBCC) follows a characteristic morphologic pattern of a length-to-width ratio that typically is at least 3 to 1.
- Linear basal cell carcinomas most commonly present in the periocular region and on the neck along relaxed skin tension lines.
- Because of the likelihood of subclinical spread, LBCC should be regarded as a high-risk subtype of basal cell carcinoma.
- Mohs micrographic surgery or excision with complete circumferential peripheral and deep-margin assessment is recommended as first-line treatment.
Noninvasive Imaging Tools in Dermatology
Traditionally, diagnosis of skin disease relies on clinical inspection, often followed by biopsy and histopathologic examination. In recent years, new noninvasive tools have emerged that can aid in clinical diagnosis and reduce the number of unnecessary benign biopsies. Although there has been a surge in noninvasive diagnostic technologies, many tools are still in research and development phases, with few tools widely adopted and used in regular clinical practice. In this article, we discuss the use of dermoscopy, reflectance confocal microscopy (RCM), and optical coherence tomography (OCT) in the diagnosis and management of skin disease.
Dermoscopy
Dermoscopy, also known as epiluminescence light microscopy and previously known as dermatoscopy, utilizes a ×10 to ×100 microscope objective with a light source to magnify and visualize structures present below the skin’s surface, such as melanin and blood vessels. There are 3 types of dermoscopy: conventional nonpolarized dermoscopy, polarized contact dermoscopy, and nonpolarized contact dermoscopy (Figure 1). Traditional nonpolarized dermoscopy requires a liquid medium and direct contact with the skin, and it relies on light reflection and refraction properties.1 Cross-polarized light sources allow visualization of deeper structures, either with or without a liquid medium and contact with the skin surface. Although there is overall concurrence among the different types of dermoscopy, subtle differences in the appearance of color, features, and structure are present.1
Dermoscopy offers many benefits for dermatologists and other providers. It can be used to aid in the diagnosis of cutaneous neoplasms and other skin diseases. Numerous low-cost dermatoscopes currently are commercially available. The handheld, easily transportable nature of dermatoscopes have resulted in widespread practice integration. Approximately 84% of attending dermatologists in US academic settings reported using dermoscopy, and many refer to the dermatoscope as “the dermatologist’s stethoscope.”2 In addition, 6% to 15% of other US providers, including family physicians, internal medicine physicians, and plastic surgeons, have reported using dermoscopy in their clinical practices. Limitations of dermoscopy include visualization of the skin surface only and not deeper structures within the tissue, the need for training for adequate interpretation of dermoscopic images, and lack of reimbursement for dermoscopic examination.3
Many dermoscopic structures that correspond well with histopathology have been described. Dermoscopy has a sensitivity of 79% to 96% and specificity of 69% to 99% in the diagnosis of melanoma.4 There is variable data on the specificity of dermoscopy in the diagnosis of melanoma, with one meta-analysis finding no statistically significant difference in specificity compared to naked eye examination,5 while other studies report increased specificity and subsequent reduction in biopsy of benign lesions.6,7 Dermoscopy also can aid in the diagnosis of keratinocytic neoplasms, and dermoscopy also results in a sensitivity of 78.6% to 100% and a specificity of 53.8% to 100% in the diagnosis of basal cell carcinoma (BCC).8 Limitations of dermoscopy include false-positive diagnoses, commonly seborrheic keratoses and nevi, resulting in unnecessary biopsies, as well as false-negative diagnoses, commonly amelanotic and nevoid melanoma, resulting in delays in skin cancer diagnosis and resultant poor outcomes.9 Dermoscopy also is used to aid in the diagnosis of inflammatory and infectious skin diseases, as well as scalp, hair, and nail disorders.10
Reflectance Confocal Microscopy
Reflectance confocal microscopy utilizes an 830-nm laser to capture horizontal en face images of the skin with high resolution. Different structures of the skin have varying indices of refraction: keratin, melanin, and collagen appear bright white, while other components appear dark, generating black-and-white RCM images.11 Currently, there are 2 reflectance confocal microscopes that are commercially available in the United States. The Vivascope 1500 (Caliber ID) is the traditional model that captures 8×8-mm images, and the Vivascope 3000 (Caliber ID) is a smaller handheld model that captures 0.5×0.5-mm images. The traditional model provides the advantages of higher-resolution images and the ability to capture larger surface areas but is best suited to image flat areas of skin to which a square window can be adhered. The handheld model allows improved contact with the varying topography of skin; does not require an adhesive window; and can be used to image cartilaginous, mucosal, and sensitive surfaces. However, it can be difficult to correlate individual images captured by the handheld RCM with the location relative to the lesion, as it is exquisitely sensitive to motion and also is operator dependent. Although complex algorithms are under development to stitch individual images to provide better correlation with the geography of the lesion, such programs are not yet widely available.12
Reflectance confocal microscopy affords many benefits for patients and providers. It is noninvasive and painless and is capable of imaging in vivo live skin as compared to clinical examination and dermoscopy, which only allow for visualization of the skin’s surface. Reflectance confocal microscopy also is time efficient, as imaging of a single lesion can be completed in 10 to 15 minutes. This technology generates high-resolution images, and RCM diagnosis has consistently demonstrated high sensitivity and specificity when compared to histopathology.13 Additionally, RCM imaging can spare biopsy and resultant scarring on cosmetically sensitive areas. Recently, RCM imaging of the skin has been granted Category I Current Procedural Terminology reimbursement codes that allow provider reimbursement and integration of RCM into daily practice14; however, private insurance coverage in the United States is variable. Limitations of RCM include a maximum depth of 200 to 300 µm, high cost to procure a reflectance confocal microscope, and the need for considerable training and practice to accurately interpret grayscale en face images.15
There has been extensive research regarding the use of RCM in the evaluation of cutaneous neoplasms and other skin diseases. Numerous features and patterns have been identified and described that correspond with different skin diseases and correspond well with histopathology (Figure 2).13,16,17 Reflectance confocal microscopy has demonstrated consistently high accuracy in the diagnosis of melanocytic lesions, with a sensitivity of 93% to 100% and a specificity of 75% to 99%.18-21 Reflectance confocal microscopy is especially useful in the evaluation of clinically or dermoscopically equivocal pigmented lesions due to greater specificity, resulting in a reduction of unnecessary biopsies.22,23 It also has high accuracy in the diagnosis of keratinocytic neoplasms, with a sensitivity of 82% to 100% and a specificity of 78% to 97% in the diagnosis of BCC,24 and a sensitivity of 74% to 100% and specificity of 78% to 100% in the diagnosis of squamous cell carcinoma (SCC).25,26 Evaluation of SCC and actinic keratosis (AK) using RCM may be limited by considerable hyperkeratosis and ulceration. In addition, it can be challenging to differentiate AK and SCC on RCM, and considerable expertise is required to accurately grade cytologic and architectural atypia.27 However, RCM has been used to discriminate between in situ and invasive proliferations.28 Reflectance confocal microscopy has wide applications in the diagnosis and management of cutaneous infections29,30 and inflammatory skin diseases.29,31-33 Recent RCM research explored the use of RCM to identify biopsy sites,34 delineate presurgical tumor margins,35,36 and monitor response to noninvasive treatments.37,38
Optical Coherence Tomography
Optical coherence tomography is an imaging modality that utilizes light backscatter from infrared light to produce grayscale cross-sectional or vertical images and horizontal en face images.39 Optical coherence tomography can visualize structures in the epidermis, dermoepidermal junction, and upper dermis.40 It can image boundaries of structures but cannot visualize individual cells.
There are different types of OCT devices available, including frequency-domain OCT (FD-OCT), or conventional OCT, and high-definition OCT (HD-OCT). With FD-OCT, images are captured at a maximum depth of 1 to 2 mm but with limited resolution. High-definition OCT has superior resolution compared to FD-OCT but is restricted to a shallower depth of 750 μm.39 The main advantage of OCT is the ability to noninvasively image live tissue and visualize 2- to 5-times greater depth as compared to RCM. Several OCT devices have obtained US Food and Drug Administration approval; however, OCT has not been widely adopted into clinical practice and is available only in tertiary academic centers. Additionally, OCT imaging in dermatology is rarely reimbursed. Other limitations of OCT include poor resolution of images, high cost to procure an OCT device, and the need for advanced training and experience to accurately interpret images.40,41
Optical coherence tomography primarily is used to diagnose cutaneous neoplasms. The best evidence of the diagnostic accuracy of OCT is in the setting of BCC, with a recent systematic review reporting a sensitivity of 66% to 96% and a specificity of 75% to 86% for conventional FD-OCT.42 The use of FD-OCT results in an increase in specificity without a significant change in sensitivity when compared to dermoscopy in the diagnosis of BCC.43 Melanoma is difficult to diagnose via FD-OCT, as the visualization of architectural features often is limited by poor resolution.44 A study of HD-OCT in the diagnosis of melanoma with a limited sample size reported a sensitivity of 74% to 80% and a specificity of 92% to 93%.45 Similarly, a study of HD-OCT used in the diagnosis of AK and SCC revealed a sensitivity and specificity of 81.6% and 92.6%, respectively, for AK and 93.8% and 98.9%, respectively, for SCC.46
Numerous algorithms and scoring systems have been developed to further explore the utility of OCT in the diagnosis of cutaneous neoplasms.47,48 Recent research investigated the utility of dynamic OCT, which can evaluate microvasculature in the diagnosis of cutaneous neoplasms (Figure 3)49; the combination of OCT with other imaging modalities50,51; the use of OCT to delineate presurgical margins52,53; and the role of OCT in the diagnosis and monitoring of inflammatory and infectious skin diseases.54,55
Final Thoughts
In recent years, there has been a surge of interest in noninvasive techniques for diagnosis and management of skin diseases; however, noninvasive tools exist on a spectrum in dermatology. Dermoscopy provides low-cost imaging of the skin’s surface and has been widely adopted by dermatologists and other providers to aid in clinical diagnosis. Reflectance confocal microscopy provides reimbursable in vivo imaging of live tissue with cellular-level resolution but is limited by depth, cost, and need for advanced training; thus, RCM has only been adopted in some clinical practices. Optical coherence tomography offers in vivo imaging of live tissue with substantial depth but poor resolution, high cost, need for advanced training, and rare reimbursement for providers. Future directions include combination of complementary imaging modalities, increased clinical practice integration, and education and reimbursement for providers.
- Benvenuto-Andrade C, Dusza SW, Agero AL, et al. Differences between polarized light dermoscopy and immersion contact dermoscopy for the evaluation of skin lesions. Arch Dermatol. 2007;143:329-338.
- Terushkin V, Oliveria SA, Marghoob AA, et al. Use of and beliefs about total body photography and dermatoscopy among US dermatology training programs: an update. J Am Acad Dermatol. 2010;62:794-803.
- Morris JB, Alfonso SV, Hernandez N, et al. Use of and intentions to use dermoscopy among physicians in the United States. Dermatol Pract Concept. 2017;7:7-16.
- Yélamos O, Braun RP, Liopyris K, et al. Dermoscopy and dermatopathology correlates of cutaneous neoplasms. J Am Acad Dermatol. 2019;80:341-363.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Carli P, de Giorgi V, Chiarugi A, et al. Addition of dermoscopy to conventional naked-eye examination in melanoma screening: a randomized study. J Am Acad Dermatol. 2004;50:683-668.
- Lallas A, Zalaudek I, Argenziano G, et al. Dermoscopy in general dermatology. Dermatol Clin. 2013;31:679-694.
- Reiter O, Mimouni I, Gdalvevich M, et al. The diagnostic accuracy of dermoscopy for basal cell carcinoma: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:1380-1388.
- Papageorgiou V, Apalla Z, Sotiriou E, et al. The limitations of dermoscopy: false-positive and false-negative tumours. J Eur Acad Dermatol Venereol. 2018;32:879-888.
- Micali G, Verzì AE, Lacarrubba F. Alternative uses of dermoscopy in daily clinical practice: an update. J Am Acad Dermatol. 2018;79:1117-1132.e1.
- Rajadhyaksha M, Grossman M, Esterowitz D, et al. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol. 1995;104:946-952.
- Kose K, Gou M, Yélamos O, et al. Automated video-mosaicking approach for confocal microscopic imaging in vivo: an approach to address challenges in imaging living tissue and extend field of view. Sci Rep. 2017;7:10759.
- Rao BK, John AM, Francisco G, et al. Diagnostic accuracy of reflectance confocal microscopy for diagnosis of skin lesions [published online October 8, 2018]. Arch Pathol Lab Med. 2019;143:326-329.
- Current Procedural Terminology, Professional Edition. Chicago IL: American Medical Association; 2016. The preliminary physician fee schedule for 2017 is available at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices-Items/CMS-1654-P.html.
- Jain M, Pulijal SV, Rajadhyaksha M, et al. Evaluation of bedside diagnostic accuracy, learning curve, and challenges for a novice reflectance confocal microscopy reader for skin cancer detection in vivo. JAMA Dermatol. 2018;154:962-965.
- Rao BK, Pellacani G. Atlas of Confocal Microscopy in Dermatology: Clinical, Confocal, and Histological Images. New York, NY: NIDIskin LLC; 2013.
- Scope A, Benvenuto-Andrande C, Agero AL, et al. In vivo reflectance confocal microscopy imaging of melanocytic skin lesions: consensus terminology glossary and illustrative images. J Am Acad Dermatol. 2007;57:644-658.
- Gerger A, Hofmann-Wellenhof R, Langsenlehner U, et al. In vivo confocal laser scanning microscopy of melanocytic skin tumours: diagnostic applicability using unselected tumour images. Br J Dermatol. 2008;158:329-333.
- Stevenson AD, Mickan S, Mallett S, et al. Systematic review of diagnostic accuracy of reflectance confocal microscopy for melanoma diagnosis in patients with clinically equivocal skin lesions. Dermatol Pract Concept. 2013;3:19-27.
- Alarcon I, Carrera C, Palou J, et al. Impact of in vivo reflectance confocal microscopy on the number needed to treat melanoma in doubtful lesions. Br J Dermatol. 2014;170:802-808.
- Lovatto L, Carrera C, Salerni G, et al. In vivo reflectance confocal microscopy of equivocal melanocytic lesions detected by digital dermoscopy follow-up. J Eur Acad Dermatol Venereol. 2015;29:1918-1925.
- Guitera P, Pellacani G, Longo C, et al. In vivo reflectance confocal microscopy enhances secondary evaluation of melanocytic lesions. J Invest Dermatol. 2009;129:131-138.
- Xiong YQ, Ma SJ, Mo Y, et al. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: a meta-analysis. J Cancer Res Clin Oncol. 2017;143:1627-1635.
- Kadouch DJ, Schram ME, Leeflang MM, et al. In vivo confocal microscopy of basal cell carcinoma: a systematic review of diagnostic accuracy. J Eur Acad Dermatol Venereol. 2015;29:1890-1897.
- Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Reflectance confocal microscopy for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018;12:CD013191.
- Nguyen KP, Peppelman M, Hoogedoorn L, et al. The current role of in vivo reflectance confocal microscopy within the continuum of actinic keratosis and squamous cell carcinoma: a systematic review. Eur J Dermatol. 2016;26:549-565.
- Pellacani G, Ulrich M, Casari A, et al. Grading keratinocyte atypia in actinic keratosis: a correlation of reflectance confocal microscopy and histopathology. J Eur Acad Dermatol Venereol. 2015;29:2216-2221.
- Manfredini M, Longo C, Ferrari B, et al. Dermoscopic and reflectance confocal microscopy features of cutaneous squamous cell carcinoma. J Eur Acad Dermatol Venereol. 2017;31:1828-1833.
- Hoogedoorn L, Peppelman M, van de Kerkhof PC, et al. The value of in vivo reflectance confocal microscopy in the diagnosis and monitoring of inflammatory and infectious skin diseases: a systematic review. Br J Dermatol. 2015;172:1222-1248.
- Cinotti E, Perrot JL, Labeille B, et al. Reflectance confocal microscopy for cutaneous infections and infestations. J Eur Acad Dermatol Venereol. 2016;30:754-763.
- Ardigo M, Longo C, Gonzalez S; International Confocal Working Group Inflammatory Skin Diseases Project. Multicentre study on inflammatory skin diseases from The International Confocal Working Group: specific confocal microscopy features and an algorithmic method of diagnosis. Br J Dermatol. 2016;175:364-374.
- Ardigo M, Agozzino M, Franceschini C, et al. Reflectance confocal microscopy algorithms for inflammatory and hair diseases. Dermatol Clin. 2016;34:487-496.
- Manfredini M, Bettoli V, Sacripanti G, et al. The evolution of healthy skin to acne lesions: a longitudinal, in vivo evaluation with reflectance confocal microscopy and optical coherence tomography [published online April 26, 2019]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.15641.
- Navarrete-Dechent C, Mori S, Cordova M, et al. Reflectance confocal microscopy as a novel tool for presurgical identification of basal cell carcinoma biopsy site. J Am Acad Dermatol. 2019;80:e7-e8.
- Pan ZY, Lin JR, Cheng TT, et al. In vivo reflectance confocal microscopy of basal cell carcinoma: feasibility of preoperative mapping of cancer margins. Dermatol Surg. 2012;38:1945-1950.
- Venturini M, Gualdi G, Zanca A, et al. A new approach for presurgical margin assessment by reflectance confocal microscopy of basal cell carcinoma. Br J Dermatol. 2016;174:380-385.
- Sierra H, Yélamos O, Cordova M, et al. Reflectance confocal microscopy‐guided laser ablation of basal cell carcinomas: initial clinical experience. J Biomed Opt. 2017;22:1-13.
- Maier T, Kulichova D, Ruzicka T, et al. Noninvasive monitoring of basal cell carcinomas treated with systemic hedgehog inhibitors: pseudocysts as a sign of tumor regression. J Am Acad Dermatol. 2014;71:725-730.
- Levine A, Wang K, Markowitz O. Optical coherence tomography in the diagnosis of skin cancer. Dermatol Clin. 2017;35:465-488.
- Schneider SL, Kohli I, Hamzavi IH, et al. Emerging imaging technologies in dermatology: part I: basic principles. J Am Acad Dermatol. 2019;80:1114-1120.
- Mogensen M, Joergensen TM, Nümberg BM, et al. Assessment of optical coherence tomography imaging in the diagnosis of non‐melanoma skin cancer and benign lesions versus normal skin: observer‐blinded evaluation by dermatologists and pathologists. Dermatol Surg. 2009;35:965-972.
- Ferrante di Ruffano L, Dinnes J, Deeks JJ, et al. Optical coherence tomography for diagnosing skin cancer in adults. Cochrane Database Syst Rev. 2018;12:CD013189.
- Ulrich M, von Braunmuehl T, Kurzen H, et al. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: an observational study. Br J Dermatol. 2015;173:428-435.
- Wessels R, de Bruin DM, Relyveld GM, et al. Functional optical coherence tomography of pigmented lesions. J Eur Acad Dermatol Venereol. 2015;29:738‐744.
- Gambichler T, Schmid-Wendtner MH, Plura I, et al. A multicentre pilot study investigating high‐definition optical coherence tomography in the differentiation of cutaneous melanoma and melanocytic naevi. J Eur Acad Dermatol Venereol. 2015;29:537‐541.
- Marneffe A, Suppa M, Miyamoto M, et al. Validation of a diagnostic algorithm for the discrimination of actinic keratosis from normal skin and squamous cell carcinoma by means of high-definition optical coherence tomography. Exp Dermatol. 2016;25:684-687.
- Boone MA, Suppa M, Dhaenens F, et al. In vivo assessment of optical properties of melanocytic skin lesions and differentiation of melanoma from non-malignant lesions by high-definition optical coherence tomography. Arch Dermatol Res. 2016;308:7-20.
- Boone MA, Suppa M, Marneffe A, et al. A new algorithm for the discrimination of actinic keratosis from normal skin and squamous cell carcinoma based on in vivo analysis of optical properties by high-definition optical coherence tomography. J Eur Acad Dermatol Venereol. 2016;30:1714-1725.
- Themstrup L, Pellacani G, Welzel J, et al. In vivo microvascular imaging of cutaneous actinic keratosis, Bowen’s disease and squamous cell carcinoma using dynamic optical coherence tomography. J Eur Acad Dermatol Venereol. 2017;31:1655-1662.
- Alex A, Weingast J, Weinigel M, et al. Three-dimensional multiphoton/optical coherence tomography for diagnostic applications in dermatology. J Biophotonics. 2013;6:352-362.
- Iftimia N, Yélamos O, Chen CJ, et al. Handheld optical coherence tomography-reflectance confocal microscopy probe for detection of basal cell carcinoma and delineation of margins. J Biomed Opt. 2017;22:76006.
- Wang KX, Meekings A, Fluhr JW, et al. Optical coherence tomography-based optimization of Mohs micrographic surgery of basal cell carcinoma: a pilot study. Dermatol Surg. 2013;39:627-633.
- Chan CS, Rohrer TE. Optical coherence tomography and its role in Mohs micrographic surgery: a case report. Case Rep Dermatol. 2012;4:269-274.
- Gambichler T, Jaedicke V, Terras S. Optical coherence tomography in dermatology: technical and clinical aspects. Arch Dermatol Res. 2011;303:457-473.
- Manfredini M, Greco M, Farnetani F, et al. Acne: morphologic and vascular study of lesions and surrounding skin by means of optical coherence tomography. J Eur Acad Dermatol Venereol. 2017;31:1541-1546.
Traditionally, diagnosis of skin disease relies on clinical inspection, often followed by biopsy and histopathologic examination. In recent years, new noninvasive tools have emerged that can aid in clinical diagnosis and reduce the number of unnecessary benign biopsies. Although there has been a surge in noninvasive diagnostic technologies, many tools are still in research and development phases, with few tools widely adopted and used in regular clinical practice. In this article, we discuss the use of dermoscopy, reflectance confocal microscopy (RCM), and optical coherence tomography (OCT) in the diagnosis and management of skin disease.
Dermoscopy
Dermoscopy, also known as epiluminescence light microscopy and previously known as dermatoscopy, utilizes a ×10 to ×100 microscope objective with a light source to magnify and visualize structures present below the skin’s surface, such as melanin and blood vessels. There are 3 types of dermoscopy: conventional nonpolarized dermoscopy, polarized contact dermoscopy, and nonpolarized contact dermoscopy (Figure 1). Traditional nonpolarized dermoscopy requires a liquid medium and direct contact with the skin, and it relies on light reflection and refraction properties.1 Cross-polarized light sources allow visualization of deeper structures, either with or without a liquid medium and contact with the skin surface. Although there is overall concurrence among the different types of dermoscopy, subtle differences in the appearance of color, features, and structure are present.1

Dermoscopy offers many benefits for dermatologists and other providers. It can be used to aid in the diagnosis of cutaneous neoplasms and other skin diseases. Numerous low-cost dermatoscopes currently are commercially available. The handheld, easily transportable nature of dermatoscopes have resulted in widespread practice integration. Approximately 84% of attending dermatologists in US academic settings reported using dermoscopy, and many refer to the dermatoscope as “the dermatologist’s stethoscope.”2 In addition, 6% to 15% of other US providers, including family physicians, internal medicine physicians, and plastic surgeons, have reported using dermoscopy in their clinical practices. Limitations of dermoscopy include visualization of the skin surface only and not deeper structures within the tissue, the need for training for adequate interpretation of dermoscopic images, and lack of reimbursement for dermoscopic examination.3
Many dermoscopic structures that correspond well with histopathology have been described. Dermoscopy has a sensitivity of 79% to 96% and specificity of 69% to 99% in the diagnosis of melanoma.4 There is variable data on the specificity of dermoscopy in the diagnosis of melanoma, with one meta-analysis finding no statistically significant difference in specificity compared to naked eye examination,5 while other studies report increased specificity and subsequent reduction in biopsy of benign lesions.6,7 Dermoscopy also can aid in the diagnosis of keratinocytic neoplasms, and dermoscopy also results in a sensitivity of 78.6% to 100% and a specificity of 53.8% to 100% in the diagnosis of basal cell carcinoma (BCC).8 Limitations of dermoscopy include false-positive diagnoses, commonly seborrheic keratoses and nevi, resulting in unnecessary biopsies, as well as false-negative diagnoses, commonly amelanotic and nevoid melanoma, resulting in delays in skin cancer diagnosis and resultant poor outcomes.9 Dermoscopy also is used to aid in the diagnosis of inflammatory and infectious skin diseases, as well as scalp, hair, and nail disorders.10
Reflectance Confocal Microscopy
Reflectance confocal microscopy utilizes an 830-nm laser to capture horizontal en face images of the skin with high resolution. Different structures of the skin have varying indices of refraction: keratin, melanin, and collagen appear bright white, while other components appear dark, generating black-and-white RCM images.11 Currently, there are 2 reflectance confocal microscopes that are commercially available in the United States. The Vivascope 1500 (Caliber ID) is the traditional model that captures 8×8-mm images, and the Vivascope 3000 (Caliber ID) is a smaller handheld model that captures 0.5×0.5-mm images. The traditional model provides the advantages of higher-resolution images and the ability to capture larger surface areas but is best suited to image flat areas of skin to which a square window can be adhered. The handheld model allows improved contact with the varying topography of skin; does not require an adhesive window; and can be used to image cartilaginous, mucosal, and sensitive surfaces. However, it can be difficult to correlate individual images captured by the handheld RCM with the location relative to the lesion, as it is exquisitely sensitive to motion and also is operator dependent. Although complex algorithms are under development to stitch individual images to provide better correlation with the geography of the lesion, such programs are not yet widely available.12
Reflectance confocal microscopy affords many benefits for patients and providers. It is noninvasive and painless and is capable of imaging in vivo live skin as compared to clinical examination and dermoscopy, which only allow for visualization of the skin’s surface. Reflectance confocal microscopy also is time efficient, as imaging of a single lesion can be completed in 10 to 15 minutes. This technology generates high-resolution images, and RCM diagnosis has consistently demonstrated high sensitivity and specificity when compared to histopathology.13 Additionally, RCM imaging can spare biopsy and resultant scarring on cosmetically sensitive areas. Recently, RCM imaging of the skin has been granted Category I Current Procedural Terminology reimbursement codes that allow provider reimbursement and integration of RCM into daily practice14; however, private insurance coverage in the United States is variable. Limitations of RCM include a maximum depth of 200 to 300 µm, high cost to procure a reflectance confocal microscope, and the need for considerable training and practice to accurately interpret grayscale en face images.15
There has been extensive research regarding the use of RCM in the evaluation of cutaneous neoplasms and other skin diseases. Numerous features and patterns have been identified and described that correspond with different skin diseases and correspond well with histopathology (Figure 2).13,16,17 Reflectance confocal microscopy has demonstrated consistently high accuracy in the diagnosis of melanocytic lesions, with a sensitivity of 93% to 100% and a specificity of 75% to 99%.18-21 Reflectance confocal microscopy is especially useful in the evaluation of clinically or dermoscopically equivocal pigmented lesions due to greater specificity, resulting in a reduction of unnecessary biopsies.22,23 It also has high accuracy in the diagnosis of keratinocytic neoplasms, with a sensitivity of 82% to 100% and a specificity of 78% to 97% in the diagnosis of BCC,24 and a sensitivity of 74% to 100% and specificity of 78% to 100% in the diagnosis of squamous cell carcinoma (SCC).25,26 Evaluation of SCC and actinic keratosis (AK) using RCM may be limited by considerable hyperkeratosis and ulceration. In addition, it can be challenging to differentiate AK and SCC on RCM, and considerable expertise is required to accurately grade cytologic and architectural atypia.27 However, RCM has been used to discriminate between in situ and invasive proliferations.28 Reflectance confocal microscopy has wide applications in the diagnosis and management of cutaneous infections29,30 and inflammatory skin diseases.29,31-33 Recent RCM research explored the use of RCM to identify biopsy sites,34 delineate presurgical tumor margins,35,36 and monitor response to noninvasive treatments.37,38

Optical Coherence Tomography
Optical coherence tomography is an imaging modality that utilizes light backscatter from infrared light to produce grayscale cross-sectional or vertical images and horizontal en face images.39 Optical coherence tomography can visualize structures in the epidermis, dermoepidermal junction, and upper dermis.40 It can image boundaries of structures but cannot visualize individual cells.
There are different types of OCT devices available, including frequency-domain OCT (FD-OCT), or conventional OCT, and high-definition OCT (HD-OCT). With FD-OCT, images are captured at a maximum depth of 1 to 2 mm but with limited resolution. High-definition OCT has superior resolution compared to FD-OCT but is restricted to a shallower depth of 750 μm.39 The main advantage of OCT is the ability to noninvasively image live tissue and visualize 2- to 5-times greater depth as compared to RCM. Several OCT devices have obtained US Food and Drug Administration approval; however, OCT has not been widely adopted into clinical practice and is available only in tertiary academic centers. Additionally, OCT imaging in dermatology is rarely reimbursed. Other limitations of OCT include poor resolution of images, high cost to procure an OCT device, and the need for advanced training and experience to accurately interpret images.40,41
Optical coherence tomography primarily is used to diagnose cutaneous neoplasms. The best evidence of the diagnostic accuracy of OCT is in the setting of BCC, with a recent systematic review reporting a sensitivity of 66% to 96% and a specificity of 75% to 86% for conventional FD-OCT.42 The use of FD-OCT results in an increase in specificity without a significant change in sensitivity when compared to dermoscopy in the diagnosis of BCC.43 Melanoma is difficult to diagnose via FD-OCT, as the visualization of architectural features often is limited by poor resolution.44 A study of HD-OCT in the diagnosis of melanoma with a limited sample size reported a sensitivity of 74% to 80% and a specificity of 92% to 93%.45 Similarly, a study of HD-OCT used in the diagnosis of AK and SCC revealed a sensitivity and specificity of 81.6% and 92.6%, respectively, for AK and 93.8% and 98.9%, respectively, for SCC.46
Numerous algorithms and scoring systems have been developed to further explore the utility of OCT in the diagnosis of cutaneous neoplasms.47,48 Recent research investigated the utility of dynamic OCT, which can evaluate microvasculature in the diagnosis of cutaneous neoplasms (Figure 3)49; the combination of OCT with other imaging modalities50,51; the use of OCT to delineate presurgical margins52,53; and the role of OCT in the diagnosis and monitoring of inflammatory and infectious skin diseases.54,55

Final Thoughts
In recent years, there has been a surge of interest in noninvasive techniques for diagnosis and management of skin diseases; however, noninvasive tools exist on a spectrum in dermatology. Dermoscopy provides low-cost imaging of the skin’s surface and has been widely adopted by dermatologists and other providers to aid in clinical diagnosis. Reflectance confocal microscopy provides reimbursable in vivo imaging of live tissue with cellular-level resolution but is limited by depth, cost, and need for advanced training; thus, RCM has only been adopted in some clinical practices. Optical coherence tomography offers in vivo imaging of live tissue with substantial depth but poor resolution, high cost, need for advanced training, and rare reimbursement for providers. Future directions include combination of complementary imaging modalities, increased clinical practice integration, and education and reimbursement for providers.
Traditionally, diagnosis of skin disease relies on clinical inspection, often followed by biopsy and histopathologic examination. In recent years, new noninvasive tools have emerged that can aid in clinical diagnosis and reduce the number of unnecessary benign biopsies. Although there has been a surge in noninvasive diagnostic technologies, many tools are still in research and development phases, with few tools widely adopted and used in regular clinical practice. In this article, we discuss the use of dermoscopy, reflectance confocal microscopy (RCM), and optical coherence tomography (OCT) in the diagnosis and management of skin disease.
Dermoscopy
Dermoscopy, also known as epiluminescence light microscopy and previously known as dermatoscopy, utilizes a ×10 to ×100 microscope objective with a light source to magnify and visualize structures present below the skin’s surface, such as melanin and blood vessels. There are 3 types of dermoscopy: conventional nonpolarized dermoscopy, polarized contact dermoscopy, and nonpolarized contact dermoscopy (Figure 1). Traditional nonpolarized dermoscopy requires a liquid medium and direct contact with the skin, and it relies on light reflection and refraction properties.1 Cross-polarized light sources allow visualization of deeper structures, either with or without a liquid medium and contact with the skin surface. Although there is overall concurrence among the different types of dermoscopy, subtle differences in the appearance of color, features, and structure are present.1

Dermoscopy offers many benefits for dermatologists and other providers. It can be used to aid in the diagnosis of cutaneous neoplasms and other skin diseases. Numerous low-cost dermatoscopes currently are commercially available. The handheld, easily transportable nature of dermatoscopes have resulted in widespread practice integration. Approximately 84% of attending dermatologists in US academic settings reported using dermoscopy, and many refer to the dermatoscope as “the dermatologist’s stethoscope.”2 In addition, 6% to 15% of other US providers, including family physicians, internal medicine physicians, and plastic surgeons, have reported using dermoscopy in their clinical practices. Limitations of dermoscopy include visualization of the skin surface only and not deeper structures within the tissue, the need for training for adequate interpretation of dermoscopic images, and lack of reimbursement for dermoscopic examination.3
Many dermoscopic structures that correspond well with histopathology have been described. Dermoscopy has a sensitivity of 79% to 96% and specificity of 69% to 99% in the diagnosis of melanoma.4 There is variable data on the specificity of dermoscopy in the diagnosis of melanoma, with one meta-analysis finding no statistically significant difference in specificity compared to naked eye examination,5 while other studies report increased specificity and subsequent reduction in biopsy of benign lesions.6,7 Dermoscopy also can aid in the diagnosis of keratinocytic neoplasms, and dermoscopy also results in a sensitivity of 78.6% to 100% and a specificity of 53.8% to 100% in the diagnosis of basal cell carcinoma (BCC).8 Limitations of dermoscopy include false-positive diagnoses, commonly seborrheic keratoses and nevi, resulting in unnecessary biopsies, as well as false-negative diagnoses, commonly amelanotic and nevoid melanoma, resulting in delays in skin cancer diagnosis and resultant poor outcomes.9 Dermoscopy also is used to aid in the diagnosis of inflammatory and infectious skin diseases, as well as scalp, hair, and nail disorders.10
Reflectance Confocal Microscopy
Reflectance confocal microscopy utilizes an 830-nm laser to capture horizontal en face images of the skin with high resolution. Different structures of the skin have varying indices of refraction: keratin, melanin, and collagen appear bright white, while other components appear dark, generating black-and-white RCM images.11 Currently, there are 2 reflectance confocal microscopes that are commercially available in the United States. The Vivascope 1500 (Caliber ID) is the traditional model that captures 8×8-mm images, and the Vivascope 3000 (Caliber ID) is a smaller handheld model that captures 0.5×0.5-mm images. The traditional model provides the advantages of higher-resolution images and the ability to capture larger surface areas but is best suited to image flat areas of skin to which a square window can be adhered. The handheld model allows improved contact with the varying topography of skin; does not require an adhesive window; and can be used to image cartilaginous, mucosal, and sensitive surfaces. However, it can be difficult to correlate individual images captured by the handheld RCM with the location relative to the lesion, as it is exquisitely sensitive to motion and also is operator dependent. Although complex algorithms are under development to stitch individual images to provide better correlation with the geography of the lesion, such programs are not yet widely available.12
Reflectance confocal microscopy affords many benefits for patients and providers. It is noninvasive and painless and is capable of imaging in vivo live skin as compared to clinical examination and dermoscopy, which only allow for visualization of the skin’s surface. Reflectance confocal microscopy also is time efficient, as imaging of a single lesion can be completed in 10 to 15 minutes. This technology generates high-resolution images, and RCM diagnosis has consistently demonstrated high sensitivity and specificity when compared to histopathology.13 Additionally, RCM imaging can spare biopsy and resultant scarring on cosmetically sensitive areas. Recently, RCM imaging of the skin has been granted Category I Current Procedural Terminology reimbursement codes that allow provider reimbursement and integration of RCM into daily practice14; however, private insurance coverage in the United States is variable. Limitations of RCM include a maximum depth of 200 to 300 µm, high cost to procure a reflectance confocal microscope, and the need for considerable training and practice to accurately interpret grayscale en face images.15
There has been extensive research regarding the use of RCM in the evaluation of cutaneous neoplasms and other skin diseases. Numerous features and patterns have been identified and described that correspond with different skin diseases and correspond well with histopathology (Figure 2).13,16,17 Reflectance confocal microscopy has demonstrated consistently high accuracy in the diagnosis of melanocytic lesions, with a sensitivity of 93% to 100% and a specificity of 75% to 99%.18-21 Reflectance confocal microscopy is especially useful in the evaluation of clinically or dermoscopically equivocal pigmented lesions due to greater specificity, resulting in a reduction of unnecessary biopsies.22,23 It also has high accuracy in the diagnosis of keratinocytic neoplasms, with a sensitivity of 82% to 100% and a specificity of 78% to 97% in the diagnosis of BCC,24 and a sensitivity of 74% to 100% and specificity of 78% to 100% in the diagnosis of squamous cell carcinoma (SCC).25,26 Evaluation of SCC and actinic keratosis (AK) using RCM may be limited by considerable hyperkeratosis and ulceration. In addition, it can be challenging to differentiate AK and SCC on RCM, and considerable expertise is required to accurately grade cytologic and architectural atypia.27 However, RCM has been used to discriminate between in situ and invasive proliferations.28 Reflectance confocal microscopy has wide applications in the diagnosis and management of cutaneous infections29,30 and inflammatory skin diseases.29,31-33 Recent RCM research explored the use of RCM to identify biopsy sites,34 delineate presurgical tumor margins,35,36 and monitor response to noninvasive treatments.37,38

Optical Coherence Tomography
Optical coherence tomography is an imaging modality that utilizes light backscatter from infrared light to produce grayscale cross-sectional or vertical images and horizontal en face images.39 Optical coherence tomography can visualize structures in the epidermis, dermoepidermal junction, and upper dermis.40 It can image boundaries of structures but cannot visualize individual cells.
There are different types of OCT devices available, including frequency-domain OCT (FD-OCT), or conventional OCT, and high-definition OCT (HD-OCT). With FD-OCT, images are captured at a maximum depth of 1 to 2 mm but with limited resolution. High-definition OCT has superior resolution compared to FD-OCT but is restricted to a shallower depth of 750 μm.39 The main advantage of OCT is the ability to noninvasively image live tissue and visualize 2- to 5-times greater depth as compared to RCM. Several OCT devices have obtained US Food and Drug Administration approval; however, OCT has not been widely adopted into clinical practice and is available only in tertiary academic centers. Additionally, OCT imaging in dermatology is rarely reimbursed. Other limitations of OCT include poor resolution of images, high cost to procure an OCT device, and the need for advanced training and experience to accurately interpret images.40,41
Optical coherence tomography primarily is used to diagnose cutaneous neoplasms. The best evidence of the diagnostic accuracy of OCT is in the setting of BCC, with a recent systematic review reporting a sensitivity of 66% to 96% and a specificity of 75% to 86% for conventional FD-OCT.42 The use of FD-OCT results in an increase in specificity without a significant change in sensitivity when compared to dermoscopy in the diagnosis of BCC.43 Melanoma is difficult to diagnose via FD-OCT, as the visualization of architectural features often is limited by poor resolution.44 A study of HD-OCT in the diagnosis of melanoma with a limited sample size reported a sensitivity of 74% to 80% and a specificity of 92% to 93%.45 Similarly, a study of HD-OCT used in the diagnosis of AK and SCC revealed a sensitivity and specificity of 81.6% and 92.6%, respectively, for AK and 93.8% and 98.9%, respectively, for SCC.46
Numerous algorithms and scoring systems have been developed to further explore the utility of OCT in the diagnosis of cutaneous neoplasms.47,48 Recent research investigated the utility of dynamic OCT, which can evaluate microvasculature in the diagnosis of cutaneous neoplasms (Figure 3)49; the combination of OCT with other imaging modalities50,51; the use of OCT to delineate presurgical margins52,53; and the role of OCT in the diagnosis and monitoring of inflammatory and infectious skin diseases.54,55

Final Thoughts
In recent years, there has been a surge of interest in noninvasive techniques for diagnosis and management of skin diseases; however, noninvasive tools exist on a spectrum in dermatology. Dermoscopy provides low-cost imaging of the skin’s surface and has been widely adopted by dermatologists and other providers to aid in clinical diagnosis. Reflectance confocal microscopy provides reimbursable in vivo imaging of live tissue with cellular-level resolution but is limited by depth, cost, and need for advanced training; thus, RCM has only been adopted in some clinical practices. Optical coherence tomography offers in vivo imaging of live tissue with substantial depth but poor resolution, high cost, need for advanced training, and rare reimbursement for providers. Future directions include combination of complementary imaging modalities, increased clinical practice integration, and education and reimbursement for providers.
- Benvenuto-Andrade C, Dusza SW, Agero AL, et al. Differences between polarized light dermoscopy and immersion contact dermoscopy for the evaluation of skin lesions. Arch Dermatol. 2007;143:329-338.
- Terushkin V, Oliveria SA, Marghoob AA, et al. Use of and beliefs about total body photography and dermatoscopy among US dermatology training programs: an update. J Am Acad Dermatol. 2010;62:794-803.
- Morris JB, Alfonso SV, Hernandez N, et al. Use of and intentions to use dermoscopy among physicians in the United States. Dermatol Pract Concept. 2017;7:7-16.
- Yélamos O, Braun RP, Liopyris K, et al. Dermoscopy and dermatopathology correlates of cutaneous neoplasms. J Am Acad Dermatol. 2019;80:341-363.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Carli P, de Giorgi V, Chiarugi A, et al. Addition of dermoscopy to conventional naked-eye examination in melanoma screening: a randomized study. J Am Acad Dermatol. 2004;50:683-668.
- Lallas A, Zalaudek I, Argenziano G, et al. Dermoscopy in general dermatology. Dermatol Clin. 2013;31:679-694.
- Reiter O, Mimouni I, Gdalvevich M, et al. The diagnostic accuracy of dermoscopy for basal cell carcinoma: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:1380-1388.
- Papageorgiou V, Apalla Z, Sotiriou E, et al. The limitations of dermoscopy: false-positive and false-negative tumours. J Eur Acad Dermatol Venereol. 2018;32:879-888.
- Micali G, Verzì AE, Lacarrubba F. Alternative uses of dermoscopy in daily clinical practice: an update. J Am Acad Dermatol. 2018;79:1117-1132.e1.
- Rajadhyaksha M, Grossman M, Esterowitz D, et al. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol. 1995;104:946-952.
- Kose K, Gou M, Yélamos O, et al. Automated video-mosaicking approach for confocal microscopic imaging in vivo: an approach to address challenges in imaging living tissue and extend field of view. Sci Rep. 2017;7:10759.
- Rao BK, John AM, Francisco G, et al. Diagnostic accuracy of reflectance confocal microscopy for diagnosis of skin lesions [published online October 8, 2018]. Arch Pathol Lab Med. 2019;143:326-329.
- Current Procedural Terminology, Professional Edition. Chicago IL: American Medical Association; 2016. The preliminary physician fee schedule for 2017 is available at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices-Items/CMS-1654-P.html.
- Jain M, Pulijal SV, Rajadhyaksha M, et al. Evaluation of bedside diagnostic accuracy, learning curve, and challenges for a novice reflectance confocal microscopy reader for skin cancer detection in vivo. JAMA Dermatol. 2018;154:962-965.
- Rao BK, Pellacani G. Atlas of Confocal Microscopy in Dermatology: Clinical, Confocal, and Histological Images. New York, NY: NIDIskin LLC; 2013.
- Scope A, Benvenuto-Andrande C, Agero AL, et al. In vivo reflectance confocal microscopy imaging of melanocytic skin lesions: consensus terminology glossary and illustrative images. J Am Acad Dermatol. 2007;57:644-658.
- Gerger A, Hofmann-Wellenhof R, Langsenlehner U, et al. In vivo confocal laser scanning microscopy of melanocytic skin tumours: diagnostic applicability using unselected tumour images. Br J Dermatol. 2008;158:329-333.
- Stevenson AD, Mickan S, Mallett S, et al. Systematic review of diagnostic accuracy of reflectance confocal microscopy for melanoma diagnosis in patients with clinically equivocal skin lesions. Dermatol Pract Concept. 2013;3:19-27.
- Alarcon I, Carrera C, Palou J, et al. Impact of in vivo reflectance confocal microscopy on the number needed to treat melanoma in doubtful lesions. Br J Dermatol. 2014;170:802-808.
- Lovatto L, Carrera C, Salerni G, et al. In vivo reflectance confocal microscopy of equivocal melanocytic lesions detected by digital dermoscopy follow-up. J Eur Acad Dermatol Venereol. 2015;29:1918-1925.
- Guitera P, Pellacani G, Longo C, et al. In vivo reflectance confocal microscopy enhances secondary evaluation of melanocytic lesions. J Invest Dermatol. 2009;129:131-138.
- Xiong YQ, Ma SJ, Mo Y, et al. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: a meta-analysis. J Cancer Res Clin Oncol. 2017;143:1627-1635.
- Kadouch DJ, Schram ME, Leeflang MM, et al. In vivo confocal microscopy of basal cell carcinoma: a systematic review of diagnostic accuracy. J Eur Acad Dermatol Venereol. 2015;29:1890-1897.
- Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Reflectance confocal microscopy for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018;12:CD013191.
- Nguyen KP, Peppelman M, Hoogedoorn L, et al. The current role of in vivo reflectance confocal microscopy within the continuum of actinic keratosis and squamous cell carcinoma: a systematic review. Eur J Dermatol. 2016;26:549-565.
- Pellacani G, Ulrich M, Casari A, et al. Grading keratinocyte atypia in actinic keratosis: a correlation of reflectance confocal microscopy and histopathology. J Eur Acad Dermatol Venereol. 2015;29:2216-2221.
- Manfredini M, Longo C, Ferrari B, et al. Dermoscopic and reflectance confocal microscopy features of cutaneous squamous cell carcinoma. J Eur Acad Dermatol Venereol. 2017;31:1828-1833.
- Hoogedoorn L, Peppelman M, van de Kerkhof PC, et al. The value of in vivo reflectance confocal microscopy in the diagnosis and monitoring of inflammatory and infectious skin diseases: a systematic review. Br J Dermatol. 2015;172:1222-1248.
- Cinotti E, Perrot JL, Labeille B, et al. Reflectance confocal microscopy for cutaneous infections and infestations. J Eur Acad Dermatol Venereol. 2016;30:754-763.
- Ardigo M, Longo C, Gonzalez S; International Confocal Working Group Inflammatory Skin Diseases Project. Multicentre study on inflammatory skin diseases from The International Confocal Working Group: specific confocal microscopy features and an algorithmic method of diagnosis. Br J Dermatol. 2016;175:364-374.
- Ardigo M, Agozzino M, Franceschini C, et al. Reflectance confocal microscopy algorithms for inflammatory and hair diseases. Dermatol Clin. 2016;34:487-496.
- Manfredini M, Bettoli V, Sacripanti G, et al. The evolution of healthy skin to acne lesions: a longitudinal, in vivo evaluation with reflectance confocal microscopy and optical coherence tomography [published online April 26, 2019]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.15641.
- Navarrete-Dechent C, Mori S, Cordova M, et al. Reflectance confocal microscopy as a novel tool for presurgical identification of basal cell carcinoma biopsy site. J Am Acad Dermatol. 2019;80:e7-e8.
- Pan ZY, Lin JR, Cheng TT, et al. In vivo reflectance confocal microscopy of basal cell carcinoma: feasibility of preoperative mapping of cancer margins. Dermatol Surg. 2012;38:1945-1950.
- Venturini M, Gualdi G, Zanca A, et al. A new approach for presurgical margin assessment by reflectance confocal microscopy of basal cell carcinoma. Br J Dermatol. 2016;174:380-385.
- Sierra H, Yélamos O, Cordova M, et al. Reflectance confocal microscopy‐guided laser ablation of basal cell carcinomas: initial clinical experience. J Biomed Opt. 2017;22:1-13.
- Maier T, Kulichova D, Ruzicka T, et al. Noninvasive monitoring of basal cell carcinomas treated with systemic hedgehog inhibitors: pseudocysts as a sign of tumor regression. J Am Acad Dermatol. 2014;71:725-730.
- Levine A, Wang K, Markowitz O. Optical coherence tomography in the diagnosis of skin cancer. Dermatol Clin. 2017;35:465-488.
- Schneider SL, Kohli I, Hamzavi IH, et al. Emerging imaging technologies in dermatology: part I: basic principles. J Am Acad Dermatol. 2019;80:1114-1120.
- Mogensen M, Joergensen TM, Nümberg BM, et al. Assessment of optical coherence tomography imaging in the diagnosis of non‐melanoma skin cancer and benign lesions versus normal skin: observer‐blinded evaluation by dermatologists and pathologists. Dermatol Surg. 2009;35:965-972.
- Ferrante di Ruffano L, Dinnes J, Deeks JJ, et al. Optical coherence tomography for diagnosing skin cancer in adults. Cochrane Database Syst Rev. 2018;12:CD013189.
- Ulrich M, von Braunmuehl T, Kurzen H, et al. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: an observational study. Br J Dermatol. 2015;173:428-435.
- Wessels R, de Bruin DM, Relyveld GM, et al. Functional optical coherence tomography of pigmented lesions. J Eur Acad Dermatol Venereol. 2015;29:738‐744.
- Gambichler T, Schmid-Wendtner MH, Plura I, et al. A multicentre pilot study investigating high‐definition optical coherence tomography in the differentiation of cutaneous melanoma and melanocytic naevi. J Eur Acad Dermatol Venereol. 2015;29:537‐541.
- Marneffe A, Suppa M, Miyamoto M, et al. Validation of a diagnostic algorithm for the discrimination of actinic keratosis from normal skin and squamous cell carcinoma by means of high-definition optical coherence tomography. Exp Dermatol. 2016;25:684-687.
- Boone MA, Suppa M, Dhaenens F, et al. In vivo assessment of optical properties of melanocytic skin lesions and differentiation of melanoma from non-malignant lesions by high-definition optical coherence tomography. Arch Dermatol Res. 2016;308:7-20.
- Boone MA, Suppa M, Marneffe A, et al. A new algorithm for the discrimination of actinic keratosis from normal skin and squamous cell carcinoma based on in vivo analysis of optical properties by high-definition optical coherence tomography. J Eur Acad Dermatol Venereol. 2016;30:1714-1725.
- Themstrup L, Pellacani G, Welzel J, et al. In vivo microvascular imaging of cutaneous actinic keratosis, Bowen’s disease and squamous cell carcinoma using dynamic optical coherence tomography. J Eur Acad Dermatol Venereol. 2017;31:1655-1662.
- Alex A, Weingast J, Weinigel M, et al. Three-dimensional multiphoton/optical coherence tomography for diagnostic applications in dermatology. J Biophotonics. 2013;6:352-362.
- Iftimia N, Yélamos O, Chen CJ, et al. Handheld optical coherence tomography-reflectance confocal microscopy probe for detection of basal cell carcinoma and delineation of margins. J Biomed Opt. 2017;22:76006.
- Wang KX, Meekings A, Fluhr JW, et al. Optical coherence tomography-based optimization of Mohs micrographic surgery of basal cell carcinoma: a pilot study. Dermatol Surg. 2013;39:627-633.
- Chan CS, Rohrer TE. Optical coherence tomography and its role in Mohs micrographic surgery: a case report. Case Rep Dermatol. 2012;4:269-274.
- Gambichler T, Jaedicke V, Terras S. Optical coherence tomography in dermatology: technical and clinical aspects. Arch Dermatol Res. 2011;303:457-473.
- Manfredini M, Greco M, Farnetani F, et al. Acne: morphologic and vascular study of lesions and surrounding skin by means of optical coherence tomography. J Eur Acad Dermatol Venereol. 2017;31:1541-1546.
- Benvenuto-Andrade C, Dusza SW, Agero AL, et al. Differences between polarized light dermoscopy and immersion contact dermoscopy for the evaluation of skin lesions. Arch Dermatol. 2007;143:329-338.
- Terushkin V, Oliveria SA, Marghoob AA, et al. Use of and beliefs about total body photography and dermatoscopy among US dermatology training programs: an update. J Am Acad Dermatol. 2010;62:794-803.
- Morris JB, Alfonso SV, Hernandez N, et al. Use of and intentions to use dermoscopy among physicians in the United States. Dermatol Pract Concept. 2017;7:7-16.
- Yélamos O, Braun RP, Liopyris K, et al. Dermoscopy and dermatopathology correlates of cutaneous neoplasms. J Am Acad Dermatol. 2019;80:341-363.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Carli P, de Giorgi V, Chiarugi A, et al. Addition of dermoscopy to conventional naked-eye examination in melanoma screening: a randomized study. J Am Acad Dermatol. 2004;50:683-668.
- Lallas A, Zalaudek I, Argenziano G, et al. Dermoscopy in general dermatology. Dermatol Clin. 2013;31:679-694.
- Reiter O, Mimouni I, Gdalvevich M, et al. The diagnostic accuracy of dermoscopy for basal cell carcinoma: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:1380-1388.
- Papageorgiou V, Apalla Z, Sotiriou E, et al. The limitations of dermoscopy: false-positive and false-negative tumours. J Eur Acad Dermatol Venereol. 2018;32:879-888.
- Micali G, Verzì AE, Lacarrubba F. Alternative uses of dermoscopy in daily clinical practice: an update. J Am Acad Dermatol. 2018;79:1117-1132.e1.
- Rajadhyaksha M, Grossman M, Esterowitz D, et al. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol. 1995;104:946-952.
- Kose K, Gou M, Yélamos O, et al. Automated video-mosaicking approach for confocal microscopic imaging in vivo: an approach to address challenges in imaging living tissue and extend field of view. Sci Rep. 2017;7:10759.
- Rao BK, John AM, Francisco G, et al. Diagnostic accuracy of reflectance confocal microscopy for diagnosis of skin lesions [published online October 8, 2018]. Arch Pathol Lab Med. 2019;143:326-329.
- Current Procedural Terminology, Professional Edition. Chicago IL: American Medical Association; 2016. The preliminary physician fee schedule for 2017 is available at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices-Items/CMS-1654-P.html.
- Jain M, Pulijal SV, Rajadhyaksha M, et al. Evaluation of bedside diagnostic accuracy, learning curve, and challenges for a novice reflectance confocal microscopy reader for skin cancer detection in vivo. JAMA Dermatol. 2018;154:962-965.
- Rao BK, Pellacani G. Atlas of Confocal Microscopy in Dermatology: Clinical, Confocal, and Histological Images. New York, NY: NIDIskin LLC; 2013.
- Scope A, Benvenuto-Andrande C, Agero AL, et al. In vivo reflectance confocal microscopy imaging of melanocytic skin lesions: consensus terminology glossary and illustrative images. J Am Acad Dermatol. 2007;57:644-658.
- Gerger A, Hofmann-Wellenhof R, Langsenlehner U, et al. In vivo confocal laser scanning microscopy of melanocytic skin tumours: diagnostic applicability using unselected tumour images. Br J Dermatol. 2008;158:329-333.
- Stevenson AD, Mickan S, Mallett S, et al. Systematic review of diagnostic accuracy of reflectance confocal microscopy for melanoma diagnosis in patients with clinically equivocal skin lesions. Dermatol Pract Concept. 2013;3:19-27.
- Alarcon I, Carrera C, Palou J, et al. Impact of in vivo reflectance confocal microscopy on the number needed to treat melanoma in doubtful lesions. Br J Dermatol. 2014;170:802-808.
- Lovatto L, Carrera C, Salerni G, et al. In vivo reflectance confocal microscopy of equivocal melanocytic lesions detected by digital dermoscopy follow-up. J Eur Acad Dermatol Venereol. 2015;29:1918-1925.
- Guitera P, Pellacani G, Longo C, et al. In vivo reflectance confocal microscopy enhances secondary evaluation of melanocytic lesions. J Invest Dermatol. 2009;129:131-138.
- Xiong YQ, Ma SJ, Mo Y, et al. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: a meta-analysis. J Cancer Res Clin Oncol. 2017;143:1627-1635.
- Kadouch DJ, Schram ME, Leeflang MM, et al. In vivo confocal microscopy of basal cell carcinoma: a systematic review of diagnostic accuracy. J Eur Acad Dermatol Venereol. 2015;29:1890-1897.
- Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Reflectance confocal microscopy for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018;12:CD013191.
- Nguyen KP, Peppelman M, Hoogedoorn L, et al. The current role of in vivo reflectance confocal microscopy within the continuum of actinic keratosis and squamous cell carcinoma: a systematic review. Eur J Dermatol. 2016;26:549-565.
- Pellacani G, Ulrich M, Casari A, et al. Grading keratinocyte atypia in actinic keratosis: a correlation of reflectance confocal microscopy and histopathology. J Eur Acad Dermatol Venereol. 2015;29:2216-2221.
- Manfredini M, Longo C, Ferrari B, et al. Dermoscopic and reflectance confocal microscopy features of cutaneous squamous cell carcinoma. J Eur Acad Dermatol Venereol. 2017;31:1828-1833.
- Hoogedoorn L, Peppelman M, van de Kerkhof PC, et al. The value of in vivo reflectance confocal microscopy in the diagnosis and monitoring of inflammatory and infectious skin diseases: a systematic review. Br J Dermatol. 2015;172:1222-1248.
- Cinotti E, Perrot JL, Labeille B, et al. Reflectance confocal microscopy for cutaneous infections and infestations. J Eur Acad Dermatol Venereol. 2016;30:754-763.
- Ardigo M, Longo C, Gonzalez S; International Confocal Working Group Inflammatory Skin Diseases Project. Multicentre study on inflammatory skin diseases from The International Confocal Working Group: specific confocal microscopy features and an algorithmic method of diagnosis. Br J Dermatol. 2016;175:364-374.
- Ardigo M, Agozzino M, Franceschini C, et al. Reflectance confocal microscopy algorithms for inflammatory and hair diseases. Dermatol Clin. 2016;34:487-496.
- Manfredini M, Bettoli V, Sacripanti G, et al. The evolution of healthy skin to acne lesions: a longitudinal, in vivo evaluation with reflectance confocal microscopy and optical coherence tomography [published online April 26, 2019]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.15641.
- Navarrete-Dechent C, Mori S, Cordova M, et al. Reflectance confocal microscopy as a novel tool for presurgical identification of basal cell carcinoma biopsy site. J Am Acad Dermatol. 2019;80:e7-e8.
- Pan ZY, Lin JR, Cheng TT, et al. In vivo reflectance confocal microscopy of basal cell carcinoma: feasibility of preoperative mapping of cancer margins. Dermatol Surg. 2012;38:1945-1950.
- Venturini M, Gualdi G, Zanca A, et al. A new approach for presurgical margin assessment by reflectance confocal microscopy of basal cell carcinoma. Br J Dermatol. 2016;174:380-385.
- Sierra H, Yélamos O, Cordova M, et al. Reflectance confocal microscopy‐guided laser ablation of basal cell carcinomas: initial clinical experience. J Biomed Opt. 2017;22:1-13.
- Maier T, Kulichova D, Ruzicka T, et al. Noninvasive monitoring of basal cell carcinomas treated with systemic hedgehog inhibitors: pseudocysts as a sign of tumor regression. J Am Acad Dermatol. 2014;71:725-730.
- Levine A, Wang K, Markowitz O. Optical coherence tomography in the diagnosis of skin cancer. Dermatol Clin. 2017;35:465-488.
- Schneider SL, Kohli I, Hamzavi IH, et al. Emerging imaging technologies in dermatology: part I: basic principles. J Am Acad Dermatol. 2019;80:1114-1120.
- Mogensen M, Joergensen TM, Nümberg BM, et al. Assessment of optical coherence tomography imaging in the diagnosis of non‐melanoma skin cancer and benign lesions versus normal skin: observer‐blinded evaluation by dermatologists and pathologists. Dermatol Surg. 2009;35:965-972.
- Ferrante di Ruffano L, Dinnes J, Deeks JJ, et al. Optical coherence tomography for diagnosing skin cancer in adults. Cochrane Database Syst Rev. 2018;12:CD013189.
- Ulrich M, von Braunmuehl T, Kurzen H, et al. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: an observational study. Br J Dermatol. 2015;173:428-435.
- Wessels R, de Bruin DM, Relyveld GM, et al. Functional optical coherence tomography of pigmented lesions. J Eur Acad Dermatol Venereol. 2015;29:738‐744.
- Gambichler T, Schmid-Wendtner MH, Plura I, et al. A multicentre pilot study investigating high‐definition optical coherence tomography in the differentiation of cutaneous melanoma and melanocytic naevi. J Eur Acad Dermatol Venereol. 2015;29:537‐541.
- Marneffe A, Suppa M, Miyamoto M, et al. Validation of a diagnostic algorithm for the discrimination of actinic keratosis from normal skin and squamous cell carcinoma by means of high-definition optical coherence tomography. Exp Dermatol. 2016;25:684-687.
- Boone MA, Suppa M, Dhaenens F, et al. In vivo assessment of optical properties of melanocytic skin lesions and differentiation of melanoma from non-malignant lesions by high-definition optical coherence tomography. Arch Dermatol Res. 2016;308:7-20.
- Boone MA, Suppa M, Marneffe A, et al. A new algorithm for the discrimination of actinic keratosis from normal skin and squamous cell carcinoma based on in vivo analysis of optical properties by high-definition optical coherence tomography. J Eur Acad Dermatol Venereol. 2016;30:1714-1725.
- Themstrup L, Pellacani G, Welzel J, et al. In vivo microvascular imaging of cutaneous actinic keratosis, Bowen’s disease and squamous cell carcinoma using dynamic optical coherence tomography. J Eur Acad Dermatol Venereol. 2017;31:1655-1662.
- Alex A, Weingast J, Weinigel M, et al. Three-dimensional multiphoton/optical coherence tomography for diagnostic applications in dermatology. J Biophotonics. 2013;6:352-362.
- Iftimia N, Yélamos O, Chen CJ, et al. Handheld optical coherence tomography-reflectance confocal microscopy probe for detection of basal cell carcinoma and delineation of margins. J Biomed Opt. 2017;22:76006.
- Wang KX, Meekings A, Fluhr JW, et al. Optical coherence tomography-based optimization of Mohs micrographic surgery of basal cell carcinoma: a pilot study. Dermatol Surg. 2013;39:627-633.
- Chan CS, Rohrer TE. Optical coherence tomography and its role in Mohs micrographic surgery: a case report. Case Rep Dermatol. 2012;4:269-274.
- Gambichler T, Jaedicke V, Terras S. Optical coherence tomography in dermatology: technical and clinical aspects. Arch Dermatol Res. 2011;303:457-473.
- Manfredini M, Greco M, Farnetani F, et al. Acne: morphologic and vascular study of lesions and surrounding skin by means of optical coherence tomography. J Eur Acad Dermatol Venereol. 2017;31:1541-1546.
Practice Points
- There are several new noninvasive imaging tools in dermatology that can be utilized to aid in the diagnosis and management of skin disease, including dermoscopy, reflectance confocal microscopy, and optical coherence tomography.
- Among these tools, there are several differences in cost, clinical integration, reimbursement, and accuracy.
Barriers and Job Satisfaction Among Dermatology Hospitalists
Consultative dermatologists, or dermatology hospitalists (DHs), perform a critical role in the care of inpatients with skin disease, providing efficient diagnosis and management of patients with complex skin conditions as well as education of patients and trainees in the hospital setting.1 In 2013, 27% of the US population was seen by a physician for a skin disease.2 In 2014, there were nearly 650,000 hospital admissions principally for skin disease.3 Input by dermatologists facilitates accurate diagnosis and management of inpatients with skin disease,4 including a substantial number of cutaneous malignancies diagnosed in the inpatient setting.5 Several studies have highlighted the generally low level of diagnostic concordance between referring services and dermatology consultants,4,6 with dermatology consultants frequently noting diagnoses not considered by referring services,7 reinforcing the importance of having access to dermatologists in the hospital setting.
The care of skin disease in the inpatient setting has become increasingly complex. The Society for Dermatology Hospitalists (SDH) was created in 2009 to address this complexity, with the goal to “strive to develop the highest standards of clinical care of hospitalized patients with skin disease.”8 A recent survey found that 50% of DHs spend between 41 to 52 weeks per year on service.9 Despite this degree of commitment, there are considerable barriers that prevent the majority of dermatologists from efficiently providing inpatient consultative care. The inpatient and outpatient provision of dermatology care varies greatly, including the variety of ethical situations encountered and the diversity of skin conditions treated.10-12 Additionally, the transition between inpatient and outpatient care can be challenging for providers.13
The goal of this study was to evaluate the overall job satisfaction of DHs and further describe potential barriers to inpatient dermatology consultations.
Methods
An anonymous 31-question electronic survey was sent via email to all current members of the SDH from November 20 to December 10, 2018. The study was reviewed and determined to be exempt from federal human subjects regulations by the University of Washington Human Subjects Division (Seattle, Washington)(STUDY00005832).
Results
At the time of survey distribution, the SDH had 145 members, including attending-level dermatologists and resident members. Thirty-seven self-identified DHs (46% [17/37] women; 54% [20/37] men) completed the survey. The majority of respondents were junior faculty, with 46% (17/37) assistant professors, 5% (2/37) acting instructors, 32% (12/37) associate professors, and 16% (6/37) professors. All regions of the United States were represented.
Time Dedicated to Providing Inpatient Dermatology Consultations
The majority of those surveyed were satisfied or very satisfied (68% [25/37]) with the amount of time allotted for inpatient dermatology consultations, while 14% (5/37) were unsatisfied or very unsatisfied. Of those surveyed, 46% (17/37) reported that 21% to 50% of their time is dedicated to inpatient dermatology consultations. The majority (57% [21/37]) reported that their outpatient clinic efforts are reduced when providing dermatology inpatient consultations.
Regarding travel to the inpatient practice site, 60% (22/37) rated their travel time/effort as very easy, with 38% (14/37) reporting that the sites at which they provide inpatient dermatology consultations and their main outpatient clinics are the same physical location; 38% (14/37) reported travel times of less than 15 minutes between clinical practice sites.
Eighty-nine percent (33/37) of respondents said they are able to spend more time teaching trainees when providing inpatient dermatology consultations compared to their time spent in clinic. Similarly, 70% (26/37) said they are able to spend more time learning about patients and their conditions when providing inpatient dermatology consultations. Respondents also reported additional time expenditures because of inpatient dermatology consultations, primarily additional teaching requirements (49% [18/37]), additional electronic medical record training (35% [13/37]), and credentialing requirements (24% [9/37]).
Infrastructure for Providing Inpatient Dermatology Consultations
For many respondents (30% [11/37]), only 2 faculty dermatologists regularly provide inpatient dermatology consultations at their institutions. Four respondents reported having at least 5 faculty dermatologists who regularly provide inpatient dermatology consultations; excluding these, the average number of DHs was 2.42 faculty per institution.
Most respondents (57% [21/37]) reported their institutions support inpatient dermatology services by providing salary support for residents to cover services. Other methods of support included dedicated office spaces (30% [11/37]), free hospital parking while providing inpatient consultations (24% [9/37]), and administrative support (11% [4/37]).
Consultation Composition
Respondents indicated that requests for DH consultations most often come from medical services, including medical intensive care, internal medicine, and family medicine (95% [35/37]); the emergency department (95% [35/37]); surgical services (92% [34/37]); and hematology/oncology (89% [33/37]). Fewer DHs reported receiving consultation requests from pediatrics (70% [26/37]).
Many respondents (49% [18/37]) reported consulting for patients with skin disorders that they considered to be life-threatening or potentially life-threatening either very frequently (daily) or frequently (several times weekly), with only 16% (6/37) responding that they see such patients about once per month.
Compensation for Inpatient Dermatology Consultation
The most commonly reported compensation models for DHs were fixed salary plus productivity or performance incentives and fixed salary only models (49% [18/37] and 32% [12/37], respectively), with relative value unit (RVU) models and other models less frequently reported (16% [6/37] and 3% [1/37], respectively). Only 46% (17/37) of respondents were satisfied or very satisfied with their institutions’ compensation models; the remainder (54% [20/37]) were either neutral, unsatisfied, or very unsatisfied regarding their institutions’ compensation models. Overall compensation satisfaction was higher, with 60% (22/37) of DHs reporting they were satisfied or very satisfied with their salaries and 41% (15/37) reporting they were either neutral or not satisfied. The majority (60% [2/37]) of respondents felt that fixed salary plus productivity or performance incentives models would be the ideal compensation model for DHs.
Of the DHs whose compensations models were RVU based (6/37 [16%]), 67% (4/6) said they receive incentive pay upon meeting their RVU targets. No respondents reported that they were expected to generate an equivalent number of RVUs when performing inpatient consultations as compared to an outpatient session. Only 32% (12/37) of respondents reported keeping the revenue/RVUs generated by inpatient dermatology consultations; most (57% [21/37]) noted that their dermatology divisions/departments keep the revenue/RVUs, followed by university hospitals (27% [10/37]), schools of medicine (11% [4/37]), and departments of medicine (3% [1/37]). The remainder of respondents (22% [8/37]) were unsure who keeps the revenue/RVUs generated by inpatient dermatology consultations.
Most respondents (70% [26/37]) reported that the revenue (or RVU equivalent) generated by inpatient dermatology consultations does not fully support their salary for the time spent as consultants. Rather, these DHs noted sources of additional financial support, primarily the DHs themselves (69% [18/26]), followed by dermatology divisions/departments (50% [13/26]), departments of medicine (23% [6/26]), university hospitals (23% [6/26]), and schools of medicine (12% [3/26]).
Job Fulfillment Among DHs
Most respondents said they choose to provide inpatient dermatology consultations due to their interest in complex medical dermatology and their desire to work with other medical teams and specialties (92% [34/37] and 76% [28/37], respectively). Seventy percent (26/37) said they choose to provide inpatient consultations to be able to teach medical students and residents as well as to take advantage of the added opportunities to practice in a variety of settings beyond their outpatient clinics (57% [21/37]). Only 3% (1/37) of respondents reported that they provide inpatient dermatology consultations because they are “required to do so.”
Most DHs (84% [31/37]) said they feel their institutions as well as their dermatology divisions/departments value having access to inpatient dermatology services, though some did not feel this way (16% [6/37] neutral or strongly disagree). Nearly all respondents (97% [36/37]) felt they provide a critical service when performing inpatient dermatology consultations. All respondents (100%) said they found providing inpatient dermatology consultations fulfilling, and 65% (24/37) said they prefer providing inpatient dermatology consultations to spending time in clinic. Of the DHs who were surveyed, 68% (25/37) said they were satisfied with the balance of outpatient and inpatient services in their clinical practice and 30% (11/37) said they were not.
Comment
Factors such as patient care, hospital infrastructure, and procedural support have all been cited by DHs as crucial aspects of their contributions to the care of hospitalized pa
Dermatology is primarily an outpatient specialty, and our study highlighted several important challenges for providers performing inpatient dermatology consultations. A major issue is time expenditures, including additional teaching requirements, additional electronic medical record training, and credentialing requirements. Travel time to inpatient hospital sites does not appear to be one of these hindering factors; nearly 60% of respondents rated their travel time/effort as very easy, with approximately 75% of respondents’ consultation locations being either at the same physical location as their main outpatient clinic or less than 15 minutes away. Maintaining easy travel between outpatient and inpatient settings is important to the success of the DH.
Our data suggest that compensation of DHs is a potential limitation to providing inpatient dermatology care. Our survey reinforced that providers who do inpatient dermatology consultations generally do not generate the revenue necessary to cover these efforts. More than 40% of DH respondents said they either feel neutral about or unsatisfied with their overall salary, and more than half said they feel similarly regarding their institutions’ compensation models. Most respondents said that a fixed salary model plus productivity or performance incentives is the ideal compensation model for those providing inpatient dermatology consultations, though only half said they actually are compensated according to this model. This discrepancy highlights the disconnect between the current accepted compensation models and the DH’s ideal model and provides direction for dermatology chairs and division heads as to what compensation model is preferable to support the success of DHs at their institutions.
Despite the barriers and compensation constraints we identified, DHs report high job satisfaction, which we hypothesize could combat burnout. In our study, all DHs surveyed say they find providing inpatient dermatology consultations fulfilling, and most were satisfied with the amount of time allotted for consultations. Some of the possible reasons why DHs may find their work fulfilling include increased time for teaching trainees and learning about patients and their conditions while consulting, as well as a preference for providing inpatient dermatology consultations to spending time in clinic. Most DHs said they choose to provide inpatient dermatology consultation rather than do so as a requirement, primarily due to their interest in complex medical dermatology and their desire to work with other medical teams/specialties; thankfully, only a small percentage said they provide these consultations because they are required to do so.
This study was conducted to analyze job satisfaction among DHs who provided inpatient dermatology consultations and determine common barriers and obstacles to their job satisfaction. Limitations to our study included the small sample size and the possibly limited representation of the intended population, as only the members of the SDH were surveyed, potentially excluding providers who regularly perform inpatient dermatology consultations but are not members of the SDH. Further limitations included recall bias and the qualitative nature of the survey instrument.
Final Thoughts
There was near-unanimous agreement among the DHs we surveyed regarding the importance of the role they play in their divisions/departments, but there are clear barriers to provision of inpatient dermatology consultation, specifically relating to extraneous time expenditures and compensation. Despite these barriers, the majority of respondents said they are very satisfied with the role they play in the inpatient setting and feel that their contributions are valued by the institutions where they work. Protecting these benefits of providing dermatology hospital consultations will be critical for maintaining this high job satisfaction and balancing out the barriers to providing these consultations. Protecting the time required to provide consultations is paramount so DHs continue to gain fulfillment from teaching trainees, caring for complex patients, and maintaining their place as valuable colleagues in the hospital setting.
Acknowledgment
The authors thank the members of the SDH for their participation in this survey.
- Biesbroeck LK, Shinohara MM. Inpatient consultative dermatology. Med Clin North Am. 2015;99:1349-1364.
- Lim HW, Collins SAB, Resneck JS Jr, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958-972.e2.
- Arnold JD, Yoon SJ, Kirkorian AY. The national burden of inpatient dermatology in adults. J Am Acad Dermatol. 2018;80:425-432.
- Mancusi S, Festa Neto C. Inpatient dermatological consultations in a university hospital. Clinics (Sao Paulo). 2010;65:851-855.
- Tsai S, Scott JF, Keller JJ, et al. Cutaneous malignancies identified in an inpatient dermatology consultation service. Br J Dermatol. 2017;177:e116-e118.
- Pereira AR, Porro AM, Seque CA, et al. Inpatient dermatology consultations in renal transplant recipients. Actas Dermosifiliogr. 2018;109:900-907.
- Tracey EH, Forrestel A, Rosenbach M, et al. Inpatient dermatology consultation in patients with hematologic malignancies. J Am Acad Dermatol. 2016;75:835-836.
- Fox LP, Cotliar J, Hughey L, et al. Hospitalist dermatology. J Am Acad Dermatol. 2009;61:153-154.
- Ko LN, Kroshinsky D. Dermatology hospitalists: a multicenter survey study characterizing the infrastructure of consultative dermatology in select American hospitals. Int J Dermatol. 2018;57:553-558.
- Hansra NK, Shinkai K, Fox LP. Ethical issues in inpatient consultative dermatology. Clin Dermatol. 2012;30:496-500.
- El-Azhary R, Weenig RH, Gibson LE. The dermatology hospitalist: creating value by rapid clinical pathologic correlation in a patient-centered care model. Int J Dermatol. 2012;51:1461-1466.
- Ahronowitz I, Fox LP. Herpes zoster in hospitalized adults: practice gaps, new evidence, and remaining questions. J Am Acad Dermatol. 2018;78:223-230.e3.
- Rosenbach M. The logistics of an inpatient dermatology service. Semin Cutan Med Surg. 2017;36:3-8.
- Ackerman L, Kessler M. The efficient, effective community hospital inpatient dermatology consult. Semin Cutan Med Surg. 2017;36:9-11.
Consultative dermatologists, or dermatology hospitalists (DHs), perform a critical role in the care of inpatients with skin disease, providing efficient diagnosis and management of patients with complex skin conditions as well as education of patients and trainees in the hospital setting.1 In 2013, 27% of the US population was seen by a physician for a skin disease.2 In 2014, there were nearly 650,000 hospital admissions principally for skin disease.3 Input by dermatologists facilitates accurate diagnosis and management of inpatients with skin disease,4 including a substantial number of cutaneous malignancies diagnosed in the inpatient setting.5 Several studies have highlighted the generally low level of diagnostic concordance between referring services and dermatology consultants,4,6 with dermatology consultants frequently noting diagnoses not considered by referring services,7 reinforcing the importance of having access to dermatologists in the hospital setting.
The care of skin disease in the inpatient setting has become increasingly complex. The Society for Dermatology Hospitalists (SDH) was created in 2009 to address this complexity, with the goal to “strive to develop the highest standards of clinical care of hospitalized patients with skin disease.”8 A recent survey found that 50% of DHs spend between 41 to 52 weeks per year on service.9 Despite this degree of commitment, there are considerable barriers that prevent the majority of dermatologists from efficiently providing inpatient consultative care. The inpatient and outpatient provision of dermatology care varies greatly, including the variety of ethical situations encountered and the diversity of skin conditions treated.10-12 Additionally, the transition between inpatient and outpatient care can be challenging for providers.13
The goal of this study was to evaluate the overall job satisfaction of DHs and further describe potential barriers to inpatient dermatology consultations.
Methods
An anonymous 31-question electronic survey was sent via email to all current members of the SDH from November 20 to December 10, 2018. The study was reviewed and determined to be exempt from federal human subjects regulations by the University of Washington Human Subjects Division (Seattle, Washington)(STUDY00005832).
Results
At the time of survey distribution, the SDH had 145 members, including attending-level dermatologists and resident members. Thirty-seven self-identified DHs (46% [17/37] women; 54% [20/37] men) completed the survey. The majority of respondents were junior faculty, with 46% (17/37) assistant professors, 5% (2/37) acting instructors, 32% (12/37) associate professors, and 16% (6/37) professors. All regions of the United States were represented.
Time Dedicated to Providing Inpatient Dermatology Consultations
The majority of those surveyed were satisfied or very satisfied (68% [25/37]) with the amount of time allotted for inpatient dermatology consultations, while 14% (5/37) were unsatisfied or very unsatisfied. Of those surveyed, 46% (17/37) reported that 21% to 50% of their time is dedicated to inpatient dermatology consultations. The majority (57% [21/37]) reported that their outpatient clinic efforts are reduced when providing dermatology inpatient consultations.
Regarding travel to the inpatient practice site, 60% (22/37) rated their travel time/effort as very easy, with 38% (14/37) reporting that the sites at which they provide inpatient dermatology consultations and their main outpatient clinics are the same physical location; 38% (14/37) reported travel times of less than 15 minutes between clinical practice sites.
Eighty-nine percent (33/37) of respondents said they are able to spend more time teaching trainees when providing inpatient dermatology consultations compared to their time spent in clinic. Similarly, 70% (26/37) said they are able to spend more time learning about patients and their conditions when providing inpatient dermatology consultations. Respondents also reported additional time expenditures because of inpatient dermatology consultations, primarily additional teaching requirements (49% [18/37]), additional electronic medical record training (35% [13/37]), and credentialing requirements (24% [9/37]).
Infrastructure for Providing Inpatient Dermatology Consultations
For many respondents (30% [11/37]), only 2 faculty dermatologists regularly provide inpatient dermatology consultations at their institutions. Four respondents reported having at least 5 faculty dermatologists who regularly provide inpatient dermatology consultations; excluding these, the average number of DHs was 2.42 faculty per institution.
Most respondents (57% [21/37]) reported their institutions support inpatient dermatology services by providing salary support for residents to cover services. Other methods of support included dedicated office spaces (30% [11/37]), free hospital parking while providing inpatient consultations (24% [9/37]), and administrative support (11% [4/37]).
Consultation Composition
Respondents indicated that requests for DH consultations most often come from medical services, including medical intensive care, internal medicine, and family medicine (95% [35/37]); the emergency department (95% [35/37]); surgical services (92% [34/37]); and hematology/oncology (89% [33/37]). Fewer DHs reported receiving consultation requests from pediatrics (70% [26/37]).
Many respondents (49% [18/37]) reported consulting for patients with skin disorders that they considered to be life-threatening or potentially life-threatening either very frequently (daily) or frequently (several times weekly), with only 16% (6/37) responding that they see such patients about once per month.
Compensation for Inpatient Dermatology Consultation
The most commonly reported compensation models for DHs were fixed salary plus productivity or performance incentives and fixed salary only models (49% [18/37] and 32% [12/37], respectively), with relative value unit (RVU) models and other models less frequently reported (16% [6/37] and 3% [1/37], respectively). Only 46% (17/37) of respondents were satisfied or very satisfied with their institutions’ compensation models; the remainder (54% [20/37]) were either neutral, unsatisfied, or very unsatisfied regarding their institutions’ compensation models. Overall compensation satisfaction was higher, with 60% (22/37) of DHs reporting they were satisfied or very satisfied with their salaries and 41% (15/37) reporting they were either neutral or not satisfied. The majority (60% [2/37]) of respondents felt that fixed salary plus productivity or performance incentives models would be the ideal compensation model for DHs.
Of the DHs whose compensations models were RVU based (6/37 [16%]), 67% (4/6) said they receive incentive pay upon meeting their RVU targets. No respondents reported that they were expected to generate an equivalent number of RVUs when performing inpatient consultations as compared to an outpatient session. Only 32% (12/37) of respondents reported keeping the revenue/RVUs generated by inpatient dermatology consultations; most (57% [21/37]) noted that their dermatology divisions/departments keep the revenue/RVUs, followed by university hospitals (27% [10/37]), schools of medicine (11% [4/37]), and departments of medicine (3% [1/37]). The remainder of respondents (22% [8/37]) were unsure who keeps the revenue/RVUs generated by inpatient dermatology consultations.
Most respondents (70% [26/37]) reported that the revenue (or RVU equivalent) generated by inpatient dermatology consultations does not fully support their salary for the time spent as consultants. Rather, these DHs noted sources of additional financial support, primarily the DHs themselves (69% [18/26]), followed by dermatology divisions/departments (50% [13/26]), departments of medicine (23% [6/26]), university hospitals (23% [6/26]), and schools of medicine (12% [3/26]).
Job Fulfillment Among DHs
Most respondents said they choose to provide inpatient dermatology consultations due to their interest in complex medical dermatology and their desire to work with other medical teams and specialties (92% [34/37] and 76% [28/37], respectively). Seventy percent (26/37) said they choose to provide inpatient consultations to be able to teach medical students and residents as well as to take advantage of the added opportunities to practice in a variety of settings beyond their outpatient clinics (57% [21/37]). Only 3% (1/37) of respondents reported that they provide inpatient dermatology consultations because they are “required to do so.”
Most DHs (84% [31/37]) said they feel their institutions as well as their dermatology divisions/departments value having access to inpatient dermatology services, though some did not feel this way (16% [6/37] neutral or strongly disagree). Nearly all respondents (97% [36/37]) felt they provide a critical service when performing inpatient dermatology consultations. All respondents (100%) said they found providing inpatient dermatology consultations fulfilling, and 65% (24/37) said they prefer providing inpatient dermatology consultations to spending time in clinic. Of the DHs who were surveyed, 68% (25/37) said they were satisfied with the balance of outpatient and inpatient services in their clinical practice and 30% (11/37) said they were not.
Comment
Factors such as patient care, hospital infrastructure, and procedural support have all been cited by DHs as crucial aspects of their contributions to the care of hospitalized pa
Dermatology is primarily an outpatient specialty, and our study highlighted several important challenges for providers performing inpatient dermatology consultations. A major issue is time expenditures, including additional teaching requirements, additional electronic medical record training, and credentialing requirements. Travel time to inpatient hospital sites does not appear to be one of these hindering factors; nearly 60% of respondents rated their travel time/effort as very easy, with approximately 75% of respondents’ consultation locations being either at the same physical location as their main outpatient clinic or less than 15 minutes away. Maintaining easy travel between outpatient and inpatient settings is important to the success of the DH.
Our data suggest that compensation of DHs is a potential limitation to providing inpatient dermatology care. Our survey reinforced that providers who do inpatient dermatology consultations generally do not generate the revenue necessary to cover these efforts. More than 40% of DH respondents said they either feel neutral about or unsatisfied with their overall salary, and more than half said they feel similarly regarding their institutions’ compensation models. Most respondents said that a fixed salary model plus productivity or performance incentives is the ideal compensation model for those providing inpatient dermatology consultations, though only half said they actually are compensated according to this model. This discrepancy highlights the disconnect between the current accepted compensation models and the DH’s ideal model and provides direction for dermatology chairs and division heads as to what compensation model is preferable to support the success of DHs at their institutions.
Despite the barriers and compensation constraints we identified, DHs report high job satisfaction, which we hypothesize could combat burnout. In our study, all DHs surveyed say they find providing inpatient dermatology consultations fulfilling, and most were satisfied with the amount of time allotted for consultations. Some of the possible reasons why DHs may find their work fulfilling include increased time for teaching trainees and learning about patients and their conditions while consulting, as well as a preference for providing inpatient dermatology consultations to spending time in clinic. Most DHs said they choose to provide inpatient dermatology consultation rather than do so as a requirement, primarily due to their interest in complex medical dermatology and their desire to work with other medical teams/specialties; thankfully, only a small percentage said they provide these consultations because they are required to do so.
This study was conducted to analyze job satisfaction among DHs who provided inpatient dermatology consultations and determine common barriers and obstacles to their job satisfaction. Limitations to our study included the small sample size and the possibly limited representation of the intended population, as only the members of the SDH were surveyed, potentially excluding providers who regularly perform inpatient dermatology consultations but are not members of the SDH. Further limitations included recall bias and the qualitative nature of the survey instrument.
Final Thoughts
There was near-unanimous agreement among the DHs we surveyed regarding the importance of the role they play in their divisions/departments, but there are clear barriers to provision of inpatient dermatology consultation, specifically relating to extraneous time expenditures and compensation. Despite these barriers, the majority of respondents said they are very satisfied with the role they play in the inpatient setting and feel that their contributions are valued by the institutions where they work. Protecting these benefits of providing dermatology hospital consultations will be critical for maintaining this high job satisfaction and balancing out the barriers to providing these consultations. Protecting the time required to provide consultations is paramount so DHs continue to gain fulfillment from teaching trainees, caring for complex patients, and maintaining their place as valuable colleagues in the hospital setting.
Acknowledgment
The authors thank the members of the SDH for their participation in this survey.
Consultative dermatologists, or dermatology hospitalists (DHs), perform a critical role in the care of inpatients with skin disease, providing efficient diagnosis and management of patients with complex skin conditions as well as education of patients and trainees in the hospital setting.1 In 2013, 27% of the US population was seen by a physician for a skin disease.2 In 2014, there were nearly 650,000 hospital admissions principally for skin disease.3 Input by dermatologists facilitates accurate diagnosis and management of inpatients with skin disease,4 including a substantial number of cutaneous malignancies diagnosed in the inpatient setting.5 Several studies have highlighted the generally low level of diagnostic concordance between referring services and dermatology consultants,4,6 with dermatology consultants frequently noting diagnoses not considered by referring services,7 reinforcing the importance of having access to dermatologists in the hospital setting.
The care of skin disease in the inpatient setting has become increasingly complex. The Society for Dermatology Hospitalists (SDH) was created in 2009 to address this complexity, with the goal to “strive to develop the highest standards of clinical care of hospitalized patients with skin disease.”8 A recent survey found that 50% of DHs spend between 41 to 52 weeks per year on service.9 Despite this degree of commitment, there are considerable barriers that prevent the majority of dermatologists from efficiently providing inpatient consultative care. The inpatient and outpatient provision of dermatology care varies greatly, including the variety of ethical situations encountered and the diversity of skin conditions treated.10-12 Additionally, the transition between inpatient and outpatient care can be challenging for providers.13
The goal of this study was to evaluate the overall job satisfaction of DHs and further describe potential barriers to inpatient dermatology consultations.
Methods
An anonymous 31-question electronic survey was sent via email to all current members of the SDH from November 20 to December 10, 2018. The study was reviewed and determined to be exempt from federal human subjects regulations by the University of Washington Human Subjects Division (Seattle, Washington)(STUDY00005832).
Results
At the time of survey distribution, the SDH had 145 members, including attending-level dermatologists and resident members. Thirty-seven self-identified DHs (46% [17/37] women; 54% [20/37] men) completed the survey. The majority of respondents were junior faculty, with 46% (17/37) assistant professors, 5% (2/37) acting instructors, 32% (12/37) associate professors, and 16% (6/37) professors. All regions of the United States were represented.
Time Dedicated to Providing Inpatient Dermatology Consultations
The majority of those surveyed were satisfied or very satisfied (68% [25/37]) with the amount of time allotted for inpatient dermatology consultations, while 14% (5/37) were unsatisfied or very unsatisfied. Of those surveyed, 46% (17/37) reported that 21% to 50% of their time is dedicated to inpatient dermatology consultations. The majority (57% [21/37]) reported that their outpatient clinic efforts are reduced when providing dermatology inpatient consultations.
Regarding travel to the inpatient practice site, 60% (22/37) rated their travel time/effort as very easy, with 38% (14/37) reporting that the sites at which they provide inpatient dermatology consultations and their main outpatient clinics are the same physical location; 38% (14/37) reported travel times of less than 15 minutes between clinical practice sites.
Eighty-nine percent (33/37) of respondents said they are able to spend more time teaching trainees when providing inpatient dermatology consultations compared to their time spent in clinic. Similarly, 70% (26/37) said they are able to spend more time learning about patients and their conditions when providing inpatient dermatology consultations. Respondents also reported additional time expenditures because of inpatient dermatology consultations, primarily additional teaching requirements (49% [18/37]), additional electronic medical record training (35% [13/37]), and credentialing requirements (24% [9/37]).
Infrastructure for Providing Inpatient Dermatology Consultations
For many respondents (30% [11/37]), only 2 faculty dermatologists regularly provide inpatient dermatology consultations at their institutions. Four respondents reported having at least 5 faculty dermatologists who regularly provide inpatient dermatology consultations; excluding these, the average number of DHs was 2.42 faculty per institution.
Most respondents (57% [21/37]) reported their institutions support inpatient dermatology services by providing salary support for residents to cover services. Other methods of support included dedicated office spaces (30% [11/37]), free hospital parking while providing inpatient consultations (24% [9/37]), and administrative support (11% [4/37]).
Consultation Composition
Respondents indicated that requests for DH consultations most often come from medical services, including medical intensive care, internal medicine, and family medicine (95% [35/37]); the emergency department (95% [35/37]); surgical services (92% [34/37]); and hematology/oncology (89% [33/37]). Fewer DHs reported receiving consultation requests from pediatrics (70% [26/37]).
Many respondents (49% [18/37]) reported consulting for patients with skin disorders that they considered to be life-threatening or potentially life-threatening either very frequently (daily) or frequently (several times weekly), with only 16% (6/37) responding that they see such patients about once per month.
Compensation for Inpatient Dermatology Consultation
The most commonly reported compensation models for DHs were fixed salary plus productivity or performance incentives and fixed salary only models (49% [18/37] and 32% [12/37], respectively), with relative value unit (RVU) models and other models less frequently reported (16% [6/37] and 3% [1/37], respectively). Only 46% (17/37) of respondents were satisfied or very satisfied with their institutions’ compensation models; the remainder (54% [20/37]) were either neutral, unsatisfied, or very unsatisfied regarding their institutions’ compensation models. Overall compensation satisfaction was higher, with 60% (22/37) of DHs reporting they were satisfied or very satisfied with their salaries and 41% (15/37) reporting they were either neutral or not satisfied. The majority (60% [2/37]) of respondents felt that fixed salary plus productivity or performance incentives models would be the ideal compensation model for DHs.
Of the DHs whose compensations models were RVU based (6/37 [16%]), 67% (4/6) said they receive incentive pay upon meeting their RVU targets. No respondents reported that they were expected to generate an equivalent number of RVUs when performing inpatient consultations as compared to an outpatient session. Only 32% (12/37) of respondents reported keeping the revenue/RVUs generated by inpatient dermatology consultations; most (57% [21/37]) noted that their dermatology divisions/departments keep the revenue/RVUs, followed by university hospitals (27% [10/37]), schools of medicine (11% [4/37]), and departments of medicine (3% [1/37]). The remainder of respondents (22% [8/37]) were unsure who keeps the revenue/RVUs generated by inpatient dermatology consultations.
Most respondents (70% [26/37]) reported that the revenue (or RVU equivalent) generated by inpatient dermatology consultations does not fully support their salary for the time spent as consultants. Rather, these DHs noted sources of additional financial support, primarily the DHs themselves (69% [18/26]), followed by dermatology divisions/departments (50% [13/26]), departments of medicine (23% [6/26]), university hospitals (23% [6/26]), and schools of medicine (12% [3/26]).
Job Fulfillment Among DHs
Most respondents said they choose to provide inpatient dermatology consultations due to their interest in complex medical dermatology and their desire to work with other medical teams and specialties (92% [34/37] and 76% [28/37], respectively). Seventy percent (26/37) said they choose to provide inpatient consultations to be able to teach medical students and residents as well as to take advantage of the added opportunities to practice in a variety of settings beyond their outpatient clinics (57% [21/37]). Only 3% (1/37) of respondents reported that they provide inpatient dermatology consultations because they are “required to do so.”
Most DHs (84% [31/37]) said they feel their institutions as well as their dermatology divisions/departments value having access to inpatient dermatology services, though some did not feel this way (16% [6/37] neutral or strongly disagree). Nearly all respondents (97% [36/37]) felt they provide a critical service when performing inpatient dermatology consultations. All respondents (100%) said they found providing inpatient dermatology consultations fulfilling, and 65% (24/37) said they prefer providing inpatient dermatology consultations to spending time in clinic. Of the DHs who were surveyed, 68% (25/37) said they were satisfied with the balance of outpatient and inpatient services in their clinical practice and 30% (11/37) said they were not.
Comment
Factors such as patient care, hospital infrastructure, and procedural support have all been cited by DHs as crucial aspects of their contributions to the care of hospitalized pa
Dermatology is primarily an outpatient specialty, and our study highlighted several important challenges for providers performing inpatient dermatology consultations. A major issue is time expenditures, including additional teaching requirements, additional electronic medical record training, and credentialing requirements. Travel time to inpatient hospital sites does not appear to be one of these hindering factors; nearly 60% of respondents rated their travel time/effort as very easy, with approximately 75% of respondents’ consultation locations being either at the same physical location as their main outpatient clinic or less than 15 minutes away. Maintaining easy travel between outpatient and inpatient settings is important to the success of the DH.
Our data suggest that compensation of DHs is a potential limitation to providing inpatient dermatology care. Our survey reinforced that providers who do inpatient dermatology consultations generally do not generate the revenue necessary to cover these efforts. More than 40% of DH respondents said they either feel neutral about or unsatisfied with their overall salary, and more than half said they feel similarly regarding their institutions’ compensation models. Most respondents said that a fixed salary model plus productivity or performance incentives is the ideal compensation model for those providing inpatient dermatology consultations, though only half said they actually are compensated according to this model. This discrepancy highlights the disconnect between the current accepted compensation models and the DH’s ideal model and provides direction for dermatology chairs and division heads as to what compensation model is preferable to support the success of DHs at their institutions.
Despite the barriers and compensation constraints we identified, DHs report high job satisfaction, which we hypothesize could combat burnout. In our study, all DHs surveyed say they find providing inpatient dermatology consultations fulfilling, and most were satisfied with the amount of time allotted for consultations. Some of the possible reasons why DHs may find their work fulfilling include increased time for teaching trainees and learning about patients and their conditions while consulting, as well as a preference for providing inpatient dermatology consultations to spending time in clinic. Most DHs said they choose to provide inpatient dermatology consultation rather than do so as a requirement, primarily due to their interest in complex medical dermatology and their desire to work with other medical teams/specialties; thankfully, only a small percentage said they provide these consultations because they are required to do so.
This study was conducted to analyze job satisfaction among DHs who provided inpatient dermatology consultations and determine common barriers and obstacles to their job satisfaction. Limitations to our study included the small sample size and the possibly limited representation of the intended population, as only the members of the SDH were surveyed, potentially excluding providers who regularly perform inpatient dermatology consultations but are not members of the SDH. Further limitations included recall bias and the qualitative nature of the survey instrument.
Final Thoughts
There was near-unanimous agreement among the DHs we surveyed regarding the importance of the role they play in their divisions/departments, but there are clear barriers to provision of inpatient dermatology consultation, specifically relating to extraneous time expenditures and compensation. Despite these barriers, the majority of respondents said they are very satisfied with the role they play in the inpatient setting and feel that their contributions are valued by the institutions where they work. Protecting these benefits of providing dermatology hospital consultations will be critical for maintaining this high job satisfaction and balancing out the barriers to providing these consultations. Protecting the time required to provide consultations is paramount so DHs continue to gain fulfillment from teaching trainees, caring for complex patients, and maintaining their place as valuable colleagues in the hospital setting.
Acknowledgment
The authors thank the members of the SDH for their participation in this survey.
- Biesbroeck LK, Shinohara MM. Inpatient consultative dermatology. Med Clin North Am. 2015;99:1349-1364.
- Lim HW, Collins SAB, Resneck JS Jr, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958-972.e2.
- Arnold JD, Yoon SJ, Kirkorian AY. The national burden of inpatient dermatology in adults. J Am Acad Dermatol. 2018;80:425-432.
- Mancusi S, Festa Neto C. Inpatient dermatological consultations in a university hospital. Clinics (Sao Paulo). 2010;65:851-855.
- Tsai S, Scott JF, Keller JJ, et al. Cutaneous malignancies identified in an inpatient dermatology consultation service. Br J Dermatol. 2017;177:e116-e118.
- Pereira AR, Porro AM, Seque CA, et al. Inpatient dermatology consultations in renal transplant recipients. Actas Dermosifiliogr. 2018;109:900-907.
- Tracey EH, Forrestel A, Rosenbach M, et al. Inpatient dermatology consultation in patients with hematologic malignancies. J Am Acad Dermatol. 2016;75:835-836.
- Fox LP, Cotliar J, Hughey L, et al. Hospitalist dermatology. J Am Acad Dermatol. 2009;61:153-154.
- Ko LN, Kroshinsky D. Dermatology hospitalists: a multicenter survey study characterizing the infrastructure of consultative dermatology in select American hospitals. Int J Dermatol. 2018;57:553-558.
- Hansra NK, Shinkai K, Fox LP. Ethical issues in inpatient consultative dermatology. Clin Dermatol. 2012;30:496-500.
- El-Azhary R, Weenig RH, Gibson LE. The dermatology hospitalist: creating value by rapid clinical pathologic correlation in a patient-centered care model. Int J Dermatol. 2012;51:1461-1466.
- Ahronowitz I, Fox LP. Herpes zoster in hospitalized adults: practice gaps, new evidence, and remaining questions. J Am Acad Dermatol. 2018;78:223-230.e3.
- Rosenbach M. The logistics of an inpatient dermatology service. Semin Cutan Med Surg. 2017;36:3-8.
- Ackerman L, Kessler M. The efficient, effective community hospital inpatient dermatology consult. Semin Cutan Med Surg. 2017;36:9-11.
- Biesbroeck LK, Shinohara MM. Inpatient consultative dermatology. Med Clin North Am. 2015;99:1349-1364.
- Lim HW, Collins SAB, Resneck JS Jr, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958-972.e2.
- Arnold JD, Yoon SJ, Kirkorian AY. The national burden of inpatient dermatology in adults. J Am Acad Dermatol. 2018;80:425-432.
- Mancusi S, Festa Neto C. Inpatient dermatological consultations in a university hospital. Clinics (Sao Paulo). 2010;65:851-855.
- Tsai S, Scott JF, Keller JJ, et al. Cutaneous malignancies identified in an inpatient dermatology consultation service. Br J Dermatol. 2017;177:e116-e118.
- Pereira AR, Porro AM, Seque CA, et al. Inpatient dermatology consultations in renal transplant recipients. Actas Dermosifiliogr. 2018;109:900-907.
- Tracey EH, Forrestel A, Rosenbach M, et al. Inpatient dermatology consultation in patients with hematologic malignancies. J Am Acad Dermatol. 2016;75:835-836.
- Fox LP, Cotliar J, Hughey L, et al. Hospitalist dermatology. J Am Acad Dermatol. 2009;61:153-154.
- Ko LN, Kroshinsky D. Dermatology hospitalists: a multicenter survey study characterizing the infrastructure of consultative dermatology in select American hospitals. Int J Dermatol. 2018;57:553-558.
- Hansra NK, Shinkai K, Fox LP. Ethical issues in inpatient consultative dermatology. Clin Dermatol. 2012;30:496-500.
- El-Azhary R, Weenig RH, Gibson LE. The dermatology hospitalist: creating value by rapid clinical pathologic correlation in a patient-centered care model. Int J Dermatol. 2012;51:1461-1466.
- Ahronowitz I, Fox LP. Herpes zoster in hospitalized adults: practice gaps, new evidence, and remaining questions. J Am Acad Dermatol. 2018;78:223-230.e3.
- Rosenbach M. The logistics of an inpatient dermatology service. Semin Cutan Med Surg. 2017;36:3-8.
- Ackerman L, Kessler M. The efficient, effective community hospital inpatient dermatology consult. Semin Cutan Med Surg. 2017;36:9-11.
Practice Points
• Dermatology hospitalists play a critical role in the specialized care of hospitalized patients with
skin conditions.
• Dermatology hospitalists have high job satisfaction, with opportunities to teach trainees and practice complex medical dermatology.
• Most dermatology hospitalists do not generate sufficient revenue providing inpatient dermatology consultations to fully support their salary for the time spent as consultants; alternate payment models are needed to maintain dermatology’s presence in the hospital.
Cardiovascular complications most common with carfilzomib in relapsed myeloma
Cardiovascular (CV) adverse events were common in patients receiving proteasome inhibitor therapy for relapsed multiple myeloma, especially with carfilzomib-based therapy, according to results from the PROTECT study.
While prior studies have shown an increased risk for CV toxicities with proteasome inhibitor therapy, detailed descriptions of the events and risk factors have been lacking. “Furthermore, there is no validated protocol to help determine which patients are at highest risk of CV toxicity during therapy, nor is there management guidance for patients who experience a [CV adverse event],” wrote Robert F. Cornell, MD, of Vanderbilt University, Nashville, Tenn., and colleagues in the Journal of Clinical Oncology.
The PROTECT (Prospective Observation of Cardiac Safety with Proteasome Inhibitor) study was conducted at Vanderbilt University Medical Center and the University of Pennsylvania Abramson Cancer Center, Philadelphia, between September 2015 and March 2018.
Researchers followed 95 patients with relapsed multiple myeloma who were treated with either bortezomib or carfilzomib for a total duration of 18 months. A total of 65 patients received a carfilzomib-based therapy and 30 patients received a bortezomib-based therapy.
Study patients received a CV assessment at baseline and at the beginning of each treatment cycle for the initial six cycles of proteasome inhibitor therapy. Subsequently, patients were monitored for the development of CV adverse events. CV assessments included ECG, echocardiography, and measurement of other cardiac biomarkers, such as NTproBNP and troponin I or T.
CV toxicities were reported among 5 patients (16.7%) of patients treated with bortezomib and 33 patients (50.7%) treated with carfilzomib (P = .005).
In total, there were 64 CV adverse events reported, most of which were grade 2 or 3, and 56 of which occurred while on carfilzomib-based therapy. For carfilzomib, the most common complications were heart failure (23 cases), followed by grade 3 or 4 hypertension (13 cases). Cardiac chest pain, atrial fibrillation, and acute coronary syndrome were reported in fewer cases.
The researchers also found that elevated natriuretic peptides that occurred before starting carfilzomib therapy or within the first 3 weeks of carfilzomib therapy were associated with a substantially higher risk of CV adverse events.
Patients who have multiple CV risk factors, and especially patients with a history of CV complications and elevated baseline natriuretic peptides, should be referred for a comprehensive cardiac evaluation, the researchers advised. “Such patients are at highest risk of CV [adverse events] with carfilzomib-based therapy, and optimization of CV therapy seems to improve overall care, allow continuation of potentially lifesaving cancer treatment, and affect severity or development of CV [adverse events],” they wrote.
A key limitation of the study was the lack of standardized treatment regimens. As a result, there was a broad dosing range for carfilzomib, in comparison to bortezomib.
Some authors reported financial relationships with carfilzomib maker Amgen and bortezomib maker Takeda, as well as with other companies.
SOURCE: Cornell RF et al. J Clin Oncol. 2019 Jun 12. doi: 10.1200/JCO.19.00231.
Cardiovascular (CV) adverse events were common in patients receiving proteasome inhibitor therapy for relapsed multiple myeloma, especially with carfilzomib-based therapy, according to results from the PROTECT study.
While prior studies have shown an increased risk for CV toxicities with proteasome inhibitor therapy, detailed descriptions of the events and risk factors have been lacking. “Furthermore, there is no validated protocol to help determine which patients are at highest risk of CV toxicity during therapy, nor is there management guidance for patients who experience a [CV adverse event],” wrote Robert F. Cornell, MD, of Vanderbilt University, Nashville, Tenn., and colleagues in the Journal of Clinical Oncology.
The PROTECT (Prospective Observation of Cardiac Safety with Proteasome Inhibitor) study was conducted at Vanderbilt University Medical Center and the University of Pennsylvania Abramson Cancer Center, Philadelphia, between September 2015 and March 2018.
Researchers followed 95 patients with relapsed multiple myeloma who were treated with either bortezomib or carfilzomib for a total duration of 18 months. A total of 65 patients received a carfilzomib-based therapy and 30 patients received a bortezomib-based therapy.
Study patients received a CV assessment at baseline and at the beginning of each treatment cycle for the initial six cycles of proteasome inhibitor therapy. Subsequently, patients were monitored for the development of CV adverse events. CV assessments included ECG, echocardiography, and measurement of other cardiac biomarkers, such as NTproBNP and troponin I or T.
CV toxicities were reported among 5 patients (16.7%) of patients treated with bortezomib and 33 patients (50.7%) treated with carfilzomib (P = .005).
In total, there were 64 CV adverse events reported, most of which were grade 2 or 3, and 56 of which occurred while on carfilzomib-based therapy. For carfilzomib, the most common complications were heart failure (23 cases), followed by grade 3 or 4 hypertension (13 cases). Cardiac chest pain, atrial fibrillation, and acute coronary syndrome were reported in fewer cases.
The researchers also found that elevated natriuretic peptides that occurred before starting carfilzomib therapy or within the first 3 weeks of carfilzomib therapy were associated with a substantially higher risk of CV adverse events.
Patients who have multiple CV risk factors, and especially patients with a history of CV complications and elevated baseline natriuretic peptides, should be referred for a comprehensive cardiac evaluation, the researchers advised. “Such patients are at highest risk of CV [adverse events] with carfilzomib-based therapy, and optimization of CV therapy seems to improve overall care, allow continuation of potentially lifesaving cancer treatment, and affect severity or development of CV [adverse events],” they wrote.
A key limitation of the study was the lack of standardized treatment regimens. As a result, there was a broad dosing range for carfilzomib, in comparison to bortezomib.
Some authors reported financial relationships with carfilzomib maker Amgen and bortezomib maker Takeda, as well as with other companies.
SOURCE: Cornell RF et al. J Clin Oncol. 2019 Jun 12. doi: 10.1200/JCO.19.00231.
Cardiovascular (CV) adverse events were common in patients receiving proteasome inhibitor therapy for relapsed multiple myeloma, especially with carfilzomib-based therapy, according to results from the PROTECT study.
While prior studies have shown an increased risk for CV toxicities with proteasome inhibitor therapy, detailed descriptions of the events and risk factors have been lacking. “Furthermore, there is no validated protocol to help determine which patients are at highest risk of CV toxicity during therapy, nor is there management guidance for patients who experience a [CV adverse event],” wrote Robert F. Cornell, MD, of Vanderbilt University, Nashville, Tenn., and colleagues in the Journal of Clinical Oncology.
The PROTECT (Prospective Observation of Cardiac Safety with Proteasome Inhibitor) study was conducted at Vanderbilt University Medical Center and the University of Pennsylvania Abramson Cancer Center, Philadelphia, between September 2015 and March 2018.
Researchers followed 95 patients with relapsed multiple myeloma who were treated with either bortezomib or carfilzomib for a total duration of 18 months. A total of 65 patients received a carfilzomib-based therapy and 30 patients received a bortezomib-based therapy.
Study patients received a CV assessment at baseline and at the beginning of each treatment cycle for the initial six cycles of proteasome inhibitor therapy. Subsequently, patients were monitored for the development of CV adverse events. CV assessments included ECG, echocardiography, and measurement of other cardiac biomarkers, such as NTproBNP and troponin I or T.
CV toxicities were reported among 5 patients (16.7%) of patients treated with bortezomib and 33 patients (50.7%) treated with carfilzomib (P = .005).
In total, there were 64 CV adverse events reported, most of which were grade 2 or 3, and 56 of which occurred while on carfilzomib-based therapy. For carfilzomib, the most common complications were heart failure (23 cases), followed by grade 3 or 4 hypertension (13 cases). Cardiac chest pain, atrial fibrillation, and acute coronary syndrome were reported in fewer cases.
The researchers also found that elevated natriuretic peptides that occurred before starting carfilzomib therapy or within the first 3 weeks of carfilzomib therapy were associated with a substantially higher risk of CV adverse events.
Patients who have multiple CV risk factors, and especially patients with a history of CV complications and elevated baseline natriuretic peptides, should be referred for a comprehensive cardiac evaluation, the researchers advised. “Such patients are at highest risk of CV [adverse events] with carfilzomib-based therapy, and optimization of CV therapy seems to improve overall care, allow continuation of potentially lifesaving cancer treatment, and affect severity or development of CV [adverse events],” they wrote.
A key limitation of the study was the lack of standardized treatment regimens. As a result, there was a broad dosing range for carfilzomib, in comparison to bortezomib.
Some authors reported financial relationships with carfilzomib maker Amgen and bortezomib maker Takeda, as well as with other companies.
SOURCE: Cornell RF et al. J Clin Oncol. 2019 Jun 12. doi: 10.1200/JCO.19.00231.
FROM THE JOURNAL OF CLINICAL ONCOLOGY