User login
Staying Awake to Evade Death
ANSWER
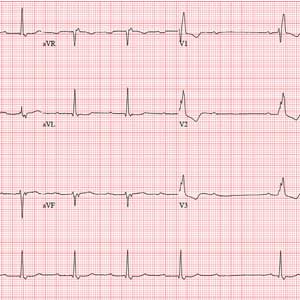
The correct answer includes sinus rhythm with second-degree atrioventricular (AV) block (Mobitz I) and a right bundle branch block (RBBB).
Sinus rhythm is evidenced by the consistent P-P intervals (best measured in the lead I rhythm strip). Mobitz I block is distinguished from Mobitz II block by the varying PR interval.
Remember that in Mobitz I (Wenckebach) block, the PR interval becomes progressively longer as the AV node fatigues, until there is blocked conduction between the atria and ventricles resulting in a P wave with no associated QRS complex. This is evident in the pause between the 6th and 7th QRS complexes. Notice also that the very next PR interval following the dropped QRS complex is much shorter than the PR interval immediately prior to that complex. In this example, the increasing PR interval before the pause is subtle compared to the PR intervals following the pause.
The skipped beats the patient feels are these pauses. Increased parasympathetic (vagal) activity occurs when a person is falling asleep, resulting in a reduction in heart rate and AV nodal conduction variability.
Finally, don’t forget the RBBB. It is indicated by a QRS duration > 120 ms, rSR’ complexes in precordial leads V1 to V3, and slurred S waves in leads I and aVF.
ANSWER
The correct answer includes sinus rhythm with second-degree atrioventricular (AV) block (Mobitz I) and a right bundle branch block (RBBB).
Sinus rhythm is evidenced by the consistent P-P intervals (best measured in the lead I rhythm strip). Mobitz I block is distinguished from Mobitz II block by the varying PR interval.
Remember that in Mobitz I (Wenckebach) block, the PR interval becomes progressively longer as the AV node fatigues, until there is blocked conduction between the atria and ventricles resulting in a P wave with no associated QRS complex. This is evident in the pause between the 6th and 7th QRS complexes. Notice also that the very next PR interval following the dropped QRS complex is much shorter than the PR interval immediately prior to that complex. In this example, the increasing PR interval before the pause is subtle compared to the PR intervals following the pause.
The skipped beats the patient feels are these pauses. Increased parasympathetic (vagal) activity occurs when a person is falling asleep, resulting in a reduction in heart rate and AV nodal conduction variability.
Finally, don’t forget the RBBB. It is indicated by a QRS duration > 120 ms, rSR’ complexes in precordial leads V1 to V3, and slurred S waves in leads I and aVF.
ANSWER
The correct answer includes sinus rhythm with second-degree atrioventricular (AV) block (Mobitz I) and a right bundle branch block (RBBB).
Sinus rhythm is evidenced by the consistent P-P intervals (best measured in the lead I rhythm strip). Mobitz I block is distinguished from Mobitz II block by the varying PR interval.
Remember that in Mobitz I (Wenckebach) block, the PR interval becomes progressively longer as the AV node fatigues, until there is blocked conduction between the atria and ventricles resulting in a P wave with no associated QRS complex. This is evident in the pause between the 6th and 7th QRS complexes. Notice also that the very next PR interval following the dropped QRS complex is much shorter than the PR interval immediately prior to that complex. In this example, the increasing PR interval before the pause is subtle compared to the PR intervals following the pause.
The skipped beats the patient feels are these pauses. Increased parasympathetic (vagal) activity occurs when a person is falling asleep, resulting in a reduction in heart rate and AV nodal conduction variability.
Finally, don’t forget the RBBB. It is indicated by a QRS duration > 120 ms, rSR’ complexes in precordial leads V1 to V3, and slurred S waves in leads I and aVF.
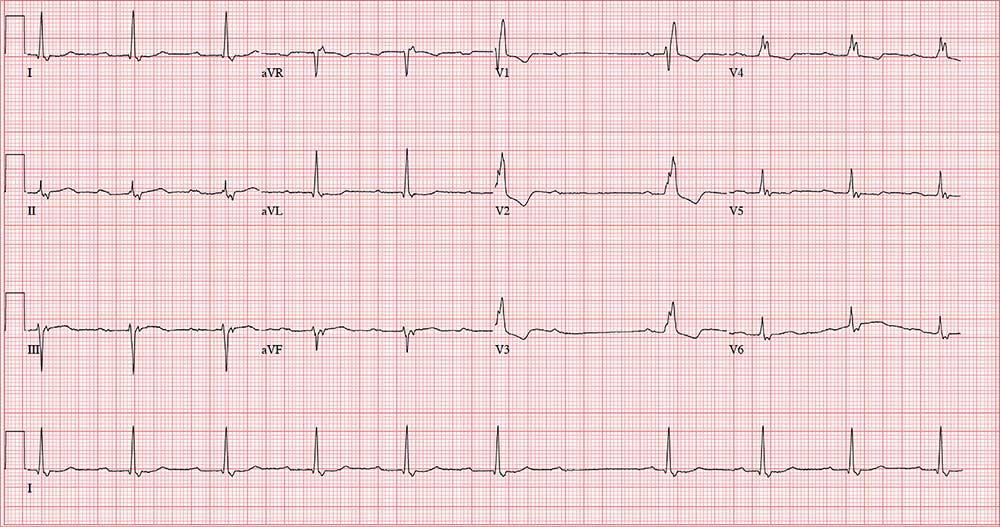
A 64-year-old man presents with a 6-month history of skipped heart beats. He says this occurs most often in the evenings, when he’s resting, and he has been awakened by it. He denies chest pain, dyspnea with exertion, syncope, or near-syncope. He has been otherwise asymptomatic from a cardiac and pulmonary standpoint.
However, he is increasingly concerned that his heart may “just stop beating” when he is asleep. When pressed further, he reveals he is afraid to go to sleep at night, knowing “my heart will start skipping beats and I’ll die!” He has discussed his fear of dying in his sleep with a psychologist, who told him that although he experiences fatigue and loss of energy and has recurrent thoughts of death, he does not meet DSM-5 criteria for major depressive disorder. The psychologist obtained an ECG and noticed pauses, which prompted referral of the patient to you.
Past medical history is remarkable for hypertension, cirrhosis, and osteoarthritis. Surgical history includes bilateral knee and left hip replacements. A screening colonoscopy 1 month ago yielded a diagnosis of stage 1 colorectal cancer.
The patient’s medication list includes hydrochlorothiazide and celecoxib. He is allergic to oxycodone (anaphylaxis).
The patient is divorced, which he attributes to his ongoing alcoholism. He drinks half a bottle of whiskey daily to “help with my nerves” and “get drunk enough to sleep.” He reports a lifetime of heavy drinking; he has tried Alcoholics Anonymous but kept dropping out to drink. He also smokes up to 1.5 packs/d of cigarettes. He has no children. He worked as a machinist in a factory but was laid off a year ago when the company downsized.
Family history is positive for alcoholism (father, 2 brothers) and hypothyroidism (mother).
Review of systems is positive for a chronic smoker’s cough, lower abdominal cramping following his colonoscopy, and arthritic pain in his wrists and ankles.
Vital signs include a blood pressure of 174/92 mm Hg; pulse, 74 beats/min; respiratory rate, 14 breaths/min; and O2 saturation, 97% on room air. His weight is 247 lb and his height, 69 in. His BMI is 36.5.
Physical exam reveals a somewhat disheveled male in no apparent distress. He wears corrective lenses and has tobacco stains on his mustache. His HEENT exam is surprisingly normal, and there is no evidence of rhinophyma. There is no jugular venous distention, thyromegaly, or lymphadenopathy. There are crackles and late expiratory wheezes in both lower lung fields.
The cardiac exam reveals a regular rate and rhythm of 74 beats/min, with no murmurs, rubs, or extra heart sounds. Peripheral pulses are strong and equal bilaterally. The abdomen is obese but nontender, and there are no palpable masses. There are surgical scars over both knees and the left hip. Arthritic changes are noticeable in the fingers on both hands. There are no neurologic deficits.
Through the electronic medical record, you access the ECG taken in the psychologist’s office. It shows a ventricular rate of 56 beats/min; no measurable PR interval; QRS duration, 144 ms; QT/QTc interval, 438/422 ms; P axis, 47°; R axis, –24°; and T axis, 55°. What is your interpretation of this ECG?
State and federal efforts address rheumatology workforce issues
LAKE BUENA VISTA, FLA. – Workforce gaps loom large in rheumatology, but efforts on both the federal and state levels address the problem, according to the chair of the American College of Rheumatology’s Government Affairs Committee, Angus B. Worthing, MD.
“We have a workforce gap that’s growing in adult arthritis; demand is increasing, and supply is decreasing,” Dr. Worthing said during an update on the committee’s activities at the annual meeting of the Florida Society of Rheumatology.
A similar “grave discrepancy” plagues pediatric rheumatology, said Dr. Worthing, a partner in a private rheumatology practice in the Washington, D.C., area.
“But these are fundamentally different,” he said, explaining that there is an oversupply of applicants for adult rheumatology fellowship spots, whereas only half of the available spots in pediatric rheumatology are being filled.
“We’re unfortunately having to turn away highly qualified [adult rheumatology] applicants, because we don’t have enough money to fund fellowship positions in the United States; about 100 doctors a year who wanted to be rheumatologists are going into other specialties,” he said. “It’s a different problem in pediatric rheumatology where you spend 2-3 extra years to earn less money than you would as a general pediatrician.”
The American College of Rheumatology is working to “find those dollars,” to alleviate the problems, he said, encouraging those who are concerned about the workforce issues to consider investing in the Rheumatology Research Foundation, which is a “huge supporter of rheumatology fellowships.”
Another proposal involves loan repayment plans for health professionals who agree to work at least 2 years in pediatric medicine.
“There’s an active bill that you can send an e-mail on right now,” Dr. Worthing said.
The bill, titled the “Educating Medical Professionals and Optimizing Workforce Efficiency and Readiness [EMPOWER] for Health Act,” represents an effort on the federal level to increase access to pediatric medical subspecialists by increasing the number who practice in underserved areas.
“It was introduced the day after we spoke on the Hill in May to leaders about this [issue],” he said.
Another effort is underway in Georgia, where a legislator who has lupus is working with the ACR on legislation that would allow the state to repay up to $25,000 on loans for cognitive specialists who agree to work in the state for a period of time.
The ACR is also working to maintain Deferred Action for Childhood Arrivals (DACA) protections for recipients pursuing medical education, who could potentially help to alleviate the shortages, he noted.
The problem of workforce issues is multifaceted and it requires a multipronged approach, Dr. Worthing said.
“It will not be solved by the American College of Rheumatology alone; I think it will end up being solved by people on the ground working with their primary care physicians and referring doctors to try to close the gap and try to see patients when they’re needed,” he said.
Dr. Worthing reported having no disclosures.
LAKE BUENA VISTA, FLA. – Workforce gaps loom large in rheumatology, but efforts on both the federal and state levels address the problem, according to the chair of the American College of Rheumatology’s Government Affairs Committee, Angus B. Worthing, MD.
“We have a workforce gap that’s growing in adult arthritis; demand is increasing, and supply is decreasing,” Dr. Worthing said during an update on the committee’s activities at the annual meeting of the Florida Society of Rheumatology.
A similar “grave discrepancy” plagues pediatric rheumatology, said Dr. Worthing, a partner in a private rheumatology practice in the Washington, D.C., area.
“But these are fundamentally different,” he said, explaining that there is an oversupply of applicants for adult rheumatology fellowship spots, whereas only half of the available spots in pediatric rheumatology are being filled.
“We’re unfortunately having to turn away highly qualified [adult rheumatology] applicants, because we don’t have enough money to fund fellowship positions in the United States; about 100 doctors a year who wanted to be rheumatologists are going into other specialties,” he said. “It’s a different problem in pediatric rheumatology where you spend 2-3 extra years to earn less money than you would as a general pediatrician.”
The American College of Rheumatology is working to “find those dollars,” to alleviate the problems, he said, encouraging those who are concerned about the workforce issues to consider investing in the Rheumatology Research Foundation, which is a “huge supporter of rheumatology fellowships.”
Another proposal involves loan repayment plans for health professionals who agree to work at least 2 years in pediatric medicine.
“There’s an active bill that you can send an e-mail on right now,” Dr. Worthing said.
The bill, titled the “Educating Medical Professionals and Optimizing Workforce Efficiency and Readiness [EMPOWER] for Health Act,” represents an effort on the federal level to increase access to pediatric medical subspecialists by increasing the number who practice in underserved areas.
“It was introduced the day after we spoke on the Hill in May to leaders about this [issue],” he said.
Another effort is underway in Georgia, where a legislator who has lupus is working with the ACR on legislation that would allow the state to repay up to $25,000 on loans for cognitive specialists who agree to work in the state for a period of time.
The ACR is also working to maintain Deferred Action for Childhood Arrivals (DACA) protections for recipients pursuing medical education, who could potentially help to alleviate the shortages, he noted.
The problem of workforce issues is multifaceted and it requires a multipronged approach, Dr. Worthing said.
“It will not be solved by the American College of Rheumatology alone; I think it will end up being solved by people on the ground working with their primary care physicians and referring doctors to try to close the gap and try to see patients when they’re needed,” he said.
Dr. Worthing reported having no disclosures.
LAKE BUENA VISTA, FLA. – Workforce gaps loom large in rheumatology, but efforts on both the federal and state levels address the problem, according to the chair of the American College of Rheumatology’s Government Affairs Committee, Angus B. Worthing, MD.
“We have a workforce gap that’s growing in adult arthritis; demand is increasing, and supply is decreasing,” Dr. Worthing said during an update on the committee’s activities at the annual meeting of the Florida Society of Rheumatology.
A similar “grave discrepancy” plagues pediatric rheumatology, said Dr. Worthing, a partner in a private rheumatology practice in the Washington, D.C., area.
“But these are fundamentally different,” he said, explaining that there is an oversupply of applicants for adult rheumatology fellowship spots, whereas only half of the available spots in pediatric rheumatology are being filled.
“We’re unfortunately having to turn away highly qualified [adult rheumatology] applicants, because we don’t have enough money to fund fellowship positions in the United States; about 100 doctors a year who wanted to be rheumatologists are going into other specialties,” he said. “It’s a different problem in pediatric rheumatology where you spend 2-3 extra years to earn less money than you would as a general pediatrician.”
The American College of Rheumatology is working to “find those dollars,” to alleviate the problems, he said, encouraging those who are concerned about the workforce issues to consider investing in the Rheumatology Research Foundation, which is a “huge supporter of rheumatology fellowships.”
Another proposal involves loan repayment plans for health professionals who agree to work at least 2 years in pediatric medicine.
“There’s an active bill that you can send an e-mail on right now,” Dr. Worthing said.
The bill, titled the “Educating Medical Professionals and Optimizing Workforce Efficiency and Readiness [EMPOWER] for Health Act,” represents an effort on the federal level to increase access to pediatric medical subspecialists by increasing the number who practice in underserved areas.
“It was introduced the day after we spoke on the Hill in May to leaders about this [issue],” he said.
Another effort is underway in Georgia, where a legislator who has lupus is working with the ACR on legislation that would allow the state to repay up to $25,000 on loans for cognitive specialists who agree to work in the state for a period of time.
The ACR is also working to maintain Deferred Action for Childhood Arrivals (DACA) protections for recipients pursuing medical education, who could potentially help to alleviate the shortages, he noted.
The problem of workforce issues is multifaceted and it requires a multipronged approach, Dr. Worthing said.
“It will not be solved by the American College of Rheumatology alone; I think it will end up being solved by people on the ground working with their primary care physicians and referring doctors to try to close the gap and try to see patients when they’re needed,” he said.
Dr. Worthing reported having no disclosures.
REPORTING FROM FSR 2019
Immune-related toxicities, hospitalization common with checkpoint inhibitor therapy
, according to a retrospective cohort study.
In addition, the majority of the immune-related toxicities were high-grade events of grade 3 or higher (65%), necessitated multidisciplinary care (91%), and eventually improved or resolved (65%). The results highlight potential risk factors for hospitalizations due to immune-related toxicities in oncology patients.
“[We aimed to] characterize the spectrum of toxicities, management, and outcomes of hospitalizations for immune-related adverse events,” wrote Aanika Balaji, BS, of Johns Hopkins University, Baltimore, and colleagues. The findings were reported in the Journal of Oncology Practice.
The researchers studied 443 patients admitted to solid tumor oncology service at an oncology center over a period of 6-months. Of these, 100 patients had at any point received checkpoint inhibitor therapy.
The proportion of hospital admissions for patients with confirmed immune-related toxicities and associations between hospitalizations due to immune-related toxicity and patient characteristics were assessed by the team. Nearly half of the patients admitted with immune-related toxicities had thoracic or head and neck cancers.
In the analysis, patients treated with combination checkpoint inhibitor therapy (odds ratio, 6.8; 95% confidence interval, 2.0-23.2), in addition to those aged 65 years and over (OR, 5.4; 95% CI, 1.6-17.8), were more likely to be hospitalized for immune-related toxicities.
Overall, 5% of all hospitalizations were the result of immune-related toxicities. Furthermore, 87% of patients discontinued checkpoint inhibitor therapy post discharge.
“We found that the most common immune-related adverse events warranting hospital admission were pneumonitis (26%) and colitis (17%),” they wrote.
The researchers acknowledged two key limitations of the study were the small sample size and lack of generalizability in community settings. Future studies that include patients from community oncology settings could improve the generalizability of the results.
“These data indicate potential risk factors for immune-related adverse event hospitalization and are likely to indicate future service needs” they concluded.
Financial support was provided by Jarushka Naidoo. The authors reported financial affiliations with AstraZeneca, Bristol-Myers Squibb, Compugen, Genentech, GlaxoSmithKline, Exelixis, MedImmune, and several others.
SOURCE: Balaji A et al. J Oncol Pract. 2019 Aug 6. doi: 10.1200/JOP.18.00703.
, according to a retrospective cohort study.
In addition, the majority of the immune-related toxicities were high-grade events of grade 3 or higher (65%), necessitated multidisciplinary care (91%), and eventually improved or resolved (65%). The results highlight potential risk factors for hospitalizations due to immune-related toxicities in oncology patients.
“[We aimed to] characterize the spectrum of toxicities, management, and outcomes of hospitalizations for immune-related adverse events,” wrote Aanika Balaji, BS, of Johns Hopkins University, Baltimore, and colleagues. The findings were reported in the Journal of Oncology Practice.
The researchers studied 443 patients admitted to solid tumor oncology service at an oncology center over a period of 6-months. Of these, 100 patients had at any point received checkpoint inhibitor therapy.
The proportion of hospital admissions for patients with confirmed immune-related toxicities and associations between hospitalizations due to immune-related toxicity and patient characteristics were assessed by the team. Nearly half of the patients admitted with immune-related toxicities had thoracic or head and neck cancers.
In the analysis, patients treated with combination checkpoint inhibitor therapy (odds ratio, 6.8; 95% confidence interval, 2.0-23.2), in addition to those aged 65 years and over (OR, 5.4; 95% CI, 1.6-17.8), were more likely to be hospitalized for immune-related toxicities.
Overall, 5% of all hospitalizations were the result of immune-related toxicities. Furthermore, 87% of patients discontinued checkpoint inhibitor therapy post discharge.
“We found that the most common immune-related adverse events warranting hospital admission were pneumonitis (26%) and colitis (17%),” they wrote.
The researchers acknowledged two key limitations of the study were the small sample size and lack of generalizability in community settings. Future studies that include patients from community oncology settings could improve the generalizability of the results.
“These data indicate potential risk factors for immune-related adverse event hospitalization and are likely to indicate future service needs” they concluded.
Financial support was provided by Jarushka Naidoo. The authors reported financial affiliations with AstraZeneca, Bristol-Myers Squibb, Compugen, Genentech, GlaxoSmithKline, Exelixis, MedImmune, and several others.
SOURCE: Balaji A et al. J Oncol Pract. 2019 Aug 6. doi: 10.1200/JOP.18.00703.
, according to a retrospective cohort study.
In addition, the majority of the immune-related toxicities were high-grade events of grade 3 or higher (65%), necessitated multidisciplinary care (91%), and eventually improved or resolved (65%). The results highlight potential risk factors for hospitalizations due to immune-related toxicities in oncology patients.
“[We aimed to] characterize the spectrum of toxicities, management, and outcomes of hospitalizations for immune-related adverse events,” wrote Aanika Balaji, BS, of Johns Hopkins University, Baltimore, and colleagues. The findings were reported in the Journal of Oncology Practice.
The researchers studied 443 patients admitted to solid tumor oncology service at an oncology center over a period of 6-months. Of these, 100 patients had at any point received checkpoint inhibitor therapy.
The proportion of hospital admissions for patients with confirmed immune-related toxicities and associations between hospitalizations due to immune-related toxicity and patient characteristics were assessed by the team. Nearly half of the patients admitted with immune-related toxicities had thoracic or head and neck cancers.
In the analysis, patients treated with combination checkpoint inhibitor therapy (odds ratio, 6.8; 95% confidence interval, 2.0-23.2), in addition to those aged 65 years and over (OR, 5.4; 95% CI, 1.6-17.8), were more likely to be hospitalized for immune-related toxicities.
Overall, 5% of all hospitalizations were the result of immune-related toxicities. Furthermore, 87% of patients discontinued checkpoint inhibitor therapy post discharge.
“We found that the most common immune-related adverse events warranting hospital admission were pneumonitis (26%) and colitis (17%),” they wrote.
The researchers acknowledged two key limitations of the study were the small sample size and lack of generalizability in community settings. Future studies that include patients from community oncology settings could improve the generalizability of the results.
“These data indicate potential risk factors for immune-related adverse event hospitalization and are likely to indicate future service needs” they concluded.
Financial support was provided by Jarushka Naidoo. The authors reported financial affiliations with AstraZeneca, Bristol-Myers Squibb, Compugen, Genentech, GlaxoSmithKline, Exelixis, MedImmune, and several others.
SOURCE: Balaji A et al. J Oncol Pract. 2019 Aug 6. doi: 10.1200/JOP.18.00703.
FROM JOURNAL OF ONCOLOGY PRACTICE
Oral semaglutide monotherapy delivers HbA1c improvements in type 2 diabetes
Oral semaglutide monotherapy was superior to placebo for improving glycated hemoglobin (HbA1c) levels at all doses tested in adults with type 2 diabetes who had been previously insufficiently managed with diet and exercise, according to findings from a global, randomized trial.
The drug also showed dose-dependent weight loss, with a statistically significant effect on body weight, compared with placebo, at higher doses.
To date, the glucagon-like peptide–1 receptor agonist has been available as weekly subcutaneous shots for patients with type 2 diabetes, and in that form they have been shown to be effective in reducing HbA1c, inducing weight loss, and lowering the risk of cardiovascular events in patients with cardiovascular disease or those who are at high risk for it, wrote Vanita R. Aroda, MD, of Brigham and Women’s Hospital, Boston, and colleagues. The report is in Diabetes Care.
The novel oral semaglutide tablet is designed to enhance medication absorption, and the pharmacokinetics and dosage were established in phase 2 studies, they noted.
In the phase 3 Peptide Innovation for Early Diabetes Treatment 1 (PIONEER 1) study, Dr. Aroda and colleagues randomized 703 adults with type 2 diabetes to receive either 3 mg, 7 mg, or 14 mg of oral semaglutide daily, or placebo. The average age of the patients was 55 years, about half were women, and the average baseline HbA1c was 8.0% (64 mmol/mol). The primary endpoint was change in HbA1c level from baseline to week 26, and the secondary endpoint was change in body weight over the same period.
After 26 weeks of once-daily treatment, patients in semaglutide group showed significant reductions in HbA1c from baseline with all three doses: –0.6% (3 mg), –0.9% (7 mg), and –1.1% (14 mg), with P less than .001 for all, based on an intention-to-treat analysis. Similar results occurred using an on-treatment analysis, with differences of –0.7%, –1.2%, and –1.4%, respectively, for the three doses.
In addition, patients in all dose groups achieved the secondary endpoint of reduction in body weight, compared with placebo, from baseline to 26 weeks based on both types of analyses. “Significantly more patients achieved body weight loss of at least 5% with oral semaglutide at 7 mg and 14 mg, compared with placebo,” Dr. Aroda and colleagues wrote (intention-to-treat: –0.1 for 3 mg daily [P = .87], –0.9 for 7 mg [P = .09], –2.3 for 14 mg [P less than .001]; and on-treatment: –0.2 for 3 mg [P = .71], –1.0 for 7 mg [P = .01], –2.6 for 14 mg [P less than .001]).
The overall incidence of adverse events and serious adverse events was similar in the treatment and placebo groups, with the most frequent being nausea and diarrhea. No deaths occurred among patients on the medication.
The findings were limited by several factors, including a patient population that had a relatively short duration of diabetes (mean, 3.5 years) and that the oral semaglutide was used as first-line monotherapy, without first using metformin, the researchers noted. However, oral semaglutide “achieved clinically meaningful and superior glucose lowering,” compared with placebo, at all three doses, they wrote.
“Ongoing additional studies in the PIONEER program will further define the effect when used in combination with other glucose-lowering therapies and in other populations of interest, such as those with high cardiovascular risk or renal impairment,” they emphasized
Novo Nordisk funded the study. The lead author disclosed relationships with Novo Nordisk, and several coauthors disclosed relationships with or employment by the company.
SOURCE: Aroda VR et al. Diabetes Care. 2019 Jul. doi: 10.2337/dc19-0749.
Oral semaglutide monotherapy was superior to placebo for improving glycated hemoglobin (HbA1c) levels at all doses tested in adults with type 2 diabetes who had been previously insufficiently managed with diet and exercise, according to findings from a global, randomized trial.
The drug also showed dose-dependent weight loss, with a statistically significant effect on body weight, compared with placebo, at higher doses.
To date, the glucagon-like peptide–1 receptor agonist has been available as weekly subcutaneous shots for patients with type 2 diabetes, and in that form they have been shown to be effective in reducing HbA1c, inducing weight loss, and lowering the risk of cardiovascular events in patients with cardiovascular disease or those who are at high risk for it, wrote Vanita R. Aroda, MD, of Brigham and Women’s Hospital, Boston, and colleagues. The report is in Diabetes Care.
The novel oral semaglutide tablet is designed to enhance medication absorption, and the pharmacokinetics and dosage were established in phase 2 studies, they noted.
In the phase 3 Peptide Innovation for Early Diabetes Treatment 1 (PIONEER 1) study, Dr. Aroda and colleagues randomized 703 adults with type 2 diabetes to receive either 3 mg, 7 mg, or 14 mg of oral semaglutide daily, or placebo. The average age of the patients was 55 years, about half were women, and the average baseline HbA1c was 8.0% (64 mmol/mol). The primary endpoint was change in HbA1c level from baseline to week 26, and the secondary endpoint was change in body weight over the same period.
After 26 weeks of once-daily treatment, patients in semaglutide group showed significant reductions in HbA1c from baseline with all three doses: –0.6% (3 mg), –0.9% (7 mg), and –1.1% (14 mg), with P less than .001 for all, based on an intention-to-treat analysis. Similar results occurred using an on-treatment analysis, with differences of –0.7%, –1.2%, and –1.4%, respectively, for the three doses.
In addition, patients in all dose groups achieved the secondary endpoint of reduction in body weight, compared with placebo, from baseline to 26 weeks based on both types of analyses. “Significantly more patients achieved body weight loss of at least 5% with oral semaglutide at 7 mg and 14 mg, compared with placebo,” Dr. Aroda and colleagues wrote (intention-to-treat: –0.1 for 3 mg daily [P = .87], –0.9 for 7 mg [P = .09], –2.3 for 14 mg [P less than .001]; and on-treatment: –0.2 for 3 mg [P = .71], –1.0 for 7 mg [P = .01], –2.6 for 14 mg [P less than .001]).
The overall incidence of adverse events and serious adverse events was similar in the treatment and placebo groups, with the most frequent being nausea and diarrhea. No deaths occurred among patients on the medication.
The findings were limited by several factors, including a patient population that had a relatively short duration of diabetes (mean, 3.5 years) and that the oral semaglutide was used as first-line monotherapy, without first using metformin, the researchers noted. However, oral semaglutide “achieved clinically meaningful and superior glucose lowering,” compared with placebo, at all three doses, they wrote.
“Ongoing additional studies in the PIONEER program will further define the effect when used in combination with other glucose-lowering therapies and in other populations of interest, such as those with high cardiovascular risk or renal impairment,” they emphasized
Novo Nordisk funded the study. The lead author disclosed relationships with Novo Nordisk, and several coauthors disclosed relationships with or employment by the company.
SOURCE: Aroda VR et al. Diabetes Care. 2019 Jul. doi: 10.2337/dc19-0749.
Oral semaglutide monotherapy was superior to placebo for improving glycated hemoglobin (HbA1c) levels at all doses tested in adults with type 2 diabetes who had been previously insufficiently managed with diet and exercise, according to findings from a global, randomized trial.
The drug also showed dose-dependent weight loss, with a statistically significant effect on body weight, compared with placebo, at higher doses.
To date, the glucagon-like peptide–1 receptor agonist has been available as weekly subcutaneous shots for patients with type 2 diabetes, and in that form they have been shown to be effective in reducing HbA1c, inducing weight loss, and lowering the risk of cardiovascular events in patients with cardiovascular disease or those who are at high risk for it, wrote Vanita R. Aroda, MD, of Brigham and Women’s Hospital, Boston, and colleagues. The report is in Diabetes Care.
The novel oral semaglutide tablet is designed to enhance medication absorption, and the pharmacokinetics and dosage were established in phase 2 studies, they noted.
In the phase 3 Peptide Innovation for Early Diabetes Treatment 1 (PIONEER 1) study, Dr. Aroda and colleagues randomized 703 adults with type 2 diabetes to receive either 3 mg, 7 mg, or 14 mg of oral semaglutide daily, or placebo. The average age of the patients was 55 years, about half were women, and the average baseline HbA1c was 8.0% (64 mmol/mol). The primary endpoint was change in HbA1c level from baseline to week 26, and the secondary endpoint was change in body weight over the same period.
After 26 weeks of once-daily treatment, patients in semaglutide group showed significant reductions in HbA1c from baseline with all three doses: –0.6% (3 mg), –0.9% (7 mg), and –1.1% (14 mg), with P less than .001 for all, based on an intention-to-treat analysis. Similar results occurred using an on-treatment analysis, with differences of –0.7%, –1.2%, and –1.4%, respectively, for the three doses.
In addition, patients in all dose groups achieved the secondary endpoint of reduction in body weight, compared with placebo, from baseline to 26 weeks based on both types of analyses. “Significantly more patients achieved body weight loss of at least 5% with oral semaglutide at 7 mg and 14 mg, compared with placebo,” Dr. Aroda and colleagues wrote (intention-to-treat: –0.1 for 3 mg daily [P = .87], –0.9 for 7 mg [P = .09], –2.3 for 14 mg [P less than .001]; and on-treatment: –0.2 for 3 mg [P = .71], –1.0 for 7 mg [P = .01], –2.6 for 14 mg [P less than .001]).
The overall incidence of adverse events and serious adverse events was similar in the treatment and placebo groups, with the most frequent being nausea and diarrhea. No deaths occurred among patients on the medication.
The findings were limited by several factors, including a patient population that had a relatively short duration of diabetes (mean, 3.5 years) and that the oral semaglutide was used as first-line monotherapy, without first using metformin, the researchers noted. However, oral semaglutide “achieved clinically meaningful and superior glucose lowering,” compared with placebo, at all three doses, they wrote.
“Ongoing additional studies in the PIONEER program will further define the effect when used in combination with other glucose-lowering therapies and in other populations of interest, such as those with high cardiovascular risk or renal impairment,” they emphasized
Novo Nordisk funded the study. The lead author disclosed relationships with Novo Nordisk, and several coauthors disclosed relationships with or employment by the company.
SOURCE: Aroda VR et al. Diabetes Care. 2019 Jul. doi: 10.2337/dc19-0749.
FROM DIABETES CARE
Cancer centers announce new faculty
Jorge E. Cortes, MD, is set to become director of the Georgia Cancer Center in Augusta on Sept. 1. In this new position, Dr. Cortes will further the center’s missions of obtaining National Cancer Institute designation and reducing the burden of cancer in Georgia.
Dr. Cortes was previously deputy chair and professor of medicine in the department of leukemia at MD Anderson Cancer Center in Houston. He received his medical degree in 1986 and began working at MD Anderson in 1991. His clinical interests include acute and chronic leukemias, myelodysplastic syndromes, and myeloproliferative neoplasms.
Fern M. Anari, MD, joined Fox Chase Cancer Center in Philadelphia in August. Dr. Anari is now an assistant professor in the division of genitourinary medical oncology within the department of hematology/oncology.
Dr. Anari earned her medical degree from Robert Wood Johnson Medical School in New Brunswick, N.J., and completed an internship and residency at Thomas Jefferson University Hospital in Philadelphia. She joined Fox Chase/Temple University for a 3-year fellowship in 2016.
Another new hire in the department of hematology/oncology at Fox Chase is Iberia Romina Sosa, MD, PhD. Dr. Sosa became an assistant professor in the clinical investigator track in August.
Dr. Sosa, who is a member of the Hematology News editorial advisory board, was previously an assistant professor in the department of hematology/oncology at Baylor College of Medicine in Houston. She received her MD and PhD from the University of Minnesota in Minneapolis and completed a residency and fellowship at Vanderbilt University in Nashville, Tenn.
Finally, John Migliano, MD, has joined The Oncology Institute of Hope and Innovation, which has locations in Southern California, Arizona, and Nevada. Dr. Migliano serves patients at the Arizona locations in Tucson, Oro Valley, and Marana.
Dr. Migliano received his medical degree from the University of Medicine and Health Sciences in Saint Kitts and completed his residency in internal medicine at the University of Arizona in Tucson. He was a hematology/oncology fellow at Wake Forest University Baptist Medical Center in Winston-Salem, N.C.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at [email protected], and you could be featured in Movers in Medicine.
Jorge E. Cortes, MD, is set to become director of the Georgia Cancer Center in Augusta on Sept. 1. In this new position, Dr. Cortes will further the center’s missions of obtaining National Cancer Institute designation and reducing the burden of cancer in Georgia.
Dr. Cortes was previously deputy chair and professor of medicine in the department of leukemia at MD Anderson Cancer Center in Houston. He received his medical degree in 1986 and began working at MD Anderson in 1991. His clinical interests include acute and chronic leukemias, myelodysplastic syndromes, and myeloproliferative neoplasms.
Fern M. Anari, MD, joined Fox Chase Cancer Center in Philadelphia in August. Dr. Anari is now an assistant professor in the division of genitourinary medical oncology within the department of hematology/oncology.
Dr. Anari earned her medical degree from Robert Wood Johnson Medical School in New Brunswick, N.J., and completed an internship and residency at Thomas Jefferson University Hospital in Philadelphia. She joined Fox Chase/Temple University for a 3-year fellowship in 2016.
Another new hire in the department of hematology/oncology at Fox Chase is Iberia Romina Sosa, MD, PhD. Dr. Sosa became an assistant professor in the clinical investigator track in August.
Dr. Sosa, who is a member of the Hematology News editorial advisory board, was previously an assistant professor in the department of hematology/oncology at Baylor College of Medicine in Houston. She received her MD and PhD from the University of Minnesota in Minneapolis and completed a residency and fellowship at Vanderbilt University in Nashville, Tenn.
Finally, John Migliano, MD, has joined The Oncology Institute of Hope and Innovation, which has locations in Southern California, Arizona, and Nevada. Dr. Migliano serves patients at the Arizona locations in Tucson, Oro Valley, and Marana.
Dr. Migliano received his medical degree from the University of Medicine and Health Sciences in Saint Kitts and completed his residency in internal medicine at the University of Arizona in Tucson. He was a hematology/oncology fellow at Wake Forest University Baptist Medical Center in Winston-Salem, N.C.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at [email protected], and you could be featured in Movers in Medicine.
Jorge E. Cortes, MD, is set to become director of the Georgia Cancer Center in Augusta on Sept. 1. In this new position, Dr. Cortes will further the center’s missions of obtaining National Cancer Institute designation and reducing the burden of cancer in Georgia.
Dr. Cortes was previously deputy chair and professor of medicine in the department of leukemia at MD Anderson Cancer Center in Houston. He received his medical degree in 1986 and began working at MD Anderson in 1991. His clinical interests include acute and chronic leukemias, myelodysplastic syndromes, and myeloproliferative neoplasms.
Fern M. Anari, MD, joined Fox Chase Cancer Center in Philadelphia in August. Dr. Anari is now an assistant professor in the division of genitourinary medical oncology within the department of hematology/oncology.
Dr. Anari earned her medical degree from Robert Wood Johnson Medical School in New Brunswick, N.J., and completed an internship and residency at Thomas Jefferson University Hospital in Philadelphia. She joined Fox Chase/Temple University for a 3-year fellowship in 2016.
Another new hire in the department of hematology/oncology at Fox Chase is Iberia Romina Sosa, MD, PhD. Dr. Sosa became an assistant professor in the clinical investigator track in August.
Dr. Sosa, who is a member of the Hematology News editorial advisory board, was previously an assistant professor in the department of hematology/oncology at Baylor College of Medicine in Houston. She received her MD and PhD from the University of Minnesota in Minneapolis and completed a residency and fellowship at Vanderbilt University in Nashville, Tenn.
Finally, John Migliano, MD, has joined The Oncology Institute of Hope and Innovation, which has locations in Southern California, Arizona, and Nevada. Dr. Migliano serves patients at the Arizona locations in Tucson, Oro Valley, and Marana.
Dr. Migliano received his medical degree from the University of Medicine and Health Sciences in Saint Kitts and completed his residency in internal medicine at the University of Arizona in Tucson. He was a hematology/oncology fellow at Wake Forest University Baptist Medical Center in Winston-Salem, N.C.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at [email protected], and you could be featured in Movers in Medicine.
AHA highlights limitations of perfusion testing for critical limb ischemia
A new assessment statement from the American Heart Association reviewed the strengths and limitations of current imaging techniques for critical limb ischemia (CLI), the severest form of peripheral arterial disease (PAD).
The main techniques discussed were the ankle-brachial index (ABI), toe-brachial index (TBI), toe systolic pressure, transcutaneous oximetry (TcPO2) and skin perfusion pressure (SPP). The literature review also identified what the authors saw as opportunities for technology improvement.
“No single vascular test has been identified as the most important predictor of wound healing or major amputation for the threatened limb,” wrote Sanjay Misra, MD, of the Mayo Clinic in Rochester, Minn., and colleagues, on behalf of the American Heart Association Council on Peripheral Vascular Disease, the Council on Clinical Cardiology, and the Council on Cardiovascular and Stroke Nursing.
Of particular concern were limitations seen in the use of ABI, the most widely used assessment method. “Although ABI was first described to diagnose PAD, it has not been shown to be an accurate predictor of wound healing or major adverse limb events. Clearly, the ABI provides important prognostic information, including the risk of death, myocardial infarction, and stroke ... and should be performed in all patients suspected of having PAD,” but in about 30% of patients with angiographically documented CLI, the ABI is normal or noncompressible, the authors wrote.
And, although recent data indicate that toe pressure may be a better predictor of major adverse limb events and tibial disease in patients with CLI, especially among those with isolated below-knee disease, there was no solid evidence that ABI or TBI have the sensitivity or specificity to be used as perfusion tools to assess wound healing or limb salvage, the authors stated.
However, there may be some technological improvements on the horizon for assessing limb perfusion that might provide eventual benefits, according to the reviewers. These include the use of indigo carmine angiography to evaluate microcirculation and angiosomal revascularization, the use of CT perfusion or MRI to quantify perfusion and monitor treatment response, the use of contrast-enhanced ultrasound to assess calf muscle perfusion, and hyperspectral imaging.
Among the other issues of concern raised in the AHA statement were the significant demographic disparities that occur in detection and treatment of CLI. The authors noted differences in how CLI is diagnosed, the coexisting conditions that were present, and the disparities in treatment given based on sex and racial differences. For example, women were more likely to experience emergency hospitalization, have differences in blood flow, and have higher disability and death rates.
As for racial disparities, the reviewers found that black and Hispanic patients with CLI were more likely to have diabetes and chronic kidney disease, and were more likely to develop gangrene, compared with white patients, who were more likely to have ulcers and pain in their legs while at rest.
In terms of treatment, black patients were 78% more likely to receive lower extremity amputation for CLI, compared with their white peers, even after adjustment for socioeconomic status, access to facilities with revascularization services, and other factors, according to the report, which was published online in Circulation.
“CLI is a complex disease process with great morbidity. This statement highlights the importance of incorporating perfusion assessment into the care of CLI patients. Despite the high prevalence of CLI, strategies for perfusion assessment remain limited. New technologies offer potential opportunities to improve the precision and quality of CLI management,” the researchers concluded.
Dr. Misra and the majority of the authors reported having no relevant disclosures. Several authors reported receiving funding from the pharmaceutical and medical device industries.
SOURCE: Chandra S. et al. Circulation. 2019;140:00-00. doi: 10.1161/CIR.0000000000000708.
A new assessment statement from the American Heart Association reviewed the strengths and limitations of current imaging techniques for critical limb ischemia (CLI), the severest form of peripheral arterial disease (PAD).
The main techniques discussed were the ankle-brachial index (ABI), toe-brachial index (TBI), toe systolic pressure, transcutaneous oximetry (TcPO2) and skin perfusion pressure (SPP). The literature review also identified what the authors saw as opportunities for technology improvement.
“No single vascular test has been identified as the most important predictor of wound healing or major amputation for the threatened limb,” wrote Sanjay Misra, MD, of the Mayo Clinic in Rochester, Minn., and colleagues, on behalf of the American Heart Association Council on Peripheral Vascular Disease, the Council on Clinical Cardiology, and the Council on Cardiovascular and Stroke Nursing.
Of particular concern were limitations seen in the use of ABI, the most widely used assessment method. “Although ABI was first described to diagnose PAD, it has not been shown to be an accurate predictor of wound healing or major adverse limb events. Clearly, the ABI provides important prognostic information, including the risk of death, myocardial infarction, and stroke ... and should be performed in all patients suspected of having PAD,” but in about 30% of patients with angiographically documented CLI, the ABI is normal or noncompressible, the authors wrote.
And, although recent data indicate that toe pressure may be a better predictor of major adverse limb events and tibial disease in patients with CLI, especially among those with isolated below-knee disease, there was no solid evidence that ABI or TBI have the sensitivity or specificity to be used as perfusion tools to assess wound healing or limb salvage, the authors stated.
However, there may be some technological improvements on the horizon for assessing limb perfusion that might provide eventual benefits, according to the reviewers. These include the use of indigo carmine angiography to evaluate microcirculation and angiosomal revascularization, the use of CT perfusion or MRI to quantify perfusion and monitor treatment response, the use of contrast-enhanced ultrasound to assess calf muscle perfusion, and hyperspectral imaging.
Among the other issues of concern raised in the AHA statement were the significant demographic disparities that occur in detection and treatment of CLI. The authors noted differences in how CLI is diagnosed, the coexisting conditions that were present, and the disparities in treatment given based on sex and racial differences. For example, women were more likely to experience emergency hospitalization, have differences in blood flow, and have higher disability and death rates.
As for racial disparities, the reviewers found that black and Hispanic patients with CLI were more likely to have diabetes and chronic kidney disease, and were more likely to develop gangrene, compared with white patients, who were more likely to have ulcers and pain in their legs while at rest.
In terms of treatment, black patients were 78% more likely to receive lower extremity amputation for CLI, compared with their white peers, even after adjustment for socioeconomic status, access to facilities with revascularization services, and other factors, according to the report, which was published online in Circulation.
“CLI is a complex disease process with great morbidity. This statement highlights the importance of incorporating perfusion assessment into the care of CLI patients. Despite the high prevalence of CLI, strategies for perfusion assessment remain limited. New technologies offer potential opportunities to improve the precision and quality of CLI management,” the researchers concluded.
Dr. Misra and the majority of the authors reported having no relevant disclosures. Several authors reported receiving funding from the pharmaceutical and medical device industries.
SOURCE: Chandra S. et al. Circulation. 2019;140:00-00. doi: 10.1161/CIR.0000000000000708.
A new assessment statement from the American Heart Association reviewed the strengths and limitations of current imaging techniques for critical limb ischemia (CLI), the severest form of peripheral arterial disease (PAD).
The main techniques discussed were the ankle-brachial index (ABI), toe-brachial index (TBI), toe systolic pressure, transcutaneous oximetry (TcPO2) and skin perfusion pressure (SPP). The literature review also identified what the authors saw as opportunities for technology improvement.
“No single vascular test has been identified as the most important predictor of wound healing or major amputation for the threatened limb,” wrote Sanjay Misra, MD, of the Mayo Clinic in Rochester, Minn., and colleagues, on behalf of the American Heart Association Council on Peripheral Vascular Disease, the Council on Clinical Cardiology, and the Council on Cardiovascular and Stroke Nursing.
Of particular concern were limitations seen in the use of ABI, the most widely used assessment method. “Although ABI was first described to diagnose PAD, it has not been shown to be an accurate predictor of wound healing or major adverse limb events. Clearly, the ABI provides important prognostic information, including the risk of death, myocardial infarction, and stroke ... and should be performed in all patients suspected of having PAD,” but in about 30% of patients with angiographically documented CLI, the ABI is normal or noncompressible, the authors wrote.
And, although recent data indicate that toe pressure may be a better predictor of major adverse limb events and tibial disease in patients with CLI, especially among those with isolated below-knee disease, there was no solid evidence that ABI or TBI have the sensitivity or specificity to be used as perfusion tools to assess wound healing or limb salvage, the authors stated.
However, there may be some technological improvements on the horizon for assessing limb perfusion that might provide eventual benefits, according to the reviewers. These include the use of indigo carmine angiography to evaluate microcirculation and angiosomal revascularization, the use of CT perfusion or MRI to quantify perfusion and monitor treatment response, the use of contrast-enhanced ultrasound to assess calf muscle perfusion, and hyperspectral imaging.
Among the other issues of concern raised in the AHA statement were the significant demographic disparities that occur in detection and treatment of CLI. The authors noted differences in how CLI is diagnosed, the coexisting conditions that were present, and the disparities in treatment given based on sex and racial differences. For example, women were more likely to experience emergency hospitalization, have differences in blood flow, and have higher disability and death rates.
As for racial disparities, the reviewers found that black and Hispanic patients with CLI were more likely to have diabetes and chronic kidney disease, and were more likely to develop gangrene, compared with white patients, who were more likely to have ulcers and pain in their legs while at rest.
In terms of treatment, black patients were 78% more likely to receive lower extremity amputation for CLI, compared with their white peers, even after adjustment for socioeconomic status, access to facilities with revascularization services, and other factors, according to the report, which was published online in Circulation.
“CLI is a complex disease process with great morbidity. This statement highlights the importance of incorporating perfusion assessment into the care of CLI patients. Despite the high prevalence of CLI, strategies for perfusion assessment remain limited. New technologies offer potential opportunities to improve the precision and quality of CLI management,” the researchers concluded.
Dr. Misra and the majority of the authors reported having no relevant disclosures. Several authors reported receiving funding from the pharmaceutical and medical device industries.
SOURCE: Chandra S. et al. Circulation. 2019;140:00-00. doi: 10.1161/CIR.0000000000000708.
FROM CIRCULATION
Advances in Hematology and Oncology (August 2019)
Click here to access August 2019 Advances in Hematology and Oncology
Table of Contents
- Partners in Oncology Care: Coordinated Follicular Lymphoma Management
- Accuracy of Endoscopic Ultrasound in Staging of Early Rectal Cancer
- Treatment Facility: An Important Prognostic Factor for Dedifferentiated Liposarcoma Survival
- Review of Radiologic Considerations in an Immunocompetent Patient With Primary Central Nervous System Lymphoma
- Use of Mobile Messaging System for Self-Management of Chemotherapy Symptoms
- Timely Diagnosis of Lung Cancer in a Dedicated VA Referral Unit With Endobronchial Ultrasound
- Research News: Darolutamide Approved for Nonmetastatic CRPC
- Roundtable: Genomic Medicine and Genetic Counseling in the VA and DoD

Click here to access August 2019 Advances in Hematology and Oncology
Table of Contents
- Partners in Oncology Care: Coordinated Follicular Lymphoma Management
- Accuracy of Endoscopic Ultrasound in Staging of Early Rectal Cancer
- Treatment Facility: An Important Prognostic Factor for Dedifferentiated Liposarcoma Survival
- Review of Radiologic Considerations in an Immunocompetent Patient With Primary Central Nervous System Lymphoma
- Use of Mobile Messaging System for Self-Management of Chemotherapy Symptoms
- Timely Diagnosis of Lung Cancer in a Dedicated VA Referral Unit With Endobronchial Ultrasound
- Research News: Darolutamide Approved for Nonmetastatic CRPC
- Roundtable: Genomic Medicine and Genetic Counseling in the VA and DoD

Click here to access August 2019 Advances in Hematology and Oncology
Table of Contents
- Partners in Oncology Care: Coordinated Follicular Lymphoma Management
- Accuracy of Endoscopic Ultrasound in Staging of Early Rectal Cancer
- Treatment Facility: An Important Prognostic Factor for Dedifferentiated Liposarcoma Survival
- Review of Radiologic Considerations in an Immunocompetent Patient With Primary Central Nervous System Lymphoma
- Use of Mobile Messaging System for Self-Management of Chemotherapy Symptoms
- Timely Diagnosis of Lung Cancer in a Dedicated VA Referral Unit With Endobronchial Ultrasound
- Research News: Darolutamide Approved for Nonmetastatic CRPC
- Roundtable: Genomic Medicine and Genetic Counseling in the VA and DoD

Aspirin interacts with epigenetics to influence breast cancer mortality
The impact of prediagnosis aspirin use on mortality in women with breast cancer is significantly tied to epigenetic changes in certain breast cancer-related genes, investigators reported.
While studies have shown aspirin reduces the risk of breast cancer development, there is limited and inconsistent data on the effect of aspirin on prognosis and mortality after a diagnosis of breast cancer, Tengteng Wang, PhD, from the department of epidemiology at the University of North Carolina at Chapel Hill and coauthors wrote in Cancer.
To address this, they analyzed data from 1,508 women who had a first diagnosis of primary breast cancer and were involved in the Long Island Breast Cancer Study Project; they then looked at the women’s methylation status, which is a mechanism of epigenetic change.
Around one in five participants reported ever using aspirin, and the analysis showed that ever use of aspirin was associated with an overall 13% decrease in breast cancer–specific mortality.
However researchers saw significant interactions between aspirin use and LINE-1 methylation status – which is a marker of methylation of genetic elements that play key roles in maintaining genomic stability – and breast cancer–specific genes.
They found that aspirin use in women with LINE-1 hypomethylation was associated with a risk of breast cancer–specific mortality that was 45% higher than that of nonusers (P = .05).
Compared with nonusers, aspirin users with methylated tumor BRCA1 promoter had significant 16% higher breast cancer mortality (P = .04) and 67% higher all-cause mortality (P = .02). However the study showed aspirin did not affect mortality in women with unmethylated BRCA1 promoter.
Among women with the PR breast cancer gene, aspirin use by those with methylation of the PR promoter was associated with a 63% higher breast cancer–specific mortality, but methylation showed no statistically significant effect on all-cause mortality, compared with nonusers.
The study found no significant change when they restricted the analysis to receptor-positive or invasive breast cancer, and the associations remained consistent even after adjusting for global methylation.
“Our findings suggest that the association between aspirin use and mortality after breast cancer may depend on methylation profiles and warrant further investigation,” the authors wrote. “These findings, if confirmed, may provide new biological insights into the association between aspirin use and breast cancer prognosis, may affect clinical decision making by identifying a subgroup of patients with breast cancer using epigenetic markers for whom prediagnosis aspirin use affects subsequent mortality, and may help refine risk-reduction strategies to improve survival among women with breast cancer.”
The study was partly supported by the National Institutes of Health. One author declared personal fees from the private sector outside the submitted work.
SOURCE: Wang T et al. Cancer. 2019 Aug 12. doi: 10.1002/cncr.32364.
This study offers new insights into the intersection of epigenetics, prediagnosis aspirin use, and breast cancer survival at a time when there is an urgent need to understand why some women respond differently to treatment and to find cost-effective therapies for the disease.
Epigenetics is a promising avenue of investigation because epigenetic shifts, such as DNA methylation, that impact the genes responsible for cell behavior and DNA damage and repair are known to contribute to and exacerbate cancer. These epigenetic signatures could act as biomarkers for risk in cancer and also aid with more effective treatment approaches. For example, aspirin is known to affect DNA methylation at certain sites in colon cancer, hence this study’s hypothesis that pre–cancer diagnosis aspirin use would interact with epigenetic signatures and influence breast cancer outcomes.
Kristen M. C. Malecki, PhD, is from the department of population health sciences in the School of Medicine and Public Health at the University of Wisconsin, Madison. The comments are adapted from an accompanying editorial (Cancer. 2019 Aug 12. doi: 10.1002/cncr.32365). Dr. Malecki declared support from the National Institutes of Health, National Institute for Environmental Health Sciences Breast Cancer, and the Environment Research Program.
This study offers new insights into the intersection of epigenetics, prediagnosis aspirin use, and breast cancer survival at a time when there is an urgent need to understand why some women respond differently to treatment and to find cost-effective therapies for the disease.
Epigenetics is a promising avenue of investigation because epigenetic shifts, such as DNA methylation, that impact the genes responsible for cell behavior and DNA damage and repair are known to contribute to and exacerbate cancer. These epigenetic signatures could act as biomarkers for risk in cancer and also aid with more effective treatment approaches. For example, aspirin is known to affect DNA methylation at certain sites in colon cancer, hence this study’s hypothesis that pre–cancer diagnosis aspirin use would interact with epigenetic signatures and influence breast cancer outcomes.
Kristen M. C. Malecki, PhD, is from the department of population health sciences in the School of Medicine and Public Health at the University of Wisconsin, Madison. The comments are adapted from an accompanying editorial (Cancer. 2019 Aug 12. doi: 10.1002/cncr.32365). Dr. Malecki declared support from the National Institutes of Health, National Institute for Environmental Health Sciences Breast Cancer, and the Environment Research Program.
This study offers new insights into the intersection of epigenetics, prediagnosis aspirin use, and breast cancer survival at a time when there is an urgent need to understand why some women respond differently to treatment and to find cost-effective therapies for the disease.
Epigenetics is a promising avenue of investigation because epigenetic shifts, such as DNA methylation, that impact the genes responsible for cell behavior and DNA damage and repair are known to contribute to and exacerbate cancer. These epigenetic signatures could act as biomarkers for risk in cancer and also aid with more effective treatment approaches. For example, aspirin is known to affect DNA methylation at certain sites in colon cancer, hence this study’s hypothesis that pre–cancer diagnosis aspirin use would interact with epigenetic signatures and influence breast cancer outcomes.
Kristen M. C. Malecki, PhD, is from the department of population health sciences in the School of Medicine and Public Health at the University of Wisconsin, Madison. The comments are adapted from an accompanying editorial (Cancer. 2019 Aug 12. doi: 10.1002/cncr.32365). Dr. Malecki declared support from the National Institutes of Health, National Institute for Environmental Health Sciences Breast Cancer, and the Environment Research Program.
The impact of prediagnosis aspirin use on mortality in women with breast cancer is significantly tied to epigenetic changes in certain breast cancer-related genes, investigators reported.
While studies have shown aspirin reduces the risk of breast cancer development, there is limited and inconsistent data on the effect of aspirin on prognosis and mortality after a diagnosis of breast cancer, Tengteng Wang, PhD, from the department of epidemiology at the University of North Carolina at Chapel Hill and coauthors wrote in Cancer.
To address this, they analyzed data from 1,508 women who had a first diagnosis of primary breast cancer and were involved in the Long Island Breast Cancer Study Project; they then looked at the women’s methylation status, which is a mechanism of epigenetic change.
Around one in five participants reported ever using aspirin, and the analysis showed that ever use of aspirin was associated with an overall 13% decrease in breast cancer–specific mortality.
However researchers saw significant interactions between aspirin use and LINE-1 methylation status – which is a marker of methylation of genetic elements that play key roles in maintaining genomic stability – and breast cancer–specific genes.
They found that aspirin use in women with LINE-1 hypomethylation was associated with a risk of breast cancer–specific mortality that was 45% higher than that of nonusers (P = .05).
Compared with nonusers, aspirin users with methylated tumor BRCA1 promoter had significant 16% higher breast cancer mortality (P = .04) and 67% higher all-cause mortality (P = .02). However the study showed aspirin did not affect mortality in women with unmethylated BRCA1 promoter.
Among women with the PR breast cancer gene, aspirin use by those with methylation of the PR promoter was associated with a 63% higher breast cancer–specific mortality, but methylation showed no statistically significant effect on all-cause mortality, compared with nonusers.
The study found no significant change when they restricted the analysis to receptor-positive or invasive breast cancer, and the associations remained consistent even after adjusting for global methylation.
“Our findings suggest that the association between aspirin use and mortality after breast cancer may depend on methylation profiles and warrant further investigation,” the authors wrote. “These findings, if confirmed, may provide new biological insights into the association between aspirin use and breast cancer prognosis, may affect clinical decision making by identifying a subgroup of patients with breast cancer using epigenetic markers for whom prediagnosis aspirin use affects subsequent mortality, and may help refine risk-reduction strategies to improve survival among women with breast cancer.”
The study was partly supported by the National Institutes of Health. One author declared personal fees from the private sector outside the submitted work.
SOURCE: Wang T et al. Cancer. 2019 Aug 12. doi: 10.1002/cncr.32364.
The impact of prediagnosis aspirin use on mortality in women with breast cancer is significantly tied to epigenetic changes in certain breast cancer-related genes, investigators reported.
While studies have shown aspirin reduces the risk of breast cancer development, there is limited and inconsistent data on the effect of aspirin on prognosis and mortality after a diagnosis of breast cancer, Tengteng Wang, PhD, from the department of epidemiology at the University of North Carolina at Chapel Hill and coauthors wrote in Cancer.
To address this, they analyzed data from 1,508 women who had a first diagnosis of primary breast cancer and were involved in the Long Island Breast Cancer Study Project; they then looked at the women’s methylation status, which is a mechanism of epigenetic change.
Around one in five participants reported ever using aspirin, and the analysis showed that ever use of aspirin was associated with an overall 13% decrease in breast cancer–specific mortality.
However researchers saw significant interactions between aspirin use and LINE-1 methylation status – which is a marker of methylation of genetic elements that play key roles in maintaining genomic stability – and breast cancer–specific genes.
They found that aspirin use in women with LINE-1 hypomethylation was associated with a risk of breast cancer–specific mortality that was 45% higher than that of nonusers (P = .05).
Compared with nonusers, aspirin users with methylated tumor BRCA1 promoter had significant 16% higher breast cancer mortality (P = .04) and 67% higher all-cause mortality (P = .02). However the study showed aspirin did not affect mortality in women with unmethylated BRCA1 promoter.
Among women with the PR breast cancer gene, aspirin use by those with methylation of the PR promoter was associated with a 63% higher breast cancer–specific mortality, but methylation showed no statistically significant effect on all-cause mortality, compared with nonusers.
The study found no significant change when they restricted the analysis to receptor-positive or invasive breast cancer, and the associations remained consistent even after adjusting for global methylation.
“Our findings suggest that the association between aspirin use and mortality after breast cancer may depend on methylation profiles and warrant further investigation,” the authors wrote. “These findings, if confirmed, may provide new biological insights into the association between aspirin use and breast cancer prognosis, may affect clinical decision making by identifying a subgroup of patients with breast cancer using epigenetic markers for whom prediagnosis aspirin use affects subsequent mortality, and may help refine risk-reduction strategies to improve survival among women with breast cancer.”
The study was partly supported by the National Institutes of Health. One author declared personal fees from the private sector outside the submitted work.
SOURCE: Wang T et al. Cancer. 2019 Aug 12. doi: 10.1002/cncr.32364.
FROM CANCER
Register for the 2019 Coding and Reimbursement Course
The vascular specialty has had many coding changes over the past five to six years. It is sometimes difficult to keep up with the changes from year to year, but attending the SVS Coding & Reimbursement workshop, to be held on Sept. 20-21 in Rosemont, Ill., is sure to help. Coding is critical in every practice model – whether it be private, academic or employed. If it is not done accurately, money will be lost. This intensive course will give attendees the knowledge they need to make a positive impact on their coding procedures. All those who wish to improve and expand their knowledge of accurate coding and reimbursement for vascular surgery should attend. Learn more and register here.
The vascular specialty has had many coding changes over the past five to six years. It is sometimes difficult to keep up with the changes from year to year, but attending the SVS Coding & Reimbursement workshop, to be held on Sept. 20-21 in Rosemont, Ill., is sure to help. Coding is critical in every practice model – whether it be private, academic or employed. If it is not done accurately, money will be lost. This intensive course will give attendees the knowledge they need to make a positive impact on their coding procedures. All those who wish to improve and expand their knowledge of accurate coding and reimbursement for vascular surgery should attend. Learn more and register here.
The vascular specialty has had many coding changes over the past five to six years. It is sometimes difficult to keep up with the changes from year to year, but attending the SVS Coding & Reimbursement workshop, to be held on Sept. 20-21 in Rosemont, Ill., is sure to help. Coding is critical in every practice model – whether it be private, academic or employed. If it is not done accurately, money will be lost. This intensive course will give attendees the knowledge they need to make a positive impact on their coding procedures. All those who wish to improve and expand their knowledge of accurate coding and reimbursement for vascular surgery should attend. Learn more and register here.
Psoriasis Treatments Could Have Bonus Benefits
Psoriasis is associated with systemic inflammation, which heightens the risk of blood vessel disease and diabetes. Therefore, the finding, while notable, may not have been entirely unexpected. Biologic therapy (BT) for psoriasis was already found to be favorably associated with luminal coronary plaque, the researchers say, but it was not clear whether those associations were attributable to direct anti-inflammatory effects on the coronary arteries. They wanted to find out whether the perivascular fat attenuation index (FAI) would offer clues. FAI is a new method of analyzing CT scans by assessing whether the fat tissue surrounding arteries becomes attenuated, or less fatty.
The researchers investigated their premise in 134 participants from an ongoing NIH study, the Psoriasis Atherosclerosis Cardiometabolic Initiative cohort. Of the participants, 82 had been receiving anti-tumor necrosis factor α, anti-interleukin (IL) 12/23, or anti-IL-17 for 1 year. The remaining 52 had not received any BT, and given topical or light therapy. The patients underwent CT scans at the start of the study and 1 year later. All of the patients had low cardiovascular risk. At baseline, 27 in the treated group and 19 in the untreated group had a focal coronary atherosclerotic plaque.
The study found that an abnormal perivascular FAI was linked to a 6- to 9-fold increased risk of major adverse cardiovascular events, study coauthor Charalambos Antoniades, MD, says. Patients on BT had a significant decrease in FAI at 1 year, as well as improved psoriasis symptoms. Even patients with preexisting coronary artery plaque had a reduction in coronary inflammation after BT. No change was seen in the untreated patients. The associations with FAI were independent of the presence of coronary plaque and were consistent among patients receiving different biologic agents.
The researchers say their findings have implications for other chronic inflammatory diseases, such as lupus and rheumatoid arthritis, which are known to raise the risk for heart attacks and stroke.
Psoriasis is associated with systemic inflammation, which heightens the risk of blood vessel disease and diabetes. Therefore, the finding, while notable, may not have been entirely unexpected. Biologic therapy (BT) for psoriasis was already found to be favorably associated with luminal coronary plaque, the researchers say, but it was not clear whether those associations were attributable to direct anti-inflammatory effects on the coronary arteries. They wanted to find out whether the perivascular fat attenuation index (FAI) would offer clues. FAI is a new method of analyzing CT scans by assessing whether the fat tissue surrounding arteries becomes attenuated, or less fatty.
The researchers investigated their premise in 134 participants from an ongoing NIH study, the Psoriasis Atherosclerosis Cardiometabolic Initiative cohort. Of the participants, 82 had been receiving anti-tumor necrosis factor α, anti-interleukin (IL) 12/23, or anti-IL-17 for 1 year. The remaining 52 had not received any BT, and given topical or light therapy. The patients underwent CT scans at the start of the study and 1 year later. All of the patients had low cardiovascular risk. At baseline, 27 in the treated group and 19 in the untreated group had a focal coronary atherosclerotic plaque.
The study found that an abnormal perivascular FAI was linked to a 6- to 9-fold increased risk of major adverse cardiovascular events, study coauthor Charalambos Antoniades, MD, says. Patients on BT had a significant decrease in FAI at 1 year, as well as improved psoriasis symptoms. Even patients with preexisting coronary artery plaque had a reduction in coronary inflammation after BT. No change was seen in the untreated patients. The associations with FAI were independent of the presence of coronary plaque and were consistent among patients receiving different biologic agents.
The researchers say their findings have implications for other chronic inflammatory diseases, such as lupus and rheumatoid arthritis, which are known to raise the risk for heart attacks and stroke.
Psoriasis is associated with systemic inflammation, which heightens the risk of blood vessel disease and diabetes. Therefore, the finding, while notable, may not have been entirely unexpected. Biologic therapy (BT) for psoriasis was already found to be favorably associated with luminal coronary plaque, the researchers say, but it was not clear whether those associations were attributable to direct anti-inflammatory effects on the coronary arteries. They wanted to find out whether the perivascular fat attenuation index (FAI) would offer clues. FAI is a new method of analyzing CT scans by assessing whether the fat tissue surrounding arteries becomes attenuated, or less fatty.
The researchers investigated their premise in 134 participants from an ongoing NIH study, the Psoriasis Atherosclerosis Cardiometabolic Initiative cohort. Of the participants, 82 had been receiving anti-tumor necrosis factor α, anti-interleukin (IL) 12/23, or anti-IL-17 for 1 year. The remaining 52 had not received any BT, and given topical or light therapy. The patients underwent CT scans at the start of the study and 1 year later. All of the patients had low cardiovascular risk. At baseline, 27 in the treated group and 19 in the untreated group had a focal coronary atherosclerotic plaque.
The study found that an abnormal perivascular FAI was linked to a 6- to 9-fold increased risk of major adverse cardiovascular events, study coauthor Charalambos Antoniades, MD, says. Patients on BT had a significant decrease in FAI at 1 year, as well as improved psoriasis symptoms. Even patients with preexisting coronary artery plaque had a reduction in coronary inflammation after BT. No change was seen in the untreated patients. The associations with FAI were independent of the presence of coronary plaque and were consistent among patients receiving different biologic agents.
The researchers say their findings have implications for other chronic inflammatory diseases, such as lupus and rheumatoid arthritis, which are known to raise the risk for heart attacks and stroke.