User login
Why do so many women aged 65 years and older die of cervical cancer?
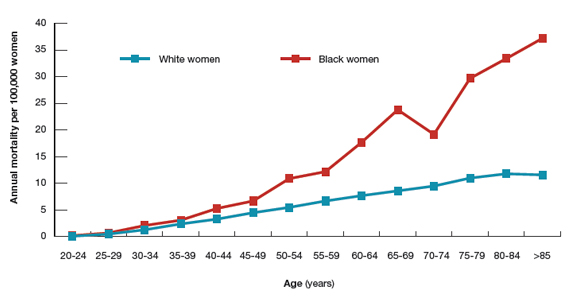
Surprisingly, the cervical cancer death rate is greater among women aged >65 years than among younger women1,2 (FIGURE). Paradoxically, most of our screening programs focus on women <65 years of age. A nationwide study from Denmark estimated that the cervical cancer death rate per 100,000 women at ages 40 to 44 and 65 to 69 was 3.8 and 9.0, respectively.1 In other words, the cervical cancer death rate at age 65 to 69 years was 2.36 times higher than at age 40 to 44 years.1
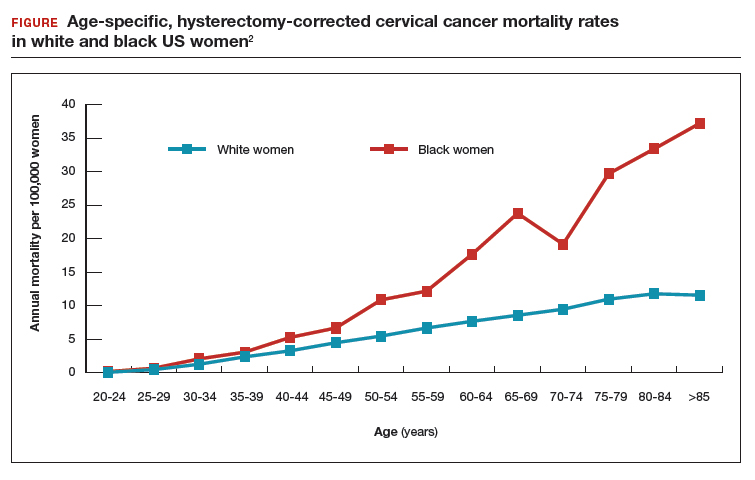
A study from the United States estimated that the cervical cancer death rate per 100,000 white women at ages 40 to 44 and 65 to 69 was 3.3 and 8.6, respectively,2 very similar to the findings from Denmark. The same US study estimated that the cervical cancer death rate per 100,000 black women at ages 40 to 44 and 65 to 69 was 5.3 and 23.8, highlighting the fact that, in the United States, cervical cancer disease burden is disproportionately greater among black than among white women.2 In addition, the cervical cancer death rate among black women at age 65 to 69 was 4.49 times higher than at age 40 to 44 years.2
Given the high death rate from cervical cancer in women >65 years of age, it is paradoxical that most professional society guidelines recommend discontinuing cervical cancer screening at 65 years of age, if previous cervical cancer screening is normal.3,4 Is the problem due to an inability to implement the current guidelines? Or is the problem that the guidelines are not optimally designed to reduce cervical cancer risk in women >65 years of age?
The American College of Obstetricians and Gynecologists (ACOG) and the US Preventive Services Task Force (USPSTF) recommend against cervical cancer screening in women >65 years of age who have had adequate prior screening and are not otherwise at high risk for cervical cancer. However, ACOG and the USPSTF caution that there are many groups of women that may benefit from continued screening after 65 years of age, including women with HIV infection, a compromised immune system, or previous high-grade precancerous lesion or cervicalcancer; women with limited access to care; women from racial/ethnic minority groups; and migrant women.4 Many clinicians remember the guidance, “discontinue cervical cancer screening at 65 years” but do not recall all the clinical factors that might warrant continued screening past age 65. Of special concern is that black,2 Hispanic,5 and migrant women6 are at much higher risk for invasive cervical cancer than white or US-born women.
The optimal implementation of the ACOG and USPSTF guidelines are undermined by a fractured health care system, where key pieces of information may be unavailable to the clinician tasked with making a decision about discontinuing cervical cancer screening. Imagine the case in which a 65-year-old woman pre‑sents to her primary care physician for cervical cancer screening. The clinician performs a cervical cytology test and obtains a report of “no intraepithelial lesion or malignancy.” The clinician then recommends that the patient discontinue cervical cancer screening. Unbeknownst to the clinician, the patient had a positive HPV 16/18/45 test within the past 10 years in another health system. In this case, it would be inappropriate to terminate the patient from cervical cancer screening.
Continue to: Testing for hrHPV is superior to cervical cytology in women >65 years...
Testing for hrHPV is superior to cervical cytology in women >65 years
In Sweden, about 30% of cervical cancer cases occur in women aged >60 years.7 To assess the prevalence of oncogenic high-risk HPV (hrHPV), women at ages 60, 65, 70, and 75 years were invited to send sequential self-collected vaginal samples for nucleic acid testing for hrHPV. The prevalence of hrHPV was found to be 4.4%. Women with a second positive, self-collected, hrHPV test were invited for colposcopy, cervical biopsy, and cytology testing. Among the women with two positive hrHPV tests, cervical biopsy revealed 7 cases of cervical intraepithelial neoplasia grade 2 (CIN2), 6 cases of CIN1, and 4 biopsies without CIN. In these women 94% of the cervical cytology samples returned, “no intraepithelial lesion or malignancy” and 6% revealed atypical squamous cells of undetermined significance. This study suggests that, in women aged >65 years, cervical cytology may have a high rate of false-negative results, possibly due to epithelial atrophy. An evolving clinical pearl is that, when using the current cervical cancer screening guidelines, the final screen for cervical cancer must include a nucleic acid test for hrHPV.
In women 65 to 90 years, the prevalence of hrHPV is approximately 5%
In a study of 40,382 women aged 14 to 95 years, the prevalence of hrHPV was 46% in 20- to 23-year-old women and 5.7% in women older than 65 years of age.8 In a study of more than 108,000 women aged 69 to >89 years the prevalence of hrHPV was 4.3%, and similar prevalence rates were seen across all ages from 69 to >89 years.9 The carcinogenic role of persistent hrHPV infection in women >65 years is an important area for future research.
Latent HPV virus infection
Following a primary varicella-zoster infection (chickenpox), the virus may remain in a latent state in sensory ganglia, reactivating later in life to cause shingles. Thirty percent of people who have a primary chickenpox infection eventually will develop a case of shingles. Immunocompromised populations are at an increased risk of developing shingles because of reduced T-cell mediated immunity.
A recent hypothesis is that in immunocompromised and older women, latent HPV can reactivate and cause clinically significant infection.10 Following renal transplantation investigators have reported a significant increase in the prevalence of genital HPV, without a change in sexual behavior.11 In cervical tissue from women with no evidence of active HPV infection, highly sensitive PCR-based assays detected HPV16 virus in a latent state in some women, possibly due to disruption of the viral E2 gene.12 If latent HPV infection is a valid biological concept, it suggests that there is no “safe age” at which to discontinue screening for HPV infection because the virus cannot be detected in screening samples while it is latent.
Options for cervical cancer screening in women >65 years
Three options might reduce the morbidity and mortality associated with cervical cancer in women >65 years.
Option 1: Double-down on trying to effectively implement current guidelines. The high rate of cervical cancer mortality in women >65 years of age indicates that the current guidelines, as implemented in real clinical practice, are not working. A problem with the current screening guidelines is that clinicians are expected to be capable of finding all relevant cervical cancer test results and properly interpreting the results. Clinicians are over-taxed and fallible, and the current approach is not likely to be successful unless additional information technology solutions are implemented.
Continue to: Health systems could use information...
Health systems could use information technology to mitigate these problems. For example, health systems could deploy software to assemble every cervical screening result on each woman and pre‑sent those results to clinicians in a single integrated view in the electronic record. Additionally, once all lifetime screening results are consolidated in one view, artificial intelligence systems could be used to analyze the totality of results and identify women who would benefit by continued screening past age 65 and women who could safely discontinue screening.
Option 2: Adopt the Australian approach to cervical cancer screening. The current Australian approach to cervical cancer screening is built on 3 pillars: 1) school-based vaccination of all children against hrHPV, 2) screening all women from 25 to 74 years of age every 5 years using nucleic acid testing for hrHPV, and 3) providing a system for the testing of samples self-collected by women who are reluctant to visit a clinician for screening.13 Australia has one of the lowest cervical cancer death rates in the world.
Option 3: Continue screening most women past age 65. Women >65 years of age are known to be infected with hrHPV genotypes. hrHPV infection causes cervical cancer. Cervical cancer causes many deaths in women aged >65 years. There is no strong rationale for ignoring these three facts. hrHPV screening every 5 years as long as the woman is healthy and has a reasonable life expectancy is an option that could be evaluated in randomized studies.
Given the high rate of cervical cancer death in women >65 years of age, I plan to be very cautious about discontinuing cervical cancer screening until I can personally ensure that my patient has no evidence of hrHPV infection.
In 2008, Harald zur Hausen, MD, received the Nobel Prize in Physiology or Medicine for discovering that human papilloma virus (HPV) caused cervical cancer. In a recent study, 74% of cervical cancers were associated with HPV 16 or 18 infections. A total of 89% of the cancers were associated with one of the high-risk HPV genotypes, including HPV 16/18/31/33/45/52/58.1
Recently, HPV has been shown to be a major cause of oropharyngeal cancer. The Centers for Disease Control and Prevention calculated that in CY2015 in the United States there were 18,917 cases of HPV-associated oropharyngeal squamous cell cancer and 11,788 cases of cervical cancer.2 Most cases of HPV-associated oropharyngeal cancer occur in men, and HPV vaccination of boys may help to prevent this cancer type. Oncogenic HPV produce two proteins (E6 and E7) that promote viral replication and squamous cell growth by inhibiting the function of p53 and retinoblastoma protein. The immortalized HeLa cell line, derived from Ms. Henrietta Lack's cervical cancer, contains integrated HPV18 nucleic acid sequences.3,4
The discovery that HPV causes cancer catalyzed the development of nucleic acid tests to identify high-risk oncogenic HPV and vaccines against high-risk oncogenic HPV genotypes that prevent cervical cancer. From a public health perspective, it is more effective to vaccinate the population against oncogenic HPV genotypes than to screen and treat cancer. In the United States, vaccination rates range from a high of 92% (District of Columbia) and 89% (Rhode Island) to a low of 47% (Wyoming) and 50% (Kentucky and Mississippi).5 To reduce HPV-associated cancer mortality, the gap in vaccination compliance must be closed.
References
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Van Dyne EA, Henley SJ, Saraiya M, et al. Trends in human papillomavirus-associated cancers - United States, 1999-2015. MMWR Morb Mortal Wkly Rep. 2018;67:918-924.
- Rosl F, Westphal EM, zur Hausen H. Chromatin structure and transcriptional regulation of human papillomavirus type 18 DNA in HeLa cells. Mol Carcinog. 1989;2:72-80.
- Adey A, Burton JN, Kitzman, et al. The haplotype-resolved genome and epigenome of the aneuploid HeLa cancer cell line. Nature. 2013;500:207-211.
- Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
- Hammer A, Kahlert J, Gravitt PE, et al. Hysterectomy-corrected cervical cancer mortality rates in Denmark during 2002-2015: a registry-based cohort study. Acta Obstet Gynecol Scand. 2019;98:1063-1069.
- Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123:1044-1050.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol. 2016;128:e111-30.
- Curry SJ, Krist AH, Owens DK, et al; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Stang A, Hawk H, Knowlton R, et al. Hysterectomy-corrected incidence rates of cervical and uterine cancers in Massachusetts, 1995-2010. Ann Epidemiol. 2014;24:849-854.
- Hallowell BD, Endeshaw M, McKenna MT, et al. Cervical cancer death rates among U.S.- and foreign-born women: U.S., 2005-2014. Am J Prev Med. 2019;56:869-874.
- Lindström AK, Hermansson RS, Gustavsson I, et al. Cervical dysplasia in elderly women performing repeated self-sampling for HPV testing. PLoS One. 2018;13:e0207714.
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Andersen B, Christensen BS, Christensen J, et al. HPV-prevalence in elderly women in Denmark. Gynecol Oncol. 2019;154:118-123.
- Gravitt PE, Winer RL. Natural history of HPV infection across the lifespan: role of viral latency. Viruses. 2017;9:E267.
- Hinten F, Hilbrands LB, Meeuwis KAP, et al. Reactivation of latent HPV infections after renal transplantation. Am J Transplant. 2017;17:1563-1573.
- Leonard SM, Pereira M, Roberts S, et al. Evidence of disrupted high-risk human papillomavirus DNA in morphologically normal cervices of older women. Sci Rep. 2016;6:20847.
- Cervical cancer screening. Cancer Council website. https://www.cancer.org.au/about-cancer/early-detection/screening-programs/cervical-cancer-screening.html. Updated March 15, 2019. Accessed July 23, 2019.
Surprisingly, the cervical cancer death rate is greater among women aged >65 years than among younger women1,2 (FIGURE). Paradoxically, most of our screening programs focus on women <65 years of age. A nationwide study from Denmark estimated that the cervical cancer death rate per 100,000 women at ages 40 to 44 and 65 to 69 was 3.8 and 9.0, respectively.1 In other words, the cervical cancer death rate at age 65 to 69 years was 2.36 times higher than at age 40 to 44 years.1

A study from the United States estimated that the cervical cancer death rate per 100,000 white women at ages 40 to 44 and 65 to 69 was 3.3 and 8.6, respectively,2 very similar to the findings from Denmark. The same US study estimated that the cervical cancer death rate per 100,000 black women at ages 40 to 44 and 65 to 69 was 5.3 and 23.8, highlighting the fact that, in the United States, cervical cancer disease burden is disproportionately greater among black than among white women.2 In addition, the cervical cancer death rate among black women at age 65 to 69 was 4.49 times higher than at age 40 to 44 years.2
Given the high death rate from cervical cancer in women >65 years of age, it is paradoxical that most professional society guidelines recommend discontinuing cervical cancer screening at 65 years of age, if previous cervical cancer screening is normal.3,4 Is the problem due to an inability to implement the current guidelines? Or is the problem that the guidelines are not optimally designed to reduce cervical cancer risk in women >65 years of age?
The American College of Obstetricians and Gynecologists (ACOG) and the US Preventive Services Task Force (USPSTF) recommend against cervical cancer screening in women >65 years of age who have had adequate prior screening and are not otherwise at high risk for cervical cancer. However, ACOG and the USPSTF caution that there are many groups of women that may benefit from continued screening after 65 years of age, including women with HIV infection, a compromised immune system, or previous high-grade precancerous lesion or cervicalcancer; women with limited access to care; women from racial/ethnic minority groups; and migrant women.4 Many clinicians remember the guidance, “discontinue cervical cancer screening at 65 years” but do not recall all the clinical factors that might warrant continued screening past age 65. Of special concern is that black,2 Hispanic,5 and migrant women6 are at much higher risk for invasive cervical cancer than white or US-born women.
The optimal implementation of the ACOG and USPSTF guidelines are undermined by a fractured health care system, where key pieces of information may be unavailable to the clinician tasked with making a decision about discontinuing cervical cancer screening. Imagine the case in which a 65-year-old woman pre‑sents to her primary care physician for cervical cancer screening. The clinician performs a cervical cytology test and obtains a report of “no intraepithelial lesion or malignancy.” The clinician then recommends that the patient discontinue cervical cancer screening. Unbeknownst to the clinician, the patient had a positive HPV 16/18/45 test within the past 10 years in another health system. In this case, it would be inappropriate to terminate the patient from cervical cancer screening.
Continue to: Testing for hrHPV is superior to cervical cytology in women >65 years...
Testing for hrHPV is superior to cervical cytology in women >65 years
In Sweden, about 30% of cervical cancer cases occur in women aged >60 years.7 To assess the prevalence of oncogenic high-risk HPV (hrHPV), women at ages 60, 65, 70, and 75 years were invited to send sequential self-collected vaginal samples for nucleic acid testing for hrHPV. The prevalence of hrHPV was found to be 4.4%. Women with a second positive, self-collected, hrHPV test were invited for colposcopy, cervical biopsy, and cytology testing. Among the women with two positive hrHPV tests, cervical biopsy revealed 7 cases of cervical intraepithelial neoplasia grade 2 (CIN2), 6 cases of CIN1, and 4 biopsies without CIN. In these women 94% of the cervical cytology samples returned, “no intraepithelial lesion or malignancy” and 6% revealed atypical squamous cells of undetermined significance. This study suggests that, in women aged >65 years, cervical cytology may have a high rate of false-negative results, possibly due to epithelial atrophy. An evolving clinical pearl is that, when using the current cervical cancer screening guidelines, the final screen for cervical cancer must include a nucleic acid test for hrHPV.
In women 65 to 90 years, the prevalence of hrHPV is approximately 5%
In a study of 40,382 women aged 14 to 95 years, the prevalence of hrHPV was 46% in 20- to 23-year-old women and 5.7% in women older than 65 years of age.8 In a study of more than 108,000 women aged 69 to >89 years the prevalence of hrHPV was 4.3%, and similar prevalence rates were seen across all ages from 69 to >89 years.9 The carcinogenic role of persistent hrHPV infection in women >65 years is an important area for future research.
Latent HPV virus infection
Following a primary varicella-zoster infection (chickenpox), the virus may remain in a latent state in sensory ganglia, reactivating later in life to cause shingles. Thirty percent of people who have a primary chickenpox infection eventually will develop a case of shingles. Immunocompromised populations are at an increased risk of developing shingles because of reduced T-cell mediated immunity.
A recent hypothesis is that in immunocompromised and older women, latent HPV can reactivate and cause clinically significant infection.10 Following renal transplantation investigators have reported a significant increase in the prevalence of genital HPV, without a change in sexual behavior.11 In cervical tissue from women with no evidence of active HPV infection, highly sensitive PCR-based assays detected HPV16 virus in a latent state in some women, possibly due to disruption of the viral E2 gene.12 If latent HPV infection is a valid biological concept, it suggests that there is no “safe age” at which to discontinue screening for HPV infection because the virus cannot be detected in screening samples while it is latent.
Options for cervical cancer screening in women >65 years
Three options might reduce the morbidity and mortality associated with cervical cancer in women >65 years.
Option 1: Double-down on trying to effectively implement current guidelines. The high rate of cervical cancer mortality in women >65 years of age indicates that the current guidelines, as implemented in real clinical practice, are not working. A problem with the current screening guidelines is that clinicians are expected to be capable of finding all relevant cervical cancer test results and properly interpreting the results. Clinicians are over-taxed and fallible, and the current approach is not likely to be successful unless additional information technology solutions are implemented.
Continue to: Health systems could use information...
Health systems could use information technology to mitigate these problems. For example, health systems could deploy software to assemble every cervical screening result on each woman and pre‑sent those results to clinicians in a single integrated view in the electronic record. Additionally, once all lifetime screening results are consolidated in one view, artificial intelligence systems could be used to analyze the totality of results and identify women who would benefit by continued screening past age 65 and women who could safely discontinue screening.
Option 2: Adopt the Australian approach to cervical cancer screening. The current Australian approach to cervical cancer screening is built on 3 pillars: 1) school-based vaccination of all children against hrHPV, 2) screening all women from 25 to 74 years of age every 5 years using nucleic acid testing for hrHPV, and 3) providing a system for the testing of samples self-collected by women who are reluctant to visit a clinician for screening.13 Australia has one of the lowest cervical cancer death rates in the world.
Option 3: Continue screening most women past age 65. Women >65 years of age are known to be infected with hrHPV genotypes. hrHPV infection causes cervical cancer. Cervical cancer causes many deaths in women aged >65 years. There is no strong rationale for ignoring these three facts. hrHPV screening every 5 years as long as the woman is healthy and has a reasonable life expectancy is an option that could be evaluated in randomized studies.
Given the high rate of cervical cancer death in women >65 years of age, I plan to be very cautious about discontinuing cervical cancer screening until I can personally ensure that my patient has no evidence of hrHPV infection.
In 2008, Harald zur Hausen, MD, received the Nobel Prize in Physiology or Medicine for discovering that human papilloma virus (HPV) caused cervical cancer. In a recent study, 74% of cervical cancers were associated with HPV 16 or 18 infections. A total of 89% of the cancers were associated with one of the high-risk HPV genotypes, including HPV 16/18/31/33/45/52/58.1
Recently, HPV has been shown to be a major cause of oropharyngeal cancer. The Centers for Disease Control and Prevention calculated that in CY2015 in the United States there were 18,917 cases of HPV-associated oropharyngeal squamous cell cancer and 11,788 cases of cervical cancer.2 Most cases of HPV-associated oropharyngeal cancer occur in men, and HPV vaccination of boys may help to prevent this cancer type. Oncogenic HPV produce two proteins (E6 and E7) that promote viral replication and squamous cell growth by inhibiting the function of p53 and retinoblastoma protein. The immortalized HeLa cell line, derived from Ms. Henrietta Lack's cervical cancer, contains integrated HPV18 nucleic acid sequences.3,4
The discovery that HPV causes cancer catalyzed the development of nucleic acid tests to identify high-risk oncogenic HPV and vaccines against high-risk oncogenic HPV genotypes that prevent cervical cancer. From a public health perspective, it is more effective to vaccinate the population against oncogenic HPV genotypes than to screen and treat cancer. In the United States, vaccination rates range from a high of 92% (District of Columbia) and 89% (Rhode Island) to a low of 47% (Wyoming) and 50% (Kentucky and Mississippi).5 To reduce HPV-associated cancer mortality, the gap in vaccination compliance must be closed.
References
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Van Dyne EA, Henley SJ, Saraiya M, et al. Trends in human papillomavirus-associated cancers - United States, 1999-2015. MMWR Morb Mortal Wkly Rep. 2018;67:918-924.
- Rosl F, Westphal EM, zur Hausen H. Chromatin structure and transcriptional regulation of human papillomavirus type 18 DNA in HeLa cells. Mol Carcinog. 1989;2:72-80.
- Adey A, Burton JN, Kitzman, et al. The haplotype-resolved genome and epigenome of the aneuploid HeLa cancer cell line. Nature. 2013;500:207-211.
- Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
Surprisingly, the cervical cancer death rate is greater among women aged >65 years than among younger women1,2 (FIGURE). Paradoxically, most of our screening programs focus on women <65 years of age. A nationwide study from Denmark estimated that the cervical cancer death rate per 100,000 women at ages 40 to 44 and 65 to 69 was 3.8 and 9.0, respectively.1 In other words, the cervical cancer death rate at age 65 to 69 years was 2.36 times higher than at age 40 to 44 years.1

A study from the United States estimated that the cervical cancer death rate per 100,000 white women at ages 40 to 44 and 65 to 69 was 3.3 and 8.6, respectively,2 very similar to the findings from Denmark. The same US study estimated that the cervical cancer death rate per 100,000 black women at ages 40 to 44 and 65 to 69 was 5.3 and 23.8, highlighting the fact that, in the United States, cervical cancer disease burden is disproportionately greater among black than among white women.2 In addition, the cervical cancer death rate among black women at age 65 to 69 was 4.49 times higher than at age 40 to 44 years.2
Given the high death rate from cervical cancer in women >65 years of age, it is paradoxical that most professional society guidelines recommend discontinuing cervical cancer screening at 65 years of age, if previous cervical cancer screening is normal.3,4 Is the problem due to an inability to implement the current guidelines? Or is the problem that the guidelines are not optimally designed to reduce cervical cancer risk in women >65 years of age?
The American College of Obstetricians and Gynecologists (ACOG) and the US Preventive Services Task Force (USPSTF) recommend against cervical cancer screening in women >65 years of age who have had adequate prior screening and are not otherwise at high risk for cervical cancer. However, ACOG and the USPSTF caution that there are many groups of women that may benefit from continued screening after 65 years of age, including women with HIV infection, a compromised immune system, or previous high-grade precancerous lesion or cervicalcancer; women with limited access to care; women from racial/ethnic minority groups; and migrant women.4 Many clinicians remember the guidance, “discontinue cervical cancer screening at 65 years” but do not recall all the clinical factors that might warrant continued screening past age 65. Of special concern is that black,2 Hispanic,5 and migrant women6 are at much higher risk for invasive cervical cancer than white or US-born women.
The optimal implementation of the ACOG and USPSTF guidelines are undermined by a fractured health care system, where key pieces of information may be unavailable to the clinician tasked with making a decision about discontinuing cervical cancer screening. Imagine the case in which a 65-year-old woman pre‑sents to her primary care physician for cervical cancer screening. The clinician performs a cervical cytology test and obtains a report of “no intraepithelial lesion or malignancy.” The clinician then recommends that the patient discontinue cervical cancer screening. Unbeknownst to the clinician, the patient had a positive HPV 16/18/45 test within the past 10 years in another health system. In this case, it would be inappropriate to terminate the patient from cervical cancer screening.
Continue to: Testing for hrHPV is superior to cervical cytology in women >65 years...
Testing for hrHPV is superior to cervical cytology in women >65 years
In Sweden, about 30% of cervical cancer cases occur in women aged >60 years.7 To assess the prevalence of oncogenic high-risk HPV (hrHPV), women at ages 60, 65, 70, and 75 years were invited to send sequential self-collected vaginal samples for nucleic acid testing for hrHPV. The prevalence of hrHPV was found to be 4.4%. Women with a second positive, self-collected, hrHPV test were invited for colposcopy, cervical biopsy, and cytology testing. Among the women with two positive hrHPV tests, cervical biopsy revealed 7 cases of cervical intraepithelial neoplasia grade 2 (CIN2), 6 cases of CIN1, and 4 biopsies without CIN. In these women 94% of the cervical cytology samples returned, “no intraepithelial lesion or malignancy” and 6% revealed atypical squamous cells of undetermined significance. This study suggests that, in women aged >65 years, cervical cytology may have a high rate of false-negative results, possibly due to epithelial atrophy. An evolving clinical pearl is that, when using the current cervical cancer screening guidelines, the final screen for cervical cancer must include a nucleic acid test for hrHPV.
In women 65 to 90 years, the prevalence of hrHPV is approximately 5%
In a study of 40,382 women aged 14 to 95 years, the prevalence of hrHPV was 46% in 20- to 23-year-old women and 5.7% in women older than 65 years of age.8 In a study of more than 108,000 women aged 69 to >89 years the prevalence of hrHPV was 4.3%, and similar prevalence rates were seen across all ages from 69 to >89 years.9 The carcinogenic role of persistent hrHPV infection in women >65 years is an important area for future research.
Latent HPV virus infection
Following a primary varicella-zoster infection (chickenpox), the virus may remain in a latent state in sensory ganglia, reactivating later in life to cause shingles. Thirty percent of people who have a primary chickenpox infection eventually will develop a case of shingles. Immunocompromised populations are at an increased risk of developing shingles because of reduced T-cell mediated immunity.
A recent hypothesis is that in immunocompromised and older women, latent HPV can reactivate and cause clinically significant infection.10 Following renal transplantation investigators have reported a significant increase in the prevalence of genital HPV, without a change in sexual behavior.11 In cervical tissue from women with no evidence of active HPV infection, highly sensitive PCR-based assays detected HPV16 virus in a latent state in some women, possibly due to disruption of the viral E2 gene.12 If latent HPV infection is a valid biological concept, it suggests that there is no “safe age” at which to discontinue screening for HPV infection because the virus cannot be detected in screening samples while it is latent.
Options for cervical cancer screening in women >65 years
Three options might reduce the morbidity and mortality associated with cervical cancer in women >65 years.
Option 1: Double-down on trying to effectively implement current guidelines. The high rate of cervical cancer mortality in women >65 years of age indicates that the current guidelines, as implemented in real clinical practice, are not working. A problem with the current screening guidelines is that clinicians are expected to be capable of finding all relevant cervical cancer test results and properly interpreting the results. Clinicians are over-taxed and fallible, and the current approach is not likely to be successful unless additional information technology solutions are implemented.
Continue to: Health systems could use information...
Health systems could use information technology to mitigate these problems. For example, health systems could deploy software to assemble every cervical screening result on each woman and pre‑sent those results to clinicians in a single integrated view in the electronic record. Additionally, once all lifetime screening results are consolidated in one view, artificial intelligence systems could be used to analyze the totality of results and identify women who would benefit by continued screening past age 65 and women who could safely discontinue screening.
Option 2: Adopt the Australian approach to cervical cancer screening. The current Australian approach to cervical cancer screening is built on 3 pillars: 1) school-based vaccination of all children against hrHPV, 2) screening all women from 25 to 74 years of age every 5 years using nucleic acid testing for hrHPV, and 3) providing a system for the testing of samples self-collected by women who are reluctant to visit a clinician for screening.13 Australia has one of the lowest cervical cancer death rates in the world.
Option 3: Continue screening most women past age 65. Women >65 years of age are known to be infected with hrHPV genotypes. hrHPV infection causes cervical cancer. Cervical cancer causes many deaths in women aged >65 years. There is no strong rationale for ignoring these three facts. hrHPV screening every 5 years as long as the woman is healthy and has a reasonable life expectancy is an option that could be evaluated in randomized studies.
Given the high rate of cervical cancer death in women >65 years of age, I plan to be very cautious about discontinuing cervical cancer screening until I can personally ensure that my patient has no evidence of hrHPV infection.
In 2008, Harald zur Hausen, MD, received the Nobel Prize in Physiology or Medicine for discovering that human papilloma virus (HPV) caused cervical cancer. In a recent study, 74% of cervical cancers were associated with HPV 16 or 18 infections. A total of 89% of the cancers were associated with one of the high-risk HPV genotypes, including HPV 16/18/31/33/45/52/58.1
Recently, HPV has been shown to be a major cause of oropharyngeal cancer. The Centers for Disease Control and Prevention calculated that in CY2015 in the United States there were 18,917 cases of HPV-associated oropharyngeal squamous cell cancer and 11,788 cases of cervical cancer.2 Most cases of HPV-associated oropharyngeal cancer occur in men, and HPV vaccination of boys may help to prevent this cancer type. Oncogenic HPV produce two proteins (E6 and E7) that promote viral replication and squamous cell growth by inhibiting the function of p53 and retinoblastoma protein. The immortalized HeLa cell line, derived from Ms. Henrietta Lack's cervical cancer, contains integrated HPV18 nucleic acid sequences.3,4
The discovery that HPV causes cancer catalyzed the development of nucleic acid tests to identify high-risk oncogenic HPV and vaccines against high-risk oncogenic HPV genotypes that prevent cervical cancer. From a public health perspective, it is more effective to vaccinate the population against oncogenic HPV genotypes than to screen and treat cancer. In the United States, vaccination rates range from a high of 92% (District of Columbia) and 89% (Rhode Island) to a low of 47% (Wyoming) and 50% (Kentucky and Mississippi).5 To reduce HPV-associated cancer mortality, the gap in vaccination compliance must be closed.
References
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Van Dyne EA, Henley SJ, Saraiya M, et al. Trends in human papillomavirus-associated cancers - United States, 1999-2015. MMWR Morb Mortal Wkly Rep. 2018;67:918-924.
- Rosl F, Westphal EM, zur Hausen H. Chromatin structure and transcriptional regulation of human papillomavirus type 18 DNA in HeLa cells. Mol Carcinog. 1989;2:72-80.
- Adey A, Burton JN, Kitzman, et al. The haplotype-resolved genome and epigenome of the aneuploid HeLa cancer cell line. Nature. 2013;500:207-211.
- Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
- Hammer A, Kahlert J, Gravitt PE, et al. Hysterectomy-corrected cervical cancer mortality rates in Denmark during 2002-2015: a registry-based cohort study. Acta Obstet Gynecol Scand. 2019;98:1063-1069.
- Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123:1044-1050.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol. 2016;128:e111-30.
- Curry SJ, Krist AH, Owens DK, et al; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Stang A, Hawk H, Knowlton R, et al. Hysterectomy-corrected incidence rates of cervical and uterine cancers in Massachusetts, 1995-2010. Ann Epidemiol. 2014;24:849-854.
- Hallowell BD, Endeshaw M, McKenna MT, et al. Cervical cancer death rates among U.S.- and foreign-born women: U.S., 2005-2014. Am J Prev Med. 2019;56:869-874.
- Lindström AK, Hermansson RS, Gustavsson I, et al. Cervical dysplasia in elderly women performing repeated self-sampling for HPV testing. PLoS One. 2018;13:e0207714.
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Andersen B, Christensen BS, Christensen J, et al. HPV-prevalence in elderly women in Denmark. Gynecol Oncol. 2019;154:118-123.
- Gravitt PE, Winer RL. Natural history of HPV infection across the lifespan: role of viral latency. Viruses. 2017;9:E267.
- Hinten F, Hilbrands LB, Meeuwis KAP, et al. Reactivation of latent HPV infections after renal transplantation. Am J Transplant. 2017;17:1563-1573.
- Leonard SM, Pereira M, Roberts S, et al. Evidence of disrupted high-risk human papillomavirus DNA in morphologically normal cervices of older women. Sci Rep. 2016;6:20847.
- Cervical cancer screening. Cancer Council website. https://www.cancer.org.au/about-cancer/early-detection/screening-programs/cervical-cancer-screening.html. Updated March 15, 2019. Accessed July 23, 2019.
- Hammer A, Kahlert J, Gravitt PE, et al. Hysterectomy-corrected cervical cancer mortality rates in Denmark during 2002-2015: a registry-based cohort study. Acta Obstet Gynecol Scand. 2019;98:1063-1069.
- Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123:1044-1050.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol. 2016;128:e111-30.
- Curry SJ, Krist AH, Owens DK, et al; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Stang A, Hawk H, Knowlton R, et al. Hysterectomy-corrected incidence rates of cervical and uterine cancers in Massachusetts, 1995-2010. Ann Epidemiol. 2014;24:849-854.
- Hallowell BD, Endeshaw M, McKenna MT, et al. Cervical cancer death rates among U.S.- and foreign-born women: U.S., 2005-2014. Am J Prev Med. 2019;56:869-874.
- Lindström AK, Hermansson RS, Gustavsson I, et al. Cervical dysplasia in elderly women performing repeated self-sampling for HPV testing. PLoS One. 2018;13:e0207714.
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Andersen B, Christensen BS, Christensen J, et al. HPV-prevalence in elderly women in Denmark. Gynecol Oncol. 2019;154:118-123.
- Gravitt PE, Winer RL. Natural history of HPV infection across the lifespan: role of viral latency. Viruses. 2017;9:E267.
- Hinten F, Hilbrands LB, Meeuwis KAP, et al. Reactivation of latent HPV infections after renal transplantation. Am J Transplant. 2017;17:1563-1573.
- Leonard SM, Pereira M, Roberts S, et al. Evidence of disrupted high-risk human papillomavirus DNA in morphologically normal cervices of older women. Sci Rep. 2016;6:20847.
- Cervical cancer screening. Cancer Council website. https://www.cancer.org.au/about-cancer/early-detection/screening-programs/cervical-cancer-screening.html. Updated March 15, 2019. Accessed July 23, 2019.
August 2019 - Quick Quiz Question 2
Q2. Correct answer: E
Rationale:
Radiographic evaluation is commonly employed in the diagnosis and management of patients with lower GI bleeding. CT scans, tagged red blood cell scintigraphy, and angiography all have roles in the care of these patients. Though tagged red blood cell scintigraphy is the most sensitive modality at detecting active bleeding, requiring rates from 0.05-0.1 cc/min, it is relatively poor at localizing the bleeding, accurately predicting the location in only 60%-70% of cases. CT scans have the advantage of being quickly performed and are widely available. If extravasation is seen, its location is also accurately determined. It is not as sensitive as red blood cell scintigraphy, however, and requires bleeding rates of 0.3-0.5 cc/min to be positive. Angiography has the advantage of being both diagnostic and potentially therapeutic. It is best performed in sicker patients with hypotension and high transfusion demands as it is higher yield in these situations. Angiography is the least sensitive of these modalities, requiring bleeding rates between 0.5 and 1 cc/min to be positive.
Reference:
1. Strate LL, Naumann CR. The role of colonoscopy and radiological procedures in the management of acute lower intestinal bleeding. Clin Gastroenterol Hepatol. 2010 Apr;8(4):333-43.
Q2. Correct answer: E
Rationale:
Radiographic evaluation is commonly employed in the diagnosis and management of patients with lower GI bleeding. CT scans, tagged red blood cell scintigraphy, and angiography all have roles in the care of these patients. Though tagged red blood cell scintigraphy is the most sensitive modality at detecting active bleeding, requiring rates from 0.05-0.1 cc/min, it is relatively poor at localizing the bleeding, accurately predicting the location in only 60%-70% of cases. CT scans have the advantage of being quickly performed and are widely available. If extravasation is seen, its location is also accurately determined. It is not as sensitive as red blood cell scintigraphy, however, and requires bleeding rates of 0.3-0.5 cc/min to be positive. Angiography has the advantage of being both diagnostic and potentially therapeutic. It is best performed in sicker patients with hypotension and high transfusion demands as it is higher yield in these situations. Angiography is the least sensitive of these modalities, requiring bleeding rates between 0.5 and 1 cc/min to be positive.
Reference:
1. Strate LL, Naumann CR. The role of colonoscopy and radiological procedures in the management of acute lower intestinal bleeding. Clin Gastroenterol Hepatol. 2010 Apr;8(4):333-43.
Q2. Correct answer: E
Rationale:
Radiographic evaluation is commonly employed in the diagnosis and management of patients with lower GI bleeding. CT scans, tagged red blood cell scintigraphy, and angiography all have roles in the care of these patients. Though tagged red blood cell scintigraphy is the most sensitive modality at detecting active bleeding, requiring rates from 0.05-0.1 cc/min, it is relatively poor at localizing the bleeding, accurately predicting the location in only 60%-70% of cases. CT scans have the advantage of being quickly performed and are widely available. If extravasation is seen, its location is also accurately determined. It is not as sensitive as red blood cell scintigraphy, however, and requires bleeding rates of 0.3-0.5 cc/min to be positive. Angiography has the advantage of being both diagnostic and potentially therapeutic. It is best performed in sicker patients with hypotension and high transfusion demands as it is higher yield in these situations. Angiography is the least sensitive of these modalities, requiring bleeding rates between 0.5 and 1 cc/min to be positive.
Reference:
1. Strate LL, Naumann CR. The role of colonoscopy and radiological procedures in the management of acute lower intestinal bleeding. Clin Gastroenterol Hepatol. 2010 Apr;8(4):333-43.
Q2:
August 2019 - Question 1
Q1. Correct answer: C
Rationale:
The two standard treatment regimens for AIH include corticosteroids (prednisone or prednisolone) alone, or corticosteroids combined with azathioprine. The combination regimen allows for a lower dose of steroids and fewer side effects with the same therapeutic efficacy. This patient appears to have developed azathioprine-induced pancreatitis, which is a rare complication more often seen in patients with Crohn's disease treated with azathioprine. In patients who are intolerant of azathioprine, mycophenolate mofetil and calcineurin inhibitors have been used with success.
There are data supporting the use of budesonide in place of prednisone, but this regimen is not as effective in patients with cirrhosis or advanced fibrosis, so it is reserved for patients with lesser degrees of liver fibrosis. The TNF-alpha inhibitors are not used to treat AIH, nor is the IL-1 inhibitor anakinra.
References:
1. Czaja AJ. Diagnosis and management of autoimmune hepatitis: Current status and future directions. Gut Liver. 2016;10:177-203.
2. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Autoimmune Hepatitis. J Hepatol. 2015:63:971-1004.
3. Manns MP, et al. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51:1-31.
Q1. Correct answer: C
Rationale:
The two standard treatment regimens for AIH include corticosteroids (prednisone or prednisolone) alone, or corticosteroids combined with azathioprine. The combination regimen allows for a lower dose of steroids and fewer side effects with the same therapeutic efficacy. This patient appears to have developed azathioprine-induced pancreatitis, which is a rare complication more often seen in patients with Crohn's disease treated with azathioprine. In patients who are intolerant of azathioprine, mycophenolate mofetil and calcineurin inhibitors have been used with success.
There are data supporting the use of budesonide in place of prednisone, but this regimen is not as effective in patients with cirrhosis or advanced fibrosis, so it is reserved for patients with lesser degrees of liver fibrosis. The TNF-alpha inhibitors are not used to treat AIH, nor is the IL-1 inhibitor anakinra.
References:
1. Czaja AJ. Diagnosis and management of autoimmune hepatitis: Current status and future directions. Gut Liver. 2016;10:177-203.
2. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Autoimmune Hepatitis. J Hepatol. 2015:63:971-1004.
3. Manns MP, et al. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51:1-31.
Q1. Correct answer: C
Rationale:
The two standard treatment regimens for AIH include corticosteroids (prednisone or prednisolone) alone, or corticosteroids combined with azathioprine. The combination regimen allows for a lower dose of steroids and fewer side effects with the same therapeutic efficacy. This patient appears to have developed azathioprine-induced pancreatitis, which is a rare complication more often seen in patients with Crohn's disease treated with azathioprine. In patients who are intolerant of azathioprine, mycophenolate mofetil and calcineurin inhibitors have been used with success.
There are data supporting the use of budesonide in place of prednisone, but this regimen is not as effective in patients with cirrhosis or advanced fibrosis, so it is reserved for patients with lesser degrees of liver fibrosis. The TNF-alpha inhibitors are not used to treat AIH, nor is the IL-1 inhibitor anakinra.
References:
1. Czaja AJ. Diagnosis and management of autoimmune hepatitis: Current status and future directions. Gut Liver. 2016;10:177-203.
2. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Autoimmune Hepatitis. J Hepatol. 2015:63:971-1004.
3. Manns MP, et al. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51:1-31.
A 21-year-old woman is diagnosed with autoimmune hepatitis and is started on prednisone and azathioprine. Within a week, she develops mid-abdominal pain, radiating to the back, and her lipase level is 537 U/L.
How do new BP guidelines affect identifying risk for hypertensive disorders of pregnancy?
Hauspurg A, Parry S, Mercer BM, et al. Blood pressure trajectory and category and risk of hypertensive disorders of pregnancy in nulliparous women. Am J Obstet Gynecol. 2019. pii: S0002-9378(19)30807-5. doi: 10.1016/j.ajog.2019.06.031.
EXPERT COMMENTARY
Hauspurg and colleagues set out to determine whether redefined BP category (normal, < 120/80 mm Hg) and trajectory (a difference of ≥ 5 mm Hg systolic, diastolic, or mean arterial pressure between the first and second prenatal visit) helps to identify women at increased risk for developing hypertensive disorders of pregnancy or preeclampsia.
With respect to the former variable, such an association was demonstrated in the first National Institutes of Health–funded preeclampsia prevention trial published in 1993, which used low-dose aspirin.1 In that trial, low-dose aspirin was not found to be effective in preventing preeclampsia in young, healthy nulliparous women. Interestingly, the 2 factors most associated with developing preeclampsia were an initial systolic BP of 120 to 134 mm Hg and an initial weight of >60 kg. For most clinicians, these findings would not be helpful in trying to better identify a high-risk group.
Details of the study
The idea of BP “trajectory” is interesting in the Hauspurg and colleagues’ study. The authors analyzed data from the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be (nuMoM2b), a prospective cohort study, and included a very large population of almost 9,000 women in the analysis. Participants were classified according to their BP measurement at the first study visit, with BP categories based on updated American College of Cardiology/American Heart Association guidelines. The primary outcome was the risk of hypertensive disorders of pregnancy, including gestational hypertension and preeclampsia.
The data analysis found that elevated BP was associated with an adjusted risk ratio (aRR) of 1.54 (95% confidence interval [CI], 1.18–2.02). Stage 1 hypertension was associated with an aRR of 2.16 (95% CI, 1.31–3.57). Compared with women whose BP had a downward systolic trajectory, women with normal BP and an upward systolic trajectory had a 41% increased risk of any hypertensive disorder of pregnancy (aRR, 1.41; 95% CI, 1.20–1.65).
Study strengths and limitations
While the large study population is a strength of this study, there are a number of limitations, such as the use of BP measurements during pregnancy only, without having pre-pregnancy measurements available. Further, a single BP measurement during each visit is also a drawback, although the standardized measurement by study staff is a strength.
Anticlimactic conclusions. The conclusions of the study, however, are either not surprising, not clinically meaningful, or of little value to clinicians at present, at least with respect to patient management.
Continue to: Conclusions that were not surprising included...
Conclusions that were not surprising included a statistically lower chance of indicated preterm delivery in the normal BP group than in the elevated BP or stage 1 hypertension groups. Conclusions that were not meaningful included a statistically significant lower birthweight in the elevated BP group (3,269 g) and in the stage 1 hypertension group (3,258 g) compared with the normal BP group (3,279 g), but the clinical significance of these differences is arguable.
Lastly is the issue of what these data mean for clinical practice. The idea of identifying high-risk groups is attractive, provided that there are effective intervention strategies available. If one follows the United States Preventive Services Task Force (USPSTF) recommendations for preeclampsia prevention,2 then virtually every nulliparous woman is a candidate for low-dose aspirin for preeclampsia prophylaxis. Beyond that, the current data do not support any change in the standard clinical practice of managing these “now identified” high-risk women. Increasing prenatal visits, using biomarkers to further delineate risk, and using uterine artery Doppler studies are all strategies that have been or are being investigated, but as yet they are not supported by conclusive data documenting improved outcomes—a sentiment supported by both the USPSTF3 and the authors of the study.
Until further data are available, my advice to clinicians is to pay close attention to all risk factors for any of the hypertensive disorders of pregnancy. Initial BP and BP trajectory are important but probably something that sound clinical judgment would identify anyway. My recommendation is to continue to use those methods of prophylaxis, fetal surveillance, and indications for delivery that are supported by current data and await the additional investigations that Hauspurg and colleagues suggest need to be done before altering your management of women at increased risk for any of the hypertensive disorders of pregnancy.
JOHN T. REPKE, MD
- Sibai BM, Caritis SN, Thom E, et al; National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine. Prevention of preeclampsia with low-dose aspirin in healthy nulliparous pregnant women. N Engl J Med. 1993;329:1213-1218.
- United States Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: preventive medication. September 2014. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-from-preeclampsia-preventive-medication. Accessed July 30, 2019.
- United States Preventive Service Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for preeclampsia: US Preventive Services Task Force recommendation statement. JAMA. 2017;387:1661-1667.
Hauspurg A, Parry S, Mercer BM, et al. Blood pressure trajectory and category and risk of hypertensive disorders of pregnancy in nulliparous women. Am J Obstet Gynecol. 2019. pii: S0002-9378(19)30807-5. doi: 10.1016/j.ajog.2019.06.031.
EXPERT COMMENTARY
Hauspurg and colleagues set out to determine whether redefined BP category (normal, < 120/80 mm Hg) and trajectory (a difference of ≥ 5 mm Hg systolic, diastolic, or mean arterial pressure between the first and second prenatal visit) helps to identify women at increased risk for developing hypertensive disorders of pregnancy or preeclampsia.
With respect to the former variable, such an association was demonstrated in the first National Institutes of Health–funded preeclampsia prevention trial published in 1993, which used low-dose aspirin.1 In that trial, low-dose aspirin was not found to be effective in preventing preeclampsia in young, healthy nulliparous women. Interestingly, the 2 factors most associated with developing preeclampsia were an initial systolic BP of 120 to 134 mm Hg and an initial weight of >60 kg. For most clinicians, these findings would not be helpful in trying to better identify a high-risk group.
Details of the study
The idea of BP “trajectory” is interesting in the Hauspurg and colleagues’ study. The authors analyzed data from the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be (nuMoM2b), a prospective cohort study, and included a very large population of almost 9,000 women in the analysis. Participants were classified according to their BP measurement at the first study visit, with BP categories based on updated American College of Cardiology/American Heart Association guidelines. The primary outcome was the risk of hypertensive disorders of pregnancy, including gestational hypertension and preeclampsia.
The data analysis found that elevated BP was associated with an adjusted risk ratio (aRR) of 1.54 (95% confidence interval [CI], 1.18–2.02). Stage 1 hypertension was associated with an aRR of 2.16 (95% CI, 1.31–3.57). Compared with women whose BP had a downward systolic trajectory, women with normal BP and an upward systolic trajectory had a 41% increased risk of any hypertensive disorder of pregnancy (aRR, 1.41; 95% CI, 1.20–1.65).
Study strengths and limitations
While the large study population is a strength of this study, there are a number of limitations, such as the use of BP measurements during pregnancy only, without having pre-pregnancy measurements available. Further, a single BP measurement during each visit is also a drawback, although the standardized measurement by study staff is a strength.
Anticlimactic conclusions. The conclusions of the study, however, are either not surprising, not clinically meaningful, or of little value to clinicians at present, at least with respect to patient management.
Continue to: Conclusions that were not surprising included...
Conclusions that were not surprising included a statistically lower chance of indicated preterm delivery in the normal BP group than in the elevated BP or stage 1 hypertension groups. Conclusions that were not meaningful included a statistically significant lower birthweight in the elevated BP group (3,269 g) and in the stage 1 hypertension group (3,258 g) compared with the normal BP group (3,279 g), but the clinical significance of these differences is arguable.
Lastly is the issue of what these data mean for clinical practice. The idea of identifying high-risk groups is attractive, provided that there are effective intervention strategies available. If one follows the United States Preventive Services Task Force (USPSTF) recommendations for preeclampsia prevention,2 then virtually every nulliparous woman is a candidate for low-dose aspirin for preeclampsia prophylaxis. Beyond that, the current data do not support any change in the standard clinical practice of managing these “now identified” high-risk women. Increasing prenatal visits, using biomarkers to further delineate risk, and using uterine artery Doppler studies are all strategies that have been or are being investigated, but as yet they are not supported by conclusive data documenting improved outcomes—a sentiment supported by both the USPSTF3 and the authors of the study.
Until further data are available, my advice to clinicians is to pay close attention to all risk factors for any of the hypertensive disorders of pregnancy. Initial BP and BP trajectory are important but probably something that sound clinical judgment would identify anyway. My recommendation is to continue to use those methods of prophylaxis, fetal surveillance, and indications for delivery that are supported by current data and await the additional investigations that Hauspurg and colleagues suggest need to be done before altering your management of women at increased risk for any of the hypertensive disorders of pregnancy.
JOHN T. REPKE, MD
Hauspurg A, Parry S, Mercer BM, et al. Blood pressure trajectory and category and risk of hypertensive disorders of pregnancy in nulliparous women. Am J Obstet Gynecol. 2019. pii: S0002-9378(19)30807-5. doi: 10.1016/j.ajog.2019.06.031.
EXPERT COMMENTARY
Hauspurg and colleagues set out to determine whether redefined BP category (normal, < 120/80 mm Hg) and trajectory (a difference of ≥ 5 mm Hg systolic, diastolic, or mean arterial pressure between the first and second prenatal visit) helps to identify women at increased risk for developing hypertensive disorders of pregnancy or preeclampsia.
With respect to the former variable, such an association was demonstrated in the first National Institutes of Health–funded preeclampsia prevention trial published in 1993, which used low-dose aspirin.1 In that trial, low-dose aspirin was not found to be effective in preventing preeclampsia in young, healthy nulliparous women. Interestingly, the 2 factors most associated with developing preeclampsia were an initial systolic BP of 120 to 134 mm Hg and an initial weight of >60 kg. For most clinicians, these findings would not be helpful in trying to better identify a high-risk group.
Details of the study
The idea of BP “trajectory” is interesting in the Hauspurg and colleagues’ study. The authors analyzed data from the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be (nuMoM2b), a prospective cohort study, and included a very large population of almost 9,000 women in the analysis. Participants were classified according to their BP measurement at the first study visit, with BP categories based on updated American College of Cardiology/American Heart Association guidelines. The primary outcome was the risk of hypertensive disorders of pregnancy, including gestational hypertension and preeclampsia.
The data analysis found that elevated BP was associated with an adjusted risk ratio (aRR) of 1.54 (95% confidence interval [CI], 1.18–2.02). Stage 1 hypertension was associated with an aRR of 2.16 (95% CI, 1.31–3.57). Compared with women whose BP had a downward systolic trajectory, women with normal BP and an upward systolic trajectory had a 41% increased risk of any hypertensive disorder of pregnancy (aRR, 1.41; 95% CI, 1.20–1.65).
Study strengths and limitations
While the large study population is a strength of this study, there are a number of limitations, such as the use of BP measurements during pregnancy only, without having pre-pregnancy measurements available. Further, a single BP measurement during each visit is also a drawback, although the standardized measurement by study staff is a strength.
Anticlimactic conclusions. The conclusions of the study, however, are either not surprising, not clinically meaningful, or of little value to clinicians at present, at least with respect to patient management.
Continue to: Conclusions that were not surprising included...
Conclusions that were not surprising included a statistically lower chance of indicated preterm delivery in the normal BP group than in the elevated BP or stage 1 hypertension groups. Conclusions that were not meaningful included a statistically significant lower birthweight in the elevated BP group (3,269 g) and in the stage 1 hypertension group (3,258 g) compared with the normal BP group (3,279 g), but the clinical significance of these differences is arguable.
Lastly is the issue of what these data mean for clinical practice. The idea of identifying high-risk groups is attractive, provided that there are effective intervention strategies available. If one follows the United States Preventive Services Task Force (USPSTF) recommendations for preeclampsia prevention,2 then virtually every nulliparous woman is a candidate for low-dose aspirin for preeclampsia prophylaxis. Beyond that, the current data do not support any change in the standard clinical practice of managing these “now identified” high-risk women. Increasing prenatal visits, using biomarkers to further delineate risk, and using uterine artery Doppler studies are all strategies that have been or are being investigated, but as yet they are not supported by conclusive data documenting improved outcomes—a sentiment supported by both the USPSTF3 and the authors of the study.
Until further data are available, my advice to clinicians is to pay close attention to all risk factors for any of the hypertensive disorders of pregnancy. Initial BP and BP trajectory are important but probably something that sound clinical judgment would identify anyway. My recommendation is to continue to use those methods of prophylaxis, fetal surveillance, and indications for delivery that are supported by current data and await the additional investigations that Hauspurg and colleagues suggest need to be done before altering your management of women at increased risk for any of the hypertensive disorders of pregnancy.
JOHN T. REPKE, MD
- Sibai BM, Caritis SN, Thom E, et al; National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine. Prevention of preeclampsia with low-dose aspirin in healthy nulliparous pregnant women. N Engl J Med. 1993;329:1213-1218.
- United States Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: preventive medication. September 2014. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-from-preeclampsia-preventive-medication. Accessed July 30, 2019.
- United States Preventive Service Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for preeclampsia: US Preventive Services Task Force recommendation statement. JAMA. 2017;387:1661-1667.
- Sibai BM, Caritis SN, Thom E, et al; National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine. Prevention of preeclampsia with low-dose aspirin in healthy nulliparous pregnant women. N Engl J Med. 1993;329:1213-1218.
- United States Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: preventive medication. September 2014. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-from-preeclampsia-preventive-medication. Accessed July 30, 2019.
- United States Preventive Service Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for preeclampsia: US Preventive Services Task Force recommendation statement. JAMA. 2017;387:1661-1667.
Gastrointestinal Stromal Tumors: Management of Localized Disease
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumor of the gastrointestinal (GI) tract and arise from the interstitial cells of Cajal of the myenteric plexus. These tumors are rare, with about 1 case per 100,000 persons diagnosed in the United States annually, but may be incidentally discovered in up to 1 in 5 autopsy specimens of older adults.1,2 Epidemiologic risk factors include increasing age, with a peak incidence between age 60 and 65 years, male gender, black race, and non-Hispanic white ethnicity. Germline predisposition can also increase the risk of developing GISTs; molecular drivers of GIST include gain-of-function mutations in the KIT proto-oncogene and platelet-derived growth factor receptor α (PDGFRA) gene, which both encode structurally similar tyrosine kinase receptors; germline mutations of succinate dehydrogenase (SDH) subunit genes; and mutations associated with neurofibromatosis type 1.
GISTs most commonly involve the stomach, followed by the small intestine, but can arise anywhere within the GI tract (esophagus, colon, rectum, and anus). They can also develop outside the GI tract, arising from the mesentery, omentum, and retroperitoneum. The majority of cases are localized or locoregional, whereas about 20% are metastatic at presentation.1 GISTs can occur in children, adolescents, and young adults. Pediatric GISTs represent a distinct subset marked by female predominance and gastric origin, are often multifocal, can sometimes have lymph node involvement, and typically lack mutations in the KIT and PDGFRA genes.
This review is the first of 2 articles focusing on the diagnosis and management of GISTs. Here, we review the evaluation and diagnosis of GISTs along with management of localized disease. Management of advanced disease is reviewed in a separate article.
Case Presentation
A 64-year-old African American man with progressive iron deficiency and abdominal discomfort undergoes upper and lower endoscopy and is found to have a bulging mass within his abdominal cavity. He undergoes a computed tomography (CT) evaluation of the chest, abdomen, and pelvis with contrast, which reveals the presence of a 10-cm gastric mass, with no other lesions identified. He undergoes surgical resection of the mass and presents for review of his pathology and to discuss his treatment plan.
What histopathologic features are consistent with GIST?
What factors are used for risk stratification and to predict likelihood of recurrence?
Clinical Presentation and Diagnosis
Most patients present with symptoms of overt or occult GI bleeding or abdominal discomfort, but a significant proportion of GISTs are discovered incidentally. Lymph node involvement is not typical, except for GISTs occurring in children and/or with rare syndromes. Most syndromic GISTs are multifocal and multicentric. After surgical resection, GISTs usually recur or metastasize within the abdominal cavity, including the omentum, peritoneum, or liver. These tumors rarely spread to the lungs, brain, or bones; when tumor spread does occur, it tends to be in heavily pre-treated patients with advanced disease who have been on multiple lines of therapy for a long duration of time.
The diagnosis usually can be made by histopathology. Specimens can be obtained by endoscopic ultrasound (EUS)– or CT-guided methods, the latter of which carries a very small risk of contamination from percutaneous biopsy. In terms of morphology, GISTs can be spindle cell, epithelioid, or mixed neoplasms. Epithelioid tumors are more commonly seen in the stomach and are often PDGFRA-mutated or SDH-deficient. The differential diagnosis includes other soft-tissue GI wall tumors such as leiomyosarcomas/leiomyomas, germ cell tumors, lymphomas, fibromatosis, and neuroendocrine and neurogenic tumors. A unique feature of GISTs that differentiates them from leiomyomas is near universal expression of CD117 by immunohistochemistry (IHC); this characteristic has allowed pathologists and providers to accurately distinguish true GISTs from other GI mesenchymal tumors.3 Recently, DOG1 (discovered on GIST1) immunoreactivity has been found to be helpful in identifying patients with CD117-negative GISTs. Initially identified through gene expression analysis of GISTs, DOG1 IHC can identify the common mutant c-Kit-driven CD117-positive GISTs as well as the rare CD117-negative GISTs, which are often driven by mutated PDGFRA.4 Importantly, IHC for KIT and DOG1 are not surrogates for mutational status, nor are they predictive of tyrosine kinase inhibitor (TKI) sensitivity. If IHC of a tumor specimen is CD117- and DOG1-negative, the specimen can be sent for KIT and PDGFRA mutational analysis to confirm the diagnosis. If analysis reveals that these genes are wild-type, then IHC staining for SDH B (SDHB) should follow to assess for an SDH-deficient GIST (negative staining).
Risk Stratification for Recurrence
The clinical behavior of GISTs can be variable. Some are indolent, while others behave more aggressively, with a greater malignant potential and a higher propensity to recur and metastasize. Clinical and pathologic features can provide important prognostic information that allows providers to risk-stratify patients. Various institutions have assessed prognostic variables for GISTs. In 2001, the National Institutes of Health (NIH) held a GIST workshop that proposed an approach to estimating metastatic risk based on tumor size and mitotic index (NIH or Fletcher criteria).5 Joensuu et al later proposed a modification of the NIH risk classification to include tumor location and tumor rupture (modified NIH criteria or Joensuu criteria).6-8 Similarly, the Armed Forces Institute of Pathology (AFIP) identified tumor site as a prognostic factor, with gastric GISTs having the best prognosis (AFIP-Miettinen criteria).9-11 Tabular schemes were designed which stratified patients into discrete groups with ranges for mitotic rate and tumor size. Nomograms for ease of use were then constructed utilizing a bimodal mitotic rate and included tumor site and size.12 Finally, contour maps were developed, which have the advantage of evaluating mitotic rate and tumor size as continuous nonlinear variables and also include tumor site and rupture (associated with a high risk of peritoneal metastasis) separately, further improving risk assessment. These contour maps have been validated against pooled data from 10 series (2560 patients).13 High-risk features identified from these studies include tumor location, size, mitotic rate and tumor rupture and are now used for deciding on the use of adjuvant imatinib and as requirements to enter clinical trials assessing adjuvant therapy for resected GISTs.
Case Continued
The patient’s operative and pathology reports indicate that the tumor is a spindle cell neoplasm of the stomach that is positive for CD117, DOG1, and CD34 and negative for smooth muscle actin and S-100, consistent with a diagnosis of GIST. Resection margins are negative. There are 10 mitoses per 50 high-power fields (HPF). Per the operative report, there was no intraoperative or intraperitoneal tumor rupture. Thus, while his GIST was gastric, which generally has a more favorable prognosis, the tumor harbors high-risk features based on its size and mitotic index.
What further testing should be requested?
Molecular Alterations
It is recommended that a mutational analysis be performed as part of the diagnostic work-up of all GISTs.14 Mutational analysis can provide prognostic and predictive information for sensitivity to imatinib and should be considered standard of care. It may also be useful for confirming a GIST diagnosis, or, if negative, lead to further evaluation with an IHC stain for SDHB. The c-Kit receptor is a member of the tyrosine kinase family and, through direct interactions with stem cell factor (SCF), can upregulate the PI3K/AKT/mTOR, Ras/Raf/MEK/ERK, and JAK-STAT pathways, resulting in transcription and translation of genes that enhance cell growth and survival.15 The cell of origin of GISTs, the interstitial cells of Cajal, are dependent on the SCF–c-Kit interaction for development.16 Likewise, the large majority of GISTs (about 70%) are driven by upregulation and constitutive activation of c-Kit, which is normally autoinhibited. About 80% of KIT mutations involve exon 11; these GISTs are most often associated with a gastric location and are associated with a favorable recurrence-free survival (RFS) rate with surgery alone.17 KIT exon 9 mutations are much less common, encompassing only about 10% of GIST KIT mutations, and GISTs with these mutations are more likely to arise from the small bowel.17
About 8% of GISTs harbor gain-of-function PDGFRA driver mutations rendering constitutively active PDGFRA.18 PDGFRA mutations are mutually exclusive from KIT mutations, and PDGFRA-mutated tumors most often occur in the stomach. PDGFRA mutations generally are associated with a lower mitotic rate and gastric location. Identification of the PDGFRA D842V mutation on exon 18, which is the most common, is important, as it is associated with imatinib resistance, and these patients should not be offered imatinib.19
Several other mutations associated with GISTs outside of the KIT and PDGFRA spectrum have been identified. About 10% of GISTs are wildtype for KIT and PDGFRA, and not all KIT/PDGFRA-wildtype GISTs are imatinib-sensitive and/or respond to other TKIs.18 These tumors may harbor aberrations in SDH and NF1, or less commonly, BRAF V600E, FGFR, and NTRK.20,21 SDH subunits B, C and D play a role in the Krebs cycle and electron transport chain. Germline mutations in these SDH subunits can result in the Carney-Stratakis syndrome characterized by the dyad of multifocal GISTs and multicentric paragangliomas.22 This syndrome is most likely to manifest in the pediatric or young adult population. In contradistinction is the Carney triad, which is associated with acquired loss of function of the SDHC gene due to promoter hypermethylation. This syndrome classically occurs in young women and is characterized by an indolent-behaving triad of multicentric GISTs, non-adrenal paragangliomas, and pulmonary chondromas.23 Like PDGFRA D842V–mutated GISTs, SDH-deficient and NF1-associated GISTs are considered imatinib resistant, and these patients should not be offered imatinib therapy.14
Case Continued
The patient’s GIST is found to harbor a KIT exon 11 single codon deletion. He appears anxious and asks to have everything done to prevent his GIST from coming back and to improve his lifespan.
What are the next steps in the management of this patient?
Management
A multidisciplinary team approach to the management of all GISTs is essential and includes input from radiology, gastroenterology, pathology, medical and surgical oncology, nuclear medicine, and nursing.
Surgical Resection
Small esophagogastric and duodenal GISTs ≤ 2 cm can be asymptomatic and managed with serial endoscopic surveillance, typically every 6 to 12 months, with biopsies if the tumors increase in size. GISTs larger than 2 cm require surgical resection, with resection of the full pseudocapsule and an R0 resection, if possible, since larger GISTs carry a higher risk of growth and recurrence. If an R0 resection would lead to significant morbidity or functional sequelae, an R1 may suffice. Rectal GISTs are an exception, where microscopic margins have been shown to be associated with an increased risk of local failure.24 It is important to explore the abdomen thoroughly for peritoneal, rectovaginal, and vesicular implants and metastasis to the liver. A formal lymph node dissection is not necessary because lymph nodes are rarely involved and should only be removed when clinically suspicious. Tumor rupture must be avoided. A laparoscopic approach should only be considered for smaller tumors, since there is a risk of tumor rupture with larger tumors.14
When is adjuvant imatinib indicated?
Adjuvant Imatinib
Among patients with local or locally advanced GISTs, the risk of death from recurrence with surgery alone can be high, with a historical 5-year overall survival (OS) of about 35%.25 As a result, multiple studies have assessed the benefit of adjuvant imatinib, which is now considered standard of care for patients with imatinib-sensitive, high-risk GISTs. In addition to inhibiting BCR-ABL, imatinib mesylate inhibits multiple other receptor tyrosine kinases, including PDGFR, SCF and c-Kit. As a result, imatinib has demonstrated in vitro inhibition of cell proliferation and apoptosis and clinical activity against GISTs expressing CD117.26 Importantly, adjuvant imatinib should only be offered to patients with imatinib-sensitive mutations, such as KIT exon 11 and KIT exon 9 mutations. Adjuvant imatinib should not be offered to patients with imatinib-insensitive mutations such as PDGFR 842V, NF1, or BRAF-related or SDH-deficient GISTs.
The ACOSOG Z9000 was the first study of adjuvant imatinib in patients with resected GISTs.25 This was a single-arm, phase 2 study involving 106 patients with surgically resected GISTs deemed high-risk for recurrence, defined as size > 10 cm, tumor rupture, or up to 4 peritoneal implants. Patients were treated with imatinib 400 mg daily for 1 year. The primary and secondary endpoints were OS and RFS, respectively. Long-term follow-up of this study demonstrated 1-, 3-, and 5-year OS of 99%, 97%, and 83%, and 1-, 3-, and 5-year RFS of 96%, 60%, and 40%, which compared favorably with historical controls. In a multivariable analysis, increasing tumor size, small bowel location, KIT exon 9 mutation, high mitotic rate, and older age were independent risk factors for a poor RFS.25 It is important to note that the benefit of adjuvant imatinib waned after discontinuation of therapy, creating a rationale to study adjuvant imatinib for longer periods of time.
As a result of the promising phase 2 data, ACOSOG opened a phase 3 randomized trial (Z9001) comparing 1 year of adjuvant imatinib to placebo among patients with surgically resected GISTs that were > 3 cm in size and that stained positive for CD117 on pathology. The trial accrued 713 patients and was stopped early at a planned interim analysis, which revealed a 1-year RFS of 98% for imatinib versus 83% for placebo (hazard ratio [HR], 0.35; P < 0.001). The 1-year OS did not differ between the 2 arms (92.2% vs 99.7%; HR, 0.66; P = 0.47).27 When comparing the 2 arms, imatinib was associated with a higher RFS among patients with a KIT exon 11 deletion, but not among patients with other KIT mutation types, PDGFRA mutations, or who were KIT/PDGFRA wildtype.28 Imatinib was granted approval by the US Food and Drug Administration (FDA) for the adjuvant treatment of high-risk GISTs based on the results of the ACOSOG Z9001 trial.
The EORTC 62024 study was a randomized placebo-controlled trial assessing the benefit of 2 years of adjuvant imatinib.29 Patients had to be considered intermediate or high risk per the 2002 NIH consensus classification to be eligible. The trial enrolled 918 patients. The 5-year OS rate, the original primary endpoint, did not differ between the 2 groups (100% vs 99%). The 3-year and 5-year RFS rates, secondary endpoints, were significantly longer among patients treated with imatinib (84% vs 66% and 69% vs 63%, respectively). Again, it was noted that the benefit of imatinib waned over time after treatment discontinuation.
The Scandinavian Sarcoma Group (SSG XVIII) trial was a prospective randomized phase 3 trial that compared 3 years versus 1 year of adjuvant imatinib.30 Patients had to be enrolled within 12 weeks of the postoperative period and had to have GISTs that were CD117-positive and with a high estimated risk of recurrence, per the modified NIH consensus criteria (size > 10 cm, > 10 mitoses per 50 HPF, diameter > 5 cm with mitotic count > 5, or tumor rupture before or at surgery). Three years of adjuvant imatinib was associated with a 54% reduction in the hazard for recurrence at 5 years (65.6% vs 47.9%; HR, 0.46; P < 0.001) and a 55% reduction in the hazard for death at 5 years (OS 92% vs 81.7%; HR, 0.45; P = 0.02). Based on the results of this study, the FDA granted approval for the use of 3 years of adjuvant imatinib in patients with high-risk resected GISTs.
The observation that a longer duration of adjuvant imatinib was associated with superior RFS and OS led to studies to further explore longer durations of adjuvant imatinib. The PERSIST-5 (Postresection Evaluation of Recurrence-free Survival for Gastrointestinal Stromal Tumors With 5 Years of Adjuvant Imatinib) was a multicenter, single-arm, phase 2 prospective study of adjuvant imatinib with a primary endpoint of RFS after 5 years.31 Patients had to have an intermediate or high risk of recurrence, which included GISTs at any site > 2 cm with > 5 mitoses per 50 HPF or nongastric GISTs that were ≥ 5 cm. With 91 patients enrolled, the estimated 5-year RFS was 90% and the OS was 95%. Of note, about half of the patients stopped treatment early due to a variety of reasons, including patient choice or adverse events. Importantly, there were no recurrences in patients with imatinib-sensitive mutations while on therapy. We know that in patients at high risk of relapse, adjuvant imatinib delays recurrence and improves survival, but whether any patients are cured, or their survival curves are just shifted to the right, is unknown. Only longer follow-up of existing studies, and the results of newer trials utilizing longer durations of adjuvant treatment, will help to determine the real value of adjuvant therapy for GIST patients.32 Based on this study, it would be reasonable to discuss a longer duration of imatinib with patients deemed to be at very high risk of recurrence and who are tolerating therapy well. We are awaiting the data from the randomized phase 3 Scandinavian Sarcoma Group XII trial comparing 5 years versus 3 years of adjuvant imatinib therapy, and from the French ImadGIST trial of adjuvant imatinib for 3 versus 6 years. A summary of the aforementioned key adjuvant trials is shown in the Table.

When imatinib is commenced, careful monitoring for treatment toxicities and drug interactions should ensue in order to improve compliance. Dose density should be maintained if possible, as retrospective studies suggest suboptimal plasma levels are associated with a worse outcome.33
When should neoadjuvant imatinib be considered?
Neoadjuvant Imatinib
Neoadjuvant imatinib should be considered for patients requiring total gastrectomy, esophagectomy, or abdominoperineal resection of the rectum in order to reduce tumor size, limit subsequent surgical morbidity, mitigate tumor bleeding and rupture, and aid with organ preservation. Patients with rectal GISTs that may otherwise warrant an abdominoperineal resection should be offered a trial of imatinib in the neoadjuvant setting. There is no evidence for the use of any other TKI aside from imatinib in the neoadjuvant or adjuvant setting. With neoadjuvant imatinib, it is difficult to accurately assess the mitotic rate in the resected tumor specimen.
The RTOG 0132/ACRIN 6665 trial was a prospective phase 2 study evaluating the efficacy of imatinib 600 mg daily in the perioperative setting.34 The trial enrolled 50 patients, 30 with primary GISTs (group A) and 22 with recurrent metastatic GISTs (group B). Based on data from the metastatic setting revealing a time to treatment response of about 2.5 months, patients were treated with 8 to 12 weeks of preoperative imatinib followed by 2 years of adjuvant imatinib. Imatinib was stopped 24 hours preoperatively and resumed as soon as possible postoperatively. In group A, 7% of patients achieved a partial response (PR), 83% achieved stable disease, and 2-year progression-free survival (PFS) and OS were 83% and 93%, respectively. In group B, 4.5% of patients achieved a PR, 91% achieved stable disease, and 4.5% experienced progressive disease in the preoperative period; the 2-year PFS and OS were 77% and 91%, respectively. The results of this trial demonstrated the feasibility of using perioperative imatinib with minimal effects on surgical outcomes and set the rationale to use neoadjuvant imatinib in select patients with borderline resectable or rectal GISTs. Another EORTC pooled analysis from 10 sarcoma centers revealed that after a median of 10 months of neoadjuvant imatinib, 83.2% of patients achieved an R0 resection and only 1% progressed during treatment.35 After a median follow-up of 46 months, the 5-year disease-free survival and OS were 65% and 87%, respectively.
Mutational testing should be performed beforehand to ensure the tumor is imatinib-sensitive. If a KIT exon 9 mutation is identified, then 400 mg twice daily should be considered (given the benefit seen with 800 mg imatinib for advanced GIST patients), although there are no studies to confirm this practice. Neoadjuvant imatinib is recommended for a total of 6 to 12 months to ensure maximal tumor debulking, but with very close monitoring and surgical input for disease resistance and growth.14 Imatinib should be stopped 1 to 2 days preoperatively and resumed once the patient has recovered from surgery for a total of 3 years (pre-/postoperatively combined). Neoadjuvant therapy has been shown to be safe and effective, but there have been no randomized trials to assess survival.
What is appropriate surveillance for resected GISTs?
Surveillance
There have been no randomized studies to guide the management of surveillance after surgical resection and adjuvant therapy. There is no known optimal follow-up schedule, but several have been proposed.13,36 Among high-risk patients, it is suggested to image every 3 to 6 months during adjuvant therapy, followed by every 3 months for 2 years after discontinuing therapy, then every 6 months for another 3 years and annually thereafter for an additional 5 years. High-risk patients usually relapse within 1 to 3 years after finishing adjuvant therapy, while low-risk patients can relapse later given that their disease can be slower growing. It has been recommended that low-risk patients undergo imaging every 6 months for 5 years, with follow-up individualized thereafter. Very-low-risk patients may not require more than annual imaging. Because most relapses occur within the peritoneum or liver, imaging should encompass the abdomen and pelvis. Surveillance imaging usually consists of CT scans of the abdomen and pelvis. MRI scans can be utilized for patients at lower risk or who are out several years in order to avoid excess radiation exposure. MRI is also specifically helpful for rectal and esophageal lesions. Chest CT or chest radiograph and bone scan are not routinely required for follow-up.
Case Conclusion
The patient receives adjuvant imatinib and experiences grade 2 myalgias, periorbital edema, and macrocytic anemia, which result in imatinib discontinuation after 3 years of treatment. He is seen every 3 to 6 months and a contrast CT abdomen and pelvis is obtained every 6 months for 5 years. During this 5-year follow-up period, he does not have any clinical or radiographic evidence of disease recurrence.
Further follow-up of this patient is presented in the second article in this 2-part review of management of GISTs.
Key Points
- GISTs are the most common mesenchymal neoplasms of the GI tract and can occasionally occur in extragastrointestinal locations as well.
- GISTs encompass a heterogeneous family of tumor subsets with different natural histories, mutations, and TKI responsiveness.
- Surgery is the mainstay of treatment for localized GISTs, with cure rates greater than 50%.
- For very small (< 2 cm) esophagogastric GISTs, endoscopic ultrasound evaluation and follow-up is recommended.
- For tumors ≥ 2 cm, biopsy and excision is the standard approach.
- For localized GISTs, complete surgical resection (R0) is standard treatment, with no lymphadenectomy for clinically negative lymph nodes.
- Mutational analysis should be considered standard of practice. It can be helpful for confirming the diagnosis and can be predictive and prognostic in determining specific TKI therapy and dose.
- Adjuvant imatinib at a dose of 400 mg for 3 years is standard of care for GISTs that are at high risk of relapse and are imatinib-sensitive, and it is the only TKI approved for adjuvant therapy. Patients with PDGFRA D842V, NF1, BRAF or SDH-deficient GISTs should not receive adjuvant imatinib therapy.
- Neoadjuvant therapy can be utilized for sites where extensive resection would lead to significant morbidity. It should be given for 6 to 12 months, but patients need to be monitored closely for tumor growth.
1. Ma GL, Murphy JD, Martinez ME et al. Epidemiology of gastrointestinal stromal tumors in the era of histology codes: results of a population-based study. Cancer Epidemiol Biomarkers Prev. 2015;24:298-302.
2. Agaimy A, Wunsch PH, Hofstaedter F, et al. Minute gastric sclerosing stromal tumors (GIST tumorlets) are common in adults and frequently show c-KIT mutations. Am J Surg Pathol. 2007;31:113-120.
3. Miettinen M, Sobin LH, Sarlomo-Rikala M. Immunohistochemical spectrum of GISTs at different sites and their differential diagnosis with a reference to CD117 (KIT). Mod Pathol. 2000;13:1134-1142.
4. West RB, Corless CL, Chen X, et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutational status. Am J Pathol. 2004;165:107-113.
5. Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Int J Surg Pathol. 2002;10:81-89.
6. Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411-1419.
7. Hohenberger P, Ronellenfitsch U, Oladeji O, et al. Pattern of recurrence in patients with ruptured primary gastrointestinal stromal tumor. Br J Surg. 2010;97:1854-1859.
8. Holmenbakk T, Bjerkehagen B, Boye K, et al. Definition and clinical significance of tumor rupture in gastrointestinal stromal tumours of the small intestine. Br J Surg. 2016;103:684-691.
9. Emory TS, Sobin LH, Lukes L, et al. Prognosis of gastrointestinal smooth-muscle (stromal) tumors: dependence on anatomic site. Am J Surg Pathol. 1999;23:82-87.
10. Miettinen M, Makhlouf H, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am J Surg Pathol. 2006;30:477-489.
11. Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52-68.
12. Gold JS, Gonen M, Gutierrez A, et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localized primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol. 2009;10:1045-1052.
13. Joensuu H, Vehtari A, Rihimaki J et al. Risk of recurrence of gastrointestinal stromal tumor after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13:265-274.
14. Casali PG, Abecassis N, Bauer S, et al. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow up. Ann Oncol. 2018;29(Supplement_4): iv267.
15. Jing L, Yan-Ling W, Bing-Jia C, et al. The c-kit receptor-mediated signal transduction and tumor-related diseases. Int J Biol Sci. 2013;9:435-443.
16. Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577-580.
17. Joensuu H, Rutkowski P, Nishida T, et al. KIT and PDGFRA mutations and the risk of GI stromal tumor recurrence. J Clin Oncol. 2015;33:634-642.
18. Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004;22:3813-3825.
19. Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342-4349.
20. Huss S, Pasternack H, Ihle MA, et al. Clinicopathological and molecular features of a large cohort of gastrointestinal stromal tumors (GISTs) and review of the literature: BRAF mutations in KIT/PDGFRA wild-type GISTs are rare events. Hum Pathol. 2017;62:206-214.
21. Shi E, Chmielecki J, Tang CM, et al. FGFR1 and NTRK3 actionable alterations in “Wild-Type” gastrointestinal stromal tumors. J Transl Med. 2016;14:339.
22. Carney JA, Stratakis CA. Familial paraganglioma and gastric stromal sarcoma: a new syndrome distinct from the Carney triad. Am J Med Genet. 2002;108:132-139.
23. Carney JA. Gastric stromal sarcoma, pulmonary chondroma, and extra-adrenal paraganglioma (Carney Triad): natural history, adrenocortical component, and possible familial occurrence. Mayo Clin Proc. 1999;74:543-552.
24. Jakob J, Mussi C, Ronellenfitsch U, et al. Gastrointestinal stromal tumor of the rectum: results of surgical and multimodality therapy in the era of imatinib. Ann Surg Oncol. 2013;20:586-592.
25. DeMatteo RP, Ballman KV, Antonescu CR, et al. Long-term results of adjuvant imatinib mesylate in localized, high-risk, primary gastrointestinal stromal tumor (GIST): ACOSOG Z9000 (Alliance) intergroup phase 2 trial. Ann Surg. 2013;258:422-429.
26. Gleevac (imatinib) [package insert]. East Hanover, NJ: Novartis Pharmaceuticals; 2016.
27. DeMatteo RP, Ballman KV, Antonescu CR, et al. Placebo-controlled randomized trial of adjuvant imatinib mesylate following the resection of localized, primary gastrointestinal stromal tumor (GIST). Lancet. 2009;373:1097-1104.
28. Corless CL, Ballman KV, Antonescu CR, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol. 2014;32:1563-1570.
29. Casali PG, Le Cesne A, Poveda Velasco A, et al. Imatinib failure-free survival (IFS) in patients with localized gastrointestinal stromal tumors (GIST) treated with adjuvant imatinib (IM): the EORTC/AGITG/FSG/GEIS/ISG randomized controlled phase III trial. J Clin Oncol. 2013;31. Abstract 10500.
30. Joensuu H, Eriksson M, Sundby HK, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012;307:1265-1272.
31. Raut CP, Espat NJ, Maki RG, et al. Efficacy and tolerability of 5-year adjuvant imatinib treatment for patients with resected intermediate- or high-risk primary gastrointestinal stromal tumor: The PERSIST-5 Clinical Trial. JAMA Oncol. 2018: e184060.
32. Benjamin RS, Casali PG. Adjuvant imatinib for GI stromal tumors: when and for how long? J Clin Oncol. 2016;34:215-218.
33. Demetri GD, Wang Y, Wehrle E, et al. Imatinib plasma levels are correlated with clinical benefit in patients with unresectable/metastatic gastrointestinal stromal tumors. J Clin Oncol. 2009;27:3141-3147.
34. Eisenberg BL, Harris J, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol. 2009;99:42-47.
35. Rutkowski P, Gronchi A, Hohenberger P, et al. Neoadjuvant imatinib in locally advanced gastrointestinal stromal tumors (GIST): the EORTC STBSG experience. Ann Surg Oncol. 2013;20:2937-2943.
36. Joensuu H, Martin-Broto J, Nishida T, et al. Follow-up strategies for patients with gastrointestinal stromal tumour treated with or without adjuvant imatinib after surgery. Eur J Cancer. 2015;51:1611-1617.
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumor of the gastrointestinal (GI) tract and arise from the interstitial cells of Cajal of the myenteric plexus. These tumors are rare, with about 1 case per 100,000 persons diagnosed in the United States annually, but may be incidentally discovered in up to 1 in 5 autopsy specimens of older adults.1,2 Epidemiologic risk factors include increasing age, with a peak incidence between age 60 and 65 years, male gender, black race, and non-Hispanic white ethnicity. Germline predisposition can also increase the risk of developing GISTs; molecular drivers of GIST include gain-of-function mutations in the KIT proto-oncogene and platelet-derived growth factor receptor α (PDGFRA) gene, which both encode structurally similar tyrosine kinase receptors; germline mutations of succinate dehydrogenase (SDH) subunit genes; and mutations associated with neurofibromatosis type 1.
GISTs most commonly involve the stomach, followed by the small intestine, but can arise anywhere within the GI tract (esophagus, colon, rectum, and anus). They can also develop outside the GI tract, arising from the mesentery, omentum, and retroperitoneum. The majority of cases are localized or locoregional, whereas about 20% are metastatic at presentation.1 GISTs can occur in children, adolescents, and young adults. Pediatric GISTs represent a distinct subset marked by female predominance and gastric origin, are often multifocal, can sometimes have lymph node involvement, and typically lack mutations in the KIT and PDGFRA genes.
This review is the first of 2 articles focusing on the diagnosis and management of GISTs. Here, we review the evaluation and diagnosis of GISTs along with management of localized disease. Management of advanced disease is reviewed in a separate article.
Case Presentation
A 64-year-old African American man with progressive iron deficiency and abdominal discomfort undergoes upper and lower endoscopy and is found to have a bulging mass within his abdominal cavity. He undergoes a computed tomography (CT) evaluation of the chest, abdomen, and pelvis with contrast, which reveals the presence of a 10-cm gastric mass, with no other lesions identified. He undergoes surgical resection of the mass and presents for review of his pathology and to discuss his treatment plan.
What histopathologic features are consistent with GIST?
What factors are used for risk stratification and to predict likelihood of recurrence?
Clinical Presentation and Diagnosis
Most patients present with symptoms of overt or occult GI bleeding or abdominal discomfort, but a significant proportion of GISTs are discovered incidentally. Lymph node involvement is not typical, except for GISTs occurring in children and/or with rare syndromes. Most syndromic GISTs are multifocal and multicentric. After surgical resection, GISTs usually recur or metastasize within the abdominal cavity, including the omentum, peritoneum, or liver. These tumors rarely spread to the lungs, brain, or bones; when tumor spread does occur, it tends to be in heavily pre-treated patients with advanced disease who have been on multiple lines of therapy for a long duration of time.
The diagnosis usually can be made by histopathology. Specimens can be obtained by endoscopic ultrasound (EUS)– or CT-guided methods, the latter of which carries a very small risk of contamination from percutaneous biopsy. In terms of morphology, GISTs can be spindle cell, epithelioid, or mixed neoplasms. Epithelioid tumors are more commonly seen in the stomach and are often PDGFRA-mutated or SDH-deficient. The differential diagnosis includes other soft-tissue GI wall tumors such as leiomyosarcomas/leiomyomas, germ cell tumors, lymphomas, fibromatosis, and neuroendocrine and neurogenic tumors. A unique feature of GISTs that differentiates them from leiomyomas is near universal expression of CD117 by immunohistochemistry (IHC); this characteristic has allowed pathologists and providers to accurately distinguish true GISTs from other GI mesenchymal tumors.3 Recently, DOG1 (discovered on GIST1) immunoreactivity has been found to be helpful in identifying patients with CD117-negative GISTs. Initially identified through gene expression analysis of GISTs, DOG1 IHC can identify the common mutant c-Kit-driven CD117-positive GISTs as well as the rare CD117-negative GISTs, which are often driven by mutated PDGFRA.4 Importantly, IHC for KIT and DOG1 are not surrogates for mutational status, nor are they predictive of tyrosine kinase inhibitor (TKI) sensitivity. If IHC of a tumor specimen is CD117- and DOG1-negative, the specimen can be sent for KIT and PDGFRA mutational analysis to confirm the diagnosis. If analysis reveals that these genes are wild-type, then IHC staining for SDH B (SDHB) should follow to assess for an SDH-deficient GIST (negative staining).
Risk Stratification for Recurrence
The clinical behavior of GISTs can be variable. Some are indolent, while others behave more aggressively, with a greater malignant potential and a higher propensity to recur and metastasize. Clinical and pathologic features can provide important prognostic information that allows providers to risk-stratify patients. Various institutions have assessed prognostic variables for GISTs. In 2001, the National Institutes of Health (NIH) held a GIST workshop that proposed an approach to estimating metastatic risk based on tumor size and mitotic index (NIH or Fletcher criteria).5 Joensuu et al later proposed a modification of the NIH risk classification to include tumor location and tumor rupture (modified NIH criteria or Joensuu criteria).6-8 Similarly, the Armed Forces Institute of Pathology (AFIP) identified tumor site as a prognostic factor, with gastric GISTs having the best prognosis (AFIP-Miettinen criteria).9-11 Tabular schemes were designed which stratified patients into discrete groups with ranges for mitotic rate and tumor size. Nomograms for ease of use were then constructed utilizing a bimodal mitotic rate and included tumor site and size.12 Finally, contour maps were developed, which have the advantage of evaluating mitotic rate and tumor size as continuous nonlinear variables and also include tumor site and rupture (associated with a high risk of peritoneal metastasis) separately, further improving risk assessment. These contour maps have been validated against pooled data from 10 series (2560 patients).13 High-risk features identified from these studies include tumor location, size, mitotic rate and tumor rupture and are now used for deciding on the use of adjuvant imatinib and as requirements to enter clinical trials assessing adjuvant therapy for resected GISTs.
Case Continued
The patient’s operative and pathology reports indicate that the tumor is a spindle cell neoplasm of the stomach that is positive for CD117, DOG1, and CD34 and negative for smooth muscle actin and S-100, consistent with a diagnosis of GIST. Resection margins are negative. There are 10 mitoses per 50 high-power fields (HPF). Per the operative report, there was no intraoperative or intraperitoneal tumor rupture. Thus, while his GIST was gastric, which generally has a more favorable prognosis, the tumor harbors high-risk features based on its size and mitotic index.
What further testing should be requested?
Molecular Alterations
It is recommended that a mutational analysis be performed as part of the diagnostic work-up of all GISTs.14 Mutational analysis can provide prognostic and predictive information for sensitivity to imatinib and should be considered standard of care. It may also be useful for confirming a GIST diagnosis, or, if negative, lead to further evaluation with an IHC stain for SDHB. The c-Kit receptor is a member of the tyrosine kinase family and, through direct interactions with stem cell factor (SCF), can upregulate the PI3K/AKT/mTOR, Ras/Raf/MEK/ERK, and JAK-STAT pathways, resulting in transcription and translation of genes that enhance cell growth and survival.15 The cell of origin of GISTs, the interstitial cells of Cajal, are dependent on the SCF–c-Kit interaction for development.16 Likewise, the large majority of GISTs (about 70%) are driven by upregulation and constitutive activation of c-Kit, which is normally autoinhibited. About 80% of KIT mutations involve exon 11; these GISTs are most often associated with a gastric location and are associated with a favorable recurrence-free survival (RFS) rate with surgery alone.17 KIT exon 9 mutations are much less common, encompassing only about 10% of GIST KIT mutations, and GISTs with these mutations are more likely to arise from the small bowel.17
About 8% of GISTs harbor gain-of-function PDGFRA driver mutations rendering constitutively active PDGFRA.18 PDGFRA mutations are mutually exclusive from KIT mutations, and PDGFRA-mutated tumors most often occur in the stomach. PDGFRA mutations generally are associated with a lower mitotic rate and gastric location. Identification of the PDGFRA D842V mutation on exon 18, which is the most common, is important, as it is associated with imatinib resistance, and these patients should not be offered imatinib.19
Several other mutations associated with GISTs outside of the KIT and PDGFRA spectrum have been identified. About 10% of GISTs are wildtype for KIT and PDGFRA, and not all KIT/PDGFRA-wildtype GISTs are imatinib-sensitive and/or respond to other TKIs.18 These tumors may harbor aberrations in SDH and NF1, or less commonly, BRAF V600E, FGFR, and NTRK.20,21 SDH subunits B, C and D play a role in the Krebs cycle and electron transport chain. Germline mutations in these SDH subunits can result in the Carney-Stratakis syndrome characterized by the dyad of multifocal GISTs and multicentric paragangliomas.22 This syndrome is most likely to manifest in the pediatric or young adult population. In contradistinction is the Carney triad, which is associated with acquired loss of function of the SDHC gene due to promoter hypermethylation. This syndrome classically occurs in young women and is characterized by an indolent-behaving triad of multicentric GISTs, non-adrenal paragangliomas, and pulmonary chondromas.23 Like PDGFRA D842V–mutated GISTs, SDH-deficient and NF1-associated GISTs are considered imatinib resistant, and these patients should not be offered imatinib therapy.14
Case Continued
The patient’s GIST is found to harbor a KIT exon 11 single codon deletion. He appears anxious and asks to have everything done to prevent his GIST from coming back and to improve his lifespan.
What are the next steps in the management of this patient?
Management
A multidisciplinary team approach to the management of all GISTs is essential and includes input from radiology, gastroenterology, pathology, medical and surgical oncology, nuclear medicine, and nursing.
Surgical Resection
Small esophagogastric and duodenal GISTs ≤ 2 cm can be asymptomatic and managed with serial endoscopic surveillance, typically every 6 to 12 months, with biopsies if the tumors increase in size. GISTs larger than 2 cm require surgical resection, with resection of the full pseudocapsule and an R0 resection, if possible, since larger GISTs carry a higher risk of growth and recurrence. If an R0 resection would lead to significant morbidity or functional sequelae, an R1 may suffice. Rectal GISTs are an exception, where microscopic margins have been shown to be associated with an increased risk of local failure.24 It is important to explore the abdomen thoroughly for peritoneal, rectovaginal, and vesicular implants and metastasis to the liver. A formal lymph node dissection is not necessary because lymph nodes are rarely involved and should only be removed when clinically suspicious. Tumor rupture must be avoided. A laparoscopic approach should only be considered for smaller tumors, since there is a risk of tumor rupture with larger tumors.14
When is adjuvant imatinib indicated?
Adjuvant Imatinib
Among patients with local or locally advanced GISTs, the risk of death from recurrence with surgery alone can be high, with a historical 5-year overall survival (OS) of about 35%.25 As a result, multiple studies have assessed the benefit of adjuvant imatinib, which is now considered standard of care for patients with imatinib-sensitive, high-risk GISTs. In addition to inhibiting BCR-ABL, imatinib mesylate inhibits multiple other receptor tyrosine kinases, including PDGFR, SCF and c-Kit. As a result, imatinib has demonstrated in vitro inhibition of cell proliferation and apoptosis and clinical activity against GISTs expressing CD117.26 Importantly, adjuvant imatinib should only be offered to patients with imatinib-sensitive mutations, such as KIT exon 11 and KIT exon 9 mutations. Adjuvant imatinib should not be offered to patients with imatinib-insensitive mutations such as PDGFR 842V, NF1, or BRAF-related or SDH-deficient GISTs.
The ACOSOG Z9000 was the first study of adjuvant imatinib in patients with resected GISTs.25 This was a single-arm, phase 2 study involving 106 patients with surgically resected GISTs deemed high-risk for recurrence, defined as size > 10 cm, tumor rupture, or up to 4 peritoneal implants. Patients were treated with imatinib 400 mg daily for 1 year. The primary and secondary endpoints were OS and RFS, respectively. Long-term follow-up of this study demonstrated 1-, 3-, and 5-year OS of 99%, 97%, and 83%, and 1-, 3-, and 5-year RFS of 96%, 60%, and 40%, which compared favorably with historical controls. In a multivariable analysis, increasing tumor size, small bowel location, KIT exon 9 mutation, high mitotic rate, and older age were independent risk factors for a poor RFS.25 It is important to note that the benefit of adjuvant imatinib waned after discontinuation of therapy, creating a rationale to study adjuvant imatinib for longer periods of time.
As a result of the promising phase 2 data, ACOSOG opened a phase 3 randomized trial (Z9001) comparing 1 year of adjuvant imatinib to placebo among patients with surgically resected GISTs that were > 3 cm in size and that stained positive for CD117 on pathology. The trial accrued 713 patients and was stopped early at a planned interim analysis, which revealed a 1-year RFS of 98% for imatinib versus 83% for placebo (hazard ratio [HR], 0.35; P < 0.001). The 1-year OS did not differ between the 2 arms (92.2% vs 99.7%; HR, 0.66; P = 0.47).27 When comparing the 2 arms, imatinib was associated with a higher RFS among patients with a KIT exon 11 deletion, but not among patients with other KIT mutation types, PDGFRA mutations, or who were KIT/PDGFRA wildtype.28 Imatinib was granted approval by the US Food and Drug Administration (FDA) for the adjuvant treatment of high-risk GISTs based on the results of the ACOSOG Z9001 trial.
The EORTC 62024 study was a randomized placebo-controlled trial assessing the benefit of 2 years of adjuvant imatinib.29 Patients had to be considered intermediate or high risk per the 2002 NIH consensus classification to be eligible. The trial enrolled 918 patients. The 5-year OS rate, the original primary endpoint, did not differ between the 2 groups (100% vs 99%). The 3-year and 5-year RFS rates, secondary endpoints, were significantly longer among patients treated with imatinib (84% vs 66% and 69% vs 63%, respectively). Again, it was noted that the benefit of imatinib waned over time after treatment discontinuation.
The Scandinavian Sarcoma Group (SSG XVIII) trial was a prospective randomized phase 3 trial that compared 3 years versus 1 year of adjuvant imatinib.30 Patients had to be enrolled within 12 weeks of the postoperative period and had to have GISTs that were CD117-positive and with a high estimated risk of recurrence, per the modified NIH consensus criteria (size > 10 cm, > 10 mitoses per 50 HPF, diameter > 5 cm with mitotic count > 5, or tumor rupture before or at surgery). Three years of adjuvant imatinib was associated with a 54% reduction in the hazard for recurrence at 5 years (65.6% vs 47.9%; HR, 0.46; P < 0.001) and a 55% reduction in the hazard for death at 5 years (OS 92% vs 81.7%; HR, 0.45; P = 0.02). Based on the results of this study, the FDA granted approval for the use of 3 years of adjuvant imatinib in patients with high-risk resected GISTs.
The observation that a longer duration of adjuvant imatinib was associated with superior RFS and OS led to studies to further explore longer durations of adjuvant imatinib. The PERSIST-5 (Postresection Evaluation of Recurrence-free Survival for Gastrointestinal Stromal Tumors With 5 Years of Adjuvant Imatinib) was a multicenter, single-arm, phase 2 prospective study of adjuvant imatinib with a primary endpoint of RFS after 5 years.31 Patients had to have an intermediate or high risk of recurrence, which included GISTs at any site > 2 cm with > 5 mitoses per 50 HPF or nongastric GISTs that were ≥ 5 cm. With 91 patients enrolled, the estimated 5-year RFS was 90% and the OS was 95%. Of note, about half of the patients stopped treatment early due to a variety of reasons, including patient choice or adverse events. Importantly, there were no recurrences in patients with imatinib-sensitive mutations while on therapy. We know that in patients at high risk of relapse, adjuvant imatinib delays recurrence and improves survival, but whether any patients are cured, or their survival curves are just shifted to the right, is unknown. Only longer follow-up of existing studies, and the results of newer trials utilizing longer durations of adjuvant treatment, will help to determine the real value of adjuvant therapy for GIST patients.32 Based on this study, it would be reasonable to discuss a longer duration of imatinib with patients deemed to be at very high risk of recurrence and who are tolerating therapy well. We are awaiting the data from the randomized phase 3 Scandinavian Sarcoma Group XII trial comparing 5 years versus 3 years of adjuvant imatinib therapy, and from the French ImadGIST trial of adjuvant imatinib for 3 versus 6 years. A summary of the aforementioned key adjuvant trials is shown in the Table.

When imatinib is commenced, careful monitoring for treatment toxicities and drug interactions should ensue in order to improve compliance. Dose density should be maintained if possible, as retrospective studies suggest suboptimal plasma levels are associated with a worse outcome.33
When should neoadjuvant imatinib be considered?
Neoadjuvant Imatinib
Neoadjuvant imatinib should be considered for patients requiring total gastrectomy, esophagectomy, or abdominoperineal resection of the rectum in order to reduce tumor size, limit subsequent surgical morbidity, mitigate tumor bleeding and rupture, and aid with organ preservation. Patients with rectal GISTs that may otherwise warrant an abdominoperineal resection should be offered a trial of imatinib in the neoadjuvant setting. There is no evidence for the use of any other TKI aside from imatinib in the neoadjuvant or adjuvant setting. With neoadjuvant imatinib, it is difficult to accurately assess the mitotic rate in the resected tumor specimen.
The RTOG 0132/ACRIN 6665 trial was a prospective phase 2 study evaluating the efficacy of imatinib 600 mg daily in the perioperative setting.34 The trial enrolled 50 patients, 30 with primary GISTs (group A) and 22 with recurrent metastatic GISTs (group B). Based on data from the metastatic setting revealing a time to treatment response of about 2.5 months, patients were treated with 8 to 12 weeks of preoperative imatinib followed by 2 years of adjuvant imatinib. Imatinib was stopped 24 hours preoperatively and resumed as soon as possible postoperatively. In group A, 7% of patients achieved a partial response (PR), 83% achieved stable disease, and 2-year progression-free survival (PFS) and OS were 83% and 93%, respectively. In group B, 4.5% of patients achieved a PR, 91% achieved stable disease, and 4.5% experienced progressive disease in the preoperative period; the 2-year PFS and OS were 77% and 91%, respectively. The results of this trial demonstrated the feasibility of using perioperative imatinib with minimal effects on surgical outcomes and set the rationale to use neoadjuvant imatinib in select patients with borderline resectable or rectal GISTs. Another EORTC pooled analysis from 10 sarcoma centers revealed that after a median of 10 months of neoadjuvant imatinib, 83.2% of patients achieved an R0 resection and only 1% progressed during treatment.35 After a median follow-up of 46 months, the 5-year disease-free survival and OS were 65% and 87%, respectively.
Mutational testing should be performed beforehand to ensure the tumor is imatinib-sensitive. If a KIT exon 9 mutation is identified, then 400 mg twice daily should be considered (given the benefit seen with 800 mg imatinib for advanced GIST patients), although there are no studies to confirm this practice. Neoadjuvant imatinib is recommended for a total of 6 to 12 months to ensure maximal tumor debulking, but with very close monitoring and surgical input for disease resistance and growth.14 Imatinib should be stopped 1 to 2 days preoperatively and resumed once the patient has recovered from surgery for a total of 3 years (pre-/postoperatively combined). Neoadjuvant therapy has been shown to be safe and effective, but there have been no randomized trials to assess survival.
What is appropriate surveillance for resected GISTs?
Surveillance
There have been no randomized studies to guide the management of surveillance after surgical resection and adjuvant therapy. There is no known optimal follow-up schedule, but several have been proposed.13,36 Among high-risk patients, it is suggested to image every 3 to 6 months during adjuvant therapy, followed by every 3 months for 2 years after discontinuing therapy, then every 6 months for another 3 years and annually thereafter for an additional 5 years. High-risk patients usually relapse within 1 to 3 years after finishing adjuvant therapy, while low-risk patients can relapse later given that their disease can be slower growing. It has been recommended that low-risk patients undergo imaging every 6 months for 5 years, with follow-up individualized thereafter. Very-low-risk patients may not require more than annual imaging. Because most relapses occur within the peritoneum or liver, imaging should encompass the abdomen and pelvis. Surveillance imaging usually consists of CT scans of the abdomen and pelvis. MRI scans can be utilized for patients at lower risk or who are out several years in order to avoid excess radiation exposure. MRI is also specifically helpful for rectal and esophageal lesions. Chest CT or chest radiograph and bone scan are not routinely required for follow-up.
Case Conclusion
The patient receives adjuvant imatinib and experiences grade 2 myalgias, periorbital edema, and macrocytic anemia, which result in imatinib discontinuation after 3 years of treatment. He is seen every 3 to 6 months and a contrast CT abdomen and pelvis is obtained every 6 months for 5 years. During this 5-year follow-up period, he does not have any clinical or radiographic evidence of disease recurrence.
Further follow-up of this patient is presented in the second article in this 2-part review of management of GISTs.
Key Points
- GISTs are the most common mesenchymal neoplasms of the GI tract and can occasionally occur in extragastrointestinal locations as well.
- GISTs encompass a heterogeneous family of tumor subsets with different natural histories, mutations, and TKI responsiveness.
- Surgery is the mainstay of treatment for localized GISTs, with cure rates greater than 50%.
- For very small (< 2 cm) esophagogastric GISTs, endoscopic ultrasound evaluation and follow-up is recommended.
- For tumors ≥ 2 cm, biopsy and excision is the standard approach.
- For localized GISTs, complete surgical resection (R0) is standard treatment, with no lymphadenectomy for clinically negative lymph nodes.
- Mutational analysis should be considered standard of practice. It can be helpful for confirming the diagnosis and can be predictive and prognostic in determining specific TKI therapy and dose.
- Adjuvant imatinib at a dose of 400 mg for 3 years is standard of care for GISTs that are at high risk of relapse and are imatinib-sensitive, and it is the only TKI approved for adjuvant therapy. Patients with PDGFRA D842V, NF1, BRAF or SDH-deficient GISTs should not receive adjuvant imatinib therapy.
- Neoadjuvant therapy can be utilized for sites where extensive resection would lead to significant morbidity. It should be given for 6 to 12 months, but patients need to be monitored closely for tumor growth.
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumor of the gastrointestinal (GI) tract and arise from the interstitial cells of Cajal of the myenteric plexus. These tumors are rare, with about 1 case per 100,000 persons diagnosed in the United States annually, but may be incidentally discovered in up to 1 in 5 autopsy specimens of older adults.1,2 Epidemiologic risk factors include increasing age, with a peak incidence between age 60 and 65 years, male gender, black race, and non-Hispanic white ethnicity. Germline predisposition can also increase the risk of developing GISTs; molecular drivers of GIST include gain-of-function mutations in the KIT proto-oncogene and platelet-derived growth factor receptor α (PDGFRA) gene, which both encode structurally similar tyrosine kinase receptors; germline mutations of succinate dehydrogenase (SDH) subunit genes; and mutations associated with neurofibromatosis type 1.
GISTs most commonly involve the stomach, followed by the small intestine, but can arise anywhere within the GI tract (esophagus, colon, rectum, and anus). They can also develop outside the GI tract, arising from the mesentery, omentum, and retroperitoneum. The majority of cases are localized or locoregional, whereas about 20% are metastatic at presentation.1 GISTs can occur in children, adolescents, and young adults. Pediatric GISTs represent a distinct subset marked by female predominance and gastric origin, are often multifocal, can sometimes have lymph node involvement, and typically lack mutations in the KIT and PDGFRA genes.
This review is the first of 2 articles focusing on the diagnosis and management of GISTs. Here, we review the evaluation and diagnosis of GISTs along with management of localized disease. Management of advanced disease is reviewed in a separate article.
Case Presentation
A 64-year-old African American man with progressive iron deficiency and abdominal discomfort undergoes upper and lower endoscopy and is found to have a bulging mass within his abdominal cavity. He undergoes a computed tomography (CT) evaluation of the chest, abdomen, and pelvis with contrast, which reveals the presence of a 10-cm gastric mass, with no other lesions identified. He undergoes surgical resection of the mass and presents for review of his pathology and to discuss his treatment plan.
What histopathologic features are consistent with GIST?
What factors are used for risk stratification and to predict likelihood of recurrence?
Clinical Presentation and Diagnosis
Most patients present with symptoms of overt or occult GI bleeding or abdominal discomfort, but a significant proportion of GISTs are discovered incidentally. Lymph node involvement is not typical, except for GISTs occurring in children and/or with rare syndromes. Most syndromic GISTs are multifocal and multicentric. After surgical resection, GISTs usually recur or metastasize within the abdominal cavity, including the omentum, peritoneum, or liver. These tumors rarely spread to the lungs, brain, or bones; when tumor spread does occur, it tends to be in heavily pre-treated patients with advanced disease who have been on multiple lines of therapy for a long duration of time.
The diagnosis usually can be made by histopathology. Specimens can be obtained by endoscopic ultrasound (EUS)– or CT-guided methods, the latter of which carries a very small risk of contamination from percutaneous biopsy. In terms of morphology, GISTs can be spindle cell, epithelioid, or mixed neoplasms. Epithelioid tumors are more commonly seen in the stomach and are often PDGFRA-mutated or SDH-deficient. The differential diagnosis includes other soft-tissue GI wall tumors such as leiomyosarcomas/leiomyomas, germ cell tumors, lymphomas, fibromatosis, and neuroendocrine and neurogenic tumors. A unique feature of GISTs that differentiates them from leiomyomas is near universal expression of CD117 by immunohistochemistry (IHC); this characteristic has allowed pathologists and providers to accurately distinguish true GISTs from other GI mesenchymal tumors.3 Recently, DOG1 (discovered on GIST1) immunoreactivity has been found to be helpful in identifying patients with CD117-negative GISTs. Initially identified through gene expression analysis of GISTs, DOG1 IHC can identify the common mutant c-Kit-driven CD117-positive GISTs as well as the rare CD117-negative GISTs, which are often driven by mutated PDGFRA.4 Importantly, IHC for KIT and DOG1 are not surrogates for mutational status, nor are they predictive of tyrosine kinase inhibitor (TKI) sensitivity. If IHC of a tumor specimen is CD117- and DOG1-negative, the specimen can be sent for KIT and PDGFRA mutational analysis to confirm the diagnosis. If analysis reveals that these genes are wild-type, then IHC staining for SDH B (SDHB) should follow to assess for an SDH-deficient GIST (negative staining).
Risk Stratification for Recurrence
The clinical behavior of GISTs can be variable. Some are indolent, while others behave more aggressively, with a greater malignant potential and a higher propensity to recur and metastasize. Clinical and pathologic features can provide important prognostic information that allows providers to risk-stratify patients. Various institutions have assessed prognostic variables for GISTs. In 2001, the National Institutes of Health (NIH) held a GIST workshop that proposed an approach to estimating metastatic risk based on tumor size and mitotic index (NIH or Fletcher criteria).5 Joensuu et al later proposed a modification of the NIH risk classification to include tumor location and tumor rupture (modified NIH criteria or Joensuu criteria).6-8 Similarly, the Armed Forces Institute of Pathology (AFIP) identified tumor site as a prognostic factor, with gastric GISTs having the best prognosis (AFIP-Miettinen criteria).9-11 Tabular schemes were designed which stratified patients into discrete groups with ranges for mitotic rate and tumor size. Nomograms for ease of use were then constructed utilizing a bimodal mitotic rate and included tumor site and size.12 Finally, contour maps were developed, which have the advantage of evaluating mitotic rate and tumor size as continuous nonlinear variables and also include tumor site and rupture (associated with a high risk of peritoneal metastasis) separately, further improving risk assessment. These contour maps have been validated against pooled data from 10 series (2560 patients).13 High-risk features identified from these studies include tumor location, size, mitotic rate and tumor rupture and are now used for deciding on the use of adjuvant imatinib and as requirements to enter clinical trials assessing adjuvant therapy for resected GISTs.
Case Continued
The patient’s operative and pathology reports indicate that the tumor is a spindle cell neoplasm of the stomach that is positive for CD117, DOG1, and CD34 and negative for smooth muscle actin and S-100, consistent with a diagnosis of GIST. Resection margins are negative. There are 10 mitoses per 50 high-power fields (HPF). Per the operative report, there was no intraoperative or intraperitoneal tumor rupture. Thus, while his GIST was gastric, which generally has a more favorable prognosis, the tumor harbors high-risk features based on its size and mitotic index.
What further testing should be requested?
Molecular Alterations
It is recommended that a mutational analysis be performed as part of the diagnostic work-up of all GISTs.14 Mutational analysis can provide prognostic and predictive information for sensitivity to imatinib and should be considered standard of care. It may also be useful for confirming a GIST diagnosis, or, if negative, lead to further evaluation with an IHC stain for SDHB. The c-Kit receptor is a member of the tyrosine kinase family and, through direct interactions with stem cell factor (SCF), can upregulate the PI3K/AKT/mTOR, Ras/Raf/MEK/ERK, and JAK-STAT pathways, resulting in transcription and translation of genes that enhance cell growth and survival.15 The cell of origin of GISTs, the interstitial cells of Cajal, are dependent on the SCF–c-Kit interaction for development.16 Likewise, the large majority of GISTs (about 70%) are driven by upregulation and constitutive activation of c-Kit, which is normally autoinhibited. About 80% of KIT mutations involve exon 11; these GISTs are most often associated with a gastric location and are associated with a favorable recurrence-free survival (RFS) rate with surgery alone.17 KIT exon 9 mutations are much less common, encompassing only about 10% of GIST KIT mutations, and GISTs with these mutations are more likely to arise from the small bowel.17
About 8% of GISTs harbor gain-of-function PDGFRA driver mutations rendering constitutively active PDGFRA.18 PDGFRA mutations are mutually exclusive from KIT mutations, and PDGFRA-mutated tumors most often occur in the stomach. PDGFRA mutations generally are associated with a lower mitotic rate and gastric location. Identification of the PDGFRA D842V mutation on exon 18, which is the most common, is important, as it is associated with imatinib resistance, and these patients should not be offered imatinib.19
Several other mutations associated with GISTs outside of the KIT and PDGFRA spectrum have been identified. About 10% of GISTs are wildtype for KIT and PDGFRA, and not all KIT/PDGFRA-wildtype GISTs are imatinib-sensitive and/or respond to other TKIs.18 These tumors may harbor aberrations in SDH and NF1, or less commonly, BRAF V600E, FGFR, and NTRK.20,21 SDH subunits B, C and D play a role in the Krebs cycle and electron transport chain. Germline mutations in these SDH subunits can result in the Carney-Stratakis syndrome characterized by the dyad of multifocal GISTs and multicentric paragangliomas.22 This syndrome is most likely to manifest in the pediatric or young adult population. In contradistinction is the Carney triad, which is associated with acquired loss of function of the SDHC gene due to promoter hypermethylation. This syndrome classically occurs in young women and is characterized by an indolent-behaving triad of multicentric GISTs, non-adrenal paragangliomas, and pulmonary chondromas.23 Like PDGFRA D842V–mutated GISTs, SDH-deficient and NF1-associated GISTs are considered imatinib resistant, and these patients should not be offered imatinib therapy.14
Case Continued
The patient’s GIST is found to harbor a KIT exon 11 single codon deletion. He appears anxious and asks to have everything done to prevent his GIST from coming back and to improve his lifespan.
What are the next steps in the management of this patient?
Management
A multidisciplinary team approach to the management of all GISTs is essential and includes input from radiology, gastroenterology, pathology, medical and surgical oncology, nuclear medicine, and nursing.
Surgical Resection
Small esophagogastric and duodenal GISTs ≤ 2 cm can be asymptomatic and managed with serial endoscopic surveillance, typically every 6 to 12 months, with biopsies if the tumors increase in size. GISTs larger than 2 cm require surgical resection, with resection of the full pseudocapsule and an R0 resection, if possible, since larger GISTs carry a higher risk of growth and recurrence. If an R0 resection would lead to significant morbidity or functional sequelae, an R1 may suffice. Rectal GISTs are an exception, where microscopic margins have been shown to be associated with an increased risk of local failure.24 It is important to explore the abdomen thoroughly for peritoneal, rectovaginal, and vesicular implants and metastasis to the liver. A formal lymph node dissection is not necessary because lymph nodes are rarely involved and should only be removed when clinically suspicious. Tumor rupture must be avoided. A laparoscopic approach should only be considered for smaller tumors, since there is a risk of tumor rupture with larger tumors.14
When is adjuvant imatinib indicated?
Adjuvant Imatinib
Among patients with local or locally advanced GISTs, the risk of death from recurrence with surgery alone can be high, with a historical 5-year overall survival (OS) of about 35%.25 As a result, multiple studies have assessed the benefit of adjuvant imatinib, which is now considered standard of care for patients with imatinib-sensitive, high-risk GISTs. In addition to inhibiting BCR-ABL, imatinib mesylate inhibits multiple other receptor tyrosine kinases, including PDGFR, SCF and c-Kit. As a result, imatinib has demonstrated in vitro inhibition of cell proliferation and apoptosis and clinical activity against GISTs expressing CD117.26 Importantly, adjuvant imatinib should only be offered to patients with imatinib-sensitive mutations, such as KIT exon 11 and KIT exon 9 mutations. Adjuvant imatinib should not be offered to patients with imatinib-insensitive mutations such as PDGFR 842V, NF1, or BRAF-related or SDH-deficient GISTs.
The ACOSOG Z9000 was the first study of adjuvant imatinib in patients with resected GISTs.25 This was a single-arm, phase 2 study involving 106 patients with surgically resected GISTs deemed high-risk for recurrence, defined as size > 10 cm, tumor rupture, or up to 4 peritoneal implants. Patients were treated with imatinib 400 mg daily for 1 year. The primary and secondary endpoints were OS and RFS, respectively. Long-term follow-up of this study demonstrated 1-, 3-, and 5-year OS of 99%, 97%, and 83%, and 1-, 3-, and 5-year RFS of 96%, 60%, and 40%, which compared favorably with historical controls. In a multivariable analysis, increasing tumor size, small bowel location, KIT exon 9 mutation, high mitotic rate, and older age were independent risk factors for a poor RFS.25 It is important to note that the benefit of adjuvant imatinib waned after discontinuation of therapy, creating a rationale to study adjuvant imatinib for longer periods of time.
As a result of the promising phase 2 data, ACOSOG opened a phase 3 randomized trial (Z9001) comparing 1 year of adjuvant imatinib to placebo among patients with surgically resected GISTs that were > 3 cm in size and that stained positive for CD117 on pathology. The trial accrued 713 patients and was stopped early at a planned interim analysis, which revealed a 1-year RFS of 98% for imatinib versus 83% for placebo (hazard ratio [HR], 0.35; P < 0.001). The 1-year OS did not differ between the 2 arms (92.2% vs 99.7%; HR, 0.66; P = 0.47).27 When comparing the 2 arms, imatinib was associated with a higher RFS among patients with a KIT exon 11 deletion, but not among patients with other KIT mutation types, PDGFRA mutations, or who were KIT/PDGFRA wildtype.28 Imatinib was granted approval by the US Food and Drug Administration (FDA) for the adjuvant treatment of high-risk GISTs based on the results of the ACOSOG Z9001 trial.
The EORTC 62024 study was a randomized placebo-controlled trial assessing the benefit of 2 years of adjuvant imatinib.29 Patients had to be considered intermediate or high risk per the 2002 NIH consensus classification to be eligible. The trial enrolled 918 patients. The 5-year OS rate, the original primary endpoint, did not differ between the 2 groups (100% vs 99%). The 3-year and 5-year RFS rates, secondary endpoints, were significantly longer among patients treated with imatinib (84% vs 66% and 69% vs 63%, respectively). Again, it was noted that the benefit of imatinib waned over time after treatment discontinuation.
The Scandinavian Sarcoma Group (SSG XVIII) trial was a prospective randomized phase 3 trial that compared 3 years versus 1 year of adjuvant imatinib.30 Patients had to be enrolled within 12 weeks of the postoperative period and had to have GISTs that were CD117-positive and with a high estimated risk of recurrence, per the modified NIH consensus criteria (size > 10 cm, > 10 mitoses per 50 HPF, diameter > 5 cm with mitotic count > 5, or tumor rupture before or at surgery). Three years of adjuvant imatinib was associated with a 54% reduction in the hazard for recurrence at 5 years (65.6% vs 47.9%; HR, 0.46; P < 0.001) and a 55% reduction in the hazard for death at 5 years (OS 92% vs 81.7%; HR, 0.45; P = 0.02). Based on the results of this study, the FDA granted approval for the use of 3 years of adjuvant imatinib in patients with high-risk resected GISTs.
The observation that a longer duration of adjuvant imatinib was associated with superior RFS and OS led to studies to further explore longer durations of adjuvant imatinib. The PERSIST-5 (Postresection Evaluation of Recurrence-free Survival for Gastrointestinal Stromal Tumors With 5 Years of Adjuvant Imatinib) was a multicenter, single-arm, phase 2 prospective study of adjuvant imatinib with a primary endpoint of RFS after 5 years.31 Patients had to have an intermediate or high risk of recurrence, which included GISTs at any site > 2 cm with > 5 mitoses per 50 HPF or nongastric GISTs that were ≥ 5 cm. With 91 patients enrolled, the estimated 5-year RFS was 90% and the OS was 95%. Of note, about half of the patients stopped treatment early due to a variety of reasons, including patient choice or adverse events. Importantly, there were no recurrences in patients with imatinib-sensitive mutations while on therapy. We know that in patients at high risk of relapse, adjuvant imatinib delays recurrence and improves survival, but whether any patients are cured, or their survival curves are just shifted to the right, is unknown. Only longer follow-up of existing studies, and the results of newer trials utilizing longer durations of adjuvant treatment, will help to determine the real value of adjuvant therapy for GIST patients.32 Based on this study, it would be reasonable to discuss a longer duration of imatinib with patients deemed to be at very high risk of recurrence and who are tolerating therapy well. We are awaiting the data from the randomized phase 3 Scandinavian Sarcoma Group XII trial comparing 5 years versus 3 years of adjuvant imatinib therapy, and from the French ImadGIST trial of adjuvant imatinib for 3 versus 6 years. A summary of the aforementioned key adjuvant trials is shown in the Table.

When imatinib is commenced, careful monitoring for treatment toxicities and drug interactions should ensue in order to improve compliance. Dose density should be maintained if possible, as retrospective studies suggest suboptimal plasma levels are associated with a worse outcome.33
When should neoadjuvant imatinib be considered?
Neoadjuvant Imatinib
Neoadjuvant imatinib should be considered for patients requiring total gastrectomy, esophagectomy, or abdominoperineal resection of the rectum in order to reduce tumor size, limit subsequent surgical morbidity, mitigate tumor bleeding and rupture, and aid with organ preservation. Patients with rectal GISTs that may otherwise warrant an abdominoperineal resection should be offered a trial of imatinib in the neoadjuvant setting. There is no evidence for the use of any other TKI aside from imatinib in the neoadjuvant or adjuvant setting. With neoadjuvant imatinib, it is difficult to accurately assess the mitotic rate in the resected tumor specimen.
The RTOG 0132/ACRIN 6665 trial was a prospective phase 2 study evaluating the efficacy of imatinib 600 mg daily in the perioperative setting.34 The trial enrolled 50 patients, 30 with primary GISTs (group A) and 22 with recurrent metastatic GISTs (group B). Based on data from the metastatic setting revealing a time to treatment response of about 2.5 months, patients were treated with 8 to 12 weeks of preoperative imatinib followed by 2 years of adjuvant imatinib. Imatinib was stopped 24 hours preoperatively and resumed as soon as possible postoperatively. In group A, 7% of patients achieved a partial response (PR), 83% achieved stable disease, and 2-year progression-free survival (PFS) and OS were 83% and 93%, respectively. In group B, 4.5% of patients achieved a PR, 91% achieved stable disease, and 4.5% experienced progressive disease in the preoperative period; the 2-year PFS and OS were 77% and 91%, respectively. The results of this trial demonstrated the feasibility of using perioperative imatinib with minimal effects on surgical outcomes and set the rationale to use neoadjuvant imatinib in select patients with borderline resectable or rectal GISTs. Another EORTC pooled analysis from 10 sarcoma centers revealed that after a median of 10 months of neoadjuvant imatinib, 83.2% of patients achieved an R0 resection and only 1% progressed during treatment.35 After a median follow-up of 46 months, the 5-year disease-free survival and OS were 65% and 87%, respectively.
Mutational testing should be performed beforehand to ensure the tumor is imatinib-sensitive. If a KIT exon 9 mutation is identified, then 400 mg twice daily should be considered (given the benefit seen with 800 mg imatinib for advanced GIST patients), although there are no studies to confirm this practice. Neoadjuvant imatinib is recommended for a total of 6 to 12 months to ensure maximal tumor debulking, but with very close monitoring and surgical input for disease resistance and growth.14 Imatinib should be stopped 1 to 2 days preoperatively and resumed once the patient has recovered from surgery for a total of 3 years (pre-/postoperatively combined). Neoadjuvant therapy has been shown to be safe and effective, but there have been no randomized trials to assess survival.
What is appropriate surveillance for resected GISTs?
Surveillance
There have been no randomized studies to guide the management of surveillance after surgical resection and adjuvant therapy. There is no known optimal follow-up schedule, but several have been proposed.13,36 Among high-risk patients, it is suggested to image every 3 to 6 months during adjuvant therapy, followed by every 3 months for 2 years after discontinuing therapy, then every 6 months for another 3 years and annually thereafter for an additional 5 years. High-risk patients usually relapse within 1 to 3 years after finishing adjuvant therapy, while low-risk patients can relapse later given that their disease can be slower growing. It has been recommended that low-risk patients undergo imaging every 6 months for 5 years, with follow-up individualized thereafter. Very-low-risk patients may not require more than annual imaging. Because most relapses occur within the peritoneum or liver, imaging should encompass the abdomen and pelvis. Surveillance imaging usually consists of CT scans of the abdomen and pelvis. MRI scans can be utilized for patients at lower risk or who are out several years in order to avoid excess radiation exposure. MRI is also specifically helpful for rectal and esophageal lesions. Chest CT or chest radiograph and bone scan are not routinely required for follow-up.
Case Conclusion
The patient receives adjuvant imatinib and experiences grade 2 myalgias, periorbital edema, and macrocytic anemia, which result in imatinib discontinuation after 3 years of treatment. He is seen every 3 to 6 months and a contrast CT abdomen and pelvis is obtained every 6 months for 5 years. During this 5-year follow-up period, he does not have any clinical or radiographic evidence of disease recurrence.
Further follow-up of this patient is presented in the second article in this 2-part review of management of GISTs.
Key Points
- GISTs are the most common mesenchymal neoplasms of the GI tract and can occasionally occur in extragastrointestinal locations as well.
- GISTs encompass a heterogeneous family of tumor subsets with different natural histories, mutations, and TKI responsiveness.
- Surgery is the mainstay of treatment for localized GISTs, with cure rates greater than 50%.
- For very small (< 2 cm) esophagogastric GISTs, endoscopic ultrasound evaluation and follow-up is recommended.
- For tumors ≥ 2 cm, biopsy and excision is the standard approach.
- For localized GISTs, complete surgical resection (R0) is standard treatment, with no lymphadenectomy for clinically negative lymph nodes.
- Mutational analysis should be considered standard of practice. It can be helpful for confirming the diagnosis and can be predictive and prognostic in determining specific TKI therapy and dose.
- Adjuvant imatinib at a dose of 400 mg for 3 years is standard of care for GISTs that are at high risk of relapse and are imatinib-sensitive, and it is the only TKI approved for adjuvant therapy. Patients with PDGFRA D842V, NF1, BRAF or SDH-deficient GISTs should not receive adjuvant imatinib therapy.
- Neoadjuvant therapy can be utilized for sites where extensive resection would lead to significant morbidity. It should be given for 6 to 12 months, but patients need to be monitored closely for tumor growth.
1. Ma GL, Murphy JD, Martinez ME et al. Epidemiology of gastrointestinal stromal tumors in the era of histology codes: results of a population-based study. Cancer Epidemiol Biomarkers Prev. 2015;24:298-302.
2. Agaimy A, Wunsch PH, Hofstaedter F, et al. Minute gastric sclerosing stromal tumors (GIST tumorlets) are common in adults and frequently show c-KIT mutations. Am J Surg Pathol. 2007;31:113-120.
3. Miettinen M, Sobin LH, Sarlomo-Rikala M. Immunohistochemical spectrum of GISTs at different sites and their differential diagnosis with a reference to CD117 (KIT). Mod Pathol. 2000;13:1134-1142.
4. West RB, Corless CL, Chen X, et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutational status. Am J Pathol. 2004;165:107-113.
5. Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Int J Surg Pathol. 2002;10:81-89.
6. Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411-1419.
7. Hohenberger P, Ronellenfitsch U, Oladeji O, et al. Pattern of recurrence in patients with ruptured primary gastrointestinal stromal tumor. Br J Surg. 2010;97:1854-1859.
8. Holmenbakk T, Bjerkehagen B, Boye K, et al. Definition and clinical significance of tumor rupture in gastrointestinal stromal tumours of the small intestine. Br J Surg. 2016;103:684-691.
9. Emory TS, Sobin LH, Lukes L, et al. Prognosis of gastrointestinal smooth-muscle (stromal) tumors: dependence on anatomic site. Am J Surg Pathol. 1999;23:82-87.
10. Miettinen M, Makhlouf H, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am J Surg Pathol. 2006;30:477-489.
11. Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52-68.
12. Gold JS, Gonen M, Gutierrez A, et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localized primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol. 2009;10:1045-1052.
13. Joensuu H, Vehtari A, Rihimaki J et al. Risk of recurrence of gastrointestinal stromal tumor after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13:265-274.
14. Casali PG, Abecassis N, Bauer S, et al. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow up. Ann Oncol. 2018;29(Supplement_4): iv267.
15. Jing L, Yan-Ling W, Bing-Jia C, et al. The c-kit receptor-mediated signal transduction and tumor-related diseases. Int J Biol Sci. 2013;9:435-443.
16. Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577-580.
17. Joensuu H, Rutkowski P, Nishida T, et al. KIT and PDGFRA mutations and the risk of GI stromal tumor recurrence. J Clin Oncol. 2015;33:634-642.
18. Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004;22:3813-3825.
19. Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342-4349.
20. Huss S, Pasternack H, Ihle MA, et al. Clinicopathological and molecular features of a large cohort of gastrointestinal stromal tumors (GISTs) and review of the literature: BRAF mutations in KIT/PDGFRA wild-type GISTs are rare events. Hum Pathol. 2017;62:206-214.
21. Shi E, Chmielecki J, Tang CM, et al. FGFR1 and NTRK3 actionable alterations in “Wild-Type” gastrointestinal stromal tumors. J Transl Med. 2016;14:339.
22. Carney JA, Stratakis CA. Familial paraganglioma and gastric stromal sarcoma: a new syndrome distinct from the Carney triad. Am J Med Genet. 2002;108:132-139.
23. Carney JA. Gastric stromal sarcoma, pulmonary chondroma, and extra-adrenal paraganglioma (Carney Triad): natural history, adrenocortical component, and possible familial occurrence. Mayo Clin Proc. 1999;74:543-552.
24. Jakob J, Mussi C, Ronellenfitsch U, et al. Gastrointestinal stromal tumor of the rectum: results of surgical and multimodality therapy in the era of imatinib. Ann Surg Oncol. 2013;20:586-592.
25. DeMatteo RP, Ballman KV, Antonescu CR, et al. Long-term results of adjuvant imatinib mesylate in localized, high-risk, primary gastrointestinal stromal tumor (GIST): ACOSOG Z9000 (Alliance) intergroup phase 2 trial. Ann Surg. 2013;258:422-429.
26. Gleevac (imatinib) [package insert]. East Hanover, NJ: Novartis Pharmaceuticals; 2016.
27. DeMatteo RP, Ballman KV, Antonescu CR, et al. Placebo-controlled randomized trial of adjuvant imatinib mesylate following the resection of localized, primary gastrointestinal stromal tumor (GIST). Lancet. 2009;373:1097-1104.
28. Corless CL, Ballman KV, Antonescu CR, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol. 2014;32:1563-1570.
29. Casali PG, Le Cesne A, Poveda Velasco A, et al. Imatinib failure-free survival (IFS) in patients with localized gastrointestinal stromal tumors (GIST) treated with adjuvant imatinib (IM): the EORTC/AGITG/FSG/GEIS/ISG randomized controlled phase III trial. J Clin Oncol. 2013;31. Abstract 10500.
30. Joensuu H, Eriksson M, Sundby HK, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012;307:1265-1272.
31. Raut CP, Espat NJ, Maki RG, et al. Efficacy and tolerability of 5-year adjuvant imatinib treatment for patients with resected intermediate- or high-risk primary gastrointestinal stromal tumor: The PERSIST-5 Clinical Trial. JAMA Oncol. 2018: e184060.
32. Benjamin RS, Casali PG. Adjuvant imatinib for GI stromal tumors: when and for how long? J Clin Oncol. 2016;34:215-218.
33. Demetri GD, Wang Y, Wehrle E, et al. Imatinib plasma levels are correlated with clinical benefit in patients with unresectable/metastatic gastrointestinal stromal tumors. J Clin Oncol. 2009;27:3141-3147.
34. Eisenberg BL, Harris J, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol. 2009;99:42-47.
35. Rutkowski P, Gronchi A, Hohenberger P, et al. Neoadjuvant imatinib in locally advanced gastrointestinal stromal tumors (GIST): the EORTC STBSG experience. Ann Surg Oncol. 2013;20:2937-2943.
36. Joensuu H, Martin-Broto J, Nishida T, et al. Follow-up strategies for patients with gastrointestinal stromal tumour treated with or without adjuvant imatinib after surgery. Eur J Cancer. 2015;51:1611-1617.
1. Ma GL, Murphy JD, Martinez ME et al. Epidemiology of gastrointestinal stromal tumors in the era of histology codes: results of a population-based study. Cancer Epidemiol Biomarkers Prev. 2015;24:298-302.
2. Agaimy A, Wunsch PH, Hofstaedter F, et al. Minute gastric sclerosing stromal tumors (GIST tumorlets) are common in adults and frequently show c-KIT mutations. Am J Surg Pathol. 2007;31:113-120.
3. Miettinen M, Sobin LH, Sarlomo-Rikala M. Immunohistochemical spectrum of GISTs at different sites and their differential diagnosis with a reference to CD117 (KIT). Mod Pathol. 2000;13:1134-1142.
4. West RB, Corless CL, Chen X, et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutational status. Am J Pathol. 2004;165:107-113.
5. Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Int J Surg Pathol. 2002;10:81-89.
6. Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411-1419.
7. Hohenberger P, Ronellenfitsch U, Oladeji O, et al. Pattern of recurrence in patients with ruptured primary gastrointestinal stromal tumor. Br J Surg. 2010;97:1854-1859.
8. Holmenbakk T, Bjerkehagen B, Boye K, et al. Definition and clinical significance of tumor rupture in gastrointestinal stromal tumours of the small intestine. Br J Surg. 2016;103:684-691.
9. Emory TS, Sobin LH, Lukes L, et al. Prognosis of gastrointestinal smooth-muscle (stromal) tumors: dependence on anatomic site. Am J Surg Pathol. 1999;23:82-87.
10. Miettinen M, Makhlouf H, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am J Surg Pathol. 2006;30:477-489.
11. Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52-68.
12. Gold JS, Gonen M, Gutierrez A, et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localized primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol. 2009;10:1045-1052.
13. Joensuu H, Vehtari A, Rihimaki J et al. Risk of recurrence of gastrointestinal stromal tumor after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13:265-274.
14. Casali PG, Abecassis N, Bauer S, et al. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow up. Ann Oncol. 2018;29(Supplement_4): iv267.
15. Jing L, Yan-Ling W, Bing-Jia C, et al. The c-kit receptor-mediated signal transduction and tumor-related diseases. Int J Biol Sci. 2013;9:435-443.
16. Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577-580.
17. Joensuu H, Rutkowski P, Nishida T, et al. KIT and PDGFRA mutations and the risk of GI stromal tumor recurrence. J Clin Oncol. 2015;33:634-642.
18. Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004;22:3813-3825.
19. Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342-4349.
20. Huss S, Pasternack H, Ihle MA, et al. Clinicopathological and molecular features of a large cohort of gastrointestinal stromal tumors (GISTs) and review of the literature: BRAF mutations in KIT/PDGFRA wild-type GISTs are rare events. Hum Pathol. 2017;62:206-214.
21. Shi E, Chmielecki J, Tang CM, et al. FGFR1 and NTRK3 actionable alterations in “Wild-Type” gastrointestinal stromal tumors. J Transl Med. 2016;14:339.
22. Carney JA, Stratakis CA. Familial paraganglioma and gastric stromal sarcoma: a new syndrome distinct from the Carney triad. Am J Med Genet. 2002;108:132-139.
23. Carney JA. Gastric stromal sarcoma, pulmonary chondroma, and extra-adrenal paraganglioma (Carney Triad): natural history, adrenocortical component, and possible familial occurrence. Mayo Clin Proc. 1999;74:543-552.
24. Jakob J, Mussi C, Ronellenfitsch U, et al. Gastrointestinal stromal tumor of the rectum: results of surgical and multimodality therapy in the era of imatinib. Ann Surg Oncol. 2013;20:586-592.
25. DeMatteo RP, Ballman KV, Antonescu CR, et al. Long-term results of adjuvant imatinib mesylate in localized, high-risk, primary gastrointestinal stromal tumor (GIST): ACOSOG Z9000 (Alliance) intergroup phase 2 trial. Ann Surg. 2013;258:422-429.
26. Gleevac (imatinib) [package insert]. East Hanover, NJ: Novartis Pharmaceuticals; 2016.
27. DeMatteo RP, Ballman KV, Antonescu CR, et al. Placebo-controlled randomized trial of adjuvant imatinib mesylate following the resection of localized, primary gastrointestinal stromal tumor (GIST). Lancet. 2009;373:1097-1104.
28. Corless CL, Ballman KV, Antonescu CR, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol. 2014;32:1563-1570.
29. Casali PG, Le Cesne A, Poveda Velasco A, et al. Imatinib failure-free survival (IFS) in patients with localized gastrointestinal stromal tumors (GIST) treated with adjuvant imatinib (IM): the EORTC/AGITG/FSG/GEIS/ISG randomized controlled phase III trial. J Clin Oncol. 2013;31. Abstract 10500.
30. Joensuu H, Eriksson M, Sundby HK, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012;307:1265-1272.
31. Raut CP, Espat NJ, Maki RG, et al. Efficacy and tolerability of 5-year adjuvant imatinib treatment for patients with resected intermediate- or high-risk primary gastrointestinal stromal tumor: The PERSIST-5 Clinical Trial. JAMA Oncol. 2018: e184060.
32. Benjamin RS, Casali PG. Adjuvant imatinib for GI stromal tumors: when and for how long? J Clin Oncol. 2016;34:215-218.
33. Demetri GD, Wang Y, Wehrle E, et al. Imatinib plasma levels are correlated with clinical benefit in patients with unresectable/metastatic gastrointestinal stromal tumors. J Clin Oncol. 2009;27:3141-3147.
34. Eisenberg BL, Harris J, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol. 2009;99:42-47.
35. Rutkowski P, Gronchi A, Hohenberger P, et al. Neoadjuvant imatinib in locally advanced gastrointestinal stromal tumors (GIST): the EORTC STBSG experience. Ann Surg Oncol. 2013;20:2937-2943.
36. Joensuu H, Martin-Broto J, Nishida T, et al. Follow-up strategies for patients with gastrointestinal stromal tumour treated with or without adjuvant imatinib after surgery. Eur J Cancer. 2015;51:1611-1617.
Efficacy of erenumab is sustained over more than 4 years of treatment
PHILADELPHIA – “Erenumab was well tolerated and safe, with no safety signals detected over this period,” said Messoud Ashina, MD, PhD, professor of neurology at the University of Copenhagen. Dr. Ashina presented the interim data from a 5-year, open-label extension study of erenumab at the annual meeting of the American Headache Society.
Erenumab is a monoclonal antibody that targets and blocks the calcitonin gene-related peptide (CGRP) receptor. In May 2018, the Food and Drug Administration approved erenumab for the preventive treatment of migraine in adults. The treatment, marketed as Aimovig, is administered once monthly by self-injection.
During the open-label study, patients initially received 70 mg of erenumab monthly. After approximately 2 years, patients switched to 140 mg of erenumab monthly. The researchers’ interim efficacy analysis included all patients on 140 mg of erenumab with data about monthly migraine days after more than 4 years of treatment. The safety analysis included all patients who enrolled in the open-label treatment period and received at least one dose of erenumab.
Of 250 patients who increased the erenumab dose from 70 mg to 140 mg, a total of 221 (88%) completed the open-label treatment period or remained on 140 mg after more than 4 years. Patients’ average number of monthly migraine days at study baseline was 8.7, and the average change from baseline to the most recent month in the interim analysis was –5.8.
During the most recent month of assessment, 77% of patients had at least a 50% reduction in monthly migraine days from baseline, 56% had at least a 75% reduction, and 33% had a 100% reduction.
Mean change from baseline in acute migraine‐specific medication treatment days was –4.6, from a baseline of 6.1.
Among the 383 patients who entered the open-label treatment period and received at least one dose of erenumab (mean age, 41.3; 79% female), the median erenumab exposure was 58.5 months. The exposure‐adjusted incidence of adverse events per 100 patient‐years was 124.9, and the three most frequent adverse events (per 100 patient-years) were nasopharyngitis (10.9), upper respiratory tract infection (6.8), and influenza (4.7). The exposure‐adjusted incidence rate per 100 patient‐years for constipation was 1.3 (9/383) for 70-mg erenumab and 2.6 (15/250) for 140-mg erenumab.
“The exposure‐adjusted incidence rate per 100 patient‐years of serious adverse events was 3.8, similar to the rate observed for erenumab and placebo during the placebo‐controlled periods of studies,” the researchers said.
The study was sponsored by Amgen, and several study authors are employees of Amgen or Novartis, the companies that market erenumab. Dr. Ashina is a consultant for Amgen, Novartis, and other companies.
SOURCE: Ashina M et al. AHS 2019, Abstract IOR10.
PHILADELPHIA – “Erenumab was well tolerated and safe, with no safety signals detected over this period,” said Messoud Ashina, MD, PhD, professor of neurology at the University of Copenhagen. Dr. Ashina presented the interim data from a 5-year, open-label extension study of erenumab at the annual meeting of the American Headache Society.
Erenumab is a monoclonal antibody that targets and blocks the calcitonin gene-related peptide (CGRP) receptor. In May 2018, the Food and Drug Administration approved erenumab for the preventive treatment of migraine in adults. The treatment, marketed as Aimovig, is administered once monthly by self-injection.
During the open-label study, patients initially received 70 mg of erenumab monthly. After approximately 2 years, patients switched to 140 mg of erenumab monthly. The researchers’ interim efficacy analysis included all patients on 140 mg of erenumab with data about monthly migraine days after more than 4 years of treatment. The safety analysis included all patients who enrolled in the open-label treatment period and received at least one dose of erenumab.
Of 250 patients who increased the erenumab dose from 70 mg to 140 mg, a total of 221 (88%) completed the open-label treatment period or remained on 140 mg after more than 4 years. Patients’ average number of monthly migraine days at study baseline was 8.7, and the average change from baseline to the most recent month in the interim analysis was –5.8.
During the most recent month of assessment, 77% of patients had at least a 50% reduction in monthly migraine days from baseline, 56% had at least a 75% reduction, and 33% had a 100% reduction.
Mean change from baseline in acute migraine‐specific medication treatment days was –4.6, from a baseline of 6.1.
Among the 383 patients who entered the open-label treatment period and received at least one dose of erenumab (mean age, 41.3; 79% female), the median erenumab exposure was 58.5 months. The exposure‐adjusted incidence of adverse events per 100 patient‐years was 124.9, and the three most frequent adverse events (per 100 patient-years) were nasopharyngitis (10.9), upper respiratory tract infection (6.8), and influenza (4.7). The exposure‐adjusted incidence rate per 100 patient‐years for constipation was 1.3 (9/383) for 70-mg erenumab and 2.6 (15/250) for 140-mg erenumab.
“The exposure‐adjusted incidence rate per 100 patient‐years of serious adverse events was 3.8, similar to the rate observed for erenumab and placebo during the placebo‐controlled periods of studies,” the researchers said.
The study was sponsored by Amgen, and several study authors are employees of Amgen or Novartis, the companies that market erenumab. Dr. Ashina is a consultant for Amgen, Novartis, and other companies.
SOURCE: Ashina M et al. AHS 2019, Abstract IOR10.
PHILADELPHIA – “Erenumab was well tolerated and safe, with no safety signals detected over this period,” said Messoud Ashina, MD, PhD, professor of neurology at the University of Copenhagen. Dr. Ashina presented the interim data from a 5-year, open-label extension study of erenumab at the annual meeting of the American Headache Society.
Erenumab is a monoclonal antibody that targets and blocks the calcitonin gene-related peptide (CGRP) receptor. In May 2018, the Food and Drug Administration approved erenumab for the preventive treatment of migraine in adults. The treatment, marketed as Aimovig, is administered once monthly by self-injection.
During the open-label study, patients initially received 70 mg of erenumab monthly. After approximately 2 years, patients switched to 140 mg of erenumab monthly. The researchers’ interim efficacy analysis included all patients on 140 mg of erenumab with data about monthly migraine days after more than 4 years of treatment. The safety analysis included all patients who enrolled in the open-label treatment period and received at least one dose of erenumab.
Of 250 patients who increased the erenumab dose from 70 mg to 140 mg, a total of 221 (88%) completed the open-label treatment period or remained on 140 mg after more than 4 years. Patients’ average number of monthly migraine days at study baseline was 8.7, and the average change from baseline to the most recent month in the interim analysis was –5.8.
During the most recent month of assessment, 77% of patients had at least a 50% reduction in monthly migraine days from baseline, 56% had at least a 75% reduction, and 33% had a 100% reduction.
Mean change from baseline in acute migraine‐specific medication treatment days was –4.6, from a baseline of 6.1.
Among the 383 patients who entered the open-label treatment period and received at least one dose of erenumab (mean age, 41.3; 79% female), the median erenumab exposure was 58.5 months. The exposure‐adjusted incidence of adverse events per 100 patient‐years was 124.9, and the three most frequent adverse events (per 100 patient-years) were nasopharyngitis (10.9), upper respiratory tract infection (6.8), and influenza (4.7). The exposure‐adjusted incidence rate per 100 patient‐years for constipation was 1.3 (9/383) for 70-mg erenumab and 2.6 (15/250) for 140-mg erenumab.
“The exposure‐adjusted incidence rate per 100 patient‐years of serious adverse events was 3.8, similar to the rate observed for erenumab and placebo during the placebo‐controlled periods of studies,” the researchers said.
The study was sponsored by Amgen, and several study authors are employees of Amgen or Novartis, the companies that market erenumab. Dr. Ashina is a consultant for Amgen, Novartis, and other companies.
SOURCE: Ashina M et al. AHS 2019, Abstract IOR10.
REPORTING FROM AHS 2019
Semen cryopreservation viable for young transgender patients in small study
as transgender women, according to results of a small retrospective cohort study.
The lack of data on this topic, however, makes it difficult to determine how long an individual must be off gender-affirming therapy before spermatogenesis resumes, if it resumes, and what the long-term effects of gender-affirming therapy are.
“This information is critical to address as part of a multidisciplinary fertility discussion with youth and their guardians so that an informed decision can be made regarding fertility preservation use,” wrote Emily P. Barnard, DO, of UPMC Magee-Womens Hospital in Pittsburgh and her associates.
The researchers retrospectively collected data on transgender patients who sought fertility preservation between 2015 and 2018.
The 11 white transgender women (sex assigned male at birth) who followed up on adolescent medicine or pediatric endocrinology referrals for fertility preservation received their consultations between ages 16 and 24, with 19 years having been the median age at which they occurred. Gender dysphoria onset happened at a median age of 12 for the patients, who were evaluated for it at a median age of 17.
All but one patient submitted at least one semen sample, and eight ultimately cryopreserved their semen.
The eight samples from gender-affirming therapy–naive patients had abnormal morphology, with the median morphology having been 6% versus the normal range of greater than 13.0%. Otherwise, the samples collected were normal, but the authors noted that established semen analysis parameters don’t exist for adolescents and young adults.
All eight patients who had their semen cryopreserved, began gonadotropin-releasing hormone (GnRH) agonist therapy after cryopreservation, and four of those patients concurrently began taking estradiol.
One patient had already been taking intramuscular leuprolide acetate every 6 months and discontinued it to attempt fertility preservation. Spermatogenesis returned after 5 months of azoospermia, albeit with abnormal morphology (9%).
Another patient had been taking spironolactone and estradiol for 26 months before ceasing therapy to attempt fertility preservation. She remained azoospermic 4 months after stopping therapy and then moved forward with an orchiectomy.
“For many transgender patients, the potential need to discontinue GnRH agonist or gender-affirming therapy to allow for resumption of spermatogenesis may be a significant barrier to pursuing fertility preservation because cessation of therapy may result in exacerbation of gender dysphoria and progression of undesired male secondary sex characteristics,” the researchers wrote. “For individuals for whom this risk is not acceptable or if azoospermia is noted on semen analysis, there are several alternate options, including electroejaculation, testicular sperm extraction, and testicular tissue cryopreservation,” they continued. Electroejaculation with a transrectal probe is an option particularly for those who cannot masturbate or feel uncomfortable doing so, the authors explained.
For those who have not previously received gender-affirming therapy, the fertility preservation “process can be completed quickly, with collections occurring every 2 to 3 days to preserve several samples before initiating GnRH agonist or gender-affirming therapy,” they concluded.
SOURCE: Barnard EP et al. Pediatrics. 2019 Aug 5. doi: 10.1542/peds.2018-3943.
The lack of long-term data on various gender-affirming medical interventions, particularly hormone therapies, for transgender adolescents and young adults has led professional medical organizations to recommend patients receive fertility counseling before beginning any such therapies.
Yet few data exist on fertility preservation either. The study by Barnard et al. is the first to examine semen cryopreservation outcomes in adolescents and young adults assigned male at birth and asserting a female gender identity.
“There is often urgency to start medical affirming interventions (MAI) among transgender and gender-diverse adolescents and young adults (TGD-AYA) due to gender dysphoria and related psychological sequelae,” wrote Jason Rafferty, MD, MPH, in an accompanying editorial. “However, starting MAI immediately and delaying fertility services may lead to increased overall morbidity for some patients.”
Although multiple professional organizations recommend fertility counseling before MAI initiation, many transgender patients are not following this advice. Dr. Rafferty noted one study found only 20% of TGD-AYA discussed fertility with their physicians before beginning MAI, and only 13% discussed possible effects of MAI on fertility – yet 60% wanted to learn more.
“Barnard et al. review data suggesting TGD-AYA have low interest in fertility services, but many TGD-AYA questioned whether this may later change,” Dr. Rafferty wrote. “After starting MAIs, TGD-AYA report being more emotionally capable of considering future parenting because of increasing comfort with their bodies and romantic relationships.”
Various barriers also exist for TGD-AYA interested in fertility services, such as cost, lack of insurance coverage, low availability of services, increased dysphoria from the procedures, stereotypes, stigma, and interest in starting MAI as soon as possible.
“Under a reproductive justice framework, autonomy around family planning is a right that should not be limited by structural or systemic barriers,” Dr. Rafferty wrote. “Overall, there is a clinical and ethical imperative to better understand and provide access to fertility services for TGD-AYA.”
Jason Rafferty, MD, MPH, is a pediatrician and child psychiatrist who practices at the gender and sexuality clinic in Riverside and at the Adolescent Healthcare Center at Hasbro Children’s Hospital in Providence, R.I. His comments are summarized from an accompanying editorial (Pediatrics 2019 Aug 5. doi: 10.1542/peds.2019-2000).
The lack of long-term data on various gender-affirming medical interventions, particularly hormone therapies, for transgender adolescents and young adults has led professional medical organizations to recommend patients receive fertility counseling before beginning any such therapies.
Yet few data exist on fertility preservation either. The study by Barnard et al. is the first to examine semen cryopreservation outcomes in adolescents and young adults assigned male at birth and asserting a female gender identity.
“There is often urgency to start medical affirming interventions (MAI) among transgender and gender-diverse adolescents and young adults (TGD-AYA) due to gender dysphoria and related psychological sequelae,” wrote Jason Rafferty, MD, MPH, in an accompanying editorial. “However, starting MAI immediately and delaying fertility services may lead to increased overall morbidity for some patients.”
Although multiple professional organizations recommend fertility counseling before MAI initiation, many transgender patients are not following this advice. Dr. Rafferty noted one study found only 20% of TGD-AYA discussed fertility with their physicians before beginning MAI, and only 13% discussed possible effects of MAI on fertility – yet 60% wanted to learn more.
“Barnard et al. review data suggesting TGD-AYA have low interest in fertility services, but many TGD-AYA questioned whether this may later change,” Dr. Rafferty wrote. “After starting MAIs, TGD-AYA report being more emotionally capable of considering future parenting because of increasing comfort with their bodies and romantic relationships.”
Various barriers also exist for TGD-AYA interested in fertility services, such as cost, lack of insurance coverage, low availability of services, increased dysphoria from the procedures, stereotypes, stigma, and interest in starting MAI as soon as possible.
“Under a reproductive justice framework, autonomy around family planning is a right that should not be limited by structural or systemic barriers,” Dr. Rafferty wrote. “Overall, there is a clinical and ethical imperative to better understand and provide access to fertility services for TGD-AYA.”
Jason Rafferty, MD, MPH, is a pediatrician and child psychiatrist who practices at the gender and sexuality clinic in Riverside and at the Adolescent Healthcare Center at Hasbro Children’s Hospital in Providence, R.I. His comments are summarized from an accompanying editorial (Pediatrics 2019 Aug 5. doi: 10.1542/peds.2019-2000).
The lack of long-term data on various gender-affirming medical interventions, particularly hormone therapies, for transgender adolescents and young adults has led professional medical organizations to recommend patients receive fertility counseling before beginning any such therapies.
Yet few data exist on fertility preservation either. The study by Barnard et al. is the first to examine semen cryopreservation outcomes in adolescents and young adults assigned male at birth and asserting a female gender identity.
“There is often urgency to start medical affirming interventions (MAI) among transgender and gender-diverse adolescents and young adults (TGD-AYA) due to gender dysphoria and related psychological sequelae,” wrote Jason Rafferty, MD, MPH, in an accompanying editorial. “However, starting MAI immediately and delaying fertility services may lead to increased overall morbidity for some patients.”
Although multiple professional organizations recommend fertility counseling before MAI initiation, many transgender patients are not following this advice. Dr. Rafferty noted one study found only 20% of TGD-AYA discussed fertility with their physicians before beginning MAI, and only 13% discussed possible effects of MAI on fertility – yet 60% wanted to learn more.
“Barnard et al. review data suggesting TGD-AYA have low interest in fertility services, but many TGD-AYA questioned whether this may later change,” Dr. Rafferty wrote. “After starting MAIs, TGD-AYA report being more emotionally capable of considering future parenting because of increasing comfort with their bodies and romantic relationships.”
Various barriers also exist for TGD-AYA interested in fertility services, such as cost, lack of insurance coverage, low availability of services, increased dysphoria from the procedures, stereotypes, stigma, and interest in starting MAI as soon as possible.
“Under a reproductive justice framework, autonomy around family planning is a right that should not be limited by structural or systemic barriers,” Dr. Rafferty wrote. “Overall, there is a clinical and ethical imperative to better understand and provide access to fertility services for TGD-AYA.”
Jason Rafferty, MD, MPH, is a pediatrician and child psychiatrist who practices at the gender and sexuality clinic in Riverside and at the Adolescent Healthcare Center at Hasbro Children’s Hospital in Providence, R.I. His comments are summarized from an accompanying editorial (Pediatrics 2019 Aug 5. doi: 10.1542/peds.2019-2000).
as transgender women, according to results of a small retrospective cohort study.
The lack of data on this topic, however, makes it difficult to determine how long an individual must be off gender-affirming therapy before spermatogenesis resumes, if it resumes, and what the long-term effects of gender-affirming therapy are.
“This information is critical to address as part of a multidisciplinary fertility discussion with youth and their guardians so that an informed decision can be made regarding fertility preservation use,” wrote Emily P. Barnard, DO, of UPMC Magee-Womens Hospital in Pittsburgh and her associates.
The researchers retrospectively collected data on transgender patients who sought fertility preservation between 2015 and 2018.
The 11 white transgender women (sex assigned male at birth) who followed up on adolescent medicine or pediatric endocrinology referrals for fertility preservation received their consultations between ages 16 and 24, with 19 years having been the median age at which they occurred. Gender dysphoria onset happened at a median age of 12 for the patients, who were evaluated for it at a median age of 17.
All but one patient submitted at least one semen sample, and eight ultimately cryopreserved their semen.
The eight samples from gender-affirming therapy–naive patients had abnormal morphology, with the median morphology having been 6% versus the normal range of greater than 13.0%. Otherwise, the samples collected were normal, but the authors noted that established semen analysis parameters don’t exist for adolescents and young adults.
All eight patients who had their semen cryopreserved, began gonadotropin-releasing hormone (GnRH) agonist therapy after cryopreservation, and four of those patients concurrently began taking estradiol.
One patient had already been taking intramuscular leuprolide acetate every 6 months and discontinued it to attempt fertility preservation. Spermatogenesis returned after 5 months of azoospermia, albeit with abnormal morphology (9%).
Another patient had been taking spironolactone and estradiol for 26 months before ceasing therapy to attempt fertility preservation. She remained azoospermic 4 months after stopping therapy and then moved forward with an orchiectomy.
“For many transgender patients, the potential need to discontinue GnRH agonist or gender-affirming therapy to allow for resumption of spermatogenesis may be a significant barrier to pursuing fertility preservation because cessation of therapy may result in exacerbation of gender dysphoria and progression of undesired male secondary sex characteristics,” the researchers wrote. “For individuals for whom this risk is not acceptable or if azoospermia is noted on semen analysis, there are several alternate options, including electroejaculation, testicular sperm extraction, and testicular tissue cryopreservation,” they continued. Electroejaculation with a transrectal probe is an option particularly for those who cannot masturbate or feel uncomfortable doing so, the authors explained.
For those who have not previously received gender-affirming therapy, the fertility preservation “process can be completed quickly, with collections occurring every 2 to 3 days to preserve several samples before initiating GnRH agonist or gender-affirming therapy,” they concluded.
SOURCE: Barnard EP et al. Pediatrics. 2019 Aug 5. doi: 10.1542/peds.2018-3943.
as transgender women, according to results of a small retrospective cohort study.
The lack of data on this topic, however, makes it difficult to determine how long an individual must be off gender-affirming therapy before spermatogenesis resumes, if it resumes, and what the long-term effects of gender-affirming therapy are.
“This information is critical to address as part of a multidisciplinary fertility discussion with youth and their guardians so that an informed decision can be made regarding fertility preservation use,” wrote Emily P. Barnard, DO, of UPMC Magee-Womens Hospital in Pittsburgh and her associates.
The researchers retrospectively collected data on transgender patients who sought fertility preservation between 2015 and 2018.
The 11 white transgender women (sex assigned male at birth) who followed up on adolescent medicine or pediatric endocrinology referrals for fertility preservation received their consultations between ages 16 and 24, with 19 years having been the median age at which they occurred. Gender dysphoria onset happened at a median age of 12 for the patients, who were evaluated for it at a median age of 17.
All but one patient submitted at least one semen sample, and eight ultimately cryopreserved their semen.
The eight samples from gender-affirming therapy–naive patients had abnormal morphology, with the median morphology having been 6% versus the normal range of greater than 13.0%. Otherwise, the samples collected were normal, but the authors noted that established semen analysis parameters don’t exist for adolescents and young adults.
All eight patients who had their semen cryopreserved, began gonadotropin-releasing hormone (GnRH) agonist therapy after cryopreservation, and four of those patients concurrently began taking estradiol.
One patient had already been taking intramuscular leuprolide acetate every 6 months and discontinued it to attempt fertility preservation. Spermatogenesis returned after 5 months of azoospermia, albeit with abnormal morphology (9%).
Another patient had been taking spironolactone and estradiol for 26 months before ceasing therapy to attempt fertility preservation. She remained azoospermic 4 months after stopping therapy and then moved forward with an orchiectomy.
“For many transgender patients, the potential need to discontinue GnRH agonist or gender-affirming therapy to allow for resumption of spermatogenesis may be a significant barrier to pursuing fertility preservation because cessation of therapy may result in exacerbation of gender dysphoria and progression of undesired male secondary sex characteristics,” the researchers wrote. “For individuals for whom this risk is not acceptable or if azoospermia is noted on semen analysis, there are several alternate options, including electroejaculation, testicular sperm extraction, and testicular tissue cryopreservation,” they continued. Electroejaculation with a transrectal probe is an option particularly for those who cannot masturbate or feel uncomfortable doing so, the authors explained.
For those who have not previously received gender-affirming therapy, the fertility preservation “process can be completed quickly, with collections occurring every 2 to 3 days to preserve several samples before initiating GnRH agonist or gender-affirming therapy,” they concluded.
SOURCE: Barnard EP et al. Pediatrics. 2019 Aug 5. doi: 10.1542/peds.2018-3943.
FROM PEDIATRICS
COPD adds complexity to shared decision making for LDCT lung cancer screening
research suggests.
Jonathan M. Iaccarino, MD, of the pulmonary center at the Boston University, and coauthors reported the results of a secondary analysis of patient-level outcomes from 75,138 low-dose CT (LDCT) scans in 26,453 participants in the National Lung Screening Trial (Chest 2019 Jul 5. doi: 10.1016/j.chest.2019.06.016).
Currently, LDCT screening is recommended annually for high-risk smokers aged 55-80 years. The National Lung Screening Trial showed that this screening achieved a 20% relative reduction in lung cancer mortality and 6.7% relative reduction in overall mortality in this group. The guidelines stress the importance of shared decision making, with discussion of the risks and benefits of screening.
Dr. Iaccarino and colleagues point out that decision aids for shared decision making need to include important baseline characteristics, such as the presence of COPD, as these can complicate the risk and benefit analysis.
In this study, they found that 14.2% of LDCT scans performed led to a subsequent diagnostic study and 1.5% resulted in an invasive procedure. In addition, 0.3% of scans resulted in a procedure-related complication, and in 89 cases (0.1%), this procedure-related complication was serious.
At the patient level, nearly one-third (30.5%) received a diagnostic study, 4.2% underwent an invasive procedure – 41% of whom ultimately were found not to have lung cancer – 0.9% had a procedure-related complication, and 0.3% had a serious procedure related complication. Furthermore, among those who experienced a serious complication, 12.5% were found not to have lung cancer.
“Our study analyzes cumulative outcomes at the level of the individual patient over the three years of LDCT screening during the NLST, showing higher rates of diagnostic procedures, invasive procedures, complications and serious complications than apparent when data is presented at the level of the individual test,” the authors wrote.
The 4,632 participants with COPD were significantly more likely to undergo diagnostic studies (36.2%), have an invasive procedure (6%), experience a procedure-related complication (1.5%) and experience a serious procedure-related complication (0.6%) than were participants without COPD. However, they also had a significantly higher incidence of lung cancer diagnosis than did participants without COPD (6.1% vs. 3.6%).
“While most decision aids note the risks of screening may be increased in those with COPD, our study helps quantify these increased risks as well as the increased likelihood of a lung cancer diagnosis, a critical advance given that providing personalized (rather than generic) information results in more accurate risk perception and more informed choices among individuals considering screening,” the authors wrote. “With the significant change in the balance of benefits and risks of screening in patients with COPD, it is critical to adjust the shared decision-making discussions accordingly.”
They also noted that other comorbidities, such as heart disease, vascular disease, and other lung diseases, would likely affect the balance of risk and benefit of LDCT screening, and that there was a need for further exploration of screening in these patients.
Noting the study’s limitations, the authors pointed that their analysis focused on outcomes that were not the primary outcomes of the National Lung Screening trial, and that they relied on self-reported COPD diagnoses.
The study was supported by the American Society of Clinical Oncology, the Charles A. King Trust, and Edith Nourse Rogers Memorial Veterans Hospital. No conflicts of interest were declared.
SOURCE: Iaccarino JM et al. CHEST 2019 Jul 5. doi: 10.1016/j.chest.2019.06.016.
research suggests.
Jonathan M. Iaccarino, MD, of the pulmonary center at the Boston University, and coauthors reported the results of a secondary analysis of patient-level outcomes from 75,138 low-dose CT (LDCT) scans in 26,453 participants in the National Lung Screening Trial (Chest 2019 Jul 5. doi: 10.1016/j.chest.2019.06.016).
Currently, LDCT screening is recommended annually for high-risk smokers aged 55-80 years. The National Lung Screening Trial showed that this screening achieved a 20% relative reduction in lung cancer mortality and 6.7% relative reduction in overall mortality in this group. The guidelines stress the importance of shared decision making, with discussion of the risks and benefits of screening.
Dr. Iaccarino and colleagues point out that decision aids for shared decision making need to include important baseline characteristics, such as the presence of COPD, as these can complicate the risk and benefit analysis.
In this study, they found that 14.2% of LDCT scans performed led to a subsequent diagnostic study and 1.5% resulted in an invasive procedure. In addition, 0.3% of scans resulted in a procedure-related complication, and in 89 cases (0.1%), this procedure-related complication was serious.
At the patient level, nearly one-third (30.5%) received a diagnostic study, 4.2% underwent an invasive procedure – 41% of whom ultimately were found not to have lung cancer – 0.9% had a procedure-related complication, and 0.3% had a serious procedure related complication. Furthermore, among those who experienced a serious complication, 12.5% were found not to have lung cancer.
“Our study analyzes cumulative outcomes at the level of the individual patient over the three years of LDCT screening during the NLST, showing higher rates of diagnostic procedures, invasive procedures, complications and serious complications than apparent when data is presented at the level of the individual test,” the authors wrote.
The 4,632 participants with COPD were significantly more likely to undergo diagnostic studies (36.2%), have an invasive procedure (6%), experience a procedure-related complication (1.5%) and experience a serious procedure-related complication (0.6%) than were participants without COPD. However, they also had a significantly higher incidence of lung cancer diagnosis than did participants without COPD (6.1% vs. 3.6%).
“While most decision aids note the risks of screening may be increased in those with COPD, our study helps quantify these increased risks as well as the increased likelihood of a lung cancer diagnosis, a critical advance given that providing personalized (rather than generic) information results in more accurate risk perception and more informed choices among individuals considering screening,” the authors wrote. “With the significant change in the balance of benefits and risks of screening in patients with COPD, it is critical to adjust the shared decision-making discussions accordingly.”
They also noted that other comorbidities, such as heart disease, vascular disease, and other lung diseases, would likely affect the balance of risk and benefit of LDCT screening, and that there was a need for further exploration of screening in these patients.
Noting the study’s limitations, the authors pointed that their analysis focused on outcomes that were not the primary outcomes of the National Lung Screening trial, and that they relied on self-reported COPD diagnoses.
The study was supported by the American Society of Clinical Oncology, the Charles A. King Trust, and Edith Nourse Rogers Memorial Veterans Hospital. No conflicts of interest were declared.
SOURCE: Iaccarino JM et al. CHEST 2019 Jul 5. doi: 10.1016/j.chest.2019.06.016.
research suggests.
Jonathan M. Iaccarino, MD, of the pulmonary center at the Boston University, and coauthors reported the results of a secondary analysis of patient-level outcomes from 75,138 low-dose CT (LDCT) scans in 26,453 participants in the National Lung Screening Trial (Chest 2019 Jul 5. doi: 10.1016/j.chest.2019.06.016).
Currently, LDCT screening is recommended annually for high-risk smokers aged 55-80 years. The National Lung Screening Trial showed that this screening achieved a 20% relative reduction in lung cancer mortality and 6.7% relative reduction in overall mortality in this group. The guidelines stress the importance of shared decision making, with discussion of the risks and benefits of screening.
Dr. Iaccarino and colleagues point out that decision aids for shared decision making need to include important baseline characteristics, such as the presence of COPD, as these can complicate the risk and benefit analysis.
In this study, they found that 14.2% of LDCT scans performed led to a subsequent diagnostic study and 1.5% resulted in an invasive procedure. In addition, 0.3% of scans resulted in a procedure-related complication, and in 89 cases (0.1%), this procedure-related complication was serious.
At the patient level, nearly one-third (30.5%) received a diagnostic study, 4.2% underwent an invasive procedure – 41% of whom ultimately were found not to have lung cancer – 0.9% had a procedure-related complication, and 0.3% had a serious procedure related complication. Furthermore, among those who experienced a serious complication, 12.5% were found not to have lung cancer.
“Our study analyzes cumulative outcomes at the level of the individual patient over the three years of LDCT screening during the NLST, showing higher rates of diagnostic procedures, invasive procedures, complications and serious complications than apparent when data is presented at the level of the individual test,” the authors wrote.
The 4,632 participants with COPD were significantly more likely to undergo diagnostic studies (36.2%), have an invasive procedure (6%), experience a procedure-related complication (1.5%) and experience a serious procedure-related complication (0.6%) than were participants without COPD. However, they also had a significantly higher incidence of lung cancer diagnosis than did participants without COPD (6.1% vs. 3.6%).
“While most decision aids note the risks of screening may be increased in those with COPD, our study helps quantify these increased risks as well as the increased likelihood of a lung cancer diagnosis, a critical advance given that providing personalized (rather than generic) information results in more accurate risk perception and more informed choices among individuals considering screening,” the authors wrote. “With the significant change in the balance of benefits and risks of screening in patients with COPD, it is critical to adjust the shared decision-making discussions accordingly.”
They also noted that other comorbidities, such as heart disease, vascular disease, and other lung diseases, would likely affect the balance of risk and benefit of LDCT screening, and that there was a need for further exploration of screening in these patients.
Noting the study’s limitations, the authors pointed that their analysis focused on outcomes that were not the primary outcomes of the National Lung Screening trial, and that they relied on self-reported COPD diagnoses.
The study was supported by the American Society of Clinical Oncology, the Charles A. King Trust, and Edith Nourse Rogers Memorial Veterans Hospital. No conflicts of interest were declared.
SOURCE: Iaccarino JM et al. CHEST 2019 Jul 5. doi: 10.1016/j.chest.2019.06.016.
FROM CHEST
Gastrointestinal Stromal Tumors: Management of Advanced Disease
Most advanced gastrointestinal stromal tumors (GISTs) are due to a recurrence of localized disease, with only a small minority presenting with metastatic disease.1 Compared with chemotherapy, tyrosine kinase inhibitors (TKIs) have significantly improved the natural history of the disease, with median overall survival (OS) increasing from less than 1 year to about 5 years and approximately 1 in 5 patients achieving long-term survival.2 In addition, newer drugs in development and in clinical trials appear promising and have the potential to improve outcomes even further. This article reviews current evidence on options for treating metastatic or recurrent GISTs and GISTs that have progressed following initial therapy. The evaluation and diagnosis of GIST along with management of localized disease are reviewed in a separate article.
Case Presentation
A 64-year-old African American man underwent surgical resection of a 10-cm gastric mass, which pathology reported was positive for CD117, DOG1, and CD34 and negative for smooth muscle actin and S-100, consistent with a diagnosis of GIST. There were 10 mitoses per 50 HPF, and there was no intraoperative or intraperitoneal tumor rupture. The patient was treated with adjuvant imatinib, which was discontinued after 3 years due to grade 2 myalgias, periorbital edema, and macrocytic anemia. Surveillance included office visits every 3 to 6 months and a contrast CT abdomen and pelvis every 6 months. For the past 5 years, he has not had any clinical or radiographic evidence of disease recurrence. New imaging reveals multiple liver metastases and peritoneal implants. He feels fatigued and has lost about 10 lb since his last visit. He is 5 years out from his initial diagnosis and 2 years out from last receiving imatinib. His original tumor harbored a KIT exon 11 deletion.
What treatment should you recommend now?
Imatinib for Advanced GISTs
Before the first report of the efficacy of imatinib for metastatic GISTs in 2002, patients with advanced unresectable or metastatic GISTs were routinely treated with doxorubicin-based chemotherapy regimens, which were largely ineffective, with response rates (RRs) of around 5% and a median overall survival (OS) of less than 1 year.3,4 In 2002 a landmark phase 2 study revealed imatinib’s significant efficacy profile in advanced or metastatic GISTs, resulting in its approval by the US Food and Drug Administration (FDA).5 In this study, 147 patients with CD117-positive GISTs were randomly assigned to receive daily imatinib 400 mg or 600 mg for up to 36 months. The RRs were similar between the 2 groups (68.5% vs 67.6%), with a median time to response of 12 weeks and median duration of response of 118 days. Results of this study were much more favorable when compared to doxorubicin, rendering imatinib the new standard of care for advanced GISTs. A long-term follow-up of this study after a median of 63 months confirmed near identical RRs, progression-free survival (PFS), and median survival of 57 months among the 2 groups.6
Imatinib Daily Dosing
Although 400 mg of daily imatinib proved to be efficacious, it was unclear if a dose-response relationship existed for imatinib. An EORTC phase 2 study demonstrated a benefit of using a higher dose of imatinib at 400 mg twice daily, producing a RR of 71% (4% complete , 67% partial) and 1-year PFS of 73%, which appeared favorable compared with once-daily dosing and set the framework for larger phase 3 studies.7 Two phase 3 studies compared imatinib 400 mg once daily versus twice daily (until disease progression or unacceptable toxicity) among patients with CD117-positive advanced or metastatic GISTs. These studies were eventually combined into a meta-analysis (metaGIST) to compare RR, PFS and OS between the treatment groups. Both studies allowed cross-over to the 800 mg dose for patients who progressed on 400 mg daily.
The first study, conducted jointly by the EORTC, Italian Sarcoma Group, and Australasian Gastro-Intestinal Trials Group (EU-AUS),8 randomly assigned 946 patients to 400 mg once daily or twice daily. There were no differences in response rates between the groups, but the twice-daily group had a predicted 18% reduction in the hazard for progression compared with the once-daily group (estimated HR, 0.82; P = 0.026), which came at the expense of greater toxicities warranting dose reductions (60%) and treatment interruptions (64%). Cross-over to high-dose imatinib was feasible and safe, producing a partial response in 2%, stable disease in 27%, and a median PFS of 81 days. The second study was an intergroup study conducted jointly by SWOG, CALGB, NCI-C, and ECOG (S0033, US-CDN), with a nearly identical study design as the EU-AUS trial.9 The trial enrolled 746 patients. After a median follow up of 4.5 years, the median PFS and OS were not statistically different (18 vs 20 months and 55 vs 51 months, respectively). There were also no differences in response rates. One third of patients initially placed on the once-daily arm who crossed over after progression achieved a treatment response or stable disease.
The combined EU-AUS and US-CDN analysis (metaGIST) included 1640 patients with a median age of 60 years and 58% of whom were men; 818 and 822 patients were assigned to the 400 mg and 800 mg total daily doses, respectively.10 The median follow-up was 37.5 months. There were no differences in OS (49 vs 48.7 months), median PFS (18.9 vs 23.2 months), or overall response rates (51.4% vs 53.9%). Patients who had crossed over (n = 347) to the 800 mg total daily dose arm had a 7.7-month average PFS while on the higher daily dose. An analysis was performed on 377 patients in the EU-AUS trial assessing the impact of mutational status on clinical outcomes among imatinib-treated patients. KIT exon 9 activating mutations were found to be a significant independent prognostic factor for death when compared with KIT exon 11 mutations. However, the adverse prognostic value of KIT exon 9 mutations was partially overcome with higher doses of imatinib, as those who received 800 mg total had a significantly better PFS, with a 61% relative risk reduction, than those who received 400 mg. Altogether, it was concluded that imatinib 400 mg once daily should be the standard-of-care first-line treatment for advanced or metastatic GISTs, unless a KIT exon 9 mutation is present, in which case imatinib 800 mg should be considered, if 400 mg is well tolerated. In addition, patients treated with frontline imatinib at 400 mg once daily, if tolerated well, should be considered for imatinib 800 mg upon progression of disease.
Despite there being problems with secondary resistance, significant progress has occurred in the treatment of metastatic disease over a short period of time. Prior to 2000, median OS for patients with metastatic GISTs was 9 months. With the introduction of imatinib and other TKIs, the median OS has increased to 5 years, with an estimated 10-year OS rate of approximately 20%.2
Imatinib Interruption
Since at this point, imatinib was a well-established standard of care for advanced GISTs, it was questioned whether imatinib therapy could be interrupted. At this time, treatment interruption in a stop-and-go fashion was deemed feasible in other metastatic solid tumors such as colorectal cancer (OPTIMOX1).11 The BFR French trial showed that stopping imatinib therapy in patients who had a response or stable disease after 1, 3, or 5 years was generally followed by relatively rapid tumor progression (approximately 50% of patients within 6 months), even when tumors were previously removed.12 Therefore, it is recommended that treatment in the metastatic setting should be continued indefinitely, unless there is disease progression. Hence, unlike with colorectal cancer or chronic myelogenous leukemia, as of now there is no role for imatinib interruption in metastatic GISTs.
Case Continued
The patient is started on imatinib 400 mg daily, and overall he tolerates therapy well. Interval CT imaging reveals a treatment response. Two years later, imaging reveals an increase in the tumor size and density with a new nodule present within a preexisting mass. There are no clinical trials in the area.
What defines tumor progression?
Disease Progression
When GISTs are responding to treatment, on imaging the tumors can become more cystic and less dense but with an increase in size. In addition, tumor progression may not always be associated with increased size—increased density of the tumor or a nodule within a mass that may indicate progression. If CT imaging is equivocal for progression, positron emission tomography (PET) can play a role in identifying true progression. It is critically important that tumor size and density are carefully assessed when performing interval imaging. Of note, radiofrequency ablation, cryotherapy, or chemoembolization can be used for symptomatic liver metastases or oligometastatic disease. When evaluating for progression, one needs to ask patients about compliance (ie, maintaining dose intensity related to side effects of therapy as well as the financial burden of treatment—copay toxicity).
What are mechanisms of secondary imatinib resistance?
Imatinib resistance can be subtle in patients with GISTs, manifesting with new nodular, enhancing foci enclosed within a preexisting mass (resistant clonal nodule), or can be clinically or radiographically overt.13 Imatinib resistance occurs through multiple mechanisms including acquisition of secondary activating KIT mutations in the intracellular ATP-binding domain (exons 13 and 14) and the activation loop (exons 17 and 18).14
What are the treatment options for this patient?
Second-line Therapy
Sunitinib malate is a multitargeted TKI that not only targets c-Kit and PDGFRA, but also has anti-angiogenic activity through inhibition of vascular endothelial growth factor receptors (VEGFR). Sunitinib gained FDA approval for the second-line treatment of advanced GISTs based on an international double-blind trial that randomized 312 patients with imatinib-resistant metastatic GISTs in a 2:1 fashion to receive sunitinib 50 mg daily for 4 weeks on and 2 weeks off or placebo.15,16 The trial was unblinded early at the planned interim analysis, which revealed a marked benefit, producing a 66% reduction in the hazard risk of progression (27.3 vs 6.4 weeks, HR, 0.33; P < 0.001). The most common treatment-related adverse events were fatigue, diarrhea, skin discoloration, nausea, and hand-foot syndrome. Another open-label phase 2 study assessed a continuous dosing schema of sunitinib 37.5 mg daily, which has been shown to be effective with less toxicity.17 Among the 60 patients enrolled, the primary endpoint of clinical benefit rate at 24 weeks was reached in 53%, which consisted of 13% partial responses and 40% stable disease. Most toxicities were grade 1 or 2 and easily manageable through standard interventions. This has been recommended as an alternative to the initial scheduled regimen.18 Part of sunitinib’s success is its activity against GISTs harboring secondary KIT exon 13 and 14 mutations, and possibly its anti-angiogenic activity.19 Sunitinib is particularly efficacious among GISTs harboring KIT exon 9 mutations.
Third-line Therapy
Patients who have progressed on prior imatinib and sunitinib can receive third-line regorafenib, a multi-TKI that differs chemically from sorafenib by a fluorouracil group (fluoro-sorafenib). FDA approval of regorafenib was based on the phase 3 GRID (GIST Regorafenib In progressive Disease) multicenter international trial.20 This trial randomly assigned 199 patients in a 2:1 fashion to receive regorafenib 160 mg daily for 21 days out of 28-day cycles plus best supportive care (BSC) versus placebo plus BSC. Cross-over was allowed. Regorafenib significantly reduced the hazard risk of progression by 73% compared with placebo (4.8 vs 0.9 months; HR, 0.27; P < 0.001). There was no difference in OS, which may be because of cross-over (median OS, 17.4 months in both arms). As a result, regorafenib is now considered standard third-line treatment for patients with metastatic GISTs. It has a less favorable toxicity profile than imatinib, with hand-foot syndrome, transaminitis, hypertension and fatigue being the most common treatment toxicities. In order to avoid noncompliance, it is recommended to start at 80 mg and carefully titrate upwards to the 160 mg dose.
A list of landmark studies for advanced GISTs is provided in Table 1.
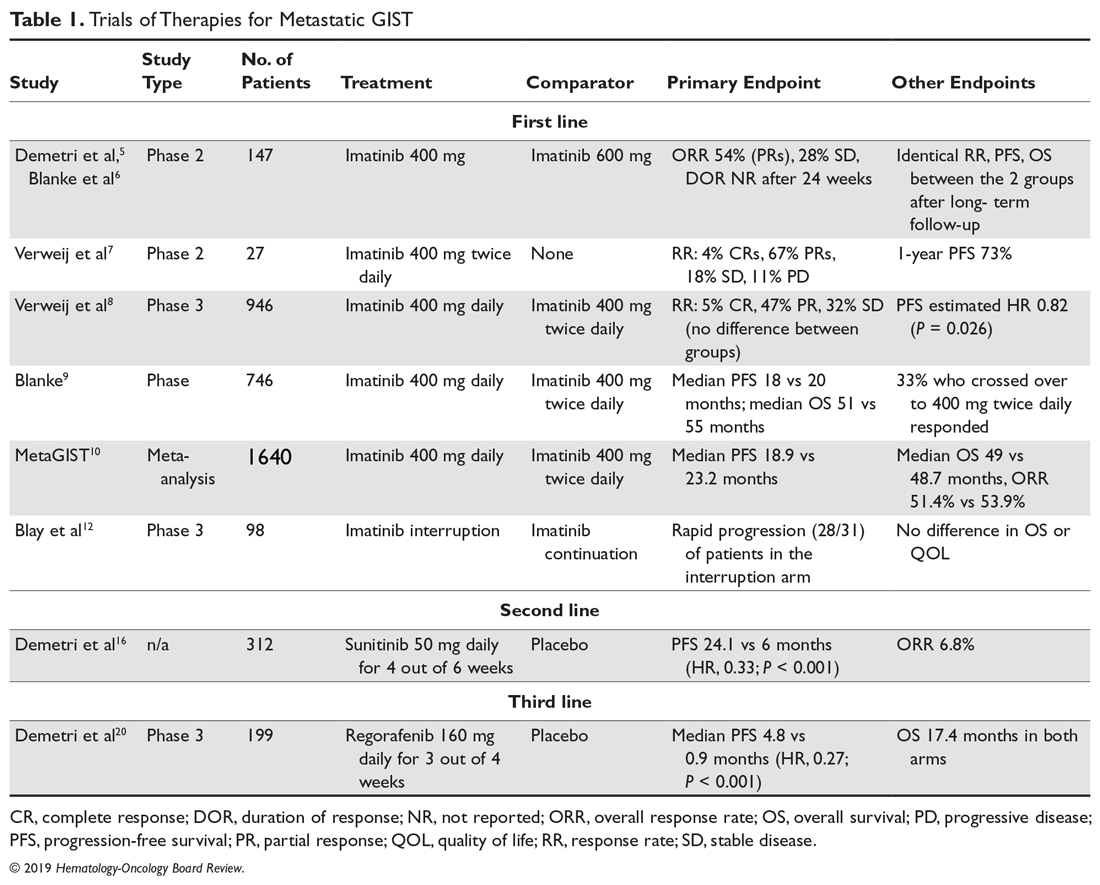
A summary of FDA-approved drugs for treating GISTs is provided in Table 2.
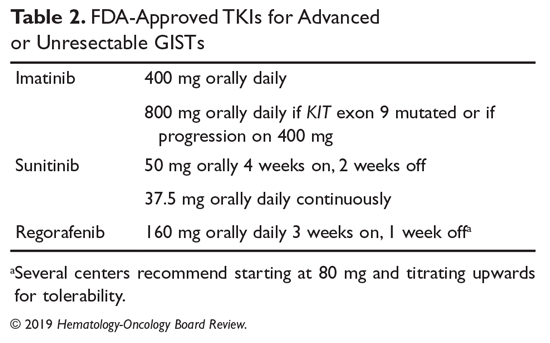
Clinical Trials
Clinical trial enrollment should be considered for all patients with advanced or unresectable GISTs throughout their treatment continuum. Owing to significant advances in genomic profiling through next-generation sequencing, multiple driver mutations have recently been identified, and targeted therapies are being explored in clinical trials.21 For example, the neurotrophic receptor tyrosine kinase (NTRK) gene appears to be mutated in a small number of advanced GISTs, and these can respond to the highly selective TRK inhibitor larotrectinib.22 Additionally, ongoing studies are assessing immunotherapies for sporadic GISTs and treatment for familial GISTs (Table 3). Some notable studies include those assessing the efficacy of agents that target KIT and PDGFR secondary mutations, including avapritinib (BLU-285) and DCC-2618, MEK inhibitors, and the multi-kinase inhibitor crenolanib for GISTs harboring the imatinib-resistant PDGFRA D842V mutation. There are also studies utilizing checkpoint inhibitors alone or in combination with imatinib.
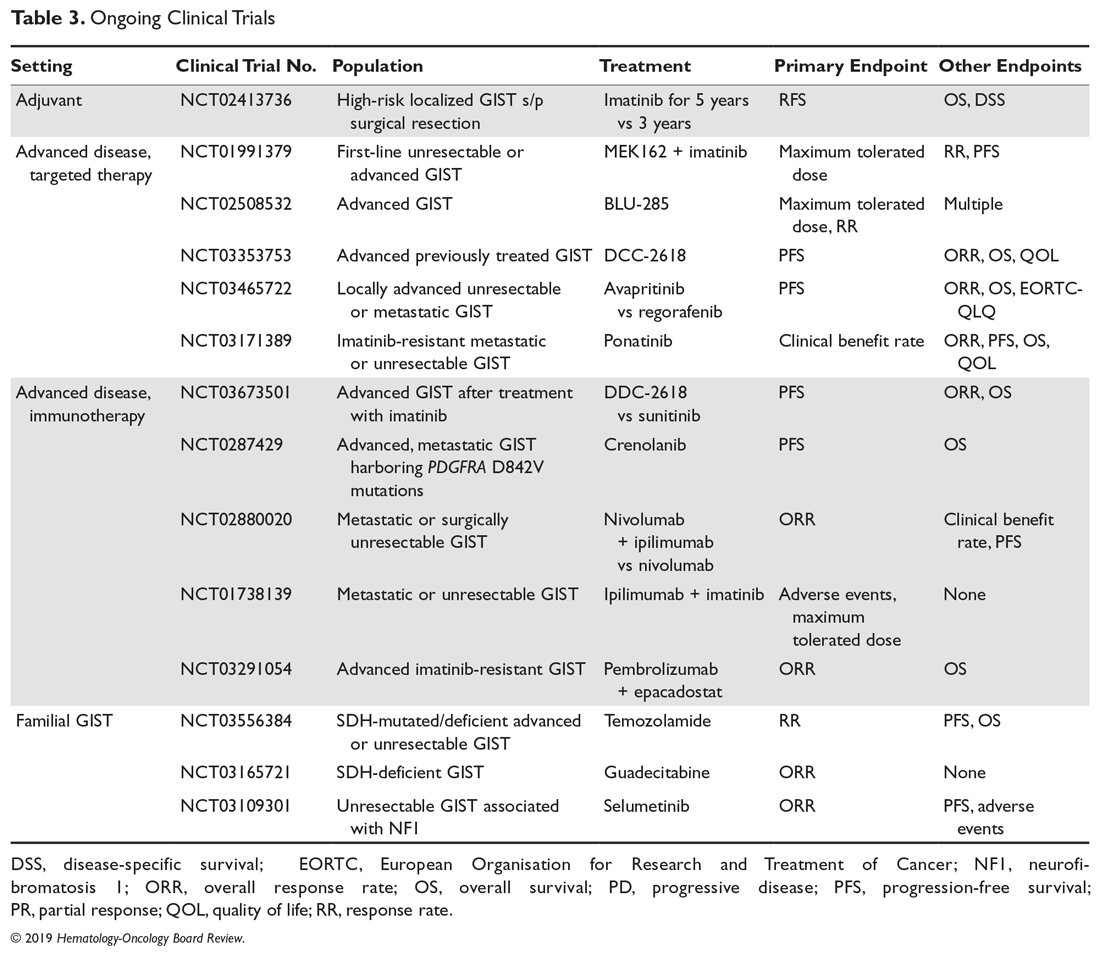
Case Conclusion
Given the patient’s progression on imatinib, he is started on second-line sunitinib malate. He experiences grade 1 fatigue and hand-foot syndrome, which are managed supportively. After he has been on sunitinib for approximately 8 months, his disease progresses. He subsequently undergoes genomic profiling of his tumor and starts BLU-285 on a clinical trial.
Key Points
- For advanced and metastatic disease, TKIs have substantially improved the prognosis of KIT mutated GISTs, with 3 FDA-approved drugs: imatinib, sunitinib, and regorafenib. Imatinib 400 mg is the standard-of-care frontline therapy for locally advanced, unresectable, or metastatic imatinib-sensitive GISTs. If a patient has a KIT exon 9 mutation and 400 mg is well-tolerated, increasing to 800 mg is recommended. Imatinib should be continued indefinitely unless there is intolerance, a specific patient request for interruption, or progression of disease.
- When there is progression of disease in a patient with a sensitive mutation on 400 mg of imatinib, the dose can be increased to 800 mg.
- For patients who are imatinib-intolerant or have progression, standard second line is sunitinib.
- For patients who further progress or are sunitinib-intolerant, regorafenib is the standard third-line treatment.
- There needs to be close attention to side effects, drug and food interactions, and patient copay costs in order to maintain patient compliance while on TKI therapy.
- There are still major limitations in the systemic treatment of GISTs marked by their inherent genetic heterogeneity and secondary resistance. Continued translational and clinical research is needed in order to improve treatment for patients who develop secondary resistance or who have less common primary resistant mutations. Patients are encouraged to participate in clinical trials of new therapies.
Summary
GISTs are the most common mesenchymal tumors of the GI tract. They comprise an expanding landscape of tumors that are heterogenous in terms of natural history, mutations, and response to systemic treatments. The mainstay of treatment for localized GISTs is surgical resection followed by at least 3-years of adjuvant imatinib for patients with high-risk features who are imatinib-sensitive. Patients with GISTs harboring resistance mutations such as PDGFRA D842V or with SDH-deficient or NF1-associated GISTs should not receive adjuvant imatinib. Patients with more advanced GISTs and/or in difficult to resect sites harboring a sensitive mutation can be considered for neoadjuvant imatinib. Those with metastatic GISTs can receive first-, second-, and third-line imatinib, sunitinib, or regorafenib, respectively. Clinical trial enrollment should be encouraged for patients whose GISTs harbor primary imatinib-resistant mutations, and those with advanced or unresectable GISTs with secondary resistance.
1. Ma GL, Murphy JD, Martinez ME et al. Epidemiology of gastrointestinal stromal tumors in the era of histology codes: results of a population-based study. Cancer Epidemiol Biomarkers Prev. 2015;24:298-302.
2. Heinrich MC, Rankin C, Blanke CD, et al. Correlation of long-term results of imatinib in advanced gastrointestinal stromal tumors with next-generation sequencing results: analysis of phase 3 SWOG Intergroup Trial S0033. JAMA Oncol. 2017;3:944-952.
3. DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51-58.
4. Goss GA, Merriam P, Manola J, et al. Clinical and pathological characteristics of gastrointestinal stromal tumors (GIST). Prog Proc Am Soc Clin Oncol. 2000;19:599a.
5. Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002; 347:472-480.
6. Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase ii trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26:620-625.
7. Verweij J, van Oosterom A, Blay JY, et al. Imatinib mesylate (STI-571 Glivec, Gleevac) is an active agent for gastrointestinal stromal tumours, but does not yield responses in other soft-tissue sarcomas that are unselected for a molecular target. Results from an EORTC Soft Tissue and Bone Sarcoma Group phase II study. Eur J Cancer. 2003;39:2006-2011.
8. Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomized trial. Lancet. 2004;364:1127-1134.
9. Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626-632.
10. Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST). Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol. 2010;28:1247-1253.
11. Tournigand C, Cervantes A, Figer A, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer –a GERCOR study. J Clin Oncol. 2006;24:394-400.
12. Blay JV, Cesne AL, Ray-Coquard I, et al. Prospective multicentric randomized phase iii study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: The French Sarcoma Group. J Clin Oncol. 2007;25:1107-1113.
13. Desai J, Shankar S, Heinrich MC, et al. Clonal evolution of resistance to imatinib in patients with metastatic gastrointestinal stromal tumors. Clin Cancer Res. 2007;13(18 Pt 1): 5398-5405.
14. Gramza AW, Corless CL, Heinrich MC. Resistance to tyrosine kinase inhibitors in gastrointestinal stromal tumors. Clin Cancer Res. 2009;15:7510-7518.
15. Sutent (sunitinib malate) [package insert]. New York, NY: Pfizer Labs; 2017.
16. Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomized controlled trial. Lancet. 2006;368:1329-1338.
17. George S, Blay JY, Casali PG, et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer. 2009;45:1959-1968.
18. Brennan MF, Antonescu CR, Maki RG. Management of Soft Tissue Sarcomas. Switzerland: Springer International Publishing; 2013.
19. Heinrich MC, Maki RG, Corless CL, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumors. J Clin Oncol. 2008;26:5352-5359.
20. Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:295-302.
21. Wilky BA, Villalobos VM. Emerging role for precision therapy through next-generation sequencing for sarcomas. JCO Precision Oncology. 2018;2:1-4.
22. Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in trk fusion-positive cancers in adults and children. N Engl J Med. 2018;378:731-739.
Most advanced gastrointestinal stromal tumors (GISTs) are due to a recurrence of localized disease, with only a small minority presenting with metastatic disease.1 Compared with chemotherapy, tyrosine kinase inhibitors (TKIs) have significantly improved the natural history of the disease, with median overall survival (OS) increasing from less than 1 year to about 5 years and approximately 1 in 5 patients achieving long-term survival.2 In addition, newer drugs in development and in clinical trials appear promising and have the potential to improve outcomes even further. This article reviews current evidence on options for treating metastatic or recurrent GISTs and GISTs that have progressed following initial therapy. The evaluation and diagnosis of GIST along with management of localized disease are reviewed in a separate article.
Case Presentation
A 64-year-old African American man underwent surgical resection of a 10-cm gastric mass, which pathology reported was positive for CD117, DOG1, and CD34 and negative for smooth muscle actin and S-100, consistent with a diagnosis of GIST. There were 10 mitoses per 50 HPF, and there was no intraoperative or intraperitoneal tumor rupture. The patient was treated with adjuvant imatinib, which was discontinued after 3 years due to grade 2 myalgias, periorbital edema, and macrocytic anemia. Surveillance included office visits every 3 to 6 months and a contrast CT abdomen and pelvis every 6 months. For the past 5 years, he has not had any clinical or radiographic evidence of disease recurrence. New imaging reveals multiple liver metastases and peritoneal implants. He feels fatigued and has lost about 10 lb since his last visit. He is 5 years out from his initial diagnosis and 2 years out from last receiving imatinib. His original tumor harbored a KIT exon 11 deletion.
What treatment should you recommend now?
Imatinib for Advanced GISTs
Before the first report of the efficacy of imatinib for metastatic GISTs in 2002, patients with advanced unresectable or metastatic GISTs were routinely treated with doxorubicin-based chemotherapy regimens, which were largely ineffective, with response rates (RRs) of around 5% and a median overall survival (OS) of less than 1 year.3,4 In 2002 a landmark phase 2 study revealed imatinib’s significant efficacy profile in advanced or metastatic GISTs, resulting in its approval by the US Food and Drug Administration (FDA).5 In this study, 147 patients with CD117-positive GISTs were randomly assigned to receive daily imatinib 400 mg or 600 mg for up to 36 months. The RRs were similar between the 2 groups (68.5% vs 67.6%), with a median time to response of 12 weeks and median duration of response of 118 days. Results of this study were much more favorable when compared to doxorubicin, rendering imatinib the new standard of care for advanced GISTs. A long-term follow-up of this study after a median of 63 months confirmed near identical RRs, progression-free survival (PFS), and median survival of 57 months among the 2 groups.6
Imatinib Daily Dosing
Although 400 mg of daily imatinib proved to be efficacious, it was unclear if a dose-response relationship existed for imatinib. An EORTC phase 2 study demonstrated a benefit of using a higher dose of imatinib at 400 mg twice daily, producing a RR of 71% (4% complete , 67% partial) and 1-year PFS of 73%, which appeared favorable compared with once-daily dosing and set the framework for larger phase 3 studies.7 Two phase 3 studies compared imatinib 400 mg once daily versus twice daily (until disease progression or unacceptable toxicity) among patients with CD117-positive advanced or metastatic GISTs. These studies were eventually combined into a meta-analysis (metaGIST) to compare RR, PFS and OS between the treatment groups. Both studies allowed cross-over to the 800 mg dose for patients who progressed on 400 mg daily.
The first study, conducted jointly by the EORTC, Italian Sarcoma Group, and Australasian Gastro-Intestinal Trials Group (EU-AUS),8 randomly assigned 946 patients to 400 mg once daily or twice daily. There were no differences in response rates between the groups, but the twice-daily group had a predicted 18% reduction in the hazard for progression compared with the once-daily group (estimated HR, 0.82; P = 0.026), which came at the expense of greater toxicities warranting dose reductions (60%) and treatment interruptions (64%). Cross-over to high-dose imatinib was feasible and safe, producing a partial response in 2%, stable disease in 27%, and a median PFS of 81 days. The second study was an intergroup study conducted jointly by SWOG, CALGB, NCI-C, and ECOG (S0033, US-CDN), with a nearly identical study design as the EU-AUS trial.9 The trial enrolled 746 patients. After a median follow up of 4.5 years, the median PFS and OS were not statistically different (18 vs 20 months and 55 vs 51 months, respectively). There were also no differences in response rates. One third of patients initially placed on the once-daily arm who crossed over after progression achieved a treatment response or stable disease.
The combined EU-AUS and US-CDN analysis (metaGIST) included 1640 patients with a median age of 60 years and 58% of whom were men; 818 and 822 patients were assigned to the 400 mg and 800 mg total daily doses, respectively.10 The median follow-up was 37.5 months. There were no differences in OS (49 vs 48.7 months), median PFS (18.9 vs 23.2 months), or overall response rates (51.4% vs 53.9%). Patients who had crossed over (n = 347) to the 800 mg total daily dose arm had a 7.7-month average PFS while on the higher daily dose. An analysis was performed on 377 patients in the EU-AUS trial assessing the impact of mutational status on clinical outcomes among imatinib-treated patients. KIT exon 9 activating mutations were found to be a significant independent prognostic factor for death when compared with KIT exon 11 mutations. However, the adverse prognostic value of KIT exon 9 mutations was partially overcome with higher doses of imatinib, as those who received 800 mg total had a significantly better PFS, with a 61% relative risk reduction, than those who received 400 mg. Altogether, it was concluded that imatinib 400 mg once daily should be the standard-of-care first-line treatment for advanced or metastatic GISTs, unless a KIT exon 9 mutation is present, in which case imatinib 800 mg should be considered, if 400 mg is well tolerated. In addition, patients treated with frontline imatinib at 400 mg once daily, if tolerated well, should be considered for imatinib 800 mg upon progression of disease.
Despite there being problems with secondary resistance, significant progress has occurred in the treatment of metastatic disease over a short period of time. Prior to 2000, median OS for patients with metastatic GISTs was 9 months. With the introduction of imatinib and other TKIs, the median OS has increased to 5 years, with an estimated 10-year OS rate of approximately 20%.2
Imatinib Interruption
Since at this point, imatinib was a well-established standard of care for advanced GISTs, it was questioned whether imatinib therapy could be interrupted. At this time, treatment interruption in a stop-and-go fashion was deemed feasible in other metastatic solid tumors such as colorectal cancer (OPTIMOX1).11 The BFR French trial showed that stopping imatinib therapy in patients who had a response or stable disease after 1, 3, or 5 years was generally followed by relatively rapid tumor progression (approximately 50% of patients within 6 months), even when tumors were previously removed.12 Therefore, it is recommended that treatment in the metastatic setting should be continued indefinitely, unless there is disease progression. Hence, unlike with colorectal cancer or chronic myelogenous leukemia, as of now there is no role for imatinib interruption in metastatic GISTs.
Case Continued
The patient is started on imatinib 400 mg daily, and overall he tolerates therapy well. Interval CT imaging reveals a treatment response. Two years later, imaging reveals an increase in the tumor size and density with a new nodule present within a preexisting mass. There are no clinical trials in the area.
What defines tumor progression?
Disease Progression
When GISTs are responding to treatment, on imaging the tumors can become more cystic and less dense but with an increase in size. In addition, tumor progression may not always be associated with increased size—increased density of the tumor or a nodule within a mass that may indicate progression. If CT imaging is equivocal for progression, positron emission tomography (PET) can play a role in identifying true progression. It is critically important that tumor size and density are carefully assessed when performing interval imaging. Of note, radiofrequency ablation, cryotherapy, or chemoembolization can be used for symptomatic liver metastases or oligometastatic disease. When evaluating for progression, one needs to ask patients about compliance (ie, maintaining dose intensity related to side effects of therapy as well as the financial burden of treatment—copay toxicity).
What are mechanisms of secondary imatinib resistance?
Imatinib resistance can be subtle in patients with GISTs, manifesting with new nodular, enhancing foci enclosed within a preexisting mass (resistant clonal nodule), or can be clinically or radiographically overt.13 Imatinib resistance occurs through multiple mechanisms including acquisition of secondary activating KIT mutations in the intracellular ATP-binding domain (exons 13 and 14) and the activation loop (exons 17 and 18).14
What are the treatment options for this patient?
Second-line Therapy
Sunitinib malate is a multitargeted TKI that not only targets c-Kit and PDGFRA, but also has anti-angiogenic activity through inhibition of vascular endothelial growth factor receptors (VEGFR). Sunitinib gained FDA approval for the second-line treatment of advanced GISTs based on an international double-blind trial that randomized 312 patients with imatinib-resistant metastatic GISTs in a 2:1 fashion to receive sunitinib 50 mg daily for 4 weeks on and 2 weeks off or placebo.15,16 The trial was unblinded early at the planned interim analysis, which revealed a marked benefit, producing a 66% reduction in the hazard risk of progression (27.3 vs 6.4 weeks, HR, 0.33; P < 0.001). The most common treatment-related adverse events were fatigue, diarrhea, skin discoloration, nausea, and hand-foot syndrome. Another open-label phase 2 study assessed a continuous dosing schema of sunitinib 37.5 mg daily, which has been shown to be effective with less toxicity.17 Among the 60 patients enrolled, the primary endpoint of clinical benefit rate at 24 weeks was reached in 53%, which consisted of 13% partial responses and 40% stable disease. Most toxicities were grade 1 or 2 and easily manageable through standard interventions. This has been recommended as an alternative to the initial scheduled regimen.18 Part of sunitinib’s success is its activity against GISTs harboring secondary KIT exon 13 and 14 mutations, and possibly its anti-angiogenic activity.19 Sunitinib is particularly efficacious among GISTs harboring KIT exon 9 mutations.
Third-line Therapy
Patients who have progressed on prior imatinib and sunitinib can receive third-line regorafenib, a multi-TKI that differs chemically from sorafenib by a fluorouracil group (fluoro-sorafenib). FDA approval of regorafenib was based on the phase 3 GRID (GIST Regorafenib In progressive Disease) multicenter international trial.20 This trial randomly assigned 199 patients in a 2:1 fashion to receive regorafenib 160 mg daily for 21 days out of 28-day cycles plus best supportive care (BSC) versus placebo plus BSC. Cross-over was allowed. Regorafenib significantly reduced the hazard risk of progression by 73% compared with placebo (4.8 vs 0.9 months; HR, 0.27; P < 0.001). There was no difference in OS, which may be because of cross-over (median OS, 17.4 months in both arms). As a result, regorafenib is now considered standard third-line treatment for patients with metastatic GISTs. It has a less favorable toxicity profile than imatinib, with hand-foot syndrome, transaminitis, hypertension and fatigue being the most common treatment toxicities. In order to avoid noncompliance, it is recommended to start at 80 mg and carefully titrate upwards to the 160 mg dose.
A list of landmark studies for advanced GISTs is provided in Table 1.

A summary of FDA-approved drugs for treating GISTs is provided in Table 2.

Clinical Trials
Clinical trial enrollment should be considered for all patients with advanced or unresectable GISTs throughout their treatment continuum. Owing to significant advances in genomic profiling through next-generation sequencing, multiple driver mutations have recently been identified, and targeted therapies are being explored in clinical trials.21 For example, the neurotrophic receptor tyrosine kinase (NTRK) gene appears to be mutated in a small number of advanced GISTs, and these can respond to the highly selective TRK inhibitor larotrectinib.22 Additionally, ongoing studies are assessing immunotherapies for sporadic GISTs and treatment for familial GISTs (Table 3). Some notable studies include those assessing the efficacy of agents that target KIT and PDGFR secondary mutations, including avapritinib (BLU-285) and DCC-2618, MEK inhibitors, and the multi-kinase inhibitor crenolanib for GISTs harboring the imatinib-resistant PDGFRA D842V mutation. There are also studies utilizing checkpoint inhibitors alone or in combination with imatinib.

Case Conclusion
Given the patient’s progression on imatinib, he is started on second-line sunitinib malate. He experiences grade 1 fatigue and hand-foot syndrome, which are managed supportively. After he has been on sunitinib for approximately 8 months, his disease progresses. He subsequently undergoes genomic profiling of his tumor and starts BLU-285 on a clinical trial.
Key Points
- For advanced and metastatic disease, TKIs have substantially improved the prognosis of KIT mutated GISTs, with 3 FDA-approved drugs: imatinib, sunitinib, and regorafenib. Imatinib 400 mg is the standard-of-care frontline therapy for locally advanced, unresectable, or metastatic imatinib-sensitive GISTs. If a patient has a KIT exon 9 mutation and 400 mg is well-tolerated, increasing to 800 mg is recommended. Imatinib should be continued indefinitely unless there is intolerance, a specific patient request for interruption, or progression of disease.
- When there is progression of disease in a patient with a sensitive mutation on 400 mg of imatinib, the dose can be increased to 800 mg.
- For patients who are imatinib-intolerant or have progression, standard second line is sunitinib.
- For patients who further progress or are sunitinib-intolerant, regorafenib is the standard third-line treatment.
- There needs to be close attention to side effects, drug and food interactions, and patient copay costs in order to maintain patient compliance while on TKI therapy.
- There are still major limitations in the systemic treatment of GISTs marked by their inherent genetic heterogeneity and secondary resistance. Continued translational and clinical research is needed in order to improve treatment for patients who develop secondary resistance or who have less common primary resistant mutations. Patients are encouraged to participate in clinical trials of new therapies.
Summary
GISTs are the most common mesenchymal tumors of the GI tract. They comprise an expanding landscape of tumors that are heterogenous in terms of natural history, mutations, and response to systemic treatments. The mainstay of treatment for localized GISTs is surgical resection followed by at least 3-years of adjuvant imatinib for patients with high-risk features who are imatinib-sensitive. Patients with GISTs harboring resistance mutations such as PDGFRA D842V or with SDH-deficient or NF1-associated GISTs should not receive adjuvant imatinib. Patients with more advanced GISTs and/or in difficult to resect sites harboring a sensitive mutation can be considered for neoadjuvant imatinib. Those with metastatic GISTs can receive first-, second-, and third-line imatinib, sunitinib, or regorafenib, respectively. Clinical trial enrollment should be encouraged for patients whose GISTs harbor primary imatinib-resistant mutations, and those with advanced or unresectable GISTs with secondary resistance.
Most advanced gastrointestinal stromal tumors (GISTs) are due to a recurrence of localized disease, with only a small minority presenting with metastatic disease.1 Compared with chemotherapy, tyrosine kinase inhibitors (TKIs) have significantly improved the natural history of the disease, with median overall survival (OS) increasing from less than 1 year to about 5 years and approximately 1 in 5 patients achieving long-term survival.2 In addition, newer drugs in development and in clinical trials appear promising and have the potential to improve outcomes even further. This article reviews current evidence on options for treating metastatic or recurrent GISTs and GISTs that have progressed following initial therapy. The evaluation and diagnosis of GIST along with management of localized disease are reviewed in a separate article.
Case Presentation
A 64-year-old African American man underwent surgical resection of a 10-cm gastric mass, which pathology reported was positive for CD117, DOG1, and CD34 and negative for smooth muscle actin and S-100, consistent with a diagnosis of GIST. There were 10 mitoses per 50 HPF, and there was no intraoperative or intraperitoneal tumor rupture. The patient was treated with adjuvant imatinib, which was discontinued after 3 years due to grade 2 myalgias, periorbital edema, and macrocytic anemia. Surveillance included office visits every 3 to 6 months and a contrast CT abdomen and pelvis every 6 months. For the past 5 years, he has not had any clinical or radiographic evidence of disease recurrence. New imaging reveals multiple liver metastases and peritoneal implants. He feels fatigued and has lost about 10 lb since his last visit. He is 5 years out from his initial diagnosis and 2 years out from last receiving imatinib. His original tumor harbored a KIT exon 11 deletion.
What treatment should you recommend now?
Imatinib for Advanced GISTs
Before the first report of the efficacy of imatinib for metastatic GISTs in 2002, patients with advanced unresectable or metastatic GISTs were routinely treated with doxorubicin-based chemotherapy regimens, which were largely ineffective, with response rates (RRs) of around 5% and a median overall survival (OS) of less than 1 year.3,4 In 2002 a landmark phase 2 study revealed imatinib’s significant efficacy profile in advanced or metastatic GISTs, resulting in its approval by the US Food and Drug Administration (FDA).5 In this study, 147 patients with CD117-positive GISTs were randomly assigned to receive daily imatinib 400 mg or 600 mg for up to 36 months. The RRs were similar between the 2 groups (68.5% vs 67.6%), with a median time to response of 12 weeks and median duration of response of 118 days. Results of this study were much more favorable when compared to doxorubicin, rendering imatinib the new standard of care for advanced GISTs. A long-term follow-up of this study after a median of 63 months confirmed near identical RRs, progression-free survival (PFS), and median survival of 57 months among the 2 groups.6
Imatinib Daily Dosing
Although 400 mg of daily imatinib proved to be efficacious, it was unclear if a dose-response relationship existed for imatinib. An EORTC phase 2 study demonstrated a benefit of using a higher dose of imatinib at 400 mg twice daily, producing a RR of 71% (4% complete , 67% partial) and 1-year PFS of 73%, which appeared favorable compared with once-daily dosing and set the framework for larger phase 3 studies.7 Two phase 3 studies compared imatinib 400 mg once daily versus twice daily (until disease progression or unacceptable toxicity) among patients with CD117-positive advanced or metastatic GISTs. These studies were eventually combined into a meta-analysis (metaGIST) to compare RR, PFS and OS between the treatment groups. Both studies allowed cross-over to the 800 mg dose for patients who progressed on 400 mg daily.
The first study, conducted jointly by the EORTC, Italian Sarcoma Group, and Australasian Gastro-Intestinal Trials Group (EU-AUS),8 randomly assigned 946 patients to 400 mg once daily or twice daily. There were no differences in response rates between the groups, but the twice-daily group had a predicted 18% reduction in the hazard for progression compared with the once-daily group (estimated HR, 0.82; P = 0.026), which came at the expense of greater toxicities warranting dose reductions (60%) and treatment interruptions (64%). Cross-over to high-dose imatinib was feasible and safe, producing a partial response in 2%, stable disease in 27%, and a median PFS of 81 days. The second study was an intergroup study conducted jointly by SWOG, CALGB, NCI-C, and ECOG (S0033, US-CDN), with a nearly identical study design as the EU-AUS trial.9 The trial enrolled 746 patients. After a median follow up of 4.5 years, the median PFS and OS were not statistically different (18 vs 20 months and 55 vs 51 months, respectively). There were also no differences in response rates. One third of patients initially placed on the once-daily arm who crossed over after progression achieved a treatment response or stable disease.
The combined EU-AUS and US-CDN analysis (metaGIST) included 1640 patients with a median age of 60 years and 58% of whom were men; 818 and 822 patients were assigned to the 400 mg and 800 mg total daily doses, respectively.10 The median follow-up was 37.5 months. There were no differences in OS (49 vs 48.7 months), median PFS (18.9 vs 23.2 months), or overall response rates (51.4% vs 53.9%). Patients who had crossed over (n = 347) to the 800 mg total daily dose arm had a 7.7-month average PFS while on the higher daily dose. An analysis was performed on 377 patients in the EU-AUS trial assessing the impact of mutational status on clinical outcomes among imatinib-treated patients. KIT exon 9 activating mutations were found to be a significant independent prognostic factor for death when compared with KIT exon 11 mutations. However, the adverse prognostic value of KIT exon 9 mutations was partially overcome with higher doses of imatinib, as those who received 800 mg total had a significantly better PFS, with a 61% relative risk reduction, than those who received 400 mg. Altogether, it was concluded that imatinib 400 mg once daily should be the standard-of-care first-line treatment for advanced or metastatic GISTs, unless a KIT exon 9 mutation is present, in which case imatinib 800 mg should be considered, if 400 mg is well tolerated. In addition, patients treated with frontline imatinib at 400 mg once daily, if tolerated well, should be considered for imatinib 800 mg upon progression of disease.
Despite there being problems with secondary resistance, significant progress has occurred in the treatment of metastatic disease over a short period of time. Prior to 2000, median OS for patients with metastatic GISTs was 9 months. With the introduction of imatinib and other TKIs, the median OS has increased to 5 years, with an estimated 10-year OS rate of approximately 20%.2
Imatinib Interruption
Since at this point, imatinib was a well-established standard of care for advanced GISTs, it was questioned whether imatinib therapy could be interrupted. At this time, treatment interruption in a stop-and-go fashion was deemed feasible in other metastatic solid tumors such as colorectal cancer (OPTIMOX1).11 The BFR French trial showed that stopping imatinib therapy in patients who had a response or stable disease after 1, 3, or 5 years was generally followed by relatively rapid tumor progression (approximately 50% of patients within 6 months), even when tumors were previously removed.12 Therefore, it is recommended that treatment in the metastatic setting should be continued indefinitely, unless there is disease progression. Hence, unlike with colorectal cancer or chronic myelogenous leukemia, as of now there is no role for imatinib interruption in metastatic GISTs.
Case Continued
The patient is started on imatinib 400 mg daily, and overall he tolerates therapy well. Interval CT imaging reveals a treatment response. Two years later, imaging reveals an increase in the tumor size and density with a new nodule present within a preexisting mass. There are no clinical trials in the area.
What defines tumor progression?
Disease Progression
When GISTs are responding to treatment, on imaging the tumors can become more cystic and less dense but with an increase in size. In addition, tumor progression may not always be associated with increased size—increased density of the tumor or a nodule within a mass that may indicate progression. If CT imaging is equivocal for progression, positron emission tomography (PET) can play a role in identifying true progression. It is critically important that tumor size and density are carefully assessed when performing interval imaging. Of note, radiofrequency ablation, cryotherapy, or chemoembolization can be used for symptomatic liver metastases or oligometastatic disease. When evaluating for progression, one needs to ask patients about compliance (ie, maintaining dose intensity related to side effects of therapy as well as the financial burden of treatment—copay toxicity).
What are mechanisms of secondary imatinib resistance?
Imatinib resistance can be subtle in patients with GISTs, manifesting with new nodular, enhancing foci enclosed within a preexisting mass (resistant clonal nodule), or can be clinically or radiographically overt.13 Imatinib resistance occurs through multiple mechanisms including acquisition of secondary activating KIT mutations in the intracellular ATP-binding domain (exons 13 and 14) and the activation loop (exons 17 and 18).14
What are the treatment options for this patient?
Second-line Therapy
Sunitinib malate is a multitargeted TKI that not only targets c-Kit and PDGFRA, but also has anti-angiogenic activity through inhibition of vascular endothelial growth factor receptors (VEGFR). Sunitinib gained FDA approval for the second-line treatment of advanced GISTs based on an international double-blind trial that randomized 312 patients with imatinib-resistant metastatic GISTs in a 2:1 fashion to receive sunitinib 50 mg daily for 4 weeks on and 2 weeks off or placebo.15,16 The trial was unblinded early at the planned interim analysis, which revealed a marked benefit, producing a 66% reduction in the hazard risk of progression (27.3 vs 6.4 weeks, HR, 0.33; P < 0.001). The most common treatment-related adverse events were fatigue, diarrhea, skin discoloration, nausea, and hand-foot syndrome. Another open-label phase 2 study assessed a continuous dosing schema of sunitinib 37.5 mg daily, which has been shown to be effective with less toxicity.17 Among the 60 patients enrolled, the primary endpoint of clinical benefit rate at 24 weeks was reached in 53%, which consisted of 13% partial responses and 40% stable disease. Most toxicities were grade 1 or 2 and easily manageable through standard interventions. This has been recommended as an alternative to the initial scheduled regimen.18 Part of sunitinib’s success is its activity against GISTs harboring secondary KIT exon 13 and 14 mutations, and possibly its anti-angiogenic activity.19 Sunitinib is particularly efficacious among GISTs harboring KIT exon 9 mutations.
Third-line Therapy
Patients who have progressed on prior imatinib and sunitinib can receive third-line regorafenib, a multi-TKI that differs chemically from sorafenib by a fluorouracil group (fluoro-sorafenib). FDA approval of regorafenib was based on the phase 3 GRID (GIST Regorafenib In progressive Disease) multicenter international trial.20 This trial randomly assigned 199 patients in a 2:1 fashion to receive regorafenib 160 mg daily for 21 days out of 28-day cycles plus best supportive care (BSC) versus placebo plus BSC. Cross-over was allowed. Regorafenib significantly reduced the hazard risk of progression by 73% compared with placebo (4.8 vs 0.9 months; HR, 0.27; P < 0.001). There was no difference in OS, which may be because of cross-over (median OS, 17.4 months in both arms). As a result, regorafenib is now considered standard third-line treatment for patients with metastatic GISTs. It has a less favorable toxicity profile than imatinib, with hand-foot syndrome, transaminitis, hypertension and fatigue being the most common treatment toxicities. In order to avoid noncompliance, it is recommended to start at 80 mg and carefully titrate upwards to the 160 mg dose.
A list of landmark studies for advanced GISTs is provided in Table 1.

A summary of FDA-approved drugs for treating GISTs is provided in Table 2.

Clinical Trials
Clinical trial enrollment should be considered for all patients with advanced or unresectable GISTs throughout their treatment continuum. Owing to significant advances in genomic profiling through next-generation sequencing, multiple driver mutations have recently been identified, and targeted therapies are being explored in clinical trials.21 For example, the neurotrophic receptor tyrosine kinase (NTRK) gene appears to be mutated in a small number of advanced GISTs, and these can respond to the highly selective TRK inhibitor larotrectinib.22 Additionally, ongoing studies are assessing immunotherapies for sporadic GISTs and treatment for familial GISTs (Table 3). Some notable studies include those assessing the efficacy of agents that target KIT and PDGFR secondary mutations, including avapritinib (BLU-285) and DCC-2618, MEK inhibitors, and the multi-kinase inhibitor crenolanib for GISTs harboring the imatinib-resistant PDGFRA D842V mutation. There are also studies utilizing checkpoint inhibitors alone or in combination with imatinib.

Case Conclusion
Given the patient’s progression on imatinib, he is started on second-line sunitinib malate. He experiences grade 1 fatigue and hand-foot syndrome, which are managed supportively. After he has been on sunitinib for approximately 8 months, his disease progresses. He subsequently undergoes genomic profiling of his tumor and starts BLU-285 on a clinical trial.
Key Points
- For advanced and metastatic disease, TKIs have substantially improved the prognosis of KIT mutated GISTs, with 3 FDA-approved drugs: imatinib, sunitinib, and regorafenib. Imatinib 400 mg is the standard-of-care frontline therapy for locally advanced, unresectable, or metastatic imatinib-sensitive GISTs. If a patient has a KIT exon 9 mutation and 400 mg is well-tolerated, increasing to 800 mg is recommended. Imatinib should be continued indefinitely unless there is intolerance, a specific patient request for interruption, or progression of disease.
- When there is progression of disease in a patient with a sensitive mutation on 400 mg of imatinib, the dose can be increased to 800 mg.
- For patients who are imatinib-intolerant or have progression, standard second line is sunitinib.
- For patients who further progress or are sunitinib-intolerant, regorafenib is the standard third-line treatment.
- There needs to be close attention to side effects, drug and food interactions, and patient copay costs in order to maintain patient compliance while on TKI therapy.
- There are still major limitations in the systemic treatment of GISTs marked by their inherent genetic heterogeneity and secondary resistance. Continued translational and clinical research is needed in order to improve treatment for patients who develop secondary resistance or who have less common primary resistant mutations. Patients are encouraged to participate in clinical trials of new therapies.
Summary
GISTs are the most common mesenchymal tumors of the GI tract. They comprise an expanding landscape of tumors that are heterogenous in terms of natural history, mutations, and response to systemic treatments. The mainstay of treatment for localized GISTs is surgical resection followed by at least 3-years of adjuvant imatinib for patients with high-risk features who are imatinib-sensitive. Patients with GISTs harboring resistance mutations such as PDGFRA D842V or with SDH-deficient or NF1-associated GISTs should not receive adjuvant imatinib. Patients with more advanced GISTs and/or in difficult to resect sites harboring a sensitive mutation can be considered for neoadjuvant imatinib. Those with metastatic GISTs can receive first-, second-, and third-line imatinib, sunitinib, or regorafenib, respectively. Clinical trial enrollment should be encouraged for patients whose GISTs harbor primary imatinib-resistant mutations, and those with advanced or unresectable GISTs with secondary resistance.
1. Ma GL, Murphy JD, Martinez ME et al. Epidemiology of gastrointestinal stromal tumors in the era of histology codes: results of a population-based study. Cancer Epidemiol Biomarkers Prev. 2015;24:298-302.
2. Heinrich MC, Rankin C, Blanke CD, et al. Correlation of long-term results of imatinib in advanced gastrointestinal stromal tumors with next-generation sequencing results: analysis of phase 3 SWOG Intergroup Trial S0033. JAMA Oncol. 2017;3:944-952.
3. DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51-58.
4. Goss GA, Merriam P, Manola J, et al. Clinical and pathological characteristics of gastrointestinal stromal tumors (GIST). Prog Proc Am Soc Clin Oncol. 2000;19:599a.
5. Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002; 347:472-480.
6. Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase ii trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26:620-625.
7. Verweij J, van Oosterom A, Blay JY, et al. Imatinib mesylate (STI-571 Glivec, Gleevac) is an active agent for gastrointestinal stromal tumours, but does not yield responses in other soft-tissue sarcomas that are unselected for a molecular target. Results from an EORTC Soft Tissue and Bone Sarcoma Group phase II study. Eur J Cancer. 2003;39:2006-2011.
8. Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomized trial. Lancet. 2004;364:1127-1134.
9. Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626-632.
10. Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST). Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol. 2010;28:1247-1253.
11. Tournigand C, Cervantes A, Figer A, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer –a GERCOR study. J Clin Oncol. 2006;24:394-400.
12. Blay JV, Cesne AL, Ray-Coquard I, et al. Prospective multicentric randomized phase iii study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: The French Sarcoma Group. J Clin Oncol. 2007;25:1107-1113.
13. Desai J, Shankar S, Heinrich MC, et al. Clonal evolution of resistance to imatinib in patients with metastatic gastrointestinal stromal tumors. Clin Cancer Res. 2007;13(18 Pt 1): 5398-5405.
14. Gramza AW, Corless CL, Heinrich MC. Resistance to tyrosine kinase inhibitors in gastrointestinal stromal tumors. Clin Cancer Res. 2009;15:7510-7518.
15. Sutent (sunitinib malate) [package insert]. New York, NY: Pfizer Labs; 2017.
16. Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomized controlled trial. Lancet. 2006;368:1329-1338.
17. George S, Blay JY, Casali PG, et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer. 2009;45:1959-1968.
18. Brennan MF, Antonescu CR, Maki RG. Management of Soft Tissue Sarcomas. Switzerland: Springer International Publishing; 2013.
19. Heinrich MC, Maki RG, Corless CL, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumors. J Clin Oncol. 2008;26:5352-5359.
20. Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:295-302.
21. Wilky BA, Villalobos VM. Emerging role for precision therapy through next-generation sequencing for sarcomas. JCO Precision Oncology. 2018;2:1-4.
22. Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in trk fusion-positive cancers in adults and children. N Engl J Med. 2018;378:731-739.
1. Ma GL, Murphy JD, Martinez ME et al. Epidemiology of gastrointestinal stromal tumors in the era of histology codes: results of a population-based study. Cancer Epidemiol Biomarkers Prev. 2015;24:298-302.
2. Heinrich MC, Rankin C, Blanke CD, et al. Correlation of long-term results of imatinib in advanced gastrointestinal stromal tumors with next-generation sequencing results: analysis of phase 3 SWOG Intergroup Trial S0033. JAMA Oncol. 2017;3:944-952.
3. DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51-58.
4. Goss GA, Merriam P, Manola J, et al. Clinical and pathological characteristics of gastrointestinal stromal tumors (GIST). Prog Proc Am Soc Clin Oncol. 2000;19:599a.
5. Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002; 347:472-480.
6. Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase ii trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26:620-625.
7. Verweij J, van Oosterom A, Blay JY, et al. Imatinib mesylate (STI-571 Glivec, Gleevac) is an active agent for gastrointestinal stromal tumours, but does not yield responses in other soft-tissue sarcomas that are unselected for a molecular target. Results from an EORTC Soft Tissue and Bone Sarcoma Group phase II study. Eur J Cancer. 2003;39:2006-2011.
8. Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomized trial. Lancet. 2004;364:1127-1134.
9. Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626-632.
10. Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST). Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol. 2010;28:1247-1253.
11. Tournigand C, Cervantes A, Figer A, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer –a GERCOR study. J Clin Oncol. 2006;24:394-400.
12. Blay JV, Cesne AL, Ray-Coquard I, et al. Prospective multicentric randomized phase iii study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: The French Sarcoma Group. J Clin Oncol. 2007;25:1107-1113.
13. Desai J, Shankar S, Heinrich MC, et al. Clonal evolution of resistance to imatinib in patients with metastatic gastrointestinal stromal tumors. Clin Cancer Res. 2007;13(18 Pt 1): 5398-5405.
14. Gramza AW, Corless CL, Heinrich MC. Resistance to tyrosine kinase inhibitors in gastrointestinal stromal tumors. Clin Cancer Res. 2009;15:7510-7518.
15. Sutent (sunitinib malate) [package insert]. New York, NY: Pfizer Labs; 2017.
16. Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomized controlled trial. Lancet. 2006;368:1329-1338.
17. George S, Blay JY, Casali PG, et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer. 2009;45:1959-1968.
18. Brennan MF, Antonescu CR, Maki RG. Management of Soft Tissue Sarcomas. Switzerland: Springer International Publishing; 2013.
19. Heinrich MC, Maki RG, Corless CL, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumors. J Clin Oncol. 2008;26:5352-5359.
20. Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:295-302.
21. Wilky BA, Villalobos VM. Emerging role for precision therapy through next-generation sequencing for sarcomas. JCO Precision Oncology. 2018;2:1-4.
22. Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in trk fusion-positive cancers in adults and children. N Engl J Med. 2018;378:731-739.
Abbott issues recall on Ellipse ICDs
Abbott Laboratories has issued a recall of all Ellipse Implantable Cardioverter Defibrillators manufactured between April 5, 2019, and May 29, 2019, because of exposed aluminum wires within the device, potentially preventing defibrillation.
The Ellipse Implantable Cardioverter Defibrillators, formerly manufactured by St. Jude Medical (now a wholly-owned subsidiary of Abbott), provide pacing for patients with bradycardia and electric shock or pacing for patients with tachycardia. The device is implanted under the skin in the upper chest area with leads running into the heart.
The recall has been issued because electrical failures have occurred; Abbott has determined that these failures are caused by a faulty manufacturing process that left some aluminum wires in the leads partially exposed. Wires without proper insulation are likely to short, leaving the device without the ability to provide high voltage therapy.
Abbott is aware of no related reports of electrical failure in any of the devices that have already been implanted, the Food and Drug Administration announced, and no reports of patient harm, adverse events, or death have occurred. All affected devices that were implanted have either been replaced or are scheduled to be replaced, the agency said.
Abbott Laboratories has issued a recall of all Ellipse Implantable Cardioverter Defibrillators manufactured between April 5, 2019, and May 29, 2019, because of exposed aluminum wires within the device, potentially preventing defibrillation.
The Ellipse Implantable Cardioverter Defibrillators, formerly manufactured by St. Jude Medical (now a wholly-owned subsidiary of Abbott), provide pacing for patients with bradycardia and electric shock or pacing for patients with tachycardia. The device is implanted under the skin in the upper chest area with leads running into the heart.
The recall has been issued because electrical failures have occurred; Abbott has determined that these failures are caused by a faulty manufacturing process that left some aluminum wires in the leads partially exposed. Wires without proper insulation are likely to short, leaving the device without the ability to provide high voltage therapy.
Abbott is aware of no related reports of electrical failure in any of the devices that have already been implanted, the Food and Drug Administration announced, and no reports of patient harm, adverse events, or death have occurred. All affected devices that were implanted have either been replaced or are scheduled to be replaced, the agency said.
Abbott Laboratories has issued a recall of all Ellipse Implantable Cardioverter Defibrillators manufactured between April 5, 2019, and May 29, 2019, because of exposed aluminum wires within the device, potentially preventing defibrillation.
The Ellipse Implantable Cardioverter Defibrillators, formerly manufactured by St. Jude Medical (now a wholly-owned subsidiary of Abbott), provide pacing for patients with bradycardia and electric shock or pacing for patients with tachycardia. The device is implanted under the skin in the upper chest area with leads running into the heart.
The recall has been issued because electrical failures have occurred; Abbott has determined that these failures are caused by a faulty manufacturing process that left some aluminum wires in the leads partially exposed. Wires without proper insulation are likely to short, leaving the device without the ability to provide high voltage therapy.
Abbott is aware of no related reports of electrical failure in any of the devices that have already been implanted, the Food and Drug Administration announced, and no reports of patient harm, adverse events, or death have occurred. All affected devices that were implanted have either been replaced or are scheduled to be replaced, the agency said.