User login
Seeing former patients’ graves at the cemetery gives perspective
Around once a month I go to the cemetery to visit my Dad. Although this was difficult 6 years ago when I started, it’s become easier thanks to that great healer, time.
His grave is a short distance from the parking spot, so I have to walk past a number of others to get there. As a result you see these change over time.
A few times in the last several years I’ve noticed a new grave marker that, to my surprise, has the name of one of my (former) patients on it. Granted, a lot of my practice is the above-75 crowd, and they wouldn’t be coming to me if they didn’t have health issues.
But still, it jolts me a bit when it happens. I may not have thought about them for a while, but suddenly I see the marker and realize why that person hadn’t been in recently. I can usually picture them, too, and remember something they may have said that concerned me or just made me laugh.
Obviously, regardless of age we all end up there, and I certainly don’t consider this a personal medical failing on my part. It’s the nature of life on Earth, no matter how good a job we do as physicians.
But it still surprises me. If it was a patient I have fond memories of, I’ll often stop and say a few words to them, too. To date no one has answered, but I suspect there’s something therapeutic for me in doing so. The cemetery is generally peaceful, and certainly not a place where I feel rushed.
Besides getting to talk to my Dad, the occasional patient visit there is a reminder of the limits of being a physician, and of our own lives. If nothing else it helps keep a perspective on those things, such as family and health, that are truly important.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Around once a month I go to the cemetery to visit my Dad. Although this was difficult 6 years ago when I started, it’s become easier thanks to that great healer, time.
His grave is a short distance from the parking spot, so I have to walk past a number of others to get there. As a result you see these change over time.
A few times in the last several years I’ve noticed a new grave marker that, to my surprise, has the name of one of my (former) patients on it. Granted, a lot of my practice is the above-75 crowd, and they wouldn’t be coming to me if they didn’t have health issues.
But still, it jolts me a bit when it happens. I may not have thought about them for a while, but suddenly I see the marker and realize why that person hadn’t been in recently. I can usually picture them, too, and remember something they may have said that concerned me or just made me laugh.
Obviously, regardless of age we all end up there, and I certainly don’t consider this a personal medical failing on my part. It’s the nature of life on Earth, no matter how good a job we do as physicians.
But it still surprises me. If it was a patient I have fond memories of, I’ll often stop and say a few words to them, too. To date no one has answered, but I suspect there’s something therapeutic for me in doing so. The cemetery is generally peaceful, and certainly not a place where I feel rushed.
Besides getting to talk to my Dad, the occasional patient visit there is a reminder of the limits of being a physician, and of our own lives. If nothing else it helps keep a perspective on those things, such as family and health, that are truly important.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Around once a month I go to the cemetery to visit my Dad. Although this was difficult 6 years ago when I started, it’s become easier thanks to that great healer, time.
His grave is a short distance from the parking spot, so I have to walk past a number of others to get there. As a result you see these change over time.
A few times in the last several years I’ve noticed a new grave marker that, to my surprise, has the name of one of my (former) patients on it. Granted, a lot of my practice is the above-75 crowd, and they wouldn’t be coming to me if they didn’t have health issues.
But still, it jolts me a bit when it happens. I may not have thought about them for a while, but suddenly I see the marker and realize why that person hadn’t been in recently. I can usually picture them, too, and remember something they may have said that concerned me or just made me laugh.
Obviously, regardless of age we all end up there, and I certainly don’t consider this a personal medical failing on my part. It’s the nature of life on Earth, no matter how good a job we do as physicians.
But it still surprises me. If it was a patient I have fond memories of, I’ll often stop and say a few words to them, too. To date no one has answered, but I suspect there’s something therapeutic for me in doing so. The cemetery is generally peaceful, and certainly not a place where I feel rushed.
Besides getting to talk to my Dad, the occasional patient visit there is a reminder of the limits of being a physician, and of our own lives. If nothing else it helps keep a perspective on those things, such as family and health, that are truly important.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Burn-Pit Research Gets Renewed Focus
During Operations Iraqi and Enduring Freedom, everything from old uniforms to plastic, aerosol cans, electronic equipment, human waste, tires, and batteries were thrown into open pits, often doused with jet fuel, and set on fire.
Many deployed soldiers were exposed to smoke from these open-air burn pits, putting them at risk for cancer, neurologic effects, reproductive effects, respiratory toxicity, and cardiovascular toxicity. Veterans who were close to burn pits have reported eye irritation, itching, rashes, and respiratory problems, such as bronchitis, asthma, and emphysema.
In May 2019, the VA redesignated the Airborne Hazards Center of Excellence (AHCE), established in 2013, as the Airborne Hazards and Burn Pits Center of Excellence (AHBPCE). The redesignation was a consequence of the Helping Veterans Exposed to Burn Pits Act, which stemmed from an 18-month bipartisan effort to prevent burn pits from becoming “the Agent Orange of this generation of soldiers.” Senator Amy Klobuchar (D-MN), who cosponsored the legislation with Thom Tillis (R-NC) said, “After the Vietnam War, it took the US government years to recognize that there was a link between Agent Orange and its devastating health effects on our soldiers. … [W]e can’t make that same tragic mistake again by failing to identify the devastating health effects associated with burn pits.”
The AHCE was responsible for assessing veterans’ cardiopulmonary function, military/ nonmilitary exposures, and health-related symptoms for those with airborne hazard concerns. The AHBPCE will specialize in clinical and transitional research, focusing on expanding understanding of health outcomes and treatments for burn pit–related issues.
VA providers can consult with the AHBPCE about assessment and treatment. When appropriate, veterans may be invited for a comprehensive, multiday health evaluation from a specialized team. The examination includes state-of-the-art assessments of lung function and exercise capacity. The findings are used to develop recommendations, which are shared with the veteran and referring provider for follow-up care. The findings also are used by researchers at the center and throughout the VA to develop research questions to investigate and potentially improve clinical practice.
Veterans (including those who receive VA-authorized care in the community) with complex clinical presentations who are unable to be diagnosed locally may be referred for consultation or examination.
AHBPCE, which is located at the New Jersey War Related Illness and Injury Study Center (WRIISC), also provides the AHBPCE-WRIISC Airborne hazards Registry (AWARE) program, designed for veterans who complete the Airborne Hazards and Open Burn Pit Registry online questionnaire, report chronic respiratory symptoms, and meet other eligibility criteria. AHBPCE’s mandate also includes analyzing registry data to monitor the VA’s overall clinical response to exposure concerns.
During Operations Iraqi and Enduring Freedom, everything from old uniforms to plastic, aerosol cans, electronic equipment, human waste, tires, and batteries were thrown into open pits, often doused with jet fuel, and set on fire.
Many deployed soldiers were exposed to smoke from these open-air burn pits, putting them at risk for cancer, neurologic effects, reproductive effects, respiratory toxicity, and cardiovascular toxicity. Veterans who were close to burn pits have reported eye irritation, itching, rashes, and respiratory problems, such as bronchitis, asthma, and emphysema.
In May 2019, the VA redesignated the Airborne Hazards Center of Excellence (AHCE), established in 2013, as the Airborne Hazards and Burn Pits Center of Excellence (AHBPCE). The redesignation was a consequence of the Helping Veterans Exposed to Burn Pits Act, which stemmed from an 18-month bipartisan effort to prevent burn pits from becoming “the Agent Orange of this generation of soldiers.” Senator Amy Klobuchar (D-MN), who cosponsored the legislation with Thom Tillis (R-NC) said, “After the Vietnam War, it took the US government years to recognize that there was a link between Agent Orange and its devastating health effects on our soldiers. … [W]e can’t make that same tragic mistake again by failing to identify the devastating health effects associated with burn pits.”
The AHCE was responsible for assessing veterans’ cardiopulmonary function, military/ nonmilitary exposures, and health-related symptoms for those with airborne hazard concerns. The AHBPCE will specialize in clinical and transitional research, focusing on expanding understanding of health outcomes and treatments for burn pit–related issues.
VA providers can consult with the AHBPCE about assessment and treatment. When appropriate, veterans may be invited for a comprehensive, multiday health evaluation from a specialized team. The examination includes state-of-the-art assessments of lung function and exercise capacity. The findings are used to develop recommendations, which are shared with the veteran and referring provider for follow-up care. The findings also are used by researchers at the center and throughout the VA to develop research questions to investigate and potentially improve clinical practice.
Veterans (including those who receive VA-authorized care in the community) with complex clinical presentations who are unable to be diagnosed locally may be referred for consultation or examination.
AHBPCE, which is located at the New Jersey War Related Illness and Injury Study Center (WRIISC), also provides the AHBPCE-WRIISC Airborne hazards Registry (AWARE) program, designed for veterans who complete the Airborne Hazards and Open Burn Pit Registry online questionnaire, report chronic respiratory symptoms, and meet other eligibility criteria. AHBPCE’s mandate also includes analyzing registry data to monitor the VA’s overall clinical response to exposure concerns.
During Operations Iraqi and Enduring Freedom, everything from old uniforms to plastic, aerosol cans, electronic equipment, human waste, tires, and batteries were thrown into open pits, often doused with jet fuel, and set on fire.
Many deployed soldiers were exposed to smoke from these open-air burn pits, putting them at risk for cancer, neurologic effects, reproductive effects, respiratory toxicity, and cardiovascular toxicity. Veterans who were close to burn pits have reported eye irritation, itching, rashes, and respiratory problems, such as bronchitis, asthma, and emphysema.
In May 2019, the VA redesignated the Airborne Hazards Center of Excellence (AHCE), established in 2013, as the Airborne Hazards and Burn Pits Center of Excellence (AHBPCE). The redesignation was a consequence of the Helping Veterans Exposed to Burn Pits Act, which stemmed from an 18-month bipartisan effort to prevent burn pits from becoming “the Agent Orange of this generation of soldiers.” Senator Amy Klobuchar (D-MN), who cosponsored the legislation with Thom Tillis (R-NC) said, “After the Vietnam War, it took the US government years to recognize that there was a link between Agent Orange and its devastating health effects on our soldiers. … [W]e can’t make that same tragic mistake again by failing to identify the devastating health effects associated with burn pits.”
The AHCE was responsible for assessing veterans’ cardiopulmonary function, military/ nonmilitary exposures, and health-related symptoms for those with airborne hazard concerns. The AHBPCE will specialize in clinical and transitional research, focusing on expanding understanding of health outcomes and treatments for burn pit–related issues.
VA providers can consult with the AHBPCE about assessment and treatment. When appropriate, veterans may be invited for a comprehensive, multiday health evaluation from a specialized team. The examination includes state-of-the-art assessments of lung function and exercise capacity. The findings are used to develop recommendations, which are shared with the veteran and referring provider for follow-up care. The findings also are used by researchers at the center and throughout the VA to develop research questions to investigate and potentially improve clinical practice.
Veterans (including those who receive VA-authorized care in the community) with complex clinical presentations who are unable to be diagnosed locally may be referred for consultation or examination.
AHBPCE, which is located at the New Jersey War Related Illness and Injury Study Center (WRIISC), also provides the AHBPCE-WRIISC Airborne hazards Registry (AWARE) program, designed for veterans who complete the Airborne Hazards and Open Burn Pit Registry online questionnaire, report chronic respiratory symptoms, and meet other eligibility criteria. AHBPCE’s mandate also includes analyzing registry data to monitor the VA’s overall clinical response to exposure concerns.
Click for Credit: Predicting preeclampsia; MI & stroke post-cancer Dx; more
Here are 5 articles from the August issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Smoking cessation could delay or prevent rheumatoid arthritis
To take the posttest, go to: https://bit.ly/2YguN2r
Expires February 22, 2020
2. No increased pregnancy loss risk for women conceiving soon after stillbirth
To take the posttest, go to: https://bit.ly/2ZnMaLc
Expires March 4, 2020
3. Total plasma tau correlates with dementia onset, Alzheimer’s disease
To take the posttest, go to: https://bit.ly/2YeglYV
Expires March 9, 2020
4. MI, strokes spike during 30 days after cancer diagnosis
To take the posttest, go to: https://bit.ly/2GCKZAv
Expires March 12, 2020
5. Combination model predicts imminent preeclampsia
To take the posttest, go to: https://bit.ly/2LTohrO
Expires February 21, 2020
Here are 5 articles from the August issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Smoking cessation could delay or prevent rheumatoid arthritis
To take the posttest, go to: https://bit.ly/2YguN2r
Expires February 22, 2020
2. No increased pregnancy loss risk for women conceiving soon after stillbirth
To take the posttest, go to: https://bit.ly/2ZnMaLc
Expires March 4, 2020
3. Total plasma tau correlates with dementia onset, Alzheimer’s disease
To take the posttest, go to: https://bit.ly/2YeglYV
Expires March 9, 2020
4. MI, strokes spike during 30 days after cancer diagnosis
To take the posttest, go to: https://bit.ly/2GCKZAv
Expires March 12, 2020
5. Combination model predicts imminent preeclampsia
To take the posttest, go to: https://bit.ly/2LTohrO
Expires February 21, 2020
Here are 5 articles from the August issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Smoking cessation could delay or prevent rheumatoid arthritis
To take the posttest, go to: https://bit.ly/2YguN2r
Expires February 22, 2020
2. No increased pregnancy loss risk for women conceiving soon after stillbirth
To take the posttest, go to: https://bit.ly/2ZnMaLc
Expires March 4, 2020
3. Total plasma tau correlates with dementia onset, Alzheimer’s disease
To take the posttest, go to: https://bit.ly/2YeglYV
Expires March 9, 2020
4. MI, strokes spike during 30 days after cancer diagnosis
To take the posttest, go to: https://bit.ly/2GCKZAv
Expires March 12, 2020
5. Combination model predicts imminent preeclampsia
To take the posttest, go to: https://bit.ly/2LTohrO
Expires February 21, 2020
FDA approves Turalio for symptomatic tenosynovial giant cell tumor
Turalio (pexidartinib) capsules have been approved for the treatment of adult patients with symptomatic tenosynovial giant cell tumor (TGCT) that is associated with severe morbidity or functional limitations not responsive to improvement with surgery, the U.S. Food and Drug Administration announced.
Turalio is the first therapy to be approved for the rare joint tumor and is available only through the Turalio Risk Evaluation and Mitigation Strategy (REMS) Program. The FDA granted the approval of Turalio to Daiichi Sankyo.
“TGCT can cause debilitating symptoms for patients such as pain, stiffness and limitation of movement,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in a statement. “Surgery is the primary treatment option, but some patients are not eligible for surgery, and tumors can recur, even after the procedure.”
The approval was based on results of a study of 120 patients, 59 of whom received placebo. After 25 weeks of treatment, the overall response rate was 38% (15% complete responses and 23% partial responses) in those who received pexidartinib; no responses occurred in patients who received placebo. The response persisted in 22 of 23 responders who had been followed for a minimum of 6 months, and in 13 of 13 responders who had been followed for a minimum of 12 months.
Turalio comes with a Boxed Warning about the risk of serious and potentially fatal liver injury. Liver tests should be performed prior to beginning treatment and the results monitored at specified intervals during treatment. Patients who develop abnormal results may need to withhold therapy, reduce the dose, or discontinue therapy depending on the severity of the liver injury.
Common side effects for patients were increased levels of lactate dehydrogenase, aspartate aminotransferase, alanine aminotransferase, and cholesterol. Loss of hair color also occurred in some patients.
Additional side effects included neutropenia, increased alkaline phosphatase levels, decreased lymphocytes, eye edema, decreased hemoglobin levels, rash, dysgeusia, and decreased phosphate levels.
Females of reproductive age and males with a female partner of reproductive potential should use effective contraception during treatment with pexidartinib. Pexidartinib may cause harm to a developing fetus or newborn baby.
Pexidartinib must be dispensed with a patient Medication Guide that describes important information about the drug’s uses and risks.
Turalio (pexidartinib) capsules have been approved for the treatment of adult patients with symptomatic tenosynovial giant cell tumor (TGCT) that is associated with severe morbidity or functional limitations not responsive to improvement with surgery, the U.S. Food and Drug Administration announced.
Turalio is the first therapy to be approved for the rare joint tumor and is available only through the Turalio Risk Evaluation and Mitigation Strategy (REMS) Program. The FDA granted the approval of Turalio to Daiichi Sankyo.
“TGCT can cause debilitating symptoms for patients such as pain, stiffness and limitation of movement,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in a statement. “Surgery is the primary treatment option, but some patients are not eligible for surgery, and tumors can recur, even after the procedure.”
The approval was based on results of a study of 120 patients, 59 of whom received placebo. After 25 weeks of treatment, the overall response rate was 38% (15% complete responses and 23% partial responses) in those who received pexidartinib; no responses occurred in patients who received placebo. The response persisted in 22 of 23 responders who had been followed for a minimum of 6 months, and in 13 of 13 responders who had been followed for a minimum of 12 months.
Turalio comes with a Boxed Warning about the risk of serious and potentially fatal liver injury. Liver tests should be performed prior to beginning treatment and the results monitored at specified intervals during treatment. Patients who develop abnormal results may need to withhold therapy, reduce the dose, or discontinue therapy depending on the severity of the liver injury.
Common side effects for patients were increased levels of lactate dehydrogenase, aspartate aminotransferase, alanine aminotransferase, and cholesterol. Loss of hair color also occurred in some patients.
Additional side effects included neutropenia, increased alkaline phosphatase levels, decreased lymphocytes, eye edema, decreased hemoglobin levels, rash, dysgeusia, and decreased phosphate levels.
Females of reproductive age and males with a female partner of reproductive potential should use effective contraception during treatment with pexidartinib. Pexidartinib may cause harm to a developing fetus or newborn baby.
Pexidartinib must be dispensed with a patient Medication Guide that describes important information about the drug’s uses and risks.
Turalio (pexidartinib) capsules have been approved for the treatment of adult patients with symptomatic tenosynovial giant cell tumor (TGCT) that is associated with severe morbidity or functional limitations not responsive to improvement with surgery, the U.S. Food and Drug Administration announced.
Turalio is the first therapy to be approved for the rare joint tumor and is available only through the Turalio Risk Evaluation and Mitigation Strategy (REMS) Program. The FDA granted the approval of Turalio to Daiichi Sankyo.
“TGCT can cause debilitating symptoms for patients such as pain, stiffness and limitation of movement,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in a statement. “Surgery is the primary treatment option, but some patients are not eligible for surgery, and tumors can recur, even after the procedure.”
The approval was based on results of a study of 120 patients, 59 of whom received placebo. After 25 weeks of treatment, the overall response rate was 38% (15% complete responses and 23% partial responses) in those who received pexidartinib; no responses occurred in patients who received placebo. The response persisted in 22 of 23 responders who had been followed for a minimum of 6 months, and in 13 of 13 responders who had been followed for a minimum of 12 months.
Turalio comes with a Boxed Warning about the risk of serious and potentially fatal liver injury. Liver tests should be performed prior to beginning treatment and the results monitored at specified intervals during treatment. Patients who develop abnormal results may need to withhold therapy, reduce the dose, or discontinue therapy depending on the severity of the liver injury.
Common side effects for patients were increased levels of lactate dehydrogenase, aspartate aminotransferase, alanine aminotransferase, and cholesterol. Loss of hair color also occurred in some patients.
Additional side effects included neutropenia, increased alkaline phosphatase levels, decreased lymphocytes, eye edema, decreased hemoglobin levels, rash, dysgeusia, and decreased phosphate levels.
Females of reproductive age and males with a female partner of reproductive potential should use effective contraception during treatment with pexidartinib. Pexidartinib may cause harm to a developing fetus or newborn baby.
Pexidartinib must be dispensed with a patient Medication Guide that describes important information about the drug’s uses and risks.
Technology, counseling, and CBT apps for primary care
There is probably no area where human contact is more important than in the area of counseling and psychotherapy. Or so most of us have thought. It turns out that, even in behavioral medicine, technology has made fantastic inroads in helping patients achieve real improvement in troublesome behavioral symptoms. We will not go over that evidence in this column, other than to say that the evidence is there, but rather we will review some of the best apps that those of us in primary care can utilize in the care of our patients. It is our opinion that these apps are best used in conjunction with our care to supplement the counseling we are giving our patients in the office. Many of the apps listed may be used for both anxiety and depression, as well as in areas related to problem solving, self-esteem, anger management, creating lifestyle changes, and coping with uncertainty.
MoodKit
MoodKit is a CBT app with four main tools: a collection of activities focused on coping self-efficacy (a person’s belief in success in specific situations) that includes individual productivity, social relationships, physical activity, and healthy habits; a thought checker; mood tracker; and journal. MoodKit is accessed in an unstructured way and can be used as an unguided self-help app. It is useful in patient interactions to access interventions in areas such as social engagement and options for choosing a healthy lifestyle. It is available in Apple’s App Store, and it costs $4.99.
Moodnotes
Based on CBT and positive psychology, Moodnotes assists in recognizing and learning about “traps” in thinking, as well as emphasizing healthier thinking habits. Traps in thinking include “catastrophic thinking” where patients with depression may think that a small error or behavioral indiscretion may lead to a consequence that far exceeds what is likely, or “mind-reading” where a person assumes that others are critical of them without actually having evidence that this is the case. Moodnotes tracks mood over a period of time while identifying factors that influence it. It is helpful in between visits to aid clinicians in gaining perspective on mood patterns. It is available in the App Store; it costs $4.99.
MoodMission
This app recommends strategies based in CBT after input of low moods or feelings of anxiety. MoodMission provides five “missions” to engage in that promote confidence in handling stressors and promotes coping self-efficacy. The app learns what style works best and tailors techniques according to when a patient uses it most frequently. Rewards in the app are used to promote motivation and to increase pleasure and self-confidence. It is useful for patients who could use a lift in mood or decrease in symptoms of anxiety and depression. It available in the App Store and Google Play, and it’s free.
What’s Up
In line with its development based on principles from CBT and Acceptance and Commitment Therapy (ACT), What’s Up identifies common negative thinking patterns and methods to overcome them with useful metaphors, a catastrophe scale, grounding techniques, and breathing exercises. What’s Up syncs data across multiple devices and uses a unique passcode to protect this information. One of the abilities that separates it from other apps is that it can become active in forums where people discuss similar feelings and strategies that have been useful for them. It is available in the App Store and Google Play, and it’s free.
Moodpath
Moodpath uses daily screenings to create better understanding of thoughts, feelings, and emotions. If needed, it provides a discussion guide to talking with a medical professional based on answers to its daily screenings. Included in the app are over 150 psychological exercises and videos to promote and strengthen overall mental health. It is useful in introducing how to discuss mental health with a professional. It is available in the App Store and Google Play free of cost.
MindShift CBT
Designed to assist youth and young adults in coping with anxiety, MindShift constructs an individualized toolbox to help individuals deal with test anxiety, perfectionism, social anxiety, worry, panic, and conflict. The app includes directions on how to construct “belief experiments” to test common beliefs that fuel anxiety, guided relaxation, as well as tools and tips to help set and accomplish goals. It is useful in helping teens and young adults learn about helpful and unhelpful anxiety, as well as to overcome fears by gradually facing them in manageable steps. It is available in the App Store and Google Play for free.
CBT-i Coach
CBT-i Coach, based on principles of cognitive behavioral therapy for insomnia (CBT-i), is a structured program to learn about sleep, develop positive sleep routines, and improve sleep environment. The CBT methods used attempt to change behaviors, which in turn provides confidence that patients will sleep better on a regular basis. It useful as a first-line intervention in treating symptoms of insomnia. It is available in the App Store and Google Play for no cost.
Getselfhelp.co.uk
This website provides free self-help and therapy resources grounded in methods that teach the change agents in CBT that can influence negative and destructive thought patterns. Negative thought patterns include thinking in terms of all or nothing: “Nothing ever works out for me,” fortune telling: “I shouldn’t even try,” and overgeneralization: “This didn’t work so this will not either.” Getselfhelp.co.uk provides handouts on a wide array of symptoms related to anxiety, depression, low self-esteem, panic attacks, social disorder, and more. The solution section of the website supplies interventions that can be printed and saved for future use. It is helpful for clinicians and patients in identifying an area of need and creating an action plan. It is also useful for clinicians to have as an augmented supplement for counseling and is free of cost.
The bottom line
When used correctly the resources that we have reviewed can essentially be deployed in a manner similar to how we use finger-stick blood sugar monitoring in the treatment of diabetes. Each of these technologies works best when combined with clinician input and periodic review. When used to supplement clinician counseling, the apps may help sustain motivation and provide insights and exercises that improve patient engagement and supplement the effect of counseling and/or medications that are prescribed in the office.
Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Aaron Sutton is a behavioral health consultant and faculty member in the family medicine residency program at Abington Jefferson Health.
There is probably no area where human contact is more important than in the area of counseling and psychotherapy. Or so most of us have thought. It turns out that, even in behavioral medicine, technology has made fantastic inroads in helping patients achieve real improvement in troublesome behavioral symptoms. We will not go over that evidence in this column, other than to say that the evidence is there, but rather we will review some of the best apps that those of us in primary care can utilize in the care of our patients. It is our opinion that these apps are best used in conjunction with our care to supplement the counseling we are giving our patients in the office. Many of the apps listed may be used for both anxiety and depression, as well as in areas related to problem solving, self-esteem, anger management, creating lifestyle changes, and coping with uncertainty.
MoodKit
MoodKit is a CBT app with four main tools: a collection of activities focused on coping self-efficacy (a person’s belief in success in specific situations) that includes individual productivity, social relationships, physical activity, and healthy habits; a thought checker; mood tracker; and journal. MoodKit is accessed in an unstructured way and can be used as an unguided self-help app. It is useful in patient interactions to access interventions in areas such as social engagement and options for choosing a healthy lifestyle. It is available in Apple’s App Store, and it costs $4.99.
Moodnotes
Based on CBT and positive psychology, Moodnotes assists in recognizing and learning about “traps” in thinking, as well as emphasizing healthier thinking habits. Traps in thinking include “catastrophic thinking” where patients with depression may think that a small error or behavioral indiscretion may lead to a consequence that far exceeds what is likely, or “mind-reading” where a person assumes that others are critical of them without actually having evidence that this is the case. Moodnotes tracks mood over a period of time while identifying factors that influence it. It is helpful in between visits to aid clinicians in gaining perspective on mood patterns. It is available in the App Store; it costs $4.99.
MoodMission
This app recommends strategies based in CBT after input of low moods or feelings of anxiety. MoodMission provides five “missions” to engage in that promote confidence in handling stressors and promotes coping self-efficacy. The app learns what style works best and tailors techniques according to when a patient uses it most frequently. Rewards in the app are used to promote motivation and to increase pleasure and self-confidence. It is useful for patients who could use a lift in mood or decrease in symptoms of anxiety and depression. It available in the App Store and Google Play, and it’s free.
What’s Up
In line with its development based on principles from CBT and Acceptance and Commitment Therapy (ACT), What’s Up identifies common negative thinking patterns and methods to overcome them with useful metaphors, a catastrophe scale, grounding techniques, and breathing exercises. What’s Up syncs data across multiple devices and uses a unique passcode to protect this information. One of the abilities that separates it from other apps is that it can become active in forums where people discuss similar feelings and strategies that have been useful for them. It is available in the App Store and Google Play, and it’s free.
Moodpath
Moodpath uses daily screenings to create better understanding of thoughts, feelings, and emotions. If needed, it provides a discussion guide to talking with a medical professional based on answers to its daily screenings. Included in the app are over 150 psychological exercises and videos to promote and strengthen overall mental health. It is useful in introducing how to discuss mental health with a professional. It is available in the App Store and Google Play free of cost.
MindShift CBT
Designed to assist youth and young adults in coping with anxiety, MindShift constructs an individualized toolbox to help individuals deal with test anxiety, perfectionism, social anxiety, worry, panic, and conflict. The app includes directions on how to construct “belief experiments” to test common beliefs that fuel anxiety, guided relaxation, as well as tools and tips to help set and accomplish goals. It is useful in helping teens and young adults learn about helpful and unhelpful anxiety, as well as to overcome fears by gradually facing them in manageable steps. It is available in the App Store and Google Play for free.
CBT-i Coach
CBT-i Coach, based on principles of cognitive behavioral therapy for insomnia (CBT-i), is a structured program to learn about sleep, develop positive sleep routines, and improve sleep environment. The CBT methods used attempt to change behaviors, which in turn provides confidence that patients will sleep better on a regular basis. It useful as a first-line intervention in treating symptoms of insomnia. It is available in the App Store and Google Play for no cost.
Getselfhelp.co.uk
This website provides free self-help and therapy resources grounded in methods that teach the change agents in CBT that can influence negative and destructive thought patterns. Negative thought patterns include thinking in terms of all or nothing: “Nothing ever works out for me,” fortune telling: “I shouldn’t even try,” and overgeneralization: “This didn’t work so this will not either.” Getselfhelp.co.uk provides handouts on a wide array of symptoms related to anxiety, depression, low self-esteem, panic attacks, social disorder, and more. The solution section of the website supplies interventions that can be printed and saved for future use. It is helpful for clinicians and patients in identifying an area of need and creating an action plan. It is also useful for clinicians to have as an augmented supplement for counseling and is free of cost.
The bottom line
When used correctly the resources that we have reviewed can essentially be deployed in a manner similar to how we use finger-stick blood sugar monitoring in the treatment of diabetes. Each of these technologies works best when combined with clinician input and periodic review. When used to supplement clinician counseling, the apps may help sustain motivation and provide insights and exercises that improve patient engagement and supplement the effect of counseling and/or medications that are prescribed in the office.
Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Aaron Sutton is a behavioral health consultant and faculty member in the family medicine residency program at Abington Jefferson Health.
There is probably no area where human contact is more important than in the area of counseling and psychotherapy. Or so most of us have thought. It turns out that, even in behavioral medicine, technology has made fantastic inroads in helping patients achieve real improvement in troublesome behavioral symptoms. We will not go over that evidence in this column, other than to say that the evidence is there, but rather we will review some of the best apps that those of us in primary care can utilize in the care of our patients. It is our opinion that these apps are best used in conjunction with our care to supplement the counseling we are giving our patients in the office. Many of the apps listed may be used for both anxiety and depression, as well as in areas related to problem solving, self-esteem, anger management, creating lifestyle changes, and coping with uncertainty.
MoodKit
MoodKit is a CBT app with four main tools: a collection of activities focused on coping self-efficacy (a person’s belief in success in specific situations) that includes individual productivity, social relationships, physical activity, and healthy habits; a thought checker; mood tracker; and journal. MoodKit is accessed in an unstructured way and can be used as an unguided self-help app. It is useful in patient interactions to access interventions in areas such as social engagement and options for choosing a healthy lifestyle. It is available in Apple’s App Store, and it costs $4.99.
Moodnotes
Based on CBT and positive psychology, Moodnotes assists in recognizing and learning about “traps” in thinking, as well as emphasizing healthier thinking habits. Traps in thinking include “catastrophic thinking” where patients with depression may think that a small error or behavioral indiscretion may lead to a consequence that far exceeds what is likely, or “mind-reading” where a person assumes that others are critical of them without actually having evidence that this is the case. Moodnotes tracks mood over a period of time while identifying factors that influence it. It is helpful in between visits to aid clinicians in gaining perspective on mood patterns. It is available in the App Store; it costs $4.99.
MoodMission
This app recommends strategies based in CBT after input of low moods or feelings of anxiety. MoodMission provides five “missions” to engage in that promote confidence in handling stressors and promotes coping self-efficacy. The app learns what style works best and tailors techniques according to when a patient uses it most frequently. Rewards in the app are used to promote motivation and to increase pleasure and self-confidence. It is useful for patients who could use a lift in mood or decrease in symptoms of anxiety and depression. It available in the App Store and Google Play, and it’s free.
What’s Up
In line with its development based on principles from CBT and Acceptance and Commitment Therapy (ACT), What’s Up identifies common negative thinking patterns and methods to overcome them with useful metaphors, a catastrophe scale, grounding techniques, and breathing exercises. What’s Up syncs data across multiple devices and uses a unique passcode to protect this information. One of the abilities that separates it from other apps is that it can become active in forums where people discuss similar feelings and strategies that have been useful for them. It is available in the App Store and Google Play, and it’s free.
Moodpath
Moodpath uses daily screenings to create better understanding of thoughts, feelings, and emotions. If needed, it provides a discussion guide to talking with a medical professional based on answers to its daily screenings. Included in the app are over 150 psychological exercises and videos to promote and strengthen overall mental health. It is useful in introducing how to discuss mental health with a professional. It is available in the App Store and Google Play free of cost.
MindShift CBT
Designed to assist youth and young adults in coping with anxiety, MindShift constructs an individualized toolbox to help individuals deal with test anxiety, perfectionism, social anxiety, worry, panic, and conflict. The app includes directions on how to construct “belief experiments” to test common beliefs that fuel anxiety, guided relaxation, as well as tools and tips to help set and accomplish goals. It is useful in helping teens and young adults learn about helpful and unhelpful anxiety, as well as to overcome fears by gradually facing them in manageable steps. It is available in the App Store and Google Play for free.
CBT-i Coach
CBT-i Coach, based on principles of cognitive behavioral therapy for insomnia (CBT-i), is a structured program to learn about sleep, develop positive sleep routines, and improve sleep environment. The CBT methods used attempt to change behaviors, which in turn provides confidence that patients will sleep better on a regular basis. It useful as a first-line intervention in treating symptoms of insomnia. It is available in the App Store and Google Play for no cost.
Getselfhelp.co.uk
This website provides free self-help and therapy resources grounded in methods that teach the change agents in CBT that can influence negative and destructive thought patterns. Negative thought patterns include thinking in terms of all or nothing: “Nothing ever works out for me,” fortune telling: “I shouldn’t even try,” and overgeneralization: “This didn’t work so this will not either.” Getselfhelp.co.uk provides handouts on a wide array of symptoms related to anxiety, depression, low self-esteem, panic attacks, social disorder, and more. The solution section of the website supplies interventions that can be printed and saved for future use. It is helpful for clinicians and patients in identifying an area of need and creating an action plan. It is also useful for clinicians to have as an augmented supplement for counseling and is free of cost.
The bottom line
When used correctly the resources that we have reviewed can essentially be deployed in a manner similar to how we use finger-stick blood sugar monitoring in the treatment of diabetes. Each of these technologies works best when combined with clinician input and periodic review. When used to supplement clinician counseling, the apps may help sustain motivation and provide insights and exercises that improve patient engagement and supplement the effect of counseling and/or medications that are prescribed in the office.
Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Aaron Sutton is a behavioral health consultant and faculty member in the family medicine residency program at Abington Jefferson Health.
Older patients who stop statins may be increasing their cardiovascular risk
Discontinuing statins was associated with an increased risk of hospital admission for a cardiovascular event, according to a study of elderly French patients with no history of heart disease.
“The results of this study suggest potential cardiovascular risk reduction associated with continuing statin therapy after the age of 75 years in persons already taking these drugs for primary prevention,” wrote Philippe Giral, MD, of Hôpital La Pitié Salpêtrière (France) and coauthors. The study was published in the European Heart Journal.
To determine if statins are a cardiovascular benefit or detriment to older people, the researchers reviewed data from 120,173 patients in French health care databases who turned 75 during 2012-2014. Patients with a diagnosis of cardiovascular disease in the previous 2 years were excluded, and all eligible patients were required to have a statin medication possession ratio of at least 80% in each of the previous 2 years.
Over a follow-up period that averaged 2.4 years, 17,204 patients (14.3%) discontinued statins and 5,396 (4.5%) were admitted for a cardiovascular event. The adjusted hazard ratios for admissions after statin discontinuation were 1.33 (95% confidence interval, 1.18-1.50) for a cardiovascular event, 1.46 (95% CI, 1.21-1.75) for a coronary event, 1.26 (95% CI, 1.05-1.51) for a cerebrovascular event, and 1.02 (95% CI, 0.74-1.40) for other vascular events, respectively.
The coauthors acknowledged their study’s limitations, including being unable to account for certain cardiovascular risk factors such as baseline LDL cholesterol level, tobacco use, obesity, and frailty markers. In addition, no information was available as to why patients discontinued statins. However, the presence of other major cardiovascular risk factors was investigated and accounted for, as was discontinuation of other cardiovascular drug therapies.
The study was not funded, and the authors declared no conflicts of interest.
SOURCE: Giral P at al. Eur Heart J. 2019 July 31. doi: 10.1093/eurheartj/ehz458.
Discontinuing statins was associated with an increased risk of hospital admission for a cardiovascular event, according to a study of elderly French patients with no history of heart disease.
“The results of this study suggest potential cardiovascular risk reduction associated with continuing statin therapy after the age of 75 years in persons already taking these drugs for primary prevention,” wrote Philippe Giral, MD, of Hôpital La Pitié Salpêtrière (France) and coauthors. The study was published in the European Heart Journal.
To determine if statins are a cardiovascular benefit or detriment to older people, the researchers reviewed data from 120,173 patients in French health care databases who turned 75 during 2012-2014. Patients with a diagnosis of cardiovascular disease in the previous 2 years were excluded, and all eligible patients were required to have a statin medication possession ratio of at least 80% in each of the previous 2 years.
Over a follow-up period that averaged 2.4 years, 17,204 patients (14.3%) discontinued statins and 5,396 (4.5%) were admitted for a cardiovascular event. The adjusted hazard ratios for admissions after statin discontinuation were 1.33 (95% confidence interval, 1.18-1.50) for a cardiovascular event, 1.46 (95% CI, 1.21-1.75) for a coronary event, 1.26 (95% CI, 1.05-1.51) for a cerebrovascular event, and 1.02 (95% CI, 0.74-1.40) for other vascular events, respectively.
The coauthors acknowledged their study’s limitations, including being unable to account for certain cardiovascular risk factors such as baseline LDL cholesterol level, tobacco use, obesity, and frailty markers. In addition, no information was available as to why patients discontinued statins. However, the presence of other major cardiovascular risk factors was investigated and accounted for, as was discontinuation of other cardiovascular drug therapies.
The study was not funded, and the authors declared no conflicts of interest.
SOURCE: Giral P at al. Eur Heart J. 2019 July 31. doi: 10.1093/eurheartj/ehz458.
Discontinuing statins was associated with an increased risk of hospital admission for a cardiovascular event, according to a study of elderly French patients with no history of heart disease.
“The results of this study suggest potential cardiovascular risk reduction associated with continuing statin therapy after the age of 75 years in persons already taking these drugs for primary prevention,” wrote Philippe Giral, MD, of Hôpital La Pitié Salpêtrière (France) and coauthors. The study was published in the European Heart Journal.
To determine if statins are a cardiovascular benefit or detriment to older people, the researchers reviewed data from 120,173 patients in French health care databases who turned 75 during 2012-2014. Patients with a diagnosis of cardiovascular disease in the previous 2 years were excluded, and all eligible patients were required to have a statin medication possession ratio of at least 80% in each of the previous 2 years.
Over a follow-up period that averaged 2.4 years, 17,204 patients (14.3%) discontinued statins and 5,396 (4.5%) were admitted for a cardiovascular event. The adjusted hazard ratios for admissions after statin discontinuation were 1.33 (95% confidence interval, 1.18-1.50) for a cardiovascular event, 1.46 (95% CI, 1.21-1.75) for a coronary event, 1.26 (95% CI, 1.05-1.51) for a cerebrovascular event, and 1.02 (95% CI, 0.74-1.40) for other vascular events, respectively.
The coauthors acknowledged their study’s limitations, including being unable to account for certain cardiovascular risk factors such as baseline LDL cholesterol level, tobacco use, obesity, and frailty markers. In addition, no information was available as to why patients discontinued statins. However, the presence of other major cardiovascular risk factors was investigated and accounted for, as was discontinuation of other cardiovascular drug therapies.
The study was not funded, and the authors declared no conflicts of interest.
SOURCE: Giral P at al. Eur Heart J. 2019 July 31. doi: 10.1093/eurheartj/ehz458.
FROM THE EUROPEAN HEART JOURNAL
Too many blood cultures ordered for pediatric SSTIs
SEATTLE – Blood cultures were ordered for over half of pediatric skin infection encounters across 38 children’s hospitals, with rates varying from about 20% to 80% between hospitals, according to a review of almost 50,000 encounters in the Pediatric Health Information System database.
It was a surprising finding, because current guidelines from the Infectious Diseases Society of America do not recommend blood cultures as part of the routine evaluation of uncomplicated pediatric skin and soft-tissue infections (SSTIs), meaning infections in children who are otherwise healthy without neutropenia or other complicating factors.
Just 0.6% of the cultures were positive in the review, and it’s likely some of those were caused by contamination. After adjustment for demographics, complex chronic conditions, and severity of illness, culture draws were associated with a 20% increase in hospital length of stay (LOS), hospital costs, and 30-day readmission rates.
“Our data provide more evidence that [routine] blood cultures for children with SSTI represents low-value practice and should be avoided,” said lead investigator John Stephens, MD, a pediatrics professor and hospitalist at the University of North Carolina at Chapel Hill.
Dr. Stephens became curious about how common the practice was across hospitals after he and a friend penned an article about the issue for the Journal of Hospital Medicine’s “Things We Do for No Reason” series. The single-center studies they reviewed showed similarly high rates of both testing and negative cultures (J Hosp Med. 2018 Jul;13[7]:496-9).
Dr. Stephens and his team queried the Pediatric Health Information System database for encounters in children aged 2 months to 18 years with the diagnostic code 383, “cellulitis and other skin infections,” from 2012 to 2017, during which time “there really wasn’t a change” in IDSA guidance, he noted. Transfers, encounters with ICU care, and immunocompromised children were excluded.
Hospital admissions were included in the review if they had an additional code for erysipelas, cellulitis, impetigo, or other localized skin infection. The rate of positive cultures was inferred from subsequent codes for bacteremia or septicemia.
Across 49,291 encounters, the median rate of blood culture for skin infection was 51.6%, with tremendous variation between hospitals. With blood cultures, the hospital LOS was about 1.9 days, the hospital cost was $4,030, and the 30-day readmission rate was 1.3%. Without cultures, LOS was 1.6 days, the cost was $3,291, and the readmission rate was 1%.
Although infrequent, it’s likely that positive cultures triggered additional work-up, time in the hospital, and other measures, which might help account for the increase in LOS and costs.
As for why blood testing was so common, especially in some hospitals, “I think it’s just institutional culture. No amount of clinical variation in patient population could explain” a 20%-80% “variation across hospitals. It’s really just ingrained habits,” Dr. Stephens said at Pediatric Hospital Medicine.
“The rate of positive blood culture was really low, and the association was for higher cost and utilization. I think this really reinforces the IDSA guidelines. We need to focus on quality improvement efforts to do this better,” he said, noting that he hopes to do so at his own institution.
“I’d also like to know more on the positives. In the single center studies, we know more than half of them are contaminants. Often, there’s more contamination than true positives,” he said at the meeting sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Instead of routine blood culture, Dr. Stephens recommended in his article to send pus for a Gram stain and culture and sensitivity, while noting that blood cultures remain reasonable for complicated infections, immunocompromised patients, and neonates.
There was no external funding, and Dr. Stephens didn’t report any disclosures.
SEATTLE – Blood cultures were ordered for over half of pediatric skin infection encounters across 38 children’s hospitals, with rates varying from about 20% to 80% between hospitals, according to a review of almost 50,000 encounters in the Pediatric Health Information System database.
It was a surprising finding, because current guidelines from the Infectious Diseases Society of America do not recommend blood cultures as part of the routine evaluation of uncomplicated pediatric skin and soft-tissue infections (SSTIs), meaning infections in children who are otherwise healthy without neutropenia or other complicating factors.
Just 0.6% of the cultures were positive in the review, and it’s likely some of those were caused by contamination. After adjustment for demographics, complex chronic conditions, and severity of illness, culture draws were associated with a 20% increase in hospital length of stay (LOS), hospital costs, and 30-day readmission rates.
“Our data provide more evidence that [routine] blood cultures for children with SSTI represents low-value practice and should be avoided,” said lead investigator John Stephens, MD, a pediatrics professor and hospitalist at the University of North Carolina at Chapel Hill.
Dr. Stephens became curious about how common the practice was across hospitals after he and a friend penned an article about the issue for the Journal of Hospital Medicine’s “Things We Do for No Reason” series. The single-center studies they reviewed showed similarly high rates of both testing and negative cultures (J Hosp Med. 2018 Jul;13[7]:496-9).
Dr. Stephens and his team queried the Pediatric Health Information System database for encounters in children aged 2 months to 18 years with the diagnostic code 383, “cellulitis and other skin infections,” from 2012 to 2017, during which time “there really wasn’t a change” in IDSA guidance, he noted. Transfers, encounters with ICU care, and immunocompromised children were excluded.
Hospital admissions were included in the review if they had an additional code for erysipelas, cellulitis, impetigo, or other localized skin infection. The rate of positive cultures was inferred from subsequent codes for bacteremia or septicemia.
Across 49,291 encounters, the median rate of blood culture for skin infection was 51.6%, with tremendous variation between hospitals. With blood cultures, the hospital LOS was about 1.9 days, the hospital cost was $4,030, and the 30-day readmission rate was 1.3%. Without cultures, LOS was 1.6 days, the cost was $3,291, and the readmission rate was 1%.
Although infrequent, it’s likely that positive cultures triggered additional work-up, time in the hospital, and other measures, which might help account for the increase in LOS and costs.
As for why blood testing was so common, especially in some hospitals, “I think it’s just institutional culture. No amount of clinical variation in patient population could explain” a 20%-80% “variation across hospitals. It’s really just ingrained habits,” Dr. Stephens said at Pediatric Hospital Medicine.
“The rate of positive blood culture was really low, and the association was for higher cost and utilization. I think this really reinforces the IDSA guidelines. We need to focus on quality improvement efforts to do this better,” he said, noting that he hopes to do so at his own institution.
“I’d also like to know more on the positives. In the single center studies, we know more than half of them are contaminants. Often, there’s more contamination than true positives,” he said at the meeting sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Instead of routine blood culture, Dr. Stephens recommended in his article to send pus for a Gram stain and culture and sensitivity, while noting that blood cultures remain reasonable for complicated infections, immunocompromised patients, and neonates.
There was no external funding, and Dr. Stephens didn’t report any disclosures.
SEATTLE – Blood cultures were ordered for over half of pediatric skin infection encounters across 38 children’s hospitals, with rates varying from about 20% to 80% between hospitals, according to a review of almost 50,000 encounters in the Pediatric Health Information System database.
It was a surprising finding, because current guidelines from the Infectious Diseases Society of America do not recommend blood cultures as part of the routine evaluation of uncomplicated pediatric skin and soft-tissue infections (SSTIs), meaning infections in children who are otherwise healthy without neutropenia or other complicating factors.
Just 0.6% of the cultures were positive in the review, and it’s likely some of those were caused by contamination. After adjustment for demographics, complex chronic conditions, and severity of illness, culture draws were associated with a 20% increase in hospital length of stay (LOS), hospital costs, and 30-day readmission rates.
“Our data provide more evidence that [routine] blood cultures for children with SSTI represents low-value practice and should be avoided,” said lead investigator John Stephens, MD, a pediatrics professor and hospitalist at the University of North Carolina at Chapel Hill.
Dr. Stephens became curious about how common the practice was across hospitals after he and a friend penned an article about the issue for the Journal of Hospital Medicine’s “Things We Do for No Reason” series. The single-center studies they reviewed showed similarly high rates of both testing and negative cultures (J Hosp Med. 2018 Jul;13[7]:496-9).
Dr. Stephens and his team queried the Pediatric Health Information System database for encounters in children aged 2 months to 18 years with the diagnostic code 383, “cellulitis and other skin infections,” from 2012 to 2017, during which time “there really wasn’t a change” in IDSA guidance, he noted. Transfers, encounters with ICU care, and immunocompromised children were excluded.
Hospital admissions were included in the review if they had an additional code for erysipelas, cellulitis, impetigo, or other localized skin infection. The rate of positive cultures was inferred from subsequent codes for bacteremia or septicemia.
Across 49,291 encounters, the median rate of blood culture for skin infection was 51.6%, with tremendous variation between hospitals. With blood cultures, the hospital LOS was about 1.9 days, the hospital cost was $4,030, and the 30-day readmission rate was 1.3%. Without cultures, LOS was 1.6 days, the cost was $3,291, and the readmission rate was 1%.
Although infrequent, it’s likely that positive cultures triggered additional work-up, time in the hospital, and other measures, which might help account for the increase in LOS and costs.
As for why blood testing was so common, especially in some hospitals, “I think it’s just institutional culture. No amount of clinical variation in patient population could explain” a 20%-80% “variation across hospitals. It’s really just ingrained habits,” Dr. Stephens said at Pediatric Hospital Medicine.
“The rate of positive blood culture was really low, and the association was for higher cost and utilization. I think this really reinforces the IDSA guidelines. We need to focus on quality improvement efforts to do this better,” he said, noting that he hopes to do so at his own institution.
“I’d also like to know more on the positives. In the single center studies, we know more than half of them are contaminants. Often, there’s more contamination than true positives,” he said at the meeting sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Instead of routine blood culture, Dr. Stephens recommended in his article to send pus for a Gram stain and culture and sensitivity, while noting that blood cultures remain reasonable for complicated infections, immunocompromised patients, and neonates.
There was no external funding, and Dr. Stephens didn’t report any disclosures.
REPORTING FROM PHM 2019
Poll: Medicare-for-all sees slight drop in support
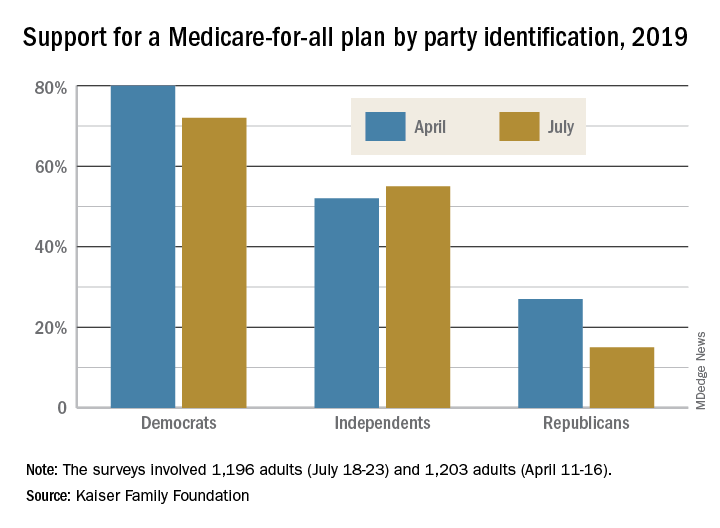
The two polls showed that Americans’ overall favorability for a national Medicare-for-all plan declined from 56% in April to 51% in July. That drop came from Democratic respondents, whose support went from 80% to 72%, and from Republicans, whose support declined from 27% to 15%. Overall favorability increased from 52% to 55% among independents, Kaiser reported in its latest Health Tracking Poll.
“The small dip in Medicare-for-all support may reflect recent debate over the role of private insurance, including employer-sponsored coverage, which would largely disappear under the leading Medicare-for-all plans but would continue under a public option,” Kaiser said in a statement accompanying the report.
When given a choice, 55% of Democrats and Democratic-leaning Independents (Republicans were not asked) would rather build on the existing Affordable Care Act, compared with 39% who want to replace it with Medicare-for-all, the Kaiser investigators said.
Support is currently greater for a public option that would give all residents the ability to choose a government-sponsored insurance plan. Almost two-thirds of those surveyed said that they favor such a proposal, with support at 85% for Democrats, 68% for independents, and 36% for Republicans, the report’s authors said.
The two polls were conducted among 1,196 adults during July 18-23, 2019, and 1,203 adults during April 11-16, 2019. The margin of sampling error for both polls was plus or minus 3 percentage points.

The two polls showed that Americans’ overall favorability for a national Medicare-for-all plan declined from 56% in April to 51% in July. That drop came from Democratic respondents, whose support went from 80% to 72%, and from Republicans, whose support declined from 27% to 15%. Overall favorability increased from 52% to 55% among independents, Kaiser reported in its latest Health Tracking Poll.
“The small dip in Medicare-for-all support may reflect recent debate over the role of private insurance, including employer-sponsored coverage, which would largely disappear under the leading Medicare-for-all plans but would continue under a public option,” Kaiser said in a statement accompanying the report.
When given a choice, 55% of Democrats and Democratic-leaning Independents (Republicans were not asked) would rather build on the existing Affordable Care Act, compared with 39% who want to replace it with Medicare-for-all, the Kaiser investigators said.
Support is currently greater for a public option that would give all residents the ability to choose a government-sponsored insurance plan. Almost two-thirds of those surveyed said that they favor such a proposal, with support at 85% for Democrats, 68% for independents, and 36% for Republicans, the report’s authors said.
The two polls were conducted among 1,196 adults during July 18-23, 2019, and 1,203 adults during April 11-16, 2019. The margin of sampling error for both polls was plus or minus 3 percentage points.

The two polls showed that Americans’ overall favorability for a national Medicare-for-all plan declined from 56% in April to 51% in July. That drop came from Democratic respondents, whose support went from 80% to 72%, and from Republicans, whose support declined from 27% to 15%. Overall favorability increased from 52% to 55% among independents, Kaiser reported in its latest Health Tracking Poll.
“The small dip in Medicare-for-all support may reflect recent debate over the role of private insurance, including employer-sponsored coverage, which would largely disappear under the leading Medicare-for-all plans but would continue under a public option,” Kaiser said in a statement accompanying the report.
When given a choice, 55% of Democrats and Democratic-leaning Independents (Republicans were not asked) would rather build on the existing Affordable Care Act, compared with 39% who want to replace it with Medicare-for-all, the Kaiser investigators said.
Support is currently greater for a public option that would give all residents the ability to choose a government-sponsored insurance plan. Almost two-thirds of those surveyed said that they favor such a proposal, with support at 85% for Democrats, 68% for independents, and 36% for Republicans, the report’s authors said.
The two polls were conducted among 1,196 adults during July 18-23, 2019, and 1,203 adults during April 11-16, 2019. The margin of sampling error for both polls was plus or minus 3 percentage points.
Dispatch from HM19: COPD updates
Session presenter
Cathy Grossman MD, FCCP, CHSE
Session title
COPD Updates 2019
Session summary
Chronic obstructive pulmonary disease (COPD) is the third most common cause of death in the United States and accounts for close to 730,000 admissions and 120,000 deaths per year.1 That correlates to one death every 4 minutes. By 2020, the adjusted cost of COPD in the United States was projected to be approximately $50 billion.2
Every COPD exacerbation is associated with economic, social, and mortality burdens. The probability of survival decreases to 20% by the end of 5 years in patients with frequent readmissions, compared with patients with no acute exacerbations of COPD.3 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) recently released its 2019 report and gave fresh guidance on medication changes to consider in patients who have had a COPD exacerbation.
At HM19, Cathy Grossman, MD, assistant professor of medicine in the division of pulmonary and critical care medicine at Virginia Commonwealth University, Richmond, discussed the updates. She explained that most of the patients who are treated by hospitalists are GOLD group C or group D, and stressed the importance of involving the pulmonology team in the care of these patients.
Dr. Grossman explained that GOLD 2019 recommended using eosinophil counts to predict the effect of inhaled corticosteroids (ICS), added to regular maintenance bronchodilator treatment, in preventing future exacerbations. These effects are observed to be incrementally increasing at higher eosinophil counts. For patients who are taking a long-acting beta2-agonist or muscarinic antagonist (LABA or LAMA), and have a high eosinophil count (at least 300 cells/mcL, or at least 100 cells/mcL plus a history of several exacerbations), one could consider adding an ICS.4 For patients who don’t fulfill these criteria, one could try a LABA plus a LAMA. However, one has to be cautious as some of these patients get intravenous dexamethasone by EMS and admission labs may not show eosinophils.
A caveat to using ICS is that, in some of these of the patients, ICS may lead to bacterial overgrowth and therefore more pneumonias, and that may be contributing to frequent admissions of these patients. In such patients, discontinuation might be a viable option. The guidelines recommend starting GOLD group C and D patients with LAMA or LAMA/LABA combination inhalers, and ICS if they have high eosinophil counts. If patients are already on triple therapy, one could add roflumilast5 or a macrolide.
The effectiveness of noninvasive positive-pressure ventilation (NIV) in COPD patients with prolonged hypercapnia after ventilatory support for acute respiratory failure remains unclear, although there is some data to support the use of home NIV in patients with COPD and obstructive sleep apnea, both with and without hypercapnia. Dr. Grossman mentioned that there are still many unanswered questions, like identifying the right patient, right time, and right settings, and more studies are underway.
Dr. Grossman concluded that bread-and-butter topics like smoking cessation counseling, inhaler instruction, and referral to pulmonary rehab are still the most important tools to decrease COPD exacerbations.
Dr. Jonnalagadda is a physician advisor, and Dr. Medarametla is medical director, of hospital medicine at Baystate Medical Center in Springfield, Mass.
References
1. Guarascio AJ et al. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res. 2013 Jun 17;5:235-45.
2. Morbidity & Mortality: 2012 Chart Book on Cardiovascular, Lung, and Blood Disease. National Institutes of Health and National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/files/docs/research/2012_ChartBook_508.pdf.
3. Soler-Cataluña JJ et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease; Thorax. 2005;60:925-31.
4. Cheng SL. Blood eosinophils and inhaled corticosteroids in patients with COPD: Systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2018 Sept 6;13:2775-84.
5. FJ Martinez et al. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): A multicenter, randomized, controlled trial. Lancet. 2015;385(9971):857-66.
Session presenter
Cathy Grossman MD, FCCP, CHSE
Session title
COPD Updates 2019
Session summary
Chronic obstructive pulmonary disease (COPD) is the third most common cause of death in the United States and accounts for close to 730,000 admissions and 120,000 deaths per year.1 That correlates to one death every 4 minutes. By 2020, the adjusted cost of COPD in the United States was projected to be approximately $50 billion.2
Every COPD exacerbation is associated with economic, social, and mortality burdens. The probability of survival decreases to 20% by the end of 5 years in patients with frequent readmissions, compared with patients with no acute exacerbations of COPD.3 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) recently released its 2019 report and gave fresh guidance on medication changes to consider in patients who have had a COPD exacerbation.
At HM19, Cathy Grossman, MD, assistant professor of medicine in the division of pulmonary and critical care medicine at Virginia Commonwealth University, Richmond, discussed the updates. She explained that most of the patients who are treated by hospitalists are GOLD group C or group D, and stressed the importance of involving the pulmonology team in the care of these patients.
Dr. Grossman explained that GOLD 2019 recommended using eosinophil counts to predict the effect of inhaled corticosteroids (ICS), added to regular maintenance bronchodilator treatment, in preventing future exacerbations. These effects are observed to be incrementally increasing at higher eosinophil counts. For patients who are taking a long-acting beta2-agonist or muscarinic antagonist (LABA or LAMA), and have a high eosinophil count (at least 300 cells/mcL, or at least 100 cells/mcL plus a history of several exacerbations), one could consider adding an ICS.4 For patients who don’t fulfill these criteria, one could try a LABA plus a LAMA. However, one has to be cautious as some of these patients get intravenous dexamethasone by EMS and admission labs may not show eosinophils.
A caveat to using ICS is that, in some of these of the patients, ICS may lead to bacterial overgrowth and therefore more pneumonias, and that may be contributing to frequent admissions of these patients. In such patients, discontinuation might be a viable option. The guidelines recommend starting GOLD group C and D patients with LAMA or LAMA/LABA combination inhalers, and ICS if they have high eosinophil counts. If patients are already on triple therapy, one could add roflumilast5 or a macrolide.
The effectiveness of noninvasive positive-pressure ventilation (NIV) in COPD patients with prolonged hypercapnia after ventilatory support for acute respiratory failure remains unclear, although there is some data to support the use of home NIV in patients with COPD and obstructive sleep apnea, both with and without hypercapnia. Dr. Grossman mentioned that there are still many unanswered questions, like identifying the right patient, right time, and right settings, and more studies are underway.
Dr. Grossman concluded that bread-and-butter topics like smoking cessation counseling, inhaler instruction, and referral to pulmonary rehab are still the most important tools to decrease COPD exacerbations.
Dr. Jonnalagadda is a physician advisor, and Dr. Medarametla is medical director, of hospital medicine at Baystate Medical Center in Springfield, Mass.
References
1. Guarascio AJ et al. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res. 2013 Jun 17;5:235-45.
2. Morbidity & Mortality: 2012 Chart Book on Cardiovascular, Lung, and Blood Disease. National Institutes of Health and National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/files/docs/research/2012_ChartBook_508.pdf.
3. Soler-Cataluña JJ et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease; Thorax. 2005;60:925-31.
4. Cheng SL. Blood eosinophils and inhaled corticosteroids in patients with COPD: Systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2018 Sept 6;13:2775-84.
5. FJ Martinez et al. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): A multicenter, randomized, controlled trial. Lancet. 2015;385(9971):857-66.
Session presenter
Cathy Grossman MD, FCCP, CHSE
Session title
COPD Updates 2019
Session summary
Chronic obstructive pulmonary disease (COPD) is the third most common cause of death in the United States and accounts for close to 730,000 admissions and 120,000 deaths per year.1 That correlates to one death every 4 minutes. By 2020, the adjusted cost of COPD in the United States was projected to be approximately $50 billion.2
Every COPD exacerbation is associated with economic, social, and mortality burdens. The probability of survival decreases to 20% by the end of 5 years in patients with frequent readmissions, compared with patients with no acute exacerbations of COPD.3 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) recently released its 2019 report and gave fresh guidance on medication changes to consider in patients who have had a COPD exacerbation.
At HM19, Cathy Grossman, MD, assistant professor of medicine in the division of pulmonary and critical care medicine at Virginia Commonwealth University, Richmond, discussed the updates. She explained that most of the patients who are treated by hospitalists are GOLD group C or group D, and stressed the importance of involving the pulmonology team in the care of these patients.
Dr. Grossman explained that GOLD 2019 recommended using eosinophil counts to predict the effect of inhaled corticosteroids (ICS), added to regular maintenance bronchodilator treatment, in preventing future exacerbations. These effects are observed to be incrementally increasing at higher eosinophil counts. For patients who are taking a long-acting beta2-agonist or muscarinic antagonist (LABA or LAMA), and have a high eosinophil count (at least 300 cells/mcL, or at least 100 cells/mcL plus a history of several exacerbations), one could consider adding an ICS.4 For patients who don’t fulfill these criteria, one could try a LABA plus a LAMA. However, one has to be cautious as some of these patients get intravenous dexamethasone by EMS and admission labs may not show eosinophils.
A caveat to using ICS is that, in some of these of the patients, ICS may lead to bacterial overgrowth and therefore more pneumonias, and that may be contributing to frequent admissions of these patients. In such patients, discontinuation might be a viable option. The guidelines recommend starting GOLD group C and D patients with LAMA or LAMA/LABA combination inhalers, and ICS if they have high eosinophil counts. If patients are already on triple therapy, one could add roflumilast5 or a macrolide.
The effectiveness of noninvasive positive-pressure ventilation (NIV) in COPD patients with prolonged hypercapnia after ventilatory support for acute respiratory failure remains unclear, although there is some data to support the use of home NIV in patients with COPD and obstructive sleep apnea, both with and without hypercapnia. Dr. Grossman mentioned that there are still many unanswered questions, like identifying the right patient, right time, and right settings, and more studies are underway.
Dr. Grossman concluded that bread-and-butter topics like smoking cessation counseling, inhaler instruction, and referral to pulmonary rehab are still the most important tools to decrease COPD exacerbations.
Dr. Jonnalagadda is a physician advisor, and Dr. Medarametla is medical director, of hospital medicine at Baystate Medical Center in Springfield, Mass.
References
1. Guarascio AJ et al. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res. 2013 Jun 17;5:235-45.
2. Morbidity & Mortality: 2012 Chart Book on Cardiovascular, Lung, and Blood Disease. National Institutes of Health and National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/files/docs/research/2012_ChartBook_508.pdf.
3. Soler-Cataluña JJ et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease; Thorax. 2005;60:925-31.
4. Cheng SL. Blood eosinophils and inhaled corticosteroids in patients with COPD: Systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2018 Sept 6;13:2775-84.
5. FJ Martinez et al. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): A multicenter, randomized, controlled trial. Lancet. 2015;385(9971):857-66.
AI technology meets AFib detection
An artificial intelligence-enabled ECG model identified patients with intermittent atrial fibrillation in a 10-second test with 83% accuracy, based on data from more than 180,000 individuals.
“We have previously shown convolution neural networks can evaluate the resting ECG for detection of antiarrhythmic drug levels, abnormal electrolytes levels, and detection of asymptomatic left ventricular dysfunction, providing proof of concept that clinically important phenomena can be detected with artificial intelligence (AI) applications to the ECG,” wrote Zachi I. Attia, an electrical engineer and a primary author of the study, is with the Mayo Clinic, Rochester, Minn., and colleagues.
In a study published in the Lancet, the researchers reviewed data from 649,931 normal sinus rhythm ECGs collected from 180,922 adults between December 1993 and July 2017.
The ECGs were divided into three groups: training (454,789 ECGs from 126,526 patients) internal validation (64,340 ECGs from 18,116 patients) and testing (130,802 ECGs from 36,280 patients). The primary outcome was whether the AI-programmed ECG could identify AFib in a total of 3,051 patients in the testing data set who had verified AFib before being tested with the AI device. The AI-enabled ECG was designed to detect subtle changes using neural network technology previously used by the researchers to identify ventricular dysfunction.
Overall, a single ECG scan identified AFib with an accuracy of 79.4%, an area under the curve (AUC) of 0.87, sensitivity of 79.0%, and specificity of 79.5%. When researchers reviewed multiple ECGs from a 1-month window of either the study start date or 31 days before the first AFib, the accuracy increased to 83.3%, with an AUC of 0.90, sensitivity of 82.3%, and specificity of 83.4%.
The results support the use of subtle changes on normal sinus rhythm ECG to identify patient with potentially undetected AFib, and suggest that AI-enabled ECGs could be used at the point of care to identify patients at risk after unexplained strokes, also known as embolic stroke of undetermined source (ESUS), or heart failure, the researchers noted.
“Although it would require further study, it is possible that this algorithm could identify a high-risk subset of patients with ESUS who could benefit from empirical anticoagulation,” the researchers said.
The study findings were limited by several factors, including possible mislabeling of patients with unidentified atrial fibrillation who were classified negative. In addition, the prevalence of AFib in the study population may be higher than in the general population, they said.
However, the results suggest that use a noninvasive, widely available, point of care test to identify AFib “could have important implications for atrial fibrillation screening and for the management of patients with unexplained stroke,” they concluded.
This study was funded by internal resources of the Mayo Clinic. The researchers had no financial conflicts to disclose.
SOURCE: Attia ZI et al. Lancet. 2019 Aug 1. doi. org/10.1016/S0140-6736(19)31721-0.
This artificial intelligence-enabled ECG interpretation is groundbreaking in creating an algorithm to reveal the likelihood of atrial fibrillation in ECGs showing sinus rhythm.
AFib is now considered a global pandemic and needs to be detected not only to manage the arrhythmia but also to prevent comorbidities and death.
A 10-second, 12-lead ECG in current clinical practice is unlikely to reveal possible AFib if not present in this short monitoring time. However, the findings have clinical importance, particularly in identifying silent AFib and may have important implications for secondary prevention of patients with embolic stroke of undetermined source in terms of providing appropriate oral anticoagulation to prevent recurrences of stroke. The AI-enabled algorithm would require further validation in a different patient cohort, testing a healthier out-of-hospital population, as well as a rigorous prospective clinical trial assessment.
Future research areas include combining ECG algorithms with demographic variables, clinical features, and biomarkers, as well as exploring the use of wearable devices linking these variables and AI for smart monitoring to diagnose AFib.
Jeroen Hendriks, MD, of the University of Adelaide (Australia), and Larissa Fabritz, MD, of the University of Birmingham (England), made these comments in an accompanying editorial. Dr. Hendriks disclosed lecture or consulting fees from Medtronic and Pfizer/Bristol-Myers Squibb. Dr. Fabritz is the inventor of two patents and disclosed research grants and nonfinancial support from European research institutions and Gilead.
This artificial intelligence-enabled ECG interpretation is groundbreaking in creating an algorithm to reveal the likelihood of atrial fibrillation in ECGs showing sinus rhythm.
AFib is now considered a global pandemic and needs to be detected not only to manage the arrhythmia but also to prevent comorbidities and death.
A 10-second, 12-lead ECG in current clinical practice is unlikely to reveal possible AFib if not present in this short monitoring time. However, the findings have clinical importance, particularly in identifying silent AFib and may have important implications for secondary prevention of patients with embolic stroke of undetermined source in terms of providing appropriate oral anticoagulation to prevent recurrences of stroke. The AI-enabled algorithm would require further validation in a different patient cohort, testing a healthier out-of-hospital population, as well as a rigorous prospective clinical trial assessment.
Future research areas include combining ECG algorithms with demographic variables, clinical features, and biomarkers, as well as exploring the use of wearable devices linking these variables and AI for smart monitoring to diagnose AFib.
Jeroen Hendriks, MD, of the University of Adelaide (Australia), and Larissa Fabritz, MD, of the University of Birmingham (England), made these comments in an accompanying editorial. Dr. Hendriks disclosed lecture or consulting fees from Medtronic and Pfizer/Bristol-Myers Squibb. Dr. Fabritz is the inventor of two patents and disclosed research grants and nonfinancial support from European research institutions and Gilead.
This artificial intelligence-enabled ECG interpretation is groundbreaking in creating an algorithm to reveal the likelihood of atrial fibrillation in ECGs showing sinus rhythm.
AFib is now considered a global pandemic and needs to be detected not only to manage the arrhythmia but also to prevent comorbidities and death.
A 10-second, 12-lead ECG in current clinical practice is unlikely to reveal possible AFib if not present in this short monitoring time. However, the findings have clinical importance, particularly in identifying silent AFib and may have important implications for secondary prevention of patients with embolic stroke of undetermined source in terms of providing appropriate oral anticoagulation to prevent recurrences of stroke. The AI-enabled algorithm would require further validation in a different patient cohort, testing a healthier out-of-hospital population, as well as a rigorous prospective clinical trial assessment.
Future research areas include combining ECG algorithms with demographic variables, clinical features, and biomarkers, as well as exploring the use of wearable devices linking these variables and AI for smart monitoring to diagnose AFib.
Jeroen Hendriks, MD, of the University of Adelaide (Australia), and Larissa Fabritz, MD, of the University of Birmingham (England), made these comments in an accompanying editorial. Dr. Hendriks disclosed lecture or consulting fees from Medtronic and Pfizer/Bristol-Myers Squibb. Dr. Fabritz is the inventor of two patents and disclosed research grants and nonfinancial support from European research institutions and Gilead.
An artificial intelligence-enabled ECG model identified patients with intermittent atrial fibrillation in a 10-second test with 83% accuracy, based on data from more than 180,000 individuals.
“We have previously shown convolution neural networks can evaluate the resting ECG for detection of antiarrhythmic drug levels, abnormal electrolytes levels, and detection of asymptomatic left ventricular dysfunction, providing proof of concept that clinically important phenomena can be detected with artificial intelligence (AI) applications to the ECG,” wrote Zachi I. Attia, an electrical engineer and a primary author of the study, is with the Mayo Clinic, Rochester, Minn., and colleagues.
In a study published in the Lancet, the researchers reviewed data from 649,931 normal sinus rhythm ECGs collected from 180,922 adults between December 1993 and July 2017.
The ECGs were divided into three groups: training (454,789 ECGs from 126,526 patients) internal validation (64,340 ECGs from 18,116 patients) and testing (130,802 ECGs from 36,280 patients). The primary outcome was whether the AI-programmed ECG could identify AFib in a total of 3,051 patients in the testing data set who had verified AFib before being tested with the AI device. The AI-enabled ECG was designed to detect subtle changes using neural network technology previously used by the researchers to identify ventricular dysfunction.
Overall, a single ECG scan identified AFib with an accuracy of 79.4%, an area under the curve (AUC) of 0.87, sensitivity of 79.0%, and specificity of 79.5%. When researchers reviewed multiple ECGs from a 1-month window of either the study start date or 31 days before the first AFib, the accuracy increased to 83.3%, with an AUC of 0.90, sensitivity of 82.3%, and specificity of 83.4%.
The results support the use of subtle changes on normal sinus rhythm ECG to identify patient with potentially undetected AFib, and suggest that AI-enabled ECGs could be used at the point of care to identify patients at risk after unexplained strokes, also known as embolic stroke of undetermined source (ESUS), or heart failure, the researchers noted.
“Although it would require further study, it is possible that this algorithm could identify a high-risk subset of patients with ESUS who could benefit from empirical anticoagulation,” the researchers said.
The study findings were limited by several factors, including possible mislabeling of patients with unidentified atrial fibrillation who were classified negative. In addition, the prevalence of AFib in the study population may be higher than in the general population, they said.
However, the results suggest that use a noninvasive, widely available, point of care test to identify AFib “could have important implications for atrial fibrillation screening and for the management of patients with unexplained stroke,” they concluded.
This study was funded by internal resources of the Mayo Clinic. The researchers had no financial conflicts to disclose.
SOURCE: Attia ZI et al. Lancet. 2019 Aug 1. doi. org/10.1016/S0140-6736(19)31721-0.
An artificial intelligence-enabled ECG model identified patients with intermittent atrial fibrillation in a 10-second test with 83% accuracy, based on data from more than 180,000 individuals.
“We have previously shown convolution neural networks can evaluate the resting ECG for detection of antiarrhythmic drug levels, abnormal electrolytes levels, and detection of asymptomatic left ventricular dysfunction, providing proof of concept that clinically important phenomena can be detected with artificial intelligence (AI) applications to the ECG,” wrote Zachi I. Attia, an electrical engineer and a primary author of the study, is with the Mayo Clinic, Rochester, Minn., and colleagues.
In a study published in the Lancet, the researchers reviewed data from 649,931 normal sinus rhythm ECGs collected from 180,922 adults between December 1993 and July 2017.
The ECGs were divided into three groups: training (454,789 ECGs from 126,526 patients) internal validation (64,340 ECGs from 18,116 patients) and testing (130,802 ECGs from 36,280 patients). The primary outcome was whether the AI-programmed ECG could identify AFib in a total of 3,051 patients in the testing data set who had verified AFib before being tested with the AI device. The AI-enabled ECG was designed to detect subtle changes using neural network technology previously used by the researchers to identify ventricular dysfunction.
Overall, a single ECG scan identified AFib with an accuracy of 79.4%, an area under the curve (AUC) of 0.87, sensitivity of 79.0%, and specificity of 79.5%. When researchers reviewed multiple ECGs from a 1-month window of either the study start date or 31 days before the first AFib, the accuracy increased to 83.3%, with an AUC of 0.90, sensitivity of 82.3%, and specificity of 83.4%.
The results support the use of subtle changes on normal sinus rhythm ECG to identify patient with potentially undetected AFib, and suggest that AI-enabled ECGs could be used at the point of care to identify patients at risk after unexplained strokes, also known as embolic stroke of undetermined source (ESUS), or heart failure, the researchers noted.
“Although it would require further study, it is possible that this algorithm could identify a high-risk subset of patients with ESUS who could benefit from empirical anticoagulation,” the researchers said.
The study findings were limited by several factors, including possible mislabeling of patients with unidentified atrial fibrillation who were classified negative. In addition, the prevalence of AFib in the study population may be higher than in the general population, they said.
However, the results suggest that use a noninvasive, widely available, point of care test to identify AFib “could have important implications for atrial fibrillation screening and for the management of patients with unexplained stroke,” they concluded.
This study was funded by internal resources of the Mayo Clinic. The researchers had no financial conflicts to disclose.
SOURCE: Attia ZI et al. Lancet. 2019 Aug 1. doi. org/10.1016/S0140-6736(19)31721-0.
FROM THE LANCET














