User login
FDA approves pembrolizumab as second-line for advanced ESCC
The Food and Drug Administration has approved pembrolizumab (Keytruda) for patients with recurrent, locally advanced, or metastatic esophageal squamous cell carcinoma (ESCC) whose tumors express PD-L1, as determined by an FDA-approved test, with disease progression after one or more prior lines of systemic therapy.
FDA approval was based on results of two clinical trials: KEYNOTE-180 and KEYNOTE-181. KEYNOTE-181 was a randomized, open-label, active-controlled trial of 628 patients with recurrent, locally advanced, or metastatic esophageal cancer who progressed on or after one prior line of systemic treatment for advanced or metastatic disease. Patients who received pembrolizumab had a median overall survival of 10.3 months, compared with 6.7 months for patients who received control drugs.
In KEYNOTE-180, a single-arm, open-label trial of 121 patients with esophageal cancer who progressed after two prior lines of treatment, patients who had a PD-L1 combined positive score of at least 10 had an overall response rate of 20%, with response durations ranging from 4.2 to over 25.1 months, and with 71% of those patients having a response time over 6 months.
Adverse reactions reported in KEYNOTE-180 and –181 were similar to those in previous trials involving pembrolizumab in patients with melanoma and non–small cell lung cancer. The most common reactions were fatigue, musculoskeletal pain, decreased appetite, pruritus, diarrhea, nausea, rash, pyrexia, cough, dyspnea, constipation, pain, and abdominal pain.
The PD-L1 IHC 22C3 pharmDx kit was approved as the companion diagnostic device, the FDA said.
Find the full press release on the FDA website.
The Food and Drug Administration has approved pembrolizumab (Keytruda) for patients with recurrent, locally advanced, or metastatic esophageal squamous cell carcinoma (ESCC) whose tumors express PD-L1, as determined by an FDA-approved test, with disease progression after one or more prior lines of systemic therapy.
FDA approval was based on results of two clinical trials: KEYNOTE-180 and KEYNOTE-181. KEYNOTE-181 was a randomized, open-label, active-controlled trial of 628 patients with recurrent, locally advanced, or metastatic esophageal cancer who progressed on or after one prior line of systemic treatment for advanced or metastatic disease. Patients who received pembrolizumab had a median overall survival of 10.3 months, compared with 6.7 months for patients who received control drugs.
In KEYNOTE-180, a single-arm, open-label trial of 121 patients with esophageal cancer who progressed after two prior lines of treatment, patients who had a PD-L1 combined positive score of at least 10 had an overall response rate of 20%, with response durations ranging from 4.2 to over 25.1 months, and with 71% of those patients having a response time over 6 months.
Adverse reactions reported in KEYNOTE-180 and –181 were similar to those in previous trials involving pembrolizumab in patients with melanoma and non–small cell lung cancer. The most common reactions were fatigue, musculoskeletal pain, decreased appetite, pruritus, diarrhea, nausea, rash, pyrexia, cough, dyspnea, constipation, pain, and abdominal pain.
The PD-L1 IHC 22C3 pharmDx kit was approved as the companion diagnostic device, the FDA said.
Find the full press release on the FDA website.
The Food and Drug Administration has approved pembrolizumab (Keytruda) for patients with recurrent, locally advanced, or metastatic esophageal squamous cell carcinoma (ESCC) whose tumors express PD-L1, as determined by an FDA-approved test, with disease progression after one or more prior lines of systemic therapy.
FDA approval was based on results of two clinical trials: KEYNOTE-180 and KEYNOTE-181. KEYNOTE-181 was a randomized, open-label, active-controlled trial of 628 patients with recurrent, locally advanced, or metastatic esophageal cancer who progressed on or after one prior line of systemic treatment for advanced or metastatic disease. Patients who received pembrolizumab had a median overall survival of 10.3 months, compared with 6.7 months for patients who received control drugs.
In KEYNOTE-180, a single-arm, open-label trial of 121 patients with esophageal cancer who progressed after two prior lines of treatment, patients who had a PD-L1 combined positive score of at least 10 had an overall response rate of 20%, with response durations ranging from 4.2 to over 25.1 months, and with 71% of those patients having a response time over 6 months.
Adverse reactions reported in KEYNOTE-180 and –181 were similar to those in previous trials involving pembrolizumab in patients with melanoma and non–small cell lung cancer. The most common reactions were fatigue, musculoskeletal pain, decreased appetite, pruritus, diarrhea, nausea, rash, pyrexia, cough, dyspnea, constipation, pain, and abdominal pain.
The PD-L1 IHC 22C3 pharmDx kit was approved as the companion diagnostic device, the FDA said.
Find the full press release on the FDA website.
ctDNA may predict relapse risk in early breast cancer
Following therapy with curative intent for early-stage primary breast care, the presence of circulating tumor DNA may identify those patients at high risk for relapse, investigators reported.
Among 101 women treated for early-stage breast cancer and followed for a median of nearly 3 years, detection of circulating tumor DNA (ctDNA) during follow-up was associated with a 2,400% increased risk for relapse, and detection of ctDNA at diagnosis but before treatment was associated with a nearly 500% risk, wrote Isaac Garcia-Murillas, PhD, of the Institute of Cancer Research, London, and colleagues.
“Prospective clinical trials are now required to assess whether detection of ctDNA can improve outcomes in patients, and a phase 2 interventional trial in TNBC [triple-negative breast cancer] has been initiated. This trial may develop a new treatment paradigm for treating breast cancer, in which treatment is initiated at molecular relapse without waiting for symptomatic incurable metastatic disease to develop,” they wrote in JAMA Oncology.
The investigators conducted a prospective, multicenter validation study of samples collected from women with early-stage breast cancer irrespective of hormone-receptor or HER2 status. The patients were scheduled for neoadjuvant chemotherapy followed by surgery, or surgery followed by adjuvant therapy.
Of 170 women recruited, 101 had tumors with identified mutations and were included in the main cohort. The investigators also conducted secondary analyses with patients in this cohort plus an additional 43 women who had participated in a previous proof-of-principle study.
They first sequenced tumor DNA to identify somatic mutations in primary tumors that could then be tracked using a breast cancer driver gene panel. For each sample, a personalized digital polymerase chain reaction (dPCR) assay was created to identify the mutations in plasma samples.
The plasma samples were collected every 3 months for the first year of follow-up, then every 6 months thereafter.
In the main cohort, the median age was 54 years, and the median follow-up was 35.5 months. The investigators found that, for the primary endpoint of relapse-free survival, ctDNA was associated with a hazard ratio for relapse of 25.2 (P less than .001). Detection of ctDNA in samples taken at the time of diagnosis was also associated with worse relapse-free survival, with an HR of 5.8 (P = .01).
In a secondary analysis, ctDNA detection preceded clinical relapse by a median of 10.7 months, and was associated with relapse in all breast cancer subtypes.
Of 29 patients who experienced a relapse, 22 of 23 with extracranial distant metastatic relapse had prior ctDNA detection.
The remaining six patients experienced relapse without ctDNA detection either before or at the time of relapse. Each of these six patients had a relapse at a single site: in the brain in three patients (with no extracranial relapses), in the ovaries in one patient, and solitary locoregional relapses in two patients.
The investigators acknowledged that the results “demonstrate clinical validity for ctDNA mutation tracking with dPCR but do not demonstrate clinical utility. Without evidence that mutation tracking can improve patient outcome, our results should not be recommended yet for routine clinical practice.”
The study was funded by Breast Cancer Now, Le Cure, and National Institute for Health Research funding to the Biomedical Research Centre at the Royal Marsden Hospital and the Institute of Cancer Research. Dr. Garcia-Murillas had no disclosures. Multiple coauthors reported grants and/or fees from various pharmaceutical companies.
SOURCE: Garcia-Murillias I et al. JAMA Oncol. 2019 Aug 1. doi: 10.1001/jamaoncol.2019.1838.
Although a strength of the study is the inclusion of all subtypes of breast cancer, Garcia-Murillas et al. found that the ability to detect circulating tumor DNA (ctDNA) was likely influenced by biologic factors, including receptor subtypes. The study had a median follow-up of 36.3 months (in the combined cohorts); however, because the risk of relapse for luminal estrogen receptor–positive breast cancers is known to persist for decades, these data cannot be applied to late recurrences, which are largely derived from luminal estrogen receptor–positive disease. Longer-term follow-up with serial sampling of ctDNA will be required to demonstrate validation for this patient population.
As addressed by the authors, the clinical utility for ctDNA detection in early-stage breast cancer is still unknown. Proof of clinical utility can be accomplished through prospective, multi-institutional trials randomizing ctDNA-positive patients to therapy versus control and demonstrating reductions in disease-free and overall survival. The use of real-time testing and rapid turnaround time may prove to be challenging if we are to implement ctDNA testing as an integral biomarker for clinical decision making. However, the study by Garcia-Murillas et al. is a major step forward in reaching this goal because the results suggest the feasibility and clinical validation of ctDNA for patients with early-stage disease.
Remarks from Swathi Karthikeyan, MS, of Johns Hopkins University, Baltimore, and Ben Ho Park, MD, PhD, of Johns Hopkins and Vanderbilt University, Nashville, Tenn., are condensed and adapted from an editorial accompanying the study by Garcia-Murillas et al. Dr. Park reported royalties from Horizon Discovery, serving as a scientific advisory board member for Loxo Oncology, having an ownership interest in Loxo Oncology, serving as a recent paid consultant for Foundation Medicine, Jackson Laboratories, H3 Biomedicine, Casdin Capital, Roche, Eli Lilly, and Astra Zeneca, and having research contracts with Abbvie, Foundation Medicine, and Pfizer. No other disclosures were reported.
Although a strength of the study is the inclusion of all subtypes of breast cancer, Garcia-Murillas et al. found that the ability to detect circulating tumor DNA (ctDNA) was likely influenced by biologic factors, including receptor subtypes. The study had a median follow-up of 36.3 months (in the combined cohorts); however, because the risk of relapse for luminal estrogen receptor–positive breast cancers is known to persist for decades, these data cannot be applied to late recurrences, which are largely derived from luminal estrogen receptor–positive disease. Longer-term follow-up with serial sampling of ctDNA will be required to demonstrate validation for this patient population.
As addressed by the authors, the clinical utility for ctDNA detection in early-stage breast cancer is still unknown. Proof of clinical utility can be accomplished through prospective, multi-institutional trials randomizing ctDNA-positive patients to therapy versus control and demonstrating reductions in disease-free and overall survival. The use of real-time testing and rapid turnaround time may prove to be challenging if we are to implement ctDNA testing as an integral biomarker for clinical decision making. However, the study by Garcia-Murillas et al. is a major step forward in reaching this goal because the results suggest the feasibility and clinical validation of ctDNA for patients with early-stage disease.
Remarks from Swathi Karthikeyan, MS, of Johns Hopkins University, Baltimore, and Ben Ho Park, MD, PhD, of Johns Hopkins and Vanderbilt University, Nashville, Tenn., are condensed and adapted from an editorial accompanying the study by Garcia-Murillas et al. Dr. Park reported royalties from Horizon Discovery, serving as a scientific advisory board member for Loxo Oncology, having an ownership interest in Loxo Oncology, serving as a recent paid consultant for Foundation Medicine, Jackson Laboratories, H3 Biomedicine, Casdin Capital, Roche, Eli Lilly, and Astra Zeneca, and having research contracts with Abbvie, Foundation Medicine, and Pfizer. No other disclosures were reported.
Although a strength of the study is the inclusion of all subtypes of breast cancer, Garcia-Murillas et al. found that the ability to detect circulating tumor DNA (ctDNA) was likely influenced by biologic factors, including receptor subtypes. The study had a median follow-up of 36.3 months (in the combined cohorts); however, because the risk of relapse for luminal estrogen receptor–positive breast cancers is known to persist for decades, these data cannot be applied to late recurrences, which are largely derived from luminal estrogen receptor–positive disease. Longer-term follow-up with serial sampling of ctDNA will be required to demonstrate validation for this patient population.
As addressed by the authors, the clinical utility for ctDNA detection in early-stage breast cancer is still unknown. Proof of clinical utility can be accomplished through prospective, multi-institutional trials randomizing ctDNA-positive patients to therapy versus control and demonstrating reductions in disease-free and overall survival. The use of real-time testing and rapid turnaround time may prove to be challenging if we are to implement ctDNA testing as an integral biomarker for clinical decision making. However, the study by Garcia-Murillas et al. is a major step forward in reaching this goal because the results suggest the feasibility and clinical validation of ctDNA for patients with early-stage disease.
Remarks from Swathi Karthikeyan, MS, of Johns Hopkins University, Baltimore, and Ben Ho Park, MD, PhD, of Johns Hopkins and Vanderbilt University, Nashville, Tenn., are condensed and adapted from an editorial accompanying the study by Garcia-Murillas et al. Dr. Park reported royalties from Horizon Discovery, serving as a scientific advisory board member for Loxo Oncology, having an ownership interest in Loxo Oncology, serving as a recent paid consultant for Foundation Medicine, Jackson Laboratories, H3 Biomedicine, Casdin Capital, Roche, Eli Lilly, and Astra Zeneca, and having research contracts with Abbvie, Foundation Medicine, and Pfizer. No other disclosures were reported.
Following therapy with curative intent for early-stage primary breast care, the presence of circulating tumor DNA may identify those patients at high risk for relapse, investigators reported.
Among 101 women treated for early-stage breast cancer and followed for a median of nearly 3 years, detection of circulating tumor DNA (ctDNA) during follow-up was associated with a 2,400% increased risk for relapse, and detection of ctDNA at diagnosis but before treatment was associated with a nearly 500% risk, wrote Isaac Garcia-Murillas, PhD, of the Institute of Cancer Research, London, and colleagues.
“Prospective clinical trials are now required to assess whether detection of ctDNA can improve outcomes in patients, and a phase 2 interventional trial in TNBC [triple-negative breast cancer] has been initiated. This trial may develop a new treatment paradigm for treating breast cancer, in which treatment is initiated at molecular relapse without waiting for symptomatic incurable metastatic disease to develop,” they wrote in JAMA Oncology.
The investigators conducted a prospective, multicenter validation study of samples collected from women with early-stage breast cancer irrespective of hormone-receptor or HER2 status. The patients were scheduled for neoadjuvant chemotherapy followed by surgery, or surgery followed by adjuvant therapy.
Of 170 women recruited, 101 had tumors with identified mutations and were included in the main cohort. The investigators also conducted secondary analyses with patients in this cohort plus an additional 43 women who had participated in a previous proof-of-principle study.
They first sequenced tumor DNA to identify somatic mutations in primary tumors that could then be tracked using a breast cancer driver gene panel. For each sample, a personalized digital polymerase chain reaction (dPCR) assay was created to identify the mutations in plasma samples.
The plasma samples were collected every 3 months for the first year of follow-up, then every 6 months thereafter.
In the main cohort, the median age was 54 years, and the median follow-up was 35.5 months. The investigators found that, for the primary endpoint of relapse-free survival, ctDNA was associated with a hazard ratio for relapse of 25.2 (P less than .001). Detection of ctDNA in samples taken at the time of diagnosis was also associated with worse relapse-free survival, with an HR of 5.8 (P = .01).
In a secondary analysis, ctDNA detection preceded clinical relapse by a median of 10.7 months, and was associated with relapse in all breast cancer subtypes.
Of 29 patients who experienced a relapse, 22 of 23 with extracranial distant metastatic relapse had prior ctDNA detection.
The remaining six patients experienced relapse without ctDNA detection either before or at the time of relapse. Each of these six patients had a relapse at a single site: in the brain in three patients (with no extracranial relapses), in the ovaries in one patient, and solitary locoregional relapses in two patients.
The investigators acknowledged that the results “demonstrate clinical validity for ctDNA mutation tracking with dPCR but do not demonstrate clinical utility. Without evidence that mutation tracking can improve patient outcome, our results should not be recommended yet for routine clinical practice.”
The study was funded by Breast Cancer Now, Le Cure, and National Institute for Health Research funding to the Biomedical Research Centre at the Royal Marsden Hospital and the Institute of Cancer Research. Dr. Garcia-Murillas had no disclosures. Multiple coauthors reported grants and/or fees from various pharmaceutical companies.
SOURCE: Garcia-Murillias I et al. JAMA Oncol. 2019 Aug 1. doi: 10.1001/jamaoncol.2019.1838.
Following therapy with curative intent for early-stage primary breast care, the presence of circulating tumor DNA may identify those patients at high risk for relapse, investigators reported.
Among 101 women treated for early-stage breast cancer and followed for a median of nearly 3 years, detection of circulating tumor DNA (ctDNA) during follow-up was associated with a 2,400% increased risk for relapse, and detection of ctDNA at diagnosis but before treatment was associated with a nearly 500% risk, wrote Isaac Garcia-Murillas, PhD, of the Institute of Cancer Research, London, and colleagues.
“Prospective clinical trials are now required to assess whether detection of ctDNA can improve outcomes in patients, and a phase 2 interventional trial in TNBC [triple-negative breast cancer] has been initiated. This trial may develop a new treatment paradigm for treating breast cancer, in which treatment is initiated at molecular relapse without waiting for symptomatic incurable metastatic disease to develop,” they wrote in JAMA Oncology.
The investigators conducted a prospective, multicenter validation study of samples collected from women with early-stage breast cancer irrespective of hormone-receptor or HER2 status. The patients were scheduled for neoadjuvant chemotherapy followed by surgery, or surgery followed by adjuvant therapy.
Of 170 women recruited, 101 had tumors with identified mutations and were included in the main cohort. The investigators also conducted secondary analyses with patients in this cohort plus an additional 43 women who had participated in a previous proof-of-principle study.
They first sequenced tumor DNA to identify somatic mutations in primary tumors that could then be tracked using a breast cancer driver gene panel. For each sample, a personalized digital polymerase chain reaction (dPCR) assay was created to identify the mutations in plasma samples.
The plasma samples were collected every 3 months for the first year of follow-up, then every 6 months thereafter.
In the main cohort, the median age was 54 years, and the median follow-up was 35.5 months. The investigators found that, for the primary endpoint of relapse-free survival, ctDNA was associated with a hazard ratio for relapse of 25.2 (P less than .001). Detection of ctDNA in samples taken at the time of diagnosis was also associated with worse relapse-free survival, with an HR of 5.8 (P = .01).
In a secondary analysis, ctDNA detection preceded clinical relapse by a median of 10.7 months, and was associated with relapse in all breast cancer subtypes.
Of 29 patients who experienced a relapse, 22 of 23 with extracranial distant metastatic relapse had prior ctDNA detection.
The remaining six patients experienced relapse without ctDNA detection either before or at the time of relapse. Each of these six patients had a relapse at a single site: in the brain in three patients (with no extracranial relapses), in the ovaries in one patient, and solitary locoregional relapses in two patients.
The investigators acknowledged that the results “demonstrate clinical validity for ctDNA mutation tracking with dPCR but do not demonstrate clinical utility. Without evidence that mutation tracking can improve patient outcome, our results should not be recommended yet for routine clinical practice.”
The study was funded by Breast Cancer Now, Le Cure, and National Institute for Health Research funding to the Biomedical Research Centre at the Royal Marsden Hospital and the Institute of Cancer Research. Dr. Garcia-Murillas had no disclosures. Multiple coauthors reported grants and/or fees from various pharmaceutical companies.
SOURCE: Garcia-Murillias I et al. JAMA Oncol. 2019 Aug 1. doi: 10.1001/jamaoncol.2019.1838.
FROM JAMA ONCOLOGY
Functional GI Disorders Common in MS
Key clinical point: Managing comorbid psychiatric disorders in patients with MS could reduce the burden of functional GI disorders.
Major finding: Approximately 42% of patients with MS report functional GI disorders.
Study details: A survey of 6,312 participants in the North American Research Committee on MS Registry.
Disclosures: The study had no sponsor. Dr. Marrie had no disclosures, but other researchers had financial relationships with pharmaceutical companies, such as Merck, Novartis, Roche, Sanofi-Aventis, and Teva.
Citation: Marrie RA et al. CMSC 2019, Abstract QOL13.
Key clinical point: Managing comorbid psychiatric disorders in patients with MS could reduce the burden of functional GI disorders.
Major finding: Approximately 42% of patients with MS report functional GI disorders.
Study details: A survey of 6,312 participants in the North American Research Committee on MS Registry.
Disclosures: The study had no sponsor. Dr. Marrie had no disclosures, but other researchers had financial relationships with pharmaceutical companies, such as Merck, Novartis, Roche, Sanofi-Aventis, and Teva.
Citation: Marrie RA et al. CMSC 2019, Abstract QOL13.
Key clinical point: Managing comorbid psychiatric disorders in patients with MS could reduce the burden of functional GI disorders.
Major finding: Approximately 42% of patients with MS report functional GI disorders.
Study details: A survey of 6,312 participants in the North American Research Committee on MS Registry.
Disclosures: The study had no sponsor. Dr. Marrie had no disclosures, but other researchers had financial relationships with pharmaceutical companies, such as Merck, Novartis, Roche, Sanofi-Aventis, and Teva.
Citation: Marrie RA et al. CMSC 2019, Abstract QOL13.
Age Does Not Influence Cladribine’s Efficacy in MS
Key clinical point: Patients with relapsing-remitting MS gain comparable benefits from cladribine therapy, regardless of age.
Major finding: The annual rate of qualifying relapses was 0.14 for treated patients older than 45 years and 0.15 for treated patients aged 45 or younger.
Study details: A post hoc analysis of data from the CLARITY and CLARITY extension studies, which included 870 patients.
Disclosures: Merck KGaA, which manufactures and markets cladribine, supported the study. Several of the investigators have received speaker honoraria, consulting fees, or other funding from Merck KGaA.
Citation: REPORTING FROM CMSC 2019
Key clinical point: Patients with relapsing-remitting MS gain comparable benefits from cladribine therapy, regardless of age.
Major finding: The annual rate of qualifying relapses was 0.14 for treated patients older than 45 years and 0.15 for treated patients aged 45 or younger.
Study details: A post hoc analysis of data from the CLARITY and CLARITY extension studies, which included 870 patients.
Disclosures: Merck KGaA, which manufactures and markets cladribine, supported the study. Several of the investigators have received speaker honoraria, consulting fees, or other funding from Merck KGaA.
Citation: REPORTING FROM CMSC 2019
Key clinical point: Patients with relapsing-remitting MS gain comparable benefits from cladribine therapy, regardless of age.
Major finding: The annual rate of qualifying relapses was 0.14 for treated patients older than 45 years and 0.15 for treated patients aged 45 or younger.
Study details: A post hoc analysis of data from the CLARITY and CLARITY extension studies, which included 870 patients.
Disclosures: Merck KGaA, which manufactures and markets cladribine, supported the study. Several of the investigators have received speaker honoraria, consulting fees, or other funding from Merck KGaA.
Citation: REPORTING FROM CMSC 2019
Evobrutinib Demonstrates Efficacy, Safety in Relapsing MS
Key clinical point: Treatment with evobrutinib reduced the number of enhancing lesions versus placebo in patients with relapsing multiple sclerosis, supporting further development of this Bruton’s tyrosine kinase (BTK) inhibitor.
Major finding: The cumulative number of MRI-assessed T1 Gd+ lesions from weeks 12-24 was significantly reduced versus placebo; lesion rate ratios were 0.30 for the 75-mg daily arm, and 0.44 for the 75-mg twice-daily arm, with unadjusted P values of .002 and .031, respectively.
Study details: Forty-eight-week results of a randomized, phase 2, placebo-controlled study of 267 patients with relapsing multiple sclerosis.
Disclosures: Dr. Montalban provided disclosures related to Biogen, Merck Serono, Genentech, Genzyme, Novartis, Sanofi-Aventis, Teva Pharmaceuticals, Roche, Celgene, Actelion, National Multiple Sclerosis Society, and Multiple Sclerosis International Federation.
Citation: Montalban X et al. AAN 2019. Abstract S56.004.
Key clinical point: Treatment with evobrutinib reduced the number of enhancing lesions versus placebo in patients with relapsing multiple sclerosis, supporting further development of this Bruton’s tyrosine kinase (BTK) inhibitor.
Major finding: The cumulative number of MRI-assessed T1 Gd+ lesions from weeks 12-24 was significantly reduced versus placebo; lesion rate ratios were 0.30 for the 75-mg daily arm, and 0.44 for the 75-mg twice-daily arm, with unadjusted P values of .002 and .031, respectively.
Study details: Forty-eight-week results of a randomized, phase 2, placebo-controlled study of 267 patients with relapsing multiple sclerosis.
Disclosures: Dr. Montalban provided disclosures related to Biogen, Merck Serono, Genentech, Genzyme, Novartis, Sanofi-Aventis, Teva Pharmaceuticals, Roche, Celgene, Actelion, National Multiple Sclerosis Society, and Multiple Sclerosis International Federation.
Citation: Montalban X et al. AAN 2019. Abstract S56.004.
Key clinical point: Treatment with evobrutinib reduced the number of enhancing lesions versus placebo in patients with relapsing multiple sclerosis, supporting further development of this Bruton’s tyrosine kinase (BTK) inhibitor.
Major finding: The cumulative number of MRI-assessed T1 Gd+ lesions from weeks 12-24 was significantly reduced versus placebo; lesion rate ratios were 0.30 for the 75-mg daily arm, and 0.44 for the 75-mg twice-daily arm, with unadjusted P values of .002 and .031, respectively.
Study details: Forty-eight-week results of a randomized, phase 2, placebo-controlled study of 267 patients with relapsing multiple sclerosis.
Disclosures: Dr. Montalban provided disclosures related to Biogen, Merck Serono, Genentech, Genzyme, Novartis, Sanofi-Aventis, Teva Pharmaceuticals, Roche, Celgene, Actelion, National Multiple Sclerosis Society, and Multiple Sclerosis International Federation.
Citation: Montalban X et al. AAN 2019. Abstract S56.004.
Sinusitis treatment depends on classification, duration of symptoms
ORLANDO – according to a speaker at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
The major signs and symptoms of sinusitis are pressure and pain on the anterior side of the face or in a localized headache, nasal obstruction, and pus observed at exam that is clouded or colored. Patients may also present with a feeling of facial congestion or fullness, nasal discharge, and fever, noted Brian Bizik, MS, PA-C, from Asthma & Allergy of Idaho and Nevada. The condition can present as acute (up to 4 weeks), subacute (4-12 weeks, with resolution of symptoms), chronic (12 weeks or more), and recurrent acute chronic sinusitis. Most cases of sinusitis are accompanied with contiguous nasal mucosa inflammation, and therefore the term rhinosinusitis is preferred.
To diagnose sinusitis, “you want patients to tell you where they’re hurting, and where their pressure is,” Mr. Bizik said, noting that he instructs patients to “point with one finger and tell me how you feel without using the word ‘sinus.’ ” Clinicians should ask whether a patient’s pain is continuous or cyclic, if they have bad breath even after brushing their teeth, if they have a chronic cough as opposed to postnasal drip, whether they have pain when they chew or walk, and if they feel like they are always tired.
According to guidelines from the Infectious Diseases Society of America, if symptoms last longer than 10 days and patients have a fever above 39° C (102.2° F), it is more likely bacterial rather than viral. Another sign of bacterial infection is when patients get better after a few days before worsening again later, said Mr. Bizik. In patients where clinicians suspect bacterial infection, the IDSA recommends amoxicillin/clavulanate over amoxicillin alone because some acute bacterial rhinosinusitis could be Haemophilus influenzae, and up to 30% of these infections can produce beta-lactamase. Patients with an amoxicillin allergy should take doxycycline, which is the only currently recommended antibiotic for patients with acute bacterial rhinosinusitis.
In general, clinicians should treat acute bacterial rhinosinusitis based on whether the patient has the most severe disease, said Mr. Bizik. “Use those three criteria: fever, symptoms longer than 10 days, purulence, and feeling lousy. If you find these people are in the high-risk group, [the guidelines] recommend antibiotic treatment.”
In addition to antibiotics, patients can likely benefit from use of topical corticosteroids such as mometasone, fluticasone, flunisolide, and beclomethasone. “It comes down to simply what you like and what works well for you,” he said. With regard to oral steroids, patients with severe pain can benefit from medication like prednisone. Finally, decongestants and relief with sinus irrigation treatments like Neti pots can help relieve symptoms and promote healthy mucosal function.
On the other hand, sinusitis with a viral origin tends to have “light” flu symptoms that do not worsen over time and almost always resolve within 10 days. “If they fit the viral mold, we’re going to do everything the same [as bacterial sinusitis]; just skip the antibiotics,” he said.
In patients with chronic rhinosinusitis (CRS), the symptoms persist over a longer period of time. CRS has a large number of associated conditions, such as allergic rhinitis and gastroesophageal reflux, as well as environmental factors like cigarette smoke, viral illness, and rebound rhinitis. If a patient’s CRS is caused by allergies, treating the allergies aggressively will improve CRS symptoms. “If they have an allergic component, you really have to have a reason not to put them on montelukast. I would encourage you to do that,” said Mr. Bizik. “Cetirizine and montelukast at bedtime works very well. They’re cheap, effective, generic, and nonsteroidal.”
Other methods for treating symptoms of CRS include saline irrigation to increase mucociliary flow rates, high doses of mucolytics, and first- and second-generation antihistamines, which can take up to 10 days to see the full effect. “I have a 10-day reminder, and I call them on day 11,” said Mr. Bizik. “If they stick with it, they say it really did help. It’s a great way to avoid antibiotics.”
Intranasal corticosteroids are also effective first-line therapies for CRS. However, technique is important when using these medications. In his presentation, Mr. Bizik described the “opposite-hand” technique he teaches to patients to reduce some of the side effects patients experience when using intranasal corticosteroids, including nosebleeds.
“You insert it in the nose, you go in all the way until you just feel your fingers touching your nose, and you point it towards the earlobe so the left nostril goes to the left earlobe [and vice versa], and you just spray,” once or twice a day depending on indication, he said. “Using those consistently, when you do this, the flower smell is less, it doesn’t bother you, less goes down your throat, and it’s very effective.”
Dr. Bizik reports being a speaking and consultant for Grifols, Boehringer Ingelheim, Meda Pharmaceuticals, and an advisory board member for Circassia Pharmaceuticals.
Global Academy for Medical Education and this news organization are owned by the same parent company.
ORLANDO – according to a speaker at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
The major signs and symptoms of sinusitis are pressure and pain on the anterior side of the face or in a localized headache, nasal obstruction, and pus observed at exam that is clouded or colored. Patients may also present with a feeling of facial congestion or fullness, nasal discharge, and fever, noted Brian Bizik, MS, PA-C, from Asthma & Allergy of Idaho and Nevada. The condition can present as acute (up to 4 weeks), subacute (4-12 weeks, with resolution of symptoms), chronic (12 weeks or more), and recurrent acute chronic sinusitis. Most cases of sinusitis are accompanied with contiguous nasal mucosa inflammation, and therefore the term rhinosinusitis is preferred.
To diagnose sinusitis, “you want patients to tell you where they’re hurting, and where their pressure is,” Mr. Bizik said, noting that he instructs patients to “point with one finger and tell me how you feel without using the word ‘sinus.’ ” Clinicians should ask whether a patient’s pain is continuous or cyclic, if they have bad breath even after brushing their teeth, if they have a chronic cough as opposed to postnasal drip, whether they have pain when they chew or walk, and if they feel like they are always tired.
According to guidelines from the Infectious Diseases Society of America, if symptoms last longer than 10 days and patients have a fever above 39° C (102.2° F), it is more likely bacterial rather than viral. Another sign of bacterial infection is when patients get better after a few days before worsening again later, said Mr. Bizik. In patients where clinicians suspect bacterial infection, the IDSA recommends amoxicillin/clavulanate over amoxicillin alone because some acute bacterial rhinosinusitis could be Haemophilus influenzae, and up to 30% of these infections can produce beta-lactamase. Patients with an amoxicillin allergy should take doxycycline, which is the only currently recommended antibiotic for patients with acute bacterial rhinosinusitis.
In general, clinicians should treat acute bacterial rhinosinusitis based on whether the patient has the most severe disease, said Mr. Bizik. “Use those three criteria: fever, symptoms longer than 10 days, purulence, and feeling lousy. If you find these people are in the high-risk group, [the guidelines] recommend antibiotic treatment.”
In addition to antibiotics, patients can likely benefit from use of topical corticosteroids such as mometasone, fluticasone, flunisolide, and beclomethasone. “It comes down to simply what you like and what works well for you,” he said. With regard to oral steroids, patients with severe pain can benefit from medication like prednisone. Finally, decongestants and relief with sinus irrigation treatments like Neti pots can help relieve symptoms and promote healthy mucosal function.
On the other hand, sinusitis with a viral origin tends to have “light” flu symptoms that do not worsen over time and almost always resolve within 10 days. “If they fit the viral mold, we’re going to do everything the same [as bacterial sinusitis]; just skip the antibiotics,” he said.
In patients with chronic rhinosinusitis (CRS), the symptoms persist over a longer period of time. CRS has a large number of associated conditions, such as allergic rhinitis and gastroesophageal reflux, as well as environmental factors like cigarette smoke, viral illness, and rebound rhinitis. If a patient’s CRS is caused by allergies, treating the allergies aggressively will improve CRS symptoms. “If they have an allergic component, you really have to have a reason not to put them on montelukast. I would encourage you to do that,” said Mr. Bizik. “Cetirizine and montelukast at bedtime works very well. They’re cheap, effective, generic, and nonsteroidal.”
Other methods for treating symptoms of CRS include saline irrigation to increase mucociliary flow rates, high doses of mucolytics, and first- and second-generation antihistamines, which can take up to 10 days to see the full effect. “I have a 10-day reminder, and I call them on day 11,” said Mr. Bizik. “If they stick with it, they say it really did help. It’s a great way to avoid antibiotics.”
Intranasal corticosteroids are also effective first-line therapies for CRS. However, technique is important when using these medications. In his presentation, Mr. Bizik described the “opposite-hand” technique he teaches to patients to reduce some of the side effects patients experience when using intranasal corticosteroids, including nosebleeds.
“You insert it in the nose, you go in all the way until you just feel your fingers touching your nose, and you point it towards the earlobe so the left nostril goes to the left earlobe [and vice versa], and you just spray,” once or twice a day depending on indication, he said. “Using those consistently, when you do this, the flower smell is less, it doesn’t bother you, less goes down your throat, and it’s very effective.”
Dr. Bizik reports being a speaking and consultant for Grifols, Boehringer Ingelheim, Meda Pharmaceuticals, and an advisory board member for Circassia Pharmaceuticals.
Global Academy for Medical Education and this news organization are owned by the same parent company.
ORLANDO – according to a speaker at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
The major signs and symptoms of sinusitis are pressure and pain on the anterior side of the face or in a localized headache, nasal obstruction, and pus observed at exam that is clouded or colored. Patients may also present with a feeling of facial congestion or fullness, nasal discharge, and fever, noted Brian Bizik, MS, PA-C, from Asthma & Allergy of Idaho and Nevada. The condition can present as acute (up to 4 weeks), subacute (4-12 weeks, with resolution of symptoms), chronic (12 weeks or more), and recurrent acute chronic sinusitis. Most cases of sinusitis are accompanied with contiguous nasal mucosa inflammation, and therefore the term rhinosinusitis is preferred.
To diagnose sinusitis, “you want patients to tell you where they’re hurting, and where their pressure is,” Mr. Bizik said, noting that he instructs patients to “point with one finger and tell me how you feel without using the word ‘sinus.’ ” Clinicians should ask whether a patient’s pain is continuous or cyclic, if they have bad breath even after brushing their teeth, if they have a chronic cough as opposed to postnasal drip, whether they have pain when they chew or walk, and if they feel like they are always tired.
According to guidelines from the Infectious Diseases Society of America, if symptoms last longer than 10 days and patients have a fever above 39° C (102.2° F), it is more likely bacterial rather than viral. Another sign of bacterial infection is when patients get better after a few days before worsening again later, said Mr. Bizik. In patients where clinicians suspect bacterial infection, the IDSA recommends amoxicillin/clavulanate over amoxicillin alone because some acute bacterial rhinosinusitis could be Haemophilus influenzae, and up to 30% of these infections can produce beta-lactamase. Patients with an amoxicillin allergy should take doxycycline, which is the only currently recommended antibiotic for patients with acute bacterial rhinosinusitis.
In general, clinicians should treat acute bacterial rhinosinusitis based on whether the patient has the most severe disease, said Mr. Bizik. “Use those three criteria: fever, symptoms longer than 10 days, purulence, and feeling lousy. If you find these people are in the high-risk group, [the guidelines] recommend antibiotic treatment.”
In addition to antibiotics, patients can likely benefit from use of topical corticosteroids such as mometasone, fluticasone, flunisolide, and beclomethasone. “It comes down to simply what you like and what works well for you,” he said. With regard to oral steroids, patients with severe pain can benefit from medication like prednisone. Finally, decongestants and relief with sinus irrigation treatments like Neti pots can help relieve symptoms and promote healthy mucosal function.
On the other hand, sinusitis with a viral origin tends to have “light” flu symptoms that do not worsen over time and almost always resolve within 10 days. “If they fit the viral mold, we’re going to do everything the same [as bacterial sinusitis]; just skip the antibiotics,” he said.
In patients with chronic rhinosinusitis (CRS), the symptoms persist over a longer period of time. CRS has a large number of associated conditions, such as allergic rhinitis and gastroesophageal reflux, as well as environmental factors like cigarette smoke, viral illness, and rebound rhinitis. If a patient’s CRS is caused by allergies, treating the allergies aggressively will improve CRS symptoms. “If they have an allergic component, you really have to have a reason not to put them on montelukast. I would encourage you to do that,” said Mr. Bizik. “Cetirizine and montelukast at bedtime works very well. They’re cheap, effective, generic, and nonsteroidal.”
Other methods for treating symptoms of CRS include saline irrigation to increase mucociliary flow rates, high doses of mucolytics, and first- and second-generation antihistamines, which can take up to 10 days to see the full effect. “I have a 10-day reminder, and I call them on day 11,” said Mr. Bizik. “If they stick with it, they say it really did help. It’s a great way to avoid antibiotics.”
Intranasal corticosteroids are also effective first-line therapies for CRS. However, technique is important when using these medications. In his presentation, Mr. Bizik described the “opposite-hand” technique he teaches to patients to reduce some of the side effects patients experience when using intranasal corticosteroids, including nosebleeds.
“You insert it in the nose, you go in all the way until you just feel your fingers touching your nose, and you point it towards the earlobe so the left nostril goes to the left earlobe [and vice versa], and you just spray,” once or twice a day depending on indication, he said. “Using those consistently, when you do this, the flower smell is less, it doesn’t bother you, less goes down your throat, and it’s very effective.”
Dr. Bizik reports being a speaking and consultant for Grifols, Boehringer Ingelheim, Meda Pharmaceuticals, and an advisory board member for Circassia Pharmaceuticals.
Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM CARPS 2019
Machine-learning model predicts anti-TNF nonresponse in RA patients
A machine-learning model that uses clinical profiles and genetic information has shown promise in predicting which rheumatoid arthritis patients respond to anti–tumor necrosis factor drugs in a patient population of European descent.
The model can “help up to 40% of European-descent anti–tumor necrosis factor [TNF] nonresponders avoid ineffective treatments” when compared with the usual “trial-and-error practice,” according to the authors led by Yuanfang Guan, PhD, of the department of computational medicine and bioinformatics at the University of Michigan, Ann Arbor.
The ability to accurately predict rheumatoid arthritis patients’ response to treatments would provide valuable information for optimal drug selection and would help potential nonresponders avoid drug expenses and side effects, such as an increased risk of infections, Dr. Guan and coauthors noted in Arthritis & Rheumatology.
The investigators used a modeling technique called Gaussian process regression (GPR) to predict anti-TNF drug responses. “GPR is designed to predict the unknown dependent variable for any given independent variables based on known but noisy observations of the dependent and independent variables,” they explained.
The model they used won first place in the Dialogue on Reverse Engineering Assessment and Methods: Rheumatoid Arthritis Responder Challenge, which used a crowd-based competition framework to develop a validated molecular predictor of anti-TNF response in RA.
The model was developed and cross-validated using 1,892 patients randomly selected from a training data set of 2,706 individuals of European ancestry compiled from 13 patient cohorts. All patients met 1987 American College of Rheumatology criteria for RA or were diagnosed by a board-certified rheumatologist. In addition, patients were required to have at least moderate disease activity at baseline, based on a 28-joint Disease Activity Score (DAS28) greater than 3.2.
The research team also evaluated the model using an independent dataset of 680 patients from the CERTAIN (Comparative Effectiveness Registry to study Therapies for Arthritis and Inflammatory Conditions) study.
The model combined demographic, clinical, and genetic markers to predict patients’ changes in DAS28 24 months after their baseline assessment, and identify nonresponders to anti-TNF treatments, the authors explained.
“Specifically, the [model] predicts the changes in [DAS28] of patients who have taken 12 months of anti-TNF treatments, and also classifies the patients’ responses based on the EULAR response metric,” they wrote.
Results showed that, in cross-validation tests, the model predicted changes in DAS28 with a correlation coefficient of 0.406, correctly classifying responses of 78% of subjects, with an area under the receiver operating characteristic curve (AUROC) of about 0.66.
In the independent test, the method achieved a Pearson correlation coefficient of 0.393 in predicting the change in DAS28.
Genetic SNP biomarkers provided a small additional contribution to the prediction on top of the clinical models, the authors noted.
“Compared to traditional trial-and-error practice, our model can help up to 40% of European-descent anti-TNF nonresponders avoid ineffective treatments. The model performance is even comparable to some published models utilizing additional biomarker data, whose AUROC ranges from 55% to 74% over various testing sets,” they wrote.
The GPR model has practical advantages in clinical application, unlike many sophisticated machine-learning algorithms, according to the authors. For example, GPR is a well-studied statistical model, its similarity-modeling approach is intuitive, and its results are easy to interpret.
“Our GPR model can predict subpopulations that do not respond to the treatment. This can help physicians tailor treatments for individual patients based on their conditions. ... The model can also estimate confidence intervals for its predictions, allowing physicians to judge how confident the predictions are,” the study authors wrote.
However, they cautioned that because the model was built using patients of European descent they did not expect it to achieve a similar performance in other populations. “Extension of the model over other populations requires new patient data and separate feature selection.”
The research was supported by the National Science Foundation and the National Natural Science Foundation of China. Several of the researchers reported financial relationships with pharmaceutical or technology companies.
SOURCE: Guan Y et al. Arthritis Rheumatol. 2019 Jul 24. doi: 10.1002/art.41056.
A machine-learning model that uses clinical profiles and genetic information has shown promise in predicting which rheumatoid arthritis patients respond to anti–tumor necrosis factor drugs in a patient population of European descent.
The model can “help up to 40% of European-descent anti–tumor necrosis factor [TNF] nonresponders avoid ineffective treatments” when compared with the usual “trial-and-error practice,” according to the authors led by Yuanfang Guan, PhD, of the department of computational medicine and bioinformatics at the University of Michigan, Ann Arbor.
The ability to accurately predict rheumatoid arthritis patients’ response to treatments would provide valuable information for optimal drug selection and would help potential nonresponders avoid drug expenses and side effects, such as an increased risk of infections, Dr. Guan and coauthors noted in Arthritis & Rheumatology.
The investigators used a modeling technique called Gaussian process regression (GPR) to predict anti-TNF drug responses. “GPR is designed to predict the unknown dependent variable for any given independent variables based on known but noisy observations of the dependent and independent variables,” they explained.
The model they used won first place in the Dialogue on Reverse Engineering Assessment and Methods: Rheumatoid Arthritis Responder Challenge, which used a crowd-based competition framework to develop a validated molecular predictor of anti-TNF response in RA.
The model was developed and cross-validated using 1,892 patients randomly selected from a training data set of 2,706 individuals of European ancestry compiled from 13 patient cohorts. All patients met 1987 American College of Rheumatology criteria for RA or were diagnosed by a board-certified rheumatologist. In addition, patients were required to have at least moderate disease activity at baseline, based on a 28-joint Disease Activity Score (DAS28) greater than 3.2.
The research team also evaluated the model using an independent dataset of 680 patients from the CERTAIN (Comparative Effectiveness Registry to study Therapies for Arthritis and Inflammatory Conditions) study.
The model combined demographic, clinical, and genetic markers to predict patients’ changes in DAS28 24 months after their baseline assessment, and identify nonresponders to anti-TNF treatments, the authors explained.
“Specifically, the [model] predicts the changes in [DAS28] of patients who have taken 12 months of anti-TNF treatments, and also classifies the patients’ responses based on the EULAR response metric,” they wrote.
Results showed that, in cross-validation tests, the model predicted changes in DAS28 with a correlation coefficient of 0.406, correctly classifying responses of 78% of subjects, with an area under the receiver operating characteristic curve (AUROC) of about 0.66.
In the independent test, the method achieved a Pearson correlation coefficient of 0.393 in predicting the change in DAS28.
Genetic SNP biomarkers provided a small additional contribution to the prediction on top of the clinical models, the authors noted.
“Compared to traditional trial-and-error practice, our model can help up to 40% of European-descent anti-TNF nonresponders avoid ineffective treatments. The model performance is even comparable to some published models utilizing additional biomarker data, whose AUROC ranges from 55% to 74% over various testing sets,” they wrote.
The GPR model has practical advantages in clinical application, unlike many sophisticated machine-learning algorithms, according to the authors. For example, GPR is a well-studied statistical model, its similarity-modeling approach is intuitive, and its results are easy to interpret.
“Our GPR model can predict subpopulations that do not respond to the treatment. This can help physicians tailor treatments for individual patients based on their conditions. ... The model can also estimate confidence intervals for its predictions, allowing physicians to judge how confident the predictions are,” the study authors wrote.
However, they cautioned that because the model was built using patients of European descent they did not expect it to achieve a similar performance in other populations. “Extension of the model over other populations requires new patient data and separate feature selection.”
The research was supported by the National Science Foundation and the National Natural Science Foundation of China. Several of the researchers reported financial relationships with pharmaceutical or technology companies.
SOURCE: Guan Y et al. Arthritis Rheumatol. 2019 Jul 24. doi: 10.1002/art.41056.
A machine-learning model that uses clinical profiles and genetic information has shown promise in predicting which rheumatoid arthritis patients respond to anti–tumor necrosis factor drugs in a patient population of European descent.
The model can “help up to 40% of European-descent anti–tumor necrosis factor [TNF] nonresponders avoid ineffective treatments” when compared with the usual “trial-and-error practice,” according to the authors led by Yuanfang Guan, PhD, of the department of computational medicine and bioinformatics at the University of Michigan, Ann Arbor.
The ability to accurately predict rheumatoid arthritis patients’ response to treatments would provide valuable information for optimal drug selection and would help potential nonresponders avoid drug expenses and side effects, such as an increased risk of infections, Dr. Guan and coauthors noted in Arthritis & Rheumatology.
The investigators used a modeling technique called Gaussian process regression (GPR) to predict anti-TNF drug responses. “GPR is designed to predict the unknown dependent variable for any given independent variables based on known but noisy observations of the dependent and independent variables,” they explained.
The model they used won first place in the Dialogue on Reverse Engineering Assessment and Methods: Rheumatoid Arthritis Responder Challenge, which used a crowd-based competition framework to develop a validated molecular predictor of anti-TNF response in RA.
The model was developed and cross-validated using 1,892 patients randomly selected from a training data set of 2,706 individuals of European ancestry compiled from 13 patient cohorts. All patients met 1987 American College of Rheumatology criteria for RA or were diagnosed by a board-certified rheumatologist. In addition, patients were required to have at least moderate disease activity at baseline, based on a 28-joint Disease Activity Score (DAS28) greater than 3.2.
The research team also evaluated the model using an independent dataset of 680 patients from the CERTAIN (Comparative Effectiveness Registry to study Therapies for Arthritis and Inflammatory Conditions) study.
The model combined demographic, clinical, and genetic markers to predict patients’ changes in DAS28 24 months after their baseline assessment, and identify nonresponders to anti-TNF treatments, the authors explained.
“Specifically, the [model] predicts the changes in [DAS28] of patients who have taken 12 months of anti-TNF treatments, and also classifies the patients’ responses based on the EULAR response metric,” they wrote.
Results showed that, in cross-validation tests, the model predicted changes in DAS28 with a correlation coefficient of 0.406, correctly classifying responses of 78% of subjects, with an area under the receiver operating characteristic curve (AUROC) of about 0.66.
In the independent test, the method achieved a Pearson correlation coefficient of 0.393 in predicting the change in DAS28.
Genetic SNP biomarkers provided a small additional contribution to the prediction on top of the clinical models, the authors noted.
“Compared to traditional trial-and-error practice, our model can help up to 40% of European-descent anti-TNF nonresponders avoid ineffective treatments. The model performance is even comparable to some published models utilizing additional biomarker data, whose AUROC ranges from 55% to 74% over various testing sets,” they wrote.
The GPR model has practical advantages in clinical application, unlike many sophisticated machine-learning algorithms, according to the authors. For example, GPR is a well-studied statistical model, its similarity-modeling approach is intuitive, and its results are easy to interpret.
“Our GPR model can predict subpopulations that do not respond to the treatment. This can help physicians tailor treatments for individual patients based on their conditions. ... The model can also estimate confidence intervals for its predictions, allowing physicians to judge how confident the predictions are,” the study authors wrote.
However, they cautioned that because the model was built using patients of European descent they did not expect it to achieve a similar performance in other populations. “Extension of the model over other populations requires new patient data and separate feature selection.”
The research was supported by the National Science Foundation and the National Natural Science Foundation of China. Several of the researchers reported financial relationships with pharmaceutical or technology companies.
SOURCE: Guan Y et al. Arthritis Rheumatol. 2019 Jul 24. doi: 10.1002/art.41056.
FROM ARTHRITIS & RHEUMATOLOGY
Did You Know? Psoriasis and cardiovascular disease



Infective endocarditis: Beyond the usual tests
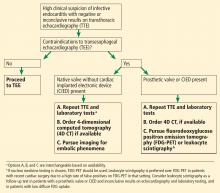
Prompt diagnois of infective endocarditis is critical. Potential consequences of missed or delayed diagnosis, including heart failure, stroke, intracardiac abscess, conduction delays, prosthesis dysfunction, and cerebral emboli, are often catastrophic. Echocardiography is the test used most frequently to evaluate for infective endocarditis, but it misses the diagnosis in almost one-third of cases, and even more often if the patient has a prosthetic valve.
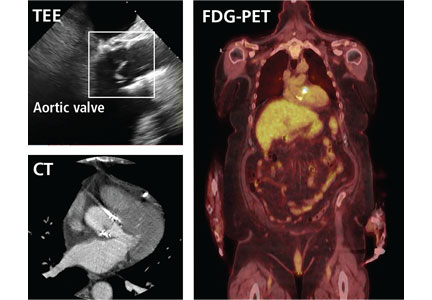
But now, several sophisticated imaging tests are available that complement echocardiography in diagnosing and assessing infective endocarditis; these include 4-dimensional computed tomography (4D CT), fluorodeoxyglucose positron emission tomography (FDG-PET), and leukocyte scintigraphy. These tests have greatly improved our ability not only to diagnose infective endocarditis, but also to determine the extent and spread of infection, and they aid in perioperative assessment. Abnormal findings on these tests have been incorporated into the European Society of Cardiology’s 2015 modified diagnostic criteria for infective endocarditis.1
This article details the indications, advantages, and limitations of the various imaging tests for diagnosing and evaluating infective endocarditis (Table 1).
INFECTIVE ENDOCARDITIS IS DIFFICULT TO DIAGNOSE AND TREAT
Infective endocarditis is difficult to diagnose and treat. Clinical and imaging clues can be subtle, and the diagnosis requires a high level of suspicion and visualization of cardiac structures.
Further, the incidence of infective endocarditis is on the rise in the United States, particularly in women and young adults, likely due to intravenous drug use.2,3
ECHOCARDIOGRAPHY HAS AN IMPORTANT ROLE, BUT IS LIMITED
Echocardiography remains the most commonly performed study for diagnosing infective endocarditis, as it is fast, widely accessible, and less expensive than other imaging tests.
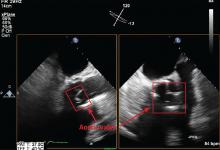
Transthoracic echocardiography (TTE) is often the first choice for testing. However, its sensitivity is only about 70% for detecting vegetations on native valves and 50% for detecting vegetations on prosthetic valves.1 It is inherently constrained by the limited number of views by which a comprehensive external evaluation of the heart can be achieved. Using a 2-dimensional instrument to view a 3-dimensional object is difficult, and depending on several factors, it can be hard to see vegetations and abscesses that are associated with infective endocarditis. Further, TTE is impeded by obesity and by hyperinflated lungs from obstructive pulmonary disease or mechanical ventilation. It has poor sensitivity for detecting small vegetations and for detecting vegetations and paravalvular complications in patients who have a prosthetic valve or a cardiac implanted electronic device.
Transesophageal echocardiography (TEE) is the recommended first-line imaging test for patients with prosthetic valves and no contraindications to the test. Otherwise, it should be done after TTE if the results of TTE are negative but clinical suspicion for infective endocarditis remains high (eg, because the patient uses intravenous drugs). But although TEE has a higher sensitivity than TTE (up to 96% for vegetations on native valves and 92% for those on prosthetic valves, if performed by an experienced sonographer), it can still miss infective endocarditis. Also, TEE does not provide a significant advantage over TTE in patients who have a cardiac implanted electronic device.1,4,5
Regardless of whether TTE or TEE is used, they are estimated to miss up to 30% of cases of infective endocarditis and its sequelae.4 False-negative findings are likelier in patients who have preexisting severe valvular lesions, prosthetic valves, cardiac implanted electronic devices, small vegetations, or abscesses, or if a vegetation has already broken free and embolized. Furthermore, distinguishing between vegetations and thrombi, cardiac tumors, and myxomatous changes using echocardiography is difficult.
CARDIAC CT
For patients who have inconclusive results on echocardiography, contraindications to TEE, or poor sonic windows, cardiac CT can be an excellent alternative. It is especially useful in the setting of a prosthetic valve.
Synchronized (“gated”) with the patient’s heart rate and rhythm, CT machines can acquire images during diastole, reducing motion artifact, and can create 3D images of the heart. In addition, newer machines can acquire several images at different points in the heart cycle to add a fourth dimension—time. The resulting 4D images play like short video loops of the beating heart and allow noninvasive assessment of cardiac anatomy with remarkable detail and resolution.
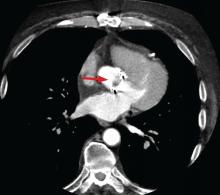
4D CT is increasingly being used in infective endocarditis, and growing evidence indicates that its accuracy is similar to that of TEE in the preoperative evaluation of patients with aortic prosthetic valve endocarditis.6 In a study of 28 patients, complementary use of CT angiography led to a change in treatment strategy in 7 (25%) compared with routine clinical workup.7 Several studies have found no difference between 4D CT and preoperative TEE in detecting pseudoaneurysm, abscess, or valve dehiscence. TEE and 4D CT also have similar sensitivities for detecting infective endocarditis in native and prosthetic valves.8,9
Coupled with CT angiography, 4D CT is also an excellent noninvasive way to perioperatively evaluate the coronary arteries without the risks associated with catheterization in those requiring nonemergency surgery (Figure 1A, B, and C).
4D CT performs well for detecting abscess and pseudoaneurysm but has slightly lower sensitivity for vegetations than TEE (91% vs 99%).9
Gated CT, PET, or both may be useful in cases of suspected prosthetic aortic valve endocarditis when TEE is negative. Pseudoaneurysms are not well visualized with TEE, and the atrial mitral curtain area is often thickened on TEE in cases of aortic prosthetic valve infective endocarditis that do not definitely involve abscesses. Gated CT and PET show this area better.8 This information is important in cases in which a surgeon may be unconvinced that the patient has prosthetic valve endocarditis.
Limitations of 4D cardiac CT
4D CT with or without angiography has limitations. It requires a wide-volume scanner and an experienced reader.
Patients with irregular heart rhythms or uncontrolled tachycardia pose technical problems for image acquisition. Cardiac CT is typically gated (ie, images are obtained within a defined time period) to acquire images during diastole. Ideally, images are acquired when the heart is in mid to late diastole, a time of minimal cardiac motion, so that motion artifact is minimized. To estimate the timing of image acquisition, the cardiac cycle must be predictable, and its duration should be as long as possible. Tachycardia or irregular rhythms such as frequent ectopic beats or atrial fibrillation make acquisition timing difficult, and thus make it nearly impossible to accurately obtain images when the heart is at minimum motion, limiting assessment of cardiac structures or the coronary tree.4,10
Extensive coronary calcification can hinder assessment of the coronary tree by CT coronary angiography.
Contrast exposure may limit the use of CT in some patients (eg, those with contrast allergies or renal dysfunction). However, modern scanners allow for much smaller contrast boluses without decreasing sensitivity.
4D CT involves radiation exposure, especially when done with angiography, although modern scanners have greatly reduced exposure. The average radiation dose in CT coronary angiography is 2.9 to 5.9 mSv11 compared with 7 mSv in diagnostic cardiac catheterization (without angioplasty or stenting) or 16 mSv in routine CT of the abdomen and pelvis with contrast.12,13 In view of the morbidity and mortality risks associated with infective endocarditis, especially if the diagnosis is delayed, this small radiation exposure may be justifiable.
Bottom line for cardiac CT
4D CT is an excellent alternative to echocardiography for select patients. Clinicians should strongly consider this study in the following situations:
- Patients with a prosthetic valve
- Patients who are strongly suspected of having infective endocarditis but who have a poor sonic window on TTE or TEE, as can occur with chronic obstructive lung disease, morbid obesity, or previous thoracic or cardiovascular surgery
- Patients who meet clinical indications for TEE, such as having a prosthetic valve or a high suspicion for native valve infective endocarditis with negative TTE, but who have contraindications to TEE
- As an alternative to TEE for preoperative evaluation in patients with known infective endocarditis.
Patients with tachycardia or irregular heart rhythms are not good candidates for this test.
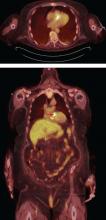
FDG-PET AND LEUKOCYTE SCINTIGRAPHY
FDG-PET and leukocyte scintigraphy are other options for diagnosing infective endocarditis and determining the presence and extent of intra- and extracardiac infection. They are more sensitive than echocardiography for detecting infection of cardiac implanted electronic devices such as ventricular assist devices, pacemakers, implanted cardiac defibrillators, and cardiac resynchronization therapy devices.14–16
The utility of FDG-PET is founded on the uptake of 18F-fluorodeoxyglucose by cells, with higher uptake taking place in cells with higher metabolic activity (such as in areas of inflammation). Similarly, leukocyte scintigraphy relies on the use of radiolabeled leukocytes (ie, leukocytes previously extracted from the patient, labelled, and re-introduced into the patient) to allow for localization of inflamed tissue.
The most significant contribution of FDG-PET may be the ability to detect infective endocarditis early, when echocardiography is initially negative. When abnormal FDG uptake was included in the modified Duke criteria, it increased the sensitivity to 97% for detecting infective endocarditis on admission, leading some to propose its incorporation as a major criterion.17 In patients with prosthetic valves and suspected infective endocarditis, FDG-PET was found in one study to have a sensitivity of up to 91% and a specificity of up to 95%.18
Both FDG-PET and leukocyte scintigraphy have a high sensitivity, specificity, and negative predictive value for cardiac implanted electronic device infection, and should be strongly considered in patients in whom it is suspected but who have negative or inconclusive findings on echocardiography.14,15
In addition, a common conundrum faced by clinicians with use of echocardiography is the difficulty of differentiating thrombus from infected vegetation on valves or device lead wires. Some evidence indicates that FDG-PET may help to discriminate between vegetation and thrombus, although more rigorous studies are needed before its use for that purpose can be recommended.19
Limitations of nuclear studies
Both FDG-PET and leukocyte scintigraphy perform poorly for detecting native-valve infective endocarditis. In a study in which 90% of the patients had native-valve infective endocarditis according to the Duke criteria, FDG-PET had a specificity of 93% but a sensitivity of only 39%.20
Both studies can be cumbersome, laborious, and time-consuming for patients. FDG-PET requires a fasting or glucose-restricted diet before testing, and the test itself can be complicated by development of hyperglycemia, although this is rare.
While FDG-PET is most effective in detecting infections of prosthetic valves and cardiac implanted electronic devices, the results can be falsely positive in patients with a history of recent cardiac surgery (due to ongoing tissue healing), as well as maladies other than infective endocarditis that lead to inflammation, such as vasculitis or malignancy. Similarly, for unclear reasons, leukocyte scintigraphy can yield false-negative results in patients with enterococcal or candidal infective endocarditis.21
FDG-PET and leukocyte scintigraphy are more expensive than TEE and cardiac CT22 and are not widely available.
Both tests entail radiation exposure, with the average dose ranging from 7 to 14 mSv. However, this is less than the average amount acquired during percutaneous coronary intervention (16 mSv), and overlaps with the amount in chest CT with contrast when assessing for pulmonary embolism (7 to 9 mSv). Lower doses are possible with optimized protocols.12,13,15,23
Bottom line for nuclear studies
FDG-PET and leukocyte scintigraphy are especially useful for patients with a prosthetic valve or cardiac implanted electronic device. However, limitations must be kept in mind.
A suggested algorithm for testing with nuclear imaging is shown in Figure 2.1,4
CEREBRAL MAGNETIC RESONANCE IMAGING
Cerebral magnetic resonance imaging (MRI) is more sensitive than cerebral CT for detecting emboli in the brain. According to American Heart Association guidelines, cerebral MRI should be done in patients with known or suspected infective endocarditis and neurologic impairment, defined as headaches, meningeal symptoms, or neurologic deficits. It is also often used in neurologically asymptomatic patients with infective endocarditis who have indications for valve surgery to assess for mycotic aneurysms, which are associated with increased intracranial bleeding during surgery.
MRI use in other asymptomatic patients remains controversial.24 In cases with high clinical suspicion for infective endocarditis and no findings on echocardiography, cerebral MRI can increase the sensitivity of the Duke criteria by adding a minor criterion. Some have argued that, in patients with definite infective endocarditis, detecting silent cerebral complications can lead to management changes. However, more studies are needed to determine if there is indeed a group of neurologically asymptomatic infective endocarditis patients for whom cerebral MRI leads to improved outcomes.
Limitations of cerebral MRI
Cerebral MRI cannot be used in patients with non-MRI-compatible implanted hardware.
Gadolinium, the contrast agent typically used, can cause nephrogenic systemic fibrosis in patients who have poor renal function. This rare but serious adverse effect is characterized by irreversible systemic fibrosis affecting skin, muscles, and even visceral tissue such as lungs. The American College of Radiology allows for gadolinium use in patients without acute kidney injury and patients with stable chronic kidney disease with a glomerular filtration rate of at least 30 mL/min/1.73 m2. Its use should be avoided in patients with renal failure on replacement therapy, with advanced chronic kidney disease (glomerular filtration rate < 30 mL/min/1.73 m2), or with acute kidney injury, even if they do not need renal replacement therapy.25
Concerns have also been raised about gadolinium retention in the brain, even in patients with normal renal function.26–28 Thus far, no conclusive clinical adverse effects of retention have been found, although more study is warranted. Nevertheless, the US Food and Drug Administration now requires a black-box warning about this possibility and advises clinicians to counsel patients appropriately.
Bottom line on cerebral MRI
Cerebral MRI should be obtained when a patient presents with definite or possible infective endocarditis with neurologic impairment, such as new headaches, meningismus, or focal neurologic deficits. Routine brain MRI in patients with confirmed infective endocarditis without neurologic symptoms, or those without definite infective endocarditis, is discouraged.
CARDIAC MRI
Cardiac MRI, typically obtained with gadolinium contrast, allows for better 3D assessment of cardiac structures and morphology than echocardiography or CT, and can detect infiltrative cardiac disease, myopericarditis, and much more. It is increasingly used in the field of structural cardiology, but its role for evaluating infective endocarditis remains unclear.
Cardiac MRI does not appear to be better than echocardiography for diagnosing infective endocarditis. However, it may prove helpful in the evaluation of patients known to have infective endocarditis but who cannot be properly evaluated for disease extent because of poor image quality on echocardiography and contraindications to CT.1,29 Its role is limited in patients with cardiac implanted electronic devices, as most devices are incompatible with MRI use, although newer devices obviate this concern. But even for devices that are MRI-compatible, results are diminished due to an eclipsing effect, wherein the device parts can make it hard to see structures clearly because the “brightness” basically eclipses the surrounding area.4
Concerns regarding use of gadolinium as described above need also be considered.
The role of cardiac MRI in diagnosing and managing infective endocarditis may evolve, but at present, the 2017 American College of Cardiology and American Heart Association appropriate-use criteria discourage its use for these purposes.16
Bottom line for cardiac MRI
Cardiac MRI to evaluate a patient for suspected infective endocarditis is not recommended due to lack of superiority compared with echocardiography or CT, and the risk of nephrogenic systemic fibrosis from gadolinium in patients with renal compromise.
- Habib G, Lancellotti P, Antunes MJ, et al; ESC Scientific Document Group. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Durante-Mangoni E, Bradley S, Selton-Suty C, et al; International Collaboration on Endocarditis Prospective Cohort Study Group. Current features of infective endocarditis in elderly patients: results of the International Collaboration on Endocarditis Prospective Cohort Study. Arch Intern Med 2008; 168(19):2095–2103. doi:10.1001/archinte.168.19.2095
- Wurcel AG, Anderson JE, Chui KK, et al. Increasing infectious endocarditis admissions among young people who inject drugs. Open Forum Infect Dis 2016; 3(3):ofw157. doi:10.1093/ofid/ofw157
- Gomes A, Glaudemans AW, Touw DJ, et al. Diagnostic value of imaging in infective endocarditis: a systematic review. Lancet Infect Dis 2017; 17(1):e1–e14. doi:10.1016/S1473-3099(16)30141-4
- Cahill TJ, Baddour LM, Habib G, et al. Challenges in infective endocarditis. J Am Coll Cardiol 2017; 69(3):325–344. doi:10.1016/j.jacc.2016.10.066
- Fagman E, Perrotta S, Bech-Hanssen O, et al. ECG-gated computed tomography: a new role for patients with suspected aortic prosthetic valve endocarditis. Eur Radiol 2012; 22(11):2407–2414. doi:10.1007/s00330-012-2491-5
- Habets J, Tanis W, van Herwerden LA, et al. Cardiac computed tomography angiography results in diagnostic and therapeutic change in prosthetic heart valve endocarditis. Int J Cardiovasc Imaging 2014; 30(2):377–387. doi:10.1007/s10554-013-0335-2
- Koneru S, Huang SS, Oldan J, et al. Role of preoperative cardiac CT in the evaluation of infective endocarditis: comparison with transesophageal echocardiography and surgical findings. Cardiovasc Diagn Ther 2018; 8(4):439–449. doi:10.21037/cdt.2018.07.07
- Koo HJ, Yang DH, Kang J, et al. Demonstration of infective endocarditis by cardiac CT and transoesophageal echocardiography: comparison with intra-operative findings. Eur Heart J Cardiovasc Imaging 2018; 19(2):199–207. doi:10.1093/ehjci/jex010
- Feuchtner GM, Stolzmann P, Dichtl W, et al. Multislice computed tomography in infective endocarditis: comparison with transesophageal echocardiography and intraoperative findings. J Am Coll Cardiol 2009; 53(5):436–444. doi:10.1016/j.jacc.2008.01.077
- Castellano IA, Nicol ED, Bull RK, Roobottom CA, Williams MC, Harden SP. A prospective national survey of coronary CT angiography radiation doses in the United Kingdom. J Cardiovasc Comput Tomogr 2017; 11(4):268–273. doi:10.1016/j.jcct.2017.05.002
- Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 2008; 248(1):254–263. doi:10.1148/radiol.2481071451
- Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 2009; 169(22):2078–2086. doi:10.1001/archinternmed.2009.427
- Ploux S, Riviere A, Amraoui S, et al. Positron emission tomography in patients with suspected pacing system infections may play a critical role in difficult cases. Heart Rhythm 2011; 8(9):1478–1481. doi:10.1016/j.hrthm.2011.03.062
- Sarrazin J, Philippon F, Tessier M, et al. Usefulness of fluorine-18 positron emission tomography/computed tomography for identification of cardiovascular implantable electronic device infections. J Am Coll Cardiol 2012; 59(18):1616–1625. doi:10.1016/j.jacc.2011.11.059
- Doherty JU, Kort S, Mehran R, Schoenhagen P, Soman P; Rating Panel Members; Appropriate Use Criteria Task Force. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2017 Appropriate use criteria for multimodality imaging in valvular heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Nucl Cardiol 2017; 24(6):2043–2063. doi:10.1007/s12350-017-1070-1
- Saby L, Laas O, Habib G, et al. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: increased valvular 18F-fluorodeoxyglucose uptake as a novel major criterion. J Am Coll Cardiol 2013; 61(23):2374–2382. doi:10.1016/j.jacc.2013.01.092
- Swart LE, Gomes A, Scholtens AM, et al. Improving the diagnostic performance of 18F-fluorodeoxyglucose positron-emission tomography/computed tomography in prosthetic heart valve endocarditis. Circulation 2018; 138(14):1412–1427. doi:10.1161/CIRCULATIONAHA.118.035032
- Graziosi M, Nanni C, Lorenzini M, et al. Role of 18F-FDG PET/CT in the diagnosis of infective endocarditis in patients with an implanted cardiac device: a prospective study. Eur J Nucl Med Mol Imaging 2014; 41(8):1617–1623. doi:10.1007/s00259-014-2773-z
- Kouijzer IJ, Vos FJ, Janssen MJ, van Dijk AP, Oyen WJ, Bleeker-Rovers CP. The value of 18F-FDG PET/CT in diagnosing infectious endocarditis. Eur J Nucl Med Mol Imaging 2013; 40(7):1102–1107. doi:10.1007/s00259-013-2376-0
- Wong D, Rubinshtein R, Keynan Y. Alternative cardiac imaging modalities to echocardiography for the diagnosis of infective endocarditis. Am J Cardiol 2016; 118(9):1410–1418. doi:10.1016/j.amjcard.2016.07.053
- Vos FJ, Bleeker-Rovers CP, Kullberg BJ, Adang EM, Oyen WJ. Cost-effectiveness of routine (18)F-FDG PET/CT in high-risk patients with gram-positive bacteremia. J Nucl Med 2011; 52(11):1673–1678. doi:10.2967/jnumed.111.089714
- McCollough CH, Bushberg JT, Fletcher JG, Eckel LJ. Answers to common questions about the use and safety of CT scans. Mayo Clin Proc 2015; 90(10):1380–1392. doi:10.1016/j.mayocp.2015.07.011
- Duval X, Iung B, Klein I, et al; IMAGE (Resonance Magnetic Imaging at the Acute Phase of Endocarditis) Study Group. Effect of early cerebral magnetic resonance imaging on clinical decisions in infective endocarditis: a prospective study. Ann Intern Med 2010; 152(8):497–504, W175. doi:10.7326/0003-4819-152-8-201004200-00006
- ACR Committee on Drugs and Contrast Media. ACR Manual on Contrast Media: 2018. www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf. Accessed July 19, 2019.
- Kanda T, Fukusato T, Matsuda M, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 2015; 276(1):228–232. doi:10.1148/radiol.2015142690
- McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 2015; 275(3):772–782. doi:10.1148/radiol.15150025
- Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014; 270(3):834–841. doi:10.1148/radiol.13131669
- Expert Panel on Pediatric Imaging; Hayes LL, Palasis S, Bartel TB, et al. ACR appropriateness criteria headache-child. J Am Coll Radiol 2018; 15(5S):S78–S90. doi:10.1016/j.jacr.2018.03.017
Prompt diagnois of infective endocarditis is critical. Potential consequences of missed or delayed diagnosis, including heart failure, stroke, intracardiac abscess, conduction delays, prosthesis dysfunction, and cerebral emboli, are often catastrophic. Echocardiography is the test used most frequently to evaluate for infective endocarditis, but it misses the diagnosis in almost one-third of cases, and even more often if the patient has a prosthetic valve.
But now, several sophisticated imaging tests are available that complement echocardiography in diagnosing and assessing infective endocarditis; these include 4-dimensional computed tomography (4D CT), fluorodeoxyglucose positron emission tomography (FDG-PET), and leukocyte scintigraphy. These tests have greatly improved our ability not only to diagnose infective endocarditis, but also to determine the extent and spread of infection, and they aid in perioperative assessment. Abnormal findings on these tests have been incorporated into the European Society of Cardiology’s 2015 modified diagnostic criteria for infective endocarditis.1
This article details the indications, advantages, and limitations of the various imaging tests for diagnosing and evaluating infective endocarditis (Table 1).
INFECTIVE ENDOCARDITIS IS DIFFICULT TO DIAGNOSE AND TREAT
Infective endocarditis is difficult to diagnose and treat. Clinical and imaging clues can be subtle, and the diagnosis requires a high level of suspicion and visualization of cardiac structures.
Further, the incidence of infective endocarditis is on the rise in the United States, particularly in women and young adults, likely due to intravenous drug use.2,3
ECHOCARDIOGRAPHY HAS AN IMPORTANT ROLE, BUT IS LIMITED
Echocardiography remains the most commonly performed study for diagnosing infective endocarditis, as it is fast, widely accessible, and less expensive than other imaging tests.
Transthoracic echocardiography (TTE) is often the first choice for testing. However, its sensitivity is only about 70% for detecting vegetations on native valves and 50% for detecting vegetations on prosthetic valves.1 It is inherently constrained by the limited number of views by which a comprehensive external evaluation of the heart can be achieved. Using a 2-dimensional instrument to view a 3-dimensional object is difficult, and depending on several factors, it can be hard to see vegetations and abscesses that are associated with infective endocarditis. Further, TTE is impeded by obesity and by hyperinflated lungs from obstructive pulmonary disease or mechanical ventilation. It has poor sensitivity for detecting small vegetations and for detecting vegetations and paravalvular complications in patients who have a prosthetic valve or a cardiac implanted electronic device.
Transesophageal echocardiography (TEE) is the recommended first-line imaging test for patients with prosthetic valves and no contraindications to the test. Otherwise, it should be done after TTE if the results of TTE are negative but clinical suspicion for infective endocarditis remains high (eg, because the patient uses intravenous drugs). But although TEE has a higher sensitivity than TTE (up to 96% for vegetations on native valves and 92% for those on prosthetic valves, if performed by an experienced sonographer), it can still miss infective endocarditis. Also, TEE does not provide a significant advantage over TTE in patients who have a cardiac implanted electronic device.1,4,5
Regardless of whether TTE or TEE is used, they are estimated to miss up to 30% of cases of infective endocarditis and its sequelae.4 False-negative findings are likelier in patients who have preexisting severe valvular lesions, prosthetic valves, cardiac implanted electronic devices, small vegetations, or abscesses, or if a vegetation has already broken free and embolized. Furthermore, distinguishing between vegetations and thrombi, cardiac tumors, and myxomatous changes using echocardiography is difficult.
CARDIAC CT
For patients who have inconclusive results on echocardiography, contraindications to TEE, or poor sonic windows, cardiac CT can be an excellent alternative. It is especially useful in the setting of a prosthetic valve.
Synchronized (“gated”) with the patient’s heart rate and rhythm, CT machines can acquire images during diastole, reducing motion artifact, and can create 3D images of the heart. In addition, newer machines can acquire several images at different points in the heart cycle to add a fourth dimension—time. The resulting 4D images play like short video loops of the beating heart and allow noninvasive assessment of cardiac anatomy with remarkable detail and resolution.
4D CT is increasingly being used in infective endocarditis, and growing evidence indicates that its accuracy is similar to that of TEE in the preoperative evaluation of patients with aortic prosthetic valve endocarditis.6 In a study of 28 patients, complementary use of CT angiography led to a change in treatment strategy in 7 (25%) compared with routine clinical workup.7 Several studies have found no difference between 4D CT and preoperative TEE in detecting pseudoaneurysm, abscess, or valve dehiscence. TEE and 4D CT also have similar sensitivities for detecting infective endocarditis in native and prosthetic valves.8,9
Coupled with CT angiography, 4D CT is also an excellent noninvasive way to perioperatively evaluate the coronary arteries without the risks associated with catheterization in those requiring nonemergency surgery (Figure 1A, B, and C).
4D CT performs well for detecting abscess and pseudoaneurysm but has slightly lower sensitivity for vegetations than TEE (91% vs 99%).9
Gated CT, PET, or both may be useful in cases of suspected prosthetic aortic valve endocarditis when TEE is negative. Pseudoaneurysms are not well visualized with TEE, and the atrial mitral curtain area is often thickened on TEE in cases of aortic prosthetic valve infective endocarditis that do not definitely involve abscesses. Gated CT and PET show this area better.8 This information is important in cases in which a surgeon may be unconvinced that the patient has prosthetic valve endocarditis.
Limitations of 4D cardiac CT
4D CT with or without angiography has limitations. It requires a wide-volume scanner and an experienced reader.
Patients with irregular heart rhythms or uncontrolled tachycardia pose technical problems for image acquisition. Cardiac CT is typically gated (ie, images are obtained within a defined time period) to acquire images during diastole. Ideally, images are acquired when the heart is in mid to late diastole, a time of minimal cardiac motion, so that motion artifact is minimized. To estimate the timing of image acquisition, the cardiac cycle must be predictable, and its duration should be as long as possible. Tachycardia or irregular rhythms such as frequent ectopic beats or atrial fibrillation make acquisition timing difficult, and thus make it nearly impossible to accurately obtain images when the heart is at minimum motion, limiting assessment of cardiac structures or the coronary tree.4,10
Extensive coronary calcification can hinder assessment of the coronary tree by CT coronary angiography.
Contrast exposure may limit the use of CT in some patients (eg, those with contrast allergies or renal dysfunction). However, modern scanners allow for much smaller contrast boluses without decreasing sensitivity.
4D CT involves radiation exposure, especially when done with angiography, although modern scanners have greatly reduced exposure. The average radiation dose in CT coronary angiography is 2.9 to 5.9 mSv11 compared with 7 mSv in diagnostic cardiac catheterization (without angioplasty or stenting) or 16 mSv in routine CT of the abdomen and pelvis with contrast.12,13 In view of the morbidity and mortality risks associated with infective endocarditis, especially if the diagnosis is delayed, this small radiation exposure may be justifiable.
Bottom line for cardiac CT
4D CT is an excellent alternative to echocardiography for select patients. Clinicians should strongly consider this study in the following situations:
- Patients with a prosthetic valve
- Patients who are strongly suspected of having infective endocarditis but who have a poor sonic window on TTE or TEE, as can occur with chronic obstructive lung disease, morbid obesity, or previous thoracic or cardiovascular surgery
- Patients who meet clinical indications for TEE, such as having a prosthetic valve or a high suspicion for native valve infective endocarditis with negative TTE, but who have contraindications to TEE
- As an alternative to TEE for preoperative evaluation in patients with known infective endocarditis.
Patients with tachycardia or irregular heart rhythms are not good candidates for this test.
FDG-PET AND LEUKOCYTE SCINTIGRAPHY
FDG-PET and leukocyte scintigraphy are other options for diagnosing infective endocarditis and determining the presence and extent of intra- and extracardiac infection. They are more sensitive than echocardiography for detecting infection of cardiac implanted electronic devices such as ventricular assist devices, pacemakers, implanted cardiac defibrillators, and cardiac resynchronization therapy devices.14–16
The utility of FDG-PET is founded on the uptake of 18F-fluorodeoxyglucose by cells, with higher uptake taking place in cells with higher metabolic activity (such as in areas of inflammation). Similarly, leukocyte scintigraphy relies on the use of radiolabeled leukocytes (ie, leukocytes previously extracted from the patient, labelled, and re-introduced into the patient) to allow for localization of inflamed tissue.
The most significant contribution of FDG-PET may be the ability to detect infective endocarditis early, when echocardiography is initially negative. When abnormal FDG uptake was included in the modified Duke criteria, it increased the sensitivity to 97% for detecting infective endocarditis on admission, leading some to propose its incorporation as a major criterion.17 In patients with prosthetic valves and suspected infective endocarditis, FDG-PET was found in one study to have a sensitivity of up to 91% and a specificity of up to 95%.18
Both FDG-PET and leukocyte scintigraphy have a high sensitivity, specificity, and negative predictive value for cardiac implanted electronic device infection, and should be strongly considered in patients in whom it is suspected but who have negative or inconclusive findings on echocardiography.14,15
In addition, a common conundrum faced by clinicians with use of echocardiography is the difficulty of differentiating thrombus from infected vegetation on valves or device lead wires. Some evidence indicates that FDG-PET may help to discriminate between vegetation and thrombus, although more rigorous studies are needed before its use for that purpose can be recommended.19
Limitations of nuclear studies
Both FDG-PET and leukocyte scintigraphy perform poorly for detecting native-valve infective endocarditis. In a study in which 90% of the patients had native-valve infective endocarditis according to the Duke criteria, FDG-PET had a specificity of 93% but a sensitivity of only 39%.20
Both studies can be cumbersome, laborious, and time-consuming for patients. FDG-PET requires a fasting or glucose-restricted diet before testing, and the test itself can be complicated by development of hyperglycemia, although this is rare.
While FDG-PET is most effective in detecting infections of prosthetic valves and cardiac implanted electronic devices, the results can be falsely positive in patients with a history of recent cardiac surgery (due to ongoing tissue healing), as well as maladies other than infective endocarditis that lead to inflammation, such as vasculitis or malignancy. Similarly, for unclear reasons, leukocyte scintigraphy can yield false-negative results in patients with enterococcal or candidal infective endocarditis.21
FDG-PET and leukocyte scintigraphy are more expensive than TEE and cardiac CT22 and are not widely available.
Both tests entail radiation exposure, with the average dose ranging from 7 to 14 mSv. However, this is less than the average amount acquired during percutaneous coronary intervention (16 mSv), and overlaps with the amount in chest CT with contrast when assessing for pulmonary embolism (7 to 9 mSv). Lower doses are possible with optimized protocols.12,13,15,23
Bottom line for nuclear studies
FDG-PET and leukocyte scintigraphy are especially useful for patients with a prosthetic valve or cardiac implanted electronic device. However, limitations must be kept in mind.
A suggested algorithm for testing with nuclear imaging is shown in Figure 2.1,4
CEREBRAL MAGNETIC RESONANCE IMAGING
Cerebral magnetic resonance imaging (MRI) is more sensitive than cerebral CT for detecting emboli in the brain. According to American Heart Association guidelines, cerebral MRI should be done in patients with known or suspected infective endocarditis and neurologic impairment, defined as headaches, meningeal symptoms, or neurologic deficits. It is also often used in neurologically asymptomatic patients with infective endocarditis who have indications for valve surgery to assess for mycotic aneurysms, which are associated with increased intracranial bleeding during surgery.
MRI use in other asymptomatic patients remains controversial.24 In cases with high clinical suspicion for infective endocarditis and no findings on echocardiography, cerebral MRI can increase the sensitivity of the Duke criteria by adding a minor criterion. Some have argued that, in patients with definite infective endocarditis, detecting silent cerebral complications can lead to management changes. However, more studies are needed to determine if there is indeed a group of neurologically asymptomatic infective endocarditis patients for whom cerebral MRI leads to improved outcomes.
Limitations of cerebral MRI
Cerebral MRI cannot be used in patients with non-MRI-compatible implanted hardware.
Gadolinium, the contrast agent typically used, can cause nephrogenic systemic fibrosis in patients who have poor renal function. This rare but serious adverse effect is characterized by irreversible systemic fibrosis affecting skin, muscles, and even visceral tissue such as lungs. The American College of Radiology allows for gadolinium use in patients without acute kidney injury and patients with stable chronic kidney disease with a glomerular filtration rate of at least 30 mL/min/1.73 m2. Its use should be avoided in patients with renal failure on replacement therapy, with advanced chronic kidney disease (glomerular filtration rate < 30 mL/min/1.73 m2), or with acute kidney injury, even if they do not need renal replacement therapy.25
Concerns have also been raised about gadolinium retention in the brain, even in patients with normal renal function.26–28 Thus far, no conclusive clinical adverse effects of retention have been found, although more study is warranted. Nevertheless, the US Food and Drug Administration now requires a black-box warning about this possibility and advises clinicians to counsel patients appropriately.
Bottom line on cerebral MRI
Cerebral MRI should be obtained when a patient presents with definite or possible infective endocarditis with neurologic impairment, such as new headaches, meningismus, or focal neurologic deficits. Routine brain MRI in patients with confirmed infective endocarditis without neurologic symptoms, or those without definite infective endocarditis, is discouraged.
CARDIAC MRI
Cardiac MRI, typically obtained with gadolinium contrast, allows for better 3D assessment of cardiac structures and morphology than echocardiography or CT, and can detect infiltrative cardiac disease, myopericarditis, and much more. It is increasingly used in the field of structural cardiology, but its role for evaluating infective endocarditis remains unclear.
Cardiac MRI does not appear to be better than echocardiography for diagnosing infective endocarditis. However, it may prove helpful in the evaluation of patients known to have infective endocarditis but who cannot be properly evaluated for disease extent because of poor image quality on echocardiography and contraindications to CT.1,29 Its role is limited in patients with cardiac implanted electronic devices, as most devices are incompatible with MRI use, although newer devices obviate this concern. But even for devices that are MRI-compatible, results are diminished due to an eclipsing effect, wherein the device parts can make it hard to see structures clearly because the “brightness” basically eclipses the surrounding area.4
Concerns regarding use of gadolinium as described above need also be considered.
The role of cardiac MRI in diagnosing and managing infective endocarditis may evolve, but at present, the 2017 American College of Cardiology and American Heart Association appropriate-use criteria discourage its use for these purposes.16
Bottom line for cardiac MRI
Cardiac MRI to evaluate a patient for suspected infective endocarditis is not recommended due to lack of superiority compared with echocardiography or CT, and the risk of nephrogenic systemic fibrosis from gadolinium in patients with renal compromise.
Prompt diagnois of infective endocarditis is critical. Potential consequences of missed or delayed diagnosis, including heart failure, stroke, intracardiac abscess, conduction delays, prosthesis dysfunction, and cerebral emboli, are often catastrophic. Echocardiography is the test used most frequently to evaluate for infective endocarditis, but it misses the diagnosis in almost one-third of cases, and even more often if the patient has a prosthetic valve.
But now, several sophisticated imaging tests are available that complement echocardiography in diagnosing and assessing infective endocarditis; these include 4-dimensional computed tomography (4D CT), fluorodeoxyglucose positron emission tomography (FDG-PET), and leukocyte scintigraphy. These tests have greatly improved our ability not only to diagnose infective endocarditis, but also to determine the extent and spread of infection, and they aid in perioperative assessment. Abnormal findings on these tests have been incorporated into the European Society of Cardiology’s 2015 modified diagnostic criteria for infective endocarditis.1
This article details the indications, advantages, and limitations of the various imaging tests for diagnosing and evaluating infective endocarditis (Table 1).
INFECTIVE ENDOCARDITIS IS DIFFICULT TO DIAGNOSE AND TREAT
Infective endocarditis is difficult to diagnose and treat. Clinical and imaging clues can be subtle, and the diagnosis requires a high level of suspicion and visualization of cardiac structures.
Further, the incidence of infective endocarditis is on the rise in the United States, particularly in women and young adults, likely due to intravenous drug use.2,3
ECHOCARDIOGRAPHY HAS AN IMPORTANT ROLE, BUT IS LIMITED
Echocardiography remains the most commonly performed study for diagnosing infective endocarditis, as it is fast, widely accessible, and less expensive than other imaging tests.
Transthoracic echocardiography (TTE) is often the first choice for testing. However, its sensitivity is only about 70% for detecting vegetations on native valves and 50% for detecting vegetations on prosthetic valves.1 It is inherently constrained by the limited number of views by which a comprehensive external evaluation of the heart can be achieved. Using a 2-dimensional instrument to view a 3-dimensional object is difficult, and depending on several factors, it can be hard to see vegetations and abscesses that are associated with infective endocarditis. Further, TTE is impeded by obesity and by hyperinflated lungs from obstructive pulmonary disease or mechanical ventilation. It has poor sensitivity for detecting small vegetations and for detecting vegetations and paravalvular complications in patients who have a prosthetic valve or a cardiac implanted electronic device.
Transesophageal echocardiography (TEE) is the recommended first-line imaging test for patients with prosthetic valves and no contraindications to the test. Otherwise, it should be done after TTE if the results of TTE are negative but clinical suspicion for infective endocarditis remains high (eg, because the patient uses intravenous drugs). But although TEE has a higher sensitivity than TTE (up to 96% for vegetations on native valves and 92% for those on prosthetic valves, if performed by an experienced sonographer), it can still miss infective endocarditis. Also, TEE does not provide a significant advantage over TTE in patients who have a cardiac implanted electronic device.1,4,5
Regardless of whether TTE or TEE is used, they are estimated to miss up to 30% of cases of infective endocarditis and its sequelae.4 False-negative findings are likelier in patients who have preexisting severe valvular lesions, prosthetic valves, cardiac implanted electronic devices, small vegetations, or abscesses, or if a vegetation has already broken free and embolized. Furthermore, distinguishing between vegetations and thrombi, cardiac tumors, and myxomatous changes using echocardiography is difficult.
CARDIAC CT
For patients who have inconclusive results on echocardiography, contraindications to TEE, or poor sonic windows, cardiac CT can be an excellent alternative. It is especially useful in the setting of a prosthetic valve.
Synchronized (“gated”) with the patient’s heart rate and rhythm, CT machines can acquire images during diastole, reducing motion artifact, and can create 3D images of the heart. In addition, newer machines can acquire several images at different points in the heart cycle to add a fourth dimension—time. The resulting 4D images play like short video loops of the beating heart and allow noninvasive assessment of cardiac anatomy with remarkable detail and resolution.
4D CT is increasingly being used in infective endocarditis, and growing evidence indicates that its accuracy is similar to that of TEE in the preoperative evaluation of patients with aortic prosthetic valve endocarditis.6 In a study of 28 patients, complementary use of CT angiography led to a change in treatment strategy in 7 (25%) compared with routine clinical workup.7 Several studies have found no difference between 4D CT and preoperative TEE in detecting pseudoaneurysm, abscess, or valve dehiscence. TEE and 4D CT also have similar sensitivities for detecting infective endocarditis in native and prosthetic valves.8,9
Coupled with CT angiography, 4D CT is also an excellent noninvasive way to perioperatively evaluate the coronary arteries without the risks associated with catheterization in those requiring nonemergency surgery (Figure 1A, B, and C).
4D CT performs well for detecting abscess and pseudoaneurysm but has slightly lower sensitivity for vegetations than TEE (91% vs 99%).9
Gated CT, PET, or both may be useful in cases of suspected prosthetic aortic valve endocarditis when TEE is negative. Pseudoaneurysms are not well visualized with TEE, and the atrial mitral curtain area is often thickened on TEE in cases of aortic prosthetic valve infective endocarditis that do not definitely involve abscesses. Gated CT and PET show this area better.8 This information is important in cases in which a surgeon may be unconvinced that the patient has prosthetic valve endocarditis.
Limitations of 4D cardiac CT
4D CT with or without angiography has limitations. It requires a wide-volume scanner and an experienced reader.
Patients with irregular heart rhythms or uncontrolled tachycardia pose technical problems for image acquisition. Cardiac CT is typically gated (ie, images are obtained within a defined time period) to acquire images during diastole. Ideally, images are acquired when the heart is in mid to late diastole, a time of minimal cardiac motion, so that motion artifact is minimized. To estimate the timing of image acquisition, the cardiac cycle must be predictable, and its duration should be as long as possible. Tachycardia or irregular rhythms such as frequent ectopic beats or atrial fibrillation make acquisition timing difficult, and thus make it nearly impossible to accurately obtain images when the heart is at minimum motion, limiting assessment of cardiac structures or the coronary tree.4,10
Extensive coronary calcification can hinder assessment of the coronary tree by CT coronary angiography.
Contrast exposure may limit the use of CT in some patients (eg, those with contrast allergies or renal dysfunction). However, modern scanners allow for much smaller contrast boluses without decreasing sensitivity.
4D CT involves radiation exposure, especially when done with angiography, although modern scanners have greatly reduced exposure. The average radiation dose in CT coronary angiography is 2.9 to 5.9 mSv11 compared with 7 mSv in diagnostic cardiac catheterization (without angioplasty or stenting) or 16 mSv in routine CT of the abdomen and pelvis with contrast.12,13 In view of the morbidity and mortality risks associated with infective endocarditis, especially if the diagnosis is delayed, this small radiation exposure may be justifiable.
Bottom line for cardiac CT
4D CT is an excellent alternative to echocardiography for select patients. Clinicians should strongly consider this study in the following situations:
- Patients with a prosthetic valve
- Patients who are strongly suspected of having infective endocarditis but who have a poor sonic window on TTE or TEE, as can occur with chronic obstructive lung disease, morbid obesity, or previous thoracic or cardiovascular surgery
- Patients who meet clinical indications for TEE, such as having a prosthetic valve or a high suspicion for native valve infective endocarditis with negative TTE, but who have contraindications to TEE
- As an alternative to TEE for preoperative evaluation in patients with known infective endocarditis.
Patients with tachycardia or irregular heart rhythms are not good candidates for this test.
FDG-PET AND LEUKOCYTE SCINTIGRAPHY
FDG-PET and leukocyte scintigraphy are other options for diagnosing infective endocarditis and determining the presence and extent of intra- and extracardiac infection. They are more sensitive than echocardiography for detecting infection of cardiac implanted electronic devices such as ventricular assist devices, pacemakers, implanted cardiac defibrillators, and cardiac resynchronization therapy devices.14–16
The utility of FDG-PET is founded on the uptake of 18F-fluorodeoxyglucose by cells, with higher uptake taking place in cells with higher metabolic activity (such as in areas of inflammation). Similarly, leukocyte scintigraphy relies on the use of radiolabeled leukocytes (ie, leukocytes previously extracted from the patient, labelled, and re-introduced into the patient) to allow for localization of inflamed tissue.
The most significant contribution of FDG-PET may be the ability to detect infective endocarditis early, when echocardiography is initially negative. When abnormal FDG uptake was included in the modified Duke criteria, it increased the sensitivity to 97% for detecting infective endocarditis on admission, leading some to propose its incorporation as a major criterion.17 In patients with prosthetic valves and suspected infective endocarditis, FDG-PET was found in one study to have a sensitivity of up to 91% and a specificity of up to 95%.18
Both FDG-PET and leukocyte scintigraphy have a high sensitivity, specificity, and negative predictive value for cardiac implanted electronic device infection, and should be strongly considered in patients in whom it is suspected but who have negative or inconclusive findings on echocardiography.14,15
In addition, a common conundrum faced by clinicians with use of echocardiography is the difficulty of differentiating thrombus from infected vegetation on valves or device lead wires. Some evidence indicates that FDG-PET may help to discriminate between vegetation and thrombus, although more rigorous studies are needed before its use for that purpose can be recommended.19
Limitations of nuclear studies
Both FDG-PET and leukocyte scintigraphy perform poorly for detecting native-valve infective endocarditis. In a study in which 90% of the patients had native-valve infective endocarditis according to the Duke criteria, FDG-PET had a specificity of 93% but a sensitivity of only 39%.20
Both studies can be cumbersome, laborious, and time-consuming for patients. FDG-PET requires a fasting or glucose-restricted diet before testing, and the test itself can be complicated by development of hyperglycemia, although this is rare.
While FDG-PET is most effective in detecting infections of prosthetic valves and cardiac implanted electronic devices, the results can be falsely positive in patients with a history of recent cardiac surgery (due to ongoing tissue healing), as well as maladies other than infective endocarditis that lead to inflammation, such as vasculitis or malignancy. Similarly, for unclear reasons, leukocyte scintigraphy can yield false-negative results in patients with enterococcal or candidal infective endocarditis.21
FDG-PET and leukocyte scintigraphy are more expensive than TEE and cardiac CT22 and are not widely available.
Both tests entail radiation exposure, with the average dose ranging from 7 to 14 mSv. However, this is less than the average amount acquired during percutaneous coronary intervention (16 mSv), and overlaps with the amount in chest CT with contrast when assessing for pulmonary embolism (7 to 9 mSv). Lower doses are possible with optimized protocols.12,13,15,23
Bottom line for nuclear studies
FDG-PET and leukocyte scintigraphy are especially useful for patients with a prosthetic valve or cardiac implanted electronic device. However, limitations must be kept in mind.
A suggested algorithm for testing with nuclear imaging is shown in Figure 2.1,4
CEREBRAL MAGNETIC RESONANCE IMAGING
Cerebral magnetic resonance imaging (MRI) is more sensitive than cerebral CT for detecting emboli in the brain. According to American Heart Association guidelines, cerebral MRI should be done in patients with known or suspected infective endocarditis and neurologic impairment, defined as headaches, meningeal symptoms, or neurologic deficits. It is also often used in neurologically asymptomatic patients with infective endocarditis who have indications for valve surgery to assess for mycotic aneurysms, which are associated with increased intracranial bleeding during surgery.
MRI use in other asymptomatic patients remains controversial.24 In cases with high clinical suspicion for infective endocarditis and no findings on echocardiography, cerebral MRI can increase the sensitivity of the Duke criteria by adding a minor criterion. Some have argued that, in patients with definite infective endocarditis, detecting silent cerebral complications can lead to management changes. However, more studies are needed to determine if there is indeed a group of neurologically asymptomatic infective endocarditis patients for whom cerebral MRI leads to improved outcomes.
Limitations of cerebral MRI
Cerebral MRI cannot be used in patients with non-MRI-compatible implanted hardware.
Gadolinium, the contrast agent typically used, can cause nephrogenic systemic fibrosis in patients who have poor renal function. This rare but serious adverse effect is characterized by irreversible systemic fibrosis affecting skin, muscles, and even visceral tissue such as lungs. The American College of Radiology allows for gadolinium use in patients without acute kidney injury and patients with stable chronic kidney disease with a glomerular filtration rate of at least 30 mL/min/1.73 m2. Its use should be avoided in patients with renal failure on replacement therapy, with advanced chronic kidney disease (glomerular filtration rate < 30 mL/min/1.73 m2), or with acute kidney injury, even if they do not need renal replacement therapy.25
Concerns have also been raised about gadolinium retention in the brain, even in patients with normal renal function.26–28 Thus far, no conclusive clinical adverse effects of retention have been found, although more study is warranted. Nevertheless, the US Food and Drug Administration now requires a black-box warning about this possibility and advises clinicians to counsel patients appropriately.
Bottom line on cerebral MRI
Cerebral MRI should be obtained when a patient presents with definite or possible infective endocarditis with neurologic impairment, such as new headaches, meningismus, or focal neurologic deficits. Routine brain MRI in patients with confirmed infective endocarditis without neurologic symptoms, or those without definite infective endocarditis, is discouraged.
CARDIAC MRI
Cardiac MRI, typically obtained with gadolinium contrast, allows for better 3D assessment of cardiac structures and morphology than echocardiography or CT, and can detect infiltrative cardiac disease, myopericarditis, and much more. It is increasingly used in the field of structural cardiology, but its role for evaluating infective endocarditis remains unclear.
Cardiac MRI does not appear to be better than echocardiography for diagnosing infective endocarditis. However, it may prove helpful in the evaluation of patients known to have infective endocarditis but who cannot be properly evaluated for disease extent because of poor image quality on echocardiography and contraindications to CT.1,29 Its role is limited in patients with cardiac implanted electronic devices, as most devices are incompatible with MRI use, although newer devices obviate this concern. But even for devices that are MRI-compatible, results are diminished due to an eclipsing effect, wherein the device parts can make it hard to see structures clearly because the “brightness” basically eclipses the surrounding area.4
Concerns regarding use of gadolinium as described above need also be considered.
The role of cardiac MRI in diagnosing and managing infective endocarditis may evolve, but at present, the 2017 American College of Cardiology and American Heart Association appropriate-use criteria discourage its use for these purposes.16
Bottom line for cardiac MRI
Cardiac MRI to evaluate a patient for suspected infective endocarditis is not recommended due to lack of superiority compared with echocardiography or CT, and the risk of nephrogenic systemic fibrosis from gadolinium in patients with renal compromise.
- Habib G, Lancellotti P, Antunes MJ, et al; ESC Scientific Document Group. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Durante-Mangoni E, Bradley S, Selton-Suty C, et al; International Collaboration on Endocarditis Prospective Cohort Study Group. Current features of infective endocarditis in elderly patients: results of the International Collaboration on Endocarditis Prospective Cohort Study. Arch Intern Med 2008; 168(19):2095–2103. doi:10.1001/archinte.168.19.2095
- Wurcel AG, Anderson JE, Chui KK, et al. Increasing infectious endocarditis admissions among young people who inject drugs. Open Forum Infect Dis 2016; 3(3):ofw157. doi:10.1093/ofid/ofw157
- Gomes A, Glaudemans AW, Touw DJ, et al. Diagnostic value of imaging in infective endocarditis: a systematic review. Lancet Infect Dis 2017; 17(1):e1–e14. doi:10.1016/S1473-3099(16)30141-4
- Cahill TJ, Baddour LM, Habib G, et al. Challenges in infective endocarditis. J Am Coll Cardiol 2017; 69(3):325–344. doi:10.1016/j.jacc.2016.10.066
- Fagman E, Perrotta S, Bech-Hanssen O, et al. ECG-gated computed tomography: a new role for patients with suspected aortic prosthetic valve endocarditis. Eur Radiol 2012; 22(11):2407–2414. doi:10.1007/s00330-012-2491-5
- Habets J, Tanis W, van Herwerden LA, et al. Cardiac computed tomography angiography results in diagnostic and therapeutic change in prosthetic heart valve endocarditis. Int J Cardiovasc Imaging 2014; 30(2):377–387. doi:10.1007/s10554-013-0335-2
- Koneru S, Huang SS, Oldan J, et al. Role of preoperative cardiac CT in the evaluation of infective endocarditis: comparison with transesophageal echocardiography and surgical findings. Cardiovasc Diagn Ther 2018; 8(4):439–449. doi:10.21037/cdt.2018.07.07
- Koo HJ, Yang DH, Kang J, et al. Demonstration of infective endocarditis by cardiac CT and transoesophageal echocardiography: comparison with intra-operative findings. Eur Heart J Cardiovasc Imaging 2018; 19(2):199–207. doi:10.1093/ehjci/jex010
- Feuchtner GM, Stolzmann P, Dichtl W, et al. Multislice computed tomography in infective endocarditis: comparison with transesophageal echocardiography and intraoperative findings. J Am Coll Cardiol 2009; 53(5):436–444. doi:10.1016/j.jacc.2008.01.077
- Castellano IA, Nicol ED, Bull RK, Roobottom CA, Williams MC, Harden SP. A prospective national survey of coronary CT angiography radiation doses in the United Kingdom. J Cardiovasc Comput Tomogr 2017; 11(4):268–273. doi:10.1016/j.jcct.2017.05.002
- Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 2008; 248(1):254–263. doi:10.1148/radiol.2481071451
- Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 2009; 169(22):2078–2086. doi:10.1001/archinternmed.2009.427
- Ploux S, Riviere A, Amraoui S, et al. Positron emission tomography in patients with suspected pacing system infections may play a critical role in difficult cases. Heart Rhythm 2011; 8(9):1478–1481. doi:10.1016/j.hrthm.2011.03.062
- Sarrazin J, Philippon F, Tessier M, et al. Usefulness of fluorine-18 positron emission tomography/computed tomography for identification of cardiovascular implantable electronic device infections. J Am Coll Cardiol 2012; 59(18):1616–1625. doi:10.1016/j.jacc.2011.11.059
- Doherty JU, Kort S, Mehran R, Schoenhagen P, Soman P; Rating Panel Members; Appropriate Use Criteria Task Force. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2017 Appropriate use criteria for multimodality imaging in valvular heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Nucl Cardiol 2017; 24(6):2043–2063. doi:10.1007/s12350-017-1070-1
- Saby L, Laas O, Habib G, et al. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: increased valvular 18F-fluorodeoxyglucose uptake as a novel major criterion. J Am Coll Cardiol 2013; 61(23):2374–2382. doi:10.1016/j.jacc.2013.01.092
- Swart LE, Gomes A, Scholtens AM, et al. Improving the diagnostic performance of 18F-fluorodeoxyglucose positron-emission tomography/computed tomography in prosthetic heart valve endocarditis. Circulation 2018; 138(14):1412–1427. doi:10.1161/CIRCULATIONAHA.118.035032
- Graziosi M, Nanni C, Lorenzini M, et al. Role of 18F-FDG PET/CT in the diagnosis of infective endocarditis in patients with an implanted cardiac device: a prospective study. Eur J Nucl Med Mol Imaging 2014; 41(8):1617–1623. doi:10.1007/s00259-014-2773-z
- Kouijzer IJ, Vos FJ, Janssen MJ, van Dijk AP, Oyen WJ, Bleeker-Rovers CP. The value of 18F-FDG PET/CT in diagnosing infectious endocarditis. Eur J Nucl Med Mol Imaging 2013; 40(7):1102–1107. doi:10.1007/s00259-013-2376-0
- Wong D, Rubinshtein R, Keynan Y. Alternative cardiac imaging modalities to echocardiography for the diagnosis of infective endocarditis. Am J Cardiol 2016; 118(9):1410–1418. doi:10.1016/j.amjcard.2016.07.053
- Vos FJ, Bleeker-Rovers CP, Kullberg BJ, Adang EM, Oyen WJ. Cost-effectiveness of routine (18)F-FDG PET/CT in high-risk patients with gram-positive bacteremia. J Nucl Med 2011; 52(11):1673–1678. doi:10.2967/jnumed.111.089714
- McCollough CH, Bushberg JT, Fletcher JG, Eckel LJ. Answers to common questions about the use and safety of CT scans. Mayo Clin Proc 2015; 90(10):1380–1392. doi:10.1016/j.mayocp.2015.07.011
- Duval X, Iung B, Klein I, et al; IMAGE (Resonance Magnetic Imaging at the Acute Phase of Endocarditis) Study Group. Effect of early cerebral magnetic resonance imaging on clinical decisions in infective endocarditis: a prospective study. Ann Intern Med 2010; 152(8):497–504, W175. doi:10.7326/0003-4819-152-8-201004200-00006
- ACR Committee on Drugs and Contrast Media. ACR Manual on Contrast Media: 2018. www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf. Accessed July 19, 2019.
- Kanda T, Fukusato T, Matsuda M, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 2015; 276(1):228–232. doi:10.1148/radiol.2015142690
- McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 2015; 275(3):772–782. doi:10.1148/radiol.15150025
- Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014; 270(3):834–841. doi:10.1148/radiol.13131669
- Expert Panel on Pediatric Imaging; Hayes LL, Palasis S, Bartel TB, et al. ACR appropriateness criteria headache-child. J Am Coll Radiol 2018; 15(5S):S78–S90. doi:10.1016/j.jacr.2018.03.017
- Habib G, Lancellotti P, Antunes MJ, et al; ESC Scientific Document Group. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Durante-Mangoni E, Bradley S, Selton-Suty C, et al; International Collaboration on Endocarditis Prospective Cohort Study Group. Current features of infective endocarditis in elderly patients: results of the International Collaboration on Endocarditis Prospective Cohort Study. Arch Intern Med 2008; 168(19):2095–2103. doi:10.1001/archinte.168.19.2095
- Wurcel AG, Anderson JE, Chui KK, et al. Increasing infectious endocarditis admissions among young people who inject drugs. Open Forum Infect Dis 2016; 3(3):ofw157. doi:10.1093/ofid/ofw157
- Gomes A, Glaudemans AW, Touw DJ, et al. Diagnostic value of imaging in infective endocarditis: a systematic review. Lancet Infect Dis 2017; 17(1):e1–e14. doi:10.1016/S1473-3099(16)30141-4
- Cahill TJ, Baddour LM, Habib G, et al. Challenges in infective endocarditis. J Am Coll Cardiol 2017; 69(3):325–344. doi:10.1016/j.jacc.2016.10.066
- Fagman E, Perrotta S, Bech-Hanssen O, et al. ECG-gated computed tomography: a new role for patients with suspected aortic prosthetic valve endocarditis. Eur Radiol 2012; 22(11):2407–2414. doi:10.1007/s00330-012-2491-5
- Habets J, Tanis W, van Herwerden LA, et al. Cardiac computed tomography angiography results in diagnostic and therapeutic change in prosthetic heart valve endocarditis. Int J Cardiovasc Imaging 2014; 30(2):377–387. doi:10.1007/s10554-013-0335-2
- Koneru S, Huang SS, Oldan J, et al. Role of preoperative cardiac CT in the evaluation of infective endocarditis: comparison with transesophageal echocardiography and surgical findings. Cardiovasc Diagn Ther 2018; 8(4):439–449. doi:10.21037/cdt.2018.07.07
- Koo HJ, Yang DH, Kang J, et al. Demonstration of infective endocarditis by cardiac CT and transoesophageal echocardiography: comparison with intra-operative findings. Eur Heart J Cardiovasc Imaging 2018; 19(2):199–207. doi:10.1093/ehjci/jex010
- Feuchtner GM, Stolzmann P, Dichtl W, et al. Multislice computed tomography in infective endocarditis: comparison with transesophageal echocardiography and intraoperative findings. J Am Coll Cardiol 2009; 53(5):436–444. doi:10.1016/j.jacc.2008.01.077
- Castellano IA, Nicol ED, Bull RK, Roobottom CA, Williams MC, Harden SP. A prospective national survey of coronary CT angiography radiation doses in the United Kingdom. J Cardiovasc Comput Tomogr 2017; 11(4):268–273. doi:10.1016/j.jcct.2017.05.002
- Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 2008; 248(1):254–263. doi:10.1148/radiol.2481071451
- Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 2009; 169(22):2078–2086. doi:10.1001/archinternmed.2009.427
- Ploux S, Riviere A, Amraoui S, et al. Positron emission tomography in patients with suspected pacing system infections may play a critical role in difficult cases. Heart Rhythm 2011; 8(9):1478–1481. doi:10.1016/j.hrthm.2011.03.062
- Sarrazin J, Philippon F, Tessier M, et al. Usefulness of fluorine-18 positron emission tomography/computed tomography for identification of cardiovascular implantable electronic device infections. J Am Coll Cardiol 2012; 59(18):1616–1625. doi:10.1016/j.jacc.2011.11.059
- Doherty JU, Kort S, Mehran R, Schoenhagen P, Soman P; Rating Panel Members; Appropriate Use Criteria Task Force. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2017 Appropriate use criteria for multimodality imaging in valvular heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Nucl Cardiol 2017; 24(6):2043–2063. doi:10.1007/s12350-017-1070-1
- Saby L, Laas O, Habib G, et al. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: increased valvular 18F-fluorodeoxyglucose uptake as a novel major criterion. J Am Coll Cardiol 2013; 61(23):2374–2382. doi:10.1016/j.jacc.2013.01.092
- Swart LE, Gomes A, Scholtens AM, et al. Improving the diagnostic performance of 18F-fluorodeoxyglucose positron-emission tomography/computed tomography in prosthetic heart valve endocarditis. Circulation 2018; 138(14):1412–1427. doi:10.1161/CIRCULATIONAHA.118.035032
- Graziosi M, Nanni C, Lorenzini M, et al. Role of 18F-FDG PET/CT in the diagnosis of infective endocarditis in patients with an implanted cardiac device: a prospective study. Eur J Nucl Med Mol Imaging 2014; 41(8):1617–1623. doi:10.1007/s00259-014-2773-z
- Kouijzer IJ, Vos FJ, Janssen MJ, van Dijk AP, Oyen WJ, Bleeker-Rovers CP. The value of 18F-FDG PET/CT in diagnosing infectious endocarditis. Eur J Nucl Med Mol Imaging 2013; 40(7):1102–1107. doi:10.1007/s00259-013-2376-0
- Wong D, Rubinshtein R, Keynan Y. Alternative cardiac imaging modalities to echocardiography for the diagnosis of infective endocarditis. Am J Cardiol 2016; 118(9):1410–1418. doi:10.1016/j.amjcard.2016.07.053
- Vos FJ, Bleeker-Rovers CP, Kullberg BJ, Adang EM, Oyen WJ. Cost-effectiveness of routine (18)F-FDG PET/CT in high-risk patients with gram-positive bacteremia. J Nucl Med 2011; 52(11):1673–1678. doi:10.2967/jnumed.111.089714
- McCollough CH, Bushberg JT, Fletcher JG, Eckel LJ. Answers to common questions about the use and safety of CT scans. Mayo Clin Proc 2015; 90(10):1380–1392. doi:10.1016/j.mayocp.2015.07.011
- Duval X, Iung B, Klein I, et al; IMAGE (Resonance Magnetic Imaging at the Acute Phase of Endocarditis) Study Group. Effect of early cerebral magnetic resonance imaging on clinical decisions in infective endocarditis: a prospective study. Ann Intern Med 2010; 152(8):497–504, W175. doi:10.7326/0003-4819-152-8-201004200-00006
- ACR Committee on Drugs and Contrast Media. ACR Manual on Contrast Media: 2018. www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf. Accessed July 19, 2019.
- Kanda T, Fukusato T, Matsuda M, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 2015; 276(1):228–232. doi:10.1148/radiol.2015142690
- McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 2015; 275(3):772–782. doi:10.1148/radiol.15150025
- Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014; 270(3):834–841. doi:10.1148/radiol.13131669
- Expert Panel on Pediatric Imaging; Hayes LL, Palasis S, Bartel TB, et al. ACR appropriateness criteria headache-child. J Am Coll Radiol 2018; 15(5S):S78–S90. doi:10.1016/j.jacr.2018.03.017
KEY POINTS
- Echocardiography can produce false-negative results in native-valve infective endocarditis and is even less sensitive in patients with a prosthetic valve or cardiac implanted electronic device.
- 4D CT is a reasonable alternative to transesophageal echocardiography. It can also be used as a second test if echocardiography is inconclusive. Coupled with angiography, it also provides a noninvasive method to evaluate coronary arteries perioperatively.
- Nuclear imaging tests—FDG-PET and leukocyte scintigraphy—increase the sensitivity of the Duke criteria for diagnosing infective endocarditis. They should be considered for evaluating suspected infective endocarditis in all patients who have a prosthetic valve or cardiac implanted electronic device, and whenever echocardiography is inconclusive and clinical suspicion remains high.
Adults with autism spectrum disorder: Updated considerations for healthcare providers
Autism spectrum disorder (ASD) has increased significantly over the past 40 years. Even in the past 2 decades, the prevalence increased from 6.7 per 1,000 in 20001 to 14.6 per 1,000 in 2012—1 in 59 people.2 Of those with ASD, 46% have an intelligence quotient (IQ) greater than 85, meaning they are of average or above-average intelligence.1
As more children with autism become adults, understanding this condition across the life span grows paramount. While many studies have focused on understanding how diagnosis and treatment can help young children, few have focused on adults with autism and how primary care teams can better assist these individuals. However, this is changing, with studies of the benefits of employment programs and pharmacologic treatment, and reproductive health needs of adults with ASD. Here we provide an updated review of ASD in adult patients.
NO MORE ASPERGER SYNDROME— IT’S ON THE SPECTRUM NOW
As the scientific understanding of autism has expanded, revisions in the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5),3 published in 2013, have paralleled these advances. For many adult patients with autism who were evaluated as children, these revisions have led to changes in diagnosis and available services.
In the previous edition (DSM-IV-TR, published in 2000),4 autistic disorder and Asperger syndrome were separate (Table 1). However, DSM-5 lumped autistic disorder and Asperger disorder together under the diagnosis of ASD; this leaves it to the clinician to specify whether the patient with ASD has accompanying intellectual or language impairment and to assign a level of severity based on communication deficits and restrictive behaviors.
The shift in diagnosis was worrisome for some, particularly for clinicians treating patients with DSM-IV Asperger syndrome, who lost this diagnostic label. Concerns that patients with Asperger syndrome may not meet the DSM-5 criteria for ASD were validated by a systematic review showing that only 50% to 75% of patients with DSM-IV autistic disorder, Asperger syndrome, or pervasive developmental disorder not otherwise specified (PDD-NOS) met the DSM-5 criteria for ASD.5 Most of those who no longer met the criteria for ASD carried a DSM-IV diagnosis of Asperger syndrome or PDD-NOS or had an IQ over 70.5 Nevertheless, these individuals may struggle with impairing symptoms related to repetitive behaviors or communication or may be affected by learning or social-emotional disabilities. Additionally, even if they meet the criteria for ASD, some may identify with the Asperger syndrome label and fear they will be stigmatized should they be classified as having the more general ASD.6,7
Although future revisions to the DSM may include further changes in classification, grouping adults with ASD according to their functional and cognitive ability may allow for pragmatic characterization of their needs. At least 3 informal groupings of autistic adults have been described that integrate cognitive ability and independence8:
- Those with low cognitive and social abilities, who need lifelong support
- Those with midrange cognitive and social limitations but who can complete their work in special education classes; they often find employment in supervised workshops or other work with repetitive tasks
- Those who have greater cognitive ability and some social skills; they may proceed to college and employment and live independently.
UNCERTAIN PROGNOSIS
Prognostication for people with ASD remains an area of research. Some adults experience a reduction in symptoms as they age, with significant improvements in speech and, sometimes, modest improvements in restrictive and repetitive behaviors.9,10
Nevertheless, autism remains a lifelong disorder for many. Adults may still require significant support and may experience impairment, particularly in social interaction.10 In longitudinal studies, only 15% to 27% of patients with ASD are characterized as having a positive outcome (often defined as variables related to independent function, near-normal relationships, employment, or a quantified reduction in core symptoms), and many experience significant dependency into adulthood.10–13
IQ has been cited as a possible prognostic factor,10,13 with an IQ below 70 associated with poorer outcome, although an IQ above 70 does not necessarily confer a positive outcome. Less-severe impairment in speech at baseline in early childhood also suggests better outcomes in adulthood.10
As we see more adults with autism, studies that include both children and adults, such as the Longitudinal European Autism Cohort, will be important to characterize the natural history, comorbidities, and genetics of ASD and may help provide more specific predictors of disease course into adulthood.14
ACHIEVING A DIAGNOSIS FOR ADULT PATIENTS WITH SUSPECTED AUTISM
While many patients are recognized as having autism in early to mid-childhood, some adults may not receive a formal diagnosis until much later in life. Those with fluent language and normal-range IQ are likely to be overlooked.15 People with ASD may have had mild symptoms during childhood that did not impair their functioning until demands of daily life exceeded their capacities in adulthood. Alternatively, parents of a child with newly diagnosed ASD may realize that they themselves or another adult family member also show signs of it.
The UK National Institute of Health and Care Excellence suggests that assessment should be considered if the patient meets psychiatric diagnostic criteria and one of the following:
- Difficulty obtaining or sustaining employment or education
- Difficulty initiating or sustaining social relationships
- Past or current contact with mental health or learning disability services
- History of a neurodevelopmental or mental health disorder.15,16
Currently, diagnosis typically involves a multidisciplinary approach, with psychiatric assessment, neuropsychological testing, and speech and language evaluation.17 Providers may need to refer patients for these services, sometimes at the patient’s request, if previous mental health misdiagnoses are suspected, if patients report symptoms or impairment consistent with ASD, or if benefits, services, or accommodations, such as a coach in the workplace, are needed.
Diagnosing ASD in adults can be difficult, given that the gold-standard diagnostic tests such as the Autism Diagnostic Observation Schedule-2 (ADOS-2)18 and the Autism Diagnostic Interview-Revised (ADI-R)19 are typically used to diagnose autism in children. However, Module 4 in the ADOS-2 was developed for adolescents and older patients with fluent language and has shown at least moderate power to distinguish adults with ASD from those without ASD.18,20
An initial psychiatric assessment should include a thorough history taken from the patient and, if applicable, the patient’s caregiver, as well as a psychiatric interview of the patient. Neuropsychological testing should include evaluation of cognitive function, social functioning (using the ADOS-2 for adults without intellectual disability, the ADI-R, or both), and adaptive functioning (using the Vineland Adaptive Behavior Scales, second edition21).
Evaluation of speech and language is particularly important in patients with limited language ability and should include both expressive and receptive language abilities. Serial testing every few years, as is often recommended in childhood, may help establish the pattern of impairment over time.
Comorbid psychiatric disorders are common
Many people with ASD also have other psychiatric disorders,17,22 which clinicians should keep in mind when seeing an adult seeking evaluation for ASD.
Attention-deficit/hyperactivity disorder is present at higher rates in patients of average intellectual function with ASD than in the general population.23
Anxiety disorders, including obsessive-compulsive disorder, were found to often coexist with autism in a sample of adults with autism without intellectual disability,24,25 and approximately 40% of youths with ASD have at least 1 comorbid anxiety disorder.26
Mood disorders are also prevalent in adults with ASD, with a small study showing that 70% of adults with DSM-IV Asperger syndrome had at least 1 depressive episode in their lifetime.27
BEHAVIORAL AND PHARMACOLOGIC THERAPIES FOR THE ADULT PATIENT
Services and medications for adults with ASD are discussed below. These will vary by individual, and services available may vary by region.
Historically, vocational and social outcomes have been poor for adults with ASD. It is estimated that most larger universities may be home to 100 to 300 students with ASD. To combat isolation, the University of California, Los Angeles, the University of Alabama, and others provide special support services, including group social activities such as board games and individual coaching.8 Nevertheless, half of the students with autism who attend institutions of higher learning leave without completing their intended degree.29 Many still struggle to establish meaningful friendships or romantic relationships.29
Planning for a transition of care
Healthcare transition planning is important but is strikingly underused.30 Individual providers, including adult psychiatrists, vary in their level of training and comfort in diagnosing, treating, and monitoring adults with autism. Youths with ASD are half as likely to receive healthcare transition services as other youths with special healthcare needs.31
Pediatric providers, including pediatric psychiatrists, developmental behavioral specialists, and pediatric neurologists, may be best equipped to treat young adult patients or to refer patients to appropriate generalists and specialists comfortable with autism-specific transition of care. The question of eligibility for services is important to patients and families during the transition period, with many parents and professionals unaware of services available to them.32 Receiving adequate transition services is enabled by having a medical home during childhood—that is, a comprehensive, centralized medical record, culturally competent care, interaction with schools, and patient access to clear, unbiased information.31
Ideally, in our experience, transitioning should be discussed well before the child ages out of the pediatric provider’s practice. If necessary, healthcare transition services should include 4 components:
- Discussing the switch to a new physician who treats adults
- Discussing changing healthcare needs as an adult
- Planning insurance coverage as an adult
- Encouragement by the physician for the child to take age-appropriate responsibility for his or her healthcare.31,33
Tools such as the Got Transition checklist from the National Health Care Transition Center can provide support during this process.34
Other services
Other services provided as an extension or adjunct to the medical home in early adulthood may include customized vocational or employment training, specialized mentorship or support in a college setting, housing support, and psychological services.35
Community-based programs that emphasize leisure have been shown to improve participants’ independence and quality of life.36 Similarly, participants in programs that emphasized supported employment, with a job coach, on-the-job support, collaboration with the participant’s larger social support network, and selection of tasks to match an individual’s abilities and strengths, demonstrated improved cognitive performance, particularly executive functioning,37 and employment.38,39 These programs work best for patients who have mild to moderate symptoms.37,39
Patients with symptoms that are more severe may do better in a residential program. Many of these programs maintain an emphasis on vocational and social skills development. One such long-standing program is Bittersweet Farms, a rural farming community in Ohio for adults with ASD, where individuals with moderate to low function live in a group setting, with emphasis on scheduled, meaningful work including horticulture, animal care, carpentry; and activities of daily living.40
Studies of patients across the autism spectrum have generally found better outcomes when vocational support is given, but larger and randomized studies are needed to characterize how to best support these individuals after they leave high school.41
Psychological services such as applied behavioral therapy, social cognition training, cognitive behavioral therapy, and mindfulness training may be particularly useful in adults.42–44
Some versions of applied behavioral therapy, such as the Early Start Denver Model,45 have been found to be cost-effective and offset some expenses in the care of children with autism, using play-based and relationship-based interventions to promote development across domains while reducing symptoms.
In randomized controlled trials, modified cognitive behavioral therapy43 and mindfulness44 were shown to reduce symptoms of anxiety, obsessive-compulsive disorder, and depression.
Dialectical behavior therapy, used to find a balance between accepting oneself and desiring to change, may help in some circumstances to regulate emotions and reduce reactivity and lability, although large randomized clinical trials have not been conducted in the ASD population.46
Drug therapy
Medications may be appropriate to manage symptoms or comorbid conditions in adults with ASD. Over 75% adults with ASD have been found to use psychotropic medications.47 However, although these drugs have been approved for treating behaviors commonly associated with ASD, none of them provide definitive treatment for this disorder, and they have not been rigorously tested or approved for use in adults with ASD.48
Irritability and aggression associated with ASD can be treated with risperidone (approved for children over age 5), aripiprazole (approved for children ages 6–17), clozapine, or haloperidol.49
Aberrant social behavior can be treated with risperidone.50 Treatments under investigation include oxytocin and secretin.49
While no approved drug has been shown to improve social communication,51 balovaptan, a vasopressin V1a agonist, has shown potential and has been granted breakthrough status by the US Food and Drug Administration for treating challenging behaviors in adults, with additional studies ongoing in children.52,53
Repetitive behaviors, if the patient finds them impairing, can be managed with selective serotonin reuptake inhibitors.49
Much more study of drug therapy in adults with ASD is needed to fully understand the best approaches to psychotropic medication use, including appropriate classes and effective dosage, in this population.
SEX: UNEXPLORED TERRITORY
The reproductive health needs of people with autism remain largely underexplored.54 Historically, individuals with ASD were thought to have little interest in sexual activity or parenthood, owing to the nature of the core symptoms of the disorder. This has been shown to be untrue, particularly as studies on this topic began to engage in direct interviews with people with ASD, rather than solely gathering information from caregivers or parents. The findings reinforce the importance of broaching this component of health in this population, for the following reasons:
Adults with ASD are at increased risk of sexual victimization, with nearly 4 out of 5 reporting unwanted sexual advances, coercion, or rape.55
They have a smaller pool of knowledge with respect to sexual health. They report56 that they learned about sex from television and from “making mistakes.” They use fewer sources. They are less likely to speak to peers and figures of authority to gain knowledge about sexually transmitted infections, sexual behaviors, and contraception. And they are more likely to use forms of nonsocial media, such as television, for information.55
They report more concerns about the future with respect to sexual behavior, suggesting the need for targeted sexual education programs.56
College-age young adults with ASD who misread communication may be particularly affected by Title IX, which requires schools to promptly investigate reports of sexual harassment and sexual assault, should they struggle to comport themselves appropriately.57 Early and frank conversations about issues of consent and appropriate displays of interest and affection may better equip youth to navigate new social scenarios as they plan to leave a supervised home environment for college or the workforce.
Gender identification: Male, female, other
In one study, 77.8% of birth-sex males with ASD said they identified as men, and 67.1% of birth-sex females identified as women, compared with 93.1% of birth-sex males and 87.3% of birth-sex females without ASD. Many of the remaining individuals with ASD reported a transgender, genderqueer, or other gender identity.58 Some studies have found females with ASD report a gay or bisexual orientation more often than males with ASD.59–61
Adolescents and young adults may be exploring their changing bodies, sexual preferences, and gender roles, and as for all people at this age, these roles emerge against a backdrop of familial and societal expectations that may or may not be concordant with their own projected path regarding sexuality and reproductive health.62
Having the conversation
As with non-ASD patients, a thorough sexual history should be collected via open-ended questions when possible to determine types of sexual activity and partners.
Education of the patient, alongside caregivers and parents, about healthy and safe sexual practices, screening for sexual violence, and hormonal and nonhormonal contraception options are important components of care for this population.
CAREGIVER STRESS MAY PERSIST INTO PATIENT’S ADULTHOOD
Caregiver burden is a monumental concern for parents or others who may have lifelong primary responsibility for these neurodiverse adults.63 Family members may feel isolated and may feel they have encountered many barriers to services.64 Remaining sensitive, knowledgeable, and inquisitive about the types of support that are needed may help forge a trusting relationship between the provider and the family.
Parents of children with ASD have been reported to experience worse physical and emotional health than parents whose children do not have developmental disabilities.63,65 These disparities have been found to persist as their children enter adolescence and young adulthood.66,67 Parents of children with ASD report more anxiety, depression, and distress compared with parents of children without ASD,63 and parents themselves may be affected by ASD symptoms, which has been linked to increased parenting stress.68 Some studies have found blunted cortisol responses,63,69,70 and some,71 but not all,63 have found elevated blood pressure in caregivers of children with developmental disabilities. Headache, backache, muscle soreness, and fatigue may also be commonly reported.67
In our experience, caregivers are tremendously appreciative when provided connections to adult ASD services and support systems as their child ages. The school system and other formal support systems often assist until the time of transition into adulthood. This transition can be stressful for the adolescent and family alike, and informal support systems such as friends and family may become increasingly crucial, particularly if the adolescent still lives at home.72,73
The affected young adult’s unmet needs, as perceived by the caregiver, have been found to be significantly associated with caregiver burden, whereas the severity of the adult patient’s ASD symptoms has not.66 Therefore, it may be helpful to ask caregivers whether they perceive any unmet needs, regardless of the clinician’s perception of the severity of the patient’s ASD symptoms. Providing support to address these needs, particularly those relating to the child’s mood disorders, communication, social needs, safety, and daytime activities, may be the domains of support that most effectively reduce the caregiver burden in this population.66
Caregiver positivity, lower stress levels, and increased social support, particularly in the form of friends and family members providing no-cost assistance to caregivers whose children do not live independently,74 have been linked to better outcomes for caregivers.70,74,75 Rigorous studies that examine caregiver burden as individuals with ASD enter mid- and late-adulthood are limited.
THE ROLE OF THE INTERNIST IN CARING FOR ADULTS WITH AUTISM
A major challenge for many adults with ASD is the transition from services provided during childhood to those provided in adulthood. While children with autism have subspecialty providers who diagnose and manage their condition, including developmental-behavioral pediatricians, pediatric neurologists, and child psychiatrists, adults with autism may have fewer options.
Autism centers are becoming more available across the nation, and many provide care across the life span. However, depending on a patient’s needs, the primary care provider may need to manage residual symptoms as the patient transitions from pediatric to adult care, ultimately deciding when and where to refer the patient.
The patient’s family should pay close attention to function and mood around the time the patient leaves the structure of high school, and they should build rapport with a primary care provider they can turn to if problems persist or arise. Referrals for behavioral therapy and for social work, job training, and vocational support can greatly benefit patients as they transition to young adulthood. Referrals and suggestions for social support can also help caregivers.
Medical care
Deciding when and how to medicate the patient for symptoms of autism and related behaviors necessitates consideration of the patient’s impairment, side effects of the medication, and the impact medications may have on the patient’s other conditions. Disordered eating, mood problems, anxiety, and attention-deficit/hyperactivity disorder should be considered, and, as in all patients, regular screenings of mental health status should be conducted.76,77
Comorbid medical conditions may cause worsening of a patient’s known behavioral symptoms or may precipitate new behaviors or aggression as a result of pain or discomfort, particularly in patients with limited speech. A change in stereotypes or increased irritability warrants a thoughtful investigation for a cause other than ASD before adding or increasing behavioral medications. Common comorbid conditions include gastrointestinal distress, most commonly constipation and diarrhea in an idiopathic ASD population, with increasing ASD symptom severity correlating with increased odds of a gastrointestinal problem.78 Allergies, sleep disorders, seizures, and other psychiatric conditions are also frequent.79
Preventive care, including vaccinations, should be given as scheduled. Caregivers and patients can be reminded if needed that vaccines do not cause or worsen autism, and vaccination is intended to improve the safety of the patient and those around them, protecting against potentially life-threatening disease. Regular dental care visits, particularly for patients who are using medications that may affect tooth or gingival health,80 and regular visits to an optometrist or ophthalmologist for screening of vision are also advised.
Adverse effects. Weight gain and metabolic syndrome are common adverse effects of medications used for behavioral management, and the primary care physician may uncover diabetes, cardiac disorders, and hyperlipidemia. Patients with ASD may be particularly sensitive to the effects of medications and therefore may require a lower dose or a slower titration than other patients. Working with a behavioral team, careful weaning of psychiatric medications to the minimum needed is strongly recommended whenever possible.81
TAKE-HOME POINTS
As more adults with autism enter society, they may require varying levels of support from the healthcare community to ensure that therapeutic gains from childhood persist, allowing them to achieve maximal functional potential.
Adults with ASD may have a high, normal, or low IQ and intellectual capability. Knowledge of this and of the patient’s symptom severity and presence of comorbid psychiatric and other health conditions can help the clinician guide the patient to appropriate social services and pharmacologic treatments.
Individualized support in the workplace, as well as education regarding sexual health, can help improve outcomes for affected individuals.
Caregiver burden for individuals with autism can be high, but it can be mitigated by social support.
Further research regarding appropriate diagnostic instruments in adulthood and appropriate treatments for impairing autism-related symptoms across the life span may be particularly helpful in supporting this patient population.
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2000 Principal Investigators; Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders—autism and developmental disabilities monitoring network, six sites, United States, 2000. MMWR Surveill Summ 2007; 56(1):1–11. pmid:17287714
- Christensen DL. Prevalence and characteristics of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2012. MMWR Surveill Summ 2016; 65(13):1–23. doi:10.15585/mmwr.ss6503a1
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edition. Washington, D.C: American Psychiatric Association; 2013.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision. Washington, DC: American Psychiatric Association; 2000.
- Smith IC, Reichow B, Volkmar FR. The effects of DSM-5 criteria on number of individuals diagnosed with autism spectrum disorder: a systematic review. J Autism Dev Disord 2015; 45(8):2541–2552. doi:10.1007/s10803-015-2423-8
- Barahona-Corrêa JB, Filipe CN. A concise history of Asperger syndrome: the short reign of a troublesome diagnosis. Front Psychol 2015; 6:2024. doi:10.3389/fpsyg.2015.02024
- Kite DM, Gullifer J, Tyson GA. Views on the diagnostic labels of autism and Asperger’s disorder and the proposed changes in the DSM. J Autism Dev Disord 2013; 43(7):1692–1700. doi:10.1007/s10803-012-1718-2
- Kuo AA. Autism in adults: an update. Presented at the: American College of Physicians Internal Medicine Meeting, New Orleans, LA, April 17–21, 2018.
- Shattuck PT, Seltzer MM, Greenberg JS, et al. Change in autism symptoms and maladaptive behaviors in adolescents and adults with an autism spectrum disorder. J Autism Dev Disord 2007; 37(9):1735–1747. doi:10.1007/s10803-006-0307-7
- Seltzer MM, Shattuck P, Abbeduto L, Greenberg JS. Trajectory of development in adolescents and adults with autism. Ment Retard Dev Disabil Res Rev 2004; 10(4):234–247. doi:10.1002/mrdd.20038
- Billstedt E, Carina Gillberg I, Gillberg C. Autism in adults: symptom patterns and early childhood predictors. Use of the DISCO in a community sample followed from childhood. J Child Psychol Psychiatry 2007; 48(11):1102–1110. doi:10.1111/j.1469-7610.2007.01774.x
- Howlin P, Goode S, Hutton J, Rutter M. Adult outcome for children with autism. J Child Psychol Psychiatry 2004; 45(2):212–229. pmid:14982237
- Marriage S, Wolverton A, Marriage K. Autism spectrum disorder grown up: a chart review of adult functioning. J Can Acad Child Adolesc Psychiatry 2009; 18(4):322–328. pmid: 19881941
- Isaksson J, Tammimies K, Neufeld J, et al. EU-AIMS Longitudinal European Autism Project (LEAP): the autism twin cohort. Mol Autism 2018; 9(1):26. doi:10.1186/s13229-018-0212-x
- Lai M-C, Baron-Cohen S. Identifying the lost generation of adults with autism spectrum conditions. Lancet Psychiatry 2015; 2(11):1013–1027. doi:10.1016/S2215-0366(15)00277-1
- National Institute for Health and Clinical Excellence. Autism: recognition, referral, diagnosis and management of adults on the autism spectrum. NICE clinical guideline 142. June 2012. https://grand.tghn.org/site_media/media/medialibrary/2015/03/ASD_NICE_3_.pdf. Accessed July 9, 2019.
- Wolf JM, Ventola P. Assessment and treatment planning in adults with autism spectrum disorders. In: Adolescents and Adults with Autism Spectrum Disorders. Springer, New York, NY; 2014:283–298.
- Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop SL. Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) manual. Torrance, CA: Western Psychological Services, 2012.
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 1994; 24(5):659–685.
- Hus V, Lord C. The autism diagnostic observation schedule, module 4: revised algorithm and standardized severity scores. J Autism Dev Disord 2014; 44(8):1996–2012. doi:10.1007/s10803-014-2080-3>
- Sparrow S, Balla D, Cicchetti D, Harrison P, Doll E. Vineland Adaptive Behavior Scales. Circle Pines, MN: American Guidance Service, 1984.
- Happé FG, Mansour H, Barrett P, Brown T, Abbott P, Charlton RA. Demographic and cognitive profile of individuals seeking a diagnosis of autism spectrum disorder in adulthood. J Autism Dev Disord 2016; 46(11):3469–3480. doi:10.1007/s10803-016-2886-2
- Johnston K, Dittner A, Bramham J, Murphy C, Knight A, Russell A. Attention deficit hyperactivity disorder symptoms in adults with autism spectrum disorders. Autism Res Off J Int Soc Autism Res 2013; 6(4):225–236. doi:10.1002/aur.1283
- Cadman T, Spain D, Johnston P, et al. Obsessive-compulsive disorder in adults with high-functioning autism spectrum disorder: what does self-report with the OCI-R tell us? Autism Res Off J Int Soc Autism Res 2015; 8(5):477–485. doi:10.1002/aur.1461
- Russell AJ, Mataix-Cols D, Anson M, Murphy DGM. Obsessions and compulsions in Asperger syndrome and high-functioning autism. Br J Psychiatry J Ment Sci 2005; 186:525–528. doi:10.1192/bjp.186.6.525
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry 2008; 47(8):921–929. doi:10.1097/CHI.0b013e318179964f
- Lugnegård T, Hallerbäck MU, Gillberg C. Psychiatric comorbidity in young adults with a clinical diagnosis of Asperger syndrome. Res Dev Disabil 2011; 32(5):1910–1917. doi:10.1016/j.ridd.2011.03.025
- Howlin P, Moss P. Adults with autism spectrum disorders. Can J Psychiatry 2012; 57(5):275–283. doi:10.1177/070674371205700502
- Levy A, Perry A. Outcomes in adolescents and adults with autism: a review of the literature. Res Autism Spectr Disord 2011; 5(4):1271–1282. doi:10.1016/J.RASD.2011.01.023
- Cheak-Zamora NC, Yang X, Farmer JE, Clark M. Disparities in transition planning for youth with autism spectrum disorder. Pediatrics 2013; 131(3):447–454. doi:10.1542/peds.2012-1572
- Rast JE, Shattuck PT, Roux AM, Anderson KA, Kuo A. The medical home and health care transition for youth with autism. Pediatrics 2018; 141(suppl 4):S328–S334. doi:10.1542/peds.2016-4300J
- Belling R, McLaren S, Paul M, et al. The effect of organisational resources and eligibility issues on transition from child and adolescent to adult mental health services. J Health Serv Res Policy 2014; 19(3):169–176. doi:10.1177/1355819614527439
- Data Resource Center for Child & Adolescent Health. 2009–2010 National Survey of Children with Special Health Care Needs. www.childhealthdata.org/docs/drc/200910-cshcn-spss-codebook_final_051012.pdf?sfvrsn=1. Accessed July 9, 2019.
- Got Transition Center for Health Care Transition Improvement. Six core elements of health care transition 2.0. Transitioning youth to an adult health care provider. For use by pediatric, family medicine, and med-peds providers. www.gottransition.org/resourceGet.cfm?id=208. Accessed July 9, 2019.
- Murphy CM, Wilson CE, Robertson DM, et al. Autism spectrum disorder in adults: diagnosis, management, and health services development. Neuropsychiatr Dis Treat 2016; 12:1669–1686. doi:10.2147/NDT.S65455
- García-Villamisar DA, Dattilo J. Effects of a leisure programme on quality of life and stress of individuals with ASD. J Intellect Disabil Res 2010; 54(7):611–619. doi:10.1111/j.1365-2788.2010.01289.x
- García-Villamisar D, Hughes C. Supported employment improves cognitive performance in adults with autism. J Intellect Disabil Res 2007; 51(pt 2):142–150. doi:10.1111/j.1365-2788.2006.00854.x
- Lawer L, Brusilovskiy E, Salzer MS, Mandell DS. Use of vocational rehabilitative services among adults with autism. J Autism Dev Disord 2009; 39(3):487–494. doi:10.1007/s10803-008-0649-4
- Howlin P, Alcock J, Burkin C. An 8 year follow-up of a specialist supported employment service for high-ability adults with autism or Asperger syndrome. Autism 2005; 9(5):533–549. doi:10.1177/1362361305057871
- Kay BR. Bittersweet Farms. J Autism Dev Disord 1990; 20(3):309–321. http://www.ncbi.nlm.nih.gov/pubmed/2228914. Accessed July 9, 2019.
- Taylor JL, McPheeters ML, Sathe NA, Dove D, Veenstra-Vanderweele J, Warren Z. A systematic review of vocational interventions for young adults with autism spectrum disorders. Pediatrics 2012; 130(3):531–538. doi:10.1542/peds.2012-0682
- Bishop-Fitzpatrick L, Minshew NJ, Eack SM. A systematic review of psychosocial interventions for adults with autism spectrum disorders. J Autism Dev Disord 2013; 43(3):687–694. doi:10.1007/s10803-012-1615-8
- Russell AJ, Jassi A, Fullana MA, et al. Cognitive behavior therapy for comorbid obsessive-compulsive disorder in high-functioning autism spectrum disorders: a randomized controlled trial. Depress Anxiety 2013; 30(8):697–708. doi:10.1002/da.22053
- Spek AA, van Ham NC, Nyklícek I. Mindfulness-based therapy in adults with an autism spectrum disorder: a randomized controlled trial. Res Dev Disabil 2013; 34(1):246–253. doi:10.1016/j.ridd.2012.08.009
- Eapen V, Crncec R, Walter A. Clinical outcomes of an early intervention program for preschool children with autism spectrum disorder in a community group setting. BMC Pediatr 2013; 13(1):3. doi:10.1186/1471-2431-13-3
- Mazefsky CA, White SW. Emotion regulation: concepts & practice in autism spectrum disorder. Child Adolesc Psychiatr Clin North Am 2014; 23(1):15–24. doi:10.1016/J.CHC.2013.07.002
- Esbensen AJ, Greenberg JS, Seltzer MM, Aman MG. A longitudinal investigation of psychotropic and non-psychotropic medication use among adolescents and adults with autism spectrum disorders. J Autism Dev Disord 2009; 39(9):1339–1349. doi:10.1007/s10803-009-0750-3
- Dove D, Warren Z, McPheeters ML, Taylor JL, Sathe NA, Veenstra-VanderWeele J. Medications for adolescents and young adults with autism spectrum disorders: a systematic review. Pediatrics 2012; 130(4):717–726. doi:10.1542/peds.2012-0683
- LeClerc S, Easley D. Pharmacological therapies for autism spectrum disorder: a review. Pharm Ther 2015; 40(6):389–397.
- Miral S, Gencer O, Inal-Emiroglu FN, Baykara B, Baykara A, Dirik E. Risperidone versus haloperidol in children and adolescents with AD: a randomized, controlled, double-blind trial. Eur Child Adolesc Psychiatry 2008; 17(1):1–8. doi:10.1007/s00787-007-0620-5
- Lai M-C, Lombardo MV, Baron-Cohen S. Autism. Lancet 2014; 383(9920):896–910. doi:10.1016/S0140-6736(13)61539-1
- Ratni H, Rogers-Evans M, Bissantz C, et al. Discovery of highly selective brain-penetrant vasopressin 1a antagonists for the potential treatment of autism via a chemogenomic and scaffold hopping approach. J Med Chem 2015; 58(5):2275–2289. doi:10.1021/jm501745f
- Umbricht D, Del Valle Rubido M, Hollander E, et al. A single dose, randomized, controlled proof-of-mechanism study of a novel vasopressin 1a receptor antagonist (RG7713) in high-functioning adults with autism spectrum disorder. Neuropsychopharmacology 2017; 42(9):1914–1923. doi:10.1038/npp.2016.232>
- Kellaher DC. Sexual behavior and autism spectrum disorders: an update and discussion. Curr Psychiatry Rep 2015; 17(4):25. doi:10.1007/s11920-015-0562-4
- Brown-Lavoie SM, Viecili MA, Weiss JA. Sexual knowledge and victimization in adults with autism spectrum disorders. J Autism Dev Disord 2014; 44(9):2185–2196. doi:10.1007/s10803-014-2093-y
- Mehzabin P, Stokes MA. Self-assessed sexuality in young adults with high-functioning autism. Res Autism Spectr Disord 2011; 5(1):614–621. doi:10.1016/J.RASD.2010.07.006>
- Brown KR. Accessibility for students with ASD: legal perspectives in the United States. In: Alphin HC Jr. Exploring the Future of Accessibility in Higher Education. Hershey, PA: IGI Global; 2017.
- George R, Stokes MA. Gender identity and sexual orientation in autism spectrum disorder. Autism 2018; 22(8):970–982. doi:10.1177/1362361317714587
- Byers ES, Nichols S, Voyer SD. Challenging stereotypes: sexual functioning of single adults with high functioning autism spectrum disorder. J Autism Dev Disord 2013; 43(11):2617–2627. doi:10.1007/s10803-013-1813-z
- Gilmour L, Schalomon PM, Smith V. Sexuality in a community based sample of adults with autism spectrum disorder. Res Autism Spectr Disord 2012; 6(1):313–318. doi:10.1016/J.RASD.2011.06.003
- Bejerot S, Eriksson JM. Sexuality and gender role in autism spectrum disorder: a case control study. Schmitz C, ed. PLoS One 2014; 9(1):e87961. doi:10.1371/journal.pone.0087961>
- Navot N, Jorgenson AG, Webb SJ. Maternal experience raising girls with autism spectrum disorder: a qualitative study. Child Care Health Dev 2017; 43(4):536–545. doi:10.1111/cch.12470
- Padden C, James JE. Stress among parents of children with and without autism spectrum disorder: a comparison involving physiological indicators and parent self-reports. J Dev Phys Disabil 2017; 29(4):567–586. doi:10.1007/s10882-017-9547-z
- Woodgate RL, Ateah C, Secco L. Living in a world of our own: the experience of parents who have a child with autism. Qual Health Res 2008; 18(8):1075–1083. doi:10.1177/1049732308320112
- Hayes SA, Watson SL. The impact of parenting stress: a meta-analysis of studies comparing the experience of parenting stress in parents of children with and without autism spectrum disorder. J Autism Dev Disord 2013; 43(3):629–642. doi:10.1007/s10803-012-1604-y
- Cadman T, Eklund H, Howley D, et al. Caregiver burden as people with autism spectrum disorder and attention-deficit/hyperactivity disorder transition into adolescence and adulthood in the United Kingdom. J Am Acad Child Adolesc Psychiatry 2012; 51(9):879–888. doi:10.1016/j.jaac.2012.06.017
- Smith LE, Seltzer MM, Greenberg JS. Daily health symptoms of mothers of adolescents and adults with fragile x syndrome and mothers of adolescents and adults with autism spectrum disorder. J Autism Dev Disord 2012; 42(9):1836–1846. doi:10.1007/s10803-011-1422-7
- van Steijn DJ, Oerlemans AM, van Aken MAG, Buitelaar JK, Rommelse NNJ. The reciprocal relationship of ASD, ADHD, depressive symptoms and stress in parents of children with ASD and/or ADHD. J Autism Dev Disord 2014; 44(5):1064–1076. doi:10.1007/s10803-013-1958-9
- Seltzer MM, Greenberg JS, Hong J, et al. Maternal cortisol levels and behavior problems in adolescents and adults with ASD. J Autism Dev Disord 2010; 40(4):457–469. doi:10.1007/S10803-009-0887-0
- Lovell B, Moss M, Wetherell MA. With a little help from my friends: psychological, endocrine and health corollaries of social support in parental caregivers of children with autism or ADHD. Res Dev Disabil 2012; 33(2):682–687. doi:10.1016/j.ridd.2011.11.014
- Gallagher S, Whiteley J. Social support is associated with blood pressure responses in parents caring for children with developmental disabilities. Res Dev Disabil 2012; 33(6):2099–2105. doi:10.1016/j.ridd.2012.06.007
- Baker JK, Smith LE, Greenberg JS, Seltzer MM, Taylor JL. Change in maternal criticism and behavior problems in adolescents and adults with autism across a 7-year period. J Abnorm Psychol 2011; 120(2):465–475. doi:10.1037/a0021900
- Marsack CN, Samuel PS. Mediating effects of social support on quality of life for parents of adults with autism. J Autism Dev Disord 2017; 47(8):2378–2389. doi:10.1007/s10803-017-3157-6
- Trute B, Benzies KM, Worthington C, Reddon JR, Moore M. Accentuate the positive to mitigate the negative: mother psychological coping resources and family adjustment in childhood disability. J Intellect Dev Disabil 2010; 35(1):36–43. doi:10.3109/13668250903496328
- Cantwell J, Muldoon OT, Gallagher S. Social support and mastery influence the association between stress and poor physical health in parents caring for children with developmental disabilities. Res Dev Disabil 2014; 35(9):2215–2223. doi:10.1016/j.ridd.2014.05.012
- Carton AM, Smith AD. Assessing the relationship between eating disorder psychopathology and autistic traits in a non-clinical adult population. Eat Weight Disord - Stud Anorexia, Bulim Obes 2014; 19(3):285–293. doi:10.1007/s40519-013-0086-z
- De Alwis D, Agrawal A, Reiersen AM, et al. ADHD symptoms, autistic traits, and substance use and misuse in adult Australian twins. J Stud Alcohol Drugs 2014; 75(2):211–221. doi:10.15288/jsad.2014.75.211
- Wang LW, Tancredi DJ, Thomas DW. The prevalence of gastrointestinal problems in children across the United States with autism spectrum disorders from families with multiple affected members. J Dev Behav Pediatr 2011; 32(5):351–360. doi:10.1097/DBP.0b013e31821bd06a
- Croen LA, Zerbo O, Qian Y, et al. The health status of adults on the autism spectrum. Autism 2015; 19(7):814–823. doi:10.1177/1362361315577517
- Kalyoncu IÖ, Tanboga I. Oral health status of children with autistic spectrum disorder compared with non-authentic peers. Iran J Public Health 2017; 46(11):1591–1593. www.ncbi.nlm.nih.gov/pmc/articles/PMC5696703. Accessed July 9, 2019.
- McGuire K, Fung LK, Hagopian L, et al. Irritability and problem behavior in autism spectrum disorder: a practice pathway for pediatric primary care. Pediatrics 2016; 137(suppl 2):S136–S148. doi:10.1542/peds.2015-2851L
Autism spectrum disorder (ASD) has increased significantly over the past 40 years. Even in the past 2 decades, the prevalence increased from 6.7 per 1,000 in 20001 to 14.6 per 1,000 in 2012—1 in 59 people.2 Of those with ASD, 46% have an intelligence quotient (IQ) greater than 85, meaning they are of average or above-average intelligence.1
As more children with autism become adults, understanding this condition across the life span grows paramount. While many studies have focused on understanding how diagnosis and treatment can help young children, few have focused on adults with autism and how primary care teams can better assist these individuals. However, this is changing, with studies of the benefits of employment programs and pharmacologic treatment, and reproductive health needs of adults with ASD. Here we provide an updated review of ASD in adult patients.
NO MORE ASPERGER SYNDROME— IT’S ON THE SPECTRUM NOW
As the scientific understanding of autism has expanded, revisions in the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5),3 published in 2013, have paralleled these advances. For many adult patients with autism who were evaluated as children, these revisions have led to changes in diagnosis and available services.
In the previous edition (DSM-IV-TR, published in 2000),4 autistic disorder and Asperger syndrome were separate (Table 1). However, DSM-5 lumped autistic disorder and Asperger disorder together under the diagnosis of ASD; this leaves it to the clinician to specify whether the patient with ASD has accompanying intellectual or language impairment and to assign a level of severity based on communication deficits and restrictive behaviors.
The shift in diagnosis was worrisome for some, particularly for clinicians treating patients with DSM-IV Asperger syndrome, who lost this diagnostic label. Concerns that patients with Asperger syndrome may not meet the DSM-5 criteria for ASD were validated by a systematic review showing that only 50% to 75% of patients with DSM-IV autistic disorder, Asperger syndrome, or pervasive developmental disorder not otherwise specified (PDD-NOS) met the DSM-5 criteria for ASD.5 Most of those who no longer met the criteria for ASD carried a DSM-IV diagnosis of Asperger syndrome or PDD-NOS or had an IQ over 70.5 Nevertheless, these individuals may struggle with impairing symptoms related to repetitive behaviors or communication or may be affected by learning or social-emotional disabilities. Additionally, even if they meet the criteria for ASD, some may identify with the Asperger syndrome label and fear they will be stigmatized should they be classified as having the more general ASD.6,7
Although future revisions to the DSM may include further changes in classification, grouping adults with ASD according to their functional and cognitive ability may allow for pragmatic characterization of their needs. At least 3 informal groupings of autistic adults have been described that integrate cognitive ability and independence8:
- Those with low cognitive and social abilities, who need lifelong support
- Those with midrange cognitive and social limitations but who can complete their work in special education classes; they often find employment in supervised workshops or other work with repetitive tasks
- Those who have greater cognitive ability and some social skills; they may proceed to college and employment and live independently.
UNCERTAIN PROGNOSIS
Prognostication for people with ASD remains an area of research. Some adults experience a reduction in symptoms as they age, with significant improvements in speech and, sometimes, modest improvements in restrictive and repetitive behaviors.9,10
Nevertheless, autism remains a lifelong disorder for many. Adults may still require significant support and may experience impairment, particularly in social interaction.10 In longitudinal studies, only 15% to 27% of patients with ASD are characterized as having a positive outcome (often defined as variables related to independent function, near-normal relationships, employment, or a quantified reduction in core symptoms), and many experience significant dependency into adulthood.10–13
IQ has been cited as a possible prognostic factor,10,13 with an IQ below 70 associated with poorer outcome, although an IQ above 70 does not necessarily confer a positive outcome. Less-severe impairment in speech at baseline in early childhood also suggests better outcomes in adulthood.10
As we see more adults with autism, studies that include both children and adults, such as the Longitudinal European Autism Cohort, will be important to characterize the natural history, comorbidities, and genetics of ASD and may help provide more specific predictors of disease course into adulthood.14
ACHIEVING A DIAGNOSIS FOR ADULT PATIENTS WITH SUSPECTED AUTISM
While many patients are recognized as having autism in early to mid-childhood, some adults may not receive a formal diagnosis until much later in life. Those with fluent language and normal-range IQ are likely to be overlooked.15 People with ASD may have had mild symptoms during childhood that did not impair their functioning until demands of daily life exceeded their capacities in adulthood. Alternatively, parents of a child with newly diagnosed ASD may realize that they themselves or another adult family member also show signs of it.
The UK National Institute of Health and Care Excellence suggests that assessment should be considered if the patient meets psychiatric diagnostic criteria and one of the following:
- Difficulty obtaining or sustaining employment or education
- Difficulty initiating or sustaining social relationships
- Past or current contact with mental health or learning disability services
- History of a neurodevelopmental or mental health disorder.15,16
Currently, diagnosis typically involves a multidisciplinary approach, with psychiatric assessment, neuropsychological testing, and speech and language evaluation.17 Providers may need to refer patients for these services, sometimes at the patient’s request, if previous mental health misdiagnoses are suspected, if patients report symptoms or impairment consistent with ASD, or if benefits, services, or accommodations, such as a coach in the workplace, are needed.
Diagnosing ASD in adults can be difficult, given that the gold-standard diagnostic tests such as the Autism Diagnostic Observation Schedule-2 (ADOS-2)18 and the Autism Diagnostic Interview-Revised (ADI-R)19 are typically used to diagnose autism in children. However, Module 4 in the ADOS-2 was developed for adolescents and older patients with fluent language and has shown at least moderate power to distinguish adults with ASD from those without ASD.18,20
An initial psychiatric assessment should include a thorough history taken from the patient and, if applicable, the patient’s caregiver, as well as a psychiatric interview of the patient. Neuropsychological testing should include evaluation of cognitive function, social functioning (using the ADOS-2 for adults without intellectual disability, the ADI-R, or both), and adaptive functioning (using the Vineland Adaptive Behavior Scales, second edition21).
Evaluation of speech and language is particularly important in patients with limited language ability and should include both expressive and receptive language abilities. Serial testing every few years, as is often recommended in childhood, may help establish the pattern of impairment over time.
Comorbid psychiatric disorders are common
Many people with ASD also have other psychiatric disorders,17,22 which clinicians should keep in mind when seeing an adult seeking evaluation for ASD.
Attention-deficit/hyperactivity disorder is present at higher rates in patients of average intellectual function with ASD than in the general population.23
Anxiety disorders, including obsessive-compulsive disorder, were found to often coexist with autism in a sample of adults with autism without intellectual disability,24,25 and approximately 40% of youths with ASD have at least 1 comorbid anxiety disorder.26
Mood disorders are also prevalent in adults with ASD, with a small study showing that 70% of adults with DSM-IV Asperger syndrome had at least 1 depressive episode in their lifetime.27
BEHAVIORAL AND PHARMACOLOGIC THERAPIES FOR THE ADULT PATIENT
Services and medications for adults with ASD are discussed below. These will vary by individual, and services available may vary by region.
Historically, vocational and social outcomes have been poor for adults with ASD. It is estimated that most larger universities may be home to 100 to 300 students with ASD. To combat isolation, the University of California, Los Angeles, the University of Alabama, and others provide special support services, including group social activities such as board games and individual coaching.8 Nevertheless, half of the students with autism who attend institutions of higher learning leave without completing their intended degree.29 Many still struggle to establish meaningful friendships or romantic relationships.29
Planning for a transition of care
Healthcare transition planning is important but is strikingly underused.30 Individual providers, including adult psychiatrists, vary in their level of training and comfort in diagnosing, treating, and monitoring adults with autism. Youths with ASD are half as likely to receive healthcare transition services as other youths with special healthcare needs.31
Pediatric providers, including pediatric psychiatrists, developmental behavioral specialists, and pediatric neurologists, may be best equipped to treat young adult patients or to refer patients to appropriate generalists and specialists comfortable with autism-specific transition of care. The question of eligibility for services is important to patients and families during the transition period, with many parents and professionals unaware of services available to them.32 Receiving adequate transition services is enabled by having a medical home during childhood—that is, a comprehensive, centralized medical record, culturally competent care, interaction with schools, and patient access to clear, unbiased information.31
Ideally, in our experience, transitioning should be discussed well before the child ages out of the pediatric provider’s practice. If necessary, healthcare transition services should include 4 components:
- Discussing the switch to a new physician who treats adults
- Discussing changing healthcare needs as an adult
- Planning insurance coverage as an adult
- Encouragement by the physician for the child to take age-appropriate responsibility for his or her healthcare.31,33
Tools such as the Got Transition checklist from the National Health Care Transition Center can provide support during this process.34
Other services
Other services provided as an extension or adjunct to the medical home in early adulthood may include customized vocational or employment training, specialized mentorship or support in a college setting, housing support, and psychological services.35
Community-based programs that emphasize leisure have been shown to improve participants’ independence and quality of life.36 Similarly, participants in programs that emphasized supported employment, with a job coach, on-the-job support, collaboration with the participant’s larger social support network, and selection of tasks to match an individual’s abilities and strengths, demonstrated improved cognitive performance, particularly executive functioning,37 and employment.38,39 These programs work best for patients who have mild to moderate symptoms.37,39
Patients with symptoms that are more severe may do better in a residential program. Many of these programs maintain an emphasis on vocational and social skills development. One such long-standing program is Bittersweet Farms, a rural farming community in Ohio for adults with ASD, where individuals with moderate to low function live in a group setting, with emphasis on scheduled, meaningful work including horticulture, animal care, carpentry; and activities of daily living.40
Studies of patients across the autism spectrum have generally found better outcomes when vocational support is given, but larger and randomized studies are needed to characterize how to best support these individuals after they leave high school.41
Psychological services such as applied behavioral therapy, social cognition training, cognitive behavioral therapy, and mindfulness training may be particularly useful in adults.42–44
Some versions of applied behavioral therapy, such as the Early Start Denver Model,45 have been found to be cost-effective and offset some expenses in the care of children with autism, using play-based and relationship-based interventions to promote development across domains while reducing symptoms.
In randomized controlled trials, modified cognitive behavioral therapy43 and mindfulness44 were shown to reduce symptoms of anxiety, obsessive-compulsive disorder, and depression.
Dialectical behavior therapy, used to find a balance between accepting oneself and desiring to change, may help in some circumstances to regulate emotions and reduce reactivity and lability, although large randomized clinical trials have not been conducted in the ASD population.46
Drug therapy
Medications may be appropriate to manage symptoms or comorbid conditions in adults with ASD. Over 75% adults with ASD have been found to use psychotropic medications.47 However, although these drugs have been approved for treating behaviors commonly associated with ASD, none of them provide definitive treatment for this disorder, and they have not been rigorously tested or approved for use in adults with ASD.48
Irritability and aggression associated with ASD can be treated with risperidone (approved for children over age 5), aripiprazole (approved for children ages 6–17), clozapine, or haloperidol.49
Aberrant social behavior can be treated with risperidone.50 Treatments under investigation include oxytocin and secretin.49
While no approved drug has been shown to improve social communication,51 balovaptan, a vasopressin V1a agonist, has shown potential and has been granted breakthrough status by the US Food and Drug Administration for treating challenging behaviors in adults, with additional studies ongoing in children.52,53
Repetitive behaviors, if the patient finds them impairing, can be managed with selective serotonin reuptake inhibitors.49
Much more study of drug therapy in adults with ASD is needed to fully understand the best approaches to psychotropic medication use, including appropriate classes and effective dosage, in this population.
SEX: UNEXPLORED TERRITORY
The reproductive health needs of people with autism remain largely underexplored.54 Historically, individuals with ASD were thought to have little interest in sexual activity or parenthood, owing to the nature of the core symptoms of the disorder. This has been shown to be untrue, particularly as studies on this topic began to engage in direct interviews with people with ASD, rather than solely gathering information from caregivers or parents. The findings reinforce the importance of broaching this component of health in this population, for the following reasons:
Adults with ASD are at increased risk of sexual victimization, with nearly 4 out of 5 reporting unwanted sexual advances, coercion, or rape.55
They have a smaller pool of knowledge with respect to sexual health. They report56 that they learned about sex from television and from “making mistakes.” They use fewer sources. They are less likely to speak to peers and figures of authority to gain knowledge about sexually transmitted infections, sexual behaviors, and contraception. And they are more likely to use forms of nonsocial media, such as television, for information.55
They report more concerns about the future with respect to sexual behavior, suggesting the need for targeted sexual education programs.56
College-age young adults with ASD who misread communication may be particularly affected by Title IX, which requires schools to promptly investigate reports of sexual harassment and sexual assault, should they struggle to comport themselves appropriately.57 Early and frank conversations about issues of consent and appropriate displays of interest and affection may better equip youth to navigate new social scenarios as they plan to leave a supervised home environment for college or the workforce.
Gender identification: Male, female, other
In one study, 77.8% of birth-sex males with ASD said they identified as men, and 67.1% of birth-sex females identified as women, compared with 93.1% of birth-sex males and 87.3% of birth-sex females without ASD. Many of the remaining individuals with ASD reported a transgender, genderqueer, or other gender identity.58 Some studies have found females with ASD report a gay or bisexual orientation more often than males with ASD.59–61
Adolescents and young adults may be exploring their changing bodies, sexual preferences, and gender roles, and as for all people at this age, these roles emerge against a backdrop of familial and societal expectations that may or may not be concordant with their own projected path regarding sexuality and reproductive health.62
Having the conversation
As with non-ASD patients, a thorough sexual history should be collected via open-ended questions when possible to determine types of sexual activity and partners.
Education of the patient, alongside caregivers and parents, about healthy and safe sexual practices, screening for sexual violence, and hormonal and nonhormonal contraception options are important components of care for this population.
CAREGIVER STRESS MAY PERSIST INTO PATIENT’S ADULTHOOD
Caregiver burden is a monumental concern for parents or others who may have lifelong primary responsibility for these neurodiverse adults.63 Family members may feel isolated and may feel they have encountered many barriers to services.64 Remaining sensitive, knowledgeable, and inquisitive about the types of support that are needed may help forge a trusting relationship between the provider and the family.
Parents of children with ASD have been reported to experience worse physical and emotional health than parents whose children do not have developmental disabilities.63,65 These disparities have been found to persist as their children enter adolescence and young adulthood.66,67 Parents of children with ASD report more anxiety, depression, and distress compared with parents of children without ASD,63 and parents themselves may be affected by ASD symptoms, which has been linked to increased parenting stress.68 Some studies have found blunted cortisol responses,63,69,70 and some,71 but not all,63 have found elevated blood pressure in caregivers of children with developmental disabilities. Headache, backache, muscle soreness, and fatigue may also be commonly reported.67
In our experience, caregivers are tremendously appreciative when provided connections to adult ASD services and support systems as their child ages. The school system and other formal support systems often assist until the time of transition into adulthood. This transition can be stressful for the adolescent and family alike, and informal support systems such as friends and family may become increasingly crucial, particularly if the adolescent still lives at home.72,73
The affected young adult’s unmet needs, as perceived by the caregiver, have been found to be significantly associated with caregiver burden, whereas the severity of the adult patient’s ASD symptoms has not.66 Therefore, it may be helpful to ask caregivers whether they perceive any unmet needs, regardless of the clinician’s perception of the severity of the patient’s ASD symptoms. Providing support to address these needs, particularly those relating to the child’s mood disorders, communication, social needs, safety, and daytime activities, may be the domains of support that most effectively reduce the caregiver burden in this population.66
Caregiver positivity, lower stress levels, and increased social support, particularly in the form of friends and family members providing no-cost assistance to caregivers whose children do not live independently,74 have been linked to better outcomes for caregivers.70,74,75 Rigorous studies that examine caregiver burden as individuals with ASD enter mid- and late-adulthood are limited.
THE ROLE OF THE INTERNIST IN CARING FOR ADULTS WITH AUTISM
A major challenge for many adults with ASD is the transition from services provided during childhood to those provided in adulthood. While children with autism have subspecialty providers who diagnose and manage their condition, including developmental-behavioral pediatricians, pediatric neurologists, and child psychiatrists, adults with autism may have fewer options.
Autism centers are becoming more available across the nation, and many provide care across the life span. However, depending on a patient’s needs, the primary care provider may need to manage residual symptoms as the patient transitions from pediatric to adult care, ultimately deciding when and where to refer the patient.
The patient’s family should pay close attention to function and mood around the time the patient leaves the structure of high school, and they should build rapport with a primary care provider they can turn to if problems persist or arise. Referrals for behavioral therapy and for social work, job training, and vocational support can greatly benefit patients as they transition to young adulthood. Referrals and suggestions for social support can also help caregivers.
Medical care
Deciding when and how to medicate the patient for symptoms of autism and related behaviors necessitates consideration of the patient’s impairment, side effects of the medication, and the impact medications may have on the patient’s other conditions. Disordered eating, mood problems, anxiety, and attention-deficit/hyperactivity disorder should be considered, and, as in all patients, regular screenings of mental health status should be conducted.76,77
Comorbid medical conditions may cause worsening of a patient’s known behavioral symptoms or may precipitate new behaviors or aggression as a result of pain or discomfort, particularly in patients with limited speech. A change in stereotypes or increased irritability warrants a thoughtful investigation for a cause other than ASD before adding or increasing behavioral medications. Common comorbid conditions include gastrointestinal distress, most commonly constipation and diarrhea in an idiopathic ASD population, with increasing ASD symptom severity correlating with increased odds of a gastrointestinal problem.78 Allergies, sleep disorders, seizures, and other psychiatric conditions are also frequent.79
Preventive care, including vaccinations, should be given as scheduled. Caregivers and patients can be reminded if needed that vaccines do not cause or worsen autism, and vaccination is intended to improve the safety of the patient and those around them, protecting against potentially life-threatening disease. Regular dental care visits, particularly for patients who are using medications that may affect tooth or gingival health,80 and regular visits to an optometrist or ophthalmologist for screening of vision are also advised.
Adverse effects. Weight gain and metabolic syndrome are common adverse effects of medications used for behavioral management, and the primary care physician may uncover diabetes, cardiac disorders, and hyperlipidemia. Patients with ASD may be particularly sensitive to the effects of medications and therefore may require a lower dose or a slower titration than other patients. Working with a behavioral team, careful weaning of psychiatric medications to the minimum needed is strongly recommended whenever possible.81
TAKE-HOME POINTS
As more adults with autism enter society, they may require varying levels of support from the healthcare community to ensure that therapeutic gains from childhood persist, allowing them to achieve maximal functional potential.
Adults with ASD may have a high, normal, or low IQ and intellectual capability. Knowledge of this and of the patient’s symptom severity and presence of comorbid psychiatric and other health conditions can help the clinician guide the patient to appropriate social services and pharmacologic treatments.
Individualized support in the workplace, as well as education regarding sexual health, can help improve outcomes for affected individuals.
Caregiver burden for individuals with autism can be high, but it can be mitigated by social support.
Further research regarding appropriate diagnostic instruments in adulthood and appropriate treatments for impairing autism-related symptoms across the life span may be particularly helpful in supporting this patient population.
Autism spectrum disorder (ASD) has increased significantly over the past 40 years. Even in the past 2 decades, the prevalence increased from 6.7 per 1,000 in 20001 to 14.6 per 1,000 in 2012—1 in 59 people.2 Of those with ASD, 46% have an intelligence quotient (IQ) greater than 85, meaning they are of average or above-average intelligence.1
As more children with autism become adults, understanding this condition across the life span grows paramount. While many studies have focused on understanding how diagnosis and treatment can help young children, few have focused on adults with autism and how primary care teams can better assist these individuals. However, this is changing, with studies of the benefits of employment programs and pharmacologic treatment, and reproductive health needs of adults with ASD. Here we provide an updated review of ASD in adult patients.
NO MORE ASPERGER SYNDROME— IT’S ON THE SPECTRUM NOW
As the scientific understanding of autism has expanded, revisions in the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5),3 published in 2013, have paralleled these advances. For many adult patients with autism who were evaluated as children, these revisions have led to changes in diagnosis and available services.
In the previous edition (DSM-IV-TR, published in 2000),4 autistic disorder and Asperger syndrome were separate (Table 1). However, DSM-5 lumped autistic disorder and Asperger disorder together under the diagnosis of ASD; this leaves it to the clinician to specify whether the patient with ASD has accompanying intellectual or language impairment and to assign a level of severity based on communication deficits and restrictive behaviors.
The shift in diagnosis was worrisome for some, particularly for clinicians treating patients with DSM-IV Asperger syndrome, who lost this diagnostic label. Concerns that patients with Asperger syndrome may not meet the DSM-5 criteria for ASD were validated by a systematic review showing that only 50% to 75% of patients with DSM-IV autistic disorder, Asperger syndrome, or pervasive developmental disorder not otherwise specified (PDD-NOS) met the DSM-5 criteria for ASD.5 Most of those who no longer met the criteria for ASD carried a DSM-IV diagnosis of Asperger syndrome or PDD-NOS or had an IQ over 70.5 Nevertheless, these individuals may struggle with impairing symptoms related to repetitive behaviors or communication or may be affected by learning or social-emotional disabilities. Additionally, even if they meet the criteria for ASD, some may identify with the Asperger syndrome label and fear they will be stigmatized should they be classified as having the more general ASD.6,7
Although future revisions to the DSM may include further changes in classification, grouping adults with ASD according to their functional and cognitive ability may allow for pragmatic characterization of their needs. At least 3 informal groupings of autistic adults have been described that integrate cognitive ability and independence8:
- Those with low cognitive and social abilities, who need lifelong support
- Those with midrange cognitive and social limitations but who can complete their work in special education classes; they often find employment in supervised workshops or other work with repetitive tasks
- Those who have greater cognitive ability and some social skills; they may proceed to college and employment and live independently.
UNCERTAIN PROGNOSIS
Prognostication for people with ASD remains an area of research. Some adults experience a reduction in symptoms as they age, with significant improvements in speech and, sometimes, modest improvements in restrictive and repetitive behaviors.9,10
Nevertheless, autism remains a lifelong disorder for many. Adults may still require significant support and may experience impairment, particularly in social interaction.10 In longitudinal studies, only 15% to 27% of patients with ASD are characterized as having a positive outcome (often defined as variables related to independent function, near-normal relationships, employment, or a quantified reduction in core symptoms), and many experience significant dependency into adulthood.10–13
IQ has been cited as a possible prognostic factor,10,13 with an IQ below 70 associated with poorer outcome, although an IQ above 70 does not necessarily confer a positive outcome. Less-severe impairment in speech at baseline in early childhood also suggests better outcomes in adulthood.10
As we see more adults with autism, studies that include both children and adults, such as the Longitudinal European Autism Cohort, will be important to characterize the natural history, comorbidities, and genetics of ASD and may help provide more specific predictors of disease course into adulthood.14
ACHIEVING A DIAGNOSIS FOR ADULT PATIENTS WITH SUSPECTED AUTISM
While many patients are recognized as having autism in early to mid-childhood, some adults may not receive a formal diagnosis until much later in life. Those with fluent language and normal-range IQ are likely to be overlooked.15 People with ASD may have had mild symptoms during childhood that did not impair their functioning until demands of daily life exceeded their capacities in adulthood. Alternatively, parents of a child with newly diagnosed ASD may realize that they themselves or another adult family member also show signs of it.
The UK National Institute of Health and Care Excellence suggests that assessment should be considered if the patient meets psychiatric diagnostic criteria and one of the following:
- Difficulty obtaining or sustaining employment or education
- Difficulty initiating or sustaining social relationships
- Past or current contact with mental health or learning disability services
- History of a neurodevelopmental or mental health disorder.15,16
Currently, diagnosis typically involves a multidisciplinary approach, with psychiatric assessment, neuropsychological testing, and speech and language evaluation.17 Providers may need to refer patients for these services, sometimes at the patient’s request, if previous mental health misdiagnoses are suspected, if patients report symptoms or impairment consistent with ASD, or if benefits, services, or accommodations, such as a coach in the workplace, are needed.
Diagnosing ASD in adults can be difficult, given that the gold-standard diagnostic tests such as the Autism Diagnostic Observation Schedule-2 (ADOS-2)18 and the Autism Diagnostic Interview-Revised (ADI-R)19 are typically used to diagnose autism in children. However, Module 4 in the ADOS-2 was developed for adolescents and older patients with fluent language and has shown at least moderate power to distinguish adults with ASD from those without ASD.18,20
An initial psychiatric assessment should include a thorough history taken from the patient and, if applicable, the patient’s caregiver, as well as a psychiatric interview of the patient. Neuropsychological testing should include evaluation of cognitive function, social functioning (using the ADOS-2 for adults without intellectual disability, the ADI-R, or both), and adaptive functioning (using the Vineland Adaptive Behavior Scales, second edition21).
Evaluation of speech and language is particularly important in patients with limited language ability and should include both expressive and receptive language abilities. Serial testing every few years, as is often recommended in childhood, may help establish the pattern of impairment over time.
Comorbid psychiatric disorders are common
Many people with ASD also have other psychiatric disorders,17,22 which clinicians should keep in mind when seeing an adult seeking evaluation for ASD.
Attention-deficit/hyperactivity disorder is present at higher rates in patients of average intellectual function with ASD than in the general population.23
Anxiety disorders, including obsessive-compulsive disorder, were found to often coexist with autism in a sample of adults with autism without intellectual disability,24,25 and approximately 40% of youths with ASD have at least 1 comorbid anxiety disorder.26
Mood disorders are also prevalent in adults with ASD, with a small study showing that 70% of adults with DSM-IV Asperger syndrome had at least 1 depressive episode in their lifetime.27
BEHAVIORAL AND PHARMACOLOGIC THERAPIES FOR THE ADULT PATIENT
Services and medications for adults with ASD are discussed below. These will vary by individual, and services available may vary by region.
Historically, vocational and social outcomes have been poor for adults with ASD. It is estimated that most larger universities may be home to 100 to 300 students with ASD. To combat isolation, the University of California, Los Angeles, the University of Alabama, and others provide special support services, including group social activities such as board games and individual coaching.8 Nevertheless, half of the students with autism who attend institutions of higher learning leave without completing their intended degree.29 Many still struggle to establish meaningful friendships or romantic relationships.29
Planning for a transition of care
Healthcare transition planning is important but is strikingly underused.30 Individual providers, including adult psychiatrists, vary in their level of training and comfort in diagnosing, treating, and monitoring adults with autism. Youths with ASD are half as likely to receive healthcare transition services as other youths with special healthcare needs.31
Pediatric providers, including pediatric psychiatrists, developmental behavioral specialists, and pediatric neurologists, may be best equipped to treat young adult patients or to refer patients to appropriate generalists and specialists comfortable with autism-specific transition of care. The question of eligibility for services is important to patients and families during the transition period, with many parents and professionals unaware of services available to them.32 Receiving adequate transition services is enabled by having a medical home during childhood—that is, a comprehensive, centralized medical record, culturally competent care, interaction with schools, and patient access to clear, unbiased information.31
Ideally, in our experience, transitioning should be discussed well before the child ages out of the pediatric provider’s practice. If necessary, healthcare transition services should include 4 components:
- Discussing the switch to a new physician who treats adults
- Discussing changing healthcare needs as an adult
- Planning insurance coverage as an adult
- Encouragement by the physician for the child to take age-appropriate responsibility for his or her healthcare.31,33
Tools such as the Got Transition checklist from the National Health Care Transition Center can provide support during this process.34
Other services
Other services provided as an extension or adjunct to the medical home in early adulthood may include customized vocational or employment training, specialized mentorship or support in a college setting, housing support, and psychological services.35
Community-based programs that emphasize leisure have been shown to improve participants’ independence and quality of life.36 Similarly, participants in programs that emphasized supported employment, with a job coach, on-the-job support, collaboration with the participant’s larger social support network, and selection of tasks to match an individual’s abilities and strengths, demonstrated improved cognitive performance, particularly executive functioning,37 and employment.38,39 These programs work best for patients who have mild to moderate symptoms.37,39
Patients with symptoms that are more severe may do better in a residential program. Many of these programs maintain an emphasis on vocational and social skills development. One such long-standing program is Bittersweet Farms, a rural farming community in Ohio for adults with ASD, where individuals with moderate to low function live in a group setting, with emphasis on scheduled, meaningful work including horticulture, animal care, carpentry; and activities of daily living.40
Studies of patients across the autism spectrum have generally found better outcomes when vocational support is given, but larger and randomized studies are needed to characterize how to best support these individuals after they leave high school.41
Psychological services such as applied behavioral therapy, social cognition training, cognitive behavioral therapy, and mindfulness training may be particularly useful in adults.42–44
Some versions of applied behavioral therapy, such as the Early Start Denver Model,45 have been found to be cost-effective and offset some expenses in the care of children with autism, using play-based and relationship-based interventions to promote development across domains while reducing symptoms.
In randomized controlled trials, modified cognitive behavioral therapy43 and mindfulness44 were shown to reduce symptoms of anxiety, obsessive-compulsive disorder, and depression.
Dialectical behavior therapy, used to find a balance between accepting oneself and desiring to change, may help in some circumstances to regulate emotions and reduce reactivity and lability, although large randomized clinical trials have not been conducted in the ASD population.46
Drug therapy
Medications may be appropriate to manage symptoms or comorbid conditions in adults with ASD. Over 75% adults with ASD have been found to use psychotropic medications.47 However, although these drugs have been approved for treating behaviors commonly associated with ASD, none of them provide definitive treatment for this disorder, and they have not been rigorously tested or approved for use in adults with ASD.48
Irritability and aggression associated with ASD can be treated with risperidone (approved for children over age 5), aripiprazole (approved for children ages 6–17), clozapine, or haloperidol.49
Aberrant social behavior can be treated with risperidone.50 Treatments under investigation include oxytocin and secretin.49
While no approved drug has been shown to improve social communication,51 balovaptan, a vasopressin V1a agonist, has shown potential and has been granted breakthrough status by the US Food and Drug Administration for treating challenging behaviors in adults, with additional studies ongoing in children.52,53
Repetitive behaviors, if the patient finds them impairing, can be managed with selective serotonin reuptake inhibitors.49
Much more study of drug therapy in adults with ASD is needed to fully understand the best approaches to psychotropic medication use, including appropriate classes and effective dosage, in this population.
SEX: UNEXPLORED TERRITORY
The reproductive health needs of people with autism remain largely underexplored.54 Historically, individuals with ASD were thought to have little interest in sexual activity or parenthood, owing to the nature of the core symptoms of the disorder. This has been shown to be untrue, particularly as studies on this topic began to engage in direct interviews with people with ASD, rather than solely gathering information from caregivers or parents. The findings reinforce the importance of broaching this component of health in this population, for the following reasons:
Adults with ASD are at increased risk of sexual victimization, with nearly 4 out of 5 reporting unwanted sexual advances, coercion, or rape.55
They have a smaller pool of knowledge with respect to sexual health. They report56 that they learned about sex from television and from “making mistakes.” They use fewer sources. They are less likely to speak to peers and figures of authority to gain knowledge about sexually transmitted infections, sexual behaviors, and contraception. And they are more likely to use forms of nonsocial media, such as television, for information.55
They report more concerns about the future with respect to sexual behavior, suggesting the need for targeted sexual education programs.56
College-age young adults with ASD who misread communication may be particularly affected by Title IX, which requires schools to promptly investigate reports of sexual harassment and sexual assault, should they struggle to comport themselves appropriately.57 Early and frank conversations about issues of consent and appropriate displays of interest and affection may better equip youth to navigate new social scenarios as they plan to leave a supervised home environment for college or the workforce.
Gender identification: Male, female, other
In one study, 77.8% of birth-sex males with ASD said they identified as men, and 67.1% of birth-sex females identified as women, compared with 93.1% of birth-sex males and 87.3% of birth-sex females without ASD. Many of the remaining individuals with ASD reported a transgender, genderqueer, or other gender identity.58 Some studies have found females with ASD report a gay or bisexual orientation more often than males with ASD.59–61
Adolescents and young adults may be exploring their changing bodies, sexual preferences, and gender roles, and as for all people at this age, these roles emerge against a backdrop of familial and societal expectations that may or may not be concordant with their own projected path regarding sexuality and reproductive health.62
Having the conversation
As with non-ASD patients, a thorough sexual history should be collected via open-ended questions when possible to determine types of sexual activity and partners.
Education of the patient, alongside caregivers and parents, about healthy and safe sexual practices, screening for sexual violence, and hormonal and nonhormonal contraception options are important components of care for this population.
CAREGIVER STRESS MAY PERSIST INTO PATIENT’S ADULTHOOD
Caregiver burden is a monumental concern for parents or others who may have lifelong primary responsibility for these neurodiverse adults.63 Family members may feel isolated and may feel they have encountered many barriers to services.64 Remaining sensitive, knowledgeable, and inquisitive about the types of support that are needed may help forge a trusting relationship between the provider and the family.
Parents of children with ASD have been reported to experience worse physical and emotional health than parents whose children do not have developmental disabilities.63,65 These disparities have been found to persist as their children enter adolescence and young adulthood.66,67 Parents of children with ASD report more anxiety, depression, and distress compared with parents of children without ASD,63 and parents themselves may be affected by ASD symptoms, which has been linked to increased parenting stress.68 Some studies have found blunted cortisol responses,63,69,70 and some,71 but not all,63 have found elevated blood pressure in caregivers of children with developmental disabilities. Headache, backache, muscle soreness, and fatigue may also be commonly reported.67
In our experience, caregivers are tremendously appreciative when provided connections to adult ASD services and support systems as their child ages. The school system and other formal support systems often assist until the time of transition into adulthood. This transition can be stressful for the adolescent and family alike, and informal support systems such as friends and family may become increasingly crucial, particularly if the adolescent still lives at home.72,73
The affected young adult’s unmet needs, as perceived by the caregiver, have been found to be significantly associated with caregiver burden, whereas the severity of the adult patient’s ASD symptoms has not.66 Therefore, it may be helpful to ask caregivers whether they perceive any unmet needs, regardless of the clinician’s perception of the severity of the patient’s ASD symptoms. Providing support to address these needs, particularly those relating to the child’s mood disorders, communication, social needs, safety, and daytime activities, may be the domains of support that most effectively reduce the caregiver burden in this population.66
Caregiver positivity, lower stress levels, and increased social support, particularly in the form of friends and family members providing no-cost assistance to caregivers whose children do not live independently,74 have been linked to better outcomes for caregivers.70,74,75 Rigorous studies that examine caregiver burden as individuals with ASD enter mid- and late-adulthood are limited.
THE ROLE OF THE INTERNIST IN CARING FOR ADULTS WITH AUTISM
A major challenge for many adults with ASD is the transition from services provided during childhood to those provided in adulthood. While children with autism have subspecialty providers who diagnose and manage their condition, including developmental-behavioral pediatricians, pediatric neurologists, and child psychiatrists, adults with autism may have fewer options.
Autism centers are becoming more available across the nation, and many provide care across the life span. However, depending on a patient’s needs, the primary care provider may need to manage residual symptoms as the patient transitions from pediatric to adult care, ultimately deciding when and where to refer the patient.
The patient’s family should pay close attention to function and mood around the time the patient leaves the structure of high school, and they should build rapport with a primary care provider they can turn to if problems persist or arise. Referrals for behavioral therapy and for social work, job training, and vocational support can greatly benefit patients as they transition to young adulthood. Referrals and suggestions for social support can also help caregivers.
Medical care
Deciding when and how to medicate the patient for symptoms of autism and related behaviors necessitates consideration of the patient’s impairment, side effects of the medication, and the impact medications may have on the patient’s other conditions. Disordered eating, mood problems, anxiety, and attention-deficit/hyperactivity disorder should be considered, and, as in all patients, regular screenings of mental health status should be conducted.76,77
Comorbid medical conditions may cause worsening of a patient’s known behavioral symptoms or may precipitate new behaviors or aggression as a result of pain or discomfort, particularly in patients with limited speech. A change in stereotypes or increased irritability warrants a thoughtful investigation for a cause other than ASD before adding or increasing behavioral medications. Common comorbid conditions include gastrointestinal distress, most commonly constipation and diarrhea in an idiopathic ASD population, with increasing ASD symptom severity correlating with increased odds of a gastrointestinal problem.78 Allergies, sleep disorders, seizures, and other psychiatric conditions are also frequent.79
Preventive care, including vaccinations, should be given as scheduled. Caregivers and patients can be reminded if needed that vaccines do not cause or worsen autism, and vaccination is intended to improve the safety of the patient and those around them, protecting against potentially life-threatening disease. Regular dental care visits, particularly for patients who are using medications that may affect tooth or gingival health,80 and regular visits to an optometrist or ophthalmologist for screening of vision are also advised.
Adverse effects. Weight gain and metabolic syndrome are common adverse effects of medications used for behavioral management, and the primary care physician may uncover diabetes, cardiac disorders, and hyperlipidemia. Patients with ASD may be particularly sensitive to the effects of medications and therefore may require a lower dose or a slower titration than other patients. Working with a behavioral team, careful weaning of psychiatric medications to the minimum needed is strongly recommended whenever possible.81
TAKE-HOME POINTS
As more adults with autism enter society, they may require varying levels of support from the healthcare community to ensure that therapeutic gains from childhood persist, allowing them to achieve maximal functional potential.
Adults with ASD may have a high, normal, or low IQ and intellectual capability. Knowledge of this and of the patient’s symptom severity and presence of comorbid psychiatric and other health conditions can help the clinician guide the patient to appropriate social services and pharmacologic treatments.
Individualized support in the workplace, as well as education regarding sexual health, can help improve outcomes for affected individuals.
Caregiver burden for individuals with autism can be high, but it can be mitigated by social support.
Further research regarding appropriate diagnostic instruments in adulthood and appropriate treatments for impairing autism-related symptoms across the life span may be particularly helpful in supporting this patient population.
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2000 Principal Investigators; Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders—autism and developmental disabilities monitoring network, six sites, United States, 2000. MMWR Surveill Summ 2007; 56(1):1–11. pmid:17287714
- Christensen DL. Prevalence and characteristics of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2012. MMWR Surveill Summ 2016; 65(13):1–23. doi:10.15585/mmwr.ss6503a1
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edition. Washington, D.C: American Psychiatric Association; 2013.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision. Washington, DC: American Psychiatric Association; 2000.
- Smith IC, Reichow B, Volkmar FR. The effects of DSM-5 criteria on number of individuals diagnosed with autism spectrum disorder: a systematic review. J Autism Dev Disord 2015; 45(8):2541–2552. doi:10.1007/s10803-015-2423-8
- Barahona-Corrêa JB, Filipe CN. A concise history of Asperger syndrome: the short reign of a troublesome diagnosis. Front Psychol 2015; 6:2024. doi:10.3389/fpsyg.2015.02024
- Kite DM, Gullifer J, Tyson GA. Views on the diagnostic labels of autism and Asperger’s disorder and the proposed changes in the DSM. J Autism Dev Disord 2013; 43(7):1692–1700. doi:10.1007/s10803-012-1718-2
- Kuo AA. Autism in adults: an update. Presented at the: American College of Physicians Internal Medicine Meeting, New Orleans, LA, April 17–21, 2018.
- Shattuck PT, Seltzer MM, Greenberg JS, et al. Change in autism symptoms and maladaptive behaviors in adolescents and adults with an autism spectrum disorder. J Autism Dev Disord 2007; 37(9):1735–1747. doi:10.1007/s10803-006-0307-7
- Seltzer MM, Shattuck P, Abbeduto L, Greenberg JS. Trajectory of development in adolescents and adults with autism. Ment Retard Dev Disabil Res Rev 2004; 10(4):234–247. doi:10.1002/mrdd.20038
- Billstedt E, Carina Gillberg I, Gillberg C. Autism in adults: symptom patterns and early childhood predictors. Use of the DISCO in a community sample followed from childhood. J Child Psychol Psychiatry 2007; 48(11):1102–1110. doi:10.1111/j.1469-7610.2007.01774.x
- Howlin P, Goode S, Hutton J, Rutter M. Adult outcome for children with autism. J Child Psychol Psychiatry 2004; 45(2):212–229. pmid:14982237
- Marriage S, Wolverton A, Marriage K. Autism spectrum disorder grown up: a chart review of adult functioning. J Can Acad Child Adolesc Psychiatry 2009; 18(4):322–328. pmid: 19881941
- Isaksson J, Tammimies K, Neufeld J, et al. EU-AIMS Longitudinal European Autism Project (LEAP): the autism twin cohort. Mol Autism 2018; 9(1):26. doi:10.1186/s13229-018-0212-x
- Lai M-C, Baron-Cohen S. Identifying the lost generation of adults with autism spectrum conditions. Lancet Psychiatry 2015; 2(11):1013–1027. doi:10.1016/S2215-0366(15)00277-1
- National Institute for Health and Clinical Excellence. Autism: recognition, referral, diagnosis and management of adults on the autism spectrum. NICE clinical guideline 142. June 2012. https://grand.tghn.org/site_media/media/medialibrary/2015/03/ASD_NICE_3_.pdf. Accessed July 9, 2019.
- Wolf JM, Ventola P. Assessment and treatment planning in adults with autism spectrum disorders. In: Adolescents and Adults with Autism Spectrum Disorders. Springer, New York, NY; 2014:283–298.
- Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop SL. Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) manual. Torrance, CA: Western Psychological Services, 2012.
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 1994; 24(5):659–685.
- Hus V, Lord C. The autism diagnostic observation schedule, module 4: revised algorithm and standardized severity scores. J Autism Dev Disord 2014; 44(8):1996–2012. doi:10.1007/s10803-014-2080-3>
- Sparrow S, Balla D, Cicchetti D, Harrison P, Doll E. Vineland Adaptive Behavior Scales. Circle Pines, MN: American Guidance Service, 1984.
- Happé FG, Mansour H, Barrett P, Brown T, Abbott P, Charlton RA. Demographic and cognitive profile of individuals seeking a diagnosis of autism spectrum disorder in adulthood. J Autism Dev Disord 2016; 46(11):3469–3480. doi:10.1007/s10803-016-2886-2
- Johnston K, Dittner A, Bramham J, Murphy C, Knight A, Russell A. Attention deficit hyperactivity disorder symptoms in adults with autism spectrum disorders. Autism Res Off J Int Soc Autism Res 2013; 6(4):225–236. doi:10.1002/aur.1283
- Cadman T, Spain D, Johnston P, et al. Obsessive-compulsive disorder in adults with high-functioning autism spectrum disorder: what does self-report with the OCI-R tell us? Autism Res Off J Int Soc Autism Res 2015; 8(5):477–485. doi:10.1002/aur.1461
- Russell AJ, Mataix-Cols D, Anson M, Murphy DGM. Obsessions and compulsions in Asperger syndrome and high-functioning autism. Br J Psychiatry J Ment Sci 2005; 186:525–528. doi:10.1192/bjp.186.6.525
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry 2008; 47(8):921–929. doi:10.1097/CHI.0b013e318179964f
- Lugnegård T, Hallerbäck MU, Gillberg C. Psychiatric comorbidity in young adults with a clinical diagnosis of Asperger syndrome. Res Dev Disabil 2011; 32(5):1910–1917. doi:10.1016/j.ridd.2011.03.025
- Howlin P, Moss P. Adults with autism spectrum disorders. Can J Psychiatry 2012; 57(5):275–283. doi:10.1177/070674371205700502
- Levy A, Perry A. Outcomes in adolescents and adults with autism: a review of the literature. Res Autism Spectr Disord 2011; 5(4):1271–1282. doi:10.1016/J.RASD.2011.01.023
- Cheak-Zamora NC, Yang X, Farmer JE, Clark M. Disparities in transition planning for youth with autism spectrum disorder. Pediatrics 2013; 131(3):447–454. doi:10.1542/peds.2012-1572
- Rast JE, Shattuck PT, Roux AM, Anderson KA, Kuo A. The medical home and health care transition for youth with autism. Pediatrics 2018; 141(suppl 4):S328–S334. doi:10.1542/peds.2016-4300J
- Belling R, McLaren S, Paul M, et al. The effect of organisational resources and eligibility issues on transition from child and adolescent to adult mental health services. J Health Serv Res Policy 2014; 19(3):169–176. doi:10.1177/1355819614527439
- Data Resource Center for Child & Adolescent Health. 2009–2010 National Survey of Children with Special Health Care Needs. www.childhealthdata.org/docs/drc/200910-cshcn-spss-codebook_final_051012.pdf?sfvrsn=1. Accessed July 9, 2019.
- Got Transition Center for Health Care Transition Improvement. Six core elements of health care transition 2.0. Transitioning youth to an adult health care provider. For use by pediatric, family medicine, and med-peds providers. www.gottransition.org/resourceGet.cfm?id=208. Accessed July 9, 2019.
- Murphy CM, Wilson CE, Robertson DM, et al. Autism spectrum disorder in adults: diagnosis, management, and health services development. Neuropsychiatr Dis Treat 2016; 12:1669–1686. doi:10.2147/NDT.S65455
- García-Villamisar DA, Dattilo J. Effects of a leisure programme on quality of life and stress of individuals with ASD. J Intellect Disabil Res 2010; 54(7):611–619. doi:10.1111/j.1365-2788.2010.01289.x
- García-Villamisar D, Hughes C. Supported employment improves cognitive performance in adults with autism. J Intellect Disabil Res 2007; 51(pt 2):142–150. doi:10.1111/j.1365-2788.2006.00854.x
- Lawer L, Brusilovskiy E, Salzer MS, Mandell DS. Use of vocational rehabilitative services among adults with autism. J Autism Dev Disord 2009; 39(3):487–494. doi:10.1007/s10803-008-0649-4
- Howlin P, Alcock J, Burkin C. An 8 year follow-up of a specialist supported employment service for high-ability adults with autism or Asperger syndrome. Autism 2005; 9(5):533–549. doi:10.1177/1362361305057871
- Kay BR. Bittersweet Farms. J Autism Dev Disord 1990; 20(3):309–321. http://www.ncbi.nlm.nih.gov/pubmed/2228914. Accessed July 9, 2019.
- Taylor JL, McPheeters ML, Sathe NA, Dove D, Veenstra-Vanderweele J, Warren Z. A systematic review of vocational interventions for young adults with autism spectrum disorders. Pediatrics 2012; 130(3):531–538. doi:10.1542/peds.2012-0682
- Bishop-Fitzpatrick L, Minshew NJ, Eack SM. A systematic review of psychosocial interventions for adults with autism spectrum disorders. J Autism Dev Disord 2013; 43(3):687–694. doi:10.1007/s10803-012-1615-8
- Russell AJ, Jassi A, Fullana MA, et al. Cognitive behavior therapy for comorbid obsessive-compulsive disorder in high-functioning autism spectrum disorders: a randomized controlled trial. Depress Anxiety 2013; 30(8):697–708. doi:10.1002/da.22053
- Spek AA, van Ham NC, Nyklícek I. Mindfulness-based therapy in adults with an autism spectrum disorder: a randomized controlled trial. Res Dev Disabil 2013; 34(1):246–253. doi:10.1016/j.ridd.2012.08.009
- Eapen V, Crncec R, Walter A. Clinical outcomes of an early intervention program for preschool children with autism spectrum disorder in a community group setting. BMC Pediatr 2013; 13(1):3. doi:10.1186/1471-2431-13-3
- Mazefsky CA, White SW. Emotion regulation: concepts & practice in autism spectrum disorder. Child Adolesc Psychiatr Clin North Am 2014; 23(1):15–24. doi:10.1016/J.CHC.2013.07.002
- Esbensen AJ, Greenberg JS, Seltzer MM, Aman MG. A longitudinal investigation of psychotropic and non-psychotropic medication use among adolescents and adults with autism spectrum disorders. J Autism Dev Disord 2009; 39(9):1339–1349. doi:10.1007/s10803-009-0750-3
- Dove D, Warren Z, McPheeters ML, Taylor JL, Sathe NA, Veenstra-VanderWeele J. Medications for adolescents and young adults with autism spectrum disorders: a systematic review. Pediatrics 2012; 130(4):717–726. doi:10.1542/peds.2012-0683
- LeClerc S, Easley D. Pharmacological therapies for autism spectrum disorder: a review. Pharm Ther 2015; 40(6):389–397.
- Miral S, Gencer O, Inal-Emiroglu FN, Baykara B, Baykara A, Dirik E. Risperidone versus haloperidol in children and adolescents with AD: a randomized, controlled, double-blind trial. Eur Child Adolesc Psychiatry 2008; 17(1):1–8. doi:10.1007/s00787-007-0620-5
- Lai M-C, Lombardo MV, Baron-Cohen S. Autism. Lancet 2014; 383(9920):896–910. doi:10.1016/S0140-6736(13)61539-1
- Ratni H, Rogers-Evans M, Bissantz C, et al. Discovery of highly selective brain-penetrant vasopressin 1a antagonists for the potential treatment of autism via a chemogenomic and scaffold hopping approach. J Med Chem 2015; 58(5):2275–2289. doi:10.1021/jm501745f
- Umbricht D, Del Valle Rubido M, Hollander E, et al. A single dose, randomized, controlled proof-of-mechanism study of a novel vasopressin 1a receptor antagonist (RG7713) in high-functioning adults with autism spectrum disorder. Neuropsychopharmacology 2017; 42(9):1914–1923. doi:10.1038/npp.2016.232>
- Kellaher DC. Sexual behavior and autism spectrum disorders: an update and discussion. Curr Psychiatry Rep 2015; 17(4):25. doi:10.1007/s11920-015-0562-4
- Brown-Lavoie SM, Viecili MA, Weiss JA. Sexual knowledge and victimization in adults with autism spectrum disorders. J Autism Dev Disord 2014; 44(9):2185–2196. doi:10.1007/s10803-014-2093-y
- Mehzabin P, Stokes MA. Self-assessed sexuality in young adults with high-functioning autism. Res Autism Spectr Disord 2011; 5(1):614–621. doi:10.1016/J.RASD.2010.07.006>
- Brown KR. Accessibility for students with ASD: legal perspectives in the United States. In: Alphin HC Jr. Exploring the Future of Accessibility in Higher Education. Hershey, PA: IGI Global; 2017.
- George R, Stokes MA. Gender identity and sexual orientation in autism spectrum disorder. Autism 2018; 22(8):970–982. doi:10.1177/1362361317714587
- Byers ES, Nichols S, Voyer SD. Challenging stereotypes: sexual functioning of single adults with high functioning autism spectrum disorder. J Autism Dev Disord 2013; 43(11):2617–2627. doi:10.1007/s10803-013-1813-z
- Gilmour L, Schalomon PM, Smith V. Sexuality in a community based sample of adults with autism spectrum disorder. Res Autism Spectr Disord 2012; 6(1):313–318. doi:10.1016/J.RASD.2011.06.003
- Bejerot S, Eriksson JM. Sexuality and gender role in autism spectrum disorder: a case control study. Schmitz C, ed. PLoS One 2014; 9(1):e87961. doi:10.1371/journal.pone.0087961>
- Navot N, Jorgenson AG, Webb SJ. Maternal experience raising girls with autism spectrum disorder: a qualitative study. Child Care Health Dev 2017; 43(4):536–545. doi:10.1111/cch.12470
- Padden C, James JE. Stress among parents of children with and without autism spectrum disorder: a comparison involving physiological indicators and parent self-reports. J Dev Phys Disabil 2017; 29(4):567–586. doi:10.1007/s10882-017-9547-z
- Woodgate RL, Ateah C, Secco L. Living in a world of our own: the experience of parents who have a child with autism. Qual Health Res 2008; 18(8):1075–1083. doi:10.1177/1049732308320112
- Hayes SA, Watson SL. The impact of parenting stress: a meta-analysis of studies comparing the experience of parenting stress in parents of children with and without autism spectrum disorder. J Autism Dev Disord 2013; 43(3):629–642. doi:10.1007/s10803-012-1604-y
- Cadman T, Eklund H, Howley D, et al. Caregiver burden as people with autism spectrum disorder and attention-deficit/hyperactivity disorder transition into adolescence and adulthood in the United Kingdom. J Am Acad Child Adolesc Psychiatry 2012; 51(9):879–888. doi:10.1016/j.jaac.2012.06.017
- Smith LE, Seltzer MM, Greenberg JS. Daily health symptoms of mothers of adolescents and adults with fragile x syndrome and mothers of adolescents and adults with autism spectrum disorder. J Autism Dev Disord 2012; 42(9):1836–1846. doi:10.1007/s10803-011-1422-7
- van Steijn DJ, Oerlemans AM, van Aken MAG, Buitelaar JK, Rommelse NNJ. The reciprocal relationship of ASD, ADHD, depressive symptoms and stress in parents of children with ASD and/or ADHD. J Autism Dev Disord 2014; 44(5):1064–1076. doi:10.1007/s10803-013-1958-9
- Seltzer MM, Greenberg JS, Hong J, et al. Maternal cortisol levels and behavior problems in adolescents and adults with ASD. J Autism Dev Disord 2010; 40(4):457–469. doi:10.1007/S10803-009-0887-0
- Lovell B, Moss M, Wetherell MA. With a little help from my friends: psychological, endocrine and health corollaries of social support in parental caregivers of children with autism or ADHD. Res Dev Disabil 2012; 33(2):682–687. doi:10.1016/j.ridd.2011.11.014
- Gallagher S, Whiteley J. Social support is associated with blood pressure responses in parents caring for children with developmental disabilities. Res Dev Disabil 2012; 33(6):2099–2105. doi:10.1016/j.ridd.2012.06.007
- Baker JK, Smith LE, Greenberg JS, Seltzer MM, Taylor JL. Change in maternal criticism and behavior problems in adolescents and adults with autism across a 7-year period. J Abnorm Psychol 2011; 120(2):465–475. doi:10.1037/a0021900
- Marsack CN, Samuel PS. Mediating effects of social support on quality of life for parents of adults with autism. J Autism Dev Disord 2017; 47(8):2378–2389. doi:10.1007/s10803-017-3157-6
- Trute B, Benzies KM, Worthington C, Reddon JR, Moore M. Accentuate the positive to mitigate the negative: mother psychological coping resources and family adjustment in childhood disability. J Intellect Dev Disabil 2010; 35(1):36–43. doi:10.3109/13668250903496328
- Cantwell J, Muldoon OT, Gallagher S. Social support and mastery influence the association between stress and poor physical health in parents caring for children with developmental disabilities. Res Dev Disabil 2014; 35(9):2215–2223. doi:10.1016/j.ridd.2014.05.012
- Carton AM, Smith AD. Assessing the relationship between eating disorder psychopathology and autistic traits in a non-clinical adult population. Eat Weight Disord - Stud Anorexia, Bulim Obes 2014; 19(3):285–293. doi:10.1007/s40519-013-0086-z
- De Alwis D, Agrawal A, Reiersen AM, et al. ADHD symptoms, autistic traits, and substance use and misuse in adult Australian twins. J Stud Alcohol Drugs 2014; 75(2):211–221. doi:10.15288/jsad.2014.75.211
- Wang LW, Tancredi DJ, Thomas DW. The prevalence of gastrointestinal problems in children across the United States with autism spectrum disorders from families with multiple affected members. J Dev Behav Pediatr 2011; 32(5):351–360. doi:10.1097/DBP.0b013e31821bd06a
- Croen LA, Zerbo O, Qian Y, et al. The health status of adults on the autism spectrum. Autism 2015; 19(7):814–823. doi:10.1177/1362361315577517
- Kalyoncu IÖ, Tanboga I. Oral health status of children with autistic spectrum disorder compared with non-authentic peers. Iran J Public Health 2017; 46(11):1591–1593. www.ncbi.nlm.nih.gov/pmc/articles/PMC5696703. Accessed July 9, 2019.
- McGuire K, Fung LK, Hagopian L, et al. Irritability and problem behavior in autism spectrum disorder: a practice pathway for pediatric primary care. Pediatrics 2016; 137(suppl 2):S136–S148. doi:10.1542/peds.2015-2851L
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2000 Principal Investigators; Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders—autism and developmental disabilities monitoring network, six sites, United States, 2000. MMWR Surveill Summ 2007; 56(1):1–11. pmid:17287714
- Christensen DL. Prevalence and characteristics of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2012. MMWR Surveill Summ 2016; 65(13):1–23. doi:10.15585/mmwr.ss6503a1
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edition. Washington, D.C: American Psychiatric Association; 2013.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision. Washington, DC: American Psychiatric Association; 2000.
- Smith IC, Reichow B, Volkmar FR. The effects of DSM-5 criteria on number of individuals diagnosed with autism spectrum disorder: a systematic review. J Autism Dev Disord 2015; 45(8):2541–2552. doi:10.1007/s10803-015-2423-8
- Barahona-Corrêa JB, Filipe CN. A concise history of Asperger syndrome: the short reign of a troublesome diagnosis. Front Psychol 2015; 6:2024. doi:10.3389/fpsyg.2015.02024
- Kite DM, Gullifer J, Tyson GA. Views on the diagnostic labels of autism and Asperger’s disorder and the proposed changes in the DSM. J Autism Dev Disord 2013; 43(7):1692–1700. doi:10.1007/s10803-012-1718-2
- Kuo AA. Autism in adults: an update. Presented at the: American College of Physicians Internal Medicine Meeting, New Orleans, LA, April 17–21, 2018.
- Shattuck PT, Seltzer MM, Greenberg JS, et al. Change in autism symptoms and maladaptive behaviors in adolescents and adults with an autism spectrum disorder. J Autism Dev Disord 2007; 37(9):1735–1747. doi:10.1007/s10803-006-0307-7
- Seltzer MM, Shattuck P, Abbeduto L, Greenberg JS. Trajectory of development in adolescents and adults with autism. Ment Retard Dev Disabil Res Rev 2004; 10(4):234–247. doi:10.1002/mrdd.20038
- Billstedt E, Carina Gillberg I, Gillberg C. Autism in adults: symptom patterns and early childhood predictors. Use of the DISCO in a community sample followed from childhood. J Child Psychol Psychiatry 2007; 48(11):1102–1110. doi:10.1111/j.1469-7610.2007.01774.x
- Howlin P, Goode S, Hutton J, Rutter M. Adult outcome for children with autism. J Child Psychol Psychiatry 2004; 45(2):212–229. pmid:14982237
- Marriage S, Wolverton A, Marriage K. Autism spectrum disorder grown up: a chart review of adult functioning. J Can Acad Child Adolesc Psychiatry 2009; 18(4):322–328. pmid: 19881941
- Isaksson J, Tammimies K, Neufeld J, et al. EU-AIMS Longitudinal European Autism Project (LEAP): the autism twin cohort. Mol Autism 2018; 9(1):26. doi:10.1186/s13229-018-0212-x
- Lai M-C, Baron-Cohen S. Identifying the lost generation of adults with autism spectrum conditions. Lancet Psychiatry 2015; 2(11):1013–1027. doi:10.1016/S2215-0366(15)00277-1
- National Institute for Health and Clinical Excellence. Autism: recognition, referral, diagnosis and management of adults on the autism spectrum. NICE clinical guideline 142. June 2012. https://grand.tghn.org/site_media/media/medialibrary/2015/03/ASD_NICE_3_.pdf. Accessed July 9, 2019.
- Wolf JM, Ventola P. Assessment and treatment planning in adults with autism spectrum disorders. In: Adolescents and Adults with Autism Spectrum Disorders. Springer, New York, NY; 2014:283–298.
- Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop SL. Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) manual. Torrance, CA: Western Psychological Services, 2012.
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 1994; 24(5):659–685.
- Hus V, Lord C. The autism diagnostic observation schedule, module 4: revised algorithm and standardized severity scores. J Autism Dev Disord 2014; 44(8):1996–2012. doi:10.1007/s10803-014-2080-3>
- Sparrow S, Balla D, Cicchetti D, Harrison P, Doll E. Vineland Adaptive Behavior Scales. Circle Pines, MN: American Guidance Service, 1984.
- Happé FG, Mansour H, Barrett P, Brown T, Abbott P, Charlton RA. Demographic and cognitive profile of individuals seeking a diagnosis of autism spectrum disorder in adulthood. J Autism Dev Disord 2016; 46(11):3469–3480. doi:10.1007/s10803-016-2886-2
- Johnston K, Dittner A, Bramham J, Murphy C, Knight A, Russell A. Attention deficit hyperactivity disorder symptoms in adults with autism spectrum disorders. Autism Res Off J Int Soc Autism Res 2013; 6(4):225–236. doi:10.1002/aur.1283
- Cadman T, Spain D, Johnston P, et al. Obsessive-compulsive disorder in adults with high-functioning autism spectrum disorder: what does self-report with the OCI-R tell us? Autism Res Off J Int Soc Autism Res 2015; 8(5):477–485. doi:10.1002/aur.1461
- Russell AJ, Mataix-Cols D, Anson M, Murphy DGM. Obsessions and compulsions in Asperger syndrome and high-functioning autism. Br J Psychiatry J Ment Sci 2005; 186:525–528. doi:10.1192/bjp.186.6.525
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry 2008; 47(8):921–929. doi:10.1097/CHI.0b013e318179964f
- Lugnegård T, Hallerbäck MU, Gillberg C. Psychiatric comorbidity in young adults with a clinical diagnosis of Asperger syndrome. Res Dev Disabil 2011; 32(5):1910–1917. doi:10.1016/j.ridd.2011.03.025
- Howlin P, Moss P. Adults with autism spectrum disorders. Can J Psychiatry 2012; 57(5):275–283. doi:10.1177/070674371205700502
- Levy A, Perry A. Outcomes in adolescents and adults with autism: a review of the literature. Res Autism Spectr Disord 2011; 5(4):1271–1282. doi:10.1016/J.RASD.2011.01.023
- Cheak-Zamora NC, Yang X, Farmer JE, Clark M. Disparities in transition planning for youth with autism spectrum disorder. Pediatrics 2013; 131(3):447–454. doi:10.1542/peds.2012-1572
- Rast JE, Shattuck PT, Roux AM, Anderson KA, Kuo A. The medical home and health care transition for youth with autism. Pediatrics 2018; 141(suppl 4):S328–S334. doi:10.1542/peds.2016-4300J
- Belling R, McLaren S, Paul M, et al. The effect of organisational resources and eligibility issues on transition from child and adolescent to adult mental health services. J Health Serv Res Policy 2014; 19(3):169–176. doi:10.1177/1355819614527439
- Data Resource Center for Child & Adolescent Health. 2009–2010 National Survey of Children with Special Health Care Needs. www.childhealthdata.org/docs/drc/200910-cshcn-spss-codebook_final_051012.pdf?sfvrsn=1. Accessed July 9, 2019.
- Got Transition Center for Health Care Transition Improvement. Six core elements of health care transition 2.0. Transitioning youth to an adult health care provider. For use by pediatric, family medicine, and med-peds providers. www.gottransition.org/resourceGet.cfm?id=208. Accessed July 9, 2019.
- Murphy CM, Wilson CE, Robertson DM, et al. Autism spectrum disorder in adults: diagnosis, management, and health services development. Neuropsychiatr Dis Treat 2016; 12:1669–1686. doi:10.2147/NDT.S65455
- García-Villamisar DA, Dattilo J. Effects of a leisure programme on quality of life and stress of individuals with ASD. J Intellect Disabil Res 2010; 54(7):611–619. doi:10.1111/j.1365-2788.2010.01289.x
- García-Villamisar D, Hughes C. Supported employment improves cognitive performance in adults with autism. J Intellect Disabil Res 2007; 51(pt 2):142–150. doi:10.1111/j.1365-2788.2006.00854.x
- Lawer L, Brusilovskiy E, Salzer MS, Mandell DS. Use of vocational rehabilitative services among adults with autism. J Autism Dev Disord 2009; 39(3):487–494. doi:10.1007/s10803-008-0649-4
- Howlin P, Alcock J, Burkin C. An 8 year follow-up of a specialist supported employment service for high-ability adults with autism or Asperger syndrome. Autism 2005; 9(5):533–549. doi:10.1177/1362361305057871
- Kay BR. Bittersweet Farms. J Autism Dev Disord 1990; 20(3):309–321. http://www.ncbi.nlm.nih.gov/pubmed/2228914. Accessed July 9, 2019.
- Taylor JL, McPheeters ML, Sathe NA, Dove D, Veenstra-Vanderweele J, Warren Z. A systematic review of vocational interventions for young adults with autism spectrum disorders. Pediatrics 2012; 130(3):531–538. doi:10.1542/peds.2012-0682
- Bishop-Fitzpatrick L, Minshew NJ, Eack SM. A systematic review of psychosocial interventions for adults with autism spectrum disorders. J Autism Dev Disord 2013; 43(3):687–694. doi:10.1007/s10803-012-1615-8
- Russell AJ, Jassi A, Fullana MA, et al. Cognitive behavior therapy for comorbid obsessive-compulsive disorder in high-functioning autism spectrum disorders: a randomized controlled trial. Depress Anxiety 2013; 30(8):697–708. doi:10.1002/da.22053
- Spek AA, van Ham NC, Nyklícek I. Mindfulness-based therapy in adults with an autism spectrum disorder: a randomized controlled trial. Res Dev Disabil 2013; 34(1):246–253. doi:10.1016/j.ridd.2012.08.009
- Eapen V, Crncec R, Walter A. Clinical outcomes of an early intervention program for preschool children with autism spectrum disorder in a community group setting. BMC Pediatr 2013; 13(1):3. doi:10.1186/1471-2431-13-3
- Mazefsky CA, White SW. Emotion regulation: concepts & practice in autism spectrum disorder. Child Adolesc Psychiatr Clin North Am 2014; 23(1):15–24. doi:10.1016/J.CHC.2013.07.002
- Esbensen AJ, Greenberg JS, Seltzer MM, Aman MG. A longitudinal investigation of psychotropic and non-psychotropic medication use among adolescents and adults with autism spectrum disorders. J Autism Dev Disord 2009; 39(9):1339–1349. doi:10.1007/s10803-009-0750-3
- Dove D, Warren Z, McPheeters ML, Taylor JL, Sathe NA, Veenstra-VanderWeele J. Medications for adolescents and young adults with autism spectrum disorders: a systematic review. Pediatrics 2012; 130(4):717–726. doi:10.1542/peds.2012-0683
- LeClerc S, Easley D. Pharmacological therapies for autism spectrum disorder: a review. Pharm Ther 2015; 40(6):389–397.
- Miral S, Gencer O, Inal-Emiroglu FN, Baykara B, Baykara A, Dirik E. Risperidone versus haloperidol in children and adolescents with AD: a randomized, controlled, double-blind trial. Eur Child Adolesc Psychiatry 2008; 17(1):1–8. doi:10.1007/s00787-007-0620-5
- Lai M-C, Lombardo MV, Baron-Cohen S. Autism. Lancet 2014; 383(9920):896–910. doi:10.1016/S0140-6736(13)61539-1
- Ratni H, Rogers-Evans M, Bissantz C, et al. Discovery of highly selective brain-penetrant vasopressin 1a antagonists for the potential treatment of autism via a chemogenomic and scaffold hopping approach. J Med Chem 2015; 58(5):2275–2289. doi:10.1021/jm501745f
- Umbricht D, Del Valle Rubido M, Hollander E, et al. A single dose, randomized, controlled proof-of-mechanism study of a novel vasopressin 1a receptor antagonist (RG7713) in high-functioning adults with autism spectrum disorder. Neuropsychopharmacology 2017; 42(9):1914–1923. doi:10.1038/npp.2016.232>
- Kellaher DC. Sexual behavior and autism spectrum disorders: an update and discussion. Curr Psychiatry Rep 2015; 17(4):25. doi:10.1007/s11920-015-0562-4
- Brown-Lavoie SM, Viecili MA, Weiss JA. Sexual knowledge and victimization in adults with autism spectrum disorders. J Autism Dev Disord 2014; 44(9):2185–2196. doi:10.1007/s10803-014-2093-y
- Mehzabin P, Stokes MA. Self-assessed sexuality in young adults with high-functioning autism. Res Autism Spectr Disord 2011; 5(1):614–621. doi:10.1016/J.RASD.2010.07.006>
- Brown KR. Accessibility for students with ASD: legal perspectives in the United States. In: Alphin HC Jr. Exploring the Future of Accessibility in Higher Education. Hershey, PA: IGI Global; 2017.
- George R, Stokes MA. Gender identity and sexual orientation in autism spectrum disorder. Autism 2018; 22(8):970–982. doi:10.1177/1362361317714587
- Byers ES, Nichols S, Voyer SD. Challenging stereotypes: sexual functioning of single adults with high functioning autism spectrum disorder. J Autism Dev Disord 2013; 43(11):2617–2627. doi:10.1007/s10803-013-1813-z
- Gilmour L, Schalomon PM, Smith V. Sexuality in a community based sample of adults with autism spectrum disorder. Res Autism Spectr Disord 2012; 6(1):313–318. doi:10.1016/J.RASD.2011.06.003
- Bejerot S, Eriksson JM. Sexuality and gender role in autism spectrum disorder: a case control study. Schmitz C, ed. PLoS One 2014; 9(1):e87961. doi:10.1371/journal.pone.0087961>
- Navot N, Jorgenson AG, Webb SJ. Maternal experience raising girls with autism spectrum disorder: a qualitative study. Child Care Health Dev 2017; 43(4):536–545. doi:10.1111/cch.12470
- Padden C, James JE. Stress among parents of children with and without autism spectrum disorder: a comparison involving physiological indicators and parent self-reports. J Dev Phys Disabil 2017; 29(4):567–586. doi:10.1007/s10882-017-9547-z
- Woodgate RL, Ateah C, Secco L. Living in a world of our own: the experience of parents who have a child with autism. Qual Health Res 2008; 18(8):1075–1083. doi:10.1177/1049732308320112
- Hayes SA, Watson SL. The impact of parenting stress: a meta-analysis of studies comparing the experience of parenting stress in parents of children with and without autism spectrum disorder. J Autism Dev Disord 2013; 43(3):629–642. doi:10.1007/s10803-012-1604-y
- Cadman T, Eklund H, Howley D, et al. Caregiver burden as people with autism spectrum disorder and attention-deficit/hyperactivity disorder transition into adolescence and adulthood in the United Kingdom. J Am Acad Child Adolesc Psychiatry 2012; 51(9):879–888. doi:10.1016/j.jaac.2012.06.017
- Smith LE, Seltzer MM, Greenberg JS. Daily health symptoms of mothers of adolescents and adults with fragile x syndrome and mothers of adolescents and adults with autism spectrum disorder. J Autism Dev Disord 2012; 42(9):1836–1846. doi:10.1007/s10803-011-1422-7
- van Steijn DJ, Oerlemans AM, van Aken MAG, Buitelaar JK, Rommelse NNJ. The reciprocal relationship of ASD, ADHD, depressive symptoms and stress in parents of children with ASD and/or ADHD. J Autism Dev Disord 2014; 44(5):1064–1076. doi:10.1007/s10803-013-1958-9
- Seltzer MM, Greenberg JS, Hong J, et al. Maternal cortisol levels and behavior problems in adolescents and adults with ASD. J Autism Dev Disord 2010; 40(4):457–469. doi:10.1007/S10803-009-0887-0
- Lovell B, Moss M, Wetherell MA. With a little help from my friends: psychological, endocrine and health corollaries of social support in parental caregivers of children with autism or ADHD. Res Dev Disabil 2012; 33(2):682–687. doi:10.1016/j.ridd.2011.11.014
- Gallagher S, Whiteley J. Social support is associated with blood pressure responses in parents caring for children with developmental disabilities. Res Dev Disabil 2012; 33(6):2099–2105. doi:10.1016/j.ridd.2012.06.007
- Baker JK, Smith LE, Greenberg JS, Seltzer MM, Taylor JL. Change in maternal criticism and behavior problems in adolescents and adults with autism across a 7-year period. J Abnorm Psychol 2011; 120(2):465–475. doi:10.1037/a0021900
- Marsack CN, Samuel PS. Mediating effects of social support on quality of life for parents of adults with autism. J Autism Dev Disord 2017; 47(8):2378–2389. doi:10.1007/s10803-017-3157-6
- Trute B, Benzies KM, Worthington C, Reddon JR, Moore M. Accentuate the positive to mitigate the negative: mother psychological coping resources and family adjustment in childhood disability. J Intellect Dev Disabil 2010; 35(1):36–43. doi:10.3109/13668250903496328
- Cantwell J, Muldoon OT, Gallagher S. Social support and mastery influence the association between stress and poor physical health in parents caring for children with developmental disabilities. Res Dev Disabil 2014; 35(9):2215–2223. doi:10.1016/j.ridd.2014.05.012
- Carton AM, Smith AD. Assessing the relationship between eating disorder psychopathology and autistic traits in a non-clinical adult population. Eat Weight Disord - Stud Anorexia, Bulim Obes 2014; 19(3):285–293. doi:10.1007/s40519-013-0086-z
- De Alwis D, Agrawal A, Reiersen AM, et al. ADHD symptoms, autistic traits, and substance use and misuse in adult Australian twins. J Stud Alcohol Drugs 2014; 75(2):211–221. doi:10.15288/jsad.2014.75.211
- Wang LW, Tancredi DJ, Thomas DW. The prevalence of gastrointestinal problems in children across the United States with autism spectrum disorders from families with multiple affected members. J Dev Behav Pediatr 2011; 32(5):351–360. doi:10.1097/DBP.0b013e31821bd06a
- Croen LA, Zerbo O, Qian Y, et al. The health status of adults on the autism spectrum. Autism 2015; 19(7):814–823. doi:10.1177/1362361315577517
- Kalyoncu IÖ, Tanboga I. Oral health status of children with autistic spectrum disorder compared with non-authentic peers. Iran J Public Health 2017; 46(11):1591–1593. www.ncbi.nlm.nih.gov/pmc/articles/PMC5696703. Accessed July 9, 2019.
- McGuire K, Fung LK, Hagopian L, et al. Irritability and problem behavior in autism spectrum disorder: a practice pathway for pediatric primary care. Pediatrics 2016; 137(suppl 2):S136–S148. doi:10.1542/peds.2015-2851L
KEY POINTS
- Autism is becoming more common, with most recent statistics showing at least 1 in 59 children affected.
- Asperger syndrome is now included in the category of ASD, with possible implications for coverage of care.
- Some children with ASD get better as they get older, but many do not, and some do not receive a diagnosis until adulthood.
- Diagnosing ASD in adults can be difficult and involves specialists from multiple disciplines.
- Social support is important. Community programs and behavioral therapies can help. Drug therapy has not been rigorously tested and is not approved for use in adults with ASD. Caregivers may also need support.