User login
How to avoid ‘checklist’ psychiatry
To determine whether a patient meets the criteria for a DSM-5 diagnosis, we rely on objective data, direct observations, and individual biopsychosocial factors as well as our patient’s subjective report of symptoms. However, because the line differentiating normal from abnormal emotional responses can sometimes be blurred, we should be prudent when establishing a diagnosis. Specifically, we need to avoid falling into the trap of “checklist” psychiatry—relegating diagnostic assessments to robotic statements about whether patients meet DSM criteria—because this can lead to making diagnoses too quickly or inaccurately.1 Potential consequences of checklist psychiatry include1,2:
- becoming so “married” to a particular diagnosis that you don’t consider alternative diagnoses
- labeling patients with a diagnosis that many clinicians may view as pejorative (eg, antisocial personality disorder), which might affect their ability to receive future treatment
- developing ineffective treatment plans based on an incorrect diagnosis, including exposing patients to medications that could have serious adverse effects
- performing suicide or violence risk assessments based on inaccurate diagnoses, thereby over- or underestimating the possible risk for an adverse outcome
- leading patients to assume the identity of the inaccurate diagnosis and possibly viewing themselves as dysfunctional or impaired.
When you are uncertain whether your patient has a diagnosable condition, it can be useful to use the terms “no diagnosis” or “diagnosis deferred.” However, many insurance companies will not reimburse without an actual diagnosis. Therefore, the following tips may be helpful in establishing an accurate diagnosis while avoiding checklist psychiatry.1,2
Ask patients about the degree and duration of impairment in functioning. Although impairment in functioning is a criterion of almost all DSM-5 diagnoses, not all endorsed symptoms warrant a diagnosis. Mild symptoms often resolve spontaneously over time without the need for diagnostic labels or interventions.
Make longitudinal observations. Interviewing patients over a long period of time and on multiple occasions can provide data on the consistency of reported symptoms, the presence or absence of behavioral correlates to reported symptomatology, the degree of impairment from the reported symptoms, and the evolution of symptoms.
Collect collateral information. Although we often rely on our patients’ reports of symptoms to establish a diagnosis, this information should not be the sole source. We can obtain a more complete picture if we approach a patient’s family members for their input, including asking about a family history of mental illness or substance use disorders. We can also review prior treatment records and gather observations from clinic or inpatient staff for additional information.
Order laboratory studies. Serum studies and urine toxicology screens provide information that can help form an accurate diagnosis. This information is helpful because certain medical conditions, substance intoxication, and substance withdrawal can mimic psychiatric symptoms.
Continuously re-evaluate your diagnoses. As clinicians, we’d like to provide an accurate diagnosis at the onset of treatment; however, this may not be realistic because the patient’s presentation might change over time. It is paramount that we view diagnoses as evolving, so that we can more readily adjust our approach to treatment, especially when the patient is not benefitting from a well-formulated and comprehensive treatment plan.
Our patients are best served when we take the necessary time to use all resources to conceptualize them as more than a checklist of symptoms.
1. Kontos N, Freudenreich O, Querques J. Thoughtful diagnoses: not ‘checklist’ psychiatry. Current Psychiatry. 2007;6(3):112.
2. Frances A. My 12 best tips on psychiatric diagnosis. Psychiatric Times. http://www.psychiatrictimes.com/dsm-5/my-12-best-tips-psychiatric-diagnosis. Published June 17, 2013. Accessed July 19, 2019.
To determine whether a patient meets the criteria for a DSM-5 diagnosis, we rely on objective data, direct observations, and individual biopsychosocial factors as well as our patient’s subjective report of symptoms. However, because the line differentiating normal from abnormal emotional responses can sometimes be blurred, we should be prudent when establishing a diagnosis. Specifically, we need to avoid falling into the trap of “checklist” psychiatry—relegating diagnostic assessments to robotic statements about whether patients meet DSM criteria—because this can lead to making diagnoses too quickly or inaccurately.1 Potential consequences of checklist psychiatry include1,2:
- becoming so “married” to a particular diagnosis that you don’t consider alternative diagnoses
- labeling patients with a diagnosis that many clinicians may view as pejorative (eg, antisocial personality disorder), which might affect their ability to receive future treatment
- developing ineffective treatment plans based on an incorrect diagnosis, including exposing patients to medications that could have serious adverse effects
- performing suicide or violence risk assessments based on inaccurate diagnoses, thereby over- or underestimating the possible risk for an adverse outcome
- leading patients to assume the identity of the inaccurate diagnosis and possibly viewing themselves as dysfunctional or impaired.
When you are uncertain whether your patient has a diagnosable condition, it can be useful to use the terms “no diagnosis” or “diagnosis deferred.” However, many insurance companies will not reimburse without an actual diagnosis. Therefore, the following tips may be helpful in establishing an accurate diagnosis while avoiding checklist psychiatry.1,2
Ask patients about the degree and duration of impairment in functioning. Although impairment in functioning is a criterion of almost all DSM-5 diagnoses, not all endorsed symptoms warrant a diagnosis. Mild symptoms often resolve spontaneously over time without the need for diagnostic labels or interventions.
Make longitudinal observations. Interviewing patients over a long period of time and on multiple occasions can provide data on the consistency of reported symptoms, the presence or absence of behavioral correlates to reported symptomatology, the degree of impairment from the reported symptoms, and the evolution of symptoms.
Collect collateral information. Although we often rely on our patients’ reports of symptoms to establish a diagnosis, this information should not be the sole source. We can obtain a more complete picture if we approach a patient’s family members for their input, including asking about a family history of mental illness or substance use disorders. We can also review prior treatment records and gather observations from clinic or inpatient staff for additional information.
Order laboratory studies. Serum studies and urine toxicology screens provide information that can help form an accurate diagnosis. This information is helpful because certain medical conditions, substance intoxication, and substance withdrawal can mimic psychiatric symptoms.
Continuously re-evaluate your diagnoses. As clinicians, we’d like to provide an accurate diagnosis at the onset of treatment; however, this may not be realistic because the patient’s presentation might change over time. It is paramount that we view diagnoses as evolving, so that we can more readily adjust our approach to treatment, especially when the patient is not benefitting from a well-formulated and comprehensive treatment plan.
Our patients are best served when we take the necessary time to use all resources to conceptualize them as more than a checklist of symptoms.
To determine whether a patient meets the criteria for a DSM-5 diagnosis, we rely on objective data, direct observations, and individual biopsychosocial factors as well as our patient’s subjective report of symptoms. However, because the line differentiating normal from abnormal emotional responses can sometimes be blurred, we should be prudent when establishing a diagnosis. Specifically, we need to avoid falling into the trap of “checklist” psychiatry—relegating diagnostic assessments to robotic statements about whether patients meet DSM criteria—because this can lead to making diagnoses too quickly or inaccurately.1 Potential consequences of checklist psychiatry include1,2:
- becoming so “married” to a particular diagnosis that you don’t consider alternative diagnoses
- labeling patients with a diagnosis that many clinicians may view as pejorative (eg, antisocial personality disorder), which might affect their ability to receive future treatment
- developing ineffective treatment plans based on an incorrect diagnosis, including exposing patients to medications that could have serious adverse effects
- performing suicide or violence risk assessments based on inaccurate diagnoses, thereby over- or underestimating the possible risk for an adverse outcome
- leading patients to assume the identity of the inaccurate diagnosis and possibly viewing themselves as dysfunctional or impaired.
When you are uncertain whether your patient has a diagnosable condition, it can be useful to use the terms “no diagnosis” or “diagnosis deferred.” However, many insurance companies will not reimburse without an actual diagnosis. Therefore, the following tips may be helpful in establishing an accurate diagnosis while avoiding checklist psychiatry.1,2
Ask patients about the degree and duration of impairment in functioning. Although impairment in functioning is a criterion of almost all DSM-5 diagnoses, not all endorsed symptoms warrant a diagnosis. Mild symptoms often resolve spontaneously over time without the need for diagnostic labels or interventions.
Make longitudinal observations. Interviewing patients over a long period of time and on multiple occasions can provide data on the consistency of reported symptoms, the presence or absence of behavioral correlates to reported symptomatology, the degree of impairment from the reported symptoms, and the evolution of symptoms.
Collect collateral information. Although we often rely on our patients’ reports of symptoms to establish a diagnosis, this information should not be the sole source. We can obtain a more complete picture if we approach a patient’s family members for their input, including asking about a family history of mental illness or substance use disorders. We can also review prior treatment records and gather observations from clinic or inpatient staff for additional information.
Order laboratory studies. Serum studies and urine toxicology screens provide information that can help form an accurate diagnosis. This information is helpful because certain medical conditions, substance intoxication, and substance withdrawal can mimic psychiatric symptoms.
Continuously re-evaluate your diagnoses. As clinicians, we’d like to provide an accurate diagnosis at the onset of treatment; however, this may not be realistic because the patient’s presentation might change over time. It is paramount that we view diagnoses as evolving, so that we can more readily adjust our approach to treatment, especially when the patient is not benefitting from a well-formulated and comprehensive treatment plan.
Our patients are best served when we take the necessary time to use all resources to conceptualize them as more than a checklist of symptoms.
1. Kontos N, Freudenreich O, Querques J. Thoughtful diagnoses: not ‘checklist’ psychiatry. Current Psychiatry. 2007;6(3):112.
2. Frances A. My 12 best tips on psychiatric diagnosis. Psychiatric Times. http://www.psychiatrictimes.com/dsm-5/my-12-best-tips-psychiatric-diagnosis. Published June 17, 2013. Accessed July 19, 2019.
1. Kontos N, Freudenreich O, Querques J. Thoughtful diagnoses: not ‘checklist’ psychiatry. Current Psychiatry. 2007;6(3):112.
2. Frances A. My 12 best tips on psychiatric diagnosis. Psychiatric Times. http://www.psychiatrictimes.com/dsm-5/my-12-best-tips-psychiatric-diagnosis. Published June 17, 2013. Accessed July 19, 2019.
Child trafficking: How to recognize the signs
Child trafficking—a modern-day form of slavery that continues to destroy many lives—often is hidden, even from the clinicians who see its victims. Traffickers typically exploit children for labor or commercial sexual work. The signs and symptoms that suggest a child is being trafficked may be less clear than those of the psychiatric illnesses we usually diagnose and treat. In this article, I summarize characteristics that could be helpful to note when you suspect a child is being trafficked, and offer some resources for helping victims.
How to identify possible victims
Children can be trafficked anywhere. The concept of a child being picked up off a street corner is outdated. Trafficking occurs in cities, suburbs, and rural areas. It happens in hotel rooms, at truck stops, on quiet residential streets, and in expensive homes. The internet has made it easier for traffickers to find victims.
Traffickers typically target youth who are emotionally and physically vulnerable. They often seek out teenagers who are undergoing financial hardships, experiencing family conflict, or have survived natural disasters. Many victims are runaways. In 2016, 1 in 6 child runaways reported to the National Center for Missing and Exploited Children were likely victims of trafficking.1 Of those children, 86% were receiving social services support or living in foster homes.
Traffickers are adept at emotional manipulation, which may explain why a child or adolescent might minimize the abuse during a clinical visit. Traffickers shroud the realities of trafficking with notions of love and inclusion. They use several physical and mental schemes to keep children and adolescents in their grip, such as withholding food, sleep, or medical care. Therefore, we should check for signs and symptoms of chronic medical conditions that have gone untreated, malnutrition, or bruises in various stages of healing.
Connecting risk factors for trafficking to dramatic changes in a young patient’s behavior is challenging. These youth often have dropped out of school, lack consistent family support, and spend their nights in search of a warm place to sleep. Their lives are upended. A child who once was more social may be forced into isolation and make excuses for why she no longer spends time with her friends.
In a study of 106 survivors of domestic sex trafficking, approximately 89% of respondents reported depression during depression. Many respondents reported experiencing anxiety (76.4%), nightmares (73.6%), flashbacks (68%), low self-esteem (81.1%), or feelings of shame or guilt (82.1%).2 Almost 88% of respondents said that they saw a doctor or other clinician while being trafficked, but their clinicians were unable to recognize the signs of trafficking. Part of the challenge is that many children and adolescents are not comfortable discussing their situations with clinicians because they may struggle with shame and guilt. Their traffickers also might have convinced them that they are criminals, not victims. These patients also may have an overwhelming fear of their trafficker, being reported to child welfare authorities, being arrested, being deported, or having their traffickers retaliate against their families. Gaining the trust of a patient who is being trafficked is critical, but not easy, because children may be skeptical of a clinician’s promise of confidentiality.
Some signs of trafficking overlap with the psychiatric presentations with which we are more familiar. These patients may abuse drugs or alcohol as means of escape or because their traffickers force them to use substances.2 They may show symptoms of depression or posttraumatic stress disorder (PTSD) and may be disoriented. Other indicators may be more telling, such as if a child or adolescent describes:
- having no control of their schedules or forms of identification
- having to work excessively long hours, often to pay off an overwhelming debt
- having high security measures installed in their place of residence (such as cameras or barred windows).
Continue to: Also, they may be...
Also, they may be dressed inappropriately for the weather.
We should be concerned when patients’ responses seem coached, if they say they are isolated from their family and community, or if they are submissive or overly timid. In addition, our suspicions should be raised if an accompanying adult guardian insists on sitting in on the appointment or translating for the child. In such instances, we may request that the guardian remain in the waiting area during the appointment so the child will have the opportunity to speak freely.2
How to help a suspected victim
Several local and national organizations help trafficking victims. These organizations provide educational materials and training opportunities for clinicians, as well as direct support for victims. The Homeland Security Blue Campaign advises against confronting a suspected trafficker directly and encourages clinicians to instead report suspected cases to 1-866-347-2423.3
Clinicians can better help children who are trafficked by taking the following 5 steps:
- Learn about the risk factors and signs of child trafficking.
- Post the National Human Trafficking Hotline (1-888-373-7888) in your waiting room.
- Determine if your patient is in danger and needs to be moved to a safe place.
- Connect the patient to social service agencies that can provide financial support and housing assistance so he/she doesn’t feel trapped by financial burdens.
- Work to rebuild their emotional and physical well-being while treating depression, PTSD, substance abuse, or any other mental illness.
1. National Center for Missing and Exploited Childr en. Missing children, state care, and child sex trafficking. http://www.missingkids.com/content/dam/missingkids/pdfs/publications/missingchildrenstatecare.pdf. Accessed June 10, 2019.
2. Lederer LJ, Wetzel CA. The health consequences of sex trafficking and their implications for identifying victims in healthcare facilities. Ann Health Law. 2014;23(1):61-91.
3. Blue Campaign. Identify a victim. US Department of Homeland Security. https://www.dhs.gov/blue-campaign/identify-victim. Accessed June 10, 2019.
Child trafficking—a modern-day form of slavery that continues to destroy many lives—often is hidden, even from the clinicians who see its victims. Traffickers typically exploit children for labor or commercial sexual work. The signs and symptoms that suggest a child is being trafficked may be less clear than those of the psychiatric illnesses we usually diagnose and treat. In this article, I summarize characteristics that could be helpful to note when you suspect a child is being trafficked, and offer some resources for helping victims.
How to identify possible victims
Children can be trafficked anywhere. The concept of a child being picked up off a street corner is outdated. Trafficking occurs in cities, suburbs, and rural areas. It happens in hotel rooms, at truck stops, on quiet residential streets, and in expensive homes. The internet has made it easier for traffickers to find victims.
Traffickers typically target youth who are emotionally and physically vulnerable. They often seek out teenagers who are undergoing financial hardships, experiencing family conflict, or have survived natural disasters. Many victims are runaways. In 2016, 1 in 6 child runaways reported to the National Center for Missing and Exploited Children were likely victims of trafficking.1 Of those children, 86% were receiving social services support or living in foster homes.
Traffickers are adept at emotional manipulation, which may explain why a child or adolescent might minimize the abuse during a clinical visit. Traffickers shroud the realities of trafficking with notions of love and inclusion. They use several physical and mental schemes to keep children and adolescents in their grip, such as withholding food, sleep, or medical care. Therefore, we should check for signs and symptoms of chronic medical conditions that have gone untreated, malnutrition, or bruises in various stages of healing.
Connecting risk factors for trafficking to dramatic changes in a young patient’s behavior is challenging. These youth often have dropped out of school, lack consistent family support, and spend their nights in search of a warm place to sleep. Their lives are upended. A child who once was more social may be forced into isolation and make excuses for why she no longer spends time with her friends.
In a study of 106 survivors of domestic sex trafficking, approximately 89% of respondents reported depression during depression. Many respondents reported experiencing anxiety (76.4%), nightmares (73.6%), flashbacks (68%), low self-esteem (81.1%), or feelings of shame or guilt (82.1%).2 Almost 88% of respondents said that they saw a doctor or other clinician while being trafficked, but their clinicians were unable to recognize the signs of trafficking. Part of the challenge is that many children and adolescents are not comfortable discussing their situations with clinicians because they may struggle with shame and guilt. Their traffickers also might have convinced them that they are criminals, not victims. These patients also may have an overwhelming fear of their trafficker, being reported to child welfare authorities, being arrested, being deported, or having their traffickers retaliate against their families. Gaining the trust of a patient who is being trafficked is critical, but not easy, because children may be skeptical of a clinician’s promise of confidentiality.
Some signs of trafficking overlap with the psychiatric presentations with which we are more familiar. These patients may abuse drugs or alcohol as means of escape or because their traffickers force them to use substances.2 They may show symptoms of depression or posttraumatic stress disorder (PTSD) and may be disoriented. Other indicators may be more telling, such as if a child or adolescent describes:
- having no control of their schedules or forms of identification
- having to work excessively long hours, often to pay off an overwhelming debt
- having high security measures installed in their place of residence (such as cameras or barred windows).
Continue to: Also, they may be...
Also, they may be dressed inappropriately for the weather.
We should be concerned when patients’ responses seem coached, if they say they are isolated from their family and community, or if they are submissive or overly timid. In addition, our suspicions should be raised if an accompanying adult guardian insists on sitting in on the appointment or translating for the child. In such instances, we may request that the guardian remain in the waiting area during the appointment so the child will have the opportunity to speak freely.2
How to help a suspected victim
Several local and national organizations help trafficking victims. These organizations provide educational materials and training opportunities for clinicians, as well as direct support for victims. The Homeland Security Blue Campaign advises against confronting a suspected trafficker directly and encourages clinicians to instead report suspected cases to 1-866-347-2423.3
Clinicians can better help children who are trafficked by taking the following 5 steps:
- Learn about the risk factors and signs of child trafficking.
- Post the National Human Trafficking Hotline (1-888-373-7888) in your waiting room.
- Determine if your patient is in danger and needs to be moved to a safe place.
- Connect the patient to social service agencies that can provide financial support and housing assistance so he/she doesn’t feel trapped by financial burdens.
- Work to rebuild their emotional and physical well-being while treating depression, PTSD, substance abuse, or any other mental illness.
Child trafficking—a modern-day form of slavery that continues to destroy many lives—often is hidden, even from the clinicians who see its victims. Traffickers typically exploit children for labor or commercial sexual work. The signs and symptoms that suggest a child is being trafficked may be less clear than those of the psychiatric illnesses we usually diagnose and treat. In this article, I summarize characteristics that could be helpful to note when you suspect a child is being trafficked, and offer some resources for helping victims.
How to identify possible victims
Children can be trafficked anywhere. The concept of a child being picked up off a street corner is outdated. Trafficking occurs in cities, suburbs, and rural areas. It happens in hotel rooms, at truck stops, on quiet residential streets, and in expensive homes. The internet has made it easier for traffickers to find victims.
Traffickers typically target youth who are emotionally and physically vulnerable. They often seek out teenagers who are undergoing financial hardships, experiencing family conflict, or have survived natural disasters. Many victims are runaways. In 2016, 1 in 6 child runaways reported to the National Center for Missing and Exploited Children were likely victims of trafficking.1 Of those children, 86% were receiving social services support or living in foster homes.
Traffickers are adept at emotional manipulation, which may explain why a child or adolescent might minimize the abuse during a clinical visit. Traffickers shroud the realities of trafficking with notions of love and inclusion. They use several physical and mental schemes to keep children and adolescents in their grip, such as withholding food, sleep, or medical care. Therefore, we should check for signs and symptoms of chronic medical conditions that have gone untreated, malnutrition, or bruises in various stages of healing.
Connecting risk factors for trafficking to dramatic changes in a young patient’s behavior is challenging. These youth often have dropped out of school, lack consistent family support, and spend their nights in search of a warm place to sleep. Their lives are upended. A child who once was more social may be forced into isolation and make excuses for why she no longer spends time with her friends.
In a study of 106 survivors of domestic sex trafficking, approximately 89% of respondents reported depression during depression. Many respondents reported experiencing anxiety (76.4%), nightmares (73.6%), flashbacks (68%), low self-esteem (81.1%), or feelings of shame or guilt (82.1%).2 Almost 88% of respondents said that they saw a doctor or other clinician while being trafficked, but their clinicians were unable to recognize the signs of trafficking. Part of the challenge is that many children and adolescents are not comfortable discussing their situations with clinicians because they may struggle with shame and guilt. Their traffickers also might have convinced them that they are criminals, not victims. These patients also may have an overwhelming fear of their trafficker, being reported to child welfare authorities, being arrested, being deported, or having their traffickers retaliate against their families. Gaining the trust of a patient who is being trafficked is critical, but not easy, because children may be skeptical of a clinician’s promise of confidentiality.
Some signs of trafficking overlap with the psychiatric presentations with which we are more familiar. These patients may abuse drugs or alcohol as means of escape or because their traffickers force them to use substances.2 They may show symptoms of depression or posttraumatic stress disorder (PTSD) and may be disoriented. Other indicators may be more telling, such as if a child or adolescent describes:
- having no control of their schedules or forms of identification
- having to work excessively long hours, often to pay off an overwhelming debt
- having high security measures installed in their place of residence (such as cameras or barred windows).
Continue to: Also, they may be...
Also, they may be dressed inappropriately for the weather.
We should be concerned when patients’ responses seem coached, if they say they are isolated from their family and community, or if they are submissive or overly timid. In addition, our suspicions should be raised if an accompanying adult guardian insists on sitting in on the appointment or translating for the child. In such instances, we may request that the guardian remain in the waiting area during the appointment so the child will have the opportunity to speak freely.2
How to help a suspected victim
Several local and national organizations help trafficking victims. These organizations provide educational materials and training opportunities for clinicians, as well as direct support for victims. The Homeland Security Blue Campaign advises against confronting a suspected trafficker directly and encourages clinicians to instead report suspected cases to 1-866-347-2423.3
Clinicians can better help children who are trafficked by taking the following 5 steps:
- Learn about the risk factors and signs of child trafficking.
- Post the National Human Trafficking Hotline (1-888-373-7888) in your waiting room.
- Determine if your patient is in danger and needs to be moved to a safe place.
- Connect the patient to social service agencies that can provide financial support and housing assistance so he/she doesn’t feel trapped by financial burdens.
- Work to rebuild their emotional and physical well-being while treating depression, PTSD, substance abuse, or any other mental illness.
1. National Center for Missing and Exploited Childr en. Missing children, state care, and child sex trafficking. http://www.missingkids.com/content/dam/missingkids/pdfs/publications/missingchildrenstatecare.pdf. Accessed June 10, 2019.
2. Lederer LJ, Wetzel CA. The health consequences of sex trafficking and their implications for identifying victims in healthcare facilities. Ann Health Law. 2014;23(1):61-91.
3. Blue Campaign. Identify a victim. US Department of Homeland Security. https://www.dhs.gov/blue-campaign/identify-victim. Accessed June 10, 2019.
1. National Center for Missing and Exploited Childr en. Missing children, state care, and child sex trafficking. http://www.missingkids.com/content/dam/missingkids/pdfs/publications/missingchildrenstatecare.pdf. Accessed June 10, 2019.
2. Lederer LJ, Wetzel CA. The health consequences of sex trafficking and their implications for identifying victims in healthcare facilities. Ann Health Law. 2014;23(1):61-91.
3. Blue Campaign. Identify a victim. US Department of Homeland Security. https://www.dhs.gov/blue-campaign/identify-victim. Accessed June 10, 2019.
Rash on both palms
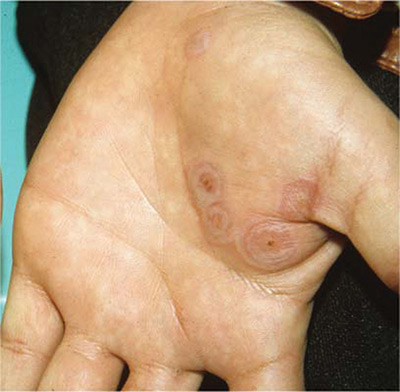
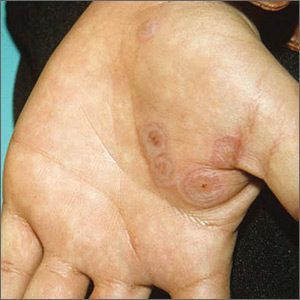
The FP diagnosed erythema multiforme (EM) in this patient based on the target lesions with central epithelial disruption on his palms. In this case, the EM was due to the herpes simplex outbreak on the patient’s lips (herpes labialis) that had occurred about a week earlier.
EM is a hypersensitivity reaction that is often secondary to infections or medications. Herpes simplex viruses (HSVI and HSV2) are the most common causative agents and have been implicated in ≥ 60% of cases.
The patient did not know that cold sores were due to herpes simplex and most oral HSV is due to HSV1 infection. He acknowledged that he experienced cold sores about every 2 months that were usually related to stress or exposure to intense sunlight. The FP recommended that the patient avoid intense sunlight (midday sun avoidance; wearing sunscreen and hats) and use lip protection with at least an SPF of 15. As the lip lesions were > 90% healed, there was no reason for the FP to prescribe an antiviral agent. The FP did, however, offer a prescription for valacyclovir to be used at the first signs of an oral herpes outbreak to avoid another case of EM (2000 mg by mouth every 12 hours x 2 doses). For symptomatic relief of the EM, the physician prescribed a 15 g tube of 0.1% triamcinolone cream to be applied to the lesions twice daily.
Photo courtesy of the University of Texas Health Sciences Center, Division of Dermatology and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Milana C, Smith M. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1161-1168.
To learn more about the 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP diagnosed erythema multiforme (EM) in this patient based on the target lesions with central epithelial disruption on his palms. In this case, the EM was due to the herpes simplex outbreak on the patient’s lips (herpes labialis) that had occurred about a week earlier.
EM is a hypersensitivity reaction that is often secondary to infections or medications. Herpes simplex viruses (HSVI and HSV2) are the most common causative agents and have been implicated in ≥ 60% of cases.
The patient did not know that cold sores were due to herpes simplex and most oral HSV is due to HSV1 infection. He acknowledged that he experienced cold sores about every 2 months that were usually related to stress or exposure to intense sunlight. The FP recommended that the patient avoid intense sunlight (midday sun avoidance; wearing sunscreen and hats) and use lip protection with at least an SPF of 15. As the lip lesions were > 90% healed, there was no reason for the FP to prescribe an antiviral agent. The FP did, however, offer a prescription for valacyclovir to be used at the first signs of an oral herpes outbreak to avoid another case of EM (2000 mg by mouth every 12 hours x 2 doses). For symptomatic relief of the EM, the physician prescribed a 15 g tube of 0.1% triamcinolone cream to be applied to the lesions twice daily.
Photo courtesy of the University of Texas Health Sciences Center, Division of Dermatology and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Milana C, Smith M. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1161-1168.
To learn more about the 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP diagnosed erythema multiforme (EM) in this patient based on the target lesions with central epithelial disruption on his palms. In this case, the EM was due to the herpes simplex outbreak on the patient’s lips (herpes labialis) that had occurred about a week earlier.
EM is a hypersensitivity reaction that is often secondary to infections or medications. Herpes simplex viruses (HSVI and HSV2) are the most common causative agents and have been implicated in ≥ 60% of cases.
The patient did not know that cold sores were due to herpes simplex and most oral HSV is due to HSV1 infection. He acknowledged that he experienced cold sores about every 2 months that were usually related to stress or exposure to intense sunlight. The FP recommended that the patient avoid intense sunlight (midday sun avoidance; wearing sunscreen and hats) and use lip protection with at least an SPF of 15. As the lip lesions were > 90% healed, there was no reason for the FP to prescribe an antiviral agent. The FP did, however, offer a prescription for valacyclovir to be used at the first signs of an oral herpes outbreak to avoid another case of EM (2000 mg by mouth every 12 hours x 2 doses). For symptomatic relief of the EM, the physician prescribed a 15 g tube of 0.1% triamcinolone cream to be applied to the lesions twice daily.
Photo courtesy of the University of Texas Health Sciences Center, Division of Dermatology and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Milana C, Smith M. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1161-1168.
To learn more about the 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
Diagnosis and management of gastric intestinal metaplasia in the United States
Introduction
Despite a global decline in the incidence of gastric cancer over the past 3 decades, it remains the fifth most commonly diagnosed cancer and the third most common cause of cancer deaths worldwide.1 In the United States it is the fourth most commonly diagnosed GI malignancy, after colorectal, pancreas, and liver cancer. The prevalence remains high in Latin America and Asia, which has implications in the United States because of growing Hispanic and Asian populations.2,3 In recent years, a change in the trend of gastric cancer among non-Hispanic whites has been observed, particularly in women younger than 50 years old.4 5
Etiology
Gastric adenocarcinomas are classified into two subcategories based on location (cardia and noncardia) and histology (intestinal and diffuse types).6,7 Atrophic gastritis and gastric intestinal metaplasia (GIM) are considered precursors of intestinal-type noncardia gastric adenocarcinoma. The Correa cascade is a commonly accepted precancer sequence for noncardia gastric adenocarcinoma that describes mucosal changes from inflammation to atrophy to metaplasia to intraepithelial neoplasia and culminating in carcinoma.8,9 It has been observed that GIM may be the histologic change prior to the development of dysplasia and over 50% of patients with high-grade dysplasia will progress to adenocarcinoma.10-12 In the United States, GIM has the highest prevalence in African Americans, Hispanics, and East Asians, with the overall GIM prevalence regardless of ethnicity reported from 3.05% to 19.2%.5,13
Risk factors and subclassification
Replacement of the foveolar and/or glandular epithelium in the oxyntic and antral mucosa by intestinal epithelium results in GIM. It can be focal when limited to one region of the stomach or extensive when two or more regions are involved.14 The main risk factors for GIM development are Helicobacter pylori infection, tobacco, alcohol consumption, high salt intake, and chronic bile reflux.15,16 Additional risks for developing gastric cancer include older age, certain ethnicities, and male sex.17
CagA strains of H. pylori can promote carcinogenesis by inducing a mitogenic cellular response and downregulating cell adhesion.18,19 Less carcinogenic risk is associated with H. pylori Cag-A negative strains; however, they also have oncogenic potential mediated by expression of babA2 and vacA genes.20 Hence, the combination of multiple virulent factors encoded in babA2, CagA, and vacA genes has been associated with increased risk of GIM, inflammation, and development of gastric cancer.15 The clinical usefulness of genotyping H. pylori strains specifically to survey precancerous gastric lesions remains to be seen because of a lack of sufficient clinical studies. In addition, genotyping H. pylori is not commonly performed as part of clinical practice.
The loss of parietal cells seen in atrophic gastritis due to chronic H. pylori infection has been linked to the development of metaplasia due to possible loss of differentiation-promoting factors. As a result, metaplastic cells emerge that express spasmolytic polypeptide (SP or TFF2); hence, this type of metaplasia is referred to as spasmolytic polypeptide–expressing metaplasia (SPEM). The cellular mechanism that may explain a precursor role of SPEM in the development of GIM remains unknown.14 A second competing theory for the development of GIM is the clonal expansion of stem cells in the gastric isthmus that can lead to dysplasia and cancer development.14
On the basis of histological similarities with small intestinal or colonic epithelium, GIM can be further classified into complete or incomplete intestinal metaplasia.21 Complete intestinal metaplasia most closely resembles small intestinal epithelium with a brush border and goblet cells. Incomplete intestinal metaplasia resembles the colonic epithelium and lacks a brush border. A second classification further classifies GIM into three subtypes: Type I contains nonsecretory absorptive cells and sialomucin secreting goblet cells; type II has few absorptive cells, columnar cells secreting sialomucin, goblet cells secreting mainly sialomucin but some sulphomucin, and presence of Paneth cells; and type III consists of columnar cells secreting predominantly sulphomucin, goblet cells secreting sialomucin or sulphomucin, and absence of Paneth cells.15,22 In this subclassification, type I GIM is known as complete GIM and types II and III as incomplete GIM.23-25
Multiple studies performed outside of the United States have shown a higher progression risk to gastric adenocarcinoma in incomplete intestinal metaplasia, or type III intestinal metaplasia.26-32 Also, the risk of gastric cancer has been demonstrated to be higher among patients with a greater area of metaplasia and extensive intestinal metaplasia, defined as GIM in both the antrum and corpus.33,34 Hence, the extent of the metaplasia determined with mapping biopsies, regardless of the subtype, should also be incorporated into the risk assessment of the patient. Currently, a major limitation in the United States is a standardized method of pathologic reporting including subclassification of incomplete versus complete intestinal metaplasia.
Which patients to screen
Understanding this sequence of carcinogenesis offers a potential window for screening and surveillance. Subsequently, early detection of precancerous mucosal changes would be more amenable for endoscopic submucosal dissection (ESD).35,36 Currently, U.S. society guidelines do not specifically address the management of GIM. The American Society for Gastrointestinal Endoscopy (ASGE) guidelines for management of premalignant and malignant conditions of the stomach recommend surveillance in individuals with a family history of gastric cancer or of high-risk ethnic background but with no specific optimal surveillance interval.37 Also, H. pylori treatment is recommended if identified, but empiric treatment in GIM was felt to be controversial. The AGA recently sought comments on a proposed new guideline for the management of GIM. This guideline should be released after the comment period and help address management of GIM in the United States. In April of 2019, the European Society of Gastrointestinal Endoscopy (ESGE) updated the management of epithelial precancerous conditions and lesions in the stomach (MAPS II) guideline.38 The MAPS II guideline identifies atrophic gastritis and intestinal metaplasia as precancerous lesions. In patients with moderate to marked atrophy or GIM affecting both antral and body mucosa, ESGE recommends endoscopic surveillance with high-definition chromoendoscopy, mapping, and guided biopsies or at least two biopsies taken separately at the lesser and greater curvature of the antrum and body. H. pylori eradication was recommended if the patient tested positive.
Furthermore, MAPS II proposed replacing atrophic gastritis (AG) in the Operative Link on Gastritis Assessment (OLGA) staging by GIM (OLGIM) as it is considered a more reliable predictor of an individual’s gastric neoplasia risk, based on the interobserver agreement kappa value 0.6 for AG versus 0.9 for GIM.39 Five biopsies (two from the antrum, two from the corpus, and one from the incisura angularis) are needed for the OLGA/OLGIM score system to be considered an accurate predictor of this risk.39 This is supported by the early findings of gastric atrophy and GIM in the incisura angularis.23 In addition, for patients with GIM only in either the antrum or the body, a family history of gastric cancer, incomplete GIM, autoimmune gastritis, or persistent H. pylori infection was felt to increase the risk to warrant surveillance every 3 years. In those patients with atrophy or GIM in both the antrum and body with a first-degree relative with gastric cancer, surveillance was recommended every 1-2 years. Patients with any dysplasia and a visible lesion should have staging and resection. With no visible lesion, a follow-up endoscopy should be performed in 6 months with high-grade dysplasia and with low-grade dysplasia a repeat in 12 months. Patients with mild to moderate atrophy in the antrum and no intestinal metaplasia were not felt to warrant any further surveillance. (See Figure 1.)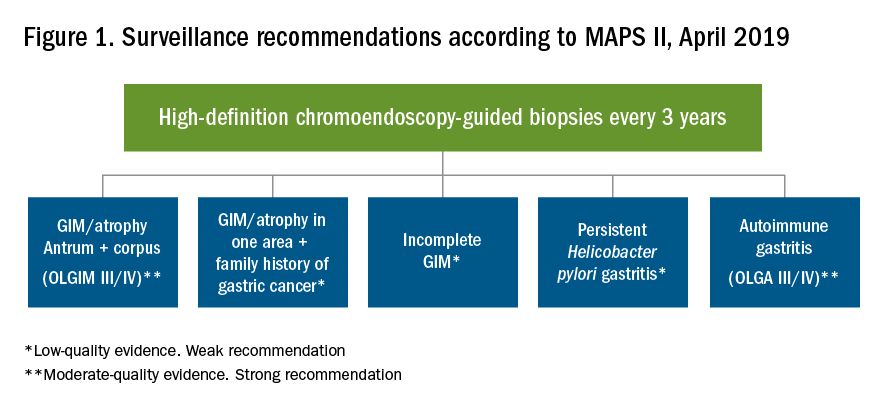
How to screen
Previous studies have found a poor correlation between the endoscopic determination of gastric atrophy and the histologic diagnosis.42 Several studies also found that gastric cancer was missed on initial endoscopic examinations. Sensitivity of endoscopy to detect gastric cancer has ranged from 77% to 93%.43,44 In the United States, there is a lack of standardized quality indicators for upper endoscopy exams. The ESGE has suggested several performance measures to ensure a quality endoscopy exam, including accurate photo documentation, sufficient procedure time of at least 7 minutes, adherence to biopsy protocols, and low complication rates.45 In Asia, a systematic screening protocol is used for photo documentation, and simple techniques such as adequate air insufflation and irrigation to remove mucus are routinely used to improve the endoscopy exam.46,47 The mean time of an endoscopy exam has also been found to increase the detection of neoplastic lesions, as slow endoscopists – with a mean exam duration of 8.6 ± 4.2 min during upper endoscopy – detected threefold more neoplastic lesions than did fast endoscopists.48
A standardized biopsy approach is also important when screening patients. The updated Sydney protocol has been suggested for mapping the stomach to screen for atrophy and GIM. This protocol recommends two biopsies from the antrum (at the lesser and greater curvature), two from the body (at the lesser and greater curvature), and one from the incisura.23 This biopsy protocol was also suggested in the recent MAPS II update, with the biopsy of the incisura felt to be an additional biopsy left to the discretion of the endoscopist. Notably, abnormal appearing mucosal areas should be biopsied separately from the mapping biopsies.
High-definition endoscopy with virtual chromoendoscopy is felt to be better than white-light endoscopy alone at detecting precancerous gastric lesions.38 (See Figure 2.)
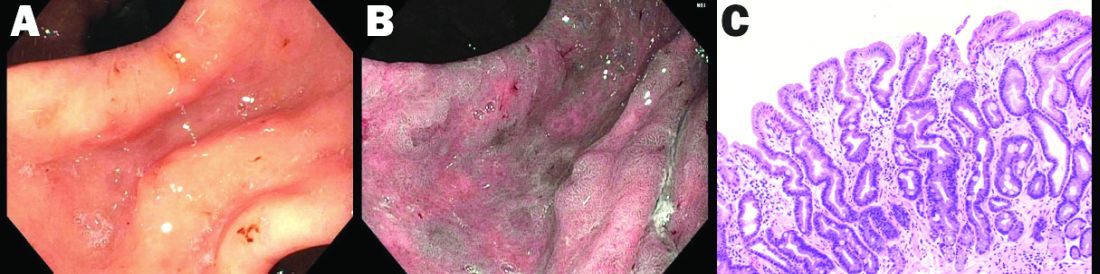
In particular, narrow-band imaging (NBI) has been studied and found to increase the diagnostic yield of GIM and dysplasia compared with white light alone.49 Several studies have shown an increased accuracy for the detection of GIM with magnification NBI.50-52 An unfortunate limitation is the geographic availability of magnification NBI: It is not available in the United States. A multicenter study in Portugal developed a new classification system for the appearance of precancerous lesions with NBI and tested its accuracy in endoscopists with a wide range of NBI experience. An abnormal mucosal pattern that showed light blue crests/regular ridge or a tubulovillous appearance and a regular mucosal pattern was found with GIM. An irregular vascular pattern with a white opaque substance and an absent or irregular mucosal pattern was most often found with dysplasia. Furthermore, the reproducibility of these patterns was high between endoscopists.53 Multiple studies have been performed on additional imaging technologies to enhance the detection of gastric neoplasia; however, these technologies are still investigational and currently not recommended for screening.54-57
Serum pepsinogens have been studied in Europe and Asia as noninvasive indicators of gastric atrophy to determine who should be screened with endoscopy.58 A low serum pepsinogen I level below 70 ng/mL and pepsinogen I/II ratio below 3 has generally been used to detect atrophic gastritis and at-risk populations. However, the studies performed in Europe and Asia used different methods for quantifying pepsinogen levels. Therefore, cutoff values cannot be generalized for all assays and should be validated for the specific tests used.38
Summary
Gastric atrophy and gastric intestinal metaplasia are considered precancerous lesions with an increased risk of development of gastric cancer. H. pylori is a major risk factor for the development of GIM. The extent of GIM as well as the presence of incomplete intestinal metaplasia, or type III intestinal metaplasia has been found to have the highest gastric cancer risk. Currently, in the United States, specific guidelines on endoscopic screening and surveillance for noncardia gastric adenocarcinoma based on histological subtype of GIM, location, and extension are lacking. The ESGE recently updated guidelines that recommend surveillance of patients with extensive atrophy and intestinal metaplasia or with a significant family history. Location and extension of intestinal metaplasia plays a role in increased risk. Screening should include a standardized upper endoscopy approach with high-definition white- light endoscopy and NBI, at least a 7-minute examination, adequate insufflation and cleaning, adequate photo documentation, and a standardized biopsy protocol. Further studies are needed to determine an appropriate surveillance interval and standardized pathology reporting approach as well.
Diana Curras-Martin MD, is an internal medicine resident at Hackensack Meridian Jersey Shore University Medical Center. Susana Gonzalez, MD, is assistant professor of medicine in the division of gastroenterology and hepatology (@WCM_GI), Weill Cornell Medicine, New York Presbyterian Hospital–Cornell.
References
1. Bray F et al. CA Cancer J Clin. 2018;68(6):394-424.
2. Global Burden of Disease Cancer Collaboration et al. JAMA Oncol. 2018;4(11):1553-68.
3. Balakrishnan M et al. Curr Gastroenterol Rep. 2017;19(8):36.
4. Anderson WF et al. J Natl Cancer Inst. 2018;110(6):608-15.
5. Trieu JA et al. Dig Dis Sci. 2019;64(5):1079-88.
6. Lauren P. Acta Pathol Microbiol Scand. 1965;64:31-49.
7. Correa P, Schneider BG. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1865-8.
8. Correa P. Cancer Res. 1992;52(24):6735-40.
9. Correa P, Piazuelo MB. J Dig Dis. 2012;13(1):2-9.
10. Correa P et al. J Natl Cancer Inst. 1970;44(2):297-306.
11. Correa P. Semin Oncol. 1985;12(1):2-10.
12. Rugge M et al. Hum Pathol. 1991;22(10):1002-8.
13. Simko V et al. Bratisl Lek Listy. 2015;116(1):3-8.
14. Giroux V, Rustgi AK. Nat Rev Cancer. 2017;17(10):594-604.
15. Jencks DS et al. Gastroenterol Hepatol (N Y). 2018;14(2):92-101.
16. Amieva M, Peek RM Jr. Gastroenterology. 2016;150(1):64-78.
17. Karimi P et al. Cancer Epidemiol Biomarkers Prev. 2014;23(5):700-13.
18. Hatakeyama M. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(4):196-219.
19. Tsutsumi R et al. Mol Cell Biol. 2006;26(1):261-76.
20. Kikuchi S et al. Am J Gastroenterol. 1999;94(12):3455-9.
21. Jass JR, Filipe MI. Histopathology. 1980;4(3):271-9.
22. Jass JR, Filipe MI. Histochem J. 1981;13(6):931-9.
23. Dixon MF et al. Am J Surg Pathol. 1996;20(10):1161-81.
24. Kang KP et al. J Gastroenterol Hepatol. 2009;24(1):140-8.
25. Gonzalez CA et al. Int J Cancer. 2010;127(11):2654-60.
26. Filipe MI et al. Gut. 1985;26(12):1319-26.
27. Filipe MI et al. Int J Cancer. 1994;57(3):324-9.
28. Gonzalez CA et al. J Gastroenterol Hepatol. 2016;31(5):953-8.
29. Cassaro M et al. Am J Gastroenterol. 2000;95(6):1431-8.
30. Shao L et al. Int J Cancer. Apr 29. 2018.
31. Stemmermann GN. Cancer. 1994;74(2):556-64.
32. Gonzalez CA et al. Int J Cancer. 2013;133(5):1023-32.
33. Reddy KM et al. Clin Gastroenterol Hepatol. 2016;14(10):1420-5.
34. Tava F et al. Hum Pathol. 2006;37(11):1489-97.
35. Fernandez-Esparrach G et al. Rev Esp Enferm Dig. 2014;106(2):120-32.
36. Ono H et al. Dig Endosc. 2016;28(1):3-15.
37. Evans JA, DeWitt JM. Gastrointest Endosc. 2016;83(1):274.
38. Pimentel-Nunes P et al. Endoscopy. 2019;51(4):365-88.
39. Capelle LG et al. Gastrointest Endosc. 2010;71(7):1150-8.
40. Saumoy M et al. Gastroenterology. 2018;155(3):648-60.
41. Gupta N et al. Gastrointest Endosc. 2011;74(3):610-24 e612.
42. Eshmuratov A et al. Dig Dis Sci. 2010;55(5):1364-75.
43. Nam JH et al. Cancer. 2012;118(20):4953-60.
44. Amin A et al. J R Coll Surg Edinb. 2002;47(5):681-4.
45. Bisschops R et al. United European Gastroenterol J. 2016;4(5):629-56.
46. Uedo N et al. Gastroenterol Clin North Am. 2013;42(2):317-35.
47. Yao K. Ann Gastroenterol. 2013;26(1):11-22.
48. Teh JL et al. Clin Gastroenterol Hepatol. 2015;13(3):480-7 e482.
49. Capelle LG et al. Dig Dis Sci. 2010;55(12):3442-8.
50. Bansal A et al. Gastrointest Endosc. 2008;67(2):210-6.
51. Tahara T et al. Gastrointest Endosc. 2009;70(2):246-53.
52. Uedo N et al. Endoscopy. 2006;38(8):819-24.
53. Pimentel-Nunes P et al. Endoscopy. 2012;44(3):236-46.
54. Kato M et al. Gastrointest Endosc. 2009;70(5):899-906.
55. Nishimura J et al. Gastroenterol Res Pract. 2014;2014:819395.
56. Dohi O et al. Gastrointest Endosc. 2019;89(1):47-57.
57. Osawa H et al. World J Gastrointest Endosc. 2012;4(8):356-61.
58. Pasechnikov V et al. World J Gastroenterol. 2014;20(38):13842-62.
Introduction
Despite a global decline in the incidence of gastric cancer over the past 3 decades, it remains the fifth most commonly diagnosed cancer and the third most common cause of cancer deaths worldwide.1 In the United States it is the fourth most commonly diagnosed GI malignancy, after colorectal, pancreas, and liver cancer. The prevalence remains high in Latin America and Asia, which has implications in the United States because of growing Hispanic and Asian populations.2,3 In recent years, a change in the trend of gastric cancer among non-Hispanic whites has been observed, particularly in women younger than 50 years old.4 5
Etiology
Gastric adenocarcinomas are classified into two subcategories based on location (cardia and noncardia) and histology (intestinal and diffuse types).6,7 Atrophic gastritis and gastric intestinal metaplasia (GIM) are considered precursors of intestinal-type noncardia gastric adenocarcinoma. The Correa cascade is a commonly accepted precancer sequence for noncardia gastric adenocarcinoma that describes mucosal changes from inflammation to atrophy to metaplasia to intraepithelial neoplasia and culminating in carcinoma.8,9 It has been observed that GIM may be the histologic change prior to the development of dysplasia and over 50% of patients with high-grade dysplasia will progress to adenocarcinoma.10-12 In the United States, GIM has the highest prevalence in African Americans, Hispanics, and East Asians, with the overall GIM prevalence regardless of ethnicity reported from 3.05% to 19.2%.5,13
Risk factors and subclassification
Replacement of the foveolar and/or glandular epithelium in the oxyntic and antral mucosa by intestinal epithelium results in GIM. It can be focal when limited to one region of the stomach or extensive when two or more regions are involved.14 The main risk factors for GIM development are Helicobacter pylori infection, tobacco, alcohol consumption, high salt intake, and chronic bile reflux.15,16 Additional risks for developing gastric cancer include older age, certain ethnicities, and male sex.17
CagA strains of H. pylori can promote carcinogenesis by inducing a mitogenic cellular response and downregulating cell adhesion.18,19 Less carcinogenic risk is associated with H. pylori Cag-A negative strains; however, they also have oncogenic potential mediated by expression of babA2 and vacA genes.20 Hence, the combination of multiple virulent factors encoded in babA2, CagA, and vacA genes has been associated with increased risk of GIM, inflammation, and development of gastric cancer.15 The clinical usefulness of genotyping H. pylori strains specifically to survey precancerous gastric lesions remains to be seen because of a lack of sufficient clinical studies. In addition, genotyping H. pylori is not commonly performed as part of clinical practice.
The loss of parietal cells seen in atrophic gastritis due to chronic H. pylori infection has been linked to the development of metaplasia due to possible loss of differentiation-promoting factors. As a result, metaplastic cells emerge that express spasmolytic polypeptide (SP or TFF2); hence, this type of metaplasia is referred to as spasmolytic polypeptide–expressing metaplasia (SPEM). The cellular mechanism that may explain a precursor role of SPEM in the development of GIM remains unknown.14 A second competing theory for the development of GIM is the clonal expansion of stem cells in the gastric isthmus that can lead to dysplasia and cancer development.14
On the basis of histological similarities with small intestinal or colonic epithelium, GIM can be further classified into complete or incomplete intestinal metaplasia.21 Complete intestinal metaplasia most closely resembles small intestinal epithelium with a brush border and goblet cells. Incomplete intestinal metaplasia resembles the colonic epithelium and lacks a brush border. A second classification further classifies GIM into three subtypes: Type I contains nonsecretory absorptive cells and sialomucin secreting goblet cells; type II has few absorptive cells, columnar cells secreting sialomucin, goblet cells secreting mainly sialomucin but some sulphomucin, and presence of Paneth cells; and type III consists of columnar cells secreting predominantly sulphomucin, goblet cells secreting sialomucin or sulphomucin, and absence of Paneth cells.15,22 In this subclassification, type I GIM is known as complete GIM and types II and III as incomplete GIM.23-25
Multiple studies performed outside of the United States have shown a higher progression risk to gastric adenocarcinoma in incomplete intestinal metaplasia, or type III intestinal metaplasia.26-32 Also, the risk of gastric cancer has been demonstrated to be higher among patients with a greater area of metaplasia and extensive intestinal metaplasia, defined as GIM in both the antrum and corpus.33,34 Hence, the extent of the metaplasia determined with mapping biopsies, regardless of the subtype, should also be incorporated into the risk assessment of the patient. Currently, a major limitation in the United States is a standardized method of pathologic reporting including subclassification of incomplete versus complete intestinal metaplasia.
Which patients to screen
Understanding this sequence of carcinogenesis offers a potential window for screening and surveillance. Subsequently, early detection of precancerous mucosal changes would be more amenable for endoscopic submucosal dissection (ESD).35,36 Currently, U.S. society guidelines do not specifically address the management of GIM. The American Society for Gastrointestinal Endoscopy (ASGE) guidelines for management of premalignant and malignant conditions of the stomach recommend surveillance in individuals with a family history of gastric cancer or of high-risk ethnic background but with no specific optimal surveillance interval.37 Also, H. pylori treatment is recommended if identified, but empiric treatment in GIM was felt to be controversial. The AGA recently sought comments on a proposed new guideline for the management of GIM. This guideline should be released after the comment period and help address management of GIM in the United States. In April of 2019, the European Society of Gastrointestinal Endoscopy (ESGE) updated the management of epithelial precancerous conditions and lesions in the stomach (MAPS II) guideline.38 The MAPS II guideline identifies atrophic gastritis and intestinal metaplasia as precancerous lesions. In patients with moderate to marked atrophy or GIM affecting both antral and body mucosa, ESGE recommends endoscopic surveillance with high-definition chromoendoscopy, mapping, and guided biopsies or at least two biopsies taken separately at the lesser and greater curvature of the antrum and body. H. pylori eradication was recommended if the patient tested positive.
Furthermore, MAPS II proposed replacing atrophic gastritis (AG) in the Operative Link on Gastritis Assessment (OLGA) staging by GIM (OLGIM) as it is considered a more reliable predictor of an individual’s gastric neoplasia risk, based on the interobserver agreement kappa value 0.6 for AG versus 0.9 for GIM.39 Five biopsies (two from the antrum, two from the corpus, and one from the incisura angularis) are needed for the OLGA/OLGIM score system to be considered an accurate predictor of this risk.39 This is supported by the early findings of gastric atrophy and GIM in the incisura angularis.23 In addition, for patients with GIM only in either the antrum or the body, a family history of gastric cancer, incomplete GIM, autoimmune gastritis, or persistent H. pylori infection was felt to increase the risk to warrant surveillance every 3 years. In those patients with atrophy or GIM in both the antrum and body with a first-degree relative with gastric cancer, surveillance was recommended every 1-2 years. Patients with any dysplasia and a visible lesion should have staging and resection. With no visible lesion, a follow-up endoscopy should be performed in 6 months with high-grade dysplasia and with low-grade dysplasia a repeat in 12 months. Patients with mild to moderate atrophy in the antrum and no intestinal metaplasia were not felt to warrant any further surveillance. (See Figure 1.)
How to screen
Previous studies have found a poor correlation between the endoscopic determination of gastric atrophy and the histologic diagnosis.42 Several studies also found that gastric cancer was missed on initial endoscopic examinations. Sensitivity of endoscopy to detect gastric cancer has ranged from 77% to 93%.43,44 In the United States, there is a lack of standardized quality indicators for upper endoscopy exams. The ESGE has suggested several performance measures to ensure a quality endoscopy exam, including accurate photo documentation, sufficient procedure time of at least 7 minutes, adherence to biopsy protocols, and low complication rates.45 In Asia, a systematic screening protocol is used for photo documentation, and simple techniques such as adequate air insufflation and irrigation to remove mucus are routinely used to improve the endoscopy exam.46,47 The mean time of an endoscopy exam has also been found to increase the detection of neoplastic lesions, as slow endoscopists – with a mean exam duration of 8.6 ± 4.2 min during upper endoscopy – detected threefold more neoplastic lesions than did fast endoscopists.48
A standardized biopsy approach is also important when screening patients. The updated Sydney protocol has been suggested for mapping the stomach to screen for atrophy and GIM. This protocol recommends two biopsies from the antrum (at the lesser and greater curvature), two from the body (at the lesser and greater curvature), and one from the incisura.23 This biopsy protocol was also suggested in the recent MAPS II update, with the biopsy of the incisura felt to be an additional biopsy left to the discretion of the endoscopist. Notably, abnormal appearing mucosal areas should be biopsied separately from the mapping biopsies.
High-definition endoscopy with virtual chromoendoscopy is felt to be better than white-light endoscopy alone at detecting precancerous gastric lesions.38 (See Figure 2.)

In particular, narrow-band imaging (NBI) has been studied and found to increase the diagnostic yield of GIM and dysplasia compared with white light alone.49 Several studies have shown an increased accuracy for the detection of GIM with magnification NBI.50-52 An unfortunate limitation is the geographic availability of magnification NBI: It is not available in the United States. A multicenter study in Portugal developed a new classification system for the appearance of precancerous lesions with NBI and tested its accuracy in endoscopists with a wide range of NBI experience. An abnormal mucosal pattern that showed light blue crests/regular ridge or a tubulovillous appearance and a regular mucosal pattern was found with GIM. An irregular vascular pattern with a white opaque substance and an absent or irregular mucosal pattern was most often found with dysplasia. Furthermore, the reproducibility of these patterns was high between endoscopists.53 Multiple studies have been performed on additional imaging technologies to enhance the detection of gastric neoplasia; however, these technologies are still investigational and currently not recommended for screening.54-57
Serum pepsinogens have been studied in Europe and Asia as noninvasive indicators of gastric atrophy to determine who should be screened with endoscopy.58 A low serum pepsinogen I level below 70 ng/mL and pepsinogen I/II ratio below 3 has generally been used to detect atrophic gastritis and at-risk populations. However, the studies performed in Europe and Asia used different methods for quantifying pepsinogen levels. Therefore, cutoff values cannot be generalized for all assays and should be validated for the specific tests used.38
Summary
Gastric atrophy and gastric intestinal metaplasia are considered precancerous lesions with an increased risk of development of gastric cancer. H. pylori is a major risk factor for the development of GIM. The extent of GIM as well as the presence of incomplete intestinal metaplasia, or type III intestinal metaplasia has been found to have the highest gastric cancer risk. Currently, in the United States, specific guidelines on endoscopic screening and surveillance for noncardia gastric adenocarcinoma based on histological subtype of GIM, location, and extension are lacking. The ESGE recently updated guidelines that recommend surveillance of patients with extensive atrophy and intestinal metaplasia or with a significant family history. Location and extension of intestinal metaplasia plays a role in increased risk. Screening should include a standardized upper endoscopy approach with high-definition white- light endoscopy and NBI, at least a 7-minute examination, adequate insufflation and cleaning, adequate photo documentation, and a standardized biopsy protocol. Further studies are needed to determine an appropriate surveillance interval and standardized pathology reporting approach as well.
Diana Curras-Martin MD, is an internal medicine resident at Hackensack Meridian Jersey Shore University Medical Center. Susana Gonzalez, MD, is assistant professor of medicine in the division of gastroenterology and hepatology (@WCM_GI), Weill Cornell Medicine, New York Presbyterian Hospital–Cornell.
References
1. Bray F et al. CA Cancer J Clin. 2018;68(6):394-424.
2. Global Burden of Disease Cancer Collaboration et al. JAMA Oncol. 2018;4(11):1553-68.
3. Balakrishnan M et al. Curr Gastroenterol Rep. 2017;19(8):36.
4. Anderson WF et al. J Natl Cancer Inst. 2018;110(6):608-15.
5. Trieu JA et al. Dig Dis Sci. 2019;64(5):1079-88.
6. Lauren P. Acta Pathol Microbiol Scand. 1965;64:31-49.
7. Correa P, Schneider BG. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1865-8.
8. Correa P. Cancer Res. 1992;52(24):6735-40.
9. Correa P, Piazuelo MB. J Dig Dis. 2012;13(1):2-9.
10. Correa P et al. J Natl Cancer Inst. 1970;44(2):297-306.
11. Correa P. Semin Oncol. 1985;12(1):2-10.
12. Rugge M et al. Hum Pathol. 1991;22(10):1002-8.
13. Simko V et al. Bratisl Lek Listy. 2015;116(1):3-8.
14. Giroux V, Rustgi AK. Nat Rev Cancer. 2017;17(10):594-604.
15. Jencks DS et al. Gastroenterol Hepatol (N Y). 2018;14(2):92-101.
16. Amieva M, Peek RM Jr. Gastroenterology. 2016;150(1):64-78.
17. Karimi P et al. Cancer Epidemiol Biomarkers Prev. 2014;23(5):700-13.
18. Hatakeyama M. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(4):196-219.
19. Tsutsumi R et al. Mol Cell Biol. 2006;26(1):261-76.
20. Kikuchi S et al. Am J Gastroenterol. 1999;94(12):3455-9.
21. Jass JR, Filipe MI. Histopathology. 1980;4(3):271-9.
22. Jass JR, Filipe MI. Histochem J. 1981;13(6):931-9.
23. Dixon MF et al. Am J Surg Pathol. 1996;20(10):1161-81.
24. Kang KP et al. J Gastroenterol Hepatol. 2009;24(1):140-8.
25. Gonzalez CA et al. Int J Cancer. 2010;127(11):2654-60.
26. Filipe MI et al. Gut. 1985;26(12):1319-26.
27. Filipe MI et al. Int J Cancer. 1994;57(3):324-9.
28. Gonzalez CA et al. J Gastroenterol Hepatol. 2016;31(5):953-8.
29. Cassaro M et al. Am J Gastroenterol. 2000;95(6):1431-8.
30. Shao L et al. Int J Cancer. Apr 29. 2018.
31. Stemmermann GN. Cancer. 1994;74(2):556-64.
32. Gonzalez CA et al. Int J Cancer. 2013;133(5):1023-32.
33. Reddy KM et al. Clin Gastroenterol Hepatol. 2016;14(10):1420-5.
34. Tava F et al. Hum Pathol. 2006;37(11):1489-97.
35. Fernandez-Esparrach G et al. Rev Esp Enferm Dig. 2014;106(2):120-32.
36. Ono H et al. Dig Endosc. 2016;28(1):3-15.
37. Evans JA, DeWitt JM. Gastrointest Endosc. 2016;83(1):274.
38. Pimentel-Nunes P et al. Endoscopy. 2019;51(4):365-88.
39. Capelle LG et al. Gastrointest Endosc. 2010;71(7):1150-8.
40. Saumoy M et al. Gastroenterology. 2018;155(3):648-60.
41. Gupta N et al. Gastrointest Endosc. 2011;74(3):610-24 e612.
42. Eshmuratov A et al. Dig Dis Sci. 2010;55(5):1364-75.
43. Nam JH et al. Cancer. 2012;118(20):4953-60.
44. Amin A et al. J R Coll Surg Edinb. 2002;47(5):681-4.
45. Bisschops R et al. United European Gastroenterol J. 2016;4(5):629-56.
46. Uedo N et al. Gastroenterol Clin North Am. 2013;42(2):317-35.
47. Yao K. Ann Gastroenterol. 2013;26(1):11-22.
48. Teh JL et al. Clin Gastroenterol Hepatol. 2015;13(3):480-7 e482.
49. Capelle LG et al. Dig Dis Sci. 2010;55(12):3442-8.
50. Bansal A et al. Gastrointest Endosc. 2008;67(2):210-6.
51. Tahara T et al. Gastrointest Endosc. 2009;70(2):246-53.
52. Uedo N et al. Endoscopy. 2006;38(8):819-24.
53. Pimentel-Nunes P et al. Endoscopy. 2012;44(3):236-46.
54. Kato M et al. Gastrointest Endosc. 2009;70(5):899-906.
55. Nishimura J et al. Gastroenterol Res Pract. 2014;2014:819395.
56. Dohi O et al. Gastrointest Endosc. 2019;89(1):47-57.
57. Osawa H et al. World J Gastrointest Endosc. 2012;4(8):356-61.
58. Pasechnikov V et al. World J Gastroenterol. 2014;20(38):13842-62.
Introduction
Despite a global decline in the incidence of gastric cancer over the past 3 decades, it remains the fifth most commonly diagnosed cancer and the third most common cause of cancer deaths worldwide.1 In the United States it is the fourth most commonly diagnosed GI malignancy, after colorectal, pancreas, and liver cancer. The prevalence remains high in Latin America and Asia, which has implications in the United States because of growing Hispanic and Asian populations.2,3 In recent years, a change in the trend of gastric cancer among non-Hispanic whites has been observed, particularly in women younger than 50 years old.4 5
Etiology
Gastric adenocarcinomas are classified into two subcategories based on location (cardia and noncardia) and histology (intestinal and diffuse types).6,7 Atrophic gastritis and gastric intestinal metaplasia (GIM) are considered precursors of intestinal-type noncardia gastric adenocarcinoma. The Correa cascade is a commonly accepted precancer sequence for noncardia gastric adenocarcinoma that describes mucosal changes from inflammation to atrophy to metaplasia to intraepithelial neoplasia and culminating in carcinoma.8,9 It has been observed that GIM may be the histologic change prior to the development of dysplasia and over 50% of patients with high-grade dysplasia will progress to adenocarcinoma.10-12 In the United States, GIM has the highest prevalence in African Americans, Hispanics, and East Asians, with the overall GIM prevalence regardless of ethnicity reported from 3.05% to 19.2%.5,13
Risk factors and subclassification
Replacement of the foveolar and/or glandular epithelium in the oxyntic and antral mucosa by intestinal epithelium results in GIM. It can be focal when limited to one region of the stomach or extensive when two or more regions are involved.14 The main risk factors for GIM development are Helicobacter pylori infection, tobacco, alcohol consumption, high salt intake, and chronic bile reflux.15,16 Additional risks for developing gastric cancer include older age, certain ethnicities, and male sex.17
CagA strains of H. pylori can promote carcinogenesis by inducing a mitogenic cellular response and downregulating cell adhesion.18,19 Less carcinogenic risk is associated with H. pylori Cag-A negative strains; however, they also have oncogenic potential mediated by expression of babA2 and vacA genes.20 Hence, the combination of multiple virulent factors encoded in babA2, CagA, and vacA genes has been associated with increased risk of GIM, inflammation, and development of gastric cancer.15 The clinical usefulness of genotyping H. pylori strains specifically to survey precancerous gastric lesions remains to be seen because of a lack of sufficient clinical studies. In addition, genotyping H. pylori is not commonly performed as part of clinical practice.
The loss of parietal cells seen in atrophic gastritis due to chronic H. pylori infection has been linked to the development of metaplasia due to possible loss of differentiation-promoting factors. As a result, metaplastic cells emerge that express spasmolytic polypeptide (SP or TFF2); hence, this type of metaplasia is referred to as spasmolytic polypeptide–expressing metaplasia (SPEM). The cellular mechanism that may explain a precursor role of SPEM in the development of GIM remains unknown.14 A second competing theory for the development of GIM is the clonal expansion of stem cells in the gastric isthmus that can lead to dysplasia and cancer development.14
On the basis of histological similarities with small intestinal or colonic epithelium, GIM can be further classified into complete or incomplete intestinal metaplasia.21 Complete intestinal metaplasia most closely resembles small intestinal epithelium with a brush border and goblet cells. Incomplete intestinal metaplasia resembles the colonic epithelium and lacks a brush border. A second classification further classifies GIM into three subtypes: Type I contains nonsecretory absorptive cells and sialomucin secreting goblet cells; type II has few absorptive cells, columnar cells secreting sialomucin, goblet cells secreting mainly sialomucin but some sulphomucin, and presence of Paneth cells; and type III consists of columnar cells secreting predominantly sulphomucin, goblet cells secreting sialomucin or sulphomucin, and absence of Paneth cells.15,22 In this subclassification, type I GIM is known as complete GIM and types II and III as incomplete GIM.23-25
Multiple studies performed outside of the United States have shown a higher progression risk to gastric adenocarcinoma in incomplete intestinal metaplasia, or type III intestinal metaplasia.26-32 Also, the risk of gastric cancer has been demonstrated to be higher among patients with a greater area of metaplasia and extensive intestinal metaplasia, defined as GIM in both the antrum and corpus.33,34 Hence, the extent of the metaplasia determined with mapping biopsies, regardless of the subtype, should also be incorporated into the risk assessment of the patient. Currently, a major limitation in the United States is a standardized method of pathologic reporting including subclassification of incomplete versus complete intestinal metaplasia.
Which patients to screen
Understanding this sequence of carcinogenesis offers a potential window for screening and surveillance. Subsequently, early detection of precancerous mucosal changes would be more amenable for endoscopic submucosal dissection (ESD).35,36 Currently, U.S. society guidelines do not specifically address the management of GIM. The American Society for Gastrointestinal Endoscopy (ASGE) guidelines for management of premalignant and malignant conditions of the stomach recommend surveillance in individuals with a family history of gastric cancer or of high-risk ethnic background but with no specific optimal surveillance interval.37 Also, H. pylori treatment is recommended if identified, but empiric treatment in GIM was felt to be controversial. The AGA recently sought comments on a proposed new guideline for the management of GIM. This guideline should be released after the comment period and help address management of GIM in the United States. In April of 2019, the European Society of Gastrointestinal Endoscopy (ESGE) updated the management of epithelial precancerous conditions and lesions in the stomach (MAPS II) guideline.38 The MAPS II guideline identifies atrophic gastritis and intestinal metaplasia as precancerous lesions. In patients with moderate to marked atrophy or GIM affecting both antral and body mucosa, ESGE recommends endoscopic surveillance with high-definition chromoendoscopy, mapping, and guided biopsies or at least two biopsies taken separately at the lesser and greater curvature of the antrum and body. H. pylori eradication was recommended if the patient tested positive.
Furthermore, MAPS II proposed replacing atrophic gastritis (AG) in the Operative Link on Gastritis Assessment (OLGA) staging by GIM (OLGIM) as it is considered a more reliable predictor of an individual’s gastric neoplasia risk, based on the interobserver agreement kappa value 0.6 for AG versus 0.9 for GIM.39 Five biopsies (two from the antrum, two from the corpus, and one from the incisura angularis) are needed for the OLGA/OLGIM score system to be considered an accurate predictor of this risk.39 This is supported by the early findings of gastric atrophy and GIM in the incisura angularis.23 In addition, for patients with GIM only in either the antrum or the body, a family history of gastric cancer, incomplete GIM, autoimmune gastritis, or persistent H. pylori infection was felt to increase the risk to warrant surveillance every 3 years. In those patients with atrophy or GIM in both the antrum and body with a first-degree relative with gastric cancer, surveillance was recommended every 1-2 years. Patients with any dysplasia and a visible lesion should have staging and resection. With no visible lesion, a follow-up endoscopy should be performed in 6 months with high-grade dysplasia and with low-grade dysplasia a repeat in 12 months. Patients with mild to moderate atrophy in the antrum and no intestinal metaplasia were not felt to warrant any further surveillance. (See Figure 1.)
How to screen
Previous studies have found a poor correlation between the endoscopic determination of gastric atrophy and the histologic diagnosis.42 Several studies also found that gastric cancer was missed on initial endoscopic examinations. Sensitivity of endoscopy to detect gastric cancer has ranged from 77% to 93%.43,44 In the United States, there is a lack of standardized quality indicators for upper endoscopy exams. The ESGE has suggested several performance measures to ensure a quality endoscopy exam, including accurate photo documentation, sufficient procedure time of at least 7 minutes, adherence to biopsy protocols, and low complication rates.45 In Asia, a systematic screening protocol is used for photo documentation, and simple techniques such as adequate air insufflation and irrigation to remove mucus are routinely used to improve the endoscopy exam.46,47 The mean time of an endoscopy exam has also been found to increase the detection of neoplastic lesions, as slow endoscopists – with a mean exam duration of 8.6 ± 4.2 min during upper endoscopy – detected threefold more neoplastic lesions than did fast endoscopists.48
A standardized biopsy approach is also important when screening patients. The updated Sydney protocol has been suggested for mapping the stomach to screen for atrophy and GIM. This protocol recommends two biopsies from the antrum (at the lesser and greater curvature), two from the body (at the lesser and greater curvature), and one from the incisura.23 This biopsy protocol was also suggested in the recent MAPS II update, with the biopsy of the incisura felt to be an additional biopsy left to the discretion of the endoscopist. Notably, abnormal appearing mucosal areas should be biopsied separately from the mapping biopsies.
High-definition endoscopy with virtual chromoendoscopy is felt to be better than white-light endoscopy alone at detecting precancerous gastric lesions.38 (See Figure 2.)

In particular, narrow-band imaging (NBI) has been studied and found to increase the diagnostic yield of GIM and dysplasia compared with white light alone.49 Several studies have shown an increased accuracy for the detection of GIM with magnification NBI.50-52 An unfortunate limitation is the geographic availability of magnification NBI: It is not available in the United States. A multicenter study in Portugal developed a new classification system for the appearance of precancerous lesions with NBI and tested its accuracy in endoscopists with a wide range of NBI experience. An abnormal mucosal pattern that showed light blue crests/regular ridge or a tubulovillous appearance and a regular mucosal pattern was found with GIM. An irregular vascular pattern with a white opaque substance and an absent or irregular mucosal pattern was most often found with dysplasia. Furthermore, the reproducibility of these patterns was high between endoscopists.53 Multiple studies have been performed on additional imaging technologies to enhance the detection of gastric neoplasia; however, these technologies are still investigational and currently not recommended for screening.54-57
Serum pepsinogens have been studied in Europe and Asia as noninvasive indicators of gastric atrophy to determine who should be screened with endoscopy.58 A low serum pepsinogen I level below 70 ng/mL and pepsinogen I/II ratio below 3 has generally been used to detect atrophic gastritis and at-risk populations. However, the studies performed in Europe and Asia used different methods for quantifying pepsinogen levels. Therefore, cutoff values cannot be generalized for all assays and should be validated for the specific tests used.38
Summary
Gastric atrophy and gastric intestinal metaplasia are considered precancerous lesions with an increased risk of development of gastric cancer. H. pylori is a major risk factor for the development of GIM. The extent of GIM as well as the presence of incomplete intestinal metaplasia, or type III intestinal metaplasia has been found to have the highest gastric cancer risk. Currently, in the United States, specific guidelines on endoscopic screening and surveillance for noncardia gastric adenocarcinoma based on histological subtype of GIM, location, and extension are lacking. The ESGE recently updated guidelines that recommend surveillance of patients with extensive atrophy and intestinal metaplasia or with a significant family history. Location and extension of intestinal metaplasia plays a role in increased risk. Screening should include a standardized upper endoscopy approach with high-definition white- light endoscopy and NBI, at least a 7-minute examination, adequate insufflation and cleaning, adequate photo documentation, and a standardized biopsy protocol. Further studies are needed to determine an appropriate surveillance interval and standardized pathology reporting approach as well.
Diana Curras-Martin MD, is an internal medicine resident at Hackensack Meridian Jersey Shore University Medical Center. Susana Gonzalez, MD, is assistant professor of medicine in the division of gastroenterology and hepatology (@WCM_GI), Weill Cornell Medicine, New York Presbyterian Hospital–Cornell.
References
1. Bray F et al. CA Cancer J Clin. 2018;68(6):394-424.
2. Global Burden of Disease Cancer Collaboration et al. JAMA Oncol. 2018;4(11):1553-68.
3. Balakrishnan M et al. Curr Gastroenterol Rep. 2017;19(8):36.
4. Anderson WF et al. J Natl Cancer Inst. 2018;110(6):608-15.
5. Trieu JA et al. Dig Dis Sci. 2019;64(5):1079-88.
6. Lauren P. Acta Pathol Microbiol Scand. 1965;64:31-49.
7. Correa P, Schneider BG. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1865-8.
8. Correa P. Cancer Res. 1992;52(24):6735-40.
9. Correa P, Piazuelo MB. J Dig Dis. 2012;13(1):2-9.
10. Correa P et al. J Natl Cancer Inst. 1970;44(2):297-306.
11. Correa P. Semin Oncol. 1985;12(1):2-10.
12. Rugge M et al. Hum Pathol. 1991;22(10):1002-8.
13. Simko V et al. Bratisl Lek Listy. 2015;116(1):3-8.
14. Giroux V, Rustgi AK. Nat Rev Cancer. 2017;17(10):594-604.
15. Jencks DS et al. Gastroenterol Hepatol (N Y). 2018;14(2):92-101.
16. Amieva M, Peek RM Jr. Gastroenterology. 2016;150(1):64-78.
17. Karimi P et al. Cancer Epidemiol Biomarkers Prev. 2014;23(5):700-13.
18. Hatakeyama M. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(4):196-219.
19. Tsutsumi R et al. Mol Cell Biol. 2006;26(1):261-76.
20. Kikuchi S et al. Am J Gastroenterol. 1999;94(12):3455-9.
21. Jass JR, Filipe MI. Histopathology. 1980;4(3):271-9.
22. Jass JR, Filipe MI. Histochem J. 1981;13(6):931-9.
23. Dixon MF et al. Am J Surg Pathol. 1996;20(10):1161-81.
24. Kang KP et al. J Gastroenterol Hepatol. 2009;24(1):140-8.
25. Gonzalez CA et al. Int J Cancer. 2010;127(11):2654-60.
26. Filipe MI et al. Gut. 1985;26(12):1319-26.
27. Filipe MI et al. Int J Cancer. 1994;57(3):324-9.
28. Gonzalez CA et al. J Gastroenterol Hepatol. 2016;31(5):953-8.
29. Cassaro M et al. Am J Gastroenterol. 2000;95(6):1431-8.
30. Shao L et al. Int J Cancer. Apr 29. 2018.
31. Stemmermann GN. Cancer. 1994;74(2):556-64.
32. Gonzalez CA et al. Int J Cancer. 2013;133(5):1023-32.
33. Reddy KM et al. Clin Gastroenterol Hepatol. 2016;14(10):1420-5.
34. Tava F et al. Hum Pathol. 2006;37(11):1489-97.
35. Fernandez-Esparrach G et al. Rev Esp Enferm Dig. 2014;106(2):120-32.
36. Ono H et al. Dig Endosc. 2016;28(1):3-15.
37. Evans JA, DeWitt JM. Gastrointest Endosc. 2016;83(1):274.
38. Pimentel-Nunes P et al. Endoscopy. 2019;51(4):365-88.
39. Capelle LG et al. Gastrointest Endosc. 2010;71(7):1150-8.
40. Saumoy M et al. Gastroenterology. 2018;155(3):648-60.
41. Gupta N et al. Gastrointest Endosc. 2011;74(3):610-24 e612.
42. Eshmuratov A et al. Dig Dis Sci. 2010;55(5):1364-75.
43. Nam JH et al. Cancer. 2012;118(20):4953-60.
44. Amin A et al. J R Coll Surg Edinb. 2002;47(5):681-4.
45. Bisschops R et al. United European Gastroenterol J. 2016;4(5):629-56.
46. Uedo N et al. Gastroenterol Clin North Am. 2013;42(2):317-35.
47. Yao K. Ann Gastroenterol. 2013;26(1):11-22.
48. Teh JL et al. Clin Gastroenterol Hepatol. 2015;13(3):480-7 e482.
49. Capelle LG et al. Dig Dis Sci. 2010;55(12):3442-8.
50. Bansal A et al. Gastrointest Endosc. 2008;67(2):210-6.
51. Tahara T et al. Gastrointest Endosc. 2009;70(2):246-53.
52. Uedo N et al. Endoscopy. 2006;38(8):819-24.
53. Pimentel-Nunes P et al. Endoscopy. 2012;44(3):236-46.
54. Kato M et al. Gastrointest Endosc. 2009;70(5):899-906.
55. Nishimura J et al. Gastroenterol Res Pract. 2014;2014:819395.
56. Dohi O et al. Gastrointest Endosc. 2019;89(1):47-57.
57. Osawa H et al. World J Gastrointest Endosc. 2012;4(8):356-61.
58. Pasechnikov V et al. World J Gastroenterol. 2014;20(38):13842-62.
We owe a lot to scientists like Henry Lynch
It is with great sadness that we note the passing (June 2, 2019; age 91) of Dr. Henry Lynch. Dr. Lynch almost singlehandedly brought attention to the genetic syndrome that bears his name. In 1913 Aldred Warthin (pathology chair at the University of Michigan) first described family “G”, the family of his seamstress who had told him that her family all dies of cancer. She herself succumbed to endometrial cancer. A plaque commemorating Dr. Warthin hangs down the hallway from my office at Michigan. His report fell into obscurity until the 1960s when Dr. Lynch arranged a reunion of family G in Ann Arbor, leading to a detailed update of the family in 1971. He recognized the autosomal dominance of the pedigree pattern.
In 1973, C. Richard Boland, MD, AGAF (past AGA President), wrote a medical school thesis entitled “A Familial Cancer Syndrome” and subsequently published two papers in which he first used the term “Lynch syndrome (I and II). Dr. Boland (whose family also carried a Lynch syndrome variant) spent his career adding to our molecular and clinical knowledge about nonpolyposis colon cancer syndromes. In the 1990s Vogelstein and others first described the molecular pathways that lead to colon cancer – and the rest is history.
I was a young faculty gastroenterologist at the Minneapolis VA Medical Center when one day my phone rang; it was Henry Lynch. He wanted to alert me that one of his patients was coming to me for surveillance colonoscopy. He explained the importance of what I was to do and how I should follow this man. I was overwhelmed by his attention to his patient (one of thousands) and his kindness to me. I had the privilege of traveling with him as visiting professors on a trip to South America. He was one of the kindest, most intelligent, and gracious persons I had ever met. I never forgot that experience.
We owe a lot to scientists, clinicians, and thought leaders like Henry Lynch who provide us the scientific basis of the care we give our patients.
John I. Allen, MD, MBA, AGAF
Editor in Chief
It is with great sadness that we note the passing (June 2, 2019; age 91) of Dr. Henry Lynch. Dr. Lynch almost singlehandedly brought attention to the genetic syndrome that bears his name. In 1913 Aldred Warthin (pathology chair at the University of Michigan) first described family “G”, the family of his seamstress who had told him that her family all dies of cancer. She herself succumbed to endometrial cancer. A plaque commemorating Dr. Warthin hangs down the hallway from my office at Michigan. His report fell into obscurity until the 1960s when Dr. Lynch arranged a reunion of family G in Ann Arbor, leading to a detailed update of the family in 1971. He recognized the autosomal dominance of the pedigree pattern.
In 1973, C. Richard Boland, MD, AGAF (past AGA President), wrote a medical school thesis entitled “A Familial Cancer Syndrome” and subsequently published two papers in which he first used the term “Lynch syndrome (I and II). Dr. Boland (whose family also carried a Lynch syndrome variant) spent his career adding to our molecular and clinical knowledge about nonpolyposis colon cancer syndromes. In the 1990s Vogelstein and others first described the molecular pathways that lead to colon cancer – and the rest is history.
I was a young faculty gastroenterologist at the Minneapolis VA Medical Center when one day my phone rang; it was Henry Lynch. He wanted to alert me that one of his patients was coming to me for surveillance colonoscopy. He explained the importance of what I was to do and how I should follow this man. I was overwhelmed by his attention to his patient (one of thousands) and his kindness to me. I had the privilege of traveling with him as visiting professors on a trip to South America. He was one of the kindest, most intelligent, and gracious persons I had ever met. I never forgot that experience.
We owe a lot to scientists, clinicians, and thought leaders like Henry Lynch who provide us the scientific basis of the care we give our patients.
John I. Allen, MD, MBA, AGAF
Editor in Chief
It is with great sadness that we note the passing (June 2, 2019; age 91) of Dr. Henry Lynch. Dr. Lynch almost singlehandedly brought attention to the genetic syndrome that bears his name. In 1913 Aldred Warthin (pathology chair at the University of Michigan) first described family “G”, the family of his seamstress who had told him that her family all dies of cancer. She herself succumbed to endometrial cancer. A plaque commemorating Dr. Warthin hangs down the hallway from my office at Michigan. His report fell into obscurity until the 1960s when Dr. Lynch arranged a reunion of family G in Ann Arbor, leading to a detailed update of the family in 1971. He recognized the autosomal dominance of the pedigree pattern.
In 1973, C. Richard Boland, MD, AGAF (past AGA President), wrote a medical school thesis entitled “A Familial Cancer Syndrome” and subsequently published two papers in which he first used the term “Lynch syndrome (I and II). Dr. Boland (whose family also carried a Lynch syndrome variant) spent his career adding to our molecular and clinical knowledge about nonpolyposis colon cancer syndromes. In the 1990s Vogelstein and others first described the molecular pathways that lead to colon cancer – and the rest is history.
I was a young faculty gastroenterologist at the Minneapolis VA Medical Center when one day my phone rang; it was Henry Lynch. He wanted to alert me that one of his patients was coming to me for surveillance colonoscopy. He explained the importance of what I was to do and how I should follow this man. I was overwhelmed by his attention to his patient (one of thousands) and his kindness to me. I had the privilege of traveling with him as visiting professors on a trip to South America. He was one of the kindest, most intelligent, and gracious persons I had ever met. I never forgot that experience.
We owe a lot to scientists, clinicians, and thought leaders like Henry Lynch who provide us the scientific basis of the care we give our patients.
John I. Allen, MD, MBA, AGAF
Editor in Chief
“Cupping” With Pain
A 30-year-old woman with a history of chronic overexposure to UV light presents to dermatology for a routine skin exam. The patient has a history of poor toleration to UV light, especially as a child, but participated in regular tanning as a teen. However, she stopped tanning when her sister developed a melanoma.
Additionally, the patient has been experiencing upper back pain, for which she has seen a variety of providers. Most recently, she consulted a naturopath, who recommended cupping therapy. Although the patient believes the therapy is alleviating her pain, she is distressed by the subsequent formation of large blemishes on her back and asks about possible treatment.
EXAMINATION
There are 10 large round patches, each measuring 7 cm in diameter, on the patient’s back. These patches consist of multiple petechiae and brown hyperpigmentation. On palpation, there is no surface disturbance or tenderness. The discoloration is nonblanchable. The size, shape, and configuration of the lesions is consistent with the patient's description of the cupping procedures she has undergone on several occasions.
Notably, the patient's skin is categorized as type II on the Fitzpatrick scale, with advanced dermatoheliosis.
What’s the diagnosis?
DISCUSSION
"Cupping," as medical therapy, was first described in ancient texts 3000 to 4000 years ago. The application of cups to the patient’s skin was intended to draw out substances (eg, toxins and fluids) inside the body that were believed to cause a variety of ailments. Though its use has long since been discarded in mainstream medicine, it is still used routinely in both Chinese and alternative medicine.
Cupping has been evaluated by numerous medical individuals and organizations, who uniformly dismiss any benefit it might offer, even as a placebo. From a pathophysiologic standpoint, cupping causes localized dilation of blood and lymph vessels, thus creating telangiectasia that, as they resolve, leave behind postinflammatory hyperpigmentation and edema. (Excessive production of telangiectasia might indicate pathologic capillary fragility, possibly secondary to Rumpel-Leede phenomenon.)
The patient's skin type can affect the rate of resolution (longer for those with darker skin, shorter for those with fair skin); there is little we can do to speed up this process. Although the case patient was disappointed with the lack of available treatment for her blemishes, she was insistent about continuing the cupping therapy.
Interestingly, there is a differential diagnosis for such lesions; it includes injury from tennis balls, racquetballs, paintballs, or even baseballs—though the associated lesions are usually solitary.
TAKE-HOME LEARNING POINTS
- Cupping, as medical therapy, has been around for thousands of years and is still routinely used in both Chinese and alternative medicine.
- The intention of its use is to draw out noxious substances that purportedly cause the patient's complaint—however, according to numerous medical authorities, the practice is totally ineffective.
- The suction effect of cupping induces edema and telangiectasia, which in turn results in postinflammatory hyperpigmentation that clears slowly.
- Similar lesions can result from being struck by paintballs, racquetballs, tennis balls, and baseballs.
A 30-year-old woman with a history of chronic overexposure to UV light presents to dermatology for a routine skin exam. The patient has a history of poor toleration to UV light, especially as a child, but participated in regular tanning as a teen. However, she stopped tanning when her sister developed a melanoma.
Additionally, the patient has been experiencing upper back pain, for which she has seen a variety of providers. Most recently, she consulted a naturopath, who recommended cupping therapy. Although the patient believes the therapy is alleviating her pain, she is distressed by the subsequent formation of large blemishes on her back and asks about possible treatment.

EXAMINATION
There are 10 large round patches, each measuring 7 cm in diameter, on the patient’s back. These patches consist of multiple petechiae and brown hyperpigmentation. On palpation, there is no surface disturbance or tenderness. The discoloration is nonblanchable. The size, shape, and configuration of the lesions is consistent with the patient's description of the cupping procedures she has undergone on several occasions.
Notably, the patient's skin is categorized as type II on the Fitzpatrick scale, with advanced dermatoheliosis.
What’s the diagnosis?
DISCUSSION
"Cupping," as medical therapy, was first described in ancient texts 3000 to 4000 years ago. The application of cups to the patient’s skin was intended to draw out substances (eg, toxins and fluids) inside the body that were believed to cause a variety of ailments. Though its use has long since been discarded in mainstream medicine, it is still used routinely in both Chinese and alternative medicine.
Cupping has been evaluated by numerous medical individuals and organizations, who uniformly dismiss any benefit it might offer, even as a placebo. From a pathophysiologic standpoint, cupping causes localized dilation of blood and lymph vessels, thus creating telangiectasia that, as they resolve, leave behind postinflammatory hyperpigmentation and edema. (Excessive production of telangiectasia might indicate pathologic capillary fragility, possibly secondary to Rumpel-Leede phenomenon.)
The patient's skin type can affect the rate of resolution (longer for those with darker skin, shorter for those with fair skin); there is little we can do to speed up this process. Although the case patient was disappointed with the lack of available treatment for her blemishes, she was insistent about continuing the cupping therapy.
Interestingly, there is a differential diagnosis for such lesions; it includes injury from tennis balls, racquetballs, paintballs, or even baseballs—though the associated lesions are usually solitary.
TAKE-HOME LEARNING POINTS
- Cupping, as medical therapy, has been around for thousands of years and is still routinely used in both Chinese and alternative medicine.
- The intention of its use is to draw out noxious substances that purportedly cause the patient's complaint—however, according to numerous medical authorities, the practice is totally ineffective.
- The suction effect of cupping induces edema and telangiectasia, which in turn results in postinflammatory hyperpigmentation that clears slowly.
- Similar lesions can result from being struck by paintballs, racquetballs, tennis balls, and baseballs.
A 30-year-old woman with a history of chronic overexposure to UV light presents to dermatology for a routine skin exam. The patient has a history of poor toleration to UV light, especially as a child, but participated in regular tanning as a teen. However, she stopped tanning when her sister developed a melanoma.
Additionally, the patient has been experiencing upper back pain, for which she has seen a variety of providers. Most recently, she consulted a naturopath, who recommended cupping therapy. Although the patient believes the therapy is alleviating her pain, she is distressed by the subsequent formation of large blemishes on her back and asks about possible treatment.

EXAMINATION
There are 10 large round patches, each measuring 7 cm in diameter, on the patient’s back. These patches consist of multiple petechiae and brown hyperpigmentation. On palpation, there is no surface disturbance or tenderness. The discoloration is nonblanchable. The size, shape, and configuration of the lesions is consistent with the patient's description of the cupping procedures she has undergone on several occasions.
Notably, the patient's skin is categorized as type II on the Fitzpatrick scale, with advanced dermatoheliosis.
What’s the diagnosis?
DISCUSSION
"Cupping," as medical therapy, was first described in ancient texts 3000 to 4000 years ago. The application of cups to the patient’s skin was intended to draw out substances (eg, toxins and fluids) inside the body that were believed to cause a variety of ailments. Though its use has long since been discarded in mainstream medicine, it is still used routinely in both Chinese and alternative medicine.
Cupping has been evaluated by numerous medical individuals and organizations, who uniformly dismiss any benefit it might offer, even as a placebo. From a pathophysiologic standpoint, cupping causes localized dilation of blood and lymph vessels, thus creating telangiectasia that, as they resolve, leave behind postinflammatory hyperpigmentation and edema. (Excessive production of telangiectasia might indicate pathologic capillary fragility, possibly secondary to Rumpel-Leede phenomenon.)
The patient's skin type can affect the rate of resolution (longer for those with darker skin, shorter for those with fair skin); there is little we can do to speed up this process. Although the case patient was disappointed with the lack of available treatment for her blemishes, she was insistent about continuing the cupping therapy.
Interestingly, there is a differential diagnosis for such lesions; it includes injury from tennis balls, racquetballs, paintballs, or even baseballs—though the associated lesions are usually solitary.
TAKE-HOME LEARNING POINTS
- Cupping, as medical therapy, has been around for thousands of years and is still routinely used in both Chinese and alternative medicine.
- The intention of its use is to draw out noxious substances that purportedly cause the patient's complaint—however, according to numerous medical authorities, the practice is totally ineffective.
- The suction effect of cupping induces edema and telangiectasia, which in turn results in postinflammatory hyperpigmentation that clears slowly.
- Similar lesions can result from being struck by paintballs, racquetballs, tennis balls, and baseballs.
A solution for reducing referrals (and malpractice suits)
I agree with Dr. Hickner’s editorial “To refer—or not?” (J Fam Pract. 2019;68:8) that family physicians could manage about 30% of the patients they refer to specialists. Still, it’s worth noting that many referrals are motivated by the threat of unmerited malpractice suits. Until the medical liability system becomes less adversarial and unmerited suits are eliminated, all primary care doctors—not just family physicians—will continue to send patients to specialists—even when these physicians are themselves capable of treating such patients.
What might help mitigate malpractice suits? There could be benefit from oversight of health courts, which would be presided over by judges with special training in medical malpractice. Being nonadversarial, health courts would cut down on legal wrangling, settle suits, and get awards to patients quicker. They would also cut down on attorney and court fees, which account for almost half of the total amount spent on litigation. These courts wouldn’t completely eliminate unnecessary referrals to specialists, but they could help make a difference.
Edward Volpintesta, MD
Bethel, Conn
I agree with Dr. Hickner’s editorial “To refer—or not?” (J Fam Pract. 2019;68:8) that family physicians could manage about 30% of the patients they refer to specialists. Still, it’s worth noting that many referrals are motivated by the threat of unmerited malpractice suits. Until the medical liability system becomes less adversarial and unmerited suits are eliminated, all primary care doctors—not just family physicians—will continue to send patients to specialists—even when these physicians are themselves capable of treating such patients.
What might help mitigate malpractice suits? There could be benefit from oversight of health courts, which would be presided over by judges with special training in medical malpractice. Being nonadversarial, health courts would cut down on legal wrangling, settle suits, and get awards to patients quicker. They would also cut down on attorney and court fees, which account for almost half of the total amount spent on litigation. These courts wouldn’t completely eliminate unnecessary referrals to specialists, but they could help make a difference.
Edward Volpintesta, MD
Bethel, Conn
I agree with Dr. Hickner’s editorial “To refer—or not?” (J Fam Pract. 2019;68:8) that family physicians could manage about 30% of the patients they refer to specialists. Still, it’s worth noting that many referrals are motivated by the threat of unmerited malpractice suits. Until the medical liability system becomes less adversarial and unmerited suits are eliminated, all primary care doctors—not just family physicians—will continue to send patients to specialists—even when these physicians are themselves capable of treating such patients.
What might help mitigate malpractice suits? There could be benefit from oversight of health courts, which would be presided over by judges with special training in medical malpractice. Being nonadversarial, health courts would cut down on legal wrangling, settle suits, and get awards to patients quicker. They would also cut down on attorney and court fees, which account for almost half of the total amount spent on litigation. These courts wouldn’t completely eliminate unnecessary referrals to specialists, but they could help make a difference.
Edward Volpintesta, MD
Bethel, Conn
Acute hearing loss, tinnitus, and fullness in the left ear • Weber test lateralized to the right ear • Positive Rinne test and normal tympanometry • Dx?
THE CASE
A healthy 48-year-old man presented to our otolaryngology clinic with a 2-hour history of hearing loss, tinnitus, and fullness in the left ear. He denied any vertigo, nausea, vomiting, otalgia, or otorrhea. He had noticed signs of a possible upper respiratory infection, including a sore throat and headache, the day before his symptoms started. His medical history was unremarkable. He denied any history of otologic surgery, trauma, or vision problems, and he was not taking any medications.
The patient was afebrile on physical examination with a heart rate of 48 beats/min and blood pressure of 117/68 mm Hg. A Weber test performed using a 512-Hz tuning fork lateralized to the right ear. A Rinne test showed air conduction was louder than bone conduction in the affected left ear—a normal finding. Tympanometry and otoscopic examination showed the bilateral tympanic membranes were normal.
THE DIAGNOSIS
Pure tone audiometry showed severe sensorineural hearing loss in the left ear and a poor speech discrimination score. The Weber test confirmed the hearing loss was sensorineural and not conductive, ruling out a middle ear effusion. Additionally, the normal tympanogram made conductive hearing loss from a middle ear effusion or tympanic membrane perforation unlikely. The positive Rinne test was consistent with a diagnosis of idiopathic sudden sensorineural hearing loss (SSNHL).
DISCUSSION
SSNHL is defined by hearing loss of more than 30 dB in at least 3 consecutive frequencies with acute onset of less than 72 hours.1,2 The most common symptoms include acute hearing loss, tinnitus, and fullness in the affected ear.1 The majority of cases of SSNHL are unilateral. The typical age of onset is in the fourth and fifth decades, occurring with equal distribution in both sexes.
Etiology.
Diagnosis. The initial evaluation should include an otoscopic examination, tuning fork tests, and pure tone audiometry.1-3 Weber and Rinne tests are essential when evaluating patients for unilateral hearing loss and determining the type of loss (ie, sensorineural vs conductive). The Weber test (ideally using a 512-Hz tuning fork) can detect either conductive or sensorineural hearing loss. In a normal Weber test, the patient should hear the vibration of the tuning fork equally in both ears. The tuning fork will be heard in both ears in conductive hearing loss but will only be heard in the unaffected hear if sensorineural hearing loss is present. So, for instance, if a patient has a perforation in the right tympanic membrane causing conductive hearing loss in the right hear, the tuning fork would be heard in both ears. If the patient has sensorineural hearing loss in the right ear, the tuning fork would only be heard in the left ear.
The Rinne test compares the perception of sound waves transmitted by air conduction vs bone conduction and serves as a rapid screen for conductive hearing loss.
Continue to: Magnetic resonance imaging...
Magnetic resonance imaging (MRI) of the brain and brainstem with gadolinium contrast can reveal vascular events (thrombotic or hemorrhagic), demyelinating disorders, or retrocochlear lesions such as vestibular schwannoma and is indicated in all cases of suspected SSNHL.4,5
Treatment and management. The current standard of care for treatment of idiopathic SSNHL is systemic steroids.1,2 Although the gold standard currently is oral prednisolone or methylprednisolone (1 mg/kg/d for 10 to 14 days with a taper,1,2 the evidence for this regimen stems from a single placebo-controlled trial (N = 67) that demonstrated greater improvement in the steroid group compared with the placebo group (61% vs 32%).6 A Cochrane review and other systematic analyses have not demonstrated clear efficacy of corticosteroid treatment for the management of idiopathic SSNHL.7,8
Because of the potential systemic adverse effects associated with oral corticosteroids, intratympanic (IT) corticosteroids have been advocated as an alternative treatment option. A prospective, randomized, noninferiority trial comparing the efficacy of oral vs IT corticosteroids for idiopathic SSNHL found IT corticosteroids to be noninferior to systemic treatment.9 IT treatment also has been advocated as a rescue therapy for patients who do not respond to systemic treatment.10
A combination of oral and IT corticosteroids was investigated in a retrospective study analyzing multiple treatment modalities.10 Researchers first compared 122 patients receiving one of 3 treatments: (1) IT corticosteroids, (2) oral corticosteroids, and (3) combination treatment (IT + oral corticosteroids). There was no difference in hearing recovery among any of the treatments. Fifty-eight patients who were refractory to initial treatment were then included in a second analysis in which they were divided into those who received additional IT corticosteroids (salvage treatment) vs no treatment (control). There was no difference in hearing recovery between the 2 groups. The authors concluded that IT corticosteroids were as effective as oral treatment and that salvage IT treatment did not add any benefit.10
The American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) recently published guidelines on the diagnosis and management of SSNHL.11 The guidelines state that IT steroids should be considered in patients who cannot tolerate oral steroids, such as patients with diabetes. It is important to note, however, that the high cost of IT treatment (~$2000 for dexamethasone or methylprednisolone vs < $10 for oral prednisolone) is an issue that needs to be considered as health care costs continue to rise.
Continue to: Antivirals
Antivirals. Because an underlying viral etiology has been speculated as a potential cause of idiopathic SSNHL, antiviral agents such as valacyclovir or famciclovir also are potential treatment agents.12 Antiviral medications have minimal adverse effects and are relatively inexpensive, but the benefits have not yet been proven in randomized controlled trials,and they currently are not endorsed by the AAO-HNS in their guidelines for the management of SSNHL.11
Spontaneous recovery occurs in up to 40% of patients with idiopathic SSNHL. As many as 65% of those who experience recovery do so within 2 weeks of the onset of symptoms, regardless of treatment.1,2 Treatment beyond 2 weeks after onset of symptoms is unlikely to be of any benefit, although some otolaryngologists will treat for up to 6 weeks after the onset of hearing loss.
A substantial number of patients with SSNHL may not recover. Management of these patients begins with referral to an appropriate specialist to initiate counseling and lifestyle changes. Depending on the degree of hearing loss, audiologic rehabilitation may include use of a traditional or bone-anchored hearing aid or a frequency-modulation system.1,2,11 Tinnitus retraining therapy might be of benefit for patients with persistent tinnitus.11
Our patient. After a discussion of his treatment options, our patient decided on a combination of oral prednisolone (60 mg once daily for 9 days followed by a taper for 5 days) and intratympanic dexamethasone injections (1 mL [10 mg/mL] once weekly for 3 weeks).
The rationale for this approach was the minimal adverse effects associated with short-term (ie, days to 1–2 weeks) use of high-dose (ie, > 30 mg/d) corticosteroids. Although steroid therapy has been associated with adverse effects such as aseptic necrosis of the hip, these complications usually arise after longer periods (ie, months to years) of high-dose steroid therapy with a mean cumulative dose much higher than what was used in our patient.13
Continue to: Our patient...
Our patient noticed slight improvement within 48 hours of the initial onset of symptoms that continued for the next several weeks until full recovery was attained. An MRI performed 5 days after the onset of symptoms was negative for retrocochlear pathology.
THE TAKEAWAY
SSNHL is a medical emergency that requires prompt recognition and diagnosis. The steps in evaluating sudden hearing loss include: (1) appropriate history and physical examination (eg, otoscopic examination, tuning fork tests), (2) urgent audiometry to confirm hearing loss, (3) immediate referral to an otolaryngologist for further testing (eg, tympanometry, blood tests, MRI), and (4) initiation of treatment.
If a specific etiology is identified (eg, vestibular schwannoma), the patient should be referred to a specialist for appropriate treatment. If there is no identifiable cause (idiopathic SSNHL), the patient should be treated with oral and/or intratympanic steroids. Patients who do not recover following treatment should be offered audiologic rehabilitation.
CORRESPONDENCE
Sergio Huerta, MD, UT Southwestern Medical Center, 4500 S Lancaster Road #112L, Dallas, TX 75216; [email protected]
1. Schreiber BE, Agrup C, Haskard DO, et al. Sudden sensorineural hearing loss. Lancet. 2010;375:1203-1211.
2. Rauch SD. Clinical practice. Idiopathic sudden sensorineural hearing loss. N Engl J Med. 2008;359:833-840.
3. Paul BC, Roland JT Jr. An abnormal audiogram. JAMA. 2015;313:85-86.
4. Aarnisalo AA, Suoranta H, Ylikoski J. Magnetic resonance imaging findings in the auditory pathway of patients with sudden deafness. Otol Neurotol. 2004;25:245-249.
5. Cadoni G, Cianfoni A, Agostino S, et al. Magnetic resonance imaging findings in sudden sensorineural hearing loss. J Otolaryngol. 2006;35:310-316.
6. Wilson WR, Byl FM, Laird N. The efficacy of steroids in the treatment of idiopathic sudden hearing loss. A double-blind clinical study. Arch Otolaryngol. 1980;106:772-776.
7. Wei BPC, Stathopoulos D, O’Leary S. Steroids for idiopathic sudden sensorineural hearing loss. Cochrane Database Syst Rev. 2013. doi:10.1002/14651858.CD003998.pub3.
8. Conlin AE, Parnes LS. Treatment of sudden sensorineural hearing loss: II. a meta-analysis. Arch Otolaryngol Head Neck Surg. 2007;133:582-586.
9. Rauch SD, Halpin CF, Antonelli PJ, et al. Oral vs intratympanic corticosteroid therapy for idiopathic sudden sensorineural hearing loss: a randomized trial. JAMA. 2011;305:2071-2079.
10. Lee KH, Ryu SH, Lee HM, et al. Is intratympanic dexamethasone injection effective for the treatment of idiopathic sudden sensorineural hearing loss? J Audiol Otol. 2015;19:154-158.
11. Stachler RJ, Chandrasekhar SS, Archer SM, et al. Clinical practice guideline: sudden hearing loss. Otolaryngol Head Neck Surg. 2012;146(3 suppl):S1-S35.
12. Westerlaken BO, Stokroos RJ, Dhooge IJ, et al. Treatment of idiopathic sudden sensorineural hearing loss with antiviral therapy: a prospective, randomized, double-blind clinical trial. Ann Otol Rhinol Laryngol. 2003;112:993-1000.
13. Nowak DA, Yeung J. Steroid-induced osteonecrosis in dermatology: a review [published online March 30, 2015]. J Cutan Med Surg. 2015;19:358-360.
THE CASE
A healthy 48-year-old man presented to our otolaryngology clinic with a 2-hour history of hearing loss, tinnitus, and fullness in the left ear. He denied any vertigo, nausea, vomiting, otalgia, or otorrhea. He had noticed signs of a possible upper respiratory infection, including a sore throat and headache, the day before his symptoms started. His medical history was unremarkable. He denied any history of otologic surgery, trauma, or vision problems, and he was not taking any medications.
The patient was afebrile on physical examination with a heart rate of 48 beats/min and blood pressure of 117/68 mm Hg. A Weber test performed using a 512-Hz tuning fork lateralized to the right ear. A Rinne test showed air conduction was louder than bone conduction in the affected left ear—a normal finding. Tympanometry and otoscopic examination showed the bilateral tympanic membranes were normal.
THE DIAGNOSIS
Pure tone audiometry showed severe sensorineural hearing loss in the left ear and a poor speech discrimination score. The Weber test confirmed the hearing loss was sensorineural and not conductive, ruling out a middle ear effusion. Additionally, the normal tympanogram made conductive hearing loss from a middle ear effusion or tympanic membrane perforation unlikely. The positive Rinne test was consistent with a diagnosis of idiopathic sudden sensorineural hearing loss (SSNHL).
DISCUSSION
SSNHL is defined by hearing loss of more than 30 dB in at least 3 consecutive frequencies with acute onset of less than 72 hours.1,2 The most common symptoms include acute hearing loss, tinnitus, and fullness in the affected ear.1 The majority of cases of SSNHL are unilateral. The typical age of onset is in the fourth and fifth decades, occurring with equal distribution in both sexes.
Etiology.
Diagnosis. The initial evaluation should include an otoscopic examination, tuning fork tests, and pure tone audiometry.1-3 Weber and Rinne tests are essential when evaluating patients for unilateral hearing loss and determining the type of loss (ie, sensorineural vs conductive). The Weber test (ideally using a 512-Hz tuning fork) can detect either conductive or sensorineural hearing loss. In a normal Weber test, the patient should hear the vibration of the tuning fork equally in both ears. The tuning fork will be heard in both ears in conductive hearing loss but will only be heard in the unaffected hear if sensorineural hearing loss is present. So, for instance, if a patient has a perforation in the right tympanic membrane causing conductive hearing loss in the right hear, the tuning fork would be heard in both ears. If the patient has sensorineural hearing loss in the right ear, the tuning fork would only be heard in the left ear.
The Rinne test compares the perception of sound waves transmitted by air conduction vs bone conduction and serves as a rapid screen for conductive hearing loss.
Continue to: Magnetic resonance imaging...
Magnetic resonance imaging (MRI) of the brain and brainstem with gadolinium contrast can reveal vascular events (thrombotic or hemorrhagic), demyelinating disorders, or retrocochlear lesions such as vestibular schwannoma and is indicated in all cases of suspected SSNHL.4,5
Treatment and management. The current standard of care for treatment of idiopathic SSNHL is systemic steroids.1,2 Although the gold standard currently is oral prednisolone or methylprednisolone (1 mg/kg/d for 10 to 14 days with a taper,1,2 the evidence for this regimen stems from a single placebo-controlled trial (N = 67) that demonstrated greater improvement in the steroid group compared with the placebo group (61% vs 32%).6 A Cochrane review and other systematic analyses have not demonstrated clear efficacy of corticosteroid treatment for the management of idiopathic SSNHL.7,8
Because of the potential systemic adverse effects associated with oral corticosteroids, intratympanic (IT) corticosteroids have been advocated as an alternative treatment option. A prospective, randomized, noninferiority trial comparing the efficacy of oral vs IT corticosteroids for idiopathic SSNHL found IT corticosteroids to be noninferior to systemic treatment.9 IT treatment also has been advocated as a rescue therapy for patients who do not respond to systemic treatment.10
A combination of oral and IT corticosteroids was investigated in a retrospective study analyzing multiple treatment modalities.10 Researchers first compared 122 patients receiving one of 3 treatments: (1) IT corticosteroids, (2) oral corticosteroids, and (3) combination treatment (IT + oral corticosteroids). There was no difference in hearing recovery among any of the treatments. Fifty-eight patients who were refractory to initial treatment were then included in a second analysis in which they were divided into those who received additional IT corticosteroids (salvage treatment) vs no treatment (control). There was no difference in hearing recovery between the 2 groups. The authors concluded that IT corticosteroids were as effective as oral treatment and that salvage IT treatment did not add any benefit.10
The American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) recently published guidelines on the diagnosis and management of SSNHL.11 The guidelines state that IT steroids should be considered in patients who cannot tolerate oral steroids, such as patients with diabetes. It is important to note, however, that the high cost of IT treatment (~$2000 for dexamethasone or methylprednisolone vs < $10 for oral prednisolone) is an issue that needs to be considered as health care costs continue to rise.
Continue to: Antivirals
Antivirals. Because an underlying viral etiology has been speculated as a potential cause of idiopathic SSNHL, antiviral agents such as valacyclovir or famciclovir also are potential treatment agents.12 Antiviral medications have minimal adverse effects and are relatively inexpensive, but the benefits have not yet been proven in randomized controlled trials,and they currently are not endorsed by the AAO-HNS in their guidelines for the management of SSNHL.11
Spontaneous recovery occurs in up to 40% of patients with idiopathic SSNHL. As many as 65% of those who experience recovery do so within 2 weeks of the onset of symptoms, regardless of treatment.1,2 Treatment beyond 2 weeks after onset of symptoms is unlikely to be of any benefit, although some otolaryngologists will treat for up to 6 weeks after the onset of hearing loss.
A substantial number of patients with SSNHL may not recover. Management of these patients begins with referral to an appropriate specialist to initiate counseling and lifestyle changes. Depending on the degree of hearing loss, audiologic rehabilitation may include use of a traditional or bone-anchored hearing aid or a frequency-modulation system.1,2,11 Tinnitus retraining therapy might be of benefit for patients with persistent tinnitus.11
Our patient. After a discussion of his treatment options, our patient decided on a combination of oral prednisolone (60 mg once daily for 9 days followed by a taper for 5 days) and intratympanic dexamethasone injections (1 mL [10 mg/mL] once weekly for 3 weeks).
The rationale for this approach was the minimal adverse effects associated with short-term (ie, days to 1–2 weeks) use of high-dose (ie, > 30 mg/d) corticosteroids. Although steroid therapy has been associated with adverse effects such as aseptic necrosis of the hip, these complications usually arise after longer periods (ie, months to years) of high-dose steroid therapy with a mean cumulative dose much higher than what was used in our patient.13
Continue to: Our patient...
Our patient noticed slight improvement within 48 hours of the initial onset of symptoms that continued for the next several weeks until full recovery was attained. An MRI performed 5 days after the onset of symptoms was negative for retrocochlear pathology.
THE TAKEAWAY
SSNHL is a medical emergency that requires prompt recognition and diagnosis. The steps in evaluating sudden hearing loss include: (1) appropriate history and physical examination (eg, otoscopic examination, tuning fork tests), (2) urgent audiometry to confirm hearing loss, (3) immediate referral to an otolaryngologist for further testing (eg, tympanometry, blood tests, MRI), and (4) initiation of treatment.
If a specific etiology is identified (eg, vestibular schwannoma), the patient should be referred to a specialist for appropriate treatment. If there is no identifiable cause (idiopathic SSNHL), the patient should be treated with oral and/or intratympanic steroids. Patients who do not recover following treatment should be offered audiologic rehabilitation.
CORRESPONDENCE
Sergio Huerta, MD, UT Southwestern Medical Center, 4500 S Lancaster Road #112L, Dallas, TX 75216; [email protected]
THE CASE
A healthy 48-year-old man presented to our otolaryngology clinic with a 2-hour history of hearing loss, tinnitus, and fullness in the left ear. He denied any vertigo, nausea, vomiting, otalgia, or otorrhea. He had noticed signs of a possible upper respiratory infection, including a sore throat and headache, the day before his symptoms started. His medical history was unremarkable. He denied any history of otologic surgery, trauma, or vision problems, and he was not taking any medications.
The patient was afebrile on physical examination with a heart rate of 48 beats/min and blood pressure of 117/68 mm Hg. A Weber test performed using a 512-Hz tuning fork lateralized to the right ear. A Rinne test showed air conduction was louder than bone conduction in the affected left ear—a normal finding. Tympanometry and otoscopic examination showed the bilateral tympanic membranes were normal.
THE DIAGNOSIS
Pure tone audiometry showed severe sensorineural hearing loss in the left ear and a poor speech discrimination score. The Weber test confirmed the hearing loss was sensorineural and not conductive, ruling out a middle ear effusion. Additionally, the normal tympanogram made conductive hearing loss from a middle ear effusion or tympanic membrane perforation unlikely. The positive Rinne test was consistent with a diagnosis of idiopathic sudden sensorineural hearing loss (SSNHL).
DISCUSSION
SSNHL is defined by hearing loss of more than 30 dB in at least 3 consecutive frequencies with acute onset of less than 72 hours.1,2 The most common symptoms include acute hearing loss, tinnitus, and fullness in the affected ear.1 The majority of cases of SSNHL are unilateral. The typical age of onset is in the fourth and fifth decades, occurring with equal distribution in both sexes.
Etiology.
Diagnosis. The initial evaluation should include an otoscopic examination, tuning fork tests, and pure tone audiometry.1-3 Weber and Rinne tests are essential when evaluating patients for unilateral hearing loss and determining the type of loss (ie, sensorineural vs conductive). The Weber test (ideally using a 512-Hz tuning fork) can detect either conductive or sensorineural hearing loss. In a normal Weber test, the patient should hear the vibration of the tuning fork equally in both ears. The tuning fork will be heard in both ears in conductive hearing loss but will only be heard in the unaffected hear if sensorineural hearing loss is present. So, for instance, if a patient has a perforation in the right tympanic membrane causing conductive hearing loss in the right hear, the tuning fork would be heard in both ears. If the patient has sensorineural hearing loss in the right ear, the tuning fork would only be heard in the left ear.
The Rinne test compares the perception of sound waves transmitted by air conduction vs bone conduction and serves as a rapid screen for conductive hearing loss.
Continue to: Magnetic resonance imaging...
Magnetic resonance imaging (MRI) of the brain and brainstem with gadolinium contrast can reveal vascular events (thrombotic or hemorrhagic), demyelinating disorders, or retrocochlear lesions such as vestibular schwannoma and is indicated in all cases of suspected SSNHL.4,5
Treatment and management. The current standard of care for treatment of idiopathic SSNHL is systemic steroids.1,2 Although the gold standard currently is oral prednisolone or methylprednisolone (1 mg/kg/d for 10 to 14 days with a taper,1,2 the evidence for this regimen stems from a single placebo-controlled trial (N = 67) that demonstrated greater improvement in the steroid group compared with the placebo group (61% vs 32%).6 A Cochrane review and other systematic analyses have not demonstrated clear efficacy of corticosteroid treatment for the management of idiopathic SSNHL.7,8
Because of the potential systemic adverse effects associated with oral corticosteroids, intratympanic (IT) corticosteroids have been advocated as an alternative treatment option. A prospective, randomized, noninferiority trial comparing the efficacy of oral vs IT corticosteroids for idiopathic SSNHL found IT corticosteroids to be noninferior to systemic treatment.9 IT treatment also has been advocated as a rescue therapy for patients who do not respond to systemic treatment.10
A combination of oral and IT corticosteroids was investigated in a retrospective study analyzing multiple treatment modalities.10 Researchers first compared 122 patients receiving one of 3 treatments: (1) IT corticosteroids, (2) oral corticosteroids, and (3) combination treatment (IT + oral corticosteroids). There was no difference in hearing recovery among any of the treatments. Fifty-eight patients who were refractory to initial treatment were then included in a second analysis in which they were divided into those who received additional IT corticosteroids (salvage treatment) vs no treatment (control). There was no difference in hearing recovery between the 2 groups. The authors concluded that IT corticosteroids were as effective as oral treatment and that salvage IT treatment did not add any benefit.10
The American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) recently published guidelines on the diagnosis and management of SSNHL.11 The guidelines state that IT steroids should be considered in patients who cannot tolerate oral steroids, such as patients with diabetes. It is important to note, however, that the high cost of IT treatment (~$2000 for dexamethasone or methylprednisolone vs < $10 for oral prednisolone) is an issue that needs to be considered as health care costs continue to rise.
Continue to: Antivirals
Antivirals. Because an underlying viral etiology has been speculated as a potential cause of idiopathic SSNHL, antiviral agents such as valacyclovir or famciclovir also are potential treatment agents.12 Antiviral medications have minimal adverse effects and are relatively inexpensive, but the benefits have not yet been proven in randomized controlled trials,and they currently are not endorsed by the AAO-HNS in their guidelines for the management of SSNHL.11
Spontaneous recovery occurs in up to 40% of patients with idiopathic SSNHL. As many as 65% of those who experience recovery do so within 2 weeks of the onset of symptoms, regardless of treatment.1,2 Treatment beyond 2 weeks after onset of symptoms is unlikely to be of any benefit, although some otolaryngologists will treat for up to 6 weeks after the onset of hearing loss.
A substantial number of patients with SSNHL may not recover. Management of these patients begins with referral to an appropriate specialist to initiate counseling and lifestyle changes. Depending on the degree of hearing loss, audiologic rehabilitation may include use of a traditional or bone-anchored hearing aid or a frequency-modulation system.1,2,11 Tinnitus retraining therapy might be of benefit for patients with persistent tinnitus.11
Our patient. After a discussion of his treatment options, our patient decided on a combination of oral prednisolone (60 mg once daily for 9 days followed by a taper for 5 days) and intratympanic dexamethasone injections (1 mL [10 mg/mL] once weekly for 3 weeks).
The rationale for this approach was the minimal adverse effects associated with short-term (ie, days to 1–2 weeks) use of high-dose (ie, > 30 mg/d) corticosteroids. Although steroid therapy has been associated with adverse effects such as aseptic necrosis of the hip, these complications usually arise after longer periods (ie, months to years) of high-dose steroid therapy with a mean cumulative dose much higher than what was used in our patient.13
Continue to: Our patient...
Our patient noticed slight improvement within 48 hours of the initial onset of symptoms that continued for the next several weeks until full recovery was attained. An MRI performed 5 days after the onset of symptoms was negative for retrocochlear pathology.
THE TAKEAWAY
SSNHL is a medical emergency that requires prompt recognition and diagnosis. The steps in evaluating sudden hearing loss include: (1) appropriate history and physical examination (eg, otoscopic examination, tuning fork tests), (2) urgent audiometry to confirm hearing loss, (3) immediate referral to an otolaryngologist for further testing (eg, tympanometry, blood tests, MRI), and (4) initiation of treatment.
If a specific etiology is identified (eg, vestibular schwannoma), the patient should be referred to a specialist for appropriate treatment. If there is no identifiable cause (idiopathic SSNHL), the patient should be treated with oral and/or intratympanic steroids. Patients who do not recover following treatment should be offered audiologic rehabilitation.
CORRESPONDENCE
Sergio Huerta, MD, UT Southwestern Medical Center, 4500 S Lancaster Road #112L, Dallas, TX 75216; [email protected]
1. Schreiber BE, Agrup C, Haskard DO, et al. Sudden sensorineural hearing loss. Lancet. 2010;375:1203-1211.
2. Rauch SD. Clinical practice. Idiopathic sudden sensorineural hearing loss. N Engl J Med. 2008;359:833-840.
3. Paul BC, Roland JT Jr. An abnormal audiogram. JAMA. 2015;313:85-86.
4. Aarnisalo AA, Suoranta H, Ylikoski J. Magnetic resonance imaging findings in the auditory pathway of patients with sudden deafness. Otol Neurotol. 2004;25:245-249.
5. Cadoni G, Cianfoni A, Agostino S, et al. Magnetic resonance imaging findings in sudden sensorineural hearing loss. J Otolaryngol. 2006;35:310-316.
6. Wilson WR, Byl FM, Laird N. The efficacy of steroids in the treatment of idiopathic sudden hearing loss. A double-blind clinical study. Arch Otolaryngol. 1980;106:772-776.
7. Wei BPC, Stathopoulos D, O’Leary S. Steroids for idiopathic sudden sensorineural hearing loss. Cochrane Database Syst Rev. 2013. doi:10.1002/14651858.CD003998.pub3.
8. Conlin AE, Parnes LS. Treatment of sudden sensorineural hearing loss: II. a meta-analysis. Arch Otolaryngol Head Neck Surg. 2007;133:582-586.
9. Rauch SD, Halpin CF, Antonelli PJ, et al. Oral vs intratympanic corticosteroid therapy for idiopathic sudden sensorineural hearing loss: a randomized trial. JAMA. 2011;305:2071-2079.
10. Lee KH, Ryu SH, Lee HM, et al. Is intratympanic dexamethasone injection effective for the treatment of idiopathic sudden sensorineural hearing loss? J Audiol Otol. 2015;19:154-158.
11. Stachler RJ, Chandrasekhar SS, Archer SM, et al. Clinical practice guideline: sudden hearing loss. Otolaryngol Head Neck Surg. 2012;146(3 suppl):S1-S35.
12. Westerlaken BO, Stokroos RJ, Dhooge IJ, et al. Treatment of idiopathic sudden sensorineural hearing loss with antiviral therapy: a prospective, randomized, double-blind clinical trial. Ann Otol Rhinol Laryngol. 2003;112:993-1000.
13. Nowak DA, Yeung J. Steroid-induced osteonecrosis in dermatology: a review [published online March 30, 2015]. J Cutan Med Surg. 2015;19:358-360.
1. Schreiber BE, Agrup C, Haskard DO, et al. Sudden sensorineural hearing loss. Lancet. 2010;375:1203-1211.
2. Rauch SD. Clinical practice. Idiopathic sudden sensorineural hearing loss. N Engl J Med. 2008;359:833-840.
3. Paul BC, Roland JT Jr. An abnormal audiogram. JAMA. 2015;313:85-86.
4. Aarnisalo AA, Suoranta H, Ylikoski J. Magnetic resonance imaging findings in the auditory pathway of patients with sudden deafness. Otol Neurotol. 2004;25:245-249.
5. Cadoni G, Cianfoni A, Agostino S, et al. Magnetic resonance imaging findings in sudden sensorineural hearing loss. J Otolaryngol. 2006;35:310-316.
6. Wilson WR, Byl FM, Laird N. The efficacy of steroids in the treatment of idiopathic sudden hearing loss. A double-blind clinical study. Arch Otolaryngol. 1980;106:772-776.
7. Wei BPC, Stathopoulos D, O’Leary S. Steroids for idiopathic sudden sensorineural hearing loss. Cochrane Database Syst Rev. 2013. doi:10.1002/14651858.CD003998.pub3.
8. Conlin AE, Parnes LS. Treatment of sudden sensorineural hearing loss: II. a meta-analysis. Arch Otolaryngol Head Neck Surg. 2007;133:582-586.
9. Rauch SD, Halpin CF, Antonelli PJ, et al. Oral vs intratympanic corticosteroid therapy for idiopathic sudden sensorineural hearing loss: a randomized trial. JAMA. 2011;305:2071-2079.
10. Lee KH, Ryu SH, Lee HM, et al. Is intratympanic dexamethasone injection effective for the treatment of idiopathic sudden sensorineural hearing loss? J Audiol Otol. 2015;19:154-158.
11. Stachler RJ, Chandrasekhar SS, Archer SM, et al. Clinical practice guideline: sudden hearing loss. Otolaryngol Head Neck Surg. 2012;146(3 suppl):S1-S35.
12. Westerlaken BO, Stokroos RJ, Dhooge IJ, et al. Treatment of idiopathic sudden sensorineural hearing loss with antiviral therapy: a prospective, randomized, double-blind clinical trial. Ann Otol Rhinol Laryngol. 2003;112:993-1000.
13. Nowak DA, Yeung J. Steroid-induced osteonecrosis in dermatology: a review [published online March 30, 2015]. J Cutan Med Surg. 2015;19:358-360.
Can unintended pregnancies be reduced by dispensing a year’s worth of hormonal contraception?
EVIDENCE SUMMARY
A 2013 systematic review studied the effect of dispensing a larger amount of pills on pregnancy rate, abortion rate, and overall cost to the health care system.1 Three of the 4 studies analyzed found lower rates of pregnancy and abortion, as well as lower cost despite increased pill wastage, in the groups that received more medication. The 1 study that didn’t show a significant difference between groups compared only short durations (1 vs 4 months).
The systematic review included a large retrospective cohort study from 2011 that examined public insurance data from more than 84,000 patients to compare pregnancy rates in women who were given a 1-year supply of oral contraceptives (12 or 13 packs) vs those given 1 or 3 packs at a time.2 The study found pregnancy rates of 2.9%, 3.3%, and 1.2% for 1, 3, and 12 or 13 months, respectively (P < .05; absolute risk reduction [ARR] = 1.7%; number needed to treat [NNT] = 59; relative risk reduction = 41%).
More pills lead to longer use of contraception
The systematic review also included a 2011 trial of 700 women starting oral contraceptives.3 It randomized them to receive a 7- or 3-month supply at their initial visit, then evaluated use of oral contraception at 6 months. All women were invited back for a 3-month follow-up visit, at which time the 3-month supply group would receive additional medication.
Fifty-one percent of the 7-month group were still using oral contraceptives at 6 months compared with 35% of the 3-month group (P < .001; NNT = 7). The contrast was starker for women younger than 18 years (49% vs 12%; NNT = 3). Notably, of the women who stopped using contraception, more in the 3-month group stopped because they ran out of medication (P = .02). Subjects in the 7-month group were more likely to have given birth and more likely to have 2 or more children.
A 2017 case study examined proposed legislation in California that required health plans to cover a 12-month supply of combined hormonal contraceptives.4 The California Health Benefits Review Program surveyed health insurers and reviewed contraception usage patterns. They found that, if the legislation passed, the state could expect a 30% reduction in unintended pregnancy (ARR = 2%; NNT = 50), resulting in 6000 fewer live births and 7000 fewer abortions per year.
RECOMMENDATIONS
The Centers for Disease Control and Prevention (CDC)’s Selected Practice Recommendations for Contraceptive Use recommend prescribing or providing as much as a 1-year supply of combined hormonal contraceptives at the initial visit and each return visit.5
The American College of Obstetricians and Gynecologists (ACOG) supports over-the-counter access to oral contraceptives, effectively allowing an unlimited supply.6
EDITOR’S TAKEAWAY
Adequate evidence of benefits and strong support from the CDC and ACOG should encourage us to offer 1-year supplies of combined oral contraceptives. Even though the higher-quality studies reviewed also showed a cost savings, up-front patient expense may remain a challenge.
1. Steenland MW, Rodriguez MI, Marchbanks PA, et al. How does the number of oral contraceptive pill packs dispensed or prescribed affect continuation and other measures of consistent and correct use? A systematic review. Contraception. 2013;87:605-610.
2. Foster DG, Hulett D, Bradsberry M, et al. Number of oral contraceptive pill packages dispensed and subsequent unintended pregnancies. Obstet Gynecol. 2011;117:566-572.
3. White KO, Westhoff C. The effect of pack supply on oral contraceptive pill continuation: a randomized controlled trial. Obstet Gynecol. 2011;118:615-622.
4. McMenamin SB, Charles SA, Tabatabaeepour N, et al. Implications of dispensing self-administered hormonal contraceptives in a 1-year supply: a California case study. Contraception. 2017;95:449-451.
5. Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-66.
6. Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. Committee Opinion No. 544: Over-the-counter access to oral contraceptives. Obstet Gynecol. 2012;120:1527-1531.
EVIDENCE SUMMARY
A 2013 systematic review studied the effect of dispensing a larger amount of pills on pregnancy rate, abortion rate, and overall cost to the health care system.1 Three of the 4 studies analyzed found lower rates of pregnancy and abortion, as well as lower cost despite increased pill wastage, in the groups that received more medication. The 1 study that didn’t show a significant difference between groups compared only short durations (1 vs 4 months).
The systematic review included a large retrospective cohort study from 2011 that examined public insurance data from more than 84,000 patients to compare pregnancy rates in women who were given a 1-year supply of oral contraceptives (12 or 13 packs) vs those given 1 or 3 packs at a time.2 The study found pregnancy rates of 2.9%, 3.3%, and 1.2% for 1, 3, and 12 or 13 months, respectively (P < .05; absolute risk reduction [ARR] = 1.7%; number needed to treat [NNT] = 59; relative risk reduction = 41%).
More pills lead to longer use of contraception
The systematic review also included a 2011 trial of 700 women starting oral contraceptives.3 It randomized them to receive a 7- or 3-month supply at their initial visit, then evaluated use of oral contraception at 6 months. All women were invited back for a 3-month follow-up visit, at which time the 3-month supply group would receive additional medication.
Fifty-one percent of the 7-month group were still using oral contraceptives at 6 months compared with 35% of the 3-month group (P < .001; NNT = 7). The contrast was starker for women younger than 18 years (49% vs 12%; NNT = 3). Notably, of the women who stopped using contraception, more in the 3-month group stopped because they ran out of medication (P = .02). Subjects in the 7-month group were more likely to have given birth and more likely to have 2 or more children.
A 2017 case study examined proposed legislation in California that required health plans to cover a 12-month supply of combined hormonal contraceptives.4 The California Health Benefits Review Program surveyed health insurers and reviewed contraception usage patterns. They found that, if the legislation passed, the state could expect a 30% reduction in unintended pregnancy (ARR = 2%; NNT = 50), resulting in 6000 fewer live births and 7000 fewer abortions per year.
RECOMMENDATIONS
The Centers for Disease Control and Prevention (CDC)’s Selected Practice Recommendations for Contraceptive Use recommend prescribing or providing as much as a 1-year supply of combined hormonal contraceptives at the initial visit and each return visit.5
The American College of Obstetricians and Gynecologists (ACOG) supports over-the-counter access to oral contraceptives, effectively allowing an unlimited supply.6
EDITOR’S TAKEAWAY
Adequate evidence of benefits and strong support from the CDC and ACOG should encourage us to offer 1-year supplies of combined oral contraceptives. Even though the higher-quality studies reviewed also showed a cost savings, up-front patient expense may remain a challenge.
EVIDENCE SUMMARY
A 2013 systematic review studied the effect of dispensing a larger amount of pills on pregnancy rate, abortion rate, and overall cost to the health care system.1 Three of the 4 studies analyzed found lower rates of pregnancy and abortion, as well as lower cost despite increased pill wastage, in the groups that received more medication. The 1 study that didn’t show a significant difference between groups compared only short durations (1 vs 4 months).
The systematic review included a large retrospective cohort study from 2011 that examined public insurance data from more than 84,000 patients to compare pregnancy rates in women who were given a 1-year supply of oral contraceptives (12 or 13 packs) vs those given 1 or 3 packs at a time.2 The study found pregnancy rates of 2.9%, 3.3%, and 1.2% for 1, 3, and 12 or 13 months, respectively (P < .05; absolute risk reduction [ARR] = 1.7%; number needed to treat [NNT] = 59; relative risk reduction = 41%).
More pills lead to longer use of contraception
The systematic review also included a 2011 trial of 700 women starting oral contraceptives.3 It randomized them to receive a 7- or 3-month supply at their initial visit, then evaluated use of oral contraception at 6 months. All women were invited back for a 3-month follow-up visit, at which time the 3-month supply group would receive additional medication.
Fifty-one percent of the 7-month group were still using oral contraceptives at 6 months compared with 35% of the 3-month group (P < .001; NNT = 7). The contrast was starker for women younger than 18 years (49% vs 12%; NNT = 3). Notably, of the women who stopped using contraception, more in the 3-month group stopped because they ran out of medication (P = .02). Subjects in the 7-month group were more likely to have given birth and more likely to have 2 or more children.
A 2017 case study examined proposed legislation in California that required health plans to cover a 12-month supply of combined hormonal contraceptives.4 The California Health Benefits Review Program surveyed health insurers and reviewed contraception usage patterns. They found that, if the legislation passed, the state could expect a 30% reduction in unintended pregnancy (ARR = 2%; NNT = 50), resulting in 6000 fewer live births and 7000 fewer abortions per year.
RECOMMENDATIONS
The Centers for Disease Control and Prevention (CDC)’s Selected Practice Recommendations for Contraceptive Use recommend prescribing or providing as much as a 1-year supply of combined hormonal contraceptives at the initial visit and each return visit.5
The American College of Obstetricians and Gynecologists (ACOG) supports over-the-counter access to oral contraceptives, effectively allowing an unlimited supply.6
EDITOR’S TAKEAWAY
Adequate evidence of benefits and strong support from the CDC and ACOG should encourage us to offer 1-year supplies of combined oral contraceptives. Even though the higher-quality studies reviewed also showed a cost savings, up-front patient expense may remain a challenge.
1. Steenland MW, Rodriguez MI, Marchbanks PA, et al. How does the number of oral contraceptive pill packs dispensed or prescribed affect continuation and other measures of consistent and correct use? A systematic review. Contraception. 2013;87:605-610.
2. Foster DG, Hulett D, Bradsberry M, et al. Number of oral contraceptive pill packages dispensed and subsequent unintended pregnancies. Obstet Gynecol. 2011;117:566-572.
3. White KO, Westhoff C. The effect of pack supply on oral contraceptive pill continuation: a randomized controlled trial. Obstet Gynecol. 2011;118:615-622.
4. McMenamin SB, Charles SA, Tabatabaeepour N, et al. Implications of dispensing self-administered hormonal contraceptives in a 1-year supply: a California case study. Contraception. 2017;95:449-451.
5. Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-66.
6. Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. Committee Opinion No. 544: Over-the-counter access to oral contraceptives. Obstet Gynecol. 2012;120:1527-1531.
1. Steenland MW, Rodriguez MI, Marchbanks PA, et al. How does the number of oral contraceptive pill packs dispensed or prescribed affect continuation and other measures of consistent and correct use? A systematic review. Contraception. 2013;87:605-610.
2. Foster DG, Hulett D, Bradsberry M, et al. Number of oral contraceptive pill packages dispensed and subsequent unintended pregnancies. Obstet Gynecol. 2011;117:566-572.
3. White KO, Westhoff C. The effect of pack supply on oral contraceptive pill continuation: a randomized controlled trial. Obstet Gynecol. 2011;118:615-622.
4. McMenamin SB, Charles SA, Tabatabaeepour N, et al. Implications of dispensing self-administered hormonal contraceptives in a 1-year supply: a California case study. Contraception. 2017;95:449-451.
5. Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-66.
6. Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. Committee Opinion No. 544: Over-the-counter access to oral contraceptives. Obstet Gynecol. 2012;120:1527-1531.
EVIDENCE-BASED ANSWER:
Probably, although studies that looked directly at this outcome are limited. A systematic review showed that women who received a larger number of pills at one time were more likely to continue using combined hormonal contraception 7 to 15 months later (strength of recommendation [SOR]: A, consistent evidence from 2 cohort studies and 1 randomized, controlled trial), which might be extrapolated to indicate lower unintended pregnancy rates.
One of the large retrospective cohort studies included in the review demonstrated a significantly lower rate of pregnancy among women who received 12 or 13 packs of oral contraceptives at an office visit compared with 1 or 3 packs (SOR: B, large retrospective cohort study).
Do A-fib patients continue to benefit from vitamin K antagonists with advancing age?
EVIDENCE SUMMARY
A meta-analysis of 12 randomized trials of stroke prevention in patients with atrial fibrillation (8932 patients, 63% male, mean age 72 years, 19.6% ≥ 80 years) examined outcomes of ischemic stroke, serious bleeding (systemic or intracranial hemorrhages requiring hospitalization, transfusion, or surgery) and cardiovascular events (ischemic stroke, myocardial infarction, systemic emboli, and vascular death).1 Patients were randomized to oral anticoagulants (3430 patients), antiplatelet therapy (3531 patients), or no therapy (1971 patients).
Warfarin target international normalized ratios (INRs) ranged from 1.5 to 4.2. Previous stoke or transient ischemic attack varied across studies but averaged 22% (patient baseline characteristics were evenly distributed among all arms of all 12 studies, suggesting appropriate randomizations). Fifteen percent of patients had diabetes, 50% had hypertension, and 20% had congestive heart failure. They were followed for a mean of 2 years.
Overall, patients experienced 623 ischemic strokes, 289 serious bleeds, and 1210 cardiovascular events. After adjusting for treatment and covariates, age was independently associated with higher risk for each outcome. For every decade increase in age, the hazard ratio (HR) for ischemic stroke was 1.45 (95% confidence interval [CI], 1.26-1.66); serious hemorrhage, 1.61 (95% CI, 1.47-1.77); and cardiovascular events, 1.43 (95% CI, 1.33-1.53).
Benefits of warfarin outweigh increased risk of hemorrhage
Treatment with vitamin K antagonists, compared with placebo, reduced ischemic strokes (HR = 0.36; 95% CI, 0.29-0.45) and cardiovascular events (HR = 0.59; 95% CI, 0.52-0.66) but increased the risk of serious hemorrhage (HR = 1.56; 95% CI, 1.03-2.37) in patients from 50 to 90 years of age. The benefits of decreased ischemic strokes and cardiovascular events consistently surpassed the increased risk of hemorrhage, however.
Across all age groups, the absolute risk reductions (ARRs) for ischemic stroke and cardiovascular events were 2% to 3% and 3% to 8%, respectively, whereas the absolute risk increase for serious hemorrhage was 0.5% to 1%. For those ages 70 to 75, for example, warfarin decreased the rate of ischemic stroke by 3% per year (number needed to treat [NNT] = 34; rates estimated from graphs) and the rate of cardiovascular events by 7% (NNT = 14) but increased the risk of serious hemorrhage by approximately 0.5% per year (number need to harm = 200).
Warfarin prevents major strokes more effectively than aspirin
A randomized open-label trial with blind assessment of endpoints, included in the meta-analysis, followed 973 patients older than 75 years (mean 81.5 years) with atrial fibrillation for 2 to 7 years.2 Researchers evaluated warfarin compared with aspirin for the outcomes of major stroke, arterial embolism, and intracranial hemorrhage. Major strokes comprised fatal or disabling strokes. Researchers excluded patients with minor strokes, rheumatic heart disease, a major nontraumatic hemorrhage within the previous 5 years, intracranial hemorrhage, peptic ulcer disease, esophageal varices, or a terminal illness.
Compared with aspirin, warfarin significantly reduced all primary events (ARR = 1.8% vs 3.8%; relative risk reduction [RRR] = 0.48; 95% CI, 0.28-0.80; NNT = 50). Warfarin decreased major strokes more than aspirin (21 vs 44 strokes; ARR = 1.8%; relative risk [RR] = 0.46; 95% CI, 0.26-0.79; NNT = 56) but didn’t alter the risk of hemorrhagic strokes (6 vs 5 absolute events, respectively; RRR = 1.15, 95% CI, 0.29-4.77) or other intracranial hemorrhages (2 vs 1 event, respectively; RR = 1.92; 95% CI, 0.10-113.3). Wide confidence intervals and the small number of hemorrhagic events suggest that the study wasn’t powered to detect a significant difference in hemorrhagic events.
Continue to: Large study finds net benefit for warfarin treatment
Large study finds net benefit for warfarin treatment
A retrospective cohort including all 182,678 Swedish Hospital Discharge Register patients with atrial fibrillation (260,000 patient-years) evaluated the net benefit of anticoagulation treatment decisions over an average of 1.5 years.3 The Swedish National Prescribed Drugs Registry, which includes all Swedish pharmacies, identified all patients who were prescribed warfarin during the study years of July 2005 through December 2008. The patients were divided into 2 groups, warfarin or no warfarin, and assigned risk scores using CHA2DS2-VASc and HAS-BLED.4,5
Researchers defined net benefit as the number of ischemic strokes avoided in patients taking warfarin, minus the number of excess intracranial bleeds. They assigned a weight of 1.5 to intracranial bleeds vs 1 for ischemic strokes to compensate for the generally more severe outcomes of intracranial bleeding.
Warfarin produced a net benefit at every CHA2DS2-VASc score greater than 0 (aggregate result of 3.9 fewer events per 100 patient-years; 95% CI, 3.8-4.1; NNT = 26). Kaplan-Meier composite plots of all-cause mortality, ischemic stroke, and intracranial bleeds showed a net benefit favoring warfarin use for all combinations of CHA2DS2-VASc greater than 0 (patients older than 65 years never have a CHA2DS2-VASc score of 0 because they’re assigned 1 point at ages 65 to 74 years and 2 points at 75 years and older) and HAS-BLED scores (all curves P < .00001).
Hazard ratios (HRs) of every combination of scores favored warfarin use (HRs ranged from 0.26-0.72; 95% CIs, less than 1 for all HRs; aggregate benefit at all risk scores: HR = 0.51; 95% CI, 0.50-0.52,). The risk of intracranial bleed, or any bleed, on warfarin at all risk strata was less than the corresponding risk of ischemic stroke (or thromboembolic event) without warfarin except among the lowest risk patients (CHA2DS2-VASc = 0). The difference between thromboses and hemorrhages increased as the CHA2DS2-VASc score increased. Of note, a smaller percentage of the highest risk patients were on warfarin.
EDITOR’S TAKEAWAY
We have solid evidence that, although the risks of systemic and intracranial bleeding from warfarin therapy in older patients with atrial fibrillation increase steadily with advancing age, so do the benefits in reduced ischemic stroke, myocardial infarction, thrombotic emboli, and overall cardiovascular death. Most important, the benefits continue to outweigh the risks by a factor of 2 to 4, even in the oldest age groups.
1. van Walraven C, Hart R, et al. Effect of age on stroke prevention therapy in patients with atrial fibrillation. Stroke. 2009;40:1410-1416.
2. Mant J, Hobbs FD. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study): a randomised controlled trial. Lancet. 2007;370:493–503.
3. Friberg L, Rosenqvist M, Lip GY. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation. 2012;125:2298-2307.
4. Friberg L, Rosenqvist M, Lip G. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182,678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33:1500-1510.
5. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest. 2010;138:1093-1100.
EVIDENCE SUMMARY
A meta-analysis of 12 randomized trials of stroke prevention in patients with atrial fibrillation (8932 patients, 63% male, mean age 72 years, 19.6% ≥ 80 years) examined outcomes of ischemic stroke, serious bleeding (systemic or intracranial hemorrhages requiring hospitalization, transfusion, or surgery) and cardiovascular events (ischemic stroke, myocardial infarction, systemic emboli, and vascular death).1 Patients were randomized to oral anticoagulants (3430 patients), antiplatelet therapy (3531 patients), or no therapy (1971 patients).
Warfarin target international normalized ratios (INRs) ranged from 1.5 to 4.2. Previous stoke or transient ischemic attack varied across studies but averaged 22% (patient baseline characteristics were evenly distributed among all arms of all 12 studies, suggesting appropriate randomizations). Fifteen percent of patients had diabetes, 50% had hypertension, and 20% had congestive heart failure. They were followed for a mean of 2 years.
Overall, patients experienced 623 ischemic strokes, 289 serious bleeds, and 1210 cardiovascular events. After adjusting for treatment and covariates, age was independently associated with higher risk for each outcome. For every decade increase in age, the hazard ratio (HR) for ischemic stroke was 1.45 (95% confidence interval [CI], 1.26-1.66); serious hemorrhage, 1.61 (95% CI, 1.47-1.77); and cardiovascular events, 1.43 (95% CI, 1.33-1.53).
Benefits of warfarin outweigh increased risk of hemorrhage
Treatment with vitamin K antagonists, compared with placebo, reduced ischemic strokes (HR = 0.36; 95% CI, 0.29-0.45) and cardiovascular events (HR = 0.59; 95% CI, 0.52-0.66) but increased the risk of serious hemorrhage (HR = 1.56; 95% CI, 1.03-2.37) in patients from 50 to 90 years of age. The benefits of decreased ischemic strokes and cardiovascular events consistently surpassed the increased risk of hemorrhage, however.
Across all age groups, the absolute risk reductions (ARRs) for ischemic stroke and cardiovascular events were 2% to 3% and 3% to 8%, respectively, whereas the absolute risk increase for serious hemorrhage was 0.5% to 1%. For those ages 70 to 75, for example, warfarin decreased the rate of ischemic stroke by 3% per year (number needed to treat [NNT] = 34; rates estimated from graphs) and the rate of cardiovascular events by 7% (NNT = 14) but increased the risk of serious hemorrhage by approximately 0.5% per year (number need to harm = 200).
Warfarin prevents major strokes more effectively than aspirin
A randomized open-label trial with blind assessment of endpoints, included in the meta-analysis, followed 973 patients older than 75 years (mean 81.5 years) with atrial fibrillation for 2 to 7 years.2 Researchers evaluated warfarin compared with aspirin for the outcomes of major stroke, arterial embolism, and intracranial hemorrhage. Major strokes comprised fatal or disabling strokes. Researchers excluded patients with minor strokes, rheumatic heart disease, a major nontraumatic hemorrhage within the previous 5 years, intracranial hemorrhage, peptic ulcer disease, esophageal varices, or a terminal illness.
Compared with aspirin, warfarin significantly reduced all primary events (ARR = 1.8% vs 3.8%; relative risk reduction [RRR] = 0.48; 95% CI, 0.28-0.80; NNT = 50). Warfarin decreased major strokes more than aspirin (21 vs 44 strokes; ARR = 1.8%; relative risk [RR] = 0.46; 95% CI, 0.26-0.79; NNT = 56) but didn’t alter the risk of hemorrhagic strokes (6 vs 5 absolute events, respectively; RRR = 1.15, 95% CI, 0.29-4.77) or other intracranial hemorrhages (2 vs 1 event, respectively; RR = 1.92; 95% CI, 0.10-113.3). Wide confidence intervals and the small number of hemorrhagic events suggest that the study wasn’t powered to detect a significant difference in hemorrhagic events.
Continue to: Large study finds net benefit for warfarin treatment
Large study finds net benefit for warfarin treatment
A retrospective cohort including all 182,678 Swedish Hospital Discharge Register patients with atrial fibrillation (260,000 patient-years) evaluated the net benefit of anticoagulation treatment decisions over an average of 1.5 years.3 The Swedish National Prescribed Drugs Registry, which includes all Swedish pharmacies, identified all patients who were prescribed warfarin during the study years of July 2005 through December 2008. The patients were divided into 2 groups, warfarin or no warfarin, and assigned risk scores using CHA2DS2-VASc and HAS-BLED.4,5
Researchers defined net benefit as the number of ischemic strokes avoided in patients taking warfarin, minus the number of excess intracranial bleeds. They assigned a weight of 1.5 to intracranial bleeds vs 1 for ischemic strokes to compensate for the generally more severe outcomes of intracranial bleeding.
Warfarin produced a net benefit at every CHA2DS2-VASc score greater than 0 (aggregate result of 3.9 fewer events per 100 patient-years; 95% CI, 3.8-4.1; NNT = 26). Kaplan-Meier composite plots of all-cause mortality, ischemic stroke, and intracranial bleeds showed a net benefit favoring warfarin use for all combinations of CHA2DS2-VASc greater than 0 (patients older than 65 years never have a CHA2DS2-VASc score of 0 because they’re assigned 1 point at ages 65 to 74 years and 2 points at 75 years and older) and HAS-BLED scores (all curves P < .00001).
Hazard ratios (HRs) of every combination of scores favored warfarin use (HRs ranged from 0.26-0.72; 95% CIs, less than 1 for all HRs; aggregate benefit at all risk scores: HR = 0.51; 95% CI, 0.50-0.52,). The risk of intracranial bleed, or any bleed, on warfarin at all risk strata was less than the corresponding risk of ischemic stroke (or thromboembolic event) without warfarin except among the lowest risk patients (CHA2DS2-VASc = 0). The difference between thromboses and hemorrhages increased as the CHA2DS2-VASc score increased. Of note, a smaller percentage of the highest risk patients were on warfarin.
EDITOR’S TAKEAWAY
We have solid evidence that, although the risks of systemic and intracranial bleeding from warfarin therapy in older patients with atrial fibrillation increase steadily with advancing age, so do the benefits in reduced ischemic stroke, myocardial infarction, thrombotic emboli, and overall cardiovascular death. Most important, the benefits continue to outweigh the risks by a factor of 2 to 4, even in the oldest age groups.
EVIDENCE SUMMARY
A meta-analysis of 12 randomized trials of stroke prevention in patients with atrial fibrillation (8932 patients, 63% male, mean age 72 years, 19.6% ≥ 80 years) examined outcomes of ischemic stroke, serious bleeding (systemic or intracranial hemorrhages requiring hospitalization, transfusion, or surgery) and cardiovascular events (ischemic stroke, myocardial infarction, systemic emboli, and vascular death).1 Patients were randomized to oral anticoagulants (3430 patients), antiplatelet therapy (3531 patients), or no therapy (1971 patients).
Warfarin target international normalized ratios (INRs) ranged from 1.5 to 4.2. Previous stoke or transient ischemic attack varied across studies but averaged 22% (patient baseline characteristics were evenly distributed among all arms of all 12 studies, suggesting appropriate randomizations). Fifteen percent of patients had diabetes, 50% had hypertension, and 20% had congestive heart failure. They were followed for a mean of 2 years.
Overall, patients experienced 623 ischemic strokes, 289 serious bleeds, and 1210 cardiovascular events. After adjusting for treatment and covariates, age was independently associated with higher risk for each outcome. For every decade increase in age, the hazard ratio (HR) for ischemic stroke was 1.45 (95% confidence interval [CI], 1.26-1.66); serious hemorrhage, 1.61 (95% CI, 1.47-1.77); and cardiovascular events, 1.43 (95% CI, 1.33-1.53).
Benefits of warfarin outweigh increased risk of hemorrhage
Treatment with vitamin K antagonists, compared with placebo, reduced ischemic strokes (HR = 0.36; 95% CI, 0.29-0.45) and cardiovascular events (HR = 0.59; 95% CI, 0.52-0.66) but increased the risk of serious hemorrhage (HR = 1.56; 95% CI, 1.03-2.37) in patients from 50 to 90 years of age. The benefits of decreased ischemic strokes and cardiovascular events consistently surpassed the increased risk of hemorrhage, however.
Across all age groups, the absolute risk reductions (ARRs) for ischemic stroke and cardiovascular events were 2% to 3% and 3% to 8%, respectively, whereas the absolute risk increase for serious hemorrhage was 0.5% to 1%. For those ages 70 to 75, for example, warfarin decreased the rate of ischemic stroke by 3% per year (number needed to treat [NNT] = 34; rates estimated from graphs) and the rate of cardiovascular events by 7% (NNT = 14) but increased the risk of serious hemorrhage by approximately 0.5% per year (number need to harm = 200).
Warfarin prevents major strokes more effectively than aspirin
A randomized open-label trial with blind assessment of endpoints, included in the meta-analysis, followed 973 patients older than 75 years (mean 81.5 years) with atrial fibrillation for 2 to 7 years.2 Researchers evaluated warfarin compared with aspirin for the outcomes of major stroke, arterial embolism, and intracranial hemorrhage. Major strokes comprised fatal or disabling strokes. Researchers excluded patients with minor strokes, rheumatic heart disease, a major nontraumatic hemorrhage within the previous 5 years, intracranial hemorrhage, peptic ulcer disease, esophageal varices, or a terminal illness.
Compared with aspirin, warfarin significantly reduced all primary events (ARR = 1.8% vs 3.8%; relative risk reduction [RRR] = 0.48; 95% CI, 0.28-0.80; NNT = 50). Warfarin decreased major strokes more than aspirin (21 vs 44 strokes; ARR = 1.8%; relative risk [RR] = 0.46; 95% CI, 0.26-0.79; NNT = 56) but didn’t alter the risk of hemorrhagic strokes (6 vs 5 absolute events, respectively; RRR = 1.15, 95% CI, 0.29-4.77) or other intracranial hemorrhages (2 vs 1 event, respectively; RR = 1.92; 95% CI, 0.10-113.3). Wide confidence intervals and the small number of hemorrhagic events suggest that the study wasn’t powered to detect a significant difference in hemorrhagic events.
Continue to: Large study finds net benefit for warfarin treatment
Large study finds net benefit for warfarin treatment
A retrospective cohort including all 182,678 Swedish Hospital Discharge Register patients with atrial fibrillation (260,000 patient-years) evaluated the net benefit of anticoagulation treatment decisions over an average of 1.5 years.3 The Swedish National Prescribed Drugs Registry, which includes all Swedish pharmacies, identified all patients who were prescribed warfarin during the study years of July 2005 through December 2008. The patients were divided into 2 groups, warfarin or no warfarin, and assigned risk scores using CHA2DS2-VASc and HAS-BLED.4,5
Researchers defined net benefit as the number of ischemic strokes avoided in patients taking warfarin, minus the number of excess intracranial bleeds. They assigned a weight of 1.5 to intracranial bleeds vs 1 for ischemic strokes to compensate for the generally more severe outcomes of intracranial bleeding.
Warfarin produced a net benefit at every CHA2DS2-VASc score greater than 0 (aggregate result of 3.9 fewer events per 100 patient-years; 95% CI, 3.8-4.1; NNT = 26). Kaplan-Meier composite plots of all-cause mortality, ischemic stroke, and intracranial bleeds showed a net benefit favoring warfarin use for all combinations of CHA2DS2-VASc greater than 0 (patients older than 65 years never have a CHA2DS2-VASc score of 0 because they’re assigned 1 point at ages 65 to 74 years and 2 points at 75 years and older) and HAS-BLED scores (all curves P < .00001).
Hazard ratios (HRs) of every combination of scores favored warfarin use (HRs ranged from 0.26-0.72; 95% CIs, less than 1 for all HRs; aggregate benefit at all risk scores: HR = 0.51; 95% CI, 0.50-0.52,). The risk of intracranial bleed, or any bleed, on warfarin at all risk strata was less than the corresponding risk of ischemic stroke (or thromboembolic event) without warfarin except among the lowest risk patients (CHA2DS2-VASc = 0). The difference between thromboses and hemorrhages increased as the CHA2DS2-VASc score increased. Of note, a smaller percentage of the highest risk patients were on warfarin.
EDITOR’S TAKEAWAY
We have solid evidence that, although the risks of systemic and intracranial bleeding from warfarin therapy in older patients with atrial fibrillation increase steadily with advancing age, so do the benefits in reduced ischemic stroke, myocardial infarction, thrombotic emboli, and overall cardiovascular death. Most important, the benefits continue to outweigh the risks by a factor of 2 to 4, even in the oldest age groups.
1. van Walraven C, Hart R, et al. Effect of age on stroke prevention therapy in patients with atrial fibrillation. Stroke. 2009;40:1410-1416.
2. Mant J, Hobbs FD. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study): a randomised controlled trial. Lancet. 2007;370:493–503.
3. Friberg L, Rosenqvist M, Lip GY. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation. 2012;125:2298-2307.
4. Friberg L, Rosenqvist M, Lip G. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182,678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33:1500-1510.
5. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest. 2010;138:1093-1100.
1. van Walraven C, Hart R, et al. Effect of age on stroke prevention therapy in patients with atrial fibrillation. Stroke. 2009;40:1410-1416.
2. Mant J, Hobbs FD. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study): a randomised controlled trial. Lancet. 2007;370:493–503.
3. Friberg L, Rosenqvist M, Lip GY. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation. 2012;125:2298-2307.
4. Friberg L, Rosenqvist M, Lip G. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182,678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33:1500-1510.
5. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest. 2010;138:1093-1100.
EVIDENCE-BASED ANSWER:
Yes, patients with atrial fibrilla- tion who are between the ages of 50 and 90 years continue to benefit from vitamin K antagonist therapy (warfarin) (strength of recommendation [SOR]: A, meta-analysis of randomized controlled trials [RCTs] and large cohorts). Regardless of age, warfarin produces a reduction in risk of thrombotic events that is 2- to 4-fold greater than the risk of hemorrhagic events.