User login
Discharge Medical Complexity, Change in Medical Complexity and Pediatric 30-day Readmission
Hospitalizations are disruptive, stressful, and costly for patients and families.1-5 Hospital readmissions subject families to the additional morbidity inherent to hospitalization and place patients at additional risk of hospital-acquired conditions or other harm.6-9 In pediatrics, hospital readmissions are common for specific conditions;10 with rates varying across institutions;10,11 and as many as one-third of unplanned pediatric readmissions are potentially preventable.12
Reducing pediatric readmissions requires a deeper understanding of the mechanisms through which readmissions occur. Medical complexity—specifically chronic conditions and use of medical technology—is associated with increased risk of readmission.13,14 Polypharmacy at discharge has also been associated with readmission.15,16 However, prior studies on polypharmacy and readmission risk examined the count of total medications and did not consider the nuances of scheduled versus as-needed medications, or the frequency of doses. These nuances may be critical to caregivers as discharge medical complexity can be overwhelming, even in diagnoses which are not traditionally considered complex.17 Finally, of potentially greater importance than medical complexity at discharge is a change in medical complexity during a hospitalization—for example, new diagnoses or new technologies that require additional education in hospital and management at home.
We sought to further understand the relationship between discharge medical complexity and readmission risk with regards to polypharmacy and home healthcare referrals at discharge. Specifically, we hypothesized that a change in medical complexity during an admission—ie, a new chronic diagnosis or new technology—would be a more prominent risk factor for readmission than discharge complexity alone. We examined these factors in the context of length of stay (LOS) since this is a marker of in-hospital severity of illness and a potentially modifiable function of time allowed for in-hospital teaching and discharge preparation.
METHODS
We conducted a retrospective, case-control study of pediatric hospitalizations at one tertiary care children’s hospital. Children <18 years were eligible for inclusion. Normal birth hospitalizations were excluded. We randomly selected one hospitalization from each child as the index visit. We identified cases, hospitalizations at C.S. Mott Children’s Hospital between 2008 and 2012 with a subsequent unplanned 30-day readmission,18 and matched them one to one with hospitalizations at the same hospital during the same period without subsequent readmission. We matched cases to controls based on the month of admission to account for seasonality of certain illnesses. We also matched on distance and direction from the hospital to the patient’s home to account for the potential to have readmissions to other institutions. We utilized both distance and direction recognizing that a family living 30 miles in one direction would be closer to an urban area with access to more facilities, as opposed to 30 miles in another direction in a rural area without additional access. We subsequently performed medical record review to abstract relevant covariates.
Primary Predictors
Medical Complexity Models (Models 1 and 2):
We evaluated three attributes of discharge medical complexity abstracted by medical record review—discharge medications, technology assistance (ie, tracheostomy, cerebral spinal fluid ventricular shunt, enteral feeding tube, central line), and the need for home healthcare after discharge. We counted discharge medications based on the number of medications listed on the discharge summary separated into scheduled or as needed.19 We also considered the number of scheduled doses to be administered in a 24-hour period (see Appendix methods for more information on counting discharge medications). For assistance by technology, we considered the presence of tracheostomy, cerebral spinal fluid ventricular shunt, enteral feeding tube, and central lines. While we describe these technologies separately, for multivariable analyses we considered the presence of any of the four types of technology.
Change in Medical Complexity Models (Models 3 and 4)
We examined two aspects of change in medical complexity—the presence of a new complex chronic condition (CCC)20 diagnosed during the hospitalization, and a new reliance on medical technology. The presence of new CCC was determined by comparing discharge diagnoses to past medical history abstracted by medical record review. A new CCC was defined as any complex chronic condition that was captured in the discharge diagnoses but was not evident in the past medical history. By definition, all CCCs coded during birth hospitalization (eg, at discharge from the neonatal intensive care unit) were assigned to “new” CCC. We calculated a kappa statistic to determine interrater reliability in determining the designation of new CCC. A sensitivity analysis examining these birth CCCs was also performed comparing no new CCC, new CCC, and new CCC after birth hospitalization. The methods appendix provides additional information on considering new CCCs. New technology, abstracted from chart review, was defined as technology placed during hospitalization that remained in place at discharge. If a child with existing technology had additional technology placed during the hospitalization (eg, a new tracheostomy in a child with a previously placed enteral feeding tube), the encounter was considered as having new technology placed.
Covariates
We created different sets of multivariable models to account for patient/hospitalization characteristics.
Statistical Analysis
A review of 600 cases and 600 controls yields 89% power to detect statistical significance for covariates with an odds ratio of 1.25 (β = 0.22) if the candidate covariate has low to moderate correlation with other covariates (<0.3). If a candidate covariate has a moderate correlation with other covariates (0.6), we have 89% power to detect an odds ratio of 1.35 (β = 0.30).21 We calculated odds of 30-days unplanned readmission using conditional logistic regression to account for matched case-control design. All the analyses were performed using STATA 13 (Stata Corp., College Station, Texas).
RESULTS
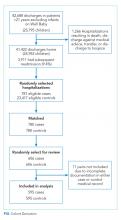
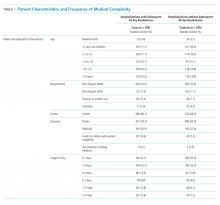
Of the 41,422 eligible index hospitalizations during the study period, 9.4% resulted in a 30-day unplanned readmission. After randomly selecting one hospitalization per child, there were 781 eligible cases. We subsequent matched all but one eligible case to a control. We randomly selected encounters for medical record review, reviewing a total of 1,212 encounters. After excluding pairs with incomplete records, we included 595 cases and 595 controls in this analysis (Figure). Patient/hospitalization characteristics are displayed in Table 1. The most frequent primary discharge diagnoses are displayed in Appendix Table 1.
Models of Medical Complexity at Discharge
Polypharmacy after discharge was common for both readmitted and nonreadmitted patients. Children who experienced unplanned readmission in 30 days were discharged with a median of four different scheduled medications (interquartile range [IQR] 2,7) which translated into a median of six (IQR 3,12) scheduled doses in a 24-hour period. In comparison, children without an unplanned readmission had a median of two different scheduled medications (IQR 1,3) with a median of three (IQR 0,7) scheduled doses in a 24-hour period. Medical technology was more common in case children (42%) than in control children (14%). Central lines and enteral tubes were the most common forms of medical technology in both cases and controls. Home health referral was common in both cases (44%) and controls (23%; Table 1).
In Model 1 (adjusting only for patient characteristics; Table 3), being discharged on two or more scheduled medications was associated with higher odds of readmission compared to being discharged without medications, with additional medications associated with even higher odds of readmission. Children with any technology had higher odds of readmission than children without medical technology. Likewise, home healthcare visits after discharge were associated with elevated odds of readmission in multivariable analyses without LOS. However, after adding LOS to the model (Model 2), home healthcare visits were no longer significantly associated with readmission.
Change in Medical Complexity Models
The adjudication of new CCCs had good reliability (Κ = 0.72). New CCCs occurred in 18% and new technologies occurred in 17% of cases. Comparatively, new CCCs occurred in 10% and new technologies in 7% of hospitalizations in control children (Table 1). In bivariate analyses, both aspects of change in medical complexity were associated with higher odds of readmission (Table 2). In multivariate analysis with patient characteristics (Model 3; Table 3), all aspects of change in complexity were associated with elevated odds of readmission. A new CCC was associated with higher odds of readmission (adjusted OR (AOR) 1.75, 95% CI: 1.11-2.75) as was new technology during admission (AOR 1.84, 95%CI: 1.09-3.10). Furthermore, the odds of readmission for medical complexity variables (polypharmacy and home healthcare need) remained largely unchanged when adding the change in medical complexity variables (ie, comparing Model 1 and Model 3). However, when accounting for LOS (Model 4), neither the acquisition of a new CCC nor the addition of new technology was associated with readmission. The most common form of new technology was central line followed by nonsurgically placed enteral tube (Appendix Table 2). Finally, in sensitivity analyses (results not detailed), separating new CCC acquired at birth and new CCCs in nonbirth hospitalizations, compared to hospitalizations with no new CCC, yielded similar results as the primary analyses.
DISCUSSION
We examined multiple attributes of polypharmacy—the number of scheduled medications, number of as-needed medications, and number of scheduled doses per 24 hours. Interestingly, only the scheduled medications (count of medication and number of doses) were associated with elevated readmission risk. As-needed medications have heterogeneity in the level of importance from critical (eg, seizure rescue) to discretionary (eg, antipyretics, creams). The burden of managing these types of medications may still be high (ie, parents must decide when to administer a critical medication); however, this burden does not translate into increased readmission risk in this population.
Not surprisingly, greater medical complexity—as defined by higher numbers of scheduled discharge medications and technology assistance—is associated with 30-day readmission risk. Our analyses do not allow us to determine how much of the increased risk is due to additional care burden and risks of polypharmacy versus the inherent increase in complexity and severity of illness for which polypharmacy is a marker. Tailoring discharge regimens to the realities of daily life, with the goal of “minimally disruptive medicine”22,23 (eg, integrating manageable discharge medication routines into school and work schedules), is not a common feature of pediatric discharge planning. For adult patients with complex medical conditions, tailoring medication regimens in a minimally disruptive way is known to improve outcomes.24 Similarly, adopting minimally disruptive techniques to integrate the polypharmacy inherent in discharge could potentially mitigate some of the readmission risks for children and adolescents.
Contrary to our hypothesis, new technologies and new diagnoses did not confer additional readmission risk when accounting for LOS and patient characteristics. One potential explanation is varying risks conveyed by different types of new technologies placed during hospitalization. Central lines, the most common form of new technology, is associated with higher odds of reutilization in unadjusted analyses. However, the second most common form of new technology, nonsurgically placed enteral feeding tube, was not. Further analyses of the differential effects of new technology should be further examined in larger datasets. Additionally, the lack of additional readmission risk from new technology may relate to additional teaching and support provided to families of patients undergoing unfamiliar procedures offsets the risks inherent of greater complexity. If so, it may be that the more intensive teaching and postdischarge support provided to families with new technology or a new diagnosis could be replicated through refresher teaching during hospitalizations, when a patient’s state of health is status quo for the family (ie, the child was admitted and discharged with the same technology and diagnoses). This notion is supported by prior work that demonstrated successful readmission reduction interventions for children with chronic conditions often rely on enhanced education or coaching.25,26
We elected to present models both with and without LOS as a confounder because it is a potentially modifiable attribute of hospitalization. Change in medical complexity aspects were significantly associated with readmission in multivariable models without LOS. However, with the addition of LOS, they were no longer significant. Thus, the readmission risk of new complexity is accounted for by the readmission risk inherent in a longer LOS. This finding prompts additional questions that merit further study: is it that LOS is a general marker for heightened complexity, or is it that a longer LOS can modify readmission risk through additional in-hospital care and time for enhanced education?
Our study has several strengths. We were able to discern true complexity at the time of discharge through medical record review. For example, if a child had a peripherally inserted central catheter placed during hospitalization, it cannot be ascertained through administrative data without medical record review if the technology was removed or in place at discharge. Likewise, medical record review allows for identification of medical technology which is not surgically implanted (eg, nasogastric feeding tubes). Given the “fog” families report as part of their in-hospital experience and its threats to education and postdischarge contingency planning,17 we felt it important to evaluate medical technology regardless of whether or not it was surgically placed. Additionally, the more detailed and nuanced understanding gained of polypharmacy burden can better inform both risk prediction models and interventions to improve the transition from hospital to home.
This study
CONCLUSION
Medical complexity at discharge is associated with pediatric readmission risk. Contrary to our hypothesis, the addition of new technologies and new CCC diagnoses are not associated with pediatric readmission, after accounting for patient and hospitalization factors including LOS. The dynamics of LOS as a risk factor for readmission for children with medical complexity are likely multifaceted and merit further investigation in a multi-institutional study.
Disclosures
The authors report no potential conflicts of interest.
Funding
This work was supported by a grant from the Agency for Healthcare Research and Quality (1K08HS204735-01A1) and a grant from the Blue Cross Blue Shield of Michigan Foundation.
1. Diaz-Caneja A, Gledhill J, Weaver T, Nadel S, Garralda E. A child’s admission to hospital: a qualitative study examining the experiences of parents. Intensive Care Med. 2005;31(9):1248-1254. https://doi.org/10.1007/s00134-005-2728-8.
2. Lapillonne A, Regnault A, Gournay V, et al. Impact on parents of bronchiolitis hospitalization of full-term, preterm and congenital heart disease infants. BMC Pediatrics. 2012;12:171. https://doi.org/10.1186/1471-2431-12-171.
3. Leader S, Jacobson P, Marcin J, Vardis R, Sorrentino M, Murray D. A method for identifying the financial burden of hospitalized infants on families. Value Health. 2002;5(1):55-59. https://doi.org/10.1046/j.1524-4733.2002.51076.x.
4. Leidy NK, Margolis MK, Marcin JP, et al. The impact of severe respiratory syncytial virus on the child, caregiver, and family during hospitalization and recovery. Pediatrics. 2005;115(6):1536-1546. https://doi.org/10.1542/peds.2004-1149.
5. Rennick JE, Johnston CC, Dougherty G, Platt R, Ritchie JA. Children’s psychological responses after critical illness and exposure to invasive technology. J Dev Behav Pediatr. 2002;23(3):133-144. PubMed
6. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. https://doi.org/10.1056/NEJM199102073240604.
7. Kohn LT, Corrigan J, Donaldson MS. To err is human: building a safer health system. Washington DC: National Academy Press; 2000.
8. Landrigan CP, Parry GJ, Bones CB, Hackbarth AD, Goldmann DA, Sharek PJ. Temporal trends in rates of patient harm resulting from medical care. N Engl J Med. 2010;363(22):2124-2134. https://doi.org/10.1056/NEJMsa1004404.
9. Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of healthcare-associated infections. N Engl J Med. 2014;370(13):1198-1208. https://doi.org/10.1056/NEJMoa1306801.
10. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351.
11. Bardach NS, Vittinghoff E, Asteria-Penaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. https://doi.org/10.1542/peds.2012-3527.
12. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-day hospital readmissions at a children’s hospital. Pediatrics. 2016;138(2):pii: e20154182. https://doi.org/10.1542/peds.2015-4182.
13. Bucholz EM, Gay JC, Hall M, Harris M, Berry JG. Timing and causes of common pediatric readmissions. J Pediatr. 2018;200:240-248. https://doi.org/10.1016/j.jpeds.2018.04.044.
14. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. https://doi.org/10.1001/jama.2011.122.
15. Winer JC, Aragona E, Fields AI, Stockwell DC. Comparison of clinical risk factors among pediatric patients with single admission, multiple admissions (without any 7-day readmissions), and 7-day readmission. Hosp Pediatr. 2016;6(3):119-125. https://doi.org/10.1542/hpeds.2015-0110.
16. Brittan MS, Martin S, Anderson L, Moss A, Torok MR. An electronic health record tool designed to improve pediatric hospital discharge has low predictive utility for readmissions. J Hosp Med. 2018;13(11):779-782. https://doi.org/10.12788/jhm.3043.
17. Solan LG, Beck AF, Brunswick SA, et al. The family perspective on hospital to home transitions: a qualitative study. Pediatrics. 2015;136(6):e1539-e1549. https://doi.org/10.1542/peds.2015-2098.
18. Auger KA, Mueller EL, Weinberg SH, et al. A validated method for identifying unplanned pediatric readmission. J Pediatr. 2016;170:105-112. https://doi.org/10.1016/j.jpeds.2015.11.051.
19. Auger KA, Shah SS, Davis MD, Brady PW. Counting the Ways to Count Medications: The Challenges of Defining Pediatric Polypharmacy. J Hosp Med. 2019;14(8):506-507. https://doi.org/10.12788/jhm.3213.
20. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatrics. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199.
21. Hsieh FY. Sample size tables for logistic regression. Stat Med. 1989;8(7):795-802. https://doi.org/10.1002/sim.4780080704.
22. May C, Montori VM, Mair FS. We need minimally disruptive medicine. BMJ. 2009;339:b2803. https://doi.org/10.1136/bmj.b2803.
23. Leppin AL, Montori VM, Gionfriddo MR. Minimally disruptive medicine: a pragmatically comprehensive model for delivering care to patients with multiple chronic conditions. Healthcare (Basel). 2015;3(1):50-63. https://doi.org/10.3390/healthcare3010050.
24. Serrano V, Spencer-Bonilla G, Boehmer KR, Montori VM. Minimally disruptive medicine for patients with diabetes. Curr Diab Rep. 2017;17(11):104. https://doi.org/10.1007/s11892-017-0935-7.
25. Auger KA, Kenyon CC, Feudtner C, Davis MM. Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2013;9(4):251-260. https://doi.org/10.1002/jhm.2134.
26. Coller RJ, Klitzner TS, Lerner CF, et al. Complex care hospital use and postdischarge coaching: a randomized controlled trial. Pediatrics. 2018;142(2):pii: e20174278. https://doi.org/10.1542/peds.2017-4278.
27. Hain PD, Gay JC, Berutti TW, Whitney GM, Wang W, Saville BR. Preventability of early readmissions at a children’s hospital. Pediatrics. 2013;131(1):e171-e181. https://doi.org/10.1542/peds.2012-0820.
28. Auger KA, Teufel RJ, 2nd, Harris JM, 2nd, et al. Children’s hospital characteristics and readmission metrics. Pediatrics. 2017;139(2). https://doi.org/10.1542/peds.2016-1720.
29. Gay JC, Agrawal R, Auger KA, et al. Rates and impact of potentially preventable readmissions at children’s hospitals. J Pediatr. 2015;166(3):613-619 e615. https://doi.org/10.1016/j.jpeds.2014.10.052.
Hospitalizations are disruptive, stressful, and costly for patients and families.1-5 Hospital readmissions subject families to the additional morbidity inherent to hospitalization and place patients at additional risk of hospital-acquired conditions or other harm.6-9 In pediatrics, hospital readmissions are common for specific conditions;10 with rates varying across institutions;10,11 and as many as one-third of unplanned pediatric readmissions are potentially preventable.12
Reducing pediatric readmissions requires a deeper understanding of the mechanisms through which readmissions occur. Medical complexity—specifically chronic conditions and use of medical technology—is associated with increased risk of readmission.13,14 Polypharmacy at discharge has also been associated with readmission.15,16 However, prior studies on polypharmacy and readmission risk examined the count of total medications and did not consider the nuances of scheduled versus as-needed medications, or the frequency of doses. These nuances may be critical to caregivers as discharge medical complexity can be overwhelming, even in diagnoses which are not traditionally considered complex.17 Finally, of potentially greater importance than medical complexity at discharge is a change in medical complexity during a hospitalization—for example, new diagnoses or new technologies that require additional education in hospital and management at home.
We sought to further understand the relationship between discharge medical complexity and readmission risk with regards to polypharmacy and home healthcare referrals at discharge. Specifically, we hypothesized that a change in medical complexity during an admission—ie, a new chronic diagnosis or new technology—would be a more prominent risk factor for readmission than discharge complexity alone. We examined these factors in the context of length of stay (LOS) since this is a marker of in-hospital severity of illness and a potentially modifiable function of time allowed for in-hospital teaching and discharge preparation.
METHODS
We conducted a retrospective, case-control study of pediatric hospitalizations at one tertiary care children’s hospital. Children <18 years were eligible for inclusion. Normal birth hospitalizations were excluded. We randomly selected one hospitalization from each child as the index visit. We identified cases, hospitalizations at C.S. Mott Children’s Hospital between 2008 and 2012 with a subsequent unplanned 30-day readmission,18 and matched them one to one with hospitalizations at the same hospital during the same period without subsequent readmission. We matched cases to controls based on the month of admission to account for seasonality of certain illnesses. We also matched on distance and direction from the hospital to the patient’s home to account for the potential to have readmissions to other institutions. We utilized both distance and direction recognizing that a family living 30 miles in one direction would be closer to an urban area with access to more facilities, as opposed to 30 miles in another direction in a rural area without additional access. We subsequently performed medical record review to abstract relevant covariates.
Primary Predictors
Medical Complexity Models (Models 1 and 2):
We evaluated three attributes of discharge medical complexity abstracted by medical record review—discharge medications, technology assistance (ie, tracheostomy, cerebral spinal fluid ventricular shunt, enteral feeding tube, central line), and the need for home healthcare after discharge. We counted discharge medications based on the number of medications listed on the discharge summary separated into scheduled or as needed.19 We also considered the number of scheduled doses to be administered in a 24-hour period (see Appendix methods for more information on counting discharge medications). For assistance by technology, we considered the presence of tracheostomy, cerebral spinal fluid ventricular shunt, enteral feeding tube, and central lines. While we describe these technologies separately, for multivariable analyses we considered the presence of any of the four types of technology.
Change in Medical Complexity Models (Models 3 and 4)
We examined two aspects of change in medical complexity—the presence of a new complex chronic condition (CCC)20 diagnosed during the hospitalization, and a new reliance on medical technology. The presence of new CCC was determined by comparing discharge diagnoses to past medical history abstracted by medical record review. A new CCC was defined as any complex chronic condition that was captured in the discharge diagnoses but was not evident in the past medical history. By definition, all CCCs coded during birth hospitalization (eg, at discharge from the neonatal intensive care unit) were assigned to “new” CCC. We calculated a kappa statistic to determine interrater reliability in determining the designation of new CCC. A sensitivity analysis examining these birth CCCs was also performed comparing no new CCC, new CCC, and new CCC after birth hospitalization. The methods appendix provides additional information on considering new CCCs. New technology, abstracted from chart review, was defined as technology placed during hospitalization that remained in place at discharge. If a child with existing technology had additional technology placed during the hospitalization (eg, a new tracheostomy in a child with a previously placed enteral feeding tube), the encounter was considered as having new technology placed.
Covariates
We created different sets of multivariable models to account for patient/hospitalization characteristics.
Statistical Analysis
A review of 600 cases and 600 controls yields 89% power to detect statistical significance for covariates with an odds ratio of 1.25 (β = 0.22) if the candidate covariate has low to moderate correlation with other covariates (<0.3). If a candidate covariate has a moderate correlation with other covariates (0.6), we have 89% power to detect an odds ratio of 1.35 (β = 0.30).21 We calculated odds of 30-days unplanned readmission using conditional logistic regression to account for matched case-control design. All the analyses were performed using STATA 13 (Stata Corp., College Station, Texas).
RESULTS
Of the 41,422 eligible index hospitalizations during the study period, 9.4% resulted in a 30-day unplanned readmission. After randomly selecting one hospitalization per child, there were 781 eligible cases. We subsequent matched all but one eligible case to a control. We randomly selected encounters for medical record review, reviewing a total of 1,212 encounters. After excluding pairs with incomplete records, we included 595 cases and 595 controls in this analysis (Figure). Patient/hospitalization characteristics are displayed in Table 1. The most frequent primary discharge diagnoses are displayed in Appendix Table 1.
Models of Medical Complexity at Discharge
Polypharmacy after discharge was common for both readmitted and nonreadmitted patients. Children who experienced unplanned readmission in 30 days were discharged with a median of four different scheduled medications (interquartile range [IQR] 2,7) which translated into a median of six (IQR 3,12) scheduled doses in a 24-hour period. In comparison, children without an unplanned readmission had a median of two different scheduled medications (IQR 1,3) with a median of three (IQR 0,7) scheduled doses in a 24-hour period. Medical technology was more common in case children (42%) than in control children (14%). Central lines and enteral tubes were the most common forms of medical technology in both cases and controls. Home health referral was common in both cases (44%) and controls (23%; Table 1).
In Model 1 (adjusting only for patient characteristics; Table 3), being discharged on two or more scheduled medications was associated with higher odds of readmission compared to being discharged without medications, with additional medications associated with even higher odds of readmission. Children with any technology had higher odds of readmission than children without medical technology. Likewise, home healthcare visits after discharge were associated with elevated odds of readmission in multivariable analyses without LOS. However, after adding LOS to the model (Model 2), home healthcare visits were no longer significantly associated with readmission.
Change in Medical Complexity Models
The adjudication of new CCCs had good reliability (Κ = 0.72). New CCCs occurred in 18% and new technologies occurred in 17% of cases. Comparatively, new CCCs occurred in 10% and new technologies in 7% of hospitalizations in control children (Table 1). In bivariate analyses, both aspects of change in medical complexity were associated with higher odds of readmission (Table 2). In multivariate analysis with patient characteristics (Model 3; Table 3), all aspects of change in complexity were associated with elevated odds of readmission. A new CCC was associated with higher odds of readmission (adjusted OR (AOR) 1.75, 95% CI: 1.11-2.75) as was new technology during admission (AOR 1.84, 95%CI: 1.09-3.10). Furthermore, the odds of readmission for medical complexity variables (polypharmacy and home healthcare need) remained largely unchanged when adding the change in medical complexity variables (ie, comparing Model 1 and Model 3). However, when accounting for LOS (Model 4), neither the acquisition of a new CCC nor the addition of new technology was associated with readmission. The most common form of new technology was central line followed by nonsurgically placed enteral tube (Appendix Table 2). Finally, in sensitivity analyses (results not detailed), separating new CCC acquired at birth and new CCCs in nonbirth hospitalizations, compared to hospitalizations with no new CCC, yielded similar results as the primary analyses.
DISCUSSION
We examined multiple attributes of polypharmacy—the number of scheduled medications, number of as-needed medications, and number of scheduled doses per 24 hours. Interestingly, only the scheduled medications (count of medication and number of doses) were associated with elevated readmission risk. As-needed medications have heterogeneity in the level of importance from critical (eg, seizure rescue) to discretionary (eg, antipyretics, creams). The burden of managing these types of medications may still be high (ie, parents must decide when to administer a critical medication); however, this burden does not translate into increased readmission risk in this population.
Not surprisingly, greater medical complexity—as defined by higher numbers of scheduled discharge medications and technology assistance—is associated with 30-day readmission risk. Our analyses do not allow us to determine how much of the increased risk is due to additional care burden and risks of polypharmacy versus the inherent increase in complexity and severity of illness for which polypharmacy is a marker. Tailoring discharge regimens to the realities of daily life, with the goal of “minimally disruptive medicine”22,23 (eg, integrating manageable discharge medication routines into school and work schedules), is not a common feature of pediatric discharge planning. For adult patients with complex medical conditions, tailoring medication regimens in a minimally disruptive way is known to improve outcomes.24 Similarly, adopting minimally disruptive techniques to integrate the polypharmacy inherent in discharge could potentially mitigate some of the readmission risks for children and adolescents.
Contrary to our hypothesis, new technologies and new diagnoses did not confer additional readmission risk when accounting for LOS and patient characteristics. One potential explanation is varying risks conveyed by different types of new technologies placed during hospitalization. Central lines, the most common form of new technology, is associated with higher odds of reutilization in unadjusted analyses. However, the second most common form of new technology, nonsurgically placed enteral feeding tube, was not. Further analyses of the differential effects of new technology should be further examined in larger datasets. Additionally, the lack of additional readmission risk from new technology may relate to additional teaching and support provided to families of patients undergoing unfamiliar procedures offsets the risks inherent of greater complexity. If so, it may be that the more intensive teaching and postdischarge support provided to families with new technology or a new diagnosis could be replicated through refresher teaching during hospitalizations, when a patient’s state of health is status quo for the family (ie, the child was admitted and discharged with the same technology and diagnoses). This notion is supported by prior work that demonstrated successful readmission reduction interventions for children with chronic conditions often rely on enhanced education or coaching.25,26
We elected to present models both with and without LOS as a confounder because it is a potentially modifiable attribute of hospitalization. Change in medical complexity aspects were significantly associated with readmission in multivariable models without LOS. However, with the addition of LOS, they were no longer significant. Thus, the readmission risk of new complexity is accounted for by the readmission risk inherent in a longer LOS. This finding prompts additional questions that merit further study: is it that LOS is a general marker for heightened complexity, or is it that a longer LOS can modify readmission risk through additional in-hospital care and time for enhanced education?
Our study has several strengths. We were able to discern true complexity at the time of discharge through medical record review. For example, if a child had a peripherally inserted central catheter placed during hospitalization, it cannot be ascertained through administrative data without medical record review if the technology was removed or in place at discharge. Likewise, medical record review allows for identification of medical technology which is not surgically implanted (eg, nasogastric feeding tubes). Given the “fog” families report as part of their in-hospital experience and its threats to education and postdischarge contingency planning,17 we felt it important to evaluate medical technology regardless of whether or not it was surgically placed. Additionally, the more detailed and nuanced understanding gained of polypharmacy burden can better inform both risk prediction models and interventions to improve the transition from hospital to home.
This study
CONCLUSION
Medical complexity at discharge is associated with pediatric readmission risk. Contrary to our hypothesis, the addition of new technologies and new CCC diagnoses are not associated with pediatric readmission, after accounting for patient and hospitalization factors including LOS. The dynamics of LOS as a risk factor for readmission for children with medical complexity are likely multifaceted and merit further investigation in a multi-institutional study.
Disclosures
The authors report no potential conflicts of interest.
Funding
This work was supported by a grant from the Agency for Healthcare Research and Quality (1K08HS204735-01A1) and a grant from the Blue Cross Blue Shield of Michigan Foundation.
Hospitalizations are disruptive, stressful, and costly for patients and families.1-5 Hospital readmissions subject families to the additional morbidity inherent to hospitalization and place patients at additional risk of hospital-acquired conditions or other harm.6-9 In pediatrics, hospital readmissions are common for specific conditions;10 with rates varying across institutions;10,11 and as many as one-third of unplanned pediatric readmissions are potentially preventable.12
Reducing pediatric readmissions requires a deeper understanding of the mechanisms through which readmissions occur. Medical complexity—specifically chronic conditions and use of medical technology—is associated with increased risk of readmission.13,14 Polypharmacy at discharge has also been associated with readmission.15,16 However, prior studies on polypharmacy and readmission risk examined the count of total medications and did not consider the nuances of scheduled versus as-needed medications, or the frequency of doses. These nuances may be critical to caregivers as discharge medical complexity can be overwhelming, even in diagnoses which are not traditionally considered complex.17 Finally, of potentially greater importance than medical complexity at discharge is a change in medical complexity during a hospitalization—for example, new diagnoses or new technologies that require additional education in hospital and management at home.
We sought to further understand the relationship between discharge medical complexity and readmission risk with regards to polypharmacy and home healthcare referrals at discharge. Specifically, we hypothesized that a change in medical complexity during an admission—ie, a new chronic diagnosis or new technology—would be a more prominent risk factor for readmission than discharge complexity alone. We examined these factors in the context of length of stay (LOS) since this is a marker of in-hospital severity of illness and a potentially modifiable function of time allowed for in-hospital teaching and discharge preparation.
METHODS
We conducted a retrospective, case-control study of pediatric hospitalizations at one tertiary care children’s hospital. Children <18 years were eligible for inclusion. Normal birth hospitalizations were excluded. We randomly selected one hospitalization from each child as the index visit. We identified cases, hospitalizations at C.S. Mott Children’s Hospital between 2008 and 2012 with a subsequent unplanned 30-day readmission,18 and matched them one to one with hospitalizations at the same hospital during the same period without subsequent readmission. We matched cases to controls based on the month of admission to account for seasonality of certain illnesses. We also matched on distance and direction from the hospital to the patient’s home to account for the potential to have readmissions to other institutions. We utilized both distance and direction recognizing that a family living 30 miles in one direction would be closer to an urban area with access to more facilities, as opposed to 30 miles in another direction in a rural area without additional access. We subsequently performed medical record review to abstract relevant covariates.
Primary Predictors
Medical Complexity Models (Models 1 and 2):
We evaluated three attributes of discharge medical complexity abstracted by medical record review—discharge medications, technology assistance (ie, tracheostomy, cerebral spinal fluid ventricular shunt, enteral feeding tube, central line), and the need for home healthcare after discharge. We counted discharge medications based on the number of medications listed on the discharge summary separated into scheduled or as needed.19 We also considered the number of scheduled doses to be administered in a 24-hour period (see Appendix methods for more information on counting discharge medications). For assistance by technology, we considered the presence of tracheostomy, cerebral spinal fluid ventricular shunt, enteral feeding tube, and central lines. While we describe these technologies separately, for multivariable analyses we considered the presence of any of the four types of technology.
Change in Medical Complexity Models (Models 3 and 4)
We examined two aspects of change in medical complexity—the presence of a new complex chronic condition (CCC)20 diagnosed during the hospitalization, and a new reliance on medical technology. The presence of new CCC was determined by comparing discharge diagnoses to past medical history abstracted by medical record review. A new CCC was defined as any complex chronic condition that was captured in the discharge diagnoses but was not evident in the past medical history. By definition, all CCCs coded during birth hospitalization (eg, at discharge from the neonatal intensive care unit) were assigned to “new” CCC. We calculated a kappa statistic to determine interrater reliability in determining the designation of new CCC. A sensitivity analysis examining these birth CCCs was also performed comparing no new CCC, new CCC, and new CCC after birth hospitalization. The methods appendix provides additional information on considering new CCCs. New technology, abstracted from chart review, was defined as technology placed during hospitalization that remained in place at discharge. If a child with existing technology had additional technology placed during the hospitalization (eg, a new tracheostomy in a child with a previously placed enteral feeding tube), the encounter was considered as having new technology placed.
Covariates
We created different sets of multivariable models to account for patient/hospitalization characteristics.
Statistical Analysis
A review of 600 cases and 600 controls yields 89% power to detect statistical significance for covariates with an odds ratio of 1.25 (β = 0.22) if the candidate covariate has low to moderate correlation with other covariates (<0.3). If a candidate covariate has a moderate correlation with other covariates (0.6), we have 89% power to detect an odds ratio of 1.35 (β = 0.30).21 We calculated odds of 30-days unplanned readmission using conditional logistic regression to account for matched case-control design. All the analyses were performed using STATA 13 (Stata Corp., College Station, Texas).
RESULTS
Of the 41,422 eligible index hospitalizations during the study period, 9.4% resulted in a 30-day unplanned readmission. After randomly selecting one hospitalization per child, there were 781 eligible cases. We subsequent matched all but one eligible case to a control. We randomly selected encounters for medical record review, reviewing a total of 1,212 encounters. After excluding pairs with incomplete records, we included 595 cases and 595 controls in this analysis (Figure). Patient/hospitalization characteristics are displayed in Table 1. The most frequent primary discharge diagnoses are displayed in Appendix Table 1.
Models of Medical Complexity at Discharge
Polypharmacy after discharge was common for both readmitted and nonreadmitted patients. Children who experienced unplanned readmission in 30 days were discharged with a median of four different scheduled medications (interquartile range [IQR] 2,7) which translated into a median of six (IQR 3,12) scheduled doses in a 24-hour period. In comparison, children without an unplanned readmission had a median of two different scheduled medications (IQR 1,3) with a median of three (IQR 0,7) scheduled doses in a 24-hour period. Medical technology was more common in case children (42%) than in control children (14%). Central lines and enteral tubes were the most common forms of medical technology in both cases and controls. Home health referral was common in both cases (44%) and controls (23%; Table 1).
In Model 1 (adjusting only for patient characteristics; Table 3), being discharged on two or more scheduled medications was associated with higher odds of readmission compared to being discharged without medications, with additional medications associated with even higher odds of readmission. Children with any technology had higher odds of readmission than children without medical technology. Likewise, home healthcare visits after discharge were associated with elevated odds of readmission in multivariable analyses without LOS. However, after adding LOS to the model (Model 2), home healthcare visits were no longer significantly associated with readmission.
Change in Medical Complexity Models
The adjudication of new CCCs had good reliability (Κ = 0.72). New CCCs occurred in 18% and new technologies occurred in 17% of cases. Comparatively, new CCCs occurred in 10% and new technologies in 7% of hospitalizations in control children (Table 1). In bivariate analyses, both aspects of change in medical complexity were associated with higher odds of readmission (Table 2). In multivariate analysis with patient characteristics (Model 3; Table 3), all aspects of change in complexity were associated with elevated odds of readmission. A new CCC was associated with higher odds of readmission (adjusted OR (AOR) 1.75, 95% CI: 1.11-2.75) as was new technology during admission (AOR 1.84, 95%CI: 1.09-3.10). Furthermore, the odds of readmission for medical complexity variables (polypharmacy and home healthcare need) remained largely unchanged when adding the change in medical complexity variables (ie, comparing Model 1 and Model 3). However, when accounting for LOS (Model 4), neither the acquisition of a new CCC nor the addition of new technology was associated with readmission. The most common form of new technology was central line followed by nonsurgically placed enteral tube (Appendix Table 2). Finally, in sensitivity analyses (results not detailed), separating new CCC acquired at birth and new CCCs in nonbirth hospitalizations, compared to hospitalizations with no new CCC, yielded similar results as the primary analyses.
DISCUSSION
We examined multiple attributes of polypharmacy—the number of scheduled medications, number of as-needed medications, and number of scheduled doses per 24 hours. Interestingly, only the scheduled medications (count of medication and number of doses) were associated with elevated readmission risk. As-needed medications have heterogeneity in the level of importance from critical (eg, seizure rescue) to discretionary (eg, antipyretics, creams). The burden of managing these types of medications may still be high (ie, parents must decide when to administer a critical medication); however, this burden does not translate into increased readmission risk in this population.
Not surprisingly, greater medical complexity—as defined by higher numbers of scheduled discharge medications and technology assistance—is associated with 30-day readmission risk. Our analyses do not allow us to determine how much of the increased risk is due to additional care burden and risks of polypharmacy versus the inherent increase in complexity and severity of illness for which polypharmacy is a marker. Tailoring discharge regimens to the realities of daily life, with the goal of “minimally disruptive medicine”22,23 (eg, integrating manageable discharge medication routines into school and work schedules), is not a common feature of pediatric discharge planning. For adult patients with complex medical conditions, tailoring medication regimens in a minimally disruptive way is known to improve outcomes.24 Similarly, adopting minimally disruptive techniques to integrate the polypharmacy inherent in discharge could potentially mitigate some of the readmission risks for children and adolescents.
Contrary to our hypothesis, new technologies and new diagnoses did not confer additional readmission risk when accounting for LOS and patient characteristics. One potential explanation is varying risks conveyed by different types of new technologies placed during hospitalization. Central lines, the most common form of new technology, is associated with higher odds of reutilization in unadjusted analyses. However, the second most common form of new technology, nonsurgically placed enteral feeding tube, was not. Further analyses of the differential effects of new technology should be further examined in larger datasets. Additionally, the lack of additional readmission risk from new technology may relate to additional teaching and support provided to families of patients undergoing unfamiliar procedures offsets the risks inherent of greater complexity. If so, it may be that the more intensive teaching and postdischarge support provided to families with new technology or a new diagnosis could be replicated through refresher teaching during hospitalizations, when a patient’s state of health is status quo for the family (ie, the child was admitted and discharged with the same technology and diagnoses). This notion is supported by prior work that demonstrated successful readmission reduction interventions for children with chronic conditions often rely on enhanced education or coaching.25,26
We elected to present models both with and without LOS as a confounder because it is a potentially modifiable attribute of hospitalization. Change in medical complexity aspects were significantly associated with readmission in multivariable models without LOS. However, with the addition of LOS, they were no longer significant. Thus, the readmission risk of new complexity is accounted for by the readmission risk inherent in a longer LOS. This finding prompts additional questions that merit further study: is it that LOS is a general marker for heightened complexity, or is it that a longer LOS can modify readmission risk through additional in-hospital care and time for enhanced education?
Our study has several strengths. We were able to discern true complexity at the time of discharge through medical record review. For example, if a child had a peripherally inserted central catheter placed during hospitalization, it cannot be ascertained through administrative data without medical record review if the technology was removed or in place at discharge. Likewise, medical record review allows for identification of medical technology which is not surgically implanted (eg, nasogastric feeding tubes). Given the “fog” families report as part of their in-hospital experience and its threats to education and postdischarge contingency planning,17 we felt it important to evaluate medical technology regardless of whether or not it was surgically placed. Additionally, the more detailed and nuanced understanding gained of polypharmacy burden can better inform both risk prediction models and interventions to improve the transition from hospital to home.
This study
CONCLUSION
Medical complexity at discharge is associated with pediatric readmission risk. Contrary to our hypothesis, the addition of new technologies and new CCC diagnoses are not associated with pediatric readmission, after accounting for patient and hospitalization factors including LOS. The dynamics of LOS as a risk factor for readmission for children with medical complexity are likely multifaceted and merit further investigation in a multi-institutional study.
Disclosures
The authors report no potential conflicts of interest.
Funding
This work was supported by a grant from the Agency for Healthcare Research and Quality (1K08HS204735-01A1) and a grant from the Blue Cross Blue Shield of Michigan Foundation.
1. Diaz-Caneja A, Gledhill J, Weaver T, Nadel S, Garralda E. A child’s admission to hospital: a qualitative study examining the experiences of parents. Intensive Care Med. 2005;31(9):1248-1254. https://doi.org/10.1007/s00134-005-2728-8.
2. Lapillonne A, Regnault A, Gournay V, et al. Impact on parents of bronchiolitis hospitalization of full-term, preterm and congenital heart disease infants. BMC Pediatrics. 2012;12:171. https://doi.org/10.1186/1471-2431-12-171.
3. Leader S, Jacobson P, Marcin J, Vardis R, Sorrentino M, Murray D. A method for identifying the financial burden of hospitalized infants on families. Value Health. 2002;5(1):55-59. https://doi.org/10.1046/j.1524-4733.2002.51076.x.
4. Leidy NK, Margolis MK, Marcin JP, et al. The impact of severe respiratory syncytial virus on the child, caregiver, and family during hospitalization and recovery. Pediatrics. 2005;115(6):1536-1546. https://doi.org/10.1542/peds.2004-1149.
5. Rennick JE, Johnston CC, Dougherty G, Platt R, Ritchie JA. Children’s psychological responses after critical illness and exposure to invasive technology. J Dev Behav Pediatr. 2002;23(3):133-144. PubMed
6. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. https://doi.org/10.1056/NEJM199102073240604.
7. Kohn LT, Corrigan J, Donaldson MS. To err is human: building a safer health system. Washington DC: National Academy Press; 2000.
8. Landrigan CP, Parry GJ, Bones CB, Hackbarth AD, Goldmann DA, Sharek PJ. Temporal trends in rates of patient harm resulting from medical care. N Engl J Med. 2010;363(22):2124-2134. https://doi.org/10.1056/NEJMsa1004404.
9. Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of healthcare-associated infections. N Engl J Med. 2014;370(13):1198-1208. https://doi.org/10.1056/NEJMoa1306801.
10. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351.
11. Bardach NS, Vittinghoff E, Asteria-Penaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. https://doi.org/10.1542/peds.2012-3527.
12. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-day hospital readmissions at a children’s hospital. Pediatrics. 2016;138(2):pii: e20154182. https://doi.org/10.1542/peds.2015-4182.
13. Bucholz EM, Gay JC, Hall M, Harris M, Berry JG. Timing and causes of common pediatric readmissions. J Pediatr. 2018;200:240-248. https://doi.org/10.1016/j.jpeds.2018.04.044.
14. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. https://doi.org/10.1001/jama.2011.122.
15. Winer JC, Aragona E, Fields AI, Stockwell DC. Comparison of clinical risk factors among pediatric patients with single admission, multiple admissions (without any 7-day readmissions), and 7-day readmission. Hosp Pediatr. 2016;6(3):119-125. https://doi.org/10.1542/hpeds.2015-0110.
16. Brittan MS, Martin S, Anderson L, Moss A, Torok MR. An electronic health record tool designed to improve pediatric hospital discharge has low predictive utility for readmissions. J Hosp Med. 2018;13(11):779-782. https://doi.org/10.12788/jhm.3043.
17. Solan LG, Beck AF, Brunswick SA, et al. The family perspective on hospital to home transitions: a qualitative study. Pediatrics. 2015;136(6):e1539-e1549. https://doi.org/10.1542/peds.2015-2098.
18. Auger KA, Mueller EL, Weinberg SH, et al. A validated method for identifying unplanned pediatric readmission. J Pediatr. 2016;170:105-112. https://doi.org/10.1016/j.jpeds.2015.11.051.
19. Auger KA, Shah SS, Davis MD, Brady PW. Counting the Ways to Count Medications: The Challenges of Defining Pediatric Polypharmacy. J Hosp Med. 2019;14(8):506-507. https://doi.org/10.12788/jhm.3213.
20. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatrics. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199.
21. Hsieh FY. Sample size tables for logistic regression. Stat Med. 1989;8(7):795-802. https://doi.org/10.1002/sim.4780080704.
22. May C, Montori VM, Mair FS. We need minimally disruptive medicine. BMJ. 2009;339:b2803. https://doi.org/10.1136/bmj.b2803.
23. Leppin AL, Montori VM, Gionfriddo MR. Minimally disruptive medicine: a pragmatically comprehensive model for delivering care to patients with multiple chronic conditions. Healthcare (Basel). 2015;3(1):50-63. https://doi.org/10.3390/healthcare3010050.
24. Serrano V, Spencer-Bonilla G, Boehmer KR, Montori VM. Minimally disruptive medicine for patients with diabetes. Curr Diab Rep. 2017;17(11):104. https://doi.org/10.1007/s11892-017-0935-7.
25. Auger KA, Kenyon CC, Feudtner C, Davis MM. Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2013;9(4):251-260. https://doi.org/10.1002/jhm.2134.
26. Coller RJ, Klitzner TS, Lerner CF, et al. Complex care hospital use and postdischarge coaching: a randomized controlled trial. Pediatrics. 2018;142(2):pii: e20174278. https://doi.org/10.1542/peds.2017-4278.
27. Hain PD, Gay JC, Berutti TW, Whitney GM, Wang W, Saville BR. Preventability of early readmissions at a children’s hospital. Pediatrics. 2013;131(1):e171-e181. https://doi.org/10.1542/peds.2012-0820.
28. Auger KA, Teufel RJ, 2nd, Harris JM, 2nd, et al. Children’s hospital characteristics and readmission metrics. Pediatrics. 2017;139(2). https://doi.org/10.1542/peds.2016-1720.
29. Gay JC, Agrawal R, Auger KA, et al. Rates and impact of potentially preventable readmissions at children’s hospitals. J Pediatr. 2015;166(3):613-619 e615. https://doi.org/10.1016/j.jpeds.2014.10.052.
1. Diaz-Caneja A, Gledhill J, Weaver T, Nadel S, Garralda E. A child’s admission to hospital: a qualitative study examining the experiences of parents. Intensive Care Med. 2005;31(9):1248-1254. https://doi.org/10.1007/s00134-005-2728-8.
2. Lapillonne A, Regnault A, Gournay V, et al. Impact on parents of bronchiolitis hospitalization of full-term, preterm and congenital heart disease infants. BMC Pediatrics. 2012;12:171. https://doi.org/10.1186/1471-2431-12-171.
3. Leader S, Jacobson P, Marcin J, Vardis R, Sorrentino M, Murray D. A method for identifying the financial burden of hospitalized infants on families. Value Health. 2002;5(1):55-59. https://doi.org/10.1046/j.1524-4733.2002.51076.x.
4. Leidy NK, Margolis MK, Marcin JP, et al. The impact of severe respiratory syncytial virus on the child, caregiver, and family during hospitalization and recovery. Pediatrics. 2005;115(6):1536-1546. https://doi.org/10.1542/peds.2004-1149.
5. Rennick JE, Johnston CC, Dougherty G, Platt R, Ritchie JA. Children’s psychological responses after critical illness and exposure to invasive technology. J Dev Behav Pediatr. 2002;23(3):133-144. PubMed
6. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. https://doi.org/10.1056/NEJM199102073240604.
7. Kohn LT, Corrigan J, Donaldson MS. To err is human: building a safer health system. Washington DC: National Academy Press; 2000.
8. Landrigan CP, Parry GJ, Bones CB, Hackbarth AD, Goldmann DA, Sharek PJ. Temporal trends in rates of patient harm resulting from medical care. N Engl J Med. 2010;363(22):2124-2134. https://doi.org/10.1056/NEJMsa1004404.
9. Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of healthcare-associated infections. N Engl J Med. 2014;370(13):1198-1208. https://doi.org/10.1056/NEJMoa1306801.
10. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351.
11. Bardach NS, Vittinghoff E, Asteria-Penaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. https://doi.org/10.1542/peds.2012-3527.
12. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-day hospital readmissions at a children’s hospital. Pediatrics. 2016;138(2):pii: e20154182. https://doi.org/10.1542/peds.2015-4182.
13. Bucholz EM, Gay JC, Hall M, Harris M, Berry JG. Timing and causes of common pediatric readmissions. J Pediatr. 2018;200:240-248. https://doi.org/10.1016/j.jpeds.2018.04.044.
14. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. https://doi.org/10.1001/jama.2011.122.
15. Winer JC, Aragona E, Fields AI, Stockwell DC. Comparison of clinical risk factors among pediatric patients with single admission, multiple admissions (without any 7-day readmissions), and 7-day readmission. Hosp Pediatr. 2016;6(3):119-125. https://doi.org/10.1542/hpeds.2015-0110.
16. Brittan MS, Martin S, Anderson L, Moss A, Torok MR. An electronic health record tool designed to improve pediatric hospital discharge has low predictive utility for readmissions. J Hosp Med. 2018;13(11):779-782. https://doi.org/10.12788/jhm.3043.
17. Solan LG, Beck AF, Brunswick SA, et al. The family perspective on hospital to home transitions: a qualitative study. Pediatrics. 2015;136(6):e1539-e1549. https://doi.org/10.1542/peds.2015-2098.
18. Auger KA, Mueller EL, Weinberg SH, et al. A validated method for identifying unplanned pediatric readmission. J Pediatr. 2016;170:105-112. https://doi.org/10.1016/j.jpeds.2015.11.051.
19. Auger KA, Shah SS, Davis MD, Brady PW. Counting the Ways to Count Medications: The Challenges of Defining Pediatric Polypharmacy. J Hosp Med. 2019;14(8):506-507. https://doi.org/10.12788/jhm.3213.
20. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatrics. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199.
21. Hsieh FY. Sample size tables for logistic regression. Stat Med. 1989;8(7):795-802. https://doi.org/10.1002/sim.4780080704.
22. May C, Montori VM, Mair FS. We need minimally disruptive medicine. BMJ. 2009;339:b2803. https://doi.org/10.1136/bmj.b2803.
23. Leppin AL, Montori VM, Gionfriddo MR. Minimally disruptive medicine: a pragmatically comprehensive model for delivering care to patients with multiple chronic conditions. Healthcare (Basel). 2015;3(1):50-63. https://doi.org/10.3390/healthcare3010050.
24. Serrano V, Spencer-Bonilla G, Boehmer KR, Montori VM. Minimally disruptive medicine for patients with diabetes. Curr Diab Rep. 2017;17(11):104. https://doi.org/10.1007/s11892-017-0935-7.
25. Auger KA, Kenyon CC, Feudtner C, Davis MM. Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2013;9(4):251-260. https://doi.org/10.1002/jhm.2134.
26. Coller RJ, Klitzner TS, Lerner CF, et al. Complex care hospital use and postdischarge coaching: a randomized controlled trial. Pediatrics. 2018;142(2):pii: e20174278. https://doi.org/10.1542/peds.2017-4278.
27. Hain PD, Gay JC, Berutti TW, Whitney GM, Wang W, Saville BR. Preventability of early readmissions at a children’s hospital. Pediatrics. 2013;131(1):e171-e181. https://doi.org/10.1542/peds.2012-0820.
28. Auger KA, Teufel RJ, 2nd, Harris JM, 2nd, et al. Children’s hospital characteristics and readmission metrics. Pediatrics. 2017;139(2). https://doi.org/10.1542/peds.2016-1720.
29. Gay JC, Agrawal R, Auger KA, et al. Rates and impact of potentially preventable readmissions at children’s hospitals. J Pediatr. 2015;166(3):613-619 e615. https://doi.org/10.1016/j.jpeds.2014.10.052.
© 2019 Society of Hospital Medicine
HHS proposes pathways for drug importation
Officials at the U.S. Department of Health and Human Services have announced a new plan that they say would lay the foundation for safe importation of certain medications, with the aim of expanding drug access and lowering prescription costs for patients.
The action plan, unveiled July 31, outlines two pathways for drug importation from foreign markets. The first route would authorize states, wholesalers, or pharmacists to propose pilot demonstrations on how they would import drugs from Canada into the United States, provided these are versions of drugs already approved by the Food and Drug Administration. Similarly, a second pathway would allow manufacturers that sell in foreign countries the opportunity to import drugs that are versions of FDA-approved medications.
HHS Secretary Alex M. Azar II said the action plan is part of President Trump’s drug-pricing blueprint and is intended to combat the sky-high price tags on many prescription medications.
“President Trump has been clear: For too long American patients have been paying exorbitantly high prices for prescription drugs that are made available to other countries at lower prices,” Mr. Azar said in a statement. “[The] announcement outlines the pathways the administration intends to explore to allow safe importation of certain prescription drugs to lower prices and reduce out of pocket costs for American patients. This is the next important step in the administration’s work to end foreign freeloading and put American patients first.”
Under the first pathway, HHS would review plans submitted by states, pharmacists, or drugmakers that outline how the entities would import Health Canada–approved drugs that are in compliance with the federal Food, Drug, and Cosmetic Act. The importation would occur in a manner that assures the drug’s validity and meets the cost requirements of federal rule making, according to an HHS fact sheet.
Demonstration projects would be time-limited and require regular reporting to ensure safety and cost conditions are being met.
Under the second pathway, manufacturers of FDA-approved drug products would be able to import versions of those drugs that they sell in foreign countries through a special process to be outlined by the agency. As part of the process, drugmakers would need to establish that the foreign version is the same as the U.S. version. The FDA would then allow the drug to be labeled for sale in the U.S. and imported, according to the fact sheet. HHS officials said they believe that manufacturers would use this pathway to offer U.S. patients lower-cost versions of their drugs and the medications affected could potentially include those used to treat diabetes, rheumatoid arthritis, cardiovascular disorders, and cancer.
“In recent years, multiple manufacturers have stated (either publicly or in statements to the Administration) that they wanted to offer lower cost versions but could not readily do so because they were locked into contracts with other parties in the supply chain,” HHS officials stated in the fact sheet. “This pathway would highlight an opportunity for manufacturers to use importation to offer lower-cost versions of their drugs.”
HHS plans to introduce its action plan through a formal notice of proposed rulemaking, which has not yet been finalized. Some elements of the final proposal may differ from its initial descriptions to reflect further consideration of the relevant issues, the agency noted.
Acting FDA Commissioner Ned Sharpless, MD, said the agency has a unique role to play in promoting competition that can help reduce drug prices and improve access to medicine for Americans.
“Driving down drug prices requires a comprehensive approach and we must continue to look at all innovative solutions to this challenge,” Dr. Sharpless said in a statement. “[The] proposal is the result of the hard work by the dedicated staff of the FDA, in close collaboration with HHS and the White House, to identify potential pathways we can pursue to support the safe importation of certain prescription drugs.”
Sen. Lamar Alexander (R-Tenn.), chair of the Health, Education, Labor and Pensions committee, said the administration’s proposal sounds promising as long as the plan ensures the safety and efficacy of imported medications.
“This is the first administration to take concrete steps to allow importation of prescription drugs to reduce their cost and I welcome it,” Sen. Alexander said in a statement. “The key for me is whether this plan preserves the Food and Drug Administration’s gold standard for safety and effectiveness. Millions of Americans every day buy prescription drugs relying on the FDA’s guarantee of quality.”
Officials at the U.S. Department of Health and Human Services have announced a new plan that they say would lay the foundation for safe importation of certain medications, with the aim of expanding drug access and lowering prescription costs for patients.
The action plan, unveiled July 31, outlines two pathways for drug importation from foreign markets. The first route would authorize states, wholesalers, or pharmacists to propose pilot demonstrations on how they would import drugs from Canada into the United States, provided these are versions of drugs already approved by the Food and Drug Administration. Similarly, a second pathway would allow manufacturers that sell in foreign countries the opportunity to import drugs that are versions of FDA-approved medications.
HHS Secretary Alex M. Azar II said the action plan is part of President Trump’s drug-pricing blueprint and is intended to combat the sky-high price tags on many prescription medications.
“President Trump has been clear: For too long American patients have been paying exorbitantly high prices for prescription drugs that are made available to other countries at lower prices,” Mr. Azar said in a statement. “[The] announcement outlines the pathways the administration intends to explore to allow safe importation of certain prescription drugs to lower prices and reduce out of pocket costs for American patients. This is the next important step in the administration’s work to end foreign freeloading and put American patients first.”
Under the first pathway, HHS would review plans submitted by states, pharmacists, or drugmakers that outline how the entities would import Health Canada–approved drugs that are in compliance with the federal Food, Drug, and Cosmetic Act. The importation would occur in a manner that assures the drug’s validity and meets the cost requirements of federal rule making, according to an HHS fact sheet.
Demonstration projects would be time-limited and require regular reporting to ensure safety and cost conditions are being met.
Under the second pathway, manufacturers of FDA-approved drug products would be able to import versions of those drugs that they sell in foreign countries through a special process to be outlined by the agency. As part of the process, drugmakers would need to establish that the foreign version is the same as the U.S. version. The FDA would then allow the drug to be labeled for sale in the U.S. and imported, according to the fact sheet. HHS officials said they believe that manufacturers would use this pathway to offer U.S. patients lower-cost versions of their drugs and the medications affected could potentially include those used to treat diabetes, rheumatoid arthritis, cardiovascular disorders, and cancer.
“In recent years, multiple manufacturers have stated (either publicly or in statements to the Administration) that they wanted to offer lower cost versions but could not readily do so because they were locked into contracts with other parties in the supply chain,” HHS officials stated in the fact sheet. “This pathway would highlight an opportunity for manufacturers to use importation to offer lower-cost versions of their drugs.”
HHS plans to introduce its action plan through a formal notice of proposed rulemaking, which has not yet been finalized. Some elements of the final proposal may differ from its initial descriptions to reflect further consideration of the relevant issues, the agency noted.
Acting FDA Commissioner Ned Sharpless, MD, said the agency has a unique role to play in promoting competition that can help reduce drug prices and improve access to medicine for Americans.
“Driving down drug prices requires a comprehensive approach and we must continue to look at all innovative solutions to this challenge,” Dr. Sharpless said in a statement. “[The] proposal is the result of the hard work by the dedicated staff of the FDA, in close collaboration with HHS and the White House, to identify potential pathways we can pursue to support the safe importation of certain prescription drugs.”
Sen. Lamar Alexander (R-Tenn.), chair of the Health, Education, Labor and Pensions committee, said the administration’s proposal sounds promising as long as the plan ensures the safety and efficacy of imported medications.
“This is the first administration to take concrete steps to allow importation of prescription drugs to reduce their cost and I welcome it,” Sen. Alexander said in a statement. “The key for me is whether this plan preserves the Food and Drug Administration’s gold standard for safety and effectiveness. Millions of Americans every day buy prescription drugs relying on the FDA’s guarantee of quality.”
Officials at the U.S. Department of Health and Human Services have announced a new plan that they say would lay the foundation for safe importation of certain medications, with the aim of expanding drug access and lowering prescription costs for patients.
The action plan, unveiled July 31, outlines two pathways for drug importation from foreign markets. The first route would authorize states, wholesalers, or pharmacists to propose pilot demonstrations on how they would import drugs from Canada into the United States, provided these are versions of drugs already approved by the Food and Drug Administration. Similarly, a second pathway would allow manufacturers that sell in foreign countries the opportunity to import drugs that are versions of FDA-approved medications.
HHS Secretary Alex M. Azar II said the action plan is part of President Trump’s drug-pricing blueprint and is intended to combat the sky-high price tags on many prescription medications.
“President Trump has been clear: For too long American patients have been paying exorbitantly high prices for prescription drugs that are made available to other countries at lower prices,” Mr. Azar said in a statement. “[The] announcement outlines the pathways the administration intends to explore to allow safe importation of certain prescription drugs to lower prices and reduce out of pocket costs for American patients. This is the next important step in the administration’s work to end foreign freeloading and put American patients first.”
Under the first pathway, HHS would review plans submitted by states, pharmacists, or drugmakers that outline how the entities would import Health Canada–approved drugs that are in compliance with the federal Food, Drug, and Cosmetic Act. The importation would occur in a manner that assures the drug’s validity and meets the cost requirements of federal rule making, according to an HHS fact sheet.
Demonstration projects would be time-limited and require regular reporting to ensure safety and cost conditions are being met.
Under the second pathway, manufacturers of FDA-approved drug products would be able to import versions of those drugs that they sell in foreign countries through a special process to be outlined by the agency. As part of the process, drugmakers would need to establish that the foreign version is the same as the U.S. version. The FDA would then allow the drug to be labeled for sale in the U.S. and imported, according to the fact sheet. HHS officials said they believe that manufacturers would use this pathway to offer U.S. patients lower-cost versions of their drugs and the medications affected could potentially include those used to treat diabetes, rheumatoid arthritis, cardiovascular disorders, and cancer.
“In recent years, multiple manufacturers have stated (either publicly or in statements to the Administration) that they wanted to offer lower cost versions but could not readily do so because they were locked into contracts with other parties in the supply chain,” HHS officials stated in the fact sheet. “This pathway would highlight an opportunity for manufacturers to use importation to offer lower-cost versions of their drugs.”
HHS plans to introduce its action plan through a formal notice of proposed rulemaking, which has not yet been finalized. Some elements of the final proposal may differ from its initial descriptions to reflect further consideration of the relevant issues, the agency noted.
Acting FDA Commissioner Ned Sharpless, MD, said the agency has a unique role to play in promoting competition that can help reduce drug prices and improve access to medicine for Americans.
“Driving down drug prices requires a comprehensive approach and we must continue to look at all innovative solutions to this challenge,” Dr. Sharpless said in a statement. “[The] proposal is the result of the hard work by the dedicated staff of the FDA, in close collaboration with HHS and the White House, to identify potential pathways we can pursue to support the safe importation of certain prescription drugs.”
Sen. Lamar Alexander (R-Tenn.), chair of the Health, Education, Labor and Pensions committee, said the administration’s proposal sounds promising as long as the plan ensures the safety and efficacy of imported medications.
“This is the first administration to take concrete steps to allow importation of prescription drugs to reduce their cost and I welcome it,” Sen. Alexander said in a statement. “The key for me is whether this plan preserves the Food and Drug Administration’s gold standard for safety and effectiveness. Millions of Americans every day buy prescription drugs relying on the FDA’s guarantee of quality.”
Critics say hospital price transparency proposal ‘misses the mark’
A proposal by the Centers for Medicare & Medicaid Services to require full price transparency, including the disclosure of both list prices and payer-negotiated prices, is already receiving pushback.
Rick Pollack, president and CEO of the American Hospital Association, said in a statement that “mandating disclosure of negotiated rates between insurers and hospitals is the wrong approach,” adding that it “could seriously limit the choices available to patients in the private market and fuel anticompetitive behavior among commercial health insurers in an already highly concentrated insurance industry.”
The requirement for hospital price transparency was posted online July 29 as part of the proposed annual update to the hospital outpatient prospective payment system (OPPS) for 2020. It is scheduled for publication in the Federal Register on Aug. 9.
CMS is proposing, beginning in calendar year 2020, that hospitals make publicly available their “standard charges,” defined as the gross – or list – price of for all services provided by the hospital, as well as payer-specific negotiated prices. To allow for price comparisons, prices would be posted on the Internet in a machine-readable file that includes common billing or accounting codes and a description of the item of service being delivered.
Additionally, hospitals must make payer-specific negotiated prices for “shoppable” services, defined as services that can be scheduled in advance – such as x-rays, outpatient visits, imaging and laboratory tests, or bundled services like a cesarean delivery with pre- and postdelivery care – in a consumer-friendly manner.
“As deductibles rise and with 29 million uninsured, patients have the right to know the price of health care services so they can shop around for the best deal,” CMS Administrator Seema Verma said during a July 29 press conference. “In fact, a recent poll showed that the majority of Americans have tried to get pricing information before getting care, but have found it challenging to find that information.”
She noted that patients may see prices that range from 150% of Medicare rates to more than 400% for the same service.
Hospitals will need to display at least 300 shoppable services, including 70 that are CMS selected and 230 that are hospital selected, according to a fact sheet outlining this and other proposed OPPS updates for 2020.
“If a hospital does not provide one or more of the 70 CMS selected shoppable services, the hospital must select additional shoppable services such that the total number of shoppable services is at least 300,” the fact sheet states.
Information on pricing will be required to be updated at least annually.
CMS is including enforcement tools as part of the proposal, including fines to hospitals for noncompliance.
“Price transparency creates a marketplace where providers compete on the basis of cost and quality that will lower cost,” Ms. Verma said.
However, that notion has been challenged by America’s Health Insurance Plans (AHIP).
Matt Eyles, president and CEO of AHIP said in a statement that “multiple experts, including the Federal Trade Commission, agree that disclosing privately negotiated rates will make it harder to bargain for lower rates, creating a floor, not a ceiling, for the prices that hospitals would be willing to accept. Publicly disclosing competitively negotiated, proprietary rates will push prices and premiums higher, not lower, for consumers, patients, and taxpayers.”
Mr. Pollack of the American Hospital Association agreed. “While we support transparency, [this] proposal misses the mark, exceeds the Administration’s legal authority, and should be abandoned.”
Ms. Verma said she believed the agency had legal authority to impose this requirement and is not worried about possible lawsuits that could challenge this provision.
“This administration is not afraid of those things,” she said. “We are not about protecting the status quo when it doesn’t work for patients.”
A proposal by the Centers for Medicare & Medicaid Services to require full price transparency, including the disclosure of both list prices and payer-negotiated prices, is already receiving pushback.
Rick Pollack, president and CEO of the American Hospital Association, said in a statement that “mandating disclosure of negotiated rates between insurers and hospitals is the wrong approach,” adding that it “could seriously limit the choices available to patients in the private market and fuel anticompetitive behavior among commercial health insurers in an already highly concentrated insurance industry.”
The requirement for hospital price transparency was posted online July 29 as part of the proposed annual update to the hospital outpatient prospective payment system (OPPS) for 2020. It is scheduled for publication in the Federal Register on Aug. 9.
CMS is proposing, beginning in calendar year 2020, that hospitals make publicly available their “standard charges,” defined as the gross – or list – price of for all services provided by the hospital, as well as payer-specific negotiated prices. To allow for price comparisons, prices would be posted on the Internet in a machine-readable file that includes common billing or accounting codes and a description of the item of service being delivered.
Additionally, hospitals must make payer-specific negotiated prices for “shoppable” services, defined as services that can be scheduled in advance – such as x-rays, outpatient visits, imaging and laboratory tests, or bundled services like a cesarean delivery with pre- and postdelivery care – in a consumer-friendly manner.
“As deductibles rise and with 29 million uninsured, patients have the right to know the price of health care services so they can shop around for the best deal,” CMS Administrator Seema Verma said during a July 29 press conference. “In fact, a recent poll showed that the majority of Americans have tried to get pricing information before getting care, but have found it challenging to find that information.”
She noted that patients may see prices that range from 150% of Medicare rates to more than 400% for the same service.
Hospitals will need to display at least 300 shoppable services, including 70 that are CMS selected and 230 that are hospital selected, according to a fact sheet outlining this and other proposed OPPS updates for 2020.
“If a hospital does not provide one or more of the 70 CMS selected shoppable services, the hospital must select additional shoppable services such that the total number of shoppable services is at least 300,” the fact sheet states.
Information on pricing will be required to be updated at least annually.
CMS is including enforcement tools as part of the proposal, including fines to hospitals for noncompliance.
“Price transparency creates a marketplace where providers compete on the basis of cost and quality that will lower cost,” Ms. Verma said.
However, that notion has been challenged by America’s Health Insurance Plans (AHIP).
Matt Eyles, president and CEO of AHIP said in a statement that “multiple experts, including the Federal Trade Commission, agree that disclosing privately negotiated rates will make it harder to bargain for lower rates, creating a floor, not a ceiling, for the prices that hospitals would be willing to accept. Publicly disclosing competitively negotiated, proprietary rates will push prices and premiums higher, not lower, for consumers, patients, and taxpayers.”
Mr. Pollack of the American Hospital Association agreed. “While we support transparency, [this] proposal misses the mark, exceeds the Administration’s legal authority, and should be abandoned.”
Ms. Verma said she believed the agency had legal authority to impose this requirement and is not worried about possible lawsuits that could challenge this provision.
“This administration is not afraid of those things,” she said. “We are not about protecting the status quo when it doesn’t work for patients.”
A proposal by the Centers for Medicare & Medicaid Services to require full price transparency, including the disclosure of both list prices and payer-negotiated prices, is already receiving pushback.
Rick Pollack, president and CEO of the American Hospital Association, said in a statement that “mandating disclosure of negotiated rates between insurers and hospitals is the wrong approach,” adding that it “could seriously limit the choices available to patients in the private market and fuel anticompetitive behavior among commercial health insurers in an already highly concentrated insurance industry.”
The requirement for hospital price transparency was posted online July 29 as part of the proposed annual update to the hospital outpatient prospective payment system (OPPS) for 2020. It is scheduled for publication in the Federal Register on Aug. 9.
CMS is proposing, beginning in calendar year 2020, that hospitals make publicly available their “standard charges,” defined as the gross – or list – price of for all services provided by the hospital, as well as payer-specific negotiated prices. To allow for price comparisons, prices would be posted on the Internet in a machine-readable file that includes common billing or accounting codes and a description of the item of service being delivered.
Additionally, hospitals must make payer-specific negotiated prices for “shoppable” services, defined as services that can be scheduled in advance – such as x-rays, outpatient visits, imaging and laboratory tests, or bundled services like a cesarean delivery with pre- and postdelivery care – in a consumer-friendly manner.
“As deductibles rise and with 29 million uninsured, patients have the right to know the price of health care services so they can shop around for the best deal,” CMS Administrator Seema Verma said during a July 29 press conference. “In fact, a recent poll showed that the majority of Americans have tried to get pricing information before getting care, but have found it challenging to find that information.”
She noted that patients may see prices that range from 150% of Medicare rates to more than 400% for the same service.
Hospitals will need to display at least 300 shoppable services, including 70 that are CMS selected and 230 that are hospital selected, according to a fact sheet outlining this and other proposed OPPS updates for 2020.
“If a hospital does not provide one or more of the 70 CMS selected shoppable services, the hospital must select additional shoppable services such that the total number of shoppable services is at least 300,” the fact sheet states.
Information on pricing will be required to be updated at least annually.
CMS is including enforcement tools as part of the proposal, including fines to hospitals for noncompliance.
“Price transparency creates a marketplace where providers compete on the basis of cost and quality that will lower cost,” Ms. Verma said.
However, that notion has been challenged by America’s Health Insurance Plans (AHIP).
Matt Eyles, president and CEO of AHIP said in a statement that “multiple experts, including the Federal Trade Commission, agree that disclosing privately negotiated rates will make it harder to bargain for lower rates, creating a floor, not a ceiling, for the prices that hospitals would be willing to accept. Publicly disclosing competitively negotiated, proprietary rates will push prices and premiums higher, not lower, for consumers, patients, and taxpayers.”
Mr. Pollack of the American Hospital Association agreed. “While we support transparency, [this] proposal misses the mark, exceeds the Administration’s legal authority, and should be abandoned.”
Ms. Verma said she believed the agency had legal authority to impose this requirement and is not worried about possible lawsuits that could challenge this provision.
“This administration is not afraid of those things,” she said. “We are not about protecting the status quo when it doesn’t work for patients.”
Key clinical point: CMS proposes complete transparency in hospital prices.
Major finding: Hospitals would be required to make public the list prices, as well as all payer-negotiated prices.
Study details: CMS asserts that the disclosure of pricing data will lead to reduced prices through market competition.
Disclosures: CMS, as issuer of the proposed rule, makes no disclosures.
Source: Proposed rule updating the hospital outpatient prospective payment system for 2020.
Timing, volume of transfusion may not matter in children with severe anemia
Trial results suggest African children with uncomplicated, severe anemia may not require immediate blood transfusion, and the volume of transfusion may only matter in the context of fever.
The TRACT trial showed no significant differences in 28-day mortality or other clinical outcomes between children who received immediate transfusions and those who did not.
Similarly, there was no significant difference in 28-day mortality among children who received transfusions of 20 mL/kg and those who received transfusions of 30 mL/kg. There was evidence to suggest a higher transfusion volume may benefit children without fevers, but this was an exploratory endpoint. The findings were published in the New England Journal of Medicine.
These results suggest “there is no credible reason to transfuse immediately or to transfuse a higher volume of blood, at least in pediatric populations in regions such as these two sub-Saharan countries [Uganda and Malawi],” Julie R. Ingelfinger, MD, of Massachusetts General Hospital in Boston, wrote in an accompanying editorial, also published in the New England Journal of Medicine (2019;381:475-6).
“The possible effect of higher volume transfusion in patients with fever may trigger additional and potentially useful studies,” she added.
Immediate transfusion
One goal of the TRACT trial was to determine if blood transfusion is the best treatment for children with severe anemia. With this in mind, Kathryn Maitland, MD, PhD, of Imperial College London and colleagues evaluated 1,565 Ugandan and Malawian children with uncomplicated, severe anemia. The patients’ median age was 26 months, and 984 (62.9%) had malaria.
The children were randomized to immediate transfusion (n = 778) or no immediate transfusion (n = 787). Children who did not have an immediate transfusion (control group) could receive a transfusion if they exhibited new signs of clinical severity or had their hemoglobin decrease to below 4 g/dL.
All children in the immediate-transfusion group received a transfusion, as did 386 (49.0%) in the control group. The median time to transfusion was 1.3 hours in the immediate group and 24.9 hours in the control group. The mean total blood volume transfused per child was 314 plus or minus 228 mL and 142 plus or minus 224, respectively. The follow-up period was 180 days, and 4.5% of patients (n = 71) were lost to follow-up.
The researchers found no significant difference between the treatment groups with regard to mortality, other clinical outcomes, or the cost of care.
The 28-day mortality rate was 0.9% in the immediate-transfusion group and 1.7% in the control group (hazard ratio, 0.54; 95% confidence interval, 0.22-1.36; P = .19). The 180-day mortality was 4.5% and 6.0%, respectively (HR, 0.75; 95% CI, 0.48-1.15).
Transfusion volume
To assess the effects of transfusion volume, Dr. Maitland and colleagues evaluated 3,196 Ugandan and Malawian children with severe anemia. The median age of the children was 37 months, and 2,050 (64.1%) had malaria.
The children received a transfusion of 30 mL/kg (n = 1,592) or 20 mL/kg (n = 1,596) at a median of 1.2 hours after randomization. Some children – 197 in the 30-mL/kg group and 300 in the 20-mL/kg group – received additional transfusions. The mean volume of total blood transfused per child was 475 plus or minus 385 mL, and 353 plus or minus 348 mL, respectively.
Overall, there was no significant between-group difference with regard to mortality. The 28-day mortality rate was 3.4% in the 30 mL/kg group and 4.5% in the 20 mL/kg group (HR = 0.76; 95% CI, 0.54 to 1.08; P = .12).
However, the 28-day mortality rate did differ according to the presence of fever at screening. The mortality rate was lower in the 30 mL/kg group for children without fevers (HR = 0.43; 95% CI, 0.27 to 0.69) but higher in the 30 mL/kg group for febrile children (HR = 1.91; 95% CI, 1.04 to 3.49).
For other outcomes, including readmissions and serious adverse events, the researchers found no significant between-group differences.
This trial was supported by a grant from the United Kingdom Medical Research Council through a concordat with the Department for International Development. One researcher has a Wellcome Senior Research Fellowship, and another is a National Institute for Health Research Senior Investigator. Dr. Ingelfinger is a deputy editor at the New England Journal of Medicine. No other relevant conflicts of interest were reported.
SOURCES: Maitland K et al. N Engl J Med. 2019;381:407-19. Maitland K et al. N Engl J Med. 2019;381:420-31.
Trial results suggest African children with uncomplicated, severe anemia may not require immediate blood transfusion, and the volume of transfusion may only matter in the context of fever.
The TRACT trial showed no significant differences in 28-day mortality or other clinical outcomes between children who received immediate transfusions and those who did not.
Similarly, there was no significant difference in 28-day mortality among children who received transfusions of 20 mL/kg and those who received transfusions of 30 mL/kg. There was evidence to suggest a higher transfusion volume may benefit children without fevers, but this was an exploratory endpoint. The findings were published in the New England Journal of Medicine.
These results suggest “there is no credible reason to transfuse immediately or to transfuse a higher volume of blood, at least in pediatric populations in regions such as these two sub-Saharan countries [Uganda and Malawi],” Julie R. Ingelfinger, MD, of Massachusetts General Hospital in Boston, wrote in an accompanying editorial, also published in the New England Journal of Medicine (2019;381:475-6).
“The possible effect of higher volume transfusion in patients with fever may trigger additional and potentially useful studies,” she added.
Immediate transfusion
One goal of the TRACT trial was to determine if blood transfusion is the best treatment for children with severe anemia. With this in mind, Kathryn Maitland, MD, PhD, of Imperial College London and colleagues evaluated 1,565 Ugandan and Malawian children with uncomplicated, severe anemia. The patients’ median age was 26 months, and 984 (62.9%) had malaria.
The children were randomized to immediate transfusion (n = 778) or no immediate transfusion (n = 787). Children who did not have an immediate transfusion (control group) could receive a transfusion if they exhibited new signs of clinical severity or had their hemoglobin decrease to below 4 g/dL.
All children in the immediate-transfusion group received a transfusion, as did 386 (49.0%) in the control group. The median time to transfusion was 1.3 hours in the immediate group and 24.9 hours in the control group. The mean total blood volume transfused per child was 314 plus or minus 228 mL and 142 plus or minus 224, respectively. The follow-up period was 180 days, and 4.5% of patients (n = 71) were lost to follow-up.
The researchers found no significant difference between the treatment groups with regard to mortality, other clinical outcomes, or the cost of care.
The 28-day mortality rate was 0.9% in the immediate-transfusion group and 1.7% in the control group (hazard ratio, 0.54; 95% confidence interval, 0.22-1.36; P = .19). The 180-day mortality was 4.5% and 6.0%, respectively (HR, 0.75; 95% CI, 0.48-1.15).
Transfusion volume
To assess the effects of transfusion volume, Dr. Maitland and colleagues evaluated 3,196 Ugandan and Malawian children with severe anemia. The median age of the children was 37 months, and 2,050 (64.1%) had malaria.
The children received a transfusion of 30 mL/kg (n = 1,592) or 20 mL/kg (n = 1,596) at a median of 1.2 hours after randomization. Some children – 197 in the 30-mL/kg group and 300 in the 20-mL/kg group – received additional transfusions. The mean volume of total blood transfused per child was 475 plus or minus 385 mL, and 353 plus or minus 348 mL, respectively.
Overall, there was no significant between-group difference with regard to mortality. The 28-day mortality rate was 3.4% in the 30 mL/kg group and 4.5% in the 20 mL/kg group (HR = 0.76; 95% CI, 0.54 to 1.08; P = .12).
However, the 28-day mortality rate did differ according to the presence of fever at screening. The mortality rate was lower in the 30 mL/kg group for children without fevers (HR = 0.43; 95% CI, 0.27 to 0.69) but higher in the 30 mL/kg group for febrile children (HR = 1.91; 95% CI, 1.04 to 3.49).
For other outcomes, including readmissions and serious adverse events, the researchers found no significant between-group differences.
This trial was supported by a grant from the United Kingdom Medical Research Council through a concordat with the Department for International Development. One researcher has a Wellcome Senior Research Fellowship, and another is a National Institute for Health Research Senior Investigator. Dr. Ingelfinger is a deputy editor at the New England Journal of Medicine. No other relevant conflicts of interest were reported.
SOURCES: Maitland K et al. N Engl J Med. 2019;381:407-19. Maitland K et al. N Engl J Med. 2019;381:420-31.
Trial results suggest African children with uncomplicated, severe anemia may not require immediate blood transfusion, and the volume of transfusion may only matter in the context of fever.
The TRACT trial showed no significant differences in 28-day mortality or other clinical outcomes between children who received immediate transfusions and those who did not.
Similarly, there was no significant difference in 28-day mortality among children who received transfusions of 20 mL/kg and those who received transfusions of 30 mL/kg. There was evidence to suggest a higher transfusion volume may benefit children without fevers, but this was an exploratory endpoint. The findings were published in the New England Journal of Medicine.
These results suggest “there is no credible reason to transfuse immediately or to transfuse a higher volume of blood, at least in pediatric populations in regions such as these two sub-Saharan countries [Uganda and Malawi],” Julie R. Ingelfinger, MD, of Massachusetts General Hospital in Boston, wrote in an accompanying editorial, also published in the New England Journal of Medicine (2019;381:475-6).
“The possible effect of higher volume transfusion in patients with fever may trigger additional and potentially useful studies,” she added.
Immediate transfusion
One goal of the TRACT trial was to determine if blood transfusion is the best treatment for children with severe anemia. With this in mind, Kathryn Maitland, MD, PhD, of Imperial College London and colleagues evaluated 1,565 Ugandan and Malawian children with uncomplicated, severe anemia. The patients’ median age was 26 months, and 984 (62.9%) had malaria.
The children were randomized to immediate transfusion (n = 778) or no immediate transfusion (n = 787). Children who did not have an immediate transfusion (control group) could receive a transfusion if they exhibited new signs of clinical severity or had their hemoglobin decrease to below 4 g/dL.
All children in the immediate-transfusion group received a transfusion, as did 386 (49.0%) in the control group. The median time to transfusion was 1.3 hours in the immediate group and 24.9 hours in the control group. The mean total blood volume transfused per child was 314 plus or minus 228 mL and 142 plus or minus 224, respectively. The follow-up period was 180 days, and 4.5% of patients (n = 71) were lost to follow-up.
The researchers found no significant difference between the treatment groups with regard to mortality, other clinical outcomes, or the cost of care.
The 28-day mortality rate was 0.9% in the immediate-transfusion group and 1.7% in the control group (hazard ratio, 0.54; 95% confidence interval, 0.22-1.36; P = .19). The 180-day mortality was 4.5% and 6.0%, respectively (HR, 0.75; 95% CI, 0.48-1.15).
Transfusion volume
To assess the effects of transfusion volume, Dr. Maitland and colleagues evaluated 3,196 Ugandan and Malawian children with severe anemia. The median age of the children was 37 months, and 2,050 (64.1%) had malaria.
The children received a transfusion of 30 mL/kg (n = 1,592) or 20 mL/kg (n = 1,596) at a median of 1.2 hours after randomization. Some children – 197 in the 30-mL/kg group and 300 in the 20-mL/kg group – received additional transfusions. The mean volume of total blood transfused per child was 475 plus or minus 385 mL, and 353 plus or minus 348 mL, respectively.
Overall, there was no significant between-group difference with regard to mortality. The 28-day mortality rate was 3.4% in the 30 mL/kg group and 4.5% in the 20 mL/kg group (HR = 0.76; 95% CI, 0.54 to 1.08; P = .12).
However, the 28-day mortality rate did differ according to the presence of fever at screening. The mortality rate was lower in the 30 mL/kg group for children without fevers (HR = 0.43; 95% CI, 0.27 to 0.69) but higher in the 30 mL/kg group for febrile children (HR = 1.91; 95% CI, 1.04 to 3.49).
For other outcomes, including readmissions and serious adverse events, the researchers found no significant between-group differences.
This trial was supported by a grant from the United Kingdom Medical Research Council through a concordat with the Department for International Development. One researcher has a Wellcome Senior Research Fellowship, and another is a National Institute for Health Research Senior Investigator. Dr. Ingelfinger is a deputy editor at the New England Journal of Medicine. No other relevant conflicts of interest were reported.
SOURCES: Maitland K et al. N Engl J Med. 2019;381:407-19. Maitland K et al. N Engl J Med. 2019;381:420-31.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: The 28-day mortality was 0.9% in patients who had immediate transfusions and 1.7% in those who did not (hazard ratio, 0.54; P = .19). The 28-day mortality rate was 3.4% in patients who received transfusions of 30 mL/kg and 4.5% in those who received transfusions of 20 mL/kg (HR, 0.76; P = .12). However, the mortality rate was lower in the 30-mL/kg group for children without fevers (HR, 0.43) and higher in the 30-mL/kg group for febrile children (HR, 1.91).
Study details: A phase 3 trial of African children with severe anemia who were randomized to immediate transfusion (n = 778) or no immediate transfusion (n = 787) and transfusions of 30 mL/kg (n = 1,592) or 20 mL/kg (n = 1,596)
Disclosures: The trial was supported by a grant from the United Kingdom Medical Research Council through a concordat with the Department for International Development. One researcher has a Wellcome Senior Research Fellowship, and another is a National Institute for Health Research Senior Investigator.
Sources: Maitland K et al. N Engl J Med. 2019;381:407-19. Maitland K et al. N Engl J Med. 2019;381:420-31.
ICYMI: Ibrutinib/rituximab combo improves CLL survival
Patients with previously untreated chronic lymphocytic leukemia (CLL) aged 70 years or younger who received ibrutinib/rituximab therapy experienced significantly greater progression-free survival, compared with those who received standard chemotherapy with fludarabine, cyclophosphamide, and rituximab (89.4% vs. 72.9% at 3 years; hazard ratio, 0.35; 95% confidence interval, 0.22-0.56; P less than .001), according to results from a randomized, phase 3 trial published in the New England Journal of Medicine (2019;381:432-43).
We first reported on the results of this trial when they were presented at the annual meeting of the American Society of Hematology. Find our coverage at the link below.
Patients with previously untreated chronic lymphocytic leukemia (CLL) aged 70 years or younger who received ibrutinib/rituximab therapy experienced significantly greater progression-free survival, compared with those who received standard chemotherapy with fludarabine, cyclophosphamide, and rituximab (89.4% vs. 72.9% at 3 years; hazard ratio, 0.35; 95% confidence interval, 0.22-0.56; P less than .001), according to results from a randomized, phase 3 trial published in the New England Journal of Medicine (2019;381:432-43).
We first reported on the results of this trial when they were presented at the annual meeting of the American Society of Hematology. Find our coverage at the link below.
Patients with previously untreated chronic lymphocytic leukemia (CLL) aged 70 years or younger who received ibrutinib/rituximab therapy experienced significantly greater progression-free survival, compared with those who received standard chemotherapy with fludarabine, cyclophosphamide, and rituximab (89.4% vs. 72.9% at 3 years; hazard ratio, 0.35; 95% confidence interval, 0.22-0.56; P less than .001), according to results from a randomized, phase 3 trial published in the New England Journal of Medicine (2019;381:432-43).
We first reported on the results of this trial when they were presented at the annual meeting of the American Society of Hematology. Find our coverage at the link below.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Hemoglobin levels are associated with long-term dementia risk
This U-shaped association “may relate to differences in white matter integrity and cerebral perfusion,” the researchers wrote in Neurology.
“With around 10% of people over age 65 having anemia in the Americas and Europe and up to 45% in African and southeast Asian countries, these results could have important implications for the burden of dementia,” said study author M. Arfan Ikram, MD, PhD, in a news release. Dr. Ikram is a professor of epidemiology at Erasmus Medical Center in Rotterdam, the Netherlands.
Prior studies have found that low hemoglobin levels are associated with adverse health outcomes, such as coronary heart disease, stroke, and mortality, but data about the relationship between hemoglobin levels and dementia risk have been limited.
A population-based cohort study
To examine the long-term association of hemoglobin levels and anemia with risk of dementia, Dr. Ikram and coauthors analyzed data from the Rotterdam Study, an ongoing population-based cohort study in the Netherlands that started in 1990. Their analysis included data from 12,305 participants without dementia who had serum hemoglobin measured at baseline (mean age, 64.6 years; 57.7% women).
During a mean follow-up of 12.1 years, 1,520 participants developed dementia, 1,194 of whom had Alzheimer’s disease.
“Both low and high hemoglobin levels were associated with increased dementia risk,” the authors wrote. Compared with participants in the middle quintile of hemoglobin levels (8.57-8.99 mmol/L), participants in the lowest quintile (less than 8.11 mmol/L) had a hazard ratio of dementia of 1.29, and participants in the highest quintile (greater than 9.40 mmol/L) had an HR of 1.20.
About 6% of the participants had anemia – that is, a hemoglobin level of less than 8.1 mmol/L for men and less than 7.5 mmol/L for women. Anemia was associated with a 34% increased risk of dementia and a 41% increased risk of Alzheimer’s disease.
Of the 745 people with anemia, 128 developed dementia, compared with 1,392 of the 11,560 people who did not have anemia (17% vs. 12%).
A U-shaped association
The researchers also examined hemoglobin in relation to vascular brain disease, structural connectivity, and global cerebral perfusion among 5,267 participants without dementia who had brain MRI. White matter hyperintensity volume and hemoglobin had a U-shaped association, similar to that for dementia and hemoglobin. In addition, hemoglobin inversely correlated to cerebral perfusion.
The results remained consistent after adjustment for factors such as smoking, high blood pressure, high cholesterol, and alcohol use.
A limitation of the study is that the participants lived in the Netherlands and were primarily of European descent, so the results may not apply to other populations, the authors wrote.
Dr. Ikram noted that the study does not prove that low or high hemoglobin levels cause dementia. “More research is needed to determine whether hemoglobin levels play a direct role in this increased risk or whether these associations can be explained by underlying issues or other vascular or metabolic changes.”
The study was supported by the Netherlands Cardiovascular Research Initiative; Erasmus Medical Centre; Erasmus University Rotterdam; Netherlands Organization for Scientific Research; Netherlands Organization for Health Research and Development; Research Institute for Diseases in the Elderly; Netherlands Genomic Initiative; Dutch Ministry of Education, Culture, and Science; Dutch Ministry of Health, Welfare, and Sports; European Commission; Municipality of Rotterdam; Netherlands Consortium for Healthy Aging; and Dutch Heart Foundation. The authors reported no relevant disclosures.
SOURCE: Ikram MA et al. Neurology. 2019 Jul 31. doi: 10.1212/WNL.0000000000008003.
This U-shaped association “may relate to differences in white matter integrity and cerebral perfusion,” the researchers wrote in Neurology.
“With around 10% of people over age 65 having anemia in the Americas and Europe and up to 45% in African and southeast Asian countries, these results could have important implications for the burden of dementia,” said study author M. Arfan Ikram, MD, PhD, in a news release. Dr. Ikram is a professor of epidemiology at Erasmus Medical Center in Rotterdam, the Netherlands.
Prior studies have found that low hemoglobin levels are associated with adverse health outcomes, such as coronary heart disease, stroke, and mortality, but data about the relationship between hemoglobin levels and dementia risk have been limited.
A population-based cohort study
To examine the long-term association of hemoglobin levels and anemia with risk of dementia, Dr. Ikram and coauthors analyzed data from the Rotterdam Study, an ongoing population-based cohort study in the Netherlands that started in 1990. Their analysis included data from 12,305 participants without dementia who had serum hemoglobin measured at baseline (mean age, 64.6 years; 57.7% women).
During a mean follow-up of 12.1 years, 1,520 participants developed dementia, 1,194 of whom had Alzheimer’s disease.
“Both low and high hemoglobin levels were associated with increased dementia risk,” the authors wrote. Compared with participants in the middle quintile of hemoglobin levels (8.57-8.99 mmol/L), participants in the lowest quintile (less than 8.11 mmol/L) had a hazard ratio of dementia of 1.29, and participants in the highest quintile (greater than 9.40 mmol/L) had an HR of 1.20.
About 6% of the participants had anemia – that is, a hemoglobin level of less than 8.1 mmol/L for men and less than 7.5 mmol/L for women. Anemia was associated with a 34% increased risk of dementia and a 41% increased risk of Alzheimer’s disease.
Of the 745 people with anemia, 128 developed dementia, compared with 1,392 of the 11,560 people who did not have anemia (17% vs. 12%).
A U-shaped association
The researchers also examined hemoglobin in relation to vascular brain disease, structural connectivity, and global cerebral perfusion among 5,267 participants without dementia who had brain MRI. White matter hyperintensity volume and hemoglobin had a U-shaped association, similar to that for dementia and hemoglobin. In addition, hemoglobin inversely correlated to cerebral perfusion.
The results remained consistent after adjustment for factors such as smoking, high blood pressure, high cholesterol, and alcohol use.
A limitation of the study is that the participants lived in the Netherlands and were primarily of European descent, so the results may not apply to other populations, the authors wrote.
Dr. Ikram noted that the study does not prove that low or high hemoglobin levels cause dementia. “More research is needed to determine whether hemoglobin levels play a direct role in this increased risk or whether these associations can be explained by underlying issues or other vascular or metabolic changes.”
The study was supported by the Netherlands Cardiovascular Research Initiative; Erasmus Medical Centre; Erasmus University Rotterdam; Netherlands Organization for Scientific Research; Netherlands Organization for Health Research and Development; Research Institute for Diseases in the Elderly; Netherlands Genomic Initiative; Dutch Ministry of Education, Culture, and Science; Dutch Ministry of Health, Welfare, and Sports; European Commission; Municipality of Rotterdam; Netherlands Consortium for Healthy Aging; and Dutch Heart Foundation. The authors reported no relevant disclosures.
SOURCE: Ikram MA et al. Neurology. 2019 Jul 31. doi: 10.1212/WNL.0000000000008003.
This U-shaped association “may relate to differences in white matter integrity and cerebral perfusion,” the researchers wrote in Neurology.
“With around 10% of people over age 65 having anemia in the Americas and Europe and up to 45% in African and southeast Asian countries, these results could have important implications for the burden of dementia,” said study author M. Arfan Ikram, MD, PhD, in a news release. Dr. Ikram is a professor of epidemiology at Erasmus Medical Center in Rotterdam, the Netherlands.
Prior studies have found that low hemoglobin levels are associated with adverse health outcomes, such as coronary heart disease, stroke, and mortality, but data about the relationship between hemoglobin levels and dementia risk have been limited.
A population-based cohort study
To examine the long-term association of hemoglobin levels and anemia with risk of dementia, Dr. Ikram and coauthors analyzed data from the Rotterdam Study, an ongoing population-based cohort study in the Netherlands that started in 1990. Their analysis included data from 12,305 participants without dementia who had serum hemoglobin measured at baseline (mean age, 64.6 years; 57.7% women).
During a mean follow-up of 12.1 years, 1,520 participants developed dementia, 1,194 of whom had Alzheimer’s disease.
“Both low and high hemoglobin levels were associated with increased dementia risk,” the authors wrote. Compared with participants in the middle quintile of hemoglobin levels (8.57-8.99 mmol/L), participants in the lowest quintile (less than 8.11 mmol/L) had a hazard ratio of dementia of 1.29, and participants in the highest quintile (greater than 9.40 mmol/L) had an HR of 1.20.
About 6% of the participants had anemia – that is, a hemoglobin level of less than 8.1 mmol/L for men and less than 7.5 mmol/L for women. Anemia was associated with a 34% increased risk of dementia and a 41% increased risk of Alzheimer’s disease.
Of the 745 people with anemia, 128 developed dementia, compared with 1,392 of the 11,560 people who did not have anemia (17% vs. 12%).
A U-shaped association
The researchers also examined hemoglobin in relation to vascular brain disease, structural connectivity, and global cerebral perfusion among 5,267 participants without dementia who had brain MRI. White matter hyperintensity volume and hemoglobin had a U-shaped association, similar to that for dementia and hemoglobin. In addition, hemoglobin inversely correlated to cerebral perfusion.
The results remained consistent after adjustment for factors such as smoking, high blood pressure, high cholesterol, and alcohol use.
A limitation of the study is that the participants lived in the Netherlands and were primarily of European descent, so the results may not apply to other populations, the authors wrote.
Dr. Ikram noted that the study does not prove that low or high hemoglobin levels cause dementia. “More research is needed to determine whether hemoglobin levels play a direct role in this increased risk or whether these associations can be explained by underlying issues or other vascular or metabolic changes.”
The study was supported by the Netherlands Cardiovascular Research Initiative; Erasmus Medical Centre; Erasmus University Rotterdam; Netherlands Organization for Scientific Research; Netherlands Organization for Health Research and Development; Research Institute for Diseases in the Elderly; Netherlands Genomic Initiative; Dutch Ministry of Education, Culture, and Science; Dutch Ministry of Health, Welfare, and Sports; European Commission; Municipality of Rotterdam; Netherlands Consortium for Healthy Aging; and Dutch Heart Foundation. The authors reported no relevant disclosures.
SOURCE: Ikram MA et al. Neurology. 2019 Jul 31. doi: 10.1212/WNL.0000000000008003.
FROM NEUROLOGY
Key clinical point: Adults with low levels of hemoglobin and adults with high levels of hemoglobin may have an increased risk of dementia.
Major finding: Compared with participants in the middle quintile of hemoglobin levels (8.57-8.99 mmol/L), participants in the lowest quintile (less than 8.11 mmol/L) had a hazard ratio of dementia of 1.29, and participants in the highest quintile (greater than 9.40 mmol/L) had an HR of 1.20.
Study details: An analysis of data from 12,305 participants in the Rotterdam Study, a population-based cohort study in the Netherlands, who were followed up for an average of 12 years.
Disclosures: The study was supported by the Netherlands Cardiovascular Research Initiative; Erasmus Medical Centre; Erasmus University Rotterdam; Netherlands Organization for Scientific Research; Netherlands Organization for Health Research and Development; Research Institute for Diseases in the Elderly; Netherlands Genomic Initiative; Dutch Ministry of Education, Culture, and Science; Dutch Ministry of Health, Welfare, and Sports; European Commission; Municipality of Rotterdam; Netherlands Consortium for Healthy Aging; and Dutch Heart Foundation. The authors reported no relevant disclosures.
Source: Ikram MA et al. Neurology. 2019 Jul 31. doi: 10.1212/WNL.0000000000008003.
Predatory journals and HULLK’s prostate
The incredible HULLK
HULLK TYPE OF RNA THAT CONTROLS GROWTH OF PROSTATE CANCER CELLS. HULLK IS STRONGEST RNA THERE IS. HULLK SMASH CANCER!
Is everyone’s favorite not-so-jolly green giant the key to crushing prostate cancer? Not exactly: HULLK is a noncoding type of RNA. Which means, instead of coding a protein, it helps regulate cellular processes.
Cancer researchers from the University of Virginia found there was more HULLK in tumors from patients with advanced prostate cancer. They also found that decreasing the incredible RNA slows tumor cell growth.
In other words, we need a little more Bruce Banner, a little less HULLK.
The scientists who identified HULLK are hopeful that it can function as a biomarker and a therapeutic target in the future, and could be an integral discovery on the way to curing prostate cancer. HULLK LIKE.
Trading places with Sigmund Freud
Sometimes, it can be helpful to talk to someone about your problems. We’re not really going out on a limb here. That is, after all, pretty much the basis of psychotherapy.
But what if that someone else was really you, disguised as Sigmund Freud?
That was the premise for a recent study involving body swapping and immersive virtual reality. The investigators scanned each subject to create a 3D avatar that functioned as an online representation and moved as he or she moved during the experiment. In virtual reality, the subject’s avatar sat across a table from Sigmund Freud, who was the variable element in the study.
For the control group, Freud responded with prescripted questions and comments about the subject’s problem. The other group, however, was able to swap virtual bodies and respond to their own bodies as Freud. In other words, they could have a conversation with themselves, but it looked like they were talking to Freud.
A week after the virtual conversations, more than 80% of those in the body-swapping group experienced some sort of change with respect to their problem, compared with less than 50% of controls.
“We found that those in the body-swapping group got better knowledge, understanding, control, and new ideas about their problem, compared to the control group,” one of the investigators said.
We’re just wondering about their choice of Freud. It kind of makes sense, because that was his line of work; but would another person have been even more helpful?
How about Dr. Phil? Or maybe Oprah? Seems like Dwayne “the Rock” Johnson is everywhere else, so why not virtual reality? It would be hard to go wrong with some type of Kardashian, right?
Cue the ‘Jaws’ music
Doctors with freshly written studies beware, for you are not alone. Lurking in the undergrowth, stalking your every move, are the predatory journals. They’ve come for you. And they pose a danger not only to you, but to the entire field of medical literature.
However, there’s no need to fear, as you have a guide through the great Serengeti of the publication process: a new guideline on avoiding predatory journals from the American Medical Writers Association, European Medical Writers Association, and International Society for Medical Publication Professionals.
According to the guideline, some telltale characteristics of these journals include a lack of information, poorly made websites, a lack of indexing in a recognized system, promises of unrealistically quick peer review, and an insatiable thirst for your blood. We may have made that last one up.
The guideline authors call for all medical authors to conduct research while submitting to journals, and only submit to those journals that conduct a full peer review process and that genuinely seek to contribute to medical literature.
Failure to do so may result in a damaged reputation, being unwittingly appointed to an editorial board, losing your paper, or having your liver eaten by an eagle day after day until Hercules finally frees you from your torture.
We may have made that one up as well.
The incredible HULLK
HULLK TYPE OF RNA THAT CONTROLS GROWTH OF PROSTATE CANCER CELLS. HULLK IS STRONGEST RNA THERE IS. HULLK SMASH CANCER!
Is everyone’s favorite not-so-jolly green giant the key to crushing prostate cancer? Not exactly: HULLK is a noncoding type of RNA. Which means, instead of coding a protein, it helps regulate cellular processes.
Cancer researchers from the University of Virginia found there was more HULLK in tumors from patients with advanced prostate cancer. They also found that decreasing the incredible RNA slows tumor cell growth.
In other words, we need a little more Bruce Banner, a little less HULLK.
The scientists who identified HULLK are hopeful that it can function as a biomarker and a therapeutic target in the future, and could be an integral discovery on the way to curing prostate cancer. HULLK LIKE.
Trading places with Sigmund Freud
Sometimes, it can be helpful to talk to someone about your problems. We’re not really going out on a limb here. That is, after all, pretty much the basis of psychotherapy.
But what if that someone else was really you, disguised as Sigmund Freud?
That was the premise for a recent study involving body swapping and immersive virtual reality. The investigators scanned each subject to create a 3D avatar that functioned as an online representation and moved as he or she moved during the experiment. In virtual reality, the subject’s avatar sat across a table from Sigmund Freud, who was the variable element in the study.
For the control group, Freud responded with prescripted questions and comments about the subject’s problem. The other group, however, was able to swap virtual bodies and respond to their own bodies as Freud. In other words, they could have a conversation with themselves, but it looked like they were talking to Freud.
A week after the virtual conversations, more than 80% of those in the body-swapping group experienced some sort of change with respect to their problem, compared with less than 50% of controls.
“We found that those in the body-swapping group got better knowledge, understanding, control, and new ideas about their problem, compared to the control group,” one of the investigators said.
We’re just wondering about their choice of Freud. It kind of makes sense, because that was his line of work; but would another person have been even more helpful?
How about Dr. Phil? Or maybe Oprah? Seems like Dwayne “the Rock” Johnson is everywhere else, so why not virtual reality? It would be hard to go wrong with some type of Kardashian, right?
Cue the ‘Jaws’ music
Doctors with freshly written studies beware, for you are not alone. Lurking in the undergrowth, stalking your every move, are the predatory journals. They’ve come for you. And they pose a danger not only to you, but to the entire field of medical literature.
However, there’s no need to fear, as you have a guide through the great Serengeti of the publication process: a new guideline on avoiding predatory journals from the American Medical Writers Association, European Medical Writers Association, and International Society for Medical Publication Professionals.
According to the guideline, some telltale characteristics of these journals include a lack of information, poorly made websites, a lack of indexing in a recognized system, promises of unrealistically quick peer review, and an insatiable thirst for your blood. We may have made that last one up.
The guideline authors call for all medical authors to conduct research while submitting to journals, and only submit to those journals that conduct a full peer review process and that genuinely seek to contribute to medical literature.
Failure to do so may result in a damaged reputation, being unwittingly appointed to an editorial board, losing your paper, or having your liver eaten by an eagle day after day until Hercules finally frees you from your torture.
We may have made that one up as well.

The incredible HULLK
HULLK TYPE OF RNA THAT CONTROLS GROWTH OF PROSTATE CANCER CELLS. HULLK IS STRONGEST RNA THERE IS. HULLK SMASH CANCER!
Is everyone’s favorite not-so-jolly green giant the key to crushing prostate cancer? Not exactly: HULLK is a noncoding type of RNA. Which means, instead of coding a protein, it helps regulate cellular processes.
Cancer researchers from the University of Virginia found there was more HULLK in tumors from patients with advanced prostate cancer. They also found that decreasing the incredible RNA slows tumor cell growth.
In other words, we need a little more Bruce Banner, a little less HULLK.
The scientists who identified HULLK are hopeful that it can function as a biomarker and a therapeutic target in the future, and could be an integral discovery on the way to curing prostate cancer. HULLK LIKE.
Trading places with Sigmund Freud
Sometimes, it can be helpful to talk to someone about your problems. We’re not really going out on a limb here. That is, after all, pretty much the basis of psychotherapy.
But what if that someone else was really you, disguised as Sigmund Freud?
That was the premise for a recent study involving body swapping and immersive virtual reality. The investigators scanned each subject to create a 3D avatar that functioned as an online representation and moved as he or she moved during the experiment. In virtual reality, the subject’s avatar sat across a table from Sigmund Freud, who was the variable element in the study.
For the control group, Freud responded with prescripted questions and comments about the subject’s problem. The other group, however, was able to swap virtual bodies and respond to their own bodies as Freud. In other words, they could have a conversation with themselves, but it looked like they were talking to Freud.
A week after the virtual conversations, more than 80% of those in the body-swapping group experienced some sort of change with respect to their problem, compared with less than 50% of controls.
“We found that those in the body-swapping group got better knowledge, understanding, control, and new ideas about their problem, compared to the control group,” one of the investigators said.
We’re just wondering about their choice of Freud. It kind of makes sense, because that was his line of work; but would another person have been even more helpful?
How about Dr. Phil? Or maybe Oprah? Seems like Dwayne “the Rock” Johnson is everywhere else, so why not virtual reality? It would be hard to go wrong with some type of Kardashian, right?
Cue the ‘Jaws’ music
Doctors with freshly written studies beware, for you are not alone. Lurking in the undergrowth, stalking your every move, are the predatory journals. They’ve come for you. And they pose a danger not only to you, but to the entire field of medical literature.
However, there’s no need to fear, as you have a guide through the great Serengeti of the publication process: a new guideline on avoiding predatory journals from the American Medical Writers Association, European Medical Writers Association, and International Society for Medical Publication Professionals.
According to the guideline, some telltale characteristics of these journals include a lack of information, poorly made websites, a lack of indexing in a recognized system, promises of unrealistically quick peer review, and an insatiable thirst for your blood. We may have made that last one up.
The guideline authors call for all medical authors to conduct research while submitting to journals, and only submit to those journals that conduct a full peer review process and that genuinely seek to contribute to medical literature.
Failure to do so may result in a damaged reputation, being unwittingly appointed to an editorial board, losing your paper, or having your liver eaten by an eagle day after day until Hercules finally frees you from your torture.
We may have made that one up as well.

GLP-1 agonists, SGLT2 inhibitors offer more options in diabetes management
ORLANDO – The big news in diabetes management this year is “happy cardiologists and nephrologists.”
According to Christine Kessler, MN, ANP-C, CNS, BC-ADM, FAANP, founder of Metabolic Medicine Associates in King George, Va., these specialists are happy because the American College of Cardiology and the American Diabetes Association both recently updated their respective societies’ guidelines to include evidence that treating patients with type 2 diabetes with glucagonlike peptide-1 (GLP-1) agonists, sodium-glucose cotransporter 2 (SGLT2) inhibitors, or metformin can lower risk of cardiovascular disease and chronic kidney disease.
“Finally, the ACC is aligned with the ADA,” Ms. Kessler said in her presentation. “This is amazing, and it’s good news.”
Recent innovations in diabetes management technology, such as continuous glucose monitors, are also helping to make diabetes management easier. “If you’re not using some of this technology in your primary care practice, it’s coming to you, and it’s amazing the data it can provide to us,” said Ms. Kessler at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
In endocrinology, diabetes is thought of in terms of macrovascular and microvascular disease, she said. Macrovascular disease is cardiovascular disease and stroke, while microvascular disease is nephropathy, neuropathy, and retinopathy. Diabetes is a cardiovascular risk factor and puts patients at higher risk for cardiovascular death, all-cause mortality, and hospitalization because of myocardial infarction or stroke, compared with patients who do not have type 2 diabetes. There is also a higher risk of kidney disease, nerve damage, blindness, nonalcoholic fatty liver disease, depression, complications during pregnancy, periodontal disease, and erectile dysfunction, said Ms. Kessler, who also is a nurse practitioner and researcher.
However, including getting enough sleep, dietary interventions that target weight loss and blood glucose control, and increasing physical activity that has cardiopulmonary benefits. Clinicians should also treat underlying conditions that contribute to increased cardiovascular risk, such as obesity, dyslipidemia, hypertension, and nonalcoholic fatty liver disease.
Addressing insulin resistance and hyperglycemia are also important, but patients must avoid hypoglycemia. “Any patient with diabetes, we don’t want to drive them there because that’s a cardiac risk,” said Ms. Kessler. The endothelial microvascular and macrovascular damage is believed to be caused by glycemic swings, she added.
For pharmacologic therapy, patients with type 2 diabetes should stay on metformin if they are already on the drug, and it can even be used in cases where patients have reduced kidney function, with a glomerular filtration rate (GFR) between 30 and 60 mL/min per 1.73 m2, with a lower dose used between 30 and 45 mL/min per 1.73 m2. To treat patients with atherosclerotic cardiovascular disease, recent evidence has shown GLP-1 agonists are beneficial and can also promote appetite satiety, prandial support, and reduce a patient’s weight, but the drug is expensive, and about 15% of patients will not see therapeutic benefit while on the medication, said Ms. Kessler. Clinicians should also watch for increased risk of pancreatitis while patients use GLP-1 agonists, and it should not be prescribed in patients with a history of thyroid medullary cancer or multiple endocrine neoplasia type 2 (MEN2).
SGLT2 inhibitors can benefit type 1 diabetes and type 2 diabetes patients with heart failure and diabetic kidney disease, but should be the second or third choice in therapy. The dosage of SGLT2 inhibitors should be cut in half when used with insulin and sulfonylurea, and the drug can also increase LDL cholesterol.
Ms. Kessler noted that while GLP-1 agonists and SGLT2 inhibitors prevent or reduce cardiovascular risk, they are not currently approved to treat cardiovascular disease.
Ms. Kessler reports being an advisor and speaker for Novo Nordisk on the subject of obesity. Global Academy for Medical Education and this news organization are owned by the same parent company.
ORLANDO – The big news in diabetes management this year is “happy cardiologists and nephrologists.”
According to Christine Kessler, MN, ANP-C, CNS, BC-ADM, FAANP, founder of Metabolic Medicine Associates in King George, Va., these specialists are happy because the American College of Cardiology and the American Diabetes Association both recently updated their respective societies’ guidelines to include evidence that treating patients with type 2 diabetes with glucagonlike peptide-1 (GLP-1) agonists, sodium-glucose cotransporter 2 (SGLT2) inhibitors, or metformin can lower risk of cardiovascular disease and chronic kidney disease.
“Finally, the ACC is aligned with the ADA,” Ms. Kessler said in her presentation. “This is amazing, and it’s good news.”
Recent innovations in diabetes management technology, such as continuous glucose monitors, are also helping to make diabetes management easier. “If you’re not using some of this technology in your primary care practice, it’s coming to you, and it’s amazing the data it can provide to us,” said Ms. Kessler at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
In endocrinology, diabetes is thought of in terms of macrovascular and microvascular disease, she said. Macrovascular disease is cardiovascular disease and stroke, while microvascular disease is nephropathy, neuropathy, and retinopathy. Diabetes is a cardiovascular risk factor and puts patients at higher risk for cardiovascular death, all-cause mortality, and hospitalization because of myocardial infarction or stroke, compared with patients who do not have type 2 diabetes. There is also a higher risk of kidney disease, nerve damage, blindness, nonalcoholic fatty liver disease, depression, complications during pregnancy, periodontal disease, and erectile dysfunction, said Ms. Kessler, who also is a nurse practitioner and researcher.
However, including getting enough sleep, dietary interventions that target weight loss and blood glucose control, and increasing physical activity that has cardiopulmonary benefits. Clinicians should also treat underlying conditions that contribute to increased cardiovascular risk, such as obesity, dyslipidemia, hypertension, and nonalcoholic fatty liver disease.
Addressing insulin resistance and hyperglycemia are also important, but patients must avoid hypoglycemia. “Any patient with diabetes, we don’t want to drive them there because that’s a cardiac risk,” said Ms. Kessler. The endothelial microvascular and macrovascular damage is believed to be caused by glycemic swings, she added.
For pharmacologic therapy, patients with type 2 diabetes should stay on metformin if they are already on the drug, and it can even be used in cases where patients have reduced kidney function, with a glomerular filtration rate (GFR) between 30 and 60 mL/min per 1.73 m2, with a lower dose used between 30 and 45 mL/min per 1.73 m2. To treat patients with atherosclerotic cardiovascular disease, recent evidence has shown GLP-1 agonists are beneficial and can also promote appetite satiety, prandial support, and reduce a patient’s weight, but the drug is expensive, and about 15% of patients will not see therapeutic benefit while on the medication, said Ms. Kessler. Clinicians should also watch for increased risk of pancreatitis while patients use GLP-1 agonists, and it should not be prescribed in patients with a history of thyroid medullary cancer or multiple endocrine neoplasia type 2 (MEN2).
SGLT2 inhibitors can benefit type 1 diabetes and type 2 diabetes patients with heart failure and diabetic kidney disease, but should be the second or third choice in therapy. The dosage of SGLT2 inhibitors should be cut in half when used with insulin and sulfonylurea, and the drug can also increase LDL cholesterol.
Ms. Kessler noted that while GLP-1 agonists and SGLT2 inhibitors prevent or reduce cardiovascular risk, they are not currently approved to treat cardiovascular disease.
Ms. Kessler reports being an advisor and speaker for Novo Nordisk on the subject of obesity. Global Academy for Medical Education and this news organization are owned by the same parent company.
ORLANDO – The big news in diabetes management this year is “happy cardiologists and nephrologists.”
According to Christine Kessler, MN, ANP-C, CNS, BC-ADM, FAANP, founder of Metabolic Medicine Associates in King George, Va., these specialists are happy because the American College of Cardiology and the American Diabetes Association both recently updated their respective societies’ guidelines to include evidence that treating patients with type 2 diabetes with glucagonlike peptide-1 (GLP-1) agonists, sodium-glucose cotransporter 2 (SGLT2) inhibitors, or metformin can lower risk of cardiovascular disease and chronic kidney disease.
“Finally, the ACC is aligned with the ADA,” Ms. Kessler said in her presentation. “This is amazing, and it’s good news.”
Recent innovations in diabetes management technology, such as continuous glucose monitors, are also helping to make diabetes management easier. “If you’re not using some of this technology in your primary care practice, it’s coming to you, and it’s amazing the data it can provide to us,” said Ms. Kessler at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
In endocrinology, diabetes is thought of in terms of macrovascular and microvascular disease, she said. Macrovascular disease is cardiovascular disease and stroke, while microvascular disease is nephropathy, neuropathy, and retinopathy. Diabetes is a cardiovascular risk factor and puts patients at higher risk for cardiovascular death, all-cause mortality, and hospitalization because of myocardial infarction or stroke, compared with patients who do not have type 2 diabetes. There is also a higher risk of kidney disease, nerve damage, blindness, nonalcoholic fatty liver disease, depression, complications during pregnancy, periodontal disease, and erectile dysfunction, said Ms. Kessler, who also is a nurse practitioner and researcher.
However, including getting enough sleep, dietary interventions that target weight loss and blood glucose control, and increasing physical activity that has cardiopulmonary benefits. Clinicians should also treat underlying conditions that contribute to increased cardiovascular risk, such as obesity, dyslipidemia, hypertension, and nonalcoholic fatty liver disease.
Addressing insulin resistance and hyperglycemia are also important, but patients must avoid hypoglycemia. “Any patient with diabetes, we don’t want to drive them there because that’s a cardiac risk,” said Ms. Kessler. The endothelial microvascular and macrovascular damage is believed to be caused by glycemic swings, she added.
For pharmacologic therapy, patients with type 2 diabetes should stay on metformin if they are already on the drug, and it can even be used in cases where patients have reduced kidney function, with a glomerular filtration rate (GFR) between 30 and 60 mL/min per 1.73 m2, with a lower dose used between 30 and 45 mL/min per 1.73 m2. To treat patients with atherosclerotic cardiovascular disease, recent evidence has shown GLP-1 agonists are beneficial and can also promote appetite satiety, prandial support, and reduce a patient’s weight, but the drug is expensive, and about 15% of patients will not see therapeutic benefit while on the medication, said Ms. Kessler. Clinicians should also watch for increased risk of pancreatitis while patients use GLP-1 agonists, and it should not be prescribed in patients with a history of thyroid medullary cancer or multiple endocrine neoplasia type 2 (MEN2).
SGLT2 inhibitors can benefit type 1 diabetes and type 2 diabetes patients with heart failure and diabetic kidney disease, but should be the second or third choice in therapy. The dosage of SGLT2 inhibitors should be cut in half when used with insulin and sulfonylurea, and the drug can also increase LDL cholesterol.
Ms. Kessler noted that while GLP-1 agonists and SGLT2 inhibitors prevent or reduce cardiovascular risk, they are not currently approved to treat cardiovascular disease.
Ms. Kessler reports being an advisor and speaker for Novo Nordisk on the subject of obesity. Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM CARPS 2019
Endocrine Society advises on diabetes care for older adults
according to a new guideline on diabetes care for older adults issued by the Endocrine Society.
“The prevalence of diabetes in the United States is projected to increase dramatically during the next 3 decades as the population ages, the numbers of higher-risk minority groups increase, and people with diabetes live longer because of decreasing rates of cardiovascular deaths,” wrote Derek LeRoith, MD, of Icahn School of Medicine at Mount Sinai, New York, and his writing committee colleagues. They said their goal was to provide health care providers with guidance for the management of type 1 or type 2 diabetes in older patients, with a focus on simplifying medication regimens and management strategies to avoid “unnecessary and/or harmful adverse effects.”
The guideline, published in the Journal of Clinical Endocrinology & Metabolism, is based mainly on evidence from controlled trials in two systematic reviews that specifically focused on adults aged 65 years and older. The guideline addresses six areas of consideration for this patient population:
- Role of the endocrinologist and diabetes care specialist.
- Screening for diabetes and prediabetes, and diabetes prevention.
- Assessment of older patients with diabetes.
- Treatment of hyperglycemia.
- Treating complications of diabetes.
- Special settings and populations.
Partnerships and screening
The guideline recommends that primary care providers partner with an endocrinologist or diabetes specialist in the care of patients aged 65 and older with newly diagnosed diabetes, and that the specialist take primary responsibility for diabetes care of patients with type 1 diabetes or those who need more complex intervention to achieve treatment goals.
Screening for diabetes in adults aged 65 years and older using fasting plasma glucose and/or hemoglobin A1c should occur every 2 years, but that schedule should be adjusted based on shared decision making with the patient, the committee said. Providers are advised to assess the patient’s overall health and personal values before settling on treatment goals and strategies. The writing group also recommends periodic cognitive screening and that medication regimens be simplified as much as possible.
Tackling hyperglycemia
For treatment of hyperglycemia, the guideline recommends outpatient strategies to minimize hypoglycemia and periodic or continuous glucose monitoring. The strategies include lifestyle modifications as a first-line intervention for ambulatory patients, as well as nutritional assessment. A high-protein diet is recommended for older patients with frailty, but no restrictions on diet are advised for patients who cannot meet glycemic targets with lifestyle modification and who are at risk for malnutrition.
Metformin is the first-choice recommendation for patients with diabetes aged 65 and older who need medical management in addition to lifestyle modification, but it is not recommended for individuals with impaired kidney function or gastrointestinal intolerance, according to the guideline. Oral and injectable drugs and/or insulin are recommended if metformin and lifestyle changes are insufficient to meet glycemic targets, the writers noted.
Managing complications
Hypertension is among the diabetes-related complications that need to be managed in older adults, and the guideline recommends a target blood pressure of 140/90 mm Hg, but other targets – based on patient-provider shared decision making – may be considered for patients in high-risk groups.
The guideline calls for management of hyperlipidemia with statin therapy and “use of an annual lipid profile to achieve the recommended levels for reducing absolute cardiovascular disease events and all-cause mortality.” The committee does not specify low-density lipoprotein cholesterol targets because of insufficient evidence, but recommends alternative treatments, including ezetimibe or proprotein convertase subtilisin/kexin type 9 inhibitors, if statin therapy is not enough to help the patients meet goals. The writers also advocate fish oil and/or fenofibrate for patients with fasting triglycerides of more than 500 mg/dL.
To manage congestive heart failure in older patients with diabetes, the guideline recommends following standard clinical practice guidelines for the condition, and cautious use of oral hypoglycemic agents, including glinides, rosiglitazone, pioglitazone, and dipeptidyl peptidase–4 inhibitors. The writers noted that low-dose aspirin is recommended for patients with diabetes with a history of atherosclerotic cardiovascular disease.
The committee also recommends an annual comprehensive eye exam for patients with diabetes aged 65 years and older to identify retinal disease and suggests that actions, such as physical therapy and reduced use of sedatives, be taken to minimize the risk of falls in patients with neuropathy or problems with balance and gait.
Older patients with diabetes also should be screened annually for chronic kidney disease, and the dosage of diabetes medications should be adjusted to minimize side effects in patients with kidney problems.
Tailoring care to setting
Finally, the guideline addresses special settings and populations, including managing diabetes in hospitals or nursing homes, or in patients who are transitioning to homes or long-term care facilities. Recommendations in this category include simplifying medications for older adults with terminal illness or severe comorbidities, as well as setting glycemic targets as part of a hospital discharge plan.
“The most important aspect of successful transition is effective, detailed, and thorough bidirectional communication between the discharging and receiving teams of health care providers,” the writers emphasized.
The guideline is cosponsored by the European Society of Endocrinology, the Gerontological Society of America, and the Obesity Society. The chair of the committee had no relevant financial conflicts to disclose, and at least 50% of the committee members were free of relevant conflicts of interest.
SOURCE: LeRoith D et al. J Clin Endocrinol Metab. 2019;104:1520-74.
according to a new guideline on diabetes care for older adults issued by the Endocrine Society.
“The prevalence of diabetes in the United States is projected to increase dramatically during the next 3 decades as the population ages, the numbers of higher-risk minority groups increase, and people with diabetes live longer because of decreasing rates of cardiovascular deaths,” wrote Derek LeRoith, MD, of Icahn School of Medicine at Mount Sinai, New York, and his writing committee colleagues. They said their goal was to provide health care providers with guidance for the management of type 1 or type 2 diabetes in older patients, with a focus on simplifying medication regimens and management strategies to avoid “unnecessary and/or harmful adverse effects.”
The guideline, published in the Journal of Clinical Endocrinology & Metabolism, is based mainly on evidence from controlled trials in two systematic reviews that specifically focused on adults aged 65 years and older. The guideline addresses six areas of consideration for this patient population:
- Role of the endocrinologist and diabetes care specialist.
- Screening for diabetes and prediabetes, and diabetes prevention.
- Assessment of older patients with diabetes.
- Treatment of hyperglycemia.
- Treating complications of diabetes.
- Special settings and populations.
Partnerships and screening
The guideline recommends that primary care providers partner with an endocrinologist or diabetes specialist in the care of patients aged 65 and older with newly diagnosed diabetes, and that the specialist take primary responsibility for diabetes care of patients with type 1 diabetes or those who need more complex intervention to achieve treatment goals.
Screening for diabetes in adults aged 65 years and older using fasting plasma glucose and/or hemoglobin A1c should occur every 2 years, but that schedule should be adjusted based on shared decision making with the patient, the committee said. Providers are advised to assess the patient’s overall health and personal values before settling on treatment goals and strategies. The writing group also recommends periodic cognitive screening and that medication regimens be simplified as much as possible.
Tackling hyperglycemia
For treatment of hyperglycemia, the guideline recommends outpatient strategies to minimize hypoglycemia and periodic or continuous glucose monitoring. The strategies include lifestyle modifications as a first-line intervention for ambulatory patients, as well as nutritional assessment. A high-protein diet is recommended for older patients with frailty, but no restrictions on diet are advised for patients who cannot meet glycemic targets with lifestyle modification and who are at risk for malnutrition.
Metformin is the first-choice recommendation for patients with diabetes aged 65 and older who need medical management in addition to lifestyle modification, but it is not recommended for individuals with impaired kidney function or gastrointestinal intolerance, according to the guideline. Oral and injectable drugs and/or insulin are recommended if metformin and lifestyle changes are insufficient to meet glycemic targets, the writers noted.
Managing complications
Hypertension is among the diabetes-related complications that need to be managed in older adults, and the guideline recommends a target blood pressure of 140/90 mm Hg, but other targets – based on patient-provider shared decision making – may be considered for patients in high-risk groups.
The guideline calls for management of hyperlipidemia with statin therapy and “use of an annual lipid profile to achieve the recommended levels for reducing absolute cardiovascular disease events and all-cause mortality.” The committee does not specify low-density lipoprotein cholesterol targets because of insufficient evidence, but recommends alternative treatments, including ezetimibe or proprotein convertase subtilisin/kexin type 9 inhibitors, if statin therapy is not enough to help the patients meet goals. The writers also advocate fish oil and/or fenofibrate for patients with fasting triglycerides of more than 500 mg/dL.
To manage congestive heart failure in older patients with diabetes, the guideline recommends following standard clinical practice guidelines for the condition, and cautious use of oral hypoglycemic agents, including glinides, rosiglitazone, pioglitazone, and dipeptidyl peptidase–4 inhibitors. The writers noted that low-dose aspirin is recommended for patients with diabetes with a history of atherosclerotic cardiovascular disease.
The committee also recommends an annual comprehensive eye exam for patients with diabetes aged 65 years and older to identify retinal disease and suggests that actions, such as physical therapy and reduced use of sedatives, be taken to minimize the risk of falls in patients with neuropathy or problems with balance and gait.
Older patients with diabetes also should be screened annually for chronic kidney disease, and the dosage of diabetes medications should be adjusted to minimize side effects in patients with kidney problems.
Tailoring care to setting
Finally, the guideline addresses special settings and populations, including managing diabetes in hospitals or nursing homes, or in patients who are transitioning to homes or long-term care facilities. Recommendations in this category include simplifying medications for older adults with terminal illness or severe comorbidities, as well as setting glycemic targets as part of a hospital discharge plan.
“The most important aspect of successful transition is effective, detailed, and thorough bidirectional communication between the discharging and receiving teams of health care providers,” the writers emphasized.
The guideline is cosponsored by the European Society of Endocrinology, the Gerontological Society of America, and the Obesity Society. The chair of the committee had no relevant financial conflicts to disclose, and at least 50% of the committee members were free of relevant conflicts of interest.
SOURCE: LeRoith D et al. J Clin Endocrinol Metab. 2019;104:1520-74.
according to a new guideline on diabetes care for older adults issued by the Endocrine Society.
“The prevalence of diabetes in the United States is projected to increase dramatically during the next 3 decades as the population ages, the numbers of higher-risk minority groups increase, and people with diabetes live longer because of decreasing rates of cardiovascular deaths,” wrote Derek LeRoith, MD, of Icahn School of Medicine at Mount Sinai, New York, and his writing committee colleagues. They said their goal was to provide health care providers with guidance for the management of type 1 or type 2 diabetes in older patients, with a focus on simplifying medication regimens and management strategies to avoid “unnecessary and/or harmful adverse effects.”
The guideline, published in the Journal of Clinical Endocrinology & Metabolism, is based mainly on evidence from controlled trials in two systematic reviews that specifically focused on adults aged 65 years and older. The guideline addresses six areas of consideration for this patient population:
- Role of the endocrinologist and diabetes care specialist.
- Screening for diabetes and prediabetes, and diabetes prevention.
- Assessment of older patients with diabetes.
- Treatment of hyperglycemia.
- Treating complications of diabetes.
- Special settings and populations.
Partnerships and screening
The guideline recommends that primary care providers partner with an endocrinologist or diabetes specialist in the care of patients aged 65 and older with newly diagnosed diabetes, and that the specialist take primary responsibility for diabetes care of patients with type 1 diabetes or those who need more complex intervention to achieve treatment goals.
Screening for diabetes in adults aged 65 years and older using fasting plasma glucose and/or hemoglobin A1c should occur every 2 years, but that schedule should be adjusted based on shared decision making with the patient, the committee said. Providers are advised to assess the patient’s overall health and personal values before settling on treatment goals and strategies. The writing group also recommends periodic cognitive screening and that medication regimens be simplified as much as possible.
Tackling hyperglycemia
For treatment of hyperglycemia, the guideline recommends outpatient strategies to minimize hypoglycemia and periodic or continuous glucose monitoring. The strategies include lifestyle modifications as a first-line intervention for ambulatory patients, as well as nutritional assessment. A high-protein diet is recommended for older patients with frailty, but no restrictions on diet are advised for patients who cannot meet glycemic targets with lifestyle modification and who are at risk for malnutrition.
Metformin is the first-choice recommendation for patients with diabetes aged 65 and older who need medical management in addition to lifestyle modification, but it is not recommended for individuals with impaired kidney function or gastrointestinal intolerance, according to the guideline. Oral and injectable drugs and/or insulin are recommended if metformin and lifestyle changes are insufficient to meet glycemic targets, the writers noted.
Managing complications
Hypertension is among the diabetes-related complications that need to be managed in older adults, and the guideline recommends a target blood pressure of 140/90 mm Hg, but other targets – based on patient-provider shared decision making – may be considered for patients in high-risk groups.
The guideline calls for management of hyperlipidemia with statin therapy and “use of an annual lipid profile to achieve the recommended levels for reducing absolute cardiovascular disease events and all-cause mortality.” The committee does not specify low-density lipoprotein cholesterol targets because of insufficient evidence, but recommends alternative treatments, including ezetimibe or proprotein convertase subtilisin/kexin type 9 inhibitors, if statin therapy is not enough to help the patients meet goals. The writers also advocate fish oil and/or fenofibrate for patients with fasting triglycerides of more than 500 mg/dL.
To manage congestive heart failure in older patients with diabetes, the guideline recommends following standard clinical practice guidelines for the condition, and cautious use of oral hypoglycemic agents, including glinides, rosiglitazone, pioglitazone, and dipeptidyl peptidase–4 inhibitors. The writers noted that low-dose aspirin is recommended for patients with diabetes with a history of atherosclerotic cardiovascular disease.
The committee also recommends an annual comprehensive eye exam for patients with diabetes aged 65 years and older to identify retinal disease and suggests that actions, such as physical therapy and reduced use of sedatives, be taken to minimize the risk of falls in patients with neuropathy or problems with balance and gait.
Older patients with diabetes also should be screened annually for chronic kidney disease, and the dosage of diabetes medications should be adjusted to minimize side effects in patients with kidney problems.
Tailoring care to setting
Finally, the guideline addresses special settings and populations, including managing diabetes in hospitals or nursing homes, or in patients who are transitioning to homes or long-term care facilities. Recommendations in this category include simplifying medications for older adults with terminal illness or severe comorbidities, as well as setting glycemic targets as part of a hospital discharge plan.
“The most important aspect of successful transition is effective, detailed, and thorough bidirectional communication between the discharging and receiving teams of health care providers,” the writers emphasized.
The guideline is cosponsored by the European Society of Endocrinology, the Gerontological Society of America, and the Obesity Society. The chair of the committee had no relevant financial conflicts to disclose, and at least 50% of the committee members were free of relevant conflicts of interest.
SOURCE: LeRoith D et al. J Clin Endocrinol Metab. 2019;104:1520-74.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
FDA approves darolutamide for nonmetastatic CRPC
The Food and Drug Administration has approved darolutamide for nonmetastatic, castration-resistant prostate cancer.
The approval was based on improved metastasis-free survival (MFS) in the randomized ARAMIS trial of 1,509 patients with nonmetastatic, castration-resistant prostate cancer.
Median MFS was 40.4 months (95% confidence interval, 34.3 months to not reached) for patients treated with darolutamide, compared with 18.4 months (95% CI, 15.5-22.3 months) for those receiving placebo (hazard ratio, 0.41; 95% CI, 0.34-0.50; P less than .0001), according to the FDA.
MFS is defined as the time from randomization to first evidence of distant metastasis or death from any cause within 33 weeks after the last evaluable scan, whichever occurred first.
In ARAMIS, patients were randomized 2:1 to receive either 600 mg darolutamide orally twice daily (n = 955) or matching placebo (n = 554). All patients received a gonadotropin-releasing hormone analog concurrently or had a previous bilateral orchiectomy. Twelve patients with previous seizure histories were treated on the darolutamide arm.
Overall survival data is not yet mature, the FDA said.
The most common adverse reactions in patients who received darolutamide were fatigue, extremity pain, and rash. Ischemic heart disease (4.3%) and heart failure (2.1%) were more common on the darolutamide arm, while seizure incidence was similar in the two arms (0.2%).
The recommended darolutamide dose is 600 mg (two 300-mg tablets) administered orally twice daily with food. Patients should also receive a gonadotropin-releasing hormone analog concurrently or should have had bilateral orchiectomy, the FDA said.
Darolutamide is marketed as Nubeqa by Bayer HealthCare Pharmaceuticals.
The Food and Drug Administration has approved darolutamide for nonmetastatic, castration-resistant prostate cancer.
The approval was based on improved metastasis-free survival (MFS) in the randomized ARAMIS trial of 1,509 patients with nonmetastatic, castration-resistant prostate cancer.
Median MFS was 40.4 months (95% confidence interval, 34.3 months to not reached) for patients treated with darolutamide, compared with 18.4 months (95% CI, 15.5-22.3 months) for those receiving placebo (hazard ratio, 0.41; 95% CI, 0.34-0.50; P less than .0001), according to the FDA.
MFS is defined as the time from randomization to first evidence of distant metastasis or death from any cause within 33 weeks after the last evaluable scan, whichever occurred first.
In ARAMIS, patients were randomized 2:1 to receive either 600 mg darolutamide orally twice daily (n = 955) or matching placebo (n = 554). All patients received a gonadotropin-releasing hormone analog concurrently or had a previous bilateral orchiectomy. Twelve patients with previous seizure histories were treated on the darolutamide arm.
Overall survival data is not yet mature, the FDA said.
The most common adverse reactions in patients who received darolutamide were fatigue, extremity pain, and rash. Ischemic heart disease (4.3%) and heart failure (2.1%) were more common on the darolutamide arm, while seizure incidence was similar in the two arms (0.2%).
The recommended darolutamide dose is 600 mg (two 300-mg tablets) administered orally twice daily with food. Patients should also receive a gonadotropin-releasing hormone analog concurrently or should have had bilateral orchiectomy, the FDA said.
Darolutamide is marketed as Nubeqa by Bayer HealthCare Pharmaceuticals.
The Food and Drug Administration has approved darolutamide for nonmetastatic, castration-resistant prostate cancer.
The approval was based on improved metastasis-free survival (MFS) in the randomized ARAMIS trial of 1,509 patients with nonmetastatic, castration-resistant prostate cancer.
Median MFS was 40.4 months (95% confidence interval, 34.3 months to not reached) for patients treated with darolutamide, compared with 18.4 months (95% CI, 15.5-22.3 months) for those receiving placebo (hazard ratio, 0.41; 95% CI, 0.34-0.50; P less than .0001), according to the FDA.
MFS is defined as the time from randomization to first evidence of distant metastasis or death from any cause within 33 weeks after the last evaluable scan, whichever occurred first.
In ARAMIS, patients were randomized 2:1 to receive either 600 mg darolutamide orally twice daily (n = 955) or matching placebo (n = 554). All patients received a gonadotropin-releasing hormone analog concurrently or had a previous bilateral orchiectomy. Twelve patients with previous seizure histories were treated on the darolutamide arm.
Overall survival data is not yet mature, the FDA said.
The most common adverse reactions in patients who received darolutamide were fatigue, extremity pain, and rash. Ischemic heart disease (4.3%) and heart failure (2.1%) were more common on the darolutamide arm, while seizure incidence was similar in the two arms (0.2%).
The recommended darolutamide dose is 600 mg (two 300-mg tablets) administered orally twice daily with food. Patients should also receive a gonadotropin-releasing hormone analog concurrently or should have had bilateral orchiectomy, the FDA said.
Darolutamide is marketed as Nubeqa by Bayer HealthCare Pharmaceuticals.