User login
Lumateperone schizophrenia drug seems to hit snag
FDA cancels lumateperone advisory panel
U.S. regulators canceled a July 31, 2019, advisory committee about lumateperone, an experimental schizophrenia drug that has had some mixed results in testing.
On July 23, the Food and Drug Administration announced the cancellation of the Psychopharmacologic Drugs Advisory Committee meeting it had previously called for to review the new drug application for lumateperone. The agency said the meeting was canceled because of “new information regarding the application.” The FDA said it was continuing to evaluate the application and would, as needed, announce a future meeting on it.
The developer of lumateperone, Intra-Cellular Therapies, issued its own statement on July 23, noting that a meeting had been scheduled with the FDA “shortly” and that an update would be provided after the meeting. The New York–based firm also said it recently had provided additional information to the FDA to meet agency requests. This information was related to “nonclinical studies.”
“The FDA canceled the advisory committee meeting to allow sufficient time to review this new and any forthcoming information as they continue” to review the new drug application for lumateperone, Intra-Cellular said in the July 23 statement. The company also said there may be an extension of the FDA’s Sept. 27, 2019, target action date on the lumateperone application.
Investors viewed this as bad news. Shares of Intra-Cellular on July 23 dropped from an opening price of $11.90 to a closing one of $8.19. On July 29, they closed at $8.12.
Still, it is unclear how the FDA will decide on the lumateperone application and whether the agency will call another advisory committee meeting on it.
Last year, the FDA accepted the application for lumateperone, a once-daily treatment, Intra-Cellular said. The agency had in 2017 given a fast-track designation to lumateperone for the treatment of schizophrenia.
Lumateperone is the lead product for the company.
On the company’s website, Intra-Cellular says three large randomized, double-blind, placebo-controlled trials have been done for lumateperone as a schizophrenia drug. In two of these studies, results for lumateperone at a 60-mg dose showed a “statistically significant separation from placebo on the primary endpoint, the Positive and Negative Syndrome Scale or PANSS total score.”
In a recent routine filing with the Securities and Exchange Commission, Intra-Cellular said it was having an “ongoing dialogue” with the FDA about lumateperone. The company in 2016 had announced that, in a phase 3 study known as ITI-007-302, lumateperone had not separated from placebo on the primary endpoint, change from baseline on the PANSS total score, in the predefined patient population. The active control for ITI-007-302, risperidone, did separate from placebo.
In the recent SEC filing, Intra-Cellular said the FDA already has confirmed that the results of ITI-007-302 did not preclude the submission of a new drug application.
Intra-Cellular also said “lumateperone was statistically significantly better than risperidone on key safety and tolerability parameters, and exhibited a safety profile similar to placebo” in the 302 study. Lumateperone’s failure to best placebo in the 302 test was “in part due to an unusually high placebo response at certain sites.”
FDA cancels lumateperone advisory panel
FDA cancels lumateperone advisory panel
U.S. regulators canceled a July 31, 2019, advisory committee about lumateperone, an experimental schizophrenia drug that has had some mixed results in testing.
On July 23, the Food and Drug Administration announced the cancellation of the Psychopharmacologic Drugs Advisory Committee meeting it had previously called for to review the new drug application for lumateperone. The agency said the meeting was canceled because of “new information regarding the application.” The FDA said it was continuing to evaluate the application and would, as needed, announce a future meeting on it.
The developer of lumateperone, Intra-Cellular Therapies, issued its own statement on July 23, noting that a meeting had been scheduled with the FDA “shortly” and that an update would be provided after the meeting. The New York–based firm also said it recently had provided additional information to the FDA to meet agency requests. This information was related to “nonclinical studies.”
“The FDA canceled the advisory committee meeting to allow sufficient time to review this new and any forthcoming information as they continue” to review the new drug application for lumateperone, Intra-Cellular said in the July 23 statement. The company also said there may be an extension of the FDA’s Sept. 27, 2019, target action date on the lumateperone application.
Investors viewed this as bad news. Shares of Intra-Cellular on July 23 dropped from an opening price of $11.90 to a closing one of $8.19. On July 29, they closed at $8.12.
Still, it is unclear how the FDA will decide on the lumateperone application and whether the agency will call another advisory committee meeting on it.
Last year, the FDA accepted the application for lumateperone, a once-daily treatment, Intra-Cellular said. The agency had in 2017 given a fast-track designation to lumateperone for the treatment of schizophrenia.
Lumateperone is the lead product for the company.
On the company’s website, Intra-Cellular says three large randomized, double-blind, placebo-controlled trials have been done for lumateperone as a schizophrenia drug. In two of these studies, results for lumateperone at a 60-mg dose showed a “statistically significant separation from placebo on the primary endpoint, the Positive and Negative Syndrome Scale or PANSS total score.”
In a recent routine filing with the Securities and Exchange Commission, Intra-Cellular said it was having an “ongoing dialogue” with the FDA about lumateperone. The company in 2016 had announced that, in a phase 3 study known as ITI-007-302, lumateperone had not separated from placebo on the primary endpoint, change from baseline on the PANSS total score, in the predefined patient population. The active control for ITI-007-302, risperidone, did separate from placebo.
In the recent SEC filing, Intra-Cellular said the FDA already has confirmed that the results of ITI-007-302 did not preclude the submission of a new drug application.
Intra-Cellular also said “lumateperone was statistically significantly better than risperidone on key safety and tolerability parameters, and exhibited a safety profile similar to placebo” in the 302 study. Lumateperone’s failure to best placebo in the 302 test was “in part due to an unusually high placebo response at certain sites.”
U.S. regulators canceled a July 31, 2019, advisory committee about lumateperone, an experimental schizophrenia drug that has had some mixed results in testing.
On July 23, the Food and Drug Administration announced the cancellation of the Psychopharmacologic Drugs Advisory Committee meeting it had previously called for to review the new drug application for lumateperone. The agency said the meeting was canceled because of “new information regarding the application.” The FDA said it was continuing to evaluate the application and would, as needed, announce a future meeting on it.
The developer of lumateperone, Intra-Cellular Therapies, issued its own statement on July 23, noting that a meeting had been scheduled with the FDA “shortly” and that an update would be provided after the meeting. The New York–based firm also said it recently had provided additional information to the FDA to meet agency requests. This information was related to “nonclinical studies.”
“The FDA canceled the advisory committee meeting to allow sufficient time to review this new and any forthcoming information as they continue” to review the new drug application for lumateperone, Intra-Cellular said in the July 23 statement. The company also said there may be an extension of the FDA’s Sept. 27, 2019, target action date on the lumateperone application.
Investors viewed this as bad news. Shares of Intra-Cellular on July 23 dropped from an opening price of $11.90 to a closing one of $8.19. On July 29, they closed at $8.12.
Still, it is unclear how the FDA will decide on the lumateperone application and whether the agency will call another advisory committee meeting on it.
Last year, the FDA accepted the application for lumateperone, a once-daily treatment, Intra-Cellular said. The agency had in 2017 given a fast-track designation to lumateperone for the treatment of schizophrenia.
Lumateperone is the lead product for the company.
On the company’s website, Intra-Cellular says three large randomized, double-blind, placebo-controlled trials have been done for lumateperone as a schizophrenia drug. In two of these studies, results for lumateperone at a 60-mg dose showed a “statistically significant separation from placebo on the primary endpoint, the Positive and Negative Syndrome Scale or PANSS total score.”
In a recent routine filing with the Securities and Exchange Commission, Intra-Cellular said it was having an “ongoing dialogue” with the FDA about lumateperone. The company in 2016 had announced that, in a phase 3 study known as ITI-007-302, lumateperone had not separated from placebo on the primary endpoint, change from baseline on the PANSS total score, in the predefined patient population. The active control for ITI-007-302, risperidone, did separate from placebo.
In the recent SEC filing, Intra-Cellular said the FDA already has confirmed that the results of ITI-007-302 did not preclude the submission of a new drug application.
Intra-Cellular also said “lumateperone was statistically significantly better than risperidone on key safety and tolerability parameters, and exhibited a safety profile similar to placebo” in the 302 study. Lumateperone’s failure to best placebo in the 302 test was “in part due to an unusually high placebo response at certain sites.”
Pantoprazole not needed for most patients on anticoagulant/antiplatelet therapies
For most patients taking antiplatelet and/or anticoagulant therapies, the proton pump inhibitor (PPI) pantoprazole is unnecessary, based on findings from the prospective COMPASS trial, which involved more than 17,000 participants.
Pantoprazole may reduce the risk of bleeding from gastroduodenal lesions, but it is unlikely to prevent upper-gastrointestinal events, reported lead author Paul Moayyedi, MB ChB, PhD, of McMaster University in Hamilton, Canada, and colleagues.
The investigators wrote in Gastroenterology, “Guidelines suggest that patients receiving the combination of antiplatelet and anticoagulant therapy should receive PPIs to reduce the risk of upper-GI bleeding. However … there are no randomized data to support the use of PPI therapy in patients taking oral anticoagulants, and a paucity of data relating to aspirin.”
To fill this knowledge gap, the investigators recruited 17,598 participants from 33 countries who had stable peripheral artery disease and cardiovascular disease. Participants were randomized to one of three groups: 100-mg aspirin once daily, 5-mg rivaroxaban twice daily, or a combination of 2.5-mg rivaroxaban twice daily with 100-mg aspirin once daily. This part of the trial was discontinued before completion because of early cardiovascular advantages associated with combination therapy over aspirin alone, and related findings were reported previously. While combination therapy did reduce cardiovascular risks, it had less favorable effects on gut health, highlighted by an associated increase in major GI bleeding events. Despite early cessation of the cardiovascular portion of the trial, the pantoprazole regimen was continued, offering a look at the effect of long-term PPI use on gut health.
At baseline, about two-thirds of participants (64%) were not taking a PPI, requiring randomization to either 40-mg pantoprazole once daily or matching placebo. The primary efficacy outcome was time to first upper-GI clinical event, defined as a composite of the following: upper-GI obstruction, perforation, at least five gastroduodenal erosions with at least 3 days of GI pain, symptomatic gastroduodenal ulcer involving at least 3 days of GI pain, overt upper-GI bleeding of unknown origin, occult bleeding (drop in hemoglobin of at least 2 g/dL), overt bleeding with a gastroduodenal lesion (active bleeding during endoscopy), or a symptomatic gastroduodenal ulcer involving at least 3 days of GI pain. In addition to this measure, the investigators evaluated a post-hoc endpoint with a looser definition of peptic ulcer events, most notably eliminating the requirement that a lesion be actively bleeding during endoscopy.
Most patients in the trial (78%) were male, and 23% were current smokers. Smaller proportions of the population were taking a nonsteroidal anti-inflammatory drug (5%) and/or had a history of peptic ulcer disease (2.6%). The median follow-up was 3.01 years, ranging from 2.49 to 3.59 years. Permanent discontinuations occurred at approximately equal rates in the pantoprazole (21%) and placebo (22%) group, after a median of 11 months (338 days). In both groups, more than 96% of participants who continued treatment took their medications as prescribed at least 80% of the time.
Analysis showed that upper-GI events occurred marginally less often in the pantoprazole group than the placebo group, but without statistical significance (1.2% vs. 1.3%; P = .35). Of the outcomes measured, only overt bleeding of gastroduodenal origin detected by radiography or endoscopy was statistically less common in the pantoprazole group than the placebo group, with a 48% reduced rate (0.2% vs. 0.4%; P = .03). No statistical efficacy differences or statistical interactions were detected between population subgroups.
“The data suggest that routine use of PPI therapy is not warranted for patients receiving low-dose rivaroxaban with or without aspirin for the prevention of atherothrombotic events in patients with stable coronary artery disease or symptomatic peripheral artery disease, as there was no overall impact on clinical upper-GI events or upper-GI bleeding,” the investigators wrote. “This is in contrast to previous systematic reviews of randomized trials reporting that PPIs were associated with a 50%-70% reduction in bleeding and symptomatic peptic ulcers related to nonsteroidal anti-inflammatory drugs, including in the critical care setting.”
Post-hoc analysis, which allowed for a broader definition of upper-GI events related to gastroduodenal ulcers, revealed a slightly greater reduction in risk of bleeding lesions in patients taking pantoprazole, compared with placebo (hazard ratio, 0.45), and additional risk reductions for peptic ulcers (HR, 0.46) and erosions (HR, 0.33). Ultimately, pantoprazole reduced the combined rate of post-hoc events by 56%.
The investigators noted that these ulcer- and erosion-reducing effects of pantoprazole align with previous reports. “It is therefore possible that PPIs might be beneficial for patients at particularly high risk for peptic ulcer disease who are also taking aspirin and/or anticoagulants,” the investigators concluded.
The COMPASS trial was funded by Bayer AG. The investigators disclosed additional relationships with Allergan, Takeda, Janssen, and others.
SOURCE: Moayyedi P et al. Gastro. 2019 May 2. doi: 10.1053/j.gastro.2019.04.041.
For most patients taking antiplatelet and/or anticoagulant therapies, the proton pump inhibitor (PPI) pantoprazole is unnecessary, based on findings from the prospective COMPASS trial, which involved more than 17,000 participants.
Pantoprazole may reduce the risk of bleeding from gastroduodenal lesions, but it is unlikely to prevent upper-gastrointestinal events, reported lead author Paul Moayyedi, MB ChB, PhD, of McMaster University in Hamilton, Canada, and colleagues.
The investigators wrote in Gastroenterology, “Guidelines suggest that patients receiving the combination of antiplatelet and anticoagulant therapy should receive PPIs to reduce the risk of upper-GI bleeding. However … there are no randomized data to support the use of PPI therapy in patients taking oral anticoagulants, and a paucity of data relating to aspirin.”
To fill this knowledge gap, the investigators recruited 17,598 participants from 33 countries who had stable peripheral artery disease and cardiovascular disease. Participants were randomized to one of three groups: 100-mg aspirin once daily, 5-mg rivaroxaban twice daily, or a combination of 2.5-mg rivaroxaban twice daily with 100-mg aspirin once daily. This part of the trial was discontinued before completion because of early cardiovascular advantages associated with combination therapy over aspirin alone, and related findings were reported previously. While combination therapy did reduce cardiovascular risks, it had less favorable effects on gut health, highlighted by an associated increase in major GI bleeding events. Despite early cessation of the cardiovascular portion of the trial, the pantoprazole regimen was continued, offering a look at the effect of long-term PPI use on gut health.
At baseline, about two-thirds of participants (64%) were not taking a PPI, requiring randomization to either 40-mg pantoprazole once daily or matching placebo. The primary efficacy outcome was time to first upper-GI clinical event, defined as a composite of the following: upper-GI obstruction, perforation, at least five gastroduodenal erosions with at least 3 days of GI pain, symptomatic gastroduodenal ulcer involving at least 3 days of GI pain, overt upper-GI bleeding of unknown origin, occult bleeding (drop in hemoglobin of at least 2 g/dL), overt bleeding with a gastroduodenal lesion (active bleeding during endoscopy), or a symptomatic gastroduodenal ulcer involving at least 3 days of GI pain. In addition to this measure, the investigators evaluated a post-hoc endpoint with a looser definition of peptic ulcer events, most notably eliminating the requirement that a lesion be actively bleeding during endoscopy.
Most patients in the trial (78%) were male, and 23% were current smokers. Smaller proportions of the population were taking a nonsteroidal anti-inflammatory drug (5%) and/or had a history of peptic ulcer disease (2.6%). The median follow-up was 3.01 years, ranging from 2.49 to 3.59 years. Permanent discontinuations occurred at approximately equal rates in the pantoprazole (21%) and placebo (22%) group, after a median of 11 months (338 days). In both groups, more than 96% of participants who continued treatment took their medications as prescribed at least 80% of the time.
Analysis showed that upper-GI events occurred marginally less often in the pantoprazole group than the placebo group, but without statistical significance (1.2% vs. 1.3%; P = .35). Of the outcomes measured, only overt bleeding of gastroduodenal origin detected by radiography or endoscopy was statistically less common in the pantoprazole group than the placebo group, with a 48% reduced rate (0.2% vs. 0.4%; P = .03). No statistical efficacy differences or statistical interactions were detected between population subgroups.
“The data suggest that routine use of PPI therapy is not warranted for patients receiving low-dose rivaroxaban with or without aspirin for the prevention of atherothrombotic events in patients with stable coronary artery disease or symptomatic peripheral artery disease, as there was no overall impact on clinical upper-GI events or upper-GI bleeding,” the investigators wrote. “This is in contrast to previous systematic reviews of randomized trials reporting that PPIs were associated with a 50%-70% reduction in bleeding and symptomatic peptic ulcers related to nonsteroidal anti-inflammatory drugs, including in the critical care setting.”
Post-hoc analysis, which allowed for a broader definition of upper-GI events related to gastroduodenal ulcers, revealed a slightly greater reduction in risk of bleeding lesions in patients taking pantoprazole, compared with placebo (hazard ratio, 0.45), and additional risk reductions for peptic ulcers (HR, 0.46) and erosions (HR, 0.33). Ultimately, pantoprazole reduced the combined rate of post-hoc events by 56%.
The investigators noted that these ulcer- and erosion-reducing effects of pantoprazole align with previous reports. “It is therefore possible that PPIs might be beneficial for patients at particularly high risk for peptic ulcer disease who are also taking aspirin and/or anticoagulants,” the investigators concluded.
The COMPASS trial was funded by Bayer AG. The investigators disclosed additional relationships with Allergan, Takeda, Janssen, and others.
SOURCE: Moayyedi P et al. Gastro. 2019 May 2. doi: 10.1053/j.gastro.2019.04.041.
For most patients taking antiplatelet and/or anticoagulant therapies, the proton pump inhibitor (PPI) pantoprazole is unnecessary, based on findings from the prospective COMPASS trial, which involved more than 17,000 participants.
Pantoprazole may reduce the risk of bleeding from gastroduodenal lesions, but it is unlikely to prevent upper-gastrointestinal events, reported lead author Paul Moayyedi, MB ChB, PhD, of McMaster University in Hamilton, Canada, and colleagues.
The investigators wrote in Gastroenterology, “Guidelines suggest that patients receiving the combination of antiplatelet and anticoagulant therapy should receive PPIs to reduce the risk of upper-GI bleeding. However … there are no randomized data to support the use of PPI therapy in patients taking oral anticoagulants, and a paucity of data relating to aspirin.”
To fill this knowledge gap, the investigators recruited 17,598 participants from 33 countries who had stable peripheral artery disease and cardiovascular disease. Participants were randomized to one of three groups: 100-mg aspirin once daily, 5-mg rivaroxaban twice daily, or a combination of 2.5-mg rivaroxaban twice daily with 100-mg aspirin once daily. This part of the trial was discontinued before completion because of early cardiovascular advantages associated with combination therapy over aspirin alone, and related findings were reported previously. While combination therapy did reduce cardiovascular risks, it had less favorable effects on gut health, highlighted by an associated increase in major GI bleeding events. Despite early cessation of the cardiovascular portion of the trial, the pantoprazole regimen was continued, offering a look at the effect of long-term PPI use on gut health.
At baseline, about two-thirds of participants (64%) were not taking a PPI, requiring randomization to either 40-mg pantoprazole once daily or matching placebo. The primary efficacy outcome was time to first upper-GI clinical event, defined as a composite of the following: upper-GI obstruction, perforation, at least five gastroduodenal erosions with at least 3 days of GI pain, symptomatic gastroduodenal ulcer involving at least 3 days of GI pain, overt upper-GI bleeding of unknown origin, occult bleeding (drop in hemoglobin of at least 2 g/dL), overt bleeding with a gastroduodenal lesion (active bleeding during endoscopy), or a symptomatic gastroduodenal ulcer involving at least 3 days of GI pain. In addition to this measure, the investigators evaluated a post-hoc endpoint with a looser definition of peptic ulcer events, most notably eliminating the requirement that a lesion be actively bleeding during endoscopy.
Most patients in the trial (78%) were male, and 23% were current smokers. Smaller proportions of the population were taking a nonsteroidal anti-inflammatory drug (5%) and/or had a history of peptic ulcer disease (2.6%). The median follow-up was 3.01 years, ranging from 2.49 to 3.59 years. Permanent discontinuations occurred at approximately equal rates in the pantoprazole (21%) and placebo (22%) group, after a median of 11 months (338 days). In both groups, more than 96% of participants who continued treatment took their medications as prescribed at least 80% of the time.
Analysis showed that upper-GI events occurred marginally less often in the pantoprazole group than the placebo group, but without statistical significance (1.2% vs. 1.3%; P = .35). Of the outcomes measured, only overt bleeding of gastroduodenal origin detected by radiography or endoscopy was statistically less common in the pantoprazole group than the placebo group, with a 48% reduced rate (0.2% vs. 0.4%; P = .03). No statistical efficacy differences or statistical interactions were detected between population subgroups.
“The data suggest that routine use of PPI therapy is not warranted for patients receiving low-dose rivaroxaban with or without aspirin for the prevention of atherothrombotic events in patients with stable coronary artery disease or symptomatic peripheral artery disease, as there was no overall impact on clinical upper-GI events or upper-GI bleeding,” the investigators wrote. “This is in contrast to previous systematic reviews of randomized trials reporting that PPIs were associated with a 50%-70% reduction in bleeding and symptomatic peptic ulcers related to nonsteroidal anti-inflammatory drugs, including in the critical care setting.”
Post-hoc analysis, which allowed for a broader definition of upper-GI events related to gastroduodenal ulcers, revealed a slightly greater reduction in risk of bleeding lesions in patients taking pantoprazole, compared with placebo (hazard ratio, 0.45), and additional risk reductions for peptic ulcers (HR, 0.46) and erosions (HR, 0.33). Ultimately, pantoprazole reduced the combined rate of post-hoc events by 56%.
The investigators noted that these ulcer- and erosion-reducing effects of pantoprazole align with previous reports. “It is therefore possible that PPIs might be beneficial for patients at particularly high risk for peptic ulcer disease who are also taking aspirin and/or anticoagulants,” the investigators concluded.
The COMPASS trial was funded by Bayer AG. The investigators disclosed additional relationships with Allergan, Takeda, Janssen, and others.
SOURCE: Moayyedi P et al. Gastro. 2019 May 2. doi: 10.1053/j.gastro.2019.04.041.
FROM GASTROENTEROLOGY
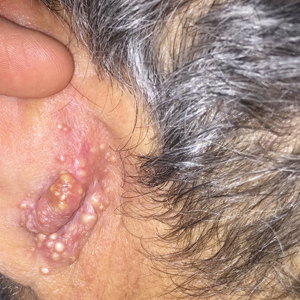
Rapidly Growing Retroauricular Tumor
The Diagnosis: Milia En Plaque
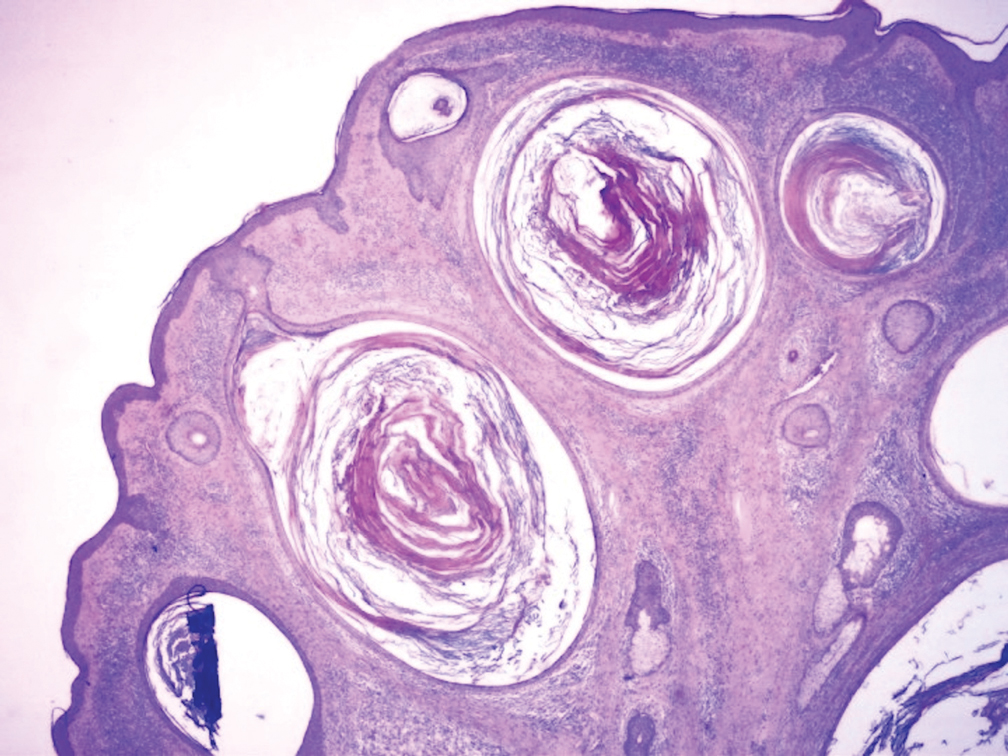
Biopsy results revealed a normal epidermis; the dermis showed multiple small cystic structures lined by a stratified squamous epithelium containing eosinophilic keratin surrounded by a mononuclear cell infiltrate and some melanophages (Figure).
Milia en plaque was first described in 1903 by Balzer and Fouquet.1 In 1978, Hubler et al2 presented 2 cases with an asymptomatic, erythematous, and edematous plaque and white milialike lesions. On histopathology, they showed multiple cystic structures characterized by central laminated keratin and an intense polymorphic inflammatory reaction surrounding the cyst and epidermal appendages. Both patients were treated with topical tretinoin with complete response at 3 months. The authors suggested the term milia en plaque to describe this clinical entity.2
Milia en plaque is described as an infrequent condition that more often presents on the head, neck, and trunk, as well as the periocular, periauricular, and perinasal areas. It has been reported to occur at any age3 but appears more frequently in middle-aged adults and females. A congenital case also has been reported.4 It has been associated with pseudoxanthoma elasticum, lichen planus, trauma, kidney transplant, and cyclosporine use, but it also can present in healthy individuals,3 as in our patient. No clear cause has been identified.
Pathology is characteristic, with multiple cysts filled with keratin and surrounded by 2 or 3 layers of epithelial cells, associated with a mononuclear, nonlichenoid, mononuclear infiltrate.5 Structures similar to follicular infundibular tumors have been described, suggesting a common origin of follicular lesions as milia en plaque.6
Treatment includes surgical excision, cryosurgery, dermabrasion, electrodesiccation, trichloroacetic acid, photodynamic therapy, CO2 and erbium lasers, topical retinoids, minocycline, and etretinate.7 We performed a complete surgical excision in our patient.
In acneform reactions, erythematous papules and pustules can be found on the cheeks and forehead. Nevus comedonicus appears during childhood and presents with multiple open comedones. Postinflammatory milia is present in chronic inflammatory pathologies such as porphyria cutanea tarda. Histopathologic findings in adnexal tumors show a benign proliferation of any cellular type of a cutaneous annex.
Milia en plaque is an unusual but benign condition that is distinguished clinically by its characteristic presentation.
- Balzer F, Fouquet C. Milium confluent retroauricularies bilateral. Bull Soc Fr Dermatol Syphiligr. 1903;14:361.
- Hubler WR, Rudolph AH, Kelleher RM. Milia en plaque. Cutis. 1978;22:67-70.
- Berk DR, Bayliss SJ. Milia: a review and classification. J Am Acad Dermatol. 2008;59:1050-1063.
- Wang AR, Bercovitch L. Congenital milia en plaque. Pediatr Dermatol. 2016;33:258-259.
- Muñoz-Martínez R, Santamarina-Albertos A, Sanz-Muñoz C, et al. Milia en plaque. Actas Dermosifiliogr. 2013;104:638-640.
- Terui H, Hashimoto A, Yamasaki K, et al. Milia en plaque as a distinct follicular hamartoma with cystic trichoepitheliomatous features. Am J Dermatopathol. 2016;38:212-217.
- Tenna S, Filoni A, Pagliarello C, et al. Eyelid milia en plaque: a treatment challenge with a new CO2 fractional laser. Dermatol Ther. 2014;27:65-67.
The Diagnosis: Milia En Plaque
Biopsy results revealed a normal epidermis; the dermis showed multiple small cystic structures lined by a stratified squamous epithelium containing eosinophilic keratin surrounded by a mononuclear cell infiltrate and some melanophages (Figure).

Milia en plaque was first described in 1903 by Balzer and Fouquet.1 In 1978, Hubler et al2 presented 2 cases with an asymptomatic, erythematous, and edematous plaque and white milialike lesions. On histopathology, they showed multiple cystic structures characterized by central laminated keratin and an intense polymorphic inflammatory reaction surrounding the cyst and epidermal appendages. Both patients were treated with topical tretinoin with complete response at 3 months. The authors suggested the term milia en plaque to describe this clinical entity.2
Milia en plaque is described as an infrequent condition that more often presents on the head, neck, and trunk, as well as the periocular, periauricular, and perinasal areas. It has been reported to occur at any age3 but appears more frequently in middle-aged adults and females. A congenital case also has been reported.4 It has been associated with pseudoxanthoma elasticum, lichen planus, trauma, kidney transplant, and cyclosporine use, but it also can present in healthy individuals,3 as in our patient. No clear cause has been identified.
Pathology is characteristic, with multiple cysts filled with keratin and surrounded by 2 or 3 layers of epithelial cells, associated with a mononuclear, nonlichenoid, mononuclear infiltrate.5 Structures similar to follicular infundibular tumors have been described, suggesting a common origin of follicular lesions as milia en plaque.6
Treatment includes surgical excision, cryosurgery, dermabrasion, electrodesiccation, trichloroacetic acid, photodynamic therapy, CO2 and erbium lasers, topical retinoids, minocycline, and etretinate.7 We performed a complete surgical excision in our patient.
In acneform reactions, erythematous papules and pustules can be found on the cheeks and forehead. Nevus comedonicus appears during childhood and presents with multiple open comedones. Postinflammatory milia is present in chronic inflammatory pathologies such as porphyria cutanea tarda. Histopathologic findings in adnexal tumors show a benign proliferation of any cellular type of a cutaneous annex.
Milia en plaque is an unusual but benign condition that is distinguished clinically by its characteristic presentation.
The Diagnosis: Milia En Plaque
Biopsy results revealed a normal epidermis; the dermis showed multiple small cystic structures lined by a stratified squamous epithelium containing eosinophilic keratin surrounded by a mononuclear cell infiltrate and some melanophages (Figure).

Milia en plaque was first described in 1903 by Balzer and Fouquet.1 In 1978, Hubler et al2 presented 2 cases with an asymptomatic, erythematous, and edematous plaque and white milialike lesions. On histopathology, they showed multiple cystic structures characterized by central laminated keratin and an intense polymorphic inflammatory reaction surrounding the cyst and epidermal appendages. Both patients were treated with topical tretinoin with complete response at 3 months. The authors suggested the term milia en plaque to describe this clinical entity.2
Milia en plaque is described as an infrequent condition that more often presents on the head, neck, and trunk, as well as the periocular, periauricular, and perinasal areas. It has been reported to occur at any age3 but appears more frequently in middle-aged adults and females. A congenital case also has been reported.4 It has been associated with pseudoxanthoma elasticum, lichen planus, trauma, kidney transplant, and cyclosporine use, but it also can present in healthy individuals,3 as in our patient. No clear cause has been identified.
Pathology is characteristic, with multiple cysts filled with keratin and surrounded by 2 or 3 layers of epithelial cells, associated with a mononuclear, nonlichenoid, mononuclear infiltrate.5 Structures similar to follicular infundibular tumors have been described, suggesting a common origin of follicular lesions as milia en plaque.6
Treatment includes surgical excision, cryosurgery, dermabrasion, electrodesiccation, trichloroacetic acid, photodynamic therapy, CO2 and erbium lasers, topical retinoids, minocycline, and etretinate.7 We performed a complete surgical excision in our patient.
In acneform reactions, erythematous papules and pustules can be found on the cheeks and forehead. Nevus comedonicus appears during childhood and presents with multiple open comedones. Postinflammatory milia is present in chronic inflammatory pathologies such as porphyria cutanea tarda. Histopathologic findings in adnexal tumors show a benign proliferation of any cellular type of a cutaneous annex.
Milia en plaque is an unusual but benign condition that is distinguished clinically by its characteristic presentation.
- Balzer F, Fouquet C. Milium confluent retroauricularies bilateral. Bull Soc Fr Dermatol Syphiligr. 1903;14:361.
- Hubler WR, Rudolph AH, Kelleher RM. Milia en plaque. Cutis. 1978;22:67-70.
- Berk DR, Bayliss SJ. Milia: a review and classification. J Am Acad Dermatol. 2008;59:1050-1063.
- Wang AR, Bercovitch L. Congenital milia en plaque. Pediatr Dermatol. 2016;33:258-259.
- Muñoz-Martínez R, Santamarina-Albertos A, Sanz-Muñoz C, et al. Milia en plaque. Actas Dermosifiliogr. 2013;104:638-640.
- Terui H, Hashimoto A, Yamasaki K, et al. Milia en plaque as a distinct follicular hamartoma with cystic trichoepitheliomatous features. Am J Dermatopathol. 2016;38:212-217.
- Tenna S, Filoni A, Pagliarello C, et al. Eyelid milia en plaque: a treatment challenge with a new CO2 fractional laser. Dermatol Ther. 2014;27:65-67.
- Balzer F, Fouquet C. Milium confluent retroauricularies bilateral. Bull Soc Fr Dermatol Syphiligr. 1903;14:361.
- Hubler WR, Rudolph AH, Kelleher RM. Milia en plaque. Cutis. 1978;22:67-70.
- Berk DR, Bayliss SJ. Milia: a review and classification. J Am Acad Dermatol. 2008;59:1050-1063.
- Wang AR, Bercovitch L. Congenital milia en plaque. Pediatr Dermatol. 2016;33:258-259.
- Muñoz-Martínez R, Santamarina-Albertos A, Sanz-Muñoz C, et al. Milia en plaque. Actas Dermosifiliogr. 2013;104:638-640.
- Terui H, Hashimoto A, Yamasaki K, et al. Milia en plaque as a distinct follicular hamartoma with cystic trichoepitheliomatous features. Am J Dermatopathol. 2016;38:212-217.
- Tenna S, Filoni A, Pagliarello C, et al. Eyelid milia en plaque: a treatment challenge with a new CO2 fractional laser. Dermatol Ther. 2014;27:65-67.
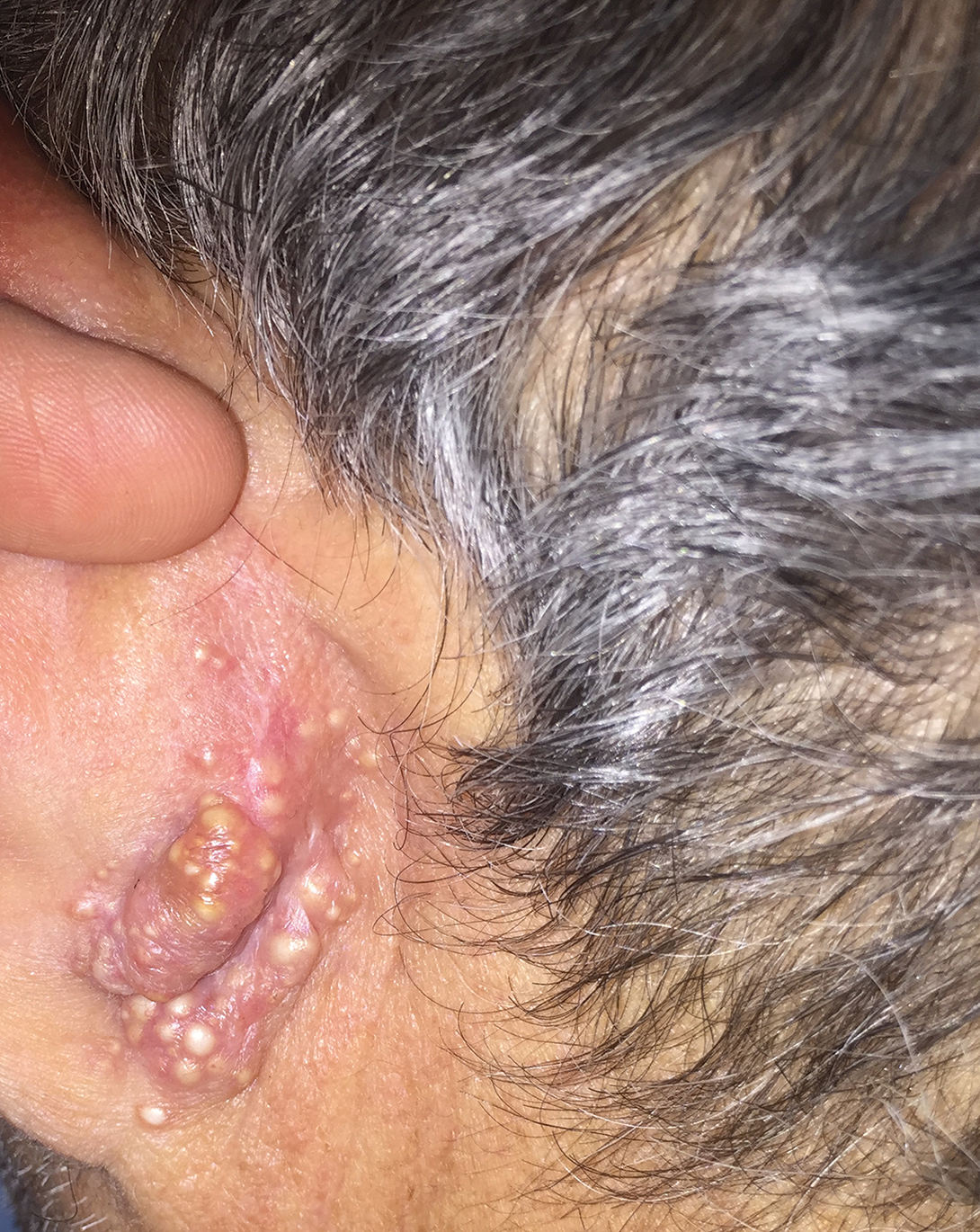
A 72-year-old man with a history of hypertension presented with a rapidly growing left retroauricular tumor of 3 months' duration. When manipulated, whitish material with a foul-smelling odor was expressed from the lesion. Physical examination showed an erythematous 3.2 ×1-cm tumor on the left posterior ear with multiple 1- to 2-mm white-yellow papules on its surface. A biopsy of the lesion was performed.
Don’t Mix Off-label Use With Off-the-rack Pills
A pregnant woman in Wisconsin received prenatal care from a family practitioner. The patient had hypertension, so at about 38 weeks’ gestation, the decision was made to induce labor.
On May 15, 2012, the family practitioner used misoprostol to induce labor. The patient received 100 mcg vaginally at 12:24
At 1:28
Variable late decelerations occurred at 11:36
Although the family practitioner was present at the bedside at 12:40
The on-call physician accomplished a vacuum delivery at 1:30
The child now has severe cerebral palsy, with gross motor involvement in the arms and legs. He can communicate through augmentative communication devices but cannot actually speak. He will require full-time care for the rest of his life.
Continue to: The defense took the position...
The defense took the position that while the dosage of misoprostol was excessive, the drug was no longer active in the mother’s body, based on its half-life, when the fetal distress occurred.
VERDICT
Four days before trial, the case was settled for $9 million.
COMMENTARY
I suspect many of you have made a pot roast—and at least some of you have used the simple, tried-and-true method of putting the meat into the slow cooker with a packet of onion soup mix. It makes a tasty dinner with minimal effort. But onion soup packets are for making onion soup—not seasoning pot roast. Guess what? You just used that soup mix off-label!
As clinicians, we all use medications for clinical indications that haven’t been specifically authorized by the FDA—and we shouldn’t stop. Off-label prescribing is legal, common, and often supported by the standard of care.
But there is a risk: The pill or tablet prepared by the manufacturer is generally aimed at the intended on-label use, not off-label uses. In this case, misoprostol (brand name, Cytotec) is approved by the FDA for prevention and treatment of gastrointestinal ulcers and peptic ulcer disease. The package insert describes dosing as follows:
The recommended adult oral dose of Cytotec for reducing the risk of NSAID-induced gastric ulcers is 200 mcg four times daily with food. If this dose cannot be tolerated, a dose of 100 mcg can be used.1
Continue to: We should not be shocked...
We should not be shocked, then, that Cytotec is supplied as 100- and 200-mcg round white tablets. However, it is frequently used off label for cervical ripening during labor at a dose of “25 mcg inserted into the posterior vaginal fornix.”2
This brings us to the malpractice trap. While off-label use may be appropriate, off-label uses may not neatly “fit” with the substance prepared by the manufacturer. To be properly administered for cervical ripening, the available tablet of misoprostol must be cut with a pill cutter or razor prior to administration.3 Furthermore, dosage is more accurate if the tablet fragments are individually weighed after cutting.3
In this case, the discrepancy between the pill prepared by the manufacturer (100 mcg) and the dosage needed (25 mcg) appears to have caught the defendant family practitioner off guard. So the take-home message is: Use medications as supported by the standard of care—but when using a drug off label, do not assume the product supplied by the manufacturer is appropriate for use as is.
Another interesting aspect of this case is the defense strategy. Most clinicians are aware that the tort of negligence involves (1) duty, (2) breach, (3) causation, and (4) harm. However, it is more logically consistent to think of the elements in this way: (1) duty, (2) breach, (3) harm, and if harm has occurred, (4) examine causation (ie, the logical connection between breach and harm).
In malpractice cases, attorneys frequently focus on one of these specific elements. In this case, the physician’s duty of care and the harm stemming from cerebral palsy are clearly established. Thus, breach and causation take center stage.
Continue to: The defense lawyers...
The defense lawyers acknowledged there was a breach, noting the dosage was “excessive.” However, they argued that this error didn’t matter because the drug was no longer active in the patient’s body. In other words, there was no causal connection between the inappropriately high dose and the resultant uterine tachysystole and fetal distress. This is a difficult road for several reasons.
First, the chief danger of using misoprostol is uterine hyperstimulation and fetal distress. The defense would have to argue the hyperstimulation and fetal distress were coincidental and unrelated to the misoprostol—which carries a black box warning for these very adverse effects. The plaintiff’s attorney is sure to make a big deal out of the black box warning in front of the jury—noting any reasonable clinician practicing obstetrics should be aware of the risks that come with misoprostol’s use. You can almost hear the argument in summation: “It is so important, they drew a warning box around it.”
Furthermore, making the argument that the misoprostol was not in the mother’s system at the time the fetal distress started would entail dueling expert testimony about pharmacokinetics and bioavailability—concepts that are difficult for lay jurors to understand. Misoprostol has a half-life of about 20 to 40 min when administered orally and about 60 min when administered vaginally.4 We know the mother received the overdose of misoprostol at 12:24
The plaintiff’s team might counter with an expert’s explanation that misoprostol’s bioavailability is increased 2- to 3-fold with vaginal versus oral administration. It would also be observed that compared with oral administration, vaginal administration of misoprostol is associated with a slower increase in plasma concentrations but longer elevations (peaking about 1-2 hours after vaginal administration).5
At best, the defense expert would be able to argue that the serum level likely peaked 1 to 2 hours after administration (1:24-2:24
Continue to: Most jurors would...
Most jurors would take a skeptical view of the defendant’s argument that the negative outcome in this case was coincidental. Some might even be angered by it. This realization likely prompted the defense to settle this case for $9 million.
IN SUMMARY
Onion soup mix makes great soup, but it’s an even better seasoning for pot roast. Similarly, there are pharmacologic agents that are effective for conditions for which they are not formally indicated. Do not withhold judicious off-label use of medications when appropriate. However, be aware that off-label uses may require extra attention, and dosing and administration may not be consistent with the product you have on hand. Don’t hesitate to seek guidance from pharmacy colleagues when you have questions—they are an underutilized resource and are generally happy to share their expertise.
1. Cytotec [package insert]. New York, NY: Pfizer Inc; 2009.
2. Misoprostol: dosing considerations. PDR: Prescribers’ Digital Reference. www.pdr.net/drug-summary/Cytotec-misoprostol-1044#8. Accessed July 29, 2019.
3. Williams MC, Tsibris JC, Davis G, et al. Dose variation that is associated with approximated one-quarter tablet doses of misoprostol. Am J Obstet Gynecol. 2002;187(3):615-619.
4. Yount SM, Lassiter N. The pharmacology of prostaglandins for induction of labor. J Midwifery Womens Health. 2013;58(2):133-144; quiz 238-239.
5. Danielsson KG, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93(2):275-280.
A pregnant woman in Wisconsin received prenatal care from a family practitioner. The patient had hypertension, so at about 38 weeks’ gestation, the decision was made to induce labor.
On May 15, 2012, the family practitioner used misoprostol to induce labor. The patient received 100 mcg vaginally at 12:24
At 1:28
Variable late decelerations occurred at 11:36
Although the family practitioner was present at the bedside at 12:40
The on-call physician accomplished a vacuum delivery at 1:30
The child now has severe cerebral palsy, with gross motor involvement in the arms and legs. He can communicate through augmentative communication devices but cannot actually speak. He will require full-time care for the rest of his life.
Continue to: The defense took the position...
The defense took the position that while the dosage of misoprostol was excessive, the drug was no longer active in the mother’s body, based on its half-life, when the fetal distress occurred.
VERDICT
Four days before trial, the case was settled for $9 million.
COMMENTARY
I suspect many of you have made a pot roast—and at least some of you have used the simple, tried-and-true method of putting the meat into the slow cooker with a packet of onion soup mix. It makes a tasty dinner with minimal effort. But onion soup packets are for making onion soup—not seasoning pot roast. Guess what? You just used that soup mix off-label!
As clinicians, we all use medications for clinical indications that haven’t been specifically authorized by the FDA—and we shouldn’t stop. Off-label prescribing is legal, common, and often supported by the standard of care.
But there is a risk: The pill or tablet prepared by the manufacturer is generally aimed at the intended on-label use, not off-label uses. In this case, misoprostol (brand name, Cytotec) is approved by the FDA for prevention and treatment of gastrointestinal ulcers and peptic ulcer disease. The package insert describes dosing as follows:
The recommended adult oral dose of Cytotec for reducing the risk of NSAID-induced gastric ulcers is 200 mcg four times daily with food. If this dose cannot be tolerated, a dose of 100 mcg can be used.1
Continue to: We should not be shocked...
We should not be shocked, then, that Cytotec is supplied as 100- and 200-mcg round white tablets. However, it is frequently used off label for cervical ripening during labor at a dose of “25 mcg inserted into the posterior vaginal fornix.”2
This brings us to the malpractice trap. While off-label use may be appropriate, off-label uses may not neatly “fit” with the substance prepared by the manufacturer. To be properly administered for cervical ripening, the available tablet of misoprostol must be cut with a pill cutter or razor prior to administration.3 Furthermore, dosage is more accurate if the tablet fragments are individually weighed after cutting.3
In this case, the discrepancy between the pill prepared by the manufacturer (100 mcg) and the dosage needed (25 mcg) appears to have caught the defendant family practitioner off guard. So the take-home message is: Use medications as supported by the standard of care—but when using a drug off label, do not assume the product supplied by the manufacturer is appropriate for use as is.
Another interesting aspect of this case is the defense strategy. Most clinicians are aware that the tort of negligence involves (1) duty, (2) breach, (3) causation, and (4) harm. However, it is more logically consistent to think of the elements in this way: (1) duty, (2) breach, (3) harm, and if harm has occurred, (4) examine causation (ie, the logical connection between breach and harm).
In malpractice cases, attorneys frequently focus on one of these specific elements. In this case, the physician’s duty of care and the harm stemming from cerebral palsy are clearly established. Thus, breach and causation take center stage.
Continue to: The defense lawyers...
The defense lawyers acknowledged there was a breach, noting the dosage was “excessive.” However, they argued that this error didn’t matter because the drug was no longer active in the patient’s body. In other words, there was no causal connection between the inappropriately high dose and the resultant uterine tachysystole and fetal distress. This is a difficult road for several reasons.
First, the chief danger of using misoprostol is uterine hyperstimulation and fetal distress. The defense would have to argue the hyperstimulation and fetal distress were coincidental and unrelated to the misoprostol—which carries a black box warning for these very adverse effects. The plaintiff’s attorney is sure to make a big deal out of the black box warning in front of the jury—noting any reasonable clinician practicing obstetrics should be aware of the risks that come with misoprostol’s use. You can almost hear the argument in summation: “It is so important, they drew a warning box around it.”
Furthermore, making the argument that the misoprostol was not in the mother’s system at the time the fetal distress started would entail dueling expert testimony about pharmacokinetics and bioavailability—concepts that are difficult for lay jurors to understand. Misoprostol has a half-life of about 20 to 40 min when administered orally and about 60 min when administered vaginally.4 We know the mother received the overdose of misoprostol at 12:24
The plaintiff’s team might counter with an expert’s explanation that misoprostol’s bioavailability is increased 2- to 3-fold with vaginal versus oral administration. It would also be observed that compared with oral administration, vaginal administration of misoprostol is associated with a slower increase in plasma concentrations but longer elevations (peaking about 1-2 hours after vaginal administration).5
At best, the defense expert would be able to argue that the serum level likely peaked 1 to 2 hours after administration (1:24-2:24
Continue to: Most jurors would...
Most jurors would take a skeptical view of the defendant’s argument that the negative outcome in this case was coincidental. Some might even be angered by it. This realization likely prompted the defense to settle this case for $9 million.
IN SUMMARY
Onion soup mix makes great soup, but it’s an even better seasoning for pot roast. Similarly, there are pharmacologic agents that are effective for conditions for which they are not formally indicated. Do not withhold judicious off-label use of medications when appropriate. However, be aware that off-label uses may require extra attention, and dosing and administration may not be consistent with the product you have on hand. Don’t hesitate to seek guidance from pharmacy colleagues when you have questions—they are an underutilized resource and are generally happy to share their expertise.
A pregnant woman in Wisconsin received prenatal care from a family practitioner. The patient had hypertension, so at about 38 weeks’ gestation, the decision was made to induce labor.
On May 15, 2012, the family practitioner used misoprostol to induce labor. The patient received 100 mcg vaginally at 12:24
At 1:28
Variable late decelerations occurred at 11:36
Although the family practitioner was present at the bedside at 12:40
The on-call physician accomplished a vacuum delivery at 1:30
The child now has severe cerebral palsy, with gross motor involvement in the arms and legs. He can communicate through augmentative communication devices but cannot actually speak. He will require full-time care for the rest of his life.
Continue to: The defense took the position...
The defense took the position that while the dosage of misoprostol was excessive, the drug was no longer active in the mother’s body, based on its half-life, when the fetal distress occurred.
VERDICT
Four days before trial, the case was settled for $9 million.
COMMENTARY
I suspect many of you have made a pot roast—and at least some of you have used the simple, tried-and-true method of putting the meat into the slow cooker with a packet of onion soup mix. It makes a tasty dinner with minimal effort. But onion soup packets are for making onion soup—not seasoning pot roast. Guess what? You just used that soup mix off-label!
As clinicians, we all use medications for clinical indications that haven’t been specifically authorized by the FDA—and we shouldn’t stop. Off-label prescribing is legal, common, and often supported by the standard of care.
But there is a risk: The pill or tablet prepared by the manufacturer is generally aimed at the intended on-label use, not off-label uses. In this case, misoprostol (brand name, Cytotec) is approved by the FDA for prevention and treatment of gastrointestinal ulcers and peptic ulcer disease. The package insert describes dosing as follows:
The recommended adult oral dose of Cytotec for reducing the risk of NSAID-induced gastric ulcers is 200 mcg four times daily with food. If this dose cannot be tolerated, a dose of 100 mcg can be used.1
Continue to: We should not be shocked...
We should not be shocked, then, that Cytotec is supplied as 100- and 200-mcg round white tablets. However, it is frequently used off label for cervical ripening during labor at a dose of “25 mcg inserted into the posterior vaginal fornix.”2
This brings us to the malpractice trap. While off-label use may be appropriate, off-label uses may not neatly “fit” with the substance prepared by the manufacturer. To be properly administered for cervical ripening, the available tablet of misoprostol must be cut with a pill cutter or razor prior to administration.3 Furthermore, dosage is more accurate if the tablet fragments are individually weighed after cutting.3
In this case, the discrepancy between the pill prepared by the manufacturer (100 mcg) and the dosage needed (25 mcg) appears to have caught the defendant family practitioner off guard. So the take-home message is: Use medications as supported by the standard of care—but when using a drug off label, do not assume the product supplied by the manufacturer is appropriate for use as is.
Another interesting aspect of this case is the defense strategy. Most clinicians are aware that the tort of negligence involves (1) duty, (2) breach, (3) causation, and (4) harm. However, it is more logically consistent to think of the elements in this way: (1) duty, (2) breach, (3) harm, and if harm has occurred, (4) examine causation (ie, the logical connection between breach and harm).
In malpractice cases, attorneys frequently focus on one of these specific elements. In this case, the physician’s duty of care and the harm stemming from cerebral palsy are clearly established. Thus, breach and causation take center stage.
Continue to: The defense lawyers...
The defense lawyers acknowledged there was a breach, noting the dosage was “excessive.” However, they argued that this error didn’t matter because the drug was no longer active in the patient’s body. In other words, there was no causal connection between the inappropriately high dose and the resultant uterine tachysystole and fetal distress. This is a difficult road for several reasons.
First, the chief danger of using misoprostol is uterine hyperstimulation and fetal distress. The defense would have to argue the hyperstimulation and fetal distress were coincidental and unrelated to the misoprostol—which carries a black box warning for these very adverse effects. The plaintiff’s attorney is sure to make a big deal out of the black box warning in front of the jury—noting any reasonable clinician practicing obstetrics should be aware of the risks that come with misoprostol’s use. You can almost hear the argument in summation: “It is so important, they drew a warning box around it.”
Furthermore, making the argument that the misoprostol was not in the mother’s system at the time the fetal distress started would entail dueling expert testimony about pharmacokinetics and bioavailability—concepts that are difficult for lay jurors to understand. Misoprostol has a half-life of about 20 to 40 min when administered orally and about 60 min when administered vaginally.4 We know the mother received the overdose of misoprostol at 12:24
The plaintiff’s team might counter with an expert’s explanation that misoprostol’s bioavailability is increased 2- to 3-fold with vaginal versus oral administration. It would also be observed that compared with oral administration, vaginal administration of misoprostol is associated with a slower increase in plasma concentrations but longer elevations (peaking about 1-2 hours after vaginal administration).5
At best, the defense expert would be able to argue that the serum level likely peaked 1 to 2 hours after administration (1:24-2:24
Continue to: Most jurors would...
Most jurors would take a skeptical view of the defendant’s argument that the negative outcome in this case was coincidental. Some might even be angered by it. This realization likely prompted the defense to settle this case for $9 million.
IN SUMMARY
Onion soup mix makes great soup, but it’s an even better seasoning for pot roast. Similarly, there are pharmacologic agents that are effective for conditions for which they are not formally indicated. Do not withhold judicious off-label use of medications when appropriate. However, be aware that off-label uses may require extra attention, and dosing and administration may not be consistent with the product you have on hand. Don’t hesitate to seek guidance from pharmacy colleagues when you have questions—they are an underutilized resource and are generally happy to share their expertise.
1. Cytotec [package insert]. New York, NY: Pfizer Inc; 2009.
2. Misoprostol: dosing considerations. PDR: Prescribers’ Digital Reference. www.pdr.net/drug-summary/Cytotec-misoprostol-1044#8. Accessed July 29, 2019.
3. Williams MC, Tsibris JC, Davis G, et al. Dose variation that is associated with approximated one-quarter tablet doses of misoprostol. Am J Obstet Gynecol. 2002;187(3):615-619.
4. Yount SM, Lassiter N. The pharmacology of prostaglandins for induction of labor. J Midwifery Womens Health. 2013;58(2):133-144; quiz 238-239.
5. Danielsson KG, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93(2):275-280.
1. Cytotec [package insert]. New York, NY: Pfizer Inc; 2009.
2. Misoprostol: dosing considerations. PDR: Prescribers’ Digital Reference. www.pdr.net/drug-summary/Cytotec-misoprostol-1044#8. Accessed July 29, 2019.
3. Williams MC, Tsibris JC, Davis G, et al. Dose variation that is associated with approximated one-quarter tablet doses of misoprostol. Am J Obstet Gynecol. 2002;187(3):615-619.
4. Yount SM, Lassiter N. The pharmacology of prostaglandins for induction of labor. J Midwifery Womens Health. 2013;58(2):133-144; quiz 238-239.
5. Danielsson KG, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93(2):275-280.
Short Takes
Comparison of the number of major complications of laparoscopic cholecystectomy versus percutaneous catheter drainage in the treatment of acute cholecystitis
This randomized, controlled trial showed that 65% of high-risk patients (APACHE II score of at least 7) with acute cholecystitis experienced major complications after undergoing percutaneous catheter drainage, compared with 12% of patients who underwent laparoscopic cholecystectomy. Major complications included reintervention and recurrent biliary disease. The rate of death was the same in both groups.
Citation: Loozen CS et al. Laparoscopic cholecystectomy versus percutaneous catheter drainage for acute cholecystitis in high-risk patients (CHOCOLATE): Multicentre randomised clinical trial. BMJ. 2018 Aug 28;363:k3965.
Food and Drug Administration approves new drug to treat influenza
Two randomized, controlled trials showed that Xofluza (baloxavir marboxil) taken as a single dose decreased symptoms in uncomplicated influenza, compared with placebo. The medication also was associated with a lower viral load on day 1 after administration, compared with both placebo and oseltamivir, the most commonly used medication to treat influenza.
Citation: Hayden FG et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Eng J Med. 2018 Sep 6;379:913-23.
Effects of missed hemodialysis treatments
Researchers used a prospective cohort of 8,501 patients from hemodialysis (HD) centers in 20 countries to identify patients who missed one or more HD sessions in 4 months. In the United States, 24% of HD patients missed one or more sessions in 4 months, compared with 10% in Canada and 9% in the United Kingdom. Moreover, 12.2% of U.S. HD patients missed at least one session per month. All-cause mortality was 68% higher in patients who missed one or more sessions in 4 months. Risk factors associated with missing dialysis treatments were travel time of more than 1 hour to the facility, depression, younger age, being on dialysis for a shorter vintage, lower perceived burden of kidney disease, and shorter treatment times.
Citation: Al Salmi I et al. Missed hemodialysis treatments: International variation, predictors, and outcomes in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2018 Nov;72(5)634-43.
Do in situ mock codes affect in-hospital cardiac arrest mortality?
This ecological study included multiple hospital systems and showed that hospitals with a higher proportion of in situ mock codes had an in-hospital cardiac arrest survival rate of 42.8% versus 31.8% in hospitals with fewer mock codes (P greater than .0001).
Citation: Josey K et al. Hospitals with more-active participation in conducting standardized in-situ mock codes have improved survival after in-hospital cardiopulmonary arrest. Resuscitation. 2018 Dec;133:47-52.
New oxygen guidelines
In patients admitted with acute stroke or MI, an international expert panel made a strong recommendation against initiating supplemental oxygen when the SpO2 is greater than 92% and a weak recommendation against initiating supplemental oxygen when the SpO2 is 90%-92%. In acutely ill medical patients receiving supplemental oxygen, the panel makes a strong recommendation to maintain an upper limit oxygen saturation of less than 96%.
Citation: Siemieniuk RAC et al. Oxygen therapy for acutely ill medical patients: A clinical practice guideline. BMJ. 2018 Oct 24;363:k4169.
Comparison of the number of major complications of laparoscopic cholecystectomy versus percutaneous catheter drainage in the treatment of acute cholecystitis
This randomized, controlled trial showed that 65% of high-risk patients (APACHE II score of at least 7) with acute cholecystitis experienced major complications after undergoing percutaneous catheter drainage, compared with 12% of patients who underwent laparoscopic cholecystectomy. Major complications included reintervention and recurrent biliary disease. The rate of death was the same in both groups.
Citation: Loozen CS et al. Laparoscopic cholecystectomy versus percutaneous catheter drainage for acute cholecystitis in high-risk patients (CHOCOLATE): Multicentre randomised clinical trial. BMJ. 2018 Aug 28;363:k3965.
Food and Drug Administration approves new drug to treat influenza
Two randomized, controlled trials showed that Xofluza (baloxavir marboxil) taken as a single dose decreased symptoms in uncomplicated influenza, compared with placebo. The medication also was associated with a lower viral load on day 1 after administration, compared with both placebo and oseltamivir, the most commonly used medication to treat influenza.
Citation: Hayden FG et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Eng J Med. 2018 Sep 6;379:913-23.
Effects of missed hemodialysis treatments
Researchers used a prospective cohort of 8,501 patients from hemodialysis (HD) centers in 20 countries to identify patients who missed one or more HD sessions in 4 months. In the United States, 24% of HD patients missed one or more sessions in 4 months, compared with 10% in Canada and 9% in the United Kingdom. Moreover, 12.2% of U.S. HD patients missed at least one session per month. All-cause mortality was 68% higher in patients who missed one or more sessions in 4 months. Risk factors associated with missing dialysis treatments were travel time of more than 1 hour to the facility, depression, younger age, being on dialysis for a shorter vintage, lower perceived burden of kidney disease, and shorter treatment times.
Citation: Al Salmi I et al. Missed hemodialysis treatments: International variation, predictors, and outcomes in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2018 Nov;72(5)634-43.
Do in situ mock codes affect in-hospital cardiac arrest mortality?
This ecological study included multiple hospital systems and showed that hospitals with a higher proportion of in situ mock codes had an in-hospital cardiac arrest survival rate of 42.8% versus 31.8% in hospitals with fewer mock codes (P greater than .0001).
Citation: Josey K et al. Hospitals with more-active participation in conducting standardized in-situ mock codes have improved survival after in-hospital cardiopulmonary arrest. Resuscitation. 2018 Dec;133:47-52.
New oxygen guidelines
In patients admitted with acute stroke or MI, an international expert panel made a strong recommendation against initiating supplemental oxygen when the SpO2 is greater than 92% and a weak recommendation against initiating supplemental oxygen when the SpO2 is 90%-92%. In acutely ill medical patients receiving supplemental oxygen, the panel makes a strong recommendation to maintain an upper limit oxygen saturation of less than 96%.
Citation: Siemieniuk RAC et al. Oxygen therapy for acutely ill medical patients: A clinical practice guideline. BMJ. 2018 Oct 24;363:k4169.
Comparison of the number of major complications of laparoscopic cholecystectomy versus percutaneous catheter drainage in the treatment of acute cholecystitis
This randomized, controlled trial showed that 65% of high-risk patients (APACHE II score of at least 7) with acute cholecystitis experienced major complications after undergoing percutaneous catheter drainage, compared with 12% of patients who underwent laparoscopic cholecystectomy. Major complications included reintervention and recurrent biliary disease. The rate of death was the same in both groups.
Citation: Loozen CS et al. Laparoscopic cholecystectomy versus percutaneous catheter drainage for acute cholecystitis in high-risk patients (CHOCOLATE): Multicentre randomised clinical trial. BMJ. 2018 Aug 28;363:k3965.
Food and Drug Administration approves new drug to treat influenza
Two randomized, controlled trials showed that Xofluza (baloxavir marboxil) taken as a single dose decreased symptoms in uncomplicated influenza, compared with placebo. The medication also was associated with a lower viral load on day 1 after administration, compared with both placebo and oseltamivir, the most commonly used medication to treat influenza.
Citation: Hayden FG et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Eng J Med. 2018 Sep 6;379:913-23.
Effects of missed hemodialysis treatments
Researchers used a prospective cohort of 8,501 patients from hemodialysis (HD) centers in 20 countries to identify patients who missed one or more HD sessions in 4 months. In the United States, 24% of HD patients missed one or more sessions in 4 months, compared with 10% in Canada and 9% in the United Kingdom. Moreover, 12.2% of U.S. HD patients missed at least one session per month. All-cause mortality was 68% higher in patients who missed one or more sessions in 4 months. Risk factors associated with missing dialysis treatments were travel time of more than 1 hour to the facility, depression, younger age, being on dialysis for a shorter vintage, lower perceived burden of kidney disease, and shorter treatment times.
Citation: Al Salmi I et al. Missed hemodialysis treatments: International variation, predictors, and outcomes in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2018 Nov;72(5)634-43.
Do in situ mock codes affect in-hospital cardiac arrest mortality?
This ecological study included multiple hospital systems and showed that hospitals with a higher proportion of in situ mock codes had an in-hospital cardiac arrest survival rate of 42.8% versus 31.8% in hospitals with fewer mock codes (P greater than .0001).
Citation: Josey K et al. Hospitals with more-active participation in conducting standardized in-situ mock codes have improved survival after in-hospital cardiopulmonary arrest. Resuscitation. 2018 Dec;133:47-52.
New oxygen guidelines
In patients admitted with acute stroke or MI, an international expert panel made a strong recommendation against initiating supplemental oxygen when the SpO2 is greater than 92% and a weak recommendation against initiating supplemental oxygen when the SpO2 is 90%-92%. In acutely ill medical patients receiving supplemental oxygen, the panel makes a strong recommendation to maintain an upper limit oxygen saturation of less than 96%.
Citation: Siemieniuk RAC et al. Oxygen therapy for acutely ill medical patients: A clinical practice guideline. BMJ. 2018 Oct 24;363:k4169.
Acid-suppressing drug use associated with increased antiallergy drug use
Gastric acid–suppressing medications such as proton pump inhibitors are associated with a significant increase in subsequent antiallergy medication use, particularly in older individuals.
In a population-based study of health insurance data from 8.2 million people, Austrian researchers looked for prescriptions of gastric acid inhibitors, antiallergy drugs, or other commonly prescribed (lipid-modifying and antihypertensive) drugs as controls from 2009-2013.
According to results published in Nature Communications, gastric acid–suppressing drugs were associated with an overall 96% higher rate of subsequent prescriptions for antiallergy medications than among the general population not taking gastric acid–suppressing drugs (P less than .001). Among individuals aged 60 years or older, the rate of allergy medication prescriptions after acid-suppressing treatment was more than five times higher than that in the general population.
The rate of antiallergy medication use after acid-suppressing medication prescription was threefold higher than the rate seen after lipid-modifying or antihypertensive drug prescription.
“Our findings confirm an epidemiological association between gastric acid suppression and development of allergic symptoms, in line with previous mechanistic animal trials and human observational studies,” wrote Galateja Jordakieva, PhD, of the department of physical medicine, rehabilitation, and occupational medicine at the Medical University of Vienna, and coauthors.
All groups of acid-inhibiting medications were associated with a higher rate of subsequent antiallergy medication prescriptions. The only exception was prostaglandin E2 medications, but the authors said that here the numbers were too low to draw conclusions.
The hazard rate for antiallergy medications also increased with increasing numbers of daily doses of acid-suppressing medication, the study showed. The hazard rate for the lowest quartile – up to 20 daily doses per year – was a significant 28% higher than that of the general population, while the third quartile (68-213 daily dose per year) was associated with a 2.67-fold higher hazard. A similar increase was seen for the fourth quartile of acid suppression–medication dosing.
The authors established that just six daily doses of acid-suppressing drugs in a year were associated with significantly earlier prescriptions of antiallergy medication.
Stephen Evans, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine, emphasized in an associated commentary that the findings reflect association, not causation. “There are many possible explanations for the observed association ... In fact, the data make other explanations more than likely to be the cause of allergies.”
Coprescriptions of aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs) are common among PPI users and “are among the drugs that are very well known to increase the risk of an allergic reaction.” The data show that antiallergy medicines are prescribed at a relatively similar rate at all ages, while PPI prescribing increases in the elderly.
“If the rate of prescription of PPIs shows a steep rise with age and they are a significant cause of allergies, then the anti-allergy medicines ... should also show a steep rise with age. The fact that they don’t, either means they show no relation or that any relation has a minimal effect on allergies,” Dr. Evans said. “Nearly all drugs can have very rare allergic reactions, including PPIs, but this paper does not help to show what the true rate is of these very rare reactions, or whether they are caused by PPIs alone. The design and analysis methods in the paper are likely to exaggerate their apparent occurrence.”
The study was supported by Burgenländische Gebietskrankenkasse and the Austrian Science Fund. No conflicts of interest were declared.
SOURCE: Jordakieva G et al. Nat Commun. 2019 Jul 30. doi: 10.1038/s41467-019-10914-6.
Gastric acid–suppressing medications such as proton pump inhibitors are associated with a significant increase in subsequent antiallergy medication use, particularly in older individuals.
In a population-based study of health insurance data from 8.2 million people, Austrian researchers looked for prescriptions of gastric acid inhibitors, antiallergy drugs, or other commonly prescribed (lipid-modifying and antihypertensive) drugs as controls from 2009-2013.
According to results published in Nature Communications, gastric acid–suppressing drugs were associated with an overall 96% higher rate of subsequent prescriptions for antiallergy medications than among the general population not taking gastric acid–suppressing drugs (P less than .001). Among individuals aged 60 years or older, the rate of allergy medication prescriptions after acid-suppressing treatment was more than five times higher than that in the general population.
The rate of antiallergy medication use after acid-suppressing medication prescription was threefold higher than the rate seen after lipid-modifying or antihypertensive drug prescription.
“Our findings confirm an epidemiological association between gastric acid suppression and development of allergic symptoms, in line with previous mechanistic animal trials and human observational studies,” wrote Galateja Jordakieva, PhD, of the department of physical medicine, rehabilitation, and occupational medicine at the Medical University of Vienna, and coauthors.
All groups of acid-inhibiting medications were associated with a higher rate of subsequent antiallergy medication prescriptions. The only exception was prostaglandin E2 medications, but the authors said that here the numbers were too low to draw conclusions.
The hazard rate for antiallergy medications also increased with increasing numbers of daily doses of acid-suppressing medication, the study showed. The hazard rate for the lowest quartile – up to 20 daily doses per year – was a significant 28% higher than that of the general population, while the third quartile (68-213 daily dose per year) was associated with a 2.67-fold higher hazard. A similar increase was seen for the fourth quartile of acid suppression–medication dosing.
The authors established that just six daily doses of acid-suppressing drugs in a year were associated with significantly earlier prescriptions of antiallergy medication.
Stephen Evans, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine, emphasized in an associated commentary that the findings reflect association, not causation. “There are many possible explanations for the observed association ... In fact, the data make other explanations more than likely to be the cause of allergies.”
Coprescriptions of aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs) are common among PPI users and “are among the drugs that are very well known to increase the risk of an allergic reaction.” The data show that antiallergy medicines are prescribed at a relatively similar rate at all ages, while PPI prescribing increases in the elderly.
“If the rate of prescription of PPIs shows a steep rise with age and they are a significant cause of allergies, then the anti-allergy medicines ... should also show a steep rise with age. The fact that they don’t, either means they show no relation or that any relation has a minimal effect on allergies,” Dr. Evans said. “Nearly all drugs can have very rare allergic reactions, including PPIs, but this paper does not help to show what the true rate is of these very rare reactions, or whether they are caused by PPIs alone. The design and analysis methods in the paper are likely to exaggerate their apparent occurrence.”
The study was supported by Burgenländische Gebietskrankenkasse and the Austrian Science Fund. No conflicts of interest were declared.
SOURCE: Jordakieva G et al. Nat Commun. 2019 Jul 30. doi: 10.1038/s41467-019-10914-6.
Gastric acid–suppressing medications such as proton pump inhibitors are associated with a significant increase in subsequent antiallergy medication use, particularly in older individuals.
In a population-based study of health insurance data from 8.2 million people, Austrian researchers looked for prescriptions of gastric acid inhibitors, antiallergy drugs, or other commonly prescribed (lipid-modifying and antihypertensive) drugs as controls from 2009-2013.
According to results published in Nature Communications, gastric acid–suppressing drugs were associated with an overall 96% higher rate of subsequent prescriptions for antiallergy medications than among the general population not taking gastric acid–suppressing drugs (P less than .001). Among individuals aged 60 years or older, the rate of allergy medication prescriptions after acid-suppressing treatment was more than five times higher than that in the general population.
The rate of antiallergy medication use after acid-suppressing medication prescription was threefold higher than the rate seen after lipid-modifying or antihypertensive drug prescription.
“Our findings confirm an epidemiological association between gastric acid suppression and development of allergic symptoms, in line with previous mechanistic animal trials and human observational studies,” wrote Galateja Jordakieva, PhD, of the department of physical medicine, rehabilitation, and occupational medicine at the Medical University of Vienna, and coauthors.
All groups of acid-inhibiting medications were associated with a higher rate of subsequent antiallergy medication prescriptions. The only exception was prostaglandin E2 medications, but the authors said that here the numbers were too low to draw conclusions.
The hazard rate for antiallergy medications also increased with increasing numbers of daily doses of acid-suppressing medication, the study showed. The hazard rate for the lowest quartile – up to 20 daily doses per year – was a significant 28% higher than that of the general population, while the third quartile (68-213 daily dose per year) was associated with a 2.67-fold higher hazard. A similar increase was seen for the fourth quartile of acid suppression–medication dosing.
The authors established that just six daily doses of acid-suppressing drugs in a year were associated with significantly earlier prescriptions of antiallergy medication.
Stephen Evans, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine, emphasized in an associated commentary that the findings reflect association, not causation. “There are many possible explanations for the observed association ... In fact, the data make other explanations more than likely to be the cause of allergies.”
Coprescriptions of aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs) are common among PPI users and “are among the drugs that are very well known to increase the risk of an allergic reaction.” The data show that antiallergy medicines are prescribed at a relatively similar rate at all ages, while PPI prescribing increases in the elderly.
“If the rate of prescription of PPIs shows a steep rise with age and they are a significant cause of allergies, then the anti-allergy medicines ... should also show a steep rise with age. The fact that they don’t, either means they show no relation or that any relation has a minimal effect on allergies,” Dr. Evans said. “Nearly all drugs can have very rare allergic reactions, including PPIs, but this paper does not help to show what the true rate is of these very rare reactions, or whether they are caused by PPIs alone. The design and analysis methods in the paper are likely to exaggerate their apparent occurrence.”
The study was supported by Burgenländische Gebietskrankenkasse and the Austrian Science Fund. No conflicts of interest were declared.
SOURCE: Jordakieva G et al. Nat Commun. 2019 Jul 30. doi: 10.1038/s41467-019-10914-6.
FROM NATURE COMMUNICATIONS
MMR vaccine maintains effectiveness after 10 years
according to Stephane Carryn, PhD, of GlaxoSmithKline and associates.
In a phase 3, observer-blind, randomized study published in Vaccine, 1,887 children aged 12-22 months received two doses of the MMR plus varicella (MMRV) vaccine Priorix-Tetra, one dose of the MMR vaccine Priorix with one dose of the varicella vaccine Varilrix delivered separately, or two doses of the MMR vaccine. Blood samples were collected at baseline and at days 42 and 84, then at year 1, 2, 4, 6, 8, and 10.
Antimeasles and antirubella antibodies declined moderately over the 10-year study period, but seropositivity remained high at 10 years, with about 94.0% of children remaining seropositive for antimeasles antibodies and about 96.6% remaining seropositive for antirubella antibodies. Children who received Priorix-Tetra had antimeasles antibody titers twice as high as those who received Priorix throughout the study. In addition, children who received a second dose of MMR vaccine later in life saw only a transient benefit in antimeasles and antirubella titers.
Antimumps antibody titer levels remained stable over the course of the study, and the seropositivity rate was about 90.0% at 10 years. Children who received a second MMR vaccine later in life saw a boosting effect in seropositivity and antimumps antibody titer levels.
“The responses obtained after receipt of one or two doses of MMR-containing vaccines remain well above the seropositivity thresholds up to 10 years post vaccination, regardless of the vaccine given and the schedule used,” the investigators concluded.
The study was funded and supported by GlaxoSmithKline. The study authors reported being employed by GlaxoSmithKline; two also reported owning shares in the company.
SOURCE: Carryn S et al. Vaccine. 2019 Jul 22. doi: 10.1016/j.vaccine.2019.07.049.
according to Stephane Carryn, PhD, of GlaxoSmithKline and associates.
In a phase 3, observer-blind, randomized study published in Vaccine, 1,887 children aged 12-22 months received two doses of the MMR plus varicella (MMRV) vaccine Priorix-Tetra, one dose of the MMR vaccine Priorix with one dose of the varicella vaccine Varilrix delivered separately, or two doses of the MMR vaccine. Blood samples were collected at baseline and at days 42 and 84, then at year 1, 2, 4, 6, 8, and 10.
Antimeasles and antirubella antibodies declined moderately over the 10-year study period, but seropositivity remained high at 10 years, with about 94.0% of children remaining seropositive for antimeasles antibodies and about 96.6% remaining seropositive for antirubella antibodies. Children who received Priorix-Tetra had antimeasles antibody titers twice as high as those who received Priorix throughout the study. In addition, children who received a second dose of MMR vaccine later in life saw only a transient benefit in antimeasles and antirubella titers.
Antimumps antibody titer levels remained stable over the course of the study, and the seropositivity rate was about 90.0% at 10 years. Children who received a second MMR vaccine later in life saw a boosting effect in seropositivity and antimumps antibody titer levels.
“The responses obtained after receipt of one or two doses of MMR-containing vaccines remain well above the seropositivity thresholds up to 10 years post vaccination, regardless of the vaccine given and the schedule used,” the investigators concluded.
The study was funded and supported by GlaxoSmithKline. The study authors reported being employed by GlaxoSmithKline; two also reported owning shares in the company.
SOURCE: Carryn S et al. Vaccine. 2019 Jul 22. doi: 10.1016/j.vaccine.2019.07.049.
according to Stephane Carryn, PhD, of GlaxoSmithKline and associates.
In a phase 3, observer-blind, randomized study published in Vaccine, 1,887 children aged 12-22 months received two doses of the MMR plus varicella (MMRV) vaccine Priorix-Tetra, one dose of the MMR vaccine Priorix with one dose of the varicella vaccine Varilrix delivered separately, or two doses of the MMR vaccine. Blood samples were collected at baseline and at days 42 and 84, then at year 1, 2, 4, 6, 8, and 10.
Antimeasles and antirubella antibodies declined moderately over the 10-year study period, but seropositivity remained high at 10 years, with about 94.0% of children remaining seropositive for antimeasles antibodies and about 96.6% remaining seropositive for antirubella antibodies. Children who received Priorix-Tetra had antimeasles antibody titers twice as high as those who received Priorix throughout the study. In addition, children who received a second dose of MMR vaccine later in life saw only a transient benefit in antimeasles and antirubella titers.
Antimumps antibody titer levels remained stable over the course of the study, and the seropositivity rate was about 90.0% at 10 years. Children who received a second MMR vaccine later in life saw a boosting effect in seropositivity and antimumps antibody titer levels.
“The responses obtained after receipt of one or two doses of MMR-containing vaccines remain well above the seropositivity thresholds up to 10 years post vaccination, regardless of the vaccine given and the schedule used,” the investigators concluded.
The study was funded and supported by GlaxoSmithKline. The study authors reported being employed by GlaxoSmithKline; two also reported owning shares in the company.
SOURCE: Carryn S et al. Vaccine. 2019 Jul 22. doi: 10.1016/j.vaccine.2019.07.049.
FROM VACCINE
CMS proposes improved E/M payments, additional price transparency for hospitals
The Centers for Medicare & Medicaid Services is proposing improvements to physician payments and an overhaul of the Merit-based Incentive Payment System (MIPS) track of the Quality Payment Program.
In a separate proposal also released on July 29, the agency proposed that hospitals be required to make more pricing information publicly available.
The Medicare Outpatient Prospective Payment System proposed rule for the 2020 annual update would require hospitals to not only publish their gross charges, but also the negotiated price by specific payer for select services that can be scheduled by a patient in advance.
The proposal states "that hospitals make public their standard changes (both gross charges and payer-specific negotiated charges) for all items and services online in a machine-readable format" which would allow them to be included in price transparency tools and electronic health records.
"Hospitals would be required to post all their payer-specific negotiated rates, which are the prices actually paid by insurers," CMS Administrator Seema Verma said during a July 29 conference call with reporters.
As "deductibles rise and with 29 million uninsured, patients have the right to know the price of health care services so they can shop around for the best deal," she said.
The rule also comes with new enforcement tools so that CMS can ensure hospitals are complying with the rule, should it be finalized.
Hospitals would need to start publishing list prices and payer-specific negotiated prices beginning Jan. 1, 2020.
In a separate proposal to update the physician fee schedule for 2020, CMS is looking to increase Medicare payments in 2021 for evaluation and management (E/M) visits based on recommendations from the American Medical Association's Relative Value Scale Update Committee (AMA-RUC).
With this update, the agency will be "rewarding the time that doctors spend with patients," Administrator Verma said.
The fact sheet on the proposed update to the physician fee schedule also highlights improvements to case management payments, allowing physicians to get paid for case management services if the patient only has one high-risk condition.
"For 2021, we are overhauling the Merit-based Incentive Payment System, or MIPS, to reduce reporting burden, making sure the measures are relevant to clinicians as they move toward value-based care," she said, noting that clinicians would be reporting on fewer, more meaningful measures that are aligned to their specialty or practice area, "making it easier to participate in MIPS."
Look for in depth analysis of both proposals shortly on this website.
[email protected]
The Centers for Medicare & Medicaid Services is proposing improvements to physician payments and an overhaul of the Merit-based Incentive Payment System (MIPS) track of the Quality Payment Program.
In a separate proposal also released on July 29, the agency proposed that hospitals be required to make more pricing information publicly available.
The Medicare Outpatient Prospective Payment System proposed rule for the 2020 annual update would require hospitals to not only publish their gross charges, but also the negotiated price by specific payer for select services that can be scheduled by a patient in advance.
The proposal states "that hospitals make public their standard changes (both gross charges and payer-specific negotiated charges) for all items and services online in a machine-readable format" which would allow them to be included in price transparency tools and electronic health records.
"Hospitals would be required to post all their payer-specific negotiated rates, which are the prices actually paid by insurers," CMS Administrator Seema Verma said during a July 29 conference call with reporters.
As "deductibles rise and with 29 million uninsured, patients have the right to know the price of health care services so they can shop around for the best deal," she said.
The rule also comes with new enforcement tools so that CMS can ensure hospitals are complying with the rule, should it be finalized.
Hospitals would need to start publishing list prices and payer-specific negotiated prices beginning Jan. 1, 2020.
In a separate proposal to update the physician fee schedule for 2020, CMS is looking to increase Medicare payments in 2021 for evaluation and management (E/M) visits based on recommendations from the American Medical Association's Relative Value Scale Update Committee (AMA-RUC).
With this update, the agency will be "rewarding the time that doctors spend with patients," Administrator Verma said.
The fact sheet on the proposed update to the physician fee schedule also highlights improvements to case management payments, allowing physicians to get paid for case management services if the patient only has one high-risk condition.
"For 2021, we are overhauling the Merit-based Incentive Payment System, or MIPS, to reduce reporting burden, making sure the measures are relevant to clinicians as they move toward value-based care," she said, noting that clinicians would be reporting on fewer, more meaningful measures that are aligned to their specialty or practice area, "making it easier to participate in MIPS."
Look for in depth analysis of both proposals shortly on this website.
[email protected]
The Centers for Medicare & Medicaid Services is proposing improvements to physician payments and an overhaul of the Merit-based Incentive Payment System (MIPS) track of the Quality Payment Program.
In a separate proposal also released on July 29, the agency proposed that hospitals be required to make more pricing information publicly available.
The Medicare Outpatient Prospective Payment System proposed rule for the 2020 annual update would require hospitals to not only publish their gross charges, but also the negotiated price by specific payer for select services that can be scheduled by a patient in advance.
The proposal states "that hospitals make public their standard changes (both gross charges and payer-specific negotiated charges) for all items and services online in a machine-readable format" which would allow them to be included in price transparency tools and electronic health records.
"Hospitals would be required to post all their payer-specific negotiated rates, which are the prices actually paid by insurers," CMS Administrator Seema Verma said during a July 29 conference call with reporters.
As "deductibles rise and with 29 million uninsured, patients have the right to know the price of health care services so they can shop around for the best deal," she said.
The rule also comes with new enforcement tools so that CMS can ensure hospitals are complying with the rule, should it be finalized.
Hospitals would need to start publishing list prices and payer-specific negotiated prices beginning Jan. 1, 2020.
In a separate proposal to update the physician fee schedule for 2020, CMS is looking to increase Medicare payments in 2021 for evaluation and management (E/M) visits based on recommendations from the American Medical Association's Relative Value Scale Update Committee (AMA-RUC).
With this update, the agency will be "rewarding the time that doctors spend with patients," Administrator Verma said.
The fact sheet on the proposed update to the physician fee schedule also highlights improvements to case management payments, allowing physicians to get paid for case management services if the patient only has one high-risk condition.
"For 2021, we are overhauling the Merit-based Incentive Payment System, or MIPS, to reduce reporting burden, making sure the measures are relevant to clinicians as they move toward value-based care," she said, noting that clinicians would be reporting on fewer, more meaningful measures that are aligned to their specialty or practice area, "making it easier to participate in MIPS."
Look for in depth analysis of both proposals shortly on this website.
[email protected]
CMS proposes improved E/M payments, additional price transparency for hospitals
The Centers for Medicare & Medicaid Services is proposing improvements to physician payments and an overhaul of the Merit-based Incentive Payment System (MIPS) track of the Quality Payment Program.
In a separate proposal also released on July 29, the agency proposed that hospitals be required to make more pricing information publicly available.
The Medicare Outpatient Prospective Payment System proposed rule for the 2020 annual update would require hospitals to not only publish their gross charges, but also the negotiated price by specific payer for select services that can be scheduled by a patient in advance.
The proposal states “that hospitals make public their standard changes (both gross charges and payer-specific negotiated charges) for all items and services online in a machine-readable format” which would allow them to be included in price transparency tools and electronic health records.
“Hospitals would be required to post all their payer-specific negotiated rates, which are the prices actually paid by insurers,” CMS Administrator Seema Verma said during a July 29 conference call with reporters.
As “deductibles rise and with 29 million uninsured, patients have the right to know the price of health care services so they can shop around for the best deal,” she said.
The rule also comes with new enforcement tools so that CMS can ensure hospitals are complying with the rule, should it be finalized.
Hospitals would need to start publishing list prices and payer-specific negotiated prices beginning Jan. 1, 2020.
In a separate proposal to update the physician fee schedule for 2020, CMS is looking to increase Medicare payments in 2021 for evaluation and management (E/M) visits based on recommendations from the American Medical Association’s Relative Value Scale Update Committee (AMA-RUC). In fact, CMS is walking back the recently proposed plans to collapse E/M levels. The CPT code changes recommended by the AMA allow clinicians to choose the E/M visit level based on either medical decision making or time.
With this update, the agency will be “rewarding the time that doctors spend with patients,” Administrator Verma said.
In a joint statement, the American Gastroenterological Association, the American College of Gastroenterology and the American Society for Gastrointestinal Endoscopy welcomed the news as the GI societies opposed CMS’ proposal when it was announced last year. “We worked with the AMA and a coalition of specialty societies in an effort to get CMS to rethink collapsing payment for E/M code levels, and subsequently decided to support the AMA’s proposed E/M changes as they moved through the CPT and RUC processes. In the rule, CMS abandons its proposal and adopts the AMA CPT changes and RUC valuation.”
The fact sheet on the proposed update to the physician fee schedule also highlights improvements to case management payments, allowing physicians to get paid for case management services if the patient only has one high-risk condition.
“For 2021, we are overhauling the Merit-based Incentive Payment System, or MIPS, to reduce reporting burden, making sure the measures are relevant to clinicians as they move toward value-based care,” she said, noting that clinicians would be reporting on fewer, more meaningful measures that are aligned to their specialty or practice area, “making it easier to participate in MIPS.” CMS proposed removal of 2 colonoscopy measures from MIPS performance year 2022 because they do not align with MIPS scoring methodology.
The American College of Gastroenterology, the American Gastroenterological Association, and the American Society for Gastrointestinal Endoscopy are currently reviewing the details of the proposed rules and will be providing joint comments. CMS will accept comments until Sept. 27, 2019.
The Centers for Medicare & Medicaid Services is proposing improvements to physician payments and an overhaul of the Merit-based Incentive Payment System (MIPS) track of the Quality Payment Program.
In a separate proposal also released on July 29, the agency proposed that hospitals be required to make more pricing information publicly available.
The Medicare Outpatient Prospective Payment System proposed rule for the 2020 annual update would require hospitals to not only publish their gross charges, but also the negotiated price by specific payer for select services that can be scheduled by a patient in advance.
The proposal states “that hospitals make public their standard changes (both gross charges and payer-specific negotiated charges) for all items and services online in a machine-readable format” which would allow them to be included in price transparency tools and electronic health records.
“Hospitals would be required to post all their payer-specific negotiated rates, which are the prices actually paid by insurers,” CMS Administrator Seema Verma said during a July 29 conference call with reporters.
As “deductibles rise and with 29 million uninsured, patients have the right to know the price of health care services so they can shop around for the best deal,” she said.
The rule also comes with new enforcement tools so that CMS can ensure hospitals are complying with the rule, should it be finalized.
Hospitals would need to start publishing list prices and payer-specific negotiated prices beginning Jan. 1, 2020.
In a separate proposal to update the physician fee schedule for 2020, CMS is looking to increase Medicare payments in 2021 for evaluation and management (E/M) visits based on recommendations from the American Medical Association’s Relative Value Scale Update Committee (AMA-RUC). In fact, CMS is walking back the recently proposed plans to collapse E/M levels. The CPT code changes recommended by the AMA allow clinicians to choose the E/M visit level based on either medical decision making or time.
With this update, the agency will be “rewarding the time that doctors spend with patients,” Administrator Verma said.
In a joint statement, the American Gastroenterological Association, the American College of Gastroenterology and the American Society for Gastrointestinal Endoscopy welcomed the news as the GI societies opposed CMS’ proposal when it was announced last year. “We worked with the AMA and a coalition of specialty societies in an effort to get CMS to rethink collapsing payment for E/M code levels, and subsequently decided to support the AMA’s proposed E/M changes as they moved through the CPT and RUC processes. In the rule, CMS abandons its proposal and adopts the AMA CPT changes and RUC valuation.”
The fact sheet on the proposed update to the physician fee schedule also highlights improvements to case management payments, allowing physicians to get paid for case management services if the patient only has one high-risk condition.
“For 2021, we are overhauling the Merit-based Incentive Payment System, or MIPS, to reduce reporting burden, making sure the measures are relevant to clinicians as they move toward value-based care,” she said, noting that clinicians would be reporting on fewer, more meaningful measures that are aligned to their specialty or practice area, “making it easier to participate in MIPS.” CMS proposed removal of 2 colonoscopy measures from MIPS performance year 2022 because they do not align with MIPS scoring methodology.
The American College of Gastroenterology, the American Gastroenterological Association, and the American Society for Gastrointestinal Endoscopy are currently reviewing the details of the proposed rules and will be providing joint comments. CMS will accept comments until Sept. 27, 2019.
The Centers for Medicare & Medicaid Services is proposing improvements to physician payments and an overhaul of the Merit-based Incentive Payment System (MIPS) track of the Quality Payment Program.
In a separate proposal also released on July 29, the agency proposed that hospitals be required to make more pricing information publicly available.
The Medicare Outpatient Prospective Payment System proposed rule for the 2020 annual update would require hospitals to not only publish their gross charges, but also the negotiated price by specific payer for select services that can be scheduled by a patient in advance.
The proposal states “that hospitals make public their standard changes (both gross charges and payer-specific negotiated charges) for all items and services online in a machine-readable format” which would allow them to be included in price transparency tools and electronic health records.
“Hospitals would be required to post all their payer-specific negotiated rates, which are the prices actually paid by insurers,” CMS Administrator Seema Verma said during a July 29 conference call with reporters.
As “deductibles rise and with 29 million uninsured, patients have the right to know the price of health care services so they can shop around for the best deal,” she said.
The rule also comes with new enforcement tools so that CMS can ensure hospitals are complying with the rule, should it be finalized.
Hospitals would need to start publishing list prices and payer-specific negotiated prices beginning Jan. 1, 2020.
In a separate proposal to update the physician fee schedule for 2020, CMS is looking to increase Medicare payments in 2021 for evaluation and management (E/M) visits based on recommendations from the American Medical Association’s Relative Value Scale Update Committee (AMA-RUC). In fact, CMS is walking back the recently proposed plans to collapse E/M levels. The CPT code changes recommended by the AMA allow clinicians to choose the E/M visit level based on either medical decision making or time.
With this update, the agency will be “rewarding the time that doctors spend with patients,” Administrator Verma said.
In a joint statement, the American Gastroenterological Association, the American College of Gastroenterology and the American Society for Gastrointestinal Endoscopy welcomed the news as the GI societies opposed CMS’ proposal when it was announced last year. “We worked with the AMA and a coalition of specialty societies in an effort to get CMS to rethink collapsing payment for E/M code levels, and subsequently decided to support the AMA’s proposed E/M changes as they moved through the CPT and RUC processes. In the rule, CMS abandons its proposal and adopts the AMA CPT changes and RUC valuation.”
The fact sheet on the proposed update to the physician fee schedule also highlights improvements to case management payments, allowing physicians to get paid for case management services if the patient only has one high-risk condition.
“For 2021, we are overhauling the Merit-based Incentive Payment System, or MIPS, to reduce reporting burden, making sure the measures are relevant to clinicians as they move toward value-based care,” she said, noting that clinicians would be reporting on fewer, more meaningful measures that are aligned to their specialty or practice area, “making it easier to participate in MIPS.” CMS proposed removal of 2 colonoscopy measures from MIPS performance year 2022 because they do not align with MIPS scoring methodology.
The American College of Gastroenterology, the American Gastroenterological Association, and the American Society for Gastrointestinal Endoscopy are currently reviewing the details of the proposed rules and will be providing joint comments. CMS will accept comments until Sept. 27, 2019.
FDA approvals permit double-immunoassay approach to Lyme disease diagnosis
Concurrent or sequential enzyme immunoassays can now be conducted to diagnose Lyme disease, according to the U.S. Food and Drug Administration.
Four previously cleared tests are now approved by the agency for marketing with new indications as part of the revised diagnostic approach. Previously, the two-step diagnostic process consisted of an initial enzyme immunoassay followed by a Western blot test.
“With today’s action, clinicians have a new option to test for Lyme that is easier to interpret by a clinical laboratory due to the streamlined method of conducting the test. These tests may improve confidence in diagnosing a patient for a condition that requires the earliest possible treatment to ensure the best outcome for patients,” Tim Stenzel, MD, PhD, director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiologic Health, said in a press release announcing the newly approved approach.
The modified two-tier enzyme immunoassay approach was found to be as accurate for assessing exposure to Borrelia burgdorferi as the standard immunoassay followed by Western blot test in an FDA review of data from clinical studies using the following ZEUS Scientific ELISA Test Systems: Borrelia VlsE1/pepC10 IgG/IgM; Borrelia burgdorferi IgG/IgM; Borrelia burgdorferi IgM; and Borrelia burgdorferi IgG.
The recommendations of the Centers for Disease Control and Prevention should be followed for the diagnosis of Lyme disease and for determining when laboratory tests are appropriate, the FDA statement said. In 2017, the last year for which the CDC published data, a total of 42,743 confirmed and probable cases of Lyme disease were reported, an increase of 17% from 2016.
The FDA granted clearance of the ZEUS ELISA enzyme immunoassay tests to ZEUS Scientific.
Concurrent or sequential enzyme immunoassays can now be conducted to diagnose Lyme disease, according to the U.S. Food and Drug Administration.
Four previously cleared tests are now approved by the agency for marketing with new indications as part of the revised diagnostic approach. Previously, the two-step diagnostic process consisted of an initial enzyme immunoassay followed by a Western blot test.
“With today’s action, clinicians have a new option to test for Lyme that is easier to interpret by a clinical laboratory due to the streamlined method of conducting the test. These tests may improve confidence in diagnosing a patient for a condition that requires the earliest possible treatment to ensure the best outcome for patients,” Tim Stenzel, MD, PhD, director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiologic Health, said in a press release announcing the newly approved approach.
The modified two-tier enzyme immunoassay approach was found to be as accurate for assessing exposure to Borrelia burgdorferi as the standard immunoassay followed by Western blot test in an FDA review of data from clinical studies using the following ZEUS Scientific ELISA Test Systems: Borrelia VlsE1/pepC10 IgG/IgM; Borrelia burgdorferi IgG/IgM; Borrelia burgdorferi IgM; and Borrelia burgdorferi IgG.
The recommendations of the Centers for Disease Control and Prevention should be followed for the diagnosis of Lyme disease and for determining when laboratory tests are appropriate, the FDA statement said. In 2017, the last year for which the CDC published data, a total of 42,743 confirmed and probable cases of Lyme disease were reported, an increase of 17% from 2016.
The FDA granted clearance of the ZEUS ELISA enzyme immunoassay tests to ZEUS Scientific.
Concurrent or sequential enzyme immunoassays can now be conducted to diagnose Lyme disease, according to the U.S. Food and Drug Administration.
Four previously cleared tests are now approved by the agency for marketing with new indications as part of the revised diagnostic approach. Previously, the two-step diagnostic process consisted of an initial enzyme immunoassay followed by a Western blot test.
“With today’s action, clinicians have a new option to test for Lyme that is easier to interpret by a clinical laboratory due to the streamlined method of conducting the test. These tests may improve confidence in diagnosing a patient for a condition that requires the earliest possible treatment to ensure the best outcome for patients,” Tim Stenzel, MD, PhD, director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiologic Health, said in a press release announcing the newly approved approach.
The modified two-tier enzyme immunoassay approach was found to be as accurate for assessing exposure to Borrelia burgdorferi as the standard immunoassay followed by Western blot test in an FDA review of data from clinical studies using the following ZEUS Scientific ELISA Test Systems: Borrelia VlsE1/pepC10 IgG/IgM; Borrelia burgdorferi IgG/IgM; Borrelia burgdorferi IgM; and Borrelia burgdorferi IgG.
The recommendations of the Centers for Disease Control and Prevention should be followed for the diagnosis of Lyme disease and for determining when laboratory tests are appropriate, the FDA statement said. In 2017, the last year for which the CDC published data, a total of 42,743 confirmed and probable cases of Lyme disease were reported, an increase of 17% from 2016.
The FDA granted clearance of the ZEUS ELISA enzyme immunoassay tests to ZEUS Scientific.