User login
Content Analysis of Psoriasis and Eczema Direct-to-Consumer Advertisements
Direct-to-consumer (DTC) advertisements are an important and influential source of health-related information for Americans. In 1997, the US Food and Drug Administration (FDA) relaxed regulations and permitted DTC drug advertisements to be televised. Now, via television alone, the average American is exposed to more than 30 hours annually of DTC advertisements for drugs,1 which exceeds, by far, the amount of time the average American spends with his/her physician.2 The United States spends $9.6 billion on DTC advertisements per year, of which $605 million is spent exclusively on DTC advertisements for dermatologic conditions—one of the highest amounts of spending for DTC advertisements, second only to diabetes.3
The increase in advertising for dermatologic conditions is reflective of the rapid growth in the number of treatment options available for chronic skin diseases, especially psoriasis. Since 2004, 11 biologics and 1 oral medication were FDA approved for the treatment of moderate to severe psoriasis. Despite the expansion of treatment options for psoriasis, knowledge and understanding of psoriasis and its treatments generally are poor,4,5 and undertreatment of psoriasis continues to be common.6 Data also suggest existing age and racial disparities in psoriasis treatment in the United States, whereby patients who are older or Black are less likely to receive biologic therapies.7-9 Although the exact causes of these disparities remain unclear, one study found that Black patients with psoriasis were less familiar with biologics compared to White patients,10 which suggests that the racial disparity in biologic treatment of psoriasis could be due to less exposure to and thus recognition of biologics as treatments of psoriasis among Black patients.
Some data suggest that DTC advertisements may affect drug uptake by encouraging patients to request advertised medications from their medical providers.11,12 As such, DTC advertisements are a potentially important source of exposure and information for patients. However, is it possible that DTC advertisements also may contribute to widening knowledge gaps among certain populations, and thus treatment disparities, by neglecting certain groups and targeting others with their content? In an effort to answer this question, we performed an analysis of DTC advertisements for psoriasis and eczema with special attention to advertisement placement, character representation, and disease-related content. We specifically targeted advertisements for psoriasis and eczema, as advertisements for the former are rampant and advertisements for the latter are on the rise because of emerging therapies. We hypothesized that age and racial/ethnic diversity among advertisement characters is poor, and disease-related content is lacking.
Materials and Methods
Study Design and Sample
We performed a cross-sectional analysis of televised DTC advertisements for psoriasis and eczema over 14 consecutive days (July 1, 2018, to July 14, 2018). We accessed Nielsen’s top 10 lists, specifically Prime Broadcast Network TV-United States and Prime Broadcast Programs Among African-American, from June 2018 and identified the networks with the greatest potential exposure to American consumers: ABC, CBS, FOX, and NBC.13,14 Each day, programming aired from 5
The FDA identifies DTC advertisement types as product-claim, reminder, and help-seeking advertisements. Product-claim advertisements are required to include the following information for the drug of interest: name; at least 1 FDA-approved indication; the most notable risks; and reference to a toll-free telephone number, website, or print advertisement by which a detailed summary of risks and benefits can be accessed. Reminder advertisements include the name of the drug but no information about the drug’s use.15 Help-seeking advertisements describe a disease or condition without referencing a specific drug treatment. Product-claim, reminder, and help-seeking advertisements for psoriasis or eczema that aired during the recorded time frame were included for analysis; advertisements that aired during sporting events and special programming were excluded.
DTC Advertisement Coding
Advertisement placement (ie, network, day of the week, time, associated television program), type, and target disease were documented for all advertisements included in the study. The content of each unique advertisement for psoriasis and eczema also was documented electronically in REDCap (Research Electronic Data Capture) as follows: characteristics of affected individuals and disease-related content. Advertisement coding was performed independently by 2 graduate students (A.H. and C.W.). First, one-third of the advertisements were randomly selected to be coded by both students. Intercoder agreement between the 2 students was 95.3%. Coding disagreements were primarily due to misunderstanding of definitions and were resolved through consensus. Subsequently, the remaining advertisements were randomly distributed between the 2 students, and each advertisement was coded by 1 student.
Statistical Analysis
All data were summarized descriptively with counts and frequencies using Stata 15 (StataCorp).
Results
We identified 297 DTC advertisements addressing 25 different conditions during our study period. CBS, ABC, NBC, and FOX aired 44.4%, 26.3%, 24.4%, and 5.1% of advertisements, respectively. Overall, DTC advertisements were least likely to air on Saturdays and between the hours of 5
Psoriasis DTC Advertisements
There were 5 unique psoriasis DTC advertisements, all of which were product-claim advertisements, with 1 each for secukinumab (Cosentyx [Novartis]), ixekizumab (Taltz [Eli Lilly and Company]), and guselkumab (Tremfya [Janssen Biotech, Inc]), and 2 for adalimumab (Humira [AbbVie Inc]). The advertisements aired on ABC (n=5 [38.5%]), CBS (n=5 [38.5%]), and NBC (n=3 [23.1%]). Most advertisements aired on weekdays (61.5%) between 6
Psoriasis Character Portrayal and Disease-Related Content
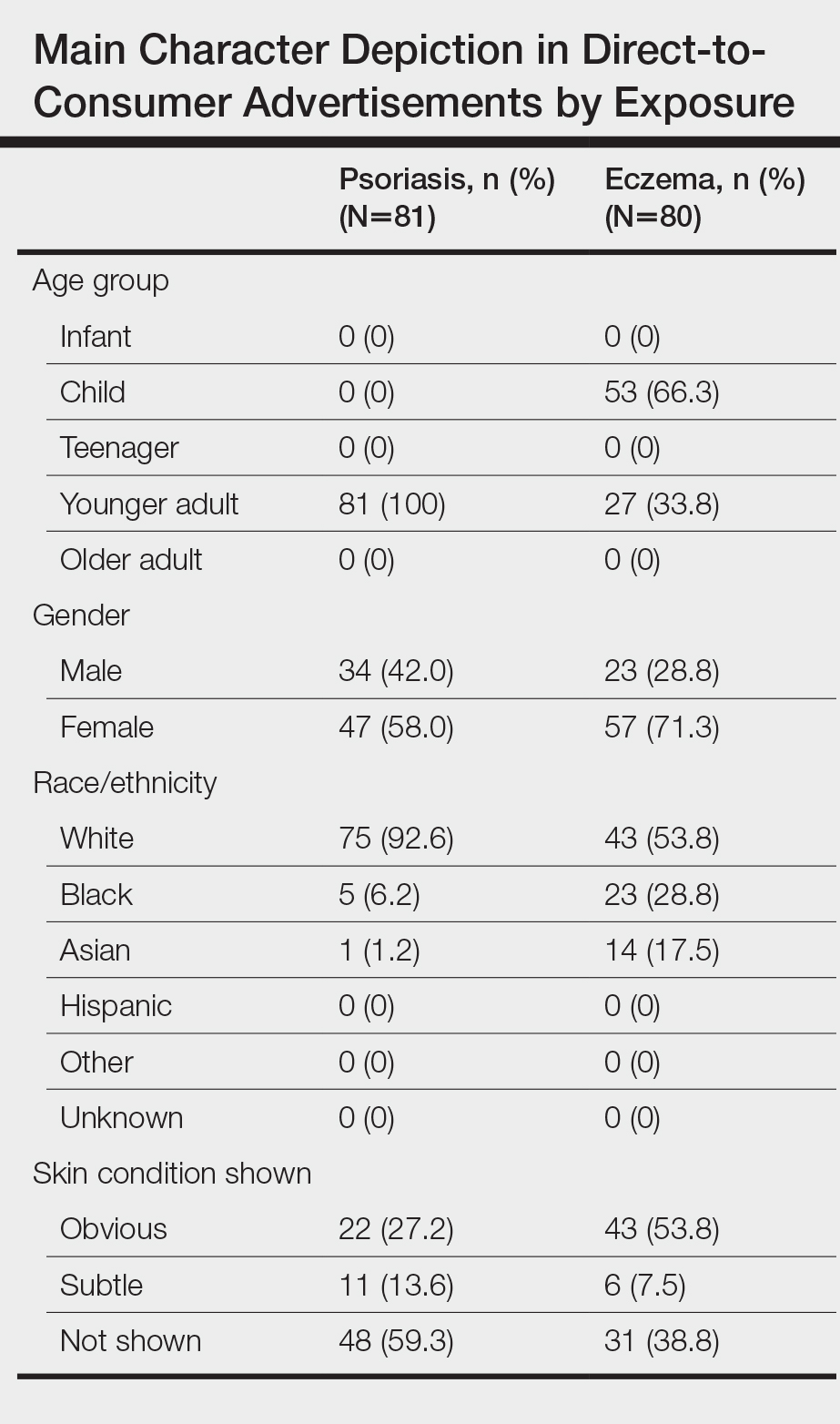
We identified 81 main characters who were depicted as having psoriasis among all advertisements. Characteristics of the affected characters are summarized in the Table. All affected characters were perceived to be younger adults, and there was a slight female predominance (58.0% [47/81]). Most characters were perceived to be White (92.6% [75/81]). Black and Asian characters only represented 6.2% (5/81) and 1.2% (1/81) of all affected individuals, respectively. Notably, the advertisements that featured only White main characters were aired 2.75 times more frequently than the advertisements that included non-White characters.
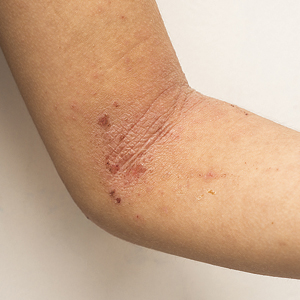
Psoriasis was shown on the skin of at least 1 character in an obvious depiction (ie, did not require more than 1 viewing) in 84.6% (11/13) of the advertisements. Symptoms of psoriasis (communicated either verbally or visually) were included in only 15.4% (2/13) of advertisements. No advertisements included information on the epidemiology of (ie, prevalence, subpopulations at risk), risk factors for, pathophysiology of, or comorbid diseases associated with psoriasis.
Eczema DTC Advertisements
Among the 27 eczema advertisements aired, there were 4 unique advertisements, of which 3 were product-claim advertisements (all for crisaborole [Eucrisa (Pfizer Inc)]), and 1 was a help-seeking advertisement that was sponsored by Sanofi Genzyme and Regeneron Pharmaceuticals. The advertisements aired on ABC (n=2 [7.4%]), CBS (n=17 [63.0%]), and NBC (n=8 [29.6%]). All advertisements aired on weekdays between 7
Eczema Character Portrayal and Disease-Related Content
We identified 80 main characters who were depicted to be affected by eczema among all advertisements. Characteristics of the affected characters are summarized in the Table. Most of the affected characters were perceived to be White (53.8% [43/80]) and female (71.3% [57/80]). Other races depicted included Black (28.8% [23/80]) and Asian (17.5% [14/80]). Each unique eczema advertisement included at least 1 non-White main character. Most eczema main characters were perceived to be children (66.3% [53/80]), followed by younger adults (33.8% [27/80]). No infants, teenagers, or older adults were shown as being affected by eczema.
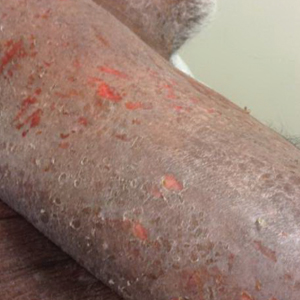
Skin manifestations of eczema were portrayed on at least 1 character in all of the advertisements; 77.8% (21/27) of the advertisements had at least 1 obvious depiction. Symptoms of eczema and the mechanism of disease (pathophysiology) were each included in 44.4% (12/27) of advertisements. This information was included exclusively in the single help-seeking advertisement, which also referenced a website for additional disease-related information. No advertisements included information on the epidemiology of, risk factors for, or comorbid diseases associated with eczema.
Comment
In our study of televised DTC advertisements for psoriasis and eczema in the United States, we identified underrepresentation of racial/ethnic minorities and specific age groups (older adults for psoriasis and all adults for eczema) across all advertisements. Although psoriasis is suggested to be less prevalent among minority patients (1.3%–1.9% among Black patients and 1.6% among Hispanic patients) compared to White patients (2%–4%),16,17 minority vs White representation in psoriasis DTC advertisements was disproportionately lower than population-based prevalence estimates. Direct-to-consumer advertisements for eczema included more minority characters than psoriasis advertisements; however, minority representation remained inadequate considering that childhood eczema is more prevalent among Black vs White children,18 and adult eczema is at least as prevalent among minority patients compared to White patients.19 Not only was minority representation in all advertisements poor, but advertisement placement also was suboptimal, particularly for reaching Black viewers. FOX network was home to 2 of the top 3 primetime broadcast programs among Black viewers around the study period,13 yet no DTC advertisements were aired on FOX.
The current literature regarding minority representation in DTC advertisements is mixed. Some studies report underrepresentation of Black and other minority patients across a variety of diseases.20 Other studies suggest that representation of Black patients, in particular, generally is adequate, except among select serious health conditions, and that advertisements depict tokenism or stereotypical roles for minorities.21 Our study provides new and specific insight about the state of racial/ethnic and age diversity, or lack thereof, in DTC advertisements for the skin conditions that currently are most commonly targeted—psoriasis and eczema. Although it remains unclear whether DTC advertisements are good or bad, existing data suggest that potential benefits of DTC advertisements include strengthening of patient-provider relationships, reduction of underdiagnosis and undertreatment of disease, and reduction of disease stigma.22 However, in our analyses, we found disease-specific factual content among all DTC advertisements to be sparse and obvious depictions of skin disease and symptoms to be uncommon, especially for psoriasis. As such, it seems unlikely that existing DTC advertisements for psoriasis and eczema can be expected to contribute to meaningful disease education, reduce underdiagnosis, and reduce the stigmatizing attitudes that have been documented for both skin diseases.23-25
Furthermore, it is important to consider our findings in light of the role that social identity theory plays in marketing. Social identity theory supports the idea that a person’s social identity (eg, age, gender, race/ethnicity) influences his/her behavior, perceptions, and performance.26 The principle of homophily—the tendency for individuals to have positive ties to those who are similar to themselves—is a critical concept in social identity theory and suggests that consumers are more likely to pay attention to and be influenced by sources perceived as similar to themselves.20 Thus, even if the potential benefits of DTC advertisements were to be realized for psoriasis and eczema, the lack of adequate minority and older adult representation raises concerns about whether these benefits would reach a diverse population and if the advertisements might further potentiate existing knowledge and treatment disparities.
Limitations
Our study is not without limitations. The sampling period was short and might not reflect advertisement content over a longer time course. We did not evaluate other potential sources of information, such as the Internet and social media. Nevertheless, televised DTC advertisements remain a major source of medical and drug information for the general public. We did not directly evaluate viewers’ reactions to the DTC advertisements of interest; however, other literature lends support to the significance of social identity theory and its impact on consumer behavior.26
Conclusion
Our study highlights a lost opportunity among psoriasis and eczema DTC advertisements for patient reach and disease education that may encourage existing and emerging knowledge and treatment disparities for both conditions. Our findings should serve as a call to action to pharmaceutical companies and other organizations involved in creating and supporting DTC advertisements for psoriasis and eczema to increase the educational content, diversify the depicted characters, and optimize advertisement placement.
- Brownfield ED, Bernhardt JM, Phan JL, et al. Direct-to-consumer drug advertisements on network television: an exploration of quantity, frequency, and placement. J Health Commun. 2004;9:491-497.
- Tai-Seale M, McGuire TG, Zhang W. Time allocation in primary care office visits. Health Serv Res. 2007;42:1871-1894.
- Schwartz LM, Woloshin S. Medical marketing in the United States, 1997-2016. JAMA. 2019;321:80-96.
- Lanigan SW, Farber EM. Patients’ knowledge of psoriasis: pilot study. Cutis. 1990;46:359-362.
- Renzi C, Di Pietro C, Tabolli S. Participation, satisfaction and knowledge level of patients with cutaneous psoriasis or psoriatic arthritis. Clin Exp Dermatol. 2011;36:885-888.
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871-881.e871-830.
- Wu JJ, Lu M, Veverka KA, et al. The journey for US psoriasis patients prescribed a topical: a retrospective database evaluation of patient progression to oral and/or biologic treatment. J Dermatolog Treat. 2019;30:446-453.
- Takeshita J, Gelfand JM, Li P, et al. Psoriasis in the US Medicare population: prevalence, treatment, and factors associated with biologic use. J Invest Dermatol. 2015;135:2955-2963.
- Kerr GS, Qaiyumi S, Richards J, et al. Psoriasis and psoriatic arthritis in African-American patients—the need to measure disease burden. Clin Rheumatol. 2015;34:1753-1759.
- Takeshita J, Eriksen WT, Raziano VT, et al. Racial differences in perceptions of psoriasis therapies: implications for racial disparities in psoriasis treatment. J Invest Dermatol. 2019;139:1672-1679.e1.
- Wu MH, Bartz D, Avorn J, et al. Trends in direct-to-consumer advertising of prescription contraceptives. Contraception. 2016;93:398-405.
- Mintzes B, Barer ML, Kravitz RL, et al. How does direct-to-consumer advertising (DTCA) affect prescribing? a survey in primary care environments with and without legal DTCA. CMAJ. 2003;169:405-412.
- Topten. Nielson website. https://www.nielsen.com/us/en/top-ten/. Accessed July 22, 2020.
- Leading ad supported broadcast and cable networks in the United States in 2019, by average number of viewers. Statistia website. https://www.statista.com/statistics/530119/tv-networks-viewers-usa/. Accessed July 22, 2020.
- Prescription drug advertisements. Electronic Code of Federal Regulations website. https://www.ecfr.gov/cgi-bin/text-idx?SID=d4f308e364578bda8e55a831638a26c6&mc=true&node=pt21.4.202&rgn=div5. Updated August 12, 2020. Accessed August 12, 2020.
- Gelfand JM, Stern RS, Nijsten T, et al. The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol. 2005;52:23-26.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Centers for Disease Control and Prevention. National Center for Health Statistics, National Health Interview Survey, 2014. https://www.cdc.gov/nchs/data/health_policy/eczema_skin_problems_tables.pdf. Accessed July 22, 2020.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Welch Cline RJ, Young HN. Marketing drugs, marketing health care relationships: a content analysis of visual cues in direct-to-consumer prescription drug advertising. Health Commun. 2004;16:131-157.
- Ball JG, Liang A, Lee WN. Representation of African Americans in direct-to-consumer pharmaceutical commercials: a content analysis with implications for health disparities. Health Mark Q. 2009;26:372-390.
- Ventola CL. Direct-to-consumer pharmaceutical advertising: therapeutic or toxic? P T. 2011;36:669-674, 681-684.
- Pearl RL, Wan MT, Takeshita J, et al. Stigmatizing attitudes toward persons with psoriasis among laypersons and medical students. J Am Acad Dermatol. 2019;80:1556-1563.
- Chernyshov PV. Stigmatization and self-perception in children with atopic dermatitis. Clin Cosmet Investig Dermatol. 2016;9:159-166.
- Wittkowski A, Richards HL, Griffiths CEM, et al. The impact of psychological and clinical factors on quality of life in individuals with atopic dermatitis. J Psychosom Res. 2004;57:195-200.
- Forehand MR, Deshpande R, Reed 2nd A. Identity salience and the influence of differential activation of the social self-schema on advertising response. J Appl Psychol. 2002;87:1086-1099.
Direct-to-consumer (DTC) advertisements are an important and influential source of health-related information for Americans. In 1997, the US Food and Drug Administration (FDA) relaxed regulations and permitted DTC drug advertisements to be televised. Now, via television alone, the average American is exposed to more than 30 hours annually of DTC advertisements for drugs,1 which exceeds, by far, the amount of time the average American spends with his/her physician.2 The United States spends $9.6 billion on DTC advertisements per year, of which $605 million is spent exclusively on DTC advertisements for dermatologic conditions—one of the highest amounts of spending for DTC advertisements, second only to diabetes.3
The increase in advertising for dermatologic conditions is reflective of the rapid growth in the number of treatment options available for chronic skin diseases, especially psoriasis. Since 2004, 11 biologics and 1 oral medication were FDA approved for the treatment of moderate to severe psoriasis. Despite the expansion of treatment options for psoriasis, knowledge and understanding of psoriasis and its treatments generally are poor,4,5 and undertreatment of psoriasis continues to be common.6 Data also suggest existing age and racial disparities in psoriasis treatment in the United States, whereby patients who are older or Black are less likely to receive biologic therapies.7-9 Although the exact causes of these disparities remain unclear, one study found that Black patients with psoriasis were less familiar with biologics compared to White patients,10 which suggests that the racial disparity in biologic treatment of psoriasis could be due to less exposure to and thus recognition of biologics as treatments of psoriasis among Black patients.
Some data suggest that DTC advertisements may affect drug uptake by encouraging patients to request advertised medications from their medical providers.11,12 As such, DTC advertisements are a potentially important source of exposure and information for patients. However, is it possible that DTC advertisements also may contribute to widening knowledge gaps among certain populations, and thus treatment disparities, by neglecting certain groups and targeting others with their content? In an effort to answer this question, we performed an analysis of DTC advertisements for psoriasis and eczema with special attention to advertisement placement, character representation, and disease-related content. We specifically targeted advertisements for psoriasis and eczema, as advertisements for the former are rampant and advertisements for the latter are on the rise because of emerging therapies. We hypothesized that age and racial/ethnic diversity among advertisement characters is poor, and disease-related content is lacking.
Materials and Methods
Study Design and Sample
We performed a cross-sectional analysis of televised DTC advertisements for psoriasis and eczema over 14 consecutive days (July 1, 2018, to July 14, 2018). We accessed Nielsen’s top 10 lists, specifically Prime Broadcast Network TV-United States and Prime Broadcast Programs Among African-American, from June 2018 and identified the networks with the greatest potential exposure to American consumers: ABC, CBS, FOX, and NBC.13,14 Each day, programming aired from 5
The FDA identifies DTC advertisement types as product-claim, reminder, and help-seeking advertisements. Product-claim advertisements are required to include the following information for the drug of interest: name; at least 1 FDA-approved indication; the most notable risks; and reference to a toll-free telephone number, website, or print advertisement by which a detailed summary of risks and benefits can be accessed. Reminder advertisements include the name of the drug but no information about the drug’s use.15 Help-seeking advertisements describe a disease or condition without referencing a specific drug treatment. Product-claim, reminder, and help-seeking advertisements for psoriasis or eczema that aired during the recorded time frame were included for analysis; advertisements that aired during sporting events and special programming were excluded.
DTC Advertisement Coding
Advertisement placement (ie, network, day of the week, time, associated television program), type, and target disease were documented for all advertisements included in the study. The content of each unique advertisement for psoriasis and eczema also was documented electronically in REDCap (Research Electronic Data Capture) as follows: characteristics of affected individuals and disease-related content. Advertisement coding was performed independently by 2 graduate students (A.H. and C.W.). First, one-third of the advertisements were randomly selected to be coded by both students. Intercoder agreement between the 2 students was 95.3%. Coding disagreements were primarily due to misunderstanding of definitions and were resolved through consensus. Subsequently, the remaining advertisements were randomly distributed between the 2 students, and each advertisement was coded by 1 student.
Statistical Analysis
All data were summarized descriptively with counts and frequencies using Stata 15 (StataCorp).
Results
We identified 297 DTC advertisements addressing 25 different conditions during our study period. CBS, ABC, NBC, and FOX aired 44.4%, 26.3%, 24.4%, and 5.1% of advertisements, respectively. Overall, DTC advertisements were least likely to air on Saturdays and between the hours of 5
Psoriasis DTC Advertisements
There were 5 unique psoriasis DTC advertisements, all of which were product-claim advertisements, with 1 each for secukinumab (Cosentyx [Novartis]), ixekizumab (Taltz [Eli Lilly and Company]), and guselkumab (Tremfya [Janssen Biotech, Inc]), and 2 for adalimumab (Humira [AbbVie Inc]). The advertisements aired on ABC (n=5 [38.5%]), CBS (n=5 [38.5%]), and NBC (n=3 [23.1%]). Most advertisements aired on weekdays (61.5%) between 6
Psoriasis Character Portrayal and Disease-Related Content
We identified 81 main characters who were depicted as having psoriasis among all advertisements. Characteristics of the affected characters are summarized in the Table. All affected characters were perceived to be younger adults, and there was a slight female predominance (58.0% [47/81]). Most characters were perceived to be White (92.6% [75/81]). Black and Asian characters only represented 6.2% (5/81) and 1.2% (1/81) of all affected individuals, respectively. Notably, the advertisements that featured only White main characters were aired 2.75 times more frequently than the advertisements that included non-White characters.

Psoriasis was shown on the skin of at least 1 character in an obvious depiction (ie, did not require more than 1 viewing) in 84.6% (11/13) of the advertisements. Symptoms of psoriasis (communicated either verbally or visually) were included in only 15.4% (2/13) of advertisements. No advertisements included information on the epidemiology of (ie, prevalence, subpopulations at risk), risk factors for, pathophysiology of, or comorbid diseases associated with psoriasis.
Eczema DTC Advertisements
Among the 27 eczema advertisements aired, there were 4 unique advertisements, of which 3 were product-claim advertisements (all for crisaborole [Eucrisa (Pfizer Inc)]), and 1 was a help-seeking advertisement that was sponsored by Sanofi Genzyme and Regeneron Pharmaceuticals. The advertisements aired on ABC (n=2 [7.4%]), CBS (n=17 [63.0%]), and NBC (n=8 [29.6%]). All advertisements aired on weekdays between 7
Eczema Character Portrayal and Disease-Related Content
We identified 80 main characters who were depicted to be affected by eczema among all advertisements. Characteristics of the affected characters are summarized in the Table. Most of the affected characters were perceived to be White (53.8% [43/80]) and female (71.3% [57/80]). Other races depicted included Black (28.8% [23/80]) and Asian (17.5% [14/80]). Each unique eczema advertisement included at least 1 non-White main character. Most eczema main characters were perceived to be children (66.3% [53/80]), followed by younger adults (33.8% [27/80]). No infants, teenagers, or older adults were shown as being affected by eczema.
Skin manifestations of eczema were portrayed on at least 1 character in all of the advertisements; 77.8% (21/27) of the advertisements had at least 1 obvious depiction. Symptoms of eczema and the mechanism of disease (pathophysiology) were each included in 44.4% (12/27) of advertisements. This information was included exclusively in the single help-seeking advertisement, which also referenced a website for additional disease-related information. No advertisements included information on the epidemiology of, risk factors for, or comorbid diseases associated with eczema.
Comment
In our study of televised DTC advertisements for psoriasis and eczema in the United States, we identified underrepresentation of racial/ethnic minorities and specific age groups (older adults for psoriasis and all adults for eczema) across all advertisements. Although psoriasis is suggested to be less prevalent among minority patients (1.3%–1.9% among Black patients and 1.6% among Hispanic patients) compared to White patients (2%–4%),16,17 minority vs White representation in psoriasis DTC advertisements was disproportionately lower than population-based prevalence estimates. Direct-to-consumer advertisements for eczema included more minority characters than psoriasis advertisements; however, minority representation remained inadequate considering that childhood eczema is more prevalent among Black vs White children,18 and adult eczema is at least as prevalent among minority patients compared to White patients.19 Not only was minority representation in all advertisements poor, but advertisement placement also was suboptimal, particularly for reaching Black viewers. FOX network was home to 2 of the top 3 primetime broadcast programs among Black viewers around the study period,13 yet no DTC advertisements were aired on FOX.
The current literature regarding minority representation in DTC advertisements is mixed. Some studies report underrepresentation of Black and other minority patients across a variety of diseases.20 Other studies suggest that representation of Black patients, in particular, generally is adequate, except among select serious health conditions, and that advertisements depict tokenism or stereotypical roles for minorities.21 Our study provides new and specific insight about the state of racial/ethnic and age diversity, or lack thereof, in DTC advertisements for the skin conditions that currently are most commonly targeted—psoriasis and eczema. Although it remains unclear whether DTC advertisements are good or bad, existing data suggest that potential benefits of DTC advertisements include strengthening of patient-provider relationships, reduction of underdiagnosis and undertreatment of disease, and reduction of disease stigma.22 However, in our analyses, we found disease-specific factual content among all DTC advertisements to be sparse and obvious depictions of skin disease and symptoms to be uncommon, especially for psoriasis. As such, it seems unlikely that existing DTC advertisements for psoriasis and eczema can be expected to contribute to meaningful disease education, reduce underdiagnosis, and reduce the stigmatizing attitudes that have been documented for both skin diseases.23-25
Furthermore, it is important to consider our findings in light of the role that social identity theory plays in marketing. Social identity theory supports the idea that a person’s social identity (eg, age, gender, race/ethnicity) influences his/her behavior, perceptions, and performance.26 The principle of homophily—the tendency for individuals to have positive ties to those who are similar to themselves—is a critical concept in social identity theory and suggests that consumers are more likely to pay attention to and be influenced by sources perceived as similar to themselves.20 Thus, even if the potential benefits of DTC advertisements were to be realized for psoriasis and eczema, the lack of adequate minority and older adult representation raises concerns about whether these benefits would reach a diverse population and if the advertisements might further potentiate existing knowledge and treatment disparities.
Limitations
Our study is not without limitations. The sampling period was short and might not reflect advertisement content over a longer time course. We did not evaluate other potential sources of information, such as the Internet and social media. Nevertheless, televised DTC advertisements remain a major source of medical and drug information for the general public. We did not directly evaluate viewers’ reactions to the DTC advertisements of interest; however, other literature lends support to the significance of social identity theory and its impact on consumer behavior.26
Conclusion
Our study highlights a lost opportunity among psoriasis and eczema DTC advertisements for patient reach and disease education that may encourage existing and emerging knowledge and treatment disparities for both conditions. Our findings should serve as a call to action to pharmaceutical companies and other organizations involved in creating and supporting DTC advertisements for psoriasis and eczema to increase the educational content, diversify the depicted characters, and optimize advertisement placement.
Direct-to-consumer (DTC) advertisements are an important and influential source of health-related information for Americans. In 1997, the US Food and Drug Administration (FDA) relaxed regulations and permitted DTC drug advertisements to be televised. Now, via television alone, the average American is exposed to more than 30 hours annually of DTC advertisements for drugs,1 which exceeds, by far, the amount of time the average American spends with his/her physician.2 The United States spends $9.6 billion on DTC advertisements per year, of which $605 million is spent exclusively on DTC advertisements for dermatologic conditions—one of the highest amounts of spending for DTC advertisements, second only to diabetes.3
The increase in advertising for dermatologic conditions is reflective of the rapid growth in the number of treatment options available for chronic skin diseases, especially psoriasis. Since 2004, 11 biologics and 1 oral medication were FDA approved for the treatment of moderate to severe psoriasis. Despite the expansion of treatment options for psoriasis, knowledge and understanding of psoriasis and its treatments generally are poor,4,5 and undertreatment of psoriasis continues to be common.6 Data also suggest existing age and racial disparities in psoriasis treatment in the United States, whereby patients who are older or Black are less likely to receive biologic therapies.7-9 Although the exact causes of these disparities remain unclear, one study found that Black patients with psoriasis were less familiar with biologics compared to White patients,10 which suggests that the racial disparity in biologic treatment of psoriasis could be due to less exposure to and thus recognition of biologics as treatments of psoriasis among Black patients.
Some data suggest that DTC advertisements may affect drug uptake by encouraging patients to request advertised medications from their medical providers.11,12 As such, DTC advertisements are a potentially important source of exposure and information for patients. However, is it possible that DTC advertisements also may contribute to widening knowledge gaps among certain populations, and thus treatment disparities, by neglecting certain groups and targeting others with their content? In an effort to answer this question, we performed an analysis of DTC advertisements for psoriasis and eczema with special attention to advertisement placement, character representation, and disease-related content. We specifically targeted advertisements for psoriasis and eczema, as advertisements for the former are rampant and advertisements for the latter are on the rise because of emerging therapies. We hypothesized that age and racial/ethnic diversity among advertisement characters is poor, and disease-related content is lacking.
Materials and Methods
Study Design and Sample
We performed a cross-sectional analysis of televised DTC advertisements for psoriasis and eczema over 14 consecutive days (July 1, 2018, to July 14, 2018). We accessed Nielsen’s top 10 lists, specifically Prime Broadcast Network TV-United States and Prime Broadcast Programs Among African-American, from June 2018 and identified the networks with the greatest potential exposure to American consumers: ABC, CBS, FOX, and NBC.13,14 Each day, programming aired from 5
The FDA identifies DTC advertisement types as product-claim, reminder, and help-seeking advertisements. Product-claim advertisements are required to include the following information for the drug of interest: name; at least 1 FDA-approved indication; the most notable risks; and reference to a toll-free telephone number, website, or print advertisement by which a detailed summary of risks and benefits can be accessed. Reminder advertisements include the name of the drug but no information about the drug’s use.15 Help-seeking advertisements describe a disease or condition without referencing a specific drug treatment. Product-claim, reminder, and help-seeking advertisements for psoriasis or eczema that aired during the recorded time frame were included for analysis; advertisements that aired during sporting events and special programming were excluded.
DTC Advertisement Coding
Advertisement placement (ie, network, day of the week, time, associated television program), type, and target disease were documented for all advertisements included in the study. The content of each unique advertisement for psoriasis and eczema also was documented electronically in REDCap (Research Electronic Data Capture) as follows: characteristics of affected individuals and disease-related content. Advertisement coding was performed independently by 2 graduate students (A.H. and C.W.). First, one-third of the advertisements were randomly selected to be coded by both students. Intercoder agreement between the 2 students was 95.3%. Coding disagreements were primarily due to misunderstanding of definitions and were resolved through consensus. Subsequently, the remaining advertisements were randomly distributed between the 2 students, and each advertisement was coded by 1 student.
Statistical Analysis
All data were summarized descriptively with counts and frequencies using Stata 15 (StataCorp).
Results
We identified 297 DTC advertisements addressing 25 different conditions during our study period. CBS, ABC, NBC, and FOX aired 44.4%, 26.3%, 24.4%, and 5.1% of advertisements, respectively. Overall, DTC advertisements were least likely to air on Saturdays and between the hours of 5
Psoriasis DTC Advertisements
There were 5 unique psoriasis DTC advertisements, all of which were product-claim advertisements, with 1 each for secukinumab (Cosentyx [Novartis]), ixekizumab (Taltz [Eli Lilly and Company]), and guselkumab (Tremfya [Janssen Biotech, Inc]), and 2 for adalimumab (Humira [AbbVie Inc]). The advertisements aired on ABC (n=5 [38.5%]), CBS (n=5 [38.5%]), and NBC (n=3 [23.1%]). Most advertisements aired on weekdays (61.5%) between 6
Psoriasis Character Portrayal and Disease-Related Content
We identified 81 main characters who were depicted as having psoriasis among all advertisements. Characteristics of the affected characters are summarized in the Table. All affected characters were perceived to be younger adults, and there was a slight female predominance (58.0% [47/81]). Most characters were perceived to be White (92.6% [75/81]). Black and Asian characters only represented 6.2% (5/81) and 1.2% (1/81) of all affected individuals, respectively. Notably, the advertisements that featured only White main characters were aired 2.75 times more frequently than the advertisements that included non-White characters.

Psoriasis was shown on the skin of at least 1 character in an obvious depiction (ie, did not require more than 1 viewing) in 84.6% (11/13) of the advertisements. Symptoms of psoriasis (communicated either verbally or visually) were included in only 15.4% (2/13) of advertisements. No advertisements included information on the epidemiology of (ie, prevalence, subpopulations at risk), risk factors for, pathophysiology of, or comorbid diseases associated with psoriasis.
Eczema DTC Advertisements
Among the 27 eczema advertisements aired, there were 4 unique advertisements, of which 3 were product-claim advertisements (all for crisaborole [Eucrisa (Pfizer Inc)]), and 1 was a help-seeking advertisement that was sponsored by Sanofi Genzyme and Regeneron Pharmaceuticals. The advertisements aired on ABC (n=2 [7.4%]), CBS (n=17 [63.0%]), and NBC (n=8 [29.6%]). All advertisements aired on weekdays between 7
Eczema Character Portrayal and Disease-Related Content
We identified 80 main characters who were depicted to be affected by eczema among all advertisements. Characteristics of the affected characters are summarized in the Table. Most of the affected characters were perceived to be White (53.8% [43/80]) and female (71.3% [57/80]). Other races depicted included Black (28.8% [23/80]) and Asian (17.5% [14/80]). Each unique eczema advertisement included at least 1 non-White main character. Most eczema main characters were perceived to be children (66.3% [53/80]), followed by younger adults (33.8% [27/80]). No infants, teenagers, or older adults were shown as being affected by eczema.
Skin manifestations of eczema were portrayed on at least 1 character in all of the advertisements; 77.8% (21/27) of the advertisements had at least 1 obvious depiction. Symptoms of eczema and the mechanism of disease (pathophysiology) were each included in 44.4% (12/27) of advertisements. This information was included exclusively in the single help-seeking advertisement, which also referenced a website for additional disease-related information. No advertisements included information on the epidemiology of, risk factors for, or comorbid diseases associated with eczema.
Comment
In our study of televised DTC advertisements for psoriasis and eczema in the United States, we identified underrepresentation of racial/ethnic minorities and specific age groups (older adults for psoriasis and all adults for eczema) across all advertisements. Although psoriasis is suggested to be less prevalent among minority patients (1.3%–1.9% among Black patients and 1.6% among Hispanic patients) compared to White patients (2%–4%),16,17 minority vs White representation in psoriasis DTC advertisements was disproportionately lower than population-based prevalence estimates. Direct-to-consumer advertisements for eczema included more minority characters than psoriasis advertisements; however, minority representation remained inadequate considering that childhood eczema is more prevalent among Black vs White children,18 and adult eczema is at least as prevalent among minority patients compared to White patients.19 Not only was minority representation in all advertisements poor, but advertisement placement also was suboptimal, particularly for reaching Black viewers. FOX network was home to 2 of the top 3 primetime broadcast programs among Black viewers around the study period,13 yet no DTC advertisements were aired on FOX.
The current literature regarding minority representation in DTC advertisements is mixed. Some studies report underrepresentation of Black and other minority patients across a variety of diseases.20 Other studies suggest that representation of Black patients, in particular, generally is adequate, except among select serious health conditions, and that advertisements depict tokenism or stereotypical roles for minorities.21 Our study provides new and specific insight about the state of racial/ethnic and age diversity, or lack thereof, in DTC advertisements for the skin conditions that currently are most commonly targeted—psoriasis and eczema. Although it remains unclear whether DTC advertisements are good or bad, existing data suggest that potential benefits of DTC advertisements include strengthening of patient-provider relationships, reduction of underdiagnosis and undertreatment of disease, and reduction of disease stigma.22 However, in our analyses, we found disease-specific factual content among all DTC advertisements to be sparse and obvious depictions of skin disease and symptoms to be uncommon, especially for psoriasis. As such, it seems unlikely that existing DTC advertisements for psoriasis and eczema can be expected to contribute to meaningful disease education, reduce underdiagnosis, and reduce the stigmatizing attitudes that have been documented for both skin diseases.23-25
Furthermore, it is important to consider our findings in light of the role that social identity theory plays in marketing. Social identity theory supports the idea that a person’s social identity (eg, age, gender, race/ethnicity) influences his/her behavior, perceptions, and performance.26 The principle of homophily—the tendency for individuals to have positive ties to those who are similar to themselves—is a critical concept in social identity theory and suggests that consumers are more likely to pay attention to and be influenced by sources perceived as similar to themselves.20 Thus, even if the potential benefits of DTC advertisements were to be realized for psoriasis and eczema, the lack of adequate minority and older adult representation raises concerns about whether these benefits would reach a diverse population and if the advertisements might further potentiate existing knowledge and treatment disparities.
Limitations
Our study is not without limitations. The sampling period was short and might not reflect advertisement content over a longer time course. We did not evaluate other potential sources of information, such as the Internet and social media. Nevertheless, televised DTC advertisements remain a major source of medical and drug information for the general public. We did not directly evaluate viewers’ reactions to the DTC advertisements of interest; however, other literature lends support to the significance of social identity theory and its impact on consumer behavior.26
Conclusion
Our study highlights a lost opportunity among psoriasis and eczema DTC advertisements for patient reach and disease education that may encourage existing and emerging knowledge and treatment disparities for both conditions. Our findings should serve as a call to action to pharmaceutical companies and other organizations involved in creating and supporting DTC advertisements for psoriasis and eczema to increase the educational content, diversify the depicted characters, and optimize advertisement placement.
- Brownfield ED, Bernhardt JM, Phan JL, et al. Direct-to-consumer drug advertisements on network television: an exploration of quantity, frequency, and placement. J Health Commun. 2004;9:491-497.
- Tai-Seale M, McGuire TG, Zhang W. Time allocation in primary care office visits. Health Serv Res. 2007;42:1871-1894.
- Schwartz LM, Woloshin S. Medical marketing in the United States, 1997-2016. JAMA. 2019;321:80-96.
- Lanigan SW, Farber EM. Patients’ knowledge of psoriasis: pilot study. Cutis. 1990;46:359-362.
- Renzi C, Di Pietro C, Tabolli S. Participation, satisfaction and knowledge level of patients with cutaneous psoriasis or psoriatic arthritis. Clin Exp Dermatol. 2011;36:885-888.
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871-881.e871-830.
- Wu JJ, Lu M, Veverka KA, et al. The journey for US psoriasis patients prescribed a topical: a retrospective database evaluation of patient progression to oral and/or biologic treatment. J Dermatolog Treat. 2019;30:446-453.
- Takeshita J, Gelfand JM, Li P, et al. Psoriasis in the US Medicare population: prevalence, treatment, and factors associated with biologic use. J Invest Dermatol. 2015;135:2955-2963.
- Kerr GS, Qaiyumi S, Richards J, et al. Psoriasis and psoriatic arthritis in African-American patients—the need to measure disease burden. Clin Rheumatol. 2015;34:1753-1759.
- Takeshita J, Eriksen WT, Raziano VT, et al. Racial differences in perceptions of psoriasis therapies: implications for racial disparities in psoriasis treatment. J Invest Dermatol. 2019;139:1672-1679.e1.
- Wu MH, Bartz D, Avorn J, et al. Trends in direct-to-consumer advertising of prescription contraceptives. Contraception. 2016;93:398-405.
- Mintzes B, Barer ML, Kravitz RL, et al. How does direct-to-consumer advertising (DTCA) affect prescribing? a survey in primary care environments with and without legal DTCA. CMAJ. 2003;169:405-412.
- Topten. Nielson website. https://www.nielsen.com/us/en/top-ten/. Accessed July 22, 2020.
- Leading ad supported broadcast and cable networks in the United States in 2019, by average number of viewers. Statistia website. https://www.statista.com/statistics/530119/tv-networks-viewers-usa/. Accessed July 22, 2020.
- Prescription drug advertisements. Electronic Code of Federal Regulations website. https://www.ecfr.gov/cgi-bin/text-idx?SID=d4f308e364578bda8e55a831638a26c6&mc=true&node=pt21.4.202&rgn=div5. Updated August 12, 2020. Accessed August 12, 2020.
- Gelfand JM, Stern RS, Nijsten T, et al. The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol. 2005;52:23-26.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Centers for Disease Control and Prevention. National Center for Health Statistics, National Health Interview Survey, 2014. https://www.cdc.gov/nchs/data/health_policy/eczema_skin_problems_tables.pdf. Accessed July 22, 2020.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Welch Cline RJ, Young HN. Marketing drugs, marketing health care relationships: a content analysis of visual cues in direct-to-consumer prescription drug advertising. Health Commun. 2004;16:131-157.
- Ball JG, Liang A, Lee WN. Representation of African Americans in direct-to-consumer pharmaceutical commercials: a content analysis with implications for health disparities. Health Mark Q. 2009;26:372-390.
- Ventola CL. Direct-to-consumer pharmaceutical advertising: therapeutic or toxic? P T. 2011;36:669-674, 681-684.
- Pearl RL, Wan MT, Takeshita J, et al. Stigmatizing attitudes toward persons with psoriasis among laypersons and medical students. J Am Acad Dermatol. 2019;80:1556-1563.
- Chernyshov PV. Stigmatization and self-perception in children with atopic dermatitis. Clin Cosmet Investig Dermatol. 2016;9:159-166.
- Wittkowski A, Richards HL, Griffiths CEM, et al. The impact of psychological and clinical factors on quality of life in individuals with atopic dermatitis. J Psychosom Res. 2004;57:195-200.
- Forehand MR, Deshpande R, Reed 2nd A. Identity salience and the influence of differential activation of the social self-schema on advertising response. J Appl Psychol. 2002;87:1086-1099.
- Brownfield ED, Bernhardt JM, Phan JL, et al. Direct-to-consumer drug advertisements on network television: an exploration of quantity, frequency, and placement. J Health Commun. 2004;9:491-497.
- Tai-Seale M, McGuire TG, Zhang W. Time allocation in primary care office visits. Health Serv Res. 2007;42:1871-1894.
- Schwartz LM, Woloshin S. Medical marketing in the United States, 1997-2016. JAMA. 2019;321:80-96.
- Lanigan SW, Farber EM. Patients’ knowledge of psoriasis: pilot study. Cutis. 1990;46:359-362.
- Renzi C, Di Pietro C, Tabolli S. Participation, satisfaction and knowledge level of patients with cutaneous psoriasis or psoriatic arthritis. Clin Exp Dermatol. 2011;36:885-888.
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871-881.e871-830.
- Wu JJ, Lu M, Veverka KA, et al. The journey for US psoriasis patients prescribed a topical: a retrospective database evaluation of patient progression to oral and/or biologic treatment. J Dermatolog Treat. 2019;30:446-453.
- Takeshita J, Gelfand JM, Li P, et al. Psoriasis in the US Medicare population: prevalence, treatment, and factors associated with biologic use. J Invest Dermatol. 2015;135:2955-2963.
- Kerr GS, Qaiyumi S, Richards J, et al. Psoriasis and psoriatic arthritis in African-American patients—the need to measure disease burden. Clin Rheumatol. 2015;34:1753-1759.
- Takeshita J, Eriksen WT, Raziano VT, et al. Racial differences in perceptions of psoriasis therapies: implications for racial disparities in psoriasis treatment. J Invest Dermatol. 2019;139:1672-1679.e1.
- Wu MH, Bartz D, Avorn J, et al. Trends in direct-to-consumer advertising of prescription contraceptives. Contraception. 2016;93:398-405.
- Mintzes B, Barer ML, Kravitz RL, et al. How does direct-to-consumer advertising (DTCA) affect prescribing? a survey in primary care environments with and without legal DTCA. CMAJ. 2003;169:405-412.
- Topten. Nielson website. https://www.nielsen.com/us/en/top-ten/. Accessed July 22, 2020.
- Leading ad supported broadcast and cable networks in the United States in 2019, by average number of viewers. Statistia website. https://www.statista.com/statistics/530119/tv-networks-viewers-usa/. Accessed July 22, 2020.
- Prescription drug advertisements. Electronic Code of Federal Regulations website. https://www.ecfr.gov/cgi-bin/text-idx?SID=d4f308e364578bda8e55a831638a26c6&mc=true&node=pt21.4.202&rgn=div5. Updated August 12, 2020. Accessed August 12, 2020.
- Gelfand JM, Stern RS, Nijsten T, et al. The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol. 2005;52:23-26.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Centers for Disease Control and Prevention. National Center for Health Statistics, National Health Interview Survey, 2014. https://www.cdc.gov/nchs/data/health_policy/eczema_skin_problems_tables.pdf. Accessed July 22, 2020.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Welch Cline RJ, Young HN. Marketing drugs, marketing health care relationships: a content analysis of visual cues in direct-to-consumer prescription drug advertising. Health Commun. 2004;16:131-157.
- Ball JG, Liang A, Lee WN. Representation of African Americans in direct-to-consumer pharmaceutical commercials: a content analysis with implications for health disparities. Health Mark Q. 2009;26:372-390.
- Ventola CL. Direct-to-consumer pharmaceutical advertising: therapeutic or toxic? P T. 2011;36:669-674, 681-684.
- Pearl RL, Wan MT, Takeshita J, et al. Stigmatizing attitudes toward persons with psoriasis among laypersons and medical students. J Am Acad Dermatol. 2019;80:1556-1563.
- Chernyshov PV. Stigmatization and self-perception in children with atopic dermatitis. Clin Cosmet Investig Dermatol. 2016;9:159-166.
- Wittkowski A, Richards HL, Griffiths CEM, et al. The impact of psychological and clinical factors on quality of life in individuals with atopic dermatitis. J Psychosom Res. 2004;57:195-200.
- Forehand MR, Deshpande R, Reed 2nd A. Identity salience and the influence of differential activation of the social self-schema on advertising response. J Appl Psychol. 2002;87:1086-1099.
Practice Points
- Racial/ethnic minorities and older adults are underrepresented in direct-to-consumer (DTC) advertisements for psoriasis and eczema.
- Character representation in psoriasis DTC advertisements, in particular, mirrors existing age and racial disparities in treatment with biologics.
- Disease-specific factual content was sparse, and obvious depictions of skin disease and symptoms were uncommon, especially among psoriasis DTC advertisements.
- Dermatologists should be aware of these deficiencies in psoriasis and eczema DTC advertisements and take care not to further reinforce existing knowledge gaps and inequitable treatment patterns among patients.
Update on Pediatric Atopic Dermatitis
Atopic dermatitis (AD) is a chronic, pruritic, inflammatory skin disease that occurs most frequently in children but also affects many adolescents and adults. There has been a tremendous evolution of knowledge in AD, with insights into pathogenesis, epidemiology, impact of disease, and new therapies. A variety of studies examine the epidemiology of AD and associated comorbidities. The broad developments in disease state research are reflected in new publication numbers of AD citations on PubMed. A PubMed search of articles indexed for MEDLINE at the end of 2010 using the term atopic dermatitis would have shown 965 citations during the preceding 1-year period. In the 1-year period of June 2019 to June 2020, there were more than 2000 articles. The large body of research includes work of great significance in pediatric AD, and in this article we review recent findings that are important in understanding the progress being made in the field.
Epidemiology and Comorbidities
The epidemiology of AD has evolved over the last few decades, with emerging trends and novel insights into the burden of disease.1 In a recent cross-sectional study on the epidemiology of AD in children aged 6 to 11 years, the 1-year diagnosed AD prevalence estimates worldwide included the following: United States, 10.0%; Canada, 13.3%; the EU5 Countries, 15.5%; Japan, 10.3%; and all countries studied, 12.2%.2 Another recent paper that analyzed data from the Fragile Families and Child Wellbeing Study showed that the prevalence and persistence of AD in urban US children was 15.0%.3Although pediatric AD may spontaneously remit over time, disease continuing into adolescence and adulthood is common. Paternoster et al4 studied the longitudinal course of AD in children from 2 birth cohort prospective studies, showing distinct AD phenotypes having differing course trajectories over time. Disease subsets included patients with early-onset-persistent and early-onset-late-resolving disease.4 Whether phenotyping or subgroup analysis can be used to predict disease course or risk for development of comorbidities is unknown, but it is interesting to consider how such work could influence tailoring of specific therapies to early disease presentation.
Atopic dermatitis poses a serious public health burden owing to its high prevalence, considerable morbidity and disability, increased health care utilization, and cost of care.1 Recent studies have found notably higher rates of multiple medical and mental health comorbidities in both children and adults with AD, including infections, atopic comorbidities (eg, allergic rhinitis, asthma, food allergies), eye diseases (eg, keratitis, conjunctivitis, keratoconus), and possible cardiovascular diseases and autoimmune disorders.1,5-9 Allergic comorbidities are quite common in pediatric AD patients.10 In a recent study examining the efficacy and safety of dupilumab monotherapy in 251 adolescents with moderate to severe inadequately controlled AD, most had comorbid type 2 diseases including asthma (53.6%), food allergies (60.8%), and allergic rhinitis (65.6%).11
Quality of Life/Life Impact of AD
Pediatric AD has a major impact on the quality of life of patients and their families.12 The well-being and development of children are strongly influenced by the physical and psychosocial health of parents/guardians. Two studies by Ramirez and colleagues13,14 published in 2019 examined sleep disturbances and exhaustion in mothers of children with AD. Data for the studies came from the Avon Longitudinal Study of Parents and Children. Children with active AD reported worse sleep quality than those without AD, with nearly 50% higher odds of sleep-quality disturbances. Analysis of the cohort data from 11,649 mother-child pairs who were followed up with a time-varying measure of child AD activity and severity as well as self-reported maternal sleep measures repeated at multiple time points for children aged 6 months to 11 years showed that mothers of children with AD reported difficulty falling asleep, subjectively insufficient sleep, and daytime exhaustion throughout the first 11 years of childhood.13,14 These data suggest that sleep disturbance may be a family affair.
A cross-sectional, real-world study on the burden of AD in children aged 6 to 11 years assessed by self-report demonstrated a substantial and multidimensional impact of AD, including itch, sleep disturbance, skin pain, and health-related quality-of-life impact, as well as comorbidities and school productivity losses. The burden associated with AD was remarkable and increased with disease severity.15
Drucker et al16 completed a comprehensive literature review on the burden of AD, summarized as a report for the National Eczema Association. Quality-of-life impact on pediatric patients included high rates of emotional distress; social isolation; depression; limitations in activities due to lesions with fear of triggers; and behavioral problems such as irritability, crying, and sleep disturbance resulting in difficulty performing at school.16 The psychological impact on children as well as emotional and behavioral difficulties may impact the ability for parents/guardians to implement treatment plans.17
There is a striking association between mental health disorders and AD in the US pediatric population, with a clear dose-dependent relationship that has been observed between the prevalence of a mental health disorder and the reported severity of the skin disease. Data suggest children with AD may be at increased risk for developing mental health disorders. The National Survey of Children’s Health found statistically significant increases in the likelihood of attention deficit hyperactivity disorder (odds ratio [OR], 1.87), depression (OR, 1.81), anxiety (OR, 1.77), conduct disorder (OR, 1.87), and autism (OR, 3.04).6
Evolving Practices and Therapies
Bathing Practices
There has long been much controversy regarding best bathing habits for patients with AD. In a 2009 study, cutaneous hydration was quantified after various bathing and moisturizing regimens.18 The study showed clear benefits of emollient application on skin hydration, either after bathing or without bathing. Bathing followed by emollient applications did not decrease skin hydration in contrast to bathing without emollient application.18
There are limited studies evaluating bathing frequency in pediatric patients, and many families receive conflicting information regarding best practice. In one study that surveyed 354 parents, more than 75% of parents/guardians who had seen multiple providers for their child’s AD reported a substantial amount of confusion and frustration from conflicting advice on bathing frequency.19 Cardona et al20 undertook a randomized clinical trial of frequent bathing and moisturizing vs less-frequent bathing and moisturizing in pediatric patients with AD aged 6 months to 11 years. Patients were divided into 2 groups: 1 being bathed twice daily with immediate moisturizer application and the other being bathed twice weekly followed by moisturization, then a switch to the other method. Patients used standardized topical corticosteroids (TCSs) in both groups. There were significant improvements in scoring AD and other objective measures during the frequent bathing time period vs infrequent bathing; in the group that bathed more frequently, SCORAD (SCORing Atopic Dermatitis) decreased by 21.2 compared with the group that bathed less frequently (95% confidence interval, 14.9-27.6; P<.0001). These findings suggest that more-frequent bathing with immediate moisturization is superior as an acute treatment intervention for improving AD disease severity in comparison to less-frequent bathing with immediate moisturization.20
Expanding Treatment Options
Topical Phosphodiesterase Inhibitors
There are several new and evolving topical therapies in AD. Crisaborole ointment 2% is a steroid-free phosphodiesterase inhibitor approved in 2016 by the US Food and Drug Administration (FDA) for mild to moderate AD in patients aged 2 years and older. A recent multicenter, open-label, single-arm study in 137 infants (CrisADe CARE 1) evaluated the pharmacokinetics and efficacy of crisaborole ointment 2% applied twice daily for 4 weeks in pediatric patients aged 3 months to less than 24 months of age with mild to moderate AD.21 The study had 2 cohorts: one with a minimum of 5% body surface area involvement and another (the pharmacokinetic cohort) with a minimum of 35% body surface area involvement. Both cohorts demonstrated similar efficacy data. From baseline to day 29, the mean percentage change in eczema area and severity index (EASI) score was −57.5%, and an investigator global assessment (IGA) score of clear or almost clear with at least a 2-grade improvement was achieved in 30.2% of patients. Crisaborole systemic exposures in infants were comparable with those in patients aged 2 years or older. Patients tolerated crisaborole well, with a 4% rate of burning, which was similar to other studies in children and adults but perhaps lower than seen in clinical practice. Pharmacokinetic studies did not show any remarkable noticeable concern with accumulation of propylene glycol absorption.21
Based on the CrisADe CARE 1 study data, in March 2020 the FDA extended the indication of crisaborole ointment 2% from a prior lower age limit of 24 months to approval for use in treating mild to moderate AD in children as young as 3 months, making it the first nonsteroidal topical anti-inflammatory medication to be approved in children younger than 2 years in the United States.
Evolving Topical Therapies
Topical Janus Kinase Inhibitors
Ruxolitinib is a potent inhibitor of Janus kinase 1 (JAK-1) and Janus kinase 2 (JAK-2) and has been developed in topical formulations. In recent phase 3 clinical trials of patients with AD aged 12 years and older with mild to moderate disease (TRuE-AD1 and TRuE-AD2), more than half of the patients treated with either ruxolitinib cream in a 0.75% or 1.5% concentration reached EASI-75 after 8 weeks of treatment.22 Additionally, more patients treated with topical ruxolitinib reached an IGA score of clear to almost clear than patients treated with vehicle at the end of treatment. Thus far, it appears to be very well tolerated, significantly decreases EASI score (P<.0001), and improves overall pruritus.22
Delgocitinib is a topical pan-JAK inhibitor that blocks several cytokine-signaling cascade pathways. It was first developed and approved in Japan in an ointment formulation for use in patients with AD aged 16 years and older.23 The efficacy and safety profile of delgocitinib is currently being evaluated in pediatric patients with AD in Japan. In a recent phase 2 clinical study of 103 Japanese patients aged 2 to 15 years with moderate to severe AD, patients were randomized to receive either delgocitinib ointment in 0.25% or 0.5% concentrations or vehicle ointment twice daily for 4 weeks. The proportion of patients with a modified EASI-75 score was 38.2% (13/34) in the 0.25% group and 50.0% (17/34) in the 0.5% group vs 8.6% (3/35) in the placebo group. More patients treated with delgocitinib ointment received an IGA score of clear or almost clear than patients treated with vehicle at the end of treatment. Overall, both delgocitinib groups demonstrated superior improvement in clinical symptoms and signs without notable side effects.24
Tapinarof
Tapinarof is a topical therapeutic aryl hydrocarbon receptor agonist. In a recent phase 2 randomized study of 2 concentrations and 2 frequencies of tapinarof cream vs vehicle in 247 randomized patients aged 12 to 65 years with moderate to severe disease, tapinarof demonstrated greater success with both concentrations than vehicle at all visits beyond week 2.25 Additionally, in patients treated with tapinarof cream 1%, nearly 50% reached an IGA score of clear to almost clear with at least a 2-grade improvement. More than 50% of patients achieved EASI-75 improvement at 12 weeks of treatment with tapinarof cream 1% used daily. These findings suggest that tapinarof may be an efficacious and well-tolerated treatment for both adolescents and adults with AD; however, large confirmation trials are needed to further investigate.25
Systemic Treatments
Oral JAK Inhibitors
Some of the most exciting novel therapies include several oral JAK inhibitors that target different combinations of kinases and have been shown to decrease AD severity and symptoms. Some of these agents have indications in other disease states, such as baricitinib and upadacitinib, which are both FDA approved for the treatment of rheumatoid arthritis, whereas others, such as abrocitinib, have been studied specifically for AD.
Although some agents have only been studied in adults to date, others have included adolescents in their core studies, such as abrocitinib, which received Breakthrough Therapy designation from the FDA for the treatment of patients with moderate to severe AD in February 2018. In recent phase 3 trials of patients aged 12 years and older with moderate to severe AD (JADE MONO-1 and JADE MONO-2), both doses of abrocitinib improved the IGA and EASI-75 outcomes compared with placebo.26 Additional studies will be conducted to further investigate the relative efficacy and safety in patients younger than 18 years.
Biologics
Dupilumab is a fully human monoclonal antibody that inhibits IL-4 and IL-13 signaling without suppressing the immune system. It is approved for use in patients aged 12 years and older with moderate to severe asthma and in adults with chronic rhinosinusitis with nasal polyposis. It is the first biologic to show positive results in the moderate to severe pediatric AD population. There are now extended data available exhibiting sustained benefit in adolescent patients who were continued on dupilumab therapy, evidenced by further improvement in EASI scores at the 1-year mark.27
Recently, dupilumab received approval for use in patients aged 6 to 11 years, making it the first biologic for AD to be approved for use in patients younger than 12 years. The expedited FDA approval was based on the phase 3 results in which the efficacy and safety of dupilumab combined with TCSs were compared to TCSs alone (N=367).28 In this trial, more than twice as many children achieved clear or almost clear skin and more than 4 times as many achieved itch reduction with dupilumab plus TCSs than with TCSs alone. Three-quarters of patients receiving dupilumab at the subsequently approved dosing achieved at least a 75% improvement in overall disease.28 An additional study is being conducted that includes pediatric patients aged 6 months to younger than 6 years (ClinicalTrials.gov Identifier NCT03346434).
Future Directions in Pediatric AD
Our review summarizes only some of the agents under clinical investigation for use in pediatric AD. Early treatment to establish excellent long-term disease control with aggressive topical regimens or with systemic agents may alter the course of AD and influence the development of comorbidities, though this has not yet been shown in clinical studies. The long-term impact of early treatment, along with many other intriguing issues, will be studied more in the near future.
- Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatol Clin. 2017;35:283-289.
- Silverberg JI, Barbarot S, Gadkari A, et al. Epidemiology of atopic dermatitis in children aged 6–11 years: a cross-sectional study in the United States (US), Canada, Europe, and Japan. Paper presented at: American Academy of Dermatology Annual Meeting; March 20-24, 2020; Denver, CO.
- McKenzie C, Silverberg JI. The prevalence and persistence of atopic dermatitis in urban United States children. Ann Allergy Asthma Immunol. 2019;123:173-178.e1.
- Paternoster L, Savenije OEM, Heron J, et al. IJ Allergy Clin Immunol. 2018;141:964-971.
- Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol. 2013;24:476-486.
- Yaghmaie P, Koudelka CW, Simpson Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol. 2013;131:428-433.
- Narla S, Silverberg JI. Association between childhood atopic dermatitis and cutaneous, extracutaneous and systemic infections. Br J Dermatol. 2018;178:1467-1468.
- al. Incidence, prevalence, and risk of selected ocular disease in adults with atopic dermatitis. J Am Acad Dermatol. 2017;77:280-286.
- Association of atopic dermatitis with cardiovascular risk factors and diseases. J Invest Dermatol. 2017;137:1074-1081.
- Major comorbidities of atopic dermatitis: beyond allergic disorders. Am J Clin Dermatol. 2018;19:821-838.
- Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis.
- Quality of life in families with children with atopic dermatitis. Pediatr Dermatol. 2016;33:28-32.
- Assessment of sleep disturbances and exhaustion in mothers of children with atopic dermatitis. JAMA Dermatol. 2019;155:556-563.
- Association of atopic dermatitis with sleep quality in children.
- Weidinger S, Simpson EL, Eckert L, et al. The patient-reported disease burden in pediatric patients with atopic dermatitis: a cross-sectional study in the United States (US), Canada, Europe, and Japan. Paperpresented at: American Academy of Dermatology Annual Meeting; March 20-24, 2020; Denver, CO.
- The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137:26-30.
- Mitchell AE. Bidirectional relationships between psychological health and dermatological conditions in children. Psychol Res Behav Manag. 2018;11:289-298.
- Chiang C, Eichenfield LF. Quantitative assessment of combination bathing and moisturizing regimens on skin hydration in atopic dermatitis. Pediatr Dermatol. 2009;26:273-278.
- Kempe E, Jain N, Cardona I. Bathing frequency recommendations for pediatric atopic dermatitis: are we adding to parental frustration? Ann Allergy Asthma Immunol. 2013;111:298‐299.
- Cardona ID, Kempe EE, Lary C, et al. Frequent versus infrequent bathing in pediatric atopic dermatitis: a randomized clinical trial. J Allergy Clin Immunol Pract. 2020;8:1014‐1021.
- Gower , Safety, effectiveness, and pharmacokinetics of crisaborole in infants aged 3 to <24 months with mild‐to‐moderate atopic dermatitis: a phase IV open‐label study (CrisADe CARE 1). Am J Clin Dermatol. 2020;21:275-284.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment atopic dermatitis: results from two phase 3, randomized, double-blind studies. Presented at: 2nd Annual Revolutionizing Atopic Dermatitis Conference; April 5, 2020; Chicago, IL.
- Dhillon S. Delgocitinib: first approval. Drugs. 2020;80:609‐615.
- Nakagawa H, Nemoto O, Igarashi A, et al. Phase 2 clinical study of delgocitinib ointment in pediatric patients with atopic dermatitis. J Allergy Clin Immunol. 2019;144:1575‐1583.
- Peppers J, Paller AS, Maeda-Chubachi T, et al. A phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of atopic dermatitis. J Am Acad Dermatol. 2019;80:89‐98.e3.
- Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255-266.
- Cork MJ, Thaçi D, Eichenfield LF, et al. Dupilumab in adolescents with uncontrolled moderate-to-severe atopic dermatitis: results from a phase IIa open-label trial and subsequent phase III open-label extension. Br J Dermatol. 2020;182:85‐96.
- Paller AS, Siegfried EC, Thaçi D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial [published online June 20, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.054.
Atopic dermatitis (AD) is a chronic, pruritic, inflammatory skin disease that occurs most frequently in children but also affects many adolescents and adults. There has been a tremendous evolution of knowledge in AD, with insights into pathogenesis, epidemiology, impact of disease, and new therapies. A variety of studies examine the epidemiology of AD and associated comorbidities. The broad developments in disease state research are reflected in new publication numbers of AD citations on PubMed. A PubMed search of articles indexed for MEDLINE at the end of 2010 using the term atopic dermatitis would have shown 965 citations during the preceding 1-year period. In the 1-year period of June 2019 to June 2020, there were more than 2000 articles. The large body of research includes work of great significance in pediatric AD, and in this article we review recent findings that are important in understanding the progress being made in the field.
Epidemiology and Comorbidities
The epidemiology of AD has evolved over the last few decades, with emerging trends and novel insights into the burden of disease.1 In a recent cross-sectional study on the epidemiology of AD in children aged 6 to 11 years, the 1-year diagnosed AD prevalence estimates worldwide included the following: United States, 10.0%; Canada, 13.3%; the EU5 Countries, 15.5%; Japan, 10.3%; and all countries studied, 12.2%.2 Another recent paper that analyzed data from the Fragile Families and Child Wellbeing Study showed that the prevalence and persistence of AD in urban US children was 15.0%.3Although pediatric AD may spontaneously remit over time, disease continuing into adolescence and adulthood is common. Paternoster et al4 studied the longitudinal course of AD in children from 2 birth cohort prospective studies, showing distinct AD phenotypes having differing course trajectories over time. Disease subsets included patients with early-onset-persistent and early-onset-late-resolving disease.4 Whether phenotyping or subgroup analysis can be used to predict disease course or risk for development of comorbidities is unknown, but it is interesting to consider how such work could influence tailoring of specific therapies to early disease presentation.
Atopic dermatitis poses a serious public health burden owing to its high prevalence, considerable morbidity and disability, increased health care utilization, and cost of care.1 Recent studies have found notably higher rates of multiple medical and mental health comorbidities in both children and adults with AD, including infections, atopic comorbidities (eg, allergic rhinitis, asthma, food allergies), eye diseases (eg, keratitis, conjunctivitis, keratoconus), and possible cardiovascular diseases and autoimmune disorders.1,5-9 Allergic comorbidities are quite common in pediatric AD patients.10 In a recent study examining the efficacy and safety of dupilumab monotherapy in 251 adolescents with moderate to severe inadequately controlled AD, most had comorbid type 2 diseases including asthma (53.6%), food allergies (60.8%), and allergic rhinitis (65.6%).11
Quality of Life/Life Impact of AD
Pediatric AD has a major impact on the quality of life of patients and their families.12 The well-being and development of children are strongly influenced by the physical and psychosocial health of parents/guardians. Two studies by Ramirez and colleagues13,14 published in 2019 examined sleep disturbances and exhaustion in mothers of children with AD. Data for the studies came from the Avon Longitudinal Study of Parents and Children. Children with active AD reported worse sleep quality than those without AD, with nearly 50% higher odds of sleep-quality disturbances. Analysis of the cohort data from 11,649 mother-child pairs who were followed up with a time-varying measure of child AD activity and severity as well as self-reported maternal sleep measures repeated at multiple time points for children aged 6 months to 11 years showed that mothers of children with AD reported difficulty falling asleep, subjectively insufficient sleep, and daytime exhaustion throughout the first 11 years of childhood.13,14 These data suggest that sleep disturbance may be a family affair.
A cross-sectional, real-world study on the burden of AD in children aged 6 to 11 years assessed by self-report demonstrated a substantial and multidimensional impact of AD, including itch, sleep disturbance, skin pain, and health-related quality-of-life impact, as well as comorbidities and school productivity losses. The burden associated with AD was remarkable and increased with disease severity.15
Drucker et al16 completed a comprehensive literature review on the burden of AD, summarized as a report for the National Eczema Association. Quality-of-life impact on pediatric patients included high rates of emotional distress; social isolation; depression; limitations in activities due to lesions with fear of triggers; and behavioral problems such as irritability, crying, and sleep disturbance resulting in difficulty performing at school.16 The psychological impact on children as well as emotional and behavioral difficulties may impact the ability for parents/guardians to implement treatment plans.17
There is a striking association between mental health disorders and AD in the US pediatric population, with a clear dose-dependent relationship that has been observed between the prevalence of a mental health disorder and the reported severity of the skin disease. Data suggest children with AD may be at increased risk for developing mental health disorders. The National Survey of Children’s Health found statistically significant increases in the likelihood of attention deficit hyperactivity disorder (odds ratio [OR], 1.87), depression (OR, 1.81), anxiety (OR, 1.77), conduct disorder (OR, 1.87), and autism (OR, 3.04).6
Evolving Practices and Therapies
Bathing Practices
There has long been much controversy regarding best bathing habits for patients with AD. In a 2009 study, cutaneous hydration was quantified after various bathing and moisturizing regimens.18 The study showed clear benefits of emollient application on skin hydration, either after bathing or without bathing. Bathing followed by emollient applications did not decrease skin hydration in contrast to bathing without emollient application.18
There are limited studies evaluating bathing frequency in pediatric patients, and many families receive conflicting information regarding best practice. In one study that surveyed 354 parents, more than 75% of parents/guardians who had seen multiple providers for their child’s AD reported a substantial amount of confusion and frustration from conflicting advice on bathing frequency.19 Cardona et al20 undertook a randomized clinical trial of frequent bathing and moisturizing vs less-frequent bathing and moisturizing in pediatric patients with AD aged 6 months to 11 years. Patients were divided into 2 groups: 1 being bathed twice daily with immediate moisturizer application and the other being bathed twice weekly followed by moisturization, then a switch to the other method. Patients used standardized topical corticosteroids (TCSs) in both groups. There were significant improvements in scoring AD and other objective measures during the frequent bathing time period vs infrequent bathing; in the group that bathed more frequently, SCORAD (SCORing Atopic Dermatitis) decreased by 21.2 compared with the group that bathed less frequently (95% confidence interval, 14.9-27.6; P<.0001). These findings suggest that more-frequent bathing with immediate moisturization is superior as an acute treatment intervention for improving AD disease severity in comparison to less-frequent bathing with immediate moisturization.20
Expanding Treatment Options
Topical Phosphodiesterase Inhibitors
There are several new and evolving topical therapies in AD. Crisaborole ointment 2% is a steroid-free phosphodiesterase inhibitor approved in 2016 by the US Food and Drug Administration (FDA) for mild to moderate AD in patients aged 2 years and older. A recent multicenter, open-label, single-arm study in 137 infants (CrisADe CARE 1) evaluated the pharmacokinetics and efficacy of crisaborole ointment 2% applied twice daily for 4 weeks in pediatric patients aged 3 months to less than 24 months of age with mild to moderate AD.21 The study had 2 cohorts: one with a minimum of 5% body surface area involvement and another (the pharmacokinetic cohort) with a minimum of 35% body surface area involvement. Both cohorts demonstrated similar efficacy data. From baseline to day 29, the mean percentage change in eczema area and severity index (EASI) score was −57.5%, and an investigator global assessment (IGA) score of clear or almost clear with at least a 2-grade improvement was achieved in 30.2% of patients. Crisaborole systemic exposures in infants were comparable with those in patients aged 2 years or older. Patients tolerated crisaborole well, with a 4% rate of burning, which was similar to other studies in children and adults but perhaps lower than seen in clinical practice. Pharmacokinetic studies did not show any remarkable noticeable concern with accumulation of propylene glycol absorption.21
Based on the CrisADe CARE 1 study data, in March 2020 the FDA extended the indication of crisaborole ointment 2% from a prior lower age limit of 24 months to approval for use in treating mild to moderate AD in children as young as 3 months, making it the first nonsteroidal topical anti-inflammatory medication to be approved in children younger than 2 years in the United States.
Evolving Topical Therapies
Topical Janus Kinase Inhibitors
Ruxolitinib is a potent inhibitor of Janus kinase 1 (JAK-1) and Janus kinase 2 (JAK-2) and has been developed in topical formulations. In recent phase 3 clinical trials of patients with AD aged 12 years and older with mild to moderate disease (TRuE-AD1 and TRuE-AD2), more than half of the patients treated with either ruxolitinib cream in a 0.75% or 1.5% concentration reached EASI-75 after 8 weeks of treatment.22 Additionally, more patients treated with topical ruxolitinib reached an IGA score of clear to almost clear than patients treated with vehicle at the end of treatment. Thus far, it appears to be very well tolerated, significantly decreases EASI score (P<.0001), and improves overall pruritus.22
Delgocitinib is a topical pan-JAK inhibitor that blocks several cytokine-signaling cascade pathways. It was first developed and approved in Japan in an ointment formulation for use in patients with AD aged 16 years and older.23 The efficacy and safety profile of delgocitinib is currently being evaluated in pediatric patients with AD in Japan. In a recent phase 2 clinical study of 103 Japanese patients aged 2 to 15 years with moderate to severe AD, patients were randomized to receive either delgocitinib ointment in 0.25% or 0.5% concentrations or vehicle ointment twice daily for 4 weeks. The proportion of patients with a modified EASI-75 score was 38.2% (13/34) in the 0.25% group and 50.0% (17/34) in the 0.5% group vs 8.6% (3/35) in the placebo group. More patients treated with delgocitinib ointment received an IGA score of clear or almost clear than patients treated with vehicle at the end of treatment. Overall, both delgocitinib groups demonstrated superior improvement in clinical symptoms and signs without notable side effects.24
Tapinarof
Tapinarof is a topical therapeutic aryl hydrocarbon receptor agonist. In a recent phase 2 randomized study of 2 concentrations and 2 frequencies of tapinarof cream vs vehicle in 247 randomized patients aged 12 to 65 years with moderate to severe disease, tapinarof demonstrated greater success with both concentrations than vehicle at all visits beyond week 2.25 Additionally, in patients treated with tapinarof cream 1%, nearly 50% reached an IGA score of clear to almost clear with at least a 2-grade improvement. More than 50% of patients achieved EASI-75 improvement at 12 weeks of treatment with tapinarof cream 1% used daily. These findings suggest that tapinarof may be an efficacious and well-tolerated treatment for both adolescents and adults with AD; however, large confirmation trials are needed to further investigate.25
Systemic Treatments
Oral JAK Inhibitors
Some of the most exciting novel therapies include several oral JAK inhibitors that target different combinations of kinases and have been shown to decrease AD severity and symptoms. Some of these agents have indications in other disease states, such as baricitinib and upadacitinib, which are both FDA approved for the treatment of rheumatoid arthritis, whereas others, such as abrocitinib, have been studied specifically for AD.
Although some agents have only been studied in adults to date, others have included adolescents in their core studies, such as abrocitinib, which received Breakthrough Therapy designation from the FDA for the treatment of patients with moderate to severe AD in February 2018. In recent phase 3 trials of patients aged 12 years and older with moderate to severe AD (JADE MONO-1 and JADE MONO-2), both doses of abrocitinib improved the IGA and EASI-75 outcomes compared with placebo.26 Additional studies will be conducted to further investigate the relative efficacy and safety in patients younger than 18 years.
Biologics
Dupilumab is a fully human monoclonal antibody that inhibits IL-4 and IL-13 signaling without suppressing the immune system. It is approved for use in patients aged 12 years and older with moderate to severe asthma and in adults with chronic rhinosinusitis with nasal polyposis. It is the first biologic to show positive results in the moderate to severe pediatric AD population. There are now extended data available exhibiting sustained benefit in adolescent patients who were continued on dupilumab therapy, evidenced by further improvement in EASI scores at the 1-year mark.27
Recently, dupilumab received approval for use in patients aged 6 to 11 years, making it the first biologic for AD to be approved for use in patients younger than 12 years. The expedited FDA approval was based on the phase 3 results in which the efficacy and safety of dupilumab combined with TCSs were compared to TCSs alone (N=367).28 In this trial, more than twice as many children achieved clear or almost clear skin and more than 4 times as many achieved itch reduction with dupilumab plus TCSs than with TCSs alone. Three-quarters of patients receiving dupilumab at the subsequently approved dosing achieved at least a 75% improvement in overall disease.28 An additional study is being conducted that includes pediatric patients aged 6 months to younger than 6 years (ClinicalTrials.gov Identifier NCT03346434).
Future Directions in Pediatric AD
Our review summarizes only some of the agents under clinical investigation for use in pediatric AD. Early treatment to establish excellent long-term disease control with aggressive topical regimens or with systemic agents may alter the course of AD and influence the development of comorbidities, though this has not yet been shown in clinical studies. The long-term impact of early treatment, along with many other intriguing issues, will be studied more in the near future.
Atopic dermatitis (AD) is a chronic, pruritic, inflammatory skin disease that occurs most frequently in children but also affects many adolescents and adults. There has been a tremendous evolution of knowledge in AD, with insights into pathogenesis, epidemiology, impact of disease, and new therapies. A variety of studies examine the epidemiology of AD and associated comorbidities. The broad developments in disease state research are reflected in new publication numbers of AD citations on PubMed. A PubMed search of articles indexed for MEDLINE at the end of 2010 using the term atopic dermatitis would have shown 965 citations during the preceding 1-year period. In the 1-year period of June 2019 to June 2020, there were more than 2000 articles. The large body of research includes work of great significance in pediatric AD, and in this article we review recent findings that are important in understanding the progress being made in the field.
Epidemiology and Comorbidities
The epidemiology of AD has evolved over the last few decades, with emerging trends and novel insights into the burden of disease.1 In a recent cross-sectional study on the epidemiology of AD in children aged 6 to 11 years, the 1-year diagnosed AD prevalence estimates worldwide included the following: United States, 10.0%; Canada, 13.3%; the EU5 Countries, 15.5%; Japan, 10.3%; and all countries studied, 12.2%.2 Another recent paper that analyzed data from the Fragile Families and Child Wellbeing Study showed that the prevalence and persistence of AD in urban US children was 15.0%.3Although pediatric AD may spontaneously remit over time, disease continuing into adolescence and adulthood is common. Paternoster et al4 studied the longitudinal course of AD in children from 2 birth cohort prospective studies, showing distinct AD phenotypes having differing course trajectories over time. Disease subsets included patients with early-onset-persistent and early-onset-late-resolving disease.4 Whether phenotyping or subgroup analysis can be used to predict disease course or risk for development of comorbidities is unknown, but it is interesting to consider how such work could influence tailoring of specific therapies to early disease presentation.
Atopic dermatitis poses a serious public health burden owing to its high prevalence, considerable morbidity and disability, increased health care utilization, and cost of care.1 Recent studies have found notably higher rates of multiple medical and mental health comorbidities in both children and adults with AD, including infections, atopic comorbidities (eg, allergic rhinitis, asthma, food allergies), eye diseases (eg, keratitis, conjunctivitis, keratoconus), and possible cardiovascular diseases and autoimmune disorders.1,5-9 Allergic comorbidities are quite common in pediatric AD patients.10 In a recent study examining the efficacy and safety of dupilumab monotherapy in 251 adolescents with moderate to severe inadequately controlled AD, most had comorbid type 2 diseases including asthma (53.6%), food allergies (60.8%), and allergic rhinitis (65.6%).11
Quality of Life/Life Impact of AD
Pediatric AD has a major impact on the quality of life of patients and their families.12 The well-being and development of children are strongly influenced by the physical and psychosocial health of parents/guardians. Two studies by Ramirez and colleagues13,14 published in 2019 examined sleep disturbances and exhaustion in mothers of children with AD. Data for the studies came from the Avon Longitudinal Study of Parents and Children. Children with active AD reported worse sleep quality than those without AD, with nearly 50% higher odds of sleep-quality disturbances. Analysis of the cohort data from 11,649 mother-child pairs who were followed up with a time-varying measure of child AD activity and severity as well as self-reported maternal sleep measures repeated at multiple time points for children aged 6 months to 11 years showed that mothers of children with AD reported difficulty falling asleep, subjectively insufficient sleep, and daytime exhaustion throughout the first 11 years of childhood.13,14 These data suggest that sleep disturbance may be a family affair.
A cross-sectional, real-world study on the burden of AD in children aged 6 to 11 years assessed by self-report demonstrated a substantial and multidimensional impact of AD, including itch, sleep disturbance, skin pain, and health-related quality-of-life impact, as well as comorbidities and school productivity losses. The burden associated with AD was remarkable and increased with disease severity.15
Drucker et al16 completed a comprehensive literature review on the burden of AD, summarized as a report for the National Eczema Association. Quality-of-life impact on pediatric patients included high rates of emotional distress; social isolation; depression; limitations in activities due to lesions with fear of triggers; and behavioral problems such as irritability, crying, and sleep disturbance resulting in difficulty performing at school.16 The psychological impact on children as well as emotional and behavioral difficulties may impact the ability for parents/guardians to implement treatment plans.17
There is a striking association between mental health disorders and AD in the US pediatric population, with a clear dose-dependent relationship that has been observed between the prevalence of a mental health disorder and the reported severity of the skin disease. Data suggest children with AD may be at increased risk for developing mental health disorders. The National Survey of Children’s Health found statistically significant increases in the likelihood of attention deficit hyperactivity disorder (odds ratio [OR], 1.87), depression (OR, 1.81), anxiety (OR, 1.77), conduct disorder (OR, 1.87), and autism (OR, 3.04).6
Evolving Practices and Therapies
Bathing Practices
There has long been much controversy regarding best bathing habits for patients with AD. In a 2009 study, cutaneous hydration was quantified after various bathing and moisturizing regimens.18 The study showed clear benefits of emollient application on skin hydration, either after bathing or without bathing. Bathing followed by emollient applications did not decrease skin hydration in contrast to bathing without emollient application.18
There are limited studies evaluating bathing frequency in pediatric patients, and many families receive conflicting information regarding best practice. In one study that surveyed 354 parents, more than 75% of parents/guardians who had seen multiple providers for their child’s AD reported a substantial amount of confusion and frustration from conflicting advice on bathing frequency.19 Cardona et al20 undertook a randomized clinical trial of frequent bathing and moisturizing vs less-frequent bathing and moisturizing in pediatric patients with AD aged 6 months to 11 years. Patients were divided into 2 groups: 1 being bathed twice daily with immediate moisturizer application and the other being bathed twice weekly followed by moisturization, then a switch to the other method. Patients used standardized topical corticosteroids (TCSs) in both groups. There were significant improvements in scoring AD and other objective measures during the frequent bathing time period vs infrequent bathing; in the group that bathed more frequently, SCORAD (SCORing Atopic Dermatitis) decreased by 21.2 compared with the group that bathed less frequently (95% confidence interval, 14.9-27.6; P<.0001). These findings suggest that more-frequent bathing with immediate moisturization is superior as an acute treatment intervention for improving AD disease severity in comparison to less-frequent bathing with immediate moisturization.20
Expanding Treatment Options
Topical Phosphodiesterase Inhibitors
There are several new and evolving topical therapies in AD. Crisaborole ointment 2% is a steroid-free phosphodiesterase inhibitor approved in 2016 by the US Food and Drug Administration (FDA) for mild to moderate AD in patients aged 2 years and older. A recent multicenter, open-label, single-arm study in 137 infants (CrisADe CARE 1) evaluated the pharmacokinetics and efficacy of crisaborole ointment 2% applied twice daily for 4 weeks in pediatric patients aged 3 months to less than 24 months of age with mild to moderate AD.21 The study had 2 cohorts: one with a minimum of 5% body surface area involvement and another (the pharmacokinetic cohort) with a minimum of 35% body surface area involvement. Both cohorts demonstrated similar efficacy data. From baseline to day 29, the mean percentage change in eczema area and severity index (EASI) score was −57.5%, and an investigator global assessment (IGA) score of clear or almost clear with at least a 2-grade improvement was achieved in 30.2% of patients. Crisaborole systemic exposures in infants were comparable with those in patients aged 2 years or older. Patients tolerated crisaborole well, with a 4% rate of burning, which was similar to other studies in children and adults but perhaps lower than seen in clinical practice. Pharmacokinetic studies did not show any remarkable noticeable concern with accumulation of propylene glycol absorption.21
Based on the CrisADe CARE 1 study data, in March 2020 the FDA extended the indication of crisaborole ointment 2% from a prior lower age limit of 24 months to approval for use in treating mild to moderate AD in children as young as 3 months, making it the first nonsteroidal topical anti-inflammatory medication to be approved in children younger than 2 years in the United States.
Evolving Topical Therapies
Topical Janus Kinase Inhibitors
Ruxolitinib is a potent inhibitor of Janus kinase 1 (JAK-1) and Janus kinase 2 (JAK-2) and has been developed in topical formulations. In recent phase 3 clinical trials of patients with AD aged 12 years and older with mild to moderate disease (TRuE-AD1 and TRuE-AD2), more than half of the patients treated with either ruxolitinib cream in a 0.75% or 1.5% concentration reached EASI-75 after 8 weeks of treatment.22 Additionally, more patients treated with topical ruxolitinib reached an IGA score of clear to almost clear than patients treated with vehicle at the end of treatment. Thus far, it appears to be very well tolerated, significantly decreases EASI score (P<.0001), and improves overall pruritus.22
Delgocitinib is a topical pan-JAK inhibitor that blocks several cytokine-signaling cascade pathways. It was first developed and approved in Japan in an ointment formulation for use in patients with AD aged 16 years and older.23 The efficacy and safety profile of delgocitinib is currently being evaluated in pediatric patients with AD in Japan. In a recent phase 2 clinical study of 103 Japanese patients aged 2 to 15 years with moderate to severe AD, patients were randomized to receive either delgocitinib ointment in 0.25% or 0.5% concentrations or vehicle ointment twice daily for 4 weeks. The proportion of patients with a modified EASI-75 score was 38.2% (13/34) in the 0.25% group and 50.0% (17/34) in the 0.5% group vs 8.6% (3/35) in the placebo group. More patients treated with delgocitinib ointment received an IGA score of clear or almost clear than patients treated with vehicle at the end of treatment. Overall, both delgocitinib groups demonstrated superior improvement in clinical symptoms and signs without notable side effects.24
Tapinarof
Tapinarof is a topical therapeutic aryl hydrocarbon receptor agonist. In a recent phase 2 randomized study of 2 concentrations and 2 frequencies of tapinarof cream vs vehicle in 247 randomized patients aged 12 to 65 years with moderate to severe disease, tapinarof demonstrated greater success with both concentrations than vehicle at all visits beyond week 2.25 Additionally, in patients treated with tapinarof cream 1%, nearly 50% reached an IGA score of clear to almost clear with at least a 2-grade improvement. More than 50% of patients achieved EASI-75 improvement at 12 weeks of treatment with tapinarof cream 1% used daily. These findings suggest that tapinarof may be an efficacious and well-tolerated treatment for both adolescents and adults with AD; however, large confirmation trials are needed to further investigate.25
Systemic Treatments
Oral JAK Inhibitors
Some of the most exciting novel therapies include several oral JAK inhibitors that target different combinations of kinases and have been shown to decrease AD severity and symptoms. Some of these agents have indications in other disease states, such as baricitinib and upadacitinib, which are both FDA approved for the treatment of rheumatoid arthritis, whereas others, such as abrocitinib, have been studied specifically for AD.
Although some agents have only been studied in adults to date, others have included adolescents in their core studies, such as abrocitinib, which received Breakthrough Therapy designation from the FDA for the treatment of patients with moderate to severe AD in February 2018. In recent phase 3 trials of patients aged 12 years and older with moderate to severe AD (JADE MONO-1 and JADE MONO-2), both doses of abrocitinib improved the IGA and EASI-75 outcomes compared with placebo.26 Additional studies will be conducted to further investigate the relative efficacy and safety in patients younger than 18 years.
Biologics
Dupilumab is a fully human monoclonal antibody that inhibits IL-4 and IL-13 signaling without suppressing the immune system. It is approved for use in patients aged 12 years and older with moderate to severe asthma and in adults with chronic rhinosinusitis with nasal polyposis. It is the first biologic to show positive results in the moderate to severe pediatric AD population. There are now extended data available exhibiting sustained benefit in adolescent patients who were continued on dupilumab therapy, evidenced by further improvement in EASI scores at the 1-year mark.27
Recently, dupilumab received approval for use in patients aged 6 to 11 years, making it the first biologic for AD to be approved for use in patients younger than 12 years. The expedited FDA approval was based on the phase 3 results in which the efficacy and safety of dupilumab combined with TCSs were compared to TCSs alone (N=367).28 In this trial, more than twice as many children achieved clear or almost clear skin and more than 4 times as many achieved itch reduction with dupilumab plus TCSs than with TCSs alone. Three-quarters of patients receiving dupilumab at the subsequently approved dosing achieved at least a 75% improvement in overall disease.28 An additional study is being conducted that includes pediatric patients aged 6 months to younger than 6 years (ClinicalTrials.gov Identifier NCT03346434).
Future Directions in Pediatric AD
Our review summarizes only some of the agents under clinical investigation for use in pediatric AD. Early treatment to establish excellent long-term disease control with aggressive topical regimens or with systemic agents may alter the course of AD and influence the development of comorbidities, though this has not yet been shown in clinical studies. The long-term impact of early treatment, along with many other intriguing issues, will be studied more in the near future.
- Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatol Clin. 2017;35:283-289.
- Silverberg JI, Barbarot S, Gadkari A, et al. Epidemiology of atopic dermatitis in children aged 6–11 years: a cross-sectional study in the United States (US), Canada, Europe, and Japan. Paper presented at: American Academy of Dermatology Annual Meeting; March 20-24, 2020; Denver, CO.
- McKenzie C, Silverberg JI. The prevalence and persistence of atopic dermatitis in urban United States children. Ann Allergy Asthma Immunol. 2019;123:173-178.e1.
- Paternoster L, Savenije OEM, Heron J, et al. IJ Allergy Clin Immunol. 2018;141:964-971.
- Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol. 2013;24:476-486.
- Yaghmaie P, Koudelka CW, Simpson Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol. 2013;131:428-433.
- Narla S, Silverberg JI. Association between childhood atopic dermatitis and cutaneous, extracutaneous and systemic infections. Br J Dermatol. 2018;178:1467-1468.
- al. Incidence, prevalence, and risk of selected ocular disease in adults with atopic dermatitis. J Am Acad Dermatol. 2017;77:280-286.
- Association of atopic dermatitis with cardiovascular risk factors and diseases. J Invest Dermatol. 2017;137:1074-1081.
- Major comorbidities of atopic dermatitis: beyond allergic disorders. Am J Clin Dermatol. 2018;19:821-838.
- Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis.
- Quality of life in families with children with atopic dermatitis. Pediatr Dermatol. 2016;33:28-32.
- Assessment of sleep disturbances and exhaustion in mothers of children with atopic dermatitis. JAMA Dermatol. 2019;155:556-563.
- Association of atopic dermatitis with sleep quality in children.
- Weidinger S, Simpson EL, Eckert L, et al. The patient-reported disease burden in pediatric patients with atopic dermatitis: a cross-sectional study in the United States (US), Canada, Europe, and Japan. Paperpresented at: American Academy of Dermatology Annual Meeting; March 20-24, 2020; Denver, CO.
- The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137:26-30.
- Mitchell AE. Bidirectional relationships between psychological health and dermatological conditions in children. Psychol Res Behav Manag. 2018;11:289-298.
- Chiang C, Eichenfield LF. Quantitative assessment of combination bathing and moisturizing regimens on skin hydration in atopic dermatitis. Pediatr Dermatol. 2009;26:273-278.
- Kempe E, Jain N, Cardona I. Bathing frequency recommendations for pediatric atopic dermatitis: are we adding to parental frustration? Ann Allergy Asthma Immunol. 2013;111:298‐299.
- Cardona ID, Kempe EE, Lary C, et al. Frequent versus infrequent bathing in pediatric atopic dermatitis: a randomized clinical trial. J Allergy Clin Immunol Pract. 2020;8:1014‐1021.
- Gower , Safety, effectiveness, and pharmacokinetics of crisaborole in infants aged 3 to <24 months with mild‐to‐moderate atopic dermatitis: a phase IV open‐label study (CrisADe CARE 1). Am J Clin Dermatol. 2020;21:275-284.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment atopic dermatitis: results from two phase 3, randomized, double-blind studies. Presented at: 2nd Annual Revolutionizing Atopic Dermatitis Conference; April 5, 2020; Chicago, IL.
- Dhillon S. Delgocitinib: first approval. Drugs. 2020;80:609‐615.
- Nakagawa H, Nemoto O, Igarashi A, et al. Phase 2 clinical study of delgocitinib ointment in pediatric patients with atopic dermatitis. J Allergy Clin Immunol. 2019;144:1575‐1583.
- Peppers J, Paller AS, Maeda-Chubachi T, et al. A phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of atopic dermatitis. J Am Acad Dermatol. 2019;80:89‐98.e3.
- Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255-266.
- Cork MJ, Thaçi D, Eichenfield LF, et al. Dupilumab in adolescents with uncontrolled moderate-to-severe atopic dermatitis: results from a phase IIa open-label trial and subsequent phase III open-label extension. Br J Dermatol. 2020;182:85‐96.
- Paller AS, Siegfried EC, Thaçi D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial [published online June 20, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.054.
- Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatol Clin. 2017;35:283-289.
- Silverberg JI, Barbarot S, Gadkari A, et al. Epidemiology of atopic dermatitis in children aged 6–11 years: a cross-sectional study in the United States (US), Canada, Europe, and Japan. Paper presented at: American Academy of Dermatology Annual Meeting; March 20-24, 2020; Denver, CO.
- McKenzie C, Silverberg JI. The prevalence and persistence of atopic dermatitis in urban United States children. Ann Allergy Asthma Immunol. 2019;123:173-178.e1.
- Paternoster L, Savenije OEM, Heron J, et al. IJ Allergy Clin Immunol. 2018;141:964-971.
- Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol. 2013;24:476-486.
- Yaghmaie P, Koudelka CW, Simpson Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol. 2013;131:428-433.
- Narla S, Silverberg JI. Association between childhood atopic dermatitis and cutaneous, extracutaneous and systemic infections. Br J Dermatol. 2018;178:1467-1468.
- al. Incidence, prevalence, and risk of selected ocular disease in adults with atopic dermatitis. J Am Acad Dermatol. 2017;77:280-286.
- Association of atopic dermatitis with cardiovascular risk factors and diseases. J Invest Dermatol. 2017;137:1074-1081.
- Major comorbidities of atopic dermatitis: beyond allergic disorders. Am J Clin Dermatol. 2018;19:821-838.
- Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis.
- Quality of life in families with children with atopic dermatitis. Pediatr Dermatol. 2016;33:28-32.
- Assessment of sleep disturbances and exhaustion in mothers of children with atopic dermatitis. JAMA Dermatol. 2019;155:556-563.
- Association of atopic dermatitis with sleep quality in children.
- Weidinger S, Simpson EL, Eckert L, et al. The patient-reported disease burden in pediatric patients with atopic dermatitis: a cross-sectional study in the United States (US), Canada, Europe, and Japan. Paperpresented at: American Academy of Dermatology Annual Meeting; March 20-24, 2020; Denver, CO.
- The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137:26-30.
- Mitchell AE. Bidirectional relationships between psychological health and dermatological conditions in children. Psychol Res Behav Manag. 2018;11:289-298.
- Chiang C, Eichenfield LF. Quantitative assessment of combination bathing and moisturizing regimens on skin hydration in atopic dermatitis. Pediatr Dermatol. 2009;26:273-278.
- Kempe E, Jain N, Cardona I. Bathing frequency recommendations for pediatric atopic dermatitis: are we adding to parental frustration? Ann Allergy Asthma Immunol. 2013;111:298‐299.
- Cardona ID, Kempe EE, Lary C, et al. Frequent versus infrequent bathing in pediatric atopic dermatitis: a randomized clinical trial. J Allergy Clin Immunol Pract. 2020;8:1014‐1021.
- Gower , Safety, effectiveness, and pharmacokinetics of crisaborole in infants aged 3 to <24 months with mild‐to‐moderate atopic dermatitis: a phase IV open‐label study (CrisADe CARE 1). Am J Clin Dermatol. 2020;21:275-284.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment atopic dermatitis: results from two phase 3, randomized, double-blind studies. Presented at: 2nd Annual Revolutionizing Atopic Dermatitis Conference; April 5, 2020; Chicago, IL.
- Dhillon S. Delgocitinib: first approval. Drugs. 2020;80:609‐615.
- Nakagawa H, Nemoto O, Igarashi A, et al. Phase 2 clinical study of delgocitinib ointment in pediatric patients with atopic dermatitis. J Allergy Clin Immunol. 2019;144:1575‐1583.
- Peppers J, Paller AS, Maeda-Chubachi T, et al. A phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of atopic dermatitis. J Am Acad Dermatol. 2019;80:89‐98.e3.
- Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255-266.
- Cork MJ, Thaçi D, Eichenfield LF, et al. Dupilumab in adolescents with uncontrolled moderate-to-severe atopic dermatitis: results from a phase IIa open-label trial and subsequent phase III open-label extension. Br J Dermatol. 2020;182:85‐96.
- Paller AS, Siegfried EC, Thaçi D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial [published online June 20, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.054.
Practice Points
- There has been tremendous growth in our understanding of atopic dermatitis, with further insight into epidemiology, the impact on quality of life of affected individuals and their families, best bathing practices, and expanding treatment options.
- There are several novel topical and systemic agents recently approved and in late-stage clinical development programs that are evolving therapeutic approaches to pediatric disease.
Cutaneous T-cell Lymphoma and Concomitant Atopic Dermatitis Responding to Dupilumab
Patients with cutaneous T-cell lymphoma (CTCL) often are diagnosed with atopic dermatitis (AD) or psoriasis before receiving their CTCL diagnosis. The effects of new biologic therapies for AD such as dupilumab, an IL-4/IL-13 antagonist, on CTCL are unknown. Dupilumab may be beneficial in CTCL given that helper T cell (TH2) cytokines are increased in advanced CTCL.1 We present a patient with definitive CTCL and concomitant AD who was safely treated with dupilumab and experienced improvement in both CTCL and AD.
Case Report
A 68-year-old man presented with increased itching from AD and a new rash on the arms, neck, chest, back, and lower extremities (Figures 1A and 2A). He had a medical history of AD and CTCL diagnosed by biopsy and peripheral blood flow cytometry (stage IVA1 [T4N0M0B2]) that was being treated with comprehensive multimodality therapy consisting of bexarotene 375 mg daily, interferon alfa-2b 3 mIU 3 times weekly, interferon gamma-1b 2 mIU 3 times weekly, total skin electron beam therapy followed by narrowband UVB twice weekly, and extracorporeal photopheresis every 4 weeks, which resulted in a partial clinical response for 6 months. A biopsy performed at the current presentation showed focal spongiosis and features of lichen simplex chronicus with no evidence suggestive of CTCL. Peripheral blood flow cytometry showed stable B1-staged disease burden (CD4/CD8, 2.6:1); CD4+/CD7−, 12% [91/µL]; CD4+/CD26−, 21% [155/µL]). Treatment with potent and superpotent topical steroids was attempted for more than 6 months and was unsuccessful in relieving the symptoms.
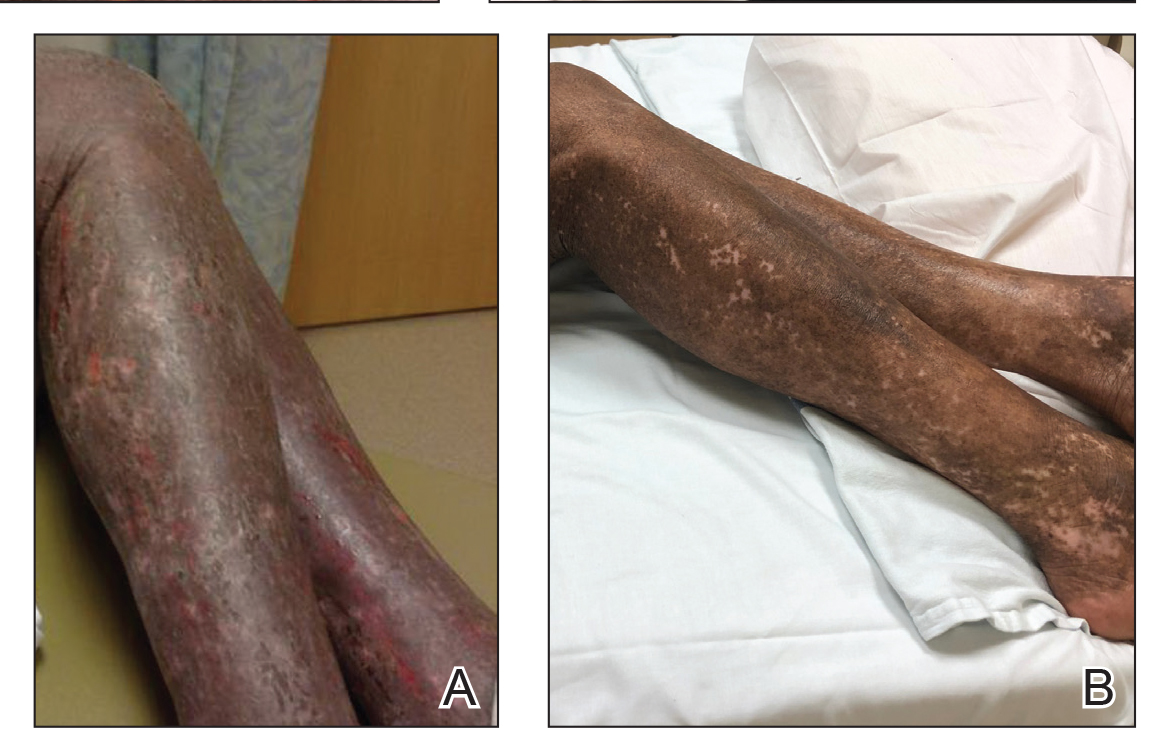
Given the recalcitrant nature of the patient’s rash and itching, dupilumab was added to his CTCL regimen. Prior to initiating dupilumab, the patient reported a numeric rating scale itch intensity of 7 out of 10. After 4 weeks of treatment with dupilumab, the patient reported a numeric rating scale itch intensity of 1. Over a 3-month period, the patient’s rash improved dramatically (Figures 1B and 2B), making it possible to decrease CTCL treatments—bexarotene decreased to 300 mg, interferon alfa-2b to 3 mIU twice weekly, interferon gamma-1b to 2 mIU twice weekly, extracorporeal photopheresis every 5 weeks, and narrowband UVB was discontinued completely. A comparison of the patient’s flow cytometry analysis from before treatment to 3 months after dupilumab showed an overall slight reduction in CTCL B1 blood involvement and normalization of the patient’s absolute eosinophil count and serum lactate dehydrogenase level. The patient tolerated the treatment well without any adverse events and has maintained clinical response for 6 months.
Comment
Cutaneous T-cell lymphomas represent a heterogeneous group of T-cell lymphoproliferative disorders involving the skin.2 The definitive diagnosis of CTCL is challenging, as the clinical and pathologic features often are nonspecific in early disease. Frequently, undiagnosed patients are treated empirically with immunosuppressive agents. Tumor necrosis factor inhibitors and cyclosporine are both associated with progression or worsening of undiagnosed CTCL.3,4 Dupilumab was the first US Food and Drug Administration–approved biologic for the treatment of moderate to severe AD. Cutaneous T-cell lymphoma has immunologic features, such as TH2 skewing, that overlap with AD; however, the effects of dupilumab in CTCL are not yet known.5,6 Our group has seen patients initially thought to have AD who received dupilumab without improvement and were subsequently diagnosed with CTCL, suggesting dupilumab did not affect CTCL tumor cells. Given these findings, there was concern that dupilumab might exacerbate undiagnosed CTCL. Our patient with definitive, severe, refractory CTCL noted marked improvement in both AD and underlying CTCL with the addition of dupilumab. No other treatments were added. The response was so dramatic that we were able to wean the doses and frequencies of several CTCL treatments. Our findings suggest that dupilumab may be beneficial in a certain subset of CTCL patients with a history of AD or known concomitant AD. Prospective studies are needed to fully investigate dupilumab safety and efficacy in CTCL and whether it has any primary effects on tumor burden in addition to benefit for itch and skin symptom relief.
- Guenova E, Watanabe R, Teague JE, et al. TH2 cytokines from malignant cells suppress TH1 responses and enforce a global TH2 bias in leukemic cutaneous T-cell lymphoma. Clin Cancer Res. 2013;19:3755-3763.
- Wilcox RA. Cutaneous T-cell lymphoma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91:151-165.
- Martinez-Escala ME, Posligua AL, Wickless H, et al. Progression of undiagnosed cutaneous lymphoma after anti-tumor necrosis factor-alpha therapy. J Am Acad Dermatol. 2018;78:1068-1076.
- Pielop JA, Jones D, Duvic M. Transient CD30+ nodal transformation of cutaneous T-cell lymphoma associated with cyclosporine treatment. Int J Dermatol. 2001;40:505-511.
- Saulite I, Hoetzenecker W, Weidinger S, et al. Sézary syndrome and atopic dermatitis: comparison of immunological aspects and targets [published online May 17, 2016]. BioMed Res Int. doi:10.1155/2016/9717530.
- Sigurdsson V, Toonstra J, Bihari IC, et al. Interleukin 4 and interferon-gamma expression of the dermal infiltrate in patients with erythroderma and mycosis fungoides. an immuno-histochemical study. J Cutan Pathol. 2000;27:429-435.
Patients with cutaneous T-cell lymphoma (CTCL) often are diagnosed with atopic dermatitis (AD) or psoriasis before receiving their CTCL diagnosis. The effects of new biologic therapies for AD such as dupilumab, an IL-4/IL-13 antagonist, on CTCL are unknown. Dupilumab may be beneficial in CTCL given that helper T cell (TH2) cytokines are increased in advanced CTCL.1 We present a patient with definitive CTCL and concomitant AD who was safely treated with dupilumab and experienced improvement in both CTCL and AD.
Case Report
A 68-year-old man presented with increased itching from AD and a new rash on the arms, neck, chest, back, and lower extremities (Figures 1A and 2A). He had a medical history of AD and CTCL diagnosed by biopsy and peripheral blood flow cytometry (stage IVA1 [T4N0M0B2]) that was being treated with comprehensive multimodality therapy consisting of bexarotene 375 mg daily, interferon alfa-2b 3 mIU 3 times weekly, interferon gamma-1b 2 mIU 3 times weekly, total skin electron beam therapy followed by narrowband UVB twice weekly, and extracorporeal photopheresis every 4 weeks, which resulted in a partial clinical response for 6 months. A biopsy performed at the current presentation showed focal spongiosis and features of lichen simplex chronicus with no evidence suggestive of CTCL. Peripheral blood flow cytometry showed stable B1-staged disease burden (CD4/CD8, 2.6:1); CD4+/CD7−, 12% [91/µL]; CD4+/CD26−, 21% [155/µL]). Treatment with potent and superpotent topical steroids was attempted for more than 6 months and was unsuccessful in relieving the symptoms.


Given the recalcitrant nature of the patient’s rash and itching, dupilumab was added to his CTCL regimen. Prior to initiating dupilumab, the patient reported a numeric rating scale itch intensity of 7 out of 10. After 4 weeks of treatment with dupilumab, the patient reported a numeric rating scale itch intensity of 1. Over a 3-month period, the patient’s rash improved dramatically (Figures 1B and 2B), making it possible to decrease CTCL treatments—bexarotene decreased to 300 mg, interferon alfa-2b to 3 mIU twice weekly, interferon gamma-1b to 2 mIU twice weekly, extracorporeal photopheresis every 5 weeks, and narrowband UVB was discontinued completely. A comparison of the patient’s flow cytometry analysis from before treatment to 3 months after dupilumab showed an overall slight reduction in CTCL B1 blood involvement and normalization of the patient’s absolute eosinophil count and serum lactate dehydrogenase level. The patient tolerated the treatment well without any adverse events and has maintained clinical response for 6 months.
Comment
Cutaneous T-cell lymphomas represent a heterogeneous group of T-cell lymphoproliferative disorders involving the skin.2 The definitive diagnosis of CTCL is challenging, as the clinical and pathologic features often are nonspecific in early disease. Frequently, undiagnosed patients are treated empirically with immunosuppressive agents. Tumor necrosis factor inhibitors and cyclosporine are both associated with progression or worsening of undiagnosed CTCL.3,4 Dupilumab was the first US Food and Drug Administration–approved biologic for the treatment of moderate to severe AD. Cutaneous T-cell lymphoma has immunologic features, such as TH2 skewing, that overlap with AD; however, the effects of dupilumab in CTCL are not yet known.5,6 Our group has seen patients initially thought to have AD who received dupilumab without improvement and were subsequently diagnosed with CTCL, suggesting dupilumab did not affect CTCL tumor cells. Given these findings, there was concern that dupilumab might exacerbate undiagnosed CTCL. Our patient with definitive, severe, refractory CTCL noted marked improvement in both AD and underlying CTCL with the addition of dupilumab. No other treatments were added. The response was so dramatic that we were able to wean the doses and frequencies of several CTCL treatments. Our findings suggest that dupilumab may be beneficial in a certain subset of CTCL patients with a history of AD or known concomitant AD. Prospective studies are needed to fully investigate dupilumab safety and efficacy in CTCL and whether it has any primary effects on tumor burden in addition to benefit for itch and skin symptom relief.
Patients with cutaneous T-cell lymphoma (CTCL) often are diagnosed with atopic dermatitis (AD) or psoriasis before receiving their CTCL diagnosis. The effects of new biologic therapies for AD such as dupilumab, an IL-4/IL-13 antagonist, on CTCL are unknown. Dupilumab may be beneficial in CTCL given that helper T cell (TH2) cytokines are increased in advanced CTCL.1 We present a patient with definitive CTCL and concomitant AD who was safely treated with dupilumab and experienced improvement in both CTCL and AD.
Case Report
A 68-year-old man presented with increased itching from AD and a new rash on the arms, neck, chest, back, and lower extremities (Figures 1A and 2A). He had a medical history of AD and CTCL diagnosed by biopsy and peripheral blood flow cytometry (stage IVA1 [T4N0M0B2]) that was being treated with comprehensive multimodality therapy consisting of bexarotene 375 mg daily, interferon alfa-2b 3 mIU 3 times weekly, interferon gamma-1b 2 mIU 3 times weekly, total skin electron beam therapy followed by narrowband UVB twice weekly, and extracorporeal photopheresis every 4 weeks, which resulted in a partial clinical response for 6 months. A biopsy performed at the current presentation showed focal spongiosis and features of lichen simplex chronicus with no evidence suggestive of CTCL. Peripheral blood flow cytometry showed stable B1-staged disease burden (CD4/CD8, 2.6:1); CD4+/CD7−, 12% [91/µL]; CD4+/CD26−, 21% [155/µL]). Treatment with potent and superpotent topical steroids was attempted for more than 6 months and was unsuccessful in relieving the symptoms.


Given the recalcitrant nature of the patient’s rash and itching, dupilumab was added to his CTCL regimen. Prior to initiating dupilumab, the patient reported a numeric rating scale itch intensity of 7 out of 10. After 4 weeks of treatment with dupilumab, the patient reported a numeric rating scale itch intensity of 1. Over a 3-month period, the patient’s rash improved dramatically (Figures 1B and 2B), making it possible to decrease CTCL treatments—bexarotene decreased to 300 mg, interferon alfa-2b to 3 mIU twice weekly, interferon gamma-1b to 2 mIU twice weekly, extracorporeal photopheresis every 5 weeks, and narrowband UVB was discontinued completely. A comparison of the patient’s flow cytometry analysis from before treatment to 3 months after dupilumab showed an overall slight reduction in CTCL B1 blood involvement and normalization of the patient’s absolute eosinophil count and serum lactate dehydrogenase level. The patient tolerated the treatment well without any adverse events and has maintained clinical response for 6 months.
Comment
Cutaneous T-cell lymphomas represent a heterogeneous group of T-cell lymphoproliferative disorders involving the skin.2 The definitive diagnosis of CTCL is challenging, as the clinical and pathologic features often are nonspecific in early disease. Frequently, undiagnosed patients are treated empirically with immunosuppressive agents. Tumor necrosis factor inhibitors and cyclosporine are both associated with progression or worsening of undiagnosed CTCL.3,4 Dupilumab was the first US Food and Drug Administration–approved biologic for the treatment of moderate to severe AD. Cutaneous T-cell lymphoma has immunologic features, such as TH2 skewing, that overlap with AD; however, the effects of dupilumab in CTCL are not yet known.5,6 Our group has seen patients initially thought to have AD who received dupilumab without improvement and were subsequently diagnosed with CTCL, suggesting dupilumab did not affect CTCL tumor cells. Given these findings, there was concern that dupilumab might exacerbate undiagnosed CTCL. Our patient with definitive, severe, refractory CTCL noted marked improvement in both AD and underlying CTCL with the addition of dupilumab. No other treatments were added. The response was so dramatic that we were able to wean the doses and frequencies of several CTCL treatments. Our findings suggest that dupilumab may be beneficial in a certain subset of CTCL patients with a history of AD or known concomitant AD. Prospective studies are needed to fully investigate dupilumab safety and efficacy in CTCL and whether it has any primary effects on tumor burden in addition to benefit for itch and skin symptom relief.
- Guenova E, Watanabe R, Teague JE, et al. TH2 cytokines from malignant cells suppress TH1 responses and enforce a global TH2 bias in leukemic cutaneous T-cell lymphoma. Clin Cancer Res. 2013;19:3755-3763.
- Wilcox RA. Cutaneous T-cell lymphoma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91:151-165.
- Martinez-Escala ME, Posligua AL, Wickless H, et al. Progression of undiagnosed cutaneous lymphoma after anti-tumor necrosis factor-alpha therapy. J Am Acad Dermatol. 2018;78:1068-1076.
- Pielop JA, Jones D, Duvic M. Transient CD30+ nodal transformation of cutaneous T-cell lymphoma associated with cyclosporine treatment. Int J Dermatol. 2001;40:505-511.
- Saulite I, Hoetzenecker W, Weidinger S, et al. Sézary syndrome and atopic dermatitis: comparison of immunological aspects and targets [published online May 17, 2016]. BioMed Res Int. doi:10.1155/2016/9717530.
- Sigurdsson V, Toonstra J, Bihari IC, et al. Interleukin 4 and interferon-gamma expression of the dermal infiltrate in patients with erythroderma and mycosis fungoides. an immuno-histochemical study. J Cutan Pathol. 2000;27:429-435.
- Guenova E, Watanabe R, Teague JE, et al. TH2 cytokines from malignant cells suppress TH1 responses and enforce a global TH2 bias in leukemic cutaneous T-cell lymphoma. Clin Cancer Res. 2013;19:3755-3763.
- Wilcox RA. Cutaneous T-cell lymphoma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91:151-165.
- Martinez-Escala ME, Posligua AL, Wickless H, et al. Progression of undiagnosed cutaneous lymphoma after anti-tumor necrosis factor-alpha therapy. J Am Acad Dermatol. 2018;78:1068-1076.
- Pielop JA, Jones D, Duvic M. Transient CD30+ nodal transformation of cutaneous T-cell lymphoma associated with cyclosporine treatment. Int J Dermatol. 2001;40:505-511.
- Saulite I, Hoetzenecker W, Weidinger S, et al. Sézary syndrome and atopic dermatitis: comparison of immunological aspects and targets [published online May 17, 2016]. BioMed Res Int. doi:10.1155/2016/9717530.
- Sigurdsson V, Toonstra J, Bihari IC, et al. Interleukin 4 and interferon-gamma expression of the dermal infiltrate in patients with erythroderma and mycosis fungoides. an immuno-histochemical study. J Cutan Pathol. 2000;27:429-435.
Practice Points
- The diagnosis of cutaneous T-cell lymphoma (CTCL), particularly early-stage disease, remains challenging and often requires a combination of serial clinical evaluations as well as laboratory diagnostic examinations.
- Dupilumab and its effect on helper T cell (TH2) skewing may play a role in the future management of CTCL.
Smoking increases risk of high plasma NfL levels in patients with MS
A new study has found that At the same time, patients who have stopped smoking have notably lower risk that correlates to how long ago they quit.
“Before, all the studies that were looking at the association between smoking and MS – especially in terms of severity – were using indications like the Expanded Disability Status Scale and the Multiple Sclerosis Severity Score,” said first author Ali Manouchehrinia, PhD, assistant professor at the Karolinska Institute, Stockholm. “Now, we have NfL as a biomarker for disease activity, and we can see the effect of smoking on that biomarker.”
The ultimate goal, he added, “is to tease out the effects of MS severity and disease activity from NfL, to make sure that changes or differences in NfL levels are truly caused by MS and nothing else.”
Dr. Manouchehrinia presented his team’s findings at the virtual annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
To determine any associations between smoking and pNfL levels, the researchers began a retrospective, population-based cohort study of 2,572 Swedish MS patients with a self-reported history of cigarette smoking. Their average age was 38.2 years, slightly more than 71% were women, and their average disease duration was 4.13 years.
Blood samples were collected at each patients’ time of diagnosis to analyze concentrations of pNfL. Three age-stratified pNfL levels were classified: above the 80th (>C80), 95th (>C95), and 99th (>C99) of 1,026 non-MS controls’ percentiles.
Of the 2,572 MS patients, 292 (11.4%) were current regular smokers and 714 (27.8%) were past regular smokers. The past smokers’ median time since quitting was 2 years. Being a current smoker was associated with much higher odds of having pNfL levels at >C99, compared with never smokers (odds ratio, 1.52; 95% confidence interval, 1.12-2.05; P = .007) and past smokers (OR, 1.42; 95% CI, 1.01-1.99; P = .043).
For past smokers who quit between 6 and 10 years ago, the risk of having pNfL levels at >C99 was considerably lower (OR, 0.53; 95% CI, 0.29-0.93; P = .032), compared with current smokers, as was the risk for past smokers who quit more than 10 years ago (OR, 0.50; 95% CI, 0.29-0.84; P = .010). The odds were also lower, though not significantly, for patients who quit 1-5 years ago (OR, 0.84; 95% CI, 0.58-1.22; P = .359).
“It looks like, after 10 years, you go back to the baseline and have the same risk as the never smokers,” Dr. Manouchehrinia said. “But the damage may have already been done. Quitting smoking is good, but it’s better to not smoke at all.”
Dr. Manouchehrinia acknowledged the study’s limitations, including the need to learn more about the role NfL levels – especially plasma NfL levels – play across MS patients, along with the complications surrounding smoking as an environmental factor. He noted that, in Sweden, many people get their nicotine from snuff rather than cigarettes. “Among our MS population, we’ve seen a recent shift toward female snuff users,” which lessens the amount they smoke and could confound the results. In fact, the study indicated that snuff users had less risk of pNfL levels at >C95, compared with nonsnuff users (OR, 0.71; 95% CI, 0.51-0.97; P = .034).
The authors reported several potential conflicts of interest, including receiving research grants and lecture honoraria and serving on advisory boards for various pharmaceutical companies.
A new study has found that At the same time, patients who have stopped smoking have notably lower risk that correlates to how long ago they quit.
“Before, all the studies that were looking at the association between smoking and MS – especially in terms of severity – were using indications like the Expanded Disability Status Scale and the Multiple Sclerosis Severity Score,” said first author Ali Manouchehrinia, PhD, assistant professor at the Karolinska Institute, Stockholm. “Now, we have NfL as a biomarker for disease activity, and we can see the effect of smoking on that biomarker.”
The ultimate goal, he added, “is to tease out the effects of MS severity and disease activity from NfL, to make sure that changes or differences in NfL levels are truly caused by MS and nothing else.”
Dr. Manouchehrinia presented his team’s findings at the virtual annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
To determine any associations between smoking and pNfL levels, the researchers began a retrospective, population-based cohort study of 2,572 Swedish MS patients with a self-reported history of cigarette smoking. Their average age was 38.2 years, slightly more than 71% were women, and their average disease duration was 4.13 years.
Blood samples were collected at each patients’ time of diagnosis to analyze concentrations of pNfL. Three age-stratified pNfL levels were classified: above the 80th (>C80), 95th (>C95), and 99th (>C99) of 1,026 non-MS controls’ percentiles.
Of the 2,572 MS patients, 292 (11.4%) were current regular smokers and 714 (27.8%) were past regular smokers. The past smokers’ median time since quitting was 2 years. Being a current smoker was associated with much higher odds of having pNfL levels at >C99, compared with never smokers (odds ratio, 1.52; 95% confidence interval, 1.12-2.05; P = .007) and past smokers (OR, 1.42; 95% CI, 1.01-1.99; P = .043).
For past smokers who quit between 6 and 10 years ago, the risk of having pNfL levels at >C99 was considerably lower (OR, 0.53; 95% CI, 0.29-0.93; P = .032), compared with current smokers, as was the risk for past smokers who quit more than 10 years ago (OR, 0.50; 95% CI, 0.29-0.84; P = .010). The odds were also lower, though not significantly, for patients who quit 1-5 years ago (OR, 0.84; 95% CI, 0.58-1.22; P = .359).
“It looks like, after 10 years, you go back to the baseline and have the same risk as the never smokers,” Dr. Manouchehrinia said. “But the damage may have already been done. Quitting smoking is good, but it’s better to not smoke at all.”
Dr. Manouchehrinia acknowledged the study’s limitations, including the need to learn more about the role NfL levels – especially plasma NfL levels – play across MS patients, along with the complications surrounding smoking as an environmental factor. He noted that, in Sweden, many people get their nicotine from snuff rather than cigarettes. “Among our MS population, we’ve seen a recent shift toward female snuff users,” which lessens the amount they smoke and could confound the results. In fact, the study indicated that snuff users had less risk of pNfL levels at >C95, compared with nonsnuff users (OR, 0.71; 95% CI, 0.51-0.97; P = .034).
The authors reported several potential conflicts of interest, including receiving research grants and lecture honoraria and serving on advisory boards for various pharmaceutical companies.
A new study has found that At the same time, patients who have stopped smoking have notably lower risk that correlates to how long ago they quit.
“Before, all the studies that were looking at the association between smoking and MS – especially in terms of severity – were using indications like the Expanded Disability Status Scale and the Multiple Sclerosis Severity Score,” said first author Ali Manouchehrinia, PhD, assistant professor at the Karolinska Institute, Stockholm. “Now, we have NfL as a biomarker for disease activity, and we can see the effect of smoking on that biomarker.”
The ultimate goal, he added, “is to tease out the effects of MS severity and disease activity from NfL, to make sure that changes or differences in NfL levels are truly caused by MS and nothing else.”
Dr. Manouchehrinia presented his team’s findings at the virtual annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
To determine any associations between smoking and pNfL levels, the researchers began a retrospective, population-based cohort study of 2,572 Swedish MS patients with a self-reported history of cigarette smoking. Their average age was 38.2 years, slightly more than 71% were women, and their average disease duration was 4.13 years.
Blood samples were collected at each patients’ time of diagnosis to analyze concentrations of pNfL. Three age-stratified pNfL levels were classified: above the 80th (>C80), 95th (>C95), and 99th (>C99) of 1,026 non-MS controls’ percentiles.
Of the 2,572 MS patients, 292 (11.4%) were current regular smokers and 714 (27.8%) were past regular smokers. The past smokers’ median time since quitting was 2 years. Being a current smoker was associated with much higher odds of having pNfL levels at >C99, compared with never smokers (odds ratio, 1.52; 95% confidence interval, 1.12-2.05; P = .007) and past smokers (OR, 1.42; 95% CI, 1.01-1.99; P = .043).
For past smokers who quit between 6 and 10 years ago, the risk of having pNfL levels at >C99 was considerably lower (OR, 0.53; 95% CI, 0.29-0.93; P = .032), compared with current smokers, as was the risk for past smokers who quit more than 10 years ago (OR, 0.50; 95% CI, 0.29-0.84; P = .010). The odds were also lower, though not significantly, for patients who quit 1-5 years ago (OR, 0.84; 95% CI, 0.58-1.22; P = .359).
“It looks like, after 10 years, you go back to the baseline and have the same risk as the never smokers,” Dr. Manouchehrinia said. “But the damage may have already been done. Quitting smoking is good, but it’s better to not smoke at all.”
Dr. Manouchehrinia acknowledged the study’s limitations, including the need to learn more about the role NfL levels – especially plasma NfL levels – play across MS patients, along with the complications surrounding smoking as an environmental factor. He noted that, in Sweden, many people get their nicotine from snuff rather than cigarettes. “Among our MS population, we’ve seen a recent shift toward female snuff users,” which lessens the amount they smoke and could confound the results. In fact, the study indicated that snuff users had less risk of pNfL levels at >C95, compared with nonsnuff users (OR, 0.71; 95% CI, 0.51-0.97; P = .034).
The authors reported several potential conflicts of interest, including receiving research grants and lecture honoraria and serving on advisory boards for various pharmaceutical companies.
FROM ECTRIMS 2020
Social distancing impacts other infectious diseases
Diagnoses of 12 common pediatric infectious diseases in a large pediatric primary care network declined significantly in the weeks after COVID-19 social distancing (SD) was enacted in Massachusetts, compared with the same time period in 2019, an analysis of EHR data has shown.
While declines in infectious disease transmission with SD are not surprising, “these data demonstrate the extent to which transmission of common pediatric infections can be altered when close contact with other children is eliminated,” Jonathan Hatoun, MD, MPH of the Pediatric Physicians’ Organization at Children’s in Brookline, Mass., and coauthors wrote in Pediatrics . “Notably, three of the studied diseases, namely, influenza, croup, and bronchiolitis, essentially disappeared with [social distancing].”
The researchers analyzed the weekly incidence of each diagnosis for similar calendar periods in 2019 and 2020. A pre-SD period was defined as week 1-9, starting on Jan. 1, and a post-SD period was defined as week 13-18. (The several-week gap represented an implementation period as social distancing was enacted in the state earlier in 2020, from a declared statewide state of emergency through school closures and stay-at-home advisories.)
To isolate the effect of widespread SD, they performed a “difference-in-differences regression analysis, with diagnosis count as a function of calendar year, time period (pre-SD versus post-SD) and the interaction between the two.” The Massachusetts pediatric network provides care for approximately 375,000 children in 100 locations around the state.
In their research brief, Dr. Hatoun and coauthors presented weekly rates expressed as diagnoses per 100,000 patients per day. The rate of bronchiolitis, for instance, was 18 and 8 in the pre- and post-SD–equivalent weeks of 2019, respectively, and 20 and 0.6 in the pre- and post-SD weeks of 2020. Their analysis showed the rate in the 2020 post-SD period to be 10 diagnoses per 100,000 patients per day lower than they would have expected based on the 2019 trend.
Rates of pneumonia, acute otitis media, and streptococcal pharyngitis were similarly 14, 85, and 31 diagnoses per 100,000 patients per day lower, respectively. The prevalence of each of the other conditions analyzed – the common cold, croup, gastroenteritis, nonstreptococcal pharyngitis, sinusitis, skin and soft tissue infections, and urinary tract infection (UTI) – also was significantly lower in the 2020 post-SD period than would be expected based on 2019 data (P < .001 for all diagnoses).
Putting things in perspective
“This study puts numbers to the sense that we have all had in pediatrics – that social distancing appears to have had a dramatic impact on the transmission of common childhood infectious diseases, especially other respiratory viral pathogens,” Audrey R. John, MD, PhD, chief of the division of pediatric infectious disease at Children’s Hospital of Philadelphia, said in an interview.
The authors acknowledged the possible role of families not seeking care, but said that a smaller decrease in diagnoses of UTI – generally not a contagious disease – “suggests that changes in care-seeking behavior had a relatively modest effect on the other observed declines.” (The rate of UTI for the pre- and post-SD periods was 3.3 and 3.7 per 100,000 patients per day in 2019, and 3.4 and 2.4 in 2020, for a difference in differences of –1.5).
In an accompanying editorial, David W. Kimberlin, MD and Erica C. Bjornstad, MD, PhD, MPH, of the University of Alabama at Birmingham, called the report “provocative” and wrote that similar observations of infections dropping during periods of isolation – namely, dramatic declines in influenza and other respiratory viruses in Seattle after a record snowstorm in 2019 – combined with findings from other modeling studies “suggest that the decline [reported in Boston] is indeed real” (Pediatrics 2020. doi: 10.1542/peds.2020-019232).
However, “we also now know that immunization rates for American children have plummeted since the onset of the SARS-CoV-2 pandemic [because of a] ... dramatic decrease in the use of health care during the first months of the pandemic,” they wrote. “Viewed through this lens,” the declines reported in Boston may reflect inflections going “undiagnosed and untreated.”
Ultimately, Dr. Kimberlin and Dr. Bjornstad said, “the verdict remains out.”
Dr. John said that she and others are “concerned about children not seeking care in a timely manner, and [concerned] that reductions in reported infections might be due to a lack of recognition rather than a lack of transmission.”
In Philadelphia, however, declines in admissions for asthma exacerbations, “which are often caused by respiratory viral infections, suggests that this may not be the case,” said Dr. John, who was asked to comment on the study.
In addition, she said, the Massachusetts data showing that UTI diagnoses “are nearly as common this year as in 2019” are “reassuring.”
Are there lessons for the future?
Coauthor Louis Vernacchio, MD, MSc, chief medical officer of the Pediatric Physicians’ Organization at Children’s network, said in an interview that beyond the pandemic, it’s likely that “more careful attention to proven infection control practices in daycares and schools could reduce the burden of common infectious diseases in children.”
Dr. John similarly sees a long-term value of quantifying the impact of social distancing. “We’ve always known [for instance] that bronchiolitis is the result of viral infection.” Findings like the Massachusetts data “will help us advise families who might be trying to protect their premature infants (at risk for severe bronchiolitis) through social distancing.”
The analysis covered both in-person and telemedicine encounters occurring on weekdays.
The authors of the research brief indicated they have no relevant financial disclosures and there was no external funding. The authors of the commentary also reported they have no relevant financial disclosures, and Dr. John said she had no relevant financial disclosures.
SOURCE: Hatoun J et al. Pediatrics. 2020. doi: 10.1542/peds.2020-006460.
Diagnoses of 12 common pediatric infectious diseases in a large pediatric primary care network declined significantly in the weeks after COVID-19 social distancing (SD) was enacted in Massachusetts, compared with the same time period in 2019, an analysis of EHR data has shown.
While declines in infectious disease transmission with SD are not surprising, “these data demonstrate the extent to which transmission of common pediatric infections can be altered when close contact with other children is eliminated,” Jonathan Hatoun, MD, MPH of the Pediatric Physicians’ Organization at Children’s in Brookline, Mass., and coauthors wrote in Pediatrics . “Notably, three of the studied diseases, namely, influenza, croup, and bronchiolitis, essentially disappeared with [social distancing].”
The researchers analyzed the weekly incidence of each diagnosis for similar calendar periods in 2019 and 2020. A pre-SD period was defined as week 1-9, starting on Jan. 1, and a post-SD period was defined as week 13-18. (The several-week gap represented an implementation period as social distancing was enacted in the state earlier in 2020, from a declared statewide state of emergency through school closures and stay-at-home advisories.)
To isolate the effect of widespread SD, they performed a “difference-in-differences regression analysis, with diagnosis count as a function of calendar year, time period (pre-SD versus post-SD) and the interaction between the two.” The Massachusetts pediatric network provides care for approximately 375,000 children in 100 locations around the state.
In their research brief, Dr. Hatoun and coauthors presented weekly rates expressed as diagnoses per 100,000 patients per day. The rate of bronchiolitis, for instance, was 18 and 8 in the pre- and post-SD–equivalent weeks of 2019, respectively, and 20 and 0.6 in the pre- and post-SD weeks of 2020. Their analysis showed the rate in the 2020 post-SD period to be 10 diagnoses per 100,000 patients per day lower than they would have expected based on the 2019 trend.
Rates of pneumonia, acute otitis media, and streptococcal pharyngitis were similarly 14, 85, and 31 diagnoses per 100,000 patients per day lower, respectively. The prevalence of each of the other conditions analyzed – the common cold, croup, gastroenteritis, nonstreptococcal pharyngitis, sinusitis, skin and soft tissue infections, and urinary tract infection (UTI) – also was significantly lower in the 2020 post-SD period than would be expected based on 2019 data (P < .001 for all diagnoses).
Putting things in perspective
“This study puts numbers to the sense that we have all had in pediatrics – that social distancing appears to have had a dramatic impact on the transmission of common childhood infectious diseases, especially other respiratory viral pathogens,” Audrey R. John, MD, PhD, chief of the division of pediatric infectious disease at Children’s Hospital of Philadelphia, said in an interview.
The authors acknowledged the possible role of families not seeking care, but said that a smaller decrease in diagnoses of UTI – generally not a contagious disease – “suggests that changes in care-seeking behavior had a relatively modest effect on the other observed declines.” (The rate of UTI for the pre- and post-SD periods was 3.3 and 3.7 per 100,000 patients per day in 2019, and 3.4 and 2.4 in 2020, for a difference in differences of –1.5).
In an accompanying editorial, David W. Kimberlin, MD and Erica C. Bjornstad, MD, PhD, MPH, of the University of Alabama at Birmingham, called the report “provocative” and wrote that similar observations of infections dropping during periods of isolation – namely, dramatic declines in influenza and other respiratory viruses in Seattle after a record snowstorm in 2019 – combined with findings from other modeling studies “suggest that the decline [reported in Boston] is indeed real” (Pediatrics 2020. doi: 10.1542/peds.2020-019232).
However, “we also now know that immunization rates for American children have plummeted since the onset of the SARS-CoV-2 pandemic [because of a] ... dramatic decrease in the use of health care during the first months of the pandemic,” they wrote. “Viewed through this lens,” the declines reported in Boston may reflect inflections going “undiagnosed and untreated.”
Ultimately, Dr. Kimberlin and Dr. Bjornstad said, “the verdict remains out.”
Dr. John said that she and others are “concerned about children not seeking care in a timely manner, and [concerned] that reductions in reported infections might be due to a lack of recognition rather than a lack of transmission.”
In Philadelphia, however, declines in admissions for asthma exacerbations, “which are often caused by respiratory viral infections, suggests that this may not be the case,” said Dr. John, who was asked to comment on the study.
In addition, she said, the Massachusetts data showing that UTI diagnoses “are nearly as common this year as in 2019” are “reassuring.”
Are there lessons for the future?
Coauthor Louis Vernacchio, MD, MSc, chief medical officer of the Pediatric Physicians’ Organization at Children’s network, said in an interview that beyond the pandemic, it’s likely that “more careful attention to proven infection control practices in daycares and schools could reduce the burden of common infectious diseases in children.”
Dr. John similarly sees a long-term value of quantifying the impact of social distancing. “We’ve always known [for instance] that bronchiolitis is the result of viral infection.” Findings like the Massachusetts data “will help us advise families who might be trying to protect their premature infants (at risk for severe bronchiolitis) through social distancing.”
The analysis covered both in-person and telemedicine encounters occurring on weekdays.
The authors of the research brief indicated they have no relevant financial disclosures and there was no external funding. The authors of the commentary also reported they have no relevant financial disclosures, and Dr. John said she had no relevant financial disclosures.
SOURCE: Hatoun J et al. Pediatrics. 2020. doi: 10.1542/peds.2020-006460.
Diagnoses of 12 common pediatric infectious diseases in a large pediatric primary care network declined significantly in the weeks after COVID-19 social distancing (SD) was enacted in Massachusetts, compared with the same time period in 2019, an analysis of EHR data has shown.
While declines in infectious disease transmission with SD are not surprising, “these data demonstrate the extent to which transmission of common pediatric infections can be altered when close contact with other children is eliminated,” Jonathan Hatoun, MD, MPH of the Pediatric Physicians’ Organization at Children’s in Brookline, Mass., and coauthors wrote in Pediatrics . “Notably, three of the studied diseases, namely, influenza, croup, and bronchiolitis, essentially disappeared with [social distancing].”
The researchers analyzed the weekly incidence of each diagnosis for similar calendar periods in 2019 and 2020. A pre-SD period was defined as week 1-9, starting on Jan. 1, and a post-SD period was defined as week 13-18. (The several-week gap represented an implementation period as social distancing was enacted in the state earlier in 2020, from a declared statewide state of emergency through school closures and stay-at-home advisories.)
To isolate the effect of widespread SD, they performed a “difference-in-differences regression analysis, with diagnosis count as a function of calendar year, time period (pre-SD versus post-SD) and the interaction between the two.” The Massachusetts pediatric network provides care for approximately 375,000 children in 100 locations around the state.
In their research brief, Dr. Hatoun and coauthors presented weekly rates expressed as diagnoses per 100,000 patients per day. The rate of bronchiolitis, for instance, was 18 and 8 in the pre- and post-SD–equivalent weeks of 2019, respectively, and 20 and 0.6 in the pre- and post-SD weeks of 2020. Their analysis showed the rate in the 2020 post-SD period to be 10 diagnoses per 100,000 patients per day lower than they would have expected based on the 2019 trend.
Rates of pneumonia, acute otitis media, and streptococcal pharyngitis were similarly 14, 85, and 31 diagnoses per 100,000 patients per day lower, respectively. The prevalence of each of the other conditions analyzed – the common cold, croup, gastroenteritis, nonstreptococcal pharyngitis, sinusitis, skin and soft tissue infections, and urinary tract infection (UTI) – also was significantly lower in the 2020 post-SD period than would be expected based on 2019 data (P < .001 for all diagnoses).
Putting things in perspective
“This study puts numbers to the sense that we have all had in pediatrics – that social distancing appears to have had a dramatic impact on the transmission of common childhood infectious diseases, especially other respiratory viral pathogens,” Audrey R. John, MD, PhD, chief of the division of pediatric infectious disease at Children’s Hospital of Philadelphia, said in an interview.
The authors acknowledged the possible role of families not seeking care, but said that a smaller decrease in diagnoses of UTI – generally not a contagious disease – “suggests that changes in care-seeking behavior had a relatively modest effect on the other observed declines.” (The rate of UTI for the pre- and post-SD periods was 3.3 and 3.7 per 100,000 patients per day in 2019, and 3.4 and 2.4 in 2020, for a difference in differences of –1.5).
In an accompanying editorial, David W. Kimberlin, MD and Erica C. Bjornstad, MD, PhD, MPH, of the University of Alabama at Birmingham, called the report “provocative” and wrote that similar observations of infections dropping during periods of isolation – namely, dramatic declines in influenza and other respiratory viruses in Seattle after a record snowstorm in 2019 – combined with findings from other modeling studies “suggest that the decline [reported in Boston] is indeed real” (Pediatrics 2020. doi: 10.1542/peds.2020-019232).
However, “we also now know that immunization rates for American children have plummeted since the onset of the SARS-CoV-2 pandemic [because of a] ... dramatic decrease in the use of health care during the first months of the pandemic,” they wrote. “Viewed through this lens,” the declines reported in Boston may reflect inflections going “undiagnosed and untreated.”
Ultimately, Dr. Kimberlin and Dr. Bjornstad said, “the verdict remains out.”
Dr. John said that she and others are “concerned about children not seeking care in a timely manner, and [concerned] that reductions in reported infections might be due to a lack of recognition rather than a lack of transmission.”
In Philadelphia, however, declines in admissions for asthma exacerbations, “which are often caused by respiratory viral infections, suggests that this may not be the case,” said Dr. John, who was asked to comment on the study.
In addition, she said, the Massachusetts data showing that UTI diagnoses “are nearly as common this year as in 2019” are “reassuring.”
Are there lessons for the future?
Coauthor Louis Vernacchio, MD, MSc, chief medical officer of the Pediatric Physicians’ Organization at Children’s network, said in an interview that beyond the pandemic, it’s likely that “more careful attention to proven infection control practices in daycares and schools could reduce the burden of common infectious diseases in children.”
Dr. John similarly sees a long-term value of quantifying the impact of social distancing. “We’ve always known [for instance] that bronchiolitis is the result of viral infection.” Findings like the Massachusetts data “will help us advise families who might be trying to protect their premature infants (at risk for severe bronchiolitis) through social distancing.”
The analysis covered both in-person and telemedicine encounters occurring on weekdays.
The authors of the research brief indicated they have no relevant financial disclosures and there was no external funding. The authors of the commentary also reported they have no relevant financial disclosures, and Dr. John said she had no relevant financial disclosures.
SOURCE: Hatoun J et al. Pediatrics. 2020. doi: 10.1542/peds.2020-006460.
FROM PEDIATRICS
Obesity-related hypoventilation increased morbidity risk after bariatric surgery
Patients with obesity-associated sleep hypoventilation had a heightened risk of postoperative morbidities after bariatric surgery, according to a retrospective study.
Reena Mehra, MD, director of sleep disorders research for the Sleep Disorders Center at the Cleveland Clinic, led the team and the findings were presented at the virtual annual meeting of the Associated Professional Sleep Societies. Her research team examined the outcomes of 1,665 patients who underwent polysomnography prior to bariatric surgery performed at the Cleveland Clinic from 2011 to 2018.
More than two-thirds – 68.5% – had obesity-associated sleep hypoventilation as defined by body mass index (BMI) of ≥30 kg/m2 and either polysomnography-based end-tidal CO2 ≥45 mm Hg or serum bicarbonate ≥27 mEq/L.
These patients represent “a subset, if you will, of obesity hypoventilation syndrome – a subset that we were able to capture from our sleep studies … [because] we do CO2 monitoring during sleep studies uniformly,” Dr. Mehra said in an interview after the meeting.
Pornprapa Chindamporn, MD, a former fellow at the center and first author on the abstract, presented the findings. Patients in the study had a mean age of 45.2 ± 12.0 years and a BMI of 48.7 ± 9.0. Approximately 20% were male and 63.6% were White.
Those with obesity-associated sleep hypoventilation were more likely to be male and have a higher BMI and higher hemoglobin A1c than those without the condition. They also had a significantly higher apnea-hypopnea index (17.0 vs. 13.8) in those without the condition, she reported.
A number of outcomes (ICU stay, intubation, tracheostomy, discharge disposition, and 30-day readmission) were compared individually and as a composite outcome between those with and without obesity-associated sleep hypoventilation. While some of these postoperative morbidities were more common in patients with the condition, the differences between those with and without OHS were not statistically significant for intubation (1.5% vs. 1.3%, P = .81) and 30-day readmission (13.8% vs. 11.3%, P = .16). However, the composite outcome was significantly higher: 18.9% vs. 14.3% (P = .021), including in multivariable analysis that considered age, gender, BMI, Apnea Hypopnea Index, and diabetes.
All-cause mortality was not significantly different between the groups, likely because of its low overall rate (hazard ratio, 1.39; 95% confidence interval, 0.56-3.42).
“In this largest sample to date of systematically phenotyped obesity-associated sleep hypoventilation in patients undergoing bariatric surgery, we identified increased postoperative morbidity,” said Dr. Chindamporn, now a pulmonologist and sleep specialist practicing in Bangkok.
Dr. Mehra said in the interview that patients considering bariatric surgery are typically assessed for obstructive sleep apnea, but “not so much obesity hypoventilation syndrome or obesity-associated sleep-related hypoventilation syndrome.” The findings, “support the notion that we should be closely examining sleep-related hypoventilation in these patients.”
At the Cleveland Clinic, “clinically, we make sure we’re identifying these individuals and communicating the findings to bariatric surgery colleagues and to anesthesia,” said Dr. Mehra, also professor of medicine at Case Western Reserve University, Cleveland.
OHS is defined, according to the 2019 American Thoracic Society clinical practice guideline on evaluation and management of OHS, by the combination of obesity, sleep-disordered breathing, and awake daytime hypercapnia, after excluding other causes for hypoventilation (Am J Respir Crit Care Med. 2019;200[3]:e6-24).
A European Respiratory Society task force has proposed severity grading for OHS, with early stages defined by sleep-related hypoventilation and the highest grade of severity defined by morbidity-associated daytime hypercapnia (Eur Respir Rev. 2019;28:180097). However, Dr. Mehra said she is “not sure that we know enough [from long-term studies of OHS] to say definitively that there’s such an evolution.”
Certainly, she said, future research on OHS should consider its heterogeneity. It is possible that a subset of patients with OHS, “maybe these individuals with sleep-related hypoventilation,” are most likely to have adverse postsurgical outcomes.
Atul Malhotra, MD, professor of medicine at the University of California, San Diego, who was asked to comment on the study, said that OHS is understudied in general and particularly in the perioperative setting. “With the obesity pandemic, issues around OHS are likely to be [increasingly] important. And with increasing [use of] bariatric surgery, strategies to minimize risks are clearly needed,” he said, adding that the potential risks of nonbariatric surgery in patients with OHS require further study.
He noted that mortality rates in good hospitals “have become quite low for many elective surgeries, making it hard to show mortality benefit to most interventions.”
The ATS guideline on OHS states that it is the most severe form of obesity-induced respiratory compromise and leads to serious sequelae, including increased rates of mortality, chronic heart failure, pulmonary hypertension, and hospitalization caused by acute-on-chronic hypercapnic respiratory failure.
Dr. Chindamporn said in her presentation that she had no disclosures. Dr. Mehra’s research program is funded by the National Institute of Health, but she has also procured funding from the American College of Chest Physicians, American Heart Association, Clinical Translational Science Collaborative, and Central Society of Clinical Research. Dr. Malhotra disclosed that he is funded by the NIH and has received income from Merck and LIvanova related to medical education.
CORRECTION 9/15/2020: The original story misstated the presenter of the study. Dr. Chindamporn presented the findings.
Patients with obesity-associated sleep hypoventilation had a heightened risk of postoperative morbidities after bariatric surgery, according to a retrospective study.
Reena Mehra, MD, director of sleep disorders research for the Sleep Disorders Center at the Cleveland Clinic, led the team and the findings were presented at the virtual annual meeting of the Associated Professional Sleep Societies. Her research team examined the outcomes of 1,665 patients who underwent polysomnography prior to bariatric surgery performed at the Cleveland Clinic from 2011 to 2018.
More than two-thirds – 68.5% – had obesity-associated sleep hypoventilation as defined by body mass index (BMI) of ≥30 kg/m2 and either polysomnography-based end-tidal CO2 ≥45 mm Hg or serum bicarbonate ≥27 mEq/L.
These patients represent “a subset, if you will, of obesity hypoventilation syndrome – a subset that we were able to capture from our sleep studies … [because] we do CO2 monitoring during sleep studies uniformly,” Dr. Mehra said in an interview after the meeting.
Pornprapa Chindamporn, MD, a former fellow at the center and first author on the abstract, presented the findings. Patients in the study had a mean age of 45.2 ± 12.0 years and a BMI of 48.7 ± 9.0. Approximately 20% were male and 63.6% were White.
Those with obesity-associated sleep hypoventilation were more likely to be male and have a higher BMI and higher hemoglobin A1c than those without the condition. They also had a significantly higher apnea-hypopnea index (17.0 vs. 13.8) in those without the condition, she reported.
A number of outcomes (ICU stay, intubation, tracheostomy, discharge disposition, and 30-day readmission) were compared individually and as a composite outcome between those with and without obesity-associated sleep hypoventilation. While some of these postoperative morbidities were more common in patients with the condition, the differences between those with and without OHS were not statistically significant for intubation (1.5% vs. 1.3%, P = .81) and 30-day readmission (13.8% vs. 11.3%, P = .16). However, the composite outcome was significantly higher: 18.9% vs. 14.3% (P = .021), including in multivariable analysis that considered age, gender, BMI, Apnea Hypopnea Index, and diabetes.
All-cause mortality was not significantly different between the groups, likely because of its low overall rate (hazard ratio, 1.39; 95% confidence interval, 0.56-3.42).
“In this largest sample to date of systematically phenotyped obesity-associated sleep hypoventilation in patients undergoing bariatric surgery, we identified increased postoperative morbidity,” said Dr. Chindamporn, now a pulmonologist and sleep specialist practicing in Bangkok.
Dr. Mehra said in the interview that patients considering bariatric surgery are typically assessed for obstructive sleep apnea, but “not so much obesity hypoventilation syndrome or obesity-associated sleep-related hypoventilation syndrome.” The findings, “support the notion that we should be closely examining sleep-related hypoventilation in these patients.”
At the Cleveland Clinic, “clinically, we make sure we’re identifying these individuals and communicating the findings to bariatric surgery colleagues and to anesthesia,” said Dr. Mehra, also professor of medicine at Case Western Reserve University, Cleveland.
OHS is defined, according to the 2019 American Thoracic Society clinical practice guideline on evaluation and management of OHS, by the combination of obesity, sleep-disordered breathing, and awake daytime hypercapnia, after excluding other causes for hypoventilation (Am J Respir Crit Care Med. 2019;200[3]:e6-24).
A European Respiratory Society task force has proposed severity grading for OHS, with early stages defined by sleep-related hypoventilation and the highest grade of severity defined by morbidity-associated daytime hypercapnia (Eur Respir Rev. 2019;28:180097). However, Dr. Mehra said she is “not sure that we know enough [from long-term studies of OHS] to say definitively that there’s such an evolution.”
Certainly, she said, future research on OHS should consider its heterogeneity. It is possible that a subset of patients with OHS, “maybe these individuals with sleep-related hypoventilation,” are most likely to have adverse postsurgical outcomes.
Atul Malhotra, MD, professor of medicine at the University of California, San Diego, who was asked to comment on the study, said that OHS is understudied in general and particularly in the perioperative setting. “With the obesity pandemic, issues around OHS are likely to be [increasingly] important. And with increasing [use of] bariatric surgery, strategies to minimize risks are clearly needed,” he said, adding that the potential risks of nonbariatric surgery in patients with OHS require further study.
He noted that mortality rates in good hospitals “have become quite low for many elective surgeries, making it hard to show mortality benefit to most interventions.”
The ATS guideline on OHS states that it is the most severe form of obesity-induced respiratory compromise and leads to serious sequelae, including increased rates of mortality, chronic heart failure, pulmonary hypertension, and hospitalization caused by acute-on-chronic hypercapnic respiratory failure.
Dr. Chindamporn said in her presentation that she had no disclosures. Dr. Mehra’s research program is funded by the National Institute of Health, but she has also procured funding from the American College of Chest Physicians, American Heart Association, Clinical Translational Science Collaborative, and Central Society of Clinical Research. Dr. Malhotra disclosed that he is funded by the NIH and has received income from Merck and LIvanova related to medical education.
CORRECTION 9/15/2020: The original story misstated the presenter of the study. Dr. Chindamporn presented the findings.
Patients with obesity-associated sleep hypoventilation had a heightened risk of postoperative morbidities after bariatric surgery, according to a retrospective study.
Reena Mehra, MD, director of sleep disorders research for the Sleep Disorders Center at the Cleveland Clinic, led the team and the findings were presented at the virtual annual meeting of the Associated Professional Sleep Societies. Her research team examined the outcomes of 1,665 patients who underwent polysomnography prior to bariatric surgery performed at the Cleveland Clinic from 2011 to 2018.
More than two-thirds – 68.5% – had obesity-associated sleep hypoventilation as defined by body mass index (BMI) of ≥30 kg/m2 and either polysomnography-based end-tidal CO2 ≥45 mm Hg or serum bicarbonate ≥27 mEq/L.
These patients represent “a subset, if you will, of obesity hypoventilation syndrome – a subset that we were able to capture from our sleep studies … [because] we do CO2 monitoring during sleep studies uniformly,” Dr. Mehra said in an interview after the meeting.
Pornprapa Chindamporn, MD, a former fellow at the center and first author on the abstract, presented the findings. Patients in the study had a mean age of 45.2 ± 12.0 years and a BMI of 48.7 ± 9.0. Approximately 20% were male and 63.6% were White.
Those with obesity-associated sleep hypoventilation were more likely to be male and have a higher BMI and higher hemoglobin A1c than those without the condition. They also had a significantly higher apnea-hypopnea index (17.0 vs. 13.8) in those without the condition, she reported.
A number of outcomes (ICU stay, intubation, tracheostomy, discharge disposition, and 30-day readmission) were compared individually and as a composite outcome between those with and without obesity-associated sleep hypoventilation. While some of these postoperative morbidities were more common in patients with the condition, the differences between those with and without OHS were not statistically significant for intubation (1.5% vs. 1.3%, P = .81) and 30-day readmission (13.8% vs. 11.3%, P = .16). However, the composite outcome was significantly higher: 18.9% vs. 14.3% (P = .021), including in multivariable analysis that considered age, gender, BMI, Apnea Hypopnea Index, and diabetes.
All-cause mortality was not significantly different between the groups, likely because of its low overall rate (hazard ratio, 1.39; 95% confidence interval, 0.56-3.42).
“In this largest sample to date of systematically phenotyped obesity-associated sleep hypoventilation in patients undergoing bariatric surgery, we identified increased postoperative morbidity,” said Dr. Chindamporn, now a pulmonologist and sleep specialist practicing in Bangkok.
Dr. Mehra said in the interview that patients considering bariatric surgery are typically assessed for obstructive sleep apnea, but “not so much obesity hypoventilation syndrome or obesity-associated sleep-related hypoventilation syndrome.” The findings, “support the notion that we should be closely examining sleep-related hypoventilation in these patients.”
At the Cleveland Clinic, “clinically, we make sure we’re identifying these individuals and communicating the findings to bariatric surgery colleagues and to anesthesia,” said Dr. Mehra, also professor of medicine at Case Western Reserve University, Cleveland.
OHS is defined, according to the 2019 American Thoracic Society clinical practice guideline on evaluation and management of OHS, by the combination of obesity, sleep-disordered breathing, and awake daytime hypercapnia, after excluding other causes for hypoventilation (Am J Respir Crit Care Med. 2019;200[3]:e6-24).
A European Respiratory Society task force has proposed severity grading for OHS, with early stages defined by sleep-related hypoventilation and the highest grade of severity defined by morbidity-associated daytime hypercapnia (Eur Respir Rev. 2019;28:180097). However, Dr. Mehra said she is “not sure that we know enough [from long-term studies of OHS] to say definitively that there’s such an evolution.”
Certainly, she said, future research on OHS should consider its heterogeneity. It is possible that a subset of patients with OHS, “maybe these individuals with sleep-related hypoventilation,” are most likely to have adverse postsurgical outcomes.
Atul Malhotra, MD, professor of medicine at the University of California, San Diego, who was asked to comment on the study, said that OHS is understudied in general and particularly in the perioperative setting. “With the obesity pandemic, issues around OHS are likely to be [increasingly] important. And with increasing [use of] bariatric surgery, strategies to minimize risks are clearly needed,” he said, adding that the potential risks of nonbariatric surgery in patients with OHS require further study.
He noted that mortality rates in good hospitals “have become quite low for many elective surgeries, making it hard to show mortality benefit to most interventions.”
The ATS guideline on OHS states that it is the most severe form of obesity-induced respiratory compromise and leads to serious sequelae, including increased rates of mortality, chronic heart failure, pulmonary hypertension, and hospitalization caused by acute-on-chronic hypercapnic respiratory failure.
Dr. Chindamporn said in her presentation that she had no disclosures. Dr. Mehra’s research program is funded by the National Institute of Health, but she has also procured funding from the American College of Chest Physicians, American Heart Association, Clinical Translational Science Collaborative, and Central Society of Clinical Research. Dr. Malhotra disclosed that he is funded by the NIH and has received income from Merck and LIvanova related to medical education.
CORRECTION 9/15/2020: The original story misstated the presenter of the study. Dr. Chindamporn presented the findings.
FROM SLEEP 2020
Transvaginal reconstructive surgery for POP: Innovative approach to graft augmentation in the post-mesh era
Pelvic organ prolapse (POP) is a common occurrence over the course of a woman’s lifetime, especially in parous women (up to 50% of women who have given birth).1 The anterior vaginal wall is the most common site of POP and has the highest recurrence rate of up to 70%.2 The risk of developing POP increases with age, obesity, White race, family history, and prior pelvic surgery, such as hysterectomy. It affects more than 3 million women in the United States alone, often negatively impacting sexual function and overall quality of life.3,4
Because women are living longer than ever before and are more active in their senior years, a long-lasting, durable surgical repair is desirable, if not necessary. To be cost-effective and to avoid general anesthesia, the surgical approach ideally should be vaginal.
Biologic and synthetic grafts to augment transvaginal repair traditionally are used to improve on the well-recognized high failure rate of native-tissue repair that is often seen at both short-term and medium-term follow-up.5 The failure rate is commonly referenced as 30% to 40% at 2-year follow-up and 61% to 70% at 5-year follow-up, well-established by the results of the OPTIMAL randomized clinical trial.6 The more recent Descent trial likewise demonstrates a higher failure rate of native-tissue repair versus transvaginal mesh repair at a shorter term of 30 to 42 months.7 Furthermore, the use of permanent versus absorbable suture in suspension of the vaginal apex is associated with lower short-term failure rates.8
Despite this Level I evidence that demonstrates a clear advantage for obtaining a longer or more durable repair with permanent materials, native-tissue repairs with absorbable suture are still performed routinely. Since the US Food and Drug Administration (FDA) ordered that the use of transvaginal surgical mesh augmentation for pelvic reconstructive surgery be discontinued, it is more important than ever to explore evolving alternative native-tissue augmentation repair techniques that hopefully can preserve the advantages and merits of vaginal surgery and achieve longer durability.9
Biologic graft augmentation use in transvaginal reconstruction
All biologic grafts, including allografts derived from human tissue and xenografts derived from animal tissue, are acellular constructs composed of extracellular matrix (ECM) that acts as scaffolding for the host tissue. The ECM is predominantly composed of collagen (types I and III) and noncollagenous fibronectin, laminin, and glycosaminoglycans in various amounts depending on the source tissue. The 3D presentation of ECM’s complex molecules allows for rapid repopulation of host cells and revascularization with eventual regeneration.
Once a biologic graft is placed surgically, the body’s response to the scaffold ECM mimics the normal wound-healing process, beginning with fibrin-rich matrix hemostasis and the subsequent innate immune response of neutrophil and M1 macrophage infiltration. M1 macrophages are proinflammatory and clear cellular debris and begin the process of graft scaffold degradation. The host tissue then begins the process of remodeling through pro-remodeling M2 macrophages and stem cell recruitment, proliferation, and differentiation.10 As the biologic graft provides initial structure and strength for pelvic repairs, the ideal ECM scaffold would not degrade before the host is able to fully undergo regeneration and maintain its structure and strength.
Biologic grafts differ in source (allograft or xenograft), type (pericardium, dermis, or bladder), developmental stage (fetal or adult), decellularization processing, and sterilization techniques. These 5 aspects determine the distinct 3D ECM scaffold structure, strength, and longevity. If the ECM scaffold is damaged or retains noncollagenous proteins during the preparation process, an inflammatory response is triggered in which the graft is degraded, resorbed, and replaced with scar tissue. Furthermore, certain processing techniques aimed at extending the ECM’s durability—that is, cross-linking collagen—results in the foreign body response in which there is no vascular infiltration or cellular penetration of the graft and a collagen capsule is created around the empty matrix.11 To avoid resorption or encapsulation of the graft, the ECM scaffolds of biologic grafts must be optimized to induce regeneration.
Continue to: Choosing surgical POP repair...
Choosing surgical POP repair
The decision to undergo surgical treatment for prolapse is a shared decision-making process between the patient and surgeon and always should be individualized. The type of procedure and the surgical approach will depend on the patient’s goals, the degree of prolapse, clinical history, risk tolerance, the surgeon’s skill set, and whether or not there is an indication or relative contraindication for uterine removal at the time of prolapse repair.
While the FDA’s order does not apply to transabdominally placed surgical mesh, such as sacrocolpopexy, not all patients are ideal candidates for an abdominal sacrocolpopexy. Most notable are women with a history of multiple prior abdominal surgeries with higher rates of intraperitoneal adhesions. Ideally, to be cost-effective and to avoid general anesthesia, the surgical approach should be vaginal whenever possible.
Biologic versus native-tissue grafts
Currently, only low-quality evidence exists that compares the outcomes of biologic grafts with traditional native-tissue repairs in POP. Studies have been limited by poor reporting of methods, inconsistency in technique and materials used, and imprecise definitions. One Cochrane Review on the surgical management of POP concluded that biologic graft augmentation was associated with a lower failure rate (18%) within 1 to 2 years when compared with a traditional anterior colporrhaphy (28%).12
Based on consideration of all Cochrane Database Reviews and recent large systematic reviews, there clearly is a paucity of information on which to draw well-defined conclusions regarding the advantage of biomaterials in prolapse surgery.12-14 This is due in part to the variation in graft material used and the surgical technique employed.
Similarly, evidence is lacking regarding the superiority of one type of biologic graft over another. Furthermore, some of the grafts previously studied are no longer on the market.15 With the FDA’s removal of all transvaginal mesh, including xenografts, only allografts are available for pelvic floor reconstruction. Currently, only 3 commercial manufacturers market allografts for pelvic floor reconstruction. Each allograft is available in various sizes and all can be trimmed at the time of the surgical procedure to customize both the size and shape to fit the individual patient.
A novel technique using Axis Dermis and polypropylene suture
One of the commercially available allografts, Axis Dermis (Coloplast), is non–cross-linked and is derived from human cadaveric dermal tissue from the back and dorsum of the upper leg. It is sterilized by a proprietary Tutoplast️ sterilization process that uses gamma irradiation to inactivate and prevent the transmission of pathogens. This unique technique involving solvent dehydration means the graft is never freeze dried; thus, the natural tissue matrix is preserved.
Additionally, the allograft is antigen-free, which decreases the risk of tissue reaction (scarring/fibrosis) and aids in the process of host tissue remodeling; invasion by growth factors, blood cells, collagen, elastin, and neovascularization. This natural tissue remodeling facilitates the anticipated “reabsorption” of the graft by the host tissue, leaving the patient with a tissue scaffold, that is, a stronger layer of “fascia” beneath the muscularis.16 As a result of this “biocompatible” graft, the host tissue remodeling has been shown in the rat model to involve early cellular infiltration and angiogenesis (in the first week after implantation), that leads to an organized cellular architecture with greater tensile strength by week 4, and ultimately inability to distinguish host collagen from the implant by 8 to 12 weeks.17,18
Continue to: Steps in performing the technique...
Steps in performing the technique
To ensure that the graft is placed adjacent to the vaginal serosa, a full-thickness dissection is carried out to enter the true vesicovaginal space, which lies below all 4 histologic layers of the vagina (nonkeratinized stratified squamous epithelium, lamina propria, muscularis, and serosa). For the anterior dissection, a Tuohy epidural needle is used to achieve an accurate and consistent depth when injecting fluid (hydrodissection) to enter this true pelvic space (FIGURE 1). Correct entry into the vesicovaginal space can be confirmed visually by the presence of adipose tissue.
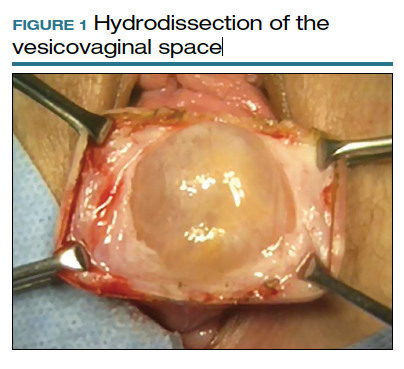
Many pelvic surgeons use the sacrospinous ligament (SSL) as a strong and reliable point of attachment for vaginal prolapse repair. It can be approached either anteriorly or posteriorly with careful dissection. Permanent suture (0-Prolene) is used to “bridge” the attachment between the SSL, the Axis Dermis graft, and the cervix (or vaginal apex). The suture is placed in the middle third and lower half of the ligament to avoid injury to nearby neurovascular structures.
While the surgeon may use any suture-capturing device, we prefer the Anchosure System (Neomedic). This device delivers a small anchor securely into the ligament through a single point of entry, minimizing the risk of postoperative pain for the patient. A 6 cm x 8 cm size Axis Dermis graft is then trimmed to meet the specifications of the patient’s anatomy.
Most commonly, we measure, mark, and trim the body of the graft to 5.5 cm in length with a width of 3 cm. The bilateral arms are approximately 1 cm in width and comprise the remaining length of the 8 cm graft (FIGURE 2). As shown in Figure 2, pre-made holes are marked and punched out using a large hollow needle. These serve as the points of attachment for the permanent suture to be “weaved” into the graft arms and delayed absorbable “tacking suture” to be attached from the pubocervical fascia at the bladder neck to the distal end of the graft. This facilitates fixation of the graft in the midline of the anterior vaginal wall, overlying any central distention-type defect.
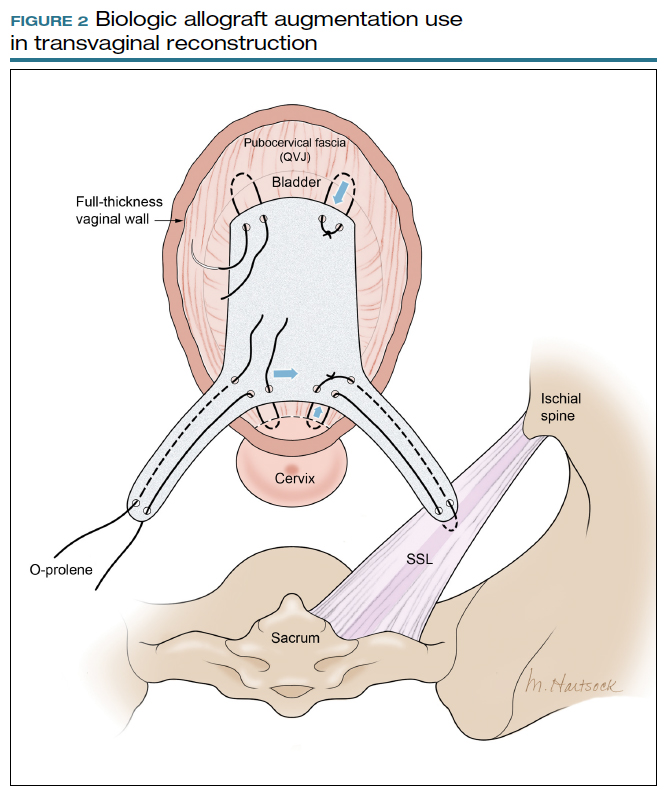
Finally, following attachment of the SSL permanent suture to the distal graft arm, this suture is then attached to the proximal U-shaped end of the graft body (in the midline), followed by a deep and secure bite through the cervix (or vaginal vault apex) and back through the proximal graft. These SSL suspension sutures are then tied such that the distal arms of the graft advance down to the ligament. Care is taken not to tie down to the SSL itself, rather until the cervix (or apex) is reduced to its normal anatomical location.
After the graft is secured in place, the full-thickness vaginal wall is closed with delayed absorbable suture. Sterile 1-inch ribbon packing is placed in the vagina immediately to close any dead space between the vagina and the graft to decrease the risk of seroma or hematoma formation.
This newly developed technique, like many surgeries for POP, requires extensive knowledge of pelvic anatomy and skill in vaginal surgery, and we recommend referral to a subspecialist in Female Pelvic Medicine and Reconstructive Surgery.

Continue to: Upcoming plans to share outcomes data...
Upcoming plans to share outcomes data
We are in the process of performing a retrospective review of all of the cases we have performed at our institution using this technique of permanent suture bridging to the SSL within the arm of the biograft. Given the relatively recent FDA announcement, we have yet to establish any long-term outcomes data. However, the preliminary results at 6-month follow-up are promising and demonstrate a low (2.6%) failure rate, without significant safety concerns. We hope to publish these data as well as more data on longitudinal outcomes in the future.
In summary
Many women are at risk for native-tissue repair failure or are not well suited for an abdominal procedure to correct their pelvic support defect and restore their quality of life. As expert pelvic surgeons, we play an important role in the search for innovative solutions for these women. There is ample opportunity for future research and clinical trials to determine the best biologic materials and their optimal use in pelvic reconstructive surgery.
Originally, polypropylene mesh was designed for use in augmenting abdominal hernia repairs and later was adapted by manufacturers for use in POP repair. The FDA removal from the market of existing transvaginal synthetic mesh kits was a unique catalyst that challenged our community to develop transvaginal repairs using biologic grafts that are genuinely tailored to the unique needs of the female pelvic anatomy. ●
- Maher C, Feiner B, Baessler K. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013:CD004014.
- Weber AM, Walters MD, Piedmonte MR, et al. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol. 2001;185:1299-1304.
- Walters MD, Ridgeway BM. Surgical treatment of vaginal apex prolapse. Obstet Gynecol. 2013;121(2 pt 1):354-374.
- Meister MRL, Sutcliffe S, Lowder JL. Definitions of apical vaginal support loss: a systematic review. Am J Obstet Gynecol. 2017;216:232. e1-232.e14.
- Cox A, Herschorn S. Evaluation of current biologic meshes in pelvic organ prolapse repair. Curr Urol Rep. 2012;13:247-255.
- Jelovsek JE, Barber M, Brubaker K, et al. Effect of uterosacral ligament suspension vs sacrospinous ligament fixation with or without perioperative behavioral therapy for pelvic organ vaginal prolapse on surgical outcomes and prolapse symptoms at 5 years in the OPTIMAL randomized clinical trial. JAMA. 2018:319:1554-1565.
- Bowen ST, Moalli P, Abramowitch S, et al. Outcomes of the defining mechanisms of anterior vaginal wall descent trial [abstract 15]. Am J Obstet Gynecol. 2020;222:S770-S771.
- Chung CP, Miskimins R, Kuehl TJ, et al. Permanent suture used in uterosacral ligament suspension offers better anatomical support than delayed absorbable suture. Int Urogynecol J. 2012;23:223-227.
- US Food and Drug Administration. FDA takes action to protect women’s health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. https://www.fda.gov/news-events/press-announcements/fda-takes-action-protect-womens-health-orders-manufacturers-surgical -mesh-intended-transvaginal. April 16, 2019. Accessed September 1, 2020.
- Londono R, Badylak SF. Biologic scaffolds for regenerative medicine: mechanisms of in vivo remodeling. Ann Biomed Eng. 2015;43:577-592.
- Cornwell KG, Landsman A, James KS. Extracellular matrix biomaterials for soft tissue repair. Clin Podiatr Med Surg. 2009;26: 507-523.
- Maher CM, Feiner B, Baessler K, et al. Surgical management of pelvic organ prolapse in women: the updated summary version Cochrane review. Int Urogynecol J. 2011;22:1445-1447.
- Maher C, Feiner B, Baessler K, et al. Surgery for women with anterior compartment prolapse. Cochrane Database Syst Rev. 2016;11:CD004014.
- Maher C, Feiner B, Baessler K, et al. Transvaginal mesh or grafts compared with native tissue repair for vaginal prolapse. Cochrane Database Syst Rev. 2016;2:CD012179.
- Rosenblatt P, Von Bargen E. Use of biologic grafts in pelvic organ prolapse surgery. Contemporary OB/GYN. 2017;62:14-19.
- Greenspan DC, Hernandez R, Faleris J. Histology of surgically implanted Tutoplast processed dermis. http://www.zimmerbiomet .co.il/images/lib_artHistologyDermis%2010.pdf. Accessed September 2, 2020.
- Williams D. Revisiting the definition of biocompatibility. Med Device Technol. 2003;14:10-13.
- Nosti PA, Carter CM, Sokol AI, et al. Transvaginal versus transabdominal placement of synthetic mesh at time of sacrocolpopexy. Female Pelvic Med Reconstr Surg. 2016;22:151-155.
Pelvic organ prolapse (POP) is a common occurrence over the course of a woman’s lifetime, especially in parous women (up to 50% of women who have given birth).1 The anterior vaginal wall is the most common site of POP and has the highest recurrence rate of up to 70%.2 The risk of developing POP increases with age, obesity, White race, family history, and prior pelvic surgery, such as hysterectomy. It affects more than 3 million women in the United States alone, often negatively impacting sexual function and overall quality of life.3,4
Because women are living longer than ever before and are more active in their senior years, a long-lasting, durable surgical repair is desirable, if not necessary. To be cost-effective and to avoid general anesthesia, the surgical approach ideally should be vaginal.
Biologic and synthetic grafts to augment transvaginal repair traditionally are used to improve on the well-recognized high failure rate of native-tissue repair that is often seen at both short-term and medium-term follow-up.5 The failure rate is commonly referenced as 30% to 40% at 2-year follow-up and 61% to 70% at 5-year follow-up, well-established by the results of the OPTIMAL randomized clinical trial.6 The more recent Descent trial likewise demonstrates a higher failure rate of native-tissue repair versus transvaginal mesh repair at a shorter term of 30 to 42 months.7 Furthermore, the use of permanent versus absorbable suture in suspension of the vaginal apex is associated with lower short-term failure rates.8
Despite this Level I evidence that demonstrates a clear advantage for obtaining a longer or more durable repair with permanent materials, native-tissue repairs with absorbable suture are still performed routinely. Since the US Food and Drug Administration (FDA) ordered that the use of transvaginal surgical mesh augmentation for pelvic reconstructive surgery be discontinued, it is more important than ever to explore evolving alternative native-tissue augmentation repair techniques that hopefully can preserve the advantages and merits of vaginal surgery and achieve longer durability.9
Biologic graft augmentation use in transvaginal reconstruction
All biologic grafts, including allografts derived from human tissue and xenografts derived from animal tissue, are acellular constructs composed of extracellular matrix (ECM) that acts as scaffolding for the host tissue. The ECM is predominantly composed of collagen (types I and III) and noncollagenous fibronectin, laminin, and glycosaminoglycans in various amounts depending on the source tissue. The 3D presentation of ECM’s complex molecules allows for rapid repopulation of host cells and revascularization with eventual regeneration.
Once a biologic graft is placed surgically, the body’s response to the scaffold ECM mimics the normal wound-healing process, beginning with fibrin-rich matrix hemostasis and the subsequent innate immune response of neutrophil and M1 macrophage infiltration. M1 macrophages are proinflammatory and clear cellular debris and begin the process of graft scaffold degradation. The host tissue then begins the process of remodeling through pro-remodeling M2 macrophages and stem cell recruitment, proliferation, and differentiation.10 As the biologic graft provides initial structure and strength for pelvic repairs, the ideal ECM scaffold would not degrade before the host is able to fully undergo regeneration and maintain its structure and strength.
Biologic grafts differ in source (allograft or xenograft), type (pericardium, dermis, or bladder), developmental stage (fetal or adult), decellularization processing, and sterilization techniques. These 5 aspects determine the distinct 3D ECM scaffold structure, strength, and longevity. If the ECM scaffold is damaged or retains noncollagenous proteins during the preparation process, an inflammatory response is triggered in which the graft is degraded, resorbed, and replaced with scar tissue. Furthermore, certain processing techniques aimed at extending the ECM’s durability—that is, cross-linking collagen—results in the foreign body response in which there is no vascular infiltration or cellular penetration of the graft and a collagen capsule is created around the empty matrix.11 To avoid resorption or encapsulation of the graft, the ECM scaffolds of biologic grafts must be optimized to induce regeneration.
Continue to: Choosing surgical POP repair...
Choosing surgical POP repair
The decision to undergo surgical treatment for prolapse is a shared decision-making process between the patient and surgeon and always should be individualized. The type of procedure and the surgical approach will depend on the patient’s goals, the degree of prolapse, clinical history, risk tolerance, the surgeon’s skill set, and whether or not there is an indication or relative contraindication for uterine removal at the time of prolapse repair.
While the FDA’s order does not apply to transabdominally placed surgical mesh, such as sacrocolpopexy, not all patients are ideal candidates for an abdominal sacrocolpopexy. Most notable are women with a history of multiple prior abdominal surgeries with higher rates of intraperitoneal adhesions. Ideally, to be cost-effective and to avoid general anesthesia, the surgical approach should be vaginal whenever possible.
Biologic versus native-tissue grafts
Currently, only low-quality evidence exists that compares the outcomes of biologic grafts with traditional native-tissue repairs in POP. Studies have been limited by poor reporting of methods, inconsistency in technique and materials used, and imprecise definitions. One Cochrane Review on the surgical management of POP concluded that biologic graft augmentation was associated with a lower failure rate (18%) within 1 to 2 years when compared with a traditional anterior colporrhaphy (28%).12
Based on consideration of all Cochrane Database Reviews and recent large systematic reviews, there clearly is a paucity of information on which to draw well-defined conclusions regarding the advantage of biomaterials in prolapse surgery.12-14 This is due in part to the variation in graft material used and the surgical technique employed.
Similarly, evidence is lacking regarding the superiority of one type of biologic graft over another. Furthermore, some of the grafts previously studied are no longer on the market.15 With the FDA’s removal of all transvaginal mesh, including xenografts, only allografts are available for pelvic floor reconstruction. Currently, only 3 commercial manufacturers market allografts for pelvic floor reconstruction. Each allograft is available in various sizes and all can be trimmed at the time of the surgical procedure to customize both the size and shape to fit the individual patient.
A novel technique using Axis Dermis and polypropylene suture
One of the commercially available allografts, Axis Dermis (Coloplast), is non–cross-linked and is derived from human cadaveric dermal tissue from the back and dorsum of the upper leg. It is sterilized by a proprietary Tutoplast️ sterilization process that uses gamma irradiation to inactivate and prevent the transmission of pathogens. This unique technique involving solvent dehydration means the graft is never freeze dried; thus, the natural tissue matrix is preserved.
Additionally, the allograft is antigen-free, which decreases the risk of tissue reaction (scarring/fibrosis) and aids in the process of host tissue remodeling; invasion by growth factors, blood cells, collagen, elastin, and neovascularization. This natural tissue remodeling facilitates the anticipated “reabsorption” of the graft by the host tissue, leaving the patient with a tissue scaffold, that is, a stronger layer of “fascia” beneath the muscularis.16 As a result of this “biocompatible” graft, the host tissue remodeling has been shown in the rat model to involve early cellular infiltration and angiogenesis (in the first week after implantation), that leads to an organized cellular architecture with greater tensile strength by week 4, and ultimately inability to distinguish host collagen from the implant by 8 to 12 weeks.17,18
Continue to: Steps in performing the technique...
Steps in performing the technique
To ensure that the graft is placed adjacent to the vaginal serosa, a full-thickness dissection is carried out to enter the true vesicovaginal space, which lies below all 4 histologic layers of the vagina (nonkeratinized stratified squamous epithelium, lamina propria, muscularis, and serosa). For the anterior dissection, a Tuohy epidural needle is used to achieve an accurate and consistent depth when injecting fluid (hydrodissection) to enter this true pelvic space (FIGURE 1). Correct entry into the vesicovaginal space can be confirmed visually by the presence of adipose tissue.

Many pelvic surgeons use the sacrospinous ligament (SSL) as a strong and reliable point of attachment for vaginal prolapse repair. It can be approached either anteriorly or posteriorly with careful dissection. Permanent suture (0-Prolene) is used to “bridge” the attachment between the SSL, the Axis Dermis graft, and the cervix (or vaginal apex). The suture is placed in the middle third and lower half of the ligament to avoid injury to nearby neurovascular structures.
While the surgeon may use any suture-capturing device, we prefer the Anchosure System (Neomedic). This device delivers a small anchor securely into the ligament through a single point of entry, minimizing the risk of postoperative pain for the patient. A 6 cm x 8 cm size Axis Dermis graft is then trimmed to meet the specifications of the patient’s anatomy.
Most commonly, we measure, mark, and trim the body of the graft to 5.5 cm in length with a width of 3 cm. The bilateral arms are approximately 1 cm in width and comprise the remaining length of the 8 cm graft (FIGURE 2). As shown in Figure 2, pre-made holes are marked and punched out using a large hollow needle. These serve as the points of attachment for the permanent suture to be “weaved” into the graft arms and delayed absorbable “tacking suture” to be attached from the pubocervical fascia at the bladder neck to the distal end of the graft. This facilitates fixation of the graft in the midline of the anterior vaginal wall, overlying any central distention-type defect.

Finally, following attachment of the SSL permanent suture to the distal graft arm, this suture is then attached to the proximal U-shaped end of the graft body (in the midline), followed by a deep and secure bite through the cervix (or vaginal vault apex) and back through the proximal graft. These SSL suspension sutures are then tied such that the distal arms of the graft advance down to the ligament. Care is taken not to tie down to the SSL itself, rather until the cervix (or apex) is reduced to its normal anatomical location.
After the graft is secured in place, the full-thickness vaginal wall is closed with delayed absorbable suture. Sterile 1-inch ribbon packing is placed in the vagina immediately to close any dead space between the vagina and the graft to decrease the risk of seroma or hematoma formation.
This newly developed technique, like many surgeries for POP, requires extensive knowledge of pelvic anatomy and skill in vaginal surgery, and we recommend referral to a subspecialist in Female Pelvic Medicine and Reconstructive Surgery.

Continue to: Upcoming plans to share outcomes data...
Upcoming plans to share outcomes data
We are in the process of performing a retrospective review of all of the cases we have performed at our institution using this technique of permanent suture bridging to the SSL within the arm of the biograft. Given the relatively recent FDA announcement, we have yet to establish any long-term outcomes data. However, the preliminary results at 6-month follow-up are promising and demonstrate a low (2.6%) failure rate, without significant safety concerns. We hope to publish these data as well as more data on longitudinal outcomes in the future.
In summary
Many women are at risk for native-tissue repair failure or are not well suited for an abdominal procedure to correct their pelvic support defect and restore their quality of life. As expert pelvic surgeons, we play an important role in the search for innovative solutions for these women. There is ample opportunity for future research and clinical trials to determine the best biologic materials and their optimal use in pelvic reconstructive surgery.
Originally, polypropylene mesh was designed for use in augmenting abdominal hernia repairs and later was adapted by manufacturers for use in POP repair. The FDA removal from the market of existing transvaginal synthetic mesh kits was a unique catalyst that challenged our community to develop transvaginal repairs using biologic grafts that are genuinely tailored to the unique needs of the female pelvic anatomy. ●
Pelvic organ prolapse (POP) is a common occurrence over the course of a woman’s lifetime, especially in parous women (up to 50% of women who have given birth).1 The anterior vaginal wall is the most common site of POP and has the highest recurrence rate of up to 70%.2 The risk of developing POP increases with age, obesity, White race, family history, and prior pelvic surgery, such as hysterectomy. It affects more than 3 million women in the United States alone, often negatively impacting sexual function and overall quality of life.3,4
Because women are living longer than ever before and are more active in their senior years, a long-lasting, durable surgical repair is desirable, if not necessary. To be cost-effective and to avoid general anesthesia, the surgical approach ideally should be vaginal.
Biologic and synthetic grafts to augment transvaginal repair traditionally are used to improve on the well-recognized high failure rate of native-tissue repair that is often seen at both short-term and medium-term follow-up.5 The failure rate is commonly referenced as 30% to 40% at 2-year follow-up and 61% to 70% at 5-year follow-up, well-established by the results of the OPTIMAL randomized clinical trial.6 The more recent Descent trial likewise demonstrates a higher failure rate of native-tissue repair versus transvaginal mesh repair at a shorter term of 30 to 42 months.7 Furthermore, the use of permanent versus absorbable suture in suspension of the vaginal apex is associated with lower short-term failure rates.8
Despite this Level I evidence that demonstrates a clear advantage for obtaining a longer or more durable repair with permanent materials, native-tissue repairs with absorbable suture are still performed routinely. Since the US Food and Drug Administration (FDA) ordered that the use of transvaginal surgical mesh augmentation for pelvic reconstructive surgery be discontinued, it is more important than ever to explore evolving alternative native-tissue augmentation repair techniques that hopefully can preserve the advantages and merits of vaginal surgery and achieve longer durability.9
Biologic graft augmentation use in transvaginal reconstruction
All biologic grafts, including allografts derived from human tissue and xenografts derived from animal tissue, are acellular constructs composed of extracellular matrix (ECM) that acts as scaffolding for the host tissue. The ECM is predominantly composed of collagen (types I and III) and noncollagenous fibronectin, laminin, and glycosaminoglycans in various amounts depending on the source tissue. The 3D presentation of ECM’s complex molecules allows for rapid repopulation of host cells and revascularization with eventual regeneration.
Once a biologic graft is placed surgically, the body’s response to the scaffold ECM mimics the normal wound-healing process, beginning with fibrin-rich matrix hemostasis and the subsequent innate immune response of neutrophil and M1 macrophage infiltration. M1 macrophages are proinflammatory and clear cellular debris and begin the process of graft scaffold degradation. The host tissue then begins the process of remodeling through pro-remodeling M2 macrophages and stem cell recruitment, proliferation, and differentiation.10 As the biologic graft provides initial structure and strength for pelvic repairs, the ideal ECM scaffold would not degrade before the host is able to fully undergo regeneration and maintain its structure and strength.
Biologic grafts differ in source (allograft or xenograft), type (pericardium, dermis, or bladder), developmental stage (fetal or adult), decellularization processing, and sterilization techniques. These 5 aspects determine the distinct 3D ECM scaffold structure, strength, and longevity. If the ECM scaffold is damaged or retains noncollagenous proteins during the preparation process, an inflammatory response is triggered in which the graft is degraded, resorbed, and replaced with scar tissue. Furthermore, certain processing techniques aimed at extending the ECM’s durability—that is, cross-linking collagen—results in the foreign body response in which there is no vascular infiltration or cellular penetration of the graft and a collagen capsule is created around the empty matrix.11 To avoid resorption or encapsulation of the graft, the ECM scaffolds of biologic grafts must be optimized to induce regeneration.
Continue to: Choosing surgical POP repair...
Choosing surgical POP repair
The decision to undergo surgical treatment for prolapse is a shared decision-making process between the patient and surgeon and always should be individualized. The type of procedure and the surgical approach will depend on the patient’s goals, the degree of prolapse, clinical history, risk tolerance, the surgeon’s skill set, and whether or not there is an indication or relative contraindication for uterine removal at the time of prolapse repair.
While the FDA’s order does not apply to transabdominally placed surgical mesh, such as sacrocolpopexy, not all patients are ideal candidates for an abdominal sacrocolpopexy. Most notable are women with a history of multiple prior abdominal surgeries with higher rates of intraperitoneal adhesions. Ideally, to be cost-effective and to avoid general anesthesia, the surgical approach should be vaginal whenever possible.
Biologic versus native-tissue grafts
Currently, only low-quality evidence exists that compares the outcomes of biologic grafts with traditional native-tissue repairs in POP. Studies have been limited by poor reporting of methods, inconsistency in technique and materials used, and imprecise definitions. One Cochrane Review on the surgical management of POP concluded that biologic graft augmentation was associated with a lower failure rate (18%) within 1 to 2 years when compared with a traditional anterior colporrhaphy (28%).12
Based on consideration of all Cochrane Database Reviews and recent large systematic reviews, there clearly is a paucity of information on which to draw well-defined conclusions regarding the advantage of biomaterials in prolapse surgery.12-14 This is due in part to the variation in graft material used and the surgical technique employed.
Similarly, evidence is lacking regarding the superiority of one type of biologic graft over another. Furthermore, some of the grafts previously studied are no longer on the market.15 With the FDA’s removal of all transvaginal mesh, including xenografts, only allografts are available for pelvic floor reconstruction. Currently, only 3 commercial manufacturers market allografts for pelvic floor reconstruction. Each allograft is available in various sizes and all can be trimmed at the time of the surgical procedure to customize both the size and shape to fit the individual patient.
A novel technique using Axis Dermis and polypropylene suture
One of the commercially available allografts, Axis Dermis (Coloplast), is non–cross-linked and is derived from human cadaveric dermal tissue from the back and dorsum of the upper leg. It is sterilized by a proprietary Tutoplast️ sterilization process that uses gamma irradiation to inactivate and prevent the transmission of pathogens. This unique technique involving solvent dehydration means the graft is never freeze dried; thus, the natural tissue matrix is preserved.
Additionally, the allograft is antigen-free, which decreases the risk of tissue reaction (scarring/fibrosis) and aids in the process of host tissue remodeling; invasion by growth factors, blood cells, collagen, elastin, and neovascularization. This natural tissue remodeling facilitates the anticipated “reabsorption” of the graft by the host tissue, leaving the patient with a tissue scaffold, that is, a stronger layer of “fascia” beneath the muscularis.16 As a result of this “biocompatible” graft, the host tissue remodeling has been shown in the rat model to involve early cellular infiltration and angiogenesis (in the first week after implantation), that leads to an organized cellular architecture with greater tensile strength by week 4, and ultimately inability to distinguish host collagen from the implant by 8 to 12 weeks.17,18
Continue to: Steps in performing the technique...
Steps in performing the technique
To ensure that the graft is placed adjacent to the vaginal serosa, a full-thickness dissection is carried out to enter the true vesicovaginal space, which lies below all 4 histologic layers of the vagina (nonkeratinized stratified squamous epithelium, lamina propria, muscularis, and serosa). For the anterior dissection, a Tuohy epidural needle is used to achieve an accurate and consistent depth when injecting fluid (hydrodissection) to enter this true pelvic space (FIGURE 1). Correct entry into the vesicovaginal space can be confirmed visually by the presence of adipose tissue.

Many pelvic surgeons use the sacrospinous ligament (SSL) as a strong and reliable point of attachment for vaginal prolapse repair. It can be approached either anteriorly or posteriorly with careful dissection. Permanent suture (0-Prolene) is used to “bridge” the attachment between the SSL, the Axis Dermis graft, and the cervix (or vaginal apex). The suture is placed in the middle third and lower half of the ligament to avoid injury to nearby neurovascular structures.
While the surgeon may use any suture-capturing device, we prefer the Anchosure System (Neomedic). This device delivers a small anchor securely into the ligament through a single point of entry, minimizing the risk of postoperative pain for the patient. A 6 cm x 8 cm size Axis Dermis graft is then trimmed to meet the specifications of the patient’s anatomy.
Most commonly, we measure, mark, and trim the body of the graft to 5.5 cm in length with a width of 3 cm. The bilateral arms are approximately 1 cm in width and comprise the remaining length of the 8 cm graft (FIGURE 2). As shown in Figure 2, pre-made holes are marked and punched out using a large hollow needle. These serve as the points of attachment for the permanent suture to be “weaved” into the graft arms and delayed absorbable “tacking suture” to be attached from the pubocervical fascia at the bladder neck to the distal end of the graft. This facilitates fixation of the graft in the midline of the anterior vaginal wall, overlying any central distention-type defect.

Finally, following attachment of the SSL permanent suture to the distal graft arm, this suture is then attached to the proximal U-shaped end of the graft body (in the midline), followed by a deep and secure bite through the cervix (or vaginal vault apex) and back through the proximal graft. These SSL suspension sutures are then tied such that the distal arms of the graft advance down to the ligament. Care is taken not to tie down to the SSL itself, rather until the cervix (or apex) is reduced to its normal anatomical location.
After the graft is secured in place, the full-thickness vaginal wall is closed with delayed absorbable suture. Sterile 1-inch ribbon packing is placed in the vagina immediately to close any dead space between the vagina and the graft to decrease the risk of seroma or hematoma formation.
This newly developed technique, like many surgeries for POP, requires extensive knowledge of pelvic anatomy and skill in vaginal surgery, and we recommend referral to a subspecialist in Female Pelvic Medicine and Reconstructive Surgery.

Continue to: Upcoming plans to share outcomes data...
Upcoming plans to share outcomes data
We are in the process of performing a retrospective review of all of the cases we have performed at our institution using this technique of permanent suture bridging to the SSL within the arm of the biograft. Given the relatively recent FDA announcement, we have yet to establish any long-term outcomes data. However, the preliminary results at 6-month follow-up are promising and demonstrate a low (2.6%) failure rate, without significant safety concerns. We hope to publish these data as well as more data on longitudinal outcomes in the future.
In summary
Many women are at risk for native-tissue repair failure or are not well suited for an abdominal procedure to correct their pelvic support defect and restore their quality of life. As expert pelvic surgeons, we play an important role in the search for innovative solutions for these women. There is ample opportunity for future research and clinical trials to determine the best biologic materials and their optimal use in pelvic reconstructive surgery.
Originally, polypropylene mesh was designed for use in augmenting abdominal hernia repairs and later was adapted by manufacturers for use in POP repair. The FDA removal from the market of existing transvaginal synthetic mesh kits was a unique catalyst that challenged our community to develop transvaginal repairs using biologic grafts that are genuinely tailored to the unique needs of the female pelvic anatomy. ●
- Maher C, Feiner B, Baessler K. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013:CD004014.
- Weber AM, Walters MD, Piedmonte MR, et al. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol. 2001;185:1299-1304.
- Walters MD, Ridgeway BM. Surgical treatment of vaginal apex prolapse. Obstet Gynecol. 2013;121(2 pt 1):354-374.
- Meister MRL, Sutcliffe S, Lowder JL. Definitions of apical vaginal support loss: a systematic review. Am J Obstet Gynecol. 2017;216:232. e1-232.e14.
- Cox A, Herschorn S. Evaluation of current biologic meshes in pelvic organ prolapse repair. Curr Urol Rep. 2012;13:247-255.
- Jelovsek JE, Barber M, Brubaker K, et al. Effect of uterosacral ligament suspension vs sacrospinous ligament fixation with or without perioperative behavioral therapy for pelvic organ vaginal prolapse on surgical outcomes and prolapse symptoms at 5 years in the OPTIMAL randomized clinical trial. JAMA. 2018:319:1554-1565.
- Bowen ST, Moalli P, Abramowitch S, et al. Outcomes of the defining mechanisms of anterior vaginal wall descent trial [abstract 15]. Am J Obstet Gynecol. 2020;222:S770-S771.
- Chung CP, Miskimins R, Kuehl TJ, et al. Permanent suture used in uterosacral ligament suspension offers better anatomical support than delayed absorbable suture. Int Urogynecol J. 2012;23:223-227.
- US Food and Drug Administration. FDA takes action to protect women’s health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. https://www.fda.gov/news-events/press-announcements/fda-takes-action-protect-womens-health-orders-manufacturers-surgical -mesh-intended-transvaginal. April 16, 2019. Accessed September 1, 2020.
- Londono R, Badylak SF. Biologic scaffolds for regenerative medicine: mechanisms of in vivo remodeling. Ann Biomed Eng. 2015;43:577-592.
- Cornwell KG, Landsman A, James KS. Extracellular matrix biomaterials for soft tissue repair. Clin Podiatr Med Surg. 2009;26: 507-523.
- Maher CM, Feiner B, Baessler K, et al. Surgical management of pelvic organ prolapse in women: the updated summary version Cochrane review. Int Urogynecol J. 2011;22:1445-1447.
- Maher C, Feiner B, Baessler K, et al. Surgery for women with anterior compartment prolapse. Cochrane Database Syst Rev. 2016;11:CD004014.
- Maher C, Feiner B, Baessler K, et al. Transvaginal mesh or grafts compared with native tissue repair for vaginal prolapse. Cochrane Database Syst Rev. 2016;2:CD012179.
- Rosenblatt P, Von Bargen E. Use of biologic grafts in pelvic organ prolapse surgery. Contemporary OB/GYN. 2017;62:14-19.
- Greenspan DC, Hernandez R, Faleris J. Histology of surgically implanted Tutoplast processed dermis. http://www.zimmerbiomet .co.il/images/lib_artHistologyDermis%2010.pdf. Accessed September 2, 2020.
- Williams D. Revisiting the definition of biocompatibility. Med Device Technol. 2003;14:10-13.
- Nosti PA, Carter CM, Sokol AI, et al. Transvaginal versus transabdominal placement of synthetic mesh at time of sacrocolpopexy. Female Pelvic Med Reconstr Surg. 2016;22:151-155.
- Maher C, Feiner B, Baessler K. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013:CD004014.
- Weber AM, Walters MD, Piedmonte MR, et al. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol. 2001;185:1299-1304.
- Walters MD, Ridgeway BM. Surgical treatment of vaginal apex prolapse. Obstet Gynecol. 2013;121(2 pt 1):354-374.
- Meister MRL, Sutcliffe S, Lowder JL. Definitions of apical vaginal support loss: a systematic review. Am J Obstet Gynecol. 2017;216:232. e1-232.e14.
- Cox A, Herschorn S. Evaluation of current biologic meshes in pelvic organ prolapse repair. Curr Urol Rep. 2012;13:247-255.
- Jelovsek JE, Barber M, Brubaker K, et al. Effect of uterosacral ligament suspension vs sacrospinous ligament fixation with or without perioperative behavioral therapy for pelvic organ vaginal prolapse on surgical outcomes and prolapse symptoms at 5 years in the OPTIMAL randomized clinical trial. JAMA. 2018:319:1554-1565.
- Bowen ST, Moalli P, Abramowitch S, et al. Outcomes of the defining mechanisms of anterior vaginal wall descent trial [abstract 15]. Am J Obstet Gynecol. 2020;222:S770-S771.
- Chung CP, Miskimins R, Kuehl TJ, et al. Permanent suture used in uterosacral ligament suspension offers better anatomical support than delayed absorbable suture. Int Urogynecol J. 2012;23:223-227.
- US Food and Drug Administration. FDA takes action to protect women’s health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. https://www.fda.gov/news-events/press-announcements/fda-takes-action-protect-womens-health-orders-manufacturers-surgical -mesh-intended-transvaginal. April 16, 2019. Accessed September 1, 2020.
- Londono R, Badylak SF. Biologic scaffolds for regenerative medicine: mechanisms of in vivo remodeling. Ann Biomed Eng. 2015;43:577-592.
- Cornwell KG, Landsman A, James KS. Extracellular matrix biomaterials for soft tissue repair. Clin Podiatr Med Surg. 2009;26: 507-523.
- Maher CM, Feiner B, Baessler K, et al. Surgical management of pelvic organ prolapse in women: the updated summary version Cochrane review. Int Urogynecol J. 2011;22:1445-1447.
- Maher C, Feiner B, Baessler K, et al. Surgery for women with anterior compartment prolapse. Cochrane Database Syst Rev. 2016;11:CD004014.
- Maher C, Feiner B, Baessler K, et al. Transvaginal mesh or grafts compared with native tissue repair for vaginal prolapse. Cochrane Database Syst Rev. 2016;2:CD012179.
- Rosenblatt P, Von Bargen E. Use of biologic grafts in pelvic organ prolapse surgery. Contemporary OB/GYN. 2017;62:14-19.
- Greenspan DC, Hernandez R, Faleris J. Histology of surgically implanted Tutoplast processed dermis. http://www.zimmerbiomet .co.il/images/lib_artHistologyDermis%2010.pdf. Accessed September 2, 2020.
- Williams D. Revisiting the definition of biocompatibility. Med Device Technol. 2003;14:10-13.
- Nosti PA, Carter CM, Sokol AI, et al. Transvaginal versus transabdominal placement of synthetic mesh at time of sacrocolpopexy. Female Pelvic Med Reconstr Surg. 2016;22:151-155.
How to build your identity as a physician online
To have a thriving business in today’s world, a functioning website is crucial to the overall business health. For a medical practice in general, and for its physicians specifically, it is one of the first steps for maintaining a practice. But to grow that practice, it is crucial to take the steps beyond just having a website. Growth requires website optimization for search engines, an expanding referral base, and the knowledge to use web tools and social media at your disposal to promote the practice and its physicians. In this roundtable, several marketing experts and web-savvy physicians discuss using available tools to best position and grow a practice.
Choosing a web upgrade
Patrick J. Culligan, MD: Peter, can you start us off by describing your relationship with Heather, and how your practice benefitted from her expertise?
Peter M. Lotze, MD: Sure. I am a urogynecologist in the competitive market of pelvic reconstructive surgery in Houston, Texas. Within that market, my main approach was to reach out to other physicians to refer patients to my practice. It generally would work, but took increasingly greater amounts of time to call these physicians up, write them letters, and maintain relationships. I felt that the large, national practice group that I am in did not have a significant web presence optimized to promote my practice, which makes it difficult for patients seeking your services to find you in their search for a doctor. It is helpful for patients to be able to understand from your website who you are, what you do, and what their experience may be like.
Glaring to me was that a web search specific for me or things that I do, would not produce our company’s results until page 2 or more on Google. This can be devastating for a practice because most people don’t go past the first page, and you can end up with fewer self-referrals, which should be a significant portion of new patients to your practice. I knew I needed guidance; I knew of Heather’s expertise given her exceptional past work building marketing strategies.
Digital go-tos for marketing
Heather Schueppert: Yes, I was pleased to work with Dr. Lotze, and at the time was a marketing consultant for practices such as his. But gone are the days of printed material—brochures, pamphlets, or even billboards—to effectively promote a business, or in this case, a practice. What still withstands the test of time, however, as the number 1 marketing referral source is word of mouth—from your trusted friend, family member, or coworker.
It is now proven that the number 2 most trusted form of advertising, the most persuasive and the most motivating, is online marketing.1 It is the “digital word of mouth”—the review. Patients are actively online, and a strong digital presence is critical to provide that direct value to retain and grow your patient base.
Continue to: Foundations of private practice reach out...
Foundations of private practice reach out
There are 3 important areas that I consider the foundation of any private practice marketing strategy (TABLE). First is an updated website that is search engine optimized (SEO). You can’t just set it and forget it, it needs to be an updated website. The algorithms for search engines are changing constantly to try to make it as fair and relevant as possible for patients or consumers to find the businesses they are searching for online.

The second area is review management, and for a physician, or even a care center, to do this on your own is a daunting task. It is a critical component, however, to making sure that your reputation out there, that online word of mouth, is as high a star rating as possible.
The third component is local search, which is basically a form of SEO that helps businesses show up in relevant local searches. We are all familiar with the search, “find a restaurant near me,” anything that pushes those search engines to find something local.
Those are what I call the effective triad: that updated website, the review management, and the local search, and all of these are tied together. I think Dr. Lotze and his practice did these effectively well, and I believe that he achieved his goals for the longer term.
Review/reputation management
Dr. Culligan: Brad, is there something that doctors may not know about Healthgrades, and are there opportunities to take full advantage of this physician-rating site?
Brad Bowman, MD: I agree with everything that Dr. Lotze and Heather have said. Start with yourself—what is it that you want to be, the one thing you want to stand for? Get your own marketing, your website right, then, the point is, once you do all that and you are number 1 in SEO, you are still only going to get about 25% of the people looking for you by name to come to your website. The other 75% are going to look at all the other different sites that are out there to provide information to consumers. So the question becomes what do you do with all these other third-party sites? Healthgrades is the most comprehensive and has the highest traffic of the third-party “find a doctor” sites. In 2020, half of all Americans who go to a doctor will use Healthgrades at some point to help select and connect with that doctor.
Physicians have their focus on the quality of the care they provide. Patients, however, focus on the quality of the entire health care experience. Did I get better? How long did I have to wait? Was the office staff helpful? Scarily enough, we still spend more time shopping for a refrigerator or mattress than we do shopping for a doctor. We still tend to think that all doctors are the same. It is the reality of how we have been trained by our insurance companies and by the health care system. That is why getting your marketing right and getting what is it that you want to be known for out there is important, so that you can get the types of patients you want.
Listings management is very important. Make sure that you are findable everywhere. There are services that will do this: Doctor.com, Reputation.com, and many others. They can help you make sure you get all your basic materials right: addresses, phone numbers, your picture. Because 75% of people are going to end up on third-party websites, if your phone number is wrong there, you could lose that patient.
Then the second piece of working with third-party sites is reputation management. Physician reviews are not a bad thing, they are the new word of mouth, as Heather pointed out. Most (80%) of the reviews are going to be positive. The others will be negative, and that is okay. It is important that you get at least 1 or 2 reviews on all the different sites. We know from Healthgrades.com that going from zero reviews to 1 review will increase your call volume by 60%. If you have the choice between 2 physicians and one practice looks like people have been there before, you will go to that one.
You can learn from reviews as well, consumers provide valid feedback. Best practice is to respond to every positive and negative review. Thank them, indicate that you have listened to them, and address any concerns as necessary.
Continue to: Dr. Lotze...
Dr. Lotze: As an example, one of the paramount things that Heather introduced me to was the third party I use to run my website. That company sends a HIPAA-compliant review out to each patient we have seen that day and gives them the opportunity to rate our services and leave comments. If a patient brings up a concern, we can respond immediately, which is important. Patients appreciate feeling that they have been heard. Typically, communicating with a patient will turn the 3-star review into a 5-star as she follows up with the practice.
Ms. Schueppert: Timeliness is important. And just to mention, there certainly is a time commitment to this (and it is a marathon versus a sprint) and there is some financial investment to get it going, but it could truly be detrimental to a practice if you decide not to do anything at all.
Dr. Bowman: Agencies can really help with the time commitment.
Handling bad reviews
Dr. Culligan: What about that person who seems to have it out for you, perhaps giving you multiple bad reviews?
Dr. Bowman: I have seen this before. At Healthgrades, we recently analyzed 8.4 million patient reviews to see what people wrote about.2 The first thing they will talk about is quality of care as they see it. Did I get better or not? You can’t “fix” every patient; there will be some that you cannot help. The next thing patients comment on is bedside manner. With negative reviews, you will see more comments about the office staff.2
A single negative review actually helps make the positive ones look more credible. But if you do believe someone is trolling you, we can flag it and will investigate to the best of our ability. (Different sites likely have different editorial policies.) For example, we look at the IP addresses of all reviews, and if multiple reviews are coming from the same location, we would only let one through, overwriting the previous review from that address.
Patients just want to be heard. We have seen people change their views, based on how their review is handled and responded to.
Dr. Lotze: Is there a response by the physician that you think tends to work better in terms of resolving the issue that can minimize a perceived caustic reaction to a patient’s criticism?
Dr. Bowman: First, just like with any stressful situation, take a deep breath and respond when you feel like you can be constructive. When you do respond, be gracious. Thank them for their feedback. Make sure you reference something about their concern: “I understand that you had to wait longer than you would have liked.” Acknowledge the problem they reference, and then just apologize: “I’m sorry we didn’t meet your expectations.” Then, if they waited too long for example, “We have a new system where no one should have to wait more than 30 minutes….” You can respond privately or publically. Generally, public responses are better as it shows other consumers that you are willing to listen and consider their point of view.
Continue to: The next phase at Healthgrades...
The next phase at Healthgrades
Dr. Culligan: Do you see changes to the way physician-rating sites are working now? Are we going to stay status quo over the next 10 years, or do you see frontiers in how your site is going to develop?
Dr. Bowman: For Healthgrades, we rely on quantitative and objective measures, not just the qualitative. We are investing heavily right now in trying to help consumers understand what are the relative volumes of different procedures or different patient types that each individual doctor sees. Orthopedics is an easy example—if you have a knee problem, you want to go to someone who specializes in knees. Our job is to help consumers easily identify, “This is a shoulder doctor, this is a knee doctor, and this is why that matters.”
In the meantime, as a physician, you can always go into our site and state your care philosophy, identifying what is the sort of patient that you like to treat. Transparency is good for everyone, and especially physicians. It helps the right patient show up for you, and it helps you do a better job providing referrals.
Social media: Avoid pitfalls, and use it to your benefit
Dr. Lotze: Branding was one of the things that I was confused about, and Heather really helped me out. As physicians, we put ourselves out there on our websites, which we try to make professional sources of information for patients. But patients often want to see what else they can find out about us, including Healthgrades and social media. I think the thing that is important to know with social media is that it is a place where people learn about you as a person. Your social media should be another avenue of promotion. Whether it is your personal or professional Facebook page, people are going to see those sites. You have an opportunity to promote yourself as a good physician and a good person with a wholesome practice that you want people to come to. If a physician is posting questionable things about themselves on any kind of social media, it could be perceived as inappropriate by the patient. That can impact how patients think of you as a person, and how they are going to grade you. If people lose sight of who you are due to a questionable social media posting, everything else (SEO, the website) can be for naught.
Dr. Culligan: What are the most important social media tools to invest your time in?
Ms. Schueppert: Before anybody jumps into social media, I firmly recommend that you make sure your local search and your Google 3-pack is set up—which is basically a method Google uses to display the top 3 results on its listings page. Then make sure you have a review management system in place. Make sure you have that updated website. Those are the foundational elements. Once you have that going, social media is the next added layer to that digital presence.
I usually recommend LinkedIn. It is huge because you are staying in contact with your colleagues, that business-to-business type of connection. It remains a way for physicians to set themselves up as experts in their level of specialty.
From there, it’s either Instagram or Facebook. If you are serving more of the younger generations, the millennials and younger, then Instagram is the way to go. If you are focusing on your 40+, 50+, they are going to be far more on Facebook.
Continue to: Dr. Lotze...
Dr. Lotze: For me, a Facebook page was a great place to start. The cost of those Google ads—the first things we see at the top of a Google search in their own separate box—is significant. If a practice has that kind of money to invest, great; it is an instant way to be first on the page during a search. But there are more cost-effective ways of doing that, especially as you are getting your name out. Facebook provides, at a smaller cost, promotion of whatever it is that you are seeking to promote. You can find people within a certain zip code, for instance, and use a Facebook ad campaign that can drive people to your Facebook page—which should have both routinely updated new posts and a link to your website. The posts should be interesting topics relevant to the patients you wish to treat (avoiding personal stories or controversial discussions). You can put a post together, or you can have a third-party service do this. People who follow your page will get reminders of you and your practice with each new post. As your page followers increase, your Facebook rank will improve, and your page will more likely be discovered by Facebook searches for your services. With an added link to your office practice website, those patients go straight to your site without getting lost in the noise of Google search results.
For Instagram, a short video or an interesting picture, along with a brief statement, are the essentials. You can add a single link. Marketing here is by direct messaging or having patients going to your website through a link. Instagram, like Facebook, offers analytics to help show you what your audience likes to read about, improving the quality of your posts and increasing number of followers.
YouTube is the number 2 search engine behind Google. A Google search for your field of medicine may be filled with pages of competitors. However, YouTube has a much lower volume of competing practices, making it easier for patients to find you. The only downside to YouTube is that it will list your video along with other competing videos, which can draw attention away from your practice.
If you want to promote your website or practice with video, using a company such as Vimeo is a better choice compared with YouTube, as YouTube gets credit for video views—which improves YouTube’s SEO and not your own website. Vimeo allows for your website to get credit each time the video is watched. Regardless of where you place your videos, make them short and to the point, with links to your website. Videos only need to be long enough to get your message across and stimulate interest in your practice.
If you can have a blog on your website, it also will help with SEO. What a search engine like Google wants to see is that a patient is on your web page and looking at something for at least 60 seconds. If so, the website is deemed to have information that is relevant, improving your SEO ranking.
Finally, Twitter also can be used for getting messaging out and for branding. The problem with it is that many people go to Twitter to follow a Hollywood celebrity, a sports star, or are looking for mass communication. There is less interest on Twitter for physician outreach.
Continue to: Measuring ROI...
Measuring ROI
Dr. Culligan: What’s the best way to track your return on investment?
Dr. Lotze: First for me was to find out what didn’t work in the office and fix that before really promoting my practice. It’s about the global experience for a patient, as Brad mentioned. As a marketing expert, Heather met with me to understand my goals. She then called my office as a patient to set up an appointment and went through that entire office experience. We identified issues needing improvement.
The next step was to develop a working relationship with my webmaster—someone who can help manage Internet image and SEO. Together, you will develop goals for what the SEO should promote specific to your practice. Once a good SEO program is in place, your website’s ranking will go up—although it can take a minimum of 6 months to see a significant increase. To help understand your website’s performance, your webmaster should provide you with reports on your site’s analytics.
As you go through this process, it is great to have a marketing expert to be the point person. You will work closely together for a while, but eventually you can back off over time. The time and expense you invest on the front end have huge rewards on the back end. Currently, I still spend a reasonable amount of money every month. I have a high self-referral base because of these efforts, however, which results in more patient surgeries and easily covers my expenses. It is money well invested. My website traffic increased by 268% over 2 years (FIGURE). I’ll propose that currently more than half of my patients are self-referrals due to online marketing.
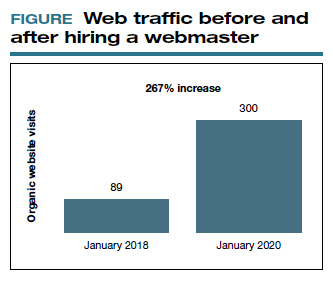
Ms. Schueppert: The only thing I would add is training your front staff. They are checking people in, taking appointments, checking your patients out. Have them be mindful that there are campaigns going on, whether it is a social media push, or a new video that went on the website. They can ask, “How did you hear about us?” when a new patient calls.
Dr. Bowman: Unless you are a large university hospital, where the analytics get significantly more advanced in terms of measuring return on investment (ROI), I think you should just be looking at your schedule and looking at your monthly billings and seeing how they change over time. You can calculate how much a new patient is worth because you can figure out how many patients you have and how much you bill and what your profits are.
Dr. Culligan: For those of us who are hospital employees, you can try to convince the hospital that you can do a detailed ROI analysis, or you can just look at it like (say it’s $3,000 per month), how many surgeries does this project have to generate before the hospital makes that back? The answer is a fraction of 1 case.
Thank you to all of you for your expertise on this roundtable.
- Anderson A. Online reviews vs. word of mouth: Which one is more important. https://www.revlocal.com/blog/reviewandreputationmanagement/onlinereviewsvswordofmouthwhichoneismoreimportant. Accessed July 17, 2020.
- 2020 Patient sentiment report. Healthgrades; Medical Group Management Association. https://www.healthgrades.com/content /patientsentimentreport. Accessed July 17, 2020
To have a thriving business in today’s world, a functioning website is crucial to the overall business health. For a medical practice in general, and for its physicians specifically, it is one of the first steps for maintaining a practice. But to grow that practice, it is crucial to take the steps beyond just having a website. Growth requires website optimization for search engines, an expanding referral base, and the knowledge to use web tools and social media at your disposal to promote the practice and its physicians. In this roundtable, several marketing experts and web-savvy physicians discuss using available tools to best position and grow a practice.
Choosing a web upgrade
Patrick J. Culligan, MD: Peter, can you start us off by describing your relationship with Heather, and how your practice benefitted from her expertise?
Peter M. Lotze, MD: Sure. I am a urogynecologist in the competitive market of pelvic reconstructive surgery in Houston, Texas. Within that market, my main approach was to reach out to other physicians to refer patients to my practice. It generally would work, but took increasingly greater amounts of time to call these physicians up, write them letters, and maintain relationships. I felt that the large, national practice group that I am in did not have a significant web presence optimized to promote my practice, which makes it difficult for patients seeking your services to find you in their search for a doctor. It is helpful for patients to be able to understand from your website who you are, what you do, and what their experience may be like.
Glaring to me was that a web search specific for me or things that I do, would not produce our company’s results until page 2 or more on Google. This can be devastating for a practice because most people don’t go past the first page, and you can end up with fewer self-referrals, which should be a significant portion of new patients to your practice. I knew I needed guidance; I knew of Heather’s expertise given her exceptional past work building marketing strategies.
Digital go-tos for marketing
Heather Schueppert: Yes, I was pleased to work with Dr. Lotze, and at the time was a marketing consultant for practices such as his. But gone are the days of printed material—brochures, pamphlets, or even billboards—to effectively promote a business, or in this case, a practice. What still withstands the test of time, however, as the number 1 marketing referral source is word of mouth—from your trusted friend, family member, or coworker.
It is now proven that the number 2 most trusted form of advertising, the most persuasive and the most motivating, is online marketing.1 It is the “digital word of mouth”—the review. Patients are actively online, and a strong digital presence is critical to provide that direct value to retain and grow your patient base.
Continue to: Foundations of private practice reach out...
Foundations of private practice reach out
There are 3 important areas that I consider the foundation of any private practice marketing strategy (TABLE). First is an updated website that is search engine optimized (SEO). You can’t just set it and forget it, it needs to be an updated website. The algorithms for search engines are changing constantly to try to make it as fair and relevant as possible for patients or consumers to find the businesses they are searching for online.

The second area is review management, and for a physician, or even a care center, to do this on your own is a daunting task. It is a critical component, however, to making sure that your reputation out there, that online word of mouth, is as high a star rating as possible.
The third component is local search, which is basically a form of SEO that helps businesses show up in relevant local searches. We are all familiar with the search, “find a restaurant near me,” anything that pushes those search engines to find something local.
Those are what I call the effective triad: that updated website, the review management, and the local search, and all of these are tied together. I think Dr. Lotze and his practice did these effectively well, and I believe that he achieved his goals for the longer term.
Review/reputation management
Dr. Culligan: Brad, is there something that doctors may not know about Healthgrades, and are there opportunities to take full advantage of this physician-rating site?
Brad Bowman, MD: I agree with everything that Dr. Lotze and Heather have said. Start with yourself—what is it that you want to be, the one thing you want to stand for? Get your own marketing, your website right, then, the point is, once you do all that and you are number 1 in SEO, you are still only going to get about 25% of the people looking for you by name to come to your website. The other 75% are going to look at all the other different sites that are out there to provide information to consumers. So the question becomes what do you do with all these other third-party sites? Healthgrades is the most comprehensive and has the highest traffic of the third-party “find a doctor” sites. In 2020, half of all Americans who go to a doctor will use Healthgrades at some point to help select and connect with that doctor.
Physicians have their focus on the quality of the care they provide. Patients, however, focus on the quality of the entire health care experience. Did I get better? How long did I have to wait? Was the office staff helpful? Scarily enough, we still spend more time shopping for a refrigerator or mattress than we do shopping for a doctor. We still tend to think that all doctors are the same. It is the reality of how we have been trained by our insurance companies and by the health care system. That is why getting your marketing right and getting what is it that you want to be known for out there is important, so that you can get the types of patients you want.
Listings management is very important. Make sure that you are findable everywhere. There are services that will do this: Doctor.com, Reputation.com, and many others. They can help you make sure you get all your basic materials right: addresses, phone numbers, your picture. Because 75% of people are going to end up on third-party websites, if your phone number is wrong there, you could lose that patient.
Then the second piece of working with third-party sites is reputation management. Physician reviews are not a bad thing, they are the new word of mouth, as Heather pointed out. Most (80%) of the reviews are going to be positive. The others will be negative, and that is okay. It is important that you get at least 1 or 2 reviews on all the different sites. We know from Healthgrades.com that going from zero reviews to 1 review will increase your call volume by 60%. If you have the choice between 2 physicians and one practice looks like people have been there before, you will go to that one.
You can learn from reviews as well, consumers provide valid feedback. Best practice is to respond to every positive and negative review. Thank them, indicate that you have listened to them, and address any concerns as necessary.
Continue to: Dr. Lotze...
Dr. Lotze: As an example, one of the paramount things that Heather introduced me to was the third party I use to run my website. That company sends a HIPAA-compliant review out to each patient we have seen that day and gives them the opportunity to rate our services and leave comments. If a patient brings up a concern, we can respond immediately, which is important. Patients appreciate feeling that they have been heard. Typically, communicating with a patient will turn the 3-star review into a 5-star as she follows up with the practice.
Ms. Schueppert: Timeliness is important. And just to mention, there certainly is a time commitment to this (and it is a marathon versus a sprint) and there is some financial investment to get it going, but it could truly be detrimental to a practice if you decide not to do anything at all.
Dr. Bowman: Agencies can really help with the time commitment.
Handling bad reviews
Dr. Culligan: What about that person who seems to have it out for you, perhaps giving you multiple bad reviews?
Dr. Bowman: I have seen this before. At Healthgrades, we recently analyzed 8.4 million patient reviews to see what people wrote about.2 The first thing they will talk about is quality of care as they see it. Did I get better or not? You can’t “fix” every patient; there will be some that you cannot help. The next thing patients comment on is bedside manner. With negative reviews, you will see more comments about the office staff.2
A single negative review actually helps make the positive ones look more credible. But if you do believe someone is trolling you, we can flag it and will investigate to the best of our ability. (Different sites likely have different editorial policies.) For example, we look at the IP addresses of all reviews, and if multiple reviews are coming from the same location, we would only let one through, overwriting the previous review from that address.
Patients just want to be heard. We have seen people change their views, based on how their review is handled and responded to.
Dr. Lotze: Is there a response by the physician that you think tends to work better in terms of resolving the issue that can minimize a perceived caustic reaction to a patient’s criticism?
Dr. Bowman: First, just like with any stressful situation, take a deep breath and respond when you feel like you can be constructive. When you do respond, be gracious. Thank them for their feedback. Make sure you reference something about their concern: “I understand that you had to wait longer than you would have liked.” Acknowledge the problem they reference, and then just apologize: “I’m sorry we didn’t meet your expectations.” Then, if they waited too long for example, “We have a new system where no one should have to wait more than 30 minutes….” You can respond privately or publically. Generally, public responses are better as it shows other consumers that you are willing to listen and consider their point of view.
Continue to: The next phase at Healthgrades...
The next phase at Healthgrades
Dr. Culligan: Do you see changes to the way physician-rating sites are working now? Are we going to stay status quo over the next 10 years, or do you see frontiers in how your site is going to develop?
Dr. Bowman: For Healthgrades, we rely on quantitative and objective measures, not just the qualitative. We are investing heavily right now in trying to help consumers understand what are the relative volumes of different procedures or different patient types that each individual doctor sees. Orthopedics is an easy example—if you have a knee problem, you want to go to someone who specializes in knees. Our job is to help consumers easily identify, “This is a shoulder doctor, this is a knee doctor, and this is why that matters.”
In the meantime, as a physician, you can always go into our site and state your care philosophy, identifying what is the sort of patient that you like to treat. Transparency is good for everyone, and especially physicians. It helps the right patient show up for you, and it helps you do a better job providing referrals.
Social media: Avoid pitfalls, and use it to your benefit
Dr. Lotze: Branding was one of the things that I was confused about, and Heather really helped me out. As physicians, we put ourselves out there on our websites, which we try to make professional sources of information for patients. But patients often want to see what else they can find out about us, including Healthgrades and social media. I think the thing that is important to know with social media is that it is a place where people learn about you as a person. Your social media should be another avenue of promotion. Whether it is your personal or professional Facebook page, people are going to see those sites. You have an opportunity to promote yourself as a good physician and a good person with a wholesome practice that you want people to come to. If a physician is posting questionable things about themselves on any kind of social media, it could be perceived as inappropriate by the patient. That can impact how patients think of you as a person, and how they are going to grade you. If people lose sight of who you are due to a questionable social media posting, everything else (SEO, the website) can be for naught.
Dr. Culligan: What are the most important social media tools to invest your time in?
Ms. Schueppert: Before anybody jumps into social media, I firmly recommend that you make sure your local search and your Google 3-pack is set up—which is basically a method Google uses to display the top 3 results on its listings page. Then make sure you have a review management system in place. Make sure you have that updated website. Those are the foundational elements. Once you have that going, social media is the next added layer to that digital presence.
I usually recommend LinkedIn. It is huge because you are staying in contact with your colleagues, that business-to-business type of connection. It remains a way for physicians to set themselves up as experts in their level of specialty.
From there, it’s either Instagram or Facebook. If you are serving more of the younger generations, the millennials and younger, then Instagram is the way to go. If you are focusing on your 40+, 50+, they are going to be far more on Facebook.
Continue to: Dr. Lotze...
Dr. Lotze: For me, a Facebook page was a great place to start. The cost of those Google ads—the first things we see at the top of a Google search in their own separate box—is significant. If a practice has that kind of money to invest, great; it is an instant way to be first on the page during a search. But there are more cost-effective ways of doing that, especially as you are getting your name out. Facebook provides, at a smaller cost, promotion of whatever it is that you are seeking to promote. You can find people within a certain zip code, for instance, and use a Facebook ad campaign that can drive people to your Facebook page—which should have both routinely updated new posts and a link to your website. The posts should be interesting topics relevant to the patients you wish to treat (avoiding personal stories or controversial discussions). You can put a post together, or you can have a third-party service do this. People who follow your page will get reminders of you and your practice with each new post. As your page followers increase, your Facebook rank will improve, and your page will more likely be discovered by Facebook searches for your services. With an added link to your office practice website, those patients go straight to your site without getting lost in the noise of Google search results.
For Instagram, a short video or an interesting picture, along with a brief statement, are the essentials. You can add a single link. Marketing here is by direct messaging or having patients going to your website through a link. Instagram, like Facebook, offers analytics to help show you what your audience likes to read about, improving the quality of your posts and increasing number of followers.
YouTube is the number 2 search engine behind Google. A Google search for your field of medicine may be filled with pages of competitors. However, YouTube has a much lower volume of competing practices, making it easier for patients to find you. The only downside to YouTube is that it will list your video along with other competing videos, which can draw attention away from your practice.
If you want to promote your website or practice with video, using a company such as Vimeo is a better choice compared with YouTube, as YouTube gets credit for video views—which improves YouTube’s SEO and not your own website. Vimeo allows for your website to get credit each time the video is watched. Regardless of where you place your videos, make them short and to the point, with links to your website. Videos only need to be long enough to get your message across and stimulate interest in your practice.
If you can have a blog on your website, it also will help with SEO. What a search engine like Google wants to see is that a patient is on your web page and looking at something for at least 60 seconds. If so, the website is deemed to have information that is relevant, improving your SEO ranking.
Finally, Twitter also can be used for getting messaging out and for branding. The problem with it is that many people go to Twitter to follow a Hollywood celebrity, a sports star, or are looking for mass communication. There is less interest on Twitter for physician outreach.
Continue to: Measuring ROI...
Measuring ROI
Dr. Culligan: What’s the best way to track your return on investment?
Dr. Lotze: First for me was to find out what didn’t work in the office and fix that before really promoting my practice. It’s about the global experience for a patient, as Brad mentioned. As a marketing expert, Heather met with me to understand my goals. She then called my office as a patient to set up an appointment and went through that entire office experience. We identified issues needing improvement.
The next step was to develop a working relationship with my webmaster—someone who can help manage Internet image and SEO. Together, you will develop goals for what the SEO should promote specific to your practice. Once a good SEO program is in place, your website’s ranking will go up—although it can take a minimum of 6 months to see a significant increase. To help understand your website’s performance, your webmaster should provide you with reports on your site’s analytics.
As you go through this process, it is great to have a marketing expert to be the point person. You will work closely together for a while, but eventually you can back off over time. The time and expense you invest on the front end have huge rewards on the back end. Currently, I still spend a reasonable amount of money every month. I have a high self-referral base because of these efforts, however, which results in more patient surgeries and easily covers my expenses. It is money well invested. My website traffic increased by 268% over 2 years (FIGURE). I’ll propose that currently more than half of my patients are self-referrals due to online marketing.

Ms. Schueppert: The only thing I would add is training your front staff. They are checking people in, taking appointments, checking your patients out. Have them be mindful that there are campaigns going on, whether it is a social media push, or a new video that went on the website. They can ask, “How did you hear about us?” when a new patient calls.
Dr. Bowman: Unless you are a large university hospital, where the analytics get significantly more advanced in terms of measuring return on investment (ROI), I think you should just be looking at your schedule and looking at your monthly billings and seeing how they change over time. You can calculate how much a new patient is worth because you can figure out how many patients you have and how much you bill and what your profits are.
Dr. Culligan: For those of us who are hospital employees, you can try to convince the hospital that you can do a detailed ROI analysis, or you can just look at it like (say it’s $3,000 per month), how many surgeries does this project have to generate before the hospital makes that back? The answer is a fraction of 1 case.
Thank you to all of you for your expertise on this roundtable.
To have a thriving business in today’s world, a functioning website is crucial to the overall business health. For a medical practice in general, and for its physicians specifically, it is one of the first steps for maintaining a practice. But to grow that practice, it is crucial to take the steps beyond just having a website. Growth requires website optimization for search engines, an expanding referral base, and the knowledge to use web tools and social media at your disposal to promote the practice and its physicians. In this roundtable, several marketing experts and web-savvy physicians discuss using available tools to best position and grow a practice.
Choosing a web upgrade
Patrick J. Culligan, MD: Peter, can you start us off by describing your relationship with Heather, and how your practice benefitted from her expertise?
Peter M. Lotze, MD: Sure. I am a urogynecologist in the competitive market of pelvic reconstructive surgery in Houston, Texas. Within that market, my main approach was to reach out to other physicians to refer patients to my practice. It generally would work, but took increasingly greater amounts of time to call these physicians up, write them letters, and maintain relationships. I felt that the large, national practice group that I am in did not have a significant web presence optimized to promote my practice, which makes it difficult for patients seeking your services to find you in their search for a doctor. It is helpful for patients to be able to understand from your website who you are, what you do, and what their experience may be like.
Glaring to me was that a web search specific for me or things that I do, would not produce our company’s results until page 2 or more on Google. This can be devastating for a practice because most people don’t go past the first page, and you can end up with fewer self-referrals, which should be a significant portion of new patients to your practice. I knew I needed guidance; I knew of Heather’s expertise given her exceptional past work building marketing strategies.
Digital go-tos for marketing
Heather Schueppert: Yes, I was pleased to work with Dr. Lotze, and at the time was a marketing consultant for practices such as his. But gone are the days of printed material—brochures, pamphlets, or even billboards—to effectively promote a business, or in this case, a practice. What still withstands the test of time, however, as the number 1 marketing referral source is word of mouth—from your trusted friend, family member, or coworker.
It is now proven that the number 2 most trusted form of advertising, the most persuasive and the most motivating, is online marketing.1 It is the “digital word of mouth”—the review. Patients are actively online, and a strong digital presence is critical to provide that direct value to retain and grow your patient base.
Continue to: Foundations of private practice reach out...
Foundations of private practice reach out
There are 3 important areas that I consider the foundation of any private practice marketing strategy (TABLE). First is an updated website that is search engine optimized (SEO). You can’t just set it and forget it, it needs to be an updated website. The algorithms for search engines are changing constantly to try to make it as fair and relevant as possible for patients or consumers to find the businesses they are searching for online.

The second area is review management, and for a physician, or even a care center, to do this on your own is a daunting task. It is a critical component, however, to making sure that your reputation out there, that online word of mouth, is as high a star rating as possible.
The third component is local search, which is basically a form of SEO that helps businesses show up in relevant local searches. We are all familiar with the search, “find a restaurant near me,” anything that pushes those search engines to find something local.
Those are what I call the effective triad: that updated website, the review management, and the local search, and all of these are tied together. I think Dr. Lotze and his practice did these effectively well, and I believe that he achieved his goals for the longer term.
Review/reputation management
Dr. Culligan: Brad, is there something that doctors may not know about Healthgrades, and are there opportunities to take full advantage of this physician-rating site?
Brad Bowman, MD: I agree with everything that Dr. Lotze and Heather have said. Start with yourself—what is it that you want to be, the one thing you want to stand for? Get your own marketing, your website right, then, the point is, once you do all that and you are number 1 in SEO, you are still only going to get about 25% of the people looking for you by name to come to your website. The other 75% are going to look at all the other different sites that are out there to provide information to consumers. So the question becomes what do you do with all these other third-party sites? Healthgrades is the most comprehensive and has the highest traffic of the third-party “find a doctor” sites. In 2020, half of all Americans who go to a doctor will use Healthgrades at some point to help select and connect with that doctor.
Physicians have their focus on the quality of the care they provide. Patients, however, focus on the quality of the entire health care experience. Did I get better? How long did I have to wait? Was the office staff helpful? Scarily enough, we still spend more time shopping for a refrigerator or mattress than we do shopping for a doctor. We still tend to think that all doctors are the same. It is the reality of how we have been trained by our insurance companies and by the health care system. That is why getting your marketing right and getting what is it that you want to be known for out there is important, so that you can get the types of patients you want.
Listings management is very important. Make sure that you are findable everywhere. There are services that will do this: Doctor.com, Reputation.com, and many others. They can help you make sure you get all your basic materials right: addresses, phone numbers, your picture. Because 75% of people are going to end up on third-party websites, if your phone number is wrong there, you could lose that patient.
Then the second piece of working with third-party sites is reputation management. Physician reviews are not a bad thing, they are the new word of mouth, as Heather pointed out. Most (80%) of the reviews are going to be positive. The others will be negative, and that is okay. It is important that you get at least 1 or 2 reviews on all the different sites. We know from Healthgrades.com that going from zero reviews to 1 review will increase your call volume by 60%. If you have the choice between 2 physicians and one practice looks like people have been there before, you will go to that one.
You can learn from reviews as well, consumers provide valid feedback. Best practice is to respond to every positive and negative review. Thank them, indicate that you have listened to them, and address any concerns as necessary.
Continue to: Dr. Lotze...
Dr. Lotze: As an example, one of the paramount things that Heather introduced me to was the third party I use to run my website. That company sends a HIPAA-compliant review out to each patient we have seen that day and gives them the opportunity to rate our services and leave comments. If a patient brings up a concern, we can respond immediately, which is important. Patients appreciate feeling that they have been heard. Typically, communicating with a patient will turn the 3-star review into a 5-star as she follows up with the practice.
Ms. Schueppert: Timeliness is important. And just to mention, there certainly is a time commitment to this (and it is a marathon versus a sprint) and there is some financial investment to get it going, but it could truly be detrimental to a practice if you decide not to do anything at all.
Dr. Bowman: Agencies can really help with the time commitment.
Handling bad reviews
Dr. Culligan: What about that person who seems to have it out for you, perhaps giving you multiple bad reviews?
Dr. Bowman: I have seen this before. At Healthgrades, we recently analyzed 8.4 million patient reviews to see what people wrote about.2 The first thing they will talk about is quality of care as they see it. Did I get better or not? You can’t “fix” every patient; there will be some that you cannot help. The next thing patients comment on is bedside manner. With negative reviews, you will see more comments about the office staff.2
A single negative review actually helps make the positive ones look more credible. But if you do believe someone is trolling you, we can flag it and will investigate to the best of our ability. (Different sites likely have different editorial policies.) For example, we look at the IP addresses of all reviews, and if multiple reviews are coming from the same location, we would only let one through, overwriting the previous review from that address.
Patients just want to be heard. We have seen people change their views, based on how their review is handled and responded to.
Dr. Lotze: Is there a response by the physician that you think tends to work better in terms of resolving the issue that can minimize a perceived caustic reaction to a patient’s criticism?
Dr. Bowman: First, just like with any stressful situation, take a deep breath and respond when you feel like you can be constructive. When you do respond, be gracious. Thank them for their feedback. Make sure you reference something about their concern: “I understand that you had to wait longer than you would have liked.” Acknowledge the problem they reference, and then just apologize: “I’m sorry we didn’t meet your expectations.” Then, if they waited too long for example, “We have a new system where no one should have to wait more than 30 minutes….” You can respond privately or publically. Generally, public responses are better as it shows other consumers that you are willing to listen and consider their point of view.
Continue to: The next phase at Healthgrades...
The next phase at Healthgrades
Dr. Culligan: Do you see changes to the way physician-rating sites are working now? Are we going to stay status quo over the next 10 years, or do you see frontiers in how your site is going to develop?
Dr. Bowman: For Healthgrades, we rely on quantitative and objective measures, not just the qualitative. We are investing heavily right now in trying to help consumers understand what are the relative volumes of different procedures or different patient types that each individual doctor sees. Orthopedics is an easy example—if you have a knee problem, you want to go to someone who specializes in knees. Our job is to help consumers easily identify, “This is a shoulder doctor, this is a knee doctor, and this is why that matters.”
In the meantime, as a physician, you can always go into our site and state your care philosophy, identifying what is the sort of patient that you like to treat. Transparency is good for everyone, and especially physicians. It helps the right patient show up for you, and it helps you do a better job providing referrals.
Social media: Avoid pitfalls, and use it to your benefit
Dr. Lotze: Branding was one of the things that I was confused about, and Heather really helped me out. As physicians, we put ourselves out there on our websites, which we try to make professional sources of information for patients. But patients often want to see what else they can find out about us, including Healthgrades and social media. I think the thing that is important to know with social media is that it is a place where people learn about you as a person. Your social media should be another avenue of promotion. Whether it is your personal or professional Facebook page, people are going to see those sites. You have an opportunity to promote yourself as a good physician and a good person with a wholesome practice that you want people to come to. If a physician is posting questionable things about themselves on any kind of social media, it could be perceived as inappropriate by the patient. That can impact how patients think of you as a person, and how they are going to grade you. If people lose sight of who you are due to a questionable social media posting, everything else (SEO, the website) can be for naught.
Dr. Culligan: What are the most important social media tools to invest your time in?
Ms. Schueppert: Before anybody jumps into social media, I firmly recommend that you make sure your local search and your Google 3-pack is set up—which is basically a method Google uses to display the top 3 results on its listings page. Then make sure you have a review management system in place. Make sure you have that updated website. Those are the foundational elements. Once you have that going, social media is the next added layer to that digital presence.
I usually recommend LinkedIn. It is huge because you are staying in contact with your colleagues, that business-to-business type of connection. It remains a way for physicians to set themselves up as experts in their level of specialty.
From there, it’s either Instagram or Facebook. If you are serving more of the younger generations, the millennials and younger, then Instagram is the way to go. If you are focusing on your 40+, 50+, they are going to be far more on Facebook.
Continue to: Dr. Lotze...
Dr. Lotze: For me, a Facebook page was a great place to start. The cost of those Google ads—the first things we see at the top of a Google search in their own separate box—is significant. If a practice has that kind of money to invest, great; it is an instant way to be first on the page during a search. But there are more cost-effective ways of doing that, especially as you are getting your name out. Facebook provides, at a smaller cost, promotion of whatever it is that you are seeking to promote. You can find people within a certain zip code, for instance, and use a Facebook ad campaign that can drive people to your Facebook page—which should have both routinely updated new posts and a link to your website. The posts should be interesting topics relevant to the patients you wish to treat (avoiding personal stories or controversial discussions). You can put a post together, or you can have a third-party service do this. People who follow your page will get reminders of you and your practice with each new post. As your page followers increase, your Facebook rank will improve, and your page will more likely be discovered by Facebook searches for your services. With an added link to your office practice website, those patients go straight to your site without getting lost in the noise of Google search results.
For Instagram, a short video or an interesting picture, along with a brief statement, are the essentials. You can add a single link. Marketing here is by direct messaging or having patients going to your website through a link. Instagram, like Facebook, offers analytics to help show you what your audience likes to read about, improving the quality of your posts and increasing number of followers.
YouTube is the number 2 search engine behind Google. A Google search for your field of medicine may be filled with pages of competitors. However, YouTube has a much lower volume of competing practices, making it easier for patients to find you. The only downside to YouTube is that it will list your video along with other competing videos, which can draw attention away from your practice.
If you want to promote your website or practice with video, using a company such as Vimeo is a better choice compared with YouTube, as YouTube gets credit for video views—which improves YouTube’s SEO and not your own website. Vimeo allows for your website to get credit each time the video is watched. Regardless of where you place your videos, make them short and to the point, with links to your website. Videos only need to be long enough to get your message across and stimulate interest in your practice.
If you can have a blog on your website, it also will help with SEO. What a search engine like Google wants to see is that a patient is on your web page and looking at something for at least 60 seconds. If so, the website is deemed to have information that is relevant, improving your SEO ranking.
Finally, Twitter also can be used for getting messaging out and for branding. The problem with it is that many people go to Twitter to follow a Hollywood celebrity, a sports star, or are looking for mass communication. There is less interest on Twitter for physician outreach.
Continue to: Measuring ROI...
Measuring ROI
Dr. Culligan: What’s the best way to track your return on investment?
Dr. Lotze: First for me was to find out what didn’t work in the office and fix that before really promoting my practice. It’s about the global experience for a patient, as Brad mentioned. As a marketing expert, Heather met with me to understand my goals. She then called my office as a patient to set up an appointment and went through that entire office experience. We identified issues needing improvement.
The next step was to develop a working relationship with my webmaster—someone who can help manage Internet image and SEO. Together, you will develop goals for what the SEO should promote specific to your practice. Once a good SEO program is in place, your website’s ranking will go up—although it can take a minimum of 6 months to see a significant increase. To help understand your website’s performance, your webmaster should provide you with reports on your site’s analytics.
As you go through this process, it is great to have a marketing expert to be the point person. You will work closely together for a while, but eventually you can back off over time. The time and expense you invest on the front end have huge rewards on the back end. Currently, I still spend a reasonable amount of money every month. I have a high self-referral base because of these efforts, however, which results in more patient surgeries and easily covers my expenses. It is money well invested. My website traffic increased by 268% over 2 years (FIGURE). I’ll propose that currently more than half of my patients are self-referrals due to online marketing.

Ms. Schueppert: The only thing I would add is training your front staff. They are checking people in, taking appointments, checking your patients out. Have them be mindful that there are campaigns going on, whether it is a social media push, or a new video that went on the website. They can ask, “How did you hear about us?” when a new patient calls.
Dr. Bowman: Unless you are a large university hospital, where the analytics get significantly more advanced in terms of measuring return on investment (ROI), I think you should just be looking at your schedule and looking at your monthly billings and seeing how they change over time. You can calculate how much a new patient is worth because you can figure out how many patients you have and how much you bill and what your profits are.
Dr. Culligan: For those of us who are hospital employees, you can try to convince the hospital that you can do a detailed ROI analysis, or you can just look at it like (say it’s $3,000 per month), how many surgeries does this project have to generate before the hospital makes that back? The answer is a fraction of 1 case.
Thank you to all of you for your expertise on this roundtable.
- Anderson A. Online reviews vs. word of mouth: Which one is more important. https://www.revlocal.com/blog/reviewandreputationmanagement/onlinereviewsvswordofmouthwhichoneismoreimportant. Accessed July 17, 2020.
- 2020 Patient sentiment report. Healthgrades; Medical Group Management Association. https://www.healthgrades.com/content /patientsentimentreport. Accessed July 17, 2020
- Anderson A. Online reviews vs. word of mouth: Which one is more important. https://www.revlocal.com/blog/reviewandreputationmanagement/onlinereviewsvswordofmouthwhichoneismoreimportant. Accessed July 17, 2020.
- 2020 Patient sentiment report. Healthgrades; Medical Group Management Association. https://www.healthgrades.com/content /patientsentimentreport. Accessed July 17, 2020
COVID-SAFE: Strategies for safeguarding your outpatient clinical practice against COVID-19
No question, the COVID-19 pandemic has been a challenging time for medical practices across the United States. Uncertainty remains regarding bringing patients and services back into our offices. One factor that distinguishes many ObGyn practices from other specialties is that our practices have remained open—in some form—since the beginning of the pandemic. In various parts of the country, gynecologic surgeries and routine office visits have been significantly reduced; however, deliveries and gynecologic emergencies have continued.
In this article, I suggest a framework of strategies and resources to provide insight for outpatient operations. Individual practices will vary across the nation depending on local conditions. Full practice capacity may take on a different look than it had prior to the pandemic, and there is opportunity to change the way we operate.
Strategy 1: Consult regulatory requirements frequently
As the local status of COVID-19 evolves quickly, it is essential to examine the frequently updated recommendations from regulatory agencies at the federal, state, and local levels. Clinical practices that function within health systems need to demonstrate alignment with hospital or university policies and procedures. The Centers for Disease Control and Prevention (CDC), Occupational Safety and Health Administration (OSHA), Centers for Medicare and Medicaid Services (CMS), and individual state departments of health provide dynamic resources that are easily accessible online.1-3
The American College of Obstetricians and Gynecologists (ACOG) continues to be an excellent medical society resource.4 Subspecialty organizations that provide up-to-date guidance include the Society for Maternal-Fetal Medicine (SMFM), Society of Gynecologic Surgeons (SGS), AAGL (American Association of Gynecologic Laparoscopists), American Society for Reproductive Medicine (ASRM), and Society of Gynecologic Oncology (SGO).5-9 These resources are updated as more information about COVID-19 emerges, and they may be modified to different local-regional conditions.
The professional liability insurance carrier is an important source of insight for a number of circumstances, including modifications to your office practice, such as returning to full-scope or part-time practice; operating outside normal clinical service arrangements (for example, assisting with emergency care); offering telehealth services; and adding extra hours or employees to accommodate the patient backlog. Business insurance coverage is a separate issue to consider. Reviewing the practice policy may protect your business from COVID-related liabilities.
Consulting with legal counselors can be helpful. They can assist with navigating various practice and personnel COVID-related changes, as well as developing a viable plan for patients who were previously insured pre–COVID-19 who are currently uninsured.
Continue to: Strategy 2: Reimagine schedule capacity...
Strategy 2: Reimagine schedule capacity
The waxing and waning of the COVID-19 crisis presents an opportunity to evaluate our office practices and make necessary and positive changes. The question becomes, do we operate our practices as usual or do we rethink our strategy for seeing patients and integrate lessons learned from the pandemic? Patients are deciding when they are comfortable to schedule elective surgeries and routine office encounters. This gives us the chance to break from the tradition of 100% in-person visits and change the way we care for women.
The coronavirus has accelerated the rise of telehealth/telemedicine and is, perhaps, a silver lining of the pandemic. Telehealth is a valuable tool for accessing health services when in-person visits are not possible. Evaluating and triaging patients for in-person versus telehealth visits is now a viable option for clinical practice and reduces exposure to COVID-19 infection.
Telemedicine is convenient, and clinicians can use it to counsel and screen for various health issues as well as to extend their reach to rural communities. Appropriate consent should be documented in the patient chart. As some areas continue to be without adequate access to WiFi, telephone contact also is currently acceptable. Telehealth does not replace the in-person visit but can be viewed as a complementary and supplementary service.
Consider a balance between telehealth and in-person visits by evaluating which visits can continue remotely and which can alternate with in-person visits. This offers tremendous flexibility and will expand delivery of essential health care to patients.10 Integrating telemedicine into clinical practice provides an additional benefit: It minimizes the exposure and transmission of COVID-19 to health care workers and patients and preserves supplies, including personal protective equipment (PPE).
Prioritize the backlog of patients who require follow-up testing, procedures, and surgeries. Communicate with patients that it is safe to be seen and important to not avoid routine and preventative visits that might reveal concerns or conditions that require treatment.
Strategy 3: Institute infection prevention and control measures
The importance of instituting and ensuring safety measures for office personnel and patients cannot be underestimated. Recently, a study from King’s College in London found that frontline health care workers with PPE still have 3 to 4 times the risk of contracting coronavirus compared with the general public.11 Health care systems should ensure adequate PPE availability and develop additional strategies to protect health care workers from COVID-19. We have to be fanatical about cleanliness and PPE. We have to be diligent about how we space ourselves and our patients. Consider adjusting workflows to ensure that visits can be conducted as quickly and safely as possible.
Communicating updated safety plans and processes are invaluable for both patients and health care workers. Patients want to be reassured that safety precautions are in place to keep the environment safe and clean. Additionally, privacy and confidentiality concerns should be addressed.
Consider a modified office schedule that can reduce the number of people in the office, person-to-person contact, and COVID-19 transmission. Social distancing is improved and PPE and other supplies are preserved.
Continue to: Employees can work on alternating days...
- Employees can work on alternating days or during different parts of the day.
- Administrative staff who do not need to be physically present in the office might work remotely.
- Expanding office hours (early morning, evening, and weekends) spreads patient visits throughout the day and minimizes high-volume in-person visits.
Institute a daily COVID-19 symptom attestation and temperature check for employees on arrival at work.
Health care personnel with symptoms of COVID-19 should be prioritized for SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) RNA testing with an approved nucleic acid or antigen detection assay. A negative result indicates that the person most likely did not have an active SARS-CoV-2 infection at the time the sample was collected. A second test for SARS-CoV-2 RNA may be performed at the discretion of the evaluating health care provider, particularly when a higher level of clinical suspicion for infection exists.
The return to work decision should be determined by an agreed on symptom-based approach to clearance. If needed on a case-by-case basis, a review can be performed with the individual’s health care provider.12
Require universal masking and appropriate protective equipment.
- All staff members, patients, and visitors must wear masks correctly in the facilities (except children under age 2).
- All clinical staff members must wear masks correctly and eye protection during every patient encounter.
Reconfigure the waiting room and patient flow.
- Configure waiting room furniture to reinforce 6 feet of physical distancing.
- Remove all books, magazines, and toys from all waiting areas.
- Laminate signage for display.
- Install plexiglass at the check-in desk to minimize virus transmission.
- If possible, ask patients to wait in their car until their appointment time or to go directly to their exam room on arrival if it is available.
- Implement virtual check-in and check-out so that patients reduce unnecessary contact with surfaces and staff.
- Limit a high volume of patients to maintain social distancing etiquette, avoid delays, and allow adequate cleaning time between patients.
- Permit visitors to accompany adult patients to their ambulatory appointments only if special assistance is required.
- Limit the number of staff members in the exam and treatment rooms and maintain at least 6 feet between people except during medical care activities.
- Consider patient flow in a one-way traffic pattern.
Focus on keeping the clinical practice clean. (Follow the instructions and disinfect with a registered disinfectant product that meets the US Environmental Protection Agency criteria for use against COVID-19.13)
- Clean waiting rooms and restrooms frequently.
- Coordinate patient appointments to allow for infection control measures.
- Frequently clean high-touch surfaces, including tables, doorknobs, light switches, countertops, handles, desks, phones, keyboards, toilets, faucets, and sinks.
- Clinicians and all medical staff members should wash their hands before and after interacting with patients.
- Clean and disinfect the exam and treatment rooms before and after each patient.
- Use products that are effective against a range of organisms and viruses, including the coronavirus that causes COVID-19.
- Place signs indicating that rooms have been cleaned; this will assure and comfort patients. Take credit for your infection control processes.
Keep abreast of isolation and precaution guidelines. Based on data available at the time of this article’s publication, the CDC recommends ending isolation and transmission-based precautions for most people with COVID-19 using a symptom-based strategy.14 This limits unnecessary prolonged isolation and use of laboratory testing resources.
Generally, repeat SARS-CoV-2 polymerase chain reaction (PCR) testing is not recommended for “COVID-19 recovered” patients. Specifically, those patients with a prior positive SARS-CoV-2 PCR test result and who have met criteria for isolation discontinuation do not need a follow-up PCR test. A test-based strategy to discontinue isolation and transmission-based precautions is required only for severely immunocompromised patients.15
Prepare for a future COVID-19 surge and review your emergency plan and responses and revise as needed. Review handling of the current pandemic and best practices plus areas of improvement.
Symptom-based criteria for discontinuing transmission-based precautions include the following:
Patients with mild to moderate illness, not severely immunocompromised:
- at least 10 days have passed since symptoms first appeared and
- at least 24 hours have passed since last fever without fever-reducing medications and
- symptoms (cough, shortness of breath) have improved.
Note: For patients who are not severely immunocompromised and are asymptomatic throughout their infection, transmission-based precautions may be discontinued when at least 10 days have passed since the date of their first positive viral diagnostic test.
Patients with severe to critical illness, severely immunocompromised:
- at least 20 days have passed since symptoms first appeared and
- at least 24 hours have passed since last fever without fever-reducing medications and
- symptoms (cough, shortness of breath) have improved.
Note: For patients who are severely immunocompromised and are asymptomatic throughout their infection, transmission-based precautions may be discontinued when at least 20 days have passed since the date of their first positive viral diagnostic test.
Continue to: Strategy 4: Implement frequent employee communication and care...
Strategy 4: Implement frequent employee communication and care
The safety and well-being of our health care workers and patients in our clinical practices is paramount. Continuing to communicate this message and developing and sharing a plan may ameliorate the obvious toll on mental and emotional well-being. Frequent and effective communication with your clinical team is vital to reinforce policies and protocols, eliminate silos, and reduce errors.
Practice communication and care with these approaches:
- Offer regular employee COVID-19 testing.
- Re-educate staff about infection control protocols to ensure buy-in.
- Communicate with staff about the plan to address staffing shortages.
- Implement regular employee team huddles that can address accomplishments, challenges, areas for improvement, and top priorities.
- Perform regular celebrations for staff appreciation.
- Address mental health and chronic stress and offer empathy and coping resources and services to staff and clinicians. This will have a valuable, long-term benefit.
Patient communication. As the COVID-19 pandemic continues and stay-at-home policies are in place, patients should be encouraged to seek medical care if they are ill or have acute or chronic conditions. Communicate regularly with patients and let them know that their safety and well-being is the top priority. Prior to in-person visits, inform them of the safety processes that are in place to protect them.
Fostering an honest clinician-patient relationship enhances communication. Despite these efforts, some patients may not be forthcoming about their COVID-19 symptoms, illness, exposure, or travel. Health care staff can be encouraged to set a tone of tolerance and compassion and treat everyone with universal precautions.
Rising to the challenges
During the coronavirus pandemic, ObGyns continue to safely care for pregnant women and also triage and treat women who require timely office care as well as emergency and cancer-related surgeries.
The COVID-19 environment rapidly changes depending on the practice location. The strategies described represent a compilation of resources from key organizations that hopefully will prove useful and can be shaped to fit your practice. Local and regional recommendations vary, and no one can predict the course of the virus.
Consider reviewing your contingency plans regularly. As we have learned over the last several months, there is a science to maintaining a COVID-SAFE environment.
Practice operations likely will change to adapt to new conditions. The pandemic has challenged us to evolve, and we have responded with new capabilities and resilience while we continue to deliver superior and compassionate care for women.
For additional strategies on how to safeguard your practice against COVID-19, see the box below. ●
Continue to: Additional strategies on how to safeguard your practice against COVID-19...
Strategy 5: Develop a resource plan for practice operations
Assess financial solvency. Because of the mitigation measures taken during the pandemic, physician practices of all sizes are facing financial hardships and instability. As the pandemic progresses, physicians in private practice and those employed by health systems may benefit from existing resources and pandemic relief to help navigate COVID-related challenges.
Frequent revision of your financial plan may safeguard cash flow in the event of fewer patient visits and elective surgeries. Many medical organizations, including ACOG, are advocating for financial relief, fair reimbursement for telehealth and in-person visits, and access to adequate PPE. ACOG provides updated information on practice management.1
The American Medical Association (AMA) has created resources for physician practices to assist in staying focused on business and financial operations. The AMA has provided a summary of the Health, Economic Assistance, Liability Protection and Schools Act (HEALS Act).2 This is the next proposed coronavirus relief fund package, which includes provisions that benefit physicians and physician practices.
Create a plan. Review available resources and establish processes to optimize your practice capacity during the ongoing COVID-19 pandemic. Develop a game plan for patient care with a phased approach to identify and address challenges. This planning will allow your practice to pivot in response to changing local COVID-19 conditions to help you anticipate and prepare for a future surge. Maintain and revise plans as the pandemic shifts. Thinking ahead avoids the need to navigate issues in real time. Communicating clearly and often with all members of the office staff and patients lets everyone know that their safety is the main priority.
Assess staffing for flexible coverage. Frequent needs assessment helps to determine the number of staff needed to maintain a safe work environment for the patient volume.
Staff shortages may occur because of COVID-19 exposure, personal or family member illness, or childcare constraints due to daycare or school availability. Staff readiness includes evaluating individual availability and willingness.
Staff members with health issues, including comorbidities and chronic medical conditions, may not be comfortable working. Nonclinical staff members with health concerns could work remotely, although some may not be able to work from home due to technology-related issues such as WiFi deficiencies.
The CDC has interim guidelines to assist employers with providing a safe workplace and employees with making the best health decisions for themselves and their families.3,4 The US Office of Personnel Management provides guidance for COVID-19–related leave and benefits for federal employees.5
To mitigate staff shortages, approaches include adjusting schedules, cross-training to perform the tasks of other positions, and hiring additional personnel. A needs assessment can help determine if existing personnel could be cross-trained for other purposes or if additional staff should be hired. Understanding the minimum number of staff required for safe and effective patient care will assist in planning for shortages as the pandemic progresses. Understanding the availability of external resources could be a critical part of an office contingency plan.6
Proactively manage your supply chain. The pandemic has caused global supply shortages. Solid supply chain management is crucial for practice operations. Take inventory of your PPE and various supplies and place orders in advance. Analyze cash flow and connect with vendors as well as local and state health agencies to understand available resources. Given ongoing PPE shortages, practices should consider preserving PPE and employ appropriate strategies for optimizing supplies of face masks.7
Certain medications and vaccines administered in the office setting may be outdated and need to be replaced. Office equipment that has not been used for several months will need to be tested. For equipment used in office electrosurgery procedures, certain safety measures can be taken to reduce the transmission of aerosolized viral particles to the health care team. While currently the risk is theoretical and more research is needed, this potential risk should be mitigated.8 Assessing availability of hospital and ambulance or transport services also is recommended as these may change depending on the local COVID-19 status.
Strategy 6: Establish and refine the patient screening process
Patients want reassurance that the health care environment is safe and that their well-being is a priority. In advance of a patient’s visit, relieve any anxiety by explaining the COVID-SAFE measures that your practice has taken.
For employee use, consider telephone and in-person scripting to ensure consistent messaging for patients.
Prescreening. At the time of appointment scheduling and on the day prior to the scheduled appointment, all patients should be screened for symptoms of COVID-19,9 fever, exposure within 14 days to someone newly diagnosed with COVID-19, and travel within 14 days from a foreign country or from a US state with a quarantine requirement.
Patients who screen positive for symptoms, exposure, or travel should be referred to a clinician. If possible, asymptomatic patients who report exposure or travel should have their in-person visit deferred until after the required 14-day quarantine.
Consider restricting visitors from accompanying adult patients to their appointment unless they are required for special assistance.
Arrival screening. At the time of presentation for the appointment, all patients and any accompanying visitors should be rescreened. The optimal location for arrival screening should be determined by the local operations team and the infection prevention and control program.
At presentation, all patients and visitors should appropriately don a surgical mask or other face covering. Patients and visitors should have their temperature checked on arrival. Patients who screen positive for symptoms, exposure to COVID-19, and/or travel should be referred to a clinician or the visit deferred and a telehealth visit considered.
Visitors who screen positive for symptoms, fever, or exposure to COVID-19 are not permitted to accompany the patient. Asymptomatic parents or guardians of pediatric patients may serve as support persons.
References
- American College of Obstetricians and Gynecologists. Financial support for physicians and practices during the COVID-19 pandemic. https://www.acog.org/practice -management/coding/coding-library/financial-support-for-physicians-and-practices-during-the-covid-19-pandemic. Accessed July 23, 2020.
- American Medical Association. HEALS Act: What physicians and medical students need to know. https://www.ama-assn.org/delivering-care/public-health/heals-act -what-physicians-and-medical-students-need-know. Accessed July 29, 2020.
- Centers for Disease Control and Prevention. Interim guidance for businesses and employers responding to coronavirus disease 2019 (COVID-19). https://www.cdc.gov /coronavirus/2019-ncov/community/guidance-business-response.html. Accessed July 12, 2020.
- Centers for Disease Control and Prevention. Criteria for return to work for healthcare personnel with SARS-CoV-2 infection (interim guidance). https://www.cdc.gov /coronavirus/2019-ncov/hcp/return-to-work.html. Accessed July 20, 2020.
- US Office of Personnel Management. Questions and answers on human resources flexibilities and authorities for coronavirus disease 2019 (COVID-19). https://www .opm.gov/policy-data-oversight/covid-19/questions-and-answers-on-human-resources-flexibilities-and-authorities-for-coronavirus-disease-2019-covid-19.pdf. Accessed July 3, 2020.
- Centers for Disease Control and Prevention. Strategies to mitigate healthcare personnel staffing shortages. https://www.cdc.gov/coronavirus/2019-ncov/hcp /mitigating-staff-shortages.html. Accessed July 17, 2020.
- Centers for Disease Control and Prevention. Strategies for optimizing the supply of facemasks. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/face -masks.html. Accessed July 3, 2020.
- Rahman S, Klebanoff J, Moawad G. Smoke evacuation in the age of COVID-19. Contemporary OB/GYN. July 2, 2020. https://www.contemporaryobgyn.net/view/smoke -evacuation-in-the-age-of-covid-19. Accessed July 3, 2020.
- Centers for Disease Control and Prevention. Symptoms of coronavirus. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed July 25, 2020.
- Centers for Disease Control and Prevention. Information for healthcare professionals about coronavirus (COVID-19). https://www.cdc.gov/coronavirus/2019-nCoV/hcp/index.html. Accessed July 22, 2020.
- US Department of Labor Occupational Safety and Health Administration. COVID-19 control and prevention: healthcare workers and employers. https://www.osha.gov/SLTC/covid-19/healthcare-workers.html. Accessed August 5, 2020.
- Centers for Medicare and Medicaid Services. COVID-19 resources for health care professionals and researchers. https://www.cms.gov/About-CMS/Agency-Information/OMH/resource-center/hcps-and-researchers. Accessed August 7, 2020.
- American College of Obstetricians and Gynecologists. COVID-19. https://www.acog.org/topics/covid-19. Accessed August 7, 2020.
- Society for Maternal-Fetal Medicine. Coronavirus (COVID-19). https://www.smfm.org/covid19. Accessed August 7, 2020.
- Society of Gynecologic Surgeons. Joint statement on re-introduction of hospital and office-based procedures in the COVID-19 climate for the practicing gynecologist. https://www.sgsonline.org/joint-statement-on-re-introduction-of-hospital-and-office-based-procedures-in-the-covid-19-climate. Accessed August 7, 2020.
- AAGL. COVID-19 articles, resources and webinars. https://www.aagl.org/covid-19/. Accessed August 7, 2020.
- American Society for Reproductive Medicine. COVID-19 updates and resources. https://www.asrm.org/news-and-publications/covid-19/. Accessed August 7, 2020.
- Society of Gynecologic Oncology. COVID-19 resources for health care practitioners. https://www.sgo.org/practice-management/covid-19/. Accessed August 7, 2020.
- Centers for Disease Control and Prevention. Using telehealth to expand access to essential health services during the COVID-19 pandemic. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html. Accessed July 3, 2020.
- Nguyen LH, Drew DA, Graham MS, et al; on behalf of the COronavirus Pandemic Epidemiology Consortium. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet. July 31, 2020. http://www.thelancet-press.com/embargo/hcwcovid.pdf. Accessed August 25, 2020.
- Centers for Disease Control and Prevention. Criteria for return to work for healthcare personnel with SARS-CoV-2 infection (interim guidance). https://www.cdc.gov/coronavirus/2019-ncov/hcp/return-to-work.html. Accessed July 20, 2020.
- US Environmental Protection Agency. List N: Disinfectants for use against SARS-CoV-2 (COVID-19). https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2-covid-19. Accessed August 24, 2020.
- Centers for Disease Control and Prevention. Duration of isolation and precautions for adults with COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html. Accessed July 23, 2020.
- Centers for Disease Control and Prevention. Discontinuation of transmission-based precautions and disposition of patients with COVID-19 in healthcare settings (interim guidance). https://www.cdc.gov/coronavirus/2019-ncov/hcp/disposition-hospitalized-patients.html#fn1. Accessed July 23, 2020.
No question, the COVID-19 pandemic has been a challenging time for medical practices across the United States. Uncertainty remains regarding bringing patients and services back into our offices. One factor that distinguishes many ObGyn practices from other specialties is that our practices have remained open—in some form—since the beginning of the pandemic. In various parts of the country, gynecologic surgeries and routine office visits have been significantly reduced; however, deliveries and gynecologic emergencies have continued.
In this article, I suggest a framework of strategies and resources to provide insight for outpatient operations. Individual practices will vary across the nation depending on local conditions. Full practice capacity may take on a different look than it had prior to the pandemic, and there is opportunity to change the way we operate.
Strategy 1: Consult regulatory requirements frequently
As the local status of COVID-19 evolves quickly, it is essential to examine the frequently updated recommendations from regulatory agencies at the federal, state, and local levels. Clinical practices that function within health systems need to demonstrate alignment with hospital or university policies and procedures. The Centers for Disease Control and Prevention (CDC), Occupational Safety and Health Administration (OSHA), Centers for Medicare and Medicaid Services (CMS), and individual state departments of health provide dynamic resources that are easily accessible online.1-3
The American College of Obstetricians and Gynecologists (ACOG) continues to be an excellent medical society resource.4 Subspecialty organizations that provide up-to-date guidance include the Society for Maternal-Fetal Medicine (SMFM), Society of Gynecologic Surgeons (SGS), AAGL (American Association of Gynecologic Laparoscopists), American Society for Reproductive Medicine (ASRM), and Society of Gynecologic Oncology (SGO).5-9 These resources are updated as more information about COVID-19 emerges, and they may be modified to different local-regional conditions.
The professional liability insurance carrier is an important source of insight for a number of circumstances, including modifications to your office practice, such as returning to full-scope or part-time practice; operating outside normal clinical service arrangements (for example, assisting with emergency care); offering telehealth services; and adding extra hours or employees to accommodate the patient backlog. Business insurance coverage is a separate issue to consider. Reviewing the practice policy may protect your business from COVID-related liabilities.
Consulting with legal counselors can be helpful. They can assist with navigating various practice and personnel COVID-related changes, as well as developing a viable plan for patients who were previously insured pre–COVID-19 who are currently uninsured.
Continue to: Strategy 2: Reimagine schedule capacity...
Strategy 2: Reimagine schedule capacity
The waxing and waning of the COVID-19 crisis presents an opportunity to evaluate our office practices and make necessary and positive changes. The question becomes, do we operate our practices as usual or do we rethink our strategy for seeing patients and integrate lessons learned from the pandemic? Patients are deciding when they are comfortable to schedule elective surgeries and routine office encounters. This gives us the chance to break from the tradition of 100% in-person visits and change the way we care for women.
The coronavirus has accelerated the rise of telehealth/telemedicine and is, perhaps, a silver lining of the pandemic. Telehealth is a valuable tool for accessing health services when in-person visits are not possible. Evaluating and triaging patients for in-person versus telehealth visits is now a viable option for clinical practice and reduces exposure to COVID-19 infection.
Telemedicine is convenient, and clinicians can use it to counsel and screen for various health issues as well as to extend their reach to rural communities. Appropriate consent should be documented in the patient chart. As some areas continue to be without adequate access to WiFi, telephone contact also is currently acceptable. Telehealth does not replace the in-person visit but can be viewed as a complementary and supplementary service.
Consider a balance between telehealth and in-person visits by evaluating which visits can continue remotely and which can alternate with in-person visits. This offers tremendous flexibility and will expand delivery of essential health care to patients.10 Integrating telemedicine into clinical practice provides an additional benefit: It minimizes the exposure and transmission of COVID-19 to health care workers and patients and preserves supplies, including personal protective equipment (PPE).
Prioritize the backlog of patients who require follow-up testing, procedures, and surgeries. Communicate with patients that it is safe to be seen and important to not avoid routine and preventative visits that might reveal concerns or conditions that require treatment.
Strategy 3: Institute infection prevention and control measures
The importance of instituting and ensuring safety measures for office personnel and patients cannot be underestimated. Recently, a study from King’s College in London found that frontline health care workers with PPE still have 3 to 4 times the risk of contracting coronavirus compared with the general public.11 Health care systems should ensure adequate PPE availability and develop additional strategies to protect health care workers from COVID-19. We have to be fanatical about cleanliness and PPE. We have to be diligent about how we space ourselves and our patients. Consider adjusting workflows to ensure that visits can be conducted as quickly and safely as possible.
Communicating updated safety plans and processes are invaluable for both patients and health care workers. Patients want to be reassured that safety precautions are in place to keep the environment safe and clean. Additionally, privacy and confidentiality concerns should be addressed.
Consider a modified office schedule that can reduce the number of people in the office, person-to-person contact, and COVID-19 transmission. Social distancing is improved and PPE and other supplies are preserved.
Continue to: Employees can work on alternating days...
- Employees can work on alternating days or during different parts of the day.
- Administrative staff who do not need to be physically present in the office might work remotely.
- Expanding office hours (early morning, evening, and weekends) spreads patient visits throughout the day and minimizes high-volume in-person visits.
Institute a daily COVID-19 symptom attestation and temperature check for employees on arrival at work.
Health care personnel with symptoms of COVID-19 should be prioritized for SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) RNA testing with an approved nucleic acid or antigen detection assay. A negative result indicates that the person most likely did not have an active SARS-CoV-2 infection at the time the sample was collected. A second test for SARS-CoV-2 RNA may be performed at the discretion of the evaluating health care provider, particularly when a higher level of clinical suspicion for infection exists.
The return to work decision should be determined by an agreed on symptom-based approach to clearance. If needed on a case-by-case basis, a review can be performed with the individual’s health care provider.12
Require universal masking and appropriate protective equipment.
- All staff members, patients, and visitors must wear masks correctly in the facilities (except children under age 2).
- All clinical staff members must wear masks correctly and eye protection during every patient encounter.
Reconfigure the waiting room and patient flow.
- Configure waiting room furniture to reinforce 6 feet of physical distancing.
- Remove all books, magazines, and toys from all waiting areas.
- Laminate signage for display.
- Install plexiglass at the check-in desk to minimize virus transmission.
- If possible, ask patients to wait in their car until their appointment time or to go directly to their exam room on arrival if it is available.
- Implement virtual check-in and check-out so that patients reduce unnecessary contact with surfaces and staff.
- Limit a high volume of patients to maintain social distancing etiquette, avoid delays, and allow adequate cleaning time between patients.
- Permit visitors to accompany adult patients to their ambulatory appointments only if special assistance is required.
- Limit the number of staff members in the exam and treatment rooms and maintain at least 6 feet between people except during medical care activities.
- Consider patient flow in a one-way traffic pattern.
Focus on keeping the clinical practice clean. (Follow the instructions and disinfect with a registered disinfectant product that meets the US Environmental Protection Agency criteria for use against COVID-19.13)
- Clean waiting rooms and restrooms frequently.
- Coordinate patient appointments to allow for infection control measures.
- Frequently clean high-touch surfaces, including tables, doorknobs, light switches, countertops, handles, desks, phones, keyboards, toilets, faucets, and sinks.
- Clinicians and all medical staff members should wash their hands before and after interacting with patients.
- Clean and disinfect the exam and treatment rooms before and after each patient.
- Use products that are effective against a range of organisms and viruses, including the coronavirus that causes COVID-19.
- Place signs indicating that rooms have been cleaned; this will assure and comfort patients. Take credit for your infection control processes.
Keep abreast of isolation and precaution guidelines. Based on data available at the time of this article’s publication, the CDC recommends ending isolation and transmission-based precautions for most people with COVID-19 using a symptom-based strategy.14 This limits unnecessary prolonged isolation and use of laboratory testing resources.
Generally, repeat SARS-CoV-2 polymerase chain reaction (PCR) testing is not recommended for “COVID-19 recovered” patients. Specifically, those patients with a prior positive SARS-CoV-2 PCR test result and who have met criteria for isolation discontinuation do not need a follow-up PCR test. A test-based strategy to discontinue isolation and transmission-based precautions is required only for severely immunocompromised patients.15
Prepare for a future COVID-19 surge and review your emergency plan and responses and revise as needed. Review handling of the current pandemic and best practices plus areas of improvement.
Symptom-based criteria for discontinuing transmission-based precautions include the following:
Patients with mild to moderate illness, not severely immunocompromised:
- at least 10 days have passed since symptoms first appeared and
- at least 24 hours have passed since last fever without fever-reducing medications and
- symptoms (cough, shortness of breath) have improved.
Note: For patients who are not severely immunocompromised and are asymptomatic throughout their infection, transmission-based precautions may be discontinued when at least 10 days have passed since the date of their first positive viral diagnostic test.
Patients with severe to critical illness, severely immunocompromised:
- at least 20 days have passed since symptoms first appeared and
- at least 24 hours have passed since last fever without fever-reducing medications and
- symptoms (cough, shortness of breath) have improved.
Note: For patients who are severely immunocompromised and are asymptomatic throughout their infection, transmission-based precautions may be discontinued when at least 20 days have passed since the date of their first positive viral diagnostic test.
Continue to: Strategy 4: Implement frequent employee communication and care...
Strategy 4: Implement frequent employee communication and care
The safety and well-being of our health care workers and patients in our clinical practices is paramount. Continuing to communicate this message and developing and sharing a plan may ameliorate the obvious toll on mental and emotional well-being. Frequent and effective communication with your clinical team is vital to reinforce policies and protocols, eliminate silos, and reduce errors.
Practice communication and care with these approaches:
- Offer regular employee COVID-19 testing.
- Re-educate staff about infection control protocols to ensure buy-in.
- Communicate with staff about the plan to address staffing shortages.
- Implement regular employee team huddles that can address accomplishments, challenges, areas for improvement, and top priorities.
- Perform regular celebrations for staff appreciation.
- Address mental health and chronic stress and offer empathy and coping resources and services to staff and clinicians. This will have a valuable, long-term benefit.
Patient communication. As the COVID-19 pandemic continues and stay-at-home policies are in place, patients should be encouraged to seek medical care if they are ill or have acute or chronic conditions. Communicate regularly with patients and let them know that their safety and well-being is the top priority. Prior to in-person visits, inform them of the safety processes that are in place to protect them.
Fostering an honest clinician-patient relationship enhances communication. Despite these efforts, some patients may not be forthcoming about their COVID-19 symptoms, illness, exposure, or travel. Health care staff can be encouraged to set a tone of tolerance and compassion and treat everyone with universal precautions.
Rising to the challenges
During the coronavirus pandemic, ObGyns continue to safely care for pregnant women and also triage and treat women who require timely office care as well as emergency and cancer-related surgeries.
The COVID-19 environment rapidly changes depending on the practice location. The strategies described represent a compilation of resources from key organizations that hopefully will prove useful and can be shaped to fit your practice. Local and regional recommendations vary, and no one can predict the course of the virus.
Consider reviewing your contingency plans regularly. As we have learned over the last several months, there is a science to maintaining a COVID-SAFE environment.
Practice operations likely will change to adapt to new conditions. The pandemic has challenged us to evolve, and we have responded with new capabilities and resilience while we continue to deliver superior and compassionate care for women.
For additional strategies on how to safeguard your practice against COVID-19, see the box below. ●
Continue to: Additional strategies on how to safeguard your practice against COVID-19...
Strategy 5: Develop a resource plan for practice operations
Assess financial solvency. Because of the mitigation measures taken during the pandemic, physician practices of all sizes are facing financial hardships and instability. As the pandemic progresses, physicians in private practice and those employed by health systems may benefit from existing resources and pandemic relief to help navigate COVID-related challenges.
Frequent revision of your financial plan may safeguard cash flow in the event of fewer patient visits and elective surgeries. Many medical organizations, including ACOG, are advocating for financial relief, fair reimbursement for telehealth and in-person visits, and access to adequate PPE. ACOG provides updated information on practice management.1
The American Medical Association (AMA) has created resources for physician practices to assist in staying focused on business and financial operations. The AMA has provided a summary of the Health, Economic Assistance, Liability Protection and Schools Act (HEALS Act).2 This is the next proposed coronavirus relief fund package, which includes provisions that benefit physicians and physician practices.
Create a plan. Review available resources and establish processes to optimize your practice capacity during the ongoing COVID-19 pandemic. Develop a game plan for patient care with a phased approach to identify and address challenges. This planning will allow your practice to pivot in response to changing local COVID-19 conditions to help you anticipate and prepare for a future surge. Maintain and revise plans as the pandemic shifts. Thinking ahead avoids the need to navigate issues in real time. Communicating clearly and often with all members of the office staff and patients lets everyone know that their safety is the main priority.
Assess staffing for flexible coverage. Frequent needs assessment helps to determine the number of staff needed to maintain a safe work environment for the patient volume.
Staff shortages may occur because of COVID-19 exposure, personal or family member illness, or childcare constraints due to daycare or school availability. Staff readiness includes evaluating individual availability and willingness.
Staff members with health issues, including comorbidities and chronic medical conditions, may not be comfortable working. Nonclinical staff members with health concerns could work remotely, although some may not be able to work from home due to technology-related issues such as WiFi deficiencies.
The CDC has interim guidelines to assist employers with providing a safe workplace and employees with making the best health decisions for themselves and their families.3,4 The US Office of Personnel Management provides guidance for COVID-19–related leave and benefits for federal employees.5
To mitigate staff shortages, approaches include adjusting schedules, cross-training to perform the tasks of other positions, and hiring additional personnel. A needs assessment can help determine if existing personnel could be cross-trained for other purposes or if additional staff should be hired. Understanding the minimum number of staff required for safe and effective patient care will assist in planning for shortages as the pandemic progresses. Understanding the availability of external resources could be a critical part of an office contingency plan.6
Proactively manage your supply chain. The pandemic has caused global supply shortages. Solid supply chain management is crucial for practice operations. Take inventory of your PPE and various supplies and place orders in advance. Analyze cash flow and connect with vendors as well as local and state health agencies to understand available resources. Given ongoing PPE shortages, practices should consider preserving PPE and employ appropriate strategies for optimizing supplies of face masks.7
Certain medications and vaccines administered in the office setting may be outdated and need to be replaced. Office equipment that has not been used for several months will need to be tested. For equipment used in office electrosurgery procedures, certain safety measures can be taken to reduce the transmission of aerosolized viral particles to the health care team. While currently the risk is theoretical and more research is needed, this potential risk should be mitigated.8 Assessing availability of hospital and ambulance or transport services also is recommended as these may change depending on the local COVID-19 status.
Strategy 6: Establish and refine the patient screening process
Patients want reassurance that the health care environment is safe and that their well-being is a priority. In advance of a patient’s visit, relieve any anxiety by explaining the COVID-SAFE measures that your practice has taken.
For employee use, consider telephone and in-person scripting to ensure consistent messaging for patients.
Prescreening. At the time of appointment scheduling and on the day prior to the scheduled appointment, all patients should be screened for symptoms of COVID-19,9 fever, exposure within 14 days to someone newly diagnosed with COVID-19, and travel within 14 days from a foreign country or from a US state with a quarantine requirement.
Patients who screen positive for symptoms, exposure, or travel should be referred to a clinician. If possible, asymptomatic patients who report exposure or travel should have their in-person visit deferred until after the required 14-day quarantine.
Consider restricting visitors from accompanying adult patients to their appointment unless they are required for special assistance.
Arrival screening. At the time of presentation for the appointment, all patients and any accompanying visitors should be rescreened. The optimal location for arrival screening should be determined by the local operations team and the infection prevention and control program.
At presentation, all patients and visitors should appropriately don a surgical mask or other face covering. Patients and visitors should have their temperature checked on arrival. Patients who screen positive for symptoms, exposure to COVID-19, and/or travel should be referred to a clinician or the visit deferred and a telehealth visit considered.
Visitors who screen positive for symptoms, fever, or exposure to COVID-19 are not permitted to accompany the patient. Asymptomatic parents or guardians of pediatric patients may serve as support persons.
References
- American College of Obstetricians and Gynecologists. Financial support for physicians and practices during the COVID-19 pandemic. https://www.acog.org/practice -management/coding/coding-library/financial-support-for-physicians-and-practices-during-the-covid-19-pandemic. Accessed July 23, 2020.
- American Medical Association. HEALS Act: What physicians and medical students need to know. https://www.ama-assn.org/delivering-care/public-health/heals-act -what-physicians-and-medical-students-need-know. Accessed July 29, 2020.
- Centers for Disease Control and Prevention. Interim guidance for businesses and employers responding to coronavirus disease 2019 (COVID-19). https://www.cdc.gov /coronavirus/2019-ncov/community/guidance-business-response.html. Accessed July 12, 2020.
- Centers for Disease Control and Prevention. Criteria for return to work for healthcare personnel with SARS-CoV-2 infection (interim guidance). https://www.cdc.gov /coronavirus/2019-ncov/hcp/return-to-work.html. Accessed July 20, 2020.
- US Office of Personnel Management. Questions and answers on human resources flexibilities and authorities for coronavirus disease 2019 (COVID-19). https://www .opm.gov/policy-data-oversight/covid-19/questions-and-answers-on-human-resources-flexibilities-and-authorities-for-coronavirus-disease-2019-covid-19.pdf. Accessed July 3, 2020.
- Centers for Disease Control and Prevention. Strategies to mitigate healthcare personnel staffing shortages. https://www.cdc.gov/coronavirus/2019-ncov/hcp /mitigating-staff-shortages.html. Accessed July 17, 2020.
- Centers for Disease Control and Prevention. Strategies for optimizing the supply of facemasks. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/face -masks.html. Accessed July 3, 2020.
- Rahman S, Klebanoff J, Moawad G. Smoke evacuation in the age of COVID-19. Contemporary OB/GYN. July 2, 2020. https://www.contemporaryobgyn.net/view/smoke -evacuation-in-the-age-of-covid-19. Accessed July 3, 2020.
- Centers for Disease Control and Prevention. Symptoms of coronavirus. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed July 25, 2020.
No question, the COVID-19 pandemic has been a challenging time for medical practices across the United States. Uncertainty remains regarding bringing patients and services back into our offices. One factor that distinguishes many ObGyn practices from other specialties is that our practices have remained open—in some form—since the beginning of the pandemic. In various parts of the country, gynecologic surgeries and routine office visits have been significantly reduced; however, deliveries and gynecologic emergencies have continued.
In this article, I suggest a framework of strategies and resources to provide insight for outpatient operations. Individual practices will vary across the nation depending on local conditions. Full practice capacity may take on a different look than it had prior to the pandemic, and there is opportunity to change the way we operate.
Strategy 1: Consult regulatory requirements frequently
As the local status of COVID-19 evolves quickly, it is essential to examine the frequently updated recommendations from regulatory agencies at the federal, state, and local levels. Clinical practices that function within health systems need to demonstrate alignment with hospital or university policies and procedures. The Centers for Disease Control and Prevention (CDC), Occupational Safety and Health Administration (OSHA), Centers for Medicare and Medicaid Services (CMS), and individual state departments of health provide dynamic resources that are easily accessible online.1-3
The American College of Obstetricians and Gynecologists (ACOG) continues to be an excellent medical society resource.4 Subspecialty organizations that provide up-to-date guidance include the Society for Maternal-Fetal Medicine (SMFM), Society of Gynecologic Surgeons (SGS), AAGL (American Association of Gynecologic Laparoscopists), American Society for Reproductive Medicine (ASRM), and Society of Gynecologic Oncology (SGO).5-9 These resources are updated as more information about COVID-19 emerges, and they may be modified to different local-regional conditions.
The professional liability insurance carrier is an important source of insight for a number of circumstances, including modifications to your office practice, such as returning to full-scope or part-time practice; operating outside normal clinical service arrangements (for example, assisting with emergency care); offering telehealth services; and adding extra hours or employees to accommodate the patient backlog. Business insurance coverage is a separate issue to consider. Reviewing the practice policy may protect your business from COVID-related liabilities.
Consulting with legal counselors can be helpful. They can assist with navigating various practice and personnel COVID-related changes, as well as developing a viable plan for patients who were previously insured pre–COVID-19 who are currently uninsured.
Continue to: Strategy 2: Reimagine schedule capacity...
Strategy 2: Reimagine schedule capacity
The waxing and waning of the COVID-19 crisis presents an opportunity to evaluate our office practices and make necessary and positive changes. The question becomes, do we operate our practices as usual or do we rethink our strategy for seeing patients and integrate lessons learned from the pandemic? Patients are deciding when they are comfortable to schedule elective surgeries and routine office encounters. This gives us the chance to break from the tradition of 100% in-person visits and change the way we care for women.
The coronavirus has accelerated the rise of telehealth/telemedicine and is, perhaps, a silver lining of the pandemic. Telehealth is a valuable tool for accessing health services when in-person visits are not possible. Evaluating and triaging patients for in-person versus telehealth visits is now a viable option for clinical practice and reduces exposure to COVID-19 infection.
Telemedicine is convenient, and clinicians can use it to counsel and screen for various health issues as well as to extend their reach to rural communities. Appropriate consent should be documented in the patient chart. As some areas continue to be without adequate access to WiFi, telephone contact also is currently acceptable. Telehealth does not replace the in-person visit but can be viewed as a complementary and supplementary service.
Consider a balance between telehealth and in-person visits by evaluating which visits can continue remotely and which can alternate with in-person visits. This offers tremendous flexibility and will expand delivery of essential health care to patients.10 Integrating telemedicine into clinical practice provides an additional benefit: It minimizes the exposure and transmission of COVID-19 to health care workers and patients and preserves supplies, including personal protective equipment (PPE).
Prioritize the backlog of patients who require follow-up testing, procedures, and surgeries. Communicate with patients that it is safe to be seen and important to not avoid routine and preventative visits that might reveal concerns or conditions that require treatment.
Strategy 3: Institute infection prevention and control measures
The importance of instituting and ensuring safety measures for office personnel and patients cannot be underestimated. Recently, a study from King’s College in London found that frontline health care workers with PPE still have 3 to 4 times the risk of contracting coronavirus compared with the general public.11 Health care systems should ensure adequate PPE availability and develop additional strategies to protect health care workers from COVID-19. We have to be fanatical about cleanliness and PPE. We have to be diligent about how we space ourselves and our patients. Consider adjusting workflows to ensure that visits can be conducted as quickly and safely as possible.
Communicating updated safety plans and processes are invaluable for both patients and health care workers. Patients want to be reassured that safety precautions are in place to keep the environment safe and clean. Additionally, privacy and confidentiality concerns should be addressed.
Consider a modified office schedule that can reduce the number of people in the office, person-to-person contact, and COVID-19 transmission. Social distancing is improved and PPE and other supplies are preserved.
Continue to: Employees can work on alternating days...
- Employees can work on alternating days or during different parts of the day.
- Administrative staff who do not need to be physically present in the office might work remotely.
- Expanding office hours (early morning, evening, and weekends) spreads patient visits throughout the day and minimizes high-volume in-person visits.
Institute a daily COVID-19 symptom attestation and temperature check for employees on arrival at work.
Health care personnel with symptoms of COVID-19 should be prioritized for SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) RNA testing with an approved nucleic acid or antigen detection assay. A negative result indicates that the person most likely did not have an active SARS-CoV-2 infection at the time the sample was collected. A second test for SARS-CoV-2 RNA may be performed at the discretion of the evaluating health care provider, particularly when a higher level of clinical suspicion for infection exists.
The return to work decision should be determined by an agreed on symptom-based approach to clearance. If needed on a case-by-case basis, a review can be performed with the individual’s health care provider.12
Require universal masking and appropriate protective equipment.
- All staff members, patients, and visitors must wear masks correctly in the facilities (except children under age 2).
- All clinical staff members must wear masks correctly and eye protection during every patient encounter.
Reconfigure the waiting room and patient flow.
- Configure waiting room furniture to reinforce 6 feet of physical distancing.
- Remove all books, magazines, and toys from all waiting areas.
- Laminate signage for display.
- Install plexiglass at the check-in desk to minimize virus transmission.
- If possible, ask patients to wait in their car until their appointment time or to go directly to their exam room on arrival if it is available.
- Implement virtual check-in and check-out so that patients reduce unnecessary contact with surfaces and staff.
- Limit a high volume of patients to maintain social distancing etiquette, avoid delays, and allow adequate cleaning time between patients.
- Permit visitors to accompany adult patients to their ambulatory appointments only if special assistance is required.
- Limit the number of staff members in the exam and treatment rooms and maintain at least 6 feet between people except during medical care activities.
- Consider patient flow in a one-way traffic pattern.
Focus on keeping the clinical practice clean. (Follow the instructions and disinfect with a registered disinfectant product that meets the US Environmental Protection Agency criteria for use against COVID-19.13)
- Clean waiting rooms and restrooms frequently.
- Coordinate patient appointments to allow for infection control measures.
- Frequently clean high-touch surfaces, including tables, doorknobs, light switches, countertops, handles, desks, phones, keyboards, toilets, faucets, and sinks.
- Clinicians and all medical staff members should wash their hands before and after interacting with patients.
- Clean and disinfect the exam and treatment rooms before and after each patient.
- Use products that are effective against a range of organisms and viruses, including the coronavirus that causes COVID-19.
- Place signs indicating that rooms have been cleaned; this will assure and comfort patients. Take credit for your infection control processes.
Keep abreast of isolation and precaution guidelines. Based on data available at the time of this article’s publication, the CDC recommends ending isolation and transmission-based precautions for most people with COVID-19 using a symptom-based strategy.14 This limits unnecessary prolonged isolation and use of laboratory testing resources.
Generally, repeat SARS-CoV-2 polymerase chain reaction (PCR) testing is not recommended for “COVID-19 recovered” patients. Specifically, those patients with a prior positive SARS-CoV-2 PCR test result and who have met criteria for isolation discontinuation do not need a follow-up PCR test. A test-based strategy to discontinue isolation and transmission-based precautions is required only for severely immunocompromised patients.15
Prepare for a future COVID-19 surge and review your emergency plan and responses and revise as needed. Review handling of the current pandemic and best practices plus areas of improvement.
Symptom-based criteria for discontinuing transmission-based precautions include the following:
Patients with mild to moderate illness, not severely immunocompromised:
- at least 10 days have passed since symptoms first appeared and
- at least 24 hours have passed since last fever without fever-reducing medications and
- symptoms (cough, shortness of breath) have improved.
Note: For patients who are not severely immunocompromised and are asymptomatic throughout their infection, transmission-based precautions may be discontinued when at least 10 days have passed since the date of their first positive viral diagnostic test.
Patients with severe to critical illness, severely immunocompromised:
- at least 20 days have passed since symptoms first appeared and
- at least 24 hours have passed since last fever without fever-reducing medications and
- symptoms (cough, shortness of breath) have improved.
Note: For patients who are severely immunocompromised and are asymptomatic throughout their infection, transmission-based precautions may be discontinued when at least 20 days have passed since the date of their first positive viral diagnostic test.
Continue to: Strategy 4: Implement frequent employee communication and care...
Strategy 4: Implement frequent employee communication and care
The safety and well-being of our health care workers and patients in our clinical practices is paramount. Continuing to communicate this message and developing and sharing a plan may ameliorate the obvious toll on mental and emotional well-being. Frequent and effective communication with your clinical team is vital to reinforce policies and protocols, eliminate silos, and reduce errors.
Practice communication and care with these approaches:
- Offer regular employee COVID-19 testing.
- Re-educate staff about infection control protocols to ensure buy-in.
- Communicate with staff about the plan to address staffing shortages.
- Implement regular employee team huddles that can address accomplishments, challenges, areas for improvement, and top priorities.
- Perform regular celebrations for staff appreciation.
- Address mental health and chronic stress and offer empathy and coping resources and services to staff and clinicians. This will have a valuable, long-term benefit.
Patient communication. As the COVID-19 pandemic continues and stay-at-home policies are in place, patients should be encouraged to seek medical care if they are ill or have acute or chronic conditions. Communicate regularly with patients and let them know that their safety and well-being is the top priority. Prior to in-person visits, inform them of the safety processes that are in place to protect them.
Fostering an honest clinician-patient relationship enhances communication. Despite these efforts, some patients may not be forthcoming about their COVID-19 symptoms, illness, exposure, or travel. Health care staff can be encouraged to set a tone of tolerance and compassion and treat everyone with universal precautions.
Rising to the challenges
During the coronavirus pandemic, ObGyns continue to safely care for pregnant women and also triage and treat women who require timely office care as well as emergency and cancer-related surgeries.
The COVID-19 environment rapidly changes depending on the practice location. The strategies described represent a compilation of resources from key organizations that hopefully will prove useful and can be shaped to fit your practice. Local and regional recommendations vary, and no one can predict the course of the virus.
Consider reviewing your contingency plans regularly. As we have learned over the last several months, there is a science to maintaining a COVID-SAFE environment.
Practice operations likely will change to adapt to new conditions. The pandemic has challenged us to evolve, and we have responded with new capabilities and resilience while we continue to deliver superior and compassionate care for women.
For additional strategies on how to safeguard your practice against COVID-19, see the box below. ●
Continue to: Additional strategies on how to safeguard your practice against COVID-19...
Strategy 5: Develop a resource plan for practice operations
Assess financial solvency. Because of the mitigation measures taken during the pandemic, physician practices of all sizes are facing financial hardships and instability. As the pandemic progresses, physicians in private practice and those employed by health systems may benefit from existing resources and pandemic relief to help navigate COVID-related challenges.
Frequent revision of your financial plan may safeguard cash flow in the event of fewer patient visits and elective surgeries. Many medical organizations, including ACOG, are advocating for financial relief, fair reimbursement for telehealth and in-person visits, and access to adequate PPE. ACOG provides updated information on practice management.1
The American Medical Association (AMA) has created resources for physician practices to assist in staying focused on business and financial operations. The AMA has provided a summary of the Health, Economic Assistance, Liability Protection and Schools Act (HEALS Act).2 This is the next proposed coronavirus relief fund package, which includes provisions that benefit physicians and physician practices.
Create a plan. Review available resources and establish processes to optimize your practice capacity during the ongoing COVID-19 pandemic. Develop a game plan for patient care with a phased approach to identify and address challenges. This planning will allow your practice to pivot in response to changing local COVID-19 conditions to help you anticipate and prepare for a future surge. Maintain and revise plans as the pandemic shifts. Thinking ahead avoids the need to navigate issues in real time. Communicating clearly and often with all members of the office staff and patients lets everyone know that their safety is the main priority.
Assess staffing for flexible coverage. Frequent needs assessment helps to determine the number of staff needed to maintain a safe work environment for the patient volume.
Staff shortages may occur because of COVID-19 exposure, personal or family member illness, or childcare constraints due to daycare or school availability. Staff readiness includes evaluating individual availability and willingness.
Staff members with health issues, including comorbidities and chronic medical conditions, may not be comfortable working. Nonclinical staff members with health concerns could work remotely, although some may not be able to work from home due to technology-related issues such as WiFi deficiencies.
The CDC has interim guidelines to assist employers with providing a safe workplace and employees with making the best health decisions for themselves and their families.3,4 The US Office of Personnel Management provides guidance for COVID-19–related leave and benefits for federal employees.5
To mitigate staff shortages, approaches include adjusting schedules, cross-training to perform the tasks of other positions, and hiring additional personnel. A needs assessment can help determine if existing personnel could be cross-trained for other purposes or if additional staff should be hired. Understanding the minimum number of staff required for safe and effective patient care will assist in planning for shortages as the pandemic progresses. Understanding the availability of external resources could be a critical part of an office contingency plan.6
Proactively manage your supply chain. The pandemic has caused global supply shortages. Solid supply chain management is crucial for practice operations. Take inventory of your PPE and various supplies and place orders in advance. Analyze cash flow and connect with vendors as well as local and state health agencies to understand available resources. Given ongoing PPE shortages, practices should consider preserving PPE and employ appropriate strategies for optimizing supplies of face masks.7
Certain medications and vaccines administered in the office setting may be outdated and need to be replaced. Office equipment that has not been used for several months will need to be tested. For equipment used in office electrosurgery procedures, certain safety measures can be taken to reduce the transmission of aerosolized viral particles to the health care team. While currently the risk is theoretical and more research is needed, this potential risk should be mitigated.8 Assessing availability of hospital and ambulance or transport services also is recommended as these may change depending on the local COVID-19 status.
Strategy 6: Establish and refine the patient screening process
Patients want reassurance that the health care environment is safe and that their well-being is a priority. In advance of a patient’s visit, relieve any anxiety by explaining the COVID-SAFE measures that your practice has taken.
For employee use, consider telephone and in-person scripting to ensure consistent messaging for patients.
Prescreening. At the time of appointment scheduling and on the day prior to the scheduled appointment, all patients should be screened for symptoms of COVID-19,9 fever, exposure within 14 days to someone newly diagnosed with COVID-19, and travel within 14 days from a foreign country or from a US state with a quarantine requirement.
Patients who screen positive for symptoms, exposure, or travel should be referred to a clinician. If possible, asymptomatic patients who report exposure or travel should have their in-person visit deferred until after the required 14-day quarantine.
Consider restricting visitors from accompanying adult patients to their appointment unless they are required for special assistance.
Arrival screening. At the time of presentation for the appointment, all patients and any accompanying visitors should be rescreened. The optimal location for arrival screening should be determined by the local operations team and the infection prevention and control program.
At presentation, all patients and visitors should appropriately don a surgical mask or other face covering. Patients and visitors should have their temperature checked on arrival. Patients who screen positive for symptoms, exposure to COVID-19, and/or travel should be referred to a clinician or the visit deferred and a telehealth visit considered.
Visitors who screen positive for symptoms, fever, or exposure to COVID-19 are not permitted to accompany the patient. Asymptomatic parents or guardians of pediatric patients may serve as support persons.
References
- American College of Obstetricians and Gynecologists. Financial support for physicians and practices during the COVID-19 pandemic. https://www.acog.org/practice -management/coding/coding-library/financial-support-for-physicians-and-practices-during-the-covid-19-pandemic. Accessed July 23, 2020.
- American Medical Association. HEALS Act: What physicians and medical students need to know. https://www.ama-assn.org/delivering-care/public-health/heals-act -what-physicians-and-medical-students-need-know. Accessed July 29, 2020.
- Centers for Disease Control and Prevention. Interim guidance for businesses and employers responding to coronavirus disease 2019 (COVID-19). https://www.cdc.gov /coronavirus/2019-ncov/community/guidance-business-response.html. Accessed July 12, 2020.
- Centers for Disease Control and Prevention. Criteria for return to work for healthcare personnel with SARS-CoV-2 infection (interim guidance). https://www.cdc.gov /coronavirus/2019-ncov/hcp/return-to-work.html. Accessed July 20, 2020.
- US Office of Personnel Management. Questions and answers on human resources flexibilities and authorities for coronavirus disease 2019 (COVID-19). https://www .opm.gov/policy-data-oversight/covid-19/questions-and-answers-on-human-resources-flexibilities-and-authorities-for-coronavirus-disease-2019-covid-19.pdf. Accessed July 3, 2020.
- Centers for Disease Control and Prevention. Strategies to mitigate healthcare personnel staffing shortages. https://www.cdc.gov/coronavirus/2019-ncov/hcp /mitigating-staff-shortages.html. Accessed July 17, 2020.
- Centers for Disease Control and Prevention. Strategies for optimizing the supply of facemasks. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/face -masks.html. Accessed July 3, 2020.
- Rahman S, Klebanoff J, Moawad G. Smoke evacuation in the age of COVID-19. Contemporary OB/GYN. July 2, 2020. https://www.contemporaryobgyn.net/view/smoke -evacuation-in-the-age-of-covid-19. Accessed July 3, 2020.
- Centers for Disease Control and Prevention. Symptoms of coronavirus. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed July 25, 2020.
- Centers for Disease Control and Prevention. Information for healthcare professionals about coronavirus (COVID-19). https://www.cdc.gov/coronavirus/2019-nCoV/hcp/index.html. Accessed July 22, 2020.
- US Department of Labor Occupational Safety and Health Administration. COVID-19 control and prevention: healthcare workers and employers. https://www.osha.gov/SLTC/covid-19/healthcare-workers.html. Accessed August 5, 2020.
- Centers for Medicare and Medicaid Services. COVID-19 resources for health care professionals and researchers. https://www.cms.gov/About-CMS/Agency-Information/OMH/resource-center/hcps-and-researchers. Accessed August 7, 2020.
- American College of Obstetricians and Gynecologists. COVID-19. https://www.acog.org/topics/covid-19. Accessed August 7, 2020.
- Society for Maternal-Fetal Medicine. Coronavirus (COVID-19). https://www.smfm.org/covid19. Accessed August 7, 2020.
- Society of Gynecologic Surgeons. Joint statement on re-introduction of hospital and office-based procedures in the COVID-19 climate for the practicing gynecologist. https://www.sgsonline.org/joint-statement-on-re-introduction-of-hospital-and-office-based-procedures-in-the-covid-19-climate. Accessed August 7, 2020.
- AAGL. COVID-19 articles, resources and webinars. https://www.aagl.org/covid-19/. Accessed August 7, 2020.
- American Society for Reproductive Medicine. COVID-19 updates and resources. https://www.asrm.org/news-and-publications/covid-19/. Accessed August 7, 2020.
- Society of Gynecologic Oncology. COVID-19 resources for health care practitioners. https://www.sgo.org/practice-management/covid-19/. Accessed August 7, 2020.
- Centers for Disease Control and Prevention. Using telehealth to expand access to essential health services during the COVID-19 pandemic. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html. Accessed July 3, 2020.
- Nguyen LH, Drew DA, Graham MS, et al; on behalf of the COronavirus Pandemic Epidemiology Consortium. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet. July 31, 2020. http://www.thelancet-press.com/embargo/hcwcovid.pdf. Accessed August 25, 2020.
- Centers for Disease Control and Prevention. Criteria for return to work for healthcare personnel with SARS-CoV-2 infection (interim guidance). https://www.cdc.gov/coronavirus/2019-ncov/hcp/return-to-work.html. Accessed July 20, 2020.
- US Environmental Protection Agency. List N: Disinfectants for use against SARS-CoV-2 (COVID-19). https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2-covid-19. Accessed August 24, 2020.
- Centers for Disease Control and Prevention. Duration of isolation and precautions for adults with COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html. Accessed July 23, 2020.
- Centers for Disease Control and Prevention. Discontinuation of transmission-based precautions and disposition of patients with COVID-19 in healthcare settings (interim guidance). https://www.cdc.gov/coronavirus/2019-ncov/hcp/disposition-hospitalized-patients.html#fn1. Accessed July 23, 2020.
- Centers for Disease Control and Prevention. Information for healthcare professionals about coronavirus (COVID-19). https://www.cdc.gov/coronavirus/2019-nCoV/hcp/index.html. Accessed July 22, 2020.
- US Department of Labor Occupational Safety and Health Administration. COVID-19 control and prevention: healthcare workers and employers. https://www.osha.gov/SLTC/covid-19/healthcare-workers.html. Accessed August 5, 2020.
- Centers for Medicare and Medicaid Services. COVID-19 resources for health care professionals and researchers. https://www.cms.gov/About-CMS/Agency-Information/OMH/resource-center/hcps-and-researchers. Accessed August 7, 2020.
- American College of Obstetricians and Gynecologists. COVID-19. https://www.acog.org/topics/covid-19. Accessed August 7, 2020.
- Society for Maternal-Fetal Medicine. Coronavirus (COVID-19). https://www.smfm.org/covid19. Accessed August 7, 2020.
- Society of Gynecologic Surgeons. Joint statement on re-introduction of hospital and office-based procedures in the COVID-19 climate for the practicing gynecologist. https://www.sgsonline.org/joint-statement-on-re-introduction-of-hospital-and-office-based-procedures-in-the-covid-19-climate. Accessed August 7, 2020.
- AAGL. COVID-19 articles, resources and webinars. https://www.aagl.org/covid-19/. Accessed August 7, 2020.
- American Society for Reproductive Medicine. COVID-19 updates and resources. https://www.asrm.org/news-and-publications/covid-19/. Accessed August 7, 2020.
- Society of Gynecologic Oncology. COVID-19 resources for health care practitioners. https://www.sgo.org/practice-management/covid-19/. Accessed August 7, 2020.
- Centers for Disease Control and Prevention. Using telehealth to expand access to essential health services during the COVID-19 pandemic. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html. Accessed July 3, 2020.
- Nguyen LH, Drew DA, Graham MS, et al; on behalf of the COronavirus Pandemic Epidemiology Consortium. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet. July 31, 2020. http://www.thelancet-press.com/embargo/hcwcovid.pdf. Accessed August 25, 2020.
- Centers for Disease Control and Prevention. Criteria for return to work for healthcare personnel with SARS-CoV-2 infection (interim guidance). https://www.cdc.gov/coronavirus/2019-ncov/hcp/return-to-work.html. Accessed July 20, 2020.
- US Environmental Protection Agency. List N: Disinfectants for use against SARS-CoV-2 (COVID-19). https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2-covid-19. Accessed August 24, 2020.
- Centers for Disease Control and Prevention. Duration of isolation and precautions for adults with COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html. Accessed July 23, 2020.
- Centers for Disease Control and Prevention. Discontinuation of transmission-based precautions and disposition of patients with COVID-19 in healthcare settings (interim guidance). https://www.cdc.gov/coronavirus/2019-ncov/hcp/disposition-hospitalized-patients.html#fn1. Accessed July 23, 2020.
Major changes in Medicare billing are planned for January 2021: Some specialties fare better than others
The Centers for Medicare and Medicaid Services (CMS) finalized an increase in the relative value of evaluation and management (E/M) service codes effective January 1, 2021, which results in an overall decrease in the payment for procedural services in the Medicare program. (Due to the mandate for budget neutrality, an increase in relative value units [RVUs] for E/M resulted in a large decrease in the conversion factor—the number of dollars per RVU). This has increased payments for endocrinologists, rheumatologists, and family medicine clinicians and decreased payments for radiologists, pathologists, and surgeons.
In a major win for physicians, CMS proposes to simplify documentation requirements for billing and focus on the complexity of the medical decision making (MDM) or the total time needed to care for the patient on the date of the service as the foundation for determining the relative value of the service. Therefore, there is no more counting bullets—ie, we don’t have to perform a comprehensive physical exam or review of systems to achieve a high level code! Prior to this change, time was only available for coding purposes when counseling and coordination of care was the predominant service (>50%), and only face-to-face time with the patient was considered. Effective January 1, for office and other outpatient services, total time on the calendar date of the encounter will be used. This acknowledges the intensity and value of non–face-to-face work.
Acting through CMS, the federal government influences greatly the US health care system. CMS is an agency in the Department of Health and Human Services that administers the Medicare program and partners with state governments to administer the Health Insurance Exchanges, Medicaid, and the Children’s Health Insurance programs (CHIP).1 In addition, CMS is responsible for enforcing quality care standards in long-term care facilities and clinical laboratories and the implementation of the Health Insurance Portability and Accountability Act.1
In January, CMS plans the following major changes to coding and documentation2,3:
- Selection of the level of E/M service will no longer require documentation of bullet points in the history, physical exam, and MDM. The simplified system allows physicians and qualified health care professionals to code either by total time (both face-to-face and non–face-to-face) on the date of the encounter or by level of MDM.
- For established office patients, 5 levels of office-based evaluation and management services will be retained. CMS had initially proposed to reduce the number of office-based E/M codes from 5 to 3, combining code levels 2, 3, and 4 into 1 code.4 However, after receiving feedback from professional societies and the public, CMS abandoned the plan for radical simplification of coding levels.2,3 Implementation of their proposal would have resulted in the same payment for treatment of a hang nail as for a complex gyn patient with multiple medical problems. Both patient advocacy groups and professional societies argued that incentives originally were misaligned.
- For new office patients, since both 99201 and 99202 require straightforward MDM, the level 1 code (99201) has been eliminated, reducing the number of code levels from 5 to 4.
- History and physical exam will no longer be used to determine code level for office E/M codes. These elements will be required only as medically appropriate. This means that documentation review will no longer focus on “bean counting” the elements in the history and physical exam.
- Following a reassessment of the actual time required to provide E/M services in real-life practice, CMS plans to markedly increase the relative value of office visits for established patients and modestly increase the relative value of office visits for new patients. CMS operates under the principle of “neutral budgeting,” meaning that an increase of the relative value of E/M codes will result in a decrease in the payment for procedural codes. The actual RVUs for procedural services do not change; however, budget neutrality requires a decrease in the dollar conversion factor. The proposed changes will increase the payment for E/M services and decrease payments for procedural services.
Continue to: Refocusing practice on MDM complexity...
Refocusing practice on MDM complexity
The practice of medicine is a calling with great rewards. Prominent among those rewards are improving the health of women, children, and the community, developing deep and trusting relationships with patients, families, and clinical colleagues. The practice of medicine is also replete with a host of punishing administrative burdens, including prior authorizations, clunky electronic medical records, poorly designed quality metrics that are applied to clinicians, and billing compliance rules that emphasize the repetitive documentation of clinical information with minimal value.
Some of the most irritating aspects of medical practice are the CMS rules governing medical record documentation required for billing ambulatory office visits. Current coding compliance focuses on counting the number of systems reviewed in the review of systems; the documentation of past history, social history, and family history; the number of organs and organ elements examined during the physical examination; and the complexity of MDM.
In January 2021, CMS plans to adopt new Current Procedural Terminology (CPT) code descriptors for the office and other outpatient E/M services that sunset most of the “bean-counting” metrics and emphasize the importance of the complexity of MDM in guiding selection of a correct code.2 Beginning in January 2021, clinicians will have the option of selecting an E/M code level based on the total amount of time required to provide the office visit service or the complexity of MDM. When selecting a code level based on MDM the new guidance emphasizes the importance of reviewing notes from other clinicians, reviewing test results, ordering of tests, and discussing and coordinating the care of the patient with other treating physicians. These changes reflect a better understanding of what is most important in good medical practice, promoting better patient care. TABLES 1 and 2 provide the initial guidance from CMS concerning selection of E/M code level based on time and MDM, respectively.2 The guidance for using MDM to select an E/M code level is likely to evolve following implementation, so stay tuned. When using MDM to select a code, 2 of the 3 general categories are required to select that level of service.
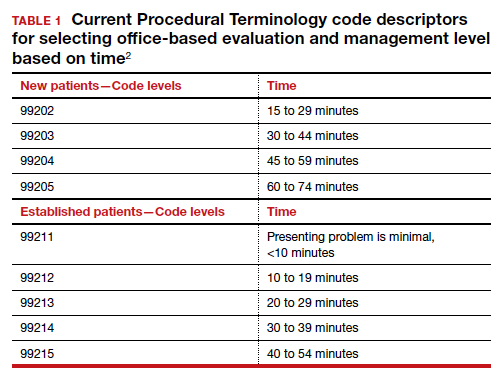
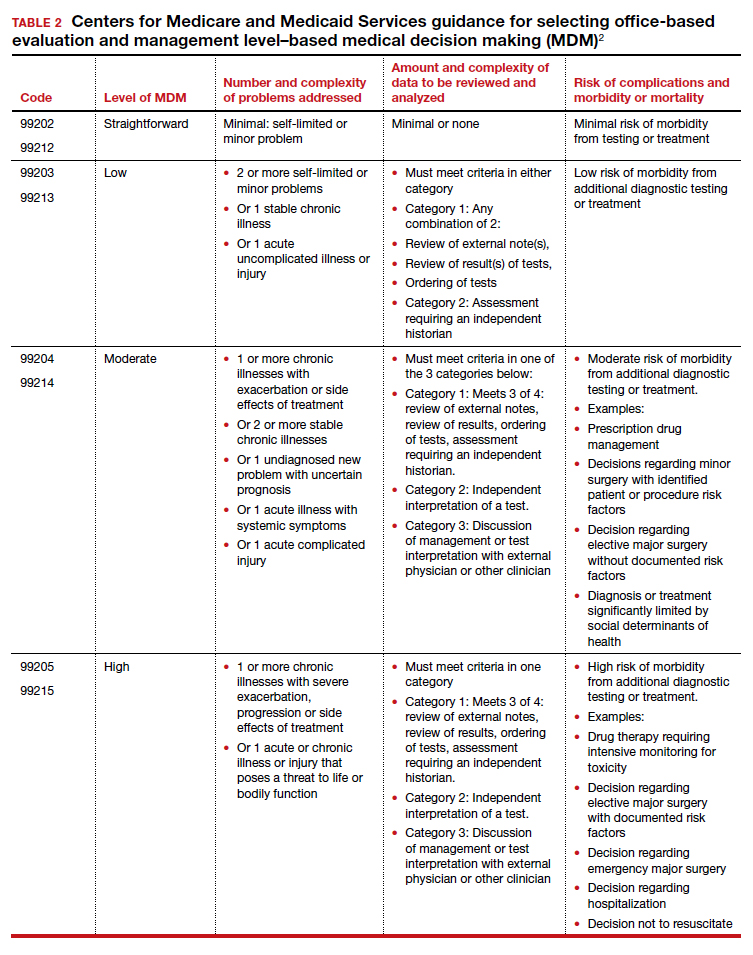
Increase in the valuation of office-based E/M services
The Medicare Physician Fee Schedule uses a resource-based relative value system to determine time and intensity of the work of clinical practice. This system recognizes 3 major factors that influence the resources required to provide a service:
- work of the clinician
- practice expense for technical components
- cost of professional liability insurance.
Many primary care professional associations have long contended that CMS has undervalued office-based E/M services relative to procedures, resulting in the devaluing of primary care practice. After the CPT code descriptors were updated by the CPT editorial panel, 52 specialty societies surveyed their members to provide inputs to CMS on the time and intensity of the office and other outpatient E/M codes as currently practiced. The American Medical Association’s Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) reviewed the surveys and provided new inputs via open comment to CMS. CMS has responded to this feedback with a review of the intensity of clinical work required to provide an ambulatory visit service. In response to the review, CMS proposes to accept the recommendations of the RUC representing the house of medicine and increase the work and practice expense relative value assigned to new and established office visit codes. Overall, the combination of changes in relative values assigned for the work of the clinician and the expense of practice, increases the total value of office-based E/M codes for new patients by 7% to 14% and for established patients from 28% to 46% (see supplemental table in the sidebar at the end of this article).
Continue to: Decreased payments for procedural services...
Decreased payments for procedural services
Medicare is required to offset increased payment in one arena of health care delivery with decreased payment in other arenas of care, thereby achieving “budget-neutrality.” As detailed above, CMS plans to increase Medicare payments for office-based E/M services. Payment for services is calculated by multiplying the total RVUs for a particular service by a “conversion factor” (ie, number of dollars per RVU). To achieve budget-neutrality, CMS has proposed substantially reducing the conversion factor for 2021 (from $36.09 to $32.26), which will effectively decrease Medicare payments for procedural services since their RVUs have not changed. While the AMA RUC and many specialty societies continue to strongly advocate for the E/M work RVU increases to be included in the E/M components of 10- and 90-day global services, CMS has proposed to implement them only for “stand alone” E/M services.
Organizations are lobbying to delay or prevent the planned decrease in conversion factor, which results in substantial declines in payment for procedural services. (See "What do the Medicare billing changes mean for the Obstetrical Bundled services?" at the end of this article.) Due to the economic and clinical practice challenges caused by the coronavirus disease 2019 (COVID-19) pandemic it would be best if CMS did not reduce payments to physicians who are experts in procedural health care, thereby avoiding the risk of reduced access to these vital services.
If the current CMS changes in payment are implemented, endocrinologists, rheumatologists, and family physicians will have an increase in payment, and radiologists, pathologists, and surgeons will have a decrease in payment (TABLE 3).6 Obstetrics and gynecology is projected to have an 8% increase in Medicare payment. However, if an obstetrician-gynecologist derives most of their Medicare payments from surgical procedures, they are likely to have a decrease in payment from Medicare. Other payers will be incorporating the new coding structure for 2021; however, their payment structures and conversion factors are likely to vary. It is important to note that the RVUs for procedures have not changed. The budget neutrality adjustment resulted in a much lower conversion factor and therefore a decrease in payment for those specialties whose RVUs did not increase.
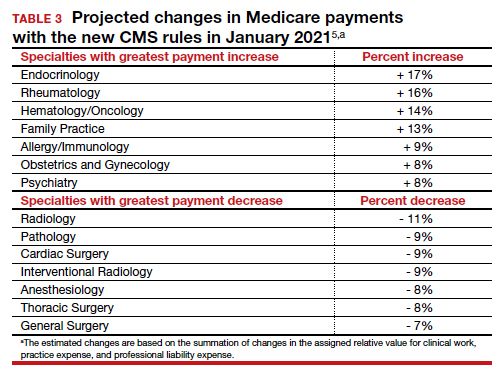
Bottom line
Working through the Medicare, Medicaid, and CHIP programs, CMS can influence greatly the practice of medicine including medical record documentation practices and payment rates for every clinical service. CMS proposes to end the onerous “bean counting” approach to billing compliance and refocus on the complexity of MDM as the foundation for selecting a billing code level. This change is long overdue, valuing the effective management of complex patients in office practice. Hopefully, CMS will reverse the planned reduction in the payment for procedural services, preserving patient access to important health care services. ●
The CY 2020 Medicare Physician Fee Schedule Final Rule was published electronically in the Federal Register on November 1, 2019. This final rule aligns the evaluation and management (E/M) coding and payment with changes recommended by the Current Procedural Terminology (CPT) Editorial Panel and American Medical Association’s (AMA) Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) for office/outpatient E/M visits. Unfortunately, the Centers for Medicare and Medicaid Services (CMS) did not agree with the RUC, AMA, and specialty societies that the E/M payment changes should be applicable across all global services that incorporate E/M visits—despite the fact that the values proposed by the RUC incorporated survey data from 52 specialties, representing most of medicine (including those specialties that predominantly perform procedures). Specifically, CMS expressed the view that the number of E/M visits within the 10- and 90-day global codes, as well as the maternity care bundle, were difficult to validate; therefore, the increased values would not be distributed to those procedural services.
Many professional societies expressed significant concerns about the resulting budget neutrality adjustments that would occur effective January 2021. The great news for ObGyns is that the American College of Obstetricians and Gynecologists (ACOG) was able to respond directly to CMS’s concerns with data to support the number of prenatal visits within the Obstetrical Bundle. Tapping into a de-identified, cloud-based data set of prenatal records—representing more than 1,100 obstetric providers with close to 30,000 recently completed pregnancies—ACOG was able to document both a mean and median number of prenatal visits across a broad geographic, payer, and patient demographic that supported the 13 prenatal visits in the Obstetrical Bundle.
With ACOG’s advocacy and ability to provide data to CMS, the proposed physician fee schedule rule for 2021 has proposed to incorporate the E/M increased reimbursement into the prenatal care codes. Now we urge the CMS to finalize this proposal. Although Medicare pays for a tiny number of pregnancies annually, we hope that all payers, including Medicaid and managed care plans, will agree with this acknowledgement of the increased work of evaluation and management that obstetricians provide during prenatal care. Join ACOG in telling CMS to finalize their proposal to increase the values of the global obstetric codes: https://acog.quorum.us/campaign/28579/.
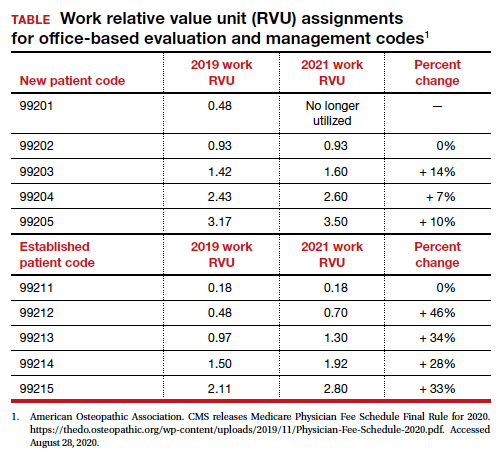
- Centers for Medicare and Medicaid Services. https://www.cms.gov/. Accessed August 28, 2020.
- American Medical Association. CPT Evaluation and Management (E/M) Office or Other Outpatient (99202-99215) and Prolonged Services (99354, 99355, 99356, 99XXX) Code and Guideline Changes. 2019. https://www.ama-assn.org /system/files/2019-06/cpt-office-prolonged-svs -code-changes.pdf. Accessed August 28, 2020.
- The American Academy of Family Physicians. Family medicine updates. Ann Fam Med. 2020;18:84-85. doi: 10.1370/afm.2508.
- Centers for Medicare and Medicaid Services. Final policy, payment and quality provisions changes to the Medicare Physician Fee Schedule for calendar year 2019. November 1, 2018. https://www.cms.gov/newsroom/fact-sheets /final-policy-payment-and-quality-provisionschanges-medicare-physician-fee-schedulecalendar-year. Accessed August 28, 2020.
- Department of Health and Human Services; Centers for Medicare and Medicaid Services. 42 CFR Parts 410, 414, 415, 423, 424, and 425. Federal Register. 2020;85(159). https://www.govinfo.gov /content/pkg/FR-2020-08-17/pdf/2020-17127 .pdf. Accessed August 28, 2020.
The Centers for Medicare and Medicaid Services (CMS) finalized an increase in the relative value of evaluation and management (E/M) service codes effective January 1, 2021, which results in an overall decrease in the payment for procedural services in the Medicare program. (Due to the mandate for budget neutrality, an increase in relative value units [RVUs] for E/M resulted in a large decrease in the conversion factor—the number of dollars per RVU). This has increased payments for endocrinologists, rheumatologists, and family medicine clinicians and decreased payments for radiologists, pathologists, and surgeons.
In a major win for physicians, CMS proposes to simplify documentation requirements for billing and focus on the complexity of the medical decision making (MDM) or the total time needed to care for the patient on the date of the service as the foundation for determining the relative value of the service. Therefore, there is no more counting bullets—ie, we don’t have to perform a comprehensive physical exam or review of systems to achieve a high level code! Prior to this change, time was only available for coding purposes when counseling and coordination of care was the predominant service (>50%), and only face-to-face time with the patient was considered. Effective January 1, for office and other outpatient services, total time on the calendar date of the encounter will be used. This acknowledges the intensity and value of non–face-to-face work.
Acting through CMS, the federal government influences greatly the US health care system. CMS is an agency in the Department of Health and Human Services that administers the Medicare program and partners with state governments to administer the Health Insurance Exchanges, Medicaid, and the Children’s Health Insurance programs (CHIP).1 In addition, CMS is responsible for enforcing quality care standards in long-term care facilities and clinical laboratories and the implementation of the Health Insurance Portability and Accountability Act.1
In January, CMS plans the following major changes to coding and documentation2,3:
- Selection of the level of E/M service will no longer require documentation of bullet points in the history, physical exam, and MDM. The simplified system allows physicians and qualified health care professionals to code either by total time (both face-to-face and non–face-to-face) on the date of the encounter or by level of MDM.
- For established office patients, 5 levels of office-based evaluation and management services will be retained. CMS had initially proposed to reduce the number of office-based E/M codes from 5 to 3, combining code levels 2, 3, and 4 into 1 code.4 However, after receiving feedback from professional societies and the public, CMS abandoned the plan for radical simplification of coding levels.2,3 Implementation of their proposal would have resulted in the same payment for treatment of a hang nail as for a complex gyn patient with multiple medical problems. Both patient advocacy groups and professional societies argued that incentives originally were misaligned.
- For new office patients, since both 99201 and 99202 require straightforward MDM, the level 1 code (99201) has been eliminated, reducing the number of code levels from 5 to 4.
- History and physical exam will no longer be used to determine code level for office E/M codes. These elements will be required only as medically appropriate. This means that documentation review will no longer focus on “bean counting” the elements in the history and physical exam.
- Following a reassessment of the actual time required to provide E/M services in real-life practice, CMS plans to markedly increase the relative value of office visits for established patients and modestly increase the relative value of office visits for new patients. CMS operates under the principle of “neutral budgeting,” meaning that an increase of the relative value of E/M codes will result in a decrease in the payment for procedural codes. The actual RVUs for procedural services do not change; however, budget neutrality requires a decrease in the dollar conversion factor. The proposed changes will increase the payment for E/M services and decrease payments for procedural services.
Continue to: Refocusing practice on MDM complexity...
Refocusing practice on MDM complexity
The practice of medicine is a calling with great rewards. Prominent among those rewards are improving the health of women, children, and the community, developing deep and trusting relationships with patients, families, and clinical colleagues. The practice of medicine is also replete with a host of punishing administrative burdens, including prior authorizations, clunky electronic medical records, poorly designed quality metrics that are applied to clinicians, and billing compliance rules that emphasize the repetitive documentation of clinical information with minimal value.
Some of the most irritating aspects of medical practice are the CMS rules governing medical record documentation required for billing ambulatory office visits. Current coding compliance focuses on counting the number of systems reviewed in the review of systems; the documentation of past history, social history, and family history; the number of organs and organ elements examined during the physical examination; and the complexity of MDM.
In January 2021, CMS plans to adopt new Current Procedural Terminology (CPT) code descriptors for the office and other outpatient E/M services that sunset most of the “bean-counting” metrics and emphasize the importance of the complexity of MDM in guiding selection of a correct code.2 Beginning in January 2021, clinicians will have the option of selecting an E/M code level based on the total amount of time required to provide the office visit service or the complexity of MDM. When selecting a code level based on MDM the new guidance emphasizes the importance of reviewing notes from other clinicians, reviewing test results, ordering of tests, and discussing and coordinating the care of the patient with other treating physicians. These changes reflect a better understanding of what is most important in good medical practice, promoting better patient care. TABLES 1 and 2 provide the initial guidance from CMS concerning selection of E/M code level based on time and MDM, respectively.2 The guidance for using MDM to select an E/M code level is likely to evolve following implementation, so stay tuned. When using MDM to select a code, 2 of the 3 general categories are required to select that level of service.


Increase in the valuation of office-based E/M services
The Medicare Physician Fee Schedule uses a resource-based relative value system to determine time and intensity of the work of clinical practice. This system recognizes 3 major factors that influence the resources required to provide a service:
- work of the clinician
- practice expense for technical components
- cost of professional liability insurance.
Many primary care professional associations have long contended that CMS has undervalued office-based E/M services relative to procedures, resulting in the devaluing of primary care practice. After the CPT code descriptors were updated by the CPT editorial panel, 52 specialty societies surveyed their members to provide inputs to CMS on the time and intensity of the office and other outpatient E/M codes as currently practiced. The American Medical Association’s Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) reviewed the surveys and provided new inputs via open comment to CMS. CMS has responded to this feedback with a review of the intensity of clinical work required to provide an ambulatory visit service. In response to the review, CMS proposes to accept the recommendations of the RUC representing the house of medicine and increase the work and practice expense relative value assigned to new and established office visit codes. Overall, the combination of changes in relative values assigned for the work of the clinician and the expense of practice, increases the total value of office-based E/M codes for new patients by 7% to 14% and for established patients from 28% to 46% (see supplemental table in the sidebar at the end of this article).
Continue to: Decreased payments for procedural services...
Decreased payments for procedural services
Medicare is required to offset increased payment in one arena of health care delivery with decreased payment in other arenas of care, thereby achieving “budget-neutrality.” As detailed above, CMS plans to increase Medicare payments for office-based E/M services. Payment for services is calculated by multiplying the total RVUs for a particular service by a “conversion factor” (ie, number of dollars per RVU). To achieve budget-neutrality, CMS has proposed substantially reducing the conversion factor for 2021 (from $36.09 to $32.26), which will effectively decrease Medicare payments for procedural services since their RVUs have not changed. While the AMA RUC and many specialty societies continue to strongly advocate for the E/M work RVU increases to be included in the E/M components of 10- and 90-day global services, CMS has proposed to implement them only for “stand alone” E/M services.
Organizations are lobbying to delay or prevent the planned decrease in conversion factor, which results in substantial declines in payment for procedural services. (See "What do the Medicare billing changes mean for the Obstetrical Bundled services?" at the end of this article.) Due to the economic and clinical practice challenges caused by the coronavirus disease 2019 (COVID-19) pandemic it would be best if CMS did not reduce payments to physicians who are experts in procedural health care, thereby avoiding the risk of reduced access to these vital services.
If the current CMS changes in payment are implemented, endocrinologists, rheumatologists, and family physicians will have an increase in payment, and radiologists, pathologists, and surgeons will have a decrease in payment (TABLE 3).6 Obstetrics and gynecology is projected to have an 8% increase in Medicare payment. However, if an obstetrician-gynecologist derives most of their Medicare payments from surgical procedures, they are likely to have a decrease in payment from Medicare. Other payers will be incorporating the new coding structure for 2021; however, their payment structures and conversion factors are likely to vary. It is important to note that the RVUs for procedures have not changed. The budget neutrality adjustment resulted in a much lower conversion factor and therefore a decrease in payment for those specialties whose RVUs did not increase.

Bottom line
Working through the Medicare, Medicaid, and CHIP programs, CMS can influence greatly the practice of medicine including medical record documentation practices and payment rates for every clinical service. CMS proposes to end the onerous “bean counting” approach to billing compliance and refocus on the complexity of MDM as the foundation for selecting a billing code level. This change is long overdue, valuing the effective management of complex patients in office practice. Hopefully, CMS will reverse the planned reduction in the payment for procedural services, preserving patient access to important health care services. ●
The CY 2020 Medicare Physician Fee Schedule Final Rule was published electronically in the Federal Register on November 1, 2019. This final rule aligns the evaluation and management (E/M) coding and payment with changes recommended by the Current Procedural Terminology (CPT) Editorial Panel and American Medical Association’s (AMA) Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) for office/outpatient E/M visits. Unfortunately, the Centers for Medicare and Medicaid Services (CMS) did not agree with the RUC, AMA, and specialty societies that the E/M payment changes should be applicable across all global services that incorporate E/M visits—despite the fact that the values proposed by the RUC incorporated survey data from 52 specialties, representing most of medicine (including those specialties that predominantly perform procedures). Specifically, CMS expressed the view that the number of E/M visits within the 10- and 90-day global codes, as well as the maternity care bundle, were difficult to validate; therefore, the increased values would not be distributed to those procedural services.
Many professional societies expressed significant concerns about the resulting budget neutrality adjustments that would occur effective January 2021. The great news for ObGyns is that the American College of Obstetricians and Gynecologists (ACOG) was able to respond directly to CMS’s concerns with data to support the number of prenatal visits within the Obstetrical Bundle. Tapping into a de-identified, cloud-based data set of prenatal records—representing more than 1,100 obstetric providers with close to 30,000 recently completed pregnancies—ACOG was able to document both a mean and median number of prenatal visits across a broad geographic, payer, and patient demographic that supported the 13 prenatal visits in the Obstetrical Bundle.
With ACOG’s advocacy and ability to provide data to CMS, the proposed physician fee schedule rule for 2021 has proposed to incorporate the E/M increased reimbursement into the prenatal care codes. Now we urge the CMS to finalize this proposal. Although Medicare pays for a tiny number of pregnancies annually, we hope that all payers, including Medicaid and managed care plans, will agree with this acknowledgement of the increased work of evaluation and management that obstetricians provide during prenatal care. Join ACOG in telling CMS to finalize their proposal to increase the values of the global obstetric codes: https://acog.quorum.us/campaign/28579/.

The Centers for Medicare and Medicaid Services (CMS) finalized an increase in the relative value of evaluation and management (E/M) service codes effective January 1, 2021, which results in an overall decrease in the payment for procedural services in the Medicare program. (Due to the mandate for budget neutrality, an increase in relative value units [RVUs] for E/M resulted in a large decrease in the conversion factor—the number of dollars per RVU). This has increased payments for endocrinologists, rheumatologists, and family medicine clinicians and decreased payments for radiologists, pathologists, and surgeons.
In a major win for physicians, CMS proposes to simplify documentation requirements for billing and focus on the complexity of the medical decision making (MDM) or the total time needed to care for the patient on the date of the service as the foundation for determining the relative value of the service. Therefore, there is no more counting bullets—ie, we don’t have to perform a comprehensive physical exam or review of systems to achieve a high level code! Prior to this change, time was only available for coding purposes when counseling and coordination of care was the predominant service (>50%), and only face-to-face time with the patient was considered. Effective January 1, for office and other outpatient services, total time on the calendar date of the encounter will be used. This acknowledges the intensity and value of non–face-to-face work.
Acting through CMS, the federal government influences greatly the US health care system. CMS is an agency in the Department of Health and Human Services that administers the Medicare program and partners with state governments to administer the Health Insurance Exchanges, Medicaid, and the Children’s Health Insurance programs (CHIP).1 In addition, CMS is responsible for enforcing quality care standards in long-term care facilities and clinical laboratories and the implementation of the Health Insurance Portability and Accountability Act.1
In January, CMS plans the following major changes to coding and documentation2,3:
- Selection of the level of E/M service will no longer require documentation of bullet points in the history, physical exam, and MDM. The simplified system allows physicians and qualified health care professionals to code either by total time (both face-to-face and non–face-to-face) on the date of the encounter or by level of MDM.
- For established office patients, 5 levels of office-based evaluation and management services will be retained. CMS had initially proposed to reduce the number of office-based E/M codes from 5 to 3, combining code levels 2, 3, and 4 into 1 code.4 However, after receiving feedback from professional societies and the public, CMS abandoned the plan for radical simplification of coding levels.2,3 Implementation of their proposal would have resulted in the same payment for treatment of a hang nail as for a complex gyn patient with multiple medical problems. Both patient advocacy groups and professional societies argued that incentives originally were misaligned.
- For new office patients, since both 99201 and 99202 require straightforward MDM, the level 1 code (99201) has been eliminated, reducing the number of code levels from 5 to 4.
- History and physical exam will no longer be used to determine code level for office E/M codes. These elements will be required only as medically appropriate. This means that documentation review will no longer focus on “bean counting” the elements in the history and physical exam.
- Following a reassessment of the actual time required to provide E/M services in real-life practice, CMS plans to markedly increase the relative value of office visits for established patients and modestly increase the relative value of office visits for new patients. CMS operates under the principle of “neutral budgeting,” meaning that an increase of the relative value of E/M codes will result in a decrease in the payment for procedural codes. The actual RVUs for procedural services do not change; however, budget neutrality requires a decrease in the dollar conversion factor. The proposed changes will increase the payment for E/M services and decrease payments for procedural services.
Continue to: Refocusing practice on MDM complexity...
Refocusing practice on MDM complexity
The practice of medicine is a calling with great rewards. Prominent among those rewards are improving the health of women, children, and the community, developing deep and trusting relationships with patients, families, and clinical colleagues. The practice of medicine is also replete with a host of punishing administrative burdens, including prior authorizations, clunky electronic medical records, poorly designed quality metrics that are applied to clinicians, and billing compliance rules that emphasize the repetitive documentation of clinical information with minimal value.
Some of the most irritating aspects of medical practice are the CMS rules governing medical record documentation required for billing ambulatory office visits. Current coding compliance focuses on counting the number of systems reviewed in the review of systems; the documentation of past history, social history, and family history; the number of organs and organ elements examined during the physical examination; and the complexity of MDM.
In January 2021, CMS plans to adopt new Current Procedural Terminology (CPT) code descriptors for the office and other outpatient E/M services that sunset most of the “bean-counting” metrics and emphasize the importance of the complexity of MDM in guiding selection of a correct code.2 Beginning in January 2021, clinicians will have the option of selecting an E/M code level based on the total amount of time required to provide the office visit service or the complexity of MDM. When selecting a code level based on MDM the new guidance emphasizes the importance of reviewing notes from other clinicians, reviewing test results, ordering of tests, and discussing and coordinating the care of the patient with other treating physicians. These changes reflect a better understanding of what is most important in good medical practice, promoting better patient care. TABLES 1 and 2 provide the initial guidance from CMS concerning selection of E/M code level based on time and MDM, respectively.2 The guidance for using MDM to select an E/M code level is likely to evolve following implementation, so stay tuned. When using MDM to select a code, 2 of the 3 general categories are required to select that level of service.


Increase in the valuation of office-based E/M services
The Medicare Physician Fee Schedule uses a resource-based relative value system to determine time and intensity of the work of clinical practice. This system recognizes 3 major factors that influence the resources required to provide a service:
- work of the clinician
- practice expense for technical components
- cost of professional liability insurance.
Many primary care professional associations have long contended that CMS has undervalued office-based E/M services relative to procedures, resulting in the devaluing of primary care practice. After the CPT code descriptors were updated by the CPT editorial panel, 52 specialty societies surveyed their members to provide inputs to CMS on the time and intensity of the office and other outpatient E/M codes as currently practiced. The American Medical Association’s Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) reviewed the surveys and provided new inputs via open comment to CMS. CMS has responded to this feedback with a review of the intensity of clinical work required to provide an ambulatory visit service. In response to the review, CMS proposes to accept the recommendations of the RUC representing the house of medicine and increase the work and practice expense relative value assigned to new and established office visit codes. Overall, the combination of changes in relative values assigned for the work of the clinician and the expense of practice, increases the total value of office-based E/M codes for new patients by 7% to 14% and for established patients from 28% to 46% (see supplemental table in the sidebar at the end of this article).
Continue to: Decreased payments for procedural services...
Decreased payments for procedural services
Medicare is required to offset increased payment in one arena of health care delivery with decreased payment in other arenas of care, thereby achieving “budget-neutrality.” As detailed above, CMS plans to increase Medicare payments for office-based E/M services. Payment for services is calculated by multiplying the total RVUs for a particular service by a “conversion factor” (ie, number of dollars per RVU). To achieve budget-neutrality, CMS has proposed substantially reducing the conversion factor for 2021 (from $36.09 to $32.26), which will effectively decrease Medicare payments for procedural services since their RVUs have not changed. While the AMA RUC and many specialty societies continue to strongly advocate for the E/M work RVU increases to be included in the E/M components of 10- and 90-day global services, CMS has proposed to implement them only for “stand alone” E/M services.
Organizations are lobbying to delay or prevent the planned decrease in conversion factor, which results in substantial declines in payment for procedural services. (See "What do the Medicare billing changes mean for the Obstetrical Bundled services?" at the end of this article.) Due to the economic and clinical practice challenges caused by the coronavirus disease 2019 (COVID-19) pandemic it would be best if CMS did not reduce payments to physicians who are experts in procedural health care, thereby avoiding the risk of reduced access to these vital services.
If the current CMS changes in payment are implemented, endocrinologists, rheumatologists, and family physicians will have an increase in payment, and radiologists, pathologists, and surgeons will have a decrease in payment (TABLE 3).6 Obstetrics and gynecology is projected to have an 8% increase in Medicare payment. However, if an obstetrician-gynecologist derives most of their Medicare payments from surgical procedures, they are likely to have a decrease in payment from Medicare. Other payers will be incorporating the new coding structure for 2021; however, their payment structures and conversion factors are likely to vary. It is important to note that the RVUs for procedures have not changed. The budget neutrality adjustment resulted in a much lower conversion factor and therefore a decrease in payment for those specialties whose RVUs did not increase.

Bottom line
Working through the Medicare, Medicaid, and CHIP programs, CMS can influence greatly the practice of medicine including medical record documentation practices and payment rates for every clinical service. CMS proposes to end the onerous “bean counting” approach to billing compliance and refocus on the complexity of MDM as the foundation for selecting a billing code level. This change is long overdue, valuing the effective management of complex patients in office practice. Hopefully, CMS will reverse the planned reduction in the payment for procedural services, preserving patient access to important health care services. ●
The CY 2020 Medicare Physician Fee Schedule Final Rule was published electronically in the Federal Register on November 1, 2019. This final rule aligns the evaluation and management (E/M) coding and payment with changes recommended by the Current Procedural Terminology (CPT) Editorial Panel and American Medical Association’s (AMA) Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) for office/outpatient E/M visits. Unfortunately, the Centers for Medicare and Medicaid Services (CMS) did not agree with the RUC, AMA, and specialty societies that the E/M payment changes should be applicable across all global services that incorporate E/M visits—despite the fact that the values proposed by the RUC incorporated survey data from 52 specialties, representing most of medicine (including those specialties that predominantly perform procedures). Specifically, CMS expressed the view that the number of E/M visits within the 10- and 90-day global codes, as well as the maternity care bundle, were difficult to validate; therefore, the increased values would not be distributed to those procedural services.
Many professional societies expressed significant concerns about the resulting budget neutrality adjustments that would occur effective January 2021. The great news for ObGyns is that the American College of Obstetricians and Gynecologists (ACOG) was able to respond directly to CMS’s concerns with data to support the number of prenatal visits within the Obstetrical Bundle. Tapping into a de-identified, cloud-based data set of prenatal records—representing more than 1,100 obstetric providers with close to 30,000 recently completed pregnancies—ACOG was able to document both a mean and median number of prenatal visits across a broad geographic, payer, and patient demographic that supported the 13 prenatal visits in the Obstetrical Bundle.
With ACOG’s advocacy and ability to provide data to CMS, the proposed physician fee schedule rule for 2021 has proposed to incorporate the E/M increased reimbursement into the prenatal care codes. Now we urge the CMS to finalize this proposal. Although Medicare pays for a tiny number of pregnancies annually, we hope that all payers, including Medicaid and managed care plans, will agree with this acknowledgement of the increased work of evaluation and management that obstetricians provide during prenatal care. Join ACOG in telling CMS to finalize their proposal to increase the values of the global obstetric codes: https://acog.quorum.us/campaign/28579/.

- Centers for Medicare and Medicaid Services. https://www.cms.gov/. Accessed August 28, 2020.
- American Medical Association. CPT Evaluation and Management (E/M) Office or Other Outpatient (99202-99215) and Prolonged Services (99354, 99355, 99356, 99XXX) Code and Guideline Changes. 2019. https://www.ama-assn.org /system/files/2019-06/cpt-office-prolonged-svs -code-changes.pdf. Accessed August 28, 2020.
- The American Academy of Family Physicians. Family medicine updates. Ann Fam Med. 2020;18:84-85. doi: 10.1370/afm.2508.
- Centers for Medicare and Medicaid Services. Final policy, payment and quality provisions changes to the Medicare Physician Fee Schedule for calendar year 2019. November 1, 2018. https://www.cms.gov/newsroom/fact-sheets /final-policy-payment-and-quality-provisionschanges-medicare-physician-fee-schedulecalendar-year. Accessed August 28, 2020.
- Department of Health and Human Services; Centers for Medicare and Medicaid Services. 42 CFR Parts 410, 414, 415, 423, 424, and 425. Federal Register. 2020;85(159). https://www.govinfo.gov /content/pkg/FR-2020-08-17/pdf/2020-17127 .pdf. Accessed August 28, 2020.
- Centers for Medicare and Medicaid Services. https://www.cms.gov/. Accessed August 28, 2020.
- American Medical Association. CPT Evaluation and Management (E/M) Office or Other Outpatient (99202-99215) and Prolonged Services (99354, 99355, 99356, 99XXX) Code and Guideline Changes. 2019. https://www.ama-assn.org /system/files/2019-06/cpt-office-prolonged-svs -code-changes.pdf. Accessed August 28, 2020.
- The American Academy of Family Physicians. Family medicine updates. Ann Fam Med. 2020;18:84-85. doi: 10.1370/afm.2508.
- Centers for Medicare and Medicaid Services. Final policy, payment and quality provisions changes to the Medicare Physician Fee Schedule for calendar year 2019. November 1, 2018. https://www.cms.gov/newsroom/fact-sheets /final-policy-payment-and-quality-provisionschanges-medicare-physician-fee-schedulecalendar-year. Accessed August 28, 2020.
- Department of Health and Human Services; Centers for Medicare and Medicaid Services. 42 CFR Parts 410, 414, 415, 423, 424, and 425. Federal Register. 2020;85(159). https://www.govinfo.gov /content/pkg/FR-2020-08-17/pdf/2020-17127 .pdf. Accessed August 28, 2020.