User login
Tools emerging to predict liver failure in cirrhosis
Systemic inflammation and portal hypertension are key predictors of acute-on-chronic liver failure (ACLF) in the 3 months after a hospital stay for acute decompensated cirrhosis and also of death after 12 months, a preliminary analysis of data from the PREDICT study shows.
“Before this, we never had any patient signatures to identify ACLF,” said Jonel Trebicka, MD, PhD, from the JW Goethe University Hospital in Frankfurt, Germany.
Now, Dr. Trebicka’s team has “characterized the phenotypes in pre-ACLF that will progress within 3 months,” he said in an interview. “Those with high levels of inflammatory proteins, white blood cell count, are more likely to develop ACLF.”
ACLF is a highly complex disorder that can lead liver, cardiovascular, renal, cerebral, pulmonary, intestinal, adrenal, and immune systems to fail, Dr. Trebicka explained when he discussed the analysis – published online in the Journal of Hepatology – during the virtual International Liver Congress (ILC) 2020.
The chance of survival after the onset of ACLF is low – the 28-day survival rate is 30% – and “the only treatment we have is liver transplant,” he said.
For their prospective observational study, Dr. Trebicka and his colleagues assessed 1071 participants from 48 European hospitals in 14 countries who were admitted for an episode of acute decompensation, defined as the development of ascites, hepatic encephalopathy, gastrointestinal hemorrhage, infection, or a combination thereof.
The researchers identified three distinct clinical courses for a patient hospitalized with acute decompensated cirrhosis that will help clinicians predict the development of ACLF.
At study enrollment, more than half of the patients at highest risk for ACLF had pre-ACLF and high-grade systemic inflammation. The patients at intermediate risk had unstable decompensated cirrhosis with low-grade systemic inflammation and complications related to severe portal hypertension. And those at lowest risk for ACLF had stable decompensated cirrhosis and no severe systemic inflammation or portal hypertension complications, and did not develop ACLF or another episode of acute decompensation in the subsequent 3 months.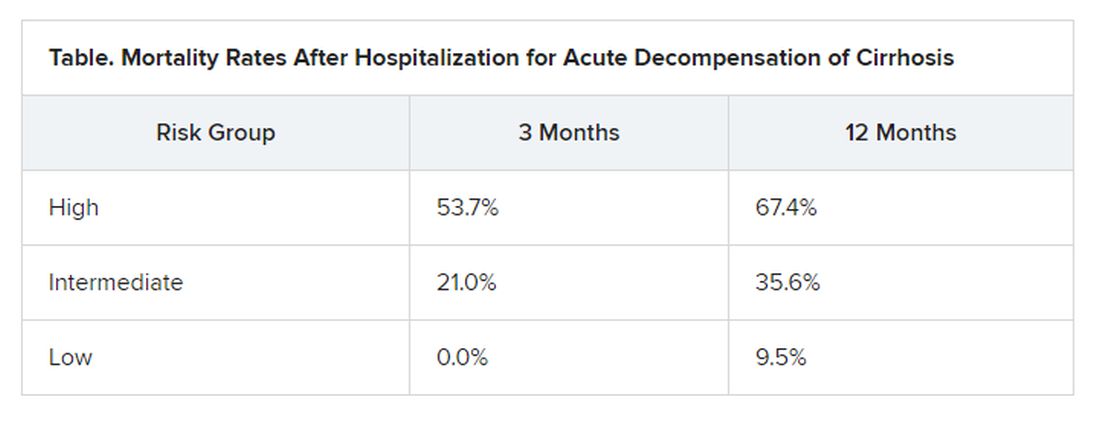
“There have been hints of possible phenotypes before – for stable and unstable ACLF – but we never had anything specific to diagnose,” Trebicka reported.
“We found that there are two main mechanisms in the development of ACLF that are most important,” he said. The first is systemic inflammation with high levels of proteins, which “leads to organ failure. This is the most striking acute mechanism.”
The second is the development of portal hypertension. “This is slower, but also very important, causing increased pressure in the portal vein, and leading to bleeding if the pressure is too great,” he said.
More tools emerging to help predict ACLF
The Albumin-functionality-test (AFT), which uses serum albumin levels to evaluate liver and kidney function, might also be useful in the prediction of ACLF and 12-month survival, according to a separate study an Italian group presented at the virtual ILC.
“Our main results are that parameters from albumin predict the development of ACLF in acute decompensated patients with the same diagnostic performance as the CLIF-AD score,” said Katja Waterstradt, PhD, from the University of Bologna in Italy.
And when the two tests are combined, diagnostic performance is increased, she added.
Dr. Trebicka has disclosed no relevant financial relationships. Dr. Waterstrand is a researcher for MedInnovation GmbH.
This article first appeared on Medscape.com.
Systemic inflammation and portal hypertension are key predictors of acute-on-chronic liver failure (ACLF) in the 3 months after a hospital stay for acute decompensated cirrhosis and also of death after 12 months, a preliminary analysis of data from the PREDICT study shows.
“Before this, we never had any patient signatures to identify ACLF,” said Jonel Trebicka, MD, PhD, from the JW Goethe University Hospital in Frankfurt, Germany.
Now, Dr. Trebicka’s team has “characterized the phenotypes in pre-ACLF that will progress within 3 months,” he said in an interview. “Those with high levels of inflammatory proteins, white blood cell count, are more likely to develop ACLF.”
ACLF is a highly complex disorder that can lead liver, cardiovascular, renal, cerebral, pulmonary, intestinal, adrenal, and immune systems to fail, Dr. Trebicka explained when he discussed the analysis – published online in the Journal of Hepatology – during the virtual International Liver Congress (ILC) 2020.
The chance of survival after the onset of ACLF is low – the 28-day survival rate is 30% – and “the only treatment we have is liver transplant,” he said.
For their prospective observational study, Dr. Trebicka and his colleagues assessed 1071 participants from 48 European hospitals in 14 countries who were admitted for an episode of acute decompensation, defined as the development of ascites, hepatic encephalopathy, gastrointestinal hemorrhage, infection, or a combination thereof.
The researchers identified three distinct clinical courses for a patient hospitalized with acute decompensated cirrhosis that will help clinicians predict the development of ACLF.
At study enrollment, more than half of the patients at highest risk for ACLF had pre-ACLF and high-grade systemic inflammation. The patients at intermediate risk had unstable decompensated cirrhosis with low-grade systemic inflammation and complications related to severe portal hypertension. And those at lowest risk for ACLF had stable decompensated cirrhosis and no severe systemic inflammation or portal hypertension complications, and did not develop ACLF or another episode of acute decompensation in the subsequent 3 months.
“There have been hints of possible phenotypes before – for stable and unstable ACLF – but we never had anything specific to diagnose,” Trebicka reported.
“We found that there are two main mechanisms in the development of ACLF that are most important,” he said. The first is systemic inflammation with high levels of proteins, which “leads to organ failure. This is the most striking acute mechanism.”
The second is the development of portal hypertension. “This is slower, but also very important, causing increased pressure in the portal vein, and leading to bleeding if the pressure is too great,” he said.
More tools emerging to help predict ACLF
The Albumin-functionality-test (AFT), which uses serum albumin levels to evaluate liver and kidney function, might also be useful in the prediction of ACLF and 12-month survival, according to a separate study an Italian group presented at the virtual ILC.
“Our main results are that parameters from albumin predict the development of ACLF in acute decompensated patients with the same diagnostic performance as the CLIF-AD score,” said Katja Waterstradt, PhD, from the University of Bologna in Italy.
And when the two tests are combined, diagnostic performance is increased, she added.
Dr. Trebicka has disclosed no relevant financial relationships. Dr. Waterstrand is a researcher for MedInnovation GmbH.
This article first appeared on Medscape.com.
Systemic inflammation and portal hypertension are key predictors of acute-on-chronic liver failure (ACLF) in the 3 months after a hospital stay for acute decompensated cirrhosis and also of death after 12 months, a preliminary analysis of data from the PREDICT study shows.
“Before this, we never had any patient signatures to identify ACLF,” said Jonel Trebicka, MD, PhD, from the JW Goethe University Hospital in Frankfurt, Germany.
Now, Dr. Trebicka’s team has “characterized the phenotypes in pre-ACLF that will progress within 3 months,” he said in an interview. “Those with high levels of inflammatory proteins, white blood cell count, are more likely to develop ACLF.”
ACLF is a highly complex disorder that can lead liver, cardiovascular, renal, cerebral, pulmonary, intestinal, adrenal, and immune systems to fail, Dr. Trebicka explained when he discussed the analysis – published online in the Journal of Hepatology – during the virtual International Liver Congress (ILC) 2020.
The chance of survival after the onset of ACLF is low – the 28-day survival rate is 30% – and “the only treatment we have is liver transplant,” he said.
For their prospective observational study, Dr. Trebicka and his colleagues assessed 1071 participants from 48 European hospitals in 14 countries who were admitted for an episode of acute decompensation, defined as the development of ascites, hepatic encephalopathy, gastrointestinal hemorrhage, infection, or a combination thereof.
The researchers identified three distinct clinical courses for a patient hospitalized with acute decompensated cirrhosis that will help clinicians predict the development of ACLF.
At study enrollment, more than half of the patients at highest risk for ACLF had pre-ACLF and high-grade systemic inflammation. The patients at intermediate risk had unstable decompensated cirrhosis with low-grade systemic inflammation and complications related to severe portal hypertension. And those at lowest risk for ACLF had stable decompensated cirrhosis and no severe systemic inflammation or portal hypertension complications, and did not develop ACLF or another episode of acute decompensation in the subsequent 3 months.
“There have been hints of possible phenotypes before – for stable and unstable ACLF – but we never had anything specific to diagnose,” Trebicka reported.
“We found that there are two main mechanisms in the development of ACLF that are most important,” he said. The first is systemic inflammation with high levels of proteins, which “leads to organ failure. This is the most striking acute mechanism.”
The second is the development of portal hypertension. “This is slower, but also very important, causing increased pressure in the portal vein, and leading to bleeding if the pressure is too great,” he said.
More tools emerging to help predict ACLF
The Albumin-functionality-test (AFT), which uses serum albumin levels to evaluate liver and kidney function, might also be useful in the prediction of ACLF and 12-month survival, according to a separate study an Italian group presented at the virtual ILC.
“Our main results are that parameters from albumin predict the development of ACLF in acute decompensated patients with the same diagnostic performance as the CLIF-AD score,” said Katja Waterstradt, PhD, from the University of Bologna in Italy.
And when the two tests are combined, diagnostic performance is increased, she added.
Dr. Trebicka has disclosed no relevant financial relationships. Dr. Waterstrand is a researcher for MedInnovation GmbH.
This article first appeared on Medscape.com.
Vascepa maker loses patent appeal, plans ‘vigorous’ fight
Amarin’s hopes of fending off generic competition for its blockbuster high-strength eicosapentaenoic acid product, icosapent ethyl (Vascepa), have dimmed following a decision by the U.S. Court of Appeals for the Federal Circuit in the company’s ongoing patent litigation.
The court upheld the March ruling by the District Court for the District of Nevada in favor of two generic companies in connection with their abbreviated new drug applications (ANDAs) for the product.
Amarin said it is currently reviewing its legal options and within 30 days expects to file a petition for an en banc review of the current decision by the full panel of 12 active judges at the Court of Appeals for the Federal Circuit.
“We are extremely disappointed with [the] ruling and plan to vigorously pursue available remedies,” John Thero, Amarin president and chief executive officer, said in a statement.
In 2012, Vascepa became the first and only prescription treatment approved by the Food and Drug Administration made up solely of the active ingredient icosapent ethyl, a unique form of eicosapentaenoic acid. It was initially approved for the reduction of very high triglyceride levels (≥500 mg/dL).
In late 2019, the FDA extended the indication to reduce the risk for cardiovascular events in people with elevated triglyceride levels and either established CV disease or diabetes with other CV risk factors.
The extended indication was based on results of the landmark REDUCE-IT trial, which showed a 25% relative risk reduction in major adverse CV events with icosapent ethyl, compared with placebo, in patients with triglyceride levels above 135 mg/dL and who had CV disease (70% of the study population), or who were high-risk primary-prevention patients with diabetes and one additional risk factor (30% of the study population).
According to Amarin, since its launch, Vascepa has been prescribed more than 8 million times.
The company said demand for the product in the United States remains “strong” and indicated that, despite the legal setback, it would continue promotional efforts. The company is also seeking additional regulatory approvals in China, Europe, and additional countries in the Middle East.
“We are particularly excited about the anticipated commercialization opportunities for Vascepa in Europe as we prepare for expected approval and launch in early 2021,” Mr. Thero said.
The company anticipates 10 years of market protection because of regulatory exclusivity in the European Union once approved, and said patent protection could extend into 2039.
Only Vascepa sold in the United States is subject to this litigation and judgment. No generic litigation is pending outside the United States, Amarin said.
A version of this article originally appeared on Medscape.com.
Amarin’s hopes of fending off generic competition for its blockbuster high-strength eicosapentaenoic acid product, icosapent ethyl (Vascepa), have dimmed following a decision by the U.S. Court of Appeals for the Federal Circuit in the company’s ongoing patent litigation.
The court upheld the March ruling by the District Court for the District of Nevada in favor of two generic companies in connection with their abbreviated new drug applications (ANDAs) for the product.
Amarin said it is currently reviewing its legal options and within 30 days expects to file a petition for an en banc review of the current decision by the full panel of 12 active judges at the Court of Appeals for the Federal Circuit.
“We are extremely disappointed with [the] ruling and plan to vigorously pursue available remedies,” John Thero, Amarin president and chief executive officer, said in a statement.
In 2012, Vascepa became the first and only prescription treatment approved by the Food and Drug Administration made up solely of the active ingredient icosapent ethyl, a unique form of eicosapentaenoic acid. It was initially approved for the reduction of very high triglyceride levels (≥500 mg/dL).
In late 2019, the FDA extended the indication to reduce the risk for cardiovascular events in people with elevated triglyceride levels and either established CV disease or diabetes with other CV risk factors.
The extended indication was based on results of the landmark REDUCE-IT trial, which showed a 25% relative risk reduction in major adverse CV events with icosapent ethyl, compared with placebo, in patients with triglyceride levels above 135 mg/dL and who had CV disease (70% of the study population), or who were high-risk primary-prevention patients with diabetes and one additional risk factor (30% of the study population).
According to Amarin, since its launch, Vascepa has been prescribed more than 8 million times.
The company said demand for the product in the United States remains “strong” and indicated that, despite the legal setback, it would continue promotional efforts. The company is also seeking additional regulatory approvals in China, Europe, and additional countries in the Middle East.
“We are particularly excited about the anticipated commercialization opportunities for Vascepa in Europe as we prepare for expected approval and launch in early 2021,” Mr. Thero said.
The company anticipates 10 years of market protection because of regulatory exclusivity in the European Union once approved, and said patent protection could extend into 2039.
Only Vascepa sold in the United States is subject to this litigation and judgment. No generic litigation is pending outside the United States, Amarin said.
A version of this article originally appeared on Medscape.com.
Amarin’s hopes of fending off generic competition for its blockbuster high-strength eicosapentaenoic acid product, icosapent ethyl (Vascepa), have dimmed following a decision by the U.S. Court of Appeals for the Federal Circuit in the company’s ongoing patent litigation.
The court upheld the March ruling by the District Court for the District of Nevada in favor of two generic companies in connection with their abbreviated new drug applications (ANDAs) for the product.
Amarin said it is currently reviewing its legal options and within 30 days expects to file a petition for an en banc review of the current decision by the full panel of 12 active judges at the Court of Appeals for the Federal Circuit.
“We are extremely disappointed with [the] ruling and plan to vigorously pursue available remedies,” John Thero, Amarin president and chief executive officer, said in a statement.
In 2012, Vascepa became the first and only prescription treatment approved by the Food and Drug Administration made up solely of the active ingredient icosapent ethyl, a unique form of eicosapentaenoic acid. It was initially approved for the reduction of very high triglyceride levels (≥500 mg/dL).
In late 2019, the FDA extended the indication to reduce the risk for cardiovascular events in people with elevated triglyceride levels and either established CV disease or diabetes with other CV risk factors.
The extended indication was based on results of the landmark REDUCE-IT trial, which showed a 25% relative risk reduction in major adverse CV events with icosapent ethyl, compared with placebo, in patients with triglyceride levels above 135 mg/dL and who had CV disease (70% of the study population), or who were high-risk primary-prevention patients with diabetes and one additional risk factor (30% of the study population).
According to Amarin, since its launch, Vascepa has been prescribed more than 8 million times.
The company said demand for the product in the United States remains “strong” and indicated that, despite the legal setback, it would continue promotional efforts. The company is also seeking additional regulatory approvals in China, Europe, and additional countries in the Middle East.
“We are particularly excited about the anticipated commercialization opportunities for Vascepa in Europe as we prepare for expected approval and launch in early 2021,” Mr. Thero said.
The company anticipates 10 years of market protection because of regulatory exclusivity in the European Union once approved, and said patent protection could extend into 2039.
Only Vascepa sold in the United States is subject to this litigation and judgment. No generic litigation is pending outside the United States, Amarin said.
A version of this article originally appeared on Medscape.com.
First U.S. trial to test aerosolized chemotherapy in advanced cancers
A team of U.S. researchers is investigating whether pressurized intraperitoneal aerosolized chemotherapy (PIPAC) can benefit patients with advanced cancer and peritoneal carcinomatosis.
The team’s phase 1 trial is the first in the United States to test PIPAC, and it will enroll patients with ovarian, uterine, colorectal, or gastric cancer who have peritoneal carcinomatosis.
Data from studies outside the United States suggest PIPAC can induce regression of peritoneal carcinomatosis, even in end-stage, therapy-resistant gastric, ovarian, and colorectal cancers (Lancet Oncol. 2019 Jul;20[7]:e368-e377).
The current study (NCT04329494) formally introduces PIPAC to the United States and serves as a launching pad for further investigation into how the treatment should be administered and which types of chemotherapies can be used.
About PIPAC
“PIPAC is a novel therapeutic approach that is minimally invasive, does not require cytoreduction, and can be repeated frequently,” said Thanh Dellinger, MD, a gynecologic oncology surgeon at City of Hope in Duarte, Calif., and co–principal investigator of the phase 1 trial.
“[PIPAC] entails accessing the abdominal cavity using standard laparoscopic techniques and relies on the increased intra-abdominal pressure (15 mm Hg) achieved with laparoscopic surgery, which generates a convective flux that forces aerosolized chemotherapy drugs from the peritoneal cavity into the subperitoneal tissue and overcomes the tumor’s interstitial pressure,” Dr. Dellinger explained in an interview.
“The surgical procedure to deliver PIPAC does not typically cause adhesive disease and allows for repeated delivery of intraperitoneal chemotherapy, objective tumor staging, and response assessment,” she noted.
Dr. Dellinger said the advantages of PIPAC include a minimally invasive approach; no debulking surgery required; deeper uptake of drugs in tumor tissues; wider, more effective drug distribution; fewer toxicities caused by lower drug dosage; repeatable administration; and palliation of peritoneal carcinomatosis symptoms, including abdominal bloating and ascites.
PIPAC achieves a deeper peritoneal nodule penetration of several millimeters with cisplatin, compared to less than 1 mm with heated intraoperative peritoneal chemotherapy (HIPEC) and other intraperitoneal methods, according to Amit Merchea, MD, an assistant professor of surgery at the Mayo Clinic in Jacksonville, Fla.
Dr. Merchea performed the first PIPAC procedure in the United States in December 2019.
Innovative therapies needed
Peritoneal carcinomatosis is often a late-stage manifestation of abdominal cancers and is usually lethal, Dr. Dellinger said. She noted that systemic chemotherapy in the palliative setting is relatively ineffective in patients with peritoneal carcinomatosis because of pharmacokinetic limitations, poor peritoneal drug uptake, and impaired local drug distribution.
“Innovative, effective therapies are urgently needed for people who have ovarian, uterine, gastric, or colorectal cancer with peritoneal carcinomatosis,” Dr. Dellinger said.
“PIPAC is a novel treatment option that has had very favorable and exciting results,” Dr. Merchea said. “It is a potential option for patients when no other treatment options exist, and it is an avenue to provide hope to patients when often they have none.”
Potential candidates for PIPAC include patients who have peritoneal carcinomatosis, have failed other standard therapies, have more than 6 months’ life expectancy, and are not candidates for cytoreduction with HIPEC. There remains very limited data on the use of PIPAC as a neoadjuvant approach to convert patients who were previously unresectable to resectable disease, Dr. Merchea noted.
“To deliver chemotherapy directly to the tumor under pressure allows PIPAC to better penetrate the peritoneal surface and tumor nodules than traditional approaches, such as HIPEC,” Dr. Merchea said. “And the drug distribution at the tissue level is better than what is often achieved by systemic chemotherapy, but without the systemic effects of chemotherapy, such as hair loss. The treatment gives essentially a real-time, quantitative assessment of response by being able to directly assess the tumor via laparoscopic visualization and repeat biopsy.”
“Importantly, patients who undergo PIPAC don’t notice a decrease in their quality of life, and some patients note improvement, particularly with respect to nausea, vomiting, appetite, fatigue, and constipation,” Dr. Merchea said.
Trial details
The phase 1 trial of PIPAC will include a maximum of 24 patients. They will receive treatment every 6 weeks for up to three cycles and be followed for up to 3 years.
Patients with ovarian, uterine, or gastric cancer will undergo PIPAC with cisplatin, followed by doxorubicin. Patients with colorectal cancer will undergo PIPAC with oxaliplatin preceded by leucovorin and fluorouracil for cycles 2 and 3.
The researchers also plan to profile patients’ tumors.
“Tumor samples will be chronologically evaluated with genomics, spatial transcriptomics, pharmacodynamics, and single-cell sequencing throughout a patient’s treatment course, thus elucidating the treatment effects and natural history of peritoneal cancers,” Dr. Dellinger said.
The trial sites include City of Hope, Mayo Clinic in Florida, Northwell Health in New York, and the National Cancer Institute in Maryland.
The trial is sponsored by City of Hope in collaboration with the National Cancer Institute. Dr. Merchea and Dr. Dellinger reported having no conflicts of interest.
A team of U.S. researchers is investigating whether pressurized intraperitoneal aerosolized chemotherapy (PIPAC) can benefit patients with advanced cancer and peritoneal carcinomatosis.

The team’s phase 1 trial is the first in the United States to test PIPAC, and it will enroll patients with ovarian, uterine, colorectal, or gastric cancer who have peritoneal carcinomatosis.
Data from studies outside the United States suggest PIPAC can induce regression of peritoneal carcinomatosis, even in end-stage, therapy-resistant gastric, ovarian, and colorectal cancers (Lancet Oncol. 2019 Jul;20[7]:e368-e377).
The current study (NCT04329494) formally introduces PIPAC to the United States and serves as a launching pad for further investigation into how the treatment should be administered and which types of chemotherapies can be used.
About PIPAC
“PIPAC is a novel therapeutic approach that is minimally invasive, does not require cytoreduction, and can be repeated frequently,” said Thanh Dellinger, MD, a gynecologic oncology surgeon at City of Hope in Duarte, Calif., and co–principal investigator of the phase 1 trial.
“[PIPAC] entails accessing the abdominal cavity using standard laparoscopic techniques and relies on the increased intra-abdominal pressure (15 mm Hg) achieved with laparoscopic surgery, which generates a convective flux that forces aerosolized chemotherapy drugs from the peritoneal cavity into the subperitoneal tissue and overcomes the tumor’s interstitial pressure,” Dr. Dellinger explained in an interview.
“The surgical procedure to deliver PIPAC does not typically cause adhesive disease and allows for repeated delivery of intraperitoneal chemotherapy, objective tumor staging, and response assessment,” she noted.
Dr. Dellinger said the advantages of PIPAC include a minimally invasive approach; no debulking surgery required; deeper uptake of drugs in tumor tissues; wider, more effective drug distribution; fewer toxicities caused by lower drug dosage; repeatable administration; and palliation of peritoneal carcinomatosis symptoms, including abdominal bloating and ascites.
PIPAC achieves a deeper peritoneal nodule penetration of several millimeters with cisplatin, compared to less than 1 mm with heated intraoperative peritoneal chemotherapy (HIPEC) and other intraperitoneal methods, according to Amit Merchea, MD, an assistant professor of surgery at the Mayo Clinic in Jacksonville, Fla.
Dr. Merchea performed the first PIPAC procedure in the United States in December 2019.
Innovative therapies needed
Peritoneal carcinomatosis is often a late-stage manifestation of abdominal cancers and is usually lethal, Dr. Dellinger said. She noted that systemic chemotherapy in the palliative setting is relatively ineffective in patients with peritoneal carcinomatosis because of pharmacokinetic limitations, poor peritoneal drug uptake, and impaired local drug distribution.
“Innovative, effective therapies are urgently needed for people who have ovarian, uterine, gastric, or colorectal cancer with peritoneal carcinomatosis,” Dr. Dellinger said.
“PIPAC is a novel treatment option that has had very favorable and exciting results,” Dr. Merchea said. “It is a potential option for patients when no other treatment options exist, and it is an avenue to provide hope to patients when often they have none.”
Potential candidates for PIPAC include patients who have peritoneal carcinomatosis, have failed other standard therapies, have more than 6 months’ life expectancy, and are not candidates for cytoreduction with HIPEC. There remains very limited data on the use of PIPAC as a neoadjuvant approach to convert patients who were previously unresectable to resectable disease, Dr. Merchea noted.
“To deliver chemotherapy directly to the tumor under pressure allows PIPAC to better penetrate the peritoneal surface and tumor nodules than traditional approaches, such as HIPEC,” Dr. Merchea said. “And the drug distribution at the tissue level is better than what is often achieved by systemic chemotherapy, but without the systemic effects of chemotherapy, such as hair loss. The treatment gives essentially a real-time, quantitative assessment of response by being able to directly assess the tumor via laparoscopic visualization and repeat biopsy.”
“Importantly, patients who undergo PIPAC don’t notice a decrease in their quality of life, and some patients note improvement, particularly with respect to nausea, vomiting, appetite, fatigue, and constipation,” Dr. Merchea said.
Trial details
The phase 1 trial of PIPAC will include a maximum of 24 patients. They will receive treatment every 6 weeks for up to three cycles and be followed for up to 3 years.
Patients with ovarian, uterine, or gastric cancer will undergo PIPAC with cisplatin, followed by doxorubicin. Patients with colorectal cancer will undergo PIPAC with oxaliplatin preceded by leucovorin and fluorouracil for cycles 2 and 3.
The researchers also plan to profile patients’ tumors.
“Tumor samples will be chronologically evaluated with genomics, spatial transcriptomics, pharmacodynamics, and single-cell sequencing throughout a patient’s treatment course, thus elucidating the treatment effects and natural history of peritoneal cancers,” Dr. Dellinger said.
The trial sites include City of Hope, Mayo Clinic in Florida, Northwell Health in New York, and the National Cancer Institute in Maryland.
The trial is sponsored by City of Hope in collaboration with the National Cancer Institute. Dr. Merchea and Dr. Dellinger reported having no conflicts of interest.
A team of U.S. researchers is investigating whether pressurized intraperitoneal aerosolized chemotherapy (PIPAC) can benefit patients with advanced cancer and peritoneal carcinomatosis.

The team’s phase 1 trial is the first in the United States to test PIPAC, and it will enroll patients with ovarian, uterine, colorectal, or gastric cancer who have peritoneal carcinomatosis.
Data from studies outside the United States suggest PIPAC can induce regression of peritoneal carcinomatosis, even in end-stage, therapy-resistant gastric, ovarian, and colorectal cancers (Lancet Oncol. 2019 Jul;20[7]:e368-e377).
The current study (NCT04329494) formally introduces PIPAC to the United States and serves as a launching pad for further investigation into how the treatment should be administered and which types of chemotherapies can be used.
About PIPAC
“PIPAC is a novel therapeutic approach that is minimally invasive, does not require cytoreduction, and can be repeated frequently,” said Thanh Dellinger, MD, a gynecologic oncology surgeon at City of Hope in Duarte, Calif., and co–principal investigator of the phase 1 trial.
“[PIPAC] entails accessing the abdominal cavity using standard laparoscopic techniques and relies on the increased intra-abdominal pressure (15 mm Hg) achieved with laparoscopic surgery, which generates a convective flux that forces aerosolized chemotherapy drugs from the peritoneal cavity into the subperitoneal tissue and overcomes the tumor’s interstitial pressure,” Dr. Dellinger explained in an interview.
“The surgical procedure to deliver PIPAC does not typically cause adhesive disease and allows for repeated delivery of intraperitoneal chemotherapy, objective tumor staging, and response assessment,” she noted.
Dr. Dellinger said the advantages of PIPAC include a minimally invasive approach; no debulking surgery required; deeper uptake of drugs in tumor tissues; wider, more effective drug distribution; fewer toxicities caused by lower drug dosage; repeatable administration; and palliation of peritoneal carcinomatosis symptoms, including abdominal bloating and ascites.
PIPAC achieves a deeper peritoneal nodule penetration of several millimeters with cisplatin, compared to less than 1 mm with heated intraoperative peritoneal chemotherapy (HIPEC) and other intraperitoneal methods, according to Amit Merchea, MD, an assistant professor of surgery at the Mayo Clinic in Jacksonville, Fla.
Dr. Merchea performed the first PIPAC procedure in the United States in December 2019.
Innovative therapies needed
Peritoneal carcinomatosis is often a late-stage manifestation of abdominal cancers and is usually lethal, Dr. Dellinger said. She noted that systemic chemotherapy in the palliative setting is relatively ineffective in patients with peritoneal carcinomatosis because of pharmacokinetic limitations, poor peritoneal drug uptake, and impaired local drug distribution.
“Innovative, effective therapies are urgently needed for people who have ovarian, uterine, gastric, or colorectal cancer with peritoneal carcinomatosis,” Dr. Dellinger said.
“PIPAC is a novel treatment option that has had very favorable and exciting results,” Dr. Merchea said. “It is a potential option for patients when no other treatment options exist, and it is an avenue to provide hope to patients when often they have none.”
Potential candidates for PIPAC include patients who have peritoneal carcinomatosis, have failed other standard therapies, have more than 6 months’ life expectancy, and are not candidates for cytoreduction with HIPEC. There remains very limited data on the use of PIPAC as a neoadjuvant approach to convert patients who were previously unresectable to resectable disease, Dr. Merchea noted.
“To deliver chemotherapy directly to the tumor under pressure allows PIPAC to better penetrate the peritoneal surface and tumor nodules than traditional approaches, such as HIPEC,” Dr. Merchea said. “And the drug distribution at the tissue level is better than what is often achieved by systemic chemotherapy, but without the systemic effects of chemotherapy, such as hair loss. The treatment gives essentially a real-time, quantitative assessment of response by being able to directly assess the tumor via laparoscopic visualization and repeat biopsy.”
“Importantly, patients who undergo PIPAC don’t notice a decrease in their quality of life, and some patients note improvement, particularly with respect to nausea, vomiting, appetite, fatigue, and constipation,” Dr. Merchea said.
Trial details
The phase 1 trial of PIPAC will include a maximum of 24 patients. They will receive treatment every 6 weeks for up to three cycles and be followed for up to 3 years.
Patients with ovarian, uterine, or gastric cancer will undergo PIPAC with cisplatin, followed by doxorubicin. Patients with colorectal cancer will undergo PIPAC with oxaliplatin preceded by leucovorin and fluorouracil for cycles 2 and 3.
The researchers also plan to profile patients’ tumors.
“Tumor samples will be chronologically evaluated with genomics, spatial transcriptomics, pharmacodynamics, and single-cell sequencing throughout a patient’s treatment course, thus elucidating the treatment effects and natural history of peritoneal cancers,” Dr. Dellinger said.
The trial sites include City of Hope, Mayo Clinic in Florida, Northwell Health in New York, and the National Cancer Institute in Maryland.
The trial is sponsored by City of Hope in collaboration with the National Cancer Institute. Dr. Merchea and Dr. Dellinger reported having no conflicts of interest.
Oxidative stress linked to cytogenetic abnormalities in CLL
Oxidative stress may play a role in pathogenesis of B-cell chronic lymphocytic leukemia (B-CLL), according to the results of a biochemical and cytogenetic study of patients published online in Experimental and Molecular Pathology.
The study evaluated the serum levels of oxidative stress biomarkers [conjugated dienes (CD), malondialdehyde (MDA), and nitrite levels] and the levels of antioxidant biomarkers [ceruloplasmin (CP) and glutathione peroxidase (GPx)] in 64 B-CLL patients. The relationship between these biomarkers and the presence of cytogenetic abnormalities was examined, according to Tatiana Zhevak, MD, of Sechenov First Moscow (Russia) State Medical University, and colleagues.
Cytogenetic abnormalities have previously been determined to be linked to a poorer prognosis in CLL patients, and factors that increase the frequency of CA have been shown to increase the risk of rapid tumor progression, Dr. Zhevak and her colleagues stated.
Oxidative stress connection
Enhanced oxidative stress was detected in B-CLL patients as shown by their increased levels of serum CD, MDA, and nitrite, as well as a demonstrated imbalance in the antioxidant defense system as shown by an increased serum CP level and decreased serum GPx activity, according to the researchers.
In addition, these metabolic changes were found to be greater in those patients whose lymphocytes harbored specific cytogenetic abnormalities, and could be predicted by the serum levels of CD. Specifically, the odds of harboring a cytogenetic abnormality increased by a factor of 1.88 (P = .004) for every one-unit increase in serum CD level (mcmol/L), according to the authors.
“Collectively, the results support our hypothesis that oxidative stress and resulting lipid peroxidation play a role in pathogenesis of B-CLL and provide a rational basis for the use of agents regulating the pro-oxidant and antioxidant activity in the treatment of B-CLL patients,” the researchers concluded.
The research was unsponsored and the authors reported having no conflicts.
SOURCE: Zhevak T et al. Exp Mol Patholo. 2020 Oct;16:104524 doi: 10.1016/j.yexmp.2020.104524.
Oxidative stress may play a role in pathogenesis of B-cell chronic lymphocytic leukemia (B-CLL), according to the results of a biochemical and cytogenetic study of patients published online in Experimental and Molecular Pathology.
The study evaluated the serum levels of oxidative stress biomarkers [conjugated dienes (CD), malondialdehyde (MDA), and nitrite levels] and the levels of antioxidant biomarkers [ceruloplasmin (CP) and glutathione peroxidase (GPx)] in 64 B-CLL patients. The relationship between these biomarkers and the presence of cytogenetic abnormalities was examined, according to Tatiana Zhevak, MD, of Sechenov First Moscow (Russia) State Medical University, and colleagues.
Cytogenetic abnormalities have previously been determined to be linked to a poorer prognosis in CLL patients, and factors that increase the frequency of CA have been shown to increase the risk of rapid tumor progression, Dr. Zhevak and her colleagues stated.
Oxidative stress connection
Enhanced oxidative stress was detected in B-CLL patients as shown by their increased levels of serum CD, MDA, and nitrite, as well as a demonstrated imbalance in the antioxidant defense system as shown by an increased serum CP level and decreased serum GPx activity, according to the researchers.
In addition, these metabolic changes were found to be greater in those patients whose lymphocytes harbored specific cytogenetic abnormalities, and could be predicted by the serum levels of CD. Specifically, the odds of harboring a cytogenetic abnormality increased by a factor of 1.88 (P = .004) for every one-unit increase in serum CD level (mcmol/L), according to the authors.
“Collectively, the results support our hypothesis that oxidative stress and resulting lipid peroxidation play a role in pathogenesis of B-CLL and provide a rational basis for the use of agents regulating the pro-oxidant and antioxidant activity in the treatment of B-CLL patients,” the researchers concluded.
The research was unsponsored and the authors reported having no conflicts.
SOURCE: Zhevak T et al. Exp Mol Patholo. 2020 Oct;16:104524 doi: 10.1016/j.yexmp.2020.104524.
Oxidative stress may play a role in pathogenesis of B-cell chronic lymphocytic leukemia (B-CLL), according to the results of a biochemical and cytogenetic study of patients published online in Experimental and Molecular Pathology.
The study evaluated the serum levels of oxidative stress biomarkers [conjugated dienes (CD), malondialdehyde (MDA), and nitrite levels] and the levels of antioxidant biomarkers [ceruloplasmin (CP) and glutathione peroxidase (GPx)] in 64 B-CLL patients. The relationship between these biomarkers and the presence of cytogenetic abnormalities was examined, according to Tatiana Zhevak, MD, of Sechenov First Moscow (Russia) State Medical University, and colleagues.
Cytogenetic abnormalities have previously been determined to be linked to a poorer prognosis in CLL patients, and factors that increase the frequency of CA have been shown to increase the risk of rapid tumor progression, Dr. Zhevak and her colleagues stated.
Oxidative stress connection
Enhanced oxidative stress was detected in B-CLL patients as shown by their increased levels of serum CD, MDA, and nitrite, as well as a demonstrated imbalance in the antioxidant defense system as shown by an increased serum CP level and decreased serum GPx activity, according to the researchers.
In addition, these metabolic changes were found to be greater in those patients whose lymphocytes harbored specific cytogenetic abnormalities, and could be predicted by the serum levels of CD. Specifically, the odds of harboring a cytogenetic abnormality increased by a factor of 1.88 (P = .004) for every one-unit increase in serum CD level (mcmol/L), according to the authors.
“Collectively, the results support our hypothesis that oxidative stress and resulting lipid peroxidation play a role in pathogenesis of B-CLL and provide a rational basis for the use of agents regulating the pro-oxidant and antioxidant activity in the treatment of B-CLL patients,” the researchers concluded.
The research was unsponsored and the authors reported having no conflicts.
SOURCE: Zhevak T et al. Exp Mol Patholo. 2020 Oct;16:104524 doi: 10.1016/j.yexmp.2020.104524.
FROM Experimental and Molecular Pathology
Deaths sky high in hospitalized COVID patients with kidney injury
More evidence indicates that the development of acute kidney injury
“This ... is the first study in the United States to report the persistence of kidney dysfunction (lack of recovery) in survivors of COVID-19–associated AKI [and] this is in marked contrast to other forms of AKI where over 80% of patients recover their renal function by 10 days,” Lili Chan, MD, of the Icahn School of Medicine at Mount Sinai, New York, and colleagues observed.
The research is a retrospective, observational cohort study published online Sept. 3 in the Journal of the American Society of Nephrology
“We may be facing an epidemic of post–COVID-19 kidney disease and that, in turn, could mean much greater numbers of patients who require kidney dialysis and even transplants,” said senior author Girish Nadkarni, MD, a nephrologist, in a statement from Mount Sinai.
Nephrologists will need to prepare for a significant uptick in patients with chronic kidney disease as a result of exposure to the SARS-CoV-2 virus that causes COVID-19, the researchers warned.
“These findings may help centers with resource planning and preparing for the increased load resulting from survivors of COVID-19–associated AKI who do not experience recovery of kidney function,” they added.
Analysis of patients from February to end of May 2020
“AKI among hospitalized patients with COVID-19 in the United States is not well described,” they noted in their article.
And so they analyzed data from five major hospitals in the Mount Sinai Health System between Feb. 27 and May 30 of this year, during which 3,993 patients were hospitalized within the system for COVID-19. The MSHS has a patient population of racially and ethnically diverse citizens from New York.
AKI was defined using Kidney Disease: Improving Global Outcomes (KDIGO) criteria. AKI occurred in 46% of the overall cohort of patients, 19% of whom required dialysis.
However, among those patients who required admission to the ICU, over three-quarters (76%) developed AKI and almost one-third of ICU patients required dialysis, the investigators said.
“The median time from hospital admission until AKI diagnoses was 1 day and the median time from AKI diagnosis to dialysis was 3 days,” they explain.
The proportion of patients with stages 1, 2, or 3 AKI among those admitted to hospital were 39%, 19%, and 42%, respectively. In patients requiring admission to ICU, 28% had stage 1 AKI, 17% had stage 2, and 56% had stage 3.
And among those who required dialysis for AKI, the median peak serum creatinine was 8.2 mg/dL, compared with 2.2 mg/dL for those who did not require dialysis.
Predictors of AKI: male sex, potassium levels, and preexisting CKD
Almost two thirds of patients (65%) had recovered from their kidney injury by the time they left hospital but 35% had acute kidney disease. Of this latter group, on follow-up, 36% had recovered from it, the investigators noted.
Conversely, of those patients who had recovered from AKI by hospital discharge, 14% went on to develop acute kidney disease at the time of follow-up.
And 30% of patients who had required dialysis at some point during their hospital care required dialysis again within 72 hours of being discharged, the investigators noted.
Predictors of severe AKI included male sex (adjusted odds ratio, 1.46), potassium levels on admission (aOR, 1.7), and preexisting chronic kidney disease (CKD) (aOR, 2.8).
Most compellingly, “in-hospital mortality in patients who experienced AKI was 50% [versus] 8% in patients without AKI (P < .001),” Dr. Nadkarni and colleagues reported.
Among those who required ICU care, 42% of patients with AKI died, compared with 7% of those in ICU who did not develop AKI, while in patients cared for outside of ICU, 62% with AKI died compared with only 13% of those who did not develop AKI.
And after adjusting for demographics, comorbidities, and laboratory values, the aOR for death was 11.4 times higher for ICU patients with AKI, compared with ICU patients without AKI, the authors emphasize.
In all patients who developed AKI, the aOR for mortality was 9.2, compared with patients who did not develop AKI, they added.
Perhaps predictably, the risk of death rose with increasing stage of AKI, and patients with stage 3 AKI who required dialysis were at highest risk of death, the authors observe.
Sheer number of AKI cases, need for dialysis unprecedented
“The sheer number of AKI cases and the overwhelming need for dialysis that we are seeing in the context of COVID-19 is unprecedented,” Dr. Nadkarni said.
“These findings bring clinical evidence to the hypothesis of lingering organ dysfunction among patients recovering from COVID-19 and serve as a reminder to hospitals around the country to be very strategic in the allocation of resources to care for patients who experience AKI,” he cautioned.
“We are grappling with a great deal of uncertainty as to how the virus will impact the kidneys in the long haul,” Dr. Nadkarni added. “We may be facing an epidemic of post–COVID-19 kidney disease, and that, in turn, could mean much greater numbers of patients who require kidney dialysis and even transplants.”
Dr. Nadkarni reported serving as a consultant and advisory board member for RenalytixAI and owns equity in the company.
This article first appeared on Medscape.com.
More evidence indicates that the development of acute kidney injury
“This ... is the first study in the United States to report the persistence of kidney dysfunction (lack of recovery) in survivors of COVID-19–associated AKI [and] this is in marked contrast to other forms of AKI where over 80% of patients recover their renal function by 10 days,” Lili Chan, MD, of the Icahn School of Medicine at Mount Sinai, New York, and colleagues observed.
The research is a retrospective, observational cohort study published online Sept. 3 in the Journal of the American Society of Nephrology
“We may be facing an epidemic of post–COVID-19 kidney disease and that, in turn, could mean much greater numbers of patients who require kidney dialysis and even transplants,” said senior author Girish Nadkarni, MD, a nephrologist, in a statement from Mount Sinai.
Nephrologists will need to prepare for a significant uptick in patients with chronic kidney disease as a result of exposure to the SARS-CoV-2 virus that causes COVID-19, the researchers warned.
“These findings may help centers with resource planning and preparing for the increased load resulting from survivors of COVID-19–associated AKI who do not experience recovery of kidney function,” they added.
Analysis of patients from February to end of May 2020
“AKI among hospitalized patients with COVID-19 in the United States is not well described,” they noted in their article.
And so they analyzed data from five major hospitals in the Mount Sinai Health System between Feb. 27 and May 30 of this year, during which 3,993 patients were hospitalized within the system for COVID-19. The MSHS has a patient population of racially and ethnically diverse citizens from New York.
AKI was defined using Kidney Disease: Improving Global Outcomes (KDIGO) criteria. AKI occurred in 46% of the overall cohort of patients, 19% of whom required dialysis.
However, among those patients who required admission to the ICU, over three-quarters (76%) developed AKI and almost one-third of ICU patients required dialysis, the investigators said.
“The median time from hospital admission until AKI diagnoses was 1 day and the median time from AKI diagnosis to dialysis was 3 days,” they explain.
The proportion of patients with stages 1, 2, or 3 AKI among those admitted to hospital were 39%, 19%, and 42%, respectively. In patients requiring admission to ICU, 28% had stage 1 AKI, 17% had stage 2, and 56% had stage 3.
And among those who required dialysis for AKI, the median peak serum creatinine was 8.2 mg/dL, compared with 2.2 mg/dL for those who did not require dialysis.
Predictors of AKI: male sex, potassium levels, and preexisting CKD
Almost two thirds of patients (65%) had recovered from their kidney injury by the time they left hospital but 35% had acute kidney disease. Of this latter group, on follow-up, 36% had recovered from it, the investigators noted.
Conversely, of those patients who had recovered from AKI by hospital discharge, 14% went on to develop acute kidney disease at the time of follow-up.
And 30% of patients who had required dialysis at some point during their hospital care required dialysis again within 72 hours of being discharged, the investigators noted.
Predictors of severe AKI included male sex (adjusted odds ratio, 1.46), potassium levels on admission (aOR, 1.7), and preexisting chronic kidney disease (CKD) (aOR, 2.8).
Most compellingly, “in-hospital mortality in patients who experienced AKI was 50% [versus] 8% in patients without AKI (P < .001),” Dr. Nadkarni and colleagues reported.
Among those who required ICU care, 42% of patients with AKI died, compared with 7% of those in ICU who did not develop AKI, while in patients cared for outside of ICU, 62% with AKI died compared with only 13% of those who did not develop AKI.
And after adjusting for demographics, comorbidities, and laboratory values, the aOR for death was 11.4 times higher for ICU patients with AKI, compared with ICU patients without AKI, the authors emphasize.
In all patients who developed AKI, the aOR for mortality was 9.2, compared with patients who did not develop AKI, they added.
Perhaps predictably, the risk of death rose with increasing stage of AKI, and patients with stage 3 AKI who required dialysis were at highest risk of death, the authors observe.
Sheer number of AKI cases, need for dialysis unprecedented
“The sheer number of AKI cases and the overwhelming need for dialysis that we are seeing in the context of COVID-19 is unprecedented,” Dr. Nadkarni said.
“These findings bring clinical evidence to the hypothesis of lingering organ dysfunction among patients recovering from COVID-19 and serve as a reminder to hospitals around the country to be very strategic in the allocation of resources to care for patients who experience AKI,” he cautioned.
“We are grappling with a great deal of uncertainty as to how the virus will impact the kidneys in the long haul,” Dr. Nadkarni added. “We may be facing an epidemic of post–COVID-19 kidney disease, and that, in turn, could mean much greater numbers of patients who require kidney dialysis and even transplants.”
Dr. Nadkarni reported serving as a consultant and advisory board member for RenalytixAI and owns equity in the company.
This article first appeared on Medscape.com.
More evidence indicates that the development of acute kidney injury
“This ... is the first study in the United States to report the persistence of kidney dysfunction (lack of recovery) in survivors of COVID-19–associated AKI [and] this is in marked contrast to other forms of AKI where over 80% of patients recover their renal function by 10 days,” Lili Chan, MD, of the Icahn School of Medicine at Mount Sinai, New York, and colleagues observed.
The research is a retrospective, observational cohort study published online Sept. 3 in the Journal of the American Society of Nephrology
“We may be facing an epidemic of post–COVID-19 kidney disease and that, in turn, could mean much greater numbers of patients who require kidney dialysis and even transplants,” said senior author Girish Nadkarni, MD, a nephrologist, in a statement from Mount Sinai.
Nephrologists will need to prepare for a significant uptick in patients with chronic kidney disease as a result of exposure to the SARS-CoV-2 virus that causes COVID-19, the researchers warned.
“These findings may help centers with resource planning and preparing for the increased load resulting from survivors of COVID-19–associated AKI who do not experience recovery of kidney function,” they added.
Analysis of patients from February to end of May 2020
“AKI among hospitalized patients with COVID-19 in the United States is not well described,” they noted in their article.
And so they analyzed data from five major hospitals in the Mount Sinai Health System between Feb. 27 and May 30 of this year, during which 3,993 patients were hospitalized within the system for COVID-19. The MSHS has a patient population of racially and ethnically diverse citizens from New York.
AKI was defined using Kidney Disease: Improving Global Outcomes (KDIGO) criteria. AKI occurred in 46% of the overall cohort of patients, 19% of whom required dialysis.
However, among those patients who required admission to the ICU, over three-quarters (76%) developed AKI and almost one-third of ICU patients required dialysis, the investigators said.
“The median time from hospital admission until AKI diagnoses was 1 day and the median time from AKI diagnosis to dialysis was 3 days,” they explain.
The proportion of patients with stages 1, 2, or 3 AKI among those admitted to hospital were 39%, 19%, and 42%, respectively. In patients requiring admission to ICU, 28% had stage 1 AKI, 17% had stage 2, and 56% had stage 3.
And among those who required dialysis for AKI, the median peak serum creatinine was 8.2 mg/dL, compared with 2.2 mg/dL for those who did not require dialysis.
Predictors of AKI: male sex, potassium levels, and preexisting CKD
Almost two thirds of patients (65%) had recovered from their kidney injury by the time they left hospital but 35% had acute kidney disease. Of this latter group, on follow-up, 36% had recovered from it, the investigators noted.
Conversely, of those patients who had recovered from AKI by hospital discharge, 14% went on to develop acute kidney disease at the time of follow-up.
And 30% of patients who had required dialysis at some point during their hospital care required dialysis again within 72 hours of being discharged, the investigators noted.
Predictors of severe AKI included male sex (adjusted odds ratio, 1.46), potassium levels on admission (aOR, 1.7), and preexisting chronic kidney disease (CKD) (aOR, 2.8).
Most compellingly, “in-hospital mortality in patients who experienced AKI was 50% [versus] 8% in patients without AKI (P < .001),” Dr. Nadkarni and colleagues reported.
Among those who required ICU care, 42% of patients with AKI died, compared with 7% of those in ICU who did not develop AKI, while in patients cared for outside of ICU, 62% with AKI died compared with only 13% of those who did not develop AKI.
And after adjusting for demographics, comorbidities, and laboratory values, the aOR for death was 11.4 times higher for ICU patients with AKI, compared with ICU patients without AKI, the authors emphasize.
In all patients who developed AKI, the aOR for mortality was 9.2, compared with patients who did not develop AKI, they added.
Perhaps predictably, the risk of death rose with increasing stage of AKI, and patients with stage 3 AKI who required dialysis were at highest risk of death, the authors observe.
Sheer number of AKI cases, need for dialysis unprecedented
“The sheer number of AKI cases and the overwhelming need for dialysis that we are seeing in the context of COVID-19 is unprecedented,” Dr. Nadkarni said.
“These findings bring clinical evidence to the hypothesis of lingering organ dysfunction among patients recovering from COVID-19 and serve as a reminder to hospitals around the country to be very strategic in the allocation of resources to care for patients who experience AKI,” he cautioned.
“We are grappling with a great deal of uncertainty as to how the virus will impact the kidneys in the long haul,” Dr. Nadkarni added. “We may be facing an epidemic of post–COVID-19 kidney disease, and that, in turn, could mean much greater numbers of patients who require kidney dialysis and even transplants.”
Dr. Nadkarni reported serving as a consultant and advisory board member for RenalytixAI and owns equity in the company.
This article first appeared on Medscape.com.
Could these old drugs help fight COVID-19 and save lives?
Early in the COVID-19 pandemic, entrepreneur and philanthropist Steve Kirsch realized that until we have a vaccine against SARS-CoV-2, we would be at the mercy of this virus. He realized that the fastest and most effective way to reduce COVID-19 fatalities would be to leverage existing drugs to treat patients at the onset of infection — before they become sick.
Medscape spoke with CETF’s chief medical advisor, Lisa Danzig, MD, about the organization’s aim to fund promising research on repurposed drugs to treat COVID-19.
What is CETF trying to do?
Two things: save lives, and get control of this pandemic.
We are facing perhaps the greatest crisis of our lifetime. Doctors who have taken care of patients with COVID are really frustrated about not having anything to offer; they just watch patients die. We want to change that. CETF was founded to find treatments that, when given early, could improve outcomes and avoid catastrophic complications in patients suffering from COVID-19. That means reducing hospitalizations, which can reduce mortality, but it also can mean reducing viral load, and that can have a profound impact on transmission within communities. We are a funding organization — a Band-Aid. We shouldn’t exist, but we do, aiming to close gaps until a coordinated response can get set up.
Tell us about drug repurposing and why you think existing drugs might have a role in mitigating COVID-19 or slowing its transmission.
This disease has two components — the viral infection, and the immunopathology. So the two promising categories of drugs are classical antivirals (or repurposed drugs with antiviral activity), and the immunomodulators. We are mechanism-agnostic. It doesn’t matter what kind of drug it is if it keeps people out of the hospital and prevents chronic morbidity and mortality.
Repurposed drugs are sort of the low-hanging fruit of clinical drugs. The QBI Coronavirus Research Group identified 69 compounds that have theoretical activity against SARS-CoV-2, 29 of which are already FDA-approved drugs. We thought, why don’t we start testing them?
Some people might call this a long shot. Does drug repurposing really work?
Drugmakers don’t test their drugs on every disease they might be effective for. Drug repurposing can work, but if we don’t look, we definitely won’t find anything. The classic repurposed drug is Viagra, a failed hypertension drug. When the studies ended because it didn’t work, the drug company asked patients to send back the unused drugs. The women all returned the drugs, but the men didn’t. And the rest is history.
There’s a long list of potential drugs that can be repurposed, but few are being tested. The famous poster child of a repurposed drug — hydroxychloroquine — has been the subject of more than 250 clinical trials, but the others weren’t getting much attention.
The beauty of a repurposed drug is that if you can get funding and start enrolling patients, you could potentially find out fairly quickly, as early as a few months, if that drug has an antiviral effect or not. These data would help prioritize drugs to be tested in larger confirmatory studies.
Your focus is on early treatment. What’s the rationale for that?
We are focusing on early treatment because it has been overlooked. The attention has been on vaccines and therapeutics for hospitalized patients. But if you are spending $20 billion on potential vaccines and billions more on diagnostics, we need to give proportional resources toward drugs that might actually work, when given early, in preventing severe disease and death.
Early treatment, if successful, would allow us to avoid the severe complications that we are seeing now. If we can find an early treatment with an existing drug, it would be the fastest, most clinically- and cost-effective way to mitigate the impact of COVID-19 and get us on the road to recovery.
How do you get from a potential repurposed drug for COVID-19 to having a therapeutic agent that will save lives?
Most of the studies we are funding are smaller outpatient studies with virologic endpoints. We are looking for a signal that the drug has antiviral activity. We want to know whether a drug works before we spend the money on questions that take a much larger sample size to answer, for example, a big postexposure prophylaxis study. We’d like to see a meaningful signal in proof-of-concept studies, so we can look at a small group of patients with positive tests and see whether their viral load dropped by more than half if they got the drug compared with those who took the placebo. If the drug had an impact on the viral load and shortened the period of infectivity and was safe, these findings would provide justification to spend a lot of money on a large clinical trial. That would probably encourage the NIH and ACTIV [Accelerating COVID-19 Therapeutic Interventions and Vaccines] collaboration to prioritize the drug for one of their big platform trials. That›s what we are aiming for.
CETF isn’t a drug developer — we are a funder for a good proposal to study a repurposed drug. We want to help move the dial — can we get an early yes or an early no? In drug development, we say, “fail fast and fail early.” It’s a numbers game. Only 10% of early candidates will become approved drugs. The value is in the data, whether they are positive or negative — it doesn’t matter. If the study is a definitive “no,” that is just as helpful as a definitive “yes.” Of course, we all want the definitive “yes,” but there are so many things to look at, the “no’s” will help us redirect resources toward what may really help.
You first announced these funding opportunities in April. How is it going so far?
As soon as the website went up, we got 40 applications. Our scientific advisory board, which has expertise from medicinal chemistry and coronavirology to translational and clinical trial expertise, reviewed the applications and prioritized 11 fundable proposals. We are using milestone-based funding; in other words, funding those who are ready to go.
Which drugs are being tested in the funded studies?
One of the earliest grants we supported was Dr David Boulaware’s randomized controlled trial of hydroxychloroquine (NCT 04308668) in 821 asymptomatic patients within 4 days after a high-risk or moderate-risk exposure. That trial did not show any benefit of hydroxychloroquine as postexposure prophylaxis against COVID-19. This trial was important for another reason. It proved the feasibility of a no-contact trial design in the setting of COVID-19, and participants enrolled themselves through a secure Internet-based survey using the Research Electronic Data Capture (REDCap) system.
Camostat, a transmembrane serine protease (TMPRSS2) inhibitor licensed for use in Japan to treat pancreatitis and esophagitis, combined with the antiandrogen bicalutamide, is being explored for early COVID-19 treatment. TMPRSS2 primes the SARS-CoV-2 spike protein to bind to the ACE2 receptor and gain entry to the cell, and has been shown to have antiviral activity. CETF has provided funding support to ongoing trials of Camostat at Yale University and Aarhus University in Denmark.
Another outpatient trial for fluvoxamine, a drug approved in the United States and routinely prescribed for depression, was also partially funded by a CETF grant to Washington University in St. Louis. Fluvoxamine is a serotonin regulator but also activates the sigma-1 receptor, which reduces the body’s immune response to prevent an overactive immune response or cytokine storm, a major cause of clinical deterioration, serious organ damage, and even death from COVID. This trial was recently completed, and the results have been submitted for publication.
Other promising drugs include niclosamide, doxazosin, favipiravir, leronlimab, interferon beta, interferon lambda, and other monoclonal antibodies. New compounds considered to have potential against COVID include a flu drug (MK-4482/EIDD-2801) and GS-441524, a metabolite of the antiviral drug, remdesivir.
Why not just put all of our resources into vaccine development?
We absolutely need a vaccine to control the outbreak and stop the pandemic. However, it’s a long road to finding an effective vaccine, and in the meantime, we need tools to keep people alive. If we can find an antiviral drug that acts early, we can reduce transmission and contribute to outbreak control. All these tools help us get back to normal while we are waiting for a vaccine. The vaccine is only good if we can give it to every susceptible person in the world — which will take longer than 3 years. And there are no guarantees. Remember, we are still waiting for an HIV vaccine.
You are calling on Americans to help. What do you want them to do?
Everyone must participate in the behavioral changes designed to control the outbreak — physical distancing, face-covering, and paying attention to case counts in local areas to enable them to take appropriate precautions. I know people are bored of that message, but we are going to repeat it until we have a vaccine or herd immunity.
This organism is ripping like wildfire through our unimmunized population. Personal behaviors might slow it down, but finding a drug that can be given to people after they’ve been exposed and test positive will have a meaningful impact on helping us get back to normal.
There’s a great spirit of volunteerism — people are constantly asking how they can help. Through us at CETF, we offer three ways that people can help. They can participate as subjects in clinical trials, many of which are ongoing, including clinical trials, surveillance studies, and follow-up studies. They can donate to our fund and help support the research needed to find an effective early treatment. We have a link on our website, TreatEarly.org. And finally, researchers can apply for funding. We think everybody can help in one of these ways by participating in trials, donating, or applying for funding. It’s an all-hands-on-deck moment for our country.
Danzig is the chief medical advisor of the COVID-19 Early Treatment Fund. She has spent more than 20 years in the pharmaceutical industry developing vaccines, diagnostics, and drugs and is currently advising companies and investors.
This article first appeared on Medscape.com.
Early in the COVID-19 pandemic, entrepreneur and philanthropist Steve Kirsch realized that until we have a vaccine against SARS-CoV-2, we would be at the mercy of this virus. He realized that the fastest and most effective way to reduce COVID-19 fatalities would be to leverage existing drugs to treat patients at the onset of infection — before they become sick.
Medscape spoke with CETF’s chief medical advisor, Lisa Danzig, MD, about the organization’s aim to fund promising research on repurposed drugs to treat COVID-19.
What is CETF trying to do?
Two things: save lives, and get control of this pandemic.
We are facing perhaps the greatest crisis of our lifetime. Doctors who have taken care of patients with COVID are really frustrated about not having anything to offer; they just watch patients die. We want to change that. CETF was founded to find treatments that, when given early, could improve outcomes and avoid catastrophic complications in patients suffering from COVID-19. That means reducing hospitalizations, which can reduce mortality, but it also can mean reducing viral load, and that can have a profound impact on transmission within communities. We are a funding organization — a Band-Aid. We shouldn’t exist, but we do, aiming to close gaps until a coordinated response can get set up.
Tell us about drug repurposing and why you think existing drugs might have a role in mitigating COVID-19 or slowing its transmission.
This disease has two components — the viral infection, and the immunopathology. So the two promising categories of drugs are classical antivirals (or repurposed drugs with antiviral activity), and the immunomodulators. We are mechanism-agnostic. It doesn’t matter what kind of drug it is if it keeps people out of the hospital and prevents chronic morbidity and mortality.
Repurposed drugs are sort of the low-hanging fruit of clinical drugs. The QBI Coronavirus Research Group identified 69 compounds that have theoretical activity against SARS-CoV-2, 29 of which are already FDA-approved drugs. We thought, why don’t we start testing them?
Some people might call this a long shot. Does drug repurposing really work?
Drugmakers don’t test their drugs on every disease they might be effective for. Drug repurposing can work, but if we don’t look, we definitely won’t find anything. The classic repurposed drug is Viagra, a failed hypertension drug. When the studies ended because it didn’t work, the drug company asked patients to send back the unused drugs. The women all returned the drugs, but the men didn’t. And the rest is history.
There’s a long list of potential drugs that can be repurposed, but few are being tested. The famous poster child of a repurposed drug — hydroxychloroquine — has been the subject of more than 250 clinical trials, but the others weren’t getting much attention.
The beauty of a repurposed drug is that if you can get funding and start enrolling patients, you could potentially find out fairly quickly, as early as a few months, if that drug has an antiviral effect or not. These data would help prioritize drugs to be tested in larger confirmatory studies.
Your focus is on early treatment. What’s the rationale for that?
We are focusing on early treatment because it has been overlooked. The attention has been on vaccines and therapeutics for hospitalized patients. But if you are spending $20 billion on potential vaccines and billions more on diagnostics, we need to give proportional resources toward drugs that might actually work, when given early, in preventing severe disease and death.
Early treatment, if successful, would allow us to avoid the severe complications that we are seeing now. If we can find an early treatment with an existing drug, it would be the fastest, most clinically- and cost-effective way to mitigate the impact of COVID-19 and get us on the road to recovery.
How do you get from a potential repurposed drug for COVID-19 to having a therapeutic agent that will save lives?
Most of the studies we are funding are smaller outpatient studies with virologic endpoints. We are looking for a signal that the drug has antiviral activity. We want to know whether a drug works before we spend the money on questions that take a much larger sample size to answer, for example, a big postexposure prophylaxis study. We’d like to see a meaningful signal in proof-of-concept studies, so we can look at a small group of patients with positive tests and see whether their viral load dropped by more than half if they got the drug compared with those who took the placebo. If the drug had an impact on the viral load and shortened the period of infectivity and was safe, these findings would provide justification to spend a lot of money on a large clinical trial. That would probably encourage the NIH and ACTIV [Accelerating COVID-19 Therapeutic Interventions and Vaccines] collaboration to prioritize the drug for one of their big platform trials. That›s what we are aiming for.
CETF isn’t a drug developer — we are a funder for a good proposal to study a repurposed drug. We want to help move the dial — can we get an early yes or an early no? In drug development, we say, “fail fast and fail early.” It’s a numbers game. Only 10% of early candidates will become approved drugs. The value is in the data, whether they are positive or negative — it doesn’t matter. If the study is a definitive “no,” that is just as helpful as a definitive “yes.” Of course, we all want the definitive “yes,” but there are so many things to look at, the “no’s” will help us redirect resources toward what may really help.
You first announced these funding opportunities in April. How is it going so far?
As soon as the website went up, we got 40 applications. Our scientific advisory board, which has expertise from medicinal chemistry and coronavirology to translational and clinical trial expertise, reviewed the applications and prioritized 11 fundable proposals. We are using milestone-based funding; in other words, funding those who are ready to go.
Which drugs are being tested in the funded studies?
One of the earliest grants we supported was Dr David Boulaware’s randomized controlled trial of hydroxychloroquine (NCT 04308668) in 821 asymptomatic patients within 4 days after a high-risk or moderate-risk exposure. That trial did not show any benefit of hydroxychloroquine as postexposure prophylaxis against COVID-19. This trial was important for another reason. It proved the feasibility of a no-contact trial design in the setting of COVID-19, and participants enrolled themselves through a secure Internet-based survey using the Research Electronic Data Capture (REDCap) system.
Camostat, a transmembrane serine protease (TMPRSS2) inhibitor licensed for use in Japan to treat pancreatitis and esophagitis, combined with the antiandrogen bicalutamide, is being explored for early COVID-19 treatment. TMPRSS2 primes the SARS-CoV-2 spike protein to bind to the ACE2 receptor and gain entry to the cell, and has been shown to have antiviral activity. CETF has provided funding support to ongoing trials of Camostat at Yale University and Aarhus University in Denmark.
Another outpatient trial for fluvoxamine, a drug approved in the United States and routinely prescribed for depression, was also partially funded by a CETF grant to Washington University in St. Louis. Fluvoxamine is a serotonin regulator but also activates the sigma-1 receptor, which reduces the body’s immune response to prevent an overactive immune response or cytokine storm, a major cause of clinical deterioration, serious organ damage, and even death from COVID. This trial was recently completed, and the results have been submitted for publication.
Other promising drugs include niclosamide, doxazosin, favipiravir, leronlimab, interferon beta, interferon lambda, and other monoclonal antibodies. New compounds considered to have potential against COVID include a flu drug (MK-4482/EIDD-2801) and GS-441524, a metabolite of the antiviral drug, remdesivir.
Why not just put all of our resources into vaccine development?
We absolutely need a vaccine to control the outbreak and stop the pandemic. However, it’s a long road to finding an effective vaccine, and in the meantime, we need tools to keep people alive. If we can find an antiviral drug that acts early, we can reduce transmission and contribute to outbreak control. All these tools help us get back to normal while we are waiting for a vaccine. The vaccine is only good if we can give it to every susceptible person in the world — which will take longer than 3 years. And there are no guarantees. Remember, we are still waiting for an HIV vaccine.
You are calling on Americans to help. What do you want them to do?
Everyone must participate in the behavioral changes designed to control the outbreak — physical distancing, face-covering, and paying attention to case counts in local areas to enable them to take appropriate precautions. I know people are bored of that message, but we are going to repeat it until we have a vaccine or herd immunity.
This organism is ripping like wildfire through our unimmunized population. Personal behaviors might slow it down, but finding a drug that can be given to people after they’ve been exposed and test positive will have a meaningful impact on helping us get back to normal.
There’s a great spirit of volunteerism — people are constantly asking how they can help. Through us at CETF, we offer three ways that people can help. They can participate as subjects in clinical trials, many of which are ongoing, including clinical trials, surveillance studies, and follow-up studies. They can donate to our fund and help support the research needed to find an effective early treatment. We have a link on our website, TreatEarly.org. And finally, researchers can apply for funding. We think everybody can help in one of these ways by participating in trials, donating, or applying for funding. It’s an all-hands-on-deck moment for our country.
Danzig is the chief medical advisor of the COVID-19 Early Treatment Fund. She has spent more than 20 years in the pharmaceutical industry developing vaccines, diagnostics, and drugs and is currently advising companies and investors.
This article first appeared on Medscape.com.
Early in the COVID-19 pandemic, entrepreneur and philanthropist Steve Kirsch realized that until we have a vaccine against SARS-CoV-2, we would be at the mercy of this virus. He realized that the fastest and most effective way to reduce COVID-19 fatalities would be to leverage existing drugs to treat patients at the onset of infection — before they become sick.
Medscape spoke with CETF’s chief medical advisor, Lisa Danzig, MD, about the organization’s aim to fund promising research on repurposed drugs to treat COVID-19.
What is CETF trying to do?
Two things: save lives, and get control of this pandemic.
We are facing perhaps the greatest crisis of our lifetime. Doctors who have taken care of patients with COVID are really frustrated about not having anything to offer; they just watch patients die. We want to change that. CETF was founded to find treatments that, when given early, could improve outcomes and avoid catastrophic complications in patients suffering from COVID-19. That means reducing hospitalizations, which can reduce mortality, but it also can mean reducing viral load, and that can have a profound impact on transmission within communities. We are a funding organization — a Band-Aid. We shouldn’t exist, but we do, aiming to close gaps until a coordinated response can get set up.
Tell us about drug repurposing and why you think existing drugs might have a role in mitigating COVID-19 or slowing its transmission.
This disease has two components — the viral infection, and the immunopathology. So the two promising categories of drugs are classical antivirals (or repurposed drugs with antiviral activity), and the immunomodulators. We are mechanism-agnostic. It doesn’t matter what kind of drug it is if it keeps people out of the hospital and prevents chronic morbidity and mortality.
Repurposed drugs are sort of the low-hanging fruit of clinical drugs. The QBI Coronavirus Research Group identified 69 compounds that have theoretical activity against SARS-CoV-2, 29 of which are already FDA-approved drugs. We thought, why don’t we start testing them?
Some people might call this a long shot. Does drug repurposing really work?
Drugmakers don’t test their drugs on every disease they might be effective for. Drug repurposing can work, but if we don’t look, we definitely won’t find anything. The classic repurposed drug is Viagra, a failed hypertension drug. When the studies ended because it didn’t work, the drug company asked patients to send back the unused drugs. The women all returned the drugs, but the men didn’t. And the rest is history.
There’s a long list of potential drugs that can be repurposed, but few are being tested. The famous poster child of a repurposed drug — hydroxychloroquine — has been the subject of more than 250 clinical trials, but the others weren’t getting much attention.
The beauty of a repurposed drug is that if you can get funding and start enrolling patients, you could potentially find out fairly quickly, as early as a few months, if that drug has an antiviral effect or not. These data would help prioritize drugs to be tested in larger confirmatory studies.
Your focus is on early treatment. What’s the rationale for that?
We are focusing on early treatment because it has been overlooked. The attention has been on vaccines and therapeutics for hospitalized patients. But if you are spending $20 billion on potential vaccines and billions more on diagnostics, we need to give proportional resources toward drugs that might actually work, when given early, in preventing severe disease and death.
Early treatment, if successful, would allow us to avoid the severe complications that we are seeing now. If we can find an early treatment with an existing drug, it would be the fastest, most clinically- and cost-effective way to mitigate the impact of COVID-19 and get us on the road to recovery.
How do you get from a potential repurposed drug for COVID-19 to having a therapeutic agent that will save lives?
Most of the studies we are funding are smaller outpatient studies with virologic endpoints. We are looking for a signal that the drug has antiviral activity. We want to know whether a drug works before we spend the money on questions that take a much larger sample size to answer, for example, a big postexposure prophylaxis study. We’d like to see a meaningful signal in proof-of-concept studies, so we can look at a small group of patients with positive tests and see whether their viral load dropped by more than half if they got the drug compared with those who took the placebo. If the drug had an impact on the viral load and shortened the period of infectivity and was safe, these findings would provide justification to spend a lot of money on a large clinical trial. That would probably encourage the NIH and ACTIV [Accelerating COVID-19 Therapeutic Interventions and Vaccines] collaboration to prioritize the drug for one of their big platform trials. That›s what we are aiming for.
CETF isn’t a drug developer — we are a funder for a good proposal to study a repurposed drug. We want to help move the dial — can we get an early yes or an early no? In drug development, we say, “fail fast and fail early.” It’s a numbers game. Only 10% of early candidates will become approved drugs. The value is in the data, whether they are positive or negative — it doesn’t matter. If the study is a definitive “no,” that is just as helpful as a definitive “yes.” Of course, we all want the definitive “yes,” but there are so many things to look at, the “no’s” will help us redirect resources toward what may really help.
You first announced these funding opportunities in April. How is it going so far?
As soon as the website went up, we got 40 applications. Our scientific advisory board, which has expertise from medicinal chemistry and coronavirology to translational and clinical trial expertise, reviewed the applications and prioritized 11 fundable proposals. We are using milestone-based funding; in other words, funding those who are ready to go.
Which drugs are being tested in the funded studies?
One of the earliest grants we supported was Dr David Boulaware’s randomized controlled trial of hydroxychloroquine (NCT 04308668) in 821 asymptomatic patients within 4 days after a high-risk or moderate-risk exposure. That trial did not show any benefit of hydroxychloroquine as postexposure prophylaxis against COVID-19. This trial was important for another reason. It proved the feasibility of a no-contact trial design in the setting of COVID-19, and participants enrolled themselves through a secure Internet-based survey using the Research Electronic Data Capture (REDCap) system.
Camostat, a transmembrane serine protease (TMPRSS2) inhibitor licensed for use in Japan to treat pancreatitis and esophagitis, combined with the antiandrogen bicalutamide, is being explored for early COVID-19 treatment. TMPRSS2 primes the SARS-CoV-2 spike protein to bind to the ACE2 receptor and gain entry to the cell, and has been shown to have antiviral activity. CETF has provided funding support to ongoing trials of Camostat at Yale University and Aarhus University in Denmark.
Another outpatient trial for fluvoxamine, a drug approved in the United States and routinely prescribed for depression, was also partially funded by a CETF grant to Washington University in St. Louis. Fluvoxamine is a serotonin regulator but also activates the sigma-1 receptor, which reduces the body’s immune response to prevent an overactive immune response or cytokine storm, a major cause of clinical deterioration, serious organ damage, and even death from COVID. This trial was recently completed, and the results have been submitted for publication.
Other promising drugs include niclosamide, doxazosin, favipiravir, leronlimab, interferon beta, interferon lambda, and other monoclonal antibodies. New compounds considered to have potential against COVID include a flu drug (MK-4482/EIDD-2801) and GS-441524, a metabolite of the antiviral drug, remdesivir.
Why not just put all of our resources into vaccine development?
We absolutely need a vaccine to control the outbreak and stop the pandemic. However, it’s a long road to finding an effective vaccine, and in the meantime, we need tools to keep people alive. If we can find an antiviral drug that acts early, we can reduce transmission and contribute to outbreak control. All these tools help us get back to normal while we are waiting for a vaccine. The vaccine is only good if we can give it to every susceptible person in the world — which will take longer than 3 years. And there are no guarantees. Remember, we are still waiting for an HIV vaccine.
You are calling on Americans to help. What do you want them to do?
Everyone must participate in the behavioral changes designed to control the outbreak — physical distancing, face-covering, and paying attention to case counts in local areas to enable them to take appropriate precautions. I know people are bored of that message, but we are going to repeat it until we have a vaccine or herd immunity.
This organism is ripping like wildfire through our unimmunized population. Personal behaviors might slow it down, but finding a drug that can be given to people after they’ve been exposed and test positive will have a meaningful impact on helping us get back to normal.
There’s a great spirit of volunteerism — people are constantly asking how they can help. Through us at CETF, we offer three ways that people can help. They can participate as subjects in clinical trials, many of which are ongoing, including clinical trials, surveillance studies, and follow-up studies. They can donate to our fund and help support the research needed to find an effective early treatment. We have a link on our website, TreatEarly.org. And finally, researchers can apply for funding. We think everybody can help in one of these ways by participating in trials, donating, or applying for funding. It’s an all-hands-on-deck moment for our country.
Danzig is the chief medical advisor of the COVID-19 Early Treatment Fund. She has spent more than 20 years in the pharmaceutical industry developing vaccines, diagnostics, and drugs and is currently advising companies and investors.
This article first appeared on Medscape.com.
MIS-C cardiac evaluation requires more than EF
Patients with multisystem inflammatory syndrome caused by COVID-19 typically seem to avoid coronary artery dilation early on, but they may be prone to cardiac injury and dysfunction longer term that requires a more discerning diagnostic approach to sort out.
The findings were revealed in a study of 28 children with COVID-19–related multisystem inflammatory syndrome (MIS-C) at Children’s Hospital of Philadelphia. The study reported that cardiac injury and dysfunction are common in these patients – even those who have preserved ejection fraction – and that diastolic dysfunction is persistent. For comparison, the study also included 20 healthy controls and 20 patients with classic Kawasaki disease (KD).
The study analyzed echocardiography findings in the patients, reporting left ventricular (LV) systolic and diastolic function were worse than in classic Kawasaki disease (KD), which MIS-C mimics. Lead author Daisuke Matsubara, MD, PhD, and colleagues reported that four markers – LV global longitudinal strain, LV circumferential strain rate, right ventricular strain, and left atrial strain – were the strongest predictors of myocardial injury in these patients. After the acute phase, systolic function tended to recover, but diastolic dysfunction persisted.
‘Strain’ measurement boosts accuracy
While echocardiography has been reported to be valuable in evaluating coronary artery function in MIS-C patients, Dr. Matsubara of the division of cardiology at CHOP, said in an interview that study is the first to use the newer echocardiography indexes, known as “strain,” to assess heart function.
“Strain is a more sensitive tool than more conventional indexes and can detect subtle decrease in heart function, even when ejection fraction is preserved,” he said. “Numerous publications have reached conclusions that strain improves the prognostic and diagnostic accuracy of echocardiography in a wide variety of cardiac pathologies causing LV dysfunction.”
Dr. Matsubara noted that the coronary arteries were mostly unaffected in the acute stage of MIS-C, as only one patient in their MIS-C cohort had coronary artery involvement, which normalized during early follow-up. “On the other hand, 20% of our classic KD patients had coronary abnormalities, including two with aneurysms.”
By using positive troponin I or elevated brain natriuretic peptide (BNP) to assess cardiac injury, they found a “high” (60%) incidence of myocardial injury in their MIS-C cohort. During early follow-up, most of the MIS-C patients showed normalization of systolic function, although diastolic dysfunction persisted.
When compared with the classic KD group, MIS-C patients had higher rates of mitral regurgitation (46% vs. 15%, P = .06), more pericardial effusion (32% vs. 15%, P = 0.46), and more pleural effusion (39% vs. 0%, P = .004). MIS-C patients with suspected myocardial injury show these findings more frequently than those with actual myocardial injury.
Compared with the healthy controls, the MIS-C patients showed both LV systolic and diastolic dysfunction as well as significantly lower left atrium (LA) strain and peak right ventricle (RV) free-wall longitudinal strain.
“In addition to the left ventricle, two other chambers of the heart, the LA and the RV that are often labeled as the ‘forgotten chambers’ of the heart, were also affected by MIS-C,” Dr. Matsubara said. “Both LA and RV strains were markedly reduced in MIS-C patients, compared to normal and KD patients.”
The study also indicates that elevated troponin I levels may not be as dire in children as they are in adults. Dr. Matsubara cited a study of more than 2,700 adult COVID-19 patients that found that even mild increases in troponin I level were associated with increased death during hospitalization (J Am Coll Cardiol. 2020;76:533-46).
However, most of the patients in the CHOP study, even those with elevated troponin I levels, recovered systolic function quickly. “We speculate that the elevation in cardiac troponins may have less dire implications in children, likely due to a more transient type of cardiac injury and less comorbidities in children,” he said. “Clearly further studies are needed before a definitive statement can be made.”
Dr. Matsubara added that recovered COVID-19 patients may be able to participate in sports as some schools reopen. “We are not saying restrict sport participation, but we are merely urging caution.”
Comprehensive LV evaluation needed
The findings reinforce that myocardial involvement is more frequent and sometimes more severe in MIS-C than previously thought, said Kevin G. Friedman, MD, a pediatrician at Harvard Medical School, Boston, and an attending physician in the department of cardiology at Boston Children’s Hospital. “We are underestimating it by using just traditional measures like ejection fraction. It requires a comprehensive evaluation of left ventricular function; it really affects all aspects of the ventricle, both the systolic function and the diastolic function.”
This study supports that MIS-C patients should have a more detailed analysis than EF on echocardiography, including strain imaging. “Probably these patients should all be followed at centers where they can evaluate a more detailed analysis of the LV and RV function,” he said. Patients with ongoing CA enlargement and LV dysfunction should have follow-up cardiac care indefinitely. Patients who have no cardiac symptoms during the acute phase probably don’t need long-term follow-up.
“We’re just trying to learn more about this disease, and it’s certainly concerning that so many kids are having cardiac involvement,” Dr. Friedman said. “Fortunately they’re getting better; we’re just trying to find out what this means for the long term.”
Dr. Matsubara and Dr. Friedman have no relevant financial disclosures.
SOURCE: Matsubara D et al. J Am Coll Cardiol. 2020 Sep 2. doi: 10.1016/j.jacc.2020.08.056.
Patients with multisystem inflammatory syndrome caused by COVID-19 typically seem to avoid coronary artery dilation early on, but they may be prone to cardiac injury and dysfunction longer term that requires a more discerning diagnostic approach to sort out.
The findings were revealed in a study of 28 children with COVID-19–related multisystem inflammatory syndrome (MIS-C) at Children’s Hospital of Philadelphia. The study reported that cardiac injury and dysfunction are common in these patients – even those who have preserved ejection fraction – and that diastolic dysfunction is persistent. For comparison, the study also included 20 healthy controls and 20 patients with classic Kawasaki disease (KD).
The study analyzed echocardiography findings in the patients, reporting left ventricular (LV) systolic and diastolic function were worse than in classic Kawasaki disease (KD), which MIS-C mimics. Lead author Daisuke Matsubara, MD, PhD, and colleagues reported that four markers – LV global longitudinal strain, LV circumferential strain rate, right ventricular strain, and left atrial strain – were the strongest predictors of myocardial injury in these patients. After the acute phase, systolic function tended to recover, but diastolic dysfunction persisted.
‘Strain’ measurement boosts accuracy
While echocardiography has been reported to be valuable in evaluating coronary artery function in MIS-C patients, Dr. Matsubara of the division of cardiology at CHOP, said in an interview that study is the first to use the newer echocardiography indexes, known as “strain,” to assess heart function.
“Strain is a more sensitive tool than more conventional indexes and can detect subtle decrease in heart function, even when ejection fraction is preserved,” he said. “Numerous publications have reached conclusions that strain improves the prognostic and diagnostic accuracy of echocardiography in a wide variety of cardiac pathologies causing LV dysfunction.”
Dr. Matsubara noted that the coronary arteries were mostly unaffected in the acute stage of MIS-C, as only one patient in their MIS-C cohort had coronary artery involvement, which normalized during early follow-up. “On the other hand, 20% of our classic KD patients had coronary abnormalities, including two with aneurysms.”
By using positive troponin I or elevated brain natriuretic peptide (BNP) to assess cardiac injury, they found a “high” (60%) incidence of myocardial injury in their MIS-C cohort. During early follow-up, most of the MIS-C patients showed normalization of systolic function, although diastolic dysfunction persisted.
When compared with the classic KD group, MIS-C patients had higher rates of mitral regurgitation (46% vs. 15%, P = .06), more pericardial effusion (32% vs. 15%, P = 0.46), and more pleural effusion (39% vs. 0%, P = .004). MIS-C patients with suspected myocardial injury show these findings more frequently than those with actual myocardial injury.
Compared with the healthy controls, the MIS-C patients showed both LV systolic and diastolic dysfunction as well as significantly lower left atrium (LA) strain and peak right ventricle (RV) free-wall longitudinal strain.
“In addition to the left ventricle, two other chambers of the heart, the LA and the RV that are often labeled as the ‘forgotten chambers’ of the heart, were also affected by MIS-C,” Dr. Matsubara said. “Both LA and RV strains were markedly reduced in MIS-C patients, compared to normal and KD patients.”
The study also indicates that elevated troponin I levels may not be as dire in children as they are in adults. Dr. Matsubara cited a study of more than 2,700 adult COVID-19 patients that found that even mild increases in troponin I level were associated with increased death during hospitalization (J Am Coll Cardiol. 2020;76:533-46).
However, most of the patients in the CHOP study, even those with elevated troponin I levels, recovered systolic function quickly. “We speculate that the elevation in cardiac troponins may have less dire implications in children, likely due to a more transient type of cardiac injury and less comorbidities in children,” he said. “Clearly further studies are needed before a definitive statement can be made.”
Dr. Matsubara added that recovered COVID-19 patients may be able to participate in sports as some schools reopen. “We are not saying restrict sport participation, but we are merely urging caution.”
Comprehensive LV evaluation needed
The findings reinforce that myocardial involvement is more frequent and sometimes more severe in MIS-C than previously thought, said Kevin G. Friedman, MD, a pediatrician at Harvard Medical School, Boston, and an attending physician in the department of cardiology at Boston Children’s Hospital. “We are underestimating it by using just traditional measures like ejection fraction. It requires a comprehensive evaluation of left ventricular function; it really affects all aspects of the ventricle, both the systolic function and the diastolic function.”
This study supports that MIS-C patients should have a more detailed analysis than EF on echocardiography, including strain imaging. “Probably these patients should all be followed at centers where they can evaluate a more detailed analysis of the LV and RV function,” he said. Patients with ongoing CA enlargement and LV dysfunction should have follow-up cardiac care indefinitely. Patients who have no cardiac symptoms during the acute phase probably don’t need long-term follow-up.
“We’re just trying to learn more about this disease, and it’s certainly concerning that so many kids are having cardiac involvement,” Dr. Friedman said. “Fortunately they’re getting better; we’re just trying to find out what this means for the long term.”
Dr. Matsubara and Dr. Friedman have no relevant financial disclosures.
SOURCE: Matsubara D et al. J Am Coll Cardiol. 2020 Sep 2. doi: 10.1016/j.jacc.2020.08.056.
Patients with multisystem inflammatory syndrome caused by COVID-19 typically seem to avoid coronary artery dilation early on, but they may be prone to cardiac injury and dysfunction longer term that requires a more discerning diagnostic approach to sort out.
The findings were revealed in a study of 28 children with COVID-19–related multisystem inflammatory syndrome (MIS-C) at Children’s Hospital of Philadelphia. The study reported that cardiac injury and dysfunction are common in these patients – even those who have preserved ejection fraction – and that diastolic dysfunction is persistent. For comparison, the study also included 20 healthy controls and 20 patients with classic Kawasaki disease (KD).
The study analyzed echocardiography findings in the patients, reporting left ventricular (LV) systolic and diastolic function were worse than in classic Kawasaki disease (KD), which MIS-C mimics. Lead author Daisuke Matsubara, MD, PhD, and colleagues reported that four markers – LV global longitudinal strain, LV circumferential strain rate, right ventricular strain, and left atrial strain – were the strongest predictors of myocardial injury in these patients. After the acute phase, systolic function tended to recover, but diastolic dysfunction persisted.
‘Strain’ measurement boosts accuracy
While echocardiography has been reported to be valuable in evaluating coronary artery function in MIS-C patients, Dr. Matsubara of the division of cardiology at CHOP, said in an interview that study is the first to use the newer echocardiography indexes, known as “strain,” to assess heart function.
“Strain is a more sensitive tool than more conventional indexes and can detect subtle decrease in heart function, even when ejection fraction is preserved,” he said. “Numerous publications have reached conclusions that strain improves the prognostic and diagnostic accuracy of echocardiography in a wide variety of cardiac pathologies causing LV dysfunction.”
Dr. Matsubara noted that the coronary arteries were mostly unaffected in the acute stage of MIS-C, as only one patient in their MIS-C cohort had coronary artery involvement, which normalized during early follow-up. “On the other hand, 20% of our classic KD patients had coronary abnormalities, including two with aneurysms.”
By using positive troponin I or elevated brain natriuretic peptide (BNP) to assess cardiac injury, they found a “high” (60%) incidence of myocardial injury in their MIS-C cohort. During early follow-up, most of the MIS-C patients showed normalization of systolic function, although diastolic dysfunction persisted.
When compared with the classic KD group, MIS-C patients had higher rates of mitral regurgitation (46% vs. 15%, P = .06), more pericardial effusion (32% vs. 15%, P = 0.46), and more pleural effusion (39% vs. 0%, P = .004). MIS-C patients with suspected myocardial injury show these findings more frequently than those with actual myocardial injury.
Compared with the healthy controls, the MIS-C patients showed both LV systolic and diastolic dysfunction as well as significantly lower left atrium (LA) strain and peak right ventricle (RV) free-wall longitudinal strain.
“In addition to the left ventricle, two other chambers of the heart, the LA and the RV that are often labeled as the ‘forgotten chambers’ of the heart, were also affected by MIS-C,” Dr. Matsubara said. “Both LA and RV strains were markedly reduced in MIS-C patients, compared to normal and KD patients.”
The study also indicates that elevated troponin I levels may not be as dire in children as they are in adults. Dr. Matsubara cited a study of more than 2,700 adult COVID-19 patients that found that even mild increases in troponin I level were associated with increased death during hospitalization (J Am Coll Cardiol. 2020;76:533-46).
However, most of the patients in the CHOP study, even those with elevated troponin I levels, recovered systolic function quickly. “We speculate that the elevation in cardiac troponins may have less dire implications in children, likely due to a more transient type of cardiac injury and less comorbidities in children,” he said. “Clearly further studies are needed before a definitive statement can be made.”
Dr. Matsubara added that recovered COVID-19 patients may be able to participate in sports as some schools reopen. “We are not saying restrict sport participation, but we are merely urging caution.”
Comprehensive LV evaluation needed
The findings reinforce that myocardial involvement is more frequent and sometimes more severe in MIS-C than previously thought, said Kevin G. Friedman, MD, a pediatrician at Harvard Medical School, Boston, and an attending physician in the department of cardiology at Boston Children’s Hospital. “We are underestimating it by using just traditional measures like ejection fraction. It requires a comprehensive evaluation of left ventricular function; it really affects all aspects of the ventricle, both the systolic function and the diastolic function.”
This study supports that MIS-C patients should have a more detailed analysis than EF on echocardiography, including strain imaging. “Probably these patients should all be followed at centers where they can evaluate a more detailed analysis of the LV and RV function,” he said. Patients with ongoing CA enlargement and LV dysfunction should have follow-up cardiac care indefinitely. Patients who have no cardiac symptoms during the acute phase probably don’t need long-term follow-up.
“We’re just trying to learn more about this disease, and it’s certainly concerning that so many kids are having cardiac involvement,” Dr. Friedman said. “Fortunately they’re getting better; we’re just trying to find out what this means for the long term.”
Dr. Matsubara and Dr. Friedman have no relevant financial disclosures.
SOURCE: Matsubara D et al. J Am Coll Cardiol. 2020 Sep 2. doi: 10.1016/j.jacc.2020.08.056.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Unexpected results in new COVID-19 ‘cytokine storm’ data
The immune system overactivation known as a “cytokine storm” does not play a major role in more severe COVID-19 outcomes, according to unexpected findings in new research. The findings stand in direct contrast to many previous reports.
“We were indeed surprised by the results of our study,” senior study author Peter Pickkers, MD, PhD, said in an interview.
In a unique approach, Dr. Pickkers and colleagues compared cytokine levels in critically ill people with COVID-19 with those in patients with bacterial sepsis, trauma, and after cardiac arrest.
“For the first time, we measured the cytokines in different diseases using the same methods. Our results convincingly show that the circulating cytokine concentrations are not higher, but lower, compared to other diseases,” said Dr. Pickkers, who is affiliated with the department of intensive care medicine at Radboud University Medical Center in Nijmegen, the Netherlands.
The team’s research was published online on Sept. 3 in a letter in JAMA.
Cytokines lower than expected
Normally, cytokines trigger inflammation and promote healing after trauma, infection, or other conditions.
Although a cytokine storm remains ill defined, the authors noted, many researchers have implicated a hyperinflammatory response involving these small proteins in the pathophysiology of COVID-19.
The question remains, however, whether all cytokine storms strike people with different conditions the same way.
Dr. Pickkers, lead author Matthijs Kox, PhD, and colleagues studied 46 people with COVID-19 and acute respiratory distress syndrome (ARDS) who were admitted to the ICU at Radboud University Medical Center. All participants underwent mechanical ventilation and were treated between March 11 and April 27, 2020.
The investigators measured plasma levels of cytokines, including tumor necrosis factor (TNF), interleukin-6, and IL-8. They compared results in this group with those in 51 patients who experienced septic shock and ARDS, 15 patients with septic shock without ARDS, 30 people with out-of-hospital cardiac arrest, and 62 people who experienced multiple traumas. They used historical data for the non–COVID-19 cohorts.
Conditional findings
Compared with patients with septic shock and ARDS, the COVID-19 cohort had lower levels of TNF, IL-6, and IL-8. The differences were statistically significant for TNF (P < .01), as well as for IL-6 and IL-8 concentrations (for both, P < .001).
In addition, the COVID-19 group had significantly lower IL-6 and IL-8 concentrations compared with the patients who had septic shock without ARDS.
The researchers likewise found lower concentrations of IL-8 in patients with COVID-19, compared with the out-of-hospital cardiac arrest patients. IL-8 levels did not differ between the COVID-19 and trauma groups.
Furthermore, the researchers found no differences in IL-6 concentrations between patients with COVID-19 and those who experienced out-of-hospital cardiac arrest or trauma.
However, levels of TNF in people with COVID-19 were higher than in trauma patients.
The small sample sizes and single-center study design are limitations.
“The findings of this preliminary analysis suggest COVID-19 may not be characterized by cytokine storm,” the researchers noted. However, they added, “whether anticytokine therapies will benefit patients with COVID-19 remains to be determined.”
Going forward, Dr. Pickkers and colleagues are investigating the effectiveness of different treatments to lower cytokine levels. They are treating people with COVID-19, for example, with the IL-1 cytokine inhibitor anakinra and steroids.
They also plan to assess the long-term effects of COVID-19 on the immune system. “Following an infection, it is known that the immune system may be suppressed for a longer period of time, and we are determining to what extent this is also present in COVID-19 patients,” Dr. Pickkers said.
Enough to cause a storm?
The study “is quite interesting, and data in this paper are consistent with our data,” Tadamitsu Kishimoto, MD, PhD, of the department of immune regulation at the Immunology Frontier Research Center at Osaka (Japan) University, said in an interview.
His study, published online August 21 in PNAS, also revealed lower serum IL-6 levels among people with COVID-19, compared with patients with bacterial ARDS or sepsis.
Dr. Kishimoto drew a distinction, however: COVID-19 patients can develop severe respiratory failure, suggesting a distinct immune reaction, compared with patients with bacterial sepsis. SARS-CoV-2 directly infects and activates endothelial cells rather than macrophages, as occurs in sepsis.
For this reason, Dr. Kishimoto said, “SARS-CoV-2 infection causes critical illness and severe dysfunction in respiratory organs and induces a cytokine storm,” even in the setting of lower but still elevated serum IL-6 levels.
Dr. Pickkers and Dr. Kishimoto reported no relevant financial relationships.
This story first appeared on Medscape.com.
The immune system overactivation known as a “cytokine storm” does not play a major role in more severe COVID-19 outcomes, according to unexpected findings in new research. The findings stand in direct contrast to many previous reports.
“We were indeed surprised by the results of our study,” senior study author Peter Pickkers, MD, PhD, said in an interview.
In a unique approach, Dr. Pickkers and colleagues compared cytokine levels in critically ill people with COVID-19 with those in patients with bacterial sepsis, trauma, and after cardiac arrest.
“For the first time, we measured the cytokines in different diseases using the same methods. Our results convincingly show that the circulating cytokine concentrations are not higher, but lower, compared to other diseases,” said Dr. Pickkers, who is affiliated with the department of intensive care medicine at Radboud University Medical Center in Nijmegen, the Netherlands.
The team’s research was published online on Sept. 3 in a letter in JAMA.
Cytokines lower than expected
Normally, cytokines trigger inflammation and promote healing after trauma, infection, or other conditions.
Although a cytokine storm remains ill defined, the authors noted, many researchers have implicated a hyperinflammatory response involving these small proteins in the pathophysiology of COVID-19.
The question remains, however, whether all cytokine storms strike people with different conditions the same way.
Dr. Pickkers, lead author Matthijs Kox, PhD, and colleagues studied 46 people with COVID-19 and acute respiratory distress syndrome (ARDS) who were admitted to the ICU at Radboud University Medical Center. All participants underwent mechanical ventilation and were treated between March 11 and April 27, 2020.
The investigators measured plasma levels of cytokines, including tumor necrosis factor (TNF), interleukin-6, and IL-8. They compared results in this group with those in 51 patients who experienced septic shock and ARDS, 15 patients with septic shock without ARDS, 30 people with out-of-hospital cardiac arrest, and 62 people who experienced multiple traumas. They used historical data for the non–COVID-19 cohorts.
Conditional findings
Compared with patients with septic shock and ARDS, the COVID-19 cohort had lower levels of TNF, IL-6, and IL-8. The differences were statistically significant for TNF (P < .01), as well as for IL-6 and IL-8 concentrations (for both, P < .001).
In addition, the COVID-19 group had significantly lower IL-6 and IL-8 concentrations compared with the patients who had septic shock without ARDS.
The researchers likewise found lower concentrations of IL-8 in patients with COVID-19, compared with the out-of-hospital cardiac arrest patients. IL-8 levels did not differ between the COVID-19 and trauma groups.
Furthermore, the researchers found no differences in IL-6 concentrations between patients with COVID-19 and those who experienced out-of-hospital cardiac arrest or trauma.
However, levels of TNF in people with COVID-19 were higher than in trauma patients.
The small sample sizes and single-center study design are limitations.
“The findings of this preliminary analysis suggest COVID-19 may not be characterized by cytokine storm,” the researchers noted. However, they added, “whether anticytokine therapies will benefit patients with COVID-19 remains to be determined.”
Going forward, Dr. Pickkers and colleagues are investigating the effectiveness of different treatments to lower cytokine levels. They are treating people with COVID-19, for example, with the IL-1 cytokine inhibitor anakinra and steroids.
They also plan to assess the long-term effects of COVID-19 on the immune system. “Following an infection, it is known that the immune system may be suppressed for a longer period of time, and we are determining to what extent this is also present in COVID-19 patients,” Dr. Pickkers said.
Enough to cause a storm?
The study “is quite interesting, and data in this paper are consistent with our data,” Tadamitsu Kishimoto, MD, PhD, of the department of immune regulation at the Immunology Frontier Research Center at Osaka (Japan) University, said in an interview.
His study, published online August 21 in PNAS, also revealed lower serum IL-6 levels among people with COVID-19, compared with patients with bacterial ARDS or sepsis.
Dr. Kishimoto drew a distinction, however: COVID-19 patients can develop severe respiratory failure, suggesting a distinct immune reaction, compared with patients with bacterial sepsis. SARS-CoV-2 directly infects and activates endothelial cells rather than macrophages, as occurs in sepsis.
For this reason, Dr. Kishimoto said, “SARS-CoV-2 infection causes critical illness and severe dysfunction in respiratory organs and induces a cytokine storm,” even in the setting of lower but still elevated serum IL-6 levels.
Dr. Pickkers and Dr. Kishimoto reported no relevant financial relationships.
This story first appeared on Medscape.com.
The immune system overactivation known as a “cytokine storm” does not play a major role in more severe COVID-19 outcomes, according to unexpected findings in new research. The findings stand in direct contrast to many previous reports.
“We were indeed surprised by the results of our study,” senior study author Peter Pickkers, MD, PhD, said in an interview.
In a unique approach, Dr. Pickkers and colleagues compared cytokine levels in critically ill people with COVID-19 with those in patients with bacterial sepsis, trauma, and after cardiac arrest.
“For the first time, we measured the cytokines in different diseases using the same methods. Our results convincingly show that the circulating cytokine concentrations are not higher, but lower, compared to other diseases,” said Dr. Pickkers, who is affiliated with the department of intensive care medicine at Radboud University Medical Center in Nijmegen, the Netherlands.
The team’s research was published online on Sept. 3 in a letter in JAMA.
Cytokines lower than expected
Normally, cytokines trigger inflammation and promote healing after trauma, infection, or other conditions.
Although a cytokine storm remains ill defined, the authors noted, many researchers have implicated a hyperinflammatory response involving these small proteins in the pathophysiology of COVID-19.
The question remains, however, whether all cytokine storms strike people with different conditions the same way.
Dr. Pickkers, lead author Matthijs Kox, PhD, and colleagues studied 46 people with COVID-19 and acute respiratory distress syndrome (ARDS) who were admitted to the ICU at Radboud University Medical Center. All participants underwent mechanical ventilation and were treated between March 11 and April 27, 2020.
The investigators measured plasma levels of cytokines, including tumor necrosis factor (TNF), interleukin-6, and IL-8. They compared results in this group with those in 51 patients who experienced septic shock and ARDS, 15 patients with septic shock without ARDS, 30 people with out-of-hospital cardiac arrest, and 62 people who experienced multiple traumas. They used historical data for the non–COVID-19 cohorts.
Conditional findings
Compared with patients with septic shock and ARDS, the COVID-19 cohort had lower levels of TNF, IL-6, and IL-8. The differences were statistically significant for TNF (P < .01), as well as for IL-6 and IL-8 concentrations (for both, P < .001).
In addition, the COVID-19 group had significantly lower IL-6 and IL-8 concentrations compared with the patients who had septic shock without ARDS.
The researchers likewise found lower concentrations of IL-8 in patients with COVID-19, compared with the out-of-hospital cardiac arrest patients. IL-8 levels did not differ between the COVID-19 and trauma groups.
Furthermore, the researchers found no differences in IL-6 concentrations between patients with COVID-19 and those who experienced out-of-hospital cardiac arrest or trauma.
However, levels of TNF in people with COVID-19 were higher than in trauma patients.
The small sample sizes and single-center study design are limitations.
“The findings of this preliminary analysis suggest COVID-19 may not be characterized by cytokine storm,” the researchers noted. However, they added, “whether anticytokine therapies will benefit patients with COVID-19 remains to be determined.”
Going forward, Dr. Pickkers and colleagues are investigating the effectiveness of different treatments to lower cytokine levels. They are treating people with COVID-19, for example, with the IL-1 cytokine inhibitor anakinra and steroids.
They also plan to assess the long-term effects of COVID-19 on the immune system. “Following an infection, it is known that the immune system may be suppressed for a longer period of time, and we are determining to what extent this is also present in COVID-19 patients,” Dr. Pickkers said.
Enough to cause a storm?
The study “is quite interesting, and data in this paper are consistent with our data,” Tadamitsu Kishimoto, MD, PhD, of the department of immune regulation at the Immunology Frontier Research Center at Osaka (Japan) University, said in an interview.
His study, published online August 21 in PNAS, also revealed lower serum IL-6 levels among people with COVID-19, compared with patients with bacterial ARDS or sepsis.
Dr. Kishimoto drew a distinction, however: COVID-19 patients can develop severe respiratory failure, suggesting a distinct immune reaction, compared with patients with bacterial sepsis. SARS-CoV-2 directly infects and activates endothelial cells rather than macrophages, as occurs in sepsis.
For this reason, Dr. Kishimoto said, “SARS-CoV-2 infection causes critical illness and severe dysfunction in respiratory organs and induces a cytokine storm,” even in the setting of lower but still elevated serum IL-6 levels.
Dr. Pickkers and Dr. Kishimoto reported no relevant financial relationships.
This story first appeared on Medscape.com.
HM20 Virtual: Improved supervision of residents
HM20 Virtual session title
Call Me Maybe: Balancing Resident Autonomy with Sensible Supervision
Presenter
Daniel Steinberg, MD, SFHM, FACP
Session summary
In this session, Dr. Steinberg, professor of medicine and medical education, associate chair for education, and residency program director in the department of medicine at Icahn School of Medicine at Mount Sinai, New York, presented key factors, techniques, and approaches to supervising residents. He discussed the important balance of resident autonomy and supervision, especially since attendings need to focus on learner education along with patient care and safety.
Dr. Steinberg stated that resident supervision is driven by three factors: what residents need, what residents want, and what the supervisor can provide. Although data is mixed on whether supervision improves patient outcomes, supervision is essential for patient care and resident education. Dr. Steinberg showcased several relevant medical education studies relating to supervision and focused on a key question: Do you trust the resident?
The review of medical education literature discussed the meaning and development of trust, oral case presentations to determine trust, and the influence of supervisor experience. One study looked at the attendings’ remote access of EMR, which allows for remote supervision as a great way to determine trust of the resident. Another study showed that attendings want more communication than what residents provide and that the saying “Page me if you need me” does not encourage communication from residents as much as attendings would desire.
Key takeaways
- Resident supervision is driven by what residents need, what residents want, and what the supervisor can provide.
- Trust can be determined from direct supervision, oral presentations, and remote access of EMR, but it is also influenced by attending experience and style.
- To increase resident communication with the attending, do not say “Page me if you need me.” Instead, an attending should specifically state when a call to an attending is required.
Dr. Tantoco is an academic med-peds hospitalist practicing at Northwestern Memorial Hospital in Chicago and Ann & Robert H. Lurie Children’s Hospital of Chicago. She is an instructor of medicine (hospital medicine) and pediatrics at Northwestern University, also in Chicago.
HM20 Virtual session title
Call Me Maybe: Balancing Resident Autonomy with Sensible Supervision
Presenter
Daniel Steinberg, MD, SFHM, FACP
Session summary
In this session, Dr. Steinberg, professor of medicine and medical education, associate chair for education, and residency program director in the department of medicine at Icahn School of Medicine at Mount Sinai, New York, presented key factors, techniques, and approaches to supervising residents. He discussed the important balance of resident autonomy and supervision, especially since attendings need to focus on learner education along with patient care and safety.
Dr. Steinberg stated that resident supervision is driven by three factors: what residents need, what residents want, and what the supervisor can provide. Although data is mixed on whether supervision improves patient outcomes, supervision is essential for patient care and resident education. Dr. Steinberg showcased several relevant medical education studies relating to supervision and focused on a key question: Do you trust the resident?
The review of medical education literature discussed the meaning and development of trust, oral case presentations to determine trust, and the influence of supervisor experience. One study looked at the attendings’ remote access of EMR, which allows for remote supervision as a great way to determine trust of the resident. Another study showed that attendings want more communication than what residents provide and that the saying “Page me if you need me” does not encourage communication from residents as much as attendings would desire.
Key takeaways
- Resident supervision is driven by what residents need, what residents want, and what the supervisor can provide.
- Trust can be determined from direct supervision, oral presentations, and remote access of EMR, but it is also influenced by attending experience and style.
- To increase resident communication with the attending, do not say “Page me if you need me.” Instead, an attending should specifically state when a call to an attending is required.
Dr. Tantoco is an academic med-peds hospitalist practicing at Northwestern Memorial Hospital in Chicago and Ann & Robert H. Lurie Children’s Hospital of Chicago. She is an instructor of medicine (hospital medicine) and pediatrics at Northwestern University, also in Chicago.
HM20 Virtual session title
Call Me Maybe: Balancing Resident Autonomy with Sensible Supervision
Presenter
Daniel Steinberg, MD, SFHM, FACP
Session summary
In this session, Dr. Steinberg, professor of medicine and medical education, associate chair for education, and residency program director in the department of medicine at Icahn School of Medicine at Mount Sinai, New York, presented key factors, techniques, and approaches to supervising residents. He discussed the important balance of resident autonomy and supervision, especially since attendings need to focus on learner education along with patient care and safety.
Dr. Steinberg stated that resident supervision is driven by three factors: what residents need, what residents want, and what the supervisor can provide. Although data is mixed on whether supervision improves patient outcomes, supervision is essential for patient care and resident education. Dr. Steinberg showcased several relevant medical education studies relating to supervision and focused on a key question: Do you trust the resident?
The review of medical education literature discussed the meaning and development of trust, oral case presentations to determine trust, and the influence of supervisor experience. One study looked at the attendings’ remote access of EMR, which allows for remote supervision as a great way to determine trust of the resident. Another study showed that attendings want more communication than what residents provide and that the saying “Page me if you need me” does not encourage communication from residents as much as attendings would desire.
Key takeaways
- Resident supervision is driven by what residents need, what residents want, and what the supervisor can provide.
- Trust can be determined from direct supervision, oral presentations, and remote access of EMR, but it is also influenced by attending experience and style.
- To increase resident communication with the attending, do not say “Page me if you need me.” Instead, an attending should specifically state when a call to an attending is required.
Dr. Tantoco is an academic med-peds hospitalist practicing at Northwestern Memorial Hospital in Chicago and Ann & Robert H. Lurie Children’s Hospital of Chicago. She is an instructor of medicine (hospital medicine) and pediatrics at Northwestern University, also in Chicago.
More U.S. states cap insulin cost, but activists will ‘fight harder’
Twelve U.S. states have now passed laws aimed at making insulin more affordable – and more than 30 are considering such legislation – but they all have gaps that still put the cost of this basic and essential medication out of reach for many with diabetes.
The laws only apply to health insurance through state-regulated plans, and not to the majority of health plans that cover most Americans: Medicare, Medicaid, the Veterans Affairs health system, or self-funded employer-sponsored plans.
Overall, Hannah Crabtree, an activist who writes the blog Data for Insulin, estimates state laws that limit copays, deductibles, or other out-of-pocket costs for insulin cover an average of 27% of people with diabetes across the United States.
And while diabetes activists have applauded state actions, most want more help for the under- and uninsured.
“Our chapter will be fighting harder next legislative session for the uninsured,” said Mindie Hooley, the leader of the Utah #insulin4all chapter, which successfully lobbied legislators to pass a bill signed by the state’s governor on March 30.
“With so many losing their jobs because of the pandemic, there’s no better time than now to fight for these patients who don’t have insurance,” Ms. Hooley said in an interview.
The American Diabetes Association has also been lobbying for state caps as one of many avenues for making insulin more affordable, said Stephen Habbe, the ADA’s director for state government affairs.
One in four insulin users report rationing the medication, Mr. Habbe said.
The state laws “can really provide important relief in terms of affordability for their insulin costs, which we know can be critical in terms of preserving their life and helping to prevent complications that can potentially be disabling or even deadly,” he said in an interview.
Activists with T1 International, which created the #insulin4all campaign, are working nationwide to convince state legislators to back measures that limit out-of-pocket costs for insulin, or for other diabetes medications and supplies.
Colorado, Connecticut, Delaware, Illinois, Maine, New Hampshire, New Mexico, New York, Utah, Virginia, Washington, and West Virginia have enacted such limits, with caps ranging from $25 to $100.
Insulin makers unfazed, blame insurers, PBMs for high prices
The three insulin manufacturers in the United States – Eli Lilly, Novo Nordisk, and Sanofi– have not overtly fought against the laws, although in July, the Pharmaceutical Research and Manufacturers of America did sue to block a related Minnesota law that provides a free emergency supply of insulin.
And the nonprofit news organization FairWarning reported in August that a lobbyist from Eli Lilly had attempted to push a Tennessee legislator to keep the uninsured from being eligible for any out-of-pocket limits.
The insulin makers have also not lowered prices in response to the mounting number of state laws.
They see no need, said Tara O’Neill Hayes, director of human welfare policy at the American Action Forum, a center right–leaning Washington, D.C., think tank.
“You’re going to do what you can get away with,” Ms. O’Neill Hayes said in an interview. “To the extent that they can keep their prices high and people are still buying, they have limited incentives to lower those costs.”
The insulin market is dysfunctional, she added. “The increasing cost of insulin seems primarily to be the result of a lack of competition in the market and convoluted drug pricing and insurance practices,” Ms. O’Neill Hayes and colleagues wrote in a report in April on federal and state attempts to address insulin affordability.
Novo Nordisk, however, maintains that drugmakers are not solely to blame.
“Everyone in the health care system has a role to play in affordability,” said Ken Inchausti, Novo Nordisk’s senior director for corporate communications. State legislation “attempts to address a systemic issue in [U.S.] health care: How benefit design can make medicines unaffordable for many, especially for those in high-deductible health plans,” he said in an interview.
“Efforts to place copay caps on insurance plans covering insulin can certainly help lower out-of-pocket costs,” said Mr. Inchausti.
Sanofi spokesperson Jon Florio said the company supports actions that increase affordable access to insulin. However, “while we support capped copays, we feel this should not be limited to just one class of medicines,” he said. Mr. Florio also noted that Sanofi provides out-of-pocket caps to anyone with commercial insurance and that anyone without insurance can buy one or multiple Sanofi insulins for a fixed price of $99 per month, up to 10 boxes of pens and/or 10-mL vials.
And Sanofi will take part in the Centers for Medicare & Medicaid Services’ new insulin demonstration program. Starting in 2021, CMS will cap insulin copays at $35 for people in Part D plans that participate.
Eli Lilly spokesperson Brad Jacklin said the company “believes in the common goal of ensuring affordable access to insulin and other life-saving medicines because nobody should have to forgo or ration because of cost.”
Lilly supports efforts “that more directly affect patients’ cost-sharing based on their health care coverage,” he said. Insurers and pharmacy benefit managers (PBMs) should pass savings on to patients, Mr. Jacklin urged. Lilly caps some insulins at $35 for the uninsured or commercially insured. The company will also participate in the CMS program.
Meanwhile, a PhRMA-sponsored website www.letstalkaboutcost.org said that, because they do not share savings, insurers and PBMs are responsible for high insulin costs.
Manufacturer assistance programs for patients with diabetes and other chronic diseases, on the other hand, can save individuals $300-$500 a year, PhRMA said in August.
PBMs point back at insulin manufacturers
PBMs, however, point back at drug companies. “PBMs have been able to moderate insulin costs for most consumers with insurance,” said J.C. Scott, president and CEO of the Pharmaceutical Care Management Association, the PBM trade group, in a statement.
The rising cost of insulin is caused by a lack of competition and overuse of patent extensions, PCMA maintains.
Health insurers, which, in tandem with PBMs, give insulins formulary preference based on a discounted price, are most likely to feel the impact of laws limiting out-of-pocket costs.
If they have to make up the shortfall from a patient’s reduced payment for a prescription, they will likely raise premiums, said Ms. O’Neill Hayes.
And if patients pay the same price for insulin – regardless of who makes it – drugmakers won’t have much incentive to offer discounts or rebates for formulary placement, she said. Again, that would likely lead to higher premiums.
David Allen, a spokesperson for America’s Health Insurance Plans, said in an interview that AHIP believes lack of competition has driven up insulin prices.
“High prices for insulin correspond with high health insurance costs for insulin,” he said. When CMS starts requiring drugmakers to discount their insulins for Medicare that will allow “health plans to use those savings to reduce out-of-pocket [costs] for seniors.”
He did not respond to a question as to why health insurers were not already passing savings on to commercially insured patients, especially in states with out-of-pocket limits.
Mr. Allen did say that AHIP’s plans “stand ready to work with state policymakers to remove barriers to lower insulin prices for Americans.”
Utah savings hopefully saving lives already
In Utah, legislators tuned out the blame game, and instead were keen to listen to patients, who had many stories about how the high cost of insulin had hurt them, said Ms. Hooley.
She noted an estimated 50,000 Utahans rely on insulin to stay alive.
Ms. Hooley and her chapter convinced legislators to pass a bill that gives insurers the option to cap patient copays at $30 per month, or to put insulin on its lowest formulary tier and waive any patient deductible. That aspect of the law does not go into effect until January 2021, but insurers are already starting to move insulin to the lowest formulary tier.
That has helped some people immediately. One state resident said her most recent insulin prescription cost $7 – instead of the usual $200.
The uninsured are not left totally high and dry either. Starting June 1, anyone in the state could buy through a state bulk-purchasing program, which guaranteed a 60% discount.
Ms. Hooley said she’d recently heard about a patient who usually spent $300 per prescription but was able to buy insulin for $100 through the program.
“Although $100 is still too much, it is nice knowing the Utah Insulin Savings Program is saving lives,” Ms. Hooley concluded.
A version of this article originally appeared on Medscape.com.
Twelve U.S. states have now passed laws aimed at making insulin more affordable – and more than 30 are considering such legislation – but they all have gaps that still put the cost of this basic and essential medication out of reach for many with diabetes.
The laws only apply to health insurance through state-regulated plans, and not to the majority of health plans that cover most Americans: Medicare, Medicaid, the Veterans Affairs health system, or self-funded employer-sponsored plans.
Overall, Hannah Crabtree, an activist who writes the blog Data for Insulin, estimates state laws that limit copays, deductibles, or other out-of-pocket costs for insulin cover an average of 27% of people with diabetes across the United States.
And while diabetes activists have applauded state actions, most want more help for the under- and uninsured.
“Our chapter will be fighting harder next legislative session for the uninsured,” said Mindie Hooley, the leader of the Utah #insulin4all chapter, which successfully lobbied legislators to pass a bill signed by the state’s governor on March 30.
“With so many losing their jobs because of the pandemic, there’s no better time than now to fight for these patients who don’t have insurance,” Ms. Hooley said in an interview.
The American Diabetes Association has also been lobbying for state caps as one of many avenues for making insulin more affordable, said Stephen Habbe, the ADA’s director for state government affairs.
One in four insulin users report rationing the medication, Mr. Habbe said.
The state laws “can really provide important relief in terms of affordability for their insulin costs, which we know can be critical in terms of preserving their life and helping to prevent complications that can potentially be disabling or even deadly,” he said in an interview.
Activists with T1 International, which created the #insulin4all campaign, are working nationwide to convince state legislators to back measures that limit out-of-pocket costs for insulin, or for other diabetes medications and supplies.
Colorado, Connecticut, Delaware, Illinois, Maine, New Hampshire, New Mexico, New York, Utah, Virginia, Washington, and West Virginia have enacted such limits, with caps ranging from $25 to $100.
Insulin makers unfazed, blame insurers, PBMs for high prices
The three insulin manufacturers in the United States – Eli Lilly, Novo Nordisk, and Sanofi– have not overtly fought against the laws, although in July, the Pharmaceutical Research and Manufacturers of America did sue to block a related Minnesota law that provides a free emergency supply of insulin.
And the nonprofit news organization FairWarning reported in August that a lobbyist from Eli Lilly had attempted to push a Tennessee legislator to keep the uninsured from being eligible for any out-of-pocket limits.
The insulin makers have also not lowered prices in response to the mounting number of state laws.
They see no need, said Tara O’Neill Hayes, director of human welfare policy at the American Action Forum, a center right–leaning Washington, D.C., think tank.
“You’re going to do what you can get away with,” Ms. O’Neill Hayes said in an interview. “To the extent that they can keep their prices high and people are still buying, they have limited incentives to lower those costs.”
The insulin market is dysfunctional, she added. “The increasing cost of insulin seems primarily to be the result of a lack of competition in the market and convoluted drug pricing and insurance practices,” Ms. O’Neill Hayes and colleagues wrote in a report in April on federal and state attempts to address insulin affordability.
Novo Nordisk, however, maintains that drugmakers are not solely to blame.
“Everyone in the health care system has a role to play in affordability,” said Ken Inchausti, Novo Nordisk’s senior director for corporate communications. State legislation “attempts to address a systemic issue in [U.S.] health care: How benefit design can make medicines unaffordable for many, especially for those in high-deductible health plans,” he said in an interview.
“Efforts to place copay caps on insurance plans covering insulin can certainly help lower out-of-pocket costs,” said Mr. Inchausti.
Sanofi spokesperson Jon Florio said the company supports actions that increase affordable access to insulin. However, “while we support capped copays, we feel this should not be limited to just one class of medicines,” he said. Mr. Florio also noted that Sanofi provides out-of-pocket caps to anyone with commercial insurance and that anyone without insurance can buy one or multiple Sanofi insulins for a fixed price of $99 per month, up to 10 boxes of pens and/or 10-mL vials.
And Sanofi will take part in the Centers for Medicare & Medicaid Services’ new insulin demonstration program. Starting in 2021, CMS will cap insulin copays at $35 for people in Part D plans that participate.
Eli Lilly spokesperson Brad Jacklin said the company “believes in the common goal of ensuring affordable access to insulin and other life-saving medicines because nobody should have to forgo or ration because of cost.”
Lilly supports efforts “that more directly affect patients’ cost-sharing based on their health care coverage,” he said. Insurers and pharmacy benefit managers (PBMs) should pass savings on to patients, Mr. Jacklin urged. Lilly caps some insulins at $35 for the uninsured or commercially insured. The company will also participate in the CMS program.
Meanwhile, a PhRMA-sponsored website www.letstalkaboutcost.org said that, because they do not share savings, insurers and PBMs are responsible for high insulin costs.
Manufacturer assistance programs for patients with diabetes and other chronic diseases, on the other hand, can save individuals $300-$500 a year, PhRMA said in August.
PBMs point back at insulin manufacturers
PBMs, however, point back at drug companies. “PBMs have been able to moderate insulin costs for most consumers with insurance,” said J.C. Scott, president and CEO of the Pharmaceutical Care Management Association, the PBM trade group, in a statement.
The rising cost of insulin is caused by a lack of competition and overuse of patent extensions, PCMA maintains.
Health insurers, which, in tandem with PBMs, give insulins formulary preference based on a discounted price, are most likely to feel the impact of laws limiting out-of-pocket costs.
If they have to make up the shortfall from a patient’s reduced payment for a prescription, they will likely raise premiums, said Ms. O’Neill Hayes.
And if patients pay the same price for insulin – regardless of who makes it – drugmakers won’t have much incentive to offer discounts or rebates for formulary placement, she said. Again, that would likely lead to higher premiums.
David Allen, a spokesperson for America’s Health Insurance Plans, said in an interview that AHIP believes lack of competition has driven up insulin prices.
“High prices for insulin correspond with high health insurance costs for insulin,” he said. When CMS starts requiring drugmakers to discount their insulins for Medicare that will allow “health plans to use those savings to reduce out-of-pocket [costs] for seniors.”
He did not respond to a question as to why health insurers were not already passing savings on to commercially insured patients, especially in states with out-of-pocket limits.
Mr. Allen did say that AHIP’s plans “stand ready to work with state policymakers to remove barriers to lower insulin prices for Americans.”
Utah savings hopefully saving lives already
In Utah, legislators tuned out the blame game, and instead were keen to listen to patients, who had many stories about how the high cost of insulin had hurt them, said Ms. Hooley.
She noted an estimated 50,000 Utahans rely on insulin to stay alive.
Ms. Hooley and her chapter convinced legislators to pass a bill that gives insurers the option to cap patient copays at $30 per month, or to put insulin on its lowest formulary tier and waive any patient deductible. That aspect of the law does not go into effect until January 2021, but insurers are already starting to move insulin to the lowest formulary tier.
That has helped some people immediately. One state resident said her most recent insulin prescription cost $7 – instead of the usual $200.
The uninsured are not left totally high and dry either. Starting June 1, anyone in the state could buy through a state bulk-purchasing program, which guaranteed a 60% discount.
Ms. Hooley said she’d recently heard about a patient who usually spent $300 per prescription but was able to buy insulin for $100 through the program.
“Although $100 is still too much, it is nice knowing the Utah Insulin Savings Program is saving lives,” Ms. Hooley concluded.
A version of this article originally appeared on Medscape.com.
Twelve U.S. states have now passed laws aimed at making insulin more affordable – and more than 30 are considering such legislation – but they all have gaps that still put the cost of this basic and essential medication out of reach for many with diabetes.
The laws only apply to health insurance through state-regulated plans, and not to the majority of health plans that cover most Americans: Medicare, Medicaid, the Veterans Affairs health system, or self-funded employer-sponsored plans.
Overall, Hannah Crabtree, an activist who writes the blog Data for Insulin, estimates state laws that limit copays, deductibles, or other out-of-pocket costs for insulin cover an average of 27% of people with diabetes across the United States.
And while diabetes activists have applauded state actions, most want more help for the under- and uninsured.
“Our chapter will be fighting harder next legislative session for the uninsured,” said Mindie Hooley, the leader of the Utah #insulin4all chapter, which successfully lobbied legislators to pass a bill signed by the state’s governor on March 30.
“With so many losing their jobs because of the pandemic, there’s no better time than now to fight for these patients who don’t have insurance,” Ms. Hooley said in an interview.
The American Diabetes Association has also been lobbying for state caps as one of many avenues for making insulin more affordable, said Stephen Habbe, the ADA’s director for state government affairs.
One in four insulin users report rationing the medication, Mr. Habbe said.
The state laws “can really provide important relief in terms of affordability for their insulin costs, which we know can be critical in terms of preserving their life and helping to prevent complications that can potentially be disabling or even deadly,” he said in an interview.
Activists with T1 International, which created the #insulin4all campaign, are working nationwide to convince state legislators to back measures that limit out-of-pocket costs for insulin, or for other diabetes medications and supplies.
Colorado, Connecticut, Delaware, Illinois, Maine, New Hampshire, New Mexico, New York, Utah, Virginia, Washington, and West Virginia have enacted such limits, with caps ranging from $25 to $100.
Insulin makers unfazed, blame insurers, PBMs for high prices
The three insulin manufacturers in the United States – Eli Lilly, Novo Nordisk, and Sanofi– have not overtly fought against the laws, although in July, the Pharmaceutical Research and Manufacturers of America did sue to block a related Minnesota law that provides a free emergency supply of insulin.
And the nonprofit news organization FairWarning reported in August that a lobbyist from Eli Lilly had attempted to push a Tennessee legislator to keep the uninsured from being eligible for any out-of-pocket limits.
The insulin makers have also not lowered prices in response to the mounting number of state laws.
They see no need, said Tara O’Neill Hayes, director of human welfare policy at the American Action Forum, a center right–leaning Washington, D.C., think tank.
“You’re going to do what you can get away with,” Ms. O’Neill Hayes said in an interview. “To the extent that they can keep their prices high and people are still buying, they have limited incentives to lower those costs.”
The insulin market is dysfunctional, she added. “The increasing cost of insulin seems primarily to be the result of a lack of competition in the market and convoluted drug pricing and insurance practices,” Ms. O’Neill Hayes and colleagues wrote in a report in April on federal and state attempts to address insulin affordability.
Novo Nordisk, however, maintains that drugmakers are not solely to blame.
“Everyone in the health care system has a role to play in affordability,” said Ken Inchausti, Novo Nordisk’s senior director for corporate communications. State legislation “attempts to address a systemic issue in [U.S.] health care: How benefit design can make medicines unaffordable for many, especially for those in high-deductible health plans,” he said in an interview.
“Efforts to place copay caps on insurance plans covering insulin can certainly help lower out-of-pocket costs,” said Mr. Inchausti.
Sanofi spokesperson Jon Florio said the company supports actions that increase affordable access to insulin. However, “while we support capped copays, we feel this should not be limited to just one class of medicines,” he said. Mr. Florio also noted that Sanofi provides out-of-pocket caps to anyone with commercial insurance and that anyone without insurance can buy one or multiple Sanofi insulins for a fixed price of $99 per month, up to 10 boxes of pens and/or 10-mL vials.
And Sanofi will take part in the Centers for Medicare & Medicaid Services’ new insulin demonstration program. Starting in 2021, CMS will cap insulin copays at $35 for people in Part D plans that participate.
Eli Lilly spokesperson Brad Jacklin said the company “believes in the common goal of ensuring affordable access to insulin and other life-saving medicines because nobody should have to forgo or ration because of cost.”
Lilly supports efforts “that more directly affect patients’ cost-sharing based on their health care coverage,” he said. Insurers and pharmacy benefit managers (PBMs) should pass savings on to patients, Mr. Jacklin urged. Lilly caps some insulins at $35 for the uninsured or commercially insured. The company will also participate in the CMS program.
Meanwhile, a PhRMA-sponsored website www.letstalkaboutcost.org said that, because they do not share savings, insurers and PBMs are responsible for high insulin costs.
Manufacturer assistance programs for patients with diabetes and other chronic diseases, on the other hand, can save individuals $300-$500 a year, PhRMA said in August.
PBMs point back at insulin manufacturers
PBMs, however, point back at drug companies. “PBMs have been able to moderate insulin costs for most consumers with insurance,” said J.C. Scott, president and CEO of the Pharmaceutical Care Management Association, the PBM trade group, in a statement.
The rising cost of insulin is caused by a lack of competition and overuse of patent extensions, PCMA maintains.
Health insurers, which, in tandem with PBMs, give insulins formulary preference based on a discounted price, are most likely to feel the impact of laws limiting out-of-pocket costs.
If they have to make up the shortfall from a patient’s reduced payment for a prescription, they will likely raise premiums, said Ms. O’Neill Hayes.
And if patients pay the same price for insulin – regardless of who makes it – drugmakers won’t have much incentive to offer discounts or rebates for formulary placement, she said. Again, that would likely lead to higher premiums.
David Allen, a spokesperson for America’s Health Insurance Plans, said in an interview that AHIP believes lack of competition has driven up insulin prices.
“High prices for insulin correspond with high health insurance costs for insulin,” he said. When CMS starts requiring drugmakers to discount their insulins for Medicare that will allow “health plans to use those savings to reduce out-of-pocket [costs] for seniors.”
He did not respond to a question as to why health insurers were not already passing savings on to commercially insured patients, especially in states with out-of-pocket limits.
Mr. Allen did say that AHIP’s plans “stand ready to work with state policymakers to remove barriers to lower insulin prices for Americans.”
Utah savings hopefully saving lives already
In Utah, legislators tuned out the blame game, and instead were keen to listen to patients, who had many stories about how the high cost of insulin had hurt them, said Ms. Hooley.
She noted an estimated 50,000 Utahans rely on insulin to stay alive.
Ms. Hooley and her chapter convinced legislators to pass a bill that gives insurers the option to cap patient copays at $30 per month, or to put insulin on its lowest formulary tier and waive any patient deductible. That aspect of the law does not go into effect until January 2021, but insurers are already starting to move insulin to the lowest formulary tier.
That has helped some people immediately. One state resident said her most recent insulin prescription cost $7 – instead of the usual $200.
The uninsured are not left totally high and dry either. Starting June 1, anyone in the state could buy through a state bulk-purchasing program, which guaranteed a 60% discount.
Ms. Hooley said she’d recently heard about a patient who usually spent $300 per prescription but was able to buy insulin for $100 through the program.
“Although $100 is still too much, it is nice knowing the Utah Insulin Savings Program is saving lives,” Ms. Hooley concluded.
A version of this article originally appeared on Medscape.com.





