User login
New schizophrenia treatment guideline released
The American Psychiatric Association has released a new evidence-based practice guideline for the treatment of schizophrenia.
The guideline focuses on assessment and treatment planning, which are integral to patient-centered care, and includes recommendations regarding pharmacotherapy, with particular focus on clozapine, as well as previously recommended and new psychosocial interventions.
“Our intention was to make recommendations to treat the whole person and take into account their family and other significant people in their lives,” George Keepers, MD, chair of the guideline writing group, said in an interview.
‘State-of-the-art methodology’
Dr. Keepers, professor of psychiatry at Oregon Health and Science University, Portland, explained the rigorous process that informs the current guideline, which was “based not solely on expert consensus but was preceded by an evidence-based review of the literature that was then discussed, digested, and distilled into specific recommendations.”
Many current recommendations are “similar to previous recommendations, but there are a few important differences,” he said.
Two experts in schizophrenia who were not involved in guideline authorship praised it for its usefulness and methodology.
Philip D. Harvey, PhD, Leonard M. Miller Professor of Psychiatry and Behavioral Sciences, University of Miami, said in an interview that the guideline “clarified the typical treatment algorithm from first episode to treatment resistance [which is] very clearly laid out for the first time.”
Christoph Correll, MD, professor of psychiatry and molecular medicine, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, N.Y., said in an interview that the guideline “followed state-of-the-art methodology.”
First steps
The guideline recommends beginning with assessment of the patient and determination of the treatment plan.
Patients should be “treated with an antipsychotic medication and monitored for effectiveness and side effects.” Even after the patient’s symptoms have improved, antipsychotic treatment should continue.
For patients whose symptoms have improved, treatment should continue with the same antipsychotic and should not be switched.
“The problem we’re addressing in this recommendation is that patients are often treated with an effective medication and then forced, by circumstances or their insurance company, to switch to another that may not be effective for them, resulting in unnecessary relapses of the illness,” said Dr. Keepers.
“ and do what’s in the best interest of the patient,” he said.
“The guideline called out that antipsychotics that are effective and tolerated should be continued, without specifying a duration of treatment, thereby indicating indirectly that there is no clear end of the recommendation for ongoing maintenance treatment in individuals with schizophrenia,” said Dr. Correll.
Clozapine underutilized
The guideline highlights the role of clozapine and recommends its use for patients with treatment-resistant schizophrenia and those at risk for suicide. Clozapine is also recommended for patients at “substantial” risk for aggressive behavior, regardless of other treatments.
“Clozapine is underutilized for treatment of schizophrenia in the U.S. and a number of other countries, but it is a really important treatment for patients who don’t respond to other antipsychotic agents,” said Dr. Keepers.
“With this recommendation, we hope that more patients will wind up receiving the medication and benefiting from it,” he added.
In addition, patients should receive treatment with a long-acting injectable antipsychotic “if they prefer such treatment or if they have a history of poor or uncertain adherence” (level of evidence, 2B).
The guideline authors “are recommending long-acting injectable medications for people who want them, not just people with poor prior adherence, which is a critical step,” said Dr. Harvey, director of the division of psychology at the University of Miami.
Managing antipsychotic side effects
The guideline offers recommendations for patients experiencing antipsychotic-induced side effects.
VMAT2s, which represent a “class of drugs that have become available since the last schizophrenia guidelines, are effective in tardive dyskinesia. It is important that patients with tardive dyskinesia have access to these drugs because they do work,” Dr. Keepers said.
Adequate funding needed
Recommended psychosocial interventions include treatment in a specialty care program for patients with schizophrenia who are experiencing a first episode of psychosis, use of cognitive-behavioral therapy for psychosis, psychoeducation, and supported employment services (2B).
“We reviewed very good data showing that patients who receive these services are more likely to be able to be employed and less likely to be rehospitalized or have a relapse,” Dr. Keepers observed.
In addition, patients with schizophrenia should receive assertive community treatment interventions if there is a “history of poor engagement with services leading to frequent relapse or social disruption.”
Family interventions are recommended for patients who have ongoing contact with their families (2B), and patients should also receive interventions “aimed at developing self-management skills and enhancing person-oriented recovery.” They should receive cognitive remediation, social skills training, and supportive psychotherapy.
Dr. Keepers pointed to “major barriers” to providing some of these psychosocial treatments. “They are beyond the scope of someone in an individual private practice situation, so they need to be delivered within the context of treatment programs that are either publicly or privately based,” he said.
“Psychiatrists can and do work closely with community and mental health centers, psychologists, and social workers who can provide these kinds of treatments,” but “many [treatments] require specialized skills and training before they can be offered, and there is a shortage of personnel to deliver them,” he noted.
“Both the national and state governments have not provided adequate funding for treatment of individuals with this condition [schizophrenia],” he added.
Dr. Keepers reports no relevant financial relationships. The other authors’ disclosures are listed in the original article. Dr. Harvey reports no relevant financial relationships. Dr. Correll disclosed ties to Acadia, Alkermes, Allergan, Angelini, Axsome, Gedeon Richter, Gerson Lehrman Group, Indivior, IntraCellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, Medscape, Merck, Mylan, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Servier, Sumitomo Dainippon, Sunovion, Supernus, Takeda, and Teva. He has received grant support from Janssen and Takeda. He is also a stock option holder of LB Pharma.
A version of this article originally appeared on Medscape.com.
The American Psychiatric Association has released a new evidence-based practice guideline for the treatment of schizophrenia.
The guideline focuses on assessment and treatment planning, which are integral to patient-centered care, and includes recommendations regarding pharmacotherapy, with particular focus on clozapine, as well as previously recommended and new psychosocial interventions.
“Our intention was to make recommendations to treat the whole person and take into account their family and other significant people in their lives,” George Keepers, MD, chair of the guideline writing group, said in an interview.
‘State-of-the-art methodology’
Dr. Keepers, professor of psychiatry at Oregon Health and Science University, Portland, explained the rigorous process that informs the current guideline, which was “based not solely on expert consensus but was preceded by an evidence-based review of the literature that was then discussed, digested, and distilled into specific recommendations.”
Many current recommendations are “similar to previous recommendations, but there are a few important differences,” he said.
Two experts in schizophrenia who were not involved in guideline authorship praised it for its usefulness and methodology.
Philip D. Harvey, PhD, Leonard M. Miller Professor of Psychiatry and Behavioral Sciences, University of Miami, said in an interview that the guideline “clarified the typical treatment algorithm from first episode to treatment resistance [which is] very clearly laid out for the first time.”
Christoph Correll, MD, professor of psychiatry and molecular medicine, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, N.Y., said in an interview that the guideline “followed state-of-the-art methodology.”
First steps
The guideline recommends beginning with assessment of the patient and determination of the treatment plan.
Patients should be “treated with an antipsychotic medication and monitored for effectiveness and side effects.” Even after the patient’s symptoms have improved, antipsychotic treatment should continue.
For patients whose symptoms have improved, treatment should continue with the same antipsychotic and should not be switched.
“The problem we’re addressing in this recommendation is that patients are often treated with an effective medication and then forced, by circumstances or their insurance company, to switch to another that may not be effective for them, resulting in unnecessary relapses of the illness,” said Dr. Keepers.
“ and do what’s in the best interest of the patient,” he said.
“The guideline called out that antipsychotics that are effective and tolerated should be continued, without specifying a duration of treatment, thereby indicating indirectly that there is no clear end of the recommendation for ongoing maintenance treatment in individuals with schizophrenia,” said Dr. Correll.
Clozapine underutilized
The guideline highlights the role of clozapine and recommends its use for patients with treatment-resistant schizophrenia and those at risk for suicide. Clozapine is also recommended for patients at “substantial” risk for aggressive behavior, regardless of other treatments.
“Clozapine is underutilized for treatment of schizophrenia in the U.S. and a number of other countries, but it is a really important treatment for patients who don’t respond to other antipsychotic agents,” said Dr. Keepers.
“With this recommendation, we hope that more patients will wind up receiving the medication and benefiting from it,” he added.
In addition, patients should receive treatment with a long-acting injectable antipsychotic “if they prefer such treatment or if they have a history of poor or uncertain adherence” (level of evidence, 2B).
The guideline authors “are recommending long-acting injectable medications for people who want them, not just people with poor prior adherence, which is a critical step,” said Dr. Harvey, director of the division of psychology at the University of Miami.
Managing antipsychotic side effects
The guideline offers recommendations for patients experiencing antipsychotic-induced side effects.
VMAT2s, which represent a “class of drugs that have become available since the last schizophrenia guidelines, are effective in tardive dyskinesia. It is important that patients with tardive dyskinesia have access to these drugs because they do work,” Dr. Keepers said.
Adequate funding needed
Recommended psychosocial interventions include treatment in a specialty care program for patients with schizophrenia who are experiencing a first episode of psychosis, use of cognitive-behavioral therapy for psychosis, psychoeducation, and supported employment services (2B).
“We reviewed very good data showing that patients who receive these services are more likely to be able to be employed and less likely to be rehospitalized or have a relapse,” Dr. Keepers observed.
In addition, patients with schizophrenia should receive assertive community treatment interventions if there is a “history of poor engagement with services leading to frequent relapse or social disruption.”
Family interventions are recommended for patients who have ongoing contact with their families (2B), and patients should also receive interventions “aimed at developing self-management skills and enhancing person-oriented recovery.” They should receive cognitive remediation, social skills training, and supportive psychotherapy.
Dr. Keepers pointed to “major barriers” to providing some of these psychosocial treatments. “They are beyond the scope of someone in an individual private practice situation, so they need to be delivered within the context of treatment programs that are either publicly or privately based,” he said.
“Psychiatrists can and do work closely with community and mental health centers, psychologists, and social workers who can provide these kinds of treatments,” but “many [treatments] require specialized skills and training before they can be offered, and there is a shortage of personnel to deliver them,” he noted.
“Both the national and state governments have not provided adequate funding for treatment of individuals with this condition [schizophrenia],” he added.
Dr. Keepers reports no relevant financial relationships. The other authors’ disclosures are listed in the original article. Dr. Harvey reports no relevant financial relationships. Dr. Correll disclosed ties to Acadia, Alkermes, Allergan, Angelini, Axsome, Gedeon Richter, Gerson Lehrman Group, Indivior, IntraCellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, Medscape, Merck, Mylan, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Servier, Sumitomo Dainippon, Sunovion, Supernus, Takeda, and Teva. He has received grant support from Janssen and Takeda. He is also a stock option holder of LB Pharma.
A version of this article originally appeared on Medscape.com.
The American Psychiatric Association has released a new evidence-based practice guideline for the treatment of schizophrenia.
The guideline focuses on assessment and treatment planning, which are integral to patient-centered care, and includes recommendations regarding pharmacotherapy, with particular focus on clozapine, as well as previously recommended and new psychosocial interventions.
“Our intention was to make recommendations to treat the whole person and take into account their family and other significant people in their lives,” George Keepers, MD, chair of the guideline writing group, said in an interview.
‘State-of-the-art methodology’
Dr. Keepers, professor of psychiatry at Oregon Health and Science University, Portland, explained the rigorous process that informs the current guideline, which was “based not solely on expert consensus but was preceded by an evidence-based review of the literature that was then discussed, digested, and distilled into specific recommendations.”
Many current recommendations are “similar to previous recommendations, but there are a few important differences,” he said.
Two experts in schizophrenia who were not involved in guideline authorship praised it for its usefulness and methodology.
Philip D. Harvey, PhD, Leonard M. Miller Professor of Psychiatry and Behavioral Sciences, University of Miami, said in an interview that the guideline “clarified the typical treatment algorithm from first episode to treatment resistance [which is] very clearly laid out for the first time.”
Christoph Correll, MD, professor of psychiatry and molecular medicine, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, N.Y., said in an interview that the guideline “followed state-of-the-art methodology.”
First steps
The guideline recommends beginning with assessment of the patient and determination of the treatment plan.
Patients should be “treated with an antipsychotic medication and monitored for effectiveness and side effects.” Even after the patient’s symptoms have improved, antipsychotic treatment should continue.
For patients whose symptoms have improved, treatment should continue with the same antipsychotic and should not be switched.
“The problem we’re addressing in this recommendation is that patients are often treated with an effective medication and then forced, by circumstances or their insurance company, to switch to another that may not be effective for them, resulting in unnecessary relapses of the illness,” said Dr. Keepers.
“ and do what’s in the best interest of the patient,” he said.
“The guideline called out that antipsychotics that are effective and tolerated should be continued, without specifying a duration of treatment, thereby indicating indirectly that there is no clear end of the recommendation for ongoing maintenance treatment in individuals with schizophrenia,” said Dr. Correll.
Clozapine underutilized
The guideline highlights the role of clozapine and recommends its use for patients with treatment-resistant schizophrenia and those at risk for suicide. Clozapine is also recommended for patients at “substantial” risk for aggressive behavior, regardless of other treatments.
“Clozapine is underutilized for treatment of schizophrenia in the U.S. and a number of other countries, but it is a really important treatment for patients who don’t respond to other antipsychotic agents,” said Dr. Keepers.
“With this recommendation, we hope that more patients will wind up receiving the medication and benefiting from it,” he added.
In addition, patients should receive treatment with a long-acting injectable antipsychotic “if they prefer such treatment or if they have a history of poor or uncertain adherence” (level of evidence, 2B).
The guideline authors “are recommending long-acting injectable medications for people who want them, not just people with poor prior adherence, which is a critical step,” said Dr. Harvey, director of the division of psychology at the University of Miami.
Managing antipsychotic side effects
The guideline offers recommendations for patients experiencing antipsychotic-induced side effects.
VMAT2s, which represent a “class of drugs that have become available since the last schizophrenia guidelines, are effective in tardive dyskinesia. It is important that patients with tardive dyskinesia have access to these drugs because they do work,” Dr. Keepers said.
Adequate funding needed
Recommended psychosocial interventions include treatment in a specialty care program for patients with schizophrenia who are experiencing a first episode of psychosis, use of cognitive-behavioral therapy for psychosis, psychoeducation, and supported employment services (2B).
“We reviewed very good data showing that patients who receive these services are more likely to be able to be employed and less likely to be rehospitalized or have a relapse,” Dr. Keepers observed.
In addition, patients with schizophrenia should receive assertive community treatment interventions if there is a “history of poor engagement with services leading to frequent relapse or social disruption.”
Family interventions are recommended for patients who have ongoing contact with their families (2B), and patients should also receive interventions “aimed at developing self-management skills and enhancing person-oriented recovery.” They should receive cognitive remediation, social skills training, and supportive psychotherapy.
Dr. Keepers pointed to “major barriers” to providing some of these psychosocial treatments. “They are beyond the scope of someone in an individual private practice situation, so they need to be delivered within the context of treatment programs that are either publicly or privately based,” he said.
“Psychiatrists can and do work closely with community and mental health centers, psychologists, and social workers who can provide these kinds of treatments,” but “many [treatments] require specialized skills and training before they can be offered, and there is a shortage of personnel to deliver them,” he noted.
“Both the national and state governments have not provided adequate funding for treatment of individuals with this condition [schizophrenia],” he added.
Dr. Keepers reports no relevant financial relationships. The other authors’ disclosures are listed in the original article. Dr. Harvey reports no relevant financial relationships. Dr. Correll disclosed ties to Acadia, Alkermes, Allergan, Angelini, Axsome, Gedeon Richter, Gerson Lehrman Group, Indivior, IntraCellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, Medscape, Merck, Mylan, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Servier, Sumitomo Dainippon, Sunovion, Supernus, Takeda, and Teva. He has received grant support from Janssen and Takeda. He is also a stock option holder of LB Pharma.
A version of this article originally appeared on Medscape.com.
Gene signature may improve prognostication in ovarian cancer
according to a study published in Annals of Oncology.
“Gene expression signature tests for prognosis are available for other cancers, such as breast cancer, and these help with treatment decisions, but no such tests are available for ovarian cancer,” senior investigator Susan J. Ramus, PhD, of Lowy Cancer Research Centre, University of NSW Sydney, commented in an interview.
Dr. Ramus and associates developed and validated their 101-gene expression signature using pretreatment tumor tissue from 3,769 women with high-grade serous ovarian cancer treated on 21 studies.
The investigators found this signature, called OTTA-SPOT (Ovarian Tumor Tissue Analysis Consortium–Stratified Prognosis of Ovarian Tumors), performed well at stratifying women according to overall survival. Median overall survival times ranged from about 2 years for patients in the top quintile of scores to more than 9 years for patients in the bottom quintile.
Moreover, OTTA-SPOT significantly improved prognostication when added to age and stage.
“This tumor test works on formalin-fixed, paraffin-embedded tumors, as collected routinely in clinical practice,” Dr. Ramus noted. “Women predicted to have poor survival using current treatments could be included in clinical trials to rapidly get alternative treatment. Many of the genes included in this test are targets of known drugs, so this information could lead to alternative targeted treatments.
“This test is not ready for routine clinical care yet,” she added. “The next step would be to include this signature as part of a clinical trial. If patients predicted to have poor survival are given alternative treatments that improve their survival, then the test could be included in treatment decisions.”
Study details
Dr. Ramus and colleagues began this work by measuring tumor expression of 513 genes selected via meta-analysis. The team then developed a gene expression assay and a prognostic signature for overall survival, which they trained on tumors from 2,702 women in 15 studies and validated on an independent set of tumors from 1,067 women in 6 studies.
In analyses adjusted for covariates, expression levels of 276 genes were associated with overall survival. The signature with the best prognostic performance contained 101 genes that were enriched in pathways having treatment implications, such as pathways involved in immune response, mitosis, and homologous recombination repair.
Adding the signature to age and stage alone improved prediction of 2- and 5-year overall survival. The area under the curve increased from 0.61 to 0.69 for 2-year overall survival and from 0.62 to 0.75 for 5-year overall survival (with nonoverlapping 95% confidence intervals for 5-year survival).
Each standard deviation increase in the gene expression score was associated with a more than doubling of the risk of death (hazard ratio, 2.35; P < .001).
The median overall survival by gene expression score quintile was 9.5 years for patients in the first quintile, 5.4 years for patients in the second, 3.8 years for patients in the third, 3.2 years for patients in the fourth, and 2.3 years for patients in the fifth.
This study was funded by the National Institutes of Health/National Cancer Institute, the Canadian Institutes for Health Research, and the Department of Defense Ovarian Cancer Research Program. Some of the authors disclosed financial relationships with a range of companies. Dr. Ramus disclosed no conflicts of interest.
SOURCE: Millstein J et al. Ann Oncol. 2020 Sep;31(9):1240-50.
according to a study published in Annals of Oncology.
“Gene expression signature tests for prognosis are available for other cancers, such as breast cancer, and these help with treatment decisions, but no such tests are available for ovarian cancer,” senior investigator Susan J. Ramus, PhD, of Lowy Cancer Research Centre, University of NSW Sydney, commented in an interview.
Dr. Ramus and associates developed and validated their 101-gene expression signature using pretreatment tumor tissue from 3,769 women with high-grade serous ovarian cancer treated on 21 studies.
The investigators found this signature, called OTTA-SPOT (Ovarian Tumor Tissue Analysis Consortium–Stratified Prognosis of Ovarian Tumors), performed well at stratifying women according to overall survival. Median overall survival times ranged from about 2 years for patients in the top quintile of scores to more than 9 years for patients in the bottom quintile.
Moreover, OTTA-SPOT significantly improved prognostication when added to age and stage.
“This tumor test works on formalin-fixed, paraffin-embedded tumors, as collected routinely in clinical practice,” Dr. Ramus noted. “Women predicted to have poor survival using current treatments could be included in clinical trials to rapidly get alternative treatment. Many of the genes included in this test are targets of known drugs, so this information could lead to alternative targeted treatments.
“This test is not ready for routine clinical care yet,” she added. “The next step would be to include this signature as part of a clinical trial. If patients predicted to have poor survival are given alternative treatments that improve their survival, then the test could be included in treatment decisions.”
Study details
Dr. Ramus and colleagues began this work by measuring tumor expression of 513 genes selected via meta-analysis. The team then developed a gene expression assay and a prognostic signature for overall survival, which they trained on tumors from 2,702 women in 15 studies and validated on an independent set of tumors from 1,067 women in 6 studies.
In analyses adjusted for covariates, expression levels of 276 genes were associated with overall survival. The signature with the best prognostic performance contained 101 genes that were enriched in pathways having treatment implications, such as pathways involved in immune response, mitosis, and homologous recombination repair.
Adding the signature to age and stage alone improved prediction of 2- and 5-year overall survival. The area under the curve increased from 0.61 to 0.69 for 2-year overall survival and from 0.62 to 0.75 for 5-year overall survival (with nonoverlapping 95% confidence intervals for 5-year survival).
Each standard deviation increase in the gene expression score was associated with a more than doubling of the risk of death (hazard ratio, 2.35; P < .001).
The median overall survival by gene expression score quintile was 9.5 years for patients in the first quintile, 5.4 years for patients in the second, 3.8 years for patients in the third, 3.2 years for patients in the fourth, and 2.3 years for patients in the fifth.
This study was funded by the National Institutes of Health/National Cancer Institute, the Canadian Institutes for Health Research, and the Department of Defense Ovarian Cancer Research Program. Some of the authors disclosed financial relationships with a range of companies. Dr. Ramus disclosed no conflicts of interest.
SOURCE: Millstein J et al. Ann Oncol. 2020 Sep;31(9):1240-50.
according to a study published in Annals of Oncology.
“Gene expression signature tests for prognosis are available for other cancers, such as breast cancer, and these help with treatment decisions, but no such tests are available for ovarian cancer,” senior investigator Susan J. Ramus, PhD, of Lowy Cancer Research Centre, University of NSW Sydney, commented in an interview.
Dr. Ramus and associates developed and validated their 101-gene expression signature using pretreatment tumor tissue from 3,769 women with high-grade serous ovarian cancer treated on 21 studies.
The investigators found this signature, called OTTA-SPOT (Ovarian Tumor Tissue Analysis Consortium–Stratified Prognosis of Ovarian Tumors), performed well at stratifying women according to overall survival. Median overall survival times ranged from about 2 years for patients in the top quintile of scores to more than 9 years for patients in the bottom quintile.
Moreover, OTTA-SPOT significantly improved prognostication when added to age and stage.
“This tumor test works on formalin-fixed, paraffin-embedded tumors, as collected routinely in clinical practice,” Dr. Ramus noted. “Women predicted to have poor survival using current treatments could be included in clinical trials to rapidly get alternative treatment. Many of the genes included in this test are targets of known drugs, so this information could lead to alternative targeted treatments.
“This test is not ready for routine clinical care yet,” she added. “The next step would be to include this signature as part of a clinical trial. If patients predicted to have poor survival are given alternative treatments that improve their survival, then the test could be included in treatment decisions.”
Study details
Dr. Ramus and colleagues began this work by measuring tumor expression of 513 genes selected via meta-analysis. The team then developed a gene expression assay and a prognostic signature for overall survival, which they trained on tumors from 2,702 women in 15 studies and validated on an independent set of tumors from 1,067 women in 6 studies.
In analyses adjusted for covariates, expression levels of 276 genes were associated with overall survival. The signature with the best prognostic performance contained 101 genes that were enriched in pathways having treatment implications, such as pathways involved in immune response, mitosis, and homologous recombination repair.
Adding the signature to age and stage alone improved prediction of 2- and 5-year overall survival. The area under the curve increased from 0.61 to 0.69 for 2-year overall survival and from 0.62 to 0.75 for 5-year overall survival (with nonoverlapping 95% confidence intervals for 5-year survival).
Each standard deviation increase in the gene expression score was associated with a more than doubling of the risk of death (hazard ratio, 2.35; P < .001).
The median overall survival by gene expression score quintile was 9.5 years for patients in the first quintile, 5.4 years for patients in the second, 3.8 years for patients in the third, 3.2 years for patients in the fourth, and 2.3 years for patients in the fifth.
This study was funded by the National Institutes of Health/National Cancer Institute, the Canadian Institutes for Health Research, and the Department of Defense Ovarian Cancer Research Program. Some of the authors disclosed financial relationships with a range of companies. Dr. Ramus disclosed no conflicts of interest.
SOURCE: Millstein J et al. Ann Oncol. 2020 Sep;31(9):1240-50.
FROM ANNALS OF ONCOLOGY
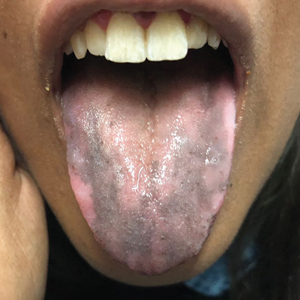
Hyperpigmentation of the Tongue
The Diagnosis: Addison Disease in the Context of Polyglandular Autoimmune Syndrome Type 2
The patient’s hormone levels as well as distinct clinical features led to a diagnosis of Addison disease in the context of polyglandular autoimmune syndrome type 2 (PAS-2). Approximately 50% of PAS-2 cases are familiar, and different modes of inheritance—autosomal recessive, autosomal dominant, and polygenic—have been reported. Women are affected up to 3 times more often than men.1,2 The age of onset ranges from infancy to late adulthood, with most cases occurring in early adulthood. Primary adrenal insufficiency (Addison disease) is the principal manifestation of PAS-2. It appears in approximately 50% of patients, occurring simultaneously with autoimmune thyroid disease or diabetes mellitus in 20% of patients and following them in 30% of patients.1,2 Autoimmune thyroid diseases such as chronic autoimmune thyroiditis and occasionally Graves disease as well as type 1 diabetes mellitus also are common. Polyglandular autoimmune syndrome type 2 with primary adrenal insufficiency and autoimmune thyroid disease was formerly referred to as Schmidt syndrome.3 It must be differentiated from polyglandular autoimmune syndrome type 1, a rare condition that also is referred to as autoimmune polyendocrinopathycandidiasis-ectodermal dystrophy syndrome.1,3 As with any other cause of adrenal insufficiency, the treatment involves hormone replacement therapy up to normal levels and then tapering according to stress levels (ie, surgery or infections that require a dose increase). Our patient was diagnosed according to hormone levels and clinical features and was started on 30 mg daily of hydrocortisone and 50 μg daily of levothyroxine. No improvement in her condition was noted after 6 months of treatment. The patient is still under yearly follow-up, and the mucous hyperpigmentation faded approximately 6 months after hormonal homeostasis was achieved.
Peutz-Jeghers syndrome is inherited in an autosomal-dominant fashion. It is characterized by multiple hamartomatous polyps in the gastrointestinal tract, mucocutaneous pigmentation, and an increased risk for gastrointestinal and nongastrointestinal cancer. Mucocutaneous pigmented macules most commonly occur on the lips and perioral region, buccal mucosa, and the palms and soles. However, mucocutaneous pigmentation usually occurs during the first 1 to 2 years of life, increases in size and number over the ensuing years, and usually fades after puberty.4
Laugier-Hunziker syndrome is an acquired benign disorder presenting in adults with lentigines on the lips and buccal mucosa. It frequently is accompaniedby longitudinal melanonychia, macular pigmentation of the genitals, and involvement of the palms and soles. The diagnosis of Laugier-Hunziker syndrome is one of exclusion and is made after ruling out other causes of oral and labial hyperpigmentation, including physiologic pigmentation seen in darker-skinned individuals as well as inherited diseases associated with lentiginosis, requiring complete physical examination, endoscopy, and colonscopy.5
A wide variety of drugs and chemicals can lead to diffuse cutaneous hyperpigmentation. Increased production of melanin and/or the deposition of drug complexes or metals in the dermis is responsible for the skin discoloration. Drugs that most often cause hyperpigmentation on mucosal surfaces are hydroxychloroquine, minocycline, nicotine, silver, and some chemotherapy agents. The hyperpigmentation usually resolves with discontinuation of the offending agent, but the course may be prolonged over months to years.6
Changes in the skin and subcutaneous tissue occur in patients with Cushing syndrome. Hyperpigmentation is induced by increased secretion of adrenocorticotropic hormone, not cortisol, and occurs most often in patients with the ectopic adrenocorticotropic hormone syndrome. Hyperpigmentation may be generalized but is more intense in areas exposed to light (eg, face, neck, dorsal aspects of the hands) or to chronic mild trauma, friction, or pressure (eg, elbows, knees, spine, knuckles). Patchy pigmentation may occur on the inner surface of the lips and the buccal mucosa along the line of dental occlusion. Acanthosis nigricans also can be present in the axillae and around the neck.7
- Ferre EM, Rose SR, Rosenzweig SD, et al. Redefined clinical features and diagnostic criteria in autoimmune polyendocrinopathycandidiasis-ectodermal dystrophy. JCI Insight. 2016;1:E88782.
- Orlova EM, Sozaeva LS, Kareva MA, et al. Expanding the phenotypic and genotypic landscape of autoimmune polyendocrine syndrome type 1. J Clin Endocrinol Metab. 2017;102:3546-3556.
- Ahonen P, Myllärniemi S, Sipilä I, et al. Clinical variation of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) in a series of 68 patients. N Engl J Med. 1990;322:1829-1836.
- Utsunomiya J, Gocho H, Miyanaga T, et al. Peutz-Jeghers syndrome: its natural course and management. Johns Hopkins Med J. 1975;136:71-82.
- Nayak RS, Kotrashetti VS, Hosmani JV. Laugier-Hunziker syndrome. J Oral Maxillofac Pathol. 2012;16:245-250.
- Krause W. Drug-induced hyperpigmentation: a systematic review. J Dtsch Dermatol Ges. 2013;11:644-651.
- Newell-Price J, Trainer P, Besser M, et al. The diagnosis and differential diagnosis of Cushing’s syndrome and pseudo-Cushing’s states. Endocr Rev. 1998;19:647-672.
The Diagnosis: Addison Disease in the Context of Polyglandular Autoimmune Syndrome Type 2
The patient’s hormone levels as well as distinct clinical features led to a diagnosis of Addison disease in the context of polyglandular autoimmune syndrome type 2 (PAS-2). Approximately 50% of PAS-2 cases are familiar, and different modes of inheritance—autosomal recessive, autosomal dominant, and polygenic—have been reported. Women are affected up to 3 times more often than men.1,2 The age of onset ranges from infancy to late adulthood, with most cases occurring in early adulthood. Primary adrenal insufficiency (Addison disease) is the principal manifestation of PAS-2. It appears in approximately 50% of patients, occurring simultaneously with autoimmune thyroid disease or diabetes mellitus in 20% of patients and following them in 30% of patients.1,2 Autoimmune thyroid diseases such as chronic autoimmune thyroiditis and occasionally Graves disease as well as type 1 diabetes mellitus also are common. Polyglandular autoimmune syndrome type 2 with primary adrenal insufficiency and autoimmune thyroid disease was formerly referred to as Schmidt syndrome.3 It must be differentiated from polyglandular autoimmune syndrome type 1, a rare condition that also is referred to as autoimmune polyendocrinopathycandidiasis-ectodermal dystrophy syndrome.1,3 As with any other cause of adrenal insufficiency, the treatment involves hormone replacement therapy up to normal levels and then tapering according to stress levels (ie, surgery or infections that require a dose increase). Our patient was diagnosed according to hormone levels and clinical features and was started on 30 mg daily of hydrocortisone and 50 μg daily of levothyroxine. No improvement in her condition was noted after 6 months of treatment. The patient is still under yearly follow-up, and the mucous hyperpigmentation faded approximately 6 months after hormonal homeostasis was achieved.
Peutz-Jeghers syndrome is inherited in an autosomal-dominant fashion. It is characterized by multiple hamartomatous polyps in the gastrointestinal tract, mucocutaneous pigmentation, and an increased risk for gastrointestinal and nongastrointestinal cancer. Mucocutaneous pigmented macules most commonly occur on the lips and perioral region, buccal mucosa, and the palms and soles. However, mucocutaneous pigmentation usually occurs during the first 1 to 2 years of life, increases in size and number over the ensuing years, and usually fades after puberty.4
Laugier-Hunziker syndrome is an acquired benign disorder presenting in adults with lentigines on the lips and buccal mucosa. It frequently is accompaniedby longitudinal melanonychia, macular pigmentation of the genitals, and involvement of the palms and soles. The diagnosis of Laugier-Hunziker syndrome is one of exclusion and is made after ruling out other causes of oral and labial hyperpigmentation, including physiologic pigmentation seen in darker-skinned individuals as well as inherited diseases associated with lentiginosis, requiring complete physical examination, endoscopy, and colonscopy.5
A wide variety of drugs and chemicals can lead to diffuse cutaneous hyperpigmentation. Increased production of melanin and/or the deposition of drug complexes or metals in the dermis is responsible for the skin discoloration. Drugs that most often cause hyperpigmentation on mucosal surfaces are hydroxychloroquine, minocycline, nicotine, silver, and some chemotherapy agents. The hyperpigmentation usually resolves with discontinuation of the offending agent, but the course may be prolonged over months to years.6
Changes in the skin and subcutaneous tissue occur in patients with Cushing syndrome. Hyperpigmentation is induced by increased secretion of adrenocorticotropic hormone, not cortisol, and occurs most often in patients with the ectopic adrenocorticotropic hormone syndrome. Hyperpigmentation may be generalized but is more intense in areas exposed to light (eg, face, neck, dorsal aspects of the hands) or to chronic mild trauma, friction, or pressure (eg, elbows, knees, spine, knuckles). Patchy pigmentation may occur on the inner surface of the lips and the buccal mucosa along the line of dental occlusion. Acanthosis nigricans also can be present in the axillae and around the neck.7
The Diagnosis: Addison Disease in the Context of Polyglandular Autoimmune Syndrome Type 2
The patient’s hormone levels as well as distinct clinical features led to a diagnosis of Addison disease in the context of polyglandular autoimmune syndrome type 2 (PAS-2). Approximately 50% of PAS-2 cases are familiar, and different modes of inheritance—autosomal recessive, autosomal dominant, and polygenic—have been reported. Women are affected up to 3 times more often than men.1,2 The age of onset ranges from infancy to late adulthood, with most cases occurring in early adulthood. Primary adrenal insufficiency (Addison disease) is the principal manifestation of PAS-2. It appears in approximately 50% of patients, occurring simultaneously with autoimmune thyroid disease or diabetes mellitus in 20% of patients and following them in 30% of patients.1,2 Autoimmune thyroid diseases such as chronic autoimmune thyroiditis and occasionally Graves disease as well as type 1 diabetes mellitus also are common. Polyglandular autoimmune syndrome type 2 with primary adrenal insufficiency and autoimmune thyroid disease was formerly referred to as Schmidt syndrome.3 It must be differentiated from polyglandular autoimmune syndrome type 1, a rare condition that also is referred to as autoimmune polyendocrinopathycandidiasis-ectodermal dystrophy syndrome.1,3 As with any other cause of adrenal insufficiency, the treatment involves hormone replacement therapy up to normal levels and then tapering according to stress levels (ie, surgery or infections that require a dose increase). Our patient was diagnosed according to hormone levels and clinical features and was started on 30 mg daily of hydrocortisone and 50 μg daily of levothyroxine. No improvement in her condition was noted after 6 months of treatment. The patient is still under yearly follow-up, and the mucous hyperpigmentation faded approximately 6 months after hormonal homeostasis was achieved.
Peutz-Jeghers syndrome is inherited in an autosomal-dominant fashion. It is characterized by multiple hamartomatous polyps in the gastrointestinal tract, mucocutaneous pigmentation, and an increased risk for gastrointestinal and nongastrointestinal cancer. Mucocutaneous pigmented macules most commonly occur on the lips and perioral region, buccal mucosa, and the palms and soles. However, mucocutaneous pigmentation usually occurs during the first 1 to 2 years of life, increases in size and number over the ensuing years, and usually fades after puberty.4
Laugier-Hunziker syndrome is an acquired benign disorder presenting in adults with lentigines on the lips and buccal mucosa. It frequently is accompaniedby longitudinal melanonychia, macular pigmentation of the genitals, and involvement of the palms and soles. The diagnosis of Laugier-Hunziker syndrome is one of exclusion and is made after ruling out other causes of oral and labial hyperpigmentation, including physiologic pigmentation seen in darker-skinned individuals as well as inherited diseases associated with lentiginosis, requiring complete physical examination, endoscopy, and colonscopy.5
A wide variety of drugs and chemicals can lead to diffuse cutaneous hyperpigmentation. Increased production of melanin and/or the deposition of drug complexes or metals in the dermis is responsible for the skin discoloration. Drugs that most often cause hyperpigmentation on mucosal surfaces are hydroxychloroquine, minocycline, nicotine, silver, and some chemotherapy agents. The hyperpigmentation usually resolves with discontinuation of the offending agent, but the course may be prolonged over months to years.6
Changes in the skin and subcutaneous tissue occur in patients with Cushing syndrome. Hyperpigmentation is induced by increased secretion of adrenocorticotropic hormone, not cortisol, and occurs most often in patients with the ectopic adrenocorticotropic hormone syndrome. Hyperpigmentation may be generalized but is more intense in areas exposed to light (eg, face, neck, dorsal aspects of the hands) or to chronic mild trauma, friction, or pressure (eg, elbows, knees, spine, knuckles). Patchy pigmentation may occur on the inner surface of the lips and the buccal mucosa along the line of dental occlusion. Acanthosis nigricans also can be present in the axillae and around the neck.7
- Ferre EM, Rose SR, Rosenzweig SD, et al. Redefined clinical features and diagnostic criteria in autoimmune polyendocrinopathycandidiasis-ectodermal dystrophy. JCI Insight. 2016;1:E88782.
- Orlova EM, Sozaeva LS, Kareva MA, et al. Expanding the phenotypic and genotypic landscape of autoimmune polyendocrine syndrome type 1. J Clin Endocrinol Metab. 2017;102:3546-3556.
- Ahonen P, Myllärniemi S, Sipilä I, et al. Clinical variation of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) in a series of 68 patients. N Engl J Med. 1990;322:1829-1836.
- Utsunomiya J, Gocho H, Miyanaga T, et al. Peutz-Jeghers syndrome: its natural course and management. Johns Hopkins Med J. 1975;136:71-82.
- Nayak RS, Kotrashetti VS, Hosmani JV. Laugier-Hunziker syndrome. J Oral Maxillofac Pathol. 2012;16:245-250.
- Krause W. Drug-induced hyperpigmentation: a systematic review. J Dtsch Dermatol Ges. 2013;11:644-651.
- Newell-Price J, Trainer P, Besser M, et al. The diagnosis and differential diagnosis of Cushing’s syndrome and pseudo-Cushing’s states. Endocr Rev. 1998;19:647-672.
- Ferre EM, Rose SR, Rosenzweig SD, et al. Redefined clinical features and diagnostic criteria in autoimmune polyendocrinopathycandidiasis-ectodermal dystrophy. JCI Insight. 2016;1:E88782.
- Orlova EM, Sozaeva LS, Kareva MA, et al. Expanding the phenotypic and genotypic landscape of autoimmune polyendocrine syndrome type 1. J Clin Endocrinol Metab. 2017;102:3546-3556.
- Ahonen P, Myllärniemi S, Sipilä I, et al. Clinical variation of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) in a series of 68 patients. N Engl J Med. 1990;322:1829-1836.
- Utsunomiya J, Gocho H, Miyanaga T, et al. Peutz-Jeghers syndrome: its natural course and management. Johns Hopkins Med J. 1975;136:71-82.
- Nayak RS, Kotrashetti VS, Hosmani JV. Laugier-Hunziker syndrome. J Oral Maxillofac Pathol. 2012;16:245-250.
- Krause W. Drug-induced hyperpigmentation: a systematic review. J Dtsch Dermatol Ges. 2013;11:644-651.
- Newell-Price J, Trainer P, Besser M, et al. The diagnosis and differential diagnosis of Cushing’s syndrome and pseudo-Cushing’s states. Endocr Rev. 1998;19:647-672.
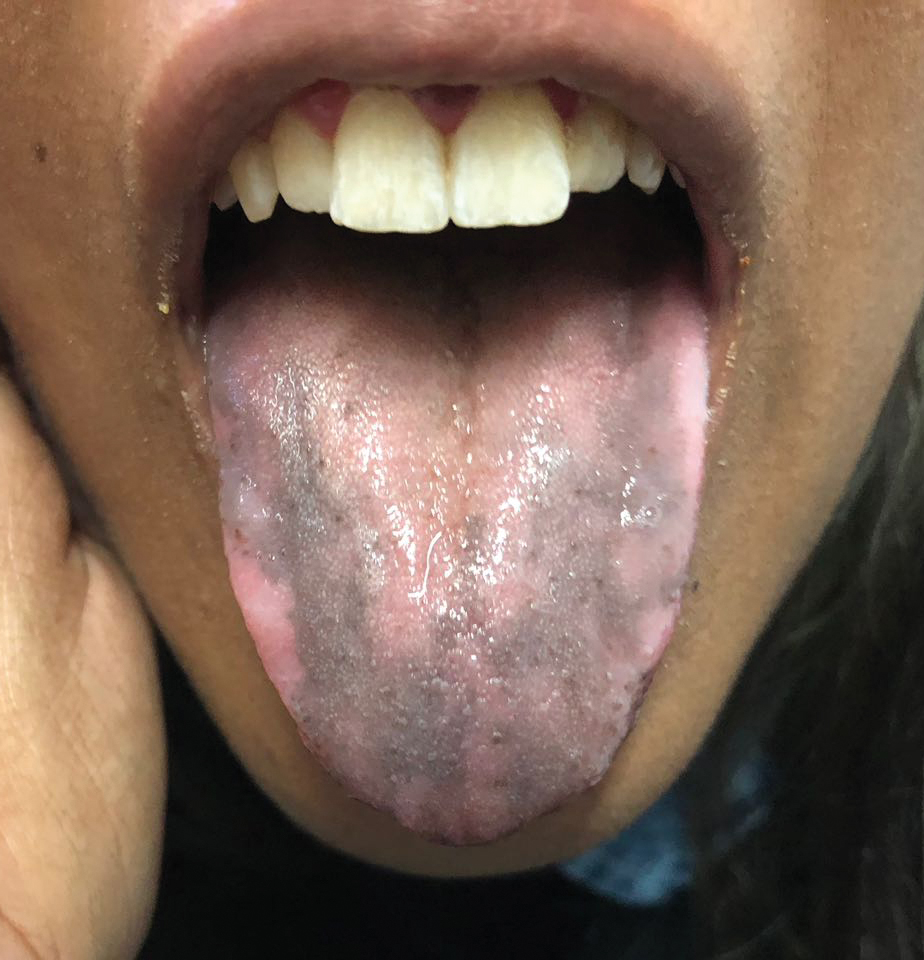
An otherwise healthy 17-year-old adolescent girl from Spain presented with hyperpigmentation on the tongue of several weeks’ duration. She denied licking graphite pencils or pens. Physical examination revealed pigmentation in the palmar creases and a slight generalized tan. The patient denied sun exposure. Neither melanonychia nor genital hyperpigmented lesions were noted. Blood tests showed overt hypothyroidism.
Smallpox Vaccination-Associated Myopericarditis
A renewed effort to vaccinate service members fighting the global war on terrorism has brought new diagnostic challenges. Vaccinations not generally given to the public are routinely given to service members when they deploy to various parts of the world. Examples include anthrax, yellow fever, Japanese encephalitis, rabies, polio, and smallpox. Every vaccination has potential for adverse effects (AEs), which can range from mild to severe life-threatening complications. These AEs often go unrecognized and untreated because physicians are not routinely screening for vaccination administration.
Background
Smallpox (Variola major) was successfully eradicated in 1977 due to worldwide vaccination efforts.1 However, the threat of bioterrorism has renewed mandatory smallpox vaccinations for high-risk individuals, such as active-duty military personnel.1,2 A notable increase in myopericarditis has been reported with the new generation of smallpox vaccination, ACAM2000.3 We present a case of a 27-year-old healthy male who presented with chest pain and diffuse ST segment elevations consistent with myopericarditis after vaccination with ACAM2000.
Case Presentation
A healthy 27-year-old soldier presented to the emergency department with sudden, new onset, sharp-stabbing, substernal chest pain, which was made worse with lying flat and better with leaning forward. Vital signs were unremarkable. He recently enlisted in the US Army and received the smallpox vaccination about 11 days before as part of a routine predeployment checklist. The patient reported he did not have any viral symptoms, such as fever, chills, nausea, vomiting, diarrhea, shortness of breath, sore throat, rhinorrhea, or sputum production. He also reported having no prior illness for the past 3 months, sick contacts at home or work, or recent travel outside the US. He reported no tobacco use, alcohol use, or illicit drug use. The patient’s family history was negative for significant cardiac disease.
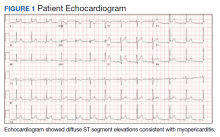
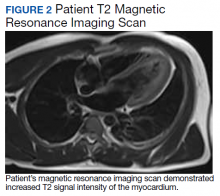
A physical examination was unremarkable. The initial laboratory report showed no leukocytosis, anemia, thrombocytopenia, electrolytes derangement, abnormal kidney function, or abnormal liver function tests. Initial troponin was 0.25 ng/mL, erythrocyte sedimentation rate (ESR) was 40 mmol/h and C-reactive protein (CRP) was 120.2 mg/L suggestive of acute inflammation. A urine drug screen was negative. D-dimer was < 0.27. An electrocardiogram (ECG) showed diffuse ST segment elevation (Figure 1). An echocardiogram showed normal left ventricle size, and function with ejection fraction 55 to 60%, normal diastolic dysfunction, and trivial pericardial effusion. Magnetic resonance imaging (MRI) showed increased T2 signal intensity of the myocardium suggestive of myopericarditis (Figure 2). A computed tomography (CT) angiogram of the coronary arteries showed no significant stenosis.
The patient was treated with ibuprofen for 2 weeks and colchicine for 3 months, and his symptoms resolved. He followed up with an appointment in the cardiology clinic 1 month later, and his ESR, CRP, and troponin results were negative. A limited echocardiogram showed ejection fraction 60 to 65%, no regional wall motion abnormalities, normal diastolic function, and resolution of the pericardial effusion.
Discussion
Smallpox was a major worldwide cause of mortality; about 30% of those infected died because of smallpox.2,4,5 Due to a worldwide vaccination effort, the World Health Organization declared smallpox was eradicated in 1977.2,4,5 However, despite successful eradication, smallpox is considered a possible bioterrorism target, which prompted a resurgence of mandatory smallpox vaccinations for active-duty personnel.2,5
Dryvax, a freeze-dried calf lymph smallpox vaccine was used extensively from the 1940s to the 1980s but was replaced in 2008 by ACAM2000, a smallpox vaccine cultured in kidney epithelial cells from African green monkeys.3,5 Myopericarditis was rarely associated with the Dryvax, with only 5 cases reported from 1955 to 1986 after millions of doses of vaccines were administered; however, in 230,734 administered ACAM2000 doses, 18 cases of myopericarditis (incidence, 7.8 per 100,000) were reported during a surveillance study in 2002 and 2003.3,5
Myopericarditis presents with a wide variety of symptoms, such as chest pain, palpitations, chills, shortness of breath, and fever.6,7 Mainstay diagnostic criteria include ECG findings consistent with myopericarditis (such as diffuse ST segment elevations) and elevated cardiac biomarkers (elevated troponins).5-7 An echocardiogram can be helpful in diagnosis, as most cases will not have regional wall motion abnormalities (to distinguish against coronary artery disease).5-7 MRI with diffuse enhancement of the myocardium can be helpful in diagnosis.5,6 The gold standard for diagnosis is an endomyocardial biopsy, which carries a significant risk of complications and is not routinely performed to diagnose myopericarditis.5,6 US military smallpox vaccination data showed that the onset of vaccine-associated myopericarditis averaged (SD) 10.4 (3.6) days after vaccination.5
Vaccine-associated myopericarditis treatment is focused on decreasing inflammation.5,6 Nonsteroidal anti-inflammatory drugs are advised for about 2 weeks with cessation of intensive cardiac activities for between 4 and 6 weeks due to risks of congestive heart failure and fatal cardiac arrhythmias.5,6
Conclusions
Since the September 11 attacks, the US needs to be continually prepared for potential terrorism on American soil and abroad. The threat of bioterrorism has renewed efforts to vaccinate or revaccinate American service members deployed to high-risk regions. These vaccinations put them at risk for vaccination-induced complications that can range from mild fever to life-threatening complications.
1. Bruner DI, Butler BS. Smallpox vaccination-associated myopericarditis is more common with the newest smallpox vaccine. J Emerg Med. 2014;46(3):e85-e87. doi:10.1016/j.jemermed.2013.06.001
2. Halsell JS, Riddle JR, Atwood JE, et al. Myopericarditis following smallpox vaccination among vaccinia-naive US military personnel. JAMA. 2003;289(24):3283-3289. doi:10.1001/jama.289.24.3283
3. Nalca A, Zumbrun EE. ACAM2000: the new smallpox vaccine for United States Strategic National Stockpile. Drug Des Devel Ther. 2010;4:71-79. doi:10.2147/dddt.s3687
4. Wollenberg A, Engler R. Smallpox, vaccination and adverse reactions to smallpox vaccine. Curr Opin Allergy Clin Immunol. 2004;4(4):271-275. doi:10.1097/01.all.0000136758.66442.28
5. Cassimatis DC, Atwood JE, Engler RM, Linz PE, Grabenstein JD, Vernalis MN. Smallpox vaccination and myopericarditis: a clinical review. J Am Coll Cardiol. 2004;43(9):1503-1510. doi:10.1016/j.jacc.2003.11.053
6. Sharma U, Tak T. A report of 2 cases of myopericarditis after Vaccinia virus (smallpox) immunization. WMJ. 2011;110(6):291-294.
7. Sarkisian SA, Hand G, Rivera VM, Smith M, Miller JA. A case series of smallpox vaccination-associated myopericarditis: effects on safety and readiness of the active duty soldier. Mil Med. 2019;184(1-2):e280-e283. doi:10.1093/milmed/usy159
A renewed effort to vaccinate service members fighting the global war on terrorism has brought new diagnostic challenges. Vaccinations not generally given to the public are routinely given to service members when they deploy to various parts of the world. Examples include anthrax, yellow fever, Japanese encephalitis, rabies, polio, and smallpox. Every vaccination has potential for adverse effects (AEs), which can range from mild to severe life-threatening complications. These AEs often go unrecognized and untreated because physicians are not routinely screening for vaccination administration.
Background
Smallpox (Variola major) was successfully eradicated in 1977 due to worldwide vaccination efforts.1 However, the threat of bioterrorism has renewed mandatory smallpox vaccinations for high-risk individuals, such as active-duty military personnel.1,2 A notable increase in myopericarditis has been reported with the new generation of smallpox vaccination, ACAM2000.3 We present a case of a 27-year-old healthy male who presented with chest pain and diffuse ST segment elevations consistent with myopericarditis after vaccination with ACAM2000.
Case Presentation
A healthy 27-year-old soldier presented to the emergency department with sudden, new onset, sharp-stabbing, substernal chest pain, which was made worse with lying flat and better with leaning forward. Vital signs were unremarkable. He recently enlisted in the US Army and received the smallpox vaccination about 11 days before as part of a routine predeployment checklist. The patient reported he did not have any viral symptoms, such as fever, chills, nausea, vomiting, diarrhea, shortness of breath, sore throat, rhinorrhea, or sputum production. He also reported having no prior illness for the past 3 months, sick contacts at home or work, or recent travel outside the US. He reported no tobacco use, alcohol use, or illicit drug use. The patient’s family history was negative for significant cardiac disease.
A physical examination was unremarkable. The initial laboratory report showed no leukocytosis, anemia, thrombocytopenia, electrolytes derangement, abnormal kidney function, or abnormal liver function tests. Initial troponin was 0.25 ng/mL, erythrocyte sedimentation rate (ESR) was 40 mmol/h and C-reactive protein (CRP) was 120.2 mg/L suggestive of acute inflammation. A urine drug screen was negative. D-dimer was < 0.27. An electrocardiogram (ECG) showed diffuse ST segment elevation (Figure 1). An echocardiogram showed normal left ventricle size, and function with ejection fraction 55 to 60%, normal diastolic dysfunction, and trivial pericardial effusion. Magnetic resonance imaging (MRI) showed increased T2 signal intensity of the myocardium suggestive of myopericarditis (Figure 2). A computed tomography (CT) angiogram of the coronary arteries showed no significant stenosis.
The patient was treated with ibuprofen for 2 weeks and colchicine for 3 months, and his symptoms resolved. He followed up with an appointment in the cardiology clinic 1 month later, and his ESR, CRP, and troponin results were negative. A limited echocardiogram showed ejection fraction 60 to 65%, no regional wall motion abnormalities, normal diastolic function, and resolution of the pericardial effusion.
Discussion
Smallpox was a major worldwide cause of mortality; about 30% of those infected died because of smallpox.2,4,5 Due to a worldwide vaccination effort, the World Health Organization declared smallpox was eradicated in 1977.2,4,5 However, despite successful eradication, smallpox is considered a possible bioterrorism target, which prompted a resurgence of mandatory smallpox vaccinations for active-duty personnel.2,5
Dryvax, a freeze-dried calf lymph smallpox vaccine was used extensively from the 1940s to the 1980s but was replaced in 2008 by ACAM2000, a smallpox vaccine cultured in kidney epithelial cells from African green monkeys.3,5 Myopericarditis was rarely associated with the Dryvax, with only 5 cases reported from 1955 to 1986 after millions of doses of vaccines were administered; however, in 230,734 administered ACAM2000 doses, 18 cases of myopericarditis (incidence, 7.8 per 100,000) were reported during a surveillance study in 2002 and 2003.3,5
Myopericarditis presents with a wide variety of symptoms, such as chest pain, palpitations, chills, shortness of breath, and fever.6,7 Mainstay diagnostic criteria include ECG findings consistent with myopericarditis (such as diffuse ST segment elevations) and elevated cardiac biomarkers (elevated troponins).5-7 An echocardiogram can be helpful in diagnosis, as most cases will not have regional wall motion abnormalities (to distinguish against coronary artery disease).5-7 MRI with diffuse enhancement of the myocardium can be helpful in diagnosis.5,6 The gold standard for diagnosis is an endomyocardial biopsy, which carries a significant risk of complications and is not routinely performed to diagnose myopericarditis.5,6 US military smallpox vaccination data showed that the onset of vaccine-associated myopericarditis averaged (SD) 10.4 (3.6) days after vaccination.5
Vaccine-associated myopericarditis treatment is focused on decreasing inflammation.5,6 Nonsteroidal anti-inflammatory drugs are advised for about 2 weeks with cessation of intensive cardiac activities for between 4 and 6 weeks due to risks of congestive heart failure and fatal cardiac arrhythmias.5,6
Conclusions
Since the September 11 attacks, the US needs to be continually prepared for potential terrorism on American soil and abroad. The threat of bioterrorism has renewed efforts to vaccinate or revaccinate American service members deployed to high-risk regions. These vaccinations put them at risk for vaccination-induced complications that can range from mild fever to life-threatening complications.
A renewed effort to vaccinate service members fighting the global war on terrorism has brought new diagnostic challenges. Vaccinations not generally given to the public are routinely given to service members when they deploy to various parts of the world. Examples include anthrax, yellow fever, Japanese encephalitis, rabies, polio, and smallpox. Every vaccination has potential for adverse effects (AEs), which can range from mild to severe life-threatening complications. These AEs often go unrecognized and untreated because physicians are not routinely screening for vaccination administration.
Background
Smallpox (Variola major) was successfully eradicated in 1977 due to worldwide vaccination efforts.1 However, the threat of bioterrorism has renewed mandatory smallpox vaccinations for high-risk individuals, such as active-duty military personnel.1,2 A notable increase in myopericarditis has been reported with the new generation of smallpox vaccination, ACAM2000.3 We present a case of a 27-year-old healthy male who presented with chest pain and diffuse ST segment elevations consistent with myopericarditis after vaccination with ACAM2000.
Case Presentation
A healthy 27-year-old soldier presented to the emergency department with sudden, new onset, sharp-stabbing, substernal chest pain, which was made worse with lying flat and better with leaning forward. Vital signs were unremarkable. He recently enlisted in the US Army and received the smallpox vaccination about 11 days before as part of a routine predeployment checklist. The patient reported he did not have any viral symptoms, such as fever, chills, nausea, vomiting, diarrhea, shortness of breath, sore throat, rhinorrhea, or sputum production. He also reported having no prior illness for the past 3 months, sick contacts at home or work, or recent travel outside the US. He reported no tobacco use, alcohol use, or illicit drug use. The patient’s family history was negative for significant cardiac disease.
A physical examination was unremarkable. The initial laboratory report showed no leukocytosis, anemia, thrombocytopenia, electrolytes derangement, abnormal kidney function, or abnormal liver function tests. Initial troponin was 0.25 ng/mL, erythrocyte sedimentation rate (ESR) was 40 mmol/h and C-reactive protein (CRP) was 120.2 mg/L suggestive of acute inflammation. A urine drug screen was negative. D-dimer was < 0.27. An electrocardiogram (ECG) showed diffuse ST segment elevation (Figure 1). An echocardiogram showed normal left ventricle size, and function with ejection fraction 55 to 60%, normal diastolic dysfunction, and trivial pericardial effusion. Magnetic resonance imaging (MRI) showed increased T2 signal intensity of the myocardium suggestive of myopericarditis (Figure 2). A computed tomography (CT) angiogram of the coronary arteries showed no significant stenosis.
The patient was treated with ibuprofen for 2 weeks and colchicine for 3 months, and his symptoms resolved. He followed up with an appointment in the cardiology clinic 1 month later, and his ESR, CRP, and troponin results were negative. A limited echocardiogram showed ejection fraction 60 to 65%, no regional wall motion abnormalities, normal diastolic function, and resolution of the pericardial effusion.
Discussion
Smallpox was a major worldwide cause of mortality; about 30% of those infected died because of smallpox.2,4,5 Due to a worldwide vaccination effort, the World Health Organization declared smallpox was eradicated in 1977.2,4,5 However, despite successful eradication, smallpox is considered a possible bioterrorism target, which prompted a resurgence of mandatory smallpox vaccinations for active-duty personnel.2,5
Dryvax, a freeze-dried calf lymph smallpox vaccine was used extensively from the 1940s to the 1980s but was replaced in 2008 by ACAM2000, a smallpox vaccine cultured in kidney epithelial cells from African green monkeys.3,5 Myopericarditis was rarely associated with the Dryvax, with only 5 cases reported from 1955 to 1986 after millions of doses of vaccines were administered; however, in 230,734 administered ACAM2000 doses, 18 cases of myopericarditis (incidence, 7.8 per 100,000) were reported during a surveillance study in 2002 and 2003.3,5
Myopericarditis presents with a wide variety of symptoms, such as chest pain, palpitations, chills, shortness of breath, and fever.6,7 Mainstay diagnostic criteria include ECG findings consistent with myopericarditis (such as diffuse ST segment elevations) and elevated cardiac biomarkers (elevated troponins).5-7 An echocardiogram can be helpful in diagnosis, as most cases will not have regional wall motion abnormalities (to distinguish against coronary artery disease).5-7 MRI with diffuse enhancement of the myocardium can be helpful in diagnosis.5,6 The gold standard for diagnosis is an endomyocardial biopsy, which carries a significant risk of complications and is not routinely performed to diagnose myopericarditis.5,6 US military smallpox vaccination data showed that the onset of vaccine-associated myopericarditis averaged (SD) 10.4 (3.6) days after vaccination.5
Vaccine-associated myopericarditis treatment is focused on decreasing inflammation.5,6 Nonsteroidal anti-inflammatory drugs are advised for about 2 weeks with cessation of intensive cardiac activities for between 4 and 6 weeks due to risks of congestive heart failure and fatal cardiac arrhythmias.5,6
Conclusions
Since the September 11 attacks, the US needs to be continually prepared for potential terrorism on American soil and abroad. The threat of bioterrorism has renewed efforts to vaccinate or revaccinate American service members deployed to high-risk regions. These vaccinations put them at risk for vaccination-induced complications that can range from mild fever to life-threatening complications.
1. Bruner DI, Butler BS. Smallpox vaccination-associated myopericarditis is more common with the newest smallpox vaccine. J Emerg Med. 2014;46(3):e85-e87. doi:10.1016/j.jemermed.2013.06.001
2. Halsell JS, Riddle JR, Atwood JE, et al. Myopericarditis following smallpox vaccination among vaccinia-naive US military personnel. JAMA. 2003;289(24):3283-3289. doi:10.1001/jama.289.24.3283
3. Nalca A, Zumbrun EE. ACAM2000: the new smallpox vaccine for United States Strategic National Stockpile. Drug Des Devel Ther. 2010;4:71-79. doi:10.2147/dddt.s3687
4. Wollenberg A, Engler R. Smallpox, vaccination and adverse reactions to smallpox vaccine. Curr Opin Allergy Clin Immunol. 2004;4(4):271-275. doi:10.1097/01.all.0000136758.66442.28
5. Cassimatis DC, Atwood JE, Engler RM, Linz PE, Grabenstein JD, Vernalis MN. Smallpox vaccination and myopericarditis: a clinical review. J Am Coll Cardiol. 2004;43(9):1503-1510. doi:10.1016/j.jacc.2003.11.053
6. Sharma U, Tak T. A report of 2 cases of myopericarditis after Vaccinia virus (smallpox) immunization. WMJ. 2011;110(6):291-294.
7. Sarkisian SA, Hand G, Rivera VM, Smith M, Miller JA. A case series of smallpox vaccination-associated myopericarditis: effects on safety and readiness of the active duty soldier. Mil Med. 2019;184(1-2):e280-e283. doi:10.1093/milmed/usy159
1. Bruner DI, Butler BS. Smallpox vaccination-associated myopericarditis is more common with the newest smallpox vaccine. J Emerg Med. 2014;46(3):e85-e87. doi:10.1016/j.jemermed.2013.06.001
2. Halsell JS, Riddle JR, Atwood JE, et al. Myopericarditis following smallpox vaccination among vaccinia-naive US military personnel. JAMA. 2003;289(24):3283-3289. doi:10.1001/jama.289.24.3283
3. Nalca A, Zumbrun EE. ACAM2000: the new smallpox vaccine for United States Strategic National Stockpile. Drug Des Devel Ther. 2010;4:71-79. doi:10.2147/dddt.s3687
4. Wollenberg A, Engler R. Smallpox, vaccination and adverse reactions to smallpox vaccine. Curr Opin Allergy Clin Immunol. 2004;4(4):271-275. doi:10.1097/01.all.0000136758.66442.28
5. Cassimatis DC, Atwood JE, Engler RM, Linz PE, Grabenstein JD, Vernalis MN. Smallpox vaccination and myopericarditis: a clinical review. J Am Coll Cardiol. 2004;43(9):1503-1510. doi:10.1016/j.jacc.2003.11.053
6. Sharma U, Tak T. A report of 2 cases of myopericarditis after Vaccinia virus (smallpox) immunization. WMJ. 2011;110(6):291-294.
7. Sarkisian SA, Hand G, Rivera VM, Smith M, Miller JA. A case series of smallpox vaccination-associated myopericarditis: effects on safety and readiness of the active duty soldier. Mil Med. 2019;184(1-2):e280-e283. doi:10.1093/milmed/usy159
Mild TBI/Concussion Clinical Tools for Providers Used Within the Department of Defense and Defense Health Agency
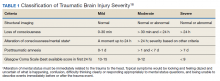
Traumatic brain injury (TBI) is a major health concern that can cause significant disability as well as economic and social burden. The Centers for Disease Control and Prevention (CDC) reported a 58% increase in the number of TBI-related emergency department visits, hospitalizations, and deaths from 2006 to 2014.1 In the CDC report, falls and motor vehicle accidents accounted for 52.3% and 20.4%, respectively, of all civilian TBI-related hospitalizations. In 2014, 56,800 TBIs in the US resulted in death. A large proportion of severe TBI survivors continue to experience long-term physical, cognitive, and psychologic disorders and require extensive rehabilitation, which may disrupt relationships and prevent return to work.2 About 37% of people with mild TBI (mTBI) cases and 51% of severe cases were unable to return to previous jobs. A study examining psychosocial burden found that people with a history of TBI reported greater feelings of loneliness compared with individuals without TBI.3
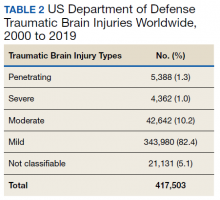
Within the US military, the Defense and Veterans Brain Injury Center (DVBIC) indicates that > 417,503 service members (SMs) have been diagnosed with TBI since November 2000.4 Of these, 82.4% were classified as having a mTBI, or concussion (Tables 1 and 2). The nature of combat and military training to which SMs are routinely exposed may increase the risk for sustaining a TBI. Specifically, the increased use of improvised explosives devices by enemy combatants in the recent military conflicts (ie, Operation Enduring Freedom, Operation Iraqi Freedom and Operation New Dawn) resulted in TBI being recognized as the signature injury of these conflicts and brought attention to the prevalence of concussion within the US military.5,6 In the military, the effects of concussion can decrease individual and unit effectiveness, emphasizing the importance of prompt diagnosis and proper management.7
Typically, patients recover from concussion within a few weeks of injury; however, some individuals experience symptoms that persist for months or years. Studies found that early intervention after concussion may aid in expediting recovery, stressing the importance of identifying concussion as promptly as possible.8,9 Active treatment is centered on patient education and symptom management, in addition to a progressive return to activities, as tolerated. Patient education may help validate the symptoms of some patients, as well as help to reattribute the symptoms to benign causes, leading to better outcomes.10 Since TBI is such a relevant health concern within the DoD, it is paramount for practitioners to understand what resources are available in order to identify and initiate treatment expeditiously.
This article focuses on the clinical tools used in evaluating and treating concussion, and best practices treatment guidelines for health care providers (HCPs) who are required to evaluate and treat military populations. While these resources are used for military SMs, they can also be used in veteran and civilian populations. This article showcases 3 DoD clinical tools that assist HCPs in evaluating and treating patients with TBI: (1) the Military Acute Concussion Evaluation 2 (MACE 2); (2) the Progressive Return to Activity (PRA) Clinical Recommendation (CR); and (3) the Concussion Management Tool (CMT). Additional DoD clinical tools and resources are discussed, and resources and links for the practitioner are provided for easy access and reference.
Military Acute Concussion Evaluation 2
Early concussion identification and evaluation are important steps in the treatment process to ensure timely recovery and return to duty for SMs. As such, DVBIC assembled a working group of military and civilian brain injury experts to create an evidence-based clinical practice guideline for the assessment and management of concussion in a military operational setting that could be learned and effectively used by corpsmen and combat medics in the battlefield to screen for a possible concussion.7 This team created the first version of the MACE, a clinical tool that prompted a systematic assessment of concussion related symptoms, neurologic signs, and cognitive deficits. The cognitive assessment portion was based on the standardized assessment of concussion (SAC) that had been reported by McCrea and colleagues in 1998.11 Soon after its creation, field utilization of the MACE for screening of concussion was mandated by the Army through an All Army Action (ALARACT 178/2008) and for all of the Services through the DoD Instruction (DoDI) 6490.11 published in 2014.12
The MACE has been updated several times since the original version. Most recently, the MACE was revised in 2018 to include a vestibular oculomotor assessment section, and red flags that immediately alert the HCP to the need for immediate triage referral and treatment of the patient possibly at a higher echelon of care or with more emergent evaluation.13-15 Additionally, the neurologic examination was expanded to increase clarity and comprehensiveness, including speech and balance testing. Updates made to the tool were intended to provide a more thorough and informative evaluation of the SM with suspected concussion.
This latest version, MACE 2, is designed to be used by any HCP who is treating SMs with a suspected or potential TBI, not just corpsmen and combat medics in theater. The MACE 2 is a comprehensive evaluation within a set of portable pocket cards designed to assist end-users in the proper triage of potentially concussed individuals. The DoD has specified 4 events that require a MACE 2 evaluation: (1) SM was in a vehicle associated with a blast event, collision, or roll over; (2) SM was within 50 meters of a blast; (3) anyone who sustained a direct blow to the head; or (4) when command provides direction (eg, repeated exposures to the events above or in accordance with protocols).12 Sleep deprivation, medications, and pain may affect MACE 2 results, in addition to deployment related stress, chronic stress, high adrenaline sustained over time, and additional comorbidities. This tool is most effective when used as close to the time of injury as possible but also may be used later (after 24 hours of rest) to reevaluate symptoms. The MACE 2 Instructor Guide, a student workbook, HCP training, and Vestibular/Ocular-Motor Screening (VOMS) for Concussion instructions can be found on the DVBIC website (Table 3).
Description
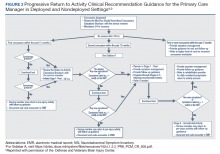
The MACE 2 is a brief multimodal screening tool that assists medics, corpsman, and primary care managers (PCMs) in the assessment and identification of a potential concussion (Figure 1). Embedded in the MACE 2 is the Standardized Assessment of Concussion (SAC), a well-validated sports concussion tool, and the VOMS tool as portions of the 2-part cognitive examination. The entirety of the tool has 5 sections: (1) red flags; (2) acute concussion screening; (3) cognitive examination, part 1; (4) neurologic examination; and (5) cognitive examination, part 2. The end of the MACE 2 includes sections on the scoring, instructions for International Classification of Diseases, Tenth Revision, TBI coding, and next steps following completion of the MACE 2. The latest version of this screening tool impacts TBI care in several noteworthy ways. First, it broadens the scope of users by expanding use to all medically trained personnel, allowing any provider to treat SMs in the field. Second, it combines state-of-the-science advances from the research field and reflects feedback from end-users collected during the development. Last, the MACE 2 is updated as changes in the field occur, and is currently undergoing research to better identify end-user utility and usability.
Screening Tools
• Red Flags. The red flags section aids in identifying potentially serious underlying conditions in patients presenting with Glasgow Coma Scale (GCS) between 13 and 15. A positive red flag prompts the practitioner to stop administering the MACE 2 and immediately consult a higher level of care and consider urgent evacuation. While the red flags are completed first, and advancement to later sections of the MACE 2 is dependent upon the absence of red flags, the red flags should be monitored throughout the completion of the MACE 2. Upon completion of patient demographics and red flags, the remaining sections of the MACE 2 are dedicated to acute concussion screening.
• Acute Concussion Screening. The acute concussion screening portion consists of 4 sections: description of the incident; alteration of consciousness or memory; a “check all that apply” symptom inventory; and a patient history that includes concussions within the past 12 months, headache disorders, and/or behavioral health concerns. The final portion of the acute concussion screening section provides an algorithm to identify a positive or negative concussion screen. When a negative screen is identified, the user is prompted to prescribe a 24-hour rest period and follow up with the SM based on the guidance in the CMT. A positive screen warrants the user to continue administration of the MACE 2.
Neurologic and CognitiveExaminations
• Cognitive Exam Part 1. The initial cognitive examination is designed to assess orientation to time (eg, What is the day of the week, day of the month, the month, the year, and the timeof day?) as well as immediate recall of a short list of concrete words (5 words total, repeated for 3 trials). These tests are based on other neuropsychological measures designed to assess cognitive/mental status and short-term memory.
• The Neurological Exam. The neurological exam section of the MACE 2 includes brief neuropsychologic tests such as speech fluency and word finding. Other sections within the neurological exam assess the
following: grip strength, vestibular function/balance (eg, tandem gait and single leg stance), as well as motor function (eg, pronator drift), autonomic nervous system function (eg, pupil response), and vestibular function (eye-tracking).
• Cognitive Exam Part 2. After completion of the first cognitive examination and the neurologic examination, the second part of the cognitive examination is initiated. Part 2 includes measures of short-term and working memory (eg, digits-reverse tasks, listing the months in reverse order, and a delayed recall task of the short list of concrete words presented in the first part). The final assessment is the administration of the VOMS, a tool developed from the sports concussion field and designed to measure vestibular-ocular function.13 It is critical to note that the VOMS is contraindicated if there is concern of an unstable cervical spine or absence of a trained HCP. An examination summary provides guidance on test scoring and yields a positive or negative indication for concussive injury. A positive test refers users to guidelines listed in the Concussion Management Tool for recommendations. The final page provides coding instructions for entering the results into the patient’s electronic medical record for documentation and future reference.
Progressive Return To Activities Clinical Recommendation
The Progressive Return to Activities Clinical Recommendation (PRA CR) also was developed by DVBIC for the DoD to assist military HCPs in managing SMs with concussion by providing systematic and evidence-based guidance to both prevent extended rest and promote return to full duty as quickly and safely as clinically indicated. The general guidance is to monitor the SM at each of the 6 stages in the process and safely and gradually increase activity to the next stage as tolerated. Daily symptoms are measured using the Neurobehavioral Symptom Inventory (NSI), which SMs self-administer every morning at each stage within the process.
Prior to initiation of the progressive return to activity, SM education using the educational brochure is strongly encouraged, as previous evidence suggests that it is an effective intervention during the acute stages of injury.10,11 Return to activity follows a 6 stage process, from stage 1 (rest) through stage 6 (unrestricted activity) (Table 4). Referral to rehabilitation providers (RPs) or higher care is left to the discretion of the PCM when (1) recovery is not progressing as anticipated; (2) progression is not being made within a 7-day period; or (3) symptoms worsen with time. The guidance outlined in the PRA CR is consistent with current policies and medical literature, and undergoes reviews as updates in the field emerge. The PRA for PCM, PRA for RP, Clinical Support Tool for PCM, Clinical Support Tool for RP, Training Slides for PCM, Training Slides for RP, Educational Brochure for PCM, and Patient Educational Tool for RP can be found on the DVBIC website (dvbic.dcoe.mil).
Description
To improve the clinical utility, 2 separate PRA CRs were developed specifically for PCMs (Figure 2) and RPs (Figure 3). The PRA CR for PCMs provides the initial framework to monitor SMs during recovery and gradually increase physical, cognitive, and vestibular/balance activities as symptoms improve in order to return to preinjury activities. The PRA CR for RPs outlines the approach for treating SMs who meet 1 of the following criteria: recovery is not progressing as anticipated, there is no progression in 7 days, symptoms are worsening, the SM is symptomatic after exertional testing following stage 5, or referral made per PCM judgment. Following the mandatory 24-hour rest period after a diagnosis of a concussion, progression through the PRA algorithm is based on history of concussion within the past 12 months (ie, 1, 2, or ≥ 3 concussions) and symptomatology, with varying treatment pathways depending on the SM’s responses to history and symptomology.
Guidelines
• One Concussion within Past 12 Months. Following the mandatory 24-hour rest period, if the SM is asymptomatic, then exertional testing (eg, activities such as push-ups, sit-ups, running in place, step aerobics, stationary bike, treadmill and/or hand crank) is performed at 65 to 85% of target heart rate for 2 minutes and symptoms are reassessed. If still asymptomatic, the SM may return to preinjury activity; however, if exertional testing provokes symptoms > 1 (mild) on the NSI, the SM should return to stage 1 with an additional 24 hours of rest. A second exertional test can be performed after stage 1, and if symptoms are provoked, progression through the remaining stages 2 to 5 is encouraged. Symptoms are continually monitored throughout each stage to determine whether the SM is recovered sufficiently to proceed to the next stage.
• Two Concussion Within Past 12 Months. Following the mandatory 24-hour rest period, no exertional testing is performed, and SMs move directly into stage 1 and remain at stage 1 or stage 2 for 7 consecutive days with no symptoms > 1 on the NSI before advancing through the remaining stages. Some defining features are longer rest periods (eg, 5 additional days of rest at stage 2) and additional patient education, symptom management, and follow-up.
• Three or more Concussions Within Past 12 Months. Following the 24 hour mandatory rest period, in cases where ≥ 3 concussions have occurred within a 12 month period, the recommendation is to provide guidance for symptom management rest and refer the SM to a higher level of care.
Concussion Management Tool
Beyond the initial assessment and concussion evaluation and the promotion of SMs’ timely return to duty, the DoD developed a tool to help endpoint users manage concussion, to include those with more protracted symptoms (Figure 4). The CMT assists HCPs and the SMs they treat in the management of symptoms before and after they return to duty. Specifically, the CMT is designed to be given in combination with guidelines issued by the DoD in the PRA CR but extends management of concussion to include those symptoms experienced more long-term, or symptoms that are not solely addressed during the timeline of the PRA CR. Together, the MACE 2, PRA CR, and the CMT provide endpoint users with a set of tools to comprehensively evaluate, treat, and manage concussions in SMs.
Description
The CMT provides step-by-step guidance for the initial and comprehensive management of concussion, once a diagnosis is made using assessments in the MACE 2. All types of HCPs, particularly those with limited training, such as Navy Hospital Corpsman and Army Combat Medics, are the intended clinical audience for the CMT. This tool was revised in 2019 to better align with the MACE 2, PRA CR, and other DVBIC CRs, and replaces the 2012 Concussion Management Algorithm and the 2014 Army Concussion Management in Garrison Setting Algorithm. The first 2 sections of the CMT are action cards, which provide management guidelines for acute injuries up to 7 days following injury and for comprehensive management beyond 1 week. Guidelines within the CMT partially overlap with those in the PRA CR; however, the PRA is designed for a more acute timeline, whereas the CMT focuses on symptom management following a more protracted recovery. The CMT clinical tool, provider training, instructor guide, and student workbook all can be found on the DVBIC website (Table 3).
Discussion
It is important for HCPs to have the skills and clinically relevant tools to optimize accurate TBI assessment. Early and accurate assessment and effective symptom management allows SMs to receive timely treatment based on clinical recommendations, and prevent and/or minimize secondary injury and prolonged recovery. Several longitudinal studies emphasize the benefits of early diagnosis and systematic follow-up.16-18 Prompt diagnosis, patient education, and early initiation to treatment may help optimize triage to care, mitigate prolonged symptoms by educating the patient on what to expect, and target specific symptoms early.8,10 Beyond the health outcomes of an individual SM, TBI recovery impacts unit readiness and consequently force readiness. As such, health outcomes and medical readiness are a priority of the Defense Health Agency (DHA).
The DHA priorities are, in part, based on DoD policy guidance for the management of concussion in the deployed setting. According to DoD instruction, “Medically documented mTBI/concussion in service members shall be clinically evaluated, treated, and managed according to the most current DoD clinical practice guidance for the deployed environment found in the Defense and Veterans Brain Injury Center (DVBIC) guidance, ‘Medical Providers: Clinical Tools.’”12 In 2018, the Deputy Secretary of Defense issued a memorandum regarding the comprehensive strategy and action plan for warfighter brain health.12 Therein, the memorandum acknowledges the enduring responsibility of the DoD to promote and protect the health and well-being of members of the nation’s armed forces. Particular emphasis was placed on issuing a response to the effects caused by concussive impacts and exposure to blast waves. This response resulted in a commitment by the DoD to understanding, preventing, diagnosing, and treating TBI in all forms. Taken together, the message from the secretary of defense and instruction from the DoD is clear and makes imperative the use of DoD clinical tools to accomplish this commitment.
Conclusion
This article showcases 3 of the DoD’s TBI clinical tools (MACE 2, PRA CR, and CMT) that assist HCPs in identifying and treating concussion. Over time, these tools undergo revisions according to the state of the science, and are adapted to meet the needs of clinicians and the SMs they treat. Studies are currently ongoing to better understand the effectiveness of these tools as well as to assist clinicians in making return-to-duty and/or medical separation decisions. These tools assist clinicians throughout the recovery process; from initial assessment and treatment (acute phase), as well as with symptom management (acute and protracted symptoms).
Concussion is not a homogenous condition and the experiences of the SM, including events that may cause emotional distress, other injuries and/or other factors, may further complicate the injury. Accordingly, there is no single clinical tool that can conclusively determine return-to-duty status; rather, these tools can help characterize injury, validate, and treat symptoms, which have been suggested to improve outcomes. More research and data are needed confirm the effectiveness of these tools to improve outcomes.
It is beyond the scope of this article to provide a more in-depth discussion on TBI prevention or longer term effects/care. However, there are additional, personalized tools for specific symptoms, deficits, or dysfunctions following concussion. These tools include the Management of Headache Following mTBI for PCM CR, Management of Sleep Disturbances Following mTBI for PCM CR, Assessment and Management of Visual Dysfunction Associated with mTBI CR, and Assessment and Management of Dizziness Associated mTBI CR. These tools enable endpoint users to evaluate and treat SMs as well as know when to elevate to higher levels of care.
The DoD commitment toward treating TBI influenced the development of the clinical tools highlighted in this article. They are the result of collective efforts among military and civilian TBI subject matter experts, data from medical literature and state-of-the-science research, and feedback from endpoint users to create the most effective, evidence-based tools. These tools undergo continuous review and revision to ensure alignment with the most up-to-date science within the field, to meet the needs of SMs and to continue the commitment to DoD concussion care.
Acknowledgments
This work was prepared under Contract (HT0014-19-C-0004) General Dynamics Information Technology and (W81XWH-16-F-0330) Credence Management Solutions, and is defined as U.S. Government work under Title 17 U.S.C.§101. Per Title 17 U.S.C.§105, copyright protection is not available for any work of the U.S. Government. For more information, please contact [email protected].
1. Centers for Disease Control and Prevention. Surveillance report of traumatic brain injury-related emergency department visits, hospitalizations, and deaths. https://www.cdc.gov/traumaticbraininjury/pdf/TBI-Surveillance-Report-FINAL_508.pdf. Published 2014. Accessed August 18, 2020.
2. Stocchetti N, Zanier ER. Chronic impact of traumatic brain injury on outcome and quality of life: a narrative review. Crit Care. 2016;20(1):148. Published 2016 Jun 21. doi:10.1186/s13054-016-1318-1
3. Kumar RG, Ornstein KA, Bollens-Lund E, et al. Lifetime history of traumatic brain injury is associated with increased loneliness in adults: A US nationally representative study. Int J Geriatr Psychiatry. 2020;35(5):553-563. doi:10.1002/gps.5271
4. Defense and Veterans Brain Injury Center. Worldwide DoD numbers for traumatic brain injury. 2020; https://dvbic.dcoe.mil/sites/default/files/tbi-numbers/DVBIC_WorldwideTotal_2000-2019.pdf. Updated March 10, 2020. Accessed August 18, 2020.
5. Kennedy JE, Lu LH, Reid MW, Leal FO, Cooper DB. Correlates of depression in U.S. military service members with a history of mild traumatic brain injury. Mil Med. 2019;184(suppl 1):148-154. doi:10.1093/milmed/usy321
6. Marshall KR, Holland SL, Meyer KS, Martin EM, Wilmore M, Grimes JB. Mild traumatic brain injury screening, diagnosis, and treatment. Mil Med. 2012;177(suppl 8):67-75. doi:10.7205/milmed-d-12-00110
7. French L, McCrea M., Baggett M. The Military Acute Concussion Evaluation. J Spec Oper Med. 2008;8(1):68-77. https://www.jsomonline.org/Publications/2008168French.pdf. Accessed August 18, 2020.
8. Kontos AP, Jorgensen-Wagers K, Trbovich AM, et al. Association of time since injury to the first clinic visit with recovery following concussion. JAMA Neurol. 2020;77(4):435-440. doi:10.1001/jamaneurol.2019.4552
9. Ponsford J, Willmott C, Rothwell A, et al. Impact of early intervention on outcome following mild head injury in adults. J Neurol Neurosurg Psychiatry. 2002;73(3):330-332. doi:10.1136/jnnp.73.3.33010.
10. Mittenberg W, Canyock EM, Condit D, Patton C. Treatment of post-concussion syndrome following mild head injury. J Clin Exp Neuropsychol. 2001;23(6):829-836. doi:10.1076/jcen.23.6.829.1022
11. McCrea M, Kelly JP, Randolph C, et al. Standardized assessment of concussion (SAC): on-site mental status evaluation of the athlete. J Head Trauma Rehabil. 1998;13(2):27-35. doi:10.1097/00001199-199804000-00005
12. US Department of Defense. Department of Defense Instruction, Number 6490.11. Policy guidance for management of mild traumatic brain injury/concussion in the deployed setting. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/649011p.pdf. Updated November 26, 2019. Accessed August 18, 2020.
13. Mucha A, Collins MW, Elbin RJ, et al. A brief Vestibular/Ocular Motor Screening (VOMS) assessment to evaluate concussions: preliminary findings. Am J Sports Med. 2014;42(10):2479-2486. doi:10.1177/0363546514543775
14. Defense and Veterans Brain Injury Center. Military Acute Concussion Evaluation 2 (MACE 2). https://dvbic.dcoe.mil/material/military-acute-concussion-evaluation-2-mace-2. Updated August 18, 2020. Accessed August 18, 2020.
15. US Department of Defense, Defense Health Agency. Defense and Veterans Brain Injury Center releases new concussion screening tool. https://www.health.mil/News/Articles/2019/03/15/Defense-and-Veterans-Brain-Injury-Center-releases-new-concussion-screening-tool. Published March 15, 2019. Accessed August 18, 2020.
16. Schwab K, Terrio HP, Brenner LA, et al. Epidemiology and prognosis of mild traumatic brain injury in returning soldiers: a cohort study. Neurology. 2017;88(16):1571-1579. doi:10.1212/WNL.0000000000003839
17. Mac Donald CL, Johnson AM, Wierzechowski L, et al. Outcome trends after US military concussive traumatic brain injury. J Neurotrauma. 2017;34(14):2206-2219. doi:10.1089/neu.2016.4434
18. Andelic N, Howe EI, Hellstrøm T, et al. Disability and quality of life 20 years after traumatic brain injury. Brain Behav. 2018;8(7):e01018. doi:10.1002/brb3.1018
Traumatic brain injury (TBI) is a major health concern that can cause significant disability as well as economic and social burden. The Centers for Disease Control and Prevention (CDC) reported a 58% increase in the number of TBI-related emergency department visits, hospitalizations, and deaths from 2006 to 2014.1 In the CDC report, falls and motor vehicle accidents accounted for 52.3% and 20.4%, respectively, of all civilian TBI-related hospitalizations. In 2014, 56,800 TBIs in the US resulted in death. A large proportion of severe TBI survivors continue to experience long-term physical, cognitive, and psychologic disorders and require extensive rehabilitation, which may disrupt relationships and prevent return to work.2 About 37% of people with mild TBI (mTBI) cases and 51% of severe cases were unable to return to previous jobs. A study examining psychosocial burden found that people with a history of TBI reported greater feelings of loneliness compared with individuals without TBI.3
Within the US military, the Defense and Veterans Brain Injury Center (DVBIC) indicates that > 417,503 service members (SMs) have been diagnosed with TBI since November 2000.4 Of these, 82.4% were classified as having a mTBI, or concussion (Tables 1 and 2). The nature of combat and military training to which SMs are routinely exposed may increase the risk for sustaining a TBI. Specifically, the increased use of improvised explosives devices by enemy combatants in the recent military conflicts (ie, Operation Enduring Freedom, Operation Iraqi Freedom and Operation New Dawn) resulted in TBI being recognized as the signature injury of these conflicts and brought attention to the prevalence of concussion within the US military.5,6 In the military, the effects of concussion can decrease individual and unit effectiveness, emphasizing the importance of prompt diagnosis and proper management.7
Typically, patients recover from concussion within a few weeks of injury; however, some individuals experience symptoms that persist for months or years. Studies found that early intervention after concussion may aid in expediting recovery, stressing the importance of identifying concussion as promptly as possible.8,9 Active treatment is centered on patient education and symptom management, in addition to a progressive return to activities, as tolerated. Patient education may help validate the symptoms of some patients, as well as help to reattribute the symptoms to benign causes, leading to better outcomes.10 Since TBI is such a relevant health concern within the DoD, it is paramount for practitioners to understand what resources are available in order to identify and initiate treatment expeditiously.
This article focuses on the clinical tools used in evaluating and treating concussion, and best practices treatment guidelines for health care providers (HCPs) who are required to evaluate and treat military populations. While these resources are used for military SMs, they can also be used in veteran and civilian populations. This article showcases 3 DoD clinical tools that assist HCPs in evaluating and treating patients with TBI: (1) the Military Acute Concussion Evaluation 2 (MACE 2); (2) the Progressive Return to Activity (PRA) Clinical Recommendation (CR); and (3) the Concussion Management Tool (CMT). Additional DoD clinical tools and resources are discussed, and resources and links for the practitioner are provided for easy access and reference.
Military Acute Concussion Evaluation 2
Early concussion identification and evaluation are important steps in the treatment process to ensure timely recovery and return to duty for SMs. As such, DVBIC assembled a working group of military and civilian brain injury experts to create an evidence-based clinical practice guideline for the assessment and management of concussion in a military operational setting that could be learned and effectively used by corpsmen and combat medics in the battlefield to screen for a possible concussion.7 This team created the first version of the MACE, a clinical tool that prompted a systematic assessment of concussion related symptoms, neurologic signs, and cognitive deficits. The cognitive assessment portion was based on the standardized assessment of concussion (SAC) that had been reported by McCrea and colleagues in 1998.11 Soon after its creation, field utilization of the MACE for screening of concussion was mandated by the Army through an All Army Action (ALARACT 178/2008) and for all of the Services through the DoD Instruction (DoDI) 6490.11 published in 2014.12
The MACE has been updated several times since the original version. Most recently, the MACE was revised in 2018 to include a vestibular oculomotor assessment section, and red flags that immediately alert the HCP to the need for immediate triage referral and treatment of the patient possibly at a higher echelon of care or with more emergent evaluation.13-15 Additionally, the neurologic examination was expanded to increase clarity and comprehensiveness, including speech and balance testing. Updates made to the tool were intended to provide a more thorough and informative evaluation of the SM with suspected concussion.
This latest version, MACE 2, is designed to be used by any HCP who is treating SMs with a suspected or potential TBI, not just corpsmen and combat medics in theater. The MACE 2 is a comprehensive evaluation within a set of portable pocket cards designed to assist end-users in the proper triage of potentially concussed individuals. The DoD has specified 4 events that require a MACE 2 evaluation: (1) SM was in a vehicle associated with a blast event, collision, or roll over; (2) SM was within 50 meters of a blast; (3) anyone who sustained a direct blow to the head; or (4) when command provides direction (eg, repeated exposures to the events above or in accordance with protocols).12 Sleep deprivation, medications, and pain may affect MACE 2 results, in addition to deployment related stress, chronic stress, high adrenaline sustained over time, and additional comorbidities. This tool is most effective when used as close to the time of injury as possible but also may be used later (after 24 hours of rest) to reevaluate symptoms. The MACE 2 Instructor Guide, a student workbook, HCP training, and Vestibular/Ocular-Motor Screening (VOMS) for Concussion instructions can be found on the DVBIC website (Table 3).
Description
The MACE 2 is a brief multimodal screening tool that assists medics, corpsman, and primary care managers (PCMs) in the assessment and identification of a potential concussion (Figure 1). Embedded in the MACE 2 is the Standardized Assessment of Concussion (SAC), a well-validated sports concussion tool, and the VOMS tool as portions of the 2-part cognitive examination. The entirety of the tool has 5 sections: (1) red flags; (2) acute concussion screening; (3) cognitive examination, part 1; (4) neurologic examination; and (5) cognitive examination, part 2. The end of the MACE 2 includes sections on the scoring, instructions for International Classification of Diseases, Tenth Revision, TBI coding, and next steps following completion of the MACE 2. The latest version of this screening tool impacts TBI care in several noteworthy ways. First, it broadens the scope of users by expanding use to all medically trained personnel, allowing any provider to treat SMs in the field. Second, it combines state-of-the-science advances from the research field and reflects feedback from end-users collected during the development. Last, the MACE 2 is updated as changes in the field occur, and is currently undergoing research to better identify end-user utility and usability.
Screening Tools
• Red Flags. The red flags section aids in identifying potentially serious underlying conditions in patients presenting with Glasgow Coma Scale (GCS) between 13 and 15. A positive red flag prompts the practitioner to stop administering the MACE 2 and immediately consult a higher level of care and consider urgent evacuation. While the red flags are completed first, and advancement to later sections of the MACE 2 is dependent upon the absence of red flags, the red flags should be monitored throughout the completion of the MACE 2. Upon completion of patient demographics and red flags, the remaining sections of the MACE 2 are dedicated to acute concussion screening.
• Acute Concussion Screening. The acute concussion screening portion consists of 4 sections: description of the incident; alteration of consciousness or memory; a “check all that apply” symptom inventory; and a patient history that includes concussions within the past 12 months, headache disorders, and/or behavioral health concerns. The final portion of the acute concussion screening section provides an algorithm to identify a positive or negative concussion screen. When a negative screen is identified, the user is prompted to prescribe a 24-hour rest period and follow up with the SM based on the guidance in the CMT. A positive screen warrants the user to continue administration of the MACE 2.
Neurologic and CognitiveExaminations
• Cognitive Exam Part 1. The initial cognitive examination is designed to assess orientation to time (eg, What is the day of the week, day of the month, the month, the year, and the timeof day?) as well as immediate recall of a short list of concrete words (5 words total, repeated for 3 trials). These tests are based on other neuropsychological measures designed to assess cognitive/mental status and short-term memory.
• The Neurological Exam. The neurological exam section of the MACE 2 includes brief neuropsychologic tests such as speech fluency and word finding. Other sections within the neurological exam assess the
following: grip strength, vestibular function/balance (eg, tandem gait and single leg stance), as well as motor function (eg, pronator drift), autonomic nervous system function (eg, pupil response), and vestibular function (eye-tracking).
• Cognitive Exam Part 2. After completion of the first cognitive examination and the neurologic examination, the second part of the cognitive examination is initiated. Part 2 includes measures of short-term and working memory (eg, digits-reverse tasks, listing the months in reverse order, and a delayed recall task of the short list of concrete words presented in the first part). The final assessment is the administration of the VOMS, a tool developed from the sports concussion field and designed to measure vestibular-ocular function.13 It is critical to note that the VOMS is contraindicated if there is concern of an unstable cervical spine or absence of a trained HCP. An examination summary provides guidance on test scoring and yields a positive or negative indication for concussive injury. A positive test refers users to guidelines listed in the Concussion Management Tool for recommendations. The final page provides coding instructions for entering the results into the patient’s electronic medical record for documentation and future reference.
Progressive Return To Activities Clinical Recommendation
The Progressive Return to Activities Clinical Recommendation (PRA CR) also was developed by DVBIC for the DoD to assist military HCPs in managing SMs with concussion by providing systematic and evidence-based guidance to both prevent extended rest and promote return to full duty as quickly and safely as clinically indicated. The general guidance is to monitor the SM at each of the 6 stages in the process and safely and gradually increase activity to the next stage as tolerated. Daily symptoms are measured using the Neurobehavioral Symptom Inventory (NSI), which SMs self-administer every morning at each stage within the process.
Prior to initiation of the progressive return to activity, SM education using the educational brochure is strongly encouraged, as previous evidence suggests that it is an effective intervention during the acute stages of injury.10,11 Return to activity follows a 6 stage process, from stage 1 (rest) through stage 6 (unrestricted activity) (Table 4). Referral to rehabilitation providers (RPs) or higher care is left to the discretion of the PCM when (1) recovery is not progressing as anticipated; (2) progression is not being made within a 7-day period; or (3) symptoms worsen with time. The guidance outlined in the PRA CR is consistent with current policies and medical literature, and undergoes reviews as updates in the field emerge. The PRA for PCM, PRA for RP, Clinical Support Tool for PCM, Clinical Support Tool for RP, Training Slides for PCM, Training Slides for RP, Educational Brochure for PCM, and Patient Educational Tool for RP can be found on the DVBIC website (dvbic.dcoe.mil).
Description
To improve the clinical utility, 2 separate PRA CRs were developed specifically for PCMs (Figure 2) and RPs (Figure 3). The PRA CR for PCMs provides the initial framework to monitor SMs during recovery and gradually increase physical, cognitive, and vestibular/balance activities as symptoms improve in order to return to preinjury activities. The PRA CR for RPs outlines the approach for treating SMs who meet 1 of the following criteria: recovery is not progressing as anticipated, there is no progression in 7 days, symptoms are worsening, the SM is symptomatic after exertional testing following stage 5, or referral made per PCM judgment. Following the mandatory 24-hour rest period after a diagnosis of a concussion, progression through the PRA algorithm is based on history of concussion within the past 12 months (ie, 1, 2, or ≥ 3 concussions) and symptomatology, with varying treatment pathways depending on the SM’s responses to history and symptomology.
Guidelines
• One Concussion within Past 12 Months. Following the mandatory 24-hour rest period, if the SM is asymptomatic, then exertional testing (eg, activities such as push-ups, sit-ups, running in place, step aerobics, stationary bike, treadmill and/or hand crank) is performed at 65 to 85% of target heart rate for 2 minutes and symptoms are reassessed. If still asymptomatic, the SM may return to preinjury activity; however, if exertional testing provokes symptoms > 1 (mild) on the NSI, the SM should return to stage 1 with an additional 24 hours of rest. A second exertional test can be performed after stage 1, and if symptoms are provoked, progression through the remaining stages 2 to 5 is encouraged. Symptoms are continually monitored throughout each stage to determine whether the SM is recovered sufficiently to proceed to the next stage.
• Two Concussion Within Past 12 Months. Following the mandatory 24-hour rest period, no exertional testing is performed, and SMs move directly into stage 1 and remain at stage 1 or stage 2 for 7 consecutive days with no symptoms > 1 on the NSI before advancing through the remaining stages. Some defining features are longer rest periods (eg, 5 additional days of rest at stage 2) and additional patient education, symptom management, and follow-up.
• Three or more Concussions Within Past 12 Months. Following the 24 hour mandatory rest period, in cases where ≥ 3 concussions have occurred within a 12 month period, the recommendation is to provide guidance for symptom management rest and refer the SM to a higher level of care.
Concussion Management Tool
Beyond the initial assessment and concussion evaluation and the promotion of SMs’ timely return to duty, the DoD developed a tool to help endpoint users manage concussion, to include those with more protracted symptoms (Figure 4). The CMT assists HCPs and the SMs they treat in the management of symptoms before and after they return to duty. Specifically, the CMT is designed to be given in combination with guidelines issued by the DoD in the PRA CR but extends management of concussion to include those symptoms experienced more long-term, or symptoms that are not solely addressed during the timeline of the PRA CR. Together, the MACE 2, PRA CR, and the CMT provide endpoint users with a set of tools to comprehensively evaluate, treat, and manage concussions in SMs.
Description
The CMT provides step-by-step guidance for the initial and comprehensive management of concussion, once a diagnosis is made using assessments in the MACE 2. All types of HCPs, particularly those with limited training, such as Navy Hospital Corpsman and Army Combat Medics, are the intended clinical audience for the CMT. This tool was revised in 2019 to better align with the MACE 2, PRA CR, and other DVBIC CRs, and replaces the 2012 Concussion Management Algorithm and the 2014 Army Concussion Management in Garrison Setting Algorithm. The first 2 sections of the CMT are action cards, which provide management guidelines for acute injuries up to 7 days following injury and for comprehensive management beyond 1 week. Guidelines within the CMT partially overlap with those in the PRA CR; however, the PRA is designed for a more acute timeline, whereas the CMT focuses on symptom management following a more protracted recovery. The CMT clinical tool, provider training, instructor guide, and student workbook all can be found on the DVBIC website (Table 3).
Discussion
It is important for HCPs to have the skills and clinically relevant tools to optimize accurate TBI assessment. Early and accurate assessment and effective symptom management allows SMs to receive timely treatment based on clinical recommendations, and prevent and/or minimize secondary injury and prolonged recovery. Several longitudinal studies emphasize the benefits of early diagnosis and systematic follow-up.16-18 Prompt diagnosis, patient education, and early initiation to treatment may help optimize triage to care, mitigate prolonged symptoms by educating the patient on what to expect, and target specific symptoms early.8,10 Beyond the health outcomes of an individual SM, TBI recovery impacts unit readiness and consequently force readiness. As such, health outcomes and medical readiness are a priority of the Defense Health Agency (DHA).
The DHA priorities are, in part, based on DoD policy guidance for the management of concussion in the deployed setting. According to DoD instruction, “Medically documented mTBI/concussion in service members shall be clinically evaluated, treated, and managed according to the most current DoD clinical practice guidance for the deployed environment found in the Defense and Veterans Brain Injury Center (DVBIC) guidance, ‘Medical Providers: Clinical Tools.’”12 In 2018, the Deputy Secretary of Defense issued a memorandum regarding the comprehensive strategy and action plan for warfighter brain health.12 Therein, the memorandum acknowledges the enduring responsibility of the DoD to promote and protect the health and well-being of members of the nation’s armed forces. Particular emphasis was placed on issuing a response to the effects caused by concussive impacts and exposure to blast waves. This response resulted in a commitment by the DoD to understanding, preventing, diagnosing, and treating TBI in all forms. Taken together, the message from the secretary of defense and instruction from the DoD is clear and makes imperative the use of DoD clinical tools to accomplish this commitment.
Conclusion
This article showcases 3 of the DoD’s TBI clinical tools (MACE 2, PRA CR, and CMT) that assist HCPs in identifying and treating concussion. Over time, these tools undergo revisions according to the state of the science, and are adapted to meet the needs of clinicians and the SMs they treat. Studies are currently ongoing to better understand the effectiveness of these tools as well as to assist clinicians in making return-to-duty and/or medical separation decisions. These tools assist clinicians throughout the recovery process; from initial assessment and treatment (acute phase), as well as with symptom management (acute and protracted symptoms).
Concussion is not a homogenous condition and the experiences of the SM, including events that may cause emotional distress, other injuries and/or other factors, may further complicate the injury. Accordingly, there is no single clinical tool that can conclusively determine return-to-duty status; rather, these tools can help characterize injury, validate, and treat symptoms, which have been suggested to improve outcomes. More research and data are needed confirm the effectiveness of these tools to improve outcomes.
It is beyond the scope of this article to provide a more in-depth discussion on TBI prevention or longer term effects/care. However, there are additional, personalized tools for specific symptoms, deficits, or dysfunctions following concussion. These tools include the Management of Headache Following mTBI for PCM CR, Management of Sleep Disturbances Following mTBI for PCM CR, Assessment and Management of Visual Dysfunction Associated with mTBI CR, and Assessment and Management of Dizziness Associated mTBI CR. These tools enable endpoint users to evaluate and treat SMs as well as know when to elevate to higher levels of care.
The DoD commitment toward treating TBI influenced the development of the clinical tools highlighted in this article. They are the result of collective efforts among military and civilian TBI subject matter experts, data from medical literature and state-of-the-science research, and feedback from endpoint users to create the most effective, evidence-based tools. These tools undergo continuous review and revision to ensure alignment with the most up-to-date science within the field, to meet the needs of SMs and to continue the commitment to DoD concussion care.
Acknowledgments
This work was prepared under Contract (HT0014-19-C-0004) General Dynamics Information Technology and (W81XWH-16-F-0330) Credence Management Solutions, and is defined as U.S. Government work under Title 17 U.S.C.§101. Per Title 17 U.S.C.§105, copyright protection is not available for any work of the U.S. Government. For more information, please contact [email protected].
Traumatic brain injury (TBI) is a major health concern that can cause significant disability as well as economic and social burden. The Centers for Disease Control and Prevention (CDC) reported a 58% increase in the number of TBI-related emergency department visits, hospitalizations, and deaths from 2006 to 2014.1 In the CDC report, falls and motor vehicle accidents accounted for 52.3% and 20.4%, respectively, of all civilian TBI-related hospitalizations. In 2014, 56,800 TBIs in the US resulted in death. A large proportion of severe TBI survivors continue to experience long-term physical, cognitive, and psychologic disorders and require extensive rehabilitation, which may disrupt relationships and prevent return to work.2 About 37% of people with mild TBI (mTBI) cases and 51% of severe cases were unable to return to previous jobs. A study examining psychosocial burden found that people with a history of TBI reported greater feelings of loneliness compared with individuals without TBI.3
Within the US military, the Defense and Veterans Brain Injury Center (DVBIC) indicates that > 417,503 service members (SMs) have been diagnosed with TBI since November 2000.4 Of these, 82.4% were classified as having a mTBI, or concussion (Tables 1 and 2). The nature of combat and military training to which SMs are routinely exposed may increase the risk for sustaining a TBI. Specifically, the increased use of improvised explosives devices by enemy combatants in the recent military conflicts (ie, Operation Enduring Freedom, Operation Iraqi Freedom and Operation New Dawn) resulted in TBI being recognized as the signature injury of these conflicts and brought attention to the prevalence of concussion within the US military.5,6 In the military, the effects of concussion can decrease individual and unit effectiveness, emphasizing the importance of prompt diagnosis and proper management.7
Typically, patients recover from concussion within a few weeks of injury; however, some individuals experience symptoms that persist for months or years. Studies found that early intervention after concussion may aid in expediting recovery, stressing the importance of identifying concussion as promptly as possible.8,9 Active treatment is centered on patient education and symptom management, in addition to a progressive return to activities, as tolerated. Patient education may help validate the symptoms of some patients, as well as help to reattribute the symptoms to benign causes, leading to better outcomes.10 Since TBI is such a relevant health concern within the DoD, it is paramount for practitioners to understand what resources are available in order to identify and initiate treatment expeditiously.
This article focuses on the clinical tools used in evaluating and treating concussion, and best practices treatment guidelines for health care providers (HCPs) who are required to evaluate and treat military populations. While these resources are used for military SMs, they can also be used in veteran and civilian populations. This article showcases 3 DoD clinical tools that assist HCPs in evaluating and treating patients with TBI: (1) the Military Acute Concussion Evaluation 2 (MACE 2); (2) the Progressive Return to Activity (PRA) Clinical Recommendation (CR); and (3) the Concussion Management Tool (CMT). Additional DoD clinical tools and resources are discussed, and resources and links for the practitioner are provided for easy access and reference.
Military Acute Concussion Evaluation 2
Early concussion identification and evaluation are important steps in the treatment process to ensure timely recovery and return to duty for SMs. As such, DVBIC assembled a working group of military and civilian brain injury experts to create an evidence-based clinical practice guideline for the assessment and management of concussion in a military operational setting that could be learned and effectively used by corpsmen and combat medics in the battlefield to screen for a possible concussion.7 This team created the first version of the MACE, a clinical tool that prompted a systematic assessment of concussion related symptoms, neurologic signs, and cognitive deficits. The cognitive assessment portion was based on the standardized assessment of concussion (SAC) that had been reported by McCrea and colleagues in 1998.11 Soon after its creation, field utilization of the MACE for screening of concussion was mandated by the Army through an All Army Action (ALARACT 178/2008) and for all of the Services through the DoD Instruction (DoDI) 6490.11 published in 2014.12
The MACE has been updated several times since the original version. Most recently, the MACE was revised in 2018 to include a vestibular oculomotor assessment section, and red flags that immediately alert the HCP to the need for immediate triage referral and treatment of the patient possibly at a higher echelon of care or with more emergent evaluation.13-15 Additionally, the neurologic examination was expanded to increase clarity and comprehensiveness, including speech and balance testing. Updates made to the tool were intended to provide a more thorough and informative evaluation of the SM with suspected concussion.
This latest version, MACE 2, is designed to be used by any HCP who is treating SMs with a suspected or potential TBI, not just corpsmen and combat medics in theater. The MACE 2 is a comprehensive evaluation within a set of portable pocket cards designed to assist end-users in the proper triage of potentially concussed individuals. The DoD has specified 4 events that require a MACE 2 evaluation: (1) SM was in a vehicle associated with a blast event, collision, or roll over; (2) SM was within 50 meters of a blast; (3) anyone who sustained a direct blow to the head; or (4) when command provides direction (eg, repeated exposures to the events above or in accordance with protocols).12 Sleep deprivation, medications, and pain may affect MACE 2 results, in addition to deployment related stress, chronic stress, high adrenaline sustained over time, and additional comorbidities. This tool is most effective when used as close to the time of injury as possible but also may be used later (after 24 hours of rest) to reevaluate symptoms. The MACE 2 Instructor Guide, a student workbook, HCP training, and Vestibular/Ocular-Motor Screening (VOMS) for Concussion instructions can be found on the DVBIC website (Table 3).
Description
The MACE 2 is a brief multimodal screening tool that assists medics, corpsman, and primary care managers (PCMs) in the assessment and identification of a potential concussion (Figure 1). Embedded in the MACE 2 is the Standardized Assessment of Concussion (SAC), a well-validated sports concussion tool, and the VOMS tool as portions of the 2-part cognitive examination. The entirety of the tool has 5 sections: (1) red flags; (2) acute concussion screening; (3) cognitive examination, part 1; (4) neurologic examination; and (5) cognitive examination, part 2. The end of the MACE 2 includes sections on the scoring, instructions for International Classification of Diseases, Tenth Revision, TBI coding, and next steps following completion of the MACE 2. The latest version of this screening tool impacts TBI care in several noteworthy ways. First, it broadens the scope of users by expanding use to all medically trained personnel, allowing any provider to treat SMs in the field. Second, it combines state-of-the-science advances from the research field and reflects feedback from end-users collected during the development. Last, the MACE 2 is updated as changes in the field occur, and is currently undergoing research to better identify end-user utility and usability.
Screening Tools
• Red Flags. The red flags section aids in identifying potentially serious underlying conditions in patients presenting with Glasgow Coma Scale (GCS) between 13 and 15. A positive red flag prompts the practitioner to stop administering the MACE 2 and immediately consult a higher level of care and consider urgent evacuation. While the red flags are completed first, and advancement to later sections of the MACE 2 is dependent upon the absence of red flags, the red flags should be monitored throughout the completion of the MACE 2. Upon completion of patient demographics and red flags, the remaining sections of the MACE 2 are dedicated to acute concussion screening.
• Acute Concussion Screening. The acute concussion screening portion consists of 4 sections: description of the incident; alteration of consciousness or memory; a “check all that apply” symptom inventory; and a patient history that includes concussions within the past 12 months, headache disorders, and/or behavioral health concerns. The final portion of the acute concussion screening section provides an algorithm to identify a positive or negative concussion screen. When a negative screen is identified, the user is prompted to prescribe a 24-hour rest period and follow up with the SM based on the guidance in the CMT. A positive screen warrants the user to continue administration of the MACE 2.
Neurologic and CognitiveExaminations
• Cognitive Exam Part 1. The initial cognitive examination is designed to assess orientation to time (eg, What is the day of the week, day of the month, the month, the year, and the timeof day?) as well as immediate recall of a short list of concrete words (5 words total, repeated for 3 trials). These tests are based on other neuropsychological measures designed to assess cognitive/mental status and short-term memory.
• The Neurological Exam. The neurological exam section of the MACE 2 includes brief neuropsychologic tests such as speech fluency and word finding. Other sections within the neurological exam assess the
following: grip strength, vestibular function/balance (eg, tandem gait and single leg stance), as well as motor function (eg, pronator drift), autonomic nervous system function (eg, pupil response), and vestibular function (eye-tracking).
• Cognitive Exam Part 2. After completion of the first cognitive examination and the neurologic examination, the second part of the cognitive examination is initiated. Part 2 includes measures of short-term and working memory (eg, digits-reverse tasks, listing the months in reverse order, and a delayed recall task of the short list of concrete words presented in the first part). The final assessment is the administration of the VOMS, a tool developed from the sports concussion field and designed to measure vestibular-ocular function.13 It is critical to note that the VOMS is contraindicated if there is concern of an unstable cervical spine or absence of a trained HCP. An examination summary provides guidance on test scoring and yields a positive or negative indication for concussive injury. A positive test refers users to guidelines listed in the Concussion Management Tool for recommendations. The final page provides coding instructions for entering the results into the patient’s electronic medical record for documentation and future reference.
Progressive Return To Activities Clinical Recommendation
The Progressive Return to Activities Clinical Recommendation (PRA CR) also was developed by DVBIC for the DoD to assist military HCPs in managing SMs with concussion by providing systematic and evidence-based guidance to both prevent extended rest and promote return to full duty as quickly and safely as clinically indicated. The general guidance is to monitor the SM at each of the 6 stages in the process and safely and gradually increase activity to the next stage as tolerated. Daily symptoms are measured using the Neurobehavioral Symptom Inventory (NSI), which SMs self-administer every morning at each stage within the process.
Prior to initiation of the progressive return to activity, SM education using the educational brochure is strongly encouraged, as previous evidence suggests that it is an effective intervention during the acute stages of injury.10,11 Return to activity follows a 6 stage process, from stage 1 (rest) through stage 6 (unrestricted activity) (Table 4). Referral to rehabilitation providers (RPs) or higher care is left to the discretion of the PCM when (1) recovery is not progressing as anticipated; (2) progression is not being made within a 7-day period; or (3) symptoms worsen with time. The guidance outlined in the PRA CR is consistent with current policies and medical literature, and undergoes reviews as updates in the field emerge. The PRA for PCM, PRA for RP, Clinical Support Tool for PCM, Clinical Support Tool for RP, Training Slides for PCM, Training Slides for RP, Educational Brochure for PCM, and Patient Educational Tool for RP can be found on the DVBIC website (dvbic.dcoe.mil).
Description
To improve the clinical utility, 2 separate PRA CRs were developed specifically for PCMs (Figure 2) and RPs (Figure 3). The PRA CR for PCMs provides the initial framework to monitor SMs during recovery and gradually increase physical, cognitive, and vestibular/balance activities as symptoms improve in order to return to preinjury activities. The PRA CR for RPs outlines the approach for treating SMs who meet 1 of the following criteria: recovery is not progressing as anticipated, there is no progression in 7 days, symptoms are worsening, the SM is symptomatic after exertional testing following stage 5, or referral made per PCM judgment. Following the mandatory 24-hour rest period after a diagnosis of a concussion, progression through the PRA algorithm is based on history of concussion within the past 12 months (ie, 1, 2, or ≥ 3 concussions) and symptomatology, with varying treatment pathways depending on the SM’s responses to history and symptomology.
Guidelines
• One Concussion within Past 12 Months. Following the mandatory 24-hour rest period, if the SM is asymptomatic, then exertional testing (eg, activities such as push-ups, sit-ups, running in place, step aerobics, stationary bike, treadmill and/or hand crank) is performed at 65 to 85% of target heart rate for 2 minutes and symptoms are reassessed. If still asymptomatic, the SM may return to preinjury activity; however, if exertional testing provokes symptoms > 1 (mild) on the NSI, the SM should return to stage 1 with an additional 24 hours of rest. A second exertional test can be performed after stage 1, and if symptoms are provoked, progression through the remaining stages 2 to 5 is encouraged. Symptoms are continually monitored throughout each stage to determine whether the SM is recovered sufficiently to proceed to the next stage.
• Two Concussion Within Past 12 Months. Following the mandatory 24-hour rest period, no exertional testing is performed, and SMs move directly into stage 1 and remain at stage 1 or stage 2 for 7 consecutive days with no symptoms > 1 on the NSI before advancing through the remaining stages. Some defining features are longer rest periods (eg, 5 additional days of rest at stage 2) and additional patient education, symptom management, and follow-up.
• Three or more Concussions Within Past 12 Months. Following the 24 hour mandatory rest period, in cases where ≥ 3 concussions have occurred within a 12 month period, the recommendation is to provide guidance for symptom management rest and refer the SM to a higher level of care.
Concussion Management Tool
Beyond the initial assessment and concussion evaluation and the promotion of SMs’ timely return to duty, the DoD developed a tool to help endpoint users manage concussion, to include those with more protracted symptoms (Figure 4). The CMT assists HCPs and the SMs they treat in the management of symptoms before and after they return to duty. Specifically, the CMT is designed to be given in combination with guidelines issued by the DoD in the PRA CR but extends management of concussion to include those symptoms experienced more long-term, or symptoms that are not solely addressed during the timeline of the PRA CR. Together, the MACE 2, PRA CR, and the CMT provide endpoint users with a set of tools to comprehensively evaluate, treat, and manage concussions in SMs.
Description
The CMT provides step-by-step guidance for the initial and comprehensive management of concussion, once a diagnosis is made using assessments in the MACE 2. All types of HCPs, particularly those with limited training, such as Navy Hospital Corpsman and Army Combat Medics, are the intended clinical audience for the CMT. This tool was revised in 2019 to better align with the MACE 2, PRA CR, and other DVBIC CRs, and replaces the 2012 Concussion Management Algorithm and the 2014 Army Concussion Management in Garrison Setting Algorithm. The first 2 sections of the CMT are action cards, which provide management guidelines for acute injuries up to 7 days following injury and for comprehensive management beyond 1 week. Guidelines within the CMT partially overlap with those in the PRA CR; however, the PRA is designed for a more acute timeline, whereas the CMT focuses on symptom management following a more protracted recovery. The CMT clinical tool, provider training, instructor guide, and student workbook all can be found on the DVBIC website (Table 3).
Discussion
It is important for HCPs to have the skills and clinically relevant tools to optimize accurate TBI assessment. Early and accurate assessment and effective symptom management allows SMs to receive timely treatment based on clinical recommendations, and prevent and/or minimize secondary injury and prolonged recovery. Several longitudinal studies emphasize the benefits of early diagnosis and systematic follow-up.16-18 Prompt diagnosis, patient education, and early initiation to treatment may help optimize triage to care, mitigate prolonged symptoms by educating the patient on what to expect, and target specific symptoms early.8,10 Beyond the health outcomes of an individual SM, TBI recovery impacts unit readiness and consequently force readiness. As such, health outcomes and medical readiness are a priority of the Defense Health Agency (DHA).
The DHA priorities are, in part, based on DoD policy guidance for the management of concussion in the deployed setting. According to DoD instruction, “Medically documented mTBI/concussion in service members shall be clinically evaluated, treated, and managed according to the most current DoD clinical practice guidance for the deployed environment found in the Defense and Veterans Brain Injury Center (DVBIC) guidance, ‘Medical Providers: Clinical Tools.’”12 In 2018, the Deputy Secretary of Defense issued a memorandum regarding the comprehensive strategy and action plan for warfighter brain health.12 Therein, the memorandum acknowledges the enduring responsibility of the DoD to promote and protect the health and well-being of members of the nation’s armed forces. Particular emphasis was placed on issuing a response to the effects caused by concussive impacts and exposure to blast waves. This response resulted in a commitment by the DoD to understanding, preventing, diagnosing, and treating TBI in all forms. Taken together, the message from the secretary of defense and instruction from the DoD is clear and makes imperative the use of DoD clinical tools to accomplish this commitment.
Conclusion
This article showcases 3 of the DoD’s TBI clinical tools (MACE 2, PRA CR, and CMT) that assist HCPs in identifying and treating concussion. Over time, these tools undergo revisions according to the state of the science, and are adapted to meet the needs of clinicians and the SMs they treat. Studies are currently ongoing to better understand the effectiveness of these tools as well as to assist clinicians in making return-to-duty and/or medical separation decisions. These tools assist clinicians throughout the recovery process; from initial assessment and treatment (acute phase), as well as with symptom management (acute and protracted symptoms).
Concussion is not a homogenous condition and the experiences of the SM, including events that may cause emotional distress, other injuries and/or other factors, may further complicate the injury. Accordingly, there is no single clinical tool that can conclusively determine return-to-duty status; rather, these tools can help characterize injury, validate, and treat symptoms, which have been suggested to improve outcomes. More research and data are needed confirm the effectiveness of these tools to improve outcomes.
It is beyond the scope of this article to provide a more in-depth discussion on TBI prevention or longer term effects/care. However, there are additional, personalized tools for specific symptoms, deficits, or dysfunctions following concussion. These tools include the Management of Headache Following mTBI for PCM CR, Management of Sleep Disturbances Following mTBI for PCM CR, Assessment and Management of Visual Dysfunction Associated with mTBI CR, and Assessment and Management of Dizziness Associated mTBI CR. These tools enable endpoint users to evaluate and treat SMs as well as know when to elevate to higher levels of care.
The DoD commitment toward treating TBI influenced the development of the clinical tools highlighted in this article. They are the result of collective efforts among military and civilian TBI subject matter experts, data from medical literature and state-of-the-science research, and feedback from endpoint users to create the most effective, evidence-based tools. These tools undergo continuous review and revision to ensure alignment with the most up-to-date science within the field, to meet the needs of SMs and to continue the commitment to DoD concussion care.
Acknowledgments
This work was prepared under Contract (HT0014-19-C-0004) General Dynamics Information Technology and (W81XWH-16-F-0330) Credence Management Solutions, and is defined as U.S. Government work under Title 17 U.S.C.§101. Per Title 17 U.S.C.§105, copyright protection is not available for any work of the U.S. Government. For more information, please contact [email protected].
1. Centers for Disease Control and Prevention. Surveillance report of traumatic brain injury-related emergency department visits, hospitalizations, and deaths. https://www.cdc.gov/traumaticbraininjury/pdf/TBI-Surveillance-Report-FINAL_508.pdf. Published 2014. Accessed August 18, 2020.
2. Stocchetti N, Zanier ER. Chronic impact of traumatic brain injury on outcome and quality of life: a narrative review. Crit Care. 2016;20(1):148. Published 2016 Jun 21. doi:10.1186/s13054-016-1318-1
3. Kumar RG, Ornstein KA, Bollens-Lund E, et al. Lifetime history of traumatic brain injury is associated with increased loneliness in adults: A US nationally representative study. Int J Geriatr Psychiatry. 2020;35(5):553-563. doi:10.1002/gps.5271
4. Defense and Veterans Brain Injury Center. Worldwide DoD numbers for traumatic brain injury. 2020; https://dvbic.dcoe.mil/sites/default/files/tbi-numbers/DVBIC_WorldwideTotal_2000-2019.pdf. Updated March 10, 2020. Accessed August 18, 2020.
5. Kennedy JE, Lu LH, Reid MW, Leal FO, Cooper DB. Correlates of depression in U.S. military service members with a history of mild traumatic brain injury. Mil Med. 2019;184(suppl 1):148-154. doi:10.1093/milmed/usy321
6. Marshall KR, Holland SL, Meyer KS, Martin EM, Wilmore M, Grimes JB. Mild traumatic brain injury screening, diagnosis, and treatment. Mil Med. 2012;177(suppl 8):67-75. doi:10.7205/milmed-d-12-00110
7. French L, McCrea M., Baggett M. The Military Acute Concussion Evaluation. J Spec Oper Med. 2008;8(1):68-77. https://www.jsomonline.org/Publications/2008168French.pdf. Accessed August 18, 2020.
8. Kontos AP, Jorgensen-Wagers K, Trbovich AM, et al. Association of time since injury to the first clinic visit with recovery following concussion. JAMA Neurol. 2020;77(4):435-440. doi:10.1001/jamaneurol.2019.4552
9. Ponsford J, Willmott C, Rothwell A, et al. Impact of early intervention on outcome following mild head injury in adults. J Neurol Neurosurg Psychiatry. 2002;73(3):330-332. doi:10.1136/jnnp.73.3.33010.
10. Mittenberg W, Canyock EM, Condit D, Patton C. Treatment of post-concussion syndrome following mild head injury. J Clin Exp Neuropsychol. 2001;23(6):829-836. doi:10.1076/jcen.23.6.829.1022
11. McCrea M, Kelly JP, Randolph C, et al. Standardized assessment of concussion (SAC): on-site mental status evaluation of the athlete. J Head Trauma Rehabil. 1998;13(2):27-35. doi:10.1097/00001199-199804000-00005
12. US Department of Defense. Department of Defense Instruction, Number 6490.11. Policy guidance for management of mild traumatic brain injury/concussion in the deployed setting. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/649011p.pdf. Updated November 26, 2019. Accessed August 18, 2020.
13. Mucha A, Collins MW, Elbin RJ, et al. A brief Vestibular/Ocular Motor Screening (VOMS) assessment to evaluate concussions: preliminary findings. Am J Sports Med. 2014;42(10):2479-2486. doi:10.1177/0363546514543775
14. Defense and Veterans Brain Injury Center. Military Acute Concussion Evaluation 2 (MACE 2). https://dvbic.dcoe.mil/material/military-acute-concussion-evaluation-2-mace-2. Updated August 18, 2020. Accessed August 18, 2020.
15. US Department of Defense, Defense Health Agency. Defense and Veterans Brain Injury Center releases new concussion screening tool. https://www.health.mil/News/Articles/2019/03/15/Defense-and-Veterans-Brain-Injury-Center-releases-new-concussion-screening-tool. Published March 15, 2019. Accessed August 18, 2020.
16. Schwab K, Terrio HP, Brenner LA, et al. Epidemiology and prognosis of mild traumatic brain injury in returning soldiers: a cohort study. Neurology. 2017;88(16):1571-1579. doi:10.1212/WNL.0000000000003839
17. Mac Donald CL, Johnson AM, Wierzechowski L, et al. Outcome trends after US military concussive traumatic brain injury. J Neurotrauma. 2017;34(14):2206-2219. doi:10.1089/neu.2016.4434
18. Andelic N, Howe EI, Hellstrøm T, et al. Disability and quality of life 20 years after traumatic brain injury. Brain Behav. 2018;8(7):e01018. doi:10.1002/brb3.1018
1. Centers for Disease Control and Prevention. Surveillance report of traumatic brain injury-related emergency department visits, hospitalizations, and deaths. https://www.cdc.gov/traumaticbraininjury/pdf/TBI-Surveillance-Report-FINAL_508.pdf. Published 2014. Accessed August 18, 2020.
2. Stocchetti N, Zanier ER. Chronic impact of traumatic brain injury on outcome and quality of life: a narrative review. Crit Care. 2016;20(1):148. Published 2016 Jun 21. doi:10.1186/s13054-016-1318-1
3. Kumar RG, Ornstein KA, Bollens-Lund E, et al. Lifetime history of traumatic brain injury is associated with increased loneliness in adults: A US nationally representative study. Int J Geriatr Psychiatry. 2020;35(5):553-563. doi:10.1002/gps.5271
4. Defense and Veterans Brain Injury Center. Worldwide DoD numbers for traumatic brain injury. 2020; https://dvbic.dcoe.mil/sites/default/files/tbi-numbers/DVBIC_WorldwideTotal_2000-2019.pdf. Updated March 10, 2020. Accessed August 18, 2020.
5. Kennedy JE, Lu LH, Reid MW, Leal FO, Cooper DB. Correlates of depression in U.S. military service members with a history of mild traumatic brain injury. Mil Med. 2019;184(suppl 1):148-154. doi:10.1093/milmed/usy321
6. Marshall KR, Holland SL, Meyer KS, Martin EM, Wilmore M, Grimes JB. Mild traumatic brain injury screening, diagnosis, and treatment. Mil Med. 2012;177(suppl 8):67-75. doi:10.7205/milmed-d-12-00110
7. French L, McCrea M., Baggett M. The Military Acute Concussion Evaluation. J Spec Oper Med. 2008;8(1):68-77. https://www.jsomonline.org/Publications/2008168French.pdf. Accessed August 18, 2020.
8. Kontos AP, Jorgensen-Wagers K, Trbovich AM, et al. Association of time since injury to the first clinic visit with recovery following concussion. JAMA Neurol. 2020;77(4):435-440. doi:10.1001/jamaneurol.2019.4552
9. Ponsford J, Willmott C, Rothwell A, et al. Impact of early intervention on outcome following mild head injury in adults. J Neurol Neurosurg Psychiatry. 2002;73(3):330-332. doi:10.1136/jnnp.73.3.33010.
10. Mittenberg W, Canyock EM, Condit D, Patton C. Treatment of post-concussion syndrome following mild head injury. J Clin Exp Neuropsychol. 2001;23(6):829-836. doi:10.1076/jcen.23.6.829.1022
11. McCrea M, Kelly JP, Randolph C, et al. Standardized assessment of concussion (SAC): on-site mental status evaluation of the athlete. J Head Trauma Rehabil. 1998;13(2):27-35. doi:10.1097/00001199-199804000-00005
12. US Department of Defense. Department of Defense Instruction, Number 6490.11. Policy guidance for management of mild traumatic brain injury/concussion in the deployed setting. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/649011p.pdf. Updated November 26, 2019. Accessed August 18, 2020.
13. Mucha A, Collins MW, Elbin RJ, et al. A brief Vestibular/Ocular Motor Screening (VOMS) assessment to evaluate concussions: preliminary findings. Am J Sports Med. 2014;42(10):2479-2486. doi:10.1177/0363546514543775
14. Defense and Veterans Brain Injury Center. Military Acute Concussion Evaluation 2 (MACE 2). https://dvbic.dcoe.mil/material/military-acute-concussion-evaluation-2-mace-2. Updated August 18, 2020. Accessed August 18, 2020.
15. US Department of Defense, Defense Health Agency. Defense and Veterans Brain Injury Center releases new concussion screening tool. https://www.health.mil/News/Articles/2019/03/15/Defense-and-Veterans-Brain-Injury-Center-releases-new-concussion-screening-tool. Published March 15, 2019. Accessed August 18, 2020.
16. Schwab K, Terrio HP, Brenner LA, et al. Epidemiology and prognosis of mild traumatic brain injury in returning soldiers: a cohort study. Neurology. 2017;88(16):1571-1579. doi:10.1212/WNL.0000000000003839
17. Mac Donald CL, Johnson AM, Wierzechowski L, et al. Outcome trends after US military concussive traumatic brain injury. J Neurotrauma. 2017;34(14):2206-2219. doi:10.1089/neu.2016.4434
18. Andelic N, Howe EI, Hellstrøm T, et al. Disability and quality of life 20 years after traumatic brain injury. Brain Behav. 2018;8(7):e01018. doi:10.1002/brb3.1018
The Brain in COVID-19: No One Is Okay
Knowing that I am a psychiatrist, my friends and colleagues recently started to ask me, “Am I losing my mind?” The symptoms underlying these concerned queries are remarkably similar: inability to concentrate, becoming easily frustrated, forgetting things, not being as productive as usual, being overly tired despite doing less, and feeling unusually irritable, among other vague somatic symptoms. This condition is to be distinguished from COVID-19 in the brain, which is the protean serious neuropsychiatric manifestations of infection with the virus ranging from strokes and seizures to encephalopathy and psychosis especially in severe cases of infection.1
As federal health care professionals (HCPs), many of us are familiar with acute high stress medical situations, which the pandemic has expanded and intensified: In New York City during the surge, the US Department of Veterans Affairs (VA) intensive care physician pushing life-sustaining resources to their limits in a valiant effort to keep alive as many people as possible; the US Public Health Service HCP working miracles without adequate supplies or staff in underserved hard-hit areas of the country; or the US Department of Defense clinician deftly trying to contain outbreaks in contained spaces like ships.
Emerging data already show that HCPs and other first responders facing these repeated episodes of acute stress are experiencing increased depression and anxiety.2 Research from prior pandemics suggests that this is only the beginning of a wave of negative mental health complications in HCPs.3
In the acute form of stress, the hypothalamic pituitary axis (HPA axis) is an evolutionary engine that coordinates multiple organ systems from lungs to liver to ensure efficient escape from primeval dinosaurs or more modern threats like viruses. Fueling that engine is the hormonal cascade that ends in excessive secretion of cortisol.
Chronic stress affects the body and brain in a different way than does acute stress. The problem is that this sympathetic nervous system surge is meant to power a sprint to survival not the marathon of uncertainty that COVID-19 has become. As long as the body stays in acute stress mode, the brain cannot downshift to the parasympathetic system that would usually moderate and regulate our neurobiologic circuits and neuropsychological processes. Like any other engine in overdrive, the stress gear erodes the machinery of our body and brain. Hence, the symptoms of psychophysical wear and tear—allostatic load—that most of us are experiencing.4
The subject of this column is the lower level of prolonged chronic stress. The mild and amorphous pertubations that can be described as “the brain in COVID-19.” It is a syndrome that affects even those who have never been infected with the actual virus. Though not usually life-threatening or disabling, it is unnerving and distressing as the queries from my colleagues and friends show. Their reports and my observations have led me to opine that “no one is okay” due to months of living under the strain of a pandemic.
The degree and scope of chronic stress that a person experiences caused by COVID-19 has to be contextualized and individualized. Those who have lost jobs, who are working while children are going to school online, who are caring for relatives, or who are in fear of losing their home face tremendous stress and challenges.5 Yet even those like me, whose biggest worry is a dog barking through important teleconference meetings, still undergo a milder form of near constant stress.
Consider that all of us have become strangers in an even stranger land. Masks have become an object of political controversy. In states where masking is mandatory, you must be mask vigilant every time you go out. In many areas of the country stores have limited hours, access, and supplies and any trip away from the house risks infection. Conversely, for those in a high-risk population, it may have been months since they have left home at all, and many sick, older, and vulnerable persons are suffering from isolation, loneliness, and boredom. The minor distractions and innocent pleasures that relieve day-to-day stress are no longer safe or available options, like eating out, attending shows, or taking trips.
Most of us are waiting for news of an effective available vaccine, some with yearning and others with dread. For George Gershwin, summertime meant that “the livin’ is easy,” but the summer of 2020 has been anything but easy and that takes its toll on the mind. Without adequate positive stimulation, attention wanders and memory fails to encode details, making even routine tasks more difficult; without meaningful social contact, emotions become sharp and ragged often hurting others. Most important, without periods in which we can relax, there is psychic exhaustion.6
At this point you may be thinking, “So, now that you told us we are all under chronic stress, are you going to tell us whether we can do anything about it?” There are many fantastic websites (including the VA) where experts far more qualified than I am offer excellent advice on coping with the pandemic.7 What I can provide is 5 reflections on managing the stress that I have used and that others with whom I shared them have found helpful.
1. Set realistic expectations. We are in a different reality in which we may need to take on smaller tasks, pace our work and take more breaks and, most of all, give ourselves a break when we are not as functional as we were before the pandemic.
2. Get out in nature. Find a green space to walk or sit, spend time with companion animals, go for a hike or bicycle near water or mountains, or watch the birds in a forest. Nothing can help restore our perspective or calm frayed nerves like the socially distanced outdoors.
3. Reach out. Even though we cannot hug or even shake hands, we can still pick up the phone or mouse and check on someone who is down. All the great traditions of the world agree that the best way to lift our own spirits is to help others.
4. Be kind. This is among the most important responses. As the epigraph suggests, everyone is engaged in an often silent and secret struggle and deserves our compassion. This call for kindness should be extended to ourselves so that we can be patient and compassionate to others.
5. Have courage and hope. This may be the most important of all. Whether we are infected or are fearing/avoiding infection, COVID-19 makes us sick in body and brain. We must have faith that there is something—the mind, the spirit—beyond the purely physical that gives us courage to outlast COVID-19 and to have hope for a postpandemic future that though not the same as before may well be in some ways better
1. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun . 2020;87:34-39. doi:10.1016/j.bbi.2020.04.027
2. Pappa S, Ntella V, Giannakas T, Giannakoulis VG, Papoutsi E, Katsaounou P. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901-907. doi:10.1016/j.bbi.2020.05.026
3. Salazar de Pablo G, Vaquerizo-Serrano J, Catalan A, et al. Impact of coronavirus syndromes on physical and mental health of health care workers: systematic review and meta-analysis. J Affect Disord. 2020;275:48-57. doi:10.1016/j.jad.2020.06.022.
4. Harkness K. Strange physical symptoms? Blame the chronic stress of life during the Covid-19 pandemic. https://the-conversation.com/strange-physical-symptoms-blame-the-chronic-stress-of-life-during-the-covid-19-pandemic-139096. Published June 11, 2020. Accessed August 29, 2020.
5. Centers for Disease Control and Prevention. Coronavirus Disease (COVID-19) 2019. Coping with stress. https://www.cdc.gov/coronavirus/2019-ncov/daily-life-coping/managing-stress-anxiety.html. Updated July 1, 2020. Accessed August 29, 2020.
6. Greenberg M. How the stress of the COVID-9 pandemic scrambles your brain. https://www.psychologytoday.com/us/blog/the-mindful-self-express/202006/how-the-stress-the-covid-19-pandemic-scrambles-your-brain. Published June 28, 2020. Accessed August 29, 2020.
7. US Department of Veterans Affairs, National Center for PTSD. Healthcare workers and responders. https://www.ptsd.va.gov/covid/list_healthcare_responders.asp. Updated August 12, 2020. Accessed August 29, 2020.
Knowing that I am a psychiatrist, my friends and colleagues recently started to ask me, “Am I losing my mind?” The symptoms underlying these concerned queries are remarkably similar: inability to concentrate, becoming easily frustrated, forgetting things, not being as productive as usual, being overly tired despite doing less, and feeling unusually irritable, among other vague somatic symptoms. This condition is to be distinguished from COVID-19 in the brain, which is the protean serious neuropsychiatric manifestations of infection with the virus ranging from strokes and seizures to encephalopathy and psychosis especially in severe cases of infection.1
As federal health care professionals (HCPs), many of us are familiar with acute high stress medical situations, which the pandemic has expanded and intensified: In New York City during the surge, the US Department of Veterans Affairs (VA) intensive care physician pushing life-sustaining resources to their limits in a valiant effort to keep alive as many people as possible; the US Public Health Service HCP working miracles without adequate supplies or staff in underserved hard-hit areas of the country; or the US Department of Defense clinician deftly trying to contain outbreaks in contained spaces like ships.
Emerging data already show that HCPs and other first responders facing these repeated episodes of acute stress are experiencing increased depression and anxiety.2 Research from prior pandemics suggests that this is only the beginning of a wave of negative mental health complications in HCPs.3
In the acute form of stress, the hypothalamic pituitary axis (HPA axis) is an evolutionary engine that coordinates multiple organ systems from lungs to liver to ensure efficient escape from primeval dinosaurs or more modern threats like viruses. Fueling that engine is the hormonal cascade that ends in excessive secretion of cortisol.
Chronic stress affects the body and brain in a different way than does acute stress. The problem is that this sympathetic nervous system surge is meant to power a sprint to survival not the marathon of uncertainty that COVID-19 has become. As long as the body stays in acute stress mode, the brain cannot downshift to the parasympathetic system that would usually moderate and regulate our neurobiologic circuits and neuropsychological processes. Like any other engine in overdrive, the stress gear erodes the machinery of our body and brain. Hence, the symptoms of psychophysical wear and tear—allostatic load—that most of us are experiencing.4
The subject of this column is the lower level of prolonged chronic stress. The mild and amorphous pertubations that can be described as “the brain in COVID-19.” It is a syndrome that affects even those who have never been infected with the actual virus. Though not usually life-threatening or disabling, it is unnerving and distressing as the queries from my colleagues and friends show. Their reports and my observations have led me to opine that “no one is okay” due to months of living under the strain of a pandemic.
The degree and scope of chronic stress that a person experiences caused by COVID-19 has to be contextualized and individualized. Those who have lost jobs, who are working while children are going to school online, who are caring for relatives, or who are in fear of losing their home face tremendous stress and challenges.5 Yet even those like me, whose biggest worry is a dog barking through important teleconference meetings, still undergo a milder form of near constant stress.
Consider that all of us have become strangers in an even stranger land. Masks have become an object of political controversy. In states where masking is mandatory, you must be mask vigilant every time you go out. In many areas of the country stores have limited hours, access, and supplies and any trip away from the house risks infection. Conversely, for those in a high-risk population, it may have been months since they have left home at all, and many sick, older, and vulnerable persons are suffering from isolation, loneliness, and boredom. The minor distractions and innocent pleasures that relieve day-to-day stress are no longer safe or available options, like eating out, attending shows, or taking trips.
Most of us are waiting for news of an effective available vaccine, some with yearning and others with dread. For George Gershwin, summertime meant that “the livin’ is easy,” but the summer of 2020 has been anything but easy and that takes its toll on the mind. Without adequate positive stimulation, attention wanders and memory fails to encode details, making even routine tasks more difficult; without meaningful social contact, emotions become sharp and ragged often hurting others. Most important, without periods in which we can relax, there is psychic exhaustion.6
At this point you may be thinking, “So, now that you told us we are all under chronic stress, are you going to tell us whether we can do anything about it?” There are many fantastic websites (including the VA) where experts far more qualified than I am offer excellent advice on coping with the pandemic.7 What I can provide is 5 reflections on managing the stress that I have used and that others with whom I shared them have found helpful.
1. Set realistic expectations. We are in a different reality in which we may need to take on smaller tasks, pace our work and take more breaks and, most of all, give ourselves a break when we are not as functional as we were before the pandemic.
2. Get out in nature. Find a green space to walk or sit, spend time with companion animals, go for a hike or bicycle near water or mountains, or watch the birds in a forest. Nothing can help restore our perspective or calm frayed nerves like the socially distanced outdoors.
3. Reach out. Even though we cannot hug or even shake hands, we can still pick up the phone or mouse and check on someone who is down. All the great traditions of the world agree that the best way to lift our own spirits is to help others.
4. Be kind. This is among the most important responses. As the epigraph suggests, everyone is engaged in an often silent and secret struggle and deserves our compassion. This call for kindness should be extended to ourselves so that we can be patient and compassionate to others.
5. Have courage and hope. This may be the most important of all. Whether we are infected or are fearing/avoiding infection, COVID-19 makes us sick in body and brain. We must have faith that there is something—the mind, the spirit—beyond the purely physical that gives us courage to outlast COVID-19 and to have hope for a postpandemic future that though not the same as before may well be in some ways better
Knowing that I am a psychiatrist, my friends and colleagues recently started to ask me, “Am I losing my mind?” The symptoms underlying these concerned queries are remarkably similar: inability to concentrate, becoming easily frustrated, forgetting things, not being as productive as usual, being overly tired despite doing less, and feeling unusually irritable, among other vague somatic symptoms. This condition is to be distinguished from COVID-19 in the brain, which is the protean serious neuropsychiatric manifestations of infection with the virus ranging from strokes and seizures to encephalopathy and psychosis especially in severe cases of infection.1
As federal health care professionals (HCPs), many of us are familiar with acute high stress medical situations, which the pandemic has expanded and intensified: In New York City during the surge, the US Department of Veterans Affairs (VA) intensive care physician pushing life-sustaining resources to their limits in a valiant effort to keep alive as many people as possible; the US Public Health Service HCP working miracles without adequate supplies or staff in underserved hard-hit areas of the country; or the US Department of Defense clinician deftly trying to contain outbreaks in contained spaces like ships.
Emerging data already show that HCPs and other first responders facing these repeated episodes of acute stress are experiencing increased depression and anxiety.2 Research from prior pandemics suggests that this is only the beginning of a wave of negative mental health complications in HCPs.3
In the acute form of stress, the hypothalamic pituitary axis (HPA axis) is an evolutionary engine that coordinates multiple organ systems from lungs to liver to ensure efficient escape from primeval dinosaurs or more modern threats like viruses. Fueling that engine is the hormonal cascade that ends in excessive secretion of cortisol.
Chronic stress affects the body and brain in a different way than does acute stress. The problem is that this sympathetic nervous system surge is meant to power a sprint to survival not the marathon of uncertainty that COVID-19 has become. As long as the body stays in acute stress mode, the brain cannot downshift to the parasympathetic system that would usually moderate and regulate our neurobiologic circuits and neuropsychological processes. Like any other engine in overdrive, the stress gear erodes the machinery of our body and brain. Hence, the symptoms of psychophysical wear and tear—allostatic load—that most of us are experiencing.4
The subject of this column is the lower level of prolonged chronic stress. The mild and amorphous pertubations that can be described as “the brain in COVID-19.” It is a syndrome that affects even those who have never been infected with the actual virus. Though not usually life-threatening or disabling, it is unnerving and distressing as the queries from my colleagues and friends show. Their reports and my observations have led me to opine that “no one is okay” due to months of living under the strain of a pandemic.
The degree and scope of chronic stress that a person experiences caused by COVID-19 has to be contextualized and individualized. Those who have lost jobs, who are working while children are going to school online, who are caring for relatives, or who are in fear of losing their home face tremendous stress and challenges.5 Yet even those like me, whose biggest worry is a dog barking through important teleconference meetings, still undergo a milder form of near constant stress.
Consider that all of us have become strangers in an even stranger land. Masks have become an object of political controversy. In states where masking is mandatory, you must be mask vigilant every time you go out. In many areas of the country stores have limited hours, access, and supplies and any trip away from the house risks infection. Conversely, for those in a high-risk population, it may have been months since they have left home at all, and many sick, older, and vulnerable persons are suffering from isolation, loneliness, and boredom. The minor distractions and innocent pleasures that relieve day-to-day stress are no longer safe or available options, like eating out, attending shows, or taking trips.
Most of us are waiting for news of an effective available vaccine, some with yearning and others with dread. For George Gershwin, summertime meant that “the livin’ is easy,” but the summer of 2020 has been anything but easy and that takes its toll on the mind. Without adequate positive stimulation, attention wanders and memory fails to encode details, making even routine tasks more difficult; without meaningful social contact, emotions become sharp and ragged often hurting others. Most important, without periods in which we can relax, there is psychic exhaustion.6
At this point you may be thinking, “So, now that you told us we are all under chronic stress, are you going to tell us whether we can do anything about it?” There are many fantastic websites (including the VA) where experts far more qualified than I am offer excellent advice on coping with the pandemic.7 What I can provide is 5 reflections on managing the stress that I have used and that others with whom I shared them have found helpful.
1. Set realistic expectations. We are in a different reality in which we may need to take on smaller tasks, pace our work and take more breaks and, most of all, give ourselves a break when we are not as functional as we were before the pandemic.
2. Get out in nature. Find a green space to walk or sit, spend time with companion animals, go for a hike or bicycle near water or mountains, or watch the birds in a forest. Nothing can help restore our perspective or calm frayed nerves like the socially distanced outdoors.
3. Reach out. Even though we cannot hug or even shake hands, we can still pick up the phone or mouse and check on someone who is down. All the great traditions of the world agree that the best way to lift our own spirits is to help others.
4. Be kind. This is among the most important responses. As the epigraph suggests, everyone is engaged in an often silent and secret struggle and deserves our compassion. This call for kindness should be extended to ourselves so that we can be patient and compassionate to others.
5. Have courage and hope. This may be the most important of all. Whether we are infected or are fearing/avoiding infection, COVID-19 makes us sick in body and brain. We must have faith that there is something—the mind, the spirit—beyond the purely physical that gives us courage to outlast COVID-19 and to have hope for a postpandemic future that though not the same as before may well be in some ways better
1. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun . 2020;87:34-39. doi:10.1016/j.bbi.2020.04.027
2. Pappa S, Ntella V, Giannakas T, Giannakoulis VG, Papoutsi E, Katsaounou P. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901-907. doi:10.1016/j.bbi.2020.05.026
3. Salazar de Pablo G, Vaquerizo-Serrano J, Catalan A, et al. Impact of coronavirus syndromes on physical and mental health of health care workers: systematic review and meta-analysis. J Affect Disord. 2020;275:48-57. doi:10.1016/j.jad.2020.06.022.
4. Harkness K. Strange physical symptoms? Blame the chronic stress of life during the Covid-19 pandemic. https://the-conversation.com/strange-physical-symptoms-blame-the-chronic-stress-of-life-during-the-covid-19-pandemic-139096. Published June 11, 2020. Accessed August 29, 2020.
5. Centers for Disease Control and Prevention. Coronavirus Disease (COVID-19) 2019. Coping with stress. https://www.cdc.gov/coronavirus/2019-ncov/daily-life-coping/managing-stress-anxiety.html. Updated July 1, 2020. Accessed August 29, 2020.
6. Greenberg M. How the stress of the COVID-9 pandemic scrambles your brain. https://www.psychologytoday.com/us/blog/the-mindful-self-express/202006/how-the-stress-the-covid-19-pandemic-scrambles-your-brain. Published June 28, 2020. Accessed August 29, 2020.
7. US Department of Veterans Affairs, National Center for PTSD. Healthcare workers and responders. https://www.ptsd.va.gov/covid/list_healthcare_responders.asp. Updated August 12, 2020. Accessed August 29, 2020.
1. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun . 2020;87:34-39. doi:10.1016/j.bbi.2020.04.027
2. Pappa S, Ntella V, Giannakas T, Giannakoulis VG, Papoutsi E, Katsaounou P. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901-907. doi:10.1016/j.bbi.2020.05.026
3. Salazar de Pablo G, Vaquerizo-Serrano J, Catalan A, et al. Impact of coronavirus syndromes on physical and mental health of health care workers: systematic review and meta-analysis. J Affect Disord. 2020;275:48-57. doi:10.1016/j.jad.2020.06.022.
4. Harkness K. Strange physical symptoms? Blame the chronic stress of life during the Covid-19 pandemic. https://the-conversation.com/strange-physical-symptoms-blame-the-chronic-stress-of-life-during-the-covid-19-pandemic-139096. Published June 11, 2020. Accessed August 29, 2020.
5. Centers for Disease Control and Prevention. Coronavirus Disease (COVID-19) 2019. Coping with stress. https://www.cdc.gov/coronavirus/2019-ncov/daily-life-coping/managing-stress-anxiety.html. Updated July 1, 2020. Accessed August 29, 2020.
6. Greenberg M. How the stress of the COVID-9 pandemic scrambles your brain. https://www.psychologytoday.com/us/blog/the-mindful-self-express/202006/how-the-stress-the-covid-19-pandemic-scrambles-your-brain. Published June 28, 2020. Accessed August 29, 2020.
7. US Department of Veterans Affairs, National Center for PTSD. Healthcare workers and responders. https://www.ptsd.va.gov/covid/list_healthcare_responders.asp. Updated August 12, 2020. Accessed August 29, 2020.
Identifying ovarian malignancy is not so easy
When an ovarian mass is anticipated or known, following evaluation of a patient’s history and physician examination, imaging via transvaginal and often abdominal ultrasound is the very next step. This evaluation likely will include both gray-scale and color Doppler examination. The initial concern always must be to identify ovarian malignancy.
Despite morphological scoring systems as well as the use of Doppler ultrasonography, there remains a lack of agreement and acceptance. In a 2008 multicenter study, Timmerman and colleagues evaluated 1,066 patients with 1,233 persistent adnexal tumors via transvaginal grayscale and Doppler ultrasound; 73% were benign tumors, and 27% were malignant tumors. Information on 42 gray-scale ultrasound variables and 6 Doppler variables was collected and evaluated to determine which variables had the highest positive predictive value for a malignant tumor and for a benign mass (Ultrasound Obstet Gynecol. 2008 Jun. doi: 10.1002/uog.5365).
Five simple rules were selected that best predict malignancy (M-rules), as follows:
- Irregular solid tumor.
- Ascites.
- At least four papillary projections.
- Irregular multilocular-solid tumor with a greatest diameter greater than or equal to 10 cm.
- Very high color content on Doppler exam.
The following five simple rules suggested that a mass is benign (B-rules):
- Unilocular cyst.
- Largest solid component less than 7 mm.
- Acoustic shadows.
- Smooth multilocular tumor less than 10 cm.
- No detectable blood flow with Doppler exam.
Unfortunately, despite a sensitivity of 93% and specificity of 90%, and a positive and negative predictive value of 80% and 97%, these 10 simple rules were applicable to only 76% of tumors.
To assist those of us who are not gynecologic oncologists and who are often faced with having to determine whether surgery is recommended, I have elicited the expertise of Jubilee Brown, MD, professor and associate director of gynecologic oncology at the Levine Cancer Institute, Carolinas HealthCare System, in Charlotte, N.C., and the current president of the AAGL, to lead us in a review of evaluating an ovarian mass.
Dr. Miller is professor of obstetrics & gynecology in the department of clinical sciences, Rosalind Franklin University, North Chicago, Ill., and director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, both in Illinois. Email him at [email protected].
When an ovarian mass is anticipated or known, following evaluation of a patient’s history and physician examination, imaging via transvaginal and often abdominal ultrasound is the very next step. This evaluation likely will include both gray-scale and color Doppler examination. The initial concern always must be to identify ovarian malignancy.
Despite morphological scoring systems as well as the use of Doppler ultrasonography, there remains a lack of agreement and acceptance. In a 2008 multicenter study, Timmerman and colleagues evaluated 1,066 patients with 1,233 persistent adnexal tumors via transvaginal grayscale and Doppler ultrasound; 73% were benign tumors, and 27% were malignant tumors. Information on 42 gray-scale ultrasound variables and 6 Doppler variables was collected and evaluated to determine which variables had the highest positive predictive value for a malignant tumor and for a benign mass (Ultrasound Obstet Gynecol. 2008 Jun. doi: 10.1002/uog.5365).
Five simple rules were selected that best predict malignancy (M-rules), as follows:
- Irregular solid tumor.
- Ascites.
- At least four papillary projections.
- Irregular multilocular-solid tumor with a greatest diameter greater than or equal to 10 cm.
- Very high color content on Doppler exam.
The following five simple rules suggested that a mass is benign (B-rules):
- Unilocular cyst.
- Largest solid component less than 7 mm.
- Acoustic shadows.
- Smooth multilocular tumor less than 10 cm.
- No detectable blood flow with Doppler exam.
Unfortunately, despite a sensitivity of 93% and specificity of 90%, and a positive and negative predictive value of 80% and 97%, these 10 simple rules were applicable to only 76% of tumors.
To assist those of us who are not gynecologic oncologists and who are often faced with having to determine whether surgery is recommended, I have elicited the expertise of Jubilee Brown, MD, professor and associate director of gynecologic oncology at the Levine Cancer Institute, Carolinas HealthCare System, in Charlotte, N.C., and the current president of the AAGL, to lead us in a review of evaluating an ovarian mass.
Dr. Miller is professor of obstetrics & gynecology in the department of clinical sciences, Rosalind Franklin University, North Chicago, Ill., and director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, both in Illinois. Email him at [email protected].
When an ovarian mass is anticipated or known, following evaluation of a patient’s history and physician examination, imaging via transvaginal and often abdominal ultrasound is the very next step. This evaluation likely will include both gray-scale and color Doppler examination. The initial concern always must be to identify ovarian malignancy.
Despite morphological scoring systems as well as the use of Doppler ultrasonography, there remains a lack of agreement and acceptance. In a 2008 multicenter study, Timmerman and colleagues evaluated 1,066 patients with 1,233 persistent adnexal tumors via transvaginal grayscale and Doppler ultrasound; 73% were benign tumors, and 27% were malignant tumors. Information on 42 gray-scale ultrasound variables and 6 Doppler variables was collected and evaluated to determine which variables had the highest positive predictive value for a malignant tumor and for a benign mass (Ultrasound Obstet Gynecol. 2008 Jun. doi: 10.1002/uog.5365).
Five simple rules were selected that best predict malignancy (M-rules), as follows:
- Irregular solid tumor.
- Ascites.
- At least four papillary projections.
- Irregular multilocular-solid tumor with a greatest diameter greater than or equal to 10 cm.
- Very high color content on Doppler exam.
The following five simple rules suggested that a mass is benign (B-rules):
- Unilocular cyst.
- Largest solid component less than 7 mm.
- Acoustic shadows.
- Smooth multilocular tumor less than 10 cm.
- No detectable blood flow with Doppler exam.
Unfortunately, despite a sensitivity of 93% and specificity of 90%, and a positive and negative predictive value of 80% and 97%, these 10 simple rules were applicable to only 76% of tumors.
To assist those of us who are not gynecologic oncologists and who are often faced with having to determine whether surgery is recommended, I have elicited the expertise of Jubilee Brown, MD, professor and associate director of gynecologic oncology at the Levine Cancer Institute, Carolinas HealthCare System, in Charlotte, N.C., and the current president of the AAGL, to lead us in a review of evaluating an ovarian mass.
Dr. Miller is professor of obstetrics & gynecology in the department of clinical sciences, Rosalind Franklin University, North Chicago, Ill., and director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, both in Illinois. Email him at [email protected].
How to evaluate a suspicious ovarian mass
Ovarian masses are common in women of all ages. It is important not to miss even one ovarian cancer, but we must also identify masses that will resolve on their own over time to avoid overtreatment. These concurrent goals of excluding malignancy while not overtreating patients are the basis for management of the pelvic mass. Additionally, fertility preservation is important when surgery is performed in a reproductive-aged woman.
An ovarian mass may be anything from a simple functional or physiologic cyst to an endometrioma to an epithelial carcinoma, a germ-cell tumor, or a stromal tumor (the latter three of which may metastasize). Across the general population, women have a 5%-10% lifetime risk of needing surgery for a suspected ovarian mass and a 1.4% (1 in 70) risk that this mass is cancerous. The majority of ovarian cysts or masses therefore are benign.
A thorough history – including family history – and physical examination with appropriate laboratory testing and directed imaging are important first steps for the ob.gyn. Fortunately, we have guidelines and criteria governing not only when observation or surgery is warranted but also when patients should be referred to a gynecologic oncologist. By following these guidelines,1 we are able to achieve the best outcomes.
Transvaginal ultrasound
A 2007 groundbreaking study led by Barbara Goff, MD, demonstrated that there are warning signs for ovarian cancer – symptoms that are significantly associated with malignancy. Dr. Goff and her coinvestigators evaluated the charts of hundreds of patients, including about 150 with ovarian cancer, and found that pelvic/abdominal pressure or pain, bloating, increase in abdominal size, and difficulty eating or feeling full were significantly and independently associated with cancer if these symptoms were present for less than a year and occurred at least 12 times per month.2
A pelvic examination is an integral part of evaluating every patient who has such concerns. That said, pelvic exams have limited ability to identify adnexal masses, especially in women who are obese – and that’s where imaging becomes especially important.
Masses generally can be considered simple or complex based on their appearance. A simple cyst is fluid-filled with thin, smooth walls and the absence of solid components or septations; it is significantly more likely to resolve on its own and is less likely to imply malignancy than a complex cyst, especially in a premenopausal woman. A complex cyst is multiseptated and/or solid – possibly with papillary projections – and is more concerning, especially if there is increased, new vascularity. Making this distinction helps us determine the risk of malignancy.
Transvaginal ultrasound (TVUS) is the preferred method for imaging, and our threshold for obtaining a TVUS should be very low. Women who have symptoms or concerns that can’t be attributed to a particular condition, and women in whom a mass can be palpated (even if asymptomatic) should have a TVUS. The imaging modality is cost effective and well tolerated by patients, does not expose the patient to ionizing radiation, and should generally be considered first-line imaging.3,4
Size is not predictive of malignancy, but it is important for determining whether surgery is warranted. In our experience, a mass of 8-10 cm or larger on TVUS is at risk of torsion and is unlikely to resolve on its own, even in a premenopausal woman. While large masses generally require surgery, patients of any age who have simple cysts smaller than 8-10 cm generally can be followed with serial exams and ultrasound; spontaneous regression is common.
Doppler ultrasonography is useful for evaluating blood flow in and around an ovarian mass and can be helpful for confirming suspected characteristics of a mass.
Recent studies from the radiology community have looked at the utility of the resistive index – a measure of the impedance and velocity of blood flow – as a predictor of ovarian malignancy. However, we caution against using Doppler to determine whether a mass is benign or malignant, or to determine the necessity of surgery. An abnormal ovary may have what is considered to be a normal resistive index, and the resistive index of a normal ovary may fall within the abnormal range. Doppler flow can be helpful, but it must be combined with other predictive features, like solid components with flow or papillary projections within a cyst, to define a decision about surgery.4,5
Magnetic resonance imaging can be useful in differentiating a fibroid from an ovarian mass, and a CT scan can be helpful in looking for disseminated disease when ovarian cancer is suspected based on ultrasound imaging, physical and history, and serum markers. A CT is useful, for instance, in a patient whose ovary is distended with ascites or who has upper abdominal complaints and a complex cyst. CT, PET, and MRI are not recommended in the initial evaluation of an ovarian mass.
The utility of serum biomarkers
Cancer antigen 125 (CA-125) testing may be helpful – in combination with other findings – for decision-making regarding the likelihood of malignancy and the need to refer patients. CA-125 is like Doppler in that a normal CA-125 cannot eliminate the possibility of cancer, and an abnormal CA-125 does not in and of itself imply malignancy. It’s far from a perfect cancer screening test.
CA-125 is a protein associated with epithelial ovarian malignancies, the type of ovarian cancer most commonly seen in postmenopausal women with genetic predispositions. Its specificity and positive predictive value are much higher in postmenopausal women than in average-risk premenopausal women (those without a family history or a known mutation that predisposes them to ovarian cancer). Levels of the marker are elevated in association with many nonmalignant conditions in premenopausal women – endometriosis, fibroids, and various inflammatory conditions, for instance – so the marker’s utility in this population is limited.
For women who have a family history of ovarian cancer or a known breast cancer gene 1 (BRCA1) or BRCA2 mutation, there are some data that suggest that monitoring with CA-125 measurements and TVUS may be a good approach to following patients prior to the age at which risk-reducing surgery can best be performed.
In an adolescent girl or a woman of reproductive age, we think less about epithelial cancer and more about germ-cell and stromal tumors. When a solid mass is palpated or visualized on imaging, we therefore will utilize a different set of markers; alpha-fetoprotein, L-lactate dehydrogenase, and beta-HCG, for instance, have much higher specificity than CA-125 does for germ-cell tumors in this age group and may be helpful in the evaluation. Similarly, in cases of a very large mass resembling a mucinous tumor, a carcinoembryonic antigen may be helpful.
A number of proprietary profiling technologies have been developed to determine the risk of a diagnosed mass being malignant. For instance, the OVA1 assay looks at five serum markers and scores the results, and the Risk of Ovarian Malignancy Algorithm (ROMA) combines the results of three serum markers with menopausal status into a numerical score. Both have Food and Drug Administration approval for use in women in whom surgery has been deemed necessary. These panels can be fairly predictive of risk and may be helpful – especially in rural areas – in determining which women should be referred to a gynecologic oncologist for surgery.
It is important to appreciate that an ovarian cyst or mass should never be biopsied or aspirated lest a malignant tumor confined to one ovary be potentially spread to the peritoneum.
Referral to a gynecologic oncologist
Postmenopausal women with a CA-125 greater than 35 U/mL should be referred, as should postmenopausal women with ascites, those with a nodular or fixed pelvic mass, and those with suspected abdominal or distant metastases (per a CT scan, for instance).
In premenopausal women, ascites, a nodular or fixed mass, and evidence of metastases also are reasons for referral to a gynecologic oncologist. CA-125, again, is much more likely to be elevated for reasons other than malignancy and therefore is not as strong a driver for referral as in postmenopausal women. Patients with markedly elevated levels, however, should probably be referred – particularly when other clinical factors also suggest the need for consultation. While there is no evidence-based threshold for CA-125 in premenopausal women, a CA-125 greater than 200 U/mL is a good cutoff for referral.
For any patient, family history of breast and/or ovarian cancer – especially in a first-degree relative – raises the risk of malignancy and should figure prominently into decision-making regarding referral. Criteria for referral are among the points discussed in the ACOG 2016 Practice Bulletin on Evaluation and Management of Adnexal Masses.1
A note on BRCA mutations
As the American College of Obstetricians and Gynecologists says in its practice bulletin, the most important personal risk factor for ovarian cancer is a strong family history of breast or ovarian cancer. Women with such a family history can undergo genetic testing for BRCA mutations and have the opportunity to prevent ovarian cancers when mutations are detected. This simple blood test can save lives.
A modeling study we recently completed – not yet published – shows that it actually would be cost effective to do population screening with BRCA testing performed on every woman at age 30 years.
According to the National Cancer Institute website (last review: 2018), it is estimated that about 44% of women who inherit a BRCA1 mutation, and about 17% of those who inherit a BRAC2 mutation, will develop ovarian cancer by the age of 80 years. By identifying those mutations, women may undergo risk-reducing surgery at designated ages after childbearing is complete and bring their risk down to under 5%.
An international take on managing adnexal masses
- Pelvic ultrasound should include the transvaginal approach. Use Doppler imaging as indicated.
- Although simple ovarian cysts are not precursor lesions to a malignant ovarian cancer, perform a high-quality examination to make sure there are no solid/papillary structures before classifying a cyst as a simple cyst. The risk of progression to malignancy is extremely low, but some follow-up is prudent.
- The most accurate method of characterizing an ovarian mass currently is real-time pattern recognition sonography in the hands of an experienced imager.
- Pattern recognition sonography or a risk model such as the International Ovarian Tumor Analysis (IOTA) Simple Rules can be used to initially characterize an ovarian mass.
- When an ovarian lesion is classified as benign, the patient may be followed conservatively, or if indicated, surgery can be performed by a general gynecologist.
- Serial sonography can be beneficial, but there are limited prospective data to support an exact interval and duration.
- Fewer surgical interventions may result in an increase in sonographic surveillance.
- When an ovarian lesion is considered indeterminate on initial sonography, and after appropriate clinical evaluation, a “second-step” evaluation may include referral to an expert sonologist, serial sonography, application of established risk-prediction models, correlation with serum biomarkers, correlation with MRI, or referral to a gynecologic oncologist for further evaluation.
From the First International Consensus Report on Adnexal Masses: Management Recommendations
Source: Glanc P et al. J Ultrasound Med. 2017 May;36(5):849-63.
Dr. Brown reported that she had received an earlier grant from Aspira Labs, the company that developed the OVA1 assay. Dr. Miller reported that he has no relevant financial disclosures.
References
1. Obstet Gynecol. 2016 Nov. doi: 10.1097/AOG.0000000000001768.
2. Cancer. 2007 Jan 15. doi: 10.1002/cncr.22371.
3. Clin Obstet Gynecol. 2015 Mar. doi: 10.1097/GRF.0000000000000083.
4. Ultrasound Q. 2013 Mar. doi: 10.1097/RUQ.0b013e3182814d9b.
5. Ultrasound Obstet Gynecol. 2008 Jun. doi: 10.1002/uog.5365.
Ovarian masses are common in women of all ages. It is important not to miss even one ovarian cancer, but we must also identify masses that will resolve on their own over time to avoid overtreatment. These concurrent goals of excluding malignancy while not overtreating patients are the basis for management of the pelvic mass. Additionally, fertility preservation is important when surgery is performed in a reproductive-aged woman.
An ovarian mass may be anything from a simple functional or physiologic cyst to an endometrioma to an epithelial carcinoma, a germ-cell tumor, or a stromal tumor (the latter three of which may metastasize). Across the general population, women have a 5%-10% lifetime risk of needing surgery for a suspected ovarian mass and a 1.4% (1 in 70) risk that this mass is cancerous. The majority of ovarian cysts or masses therefore are benign.
A thorough history – including family history – and physical examination with appropriate laboratory testing and directed imaging are important first steps for the ob.gyn. Fortunately, we have guidelines and criteria governing not only when observation or surgery is warranted but also when patients should be referred to a gynecologic oncologist. By following these guidelines,1 we are able to achieve the best outcomes.
Transvaginal ultrasound
A 2007 groundbreaking study led by Barbara Goff, MD, demonstrated that there are warning signs for ovarian cancer – symptoms that are significantly associated with malignancy. Dr. Goff and her coinvestigators evaluated the charts of hundreds of patients, including about 150 with ovarian cancer, and found that pelvic/abdominal pressure or pain, bloating, increase in abdominal size, and difficulty eating or feeling full were significantly and independently associated with cancer if these symptoms were present for less than a year and occurred at least 12 times per month.2
A pelvic examination is an integral part of evaluating every patient who has such concerns. That said, pelvic exams have limited ability to identify adnexal masses, especially in women who are obese – and that’s where imaging becomes especially important.
Masses generally can be considered simple or complex based on their appearance. A simple cyst is fluid-filled with thin, smooth walls and the absence of solid components or septations; it is significantly more likely to resolve on its own and is less likely to imply malignancy than a complex cyst, especially in a premenopausal woman. A complex cyst is multiseptated and/or solid – possibly with papillary projections – and is more concerning, especially if there is increased, new vascularity. Making this distinction helps us determine the risk of malignancy.
Transvaginal ultrasound (TVUS) is the preferred method for imaging, and our threshold for obtaining a TVUS should be very low. Women who have symptoms or concerns that can’t be attributed to a particular condition, and women in whom a mass can be palpated (even if asymptomatic) should have a TVUS. The imaging modality is cost effective and well tolerated by patients, does not expose the patient to ionizing radiation, and should generally be considered first-line imaging.3,4
Size is not predictive of malignancy, but it is important for determining whether surgery is warranted. In our experience, a mass of 8-10 cm or larger on TVUS is at risk of torsion and is unlikely to resolve on its own, even in a premenopausal woman. While large masses generally require surgery, patients of any age who have simple cysts smaller than 8-10 cm generally can be followed with serial exams and ultrasound; spontaneous regression is common.
Doppler ultrasonography is useful for evaluating blood flow in and around an ovarian mass and can be helpful for confirming suspected characteristics of a mass.
Recent studies from the radiology community have looked at the utility of the resistive index – a measure of the impedance and velocity of blood flow – as a predictor of ovarian malignancy. However, we caution against using Doppler to determine whether a mass is benign or malignant, or to determine the necessity of surgery. An abnormal ovary may have what is considered to be a normal resistive index, and the resistive index of a normal ovary may fall within the abnormal range. Doppler flow can be helpful, but it must be combined with other predictive features, like solid components with flow or papillary projections within a cyst, to define a decision about surgery.4,5
Magnetic resonance imaging can be useful in differentiating a fibroid from an ovarian mass, and a CT scan can be helpful in looking for disseminated disease when ovarian cancer is suspected based on ultrasound imaging, physical and history, and serum markers. A CT is useful, for instance, in a patient whose ovary is distended with ascites or who has upper abdominal complaints and a complex cyst. CT, PET, and MRI are not recommended in the initial evaluation of an ovarian mass.
The utility of serum biomarkers
Cancer antigen 125 (CA-125) testing may be helpful – in combination with other findings – for decision-making regarding the likelihood of malignancy and the need to refer patients. CA-125 is like Doppler in that a normal CA-125 cannot eliminate the possibility of cancer, and an abnormal CA-125 does not in and of itself imply malignancy. It’s far from a perfect cancer screening test.
CA-125 is a protein associated with epithelial ovarian malignancies, the type of ovarian cancer most commonly seen in postmenopausal women with genetic predispositions. Its specificity and positive predictive value are much higher in postmenopausal women than in average-risk premenopausal women (those without a family history or a known mutation that predisposes them to ovarian cancer). Levels of the marker are elevated in association with many nonmalignant conditions in premenopausal women – endometriosis, fibroids, and various inflammatory conditions, for instance – so the marker’s utility in this population is limited.
For women who have a family history of ovarian cancer or a known breast cancer gene 1 (BRCA1) or BRCA2 mutation, there are some data that suggest that monitoring with CA-125 measurements and TVUS may be a good approach to following patients prior to the age at which risk-reducing surgery can best be performed.
In an adolescent girl or a woman of reproductive age, we think less about epithelial cancer and more about germ-cell and stromal tumors. When a solid mass is palpated or visualized on imaging, we therefore will utilize a different set of markers; alpha-fetoprotein, L-lactate dehydrogenase, and beta-HCG, for instance, have much higher specificity than CA-125 does for germ-cell tumors in this age group and may be helpful in the evaluation. Similarly, in cases of a very large mass resembling a mucinous tumor, a carcinoembryonic antigen may be helpful.
A number of proprietary profiling technologies have been developed to determine the risk of a diagnosed mass being malignant. For instance, the OVA1 assay looks at five serum markers and scores the results, and the Risk of Ovarian Malignancy Algorithm (ROMA) combines the results of three serum markers with menopausal status into a numerical score. Both have Food and Drug Administration approval for use in women in whom surgery has been deemed necessary. These panels can be fairly predictive of risk and may be helpful – especially in rural areas – in determining which women should be referred to a gynecologic oncologist for surgery.
It is important to appreciate that an ovarian cyst or mass should never be biopsied or aspirated lest a malignant tumor confined to one ovary be potentially spread to the peritoneum.
Referral to a gynecologic oncologist
Postmenopausal women with a CA-125 greater than 35 U/mL should be referred, as should postmenopausal women with ascites, those with a nodular or fixed pelvic mass, and those with suspected abdominal or distant metastases (per a CT scan, for instance).
In premenopausal women, ascites, a nodular or fixed mass, and evidence of metastases also are reasons for referral to a gynecologic oncologist. CA-125, again, is much more likely to be elevated for reasons other than malignancy and therefore is not as strong a driver for referral as in postmenopausal women. Patients with markedly elevated levels, however, should probably be referred – particularly when other clinical factors also suggest the need for consultation. While there is no evidence-based threshold for CA-125 in premenopausal women, a CA-125 greater than 200 U/mL is a good cutoff for referral.
For any patient, family history of breast and/or ovarian cancer – especially in a first-degree relative – raises the risk of malignancy and should figure prominently into decision-making regarding referral. Criteria for referral are among the points discussed in the ACOG 2016 Practice Bulletin on Evaluation and Management of Adnexal Masses.1
A note on BRCA mutations
As the American College of Obstetricians and Gynecologists says in its practice bulletin, the most important personal risk factor for ovarian cancer is a strong family history of breast or ovarian cancer. Women with such a family history can undergo genetic testing for BRCA mutations and have the opportunity to prevent ovarian cancers when mutations are detected. This simple blood test can save lives.
A modeling study we recently completed – not yet published – shows that it actually would be cost effective to do population screening with BRCA testing performed on every woman at age 30 years.
According to the National Cancer Institute website (last review: 2018), it is estimated that about 44% of women who inherit a BRCA1 mutation, and about 17% of those who inherit a BRAC2 mutation, will develop ovarian cancer by the age of 80 years. By identifying those mutations, women may undergo risk-reducing surgery at designated ages after childbearing is complete and bring their risk down to under 5%.
An international take on managing adnexal masses
- Pelvic ultrasound should include the transvaginal approach. Use Doppler imaging as indicated.
- Although simple ovarian cysts are not precursor lesions to a malignant ovarian cancer, perform a high-quality examination to make sure there are no solid/papillary structures before classifying a cyst as a simple cyst. The risk of progression to malignancy is extremely low, but some follow-up is prudent.
- The most accurate method of characterizing an ovarian mass currently is real-time pattern recognition sonography in the hands of an experienced imager.
- Pattern recognition sonography or a risk model such as the International Ovarian Tumor Analysis (IOTA) Simple Rules can be used to initially characterize an ovarian mass.
- When an ovarian lesion is classified as benign, the patient may be followed conservatively, or if indicated, surgery can be performed by a general gynecologist.
- Serial sonography can be beneficial, but there are limited prospective data to support an exact interval and duration.
- Fewer surgical interventions may result in an increase in sonographic surveillance.
- When an ovarian lesion is considered indeterminate on initial sonography, and after appropriate clinical evaluation, a “second-step” evaluation may include referral to an expert sonologist, serial sonography, application of established risk-prediction models, correlation with serum biomarkers, correlation with MRI, or referral to a gynecologic oncologist for further evaluation.
From the First International Consensus Report on Adnexal Masses: Management Recommendations
Source: Glanc P et al. J Ultrasound Med. 2017 May;36(5):849-63.
Dr. Brown reported that she had received an earlier grant from Aspira Labs, the company that developed the OVA1 assay. Dr. Miller reported that he has no relevant financial disclosures.
References
1. Obstet Gynecol. 2016 Nov. doi: 10.1097/AOG.0000000000001768.
2. Cancer. 2007 Jan 15. doi: 10.1002/cncr.22371.
3. Clin Obstet Gynecol. 2015 Mar. doi: 10.1097/GRF.0000000000000083.
4. Ultrasound Q. 2013 Mar. doi: 10.1097/RUQ.0b013e3182814d9b.
5. Ultrasound Obstet Gynecol. 2008 Jun. doi: 10.1002/uog.5365.
Ovarian masses are common in women of all ages. It is important not to miss even one ovarian cancer, but we must also identify masses that will resolve on their own over time to avoid overtreatment. These concurrent goals of excluding malignancy while not overtreating patients are the basis for management of the pelvic mass. Additionally, fertility preservation is important when surgery is performed in a reproductive-aged woman.
An ovarian mass may be anything from a simple functional or physiologic cyst to an endometrioma to an epithelial carcinoma, a germ-cell tumor, or a stromal tumor (the latter three of which may metastasize). Across the general population, women have a 5%-10% lifetime risk of needing surgery for a suspected ovarian mass and a 1.4% (1 in 70) risk that this mass is cancerous. The majority of ovarian cysts or masses therefore are benign.
A thorough history – including family history – and physical examination with appropriate laboratory testing and directed imaging are important first steps for the ob.gyn. Fortunately, we have guidelines and criteria governing not only when observation or surgery is warranted but also when patients should be referred to a gynecologic oncologist. By following these guidelines,1 we are able to achieve the best outcomes.
Transvaginal ultrasound
A 2007 groundbreaking study led by Barbara Goff, MD, demonstrated that there are warning signs for ovarian cancer – symptoms that are significantly associated with malignancy. Dr. Goff and her coinvestigators evaluated the charts of hundreds of patients, including about 150 with ovarian cancer, and found that pelvic/abdominal pressure or pain, bloating, increase in abdominal size, and difficulty eating or feeling full were significantly and independently associated with cancer if these symptoms were present for less than a year and occurred at least 12 times per month.2
A pelvic examination is an integral part of evaluating every patient who has such concerns. That said, pelvic exams have limited ability to identify adnexal masses, especially in women who are obese – and that’s where imaging becomes especially important.
Masses generally can be considered simple or complex based on their appearance. A simple cyst is fluid-filled with thin, smooth walls and the absence of solid components or septations; it is significantly more likely to resolve on its own and is less likely to imply malignancy than a complex cyst, especially in a premenopausal woman. A complex cyst is multiseptated and/or solid – possibly with papillary projections – and is more concerning, especially if there is increased, new vascularity. Making this distinction helps us determine the risk of malignancy.
Transvaginal ultrasound (TVUS) is the preferred method for imaging, and our threshold for obtaining a TVUS should be very low. Women who have symptoms or concerns that can’t be attributed to a particular condition, and women in whom a mass can be palpated (even if asymptomatic) should have a TVUS. The imaging modality is cost effective and well tolerated by patients, does not expose the patient to ionizing radiation, and should generally be considered first-line imaging.3,4
Size is not predictive of malignancy, but it is important for determining whether surgery is warranted. In our experience, a mass of 8-10 cm or larger on TVUS is at risk of torsion and is unlikely to resolve on its own, even in a premenopausal woman. While large masses generally require surgery, patients of any age who have simple cysts smaller than 8-10 cm generally can be followed with serial exams and ultrasound; spontaneous regression is common.
Doppler ultrasonography is useful for evaluating blood flow in and around an ovarian mass and can be helpful for confirming suspected characteristics of a mass.
Recent studies from the radiology community have looked at the utility of the resistive index – a measure of the impedance and velocity of blood flow – as a predictor of ovarian malignancy. However, we caution against using Doppler to determine whether a mass is benign or malignant, or to determine the necessity of surgery. An abnormal ovary may have what is considered to be a normal resistive index, and the resistive index of a normal ovary may fall within the abnormal range. Doppler flow can be helpful, but it must be combined with other predictive features, like solid components with flow or papillary projections within a cyst, to define a decision about surgery.4,5
Magnetic resonance imaging can be useful in differentiating a fibroid from an ovarian mass, and a CT scan can be helpful in looking for disseminated disease when ovarian cancer is suspected based on ultrasound imaging, physical and history, and serum markers. A CT is useful, for instance, in a patient whose ovary is distended with ascites or who has upper abdominal complaints and a complex cyst. CT, PET, and MRI are not recommended in the initial evaluation of an ovarian mass.
The utility of serum biomarkers
Cancer antigen 125 (CA-125) testing may be helpful – in combination with other findings – for decision-making regarding the likelihood of malignancy and the need to refer patients. CA-125 is like Doppler in that a normal CA-125 cannot eliminate the possibility of cancer, and an abnormal CA-125 does not in and of itself imply malignancy. It’s far from a perfect cancer screening test.
CA-125 is a protein associated with epithelial ovarian malignancies, the type of ovarian cancer most commonly seen in postmenopausal women with genetic predispositions. Its specificity and positive predictive value are much higher in postmenopausal women than in average-risk premenopausal women (those without a family history or a known mutation that predisposes them to ovarian cancer). Levels of the marker are elevated in association with many nonmalignant conditions in premenopausal women – endometriosis, fibroids, and various inflammatory conditions, for instance – so the marker’s utility in this population is limited.
For women who have a family history of ovarian cancer or a known breast cancer gene 1 (BRCA1) or BRCA2 mutation, there are some data that suggest that monitoring with CA-125 measurements and TVUS may be a good approach to following patients prior to the age at which risk-reducing surgery can best be performed.
In an adolescent girl or a woman of reproductive age, we think less about epithelial cancer and more about germ-cell and stromal tumors. When a solid mass is palpated or visualized on imaging, we therefore will utilize a different set of markers; alpha-fetoprotein, L-lactate dehydrogenase, and beta-HCG, for instance, have much higher specificity than CA-125 does for germ-cell tumors in this age group and may be helpful in the evaluation. Similarly, in cases of a very large mass resembling a mucinous tumor, a carcinoembryonic antigen may be helpful.
A number of proprietary profiling technologies have been developed to determine the risk of a diagnosed mass being malignant. For instance, the OVA1 assay looks at five serum markers and scores the results, and the Risk of Ovarian Malignancy Algorithm (ROMA) combines the results of three serum markers with menopausal status into a numerical score. Both have Food and Drug Administration approval for use in women in whom surgery has been deemed necessary. These panels can be fairly predictive of risk and may be helpful – especially in rural areas – in determining which women should be referred to a gynecologic oncologist for surgery.
It is important to appreciate that an ovarian cyst or mass should never be biopsied or aspirated lest a malignant tumor confined to one ovary be potentially spread to the peritoneum.
Referral to a gynecologic oncologist
Postmenopausal women with a CA-125 greater than 35 U/mL should be referred, as should postmenopausal women with ascites, those with a nodular or fixed pelvic mass, and those with suspected abdominal or distant metastases (per a CT scan, for instance).
In premenopausal women, ascites, a nodular or fixed mass, and evidence of metastases also are reasons for referral to a gynecologic oncologist. CA-125, again, is much more likely to be elevated for reasons other than malignancy and therefore is not as strong a driver for referral as in postmenopausal women. Patients with markedly elevated levels, however, should probably be referred – particularly when other clinical factors also suggest the need for consultation. While there is no evidence-based threshold for CA-125 in premenopausal women, a CA-125 greater than 200 U/mL is a good cutoff for referral.
For any patient, family history of breast and/or ovarian cancer – especially in a first-degree relative – raises the risk of malignancy and should figure prominently into decision-making regarding referral. Criteria for referral are among the points discussed in the ACOG 2016 Practice Bulletin on Evaluation and Management of Adnexal Masses.1
A note on BRCA mutations
As the American College of Obstetricians and Gynecologists says in its practice bulletin, the most important personal risk factor for ovarian cancer is a strong family history of breast or ovarian cancer. Women with such a family history can undergo genetic testing for BRCA mutations and have the opportunity to prevent ovarian cancers when mutations are detected. This simple blood test can save lives.
A modeling study we recently completed – not yet published – shows that it actually would be cost effective to do population screening with BRCA testing performed on every woman at age 30 years.
According to the National Cancer Institute website (last review: 2018), it is estimated that about 44% of women who inherit a BRCA1 mutation, and about 17% of those who inherit a BRAC2 mutation, will develop ovarian cancer by the age of 80 years. By identifying those mutations, women may undergo risk-reducing surgery at designated ages after childbearing is complete and bring their risk down to under 5%.
An international take on managing adnexal masses
- Pelvic ultrasound should include the transvaginal approach. Use Doppler imaging as indicated.
- Although simple ovarian cysts are not precursor lesions to a malignant ovarian cancer, perform a high-quality examination to make sure there are no solid/papillary structures before classifying a cyst as a simple cyst. The risk of progression to malignancy is extremely low, but some follow-up is prudent.
- The most accurate method of characterizing an ovarian mass currently is real-time pattern recognition sonography in the hands of an experienced imager.
- Pattern recognition sonography or a risk model such as the International Ovarian Tumor Analysis (IOTA) Simple Rules can be used to initially characterize an ovarian mass.
- When an ovarian lesion is classified as benign, the patient may be followed conservatively, or if indicated, surgery can be performed by a general gynecologist.
- Serial sonography can be beneficial, but there are limited prospective data to support an exact interval and duration.
- Fewer surgical interventions may result in an increase in sonographic surveillance.
- When an ovarian lesion is considered indeterminate on initial sonography, and after appropriate clinical evaluation, a “second-step” evaluation may include referral to an expert sonologist, serial sonography, application of established risk-prediction models, correlation with serum biomarkers, correlation with MRI, or referral to a gynecologic oncologist for further evaluation.
From the First International Consensus Report on Adnexal Masses: Management Recommendations
Source: Glanc P et al. J Ultrasound Med. 2017 May;36(5):849-63.
Dr. Brown reported that she had received an earlier grant from Aspira Labs, the company that developed the OVA1 assay. Dr. Miller reported that he has no relevant financial disclosures.
References
1. Obstet Gynecol. 2016 Nov. doi: 10.1097/AOG.0000000000001768.
2. Cancer. 2007 Jan 15. doi: 10.1002/cncr.22371.
3. Clin Obstet Gynecol. 2015 Mar. doi: 10.1097/GRF.0000000000000083.
4. Ultrasound Q. 2013 Mar. doi: 10.1097/RUQ.0b013e3182814d9b.
5. Ultrasound Obstet Gynecol. 2008 Jun. doi: 10.1002/uog.5365.
A practical approach to utilizing cannabis as adjuvant therapy in inflammatory bowel disease
Case 1
A 30 year-old female with longstanding ulcerative colitis who has a history of medically refractory steroid-dependent disease and was able to achieve remission with vedolizumab for the last 5 years. Most recent objective assessment showed histologic remission. She has been using daily cannabis medicinally for the last year (high CBD:THC [cannabidiol:delta-9-tetracannabidol] concentration). She notes that she has felt better in the last year since introducing cannabis (improved stool frequency/formation, sleep quality). She inquires about discontinuing her biologic therapy in the hope of using cannabis alone to maintain remission.

Figure 1.
Case 2
A 22-year-old male with ileocolonic inflammatory Crohn’s disease escalated to adalimumab requiring an intensification of therapy to weekly dosing to normalize C-reactive protein (CRP). A recent colonoscopy showed endoscopic improvement (colonic normalization and rare aphthae in ileum). He notes clear clinical improvement, but he continues to experience diarrhea and abdominal cramping (no relationship to meals). Declines addition of immunomodulator (nervous about returning to college during the COVID-19 pandemic). He wonders whether cannabis could be effective in controlling his symptoms as he has had improvement in symptoms during his sporadic recreational cannabis exposure.
Discussion
These cases outline the challenges that providers face when managing patients with inflammatory bowel disease (IBD) when a patient would like to either substitute or incorporate cannabis into their treatment plan. Studies have shown a high prevalence of cannabis use among patients with IBD. With the restrictions surrounding the use of cannabis – either medically or recreationally – being liberalized in many states, these conversations are likely to become more frequent in your practice. However, one of the first challenges that providers face surrounding cannabis is that many patients who use cannabis do not disclose use to their health care team for fear of being judged negatively. In addition, many providers do not routinely ask about cannabis use during office visits. This might be directly related to being unprepared to have a knowledge-based discussion on the risks and benefits of cannabis use in IBD, with the same confidence present during discussion of biologic therapies.
For background, Cannabis sativa (cannabis) is composed of hundreds of phytocannabinoids, the two most common are THC and CBD. These cannabinoids act at the endocannabinoid receptors, which are expressed in the central and peripheral nervous systems and immune cells/tissues, and help explain the clinical changes experienced by cannabis users. Both THC and CBD have been studied in varying doses and routes of administration in patients with IBD, making it challenging to translate into real-world recommendations for patients. Some of the most common reported benefits of cannabis use (particularly in an IBD population) are improvement in pain, diarrhea, nausea, and joint pain. Some studies have shown overall improvement in quality of life (Figure 1).
Some common questions that arise surrounding cannabis use in IBD patients include:
1. Is it possible to stop traditional medical therapy and replace it with cannabis therapy?
No studies have directly addressed this exact question. The small studies, both randomized controlled trials and retrospective ones, have studied the effects of cannabis as adjuvant therapy only. None of the data available to date suggest that cannabis has any anti-inflammatory properties with absence of improvement in biomarkers or endoscopic measures of inflammation. In effect, any attempt to discontinue standard therapy with substitution of cannabis-based therapy should be seen as no different than simply discontinuing standard therapy. There exists the argument that – among those with moderate to severe disease – cannabis might suppress the investigation of mild symptoms which may herald a flare of disease, thus lulling the patient into a state of false stability. We do not advocate the substitution of cannabis products in place of standard medical therapy.
2. Is there a role for cannabis as adjuvant therapy in patients with IBD?
Studies to date have included only symptomatic patients with objective evidence of inflammation and assessed clinical, biochemical, or endoscopic endpoints. In Crohn’s disease, two studies showed no improvement in clinical remission rates but showed improvement in clinical response; a third study showed both improvement in clinical remission/response as well as improved quality of life. No study showed a change in disease markers of activity including CRP, fecal calprotectin, or endoscopic scoring. In one study, all patients relapsed shortly after cannabis discontinuation suggesting that, while there was benefit in symptom control, there was no improvement of the underlying chronic inflammation.
In patients with ulcerative colitis, there were two studies. One study showed no improvement and high rates of intolerance in the treatment group, while the other study reported improved disease activity but no objective improvement. The variation in results between disease states and between studies might be because of cannabis formulations. In patients with persistent symptoms despite current medical therapy, there might be a role in those patients for adjuvant therapy for improvement symptom control but not disease control. Optimization of medical therapy would still be indicated.
3. What dose and formulation of cannabis should I recommend to a patient as adjuvant therapy?
This is an excellent question and one that unfortunately we do not have the answer to. As mentioned previously, the studies have looked at varying formulations (THC alone, CBD:THC with varying percentages of THC, CBD alone) and varying routes of administration (sublingual, oral, inhalation). The IBD studies looking at CBD-alone formulations lacked clinical efficacy. In states where cannabis products have been accessible to IBD patients, no data on the product type (THC:CBD), method of administration, or prescriber preferences have been published.
4. What risks should I advise my patients about with cannabis use?
The challenge is that we don’t have large population-based studies in IBD looking at long-term risks of cannabis use. However, in the small RCT studies there were minimal reported side effects and no major adverse events over 8-10 weeks. Larger IBD population-based studies have shown that cannabis users were more likely to discontinue traditional medical therapy, and there is an increased risk for surgery in patients with Crohn’s disease. Larger studies in non-IBD patients have shown risk for addiction to other substances, diminished life achievement, increased motor vehicle accidents, chronic bronchitis, psychiatric disturbances and cannabis dependence, and cannabis hyperemesis syndrome (with an uncanny presentation resembling Crohn’s disease flare with partial small bowel obstruction). Patients should also be advised about legal implications of use (given its continued classification as a federal schedule 1 drug), possible drug interactions, and special considerations in pediatric patients (increased risk of addiction), elderly patients (increased risk of neuropsychological effects), and during pregnancy (with national obstetric society guidelines warning against use because of fetal exposure and increased risk of stillbirth).
5. What are the legal implications for providers? Patients?
As of July 2020, cannabis is available for recreational use in 12 states, for medicinal use in 28 states, and illegal in 11 states. So the answer really depends on what state the patient lives in. As a provider who might certify patients (in some medicinal states) or recommend cannabis to patients, you should consider legal and licensing implications. Again, this might vary state to state, and you should also take into account federal status. Providers acting in compliance with state laws are unlikely to have federal consequences. However, remember that malpractice insurance only covers FDA-approved medical therapies. Patients should be advised to consider the potential (although highly unlikely) to face federal prosecution and implications of use for employment, school, camp, or travel, and driving restrictions.
Take home points
- Inquire about cannabis to start the conversation.
- Know your state’s legalization status surrounding cannabis.
- Patients with IBD report improvement in symptoms and quality of life with adjuvant cannabis use; however, there is no change in disease activity.
- Encourage your patients to continue and optimize their maintenance therapy.
- Educate your patients about the legal considerations and known risks.
In conclusion, the use of cannabis in IBD patients has increased in recent years. It is important to be able to discuss the risks and benefits of use with your IBD patients. Focus on the lack of data showing that cannabis improves disease activity, and has shown benefit only in improving IBD-associated symptoms. In some patients there might be a role for adjuvant cannabis therapy to improve overall symptom control and quality of life.
Dr. Kinnucan is an assistant professor of medicine, division of gastroenterology, Michigan Medicine, University of Michigan, Ann Arbor; Dr. Swaminath is an associate professor of medicine, division of gastroenterology, Lenox Hill Hospital, Northwell Health, New York.
Case 1
A 30 year-old female with longstanding ulcerative colitis who has a history of medically refractory steroid-dependent disease and was able to achieve remission with vedolizumab for the last 5 years. Most recent objective assessment showed histologic remission. She has been using daily cannabis medicinally for the last year (high CBD:THC [cannabidiol:delta-9-tetracannabidol] concentration). She notes that she has felt better in the last year since introducing cannabis (improved stool frequency/formation, sleep quality). She inquires about discontinuing her biologic therapy in the hope of using cannabis alone to maintain remission.

Figure 1.
Case 2
A 22-year-old male with ileocolonic inflammatory Crohn’s disease escalated to adalimumab requiring an intensification of therapy to weekly dosing to normalize C-reactive protein (CRP). A recent colonoscopy showed endoscopic improvement (colonic normalization and rare aphthae in ileum). He notes clear clinical improvement, but he continues to experience diarrhea and abdominal cramping (no relationship to meals). Declines addition of immunomodulator (nervous about returning to college during the COVID-19 pandemic). He wonders whether cannabis could be effective in controlling his symptoms as he has had improvement in symptoms during his sporadic recreational cannabis exposure.
Discussion
These cases outline the challenges that providers face when managing patients with inflammatory bowel disease (IBD) when a patient would like to either substitute or incorporate cannabis into their treatment plan. Studies have shown a high prevalence of cannabis use among patients with IBD. With the restrictions surrounding the use of cannabis – either medically or recreationally – being liberalized in many states, these conversations are likely to become more frequent in your practice. However, one of the first challenges that providers face surrounding cannabis is that many patients who use cannabis do not disclose use to their health care team for fear of being judged negatively. In addition, many providers do not routinely ask about cannabis use during office visits. This might be directly related to being unprepared to have a knowledge-based discussion on the risks and benefits of cannabis use in IBD, with the same confidence present during discussion of biologic therapies.
For background, Cannabis sativa (cannabis) is composed of hundreds of phytocannabinoids, the two most common are THC and CBD. These cannabinoids act at the endocannabinoid receptors, which are expressed in the central and peripheral nervous systems and immune cells/tissues, and help explain the clinical changes experienced by cannabis users. Both THC and CBD have been studied in varying doses and routes of administration in patients with IBD, making it challenging to translate into real-world recommendations for patients. Some of the most common reported benefits of cannabis use (particularly in an IBD population) are improvement in pain, diarrhea, nausea, and joint pain. Some studies have shown overall improvement in quality of life (Figure 1).
Some common questions that arise surrounding cannabis use in IBD patients include:
1. Is it possible to stop traditional medical therapy and replace it with cannabis therapy?
No studies have directly addressed this exact question. The small studies, both randomized controlled trials and retrospective ones, have studied the effects of cannabis as adjuvant therapy only. None of the data available to date suggest that cannabis has any anti-inflammatory properties with absence of improvement in biomarkers or endoscopic measures of inflammation. In effect, any attempt to discontinue standard therapy with substitution of cannabis-based therapy should be seen as no different than simply discontinuing standard therapy. There exists the argument that – among those with moderate to severe disease – cannabis might suppress the investigation of mild symptoms which may herald a flare of disease, thus lulling the patient into a state of false stability. We do not advocate the substitution of cannabis products in place of standard medical therapy.
2. Is there a role for cannabis as adjuvant therapy in patients with IBD?
Studies to date have included only symptomatic patients with objective evidence of inflammation and assessed clinical, biochemical, or endoscopic endpoints. In Crohn’s disease, two studies showed no improvement in clinical remission rates but showed improvement in clinical response; a third study showed both improvement in clinical remission/response as well as improved quality of life. No study showed a change in disease markers of activity including CRP, fecal calprotectin, or endoscopic scoring. In one study, all patients relapsed shortly after cannabis discontinuation suggesting that, while there was benefit in symptom control, there was no improvement of the underlying chronic inflammation.
In patients with ulcerative colitis, there were two studies. One study showed no improvement and high rates of intolerance in the treatment group, while the other study reported improved disease activity but no objective improvement. The variation in results between disease states and between studies might be because of cannabis formulations. In patients with persistent symptoms despite current medical therapy, there might be a role in those patients for adjuvant therapy for improvement symptom control but not disease control. Optimization of medical therapy would still be indicated.
3. What dose and formulation of cannabis should I recommend to a patient as adjuvant therapy?
This is an excellent question and one that unfortunately we do not have the answer to. As mentioned previously, the studies have looked at varying formulations (THC alone, CBD:THC with varying percentages of THC, CBD alone) and varying routes of administration (sublingual, oral, inhalation). The IBD studies looking at CBD-alone formulations lacked clinical efficacy. In states where cannabis products have been accessible to IBD patients, no data on the product type (THC:CBD), method of administration, or prescriber preferences have been published.
4. What risks should I advise my patients about with cannabis use?
The challenge is that we don’t have large population-based studies in IBD looking at long-term risks of cannabis use. However, in the small RCT studies there were minimal reported side effects and no major adverse events over 8-10 weeks. Larger IBD population-based studies have shown that cannabis users were more likely to discontinue traditional medical therapy, and there is an increased risk for surgery in patients with Crohn’s disease. Larger studies in non-IBD patients have shown risk for addiction to other substances, diminished life achievement, increased motor vehicle accidents, chronic bronchitis, psychiatric disturbances and cannabis dependence, and cannabis hyperemesis syndrome (with an uncanny presentation resembling Crohn’s disease flare with partial small bowel obstruction). Patients should also be advised about legal implications of use (given its continued classification as a federal schedule 1 drug), possible drug interactions, and special considerations in pediatric patients (increased risk of addiction), elderly patients (increased risk of neuropsychological effects), and during pregnancy (with national obstetric society guidelines warning against use because of fetal exposure and increased risk of stillbirth).
5. What are the legal implications for providers? Patients?
As of July 2020, cannabis is available for recreational use in 12 states, for medicinal use in 28 states, and illegal in 11 states. So the answer really depends on what state the patient lives in. As a provider who might certify patients (in some medicinal states) or recommend cannabis to patients, you should consider legal and licensing implications. Again, this might vary state to state, and you should also take into account federal status. Providers acting in compliance with state laws are unlikely to have federal consequences. However, remember that malpractice insurance only covers FDA-approved medical therapies. Patients should be advised to consider the potential (although highly unlikely) to face federal prosecution and implications of use for employment, school, camp, or travel, and driving restrictions.
Take home points
- Inquire about cannabis to start the conversation.
- Know your state’s legalization status surrounding cannabis.
- Patients with IBD report improvement in symptoms and quality of life with adjuvant cannabis use; however, there is no change in disease activity.
- Encourage your patients to continue and optimize their maintenance therapy.
- Educate your patients about the legal considerations and known risks.
In conclusion, the use of cannabis in IBD patients has increased in recent years. It is important to be able to discuss the risks and benefits of use with your IBD patients. Focus on the lack of data showing that cannabis improves disease activity, and has shown benefit only in improving IBD-associated symptoms. In some patients there might be a role for adjuvant cannabis therapy to improve overall symptom control and quality of life.
Dr. Kinnucan is an assistant professor of medicine, division of gastroenterology, Michigan Medicine, University of Michigan, Ann Arbor; Dr. Swaminath is an associate professor of medicine, division of gastroenterology, Lenox Hill Hospital, Northwell Health, New York.
Case 1
A 30 year-old female with longstanding ulcerative colitis who has a history of medically refractory steroid-dependent disease and was able to achieve remission with vedolizumab for the last 5 years. Most recent objective assessment showed histologic remission. She has been using daily cannabis medicinally for the last year (high CBD:THC [cannabidiol:delta-9-tetracannabidol] concentration). She notes that she has felt better in the last year since introducing cannabis (improved stool frequency/formation, sleep quality). She inquires about discontinuing her biologic therapy in the hope of using cannabis alone to maintain remission.

Figure 1.
Case 2
A 22-year-old male with ileocolonic inflammatory Crohn’s disease escalated to adalimumab requiring an intensification of therapy to weekly dosing to normalize C-reactive protein (CRP). A recent colonoscopy showed endoscopic improvement (colonic normalization and rare aphthae in ileum). He notes clear clinical improvement, but he continues to experience diarrhea and abdominal cramping (no relationship to meals). Declines addition of immunomodulator (nervous about returning to college during the COVID-19 pandemic). He wonders whether cannabis could be effective in controlling his symptoms as he has had improvement in symptoms during his sporadic recreational cannabis exposure.
Discussion
These cases outline the challenges that providers face when managing patients with inflammatory bowel disease (IBD) when a patient would like to either substitute or incorporate cannabis into their treatment plan. Studies have shown a high prevalence of cannabis use among patients with IBD. With the restrictions surrounding the use of cannabis – either medically or recreationally – being liberalized in many states, these conversations are likely to become more frequent in your practice. However, one of the first challenges that providers face surrounding cannabis is that many patients who use cannabis do not disclose use to their health care team for fear of being judged negatively. In addition, many providers do not routinely ask about cannabis use during office visits. This might be directly related to being unprepared to have a knowledge-based discussion on the risks and benefits of cannabis use in IBD, with the same confidence present during discussion of biologic therapies.
For background, Cannabis sativa (cannabis) is composed of hundreds of phytocannabinoids, the two most common are THC and CBD. These cannabinoids act at the endocannabinoid receptors, which are expressed in the central and peripheral nervous systems and immune cells/tissues, and help explain the clinical changes experienced by cannabis users. Both THC and CBD have been studied in varying doses and routes of administration in patients with IBD, making it challenging to translate into real-world recommendations for patients. Some of the most common reported benefits of cannabis use (particularly in an IBD population) are improvement in pain, diarrhea, nausea, and joint pain. Some studies have shown overall improvement in quality of life (Figure 1).
Some common questions that arise surrounding cannabis use in IBD patients include:
1. Is it possible to stop traditional medical therapy and replace it with cannabis therapy?
No studies have directly addressed this exact question. The small studies, both randomized controlled trials and retrospective ones, have studied the effects of cannabis as adjuvant therapy only. None of the data available to date suggest that cannabis has any anti-inflammatory properties with absence of improvement in biomarkers or endoscopic measures of inflammation. In effect, any attempt to discontinue standard therapy with substitution of cannabis-based therapy should be seen as no different than simply discontinuing standard therapy. There exists the argument that – among those with moderate to severe disease – cannabis might suppress the investigation of mild symptoms which may herald a flare of disease, thus lulling the patient into a state of false stability. We do not advocate the substitution of cannabis products in place of standard medical therapy.
2. Is there a role for cannabis as adjuvant therapy in patients with IBD?
Studies to date have included only symptomatic patients with objective evidence of inflammation and assessed clinical, biochemical, or endoscopic endpoints. In Crohn’s disease, two studies showed no improvement in clinical remission rates but showed improvement in clinical response; a third study showed both improvement in clinical remission/response as well as improved quality of life. No study showed a change in disease markers of activity including CRP, fecal calprotectin, or endoscopic scoring. In one study, all patients relapsed shortly after cannabis discontinuation suggesting that, while there was benefit in symptom control, there was no improvement of the underlying chronic inflammation.
In patients with ulcerative colitis, there were two studies. One study showed no improvement and high rates of intolerance in the treatment group, while the other study reported improved disease activity but no objective improvement. The variation in results between disease states and between studies might be because of cannabis formulations. In patients with persistent symptoms despite current medical therapy, there might be a role in those patients for adjuvant therapy for improvement symptom control but not disease control. Optimization of medical therapy would still be indicated.
3. What dose and formulation of cannabis should I recommend to a patient as adjuvant therapy?
This is an excellent question and one that unfortunately we do not have the answer to. As mentioned previously, the studies have looked at varying formulations (THC alone, CBD:THC with varying percentages of THC, CBD alone) and varying routes of administration (sublingual, oral, inhalation). The IBD studies looking at CBD-alone formulations lacked clinical efficacy. In states where cannabis products have been accessible to IBD patients, no data on the product type (THC:CBD), method of administration, or prescriber preferences have been published.
4. What risks should I advise my patients about with cannabis use?
The challenge is that we don’t have large population-based studies in IBD looking at long-term risks of cannabis use. However, in the small RCT studies there were minimal reported side effects and no major adverse events over 8-10 weeks. Larger IBD population-based studies have shown that cannabis users were more likely to discontinue traditional medical therapy, and there is an increased risk for surgery in patients with Crohn’s disease. Larger studies in non-IBD patients have shown risk for addiction to other substances, diminished life achievement, increased motor vehicle accidents, chronic bronchitis, psychiatric disturbances and cannabis dependence, and cannabis hyperemesis syndrome (with an uncanny presentation resembling Crohn’s disease flare with partial small bowel obstruction). Patients should also be advised about legal implications of use (given its continued classification as a federal schedule 1 drug), possible drug interactions, and special considerations in pediatric patients (increased risk of addiction), elderly patients (increased risk of neuropsychological effects), and during pregnancy (with national obstetric society guidelines warning against use because of fetal exposure and increased risk of stillbirth).
5. What are the legal implications for providers? Patients?
As of July 2020, cannabis is available for recreational use in 12 states, for medicinal use in 28 states, and illegal in 11 states. So the answer really depends on what state the patient lives in. As a provider who might certify patients (in some medicinal states) or recommend cannabis to patients, you should consider legal and licensing implications. Again, this might vary state to state, and you should also take into account federal status. Providers acting in compliance with state laws are unlikely to have federal consequences. However, remember that malpractice insurance only covers FDA-approved medical therapies. Patients should be advised to consider the potential (although highly unlikely) to face federal prosecution and implications of use for employment, school, camp, or travel, and driving restrictions.
Take home points
- Inquire about cannabis to start the conversation.
- Know your state’s legalization status surrounding cannabis.
- Patients with IBD report improvement in symptoms and quality of life with adjuvant cannabis use; however, there is no change in disease activity.
- Encourage your patients to continue and optimize their maintenance therapy.
- Educate your patients about the legal considerations and known risks.
In conclusion, the use of cannabis in IBD patients has increased in recent years. It is important to be able to discuss the risks and benefits of use with your IBD patients. Focus on the lack of data showing that cannabis improves disease activity, and has shown benefit only in improving IBD-associated symptoms. In some patients there might be a role for adjuvant cannabis therapy to improve overall symptom control and quality of life.
Dr. Kinnucan is an assistant professor of medicine, division of gastroenterology, Michigan Medicine, University of Michigan, Ann Arbor; Dr. Swaminath is an associate professor of medicine, division of gastroenterology, Lenox Hill Hospital, Northwell Health, New York.
Antidepressant use shows gender, racial disparities
Women are more than twice as likely as men to use antidepressants, and use among White women is at least double that of other races/ethnicities, according to a new analysis from the National Center for Health Statistics.
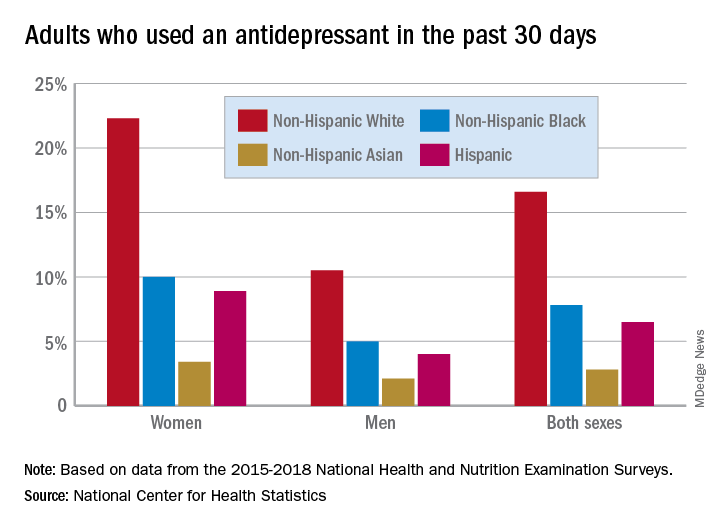
Here are the actual numbers: 17.7% of women and 8.4% of men used an antidepressant in the 30 days before being interviewed for the National Health and Nutrition Examination Survey (NHANES). Put them together, and it works out to 13.2% of all adults over the 4-year period from 2015 to 2018, Debra J. Brody, MPH, and Qiuping Gu, MD, PhD, said Sept. 4 in an NCHS data brief.
Non-Hispanic White women had a past-30-day prevalence of 22.3%, compared with 10.0% for non-Hispanic Black women, 3.4% for non-Hispanic Asian women, and 8.9% for Hispanic women, based on the NHANES data.
The order was the same for men, but the numbers are lower. Non-Hispanic Whites had the highest antidepressant use at 10.5%, followed by non-Hispanic Blacks (5.0%), non-Hispanic Asians (2.1%), and Hispanics (4.0%). All of the differences between Whites and non-Whites were significant for both women and men, the researchers noted.
A look at trends over time shows that the gap between men and women has widened in the last 10 years. Past-30-day use among women went from 13.8% in 2009-2010 to 18.6% in 2017-2018, with a corresponding increase from 7.1% to 8.7% in men. For women, that change was significant; for men, it was not, Ms. Brody and Dr. Gu said.
The sample size averaged just over 6,000 for each of the five 2-year NHANES cycles included in the analysis. The survey includes a household interview and a physical examination at a mobile exam center.
Women are more than twice as likely as men to use antidepressants, and use among White women is at least double that of other races/ethnicities, according to a new analysis from the National Center for Health Statistics.

Here are the actual numbers: 17.7% of women and 8.4% of men used an antidepressant in the 30 days before being interviewed for the National Health and Nutrition Examination Survey (NHANES). Put them together, and it works out to 13.2% of all adults over the 4-year period from 2015 to 2018, Debra J. Brody, MPH, and Qiuping Gu, MD, PhD, said Sept. 4 in an NCHS data brief.
Non-Hispanic White women had a past-30-day prevalence of 22.3%, compared with 10.0% for non-Hispanic Black women, 3.4% for non-Hispanic Asian women, and 8.9% for Hispanic women, based on the NHANES data.
The order was the same for men, but the numbers are lower. Non-Hispanic Whites had the highest antidepressant use at 10.5%, followed by non-Hispanic Blacks (5.0%), non-Hispanic Asians (2.1%), and Hispanics (4.0%). All of the differences between Whites and non-Whites were significant for both women and men, the researchers noted.
A look at trends over time shows that the gap between men and women has widened in the last 10 years. Past-30-day use among women went from 13.8% in 2009-2010 to 18.6% in 2017-2018, with a corresponding increase from 7.1% to 8.7% in men. For women, that change was significant; for men, it was not, Ms. Brody and Dr. Gu said.
The sample size averaged just over 6,000 for each of the five 2-year NHANES cycles included in the analysis. The survey includes a household interview and a physical examination at a mobile exam center.
Women are more than twice as likely as men to use antidepressants, and use among White women is at least double that of other races/ethnicities, according to a new analysis from the National Center for Health Statistics.

Here are the actual numbers: 17.7% of women and 8.4% of men used an antidepressant in the 30 days before being interviewed for the National Health and Nutrition Examination Survey (NHANES). Put them together, and it works out to 13.2% of all adults over the 4-year period from 2015 to 2018, Debra J. Brody, MPH, and Qiuping Gu, MD, PhD, said Sept. 4 in an NCHS data brief.
Non-Hispanic White women had a past-30-day prevalence of 22.3%, compared with 10.0% for non-Hispanic Black women, 3.4% for non-Hispanic Asian women, and 8.9% for Hispanic women, based on the NHANES data.
The order was the same for men, but the numbers are lower. Non-Hispanic Whites had the highest antidepressant use at 10.5%, followed by non-Hispanic Blacks (5.0%), non-Hispanic Asians (2.1%), and Hispanics (4.0%). All of the differences between Whites and non-Whites were significant for both women and men, the researchers noted.
A look at trends over time shows that the gap between men and women has widened in the last 10 years. Past-30-day use among women went from 13.8% in 2009-2010 to 18.6% in 2017-2018, with a corresponding increase from 7.1% to 8.7% in men. For women, that change was significant; for men, it was not, Ms. Brody and Dr. Gu said.
The sample size averaged just over 6,000 for each of the five 2-year NHANES cycles included in the analysis. The survey includes a household interview and a physical examination at a mobile exam center.