User login
Patients may prefer retrograde-fill voiding trials after pelvic floor surgery
Voiding trials after female pelvic floor surgery may detect similar rates of voiding dysfunction regardless of whether voiding occurs spontaneously or after the bladder is retrograde-filled with saline, according to a randomized study.
Nevertheless, patients may prefer the more common retrograde-fill approach.
In the study of 109 patients, those who underwent retrograde fill reported significantly greater satisfaction with their method of voiding evaluation, compared with patients whose voiding trials occurred spontaneously. The increased satisfaction could relate to the fact that retrograde-fill trials take less time, study investigator Patrick Popiel, MD, of Yale University, New Haven, Conn., suggested at the virtual annual scientific meeting of the Society of Gynecologic Surgeons. The exact reasons are unclear, however.
Voiding trials help identify patients who cannot sufficiently empty their bladder after surgery. Prior research has indicated that the incidence of voiding dysfunction after pelvic floor surgery is about 25%-35%. “Patients with voiding dysfunction are generally managed with an indwelling Foley catheter or clean intermittent self-catheterization,” Dr. Popiel said. “Catheterization increases the risk of urinary tract infection, increases anxiety, and decreases patient satisfaction. A large proportion of patients who are discharged home with a Foley catheter state that the catheter was the worst aspect of their experience.”
Dr. Popiel and colleagues conducted a randomized, prospective study to examine the rate of failed voiding trials that necessitate discharge home with an indwelling Foley catheter using spontaneous and retrograde-fill approaches. The study included women who required a voiding trial after surgery for pelvic organ prolapse or urinary incontinence. Patients who required prolonged catheterization after surgery, such as those with a urinary tract infection, bowel injury, or large amount of blood loss, were excluded.
Researchers analyzed data from 55 patients who were randomly assigned to the retrograde-fill group and 54 patients who were randomly assigned to the spontaneous trial group.
In the spontaneous group, patients were required to void at least 150 mL at one time within 6 hours of catheter removal to successfully complete the voiding trial.
In the retrograde-fill group, the bladder was filled in the postanesthesia care unit with 300 mL of saline or until the maximum volume tolerated by the patient (not exceeding 300 mL ) was reached. Patients in this group had to void at least 150 mL or 50% of the instilled volume at one time within 60 minutes of catheter removal to pass the trial.
The researchers documented postvoid residual (PVR) but did not use this measure to determine voiding function.
The baseline demographics of the two groups were similar, although prior hysterectomy was more common in the retrograde-fill group than in the spontaneous group (32.7% vs. 14.8%). The average age was 58.5 years in the retrograde-fill group and 61 years in the spontaneous group.
“There was no significant difference in our primary outcome,” Dr. Popiel said. “There was a 12.7% rate of failed voiding trial in the retrograde group versus 7.7% in the spontaneous group.”
No patients had urinary retention after initially passing their voiding trial. Force of stream did not differ between groups, and about 15% in each group had a postoperative urinary tract infection.
The study demonstrates that voiding assessment based on a spontaneous minimum void of 150 mL is safe and has similar pass rates, compared with the more commonly performed retrograde void trial, Dr. Popiel said. “If the voided amount is at least 150 mL, PVR is not critical to obtain. The study adds to the body of literature that supports less stringent criteria for evaluating voiding function and can limit postoperative urinary recatheterization.”
The investigators allowed patients with PVRs as high as 575 mL to return home without an alternative way to empty the bladder, C. Sage Claydon, MD, a urogynecologist who was not involved in the study, noted during a discussion after the presentation. In all, 6 patients who met the passing criteria for the spontaneous voiding trial had a PVR greater than 200 mL, with volumes ranging from 205-575 mL.
The patients received standardized counseling about postoperative voiding problems, said Dr. Popiel. “This is similar to the work done by Ingber et al. from 2011, where patients who reached a certain force of stream, greater than 5 out of 10, were discharged home regardless of PVR.”
Dr. Popiel had no relevant disclosures. Two coinvestigators disclosed ties to BlossomMed, Renovia, and ArmadaHealth.
SOURCE: Popiel P et al. SGS 2020, Abstract 14.
Voiding trials after female pelvic floor surgery may detect similar rates of voiding dysfunction regardless of whether voiding occurs spontaneously or after the bladder is retrograde-filled with saline, according to a randomized study.
Nevertheless, patients may prefer the more common retrograde-fill approach.
In the study of 109 patients, those who underwent retrograde fill reported significantly greater satisfaction with their method of voiding evaluation, compared with patients whose voiding trials occurred spontaneously. The increased satisfaction could relate to the fact that retrograde-fill trials take less time, study investigator Patrick Popiel, MD, of Yale University, New Haven, Conn., suggested at the virtual annual scientific meeting of the Society of Gynecologic Surgeons. The exact reasons are unclear, however.
Voiding trials help identify patients who cannot sufficiently empty their bladder after surgery. Prior research has indicated that the incidence of voiding dysfunction after pelvic floor surgery is about 25%-35%. “Patients with voiding dysfunction are generally managed with an indwelling Foley catheter or clean intermittent self-catheterization,” Dr. Popiel said. “Catheterization increases the risk of urinary tract infection, increases anxiety, and decreases patient satisfaction. A large proportion of patients who are discharged home with a Foley catheter state that the catheter was the worst aspect of their experience.”
Dr. Popiel and colleagues conducted a randomized, prospective study to examine the rate of failed voiding trials that necessitate discharge home with an indwelling Foley catheter using spontaneous and retrograde-fill approaches. The study included women who required a voiding trial after surgery for pelvic organ prolapse or urinary incontinence. Patients who required prolonged catheterization after surgery, such as those with a urinary tract infection, bowel injury, or large amount of blood loss, were excluded.
Researchers analyzed data from 55 patients who were randomly assigned to the retrograde-fill group and 54 patients who were randomly assigned to the spontaneous trial group.
In the spontaneous group, patients were required to void at least 150 mL at one time within 6 hours of catheter removal to successfully complete the voiding trial.
In the retrograde-fill group, the bladder was filled in the postanesthesia care unit with 300 mL of saline or until the maximum volume tolerated by the patient (not exceeding 300 mL ) was reached. Patients in this group had to void at least 150 mL or 50% of the instilled volume at one time within 60 minutes of catheter removal to pass the trial.
The researchers documented postvoid residual (PVR) but did not use this measure to determine voiding function.
The baseline demographics of the two groups were similar, although prior hysterectomy was more common in the retrograde-fill group than in the spontaneous group (32.7% vs. 14.8%). The average age was 58.5 years in the retrograde-fill group and 61 years in the spontaneous group.
“There was no significant difference in our primary outcome,” Dr. Popiel said. “There was a 12.7% rate of failed voiding trial in the retrograde group versus 7.7% in the spontaneous group.”
No patients had urinary retention after initially passing their voiding trial. Force of stream did not differ between groups, and about 15% in each group had a postoperative urinary tract infection.
The study demonstrates that voiding assessment based on a spontaneous minimum void of 150 mL is safe and has similar pass rates, compared with the more commonly performed retrograde void trial, Dr. Popiel said. “If the voided amount is at least 150 mL, PVR is not critical to obtain. The study adds to the body of literature that supports less stringent criteria for evaluating voiding function and can limit postoperative urinary recatheterization.”
The investigators allowed patients with PVRs as high as 575 mL to return home without an alternative way to empty the bladder, C. Sage Claydon, MD, a urogynecologist who was not involved in the study, noted during a discussion after the presentation. In all, 6 patients who met the passing criteria for the spontaneous voiding trial had a PVR greater than 200 mL, with volumes ranging from 205-575 mL.
The patients received standardized counseling about postoperative voiding problems, said Dr. Popiel. “This is similar to the work done by Ingber et al. from 2011, where patients who reached a certain force of stream, greater than 5 out of 10, were discharged home regardless of PVR.”
Dr. Popiel had no relevant disclosures. Two coinvestigators disclosed ties to BlossomMed, Renovia, and ArmadaHealth.
SOURCE: Popiel P et al. SGS 2020, Abstract 14.
Voiding trials after female pelvic floor surgery may detect similar rates of voiding dysfunction regardless of whether voiding occurs spontaneously or after the bladder is retrograde-filled with saline, according to a randomized study.
Nevertheless, patients may prefer the more common retrograde-fill approach.
In the study of 109 patients, those who underwent retrograde fill reported significantly greater satisfaction with their method of voiding evaluation, compared with patients whose voiding trials occurred spontaneously. The increased satisfaction could relate to the fact that retrograde-fill trials take less time, study investigator Patrick Popiel, MD, of Yale University, New Haven, Conn., suggested at the virtual annual scientific meeting of the Society of Gynecologic Surgeons. The exact reasons are unclear, however.
Voiding trials help identify patients who cannot sufficiently empty their bladder after surgery. Prior research has indicated that the incidence of voiding dysfunction after pelvic floor surgery is about 25%-35%. “Patients with voiding dysfunction are generally managed with an indwelling Foley catheter or clean intermittent self-catheterization,” Dr. Popiel said. “Catheterization increases the risk of urinary tract infection, increases anxiety, and decreases patient satisfaction. A large proportion of patients who are discharged home with a Foley catheter state that the catheter was the worst aspect of their experience.”
Dr. Popiel and colleagues conducted a randomized, prospective study to examine the rate of failed voiding trials that necessitate discharge home with an indwelling Foley catheter using spontaneous and retrograde-fill approaches. The study included women who required a voiding trial after surgery for pelvic organ prolapse or urinary incontinence. Patients who required prolonged catheterization after surgery, such as those with a urinary tract infection, bowel injury, or large amount of blood loss, were excluded.
Researchers analyzed data from 55 patients who were randomly assigned to the retrograde-fill group and 54 patients who were randomly assigned to the spontaneous trial group.
In the spontaneous group, patients were required to void at least 150 mL at one time within 6 hours of catheter removal to successfully complete the voiding trial.
In the retrograde-fill group, the bladder was filled in the postanesthesia care unit with 300 mL of saline or until the maximum volume tolerated by the patient (not exceeding 300 mL ) was reached. Patients in this group had to void at least 150 mL or 50% of the instilled volume at one time within 60 minutes of catheter removal to pass the trial.
The researchers documented postvoid residual (PVR) but did not use this measure to determine voiding function.
The baseline demographics of the two groups were similar, although prior hysterectomy was more common in the retrograde-fill group than in the spontaneous group (32.7% vs. 14.8%). The average age was 58.5 years in the retrograde-fill group and 61 years in the spontaneous group.
“There was no significant difference in our primary outcome,” Dr. Popiel said. “There was a 12.7% rate of failed voiding trial in the retrograde group versus 7.7% in the spontaneous group.”
No patients had urinary retention after initially passing their voiding trial. Force of stream did not differ between groups, and about 15% in each group had a postoperative urinary tract infection.
The study demonstrates that voiding assessment based on a spontaneous minimum void of 150 mL is safe and has similar pass rates, compared with the more commonly performed retrograde void trial, Dr. Popiel said. “If the voided amount is at least 150 mL, PVR is not critical to obtain. The study adds to the body of literature that supports less stringent criteria for evaluating voiding function and can limit postoperative urinary recatheterization.”
The investigators allowed patients with PVRs as high as 575 mL to return home without an alternative way to empty the bladder, C. Sage Claydon, MD, a urogynecologist who was not involved in the study, noted during a discussion after the presentation. In all, 6 patients who met the passing criteria for the spontaneous voiding trial had a PVR greater than 200 mL, with volumes ranging from 205-575 mL.
The patients received standardized counseling about postoperative voiding problems, said Dr. Popiel. “This is similar to the work done by Ingber et al. from 2011, where patients who reached a certain force of stream, greater than 5 out of 10, were discharged home regardless of PVR.”
Dr. Popiel had no relevant disclosures. Two coinvestigators disclosed ties to BlossomMed, Renovia, and ArmadaHealth.
SOURCE: Popiel P et al. SGS 2020, Abstract 14.
FROM SGS 2020
Minidose edoxaban may safely cut AFib stroke risk in the frail, very elderly
suggests a randomized trial conducted in Japan.
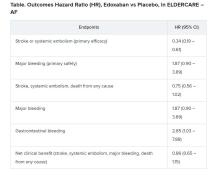
Many of the study’s 984 mostly octogenarian patients were objectively frail with poor renal function, low body weight, a history of serious bleeding, or other conditions that made them poor candidates for regular-dose oral anticoagulation. Yet those who took the factor Xa inhibitor edoxaban (Savaysa) at the off-label dosage of 15 mg once daily showed a two-thirds drop in risk for stroke or systemic embolism (P < .001), compared with patients who received placebo. There were no fatal bleeds and virtually no intracranial hemorrhages.
For such high-risk patients with nonvalvular AFib who otherwise would not be given an OAC, edoxaban 15 mg “can be an acceptable treatment option in decreasing the risk of devastating stroke”; however, “it may increase the risk of gastrointestinal bleeding, so care should be given in every patient,” said Ken Okumura, MD, PhD. Indeed, the rate of gastrointestinal bleeding tripled among the patients who received edoxaban, compared with those given placebo, at about 2.3% per year versus 0.8% per year.
Although their 87% increased risk for major bleeding did not reach significance, it hit close, with a P value of .09 in the trial, called Edoxaban Low-Dose for Elder Care Atrial Fibrillation Patients (ELDERCARE-AF).
Dr. Okumura, of Saiseikai Kumamoto (Japan) Hospital, presented the study August 30 during the virtual annual congress of the European Society of Cardiology. He is lead author of an article describing the study, which was simultaneously published in the New England Journal of Medicine.
Many patients with AFib suffer strokes if they are not given oral anticoagulation because of “fear of major bleeding caused by standard OAC therapy,” Dr. Okumura noted. Others are inappropriately administered antiplatelets or anticoagulants at conventional dosages. “There is no standard of practice in Japan for patients like those in the present trial,” Dr. Okumura said. “However, I believe the present study opens a new possible path of thromboprophylaxis in such high-risk patients.”
Even with its relatively few bleeding events, ELDERCARE-AF “does suggest that the risk of the worst types of bleeds is not that high,” said Daniel E. Singer, MD, of Massachusetts General Hospital, Boston. “Gastrointestinal bleeding is annoying, and it will probably stop people from taking their edoxaban, but for the most part it doesn’t kill people.”
Moreover, he added, the trial suggests that low-dose edoxaban, in exchange for a steep reduction in thromboembolic risk, “doesn’t add to your risk of intracranial hemorrhage!”
ELDERCARE-AF may give practitioners “yet another reason to rethink” whether a low-dose DOAC such as edoxaban 15 mg/day may well be a good approach for such patients with AFib who are not receiving standard-dose OAC because of a perceived high risk for serious bleeding, said Dr. Singer, who was not involved in the study.
The trial randomly and evenly assigned 984 patients with AF in Japan to take either edoxaban 15 mg/day or placebo. The patients, who were at least 80 years old and had a CHADS2 score of 2 or higher, were judged inappropriate candidates for OAC at dosages approved for stroke prevention.
The mean age of the patients was 86.6, more than a decade older than patients “in the previous landmark clinical trials of direct oral anticoagulants,” and were 5-10 years older than the general AFib population, reported Dr. Okumura and colleagues.
Their mean weight was 52 kg, and mean creatinine clearance was 36.3 mL/min; 41% were classified as frail according to validated assessment tools.
Of the 303 patients who did not complete the trial, 158 voluntarily withdrew for various reasons. The withdrawal rate was similar in the two treatment arms. Outcomes were analyzed by intention to treat, the report noted.
The annualized rate of stroke or systemic embolism, the primary efficacy endpoint, was 2.3% for those who received edoxaban and 6.7% for the control group. Corresponding rates for the primary safety endpoint, major bleeding as determined by International Society on Thrombosis and Hemostasis criteria, were 3.3% and 1.8%, respectively.
“The question is, can the Food and Drug Administration act on this information? I doubt it can. What will be needed is to reproduce the study in a U.S. population to see if it holds,” Dr. Singer proposed.
“Edoxaban isn’t used much in the U.S. This could heighten interest. And who knows, there may be a gold rush,” he said, if the strategy were to pan out for the other DOACs, rivaroxaban (Xarelto), apixaban (Eliquis), and dabigatran (Pradaxa).
ELDERCARE-AF was funded by Daiichi Sankyo, from which Dr. Okumura reported receiving grants and personal fees; he also disclosed personal fees from Daiichi Sankyo, Boehringer Ingelheim, Bristol-Myers Squibb, Medtronic, Johnson & Johnson, and Bayer.
A version of this article originally appeared on Medscape.com.
suggests a randomized trial conducted in Japan.
Many of the study’s 984 mostly octogenarian patients were objectively frail with poor renal function, low body weight, a history of serious bleeding, or other conditions that made them poor candidates for regular-dose oral anticoagulation. Yet those who took the factor Xa inhibitor edoxaban (Savaysa) at the off-label dosage of 15 mg once daily showed a two-thirds drop in risk for stroke or systemic embolism (P < .001), compared with patients who received placebo. There were no fatal bleeds and virtually no intracranial hemorrhages.
For such high-risk patients with nonvalvular AFib who otherwise would not be given an OAC, edoxaban 15 mg “can be an acceptable treatment option in decreasing the risk of devastating stroke”; however, “it may increase the risk of gastrointestinal bleeding, so care should be given in every patient,” said Ken Okumura, MD, PhD. Indeed, the rate of gastrointestinal bleeding tripled among the patients who received edoxaban, compared with those given placebo, at about 2.3% per year versus 0.8% per year.
Although their 87% increased risk for major bleeding did not reach significance, it hit close, with a P value of .09 in the trial, called Edoxaban Low-Dose for Elder Care Atrial Fibrillation Patients (ELDERCARE-AF).
Dr. Okumura, of Saiseikai Kumamoto (Japan) Hospital, presented the study August 30 during the virtual annual congress of the European Society of Cardiology. He is lead author of an article describing the study, which was simultaneously published in the New England Journal of Medicine.
Many patients with AFib suffer strokes if they are not given oral anticoagulation because of “fear of major bleeding caused by standard OAC therapy,” Dr. Okumura noted. Others are inappropriately administered antiplatelets or anticoagulants at conventional dosages. “There is no standard of practice in Japan for patients like those in the present trial,” Dr. Okumura said. “However, I believe the present study opens a new possible path of thromboprophylaxis in such high-risk patients.”
Even with its relatively few bleeding events, ELDERCARE-AF “does suggest that the risk of the worst types of bleeds is not that high,” said Daniel E. Singer, MD, of Massachusetts General Hospital, Boston. “Gastrointestinal bleeding is annoying, and it will probably stop people from taking their edoxaban, but for the most part it doesn’t kill people.”
Moreover, he added, the trial suggests that low-dose edoxaban, in exchange for a steep reduction in thromboembolic risk, “doesn’t add to your risk of intracranial hemorrhage!”
ELDERCARE-AF may give practitioners “yet another reason to rethink” whether a low-dose DOAC such as edoxaban 15 mg/day may well be a good approach for such patients with AFib who are not receiving standard-dose OAC because of a perceived high risk for serious bleeding, said Dr. Singer, who was not involved in the study.
The trial randomly and evenly assigned 984 patients with AF in Japan to take either edoxaban 15 mg/day or placebo. The patients, who were at least 80 years old and had a CHADS2 score of 2 or higher, were judged inappropriate candidates for OAC at dosages approved for stroke prevention.
The mean age of the patients was 86.6, more than a decade older than patients “in the previous landmark clinical trials of direct oral anticoagulants,” and were 5-10 years older than the general AFib population, reported Dr. Okumura and colleagues.
Their mean weight was 52 kg, and mean creatinine clearance was 36.3 mL/min; 41% were classified as frail according to validated assessment tools.
Of the 303 patients who did not complete the trial, 158 voluntarily withdrew for various reasons. The withdrawal rate was similar in the two treatment arms. Outcomes were analyzed by intention to treat, the report noted.
The annualized rate of stroke or systemic embolism, the primary efficacy endpoint, was 2.3% for those who received edoxaban and 6.7% for the control group. Corresponding rates for the primary safety endpoint, major bleeding as determined by International Society on Thrombosis and Hemostasis criteria, were 3.3% and 1.8%, respectively.
“The question is, can the Food and Drug Administration act on this information? I doubt it can. What will be needed is to reproduce the study in a U.S. population to see if it holds,” Dr. Singer proposed.
“Edoxaban isn’t used much in the U.S. This could heighten interest. And who knows, there may be a gold rush,” he said, if the strategy were to pan out for the other DOACs, rivaroxaban (Xarelto), apixaban (Eliquis), and dabigatran (Pradaxa).
ELDERCARE-AF was funded by Daiichi Sankyo, from which Dr. Okumura reported receiving grants and personal fees; he also disclosed personal fees from Daiichi Sankyo, Boehringer Ingelheim, Bristol-Myers Squibb, Medtronic, Johnson & Johnson, and Bayer.
A version of this article originally appeared on Medscape.com.
suggests a randomized trial conducted in Japan.
Many of the study’s 984 mostly octogenarian patients were objectively frail with poor renal function, low body weight, a history of serious bleeding, or other conditions that made them poor candidates for regular-dose oral anticoagulation. Yet those who took the factor Xa inhibitor edoxaban (Savaysa) at the off-label dosage of 15 mg once daily showed a two-thirds drop in risk for stroke or systemic embolism (P < .001), compared with patients who received placebo. There were no fatal bleeds and virtually no intracranial hemorrhages.
For such high-risk patients with nonvalvular AFib who otherwise would not be given an OAC, edoxaban 15 mg “can be an acceptable treatment option in decreasing the risk of devastating stroke”; however, “it may increase the risk of gastrointestinal bleeding, so care should be given in every patient,” said Ken Okumura, MD, PhD. Indeed, the rate of gastrointestinal bleeding tripled among the patients who received edoxaban, compared with those given placebo, at about 2.3% per year versus 0.8% per year.
Although their 87% increased risk for major bleeding did not reach significance, it hit close, with a P value of .09 in the trial, called Edoxaban Low-Dose for Elder Care Atrial Fibrillation Patients (ELDERCARE-AF).
Dr. Okumura, of Saiseikai Kumamoto (Japan) Hospital, presented the study August 30 during the virtual annual congress of the European Society of Cardiology. He is lead author of an article describing the study, which was simultaneously published in the New England Journal of Medicine.
Many patients with AFib suffer strokes if they are not given oral anticoagulation because of “fear of major bleeding caused by standard OAC therapy,” Dr. Okumura noted. Others are inappropriately administered antiplatelets or anticoagulants at conventional dosages. “There is no standard of practice in Japan for patients like those in the present trial,” Dr. Okumura said. “However, I believe the present study opens a new possible path of thromboprophylaxis in such high-risk patients.”
Even with its relatively few bleeding events, ELDERCARE-AF “does suggest that the risk of the worst types of bleeds is not that high,” said Daniel E. Singer, MD, of Massachusetts General Hospital, Boston. “Gastrointestinal bleeding is annoying, and it will probably stop people from taking their edoxaban, but for the most part it doesn’t kill people.”
Moreover, he added, the trial suggests that low-dose edoxaban, in exchange for a steep reduction in thromboembolic risk, “doesn’t add to your risk of intracranial hemorrhage!”
ELDERCARE-AF may give practitioners “yet another reason to rethink” whether a low-dose DOAC such as edoxaban 15 mg/day may well be a good approach for such patients with AFib who are not receiving standard-dose OAC because of a perceived high risk for serious bleeding, said Dr. Singer, who was not involved in the study.
The trial randomly and evenly assigned 984 patients with AF in Japan to take either edoxaban 15 mg/day or placebo. The patients, who were at least 80 years old and had a CHADS2 score of 2 or higher, were judged inappropriate candidates for OAC at dosages approved for stroke prevention.
The mean age of the patients was 86.6, more than a decade older than patients “in the previous landmark clinical trials of direct oral anticoagulants,” and were 5-10 years older than the general AFib population, reported Dr. Okumura and colleagues.
Their mean weight was 52 kg, and mean creatinine clearance was 36.3 mL/min; 41% were classified as frail according to validated assessment tools.
Of the 303 patients who did not complete the trial, 158 voluntarily withdrew for various reasons. The withdrawal rate was similar in the two treatment arms. Outcomes were analyzed by intention to treat, the report noted.
The annualized rate of stroke or systemic embolism, the primary efficacy endpoint, was 2.3% for those who received edoxaban and 6.7% for the control group. Corresponding rates for the primary safety endpoint, major bleeding as determined by International Society on Thrombosis and Hemostasis criteria, were 3.3% and 1.8%, respectively.
“The question is, can the Food and Drug Administration act on this information? I doubt it can. What will be needed is to reproduce the study in a U.S. population to see if it holds,” Dr. Singer proposed.
“Edoxaban isn’t used much in the U.S. This could heighten interest. And who knows, there may be a gold rush,” he said, if the strategy were to pan out for the other DOACs, rivaroxaban (Xarelto), apixaban (Eliquis), and dabigatran (Pradaxa).
ELDERCARE-AF was funded by Daiichi Sankyo, from which Dr. Okumura reported receiving grants and personal fees; he also disclosed personal fees from Daiichi Sankyo, Boehringer Ingelheim, Bristol-Myers Squibb, Medtronic, Johnson & Johnson, and Bayer.
A version of this article originally appeared on Medscape.com.
FROM ESC CONGRESS 2020
High schoolers prefer tobacco as vapor, not smoke
according to the Centers for Disease Control and Prevention.
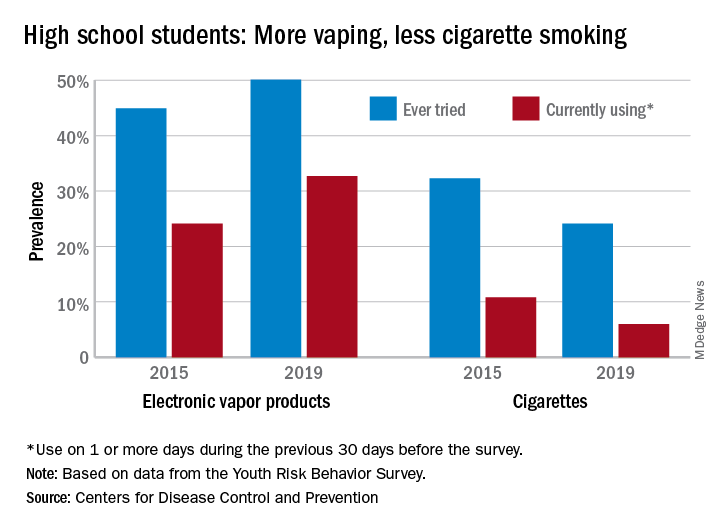
From 2015 to 2019, current use of electronic vapor products among students in grades 9-12 rose from 24.1% to 32.7%, while the same level of cigarette use – on 1 or more days in the previous 30 – dropped from 10.8% to 6.0%, based on data from the Youth Risk Behavior Survey.
Among the survey respondents, 50.1% had at least tried an electronic vapor product by 2019, up from 44.9% in 2015. Cigarettes again showed a decline, as ever use fell from 32.3% to 24.1%, or less than half of the e-product prevalence. Everyday use of vaping products was 7.2% in 2019 (up from 2.0% in 2015), compared with 1.1% for cigarettes (down from 2.3%), the YRBS data show.
“The dramatic increase in electronic vapor product use among high school students has led to increases in overall tobacco product use among U.S. youths, erasing gains made in previous years and leading the U.S. Surgeon General to declare youth e-cigarette use an epidemic in the United States,” MeLisa R. Creamer, PhD, and associates at the CDC wrote in the MMWR.
Electronic vapor products, as defined by the survey, “include e-cigarettes, vapes, vape pens, e-cigars, e-hookahs, hookah pens, and mods.”
Current use of cigarettes among high school students, as measured by the YRBS, has been declining since reaching a high of 36.4% in 1997; the prevalence of everyday use peaked at 12.8% in 1999. Current use of cigars declined as well, falling from 17.7% in 1999 to 5.7% in 2019, according to YRBS data.
“In 2019, a total of 36.5% of high school students currently used any tobacco product, with electronic vapor products being the most commonly used product,” Dr. Creamer and associates wrote in their recent analysis of the YRBS data (MMWR Supp. 2020 Aug 21;69[1]:56-63).
For the first time since the use of electronic vapor products was included in the every-other-year survey in 2015, females were more likely than males to be current users of vaping products last year, 33.5% to 32.0%. Males were heavier users of cigarettes by a margin of 6.9% to 4.9%, the CDC reported.
Geographically speaking, use of both electronic vapor products and cigarettes varied considerably among the 43 states with available data. Current use of electronic products ranged from a low of 9.7% in Utah to a high of 35.7% in West Virginia, with the two states in the same positions regarding current cigarette use: Utah (2.2%) lowest and West Virginia (13.5%) highest, based on the 2019 YRBS data.
“Tobacco product usage has evolved, and the increasing prevalence of electronic vapor product use among youths during recent years is concerning,” Dr. Creamer and associates wrote.
according to the Centers for Disease Control and Prevention.
From 2015 to 2019, current use of electronic vapor products among students in grades 9-12 rose from 24.1% to 32.7%, while the same level of cigarette use – on 1 or more days in the previous 30 – dropped from 10.8% to 6.0%, based on data from the Youth Risk Behavior Survey.
Among the survey respondents, 50.1% had at least tried an electronic vapor product by 2019, up from 44.9% in 2015. Cigarettes again showed a decline, as ever use fell from 32.3% to 24.1%, or less than half of the e-product prevalence. Everyday use of vaping products was 7.2% in 2019 (up from 2.0% in 2015), compared with 1.1% for cigarettes (down from 2.3%), the YRBS data show.
“The dramatic increase in electronic vapor product use among high school students has led to increases in overall tobacco product use among U.S. youths, erasing gains made in previous years and leading the U.S. Surgeon General to declare youth e-cigarette use an epidemic in the United States,” MeLisa R. Creamer, PhD, and associates at the CDC wrote in the MMWR.
Electronic vapor products, as defined by the survey, “include e-cigarettes, vapes, vape pens, e-cigars, e-hookahs, hookah pens, and mods.”
Current use of cigarettes among high school students, as measured by the YRBS, has been declining since reaching a high of 36.4% in 1997; the prevalence of everyday use peaked at 12.8% in 1999. Current use of cigars declined as well, falling from 17.7% in 1999 to 5.7% in 2019, according to YRBS data.
“In 2019, a total of 36.5% of high school students currently used any tobacco product, with electronic vapor products being the most commonly used product,” Dr. Creamer and associates wrote in their recent analysis of the YRBS data (MMWR Supp. 2020 Aug 21;69[1]:56-63).
For the first time since the use of electronic vapor products was included in the every-other-year survey in 2015, females were more likely than males to be current users of vaping products last year, 33.5% to 32.0%. Males were heavier users of cigarettes by a margin of 6.9% to 4.9%, the CDC reported.
Geographically speaking, use of both electronic vapor products and cigarettes varied considerably among the 43 states with available data. Current use of electronic products ranged from a low of 9.7% in Utah to a high of 35.7% in West Virginia, with the two states in the same positions regarding current cigarette use: Utah (2.2%) lowest and West Virginia (13.5%) highest, based on the 2019 YRBS data.
“Tobacco product usage has evolved, and the increasing prevalence of electronic vapor product use among youths during recent years is concerning,” Dr. Creamer and associates wrote.
according to the Centers for Disease Control and Prevention.
From 2015 to 2019, current use of electronic vapor products among students in grades 9-12 rose from 24.1% to 32.7%, while the same level of cigarette use – on 1 or more days in the previous 30 – dropped from 10.8% to 6.0%, based on data from the Youth Risk Behavior Survey.
Among the survey respondents, 50.1% had at least tried an electronic vapor product by 2019, up from 44.9% in 2015. Cigarettes again showed a decline, as ever use fell from 32.3% to 24.1%, or less than half of the e-product prevalence. Everyday use of vaping products was 7.2% in 2019 (up from 2.0% in 2015), compared with 1.1% for cigarettes (down from 2.3%), the YRBS data show.
“The dramatic increase in electronic vapor product use among high school students has led to increases in overall tobacco product use among U.S. youths, erasing gains made in previous years and leading the U.S. Surgeon General to declare youth e-cigarette use an epidemic in the United States,” MeLisa R. Creamer, PhD, and associates at the CDC wrote in the MMWR.
Electronic vapor products, as defined by the survey, “include e-cigarettes, vapes, vape pens, e-cigars, e-hookahs, hookah pens, and mods.”
Current use of cigarettes among high school students, as measured by the YRBS, has been declining since reaching a high of 36.4% in 1997; the prevalence of everyday use peaked at 12.8% in 1999. Current use of cigars declined as well, falling from 17.7% in 1999 to 5.7% in 2019, according to YRBS data.
“In 2019, a total of 36.5% of high school students currently used any tobacco product, with electronic vapor products being the most commonly used product,” Dr. Creamer and associates wrote in their recent analysis of the YRBS data (MMWR Supp. 2020 Aug 21;69[1]:56-63).
For the first time since the use of electronic vapor products was included in the every-other-year survey in 2015, females were more likely than males to be current users of vaping products last year, 33.5% to 32.0%. Males were heavier users of cigarettes by a margin of 6.9% to 4.9%, the CDC reported.
Geographically speaking, use of both electronic vapor products and cigarettes varied considerably among the 43 states with available data. Current use of electronic products ranged from a low of 9.7% in Utah to a high of 35.7% in West Virginia, with the two states in the same positions regarding current cigarette use: Utah (2.2%) lowest and West Virginia (13.5%) highest, based on the 2019 YRBS data.
“Tobacco product usage has evolved, and the increasing prevalence of electronic vapor product use among youths during recent years is concerning,” Dr. Creamer and associates wrote.
Drug Overdose and Suicide Among Veteran Enrollees in the VHA: Comparison Among Local, Regional, and National Data
Suicide is the 10th leading cause of death in the US. In 2017, there were 47,173 deaths by suicide (14 deaths per 100,000 people), representing a 33% increase from 1999.1 In 2017 veterans accounted for 13.5% of all suicide deaths among US adults, although veterans comprised only 7.9% of the adult population; the age- and sex-adjusted suicide rate was 1.5 times higher for veterans than that of nonveteran adults.2,3
Among veteran users of Veterans Health Administration (VHA) services, mental health and substance use disorders, chronic medical conditions, and chronic pain are associated with an increased risk for suicide.3 About one-half of VHA veterans have been diagnosed with chronic pain.4 A chronic pain diagnosis (eg, back pain, migraine, and psychogenic pain) increased the risk of death by suicide even after adjusting for comorbid psychiatric diagnoses, according to a study on pain and suicide among US veterans.5
One-quarter of veterans received an opioid prescription during VHA outpatient care in 2012.4 Increased prescribing of opioid medications has been associated with opioid overdose and suicides.6-10 Opioids are the most common drugs found in suicide by overdose.11 The rate of opioid-related suicide deaths is 13 times higher among individuals with opioid use disorder (OUD) than it is for those without OUD.12 The rate of OUD diagnosis among VHA users was 7 times higher than that for non-VHA users.13
In the US the age-adjusted rate of drug overdose deaths increased from 6 per 100,000 persons in 1999 to 22 per 100,000 in 2017.14 Drug overdoses accounted for 52,404 US deaths in 2015; 33,091 (63.1%) were from opioids.15 In 2017, there were 70,237 drug overdose deaths; 67.8% involved opioids (ie, 5 per 100,000 population represent prescription opioids).16
The VHA is committed to reducing opioid use and veteran suicide prevention. In 2013 the VHA launched the Opioid Safety Initiative employing 4 strategies: education, pain management, risk management, and addiction treatment.17 To address the opioid epidemic, the North Florida/South Georgia Veteran Health System (NF/SGVHS) developed and implemented a multispecialty Opioid Risk Reduction Program that is fully integrated with mental health and addiction services. The purpose of the NF/SGVHS one-stop pain addiction clinic is to provide a treatment program for chronic pain and addiction. The program includes elements of a whole health approach to pain care, including battlefield and traditional acupuncture. The focus went beyond replacing pharmacologic treatments with a complementary integrative health approach to helping veterans regain control of their lives through empowerment, skill building, shared goal setting, and reinforcing self-management.
The self-management programs include a pain school for patient education, a pain psychology program, and a yoga program, all stressing self-management offered onsite and via telehealth. Special effort was directed to identify patients with OUD and opioid dependence. Many of these patients were transitioned to buprenorphine, a potent analgesic that suppresses opioid cravings and withdrawal symptoms associated with stopping opioids. The clinic was structured so that patients could be seen often for follow-up and support. In addition, open lines of communication and referral were set up between this clinic, the interventional pain clinic, and the physical medicine and rehabilitation service. A detailed description of this program has been published elsewhere.18
The number of veterans receiving opioid prescription across the VHA system decreased by 172,000 prescriptions quarterly between 2012 and 2016.19 Fewer veterans were prescribed high doses of opioids or concomitant interacting medicines and more veterans were receiving nonopioid therapies.19 The prescription reduction across the VHA has varied. For example, from 2012 to 2017 the NF/SGVHS reported an 87% reduction of opioid prescriptions (≥ 100 mg morphine equivalents/d), compared with the VHA national average reduction of 49%.18
Vigorous opioid reduction is controversial. In a systematic review on opioid reduction, Frank and colleagues reported some beneficial effects of opioid reduction, such as increased health-related quality of life.20 However, another study suggested a risk of increased pain with opioid tapering.21 The literature findings on the association between prescription opioid use and suicide are mixed. The VHA Office of Mental Health and Suicide Prevention literature review reported that veterans were at increased risk of committing suicide within the first 6 months of discontinuing opioid therapy.22 Another study reported that veterans who discontinued long-term opioid treatment had an increased risk for suicidal ideation.23 However, higher doses of opioids were associated with an increased risk for suicide among individuals with chronic pain.10 The link between opioid tapering and the risk of suicide or overdose is uncertain.
Bohnert and Ilgen suggested that discontinuing prescription opioids leads to suicide without examining the risk factors that influenced discontinuation is ill-informed.7 Strong evidence about the association or relationship among opioid use, overdose, and suicide is needed. To increase our understanding of that association, Bohnert and Ilgen argued for multifaceted interventions that simultaneously address the shared causes and risk factors for OUD,7 such as the multispecialty Opioid Risk Reduction Program at NF/SGVHS.
Because of the reported association between robust integrated mental health and addiction, primary care pain clinic intervention, and the higher rate of opioid tapering in NF/SGVHS,18 this study aims to describe the pattern of overdose diagnosis (opioid overdose and nonopioid overdose) and pattern of suicide rates among veterans enrolled in NF/SGVHS, Veterans Integrated Service Network (VISN) 8, and the entire VA health care system during 2012 to 2016.The study reviewed and compared overdose diagnosis and suicide rates among veterans across NF/SGVHS and 2 other levels of the VA health care system to determine whether there were variances in the pattern of overdose/suicide rates and to explore these differences.
Methods
In this retrospective study, aggregate data were obtained from several sources. First, the drug overdose data were extracted from the VA Support Service Center (VSSC) medical diagnosis cube. We reviewed the literature for opioid codes reported in the literature and compared these reported opioid International Classification of Diseases, Ninth Revision (ICD-9) and International Classification of Diseases, 10th Revision (ICD-10) codes with the local facility patient-level comprehensive overdose diagnosis codes. Based on the comparison, we found 98 ICD-9 and ICD-10 overdose diagnosis codes and ran the modified codes against the VSSC national database. Overdose data were aggregated by facility and fiscal year, and the overdose rates (per 1,000) were calculated for unique veteran users at the 3 levels (NF/SGVHS, VISN 8, and VA national) as the denominator.
Each of the 18 VISNs comprise multiple VAMCs and clinics within a geographic region. VISN 8 encompasses most of Florida and portions of southern Georgia and the Caribbean (Puerto Rico, US Virgin Islands), including NF/SGVHS.
In this study, drug overdose refers to the overdose or poisoning from all drugs (ie, opioids, cocaine, amphetamines, sedatives, etc) and defined as any unintentional (accidental), deliberate, or intent undetermined drug poisoning.24 The suicide data for this study were drawn from the VA Suicide Prevention Program at 3 different levels: NF/SGVHS, VISN 8, and VHA national. Suicide is death caused by an intentional act of injuring oneself with the intent to die.25
This descriptive study compared the rate of annual drug overdoses (per 1,000 enrollees) between NF/SGVHS, VISN 8, and VHA national from 2012 to 2016. It also compared the annual rate of suicide per 100,000 enrollees across these 3 levels of the VHA. The overdose and suicide rates and numbers are mutually exclusive, meaning the VISN 8 data do not include the NF/SGVHS information, and the national data excluded data from VISN 8 and NF/SGVHS. This approach helped improve the quality of multiple level comparisons for different levels of the VHA system.
Results
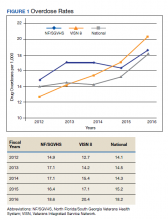
Figure 1 shows the pattern of overdose diagnosis by rates (per 1,000) across the study period (2012 to 2016) and compares patterns at 3 levels of VHA (NF/SGVHS, VISN 8, and VHA national). The average annual rate of overdose diagnoses for NF/SGVHS during the study was slightly higher (16.8 per 1,000) than that of VISN 8 (16 per 1,000) and VHA national (15.3 per 1,000), but by the end of the study period the NF/SGVHS rate (18.6 per 1,000) nearly matched the national rate (18.2 per 1,000) and was lower than the VISN 8 rate (20.4 per 1,000). Additionally, NF/SGVHS had less variability (SD, 1.34) in yearly average overdose rates compared with VISN 8 (SD, 2.96), and VHA national (SD, 1.69).
From 2013 to 2014 the overdose diagnosis rate for NF/SGVHS remained the same (17.1 per 1,000). A similar pattern was observed for the VHA national data, whereas the VISN 8 data showed a steady increase during the same period. In 2015, the NF/SGVHS had 0.7 per 1,000 decrease in overdose diagnosis rate, whereas VISN 8 and VHA national data showed 1.7 per 1,000 and 0.9 per 1,000 increases, respectively. During the last year of the study (2016), there was a dramatic increase in overdose diagnosis for all the health care systems, ranging from 2.2 per 1,000 for NF/SGVHS to 3.3 per 1,000 for VISN 8.
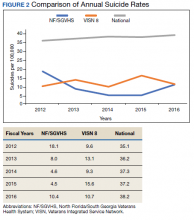
Figure 2 shows the annual rates (per 100,000 individuals) of suicide for NF/SGVHS, VISN 8, and VHA national. The suicide pattern for VISN 8 shows a cyclical acceleration and deceleration trend across the study period. From 2012 to 2014, the VHA national data show a steady increase of about 1 per 100,000 from year to year. On the contrary, NF/SGVHS shows a low suicide rate from year to year within the same period with a rate of 10 per 100,000 in 2013 compared with the previous year. Although the NF/SGVHS suicide rate increased in 2016 (10.4 per 100,000), it remained lower than that of VISN 8 (10.7 per 100,00) and VHA national (38.2 per 100,000).
This study shows that NF/SGVHS had the lowest average annual rate of suicide (9.1 per 100,000) during the study period, which was 4 times lower than that of VHA national and 2.6 times lower than VISN 8.
Discussion
This study described and compared the distribution pattern of overdose (nonopioid and opioid) and suicide rates at different levels of the VHA system. Although VHA implemented systemwide opioid tapering in 2013, little is known about the association between opioid tapering and overdose and suicide. We believe a retrospective examination regarding overdose and suicide among VHA users at 3 different levels of the system from 2012 to 2016 could contribute to the discussion regarding the potential risks and benefits of discontinuing opioids.
First, the average annual rate of overdose diagnosis for NF/SGVHS during the study period was slightly higher (16.8 per 1,000) compared with those of VISN 8 (16.0 per 1,000) and VHA national (15.3 per 1,000) with a general pattern of increase and minimum variations in the rates observed during the study period among the 3 levels of the system. These increased overdose patterns are consistent with other reports in the literature.14 By the end of the study period, the NF/SGVHS rate (18.6 per 1,000) nearly matched the national rate (18.2 per 1,000) and was lower than VISN 8 (20.4 per 1,000). During the last year of the study period (2016), there was a dramatic increase in overdose diagnosis for all health care systems ranging from 2.2 per 1,000 for NF/SGVHS to 3.3 per 1,000 for VISN 8, which might be because of the VHA systemwide change of diagnosis code from ICD-9 to ICD-10, which includes more detailed diagnosis codes.
Second, our results showed that NF/SGVHS had the lowest average annual suicide rate (9.1 per 100,000) during the study period, which is one-fourth the VHA national rate and 2.6 per 100,000 lower than the VISN 8 rate. According to Bohnert and Ilgen,programs that improve the quality of pain care, expand access to psychotherapy, and increase access to medication-assisted treatment for OUDs could reduce suicide by drug overdose.7 We suggest that the low suicide rate at NF/SGVHS and the difference in the suicide rates between the NF/SGVHS and VISN 8 and VHA national data might be associated with the practice-based biopsychosocial interventions implemented at NF/SGVHS.
Our data showed a rise in the incidence of suicide at the NF/SGVHS in 2016. We are not aware of a local change in conditions, policy, and practice that would account for this increase. Suicide is variable, and data are likely to show spikes and valleys. Based on the available data, although the incidence of suicides at the NF/SGVHS in 2016 was higher, it remained below the VISN 8 and national VHA rate. This study seems to support the practice of tapering or stopping opioids within the context of a multidisciplinary approach that offers frequent follow-up, nonopioid options, and treatment of opioid addiction/dependence.
Limitations
The research findings of this study are limited by the retrospective and descriptive nature of its design. However, the findings might provide important information for understanding variations of overdose and suicide among VHA enrollees. Studies that use more robust methodologies are warranted to clinically investigate the impact of a multispecialty opioid risk reduction program targeting chronic pain and addiction management and identify best practices of opioid reduction and any unintended consequences that might arise from opioid tapering.26 Further, we did not have access to the VA national overdose and suicide data after 2016. Similar to most retrospective data studies, ours might be limited by availability of national overdose and suicide data after 2016. It is important for future studies to cross-validate our study findings.
Conclusions
The NF/SGVHS developed and implemented a biopsychosocial model of pain treatment that includes multicomponent primary care integrated with mental health and addiction services as well as the interventional pain and physical medicine and rehabilitation services. The presence of this program, during a period when the facility was tapering opioids is likely to account for at least part of the relative reduction in suicide.
1. American Foundation for Suicide Prevention. Suicide statistics. https://afsp.org/about-suicide/suicide-statistics. Updated 2019. Accessed September 2, 2020.
2. Shane L 3rd. New veteran suicide numbers raise concerns among experts hoping for positive news. https://www.militarytimes.com/news/pentagon-congress/2019/10/09/new-veteran-suicide-numbers-raise-concerns-among-experts-hoping-for-positive-news. Published October 9, 2019. Accessed July 23, 2020.
3. Veterans Health Administration, Office of Mental Health and Suicide Prevention. Veteran suicide data report, 2005–2017. https://www.mentalhealth.va.gov/docs/data-sheets/2019/2019_National_Veteran_Suicide_Prevention_Annual_Report_508.pdf. Published September 2019. Accessed July 20, 2020.
4. Gallagher RM. Advancing the pain agenda in the veteran population. Anesthesiol Clin. 2016;34(2):357-378. doi:10.1016/j.anclin.2016.01.003
5. Ilgen MA, Kleinberg F, Ignacio RV, et al. Noncancer pain conditions and risk of suicide. JAMA Psychiatry. 2013;70(7):692-697. doi:10.1001/jamapsychiatry.2013.908
6. Frenk SM, Porter KS, Paulozzi LJ. Prescription opioid analgesic use among adults: United States, 1999-2012. National Center for Health Statistics data brief. https://www.cdc.gov/nchs/products/databriefs/db189.htm. Published February 25, 2015. Accessed July 20, 2020.
7. Bohnert ASB, Ilgen MA. Understanding links among opioid use, overdose, and suicide. N Engl J Med. 2019;380(14):71-79. doi:10.1056/NEJMc1901540
8. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85-92. doi:10.7326/0003-4819-152-2-201001190-00006
9. Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171(7):686-691. doi:10.1001/archinternmed.2011.117
10. Ilgen MA, Bohnert AS, Ganoczy D, Bair MJ, McCarthy JF, Blow FC. Opioid dose and risk of suicide. Pain. 2016;157(5):1079-1084. doi:10.1097/j.pain.0000000000000484
11. Sinyor M, Howlett A, Cheung AH, Schaffer A. Substances used in completed suicide by overdose in Toronto: an observational study of coroner’s data. Can J Psychiatry. 2012;57(3):184-191. doi:10.1177/070674371205700308
12. Wilcox HC, Conner KR, Caine ED. Association of alcohol and drug use disorders and completed suicide: an empirical review of cohort studies. Drug Alcohol Depend. 2004;76(suppl):S11-S19 doi:10.1016/j.drugalcdep.2004.08.003.
13. Baser OL, Mardekian XJ, Schaaf D, Wang L, Joshi AV. Prevalence of diagnosed opioid abuse and its economic burden in the Veterans Health Administration. Pain Pract. 2014;14(5):437-445. doi:10.1111/papr.12097
14. Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the united states, 1999-2015. National Center for Health Statistics data brief. https://www.cdc.gov/nchs/data/databriefs/db273.pdf. Published February 2017. Accessed July 20, 2020.
15. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452. doi:10.15585/mmwr.mm655051e1
16. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths—United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2019,67(5152):1419-1427. doi:10.15585/mmwr.mm675152e1
17. US Department of Veterans Affairs and Department of Defense. VA/DOD clinical practice guideline for opioid therapy for chronic pain version 3.0. https://www.healthquality.va.gov/guidelines/pain/cot. Updated March 1, 2018. Accessed July 20, 2020.
18. Vaughn IA, Beyth RJ, Ayers ML, et al. Multispecialty opioid risk reduction program targeting chronic pain and addiction management in veterans. Fed Pract. 2019;36(9):406-411.
19. Gellad WF, Good CB, Shulkin DJ. Addressing the opioid epidemic in the United States: lessons from the Department of Veterans Affairs. JAMA Intern Med. 2017;177(5):611-612. doi:10.1001/jamainternmed.2017.0147
20. Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017;167(3):181-191. doi:10.7326/M17-0598
21. Berna C, Kulich RJ, Rathmell JP. Tapering long-term opioid therapy in chronic noncancer pain: evidence and recommendations for everyday practice. Mayo Clin Proc. 2015;90(6):828-842. doi:10.1016/j.mayocp.2015.04.003
22. Veterans Health Administration, Office of Mental Health and Suicide Prevention. Opioid use and suicide risk. https://www.mentalhealth.va.gov/suicide_prevention/docs/Literature_Review_Opioid_Use_and_Suicide_Risk_508_FINAL_04-26-2019.pdf. Published April 26, 2019. Accessed July 20, 2020.
23. Demidenko MI, Dobscha SK, Morasco BJ, Meath THA, Ilgen MA, Lovejoy TI. Suicidal ideation and suicidal self-directed violence following clinician-initiated prescription opioid discontinuation among long-term opioid users. Gen Hosp Psychiatry. 2017;47:29-35. doi:10.1016/j.genhosppsych.2017.04.011
24. National Institute on Drug Abuse. Intentional versus unintentional overdose deaths. https://www.drugabuse.gov/related-topics/treatment/intentional-vs-unintentional-overdose-deaths. Updated February 13, 2017. Accessed July 20, 2020.
25. Centers for Disease Control and Prevention. Preventing suicide. https://www.cdc.gov/violenceprevention/pdf/suicide-factsheet.pdf. Published 2018. Accessed July 20, 2020.
26. Webster LR. Pain and suicide: the other side of the opioid story. Pain Med. 2014;15(3):345-346. doi:10.1111/pme.12398
Suicide is the 10th leading cause of death in the US. In 2017, there were 47,173 deaths by suicide (14 deaths per 100,000 people), representing a 33% increase from 1999.1 In 2017 veterans accounted for 13.5% of all suicide deaths among US adults, although veterans comprised only 7.9% of the adult population; the age- and sex-adjusted suicide rate was 1.5 times higher for veterans than that of nonveteran adults.2,3
Among veteran users of Veterans Health Administration (VHA) services, mental health and substance use disorders, chronic medical conditions, and chronic pain are associated with an increased risk for suicide.3 About one-half of VHA veterans have been diagnosed with chronic pain.4 A chronic pain diagnosis (eg, back pain, migraine, and psychogenic pain) increased the risk of death by suicide even after adjusting for comorbid psychiatric diagnoses, according to a study on pain and suicide among US veterans.5
One-quarter of veterans received an opioid prescription during VHA outpatient care in 2012.4 Increased prescribing of opioid medications has been associated with opioid overdose and suicides.6-10 Opioids are the most common drugs found in suicide by overdose.11 The rate of opioid-related suicide deaths is 13 times higher among individuals with opioid use disorder (OUD) than it is for those without OUD.12 The rate of OUD diagnosis among VHA users was 7 times higher than that for non-VHA users.13
In the US the age-adjusted rate of drug overdose deaths increased from 6 per 100,000 persons in 1999 to 22 per 100,000 in 2017.14 Drug overdoses accounted for 52,404 US deaths in 2015; 33,091 (63.1%) were from opioids.15 In 2017, there were 70,237 drug overdose deaths; 67.8% involved opioids (ie, 5 per 100,000 population represent prescription opioids).16
The VHA is committed to reducing opioid use and veteran suicide prevention. In 2013 the VHA launched the Opioid Safety Initiative employing 4 strategies: education, pain management, risk management, and addiction treatment.17 To address the opioid epidemic, the North Florida/South Georgia Veteran Health System (NF/SGVHS) developed and implemented a multispecialty Opioid Risk Reduction Program that is fully integrated with mental health and addiction services. The purpose of the NF/SGVHS one-stop pain addiction clinic is to provide a treatment program for chronic pain and addiction. The program includes elements of a whole health approach to pain care, including battlefield and traditional acupuncture. The focus went beyond replacing pharmacologic treatments with a complementary integrative health approach to helping veterans regain control of their lives through empowerment, skill building, shared goal setting, and reinforcing self-management.
The self-management programs include a pain school for patient education, a pain psychology program, and a yoga program, all stressing self-management offered onsite and via telehealth. Special effort was directed to identify patients with OUD and opioid dependence. Many of these patients were transitioned to buprenorphine, a potent analgesic that suppresses opioid cravings and withdrawal symptoms associated with stopping opioids. The clinic was structured so that patients could be seen often for follow-up and support. In addition, open lines of communication and referral were set up between this clinic, the interventional pain clinic, and the physical medicine and rehabilitation service. A detailed description of this program has been published elsewhere.18
The number of veterans receiving opioid prescription across the VHA system decreased by 172,000 prescriptions quarterly between 2012 and 2016.19 Fewer veterans were prescribed high doses of opioids or concomitant interacting medicines and more veterans were receiving nonopioid therapies.19 The prescription reduction across the VHA has varied. For example, from 2012 to 2017 the NF/SGVHS reported an 87% reduction of opioid prescriptions (≥ 100 mg morphine equivalents/d), compared with the VHA national average reduction of 49%.18
Vigorous opioid reduction is controversial. In a systematic review on opioid reduction, Frank and colleagues reported some beneficial effects of opioid reduction, such as increased health-related quality of life.20 However, another study suggested a risk of increased pain with opioid tapering.21 The literature findings on the association between prescription opioid use and suicide are mixed. The VHA Office of Mental Health and Suicide Prevention literature review reported that veterans were at increased risk of committing suicide within the first 6 months of discontinuing opioid therapy.22 Another study reported that veterans who discontinued long-term opioid treatment had an increased risk for suicidal ideation.23 However, higher doses of opioids were associated with an increased risk for suicide among individuals with chronic pain.10 The link between opioid tapering and the risk of suicide or overdose is uncertain.
Bohnert and Ilgen suggested that discontinuing prescription opioids leads to suicide without examining the risk factors that influenced discontinuation is ill-informed.7 Strong evidence about the association or relationship among opioid use, overdose, and suicide is needed. To increase our understanding of that association, Bohnert and Ilgen argued for multifaceted interventions that simultaneously address the shared causes and risk factors for OUD,7 such as the multispecialty Opioid Risk Reduction Program at NF/SGVHS.
Because of the reported association between robust integrated mental health and addiction, primary care pain clinic intervention, and the higher rate of opioid tapering in NF/SGVHS,18 this study aims to describe the pattern of overdose diagnosis (opioid overdose and nonopioid overdose) and pattern of suicide rates among veterans enrolled in NF/SGVHS, Veterans Integrated Service Network (VISN) 8, and the entire VA health care system during 2012 to 2016.The study reviewed and compared overdose diagnosis and suicide rates among veterans across NF/SGVHS and 2 other levels of the VA health care system to determine whether there were variances in the pattern of overdose/suicide rates and to explore these differences.
Methods
In this retrospective study, aggregate data were obtained from several sources. First, the drug overdose data were extracted from the VA Support Service Center (VSSC) medical diagnosis cube. We reviewed the literature for opioid codes reported in the literature and compared these reported opioid International Classification of Diseases, Ninth Revision (ICD-9) and International Classification of Diseases, 10th Revision (ICD-10) codes with the local facility patient-level comprehensive overdose diagnosis codes. Based on the comparison, we found 98 ICD-9 and ICD-10 overdose diagnosis codes and ran the modified codes against the VSSC national database. Overdose data were aggregated by facility and fiscal year, and the overdose rates (per 1,000) were calculated for unique veteran users at the 3 levels (NF/SGVHS, VISN 8, and VA national) as the denominator.
Each of the 18 VISNs comprise multiple VAMCs and clinics within a geographic region. VISN 8 encompasses most of Florida and portions of southern Georgia and the Caribbean (Puerto Rico, US Virgin Islands), including NF/SGVHS.
In this study, drug overdose refers to the overdose or poisoning from all drugs (ie, opioids, cocaine, amphetamines, sedatives, etc) and defined as any unintentional (accidental), deliberate, or intent undetermined drug poisoning.24 The suicide data for this study were drawn from the VA Suicide Prevention Program at 3 different levels: NF/SGVHS, VISN 8, and VHA national. Suicide is death caused by an intentional act of injuring oneself with the intent to die.25
This descriptive study compared the rate of annual drug overdoses (per 1,000 enrollees) between NF/SGVHS, VISN 8, and VHA national from 2012 to 2016. It also compared the annual rate of suicide per 100,000 enrollees across these 3 levels of the VHA. The overdose and suicide rates and numbers are mutually exclusive, meaning the VISN 8 data do not include the NF/SGVHS information, and the national data excluded data from VISN 8 and NF/SGVHS. This approach helped improve the quality of multiple level comparisons for different levels of the VHA system.
Results
Figure 1 shows the pattern of overdose diagnosis by rates (per 1,000) across the study period (2012 to 2016) and compares patterns at 3 levels of VHA (NF/SGVHS, VISN 8, and VHA national). The average annual rate of overdose diagnoses for NF/SGVHS during the study was slightly higher (16.8 per 1,000) than that of VISN 8 (16 per 1,000) and VHA national (15.3 per 1,000), but by the end of the study period the NF/SGVHS rate (18.6 per 1,000) nearly matched the national rate (18.2 per 1,000) and was lower than the VISN 8 rate (20.4 per 1,000). Additionally, NF/SGVHS had less variability (SD, 1.34) in yearly average overdose rates compared with VISN 8 (SD, 2.96), and VHA national (SD, 1.69).
From 2013 to 2014 the overdose diagnosis rate for NF/SGVHS remained the same (17.1 per 1,000). A similar pattern was observed for the VHA national data, whereas the VISN 8 data showed a steady increase during the same period. In 2015, the NF/SGVHS had 0.7 per 1,000 decrease in overdose diagnosis rate, whereas VISN 8 and VHA national data showed 1.7 per 1,000 and 0.9 per 1,000 increases, respectively. During the last year of the study (2016), there was a dramatic increase in overdose diagnosis for all the health care systems, ranging from 2.2 per 1,000 for NF/SGVHS to 3.3 per 1,000 for VISN 8.
Figure 2 shows the annual rates (per 100,000 individuals) of suicide for NF/SGVHS, VISN 8, and VHA national. The suicide pattern for VISN 8 shows a cyclical acceleration and deceleration trend across the study period. From 2012 to 2014, the VHA national data show a steady increase of about 1 per 100,000 from year to year. On the contrary, NF/SGVHS shows a low suicide rate from year to year within the same period with a rate of 10 per 100,000 in 2013 compared with the previous year. Although the NF/SGVHS suicide rate increased in 2016 (10.4 per 100,000), it remained lower than that of VISN 8 (10.7 per 100,00) and VHA national (38.2 per 100,000).
This study shows that NF/SGVHS had the lowest average annual rate of suicide (9.1 per 100,000) during the study period, which was 4 times lower than that of VHA national and 2.6 times lower than VISN 8.
Discussion
This study described and compared the distribution pattern of overdose (nonopioid and opioid) and suicide rates at different levels of the VHA system. Although VHA implemented systemwide opioid tapering in 2013, little is known about the association between opioid tapering and overdose and suicide. We believe a retrospective examination regarding overdose and suicide among VHA users at 3 different levels of the system from 2012 to 2016 could contribute to the discussion regarding the potential risks and benefits of discontinuing opioids.
First, the average annual rate of overdose diagnosis for NF/SGVHS during the study period was slightly higher (16.8 per 1,000) compared with those of VISN 8 (16.0 per 1,000) and VHA national (15.3 per 1,000) with a general pattern of increase and minimum variations in the rates observed during the study period among the 3 levels of the system. These increased overdose patterns are consistent with other reports in the literature.14 By the end of the study period, the NF/SGVHS rate (18.6 per 1,000) nearly matched the national rate (18.2 per 1,000) and was lower than VISN 8 (20.4 per 1,000). During the last year of the study period (2016), there was a dramatic increase in overdose diagnosis for all health care systems ranging from 2.2 per 1,000 for NF/SGVHS to 3.3 per 1,000 for VISN 8, which might be because of the VHA systemwide change of diagnosis code from ICD-9 to ICD-10, which includes more detailed diagnosis codes.
Second, our results showed that NF/SGVHS had the lowest average annual suicide rate (9.1 per 100,000) during the study period, which is one-fourth the VHA national rate and 2.6 per 100,000 lower than the VISN 8 rate. According to Bohnert and Ilgen,programs that improve the quality of pain care, expand access to psychotherapy, and increase access to medication-assisted treatment for OUDs could reduce suicide by drug overdose.7 We suggest that the low suicide rate at NF/SGVHS and the difference in the suicide rates between the NF/SGVHS and VISN 8 and VHA national data might be associated with the practice-based biopsychosocial interventions implemented at NF/SGVHS.
Our data showed a rise in the incidence of suicide at the NF/SGVHS in 2016. We are not aware of a local change in conditions, policy, and practice that would account for this increase. Suicide is variable, and data are likely to show spikes and valleys. Based on the available data, although the incidence of suicides at the NF/SGVHS in 2016 was higher, it remained below the VISN 8 and national VHA rate. This study seems to support the practice of tapering or stopping opioids within the context of a multidisciplinary approach that offers frequent follow-up, nonopioid options, and treatment of opioid addiction/dependence.
Limitations
The research findings of this study are limited by the retrospective and descriptive nature of its design. However, the findings might provide important information for understanding variations of overdose and suicide among VHA enrollees. Studies that use more robust methodologies are warranted to clinically investigate the impact of a multispecialty opioid risk reduction program targeting chronic pain and addiction management and identify best practices of opioid reduction and any unintended consequences that might arise from opioid tapering.26 Further, we did not have access to the VA national overdose and suicide data after 2016. Similar to most retrospective data studies, ours might be limited by availability of national overdose and suicide data after 2016. It is important for future studies to cross-validate our study findings.
Conclusions
The NF/SGVHS developed and implemented a biopsychosocial model of pain treatment that includes multicomponent primary care integrated with mental health and addiction services as well as the interventional pain and physical medicine and rehabilitation services. The presence of this program, during a period when the facility was tapering opioids is likely to account for at least part of the relative reduction in suicide.
Suicide is the 10th leading cause of death in the US. In 2017, there were 47,173 deaths by suicide (14 deaths per 100,000 people), representing a 33% increase from 1999.1 In 2017 veterans accounted for 13.5% of all suicide deaths among US adults, although veterans comprised only 7.9% of the adult population; the age- and sex-adjusted suicide rate was 1.5 times higher for veterans than that of nonveteran adults.2,3
Among veteran users of Veterans Health Administration (VHA) services, mental health and substance use disorders, chronic medical conditions, and chronic pain are associated with an increased risk for suicide.3 About one-half of VHA veterans have been diagnosed with chronic pain.4 A chronic pain diagnosis (eg, back pain, migraine, and psychogenic pain) increased the risk of death by suicide even after adjusting for comorbid psychiatric diagnoses, according to a study on pain and suicide among US veterans.5
One-quarter of veterans received an opioid prescription during VHA outpatient care in 2012.4 Increased prescribing of opioid medications has been associated with opioid overdose and suicides.6-10 Opioids are the most common drugs found in suicide by overdose.11 The rate of opioid-related suicide deaths is 13 times higher among individuals with opioid use disorder (OUD) than it is for those without OUD.12 The rate of OUD diagnosis among VHA users was 7 times higher than that for non-VHA users.13
In the US the age-adjusted rate of drug overdose deaths increased from 6 per 100,000 persons in 1999 to 22 per 100,000 in 2017.14 Drug overdoses accounted for 52,404 US deaths in 2015; 33,091 (63.1%) were from opioids.15 In 2017, there were 70,237 drug overdose deaths; 67.8% involved opioids (ie, 5 per 100,000 population represent prescription opioids).16
The VHA is committed to reducing opioid use and veteran suicide prevention. In 2013 the VHA launched the Opioid Safety Initiative employing 4 strategies: education, pain management, risk management, and addiction treatment.17 To address the opioid epidemic, the North Florida/South Georgia Veteran Health System (NF/SGVHS) developed and implemented a multispecialty Opioid Risk Reduction Program that is fully integrated with mental health and addiction services. The purpose of the NF/SGVHS one-stop pain addiction clinic is to provide a treatment program for chronic pain and addiction. The program includes elements of a whole health approach to pain care, including battlefield and traditional acupuncture. The focus went beyond replacing pharmacologic treatments with a complementary integrative health approach to helping veterans regain control of their lives through empowerment, skill building, shared goal setting, and reinforcing self-management.
The self-management programs include a pain school for patient education, a pain psychology program, and a yoga program, all stressing self-management offered onsite and via telehealth. Special effort was directed to identify patients with OUD and opioid dependence. Many of these patients were transitioned to buprenorphine, a potent analgesic that suppresses opioid cravings and withdrawal symptoms associated with stopping opioids. The clinic was structured so that patients could be seen often for follow-up and support. In addition, open lines of communication and referral were set up between this clinic, the interventional pain clinic, and the physical medicine and rehabilitation service. A detailed description of this program has been published elsewhere.18
The number of veterans receiving opioid prescription across the VHA system decreased by 172,000 prescriptions quarterly between 2012 and 2016.19 Fewer veterans were prescribed high doses of opioids or concomitant interacting medicines and more veterans were receiving nonopioid therapies.19 The prescription reduction across the VHA has varied. For example, from 2012 to 2017 the NF/SGVHS reported an 87% reduction of opioid prescriptions (≥ 100 mg morphine equivalents/d), compared with the VHA national average reduction of 49%.18
Vigorous opioid reduction is controversial. In a systematic review on opioid reduction, Frank and colleagues reported some beneficial effects of opioid reduction, such as increased health-related quality of life.20 However, another study suggested a risk of increased pain with opioid tapering.21 The literature findings on the association between prescription opioid use and suicide are mixed. The VHA Office of Mental Health and Suicide Prevention literature review reported that veterans were at increased risk of committing suicide within the first 6 months of discontinuing opioid therapy.22 Another study reported that veterans who discontinued long-term opioid treatment had an increased risk for suicidal ideation.23 However, higher doses of opioids were associated with an increased risk for suicide among individuals with chronic pain.10 The link between opioid tapering and the risk of suicide or overdose is uncertain.
Bohnert and Ilgen suggested that discontinuing prescription opioids leads to suicide without examining the risk factors that influenced discontinuation is ill-informed.7 Strong evidence about the association or relationship among opioid use, overdose, and suicide is needed. To increase our understanding of that association, Bohnert and Ilgen argued for multifaceted interventions that simultaneously address the shared causes and risk factors for OUD,7 such as the multispecialty Opioid Risk Reduction Program at NF/SGVHS.
Because of the reported association between robust integrated mental health and addiction, primary care pain clinic intervention, and the higher rate of opioid tapering in NF/SGVHS,18 this study aims to describe the pattern of overdose diagnosis (opioid overdose and nonopioid overdose) and pattern of suicide rates among veterans enrolled in NF/SGVHS, Veterans Integrated Service Network (VISN) 8, and the entire VA health care system during 2012 to 2016.The study reviewed and compared overdose diagnosis and suicide rates among veterans across NF/SGVHS and 2 other levels of the VA health care system to determine whether there were variances in the pattern of overdose/suicide rates and to explore these differences.
Methods
In this retrospective study, aggregate data were obtained from several sources. First, the drug overdose data were extracted from the VA Support Service Center (VSSC) medical diagnosis cube. We reviewed the literature for opioid codes reported in the literature and compared these reported opioid International Classification of Diseases, Ninth Revision (ICD-9) and International Classification of Diseases, 10th Revision (ICD-10) codes with the local facility patient-level comprehensive overdose diagnosis codes. Based on the comparison, we found 98 ICD-9 and ICD-10 overdose diagnosis codes and ran the modified codes against the VSSC national database. Overdose data were aggregated by facility and fiscal year, and the overdose rates (per 1,000) were calculated for unique veteran users at the 3 levels (NF/SGVHS, VISN 8, and VA national) as the denominator.
Each of the 18 VISNs comprise multiple VAMCs and clinics within a geographic region. VISN 8 encompasses most of Florida and portions of southern Georgia and the Caribbean (Puerto Rico, US Virgin Islands), including NF/SGVHS.
In this study, drug overdose refers to the overdose or poisoning from all drugs (ie, opioids, cocaine, amphetamines, sedatives, etc) and defined as any unintentional (accidental), deliberate, or intent undetermined drug poisoning.24 The suicide data for this study were drawn from the VA Suicide Prevention Program at 3 different levels: NF/SGVHS, VISN 8, and VHA national. Suicide is death caused by an intentional act of injuring oneself with the intent to die.25
This descriptive study compared the rate of annual drug overdoses (per 1,000 enrollees) between NF/SGVHS, VISN 8, and VHA national from 2012 to 2016. It also compared the annual rate of suicide per 100,000 enrollees across these 3 levels of the VHA. The overdose and suicide rates and numbers are mutually exclusive, meaning the VISN 8 data do not include the NF/SGVHS information, and the national data excluded data from VISN 8 and NF/SGVHS. This approach helped improve the quality of multiple level comparisons for different levels of the VHA system.
Results
Figure 1 shows the pattern of overdose diagnosis by rates (per 1,000) across the study period (2012 to 2016) and compares patterns at 3 levels of VHA (NF/SGVHS, VISN 8, and VHA national). The average annual rate of overdose diagnoses for NF/SGVHS during the study was slightly higher (16.8 per 1,000) than that of VISN 8 (16 per 1,000) and VHA national (15.3 per 1,000), but by the end of the study period the NF/SGVHS rate (18.6 per 1,000) nearly matched the national rate (18.2 per 1,000) and was lower than the VISN 8 rate (20.4 per 1,000). Additionally, NF/SGVHS had less variability (SD, 1.34) in yearly average overdose rates compared with VISN 8 (SD, 2.96), and VHA national (SD, 1.69).
From 2013 to 2014 the overdose diagnosis rate for NF/SGVHS remained the same (17.1 per 1,000). A similar pattern was observed for the VHA national data, whereas the VISN 8 data showed a steady increase during the same period. In 2015, the NF/SGVHS had 0.7 per 1,000 decrease in overdose diagnosis rate, whereas VISN 8 and VHA national data showed 1.7 per 1,000 and 0.9 per 1,000 increases, respectively. During the last year of the study (2016), there was a dramatic increase in overdose diagnosis for all the health care systems, ranging from 2.2 per 1,000 for NF/SGVHS to 3.3 per 1,000 for VISN 8.
Figure 2 shows the annual rates (per 100,000 individuals) of suicide for NF/SGVHS, VISN 8, and VHA national. The suicide pattern for VISN 8 shows a cyclical acceleration and deceleration trend across the study period. From 2012 to 2014, the VHA national data show a steady increase of about 1 per 100,000 from year to year. On the contrary, NF/SGVHS shows a low suicide rate from year to year within the same period with a rate of 10 per 100,000 in 2013 compared with the previous year. Although the NF/SGVHS suicide rate increased in 2016 (10.4 per 100,000), it remained lower than that of VISN 8 (10.7 per 100,00) and VHA national (38.2 per 100,000).
This study shows that NF/SGVHS had the lowest average annual rate of suicide (9.1 per 100,000) during the study period, which was 4 times lower than that of VHA national and 2.6 times lower than VISN 8.
Discussion
This study described and compared the distribution pattern of overdose (nonopioid and opioid) and suicide rates at different levels of the VHA system. Although VHA implemented systemwide opioid tapering in 2013, little is known about the association between opioid tapering and overdose and suicide. We believe a retrospective examination regarding overdose and suicide among VHA users at 3 different levels of the system from 2012 to 2016 could contribute to the discussion regarding the potential risks and benefits of discontinuing opioids.
First, the average annual rate of overdose diagnosis for NF/SGVHS during the study period was slightly higher (16.8 per 1,000) compared with those of VISN 8 (16.0 per 1,000) and VHA national (15.3 per 1,000) with a general pattern of increase and minimum variations in the rates observed during the study period among the 3 levels of the system. These increased overdose patterns are consistent with other reports in the literature.14 By the end of the study period, the NF/SGVHS rate (18.6 per 1,000) nearly matched the national rate (18.2 per 1,000) and was lower than VISN 8 (20.4 per 1,000). During the last year of the study period (2016), there was a dramatic increase in overdose diagnosis for all health care systems ranging from 2.2 per 1,000 for NF/SGVHS to 3.3 per 1,000 for VISN 8, which might be because of the VHA systemwide change of diagnosis code from ICD-9 to ICD-10, which includes more detailed diagnosis codes.
Second, our results showed that NF/SGVHS had the lowest average annual suicide rate (9.1 per 100,000) during the study period, which is one-fourth the VHA national rate and 2.6 per 100,000 lower than the VISN 8 rate. According to Bohnert and Ilgen,programs that improve the quality of pain care, expand access to psychotherapy, and increase access to medication-assisted treatment for OUDs could reduce suicide by drug overdose.7 We suggest that the low suicide rate at NF/SGVHS and the difference in the suicide rates between the NF/SGVHS and VISN 8 and VHA national data might be associated with the practice-based biopsychosocial interventions implemented at NF/SGVHS.
Our data showed a rise in the incidence of suicide at the NF/SGVHS in 2016. We are not aware of a local change in conditions, policy, and practice that would account for this increase. Suicide is variable, and data are likely to show spikes and valleys. Based on the available data, although the incidence of suicides at the NF/SGVHS in 2016 was higher, it remained below the VISN 8 and national VHA rate. This study seems to support the practice of tapering or stopping opioids within the context of a multidisciplinary approach that offers frequent follow-up, nonopioid options, and treatment of opioid addiction/dependence.
Limitations
The research findings of this study are limited by the retrospective and descriptive nature of its design. However, the findings might provide important information for understanding variations of overdose and suicide among VHA enrollees. Studies that use more robust methodologies are warranted to clinically investigate the impact of a multispecialty opioid risk reduction program targeting chronic pain and addiction management and identify best practices of opioid reduction and any unintended consequences that might arise from opioid tapering.26 Further, we did not have access to the VA national overdose and suicide data after 2016. Similar to most retrospective data studies, ours might be limited by availability of national overdose and suicide data after 2016. It is important for future studies to cross-validate our study findings.
Conclusions
The NF/SGVHS developed and implemented a biopsychosocial model of pain treatment that includes multicomponent primary care integrated with mental health and addiction services as well as the interventional pain and physical medicine and rehabilitation services. The presence of this program, during a period when the facility was tapering opioids is likely to account for at least part of the relative reduction in suicide.
1. American Foundation for Suicide Prevention. Suicide statistics. https://afsp.org/about-suicide/suicide-statistics. Updated 2019. Accessed September 2, 2020.
2. Shane L 3rd. New veteran suicide numbers raise concerns among experts hoping for positive news. https://www.militarytimes.com/news/pentagon-congress/2019/10/09/new-veteran-suicide-numbers-raise-concerns-among-experts-hoping-for-positive-news. Published October 9, 2019. Accessed July 23, 2020.
3. Veterans Health Administration, Office of Mental Health and Suicide Prevention. Veteran suicide data report, 2005–2017. https://www.mentalhealth.va.gov/docs/data-sheets/2019/2019_National_Veteran_Suicide_Prevention_Annual_Report_508.pdf. Published September 2019. Accessed July 20, 2020.
4. Gallagher RM. Advancing the pain agenda in the veteran population. Anesthesiol Clin. 2016;34(2):357-378. doi:10.1016/j.anclin.2016.01.003
5. Ilgen MA, Kleinberg F, Ignacio RV, et al. Noncancer pain conditions and risk of suicide. JAMA Psychiatry. 2013;70(7):692-697. doi:10.1001/jamapsychiatry.2013.908
6. Frenk SM, Porter KS, Paulozzi LJ. Prescription opioid analgesic use among adults: United States, 1999-2012. National Center for Health Statistics data brief. https://www.cdc.gov/nchs/products/databriefs/db189.htm. Published February 25, 2015. Accessed July 20, 2020.
7. Bohnert ASB, Ilgen MA. Understanding links among opioid use, overdose, and suicide. N Engl J Med. 2019;380(14):71-79. doi:10.1056/NEJMc1901540
8. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85-92. doi:10.7326/0003-4819-152-2-201001190-00006
9. Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171(7):686-691. doi:10.1001/archinternmed.2011.117
10. Ilgen MA, Bohnert AS, Ganoczy D, Bair MJ, McCarthy JF, Blow FC. Opioid dose and risk of suicide. Pain. 2016;157(5):1079-1084. doi:10.1097/j.pain.0000000000000484
11. Sinyor M, Howlett A, Cheung AH, Schaffer A. Substances used in completed suicide by overdose in Toronto: an observational study of coroner’s data. Can J Psychiatry. 2012;57(3):184-191. doi:10.1177/070674371205700308
12. Wilcox HC, Conner KR, Caine ED. Association of alcohol and drug use disorders and completed suicide: an empirical review of cohort studies. Drug Alcohol Depend. 2004;76(suppl):S11-S19 doi:10.1016/j.drugalcdep.2004.08.003.
13. Baser OL, Mardekian XJ, Schaaf D, Wang L, Joshi AV. Prevalence of diagnosed opioid abuse and its economic burden in the Veterans Health Administration. Pain Pract. 2014;14(5):437-445. doi:10.1111/papr.12097
14. Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the united states, 1999-2015. National Center for Health Statistics data brief. https://www.cdc.gov/nchs/data/databriefs/db273.pdf. Published February 2017. Accessed July 20, 2020.
15. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452. doi:10.15585/mmwr.mm655051e1
16. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths—United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2019,67(5152):1419-1427. doi:10.15585/mmwr.mm675152e1
17. US Department of Veterans Affairs and Department of Defense. VA/DOD clinical practice guideline for opioid therapy for chronic pain version 3.0. https://www.healthquality.va.gov/guidelines/pain/cot. Updated March 1, 2018. Accessed July 20, 2020.
18. Vaughn IA, Beyth RJ, Ayers ML, et al. Multispecialty opioid risk reduction program targeting chronic pain and addiction management in veterans. Fed Pract. 2019;36(9):406-411.
19. Gellad WF, Good CB, Shulkin DJ. Addressing the opioid epidemic in the United States: lessons from the Department of Veterans Affairs. JAMA Intern Med. 2017;177(5):611-612. doi:10.1001/jamainternmed.2017.0147
20. Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017;167(3):181-191. doi:10.7326/M17-0598
21. Berna C, Kulich RJ, Rathmell JP. Tapering long-term opioid therapy in chronic noncancer pain: evidence and recommendations for everyday practice. Mayo Clin Proc. 2015;90(6):828-842. doi:10.1016/j.mayocp.2015.04.003
22. Veterans Health Administration, Office of Mental Health and Suicide Prevention. Opioid use and suicide risk. https://www.mentalhealth.va.gov/suicide_prevention/docs/Literature_Review_Opioid_Use_and_Suicide_Risk_508_FINAL_04-26-2019.pdf. Published April 26, 2019. Accessed July 20, 2020.
23. Demidenko MI, Dobscha SK, Morasco BJ, Meath THA, Ilgen MA, Lovejoy TI. Suicidal ideation and suicidal self-directed violence following clinician-initiated prescription opioid discontinuation among long-term opioid users. Gen Hosp Psychiatry. 2017;47:29-35. doi:10.1016/j.genhosppsych.2017.04.011
24. National Institute on Drug Abuse. Intentional versus unintentional overdose deaths. https://www.drugabuse.gov/related-topics/treatment/intentional-vs-unintentional-overdose-deaths. Updated February 13, 2017. Accessed July 20, 2020.
25. Centers for Disease Control and Prevention. Preventing suicide. https://www.cdc.gov/violenceprevention/pdf/suicide-factsheet.pdf. Published 2018. Accessed July 20, 2020.
26. Webster LR. Pain and suicide: the other side of the opioid story. Pain Med. 2014;15(3):345-346. doi:10.1111/pme.12398
1. American Foundation for Suicide Prevention. Suicide statistics. https://afsp.org/about-suicide/suicide-statistics. Updated 2019. Accessed September 2, 2020.
2. Shane L 3rd. New veteran suicide numbers raise concerns among experts hoping for positive news. https://www.militarytimes.com/news/pentagon-congress/2019/10/09/new-veteran-suicide-numbers-raise-concerns-among-experts-hoping-for-positive-news. Published October 9, 2019. Accessed July 23, 2020.
3. Veterans Health Administration, Office of Mental Health and Suicide Prevention. Veteran suicide data report, 2005–2017. https://www.mentalhealth.va.gov/docs/data-sheets/2019/2019_National_Veteran_Suicide_Prevention_Annual_Report_508.pdf. Published September 2019. Accessed July 20, 2020.
4. Gallagher RM. Advancing the pain agenda in the veteran population. Anesthesiol Clin. 2016;34(2):357-378. doi:10.1016/j.anclin.2016.01.003
5. Ilgen MA, Kleinberg F, Ignacio RV, et al. Noncancer pain conditions and risk of suicide. JAMA Psychiatry. 2013;70(7):692-697. doi:10.1001/jamapsychiatry.2013.908
6. Frenk SM, Porter KS, Paulozzi LJ. Prescription opioid analgesic use among adults: United States, 1999-2012. National Center for Health Statistics data brief. https://www.cdc.gov/nchs/products/databriefs/db189.htm. Published February 25, 2015. Accessed July 20, 2020.
7. Bohnert ASB, Ilgen MA. Understanding links among opioid use, overdose, and suicide. N Engl J Med. 2019;380(14):71-79. doi:10.1056/NEJMc1901540
8. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85-92. doi:10.7326/0003-4819-152-2-201001190-00006
9. Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171(7):686-691. doi:10.1001/archinternmed.2011.117
10. Ilgen MA, Bohnert AS, Ganoczy D, Bair MJ, McCarthy JF, Blow FC. Opioid dose and risk of suicide. Pain. 2016;157(5):1079-1084. doi:10.1097/j.pain.0000000000000484
11. Sinyor M, Howlett A, Cheung AH, Schaffer A. Substances used in completed suicide by overdose in Toronto: an observational study of coroner’s data. Can J Psychiatry. 2012;57(3):184-191. doi:10.1177/070674371205700308
12. Wilcox HC, Conner KR, Caine ED. Association of alcohol and drug use disorders and completed suicide: an empirical review of cohort studies. Drug Alcohol Depend. 2004;76(suppl):S11-S19 doi:10.1016/j.drugalcdep.2004.08.003.
13. Baser OL, Mardekian XJ, Schaaf D, Wang L, Joshi AV. Prevalence of diagnosed opioid abuse and its economic burden in the Veterans Health Administration. Pain Pract. 2014;14(5):437-445. doi:10.1111/papr.12097
14. Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the united states, 1999-2015. National Center for Health Statistics data brief. https://www.cdc.gov/nchs/data/databriefs/db273.pdf. Published February 2017. Accessed July 20, 2020.
15. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452. doi:10.15585/mmwr.mm655051e1
16. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths—United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2019,67(5152):1419-1427. doi:10.15585/mmwr.mm675152e1
17. US Department of Veterans Affairs and Department of Defense. VA/DOD clinical practice guideline for opioid therapy for chronic pain version 3.0. https://www.healthquality.va.gov/guidelines/pain/cot. Updated March 1, 2018. Accessed July 20, 2020.
18. Vaughn IA, Beyth RJ, Ayers ML, et al. Multispecialty opioid risk reduction program targeting chronic pain and addiction management in veterans. Fed Pract. 2019;36(9):406-411.
19. Gellad WF, Good CB, Shulkin DJ. Addressing the opioid epidemic in the United States: lessons from the Department of Veterans Affairs. JAMA Intern Med. 2017;177(5):611-612. doi:10.1001/jamainternmed.2017.0147
20. Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017;167(3):181-191. doi:10.7326/M17-0598
21. Berna C, Kulich RJ, Rathmell JP. Tapering long-term opioid therapy in chronic noncancer pain: evidence and recommendations for everyday practice. Mayo Clin Proc. 2015;90(6):828-842. doi:10.1016/j.mayocp.2015.04.003
22. Veterans Health Administration, Office of Mental Health and Suicide Prevention. Opioid use and suicide risk. https://www.mentalhealth.va.gov/suicide_prevention/docs/Literature_Review_Opioid_Use_and_Suicide_Risk_508_FINAL_04-26-2019.pdf. Published April 26, 2019. Accessed July 20, 2020.
23. Demidenko MI, Dobscha SK, Morasco BJ, Meath THA, Ilgen MA, Lovejoy TI. Suicidal ideation and suicidal self-directed violence following clinician-initiated prescription opioid discontinuation among long-term opioid users. Gen Hosp Psychiatry. 2017;47:29-35. doi:10.1016/j.genhosppsych.2017.04.011
24. National Institute on Drug Abuse. Intentional versus unintentional overdose deaths. https://www.drugabuse.gov/related-topics/treatment/intentional-vs-unintentional-overdose-deaths. Updated February 13, 2017. Accessed July 20, 2020.
25. Centers for Disease Control and Prevention. Preventing suicide. https://www.cdc.gov/violenceprevention/pdf/suicide-factsheet.pdf. Published 2018. Accessed July 20, 2020.
26. Webster LR. Pain and suicide: the other side of the opioid story. Pain Med. 2014;15(3):345-346. doi:10.1111/pme.12398
Veterans, Firearms, and Suicide: Safe Storage Prevention Policy and the PREVENTS Roadmap
US veterans die by suicide at a higher rate than that of the civilian population, and are more likely to use a firearm as their lethal means.1 In 2017, 6,139 veterans died by suicide, about 17 per day.1 Nearly as many veterans die by suicide yearly as the total aggregate number of service members killed in action during the decades-long Iraq and Afghanistan operations.2 Veterans are more likely to own firearms than are civilians.3 Until June 2020, however, systemic efforts to address the use of firearms in suicide had been largely evaded, entangled in gun advocates’ assertion that veterans’ constitutional right to bear arms would be infringed.
That impasse changed with the President’s Roadmap to Empower Veterans and End the National Tragedy of Suicide (PREVENTS) task force report, released June 17, 2020.4 Although the US Department of Veterans Affairs (VA) has pioneered initiatives to encourage safe firearm storage for at-risk veterans, and major public health organizations have endorsed the utility of lethal means safety strategies, the policy language of the Roadmap released by the White House is unprecedented. Lethal means safety refers to efforts aimed at increasing the time and distance needed to access suicide methods.
Among the report’s 10 recommendations, the Roadmap verified the link between, and the need to address, at-risk veterans and their access to firearms (the author was a minor consultant to a PREVENTS workgroup). The document states, “The science supporting lethal means safety is robust and compelling: enhancing safety measures specific to the availability and accessibility of potential lethal means saves lives. A key component of effective suicide prevention is voluntary reduction in the ability to access lethal means with respect to time, distance, and convenience, particularly during acute suicidal crises.”4 The report recommends widespread distribution of safety education materials that encourage at-risk individuals to temporarily transfer or store their guns safely, and the expansion of free or affordable options for storing weapons, among other recommendations.
This paper reviews the literature on the intersection of veterans, firearms, and suicide, then explores existing VA prevention initiatives aimed at reducing at-risk veterans’ access to lethal means and offers policy recommendations to expand efforts in the context of the PREVENTS Roadmap.
Veteran Suicide and Firearms
Firearms are, by far, the most common lethal means used by veterans who die by suicide. About 71% of male veteran suicide deaths and 43% of female veteran suicide deaths are with firearms, rates that far exceed those of nonveterans (Table).For all age groups, veterans are more likely to complete suicide by firearm than are nonveterans.5
Veteran suicide and gun ownership rates are highest in rural areas.6,7 When compared with veterans living in urban areas, veterans in rural areas are 20% more likely to die by suicide, with the excessive risk largely attributed to suicide by firearm.8
Access to firearms at home increases the risk of suicide. Individuals with any firearm at home are 3 times more likely to die by suicide than is a person with no firearms at home. The elevated suicide risk applies to other household members as well as the firearm owner.9-18 Survivors of suicide attempts using firearms report that the availability of guns at home is the primary reason for their method choice.19,20
There is a common misperception that people who are intent on suicide and are thwarted or survive an attempt using one method will try again with another.21 Suicidal crises often represent a conflicting wish to live or die,22 and approximately two-thirds of those who survive an attempt will never try again. About 23% reattempt nonfatally, and only 10% die by suicide.23-25 However, people who attempt suicide with a firearm usually won’t get a chance at a new start, because 90% of such acts are fatal.26
Although some suicide attempts might be contemplated or planned over an extended period, the decision is impulsive for most individuals. Surveys have found that many people who survive suicide attempts began the act only minutes or hours after making the decision to end their life.27-30 The high-risk, acute phase of many suicidal crises arise quickly and is fleeting.
Limiting the ease by which at-risk individuals can access firearms has been shown to prevent suicide. In 2006, the overall suicide rate in Israel dropped 40% when the Israeli Defense Forces began requiring soldiers to store their firearms on base before going on weekend leave.Since then, the suicide rate has declined even further.31,32
Delaying Access to Firearms for At-Risk Veterans
Among veterans, 45% own ≥ 1 firearms (47% male and 24% female veterans vs 30% male and 12% female nonveterans).3 Many veteran firearm owners (34% male and 13% female) store ≥ 1 gun loaded and unlocked; 44% store a firearm either loaded or unlocked. Only 23% safely store their firearms unloaded and locked at home. Storing ≥ 1 firearm loaded and unlocked is more likely among veterans who reside in rural areas, separated from service before 2002, and report personal protection as the primary reason for ownership.33
Because evidence shows that delaying access to firearms—especially by transferring them out of the home—saves lives, many US health organizations have advocated for strategies that promote evaluation of firearm access and counseling safe storage for individuals at risk for suicide. These organizations include the US Office of the Surgeon General, National Action Alliance for Suicide Prevention, Centers for Disease Control and Prevention, and American Public Health Association.34-36
Some health care systems—notably Kaiser Permanente and Henry Ford Health Systems—implemented protocols for lethal means assessment and counseling for behavioral health patients.37,38 Washington state requires specific health professionals to enroll in suicide prevention training that includes content on the risk of imminent harm by lethal means.39 California is designing a curriculum on counseling patients to reduce firearm injury for physicians and other health care practitioners (HCPs).40
The scope of these efforts, however, pale in comparison with the VA’s comprehensive, innovative lethal means safety approach. Since 2012, VA’s Suicide Prevention Program has distributed free firearm cable locks to veterans who request them. The VA has created lethal means public service announcements, social media messages, and websites.41-44 The VA distributes firearm and medication safe storage practice resource kits to its primary care, mental health and women’s health clinics, and Vet Centers, that include brochures, large poster cards, stickers, exam room posters, and provider pocket cards. VA developed an online lethal means safety counseling training that 20,000 VA HCPs have taken, and is moving toward a revamped mandatory training for VA’s mental health, pain, primary care, and emergency department (ED) providers and Veterans Crisis Line responders. VA offers free, individualized lethal means risk management consultation to all clinicians who work with veterans.45 VA includes lethal means safety procedures in its National Strategy for Preventing Veteran Suicide,VA/DoD Clinical Practice Guideline,and VA Suicide Risk Evaluation and Suicide Prevention Safety Planrequired of clinicians.46-48
The VA also added public health strategies that promote safe storage practices for veterans through a partnership with the National Shooting Sports Foundation (NSSF; the firearm industry trade association) and the American Foundation for Suicide Prevention (AFSP).49 Collectively, these organizations cobranded an educational, training, and resource toolkit to foster community coalitions and gun retailer projects that encourage veterans to securely store firearms.50 The VA partnered with NSSF to post billboards in 8 states, encouraging storing firearms responsibly to prevent suicide. VA invited states and cities in the Governor/Mayoral Challenge to Prevent Suicide (joint VA and Substance Abuse and Mental Health Services Administration endeavors) to develop plans for messaging regarding enhanced lethal means safety processes. The VA collaborated with local firearm advocates in community prevention pilot projects and in a “Together with Veterans” dissemination of material and outreach to rural veterans.51 Along with AFSP, VA hosted conferences for HCPs, policy makers, and stakeholders about innovations related to lethal means safety.52 In May 2020, the VA cosponsored a COVID-19 suicide prevention video with the United States Concealed Carry Association, NSSF, and AFSP, including ways that the firearm industry, gun owners, and their families can help.53
These programs are promising, and the Roadmap’s emphatic endorsement of lethal means safety approaches will accelerate advances. However, the Roadmap’s omissions are consequential. By focusing on population interventions, the document is silent about VA-specific or veteran-specific firearm access strategies. The means safety work of VA’s Suicide Prevention Program Office is scarcely recognized. Further, it stops short of specific legislative initiatives, making aspirational recommendations instead.
This paper will list proposed policy actions to bolster the acceptability and practice of lethal means safety with veterans. They cover an entire range of possibilities, from putting more teeth into the Roadmap’s population-wide interventions to initiatives tailored to veterans. Responsibility for leading and funding the changes would reside in a mix of Congress and state legislatures, the VA, and health system accreditation bodies. Although there is solid evidence that lethal means safety prevents suicide, it is unknown how these approaches affect firearm storage behaviors or suicide rates;therefore, the policy actions should come with federal and state funds for rigorous evaluation.54
Recommended Actions to Further Promote Safe Storage
Develop Campaigns to Shift Cultural Norms for Firearm Storage During Crises
National campaigns have been shown to be highly effective in changing injurious behaviors. Alliances and resources with regard to lethal means safety could be assembled, including federal funds for a campaign to shift social norms for firearm storage conversations and behaviors during crises. This campaign should be modeled after the “friends don’t let friends drive drunk” and “designated driver” campaigns that empower family and friends to protect one another. Since those campaigns’ inception in 1982, two-thirds of Americans have tried to prevent someone from driving after drinking,and traffic deaths involving alcohol-impaired crashes have decreased 65%.55,56
The comparable lethal means safety enterprise would encourage friends and family to talk with those in crisis about storing firearms safely. The campaign must use spokespersons who have strong respect and credibility among firearm owners, such as the NSSF and the United States Concealed Carry Association who have developed firearm suicide prevention websites and videos.57,58
The emphasis is that it’s a personal strength—not a failing—to talk to friends, loved ones, or counselors about storing guns until a crisis passes. Some of the current phrasing includes: “Hey, let me hold your guns for a while,” “People who love guns, love you,” and “Have a brave conversation.” 59-61
The national campaign should attempt to correct the inaccurate beliefs that suicide death always is the result of mental illness and is inevitable once seriously contemplated. In fact, more than half of the individuals who die by suicide have no diagnosed mental health condition.62 Other crises, such as with finances, relationships, or physical health, might be more contributory. These myths about suicide and mental illness weaken public and policy maker interest in solutions aimed toward accessing lethal means.
Facilitate Temporary Storage Out of the Home
The PREVENTS Roadmap Supplemental Materials concluded, “Moving firearms out of the home is generally cited as the safest, most desirable option; this can include storage with another person or at a location like a firearm range, armory, pawn shop, self-storage unit, or law enforcement agency, although state laws for firearm transfers may affect what options are legal.”63 This goal could be achieved by establishing grants to gun shops and ranges to offer free lockers for voluntary safe harbor.
The creation of free community lockers was a top PREVENTS recommendation. Likewise, the congressionally chartered COVER (Creating Options for Veterans' Expedited Recovery) Commission recommended grants “to further support the development of voluntary firearm safe storage options across the country.”64 Federal and state grants might resolve hesitations cited by retailers by covering all expenses for lockers, labor, and insurance for theft/damage/liability.65,66 Locker use would be free to the user, eliminating all financial barriers, although it is unknown whether monetary incentives change storage behaviors. Many firearm owners report that private gun shops or ranges are more acceptable than police stations for storage. If retailers come on board, changes in cultural storage norms might be expedited. An additional benefit could be reduction of accidental firearm fatalities in the home. States that have legal impediments to returning firearms to their owners could modify laws to achieve popular acceptance.
Congress could consider funding a national, easily accessible, public online directory of locations for out-of-home firearm storage, with staff to update the site. Colorado, Maryland, and Washington have developed online maps showing locations of firearm outlets and law enforcement agencies willing to consider temporary storage.67 A site directory for every state would simplify the process for individuals and family members seeking to temporarily and voluntarily store guns offsite during a crisis. Online directories have been backed by firearm groups,although their effect on storage behavior is not known.68State governments should strive to make it easier to quickly transfer firearms temporarily to trusted individuals in situations of imminent suicide risk. Rapid transfer of firearms to friends or family could effectively separate lethal means from individuals during a crisis. However, some state laws that require background checks whenever a gun is transferred might delay such transfers.69 Only a few states have legal exemptions that could expedite temporary transfers when it’s potentially lifesaving.
Improve In-Home Safe Storage Options
Out-of-home transfer of firearms might not be acceptable or feasible for some veterans. Accordingly, there is need for improved options for safer in-home storage, especially because of frequent unsafe storage practices among veterans. The VA could consider sponsoring another open-innovation Gun Safety Matters Challenge like the one it held in 2018 for in-home firearm storage technology that could prevent suicide.70 Further innovation and bringing winning entries to market has great potential.
Require Enhanced Lethal Means Safety Standards and Training
Broader lethal means safety competence is needed, both in the VA where modest levels of training has been implemented and in the community among Veterans Community Care Program (VCCP) HCPs where it hasn’t. Oversight for enhanced standards and training—as well as of all lethal means initiatives and their program evaluations—might best be accomplished by establishing a separate VA Suicide Prevention Program lethal means safety team. Veteran firearm suicide is a significant problem that warrants its own discrete, permanent VA team (although joining with the US Department of Defense might be advantageous). The VA Suicide Prevention Program has been the industry leader and innovator in this field and should be conferred continued stewardship going forward.
The VA is moving toward requiring lethal means safety counseling training for mental health, pain, primary care, women’s health, ED providers, and Veterans Crisis Line responders.
VCCP HCPs, however, have no required training in lethal means safety counseling or even in basic suicide risk identification and intervention, and the Roadmap did not stipulate that this deficiency should be remedied. Surveys have revealed that community HCPs rarely screen or counsel their patients—even those at high risk—about firearm safety.71 A bill was introduced in Congress August 21, 2020, to expand VA suicide prevention training with firearms community input on cultural competency components and mandate that VA and VCCP providers, and some others with frequent contact with veterans, receive this training.72
Training should be obligatory for VA and VCCP HCPs and trainees most likely to interface with at-risk veterans, including those working in mental health, primary care, pain, women’s health, and ED. Training also should include geriatrics, extended care, and oncology providers because most older adults who die by firearm suicide have physical health problems but no known mental illness.73-75 Lethal means safety counseling training has been shown to improve HCPs’ knowledge about the relationship between access to lethal means and suicide, and confidence in and frequency of having lethal means safety counseling conversations.76 Likewise, training should include peer counselors; veterans are receptive to fellow veterans raising the topic of safe storage.56,77 If feasible, the training should include time to rehearse skills shown to motivate behavior change among patients.
The VA should aim to improve semiyearly clinical pertinence reviews and safety plans for VA and VCCP mental health providers. VA could conduct clinical pertinence reviews that ascertain whether a suicide assessment is recorded in the health record, and when a patient is at elevated risk, whether a lethal means safety assessment and plan is documented.
VA’s safety plan template, although best practice, covers only the initial steps to take when suicide potential is identified. A standard for follow-up is needed. If an at-risk patient agrees to take a safe storage action, subsequent contact HCPs need to ask and document what action was performed. This action will help ensure that at-risk patients with ready access do not fall through the cracks. This suggestion lends itself to studying changes in veterans’ storage habits after intervention.
I also recommend that health care accrediting bodies include lethal means safety assessment, counseling, and follow up as a suicide prevention standard. This recommendation applies to more than just the VA health care system and recognizes that modifying accrediting body standards is an expeditious way to drive change in health care. The accreditation standards of the Commission on Accreditation of Rehabilitation Facilities for behavioral health and opioid treatment programs, and of the Joint Commission for medical centers do not require lethal means safety assessment and intervention.78,79
Conclusions
Suicide prevention requires a multimodal approach, and attention to firearms access must become a more salient component. The high rate of veteran suicides involving firearms requires far-reaching interventions at societal, institutional, community, family, and individual levels. With the link between ready access to firearms and suicide supported by research and now firmly recognized by the PREVENTS Roadmap, we have a fresh opportunity to reduce suicide among veterans. Efforts must move vigorously forward until it is commonplace for veterans—and anyone—at risk of suicide to voluntarily reduce immediate access to firearms.
1. US Department of Veterans Affairs. Veteran suicide prevention annual report. https://www.mentalhealth.va.gov/docs/data-sheets/2019/2019_National_Veteran_Suicide_Prevention_Annual_Report_508.pdf. Published September 2019. Accessed August 20, 2020.
2. US Department of Defense. Casualty status. https://www.defense.gov/casualty.pdf. Published August 17, 2020. Accessed August 20, 2020.
3. Cleveland EC, Azreal D, Simonetti JA, Miller M. Firearm ownership among American veterans: findings from the 2015 National Firearm Survey. Inj Epidemiol. 2017;4:33. doi:10.1186/s40621-017-0130-y
4. US Department of Veterans Affairs. PREVENTS: the President’s roadmap to empower veterans and end a national tragedy of suicide. https://www.va.gov/PREVENTS/docs/PRE-007-The-PREVENTS-Roadmap-1-2_508.pdf. Published June 17, 2020. Accessed August 20, 2020.
5. Kaplan MS, McFarland BH, Huguet N. Firearm suicide among veterans in the general population: findings from the National Violent Death Reporting System. Trauma. 2009;67(3):503-507. doi:10.1097/TA.0b013e3181b36521
6. Miller M, Barber C, White RA, Azrael D. Firearms and suicide in the United States: is risk independent of underlying suicidal behavior? Am J Epidemiol. 2013;178(6):946-955. doi:10.1093/aje/kwt197
7. Ivey-Stephenson AZ, Crosby AE, Jack, SP, Haileyesus, T, Kresnow-Sedacca M. Suicide trends among and within urbanization levels by sex, race/ethnicity, age group, and mechanism of death—United States, 2001-2015. MMWR Surveill Summ. 2017;66(18):1-16. doi:10.15585/mmwr.ss6618a1
8. McCarthy JF, Blow FC, Ignacio RV, Ilgen MA, Austin KL, Valenstein M. Suicide among patients in the Veterans Affairs Health System: rural-urban differences in rates, risks and methods. Am J Public Health. 2012;102(suppl 1):S111-S117. doi:10.2105/AJPH.2011.300463
9. RAND Corporation. The relationship between firearm availability and suicide. https://www.rand.org/research/gun-policy/analysis/essays/firearm-availability-suicide.html. Published March 2, 2018. Accessed August 20, 2020.
10. Anglemyer A, Horvath T, Rutherford G. The accessibility of firearms and risk for suicide and homicide victimization among household members: a systematic review and meta-analysis. Ann Intern Med. 2014;160(2):101-110. doi:10.7326/M13-1301
11. Studdert DM, Zhang Y, Swanson SA, et al. Handgun ownership and suicide in California. N Engl J Med. 2020;382(23):2220-2229. doi:10.1056/NEJMsa1916744
12. Miller M, Hemenway D. The relationship between firearms and suicide: a review of the literature. Aggression Violent Behav. 1999;4(1):59-75. doi:10.1016/S1359-1789(97)00057-8
13. Brent DA. Firearms and suicide. Ann N Y Acad Sci. 2001;932:225-239. doi:10.1111/j.1749-6632.2001.tb05808.x
14. Conwell Y, Duberstein PR, Connor K, Eberly S, Cox C, Caine ED. Access to firearms and risk for suicide in middle aged and older adults. Am J Geriatr Psychiatry. 2002;10(4):407-416. doi:10.1176/appi.ajgp.10.4.407
15. Grossman DC, Mueller BA, Riedy C, et al. Gun storage practices and risk of youth suicide and unintentional firearm injuries. JAMA. 2005;293(6):707-714. doi:10.1001/jama.293.6.707
16. Simonetti JA, Rowhani-Rahbar A. Limiting access to firearms as a suicide prevention strategy among adults. JAMA Netw Open. 2019;2(6):e195400. doi:10.1001/jamanetworkopen.2019.5400
17. Dempsey CL, Benedek DM, Zuromski KL, et al. Association of firearm use, accessibility, and storage practices with suicide risk among US army soldiers. JAMA Netw Open. 2019;2(6):e195383. doi:10.1001/jamanetworkopen.2019.5383
18. Wiebe DJ. Homicide and suicide risks associated with firearms in the home: a national case-control study. Ann Emerg Med. 41(6):771-782. doi:10.1067/mem.2003.187
19. de Moore GM, Plew JD, Bray KM, Snars JN. Survivors of self-inflicted firearm injury: a liaison psychiatry perspective. Med J Aust. 1994;160(7):421-425. doi:10.5694/j.1326-5377.1994.tb138267.x
20. Peterson LG, Peterson M, O’Shanick GJ, Swann A. Self-inflicted gunshot wounds: lethality of method versus intent. Am J Psychiatry. 1985;142(2):228-231. doi:10.1176/ajp.142.2.228
21. Miller M, Azrael D, Hemenway D. Belief in the inevitability of suicide: results from a national survey. Suicide Life Threat Behav. 2006;36(1):1-11. doi:10.1521/suli.2006.36.1.1
22. Bryan CJ, Rudd MD, Peterson AL, Young-McCaughan S, Wertenberger, EG. The ebb and flow of the wish to live and the wish to die among suicidal military personnel. J Affect Disord. 2016;202:58-66. doi:10.1016/j.jad.2016.05.049
23. O’Donnell I, Arthur AJ, Farmer RD. A follow-up study of attempted railway suicides. Soc Sci Med. 1994;38(3):437-442. doi:10.1016/0277-9536(94)90444-8
24. Owens D, Horrocks J, House A. Fatal and nonfatal repetition of self-harm: systematic review. Br J Psychiatry. 2002;181:193-199. doi:10.1192/bjp.181.3.193
25. Seiden RH. Where are they now? A follow-up study of suicide attempters from the Golden Gate Bridge. Suicide Life Threat Behav. 1978;8(4):203-216. doi:10.1111/j.1943-278X.1978.tb00587.x
26. Conner A, Azrael D, Miller M. Suicide case fatality rates in the United States, 2007 to 2014: a nationwide population-based study. Ann Intern Med. 2019;171(12):885-895. doi:10.7326/M19-1324
27. Disenhammer EA, Ing CM, Strauss R, Kemmler G, Hinterhuber H, Weiss EM. The duration of the suicidal process: how much time is left for intervention between consideration and accomplishment of a suicide attempt? J Clin Psych. 2009;70(1):19-24. doi:10.4088/JCP.07m03904
28. Simon TR, Swann AC, Powell KE, Potter LB, Kresnow M, O’Carrol, PW. Characteristics of impulsive suicide attempts and attempters. Suicide Life Threat Behav. 2001;32(suppl 1):49-59. doi:10.1521/suli.32.1.5.49.24212
29. Williams CL, Davidson JA, Montgomery I. Impulsive suicidal behavior. J Clin Psychol. 1980;36(1):90-94. doi:10.1002/1097-4679(198001)36:1<90::aid-jclp2270360104>3.0.co;2-f
30. Drum, DJ, Brownson, CB, Denmark, AB, Smith, SE. New data on the nature of suicidal crises in college students: shifting the paradigm. Professional Psychol: Res Pract. 2009;40(3):213-222. doi:10.1037/a0014465
31. Lubin G, Werbeloff N, Halperin D, Shmushkevitch M, Weise M, Knobler H. Decrease in suicide rates after a change of policy reducing access to firearms in adolescents: a naturalistic epidemiological study. Suicide Life Threat Behav. 2010;40(5):421-424. doi:10.1521/suli.2010.40.5.421
32. Shelef L, Tatsa-Laur L, Derazne E, Mann JJ, Fruchter E. An effective suicide prevention program in the Israeli Defense Forces: a cohort study. Eur Psychiatry. 2016;31:37-43. doi:10.1016/j.eurpsy.2015.10.004

33. Simonetti JA, Azrael D, Rowhani-Rahbar A, Miller M. Firearm storage practices among American veterans. Amer J Prev Med. 2018;55(4):445-454. doi:10.1016/j.amepre.2018.04.014
34. Office of the Surgeon General, National Action Alliance for Suicide Prevention. National strategy for suicide prevention: goals and objectives for action: a report of the U.S. Surgeon General and of the National Action Alliance for Suicide Prevention. https://www.ncbi.nlm.nih.gov/pubmed/23136686 Published September 2012. Accessed August 18, 2020.
35. Stone D, Holland KM, Bartholow B, Crosby AE, Davis S, Wilkins N. Preventing suicide: a technical package of policies, programs, and practices. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2017.
36. American Public Health Association. Reducing suicides by firearms. https://www.apha.org/policies-and-advocacy/public-health-policy-statements/policy-database/2019/01/28/reducing-suicides-by-firearms. Published November 13, 2018. Accessed August 18, 2020.
37. Coffey MJ, Coffey CE, Ahmedani BK. Suicide in a health maintenance organization population. JAMA Psychiatry. 2015;72(3):294-296. doi:10.1001/jamapsychiatry.2014.2440
38. Boggs JM, Beck A, Ritzwoller DP, Battaglia C, Anderson HD, Lindrooth RC. A quasi-experimental analysis of lethal means assessment and risk for subsequent suicide attempts and deaths. J Gen Intern Med. 2020;35(6):1709-1714. doi:10.1007/s11606-020-05641-4
39. Washington State Health Assessment 2018. Suicide & safe storage of firearms https://www.doh.wa.gov/Portals/1/Documents/1000/SHA-SuicideandSafeStorageofFirearms.pdf. Accessed August 18, 2020.
40. UC Davis Health Newsroom. First-in-the-nation gun violence prevention training program for health professionals established at UC Davis Health. https://health.ucdavis.edu/health-news/newsroom/first-in-the-nation-gun-violence-prevention-training-program-for-health-professionals-established-at-uc-davis-health/2019/10. Published October 15, 2019. Accessed August 18, 2020.
41. Mental Illness Research, Education, and Clinical Center. Lethal means safety & suicide prevention. https://www.mirecc.va.gov/lethalmeanssafety/index.asp. Updated February 1, 2018. Accessed August 18, 2020.
42. US Department of Veterans Affairs. Reducing firearm & other household safety risks for veterans and their families. https://www.mentalhealth.va.gov/suicide_prevention/docs/Brochure-for-Veterans-Means-Safety-Messaging_508_CLEARED_11-15-19.pdf. Published July 2019. Accessed August 18, 2020.
43. US Department of Veterans Affairs. Means safety messaging for clinical staff. https://www.mentalhealth.va.gov/suicide_prevention/docs/Pocket-Card-for-Clinicians-Means-Safety-Messaging_508_CLEARED_9-3-19.pdf. Accessed August 18, 2020.
44. Department of Veterans Affairs and Department of Defense. Lethal means counseling: recommendations for providers. https://www.healthquality.va.gov/guidelines/MH/srb/LethalMeansProviders20200527508.pdf. Published May 2020. Accessed August 18, 2020.
45. U.S. Department of Veterans Affairs. Supporting providers who serve veterans. https://www.mirecc.va.gov/visn19/consult. Updated August 3, 2020. Accessed August 18, 2020.
46. US Department of Veterans Affairs. National strategy for preventing veteran suicide 2018-2028. https://www.mentalhealth.va.gov/suicide_prevention/docs/Office-of-Mental-Health-and-Suicide-Prevention-National-Strategy-for-Preventing-Veterans-Suicide.pdf. Accessed August 18, 2020.
47. US Department of Veterans Affairs. VA/DoD clinical practice guidelines: assessment and management of patients at risk for suicide. https://www.healthquality.va.gov/guidelines/MH/srb. Updated July 30, 2020. Accessed August 18, 2020.
48. US Department of Veterans Affairs. Developing a safety plan. https://www.mentalhealth.va.gov/docs/vasafetyplancolor.pdf. Published March 2012. Accessed August 18, 2020.
49. Lemle RB. VA forges an historic partnership with the National Shooting Sports Foundation and the American Foundation for Suicide Prevention to prevent veteran suicide. Fed Pract. 2019;36(2):18-24.
50. US Department of Veterans Affairs, American Foundation for Suicide Prevention, National Shooting Sports Foundation. Suicide prevention is everyone’s business: a toolkit for safe firearm storage in your community. https://project2025.afsp.org/wp-content/uploads/2020/03/Toolkit_Safe_Firearm_Storage_CLEARED_508_2-24-20.pdf. Accessed August 18, 2020.
51. Montheith, LL, Wendleton, L, Bahraini, NH, Matarazzo, BB, Brimner, G, Mohatt, NV. Together with veterans: VA national strategy alignment and lessons learned from community-based suicide prevention for rural veterans. Suicide Life Threat Behav. 2020;50(3):588-600. doi:10.1111/sltb.12613. Epub 2020 Jan 16
52. Gordon S. VA pioneering efforts to reduce veteran suicide from firearms. http://beyondchron.org/va-pioneering-efforts-to-reduce-veteran-suicide-from-firearms. Published March 10, 2020. Accessed August 20, 2020.
53. Johnson A. Protecting mental health and preventing suicide during COVID-19. https://www.blogs.va.gov/VAntage/76827/mental-health-and-suicide-prevention-during-covid-19. Published July 14, 2020. Accessed August 18, 2020.
54. Betz ME, Anestis MD. Firearms, pesticides, and suicide: a look back for a way forward. Prev Med. 2020;138:106144. doi:10.1016/j.ypmed.2020.106144
55. Buckley, L, Chapman, RL, and Lewis, I. A systematic review of intervening to prevent driving while intoxicated: The problem of driving while intoxicated (DWI), Substance Use & Misuse. 2016; 51(1): 104-112. doi:10.3109/10826084.2015.1090452

56. National Safety Council. Injury facts. motor vehicle safety issues. https://injuryfacts.nsc.org/motor-vehicle/motor-vehicle-safety-issues/alcohol-impaired-driving. Accessed August 2020.
57. Crifasi CK, Doucette ML, McGinty EE, Webster DW, Barry CL. Storage practices of US gun owners in 2016. Am J Public Health. 2018;108(4):532-537. doi:10.2105/AJPH.2017.304262
58. National Shooting Sports Foundation. Suicide prevention program for retailers and ranges. https://www.nssf.org/safety/suicide-prevention. Accessed August 18, 2020.
59. Pallin R, Siry B, Azrael D, et al. “Hey, let me hold your guns for a while”: a qualitative study of messaging for firearm suicide prevention. Behav Sci Law. 2019;37(3):259-269. doi:10.1002/bsl.2393
60. Oregon Firearm Safety. http://oregonfirearmsafety.org. Accessed August 18, 2020.
61. National Shooting Sports Foundation. Suicide prevention toolkit items. https://www.nssf.org/safety/suicide-prevention/suicide-prevention-toolkit. Accessed August 18, 2020.
62. Centers for Disease Control and Prevention. Suicide rising across the US. https://www.cdc.gov/vitalsigns/suicide/index.html. Updated June 7, 2018. Accessed August 18, 2020.
63. U.S Department of Veterans Affairs. PREVENTS: executive order 13861. https://www.va.gov/PREVENTS/EO-13861.asp. Updated August 13, 2020. Accessed August 18, 2020.
64. COVER Commission. Creating Options for Veterans’ Expedited Recovery (COVER) Commission Final Report. https://www.va.gov/COVER/docs/COVER-Commission-Final-Report-2020-01-24.pdf. Published January 24, 2020. Accessed August 18, 2020.
65. Pierpoint LA, Tung GJ, Brooks-Russell A, Brandspigel S, Betz M, Runyan CW. Gun retailers as storage partners for suicide prevention: what barriers need to be overcome? Inj Prev. 2019;25(suppl 1):i5-i8. doi:10.1136/injuryprev-2017-042700
66. Gibbons MJ, Fan MD, Rowhani-Rahbar A, Rivara FP. Legal liability for returning firearms to suicidal persons who voluntarily surrender them in 50 US states. Am J Public Health. 2020;110(5):685-688. doi:10.2105/AJPH.2019.305545
67. Kelly T, Brandspigel S, Polzer E, Betz ME. Firearm storage maps: a pragmatic approach to reduce firearm suicide during times of risk. Ann Intern Med. 2020;172(5):351-353. doi:10.7326/M19-2944
68. Edwards C. This new gun storage map is designed to save lives in Colorado. https://bearingarms.com/cam-e/2019/08/27/new-gun-storage-map-designed-save-lives-colorado. Published August 27, 2019. Accessed August 18, 2020.
69. McCourt AD, Vernick JS, Betz ME, Brandspigel S, Runyan CW. Temporary transfer of firearms from the home to prevent suicide: legal obstacles and recommendations. JAMA Intern Med. 2017;177(1):96-101. doi:10.1001/jamainternmed.2016.5704
70. US Department of Veterans Affairs. Aimed at suicide prevention, VA shares winners of its ‘Gun Safety Matters Challenge.” https://www.blogs.va.gov/VAntage/50233/aimed-suicide-prevention-va-shares-winners-gun-safety-matters-challenge. Published July 9, 2019. Accessed August 18, 2020.
71. Roszko PJD, Ameli J, Carter PM, Cunningham RM, Ranney ML. Clinician attitudes, screening practices, and interventions to reduce firearm-related injury. Epidemiol Rev. 2016;38(1):87-110. doi:10.1093/epirev/mxv005
72. Lethal Means Safety Training Act. HR 8084, 116th Cong. 2nd Sess (2020). https://www.congress.gov/bill/116th-congress/house-bill/8084/text. Accessed August 25, 2020.
73. Boggs JM, Simon GE, Ahmedani BK, Peterson E, Hubley S, Beck A. The association of firearm suicide with mental illness, substance use conditions, and previous suicide attempts. Ann Intern Med. 2017;167(4):287-288. doi:10.7326/L17-0111
74. Schmutte TJ, Wilkinson ST. Suicide in older adults with and without known mental illness: results from the National Violent Death Reporting System, 2003-2016. Am J Prev Med. 2020;58(4):584-590. doi:10.1016/j.amepre.2019.11.001
75. Morin RT, Li Y, Mackin RS, Whooley MA, Conwell Y, Byers AL. Comorbidity profiles identified in older primary care patients who attempt suicide. J Am Geriatr Soc. 2019;67(12):2553-2559. doi:10.1111/jgs.16126
76. Roszko PJD, Ameli J, Carter PM, Cunningham RM, Ranney, ML. Clinician attitudes, screening practices, and interventions to reduce firearm-related injury. Epidemiol Rev. 2016;38(1):87-110. doi:10.1093/epirev/mxv005
77. Iraq and Afghanistan Veterans of America. 7th annual IAVA member survey: the most comprehensive look into the lives of post-9/11. https://iava.org/wp-content/uploads/2020/02/IAVA-MemberSurvey-single-pgs1.pdf. Accessed August 18, 2020.
78. CARF International. CARF adds screening for suicide risk to its assessment standards. http://www.carf.org/universal-suicide-screening-standards. Published May 2, 2019. Accessed August 18, 2020.
79. Paul S. National Patient Safety Goal expands focus on suicide prevention. https://www.jointcommission.org/resources/news-and-multimedia/blogs/dateline-tjc/2019/01/national-patient-safety-goal-expands-focus-on-suicide-prevention/. Published January 24, 2019. Accessed August 18, 2020.
US veterans die by suicide at a higher rate than that of the civilian population, and are more likely to use a firearm as their lethal means.1 In 2017, 6,139 veterans died by suicide, about 17 per day.1 Nearly as many veterans die by suicide yearly as the total aggregate number of service members killed in action during the decades-long Iraq and Afghanistan operations.2 Veterans are more likely to own firearms than are civilians.3 Until June 2020, however, systemic efforts to address the use of firearms in suicide had been largely evaded, entangled in gun advocates’ assertion that veterans’ constitutional right to bear arms would be infringed.
That impasse changed with the President’s Roadmap to Empower Veterans and End the National Tragedy of Suicide (PREVENTS) task force report, released June 17, 2020.4 Although the US Department of Veterans Affairs (VA) has pioneered initiatives to encourage safe firearm storage for at-risk veterans, and major public health organizations have endorsed the utility of lethal means safety strategies, the policy language of the Roadmap released by the White House is unprecedented. Lethal means safety refers to efforts aimed at increasing the time and distance needed to access suicide methods.
Among the report’s 10 recommendations, the Roadmap verified the link between, and the need to address, at-risk veterans and their access to firearms (the author was a minor consultant to a PREVENTS workgroup). The document states, “The science supporting lethal means safety is robust and compelling: enhancing safety measures specific to the availability and accessibility of potential lethal means saves lives. A key component of effective suicide prevention is voluntary reduction in the ability to access lethal means with respect to time, distance, and convenience, particularly during acute suicidal crises.”4 The report recommends widespread distribution of safety education materials that encourage at-risk individuals to temporarily transfer or store their guns safely, and the expansion of free or affordable options for storing weapons, among other recommendations.
This paper reviews the literature on the intersection of veterans, firearms, and suicide, then explores existing VA prevention initiatives aimed at reducing at-risk veterans’ access to lethal means and offers policy recommendations to expand efforts in the context of the PREVENTS Roadmap.
Veteran Suicide and Firearms
Firearms are, by far, the most common lethal means used by veterans who die by suicide. About 71% of male veteran suicide deaths and 43% of female veteran suicide deaths are with firearms, rates that far exceed those of nonveterans (Table).For all age groups, veterans are more likely to complete suicide by firearm than are nonveterans.5
Veteran suicide and gun ownership rates are highest in rural areas.6,7 When compared with veterans living in urban areas, veterans in rural areas are 20% more likely to die by suicide, with the excessive risk largely attributed to suicide by firearm.8
Access to firearms at home increases the risk of suicide. Individuals with any firearm at home are 3 times more likely to die by suicide than is a person with no firearms at home. The elevated suicide risk applies to other household members as well as the firearm owner.9-18 Survivors of suicide attempts using firearms report that the availability of guns at home is the primary reason for their method choice.19,20
There is a common misperception that people who are intent on suicide and are thwarted or survive an attempt using one method will try again with another.21 Suicidal crises often represent a conflicting wish to live or die,22 and approximately two-thirds of those who survive an attempt will never try again. About 23% reattempt nonfatally, and only 10% die by suicide.23-25 However, people who attempt suicide with a firearm usually won’t get a chance at a new start, because 90% of such acts are fatal.26
Although some suicide attempts might be contemplated or planned over an extended period, the decision is impulsive for most individuals. Surveys have found that many people who survive suicide attempts began the act only minutes or hours after making the decision to end their life.27-30 The high-risk, acute phase of many suicidal crises arise quickly and is fleeting.
Limiting the ease by which at-risk individuals can access firearms has been shown to prevent suicide. In 2006, the overall suicide rate in Israel dropped 40% when the Israeli Defense Forces began requiring soldiers to store their firearms on base before going on weekend leave.Since then, the suicide rate has declined even further.31,32
Delaying Access to Firearms for At-Risk Veterans
Among veterans, 45% own ≥ 1 firearms (47% male and 24% female veterans vs 30% male and 12% female nonveterans).3 Many veteran firearm owners (34% male and 13% female) store ≥ 1 gun loaded and unlocked; 44% store a firearm either loaded or unlocked. Only 23% safely store their firearms unloaded and locked at home. Storing ≥ 1 firearm loaded and unlocked is more likely among veterans who reside in rural areas, separated from service before 2002, and report personal protection as the primary reason for ownership.33
Because evidence shows that delaying access to firearms—especially by transferring them out of the home—saves lives, many US health organizations have advocated for strategies that promote evaluation of firearm access and counseling safe storage for individuals at risk for suicide. These organizations include the US Office of the Surgeon General, National Action Alliance for Suicide Prevention, Centers for Disease Control and Prevention, and American Public Health Association.34-36
Some health care systems—notably Kaiser Permanente and Henry Ford Health Systems—implemented protocols for lethal means assessment and counseling for behavioral health patients.37,38 Washington state requires specific health professionals to enroll in suicide prevention training that includes content on the risk of imminent harm by lethal means.39 California is designing a curriculum on counseling patients to reduce firearm injury for physicians and other health care practitioners (HCPs).40
The scope of these efforts, however, pale in comparison with the VA’s comprehensive, innovative lethal means safety approach. Since 2012, VA’s Suicide Prevention Program has distributed free firearm cable locks to veterans who request them. The VA has created lethal means public service announcements, social media messages, and websites.41-44 The VA distributes firearm and medication safe storage practice resource kits to its primary care, mental health and women’s health clinics, and Vet Centers, that include brochures, large poster cards, stickers, exam room posters, and provider pocket cards. VA developed an online lethal means safety counseling training that 20,000 VA HCPs have taken, and is moving toward a revamped mandatory training for VA’s mental health, pain, primary care, and emergency department (ED) providers and Veterans Crisis Line responders. VA offers free, individualized lethal means risk management consultation to all clinicians who work with veterans.45 VA includes lethal means safety procedures in its National Strategy for Preventing Veteran Suicide,VA/DoD Clinical Practice Guideline,and VA Suicide Risk Evaluation and Suicide Prevention Safety Planrequired of clinicians.46-48
The VA also added public health strategies that promote safe storage practices for veterans through a partnership with the National Shooting Sports Foundation (NSSF; the firearm industry trade association) and the American Foundation for Suicide Prevention (AFSP).49 Collectively, these organizations cobranded an educational, training, and resource toolkit to foster community coalitions and gun retailer projects that encourage veterans to securely store firearms.50 The VA partnered with NSSF to post billboards in 8 states, encouraging storing firearms responsibly to prevent suicide. VA invited states and cities in the Governor/Mayoral Challenge to Prevent Suicide (joint VA and Substance Abuse and Mental Health Services Administration endeavors) to develop plans for messaging regarding enhanced lethal means safety processes. The VA collaborated with local firearm advocates in community prevention pilot projects and in a “Together with Veterans” dissemination of material and outreach to rural veterans.51 Along with AFSP, VA hosted conferences for HCPs, policy makers, and stakeholders about innovations related to lethal means safety.52 In May 2020, the VA cosponsored a COVID-19 suicide prevention video with the United States Concealed Carry Association, NSSF, and AFSP, including ways that the firearm industry, gun owners, and their families can help.53
These programs are promising, and the Roadmap’s emphatic endorsement of lethal means safety approaches will accelerate advances. However, the Roadmap’s omissions are consequential. By focusing on population interventions, the document is silent about VA-specific or veteran-specific firearm access strategies. The means safety work of VA’s Suicide Prevention Program Office is scarcely recognized. Further, it stops short of specific legislative initiatives, making aspirational recommendations instead.
This paper will list proposed policy actions to bolster the acceptability and practice of lethal means safety with veterans. They cover an entire range of possibilities, from putting more teeth into the Roadmap’s population-wide interventions to initiatives tailored to veterans. Responsibility for leading and funding the changes would reside in a mix of Congress and state legislatures, the VA, and health system accreditation bodies. Although there is solid evidence that lethal means safety prevents suicide, it is unknown how these approaches affect firearm storage behaviors or suicide rates;therefore, the policy actions should come with federal and state funds for rigorous evaluation.54
Recommended Actions to Further Promote Safe Storage
Develop Campaigns to Shift Cultural Norms for Firearm Storage During Crises
National campaigns have been shown to be highly effective in changing injurious behaviors. Alliances and resources with regard to lethal means safety could be assembled, including federal funds for a campaign to shift social norms for firearm storage conversations and behaviors during crises. This campaign should be modeled after the “friends don’t let friends drive drunk” and “designated driver” campaigns that empower family and friends to protect one another. Since those campaigns’ inception in 1982, two-thirds of Americans have tried to prevent someone from driving after drinking,and traffic deaths involving alcohol-impaired crashes have decreased 65%.55,56
The comparable lethal means safety enterprise would encourage friends and family to talk with those in crisis about storing firearms safely. The campaign must use spokespersons who have strong respect and credibility among firearm owners, such as the NSSF and the United States Concealed Carry Association who have developed firearm suicide prevention websites and videos.57,58
The emphasis is that it’s a personal strength—not a failing—to talk to friends, loved ones, or counselors about storing guns until a crisis passes. Some of the current phrasing includes: “Hey, let me hold your guns for a while,” “People who love guns, love you,” and “Have a brave conversation.” 59-61
The national campaign should attempt to correct the inaccurate beliefs that suicide death always is the result of mental illness and is inevitable once seriously contemplated. In fact, more than half of the individuals who die by suicide have no diagnosed mental health condition.62 Other crises, such as with finances, relationships, or physical health, might be more contributory. These myths about suicide and mental illness weaken public and policy maker interest in solutions aimed toward accessing lethal means.
Facilitate Temporary Storage Out of the Home
The PREVENTS Roadmap Supplemental Materials concluded, “Moving firearms out of the home is generally cited as the safest, most desirable option; this can include storage with another person or at a location like a firearm range, armory, pawn shop, self-storage unit, or law enforcement agency, although state laws for firearm transfers may affect what options are legal.”63 This goal could be achieved by establishing grants to gun shops and ranges to offer free lockers for voluntary safe harbor.
The creation of free community lockers was a top PREVENTS recommendation. Likewise, the congressionally chartered COVER (Creating Options for Veterans' Expedited Recovery) Commission recommended grants “to further support the development of voluntary firearm safe storage options across the country.”64 Federal and state grants might resolve hesitations cited by retailers by covering all expenses for lockers, labor, and insurance for theft/damage/liability.65,66 Locker use would be free to the user, eliminating all financial barriers, although it is unknown whether monetary incentives change storage behaviors. Many firearm owners report that private gun shops or ranges are more acceptable than police stations for storage. If retailers come on board, changes in cultural storage norms might be expedited. An additional benefit could be reduction of accidental firearm fatalities in the home. States that have legal impediments to returning firearms to their owners could modify laws to achieve popular acceptance.
Congress could consider funding a national, easily accessible, public online directory of locations for out-of-home firearm storage, with staff to update the site. Colorado, Maryland, and Washington have developed online maps showing locations of firearm outlets and law enforcement agencies willing to consider temporary storage.67 A site directory for every state would simplify the process for individuals and family members seeking to temporarily and voluntarily store guns offsite during a crisis. Online directories have been backed by firearm groups,although their effect on storage behavior is not known.68State governments should strive to make it easier to quickly transfer firearms temporarily to trusted individuals in situations of imminent suicide risk. Rapid transfer of firearms to friends or family could effectively separate lethal means from individuals during a crisis. However, some state laws that require background checks whenever a gun is transferred might delay such transfers.69 Only a few states have legal exemptions that could expedite temporary transfers when it’s potentially lifesaving.
Improve In-Home Safe Storage Options
Out-of-home transfer of firearms might not be acceptable or feasible for some veterans. Accordingly, there is need for improved options for safer in-home storage, especially because of frequent unsafe storage practices among veterans. The VA could consider sponsoring another open-innovation Gun Safety Matters Challenge like the one it held in 2018 for in-home firearm storage technology that could prevent suicide.70 Further innovation and bringing winning entries to market has great potential.
Require Enhanced Lethal Means Safety Standards and Training
Broader lethal means safety competence is needed, both in the VA where modest levels of training has been implemented and in the community among Veterans Community Care Program (VCCP) HCPs where it hasn’t. Oversight for enhanced standards and training—as well as of all lethal means initiatives and their program evaluations—might best be accomplished by establishing a separate VA Suicide Prevention Program lethal means safety team. Veteran firearm suicide is a significant problem that warrants its own discrete, permanent VA team (although joining with the US Department of Defense might be advantageous). The VA Suicide Prevention Program has been the industry leader and innovator in this field and should be conferred continued stewardship going forward.
The VA is moving toward requiring lethal means safety counseling training for mental health, pain, primary care, women’s health, ED providers, and Veterans Crisis Line responders.
VCCP HCPs, however, have no required training in lethal means safety counseling or even in basic suicide risk identification and intervention, and the Roadmap did not stipulate that this deficiency should be remedied. Surveys have revealed that community HCPs rarely screen or counsel their patients—even those at high risk—about firearm safety.71 A bill was introduced in Congress August 21, 2020, to expand VA suicide prevention training with firearms community input on cultural competency components and mandate that VA and VCCP providers, and some others with frequent contact with veterans, receive this training.72
Training should be obligatory for VA and VCCP HCPs and trainees most likely to interface with at-risk veterans, including those working in mental health, primary care, pain, women’s health, and ED. Training also should include geriatrics, extended care, and oncology providers because most older adults who die by firearm suicide have physical health problems but no known mental illness.73-75 Lethal means safety counseling training has been shown to improve HCPs’ knowledge about the relationship between access to lethal means and suicide, and confidence in and frequency of having lethal means safety counseling conversations.76 Likewise, training should include peer counselors; veterans are receptive to fellow veterans raising the topic of safe storage.56,77 If feasible, the training should include time to rehearse skills shown to motivate behavior change among patients.
The VA should aim to improve semiyearly clinical pertinence reviews and safety plans for VA and VCCP mental health providers. VA could conduct clinical pertinence reviews that ascertain whether a suicide assessment is recorded in the health record, and when a patient is at elevated risk, whether a lethal means safety assessment and plan is documented.
VA’s safety plan template, although best practice, covers only the initial steps to take when suicide potential is identified. A standard for follow-up is needed. If an at-risk patient agrees to take a safe storage action, subsequent contact HCPs need to ask and document what action was performed. This action will help ensure that at-risk patients with ready access do not fall through the cracks. This suggestion lends itself to studying changes in veterans’ storage habits after intervention.
I also recommend that health care accrediting bodies include lethal means safety assessment, counseling, and follow up as a suicide prevention standard. This recommendation applies to more than just the VA health care system and recognizes that modifying accrediting body standards is an expeditious way to drive change in health care. The accreditation standards of the Commission on Accreditation of Rehabilitation Facilities for behavioral health and opioid treatment programs, and of the Joint Commission for medical centers do not require lethal means safety assessment and intervention.78,79
Conclusions
Suicide prevention requires a multimodal approach, and attention to firearms access must become a more salient component. The high rate of veteran suicides involving firearms requires far-reaching interventions at societal, institutional, community, family, and individual levels. With the link between ready access to firearms and suicide supported by research and now firmly recognized by the PREVENTS Roadmap, we have a fresh opportunity to reduce suicide among veterans. Efforts must move vigorously forward until it is commonplace for veterans—and anyone—at risk of suicide to voluntarily reduce immediate access to firearms.
US veterans die by suicide at a higher rate than that of the civilian population, and are more likely to use a firearm as their lethal means.1 In 2017, 6,139 veterans died by suicide, about 17 per day.1 Nearly as many veterans die by suicide yearly as the total aggregate number of service members killed in action during the decades-long Iraq and Afghanistan operations.2 Veterans are more likely to own firearms than are civilians.3 Until June 2020, however, systemic efforts to address the use of firearms in suicide had been largely evaded, entangled in gun advocates’ assertion that veterans’ constitutional right to bear arms would be infringed.
That impasse changed with the President’s Roadmap to Empower Veterans and End the National Tragedy of Suicide (PREVENTS) task force report, released June 17, 2020.4 Although the US Department of Veterans Affairs (VA) has pioneered initiatives to encourage safe firearm storage for at-risk veterans, and major public health organizations have endorsed the utility of lethal means safety strategies, the policy language of the Roadmap released by the White House is unprecedented. Lethal means safety refers to efforts aimed at increasing the time and distance needed to access suicide methods.
Among the report’s 10 recommendations, the Roadmap verified the link between, and the need to address, at-risk veterans and their access to firearms (the author was a minor consultant to a PREVENTS workgroup). The document states, “The science supporting lethal means safety is robust and compelling: enhancing safety measures specific to the availability and accessibility of potential lethal means saves lives. A key component of effective suicide prevention is voluntary reduction in the ability to access lethal means with respect to time, distance, and convenience, particularly during acute suicidal crises.”4 The report recommends widespread distribution of safety education materials that encourage at-risk individuals to temporarily transfer or store their guns safely, and the expansion of free or affordable options for storing weapons, among other recommendations.
This paper reviews the literature on the intersection of veterans, firearms, and suicide, then explores existing VA prevention initiatives aimed at reducing at-risk veterans’ access to lethal means and offers policy recommendations to expand efforts in the context of the PREVENTS Roadmap.
Veteran Suicide and Firearms
Firearms are, by far, the most common lethal means used by veterans who die by suicide. About 71% of male veteran suicide deaths and 43% of female veteran suicide deaths are with firearms, rates that far exceed those of nonveterans (Table).For all age groups, veterans are more likely to complete suicide by firearm than are nonveterans.5
Veteran suicide and gun ownership rates are highest in rural areas.6,7 When compared with veterans living in urban areas, veterans in rural areas are 20% more likely to die by suicide, with the excessive risk largely attributed to suicide by firearm.8
Access to firearms at home increases the risk of suicide. Individuals with any firearm at home are 3 times more likely to die by suicide than is a person with no firearms at home. The elevated suicide risk applies to other household members as well as the firearm owner.9-18 Survivors of suicide attempts using firearms report that the availability of guns at home is the primary reason for their method choice.19,20
There is a common misperception that people who are intent on suicide and are thwarted or survive an attempt using one method will try again with another.21 Suicidal crises often represent a conflicting wish to live or die,22 and approximately two-thirds of those who survive an attempt will never try again. About 23% reattempt nonfatally, and only 10% die by suicide.23-25 However, people who attempt suicide with a firearm usually won’t get a chance at a new start, because 90% of such acts are fatal.26
Although some suicide attempts might be contemplated or planned over an extended period, the decision is impulsive for most individuals. Surveys have found that many people who survive suicide attempts began the act only minutes or hours after making the decision to end their life.27-30 The high-risk, acute phase of many suicidal crises arise quickly and is fleeting.
Limiting the ease by which at-risk individuals can access firearms has been shown to prevent suicide. In 2006, the overall suicide rate in Israel dropped 40% when the Israeli Defense Forces began requiring soldiers to store their firearms on base before going on weekend leave.Since then, the suicide rate has declined even further.31,32
Delaying Access to Firearms for At-Risk Veterans
Among veterans, 45% own ≥ 1 firearms (47% male and 24% female veterans vs 30% male and 12% female nonveterans).3 Many veteran firearm owners (34% male and 13% female) store ≥ 1 gun loaded and unlocked; 44% store a firearm either loaded or unlocked. Only 23% safely store their firearms unloaded and locked at home. Storing ≥ 1 firearm loaded and unlocked is more likely among veterans who reside in rural areas, separated from service before 2002, and report personal protection as the primary reason for ownership.33
Because evidence shows that delaying access to firearms—especially by transferring them out of the home—saves lives, many US health organizations have advocated for strategies that promote evaluation of firearm access and counseling safe storage for individuals at risk for suicide. These organizations include the US Office of the Surgeon General, National Action Alliance for Suicide Prevention, Centers for Disease Control and Prevention, and American Public Health Association.34-36
Some health care systems—notably Kaiser Permanente and Henry Ford Health Systems—implemented protocols for lethal means assessment and counseling for behavioral health patients.37,38 Washington state requires specific health professionals to enroll in suicide prevention training that includes content on the risk of imminent harm by lethal means.39 California is designing a curriculum on counseling patients to reduce firearm injury for physicians and other health care practitioners (HCPs).40
The scope of these efforts, however, pale in comparison with the VA’s comprehensive, innovative lethal means safety approach. Since 2012, VA’s Suicide Prevention Program has distributed free firearm cable locks to veterans who request them. The VA has created lethal means public service announcements, social media messages, and websites.41-44 The VA distributes firearm and medication safe storage practice resource kits to its primary care, mental health and women’s health clinics, and Vet Centers, that include brochures, large poster cards, stickers, exam room posters, and provider pocket cards. VA developed an online lethal means safety counseling training that 20,000 VA HCPs have taken, and is moving toward a revamped mandatory training for VA’s mental health, pain, primary care, and emergency department (ED) providers and Veterans Crisis Line responders. VA offers free, individualized lethal means risk management consultation to all clinicians who work with veterans.45 VA includes lethal means safety procedures in its National Strategy for Preventing Veteran Suicide,VA/DoD Clinical Practice Guideline,and VA Suicide Risk Evaluation and Suicide Prevention Safety Planrequired of clinicians.46-48
The VA also added public health strategies that promote safe storage practices for veterans through a partnership with the National Shooting Sports Foundation (NSSF; the firearm industry trade association) and the American Foundation for Suicide Prevention (AFSP).49 Collectively, these organizations cobranded an educational, training, and resource toolkit to foster community coalitions and gun retailer projects that encourage veterans to securely store firearms.50 The VA partnered with NSSF to post billboards in 8 states, encouraging storing firearms responsibly to prevent suicide. VA invited states and cities in the Governor/Mayoral Challenge to Prevent Suicide (joint VA and Substance Abuse and Mental Health Services Administration endeavors) to develop plans for messaging regarding enhanced lethal means safety processes. The VA collaborated with local firearm advocates in community prevention pilot projects and in a “Together with Veterans” dissemination of material and outreach to rural veterans.51 Along with AFSP, VA hosted conferences for HCPs, policy makers, and stakeholders about innovations related to lethal means safety.52 In May 2020, the VA cosponsored a COVID-19 suicide prevention video with the United States Concealed Carry Association, NSSF, and AFSP, including ways that the firearm industry, gun owners, and their families can help.53
These programs are promising, and the Roadmap’s emphatic endorsement of lethal means safety approaches will accelerate advances. However, the Roadmap’s omissions are consequential. By focusing on population interventions, the document is silent about VA-specific or veteran-specific firearm access strategies. The means safety work of VA’s Suicide Prevention Program Office is scarcely recognized. Further, it stops short of specific legislative initiatives, making aspirational recommendations instead.
This paper will list proposed policy actions to bolster the acceptability and practice of lethal means safety with veterans. They cover an entire range of possibilities, from putting more teeth into the Roadmap’s population-wide interventions to initiatives tailored to veterans. Responsibility for leading and funding the changes would reside in a mix of Congress and state legislatures, the VA, and health system accreditation bodies. Although there is solid evidence that lethal means safety prevents suicide, it is unknown how these approaches affect firearm storage behaviors or suicide rates;therefore, the policy actions should come with federal and state funds for rigorous evaluation.54
Recommended Actions to Further Promote Safe Storage
Develop Campaigns to Shift Cultural Norms for Firearm Storage During Crises
National campaigns have been shown to be highly effective in changing injurious behaviors. Alliances and resources with regard to lethal means safety could be assembled, including federal funds for a campaign to shift social norms for firearm storage conversations and behaviors during crises. This campaign should be modeled after the “friends don’t let friends drive drunk” and “designated driver” campaigns that empower family and friends to protect one another. Since those campaigns’ inception in 1982, two-thirds of Americans have tried to prevent someone from driving after drinking,and traffic deaths involving alcohol-impaired crashes have decreased 65%.55,56
The comparable lethal means safety enterprise would encourage friends and family to talk with those in crisis about storing firearms safely. The campaign must use spokespersons who have strong respect and credibility among firearm owners, such as the NSSF and the United States Concealed Carry Association who have developed firearm suicide prevention websites and videos.57,58
The emphasis is that it’s a personal strength—not a failing—to talk to friends, loved ones, or counselors about storing guns until a crisis passes. Some of the current phrasing includes: “Hey, let me hold your guns for a while,” “People who love guns, love you,” and “Have a brave conversation.” 59-61
The national campaign should attempt to correct the inaccurate beliefs that suicide death always is the result of mental illness and is inevitable once seriously contemplated. In fact, more than half of the individuals who die by suicide have no diagnosed mental health condition.62 Other crises, such as with finances, relationships, or physical health, might be more contributory. These myths about suicide and mental illness weaken public and policy maker interest in solutions aimed toward accessing lethal means.
Facilitate Temporary Storage Out of the Home
The PREVENTS Roadmap Supplemental Materials concluded, “Moving firearms out of the home is generally cited as the safest, most desirable option; this can include storage with another person or at a location like a firearm range, armory, pawn shop, self-storage unit, or law enforcement agency, although state laws for firearm transfers may affect what options are legal.”63 This goal could be achieved by establishing grants to gun shops and ranges to offer free lockers for voluntary safe harbor.
The creation of free community lockers was a top PREVENTS recommendation. Likewise, the congressionally chartered COVER (Creating Options for Veterans' Expedited Recovery) Commission recommended grants “to further support the development of voluntary firearm safe storage options across the country.”64 Federal and state grants might resolve hesitations cited by retailers by covering all expenses for lockers, labor, and insurance for theft/damage/liability.65,66 Locker use would be free to the user, eliminating all financial barriers, although it is unknown whether monetary incentives change storage behaviors. Many firearm owners report that private gun shops or ranges are more acceptable than police stations for storage. If retailers come on board, changes in cultural storage norms might be expedited. An additional benefit could be reduction of accidental firearm fatalities in the home. States that have legal impediments to returning firearms to their owners could modify laws to achieve popular acceptance.
Congress could consider funding a national, easily accessible, public online directory of locations for out-of-home firearm storage, with staff to update the site. Colorado, Maryland, and Washington have developed online maps showing locations of firearm outlets and law enforcement agencies willing to consider temporary storage.67 A site directory for every state would simplify the process for individuals and family members seeking to temporarily and voluntarily store guns offsite during a crisis. Online directories have been backed by firearm groups,although their effect on storage behavior is not known.68State governments should strive to make it easier to quickly transfer firearms temporarily to trusted individuals in situations of imminent suicide risk. Rapid transfer of firearms to friends or family could effectively separate lethal means from individuals during a crisis. However, some state laws that require background checks whenever a gun is transferred might delay such transfers.69 Only a few states have legal exemptions that could expedite temporary transfers when it’s potentially lifesaving.
Improve In-Home Safe Storage Options
Out-of-home transfer of firearms might not be acceptable or feasible for some veterans. Accordingly, there is need for improved options for safer in-home storage, especially because of frequent unsafe storage practices among veterans. The VA could consider sponsoring another open-innovation Gun Safety Matters Challenge like the one it held in 2018 for in-home firearm storage technology that could prevent suicide.70 Further innovation and bringing winning entries to market has great potential.
Require Enhanced Lethal Means Safety Standards and Training
Broader lethal means safety competence is needed, both in the VA where modest levels of training has been implemented and in the community among Veterans Community Care Program (VCCP) HCPs where it hasn’t. Oversight for enhanced standards and training—as well as of all lethal means initiatives and their program evaluations—might best be accomplished by establishing a separate VA Suicide Prevention Program lethal means safety team. Veteran firearm suicide is a significant problem that warrants its own discrete, permanent VA team (although joining with the US Department of Defense might be advantageous). The VA Suicide Prevention Program has been the industry leader and innovator in this field and should be conferred continued stewardship going forward.
The VA is moving toward requiring lethal means safety counseling training for mental health, pain, primary care, women’s health, ED providers, and Veterans Crisis Line responders.
VCCP HCPs, however, have no required training in lethal means safety counseling or even in basic suicide risk identification and intervention, and the Roadmap did not stipulate that this deficiency should be remedied. Surveys have revealed that community HCPs rarely screen or counsel their patients—even those at high risk—about firearm safety.71 A bill was introduced in Congress August 21, 2020, to expand VA suicide prevention training with firearms community input on cultural competency components and mandate that VA and VCCP providers, and some others with frequent contact with veterans, receive this training.72
Training should be obligatory for VA and VCCP HCPs and trainees most likely to interface with at-risk veterans, including those working in mental health, primary care, pain, women’s health, and ED. Training also should include geriatrics, extended care, and oncology providers because most older adults who die by firearm suicide have physical health problems but no known mental illness.73-75 Lethal means safety counseling training has been shown to improve HCPs’ knowledge about the relationship between access to lethal means and suicide, and confidence in and frequency of having lethal means safety counseling conversations.76 Likewise, training should include peer counselors; veterans are receptive to fellow veterans raising the topic of safe storage.56,77 If feasible, the training should include time to rehearse skills shown to motivate behavior change among patients.
The VA should aim to improve semiyearly clinical pertinence reviews and safety plans for VA and VCCP mental health providers. VA could conduct clinical pertinence reviews that ascertain whether a suicide assessment is recorded in the health record, and when a patient is at elevated risk, whether a lethal means safety assessment and plan is documented.
VA’s safety plan template, although best practice, covers only the initial steps to take when suicide potential is identified. A standard for follow-up is needed. If an at-risk patient agrees to take a safe storage action, subsequent contact HCPs need to ask and document what action was performed. This action will help ensure that at-risk patients with ready access do not fall through the cracks. This suggestion lends itself to studying changes in veterans’ storage habits after intervention.
I also recommend that health care accrediting bodies include lethal means safety assessment, counseling, and follow up as a suicide prevention standard. This recommendation applies to more than just the VA health care system and recognizes that modifying accrediting body standards is an expeditious way to drive change in health care. The accreditation standards of the Commission on Accreditation of Rehabilitation Facilities for behavioral health and opioid treatment programs, and of the Joint Commission for medical centers do not require lethal means safety assessment and intervention.78,79
Conclusions
Suicide prevention requires a multimodal approach, and attention to firearms access must become a more salient component. The high rate of veteran suicides involving firearms requires far-reaching interventions at societal, institutional, community, family, and individual levels. With the link between ready access to firearms and suicide supported by research and now firmly recognized by the PREVENTS Roadmap, we have a fresh opportunity to reduce suicide among veterans. Efforts must move vigorously forward until it is commonplace for veterans—and anyone—at risk of suicide to voluntarily reduce immediate access to firearms.
1. US Department of Veterans Affairs. Veteran suicide prevention annual report. https://www.mentalhealth.va.gov/docs/data-sheets/2019/2019_National_Veteran_Suicide_Prevention_Annual_Report_508.pdf. Published September 2019. Accessed August 20, 2020.
2. US Department of Defense. Casualty status. https://www.defense.gov/casualty.pdf. Published August 17, 2020. Accessed August 20, 2020.
3. Cleveland EC, Azreal D, Simonetti JA, Miller M. Firearm ownership among American veterans: findings from the 2015 National Firearm Survey. Inj Epidemiol. 2017;4:33. doi:10.1186/s40621-017-0130-y
4. US Department of Veterans Affairs. PREVENTS: the President’s roadmap to empower veterans and end a national tragedy of suicide. https://www.va.gov/PREVENTS/docs/PRE-007-The-PREVENTS-Roadmap-1-2_508.pdf. Published June 17, 2020. Accessed August 20, 2020.
5. Kaplan MS, McFarland BH, Huguet N. Firearm suicide among veterans in the general population: findings from the National Violent Death Reporting System. Trauma. 2009;67(3):503-507. doi:10.1097/TA.0b013e3181b36521
6. Miller M, Barber C, White RA, Azrael D. Firearms and suicide in the United States: is risk independent of underlying suicidal behavior? Am J Epidemiol. 2013;178(6):946-955. doi:10.1093/aje/kwt197
7. Ivey-Stephenson AZ, Crosby AE, Jack, SP, Haileyesus, T, Kresnow-Sedacca M. Suicide trends among and within urbanization levels by sex, race/ethnicity, age group, and mechanism of death—United States, 2001-2015. MMWR Surveill Summ. 2017;66(18):1-16. doi:10.15585/mmwr.ss6618a1
8. McCarthy JF, Blow FC, Ignacio RV, Ilgen MA, Austin KL, Valenstein M. Suicide among patients in the Veterans Affairs Health System: rural-urban differences in rates, risks and methods. Am J Public Health. 2012;102(suppl 1):S111-S117. doi:10.2105/AJPH.2011.300463
9. RAND Corporation. The relationship between firearm availability and suicide. https://www.rand.org/research/gun-policy/analysis/essays/firearm-availability-suicide.html. Published March 2, 2018. Accessed August 20, 2020.
10. Anglemyer A, Horvath T, Rutherford G. The accessibility of firearms and risk for suicide and homicide victimization among household members: a systematic review and meta-analysis. Ann Intern Med. 2014;160(2):101-110. doi:10.7326/M13-1301
11. Studdert DM, Zhang Y, Swanson SA, et al. Handgun ownership and suicide in California. N Engl J Med. 2020;382(23):2220-2229. doi:10.1056/NEJMsa1916744
12. Miller M, Hemenway D. The relationship between firearms and suicide: a review of the literature. Aggression Violent Behav. 1999;4(1):59-75. doi:10.1016/S1359-1789(97)00057-8
13. Brent DA. Firearms and suicide. Ann N Y Acad Sci. 2001;932:225-239. doi:10.1111/j.1749-6632.2001.tb05808.x
14. Conwell Y, Duberstein PR, Connor K, Eberly S, Cox C, Caine ED. Access to firearms and risk for suicide in middle aged and older adults. Am J Geriatr Psychiatry. 2002;10(4):407-416. doi:10.1176/appi.ajgp.10.4.407
15. Grossman DC, Mueller BA, Riedy C, et al. Gun storage practices and risk of youth suicide and unintentional firearm injuries. JAMA. 2005;293(6):707-714. doi:10.1001/jama.293.6.707
16. Simonetti JA, Rowhani-Rahbar A. Limiting access to firearms as a suicide prevention strategy among adults. JAMA Netw Open. 2019;2(6):e195400. doi:10.1001/jamanetworkopen.2019.5400
17. Dempsey CL, Benedek DM, Zuromski KL, et al. Association of firearm use, accessibility, and storage practices with suicide risk among US army soldiers. JAMA Netw Open. 2019;2(6):e195383. doi:10.1001/jamanetworkopen.2019.5383
18. Wiebe DJ. Homicide and suicide risks associated with firearms in the home: a national case-control study. Ann Emerg Med. 41(6):771-782. doi:10.1067/mem.2003.187
19. de Moore GM, Plew JD, Bray KM, Snars JN. Survivors of self-inflicted firearm injury: a liaison psychiatry perspective. Med J Aust. 1994;160(7):421-425. doi:10.5694/j.1326-5377.1994.tb138267.x
20. Peterson LG, Peterson M, O’Shanick GJ, Swann A. Self-inflicted gunshot wounds: lethality of method versus intent. Am J Psychiatry. 1985;142(2):228-231. doi:10.1176/ajp.142.2.228
21. Miller M, Azrael D, Hemenway D. Belief in the inevitability of suicide: results from a national survey. Suicide Life Threat Behav. 2006;36(1):1-11. doi:10.1521/suli.2006.36.1.1
22. Bryan CJ, Rudd MD, Peterson AL, Young-McCaughan S, Wertenberger, EG. The ebb and flow of the wish to live and the wish to die among suicidal military personnel. J Affect Disord. 2016;202:58-66. doi:10.1016/j.jad.2016.05.049
23. O’Donnell I, Arthur AJ, Farmer RD. A follow-up study of attempted railway suicides. Soc Sci Med. 1994;38(3):437-442. doi:10.1016/0277-9536(94)90444-8
24. Owens D, Horrocks J, House A. Fatal and nonfatal repetition of self-harm: systematic review. Br J Psychiatry. 2002;181:193-199. doi:10.1192/bjp.181.3.193
25. Seiden RH. Where are they now? A follow-up study of suicide attempters from the Golden Gate Bridge. Suicide Life Threat Behav. 1978;8(4):203-216. doi:10.1111/j.1943-278X.1978.tb00587.x
26. Conner A, Azrael D, Miller M. Suicide case fatality rates in the United States, 2007 to 2014: a nationwide population-based study. Ann Intern Med. 2019;171(12):885-895. doi:10.7326/M19-1324
27. Disenhammer EA, Ing CM, Strauss R, Kemmler G, Hinterhuber H, Weiss EM. The duration of the suicidal process: how much time is left for intervention between consideration and accomplishment of a suicide attempt? J Clin Psych. 2009;70(1):19-24. doi:10.4088/JCP.07m03904
28. Simon TR, Swann AC, Powell KE, Potter LB, Kresnow M, O’Carrol, PW. Characteristics of impulsive suicide attempts and attempters. Suicide Life Threat Behav. 2001;32(suppl 1):49-59. doi:10.1521/suli.32.1.5.49.24212
29. Williams CL, Davidson JA, Montgomery I. Impulsive suicidal behavior. J Clin Psychol. 1980;36(1):90-94. doi:10.1002/1097-4679(198001)36:1<90::aid-jclp2270360104>3.0.co;2-f
30. Drum, DJ, Brownson, CB, Denmark, AB, Smith, SE. New data on the nature of suicidal crises in college students: shifting the paradigm. Professional Psychol: Res Pract. 2009;40(3):213-222. doi:10.1037/a0014465
31. Lubin G, Werbeloff N, Halperin D, Shmushkevitch M, Weise M, Knobler H. Decrease in suicide rates after a change of policy reducing access to firearms in adolescents: a naturalistic epidemiological study. Suicide Life Threat Behav. 2010;40(5):421-424. doi:10.1521/suli.2010.40.5.421
32. Shelef L, Tatsa-Laur L, Derazne E, Mann JJ, Fruchter E. An effective suicide prevention program in the Israeli Defense Forces: a cohort study. Eur Psychiatry. 2016;31:37-43. doi:10.1016/j.eurpsy.2015.10.004

33. Simonetti JA, Azrael D, Rowhani-Rahbar A, Miller M. Firearm storage practices among American veterans. Amer J Prev Med. 2018;55(4):445-454. doi:10.1016/j.amepre.2018.04.014
34. Office of the Surgeon General, National Action Alliance for Suicide Prevention. National strategy for suicide prevention: goals and objectives for action: a report of the U.S. Surgeon General and of the National Action Alliance for Suicide Prevention. https://www.ncbi.nlm.nih.gov/pubmed/23136686 Published September 2012. Accessed August 18, 2020.
35. Stone D, Holland KM, Bartholow B, Crosby AE, Davis S, Wilkins N. Preventing suicide: a technical package of policies, programs, and practices. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2017.
36. American Public Health Association. Reducing suicides by firearms. https://www.apha.org/policies-and-advocacy/public-health-policy-statements/policy-database/2019/01/28/reducing-suicides-by-firearms. Published November 13, 2018. Accessed August 18, 2020.
37. Coffey MJ, Coffey CE, Ahmedani BK. Suicide in a health maintenance organization population. JAMA Psychiatry. 2015;72(3):294-296. doi:10.1001/jamapsychiatry.2014.2440
38. Boggs JM, Beck A, Ritzwoller DP, Battaglia C, Anderson HD, Lindrooth RC. A quasi-experimental analysis of lethal means assessment and risk for subsequent suicide attempts and deaths. J Gen Intern Med. 2020;35(6):1709-1714. doi:10.1007/s11606-020-05641-4
39. Washington State Health Assessment 2018. Suicide & safe storage of firearms https://www.doh.wa.gov/Portals/1/Documents/1000/SHA-SuicideandSafeStorageofFirearms.pdf. Accessed August 18, 2020.
40. UC Davis Health Newsroom. First-in-the-nation gun violence prevention training program for health professionals established at UC Davis Health. https://health.ucdavis.edu/health-news/newsroom/first-in-the-nation-gun-violence-prevention-training-program-for-health-professionals-established-at-uc-davis-health/2019/10. Published October 15, 2019. Accessed August 18, 2020.
41. Mental Illness Research, Education, and Clinical Center. Lethal means safety & suicide prevention. https://www.mirecc.va.gov/lethalmeanssafety/index.asp. Updated February 1, 2018. Accessed August 18, 2020.
42. US Department of Veterans Affairs. Reducing firearm & other household safety risks for veterans and their families. https://www.mentalhealth.va.gov/suicide_prevention/docs/Brochure-for-Veterans-Means-Safety-Messaging_508_CLEARED_11-15-19.pdf. Published July 2019. Accessed August 18, 2020.
43. US Department of Veterans Affairs. Means safety messaging for clinical staff. https://www.mentalhealth.va.gov/suicide_prevention/docs/Pocket-Card-for-Clinicians-Means-Safety-Messaging_508_CLEARED_9-3-19.pdf. Accessed August 18, 2020.
44. Department of Veterans Affairs and Department of Defense. Lethal means counseling: recommendations for providers. https://www.healthquality.va.gov/guidelines/MH/srb/LethalMeansProviders20200527508.pdf. Published May 2020. Accessed August 18, 2020.
45. U.S. Department of Veterans Affairs. Supporting providers who serve veterans. https://www.mirecc.va.gov/visn19/consult. Updated August 3, 2020. Accessed August 18, 2020.
46. US Department of Veterans Affairs. National strategy for preventing veteran suicide 2018-2028. https://www.mentalhealth.va.gov/suicide_prevention/docs/Office-of-Mental-Health-and-Suicide-Prevention-National-Strategy-for-Preventing-Veterans-Suicide.pdf. Accessed August 18, 2020.
47. US Department of Veterans Affairs. VA/DoD clinical practice guidelines: assessment and management of patients at risk for suicide. https://www.healthquality.va.gov/guidelines/MH/srb. Updated July 30, 2020. Accessed August 18, 2020.
48. US Department of Veterans Affairs. Developing a safety plan. https://www.mentalhealth.va.gov/docs/vasafetyplancolor.pdf. Published March 2012. Accessed August 18, 2020.
49. Lemle RB. VA forges an historic partnership with the National Shooting Sports Foundation and the American Foundation for Suicide Prevention to prevent veteran suicide. Fed Pract. 2019;36(2):18-24.
50. US Department of Veterans Affairs, American Foundation for Suicide Prevention, National Shooting Sports Foundation. Suicide prevention is everyone’s business: a toolkit for safe firearm storage in your community. https://project2025.afsp.org/wp-content/uploads/2020/03/Toolkit_Safe_Firearm_Storage_CLEARED_508_2-24-20.pdf. Accessed August 18, 2020.
51. Montheith, LL, Wendleton, L, Bahraini, NH, Matarazzo, BB, Brimner, G, Mohatt, NV. Together with veterans: VA national strategy alignment and lessons learned from community-based suicide prevention for rural veterans. Suicide Life Threat Behav. 2020;50(3):588-600. doi:10.1111/sltb.12613. Epub 2020 Jan 16
52. Gordon S. VA pioneering efforts to reduce veteran suicide from firearms. http://beyondchron.org/va-pioneering-efforts-to-reduce-veteran-suicide-from-firearms. Published March 10, 2020. Accessed August 20, 2020.
53. Johnson A. Protecting mental health and preventing suicide during COVID-19. https://www.blogs.va.gov/VAntage/76827/mental-health-and-suicide-prevention-during-covid-19. Published July 14, 2020. Accessed August 18, 2020.
54. Betz ME, Anestis MD. Firearms, pesticides, and suicide: a look back for a way forward. Prev Med. 2020;138:106144. doi:10.1016/j.ypmed.2020.106144
55. Buckley, L, Chapman, RL, and Lewis, I. A systematic review of intervening to prevent driving while intoxicated: The problem of driving while intoxicated (DWI), Substance Use & Misuse. 2016; 51(1): 104-112. doi:10.3109/10826084.2015.1090452

56. National Safety Council. Injury facts. motor vehicle safety issues. https://injuryfacts.nsc.org/motor-vehicle/motor-vehicle-safety-issues/alcohol-impaired-driving. Accessed August 2020.
57. Crifasi CK, Doucette ML, McGinty EE, Webster DW, Barry CL. Storage practices of US gun owners in 2016. Am J Public Health. 2018;108(4):532-537. doi:10.2105/AJPH.2017.304262
58. National Shooting Sports Foundation. Suicide prevention program for retailers and ranges. https://www.nssf.org/safety/suicide-prevention. Accessed August 18, 2020.
59. Pallin R, Siry B, Azrael D, et al. “Hey, let me hold your guns for a while”: a qualitative study of messaging for firearm suicide prevention. Behav Sci Law. 2019;37(3):259-269. doi:10.1002/bsl.2393
60. Oregon Firearm Safety. http://oregonfirearmsafety.org. Accessed August 18, 2020.
61. National Shooting Sports Foundation. Suicide prevention toolkit items. https://www.nssf.org/safety/suicide-prevention/suicide-prevention-toolkit. Accessed August 18, 2020.
62. Centers for Disease Control and Prevention. Suicide rising across the US. https://www.cdc.gov/vitalsigns/suicide/index.html. Updated June 7, 2018. Accessed August 18, 2020.
63. U.S Department of Veterans Affairs. PREVENTS: executive order 13861. https://www.va.gov/PREVENTS/EO-13861.asp. Updated August 13, 2020. Accessed August 18, 2020.
64. COVER Commission. Creating Options for Veterans’ Expedited Recovery (COVER) Commission Final Report. https://www.va.gov/COVER/docs/COVER-Commission-Final-Report-2020-01-24.pdf. Published January 24, 2020. Accessed August 18, 2020.
65. Pierpoint LA, Tung GJ, Brooks-Russell A, Brandspigel S, Betz M, Runyan CW. Gun retailers as storage partners for suicide prevention: what barriers need to be overcome? Inj Prev. 2019;25(suppl 1):i5-i8. doi:10.1136/injuryprev-2017-042700
66. Gibbons MJ, Fan MD, Rowhani-Rahbar A, Rivara FP. Legal liability for returning firearms to suicidal persons who voluntarily surrender them in 50 US states. Am J Public Health. 2020;110(5):685-688. doi:10.2105/AJPH.2019.305545
67. Kelly T, Brandspigel S, Polzer E, Betz ME. Firearm storage maps: a pragmatic approach to reduce firearm suicide during times of risk. Ann Intern Med. 2020;172(5):351-353. doi:10.7326/M19-2944
68. Edwards C. This new gun storage map is designed to save lives in Colorado. https://bearingarms.com/cam-e/2019/08/27/new-gun-storage-map-designed-save-lives-colorado. Published August 27, 2019. Accessed August 18, 2020.
69. McCourt AD, Vernick JS, Betz ME, Brandspigel S, Runyan CW. Temporary transfer of firearms from the home to prevent suicide: legal obstacles and recommendations. JAMA Intern Med. 2017;177(1):96-101. doi:10.1001/jamainternmed.2016.5704
70. US Department of Veterans Affairs. Aimed at suicide prevention, VA shares winners of its ‘Gun Safety Matters Challenge.” https://www.blogs.va.gov/VAntage/50233/aimed-suicide-prevention-va-shares-winners-gun-safety-matters-challenge. Published July 9, 2019. Accessed August 18, 2020.
71. Roszko PJD, Ameli J, Carter PM, Cunningham RM, Ranney ML. Clinician attitudes, screening practices, and interventions to reduce firearm-related injury. Epidemiol Rev. 2016;38(1):87-110. doi:10.1093/epirev/mxv005
72. Lethal Means Safety Training Act. HR 8084, 116th Cong. 2nd Sess (2020). https://www.congress.gov/bill/116th-congress/house-bill/8084/text. Accessed August 25, 2020.
73. Boggs JM, Simon GE, Ahmedani BK, Peterson E, Hubley S, Beck A. The association of firearm suicide with mental illness, substance use conditions, and previous suicide attempts. Ann Intern Med. 2017;167(4):287-288. doi:10.7326/L17-0111
74. Schmutte TJ, Wilkinson ST. Suicide in older adults with and without known mental illness: results from the National Violent Death Reporting System, 2003-2016. Am J Prev Med. 2020;58(4):584-590. doi:10.1016/j.amepre.2019.11.001
75. Morin RT, Li Y, Mackin RS, Whooley MA, Conwell Y, Byers AL. Comorbidity profiles identified in older primary care patients who attempt suicide. J Am Geriatr Soc. 2019;67(12):2553-2559. doi:10.1111/jgs.16126
76. Roszko PJD, Ameli J, Carter PM, Cunningham RM, Ranney, ML. Clinician attitudes, screening practices, and interventions to reduce firearm-related injury. Epidemiol Rev. 2016;38(1):87-110. doi:10.1093/epirev/mxv005
77. Iraq and Afghanistan Veterans of America. 7th annual IAVA member survey: the most comprehensive look into the lives of post-9/11. https://iava.org/wp-content/uploads/2020/02/IAVA-MemberSurvey-single-pgs1.pdf. Accessed August 18, 2020.
78. CARF International. CARF adds screening for suicide risk to its assessment standards. http://www.carf.org/universal-suicide-screening-standards. Published May 2, 2019. Accessed August 18, 2020.
79. Paul S. National Patient Safety Goal expands focus on suicide prevention. https://www.jointcommission.org/resources/news-and-multimedia/blogs/dateline-tjc/2019/01/national-patient-safety-goal-expands-focus-on-suicide-prevention/. Published January 24, 2019. Accessed August 18, 2020.
1. US Department of Veterans Affairs. Veteran suicide prevention annual report. https://www.mentalhealth.va.gov/docs/data-sheets/2019/2019_National_Veteran_Suicide_Prevention_Annual_Report_508.pdf. Published September 2019. Accessed August 20, 2020.
2. US Department of Defense. Casualty status. https://www.defense.gov/casualty.pdf. Published August 17, 2020. Accessed August 20, 2020.
3. Cleveland EC, Azreal D, Simonetti JA, Miller M. Firearm ownership among American veterans: findings from the 2015 National Firearm Survey. Inj Epidemiol. 2017;4:33. doi:10.1186/s40621-017-0130-y
4. US Department of Veterans Affairs. PREVENTS: the President’s roadmap to empower veterans and end a national tragedy of suicide. https://www.va.gov/PREVENTS/docs/PRE-007-The-PREVENTS-Roadmap-1-2_508.pdf. Published June 17, 2020. Accessed August 20, 2020.
5. Kaplan MS, McFarland BH, Huguet N. Firearm suicide among veterans in the general population: findings from the National Violent Death Reporting System. Trauma. 2009;67(3):503-507. doi:10.1097/TA.0b013e3181b36521
6. Miller M, Barber C, White RA, Azrael D. Firearms and suicide in the United States: is risk independent of underlying suicidal behavior? Am J Epidemiol. 2013;178(6):946-955. doi:10.1093/aje/kwt197
7. Ivey-Stephenson AZ, Crosby AE, Jack, SP, Haileyesus, T, Kresnow-Sedacca M. Suicide trends among and within urbanization levels by sex, race/ethnicity, age group, and mechanism of death—United States, 2001-2015. MMWR Surveill Summ. 2017;66(18):1-16. doi:10.15585/mmwr.ss6618a1
8. McCarthy JF, Blow FC, Ignacio RV, Ilgen MA, Austin KL, Valenstein M. Suicide among patients in the Veterans Affairs Health System: rural-urban differences in rates, risks and methods. Am J Public Health. 2012;102(suppl 1):S111-S117. doi:10.2105/AJPH.2011.300463
9. RAND Corporation. The relationship between firearm availability and suicide. https://www.rand.org/research/gun-policy/analysis/essays/firearm-availability-suicide.html. Published March 2, 2018. Accessed August 20, 2020.
10. Anglemyer A, Horvath T, Rutherford G. The accessibility of firearms and risk for suicide and homicide victimization among household members: a systematic review and meta-analysis. Ann Intern Med. 2014;160(2):101-110. doi:10.7326/M13-1301
11. Studdert DM, Zhang Y, Swanson SA, et al. Handgun ownership and suicide in California. N Engl J Med. 2020;382(23):2220-2229. doi:10.1056/NEJMsa1916744
12. Miller M, Hemenway D. The relationship between firearms and suicide: a review of the literature. Aggression Violent Behav. 1999;4(1):59-75. doi:10.1016/S1359-1789(97)00057-8
13. Brent DA. Firearms and suicide. Ann N Y Acad Sci. 2001;932:225-239. doi:10.1111/j.1749-6632.2001.tb05808.x
14. Conwell Y, Duberstein PR, Connor K, Eberly S, Cox C, Caine ED. Access to firearms and risk for suicide in middle aged and older adults. Am J Geriatr Psychiatry. 2002;10(4):407-416. doi:10.1176/appi.ajgp.10.4.407
15. Grossman DC, Mueller BA, Riedy C, et al. Gun storage practices and risk of youth suicide and unintentional firearm injuries. JAMA. 2005;293(6):707-714. doi:10.1001/jama.293.6.707
16. Simonetti JA, Rowhani-Rahbar A. Limiting access to firearms as a suicide prevention strategy among adults. JAMA Netw Open. 2019;2(6):e195400. doi:10.1001/jamanetworkopen.2019.5400
17. Dempsey CL, Benedek DM, Zuromski KL, et al. Association of firearm use, accessibility, and storage practices with suicide risk among US army soldiers. JAMA Netw Open. 2019;2(6):e195383. doi:10.1001/jamanetworkopen.2019.5383
18. Wiebe DJ. Homicide and suicide risks associated with firearms in the home: a national case-control study. Ann Emerg Med. 41(6):771-782. doi:10.1067/mem.2003.187
19. de Moore GM, Plew JD, Bray KM, Snars JN. Survivors of self-inflicted firearm injury: a liaison psychiatry perspective. Med J Aust. 1994;160(7):421-425. doi:10.5694/j.1326-5377.1994.tb138267.x
20. Peterson LG, Peterson M, O’Shanick GJ, Swann A. Self-inflicted gunshot wounds: lethality of method versus intent. Am J Psychiatry. 1985;142(2):228-231. doi:10.1176/ajp.142.2.228
21. Miller M, Azrael D, Hemenway D. Belief in the inevitability of suicide: results from a national survey. Suicide Life Threat Behav. 2006;36(1):1-11. doi:10.1521/suli.2006.36.1.1
22. Bryan CJ, Rudd MD, Peterson AL, Young-McCaughan S, Wertenberger, EG. The ebb and flow of the wish to live and the wish to die among suicidal military personnel. J Affect Disord. 2016;202:58-66. doi:10.1016/j.jad.2016.05.049
23. O’Donnell I, Arthur AJ, Farmer RD. A follow-up study of attempted railway suicides. Soc Sci Med. 1994;38(3):437-442. doi:10.1016/0277-9536(94)90444-8
24. Owens D, Horrocks J, House A. Fatal and nonfatal repetition of self-harm: systematic review. Br J Psychiatry. 2002;181:193-199. doi:10.1192/bjp.181.3.193
25. Seiden RH. Where are they now? A follow-up study of suicide attempters from the Golden Gate Bridge. Suicide Life Threat Behav. 1978;8(4):203-216. doi:10.1111/j.1943-278X.1978.tb00587.x
26. Conner A, Azrael D, Miller M. Suicide case fatality rates in the United States, 2007 to 2014: a nationwide population-based study. Ann Intern Med. 2019;171(12):885-895. doi:10.7326/M19-1324
27. Disenhammer EA, Ing CM, Strauss R, Kemmler G, Hinterhuber H, Weiss EM. The duration of the suicidal process: how much time is left for intervention between consideration and accomplishment of a suicide attempt? J Clin Psych. 2009;70(1):19-24. doi:10.4088/JCP.07m03904
28. Simon TR, Swann AC, Powell KE, Potter LB, Kresnow M, O’Carrol, PW. Characteristics of impulsive suicide attempts and attempters. Suicide Life Threat Behav. 2001;32(suppl 1):49-59. doi:10.1521/suli.32.1.5.49.24212
29. Williams CL, Davidson JA, Montgomery I. Impulsive suicidal behavior. J Clin Psychol. 1980;36(1):90-94. doi:10.1002/1097-4679(198001)36:1<90::aid-jclp2270360104>3.0.co;2-f
30. Drum, DJ, Brownson, CB, Denmark, AB, Smith, SE. New data on the nature of suicidal crises in college students: shifting the paradigm. Professional Psychol: Res Pract. 2009;40(3):213-222. doi:10.1037/a0014465
31. Lubin G, Werbeloff N, Halperin D, Shmushkevitch M, Weise M, Knobler H. Decrease in suicide rates after a change of policy reducing access to firearms in adolescents: a naturalistic epidemiological study. Suicide Life Threat Behav. 2010;40(5):421-424. doi:10.1521/suli.2010.40.5.421
32. Shelef L, Tatsa-Laur L, Derazne E, Mann JJ, Fruchter E. An effective suicide prevention program in the Israeli Defense Forces: a cohort study. Eur Psychiatry. 2016;31:37-43. doi:10.1016/j.eurpsy.2015.10.004

33. Simonetti JA, Azrael D, Rowhani-Rahbar A, Miller M. Firearm storage practices among American veterans. Amer J Prev Med. 2018;55(4):445-454. doi:10.1016/j.amepre.2018.04.014
34. Office of the Surgeon General, National Action Alliance for Suicide Prevention. National strategy for suicide prevention: goals and objectives for action: a report of the U.S. Surgeon General and of the National Action Alliance for Suicide Prevention. https://www.ncbi.nlm.nih.gov/pubmed/23136686 Published September 2012. Accessed August 18, 2020.
35. Stone D, Holland KM, Bartholow B, Crosby AE, Davis S, Wilkins N. Preventing suicide: a technical package of policies, programs, and practices. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2017.
36. American Public Health Association. Reducing suicides by firearms. https://www.apha.org/policies-and-advocacy/public-health-policy-statements/policy-database/2019/01/28/reducing-suicides-by-firearms. Published November 13, 2018. Accessed August 18, 2020.
37. Coffey MJ, Coffey CE, Ahmedani BK. Suicide in a health maintenance organization population. JAMA Psychiatry. 2015;72(3):294-296. doi:10.1001/jamapsychiatry.2014.2440
38. Boggs JM, Beck A, Ritzwoller DP, Battaglia C, Anderson HD, Lindrooth RC. A quasi-experimental analysis of lethal means assessment and risk for subsequent suicide attempts and deaths. J Gen Intern Med. 2020;35(6):1709-1714. doi:10.1007/s11606-020-05641-4
39. Washington State Health Assessment 2018. Suicide & safe storage of firearms https://www.doh.wa.gov/Portals/1/Documents/1000/SHA-SuicideandSafeStorageofFirearms.pdf. Accessed August 18, 2020.
40. UC Davis Health Newsroom. First-in-the-nation gun violence prevention training program for health professionals established at UC Davis Health. https://health.ucdavis.edu/health-news/newsroom/first-in-the-nation-gun-violence-prevention-training-program-for-health-professionals-established-at-uc-davis-health/2019/10. Published October 15, 2019. Accessed August 18, 2020.
41. Mental Illness Research, Education, and Clinical Center. Lethal means safety & suicide prevention. https://www.mirecc.va.gov/lethalmeanssafety/index.asp. Updated February 1, 2018. Accessed August 18, 2020.
42. US Department of Veterans Affairs. Reducing firearm & other household safety risks for veterans and their families. https://www.mentalhealth.va.gov/suicide_prevention/docs/Brochure-for-Veterans-Means-Safety-Messaging_508_CLEARED_11-15-19.pdf. Published July 2019. Accessed August 18, 2020.
43. US Department of Veterans Affairs. Means safety messaging for clinical staff. https://www.mentalhealth.va.gov/suicide_prevention/docs/Pocket-Card-for-Clinicians-Means-Safety-Messaging_508_CLEARED_9-3-19.pdf. Accessed August 18, 2020.
44. Department of Veterans Affairs and Department of Defense. Lethal means counseling: recommendations for providers. https://www.healthquality.va.gov/guidelines/MH/srb/LethalMeansProviders20200527508.pdf. Published May 2020. Accessed August 18, 2020.
45. U.S. Department of Veterans Affairs. Supporting providers who serve veterans. https://www.mirecc.va.gov/visn19/consult. Updated August 3, 2020. Accessed August 18, 2020.
46. US Department of Veterans Affairs. National strategy for preventing veteran suicide 2018-2028. https://www.mentalhealth.va.gov/suicide_prevention/docs/Office-of-Mental-Health-and-Suicide-Prevention-National-Strategy-for-Preventing-Veterans-Suicide.pdf. Accessed August 18, 2020.
47. US Department of Veterans Affairs. VA/DoD clinical practice guidelines: assessment and management of patients at risk for suicide. https://www.healthquality.va.gov/guidelines/MH/srb. Updated July 30, 2020. Accessed August 18, 2020.
48. US Department of Veterans Affairs. Developing a safety plan. https://www.mentalhealth.va.gov/docs/vasafetyplancolor.pdf. Published March 2012. Accessed August 18, 2020.
49. Lemle RB. VA forges an historic partnership with the National Shooting Sports Foundation and the American Foundation for Suicide Prevention to prevent veteran suicide. Fed Pract. 2019;36(2):18-24.
50. US Department of Veterans Affairs, American Foundation for Suicide Prevention, National Shooting Sports Foundation. Suicide prevention is everyone’s business: a toolkit for safe firearm storage in your community. https://project2025.afsp.org/wp-content/uploads/2020/03/Toolkit_Safe_Firearm_Storage_CLEARED_508_2-24-20.pdf. Accessed August 18, 2020.
51. Montheith, LL, Wendleton, L, Bahraini, NH, Matarazzo, BB, Brimner, G, Mohatt, NV. Together with veterans: VA national strategy alignment and lessons learned from community-based suicide prevention for rural veterans. Suicide Life Threat Behav. 2020;50(3):588-600. doi:10.1111/sltb.12613. Epub 2020 Jan 16
52. Gordon S. VA pioneering efforts to reduce veteran suicide from firearms. http://beyondchron.org/va-pioneering-efforts-to-reduce-veteran-suicide-from-firearms. Published March 10, 2020. Accessed August 20, 2020.
53. Johnson A. Protecting mental health and preventing suicide during COVID-19. https://www.blogs.va.gov/VAntage/76827/mental-health-and-suicide-prevention-during-covid-19. Published July 14, 2020. Accessed August 18, 2020.
54. Betz ME, Anestis MD. Firearms, pesticides, and suicide: a look back for a way forward. Prev Med. 2020;138:106144. doi:10.1016/j.ypmed.2020.106144
55. Buckley, L, Chapman, RL, and Lewis, I. A systematic review of intervening to prevent driving while intoxicated: The problem of driving while intoxicated (DWI), Substance Use & Misuse. 2016; 51(1): 104-112. doi:10.3109/10826084.2015.1090452

56. National Safety Council. Injury facts. motor vehicle safety issues. https://injuryfacts.nsc.org/motor-vehicle/motor-vehicle-safety-issues/alcohol-impaired-driving. Accessed August 2020.
57. Crifasi CK, Doucette ML, McGinty EE, Webster DW, Barry CL. Storage practices of US gun owners in 2016. Am J Public Health. 2018;108(4):532-537. doi:10.2105/AJPH.2017.304262
58. National Shooting Sports Foundation. Suicide prevention program for retailers and ranges. https://www.nssf.org/safety/suicide-prevention. Accessed August 18, 2020.
59. Pallin R, Siry B, Azrael D, et al. “Hey, let me hold your guns for a while”: a qualitative study of messaging for firearm suicide prevention. Behav Sci Law. 2019;37(3):259-269. doi:10.1002/bsl.2393
60. Oregon Firearm Safety. http://oregonfirearmsafety.org. Accessed August 18, 2020.
61. National Shooting Sports Foundation. Suicide prevention toolkit items. https://www.nssf.org/safety/suicide-prevention/suicide-prevention-toolkit. Accessed August 18, 2020.
62. Centers for Disease Control and Prevention. Suicide rising across the US. https://www.cdc.gov/vitalsigns/suicide/index.html. Updated June 7, 2018. Accessed August 18, 2020.
63. U.S Department of Veterans Affairs. PREVENTS: executive order 13861. https://www.va.gov/PREVENTS/EO-13861.asp. Updated August 13, 2020. Accessed August 18, 2020.
64. COVER Commission. Creating Options for Veterans’ Expedited Recovery (COVER) Commission Final Report. https://www.va.gov/COVER/docs/COVER-Commission-Final-Report-2020-01-24.pdf. Published January 24, 2020. Accessed August 18, 2020.
65. Pierpoint LA, Tung GJ, Brooks-Russell A, Brandspigel S, Betz M, Runyan CW. Gun retailers as storage partners for suicide prevention: what barriers need to be overcome? Inj Prev. 2019;25(suppl 1):i5-i8. doi:10.1136/injuryprev-2017-042700
66. Gibbons MJ, Fan MD, Rowhani-Rahbar A, Rivara FP. Legal liability for returning firearms to suicidal persons who voluntarily surrender them in 50 US states. Am J Public Health. 2020;110(5):685-688. doi:10.2105/AJPH.2019.305545
67. Kelly T, Brandspigel S, Polzer E, Betz ME. Firearm storage maps: a pragmatic approach to reduce firearm suicide during times of risk. Ann Intern Med. 2020;172(5):351-353. doi:10.7326/M19-2944
68. Edwards C. This new gun storage map is designed to save lives in Colorado. https://bearingarms.com/cam-e/2019/08/27/new-gun-storage-map-designed-save-lives-colorado. Published August 27, 2019. Accessed August 18, 2020.
69. McCourt AD, Vernick JS, Betz ME, Brandspigel S, Runyan CW. Temporary transfer of firearms from the home to prevent suicide: legal obstacles and recommendations. JAMA Intern Med. 2017;177(1):96-101. doi:10.1001/jamainternmed.2016.5704
70. US Department of Veterans Affairs. Aimed at suicide prevention, VA shares winners of its ‘Gun Safety Matters Challenge.” https://www.blogs.va.gov/VAntage/50233/aimed-suicide-prevention-va-shares-winners-gun-safety-matters-challenge. Published July 9, 2019. Accessed August 18, 2020.
71. Roszko PJD, Ameli J, Carter PM, Cunningham RM, Ranney ML. Clinician attitudes, screening practices, and interventions to reduce firearm-related injury. Epidemiol Rev. 2016;38(1):87-110. doi:10.1093/epirev/mxv005
72. Lethal Means Safety Training Act. HR 8084, 116th Cong. 2nd Sess (2020). https://www.congress.gov/bill/116th-congress/house-bill/8084/text. Accessed August 25, 2020.
73. Boggs JM, Simon GE, Ahmedani BK, Peterson E, Hubley S, Beck A. The association of firearm suicide with mental illness, substance use conditions, and previous suicide attempts. Ann Intern Med. 2017;167(4):287-288. doi:10.7326/L17-0111
74. Schmutte TJ, Wilkinson ST. Suicide in older adults with and without known mental illness: results from the National Violent Death Reporting System, 2003-2016. Am J Prev Med. 2020;58(4):584-590. doi:10.1016/j.amepre.2019.11.001
75. Morin RT, Li Y, Mackin RS, Whooley MA, Conwell Y, Byers AL. Comorbidity profiles identified in older primary care patients who attempt suicide. J Am Geriatr Soc. 2019;67(12):2553-2559. doi:10.1111/jgs.16126
76. Roszko PJD, Ameli J, Carter PM, Cunningham RM, Ranney, ML. Clinician attitudes, screening practices, and interventions to reduce firearm-related injury. Epidemiol Rev. 2016;38(1):87-110. doi:10.1093/epirev/mxv005
77. Iraq and Afghanistan Veterans of America. 7th annual IAVA member survey: the most comprehensive look into the lives of post-9/11. https://iava.org/wp-content/uploads/2020/02/IAVA-MemberSurvey-single-pgs1.pdf. Accessed August 18, 2020.
78. CARF International. CARF adds screening for suicide risk to its assessment standards. http://www.carf.org/universal-suicide-screening-standards. Published May 2, 2019. Accessed August 18, 2020.
79. Paul S. National Patient Safety Goal expands focus on suicide prevention. https://www.jointcommission.org/resources/news-and-multimedia/blogs/dateline-tjc/2019/01/national-patient-safety-goal-expands-focus-on-suicide-prevention/. Published January 24, 2019. Accessed August 18, 2020.
Papules on a child’s chest
Potassium hydroxide preparation of expressed contents revealed no molluscum bodies, but rather, small-caliber, trapped, and coiled hairs consistent with eruptive vellus hair cysts (EVHCs).
Vellus hairs are fine, light-colored (nonterminal) hairs that normally are found on the face, trunk, and limbs. EVHCs are typically asymptomatic papules containing an entrapped vellus hair. They form from entrapment of the hair follicle just proximal to the infundibulum, leading to hair bulb atrophy and dilation of the follicle. EVHCs typically are dome-shaped, flesh-colored soft papules that are 2 to 3 mm in size.
Diagnostic confusion can arise with molluscum contagiosum because those lesions also tend to be umbilicated and flesh colored. EVHCs can appear at any age but are more common in the first 3 decades of life. Familial types exist, presenting earlier in life than sporadic types. The differential diagnosis includes molluscum contagiosum, folliculitis, and steatocystoma multiplex. Rare genodermatoses have been associated with EVHCs, including autosomal dominant familial keratin gene mutation, anhidrotic and hidrotic ectodermal dysplasia, and pachyonychia congenita.
Up to 25% of cases spontaneously resolve, so patients can be counseled to observe the lesions. Alternative options including topical emollients or various topical keratolytics, including ammonium lactate 20% once to twice daily, topical urea, or topical salicylic acid until the lesions are flat and smooth. Topical and systemic retinoids have been reported to have limited success. Individual lesions of concern may be removed surgically by excision, curettage, cryotherapy, or laser but will likely leave a minor scar.
The patient and his family were reassured of the benign nature of the lesions and instructed to treat the papules with topical ammonium lactate 20% twice daily until the next appointment in 12 weeks. At follow-up, many, but not all, of the lesions had resolved.
Text courtesy of Tristan Reynolds, DO, Maine Dartmouth Family Medicine Residency, and Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
Torchia D, Vega J, Schachner LA. Eruptive vellus hair cysts: a systematic review. Am J Clin Dermatol. 2012;13:19-28.

Potassium hydroxide preparation of expressed contents revealed no molluscum bodies, but rather, small-caliber, trapped, and coiled hairs consistent with eruptive vellus hair cysts (EVHCs).
Vellus hairs are fine, light-colored (nonterminal) hairs that normally are found on the face, trunk, and limbs. EVHCs are typically asymptomatic papules containing an entrapped vellus hair. They form from entrapment of the hair follicle just proximal to the infundibulum, leading to hair bulb atrophy and dilation of the follicle. EVHCs typically are dome-shaped, flesh-colored soft papules that are 2 to 3 mm in size.
Diagnostic confusion can arise with molluscum contagiosum because those lesions also tend to be umbilicated and flesh colored. EVHCs can appear at any age but are more common in the first 3 decades of life. Familial types exist, presenting earlier in life than sporadic types. The differential diagnosis includes molluscum contagiosum, folliculitis, and steatocystoma multiplex. Rare genodermatoses have been associated with EVHCs, including autosomal dominant familial keratin gene mutation, anhidrotic and hidrotic ectodermal dysplasia, and pachyonychia congenita.
Up to 25% of cases spontaneously resolve, so patients can be counseled to observe the lesions. Alternative options including topical emollients or various topical keratolytics, including ammonium lactate 20% once to twice daily, topical urea, or topical salicylic acid until the lesions are flat and smooth. Topical and systemic retinoids have been reported to have limited success. Individual lesions of concern may be removed surgically by excision, curettage, cryotherapy, or laser but will likely leave a minor scar.
The patient and his family were reassured of the benign nature of the lesions and instructed to treat the papules with topical ammonium lactate 20% twice daily until the next appointment in 12 weeks. At follow-up, many, but not all, of the lesions had resolved.
Text courtesy of Tristan Reynolds, DO, Maine Dartmouth Family Medicine Residency, and Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

Potassium hydroxide preparation of expressed contents revealed no molluscum bodies, but rather, small-caliber, trapped, and coiled hairs consistent with eruptive vellus hair cysts (EVHCs).
Vellus hairs are fine, light-colored (nonterminal) hairs that normally are found on the face, trunk, and limbs. EVHCs are typically asymptomatic papules containing an entrapped vellus hair. They form from entrapment of the hair follicle just proximal to the infundibulum, leading to hair bulb atrophy and dilation of the follicle. EVHCs typically are dome-shaped, flesh-colored soft papules that are 2 to 3 mm in size.
Diagnostic confusion can arise with molluscum contagiosum because those lesions also tend to be umbilicated and flesh colored. EVHCs can appear at any age but are more common in the first 3 decades of life. Familial types exist, presenting earlier in life than sporadic types. The differential diagnosis includes molluscum contagiosum, folliculitis, and steatocystoma multiplex. Rare genodermatoses have been associated with EVHCs, including autosomal dominant familial keratin gene mutation, anhidrotic and hidrotic ectodermal dysplasia, and pachyonychia congenita.
Up to 25% of cases spontaneously resolve, so patients can be counseled to observe the lesions. Alternative options including topical emollients or various topical keratolytics, including ammonium lactate 20% once to twice daily, topical urea, or topical salicylic acid until the lesions are flat and smooth. Topical and systemic retinoids have been reported to have limited success. Individual lesions of concern may be removed surgically by excision, curettage, cryotherapy, or laser but will likely leave a minor scar.
The patient and his family were reassured of the benign nature of the lesions and instructed to treat the papules with topical ammonium lactate 20% twice daily until the next appointment in 12 weeks. At follow-up, many, but not all, of the lesions had resolved.
Text courtesy of Tristan Reynolds, DO, Maine Dartmouth Family Medicine Residency, and Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
Torchia D, Vega J, Schachner LA. Eruptive vellus hair cysts: a systematic review. Am J Clin Dermatol. 2012;13:19-28.
Torchia D, Vega J, Schachner LA. Eruptive vellus hair cysts: a systematic review. Am J Clin Dermatol. 2012;13:19-28.
High mortality rates reported in large COVID-19 study
Factors including older age and certain comorbidities have been linked to more serious COVID-19 outcomes in previous research, and now a large dataset collected from hundreds of hospitals nationwide provides more detailed data regarding risk for mechanical ventilation and death.
History of pulmonary disease or smoking, interestingly, were not.
One expert urges caution when interpreting the results, however. Although the study found a number of risk factors for ventilation and mortality, she says the dataset lacks information on race and disease severity, and the sample may not be nationally representative.
The investigators hope their level of granularity will further assist researchers searching for effective treatments and clinicians seeking to triage patients during the COVID-19 pandemic.
The study was published online August 28 in Clinical Infectious Diseases.
COVID-19 and comorbidities
“What I found most illuminating was this whole concept of comorbid conditions. This provides suggestive data about who we need to worry about most and who we may need to worry about less,” study author Robert S. Brown Jr, MD, MPH, told Medscape Medical News.
Comorbid conditions included hypertension in 47% of patients, diabetes in 28%, and cardiovascular disease in 19%. Another 16% were obese and 12% had chronic kidney disease. People with comorbid obesity, chronic kidney disease, and cardiovascular disease were more likely to receive mechanical ventilation compared to those without a history of these conditions in an adjusted, multivariable logistic analysis.
With the exception of obesity, the same factors were associated with risk for death during hospitalization.
In contrast, hypertension, history of smoking, and history of pulmonary disease were associated with a lower risk of needing mechanical ventilation and/or lower risk for mortality.
Furthermore, people with liver disease, gastrointestinal diseases, and even autoimmune diseases – which are likely associated with immunosuppression – “are not at that much of an increased risk that we noticed it in our data,” Brown said.
“As I tell many of my patients who have mild liver disease, for example, I would rather have mild liver disease and be on immunosuppressant therapy than be an older, obese male,” he added.
Assessing data for people in 38 U.S. states, and not limiting outcomes to patients in a particular COVID-19 hot spot, was a unique aspect of the research, said Brown, clinical chief of the Division of Gastroenterology and Hepatology at Weill Cornell Medicine in New York City.
Brown, lead author Michael W. Fried, MD, from TARGET PharmaSolutions in Durham, North Carolina, and colleagues studied adults from a commercially available Target Real-World Evidence (RWE) dataset of nearly 70,000 patients. They examined hospital chargemaster data and ICD-10 codes for COVID-19 inpatients between February 15 and April 20.
This population tended to be older, with 60% older than 60 years. A little more than half of participants, 53%, were men.
Key findings
A total of 21% of patients died after a median hospital length of stay of 8 days.
Older patients were significantly more likely to die, particularly those older than 60 years (P < .0001).
“This confirms some of the things we know about age and its impact on outcome,” Brown said.
The risk for mortality among patients older than 60 years was 7.2 times that of patients between 18 and 40 years in an adjusted multivariate analysis. The risk for death for those between 41 and 60 years of age was lower (odds ratio [OR], 2.6), compared with the youngest cohort.
Men were more likely to die than women (OR, 1.5).
When asked if he was surprised by the high mortality rates, Brown said, “Having worked here in New York? No, I was not.”
Mechanical ventilation and mortality
Male sex, age older than 40 years, obesity, and presence of cardiovascular or chronic kidney disease were risk factors for mechanical ventilation.
Among the nearly 2,000 hospitalized adults requiring mechanical ventilation in the current report, only 27% were discharged alive. “The outcomes of people who are mechanically ventilated are really quite sobering,” Brown said.
People who ever required mechanical ventilation were 32 times more likely to die compared with others whose highest level of oxygenation was low-flow, high-flow, or no-oxygen therapy in an analysis that controlled for demographics and comorbidities.
Furthermore, patients placed on mechanical ventilation earlier – within 24 hours of admission – tended to experience better outcomes.
COVID-19 therapies?
Brown and colleagues also evaluated outcomes in patients who were taking either remdesivir or hydroxychloroquine. A total of 48 people were treated with remdesivir.
The four individuals receiving remdesivir who died were among 11 who were taking remdesivir and also on mechanical ventilation.
“The data for remdesivir is very encouraging,” Brown said.
Many more participants were treated with hydroxychloroquine, more than 4,200 or 36% of the total study population.
A higher proportion of people treated with hydroxychloroquine received mechanical ventilation, at 25%, versus 12% not treated with hydroxychloroquine.
The unadjusted mortality rate was also higher among those treated with the agent, at 25%, compared to 20% not receiving hydroxychloroquine.
The data with hydroxychloroquine can lead to two conclusions, Brown said: “One, it doesn’t work. Or two, it doesn’t work in the way that we use it.”
The researchers cautioned that their hydroxychloroquine findings must be interpreted carefully because those treated with the agent were also more likely to have comorbidities and greater COVID-19 disease severity.
“This study greatly contributes to understanding the natural course of COVID-19 infection by describing characteristics and outcomes of patients with COVID-19 hospitalized throughout the US,” the investigators note. “It identified categories of patients at greatest risk for poor outcomes, which should be used to prioritize prevention and treatment strategies in the future.”
Some limitations
“The findings that patients with hypertension and who were smokers had lower ventilation rates, and patients with hypertension, pulmonary disease, who were smokers had lower mortality risks was very surprising,” Ninez A. Ponce, PhD, MPP, told Medscape Medical News when asked to comment on the study.
Although the study identified multiple risk factors for ventilation and mortality, “unfortunately the dataset did not have race available or disease severity,” said Ponce, director of the UCLA Center for Health Policy Research and professor in the Department of Health Policy and Management at the UCLA Fielding School of Public Health.
“These omitted variables could have a considerable effect on the significance, magnitude, and direction of point estimates provided, so I would be cautious in interpreting the results as a picture of a nationally representative sample,” she said.
On a positive note, the study and dataset could illuminate the utility of medications used to treat COVID-19, Ponce said. In addition, as the authors note, “the data will expand over time.”
Brown has reported receiving grants and consulting for Gilead. Ponce has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Factors including older age and certain comorbidities have been linked to more serious COVID-19 outcomes in previous research, and now a large dataset collected from hundreds of hospitals nationwide provides more detailed data regarding risk for mechanical ventilation and death.
History of pulmonary disease or smoking, interestingly, were not.
One expert urges caution when interpreting the results, however. Although the study found a number of risk factors for ventilation and mortality, she says the dataset lacks information on race and disease severity, and the sample may not be nationally representative.
The investigators hope their level of granularity will further assist researchers searching for effective treatments and clinicians seeking to triage patients during the COVID-19 pandemic.
The study was published online August 28 in Clinical Infectious Diseases.
COVID-19 and comorbidities
“What I found most illuminating was this whole concept of comorbid conditions. This provides suggestive data about who we need to worry about most and who we may need to worry about less,” study author Robert S. Brown Jr, MD, MPH, told Medscape Medical News.
Comorbid conditions included hypertension in 47% of patients, diabetes in 28%, and cardiovascular disease in 19%. Another 16% were obese and 12% had chronic kidney disease. People with comorbid obesity, chronic kidney disease, and cardiovascular disease were more likely to receive mechanical ventilation compared to those without a history of these conditions in an adjusted, multivariable logistic analysis.
With the exception of obesity, the same factors were associated with risk for death during hospitalization.
In contrast, hypertension, history of smoking, and history of pulmonary disease were associated with a lower risk of needing mechanical ventilation and/or lower risk for mortality.
Furthermore, people with liver disease, gastrointestinal diseases, and even autoimmune diseases – which are likely associated with immunosuppression – “are not at that much of an increased risk that we noticed it in our data,” Brown said.
“As I tell many of my patients who have mild liver disease, for example, I would rather have mild liver disease and be on immunosuppressant therapy than be an older, obese male,” he added.
Assessing data for people in 38 U.S. states, and not limiting outcomes to patients in a particular COVID-19 hot spot, was a unique aspect of the research, said Brown, clinical chief of the Division of Gastroenterology and Hepatology at Weill Cornell Medicine in New York City.
Brown, lead author Michael W. Fried, MD, from TARGET PharmaSolutions in Durham, North Carolina, and colleagues studied adults from a commercially available Target Real-World Evidence (RWE) dataset of nearly 70,000 patients. They examined hospital chargemaster data and ICD-10 codes for COVID-19 inpatients between February 15 and April 20.
This population tended to be older, with 60% older than 60 years. A little more than half of participants, 53%, were men.
Key findings
A total of 21% of patients died after a median hospital length of stay of 8 days.
Older patients were significantly more likely to die, particularly those older than 60 years (P < .0001).
“This confirms some of the things we know about age and its impact on outcome,” Brown said.
The risk for mortality among patients older than 60 years was 7.2 times that of patients between 18 and 40 years in an adjusted multivariate analysis. The risk for death for those between 41 and 60 years of age was lower (odds ratio [OR], 2.6), compared with the youngest cohort.
Men were more likely to die than women (OR, 1.5).
When asked if he was surprised by the high mortality rates, Brown said, “Having worked here in New York? No, I was not.”
Mechanical ventilation and mortality
Male sex, age older than 40 years, obesity, and presence of cardiovascular or chronic kidney disease were risk factors for mechanical ventilation.
Among the nearly 2,000 hospitalized adults requiring mechanical ventilation in the current report, only 27% were discharged alive. “The outcomes of people who are mechanically ventilated are really quite sobering,” Brown said.
People who ever required mechanical ventilation were 32 times more likely to die compared with others whose highest level of oxygenation was low-flow, high-flow, or no-oxygen therapy in an analysis that controlled for demographics and comorbidities.
Furthermore, patients placed on mechanical ventilation earlier – within 24 hours of admission – tended to experience better outcomes.
COVID-19 therapies?
Brown and colleagues also evaluated outcomes in patients who were taking either remdesivir or hydroxychloroquine. A total of 48 people were treated with remdesivir.
The four individuals receiving remdesivir who died were among 11 who were taking remdesivir and also on mechanical ventilation.
“The data for remdesivir is very encouraging,” Brown said.
Many more participants were treated with hydroxychloroquine, more than 4,200 or 36% of the total study population.
A higher proportion of people treated with hydroxychloroquine received mechanical ventilation, at 25%, versus 12% not treated with hydroxychloroquine.
The unadjusted mortality rate was also higher among those treated with the agent, at 25%, compared to 20% not receiving hydroxychloroquine.
The data with hydroxychloroquine can lead to two conclusions, Brown said: “One, it doesn’t work. Or two, it doesn’t work in the way that we use it.”
The researchers cautioned that their hydroxychloroquine findings must be interpreted carefully because those treated with the agent were also more likely to have comorbidities and greater COVID-19 disease severity.
“This study greatly contributes to understanding the natural course of COVID-19 infection by describing characteristics and outcomes of patients with COVID-19 hospitalized throughout the US,” the investigators note. “It identified categories of patients at greatest risk for poor outcomes, which should be used to prioritize prevention and treatment strategies in the future.”
Some limitations
“The findings that patients with hypertension and who were smokers had lower ventilation rates, and patients with hypertension, pulmonary disease, who were smokers had lower mortality risks was very surprising,” Ninez A. Ponce, PhD, MPP, told Medscape Medical News when asked to comment on the study.
Although the study identified multiple risk factors for ventilation and mortality, “unfortunately the dataset did not have race available or disease severity,” said Ponce, director of the UCLA Center for Health Policy Research and professor in the Department of Health Policy and Management at the UCLA Fielding School of Public Health.
“These omitted variables could have a considerable effect on the significance, magnitude, and direction of point estimates provided, so I would be cautious in interpreting the results as a picture of a nationally representative sample,” she said.
On a positive note, the study and dataset could illuminate the utility of medications used to treat COVID-19, Ponce said. In addition, as the authors note, “the data will expand over time.”
Brown has reported receiving grants and consulting for Gilead. Ponce has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Factors including older age and certain comorbidities have been linked to more serious COVID-19 outcomes in previous research, and now a large dataset collected from hundreds of hospitals nationwide provides more detailed data regarding risk for mechanical ventilation and death.
History of pulmonary disease or smoking, interestingly, were not.
One expert urges caution when interpreting the results, however. Although the study found a number of risk factors for ventilation and mortality, she says the dataset lacks information on race and disease severity, and the sample may not be nationally representative.
The investigators hope their level of granularity will further assist researchers searching for effective treatments and clinicians seeking to triage patients during the COVID-19 pandemic.
The study was published online August 28 in Clinical Infectious Diseases.
COVID-19 and comorbidities
“What I found most illuminating was this whole concept of comorbid conditions. This provides suggestive data about who we need to worry about most and who we may need to worry about less,” study author Robert S. Brown Jr, MD, MPH, told Medscape Medical News.
Comorbid conditions included hypertension in 47% of patients, diabetes in 28%, and cardiovascular disease in 19%. Another 16% were obese and 12% had chronic kidney disease. People with comorbid obesity, chronic kidney disease, and cardiovascular disease were more likely to receive mechanical ventilation compared to those without a history of these conditions in an adjusted, multivariable logistic analysis.
With the exception of obesity, the same factors were associated with risk for death during hospitalization.
In contrast, hypertension, history of smoking, and history of pulmonary disease were associated with a lower risk of needing mechanical ventilation and/or lower risk for mortality.
Furthermore, people with liver disease, gastrointestinal diseases, and even autoimmune diseases – which are likely associated with immunosuppression – “are not at that much of an increased risk that we noticed it in our data,” Brown said.
“As I tell many of my patients who have mild liver disease, for example, I would rather have mild liver disease and be on immunosuppressant therapy than be an older, obese male,” he added.
Assessing data for people in 38 U.S. states, and not limiting outcomes to patients in a particular COVID-19 hot spot, was a unique aspect of the research, said Brown, clinical chief of the Division of Gastroenterology and Hepatology at Weill Cornell Medicine in New York City.
Brown, lead author Michael W. Fried, MD, from TARGET PharmaSolutions in Durham, North Carolina, and colleagues studied adults from a commercially available Target Real-World Evidence (RWE) dataset of nearly 70,000 patients. They examined hospital chargemaster data and ICD-10 codes for COVID-19 inpatients between February 15 and April 20.
This population tended to be older, with 60% older than 60 years. A little more than half of participants, 53%, were men.
Key findings
A total of 21% of patients died after a median hospital length of stay of 8 days.
Older patients were significantly more likely to die, particularly those older than 60 years (P < .0001).
“This confirms some of the things we know about age and its impact on outcome,” Brown said.
The risk for mortality among patients older than 60 years was 7.2 times that of patients between 18 and 40 years in an adjusted multivariate analysis. The risk for death for those between 41 and 60 years of age was lower (odds ratio [OR], 2.6), compared with the youngest cohort.
Men were more likely to die than women (OR, 1.5).
When asked if he was surprised by the high mortality rates, Brown said, “Having worked here in New York? No, I was not.”
Mechanical ventilation and mortality
Male sex, age older than 40 years, obesity, and presence of cardiovascular or chronic kidney disease were risk factors for mechanical ventilation.
Among the nearly 2,000 hospitalized adults requiring mechanical ventilation in the current report, only 27% were discharged alive. “The outcomes of people who are mechanically ventilated are really quite sobering,” Brown said.
People who ever required mechanical ventilation were 32 times more likely to die compared with others whose highest level of oxygenation was low-flow, high-flow, or no-oxygen therapy in an analysis that controlled for demographics and comorbidities.
Furthermore, patients placed on mechanical ventilation earlier – within 24 hours of admission – tended to experience better outcomes.
COVID-19 therapies?
Brown and colleagues also evaluated outcomes in patients who were taking either remdesivir or hydroxychloroquine. A total of 48 people were treated with remdesivir.
The four individuals receiving remdesivir who died were among 11 who were taking remdesivir and also on mechanical ventilation.
“The data for remdesivir is very encouraging,” Brown said.
Many more participants were treated with hydroxychloroquine, more than 4,200 or 36% of the total study population.
A higher proportion of people treated with hydroxychloroquine received mechanical ventilation, at 25%, versus 12% not treated with hydroxychloroquine.
The unadjusted mortality rate was also higher among those treated with the agent, at 25%, compared to 20% not receiving hydroxychloroquine.
The data with hydroxychloroquine can lead to two conclusions, Brown said: “One, it doesn’t work. Or two, it doesn’t work in the way that we use it.”
The researchers cautioned that their hydroxychloroquine findings must be interpreted carefully because those treated with the agent were also more likely to have comorbidities and greater COVID-19 disease severity.
“This study greatly contributes to understanding the natural course of COVID-19 infection by describing characteristics and outcomes of patients with COVID-19 hospitalized throughout the US,” the investigators note. “It identified categories of patients at greatest risk for poor outcomes, which should be used to prioritize prevention and treatment strategies in the future.”
Some limitations
“The findings that patients with hypertension and who were smokers had lower ventilation rates, and patients with hypertension, pulmonary disease, who were smokers had lower mortality risks was very surprising,” Ninez A. Ponce, PhD, MPP, told Medscape Medical News when asked to comment on the study.
Although the study identified multiple risk factors for ventilation and mortality, “unfortunately the dataset did not have race available or disease severity,” said Ponce, director of the UCLA Center for Health Policy Research and professor in the Department of Health Policy and Management at the UCLA Fielding School of Public Health.
“These omitted variables could have a considerable effect on the significance, magnitude, and direction of point estimates provided, so I would be cautious in interpreting the results as a picture of a nationally representative sample,” she said.
On a positive note, the study and dataset could illuminate the utility of medications used to treat COVID-19, Ponce said. In addition, as the authors note, “the data will expand over time.”
Brown has reported receiving grants and consulting for Gilead. Ponce has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Nightmares: An independent risk factor for heart disease?
, new research shows. In what researchers describe as “surprising” findings, results from a large study of relatively young military veterans showed those who had nightmares two or more times per week had significantly increased risks for hypertension, myocardial infarction, or other heart problems.
“A diagnosis of PTSD incorporates sleep disturbance as a symptom. Thus, we were surprised to find that nightmares continued to be associated with CVD after controlling not only for PTSD and demographic factors, but also smoking and depression diagnosis,” said Christi Ulmer, PhD, of the department of psychiatry and behavioral sciences, Duke University Medical Center, Durham, N.C.
The findings were presented at the virtual annual meeting of the Associated Professional Sleep Societies.
Unclear mechanism
The study included 3,468 veterans (77% male) with a mean age of 38 years who had served one or two tours of duty since Sept. 11, 2001. Nearly one-third (31%) met criteria for PTSD, and 33% self-reported having at least one cardiovascular condition, such as heart problems, hypertension, stroke, and MI.
Nightmare frequency and severity was assessed using the Davidson Trauma Scale. Nightmares were considered frequent if they occurred two or more times per week and moderate to severe if they were at least moderately distressing. About 31% of veterans reported having frequent nightmares, and 35% reported moderately distressing nightmares over the past week.
After adjusting for age, race, and sex, frequent nightmares were associated with hypertension (odds ratio, 1.51; 95% confidence interval, 1.28-1.78), heart problems (OR, 1.50; 95% CI, 1.11-2.02), and MI (OR, 2.32; 95% CI, 1.18-4.54).
Associations between frequent nightmares and hypertension (OR, 1.43; 95% CI, 1.17-1.73) and heart problems (OR, 1.43; 95% CI, 1.00-2.05) remained significant after further adjusting for smoking, depression, and PTSD.
“Our cross-sectional findings set the stage for future research examining the possibility that nightmares may confer cardiovascular disease risks beyond those conferred by PTSD diagnosis alone,” Dr. Ulmer said in a news release.
Dr. Ulmer also said that, because the study was based on self-reported data, the findings are “very preliminary.” Before doctors adjust clinical practices, it’s important that our findings be replicated using longitudinal studies, clinically diagnosed medical conditions, and objectively assessed sleep,” she said.
She added that more research is needed to uncover mechanisms explaining these associations and determine if reducing the frequency and severity of nightmares can lead to improved cardiovascular health.
Timely research
Reached for comment, Rajkumar (Raj) Dasgupta, MD, of the University of Southern California, Los Angeles, noted “the correlation between nightmares and heart disease is a timely topic right now with COVID-19 as more people may be having nightmares.”
“If a patient mentions nightmares, I do think it’s important not to just glaze over it, but to talk more about it and document it in the patient record, especially in patients with cardiovascular disease, atrial fibrillation, diabetes, and hypertension,” said Dr. Dasgupta, who wasn’t involved in the study.
The research was supported by the Veterans Integrated Service Network 6 Mental Illness Research, Education and Clinical Center and the Department of Veterans Affairs HSR&D ADAPT Center at the Durham VA Health Care System. Dr. Ulmer and Dr. Dasgupta have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, new research shows. In what researchers describe as “surprising” findings, results from a large study of relatively young military veterans showed those who had nightmares two or more times per week had significantly increased risks for hypertension, myocardial infarction, or other heart problems.
“A diagnosis of PTSD incorporates sleep disturbance as a symptom. Thus, we were surprised to find that nightmares continued to be associated with CVD after controlling not only for PTSD and demographic factors, but also smoking and depression diagnosis,” said Christi Ulmer, PhD, of the department of psychiatry and behavioral sciences, Duke University Medical Center, Durham, N.C.
The findings were presented at the virtual annual meeting of the Associated Professional Sleep Societies.
Unclear mechanism
The study included 3,468 veterans (77% male) with a mean age of 38 years who had served one or two tours of duty since Sept. 11, 2001. Nearly one-third (31%) met criteria for PTSD, and 33% self-reported having at least one cardiovascular condition, such as heart problems, hypertension, stroke, and MI.
Nightmare frequency and severity was assessed using the Davidson Trauma Scale. Nightmares were considered frequent if they occurred two or more times per week and moderate to severe if they were at least moderately distressing. About 31% of veterans reported having frequent nightmares, and 35% reported moderately distressing nightmares over the past week.
After adjusting for age, race, and sex, frequent nightmares were associated with hypertension (odds ratio, 1.51; 95% confidence interval, 1.28-1.78), heart problems (OR, 1.50; 95% CI, 1.11-2.02), and MI (OR, 2.32; 95% CI, 1.18-4.54).
Associations between frequent nightmares and hypertension (OR, 1.43; 95% CI, 1.17-1.73) and heart problems (OR, 1.43; 95% CI, 1.00-2.05) remained significant after further adjusting for smoking, depression, and PTSD.
“Our cross-sectional findings set the stage for future research examining the possibility that nightmares may confer cardiovascular disease risks beyond those conferred by PTSD diagnosis alone,” Dr. Ulmer said in a news release.
Dr. Ulmer also said that, because the study was based on self-reported data, the findings are “very preliminary.” Before doctors adjust clinical practices, it’s important that our findings be replicated using longitudinal studies, clinically diagnosed medical conditions, and objectively assessed sleep,” she said.
She added that more research is needed to uncover mechanisms explaining these associations and determine if reducing the frequency and severity of nightmares can lead to improved cardiovascular health.
Timely research
Reached for comment, Rajkumar (Raj) Dasgupta, MD, of the University of Southern California, Los Angeles, noted “the correlation between nightmares and heart disease is a timely topic right now with COVID-19 as more people may be having nightmares.”
“If a patient mentions nightmares, I do think it’s important not to just glaze over it, but to talk more about it and document it in the patient record, especially in patients with cardiovascular disease, atrial fibrillation, diabetes, and hypertension,” said Dr. Dasgupta, who wasn’t involved in the study.
The research was supported by the Veterans Integrated Service Network 6 Mental Illness Research, Education and Clinical Center and the Department of Veterans Affairs HSR&D ADAPT Center at the Durham VA Health Care System. Dr. Ulmer and Dr. Dasgupta have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, new research shows. In what researchers describe as “surprising” findings, results from a large study of relatively young military veterans showed those who had nightmares two or more times per week had significantly increased risks for hypertension, myocardial infarction, or other heart problems.
“A diagnosis of PTSD incorporates sleep disturbance as a symptom. Thus, we were surprised to find that nightmares continued to be associated with CVD after controlling not only for PTSD and demographic factors, but also smoking and depression diagnosis,” said Christi Ulmer, PhD, of the department of psychiatry and behavioral sciences, Duke University Medical Center, Durham, N.C.
The findings were presented at the virtual annual meeting of the Associated Professional Sleep Societies.
Unclear mechanism
The study included 3,468 veterans (77% male) with a mean age of 38 years who had served one or two tours of duty since Sept. 11, 2001. Nearly one-third (31%) met criteria for PTSD, and 33% self-reported having at least one cardiovascular condition, such as heart problems, hypertension, stroke, and MI.
Nightmare frequency and severity was assessed using the Davidson Trauma Scale. Nightmares were considered frequent if they occurred two or more times per week and moderate to severe if they were at least moderately distressing. About 31% of veterans reported having frequent nightmares, and 35% reported moderately distressing nightmares over the past week.
After adjusting for age, race, and sex, frequent nightmares were associated with hypertension (odds ratio, 1.51; 95% confidence interval, 1.28-1.78), heart problems (OR, 1.50; 95% CI, 1.11-2.02), and MI (OR, 2.32; 95% CI, 1.18-4.54).
Associations between frequent nightmares and hypertension (OR, 1.43; 95% CI, 1.17-1.73) and heart problems (OR, 1.43; 95% CI, 1.00-2.05) remained significant after further adjusting for smoking, depression, and PTSD.
“Our cross-sectional findings set the stage for future research examining the possibility that nightmares may confer cardiovascular disease risks beyond those conferred by PTSD diagnosis alone,” Dr. Ulmer said in a news release.
Dr. Ulmer also said that, because the study was based on self-reported data, the findings are “very preliminary.” Before doctors adjust clinical practices, it’s important that our findings be replicated using longitudinal studies, clinically diagnosed medical conditions, and objectively assessed sleep,” she said.
She added that more research is needed to uncover mechanisms explaining these associations and determine if reducing the frequency and severity of nightmares can lead to improved cardiovascular health.
Timely research
Reached for comment, Rajkumar (Raj) Dasgupta, MD, of the University of Southern California, Los Angeles, noted “the correlation between nightmares and heart disease is a timely topic right now with COVID-19 as more people may be having nightmares.”
“If a patient mentions nightmares, I do think it’s important not to just glaze over it, but to talk more about it and document it in the patient record, especially in patients with cardiovascular disease, atrial fibrillation, diabetes, and hypertension,” said Dr. Dasgupta, who wasn’t involved in the study.
The research was supported by the Veterans Integrated Service Network 6 Mental Illness Research, Education and Clinical Center and the Department of Veterans Affairs HSR&D ADAPT Center at the Durham VA Health Care System. Dr. Ulmer and Dr. Dasgupta have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM SLEEP 2020
Fatal pediatric melanomas diverse in presentation
results of a retrospective multicenter study showed.
“The most striking thing that we learned from this study is that pediatric melanoma can present in so many different ways, and it’s distinct from the adult population in that we see more presentations associated with congenital nevi, or spitz melanoma, which is a special class of pigmented lesions that looks a little different under the microscope,” Elena B. Hawryluk, MD, PhD, of the department of dermatology at Massachusetts General Hospital (MGH) and Harvard University, Boston, said in an interview. Dr. Hawryluk is lead author of the study, which was published online ahead of print in the Journal of the American Academy of Dermatology.
Dr. Hawryluk and colleagues at MGH and 11 other centers conducted a retrospective review of all cases of fatal pediatric melanoma among patients younger than 20 years diagnosed from late 1994 through early 2017.
They identified a total of 38 fatal cases over more than 2 decades. The cases were distinguished primarily by their heterogeneous clinical presentation and by the diversity of the patients, their precursor lesions, and the tumor histopathology, she said in an interview.
“We were surprised to find that patients with each of these presentations could end up with a fatal course, it wasn’t just all the adolescents, or all the patients with giant congenital nevi; it really presented quite diversely.”
Rare malignancy
Melanoma is far less common in the pediatric population than in adults, with an annual incidence of 18 per 1 million among adolescents aged 15-18 years, and 1 per 1 million in children under 10 years, the authors noted.
“Melanoma in children and adolescents often has distinct clinical presentations such as association with a congenital melanocytic nevus (CMN), spitzoid melanoma, or amelanotic melanoma, which are more rarely observed in adult melanoma patients. Unique pediatric-specific clinical detection criteria have been proposed to highlight these differences, such as a tendency to present amelanotically,” they wrote.
Factors associated with worse prognosis, such as higher Breslow thickness and mitotic index, are more frequently present at the time of diagnosis in children compared with adults, particularly those diagnosed before age 11 years.
“It is unclear if this difference is secondary to diagnostic delays due to low clinical suspicion, atypical clinical presentations, or more rapid tumor growth rate, as many childhood melanomas are of nodular or spitzoid subtypes,” Dr. Hawryluk and her coauthors wrote.
Study details
The investigators sought to characterize the clinical and histopathologic features of fatal pediatric melanomas.
They found that 21 of the 38 patients (57%) were of White heritage, 7 (19%) were of Hispanic or Latino background, 1 (3%) was of Asian lineage, and 1 each were of Black African American or Black Hispanic background. The remaining children were classified as “other” or did not have their ethnic backgrounds recorded.
The “striking prevalence” of Hispanic patients observed in the study is consistent with surveillance reports of an increasing incidence of melanoma among children of Hispanic background, they noted.
The mean age at diagnosis was 12.7 years, and the mean age at death was 15.6 years.
Of the 16 cases with known identifiable disease subtypes, 8 (50%) were nodular, 5 (31%) were superficial spreading, and 3 (19%) were spitzoid melanomas. Of the 38 fatal melanomas, 10 were thought to have originated from congenital melanocytic nevi.
Outlook improving
Recent therapeutic breakthroughs such as targeted agents and immunotherapy with checkpoint inhibitors augur well for children diagnosed with melanoma, Dr. Hawryluk said.
“Fortunately, it’s not superaggressive in children at high frequency, so we generally use adult algorithms to inform treatment decisions,” she said. “It’s just important to note that melanomas that arise in congenital nevi tend to have different driver mutations than those that arise in older patients who may have lots of sun exposure.”
“Nowadays, we’re lucky to have a lot of extra tests and workups so that, if a patient does have metastatic or advance disease, they can have a better genetic profile that would guide our choice of medications,” she added.
The study was supported by a Pediatric Dermatology Research Alliance Study Support grant and Society for Pediatric Dermatology, Pediatric Dermatology Research Alliance Pilot award. Dr. Hawryluk is supported by the Dermatology Foundation and the Harvard Medical School Eleanor and Miles Shore Fellowship award. The authors reported no conflicts of interest.
SOURCE: Hawryluk EB et al. J Am Acad Dermatol. 2020 Jul 1. doi: 10.1016/j.jaad.2020.06.1010.
results of a retrospective multicenter study showed.
“The most striking thing that we learned from this study is that pediatric melanoma can present in so many different ways, and it’s distinct from the adult population in that we see more presentations associated with congenital nevi, or spitz melanoma, which is a special class of pigmented lesions that looks a little different under the microscope,” Elena B. Hawryluk, MD, PhD, of the department of dermatology at Massachusetts General Hospital (MGH) and Harvard University, Boston, said in an interview. Dr. Hawryluk is lead author of the study, which was published online ahead of print in the Journal of the American Academy of Dermatology.
Dr. Hawryluk and colleagues at MGH and 11 other centers conducted a retrospective review of all cases of fatal pediatric melanoma among patients younger than 20 years diagnosed from late 1994 through early 2017.
They identified a total of 38 fatal cases over more than 2 decades. The cases were distinguished primarily by their heterogeneous clinical presentation and by the diversity of the patients, their precursor lesions, and the tumor histopathology, she said in an interview.
“We were surprised to find that patients with each of these presentations could end up with a fatal course, it wasn’t just all the adolescents, or all the patients with giant congenital nevi; it really presented quite diversely.”
Rare malignancy
Melanoma is far less common in the pediatric population than in adults, with an annual incidence of 18 per 1 million among adolescents aged 15-18 years, and 1 per 1 million in children under 10 years, the authors noted.
“Melanoma in children and adolescents often has distinct clinical presentations such as association with a congenital melanocytic nevus (CMN), spitzoid melanoma, or amelanotic melanoma, which are more rarely observed in adult melanoma patients. Unique pediatric-specific clinical detection criteria have been proposed to highlight these differences, such as a tendency to present amelanotically,” they wrote.
Factors associated with worse prognosis, such as higher Breslow thickness and mitotic index, are more frequently present at the time of diagnosis in children compared with adults, particularly those diagnosed before age 11 years.
“It is unclear if this difference is secondary to diagnostic delays due to low clinical suspicion, atypical clinical presentations, or more rapid tumor growth rate, as many childhood melanomas are of nodular or spitzoid subtypes,” Dr. Hawryluk and her coauthors wrote.
Study details
The investigators sought to characterize the clinical and histopathologic features of fatal pediatric melanomas.
They found that 21 of the 38 patients (57%) were of White heritage, 7 (19%) were of Hispanic or Latino background, 1 (3%) was of Asian lineage, and 1 each were of Black African American or Black Hispanic background. The remaining children were classified as “other” or did not have their ethnic backgrounds recorded.
The “striking prevalence” of Hispanic patients observed in the study is consistent with surveillance reports of an increasing incidence of melanoma among children of Hispanic background, they noted.
The mean age at diagnosis was 12.7 years, and the mean age at death was 15.6 years.
Of the 16 cases with known identifiable disease subtypes, 8 (50%) were nodular, 5 (31%) were superficial spreading, and 3 (19%) were spitzoid melanomas. Of the 38 fatal melanomas, 10 were thought to have originated from congenital melanocytic nevi.
Outlook improving
Recent therapeutic breakthroughs such as targeted agents and immunotherapy with checkpoint inhibitors augur well for children diagnosed with melanoma, Dr. Hawryluk said.
“Fortunately, it’s not superaggressive in children at high frequency, so we generally use adult algorithms to inform treatment decisions,” she said. “It’s just important to note that melanomas that arise in congenital nevi tend to have different driver mutations than those that arise in older patients who may have lots of sun exposure.”
“Nowadays, we’re lucky to have a lot of extra tests and workups so that, if a patient does have metastatic or advance disease, they can have a better genetic profile that would guide our choice of medications,” she added.
The study was supported by a Pediatric Dermatology Research Alliance Study Support grant and Society for Pediatric Dermatology, Pediatric Dermatology Research Alliance Pilot award. Dr. Hawryluk is supported by the Dermatology Foundation and the Harvard Medical School Eleanor and Miles Shore Fellowship award. The authors reported no conflicts of interest.
SOURCE: Hawryluk EB et al. J Am Acad Dermatol. 2020 Jul 1. doi: 10.1016/j.jaad.2020.06.1010.
results of a retrospective multicenter study showed.
“The most striking thing that we learned from this study is that pediatric melanoma can present in so many different ways, and it’s distinct from the adult population in that we see more presentations associated with congenital nevi, or spitz melanoma, which is a special class of pigmented lesions that looks a little different under the microscope,” Elena B. Hawryluk, MD, PhD, of the department of dermatology at Massachusetts General Hospital (MGH) and Harvard University, Boston, said in an interview. Dr. Hawryluk is lead author of the study, which was published online ahead of print in the Journal of the American Academy of Dermatology.
Dr. Hawryluk and colleagues at MGH and 11 other centers conducted a retrospective review of all cases of fatal pediatric melanoma among patients younger than 20 years diagnosed from late 1994 through early 2017.
They identified a total of 38 fatal cases over more than 2 decades. The cases were distinguished primarily by their heterogeneous clinical presentation and by the diversity of the patients, their precursor lesions, and the tumor histopathology, she said in an interview.
“We were surprised to find that patients with each of these presentations could end up with a fatal course, it wasn’t just all the adolescents, or all the patients with giant congenital nevi; it really presented quite diversely.”
Rare malignancy
Melanoma is far less common in the pediatric population than in adults, with an annual incidence of 18 per 1 million among adolescents aged 15-18 years, and 1 per 1 million in children under 10 years, the authors noted.
“Melanoma in children and adolescents often has distinct clinical presentations such as association with a congenital melanocytic nevus (CMN), spitzoid melanoma, or amelanotic melanoma, which are more rarely observed in adult melanoma patients. Unique pediatric-specific clinical detection criteria have been proposed to highlight these differences, such as a tendency to present amelanotically,” they wrote.
Factors associated with worse prognosis, such as higher Breslow thickness and mitotic index, are more frequently present at the time of diagnosis in children compared with adults, particularly those diagnosed before age 11 years.
“It is unclear if this difference is secondary to diagnostic delays due to low clinical suspicion, atypical clinical presentations, or more rapid tumor growth rate, as many childhood melanomas are of nodular or spitzoid subtypes,” Dr. Hawryluk and her coauthors wrote.
Study details
The investigators sought to characterize the clinical and histopathologic features of fatal pediatric melanomas.
They found that 21 of the 38 patients (57%) were of White heritage, 7 (19%) were of Hispanic or Latino background, 1 (3%) was of Asian lineage, and 1 each were of Black African American or Black Hispanic background. The remaining children were classified as “other” or did not have their ethnic backgrounds recorded.
The “striking prevalence” of Hispanic patients observed in the study is consistent with surveillance reports of an increasing incidence of melanoma among children of Hispanic background, they noted.
The mean age at diagnosis was 12.7 years, and the mean age at death was 15.6 years.
Of the 16 cases with known identifiable disease subtypes, 8 (50%) were nodular, 5 (31%) were superficial spreading, and 3 (19%) were spitzoid melanomas. Of the 38 fatal melanomas, 10 were thought to have originated from congenital melanocytic nevi.
Outlook improving
Recent therapeutic breakthroughs such as targeted agents and immunotherapy with checkpoint inhibitors augur well for children diagnosed with melanoma, Dr. Hawryluk said.
“Fortunately, it’s not superaggressive in children at high frequency, so we generally use adult algorithms to inform treatment decisions,” she said. “It’s just important to note that melanomas that arise in congenital nevi tend to have different driver mutations than those that arise in older patients who may have lots of sun exposure.”
“Nowadays, we’re lucky to have a lot of extra tests and workups so that, if a patient does have metastatic or advance disease, they can have a better genetic profile that would guide our choice of medications,” she added.
The study was supported by a Pediatric Dermatology Research Alliance Study Support grant and Society for Pediatric Dermatology, Pediatric Dermatology Research Alliance Pilot award. Dr. Hawryluk is supported by the Dermatology Foundation and the Harvard Medical School Eleanor and Miles Shore Fellowship award. The authors reported no conflicts of interest.
SOURCE: Hawryluk EB et al. J Am Acad Dermatol. 2020 Jul 1. doi: 10.1016/j.jaad.2020.06.1010.
FROM JAAD