User login
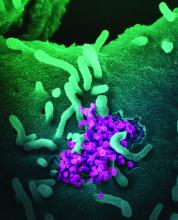
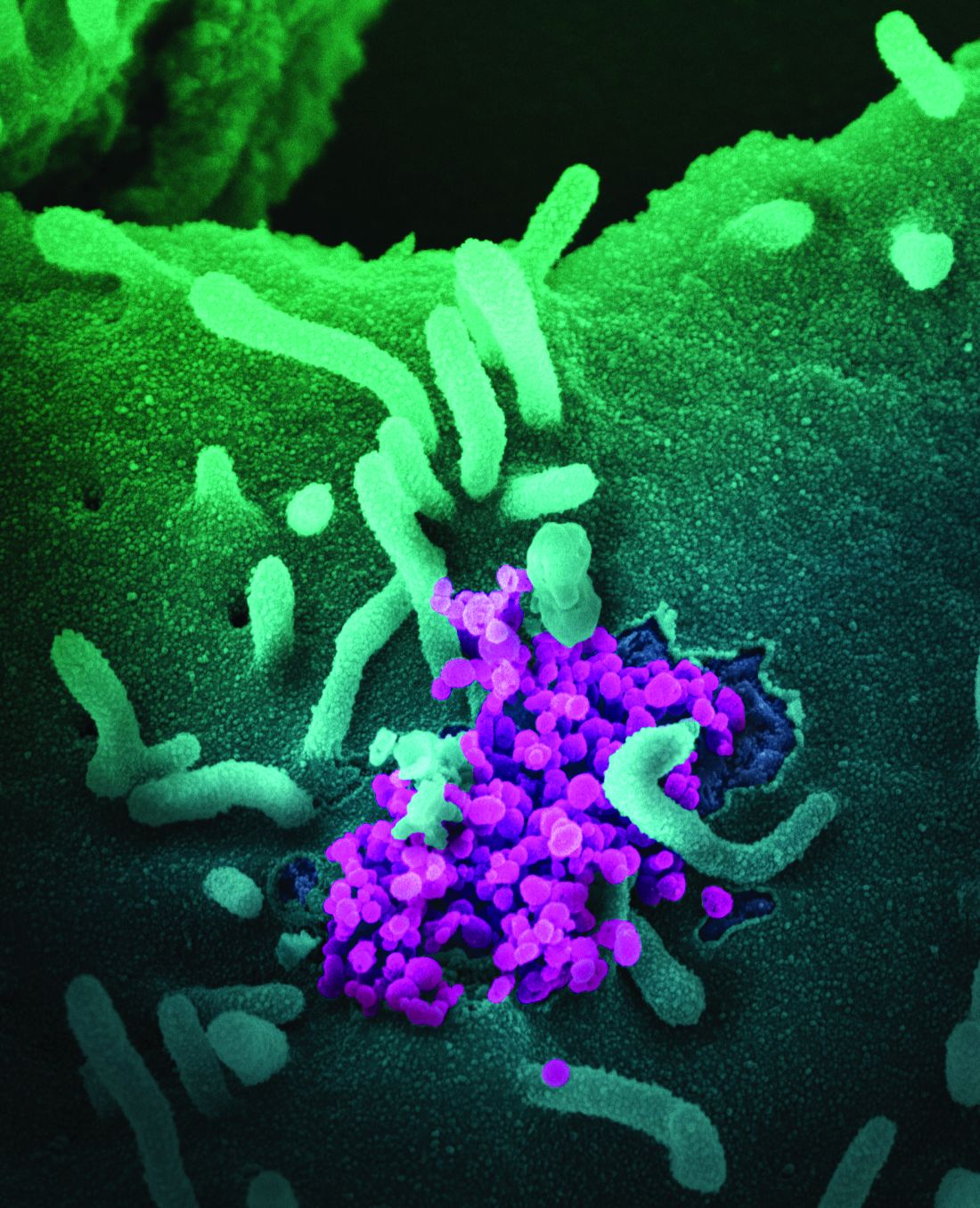
Asymptomatic SARS-CoV-2 infections in kids tied to local rates
As communities wrestle with the decision to send children back to school or opt for distance learning, a key question is how many children are likely to have asymptomatic SARS-CoV-2 infections.
“The strong association between prevalence of SARS-CoV-2 in children who are asymptomatic and contemporaneous weekly incidence of COVID-19 in the general population ... provides a simple means for institutions to estimate local pediatric asymptomatic prevalence from the publicly available Johns Hopkins University database,” researchers say in an article published online August 25 in JAMA Pediatrics.
Ana Marija Sola, BS, a researcher at the University of California, San Francisco, and colleagues examined the prevalence of SARS-CoV-2 infection among 33,041 children who underwent routine testing in April and May when hospitals resumed elective medical and surgical care. The hospitals performed reverse transcription–polymerase chain reaction tests for SARS-CoV-2 RNA before surgery, clinic visits, or hospital admissions. Pediatric otolaryngologists reported the prevalence data through May 29 as part of a quality improvement project.
In all, 250 patients tested positive for the virus, for an overall prevalence of 0.65%. Across 25 geographic areas, the prevalence ranged from 0% to 2.2%. By region, prevalence was highest in the Northeast, at 0.90%, and the Midwest, at 0.87%; prevalence was lower in the West, at 0.59%, and the South, at 0.52%.
To get a sense of how those rates compared with overall rates in the same geographic areas, the researchers used the Johns Hopkins University confirmed cases database to calculate the average weekly incidence of COVID-19 for the entire population for each geographic area.
“Asymptomatic pediatric prevalence was significantly associated with weekly incidence of COVID-19 in the general population during the 6-week period over which most testing of individuals without symptoms occurred,” Ms. Sola and colleagues reported. An analysis using additional data from 11 geographic areas demonstrated that this association persisted at a later time point.
The study provides “another window on the question of how likely is it that an asymptomatic child will be carrying coronavirus,” said Susan E. Coffin, MD, MPH, an attending physician for the division of infectious diseases at Children’s Hospital of Philadelphia. However, important related questions remain, said Dr. Coffin, who was not involved with the study.
For one, it is unclear how many children remain asymptomatic in comparison with those who were in a presymptomatic phase at the time of testing. And importantly, “what proportion of these children are infectious?” said Dr. Coffin. “There is some data to suggest that children with asymptomatic infection may be less infectious than children with symptomatic infection.”
It also could be that patients seen at children’s hospitals differ from the general pediatric population. “What does this look like if you do the exact same study in a group of randomly selected children, not children who are queueing up to have a procedure? ... And what do these numbers look like now that stay-at-home orders have been lifted?” Dr. Coffin asked.
Further studies are needed to establish that detection of COVID-19 in the general population is predictive of the prevalence of SARS-CoV-2 infection in asymptomatic children, Dr. Coffin said.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
As communities wrestle with the decision to send children back to school or opt for distance learning, a key question is how many children are likely to have asymptomatic SARS-CoV-2 infections.
“The strong association between prevalence of SARS-CoV-2 in children who are asymptomatic and contemporaneous weekly incidence of COVID-19 in the general population ... provides a simple means for institutions to estimate local pediatric asymptomatic prevalence from the publicly available Johns Hopkins University database,” researchers say in an article published online August 25 in JAMA Pediatrics.
Ana Marija Sola, BS, a researcher at the University of California, San Francisco, and colleagues examined the prevalence of SARS-CoV-2 infection among 33,041 children who underwent routine testing in April and May when hospitals resumed elective medical and surgical care. The hospitals performed reverse transcription–polymerase chain reaction tests for SARS-CoV-2 RNA before surgery, clinic visits, or hospital admissions. Pediatric otolaryngologists reported the prevalence data through May 29 as part of a quality improvement project.
In all, 250 patients tested positive for the virus, for an overall prevalence of 0.65%. Across 25 geographic areas, the prevalence ranged from 0% to 2.2%. By region, prevalence was highest in the Northeast, at 0.90%, and the Midwest, at 0.87%; prevalence was lower in the West, at 0.59%, and the South, at 0.52%.
To get a sense of how those rates compared with overall rates in the same geographic areas, the researchers used the Johns Hopkins University confirmed cases database to calculate the average weekly incidence of COVID-19 for the entire population for each geographic area.
“Asymptomatic pediatric prevalence was significantly associated with weekly incidence of COVID-19 in the general population during the 6-week period over which most testing of individuals without symptoms occurred,” Ms. Sola and colleagues reported. An analysis using additional data from 11 geographic areas demonstrated that this association persisted at a later time point.
The study provides “another window on the question of how likely is it that an asymptomatic child will be carrying coronavirus,” said Susan E. Coffin, MD, MPH, an attending physician for the division of infectious diseases at Children’s Hospital of Philadelphia. However, important related questions remain, said Dr. Coffin, who was not involved with the study.
For one, it is unclear how many children remain asymptomatic in comparison with those who were in a presymptomatic phase at the time of testing. And importantly, “what proportion of these children are infectious?” said Dr. Coffin. “There is some data to suggest that children with asymptomatic infection may be less infectious than children with symptomatic infection.”
It also could be that patients seen at children’s hospitals differ from the general pediatric population. “What does this look like if you do the exact same study in a group of randomly selected children, not children who are queueing up to have a procedure? ... And what do these numbers look like now that stay-at-home orders have been lifted?” Dr. Coffin asked.
Further studies are needed to establish that detection of COVID-19 in the general population is predictive of the prevalence of SARS-CoV-2 infection in asymptomatic children, Dr. Coffin said.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
As communities wrestle with the decision to send children back to school or opt for distance learning, a key question is how many children are likely to have asymptomatic SARS-CoV-2 infections.
“The strong association between prevalence of SARS-CoV-2 in children who are asymptomatic and contemporaneous weekly incidence of COVID-19 in the general population ... provides a simple means for institutions to estimate local pediatric asymptomatic prevalence from the publicly available Johns Hopkins University database,” researchers say in an article published online August 25 in JAMA Pediatrics.
Ana Marija Sola, BS, a researcher at the University of California, San Francisco, and colleagues examined the prevalence of SARS-CoV-2 infection among 33,041 children who underwent routine testing in April and May when hospitals resumed elective medical and surgical care. The hospitals performed reverse transcription–polymerase chain reaction tests for SARS-CoV-2 RNA before surgery, clinic visits, or hospital admissions. Pediatric otolaryngologists reported the prevalence data through May 29 as part of a quality improvement project.
In all, 250 patients tested positive for the virus, for an overall prevalence of 0.65%. Across 25 geographic areas, the prevalence ranged from 0% to 2.2%. By region, prevalence was highest in the Northeast, at 0.90%, and the Midwest, at 0.87%; prevalence was lower in the West, at 0.59%, and the South, at 0.52%.
To get a sense of how those rates compared with overall rates in the same geographic areas, the researchers used the Johns Hopkins University confirmed cases database to calculate the average weekly incidence of COVID-19 for the entire population for each geographic area.
“Asymptomatic pediatric prevalence was significantly associated with weekly incidence of COVID-19 in the general population during the 6-week period over which most testing of individuals without symptoms occurred,” Ms. Sola and colleagues reported. An analysis using additional data from 11 geographic areas demonstrated that this association persisted at a later time point.
The study provides “another window on the question of how likely is it that an asymptomatic child will be carrying coronavirus,” said Susan E. Coffin, MD, MPH, an attending physician for the division of infectious diseases at Children’s Hospital of Philadelphia. However, important related questions remain, said Dr. Coffin, who was not involved with the study.
For one, it is unclear how many children remain asymptomatic in comparison with those who were in a presymptomatic phase at the time of testing. And importantly, “what proportion of these children are infectious?” said Dr. Coffin. “There is some data to suggest that children with asymptomatic infection may be less infectious than children with symptomatic infection.”
It also could be that patients seen at children’s hospitals differ from the general pediatric population. “What does this look like if you do the exact same study in a group of randomly selected children, not children who are queueing up to have a procedure? ... And what do these numbers look like now that stay-at-home orders have been lifted?” Dr. Coffin asked.
Further studies are needed to establish that detection of COVID-19 in the general population is predictive of the prevalence of SARS-CoV-2 infection in asymptomatic children, Dr. Coffin said.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Aspirin may accelerate cancer progression in older adults
Aspirin may accelerate the progression of advanced cancers and lead to an earlier death as a result, new data from the ASPREE study suggest.
The results showed that patients 65 years and older who started taking daily low-dose aspirin had a 19% higher chance of being diagnosed with metastatic cancer, a 22% higher chance of being diagnosed with a stage 4 tumor, and a 31% increased risk of death from stage 4 cancer, when compared with patients who took a placebo.
John J. McNeil, MBBS, PhD, of Monash University in Melbourne, Australia, and colleagues detailed these findings in the Journal of the National Cancer Institute.
“If confirmed, the clinical implications of these findings could be important for the use of aspirin in an older population,” the authors wrote.
When results of the ASPREE study were first reported in 2018, they “raised important concerns,” Ernest Hawk, MD, and Karen Colbert Maresso wrote in an editorial related to the current publication.
“Unlike ARRIVE, ASCEND, and nearly all prior primary prevention CVD [cardiovascular disease] trials of aspirin, ASPREE surprisingly demonstrated increased all-cause mortality in the aspirin group, which appeared to be driven largely by an increase in cancer-related deaths,” wrote the editorialists, who are both from the University of Texas MD Anderson Cancer Center in Houston.
Even though the ASPREE investigators have now taken a deeper dive into their data, the findings “neither explain nor alleviate the concerns raised by the initial ASPREE report,” the editorialists noted.
ASPREE design and results
ASPREE is a multicenter, double-blind trial of 19,114 older adults living in Australia (n = 16,703) or the United States (n = 2,411). Most patients were 70 years or older at baseline. However, the U.S. group also included patients 65 years and older who were racial/ethnic minorities (n = 564).
Patients were randomized to receive 100 mg of enteric-coated aspirin daily (n = 9,525) or matching placebo (n = 9,589) from March 2010 through December 2014.
At inclusion, all participants were free from cardiovascular disease, dementia, or physical disability. A previous history of cancer was not used to exclude participants, and 19.1% of patients had cancer at randomization. Most patients (89%) had not used aspirin regularly before entering the trial.
At a median follow-up of 4.7 years, there were 981 incident cancer events in the aspirin-treated group and 952 in the placebo-treated group, with an overall incident cancer rate of 10.1%.
Of the 1,933 patients with newly diagnosed cancer, 65.7% had a localized cancer, 18.8% had a new metastatic cancer, 5.8% had metastatic disease from an existing cancer, and 9.7% had a new hematologic or lymphatic cancer.
A quarter of cancer patients (n = 495) died as a result of their malignancy, with 52 dying from a cancer they already had at randomization.
Aspirin was not associated with the risk of first incident cancer diagnosis or incident localized cancer diagnosis. The hazard ratios were 1.04 for all incident cancers (95% confidence interval, 0.95-1.14) and 0.99 for incident localized cancers (95% CI, 0.89-1.11).
However, aspirin was associated with an increased risk of metastatic cancer and cancer presenting at stage 4. The HR for metastatic cancer was 1.19 (95% CI, 1.00-1.43), and the HR for newly diagnosed stage 4 cancer was 1.22 (95% CI, 1.02-1.45).
Furthermore, “an increased progression to death was observed amongst those randomized to aspirin, regardless of whether the initial cancer presentation had been localized or metastatic,” the investigators wrote.
The HRs for death were 1.35 for all cancers (95% CI, 1.13-1.61), 1.47 for localized cancers (95% CI, 1.07-2.02), and 1.30 for metastatic cancers (95% CI, 1.03-1.63).
“Deaths were particularly high among those on aspirin who were diagnosed with advanced solid cancers,” study author Andrew Chan, MD, of Massachusetts General Hospital in Boston, said in a press statement.
Indeed, HRs for death in patients with solid tumors presenting at stage 3 and 4 were a respective 2.11 (95% CI, 1.03-4.33) and 1.31 (95% CI, 1.04-1.64). This suggests a possible adverse effect of aspirin on the growth of cancers once they have already developed in older adults, Dr. Chan said.
Where does that leave aspirin for cancer prevention?
“Although these results suggest that we should be cautious about starting aspirin therapy in otherwise healthy older adults, this does not mean that individuals who are already taking aspirin – particularly if they began taking it at a younger age – should stop their aspirin regimen,” Dr. Chan said.
There are decades of data supporting the use of daily aspirin to prevent multiple cancer types, particularly colorectal cancer, in individuals under the age of 70 years. In a recent meta-analysis, for example, regular aspirin use was linked to a 27% reduced risk for colorectal cancer, a 33% reduced risk for squamous cell esophageal cancer, a 39% decreased risk for adenocarcinoma of the esophagus and gastric cardia, a 36% decreased risk for stomach cancer, a 38% decreased risk for hepatobiliary tract cancer, and a 22% decreased risk for pancreatic cancer.
While these figures are mostly based on observational and case-control studies, it “reaffirms the fact that, overall, when you look at all of the ages, that there is still a benefit of aspirin for cancer,” John Cuzick, PhD, of Queen Mary University of London (England), said in an interview.
In fact, the meta-analysis goes as far as suggesting that perhaps the dose of aspirin being used is too low, with the authors noting that there was a 35% risk reduction in colorectal cancer with a dose of 325 mg daily. That’s a new finding, Dr. Cuzick said.
He noted that the ASPREE study largely consists of patients 70 years of age or older, and the authors “draw some conclusions which we can’t ignore about potential safety.”
One of the safety concerns is the increased risk for gastrointestinal bleeding, which is why Dr. Cuzick and colleagues previously recommended caution in the use of aspirin to prevent cancer in elderly patients. The group published a study in 2015 that suggested a benefit of taking aspirin daily for 5-10 years in patients aged 50-65 years, but the risk/benefit ratio was unclear for patients 70 years and older.
The ASPREE data now add to those uncertainties and suggest “there may be some side effects that we do not understand,” Dr. Cuzick said.
“I’m still optimistic that aspirin is going to be important for cancer prevention, but probably focusing on ages 50-70,” he added. “[The ASPREE data] reinforce the caution that we have to take in terms of trying to understand what the side effects are and what’s going on at these older ages.”
Dr. Cuzick is currently leading the AsCaP Project, an international effort to better understand why aspirin might work in preventing some cancer types but not others. AsCaP is supported by Cancer Research UK and also includes Dr. Chan among the researchers attempting to find out which patients may benefit the most from aspirin and which may be at greater risk of adverse effects.
The ASPREE trial was funded by grants from the National Institute on Aging, the National Cancer Institute, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Several ASPREE investigators disclosed financial relationships with Bayer Pharma. The editorialists had no conflicts of interest. Dr. Cuzick has been an advisory board member for Bayer in the past.
SOURCE: McNeil J et al. J Natl Cancer Inst. 2020 Aug 11. doi: 10.1093/jnci/djaa114.
Aspirin may accelerate the progression of advanced cancers and lead to an earlier death as a result, new data from the ASPREE study suggest.
The results showed that patients 65 years and older who started taking daily low-dose aspirin had a 19% higher chance of being diagnosed with metastatic cancer, a 22% higher chance of being diagnosed with a stage 4 tumor, and a 31% increased risk of death from stage 4 cancer, when compared with patients who took a placebo.
John J. McNeil, MBBS, PhD, of Monash University in Melbourne, Australia, and colleagues detailed these findings in the Journal of the National Cancer Institute.
“If confirmed, the clinical implications of these findings could be important for the use of aspirin in an older population,” the authors wrote.
When results of the ASPREE study were first reported in 2018, they “raised important concerns,” Ernest Hawk, MD, and Karen Colbert Maresso wrote in an editorial related to the current publication.
“Unlike ARRIVE, ASCEND, and nearly all prior primary prevention CVD [cardiovascular disease] trials of aspirin, ASPREE surprisingly demonstrated increased all-cause mortality in the aspirin group, which appeared to be driven largely by an increase in cancer-related deaths,” wrote the editorialists, who are both from the University of Texas MD Anderson Cancer Center in Houston.
Even though the ASPREE investigators have now taken a deeper dive into their data, the findings “neither explain nor alleviate the concerns raised by the initial ASPREE report,” the editorialists noted.
ASPREE design and results
ASPREE is a multicenter, double-blind trial of 19,114 older adults living in Australia (n = 16,703) or the United States (n = 2,411). Most patients were 70 years or older at baseline. However, the U.S. group also included patients 65 years and older who were racial/ethnic minorities (n = 564).
Patients were randomized to receive 100 mg of enteric-coated aspirin daily (n = 9,525) or matching placebo (n = 9,589) from March 2010 through December 2014.
At inclusion, all participants were free from cardiovascular disease, dementia, or physical disability. A previous history of cancer was not used to exclude participants, and 19.1% of patients had cancer at randomization. Most patients (89%) had not used aspirin regularly before entering the trial.
At a median follow-up of 4.7 years, there were 981 incident cancer events in the aspirin-treated group and 952 in the placebo-treated group, with an overall incident cancer rate of 10.1%.
Of the 1,933 patients with newly diagnosed cancer, 65.7% had a localized cancer, 18.8% had a new metastatic cancer, 5.8% had metastatic disease from an existing cancer, and 9.7% had a new hematologic or lymphatic cancer.
A quarter of cancer patients (n = 495) died as a result of their malignancy, with 52 dying from a cancer they already had at randomization.
Aspirin was not associated with the risk of first incident cancer diagnosis or incident localized cancer diagnosis. The hazard ratios were 1.04 for all incident cancers (95% confidence interval, 0.95-1.14) and 0.99 for incident localized cancers (95% CI, 0.89-1.11).
However, aspirin was associated with an increased risk of metastatic cancer and cancer presenting at stage 4. The HR for metastatic cancer was 1.19 (95% CI, 1.00-1.43), and the HR for newly diagnosed stage 4 cancer was 1.22 (95% CI, 1.02-1.45).
Furthermore, “an increased progression to death was observed amongst those randomized to aspirin, regardless of whether the initial cancer presentation had been localized or metastatic,” the investigators wrote.
The HRs for death were 1.35 for all cancers (95% CI, 1.13-1.61), 1.47 for localized cancers (95% CI, 1.07-2.02), and 1.30 for metastatic cancers (95% CI, 1.03-1.63).
“Deaths were particularly high among those on aspirin who were diagnosed with advanced solid cancers,” study author Andrew Chan, MD, of Massachusetts General Hospital in Boston, said in a press statement.
Indeed, HRs for death in patients with solid tumors presenting at stage 3 and 4 were a respective 2.11 (95% CI, 1.03-4.33) and 1.31 (95% CI, 1.04-1.64). This suggests a possible adverse effect of aspirin on the growth of cancers once they have already developed in older adults, Dr. Chan said.
Where does that leave aspirin for cancer prevention?
“Although these results suggest that we should be cautious about starting aspirin therapy in otherwise healthy older adults, this does not mean that individuals who are already taking aspirin – particularly if they began taking it at a younger age – should stop their aspirin regimen,” Dr. Chan said.
There are decades of data supporting the use of daily aspirin to prevent multiple cancer types, particularly colorectal cancer, in individuals under the age of 70 years. In a recent meta-analysis, for example, regular aspirin use was linked to a 27% reduced risk for colorectal cancer, a 33% reduced risk for squamous cell esophageal cancer, a 39% decreased risk for adenocarcinoma of the esophagus and gastric cardia, a 36% decreased risk for stomach cancer, a 38% decreased risk for hepatobiliary tract cancer, and a 22% decreased risk for pancreatic cancer.
While these figures are mostly based on observational and case-control studies, it “reaffirms the fact that, overall, when you look at all of the ages, that there is still a benefit of aspirin for cancer,” John Cuzick, PhD, of Queen Mary University of London (England), said in an interview.
In fact, the meta-analysis goes as far as suggesting that perhaps the dose of aspirin being used is too low, with the authors noting that there was a 35% risk reduction in colorectal cancer with a dose of 325 mg daily. That’s a new finding, Dr. Cuzick said.
He noted that the ASPREE study largely consists of patients 70 years of age or older, and the authors “draw some conclusions which we can’t ignore about potential safety.”
One of the safety concerns is the increased risk for gastrointestinal bleeding, which is why Dr. Cuzick and colleagues previously recommended caution in the use of aspirin to prevent cancer in elderly patients. The group published a study in 2015 that suggested a benefit of taking aspirin daily for 5-10 years in patients aged 50-65 years, but the risk/benefit ratio was unclear for patients 70 years and older.
The ASPREE data now add to those uncertainties and suggest “there may be some side effects that we do not understand,” Dr. Cuzick said.
“I’m still optimistic that aspirin is going to be important for cancer prevention, but probably focusing on ages 50-70,” he added. “[The ASPREE data] reinforce the caution that we have to take in terms of trying to understand what the side effects are and what’s going on at these older ages.”
Dr. Cuzick is currently leading the AsCaP Project, an international effort to better understand why aspirin might work in preventing some cancer types but not others. AsCaP is supported by Cancer Research UK and also includes Dr. Chan among the researchers attempting to find out which patients may benefit the most from aspirin and which may be at greater risk of adverse effects.
The ASPREE trial was funded by grants from the National Institute on Aging, the National Cancer Institute, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Several ASPREE investigators disclosed financial relationships with Bayer Pharma. The editorialists had no conflicts of interest. Dr. Cuzick has been an advisory board member for Bayer in the past.
SOURCE: McNeil J et al. J Natl Cancer Inst. 2020 Aug 11. doi: 10.1093/jnci/djaa114.
Aspirin may accelerate the progression of advanced cancers and lead to an earlier death as a result, new data from the ASPREE study suggest.
The results showed that patients 65 years and older who started taking daily low-dose aspirin had a 19% higher chance of being diagnosed with metastatic cancer, a 22% higher chance of being diagnosed with a stage 4 tumor, and a 31% increased risk of death from stage 4 cancer, when compared with patients who took a placebo.
John J. McNeil, MBBS, PhD, of Monash University in Melbourne, Australia, and colleagues detailed these findings in the Journal of the National Cancer Institute.
“If confirmed, the clinical implications of these findings could be important for the use of aspirin in an older population,” the authors wrote.
When results of the ASPREE study were first reported in 2018, they “raised important concerns,” Ernest Hawk, MD, and Karen Colbert Maresso wrote in an editorial related to the current publication.
“Unlike ARRIVE, ASCEND, and nearly all prior primary prevention CVD [cardiovascular disease] trials of aspirin, ASPREE surprisingly demonstrated increased all-cause mortality in the aspirin group, which appeared to be driven largely by an increase in cancer-related deaths,” wrote the editorialists, who are both from the University of Texas MD Anderson Cancer Center in Houston.
Even though the ASPREE investigators have now taken a deeper dive into their data, the findings “neither explain nor alleviate the concerns raised by the initial ASPREE report,” the editorialists noted.
ASPREE design and results
ASPREE is a multicenter, double-blind trial of 19,114 older adults living in Australia (n = 16,703) or the United States (n = 2,411). Most patients were 70 years or older at baseline. However, the U.S. group also included patients 65 years and older who were racial/ethnic minorities (n = 564).
Patients were randomized to receive 100 mg of enteric-coated aspirin daily (n = 9,525) or matching placebo (n = 9,589) from March 2010 through December 2014.
At inclusion, all participants were free from cardiovascular disease, dementia, or physical disability. A previous history of cancer was not used to exclude participants, and 19.1% of patients had cancer at randomization. Most patients (89%) had not used aspirin regularly before entering the trial.
At a median follow-up of 4.7 years, there were 981 incident cancer events in the aspirin-treated group and 952 in the placebo-treated group, with an overall incident cancer rate of 10.1%.
Of the 1,933 patients with newly diagnosed cancer, 65.7% had a localized cancer, 18.8% had a new metastatic cancer, 5.8% had metastatic disease from an existing cancer, and 9.7% had a new hematologic or lymphatic cancer.
A quarter of cancer patients (n = 495) died as a result of their malignancy, with 52 dying from a cancer they already had at randomization.
Aspirin was not associated with the risk of first incident cancer diagnosis or incident localized cancer diagnosis. The hazard ratios were 1.04 for all incident cancers (95% confidence interval, 0.95-1.14) and 0.99 for incident localized cancers (95% CI, 0.89-1.11).
However, aspirin was associated with an increased risk of metastatic cancer and cancer presenting at stage 4. The HR for metastatic cancer was 1.19 (95% CI, 1.00-1.43), and the HR for newly diagnosed stage 4 cancer was 1.22 (95% CI, 1.02-1.45).
Furthermore, “an increased progression to death was observed amongst those randomized to aspirin, regardless of whether the initial cancer presentation had been localized or metastatic,” the investigators wrote.
The HRs for death were 1.35 for all cancers (95% CI, 1.13-1.61), 1.47 for localized cancers (95% CI, 1.07-2.02), and 1.30 for metastatic cancers (95% CI, 1.03-1.63).
“Deaths were particularly high among those on aspirin who were diagnosed with advanced solid cancers,” study author Andrew Chan, MD, of Massachusetts General Hospital in Boston, said in a press statement.
Indeed, HRs for death in patients with solid tumors presenting at stage 3 and 4 were a respective 2.11 (95% CI, 1.03-4.33) and 1.31 (95% CI, 1.04-1.64). This suggests a possible adverse effect of aspirin on the growth of cancers once they have already developed in older adults, Dr. Chan said.
Where does that leave aspirin for cancer prevention?
“Although these results suggest that we should be cautious about starting aspirin therapy in otherwise healthy older adults, this does not mean that individuals who are already taking aspirin – particularly if they began taking it at a younger age – should stop their aspirin regimen,” Dr. Chan said.
There are decades of data supporting the use of daily aspirin to prevent multiple cancer types, particularly colorectal cancer, in individuals under the age of 70 years. In a recent meta-analysis, for example, regular aspirin use was linked to a 27% reduced risk for colorectal cancer, a 33% reduced risk for squamous cell esophageal cancer, a 39% decreased risk for adenocarcinoma of the esophagus and gastric cardia, a 36% decreased risk for stomach cancer, a 38% decreased risk for hepatobiliary tract cancer, and a 22% decreased risk for pancreatic cancer.
While these figures are mostly based on observational and case-control studies, it “reaffirms the fact that, overall, when you look at all of the ages, that there is still a benefit of aspirin for cancer,” John Cuzick, PhD, of Queen Mary University of London (England), said in an interview.
In fact, the meta-analysis goes as far as suggesting that perhaps the dose of aspirin being used is too low, with the authors noting that there was a 35% risk reduction in colorectal cancer with a dose of 325 mg daily. That’s a new finding, Dr. Cuzick said.
He noted that the ASPREE study largely consists of patients 70 years of age or older, and the authors “draw some conclusions which we can’t ignore about potential safety.”
One of the safety concerns is the increased risk for gastrointestinal bleeding, which is why Dr. Cuzick and colleagues previously recommended caution in the use of aspirin to prevent cancer in elderly patients. The group published a study in 2015 that suggested a benefit of taking aspirin daily for 5-10 years in patients aged 50-65 years, but the risk/benefit ratio was unclear for patients 70 years and older.
The ASPREE data now add to those uncertainties and suggest “there may be some side effects that we do not understand,” Dr. Cuzick said.
“I’m still optimistic that aspirin is going to be important for cancer prevention, but probably focusing on ages 50-70,” he added. “[The ASPREE data] reinforce the caution that we have to take in terms of trying to understand what the side effects are and what’s going on at these older ages.”
Dr. Cuzick is currently leading the AsCaP Project, an international effort to better understand why aspirin might work in preventing some cancer types but not others. AsCaP is supported by Cancer Research UK and also includes Dr. Chan among the researchers attempting to find out which patients may benefit the most from aspirin and which may be at greater risk of adverse effects.
The ASPREE trial was funded by grants from the National Institute on Aging, the National Cancer Institute, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Several ASPREE investigators disclosed financial relationships with Bayer Pharma. The editorialists had no conflicts of interest. Dr. Cuzick has been an advisory board member for Bayer in the past.
SOURCE: McNeil J et al. J Natl Cancer Inst. 2020 Aug 11. doi: 10.1093/jnci/djaa114.
FROM JOURNAL OF THE NATIONAL CANCER INSTITUTE
COVID-19: ‘Record’ spike in Internet anxiety, panic queries
Internet searches regarding acute anxiety reached an all-time high between March and May 2020, new research shows.
Investigators used data collected by Google to monitor the daily percentage of all Internet searches originating in the United States that included the terms “anxiety” or “panic” in combination with “attack” between January 2004 and May 2020.
They found an 11% increase in all acute anxiety queries between March 2020, when President Donald Trump first declared the COVID-19 pandemic a national emergency, and May 2020. This translates into approximately 375,000 more searches than expected.
Most of the increase in inquiries occurred when specific developments in COVID-19 were reported.
“We found record levels of people potentially having panic attacks, as reflected by their online queries since early in the pandemic,” lead author John W. Ayers, PhD, associate adjunct professor of medicine, school of health sciences, University of California, San Diego, said in an interview.
“There are two main take-home messages from our research – one is that we need to think about how to address acute anxiety during COVID-19, and the other is that who is also vice chief of innovation, division of infectious diseases and global public health at the University of California, San Diego.
The study was published online August 24 in JAMA Internal Medicine.
Real-time data
“There has been a lot of speculation about collateral consequences of COVID-19, especially in mental health,” Dr. Ayers said.
Most of the research has been conducted via self-report survey, but these types of surveys may miss individuals who do not participate in the surveys or do not seek care, Dr. Ayers added.
“We need a strategy that can measure behavioral health in real time so we can design interventions to meet these needs,” he said.
He explained that he and his colleagues “looked at one case study – panic attacks – because it is the most prevalent form of mental health problem driven by your surroundings, and it is socially contagious, meaning that when someone you know is having a severe acute anxiety or panic attack, you’re more likely to have one yourself.”
The researchers turned to publicly available nonidentifiable data collected via Google Trends – a feature of Google that shows how frequently a given search term is entered into Google’s search engine, relative to the site’s total search volume, over a specific period.
They monitored all searches containing their keywords over a 15-year period (Jan. 1, 2004–May 4, 2020). Search volumes between March 13, 2020 (when the national emergency was declared) and the last date of available data (May 9, 2020) were compared with the expected search volumes that would have been found had COVID-19 not occurred.
Headline-related spikes
Cumulatively, all acute anxiety searches were 11% higher than expected for the 58-day study period (95% confidence interval, 7%-14%). There was a dramatic increase in searches (375,000), or a total of 3.4 million searches, during that period.
Most of these searches took place between March 16, 2020, and April 14, 2020, when searches were cumulatively 17% higher than expected (95% CI, 13%-22%).
Several COVID-19–related milestones took place during that period.
- First imposition of social distancing guidelines occurred (March 16, 2020).
- The United States surpassed China with the most reported COVID-19 cases (March 26, 2020).
- Extension of social distancing guidelines occurred (March 29, 2020).
- The Centers for Disease Control and Prevention recommended use of face masks (April 3, 2020).
- The United States surpassed Italy for having the most COVID-19–related deaths (April 11, 2020).
The largest spike in acute anxiety queries occurred on March 28, 2020, on which date there were 52% more searches than expected. Queries returned to expected levels on April 15, 2020, and have fallen within expected ranges since then.
Dr. Ayers noted that, although other stressors have affected people in the United States, including electoral and economic issues, “the headlines around COVID-19 were driving the anxiety, and those days with dramatic headlines were associated with large spikes in queries.”
“Our messaging surrounding how COVID-19 is reported may need to change to prevent this,” Dr. Ayers said. “Headlines that hit people in the head by reporting how many people died and bury in the article how we can slow the spread may increase anxiety more than headlines reporting strategies that work right up front.”
He noted that media reporting of suicide has begun to change; there have been fewer sensationalized headlines, and there has been an increase in referrals to suicide hotlines. “We need to be thinking about similar strategies when reporting COVID-19,” said Dr. Ayers.
He also suggested tapping existing resources, such as state suicide hotlines, by training staff to assist persons experiencing acute anxiety and panic attacks.
As an example of this model, the authors point to an Illinois-based hotline, Call4Calm, which helps people cope with acute COVID-19 anxiety.
Google queries concerning panic and anxiety do not yield any links to helplines, although OneBox, a Google feature, provides information to people inquiring about suicide and addiction. “This approach could be used to promote resources about anxiety and panic and COVID-19,” Dr. Ayers suggested.
Call to action
Elspeth Cameron Ritchie, MD, chair of the department of psychiatry, Medstar Washington Hospital Center, said the study’s recommendations were “interesting and worth amplifying “ and that “headlines calling for calm” were “a good suggestion.” She was not involved with the study.
“A multifaceted, multimedia approach is needed – not only what’s on Google, but it would be helpful if politicians could acknowledge the anxiety and make available more mental health resources,” she added.
Dr. Ritchie, who is also vice chair of psychiatry at Georgetown University, Washington, suggested that it is a “simple option [during patient visits] to incorporate a question as to how your patient is being affected by the pandemic into your standard template, together with questions about sleep, appetite, sexual functioning, etc.”
Dr. Ayers said that the “call to action” that he and his colleagues are making is to use their methodology to “know what mental health needs are in the population” and to use existing frameworks and strategies to address them.
The study was supported by a grant from the University of California office of the president and by intramural support from the division of infectious diseases and the Center for Data Driven Health at the Qualcomm Institute, both with the University of California, San Diego. One coauthor was funded by Bill and Melinda Gates through the Global Good Fund. Dr. Ayers owns equity positions in Directing Medicine, Health Watcher, and Good Analytics, companies that advise on the use of digital data for public health surveillance. Dr. Ritchie reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Internet searches regarding acute anxiety reached an all-time high between March and May 2020, new research shows.
Investigators used data collected by Google to monitor the daily percentage of all Internet searches originating in the United States that included the terms “anxiety” or “panic” in combination with “attack” between January 2004 and May 2020.
They found an 11% increase in all acute anxiety queries between March 2020, when President Donald Trump first declared the COVID-19 pandemic a national emergency, and May 2020. This translates into approximately 375,000 more searches than expected.
Most of the increase in inquiries occurred when specific developments in COVID-19 were reported.
“We found record levels of people potentially having panic attacks, as reflected by their online queries since early in the pandemic,” lead author John W. Ayers, PhD, associate adjunct professor of medicine, school of health sciences, University of California, San Diego, said in an interview.
“There are two main take-home messages from our research – one is that we need to think about how to address acute anxiety during COVID-19, and the other is that who is also vice chief of innovation, division of infectious diseases and global public health at the University of California, San Diego.
The study was published online August 24 in JAMA Internal Medicine.
Real-time data
“There has been a lot of speculation about collateral consequences of COVID-19, especially in mental health,” Dr. Ayers said.
Most of the research has been conducted via self-report survey, but these types of surveys may miss individuals who do not participate in the surveys or do not seek care, Dr. Ayers added.
“We need a strategy that can measure behavioral health in real time so we can design interventions to meet these needs,” he said.
He explained that he and his colleagues “looked at one case study – panic attacks – because it is the most prevalent form of mental health problem driven by your surroundings, and it is socially contagious, meaning that when someone you know is having a severe acute anxiety or panic attack, you’re more likely to have one yourself.”
The researchers turned to publicly available nonidentifiable data collected via Google Trends – a feature of Google that shows how frequently a given search term is entered into Google’s search engine, relative to the site’s total search volume, over a specific period.
They monitored all searches containing their keywords over a 15-year period (Jan. 1, 2004–May 4, 2020). Search volumes between March 13, 2020 (when the national emergency was declared) and the last date of available data (May 9, 2020) were compared with the expected search volumes that would have been found had COVID-19 not occurred.
Headline-related spikes
Cumulatively, all acute anxiety searches were 11% higher than expected for the 58-day study period (95% confidence interval, 7%-14%). There was a dramatic increase in searches (375,000), or a total of 3.4 million searches, during that period.
Most of these searches took place between March 16, 2020, and April 14, 2020, when searches were cumulatively 17% higher than expected (95% CI, 13%-22%).
Several COVID-19–related milestones took place during that period.
- First imposition of social distancing guidelines occurred (March 16, 2020).
- The United States surpassed China with the most reported COVID-19 cases (March 26, 2020).
- Extension of social distancing guidelines occurred (March 29, 2020).
- The Centers for Disease Control and Prevention recommended use of face masks (April 3, 2020).
- The United States surpassed Italy for having the most COVID-19–related deaths (April 11, 2020).
The largest spike in acute anxiety queries occurred on March 28, 2020, on which date there were 52% more searches than expected. Queries returned to expected levels on April 15, 2020, and have fallen within expected ranges since then.
Dr. Ayers noted that, although other stressors have affected people in the United States, including electoral and economic issues, “the headlines around COVID-19 were driving the anxiety, and those days with dramatic headlines were associated with large spikes in queries.”
“Our messaging surrounding how COVID-19 is reported may need to change to prevent this,” Dr. Ayers said. “Headlines that hit people in the head by reporting how many people died and bury in the article how we can slow the spread may increase anxiety more than headlines reporting strategies that work right up front.”
He noted that media reporting of suicide has begun to change; there have been fewer sensationalized headlines, and there has been an increase in referrals to suicide hotlines. “We need to be thinking about similar strategies when reporting COVID-19,” said Dr. Ayers.
He also suggested tapping existing resources, such as state suicide hotlines, by training staff to assist persons experiencing acute anxiety and panic attacks.
As an example of this model, the authors point to an Illinois-based hotline, Call4Calm, which helps people cope with acute COVID-19 anxiety.
Google queries concerning panic and anxiety do not yield any links to helplines, although OneBox, a Google feature, provides information to people inquiring about suicide and addiction. “This approach could be used to promote resources about anxiety and panic and COVID-19,” Dr. Ayers suggested.
Call to action
Elspeth Cameron Ritchie, MD, chair of the department of psychiatry, Medstar Washington Hospital Center, said the study’s recommendations were “interesting and worth amplifying “ and that “headlines calling for calm” were “a good suggestion.” She was not involved with the study.
“A multifaceted, multimedia approach is needed – not only what’s on Google, but it would be helpful if politicians could acknowledge the anxiety and make available more mental health resources,” she added.
Dr. Ritchie, who is also vice chair of psychiatry at Georgetown University, Washington, suggested that it is a “simple option [during patient visits] to incorporate a question as to how your patient is being affected by the pandemic into your standard template, together with questions about sleep, appetite, sexual functioning, etc.”
Dr. Ayers said that the “call to action” that he and his colleagues are making is to use their methodology to “know what mental health needs are in the population” and to use existing frameworks and strategies to address them.
The study was supported by a grant from the University of California office of the president and by intramural support from the division of infectious diseases and the Center for Data Driven Health at the Qualcomm Institute, both with the University of California, San Diego. One coauthor was funded by Bill and Melinda Gates through the Global Good Fund. Dr. Ayers owns equity positions in Directing Medicine, Health Watcher, and Good Analytics, companies that advise on the use of digital data for public health surveillance. Dr. Ritchie reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Internet searches regarding acute anxiety reached an all-time high between March and May 2020, new research shows.
Investigators used data collected by Google to monitor the daily percentage of all Internet searches originating in the United States that included the terms “anxiety” or “panic” in combination with “attack” between January 2004 and May 2020.
They found an 11% increase in all acute anxiety queries between March 2020, when President Donald Trump first declared the COVID-19 pandemic a national emergency, and May 2020. This translates into approximately 375,000 more searches than expected.
Most of the increase in inquiries occurred when specific developments in COVID-19 were reported.
“We found record levels of people potentially having panic attacks, as reflected by their online queries since early in the pandemic,” lead author John W. Ayers, PhD, associate adjunct professor of medicine, school of health sciences, University of California, San Diego, said in an interview.
“There are two main take-home messages from our research – one is that we need to think about how to address acute anxiety during COVID-19, and the other is that who is also vice chief of innovation, division of infectious diseases and global public health at the University of California, San Diego.
The study was published online August 24 in JAMA Internal Medicine.
Real-time data
“There has been a lot of speculation about collateral consequences of COVID-19, especially in mental health,” Dr. Ayers said.
Most of the research has been conducted via self-report survey, but these types of surveys may miss individuals who do not participate in the surveys or do not seek care, Dr. Ayers added.
“We need a strategy that can measure behavioral health in real time so we can design interventions to meet these needs,” he said.
He explained that he and his colleagues “looked at one case study – panic attacks – because it is the most prevalent form of mental health problem driven by your surroundings, and it is socially contagious, meaning that when someone you know is having a severe acute anxiety or panic attack, you’re more likely to have one yourself.”
The researchers turned to publicly available nonidentifiable data collected via Google Trends – a feature of Google that shows how frequently a given search term is entered into Google’s search engine, relative to the site’s total search volume, over a specific period.
They monitored all searches containing their keywords over a 15-year period (Jan. 1, 2004–May 4, 2020). Search volumes between March 13, 2020 (when the national emergency was declared) and the last date of available data (May 9, 2020) were compared with the expected search volumes that would have been found had COVID-19 not occurred.
Headline-related spikes
Cumulatively, all acute anxiety searches were 11% higher than expected for the 58-day study period (95% confidence interval, 7%-14%). There was a dramatic increase in searches (375,000), or a total of 3.4 million searches, during that period.
Most of these searches took place between March 16, 2020, and April 14, 2020, when searches were cumulatively 17% higher than expected (95% CI, 13%-22%).
Several COVID-19–related milestones took place during that period.
- First imposition of social distancing guidelines occurred (March 16, 2020).
- The United States surpassed China with the most reported COVID-19 cases (March 26, 2020).
- Extension of social distancing guidelines occurred (March 29, 2020).
- The Centers for Disease Control and Prevention recommended use of face masks (April 3, 2020).
- The United States surpassed Italy for having the most COVID-19–related deaths (April 11, 2020).
The largest spike in acute anxiety queries occurred on March 28, 2020, on which date there were 52% more searches than expected. Queries returned to expected levels on April 15, 2020, and have fallen within expected ranges since then.
Dr. Ayers noted that, although other stressors have affected people in the United States, including electoral and economic issues, “the headlines around COVID-19 were driving the anxiety, and those days with dramatic headlines were associated with large spikes in queries.”
“Our messaging surrounding how COVID-19 is reported may need to change to prevent this,” Dr. Ayers said. “Headlines that hit people in the head by reporting how many people died and bury in the article how we can slow the spread may increase anxiety more than headlines reporting strategies that work right up front.”
He noted that media reporting of suicide has begun to change; there have been fewer sensationalized headlines, and there has been an increase in referrals to suicide hotlines. “We need to be thinking about similar strategies when reporting COVID-19,” said Dr. Ayers.
He also suggested tapping existing resources, such as state suicide hotlines, by training staff to assist persons experiencing acute anxiety and panic attacks.
As an example of this model, the authors point to an Illinois-based hotline, Call4Calm, which helps people cope with acute COVID-19 anxiety.
Google queries concerning panic and anxiety do not yield any links to helplines, although OneBox, a Google feature, provides information to people inquiring about suicide and addiction. “This approach could be used to promote resources about anxiety and panic and COVID-19,” Dr. Ayers suggested.
Call to action
Elspeth Cameron Ritchie, MD, chair of the department of psychiatry, Medstar Washington Hospital Center, said the study’s recommendations were “interesting and worth amplifying “ and that “headlines calling for calm” were “a good suggestion.” She was not involved with the study.
“A multifaceted, multimedia approach is needed – not only what’s on Google, but it would be helpful if politicians could acknowledge the anxiety and make available more mental health resources,” she added.
Dr. Ritchie, who is also vice chair of psychiatry at Georgetown University, Washington, suggested that it is a “simple option [during patient visits] to incorporate a question as to how your patient is being affected by the pandemic into your standard template, together with questions about sleep, appetite, sexual functioning, etc.”
Dr. Ayers said that the “call to action” that he and his colleagues are making is to use their methodology to “know what mental health needs are in the population” and to use existing frameworks and strategies to address them.
The study was supported by a grant from the University of California office of the president and by intramural support from the division of infectious diseases and the Center for Data Driven Health at the Qualcomm Institute, both with the University of California, San Diego. One coauthor was funded by Bill and Melinda Gates through the Global Good Fund. Dr. Ayers owns equity positions in Directing Medicine, Health Watcher, and Good Analytics, companies that advise on the use of digital data for public health surveillance. Dr. Ritchie reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Heart failure: Practice-changing developments for hospitalists
A recently validated, easy-to-use calculator of predicted 7-day mortality risk in patients presenting with acute decompensated heart failure is well worth incorporating into hospitalist clinical practice, Dustin T. Smith, MD, said at HM20 Virtual, hosted by the Society of Hospital Medicine.
In addition to the EHMRG, other highlights of his wide-ranging update on recent practice-changing developments in heart failure directly relevant to hospitalists included the introduction of a simple, evidence-based tool for differentiating heart failure with preserved ejection fraction from other potential causes of unexplained dyspnea on exertion in euvolemic patients, and a study debunking what has been called the potassium repletion reflex in patients with acute heart failure undergoing diuresis.
The ACUTE study
Heart failure is an area of special interest for Dr. Smith. He has been surprised to find that virtually no hospitalists, emergency medicine physicians, or cardiologists he has spoken with have heard of the EHMRG or its validation in the ACUTE (Acute Congestive Heart Failure Urgent Care Evaluation) study. Yet this is a very handy tool for hospitalists, he observed.
The EHMRG algorithm utilizes nine variables for which data is readily available for every patient who arrives at the emergency department with acute heart failure. The variables are age, arrival by ambulance, heart rate, systolic blood pressure, potassium level, oxygen saturation, troponin, serum creatine, and presence or absence of active cancer. The information is entered into a cell phone app, which spits out the patient’s estimated 7-day mortality risk. The algorithm divides patients into one of five risk groups ranging from very low to very high. With the addition of data input as to the presence or absence of ST-segment depression on the 12-lead ECG, the weighted algorithm will simultaneously generate an estimated 30-day mortality risk.
ACUTE was a prospective, observational, real-world validation study of EHMRG involving 1,983 patients seeking emergency department care for acute heart failure at nine Canadian hospitals. The actual 7-day mortality rate was 0% in the very-low-risk group, 0% in the low-risk group, 0.6% with an intermediate-risk EHMRG, 1.9% with high risk, and 3.9% in the very-high-risk group. The corresponding 30-day mortality rates were 0%, 1.9%, 3.9%, 5.9%, and 14.3%.
The University of Toronto investigators also asked participating physicians for their clinical estimates of 7-day mortality risk while blinded to the EHMRG predictions. The algorithm proved more accurate than physician predictions across the board. Indeed, physicians consistently overestimated the mortality risk for all categories except the very-high-risk one, where they underestimated the true risk (Circulation. 2019 Feb 26;139[9]:1146-56).
Given that heart failure remains year after year at the top of the list of most frequent causes for hospital admission, and that there is compelling evidence that many low-risk patients get hospitalized while potentially unsafe early discharges also occur, the EHMRG score fills an important unmet need.
“I think this can help inform us as to who with acute heart failure potentially needs to come into the hospital and who doesn’t,” Dr. Smith said. “I think the sweet spot here is that if you’re in the low- or very-low-risk category, your 7-day mortality is less than 1%; in fact, in this study it’s zero. But once you get to category 3 – the intermediate category – you’re talking about a 7-day mortality of 1%-2%, which I think is high enough to warrant hospital admission for treatment and to watch them, not just send them home.”
The H2FPEF score
Diagnosis of heart failure with preserved ejection fraction (HFpEF) is a challenge in euvolemic patients with clear lungs and dyspnea on exertion. Investigators at the Mayo Clinic have developed and subsequently validated a weighted score known as the H2FPEF score that’s of great assistance in this task. The score is based upon a set of six simple variables universally available in patients undergoing diagnostic workup for the numerous potential causes for dyspnea on exertion. Together these six variables comprise the acronym H2FPEF:
- Heavy: One point for a BMI greater than 30 kg/m2.
- Hypertension: One point for being on two or more antihypertensive drugs.
- Atrial fibrillation: Three points for paroxysmal or persistent AF.
- Pulmonary hypertension: One point for having a Doppler echocardiographic estimated pulmonary artery systolic pressure greater than 35 mm Hg.
- Elder: One point for age greater than 60 years.
- Filling pressure: One point for a Doppler echocardiographic E/e’ ratio above 9.
The total score can range from 0 to 9. (Circulation. 2018 Aug 28;138[9]:861-70).
Each 1-point increase in the score essentially doubled a patient’s risk of having HFpEF as opposed to pulmonary embolism or some other cause for the dyspnea.
“I really like this H2FPEF score. The score works very, very well. Once you get to a score of 6 or above, the probability of HFpEF is more than 90%, which is pretty powerful. I think this is worthwhile,” Dr. Smith said.
In their derivation and validation cohorts, the Mayo Clinic investigators used as their gold standard for diagnosis of HFpEF invasive hemodynamic exercise testing with a pulmonary artery catheter in place to measure pressures. A score that enables hospitalists to lessen the need for that kind of costly invasive testing is most welcome.
“Here’s how I’d use this score: With an H2FPEF score of 0-1, HFpEF is unlikely. With an intermediate score of 2-5, additional testing is warranted. If the score is high, 6-9, I think HFpEF is likely,” the hospitalist said.
Dr. Smith isn’t the only big fan of the H2FPEF score. In an editorial accompanying publication of the score’s validation study, Walter J. Paulus, MD, PhD, hailed the H2FPEF score as “a unique tour de force” which constitutes a major advance beyond the confusing diagnostic recommendations for HFpEF issued by the European Society of Cardiology and the American Society of Echocardiography, which he said have been “met by skepticism qualifying them as overcomplicated and even triggered disbelief in the existence of HFpEF.”
Particularly interesting were the variables rejected for inclusion in the H2FPEF score because they failed to achieve statistical significance as predictors, even though they’re often considered important in defining HFpEF, he noted. These included left atrial volume index, sex, and levels of circulating N-terminal probrain natriuretic peptide, wrote Dr. Paulus, professor of cardiac pathophysiology at VU University, Amsterdam.
Debunking the potassium repletion reflex
Longstanding conventional wisdom holds that patients hospitalized for heart failure need to maintain a serum potassium above 4.0 mEq/L.
“I’m sure you’ve all written orders to keep the potassium greater than 4.0 mEq/L and the magnesium above 2mEq/L about a million times, like I have,” Dr. Smith said.
But it turns out this traditional practice, which involves a huge cost in terms of time, money, and health care resources, is supported by weak evidence – and an important recent study has now debunked what the investigators termed the potassium “repletion reflex.”
The investigators at the University of Massachusetts identified 4,995 patients admitted with exacerbation of acute heart failure and a normal admission serum potassium level of 3.5-5.0 mEq/L. More than 70% received potassium repletion at least once within a 72-hour observation window, during which 2,080 patients maintained a low-normal serum potassium below 4.0 mEq/L, 2,326 had a mid-normal level of 4.0-4.5 mEq/L, and 589 had a high-normal level of more than 4.5 mEq/L but not more than 5.0 mEq/L.
The study had three endpoints: in-hospital mortality, transfer to the intensive care unit, and hospital length of stay. After statistical adjustment for comorbidities, demographics, and severity at admission, there was no difference between the low- and mid-normal serum potassium groups in any of the three endpoints. In contrast, the high-normal potassium group had a significantly longer length of stay, by a median of 0.6 extra days. The high-normal group also had a 78% increased likelihood of ICU transfer and a 51% increased risk of in-hospital mortality, although neither of these differences reached statistical significance (J Hosp Med. 2019 Dec 1;14[12]:729-36).
“A potassium greater than 4.5 mEq/L may be associated with increased risk of worse outcomes,” Dr. Smith observed. “I think the sweet spot may be 3.5-4.5 mEq/L based on this study.”
He reported having no financial conflicts regarding his presentation.
A recently validated, easy-to-use calculator of predicted 7-day mortality risk in patients presenting with acute decompensated heart failure is well worth incorporating into hospitalist clinical practice, Dustin T. Smith, MD, said at HM20 Virtual, hosted by the Society of Hospital Medicine.
In addition to the EHMRG, other highlights of his wide-ranging update on recent practice-changing developments in heart failure directly relevant to hospitalists included the introduction of a simple, evidence-based tool for differentiating heart failure with preserved ejection fraction from other potential causes of unexplained dyspnea on exertion in euvolemic patients, and a study debunking what has been called the potassium repletion reflex in patients with acute heart failure undergoing diuresis.
The ACUTE study
Heart failure is an area of special interest for Dr. Smith. He has been surprised to find that virtually no hospitalists, emergency medicine physicians, or cardiologists he has spoken with have heard of the EHMRG or its validation in the ACUTE (Acute Congestive Heart Failure Urgent Care Evaluation) study. Yet this is a very handy tool for hospitalists, he observed.
The EHMRG algorithm utilizes nine variables for which data is readily available for every patient who arrives at the emergency department with acute heart failure. The variables are age, arrival by ambulance, heart rate, systolic blood pressure, potassium level, oxygen saturation, troponin, serum creatine, and presence or absence of active cancer. The information is entered into a cell phone app, which spits out the patient’s estimated 7-day mortality risk. The algorithm divides patients into one of five risk groups ranging from very low to very high. With the addition of data input as to the presence or absence of ST-segment depression on the 12-lead ECG, the weighted algorithm will simultaneously generate an estimated 30-day mortality risk.
ACUTE was a prospective, observational, real-world validation study of EHMRG involving 1,983 patients seeking emergency department care for acute heart failure at nine Canadian hospitals. The actual 7-day mortality rate was 0% in the very-low-risk group, 0% in the low-risk group, 0.6% with an intermediate-risk EHMRG, 1.9% with high risk, and 3.9% in the very-high-risk group. The corresponding 30-day mortality rates were 0%, 1.9%, 3.9%, 5.9%, and 14.3%.
The University of Toronto investigators also asked participating physicians for their clinical estimates of 7-day mortality risk while blinded to the EHMRG predictions. The algorithm proved more accurate than physician predictions across the board. Indeed, physicians consistently overestimated the mortality risk for all categories except the very-high-risk one, where they underestimated the true risk (Circulation. 2019 Feb 26;139[9]:1146-56).
Given that heart failure remains year after year at the top of the list of most frequent causes for hospital admission, and that there is compelling evidence that many low-risk patients get hospitalized while potentially unsafe early discharges also occur, the EHMRG score fills an important unmet need.
“I think this can help inform us as to who with acute heart failure potentially needs to come into the hospital and who doesn’t,” Dr. Smith said. “I think the sweet spot here is that if you’re in the low- or very-low-risk category, your 7-day mortality is less than 1%; in fact, in this study it’s zero. But once you get to category 3 – the intermediate category – you’re talking about a 7-day mortality of 1%-2%, which I think is high enough to warrant hospital admission for treatment and to watch them, not just send them home.”
The H2FPEF score
Diagnosis of heart failure with preserved ejection fraction (HFpEF) is a challenge in euvolemic patients with clear lungs and dyspnea on exertion. Investigators at the Mayo Clinic have developed and subsequently validated a weighted score known as the H2FPEF score that’s of great assistance in this task. The score is based upon a set of six simple variables universally available in patients undergoing diagnostic workup for the numerous potential causes for dyspnea on exertion. Together these six variables comprise the acronym H2FPEF:
- Heavy: One point for a BMI greater than 30 kg/m2.
- Hypertension: One point for being on two or more antihypertensive drugs.
- Atrial fibrillation: Three points for paroxysmal or persistent AF.
- Pulmonary hypertension: One point for having a Doppler echocardiographic estimated pulmonary artery systolic pressure greater than 35 mm Hg.
- Elder: One point for age greater than 60 years.
- Filling pressure: One point for a Doppler echocardiographic E/e’ ratio above 9.
The total score can range from 0 to 9. (Circulation. 2018 Aug 28;138[9]:861-70).
Each 1-point increase in the score essentially doubled a patient’s risk of having HFpEF as opposed to pulmonary embolism or some other cause for the dyspnea.
“I really like this H2FPEF score. The score works very, very well. Once you get to a score of 6 or above, the probability of HFpEF is more than 90%, which is pretty powerful. I think this is worthwhile,” Dr. Smith said.
In their derivation and validation cohorts, the Mayo Clinic investigators used as their gold standard for diagnosis of HFpEF invasive hemodynamic exercise testing with a pulmonary artery catheter in place to measure pressures. A score that enables hospitalists to lessen the need for that kind of costly invasive testing is most welcome.
“Here’s how I’d use this score: With an H2FPEF score of 0-1, HFpEF is unlikely. With an intermediate score of 2-5, additional testing is warranted. If the score is high, 6-9, I think HFpEF is likely,” the hospitalist said.
Dr. Smith isn’t the only big fan of the H2FPEF score. In an editorial accompanying publication of the score’s validation study, Walter J. Paulus, MD, PhD, hailed the H2FPEF score as “a unique tour de force” which constitutes a major advance beyond the confusing diagnostic recommendations for HFpEF issued by the European Society of Cardiology and the American Society of Echocardiography, which he said have been “met by skepticism qualifying them as overcomplicated and even triggered disbelief in the existence of HFpEF.”
Particularly interesting were the variables rejected for inclusion in the H2FPEF score because they failed to achieve statistical significance as predictors, even though they’re often considered important in defining HFpEF, he noted. These included left atrial volume index, sex, and levels of circulating N-terminal probrain natriuretic peptide, wrote Dr. Paulus, professor of cardiac pathophysiology at VU University, Amsterdam.
Debunking the potassium repletion reflex
Longstanding conventional wisdom holds that patients hospitalized for heart failure need to maintain a serum potassium above 4.0 mEq/L.
“I’m sure you’ve all written orders to keep the potassium greater than 4.0 mEq/L and the magnesium above 2mEq/L about a million times, like I have,” Dr. Smith said.
But it turns out this traditional practice, which involves a huge cost in terms of time, money, and health care resources, is supported by weak evidence – and an important recent study has now debunked what the investigators termed the potassium “repletion reflex.”
The investigators at the University of Massachusetts identified 4,995 patients admitted with exacerbation of acute heart failure and a normal admission serum potassium level of 3.5-5.0 mEq/L. More than 70% received potassium repletion at least once within a 72-hour observation window, during which 2,080 patients maintained a low-normal serum potassium below 4.0 mEq/L, 2,326 had a mid-normal level of 4.0-4.5 mEq/L, and 589 had a high-normal level of more than 4.5 mEq/L but not more than 5.0 mEq/L.
The study had three endpoints: in-hospital mortality, transfer to the intensive care unit, and hospital length of stay. After statistical adjustment for comorbidities, demographics, and severity at admission, there was no difference between the low- and mid-normal serum potassium groups in any of the three endpoints. In contrast, the high-normal potassium group had a significantly longer length of stay, by a median of 0.6 extra days. The high-normal group also had a 78% increased likelihood of ICU transfer and a 51% increased risk of in-hospital mortality, although neither of these differences reached statistical significance (J Hosp Med. 2019 Dec 1;14[12]:729-36).
“A potassium greater than 4.5 mEq/L may be associated with increased risk of worse outcomes,” Dr. Smith observed. “I think the sweet spot may be 3.5-4.5 mEq/L based on this study.”
He reported having no financial conflicts regarding his presentation.
A recently validated, easy-to-use calculator of predicted 7-day mortality risk in patients presenting with acute decompensated heart failure is well worth incorporating into hospitalist clinical practice, Dustin T. Smith, MD, said at HM20 Virtual, hosted by the Society of Hospital Medicine.
In addition to the EHMRG, other highlights of his wide-ranging update on recent practice-changing developments in heart failure directly relevant to hospitalists included the introduction of a simple, evidence-based tool for differentiating heart failure with preserved ejection fraction from other potential causes of unexplained dyspnea on exertion in euvolemic patients, and a study debunking what has been called the potassium repletion reflex in patients with acute heart failure undergoing diuresis.
The ACUTE study
Heart failure is an area of special interest for Dr. Smith. He has been surprised to find that virtually no hospitalists, emergency medicine physicians, or cardiologists he has spoken with have heard of the EHMRG or its validation in the ACUTE (Acute Congestive Heart Failure Urgent Care Evaluation) study. Yet this is a very handy tool for hospitalists, he observed.
The EHMRG algorithm utilizes nine variables for which data is readily available for every patient who arrives at the emergency department with acute heart failure. The variables are age, arrival by ambulance, heart rate, systolic blood pressure, potassium level, oxygen saturation, troponin, serum creatine, and presence or absence of active cancer. The information is entered into a cell phone app, which spits out the patient’s estimated 7-day mortality risk. The algorithm divides patients into one of five risk groups ranging from very low to very high. With the addition of data input as to the presence or absence of ST-segment depression on the 12-lead ECG, the weighted algorithm will simultaneously generate an estimated 30-day mortality risk.
ACUTE was a prospective, observational, real-world validation study of EHMRG involving 1,983 patients seeking emergency department care for acute heart failure at nine Canadian hospitals. The actual 7-day mortality rate was 0% in the very-low-risk group, 0% in the low-risk group, 0.6% with an intermediate-risk EHMRG, 1.9% with high risk, and 3.9% in the very-high-risk group. The corresponding 30-day mortality rates were 0%, 1.9%, 3.9%, 5.9%, and 14.3%.
The University of Toronto investigators also asked participating physicians for their clinical estimates of 7-day mortality risk while blinded to the EHMRG predictions. The algorithm proved more accurate than physician predictions across the board. Indeed, physicians consistently overestimated the mortality risk for all categories except the very-high-risk one, where they underestimated the true risk (Circulation. 2019 Feb 26;139[9]:1146-56).
Given that heart failure remains year after year at the top of the list of most frequent causes for hospital admission, and that there is compelling evidence that many low-risk patients get hospitalized while potentially unsafe early discharges also occur, the EHMRG score fills an important unmet need.
“I think this can help inform us as to who with acute heart failure potentially needs to come into the hospital and who doesn’t,” Dr. Smith said. “I think the sweet spot here is that if you’re in the low- or very-low-risk category, your 7-day mortality is less than 1%; in fact, in this study it’s zero. But once you get to category 3 – the intermediate category – you’re talking about a 7-day mortality of 1%-2%, which I think is high enough to warrant hospital admission for treatment and to watch them, not just send them home.”
The H2FPEF score
Diagnosis of heart failure with preserved ejection fraction (HFpEF) is a challenge in euvolemic patients with clear lungs and dyspnea on exertion. Investigators at the Mayo Clinic have developed and subsequently validated a weighted score known as the H2FPEF score that’s of great assistance in this task. The score is based upon a set of six simple variables universally available in patients undergoing diagnostic workup for the numerous potential causes for dyspnea on exertion. Together these six variables comprise the acronym H2FPEF:
- Heavy: One point for a BMI greater than 30 kg/m2.
- Hypertension: One point for being on two or more antihypertensive drugs.
- Atrial fibrillation: Three points for paroxysmal or persistent AF.
- Pulmonary hypertension: One point for having a Doppler echocardiographic estimated pulmonary artery systolic pressure greater than 35 mm Hg.
- Elder: One point for age greater than 60 years.
- Filling pressure: One point for a Doppler echocardiographic E/e’ ratio above 9.
The total score can range from 0 to 9. (Circulation. 2018 Aug 28;138[9]:861-70).
Each 1-point increase in the score essentially doubled a patient’s risk of having HFpEF as opposed to pulmonary embolism or some other cause for the dyspnea.
“I really like this H2FPEF score. The score works very, very well. Once you get to a score of 6 or above, the probability of HFpEF is more than 90%, which is pretty powerful. I think this is worthwhile,” Dr. Smith said.
In their derivation and validation cohorts, the Mayo Clinic investigators used as their gold standard for diagnosis of HFpEF invasive hemodynamic exercise testing with a pulmonary artery catheter in place to measure pressures. A score that enables hospitalists to lessen the need for that kind of costly invasive testing is most welcome.
“Here’s how I’d use this score: With an H2FPEF score of 0-1, HFpEF is unlikely. With an intermediate score of 2-5, additional testing is warranted. If the score is high, 6-9, I think HFpEF is likely,” the hospitalist said.
Dr. Smith isn’t the only big fan of the H2FPEF score. In an editorial accompanying publication of the score’s validation study, Walter J. Paulus, MD, PhD, hailed the H2FPEF score as “a unique tour de force” which constitutes a major advance beyond the confusing diagnostic recommendations for HFpEF issued by the European Society of Cardiology and the American Society of Echocardiography, which he said have been “met by skepticism qualifying them as overcomplicated and even triggered disbelief in the existence of HFpEF.”
Particularly interesting were the variables rejected for inclusion in the H2FPEF score because they failed to achieve statistical significance as predictors, even though they’re often considered important in defining HFpEF, he noted. These included left atrial volume index, sex, and levels of circulating N-terminal probrain natriuretic peptide, wrote Dr. Paulus, professor of cardiac pathophysiology at VU University, Amsterdam.
Debunking the potassium repletion reflex
Longstanding conventional wisdom holds that patients hospitalized for heart failure need to maintain a serum potassium above 4.0 mEq/L.
“I’m sure you’ve all written orders to keep the potassium greater than 4.0 mEq/L and the magnesium above 2mEq/L about a million times, like I have,” Dr. Smith said.
But it turns out this traditional practice, which involves a huge cost in terms of time, money, and health care resources, is supported by weak evidence – and an important recent study has now debunked what the investigators termed the potassium “repletion reflex.”
The investigators at the University of Massachusetts identified 4,995 patients admitted with exacerbation of acute heart failure and a normal admission serum potassium level of 3.5-5.0 mEq/L. More than 70% received potassium repletion at least once within a 72-hour observation window, during which 2,080 patients maintained a low-normal serum potassium below 4.0 mEq/L, 2,326 had a mid-normal level of 4.0-4.5 mEq/L, and 589 had a high-normal level of more than 4.5 mEq/L but not more than 5.0 mEq/L.
The study had three endpoints: in-hospital mortality, transfer to the intensive care unit, and hospital length of stay. After statistical adjustment for comorbidities, demographics, and severity at admission, there was no difference between the low- and mid-normal serum potassium groups in any of the three endpoints. In contrast, the high-normal potassium group had a significantly longer length of stay, by a median of 0.6 extra days. The high-normal group also had a 78% increased likelihood of ICU transfer and a 51% increased risk of in-hospital mortality, although neither of these differences reached statistical significance (J Hosp Med. 2019 Dec 1;14[12]:729-36).
“A potassium greater than 4.5 mEq/L may be associated with increased risk of worse outcomes,” Dr. Smith observed. “I think the sweet spot may be 3.5-4.5 mEq/L based on this study.”
He reported having no financial conflicts regarding his presentation.
FROM HM20 VIRTUAL
The interesting history of dermatologist-developed skin care
Those of you who have visited my dermatology practice in Miami know that the art in my office is dedicated to the history of the skin care industry. I collect , and I have written this historical column in honor of the 50th anniversary of Dermatology News.
The first doctor to market his own cosmetic product, Erasmus Wilson, MD, faced scrutiny from his colleagues. Although he had contributed much to the field of dermatology, he was criticized by other dermatologists when he promoted a hair wash. The next doctor in my story, William Pusey, MD, was criticized for helping the company that manufactured Camay soap because he allowed his name to be used in Camay advertisements. The scrutiny that these two well-respected dermatologists endured from their colleagues deterred dermatologists from entering the skin care business for decades. The professional jealousy from dermatologic colleagues left the skin care field wide open for imposters, charlatans, and nondermatologists who had no concern for efficacy and patient outcomes to flourish. This is the story of a group of brilliant entrepreneurial dermatologists and one chiropractor who misrepresented himself as a dermatologist and how they influenced skin care as we know it.
Erasmus Wilson, MD1 (1809-1884): In 1840, Erasmus Wilson2 was a physician in London who chose to specialize in dermatology at a time when that specialization was frowned upon. He was a subeditor for The Lancet and wrote several books on dermatology including “Diseases of the Skin – A Practical and Theoretical Treatise,” “Portraits of the Diseases of the Skin,” and “Student’s Book on Diseases of the Skin.” He was the first professor of dermatology in the College of Surgeons and by 1869, was the leading English-speaking dermatologist in the world. He contributed much to dermatology, including his pioneering characterizations of Demodex mites, lichen planus, exfoliative dermatitis, neurotic excoriations, and roseola. Dr. Wilson was knighted in 1881 for his good works and notable generosity. (He was known for giving his poor patients money for food, endowing chairs in dermatology, and donating a famous obelisk in London).
In 1854, Dr. Wilson wrote a book for laypeople called “Healthy Skin: A Popular Treatise on the Skin and Hair, Their Preservation and Management,” in which he advocated cleanliness and bathing, which led to the popularity of Turkish baths and bathing resorts in Europe. Despite his undeniable contributions to dermatology, he was widely criticized by his colleagues for promoting a “Hair Wash” and a turtle oil soap. I cannot find any information about whether or not he developed the hair wash and turtle soap himself, but it seems that he earned income from sales of these two products, even though he was said to have donated it all to charities.
William A. Pusey MD (1865-1940): Dr. Pusey was the first chairman of dermatology at the University of Illinois College of Medicine, Chicago. He published several books, including “Care of the Skin and Hair,” “Syphilis as a Modern Problem,” “The Principles and Practices of Dermatology,” and “History of Dermatology” among others. He is best known for his work in developing the use of x-rays (roentgen rays) and phototherapy in dermatology, and in 1907, he was the first dermatologist to describe the use of solid carbon dioxide to treat skin lesions. He was president of the American Dermatological Association in 1910, president of the Chicago Medical Society in 1918, editor of the Archives of Dermatology in 1920, and president of the American Medical Association in 1924.
In the early 1920s, skin care companies were beginning to advertise their products using endorsements from celebrities and socialites, and were making misleading claims. Dr. Pusey wanted to work with these companies to help them perform evidence-based trials so they could make scientifically correct claims. Proctor & Gamble asked Dr. Pusey to advise them on how they could advertise honestly about their new soap, “Camay.” In Dr. Pusey’s words,3 “they (Proctor & Gamble) wanted to give the public authoritative advice about the use of soap and water. They suggested that I get a group of dermatologists of my selection to examine the soap and prepare instructions for bathing and the use of soap, and, if they found this soap was of high quality, to certify to that effect.” The research was performed as he suggested, and he allowed his name to be used in the Camay soap ads from 1926 to 1929. He said that he allowed them to use his name hoping to promote the need for evidence-based research, in contrast to the skin care products endorsed by socialites and celebrities that were flooding the market around that time.
Herbert Rattner, MD, at Northwestern University, Chicago, was his friend and one of the many dermatologists who criticized Dr. Pusey for allowing his name to be used in the Camay ads. Dr. Pusey’s reply to the criticism (according to Dr. Rattner) was that Proctor & Gamble was “proposing to do what the medical profession always is criticizing commercial concerns for not doing, namely, coming to physicians for information on medical matters. Could the profession hope to have any influence with business concerns if it was always eager to criticize bad commercial practices but never willing to support good ones?”3
While Dr. Pusey felt his reasons for adding his name to the Camay ads and research were justified, many of his friends stated that in hindsight, he regretted the action because of the negative response of his colleagues. It was years before dermatologists began providing input again into the skin care industry. During that time, radio, television and print ads were rampant with misleading claims – which led the way for a dermatologic imposter to make a fortune on skin care.
John Woodbury (1851-1909): John Woodbury, a chiropractor, never went to medical school, but that did not stop him from claiming he was a dermatologist and cosmetic surgeon. In 1889, he opened the John H. Woodbury Dermatological Institute in New York City, and over the next few years, opened Woodbury Dermatological Institutes in at least 5 states and employed 25 “physicians” who were not licensed to practice medicine. He came out with face soaps, tonics, and cold creams and spent a fortune on advertising these products and his institutes. In 1901, he sold his “Woodbury Soap” to the Andrew Jergens Company for $212,500 and 10% in royalties.
Multiple lawsuits occurred from 1898 to 1907 because he continued using the Woodbury name on his own products, despite having sold the “Woodbury” trademark to Jergens. He was sued for practicing medicine without a medical license and claiming to be a dermatologist when he was not. He lost most of these lawsuits, including one in 1907 in which the court ruled that corporations may not employ unlicensed professionals to practice medicine. In 1909, John Woodbury committed suicide. The Woodbury Soap company flourished in the 1930s and 1940s, as part of Jergens, until the brand was discontinued in 1970 when Jergens was acquired by American Brands.
The next dermatologists to come along did not make the same mistakes as those of their predecessors. They all made scientific discoveries through their basic science research in the laboratory, filed patents, formed skin care companies, perfected the formulations, and conducted research trials of the final product. Their marketing focused on science and efficacy and only rarely used their names and images in advertising, allowing them to maintain their reputations in the dermatology field.
Eugene Van Scott, MD (1922-present): Dermatologist Dr. Van Scott and dermatopharmacologist Ruey Yu, PhD, filed a method patent in the early 1970s on the effectiveness of alpha hydroxy acids to treat ichthyosis. They invented the abbreviation “AHA” and have continued their work on organic acids to this day. They now have more than 125 patents, which they have licensed to 60 companies in the cosmetics and pharmaceutical industries.
In 1988, 14 years after their initial publication, they founded the company they named Polystrata, which grew into today’s NeoStrata.4 Over the years, they had to defend their patents because many personal care companies used their technologies without licensing them. In 2007, they won a $41 million settlement in a patent infringement suit against Mary Kay filed in March 2005. They have both been very philanthropic in the dermatology world5 and are highly respected in the field. Among many other honors, Dr. Van Scott was named a Master Dermatologist by the American Academy of Dermatology in 1998 and received the Dermatology Foundation’s Distinguished Service Medallion in 2004.
Sheldon Pinnell, MD (1937-2013): After Dr. Pinnell completed his dermatology residency at Harvard Medical School, he spent 2 years studying collagen chemistry at the Max Planck Institute in Munich, Germany. In 1973, he returned to Duke University where he had earned his undergraduate degree before attending Yale University. He remained at Duke for the duration of his career and was professor and chief of dermatology there for many years. Early in his career, he focused on the role of vitamin C in collagen biosynthesis and discovered some of the mechanisms by which sun exposure causes photoaging. He described the use of the first (and most popular) topically applied L-ascorbic acid (vitamin C) to prevent and treat skin aging.
Dr. Pinnell’s many discoveries include showing that the addition of ascorbic acid to fibroblast cultures increases collagen production and that topically applied L-ascorbic acid penetrates into the skin best at a pH of 2-2.5. Dr. Pinnell changed the way the world uses topical antioxidants today; he was widely respected and was a member of the American Dermatological Association and an honorary member of the Society of Investigative Dermatology. He published more than 200 scientific articles and held 10 patents. He started the skin care company Skinceuticals, based on his antioxidant technologies. It was acquired by L’Oreal in 2005.
Richard Fitzpatrick, MD (1944-2014): The dermatologist affectionately known as “Fitz” is credited with being the first to use lasers for skin resurfacing. He went to medical school at Emory University and did his dermatology residency at the University of California, Los Angeles. He authored more than 130 publications and was one of the first doctors to specialize in cosmetic dermatology. He realized that fibroblast cell cultures used to produce the collagen filler CosmoPlast (no longer on the market) generated many growth factors that could rejuvenate the skin, and in 1999, he launched the skin care brand SkinMedica. In 2000, he received a patent for fibroblast-derived growth factors used topically for antiaging – a formula he called Tissue Nutrient Solution. In 2001, the popular product TNS Recovery Complex was launched based on the patented growth factor technology. It is still the most popular growth factor technology on the market.
What can we learn from these pioneers? I have had several interesting discussions about this topic with Leonard Hoenig, MD, section editor for Reflections on Dermatology: Past, Present, and Future, in Clinics in Dermatology. (Dr. Hoenig told me the interesting story that Listerine mouthwash was named in honor of Joseph Lister but accounts vary as to whether he gave permission to do so. This makes Dr. Lister the most famous physician to endorse a personal care product.) When Dr. Hoenig and I discussed the ethics of dermatologists creating a skin care line or retailing skin care in their medical practice, he stated my sentiments perfectly: “We should rely on professional, ethical, and legal guidelines to help us do what is right. Most importantly, we should have the best interests of our patients at heart when recommending any treatments.”
Dermatologists have unique knowledge, experience, and perspective on treating the skin with topical agents and have the true desire to improve skin health. If we do not discover, research, patent, and develop efficacious skin care products, someone else will do it – and I do not think they will do it as well as a dermatologist can.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Everett MA. Int J Dermatol. 1978 May;17(4):345-52.
2. Moxon RK. N Engl J Med. 1976 Apr 1;294(14):762-4.
3. Rattner H. Arch Derm Syphilol. 1937;35(1):25-66.
4. Neostrata: More than Hope, by Elaine Strauss, U.S. 1 Newspaper, Feb. 24, 1999.
5. Two legends in the field of dermatology provide $1 million gift to Temple University school of medicine’s department of dermatology, Temple University, June 5, 2015.
Those of you who have visited my dermatology practice in Miami know that the art in my office is dedicated to the history of the skin care industry. I collect , and I have written this historical column in honor of the 50th anniversary of Dermatology News.
The first doctor to market his own cosmetic product, Erasmus Wilson, MD, faced scrutiny from his colleagues. Although he had contributed much to the field of dermatology, he was criticized by other dermatologists when he promoted a hair wash. The next doctor in my story, William Pusey, MD, was criticized for helping the company that manufactured Camay soap because he allowed his name to be used in Camay advertisements. The scrutiny that these two well-respected dermatologists endured from their colleagues deterred dermatologists from entering the skin care business for decades. The professional jealousy from dermatologic colleagues left the skin care field wide open for imposters, charlatans, and nondermatologists who had no concern for efficacy and patient outcomes to flourish. This is the story of a group of brilliant entrepreneurial dermatologists and one chiropractor who misrepresented himself as a dermatologist and how they influenced skin care as we know it.
Erasmus Wilson, MD1 (1809-1884): In 1840, Erasmus Wilson2 was a physician in London who chose to specialize in dermatology at a time when that specialization was frowned upon. He was a subeditor for The Lancet and wrote several books on dermatology including “Diseases of the Skin – A Practical and Theoretical Treatise,” “Portraits of the Diseases of the Skin,” and “Student’s Book on Diseases of the Skin.” He was the first professor of dermatology in the College of Surgeons and by 1869, was the leading English-speaking dermatologist in the world. He contributed much to dermatology, including his pioneering characterizations of Demodex mites, lichen planus, exfoliative dermatitis, neurotic excoriations, and roseola. Dr. Wilson was knighted in 1881 for his good works and notable generosity. (He was known for giving his poor patients money for food, endowing chairs in dermatology, and donating a famous obelisk in London).
In 1854, Dr. Wilson wrote a book for laypeople called “Healthy Skin: A Popular Treatise on the Skin and Hair, Their Preservation and Management,” in which he advocated cleanliness and bathing, which led to the popularity of Turkish baths and bathing resorts in Europe. Despite his undeniable contributions to dermatology, he was widely criticized by his colleagues for promoting a “Hair Wash” and a turtle oil soap. I cannot find any information about whether or not he developed the hair wash and turtle soap himself, but it seems that he earned income from sales of these two products, even though he was said to have donated it all to charities.
William A. Pusey MD (1865-1940): Dr. Pusey was the first chairman of dermatology at the University of Illinois College of Medicine, Chicago. He published several books, including “Care of the Skin and Hair,” “Syphilis as a Modern Problem,” “The Principles and Practices of Dermatology,” and “History of Dermatology” among others. He is best known for his work in developing the use of x-rays (roentgen rays) and phototherapy in dermatology, and in 1907, he was the first dermatologist to describe the use of solid carbon dioxide to treat skin lesions. He was president of the American Dermatological Association in 1910, president of the Chicago Medical Society in 1918, editor of the Archives of Dermatology in 1920, and president of the American Medical Association in 1924.
In the early 1920s, skin care companies were beginning to advertise their products using endorsements from celebrities and socialites, and were making misleading claims. Dr. Pusey wanted to work with these companies to help them perform evidence-based trials so they could make scientifically correct claims. Proctor & Gamble asked Dr. Pusey to advise them on how they could advertise honestly about their new soap, “Camay.” In Dr. Pusey’s words,3 “they (Proctor & Gamble) wanted to give the public authoritative advice about the use of soap and water. They suggested that I get a group of dermatologists of my selection to examine the soap and prepare instructions for bathing and the use of soap, and, if they found this soap was of high quality, to certify to that effect.” The research was performed as he suggested, and he allowed his name to be used in the Camay soap ads from 1926 to 1929. He said that he allowed them to use his name hoping to promote the need for evidence-based research, in contrast to the skin care products endorsed by socialites and celebrities that were flooding the market around that time.
Herbert Rattner, MD, at Northwestern University, Chicago, was his friend and one of the many dermatologists who criticized Dr. Pusey for allowing his name to be used in the Camay ads. Dr. Pusey’s reply to the criticism (according to Dr. Rattner) was that Proctor & Gamble was “proposing to do what the medical profession always is criticizing commercial concerns for not doing, namely, coming to physicians for information on medical matters. Could the profession hope to have any influence with business concerns if it was always eager to criticize bad commercial practices but never willing to support good ones?”3
While Dr. Pusey felt his reasons for adding his name to the Camay ads and research were justified, many of his friends stated that in hindsight, he regretted the action because of the negative response of his colleagues. It was years before dermatologists began providing input again into the skin care industry. During that time, radio, television and print ads were rampant with misleading claims – which led the way for a dermatologic imposter to make a fortune on skin care.
John Woodbury (1851-1909): John Woodbury, a chiropractor, never went to medical school, but that did not stop him from claiming he was a dermatologist and cosmetic surgeon. In 1889, he opened the John H. Woodbury Dermatological Institute in New York City, and over the next few years, opened Woodbury Dermatological Institutes in at least 5 states and employed 25 “physicians” who were not licensed to practice medicine. He came out with face soaps, tonics, and cold creams and spent a fortune on advertising these products and his institutes. In 1901, he sold his “Woodbury Soap” to the Andrew Jergens Company for $212,500 and 10% in royalties.
Multiple lawsuits occurred from 1898 to 1907 because he continued using the Woodbury name on his own products, despite having sold the “Woodbury” trademark to Jergens. He was sued for practicing medicine without a medical license and claiming to be a dermatologist when he was not. He lost most of these lawsuits, including one in 1907 in which the court ruled that corporations may not employ unlicensed professionals to practice medicine. In 1909, John Woodbury committed suicide. The Woodbury Soap company flourished in the 1930s and 1940s, as part of Jergens, until the brand was discontinued in 1970 when Jergens was acquired by American Brands.
The next dermatologists to come along did not make the same mistakes as those of their predecessors. They all made scientific discoveries through their basic science research in the laboratory, filed patents, formed skin care companies, perfected the formulations, and conducted research trials of the final product. Their marketing focused on science and efficacy and only rarely used their names and images in advertising, allowing them to maintain their reputations in the dermatology field.
Eugene Van Scott, MD (1922-present): Dermatologist Dr. Van Scott and dermatopharmacologist Ruey Yu, PhD, filed a method patent in the early 1970s on the effectiveness of alpha hydroxy acids to treat ichthyosis. They invented the abbreviation “AHA” and have continued their work on organic acids to this day. They now have more than 125 patents, which they have licensed to 60 companies in the cosmetics and pharmaceutical industries.
In 1988, 14 years after their initial publication, they founded the company they named Polystrata, which grew into today’s NeoStrata.4 Over the years, they had to defend their patents because many personal care companies used their technologies without licensing them. In 2007, they won a $41 million settlement in a patent infringement suit against Mary Kay filed in March 2005. They have both been very philanthropic in the dermatology world5 and are highly respected in the field. Among many other honors, Dr. Van Scott was named a Master Dermatologist by the American Academy of Dermatology in 1998 and received the Dermatology Foundation’s Distinguished Service Medallion in 2004.
Sheldon Pinnell, MD (1937-2013): After Dr. Pinnell completed his dermatology residency at Harvard Medical School, he spent 2 years studying collagen chemistry at the Max Planck Institute in Munich, Germany. In 1973, he returned to Duke University where he had earned his undergraduate degree before attending Yale University. He remained at Duke for the duration of his career and was professor and chief of dermatology there for many years. Early in his career, he focused on the role of vitamin C in collagen biosynthesis and discovered some of the mechanisms by which sun exposure causes photoaging. He described the use of the first (and most popular) topically applied L-ascorbic acid (vitamin C) to prevent and treat skin aging.
Dr. Pinnell’s many discoveries include showing that the addition of ascorbic acid to fibroblast cultures increases collagen production and that topically applied L-ascorbic acid penetrates into the skin best at a pH of 2-2.5. Dr. Pinnell changed the way the world uses topical antioxidants today; he was widely respected and was a member of the American Dermatological Association and an honorary member of the Society of Investigative Dermatology. He published more than 200 scientific articles and held 10 patents. He started the skin care company Skinceuticals, based on his antioxidant technologies. It was acquired by L’Oreal in 2005.
Richard Fitzpatrick, MD (1944-2014): The dermatologist affectionately known as “Fitz” is credited with being the first to use lasers for skin resurfacing. He went to medical school at Emory University and did his dermatology residency at the University of California, Los Angeles. He authored more than 130 publications and was one of the first doctors to specialize in cosmetic dermatology. He realized that fibroblast cell cultures used to produce the collagen filler CosmoPlast (no longer on the market) generated many growth factors that could rejuvenate the skin, and in 1999, he launched the skin care brand SkinMedica. In 2000, he received a patent for fibroblast-derived growth factors used topically for antiaging – a formula he called Tissue Nutrient Solution. In 2001, the popular product TNS Recovery Complex was launched based on the patented growth factor technology. It is still the most popular growth factor technology on the market.
What can we learn from these pioneers? I have had several interesting discussions about this topic with Leonard Hoenig, MD, section editor for Reflections on Dermatology: Past, Present, and Future, in Clinics in Dermatology. (Dr. Hoenig told me the interesting story that Listerine mouthwash was named in honor of Joseph Lister but accounts vary as to whether he gave permission to do so. This makes Dr. Lister the most famous physician to endorse a personal care product.) When Dr. Hoenig and I discussed the ethics of dermatologists creating a skin care line or retailing skin care in their medical practice, he stated my sentiments perfectly: “We should rely on professional, ethical, and legal guidelines to help us do what is right. Most importantly, we should have the best interests of our patients at heart when recommending any treatments.”
Dermatologists have unique knowledge, experience, and perspective on treating the skin with topical agents and have the true desire to improve skin health. If we do not discover, research, patent, and develop efficacious skin care products, someone else will do it – and I do not think they will do it as well as a dermatologist can.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Everett MA. Int J Dermatol. 1978 May;17(4):345-52.
2. Moxon RK. N Engl J Med. 1976 Apr 1;294(14):762-4.
3. Rattner H. Arch Derm Syphilol. 1937;35(1):25-66.
4. Neostrata: More than Hope, by Elaine Strauss, U.S. 1 Newspaper, Feb. 24, 1999.
5. Two legends in the field of dermatology provide $1 million gift to Temple University school of medicine’s department of dermatology, Temple University, June 5, 2015.
Those of you who have visited my dermatology practice in Miami know that the art in my office is dedicated to the history of the skin care industry. I collect , and I have written this historical column in honor of the 50th anniversary of Dermatology News.
The first doctor to market his own cosmetic product, Erasmus Wilson, MD, faced scrutiny from his colleagues. Although he had contributed much to the field of dermatology, he was criticized by other dermatologists when he promoted a hair wash. The next doctor in my story, William Pusey, MD, was criticized for helping the company that manufactured Camay soap because he allowed his name to be used in Camay advertisements. The scrutiny that these two well-respected dermatologists endured from their colleagues deterred dermatologists from entering the skin care business for decades. The professional jealousy from dermatologic colleagues left the skin care field wide open for imposters, charlatans, and nondermatologists who had no concern for efficacy and patient outcomes to flourish. This is the story of a group of brilliant entrepreneurial dermatologists and one chiropractor who misrepresented himself as a dermatologist and how they influenced skin care as we know it.
Erasmus Wilson, MD1 (1809-1884): In 1840, Erasmus Wilson2 was a physician in London who chose to specialize in dermatology at a time when that specialization was frowned upon. He was a subeditor for The Lancet and wrote several books on dermatology including “Diseases of the Skin – A Practical and Theoretical Treatise,” “Portraits of the Diseases of the Skin,” and “Student’s Book on Diseases of the Skin.” He was the first professor of dermatology in the College of Surgeons and by 1869, was the leading English-speaking dermatologist in the world. He contributed much to dermatology, including his pioneering characterizations of Demodex mites, lichen planus, exfoliative dermatitis, neurotic excoriations, and roseola. Dr. Wilson was knighted in 1881 for his good works and notable generosity. (He was known for giving his poor patients money for food, endowing chairs in dermatology, and donating a famous obelisk in London).
In 1854, Dr. Wilson wrote a book for laypeople called “Healthy Skin: A Popular Treatise on the Skin and Hair, Their Preservation and Management,” in which he advocated cleanliness and bathing, which led to the popularity of Turkish baths and bathing resorts in Europe. Despite his undeniable contributions to dermatology, he was widely criticized by his colleagues for promoting a “Hair Wash” and a turtle oil soap. I cannot find any information about whether or not he developed the hair wash and turtle soap himself, but it seems that he earned income from sales of these two products, even though he was said to have donated it all to charities.
William A. Pusey MD (1865-1940): Dr. Pusey was the first chairman of dermatology at the University of Illinois College of Medicine, Chicago. He published several books, including “Care of the Skin and Hair,” “Syphilis as a Modern Problem,” “The Principles and Practices of Dermatology,” and “History of Dermatology” among others. He is best known for his work in developing the use of x-rays (roentgen rays) and phototherapy in dermatology, and in 1907, he was the first dermatologist to describe the use of solid carbon dioxide to treat skin lesions. He was president of the American Dermatological Association in 1910, president of the Chicago Medical Society in 1918, editor of the Archives of Dermatology in 1920, and president of the American Medical Association in 1924.
In the early 1920s, skin care companies were beginning to advertise their products using endorsements from celebrities and socialites, and were making misleading claims. Dr. Pusey wanted to work with these companies to help them perform evidence-based trials so they could make scientifically correct claims. Proctor & Gamble asked Dr. Pusey to advise them on how they could advertise honestly about their new soap, “Camay.” In Dr. Pusey’s words,3 “they (Proctor & Gamble) wanted to give the public authoritative advice about the use of soap and water. They suggested that I get a group of dermatologists of my selection to examine the soap and prepare instructions for bathing and the use of soap, and, if they found this soap was of high quality, to certify to that effect.” The research was performed as he suggested, and he allowed his name to be used in the Camay soap ads from 1926 to 1929. He said that he allowed them to use his name hoping to promote the need for evidence-based research, in contrast to the skin care products endorsed by socialites and celebrities that were flooding the market around that time.
Herbert Rattner, MD, at Northwestern University, Chicago, was his friend and one of the many dermatologists who criticized Dr. Pusey for allowing his name to be used in the Camay ads. Dr. Pusey’s reply to the criticism (according to Dr. Rattner) was that Proctor & Gamble was “proposing to do what the medical profession always is criticizing commercial concerns for not doing, namely, coming to physicians for information on medical matters. Could the profession hope to have any influence with business concerns if it was always eager to criticize bad commercial practices but never willing to support good ones?”3
While Dr. Pusey felt his reasons for adding his name to the Camay ads and research were justified, many of his friends stated that in hindsight, he regretted the action because of the negative response of his colleagues. It was years before dermatologists began providing input again into the skin care industry. During that time, radio, television and print ads were rampant with misleading claims – which led the way for a dermatologic imposter to make a fortune on skin care.
John Woodbury (1851-1909): John Woodbury, a chiropractor, never went to medical school, but that did not stop him from claiming he was a dermatologist and cosmetic surgeon. In 1889, he opened the John H. Woodbury Dermatological Institute in New York City, and over the next few years, opened Woodbury Dermatological Institutes in at least 5 states and employed 25 “physicians” who were not licensed to practice medicine. He came out with face soaps, tonics, and cold creams and spent a fortune on advertising these products and his institutes. In 1901, he sold his “Woodbury Soap” to the Andrew Jergens Company for $212,500 and 10% in royalties.
Multiple lawsuits occurred from 1898 to 1907 because he continued using the Woodbury name on his own products, despite having sold the “Woodbury” trademark to Jergens. He was sued for practicing medicine without a medical license and claiming to be a dermatologist when he was not. He lost most of these lawsuits, including one in 1907 in which the court ruled that corporations may not employ unlicensed professionals to practice medicine. In 1909, John Woodbury committed suicide. The Woodbury Soap company flourished in the 1930s and 1940s, as part of Jergens, until the brand was discontinued in 1970 when Jergens was acquired by American Brands.
The next dermatologists to come along did not make the same mistakes as those of their predecessors. They all made scientific discoveries through their basic science research in the laboratory, filed patents, formed skin care companies, perfected the formulations, and conducted research trials of the final product. Their marketing focused on science and efficacy and only rarely used their names and images in advertising, allowing them to maintain their reputations in the dermatology field.
Eugene Van Scott, MD (1922-present): Dermatologist Dr. Van Scott and dermatopharmacologist Ruey Yu, PhD, filed a method patent in the early 1970s on the effectiveness of alpha hydroxy acids to treat ichthyosis. They invented the abbreviation “AHA” and have continued their work on organic acids to this day. They now have more than 125 patents, which they have licensed to 60 companies in the cosmetics and pharmaceutical industries.
In 1988, 14 years after their initial publication, they founded the company they named Polystrata, which grew into today’s NeoStrata.4 Over the years, they had to defend their patents because many personal care companies used their technologies without licensing them. In 2007, they won a $41 million settlement in a patent infringement suit against Mary Kay filed in March 2005. They have both been very philanthropic in the dermatology world5 and are highly respected in the field. Among many other honors, Dr. Van Scott was named a Master Dermatologist by the American Academy of Dermatology in 1998 and received the Dermatology Foundation’s Distinguished Service Medallion in 2004.
Sheldon Pinnell, MD (1937-2013): After Dr. Pinnell completed his dermatology residency at Harvard Medical School, he spent 2 years studying collagen chemistry at the Max Planck Institute in Munich, Germany. In 1973, he returned to Duke University where he had earned his undergraduate degree before attending Yale University. He remained at Duke for the duration of his career and was professor and chief of dermatology there for many years. Early in his career, he focused on the role of vitamin C in collagen biosynthesis and discovered some of the mechanisms by which sun exposure causes photoaging. He described the use of the first (and most popular) topically applied L-ascorbic acid (vitamin C) to prevent and treat skin aging.
Dr. Pinnell’s many discoveries include showing that the addition of ascorbic acid to fibroblast cultures increases collagen production and that topically applied L-ascorbic acid penetrates into the skin best at a pH of 2-2.5. Dr. Pinnell changed the way the world uses topical antioxidants today; he was widely respected and was a member of the American Dermatological Association and an honorary member of the Society of Investigative Dermatology. He published more than 200 scientific articles and held 10 patents. He started the skin care company Skinceuticals, based on his antioxidant technologies. It was acquired by L’Oreal in 2005.
Richard Fitzpatrick, MD (1944-2014): The dermatologist affectionately known as “Fitz” is credited with being the first to use lasers for skin resurfacing. He went to medical school at Emory University and did his dermatology residency at the University of California, Los Angeles. He authored more than 130 publications and was one of the first doctors to specialize in cosmetic dermatology. He realized that fibroblast cell cultures used to produce the collagen filler CosmoPlast (no longer on the market) generated many growth factors that could rejuvenate the skin, and in 1999, he launched the skin care brand SkinMedica. In 2000, he received a patent for fibroblast-derived growth factors used topically for antiaging – a formula he called Tissue Nutrient Solution. In 2001, the popular product TNS Recovery Complex was launched based on the patented growth factor technology. It is still the most popular growth factor technology on the market.
What can we learn from these pioneers? I have had several interesting discussions about this topic with Leonard Hoenig, MD, section editor for Reflections on Dermatology: Past, Present, and Future, in Clinics in Dermatology. (Dr. Hoenig told me the interesting story that Listerine mouthwash was named in honor of Joseph Lister but accounts vary as to whether he gave permission to do so. This makes Dr. Lister the most famous physician to endorse a personal care product.) When Dr. Hoenig and I discussed the ethics of dermatologists creating a skin care line or retailing skin care in their medical practice, he stated my sentiments perfectly: “We should rely on professional, ethical, and legal guidelines to help us do what is right. Most importantly, we should have the best interests of our patients at heart when recommending any treatments.”
Dermatologists have unique knowledge, experience, and perspective on treating the skin with topical agents and have the true desire to improve skin health. If we do not discover, research, patent, and develop efficacious skin care products, someone else will do it – and I do not think they will do it as well as a dermatologist can.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Everett MA. Int J Dermatol. 1978 May;17(4):345-52.
2. Moxon RK. N Engl J Med. 1976 Apr 1;294(14):762-4.
3. Rattner H. Arch Derm Syphilol. 1937;35(1):25-66.
4. Neostrata: More than Hope, by Elaine Strauss, U.S. 1 Newspaper, Feb. 24, 1999.
5. Two legends in the field of dermatology provide $1 million gift to Temple University school of medicine’s department of dermatology, Temple University, June 5, 2015.
Convalescent plasma actions spark trial recruitment concerns
The agency’s move took many investigators by surprise. The EUA was announced at the White House the day after President Donald J. Trump accused the FDA of delaying approval of therapeutics to hurt his re-election chances.
In a memo describing the decision, the FDA cited data from some controlled and uncontrolled studies and, primarily, data from an open-label expanded-access protocol overseen by the Mayo Clinic.
At the White House, FDA Commissioner Stephen Hahn, MD, said that plasma had been found to save the lives of 35 out of every 100 who were treated. That figure was later found to have been erroneous, and many experts pointed out that Hahn had conflated an absolute risk reduction with a relative reduction. After a firestorm of criticism, Hahn issued an apology.
“The criticism is entirely justified,” he tweeted. “What I should have said better is that the data show a relative risk reduction not an absolute risk reduction.”
About 15 randomized controlled trials – out of 54 total studies involving convalescent plasma – are underway in the United States, according to ClinicalTrials.gov. The FDA’s Aug. 23 emergency authorization gave clinicians wide leeway to employ convalescent plasma in patients hospitalized with COVID-19.
The agency noted, however, that “adequate and well-controlled randomized trials remain necessary for a definitive demonstration of COVID-19 convalescent plasma efficacy and to determine the optimal product attributes and appropriate patient populations for its use.”
But it’s not clear that people with COVID-19, especially those who are severely ill and hospitalized, will choose to enlist in a clinical trial – where they could receive a placebo – when they instead could get plasma.
“I’ve been asked repeatedly whether the EUA will affect our ability to recruit people into our hospitalized patient trial,” said Liise-anne Pirofski, MD, FIDSA, chief of the department of medicine, infectious diseases division at Albert Einstein College of Medicine and Montefiore Medical Center in the Bronx, New York. “I do not know,” she said, on a call with reporters organized by the Infectious Diseases Society of America.
“But,” she said, “I do know that the trial will continue and that we will discuss the evidence that we have with our patients and give them all that we can to help them weigh the evidence and make up their minds.”
Pirofski said the study being conducted at Montefiore and four other sites has since late April enrolled 190 patients out of a hoped-for 300.
When the study – which compares convalescent plasma to saline in hospitalized patients – was first designed, “there was not any funding for our trial and honestly not a whole lot of interest,” Pirofski told reporters. Individual donors helped support the initial rollout in late April and the trial quickly enrolled 150 patients as the pandemic peaked in the New York City area.
The National Institutes of Health has since given funding, which allowed the study to expand to New York University, Yale University, the University of Miami, and the University of Texas at Houston.
Hopeful, but a long way to go
Shmuel Shoham, MD, FIDSA, associate director of the transplant and oncology infectious diseases center at Johns Hopkins University School of Medicine in Baltimore, said that he’s hopeful that people will continue to enroll in his trial, which is seeking to determine if plasma can prevent COVID-19 in those who’ve been recently exposed.
“Volunteers joining the study is the only way that we’re going to get to know whether this stuff works for prevention and treatment,” Shoham said on the call. He urged physicians and other healthcare workers to talk with patients about considering trial participation.
Shoham’s study is being conducted at 30 US sites and one at the Navajo Nation. It has enrolled 25 out of a hoped-for 500 participants. “We have a long way to go,” said Shoham.
Another Hopkins study to determine whether plasma is helpful in shortening illness in nonhospitalized patients, which is being conducted at the same 31 sites, has enrolled 50 out of 600.
Shoham said recruiting patients with COVID for any study had proven to be difficult. “The vast majority of people that have coronavirus do not come to centers that do clinical trials or interventional trials,” he said, adding that, in addition, most of those “who have coronavirus don’t want to be in a trial. They just want to have coronavirus and get it over with.”
But it’s important to understand how to conduct trials in a pandemic – in part to get answers quickly, he said. Researchers have been looking at convalescent plasma for months, said Shoham. “Why don’t we have the randomized clinical trial data that we want?”
Pirofski noted that trials have also been hobbled in part by “the shifting areas of the pandemic.” Fewer cases make for fewer potential plasma donors.
Both Shoham and Pirofski also said that more needed to be done to encourage plasma donors to participate.
The US Department of Health & Human Services clarified in August that hospitals, physicians, health plans, and other health care workers could contact individuals who had recovered from COVID-19 without violating the HIPAA privacy rule.
Pirofski said she believes that trial investigators know it is legal to reach out to patients. But, she said, “it probably could be better known.”
This article first appeared on Medscape.com.
The agency’s move took many investigators by surprise. The EUA was announced at the White House the day after President Donald J. Trump accused the FDA of delaying approval of therapeutics to hurt his re-election chances.
In a memo describing the decision, the FDA cited data from some controlled and uncontrolled studies and, primarily, data from an open-label expanded-access protocol overseen by the Mayo Clinic.
At the White House, FDA Commissioner Stephen Hahn, MD, said that plasma had been found to save the lives of 35 out of every 100 who were treated. That figure was later found to have been erroneous, and many experts pointed out that Hahn had conflated an absolute risk reduction with a relative reduction. After a firestorm of criticism, Hahn issued an apology.
“The criticism is entirely justified,” he tweeted. “What I should have said better is that the data show a relative risk reduction not an absolute risk reduction.”
About 15 randomized controlled trials – out of 54 total studies involving convalescent plasma – are underway in the United States, according to ClinicalTrials.gov. The FDA’s Aug. 23 emergency authorization gave clinicians wide leeway to employ convalescent plasma in patients hospitalized with COVID-19.
The agency noted, however, that “adequate and well-controlled randomized trials remain necessary for a definitive demonstration of COVID-19 convalescent plasma efficacy and to determine the optimal product attributes and appropriate patient populations for its use.”
But it’s not clear that people with COVID-19, especially those who are severely ill and hospitalized, will choose to enlist in a clinical trial – where they could receive a placebo – when they instead could get plasma.
“I’ve been asked repeatedly whether the EUA will affect our ability to recruit people into our hospitalized patient trial,” said Liise-anne Pirofski, MD, FIDSA, chief of the department of medicine, infectious diseases division at Albert Einstein College of Medicine and Montefiore Medical Center in the Bronx, New York. “I do not know,” she said, on a call with reporters organized by the Infectious Diseases Society of America.
“But,” she said, “I do know that the trial will continue and that we will discuss the evidence that we have with our patients and give them all that we can to help them weigh the evidence and make up their minds.”
Pirofski said the study being conducted at Montefiore and four other sites has since late April enrolled 190 patients out of a hoped-for 300.
When the study – which compares convalescent plasma to saline in hospitalized patients – was first designed, “there was not any funding for our trial and honestly not a whole lot of interest,” Pirofski told reporters. Individual donors helped support the initial rollout in late April and the trial quickly enrolled 150 patients as the pandemic peaked in the New York City area.
The National Institutes of Health has since given funding, which allowed the study to expand to New York University, Yale University, the University of Miami, and the University of Texas at Houston.
Hopeful, but a long way to go
Shmuel Shoham, MD, FIDSA, associate director of the transplant and oncology infectious diseases center at Johns Hopkins University School of Medicine in Baltimore, said that he’s hopeful that people will continue to enroll in his trial, which is seeking to determine if plasma can prevent COVID-19 in those who’ve been recently exposed.
“Volunteers joining the study is the only way that we’re going to get to know whether this stuff works for prevention and treatment,” Shoham said on the call. He urged physicians and other healthcare workers to talk with patients about considering trial participation.
Shoham’s study is being conducted at 30 US sites and one at the Navajo Nation. It has enrolled 25 out of a hoped-for 500 participants. “We have a long way to go,” said Shoham.
Another Hopkins study to determine whether plasma is helpful in shortening illness in nonhospitalized patients, which is being conducted at the same 31 sites, has enrolled 50 out of 600.
Shoham said recruiting patients with COVID for any study had proven to be difficult. “The vast majority of people that have coronavirus do not come to centers that do clinical trials or interventional trials,” he said, adding that, in addition, most of those “who have coronavirus don’t want to be in a trial. They just want to have coronavirus and get it over with.”
But it’s important to understand how to conduct trials in a pandemic – in part to get answers quickly, he said. Researchers have been looking at convalescent plasma for months, said Shoham. “Why don’t we have the randomized clinical trial data that we want?”
Pirofski noted that trials have also been hobbled in part by “the shifting areas of the pandemic.” Fewer cases make for fewer potential plasma donors.
Both Shoham and Pirofski also said that more needed to be done to encourage plasma donors to participate.
The US Department of Health & Human Services clarified in August that hospitals, physicians, health plans, and other health care workers could contact individuals who had recovered from COVID-19 without violating the HIPAA privacy rule.
Pirofski said she believes that trial investigators know it is legal to reach out to patients. But, she said, “it probably could be better known.”
This article first appeared on Medscape.com.
The agency’s move took many investigators by surprise. The EUA was announced at the White House the day after President Donald J. Trump accused the FDA of delaying approval of therapeutics to hurt his re-election chances.
In a memo describing the decision, the FDA cited data from some controlled and uncontrolled studies and, primarily, data from an open-label expanded-access protocol overseen by the Mayo Clinic.
At the White House, FDA Commissioner Stephen Hahn, MD, said that plasma had been found to save the lives of 35 out of every 100 who were treated. That figure was later found to have been erroneous, and many experts pointed out that Hahn had conflated an absolute risk reduction with a relative reduction. After a firestorm of criticism, Hahn issued an apology.
“The criticism is entirely justified,” he tweeted. “What I should have said better is that the data show a relative risk reduction not an absolute risk reduction.”
About 15 randomized controlled trials – out of 54 total studies involving convalescent plasma – are underway in the United States, according to ClinicalTrials.gov. The FDA’s Aug. 23 emergency authorization gave clinicians wide leeway to employ convalescent plasma in patients hospitalized with COVID-19.
The agency noted, however, that “adequate and well-controlled randomized trials remain necessary for a definitive demonstration of COVID-19 convalescent plasma efficacy and to determine the optimal product attributes and appropriate patient populations for its use.”
But it’s not clear that people with COVID-19, especially those who are severely ill and hospitalized, will choose to enlist in a clinical trial – where they could receive a placebo – when they instead could get plasma.
“I’ve been asked repeatedly whether the EUA will affect our ability to recruit people into our hospitalized patient trial,” said Liise-anne Pirofski, MD, FIDSA, chief of the department of medicine, infectious diseases division at Albert Einstein College of Medicine and Montefiore Medical Center in the Bronx, New York. “I do not know,” she said, on a call with reporters organized by the Infectious Diseases Society of America.
“But,” she said, “I do know that the trial will continue and that we will discuss the evidence that we have with our patients and give them all that we can to help them weigh the evidence and make up their minds.”
Pirofski said the study being conducted at Montefiore and four other sites has since late April enrolled 190 patients out of a hoped-for 300.
When the study – which compares convalescent plasma to saline in hospitalized patients – was first designed, “there was not any funding for our trial and honestly not a whole lot of interest,” Pirofski told reporters. Individual donors helped support the initial rollout in late April and the trial quickly enrolled 150 patients as the pandemic peaked in the New York City area.
The National Institutes of Health has since given funding, which allowed the study to expand to New York University, Yale University, the University of Miami, and the University of Texas at Houston.
Hopeful, but a long way to go
Shmuel Shoham, MD, FIDSA, associate director of the transplant and oncology infectious diseases center at Johns Hopkins University School of Medicine in Baltimore, said that he’s hopeful that people will continue to enroll in his trial, which is seeking to determine if plasma can prevent COVID-19 in those who’ve been recently exposed.
“Volunteers joining the study is the only way that we’re going to get to know whether this stuff works for prevention and treatment,” Shoham said on the call. He urged physicians and other healthcare workers to talk with patients about considering trial participation.
Shoham’s study is being conducted at 30 US sites and one at the Navajo Nation. It has enrolled 25 out of a hoped-for 500 participants. “We have a long way to go,” said Shoham.
Another Hopkins study to determine whether plasma is helpful in shortening illness in nonhospitalized patients, which is being conducted at the same 31 sites, has enrolled 50 out of 600.
Shoham said recruiting patients with COVID for any study had proven to be difficult. “The vast majority of people that have coronavirus do not come to centers that do clinical trials or interventional trials,” he said, adding that, in addition, most of those “who have coronavirus don’t want to be in a trial. They just want to have coronavirus and get it over with.”
But it’s important to understand how to conduct trials in a pandemic – in part to get answers quickly, he said. Researchers have been looking at convalescent plasma for months, said Shoham. “Why don’t we have the randomized clinical trial data that we want?”
Pirofski noted that trials have also been hobbled in part by “the shifting areas of the pandemic.” Fewer cases make for fewer potential plasma donors.
Both Shoham and Pirofski also said that more needed to be done to encourage plasma donors to participate.
The US Department of Health & Human Services clarified in August that hospitals, physicians, health plans, and other health care workers could contact individuals who had recovered from COVID-19 without violating the HIPAA privacy rule.
Pirofski said she believes that trial investigators know it is legal to reach out to patients. But, she said, “it probably could be better known.”
This article first appeared on Medscape.com.
Nine antihypertensive drugs associated with reduced risk of depression
The risk of depression is elevated in patients with cardiovascular diseases, but several specific antihypertensive therapies are associated with a reduced risk, and none appear to increase the risk, according to a population-based study that evaluated 10 years of data in nearly 4 million subjects.
“As the first study on individual antihypertensives and risk of depression, we found a decreased risk of depression with nine drugs,” reported a collaborative group of investigators from multiple institutions in Denmark where the study was undertaken.
In a study period spanning from 2005 to 2015, risk of a diagnosis of depression was evaluated in patients taking any of 41 antihypertensive therapies in four major categories. These were identified as angiotensin agents (ACE inhibitors or angiotensin II receptor blockers), calcium antagonists, beta-blockers, and diuretics.
Within these groups, agents associated with a reduced risk of depression were: two angiotensin agents, enalapril and ramipril; three calcium antagonists, amlodipine, verapamil, and verapamil combinations; and four beta-blockers, propranolol, atenolol, bisoprolol, and carvedilol. The remaining drugs in these classes and diuretics were not associated with a reduced risk of depression. However, no antihypertensive agent was linked to an increased risk of depression.
All people living in Denmark are assigned a unique personal identification number that permits health information to be tracked across multiple registers. In this study, information was linked for several registries, including the Danish Medical Register on Vital Statistics, the Medicinal Product Statistics, and the Danish Psychiatric Central Register.
Data from a total of 3.75 million patients exposed to antihypertensive therapy during the study period were evaluated. Roughly 1 million of them were exposed to angiotensin drugs and slightly more than a million were exposed to diuretics. For calcium antagonists or beta-blockers, the numbers were approximately 835,000 and 775,000, respectively.
After adjustment for such factors as concomitant somatic diagnoses, sex, age, and employment status, the hazard ratios for depression among drugs associated with protection identified a risk reduction of 10%-25% in most cases when those who had been given 6-10 prescriptions or more than 10 prescriptions were compared with those who received 2 or fewer.
At the level of 10 or more prescriptions, for example, the risk reductions were 17% for ramipril (HR, 0.83; 95% CI, 0.78-0.89), 8% for enalapril (HR, 0.92; 95% CI, 0.88-0.96), 18% for amlodipine (HR, 0.82; 95% CI, 0.79-0.86), 15% for verapamil (HR, 0.85; 95% CI, 0.79-0.83), 28% for propranolol (HR, 0.72; 95% CI, 0.67-0.77), 20% for atenolol (HR, 0.80; 95% CI, 0.74-0.86), 25% for bisoprolol (HR, 0.75; 95% CI, 0.67-0.84), and 16% for carvedilol (HR, 0.84; 95% CI, 0.75-0.95).
For verapamil combinations, the risk reduction was 67% (HR, 0.33; 95% CI, 0.17-0.63), but the investigators cautioned that only 130 individuals were exposed to verapamil combinations, limiting the reliability of this analysis.
Interpreting the findings
A study hypothesis, the observed protective effect against depression, was expected for angiotensin drugs and calcium-channel blockers, but not for beta-blockers, according to the investigators.
“The renin-angiotensin systems is one of the pathways known to modulate inflammation in the central nervous system and seems involved in the regulation of the stress response. Angiotensin agents may also exert anti-inflammatory effects,” the investigators explained. “Dysregulation of intracellular calcium is evident in depression, including receptor-regulated calcium signaling.”
In contrast, beta-blockers have been associated with increased risk of depression in some but not all studies, according to the investigators. They maintained that some clinicians avoid these agents in patients with a history of mood disorders.
In attempting to account for the variability within drug classes regarding protection and lack of protection against depression, the investigators speculated that differences in pharmacologic properties, such as relative lipophilicity or anti-inflammatory effect, might be important.
Despite the large amount of data, William B. White, MD, professor emeritus at the Calhoun Cardiology Center, University of Connecticut, Farmington, is not convinced.
“In observational studies, even those with very large samples sizes, bias and confounding are hard to extricate with controls and propensity-score matching,” Dr. White said. From his perspective, the protective effects of some but not all drugs within a class “give one the impression that the findings are likely random.”
A member of the editorial board of the journal in which this study appeared, Dr. White said he was not involved in the review of the manuscript. Ultimately, he believed that the results are difficult to interpret.
“For example, there is no plausible rationale for why 2 of the 16 ACE inhibitors or angiotensin II receptor blockers or 4 of the 15 beta-blockers or 3 of the 10 calcium-channel blockers would reduce depression while the others in the class would have no effect,” he said.
Despite the investigators’ conclusion that these data should drive drug choice for patients at risk of depression, “I would say the results of this analysis would not lead me to alter clinical practice,” Dr. White added.
According to the principal investigator of the study, Lars Vedel Kessing, MD, DSc, professor of psychiatry at the University of Copenhagen, many variables affect choice of antihypertensive drug. However, the depression risk is elevated in patients with cardiovascular or cerebrovascular disease and hypertension.
When risk of a mood disorder is a concern, use of one of the nine drugs associated with protection from depression should be considered, “especially in patients at increased risk of developing depression, including patients with prior depression or anxiety and patients with a family history of depression,” he and his coinvestigators concluded.
However, Dr. Kessing said in an interview that the data do not help with individual treatment choices. “We do not compare different antihypertensives against each other due to the risk of confounding by indications, so, no, it is not reasonable to consider relative risk among specific agents.”
The authors reported no potential conflicts of interest involving this topic.
SOURCE: Kessing LV et al. Hypertension. 2020 Aug 24. doi: 10.1161/HYPERTENSIONAHA.120.15605.
The risk of depression is elevated in patients with cardiovascular diseases, but several specific antihypertensive therapies are associated with a reduced risk, and none appear to increase the risk, according to a population-based study that evaluated 10 years of data in nearly 4 million subjects.
“As the first study on individual antihypertensives and risk of depression, we found a decreased risk of depression with nine drugs,” reported a collaborative group of investigators from multiple institutions in Denmark where the study was undertaken.
In a study period spanning from 2005 to 2015, risk of a diagnosis of depression was evaluated in patients taking any of 41 antihypertensive therapies in four major categories. These were identified as angiotensin agents (ACE inhibitors or angiotensin II receptor blockers), calcium antagonists, beta-blockers, and diuretics.
Within these groups, agents associated with a reduced risk of depression were: two angiotensin agents, enalapril and ramipril; three calcium antagonists, amlodipine, verapamil, and verapamil combinations; and four beta-blockers, propranolol, atenolol, bisoprolol, and carvedilol. The remaining drugs in these classes and diuretics were not associated with a reduced risk of depression. However, no antihypertensive agent was linked to an increased risk of depression.
All people living in Denmark are assigned a unique personal identification number that permits health information to be tracked across multiple registers. In this study, information was linked for several registries, including the Danish Medical Register on Vital Statistics, the Medicinal Product Statistics, and the Danish Psychiatric Central Register.
Data from a total of 3.75 million patients exposed to antihypertensive therapy during the study period were evaluated. Roughly 1 million of them were exposed to angiotensin drugs and slightly more than a million were exposed to diuretics. For calcium antagonists or beta-blockers, the numbers were approximately 835,000 and 775,000, respectively.
After adjustment for such factors as concomitant somatic diagnoses, sex, age, and employment status, the hazard ratios for depression among drugs associated with protection identified a risk reduction of 10%-25% in most cases when those who had been given 6-10 prescriptions or more than 10 prescriptions were compared with those who received 2 or fewer.
At the level of 10 or more prescriptions, for example, the risk reductions were 17% for ramipril (HR, 0.83; 95% CI, 0.78-0.89), 8% for enalapril (HR, 0.92; 95% CI, 0.88-0.96), 18% for amlodipine (HR, 0.82; 95% CI, 0.79-0.86), 15% for verapamil (HR, 0.85; 95% CI, 0.79-0.83), 28% for propranolol (HR, 0.72; 95% CI, 0.67-0.77), 20% for atenolol (HR, 0.80; 95% CI, 0.74-0.86), 25% for bisoprolol (HR, 0.75; 95% CI, 0.67-0.84), and 16% for carvedilol (HR, 0.84; 95% CI, 0.75-0.95).
For verapamil combinations, the risk reduction was 67% (HR, 0.33; 95% CI, 0.17-0.63), but the investigators cautioned that only 130 individuals were exposed to verapamil combinations, limiting the reliability of this analysis.
Interpreting the findings
A study hypothesis, the observed protective effect against depression, was expected for angiotensin drugs and calcium-channel blockers, but not for beta-blockers, according to the investigators.
“The renin-angiotensin systems is one of the pathways known to modulate inflammation in the central nervous system and seems involved in the regulation of the stress response. Angiotensin agents may also exert anti-inflammatory effects,” the investigators explained. “Dysregulation of intracellular calcium is evident in depression, including receptor-regulated calcium signaling.”
In contrast, beta-blockers have been associated with increased risk of depression in some but not all studies, according to the investigators. They maintained that some clinicians avoid these agents in patients with a history of mood disorders.
In attempting to account for the variability within drug classes regarding protection and lack of protection against depression, the investigators speculated that differences in pharmacologic properties, such as relative lipophilicity or anti-inflammatory effect, might be important.
Despite the large amount of data, William B. White, MD, professor emeritus at the Calhoun Cardiology Center, University of Connecticut, Farmington, is not convinced.
“In observational studies, even those with very large samples sizes, bias and confounding are hard to extricate with controls and propensity-score matching,” Dr. White said. From his perspective, the protective effects of some but not all drugs within a class “give one the impression that the findings are likely random.”
A member of the editorial board of the journal in which this study appeared, Dr. White said he was not involved in the review of the manuscript. Ultimately, he believed that the results are difficult to interpret.
“For example, there is no plausible rationale for why 2 of the 16 ACE inhibitors or angiotensin II receptor blockers or 4 of the 15 beta-blockers or 3 of the 10 calcium-channel blockers would reduce depression while the others in the class would have no effect,” he said.
Despite the investigators’ conclusion that these data should drive drug choice for patients at risk of depression, “I would say the results of this analysis would not lead me to alter clinical practice,” Dr. White added.
According to the principal investigator of the study, Lars Vedel Kessing, MD, DSc, professor of psychiatry at the University of Copenhagen, many variables affect choice of antihypertensive drug. However, the depression risk is elevated in patients with cardiovascular or cerebrovascular disease and hypertension.
When risk of a mood disorder is a concern, use of one of the nine drugs associated with protection from depression should be considered, “especially in patients at increased risk of developing depression, including patients with prior depression or anxiety and patients with a family history of depression,” he and his coinvestigators concluded.
However, Dr. Kessing said in an interview that the data do not help with individual treatment choices. “We do not compare different antihypertensives against each other due to the risk of confounding by indications, so, no, it is not reasonable to consider relative risk among specific agents.”
The authors reported no potential conflicts of interest involving this topic.
SOURCE: Kessing LV et al. Hypertension. 2020 Aug 24. doi: 10.1161/HYPERTENSIONAHA.120.15605.
The risk of depression is elevated in patients with cardiovascular diseases, but several specific antihypertensive therapies are associated with a reduced risk, and none appear to increase the risk, according to a population-based study that evaluated 10 years of data in nearly 4 million subjects.
“As the first study on individual antihypertensives and risk of depression, we found a decreased risk of depression with nine drugs,” reported a collaborative group of investigators from multiple institutions in Denmark where the study was undertaken.
In a study period spanning from 2005 to 2015, risk of a diagnosis of depression was evaluated in patients taking any of 41 antihypertensive therapies in four major categories. These were identified as angiotensin agents (ACE inhibitors or angiotensin II receptor blockers), calcium antagonists, beta-blockers, and diuretics.
Within these groups, agents associated with a reduced risk of depression were: two angiotensin agents, enalapril and ramipril; three calcium antagonists, amlodipine, verapamil, and verapamil combinations; and four beta-blockers, propranolol, atenolol, bisoprolol, and carvedilol. The remaining drugs in these classes and diuretics were not associated with a reduced risk of depression. However, no antihypertensive agent was linked to an increased risk of depression.
All people living in Denmark are assigned a unique personal identification number that permits health information to be tracked across multiple registers. In this study, information was linked for several registries, including the Danish Medical Register on Vital Statistics, the Medicinal Product Statistics, and the Danish Psychiatric Central Register.
Data from a total of 3.75 million patients exposed to antihypertensive therapy during the study period were evaluated. Roughly 1 million of them were exposed to angiotensin drugs and slightly more than a million were exposed to diuretics. For calcium antagonists or beta-blockers, the numbers were approximately 835,000 and 775,000, respectively.
After adjustment for such factors as concomitant somatic diagnoses, sex, age, and employment status, the hazard ratios for depression among drugs associated with protection identified a risk reduction of 10%-25% in most cases when those who had been given 6-10 prescriptions or more than 10 prescriptions were compared with those who received 2 or fewer.
At the level of 10 or more prescriptions, for example, the risk reductions were 17% for ramipril (HR, 0.83; 95% CI, 0.78-0.89), 8% for enalapril (HR, 0.92; 95% CI, 0.88-0.96), 18% for amlodipine (HR, 0.82; 95% CI, 0.79-0.86), 15% for verapamil (HR, 0.85; 95% CI, 0.79-0.83), 28% for propranolol (HR, 0.72; 95% CI, 0.67-0.77), 20% for atenolol (HR, 0.80; 95% CI, 0.74-0.86), 25% for bisoprolol (HR, 0.75; 95% CI, 0.67-0.84), and 16% for carvedilol (HR, 0.84; 95% CI, 0.75-0.95).
For verapamil combinations, the risk reduction was 67% (HR, 0.33; 95% CI, 0.17-0.63), but the investigators cautioned that only 130 individuals were exposed to verapamil combinations, limiting the reliability of this analysis.
Interpreting the findings
A study hypothesis, the observed protective effect against depression, was expected for angiotensin drugs and calcium-channel blockers, but not for beta-blockers, according to the investigators.
“The renin-angiotensin systems is one of the pathways known to modulate inflammation in the central nervous system and seems involved in the regulation of the stress response. Angiotensin agents may also exert anti-inflammatory effects,” the investigators explained. “Dysregulation of intracellular calcium is evident in depression, including receptor-regulated calcium signaling.”
In contrast, beta-blockers have been associated with increased risk of depression in some but not all studies, according to the investigators. They maintained that some clinicians avoid these agents in patients with a history of mood disorders.
In attempting to account for the variability within drug classes regarding protection and lack of protection against depression, the investigators speculated that differences in pharmacologic properties, such as relative lipophilicity or anti-inflammatory effect, might be important.
Despite the large amount of data, William B. White, MD, professor emeritus at the Calhoun Cardiology Center, University of Connecticut, Farmington, is not convinced.
“In observational studies, even those with very large samples sizes, bias and confounding are hard to extricate with controls and propensity-score matching,” Dr. White said. From his perspective, the protective effects of some but not all drugs within a class “give one the impression that the findings are likely random.”
A member of the editorial board of the journal in which this study appeared, Dr. White said he was not involved in the review of the manuscript. Ultimately, he believed that the results are difficult to interpret.
“For example, there is no plausible rationale for why 2 of the 16 ACE inhibitors or angiotensin II receptor blockers or 4 of the 15 beta-blockers or 3 of the 10 calcium-channel blockers would reduce depression while the others in the class would have no effect,” he said.
Despite the investigators’ conclusion that these data should drive drug choice for patients at risk of depression, “I would say the results of this analysis would not lead me to alter clinical practice,” Dr. White added.
According to the principal investigator of the study, Lars Vedel Kessing, MD, DSc, professor of psychiatry at the University of Copenhagen, many variables affect choice of antihypertensive drug. However, the depression risk is elevated in patients with cardiovascular or cerebrovascular disease and hypertension.
When risk of a mood disorder is a concern, use of one of the nine drugs associated with protection from depression should be considered, “especially in patients at increased risk of developing depression, including patients with prior depression or anxiety and patients with a family history of depression,” he and his coinvestigators concluded.
However, Dr. Kessing said in an interview that the data do not help with individual treatment choices. “We do not compare different antihypertensives against each other due to the risk of confounding by indications, so, no, it is not reasonable to consider relative risk among specific agents.”
The authors reported no potential conflicts of interest involving this topic.
SOURCE: Kessing LV et al. Hypertension. 2020 Aug 24. doi: 10.1161/HYPERTENSIONAHA.120.15605.
FROM HYPERTENSION
e-Interview With CHEST President-Elect Steven Q. Simpson, MD, FCCP
CHEST President-Elect Steven Q. Simpson, MD, FCCP, is Professor of Medicine in the Division of Pulmonary and Critical Care Medicine at the University of Kansas. He is also senior advisor to the Solving Sepsis initiative of the Biomedical Advanced Research and Development Authority (BARDA) of the US Department of Health and Human Services.
As we greet our new incoming CHEST President, we asked him for a few thoughts about his upcoming presidential year. He kindly offered these responses:
What would you like to accomplish as President of CHEST?
This is an interesting question, because a global pandemic and other developments in our world dictate that our organizational goals must adapt to a landscape that has shifted in recent months. My goals as President are somewhat different from what I stated when I ran for the office.
1. First, I will build on the efforts of my predecessors to ensure that CHEST is an inclusive and anti-racist organization. All CHEST members must have equal opportunities within our organization to advance their lives and their careers, regardless of race, ethnicity, sex, or gender. My goal is to examine our structures for participation and advancement to positions of leadership in the organization and to evaluate our educational and research offerings, all with the purpose of discovering and remedying places where we have been blind to our own systematic bias. Further, CHEST must advocate for and lead others to advocate for equality, for equal access to medical care, and for policies that promote them. We must be leaders in this arena, through both our voice and our actions.
2. We will build on CHEST’s new initiative to support the wellness of our members and to help us all perform at our best, day in and day out. I hope for our newly established Wellness Center to become a frequent stop for all CHEST members, myself included, to help us to sustain ourselves through the pandemic and beyond.
3. We must maintain both the quality and the feel of our educational and research offerings during this time when we cannot come together in person. My goal for us is that we use this time to embrace remote and nontemporally synchronous education, ie, web-based education, to make CHEST’s offerings the best anywhere. In the remainder of the 21st century, digital transformation of teaching and learning will advance tremendously, and our creative use of technology will become a norm. I hope that we never abandon in-person meetings, but using technology to improve information transfer and augmenting our members’ continuing education are clearly here to stay. My goal for us is that we maintain an atmosphere to both our in-person meetings and our remotely delivered meetings that makes generating new knowledge and learning what we generate enjoyable, even fun. I believe our digital transformation will make some interesting things possible over time.
4. My overall goal for CHEST in the coming year is not that we “make it through” the current pandemic, but that we emerge stronger, smarter, and better for the experience, and prepared for the next challenge(s).
Before COVID-19, I had goals for my presidency, and these issues have not disappeared. CHEST needs to be user- friendly for our members, from our website, to the ways in which we deliver education, to the type of research we develop and promote. On the research side, our members have long been interested in clinical research that informs and improves our patient care. My goal is to double down on promoting, supporting, and presenting research that serves exactly this purpose. We are growing our team-based education, and I have a special goal for CHEST to become the home for pulmonary, critical care, and sleep advanced practice providers. I care tremendously about our international members, and I will promote both international growth and catering of CHEST’s offerings to benefit our international members.
What do you consider to be the greatest strength of CHEST, and how will you build upon this during your Presidency?
There is zero doubt that CHEST’s greatest strength is the people who gravitate to our organization. From pure clinicians to academicians; from clinical researchers to clinical educators to outcomes mavens—all levels of the health-care team. At every level of this organization are members who all want to be better at what we do, who want to figure out the ways for doing that, who want to explore the boundaries of what that means, and who want to help others to do the same. That goes, as well, for the professional staff who support the members, and who have adopted the motto, “CRUSH lung disease,” because they share our mission and are here to help us do it better.
The absolutely most enjoyable thing about leadership is having the opportunity to survey the landscape and see who’s looking for opportunity, who’s a rising star, who’s looking for people to mentor, then matching those people with opportunities and with jobs to do. Good people who are motivated by the right principles rise to the occasion. My job as President is to help ensure that the organization via the CHEST Board of Regents is addressing the correct problems with the right vision, to identify the right talented and dedicated members for the jobs, and then to support and stay out of their way as they make the vision a reality.
What are some challenges facing CHEST, and how will you address these challenges?
The major immediate challenges facing CHEST are pandemic-related, in terms of helping to ensure the well-being of our members, and in helping them to address the inequities and disparities in care for our patients of color, who have been hardest hit by the emergence of SARS-CoV-2. I addressed these with my goals, above. To be more specific, though, our board will be using various techniques, including dialogue with our members of color, to understand and address our own implicit biases, so that we can achieve the correct vision and tone of inclusion for all of our members. Also addressed in my goals is the isolation from one another that we are all experiencing because of the pandemic. This situation makes it difficult for us to maintain the style and tone of live learning experiences that our CHEST members are accustomed to. The challenge is to develop materials that can be interactive at a distance, and this likely includes gamification of educational content and employing virtual reality. CHEST Innovations is already working in this arena, and it will be our job as member volunteers to support those efforts. The isolation affects our international members, as well, and our ability to travel to maintain relationships. The nice thing is that web conferencing works just as well for international meetings as for meetings in the US, although somebody often has to go to bed very late or get up very early in the morning to make them work! The efforts are worth our time. Again, we will be working in various arenas to maintain and grow our international relationships.
And finally, what is your charge to the members and new Fellows (FCCPs) of CHEST?
We do not yet see clearly whether to expect a massive winter surge of COVID-19 infections. However, it is a reasonably likely possibility. My charge to our members and our new Fellows is first to stay safe, yourself, and to take care of your mental and physical well-being, so that you can be present and functioning at peak levels for your patients. Make sure your family is, likewise, being safe. Secondly, keep doing what you do, which is excellent patient care, excellent teaching, excellent research to push the boundaries of our knowledge. And finally, you’ve seen my ideas of the challenges facing CHEST. I want you to survey, yourself, and tell me what you think our challenges, goals, and responsibilities should be. And if anything I’ve said resonates with you, volunteer to help us address our challenges and keep CHEST the professional home that you deserve and that you will never want to leave. CHEST wants you and needs you. We are so happy you are with us!
CHEST President-Elect Steven Q. Simpson, MD, FCCP, is Professor of Medicine in the Division of Pulmonary and Critical Care Medicine at the University of Kansas. He is also senior advisor to the Solving Sepsis initiative of the Biomedical Advanced Research and Development Authority (BARDA) of the US Department of Health and Human Services.
As we greet our new incoming CHEST President, we asked him for a few thoughts about his upcoming presidential year. He kindly offered these responses:
What would you like to accomplish as President of CHEST?
This is an interesting question, because a global pandemic and other developments in our world dictate that our organizational goals must adapt to a landscape that has shifted in recent months. My goals as President are somewhat different from what I stated when I ran for the office.
1. First, I will build on the efforts of my predecessors to ensure that CHEST is an inclusive and anti-racist organization. All CHEST members must have equal opportunities within our organization to advance their lives and their careers, regardless of race, ethnicity, sex, or gender. My goal is to examine our structures for participation and advancement to positions of leadership in the organization and to evaluate our educational and research offerings, all with the purpose of discovering and remedying places where we have been blind to our own systematic bias. Further, CHEST must advocate for and lead others to advocate for equality, for equal access to medical care, and for policies that promote them. We must be leaders in this arena, through both our voice and our actions.
2. We will build on CHEST’s new initiative to support the wellness of our members and to help us all perform at our best, day in and day out. I hope for our newly established Wellness Center to become a frequent stop for all CHEST members, myself included, to help us to sustain ourselves through the pandemic and beyond.
3. We must maintain both the quality and the feel of our educational and research offerings during this time when we cannot come together in person. My goal for us is that we use this time to embrace remote and nontemporally synchronous education, ie, web-based education, to make CHEST’s offerings the best anywhere. In the remainder of the 21st century, digital transformation of teaching and learning will advance tremendously, and our creative use of technology will become a norm. I hope that we never abandon in-person meetings, but using technology to improve information transfer and augmenting our members’ continuing education are clearly here to stay. My goal for us is that we maintain an atmosphere to both our in-person meetings and our remotely delivered meetings that makes generating new knowledge and learning what we generate enjoyable, even fun. I believe our digital transformation will make some interesting things possible over time.
4. My overall goal for CHEST in the coming year is not that we “make it through” the current pandemic, but that we emerge stronger, smarter, and better for the experience, and prepared for the next challenge(s).
Before COVID-19, I had goals for my presidency, and these issues have not disappeared. CHEST needs to be user- friendly for our members, from our website, to the ways in which we deliver education, to the type of research we develop and promote. On the research side, our members have long been interested in clinical research that informs and improves our patient care. My goal is to double down on promoting, supporting, and presenting research that serves exactly this purpose. We are growing our team-based education, and I have a special goal for CHEST to become the home for pulmonary, critical care, and sleep advanced practice providers. I care tremendously about our international members, and I will promote both international growth and catering of CHEST’s offerings to benefit our international members.
What do you consider to be the greatest strength of CHEST, and how will you build upon this during your Presidency?
There is zero doubt that CHEST’s greatest strength is the people who gravitate to our organization. From pure clinicians to academicians; from clinical researchers to clinical educators to outcomes mavens—all levels of the health-care team. At every level of this organization are members who all want to be better at what we do, who want to figure out the ways for doing that, who want to explore the boundaries of what that means, and who want to help others to do the same. That goes, as well, for the professional staff who support the members, and who have adopted the motto, “CRUSH lung disease,” because they share our mission and are here to help us do it better.
The absolutely most enjoyable thing about leadership is having the opportunity to survey the landscape and see who’s looking for opportunity, who’s a rising star, who’s looking for people to mentor, then matching those people with opportunities and with jobs to do. Good people who are motivated by the right principles rise to the occasion. My job as President is to help ensure that the organization via the CHEST Board of Regents is addressing the correct problems with the right vision, to identify the right talented and dedicated members for the jobs, and then to support and stay out of their way as they make the vision a reality.
What are some challenges facing CHEST, and how will you address these challenges?
The major immediate challenges facing CHEST are pandemic-related, in terms of helping to ensure the well-being of our members, and in helping them to address the inequities and disparities in care for our patients of color, who have been hardest hit by the emergence of SARS-CoV-2. I addressed these with my goals, above. To be more specific, though, our board will be using various techniques, including dialogue with our members of color, to understand and address our own implicit biases, so that we can achieve the correct vision and tone of inclusion for all of our members. Also addressed in my goals is the isolation from one another that we are all experiencing because of the pandemic. This situation makes it difficult for us to maintain the style and tone of live learning experiences that our CHEST members are accustomed to. The challenge is to develop materials that can be interactive at a distance, and this likely includes gamification of educational content and employing virtual reality. CHEST Innovations is already working in this arena, and it will be our job as member volunteers to support those efforts. The isolation affects our international members, as well, and our ability to travel to maintain relationships. The nice thing is that web conferencing works just as well for international meetings as for meetings in the US, although somebody often has to go to bed very late or get up very early in the morning to make them work! The efforts are worth our time. Again, we will be working in various arenas to maintain and grow our international relationships.
And finally, what is your charge to the members and new Fellows (FCCPs) of CHEST?
We do not yet see clearly whether to expect a massive winter surge of COVID-19 infections. However, it is a reasonably likely possibility. My charge to our members and our new Fellows is first to stay safe, yourself, and to take care of your mental and physical well-being, so that you can be present and functioning at peak levels for your patients. Make sure your family is, likewise, being safe. Secondly, keep doing what you do, which is excellent patient care, excellent teaching, excellent research to push the boundaries of our knowledge. And finally, you’ve seen my ideas of the challenges facing CHEST. I want you to survey, yourself, and tell me what you think our challenges, goals, and responsibilities should be. And if anything I’ve said resonates with you, volunteer to help us address our challenges and keep CHEST the professional home that you deserve and that you will never want to leave. CHEST wants you and needs you. We are so happy you are with us!
CHEST President-Elect Steven Q. Simpson, MD, FCCP, is Professor of Medicine in the Division of Pulmonary and Critical Care Medicine at the University of Kansas. He is also senior advisor to the Solving Sepsis initiative of the Biomedical Advanced Research and Development Authority (BARDA) of the US Department of Health and Human Services.
As we greet our new incoming CHEST President, we asked him for a few thoughts about his upcoming presidential year. He kindly offered these responses:
What would you like to accomplish as President of CHEST?
This is an interesting question, because a global pandemic and other developments in our world dictate that our organizational goals must adapt to a landscape that has shifted in recent months. My goals as President are somewhat different from what I stated when I ran for the office.
1. First, I will build on the efforts of my predecessors to ensure that CHEST is an inclusive and anti-racist organization. All CHEST members must have equal opportunities within our organization to advance their lives and their careers, regardless of race, ethnicity, sex, or gender. My goal is to examine our structures for participation and advancement to positions of leadership in the organization and to evaluate our educational and research offerings, all with the purpose of discovering and remedying places where we have been blind to our own systematic bias. Further, CHEST must advocate for and lead others to advocate for equality, for equal access to medical care, and for policies that promote them. We must be leaders in this arena, through both our voice and our actions.
2. We will build on CHEST’s new initiative to support the wellness of our members and to help us all perform at our best, day in and day out. I hope for our newly established Wellness Center to become a frequent stop for all CHEST members, myself included, to help us to sustain ourselves through the pandemic and beyond.
3. We must maintain both the quality and the feel of our educational and research offerings during this time when we cannot come together in person. My goal for us is that we use this time to embrace remote and nontemporally synchronous education, ie, web-based education, to make CHEST’s offerings the best anywhere. In the remainder of the 21st century, digital transformation of teaching and learning will advance tremendously, and our creative use of technology will become a norm. I hope that we never abandon in-person meetings, but using technology to improve information transfer and augmenting our members’ continuing education are clearly here to stay. My goal for us is that we maintain an atmosphere to both our in-person meetings and our remotely delivered meetings that makes generating new knowledge and learning what we generate enjoyable, even fun. I believe our digital transformation will make some interesting things possible over time.
4. My overall goal for CHEST in the coming year is not that we “make it through” the current pandemic, but that we emerge stronger, smarter, and better for the experience, and prepared for the next challenge(s).
Before COVID-19, I had goals for my presidency, and these issues have not disappeared. CHEST needs to be user- friendly for our members, from our website, to the ways in which we deliver education, to the type of research we develop and promote. On the research side, our members have long been interested in clinical research that informs and improves our patient care. My goal is to double down on promoting, supporting, and presenting research that serves exactly this purpose. We are growing our team-based education, and I have a special goal for CHEST to become the home for pulmonary, critical care, and sleep advanced practice providers. I care tremendously about our international members, and I will promote both international growth and catering of CHEST’s offerings to benefit our international members.
What do you consider to be the greatest strength of CHEST, and how will you build upon this during your Presidency?
There is zero doubt that CHEST’s greatest strength is the people who gravitate to our organization. From pure clinicians to academicians; from clinical researchers to clinical educators to outcomes mavens—all levels of the health-care team. At every level of this organization are members who all want to be better at what we do, who want to figure out the ways for doing that, who want to explore the boundaries of what that means, and who want to help others to do the same. That goes, as well, for the professional staff who support the members, and who have adopted the motto, “CRUSH lung disease,” because they share our mission and are here to help us do it better.
The absolutely most enjoyable thing about leadership is having the opportunity to survey the landscape and see who’s looking for opportunity, who’s a rising star, who’s looking for people to mentor, then matching those people with opportunities and with jobs to do. Good people who are motivated by the right principles rise to the occasion. My job as President is to help ensure that the organization via the CHEST Board of Regents is addressing the correct problems with the right vision, to identify the right talented and dedicated members for the jobs, and then to support and stay out of their way as they make the vision a reality.
What are some challenges facing CHEST, and how will you address these challenges?
The major immediate challenges facing CHEST are pandemic-related, in terms of helping to ensure the well-being of our members, and in helping them to address the inequities and disparities in care for our patients of color, who have been hardest hit by the emergence of SARS-CoV-2. I addressed these with my goals, above. To be more specific, though, our board will be using various techniques, including dialogue with our members of color, to understand and address our own implicit biases, so that we can achieve the correct vision and tone of inclusion for all of our members. Also addressed in my goals is the isolation from one another that we are all experiencing because of the pandemic. This situation makes it difficult for us to maintain the style and tone of live learning experiences that our CHEST members are accustomed to. The challenge is to develop materials that can be interactive at a distance, and this likely includes gamification of educational content and employing virtual reality. CHEST Innovations is already working in this arena, and it will be our job as member volunteers to support those efforts. The isolation affects our international members, as well, and our ability to travel to maintain relationships. The nice thing is that web conferencing works just as well for international meetings as for meetings in the US, although somebody often has to go to bed very late or get up very early in the morning to make them work! The efforts are worth our time. Again, we will be working in various arenas to maintain and grow our international relationships.
And finally, what is your charge to the members and new Fellows (FCCPs) of CHEST?
We do not yet see clearly whether to expect a massive winter surge of COVID-19 infections. However, it is a reasonably likely possibility. My charge to our members and our new Fellows is first to stay safe, yourself, and to take care of your mental and physical well-being, so that you can be present and functioning at peak levels for your patients. Make sure your family is, likewise, being safe. Secondly, keep doing what you do, which is excellent patient care, excellent teaching, excellent research to push the boundaries of our knowledge. And finally, you’ve seen my ideas of the challenges facing CHEST. I want you to survey, yourself, and tell me what you think our challenges, goals, and responsibilities should be. And if anything I’ve said resonates with you, volunteer to help us address our challenges and keep CHEST the professional home that you deserve and that you will never want to leave. CHEST wants you and needs you. We are so happy you are with us!
FDA pulls amputation boxed warning off canagliflozin label
The Food and Drug Administration has removed the boxed warning about the risk of leg and foot amputations for canagliflozin (Invokana, Invokamet, Janssen), a sodium-glucose cotransporter-2 (SGLT2) inhibitor for the treatment of type 2 diabetes, the agency announced Aug. 26.
As previously reported by Medscape Medical News, the FDA added the boxed warning to the canagliflozin label in May 2017, after an approximately doubled risk for lower-extremity amputations with the drug compared with placebo was seen during two trials.
The FDA said the decision to remove the boxed warning was made following a review of new data from three clinical trials, which demonstrated additional heart- and kidney-related benefits and led to additional approved uses for canagliflozin.
In 2018, canagliflozin was approved to reduce the risk of major adverse cardiovascular events in adults with type 2 diabetes who have established cardiovascular disease.
In 2019, canagliflozin was approved to reduce the risk of end-stage kidney disease, worsening of kidney function, cardiovascular death, and heart failure hospitalization, in adults with type 2 diabetes and diabetic kidney disease.
“Collectively, these newly identified effects of canagliflozin on heart and kidney disease show significantly enhanced benefit of this medicine,” the FDA said.
The safety information from these trials, the FDA said, suggests that the risk of amputation, “while still increased with canagliflozin, is lower than previously described, particularly when appropriately monitored.”
The agency added: “Based upon these considerations, FDA concluded that the boxed warning should be removed.”
The FDA announcement said clinicians and patients should continue to be aware of the importance of preventive foot care and to monitor for new pain, tenderness, sores, ulcers, and infections in the legs and feet. Risk factors that may predispose patients to amputation should be considered when choosing antidiabetic medicines.
Health care professionals are encouraged to report adverse reactions with canagliflozin to the FDA’s MedWatch program.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has removed the boxed warning about the risk of leg and foot amputations for canagliflozin (Invokana, Invokamet, Janssen), a sodium-glucose cotransporter-2 (SGLT2) inhibitor for the treatment of type 2 diabetes, the agency announced Aug. 26.
As previously reported by Medscape Medical News, the FDA added the boxed warning to the canagliflozin label in May 2017, after an approximately doubled risk for lower-extremity amputations with the drug compared with placebo was seen during two trials.
The FDA said the decision to remove the boxed warning was made following a review of new data from three clinical trials, which demonstrated additional heart- and kidney-related benefits and led to additional approved uses for canagliflozin.
In 2018, canagliflozin was approved to reduce the risk of major adverse cardiovascular events in adults with type 2 diabetes who have established cardiovascular disease.
In 2019, canagliflozin was approved to reduce the risk of end-stage kidney disease, worsening of kidney function, cardiovascular death, and heart failure hospitalization, in adults with type 2 diabetes and diabetic kidney disease.
“Collectively, these newly identified effects of canagliflozin on heart and kidney disease show significantly enhanced benefit of this medicine,” the FDA said.
The safety information from these trials, the FDA said, suggests that the risk of amputation, “while still increased with canagliflozin, is lower than previously described, particularly when appropriately monitored.”
The agency added: “Based upon these considerations, FDA concluded that the boxed warning should be removed.”
The FDA announcement said clinicians and patients should continue to be aware of the importance of preventive foot care and to monitor for new pain, tenderness, sores, ulcers, and infections in the legs and feet. Risk factors that may predispose patients to amputation should be considered when choosing antidiabetic medicines.
Health care professionals are encouraged to report adverse reactions with canagliflozin to the FDA’s MedWatch program.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has removed the boxed warning about the risk of leg and foot amputations for canagliflozin (Invokana, Invokamet, Janssen), a sodium-glucose cotransporter-2 (SGLT2) inhibitor for the treatment of type 2 diabetes, the agency announced Aug. 26.
As previously reported by Medscape Medical News, the FDA added the boxed warning to the canagliflozin label in May 2017, after an approximately doubled risk for lower-extremity amputations with the drug compared with placebo was seen during two trials.
The FDA said the decision to remove the boxed warning was made following a review of new data from three clinical trials, which demonstrated additional heart- and kidney-related benefits and led to additional approved uses for canagliflozin.
In 2018, canagliflozin was approved to reduce the risk of major adverse cardiovascular events in adults with type 2 diabetes who have established cardiovascular disease.
In 2019, canagliflozin was approved to reduce the risk of end-stage kidney disease, worsening of kidney function, cardiovascular death, and heart failure hospitalization, in adults with type 2 diabetes and diabetic kidney disease.
“Collectively, these newly identified effects of canagliflozin on heart and kidney disease show significantly enhanced benefit of this medicine,” the FDA said.
The safety information from these trials, the FDA said, suggests that the risk of amputation, “while still increased with canagliflozin, is lower than previously described, particularly when appropriately monitored.”
The agency added: “Based upon these considerations, FDA concluded that the boxed warning should be removed.”
The FDA announcement said clinicians and patients should continue to be aware of the importance of preventive foot care and to monitor for new pain, tenderness, sores, ulcers, and infections in the legs and feet. Risk factors that may predispose patients to amputation should be considered when choosing antidiabetic medicines.
Health care professionals are encouraged to report adverse reactions with canagliflozin to the FDA’s MedWatch program.
A version of this article originally appeared on Medscape.com.
Prognosis for rural hospitals worsens with pandemic
Jerome Antone said he is one of the lucky ones.
After becoming ill with COVID-19, Mr. Antone was hospitalized only 65 miles away from his small Alabama town. He is the mayor of Georgiana – population 1,700.
“It hit our rural community so rabid,” Mr. Antone said. The town’s hospital closed last year. If hospitals in nearby communities don’t have beds available, “you may have to go 4 or 5 hours away.”
Eighteen rural hospitals closed last year and the first 3 months of 2020 were “really big months,” said Mark Holmes, PhD, director of the Cecil G. Sheps Center for Health Services Research at the University of North Carolina at Chapel Hill. Many of the losses are in Southern states like Florida and Texas. More than 170 rural hospitals have closed nationwide since 2005, according to data collected by the Sheps Center.
It’s a dangerous scenario. “We know that a closure leads to higher mortality pretty quickly” among the populations served, said Dr. Holmes, who is also a professor at UNC Gillings School of Global Public Health. “That’s pretty clear.”
One 2019 study found that death rates in the surrounding communities increase nearly 6% after a rural hospital closes – and that’s when there’s not a pandemic.
Add to that what is known about the coronavirus: People who are obese or live with diabetes, hypertension, asthma, and other underlying health issues are more susceptible to COVID-19. Rural areas tend to have higher rates of these conditions. And rural residents are more likely to be older, sicker and poorer than those in urban areas. All this leaves rural communities particularly vulnerable to the coronavirus.
Congress approved billions in federal relief funds for health care providers. Initially, federal officials based what a hospital would get on its Medicare payments, but by late April they heeded criticism and carved out funds for rural hospitals and COVID-19 hot spots. Rural hospitals leapt at the chance to shore up already-negative budgets and prepare for the pandemic.
The funds “helped rural hospitals with the immediate storm,” said Don Williamson, MD, president of the Alabama Hospital Association. Nearly 80% of Alabama’s rural hospitals began the year with negative balance sheets and about 8 days’ worth of cash on hand.
Before the pandemic hit this year, hundreds of rural hospitals “were just trying to keep their doors open,” said Maggie Elehwany, vice president of government affairs with the National Rural Health Association. Then an estimated 70% of their income stopped as patients avoided the emergency room, doctor’s appointments, and elective surgeries.
“It was devastating,” Ms. Elehwany said.
Paul Taylor, chief executive of a 25-bed critical-access hospital and outpatient clinics in northwestern Arkansas, accepted millions in grants and loan money Congress approved this spring, largely through the CARES (Coronavirus Aid, Relief, and Economic Security) Act.
“For us, this was survival money and we spent it already,” Mr. Taylor said. With those funds, Ozarks Community Hospital increased surge capacity, expanding from 25 beds to 50 beds, adding negative pressure rooms and buying six ventilators. Taylor also ramped up COVID-19 testing at his hospital and clinics, located near some meat-processing plants.
Throughout June and July, Ozarks tested 1,000 patients a day and reported a 20% positive rate. The rate dropped to 16.9% in late July. But patients continue to avoid routine care.
Mr. Taylor said revenue is still constrained and he does not know how he will pay back $8 million that he borrowed from Medicare. The program allowed hospitals to borrow against future payments from the federal government, but stipulated that repayment would begin within 120 days.
For Mr. Taylor, this seems impossible. Medicare makes up 40% of Ozarks’ income. And he has to pay the loan back before he gets any more payments from Medicare. He’s hoping to refinance the hospital’s mortgage.
“If I get no relief and they take the money ... we won’t still be open,” Mr. Taylor said. Ozarks provides 625 jobs and serves an area with a population of about 75,000.
There are 1,300 small critical-access hospitals like Ozarks in rural America, and of those, 859 took advantage of the Medicare loans, sending about $3.1 billion into the local communities. But many rural communities have not yet experienced a surge in coronavirus cases – national leaders fear it will come as part of a new phase.
“There are pockets of rural America who say, ‘We haven’t seen a single COVID patient yet and we do not believe it’s real,’ ” Mr. Taylor said. “They will get hit sooner or later.”
Across the country, the reduced patient numbers and increased spending required to fight and prepare for the coronavirus was “like a knife cutting into a hospital’s blood supply,” said Ge Bai, PhD, associate professor of health policy and management at the Johns Hopkins Bloomberg School of Public Health in Baltimore.
Dr. Bai said the way the federal government reimbursed small rural hospitals through federal programs like Medicare before the pandemic was faulty and inefficient. “They are too weak to survive,” she said.
In rural Texas, about 2 hours from Dallas, Titus Regional Medical Center chief executive officer Terry Scoggin cut staff and furloughed workers even as his rural hospital faced down the pandemic. Titus Regional lost about $4 million last fiscal year and broke even each of the three years before that.
Mr. Scoggin said he did not cut from his clinical staff, though. Titus is now facing its second surge of the virus in the community. “The last 7 days, we’ve been testing 30% positive,” he said, including the case of his father, who contracted it at a nursing home and survived.
“It’s personal and this is real,” Mr. Scoggin said. “You know the people who are infected. You know the people who are passing away.”
Of his roughly 700 employees, 48 have tested positive for the virus and 1 has died. They are short on testing kits, medication, and supplies.
“Right now the staff is strained,” Mr. Scoggin said. “I’ve been blown away by their selflessness and unbelievable spirit. We’re resilient, we’re nimble, and we will make it. We don’t have a choice.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation, which is not affiliated with Kaiser Permanente.
Jerome Antone said he is one of the lucky ones.
After becoming ill with COVID-19, Mr. Antone was hospitalized only 65 miles away from his small Alabama town. He is the mayor of Georgiana – population 1,700.
“It hit our rural community so rabid,” Mr. Antone said. The town’s hospital closed last year. If hospitals in nearby communities don’t have beds available, “you may have to go 4 or 5 hours away.”
Eighteen rural hospitals closed last year and the first 3 months of 2020 were “really big months,” said Mark Holmes, PhD, director of the Cecil G. Sheps Center for Health Services Research at the University of North Carolina at Chapel Hill. Many of the losses are in Southern states like Florida and Texas. More than 170 rural hospitals have closed nationwide since 2005, according to data collected by the Sheps Center.
It’s a dangerous scenario. “We know that a closure leads to higher mortality pretty quickly” among the populations served, said Dr. Holmes, who is also a professor at UNC Gillings School of Global Public Health. “That’s pretty clear.”
One 2019 study found that death rates in the surrounding communities increase nearly 6% after a rural hospital closes – and that’s when there’s not a pandemic.
Add to that what is known about the coronavirus: People who are obese or live with diabetes, hypertension, asthma, and other underlying health issues are more susceptible to COVID-19. Rural areas tend to have higher rates of these conditions. And rural residents are more likely to be older, sicker and poorer than those in urban areas. All this leaves rural communities particularly vulnerable to the coronavirus.
Congress approved billions in federal relief funds for health care providers. Initially, federal officials based what a hospital would get on its Medicare payments, but by late April they heeded criticism and carved out funds for rural hospitals and COVID-19 hot spots. Rural hospitals leapt at the chance to shore up already-negative budgets and prepare for the pandemic.
The funds “helped rural hospitals with the immediate storm,” said Don Williamson, MD, president of the Alabama Hospital Association. Nearly 80% of Alabama’s rural hospitals began the year with negative balance sheets and about 8 days’ worth of cash on hand.
Before the pandemic hit this year, hundreds of rural hospitals “were just trying to keep their doors open,” said Maggie Elehwany, vice president of government affairs with the National Rural Health Association. Then an estimated 70% of their income stopped as patients avoided the emergency room, doctor’s appointments, and elective surgeries.
“It was devastating,” Ms. Elehwany said.
Paul Taylor, chief executive of a 25-bed critical-access hospital and outpatient clinics in northwestern Arkansas, accepted millions in grants and loan money Congress approved this spring, largely through the CARES (Coronavirus Aid, Relief, and Economic Security) Act.
“For us, this was survival money and we spent it already,” Mr. Taylor said. With those funds, Ozarks Community Hospital increased surge capacity, expanding from 25 beds to 50 beds, adding negative pressure rooms and buying six ventilators. Taylor also ramped up COVID-19 testing at his hospital and clinics, located near some meat-processing plants.
Throughout June and July, Ozarks tested 1,000 patients a day and reported a 20% positive rate. The rate dropped to 16.9% in late July. But patients continue to avoid routine care.
Mr. Taylor said revenue is still constrained and he does not know how he will pay back $8 million that he borrowed from Medicare. The program allowed hospitals to borrow against future payments from the federal government, but stipulated that repayment would begin within 120 days.
For Mr. Taylor, this seems impossible. Medicare makes up 40% of Ozarks’ income. And he has to pay the loan back before he gets any more payments from Medicare. He’s hoping to refinance the hospital’s mortgage.
“If I get no relief and they take the money ... we won’t still be open,” Mr. Taylor said. Ozarks provides 625 jobs and serves an area with a population of about 75,000.
There are 1,300 small critical-access hospitals like Ozarks in rural America, and of those, 859 took advantage of the Medicare loans, sending about $3.1 billion into the local communities. But many rural communities have not yet experienced a surge in coronavirus cases – national leaders fear it will come as part of a new phase.
“There are pockets of rural America who say, ‘We haven’t seen a single COVID patient yet and we do not believe it’s real,’ ” Mr. Taylor said. “They will get hit sooner or later.”
Across the country, the reduced patient numbers and increased spending required to fight and prepare for the coronavirus was “like a knife cutting into a hospital’s blood supply,” said Ge Bai, PhD, associate professor of health policy and management at the Johns Hopkins Bloomberg School of Public Health in Baltimore.
Dr. Bai said the way the federal government reimbursed small rural hospitals through federal programs like Medicare before the pandemic was faulty and inefficient. “They are too weak to survive,” she said.
In rural Texas, about 2 hours from Dallas, Titus Regional Medical Center chief executive officer Terry Scoggin cut staff and furloughed workers even as his rural hospital faced down the pandemic. Titus Regional lost about $4 million last fiscal year and broke even each of the three years before that.
Mr. Scoggin said he did not cut from his clinical staff, though. Titus is now facing its second surge of the virus in the community. “The last 7 days, we’ve been testing 30% positive,” he said, including the case of his father, who contracted it at a nursing home and survived.
“It’s personal and this is real,” Mr. Scoggin said. “You know the people who are infected. You know the people who are passing away.”
Of his roughly 700 employees, 48 have tested positive for the virus and 1 has died. They are short on testing kits, medication, and supplies.
“Right now the staff is strained,” Mr. Scoggin said. “I’ve been blown away by their selflessness and unbelievable spirit. We’re resilient, we’re nimble, and we will make it. We don’t have a choice.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation, which is not affiliated with Kaiser Permanente.
Jerome Antone said he is one of the lucky ones.
After becoming ill with COVID-19, Mr. Antone was hospitalized only 65 miles away from his small Alabama town. He is the mayor of Georgiana – population 1,700.
“It hit our rural community so rabid,” Mr. Antone said. The town’s hospital closed last year. If hospitals in nearby communities don’t have beds available, “you may have to go 4 or 5 hours away.”
Eighteen rural hospitals closed last year and the first 3 months of 2020 were “really big months,” said Mark Holmes, PhD, director of the Cecil G. Sheps Center for Health Services Research at the University of North Carolina at Chapel Hill. Many of the losses are in Southern states like Florida and Texas. More than 170 rural hospitals have closed nationwide since 2005, according to data collected by the Sheps Center.
It’s a dangerous scenario. “We know that a closure leads to higher mortality pretty quickly” among the populations served, said Dr. Holmes, who is also a professor at UNC Gillings School of Global Public Health. “That’s pretty clear.”
One 2019 study found that death rates in the surrounding communities increase nearly 6% after a rural hospital closes – and that’s when there’s not a pandemic.
Add to that what is known about the coronavirus: People who are obese or live with diabetes, hypertension, asthma, and other underlying health issues are more susceptible to COVID-19. Rural areas tend to have higher rates of these conditions. And rural residents are more likely to be older, sicker and poorer than those in urban areas. All this leaves rural communities particularly vulnerable to the coronavirus.
Congress approved billions in federal relief funds for health care providers. Initially, federal officials based what a hospital would get on its Medicare payments, but by late April they heeded criticism and carved out funds for rural hospitals and COVID-19 hot spots. Rural hospitals leapt at the chance to shore up already-negative budgets and prepare for the pandemic.
The funds “helped rural hospitals with the immediate storm,” said Don Williamson, MD, president of the Alabama Hospital Association. Nearly 80% of Alabama’s rural hospitals began the year with negative balance sheets and about 8 days’ worth of cash on hand.
Before the pandemic hit this year, hundreds of rural hospitals “were just trying to keep their doors open,” said Maggie Elehwany, vice president of government affairs with the National Rural Health Association. Then an estimated 70% of their income stopped as patients avoided the emergency room, doctor’s appointments, and elective surgeries.
“It was devastating,” Ms. Elehwany said.
Paul Taylor, chief executive of a 25-bed critical-access hospital and outpatient clinics in northwestern Arkansas, accepted millions in grants and loan money Congress approved this spring, largely through the CARES (Coronavirus Aid, Relief, and Economic Security) Act.
“For us, this was survival money and we spent it already,” Mr. Taylor said. With those funds, Ozarks Community Hospital increased surge capacity, expanding from 25 beds to 50 beds, adding negative pressure rooms and buying six ventilators. Taylor also ramped up COVID-19 testing at his hospital and clinics, located near some meat-processing plants.
Throughout June and July, Ozarks tested 1,000 patients a day and reported a 20% positive rate. The rate dropped to 16.9% in late July. But patients continue to avoid routine care.
Mr. Taylor said revenue is still constrained and he does not know how he will pay back $8 million that he borrowed from Medicare. The program allowed hospitals to borrow against future payments from the federal government, but stipulated that repayment would begin within 120 days.
For Mr. Taylor, this seems impossible. Medicare makes up 40% of Ozarks’ income. And he has to pay the loan back before he gets any more payments from Medicare. He’s hoping to refinance the hospital’s mortgage.
“If I get no relief and they take the money ... we won’t still be open,” Mr. Taylor said. Ozarks provides 625 jobs and serves an area with a population of about 75,000.
There are 1,300 small critical-access hospitals like Ozarks in rural America, and of those, 859 took advantage of the Medicare loans, sending about $3.1 billion into the local communities. But many rural communities have not yet experienced a surge in coronavirus cases – national leaders fear it will come as part of a new phase.
“There are pockets of rural America who say, ‘We haven’t seen a single COVID patient yet and we do not believe it’s real,’ ” Mr. Taylor said. “They will get hit sooner or later.”
Across the country, the reduced patient numbers and increased spending required to fight and prepare for the coronavirus was “like a knife cutting into a hospital’s blood supply,” said Ge Bai, PhD, associate professor of health policy and management at the Johns Hopkins Bloomberg School of Public Health in Baltimore.
Dr. Bai said the way the federal government reimbursed small rural hospitals through federal programs like Medicare before the pandemic was faulty and inefficient. “They are too weak to survive,” she said.
In rural Texas, about 2 hours from Dallas, Titus Regional Medical Center chief executive officer Terry Scoggin cut staff and furloughed workers even as his rural hospital faced down the pandemic. Titus Regional lost about $4 million last fiscal year and broke even each of the three years before that.
Mr. Scoggin said he did not cut from his clinical staff, though. Titus is now facing its second surge of the virus in the community. “The last 7 days, we’ve been testing 30% positive,” he said, including the case of his father, who contracted it at a nursing home and survived.
“It’s personal and this is real,” Mr. Scoggin said. “You know the people who are infected. You know the people who are passing away.”
Of his roughly 700 employees, 48 have tested positive for the virus and 1 has died. They are short on testing kits, medication, and supplies.
“Right now the staff is strained,” Mr. Scoggin said. “I’ve been blown away by their selflessness and unbelievable spirit. We’re resilient, we’re nimble, and we will make it. We don’t have a choice.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation, which is not affiliated with Kaiser Permanente.