User login
When viruses collide: Flu season during pandemic
The medical community is about to find out how prepared it is for the double whammy of influenza and COVID-19 that has been predicted for the fall of 2020. The complexities of diagnosis, management of vulnerable patients, and overflowing medical centers that have made the COVID-19 crisis so brutal may all be exacerbated by the arrival of seasonal influenza.
Lewis Jay Kaplan, MD, FCCP, a critical care surgeon at the University of Pennsylvania, Philadelphia, has seen his share of critically ill COVID-19 patients in the surgical ICU that he oversees. He’s approaching the upcoming flu season, poised to collide with the ongoing COVID-19 pandemic, ready to listen to each patient’s story to distinguish one from the other and determine treatment.
“The patients that have underlying comorbidities all have a story, and it’s up to you to figure out which chapter you’re in and how far along you happen to be,” he said. “It’s a very interesting approach to care, medical storytelling.”
With flu season closing in, pulmonologists are ruminating about how they’ll distinguish symptoms of COVID-19 and traditional influenza and how they’ll manage the most vulnerable patients, namely those with underlying respiratory disease and children. Influenza kills 12,000-61,000 people a year, according to the Centers for Disease Control, and results in 140,000-810,00 hospitalizations. Having a flu season in the midst of a pandemic of a disease with multiple overlapping symptoms threatens to overwhelm practitioners, hospitals, and the health system.
Dr. Kaplan said each patient’s story can point to the correct clinical approach. “Instead of just sharing data when you are on rounds, you’re really telling someone’s story.” It arises from a series of questions about how the disease has impacted them, specifics of their presentation, how their signs and symptoms differ from the usual, and how they responded to treatment. “It also helps you to then take what you’re doing, which can seem very, very complicated to individuals who are not medically sophisticated, and then help them to understand why you’re doing what you’re doing at this point.”
That can help get through to a patient with respiratory disease who insists he or she has or doesn’t have COVID-19 rather than the flu. “They form a different group that brings with them different fears and concerns, and you have to help them navigate that, too: all of this data and your decision-making around testing and admissions, and what you can omit doing and what you must do help them to navigate their own story,” Dr. Kaplan said.
Benjamin D. Singer, MD, a pulmonologist at Northwestern University, Chicago, authored an editorial in Science Advances that addressed four factors that will determine the scope of flu spread in the upcoming season: rate of transmission; vaccination rates; coinfection rates; and health disparities in minority populations, which are prone to higher rates of flu as well as COVID-19.
Flu vaccine ‘extra important’
The convergence of COVID-19 and influenza has the potential to overwhelm the health system, said Daniel A. Solomon, MD, of Brigham and Women’s in Boston. He coauthored a JAMA Insights clinical update on flu season during the COVID-19 pandemic that lists distinguishing and overlapping signs and symptoms of the two diseases.
The flu vaccine, he said, is “extra important this year,” especially in patients with existing respiratory disease, but COVID-19 has thrown up barriers to vaccination. Telemedicine has supplanted office visits. “People may miss that easy-touch opportunity to get the flu vaccine, so we have to be creative about making the flu vaccine highly accessible, maybe in nontraditional ways,” Dr. Solomon said. Some ideas he offered are pop-up vaccine fairs at schools and churches.
But just as COVID-19 may hinder flu vaccines, it may also be helping to mitigate flu transmission. “The interesting thing about transmission of the flu is that it’s transmitted the same way COVID is, so if we actually know how to decrease transmission of COVID, which we do – we’ve done it – we can actually decrease transmission of influenza as well,” Dr. Solomon said. Studies out of Hong Kong and Japan have reported a reduction in influenza cases during COVID-19 outbreaks in those places (Lancet Public Health. 2020;5:e279-88; JAMA. 2020;323:1969-71).
Risks of coinfection
About one in four COVID-19 patients have been diagnosed with an additional respiratory infection, including influenza (JAMA. 2020:323:2085-6). Pulmonologists must keep that in mind when managing COVID-19 suspects, said Dr. Singer.
“While it is true that most of the time COVID-19 travels alone, we have numerous examples in the literature and in our own experience that COVID-19 is accompanied by either another virus or another bacterial infection, including influenza,” Dr. Singer said. “The distinction is important. One is just for diagnostic reasons and public reporting reasons, but also because flu and COVID-19 have different requirements for how you care for patients in terms of the health system.”
Clinical suspicion for coinfection should remain high if the community spread of both COVID-19 and influenza is high, said Megan Conroy, MD, chief pulmonary and critical care fellow at Ohio State University, Columbus. “As the coronavirus first took hold in the United States in March 2020, we were at the tail end of influenza season, so it’s hard to predict what the upcoming influenza season will really look like with regards to coinfection.”
Distinguishing COVID-19 from flu
Multiple signs and symptoms between COVID-19 and the flu overlap. They include fever, chills, headache, myalgia, cough, and fatigue. Nasal congestion and sore throat are characteristic of the flu; shortness of breath and loss of the sense of smell have been widely reported in COVID-19. “While many upper respiratory infections can result in loss of smell, this may be more prevalent in COVID-19,” Dr. Conroy said. Other symptoms unique to COVID-19 are GI symptoms such as diarrhea and skin rashes such as acral ischemia.
Testing, however, is the cornerstone of the differential diagnosis. “You can’t confidently distinguish between them on symptoms alone,” Dr. Conroy added.
“I think the challenge we’ll face as clinicians, is caring for people with nonspecific symptoms of a respiratory viral illness, especially in the early phase of the illness,” said Dr. Solomon.
But even after that, symptoms can be difficult to distinguish.
“Later in the illness, COVID is more associated with a hypercoagulable state,” he said. “It is more associated with viral pneumonia on chest imaging, like the diffuse ground-glass infiltrates that we’ve all gotten used to seeing – but flu can do both of those things as well. So, without a test, it’s impossible to distinguish between the two infections in the clinic.”
But testing can have its shortcomings when flu season clashes with the COVID-19 pandemic. “Getting the test is not the same as getting the test results,” Dr. Solomon added. “Though a lot of people can get a test, if it takes 7 or 8 days to get the test result back, the result is useless.”
Widespread, rapid testing also depends on having adequate supplies of viral media transport and swabs. “I think that this is what we should be focusing on now: scaling up access to rapid turnaround testing,” he said. Distinguishing between the two is also important to preserve hospital resources. COVID-19 has more rigorous standards than flu for personal protective equipment and isolation of patients within the hospital.
Having chronic lung disease isn’t necessarily a risk factor for contracting COVID-19 or the flu, or both, Dr. Solomon said. “It’s a risk factor for having severe disease.” Again, he noted that flu vaccines are still necessary in these patients, as well as patients of advanced age and underlying medical conditions such as heart disease, diabetes, and obesity.
In managing children, it’s important to keep in mind that they communicate differently about their illnesses than adults, said Dr. Kaplan. “They may not have the words to tell you the same kind of thing that the adult tells you.” That’s where family members can help to flesh out the history. “They may present with an initially much milder form, if you will, where they’re not as critical up front, but then that small proportion of them comes back with the multi-inflammatory syndrome and then they are profoundly ill.”
Younger people make up a larger share of COVID-19 patients now, compared with the initial wave that hit the Northeast in the spring, Dr. Kaplan said. “We don’t know if that’s because the virus is a little different or the people that are getting sick are a little bit different.”
The COVID-19 strain now emerging may be less virulent than the strain that hit in early spring, he said. “That doesn’t mean that there aren’t still profoundly critical ill people with COVID of many different age ranges, that is true, but there are a lot of people that we now see will test positive, but aren’t really as profoundly ill as when it first landed here in the United States.”
That may be somewhat welcome as flu season arrives.
The physicians interviewed have no relevant disclosures.
The medical community is about to find out how prepared it is for the double whammy of influenza and COVID-19 that has been predicted for the fall of 2020. The complexities of diagnosis, management of vulnerable patients, and overflowing medical centers that have made the COVID-19 crisis so brutal may all be exacerbated by the arrival of seasonal influenza.
Lewis Jay Kaplan, MD, FCCP, a critical care surgeon at the University of Pennsylvania, Philadelphia, has seen his share of critically ill COVID-19 patients in the surgical ICU that he oversees. He’s approaching the upcoming flu season, poised to collide with the ongoing COVID-19 pandemic, ready to listen to each patient’s story to distinguish one from the other and determine treatment.
“The patients that have underlying comorbidities all have a story, and it’s up to you to figure out which chapter you’re in and how far along you happen to be,” he said. “It’s a very interesting approach to care, medical storytelling.”
With flu season closing in, pulmonologists are ruminating about how they’ll distinguish symptoms of COVID-19 and traditional influenza and how they’ll manage the most vulnerable patients, namely those with underlying respiratory disease and children. Influenza kills 12,000-61,000 people a year, according to the Centers for Disease Control, and results in 140,000-810,00 hospitalizations. Having a flu season in the midst of a pandemic of a disease with multiple overlapping symptoms threatens to overwhelm practitioners, hospitals, and the health system.
Dr. Kaplan said each patient’s story can point to the correct clinical approach. “Instead of just sharing data when you are on rounds, you’re really telling someone’s story.” It arises from a series of questions about how the disease has impacted them, specifics of their presentation, how their signs and symptoms differ from the usual, and how they responded to treatment. “It also helps you to then take what you’re doing, which can seem very, very complicated to individuals who are not medically sophisticated, and then help them to understand why you’re doing what you’re doing at this point.”
That can help get through to a patient with respiratory disease who insists he or she has or doesn’t have COVID-19 rather than the flu. “They form a different group that brings with them different fears and concerns, and you have to help them navigate that, too: all of this data and your decision-making around testing and admissions, and what you can omit doing and what you must do help them to navigate their own story,” Dr. Kaplan said.
Benjamin D. Singer, MD, a pulmonologist at Northwestern University, Chicago, authored an editorial in Science Advances that addressed four factors that will determine the scope of flu spread in the upcoming season: rate of transmission; vaccination rates; coinfection rates; and health disparities in minority populations, which are prone to higher rates of flu as well as COVID-19.
Flu vaccine ‘extra important’
The convergence of COVID-19 and influenza has the potential to overwhelm the health system, said Daniel A. Solomon, MD, of Brigham and Women’s in Boston. He coauthored a JAMA Insights clinical update on flu season during the COVID-19 pandemic that lists distinguishing and overlapping signs and symptoms of the two diseases.
The flu vaccine, he said, is “extra important this year,” especially in patients with existing respiratory disease, but COVID-19 has thrown up barriers to vaccination. Telemedicine has supplanted office visits. “People may miss that easy-touch opportunity to get the flu vaccine, so we have to be creative about making the flu vaccine highly accessible, maybe in nontraditional ways,” Dr. Solomon said. Some ideas he offered are pop-up vaccine fairs at schools and churches.
But just as COVID-19 may hinder flu vaccines, it may also be helping to mitigate flu transmission. “The interesting thing about transmission of the flu is that it’s transmitted the same way COVID is, so if we actually know how to decrease transmission of COVID, which we do – we’ve done it – we can actually decrease transmission of influenza as well,” Dr. Solomon said. Studies out of Hong Kong and Japan have reported a reduction in influenza cases during COVID-19 outbreaks in those places (Lancet Public Health. 2020;5:e279-88; JAMA. 2020;323:1969-71).
Risks of coinfection
About one in four COVID-19 patients have been diagnosed with an additional respiratory infection, including influenza (JAMA. 2020:323:2085-6). Pulmonologists must keep that in mind when managing COVID-19 suspects, said Dr. Singer.
“While it is true that most of the time COVID-19 travels alone, we have numerous examples in the literature and in our own experience that COVID-19 is accompanied by either another virus or another bacterial infection, including influenza,” Dr. Singer said. “The distinction is important. One is just for diagnostic reasons and public reporting reasons, but also because flu and COVID-19 have different requirements for how you care for patients in terms of the health system.”
Clinical suspicion for coinfection should remain high if the community spread of both COVID-19 and influenza is high, said Megan Conroy, MD, chief pulmonary and critical care fellow at Ohio State University, Columbus. “As the coronavirus first took hold in the United States in March 2020, we were at the tail end of influenza season, so it’s hard to predict what the upcoming influenza season will really look like with regards to coinfection.”
Distinguishing COVID-19 from flu
Multiple signs and symptoms between COVID-19 and the flu overlap. They include fever, chills, headache, myalgia, cough, and fatigue. Nasal congestion and sore throat are characteristic of the flu; shortness of breath and loss of the sense of smell have been widely reported in COVID-19. “While many upper respiratory infections can result in loss of smell, this may be more prevalent in COVID-19,” Dr. Conroy said. Other symptoms unique to COVID-19 are GI symptoms such as diarrhea and skin rashes such as acral ischemia.
Testing, however, is the cornerstone of the differential diagnosis. “You can’t confidently distinguish between them on symptoms alone,” Dr. Conroy added.
“I think the challenge we’ll face as clinicians, is caring for people with nonspecific symptoms of a respiratory viral illness, especially in the early phase of the illness,” said Dr. Solomon.
But even after that, symptoms can be difficult to distinguish.
“Later in the illness, COVID is more associated with a hypercoagulable state,” he said. “It is more associated with viral pneumonia on chest imaging, like the diffuse ground-glass infiltrates that we’ve all gotten used to seeing – but flu can do both of those things as well. So, without a test, it’s impossible to distinguish between the two infections in the clinic.”
But testing can have its shortcomings when flu season clashes with the COVID-19 pandemic. “Getting the test is not the same as getting the test results,” Dr. Solomon added. “Though a lot of people can get a test, if it takes 7 or 8 days to get the test result back, the result is useless.”
Widespread, rapid testing also depends on having adequate supplies of viral media transport and swabs. “I think that this is what we should be focusing on now: scaling up access to rapid turnaround testing,” he said. Distinguishing between the two is also important to preserve hospital resources. COVID-19 has more rigorous standards than flu for personal protective equipment and isolation of patients within the hospital.
Having chronic lung disease isn’t necessarily a risk factor for contracting COVID-19 or the flu, or both, Dr. Solomon said. “It’s a risk factor for having severe disease.” Again, he noted that flu vaccines are still necessary in these patients, as well as patients of advanced age and underlying medical conditions such as heart disease, diabetes, and obesity.
In managing children, it’s important to keep in mind that they communicate differently about their illnesses than adults, said Dr. Kaplan. “They may not have the words to tell you the same kind of thing that the adult tells you.” That’s where family members can help to flesh out the history. “They may present with an initially much milder form, if you will, where they’re not as critical up front, but then that small proportion of them comes back with the multi-inflammatory syndrome and then they are profoundly ill.”
Younger people make up a larger share of COVID-19 patients now, compared with the initial wave that hit the Northeast in the spring, Dr. Kaplan said. “We don’t know if that’s because the virus is a little different or the people that are getting sick are a little bit different.”
The COVID-19 strain now emerging may be less virulent than the strain that hit in early spring, he said. “That doesn’t mean that there aren’t still profoundly critical ill people with COVID of many different age ranges, that is true, but there are a lot of people that we now see will test positive, but aren’t really as profoundly ill as when it first landed here in the United States.”
That may be somewhat welcome as flu season arrives.
The physicians interviewed have no relevant disclosures.
The medical community is about to find out how prepared it is for the double whammy of influenza and COVID-19 that has been predicted for the fall of 2020. The complexities of diagnosis, management of vulnerable patients, and overflowing medical centers that have made the COVID-19 crisis so brutal may all be exacerbated by the arrival of seasonal influenza.
Lewis Jay Kaplan, MD, FCCP, a critical care surgeon at the University of Pennsylvania, Philadelphia, has seen his share of critically ill COVID-19 patients in the surgical ICU that he oversees. He’s approaching the upcoming flu season, poised to collide with the ongoing COVID-19 pandemic, ready to listen to each patient’s story to distinguish one from the other and determine treatment.
“The patients that have underlying comorbidities all have a story, and it’s up to you to figure out which chapter you’re in and how far along you happen to be,” he said. “It’s a very interesting approach to care, medical storytelling.”
With flu season closing in, pulmonologists are ruminating about how they’ll distinguish symptoms of COVID-19 and traditional influenza and how they’ll manage the most vulnerable patients, namely those with underlying respiratory disease and children. Influenza kills 12,000-61,000 people a year, according to the Centers for Disease Control, and results in 140,000-810,00 hospitalizations. Having a flu season in the midst of a pandemic of a disease with multiple overlapping symptoms threatens to overwhelm practitioners, hospitals, and the health system.
Dr. Kaplan said each patient’s story can point to the correct clinical approach. “Instead of just sharing data when you are on rounds, you’re really telling someone’s story.” It arises from a series of questions about how the disease has impacted them, specifics of their presentation, how their signs and symptoms differ from the usual, and how they responded to treatment. “It also helps you to then take what you’re doing, which can seem very, very complicated to individuals who are not medically sophisticated, and then help them to understand why you’re doing what you’re doing at this point.”
That can help get through to a patient with respiratory disease who insists he or she has or doesn’t have COVID-19 rather than the flu. “They form a different group that brings with them different fears and concerns, and you have to help them navigate that, too: all of this data and your decision-making around testing and admissions, and what you can omit doing and what you must do help them to navigate their own story,” Dr. Kaplan said.
Benjamin D. Singer, MD, a pulmonologist at Northwestern University, Chicago, authored an editorial in Science Advances that addressed four factors that will determine the scope of flu spread in the upcoming season: rate of transmission; vaccination rates; coinfection rates; and health disparities in minority populations, which are prone to higher rates of flu as well as COVID-19.
Flu vaccine ‘extra important’
The convergence of COVID-19 and influenza has the potential to overwhelm the health system, said Daniel A. Solomon, MD, of Brigham and Women’s in Boston. He coauthored a JAMA Insights clinical update on flu season during the COVID-19 pandemic that lists distinguishing and overlapping signs and symptoms of the two diseases.
The flu vaccine, he said, is “extra important this year,” especially in patients with existing respiratory disease, but COVID-19 has thrown up barriers to vaccination. Telemedicine has supplanted office visits. “People may miss that easy-touch opportunity to get the flu vaccine, so we have to be creative about making the flu vaccine highly accessible, maybe in nontraditional ways,” Dr. Solomon said. Some ideas he offered are pop-up vaccine fairs at schools and churches.
But just as COVID-19 may hinder flu vaccines, it may also be helping to mitigate flu transmission. “The interesting thing about transmission of the flu is that it’s transmitted the same way COVID is, so if we actually know how to decrease transmission of COVID, which we do – we’ve done it – we can actually decrease transmission of influenza as well,” Dr. Solomon said. Studies out of Hong Kong and Japan have reported a reduction in influenza cases during COVID-19 outbreaks in those places (Lancet Public Health. 2020;5:e279-88; JAMA. 2020;323:1969-71).
Risks of coinfection
About one in four COVID-19 patients have been diagnosed with an additional respiratory infection, including influenza (JAMA. 2020:323:2085-6). Pulmonologists must keep that in mind when managing COVID-19 suspects, said Dr. Singer.
“While it is true that most of the time COVID-19 travels alone, we have numerous examples in the literature and in our own experience that COVID-19 is accompanied by either another virus or another bacterial infection, including influenza,” Dr. Singer said. “The distinction is important. One is just for diagnostic reasons and public reporting reasons, but also because flu and COVID-19 have different requirements for how you care for patients in terms of the health system.”
Clinical suspicion for coinfection should remain high if the community spread of both COVID-19 and influenza is high, said Megan Conroy, MD, chief pulmonary and critical care fellow at Ohio State University, Columbus. “As the coronavirus first took hold in the United States in March 2020, we were at the tail end of influenza season, so it’s hard to predict what the upcoming influenza season will really look like with regards to coinfection.”
Distinguishing COVID-19 from flu
Multiple signs and symptoms between COVID-19 and the flu overlap. They include fever, chills, headache, myalgia, cough, and fatigue. Nasal congestion and sore throat are characteristic of the flu; shortness of breath and loss of the sense of smell have been widely reported in COVID-19. “While many upper respiratory infections can result in loss of smell, this may be more prevalent in COVID-19,” Dr. Conroy said. Other symptoms unique to COVID-19 are GI symptoms such as diarrhea and skin rashes such as acral ischemia.
Testing, however, is the cornerstone of the differential diagnosis. “You can’t confidently distinguish between them on symptoms alone,” Dr. Conroy added.
“I think the challenge we’ll face as clinicians, is caring for people with nonspecific symptoms of a respiratory viral illness, especially in the early phase of the illness,” said Dr. Solomon.
But even after that, symptoms can be difficult to distinguish.
“Later in the illness, COVID is more associated with a hypercoagulable state,” he said. “It is more associated with viral pneumonia on chest imaging, like the diffuse ground-glass infiltrates that we’ve all gotten used to seeing – but flu can do both of those things as well. So, without a test, it’s impossible to distinguish between the two infections in the clinic.”
But testing can have its shortcomings when flu season clashes with the COVID-19 pandemic. “Getting the test is not the same as getting the test results,” Dr. Solomon added. “Though a lot of people can get a test, if it takes 7 or 8 days to get the test result back, the result is useless.”
Widespread, rapid testing also depends on having adequate supplies of viral media transport and swabs. “I think that this is what we should be focusing on now: scaling up access to rapid turnaround testing,” he said. Distinguishing between the two is also important to preserve hospital resources. COVID-19 has more rigorous standards than flu for personal protective equipment and isolation of patients within the hospital.
Having chronic lung disease isn’t necessarily a risk factor for contracting COVID-19 or the flu, or both, Dr. Solomon said. “It’s a risk factor for having severe disease.” Again, he noted that flu vaccines are still necessary in these patients, as well as patients of advanced age and underlying medical conditions such as heart disease, diabetes, and obesity.
In managing children, it’s important to keep in mind that they communicate differently about their illnesses than adults, said Dr. Kaplan. “They may not have the words to tell you the same kind of thing that the adult tells you.” That’s where family members can help to flesh out the history. “They may present with an initially much milder form, if you will, where they’re not as critical up front, but then that small proportion of them comes back with the multi-inflammatory syndrome and then they are profoundly ill.”
Younger people make up a larger share of COVID-19 patients now, compared with the initial wave that hit the Northeast in the spring, Dr. Kaplan said. “We don’t know if that’s because the virus is a little different or the people that are getting sick are a little bit different.”
The COVID-19 strain now emerging may be less virulent than the strain that hit in early spring, he said. “That doesn’t mean that there aren’t still profoundly critical ill people with COVID of many different age ranges, that is true, but there are a lot of people that we now see will test positive, but aren’t really as profoundly ill as when it first landed here in the United States.”
That may be somewhat welcome as flu season arrives.
The physicians interviewed have no relevant disclosures.
One-off blast of RT, rather than weeks, for early breast cancer
Long-term outcomes now being reported confirm earlier reports from the same trial showing efficacy for the use of targeted intraoperative radiotherapy (TARGIT) in patients with early breast cancer.
This novel approach, which delivers a one-off blast of radiation directed at the tumor bed and is given during lumpectomy, has similar efficacy and lowers non–breast cancer mortality when compared with whole-breast external beam radiotherapy (EBRT), which is delivered in fractions over 3-6 weeks after surgery.
Giving the boost of radiation during surgery has numerous benefits, say the authors: it is more convenient for patients and saves on healthcare costs.
However, the controversy over local recurrence rates, sparked by the earlier results, still remains. The difference in the 5-year local recurrence rate between TARGIT and EBRT was within the 2.5% margin for non-inferiority: the rate was 2.11% in 1140 TARGIT recipients, compared with 0.95% in 1158 EBRT recipients, for a difference of 1.16% (13 recurrences).
The new longer-term results from the TARGIT-A trial were published August 20 in the BMJ, and confirm earlier results from this trial published in 2014. Meanwhile, other approaches to intraoperative radiotherapy (IORT) have also been reported.
Nevertheless, whole-breast radiotherapy remains the standard of care today, note the authors.
“The biggest battle the TARGIT investigator family has faced is our challenge to the conventional dogma that radiotherapy has to be given in multiple daily doses, and moreover that whole-breast radiotherapy is always essential,” said lead author Jayant Vaidya, MD, professor of surgery and oncology at University College London, UK. He was one of the team of investigators that together developed the TARGIT approach in the 1990s, as he recalls in a related BMJ blog post.
It is unclear whether the TARGIT-A long-term outcomes will change practice, Rachel Jimenez, MD, associate program director of the Harvard Radiation Oncology Residency Program and assistant professor of radiation oncology at Massachusetts General Hospital, Boston, told Medscape Medical News.
She noted there was controversy and debate over the earlier reports from TARGIT-A, and those findings “did little to change practice patterns over the past 6 years,” she said.
“With the publication of longer-term follow-up, my expectation would be that perceptions of IORT will remain unchanged in the radiation oncology community, and that those previously supportive of the TARGIT-A approach will continue to embrace it while those initially skeptical will be unlikely to change practice despite the longer-term results,” she said.
“However, despite the controversy surrounding TARGIT-A, it is heartening as a clinician who cares for breast cancer patients to see a trend within the early breast cancer clinical trials space toward the evaluation of increasingly targeted and abbreviated courses of radiation,” Jimenez commented.
Details of new long-term results
TARGIT-A is an open-label, 32-center multinational study conducted in 2298 women aged 45 years or older with early-stage invasive ductal carcinoma who were eligible for breast-conserving surgery. Between March 24, 2000, and June 25, 2012, participants were randomized 1:1 to risk-adapted TARGIT immediately after lumpectomy or to whole-breast EBRT delivered for the standard 3-6 week daily fractionated course.
At a median follow-up of 8.6 years — with some patients followed for nearly 19 years — no significant difference was seen between the treatment groups in local recurrence-free survival (167 vs 147 events; hazard ratio, 1.13); invasive local recurrence-free survival (154 vs 146 events; HR, 1.04); mastectomy-free survival (170 vs 175 events; HR, 0.96); distant disease-free survival (133 vs 148 events; HR, 0.88); overall survival (110 vs 131 events; HR, 0.82); or breast cancer mortality (6 vs 57 events; HR, 1.12).
“Mortality from other causes was significantly lower (45 vs 74 events; HR, 0.59),” the authors note.
Controversy of Earlier Results
Vaidya and colleagues comment that these new results confirm earlier findings from this trial. They were initially presented in 2012 by Vaidya at the San Antonio Breast Cancer Symposium and subsequently published in The Lancet, as previously reported by Medscape Medical News. Those earlier results showed a trend toward lower overall mortality with TARGIT (absolute difference, -1.3%; P = .01) and significantly fewer deaths from causes other than breast cancer (absolute difference, -2.1%; P = .009).
However, TARGIT was associated with slightly more same-breast recurrences at that time (3.3% vs 1.3%; P = 0.42), even though this was still within the 2.5% margin for non-inferiority.
The new longer-term results show a similar pattern.
It was this risk of same-breast recurrences that sparked the heated debate over the findings, as some breast cancer experts argued that this needs to be weighed against various potential benefits of IORT for patients: greater convenience, potentially improved mortality, and lower costs.
The extent of that “vigorous debate” was highlighted in 2015 in the International Journal of Radiation Oncology, Biology, Physics, (the Red Journal), in which editor-in-chief Anthony Zietman, MD, shared numerous letters to the editor, written in response to two editorials, that contained “passionately and articulately expressed” views from “senior investigators and breast cancer physicians from around the globe.”
At the same time, in 2015, another approach to IORT was reported at the American Society for Radiation Oncology (ASTRO) annual meeting and simultaneously published online in The Lancet. This was accelerated partial-breast irradiation (APBI) delivered directly to the tumor bed using multicatheter brachytherapy at the time of lumpectomy in women with early breast cancer. The results showed outcomes that were comparable with whole-breast irradiation, but with fewer side effects.
Those findings prompted a 2016 update to the ASTRO consensus statement on APBI to note that APBI after lumpectomy may be suitable for more women with early-stage breast cancer, including younger patients and those with ductal carcinoma in situ.
In comments to Medscape Medical News, Jimenez noted that several recent studies have shown efficacy for various IORT approaches. There have been two phase 3 non-inferiority studies, namely the NSABP B-39 and the RAPID trial, that evaluate the use of APBI in lieu of whole-breast radiation. There have also been two trials as well as the evaluation of a 5-day ultrahypofractionated whole-breast radiation course per the UK Fast and Fast-Forward trials, compared with several weeks of whole-breast radiation.
“Collectively, these studies lend support for fewer and/or more targeted radiotherapy treatments for our patients and have the potential to reduce patient burden and limit healthcare costs,” Jimenez told Medscape Medical News.
Indeed, the TARGIT-A researchers write that their long-term findings “have shown that risk-adapted single-dose TARGIT-IORT given during lumpectomy can effectively replace the mandatory use of several weeks of daily postoperative whole-breast radiotherapy in patients with breast cancer undergoing breast conservation.”
Given the numerous benefits to patients that this approach provides, the choice should ultimately rest with the patient, the authors conclude.
An extended follow-up of the trial (TARGIT-Ex) is ongoing, as is the TARGIT-B(oost) trial looking at TARGIT-IORT as a tumor bed boost with EBRT boost in younger women and those with higher-risk disease.
The TARGIT-A trial was sponsored by University College London Hospitals (UCLH) Comprehensive Biomedical Research Centre and funded by UCLH Charities, the National Institute for Health Research Health Technology Assessment program, Ninewells Cancer Campaign, the National Health and Medical Research Council, and the German Federal Ministry of Education and Research. The authors reported numerous disclosures, as detailed in the publication.
This article first appeared on Medscape.com.
Long-term outcomes now being reported confirm earlier reports from the same trial showing efficacy for the use of targeted intraoperative radiotherapy (TARGIT) in patients with early breast cancer.
This novel approach, which delivers a one-off blast of radiation directed at the tumor bed and is given during lumpectomy, has similar efficacy and lowers non–breast cancer mortality when compared with whole-breast external beam radiotherapy (EBRT), which is delivered in fractions over 3-6 weeks after surgery.
Giving the boost of radiation during surgery has numerous benefits, say the authors: it is more convenient for patients and saves on healthcare costs.
However, the controversy over local recurrence rates, sparked by the earlier results, still remains. The difference in the 5-year local recurrence rate between TARGIT and EBRT was within the 2.5% margin for non-inferiority: the rate was 2.11% in 1140 TARGIT recipients, compared with 0.95% in 1158 EBRT recipients, for a difference of 1.16% (13 recurrences).
The new longer-term results from the TARGIT-A trial were published August 20 in the BMJ, and confirm earlier results from this trial published in 2014. Meanwhile, other approaches to intraoperative radiotherapy (IORT) have also been reported.
Nevertheless, whole-breast radiotherapy remains the standard of care today, note the authors.
“The biggest battle the TARGIT investigator family has faced is our challenge to the conventional dogma that radiotherapy has to be given in multiple daily doses, and moreover that whole-breast radiotherapy is always essential,” said lead author Jayant Vaidya, MD, professor of surgery and oncology at University College London, UK. He was one of the team of investigators that together developed the TARGIT approach in the 1990s, as he recalls in a related BMJ blog post.
It is unclear whether the TARGIT-A long-term outcomes will change practice, Rachel Jimenez, MD, associate program director of the Harvard Radiation Oncology Residency Program and assistant professor of radiation oncology at Massachusetts General Hospital, Boston, told Medscape Medical News.
She noted there was controversy and debate over the earlier reports from TARGIT-A, and those findings “did little to change practice patterns over the past 6 years,” she said.
“With the publication of longer-term follow-up, my expectation would be that perceptions of IORT will remain unchanged in the radiation oncology community, and that those previously supportive of the TARGIT-A approach will continue to embrace it while those initially skeptical will be unlikely to change practice despite the longer-term results,” she said.
“However, despite the controversy surrounding TARGIT-A, it is heartening as a clinician who cares for breast cancer patients to see a trend within the early breast cancer clinical trials space toward the evaluation of increasingly targeted and abbreviated courses of radiation,” Jimenez commented.
Details of new long-term results
TARGIT-A is an open-label, 32-center multinational study conducted in 2298 women aged 45 years or older with early-stage invasive ductal carcinoma who were eligible for breast-conserving surgery. Between March 24, 2000, and June 25, 2012, participants were randomized 1:1 to risk-adapted TARGIT immediately after lumpectomy or to whole-breast EBRT delivered for the standard 3-6 week daily fractionated course.
At a median follow-up of 8.6 years — with some patients followed for nearly 19 years — no significant difference was seen between the treatment groups in local recurrence-free survival (167 vs 147 events; hazard ratio, 1.13); invasive local recurrence-free survival (154 vs 146 events; HR, 1.04); mastectomy-free survival (170 vs 175 events; HR, 0.96); distant disease-free survival (133 vs 148 events; HR, 0.88); overall survival (110 vs 131 events; HR, 0.82); or breast cancer mortality (6 vs 57 events; HR, 1.12).
“Mortality from other causes was significantly lower (45 vs 74 events; HR, 0.59),” the authors note.
Controversy of Earlier Results
Vaidya and colleagues comment that these new results confirm earlier findings from this trial. They were initially presented in 2012 by Vaidya at the San Antonio Breast Cancer Symposium and subsequently published in The Lancet, as previously reported by Medscape Medical News. Those earlier results showed a trend toward lower overall mortality with TARGIT (absolute difference, -1.3%; P = .01) and significantly fewer deaths from causes other than breast cancer (absolute difference, -2.1%; P = .009).
However, TARGIT was associated with slightly more same-breast recurrences at that time (3.3% vs 1.3%; P = 0.42), even though this was still within the 2.5% margin for non-inferiority.
The new longer-term results show a similar pattern.
It was this risk of same-breast recurrences that sparked the heated debate over the findings, as some breast cancer experts argued that this needs to be weighed against various potential benefits of IORT for patients: greater convenience, potentially improved mortality, and lower costs.
The extent of that “vigorous debate” was highlighted in 2015 in the International Journal of Radiation Oncology, Biology, Physics, (the Red Journal), in which editor-in-chief Anthony Zietman, MD, shared numerous letters to the editor, written in response to two editorials, that contained “passionately and articulately expressed” views from “senior investigators and breast cancer physicians from around the globe.”
At the same time, in 2015, another approach to IORT was reported at the American Society for Radiation Oncology (ASTRO) annual meeting and simultaneously published online in The Lancet. This was accelerated partial-breast irradiation (APBI) delivered directly to the tumor bed using multicatheter brachytherapy at the time of lumpectomy in women with early breast cancer. The results showed outcomes that were comparable with whole-breast irradiation, but with fewer side effects.
Those findings prompted a 2016 update to the ASTRO consensus statement on APBI to note that APBI after lumpectomy may be suitable for more women with early-stage breast cancer, including younger patients and those with ductal carcinoma in situ.
In comments to Medscape Medical News, Jimenez noted that several recent studies have shown efficacy for various IORT approaches. There have been two phase 3 non-inferiority studies, namely the NSABP B-39 and the RAPID trial, that evaluate the use of APBI in lieu of whole-breast radiation. There have also been two trials as well as the evaluation of a 5-day ultrahypofractionated whole-breast radiation course per the UK Fast and Fast-Forward trials, compared with several weeks of whole-breast radiation.
“Collectively, these studies lend support for fewer and/or more targeted radiotherapy treatments for our patients and have the potential to reduce patient burden and limit healthcare costs,” Jimenez told Medscape Medical News.
Indeed, the TARGIT-A researchers write that their long-term findings “have shown that risk-adapted single-dose TARGIT-IORT given during lumpectomy can effectively replace the mandatory use of several weeks of daily postoperative whole-breast radiotherapy in patients with breast cancer undergoing breast conservation.”
Given the numerous benefits to patients that this approach provides, the choice should ultimately rest with the patient, the authors conclude.
An extended follow-up of the trial (TARGIT-Ex) is ongoing, as is the TARGIT-B(oost) trial looking at TARGIT-IORT as a tumor bed boost with EBRT boost in younger women and those with higher-risk disease.
The TARGIT-A trial was sponsored by University College London Hospitals (UCLH) Comprehensive Biomedical Research Centre and funded by UCLH Charities, the National Institute for Health Research Health Technology Assessment program, Ninewells Cancer Campaign, the National Health and Medical Research Council, and the German Federal Ministry of Education and Research. The authors reported numerous disclosures, as detailed in the publication.
This article first appeared on Medscape.com.
Long-term outcomes now being reported confirm earlier reports from the same trial showing efficacy for the use of targeted intraoperative radiotherapy (TARGIT) in patients with early breast cancer.
This novel approach, which delivers a one-off blast of radiation directed at the tumor bed and is given during lumpectomy, has similar efficacy and lowers non–breast cancer mortality when compared with whole-breast external beam radiotherapy (EBRT), which is delivered in fractions over 3-6 weeks after surgery.
Giving the boost of radiation during surgery has numerous benefits, say the authors: it is more convenient for patients and saves on healthcare costs.
However, the controversy over local recurrence rates, sparked by the earlier results, still remains. The difference in the 5-year local recurrence rate between TARGIT and EBRT was within the 2.5% margin for non-inferiority: the rate was 2.11% in 1140 TARGIT recipients, compared with 0.95% in 1158 EBRT recipients, for a difference of 1.16% (13 recurrences).
The new longer-term results from the TARGIT-A trial were published August 20 in the BMJ, and confirm earlier results from this trial published in 2014. Meanwhile, other approaches to intraoperative radiotherapy (IORT) have also been reported.
Nevertheless, whole-breast radiotherapy remains the standard of care today, note the authors.
“The biggest battle the TARGIT investigator family has faced is our challenge to the conventional dogma that radiotherapy has to be given in multiple daily doses, and moreover that whole-breast radiotherapy is always essential,” said lead author Jayant Vaidya, MD, professor of surgery and oncology at University College London, UK. He was one of the team of investigators that together developed the TARGIT approach in the 1990s, as he recalls in a related BMJ blog post.
It is unclear whether the TARGIT-A long-term outcomes will change practice, Rachel Jimenez, MD, associate program director of the Harvard Radiation Oncology Residency Program and assistant professor of radiation oncology at Massachusetts General Hospital, Boston, told Medscape Medical News.
She noted there was controversy and debate over the earlier reports from TARGIT-A, and those findings “did little to change practice patterns over the past 6 years,” she said.
“With the publication of longer-term follow-up, my expectation would be that perceptions of IORT will remain unchanged in the radiation oncology community, and that those previously supportive of the TARGIT-A approach will continue to embrace it while those initially skeptical will be unlikely to change practice despite the longer-term results,” she said.
“However, despite the controversy surrounding TARGIT-A, it is heartening as a clinician who cares for breast cancer patients to see a trend within the early breast cancer clinical trials space toward the evaluation of increasingly targeted and abbreviated courses of radiation,” Jimenez commented.
Details of new long-term results
TARGIT-A is an open-label, 32-center multinational study conducted in 2298 women aged 45 years or older with early-stage invasive ductal carcinoma who were eligible for breast-conserving surgery. Between March 24, 2000, and June 25, 2012, participants were randomized 1:1 to risk-adapted TARGIT immediately after lumpectomy or to whole-breast EBRT delivered for the standard 3-6 week daily fractionated course.
At a median follow-up of 8.6 years — with some patients followed for nearly 19 years — no significant difference was seen between the treatment groups in local recurrence-free survival (167 vs 147 events; hazard ratio, 1.13); invasive local recurrence-free survival (154 vs 146 events; HR, 1.04); mastectomy-free survival (170 vs 175 events; HR, 0.96); distant disease-free survival (133 vs 148 events; HR, 0.88); overall survival (110 vs 131 events; HR, 0.82); or breast cancer mortality (6 vs 57 events; HR, 1.12).
“Mortality from other causes was significantly lower (45 vs 74 events; HR, 0.59),” the authors note.
Controversy of Earlier Results
Vaidya and colleagues comment that these new results confirm earlier findings from this trial. They were initially presented in 2012 by Vaidya at the San Antonio Breast Cancer Symposium and subsequently published in The Lancet, as previously reported by Medscape Medical News. Those earlier results showed a trend toward lower overall mortality with TARGIT (absolute difference, -1.3%; P = .01) and significantly fewer deaths from causes other than breast cancer (absolute difference, -2.1%; P = .009).
However, TARGIT was associated with slightly more same-breast recurrences at that time (3.3% vs 1.3%; P = 0.42), even though this was still within the 2.5% margin for non-inferiority.
The new longer-term results show a similar pattern.
It was this risk of same-breast recurrences that sparked the heated debate over the findings, as some breast cancer experts argued that this needs to be weighed against various potential benefits of IORT for patients: greater convenience, potentially improved mortality, and lower costs.
The extent of that “vigorous debate” was highlighted in 2015 in the International Journal of Radiation Oncology, Biology, Physics, (the Red Journal), in which editor-in-chief Anthony Zietman, MD, shared numerous letters to the editor, written in response to two editorials, that contained “passionately and articulately expressed” views from “senior investigators and breast cancer physicians from around the globe.”
At the same time, in 2015, another approach to IORT was reported at the American Society for Radiation Oncology (ASTRO) annual meeting and simultaneously published online in The Lancet. This was accelerated partial-breast irradiation (APBI) delivered directly to the tumor bed using multicatheter brachytherapy at the time of lumpectomy in women with early breast cancer. The results showed outcomes that were comparable with whole-breast irradiation, but with fewer side effects.
Those findings prompted a 2016 update to the ASTRO consensus statement on APBI to note that APBI after lumpectomy may be suitable for more women with early-stage breast cancer, including younger patients and those with ductal carcinoma in situ.
In comments to Medscape Medical News, Jimenez noted that several recent studies have shown efficacy for various IORT approaches. There have been two phase 3 non-inferiority studies, namely the NSABP B-39 and the RAPID trial, that evaluate the use of APBI in lieu of whole-breast radiation. There have also been two trials as well as the evaluation of a 5-day ultrahypofractionated whole-breast radiation course per the UK Fast and Fast-Forward trials, compared with several weeks of whole-breast radiation.
“Collectively, these studies lend support for fewer and/or more targeted radiotherapy treatments for our patients and have the potential to reduce patient burden and limit healthcare costs,” Jimenez told Medscape Medical News.
Indeed, the TARGIT-A researchers write that their long-term findings “have shown that risk-adapted single-dose TARGIT-IORT given during lumpectomy can effectively replace the mandatory use of several weeks of daily postoperative whole-breast radiotherapy in patients with breast cancer undergoing breast conservation.”
Given the numerous benefits to patients that this approach provides, the choice should ultimately rest with the patient, the authors conclude.
An extended follow-up of the trial (TARGIT-Ex) is ongoing, as is the TARGIT-B(oost) trial looking at TARGIT-IORT as a tumor bed boost with EBRT boost in younger women and those with higher-risk disease.
The TARGIT-A trial was sponsored by University College London Hospitals (UCLH) Comprehensive Biomedical Research Centre and funded by UCLH Charities, the National Institute for Health Research Health Technology Assessment program, Ninewells Cancer Campaign, the National Health and Medical Research Council, and the German Federal Ministry of Education and Research. The authors reported numerous disclosures, as detailed in the publication.
This article first appeared on Medscape.com.
As COVID-19 cases increase in children, deaths remain low
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
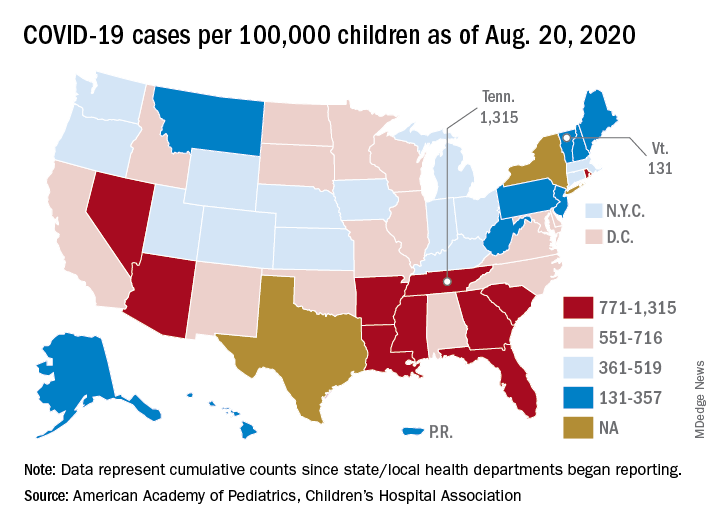
The cumulative number of pediatric cases reported up to that date was 442,785, or 9.3% of the total COVID-19 case load of more than 4.76 million among all ages. There have been only 92 pediatric deaths, however, which works out to just 0.06% of the 154,279 reported for all ages, the AAP and the CHA said Aug. 24 in their most recent update.
Child hospitalizations also were on the low side, representing 1.7% (4,062) of the cumulative total of 234,810 admissions among all ages as of Aug. 20, based on data from 21 states and New York City.
Nationally, the cumulative number of reported child cases is now up to 583 per 100,000 children, and that figure covers 49 states, Washington, D.C., Guam, New York City, and Puerto Rico.
There is some disagreement among the states, though, about the definition of “child.” Most states use an age range of 0-17, 0-18, or 0-19, but Florida and Utah go with a range of 0-14 years while South Carolina and Tennessee consider humans aged 0-20 years to be children. Other data limitations involve Texas, which has reported age distribution for only 8% of all cases, and New York, which is not reporting the age distribution of statewide cases, the AAP/CHA report noted.
The definition of child isn’t the only thing that varies between the states. The cumulative case rate for Tennessee, the highest in the country at 1,315 per 100,000 children, is 10 times that of Vermont, which is the lowest at 131 per 100,000, the AAP and CHA said. Vermont reports child COVID-19 cases using an age range of 0-19 years.
The other states with rates over 1,000 cases per 100,000 children are Arizona (1,300), which had the highest rate a week ago; South Carolina (1,214); Louisiana (1,127); Mississippi (1,120); and Nevada (1,068). Those with rates below 200 cases per 100,000 children are Maine (150), New Hampshire (175), and Hawaii (188), according to this week’s report.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The cumulative number of pediatric cases reported up to that date was 442,785, or 9.3% of the total COVID-19 case load of more than 4.76 million among all ages. There have been only 92 pediatric deaths, however, which works out to just 0.06% of the 154,279 reported for all ages, the AAP and the CHA said Aug. 24 in their most recent update.
Child hospitalizations also were on the low side, representing 1.7% (4,062) of the cumulative total of 234,810 admissions among all ages as of Aug. 20, based on data from 21 states and New York City.
Nationally, the cumulative number of reported child cases is now up to 583 per 100,000 children, and that figure covers 49 states, Washington, D.C., Guam, New York City, and Puerto Rico.
There is some disagreement among the states, though, about the definition of “child.” Most states use an age range of 0-17, 0-18, or 0-19, but Florida and Utah go with a range of 0-14 years while South Carolina and Tennessee consider humans aged 0-20 years to be children. Other data limitations involve Texas, which has reported age distribution for only 8% of all cases, and New York, which is not reporting the age distribution of statewide cases, the AAP/CHA report noted.
The definition of child isn’t the only thing that varies between the states. The cumulative case rate for Tennessee, the highest in the country at 1,315 per 100,000 children, is 10 times that of Vermont, which is the lowest at 131 per 100,000, the AAP and CHA said. Vermont reports child COVID-19 cases using an age range of 0-19 years.
The other states with rates over 1,000 cases per 100,000 children are Arizona (1,300), which had the highest rate a week ago; South Carolina (1,214); Louisiana (1,127); Mississippi (1,120); and Nevada (1,068). Those with rates below 200 cases per 100,000 children are Maine (150), New Hampshire (175), and Hawaii (188), according to this week’s report.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The cumulative number of pediatric cases reported up to that date was 442,785, or 9.3% of the total COVID-19 case load of more than 4.76 million among all ages. There have been only 92 pediatric deaths, however, which works out to just 0.06% of the 154,279 reported for all ages, the AAP and the CHA said Aug. 24 in their most recent update.
Child hospitalizations also were on the low side, representing 1.7% (4,062) of the cumulative total of 234,810 admissions among all ages as of Aug. 20, based on data from 21 states and New York City.
Nationally, the cumulative number of reported child cases is now up to 583 per 100,000 children, and that figure covers 49 states, Washington, D.C., Guam, New York City, and Puerto Rico.
There is some disagreement among the states, though, about the definition of “child.” Most states use an age range of 0-17, 0-18, or 0-19, but Florida and Utah go with a range of 0-14 years while South Carolina and Tennessee consider humans aged 0-20 years to be children. Other data limitations involve Texas, which has reported age distribution for only 8% of all cases, and New York, which is not reporting the age distribution of statewide cases, the AAP/CHA report noted.
The definition of child isn’t the only thing that varies between the states. The cumulative case rate for Tennessee, the highest in the country at 1,315 per 100,000 children, is 10 times that of Vermont, which is the lowest at 131 per 100,000, the AAP and CHA said. Vermont reports child COVID-19 cases using an age range of 0-19 years.
The other states with rates over 1,000 cases per 100,000 children are Arizona (1,300), which had the highest rate a week ago; South Carolina (1,214); Louisiana (1,127); Mississippi (1,120); and Nevada (1,068). Those with rates below 200 cases per 100,000 children are Maine (150), New Hampshire (175), and Hawaii (188), according to this week’s report.
Reflections on life before and during COVID-19
I wrote these poems in mid-March, when fear of COVID-19 struck and New York City locked down. Nearly a half-year later, the impact continues with uncertainty everywhere.
Before and After
Before – there were trees,
I hardly noticed them.
There were buses and newspapers.
Should I read a book or the Post?
Am I wasting time looking
out the window at crowds
milling into Central Park?
The tourists walk to Strawberry Fields,
and the bus turns to Central Park West.
I hardly noticed
because I had plans.
After – it ended, first slowly,
then abruptly. We sat together
in the shop, knitting,
only three of us
before the store shut.
After that –
In the park daffodils radiate gold
and grow in groups.
And the magnolia trees
flaunt their succulent petals.
The fragile cherry blossoms float flowers
Still – it is after
And before, there were trees
I hardly noticed.
War Means Nothing to Them
The birds and the trees know nothing.
They are not embarrassed.
The birds chirp, the trees flower;
War means nothing to them.
Grass grows thick and green,
welcomes the spring.
Babies too, even toddlers,
go about their infant business.
They play or coo or smile
as happy as the birds, the trees,
the grass, flush with life.
Dr. Cohen is in private practice and is a clinical assistant professor of psychiatry at Weill Cornell Medical Center of New York-Presbyterian Hospital, and psychiatric consultant at the Hospital for Special Surgery, also in New York.
I wrote these poems in mid-March, when fear of COVID-19 struck and New York City locked down. Nearly a half-year later, the impact continues with uncertainty everywhere.
Before and After
Before – there were trees,
I hardly noticed them.
There were buses and newspapers.
Should I read a book or the Post?
Am I wasting time looking
out the window at crowds
milling into Central Park?
The tourists walk to Strawberry Fields,
and the bus turns to Central Park West.
I hardly noticed
because I had plans.
After – it ended, first slowly,
then abruptly. We sat together
in the shop, knitting,
only three of us
before the store shut.
After that –
In the park daffodils radiate gold
and grow in groups.
And the magnolia trees
flaunt their succulent petals.
The fragile cherry blossoms float flowers
Still – it is after
And before, there were trees
I hardly noticed.
War Means Nothing to Them
The birds and the trees know nothing.
They are not embarrassed.
The birds chirp, the trees flower;
War means nothing to them.
Grass grows thick and green,
welcomes the spring.
Babies too, even toddlers,
go about their infant business.
They play or coo or smile
as happy as the birds, the trees,
the grass, flush with life.
Dr. Cohen is in private practice and is a clinical assistant professor of psychiatry at Weill Cornell Medical Center of New York-Presbyterian Hospital, and psychiatric consultant at the Hospital for Special Surgery, also in New York.
I wrote these poems in mid-March, when fear of COVID-19 struck and New York City locked down. Nearly a half-year later, the impact continues with uncertainty everywhere.
Before and After
Before – there were trees,
I hardly noticed them.
There were buses and newspapers.
Should I read a book or the Post?
Am I wasting time looking
out the window at crowds
milling into Central Park?
The tourists walk to Strawberry Fields,
and the bus turns to Central Park West.
I hardly noticed
because I had plans.
After – it ended, first slowly,
then abruptly. We sat together
in the shop, knitting,
only three of us
before the store shut.
After that –
In the park daffodils radiate gold
and grow in groups.
And the magnolia trees
flaunt their succulent petals.
The fragile cherry blossoms float flowers
Still – it is after
And before, there were trees
I hardly noticed.
War Means Nothing to Them
The birds and the trees know nothing.
They are not embarrassed.
The birds chirp, the trees flower;
War means nothing to them.
Grass grows thick and green,
welcomes the spring.
Babies too, even toddlers,
go about their infant business.
They play or coo or smile
as happy as the birds, the trees,
the grass, flush with life.
Dr. Cohen is in private practice and is a clinical assistant professor of psychiatry at Weill Cornell Medical Center of New York-Presbyterian Hospital, and psychiatric consultant at the Hospital for Special Surgery, also in New York.
An update on the pharmacologic treatment of hypersomnia
The hypersomnias are an etiologically diverse group of disorders of wakefulness and sleep, characterized principally by excessive daytime sleepiness (EDS), often despite sufficient or even long total sleep durations. Hypersomnolence may be severely disabling and isolating for patients, is associated with decreased quality of life and economic disadvantage, and, in some cases, may pose a personal and public health danger through drowsy driving. Though historically, management of these patients has been principally supportive and aimed at reducing daytime functional impairment, new and evolving treatments are quickly changing management paradigms in this population. This brief review highlights some of the newest pharmacotherapeutic advances in this dynamic field.
Hypersomnolence is a common presenting concern primary care and sleep clinics, with an estimated prevalence of EDS in the general adult population of as high as 6%.1 The initial diagnosis of hypersomnia is, broadly, a clinical one, with careful consideration to the patient’s report of daytime sleepiness and functional impairment, sleep/wake cycle, and any medical comorbidities. The primary hypersomnias include narcolepsy type 1 (narcolepsy with cataplexy, NT1) and narcolepsy type 2 (without cataplexy, NT2), Kleine-Levin Syndrome (KLS), and idiopathic hypersomnia. Secondary hypersomnia disorders are more commonly encountered in clinical practice and include hypersomnia attributable to another medical condition (including psychiatric and neurologic disorders), hypersomnia related to medication effects, and EDS related to behaviorally insufficient sleep. Distinguishing primary and secondary etiologies, when possible, is important as treatment pathways may vary considerably between hypersomnias.
Generally, overnight in-lab polysomnography is warranted to exclude untreated or sub-optimally treated sleep-disordered breathing or movement disorders which may undermine sleep quality. In the absence of any such findings, this is usually followed by daytime multiple sleep latency testing (MSLT). The MSLT is comprised of four to five scheduled daytime naps in the sleep lab and is designed to quantify a patient’s propensity to sleep during the day and to identify architectural sleep abnormalities which indicate narcolepsy. Specifically, narcolepsy is identified by MSLT when a patient exhibits a sleep onset latency of ≤ 8 minutes and at least two sleep-onset REM periods (SOREMPs), or, one SOREMP on MSLT with a second noted on the preceding night’s PSG. Actigraphy or sleep logs may be helpful in quantifying a patient’s total sleep time in their home environment. Adjunctive laboratory tests for narcolepsy including HLA typing and CSF hypocretin testing may sometimes be indicated.
General hypersomnia management usually consists of the use of wakefulness promoting agents, such as stimulants (eg, dexmethylphenidate) and dopamine-modulating agents (eg, modafinil, armodafinil), and adjunctive supportive strategies, including planned daytime naps and elimination of modifiable secondary causes. In those patients with hypersomnolence associated with depression or anxiety, the use of antidepressants, including SSRI, SNRI, and DNRIs, is often effective, and these medications can also improve cataplexy symptoms in narcoleptics. KLS may respond to treatment with lithium, shortening the duration of the striking hypersomnolent episodes characteristic of this rare syndrome, and there is some indication that ketamine may also be a helpful adjunctive in some cases. In treatment-refractory cases of hypersomnolence associated with GABA-A receptor potentiation, drugs such as flumazenil and clarithromycin appear to improve subjective measures of hypersomnolence.2,3 In patients with narcolepsy, sodium oxybate (available as Xyrem and, more recently, as a generic medication) has proven to be clinically very useful, reducing EDS and the frequency and severity of cataplexy and sleep disturbance associated with this condition. In July 2020, the FDA approved a new, low-sodium formulation of sodium oxybate (Xywav) for patients 7 years of age and older with a diagnosis of narcolepsy, a helpful option in those patients with cardiovascular and renal disease.
Despite this broadening armamentarium, in many cases daytime sleepiness and functional impairment is refractory to typical pharmacotherapy. In this context, we would like to highlight two newer pharmacotherapeutic options, solriamfetol and pitolisant.
Solriamfetol
Solriamfetol (Sunosi) is a Schedule IV FDA-approved medication indicated for treatment of EDS in adults with narcolepsy or obstructive sleep apnea. The precise mechanism of action is unknown, but this medication is believed to inhibit both dopamine and norepinerphrine reuptake in the brain, similar to the widely-prescribed NDRI buproprion. In a 12-week RCT study on its effects on narcolepsy in adults, solriamfetol improved important measures of wakefulness and sleepiness, without associated polysomnographic evidence of significant sleep disruption.4 In another 12-week RCT study of solriamfetol in adult patients with EDS related to OSA, there was a dose-dependent improvement in measures of wakefulness.5 Some notable side-effects seen with this medication include anxiety and elevated mood, as well as increases in blood pressure. A subsequent study of this medication found that it was efficacious at maintenance of improvements at 6 months.6 Given the theorized mechanism of action as an NDRI, future observation and studies could provide insights on its effect on depression, as well.
Pitolisant
Histaminergic neurons within the CNS play an important role in the promotion of wakefulness. Pitolisant (Wakix) is an interesting wakefulness-promoting agent for adult patients with narcolepsy. It acts as an inverse agonist and antagonist of histamine H3 receptors, resulting in a reduction of the usual feedback inhibition effected through the H3 receptor, thereby enhancing CNS release of histamine and other neurotransmitters. This medication was approved by the FDA in August 2019 and is currently indicated for adult patients with narcolepsy. The HARMONY I trial comparing pitolisant with both placebo and modafinil in adults with narcolepsy and EDS demonstrated improvement in measures of sleepiness and maintenance of wakefulness over placebo, and noninferiority to modafinil.7 In addition, pitolisant had a favorable side-effect profile compared with modafinil. Subsequent studies have reaffirmed the safety profile of pitolisant, including its minimal abuse potential. In one recent placebo-controlled trial of the use of pitolisant in a population of 268 adults with positive airway pressure (PAP) non-adherence, pitolisant was found to improve measures of EDS and related patient-reported measurements in patients with OSA who were CPAP nonadherent.8 Though generally well-tolerated by patients, in initial clinical trials pitolisant was associated with increased headache, insomnia, and nausea relative to placebo, among other less commonly reported adverse effects. Pitolisant is QT interval-prolonging, so caution must be taken when using this medication in combination other medications which may induce QT interval prolongation, including SSRIs.
Future directions
Greater awareness of the hypersomnias and their management has led to improved outcomes and access to care for these patients, yet these disorders remain burdensome and the treatments imperfect. Looking forward, novel pharmacotherapies that target underlying mechanisms rather than symptom palliation will allow for more precise treatments. Ongoing investigations of hypocretin receptor agonists seek to target one critical central mediator of wakefulness. Recent studies have highlighted the association of dysautonomia with hypersomnia, offering interesting insight into possible future targets to improve the function and quality of life of these patients.9 Similarly, understanding of the interplay between psychiatric disorders and primary and secondary hypersomnias may offer new therapeutic pathways.
As treatment plans targeting hypersomnia become more comprehensive and holistic, with an increased emphasis on self-care, sleep hygiene, and mental health awareness, in addition to mechanism-specific treatments, we hope they will ultimately provide improved symptom and burden relief for our patients.
Dr. Shih Yee-Marie Tan Gipson is a psychiatrist and Dr. Kevin Gipson is a sleep medicine specialist, both with Massachusetts General Hospital, Boston.
References
1 Dauvilliers, et al. Hypersomnia. Dialogues Clin Neurosci. 2005;7(4):347-356.
2 Trotti, et al. Clarithromycin in gamma-aminobutyric acid-related hypersomnolence: A randomized, crossover trial. Ann Neurol. 2015;78(3):454-465. doi: 10.1002/ana.24459.
3 Trotti, et al. Flumazenil for the treatment of refractory hypersomnolence: Clinical experience with 153 patients. J Clin Sleep Med. 2016;12(10):1389-1394. doi: 10.5664/jcsm.6196.
4 Thorpy, et al. A randomized study of solriamfetol for excessive sleepiness in narcolepsy. Ann Neurol. 2019; 85(3):359-370. doi: 10.1002/ana.25423.
5 Schweitzer, et al. Solriamfetol for excessive sleepiness in obstructive sleep apnea (TONES 3): A randomized controlled trial. Am J Respir Crit Care Med. 2019;199(11):1421-1431. doi: 10.1164/rccm.201806-1100OC.
6 Malhotra, et al. Long-term study of the safety and maintenance of efficacy of solriamfetol (JZP-110) in the treatment of excessive sleepiness in participants with narcolepsy or obstructive sleep apnea. Sleep. 2020; 43(2): doi: 10.1093/sleep/zsz220.
7 Dauvilliers, et al. Pitolisant versus placebo or modafinil in patients with narcolepsy: a double-blind, randomised trial. Lancet Neurol. 2013;12(11):1068-1075. doi: 10.1016/S1474-4422(13)70225-4.
8 Dauvilliers, et al. Pitolisant for daytime sleepiness in obstructive sleep apnea patients refusing CPAP: A randomized trial. Am J Respir Crit Care Med. 2020. doi: 10.1164/rccm.201907-1284OC.
9 Miglis, et al. Frequency and severity of autonomic symptoms in idiopathic hypersomnia. J Clin Sleep Med. 2020; 16(5):749-756. doi: 10.5664/jcsm.8344.
The hypersomnias are an etiologically diverse group of disorders of wakefulness and sleep, characterized principally by excessive daytime sleepiness (EDS), often despite sufficient or even long total sleep durations. Hypersomnolence may be severely disabling and isolating for patients, is associated with decreased quality of life and economic disadvantage, and, in some cases, may pose a personal and public health danger through drowsy driving. Though historically, management of these patients has been principally supportive and aimed at reducing daytime functional impairment, new and evolving treatments are quickly changing management paradigms in this population. This brief review highlights some of the newest pharmacotherapeutic advances in this dynamic field.
Hypersomnolence is a common presenting concern primary care and sleep clinics, with an estimated prevalence of EDS in the general adult population of as high as 6%.1 The initial diagnosis of hypersomnia is, broadly, a clinical one, with careful consideration to the patient’s report of daytime sleepiness and functional impairment, sleep/wake cycle, and any medical comorbidities. The primary hypersomnias include narcolepsy type 1 (narcolepsy with cataplexy, NT1) and narcolepsy type 2 (without cataplexy, NT2), Kleine-Levin Syndrome (KLS), and idiopathic hypersomnia. Secondary hypersomnia disorders are more commonly encountered in clinical practice and include hypersomnia attributable to another medical condition (including psychiatric and neurologic disorders), hypersomnia related to medication effects, and EDS related to behaviorally insufficient sleep. Distinguishing primary and secondary etiologies, when possible, is important as treatment pathways may vary considerably between hypersomnias.
Generally, overnight in-lab polysomnography is warranted to exclude untreated or sub-optimally treated sleep-disordered breathing or movement disorders which may undermine sleep quality. In the absence of any such findings, this is usually followed by daytime multiple sleep latency testing (MSLT). The MSLT is comprised of four to five scheduled daytime naps in the sleep lab and is designed to quantify a patient’s propensity to sleep during the day and to identify architectural sleep abnormalities which indicate narcolepsy. Specifically, narcolepsy is identified by MSLT when a patient exhibits a sleep onset latency of ≤ 8 minutes and at least two sleep-onset REM periods (SOREMPs), or, one SOREMP on MSLT with a second noted on the preceding night’s PSG. Actigraphy or sleep logs may be helpful in quantifying a patient’s total sleep time in their home environment. Adjunctive laboratory tests for narcolepsy including HLA typing and CSF hypocretin testing may sometimes be indicated.
General hypersomnia management usually consists of the use of wakefulness promoting agents, such as stimulants (eg, dexmethylphenidate) and dopamine-modulating agents (eg, modafinil, armodafinil), and adjunctive supportive strategies, including planned daytime naps and elimination of modifiable secondary causes. In those patients with hypersomnolence associated with depression or anxiety, the use of antidepressants, including SSRI, SNRI, and DNRIs, is often effective, and these medications can also improve cataplexy symptoms in narcoleptics. KLS may respond to treatment with lithium, shortening the duration of the striking hypersomnolent episodes characteristic of this rare syndrome, and there is some indication that ketamine may also be a helpful adjunctive in some cases. In treatment-refractory cases of hypersomnolence associated with GABA-A receptor potentiation, drugs such as flumazenil and clarithromycin appear to improve subjective measures of hypersomnolence.2,3 In patients with narcolepsy, sodium oxybate (available as Xyrem and, more recently, as a generic medication) has proven to be clinically very useful, reducing EDS and the frequency and severity of cataplexy and sleep disturbance associated with this condition. In July 2020, the FDA approved a new, low-sodium formulation of sodium oxybate (Xywav) for patients 7 years of age and older with a diagnosis of narcolepsy, a helpful option in those patients with cardiovascular and renal disease.
Despite this broadening armamentarium, in many cases daytime sleepiness and functional impairment is refractory to typical pharmacotherapy. In this context, we would like to highlight two newer pharmacotherapeutic options, solriamfetol and pitolisant.
Solriamfetol
Solriamfetol (Sunosi) is a Schedule IV FDA-approved medication indicated for treatment of EDS in adults with narcolepsy or obstructive sleep apnea. The precise mechanism of action is unknown, but this medication is believed to inhibit both dopamine and norepinerphrine reuptake in the brain, similar to the widely-prescribed NDRI buproprion. In a 12-week RCT study on its effects on narcolepsy in adults, solriamfetol improved important measures of wakefulness and sleepiness, without associated polysomnographic evidence of significant sleep disruption.4 In another 12-week RCT study of solriamfetol in adult patients with EDS related to OSA, there was a dose-dependent improvement in measures of wakefulness.5 Some notable side-effects seen with this medication include anxiety and elevated mood, as well as increases in blood pressure. A subsequent study of this medication found that it was efficacious at maintenance of improvements at 6 months.6 Given the theorized mechanism of action as an NDRI, future observation and studies could provide insights on its effect on depression, as well.
Pitolisant
Histaminergic neurons within the CNS play an important role in the promotion of wakefulness. Pitolisant (Wakix) is an interesting wakefulness-promoting agent for adult patients with narcolepsy. It acts as an inverse agonist and antagonist of histamine H3 receptors, resulting in a reduction of the usual feedback inhibition effected through the H3 receptor, thereby enhancing CNS release of histamine and other neurotransmitters. This medication was approved by the FDA in August 2019 and is currently indicated for adult patients with narcolepsy. The HARMONY I trial comparing pitolisant with both placebo and modafinil in adults with narcolepsy and EDS demonstrated improvement in measures of sleepiness and maintenance of wakefulness over placebo, and noninferiority to modafinil.7 In addition, pitolisant had a favorable side-effect profile compared with modafinil. Subsequent studies have reaffirmed the safety profile of pitolisant, including its minimal abuse potential. In one recent placebo-controlled trial of the use of pitolisant in a population of 268 adults with positive airway pressure (PAP) non-adherence, pitolisant was found to improve measures of EDS and related patient-reported measurements in patients with OSA who were CPAP nonadherent.8 Though generally well-tolerated by patients, in initial clinical trials pitolisant was associated with increased headache, insomnia, and nausea relative to placebo, among other less commonly reported adverse effects. Pitolisant is QT interval-prolonging, so caution must be taken when using this medication in combination other medications which may induce QT interval prolongation, including SSRIs.
Future directions
Greater awareness of the hypersomnias and their management has led to improved outcomes and access to care for these patients, yet these disorders remain burdensome and the treatments imperfect. Looking forward, novel pharmacotherapies that target underlying mechanisms rather than symptom palliation will allow for more precise treatments. Ongoing investigations of hypocretin receptor agonists seek to target one critical central mediator of wakefulness. Recent studies have highlighted the association of dysautonomia with hypersomnia, offering interesting insight into possible future targets to improve the function and quality of life of these patients.9 Similarly, understanding of the interplay between psychiatric disorders and primary and secondary hypersomnias may offer new therapeutic pathways.
As treatment plans targeting hypersomnia become more comprehensive and holistic, with an increased emphasis on self-care, sleep hygiene, and mental health awareness, in addition to mechanism-specific treatments, we hope they will ultimately provide improved symptom and burden relief for our patients.
Dr. Shih Yee-Marie Tan Gipson is a psychiatrist and Dr. Kevin Gipson is a sleep medicine specialist, both with Massachusetts General Hospital, Boston.
References
1 Dauvilliers, et al. Hypersomnia. Dialogues Clin Neurosci. 2005;7(4):347-356.
2 Trotti, et al. Clarithromycin in gamma-aminobutyric acid-related hypersomnolence: A randomized, crossover trial. Ann Neurol. 2015;78(3):454-465. doi: 10.1002/ana.24459.
3 Trotti, et al. Flumazenil for the treatment of refractory hypersomnolence: Clinical experience with 153 patients. J Clin Sleep Med. 2016;12(10):1389-1394. doi: 10.5664/jcsm.6196.
4 Thorpy, et al. A randomized study of solriamfetol for excessive sleepiness in narcolepsy. Ann Neurol. 2019; 85(3):359-370. doi: 10.1002/ana.25423.
5 Schweitzer, et al. Solriamfetol for excessive sleepiness in obstructive sleep apnea (TONES 3): A randomized controlled trial. Am J Respir Crit Care Med. 2019;199(11):1421-1431. doi: 10.1164/rccm.201806-1100OC.
6 Malhotra, et al. Long-term study of the safety and maintenance of efficacy of solriamfetol (JZP-110) in the treatment of excessive sleepiness in participants with narcolepsy or obstructive sleep apnea. Sleep. 2020; 43(2): doi: 10.1093/sleep/zsz220.
7 Dauvilliers, et al. Pitolisant versus placebo or modafinil in patients with narcolepsy: a double-blind, randomised trial. Lancet Neurol. 2013;12(11):1068-1075. doi: 10.1016/S1474-4422(13)70225-4.
8 Dauvilliers, et al. Pitolisant for daytime sleepiness in obstructive sleep apnea patients refusing CPAP: A randomized trial. Am J Respir Crit Care Med. 2020. doi: 10.1164/rccm.201907-1284OC.
9 Miglis, et al. Frequency and severity of autonomic symptoms in idiopathic hypersomnia. J Clin Sleep Med. 2020; 16(5):749-756. doi: 10.5664/jcsm.8344.
The hypersomnias are an etiologically diverse group of disorders of wakefulness and sleep, characterized principally by excessive daytime sleepiness (EDS), often despite sufficient or even long total sleep durations. Hypersomnolence may be severely disabling and isolating for patients, is associated with decreased quality of life and economic disadvantage, and, in some cases, may pose a personal and public health danger through drowsy driving. Though historically, management of these patients has been principally supportive and aimed at reducing daytime functional impairment, new and evolving treatments are quickly changing management paradigms in this population. This brief review highlights some of the newest pharmacotherapeutic advances in this dynamic field.
Hypersomnolence is a common presenting concern primary care and sleep clinics, with an estimated prevalence of EDS in the general adult population of as high as 6%.1 The initial diagnosis of hypersomnia is, broadly, a clinical one, with careful consideration to the patient’s report of daytime sleepiness and functional impairment, sleep/wake cycle, and any medical comorbidities. The primary hypersomnias include narcolepsy type 1 (narcolepsy with cataplexy, NT1) and narcolepsy type 2 (without cataplexy, NT2), Kleine-Levin Syndrome (KLS), and idiopathic hypersomnia. Secondary hypersomnia disorders are more commonly encountered in clinical practice and include hypersomnia attributable to another medical condition (including psychiatric and neurologic disorders), hypersomnia related to medication effects, and EDS related to behaviorally insufficient sleep. Distinguishing primary and secondary etiologies, when possible, is important as treatment pathways may vary considerably between hypersomnias.
Generally, overnight in-lab polysomnography is warranted to exclude untreated or sub-optimally treated sleep-disordered breathing or movement disorders which may undermine sleep quality. In the absence of any such findings, this is usually followed by daytime multiple sleep latency testing (MSLT). The MSLT is comprised of four to five scheduled daytime naps in the sleep lab and is designed to quantify a patient’s propensity to sleep during the day and to identify architectural sleep abnormalities which indicate narcolepsy. Specifically, narcolepsy is identified by MSLT when a patient exhibits a sleep onset latency of ≤ 8 minutes and at least two sleep-onset REM periods (SOREMPs), or, one SOREMP on MSLT with a second noted on the preceding night’s PSG. Actigraphy or sleep logs may be helpful in quantifying a patient’s total sleep time in their home environment. Adjunctive laboratory tests for narcolepsy including HLA typing and CSF hypocretin testing may sometimes be indicated.
General hypersomnia management usually consists of the use of wakefulness promoting agents, such as stimulants (eg, dexmethylphenidate) and dopamine-modulating agents (eg, modafinil, armodafinil), and adjunctive supportive strategies, including planned daytime naps and elimination of modifiable secondary causes. In those patients with hypersomnolence associated with depression or anxiety, the use of antidepressants, including SSRI, SNRI, and DNRIs, is often effective, and these medications can also improve cataplexy symptoms in narcoleptics. KLS may respond to treatment with lithium, shortening the duration of the striking hypersomnolent episodes characteristic of this rare syndrome, and there is some indication that ketamine may also be a helpful adjunctive in some cases. In treatment-refractory cases of hypersomnolence associated with GABA-A receptor potentiation, drugs such as flumazenil and clarithromycin appear to improve subjective measures of hypersomnolence.2,3 In patients with narcolepsy, sodium oxybate (available as Xyrem and, more recently, as a generic medication) has proven to be clinically very useful, reducing EDS and the frequency and severity of cataplexy and sleep disturbance associated with this condition. In July 2020, the FDA approved a new, low-sodium formulation of sodium oxybate (Xywav) for patients 7 years of age and older with a diagnosis of narcolepsy, a helpful option in those patients with cardiovascular and renal disease.
Despite this broadening armamentarium, in many cases daytime sleepiness and functional impairment is refractory to typical pharmacotherapy. In this context, we would like to highlight two newer pharmacotherapeutic options, solriamfetol and pitolisant.
Solriamfetol
Solriamfetol (Sunosi) is a Schedule IV FDA-approved medication indicated for treatment of EDS in adults with narcolepsy or obstructive sleep apnea. The precise mechanism of action is unknown, but this medication is believed to inhibit both dopamine and norepinerphrine reuptake in the brain, similar to the widely-prescribed NDRI buproprion. In a 12-week RCT study on its effects on narcolepsy in adults, solriamfetol improved important measures of wakefulness and sleepiness, without associated polysomnographic evidence of significant sleep disruption.4 In another 12-week RCT study of solriamfetol in adult patients with EDS related to OSA, there was a dose-dependent improvement in measures of wakefulness.5 Some notable side-effects seen with this medication include anxiety and elevated mood, as well as increases in blood pressure. A subsequent study of this medication found that it was efficacious at maintenance of improvements at 6 months.6 Given the theorized mechanism of action as an NDRI, future observation and studies could provide insights on its effect on depression, as well.
Pitolisant
Histaminergic neurons within the CNS play an important role in the promotion of wakefulness. Pitolisant (Wakix) is an interesting wakefulness-promoting agent for adult patients with narcolepsy. It acts as an inverse agonist and antagonist of histamine H3 receptors, resulting in a reduction of the usual feedback inhibition effected through the H3 receptor, thereby enhancing CNS release of histamine and other neurotransmitters. This medication was approved by the FDA in August 2019 and is currently indicated for adult patients with narcolepsy. The HARMONY I trial comparing pitolisant with both placebo and modafinil in adults with narcolepsy and EDS demonstrated improvement in measures of sleepiness and maintenance of wakefulness over placebo, and noninferiority to modafinil.7 In addition, pitolisant had a favorable side-effect profile compared with modafinil. Subsequent studies have reaffirmed the safety profile of pitolisant, including its minimal abuse potential. In one recent placebo-controlled trial of the use of pitolisant in a population of 268 adults with positive airway pressure (PAP) non-adherence, pitolisant was found to improve measures of EDS and related patient-reported measurements in patients with OSA who were CPAP nonadherent.8 Though generally well-tolerated by patients, in initial clinical trials pitolisant was associated with increased headache, insomnia, and nausea relative to placebo, among other less commonly reported adverse effects. Pitolisant is QT interval-prolonging, so caution must be taken when using this medication in combination other medications which may induce QT interval prolongation, including SSRIs.
Future directions
Greater awareness of the hypersomnias and their management has led to improved outcomes and access to care for these patients, yet these disorders remain burdensome and the treatments imperfect. Looking forward, novel pharmacotherapies that target underlying mechanisms rather than symptom palliation will allow for more precise treatments. Ongoing investigations of hypocretin receptor agonists seek to target one critical central mediator of wakefulness. Recent studies have highlighted the association of dysautonomia with hypersomnia, offering interesting insight into possible future targets to improve the function and quality of life of these patients.9 Similarly, understanding of the interplay between psychiatric disorders and primary and secondary hypersomnias may offer new therapeutic pathways.
As treatment plans targeting hypersomnia become more comprehensive and holistic, with an increased emphasis on self-care, sleep hygiene, and mental health awareness, in addition to mechanism-specific treatments, we hope they will ultimately provide improved symptom and burden relief for our patients.
Dr. Shih Yee-Marie Tan Gipson is a psychiatrist and Dr. Kevin Gipson is a sleep medicine specialist, both with Massachusetts General Hospital, Boston.
References
1 Dauvilliers, et al. Hypersomnia. Dialogues Clin Neurosci. 2005;7(4):347-356.
2 Trotti, et al. Clarithromycin in gamma-aminobutyric acid-related hypersomnolence: A randomized, crossover trial. Ann Neurol. 2015;78(3):454-465. doi: 10.1002/ana.24459.
3 Trotti, et al. Flumazenil for the treatment of refractory hypersomnolence: Clinical experience with 153 patients. J Clin Sleep Med. 2016;12(10):1389-1394. doi: 10.5664/jcsm.6196.
4 Thorpy, et al. A randomized study of solriamfetol for excessive sleepiness in narcolepsy. Ann Neurol. 2019; 85(3):359-370. doi: 10.1002/ana.25423.
5 Schweitzer, et al. Solriamfetol for excessive sleepiness in obstructive sleep apnea (TONES 3): A randomized controlled trial. Am J Respir Crit Care Med. 2019;199(11):1421-1431. doi: 10.1164/rccm.201806-1100OC.
6 Malhotra, et al. Long-term study of the safety and maintenance of efficacy of solriamfetol (JZP-110) in the treatment of excessive sleepiness in participants with narcolepsy or obstructive sleep apnea. Sleep. 2020; 43(2): doi: 10.1093/sleep/zsz220.
7 Dauvilliers, et al. Pitolisant versus placebo or modafinil in patients with narcolepsy: a double-blind, randomised trial. Lancet Neurol. 2013;12(11):1068-1075. doi: 10.1016/S1474-4422(13)70225-4.
8 Dauvilliers, et al. Pitolisant for daytime sleepiness in obstructive sleep apnea patients refusing CPAP: A randomized trial. Am J Respir Crit Care Med. 2020. doi: 10.1164/rccm.201907-1284OC.
9 Miglis, et al. Frequency and severity of autonomic symptoms in idiopathic hypersomnia. J Clin Sleep Med. 2020; 16(5):749-756. doi: 10.5664/jcsm.8344.
Coronavirus-associated aspergillosis increased 30-day mortality risk
Researchers are beginning to make some headway in identifying the role of secondary infections in the course and outcomes of COVID-19.
Patients who are on ventilatory support for severe COVID-19 infections appear to be at high risk for invasive pulmonary aspergillosis, which in a small prospective study was associated with a more than threefold risk for 30-day mortality. The findings were published online in Clinical Infectious Diseases.
Among 108 patients with COVID-19 on mechanical ventilation in one of three intensive care units, 30 (27.7%) were diagnosed with coronavirus-associated pulmonary aspergillosis (CAPA) based on consensus definitions similar to those used to diagnose influenza-associated pulmonary aspergillosis (IAPA).
Of the patients with CAPA, 44% died within 30 days of ICU admission, compared with 19% of patients who did not meet the criteria for aspergillosis (P = .002). This difference translated into an odds ratio (OR) for death with CAPA of 3.55 (P = .014), reported Michele Bartoletti, MD, PhD, of the infectious diseases unit at Sant’Orsola Malpighi Hospital in Bologna, Italy, and colleagues.
When the investigators applied a proposed definition of putative invasive pulmonary aspergillosis, or “PIPA” to the same patients, the 30-day mortality rate jumped to 74% vs. 26% for patients without PIPA (P < .001), with an OR of 11.60 (P < .001). “We found a high incidence of CAPA among critically ill COVID-19 patients and that its occurrence seems to change the natural history of disease,” they wrote.
“[T]he study from Bartoletti et al. alerts the clinical audience to be aware of CAPA and take appropriate (and where needed repetitive) actions that fits their clinical setting,” Roger J. Brüggemann, PharmD, of the department of pharmacy, Radboud University Medical Center, Nijmegen, the Netherlands, and colleagues wrote in an editorial accompanying the study.
Diagnosis challenging
At the best of times, the diagnosis of pulmonary aspergillosis is difficult, subject to both false-positive and false-negative results, said a critical care specialist who was not involved in the study.
“Critically ill patients are susceptible to having aspergillus, so in reading the article, my only concerns are that I don’t know how accurate the testing is, and I don’t know if their population is truly different from a general population of patients in the ICU,” Daniel R. Ouellette, MD, FCCP, associate director of medical critical care at Henry Ford Hospital in Detroit, said in an interview.
As seen in ICU patients with severe influenza or other viral infections, patients with severe COVID-19 disease are susceptible to secondary infections, he said, making it difficult to know whether the worse outcomes seen in patients with COVID-19 and presumed aspergillosis are a reflection of their being more critically ill or whether the secondary infections themselves account for the difference in mortality.
Three ICUs
Dr. Bartoletti and colleagues conducted a study on all adult patients with microbiologically confirmed COVID-19 receiving mechanical ventilation in three ICUs in Bologna.
All patients included in the study were screened for invasive pulmonary aspergillosis with bronchoalveolar lavage and galactomannan detection and cultures. The lavage was performed on ICU admission, one day from the first day of mechanical ventilation, and if patients had evidence of clinical disease progression.
Samples that tested positive for galactomannan, a component of the aspergillus cell wall, were stored and later analyzed with a commercial quantitative real-time polymerase chain reaction assay for aspergillus; these results were not reported to clinicians on the patient floors.
The investigators defined invasive pulmonary aspergillosis according to a recently proposed definition for CAPA. This definition applies to COVID-19–positive patients admitted to an ICU with pulmonary infiltrates and at least one of the following:
- A serum galactomannan > 0.5.
- Bronchoalveolar lavage galactomannan > 1.0.
- Positive aspergillus bronchoalveolar lavage culture or cavitating infiltrate not attributed to another cause in the area of the pulmonary infiltrate.
They compared the CAPA diagnostic criteria with those of PIPA criteria as described by Stijn J. Blot, PhD, and colleagues in study published in the American Journal of Respiratory and Critical Care Medicine (2012 Jul 1;186(1):56-64).
A total of 108 patients were screened for aspergillosis, with a median age of 64. The majority of patients (78%) were male. The median age-adjusted Charlson Comorbidity Index was 2.5 (range 1-4). The median Sequential Organ Failure Assessment (SOFA) score at ICU admission was 4 (range 3-5).
As noted, probable aspergillosis by CAPA criteria was diagnosed in 30 patients (27.7%), with the diagnosis made after a median of 4 days after intubation and a median of 14 days from onset of COVID-19 symptoms.
The incidence rate of probable CAPA was 38.83 per 10,000 ICU patient days.
A comparison of clinical characteristics of patients with and without probable CAPA showed that only chronic steroid therapy at ≥ 16 mg/day prednisone for at least 15 days was significantly associated with risk for CAPA (P = .02).
At a median follow-up of 31 days, 54 patients (50%) had been discharged, 44 (41%) had died, and the remaining patients were still on follow-up.
As noted before, the mortality rate with 30 days of ICU admission was 44% for patients with probable CAPA vs. 19% for patients without. Among patients deemed to have PIPA, 74% died within 30 days of admission, compared with 26% without PIPA.
In a logistic regression model, the association of CAPA with increased risk for 30-day mortality remained even after adjustment for the need for renal replacement therapy (OR 3.02, P = .015) and SOFA score at ICU admission (OR 1.38, P = .004).
In a logistic regression using the PIPA rather than CAPA definition, the OR for 30-day mortality was 11.60 (P = .001).
Prognostic marker
The investigators noted that bronchoalveolar lavage galactomannan index appeared to be predictive of death. Each 1-point increase in the index was associated with 1.41-fold increase in the risk for 30-day mortality (P = .0070), a relationship that held up after adjustment for age, need for renal replacement therapy, and SOFA score.
Sixteen patients who met the CAPA definition received antifungal therapy, primarily voriconazole. The use of voriconazole was associated with a nonsignificant trend toward lower mortality.
They noted that the heavy use of immunomodulating agents in the patients in their study may have contributed to the high prevalence of CAPA.
Dr. Ouellette agreed that many of the therapies used to treat COVID-19 in the ICU are experimental, and that agents used to suppress the cytokine storm that is believed to contribute to disease severity may increase risk for secondary infections such as invasive aspergillosis.
“Many of our treatments may be associated with adverse consequences,” he said. “There is a trend toward treating patients with COVID-19 pneumonia with corticosteroids, and certainly that could have an immunosuppressant effect and predispose patients to secondary infections.”
He noted that the World Health Organization recommendations current in March 2020, when the pandemic began in earnest in the United States, advised against the use of corticosteroids, likely because of a lack of evidence of efficacy and concerns about risk for secondary infections.
“Regardless of the strategic choice made, all efforts should be put into improving our ability to reliably identify patients that may benefit from therapeutic interventions, which include host and risk factors, clinical factors and CAPA disease markers,” Dr. Brüggemann and colleagues wrote in their editorial.
The study was performed without external funding. The authors and Dr. Ouellette reported no conflicts of interest. Dr. Brüggemann and coauthors report grants and/or personal fees from various companies outside the submitted work.
SOURCE: Bartoletti M et al. Clin Infect Dis. 2020 Jul 28. doi: 10.1093/cid/ciaa1065.
Researchers are beginning to make some headway in identifying the role of secondary infections in the course and outcomes of COVID-19.
Patients who are on ventilatory support for severe COVID-19 infections appear to be at high risk for invasive pulmonary aspergillosis, which in a small prospective study was associated with a more than threefold risk for 30-day mortality. The findings were published online in Clinical Infectious Diseases.
Among 108 patients with COVID-19 on mechanical ventilation in one of three intensive care units, 30 (27.7%) were diagnosed with coronavirus-associated pulmonary aspergillosis (CAPA) based on consensus definitions similar to those used to diagnose influenza-associated pulmonary aspergillosis (IAPA).
Of the patients with CAPA, 44% died within 30 days of ICU admission, compared with 19% of patients who did not meet the criteria for aspergillosis (P = .002). This difference translated into an odds ratio (OR) for death with CAPA of 3.55 (P = .014), reported Michele Bartoletti, MD, PhD, of the infectious diseases unit at Sant’Orsola Malpighi Hospital in Bologna, Italy, and colleagues.
When the investigators applied a proposed definition of putative invasive pulmonary aspergillosis, or “PIPA” to the same patients, the 30-day mortality rate jumped to 74% vs. 26% for patients without PIPA (P < .001), with an OR of 11.60 (P < .001). “We found a high incidence of CAPA among critically ill COVID-19 patients and that its occurrence seems to change the natural history of disease,” they wrote.
“[T]he study from Bartoletti et al. alerts the clinical audience to be aware of CAPA and take appropriate (and where needed repetitive) actions that fits their clinical setting,” Roger J. Brüggemann, PharmD, of the department of pharmacy, Radboud University Medical Center, Nijmegen, the Netherlands, and colleagues wrote in an editorial accompanying the study.
Diagnosis challenging
At the best of times, the diagnosis of pulmonary aspergillosis is difficult, subject to both false-positive and false-negative results, said a critical care specialist who was not involved in the study.
“Critically ill patients are susceptible to having aspergillus, so in reading the article, my only concerns are that I don’t know how accurate the testing is, and I don’t know if their population is truly different from a general population of patients in the ICU,” Daniel R. Ouellette, MD, FCCP, associate director of medical critical care at Henry Ford Hospital in Detroit, said in an interview.
As seen in ICU patients with severe influenza or other viral infections, patients with severe COVID-19 disease are susceptible to secondary infections, he said, making it difficult to know whether the worse outcomes seen in patients with COVID-19 and presumed aspergillosis are a reflection of their being more critically ill or whether the secondary infections themselves account for the difference in mortality.
Three ICUs
Dr. Bartoletti and colleagues conducted a study on all adult patients with microbiologically confirmed COVID-19 receiving mechanical ventilation in three ICUs in Bologna.
All patients included in the study were screened for invasive pulmonary aspergillosis with bronchoalveolar lavage and galactomannan detection and cultures. The lavage was performed on ICU admission, one day from the first day of mechanical ventilation, and if patients had evidence of clinical disease progression.
Samples that tested positive for galactomannan, a component of the aspergillus cell wall, were stored and later analyzed with a commercial quantitative real-time polymerase chain reaction assay for aspergillus; these results were not reported to clinicians on the patient floors.
The investigators defined invasive pulmonary aspergillosis according to a recently proposed definition for CAPA. This definition applies to COVID-19–positive patients admitted to an ICU with pulmonary infiltrates and at least one of the following:
- A serum galactomannan > 0.5.
- Bronchoalveolar lavage galactomannan > 1.0.
- Positive aspergillus bronchoalveolar lavage culture or cavitating infiltrate not attributed to another cause in the area of the pulmonary infiltrate.
They compared the CAPA diagnostic criteria with those of PIPA criteria as described by Stijn J. Blot, PhD, and colleagues in study published in the American Journal of Respiratory and Critical Care Medicine (2012 Jul 1;186(1):56-64).
A total of 108 patients were screened for aspergillosis, with a median age of 64. The majority of patients (78%) were male. The median age-adjusted Charlson Comorbidity Index was 2.5 (range 1-4). The median Sequential Organ Failure Assessment (SOFA) score at ICU admission was 4 (range 3-5).
As noted, probable aspergillosis by CAPA criteria was diagnosed in 30 patients (27.7%), with the diagnosis made after a median of 4 days after intubation and a median of 14 days from onset of COVID-19 symptoms.
The incidence rate of probable CAPA was 38.83 per 10,000 ICU patient days.
A comparison of clinical characteristics of patients with and without probable CAPA showed that only chronic steroid therapy at ≥ 16 mg/day prednisone for at least 15 days was significantly associated with risk for CAPA (P = .02).
At a median follow-up of 31 days, 54 patients (50%) had been discharged, 44 (41%) had died, and the remaining patients were still on follow-up.
As noted before, the mortality rate with 30 days of ICU admission was 44% for patients with probable CAPA vs. 19% for patients without. Among patients deemed to have PIPA, 74% died within 30 days of admission, compared with 26% without PIPA.
In a logistic regression model, the association of CAPA with increased risk for 30-day mortality remained even after adjustment for the need for renal replacement therapy (OR 3.02, P = .015) and SOFA score at ICU admission (OR 1.38, P = .004).
In a logistic regression using the PIPA rather than CAPA definition, the OR for 30-day mortality was 11.60 (P = .001).
Prognostic marker
The investigators noted that bronchoalveolar lavage galactomannan index appeared to be predictive of death. Each 1-point increase in the index was associated with 1.41-fold increase in the risk for 30-day mortality (P = .0070), a relationship that held up after adjustment for age, need for renal replacement therapy, and SOFA score.
Sixteen patients who met the CAPA definition received antifungal therapy, primarily voriconazole. The use of voriconazole was associated with a nonsignificant trend toward lower mortality.
They noted that the heavy use of immunomodulating agents in the patients in their study may have contributed to the high prevalence of CAPA.
Dr. Ouellette agreed that many of the therapies used to treat COVID-19 in the ICU are experimental, and that agents used to suppress the cytokine storm that is believed to contribute to disease severity may increase risk for secondary infections such as invasive aspergillosis.
“Many of our treatments may be associated with adverse consequences,” he said. “There is a trend toward treating patients with COVID-19 pneumonia with corticosteroids, and certainly that could have an immunosuppressant effect and predispose patients to secondary infections.”
He noted that the World Health Organization recommendations current in March 2020, when the pandemic began in earnest in the United States, advised against the use of corticosteroids, likely because of a lack of evidence of efficacy and concerns about risk for secondary infections.
“Regardless of the strategic choice made, all efforts should be put into improving our ability to reliably identify patients that may benefit from therapeutic interventions, which include host and risk factors, clinical factors and CAPA disease markers,” Dr. Brüggemann and colleagues wrote in their editorial.
The study was performed without external funding. The authors and Dr. Ouellette reported no conflicts of interest. Dr. Brüggemann and coauthors report grants and/or personal fees from various companies outside the submitted work.
SOURCE: Bartoletti M et al. Clin Infect Dis. 2020 Jul 28. doi: 10.1093/cid/ciaa1065.
Researchers are beginning to make some headway in identifying the role of secondary infections in the course and outcomes of COVID-19.
Patients who are on ventilatory support for severe COVID-19 infections appear to be at high risk for invasive pulmonary aspergillosis, which in a small prospective study was associated with a more than threefold risk for 30-day mortality. The findings were published online in Clinical Infectious Diseases.
Among 108 patients with COVID-19 on mechanical ventilation in one of three intensive care units, 30 (27.7%) were diagnosed with coronavirus-associated pulmonary aspergillosis (CAPA) based on consensus definitions similar to those used to diagnose influenza-associated pulmonary aspergillosis (IAPA).
Of the patients with CAPA, 44% died within 30 days of ICU admission, compared with 19% of patients who did not meet the criteria for aspergillosis (P = .002). This difference translated into an odds ratio (OR) for death with CAPA of 3.55 (P = .014), reported Michele Bartoletti, MD, PhD, of the infectious diseases unit at Sant’Orsola Malpighi Hospital in Bologna, Italy, and colleagues.
When the investigators applied a proposed definition of putative invasive pulmonary aspergillosis, or “PIPA” to the same patients, the 30-day mortality rate jumped to 74% vs. 26% for patients without PIPA (P < .001), with an OR of 11.60 (P < .001). “We found a high incidence of CAPA among critically ill COVID-19 patients and that its occurrence seems to change the natural history of disease,” they wrote.
“[T]he study from Bartoletti et al. alerts the clinical audience to be aware of CAPA and take appropriate (and where needed repetitive) actions that fits their clinical setting,” Roger J. Brüggemann, PharmD, of the department of pharmacy, Radboud University Medical Center, Nijmegen, the Netherlands, and colleagues wrote in an editorial accompanying the study.
Diagnosis challenging
At the best of times, the diagnosis of pulmonary aspergillosis is difficult, subject to both false-positive and false-negative results, said a critical care specialist who was not involved in the study.
“Critically ill patients are susceptible to having aspergillus, so in reading the article, my only concerns are that I don’t know how accurate the testing is, and I don’t know if their population is truly different from a general population of patients in the ICU,” Daniel R. Ouellette, MD, FCCP, associate director of medical critical care at Henry Ford Hospital in Detroit, said in an interview.
As seen in ICU patients with severe influenza or other viral infections, patients with severe COVID-19 disease are susceptible to secondary infections, he said, making it difficult to know whether the worse outcomes seen in patients with COVID-19 and presumed aspergillosis are a reflection of their being more critically ill or whether the secondary infections themselves account for the difference in mortality.
Three ICUs
Dr. Bartoletti and colleagues conducted a study on all adult patients with microbiologically confirmed COVID-19 receiving mechanical ventilation in three ICUs in Bologna.
All patients included in the study were screened for invasive pulmonary aspergillosis with bronchoalveolar lavage and galactomannan detection and cultures. The lavage was performed on ICU admission, one day from the first day of mechanical ventilation, and if patients had evidence of clinical disease progression.
Samples that tested positive for galactomannan, a component of the aspergillus cell wall, were stored and later analyzed with a commercial quantitative real-time polymerase chain reaction assay for aspergillus; these results were not reported to clinicians on the patient floors.
The investigators defined invasive pulmonary aspergillosis according to a recently proposed definition for CAPA. This definition applies to COVID-19–positive patients admitted to an ICU with pulmonary infiltrates and at least one of the following:
- A serum galactomannan > 0.5.
- Bronchoalveolar lavage galactomannan > 1.0.
- Positive aspergillus bronchoalveolar lavage culture or cavitating infiltrate not attributed to another cause in the area of the pulmonary infiltrate.
They compared the CAPA diagnostic criteria with those of PIPA criteria as described by Stijn J. Blot, PhD, and colleagues in study published in the American Journal of Respiratory and Critical Care Medicine (2012 Jul 1;186(1):56-64).
A total of 108 patients were screened for aspergillosis, with a median age of 64. The majority of patients (78%) were male. The median age-adjusted Charlson Comorbidity Index was 2.5 (range 1-4). The median Sequential Organ Failure Assessment (SOFA) score at ICU admission was 4 (range 3-5).
As noted, probable aspergillosis by CAPA criteria was diagnosed in 30 patients (27.7%), with the diagnosis made after a median of 4 days after intubation and a median of 14 days from onset of COVID-19 symptoms.
The incidence rate of probable CAPA was 38.83 per 10,000 ICU patient days.
A comparison of clinical characteristics of patients with and without probable CAPA showed that only chronic steroid therapy at ≥ 16 mg/day prednisone for at least 15 days was significantly associated with risk for CAPA (P = .02).
At a median follow-up of 31 days, 54 patients (50%) had been discharged, 44 (41%) had died, and the remaining patients were still on follow-up.
As noted before, the mortality rate with 30 days of ICU admission was 44% for patients with probable CAPA vs. 19% for patients without. Among patients deemed to have PIPA, 74% died within 30 days of admission, compared with 26% without PIPA.
In a logistic regression model, the association of CAPA with increased risk for 30-day mortality remained even after adjustment for the need for renal replacement therapy (OR 3.02, P = .015) and SOFA score at ICU admission (OR 1.38, P = .004).
In a logistic regression using the PIPA rather than CAPA definition, the OR for 30-day mortality was 11.60 (P = .001).
Prognostic marker
The investigators noted that bronchoalveolar lavage galactomannan index appeared to be predictive of death. Each 1-point increase in the index was associated with 1.41-fold increase in the risk for 30-day mortality (P = .0070), a relationship that held up after adjustment for age, need for renal replacement therapy, and SOFA score.
Sixteen patients who met the CAPA definition received antifungal therapy, primarily voriconazole. The use of voriconazole was associated with a nonsignificant trend toward lower mortality.
They noted that the heavy use of immunomodulating agents in the patients in their study may have contributed to the high prevalence of CAPA.
Dr. Ouellette agreed that many of the therapies used to treat COVID-19 in the ICU are experimental, and that agents used to suppress the cytokine storm that is believed to contribute to disease severity may increase risk for secondary infections such as invasive aspergillosis.
“Many of our treatments may be associated with adverse consequences,” he said. “There is a trend toward treating patients with COVID-19 pneumonia with corticosteroids, and certainly that could have an immunosuppressant effect and predispose patients to secondary infections.”
He noted that the World Health Organization recommendations current in March 2020, when the pandemic began in earnest in the United States, advised against the use of corticosteroids, likely because of a lack of evidence of efficacy and concerns about risk for secondary infections.
“Regardless of the strategic choice made, all efforts should be put into improving our ability to reliably identify patients that may benefit from therapeutic interventions, which include host and risk factors, clinical factors and CAPA disease markers,” Dr. Brüggemann and colleagues wrote in their editorial.
The study was performed without external funding. The authors and Dr. Ouellette reported no conflicts of interest. Dr. Brüggemann and coauthors report grants and/or personal fees from various companies outside the submitted work.
SOURCE: Bartoletti M et al. Clin Infect Dis. 2020 Jul 28. doi: 10.1093/cid/ciaa1065.
FROM CLINICAL INFECTIOUS DISEASES
First evidence of SARS-CoV-2 in heart cells
SARS-CoV-2 has been found in cardiac tissue of a child from Brazil with multisystem inflammatory syndrome (MIS-C) related to COVID-19 who presented with myocarditis and died of heart failure.
It’s believed to be the first evidence of direct infection of heart muscle cells by the virus; viral particles were identified in different cell lineages of the heart, including cardiomyocytes, endothelial cells, mesenchymal cells, and inflammatory cells.
The case was described in a report published online August 20 in The Lancet Child & Adolescent Health.
“The presence of the virus in various cell types of cardiac tissue, as evidenced by electron microscopy, shows that myocarditis in this case is likely a direct inflammatory response to the virus infection in the heart,” first author Marisa Dolhnikoff, MD, department of pathology, University of São Paulo, said in an interview.
There have been previous reports in adults with COVID-19 of both SARS-CoV-2 RNA by reverse transcription–polymerase chain reaction (RT-PCR) and viral particles by electron microscopy in cardiac tissue from endomyocardial specimens, the researchers noted. One of these reports, published in April by Tavazzi and colleagues, “detected viral particles in cardiac macrophages in an adult patient with acute cardiac injury associated with COVID-19; no viral particles were seen in cardiomyocytes or endothelial cells.
“Our case report is the first to our knowledge to document the presence of viral particles in the cardiac tissue of a child affected by MIS-C,” they added. “Moreover, viral particles were identified in different cell lineages of the heart, including cardiomyocytes, endothelial cells, mesenchymal cells, and inflammatory cells.”
‘Concerning’ case report
“This is a concerning report as it shows for the first time that the virus can actually invade the heart muscle cells themselves,” C. Michael Gibson, MD, CEO of the Baim Institute for Clinical Research in Boston, said in an interview.
“Previous reports of COVID-19 and the heart found that the virus was in the area outside the heart muscle cells. We do not know yet the relative contribution of the inflammatory cells invading the heart, the release of blood-borne inflammatory mediators, and the virus inside the heart muscle cells themselves to heart damage,” Dr. Gibson said.
The patient was a previously healthy 11-year-old girl of African descent with MIS-C related to COVID-19. She developed cardiac failure and died after 1 day in the hospital, despite aggressive treatment.
SARS-CoV-2 RNA was detected on a postmortem nasopharyngeal swab and in cardiac and pulmonary tissues by RT-PCR.
Postmortem ultrasound examination of the heart showed a “hyperechogenic and diffusely thickened endocardium (mean thickness, 10 mm), a thickened myocardium (18 mm thick in the left ventricle), and a small pericardial effusion,” Dr. Dolhnikoff and colleagues reported.
Histopathologic exam revealed myocarditis, pericarditis, and endocarditis characterized by infiltration of inflammatory cells. Inflammation was mainly interstitial and perivascular, associated with foci of cardiomyocyte necrosis and was mainly composed of CD68+ macrophages, a few CD45+ lymphocytes, and a few neutrophils and eosinophils.
Electron microscopy of cardiac tissue revealed spherical viral particles in shape and size consistent with the Coronaviridae family in the extracellular compartment and within cardiomyocytes, capillary endothelial cells, endocardium endothelial cells, macrophages, neutrophils, and fibroblasts.
Microthrombi in the pulmonary arterioles and renal glomerular capillaries were also seen at autopsy. SARS-CoV-2–associated pneumonia was mild.
Lymphoid depletion and signs of hemophagocytosis were observed in the spleen and lymph nodes. Acute tubular necrosis in the kidneys and hepatic centrilobular necrosis, secondary to shock, were also seen. Brain tissue showed microglial reactivity.
“Fortunately, MIS-C is a rare event and, although it can be severe and life threatening, most children recover,” Dr. Dolhnikoff commented.
“This case report comes at a time when the scientific community around the world calls attention to MIS-C and the need for it to be quickly recognized and treated by the pediatric community. Evidence of a direct relation between the virus and myocarditis confirms that MIS-C is one of the possible forms of presentation of COVID-19 and that the heart may be the target organ. It also alerts clinicians to possible cardiac sequelae in these children,” she added.
Experts weigh in
Scott Aydin, MD, medical director of pediatric cardiac intensive care, Mount Sinai Kravis Children’s Hospital in New York City, said that this case report is “unfortunately not all that surprising.
“Since the initial presentations of MIS-C several months ago, we have suspected mechanisms of direct and indirect injury to the myocardium. This important work is just the next step in further understanding the mechanisms of how COVID-19 creates havoc in the human body and the choices of possible therapies we have to treat children with COVID-19 and MIS-C,” said Dr. Aydin, who was not involved with the case report.
Anish Koka, MD, a cardiologist in private practice in Philadelphia, noted that, in these cases, endomyocardial biopsy is “rarely done because it is fairly invasive, but even when it has been done, the pathologic findings are of widespread inflammation rather than virus-induced cell necrosis.”
“While reports like this are sure to spawn viral tweets, it’s vital to understand that it’s not unusual to find widespread organ dissemination of virus in very sick patients. This does not mean that the virus is causing dysfunction of the organ it happens to be found in,” Dr. Koka said in an interview.
He noted that, in the case of the young girl who died, it took high PCR-cycle threshold values to isolate virus from the lung and heart samples.
“This means there was a low viral load in both organs, supporting the theory of SARS-CoV-2 as a potential trigger of a widespread inflammatory response that results in organ damage, rather than the virus itself infecting and destroying organs,” said Dr. Koka, who was also not associated with the case report.
This research had no specific funding. The authors declared no competing interests. Dr. Aydin disclosed no relevant financial relationships. Dr. Koka disclosed financial relationships with Boehringer Ingelheim and Jardiance.
This article first appeared on Medscape.com.
SARS-CoV-2 has been found in cardiac tissue of a child from Brazil with multisystem inflammatory syndrome (MIS-C) related to COVID-19 who presented with myocarditis and died of heart failure.
It’s believed to be the first evidence of direct infection of heart muscle cells by the virus; viral particles were identified in different cell lineages of the heart, including cardiomyocytes, endothelial cells, mesenchymal cells, and inflammatory cells.
The case was described in a report published online August 20 in The Lancet Child & Adolescent Health.
“The presence of the virus in various cell types of cardiac tissue, as evidenced by electron microscopy, shows that myocarditis in this case is likely a direct inflammatory response to the virus infection in the heart,” first author Marisa Dolhnikoff, MD, department of pathology, University of São Paulo, said in an interview.
There have been previous reports in adults with COVID-19 of both SARS-CoV-2 RNA by reverse transcription–polymerase chain reaction (RT-PCR) and viral particles by electron microscopy in cardiac tissue from endomyocardial specimens, the researchers noted. One of these reports, published in April by Tavazzi and colleagues, “detected viral particles in cardiac macrophages in an adult patient with acute cardiac injury associated with COVID-19; no viral particles were seen in cardiomyocytes or endothelial cells.
“Our case report is the first to our knowledge to document the presence of viral particles in the cardiac tissue of a child affected by MIS-C,” they added. “Moreover, viral particles were identified in different cell lineages of the heart, including cardiomyocytes, endothelial cells, mesenchymal cells, and inflammatory cells.”
‘Concerning’ case report
“This is a concerning report as it shows for the first time that the virus can actually invade the heart muscle cells themselves,” C. Michael Gibson, MD, CEO of the Baim Institute for Clinical Research in Boston, said in an interview.
“Previous reports of COVID-19 and the heart found that the virus was in the area outside the heart muscle cells. We do not know yet the relative contribution of the inflammatory cells invading the heart, the release of blood-borne inflammatory mediators, and the virus inside the heart muscle cells themselves to heart damage,” Dr. Gibson said.
The patient was a previously healthy 11-year-old girl of African descent with MIS-C related to COVID-19. She developed cardiac failure and died after 1 day in the hospital, despite aggressive treatment.
SARS-CoV-2 RNA was detected on a postmortem nasopharyngeal swab and in cardiac and pulmonary tissues by RT-PCR.
Postmortem ultrasound examination of the heart showed a “hyperechogenic and diffusely thickened endocardium (mean thickness, 10 mm), a thickened myocardium (18 mm thick in the left ventricle), and a small pericardial effusion,” Dr. Dolhnikoff and colleagues reported.
Histopathologic exam revealed myocarditis, pericarditis, and endocarditis characterized by infiltration of inflammatory cells. Inflammation was mainly interstitial and perivascular, associated with foci of cardiomyocyte necrosis and was mainly composed of CD68+ macrophages, a few CD45+ lymphocytes, and a few neutrophils and eosinophils.
Electron microscopy of cardiac tissue revealed spherical viral particles in shape and size consistent with the Coronaviridae family in the extracellular compartment and within cardiomyocytes, capillary endothelial cells, endocardium endothelial cells, macrophages, neutrophils, and fibroblasts.
Microthrombi in the pulmonary arterioles and renal glomerular capillaries were also seen at autopsy. SARS-CoV-2–associated pneumonia was mild.
Lymphoid depletion and signs of hemophagocytosis were observed in the spleen and lymph nodes. Acute tubular necrosis in the kidneys and hepatic centrilobular necrosis, secondary to shock, were also seen. Brain tissue showed microglial reactivity.
“Fortunately, MIS-C is a rare event and, although it can be severe and life threatening, most children recover,” Dr. Dolhnikoff commented.
“This case report comes at a time when the scientific community around the world calls attention to MIS-C and the need for it to be quickly recognized and treated by the pediatric community. Evidence of a direct relation between the virus and myocarditis confirms that MIS-C is one of the possible forms of presentation of COVID-19 and that the heart may be the target organ. It also alerts clinicians to possible cardiac sequelae in these children,” she added.
Experts weigh in
Scott Aydin, MD, medical director of pediatric cardiac intensive care, Mount Sinai Kravis Children’s Hospital in New York City, said that this case report is “unfortunately not all that surprising.
“Since the initial presentations of MIS-C several months ago, we have suspected mechanisms of direct and indirect injury to the myocardium. This important work is just the next step in further understanding the mechanisms of how COVID-19 creates havoc in the human body and the choices of possible therapies we have to treat children with COVID-19 and MIS-C,” said Dr. Aydin, who was not involved with the case report.
Anish Koka, MD, a cardiologist in private practice in Philadelphia, noted that, in these cases, endomyocardial biopsy is “rarely done because it is fairly invasive, but even when it has been done, the pathologic findings are of widespread inflammation rather than virus-induced cell necrosis.”
“While reports like this are sure to spawn viral tweets, it’s vital to understand that it’s not unusual to find widespread organ dissemination of virus in very sick patients. This does not mean that the virus is causing dysfunction of the organ it happens to be found in,” Dr. Koka said in an interview.
He noted that, in the case of the young girl who died, it took high PCR-cycle threshold values to isolate virus from the lung and heart samples.
“This means there was a low viral load in both organs, supporting the theory of SARS-CoV-2 as a potential trigger of a widespread inflammatory response that results in organ damage, rather than the virus itself infecting and destroying organs,” said Dr. Koka, who was also not associated with the case report.
This research had no specific funding. The authors declared no competing interests. Dr. Aydin disclosed no relevant financial relationships. Dr. Koka disclosed financial relationships with Boehringer Ingelheim and Jardiance.
This article first appeared on Medscape.com.
SARS-CoV-2 has been found in cardiac tissue of a child from Brazil with multisystem inflammatory syndrome (MIS-C) related to COVID-19 who presented with myocarditis and died of heart failure.
It’s believed to be the first evidence of direct infection of heart muscle cells by the virus; viral particles were identified in different cell lineages of the heart, including cardiomyocytes, endothelial cells, mesenchymal cells, and inflammatory cells.
The case was described in a report published online August 20 in The Lancet Child & Adolescent Health.
“The presence of the virus in various cell types of cardiac tissue, as evidenced by electron microscopy, shows that myocarditis in this case is likely a direct inflammatory response to the virus infection in the heart,” first author Marisa Dolhnikoff, MD, department of pathology, University of São Paulo, said in an interview.
There have been previous reports in adults with COVID-19 of both SARS-CoV-2 RNA by reverse transcription–polymerase chain reaction (RT-PCR) and viral particles by electron microscopy in cardiac tissue from endomyocardial specimens, the researchers noted. One of these reports, published in April by Tavazzi and colleagues, “detected viral particles in cardiac macrophages in an adult patient with acute cardiac injury associated with COVID-19; no viral particles were seen in cardiomyocytes or endothelial cells.
“Our case report is the first to our knowledge to document the presence of viral particles in the cardiac tissue of a child affected by MIS-C,” they added. “Moreover, viral particles were identified in different cell lineages of the heart, including cardiomyocytes, endothelial cells, mesenchymal cells, and inflammatory cells.”
‘Concerning’ case report
“This is a concerning report as it shows for the first time that the virus can actually invade the heart muscle cells themselves,” C. Michael Gibson, MD, CEO of the Baim Institute for Clinical Research in Boston, said in an interview.
“Previous reports of COVID-19 and the heart found that the virus was in the area outside the heart muscle cells. We do not know yet the relative contribution of the inflammatory cells invading the heart, the release of blood-borne inflammatory mediators, and the virus inside the heart muscle cells themselves to heart damage,” Dr. Gibson said.
The patient was a previously healthy 11-year-old girl of African descent with MIS-C related to COVID-19. She developed cardiac failure and died after 1 day in the hospital, despite aggressive treatment.
SARS-CoV-2 RNA was detected on a postmortem nasopharyngeal swab and in cardiac and pulmonary tissues by RT-PCR.
Postmortem ultrasound examination of the heart showed a “hyperechogenic and diffusely thickened endocardium (mean thickness, 10 mm), a thickened myocardium (18 mm thick in the left ventricle), and a small pericardial effusion,” Dr. Dolhnikoff and colleagues reported.
Histopathologic exam revealed myocarditis, pericarditis, and endocarditis characterized by infiltration of inflammatory cells. Inflammation was mainly interstitial and perivascular, associated with foci of cardiomyocyte necrosis and was mainly composed of CD68+ macrophages, a few CD45+ lymphocytes, and a few neutrophils and eosinophils.
Electron microscopy of cardiac tissue revealed spherical viral particles in shape and size consistent with the Coronaviridae family in the extracellular compartment and within cardiomyocytes, capillary endothelial cells, endocardium endothelial cells, macrophages, neutrophils, and fibroblasts.
Microthrombi in the pulmonary arterioles and renal glomerular capillaries were also seen at autopsy. SARS-CoV-2–associated pneumonia was mild.
Lymphoid depletion and signs of hemophagocytosis were observed in the spleen and lymph nodes. Acute tubular necrosis in the kidneys and hepatic centrilobular necrosis, secondary to shock, were also seen. Brain tissue showed microglial reactivity.
“Fortunately, MIS-C is a rare event and, although it can be severe and life threatening, most children recover,” Dr. Dolhnikoff commented.
“This case report comes at a time when the scientific community around the world calls attention to MIS-C and the need for it to be quickly recognized and treated by the pediatric community. Evidence of a direct relation between the virus and myocarditis confirms that MIS-C is one of the possible forms of presentation of COVID-19 and that the heart may be the target organ. It also alerts clinicians to possible cardiac sequelae in these children,” she added.
Experts weigh in
Scott Aydin, MD, medical director of pediatric cardiac intensive care, Mount Sinai Kravis Children’s Hospital in New York City, said that this case report is “unfortunately not all that surprising.
“Since the initial presentations of MIS-C several months ago, we have suspected mechanisms of direct and indirect injury to the myocardium. This important work is just the next step in further understanding the mechanisms of how COVID-19 creates havoc in the human body and the choices of possible therapies we have to treat children with COVID-19 and MIS-C,” said Dr. Aydin, who was not involved with the case report.
Anish Koka, MD, a cardiologist in private practice in Philadelphia, noted that, in these cases, endomyocardial biopsy is “rarely done because it is fairly invasive, but even when it has been done, the pathologic findings are of widespread inflammation rather than virus-induced cell necrosis.”
“While reports like this are sure to spawn viral tweets, it’s vital to understand that it’s not unusual to find widespread organ dissemination of virus in very sick patients. This does not mean that the virus is causing dysfunction of the organ it happens to be found in,” Dr. Koka said in an interview.
He noted that, in the case of the young girl who died, it took high PCR-cycle threshold values to isolate virus from the lung and heart samples.
“This means there was a low viral load in both organs, supporting the theory of SARS-CoV-2 as a potential trigger of a widespread inflammatory response that results in organ damage, rather than the virus itself infecting and destroying organs,” said Dr. Koka, who was also not associated with the case report.
This research had no specific funding. The authors declared no competing interests. Dr. Aydin disclosed no relevant financial relationships. Dr. Koka disclosed financial relationships with Boehringer Ingelheim and Jardiance.
This article first appeared on Medscape.com.
Weight-loss benefit in diabetes is similar via surgery or diet
The metabolic benefits achieved with weight loss for patients with type 2 diabetes appear to be similar whether achieved via diet or gastric bypass surgery, according to new research.
The findings, from a small study of 22 people with type 2 diabetes and obesity, were published online August 19 in the New England Journal of Medicine. The study was conducted by Mihoko Yoshino, MD, PhD, of Washington University, St. Louis, and colleagues.
Among 11 patients in each group who experienced comparable degrees of weight loss (about 18%), there were very similar benefits in multiorgan insulin sensitivity, beta-cell function, 24-hour plasma glucose and insulin profiles, and body composition.
“The results from our study underscore the profound effect that marked weight loss can have on metabolic function in people with diabetes,” Dr. Yoshino and colleagues wrote.
“The similar findings in participants in the two groups challenge the current belief that upper gastrointestinal bypass has clinically meaningful effects on key metabolic factors involved in glucose homeostasis and the pathogenesis of diabetes that are independent of weight loss,” they added.
However, they acknowledged, “the difficulty in achieving successful long-term weight loss with lifestyle therapy often renders gastric bypass surgery far more effective than diet therapy for most patients with obesity and type 2 diabetes.”
“This study confirms the pathogenic nature of obesity in driving insulin resistance and, ultimately, type 2 diabetes; furthermore, it delivers a straightforward and important message for both clinicians and patients – reducing adipose tissue volume, by whatever means, will improve blood glucose control in persons with type 2 diabetes,” stated the authors of an accompanying editorial.
Asked to comment, Matthew M. Hutter, MD, president of the American Society for Metabolic and Bariatric Surgery and professor of surgery at Harvard Medical School and Massachusetts General Hospital, both in Boston, said in an interview that the study was “very elegant” and “well designed.”
However, he pointed out, “the reality is diet alone has been shown over and over that it’s not sustainable. For a very small few it is, but for the vast majority, that’s not the case.”
Dr. Hutter also noted that the research was conducted in a laboratory setting and that the diet group was given prepackaged food. Therefore, the study “doesn’t look at how effective [dieting] is in the real world.”
Bariatric surgery, on the other hand, “is very effective for treating type 2 diabetes. It’s a good long-term solution.”
No significant differences in multiple parameters
The nonrandomized prospective cohort study began with 33 adults with obesity and type 2 diabetes, of whom 18 received weekly diet education sessions and prepackaged food, and 15 underwent Roux-en-Y gastric bypass procedures. Seven in the diet group and four in the surgery group were withdrawn from the study because of a failure to achieve the target of 16%-18% loss of body weight.
Of those remaining in the study, mean weight loss at around 4 months was 17.8% of body weight for the 11 in the diet group and 18.7% for the 11 in the surgery group. For the diet group, the mean age was 54 years and the mean duration of diabetes was 9.1 years; for the surgery group, the mean age was 49 years and the mean duration of diabetes 9.6 years.
A three-stage hyperinsulinemic euglycemic pancreatic clamp was used to control both portal and systemic plasma insulin concentrations to provide a reliable assessment of hepatic, muscle, and adipose tissue insulin sensitivity across a physiological range of plasma insulin concentrations. In both treatment groups, similar improvements were seen in hepatic, adipose tissue, and skeletal muscle insulin sensitivity
Both groups experienced a similar drop in the use of diabetes medications. Four individuals in the diet group and two in the surgery group achieved hemoglobin A1c levels below 6.0% without any medications.
After ingestion of identical mixed meals, 4-hour areas under the curve (AUC) for plasma glucose and insulin were lower after weight loss than before for both groups, although the decrease in glucose was greater in the diet group. Postprandial glucose peaks were greater in the surgery group as well, owing to an increase in the rate of glucose delivery into the circulation.
Weight loss was associated with decreased total AUC for glucose, free fatty acids, insulin, and insulin secretion to similar degrees in both groups. However, there were differences in several factors that have been attributed to the effects of bariatric surgery independently of weight loss. These include a greater decrease in 24-hour plasma branched-chain amino acid and C3 and C5 acylcarnitine concentrations after weight loss in the surgery group than in the diet group.
Plasma bile acid levels after weight loss dropped from baseline in the diet group but rose in the surgery group. Although microbiome changes occurred in both groups, they were more pronounced with weight loss following surgery.
Study limitations: Small, not randomized, long diabetes duration
The authors acknowledged some of the study’s limitations, including the fact that it wasn’t randomized and there was a large number of dropouts, although they say that the method used to assess the primary outcome can detect small differences even in a few patients.
The editorialists – endocrinologist Clifford J. Rosen, MD, director of the Center for Clinical and Translational Research at Maine Medical Center Research Institute, Scarborough, and pediatric nephrologist and deputy editor for the New England Journal of Medicine Julie R. Ingelfinger, MD, of Massachusetts General Hospital, Boston – pointed out that today more sleeve gastrectomies are performed than Roux-en-Y bypass procedures.
However, Dr. Hutter said that gastric bypass procedures are still being performed, that both procedures are effective and safe, and that for patients with diabetes the bypass tends to be somewhat more effective than sleeve gastrectomy.
He also qualified: “What phase you’re in is critical. Here, the 18% [weight loss] is still early in the track for bariatric surgery. They could still be losing weight after that. The diet groups could have been in the plateau or regain phase. Those [numbers] could look very different with time.”
In addition, he noted that the relatively long average duration of diabetes in the study participants makes the success of bariatric surgery less likely.
“The pancreas burns out, and the tissues become less insulin sensitive. I would have wanted to stratify the patients into early versus later disease, but you would need a larger sample size to do that,” he said.
Dr. Hutter noted: “I thought the study was very interesting. It just amazes me that 60 years after the gastric bypass procedure was invented, we’re still trying to figure out how it works.”
Dr. Hutter is a consultant for Vicarious Surgical and has received honoraria from Ethicon, Medtronic, and Olympus.
A version of this article originally appeared on Medscape.com.
The AGA Practice guide on Obesity and Weight management, Education and Resources (POWER) white paper provides physicians with a comprehensive, multi-disciplinary process to guide and personalize innovative obesity care for safe and effective weight management. Learn more at www.gastro.org/obesity.
The metabolic benefits achieved with weight loss for patients with type 2 diabetes appear to be similar whether achieved via diet or gastric bypass surgery, according to new research.
The findings, from a small study of 22 people with type 2 diabetes and obesity, were published online August 19 in the New England Journal of Medicine. The study was conducted by Mihoko Yoshino, MD, PhD, of Washington University, St. Louis, and colleagues.
Among 11 patients in each group who experienced comparable degrees of weight loss (about 18%), there were very similar benefits in multiorgan insulin sensitivity, beta-cell function, 24-hour plasma glucose and insulin profiles, and body composition.
“The results from our study underscore the profound effect that marked weight loss can have on metabolic function in people with diabetes,” Dr. Yoshino and colleagues wrote.
“The similar findings in participants in the two groups challenge the current belief that upper gastrointestinal bypass has clinically meaningful effects on key metabolic factors involved in glucose homeostasis and the pathogenesis of diabetes that are independent of weight loss,” they added.
However, they acknowledged, “the difficulty in achieving successful long-term weight loss with lifestyle therapy often renders gastric bypass surgery far more effective than diet therapy for most patients with obesity and type 2 diabetes.”
“This study confirms the pathogenic nature of obesity in driving insulin resistance and, ultimately, type 2 diabetes; furthermore, it delivers a straightforward and important message for both clinicians and patients – reducing adipose tissue volume, by whatever means, will improve blood glucose control in persons with type 2 diabetes,” stated the authors of an accompanying editorial.
Asked to comment, Matthew M. Hutter, MD, president of the American Society for Metabolic and Bariatric Surgery and professor of surgery at Harvard Medical School and Massachusetts General Hospital, both in Boston, said in an interview that the study was “very elegant” and “well designed.”
However, he pointed out, “the reality is diet alone has been shown over and over that it’s not sustainable. For a very small few it is, but for the vast majority, that’s not the case.”
Dr. Hutter also noted that the research was conducted in a laboratory setting and that the diet group was given prepackaged food. Therefore, the study “doesn’t look at how effective [dieting] is in the real world.”
Bariatric surgery, on the other hand, “is very effective for treating type 2 diabetes. It’s a good long-term solution.”
No significant differences in multiple parameters
The nonrandomized prospective cohort study began with 33 adults with obesity and type 2 diabetes, of whom 18 received weekly diet education sessions and prepackaged food, and 15 underwent Roux-en-Y gastric bypass procedures. Seven in the diet group and four in the surgery group were withdrawn from the study because of a failure to achieve the target of 16%-18% loss of body weight.
Of those remaining in the study, mean weight loss at around 4 months was 17.8% of body weight for the 11 in the diet group and 18.7% for the 11 in the surgery group. For the diet group, the mean age was 54 years and the mean duration of diabetes was 9.1 years; for the surgery group, the mean age was 49 years and the mean duration of diabetes 9.6 years.
A three-stage hyperinsulinemic euglycemic pancreatic clamp was used to control both portal and systemic plasma insulin concentrations to provide a reliable assessment of hepatic, muscle, and adipose tissue insulin sensitivity across a physiological range of plasma insulin concentrations. In both treatment groups, similar improvements were seen in hepatic, adipose tissue, and skeletal muscle insulin sensitivity
Both groups experienced a similar drop in the use of diabetes medications. Four individuals in the diet group and two in the surgery group achieved hemoglobin A1c levels below 6.0% without any medications.
After ingestion of identical mixed meals, 4-hour areas under the curve (AUC) for plasma glucose and insulin were lower after weight loss than before for both groups, although the decrease in glucose was greater in the diet group. Postprandial glucose peaks were greater in the surgery group as well, owing to an increase in the rate of glucose delivery into the circulation.
Weight loss was associated with decreased total AUC for glucose, free fatty acids, insulin, and insulin secretion to similar degrees in both groups. However, there were differences in several factors that have been attributed to the effects of bariatric surgery independently of weight loss. These include a greater decrease in 24-hour plasma branched-chain amino acid and C3 and C5 acylcarnitine concentrations after weight loss in the surgery group than in the diet group.
Plasma bile acid levels after weight loss dropped from baseline in the diet group but rose in the surgery group. Although microbiome changes occurred in both groups, they were more pronounced with weight loss following surgery.
Study limitations: Small, not randomized, long diabetes duration
The authors acknowledged some of the study’s limitations, including the fact that it wasn’t randomized and there was a large number of dropouts, although they say that the method used to assess the primary outcome can detect small differences even in a few patients.
The editorialists – endocrinologist Clifford J. Rosen, MD, director of the Center for Clinical and Translational Research at Maine Medical Center Research Institute, Scarborough, and pediatric nephrologist and deputy editor for the New England Journal of Medicine Julie R. Ingelfinger, MD, of Massachusetts General Hospital, Boston – pointed out that today more sleeve gastrectomies are performed than Roux-en-Y bypass procedures.
However, Dr. Hutter said that gastric bypass procedures are still being performed, that both procedures are effective and safe, and that for patients with diabetes the bypass tends to be somewhat more effective than sleeve gastrectomy.
He also qualified: “What phase you’re in is critical. Here, the 18% [weight loss] is still early in the track for bariatric surgery. They could still be losing weight after that. The diet groups could have been in the plateau or regain phase. Those [numbers] could look very different with time.”
In addition, he noted that the relatively long average duration of diabetes in the study participants makes the success of bariatric surgery less likely.
“The pancreas burns out, and the tissues become less insulin sensitive. I would have wanted to stratify the patients into early versus later disease, but you would need a larger sample size to do that,” he said.
Dr. Hutter noted: “I thought the study was very interesting. It just amazes me that 60 years after the gastric bypass procedure was invented, we’re still trying to figure out how it works.”
Dr. Hutter is a consultant for Vicarious Surgical and has received honoraria from Ethicon, Medtronic, and Olympus.
A version of this article originally appeared on Medscape.com.
The AGA Practice guide on Obesity and Weight management, Education and Resources (POWER) white paper provides physicians with a comprehensive, multi-disciplinary process to guide and personalize innovative obesity care for safe and effective weight management. Learn more at www.gastro.org/obesity.
The metabolic benefits achieved with weight loss for patients with type 2 diabetes appear to be similar whether achieved via diet or gastric bypass surgery, according to new research.
The findings, from a small study of 22 people with type 2 diabetes and obesity, were published online August 19 in the New England Journal of Medicine. The study was conducted by Mihoko Yoshino, MD, PhD, of Washington University, St. Louis, and colleagues.
Among 11 patients in each group who experienced comparable degrees of weight loss (about 18%), there were very similar benefits in multiorgan insulin sensitivity, beta-cell function, 24-hour plasma glucose and insulin profiles, and body composition.
“The results from our study underscore the profound effect that marked weight loss can have on metabolic function in people with diabetes,” Dr. Yoshino and colleagues wrote.
“The similar findings in participants in the two groups challenge the current belief that upper gastrointestinal bypass has clinically meaningful effects on key metabolic factors involved in glucose homeostasis and the pathogenesis of diabetes that are independent of weight loss,” they added.
However, they acknowledged, “the difficulty in achieving successful long-term weight loss with lifestyle therapy often renders gastric bypass surgery far more effective than diet therapy for most patients with obesity and type 2 diabetes.”
“This study confirms the pathogenic nature of obesity in driving insulin resistance and, ultimately, type 2 diabetes; furthermore, it delivers a straightforward and important message for both clinicians and patients – reducing adipose tissue volume, by whatever means, will improve blood glucose control in persons with type 2 diabetes,” stated the authors of an accompanying editorial.
Asked to comment, Matthew M. Hutter, MD, president of the American Society for Metabolic and Bariatric Surgery and professor of surgery at Harvard Medical School and Massachusetts General Hospital, both in Boston, said in an interview that the study was “very elegant” and “well designed.”
However, he pointed out, “the reality is diet alone has been shown over and over that it’s not sustainable. For a very small few it is, but for the vast majority, that’s not the case.”
Dr. Hutter also noted that the research was conducted in a laboratory setting and that the diet group was given prepackaged food. Therefore, the study “doesn’t look at how effective [dieting] is in the real world.”
Bariatric surgery, on the other hand, “is very effective for treating type 2 diabetes. It’s a good long-term solution.”
No significant differences in multiple parameters
The nonrandomized prospective cohort study began with 33 adults with obesity and type 2 diabetes, of whom 18 received weekly diet education sessions and prepackaged food, and 15 underwent Roux-en-Y gastric bypass procedures. Seven in the diet group and four in the surgery group were withdrawn from the study because of a failure to achieve the target of 16%-18% loss of body weight.
Of those remaining in the study, mean weight loss at around 4 months was 17.8% of body weight for the 11 in the diet group and 18.7% for the 11 in the surgery group. For the diet group, the mean age was 54 years and the mean duration of diabetes was 9.1 years; for the surgery group, the mean age was 49 years and the mean duration of diabetes 9.6 years.
A three-stage hyperinsulinemic euglycemic pancreatic clamp was used to control both portal and systemic plasma insulin concentrations to provide a reliable assessment of hepatic, muscle, and adipose tissue insulin sensitivity across a physiological range of plasma insulin concentrations. In both treatment groups, similar improvements were seen in hepatic, adipose tissue, and skeletal muscle insulin sensitivity
Both groups experienced a similar drop in the use of diabetes medications. Four individuals in the diet group and two in the surgery group achieved hemoglobin A1c levels below 6.0% without any medications.
After ingestion of identical mixed meals, 4-hour areas under the curve (AUC) for plasma glucose and insulin were lower after weight loss than before for both groups, although the decrease in glucose was greater in the diet group. Postprandial glucose peaks were greater in the surgery group as well, owing to an increase in the rate of glucose delivery into the circulation.
Weight loss was associated with decreased total AUC for glucose, free fatty acids, insulin, and insulin secretion to similar degrees in both groups. However, there were differences in several factors that have been attributed to the effects of bariatric surgery independently of weight loss. These include a greater decrease in 24-hour plasma branched-chain amino acid and C3 and C5 acylcarnitine concentrations after weight loss in the surgery group than in the diet group.
Plasma bile acid levels after weight loss dropped from baseline in the diet group but rose in the surgery group. Although microbiome changes occurred in both groups, they were more pronounced with weight loss following surgery.
Study limitations: Small, not randomized, long diabetes duration
The authors acknowledged some of the study’s limitations, including the fact that it wasn’t randomized and there was a large number of dropouts, although they say that the method used to assess the primary outcome can detect small differences even in a few patients.
The editorialists – endocrinologist Clifford J. Rosen, MD, director of the Center for Clinical and Translational Research at Maine Medical Center Research Institute, Scarborough, and pediatric nephrologist and deputy editor for the New England Journal of Medicine Julie R. Ingelfinger, MD, of Massachusetts General Hospital, Boston – pointed out that today more sleeve gastrectomies are performed than Roux-en-Y bypass procedures.
However, Dr. Hutter said that gastric bypass procedures are still being performed, that both procedures are effective and safe, and that for patients with diabetes the bypass tends to be somewhat more effective than sleeve gastrectomy.
He also qualified: “What phase you’re in is critical. Here, the 18% [weight loss] is still early in the track for bariatric surgery. They could still be losing weight after that. The diet groups could have been in the plateau or regain phase. Those [numbers] could look very different with time.”
In addition, he noted that the relatively long average duration of diabetes in the study participants makes the success of bariatric surgery less likely.
“The pancreas burns out, and the tissues become less insulin sensitive. I would have wanted to stratify the patients into early versus later disease, but you would need a larger sample size to do that,” he said.
Dr. Hutter noted: “I thought the study was very interesting. It just amazes me that 60 years after the gastric bypass procedure was invented, we’re still trying to figure out how it works.”
Dr. Hutter is a consultant for Vicarious Surgical and has received honoraria from Ethicon, Medtronic, and Olympus.
A version of this article originally appeared on Medscape.com.
The AGA Practice guide on Obesity and Weight management, Education and Resources (POWER) white paper provides physicians with a comprehensive, multi-disciplinary process to guide and personalize innovative obesity care for safe and effective weight management. Learn more at www.gastro.org/obesity.
Famotidine associated with benefits in hospitalized COVID patients in another trial
It also demonstrated lower levels of serum markers for severe disease.
The findings come from an observational study of 83 hospitalized patients that was published in the American Journal of Gastroenterology.
“The mechanism of exactly how famotidine works has yet to be proven,” lead study author Jeffrey F. Mather, MS, said in an interview. “There’s thought that it works directly on the virus, and there is thought that it works through inactivating certain proteases that are required for the virus infection, but I think the most interesting [hypothesis] is by Malone et al. “They’re looking at the blocking of the histamine-2 receptor causing a decrease in the amount of histamine. It’s all speculative, but it will be interesting if that gets worked out.”
In a study that largely mimicked that of an earlier, larger published observational study on the topic (doi: 10.1053/j.gastro.2020.05.053), Mr. Mather and colleagues retrospectively evaluated 878 patients who tested positive for SARS-CoV-2 and who required admission to Hartford (Conn.) Hospital between Feb. 24, 2020, and May 14, 2020. Patients were classified as receiving famotidine if they were treated with either oral or intravenous drug within 1 week of COVID-19 screening and/or hospital admission. Primary outcomes of interest were in-hospital death as recorded in the discharge of the patients, requirement for mechanical ventilation, and the composite of death or requirement for ventilation. Secondary outcomes of interest were several serum markers of disease activity including white blood cell count, lymphocyte count, and eosinophil count.
Famotidine was administered orally in 83% of the patients and intravenously in the remaining 17%. Mr. Mather, director of data management in the division of research management at Hartford Hospital, and his colleagues reported that 83 of the 878 patients studied (9.5%) received famotidine. Compared with patients not treated with famotidine, those who received the drug were slightly younger (a mean of 64 vs. 68 years, respectively; P = .021); otherwise, there were no differences between the two groups in baseline demographics or in preexisting comorbidities.
The use of famotidine was associated with a decreased risk of in-hospital mortality (odds ratio, 0.37; P = .021) as well as combined death or intubation (OR, 0.47; P = .040). The outcomes were similar when the researchers performed propensity score matching to adjust for age differences between groups.
In addition, the use of famotidine was associated with lower levels of serum markers for severe disease including lower median peak C-reactive protein levels (9.4 vs. 12.7 mg/dL; P =. 002), lower median procalcitonin levels (0.16 vs. 0.30 ng/mL; P = .004), and a nonsignificant trend to lower median mean ferritin levels (797.5 vs. 964 ng/mL; P = .076).
Logistic regression analysis revealed that use of famotidine was an independent predictor of both lower mortality and combined death/intubation. In addition, predictors of both adverse outcomes included older age, a body mass index of greater than 30 kg/m2, chronic kidney disease, the national early warning score, and a higher neutrophil-lymphocyte ratio.
“This is an important stepping stone, but until we have a randomized, controlled trial, we really can’t speak about causation; we can only speak about association, and that’s okay,” Brennan Spiegel, MD, MSHS, director of health services research at Cedars-Sinai, Los Angeles, who was not affiliated with the study, said in an interview. “There’s nothing wrong with association because finding associations can raise important hypotheses that can then be tested in prospective randomized trials, for example.”
In July 2020, Dr. Spiegel and his colleagues published a separate paper looking at proton pump inhibitors and the risk of COVID-19. “In that study we did look at H2 blockers, and we did find that they were slightly associated with a reduction in COVID-19,” he said. “It was a small effect, but it was a benefit. When we see consistency among studies, it’s a signal in the noise we can try and follow and see if there is something more to it.”
Mr. Mather acknowledged certain limitations of the study, including the fact that patients who did and did not receive famotidine were propensity-matched for age. “The risk factors that others have shown for adverse events are equivalent in the groups, but anytime you do a retrospective study like this there is the potential for underlying factors that may play a role in the outcomes that you’re not considering,” Mr. Mather said. “That’s why the gold standard is the randomized trial, to wash those effects out. There’s only an association here, and it supports the need for a randomized trial.”
Famotidine is currently being tested in a double-blind randomized clinical trial in combination with either hydroxychloroquine or remdesivir (NCT 04370262).
“It’s fascinating because famotidine is a safe medicine,” added Dr. Spiegel, who is also co–editor in chief of the American Journal of Gastroenterology. “There are very few side effects; it’s something we’ve been using for decades.”
Mr. Mather and his colleagues reported having no financial disclosures. Dr. Spiegel disclosed that he has served on advisory boards for Allergan, Alnylam Pharmaceuticals, Arena Pharmaceuticals, Ironwood Pharmaceuticals, Salix Pharmaceuticals, Synergy Pharmaceuticals, and Takeda Pharmaceuticals.
SOURCE: Mather J et al. 2020 Aug 14. Am J Gastroenterol.
It also demonstrated lower levels of serum markers for severe disease.
The findings come from an observational study of 83 hospitalized patients that was published in the American Journal of Gastroenterology.
“The mechanism of exactly how famotidine works has yet to be proven,” lead study author Jeffrey F. Mather, MS, said in an interview. “There’s thought that it works directly on the virus, and there is thought that it works through inactivating certain proteases that are required for the virus infection, but I think the most interesting [hypothesis] is by Malone et al. “They’re looking at the blocking of the histamine-2 receptor causing a decrease in the amount of histamine. It’s all speculative, but it will be interesting if that gets worked out.”
In a study that largely mimicked that of an earlier, larger published observational study on the topic (doi: 10.1053/j.gastro.2020.05.053), Mr. Mather and colleagues retrospectively evaluated 878 patients who tested positive for SARS-CoV-2 and who required admission to Hartford (Conn.) Hospital between Feb. 24, 2020, and May 14, 2020. Patients were classified as receiving famotidine if they were treated with either oral or intravenous drug within 1 week of COVID-19 screening and/or hospital admission. Primary outcomes of interest were in-hospital death as recorded in the discharge of the patients, requirement for mechanical ventilation, and the composite of death or requirement for ventilation. Secondary outcomes of interest were several serum markers of disease activity including white blood cell count, lymphocyte count, and eosinophil count.
Famotidine was administered orally in 83% of the patients and intravenously in the remaining 17%. Mr. Mather, director of data management in the division of research management at Hartford Hospital, and his colleagues reported that 83 of the 878 patients studied (9.5%) received famotidine. Compared with patients not treated with famotidine, those who received the drug were slightly younger (a mean of 64 vs. 68 years, respectively; P = .021); otherwise, there were no differences between the two groups in baseline demographics or in preexisting comorbidities.
The use of famotidine was associated with a decreased risk of in-hospital mortality (odds ratio, 0.37; P = .021) as well as combined death or intubation (OR, 0.47; P = .040). The outcomes were similar when the researchers performed propensity score matching to adjust for age differences between groups.
In addition, the use of famotidine was associated with lower levels of serum markers for severe disease including lower median peak C-reactive protein levels (9.4 vs. 12.7 mg/dL; P =. 002), lower median procalcitonin levels (0.16 vs. 0.30 ng/mL; P = .004), and a nonsignificant trend to lower median mean ferritin levels (797.5 vs. 964 ng/mL; P = .076).
Logistic regression analysis revealed that use of famotidine was an independent predictor of both lower mortality and combined death/intubation. In addition, predictors of both adverse outcomes included older age, a body mass index of greater than 30 kg/m2, chronic kidney disease, the national early warning score, and a higher neutrophil-lymphocyte ratio.
“This is an important stepping stone, but until we have a randomized, controlled trial, we really can’t speak about causation; we can only speak about association, and that’s okay,” Brennan Spiegel, MD, MSHS, director of health services research at Cedars-Sinai, Los Angeles, who was not affiliated with the study, said in an interview. “There’s nothing wrong with association because finding associations can raise important hypotheses that can then be tested in prospective randomized trials, for example.”
In July 2020, Dr. Spiegel and his colleagues published a separate paper looking at proton pump inhibitors and the risk of COVID-19. “In that study we did look at H2 blockers, and we did find that they were slightly associated with a reduction in COVID-19,” he said. “It was a small effect, but it was a benefit. When we see consistency among studies, it’s a signal in the noise we can try and follow and see if there is something more to it.”
Mr. Mather acknowledged certain limitations of the study, including the fact that patients who did and did not receive famotidine were propensity-matched for age. “The risk factors that others have shown for adverse events are equivalent in the groups, but anytime you do a retrospective study like this there is the potential for underlying factors that may play a role in the outcomes that you’re not considering,” Mr. Mather said. “That’s why the gold standard is the randomized trial, to wash those effects out. There’s only an association here, and it supports the need for a randomized trial.”
Famotidine is currently being tested in a double-blind randomized clinical trial in combination with either hydroxychloroquine or remdesivir (NCT 04370262).
“It’s fascinating because famotidine is a safe medicine,” added Dr. Spiegel, who is also co–editor in chief of the American Journal of Gastroenterology. “There are very few side effects; it’s something we’ve been using for decades.”
Mr. Mather and his colleagues reported having no financial disclosures. Dr. Spiegel disclosed that he has served on advisory boards for Allergan, Alnylam Pharmaceuticals, Arena Pharmaceuticals, Ironwood Pharmaceuticals, Salix Pharmaceuticals, Synergy Pharmaceuticals, and Takeda Pharmaceuticals.
SOURCE: Mather J et al. 2020 Aug 14. Am J Gastroenterol.
It also demonstrated lower levels of serum markers for severe disease.
The findings come from an observational study of 83 hospitalized patients that was published in the American Journal of Gastroenterology.
“The mechanism of exactly how famotidine works has yet to be proven,” lead study author Jeffrey F. Mather, MS, said in an interview. “There’s thought that it works directly on the virus, and there is thought that it works through inactivating certain proteases that are required for the virus infection, but I think the most interesting [hypothesis] is by Malone et al. “They’re looking at the blocking of the histamine-2 receptor causing a decrease in the amount of histamine. It’s all speculative, but it will be interesting if that gets worked out.”
In a study that largely mimicked that of an earlier, larger published observational study on the topic (doi: 10.1053/j.gastro.2020.05.053), Mr. Mather and colleagues retrospectively evaluated 878 patients who tested positive for SARS-CoV-2 and who required admission to Hartford (Conn.) Hospital between Feb. 24, 2020, and May 14, 2020. Patients were classified as receiving famotidine if they were treated with either oral or intravenous drug within 1 week of COVID-19 screening and/or hospital admission. Primary outcomes of interest were in-hospital death as recorded in the discharge of the patients, requirement for mechanical ventilation, and the composite of death or requirement for ventilation. Secondary outcomes of interest were several serum markers of disease activity including white blood cell count, lymphocyte count, and eosinophil count.
Famotidine was administered orally in 83% of the patients and intravenously in the remaining 17%. Mr. Mather, director of data management in the division of research management at Hartford Hospital, and his colleagues reported that 83 of the 878 patients studied (9.5%) received famotidine. Compared with patients not treated with famotidine, those who received the drug were slightly younger (a mean of 64 vs. 68 years, respectively; P = .021); otherwise, there were no differences between the two groups in baseline demographics or in preexisting comorbidities.
The use of famotidine was associated with a decreased risk of in-hospital mortality (odds ratio, 0.37; P = .021) as well as combined death or intubation (OR, 0.47; P = .040). The outcomes were similar when the researchers performed propensity score matching to adjust for age differences between groups.
In addition, the use of famotidine was associated with lower levels of serum markers for severe disease including lower median peak C-reactive protein levels (9.4 vs. 12.7 mg/dL; P =. 002), lower median procalcitonin levels (0.16 vs. 0.30 ng/mL; P = .004), and a nonsignificant trend to lower median mean ferritin levels (797.5 vs. 964 ng/mL; P = .076).
Logistic regression analysis revealed that use of famotidine was an independent predictor of both lower mortality and combined death/intubation. In addition, predictors of both adverse outcomes included older age, a body mass index of greater than 30 kg/m2, chronic kidney disease, the national early warning score, and a higher neutrophil-lymphocyte ratio.
“This is an important stepping stone, but until we have a randomized, controlled trial, we really can’t speak about causation; we can only speak about association, and that’s okay,” Brennan Spiegel, MD, MSHS, director of health services research at Cedars-Sinai, Los Angeles, who was not affiliated with the study, said in an interview. “There’s nothing wrong with association because finding associations can raise important hypotheses that can then be tested in prospective randomized trials, for example.”
In July 2020, Dr. Spiegel and his colleagues published a separate paper looking at proton pump inhibitors and the risk of COVID-19. “In that study we did look at H2 blockers, and we did find that they were slightly associated with a reduction in COVID-19,” he said. “It was a small effect, but it was a benefit. When we see consistency among studies, it’s a signal in the noise we can try and follow and see if there is something more to it.”
Mr. Mather acknowledged certain limitations of the study, including the fact that patients who did and did not receive famotidine were propensity-matched for age. “The risk factors that others have shown for adverse events are equivalent in the groups, but anytime you do a retrospective study like this there is the potential for underlying factors that may play a role in the outcomes that you’re not considering,” Mr. Mather said. “That’s why the gold standard is the randomized trial, to wash those effects out. There’s only an association here, and it supports the need for a randomized trial.”
Famotidine is currently being tested in a double-blind randomized clinical trial in combination with either hydroxychloroquine or remdesivir (NCT 04370262).
“It’s fascinating because famotidine is a safe medicine,” added Dr. Spiegel, who is also co–editor in chief of the American Journal of Gastroenterology. “There are very few side effects; it’s something we’ve been using for decades.”
Mr. Mather and his colleagues reported having no financial disclosures. Dr. Spiegel disclosed that he has served on advisory boards for Allergan, Alnylam Pharmaceuticals, Arena Pharmaceuticals, Ironwood Pharmaceuticals, Salix Pharmaceuticals, Synergy Pharmaceuticals, and Takeda Pharmaceuticals.
SOURCE: Mather J et al. 2020 Aug 14. Am J Gastroenterol.
REPORTING FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
Key clinical point: Among hospitalized COVID-19 patients, famotidine use was associated with a reduction in death and either death or intubation.
Major finding: The use of famotidine was associated with a decreased risk of in-hospital mortality (OR, 0.37; P = .021), as well as the combined endpoint of death or intubation (OR, 0.47; P = .040).
Study details: A single-center observational study of 83 patients hospitalized with COVID-19.
Disclosures: The researchers reported having no financial disclosures.
Source: Mather J et al. 2020 Aug 14. Am J Gastroenterol.
COVID-19, school reopenings, and safety: What should we tell parents?
Parents, teachers, children, and adolescents are facing stress and anxiety as K-12 school districts across the country debate whether to return to in-person instruction amid the COVID-19 pandemic. As we approach the opening of schools, the stress and anxiety seem to be heightening.
According to Education Week, which is tracking the reopening plans of public schools across the United States, 21 of the 25 largest school districts are opting to implement remote learning only as their model. I would like to see all of those districts adopt that model until we understand more about this illness, and can prevent and treat it.
Yes, it’s true – I am a psychiatrist – not an infectious disease specialist. And I realize that the American Academy of Pediatrics and the Centers for Disease Control and Prevention have taken nuanced positions on this issue. Their positions make it clear that it is within a child’s best interests – from an educational and social point of view – to attend school in person. Not only is the classroom experience important, but so is the socialization and the exercise. However, when I look at the science on children who have been exposed to the coronavirus, I worry.
For example, a study by Lael M. Yonker, MD, and associates on pediatric SARS-CoV-2 found that the children in days 0-2 of illness have far higher viral loads than adults who have been hospitalized for severe disease. “This study reveals that children may be a potential source of contagion in the SARS-CoV-2 pandemic in spite of milder disease or lack of symptoms, and immune dysregulation is implicated in severe post-infectious [multisystem inflammatory syndrome in children],” Dr. Yonker and associates wrote, referring to the illness associated with COVID-19 in children. Their study was published recently in the Journal of Pediatrics (2020 Aug 19. doi: 10.1016/j.jpeds.2020.08.037).
In my state, where positivity rates are fairly low, Gov. Andrew Cuomo admitted in an interview recently that sending children to school in New York City is a “tricky proposition.” At this point, New York City public schools are scheduled to open in mid-September using a hybrid mixture of in-person and remote learning.
And look at what happened several weeks ago in Israel, where schools reopened after the virus was beaten back. At one high school in Jerusalem, just days after the reopening, the virus spread so prolifically to students, teachers, and relatives that the schools had to be closed again. Other countries should not follow Israel’s example, Eli Weizmann, who chairs the team advising Israel’s National Security Council on the pandemic, reportedly told the New York Times. “It was a major failure.”
But I must be honest: I was worried about children returning to school before I heard about the study by Dr. Yonker and associates, Gov. Cuomo’s comments, and what happened in Israel. So far, here in the Northeast, particularly in New York, New Jersey, and Connecticut, we have managed to get COVID-19 under control. Perhaps, in this part of the country, opening classroom education might be feasible – with close monitoring and proper precautions.
But COVID-19 has taken the lives of hundreds of thousands of Americans – more than 176,000 as of this writing. A new model from the University of Washington’s Institute for Health Metrics and Evaluation projects that COVID-19 could lead to more than 300,000 U.S. deaths by Dec. 1. Thankfully, the number of COVID-19–positive children who have died has been low. But they could still pass on the virus to adults.
To get a better understanding of COVID-19, I spoke with Sheryl L. Wulkan, MD, an internist and expert in personal protective equipment (PPE) who has consulted for numerous health care agencies about these issues. Dr. Wulkan said that, in some areas with low infection rates, school openings might be appropriate. However, she said, without proper testing and contact tracing, we are at a loss of controlling the spread.
What we should tell patients, family, and friends
From a psychiatric point of view, how should we advise our patients, family, and friends about sending their children back to school? Is on-site learning better than remote learning? It is. Do our children need the socialization that a school brings? Yes, they do.
Socialization and relating to peers are, indeed, important, but today’s children socialize in many ways beyond attending school – and they have peer friendships and interactions with electronic devices at their disposal.
Can remote learning cause social isolation – an isolation so profound that school is necessary not only for learning but the psyche as well? A meta-analysis of 80 studies that looked at the impact of social isolation and loneliness on adolescents and children who were previously healthy found that the young people “are probably more likely to experience high rates of depression and probably anxiety during and after enforced isolation ends. This may increase as enforced isolation continues,” wrote Maria Elizabeth Loades, PhD, and associates (J Am Acad Child Adolesc Psychiatry. 2020 Jun 3. S0890-8567[20]30337-3).
I am concerned about young people who experience anxiety and depression, and agree with Dr. Loades that we mental health professionals need to be ready to intervene early and provide preventive support. To do this, we should encourage parents to keep us informed about how their children are doing.
So my advice is that, in the absence of a vaccine and an effective treatment like we have for influenza – such as Tamiflu – and effective testing, such the saliva-based test developed by Yale University researchers, if I had school-aged children, I would continue to keep them home from school. Ultimately, however, parents must look at the science and make their decisions based on that. My children are adults with their own children, and only they can make informed decisions about which options are best for their families.
Interestingly, Sanjay Gupta, MD, the neurosurgeon who works as chief medical correspondent of CNN, recently discussed the thought process he and his wife used to determine whether their daughters would return to the classroom. After weighing many factors, including the viral spread in Fulton County, Ga., where they live, the Guptas decided that, at this time, the risks of allowing the girls to return to the classroom outweigh the benefits. “This was not an easy decision, but one that we believe best respects the science, decreases the risk of further spread, and follows the task force criteria,” wrote Dr. Gupta, who is affiliated with Emory University in Atlanta. “After 2 weeks, we will reassess.”
I understand that parents worry about the social and psychological costs of remote learning. And I can only imagine the difficulty of those who must balance homeschooling with working. And frankly, remote learning is not an option for all students. For those less fortunate, substantial governmental aid is important to assist these people and to keep them safe and on their feet until this pandemic is done. Also, those who were under the care of a psychiatrist should continue to receive care during the pandemic. We must be prepared to step in with interventions that can address the suffering that is inevitable, such as the use of targeted cognitive-behavioral therapy.
Public TV as an educational tool
Families with Internet access and those without it could benefit from using public television as a tool.
I would advise educators and the entertainment industry to harness the wonder of TV to develop curricula that can be used to educate children. As we know, Sesame Street proved to be an effective early childhood intervention, particularly for boys (Am Econ J: Applied Economics. 2019;11[1]:318-50). I would like to see programming that goes beyond Sesame Street. Learning from watching this kind of programming would be no substitute for engaging with teachers in real, live classrooms, however.
Children and adolescents will be changed by learning remotely. They will miss their friends, teachers, and other staff members, but their lives will not be ruined. Mental health professionals should be prepared to intervene to address depression, anxiety, and other sequelae and problematic behaviors that could result from social isolation. Schools, businesses, and the economy will again flourish after we get the virus behind us but controlling and eliminating this pandemic need to come first. Let’s keep our children home – to the extent that we can – until we move beyond this pandemic.
Dr. London has been a practicing psychiatrist for 4 decades and a newspaper columnist for almost as long. He has a private practice in New York and is author of “Find Freedom Fast: Short-Term Therapy That Works” (New York: Kettlehole Publishing, 2019). Dr. London has no conflicts of interest.
Parents, teachers, children, and adolescents are facing stress and anxiety as K-12 school districts across the country debate whether to return to in-person instruction amid the COVID-19 pandemic. As we approach the opening of schools, the stress and anxiety seem to be heightening.
According to Education Week, which is tracking the reopening plans of public schools across the United States, 21 of the 25 largest school districts are opting to implement remote learning only as their model. I would like to see all of those districts adopt that model until we understand more about this illness, and can prevent and treat it.
Yes, it’s true – I am a psychiatrist – not an infectious disease specialist. And I realize that the American Academy of Pediatrics and the Centers for Disease Control and Prevention have taken nuanced positions on this issue. Their positions make it clear that it is within a child’s best interests – from an educational and social point of view – to attend school in person. Not only is the classroom experience important, but so is the socialization and the exercise. However, when I look at the science on children who have been exposed to the coronavirus, I worry.
For example, a study by Lael M. Yonker, MD, and associates on pediatric SARS-CoV-2 found that the children in days 0-2 of illness have far higher viral loads than adults who have been hospitalized for severe disease. “This study reveals that children may be a potential source of contagion in the SARS-CoV-2 pandemic in spite of milder disease or lack of symptoms, and immune dysregulation is implicated in severe post-infectious [multisystem inflammatory syndrome in children],” Dr. Yonker and associates wrote, referring to the illness associated with COVID-19 in children. Their study was published recently in the Journal of Pediatrics (2020 Aug 19. doi: 10.1016/j.jpeds.2020.08.037).
In my state, where positivity rates are fairly low, Gov. Andrew Cuomo admitted in an interview recently that sending children to school in New York City is a “tricky proposition.” At this point, New York City public schools are scheduled to open in mid-September using a hybrid mixture of in-person and remote learning.
And look at what happened several weeks ago in Israel, where schools reopened after the virus was beaten back. At one high school in Jerusalem, just days after the reopening, the virus spread so prolifically to students, teachers, and relatives that the schools had to be closed again. Other countries should not follow Israel’s example, Eli Weizmann, who chairs the team advising Israel’s National Security Council on the pandemic, reportedly told the New York Times. “It was a major failure.”
But I must be honest: I was worried about children returning to school before I heard about the study by Dr. Yonker and associates, Gov. Cuomo’s comments, and what happened in Israel. So far, here in the Northeast, particularly in New York, New Jersey, and Connecticut, we have managed to get COVID-19 under control. Perhaps, in this part of the country, opening classroom education might be feasible – with close monitoring and proper precautions.
But COVID-19 has taken the lives of hundreds of thousands of Americans – more than 176,000 as of this writing. A new model from the University of Washington’s Institute for Health Metrics and Evaluation projects that COVID-19 could lead to more than 300,000 U.S. deaths by Dec. 1. Thankfully, the number of COVID-19–positive children who have died has been low. But they could still pass on the virus to adults.
To get a better understanding of COVID-19, I spoke with Sheryl L. Wulkan, MD, an internist and expert in personal protective equipment (PPE) who has consulted for numerous health care agencies about these issues. Dr. Wulkan said that, in some areas with low infection rates, school openings might be appropriate. However, she said, without proper testing and contact tracing, we are at a loss of controlling the spread.
What we should tell patients, family, and friends
From a psychiatric point of view, how should we advise our patients, family, and friends about sending their children back to school? Is on-site learning better than remote learning? It is. Do our children need the socialization that a school brings? Yes, they do.
Socialization and relating to peers are, indeed, important, but today’s children socialize in many ways beyond attending school – and they have peer friendships and interactions with electronic devices at their disposal.
Can remote learning cause social isolation – an isolation so profound that school is necessary not only for learning but the psyche as well? A meta-analysis of 80 studies that looked at the impact of social isolation and loneliness on adolescents and children who were previously healthy found that the young people “are probably more likely to experience high rates of depression and probably anxiety during and after enforced isolation ends. This may increase as enforced isolation continues,” wrote Maria Elizabeth Loades, PhD, and associates (J Am Acad Child Adolesc Psychiatry. 2020 Jun 3. S0890-8567[20]30337-3).
I am concerned about young people who experience anxiety and depression, and agree with Dr. Loades that we mental health professionals need to be ready to intervene early and provide preventive support. To do this, we should encourage parents to keep us informed about how their children are doing.
So my advice is that, in the absence of a vaccine and an effective treatment like we have for influenza – such as Tamiflu – and effective testing, such the saliva-based test developed by Yale University researchers, if I had school-aged children, I would continue to keep them home from school. Ultimately, however, parents must look at the science and make their decisions based on that. My children are adults with their own children, and only they can make informed decisions about which options are best for their families.
Interestingly, Sanjay Gupta, MD, the neurosurgeon who works as chief medical correspondent of CNN, recently discussed the thought process he and his wife used to determine whether their daughters would return to the classroom. After weighing many factors, including the viral spread in Fulton County, Ga., where they live, the Guptas decided that, at this time, the risks of allowing the girls to return to the classroom outweigh the benefits. “This was not an easy decision, but one that we believe best respects the science, decreases the risk of further spread, and follows the task force criteria,” wrote Dr. Gupta, who is affiliated with Emory University in Atlanta. “After 2 weeks, we will reassess.”
I understand that parents worry about the social and psychological costs of remote learning. And I can only imagine the difficulty of those who must balance homeschooling with working. And frankly, remote learning is not an option for all students. For those less fortunate, substantial governmental aid is important to assist these people and to keep them safe and on their feet until this pandemic is done. Also, those who were under the care of a psychiatrist should continue to receive care during the pandemic. We must be prepared to step in with interventions that can address the suffering that is inevitable, such as the use of targeted cognitive-behavioral therapy.
Public TV as an educational tool
Families with Internet access and those without it could benefit from using public television as a tool.
I would advise educators and the entertainment industry to harness the wonder of TV to develop curricula that can be used to educate children. As we know, Sesame Street proved to be an effective early childhood intervention, particularly for boys (Am Econ J: Applied Economics. 2019;11[1]:318-50). I would like to see programming that goes beyond Sesame Street. Learning from watching this kind of programming would be no substitute for engaging with teachers in real, live classrooms, however.
Children and adolescents will be changed by learning remotely. They will miss their friends, teachers, and other staff members, but their lives will not be ruined. Mental health professionals should be prepared to intervene to address depression, anxiety, and other sequelae and problematic behaviors that could result from social isolation. Schools, businesses, and the economy will again flourish after we get the virus behind us but controlling and eliminating this pandemic need to come first. Let’s keep our children home – to the extent that we can – until we move beyond this pandemic.
Dr. London has been a practicing psychiatrist for 4 decades and a newspaper columnist for almost as long. He has a private practice in New York and is author of “Find Freedom Fast: Short-Term Therapy That Works” (New York: Kettlehole Publishing, 2019). Dr. London has no conflicts of interest.
Parents, teachers, children, and adolescents are facing stress and anxiety as K-12 school districts across the country debate whether to return to in-person instruction amid the COVID-19 pandemic. As we approach the opening of schools, the stress and anxiety seem to be heightening.
According to Education Week, which is tracking the reopening plans of public schools across the United States, 21 of the 25 largest school districts are opting to implement remote learning only as their model. I would like to see all of those districts adopt that model until we understand more about this illness, and can prevent and treat it.
Yes, it’s true – I am a psychiatrist – not an infectious disease specialist. And I realize that the American Academy of Pediatrics and the Centers for Disease Control and Prevention have taken nuanced positions on this issue. Their positions make it clear that it is within a child’s best interests – from an educational and social point of view – to attend school in person. Not only is the classroom experience important, but so is the socialization and the exercise. However, when I look at the science on children who have been exposed to the coronavirus, I worry.
For example, a study by Lael M. Yonker, MD, and associates on pediatric SARS-CoV-2 found that the children in days 0-2 of illness have far higher viral loads than adults who have been hospitalized for severe disease. “This study reveals that children may be a potential source of contagion in the SARS-CoV-2 pandemic in spite of milder disease or lack of symptoms, and immune dysregulation is implicated in severe post-infectious [multisystem inflammatory syndrome in children],” Dr. Yonker and associates wrote, referring to the illness associated with COVID-19 in children. Their study was published recently in the Journal of Pediatrics (2020 Aug 19. doi: 10.1016/j.jpeds.2020.08.037).
In my state, where positivity rates are fairly low, Gov. Andrew Cuomo admitted in an interview recently that sending children to school in New York City is a “tricky proposition.” At this point, New York City public schools are scheduled to open in mid-September using a hybrid mixture of in-person and remote learning.
And look at what happened several weeks ago in Israel, where schools reopened after the virus was beaten back. At one high school in Jerusalem, just days after the reopening, the virus spread so prolifically to students, teachers, and relatives that the schools had to be closed again. Other countries should not follow Israel’s example, Eli Weizmann, who chairs the team advising Israel’s National Security Council on the pandemic, reportedly told the New York Times. “It was a major failure.”
But I must be honest: I was worried about children returning to school before I heard about the study by Dr. Yonker and associates, Gov. Cuomo’s comments, and what happened in Israel. So far, here in the Northeast, particularly in New York, New Jersey, and Connecticut, we have managed to get COVID-19 under control. Perhaps, in this part of the country, opening classroom education might be feasible – with close monitoring and proper precautions.
But COVID-19 has taken the lives of hundreds of thousands of Americans – more than 176,000 as of this writing. A new model from the University of Washington’s Institute for Health Metrics and Evaluation projects that COVID-19 could lead to more than 300,000 U.S. deaths by Dec. 1. Thankfully, the number of COVID-19–positive children who have died has been low. But they could still pass on the virus to adults.
To get a better understanding of COVID-19, I spoke with Sheryl L. Wulkan, MD, an internist and expert in personal protective equipment (PPE) who has consulted for numerous health care agencies about these issues. Dr. Wulkan said that, in some areas with low infection rates, school openings might be appropriate. However, she said, without proper testing and contact tracing, we are at a loss of controlling the spread.
What we should tell patients, family, and friends
From a psychiatric point of view, how should we advise our patients, family, and friends about sending their children back to school? Is on-site learning better than remote learning? It is. Do our children need the socialization that a school brings? Yes, they do.
Socialization and relating to peers are, indeed, important, but today’s children socialize in many ways beyond attending school – and they have peer friendships and interactions with electronic devices at their disposal.
Can remote learning cause social isolation – an isolation so profound that school is necessary not only for learning but the psyche as well? A meta-analysis of 80 studies that looked at the impact of social isolation and loneliness on adolescents and children who were previously healthy found that the young people “are probably more likely to experience high rates of depression and probably anxiety during and after enforced isolation ends. This may increase as enforced isolation continues,” wrote Maria Elizabeth Loades, PhD, and associates (J Am Acad Child Adolesc Psychiatry. 2020 Jun 3. S0890-8567[20]30337-3).
I am concerned about young people who experience anxiety and depression, and agree with Dr. Loades that we mental health professionals need to be ready to intervene early and provide preventive support. To do this, we should encourage parents to keep us informed about how their children are doing.
So my advice is that, in the absence of a vaccine and an effective treatment like we have for influenza – such as Tamiflu – and effective testing, such the saliva-based test developed by Yale University researchers, if I had school-aged children, I would continue to keep them home from school. Ultimately, however, parents must look at the science and make their decisions based on that. My children are adults with their own children, and only they can make informed decisions about which options are best for their families.
Interestingly, Sanjay Gupta, MD, the neurosurgeon who works as chief medical correspondent of CNN, recently discussed the thought process he and his wife used to determine whether their daughters would return to the classroom. After weighing many factors, including the viral spread in Fulton County, Ga., where they live, the Guptas decided that, at this time, the risks of allowing the girls to return to the classroom outweigh the benefits. “This was not an easy decision, but one that we believe best respects the science, decreases the risk of further spread, and follows the task force criteria,” wrote Dr. Gupta, who is affiliated with Emory University in Atlanta. “After 2 weeks, we will reassess.”
I understand that parents worry about the social and psychological costs of remote learning. And I can only imagine the difficulty of those who must balance homeschooling with working. And frankly, remote learning is not an option for all students. For those less fortunate, substantial governmental aid is important to assist these people and to keep them safe and on their feet until this pandemic is done. Also, those who were under the care of a psychiatrist should continue to receive care during the pandemic. We must be prepared to step in with interventions that can address the suffering that is inevitable, such as the use of targeted cognitive-behavioral therapy.
Public TV as an educational tool
Families with Internet access and those without it could benefit from using public television as a tool.
I would advise educators and the entertainment industry to harness the wonder of TV to develop curricula that can be used to educate children. As we know, Sesame Street proved to be an effective early childhood intervention, particularly for boys (Am Econ J: Applied Economics. 2019;11[1]:318-50). I would like to see programming that goes beyond Sesame Street. Learning from watching this kind of programming would be no substitute for engaging with teachers in real, live classrooms, however.
Children and adolescents will be changed by learning remotely. They will miss their friends, teachers, and other staff members, but their lives will not be ruined. Mental health professionals should be prepared to intervene to address depression, anxiety, and other sequelae and problematic behaviors that could result from social isolation. Schools, businesses, and the economy will again flourish after we get the virus behind us but controlling and eliminating this pandemic need to come first. Let’s keep our children home – to the extent that we can – until we move beyond this pandemic.
Dr. London has been a practicing psychiatrist for 4 decades and a newspaper columnist for almost as long. He has a private practice in New York and is author of “Find Freedom Fast: Short-Term Therapy That Works” (New York: Kettlehole Publishing, 2019). Dr. London has no conflicts of interest.