User login
What Should Be Prioritized in Managing Early Diabetes?
ORLANDO, FLORIDA — What to prioritize first in managing early diabetes? That was the question debated on an expert panel at the American Diabetes Association (ADA) 84th Scientific Sessions, with impassioned responses ranging from a plea to “treat obesity first,” to a James Carville–inspired counterpoint of “it’s the glucose, stupid.”
With a focus on preventing complications and inducing remission rounding out the four positions argued,
“In clinical decision-making [for early diabetes], we are faced with weighing each of these variables for the individual patient, and while all are good options, strong arguments can be made for prioritizing each — with the potential of each choice to influence or improve all of the others,” Dr. Retnakaran told this news organization.
Which to Prioritize First?
Making the obesity first argument, Ania M. Jastreboff, MD, PhD, associate professor and director of the Yale Obesity Research Center at Yale School of Medicine, New Haven, Connecticut, noted the striking statistic that nearly 90% of people with type 2 diabetes have overweight or obesity and discussed the ever-expanding data showing the benefits of drugs including glucagon-like peptide 1 (GLP-1) receptor agonists not just in weight loss but also in kidney, cardiovascular, and, as presented at the meeting, sleep apnea improvement.
She contrasted the experiences of two patients with obesity: One treated for the obesity upon type 2 diagnosis — who had a quick normalization of lipids and hypertension soon after the obesity treatment — and the other presenting after 10 years with type 2 diabetes — who was on therapy for hypertension and hyperlipidemia but not for obesity and whose diseases were not as easily treated by that point.
“Why are we treating all the downstream effects and we’re not treating the disease that is potentially the root cause of all these other diseases?” Dr. Jastreboff said.
Complications?
Arguing in favor of focusing on complications, Roopa Mehta, MD, PhD, with the department of endocrinology and metabolism at Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INCMNSZ), Mexico City, made the case that stakes don’t get any higher in diabetes than when it comes the looming threat of potentially fatal complications.
Acute myocardial infarction, stroke, amputation, and end-stage renal disease are all on the list of unwanted outcomes and need to be considered even in the earliest stages, as data show early onset type 2 diabetes is linked to life expectancy.
“The main goal of management has always been to prevent complications,” she noted. Citing ADA guidelines, Dr. Mehta underscored the benefits of first- and second-line therapy of metformin, sodium-glucose cotransporter 2 (SGLT2) inhibitors, and GLP-1 receptor agonists for most patients.
Remission?
Discussing the priority of putting patients into disease remission, Roy Taylor, MD, professor of medicine and metabolism at Newcastle University and Newcastle Hospitals NHS in Newcastle upon Tyne, England, and author of the book Life Without Diabetes, focused on an evidence-based alternative to achieving remission — a nonpharmacologic approach that avoids costly and sometimes inaccessible drugs.
In the intervention, described in the DiRECT randomized trial and subsequently in the UK National Health Service Type 2 Diabetes Path to Remission Program, patients with overweight or obesity were placed on a highly restrictive diet of just 800-900 calories a day for 12-20 weeks, followed by maintenance for 12 months, and they not only achieved weight loss but also achieved diabetes remission, in some cases long term.
Acknowledging that “this is not for everyone,” Dr. Taylor asserted that “we have to realize there is a substantial minority of people who want to be healthy but who don’t want to be medicalized,” he said.
“They want their health, and they can do extremely well.”
Glucose?
In taking his self-titled “it’s the glucose, stupid” stand, David M. Nathan, MD, of the Diabetes Center, Massachusetts General Hospital, Harvard Medical School, in Boston, cited extensive evidence showing that early intensive blood glucose control with treatment including sulfonylureas, insulin, or metformin significantly reduced the risk for complications in type 2 diabetes 15 or more years later, including renal failure, blindness, amputation, and myocardial infarctions, in addition to a reduction in diabetes-related death.
“In many of these studies, you saw the benefit even in the setting of weight-gain,” Dr. Nathan underscored.
He further noted the “sobering” findings of the Look AHEAD study, which had to be stopped due to futility when an intensive lifestyle/weight loss intervention showed no significant benefits in terms of cardiovascular disease in people with type 2 diabetes at a median follow-up of 9.6 years.
Ultimately, “diabetes, type 1 and type 2, remains a gluco-centric disease,” Dr. Nathan asserted. “Hyperglycemia is the only universal link between all forms of diabetes and mortality, and the long-term complications of diabetes are intimately associated with hyperglycemia.”
Tackling the Caveats
The ensuing panel discussion did not fail to deliver in delving into key areas of contention, particularly in terms of GLP-1 treatment.
Regarding a lack of data on the potential long-term effects of GLP-1s: “Yes, there are a huge number of studies [on GLP-1 receptor agonists], but they are, in general, over short periods of time and driven by pharma, who get in and get out as quickly as they can and have little in the way of interest to do comparative effectiveness studies,” Dr. Nathan argued.
“Meanwhile, this is like the crack cocaine of medications — patients have to stay on it for a lifetime or they will regain the weight — are you concerned at all about a lifetime of exposure to GLP-1 [drugs]?” he asked the panel.
Dr. Jastreboff responded that the first GLP-1 receptor agonist medications were approved in 2005, nearly 20 years ago, by the US Food and Drug Administration.
“Do I think we need long-term lifetime data? Absolutely,” she said. “We need to do our due diligence, we need to be careful, we need to monitor patients, and when and if there are signals, we need to follow them.”
What about the notorious gastrointestinal side effects of the drugs? “A majority of them are mitigated by slow up-titration,” Dr. Jastreboff noted.
“If patients have nausea, I do not go up [in dose]. I invite patients to tell me if they’re having vomiting because I don’t want anybody to have it, and I can count on one hand how many of my patients do.”
Dr. Mehta added the concern that as the drugs’ popularity soars, “a lot of doctors don’t know when they need to put the brakes on [weight coming off too quickly].”
She underscored that “we are not treating obesity for weight loss or for cosmetic reasons — this is about optimizing health.”
Dr. Jastreboff noted that in her practice, “I down-titrate if they’re losing weight too quickly.”
“If the patient is losing more than 1% per week of their body weight, then I slow down to make sure they’re getting the nutrients that they need, that they have enough energy to exercise, and that they’re prioritizing protein and fruits and vegetables in their diet.
“We just need to go slow, and yes, we need to follow them long term,” she said.
Chiming in from the audience, Julio Rosenstock, MD, a recognized thought leader in type 2 diabetes, offered his own take on the issues, describing Dr. Taylor’s very low–calorie diet suggestion as “not realistic” and Dr. Nathan’s glucose-first argument to be “stuck in the past.”
Based on modern-day evidence, “there is no reason on earth to start [diabetes treatment] with only metformin,” asserted Dr. Rosenstock, director of the Velocity Clinical Research center at Medical City and clinical professor of medicine at the University of Texas Southwestern Medical Center, Dallas.
“We need to start at the very least with metformin and a sodium-glucose cotransporter 2 (SGLT2) inhibitor from day 1, and then, if it’s affordable and there is access, with a GLP-1 receptor agonist,” he said.
“There is nothing better these days than those agents that consistently have shown a reduction of cardiovascular events and slowing of kidney disease progression.”
Overall, however, “I think you are all right,” he added, a sentiment shared by most.
Noting that the discussion as a whole represents a virtual sea change from the evidence-based options that would have been discussed only a decade ago, Dr. Retnakaran summed up his take-home message: “Stay tuned.
“You could easily see things changing in the next decade to come as we get more data and evidence to support what we ultimately should prioritize an early type 2 diabetes, so this is an exciting time.”
Dr. Retnakaran disclosed ties with Novo Nordisk, Boehringer Ingelheim, Novartis, Sanofi, and Eli Lilly. Dr. Jastreboff disclosed ties with Amgen, AstraZeneca, Boehringer Ingelheim, Biohaven, Eli Lilly, Intellihealth, Novo Nordisk, Pfizer, Regeneron, Scholar Rock, Structure Therapeutics, Terms Pharmaceutical, Weight Watchers, and Zealand Pharmaceuticals. Dr. Roopa had relationships with Novo Nordisk, Boehringer Ingelheim, Amgen, AstraZeneca, Eli Lilly, Silanes, and Sanofi. Dr. Taylor received lecture fees from Novartis, Lilly, Abbott, and Nestle Health and research funding from Diabetes UK and is an advisor to Fast800. Dr. Rosenstock reported relationships with Applied Therapeutics, AstraZeneca, Biomea Fusion, Boehringer Ingelheim, Eli Lilly and Company, Hanmi, Merck, Oramed, Structure Therapeutics, Novartis, Novo Nordisk, Pfizer, Ragor, and Sanofi. Dr. Nathan had no disclosures to report.
A version of this article first appeared on Medscape.com.
ORLANDO, FLORIDA — What to prioritize first in managing early diabetes? That was the question debated on an expert panel at the American Diabetes Association (ADA) 84th Scientific Sessions, with impassioned responses ranging from a plea to “treat obesity first,” to a James Carville–inspired counterpoint of “it’s the glucose, stupid.”
With a focus on preventing complications and inducing remission rounding out the four positions argued,
“In clinical decision-making [for early diabetes], we are faced with weighing each of these variables for the individual patient, and while all are good options, strong arguments can be made for prioritizing each — with the potential of each choice to influence or improve all of the others,” Dr. Retnakaran told this news organization.
Which to Prioritize First?
Making the obesity first argument, Ania M. Jastreboff, MD, PhD, associate professor and director of the Yale Obesity Research Center at Yale School of Medicine, New Haven, Connecticut, noted the striking statistic that nearly 90% of people with type 2 diabetes have overweight or obesity and discussed the ever-expanding data showing the benefits of drugs including glucagon-like peptide 1 (GLP-1) receptor agonists not just in weight loss but also in kidney, cardiovascular, and, as presented at the meeting, sleep apnea improvement.
She contrasted the experiences of two patients with obesity: One treated for the obesity upon type 2 diagnosis — who had a quick normalization of lipids and hypertension soon after the obesity treatment — and the other presenting after 10 years with type 2 diabetes — who was on therapy for hypertension and hyperlipidemia but not for obesity and whose diseases were not as easily treated by that point.
“Why are we treating all the downstream effects and we’re not treating the disease that is potentially the root cause of all these other diseases?” Dr. Jastreboff said.
Complications?
Arguing in favor of focusing on complications, Roopa Mehta, MD, PhD, with the department of endocrinology and metabolism at Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INCMNSZ), Mexico City, made the case that stakes don’t get any higher in diabetes than when it comes the looming threat of potentially fatal complications.
Acute myocardial infarction, stroke, amputation, and end-stage renal disease are all on the list of unwanted outcomes and need to be considered even in the earliest stages, as data show early onset type 2 diabetes is linked to life expectancy.
“The main goal of management has always been to prevent complications,” she noted. Citing ADA guidelines, Dr. Mehta underscored the benefits of first- and second-line therapy of metformin, sodium-glucose cotransporter 2 (SGLT2) inhibitors, and GLP-1 receptor agonists for most patients.
Remission?
Discussing the priority of putting patients into disease remission, Roy Taylor, MD, professor of medicine and metabolism at Newcastle University and Newcastle Hospitals NHS in Newcastle upon Tyne, England, and author of the book Life Without Diabetes, focused on an evidence-based alternative to achieving remission — a nonpharmacologic approach that avoids costly and sometimes inaccessible drugs.
In the intervention, described in the DiRECT randomized trial and subsequently in the UK National Health Service Type 2 Diabetes Path to Remission Program, patients with overweight or obesity were placed on a highly restrictive diet of just 800-900 calories a day for 12-20 weeks, followed by maintenance for 12 months, and they not only achieved weight loss but also achieved diabetes remission, in some cases long term.
Acknowledging that “this is not for everyone,” Dr. Taylor asserted that “we have to realize there is a substantial minority of people who want to be healthy but who don’t want to be medicalized,” he said.
“They want their health, and they can do extremely well.”
Glucose?
In taking his self-titled “it’s the glucose, stupid” stand, David M. Nathan, MD, of the Diabetes Center, Massachusetts General Hospital, Harvard Medical School, in Boston, cited extensive evidence showing that early intensive blood glucose control with treatment including sulfonylureas, insulin, or metformin significantly reduced the risk for complications in type 2 diabetes 15 or more years later, including renal failure, blindness, amputation, and myocardial infarctions, in addition to a reduction in diabetes-related death.
“In many of these studies, you saw the benefit even in the setting of weight-gain,” Dr. Nathan underscored.
He further noted the “sobering” findings of the Look AHEAD study, which had to be stopped due to futility when an intensive lifestyle/weight loss intervention showed no significant benefits in terms of cardiovascular disease in people with type 2 diabetes at a median follow-up of 9.6 years.
Ultimately, “diabetes, type 1 and type 2, remains a gluco-centric disease,” Dr. Nathan asserted. “Hyperglycemia is the only universal link between all forms of diabetes and mortality, and the long-term complications of diabetes are intimately associated with hyperglycemia.”
Tackling the Caveats
The ensuing panel discussion did not fail to deliver in delving into key areas of contention, particularly in terms of GLP-1 treatment.
Regarding a lack of data on the potential long-term effects of GLP-1s: “Yes, there are a huge number of studies [on GLP-1 receptor agonists], but they are, in general, over short periods of time and driven by pharma, who get in and get out as quickly as they can and have little in the way of interest to do comparative effectiveness studies,” Dr. Nathan argued.
“Meanwhile, this is like the crack cocaine of medications — patients have to stay on it for a lifetime or they will regain the weight — are you concerned at all about a lifetime of exposure to GLP-1 [drugs]?” he asked the panel.
Dr. Jastreboff responded that the first GLP-1 receptor agonist medications were approved in 2005, nearly 20 years ago, by the US Food and Drug Administration.
“Do I think we need long-term lifetime data? Absolutely,” she said. “We need to do our due diligence, we need to be careful, we need to monitor patients, and when and if there are signals, we need to follow them.”
What about the notorious gastrointestinal side effects of the drugs? “A majority of them are mitigated by slow up-titration,” Dr. Jastreboff noted.
“If patients have nausea, I do not go up [in dose]. I invite patients to tell me if they’re having vomiting because I don’t want anybody to have it, and I can count on one hand how many of my patients do.”
Dr. Mehta added the concern that as the drugs’ popularity soars, “a lot of doctors don’t know when they need to put the brakes on [weight coming off too quickly].”
She underscored that “we are not treating obesity for weight loss or for cosmetic reasons — this is about optimizing health.”
Dr. Jastreboff noted that in her practice, “I down-titrate if they’re losing weight too quickly.”
“If the patient is losing more than 1% per week of their body weight, then I slow down to make sure they’re getting the nutrients that they need, that they have enough energy to exercise, and that they’re prioritizing protein and fruits and vegetables in their diet.
“We just need to go slow, and yes, we need to follow them long term,” she said.
Chiming in from the audience, Julio Rosenstock, MD, a recognized thought leader in type 2 diabetes, offered his own take on the issues, describing Dr. Taylor’s very low–calorie diet suggestion as “not realistic” and Dr. Nathan’s glucose-first argument to be “stuck in the past.”
Based on modern-day evidence, “there is no reason on earth to start [diabetes treatment] with only metformin,” asserted Dr. Rosenstock, director of the Velocity Clinical Research center at Medical City and clinical professor of medicine at the University of Texas Southwestern Medical Center, Dallas.
“We need to start at the very least with metformin and a sodium-glucose cotransporter 2 (SGLT2) inhibitor from day 1, and then, if it’s affordable and there is access, with a GLP-1 receptor agonist,” he said.
“There is nothing better these days than those agents that consistently have shown a reduction of cardiovascular events and slowing of kidney disease progression.”
Overall, however, “I think you are all right,” he added, a sentiment shared by most.
Noting that the discussion as a whole represents a virtual sea change from the evidence-based options that would have been discussed only a decade ago, Dr. Retnakaran summed up his take-home message: “Stay tuned.
“You could easily see things changing in the next decade to come as we get more data and evidence to support what we ultimately should prioritize an early type 2 diabetes, so this is an exciting time.”
Dr. Retnakaran disclosed ties with Novo Nordisk, Boehringer Ingelheim, Novartis, Sanofi, and Eli Lilly. Dr. Jastreboff disclosed ties with Amgen, AstraZeneca, Boehringer Ingelheim, Biohaven, Eli Lilly, Intellihealth, Novo Nordisk, Pfizer, Regeneron, Scholar Rock, Structure Therapeutics, Terms Pharmaceutical, Weight Watchers, and Zealand Pharmaceuticals. Dr. Roopa had relationships with Novo Nordisk, Boehringer Ingelheim, Amgen, AstraZeneca, Eli Lilly, Silanes, and Sanofi. Dr. Taylor received lecture fees from Novartis, Lilly, Abbott, and Nestle Health and research funding from Diabetes UK and is an advisor to Fast800. Dr. Rosenstock reported relationships with Applied Therapeutics, AstraZeneca, Biomea Fusion, Boehringer Ingelheim, Eli Lilly and Company, Hanmi, Merck, Oramed, Structure Therapeutics, Novartis, Novo Nordisk, Pfizer, Ragor, and Sanofi. Dr. Nathan had no disclosures to report.
A version of this article first appeared on Medscape.com.
ORLANDO, FLORIDA — What to prioritize first in managing early diabetes? That was the question debated on an expert panel at the American Diabetes Association (ADA) 84th Scientific Sessions, with impassioned responses ranging from a plea to “treat obesity first,” to a James Carville–inspired counterpoint of “it’s the glucose, stupid.”
With a focus on preventing complications and inducing remission rounding out the four positions argued,
“In clinical decision-making [for early diabetes], we are faced with weighing each of these variables for the individual patient, and while all are good options, strong arguments can be made for prioritizing each — with the potential of each choice to influence or improve all of the others,” Dr. Retnakaran told this news organization.
Which to Prioritize First?
Making the obesity first argument, Ania M. Jastreboff, MD, PhD, associate professor and director of the Yale Obesity Research Center at Yale School of Medicine, New Haven, Connecticut, noted the striking statistic that nearly 90% of people with type 2 diabetes have overweight or obesity and discussed the ever-expanding data showing the benefits of drugs including glucagon-like peptide 1 (GLP-1) receptor agonists not just in weight loss but also in kidney, cardiovascular, and, as presented at the meeting, sleep apnea improvement.
She contrasted the experiences of two patients with obesity: One treated for the obesity upon type 2 diagnosis — who had a quick normalization of lipids and hypertension soon after the obesity treatment — and the other presenting after 10 years with type 2 diabetes — who was on therapy for hypertension and hyperlipidemia but not for obesity and whose diseases were not as easily treated by that point.
“Why are we treating all the downstream effects and we’re not treating the disease that is potentially the root cause of all these other diseases?” Dr. Jastreboff said.
Complications?
Arguing in favor of focusing on complications, Roopa Mehta, MD, PhD, with the department of endocrinology and metabolism at Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INCMNSZ), Mexico City, made the case that stakes don’t get any higher in diabetes than when it comes the looming threat of potentially fatal complications.
Acute myocardial infarction, stroke, amputation, and end-stage renal disease are all on the list of unwanted outcomes and need to be considered even in the earliest stages, as data show early onset type 2 diabetes is linked to life expectancy.
“The main goal of management has always been to prevent complications,” she noted. Citing ADA guidelines, Dr. Mehta underscored the benefits of first- and second-line therapy of metformin, sodium-glucose cotransporter 2 (SGLT2) inhibitors, and GLP-1 receptor agonists for most patients.
Remission?
Discussing the priority of putting patients into disease remission, Roy Taylor, MD, professor of medicine and metabolism at Newcastle University and Newcastle Hospitals NHS in Newcastle upon Tyne, England, and author of the book Life Without Diabetes, focused on an evidence-based alternative to achieving remission — a nonpharmacologic approach that avoids costly and sometimes inaccessible drugs.
In the intervention, described in the DiRECT randomized trial and subsequently in the UK National Health Service Type 2 Diabetes Path to Remission Program, patients with overweight or obesity were placed on a highly restrictive diet of just 800-900 calories a day for 12-20 weeks, followed by maintenance for 12 months, and they not only achieved weight loss but also achieved diabetes remission, in some cases long term.
Acknowledging that “this is not for everyone,” Dr. Taylor asserted that “we have to realize there is a substantial minority of people who want to be healthy but who don’t want to be medicalized,” he said.
“They want their health, and they can do extremely well.”
Glucose?
In taking his self-titled “it’s the glucose, stupid” stand, David M. Nathan, MD, of the Diabetes Center, Massachusetts General Hospital, Harvard Medical School, in Boston, cited extensive evidence showing that early intensive blood glucose control with treatment including sulfonylureas, insulin, or metformin significantly reduced the risk for complications in type 2 diabetes 15 or more years later, including renal failure, blindness, amputation, and myocardial infarctions, in addition to a reduction in diabetes-related death.
“In many of these studies, you saw the benefit even in the setting of weight-gain,” Dr. Nathan underscored.
He further noted the “sobering” findings of the Look AHEAD study, which had to be stopped due to futility when an intensive lifestyle/weight loss intervention showed no significant benefits in terms of cardiovascular disease in people with type 2 diabetes at a median follow-up of 9.6 years.
Ultimately, “diabetes, type 1 and type 2, remains a gluco-centric disease,” Dr. Nathan asserted. “Hyperglycemia is the only universal link between all forms of diabetes and mortality, and the long-term complications of diabetes are intimately associated with hyperglycemia.”
Tackling the Caveats
The ensuing panel discussion did not fail to deliver in delving into key areas of contention, particularly in terms of GLP-1 treatment.
Regarding a lack of data on the potential long-term effects of GLP-1s: “Yes, there are a huge number of studies [on GLP-1 receptor agonists], but they are, in general, over short periods of time and driven by pharma, who get in and get out as quickly as they can and have little in the way of interest to do comparative effectiveness studies,” Dr. Nathan argued.
“Meanwhile, this is like the crack cocaine of medications — patients have to stay on it for a lifetime or they will regain the weight — are you concerned at all about a lifetime of exposure to GLP-1 [drugs]?” he asked the panel.
Dr. Jastreboff responded that the first GLP-1 receptor agonist medications were approved in 2005, nearly 20 years ago, by the US Food and Drug Administration.
“Do I think we need long-term lifetime data? Absolutely,” she said. “We need to do our due diligence, we need to be careful, we need to monitor patients, and when and if there are signals, we need to follow them.”
What about the notorious gastrointestinal side effects of the drugs? “A majority of them are mitigated by slow up-titration,” Dr. Jastreboff noted.
“If patients have nausea, I do not go up [in dose]. I invite patients to tell me if they’re having vomiting because I don’t want anybody to have it, and I can count on one hand how many of my patients do.”
Dr. Mehta added the concern that as the drugs’ popularity soars, “a lot of doctors don’t know when they need to put the brakes on [weight coming off too quickly].”
She underscored that “we are not treating obesity for weight loss or for cosmetic reasons — this is about optimizing health.”
Dr. Jastreboff noted that in her practice, “I down-titrate if they’re losing weight too quickly.”
“If the patient is losing more than 1% per week of their body weight, then I slow down to make sure they’re getting the nutrients that they need, that they have enough energy to exercise, and that they’re prioritizing protein and fruits and vegetables in their diet.
“We just need to go slow, and yes, we need to follow them long term,” she said.
Chiming in from the audience, Julio Rosenstock, MD, a recognized thought leader in type 2 diabetes, offered his own take on the issues, describing Dr. Taylor’s very low–calorie diet suggestion as “not realistic” and Dr. Nathan’s glucose-first argument to be “stuck in the past.”
Based on modern-day evidence, “there is no reason on earth to start [diabetes treatment] with only metformin,” asserted Dr. Rosenstock, director of the Velocity Clinical Research center at Medical City and clinical professor of medicine at the University of Texas Southwestern Medical Center, Dallas.
“We need to start at the very least with metformin and a sodium-glucose cotransporter 2 (SGLT2) inhibitor from day 1, and then, if it’s affordable and there is access, with a GLP-1 receptor agonist,” he said.
“There is nothing better these days than those agents that consistently have shown a reduction of cardiovascular events and slowing of kidney disease progression.”
Overall, however, “I think you are all right,” he added, a sentiment shared by most.
Noting that the discussion as a whole represents a virtual sea change from the evidence-based options that would have been discussed only a decade ago, Dr. Retnakaran summed up his take-home message: “Stay tuned.
“You could easily see things changing in the next decade to come as we get more data and evidence to support what we ultimately should prioritize an early type 2 diabetes, so this is an exciting time.”
Dr. Retnakaran disclosed ties with Novo Nordisk, Boehringer Ingelheim, Novartis, Sanofi, and Eli Lilly. Dr. Jastreboff disclosed ties with Amgen, AstraZeneca, Boehringer Ingelheim, Biohaven, Eli Lilly, Intellihealth, Novo Nordisk, Pfizer, Regeneron, Scholar Rock, Structure Therapeutics, Terms Pharmaceutical, Weight Watchers, and Zealand Pharmaceuticals. Dr. Roopa had relationships with Novo Nordisk, Boehringer Ingelheim, Amgen, AstraZeneca, Eli Lilly, Silanes, and Sanofi. Dr. Taylor received lecture fees from Novartis, Lilly, Abbott, and Nestle Health and research funding from Diabetes UK and is an advisor to Fast800. Dr. Rosenstock reported relationships with Applied Therapeutics, AstraZeneca, Biomea Fusion, Boehringer Ingelheim, Eli Lilly and Company, Hanmi, Merck, Oramed, Structure Therapeutics, Novartis, Novo Nordisk, Pfizer, Ragor, and Sanofi. Dr. Nathan had no disclosures to report.
A version of this article first appeared on Medscape.com.
Triple Therapy May Be Effective in Drug-Naive T2D
TOPLINE:
A triple combination therapy (TCT) of metformin, dapagliflozin, and saxagliptin is an effective and safe treatment option for drug-naive patients with type 2 diabetes (T2D) compared with stepwise add-on therapy.
METHODOLOGY:
- Current guidelines recommend early combination therapy to extend the time to treatment failure, reduce the risk for diabetic complications, and prevent clinical inertia in patients with T2D.
- This randomized controlled open-label trial conducted at nine sites in South Korea included 105 drug-naive patients with T2D (mean age, 49.5 years; 32.4% women) who either received triple therapy (metformin, dapagliflozin, and saxagliptin) or stepwise add-on therapy (initiated with metformin, followed by glimepiride and sitagliptin for those with baseline hemoglobin A1c levels < 9.0% or with initial dual metformin and glimepiride in those with A1c levels ≥ 9.0% followed by sitagliptin).
- The primary outcome was the proportion of patients who achieved A1c levels < 6.5% without hypoglycemia, weight gain ≥ 5%, or discontinuation of drugs because of adverse events at week 104.
- The secondary outcomes were the proportion of patients whose A1c levels dropped to < 7.0% at weeks 56 and 104 and dropped to < 6.5% at week 56, all without hypoglycemia, weight gain, nor discontinuation due to adverse events.
TAKEAWAY:
- At week 104, a higher proportion of patients in the triple therapy group achieved the primary outcome than those in the stepwise add-on therapy group (39.0% vs 17.1%; P = .027).
- In both groups, a similar proportion of patients (46.3%) achieved A1c levels < 6.5% at week 104, but the proportion of patients without hypoglycemia, weight gain, or discontinuation because of adverse events was higher in the triple therapy group than those in the stepwise add-on therapy group (83.3% vs 38.0%; P < .001).
IN PRACTICE:
The authors wrote: “Although the glycemic efficacy of each drug in the TCT was modest, the combination of these drugs resulted in a 2-year durable glycemic efficacy, with greater than a 2.5% reduction in A1c levels from baseline. The overall results of this study suggest a novel strategy for initial combination therapy in newly diagnosed T2D patients.”
SOURCE:
The study was led by Nam Hoon Kim, MD, of the Department of Internal Medicine, Korea University College of Medicine, Seoul. It was published online in Diabetes, Obesity and Metabolism.
LIMITATIONS:
The study had a relatively small sample size as compared with previous clinical trials. More people in the standard therapy group had A1c levels ≥ 9.0%, which resulted in more than double the number of people receiving dual combination therapy over monotherapy in that group. The trial duration was insufficient to evaluate the cardiovascular outcomes.
DISCLOSURES:
The study was funded by AstraZeneca. Some authors reported financial ties with AstraZeneca and other pharmaceutical and medical device companies as members of advisory boards or recipients of grants, consulting fees, honoraria, or lecture fees.
A version of this article appeared on Medscape.com.
TOPLINE:
A triple combination therapy (TCT) of metformin, dapagliflozin, and saxagliptin is an effective and safe treatment option for drug-naive patients with type 2 diabetes (T2D) compared with stepwise add-on therapy.
METHODOLOGY:
- Current guidelines recommend early combination therapy to extend the time to treatment failure, reduce the risk for diabetic complications, and prevent clinical inertia in patients with T2D.
- This randomized controlled open-label trial conducted at nine sites in South Korea included 105 drug-naive patients with T2D (mean age, 49.5 years; 32.4% women) who either received triple therapy (metformin, dapagliflozin, and saxagliptin) or stepwise add-on therapy (initiated with metformin, followed by glimepiride and sitagliptin for those with baseline hemoglobin A1c levels < 9.0% or with initial dual metformin and glimepiride in those with A1c levels ≥ 9.0% followed by sitagliptin).
- The primary outcome was the proportion of patients who achieved A1c levels < 6.5% without hypoglycemia, weight gain ≥ 5%, or discontinuation of drugs because of adverse events at week 104.
- The secondary outcomes were the proportion of patients whose A1c levels dropped to < 7.0% at weeks 56 and 104 and dropped to < 6.5% at week 56, all without hypoglycemia, weight gain, nor discontinuation due to adverse events.
TAKEAWAY:
- At week 104, a higher proportion of patients in the triple therapy group achieved the primary outcome than those in the stepwise add-on therapy group (39.0% vs 17.1%; P = .027).
- In both groups, a similar proportion of patients (46.3%) achieved A1c levels < 6.5% at week 104, but the proportion of patients without hypoglycemia, weight gain, or discontinuation because of adverse events was higher in the triple therapy group than those in the stepwise add-on therapy group (83.3% vs 38.0%; P < .001).
IN PRACTICE:
The authors wrote: “Although the glycemic efficacy of each drug in the TCT was modest, the combination of these drugs resulted in a 2-year durable glycemic efficacy, with greater than a 2.5% reduction in A1c levels from baseline. The overall results of this study suggest a novel strategy for initial combination therapy in newly diagnosed T2D patients.”
SOURCE:
The study was led by Nam Hoon Kim, MD, of the Department of Internal Medicine, Korea University College of Medicine, Seoul. It was published online in Diabetes, Obesity and Metabolism.
LIMITATIONS:
The study had a relatively small sample size as compared with previous clinical trials. More people in the standard therapy group had A1c levels ≥ 9.0%, which resulted in more than double the number of people receiving dual combination therapy over monotherapy in that group. The trial duration was insufficient to evaluate the cardiovascular outcomes.
DISCLOSURES:
The study was funded by AstraZeneca. Some authors reported financial ties with AstraZeneca and other pharmaceutical and medical device companies as members of advisory boards or recipients of grants, consulting fees, honoraria, or lecture fees.
A version of this article appeared on Medscape.com.
TOPLINE:
A triple combination therapy (TCT) of metformin, dapagliflozin, and saxagliptin is an effective and safe treatment option for drug-naive patients with type 2 diabetes (T2D) compared with stepwise add-on therapy.
METHODOLOGY:
- Current guidelines recommend early combination therapy to extend the time to treatment failure, reduce the risk for diabetic complications, and prevent clinical inertia in patients with T2D.
- This randomized controlled open-label trial conducted at nine sites in South Korea included 105 drug-naive patients with T2D (mean age, 49.5 years; 32.4% women) who either received triple therapy (metformin, dapagliflozin, and saxagliptin) or stepwise add-on therapy (initiated with metformin, followed by glimepiride and sitagliptin for those with baseline hemoglobin A1c levels < 9.0% or with initial dual metformin and glimepiride in those with A1c levels ≥ 9.0% followed by sitagliptin).
- The primary outcome was the proportion of patients who achieved A1c levels < 6.5% without hypoglycemia, weight gain ≥ 5%, or discontinuation of drugs because of adverse events at week 104.
- The secondary outcomes were the proportion of patients whose A1c levels dropped to < 7.0% at weeks 56 and 104 and dropped to < 6.5% at week 56, all without hypoglycemia, weight gain, nor discontinuation due to adverse events.
TAKEAWAY:
- At week 104, a higher proportion of patients in the triple therapy group achieved the primary outcome than those in the stepwise add-on therapy group (39.0% vs 17.1%; P = .027).
- In both groups, a similar proportion of patients (46.3%) achieved A1c levels < 6.5% at week 104, but the proportion of patients without hypoglycemia, weight gain, or discontinuation because of adverse events was higher in the triple therapy group than those in the stepwise add-on therapy group (83.3% vs 38.0%; P < .001).
IN PRACTICE:
The authors wrote: “Although the glycemic efficacy of each drug in the TCT was modest, the combination of these drugs resulted in a 2-year durable glycemic efficacy, with greater than a 2.5% reduction in A1c levels from baseline. The overall results of this study suggest a novel strategy for initial combination therapy in newly diagnosed T2D patients.”
SOURCE:
The study was led by Nam Hoon Kim, MD, of the Department of Internal Medicine, Korea University College of Medicine, Seoul. It was published online in Diabetes, Obesity and Metabolism.
LIMITATIONS:
The study had a relatively small sample size as compared with previous clinical trials. More people in the standard therapy group had A1c levels ≥ 9.0%, which resulted in more than double the number of people receiving dual combination therapy over monotherapy in that group. The trial duration was insufficient to evaluate the cardiovascular outcomes.
DISCLOSURES:
The study was funded by AstraZeneca. Some authors reported financial ties with AstraZeneca and other pharmaceutical and medical device companies as members of advisory boards or recipients of grants, consulting fees, honoraria, or lecture fees.
A version of this article appeared on Medscape.com.
Generational Differences in Isotretinoin Prescribing Habits: A Cross-Sectional Analysis
To the Editor:
Prescriptions for isotretinoin may be influenced by patient demographics, medical comorbidities, and drug safety programs.1,2 In 1982, isotretinoin was approved by the US Food and Drug Administration for treatment of severe recalcitrant nodulocystic acne that is nonresponsive to conventional therapies such as antibiotics; however, prescriber beliefs regarding the necessity of oral antibiotic failure before isotretinoin is prescribed may be influenced by the provider’s generational age.3 Currently, there is a knowledge gap regarding the impact of provider characteristics, including the year providers completed training, on isotretinoin utilization. The aim of our cross-sectional study was to characterize generational isotretinoin prescribing habits in a large-scale midwestern private practice dermatology group.
Modernizing Medicine (https://www.modmed.com), an electronic medical record software, was queried for all encounters that included both an International Classification of Diseases, Tenth Revision, Clinical Modification diagnosis code L70.0 (acne vulgaris) and a medication prescription from May 2021 to May 2022. Data were collected from a large private practice group with locations across the state of Ohio. Exclusion criteria included provider-patient prescription pairs that included non–acne medication prescriptions, patients seen by multiple providers, and providers who treated fewer than 5 patients with acne during the study period. A mixed-effect multiple logistic regression was performed to analyze whether a patient was ever prescribed isotretinoin, adjusting for individual prescriber, prescriber generation (millennial [1981–1996], Generation X [1965–1980], and baby boomer [1946–1964]),4 and patient sex; spironolactone and oral antibiotic prescriptions during the study period were included as additional covariates in a subsequent post hoc analysis. This study utilized data that was fully deidentified in accordance with the US Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule. Approval from an institutional review board was not required.
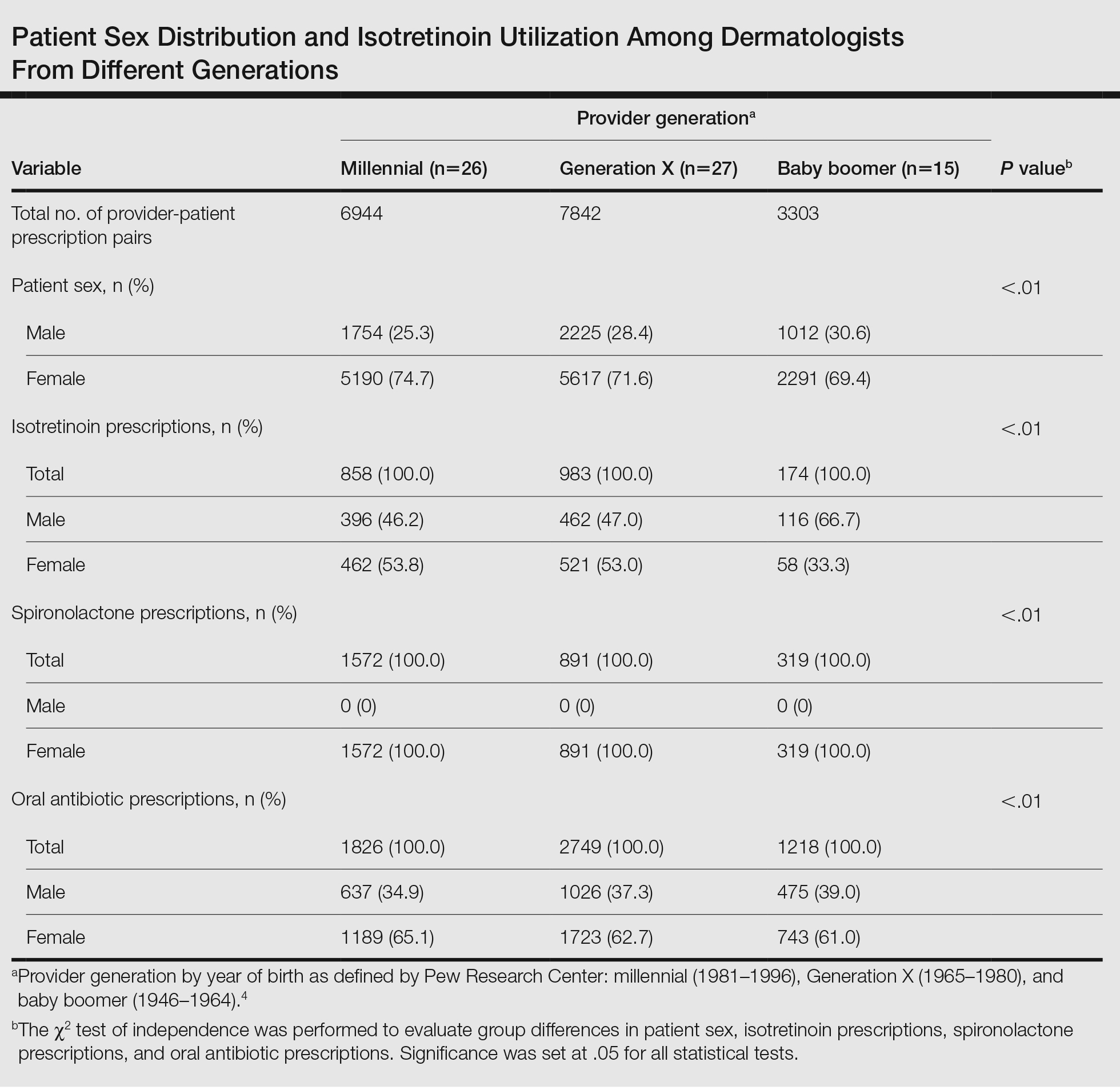
A total of 18,089 provider-patient prescription pairs were included in our analysis (Table). In our most robust model, female patients were significantly less likely to receive isotretinoin compared with male patients (adjusted OR [aOR], 0.394; P<.01). Millennial providers were significantly more likely to utilize isotretinoin in patients who did not receive antibiotics compared with patients who did receive antibiotics (aOR, 1.693; P<.01). When compared with both Generation X and baby boomers, millennial providers were more likely to prescribe isotretinoin in patients who received antibiotics (aOR, 2.227 [P=.02] and 3.638 [P<.01], respectively).
In 2018, the American Academy of Dermatology and the Global Alliance to Improve Outcomes in Acne updated thir guidelines to recommend isotretinoin as a first-line therapy for severe nodular acne, treatment-resistant moderate acne, or acne that produces scarring or psychosocial distress.5 Our study results suggest that millennial providers are adhering to these guidelines and readily prescribing isotretinoin in patients who did not receive antibiotics, which corroborates survey findings by Nagler and Orlow.3 Our results also revealed that prescriber generation may influence isotretinoin usage, with millennials utilizing isotretinoin more in patients who received oral antibiotic therapy than their older counterparts. In part, this may be due to beliefs among older generations that failure of oral antibiotics is necessary before pursuing isotretinoin.3 Additionally, this finding suggests that millennials, if utilizing antibiotics for acne, may have a lower threshold for starting isotretinoin in patients who received oral antibiotic therapy.
Generational prescribing variation appears not to be unique to isotretinoin and also may be present in the use of spironolactone. Over the past decade, utilization of spironolactone for acne treatment has increased, likely in response to new data demonstrating that routine use is safe and effective.6 Several large cohort and retrospective studies have debunked the historical concerns for tumorigenicity in those with breast cancer history as well as the need for routine laboratory monitoring for hyperkalemia.7,8 Although spironolactone use for the treatment of acne has increased, it still remains relatively underutilized,6 suggesting there may be a knowledge gap similar to that of isotretinoin, with younger generations utilizing spironolactone more readily than older generations.
Our study analyzed generational differences in isotretinoin utilization for acne over 1 calendar year. Limitations include sampling from a midwestern patient cohort and private practice–based providers. Due to limitations of our data set, we were unable to capture acne medication usage prior to May 2021, temporal sequencing of acne medication usage, and stratification of patients by acne severity. Furthermore, we were unable to capture female patients who were pregnant or planning pregnancy at the time of their encounter, which would exclude isotretinoin usage.
Overall, millennial providers may be utilizing isotretinoin more in line with the updated acne guidelines5 compared with providers from older generations. Further research is necessary to elucidate how these prescribing habits may change based on acne severity.
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22. doi:10.1001/jamadermatol.2019.3270
- Nagler AR, Orlow SJ. Dermatologists’ attitudes, prescription, and counseling patterns for isotretinoin: a questionnaire-based study. J Drugs Dermatol. 2015;14:184-189.
- Dimock M. Where Millennials end and Generation Z begins. Pew Research Center website. January 17, 2019. Accessed June 17, 2024. https://www.pewresearch.org/fact-tank/2019/01/17/where-millennials-end-and-generation-z-begins/
- Thiboutot DM, Dréno B, Abanmi A, et al. Practical management of acne for clinicians: an international consensus from the Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2018;78(2 suppl 1):S1-S23.e1. doi:10.1016/j.jaad.2017.09.078
- Guzman AK, Barbieri JS. Comparative analysis of prescribing patterns of tetracycline class antibiotics and spironolactone between advanced practice providers and physicians in the treatment of acne vulgaris. J Am Acad Dermatol. 2021;84:1119-1121. doi:10.1016/j.jaad.2020.06.044
- Wei C, Bovonratwet P, Gu A, et al. Spironolactone use does not increase the risk of female breast cancer recurrence: a retrospective analysis. J Am Acad Dermatol. 2020;83:1021-1027. doi:10.1016/j.jaad.2020.05.081
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944. doi:10.1001/jamadermatol.2015.34
To the Editor:
Prescriptions for isotretinoin may be influenced by patient demographics, medical comorbidities, and drug safety programs.1,2 In 1982, isotretinoin was approved by the US Food and Drug Administration for treatment of severe recalcitrant nodulocystic acne that is nonresponsive to conventional therapies such as antibiotics; however, prescriber beliefs regarding the necessity of oral antibiotic failure before isotretinoin is prescribed may be influenced by the provider’s generational age.3 Currently, there is a knowledge gap regarding the impact of provider characteristics, including the year providers completed training, on isotretinoin utilization. The aim of our cross-sectional study was to characterize generational isotretinoin prescribing habits in a large-scale midwestern private practice dermatology group.
Modernizing Medicine (https://www.modmed.com), an electronic medical record software, was queried for all encounters that included both an International Classification of Diseases, Tenth Revision, Clinical Modification diagnosis code L70.0 (acne vulgaris) and a medication prescription from May 2021 to May 2022. Data were collected from a large private practice group with locations across the state of Ohio. Exclusion criteria included provider-patient prescription pairs that included non–acne medication prescriptions, patients seen by multiple providers, and providers who treated fewer than 5 patients with acne during the study period. A mixed-effect multiple logistic regression was performed to analyze whether a patient was ever prescribed isotretinoin, adjusting for individual prescriber, prescriber generation (millennial [1981–1996], Generation X [1965–1980], and baby boomer [1946–1964]),4 and patient sex; spironolactone and oral antibiotic prescriptions during the study period were included as additional covariates in a subsequent post hoc analysis. This study utilized data that was fully deidentified in accordance with the US Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule. Approval from an institutional review board was not required.
A total of 18,089 provider-patient prescription pairs were included in our analysis (Table). In our most robust model, female patients were significantly less likely to receive isotretinoin compared with male patients (adjusted OR [aOR], 0.394; P<.01). Millennial providers were significantly more likely to utilize isotretinoin in patients who did not receive antibiotics compared with patients who did receive antibiotics (aOR, 1.693; P<.01). When compared with both Generation X and baby boomers, millennial providers were more likely to prescribe isotretinoin in patients who received antibiotics (aOR, 2.227 [P=.02] and 3.638 [P<.01], respectively).

In 2018, the American Academy of Dermatology and the Global Alliance to Improve Outcomes in Acne updated thir guidelines to recommend isotretinoin as a first-line therapy for severe nodular acne, treatment-resistant moderate acne, or acne that produces scarring or psychosocial distress.5 Our study results suggest that millennial providers are adhering to these guidelines and readily prescribing isotretinoin in patients who did not receive antibiotics, which corroborates survey findings by Nagler and Orlow.3 Our results also revealed that prescriber generation may influence isotretinoin usage, with millennials utilizing isotretinoin more in patients who received oral antibiotic therapy than their older counterparts. In part, this may be due to beliefs among older generations that failure of oral antibiotics is necessary before pursuing isotretinoin.3 Additionally, this finding suggests that millennials, if utilizing antibiotics for acne, may have a lower threshold for starting isotretinoin in patients who received oral antibiotic therapy.
Generational prescribing variation appears not to be unique to isotretinoin and also may be present in the use of spironolactone. Over the past decade, utilization of spironolactone for acne treatment has increased, likely in response to new data demonstrating that routine use is safe and effective.6 Several large cohort and retrospective studies have debunked the historical concerns for tumorigenicity in those with breast cancer history as well as the need for routine laboratory monitoring for hyperkalemia.7,8 Although spironolactone use for the treatment of acne has increased, it still remains relatively underutilized,6 suggesting there may be a knowledge gap similar to that of isotretinoin, with younger generations utilizing spironolactone more readily than older generations.
Our study analyzed generational differences in isotretinoin utilization for acne over 1 calendar year. Limitations include sampling from a midwestern patient cohort and private practice–based providers. Due to limitations of our data set, we were unable to capture acne medication usage prior to May 2021, temporal sequencing of acne medication usage, and stratification of patients by acne severity. Furthermore, we were unable to capture female patients who were pregnant or planning pregnancy at the time of their encounter, which would exclude isotretinoin usage.
Overall, millennial providers may be utilizing isotretinoin more in line with the updated acne guidelines5 compared with providers from older generations. Further research is necessary to elucidate how these prescribing habits may change based on acne severity.
To the Editor:
Prescriptions for isotretinoin may be influenced by patient demographics, medical comorbidities, and drug safety programs.1,2 In 1982, isotretinoin was approved by the US Food and Drug Administration for treatment of severe recalcitrant nodulocystic acne that is nonresponsive to conventional therapies such as antibiotics; however, prescriber beliefs regarding the necessity of oral antibiotic failure before isotretinoin is prescribed may be influenced by the provider’s generational age.3 Currently, there is a knowledge gap regarding the impact of provider characteristics, including the year providers completed training, on isotretinoin utilization. The aim of our cross-sectional study was to characterize generational isotretinoin prescribing habits in a large-scale midwestern private practice dermatology group.
Modernizing Medicine (https://www.modmed.com), an electronic medical record software, was queried for all encounters that included both an International Classification of Diseases, Tenth Revision, Clinical Modification diagnosis code L70.0 (acne vulgaris) and a medication prescription from May 2021 to May 2022. Data were collected from a large private practice group with locations across the state of Ohio. Exclusion criteria included provider-patient prescription pairs that included non–acne medication prescriptions, patients seen by multiple providers, and providers who treated fewer than 5 patients with acne during the study period. A mixed-effect multiple logistic regression was performed to analyze whether a patient was ever prescribed isotretinoin, adjusting for individual prescriber, prescriber generation (millennial [1981–1996], Generation X [1965–1980], and baby boomer [1946–1964]),4 and patient sex; spironolactone and oral antibiotic prescriptions during the study period were included as additional covariates in a subsequent post hoc analysis. This study utilized data that was fully deidentified in accordance with the US Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule. Approval from an institutional review board was not required.
A total of 18,089 provider-patient prescription pairs were included in our analysis (Table). In our most robust model, female patients were significantly less likely to receive isotretinoin compared with male patients (adjusted OR [aOR], 0.394; P<.01). Millennial providers were significantly more likely to utilize isotretinoin in patients who did not receive antibiotics compared with patients who did receive antibiotics (aOR, 1.693; P<.01). When compared with both Generation X and baby boomers, millennial providers were more likely to prescribe isotretinoin in patients who received antibiotics (aOR, 2.227 [P=.02] and 3.638 [P<.01], respectively).

In 2018, the American Academy of Dermatology and the Global Alliance to Improve Outcomes in Acne updated thir guidelines to recommend isotretinoin as a first-line therapy for severe nodular acne, treatment-resistant moderate acne, or acne that produces scarring or psychosocial distress.5 Our study results suggest that millennial providers are adhering to these guidelines and readily prescribing isotretinoin in patients who did not receive antibiotics, which corroborates survey findings by Nagler and Orlow.3 Our results also revealed that prescriber generation may influence isotretinoin usage, with millennials utilizing isotretinoin more in patients who received oral antibiotic therapy than their older counterparts. In part, this may be due to beliefs among older generations that failure of oral antibiotics is necessary before pursuing isotretinoin.3 Additionally, this finding suggests that millennials, if utilizing antibiotics for acne, may have a lower threshold for starting isotretinoin in patients who received oral antibiotic therapy.
Generational prescribing variation appears not to be unique to isotretinoin and also may be present in the use of spironolactone. Over the past decade, utilization of spironolactone for acne treatment has increased, likely in response to new data demonstrating that routine use is safe and effective.6 Several large cohort and retrospective studies have debunked the historical concerns for tumorigenicity in those with breast cancer history as well as the need for routine laboratory monitoring for hyperkalemia.7,8 Although spironolactone use for the treatment of acne has increased, it still remains relatively underutilized,6 suggesting there may be a knowledge gap similar to that of isotretinoin, with younger generations utilizing spironolactone more readily than older generations.
Our study analyzed generational differences in isotretinoin utilization for acne over 1 calendar year. Limitations include sampling from a midwestern patient cohort and private practice–based providers. Due to limitations of our data set, we were unable to capture acne medication usage prior to May 2021, temporal sequencing of acne medication usage, and stratification of patients by acne severity. Furthermore, we were unable to capture female patients who were pregnant or planning pregnancy at the time of their encounter, which would exclude isotretinoin usage.
Overall, millennial providers may be utilizing isotretinoin more in line with the updated acne guidelines5 compared with providers from older generations. Further research is necessary to elucidate how these prescribing habits may change based on acne severity.
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22. doi:10.1001/jamadermatol.2019.3270
- Nagler AR, Orlow SJ. Dermatologists’ attitudes, prescription, and counseling patterns for isotretinoin: a questionnaire-based study. J Drugs Dermatol. 2015;14:184-189.
- Dimock M. Where Millennials end and Generation Z begins. Pew Research Center website. January 17, 2019. Accessed June 17, 2024. https://www.pewresearch.org/fact-tank/2019/01/17/where-millennials-end-and-generation-z-begins/
- Thiboutot DM, Dréno B, Abanmi A, et al. Practical management of acne for clinicians: an international consensus from the Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2018;78(2 suppl 1):S1-S23.e1. doi:10.1016/j.jaad.2017.09.078
- Guzman AK, Barbieri JS. Comparative analysis of prescribing patterns of tetracycline class antibiotics and spironolactone between advanced practice providers and physicians in the treatment of acne vulgaris. J Am Acad Dermatol. 2021;84:1119-1121. doi:10.1016/j.jaad.2020.06.044
- Wei C, Bovonratwet P, Gu A, et al. Spironolactone use does not increase the risk of female breast cancer recurrence: a retrospective analysis. J Am Acad Dermatol. 2020;83:1021-1027. doi:10.1016/j.jaad.2020.05.081
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944. doi:10.1001/jamadermatol.2015.34
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22. doi:10.1001/jamadermatol.2019.3270
- Nagler AR, Orlow SJ. Dermatologists’ attitudes, prescription, and counseling patterns for isotretinoin: a questionnaire-based study. J Drugs Dermatol. 2015;14:184-189.
- Dimock M. Where Millennials end and Generation Z begins. Pew Research Center website. January 17, 2019. Accessed June 17, 2024. https://www.pewresearch.org/fact-tank/2019/01/17/where-millennials-end-and-generation-z-begins/
- Thiboutot DM, Dréno B, Abanmi A, et al. Practical management of acne for clinicians: an international consensus from the Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2018;78(2 suppl 1):S1-S23.e1. doi:10.1016/j.jaad.2017.09.078
- Guzman AK, Barbieri JS. Comparative analysis of prescribing patterns of tetracycline class antibiotics and spironolactone between advanced practice providers and physicians in the treatment of acne vulgaris. J Am Acad Dermatol. 2021;84:1119-1121. doi:10.1016/j.jaad.2020.06.044
- Wei C, Bovonratwet P, Gu A, et al. Spironolactone use does not increase the risk of female breast cancer recurrence: a retrospective analysis. J Am Acad Dermatol. 2020;83:1021-1027. doi:10.1016/j.jaad.2020.05.081
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944. doi:10.1001/jamadermatol.2015.34
Practice Points
- Provider generational age appears to impact utilization of isotretinoin for the treatment of acne.
- Millennial providers seem to adhere more readily to guidelines for precribing isotretinoin vs older generations and also may have a lower threshold for starting isotretinoin in patients who received oral antibiotic therapy for acne treatment.
Time Warp: Fax Machines Still Common in Oncology Practice. Why?
One minute, he’s working on sequencing a tumor genome. The next, he’s sifting through pages of disorganized data from a device that has been around for decades: the fax machine.
“If two doctors’ offices aren’t on the same electronic medical record, one of the main ways to transfer records is still by fax,” said Dr. Lewis, director of gastrointestinal oncology at Intermountain Healthcare in Murray, Utah. “I can go from cutting-edge innovation to relying on, at best, 1980s information technology. It just boggles my mind.”
Dr. Lewis, who has posted about his frustration with fax machines, is far from alone. Oncologists are among the many specialists across the country at the mercy of telecopiers.
According to a 2021 report by the Office of the National Coordinator for Health Information Technology, fax and mail continue to be the most common methods for hospitals and health systems to exchange care record summaries. In 2019, nearly 8 in 10 hospitals used mail or fax to send and receive health information, the report found.
Fax machines are still commonplace across the healthcare spectrum, said Robert Havasy, MS, senior director for informatics strategy at the Healthcare Information and Management Systems Society (HIMSS). Inertia, cost, and more pressing priorities for hospitals and medical institutions contribute to the technology sticking around, he explained.
“Post-COVID, my guess is we’re still at over 50% of healthcare practices using fax for some reason, on a daily basis,” Mr. Havasy said in an interview. “A lot of hospitals just don’t have the time, the money, or the staff to fix that problem because there’s always something a little higher up the priority chain they need to focus on.”
If, for instance, “you’re going to do a process redesign to reduce hospital total acquired infections, your fax machine replacement might be 10th or 12th on the list. It just never gets up to 1 or 2 because it’s ‘not that much of a problem,’ ” he added.
Or is it?
Administrators may not view fax machines as a top concern, but clinicians who deal with the machines daily see it differently.
“What worries me is we’re taking records out of an electronic storehouse [and] converting them to a paper medium,” Dr. Lewis said. “And then we are scanning into another electronic storehouse. The more steps, the more can be lost.”
And when information is lost, patient care can be compromised.
Slower Workflows, Care Concerns
Although there are no published data on fax machine use in oncology specifically, this outdated technology does come into play in a variety of ways along the cancer care continuum.
Radiation oncologist David R. Penberthy, MD, said patients often seek his cancer center’s expertise for second opinions, and that requires collecting patient records from many different practices.
“Ideally, it would come electronically, but sometimes it does come by fax,” said Dr. Penberthy, program director of radiation oncology at the University of Virginia School of Medicine in Charlottesville. “The quality of the fax is not always the best. Sometimes it’s literally a fax of a fax. You’re reading something that’s very difficult to read.”
Orders for new tests are also typically sent and received via fax temporarily while IT teams work to integrate them into the electronic health record (EHR), Dr. Penberthy said.
Insurers and third-party laboratories often send test results back by fax as well.
“Even if I haven’t actually sent my patient out of our institution, this crucial result may only be entered back into the record as a scanned document from a fax, which is not great because it can get lost in the other results that are reported electronically,” Dr. Lewis said. The risk here is that an ordering physician won’t see these results, which can lead to delayed or overlooked care for patients, he explained.
“To me, it’s like a blind spot,” Dr. Lewis said. “Every time we use a fax, I see it actually as an opportunity for oversight and missed opportunity to collect data.”
Dr. Penberthy said faxing can slow things down at his practice, particularly if he faxes a document to another office but receives no confirmation and has to track down what happened.
As for cybersecurity, data that are in transit during faxing are generally considered secure and compliant with the Health Insurance Portability and Accountability Act (HIPAA), said Mr. Havasy of HIMSS. However, the Privacy Rule also requires that data remain secure while at rest, which isn’t always possible, he added.
“That’s where faxes fall down, because generally fax machines are in public, if you will, or open areas in a hospital,” he said. “They just sit on a desk. I don’t know that the next nurse who comes up and looks through that stack was the nurse who was treating the patient.”
Important decisions or results can also be missed when sent by fax, creating headaches for physicians and care problems for patients.
Dr. Lewis recently experienced an insurance-related fax mishap over Memorial Day weekend. He believed his patient had access to the antinausea medication he had prescribed. When Dr. Lewis happened to check the fax machine over the weekend, he found a coverage denial for the medication from the insurer but, at that point, had no recourse to appeal because it was a long holiday weekend.
“Had the denial been sent by an electronic means that was quicker and more readily available, it would have been possible to appeal before the holiday weekend,” he said.
Hematologist Aaron Goodman, MD, encountered a similar problem after an insurer denied coverage of an expensive cancer drug for a patient and faxed over its reason for the denial. Dr. Goodman was not directly notified that the information arrived and didn’t learn about the denial for a week, he said.
“There’s no ‘ding’ in my inbox if something is faxed over and scanned,” said Dr. Goodman, associate professor of medicine at UC San Diego Health. “Once I realized it was denied, I was able to rectify it, but it wasted a week of a patient not getting a drug that I felt would be beneficial for them.”
Broader Health Policy Impacts
The use of outdated technology, such as fax machines, also creates ripple effects that burden the health system, health policy experts say.
Duplicate testing and unnecessary care are top impacts, said Julia Adler-Milstein, PhD, professor of medicine and chief of the division of clinical informatics and digital transformation at the University of California, San Francisco.
Studies show that 20%-30% of the $65 billion spent annually on lab tests is used on unnecessary duplicate tests, and another estimated $30 billion is spent each year on unnecessary duplicate medical imaging. These duplicate tests may be mitigated if hospitals adopt certified EHR technology, research shows.
Still, without EHR interoperability between institutions, new providers may be unaware that tests or past labs for patients exist, leading to repeat tests, said Dr. Adler-Milstein, who researches health IT policy with a focus on EHRs. Patients can sometimes fill in the gaps, but not always.
“Fax machines only help close information gaps if the clinician is aware of where to seek out the information and there is someone at the other organization to locate and transmit the information in a timely manner,” Dr. Adler-Milstein said.
Old technology and poor interoperability also greatly affect data collection for disease surveillance and monitoring, said Janet Hamilton, MPH, executive director for the Council of State and Territorial Epidemiologists. This issue was keenly demonstrated during the pandemic, Ms. Hamilton said.
“It was tragic, quite honestly,” she said. “There was such an immense amount of data that needed to be moved quickly, and that’s when computers are at their best.”
But, she said, “we didn’t have the level of systems in place to do it well.”
Specifically, the lack of electronic case reporting in place during the pandemic — where diagnoses are documented in the record and then immediately sent to the public health system — led to reports that were delayed, not made, or had missing or incomplete information, such as patients’ race and ethnicity or other health conditions, Ms. Hamilton said.
Incomplete or missing data hampered the ability of public health officials and researchers to understand how the virus might affect different patients.
“If you had a chronic condition like cancer, you were less likely to have a positive outcome with COVID,” Ms. Hamilton said. “But because electronic case reporting was not in place, we didn’t get some of those additional pieces of information. We didn’t have people’s underlying oncology status to then say, ‘Here are individuals with these types of characteristics, and these are the things that happen if they also have a cancer.’”
Slow, but Steady, Improvements
Efforts at the state and federal levels have targeted improved health information exchange, but progress takes time, Dr. Adler-Milstein said.
Most states have some form of health information exchange, such as statewide exchanges, regional health information organizations, or clinical data registries. Maryland is often held up as a notable example for its health information exchange, Dr. Adler-Milstein noted.
According to Maryland law, all hospitals under the jurisdiction of the Maryland Health Care Commission are required to electronically connect to the state-designated health information exchange. In 2012, Maryland became the first state to connect all its 46 acute care hospitals in the sharing of real-time data.
The Health Information Technology for Economic and Clinical Health (HITECH) Act provided federal-enhanced Medicaid matching funds to states through 2021 to support efforts to advance electronic exchange. Nearly all states used these funds, and most have identified other sources to sustain the efforts, according to a recent US Government Accountability Office (GAO) report. However, GAO found that small and rural providers are less likely to have the financial and technological resources to participate in or maintain electronic exchange capabilities.
Nationally, several recent initiatives have targeted health data interoperability, including for cancer care. The Centers for Disease Control and Prevention’s Data Modernization Initiative is a multiyear, multi–billion-dollar effort to improve data sharing across the federal and state public health landscape.
Meanwhile, in March 2024, the Biden-Harris administration launched United States Core Data for Interoperability Plus Cancer. The program will define a recommended minimum set of cancer-related data to be included in a patient’s EHR to enhance data exchange for research and clinical care.
EHR vendors are also key to improving the landscape, said Dr. Adler-Milstein. Vendors such as Epic have developed strong sharing capabilities for transmitting health information from site to site, but of course, that only helps if providers have Epic, she said.
“That’s where these national frameworks should help, because we don’t want it to break down by what EHR vendor you have,” she said. “It’s a patchwork. You can go to some places and hear success stories because they have Epic or a state health information exchange, but it’s very heterogeneous. In some places, they have nothing and are using a fax machine.”
Mr. Havasy believes fax machines will ultimately go extinct, particularly as a younger, more digitally savvy generation enters the healthcare workforce. He also foresees that the growing use of artificial intelligence will help eradicate the outdated technology.
But, Ms. Hamilton noted, “unless we have consistent, ongoing, sustained funding, it is very hard to move off [an older] technology that can work. That’s one of the biggest barriers.”
“Public health is about protecting the lives of every single person everywhere,” Ms. Hamilton said, “but when we don’t have the data that comes into the system, we can’t achieve our mission.”
A version of this article appeared on Medscape.com.
One minute, he’s working on sequencing a tumor genome. The next, he’s sifting through pages of disorganized data from a device that has been around for decades: the fax machine.
“If two doctors’ offices aren’t on the same electronic medical record, one of the main ways to transfer records is still by fax,” said Dr. Lewis, director of gastrointestinal oncology at Intermountain Healthcare in Murray, Utah. “I can go from cutting-edge innovation to relying on, at best, 1980s information technology. It just boggles my mind.”
Dr. Lewis, who has posted about his frustration with fax machines, is far from alone. Oncologists are among the many specialists across the country at the mercy of telecopiers.
According to a 2021 report by the Office of the National Coordinator for Health Information Technology, fax and mail continue to be the most common methods for hospitals and health systems to exchange care record summaries. In 2019, nearly 8 in 10 hospitals used mail or fax to send and receive health information, the report found.
Fax machines are still commonplace across the healthcare spectrum, said Robert Havasy, MS, senior director for informatics strategy at the Healthcare Information and Management Systems Society (HIMSS). Inertia, cost, and more pressing priorities for hospitals and medical institutions contribute to the technology sticking around, he explained.
“Post-COVID, my guess is we’re still at over 50% of healthcare practices using fax for some reason, on a daily basis,” Mr. Havasy said in an interview. “A lot of hospitals just don’t have the time, the money, or the staff to fix that problem because there’s always something a little higher up the priority chain they need to focus on.”
If, for instance, “you’re going to do a process redesign to reduce hospital total acquired infections, your fax machine replacement might be 10th or 12th on the list. It just never gets up to 1 or 2 because it’s ‘not that much of a problem,’ ” he added.
Or is it?
Administrators may not view fax machines as a top concern, but clinicians who deal with the machines daily see it differently.
“What worries me is we’re taking records out of an electronic storehouse [and] converting them to a paper medium,” Dr. Lewis said. “And then we are scanning into another electronic storehouse. The more steps, the more can be lost.”
And when information is lost, patient care can be compromised.
Slower Workflows, Care Concerns
Although there are no published data on fax machine use in oncology specifically, this outdated technology does come into play in a variety of ways along the cancer care continuum.
Radiation oncologist David R. Penberthy, MD, said patients often seek his cancer center’s expertise for second opinions, and that requires collecting patient records from many different practices.
“Ideally, it would come electronically, but sometimes it does come by fax,” said Dr. Penberthy, program director of radiation oncology at the University of Virginia School of Medicine in Charlottesville. “The quality of the fax is not always the best. Sometimes it’s literally a fax of a fax. You’re reading something that’s very difficult to read.”
Orders for new tests are also typically sent and received via fax temporarily while IT teams work to integrate them into the electronic health record (EHR), Dr. Penberthy said.
Insurers and third-party laboratories often send test results back by fax as well.
“Even if I haven’t actually sent my patient out of our institution, this crucial result may only be entered back into the record as a scanned document from a fax, which is not great because it can get lost in the other results that are reported electronically,” Dr. Lewis said. The risk here is that an ordering physician won’t see these results, which can lead to delayed or overlooked care for patients, he explained.
“To me, it’s like a blind spot,” Dr. Lewis said. “Every time we use a fax, I see it actually as an opportunity for oversight and missed opportunity to collect data.”
Dr. Penberthy said faxing can slow things down at his practice, particularly if he faxes a document to another office but receives no confirmation and has to track down what happened.
As for cybersecurity, data that are in transit during faxing are generally considered secure and compliant with the Health Insurance Portability and Accountability Act (HIPAA), said Mr. Havasy of HIMSS. However, the Privacy Rule also requires that data remain secure while at rest, which isn’t always possible, he added.
“That’s where faxes fall down, because generally fax machines are in public, if you will, or open areas in a hospital,” he said. “They just sit on a desk. I don’t know that the next nurse who comes up and looks through that stack was the nurse who was treating the patient.”
Important decisions or results can also be missed when sent by fax, creating headaches for physicians and care problems for patients.
Dr. Lewis recently experienced an insurance-related fax mishap over Memorial Day weekend. He believed his patient had access to the antinausea medication he had prescribed. When Dr. Lewis happened to check the fax machine over the weekend, he found a coverage denial for the medication from the insurer but, at that point, had no recourse to appeal because it was a long holiday weekend.
“Had the denial been sent by an electronic means that was quicker and more readily available, it would have been possible to appeal before the holiday weekend,” he said.
Hematologist Aaron Goodman, MD, encountered a similar problem after an insurer denied coverage of an expensive cancer drug for a patient and faxed over its reason for the denial. Dr. Goodman was not directly notified that the information arrived and didn’t learn about the denial for a week, he said.
“There’s no ‘ding’ in my inbox if something is faxed over and scanned,” said Dr. Goodman, associate professor of medicine at UC San Diego Health. “Once I realized it was denied, I was able to rectify it, but it wasted a week of a patient not getting a drug that I felt would be beneficial for them.”
Broader Health Policy Impacts
The use of outdated technology, such as fax machines, also creates ripple effects that burden the health system, health policy experts say.
Duplicate testing and unnecessary care are top impacts, said Julia Adler-Milstein, PhD, professor of medicine and chief of the division of clinical informatics and digital transformation at the University of California, San Francisco.
Studies show that 20%-30% of the $65 billion spent annually on lab tests is used on unnecessary duplicate tests, and another estimated $30 billion is spent each year on unnecessary duplicate medical imaging. These duplicate tests may be mitigated if hospitals adopt certified EHR technology, research shows.
Still, without EHR interoperability between institutions, new providers may be unaware that tests or past labs for patients exist, leading to repeat tests, said Dr. Adler-Milstein, who researches health IT policy with a focus on EHRs. Patients can sometimes fill in the gaps, but not always.
“Fax machines only help close information gaps if the clinician is aware of where to seek out the information and there is someone at the other organization to locate and transmit the information in a timely manner,” Dr. Adler-Milstein said.
Old technology and poor interoperability also greatly affect data collection for disease surveillance and monitoring, said Janet Hamilton, MPH, executive director for the Council of State and Territorial Epidemiologists. This issue was keenly demonstrated during the pandemic, Ms. Hamilton said.
“It was tragic, quite honestly,” she said. “There was such an immense amount of data that needed to be moved quickly, and that’s when computers are at their best.”
But, she said, “we didn’t have the level of systems in place to do it well.”
Specifically, the lack of electronic case reporting in place during the pandemic — where diagnoses are documented in the record and then immediately sent to the public health system — led to reports that were delayed, not made, or had missing or incomplete information, such as patients’ race and ethnicity or other health conditions, Ms. Hamilton said.
Incomplete or missing data hampered the ability of public health officials and researchers to understand how the virus might affect different patients.
“If you had a chronic condition like cancer, you were less likely to have a positive outcome with COVID,” Ms. Hamilton said. “But because electronic case reporting was not in place, we didn’t get some of those additional pieces of information. We didn’t have people’s underlying oncology status to then say, ‘Here are individuals with these types of characteristics, and these are the things that happen if they also have a cancer.’”
Slow, but Steady, Improvements
Efforts at the state and federal levels have targeted improved health information exchange, but progress takes time, Dr. Adler-Milstein said.
Most states have some form of health information exchange, such as statewide exchanges, regional health information organizations, or clinical data registries. Maryland is often held up as a notable example for its health information exchange, Dr. Adler-Milstein noted.
According to Maryland law, all hospitals under the jurisdiction of the Maryland Health Care Commission are required to electronically connect to the state-designated health information exchange. In 2012, Maryland became the first state to connect all its 46 acute care hospitals in the sharing of real-time data.
The Health Information Technology for Economic and Clinical Health (HITECH) Act provided federal-enhanced Medicaid matching funds to states through 2021 to support efforts to advance electronic exchange. Nearly all states used these funds, and most have identified other sources to sustain the efforts, according to a recent US Government Accountability Office (GAO) report. However, GAO found that small and rural providers are less likely to have the financial and technological resources to participate in or maintain electronic exchange capabilities.
Nationally, several recent initiatives have targeted health data interoperability, including for cancer care. The Centers for Disease Control and Prevention’s Data Modernization Initiative is a multiyear, multi–billion-dollar effort to improve data sharing across the federal and state public health landscape.
Meanwhile, in March 2024, the Biden-Harris administration launched United States Core Data for Interoperability Plus Cancer. The program will define a recommended minimum set of cancer-related data to be included in a patient’s EHR to enhance data exchange for research and clinical care.
EHR vendors are also key to improving the landscape, said Dr. Adler-Milstein. Vendors such as Epic have developed strong sharing capabilities for transmitting health information from site to site, but of course, that only helps if providers have Epic, she said.
“That’s where these national frameworks should help, because we don’t want it to break down by what EHR vendor you have,” she said. “It’s a patchwork. You can go to some places and hear success stories because they have Epic or a state health information exchange, but it’s very heterogeneous. In some places, they have nothing and are using a fax machine.”
Mr. Havasy believes fax machines will ultimately go extinct, particularly as a younger, more digitally savvy generation enters the healthcare workforce. He also foresees that the growing use of artificial intelligence will help eradicate the outdated technology.
But, Ms. Hamilton noted, “unless we have consistent, ongoing, sustained funding, it is very hard to move off [an older] technology that can work. That’s one of the biggest barriers.”
“Public health is about protecting the lives of every single person everywhere,” Ms. Hamilton said, “but when we don’t have the data that comes into the system, we can’t achieve our mission.”
A version of this article appeared on Medscape.com.
One minute, he’s working on sequencing a tumor genome. The next, he’s sifting through pages of disorganized data from a device that has been around for decades: the fax machine.
“If two doctors’ offices aren’t on the same electronic medical record, one of the main ways to transfer records is still by fax,” said Dr. Lewis, director of gastrointestinal oncology at Intermountain Healthcare in Murray, Utah. “I can go from cutting-edge innovation to relying on, at best, 1980s information technology. It just boggles my mind.”
Dr. Lewis, who has posted about his frustration with fax machines, is far from alone. Oncologists are among the many specialists across the country at the mercy of telecopiers.
According to a 2021 report by the Office of the National Coordinator for Health Information Technology, fax and mail continue to be the most common methods for hospitals and health systems to exchange care record summaries. In 2019, nearly 8 in 10 hospitals used mail or fax to send and receive health information, the report found.
Fax machines are still commonplace across the healthcare spectrum, said Robert Havasy, MS, senior director for informatics strategy at the Healthcare Information and Management Systems Society (HIMSS). Inertia, cost, and more pressing priorities for hospitals and medical institutions contribute to the technology sticking around, he explained.
“Post-COVID, my guess is we’re still at over 50% of healthcare practices using fax for some reason, on a daily basis,” Mr. Havasy said in an interview. “A lot of hospitals just don’t have the time, the money, or the staff to fix that problem because there’s always something a little higher up the priority chain they need to focus on.”
If, for instance, “you’re going to do a process redesign to reduce hospital total acquired infections, your fax machine replacement might be 10th or 12th on the list. It just never gets up to 1 or 2 because it’s ‘not that much of a problem,’ ” he added.
Or is it?
Administrators may not view fax machines as a top concern, but clinicians who deal with the machines daily see it differently.
“What worries me is we’re taking records out of an electronic storehouse [and] converting them to a paper medium,” Dr. Lewis said. “And then we are scanning into another electronic storehouse. The more steps, the more can be lost.”
And when information is lost, patient care can be compromised.
Slower Workflows, Care Concerns
Although there are no published data on fax machine use in oncology specifically, this outdated technology does come into play in a variety of ways along the cancer care continuum.
Radiation oncologist David R. Penberthy, MD, said patients often seek his cancer center’s expertise for second opinions, and that requires collecting patient records from many different practices.
“Ideally, it would come electronically, but sometimes it does come by fax,” said Dr. Penberthy, program director of radiation oncology at the University of Virginia School of Medicine in Charlottesville. “The quality of the fax is not always the best. Sometimes it’s literally a fax of a fax. You’re reading something that’s very difficult to read.”
Orders for new tests are also typically sent and received via fax temporarily while IT teams work to integrate them into the electronic health record (EHR), Dr. Penberthy said.
Insurers and third-party laboratories often send test results back by fax as well.
“Even if I haven’t actually sent my patient out of our institution, this crucial result may only be entered back into the record as a scanned document from a fax, which is not great because it can get lost in the other results that are reported electronically,” Dr. Lewis said. The risk here is that an ordering physician won’t see these results, which can lead to delayed or overlooked care for patients, he explained.
“To me, it’s like a blind spot,” Dr. Lewis said. “Every time we use a fax, I see it actually as an opportunity for oversight and missed opportunity to collect data.”
Dr. Penberthy said faxing can slow things down at his practice, particularly if he faxes a document to another office but receives no confirmation and has to track down what happened.
As for cybersecurity, data that are in transit during faxing are generally considered secure and compliant with the Health Insurance Portability and Accountability Act (HIPAA), said Mr. Havasy of HIMSS. However, the Privacy Rule also requires that data remain secure while at rest, which isn’t always possible, he added.
“That’s where faxes fall down, because generally fax machines are in public, if you will, or open areas in a hospital,” he said. “They just sit on a desk. I don’t know that the next nurse who comes up and looks through that stack was the nurse who was treating the patient.”
Important decisions or results can also be missed when sent by fax, creating headaches for physicians and care problems for patients.
Dr. Lewis recently experienced an insurance-related fax mishap over Memorial Day weekend. He believed his patient had access to the antinausea medication he had prescribed. When Dr. Lewis happened to check the fax machine over the weekend, he found a coverage denial for the medication from the insurer but, at that point, had no recourse to appeal because it was a long holiday weekend.
“Had the denial been sent by an electronic means that was quicker and more readily available, it would have been possible to appeal before the holiday weekend,” he said.
Hematologist Aaron Goodman, MD, encountered a similar problem after an insurer denied coverage of an expensive cancer drug for a patient and faxed over its reason for the denial. Dr. Goodman was not directly notified that the information arrived and didn’t learn about the denial for a week, he said.
“There’s no ‘ding’ in my inbox if something is faxed over and scanned,” said Dr. Goodman, associate professor of medicine at UC San Diego Health. “Once I realized it was denied, I was able to rectify it, but it wasted a week of a patient not getting a drug that I felt would be beneficial for them.”
Broader Health Policy Impacts
The use of outdated technology, such as fax machines, also creates ripple effects that burden the health system, health policy experts say.
Duplicate testing and unnecessary care are top impacts, said Julia Adler-Milstein, PhD, professor of medicine and chief of the division of clinical informatics and digital transformation at the University of California, San Francisco.
Studies show that 20%-30% of the $65 billion spent annually on lab tests is used on unnecessary duplicate tests, and another estimated $30 billion is spent each year on unnecessary duplicate medical imaging. These duplicate tests may be mitigated if hospitals adopt certified EHR technology, research shows.
Still, without EHR interoperability between institutions, new providers may be unaware that tests or past labs for patients exist, leading to repeat tests, said Dr. Adler-Milstein, who researches health IT policy with a focus on EHRs. Patients can sometimes fill in the gaps, but not always.
“Fax machines only help close information gaps if the clinician is aware of where to seek out the information and there is someone at the other organization to locate and transmit the information in a timely manner,” Dr. Adler-Milstein said.
Old technology and poor interoperability also greatly affect data collection for disease surveillance and monitoring, said Janet Hamilton, MPH, executive director for the Council of State and Territorial Epidemiologists. This issue was keenly demonstrated during the pandemic, Ms. Hamilton said.
“It was tragic, quite honestly,” she said. “There was such an immense amount of data that needed to be moved quickly, and that’s when computers are at their best.”
But, she said, “we didn’t have the level of systems in place to do it well.”
Specifically, the lack of electronic case reporting in place during the pandemic — where diagnoses are documented in the record and then immediately sent to the public health system — led to reports that were delayed, not made, or had missing or incomplete information, such as patients’ race and ethnicity or other health conditions, Ms. Hamilton said.
Incomplete or missing data hampered the ability of public health officials and researchers to understand how the virus might affect different patients.
“If you had a chronic condition like cancer, you were less likely to have a positive outcome with COVID,” Ms. Hamilton said. “But because electronic case reporting was not in place, we didn’t get some of those additional pieces of information. We didn’t have people’s underlying oncology status to then say, ‘Here are individuals with these types of characteristics, and these are the things that happen if they also have a cancer.’”
Slow, but Steady, Improvements
Efforts at the state and federal levels have targeted improved health information exchange, but progress takes time, Dr. Adler-Milstein said.
Most states have some form of health information exchange, such as statewide exchanges, regional health information organizations, or clinical data registries. Maryland is often held up as a notable example for its health information exchange, Dr. Adler-Milstein noted.
According to Maryland law, all hospitals under the jurisdiction of the Maryland Health Care Commission are required to electronically connect to the state-designated health information exchange. In 2012, Maryland became the first state to connect all its 46 acute care hospitals in the sharing of real-time data.
The Health Information Technology for Economic and Clinical Health (HITECH) Act provided federal-enhanced Medicaid matching funds to states through 2021 to support efforts to advance electronic exchange. Nearly all states used these funds, and most have identified other sources to sustain the efforts, according to a recent US Government Accountability Office (GAO) report. However, GAO found that small and rural providers are less likely to have the financial and technological resources to participate in or maintain electronic exchange capabilities.
Nationally, several recent initiatives have targeted health data interoperability, including for cancer care. The Centers for Disease Control and Prevention’s Data Modernization Initiative is a multiyear, multi–billion-dollar effort to improve data sharing across the federal and state public health landscape.
Meanwhile, in March 2024, the Biden-Harris administration launched United States Core Data for Interoperability Plus Cancer. The program will define a recommended minimum set of cancer-related data to be included in a patient’s EHR to enhance data exchange for research and clinical care.
EHR vendors are also key to improving the landscape, said Dr. Adler-Milstein. Vendors such as Epic have developed strong sharing capabilities for transmitting health information from site to site, but of course, that only helps if providers have Epic, she said.
“That’s where these national frameworks should help, because we don’t want it to break down by what EHR vendor you have,” she said. “It’s a patchwork. You can go to some places and hear success stories because they have Epic or a state health information exchange, but it’s very heterogeneous. In some places, they have nothing and are using a fax machine.”
Mr. Havasy believes fax machines will ultimately go extinct, particularly as a younger, more digitally savvy generation enters the healthcare workforce. He also foresees that the growing use of artificial intelligence will help eradicate the outdated technology.
But, Ms. Hamilton noted, “unless we have consistent, ongoing, sustained funding, it is very hard to move off [an older] technology that can work. That’s one of the biggest barriers.”
“Public health is about protecting the lives of every single person everywhere,” Ms. Hamilton said, “but when we don’t have the data that comes into the system, we can’t achieve our mission.”
A version of this article appeared on Medscape.com.
Transgender and Gender Diverse Health Care in the US Military: What Dermatologists Need to Know
People whose gender identity differs from the sex assigned at birth are referred to as transgender. For some, gender identity may not fit into the binary constructs of male and female but rather falls between, within, or outside this construct. These people often consider themselves nonbinary or gender diverse. As the terminology continues to evolve, current recommendations include referring to this patient population as transgender and gender diverse (TGD) to ensure the broadest inclusivity.1 In this article, the following terms are used as defined below:
- The terms transgender woman and trans feminine describe persons who were assigned male gender at birth but their affirmed gender is female or nonmasculine.
- The terms transgender man and trans masculine describe persons who were assigned female gender at birth but their affirmed gender is male or nonfeminine.
The US Military’s policies on the service of TGD persons have evolved considerably over the past decade. Initial military policies barred TGD service members (TSMs) from service all together, leading to challenges in accessing necessary health care. The first official memorandum explicitly allowing military service by TGD persons was released on June 30, 2016.2 The intention of this memorandum was 2-fold: (1) to allow TGD persons to serve in the military so long as they meet “the rigorous standards for military service and readiness” by fulfilling the same standards and procedures as other military service members, including medical fitness for duty, physical fitness, uniform and grooming, deployability, and retention, and (2) to direct the establishment of new or updated policies to specific departments and prescribe procedures for retention standards, separation from service, in-service transition, and medical coverage.2 Several other official policies were released following this initial memorandum that provided more specific guidance on how to implement these policies at the level of the force, unit, and individual service member.
Modifications to the original 2016 policies had varying impacts on transgender health care provision and access.3 At the time of publication of this article, the current policy—the Department of Defense Instruction 1300.284—among others, establishes standards and procedures for the process by which active and reserve TSMs may medically, socially, and legally transition genders within the military. The current policy applies to all military branches and serves as the framework by which each branch currently organizes their gender-affirmation processes (GAP).4
There currently are several different GAP models among the military branches.5 Each branch has a different model or approach to implementing the current policy, with varying service-specific processes in place for TSMs to access gender-affirming care; however, this may be changing. The Defense Health Agency is in the process of consolidating and streamlining the GAP across the Department of Defense branches in an effort to optimize costs and ensure uniformity of care. Per the Defense Health Agency Procedural Instruction Number 6025.21 published in May 2023, the proposed consolidated model likely will entail a single central transgender health center that provides oversight and guidance for several regional joint-service gender-affirming medical hubs. Patients would either be managed at the level of the hub or be referred to the central site.5
Herein, we discuss the importance of gender-affirming care and how military and civilian dermatologists can contribute. We also review disparities in health care and identify areas of improvement.
Benefits of Gender-Affirming Care
Gender-affirming procedures are critical for aligning physical appearance with gender identity. Physical appearance is essential for psychological well-being, operational readiness, and the safety of TSMs.6 It is well documented that TGD persons experience suicidal ideation, depression, stigma, discrimination and violence at higher rates than their cisgender peers.7,8 It is important to recognize that transgender identity is not a mental illness, and these elevated rates have been linked to complex trauma, societal stigma, violence, and discrimination.1 Other studies have suggested that increased access to gender-affirming interventions may ameliorate these mental health concerns.1,7-9
The major components of gender-affirming care include hormone therapy, gender confirmation surgery, and mental health care, if needed. These are covered by TRICARE, the health care program for military service members; however, at the time of publication, many of the dermatologic gender-affirming procedures are not covered by TRICARE because they are considered “cosmetic procedures,” which is a term used by insurance companies but does not accurately indicate whether a procedure is medically necessary or not. Newer literature has demonstrated that gender-affirming care positively affects the lives of TGD patients, strengthening the argument that gender-affirming care is a medical necessity and not just cosmetic.1
Aesthetic Procedures in Gender-Affirming Care
Surgeons, including those within the specialties of oto-laryngology, oral and maxillofacial surgery, urology, gynecology, and plastic surgery, provide major gender-affirming interventions; however, dermatologists may offer less invasive solutions that can serve as a temporary experience prior to undergoing more permanent procedures.Hormonally driven disorders including acne, hair loss, and melasma also are managed by dermatologists, along with scar treatment following surgeries.
Because human variation is expansive and subjective, what is considered feminine or masculine may vary by person, group, culture, and country; therefore, it is imperative to ask patients about their individual aesthetic goals and tailor their treatment accordingly. Feminine and masculine are terms that will be used to describe prototypical appearances and are not meant to define a patient’s current state or ultimate goals. The following procedures and medical interventions are where dermatologists can play an important role in TGD persons’ GAPs.
Botulinum Toxin Injections—Botulinum toxin injection is the most common nonsurgical aesthetic procedure performed around the world.10 The selective paralysis afforded by botulinum toxin has several uses for people undergoing transition. Aesthetically, the feminine eyebrow tends to be positioned above the orbital rim and is arched with its apex between the lateral limbus and lateral canthus,11 while the masculine eyebrow tends to be flatter and fuller and runs over the orbital rim without a peak. For people seeking a more feminine appearance, an eyebrow lift with botulinum toxin can help reshape the typical flatter masculine eyebrow to give it lateral lift that often is considered more feminine. The targeted muscle is the superolateral orbicularis oculi, which serves as a depressor on the eyebrow. This can be combined with purposefully avoiding total lateral frontalis paralysis, which leads to a “Spock” brow for extra lift. Conversely, a naturally arched and higher eyebrow can be flattened and lowered by selectively targeting areas of the frontalis muscle.
Broad square jawlines typically are considered a masculine feature and are another area where botulinum toxin can be used to feminize a patient’s facial features. Targeting the masseter muscle induces muscle weakness, which ultimately may result in atrophy after one or more treatment sessions. This atrophy may lead to narrowing of the lower face and thus may lead to a fuller-appearing midface or overall more heart-shaped face. Every individual’s aesthetic goals are unique and therefore should be discussed prior to any treatment.
Dermal Fillers—Dermal fillers are gel-like substances injected under the skin for subtle contouring of the face. Fillers also can be used to help promote a more masculine or feminine appearance. Filler can be placed in the lips to create a fuller, more projected, feminine-appearing lip. Malar cheek and central lower chin filler can be used to help define a heart-shaped face by accentuating the upper portion of the face and creating a more pointed chin, respectively. Alternatively, filler can be used to masculinize the chin by placing it where it can increase jawline squareness and increase anterior jaw projection. Additionally, filler at the angle of the jaw can help accentuate a square facial shape and a more defined jawline. Although not as widely practiced, lateral brow filler can create a heavier-appearing and broader forehead for a more masculine appearance. These procedures can be combined with the previously mentioned botulinum toxin procedures for a synergistic effect.
Deoxycholic Acid—Deoxycholic acid is an injectable product used to selectively remove unwanted fat. It currently is approved by the US Food and Drug Administration for submental fat, but some providers are experimenting with off-label uses. Buccal fat pad removal—or in this case reduction by dissolution—tends to give a thinner, more feminine facial appearance.12 Reducing fat around the axillae also can help promote a more masculine upper torso.13 The safety of deoxycholic acid in these areas has not been adequately tested; thus, caution should be used when discussing these off-label uses with patients.
Hair and Tattoo Removal—Hair removal may be desired by TGD persons for a variety of reasons. Because cisgender females tend to have less body hair overall, transgender people in pursuit of a more feminine appearance often desire removal of facial, neck, and body hair. Although shaving and other modalities such as waxing and chemical depilatories are readily available at-home options, they are not permanent and may lead to folliculitis or pseudofolliculitis barbae. Laser hair removal (LHR) and electrolysis are modalities provided by dermatologists that tend to be more permanent and lead to better outcomes, including less irritation and better aesthetic appearance. It is important to keep in mind that not every person and not every body site can be safely treated with LHR. Patients with lighter skin types and darker hair tend to have the most effective response with a higher margin of safety, as these features allow the laser energy to be selectively absorbed by the melanin in the hair bulb and not by the background skin pigmentation.14,15 Inappropriate patient selection or improper settings for wavelength, pulse width, or fluences can lead to burns and permanent scarring.14,15 Electrolysis is an alternative to hair removal within tattoos and is more effective for those individuals with blonde, red, or white hair.16
Another novel treatment for unwanted hair is eflornithine hydrochloride cream, which works by blocking ornithine decarboxylase, the enzyme that stimulates hair growth. It currently is approved to reduce unwanted hair on the face and adjacent areas under the chin; however the effects of this medication are modest and the medication can be expensive.17
Cosmetic hair and tattoo removal are not currently covered by TRICARE, except in cases of surgical and donor-site preparation for some GAPs. Individuals may desire removal of tattoos at surgery sites to obtain more natural-appearing skin. Currently, GAPs such as vaginoplasty, phalloplasty, and metoidioplasty—often referred to by patients as “bottom surgeries”—include insurance coverage for tattoo removal, LHR, and/or electrolysis.
Management of Hormonal Adverse Effects
Acne—Individuals on testosterone supplementation tend to develop acne for the first several years of treatment, but it may improve with time.18 Acne is treated in individuals receiving testosterone the same way as it is treated in cisgender men, with numerous options for topical and oral medications. In trans masculine persons, spironolactone therapy typically is avoided because it may interfere with the actions of exogenous testosterone administered as part of gender-affirming medical treatment and may lead to other undesired adverse effects such as impotence and gynecomastia.1
Although acne typically improves after starting estrogen therapy, patients receiving estrogens may still develop acne. Most trans feminine patients will already be on an estrogen and an antiandrogen, often spironolactone.1 Spironolactone often is used as monotherapy for acne control in cisgender women. Additionally, an important factor to consider with spironolactone is the possible adverse effect of increased micturition. Currently, the military rarely has gender-inclusive restroom options, which can create a challenge for TSMs who find themselves needing to use the restroom more frequently in the workplace.
If planning therapy with isotretinoin, dermatologists should discuss several important factors with all patients, including TGD patients. One consideration is the patient’s planned future surgeries. Although new literature shows that isotretinoin does not adversely affect wound healing,19 some surgeons still adhere to an isotretinoin washout period of 6 to 12 months prior to performing any elective procedures due to concerns about wound healing.20,21 Second, be sure to properly assess and document pregnancy potential in TGD persons. Providers should not assume that a patient is not pregnant or is not trying to become pregnant just because they are trans masculine. It also is important to note that testosterone is not a reliable birth control method.1 If a patient still has ovaries, fallopian tubes, and a uterus, they are considered medically capable of pregnancy, and providers should keep this in mind regarding all procedures in the TGD population.
Another newer acne treatment modality is the 1762-nm laser, which targets sebaceous glands.22 This device allows for targeted treatment of acne-prone areas without systemic therapy such as retinoids or antiandrogens. The 1762-nm laser is not widely available but may become a regular treatment option once its benefits are proven over time.
Alopecia and Hyperpigmentation—Androgens, whether endogenously or exogenously derived, can lead to androgenetic alopecia (AGA) in genetically susceptible individuals. Trans masculine persons and others receiving androgen therapy are at higher risk for AGA, which often is undesirable and may be considered gender affirming by some TGD persons. Standard AGA treatments for cisgender men also can be used in trans masculine persons. Some of the most common anti-AGA medications are topical minoxidil, oral finasteride, and oral minoxidil. Although Coleman et al1 recently reported that finasteride may be an appropriate treatment option in trans masculine persons experiencing alopecia, treatment with 5α-reductase inhibitors may impair clitoral growth and the development of facial and body hair. Further studies are needed to assess the efficacy and safety of 5α-reductase inhibitors in transgender populations.1 Dutasteride may be used off-label and comes with a similar potential adverse-event profile as finasteride, which includes depression, decreased libido, erectile dysfunction, ejaculation disorders, and gynecomastia.
Conversely, AGA tends to improve in trans feminine persons and others receiving estrogen and antiandrogen therapy. Natural testosterone production is suppressed by estrogens and spironolactone as well as in patients who undergo orchiectomy.1 Although spironolactone is not approved for acne, AGA, or hirsutism, it is a standard treatment of AGA in cisgender women because it functions to block the effects of androgens, including at the hair follicle. Finasteride may be used for AGA in cisgender women but it is not recommended for trans feminine persons.1
There are many other modalities available for the treatment of AGA that are less commonly used—some may be cost prohibitive or do not have robust supporting evidence, or both. One example is hair
Melasma is a hyperpigmentation disorder related to estrogens, UV light exposure, and sometimes medication use (eg, hormonal birth control, spironolactone).24 The mainstay of treatment is prevention, including sun avoidance as well as use of sun-protective clothing and broad-spectrum sunscreens. Dermatologists tend to recommend physical sunscreens containing zinc oxide, titanium dioxide, and/or iron oxide, as they cover a wider UV spectrum and also provide some protection from visible light. Once melasma is present, dermatologists still have several treatment options. Topical hydroquinone is a proven treatment; however, it must be used with caution to avoid ochronosis. With careful patient selection, chemical peels also are effective treatment options for dyspigmentation and hyperpigmentation. Energy devices such as intense pulsed light and tattoo removal lasers—Q-switched lasers and picosecond pulse widths—also can be used to treat hyperpigmentation. Oral, intralesional, and topical tranexamic acid are newer treatment options for melasma that still are being studied and have shown promising results. Further studies are needed to determine long-term safety and optimal treatment regimens.24,25
Many insurance carriers, including TRICARE, do not routinely cover medical management of AGA or melasma. Patients should be advised that they likely will have to pay for any medications prescribed and procedures undertaken for these purposes; however, some medication costs can be offset by ordering larger prescription quantities, such as a 90-day supply vs a 30-day supply, as well as utilizing pharmacy discount programs.
Scar Management Following Surgery
In TSMs who undergo gender-affirming surgeries, dermatologists play an important role when scar symptoms develop, including pruritus, tenderness, and/or paresthesia. In the military, some common treatment modalities for symptomatic scars include intralesional steroids with or without 5-fluouroruacil and the fractionated CO2 laser. There also are numerous experimental treatment options for scars, including intralesional or perilesional botulinum toxin, the pulsed dye laser, or nonablative fractionated lasers. These modalities also may be used on hypertrophic scars or keloids. Another option for keloids is scar excision followed by superficial radiation therapy.26
Mental Health Considerations
Providers must take psychological adverse effects into consideration when considering medical therapies for dermatologic conditions in TGD patients. In particular, it is important to consider the risks for increased rates of depression and suicidal ideation formerly associated with the use of isotretinoin and finasteride, though much of the evidence regarding these risks has been called into question in recent years.27,28 Nonetheless, it remains prominent in lay media and may be a more important consideration in patients at higher baseline risk.27 Although there are no known studies that have expressly assessed rates of depression or suicidal ideation in TGD patients taking isotretinoin or finasteride, it is well established that TGD persons are at higher baseline risk for depression and suicidality.1,7,8 All patients should be carefully assessed for depression and suicidal ideation as well as counseled regarding these risks prior to initiating these therapies. If concerns for untreated mental health issues arise during screening and counseling, patients should be referred for assessment by a behavioral health specialist prior to starting therapy.
Future Directions
The future of TGD health care in the military could see an expansion of covered benefits and the development of new dermatologic procedures or medications. Research and policy evolution are necessary to bridge the current gaps in care; however, it is unlikely that all procedures currently considered to be cosmetic will become covered benefits.
Facial LHR is a promising candidate for future coverage for trans feminine persons. When cisgender men develop adverse effects from mandatory daily shaving, LHR is already a covered benefit. Two arguments in support of adding LHR for TGD patients revolve around achieving and maintaining an appearance congruent with their gender along with avoiding unwanted adverse effects related to daily shaving. Visual conformity with one’s affirmed gender has been associated with improvements in well-being, quality of life, and some mental health conditions.29
Scar prevention, treatment, and reduction are additional areas under active research in which dermatologists likely will play a crucial role.30,31 As more dermatologic procedures are performed on TGD persons, the published data and collective knowledge regarding best practices in this population will continue to grow, which will lead to improved cosmetic and safety outcomes.
Final Thoughts
Although dermatologists do not directly perform gender-affirming surgeries or hormone management, they do play an important role in enhancing a TGD person’s desired appearance and managing possible adverse effects resulting from gender-affirming interventions. There have been considerable advancements in TGD health care over the past decade, but there likely are more changes on the way. As policies and understanding of TGD health care needs evolve, it is crucial that the military health care system adapts to provide comprehensive, accessible, and equitable care, which includes expanding the range of covered dermatologic treatments to fully support the health and readiness of TSMs.
Acknowledgment—We would like to extend our sincere appreciation to the invaluable contributions and editorial support provided by Allison Higgins, JD (San Antonio, Texas), throughout the writing of this article.
- Coleman E, Radix AE, Bouman WP, et al. Standards of care for the health of transgender and gender diverse people, version 8. Int J Transgend Health. 2022;23(suppl 1):S1-S260. doi:10.1080/26895269.2022.2100644
- Secretary of Defense. DTM 16-005—military service of transgender service members. June 30, 2016. Accessed June 17, 2024. https://dod.defense.gov/Portals/1/features/2016/0616_policy/DTM-16-005.pdf
- Office of the Deputy Secretary of Defense. DTM 19-004—military service by transgender persons and persons with gender dysphoria. March 17, 2020. Accessed June 17, 2024. https://health.mil/Reference-Center/Policies/2020/03/17/Military-Service-by-Transgender-Persons-and-Persons-with-Gender-Dysphoria
- Office of the Under Secretary of Defense for Personnel and Readiness. Department of Defense Instruction (DODI) 1300.28. in-service transition for transgender service members. September 4, 2020. Accessed June 17, 2024. https://health.mil/Reference-Center/Policies/2020/09/04/Military-Service-by-Transgender-Persons-and-Persons-with-Gender-Dysphoria
- Defense Health Agency Procedural Instruction Number 6025.21, Guidance for Gender-Affirming Health Care of Transgender and Gender-Diverse Active and Reserve Component Service Members, May 12, 2023. https://www.health.mil/Reference-Center/DHA-Publications/2023/05/12/DHA-PI-6015-21
- Elders MJ, Brown GR, Coleman E, et al. Medical aspects of transgender military service. Armed Forces Soc. 2015;41:199-220. doi:10.1177/0095327X14545625.
- Almazan AN, Keuroghlian AS. Association between gender-affirming surgeries and mental health outcomes. JAMA Surg. 2021;156:611-618.
- Tordoff DM, Wanta JW, Collin A, et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Netw Open. 2022;5:E220978. doi:10.1001/jamanetworkopen.2022.0978
- Olson-Kennedy J, Warus J, Okonta V, et al. Chest reconstruction and chest dysphoria in transmasculine minors and young adults: comparisons of nonsurgical and postsurgical cohorts. JAMA Pediatr. 2018;172:431-436. doi:10.1001/jamapediatrics.2017.5440
- Top non-invasive cosmetic procedures worldwide 2022. Statista website. February 8, 2024. Accessed June 13, 2024. https://www.statista.com/statistics/293449/leading-nonsurgical-cosmetic-procedures/
- Kashkouli MB, Abdolalizadeh P, Abolfathzadeh N, et al. Periorbital facial rejuvenation; applied anatomy and pre-operative assessment. J Curr Ophthalmol. 2017;29:154-168. doi:10.1016/j.joco.2017.04.001
- Thomas MK, D’Silva JA, Borole AJ. Injection lipolysis: a systematic review of literature and our experience with a combination of phosphatidylcholine and deoxycholate over a period of 14 years in 1269 patients of Indian and South East Asian origin. J Cutan Aesthet Surg. 2018;11:222-228. doi:10.4103/JCAS.JCAS_117_18
- Jegasothy SM. Deoxycholic acid injections for bra-line lipolysis. Dermatol Surg. 2018;44:757-760. doi:10.1097/DSS.0000000000001311
- Dierickx CC. Hair removal by lasers and intense pulsed light sources. Dermatol Clin. 2002;20:135-146. doi:10.1016/s0733-8635(03)00052-4
- Lepselter J, Elman M. Biological and clinical aspects in laser hair removal. J Dermatolog Treat. 2004;15:72-83. doi:10.1080/09546630310023152
- Yuan N, Feldman AT, Chin P, et al. Comparison of permanent hair removal procedures before gender-affirming vaginoplasty: why we should consider laser hair removal as a first-line treatment for patients who meet criteria. Sex Med. 2022;10:100545. doi:10.1016/j.esxm.2022.100545
- Kumar A, Naguib YW, Shi YC, et al. A method to improve the efficacy of topical eflornithine hydrochloride cream. Drug Deliv. 2016;23:1495-1501. doi:10.3109/10717544.2014.951746
- Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metabol. 2017;102:3869-3903.
- Hatami P, Balighi K, Asl HN, et al. Isotretinoin and timing of procedural interventions: clinical implications and practical points. J Cosmet Dermatol. 2023;22:2146-2149. doi:10.1111/jocd.15874
- Rubenstein R, Roenigk HH Jr, Stegman SJ, et al. Atypical keloids after dermabrasion of patients taking isotretinoin. J Am Acad Dermatol. 1986;15(2 pt 1):280-285.
- Zachariae H. Delayed wound healing and keloid formation following argon laser treatment or dermabrasion during isotretinoin treatment. Br J Dermatol. 1988;118:703-706.
- Goldberg D, Kothare A, Doucette M, et al. Selective photothermolysis with a novel 1726 nm laser beam: a safe and effective solution for acne vulgaris. J Cosmet Dermatol. 2023;22:486-496. doi:10.1111/jocd.15602
- Sun HY, Sebaratnam DF. Clascoterone as a novel treatment for androgenetic alopecia. Clin Exp Dermatol. 2020;45:913-914. doi:10.1111/ced.14292
- Bolognia JL, Schaffer JV, Cerroni L. Dermatology: 2-Volume Set. Elsevier; 2024:1130.
- Konisky H, Balazic E, Jaller JA, et al. Tranexamic acid in melasma: a focused review on drug administration routes. J Cosmet Dermatol. 2023;22:1197-1206. doi:10.1111/jocd.15589
- Walsh LA, Wu E, Pontes D, et al. Keloid treatments: an evidence-based systematic review of recent advances. Syst Rev. 2023;12:42. doi:10.1186/s13643-023-02192-7
- Kridin K, Ludwig RJ. Isotretinoin and the risk of psychiatric disturbances: a global study shedding new light on a debatable story. J Am Acad Dermatol. 2023;88:388-394. doi:10.1016/j.jaad.2022.10.031
- Dyson TE, Cantrell MA, Lund BC. Lack of association between 5α-reductase inhibitors and depression. J Urol. 2020;204:793-798. doi:10.1097/JU.0000000000001079
- To M, Zhang Q, Bradlyn A, et al. Visual conformity with affirmed gender or “passing”: its distribution and association with depression and anxiety in a cohort of transgender people. J Sex Med. 2020;17:2084-2092. doi:10.1016/j.jsxm.2020.07.019
- Fernandes MG, da Silva LP, Cerqueira MT, et al. Mechanomodulatory biomaterials prospects in scar prevention and treatment. Acta Biomater. 2022;150:22-33. doi:10.1016/j.actbio.2022.07.042
- Kolli H, Moy RL. Prevention of scarring with intraoperative erbium:YAG laser treatment. J Drugs Dermatol. 2020;19:1040-1043. doi:10.36849/JDD.2020.5244
People whose gender identity differs from the sex assigned at birth are referred to as transgender. For some, gender identity may not fit into the binary constructs of male and female but rather falls between, within, or outside this construct. These people often consider themselves nonbinary or gender diverse. As the terminology continues to evolve, current recommendations include referring to this patient population as transgender and gender diverse (TGD) to ensure the broadest inclusivity.1 In this article, the following terms are used as defined below:
- The terms transgender woman and trans feminine describe persons who were assigned male gender at birth but their affirmed gender is female or nonmasculine.
- The terms transgender man and trans masculine describe persons who were assigned female gender at birth but their affirmed gender is male or nonfeminine.
The US Military’s policies on the service of TGD persons have evolved considerably over the past decade. Initial military policies barred TGD service members (TSMs) from service all together, leading to challenges in accessing necessary health care. The first official memorandum explicitly allowing military service by TGD persons was released on June 30, 2016.2 The intention of this memorandum was 2-fold: (1) to allow TGD persons to serve in the military so long as they meet “the rigorous standards for military service and readiness” by fulfilling the same standards and procedures as other military service members, including medical fitness for duty, physical fitness, uniform and grooming, deployability, and retention, and (2) to direct the establishment of new or updated policies to specific departments and prescribe procedures for retention standards, separation from service, in-service transition, and medical coverage.2 Several other official policies were released following this initial memorandum that provided more specific guidance on how to implement these policies at the level of the force, unit, and individual service member.
Modifications to the original 2016 policies had varying impacts on transgender health care provision and access.3 At the time of publication of this article, the current policy—the Department of Defense Instruction 1300.284—among others, establishes standards and procedures for the process by which active and reserve TSMs may medically, socially, and legally transition genders within the military. The current policy applies to all military branches and serves as the framework by which each branch currently organizes their gender-affirmation processes (GAP).4
There currently are several different GAP models among the military branches.5 Each branch has a different model or approach to implementing the current policy, with varying service-specific processes in place for TSMs to access gender-affirming care; however, this may be changing. The Defense Health Agency is in the process of consolidating and streamlining the GAP across the Department of Defense branches in an effort to optimize costs and ensure uniformity of care. Per the Defense Health Agency Procedural Instruction Number 6025.21 published in May 2023, the proposed consolidated model likely will entail a single central transgender health center that provides oversight and guidance for several regional joint-service gender-affirming medical hubs. Patients would either be managed at the level of the hub or be referred to the central site.5
Herein, we discuss the importance of gender-affirming care and how military and civilian dermatologists can contribute. We also review disparities in health care and identify areas of improvement.
Benefits of Gender-Affirming Care
Gender-affirming procedures are critical for aligning physical appearance with gender identity. Physical appearance is essential for psychological well-being, operational readiness, and the safety of TSMs.6 It is well documented that TGD persons experience suicidal ideation, depression, stigma, discrimination and violence at higher rates than their cisgender peers.7,8 It is important to recognize that transgender identity is not a mental illness, and these elevated rates have been linked to complex trauma, societal stigma, violence, and discrimination.1 Other studies have suggested that increased access to gender-affirming interventions may ameliorate these mental health concerns.1,7-9
The major components of gender-affirming care include hormone therapy, gender confirmation surgery, and mental health care, if needed. These are covered by TRICARE, the health care program for military service members; however, at the time of publication, many of the dermatologic gender-affirming procedures are not covered by TRICARE because they are considered “cosmetic procedures,” which is a term used by insurance companies but does not accurately indicate whether a procedure is medically necessary or not. Newer literature has demonstrated that gender-affirming care positively affects the lives of TGD patients, strengthening the argument that gender-affirming care is a medical necessity and not just cosmetic.1
Aesthetic Procedures in Gender-Affirming Care
Surgeons, including those within the specialties of oto-laryngology, oral and maxillofacial surgery, urology, gynecology, and plastic surgery, provide major gender-affirming interventions; however, dermatologists may offer less invasive solutions that can serve as a temporary experience prior to undergoing more permanent procedures.Hormonally driven disorders including acne, hair loss, and melasma also are managed by dermatologists, along with scar treatment following surgeries.
Because human variation is expansive and subjective, what is considered feminine or masculine may vary by person, group, culture, and country; therefore, it is imperative to ask patients about their individual aesthetic goals and tailor their treatment accordingly. Feminine and masculine are terms that will be used to describe prototypical appearances and are not meant to define a patient’s current state or ultimate goals. The following procedures and medical interventions are where dermatologists can play an important role in TGD persons’ GAPs.
Botulinum Toxin Injections—Botulinum toxin injection is the most common nonsurgical aesthetic procedure performed around the world.10 The selective paralysis afforded by botulinum toxin has several uses for people undergoing transition. Aesthetically, the feminine eyebrow tends to be positioned above the orbital rim and is arched with its apex between the lateral limbus and lateral canthus,11 while the masculine eyebrow tends to be flatter and fuller and runs over the orbital rim without a peak. For people seeking a more feminine appearance, an eyebrow lift with botulinum toxin can help reshape the typical flatter masculine eyebrow to give it lateral lift that often is considered more feminine. The targeted muscle is the superolateral orbicularis oculi, which serves as a depressor on the eyebrow. This can be combined with purposefully avoiding total lateral frontalis paralysis, which leads to a “Spock” brow for extra lift. Conversely, a naturally arched and higher eyebrow can be flattened and lowered by selectively targeting areas of the frontalis muscle.
Broad square jawlines typically are considered a masculine feature and are another area where botulinum toxin can be used to feminize a patient’s facial features. Targeting the masseter muscle induces muscle weakness, which ultimately may result in atrophy after one or more treatment sessions. This atrophy may lead to narrowing of the lower face and thus may lead to a fuller-appearing midface or overall more heart-shaped face. Every individual’s aesthetic goals are unique and therefore should be discussed prior to any treatment.
Dermal Fillers—Dermal fillers are gel-like substances injected under the skin for subtle contouring of the face. Fillers also can be used to help promote a more masculine or feminine appearance. Filler can be placed in the lips to create a fuller, more projected, feminine-appearing lip. Malar cheek and central lower chin filler can be used to help define a heart-shaped face by accentuating the upper portion of the face and creating a more pointed chin, respectively. Alternatively, filler can be used to masculinize the chin by placing it where it can increase jawline squareness and increase anterior jaw projection. Additionally, filler at the angle of the jaw can help accentuate a square facial shape and a more defined jawline. Although not as widely practiced, lateral brow filler can create a heavier-appearing and broader forehead for a more masculine appearance. These procedures can be combined with the previously mentioned botulinum toxin procedures for a synergistic effect.
Deoxycholic Acid—Deoxycholic acid is an injectable product used to selectively remove unwanted fat. It currently is approved by the US Food and Drug Administration for submental fat, but some providers are experimenting with off-label uses. Buccal fat pad removal—or in this case reduction by dissolution—tends to give a thinner, more feminine facial appearance.12 Reducing fat around the axillae also can help promote a more masculine upper torso.13 The safety of deoxycholic acid in these areas has not been adequately tested; thus, caution should be used when discussing these off-label uses with patients.
Hair and Tattoo Removal—Hair removal may be desired by TGD persons for a variety of reasons. Because cisgender females tend to have less body hair overall, transgender people in pursuit of a more feminine appearance often desire removal of facial, neck, and body hair. Although shaving and other modalities such as waxing and chemical depilatories are readily available at-home options, they are not permanent and may lead to folliculitis or pseudofolliculitis barbae. Laser hair removal (LHR) and electrolysis are modalities provided by dermatologists that tend to be more permanent and lead to better outcomes, including less irritation and better aesthetic appearance. It is important to keep in mind that not every person and not every body site can be safely treated with LHR. Patients with lighter skin types and darker hair tend to have the most effective response with a higher margin of safety, as these features allow the laser energy to be selectively absorbed by the melanin in the hair bulb and not by the background skin pigmentation.14,15 Inappropriate patient selection or improper settings for wavelength, pulse width, or fluences can lead to burns and permanent scarring.14,15 Electrolysis is an alternative to hair removal within tattoos and is more effective for those individuals with blonde, red, or white hair.16
Another novel treatment for unwanted hair is eflornithine hydrochloride cream, which works by blocking ornithine decarboxylase, the enzyme that stimulates hair growth. It currently is approved to reduce unwanted hair on the face and adjacent areas under the chin; however the effects of this medication are modest and the medication can be expensive.17
Cosmetic hair and tattoo removal are not currently covered by TRICARE, except in cases of surgical and donor-site preparation for some GAPs. Individuals may desire removal of tattoos at surgery sites to obtain more natural-appearing skin. Currently, GAPs such as vaginoplasty, phalloplasty, and metoidioplasty—often referred to by patients as “bottom surgeries”—include insurance coverage for tattoo removal, LHR, and/or electrolysis.
Management of Hormonal Adverse Effects
Acne—Individuals on testosterone supplementation tend to develop acne for the first several years of treatment, but it may improve with time.18 Acne is treated in individuals receiving testosterone the same way as it is treated in cisgender men, with numerous options for topical and oral medications. In trans masculine persons, spironolactone therapy typically is avoided because it may interfere with the actions of exogenous testosterone administered as part of gender-affirming medical treatment and may lead to other undesired adverse effects such as impotence and gynecomastia.1
Although acne typically improves after starting estrogen therapy, patients receiving estrogens may still develop acne. Most trans feminine patients will already be on an estrogen and an antiandrogen, often spironolactone.1 Spironolactone often is used as monotherapy for acne control in cisgender women. Additionally, an important factor to consider with spironolactone is the possible adverse effect of increased micturition. Currently, the military rarely has gender-inclusive restroom options, which can create a challenge for TSMs who find themselves needing to use the restroom more frequently in the workplace.
If planning therapy with isotretinoin, dermatologists should discuss several important factors with all patients, including TGD patients. One consideration is the patient’s planned future surgeries. Although new literature shows that isotretinoin does not adversely affect wound healing,19 some surgeons still adhere to an isotretinoin washout period of 6 to 12 months prior to performing any elective procedures due to concerns about wound healing.20,21 Second, be sure to properly assess and document pregnancy potential in TGD persons. Providers should not assume that a patient is not pregnant or is not trying to become pregnant just because they are trans masculine. It also is important to note that testosterone is not a reliable birth control method.1 If a patient still has ovaries, fallopian tubes, and a uterus, they are considered medically capable of pregnancy, and providers should keep this in mind regarding all procedures in the TGD population.
Another newer acne treatment modality is the 1762-nm laser, which targets sebaceous glands.22 This device allows for targeted treatment of acne-prone areas without systemic therapy such as retinoids or antiandrogens. The 1762-nm laser is not widely available but may become a regular treatment option once its benefits are proven over time.
Alopecia and Hyperpigmentation—Androgens, whether endogenously or exogenously derived, can lead to androgenetic alopecia (AGA) in genetically susceptible individuals. Trans masculine persons and others receiving androgen therapy are at higher risk for AGA, which often is undesirable and may be considered gender affirming by some TGD persons. Standard AGA treatments for cisgender men also can be used in trans masculine persons. Some of the most common anti-AGA medications are topical minoxidil, oral finasteride, and oral minoxidil. Although Coleman et al1 recently reported that finasteride may be an appropriate treatment option in trans masculine persons experiencing alopecia, treatment with 5α-reductase inhibitors may impair clitoral growth and the development of facial and body hair. Further studies are needed to assess the efficacy and safety of 5α-reductase inhibitors in transgender populations.1 Dutasteride may be used off-label and comes with a similar potential adverse-event profile as finasteride, which includes depression, decreased libido, erectile dysfunction, ejaculation disorders, and gynecomastia.
Conversely, AGA tends to improve in trans feminine persons and others receiving estrogen and antiandrogen therapy. Natural testosterone production is suppressed by estrogens and spironolactone as well as in patients who undergo orchiectomy.1 Although spironolactone is not approved for acne, AGA, or hirsutism, it is a standard treatment of AGA in cisgender women because it functions to block the effects of androgens, including at the hair follicle. Finasteride may be used for AGA in cisgender women but it is not recommended for trans feminine persons.1
There are many other modalities available for the treatment of AGA that are less commonly used—some may be cost prohibitive or do not have robust supporting evidence, or both. One example is hair
Melasma is a hyperpigmentation disorder related to estrogens, UV light exposure, and sometimes medication use (eg, hormonal birth control, spironolactone).24 The mainstay of treatment is prevention, including sun avoidance as well as use of sun-protective clothing and broad-spectrum sunscreens. Dermatologists tend to recommend physical sunscreens containing zinc oxide, titanium dioxide, and/or iron oxide, as they cover a wider UV spectrum and also provide some protection from visible light. Once melasma is present, dermatologists still have several treatment options. Topical hydroquinone is a proven treatment; however, it must be used with caution to avoid ochronosis. With careful patient selection, chemical peels also are effective treatment options for dyspigmentation and hyperpigmentation. Energy devices such as intense pulsed light and tattoo removal lasers—Q-switched lasers and picosecond pulse widths—also can be used to treat hyperpigmentation. Oral, intralesional, and topical tranexamic acid are newer treatment options for melasma that still are being studied and have shown promising results. Further studies are needed to determine long-term safety and optimal treatment regimens.24,25
Many insurance carriers, including TRICARE, do not routinely cover medical management of AGA or melasma. Patients should be advised that they likely will have to pay for any medications prescribed and procedures undertaken for these purposes; however, some medication costs can be offset by ordering larger prescription quantities, such as a 90-day supply vs a 30-day supply, as well as utilizing pharmacy discount programs.
Scar Management Following Surgery
In TSMs who undergo gender-affirming surgeries, dermatologists play an important role when scar symptoms develop, including pruritus, tenderness, and/or paresthesia. In the military, some common treatment modalities for symptomatic scars include intralesional steroids with or without 5-fluouroruacil and the fractionated CO2 laser. There also are numerous experimental treatment options for scars, including intralesional or perilesional botulinum toxin, the pulsed dye laser, or nonablative fractionated lasers. These modalities also may be used on hypertrophic scars or keloids. Another option for keloids is scar excision followed by superficial radiation therapy.26
Mental Health Considerations
Providers must take psychological adverse effects into consideration when considering medical therapies for dermatologic conditions in TGD patients. In particular, it is important to consider the risks for increased rates of depression and suicidal ideation formerly associated with the use of isotretinoin and finasteride, though much of the evidence regarding these risks has been called into question in recent years.27,28 Nonetheless, it remains prominent in lay media and may be a more important consideration in patients at higher baseline risk.27 Although there are no known studies that have expressly assessed rates of depression or suicidal ideation in TGD patients taking isotretinoin or finasteride, it is well established that TGD persons are at higher baseline risk for depression and suicidality.1,7,8 All patients should be carefully assessed for depression and suicidal ideation as well as counseled regarding these risks prior to initiating these therapies. If concerns for untreated mental health issues arise during screening and counseling, patients should be referred for assessment by a behavioral health specialist prior to starting therapy.
Future Directions
The future of TGD health care in the military could see an expansion of covered benefits and the development of new dermatologic procedures or medications. Research and policy evolution are necessary to bridge the current gaps in care; however, it is unlikely that all procedures currently considered to be cosmetic will become covered benefits.
Facial LHR is a promising candidate for future coverage for trans feminine persons. When cisgender men develop adverse effects from mandatory daily shaving, LHR is already a covered benefit. Two arguments in support of adding LHR for TGD patients revolve around achieving and maintaining an appearance congruent with their gender along with avoiding unwanted adverse effects related to daily shaving. Visual conformity with one’s affirmed gender has been associated with improvements in well-being, quality of life, and some mental health conditions.29
Scar prevention, treatment, and reduction are additional areas under active research in which dermatologists likely will play a crucial role.30,31 As more dermatologic procedures are performed on TGD persons, the published data and collective knowledge regarding best practices in this population will continue to grow, which will lead to improved cosmetic and safety outcomes.
Final Thoughts
Although dermatologists do not directly perform gender-affirming surgeries or hormone management, they do play an important role in enhancing a TGD person’s desired appearance and managing possible adverse effects resulting from gender-affirming interventions. There have been considerable advancements in TGD health care over the past decade, but there likely are more changes on the way. As policies and understanding of TGD health care needs evolve, it is crucial that the military health care system adapts to provide comprehensive, accessible, and equitable care, which includes expanding the range of covered dermatologic treatments to fully support the health and readiness of TSMs.
Acknowledgment—We would like to extend our sincere appreciation to the invaluable contributions and editorial support provided by Allison Higgins, JD (San Antonio, Texas), throughout the writing of this article.
People whose gender identity differs from the sex assigned at birth are referred to as transgender. For some, gender identity may not fit into the binary constructs of male and female but rather falls between, within, or outside this construct. These people often consider themselves nonbinary or gender diverse. As the terminology continues to evolve, current recommendations include referring to this patient population as transgender and gender diverse (TGD) to ensure the broadest inclusivity.1 In this article, the following terms are used as defined below:
- The terms transgender woman and trans feminine describe persons who were assigned male gender at birth but their affirmed gender is female or nonmasculine.
- The terms transgender man and trans masculine describe persons who were assigned female gender at birth but their affirmed gender is male or nonfeminine.
The US Military’s policies on the service of TGD persons have evolved considerably over the past decade. Initial military policies barred TGD service members (TSMs) from service all together, leading to challenges in accessing necessary health care. The first official memorandum explicitly allowing military service by TGD persons was released on June 30, 2016.2 The intention of this memorandum was 2-fold: (1) to allow TGD persons to serve in the military so long as they meet “the rigorous standards for military service and readiness” by fulfilling the same standards and procedures as other military service members, including medical fitness for duty, physical fitness, uniform and grooming, deployability, and retention, and (2) to direct the establishment of new or updated policies to specific departments and prescribe procedures for retention standards, separation from service, in-service transition, and medical coverage.2 Several other official policies were released following this initial memorandum that provided more specific guidance on how to implement these policies at the level of the force, unit, and individual service member.
Modifications to the original 2016 policies had varying impacts on transgender health care provision and access.3 At the time of publication of this article, the current policy—the Department of Defense Instruction 1300.284—among others, establishes standards and procedures for the process by which active and reserve TSMs may medically, socially, and legally transition genders within the military. The current policy applies to all military branches and serves as the framework by which each branch currently organizes their gender-affirmation processes (GAP).4
There currently are several different GAP models among the military branches.5 Each branch has a different model or approach to implementing the current policy, with varying service-specific processes in place for TSMs to access gender-affirming care; however, this may be changing. The Defense Health Agency is in the process of consolidating and streamlining the GAP across the Department of Defense branches in an effort to optimize costs and ensure uniformity of care. Per the Defense Health Agency Procedural Instruction Number 6025.21 published in May 2023, the proposed consolidated model likely will entail a single central transgender health center that provides oversight and guidance for several regional joint-service gender-affirming medical hubs. Patients would either be managed at the level of the hub or be referred to the central site.5
Herein, we discuss the importance of gender-affirming care and how military and civilian dermatologists can contribute. We also review disparities in health care and identify areas of improvement.
Benefits of Gender-Affirming Care
Gender-affirming procedures are critical for aligning physical appearance with gender identity. Physical appearance is essential for psychological well-being, operational readiness, and the safety of TSMs.6 It is well documented that TGD persons experience suicidal ideation, depression, stigma, discrimination and violence at higher rates than their cisgender peers.7,8 It is important to recognize that transgender identity is not a mental illness, and these elevated rates have been linked to complex trauma, societal stigma, violence, and discrimination.1 Other studies have suggested that increased access to gender-affirming interventions may ameliorate these mental health concerns.1,7-9
The major components of gender-affirming care include hormone therapy, gender confirmation surgery, and mental health care, if needed. These are covered by TRICARE, the health care program for military service members; however, at the time of publication, many of the dermatologic gender-affirming procedures are not covered by TRICARE because they are considered “cosmetic procedures,” which is a term used by insurance companies but does not accurately indicate whether a procedure is medically necessary or not. Newer literature has demonstrated that gender-affirming care positively affects the lives of TGD patients, strengthening the argument that gender-affirming care is a medical necessity and not just cosmetic.1
Aesthetic Procedures in Gender-Affirming Care
Surgeons, including those within the specialties of oto-laryngology, oral and maxillofacial surgery, urology, gynecology, and plastic surgery, provide major gender-affirming interventions; however, dermatologists may offer less invasive solutions that can serve as a temporary experience prior to undergoing more permanent procedures.Hormonally driven disorders including acne, hair loss, and melasma also are managed by dermatologists, along with scar treatment following surgeries.
Because human variation is expansive and subjective, what is considered feminine or masculine may vary by person, group, culture, and country; therefore, it is imperative to ask patients about their individual aesthetic goals and tailor their treatment accordingly. Feminine and masculine are terms that will be used to describe prototypical appearances and are not meant to define a patient’s current state or ultimate goals. The following procedures and medical interventions are where dermatologists can play an important role in TGD persons’ GAPs.
Botulinum Toxin Injections—Botulinum toxin injection is the most common nonsurgical aesthetic procedure performed around the world.10 The selective paralysis afforded by botulinum toxin has several uses for people undergoing transition. Aesthetically, the feminine eyebrow tends to be positioned above the orbital rim and is arched with its apex between the lateral limbus and lateral canthus,11 while the masculine eyebrow tends to be flatter and fuller and runs over the orbital rim without a peak. For people seeking a more feminine appearance, an eyebrow lift with botulinum toxin can help reshape the typical flatter masculine eyebrow to give it lateral lift that often is considered more feminine. The targeted muscle is the superolateral orbicularis oculi, which serves as a depressor on the eyebrow. This can be combined with purposefully avoiding total lateral frontalis paralysis, which leads to a “Spock” brow for extra lift. Conversely, a naturally arched and higher eyebrow can be flattened and lowered by selectively targeting areas of the frontalis muscle.
Broad square jawlines typically are considered a masculine feature and are another area where botulinum toxin can be used to feminize a patient’s facial features. Targeting the masseter muscle induces muscle weakness, which ultimately may result in atrophy after one or more treatment sessions. This atrophy may lead to narrowing of the lower face and thus may lead to a fuller-appearing midface or overall more heart-shaped face. Every individual’s aesthetic goals are unique and therefore should be discussed prior to any treatment.
Dermal Fillers—Dermal fillers are gel-like substances injected under the skin for subtle contouring of the face. Fillers also can be used to help promote a more masculine or feminine appearance. Filler can be placed in the lips to create a fuller, more projected, feminine-appearing lip. Malar cheek and central lower chin filler can be used to help define a heart-shaped face by accentuating the upper portion of the face and creating a more pointed chin, respectively. Alternatively, filler can be used to masculinize the chin by placing it where it can increase jawline squareness and increase anterior jaw projection. Additionally, filler at the angle of the jaw can help accentuate a square facial shape and a more defined jawline. Although not as widely practiced, lateral brow filler can create a heavier-appearing and broader forehead for a more masculine appearance. These procedures can be combined with the previously mentioned botulinum toxin procedures for a synergistic effect.
Deoxycholic Acid—Deoxycholic acid is an injectable product used to selectively remove unwanted fat. It currently is approved by the US Food and Drug Administration for submental fat, but some providers are experimenting with off-label uses. Buccal fat pad removal—or in this case reduction by dissolution—tends to give a thinner, more feminine facial appearance.12 Reducing fat around the axillae also can help promote a more masculine upper torso.13 The safety of deoxycholic acid in these areas has not been adequately tested; thus, caution should be used when discussing these off-label uses with patients.
Hair and Tattoo Removal—Hair removal may be desired by TGD persons for a variety of reasons. Because cisgender females tend to have less body hair overall, transgender people in pursuit of a more feminine appearance often desire removal of facial, neck, and body hair. Although shaving and other modalities such as waxing and chemical depilatories are readily available at-home options, they are not permanent and may lead to folliculitis or pseudofolliculitis barbae. Laser hair removal (LHR) and electrolysis are modalities provided by dermatologists that tend to be more permanent and lead to better outcomes, including less irritation and better aesthetic appearance. It is important to keep in mind that not every person and not every body site can be safely treated with LHR. Patients with lighter skin types and darker hair tend to have the most effective response with a higher margin of safety, as these features allow the laser energy to be selectively absorbed by the melanin in the hair bulb and not by the background skin pigmentation.14,15 Inappropriate patient selection or improper settings for wavelength, pulse width, or fluences can lead to burns and permanent scarring.14,15 Electrolysis is an alternative to hair removal within tattoos and is more effective for those individuals with blonde, red, or white hair.16
Another novel treatment for unwanted hair is eflornithine hydrochloride cream, which works by blocking ornithine decarboxylase, the enzyme that stimulates hair growth. It currently is approved to reduce unwanted hair on the face and adjacent areas under the chin; however the effects of this medication are modest and the medication can be expensive.17
Cosmetic hair and tattoo removal are not currently covered by TRICARE, except in cases of surgical and donor-site preparation for some GAPs. Individuals may desire removal of tattoos at surgery sites to obtain more natural-appearing skin. Currently, GAPs such as vaginoplasty, phalloplasty, and metoidioplasty—often referred to by patients as “bottom surgeries”—include insurance coverage for tattoo removal, LHR, and/or electrolysis.
Management of Hormonal Adverse Effects
Acne—Individuals on testosterone supplementation tend to develop acne for the first several years of treatment, but it may improve with time.18 Acne is treated in individuals receiving testosterone the same way as it is treated in cisgender men, with numerous options for topical and oral medications. In trans masculine persons, spironolactone therapy typically is avoided because it may interfere with the actions of exogenous testosterone administered as part of gender-affirming medical treatment and may lead to other undesired adverse effects such as impotence and gynecomastia.1
Although acne typically improves after starting estrogen therapy, patients receiving estrogens may still develop acne. Most trans feminine patients will already be on an estrogen and an antiandrogen, often spironolactone.1 Spironolactone often is used as monotherapy for acne control in cisgender women. Additionally, an important factor to consider with spironolactone is the possible adverse effect of increased micturition. Currently, the military rarely has gender-inclusive restroom options, which can create a challenge for TSMs who find themselves needing to use the restroom more frequently in the workplace.
If planning therapy with isotretinoin, dermatologists should discuss several important factors with all patients, including TGD patients. One consideration is the patient’s planned future surgeries. Although new literature shows that isotretinoin does not adversely affect wound healing,19 some surgeons still adhere to an isotretinoin washout period of 6 to 12 months prior to performing any elective procedures due to concerns about wound healing.20,21 Second, be sure to properly assess and document pregnancy potential in TGD persons. Providers should not assume that a patient is not pregnant or is not trying to become pregnant just because they are trans masculine. It also is important to note that testosterone is not a reliable birth control method.1 If a patient still has ovaries, fallopian tubes, and a uterus, they are considered medically capable of pregnancy, and providers should keep this in mind regarding all procedures in the TGD population.
Another newer acne treatment modality is the 1762-nm laser, which targets sebaceous glands.22 This device allows for targeted treatment of acne-prone areas without systemic therapy such as retinoids or antiandrogens. The 1762-nm laser is not widely available but may become a regular treatment option once its benefits are proven over time.
Alopecia and Hyperpigmentation—Androgens, whether endogenously or exogenously derived, can lead to androgenetic alopecia (AGA) in genetically susceptible individuals. Trans masculine persons and others receiving androgen therapy are at higher risk for AGA, which often is undesirable and may be considered gender affirming by some TGD persons. Standard AGA treatments for cisgender men also can be used in trans masculine persons. Some of the most common anti-AGA medications are topical minoxidil, oral finasteride, and oral minoxidil. Although Coleman et al1 recently reported that finasteride may be an appropriate treatment option in trans masculine persons experiencing alopecia, treatment with 5α-reductase inhibitors may impair clitoral growth and the development of facial and body hair. Further studies are needed to assess the efficacy and safety of 5α-reductase inhibitors in transgender populations.1 Dutasteride may be used off-label and comes with a similar potential adverse-event profile as finasteride, which includes depression, decreased libido, erectile dysfunction, ejaculation disorders, and gynecomastia.
Conversely, AGA tends to improve in trans feminine persons and others receiving estrogen and antiandrogen therapy. Natural testosterone production is suppressed by estrogens and spironolactone as well as in patients who undergo orchiectomy.1 Although spironolactone is not approved for acne, AGA, or hirsutism, it is a standard treatment of AGA in cisgender women because it functions to block the effects of androgens, including at the hair follicle. Finasteride may be used for AGA in cisgender women but it is not recommended for trans feminine persons.1
There are many other modalities available for the treatment of AGA that are less commonly used—some may be cost prohibitive or do not have robust supporting evidence, or both. One example is hair
Melasma is a hyperpigmentation disorder related to estrogens, UV light exposure, and sometimes medication use (eg, hormonal birth control, spironolactone).24 The mainstay of treatment is prevention, including sun avoidance as well as use of sun-protective clothing and broad-spectrum sunscreens. Dermatologists tend to recommend physical sunscreens containing zinc oxide, titanium dioxide, and/or iron oxide, as they cover a wider UV spectrum and also provide some protection from visible light. Once melasma is present, dermatologists still have several treatment options. Topical hydroquinone is a proven treatment; however, it must be used with caution to avoid ochronosis. With careful patient selection, chemical peels also are effective treatment options for dyspigmentation and hyperpigmentation. Energy devices such as intense pulsed light and tattoo removal lasers—Q-switched lasers and picosecond pulse widths—also can be used to treat hyperpigmentation. Oral, intralesional, and topical tranexamic acid are newer treatment options for melasma that still are being studied and have shown promising results. Further studies are needed to determine long-term safety and optimal treatment regimens.24,25
Many insurance carriers, including TRICARE, do not routinely cover medical management of AGA or melasma. Patients should be advised that they likely will have to pay for any medications prescribed and procedures undertaken for these purposes; however, some medication costs can be offset by ordering larger prescription quantities, such as a 90-day supply vs a 30-day supply, as well as utilizing pharmacy discount programs.
Scar Management Following Surgery
In TSMs who undergo gender-affirming surgeries, dermatologists play an important role when scar symptoms develop, including pruritus, tenderness, and/or paresthesia. In the military, some common treatment modalities for symptomatic scars include intralesional steroids with or without 5-fluouroruacil and the fractionated CO2 laser. There also are numerous experimental treatment options for scars, including intralesional or perilesional botulinum toxin, the pulsed dye laser, or nonablative fractionated lasers. These modalities also may be used on hypertrophic scars or keloids. Another option for keloids is scar excision followed by superficial radiation therapy.26
Mental Health Considerations
Providers must take psychological adverse effects into consideration when considering medical therapies for dermatologic conditions in TGD patients. In particular, it is important to consider the risks for increased rates of depression and suicidal ideation formerly associated with the use of isotretinoin and finasteride, though much of the evidence regarding these risks has been called into question in recent years.27,28 Nonetheless, it remains prominent in lay media and may be a more important consideration in patients at higher baseline risk.27 Although there are no known studies that have expressly assessed rates of depression or suicidal ideation in TGD patients taking isotretinoin or finasteride, it is well established that TGD persons are at higher baseline risk for depression and suicidality.1,7,8 All patients should be carefully assessed for depression and suicidal ideation as well as counseled regarding these risks prior to initiating these therapies. If concerns for untreated mental health issues arise during screening and counseling, patients should be referred for assessment by a behavioral health specialist prior to starting therapy.
Future Directions
The future of TGD health care in the military could see an expansion of covered benefits and the development of new dermatologic procedures or medications. Research and policy evolution are necessary to bridge the current gaps in care; however, it is unlikely that all procedures currently considered to be cosmetic will become covered benefits.
Facial LHR is a promising candidate for future coverage for trans feminine persons. When cisgender men develop adverse effects from mandatory daily shaving, LHR is already a covered benefit. Two arguments in support of adding LHR for TGD patients revolve around achieving and maintaining an appearance congruent with their gender along with avoiding unwanted adverse effects related to daily shaving. Visual conformity with one’s affirmed gender has been associated with improvements in well-being, quality of life, and some mental health conditions.29
Scar prevention, treatment, and reduction are additional areas under active research in which dermatologists likely will play a crucial role.30,31 As more dermatologic procedures are performed on TGD persons, the published data and collective knowledge regarding best practices in this population will continue to grow, which will lead to improved cosmetic and safety outcomes.
Final Thoughts
Although dermatologists do not directly perform gender-affirming surgeries or hormone management, they do play an important role in enhancing a TGD person’s desired appearance and managing possible adverse effects resulting from gender-affirming interventions. There have been considerable advancements in TGD health care over the past decade, but there likely are more changes on the way. As policies and understanding of TGD health care needs evolve, it is crucial that the military health care system adapts to provide comprehensive, accessible, and equitable care, which includes expanding the range of covered dermatologic treatments to fully support the health and readiness of TSMs.
Acknowledgment—We would like to extend our sincere appreciation to the invaluable contributions and editorial support provided by Allison Higgins, JD (San Antonio, Texas), throughout the writing of this article.
- Coleman E, Radix AE, Bouman WP, et al. Standards of care for the health of transgender and gender diverse people, version 8. Int J Transgend Health. 2022;23(suppl 1):S1-S260. doi:10.1080/26895269.2022.2100644
- Secretary of Defense. DTM 16-005—military service of transgender service members. June 30, 2016. Accessed June 17, 2024. https://dod.defense.gov/Portals/1/features/2016/0616_policy/DTM-16-005.pdf
- Office of the Deputy Secretary of Defense. DTM 19-004—military service by transgender persons and persons with gender dysphoria. March 17, 2020. Accessed June 17, 2024. https://health.mil/Reference-Center/Policies/2020/03/17/Military-Service-by-Transgender-Persons-and-Persons-with-Gender-Dysphoria
- Office of the Under Secretary of Defense for Personnel and Readiness. Department of Defense Instruction (DODI) 1300.28. in-service transition for transgender service members. September 4, 2020. Accessed June 17, 2024. https://health.mil/Reference-Center/Policies/2020/09/04/Military-Service-by-Transgender-Persons-and-Persons-with-Gender-Dysphoria
- Defense Health Agency Procedural Instruction Number 6025.21, Guidance for Gender-Affirming Health Care of Transgender and Gender-Diverse Active and Reserve Component Service Members, May 12, 2023. https://www.health.mil/Reference-Center/DHA-Publications/2023/05/12/DHA-PI-6015-21
- Elders MJ, Brown GR, Coleman E, et al. Medical aspects of transgender military service. Armed Forces Soc. 2015;41:199-220. doi:10.1177/0095327X14545625.
- Almazan AN, Keuroghlian AS. Association between gender-affirming surgeries and mental health outcomes. JAMA Surg. 2021;156:611-618.
- Tordoff DM, Wanta JW, Collin A, et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Netw Open. 2022;5:E220978. doi:10.1001/jamanetworkopen.2022.0978
- Olson-Kennedy J, Warus J, Okonta V, et al. Chest reconstruction and chest dysphoria in transmasculine minors and young adults: comparisons of nonsurgical and postsurgical cohorts. JAMA Pediatr. 2018;172:431-436. doi:10.1001/jamapediatrics.2017.5440
- Top non-invasive cosmetic procedures worldwide 2022. Statista website. February 8, 2024. Accessed June 13, 2024. https://www.statista.com/statistics/293449/leading-nonsurgical-cosmetic-procedures/
- Kashkouli MB, Abdolalizadeh P, Abolfathzadeh N, et al. Periorbital facial rejuvenation; applied anatomy and pre-operative assessment. J Curr Ophthalmol. 2017;29:154-168. doi:10.1016/j.joco.2017.04.001
- Thomas MK, D’Silva JA, Borole AJ. Injection lipolysis: a systematic review of literature and our experience with a combination of phosphatidylcholine and deoxycholate over a period of 14 years in 1269 patients of Indian and South East Asian origin. J Cutan Aesthet Surg. 2018;11:222-228. doi:10.4103/JCAS.JCAS_117_18
- Jegasothy SM. Deoxycholic acid injections for bra-line lipolysis. Dermatol Surg. 2018;44:757-760. doi:10.1097/DSS.0000000000001311
- Dierickx CC. Hair removal by lasers and intense pulsed light sources. Dermatol Clin. 2002;20:135-146. doi:10.1016/s0733-8635(03)00052-4
- Lepselter J, Elman M. Biological and clinical aspects in laser hair removal. J Dermatolog Treat. 2004;15:72-83. doi:10.1080/09546630310023152
- Yuan N, Feldman AT, Chin P, et al. Comparison of permanent hair removal procedures before gender-affirming vaginoplasty: why we should consider laser hair removal as a first-line treatment for patients who meet criteria. Sex Med. 2022;10:100545. doi:10.1016/j.esxm.2022.100545
- Kumar A, Naguib YW, Shi YC, et al. A method to improve the efficacy of topical eflornithine hydrochloride cream. Drug Deliv. 2016;23:1495-1501. doi:10.3109/10717544.2014.951746
- Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metabol. 2017;102:3869-3903.
- Hatami P, Balighi K, Asl HN, et al. Isotretinoin and timing of procedural interventions: clinical implications and practical points. J Cosmet Dermatol. 2023;22:2146-2149. doi:10.1111/jocd.15874
- Rubenstein R, Roenigk HH Jr, Stegman SJ, et al. Atypical keloids after dermabrasion of patients taking isotretinoin. J Am Acad Dermatol. 1986;15(2 pt 1):280-285.
- Zachariae H. Delayed wound healing and keloid formation following argon laser treatment or dermabrasion during isotretinoin treatment. Br J Dermatol. 1988;118:703-706.
- Goldberg D, Kothare A, Doucette M, et al. Selective photothermolysis with a novel 1726 nm laser beam: a safe and effective solution for acne vulgaris. J Cosmet Dermatol. 2023;22:486-496. doi:10.1111/jocd.15602
- Sun HY, Sebaratnam DF. Clascoterone as a novel treatment for androgenetic alopecia. Clin Exp Dermatol. 2020;45:913-914. doi:10.1111/ced.14292
- Bolognia JL, Schaffer JV, Cerroni L. Dermatology: 2-Volume Set. Elsevier; 2024:1130.
- Konisky H, Balazic E, Jaller JA, et al. Tranexamic acid in melasma: a focused review on drug administration routes. J Cosmet Dermatol. 2023;22:1197-1206. doi:10.1111/jocd.15589
- Walsh LA, Wu E, Pontes D, et al. Keloid treatments: an evidence-based systematic review of recent advances. Syst Rev. 2023;12:42. doi:10.1186/s13643-023-02192-7
- Kridin K, Ludwig RJ. Isotretinoin and the risk of psychiatric disturbances: a global study shedding new light on a debatable story. J Am Acad Dermatol. 2023;88:388-394. doi:10.1016/j.jaad.2022.10.031
- Dyson TE, Cantrell MA, Lund BC. Lack of association between 5α-reductase inhibitors and depression. J Urol. 2020;204:793-798. doi:10.1097/JU.0000000000001079
- To M, Zhang Q, Bradlyn A, et al. Visual conformity with affirmed gender or “passing”: its distribution and association with depression and anxiety in a cohort of transgender people. J Sex Med. 2020;17:2084-2092. doi:10.1016/j.jsxm.2020.07.019
- Fernandes MG, da Silva LP, Cerqueira MT, et al. Mechanomodulatory biomaterials prospects in scar prevention and treatment. Acta Biomater. 2022;150:22-33. doi:10.1016/j.actbio.2022.07.042
- Kolli H, Moy RL. Prevention of scarring with intraoperative erbium:YAG laser treatment. J Drugs Dermatol. 2020;19:1040-1043. doi:10.36849/JDD.2020.5244
- Coleman E, Radix AE, Bouman WP, et al. Standards of care for the health of transgender and gender diverse people, version 8. Int J Transgend Health. 2022;23(suppl 1):S1-S260. doi:10.1080/26895269.2022.2100644
- Secretary of Defense. DTM 16-005—military service of transgender service members. June 30, 2016. Accessed June 17, 2024. https://dod.defense.gov/Portals/1/features/2016/0616_policy/DTM-16-005.pdf
- Office of the Deputy Secretary of Defense. DTM 19-004—military service by transgender persons and persons with gender dysphoria. March 17, 2020. Accessed June 17, 2024. https://health.mil/Reference-Center/Policies/2020/03/17/Military-Service-by-Transgender-Persons-and-Persons-with-Gender-Dysphoria
- Office of the Under Secretary of Defense for Personnel and Readiness. Department of Defense Instruction (DODI) 1300.28. in-service transition for transgender service members. September 4, 2020. Accessed June 17, 2024. https://health.mil/Reference-Center/Policies/2020/09/04/Military-Service-by-Transgender-Persons-and-Persons-with-Gender-Dysphoria
- Defense Health Agency Procedural Instruction Number 6025.21, Guidance for Gender-Affirming Health Care of Transgender and Gender-Diverse Active and Reserve Component Service Members, May 12, 2023. https://www.health.mil/Reference-Center/DHA-Publications/2023/05/12/DHA-PI-6015-21
- Elders MJ, Brown GR, Coleman E, et al. Medical aspects of transgender military service. Armed Forces Soc. 2015;41:199-220. doi:10.1177/0095327X14545625.
- Almazan AN, Keuroghlian AS. Association between gender-affirming surgeries and mental health outcomes. JAMA Surg. 2021;156:611-618.
- Tordoff DM, Wanta JW, Collin A, et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Netw Open. 2022;5:E220978. doi:10.1001/jamanetworkopen.2022.0978
- Olson-Kennedy J, Warus J, Okonta V, et al. Chest reconstruction and chest dysphoria in transmasculine minors and young adults: comparisons of nonsurgical and postsurgical cohorts. JAMA Pediatr. 2018;172:431-436. doi:10.1001/jamapediatrics.2017.5440
- Top non-invasive cosmetic procedures worldwide 2022. Statista website. February 8, 2024. Accessed June 13, 2024. https://www.statista.com/statistics/293449/leading-nonsurgical-cosmetic-procedures/
- Kashkouli MB, Abdolalizadeh P, Abolfathzadeh N, et al. Periorbital facial rejuvenation; applied anatomy and pre-operative assessment. J Curr Ophthalmol. 2017;29:154-168. doi:10.1016/j.joco.2017.04.001
- Thomas MK, D’Silva JA, Borole AJ. Injection lipolysis: a systematic review of literature and our experience with a combination of phosphatidylcholine and deoxycholate over a period of 14 years in 1269 patients of Indian and South East Asian origin. J Cutan Aesthet Surg. 2018;11:222-228. doi:10.4103/JCAS.JCAS_117_18
- Jegasothy SM. Deoxycholic acid injections for bra-line lipolysis. Dermatol Surg. 2018;44:757-760. doi:10.1097/DSS.0000000000001311
- Dierickx CC. Hair removal by lasers and intense pulsed light sources. Dermatol Clin. 2002;20:135-146. doi:10.1016/s0733-8635(03)00052-4
- Lepselter J, Elman M. Biological and clinical aspects in laser hair removal. J Dermatolog Treat. 2004;15:72-83. doi:10.1080/09546630310023152
- Yuan N, Feldman AT, Chin P, et al. Comparison of permanent hair removal procedures before gender-affirming vaginoplasty: why we should consider laser hair removal as a first-line treatment for patients who meet criteria. Sex Med. 2022;10:100545. doi:10.1016/j.esxm.2022.100545
- Kumar A, Naguib YW, Shi YC, et al. A method to improve the efficacy of topical eflornithine hydrochloride cream. Drug Deliv. 2016;23:1495-1501. doi:10.3109/10717544.2014.951746
- Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metabol. 2017;102:3869-3903.
- Hatami P, Balighi K, Asl HN, et al. Isotretinoin and timing of procedural interventions: clinical implications and practical points. J Cosmet Dermatol. 2023;22:2146-2149. doi:10.1111/jocd.15874
- Rubenstein R, Roenigk HH Jr, Stegman SJ, et al. Atypical keloids after dermabrasion of patients taking isotretinoin. J Am Acad Dermatol. 1986;15(2 pt 1):280-285.
- Zachariae H. Delayed wound healing and keloid formation following argon laser treatment or dermabrasion during isotretinoin treatment. Br J Dermatol. 1988;118:703-706.
- Goldberg D, Kothare A, Doucette M, et al. Selective photothermolysis with a novel 1726 nm laser beam: a safe and effective solution for acne vulgaris. J Cosmet Dermatol. 2023;22:486-496. doi:10.1111/jocd.15602
- Sun HY, Sebaratnam DF. Clascoterone as a novel treatment for androgenetic alopecia. Clin Exp Dermatol. 2020;45:913-914. doi:10.1111/ced.14292
- Bolognia JL, Schaffer JV, Cerroni L. Dermatology: 2-Volume Set. Elsevier; 2024:1130.
- Konisky H, Balazic E, Jaller JA, et al. Tranexamic acid in melasma: a focused review on drug administration routes. J Cosmet Dermatol. 2023;22:1197-1206. doi:10.1111/jocd.15589
- Walsh LA, Wu E, Pontes D, et al. Keloid treatments: an evidence-based systematic review of recent advances. Syst Rev. 2023;12:42. doi:10.1186/s13643-023-02192-7
- Kridin K, Ludwig RJ. Isotretinoin and the risk of psychiatric disturbances: a global study shedding new light on a debatable story. J Am Acad Dermatol. 2023;88:388-394. doi:10.1016/j.jaad.2022.10.031
- Dyson TE, Cantrell MA, Lund BC. Lack of association between 5α-reductase inhibitors and depression. J Urol. 2020;204:793-798. doi:10.1097/JU.0000000000001079
- To M, Zhang Q, Bradlyn A, et al. Visual conformity with affirmed gender or “passing”: its distribution and association with depression and anxiety in a cohort of transgender people. J Sex Med. 2020;17:2084-2092. doi:10.1016/j.jsxm.2020.07.019
- Fernandes MG, da Silva LP, Cerqueira MT, et al. Mechanomodulatory biomaterials prospects in scar prevention and treatment. Acta Biomater. 2022;150:22-33. doi:10.1016/j.actbio.2022.07.042
- Kolli H, Moy RL. Prevention of scarring with intraoperative erbium:YAG laser treatment. J Drugs Dermatol. 2020;19:1040-1043. doi:10.36849/JDD.2020.5244
Practice Points
- Transgender and gender diverse (TGD) health care is multidisciplinary, and both military and civilian dermatologists can serve an important role.
- Although dermatologists do not directly perform gender-affirming surgeries or hormone management, there are a number of dermatologic procedures and medical interventions that can enhance a TGD person’s desired appearance.
- Dermatologists also can help manage possible adverse effects from gender-affirming interventions.
Cancer Drug Shortages Continue in the US, Survey Finds
Nearly 90% of the 28 NCCN member centers who responded to the survey, conducted between May 28 and June 11, said they were experiencing a shortage of at least one drug.
“Many drugs that are currently in shortage form the backbones of effective multiagent regimens across both curative and palliative treatment settings,” NCCN’s CEO Crystal S. Denlinger, MD, said in an interview.
The good news is that carboplatin and cisplatin shortages have fallen dramatically since 2023. At the peak of the shortage in 2023, 93% of centers surveyed reported experiencing a shortage of carboplatin and 70% were experiencing a shortage of cisplatin, whereas in 2024, only 11% reported a carboplatin shortage and 7% reported a cisplatin shortage.
“Thankfully, the shortages for carboplatin and cisplatin are mostly resolved at this time,” Dr. Denlinger said.
However, all three NCCN surveys conducted in the past year, including the most recent one, have found shortages of various chemotherapies and supportive care medications, which suggests this is an ongoing issue affecting a significant spectrum of generic drugs.
“The acute crisis associated with the shortage of carboplatin and cisplatin was a singular event that brought the issue into the national spotlight,” but it’s “important to note that the current broad drug shortages found on this survey are not new,” said Dr. Denlinger.
In the latest survey, 89% of NCCN centers continue to report shortages of one or more drugs, and 75% said they are experiencing shortages of two or more drugs.
Overall, 57% of centers are short on vinblastine, 46% are short on etoposide, and 43% are short on topotecan. Other common chemotherapy and supportive care agents in short supply include dacarbazine (18% of centers) as well as 5-fluorouracil (5-FU) and methotrexate (14% of centers).
In 2023, however, shortages of methotrexate and 5-FU were worse, with 67% of centers reporting shortages of methotrexate and 26% of 5-FU.
In the current survey, 75% of NCCN centers also noted they were aware of drug shortages within community practices in their area, and more than one in four centers reported treatment delays requiring additional prior authorization.
Cancer drug shortages impact not only routine treatments but also clinical trials. The recent survey found that 43% of respondents said drug shortages disrupted clinical trials at their center. The biggest issues centers flagged included greater administrative burdens, lower patient enrollment, and fewer open trials.
How are centers dealing with ongoing supply issues?
Top mitigation strategies include reducing waste, limiting use of current stock, and adjusting the timing and dosage within evidence-based ranges.
“The current situation underscores the need for sustainable, long-term solutions that ensure a stable supply of high-quality cancer medications,” Alyssa Schatz, MSW, NCCN senior director of policy and advocacy, said in a news release.
Three-quarters (75%) of survey respondents said they would like to see economic incentives put in place to encourage the high-quality manufacturing of medications, especially generic versions that are often in short supply. Nearly two-thirds (64%) cited a need for a broader buffer stock payment, and the same percentage would like to see more information on user experiences with various generic suppliers to help hospitals contract with those engaging in high-quality practices.
The NCCN also continues to work with federal regulators, agencies, and lawmakers to implement long-term solutions to cancer drug shortages.
“The federal government has a key role to play in addressing this issue,” Ms. Schatz said. “Establishing economic incentives, such as tax breaks or manufacturing grants for generic drugmakers, will help support a robust and resilient supply chain — ultimately safeguarding care for people with cancer across the country.”
A version of this article appeared on Medscape.com.
Nearly 90% of the 28 NCCN member centers who responded to the survey, conducted between May 28 and June 11, said they were experiencing a shortage of at least one drug.
“Many drugs that are currently in shortage form the backbones of effective multiagent regimens across both curative and palliative treatment settings,” NCCN’s CEO Crystal S. Denlinger, MD, said in an interview.
The good news is that carboplatin and cisplatin shortages have fallen dramatically since 2023. At the peak of the shortage in 2023, 93% of centers surveyed reported experiencing a shortage of carboplatin and 70% were experiencing a shortage of cisplatin, whereas in 2024, only 11% reported a carboplatin shortage and 7% reported a cisplatin shortage.
“Thankfully, the shortages for carboplatin and cisplatin are mostly resolved at this time,” Dr. Denlinger said.
However, all three NCCN surveys conducted in the past year, including the most recent one, have found shortages of various chemotherapies and supportive care medications, which suggests this is an ongoing issue affecting a significant spectrum of generic drugs.
“The acute crisis associated with the shortage of carboplatin and cisplatin was a singular event that brought the issue into the national spotlight,” but it’s “important to note that the current broad drug shortages found on this survey are not new,” said Dr. Denlinger.
In the latest survey, 89% of NCCN centers continue to report shortages of one or more drugs, and 75% said they are experiencing shortages of two or more drugs.
Overall, 57% of centers are short on vinblastine, 46% are short on etoposide, and 43% are short on topotecan. Other common chemotherapy and supportive care agents in short supply include dacarbazine (18% of centers) as well as 5-fluorouracil (5-FU) and methotrexate (14% of centers).
In 2023, however, shortages of methotrexate and 5-FU were worse, with 67% of centers reporting shortages of methotrexate and 26% of 5-FU.
In the current survey, 75% of NCCN centers also noted they were aware of drug shortages within community practices in their area, and more than one in four centers reported treatment delays requiring additional prior authorization.
Cancer drug shortages impact not only routine treatments but also clinical trials. The recent survey found that 43% of respondents said drug shortages disrupted clinical trials at their center. The biggest issues centers flagged included greater administrative burdens, lower patient enrollment, and fewer open trials.
How are centers dealing with ongoing supply issues?
Top mitigation strategies include reducing waste, limiting use of current stock, and adjusting the timing and dosage within evidence-based ranges.
“The current situation underscores the need for sustainable, long-term solutions that ensure a stable supply of high-quality cancer medications,” Alyssa Schatz, MSW, NCCN senior director of policy and advocacy, said in a news release.
Three-quarters (75%) of survey respondents said they would like to see economic incentives put in place to encourage the high-quality manufacturing of medications, especially generic versions that are often in short supply. Nearly two-thirds (64%) cited a need for a broader buffer stock payment, and the same percentage would like to see more information on user experiences with various generic suppliers to help hospitals contract with those engaging in high-quality practices.
The NCCN also continues to work with federal regulators, agencies, and lawmakers to implement long-term solutions to cancer drug shortages.
“The federal government has a key role to play in addressing this issue,” Ms. Schatz said. “Establishing economic incentives, such as tax breaks or manufacturing grants for generic drugmakers, will help support a robust and resilient supply chain — ultimately safeguarding care for people with cancer across the country.”
A version of this article appeared on Medscape.com.
Nearly 90% of the 28 NCCN member centers who responded to the survey, conducted between May 28 and June 11, said they were experiencing a shortage of at least one drug.
“Many drugs that are currently in shortage form the backbones of effective multiagent regimens across both curative and palliative treatment settings,” NCCN’s CEO Crystal S. Denlinger, MD, said in an interview.
The good news is that carboplatin and cisplatin shortages have fallen dramatically since 2023. At the peak of the shortage in 2023, 93% of centers surveyed reported experiencing a shortage of carboplatin and 70% were experiencing a shortage of cisplatin, whereas in 2024, only 11% reported a carboplatin shortage and 7% reported a cisplatin shortage.
“Thankfully, the shortages for carboplatin and cisplatin are mostly resolved at this time,” Dr. Denlinger said.
However, all three NCCN surveys conducted in the past year, including the most recent one, have found shortages of various chemotherapies and supportive care medications, which suggests this is an ongoing issue affecting a significant spectrum of generic drugs.
“The acute crisis associated with the shortage of carboplatin and cisplatin was a singular event that brought the issue into the national spotlight,” but it’s “important to note that the current broad drug shortages found on this survey are not new,” said Dr. Denlinger.
In the latest survey, 89% of NCCN centers continue to report shortages of one or more drugs, and 75% said they are experiencing shortages of two or more drugs.
Overall, 57% of centers are short on vinblastine, 46% are short on etoposide, and 43% are short on topotecan. Other common chemotherapy and supportive care agents in short supply include dacarbazine (18% of centers) as well as 5-fluorouracil (5-FU) and methotrexate (14% of centers).
In 2023, however, shortages of methotrexate and 5-FU were worse, with 67% of centers reporting shortages of methotrexate and 26% of 5-FU.
In the current survey, 75% of NCCN centers also noted they were aware of drug shortages within community practices in their area, and more than one in four centers reported treatment delays requiring additional prior authorization.
Cancer drug shortages impact not only routine treatments but also clinical trials. The recent survey found that 43% of respondents said drug shortages disrupted clinical trials at their center. The biggest issues centers flagged included greater administrative burdens, lower patient enrollment, and fewer open trials.
How are centers dealing with ongoing supply issues?
Top mitigation strategies include reducing waste, limiting use of current stock, and adjusting the timing and dosage within evidence-based ranges.
“The current situation underscores the need for sustainable, long-term solutions that ensure a stable supply of high-quality cancer medications,” Alyssa Schatz, MSW, NCCN senior director of policy and advocacy, said in a news release.
Three-quarters (75%) of survey respondents said they would like to see economic incentives put in place to encourage the high-quality manufacturing of medications, especially generic versions that are often in short supply. Nearly two-thirds (64%) cited a need for a broader buffer stock payment, and the same percentage would like to see more information on user experiences with various generic suppliers to help hospitals contract with those engaging in high-quality practices.
The NCCN also continues to work with federal regulators, agencies, and lawmakers to implement long-term solutions to cancer drug shortages.
“The federal government has a key role to play in addressing this issue,” Ms. Schatz said. “Establishing economic incentives, such as tax breaks or manufacturing grants for generic drugmakers, will help support a robust and resilient supply chain — ultimately safeguarding care for people with cancer across the country.”
A version of this article appeared on Medscape.com.
Benzoyl Peroxide, Benzene, and Lots of Unanswered Questions: Where Are We Now?
March 2024 proved to be a busy month for benzoyl peroxide in the media! We are now at almost 4 months since Valisure, an independent analytical laboratory located in Connecticut, filed a Citizen Petition on benzene in benzoyl peroxide drug products with the US Food and Drug Administration (FDA) on March 5, 2024.1 This petition was filed shortly before the annual meeting of the American Academy of Dermatology was held in San Diego, California, creating quite a stir of concern in the dermatology world. Further information on the degradation of benzoyl peroxide with production of benzene was published in the medical literature in March 2024.2 As benzene is recognized as a human carcinogen, manufacturing regulations exist to assure that it does not appear in topical products either through contamination or degradation over the course of a product’s shelf-life.3
As anticipated, several opinions and commentaries appeared quickly, both on video and in various articles. The American Acne & Rosacea Society (AARS) released a statement on this issue on March 20, 2024.4 The safety of the public is the overarching primary concern. This AARS statement does include some general suggestions related to benzoyl peroxide use based on the best assessment to date while awaiting further guidance from the FDA on this issue. Benzoyl peroxide is approved for use by the FDA as an over-the-counter (OTC) topical product for acne and also is in several FDA-approved prescription topical products.5,6
The following reflects my personal viewpoint as both a dermatologist and a grandfather who has grandchildren who use acne products. My views are not necessarily those of AARS. Since early March 2024, I have read several documents and spoken to several dermatologists, scientists, and formulators with knowledge in this area, including contacts at Valisure. I was hoping to get to some reasonable definitive answer but have not been able to achieve this to my full satisfaction. There are many opinions and concerns, and each one makes sense based on the vantage point of the presenter. However, several unanswered questions remain related to what testing and data are currently required of companies to gain FDA approval of a benzoyl peroxide product, including:
- assessment of stability and degradation products (including benzene),
- validation of testing methods,
- the issue of benzoyl peroxide stability in commercial products, and
- the relevant magnitude of resultant benzene exposures, especially as we are all exposed to benzene from several sources each day.
I am certain that companies with benzoyl peroxide products will evaluate their already-approved products and also do further testing. However, in this situation, which impacts millions of people on so many levels, I feel there needs to be an organized approach to evaluate and resolve the issue, otherwise the likelihood of continued confusion and uncertainty is high. As the FDA is the approval body, I am hoping it will provide definitive guidance within a reasonable timeline so that clinicians, patients, and manufacturers of benzoyl peroxide can proceed with full confidence. Right now, we all remain in a state of limbo. It is time for less talk and more definitive action to sort out this issue.
- Valisure Citizen Petition on Benzene in Benzoyl Peroxide Products. March 5, 2024. Accessed June 5, 2024. https://assets-global.website-files.com/6215052733f8bb8fea016220/65e8560962ed23f744902a7b_Valisure%20Citizen%20Petition%20on%20Benzene%20in%20Benzoyl%20Peroxide%20Drug%20Products.pdf
- Kucera K, Zenzola N, Hudspeth A, et al. Benzoyl peroxide drug products form benzene. Environ Health Perspect. 2024;132:37702. doi:10.1289/EHP13984
- US Food and Drug Administration. Reformulating drug products that contain carbomers manufactured with benzene. December 2023. Accessed June 12, 2024. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/reformulating-drug-products-contain-carbomers-manufactured-benzene
- American Acne & Rosacea Society. Response Statement from the AARS to the Valisure Citizen Petition on Benzene in Benzoyl Peroxide Drug Products. March 20, 2024. Accessed June 12, 2024. https://www.einpresswire.com/article/697481595/response-statement-from-the-aars-to-the-valisure-citizen-petition-on-benzene-in-benzoyl-peroxide-drug-products
- Department of Health and Human Services. Classification of benzoyl peroxide as safe and effective and revision of labeling to drug facts format; topical acne drug products for over-the-counter human use; Final Rule. Fed Registr. 2010;75:9767-9777.
- US Food and Drug Administration. Topical acne drug products for over-the-counter human use—revision of labeling and classification of benzoyl peroxide as safe and effective. June 2011. Accessed June 12, 2024. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/topical-acne-drug-products-over-counter-human-use-revision-labeling-and-classification-benzoyl
March 2024 proved to be a busy month for benzoyl peroxide in the media! We are now at almost 4 months since Valisure, an independent analytical laboratory located in Connecticut, filed a Citizen Petition on benzene in benzoyl peroxide drug products with the US Food and Drug Administration (FDA) on March 5, 2024.1 This petition was filed shortly before the annual meeting of the American Academy of Dermatology was held in San Diego, California, creating quite a stir of concern in the dermatology world. Further information on the degradation of benzoyl peroxide with production of benzene was published in the medical literature in March 2024.2 As benzene is recognized as a human carcinogen, manufacturing regulations exist to assure that it does not appear in topical products either through contamination or degradation over the course of a product’s shelf-life.3
As anticipated, several opinions and commentaries appeared quickly, both on video and in various articles. The American Acne & Rosacea Society (AARS) released a statement on this issue on March 20, 2024.4 The safety of the public is the overarching primary concern. This AARS statement does include some general suggestions related to benzoyl peroxide use based on the best assessment to date while awaiting further guidance from the FDA on this issue. Benzoyl peroxide is approved for use by the FDA as an over-the-counter (OTC) topical product for acne and also is in several FDA-approved prescription topical products.5,6
The following reflects my personal viewpoint as both a dermatologist and a grandfather who has grandchildren who use acne products. My views are not necessarily those of AARS. Since early March 2024, I have read several documents and spoken to several dermatologists, scientists, and formulators with knowledge in this area, including contacts at Valisure. I was hoping to get to some reasonable definitive answer but have not been able to achieve this to my full satisfaction. There are many opinions and concerns, and each one makes sense based on the vantage point of the presenter. However, several unanswered questions remain related to what testing and data are currently required of companies to gain FDA approval of a benzoyl peroxide product, including:
- assessment of stability and degradation products (including benzene),
- validation of testing methods,
- the issue of benzoyl peroxide stability in commercial products, and
- the relevant magnitude of resultant benzene exposures, especially as we are all exposed to benzene from several sources each day.
I am certain that companies with benzoyl peroxide products will evaluate their already-approved products and also do further testing. However, in this situation, which impacts millions of people on so many levels, I feel there needs to be an organized approach to evaluate and resolve the issue, otherwise the likelihood of continued confusion and uncertainty is high. As the FDA is the approval body, I am hoping it will provide definitive guidance within a reasonable timeline so that clinicians, patients, and manufacturers of benzoyl peroxide can proceed with full confidence. Right now, we all remain in a state of limbo. It is time for less talk and more definitive action to sort out this issue.
March 2024 proved to be a busy month for benzoyl peroxide in the media! We are now at almost 4 months since Valisure, an independent analytical laboratory located in Connecticut, filed a Citizen Petition on benzene in benzoyl peroxide drug products with the US Food and Drug Administration (FDA) on March 5, 2024.1 This petition was filed shortly before the annual meeting of the American Academy of Dermatology was held in San Diego, California, creating quite a stir of concern in the dermatology world. Further information on the degradation of benzoyl peroxide with production of benzene was published in the medical literature in March 2024.2 As benzene is recognized as a human carcinogen, manufacturing regulations exist to assure that it does not appear in topical products either through contamination or degradation over the course of a product’s shelf-life.3
As anticipated, several opinions and commentaries appeared quickly, both on video and in various articles. The American Acne & Rosacea Society (AARS) released a statement on this issue on March 20, 2024.4 The safety of the public is the overarching primary concern. This AARS statement does include some general suggestions related to benzoyl peroxide use based on the best assessment to date while awaiting further guidance from the FDA on this issue. Benzoyl peroxide is approved for use by the FDA as an over-the-counter (OTC) topical product for acne and also is in several FDA-approved prescription topical products.5,6
The following reflects my personal viewpoint as both a dermatologist and a grandfather who has grandchildren who use acne products. My views are not necessarily those of AARS. Since early March 2024, I have read several documents and spoken to several dermatologists, scientists, and formulators with knowledge in this area, including contacts at Valisure. I was hoping to get to some reasonable definitive answer but have not been able to achieve this to my full satisfaction. There are many opinions and concerns, and each one makes sense based on the vantage point of the presenter. However, several unanswered questions remain related to what testing and data are currently required of companies to gain FDA approval of a benzoyl peroxide product, including:
- assessment of stability and degradation products (including benzene),
- validation of testing methods,
- the issue of benzoyl peroxide stability in commercial products, and
- the relevant magnitude of resultant benzene exposures, especially as we are all exposed to benzene from several sources each day.
I am certain that companies with benzoyl peroxide products will evaluate their already-approved products and also do further testing. However, in this situation, which impacts millions of people on so many levels, I feel there needs to be an organized approach to evaluate and resolve the issue, otherwise the likelihood of continued confusion and uncertainty is high. As the FDA is the approval body, I am hoping it will provide definitive guidance within a reasonable timeline so that clinicians, patients, and manufacturers of benzoyl peroxide can proceed with full confidence. Right now, we all remain in a state of limbo. It is time for less talk and more definitive action to sort out this issue.
- Valisure Citizen Petition on Benzene in Benzoyl Peroxide Products. March 5, 2024. Accessed June 5, 2024. https://assets-global.website-files.com/6215052733f8bb8fea016220/65e8560962ed23f744902a7b_Valisure%20Citizen%20Petition%20on%20Benzene%20in%20Benzoyl%20Peroxide%20Drug%20Products.pdf
- Kucera K, Zenzola N, Hudspeth A, et al. Benzoyl peroxide drug products form benzene. Environ Health Perspect. 2024;132:37702. doi:10.1289/EHP13984
- US Food and Drug Administration. Reformulating drug products that contain carbomers manufactured with benzene. December 2023. Accessed June 12, 2024. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/reformulating-drug-products-contain-carbomers-manufactured-benzene
- American Acne & Rosacea Society. Response Statement from the AARS to the Valisure Citizen Petition on Benzene in Benzoyl Peroxide Drug Products. March 20, 2024. Accessed June 12, 2024. https://www.einpresswire.com/article/697481595/response-statement-from-the-aars-to-the-valisure-citizen-petition-on-benzene-in-benzoyl-peroxide-drug-products
- Department of Health and Human Services. Classification of benzoyl peroxide as safe and effective and revision of labeling to drug facts format; topical acne drug products for over-the-counter human use; Final Rule. Fed Registr. 2010;75:9767-9777.
- US Food and Drug Administration. Topical acne drug products for over-the-counter human use—revision of labeling and classification of benzoyl peroxide as safe and effective. June 2011. Accessed June 12, 2024. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/topical-acne-drug-products-over-counter-human-use-revision-labeling-and-classification-benzoyl
- Valisure Citizen Petition on Benzene in Benzoyl Peroxide Products. March 5, 2024. Accessed June 5, 2024. https://assets-global.website-files.com/6215052733f8bb8fea016220/65e8560962ed23f744902a7b_Valisure%20Citizen%20Petition%20on%20Benzene%20in%20Benzoyl%20Peroxide%20Drug%20Products.pdf
- Kucera K, Zenzola N, Hudspeth A, et al. Benzoyl peroxide drug products form benzene. Environ Health Perspect. 2024;132:37702. doi:10.1289/EHP13984
- US Food and Drug Administration. Reformulating drug products that contain carbomers manufactured with benzene. December 2023. Accessed June 12, 2024. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/reformulating-drug-products-contain-carbomers-manufactured-benzene
- American Acne & Rosacea Society. Response Statement from the AARS to the Valisure Citizen Petition on Benzene in Benzoyl Peroxide Drug Products. March 20, 2024. Accessed June 12, 2024. https://www.einpresswire.com/article/697481595/response-statement-from-the-aars-to-the-valisure-citizen-petition-on-benzene-in-benzoyl-peroxide-drug-products
- Department of Health and Human Services. Classification of benzoyl peroxide as safe and effective and revision of labeling to drug facts format; topical acne drug products for over-the-counter human use; Final Rule. Fed Registr. 2010;75:9767-9777.
- US Food and Drug Administration. Topical acne drug products for over-the-counter human use—revision of labeling and classification of benzoyl peroxide as safe and effective. June 2011. Accessed June 12, 2024. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/topical-acne-drug-products-over-counter-human-use-revision-labeling-and-classification-benzoyl
New Tools for Monitoring Multiple Myeloma
Advances in drugs and combinations have revolutionized the landscape in multiple myeloma, thus allowing patients to live much longer, according to Bruno Paiva, PhD, director of flow cytometry and the myeloma laboratory at the University of Navarra Clinic in Pamplona, Spain.
“Much better treatment responses are achieved, with long-term remission, so tools are needed for long-term monitoring. The starting point for monitoring is the monoclonal protein secreted by the myeloma tumor cell, which can be measured in serum and urine. Complete remission is defined when that monoclonal component is not detected with routine laboratory techniques, such as immunofixation,” said Dr. Paiva.
Even if the patient may be in complete remission, minimal residual disease is sometimes detected as myeloma can infiltrate the bone marrow. Techniques for identifying minimal residual disease, like cytometry or next-generation sequencing, can detect bone marrow blood aspirate. “The detection of this minimal residual disease corresponds with a significant reduction in survival,” Dr. Paiva warned.
In addition to these techniques, PET-CT is also used. This imaging tool is “very useful for seeing disease both inside and outside the marrow,” said Dr. Paiva.
“As for the future, the FDA [Food and Drug Administration] has just approved the use of minimal residual disease as one of the trial objectives. This may allow drugs to reach patients much sooner, instead of waiting for survival data, which takes much longer to obtain,” he said.
Researchers are also learning how to use minimal residual disease and these imaging techniques to individualize the treatment of patients with myeloma. “Furthermore, since some of these techniques are invasive, such as bone marrow ones, we are trying to focus on peripheral blood. This way, monitoring is minimally invasive, much more comfortable for the patient, and more informative because it can be done many times,” said Dr. Paiva.
Dr. Paiva is extending these imaging techniques “to different scenarios, such as the precursor stages of the disease. Our laboratory is especially known for flow cytometry, and we are launching the NoMoreMGUS project, the largest ever conducted in Spain (and perhaps in Europe) on monoclonal gammopathy of undetermined significance. This is a condition that precedes myeloma. We are looking to study 5000 patients in Spain once a year for 5 years, which means analyzing 25,000 samples.
“On the other hand,” he continued, “we are taking some of these developments to other neoplasms, such as acute lymphoblastic leukemia. And we are interested in using all the potential of cytometry not only to measure tumor cells but also to characterize the immune system as another important biomarker in the pathogenesis of the disease. And, for example, to predict infections, which is very important in patients with myeloma.”
This story was translated from El Médico Interactivo, which is part of the Medscape Professional Network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
Advances in drugs and combinations have revolutionized the landscape in multiple myeloma, thus allowing patients to live much longer, according to Bruno Paiva, PhD, director of flow cytometry and the myeloma laboratory at the University of Navarra Clinic in Pamplona, Spain.
“Much better treatment responses are achieved, with long-term remission, so tools are needed for long-term monitoring. The starting point for monitoring is the monoclonal protein secreted by the myeloma tumor cell, which can be measured in serum and urine. Complete remission is defined when that monoclonal component is not detected with routine laboratory techniques, such as immunofixation,” said Dr. Paiva.
Even if the patient may be in complete remission, minimal residual disease is sometimes detected as myeloma can infiltrate the bone marrow. Techniques for identifying minimal residual disease, like cytometry or next-generation sequencing, can detect bone marrow blood aspirate. “The detection of this minimal residual disease corresponds with a significant reduction in survival,” Dr. Paiva warned.
In addition to these techniques, PET-CT is also used. This imaging tool is “very useful for seeing disease both inside and outside the marrow,” said Dr. Paiva.
“As for the future, the FDA [Food and Drug Administration] has just approved the use of minimal residual disease as one of the trial objectives. This may allow drugs to reach patients much sooner, instead of waiting for survival data, which takes much longer to obtain,” he said.
Researchers are also learning how to use minimal residual disease and these imaging techniques to individualize the treatment of patients with myeloma. “Furthermore, since some of these techniques are invasive, such as bone marrow ones, we are trying to focus on peripheral blood. This way, monitoring is minimally invasive, much more comfortable for the patient, and more informative because it can be done many times,” said Dr. Paiva.
Dr. Paiva is extending these imaging techniques “to different scenarios, such as the precursor stages of the disease. Our laboratory is especially known for flow cytometry, and we are launching the NoMoreMGUS project, the largest ever conducted in Spain (and perhaps in Europe) on monoclonal gammopathy of undetermined significance. This is a condition that precedes myeloma. We are looking to study 5000 patients in Spain once a year for 5 years, which means analyzing 25,000 samples.
“On the other hand,” he continued, “we are taking some of these developments to other neoplasms, such as acute lymphoblastic leukemia. And we are interested in using all the potential of cytometry not only to measure tumor cells but also to characterize the immune system as another important biomarker in the pathogenesis of the disease. And, for example, to predict infections, which is very important in patients with myeloma.”
This story was translated from El Médico Interactivo, which is part of the Medscape Professional Network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
Advances in drugs and combinations have revolutionized the landscape in multiple myeloma, thus allowing patients to live much longer, according to Bruno Paiva, PhD, director of flow cytometry and the myeloma laboratory at the University of Navarra Clinic in Pamplona, Spain.
“Much better treatment responses are achieved, with long-term remission, so tools are needed for long-term monitoring. The starting point for monitoring is the monoclonal protein secreted by the myeloma tumor cell, which can be measured in serum and urine. Complete remission is defined when that monoclonal component is not detected with routine laboratory techniques, such as immunofixation,” said Dr. Paiva.
Even if the patient may be in complete remission, minimal residual disease is sometimes detected as myeloma can infiltrate the bone marrow. Techniques for identifying minimal residual disease, like cytometry or next-generation sequencing, can detect bone marrow blood aspirate. “The detection of this minimal residual disease corresponds with a significant reduction in survival,” Dr. Paiva warned.
In addition to these techniques, PET-CT is also used. This imaging tool is “very useful for seeing disease both inside and outside the marrow,” said Dr. Paiva.
“As for the future, the FDA [Food and Drug Administration] has just approved the use of minimal residual disease as one of the trial objectives. This may allow drugs to reach patients much sooner, instead of waiting for survival data, which takes much longer to obtain,” he said.
Researchers are also learning how to use minimal residual disease and these imaging techniques to individualize the treatment of patients with myeloma. “Furthermore, since some of these techniques are invasive, such as bone marrow ones, we are trying to focus on peripheral blood. This way, monitoring is minimally invasive, much more comfortable for the patient, and more informative because it can be done many times,” said Dr. Paiva.
Dr. Paiva is extending these imaging techniques “to different scenarios, such as the precursor stages of the disease. Our laboratory is especially known for flow cytometry, and we are launching the NoMoreMGUS project, the largest ever conducted in Spain (and perhaps in Europe) on monoclonal gammopathy of undetermined significance. This is a condition that precedes myeloma. We are looking to study 5000 patients in Spain once a year for 5 years, which means analyzing 25,000 samples.
“On the other hand,” he continued, “we are taking some of these developments to other neoplasms, such as acute lymphoblastic leukemia. And we are interested in using all the potential of cytometry not only to measure tumor cells but also to characterize the immune system as another important biomarker in the pathogenesis of the disease. And, for example, to predict infections, which is very important in patients with myeloma.”
This story was translated from El Médico Interactivo, which is part of the Medscape Professional Network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
Evolving Treatment of Severe Alopecia
The classification of severe alopecia areata (AA) and its treatment are evolving. Dr Ali Jabbari, from the University of Iowa, discusses factors that characterize severe AA and traces the expanded treatment options provided by the advent of Janus kinase (JAK) inhibitors.
Dr Jabbari reports on a modified AA severity scale, published in the Journal of American Academy of Dermatology, in which the presence of certain factors upgrade the level of severity. Factors include eyebrow/eyelash involvement and psychosocial comorbidities such as depression, anxiety, and social phobias. Traditional treatments for severe AA have relied largely on corticosteroids. Dr Jabbari explains how this treatment can be helpful for patients with limited disease, but may be burdensome for those with severe disease, in terms of injection pain and side effects of long-term use. Another option for patients with severe AA is the use of systemic immunosuppressants, but these are not as effective as newer treatments.
Dr Jabbari looks at two FDA-approved options for JAK inhibitors: baricitinib for patients aged 18 years or older and ritlecitinib for patients aged 12 years or older. He notes some of the potential side effects of these medications but concludes that JAK inhibitors are a safe option for treating patients with severe AA.
--
Ali Jabbari, MD, PhD, Chair, DEO, Roger I. Ceilley Associate Professor, Department of Dermatology, University of Iowa Roy J. and Lucille A. Carver College of Medicine, Iowa City, Iowa
Ali Jabbari, MD, PhD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: Pfizer; Inc.; Cage Bio
Received research grant from: National Institutes of Health; Department of Veterans Affairs; Pfizer; Inc
Scientific Advisory Board for: BiologicsMD
The classification of severe alopecia areata (AA) and its treatment are evolving. Dr Ali Jabbari, from the University of Iowa, discusses factors that characterize severe AA and traces the expanded treatment options provided by the advent of Janus kinase (JAK) inhibitors.
Dr Jabbari reports on a modified AA severity scale, published in the Journal of American Academy of Dermatology, in which the presence of certain factors upgrade the level of severity. Factors include eyebrow/eyelash involvement and psychosocial comorbidities such as depression, anxiety, and social phobias. Traditional treatments for severe AA have relied largely on corticosteroids. Dr Jabbari explains how this treatment can be helpful for patients with limited disease, but may be burdensome for those with severe disease, in terms of injection pain and side effects of long-term use. Another option for patients with severe AA is the use of systemic immunosuppressants, but these are not as effective as newer treatments.
Dr Jabbari looks at two FDA-approved options for JAK inhibitors: baricitinib for patients aged 18 years or older and ritlecitinib for patients aged 12 years or older. He notes some of the potential side effects of these medications but concludes that JAK inhibitors are a safe option for treating patients with severe AA.
--
Ali Jabbari, MD, PhD, Chair, DEO, Roger I. Ceilley Associate Professor, Department of Dermatology, University of Iowa Roy J. and Lucille A. Carver College of Medicine, Iowa City, Iowa
Ali Jabbari, MD, PhD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: Pfizer; Inc.; Cage Bio
Received research grant from: National Institutes of Health; Department of Veterans Affairs; Pfizer; Inc
Scientific Advisory Board for: BiologicsMD
The classification of severe alopecia areata (AA) and its treatment are evolving. Dr Ali Jabbari, from the University of Iowa, discusses factors that characterize severe AA and traces the expanded treatment options provided by the advent of Janus kinase (JAK) inhibitors.
Dr Jabbari reports on a modified AA severity scale, published in the Journal of American Academy of Dermatology, in which the presence of certain factors upgrade the level of severity. Factors include eyebrow/eyelash involvement and psychosocial comorbidities such as depression, anxiety, and social phobias. Traditional treatments for severe AA have relied largely on corticosteroids. Dr Jabbari explains how this treatment can be helpful for patients with limited disease, but may be burdensome for those with severe disease, in terms of injection pain and side effects of long-term use. Another option for patients with severe AA is the use of systemic immunosuppressants, but these are not as effective as newer treatments.
Dr Jabbari looks at two FDA-approved options for JAK inhibitors: baricitinib for patients aged 18 years or older and ritlecitinib for patients aged 12 years or older. He notes some of the potential side effects of these medications but concludes that JAK inhibitors are a safe option for treating patients with severe AA.
--
Ali Jabbari, MD, PhD, Chair, DEO, Roger I. Ceilley Associate Professor, Department of Dermatology, University of Iowa Roy J. and Lucille A. Carver College of Medicine, Iowa City, Iowa
Ali Jabbari, MD, PhD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: Pfizer; Inc.; Cage Bio
Received research grant from: National Institutes of Health; Department of Veterans Affairs; Pfizer; Inc
Scientific Advisory Board for: BiologicsMD

Survey Highlights Real-World Use of Upadacitinib in Adults With Atopic Dermatitis
, while 7.8% rated their itch as minimally improved.
Also, 27.5% reported itch improvement within one day of taking upadacitinib (Rinvoq), an oral Janus kinase inhibitor that was approved to treat moderate to severe AD in adults and children aged ≥ 12 years in January 2022.
“We have a lot of data about upadacitinib from clinical trials, but sometimes there’s a concern that when you start using a medication in the real world, the effectiveness doesn’t match up with the efficacy observed in clinical trials,” the study’s first author, Jonathan I. Silverberg, MD, PhD, professor of dermatology at George Washington University, Washington, said in an interview after the Revolutionizing Atopic Dermatitis conference, where the study was presented during a late-breaking abstract session. “We always want to confirm or reaffirm clinical trial results with real-world data.”
In SCALE-UP, 6191 adults with moderate to severe AD participating in the patient support program for upadacitinib in the United States were invited to complete a one-time online survey about their experience with upadacitinib, including the degree of and time to itch improvement and skin clearance. The researchers reported on 204 patients who completed the survey questions, for a response rate of 3.3%. The mean age of respondents was 45.3 years, their mean age when diagnosed with AD was 30.3 years; 70.1% were women, and 37% were using topical corticosteroids. In addition, 68.6% were White individuals, 12.3% were Black individuals, 8.8% were Asian individuals or Pacific Islanders, and 0.5% were Native Americans/Alaska Natives.
Duration of upadacitinib treatment was 2-6 months for 50.5% of the patients and 7-12 months for the remaining patients. Starting upadacitinib dose was 15 mg for about 95% of patients and 30 mg for nearly 4% of patients. At the time of the survey, 79.4% of patients were receiving upadacitinib 15 mg once a day, and 19.6% were receiving upadacitinib 30 mg once a day.
Improvements in Itch, Skin Clearance
Nearly all experienced improvements in itch, with 86.8% reporting “very much” or “much” improved itch. Relief was rapid, with 87% noticing improvement in itch within 7 days and 27.5% noticing improvement within 1 day. “This is something I have clinically seen,” Dr. Silverberg said.
After receiving upadacitinib, 87% and 86% of patients indicated they were “extremely” or “very” satisfied with the degree and speed of itch improvement, respectively.
In findings related to skin clearance, 90.7% of respondents reported clearer skin after initiating upadacitinib, with 81.4% reporting “very much” or “much” clearer skin. Skin clearance occurred rapidly, with 30.8% of patients noticing clearer skin within 3 days of starting upadacitinib and 89.2% of patients noticing clearer skin within 14 days. The proportions of patients who were “extremely” or “very” satisfied with the degree and speed of skin clearance were 83.8% and 83.2%, respectively.
“What we’re seeing is that the real-world effectiveness [of upadacitinib] aligns with the clinical trial efficacy,” Dr. Silverberg told this news organization. “This study adds even more data to help inform shared decision-making discussion with our patients in trying to decide what medication is best for them.”
He acknowledged certain limitations of the survey, including the lack of a control group of other treatments for comparison, a low response rate, and the potential for response bias. “That said, I think the results remain important, but we value having even more real-world data in the future from prospective registries,” he said. “Those kinds of studies are ongoing, and we look forward to getting more real-world data readouts.”
AbbVie, the manufacturer of upadacitinib, funded the study. Dr. Silverberg reported having served as an advisor, consultant, speaker, and/or investigator for several pharmaceutical companies, including AbbVie. Two authors are AbbVie employees.
A version of this article appeared on Medscape.com.
, while 7.8% rated their itch as minimally improved.
Also, 27.5% reported itch improvement within one day of taking upadacitinib (Rinvoq), an oral Janus kinase inhibitor that was approved to treat moderate to severe AD in adults and children aged ≥ 12 years in January 2022.
“We have a lot of data about upadacitinib from clinical trials, but sometimes there’s a concern that when you start using a medication in the real world, the effectiveness doesn’t match up with the efficacy observed in clinical trials,” the study’s first author, Jonathan I. Silverberg, MD, PhD, professor of dermatology at George Washington University, Washington, said in an interview after the Revolutionizing Atopic Dermatitis conference, where the study was presented during a late-breaking abstract session. “We always want to confirm or reaffirm clinical trial results with real-world data.”
In SCALE-UP, 6191 adults with moderate to severe AD participating in the patient support program for upadacitinib in the United States were invited to complete a one-time online survey about their experience with upadacitinib, including the degree of and time to itch improvement and skin clearance. The researchers reported on 204 patients who completed the survey questions, for a response rate of 3.3%. The mean age of respondents was 45.3 years, their mean age when diagnosed with AD was 30.3 years; 70.1% were women, and 37% were using topical corticosteroids. In addition, 68.6% were White individuals, 12.3% were Black individuals, 8.8% were Asian individuals or Pacific Islanders, and 0.5% were Native Americans/Alaska Natives.
Duration of upadacitinib treatment was 2-6 months for 50.5% of the patients and 7-12 months for the remaining patients. Starting upadacitinib dose was 15 mg for about 95% of patients and 30 mg for nearly 4% of patients. At the time of the survey, 79.4% of patients were receiving upadacitinib 15 mg once a day, and 19.6% were receiving upadacitinib 30 mg once a day.
Improvements in Itch, Skin Clearance
Nearly all experienced improvements in itch, with 86.8% reporting “very much” or “much” improved itch. Relief was rapid, with 87% noticing improvement in itch within 7 days and 27.5% noticing improvement within 1 day. “This is something I have clinically seen,” Dr. Silverberg said.
After receiving upadacitinib, 87% and 86% of patients indicated they were “extremely” or “very” satisfied with the degree and speed of itch improvement, respectively.
In findings related to skin clearance, 90.7% of respondents reported clearer skin after initiating upadacitinib, with 81.4% reporting “very much” or “much” clearer skin. Skin clearance occurred rapidly, with 30.8% of patients noticing clearer skin within 3 days of starting upadacitinib and 89.2% of patients noticing clearer skin within 14 days. The proportions of patients who were “extremely” or “very” satisfied with the degree and speed of skin clearance were 83.8% and 83.2%, respectively.
“What we’re seeing is that the real-world effectiveness [of upadacitinib] aligns with the clinical trial efficacy,” Dr. Silverberg told this news organization. “This study adds even more data to help inform shared decision-making discussion with our patients in trying to decide what medication is best for them.”
He acknowledged certain limitations of the survey, including the lack of a control group of other treatments for comparison, a low response rate, and the potential for response bias. “That said, I think the results remain important, but we value having even more real-world data in the future from prospective registries,” he said. “Those kinds of studies are ongoing, and we look forward to getting more real-world data readouts.”
AbbVie, the manufacturer of upadacitinib, funded the study. Dr. Silverberg reported having served as an advisor, consultant, speaker, and/or investigator for several pharmaceutical companies, including AbbVie. Two authors are AbbVie employees.
A version of this article appeared on Medscape.com.
, while 7.8% rated their itch as minimally improved.
Also, 27.5% reported itch improvement within one day of taking upadacitinib (Rinvoq), an oral Janus kinase inhibitor that was approved to treat moderate to severe AD in adults and children aged ≥ 12 years in January 2022.
“We have a lot of data about upadacitinib from clinical trials, but sometimes there’s a concern that when you start using a medication in the real world, the effectiveness doesn’t match up with the efficacy observed in clinical trials,” the study’s first author, Jonathan I. Silverberg, MD, PhD, professor of dermatology at George Washington University, Washington, said in an interview after the Revolutionizing Atopic Dermatitis conference, where the study was presented during a late-breaking abstract session. “We always want to confirm or reaffirm clinical trial results with real-world data.”
In SCALE-UP, 6191 adults with moderate to severe AD participating in the patient support program for upadacitinib in the United States were invited to complete a one-time online survey about their experience with upadacitinib, including the degree of and time to itch improvement and skin clearance. The researchers reported on 204 patients who completed the survey questions, for a response rate of 3.3%. The mean age of respondents was 45.3 years, their mean age when diagnosed with AD was 30.3 years; 70.1% were women, and 37% were using topical corticosteroids. In addition, 68.6% were White individuals, 12.3% were Black individuals, 8.8% were Asian individuals or Pacific Islanders, and 0.5% were Native Americans/Alaska Natives.
Duration of upadacitinib treatment was 2-6 months for 50.5% of the patients and 7-12 months for the remaining patients. Starting upadacitinib dose was 15 mg for about 95% of patients and 30 mg for nearly 4% of patients. At the time of the survey, 79.4% of patients were receiving upadacitinib 15 mg once a day, and 19.6% were receiving upadacitinib 30 mg once a day.
Improvements in Itch, Skin Clearance
Nearly all experienced improvements in itch, with 86.8% reporting “very much” or “much” improved itch. Relief was rapid, with 87% noticing improvement in itch within 7 days and 27.5% noticing improvement within 1 day. “This is something I have clinically seen,” Dr. Silverberg said.
After receiving upadacitinib, 87% and 86% of patients indicated they were “extremely” or “very” satisfied with the degree and speed of itch improvement, respectively.
In findings related to skin clearance, 90.7% of respondents reported clearer skin after initiating upadacitinib, with 81.4% reporting “very much” or “much” clearer skin. Skin clearance occurred rapidly, with 30.8% of patients noticing clearer skin within 3 days of starting upadacitinib and 89.2% of patients noticing clearer skin within 14 days. The proportions of patients who were “extremely” or “very” satisfied with the degree and speed of skin clearance were 83.8% and 83.2%, respectively.
“What we’re seeing is that the real-world effectiveness [of upadacitinib] aligns with the clinical trial efficacy,” Dr. Silverberg told this news organization. “This study adds even more data to help inform shared decision-making discussion with our patients in trying to decide what medication is best for them.”
He acknowledged certain limitations of the survey, including the lack of a control group of other treatments for comparison, a low response rate, and the potential for response bias. “That said, I think the results remain important, but we value having even more real-world data in the future from prospective registries,” he said. “Those kinds of studies are ongoing, and we look forward to getting more real-world data readouts.”
AbbVie, the manufacturer of upadacitinib, funded the study. Dr. Silverberg reported having served as an advisor, consultant, speaker, and/or investigator for several pharmaceutical companies, including AbbVie. Two authors are AbbVie employees.
A version of this article appeared on Medscape.com.