User login
How Dermatologists Can Safeguard Against Malpractice Claims
for liability. Dermatologists can protect themselves by understanding malpractice trends and taking preventive steps, such as making sure NPOs have appropriate training and using a rigorous informed consent process, according to a dermatology resident and a dermatologist who have researched recent trends in dermatology lawsuits.
“It’s really important that physicians recognize their responsibility when delegating procedures to nonphysician operators and the physician’s role in supervision of these procedures,” Scott Stratman, MD, MPH, a dermatology resident at the Icahn School of Medicine at Mount Sinai, New York City, told this news organization. He led a study recently published in the Journal of the American Academy of Dermatology, which found that the majority (52%) of malpractice cases for cutaneous energy-based device procedures in the LexisNexis database from 1985 to September 2023 involved NPOs. The study did not break the data down between different types of NPOs.
Trends in Dermatology Malpractice
This follows a similar trend reported in a 2014 study led by Mathew M. Avram, MD, JD, director of the MGH Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston. The study analyzed liability claims related to cutaneous laser surgery performed by nonphysicians from January 1999 to December 2012.
“With nonphysician litigation data, we saw trend lines beginning in 2008 where the proportion of cases began to increase,” Dr. Avram said at the American Society for Laser Medicine and Surgery (ASLMS) meeting on April 12, 2024. “Over a period of 2008-2012, it went from 36% of cases to about 78%,” he said.
About a quarter (23.4%) of those were in medical offices; 76.6% were in nontraditional settings such as medical spas, he added. The proportion of NPOs was similar in a 2022 study that looked at causes of litigation in cutaneous laser surgery from 2012 to 2020, Dr. Avram said. Again, neither study broke down cases involving NPOs by specific type, but the 2014 study reported that 64% of cases by NPOs occurred outside of a traditional medical setting.
“So it seems that the location and potentially the supervision are issues that are important to patient safety,” Dr. Avram said at the meeting. While state laws regarding laser delegation vary widely, “depending on where you practice, it’s incumbent upon you to know that.”
Dr. Avram and colleagues were also the authors of a study published in June in Dermatologic Surgery that looked at the reasons behind ligations involving dermatologists in a retrospective analysis of 48 state and federal cases between 2011 and 2022. The majority of cases — 54.2% — were for unexpected harm, followed by wrong or delayed diagnoses, which accounted for a third of litigations.
Dr. Stratman’s study found that laser hair removal was the most common procedure for malpractice claims in dermatology among cutaneous energy-based device procedures. Complications from energy-based devices included burns, scarring, and pigmentation changes.
The growth of malpractice suits involving NPOs could be because NPOs are performing a greater proportion of dermatologic procedures, “particularly those practicing without direct supervision, such as in the context of a medical spa,” Dr. Stratman said in the interview. “Again, this highlights a physician’s responsibility in delegating these kinds of procedures to NPOs.”
Training Is a Must — But Not Standardized
Comprehensive training for physicians, staff, NPOs, and physicians “is all necessary and paramount in order to diminish adverse outcomes and legal risk, and then, of course, all these practitioners, be it staff or [NPOs], and, of course, physicians, are all held to the same standard of care,” Dr. Stratman said.
However, he added, “There is really no standardized training to operate these devices. That being said, it’s really important to know that both providers and facility owners have a significant obligation to their patients to make sure that their staff in their centers are appropriately trained.”
Training not only involves protocols and procedures but also how to handle patient interactions, Dr. Stratman said.
The legal concept of respondeat superior applies when nonphysicians participate in a patient’s care, Dr. Avram said at the ASLMS meeting. The physician is held liable for a nonphysician’s “negligence provided he or she is an employee receiving a salary [and] benefits and is performing within the scope of his or her duty,” regardless of whether the physician saw the patient or not at that visit, he said. Again, supervision of nonphysician laser procedures varies from state to state, he added.
“So the take-home point is to provide excellent training and appropriate supervision, and if you’re the owner of that practice, you are liable in the event of negligence even though you never were part of the treatment,” Dr. Avram said.
Ins and Outs of Informed Consent
When a patient outcome is less than desirable, or at least less than what the patient expected, a transparent and thorough informed consent process can protect the practice and physician, Dr. Avram said at the meeting.
“Malpractice and consent have nothing to do with each other,” he said. “Consent is getting permission to do a procedure. It’s needed actually for any medical intervention that you perform. What you need to do is to provide information to enable the patient or guardian or to choose knowledgeably among reasonable medical alternatives. This places the patient in control of the course of their medical treatment.”
The information conveyed to the patient should include the diagnosis, the medical causes, the nature and purpose of the treatment, and the risks and alternatives of procedure, “particularly if they’re high risk,” Dr. Avram said.
“Failure to obtain informed consent constitutes a civil battery, and the physician is liable for civil damages,” he said. “The patient need only show that he or she was not informed of the medical nature of the medical touching; physical injury is not necessary.”
A battery could occur if a procedure extends beyond the scope or area of treatment the patient agreed to — for example, extending a liposuction to an area that wasn’t originally targeted, or extending a laser procedure to an area of the body as a presumed favor to the patient. “It does not require a standard of care or an expert witness,” Dr. Avram said. “One only needs to show nonconsensual touching.”
Informed consents should include plain language, he said. “The whole idea is the patient understands what the risks and benefits are,” Dr. Avram said. “You don’t need to use medical jargon.” As an example, he suggested using the term “blisters” instead of “bullae.” If the treatment involves an off-label procedure, include that too, he said.
He also advised avoiding blanket authorizations. “Courts disfavor them,” he noted. “They need more specificity. So those are not valid.”
Dr. Stratman added that providers should think about the setting in which they obtain informed consent. “It’s really important that providers are consenting their patients in private and quiet places, free from distractions, that they accommodate patients who might have disabilities or limitations in English proficiency, using a teach-back method to help patients understand or demonstrate their understanding of the procedure in order to gauge comprehension,” he said.
Both Dr. Avram and Dr. Stratman pointed out that another strategy to prevent malpractice is to build trusting patient-provider relationships. “The patient-provider relationship is paramount not only to the success of the procedure but to the clinical visit as a whole,” Dr. Stratman said.
That’s a two-way street, he added. Patients should be able to trust that their provider provides them with the best treatment based on their own history, and providers should also be able to trust that patients are providing them with an accurate history, asking relevant questions, or expressing any level of apprehension about the procedure or visit. “The patient-provider relationship is everything,” Dr. Stratman said.
Dr. Stratman and Dr. Avram had no relevant disclosures.
A version of this article appeared on Medscape.com.
for liability. Dermatologists can protect themselves by understanding malpractice trends and taking preventive steps, such as making sure NPOs have appropriate training and using a rigorous informed consent process, according to a dermatology resident and a dermatologist who have researched recent trends in dermatology lawsuits.
“It’s really important that physicians recognize their responsibility when delegating procedures to nonphysician operators and the physician’s role in supervision of these procedures,” Scott Stratman, MD, MPH, a dermatology resident at the Icahn School of Medicine at Mount Sinai, New York City, told this news organization. He led a study recently published in the Journal of the American Academy of Dermatology, which found that the majority (52%) of malpractice cases for cutaneous energy-based device procedures in the LexisNexis database from 1985 to September 2023 involved NPOs. The study did not break the data down between different types of NPOs.
Trends in Dermatology Malpractice
This follows a similar trend reported in a 2014 study led by Mathew M. Avram, MD, JD, director of the MGH Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston. The study analyzed liability claims related to cutaneous laser surgery performed by nonphysicians from January 1999 to December 2012.
“With nonphysician litigation data, we saw trend lines beginning in 2008 where the proportion of cases began to increase,” Dr. Avram said at the American Society for Laser Medicine and Surgery (ASLMS) meeting on April 12, 2024. “Over a period of 2008-2012, it went from 36% of cases to about 78%,” he said.
About a quarter (23.4%) of those were in medical offices; 76.6% were in nontraditional settings such as medical spas, he added. The proportion of NPOs was similar in a 2022 study that looked at causes of litigation in cutaneous laser surgery from 2012 to 2020, Dr. Avram said. Again, neither study broke down cases involving NPOs by specific type, but the 2014 study reported that 64% of cases by NPOs occurred outside of a traditional medical setting.
“So it seems that the location and potentially the supervision are issues that are important to patient safety,” Dr. Avram said at the meeting. While state laws regarding laser delegation vary widely, “depending on where you practice, it’s incumbent upon you to know that.”
Dr. Avram and colleagues were also the authors of a study published in June in Dermatologic Surgery that looked at the reasons behind ligations involving dermatologists in a retrospective analysis of 48 state and federal cases between 2011 and 2022. The majority of cases — 54.2% — were for unexpected harm, followed by wrong or delayed diagnoses, which accounted for a third of litigations.
Dr. Stratman’s study found that laser hair removal was the most common procedure for malpractice claims in dermatology among cutaneous energy-based device procedures. Complications from energy-based devices included burns, scarring, and pigmentation changes.
The growth of malpractice suits involving NPOs could be because NPOs are performing a greater proportion of dermatologic procedures, “particularly those practicing without direct supervision, such as in the context of a medical spa,” Dr. Stratman said in the interview. “Again, this highlights a physician’s responsibility in delegating these kinds of procedures to NPOs.”
Training Is a Must — But Not Standardized
Comprehensive training for physicians, staff, NPOs, and physicians “is all necessary and paramount in order to diminish adverse outcomes and legal risk, and then, of course, all these practitioners, be it staff or [NPOs], and, of course, physicians, are all held to the same standard of care,” Dr. Stratman said.
However, he added, “There is really no standardized training to operate these devices. That being said, it’s really important to know that both providers and facility owners have a significant obligation to their patients to make sure that their staff in their centers are appropriately trained.”
Training not only involves protocols and procedures but also how to handle patient interactions, Dr. Stratman said.
The legal concept of respondeat superior applies when nonphysicians participate in a patient’s care, Dr. Avram said at the ASLMS meeting. The physician is held liable for a nonphysician’s “negligence provided he or she is an employee receiving a salary [and] benefits and is performing within the scope of his or her duty,” regardless of whether the physician saw the patient or not at that visit, he said. Again, supervision of nonphysician laser procedures varies from state to state, he added.
“So the take-home point is to provide excellent training and appropriate supervision, and if you’re the owner of that practice, you are liable in the event of negligence even though you never were part of the treatment,” Dr. Avram said.
Ins and Outs of Informed Consent
When a patient outcome is less than desirable, or at least less than what the patient expected, a transparent and thorough informed consent process can protect the practice and physician, Dr. Avram said at the meeting.
“Malpractice and consent have nothing to do with each other,” he said. “Consent is getting permission to do a procedure. It’s needed actually for any medical intervention that you perform. What you need to do is to provide information to enable the patient or guardian or to choose knowledgeably among reasonable medical alternatives. This places the patient in control of the course of their medical treatment.”
The information conveyed to the patient should include the diagnosis, the medical causes, the nature and purpose of the treatment, and the risks and alternatives of procedure, “particularly if they’re high risk,” Dr. Avram said.
“Failure to obtain informed consent constitutes a civil battery, and the physician is liable for civil damages,” he said. “The patient need only show that he or she was not informed of the medical nature of the medical touching; physical injury is not necessary.”
A battery could occur if a procedure extends beyond the scope or area of treatment the patient agreed to — for example, extending a liposuction to an area that wasn’t originally targeted, or extending a laser procedure to an area of the body as a presumed favor to the patient. “It does not require a standard of care or an expert witness,” Dr. Avram said. “One only needs to show nonconsensual touching.”
Informed consents should include plain language, he said. “The whole idea is the patient understands what the risks and benefits are,” Dr. Avram said. “You don’t need to use medical jargon.” As an example, he suggested using the term “blisters” instead of “bullae.” If the treatment involves an off-label procedure, include that too, he said.
He also advised avoiding blanket authorizations. “Courts disfavor them,” he noted. “They need more specificity. So those are not valid.”
Dr. Stratman added that providers should think about the setting in which they obtain informed consent. “It’s really important that providers are consenting their patients in private and quiet places, free from distractions, that they accommodate patients who might have disabilities or limitations in English proficiency, using a teach-back method to help patients understand or demonstrate their understanding of the procedure in order to gauge comprehension,” he said.
Both Dr. Avram and Dr. Stratman pointed out that another strategy to prevent malpractice is to build trusting patient-provider relationships. “The patient-provider relationship is paramount not only to the success of the procedure but to the clinical visit as a whole,” Dr. Stratman said.
That’s a two-way street, he added. Patients should be able to trust that their provider provides them with the best treatment based on their own history, and providers should also be able to trust that patients are providing them with an accurate history, asking relevant questions, or expressing any level of apprehension about the procedure or visit. “The patient-provider relationship is everything,” Dr. Stratman said.
Dr. Stratman and Dr. Avram had no relevant disclosures.
A version of this article appeared on Medscape.com.
for liability. Dermatologists can protect themselves by understanding malpractice trends and taking preventive steps, such as making sure NPOs have appropriate training and using a rigorous informed consent process, according to a dermatology resident and a dermatologist who have researched recent trends in dermatology lawsuits.
“It’s really important that physicians recognize their responsibility when delegating procedures to nonphysician operators and the physician’s role in supervision of these procedures,” Scott Stratman, MD, MPH, a dermatology resident at the Icahn School of Medicine at Mount Sinai, New York City, told this news organization. He led a study recently published in the Journal of the American Academy of Dermatology, which found that the majority (52%) of malpractice cases for cutaneous energy-based device procedures in the LexisNexis database from 1985 to September 2023 involved NPOs. The study did not break the data down between different types of NPOs.
Trends in Dermatology Malpractice
This follows a similar trend reported in a 2014 study led by Mathew M. Avram, MD, JD, director of the MGH Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston. The study analyzed liability claims related to cutaneous laser surgery performed by nonphysicians from January 1999 to December 2012.
“With nonphysician litigation data, we saw trend lines beginning in 2008 where the proportion of cases began to increase,” Dr. Avram said at the American Society for Laser Medicine and Surgery (ASLMS) meeting on April 12, 2024. “Over a period of 2008-2012, it went from 36% of cases to about 78%,” he said.
About a quarter (23.4%) of those were in medical offices; 76.6% were in nontraditional settings such as medical spas, he added. The proportion of NPOs was similar in a 2022 study that looked at causes of litigation in cutaneous laser surgery from 2012 to 2020, Dr. Avram said. Again, neither study broke down cases involving NPOs by specific type, but the 2014 study reported that 64% of cases by NPOs occurred outside of a traditional medical setting.
“So it seems that the location and potentially the supervision are issues that are important to patient safety,” Dr. Avram said at the meeting. While state laws regarding laser delegation vary widely, “depending on where you practice, it’s incumbent upon you to know that.”
Dr. Avram and colleagues were also the authors of a study published in June in Dermatologic Surgery that looked at the reasons behind ligations involving dermatologists in a retrospective analysis of 48 state and federal cases between 2011 and 2022. The majority of cases — 54.2% — were for unexpected harm, followed by wrong or delayed diagnoses, which accounted for a third of litigations.
Dr. Stratman’s study found that laser hair removal was the most common procedure for malpractice claims in dermatology among cutaneous energy-based device procedures. Complications from energy-based devices included burns, scarring, and pigmentation changes.
The growth of malpractice suits involving NPOs could be because NPOs are performing a greater proportion of dermatologic procedures, “particularly those practicing without direct supervision, such as in the context of a medical spa,” Dr. Stratman said in the interview. “Again, this highlights a physician’s responsibility in delegating these kinds of procedures to NPOs.”
Training Is a Must — But Not Standardized
Comprehensive training for physicians, staff, NPOs, and physicians “is all necessary and paramount in order to diminish adverse outcomes and legal risk, and then, of course, all these practitioners, be it staff or [NPOs], and, of course, physicians, are all held to the same standard of care,” Dr. Stratman said.
However, he added, “There is really no standardized training to operate these devices. That being said, it’s really important to know that both providers and facility owners have a significant obligation to their patients to make sure that their staff in their centers are appropriately trained.”
Training not only involves protocols and procedures but also how to handle patient interactions, Dr. Stratman said.
The legal concept of respondeat superior applies when nonphysicians participate in a patient’s care, Dr. Avram said at the ASLMS meeting. The physician is held liable for a nonphysician’s “negligence provided he or she is an employee receiving a salary [and] benefits and is performing within the scope of his or her duty,” regardless of whether the physician saw the patient or not at that visit, he said. Again, supervision of nonphysician laser procedures varies from state to state, he added.
“So the take-home point is to provide excellent training and appropriate supervision, and if you’re the owner of that practice, you are liable in the event of negligence even though you never were part of the treatment,” Dr. Avram said.
Ins and Outs of Informed Consent
When a patient outcome is less than desirable, or at least less than what the patient expected, a transparent and thorough informed consent process can protect the practice and physician, Dr. Avram said at the meeting.
“Malpractice and consent have nothing to do with each other,” he said. “Consent is getting permission to do a procedure. It’s needed actually for any medical intervention that you perform. What you need to do is to provide information to enable the patient or guardian or to choose knowledgeably among reasonable medical alternatives. This places the patient in control of the course of their medical treatment.”
The information conveyed to the patient should include the diagnosis, the medical causes, the nature and purpose of the treatment, and the risks and alternatives of procedure, “particularly if they’re high risk,” Dr. Avram said.
“Failure to obtain informed consent constitutes a civil battery, and the physician is liable for civil damages,” he said. “The patient need only show that he or she was not informed of the medical nature of the medical touching; physical injury is not necessary.”
A battery could occur if a procedure extends beyond the scope or area of treatment the patient agreed to — for example, extending a liposuction to an area that wasn’t originally targeted, or extending a laser procedure to an area of the body as a presumed favor to the patient. “It does not require a standard of care or an expert witness,” Dr. Avram said. “One only needs to show nonconsensual touching.”
Informed consents should include plain language, he said. “The whole idea is the patient understands what the risks and benefits are,” Dr. Avram said. “You don’t need to use medical jargon.” As an example, he suggested using the term “blisters” instead of “bullae.” If the treatment involves an off-label procedure, include that too, he said.
He also advised avoiding blanket authorizations. “Courts disfavor them,” he noted. “They need more specificity. So those are not valid.”
Dr. Stratman added that providers should think about the setting in which they obtain informed consent. “It’s really important that providers are consenting their patients in private and quiet places, free from distractions, that they accommodate patients who might have disabilities or limitations in English proficiency, using a teach-back method to help patients understand or demonstrate their understanding of the procedure in order to gauge comprehension,” he said.
Both Dr. Avram and Dr. Stratman pointed out that another strategy to prevent malpractice is to build trusting patient-provider relationships. “The patient-provider relationship is paramount not only to the success of the procedure but to the clinical visit as a whole,” Dr. Stratman said.
That’s a two-way street, he added. Patients should be able to trust that their provider provides them with the best treatment based on their own history, and providers should also be able to trust that patients are providing them with an accurate history, asking relevant questions, or expressing any level of apprehension about the procedure or visit. “The patient-provider relationship is everything,” Dr. Stratman said.
Dr. Stratman and Dr. Avram had no relevant disclosures.
A version of this article appeared on Medscape.com.
An Overview of Gender-Affirming Care for Children and Adolescents
As Pride Month drew to a close, the Supreme Court made a shocking announcement. For the first time in the history of the court, it is willing to hear a legal challenge regarding gender-affirming care for minors. The justices will review whether a 2023 Tennessee law, SB1, which bans hormone therapy, puberty blockers, and surgery for transgender minors, is unconstitutional. This is the first time the Supreme Court will directly weigh in on gender-affirming care.
There are few topics as politically and medically divisive as gender-affirming care for minors. When the World Professional Association for Transgender Health (WPATH) released its updated Standards of Care, SOC8, one of the noticeable changes to the document was its approach to caring for transgender children and adolescents.
Before I highlight these recommendations and the ensuing controversy, it is imperative to establish proper terminology. Unfortunately, medical and legal terms often differ. Both activists and opponents use these terms interchangeably, which makes discourse about an already emotionally charged topic extremely difficult. From a legal perspective, the terms “minor” and “child” often refer to individuals under the age of majority. In the United States, the age of majority is 18. However, the term child also has a well-established medical definition. A child is an individual between the stages of infancy and puberty. Adolescence is a transitional period marked by the onset of puberty until adulthood (typically the age of majority). As medical providers, understanding these definitions is essential to identifying misinformation pertaining to this type of healthcare.
For the purposes of this article, I will be adhering to the medical terminology. Now, I want to be very clear. WPATH does not endorse surgical procedures on children. Furthermore, surgeons are not performing gender-affirming surgeries on children. On adolescents, rarely. But children, never.
According to the updated SOC8, the only acceptable gender-affirming intervention for children is psychosocial support.1 This does not include puberty blockers, hormones, or surgery, but rather allowing a child to explore their gender identity by experimenting with different clothing, toys, hairstyles, and even an alternative name that aligns more closely with their gender identity.1
It is only after children reach adolescence that medical, and in rare cases, surgical interventions, can be considered. Puberty blockers are appropriate for patients who have started puberty and experience gender dysphoria. These medications are reversible, and their purpose is to temporarily pause puberty to allow the adolescent to further explore their gender identity.
The most significant side effect of puberty blockers is decreased bone density.1 As a result, providers typically do not prescribe these medications for more than 2-3 years. After discontinuation of the medication, bone density returns to baseline.1 If the adolescent’s gender identity is marked and sustained over time, hormone therapy, such as testosterone or estrogen is then considered. Unlike puberty blockers, these medications can have permanent side effects. Testosterone use can lead to irreversible hair growth, alopecia, clitoromegaly, and voice deepening, while estrogen can cause permanent breast growth and halt sperm production.1 Future fertility and these side effects are discussed with the patient in detail prior to the initiation of these medications.
Contrary to the current political narrative, gender-affirming care for children and adolescents is not taken lightly. These individuals often receive years of multidisciplinary assessments, with a focus on gender identity development, social development and support, and diagnostic assessment of possible co-occurring mental health or developmental concerns and capacity for decision making.1 The clinical visits also occur with parental support and consent.
WPATH SOC8 also delineates the provider qualifications for health care professionals assessing these patients. Providers must be licensed by their statutory bodies and hold a postgraduate degree by a nationally accredited statutory institution; receive theoretical and evidence-based training and develop expertise in child, adolescent, and family mental health across the developmental spectrum; receive training and have expertise in gender identity development and gender diversity in children and adolescence; have the ability to assess capacity to assent/consent; receive training and develop expertise in autism spectrum disorders and other neurodevelopmental presentations; and to continue engaging in professional development in all areas relevant to gender-diverse children, adolescents, and families.1
The most controversial aspect of gender-affirming care for children and adolescents relates to surgical treatment. While the rates of gender-affirming surgeries have increased for this age group over the years, the overall rate of gender-affirming surgery for adolescents is markedly lower compared with other adolescents seeking cosmetic surgeries and compared with transgender adults undergoing gender-affirming surgery.
In a cohort study conducted between 2016 to 2020, 48,019 patients were identified who had undergone gender-affirming surgery.2 Only 3678 or 7.7% of patients were aged between 12 and 18, with the most common procedure being chest/breast surgery.2 So, under about 1000 cases per year were gender-affirming surgeries on patients under 18.
During 2020 alone, the number of cisgender adolescents between the ages of 13 and 19 who underwent breast augmentation and breast reduction was 3233 and 4666, respectively.3 The outrage about gender-affirming surgeries on transgender youth, yet the silence on cosmetic procedures in this same age group, speaks volumes.
All surgeries on adolescents should be taken seriously and with caution, regardless of gender identity. However, current legislation disproportionately targets only transgender youth. For whatever reason, surgeries on transgender individuals are labeled as “body mutilation,” whereas surgeries on cisgender youth are not even discussed. Such inflammatory rhetoric and complete lack of empathy impedes the common goal of all parties: what is in the best interest of the minor? Unfortunately, in a few short months, the answer to this question will be determined by a group of nine justices who have no experience in medicine or transgender health care, instead of by medical experts and the parents who care for these individuals.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pennsylvania. She has no conflicts of interest.
References
1. Coleman E et al. Standards of care for the health of transgender and gender diverse people, version 8. Int J Transgender Health. 2022;23(sup):S1-S259.
2. Wright JD et al. National estimates of gender affirming surgery in the US. JAMA Netw Open. 2023 Aug 1;6(8):e2330348.
3. American Society of Plastic Surgeons. Plastic Surgery Statistics Report. ASPS National Clearinghouse of Plastic Surgery Procedural Statistics. 2020.
As Pride Month drew to a close, the Supreme Court made a shocking announcement. For the first time in the history of the court, it is willing to hear a legal challenge regarding gender-affirming care for minors. The justices will review whether a 2023 Tennessee law, SB1, which bans hormone therapy, puberty blockers, and surgery for transgender minors, is unconstitutional. This is the first time the Supreme Court will directly weigh in on gender-affirming care.
There are few topics as politically and medically divisive as gender-affirming care for minors. When the World Professional Association for Transgender Health (WPATH) released its updated Standards of Care, SOC8, one of the noticeable changes to the document was its approach to caring for transgender children and adolescents.
Before I highlight these recommendations and the ensuing controversy, it is imperative to establish proper terminology. Unfortunately, medical and legal terms often differ. Both activists and opponents use these terms interchangeably, which makes discourse about an already emotionally charged topic extremely difficult. From a legal perspective, the terms “minor” and “child” often refer to individuals under the age of majority. In the United States, the age of majority is 18. However, the term child also has a well-established medical definition. A child is an individual between the stages of infancy and puberty. Adolescence is a transitional period marked by the onset of puberty until adulthood (typically the age of majority). As medical providers, understanding these definitions is essential to identifying misinformation pertaining to this type of healthcare.
For the purposes of this article, I will be adhering to the medical terminology. Now, I want to be very clear. WPATH does not endorse surgical procedures on children. Furthermore, surgeons are not performing gender-affirming surgeries on children. On adolescents, rarely. But children, never.
According to the updated SOC8, the only acceptable gender-affirming intervention for children is psychosocial support.1 This does not include puberty blockers, hormones, or surgery, but rather allowing a child to explore their gender identity by experimenting with different clothing, toys, hairstyles, and even an alternative name that aligns more closely with their gender identity.1
It is only after children reach adolescence that medical, and in rare cases, surgical interventions, can be considered. Puberty blockers are appropriate for patients who have started puberty and experience gender dysphoria. These medications are reversible, and their purpose is to temporarily pause puberty to allow the adolescent to further explore their gender identity.
The most significant side effect of puberty blockers is decreased bone density.1 As a result, providers typically do not prescribe these medications for more than 2-3 years. After discontinuation of the medication, bone density returns to baseline.1 If the adolescent’s gender identity is marked and sustained over time, hormone therapy, such as testosterone or estrogen is then considered. Unlike puberty blockers, these medications can have permanent side effects. Testosterone use can lead to irreversible hair growth, alopecia, clitoromegaly, and voice deepening, while estrogen can cause permanent breast growth and halt sperm production.1 Future fertility and these side effects are discussed with the patient in detail prior to the initiation of these medications.
Contrary to the current political narrative, gender-affirming care for children and adolescents is not taken lightly. These individuals often receive years of multidisciplinary assessments, with a focus on gender identity development, social development and support, and diagnostic assessment of possible co-occurring mental health or developmental concerns and capacity for decision making.1 The clinical visits also occur with parental support and consent.
WPATH SOC8 also delineates the provider qualifications for health care professionals assessing these patients. Providers must be licensed by their statutory bodies and hold a postgraduate degree by a nationally accredited statutory institution; receive theoretical and evidence-based training and develop expertise in child, adolescent, and family mental health across the developmental spectrum; receive training and have expertise in gender identity development and gender diversity in children and adolescence; have the ability to assess capacity to assent/consent; receive training and develop expertise in autism spectrum disorders and other neurodevelopmental presentations; and to continue engaging in professional development in all areas relevant to gender-diverse children, adolescents, and families.1
The most controversial aspect of gender-affirming care for children and adolescents relates to surgical treatment. While the rates of gender-affirming surgeries have increased for this age group over the years, the overall rate of gender-affirming surgery for adolescents is markedly lower compared with other adolescents seeking cosmetic surgeries and compared with transgender adults undergoing gender-affirming surgery.
In a cohort study conducted between 2016 to 2020, 48,019 patients were identified who had undergone gender-affirming surgery.2 Only 3678 or 7.7% of patients were aged between 12 and 18, with the most common procedure being chest/breast surgery.2 So, under about 1000 cases per year were gender-affirming surgeries on patients under 18.
During 2020 alone, the number of cisgender adolescents between the ages of 13 and 19 who underwent breast augmentation and breast reduction was 3233 and 4666, respectively.3 The outrage about gender-affirming surgeries on transgender youth, yet the silence on cosmetic procedures in this same age group, speaks volumes.
All surgeries on adolescents should be taken seriously and with caution, regardless of gender identity. However, current legislation disproportionately targets only transgender youth. For whatever reason, surgeries on transgender individuals are labeled as “body mutilation,” whereas surgeries on cisgender youth are not even discussed. Such inflammatory rhetoric and complete lack of empathy impedes the common goal of all parties: what is in the best interest of the minor? Unfortunately, in a few short months, the answer to this question will be determined by a group of nine justices who have no experience in medicine or transgender health care, instead of by medical experts and the parents who care for these individuals.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pennsylvania. She has no conflicts of interest.
References
1. Coleman E et al. Standards of care for the health of transgender and gender diverse people, version 8. Int J Transgender Health. 2022;23(sup):S1-S259.
2. Wright JD et al. National estimates of gender affirming surgery in the US. JAMA Netw Open. 2023 Aug 1;6(8):e2330348.
3. American Society of Plastic Surgeons. Plastic Surgery Statistics Report. ASPS National Clearinghouse of Plastic Surgery Procedural Statistics. 2020.
As Pride Month drew to a close, the Supreme Court made a shocking announcement. For the first time in the history of the court, it is willing to hear a legal challenge regarding gender-affirming care for minors. The justices will review whether a 2023 Tennessee law, SB1, which bans hormone therapy, puberty blockers, and surgery for transgender minors, is unconstitutional. This is the first time the Supreme Court will directly weigh in on gender-affirming care.
There are few topics as politically and medically divisive as gender-affirming care for minors. When the World Professional Association for Transgender Health (WPATH) released its updated Standards of Care, SOC8, one of the noticeable changes to the document was its approach to caring for transgender children and adolescents.
Before I highlight these recommendations and the ensuing controversy, it is imperative to establish proper terminology. Unfortunately, medical and legal terms often differ. Both activists and opponents use these terms interchangeably, which makes discourse about an already emotionally charged topic extremely difficult. From a legal perspective, the terms “minor” and “child” often refer to individuals under the age of majority. In the United States, the age of majority is 18. However, the term child also has a well-established medical definition. A child is an individual between the stages of infancy and puberty. Adolescence is a transitional period marked by the onset of puberty until adulthood (typically the age of majority). As medical providers, understanding these definitions is essential to identifying misinformation pertaining to this type of healthcare.
For the purposes of this article, I will be adhering to the medical terminology. Now, I want to be very clear. WPATH does not endorse surgical procedures on children. Furthermore, surgeons are not performing gender-affirming surgeries on children. On adolescents, rarely. But children, never.
According to the updated SOC8, the only acceptable gender-affirming intervention for children is psychosocial support.1 This does not include puberty blockers, hormones, or surgery, but rather allowing a child to explore their gender identity by experimenting with different clothing, toys, hairstyles, and even an alternative name that aligns more closely with their gender identity.1
It is only after children reach adolescence that medical, and in rare cases, surgical interventions, can be considered. Puberty blockers are appropriate for patients who have started puberty and experience gender dysphoria. These medications are reversible, and their purpose is to temporarily pause puberty to allow the adolescent to further explore their gender identity.
The most significant side effect of puberty blockers is decreased bone density.1 As a result, providers typically do not prescribe these medications for more than 2-3 years. After discontinuation of the medication, bone density returns to baseline.1 If the adolescent’s gender identity is marked and sustained over time, hormone therapy, such as testosterone or estrogen is then considered. Unlike puberty blockers, these medications can have permanent side effects. Testosterone use can lead to irreversible hair growth, alopecia, clitoromegaly, and voice deepening, while estrogen can cause permanent breast growth and halt sperm production.1 Future fertility and these side effects are discussed with the patient in detail prior to the initiation of these medications.
Contrary to the current political narrative, gender-affirming care for children and adolescents is not taken lightly. These individuals often receive years of multidisciplinary assessments, with a focus on gender identity development, social development and support, and diagnostic assessment of possible co-occurring mental health or developmental concerns and capacity for decision making.1 The clinical visits also occur with parental support and consent.
WPATH SOC8 also delineates the provider qualifications for health care professionals assessing these patients. Providers must be licensed by their statutory bodies and hold a postgraduate degree by a nationally accredited statutory institution; receive theoretical and evidence-based training and develop expertise in child, adolescent, and family mental health across the developmental spectrum; receive training and have expertise in gender identity development and gender diversity in children and adolescence; have the ability to assess capacity to assent/consent; receive training and develop expertise in autism spectrum disorders and other neurodevelopmental presentations; and to continue engaging in professional development in all areas relevant to gender-diverse children, adolescents, and families.1
The most controversial aspect of gender-affirming care for children and adolescents relates to surgical treatment. While the rates of gender-affirming surgeries have increased for this age group over the years, the overall rate of gender-affirming surgery for adolescents is markedly lower compared with other adolescents seeking cosmetic surgeries and compared with transgender adults undergoing gender-affirming surgery.
In a cohort study conducted between 2016 to 2020, 48,019 patients were identified who had undergone gender-affirming surgery.2 Only 3678 or 7.7% of patients were aged between 12 and 18, with the most common procedure being chest/breast surgery.2 So, under about 1000 cases per year were gender-affirming surgeries on patients under 18.
During 2020 alone, the number of cisgender adolescents between the ages of 13 and 19 who underwent breast augmentation and breast reduction was 3233 and 4666, respectively.3 The outrage about gender-affirming surgeries on transgender youth, yet the silence on cosmetic procedures in this same age group, speaks volumes.
All surgeries on adolescents should be taken seriously and with caution, regardless of gender identity. However, current legislation disproportionately targets only transgender youth. For whatever reason, surgeries on transgender individuals are labeled as “body mutilation,” whereas surgeries on cisgender youth are not even discussed. Such inflammatory rhetoric and complete lack of empathy impedes the common goal of all parties: what is in the best interest of the minor? Unfortunately, in a few short months, the answer to this question will be determined by a group of nine justices who have no experience in medicine or transgender health care, instead of by medical experts and the parents who care for these individuals.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pennsylvania. She has no conflicts of interest.
References
1. Coleman E et al. Standards of care for the health of transgender and gender diverse people, version 8. Int J Transgender Health. 2022;23(sup):S1-S259.
2. Wright JD et al. National estimates of gender affirming surgery in the US. JAMA Netw Open. 2023 Aug 1;6(8):e2330348.
3. American Society of Plastic Surgeons. Plastic Surgery Statistics Report. ASPS National Clearinghouse of Plastic Surgery Procedural Statistics. 2020.
Sorafenib Plus TACE Prolongs Survival in Recurrent HCC
TOPLINE:
METHODOLOGY:
- Recurrent intermediate-stage HCC has a poor prognosis, and TACE alone has yielded “unsatisfactory survival benefits,” the study authors explained. Retrospective studies suggest that combining sorafenib and TACE may be a better therapeutic option.
- Sorafenib, an inhibitor of vascular endothelial growth factor and platelet-derived growth factor receptors, may have a synergistic effect alongside TACE after hepatectomy in patients with positive microvascular invasion.
- To investigate further, 162 patients (median age, 55 years; 93% men) with recurrent intermediate-stage HCC and positive microvascular invasion were randomly allocated to sorafenib plus TACE or TACE alone.
- The trial was conducted at five hospitals in China from October 2019 to December 2021.
TAKEAWAY:
- Median overall survival was significantly longer with sorafenib plus TACE than with TACE alone (22.2 months vs 15.1 months; hazard ratio [HR], 0.55; P < .001).
- The overall survival rate at 24 months was 44.4% in the combination group vs 24.2% in the TACE group, and the rate at 36 months was 26.9% and 13.6%, respectively.
- The combination of sorafenib and TACE also significantly prolonged progression-free survival (median 16.2 months vs 11.8 months; HR, 0.54; P < .001) and led to a significantly better objective response rate (80.2% vs 58.0%; P = .002).
- Any-grade adverse events were more common in the combination arm, but all responded well to treatment, and no unexpected adverse events or treatment-related deaths occurred. The most common grade 3 or 4 adverse events in both arms included increased alanine aminotransferase (19.8% in both) and increased aspartate aminotransferase (23.5% in the combination group vs 18.5% in the TACE arm).
IN PRACTICE:
“These findings suggest that combined [sorafenib plus TACE] treatment should be considered for patients with recurrent intermediate-stage HCC after R0 hepatectomy with positive microvascular invasion,” the authors wrote.
SOURCE:
The study, with first author Wenzhe Fan, MD, from The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China, was published online in JAMA Oncology.
LIMITATIONS:
The open-label design may introduce potential bias, although the results were confirmed by a masked independent imaging review. The study population was primarily from an endemic region with high rates of chronic hepatitis B virus infection, which may limit generalizability to populations with different etiologies of HCC, such as hepatitis C in Western countries.
DISCLOSURES:
Funding was provided by the National Natural Science Foundation of China and the Outstanding Youth Fund of the National Natural Science Foundation of China. The authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Recurrent intermediate-stage HCC has a poor prognosis, and TACE alone has yielded “unsatisfactory survival benefits,” the study authors explained. Retrospective studies suggest that combining sorafenib and TACE may be a better therapeutic option.
- Sorafenib, an inhibitor of vascular endothelial growth factor and platelet-derived growth factor receptors, may have a synergistic effect alongside TACE after hepatectomy in patients with positive microvascular invasion.
- To investigate further, 162 patients (median age, 55 years; 93% men) with recurrent intermediate-stage HCC and positive microvascular invasion were randomly allocated to sorafenib plus TACE or TACE alone.
- The trial was conducted at five hospitals in China from October 2019 to December 2021.
TAKEAWAY:
- Median overall survival was significantly longer with sorafenib plus TACE than with TACE alone (22.2 months vs 15.1 months; hazard ratio [HR], 0.55; P < .001).
- The overall survival rate at 24 months was 44.4% in the combination group vs 24.2% in the TACE group, and the rate at 36 months was 26.9% and 13.6%, respectively.
- The combination of sorafenib and TACE also significantly prolonged progression-free survival (median 16.2 months vs 11.8 months; HR, 0.54; P < .001) and led to a significantly better objective response rate (80.2% vs 58.0%; P = .002).
- Any-grade adverse events were more common in the combination arm, but all responded well to treatment, and no unexpected adverse events or treatment-related deaths occurred. The most common grade 3 or 4 adverse events in both arms included increased alanine aminotransferase (19.8% in both) and increased aspartate aminotransferase (23.5% in the combination group vs 18.5% in the TACE arm).
IN PRACTICE:
“These findings suggest that combined [sorafenib plus TACE] treatment should be considered for patients with recurrent intermediate-stage HCC after R0 hepatectomy with positive microvascular invasion,” the authors wrote.
SOURCE:
The study, with first author Wenzhe Fan, MD, from The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China, was published online in JAMA Oncology.
LIMITATIONS:
The open-label design may introduce potential bias, although the results were confirmed by a masked independent imaging review. The study population was primarily from an endemic region with high rates of chronic hepatitis B virus infection, which may limit generalizability to populations with different etiologies of HCC, such as hepatitis C in Western countries.
DISCLOSURES:
Funding was provided by the National Natural Science Foundation of China and the Outstanding Youth Fund of the National Natural Science Foundation of China. The authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Recurrent intermediate-stage HCC has a poor prognosis, and TACE alone has yielded “unsatisfactory survival benefits,” the study authors explained. Retrospective studies suggest that combining sorafenib and TACE may be a better therapeutic option.
- Sorafenib, an inhibitor of vascular endothelial growth factor and platelet-derived growth factor receptors, may have a synergistic effect alongside TACE after hepatectomy in patients with positive microvascular invasion.
- To investigate further, 162 patients (median age, 55 years; 93% men) with recurrent intermediate-stage HCC and positive microvascular invasion were randomly allocated to sorafenib plus TACE or TACE alone.
- The trial was conducted at five hospitals in China from October 2019 to December 2021.
TAKEAWAY:
- Median overall survival was significantly longer with sorafenib plus TACE than with TACE alone (22.2 months vs 15.1 months; hazard ratio [HR], 0.55; P < .001).
- The overall survival rate at 24 months was 44.4% in the combination group vs 24.2% in the TACE group, and the rate at 36 months was 26.9% and 13.6%, respectively.
- The combination of sorafenib and TACE also significantly prolonged progression-free survival (median 16.2 months vs 11.8 months; HR, 0.54; P < .001) and led to a significantly better objective response rate (80.2% vs 58.0%; P = .002).
- Any-grade adverse events were more common in the combination arm, but all responded well to treatment, and no unexpected adverse events or treatment-related deaths occurred. The most common grade 3 or 4 adverse events in both arms included increased alanine aminotransferase (19.8% in both) and increased aspartate aminotransferase (23.5% in the combination group vs 18.5% in the TACE arm).
IN PRACTICE:
“These findings suggest that combined [sorafenib plus TACE] treatment should be considered for patients with recurrent intermediate-stage HCC after R0 hepatectomy with positive microvascular invasion,” the authors wrote.
SOURCE:
The study, with first author Wenzhe Fan, MD, from The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China, was published online in JAMA Oncology.
LIMITATIONS:
The open-label design may introduce potential bias, although the results were confirmed by a masked independent imaging review. The study population was primarily from an endemic region with high rates of chronic hepatitis B virus infection, which may limit generalizability to populations with different etiologies of HCC, such as hepatitis C in Western countries.
DISCLOSURES:
Funding was provided by the National Natural Science Foundation of China and the Outstanding Youth Fund of the National Natural Science Foundation of China. The authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Cosmetic Botulinum Toxin A Doses May Differ in Sunny Climates
findings from a comparative cohort study suggested.
“Botulinum toxin A to the glabella is a popular cosmetic intervention,” researchers led by Kim L. Borsky, MD, MBBS, of the Department of Plastic and Reconstructive Surgery at Stoke Mandeville Hospital, Aylesbury, England, and colleagues wrote in their study, which was published in Plastic and Reconstructive Surgery. “Functional musculature differences may arise from chronic behavioral adjustment to high sun exposure levels, requiring greater doses. This could affect clinical practice globally.”
To investigate the effect of climate on real-world doses of the product, the researchers enrolled 523 women aged 35-60 years who received glabellar botulinum toxin treatment at two centers between 2012 and 2019: one in the United Kingdom and one in Malta. They evaluated data on 292 patients treated during the summer months at the Malta center (classified as the high sun-exposure group), and 231 patients treated during the winter months at the UK center (classified as the low sun-exposure group). The primary outcomes of interest were the required top-up doses and the total dose to achieve full paralysis. Smokers were excluded from the analysis, as were those who did not seek maximal paralysis, those documented as not compliant with posttreatment advice, and those with colds or fevers. They used univariable and multivariable analyses to compare the high vs low sun-exposure groups.
The researchers found that 68.5% of women in the high-sun group required a top-up dose to achieve full paralysis, compared with 61.5% in the low-sun group, a difference that did not reach statistical significance (P = .1032). All patients achieved full paralysis with the treatment protocol used. However, in the high-sun group, the mean top-up dose was significantly higher than that in the low-sun group (a mean of 9.30 vs 7.06 units, respectively; P = .0009), as was the mean total dose (a mean of 29.23 vs 27.25 units; P = .0031).
“Patients subject to less sun exposure require a lower dose than patients with high sun exposure, and this was present and persisted when controlling for potential confounders,” the researchers wrote. “Although robustly demonstrated, the difference in doses seen here was small, and so may not directly impact at a health economic level, as the difference would not necessarily change the number of vials used. However, it may be of relevance to training and protocolization of treatments. Rigid protocols about doses and distributions may lead to undertreatment if applied in sunnier climates.”
They acknowledged certain limitations of their study, including its unblinded design and the fact that they did not evaluate or control for ethnicity. They also characterized the population of Malta as “very homogeneous, mainly made up of Maltese with less than 5% foreigners,” while the demographics of the United Kingdom and especially London, where the injections were performed, “are much more diverse.”
Asked to comment on the results, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, DC, said that the study highlights the importance of tailoring neuromodulator treatment to the individual patient based not just on gender but also on lifestyle and climate. “The conclusion [of the study] is logical, but it’s encouraging that the data supports this,” Dr. Sodha told this news organization. “The potential confounders, such as injection technique (5 point vs 3 point), nonblinding of the evaluator, history of prior treatments, and variation in treatment effect by different botulinum toxin products may be important as well in how we consider this data in practice.”
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Neither the researchers nor Dr. Sodha reported having financial disclosures.
A version of this article appeared on Medscape.com.
findings from a comparative cohort study suggested.
“Botulinum toxin A to the glabella is a popular cosmetic intervention,” researchers led by Kim L. Borsky, MD, MBBS, of the Department of Plastic and Reconstructive Surgery at Stoke Mandeville Hospital, Aylesbury, England, and colleagues wrote in their study, which was published in Plastic and Reconstructive Surgery. “Functional musculature differences may arise from chronic behavioral adjustment to high sun exposure levels, requiring greater doses. This could affect clinical practice globally.”
To investigate the effect of climate on real-world doses of the product, the researchers enrolled 523 women aged 35-60 years who received glabellar botulinum toxin treatment at two centers between 2012 and 2019: one in the United Kingdom and one in Malta. They evaluated data on 292 patients treated during the summer months at the Malta center (classified as the high sun-exposure group), and 231 patients treated during the winter months at the UK center (classified as the low sun-exposure group). The primary outcomes of interest were the required top-up doses and the total dose to achieve full paralysis. Smokers were excluded from the analysis, as were those who did not seek maximal paralysis, those documented as not compliant with posttreatment advice, and those with colds or fevers. They used univariable and multivariable analyses to compare the high vs low sun-exposure groups.
The researchers found that 68.5% of women in the high-sun group required a top-up dose to achieve full paralysis, compared with 61.5% in the low-sun group, a difference that did not reach statistical significance (P = .1032). All patients achieved full paralysis with the treatment protocol used. However, in the high-sun group, the mean top-up dose was significantly higher than that in the low-sun group (a mean of 9.30 vs 7.06 units, respectively; P = .0009), as was the mean total dose (a mean of 29.23 vs 27.25 units; P = .0031).
“Patients subject to less sun exposure require a lower dose than patients with high sun exposure, and this was present and persisted when controlling for potential confounders,” the researchers wrote. “Although robustly demonstrated, the difference in doses seen here was small, and so may not directly impact at a health economic level, as the difference would not necessarily change the number of vials used. However, it may be of relevance to training and protocolization of treatments. Rigid protocols about doses and distributions may lead to undertreatment if applied in sunnier climates.”
They acknowledged certain limitations of their study, including its unblinded design and the fact that they did not evaluate or control for ethnicity. They also characterized the population of Malta as “very homogeneous, mainly made up of Maltese with less than 5% foreigners,” while the demographics of the United Kingdom and especially London, where the injections were performed, “are much more diverse.”
Asked to comment on the results, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, DC, said that the study highlights the importance of tailoring neuromodulator treatment to the individual patient based not just on gender but also on lifestyle and climate. “The conclusion [of the study] is logical, but it’s encouraging that the data supports this,” Dr. Sodha told this news organization. “The potential confounders, such as injection technique (5 point vs 3 point), nonblinding of the evaluator, history of prior treatments, and variation in treatment effect by different botulinum toxin products may be important as well in how we consider this data in practice.”
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Neither the researchers nor Dr. Sodha reported having financial disclosures.
A version of this article appeared on Medscape.com.
findings from a comparative cohort study suggested.
“Botulinum toxin A to the glabella is a popular cosmetic intervention,” researchers led by Kim L. Borsky, MD, MBBS, of the Department of Plastic and Reconstructive Surgery at Stoke Mandeville Hospital, Aylesbury, England, and colleagues wrote in their study, which was published in Plastic and Reconstructive Surgery. “Functional musculature differences may arise from chronic behavioral adjustment to high sun exposure levels, requiring greater doses. This could affect clinical practice globally.”
To investigate the effect of climate on real-world doses of the product, the researchers enrolled 523 women aged 35-60 years who received glabellar botulinum toxin treatment at two centers between 2012 and 2019: one in the United Kingdom and one in Malta. They evaluated data on 292 patients treated during the summer months at the Malta center (classified as the high sun-exposure group), and 231 patients treated during the winter months at the UK center (classified as the low sun-exposure group). The primary outcomes of interest were the required top-up doses and the total dose to achieve full paralysis. Smokers were excluded from the analysis, as were those who did not seek maximal paralysis, those documented as not compliant with posttreatment advice, and those with colds or fevers. They used univariable and multivariable analyses to compare the high vs low sun-exposure groups.
The researchers found that 68.5% of women in the high-sun group required a top-up dose to achieve full paralysis, compared with 61.5% in the low-sun group, a difference that did not reach statistical significance (P = .1032). All patients achieved full paralysis with the treatment protocol used. However, in the high-sun group, the mean top-up dose was significantly higher than that in the low-sun group (a mean of 9.30 vs 7.06 units, respectively; P = .0009), as was the mean total dose (a mean of 29.23 vs 27.25 units; P = .0031).
“Patients subject to less sun exposure require a lower dose than patients with high sun exposure, and this was present and persisted when controlling for potential confounders,” the researchers wrote. “Although robustly demonstrated, the difference in doses seen here was small, and so may not directly impact at a health economic level, as the difference would not necessarily change the number of vials used. However, it may be of relevance to training and protocolization of treatments. Rigid protocols about doses and distributions may lead to undertreatment if applied in sunnier climates.”
They acknowledged certain limitations of their study, including its unblinded design and the fact that they did not evaluate or control for ethnicity. They also characterized the population of Malta as “very homogeneous, mainly made up of Maltese with less than 5% foreigners,” while the demographics of the United Kingdom and especially London, where the injections were performed, “are much more diverse.”
Asked to comment on the results, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, DC, said that the study highlights the importance of tailoring neuromodulator treatment to the individual patient based not just on gender but also on lifestyle and climate. “The conclusion [of the study] is logical, but it’s encouraging that the data supports this,” Dr. Sodha told this news organization. “The potential confounders, such as injection technique (5 point vs 3 point), nonblinding of the evaluator, history of prior treatments, and variation in treatment effect by different botulinum toxin products may be important as well in how we consider this data in practice.”
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Neither the researchers nor Dr. Sodha reported having financial disclosures.
A version of this article appeared on Medscape.com.
FROM PLASTIC AND RECONSTRUCTIVE SURGERY
Draining Nodule of the Hand
The Diagnosis: Cutaneous Nocardiosis
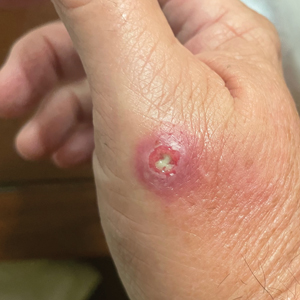
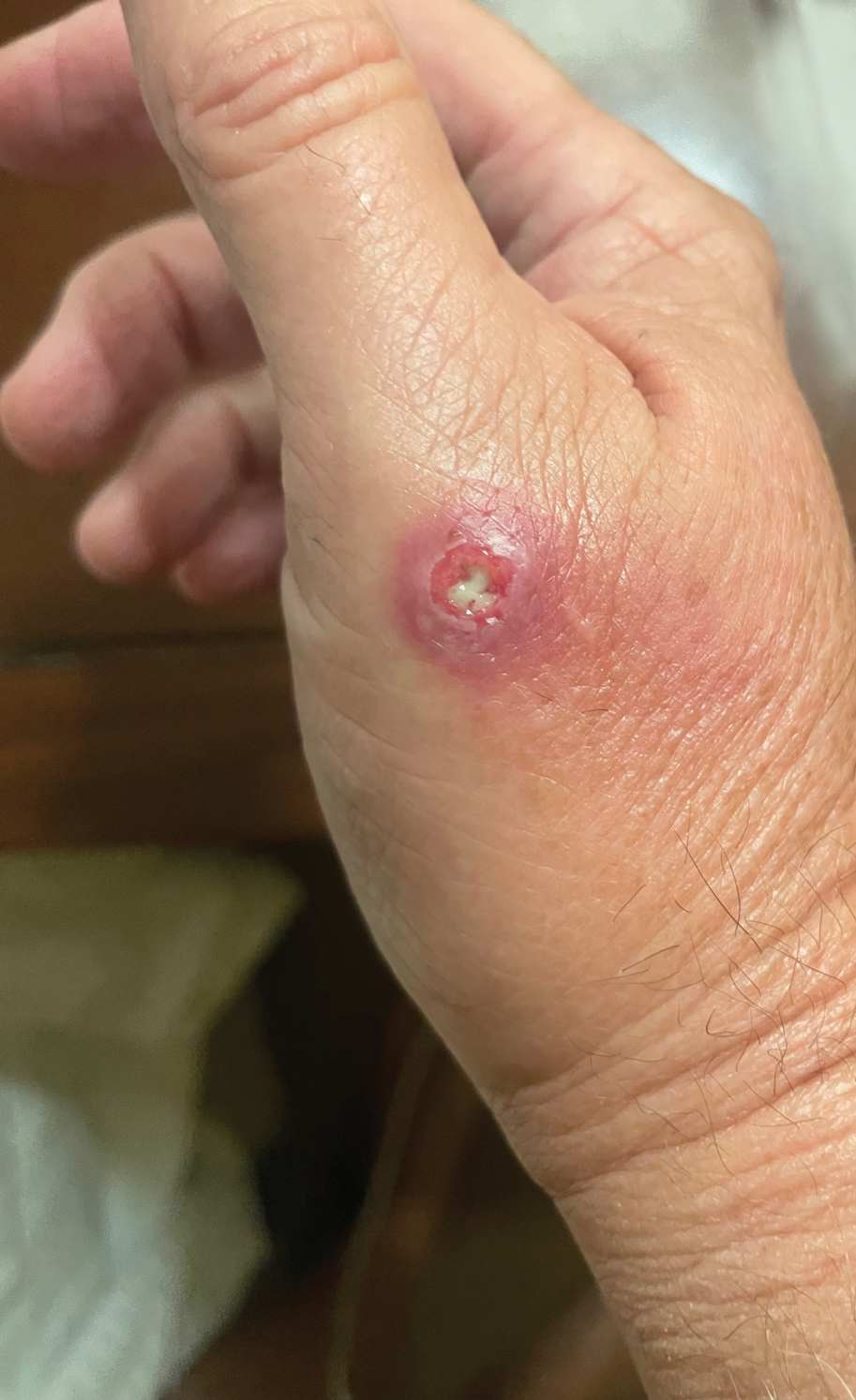
The wound culture was positive for Nocardia farcinica. The patient received a 5-day course of intravenous sulfamethoxazole-trimethoprim in the hospital and was transitioned to oral sulfamethoxazoletrimethoprim (800 mg/160 mg taken as 1 tablet twice daily) for 6 months. Complete resolution of the infection was noted at 6-month follow-up (Figure).
Nocardia is a gram-positive, aerobic bacterium that typically is found in soil, water, and decaying organic matter.1 There are more than 50 species; N farcinica, Nocardia nova, and Nocardia asteroides are the leading causes of infection in humans and animals. Nocardia asteroides is the most common cause of infection in humans.1,2 Nocardiosis is an uncommon opportunistic infection that usually targets the skin, lungs, and central nervous system.3 Although it mainly affects individuals who are immunocompromised, up to 30% of infections can be seen in immunocompetent hosts who can contract cutaneous nocardiosis after experiencing traumatic injury to the skin.1
Nocardiosis is difficult to diagnose due to its diverse clinical presentations. For example, cutaneous nocardiosis can manifest similar to mycetoma, sporotrichosis, spider bites, nontuberculous mycobacteria such as Mycobacterium marinum, or methicillin-resistant Staphylococcus aureus infections, thus making cutaneous nocardiosis one of the great imitators.1 A culture is required for definitive diagnosis, as Nocardia grows well on nonselective media such as blood or Löwenstein-Jensen agar. It grows as waxy, pigmented, cerebriform colonies 3 to 5 days following incubation.3 The bacterium can be difficult to culture, and it is important to notify the microbiology laboratory if there is a high index of clinical suspicion for infection.
A history of exposure to gardening or handling animals can increase the risk for an individual contracting Nocardia.3 Although nocardiosis can be found across the world, it is native to tropical and subtropical climates such as those found in India, Africa, Latin America, and Southeast Asia.1 Infections mostly are observed in individuals aged 20 to 40 years and tend to affect men more than women. Lesions typically are seen on the lower extremities, but localized infections also can be found on the torso, neck, and upper extremities.1
Cutaneous nocardiosis is a granulomatous infection encompassing both cutaneous and subcutaneous tissue, which ultimately can lead to injury of bone and viscera.1 Primary cutaneous nocardiosis can manifest as tumors or nodules that have a sporotrichoid pattern, in which they ascend along the lymphatics. Histopathology of infected tissue frequently shows a subcutaneous dermal infiltrate of neutrophils accompanied with abscess formation, and everlasting lesions may show signs of chronic inflammation and nonspecific granulomas.3
Treatment of nocardiosis should be guided by in vitro susceptibility tests. Sulfamethoxazole-trimethoprim 800 mg/160 mg taken as 1 tablet twice daily is the first-line option. The treatment duration is contingent on the extent, severity, and complications of infection but typically is 3 to 6 months.1
- Yu Q, Song J, Liu Y, et al. Progressive primary cutaneous nocardiosis in an immunocompetent patient. Cutis. 2023;111:E22-E25.
- Gaines RJ, Randall CJ, Ruland RT. Lymphocutaneous nocardiosis from commercially treated lumber: a case report. Cutis. 2006;78:249-251.
- Riswold KJ, Tjarks BJ, Kerkvliet AM. Cutaneous nocardiosis in an immunocompromised patient. Cutis. 2019;104:226-229.
The Diagnosis: Cutaneous Nocardiosis
The wound culture was positive for Nocardia farcinica. The patient received a 5-day course of intravenous sulfamethoxazole-trimethoprim in the hospital and was transitioned to oral sulfamethoxazoletrimethoprim (800 mg/160 mg taken as 1 tablet twice daily) for 6 months. Complete resolution of the infection was noted at 6-month follow-up (Figure).
Nocardia is a gram-positive, aerobic bacterium that typically is found in soil, water, and decaying organic matter.1 There are more than 50 species; N farcinica, Nocardia nova, and Nocardia asteroides are the leading causes of infection in humans and animals. Nocardia asteroides is the most common cause of infection in humans.1,2 Nocardiosis is an uncommon opportunistic infection that usually targets the skin, lungs, and central nervous system.3 Although it mainly affects individuals who are immunocompromised, up to 30% of infections can be seen in immunocompetent hosts who can contract cutaneous nocardiosis after experiencing traumatic injury to the skin.1
Nocardiosis is difficult to diagnose due to its diverse clinical presentations. For example, cutaneous nocardiosis can manifest similar to mycetoma, sporotrichosis, spider bites, nontuberculous mycobacteria such as Mycobacterium marinum, or methicillin-resistant Staphylococcus aureus infections, thus making cutaneous nocardiosis one of the great imitators.1 A culture is required for definitive diagnosis, as Nocardia grows well on nonselective media such as blood or Löwenstein-Jensen agar. It grows as waxy, pigmented, cerebriform colonies 3 to 5 days following incubation.3 The bacterium can be difficult to culture, and it is important to notify the microbiology laboratory if there is a high index of clinical suspicion for infection.
A history of exposure to gardening or handling animals can increase the risk for an individual contracting Nocardia.3 Although nocardiosis can be found across the world, it is native to tropical and subtropical climates such as those found in India, Africa, Latin America, and Southeast Asia.1 Infections mostly are observed in individuals aged 20 to 40 years and tend to affect men more than women. Lesions typically are seen on the lower extremities, but localized infections also can be found on the torso, neck, and upper extremities.1

Cutaneous nocardiosis is a granulomatous infection encompassing both cutaneous and subcutaneous tissue, which ultimately can lead to injury of bone and viscera.1 Primary cutaneous nocardiosis can manifest as tumors or nodules that have a sporotrichoid pattern, in which they ascend along the lymphatics. Histopathology of infected tissue frequently shows a subcutaneous dermal infiltrate of neutrophils accompanied with abscess formation, and everlasting lesions may show signs of chronic inflammation and nonspecific granulomas.3
Treatment of nocardiosis should be guided by in vitro susceptibility tests. Sulfamethoxazole-trimethoprim 800 mg/160 mg taken as 1 tablet twice daily is the first-line option. The treatment duration is contingent on the extent, severity, and complications of infection but typically is 3 to 6 months.1
The Diagnosis: Cutaneous Nocardiosis
The wound culture was positive for Nocardia farcinica. The patient received a 5-day course of intravenous sulfamethoxazole-trimethoprim in the hospital and was transitioned to oral sulfamethoxazoletrimethoprim (800 mg/160 mg taken as 1 tablet twice daily) for 6 months. Complete resolution of the infection was noted at 6-month follow-up (Figure).
Nocardia is a gram-positive, aerobic bacterium that typically is found in soil, water, and decaying organic matter.1 There are more than 50 species; N farcinica, Nocardia nova, and Nocardia asteroides are the leading causes of infection in humans and animals. Nocardia asteroides is the most common cause of infection in humans.1,2 Nocardiosis is an uncommon opportunistic infection that usually targets the skin, lungs, and central nervous system.3 Although it mainly affects individuals who are immunocompromised, up to 30% of infections can be seen in immunocompetent hosts who can contract cutaneous nocardiosis after experiencing traumatic injury to the skin.1
Nocardiosis is difficult to diagnose due to its diverse clinical presentations. For example, cutaneous nocardiosis can manifest similar to mycetoma, sporotrichosis, spider bites, nontuberculous mycobacteria such as Mycobacterium marinum, or methicillin-resistant Staphylococcus aureus infections, thus making cutaneous nocardiosis one of the great imitators.1 A culture is required for definitive diagnosis, as Nocardia grows well on nonselective media such as blood or Löwenstein-Jensen agar. It grows as waxy, pigmented, cerebriform colonies 3 to 5 days following incubation.3 The bacterium can be difficult to culture, and it is important to notify the microbiology laboratory if there is a high index of clinical suspicion for infection.
A history of exposure to gardening or handling animals can increase the risk for an individual contracting Nocardia.3 Although nocardiosis can be found across the world, it is native to tropical and subtropical climates such as those found in India, Africa, Latin America, and Southeast Asia.1 Infections mostly are observed in individuals aged 20 to 40 years and tend to affect men more than women. Lesions typically are seen on the lower extremities, but localized infections also can be found on the torso, neck, and upper extremities.1

Cutaneous nocardiosis is a granulomatous infection encompassing both cutaneous and subcutaneous tissue, which ultimately can lead to injury of bone and viscera.1 Primary cutaneous nocardiosis can manifest as tumors or nodules that have a sporotrichoid pattern, in which they ascend along the lymphatics. Histopathology of infected tissue frequently shows a subcutaneous dermal infiltrate of neutrophils accompanied with abscess formation, and everlasting lesions may show signs of chronic inflammation and nonspecific granulomas.3
Treatment of nocardiosis should be guided by in vitro susceptibility tests. Sulfamethoxazole-trimethoprim 800 mg/160 mg taken as 1 tablet twice daily is the first-line option. The treatment duration is contingent on the extent, severity, and complications of infection but typically is 3 to 6 months.1
- Yu Q, Song J, Liu Y, et al. Progressive primary cutaneous nocardiosis in an immunocompetent patient. Cutis. 2023;111:E22-E25.
- Gaines RJ, Randall CJ, Ruland RT. Lymphocutaneous nocardiosis from commercially treated lumber: a case report. Cutis. 2006;78:249-251.
- Riswold KJ, Tjarks BJ, Kerkvliet AM. Cutaneous nocardiosis in an immunocompromised patient. Cutis. 2019;104:226-229.
- Yu Q, Song J, Liu Y, et al. Progressive primary cutaneous nocardiosis in an immunocompetent patient. Cutis. 2023;111:E22-E25.
- Gaines RJ, Randall CJ, Ruland RT. Lymphocutaneous nocardiosis from commercially treated lumber: a case report. Cutis. 2006;78:249-251.
- Riswold KJ, Tjarks BJ, Kerkvliet AM. Cutaneous nocardiosis in an immunocompromised patient. Cutis. 2019;104:226-229.
A 67-year-old man presented to the emergency department with a draining nodule on the right hand of 4 days’ duration. He reported that the swelling and redness started 1 hour after handling a succulent plant. The following day, the nodule increased in size and exudated yellow pus. He presented with swelling of the thumb and hand, which resulted in a decreased range of motion. He had a history of prediabetes and denied any recent travel, allergies, or animal exposures. Physical examination revealed a draining nodule on the dorsal aspect of the right hand that measured approximately 15×15 mm with surrounding erythema and tenderness. There also was progression of ascending erythema up to the axilla. The patient was admitted to the hospital.
Weight Loss Drugs Cut Cancer Risk in Diabetes Patients
Recent research on popular weight loss drugs has uncovered surprising benefits beyond their intended use, like lowering the risk of fatal heart attacks. And now there may be another unforeseen advantage:
That’s according to a study published July 5 in JAMA Network Open where researchers studied glucagon-like peptide receptor agonists (known as GLP-1RAs), a class of drugs used to treat diabetes and obesity. Ozempic, Wegovy, Mounjaro, and Zepbound, which have become well-known recently because they are linked to rapid weight loss, contain GLP-1RAs.
For the study, they looked at electronic health records of 1.7 million patients who had type 2 diabetes, no prior diagnosis of obesity-related cancers, and had been prescribed GLP-1RAs, insulins, or metformin from March 2005 to November 2018.
The scientists found that compared to patients who took insulin, people who took GLP-1RAs had a “significant risk reduction” in 10 of 13 obesity-related cancers. Those 10 cancers were esophageal, colorectal, endometrial, gallbladder, kidney, liver, ovarian, and pancreatic cancers, as well as meningioma and multiple myeloma.
Compared with patients taking insulin, patients taking GLP-1RAs showed no statistically significant reduction in stomach cancer and no reduced risk of breast and thyroid cancers, the study said.
But the study found no decrease in cancer risk with GLP-1RAs compared with metformin.
While the study results suggest that these drugs may reduce the risk of certain obesity-related cancers better than insulins, more research is needed, they said.
A version of this article appeared on WebMD.com.
Recent research on popular weight loss drugs has uncovered surprising benefits beyond their intended use, like lowering the risk of fatal heart attacks. And now there may be another unforeseen advantage:
That’s according to a study published July 5 in JAMA Network Open where researchers studied glucagon-like peptide receptor agonists (known as GLP-1RAs), a class of drugs used to treat diabetes and obesity. Ozempic, Wegovy, Mounjaro, and Zepbound, which have become well-known recently because they are linked to rapid weight loss, contain GLP-1RAs.
For the study, they looked at electronic health records of 1.7 million patients who had type 2 diabetes, no prior diagnosis of obesity-related cancers, and had been prescribed GLP-1RAs, insulins, or metformin from March 2005 to November 2018.
The scientists found that compared to patients who took insulin, people who took GLP-1RAs had a “significant risk reduction” in 10 of 13 obesity-related cancers. Those 10 cancers were esophageal, colorectal, endometrial, gallbladder, kidney, liver, ovarian, and pancreatic cancers, as well as meningioma and multiple myeloma.
Compared with patients taking insulin, patients taking GLP-1RAs showed no statistically significant reduction in stomach cancer and no reduced risk of breast and thyroid cancers, the study said.
But the study found no decrease in cancer risk with GLP-1RAs compared with metformin.
While the study results suggest that these drugs may reduce the risk of certain obesity-related cancers better than insulins, more research is needed, they said.
A version of this article appeared on WebMD.com.
Recent research on popular weight loss drugs has uncovered surprising benefits beyond their intended use, like lowering the risk of fatal heart attacks. And now there may be another unforeseen advantage:
That’s according to a study published July 5 in JAMA Network Open where researchers studied glucagon-like peptide receptor agonists (known as GLP-1RAs), a class of drugs used to treat diabetes and obesity. Ozempic, Wegovy, Mounjaro, and Zepbound, which have become well-known recently because they are linked to rapid weight loss, contain GLP-1RAs.
For the study, they looked at electronic health records of 1.7 million patients who had type 2 diabetes, no prior diagnosis of obesity-related cancers, and had been prescribed GLP-1RAs, insulins, or metformin from March 2005 to November 2018.
The scientists found that compared to patients who took insulin, people who took GLP-1RAs had a “significant risk reduction” in 10 of 13 obesity-related cancers. Those 10 cancers were esophageal, colorectal, endometrial, gallbladder, kidney, liver, ovarian, and pancreatic cancers, as well as meningioma and multiple myeloma.
Compared with patients taking insulin, patients taking GLP-1RAs showed no statistically significant reduction in stomach cancer and no reduced risk of breast and thyroid cancers, the study said.
But the study found no decrease in cancer risk with GLP-1RAs compared with metformin.
While the study results suggest that these drugs may reduce the risk of certain obesity-related cancers better than insulins, more research is needed, they said.
A version of this article appeared on WebMD.com.
Can ChatGPT Improve Pancreatic Cancer Synoptic Reports?
TOPLINE:
GPT-4 generated highly accurate pancreatic cancer synoptic reports from original reports, outperforming GPT-3.5.
METHODOLOGY:
- Compared with original reports, structured imaging reports help surgeons assess tumor resectability in patients with pancreatic ductal adenocarcinoma. However, radiologist uptake of structured reporting remains inconsistent.
- To determine whether converting free-text (ie, original) radiology reports into structured reports can benefit surgeons, researchers evaluated how well GPT-4 and GPT-3.5 were able to generate pancreatic ductal adenocarcinoma synoptic reports from originals.
- The retrospective study included 180 consecutive pancreatic ductal adenocarcinoma staging CT reports, which were reviewed by two radiologists to establish a reference standard for 14 key findings and National Comprehensive Cancer Network resectability category.
- Researchers prompted GPT-3.5 and GPT-4 to create synoptic reports from original reports using the same criteria, and surgeons compared the precision, accuracy, and time to assess the original and artificial intelligence (AI)–generated reports.
TAKEAWAY:
- GPT-4 outperformed GPT-3.5 on all metrics evaluated. For instance, compared with GPT-3.5, GPT-4 achieved equal or higher F1 scores for all 14 key features (F1 scores help assess the precision and recall of a machine-learning model).
- GPT-4 also demonstrated greater precision than GPT-3.5 for extracting superior mesenteric artery involvement (100% vs 88.8%, respectively) and for categorizing resectability.
- Compared with original reports, AI-generated reports helped surgeons better categorize resectability (83% vs 76%, respectively; P = .03), and surgeons spent less time when using AI-generated reports.
- The AI-generated reports did lead to some clinically notable errors. GPT-4, for instance, made errors in extracting common hepatic artery involvement.
IN PRACTICE:
“In our study, GPT-4 was near-perfect at automatically creating pancreatic ductal adenocarcinoma synoptic reports from original reports, outperforming GPT-3.5 overall,” the authors wrote. This “represents a useful application that can increase standardization and improve communication between radiologists and surgeons.” However, the authors cautioned, the “presence of some clinically significant errors highlights the need for implementation in supervised and preliminary contexts, rather than being relied on for management decisions.”
SOURCE:
The study, with first author Rajesh Bhayana, MD, University Health Network in Toronto, Ontario, Canada, was published online in Radiology.
LIMITATIONS:
While GPT-4 showed high accuracy in report generation, it did lead to some errors. Researchers also relied on original reports when generating the AI reports, and the original reports can contain ambiguous descriptions and language.
DISCLOSURES:
Dr. Bhayana reported no relevant conflicts of interest. Additional disclosures are noted in the original article.
A version of this article first appeared on Medscape.com.
TOPLINE:
GPT-4 generated highly accurate pancreatic cancer synoptic reports from original reports, outperforming GPT-3.5.
METHODOLOGY:
- Compared with original reports, structured imaging reports help surgeons assess tumor resectability in patients with pancreatic ductal adenocarcinoma. However, radiologist uptake of structured reporting remains inconsistent.
- To determine whether converting free-text (ie, original) radiology reports into structured reports can benefit surgeons, researchers evaluated how well GPT-4 and GPT-3.5 were able to generate pancreatic ductal adenocarcinoma synoptic reports from originals.
- The retrospective study included 180 consecutive pancreatic ductal adenocarcinoma staging CT reports, which were reviewed by two radiologists to establish a reference standard for 14 key findings and National Comprehensive Cancer Network resectability category.
- Researchers prompted GPT-3.5 and GPT-4 to create synoptic reports from original reports using the same criteria, and surgeons compared the precision, accuracy, and time to assess the original and artificial intelligence (AI)–generated reports.
TAKEAWAY:
- GPT-4 outperformed GPT-3.5 on all metrics evaluated. For instance, compared with GPT-3.5, GPT-4 achieved equal or higher F1 scores for all 14 key features (F1 scores help assess the precision and recall of a machine-learning model).
- GPT-4 also demonstrated greater precision than GPT-3.5 for extracting superior mesenteric artery involvement (100% vs 88.8%, respectively) and for categorizing resectability.
- Compared with original reports, AI-generated reports helped surgeons better categorize resectability (83% vs 76%, respectively; P = .03), and surgeons spent less time when using AI-generated reports.
- The AI-generated reports did lead to some clinically notable errors. GPT-4, for instance, made errors in extracting common hepatic artery involvement.
IN PRACTICE:
“In our study, GPT-4 was near-perfect at automatically creating pancreatic ductal adenocarcinoma synoptic reports from original reports, outperforming GPT-3.5 overall,” the authors wrote. This “represents a useful application that can increase standardization and improve communication between radiologists and surgeons.” However, the authors cautioned, the “presence of some clinically significant errors highlights the need for implementation in supervised and preliminary contexts, rather than being relied on for management decisions.”
SOURCE:
The study, with first author Rajesh Bhayana, MD, University Health Network in Toronto, Ontario, Canada, was published online in Radiology.
LIMITATIONS:
While GPT-4 showed high accuracy in report generation, it did lead to some errors. Researchers also relied on original reports when generating the AI reports, and the original reports can contain ambiguous descriptions and language.
DISCLOSURES:
Dr. Bhayana reported no relevant conflicts of interest. Additional disclosures are noted in the original article.
A version of this article first appeared on Medscape.com.
TOPLINE:
GPT-4 generated highly accurate pancreatic cancer synoptic reports from original reports, outperforming GPT-3.5.
METHODOLOGY:
- Compared with original reports, structured imaging reports help surgeons assess tumor resectability in patients with pancreatic ductal adenocarcinoma. However, radiologist uptake of structured reporting remains inconsistent.
- To determine whether converting free-text (ie, original) radiology reports into structured reports can benefit surgeons, researchers evaluated how well GPT-4 and GPT-3.5 were able to generate pancreatic ductal adenocarcinoma synoptic reports from originals.
- The retrospective study included 180 consecutive pancreatic ductal adenocarcinoma staging CT reports, which were reviewed by two radiologists to establish a reference standard for 14 key findings and National Comprehensive Cancer Network resectability category.
- Researchers prompted GPT-3.5 and GPT-4 to create synoptic reports from original reports using the same criteria, and surgeons compared the precision, accuracy, and time to assess the original and artificial intelligence (AI)–generated reports.
TAKEAWAY:
- GPT-4 outperformed GPT-3.5 on all metrics evaluated. For instance, compared with GPT-3.5, GPT-4 achieved equal or higher F1 scores for all 14 key features (F1 scores help assess the precision and recall of a machine-learning model).
- GPT-4 also demonstrated greater precision than GPT-3.5 for extracting superior mesenteric artery involvement (100% vs 88.8%, respectively) and for categorizing resectability.
- Compared with original reports, AI-generated reports helped surgeons better categorize resectability (83% vs 76%, respectively; P = .03), and surgeons spent less time when using AI-generated reports.
- The AI-generated reports did lead to some clinically notable errors. GPT-4, for instance, made errors in extracting common hepatic artery involvement.
IN PRACTICE:
“In our study, GPT-4 was near-perfect at automatically creating pancreatic ductal adenocarcinoma synoptic reports from original reports, outperforming GPT-3.5 overall,” the authors wrote. This “represents a useful application that can increase standardization and improve communication between radiologists and surgeons.” However, the authors cautioned, the “presence of some clinically significant errors highlights the need for implementation in supervised and preliminary contexts, rather than being relied on for management decisions.”
SOURCE:
The study, with first author Rajesh Bhayana, MD, University Health Network in Toronto, Ontario, Canada, was published online in Radiology.
LIMITATIONS:
While GPT-4 showed high accuracy in report generation, it did lead to some errors. Researchers also relied on original reports when generating the AI reports, and the original reports can contain ambiguous descriptions and language.
DISCLOSURES:
Dr. Bhayana reported no relevant conflicts of interest. Additional disclosures are noted in the original article.
A version of this article first appeared on Medscape.com.
Another Social Media Snowball
Recently, the British Journal of General Practice published a paper that claimed that anxiety may be a prodromal feature of Parkinson’s disease). That news was widely picked up and spread.
The researchers certainly have some interesting data, but this sort of article, once enough general and social media websites get a hold of it, is bound to cause panic in the streets. And phone calls to my office.
An anxious-by-nature friend even emailed me the link with a laconic “Well, I’m screwed” in the subject line.
Is there a correlation between Parkinson’s disease and anxiety? Probably. Any of us practicing neurology have seen it. Some of it is likely from the anxiety of the situation, but the biochemical changes brought by the disease are also likely a big part.
But does that mean everyone with anxiety has Parkinson’s disease? Of course not. Anxiety is common, probably more common in our current era than ever before (this is why I tell patients not to watch the news and to avoid social media — they’re bad for your sanity and blood pressure).
Stories like this, once they start getting forwarded on Facebook (or another social media outlet), only raise anxiety, which results in more forwarding, and the snowball begins rolling downhill before crashing into my office (obviously this is a figure of speech, as it’s July in Phoenix).
The research is interesting. The point is valid. But the leaps the public makes are ... problematic. It’s only a matter of time before someone comes in demanding a DaT scan because they’re anxious. At $4K a test, that’s not happening.
Which raises anxiety all around.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
Recently, the British Journal of General Practice published a paper that claimed that anxiety may be a prodromal feature of Parkinson’s disease). That news was widely picked up and spread.
The researchers certainly have some interesting data, but this sort of article, once enough general and social media websites get a hold of it, is bound to cause panic in the streets. And phone calls to my office.
An anxious-by-nature friend even emailed me the link with a laconic “Well, I’m screwed” in the subject line.
Is there a correlation between Parkinson’s disease and anxiety? Probably. Any of us practicing neurology have seen it. Some of it is likely from the anxiety of the situation, but the biochemical changes brought by the disease are also likely a big part.
But does that mean everyone with anxiety has Parkinson’s disease? Of course not. Anxiety is common, probably more common in our current era than ever before (this is why I tell patients not to watch the news and to avoid social media — they’re bad for your sanity and blood pressure).
Stories like this, once they start getting forwarded on Facebook (or another social media outlet), only raise anxiety, which results in more forwarding, and the snowball begins rolling downhill before crashing into my office (obviously this is a figure of speech, as it’s July in Phoenix).
The research is interesting. The point is valid. But the leaps the public makes are ... problematic. It’s only a matter of time before someone comes in demanding a DaT scan because they’re anxious. At $4K a test, that’s not happening.
Which raises anxiety all around.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
Recently, the British Journal of General Practice published a paper that claimed that anxiety may be a prodromal feature of Parkinson’s disease). That news was widely picked up and spread.
The researchers certainly have some interesting data, but this sort of article, once enough general and social media websites get a hold of it, is bound to cause panic in the streets. And phone calls to my office.
An anxious-by-nature friend even emailed me the link with a laconic “Well, I’m screwed” in the subject line.
Is there a correlation between Parkinson’s disease and anxiety? Probably. Any of us practicing neurology have seen it. Some of it is likely from the anxiety of the situation, but the biochemical changes brought by the disease are also likely a big part.
But does that mean everyone with anxiety has Parkinson’s disease? Of course not. Anxiety is common, probably more common in our current era than ever before (this is why I tell patients not to watch the news and to avoid social media — they’re bad for your sanity and blood pressure).
Stories like this, once they start getting forwarded on Facebook (or another social media outlet), only raise anxiety, which results in more forwarding, and the snowball begins rolling downhill before crashing into my office (obviously this is a figure of speech, as it’s July in Phoenix).
The research is interesting. The point is valid. But the leaps the public makes are ... problematic. It’s only a matter of time before someone comes in demanding a DaT scan because they’re anxious. At $4K a test, that’s not happening.
Which raises anxiety all around.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
Neck Pain in Migraine Is Common, Linked to More Disability
, an international, prospective, cross-sectional study finds.
Of 51,969 respondents with headache over the past year, the 27.9% with migraine were more likely to have neck pain than those with non-migraine headache (68.3% vs 36.1%, respectively, P < .001), reported Richard B. Lipton, MD, professor of neurology at Albert Einstein College of Medicine, New York City, and colleagues in Headache.
Compared with other patients with migraine, those who also have neck pain have “greater disability, more psychiatric comorbidities, more allodynia, diminished quality of life, decreased work productivity, and reduced response to treatment,” Dr. Lipton said in an interview. “If patients don’t report [neck pain], it is probably worth asking about. And when patients have both migraine and neck pain, they may merit increased therapeutic attention.”
As Dr. Lipton noted, clinicians have long known that neck pain is common in migraine, although it’s been unclear how the two conditions are connected. “One possibility is that the neck pain is actually a manifestation of the migraine headache. Another possibility is that the neck pain is an independent factor unrelated to migraine headaches: Many people have migraine and cervical spine disease. And the third possibility is that neck pain may be an exacerbating factor, that cervical spine disease may make the migraine worse.”
Referred pain is a potential factor too, he said.
Assessing Migraine, Neck Pain, and Disability
The new study sought to better understand the role of neck pain in migraine, Dr. Lipton said.
For the CaMEO-I study, researchers surveyed 51,969 adults with headache via the Internet in Canada, France, Germany, Japan, United Kingdom, and the United States from 2021-2022. Most of the 37,477 patients with non-migraine headaches were considered to have tension headaches.
Among the 14,492 patients with migraine, demographics were statistically similar among those who had neck pain or didn’t have it (average age = 40.7 and 42.1, 68.4% and 72.5% female, and average BMIs = 26.0 and 26.4, respectively).
Among patients in the US, 71.4% of patients with migraine reported neck pain versus 35.9% of those with non-migraine headaches. In Canada, the numbers were 69.5% and 37.5%, respectively.
Among all patients with migraine, moderate-to-severe disability was more common among those with neck pain than those without neck pain (47.7% vs 28.9%, respectively, P < .001). Those with both migraine and neck pain had more symptom burden (P < .001), and 28.4% said neck pain was their most bothersome symptom. They also had a higher number of symptoms (P < .001).
Several conditions were more common among patients with migraine who reported neck pain versus those who didn’t (depression/anxiety, 40.2% vs 28.2%; anxiety, 41.2% vs 29.2%; and allodynia, 54.0% vs 36.6%, respectively, all P < 0.001). Those with neck pain were also more likely to have “poor acute treatment optimization” (61.1% vs 53.3%, respectively, P < .001).
Researchers noted limitations such as the use of self-reported data, the potential for selection bias, limitations regarding survey questions, and an inability to determine causation.
Clinical Messages
The findings suggest that patients with both migraine and neck pain have greater activation of second-order neurons in the trigeminocervical complex, Dr. Lipton said.
He added that neck pain is often part of the migraine prodrome or the migraine attack itself, suggesting that it’s “part and parcel of the migraine attack.” However, neck pain may have another cause — such as degenerative disease of the neck — if it’s not directly connected to migraine, he added.
As for clinical messages from the study, “it’s quite likely that the neck pain is a primary manifestation of migraine. Migraine may well be the explanation in the absence of a reason to look further,” Dr. Lipton said.
If neck pain heralds a migraine, treating the prodrome with CGRP receptor antagonists (“gepants”) can be helpful, he said. He highlighted other preventive options include beta blockers, anti-epilepsy drugs, and monoclonal antibodies. There’s also anecdotal support for using botulinum toxin A in patients with chronic migraine and neck pain, he said.
In an interview, Mayo Clinic Arizona associate professor of neurology Rashmi B. Halker Singh, MD, who’s familiar with the study but did not take part in it, praised the research. The findings “help us to better understand the impact of living with neck pain if you are somebody with migraine,” she said. “It alerts us that we need to be more aggressive in how we manage that in patients.”
The study also emphasizes the importance of preventive medication in appropriate patients with migraine, especially those with neck pain who may be living with greater disability, she said. “About 13% of people with migraine are on a preventive medication, but about 40% are eligible. That’s an area where we have a big gap.”
Dr. Halker Singh added that non-medication strategies such as acupuncture and physical therapy can be helpful.
AbbVie funded the study. Dr. Lipton reports support for the study from AbbVie; research support paid to his institution from the Czap Foundation, National Headache Foundation, National Institutes of Health, S&L Marx Foundation, and US Food and Drug Administration; and personal fees from AbbVie/Allergan, American Academy of Neurology, American Headache Society, Amgen, Biohaven, Biovision, Boston, Dr. Reddy’s (Promius), electroCore, Eli Lilly, GlaxoSmithKline, Grifols, Lundbeck (Alder), Merck, Pernix, Pfizer, Teva, Vector, and Vedanta Research. He holds stock/options in Axon, Biohaven, CoolTech, and Manistee. Other authors report various disclosures.
Dr. Halker Singh is deputy editor of Headache, where the study was published, but wasn’t aware of it until it was published.
, an international, prospective, cross-sectional study finds.
Of 51,969 respondents with headache over the past year, the 27.9% with migraine were more likely to have neck pain than those with non-migraine headache (68.3% vs 36.1%, respectively, P < .001), reported Richard B. Lipton, MD, professor of neurology at Albert Einstein College of Medicine, New York City, and colleagues in Headache.
Compared with other patients with migraine, those who also have neck pain have “greater disability, more psychiatric comorbidities, more allodynia, diminished quality of life, decreased work productivity, and reduced response to treatment,” Dr. Lipton said in an interview. “If patients don’t report [neck pain], it is probably worth asking about. And when patients have both migraine and neck pain, they may merit increased therapeutic attention.”
As Dr. Lipton noted, clinicians have long known that neck pain is common in migraine, although it’s been unclear how the two conditions are connected. “One possibility is that the neck pain is actually a manifestation of the migraine headache. Another possibility is that the neck pain is an independent factor unrelated to migraine headaches: Many people have migraine and cervical spine disease. And the third possibility is that neck pain may be an exacerbating factor, that cervical spine disease may make the migraine worse.”
Referred pain is a potential factor too, he said.
Assessing Migraine, Neck Pain, and Disability
The new study sought to better understand the role of neck pain in migraine, Dr. Lipton said.
For the CaMEO-I study, researchers surveyed 51,969 adults with headache via the Internet in Canada, France, Germany, Japan, United Kingdom, and the United States from 2021-2022. Most of the 37,477 patients with non-migraine headaches were considered to have tension headaches.
Among the 14,492 patients with migraine, demographics were statistically similar among those who had neck pain or didn’t have it (average age = 40.7 and 42.1, 68.4% and 72.5% female, and average BMIs = 26.0 and 26.4, respectively).
Among patients in the US, 71.4% of patients with migraine reported neck pain versus 35.9% of those with non-migraine headaches. In Canada, the numbers were 69.5% and 37.5%, respectively.
Among all patients with migraine, moderate-to-severe disability was more common among those with neck pain than those without neck pain (47.7% vs 28.9%, respectively, P < .001). Those with both migraine and neck pain had more symptom burden (P < .001), and 28.4% said neck pain was their most bothersome symptom. They also had a higher number of symptoms (P < .001).
Several conditions were more common among patients with migraine who reported neck pain versus those who didn’t (depression/anxiety, 40.2% vs 28.2%; anxiety, 41.2% vs 29.2%; and allodynia, 54.0% vs 36.6%, respectively, all P < 0.001). Those with neck pain were also more likely to have “poor acute treatment optimization” (61.1% vs 53.3%, respectively, P < .001).
Researchers noted limitations such as the use of self-reported data, the potential for selection bias, limitations regarding survey questions, and an inability to determine causation.
Clinical Messages
The findings suggest that patients with both migraine and neck pain have greater activation of second-order neurons in the trigeminocervical complex, Dr. Lipton said.
He added that neck pain is often part of the migraine prodrome or the migraine attack itself, suggesting that it’s “part and parcel of the migraine attack.” However, neck pain may have another cause — such as degenerative disease of the neck — if it’s not directly connected to migraine, he added.
As for clinical messages from the study, “it’s quite likely that the neck pain is a primary manifestation of migraine. Migraine may well be the explanation in the absence of a reason to look further,” Dr. Lipton said.
If neck pain heralds a migraine, treating the prodrome with CGRP receptor antagonists (“gepants”) can be helpful, he said. He highlighted other preventive options include beta blockers, anti-epilepsy drugs, and monoclonal antibodies. There’s also anecdotal support for using botulinum toxin A in patients with chronic migraine and neck pain, he said.
In an interview, Mayo Clinic Arizona associate professor of neurology Rashmi B. Halker Singh, MD, who’s familiar with the study but did not take part in it, praised the research. The findings “help us to better understand the impact of living with neck pain if you are somebody with migraine,” she said. “It alerts us that we need to be more aggressive in how we manage that in patients.”
The study also emphasizes the importance of preventive medication in appropriate patients with migraine, especially those with neck pain who may be living with greater disability, she said. “About 13% of people with migraine are on a preventive medication, but about 40% are eligible. That’s an area where we have a big gap.”
Dr. Halker Singh added that non-medication strategies such as acupuncture and physical therapy can be helpful.
AbbVie funded the study. Dr. Lipton reports support for the study from AbbVie; research support paid to his institution from the Czap Foundation, National Headache Foundation, National Institutes of Health, S&L Marx Foundation, and US Food and Drug Administration; and personal fees from AbbVie/Allergan, American Academy of Neurology, American Headache Society, Amgen, Biohaven, Biovision, Boston, Dr. Reddy’s (Promius), electroCore, Eli Lilly, GlaxoSmithKline, Grifols, Lundbeck (Alder), Merck, Pernix, Pfizer, Teva, Vector, and Vedanta Research. He holds stock/options in Axon, Biohaven, CoolTech, and Manistee. Other authors report various disclosures.
Dr. Halker Singh is deputy editor of Headache, where the study was published, but wasn’t aware of it until it was published.
, an international, prospective, cross-sectional study finds.
Of 51,969 respondents with headache over the past year, the 27.9% with migraine were more likely to have neck pain than those with non-migraine headache (68.3% vs 36.1%, respectively, P < .001), reported Richard B. Lipton, MD, professor of neurology at Albert Einstein College of Medicine, New York City, and colleagues in Headache.
Compared with other patients with migraine, those who also have neck pain have “greater disability, more psychiatric comorbidities, more allodynia, diminished quality of life, decreased work productivity, and reduced response to treatment,” Dr. Lipton said in an interview. “If patients don’t report [neck pain], it is probably worth asking about. And when patients have both migraine and neck pain, they may merit increased therapeutic attention.”
As Dr. Lipton noted, clinicians have long known that neck pain is common in migraine, although it’s been unclear how the two conditions are connected. “One possibility is that the neck pain is actually a manifestation of the migraine headache. Another possibility is that the neck pain is an independent factor unrelated to migraine headaches: Many people have migraine and cervical spine disease. And the third possibility is that neck pain may be an exacerbating factor, that cervical spine disease may make the migraine worse.”
Referred pain is a potential factor too, he said.
Assessing Migraine, Neck Pain, and Disability
The new study sought to better understand the role of neck pain in migraine, Dr. Lipton said.
For the CaMEO-I study, researchers surveyed 51,969 adults with headache via the Internet in Canada, France, Germany, Japan, United Kingdom, and the United States from 2021-2022. Most of the 37,477 patients with non-migraine headaches were considered to have tension headaches.
Among the 14,492 patients with migraine, demographics were statistically similar among those who had neck pain or didn’t have it (average age = 40.7 and 42.1, 68.4% and 72.5% female, and average BMIs = 26.0 and 26.4, respectively).
Among patients in the US, 71.4% of patients with migraine reported neck pain versus 35.9% of those with non-migraine headaches. In Canada, the numbers were 69.5% and 37.5%, respectively.
Among all patients with migraine, moderate-to-severe disability was more common among those with neck pain than those without neck pain (47.7% vs 28.9%, respectively, P < .001). Those with both migraine and neck pain had more symptom burden (P < .001), and 28.4% said neck pain was their most bothersome symptom. They also had a higher number of symptoms (P < .001).
Several conditions were more common among patients with migraine who reported neck pain versus those who didn’t (depression/anxiety, 40.2% vs 28.2%; anxiety, 41.2% vs 29.2%; and allodynia, 54.0% vs 36.6%, respectively, all P < 0.001). Those with neck pain were also more likely to have “poor acute treatment optimization” (61.1% vs 53.3%, respectively, P < .001).
Researchers noted limitations such as the use of self-reported data, the potential for selection bias, limitations regarding survey questions, and an inability to determine causation.
Clinical Messages
The findings suggest that patients with both migraine and neck pain have greater activation of second-order neurons in the trigeminocervical complex, Dr. Lipton said.
He added that neck pain is often part of the migraine prodrome or the migraine attack itself, suggesting that it’s “part and parcel of the migraine attack.” However, neck pain may have another cause — such as degenerative disease of the neck — if it’s not directly connected to migraine, he added.
As for clinical messages from the study, “it’s quite likely that the neck pain is a primary manifestation of migraine. Migraine may well be the explanation in the absence of a reason to look further,” Dr. Lipton said.
If neck pain heralds a migraine, treating the prodrome with CGRP receptor antagonists (“gepants”) can be helpful, he said. He highlighted other preventive options include beta blockers, anti-epilepsy drugs, and monoclonal antibodies. There’s also anecdotal support for using botulinum toxin A in patients with chronic migraine and neck pain, he said.
In an interview, Mayo Clinic Arizona associate professor of neurology Rashmi B. Halker Singh, MD, who’s familiar with the study but did not take part in it, praised the research. The findings “help us to better understand the impact of living with neck pain if you are somebody with migraine,” she said. “It alerts us that we need to be more aggressive in how we manage that in patients.”
The study also emphasizes the importance of preventive medication in appropriate patients with migraine, especially those with neck pain who may be living with greater disability, she said. “About 13% of people with migraine are on a preventive medication, but about 40% are eligible. That’s an area where we have a big gap.”
Dr. Halker Singh added that non-medication strategies such as acupuncture and physical therapy can be helpful.
AbbVie funded the study. Dr. Lipton reports support for the study from AbbVie; research support paid to his institution from the Czap Foundation, National Headache Foundation, National Institutes of Health, S&L Marx Foundation, and US Food and Drug Administration; and personal fees from AbbVie/Allergan, American Academy of Neurology, American Headache Society, Amgen, Biohaven, Biovision, Boston, Dr. Reddy’s (Promius), electroCore, Eli Lilly, GlaxoSmithKline, Grifols, Lundbeck (Alder), Merck, Pernix, Pfizer, Teva, Vector, and Vedanta Research. He holds stock/options in Axon, Biohaven, CoolTech, and Manistee. Other authors report various disclosures.
Dr. Halker Singh is deputy editor of Headache, where the study was published, but wasn’t aware of it until it was published.
FROM HEADACHE
Feds May End Hospital System’s Noncompete Contract for Part-Time Docs
Mount Sinai Health System in New York City is forcing part-time physicians to sign employment contracts that violate their labor rights, according to a June 2024 complaint by the National Labor Relations Board (NLRB).
The complaint stems from no-poaching and confidentiality clauses in the agreements required as a condition of employment, NLRB officials alleged.
according to a copy of the terms included in NLRB’s June 18 complaint.
By requiring the agreements, NLRB officials claimed, Mount Sinai is “interfering with, restraining, and coercing employees” in violation of the National Labor Relations Act. The health system’s “unfair labor practices” affects commerce as outlined under the law, according to the NLRB. The Act bans employers from burdening or obstructing commerce or the free flow of commerce.
Mount Sinai did not respond to requests for comment.
The NLRB’s complaint follows a landmark decision by the Federal Trade Commission (FTC) to ban noncompete agreements nationwide. In April 2024, the FTC voted to prohibit noncompetes indefinitely in an effort to protect workers.
“Noncompete clauses keep wages low, suppress new ideas, and rob the American economy of dynamism, including from the more than 8500 new startups that would be created a year once noncompetes are banned,” FTC Chair Lina M. Khan said in a statement. “The FTC’s final rule to ban noncompetes will ensure Americans have the freedom to pursue a new job, start a new business, or bring a new idea to market.”
Business groups and agencies have since sued to challenge against the ban, including the Chamber of Commerce. The Chamber and other business groups argue that noncompete agreements are important for companies to protect trade secrets, shield recruiting investments, and hide confidential information. The lawsuits are ongoing.
A Physician Blows the Whistle
An anonymous physician first alerted the NLRB to the contract language in November 2023. The doctor was required the sign the hospital system’s agreement for part-time physicians. The complaint does not say if the employee is still employed by the hospital system.
To remedy the unfair labor practices alleged, the NLRB seeks an order requiring the health system to rescind the contract language, stop any actions against current or former employees to enforce the provisions, and make whole any employees who suffered financial losses related to the contract terms.
The allegation against Mount Sinai is among a rising number of grievances filed with the NLRB that claim unfair labor practices. During the first 6 months of fiscal year 2024, unfair labor practice charges filed across the NLRB’s field offices increased 7% — from 9612 in 2023 to 10,278 in 2024, according to a news release.
NLRB, meanwhile has been cracking down on anticompetitive labor practices and confidentiality provisions that prevent employees from speaking out.
In a February 2023 decision for instance, NLRB ruled that an employer violates the National Labor Relations Act by offering severance agreements to workers that include restrictive confidentiality and nondisparagement terms. In 2022, the NLRB and the Federal Trade Commission forged a partnership to more widely combat unfair, anticompetitive, and deceptive business practices.
“Noncompete provisions reasonably tend to chill employees in the exercise of Section 7 rights when the provisions could reasonably be construed by employees to deny them the ability to quit or change jobs by cutting off their access to other employment opportunities that they are qualified for,” NLRB General Counsel Jennifer Abruzzo said in a 2023 release.
Ms. Abruzzo stressed in a memo that NLR Act is committed to an interagency approach to restrictions on the exercise of employee rights, “including limits to workers’ job mobility, information sharing, and referrals to other agencies.”
Mount Sinai Health System must respond to the NLRB’s complaint by July 16, and an administrative law judge is scheduled to hear the case on September 24.
A version of this article first appeared on Medscape.com.
Mount Sinai Health System in New York City is forcing part-time physicians to sign employment contracts that violate their labor rights, according to a June 2024 complaint by the National Labor Relations Board (NLRB).
The complaint stems from no-poaching and confidentiality clauses in the agreements required as a condition of employment, NLRB officials alleged.
according to a copy of the terms included in NLRB’s June 18 complaint.
By requiring the agreements, NLRB officials claimed, Mount Sinai is “interfering with, restraining, and coercing employees” in violation of the National Labor Relations Act. The health system’s “unfair labor practices” affects commerce as outlined under the law, according to the NLRB. The Act bans employers from burdening or obstructing commerce or the free flow of commerce.
Mount Sinai did not respond to requests for comment.
The NLRB’s complaint follows a landmark decision by the Federal Trade Commission (FTC) to ban noncompete agreements nationwide. In April 2024, the FTC voted to prohibit noncompetes indefinitely in an effort to protect workers.
“Noncompete clauses keep wages low, suppress new ideas, and rob the American economy of dynamism, including from the more than 8500 new startups that would be created a year once noncompetes are banned,” FTC Chair Lina M. Khan said in a statement. “The FTC’s final rule to ban noncompetes will ensure Americans have the freedom to pursue a new job, start a new business, or bring a new idea to market.”
Business groups and agencies have since sued to challenge against the ban, including the Chamber of Commerce. The Chamber and other business groups argue that noncompete agreements are important for companies to protect trade secrets, shield recruiting investments, and hide confidential information. The lawsuits are ongoing.
A Physician Blows the Whistle
An anonymous physician first alerted the NLRB to the contract language in November 2023. The doctor was required the sign the hospital system’s agreement for part-time physicians. The complaint does not say if the employee is still employed by the hospital system.
To remedy the unfair labor practices alleged, the NLRB seeks an order requiring the health system to rescind the contract language, stop any actions against current or former employees to enforce the provisions, and make whole any employees who suffered financial losses related to the contract terms.
The allegation against Mount Sinai is among a rising number of grievances filed with the NLRB that claim unfair labor practices. During the first 6 months of fiscal year 2024, unfair labor practice charges filed across the NLRB’s field offices increased 7% — from 9612 in 2023 to 10,278 in 2024, according to a news release.
NLRB, meanwhile has been cracking down on anticompetitive labor practices and confidentiality provisions that prevent employees from speaking out.
In a February 2023 decision for instance, NLRB ruled that an employer violates the National Labor Relations Act by offering severance agreements to workers that include restrictive confidentiality and nondisparagement terms. In 2022, the NLRB and the Federal Trade Commission forged a partnership to more widely combat unfair, anticompetitive, and deceptive business practices.
“Noncompete provisions reasonably tend to chill employees in the exercise of Section 7 rights when the provisions could reasonably be construed by employees to deny them the ability to quit or change jobs by cutting off their access to other employment opportunities that they are qualified for,” NLRB General Counsel Jennifer Abruzzo said in a 2023 release.
Ms. Abruzzo stressed in a memo that NLR Act is committed to an interagency approach to restrictions on the exercise of employee rights, “including limits to workers’ job mobility, information sharing, and referrals to other agencies.”
Mount Sinai Health System must respond to the NLRB’s complaint by July 16, and an administrative law judge is scheduled to hear the case on September 24.
A version of this article first appeared on Medscape.com.
Mount Sinai Health System in New York City is forcing part-time physicians to sign employment contracts that violate their labor rights, according to a June 2024 complaint by the National Labor Relations Board (NLRB).
The complaint stems from no-poaching and confidentiality clauses in the agreements required as a condition of employment, NLRB officials alleged.
according to a copy of the terms included in NLRB’s June 18 complaint.
By requiring the agreements, NLRB officials claimed, Mount Sinai is “interfering with, restraining, and coercing employees” in violation of the National Labor Relations Act. The health system’s “unfair labor practices” affects commerce as outlined under the law, according to the NLRB. The Act bans employers from burdening or obstructing commerce or the free flow of commerce.
Mount Sinai did not respond to requests for comment.
The NLRB’s complaint follows a landmark decision by the Federal Trade Commission (FTC) to ban noncompete agreements nationwide. In April 2024, the FTC voted to prohibit noncompetes indefinitely in an effort to protect workers.
“Noncompete clauses keep wages low, suppress new ideas, and rob the American economy of dynamism, including from the more than 8500 new startups that would be created a year once noncompetes are banned,” FTC Chair Lina M. Khan said in a statement. “The FTC’s final rule to ban noncompetes will ensure Americans have the freedom to pursue a new job, start a new business, or bring a new idea to market.”
Business groups and agencies have since sued to challenge against the ban, including the Chamber of Commerce. The Chamber and other business groups argue that noncompete agreements are important for companies to protect trade secrets, shield recruiting investments, and hide confidential information. The lawsuits are ongoing.
A Physician Blows the Whistle
An anonymous physician first alerted the NLRB to the contract language in November 2023. The doctor was required the sign the hospital system’s agreement for part-time physicians. The complaint does not say if the employee is still employed by the hospital system.
To remedy the unfair labor practices alleged, the NLRB seeks an order requiring the health system to rescind the contract language, stop any actions against current or former employees to enforce the provisions, and make whole any employees who suffered financial losses related to the contract terms.
The allegation against Mount Sinai is among a rising number of grievances filed with the NLRB that claim unfair labor practices. During the first 6 months of fiscal year 2024, unfair labor practice charges filed across the NLRB’s field offices increased 7% — from 9612 in 2023 to 10,278 in 2024, according to a news release.
NLRB, meanwhile has been cracking down on anticompetitive labor practices and confidentiality provisions that prevent employees from speaking out.
In a February 2023 decision for instance, NLRB ruled that an employer violates the National Labor Relations Act by offering severance agreements to workers that include restrictive confidentiality and nondisparagement terms. In 2022, the NLRB and the Federal Trade Commission forged a partnership to more widely combat unfair, anticompetitive, and deceptive business practices.
“Noncompete provisions reasonably tend to chill employees in the exercise of Section 7 rights when the provisions could reasonably be construed by employees to deny them the ability to quit or change jobs by cutting off their access to other employment opportunities that they are qualified for,” NLRB General Counsel Jennifer Abruzzo said in a 2023 release.
Ms. Abruzzo stressed in a memo that NLR Act is committed to an interagency approach to restrictions on the exercise of employee rights, “including limits to workers’ job mobility, information sharing, and referrals to other agencies.”
Mount Sinai Health System must respond to the NLRB’s complaint by July 16, and an administrative law judge is scheduled to hear the case on September 24.
A version of this article first appeared on Medscape.com.