User login
Isotretinoin-Induced Skin Fragility in an Aerialist
Isotretinoin was introduced more than 3 decades ago and marked a major advancement in the treatment of severe refractory cystic acne. The most common adverse effects linked to isotretinoin usage are mucocutaneous in nature, manifesting as xerosis and cheilitis.1 Skin fragility and poor wound healing also have been reported.2-6 Current recommendations for avoiding these adverse effects include refraining from waxing, laser procedures, and other elective cutaneous procedures for at least 6 months.7 We present a case of isotretinoin-induced cutaneous fragility resulting in blistering and erosions on the palms of a competitive aerial trapeze artist.
Case Report
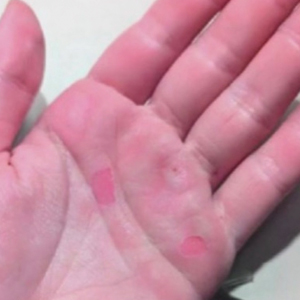
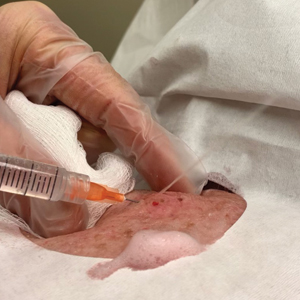
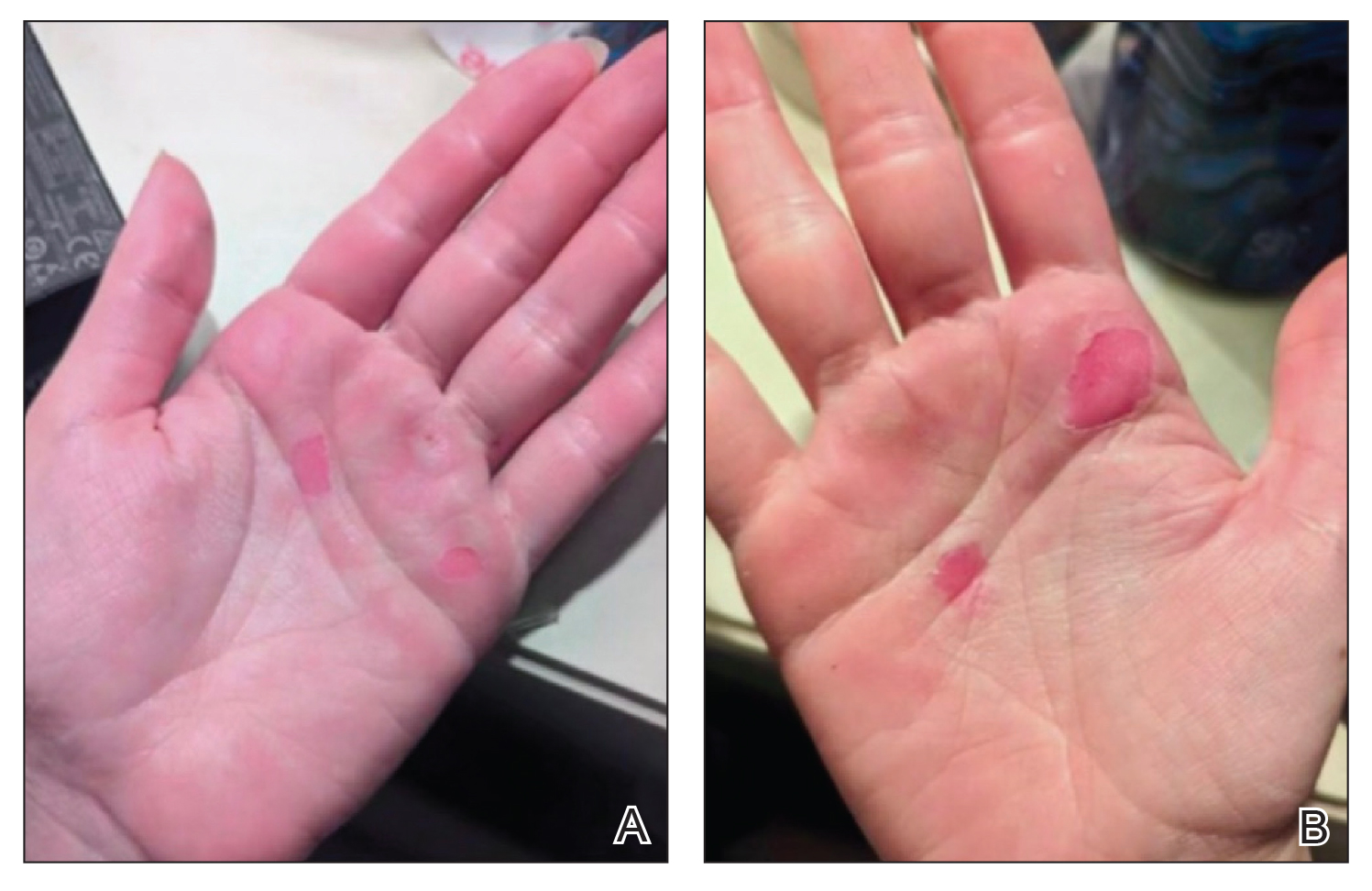
A 25-year-old woman presented for follow-up during week 12 of isotretinoin therapy (40 mg twice daily) prescribed for acne. She reported peeling of the skin on the palms following intense aerial acrobatic workouts. She had been a performing aerialist for many years and had never sustained a similar injury. The wounds were painful and led to decreased activity. She had no notable medical history. Physical examination of the palms revealed erosions in a distribution that corresponded to horizontal bar contact and friction (Figure). The patient was advised on proper wound care, application of emollients, and minimizing friction. She completed the course of isotretinoin and has continued aerialist activity without recurrence of skin fragility.
Comment
Skin fragility is a well-known adverse effect of isotretinoin therapy.8 Pavlis and Lieblich9 reported skin fragility in a young wrestler who experienced similar skin erosions due to isotretinoin therapy. The proposed mechanism of isotretinoin-induced skin fragility is multifactorial. It involves an apoptotic effect on sebocytes,5 which results in reduced stratum corneum hydration and an associated increase in transepidermal water loss.6,10,11 Retinoids also are known to cause thinning of the skin, likely due to the disadhesion of both the epidermis and the stratum corneum, which was demonstrated by the easy removal of cornified cells through tape stripping in hairless mice treated with isotretinoin.12 In further investigations, human patients and hairless mice treated with isotretinoin readily developed friction blisters through pencil eraser abrasion.13 Examination of the friction blisters using light and electron microscopy revealed fraying or loss of the stratum corneum and viable epidermis as well as loss of desmosomes and tonofilaments. Additionally, intracellular and intercellular deposits of an unidentified amorphous material were noted.13
Overall, the origin of skin fragility induced by isotretinoin is supported by its effect on sebocytes, increased transepidermal water loss, and profound disruption of the integrity of the epidermis, resulting in an elevated risk for inadvertent skin damage. Patients were encouraged to avoid cosmetic procedures in prior case reports,14-16 and because our case demonstrates the risk for cutaneous injury in athletes due to isotretinoin-induced skin fragility, we propose an extension of these warnings to encompass athletes receiving isotretinoin treatment. Offering early guidance on wound prevention is of paramount importance in maintaining athletic performance and minimizing painful injuries.
- Rajput I, Anjankar VP. Side effects of treating acne vulgaris with isotretinoin: a systematic review. Cureus. 2024;16:E55946. doi:10.7759/cureus.55946
- Hatami P, Balighi K, Asl HN, et al. Isotretinoin and timing of procedural interventions: clinical implications and practical points. J Cosmet Dermatol. 2023;22:2146-2149. doi:10.1111/jocd.15874
- McDonald KA, Shelley AJ, Alavi A. A systematic review on oral isotretinoin therapy and clinically observable wound healing in acne patients. J Cutan Med Surg. 2017;21:325-333. doi:10.1177/1203475417701419
- Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1:162-169. doi:10.4161/derm.1.3.9364
- Zouboulis CC. Isotretinoin revisited: pluripotent effects on human sebaceous gland cells. J Invest Dermatol. 2006;126:2154-2156. doi:10.1038/sj.jid.5700418
- Kmiec´ ML, Pajor A, Broniarczyk-Dyła G. Evaluation of biophysical skin parameters and assessment of hair growth in patients with acne treated with isotretinoin. Postepy Dermatol Alergol. 2013;30:343-349. doi:10.5114/pdia.2013.39432
- Waldman A, Bolotin D, Arndt KA, et al. ASDS Guidelines Task Force: Consensus recommendations regarding the safety of lasers, dermabrasion, chemical peels, energy devices, and skin surgery during and after isotretinoin use. Dermatolog Surg. 2017;43:1249-1262. doi:10.1097/DSS.0000000000001166
- Aksoy H, Aksoy B, Calikoglu E. Systemic retinoids and scar dehiscence. Indian J Dermatol. 2019;64:68. doi:10.4103/ijd.IJD_148_18
- Pavlis MB, Lieblich L. Isotretinoin-induced skin fragility in a teenaged athlete: a case report. Cutis. 2013;92:33-34.
- Herane MI, Fuenzalida H, Zegpi E, et al. Specific gel-cream as adjuvant to oral isotretinoin improved hydration and prevented TEWL increase—a double-blind, randomized, placebo-controlled study. J Cosmet Dermatol. 2009;8:181-185. doi:10.1111/j.1473-2165.2009.00455.x
- Park KY, Ko EJ, Kim IS, et al. The effect of evening primrose oil for the prevention of xerotic cheilitis in acne patients being treated with isotretinoin: a pilot study. Ann Dermatol. 2014;26:706-712. doi:10.5021/ad.2014.26.6.706
- Elias PM, Fritsch PO, Lampe M, et al. Retinoid effects on epidermal structure, differentiation, and permeability. Lab Invest. 1981;44:531-540.
- Williams ML, Elias PM. Nature of skin fragility in patients receiving retinoids for systemic effect. Arch Dermatol. 1981;117:611-619.
- Rubenstein R, Roenigk HH, Stegman SJ, et al. Atypical keloids after dermabrasion of patients taking isotretinoin. J Am Acad Dermatol. 1986;15:280-285. doi:10.1016/S0190-9622(86)70167-9
- Zachariae H. Delayed wound healing and keloid formation following argon laser treatment or dermabrasion during isotretinoin treatment. Br J Dermatol. 1988;118:703-706. doi:10.1111/j.1365-2133.1988.tb02574.x
- Katz BE, Mac Farlane DF. Atypical facial scarring after isotretinoin therapy in a patient with previous dermabrasion. J Am Acad Dermatol. 1994;30:852-853. doi:10.1016/S0190-9622(94)70096-6
Isotretinoin was introduced more than 3 decades ago and marked a major advancement in the treatment of severe refractory cystic acne. The most common adverse effects linked to isotretinoin usage are mucocutaneous in nature, manifesting as xerosis and cheilitis.1 Skin fragility and poor wound healing also have been reported.2-6 Current recommendations for avoiding these adverse effects include refraining from waxing, laser procedures, and other elective cutaneous procedures for at least 6 months.7 We present a case of isotretinoin-induced cutaneous fragility resulting in blistering and erosions on the palms of a competitive aerial trapeze artist.
Case Report
A 25-year-old woman presented for follow-up during week 12 of isotretinoin therapy (40 mg twice daily) prescribed for acne. She reported peeling of the skin on the palms following intense aerial acrobatic workouts. She had been a performing aerialist for many years and had never sustained a similar injury. The wounds were painful and led to decreased activity. She had no notable medical history. Physical examination of the palms revealed erosions in a distribution that corresponded to horizontal bar contact and friction (Figure). The patient was advised on proper wound care, application of emollients, and minimizing friction. She completed the course of isotretinoin and has continued aerialist activity without recurrence of skin fragility.
Comment
Skin fragility is a well-known adverse effect of isotretinoin therapy.8 Pavlis and Lieblich9 reported skin fragility in a young wrestler who experienced similar skin erosions due to isotretinoin therapy. The proposed mechanism of isotretinoin-induced skin fragility is multifactorial. It involves an apoptotic effect on sebocytes,5 which results in reduced stratum corneum hydration and an associated increase in transepidermal water loss.6,10,11 Retinoids also are known to cause thinning of the skin, likely due to the disadhesion of both the epidermis and the stratum corneum, which was demonstrated by the easy removal of cornified cells through tape stripping in hairless mice treated with isotretinoin.12 In further investigations, human patients and hairless mice treated with isotretinoin readily developed friction blisters through pencil eraser abrasion.13 Examination of the friction blisters using light and electron microscopy revealed fraying or loss of the stratum corneum and viable epidermis as well as loss of desmosomes and tonofilaments. Additionally, intracellular and intercellular deposits of an unidentified amorphous material were noted.13

Overall, the origin of skin fragility induced by isotretinoin is supported by its effect on sebocytes, increased transepidermal water loss, and profound disruption of the integrity of the epidermis, resulting in an elevated risk for inadvertent skin damage. Patients were encouraged to avoid cosmetic procedures in prior case reports,14-16 and because our case demonstrates the risk for cutaneous injury in athletes due to isotretinoin-induced skin fragility, we propose an extension of these warnings to encompass athletes receiving isotretinoin treatment. Offering early guidance on wound prevention is of paramount importance in maintaining athletic performance and minimizing painful injuries.
Isotretinoin was introduced more than 3 decades ago and marked a major advancement in the treatment of severe refractory cystic acne. The most common adverse effects linked to isotretinoin usage are mucocutaneous in nature, manifesting as xerosis and cheilitis.1 Skin fragility and poor wound healing also have been reported.2-6 Current recommendations for avoiding these adverse effects include refraining from waxing, laser procedures, and other elective cutaneous procedures for at least 6 months.7 We present a case of isotretinoin-induced cutaneous fragility resulting in blistering and erosions on the palms of a competitive aerial trapeze artist.
Case Report
A 25-year-old woman presented for follow-up during week 12 of isotretinoin therapy (40 mg twice daily) prescribed for acne. She reported peeling of the skin on the palms following intense aerial acrobatic workouts. She had been a performing aerialist for many years and had never sustained a similar injury. The wounds were painful and led to decreased activity. She had no notable medical history. Physical examination of the palms revealed erosions in a distribution that corresponded to horizontal bar contact and friction (Figure). The patient was advised on proper wound care, application of emollients, and minimizing friction. She completed the course of isotretinoin and has continued aerialist activity without recurrence of skin fragility.
Comment
Skin fragility is a well-known adverse effect of isotretinoin therapy.8 Pavlis and Lieblich9 reported skin fragility in a young wrestler who experienced similar skin erosions due to isotretinoin therapy. The proposed mechanism of isotretinoin-induced skin fragility is multifactorial. It involves an apoptotic effect on sebocytes,5 which results in reduced stratum corneum hydration and an associated increase in transepidermal water loss.6,10,11 Retinoids also are known to cause thinning of the skin, likely due to the disadhesion of both the epidermis and the stratum corneum, which was demonstrated by the easy removal of cornified cells through tape stripping in hairless mice treated with isotretinoin.12 In further investigations, human patients and hairless mice treated with isotretinoin readily developed friction blisters through pencil eraser abrasion.13 Examination of the friction blisters using light and electron microscopy revealed fraying or loss of the stratum corneum and viable epidermis as well as loss of desmosomes and tonofilaments. Additionally, intracellular and intercellular deposits of an unidentified amorphous material were noted.13

Overall, the origin of skin fragility induced by isotretinoin is supported by its effect on sebocytes, increased transepidermal water loss, and profound disruption of the integrity of the epidermis, resulting in an elevated risk for inadvertent skin damage. Patients were encouraged to avoid cosmetic procedures in prior case reports,14-16 and because our case demonstrates the risk for cutaneous injury in athletes due to isotretinoin-induced skin fragility, we propose an extension of these warnings to encompass athletes receiving isotretinoin treatment. Offering early guidance on wound prevention is of paramount importance in maintaining athletic performance and minimizing painful injuries.
- Rajput I, Anjankar VP. Side effects of treating acne vulgaris with isotretinoin: a systematic review. Cureus. 2024;16:E55946. doi:10.7759/cureus.55946
- Hatami P, Balighi K, Asl HN, et al. Isotretinoin and timing of procedural interventions: clinical implications and practical points. J Cosmet Dermatol. 2023;22:2146-2149. doi:10.1111/jocd.15874
- McDonald KA, Shelley AJ, Alavi A. A systematic review on oral isotretinoin therapy and clinically observable wound healing in acne patients. J Cutan Med Surg. 2017;21:325-333. doi:10.1177/1203475417701419
- Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1:162-169. doi:10.4161/derm.1.3.9364
- Zouboulis CC. Isotretinoin revisited: pluripotent effects on human sebaceous gland cells. J Invest Dermatol. 2006;126:2154-2156. doi:10.1038/sj.jid.5700418
- Kmiec´ ML, Pajor A, Broniarczyk-Dyła G. Evaluation of biophysical skin parameters and assessment of hair growth in patients with acne treated with isotretinoin. Postepy Dermatol Alergol. 2013;30:343-349. doi:10.5114/pdia.2013.39432
- Waldman A, Bolotin D, Arndt KA, et al. ASDS Guidelines Task Force: Consensus recommendations regarding the safety of lasers, dermabrasion, chemical peels, energy devices, and skin surgery during and after isotretinoin use. Dermatolog Surg. 2017;43:1249-1262. doi:10.1097/DSS.0000000000001166
- Aksoy H, Aksoy B, Calikoglu E. Systemic retinoids and scar dehiscence. Indian J Dermatol. 2019;64:68. doi:10.4103/ijd.IJD_148_18
- Pavlis MB, Lieblich L. Isotretinoin-induced skin fragility in a teenaged athlete: a case report. Cutis. 2013;92:33-34.
- Herane MI, Fuenzalida H, Zegpi E, et al. Specific gel-cream as adjuvant to oral isotretinoin improved hydration and prevented TEWL increase—a double-blind, randomized, placebo-controlled study. J Cosmet Dermatol. 2009;8:181-185. doi:10.1111/j.1473-2165.2009.00455.x
- Park KY, Ko EJ, Kim IS, et al. The effect of evening primrose oil for the prevention of xerotic cheilitis in acne patients being treated with isotretinoin: a pilot study. Ann Dermatol. 2014;26:706-712. doi:10.5021/ad.2014.26.6.706
- Elias PM, Fritsch PO, Lampe M, et al. Retinoid effects on epidermal structure, differentiation, and permeability. Lab Invest. 1981;44:531-540.
- Williams ML, Elias PM. Nature of skin fragility in patients receiving retinoids for systemic effect. Arch Dermatol. 1981;117:611-619.
- Rubenstein R, Roenigk HH, Stegman SJ, et al. Atypical keloids after dermabrasion of patients taking isotretinoin. J Am Acad Dermatol. 1986;15:280-285. doi:10.1016/S0190-9622(86)70167-9
- Zachariae H. Delayed wound healing and keloid formation following argon laser treatment or dermabrasion during isotretinoin treatment. Br J Dermatol. 1988;118:703-706. doi:10.1111/j.1365-2133.1988.tb02574.x
- Katz BE, Mac Farlane DF. Atypical facial scarring after isotretinoin therapy in a patient with previous dermabrasion. J Am Acad Dermatol. 1994;30:852-853. doi:10.1016/S0190-9622(94)70096-6
- Rajput I, Anjankar VP. Side effects of treating acne vulgaris with isotretinoin: a systematic review. Cureus. 2024;16:E55946. doi:10.7759/cureus.55946
- Hatami P, Balighi K, Asl HN, et al. Isotretinoin and timing of procedural interventions: clinical implications and practical points. J Cosmet Dermatol. 2023;22:2146-2149. doi:10.1111/jocd.15874
- McDonald KA, Shelley AJ, Alavi A. A systematic review on oral isotretinoin therapy and clinically observable wound healing in acne patients. J Cutan Med Surg. 2017;21:325-333. doi:10.1177/1203475417701419
- Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1:162-169. doi:10.4161/derm.1.3.9364
- Zouboulis CC. Isotretinoin revisited: pluripotent effects on human sebaceous gland cells. J Invest Dermatol. 2006;126:2154-2156. doi:10.1038/sj.jid.5700418
- Kmiec´ ML, Pajor A, Broniarczyk-Dyła G. Evaluation of biophysical skin parameters and assessment of hair growth in patients with acne treated with isotretinoin. Postepy Dermatol Alergol. 2013;30:343-349. doi:10.5114/pdia.2013.39432
- Waldman A, Bolotin D, Arndt KA, et al. ASDS Guidelines Task Force: Consensus recommendations regarding the safety of lasers, dermabrasion, chemical peels, energy devices, and skin surgery during and after isotretinoin use. Dermatolog Surg. 2017;43:1249-1262. doi:10.1097/DSS.0000000000001166
- Aksoy H, Aksoy B, Calikoglu E. Systemic retinoids and scar dehiscence. Indian J Dermatol. 2019;64:68. doi:10.4103/ijd.IJD_148_18
- Pavlis MB, Lieblich L. Isotretinoin-induced skin fragility in a teenaged athlete: a case report. Cutis. 2013;92:33-34.
- Herane MI, Fuenzalida H, Zegpi E, et al. Specific gel-cream as adjuvant to oral isotretinoin improved hydration and prevented TEWL increase—a double-blind, randomized, placebo-controlled study. J Cosmet Dermatol. 2009;8:181-185. doi:10.1111/j.1473-2165.2009.00455.x
- Park KY, Ko EJ, Kim IS, et al. The effect of evening primrose oil for the prevention of xerotic cheilitis in acne patients being treated with isotretinoin: a pilot study. Ann Dermatol. 2014;26:706-712. doi:10.5021/ad.2014.26.6.706
- Elias PM, Fritsch PO, Lampe M, et al. Retinoid effects on epidermal structure, differentiation, and permeability. Lab Invest. 1981;44:531-540.
- Williams ML, Elias PM. Nature of skin fragility in patients receiving retinoids for systemic effect. Arch Dermatol. 1981;117:611-619.
- Rubenstein R, Roenigk HH, Stegman SJ, et al. Atypical keloids after dermabrasion of patients taking isotretinoin. J Am Acad Dermatol. 1986;15:280-285. doi:10.1016/S0190-9622(86)70167-9
- Zachariae H. Delayed wound healing and keloid formation following argon laser treatment or dermabrasion during isotretinoin treatment. Br J Dermatol. 1988;118:703-706. doi:10.1111/j.1365-2133.1988.tb02574.x
- Katz BE, Mac Farlane DF. Atypical facial scarring after isotretinoin therapy in a patient with previous dermabrasion. J Am Acad Dermatol. 1994;30:852-853. doi:10.1016/S0190-9622(94)70096-6
Practice Points
- Isotretinoin is used to treat severe nodulocystic acne but can cause adverse effects such as skin fragility, xerosis, and poor wound healing.
- Dermatologists should inform athletes of heightened skin vulnerability while undergoing isotretinoin treatment.
- Isotretinoin-induced skin fragility involves the effects of isotretinoin on sebocytes, transepidermal water loss, and disruption of the integrity of the epidermis.
Small Melanoma In Situ: Single Center Study Finds Recurrence Low With 5-mm Margin Excisions
. This approach has the potential to reduce morbidity and cost associated with treatment “without compromising patient outcomes in a selected population of lesions,” the authors say.
“Currently, there is uncertainty regarding the optimal excision margin for MIS, with different guidelines recommending a range between 5 and 10 mm,” corresponding author Cong Sun, MD, of Mater Hospital Brisbane Raymond Terrace, South Brisbane, Queensland, Australia, and colleagues wrote in the study, which was published in JAMA Dermatology. “In addition, studies using the Mohs micrographic surgery technique have suggested that wider margins, up to 18 mm, may be required for MIS in some settings.”
To further examine the use of 5-mm margins for excision of small MIS on low-risk sites, the researchers retrospectively evaluated 351 MIS lesions diagnosed in 292 patients between January 1, 2011, and November 30, 2018. Lesions were eligible for analysis if a 5-mm excisional margin was documented on the operation report and if there was more than 5 years of site-specific follow-up after wide local excision. Lesions with undocumented margins were excluded from analysis, as were those with fewer than 5 years of follow-up, and those that required more than one wide local excision.
The mean age of patients was 60.3 years, 55.5% were female, and the mean dimensions of the lesions was 6 × 5 mm. The most common subtype of melanoma diagnosed was superficial spreading melanoma (50.4% of lesions), followed by lentigo maligna (30.5%) and lentiginous MIS (19.1%). Nearly half of the lesions were on the trunk (47.9%), followed by the upper limb (27.4%), lower limb (16.8%), neck (4%), face (3.4%), and scalp (0.6%). As for the size of lesions, 78.1% were < 10 mm long and 88.9% were < 10 mm wide.
Nearly 71% (248) of the lesions were treated with an initial excisional biopsy, and 29.3% (103) underwent an initial shave excision. Median follow-up was 7 years.
Only three of the 351 lesions (0.9%) had a local recurrence, with no regional recurrence or metastatic spread, and 99.1% had no recurrence. The recurrences were reexcised “with clear margins” and after at least 5 years of follow-up, no further recurrences were reported, the authors said.
In Mohs surgery studies, reported recurrence rates for MIS have been “between 0.26% and 1.1%, with excisional margins between 6 and 12 mm required,” the authors noted. “This study demonstrated a comparable 0.9% recurrence rate achieved with a conservative 5-mm excisional margin. This shows that using a 5-mm margin for MIS of smaller size (< 10 mm) may reduce morbidity and cost associated with treatment without compromising patient outcomes in a selected population of lesions.”
The researchers recommended additional studies to confirm their findings and acknowledged certain limitations of their analysis, including its retrospective, single-center design and the predominantly small sizes of the lesions.
In an accompanying editorial, John A. Zitelli, MD, of the University of Pittsburgh, Pittsburgh, Pennsylvania, said that the margin measurement used by the researchers was another limitation. “Before the excision with a 5-mm margin was performed, the diagnosis of MIS was obtained by shave biopsy or excisional biopsy with a 2- to 3-mm margin of clinically normal skin,” Dr. Zitelli wrote. “Therefore, in patients without a 2- to 3-mm biopsy margin, a minimum surgical margin of 7-8 mm would be required to achieve a similar true negative excision margin.”
Also, he continued, the exclusion of lesions with wide subclinical extension that required wider margins “weakens the conclusion that 5 mm would be an effective treatment for all MIS.”
Hugh Greenway, MD, head of Mohs micrographic surgery and director of cutaneous oncology at Scripps Cancer Center, San Diego, who was asked to comment on the study, said that clinicians continue to search for the optimum smaller surgical margin for MIS. “This can be challenging with the variability of MIS based on location and other factors,” Dr. Greenway told this news organization. “This Australian retrospective study notes that for selected, well-defined 6 × 5 mm lesions of low-risk body sites (mainly torso and limbs), a 5-mm surgical margin can provide a high cure rate. The authors note further studies are indicated. Thus, for selected lesions in selected locations, the 5-mm surgical margin may be appropriate for MIS.”
The study authors, Dr. Zitelli, and Dr. Greenway reported no financial disclosures.
A version of this article appeared on Medscape.com.
. This approach has the potential to reduce morbidity and cost associated with treatment “without compromising patient outcomes in a selected population of lesions,” the authors say.
“Currently, there is uncertainty regarding the optimal excision margin for MIS, with different guidelines recommending a range between 5 and 10 mm,” corresponding author Cong Sun, MD, of Mater Hospital Brisbane Raymond Terrace, South Brisbane, Queensland, Australia, and colleagues wrote in the study, which was published in JAMA Dermatology. “In addition, studies using the Mohs micrographic surgery technique have suggested that wider margins, up to 18 mm, may be required for MIS in some settings.”
To further examine the use of 5-mm margins for excision of small MIS on low-risk sites, the researchers retrospectively evaluated 351 MIS lesions diagnosed in 292 patients between January 1, 2011, and November 30, 2018. Lesions were eligible for analysis if a 5-mm excisional margin was documented on the operation report and if there was more than 5 years of site-specific follow-up after wide local excision. Lesions with undocumented margins were excluded from analysis, as were those with fewer than 5 years of follow-up, and those that required more than one wide local excision.
The mean age of patients was 60.3 years, 55.5% were female, and the mean dimensions of the lesions was 6 × 5 mm. The most common subtype of melanoma diagnosed was superficial spreading melanoma (50.4% of lesions), followed by lentigo maligna (30.5%) and lentiginous MIS (19.1%). Nearly half of the lesions were on the trunk (47.9%), followed by the upper limb (27.4%), lower limb (16.8%), neck (4%), face (3.4%), and scalp (0.6%). As for the size of lesions, 78.1% were < 10 mm long and 88.9% were < 10 mm wide.
Nearly 71% (248) of the lesions were treated with an initial excisional biopsy, and 29.3% (103) underwent an initial shave excision. Median follow-up was 7 years.
Only three of the 351 lesions (0.9%) had a local recurrence, with no regional recurrence or metastatic spread, and 99.1% had no recurrence. The recurrences were reexcised “with clear margins” and after at least 5 years of follow-up, no further recurrences were reported, the authors said.
In Mohs surgery studies, reported recurrence rates for MIS have been “between 0.26% and 1.1%, with excisional margins between 6 and 12 mm required,” the authors noted. “This study demonstrated a comparable 0.9% recurrence rate achieved with a conservative 5-mm excisional margin. This shows that using a 5-mm margin for MIS of smaller size (< 10 mm) may reduce morbidity and cost associated with treatment without compromising patient outcomes in a selected population of lesions.”
The researchers recommended additional studies to confirm their findings and acknowledged certain limitations of their analysis, including its retrospective, single-center design and the predominantly small sizes of the lesions.
In an accompanying editorial, John A. Zitelli, MD, of the University of Pittsburgh, Pittsburgh, Pennsylvania, said that the margin measurement used by the researchers was another limitation. “Before the excision with a 5-mm margin was performed, the diagnosis of MIS was obtained by shave biopsy or excisional biopsy with a 2- to 3-mm margin of clinically normal skin,” Dr. Zitelli wrote. “Therefore, in patients without a 2- to 3-mm biopsy margin, a minimum surgical margin of 7-8 mm would be required to achieve a similar true negative excision margin.”
Also, he continued, the exclusion of lesions with wide subclinical extension that required wider margins “weakens the conclusion that 5 mm would be an effective treatment for all MIS.”
Hugh Greenway, MD, head of Mohs micrographic surgery and director of cutaneous oncology at Scripps Cancer Center, San Diego, who was asked to comment on the study, said that clinicians continue to search for the optimum smaller surgical margin for MIS. “This can be challenging with the variability of MIS based on location and other factors,” Dr. Greenway told this news organization. “This Australian retrospective study notes that for selected, well-defined 6 × 5 mm lesions of low-risk body sites (mainly torso and limbs), a 5-mm surgical margin can provide a high cure rate. The authors note further studies are indicated. Thus, for selected lesions in selected locations, the 5-mm surgical margin may be appropriate for MIS.”
The study authors, Dr. Zitelli, and Dr. Greenway reported no financial disclosures.
A version of this article appeared on Medscape.com.
. This approach has the potential to reduce morbidity and cost associated with treatment “without compromising patient outcomes in a selected population of lesions,” the authors say.
“Currently, there is uncertainty regarding the optimal excision margin for MIS, with different guidelines recommending a range between 5 and 10 mm,” corresponding author Cong Sun, MD, of Mater Hospital Brisbane Raymond Terrace, South Brisbane, Queensland, Australia, and colleagues wrote in the study, which was published in JAMA Dermatology. “In addition, studies using the Mohs micrographic surgery technique have suggested that wider margins, up to 18 mm, may be required for MIS in some settings.”
To further examine the use of 5-mm margins for excision of small MIS on low-risk sites, the researchers retrospectively evaluated 351 MIS lesions diagnosed in 292 patients between January 1, 2011, and November 30, 2018. Lesions were eligible for analysis if a 5-mm excisional margin was documented on the operation report and if there was more than 5 years of site-specific follow-up after wide local excision. Lesions with undocumented margins were excluded from analysis, as were those with fewer than 5 years of follow-up, and those that required more than one wide local excision.
The mean age of patients was 60.3 years, 55.5% were female, and the mean dimensions of the lesions was 6 × 5 mm. The most common subtype of melanoma diagnosed was superficial spreading melanoma (50.4% of lesions), followed by lentigo maligna (30.5%) and lentiginous MIS (19.1%). Nearly half of the lesions were on the trunk (47.9%), followed by the upper limb (27.4%), lower limb (16.8%), neck (4%), face (3.4%), and scalp (0.6%). As for the size of lesions, 78.1% were < 10 mm long and 88.9% were < 10 mm wide.
Nearly 71% (248) of the lesions were treated with an initial excisional biopsy, and 29.3% (103) underwent an initial shave excision. Median follow-up was 7 years.
Only three of the 351 lesions (0.9%) had a local recurrence, with no regional recurrence or metastatic spread, and 99.1% had no recurrence. The recurrences were reexcised “with clear margins” and after at least 5 years of follow-up, no further recurrences were reported, the authors said.
In Mohs surgery studies, reported recurrence rates for MIS have been “between 0.26% and 1.1%, with excisional margins between 6 and 12 mm required,” the authors noted. “This study demonstrated a comparable 0.9% recurrence rate achieved with a conservative 5-mm excisional margin. This shows that using a 5-mm margin for MIS of smaller size (< 10 mm) may reduce morbidity and cost associated with treatment without compromising patient outcomes in a selected population of lesions.”
The researchers recommended additional studies to confirm their findings and acknowledged certain limitations of their analysis, including its retrospective, single-center design and the predominantly small sizes of the lesions.
In an accompanying editorial, John A. Zitelli, MD, of the University of Pittsburgh, Pittsburgh, Pennsylvania, said that the margin measurement used by the researchers was another limitation. “Before the excision with a 5-mm margin was performed, the diagnosis of MIS was obtained by shave biopsy or excisional biopsy with a 2- to 3-mm margin of clinically normal skin,” Dr. Zitelli wrote. “Therefore, in patients without a 2- to 3-mm biopsy margin, a minimum surgical margin of 7-8 mm would be required to achieve a similar true negative excision margin.”
Also, he continued, the exclusion of lesions with wide subclinical extension that required wider margins “weakens the conclusion that 5 mm would be an effective treatment for all MIS.”
Hugh Greenway, MD, head of Mohs micrographic surgery and director of cutaneous oncology at Scripps Cancer Center, San Diego, who was asked to comment on the study, said that clinicians continue to search for the optimum smaller surgical margin for MIS. “This can be challenging with the variability of MIS based on location and other factors,” Dr. Greenway told this news organization. “This Australian retrospective study notes that for selected, well-defined 6 × 5 mm lesions of low-risk body sites (mainly torso and limbs), a 5-mm surgical margin can provide a high cure rate. The authors note further studies are indicated. Thus, for selected lesions in selected locations, the 5-mm surgical margin may be appropriate for MIS.”
The study authors, Dr. Zitelli, and Dr. Greenway reported no financial disclosures.
A version of this article appeared on Medscape.com.
Study Finds Variations in Pediatric Dermatologists Who Accept Medicaid
TOPLINE:
METHODOLOGY:
- Researchers identified 352 actively practicing board-certified pediatric dermatologists using the Society for Pediatric Dermatology database and determined Medicaid acceptance status.
- They collected physician and practice characteristics from the US Census American Community Survey data and a web search.
TAKEAWAY:
- A total of 275 (78.1%) board-certified pediatric dermatologists accepted Medicaid.
- Academic practices had the highest Medicaid acceptance rate (98.7%), while private practices had the lowest (43.1%), a significant difference (P < .001).
- Acceptance rates were significantly higher in the Midwest (90.9%) than in the Northeast (71.8%) or West (71.4%; P = .005). Regional differences persisted after controlling for practice type: Midwest practice locations had greater odds of Medicaid acceptance than those in the Northeast (odds ratio [OR], 5.25; 95% confidence interval [CI], 1.76-15.65) or West (OR, 5.26; 95% CI, 1.88-14.66).
- Practices in counties with lower median household incomes and greater densities of pediatric dermatologists were associated with higher Medicaid acceptance (P = .001).
IN PRACTICE:
“While most pediatric dermatologists accept Medicaid, this study revealed differential access to care based on practice type, geographic location, and density of pediatric dermatologists per county,” the authors wrote. More research is needed on “the impact on health outcomes when specialty services are unavailable” and on “the role of administrative and reimbursement barriers limiting Medicaid acceptance among pediatric dermatologists,” they added.
SOURCE:
The study was led by Madeleine Tessier-Kay, MPH, Department of Dermatology, at the University of Connecticut Health Center in Farmington, Connecticut. It was published online in Pediatric Dermatology.
LIMITATIONS:
Limitations include potential incomplete capture of board-certified physicians, as not all board-certified pediatric dermatologists may be members of the Society for Pediatric Dermatology, and potential inaccurate capture of physician characteristics and Medicaid acceptance status.
DISCLOSURES:
The study funding source was not disclosed. One author was a consultant for AbbVie. Other authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers identified 352 actively practicing board-certified pediatric dermatologists using the Society for Pediatric Dermatology database and determined Medicaid acceptance status.
- They collected physician and practice characteristics from the US Census American Community Survey data and a web search.
TAKEAWAY:
- A total of 275 (78.1%) board-certified pediatric dermatologists accepted Medicaid.
- Academic practices had the highest Medicaid acceptance rate (98.7%), while private practices had the lowest (43.1%), a significant difference (P < .001).
- Acceptance rates were significantly higher in the Midwest (90.9%) than in the Northeast (71.8%) or West (71.4%; P = .005). Regional differences persisted after controlling for practice type: Midwest practice locations had greater odds of Medicaid acceptance than those in the Northeast (odds ratio [OR], 5.25; 95% confidence interval [CI], 1.76-15.65) or West (OR, 5.26; 95% CI, 1.88-14.66).
- Practices in counties with lower median household incomes and greater densities of pediatric dermatologists were associated with higher Medicaid acceptance (P = .001).
IN PRACTICE:
“While most pediatric dermatologists accept Medicaid, this study revealed differential access to care based on practice type, geographic location, and density of pediatric dermatologists per county,” the authors wrote. More research is needed on “the impact on health outcomes when specialty services are unavailable” and on “the role of administrative and reimbursement barriers limiting Medicaid acceptance among pediatric dermatologists,” they added.
SOURCE:
The study was led by Madeleine Tessier-Kay, MPH, Department of Dermatology, at the University of Connecticut Health Center in Farmington, Connecticut. It was published online in Pediatric Dermatology.
LIMITATIONS:
Limitations include potential incomplete capture of board-certified physicians, as not all board-certified pediatric dermatologists may be members of the Society for Pediatric Dermatology, and potential inaccurate capture of physician characteristics and Medicaid acceptance status.
DISCLOSURES:
The study funding source was not disclosed. One author was a consultant for AbbVie. Other authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers identified 352 actively practicing board-certified pediatric dermatologists using the Society for Pediatric Dermatology database and determined Medicaid acceptance status.
- They collected physician and practice characteristics from the US Census American Community Survey data and a web search.
TAKEAWAY:
- A total of 275 (78.1%) board-certified pediatric dermatologists accepted Medicaid.
- Academic practices had the highest Medicaid acceptance rate (98.7%), while private practices had the lowest (43.1%), a significant difference (P < .001).
- Acceptance rates were significantly higher in the Midwest (90.9%) than in the Northeast (71.8%) or West (71.4%; P = .005). Regional differences persisted after controlling for practice type: Midwest practice locations had greater odds of Medicaid acceptance than those in the Northeast (odds ratio [OR], 5.25; 95% confidence interval [CI], 1.76-15.65) or West (OR, 5.26; 95% CI, 1.88-14.66).
- Practices in counties with lower median household incomes and greater densities of pediatric dermatologists were associated with higher Medicaid acceptance (P = .001).
IN PRACTICE:
“While most pediatric dermatologists accept Medicaid, this study revealed differential access to care based on practice type, geographic location, and density of pediatric dermatologists per county,” the authors wrote. More research is needed on “the impact on health outcomes when specialty services are unavailable” and on “the role of administrative and reimbursement barriers limiting Medicaid acceptance among pediatric dermatologists,” they added.
SOURCE:
The study was led by Madeleine Tessier-Kay, MPH, Department of Dermatology, at the University of Connecticut Health Center in Farmington, Connecticut. It was published online in Pediatric Dermatology.
LIMITATIONS:
Limitations include potential incomplete capture of board-certified physicians, as not all board-certified pediatric dermatologists may be members of the Society for Pediatric Dermatology, and potential inaccurate capture of physician characteristics and Medicaid acceptance status.
DISCLOSURES:
The study funding source was not disclosed. One author was a consultant for AbbVie. Other authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Dermatofibrosarcoma Protuberans More Common In Black Patients, Analysis Finds
TOPLINE:
that also found that larger tumor size and older age were associated with survival outcomes.
METHODOLOGY:
- Researchers used the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) registry from 2000 through 2018 to provide a comprehensive report on the incidence of DFSP, a rare, low-grade cutaneous soft tissue sarcoma, and factors associated with metastatic progression, overall survival (OS), and cancer-specific survival.
- A total of 7748 patients (mean age, 43.5 years; 53.3% women; 52% non-Hispanic White) were diagnosed with histologically confirmed DFSP of the skin and connective tissue and were included in the study.
- DFSP incidence was reported as cases per million person-years and age-adjusted to the 2000 US Standard Population, and factors influencing metastasis were assessed.
TAKEAWAY:
- The overall DFSP incidence rate was 6.25 cases per million person-years, with a higher incidence in Black individuals than in White individuals (8.74 vs 4.53).
- The 5-year OS rate was 95.8%. Older age (≥ 60 years; hazard ratio [HR], 6.66), male gender assigned at birth (HR, 1.79), and larger tumor size (≥ 3 cm; HR, 2.02) were associated with poorer OS (P < .001 for all).
- The 1-year and 5-year DFSP-specific survival rates were 99.9% and 99.2%, respectively. Older age (HR, 3.47; P < .001) and larger tumor size (≥ 3 cm; HR, 5.34; P = .002) were associated with significantly worse cancer-specific survival.
- Large tumor size (odds ratio [OR], 2.24) and DFSP located on the head and neck (OR, 4.88), or genitalia (OR, 3.16) were significantly associated with increased metastasis risk. Higher socioeconomic status was linked to a lower risk for metastasis.
IN PRACTICE:
“Our findings highlight the increased incidence rates of DFSP among Black patients. We demonstrate the interplay between patient demographics and clinical factors in influencing DFSP metastasis, OS, and cancer-specific survival,” the authors wrote. The results, they added, “may be useful for further evaluation of proposed causes, which will ultimately lead to further understanding and prevention of this disease.”
SOURCE:
The study was led by Jalal Maghfour, MD, Department of Dermatology, Henry Ford Health, Detroit, and was published online on June 20 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Details on specific cases in the SEER registry are limited. For 1752 patients, tumor size was not included, increasing the risk for misclassification bias. Because specific pathology reports were not available, the analysis did not address histologic grade.
DISCLOSURES:
The study did not receive any funding support. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
that also found that larger tumor size and older age were associated with survival outcomes.
METHODOLOGY:
- Researchers used the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) registry from 2000 through 2018 to provide a comprehensive report on the incidence of DFSP, a rare, low-grade cutaneous soft tissue sarcoma, and factors associated with metastatic progression, overall survival (OS), and cancer-specific survival.
- A total of 7748 patients (mean age, 43.5 years; 53.3% women; 52% non-Hispanic White) were diagnosed with histologically confirmed DFSP of the skin and connective tissue and were included in the study.
- DFSP incidence was reported as cases per million person-years and age-adjusted to the 2000 US Standard Population, and factors influencing metastasis were assessed.
TAKEAWAY:
- The overall DFSP incidence rate was 6.25 cases per million person-years, with a higher incidence in Black individuals than in White individuals (8.74 vs 4.53).
- The 5-year OS rate was 95.8%. Older age (≥ 60 years; hazard ratio [HR], 6.66), male gender assigned at birth (HR, 1.79), and larger tumor size (≥ 3 cm; HR, 2.02) were associated with poorer OS (P < .001 for all).
- The 1-year and 5-year DFSP-specific survival rates were 99.9% and 99.2%, respectively. Older age (HR, 3.47; P < .001) and larger tumor size (≥ 3 cm; HR, 5.34; P = .002) were associated with significantly worse cancer-specific survival.
- Large tumor size (odds ratio [OR], 2.24) and DFSP located on the head and neck (OR, 4.88), or genitalia (OR, 3.16) were significantly associated with increased metastasis risk. Higher socioeconomic status was linked to a lower risk for metastasis.
IN PRACTICE:
“Our findings highlight the increased incidence rates of DFSP among Black patients. We demonstrate the interplay between patient demographics and clinical factors in influencing DFSP metastasis, OS, and cancer-specific survival,” the authors wrote. The results, they added, “may be useful for further evaluation of proposed causes, which will ultimately lead to further understanding and prevention of this disease.”
SOURCE:
The study was led by Jalal Maghfour, MD, Department of Dermatology, Henry Ford Health, Detroit, and was published online on June 20 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Details on specific cases in the SEER registry are limited. For 1752 patients, tumor size was not included, increasing the risk for misclassification bias. Because specific pathology reports were not available, the analysis did not address histologic grade.
DISCLOSURES:
The study did not receive any funding support. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
that also found that larger tumor size and older age were associated with survival outcomes.
METHODOLOGY:
- Researchers used the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) registry from 2000 through 2018 to provide a comprehensive report on the incidence of DFSP, a rare, low-grade cutaneous soft tissue sarcoma, and factors associated with metastatic progression, overall survival (OS), and cancer-specific survival.
- A total of 7748 patients (mean age, 43.5 years; 53.3% women; 52% non-Hispanic White) were diagnosed with histologically confirmed DFSP of the skin and connective tissue and were included in the study.
- DFSP incidence was reported as cases per million person-years and age-adjusted to the 2000 US Standard Population, and factors influencing metastasis were assessed.
TAKEAWAY:
- The overall DFSP incidence rate was 6.25 cases per million person-years, with a higher incidence in Black individuals than in White individuals (8.74 vs 4.53).
- The 5-year OS rate was 95.8%. Older age (≥ 60 years; hazard ratio [HR], 6.66), male gender assigned at birth (HR, 1.79), and larger tumor size (≥ 3 cm; HR, 2.02) were associated with poorer OS (P < .001 for all).
- The 1-year and 5-year DFSP-specific survival rates were 99.9% and 99.2%, respectively. Older age (HR, 3.47; P < .001) and larger tumor size (≥ 3 cm; HR, 5.34; P = .002) were associated with significantly worse cancer-specific survival.
- Large tumor size (odds ratio [OR], 2.24) and DFSP located on the head and neck (OR, 4.88), or genitalia (OR, 3.16) were significantly associated with increased metastasis risk. Higher socioeconomic status was linked to a lower risk for metastasis.
IN PRACTICE:
“Our findings highlight the increased incidence rates of DFSP among Black patients. We demonstrate the interplay between patient demographics and clinical factors in influencing DFSP metastasis, OS, and cancer-specific survival,” the authors wrote. The results, they added, “may be useful for further evaluation of proposed causes, which will ultimately lead to further understanding and prevention of this disease.”
SOURCE:
The study was led by Jalal Maghfour, MD, Department of Dermatology, Henry Ford Health, Detroit, and was published online on June 20 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Details on specific cases in the SEER registry are limited. For 1752 patients, tumor size was not included, increasing the risk for misclassification bias. Because specific pathology reports were not available, the analysis did not address histologic grade.
DISCLOSURES:
The study did not receive any funding support. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Debate Over Axial Involvement in Psoriatic Arthritis Still Unresolved Despite New Studies
VIENNA — While there is no doubt that some people with psoriatic arthritis (PsA) have axial symptoms, data presented at the annual European Congress of Rheumatology do not appear to add much to what is already known about axial PsA or to further the cause of differentiating it from axial spondyloarthritis (axSpA).
In both the AXIS study and Reuma.pt, around one in three patients with PsA were found to have axial involvement. Notably, the percentage of people with axial PsA was found to vary according to how imaging information was interpreted in the AXIS study. Both studies were discussed during the Axial Involvement in PsA and SpA session at EULAR 2024.
The One-Million-Dollar Question
“So, the one-million-dollar question: What is it, really?” Philippe Carron, MD, PhD, Ghent University Hospital, Ghent, Belgium, said in the presentation that started the session. Despite PsA being described more than 60 years ago, “we still have no internationally accepted definition or a consensus on how we should define these patients and how we should screen them,” he said.
“There are some believers that it is just a form of axial SpA with concomitant psoriasis, but also some people that think that the axial PsA is a typical disease, with typical characteristics which are different from axial disease,” Dr. Carron said.
The lack of consensus makes it difficult to estimate just how many people have axial PsA. Reported prevalences range from 5% to 70%, “all caused by which criteria that you’re using to define axial involvement,” Dr. Carron added.
There are, however, two things that can be agreed upon, according to Dr. Carron. First, the prevalence of axial involvement in people with early PsA is “much, much lower” than that of more established disease. Second, exclusive axial involvement is seen in “just a minority of PsA patients.” Most people with axial disease also have peripheral disease, he added.
Imaging findings in axial PsA “are quite similar to those seen in axial SpA,” although Dr. Carron also said that there were some distinct differences. Radiographic sacroiliitis occurs in around 25%-50% of people with axial PsA, and atypical syndesmophytes are more often found in people with axial PsA than in those with axSpA.
Shared Characteristics
But are axial PsA and axSpA separate diseases or part of the same disease continuum? That’s a question that is still very much open for debate, said Sofia Ramiro, MD, PhD, a senior researcher at Leiden University Medical Center, Leiden, the Netherlands, and rheumatology consultant at Zuyderland Medical Center in Heerlen, the Netherlands.
While many studies have looked to answer this question, there is a big methodological problem — the studies largely cannot be compared as they have used different definitions of axSpA.
Take a patient with inflammatory back pain, psoriasis, and oligoarthritis, Dr. Ramiro said. If the patient goes to one rheumatologist, they may get a diagnosis of axSpA, but if they go to a different rheumatologist, they may get a diagnosis of axial PsA.
“This is influenced by training, expertise, by beliefs, and by belonging to ASAS [Assessment of Spondyloarthritis International Society] or to GRAPPA [Group for Research and Assessment of Psoriasis and Psoriatic Arthritis],” Dr. Ramiro suggested. It’s “a diagnostic bias” that is very difficult to overcome and makes direct comparisons between patient populations recruited into clinical studies “extremely challenging.”
To confuse matters more, axial PsA and axSpA share common characteristics: Inflammatory back pain, HLA-B27 positivity, elevated levels of C-reactive protein (CRP) or a higher erythrocyte sedimentation rate, and structural lesions in the sacroiliac joints and spine.
AXIS Study ‘Gives Answers’
More research into factors associated with axial PsA need to be performed to try to help define the condition and enable classification and ultimately treatment guidelines. This is where the AXIS study comes in.
The AXIS study is a joint project of ASAS and GRAPPA that was started in January 2019 with the aim of defining a homogeneous subgroup of patients who could be studied.
“The objectives of the AXIS study are to determine the frequency of axial involvement in patients with PsA; to identify the frequency of active inflammatory and structural changes on imaging; and to identify factors associated with the presence of axial involvement in PsA,” Murat Torgutalp, MD, of Charité – Universitätsmedizin Berlin, Berlin, Germany, said at EULAR 2024.
The study population consisted of 409 consecutively recruited patients diagnosed with PsA according to CASPAR (Classification for Psoriatic Arthritis) criteria; all have had PsA for up to 10 years and were untreated with biologic or targeted synthetic disease modifying drugs at the time of inclusion.
Dr. Torgutalp, who is the study’s primary research coordinator, reported that a diagnosis of PsA was made in 37% of the population when local investigators considered available clinical, laboratory, and imaging data. However, patients’ imaging data were also centrally assessed, and when the local investigators were party to the expert imaging interpretations, the percentage of people diagnosed with PsA dropped to 27%.
“When we looked at the clinical characteristics, the presence of the back pain, particularly inflammatory back pain, HLA-B27 positivity, elevated CRP, and presence of active, inflammatory and structural changes in the sacroiliac joints and spine were associated with the final conclusion on the presence of axial involvement,” Dr. Torgutalp said.
Despite the title of his presentation being “The Axis Study Gives Answers,” Dr. Torgutalp presented lots of data without giving much insight into how they might be used. He concluded that “overall, there was a trend toward overestimation of the presence of imaging changes indicative of axial involvement across all imaging modalities” by the local investigators.
Dennis McGonagle, MB, MCH, BAO, PhD, of the University of Leeds, Leeds, England,said in an interview that the AXIS study “is a noble, international effort across multiple countries to try and better understand axial PsA.”
Dr. McGonagle, who was not involved in the study, added: “A lot of data are being generated, and a lot of analysis needs to be done to drill down to get a clear message that could influence practice.”
Axial PsA in the Portuguese Population
Separately, Catarina Abreu, a rheumatology intern at Hospital Garcia de Orta, Almada, Portugal, presented some real-world data on axial PsA from Reuma.pt.
Of 2304 patients, 854 (37.1%) reportedly had axial PsA, which had been defined as physician-reported spondylitis or the presence of imaging findings suggestive of axial involvement. This included radiographic- or MRI-detected sacroiliitis or syndesmophytes seen on axial x-rays.
The majority (78.2%) of those with an axial PsA diagnosis had concomitant peripheral involvement, with 8.1% having exclusive axial disease.
About 70% of the axial PsA diagnoses had been made using clinical or laboratory findings alone, and 30% of diagnoses was based on imaging results. Of the latter, Ms. Abreu noted that patients who had imaging data available were more likely to be HLA-B27 positive and less likely to have dactylitis, with respective odds ratios (ORs) of 3.10 and 2.42.
Individuals with axial PsA were more likely to have enthesitis (OR, 1.92), although no data were available on whether this was axial or peripheral enthesitis. Tobacco exposure was also linked to an increased chance of having axial PsA (OR, 1.66).
Ms. Abreu noted that the “scarce number of available imaging exams” and other missing data in Reuma.pt may have led to an underdiagnosis of axial PsA.
“The difference that we found between axial and peripheral [PsA] are similar to the differences found in other studies that compared axial psoriatic arthritis with axial spondyloarthritis,” Ms. Abreu said.
“So, we leave with the question that was already left before here: If these are different diseases or just different phenotypes of the same disease, and what implications will this have in the future?” Ms. Abreu concluded.
Dr. Carron received educational grants, speaker fees, or honoraria for other consultancy work from AbbVie, UCB, Pfizer, Eli Lilly, Novartis, Janssen, and Galapagos/Alfasigma. Dr. Ramiro is an ASAS executive committee member and received research grants or consulting/speaker fees from AbbVie, Eli Lilly, Galapagos, Janssen, Merck Sharp and Dohme, Novartis, Pfizer, Sanofi, and UCB. AXIS is supported by unrestricted research grants from AbbVie, Galapagos, Janssen, Eli Lilly, Novartis, Pfizer, and UCB. Dr. Torgutalp is the primary research coordinator for the study; he reported no financial conflicts of interest. The Reuma.pt registry was developed with the financial support of the pharmaceutical industry and is currently supported by AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp and Dohme, Novartis, Pfizer, and Sobi. Ms. Abreu reported no financial conflicts of interest.
A version of this article appeared on Medscape.com.
VIENNA — While there is no doubt that some people with psoriatic arthritis (PsA) have axial symptoms, data presented at the annual European Congress of Rheumatology do not appear to add much to what is already known about axial PsA or to further the cause of differentiating it from axial spondyloarthritis (axSpA).
In both the AXIS study and Reuma.pt, around one in three patients with PsA were found to have axial involvement. Notably, the percentage of people with axial PsA was found to vary according to how imaging information was interpreted in the AXIS study. Both studies were discussed during the Axial Involvement in PsA and SpA session at EULAR 2024.
The One-Million-Dollar Question
“So, the one-million-dollar question: What is it, really?” Philippe Carron, MD, PhD, Ghent University Hospital, Ghent, Belgium, said in the presentation that started the session. Despite PsA being described more than 60 years ago, “we still have no internationally accepted definition or a consensus on how we should define these patients and how we should screen them,” he said.
“There are some believers that it is just a form of axial SpA with concomitant psoriasis, but also some people that think that the axial PsA is a typical disease, with typical characteristics which are different from axial disease,” Dr. Carron said.
The lack of consensus makes it difficult to estimate just how many people have axial PsA. Reported prevalences range from 5% to 70%, “all caused by which criteria that you’re using to define axial involvement,” Dr. Carron added.
There are, however, two things that can be agreed upon, according to Dr. Carron. First, the prevalence of axial involvement in people with early PsA is “much, much lower” than that of more established disease. Second, exclusive axial involvement is seen in “just a minority of PsA patients.” Most people with axial disease also have peripheral disease, he added.
Imaging findings in axial PsA “are quite similar to those seen in axial SpA,” although Dr. Carron also said that there were some distinct differences. Radiographic sacroiliitis occurs in around 25%-50% of people with axial PsA, and atypical syndesmophytes are more often found in people with axial PsA than in those with axSpA.
Shared Characteristics
But are axial PsA and axSpA separate diseases or part of the same disease continuum? That’s a question that is still very much open for debate, said Sofia Ramiro, MD, PhD, a senior researcher at Leiden University Medical Center, Leiden, the Netherlands, and rheumatology consultant at Zuyderland Medical Center in Heerlen, the Netherlands.
While many studies have looked to answer this question, there is a big methodological problem — the studies largely cannot be compared as they have used different definitions of axSpA.
Take a patient with inflammatory back pain, psoriasis, and oligoarthritis, Dr. Ramiro said. If the patient goes to one rheumatologist, they may get a diagnosis of axSpA, but if they go to a different rheumatologist, they may get a diagnosis of axial PsA.
“This is influenced by training, expertise, by beliefs, and by belonging to ASAS [Assessment of Spondyloarthritis International Society] or to GRAPPA [Group for Research and Assessment of Psoriasis and Psoriatic Arthritis],” Dr. Ramiro suggested. It’s “a diagnostic bias” that is very difficult to overcome and makes direct comparisons between patient populations recruited into clinical studies “extremely challenging.”
To confuse matters more, axial PsA and axSpA share common characteristics: Inflammatory back pain, HLA-B27 positivity, elevated levels of C-reactive protein (CRP) or a higher erythrocyte sedimentation rate, and structural lesions in the sacroiliac joints and spine.
AXIS Study ‘Gives Answers’
More research into factors associated with axial PsA need to be performed to try to help define the condition and enable classification and ultimately treatment guidelines. This is where the AXIS study comes in.
The AXIS study is a joint project of ASAS and GRAPPA that was started in January 2019 with the aim of defining a homogeneous subgroup of patients who could be studied.
“The objectives of the AXIS study are to determine the frequency of axial involvement in patients with PsA; to identify the frequency of active inflammatory and structural changes on imaging; and to identify factors associated with the presence of axial involvement in PsA,” Murat Torgutalp, MD, of Charité – Universitätsmedizin Berlin, Berlin, Germany, said at EULAR 2024.
The study population consisted of 409 consecutively recruited patients diagnosed with PsA according to CASPAR (Classification for Psoriatic Arthritis) criteria; all have had PsA for up to 10 years and were untreated with biologic or targeted synthetic disease modifying drugs at the time of inclusion.
Dr. Torgutalp, who is the study’s primary research coordinator, reported that a diagnosis of PsA was made in 37% of the population when local investigators considered available clinical, laboratory, and imaging data. However, patients’ imaging data were also centrally assessed, and when the local investigators were party to the expert imaging interpretations, the percentage of people diagnosed with PsA dropped to 27%.
“When we looked at the clinical characteristics, the presence of the back pain, particularly inflammatory back pain, HLA-B27 positivity, elevated CRP, and presence of active, inflammatory and structural changes in the sacroiliac joints and spine were associated with the final conclusion on the presence of axial involvement,” Dr. Torgutalp said.
Despite the title of his presentation being “The Axis Study Gives Answers,” Dr. Torgutalp presented lots of data without giving much insight into how they might be used. He concluded that “overall, there was a trend toward overestimation of the presence of imaging changes indicative of axial involvement across all imaging modalities” by the local investigators.
Dennis McGonagle, MB, MCH, BAO, PhD, of the University of Leeds, Leeds, England,said in an interview that the AXIS study “is a noble, international effort across multiple countries to try and better understand axial PsA.”
Dr. McGonagle, who was not involved in the study, added: “A lot of data are being generated, and a lot of analysis needs to be done to drill down to get a clear message that could influence practice.”
Axial PsA in the Portuguese Population
Separately, Catarina Abreu, a rheumatology intern at Hospital Garcia de Orta, Almada, Portugal, presented some real-world data on axial PsA from Reuma.pt.
Of 2304 patients, 854 (37.1%) reportedly had axial PsA, which had been defined as physician-reported spondylitis or the presence of imaging findings suggestive of axial involvement. This included radiographic- or MRI-detected sacroiliitis or syndesmophytes seen on axial x-rays.
The majority (78.2%) of those with an axial PsA diagnosis had concomitant peripheral involvement, with 8.1% having exclusive axial disease.
About 70% of the axial PsA diagnoses had been made using clinical or laboratory findings alone, and 30% of diagnoses was based on imaging results. Of the latter, Ms. Abreu noted that patients who had imaging data available were more likely to be HLA-B27 positive and less likely to have dactylitis, with respective odds ratios (ORs) of 3.10 and 2.42.
Individuals with axial PsA were more likely to have enthesitis (OR, 1.92), although no data were available on whether this was axial or peripheral enthesitis. Tobacco exposure was also linked to an increased chance of having axial PsA (OR, 1.66).
Ms. Abreu noted that the “scarce number of available imaging exams” and other missing data in Reuma.pt may have led to an underdiagnosis of axial PsA.
“The difference that we found between axial and peripheral [PsA] are similar to the differences found in other studies that compared axial psoriatic arthritis with axial spondyloarthritis,” Ms. Abreu said.
“So, we leave with the question that was already left before here: If these are different diseases or just different phenotypes of the same disease, and what implications will this have in the future?” Ms. Abreu concluded.
Dr. Carron received educational grants, speaker fees, or honoraria for other consultancy work from AbbVie, UCB, Pfizer, Eli Lilly, Novartis, Janssen, and Galapagos/Alfasigma. Dr. Ramiro is an ASAS executive committee member and received research grants or consulting/speaker fees from AbbVie, Eli Lilly, Galapagos, Janssen, Merck Sharp and Dohme, Novartis, Pfizer, Sanofi, and UCB. AXIS is supported by unrestricted research grants from AbbVie, Galapagos, Janssen, Eli Lilly, Novartis, Pfizer, and UCB. Dr. Torgutalp is the primary research coordinator for the study; he reported no financial conflicts of interest. The Reuma.pt registry was developed with the financial support of the pharmaceutical industry and is currently supported by AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp and Dohme, Novartis, Pfizer, and Sobi. Ms. Abreu reported no financial conflicts of interest.
A version of this article appeared on Medscape.com.
VIENNA — While there is no doubt that some people with psoriatic arthritis (PsA) have axial symptoms, data presented at the annual European Congress of Rheumatology do not appear to add much to what is already known about axial PsA or to further the cause of differentiating it from axial spondyloarthritis (axSpA).
In both the AXIS study and Reuma.pt, around one in three patients with PsA were found to have axial involvement. Notably, the percentage of people with axial PsA was found to vary according to how imaging information was interpreted in the AXIS study. Both studies were discussed during the Axial Involvement in PsA and SpA session at EULAR 2024.
The One-Million-Dollar Question
“So, the one-million-dollar question: What is it, really?” Philippe Carron, MD, PhD, Ghent University Hospital, Ghent, Belgium, said in the presentation that started the session. Despite PsA being described more than 60 years ago, “we still have no internationally accepted definition or a consensus on how we should define these patients and how we should screen them,” he said.
“There are some believers that it is just a form of axial SpA with concomitant psoriasis, but also some people that think that the axial PsA is a typical disease, with typical characteristics which are different from axial disease,” Dr. Carron said.
The lack of consensus makes it difficult to estimate just how many people have axial PsA. Reported prevalences range from 5% to 70%, “all caused by which criteria that you’re using to define axial involvement,” Dr. Carron added.
There are, however, two things that can be agreed upon, according to Dr. Carron. First, the prevalence of axial involvement in people with early PsA is “much, much lower” than that of more established disease. Second, exclusive axial involvement is seen in “just a minority of PsA patients.” Most people with axial disease also have peripheral disease, he added.
Imaging findings in axial PsA “are quite similar to those seen in axial SpA,” although Dr. Carron also said that there were some distinct differences. Radiographic sacroiliitis occurs in around 25%-50% of people with axial PsA, and atypical syndesmophytes are more often found in people with axial PsA than in those with axSpA.
Shared Characteristics
But are axial PsA and axSpA separate diseases or part of the same disease continuum? That’s a question that is still very much open for debate, said Sofia Ramiro, MD, PhD, a senior researcher at Leiden University Medical Center, Leiden, the Netherlands, and rheumatology consultant at Zuyderland Medical Center in Heerlen, the Netherlands.
While many studies have looked to answer this question, there is a big methodological problem — the studies largely cannot be compared as they have used different definitions of axSpA.
Take a patient with inflammatory back pain, psoriasis, and oligoarthritis, Dr. Ramiro said. If the patient goes to one rheumatologist, they may get a diagnosis of axSpA, but if they go to a different rheumatologist, they may get a diagnosis of axial PsA.
“This is influenced by training, expertise, by beliefs, and by belonging to ASAS [Assessment of Spondyloarthritis International Society] or to GRAPPA [Group for Research and Assessment of Psoriasis and Psoriatic Arthritis],” Dr. Ramiro suggested. It’s “a diagnostic bias” that is very difficult to overcome and makes direct comparisons between patient populations recruited into clinical studies “extremely challenging.”
To confuse matters more, axial PsA and axSpA share common characteristics: Inflammatory back pain, HLA-B27 positivity, elevated levels of C-reactive protein (CRP) or a higher erythrocyte sedimentation rate, and structural lesions in the sacroiliac joints and spine.
AXIS Study ‘Gives Answers’
More research into factors associated with axial PsA need to be performed to try to help define the condition and enable classification and ultimately treatment guidelines. This is where the AXIS study comes in.
The AXIS study is a joint project of ASAS and GRAPPA that was started in January 2019 with the aim of defining a homogeneous subgroup of patients who could be studied.
“The objectives of the AXIS study are to determine the frequency of axial involvement in patients with PsA; to identify the frequency of active inflammatory and structural changes on imaging; and to identify factors associated with the presence of axial involvement in PsA,” Murat Torgutalp, MD, of Charité – Universitätsmedizin Berlin, Berlin, Germany, said at EULAR 2024.
The study population consisted of 409 consecutively recruited patients diagnosed with PsA according to CASPAR (Classification for Psoriatic Arthritis) criteria; all have had PsA for up to 10 years and were untreated with biologic or targeted synthetic disease modifying drugs at the time of inclusion.
Dr. Torgutalp, who is the study’s primary research coordinator, reported that a diagnosis of PsA was made in 37% of the population when local investigators considered available clinical, laboratory, and imaging data. However, patients’ imaging data were also centrally assessed, and when the local investigators were party to the expert imaging interpretations, the percentage of people diagnosed with PsA dropped to 27%.
“When we looked at the clinical characteristics, the presence of the back pain, particularly inflammatory back pain, HLA-B27 positivity, elevated CRP, and presence of active, inflammatory and structural changes in the sacroiliac joints and spine were associated with the final conclusion on the presence of axial involvement,” Dr. Torgutalp said.
Despite the title of his presentation being “The Axis Study Gives Answers,” Dr. Torgutalp presented lots of data without giving much insight into how they might be used. He concluded that “overall, there was a trend toward overestimation of the presence of imaging changes indicative of axial involvement across all imaging modalities” by the local investigators.
Dennis McGonagle, MB, MCH, BAO, PhD, of the University of Leeds, Leeds, England,said in an interview that the AXIS study “is a noble, international effort across multiple countries to try and better understand axial PsA.”
Dr. McGonagle, who was not involved in the study, added: “A lot of data are being generated, and a lot of analysis needs to be done to drill down to get a clear message that could influence practice.”
Axial PsA in the Portuguese Population
Separately, Catarina Abreu, a rheumatology intern at Hospital Garcia de Orta, Almada, Portugal, presented some real-world data on axial PsA from Reuma.pt.
Of 2304 patients, 854 (37.1%) reportedly had axial PsA, which had been defined as physician-reported spondylitis or the presence of imaging findings suggestive of axial involvement. This included radiographic- or MRI-detected sacroiliitis or syndesmophytes seen on axial x-rays.
The majority (78.2%) of those with an axial PsA diagnosis had concomitant peripheral involvement, with 8.1% having exclusive axial disease.
About 70% of the axial PsA diagnoses had been made using clinical or laboratory findings alone, and 30% of diagnoses was based on imaging results. Of the latter, Ms. Abreu noted that patients who had imaging data available were more likely to be HLA-B27 positive and less likely to have dactylitis, with respective odds ratios (ORs) of 3.10 and 2.42.
Individuals with axial PsA were more likely to have enthesitis (OR, 1.92), although no data were available on whether this was axial or peripheral enthesitis. Tobacco exposure was also linked to an increased chance of having axial PsA (OR, 1.66).
Ms. Abreu noted that the “scarce number of available imaging exams” and other missing data in Reuma.pt may have led to an underdiagnosis of axial PsA.
“The difference that we found between axial and peripheral [PsA] are similar to the differences found in other studies that compared axial psoriatic arthritis with axial spondyloarthritis,” Ms. Abreu said.
“So, we leave with the question that was already left before here: If these are different diseases or just different phenotypes of the same disease, and what implications will this have in the future?” Ms. Abreu concluded.
Dr. Carron received educational grants, speaker fees, or honoraria for other consultancy work from AbbVie, UCB, Pfizer, Eli Lilly, Novartis, Janssen, and Galapagos/Alfasigma. Dr. Ramiro is an ASAS executive committee member and received research grants or consulting/speaker fees from AbbVie, Eli Lilly, Galapagos, Janssen, Merck Sharp and Dohme, Novartis, Pfizer, Sanofi, and UCB. AXIS is supported by unrestricted research grants from AbbVie, Galapagos, Janssen, Eli Lilly, Novartis, Pfizer, and UCB. Dr. Torgutalp is the primary research coordinator for the study; he reported no financial conflicts of interest. The Reuma.pt registry was developed with the financial support of the pharmaceutical industry and is currently supported by AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp and Dohme, Novartis, Pfizer, and Sobi. Ms. Abreu reported no financial conflicts of interest.
A version of this article appeared on Medscape.com.
FROM EULAR 2024
Trading TV Time for Physical Activity Boosts Healthy Aging
TOPLINE:
, but substituting it with any physical activity — or even sleeping, in case of women with inadequate sleep — may lead to better overall health.
METHODOLOGY:
- Previous studies have shown that replacing sedentary behavior with physical activity may improve mortality outcomes, but whether this increased lifespan is accompanied by better overall health remains an unanswered question.
- To understand the impact of sedentary behavior and physical activity on healthy aging, researchers analyzed data from the prospective cohort Nurses’ Health Study.
- They included 45,176 women aged > 50 years in 1992 (mean age, 59.2 years) who were free of major chronic diseases and were followed up for 20 years.
- In 1992, validated questionnaires were used to record exposure to sedentary behavior, different levels of physical activity, and sleep. The time spent watching television was the primary exposure in the sedentary behavior category.
- The main outcome was healthy aging, defined as survival to ≥ 70 years of age and maintenance of four domains of health — being free of 11 main chronic diseases and having no impairment of subjective memory, physical function, or mental health.
TAKEAWAY:
- At 20 years of follow-up, 8.6% of the women achieved healthy aging, while 41.4% had none of the 11 chronic diseases, 16.1% had no physical function impairment, 44.1% had no mental health limitation, and 51.9% reported no memory impairment.
- For each increase of 2 hours per day spent sitting and watching television, the odds of healthy aging dropped by 12% (95% confidence interval [CI], 7%-17%).
- Conversely, every additional 2 hours per day of low-level physical activity at work upped the odds of healthy aging by 6% (95% CI, 3%-9%); furthermore, each extra hour per day of standardized moderate to vigorous physical activity (normal pace walking or the equivalent) was associated with 14% higher odds (95% CI, 11%-16%) of healthy aging.
- In a theoretical modeling analysis, individuals could increase their odds of healthy aging by replacing 1 hour of television time per day with low levels of physical activity at home and work or with moderate to vigorous levels of physical activity — or even sleeping, for those who slept for ≤ 7 hours.
IN PRACTICE:
“These findings expand on the literature reporting that replacing sedentary behavior with light or moderate to vigorous physical activity is associated with decreased mortality by suggesting that this increased lifespan might be accompanied by better overall health,” the authors wrote.
SOURCE:
Hongying Shi, PhD, Department of Epidemiology and Health Statistics, School of Public Health, Wenzhou Medical University, Wenzhou, China, led this study, which was published online in JAMA Network Open.
LIMITATIONS:
The measures of different behaviors were self-reported and may, therefore, be less accurate than objective measurement methods. Measurement error may have attenuated the effect of low levels of physical activity. The single exposure assessment at baseline may not reflect the long-term pattern of these activities.
DISCLOSURES:
The lead author was supported by the National Social Science Foundation Project of China and the Zhejiang Provincial Philosophy and Social Sciences Planning Project. A co-author and the Nurses’ Health Study were supported by the US National Institutes of Health. The authors declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
, but substituting it with any physical activity — or even sleeping, in case of women with inadequate sleep — may lead to better overall health.
METHODOLOGY:
- Previous studies have shown that replacing sedentary behavior with physical activity may improve mortality outcomes, but whether this increased lifespan is accompanied by better overall health remains an unanswered question.
- To understand the impact of sedentary behavior and physical activity on healthy aging, researchers analyzed data from the prospective cohort Nurses’ Health Study.
- They included 45,176 women aged > 50 years in 1992 (mean age, 59.2 years) who were free of major chronic diseases and were followed up for 20 years.
- In 1992, validated questionnaires were used to record exposure to sedentary behavior, different levels of physical activity, and sleep. The time spent watching television was the primary exposure in the sedentary behavior category.
- The main outcome was healthy aging, defined as survival to ≥ 70 years of age and maintenance of four domains of health — being free of 11 main chronic diseases and having no impairment of subjective memory, physical function, or mental health.
TAKEAWAY:
- At 20 years of follow-up, 8.6% of the women achieved healthy aging, while 41.4% had none of the 11 chronic diseases, 16.1% had no physical function impairment, 44.1% had no mental health limitation, and 51.9% reported no memory impairment.
- For each increase of 2 hours per day spent sitting and watching television, the odds of healthy aging dropped by 12% (95% confidence interval [CI], 7%-17%).
- Conversely, every additional 2 hours per day of low-level physical activity at work upped the odds of healthy aging by 6% (95% CI, 3%-9%); furthermore, each extra hour per day of standardized moderate to vigorous physical activity (normal pace walking or the equivalent) was associated with 14% higher odds (95% CI, 11%-16%) of healthy aging.
- In a theoretical modeling analysis, individuals could increase their odds of healthy aging by replacing 1 hour of television time per day with low levels of physical activity at home and work or with moderate to vigorous levels of physical activity — or even sleeping, for those who slept for ≤ 7 hours.
IN PRACTICE:
“These findings expand on the literature reporting that replacing sedentary behavior with light or moderate to vigorous physical activity is associated with decreased mortality by suggesting that this increased lifespan might be accompanied by better overall health,” the authors wrote.
SOURCE:
Hongying Shi, PhD, Department of Epidemiology and Health Statistics, School of Public Health, Wenzhou Medical University, Wenzhou, China, led this study, which was published online in JAMA Network Open.
LIMITATIONS:
The measures of different behaviors were self-reported and may, therefore, be less accurate than objective measurement methods. Measurement error may have attenuated the effect of low levels of physical activity. The single exposure assessment at baseline may not reflect the long-term pattern of these activities.
DISCLOSURES:
The lead author was supported by the National Social Science Foundation Project of China and the Zhejiang Provincial Philosophy and Social Sciences Planning Project. A co-author and the Nurses’ Health Study were supported by the US National Institutes of Health. The authors declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
, but substituting it with any physical activity — or even sleeping, in case of women with inadequate sleep — may lead to better overall health.
METHODOLOGY:
- Previous studies have shown that replacing sedentary behavior with physical activity may improve mortality outcomes, but whether this increased lifespan is accompanied by better overall health remains an unanswered question.
- To understand the impact of sedentary behavior and physical activity on healthy aging, researchers analyzed data from the prospective cohort Nurses’ Health Study.
- They included 45,176 women aged > 50 years in 1992 (mean age, 59.2 years) who were free of major chronic diseases and were followed up for 20 years.
- In 1992, validated questionnaires were used to record exposure to sedentary behavior, different levels of physical activity, and sleep. The time spent watching television was the primary exposure in the sedentary behavior category.
- The main outcome was healthy aging, defined as survival to ≥ 70 years of age and maintenance of four domains of health — being free of 11 main chronic diseases and having no impairment of subjective memory, physical function, or mental health.
TAKEAWAY:
- At 20 years of follow-up, 8.6% of the women achieved healthy aging, while 41.4% had none of the 11 chronic diseases, 16.1% had no physical function impairment, 44.1% had no mental health limitation, and 51.9% reported no memory impairment.
- For each increase of 2 hours per day spent sitting and watching television, the odds of healthy aging dropped by 12% (95% confidence interval [CI], 7%-17%).
- Conversely, every additional 2 hours per day of low-level physical activity at work upped the odds of healthy aging by 6% (95% CI, 3%-9%); furthermore, each extra hour per day of standardized moderate to vigorous physical activity (normal pace walking or the equivalent) was associated with 14% higher odds (95% CI, 11%-16%) of healthy aging.
- In a theoretical modeling analysis, individuals could increase their odds of healthy aging by replacing 1 hour of television time per day with low levels of physical activity at home and work or with moderate to vigorous levels of physical activity — or even sleeping, for those who slept for ≤ 7 hours.
IN PRACTICE:
“These findings expand on the literature reporting that replacing sedentary behavior with light or moderate to vigorous physical activity is associated with decreased mortality by suggesting that this increased lifespan might be accompanied by better overall health,” the authors wrote.
SOURCE:
Hongying Shi, PhD, Department of Epidemiology and Health Statistics, School of Public Health, Wenzhou Medical University, Wenzhou, China, led this study, which was published online in JAMA Network Open.
LIMITATIONS:
The measures of different behaviors were self-reported and may, therefore, be less accurate than objective measurement methods. Measurement error may have attenuated the effect of low levels of physical activity. The single exposure assessment at baseline may not reflect the long-term pattern of these activities.
DISCLOSURES:
The lead author was supported by the National Social Science Foundation Project of China and the Zhejiang Provincial Philosophy and Social Sciences Planning Project. A co-author and the Nurses’ Health Study were supported by the US National Institutes of Health. The authors declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
Supreme Court Ruling Overturning Chevron Could ‘Paralyze’ Health Policy Making: Experts
Larry Levitt, executive vice president for health policy at the Kaiser Family Foundation, wrote on X, “my worry is that it will paralyze policymaking in healthcare and other areas,” because “Congress will try to fill in more details, making it harder to pass legislation.” He also wrote that federal agencies “will become very cautious in using their regulatory authority.”
In their 6-3 opinion reversing the “Chevron doctrine” — which has been followed since a 1984 Court opinion — the Justices said that the judiciary should no longer have to defer to federal agency interpretations of laws. Existing federal law “requires courts to exercise their independent judgment in deciding whether an agency has acted within its statutory authority,” said the Court, in stating why Chevron should be overruled.
Writing for the majority in the combined cases — Relentless v. Department of Commerce and Loper Bright Enterprises v. Raimondo — Chief Justice John Roberts Jr. wrote that “agencies have no special competence in resolving statutory ambiguities. Courts do.”
In a dissenting opinion, Justice Elena Kagan said the decision was a judicial power grab and would result in a “jolt to the legal system.” She was joined by Justices Sonia Sotomayor and Ketanji Brown Jackson.
The opinion will have many repercussions, said lawyer and Supreme Court watcher Amy Howe. The Chevron ruling has been “one of the most important rulings on federal administrative law, cited by federal courts more than 18,000 times,” she wrote on her blog.
For example, without the long-standing deference to agencies under Chevron, healthcare providers may have more opportunities to challenge how federal officials set Medicare reimbursement for hospital procedures or prescription drugs, Baker Donelson healthcare attorneys McKenna Cloud and Thomas Barnard wrote in an analysis.
Seventeen health organizations issued a joint statement signaling their disappointment.
“We anticipate that today’s ruling will cause significant disruption to publicly funded health insurance programs, to the stability of this country’s healthcare and food and drug review systems, and to the health and well-being of the patients and consumers we serve,” wrote the organizations, which included American Academy of Pediatrics, American Cancer Society, American Cancer Society Cancer Action Network, ALS Association, American Heart Association, American Lung Association, American Public Health Association, American Thoracic Society, Bazelon Center for Mental Health Law, Campaign for Tobacco-Free Kids, Child Neurology Foundation, Epilepsy Foundation, Muscular Dystrophy Association, National Health Law Program, Physicians for Social Responsibility, The Leukemia & Lymphoma Society, and Truth Initiative.
“It’s much harder for agencies to exercise power without some power to interpret statues. This is big,” wrote Berkeley Law Professor Orin Kerr on X.
A New, Uncertain Landscape for Healthcare
In the original Chevron case, the Court ruled that federal agencies had relevant expertise and should be given deference in resolving ambiguities that Congress had not spelled out in legislation.
In Relentless and Loper Bright, the plaintiffs argued that federal agencies overstepped their authority by issuing a rule that required commercial fishing vessels to pay for professional observers to monitor their catch.
In a statement after Friday’s ruling, the Relentless plaintiffs’ attorneys said that the decision “will recalibrate the balance of power between agencies and courts” and “make it harder for those agencies to adopt regulatory programs that exceed the authority conferred on them by Congress.”
Some predicted chaos in the wake of the ruling.
“Overturning Chevron could invite legal challenges to any and all agency determinations of ambiguous statutes by any stakeholder, leaving individual courts with the impractical task of determining the ‘correct’ meaning of statutes without the benefit of requisite expertise, practical experience, or public engagement,” wrote Sahil Agrawal, MD, PhD, Joseph S. Ross, MD, and Reshma Ramachandran, MD, in JAMA in an opinion piece in March that considered the ramifications of overturning Chevron.
“The spillover effects for medicine and public health, in turn, will be consequential,” they wrote.
In an analysis published in April, the Kaiser Family Foundation noted many potential ramifications on patient and consumer protections in the health insurance market. For instance, courts could vacate current rules governing protections under the Affordable Care Act, including that health plans offer a range of free preventive health services, such as breast, cervical, colon, and lung cancer screening.
Congressional, White House Reaction
Many legal observers said the ruling will have the effect of requiring Congress to write ever-more dense and exacting legislation to prevent agencies from interpreting any gaps.
Some members of Congress welcomed the decision.
Senate Minority Leader Mitch McConnell (R-Kentucky) said in a statement, “The Constitution vests Congress with the sole authority to make law,” adding, “the Supreme Court made it clear today that our system of government leaves no room for an unelected bureaucracy to co-opt this authority for itself.”
In a post on X, Senate Majority Leader Chuck Schumer (D-New York) accused the Court of siding with “special interests and giant corporations.” Added Mr. Schumer, “Their headlong rush to overturn 40 years of precedent and impose their own radical views is appalling.”
White House Press Secretary Karine Jean-Pierre said in a statement that “While this decision undermines the ability of federal agencies to use their expertise as Congress intended to make government work for the people, the Biden-Harris Administration will not relent in our efforts to protect and serve every American.”
A version of this article first appeared on Medscape.com.
Larry Levitt, executive vice president for health policy at the Kaiser Family Foundation, wrote on X, “my worry is that it will paralyze policymaking in healthcare and other areas,” because “Congress will try to fill in more details, making it harder to pass legislation.” He also wrote that federal agencies “will become very cautious in using their regulatory authority.”
In their 6-3 opinion reversing the “Chevron doctrine” — which has been followed since a 1984 Court opinion — the Justices said that the judiciary should no longer have to defer to federal agency interpretations of laws. Existing federal law “requires courts to exercise their independent judgment in deciding whether an agency has acted within its statutory authority,” said the Court, in stating why Chevron should be overruled.
Writing for the majority in the combined cases — Relentless v. Department of Commerce and Loper Bright Enterprises v. Raimondo — Chief Justice John Roberts Jr. wrote that “agencies have no special competence in resolving statutory ambiguities. Courts do.”
In a dissenting opinion, Justice Elena Kagan said the decision was a judicial power grab and would result in a “jolt to the legal system.” She was joined by Justices Sonia Sotomayor and Ketanji Brown Jackson.
The opinion will have many repercussions, said lawyer and Supreme Court watcher Amy Howe. The Chevron ruling has been “one of the most important rulings on federal administrative law, cited by federal courts more than 18,000 times,” she wrote on her blog.
For example, without the long-standing deference to agencies under Chevron, healthcare providers may have more opportunities to challenge how federal officials set Medicare reimbursement for hospital procedures or prescription drugs, Baker Donelson healthcare attorneys McKenna Cloud and Thomas Barnard wrote in an analysis.
Seventeen health organizations issued a joint statement signaling their disappointment.
“We anticipate that today’s ruling will cause significant disruption to publicly funded health insurance programs, to the stability of this country’s healthcare and food and drug review systems, and to the health and well-being of the patients and consumers we serve,” wrote the organizations, which included American Academy of Pediatrics, American Cancer Society, American Cancer Society Cancer Action Network, ALS Association, American Heart Association, American Lung Association, American Public Health Association, American Thoracic Society, Bazelon Center for Mental Health Law, Campaign for Tobacco-Free Kids, Child Neurology Foundation, Epilepsy Foundation, Muscular Dystrophy Association, National Health Law Program, Physicians for Social Responsibility, The Leukemia & Lymphoma Society, and Truth Initiative.
“It’s much harder for agencies to exercise power without some power to interpret statues. This is big,” wrote Berkeley Law Professor Orin Kerr on X.
A New, Uncertain Landscape for Healthcare
In the original Chevron case, the Court ruled that federal agencies had relevant expertise and should be given deference in resolving ambiguities that Congress had not spelled out in legislation.
In Relentless and Loper Bright, the plaintiffs argued that federal agencies overstepped their authority by issuing a rule that required commercial fishing vessels to pay for professional observers to monitor their catch.
In a statement after Friday’s ruling, the Relentless plaintiffs’ attorneys said that the decision “will recalibrate the balance of power between agencies and courts” and “make it harder for those agencies to adopt regulatory programs that exceed the authority conferred on them by Congress.”
Some predicted chaos in the wake of the ruling.
“Overturning Chevron could invite legal challenges to any and all agency determinations of ambiguous statutes by any stakeholder, leaving individual courts with the impractical task of determining the ‘correct’ meaning of statutes without the benefit of requisite expertise, practical experience, or public engagement,” wrote Sahil Agrawal, MD, PhD, Joseph S. Ross, MD, and Reshma Ramachandran, MD, in JAMA in an opinion piece in March that considered the ramifications of overturning Chevron.
“The spillover effects for medicine and public health, in turn, will be consequential,” they wrote.
In an analysis published in April, the Kaiser Family Foundation noted many potential ramifications on patient and consumer protections in the health insurance market. For instance, courts could vacate current rules governing protections under the Affordable Care Act, including that health plans offer a range of free preventive health services, such as breast, cervical, colon, and lung cancer screening.
Congressional, White House Reaction
Many legal observers said the ruling will have the effect of requiring Congress to write ever-more dense and exacting legislation to prevent agencies from interpreting any gaps.
Some members of Congress welcomed the decision.
Senate Minority Leader Mitch McConnell (R-Kentucky) said in a statement, “The Constitution vests Congress with the sole authority to make law,” adding, “the Supreme Court made it clear today that our system of government leaves no room for an unelected bureaucracy to co-opt this authority for itself.”
In a post on X, Senate Majority Leader Chuck Schumer (D-New York) accused the Court of siding with “special interests and giant corporations.” Added Mr. Schumer, “Their headlong rush to overturn 40 years of precedent and impose their own radical views is appalling.”
White House Press Secretary Karine Jean-Pierre said in a statement that “While this decision undermines the ability of federal agencies to use their expertise as Congress intended to make government work for the people, the Biden-Harris Administration will not relent in our efforts to protect and serve every American.”
A version of this article first appeared on Medscape.com.
Larry Levitt, executive vice president for health policy at the Kaiser Family Foundation, wrote on X, “my worry is that it will paralyze policymaking in healthcare and other areas,” because “Congress will try to fill in more details, making it harder to pass legislation.” He also wrote that federal agencies “will become very cautious in using their regulatory authority.”
In their 6-3 opinion reversing the “Chevron doctrine” — which has been followed since a 1984 Court opinion — the Justices said that the judiciary should no longer have to defer to federal agency interpretations of laws. Existing federal law “requires courts to exercise their independent judgment in deciding whether an agency has acted within its statutory authority,” said the Court, in stating why Chevron should be overruled.
Writing for the majority in the combined cases — Relentless v. Department of Commerce and Loper Bright Enterprises v. Raimondo — Chief Justice John Roberts Jr. wrote that “agencies have no special competence in resolving statutory ambiguities. Courts do.”
In a dissenting opinion, Justice Elena Kagan said the decision was a judicial power grab and would result in a “jolt to the legal system.” She was joined by Justices Sonia Sotomayor and Ketanji Brown Jackson.
The opinion will have many repercussions, said lawyer and Supreme Court watcher Amy Howe. The Chevron ruling has been “one of the most important rulings on federal administrative law, cited by federal courts more than 18,000 times,” she wrote on her blog.
For example, without the long-standing deference to agencies under Chevron, healthcare providers may have more opportunities to challenge how federal officials set Medicare reimbursement for hospital procedures or prescription drugs, Baker Donelson healthcare attorneys McKenna Cloud and Thomas Barnard wrote in an analysis.
Seventeen health organizations issued a joint statement signaling their disappointment.
“We anticipate that today’s ruling will cause significant disruption to publicly funded health insurance programs, to the stability of this country’s healthcare and food and drug review systems, and to the health and well-being of the patients and consumers we serve,” wrote the organizations, which included American Academy of Pediatrics, American Cancer Society, American Cancer Society Cancer Action Network, ALS Association, American Heart Association, American Lung Association, American Public Health Association, American Thoracic Society, Bazelon Center for Mental Health Law, Campaign for Tobacco-Free Kids, Child Neurology Foundation, Epilepsy Foundation, Muscular Dystrophy Association, National Health Law Program, Physicians for Social Responsibility, The Leukemia & Lymphoma Society, and Truth Initiative.
“It’s much harder for agencies to exercise power without some power to interpret statues. This is big,” wrote Berkeley Law Professor Orin Kerr on X.
A New, Uncertain Landscape for Healthcare
In the original Chevron case, the Court ruled that federal agencies had relevant expertise and should be given deference in resolving ambiguities that Congress had not spelled out in legislation.
In Relentless and Loper Bright, the plaintiffs argued that federal agencies overstepped their authority by issuing a rule that required commercial fishing vessels to pay for professional observers to monitor their catch.
In a statement after Friday’s ruling, the Relentless plaintiffs’ attorneys said that the decision “will recalibrate the balance of power between agencies and courts” and “make it harder for those agencies to adopt regulatory programs that exceed the authority conferred on them by Congress.”
Some predicted chaos in the wake of the ruling.
“Overturning Chevron could invite legal challenges to any and all agency determinations of ambiguous statutes by any stakeholder, leaving individual courts with the impractical task of determining the ‘correct’ meaning of statutes without the benefit of requisite expertise, practical experience, or public engagement,” wrote Sahil Agrawal, MD, PhD, Joseph S. Ross, MD, and Reshma Ramachandran, MD, in JAMA in an opinion piece in March that considered the ramifications of overturning Chevron.
“The spillover effects for medicine and public health, in turn, will be consequential,” they wrote.
In an analysis published in April, the Kaiser Family Foundation noted many potential ramifications on patient and consumer protections in the health insurance market. For instance, courts could vacate current rules governing protections under the Affordable Care Act, including that health plans offer a range of free preventive health services, such as breast, cervical, colon, and lung cancer screening.
Congressional, White House Reaction
Many legal observers said the ruling will have the effect of requiring Congress to write ever-more dense and exacting legislation to prevent agencies from interpreting any gaps.
Some members of Congress welcomed the decision.
Senate Minority Leader Mitch McConnell (R-Kentucky) said in a statement, “The Constitution vests Congress with the sole authority to make law,” adding, “the Supreme Court made it clear today that our system of government leaves no room for an unelected bureaucracy to co-opt this authority for itself.”
In a post on X, Senate Majority Leader Chuck Schumer (D-New York) accused the Court of siding with “special interests and giant corporations.” Added Mr. Schumer, “Their headlong rush to overturn 40 years of precedent and impose their own radical views is appalling.”
White House Press Secretary Karine Jean-Pierre said in a statement that “While this decision undermines the ability of federal agencies to use their expertise as Congress intended to make government work for the people, the Biden-Harris Administration will not relent in our efforts to protect and serve every American.”
A version of this article first appeared on Medscape.com.
EMA Greenlights Four Drugs for Bladder and Other Cancers
Balversa
The CHMP endorsed the approval of Balversa (erdafitinib, Janssen-Cilag International N.V.), intended for the treatment of urothelial carcinoma, a type of cancer affecting the bladder and urinary system.
As a monotherapy, Balversa is indicated for the treatment of adult patients with unresectable or metastatic urothelial carcinoma harboring susceptible FGFR3 genetic alterations. These patients must have previously received at least one line of therapy containing a programmed death receptor 1 (PD-1) or programmed death-ligand 1 (PD-L1) inhibitor in the unresectable or metastatic treatment setting.
Urothelial carcinoma is the most common form of bladder cancer, the ninth most frequently diagnosed cancer worldwide. In 2022, there were approximately 614,000 new cases of bladder cancer and 220,000 deaths globally.
The highest incidence rates in both men and women are found in Southern Europe. Greece had 5800 new cases and 1537 deaths in 2018. Spain has the highest incidence rate in men globally. Since the 1990s, bladder cancer incidence trends have diverged by sex, with rates decreasing or stabilizing in men but increasing among women in certain European countries.
The CHMP recommendation is based on data from cohort 1 of the phase 3 THOR trial, which compared erdafitinib with standard-of-care chemotherapy (investigator’s choice of docetaxel or vinflunine). Cohort 1 included 266 adults with advanced urothelial cancer harboring selected FGFR3 alterations.
All patients had disease progression after one or two prior treatments, at least one of which included a PD-1 or PD-L1 inhibitor. The major efficacy endpoints were overall survival, progression free survival, and objective response rate (ORR).
Treatment with erdafitinib reduced the risk for death by 36% compared with chemotherapy (hazard ratio [HR], 0.64; P = .005). Median overall survival was 12.1 months in the erdafitinib arm vs 7.8 months in the chemotherapy arm. Median progression-free survival was 5.6 months in the erdafitinib arm vs 2.7 months in the chemotherapy arm (HR, 0.58; P = .0002). ORR was 35.3% with erdafitinib compared with 8.5% with chemotherapy.
Balversa will be available as 3-mg, 4-mg, and 5-mg film-coated tablets. Erdafitinib, the active substance in Balversa, is an antineoplastic protein kinase inhibitor that suppresses fibroblast growth factor receptor (FGFR) tyrosine kinases. Deregulation of FGFR3 signaling is implicated in the pathogenesis of urothelial cancer, and FGFR inhibition has demonstrated antitumor activity in FGFR-expressing cells.
Ordspono
The committee adopted a positive opinion for Ordspono (odronextamab, Regeneron Ireland Designated Activity Company), indicated as a monotherapy for the treatment of adult patients with:
- Relapsed or refractory follicular lymphoma (rrFL), after two or more lines of systemic therapy.
- Relapsed or refractory diffuse large B-cell lymphoma (rrDLBCL), after two or more lines of systemic therapy.
The approval recommendation is based on phase 2 trials (NCT02290951, NCT03888105), which demonstrated high ORRs in patients with rrFL and rrDLBCL.
In the DLBCL cohort, a 49% ORR was achieved in heavily pretreated patients who had not received chimeric antigen receptor T-cell therapy. A total of 31% achieved a complete response.
The FL cohort showed an 82% response rate in patients with grades I-IIIA disease, with 75% of the overall population achieving a complete response.
Ordspono will be available as a 2-mg, 80-mg, and 320-mg concentrate for solution for infusion. The active substance of Ordspono is odronextamab, a bispecific antibody that targets CD20-expressing B cells and CD3-expressing T cells. By binding to both, it induces T-cell activation and generates a polyclonal cytotoxic T-cell response, leading to the lysis of malignant B cells.
Generics
The panel also adopted positive opinions for two generic cancer medicines.
Enzalutamide Viatris (enzalutamide) is indicated for the treatment of adult men with prostate cancer in several scenarios:
- As monotherapy or with androgen-deprivation therapy for high-risk biochemical recurrent nonmetastatic hormone-sensitive prostate cancer in men unsuitable for salvage-radiotherapy.
- In combination with androgen-deprivation therapy for metastatic hormone-sensitive prostate cancer.
- For high-risk nonmetastatic castration-resistant prostate cancer (CRPC).
- For metastatic CRPC in men who are asymptomatic or mildly symptomatic after failure of androgen-deprivation therapy, where chemotherapy is not yet indicated.
- For metastatic CRPC in men whose disease has progressed on or after docetaxel therapy.
Enzalutamide Viatris is a generic version of Xtandi, authorized in the European Union since June 2013. Studies have confirmed the satisfactory quality and bioequivalence of Enzalutamide Viatris to Xtandi.
Enzalutamide Viatris will be available as 40-mg and 80-mg film-coated tablets. The active substance of Enzalutamide Viatris is enzalutamide, a hormone antagonist that blocks multiple steps in the androgen receptor–signaling pathway.
Nilotinib Accord (nilotinib) is indicated for the treatment of Philadelphia chromosome–positive chronic myelogenous leukemia (CML).
It is used in adult and pediatric patients with newly diagnosed CML in the chronic phase, adult patients with chronic phase and accelerated phase CML with resistance or intolerance to prior therapy including imatinib, and pediatric patients with CML with resistance or intolerance to prior therapy including imatinib.
Nilotinib Accord is a generic of Tasigna, authorized in the European Union since November 2007. Studies have demonstrated the satisfactory quality and bioequivalence of Nilotinib Accord to Tasigna.
Nilotinib Accord will be available as 50-mg, 150-mg, and 200-mg hard capsules. The active substance of Nilotinib Accord is nilotinib, an antineoplastic protein kinase inhibitor that targets BCR-ABL kinase and other oncogenic kinases.
A version of this article appeared on Medscape.com.
Balversa
The CHMP endorsed the approval of Balversa (erdafitinib, Janssen-Cilag International N.V.), intended for the treatment of urothelial carcinoma, a type of cancer affecting the bladder and urinary system.
As a monotherapy, Balversa is indicated for the treatment of adult patients with unresectable or metastatic urothelial carcinoma harboring susceptible FGFR3 genetic alterations. These patients must have previously received at least one line of therapy containing a programmed death receptor 1 (PD-1) or programmed death-ligand 1 (PD-L1) inhibitor in the unresectable or metastatic treatment setting.
Urothelial carcinoma is the most common form of bladder cancer, the ninth most frequently diagnosed cancer worldwide. In 2022, there were approximately 614,000 new cases of bladder cancer and 220,000 deaths globally.
The highest incidence rates in both men and women are found in Southern Europe. Greece had 5800 new cases and 1537 deaths in 2018. Spain has the highest incidence rate in men globally. Since the 1990s, bladder cancer incidence trends have diverged by sex, with rates decreasing or stabilizing in men but increasing among women in certain European countries.
The CHMP recommendation is based on data from cohort 1 of the phase 3 THOR trial, which compared erdafitinib with standard-of-care chemotherapy (investigator’s choice of docetaxel or vinflunine). Cohort 1 included 266 adults with advanced urothelial cancer harboring selected FGFR3 alterations.
All patients had disease progression after one or two prior treatments, at least one of which included a PD-1 or PD-L1 inhibitor. The major efficacy endpoints were overall survival, progression free survival, and objective response rate (ORR).
Treatment with erdafitinib reduced the risk for death by 36% compared with chemotherapy (hazard ratio [HR], 0.64; P = .005). Median overall survival was 12.1 months in the erdafitinib arm vs 7.8 months in the chemotherapy arm. Median progression-free survival was 5.6 months in the erdafitinib arm vs 2.7 months in the chemotherapy arm (HR, 0.58; P = .0002). ORR was 35.3% with erdafitinib compared with 8.5% with chemotherapy.
Balversa will be available as 3-mg, 4-mg, and 5-mg film-coated tablets. Erdafitinib, the active substance in Balversa, is an antineoplastic protein kinase inhibitor that suppresses fibroblast growth factor receptor (FGFR) tyrosine kinases. Deregulation of FGFR3 signaling is implicated in the pathogenesis of urothelial cancer, and FGFR inhibition has demonstrated antitumor activity in FGFR-expressing cells.
Ordspono
The committee adopted a positive opinion for Ordspono (odronextamab, Regeneron Ireland Designated Activity Company), indicated as a monotherapy for the treatment of adult patients with:
- Relapsed or refractory follicular lymphoma (rrFL), after two or more lines of systemic therapy.
- Relapsed or refractory diffuse large B-cell lymphoma (rrDLBCL), after two or more lines of systemic therapy.
The approval recommendation is based on phase 2 trials (NCT02290951, NCT03888105), which demonstrated high ORRs in patients with rrFL and rrDLBCL.
In the DLBCL cohort, a 49% ORR was achieved in heavily pretreated patients who had not received chimeric antigen receptor T-cell therapy. A total of 31% achieved a complete response.
The FL cohort showed an 82% response rate in patients with grades I-IIIA disease, with 75% of the overall population achieving a complete response.
Ordspono will be available as a 2-mg, 80-mg, and 320-mg concentrate for solution for infusion. The active substance of Ordspono is odronextamab, a bispecific antibody that targets CD20-expressing B cells and CD3-expressing T cells. By binding to both, it induces T-cell activation and generates a polyclonal cytotoxic T-cell response, leading to the lysis of malignant B cells.
Generics
The panel also adopted positive opinions for two generic cancer medicines.
Enzalutamide Viatris (enzalutamide) is indicated for the treatment of adult men with prostate cancer in several scenarios:
- As monotherapy or with androgen-deprivation therapy for high-risk biochemical recurrent nonmetastatic hormone-sensitive prostate cancer in men unsuitable for salvage-radiotherapy.
- In combination with androgen-deprivation therapy for metastatic hormone-sensitive prostate cancer.
- For high-risk nonmetastatic castration-resistant prostate cancer (CRPC).
- For metastatic CRPC in men who are asymptomatic or mildly symptomatic after failure of androgen-deprivation therapy, where chemotherapy is not yet indicated.
- For metastatic CRPC in men whose disease has progressed on or after docetaxel therapy.
Enzalutamide Viatris is a generic version of Xtandi, authorized in the European Union since June 2013. Studies have confirmed the satisfactory quality and bioequivalence of Enzalutamide Viatris to Xtandi.
Enzalutamide Viatris will be available as 40-mg and 80-mg film-coated tablets. The active substance of Enzalutamide Viatris is enzalutamide, a hormone antagonist that blocks multiple steps in the androgen receptor–signaling pathway.
Nilotinib Accord (nilotinib) is indicated for the treatment of Philadelphia chromosome–positive chronic myelogenous leukemia (CML).
It is used in adult and pediatric patients with newly diagnosed CML in the chronic phase, adult patients with chronic phase and accelerated phase CML with resistance or intolerance to prior therapy including imatinib, and pediatric patients with CML with resistance or intolerance to prior therapy including imatinib.
Nilotinib Accord is a generic of Tasigna, authorized in the European Union since November 2007. Studies have demonstrated the satisfactory quality and bioequivalence of Nilotinib Accord to Tasigna.
Nilotinib Accord will be available as 50-mg, 150-mg, and 200-mg hard capsules. The active substance of Nilotinib Accord is nilotinib, an antineoplastic protein kinase inhibitor that targets BCR-ABL kinase and other oncogenic kinases.
A version of this article appeared on Medscape.com.
Balversa
The CHMP endorsed the approval of Balversa (erdafitinib, Janssen-Cilag International N.V.), intended for the treatment of urothelial carcinoma, a type of cancer affecting the bladder and urinary system.
As a monotherapy, Balversa is indicated for the treatment of adult patients with unresectable or metastatic urothelial carcinoma harboring susceptible FGFR3 genetic alterations. These patients must have previously received at least one line of therapy containing a programmed death receptor 1 (PD-1) or programmed death-ligand 1 (PD-L1) inhibitor in the unresectable or metastatic treatment setting.
Urothelial carcinoma is the most common form of bladder cancer, the ninth most frequently diagnosed cancer worldwide. In 2022, there were approximately 614,000 new cases of bladder cancer and 220,000 deaths globally.
The highest incidence rates in both men and women are found in Southern Europe. Greece had 5800 new cases and 1537 deaths in 2018. Spain has the highest incidence rate in men globally. Since the 1990s, bladder cancer incidence trends have diverged by sex, with rates decreasing or stabilizing in men but increasing among women in certain European countries.
The CHMP recommendation is based on data from cohort 1 of the phase 3 THOR trial, which compared erdafitinib with standard-of-care chemotherapy (investigator’s choice of docetaxel or vinflunine). Cohort 1 included 266 adults with advanced urothelial cancer harboring selected FGFR3 alterations.
All patients had disease progression after one or two prior treatments, at least one of which included a PD-1 or PD-L1 inhibitor. The major efficacy endpoints were overall survival, progression free survival, and objective response rate (ORR).
Treatment with erdafitinib reduced the risk for death by 36% compared with chemotherapy (hazard ratio [HR], 0.64; P = .005). Median overall survival was 12.1 months in the erdafitinib arm vs 7.8 months in the chemotherapy arm. Median progression-free survival was 5.6 months in the erdafitinib arm vs 2.7 months in the chemotherapy arm (HR, 0.58; P = .0002). ORR was 35.3% with erdafitinib compared with 8.5% with chemotherapy.
Balversa will be available as 3-mg, 4-mg, and 5-mg film-coated tablets. Erdafitinib, the active substance in Balversa, is an antineoplastic protein kinase inhibitor that suppresses fibroblast growth factor receptor (FGFR) tyrosine kinases. Deregulation of FGFR3 signaling is implicated in the pathogenesis of urothelial cancer, and FGFR inhibition has demonstrated antitumor activity in FGFR-expressing cells.
Ordspono
The committee adopted a positive opinion for Ordspono (odronextamab, Regeneron Ireland Designated Activity Company), indicated as a monotherapy for the treatment of adult patients with:
- Relapsed or refractory follicular lymphoma (rrFL), after two or more lines of systemic therapy.
- Relapsed or refractory diffuse large B-cell lymphoma (rrDLBCL), after two or more lines of systemic therapy.
The approval recommendation is based on phase 2 trials (NCT02290951, NCT03888105), which demonstrated high ORRs in patients with rrFL and rrDLBCL.
In the DLBCL cohort, a 49% ORR was achieved in heavily pretreated patients who had not received chimeric antigen receptor T-cell therapy. A total of 31% achieved a complete response.
The FL cohort showed an 82% response rate in patients with grades I-IIIA disease, with 75% of the overall population achieving a complete response.
Ordspono will be available as a 2-mg, 80-mg, and 320-mg concentrate for solution for infusion. The active substance of Ordspono is odronextamab, a bispecific antibody that targets CD20-expressing B cells and CD3-expressing T cells. By binding to both, it induces T-cell activation and generates a polyclonal cytotoxic T-cell response, leading to the lysis of malignant B cells.
Generics
The panel also adopted positive opinions for two generic cancer medicines.
Enzalutamide Viatris (enzalutamide) is indicated for the treatment of adult men with prostate cancer in several scenarios:
- As monotherapy or with androgen-deprivation therapy for high-risk biochemical recurrent nonmetastatic hormone-sensitive prostate cancer in men unsuitable for salvage-radiotherapy.
- In combination with androgen-deprivation therapy for metastatic hormone-sensitive prostate cancer.
- For high-risk nonmetastatic castration-resistant prostate cancer (CRPC).
- For metastatic CRPC in men who are asymptomatic or mildly symptomatic after failure of androgen-deprivation therapy, where chemotherapy is not yet indicated.
- For metastatic CRPC in men whose disease has progressed on or after docetaxel therapy.
Enzalutamide Viatris is a generic version of Xtandi, authorized in the European Union since June 2013. Studies have confirmed the satisfactory quality and bioequivalence of Enzalutamide Viatris to Xtandi.
Enzalutamide Viatris will be available as 40-mg and 80-mg film-coated tablets. The active substance of Enzalutamide Viatris is enzalutamide, a hormone antagonist that blocks multiple steps in the androgen receptor–signaling pathway.
Nilotinib Accord (nilotinib) is indicated for the treatment of Philadelphia chromosome–positive chronic myelogenous leukemia (CML).
It is used in adult and pediatric patients with newly diagnosed CML in the chronic phase, adult patients with chronic phase and accelerated phase CML with resistance or intolerance to prior therapy including imatinib, and pediatric patients with CML with resistance or intolerance to prior therapy including imatinib.
Nilotinib Accord is a generic of Tasigna, authorized in the European Union since November 2007. Studies have demonstrated the satisfactory quality and bioequivalence of Nilotinib Accord to Tasigna.
Nilotinib Accord will be available as 50-mg, 150-mg, and 200-mg hard capsules. The active substance of Nilotinib Accord is nilotinib, an antineoplastic protein kinase inhibitor that targets BCR-ABL kinase and other oncogenic kinases.
A version of this article appeared on Medscape.com.
Two Techniques to Avoid Cyst Spray During Excision
Practice Gap
Epidermoid cysts are asymptomatic, well-circumscribed, mobile, subcutaneous masses that elevate the skin. Also known as epidermal, keratin, or infundibular cysts, epidermoid cysts are caused by proliferation of surface epidermoid cells within the dermis and can arise anywhere on the body, most commonly on the face, neck, and trunk.1 Cutaneous cysts often contain fluid or semifluid contents and can be aesthetically displeasing or cause mild pain, prompting patients to seek removal. Definitive treatment of epidermoid cysts is complete surgical removal,2 which can be performed in office in a sterile or clean manner by either dermatologists or primary care providers.
Prior to incision, a local anesthetic—commonly lidocaine with epinephrine—is injected in the region surrounding the cyst sac so as not to rupture the cyst wall. Maintaining the cyst wall throughout the procedure ensures total cyst removal and minimizes the risk for recurrence. However, it often is difficult to approximate the cyst border because it cannot be visualized prior to incision.
Throughout the duration of the procedure, cyst contents may suddenly spray out of the area and pose a risk to providers and their staff (Figure, A). Even with careful application around the periphery, either puncture or pericystic anesthesia between the cyst wall and the dermis can lead to splatter. Larger and wider peripheral anesthesia may not be possible given a shortage of lidocaine and a desire to minimize injection. Even with meticulous use of personal protective equipment in cutaneous surgery, infectious organisms found in ruptured cysts and abscesses may spray the surgical field.3 Therefore, it is in our best interest to minimize the trajectory of cyst spray contents.
The Tools
We have employed 2 simple techniques using equipment normally found on a standard surgical tray for easy safe injection of cysts. Supplies needed include 4×4-inch gauze pads, alcohol and chlorhexidine, a marker, all instruments necessary for cyst excision, and a small clear biohazard bag.
The Technique
Prior to covering the cyst, care is taken to locate the cyst opening. At times, a comedo or punctum can be seen overlying the cyst bulge. We mark the lumen and cyst opening with a surgical marker. If the pore is not easily identified, we draw an 8-mm circle around the mound of the cyst.
One option is to apply a gauze pad over the cyst to allow for stabilization of the surgical field and blanket the area from splatter (Figure, B). Then we cover the cyst using antiseptic-soaked gauze as a protective barrier to avoid potentially contaminated spray. This tool can be constructed from a 4×4-inch gauze pad with the addition of alcohol and chlorhexidine. When the cyst is covered, the surgeon can inject the lesion and surrounding tissue without biohazard splatter.
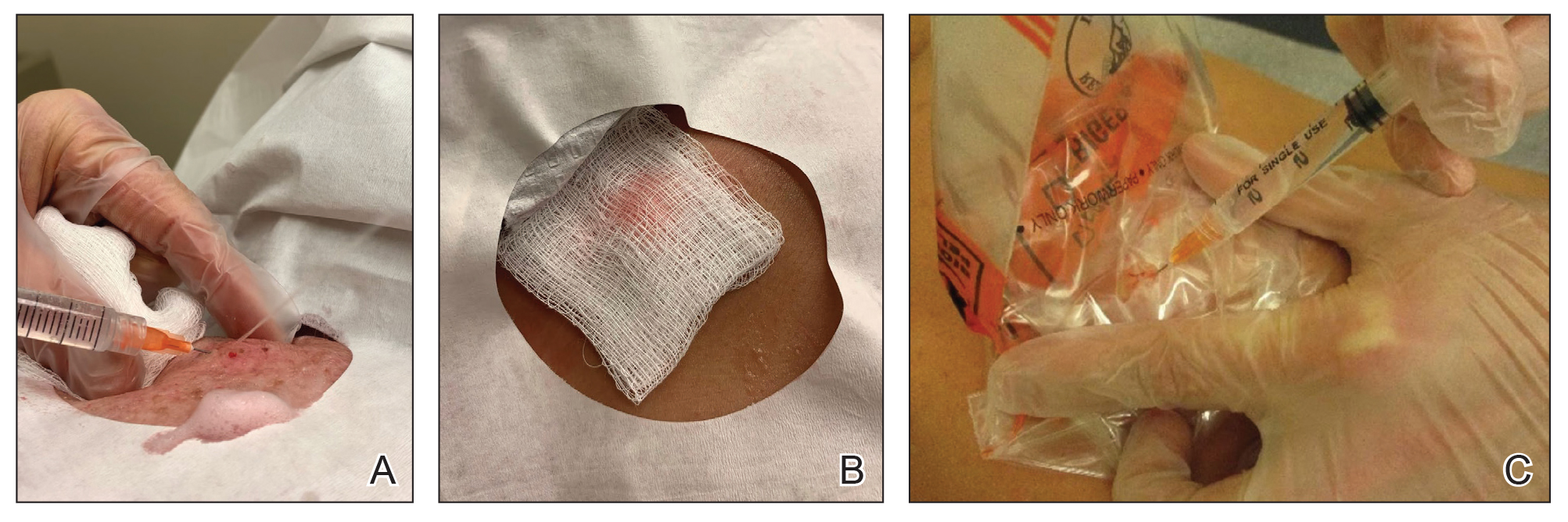
Another method is to cover the cyst with a small clear biohazard bag (Figure, C). When injecting anesthetic through the bag, the spray is captured by the bag and does not reach the surgeon or staff. This method is potentially more effective given that the cyst can still be visualized fully for more accurate injection.
Practice Implications
Outpatient surgical excision is a common effective procedure for epidermoid cysts. However, it is not uncommon for cyst contents to spray during the injection of anesthetic, posing a nuisance to the surgeon, health care staff, and patient. The technique of covering the lesion with antiseptic-soaked gauze or a small clear biohazard bag prevents cyst contents from spraying and reduces risk for contamination. In addition to these protective benefits, the use of readily available items replaces the need to order a splatter control shield.
Limitations—Although we seldom see spray using our technique, covering the cyst with gauze may disguise the region of interest and interfere with accurate incision. Marking the lesion prior to anesthesia administration or using a clear biohazard bag minimizes difficulty visualizing the cyst opening.
- Zito PM, Scharf R. Epidermoid cyst. StatPearls [Internet]. Updated August 8, 2023. Accessed June 13, 2024. https://www.ncbi.nlm.nih.gov/books/NBK499974
- Weir CB, St. Hilaire NJ. Epidermal inclusion cyst. StatPearls [Internet]. Updated August 8, 2023. Accessed June3, 2024. https://www.ncbi.nlm.nih.gov/books/NBK532310/
- Kuniyuki S, Yoshida Y, Maekawa N, et al. Bacteriological study of epidermal cysts. Acta Derm Venereol. 2018;88:23-25. doi:10.2340/00015555-0348
Practice Gap
Epidermoid cysts are asymptomatic, well-circumscribed, mobile, subcutaneous masses that elevate the skin. Also known as epidermal, keratin, or infundibular cysts, epidermoid cysts are caused by proliferation of surface epidermoid cells within the dermis and can arise anywhere on the body, most commonly on the face, neck, and trunk.1 Cutaneous cysts often contain fluid or semifluid contents and can be aesthetically displeasing or cause mild pain, prompting patients to seek removal. Definitive treatment of epidermoid cysts is complete surgical removal,2 which can be performed in office in a sterile or clean manner by either dermatologists or primary care providers.
Prior to incision, a local anesthetic—commonly lidocaine with epinephrine—is injected in the region surrounding the cyst sac so as not to rupture the cyst wall. Maintaining the cyst wall throughout the procedure ensures total cyst removal and minimizes the risk for recurrence. However, it often is difficult to approximate the cyst border because it cannot be visualized prior to incision.
Throughout the duration of the procedure, cyst contents may suddenly spray out of the area and pose a risk to providers and their staff (Figure, A). Even with careful application around the periphery, either puncture or pericystic anesthesia between the cyst wall and the dermis can lead to splatter. Larger and wider peripheral anesthesia may not be possible given a shortage of lidocaine and a desire to minimize injection. Even with meticulous use of personal protective equipment in cutaneous surgery, infectious organisms found in ruptured cysts and abscesses may spray the surgical field.3 Therefore, it is in our best interest to minimize the trajectory of cyst spray contents.
The Tools
We have employed 2 simple techniques using equipment normally found on a standard surgical tray for easy safe injection of cysts. Supplies needed include 4×4-inch gauze pads, alcohol and chlorhexidine, a marker, all instruments necessary for cyst excision, and a small clear biohazard bag.
The Technique
Prior to covering the cyst, care is taken to locate the cyst opening. At times, a comedo or punctum can be seen overlying the cyst bulge. We mark the lumen and cyst opening with a surgical marker. If the pore is not easily identified, we draw an 8-mm circle around the mound of the cyst.
One option is to apply a gauze pad over the cyst to allow for stabilization of the surgical field and blanket the area from splatter (Figure, B). Then we cover the cyst using antiseptic-soaked gauze as a protective barrier to avoid potentially contaminated spray. This tool can be constructed from a 4×4-inch gauze pad with the addition of alcohol and chlorhexidine. When the cyst is covered, the surgeon can inject the lesion and surrounding tissue without biohazard splatter.

Another method is to cover the cyst with a small clear biohazard bag (Figure, C). When injecting anesthetic through the bag, the spray is captured by the bag and does not reach the surgeon or staff. This method is potentially more effective given that the cyst can still be visualized fully for more accurate injection.
Practice Implications
Outpatient surgical excision is a common effective procedure for epidermoid cysts. However, it is not uncommon for cyst contents to spray during the injection of anesthetic, posing a nuisance to the surgeon, health care staff, and patient. The technique of covering the lesion with antiseptic-soaked gauze or a small clear biohazard bag prevents cyst contents from spraying and reduces risk for contamination. In addition to these protective benefits, the use of readily available items replaces the need to order a splatter control shield.
Limitations—Although we seldom see spray using our technique, covering the cyst with gauze may disguise the region of interest and interfere with accurate incision. Marking the lesion prior to anesthesia administration or using a clear biohazard bag minimizes difficulty visualizing the cyst opening.
Practice Gap
Epidermoid cysts are asymptomatic, well-circumscribed, mobile, subcutaneous masses that elevate the skin. Also known as epidermal, keratin, or infundibular cysts, epidermoid cysts are caused by proliferation of surface epidermoid cells within the dermis and can arise anywhere on the body, most commonly on the face, neck, and trunk.1 Cutaneous cysts often contain fluid or semifluid contents and can be aesthetically displeasing or cause mild pain, prompting patients to seek removal. Definitive treatment of epidermoid cysts is complete surgical removal,2 which can be performed in office in a sterile or clean manner by either dermatologists or primary care providers.
Prior to incision, a local anesthetic—commonly lidocaine with epinephrine—is injected in the region surrounding the cyst sac so as not to rupture the cyst wall. Maintaining the cyst wall throughout the procedure ensures total cyst removal and minimizes the risk for recurrence. However, it often is difficult to approximate the cyst border because it cannot be visualized prior to incision.
Throughout the duration of the procedure, cyst contents may suddenly spray out of the area and pose a risk to providers and their staff (Figure, A). Even with careful application around the periphery, either puncture or pericystic anesthesia between the cyst wall and the dermis can lead to splatter. Larger and wider peripheral anesthesia may not be possible given a shortage of lidocaine and a desire to minimize injection. Even with meticulous use of personal protective equipment in cutaneous surgery, infectious organisms found in ruptured cysts and abscesses may spray the surgical field.3 Therefore, it is in our best interest to minimize the trajectory of cyst spray contents.
The Tools
We have employed 2 simple techniques using equipment normally found on a standard surgical tray for easy safe injection of cysts. Supplies needed include 4×4-inch gauze pads, alcohol and chlorhexidine, a marker, all instruments necessary for cyst excision, and a small clear biohazard bag.
The Technique
Prior to covering the cyst, care is taken to locate the cyst opening. At times, a comedo or punctum can be seen overlying the cyst bulge. We mark the lumen and cyst opening with a surgical marker. If the pore is not easily identified, we draw an 8-mm circle around the mound of the cyst.
One option is to apply a gauze pad over the cyst to allow for stabilization of the surgical field and blanket the area from splatter (Figure, B). Then we cover the cyst using antiseptic-soaked gauze as a protective barrier to avoid potentially contaminated spray. This tool can be constructed from a 4×4-inch gauze pad with the addition of alcohol and chlorhexidine. When the cyst is covered, the surgeon can inject the lesion and surrounding tissue without biohazard splatter.

Another method is to cover the cyst with a small clear biohazard bag (Figure, C). When injecting anesthetic through the bag, the spray is captured by the bag and does not reach the surgeon or staff. This method is potentially more effective given that the cyst can still be visualized fully for more accurate injection.
Practice Implications
Outpatient surgical excision is a common effective procedure for epidermoid cysts. However, it is not uncommon for cyst contents to spray during the injection of anesthetic, posing a nuisance to the surgeon, health care staff, and patient. The technique of covering the lesion with antiseptic-soaked gauze or a small clear biohazard bag prevents cyst contents from spraying and reduces risk for contamination. In addition to these protective benefits, the use of readily available items replaces the need to order a splatter control shield.
Limitations—Although we seldom see spray using our technique, covering the cyst with gauze may disguise the region of interest and interfere with accurate incision. Marking the lesion prior to anesthesia administration or using a clear biohazard bag minimizes difficulty visualizing the cyst opening.
- Zito PM, Scharf R. Epidermoid cyst. StatPearls [Internet]. Updated August 8, 2023. Accessed June 13, 2024. https://www.ncbi.nlm.nih.gov/books/NBK499974
- Weir CB, St. Hilaire NJ. Epidermal inclusion cyst. StatPearls [Internet]. Updated August 8, 2023. Accessed June3, 2024. https://www.ncbi.nlm.nih.gov/books/NBK532310/
- Kuniyuki S, Yoshida Y, Maekawa N, et al. Bacteriological study of epidermal cysts. Acta Derm Venereol. 2018;88:23-25. doi:10.2340/00015555-0348
- Zito PM, Scharf R. Epidermoid cyst. StatPearls [Internet]. Updated August 8, 2023. Accessed June 13, 2024. https://www.ncbi.nlm.nih.gov/books/NBK499974
- Weir CB, St. Hilaire NJ. Epidermal inclusion cyst. StatPearls [Internet]. Updated August 8, 2023. Accessed June3, 2024. https://www.ncbi.nlm.nih.gov/books/NBK532310/
- Kuniyuki S, Yoshida Y, Maekawa N, et al. Bacteriological study of epidermal cysts. Acta Derm Venereol. 2018;88:23-25. doi:10.2340/00015555-0348
Children on Medicaid With Asthma Receive Less Specialty Care
Primary care clinicians successfully manage many children with asthma, but data on specialist care according to insurance coverage are lacking, wrote Kimberley H. Geissler, PhD, of the University of Massachusetts Chan Medical School–Baystate, Springfield, Massachusetts, and colleagues.
Despite many interventions over time, “low-income children insured by Medicaid, many of whom are from minoritized racial and ethnic groups, continue to have worse outcomes and higher rates of poorly controlled asthma than children who are privately insured,” Dr. Geissler said in an interview.
“Because differences in whether a child sees an asthma specialist could contribute to these disparities, better understanding specialist use among both groups of kids may help inform potential solutions,” she said.
In a study published in JAMA Network Open, the researchers identified children with asthma aged 2-17 years using data from the Massachusetts All-Payer Claims Database for the years 2015-2020. The study population included 198,101 children and 432,455 child-year observations from children with asthma during a year when they met at least one of three criteria with any asthma diagnosis: One or more hospital visits, two or more outpatient visits, or at least one outpatient visit and at least one asthma medication.
Outpatient Visit Outcome
The primary outcome of asthma specialist care was defined as at least one outpatient visit with any asthma diagnosis to a clinician with a code of allergy and immunology, pulmonology, or otolaryngology.
A total of 66.2% of the child-year observations involved Medicaid and 33.8% involved private insurance. Approximately 15% of the children received asthma specialist care. However, nearly twice as many children with private insurance received asthma specialty care compared with those with Medicaid (20.6% vs 11.9%). In a full logistic regression analysis, children with Medicaid insurance were 55% less likely to receive asthma specialist treatment than children with private insurance.
Allergy and immunology was the most common specialty used, and the child-years for this specialty among children with Medicaid were less than half of those among children with private insurance (7.1% vs 15.9%).
Rates of persistent asthma were 20.0% and 16.9% in children with Medicaid and private insurance, respectively. Overall, children with persistent asthma were nearly four times as likely to receive asthma specialist care (adjusted odds ratio, 3.96). However, the difference in odds of receiving specialty care based on insurance type in favor of private insurance was greater among children with persistent asthma than among those without persistent asthma (−24.0 percentage points vs −20.8 percentage points).
The researchers found a similar pattern of difference in asthma specialty care in a sensitivity analysis limiting the results to child-year observations with at least one outpatient visit with any asthma diagnosis in a calendar year, although they also found a slight narrowing of the difference between the groups over time.
“Contrary to expectations, disparities in specialist care by insurance type were even more striking in children with persistent asthma,” the researchers wrote in their discussion. Notably, the growth of specialty drugs such as biologics for moderate to severe asthma are mainly prescribed by specialists, and ensuring access to specialists for children with Medicaid may reduce disparities in asthma control for those with severe or poorly controlled disease, they added.
The study findings were limited by several factors including the use only of data from Massachusetts, which may not generalize to other states, and the use of completed specialist visits without data on referrals, the researchers noted. Other limitations included a lack of data on asthma symptom frequency or control and on the setting in which an asthma diagnosis was made.
However, the results suggest a need for more attention to disparities in asthma care by insurance type, and more research is needed to determine whether these disparities persist in subsets of children with asthma, such as those with allergies or chronic medical conditions, they concluded.
Takeaways and Next Steps
“Perhaps unsurprisingly, children with private insurance were more likely to receive asthma specialist care than children with Medicaid,” Dr. Geissler told this news organization. The researchers expected a smaller gap between insurance types among children with persistent asthma, a marker for asthma severity, she said. However, “we found that the gap between those with Medicaid and those with private insurance is actually larger” for children with persistent asthma, she added.
As improved treatments for hard-to-control asthma become more available, pediatricians and primary care clinicians should follow the latest clinical guidelines for referring children to specialists for asthma care, said Dr. Geissler.
“Additionally, asthma specialists should ensure that their practices are accessible to children with Medicaid, as these families may face higher barriers to care; for example, transportation needs or scheduling challenges,” she said. Other strategies to overcome barriers to care might include electronic consultations with specialists or primary care–oriented interdisciplinary asthma clinics, which may be useful for all children with asthma but may particularly benefit those insured by Medicaid, she noted.
“Based on data limitations, we could not examine why we observed such big differences in specialist use by insurance type; for example, whether pediatricians were referring to specialists less for Medicaid-insured kids, or whether kids with Medicaid were less likely to see a specialist after a referral was made,” Dr. Geissler said. More research is needed to examine not only these factors but also the appropriateness of specialty care based on clinical guidelines to ensure high-quality evidence-based care for children with asthma who are insured by Medicaid, she said.
Improve Access and Expand Analysis
Asthma is a chronic and prevalent disease and requires a comprehensive approach that sometimes calls for specialist care, Anne Coates, MD, a pediatric pulmonologist in Portland, Maine, said in an interview.
Dr. Coates said she was surprised by the results of the current study but commended the authors for highlighting the limitations of the study, which illustrate areas for additional research. Notably, “the authors couldn’t observe referrals to specialists from primary care physicians; they used completed visits as a proxy,” Dr. Coates said.
More studies are needed to assess the completion of referral visits regardless of children’s insurance in order to better understand and address the barriers to specialty care, she added.
The current study is important because of the extent of asthma coupled with the significant number of children across the United States who are insured by Medicaid, especially underserved populations, she said.
“The burden of asthma differentially affects people of color who are living in lower resourced areas, and it is important in further research to understanding the barriers to helping people get the care they need,” Dr. Coates told this news organization. Some alternatives might include telehealth visits or even a hybrid visit to a primary care provider (PCP) who has high-speed internet, and the specialist could then conduct a telehealth visit from the PCP’s office, with the PCP acting as on-site eyes and ears, said Dr. Coates, who has used this strategy in her practice in Maine, where many patients live far from specialist care.
The study was supported by the National Heart, Lung, and Blood Institute and the University of Massachusetts Center for Clinical and Translational Science-Biostatistics, Epidemiology & Research Design Component. Dr. Geissler and Dr. Coates had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Primary care clinicians successfully manage many children with asthma, but data on specialist care according to insurance coverage are lacking, wrote Kimberley H. Geissler, PhD, of the University of Massachusetts Chan Medical School–Baystate, Springfield, Massachusetts, and colleagues.
Despite many interventions over time, “low-income children insured by Medicaid, many of whom are from minoritized racial and ethnic groups, continue to have worse outcomes and higher rates of poorly controlled asthma than children who are privately insured,” Dr. Geissler said in an interview.
“Because differences in whether a child sees an asthma specialist could contribute to these disparities, better understanding specialist use among both groups of kids may help inform potential solutions,” she said.
In a study published in JAMA Network Open, the researchers identified children with asthma aged 2-17 years using data from the Massachusetts All-Payer Claims Database for the years 2015-2020. The study population included 198,101 children and 432,455 child-year observations from children with asthma during a year when they met at least one of three criteria with any asthma diagnosis: One or more hospital visits, two or more outpatient visits, or at least one outpatient visit and at least one asthma medication.
Outpatient Visit Outcome
The primary outcome of asthma specialist care was defined as at least one outpatient visit with any asthma diagnosis to a clinician with a code of allergy and immunology, pulmonology, or otolaryngology.
A total of 66.2% of the child-year observations involved Medicaid and 33.8% involved private insurance. Approximately 15% of the children received asthma specialist care. However, nearly twice as many children with private insurance received asthma specialty care compared with those with Medicaid (20.6% vs 11.9%). In a full logistic regression analysis, children with Medicaid insurance were 55% less likely to receive asthma specialist treatment than children with private insurance.
Allergy and immunology was the most common specialty used, and the child-years for this specialty among children with Medicaid were less than half of those among children with private insurance (7.1% vs 15.9%).
Rates of persistent asthma were 20.0% and 16.9% in children with Medicaid and private insurance, respectively. Overall, children with persistent asthma were nearly four times as likely to receive asthma specialist care (adjusted odds ratio, 3.96). However, the difference in odds of receiving specialty care based on insurance type in favor of private insurance was greater among children with persistent asthma than among those without persistent asthma (−24.0 percentage points vs −20.8 percentage points).
The researchers found a similar pattern of difference in asthma specialty care in a sensitivity analysis limiting the results to child-year observations with at least one outpatient visit with any asthma diagnosis in a calendar year, although they also found a slight narrowing of the difference between the groups over time.
“Contrary to expectations, disparities in specialist care by insurance type were even more striking in children with persistent asthma,” the researchers wrote in their discussion. Notably, the growth of specialty drugs such as biologics for moderate to severe asthma are mainly prescribed by specialists, and ensuring access to specialists for children with Medicaid may reduce disparities in asthma control for those with severe or poorly controlled disease, they added.
The study findings were limited by several factors including the use only of data from Massachusetts, which may not generalize to other states, and the use of completed specialist visits without data on referrals, the researchers noted. Other limitations included a lack of data on asthma symptom frequency or control and on the setting in which an asthma diagnosis was made.
However, the results suggest a need for more attention to disparities in asthma care by insurance type, and more research is needed to determine whether these disparities persist in subsets of children with asthma, such as those with allergies or chronic medical conditions, they concluded.
Takeaways and Next Steps
“Perhaps unsurprisingly, children with private insurance were more likely to receive asthma specialist care than children with Medicaid,” Dr. Geissler told this news organization. The researchers expected a smaller gap between insurance types among children with persistent asthma, a marker for asthma severity, she said. However, “we found that the gap between those with Medicaid and those with private insurance is actually larger” for children with persistent asthma, she added.
As improved treatments for hard-to-control asthma become more available, pediatricians and primary care clinicians should follow the latest clinical guidelines for referring children to specialists for asthma care, said Dr. Geissler.
“Additionally, asthma specialists should ensure that their practices are accessible to children with Medicaid, as these families may face higher barriers to care; for example, transportation needs or scheduling challenges,” she said. Other strategies to overcome barriers to care might include electronic consultations with specialists or primary care–oriented interdisciplinary asthma clinics, which may be useful for all children with asthma but may particularly benefit those insured by Medicaid, she noted.
“Based on data limitations, we could not examine why we observed such big differences in specialist use by insurance type; for example, whether pediatricians were referring to specialists less for Medicaid-insured kids, or whether kids with Medicaid were less likely to see a specialist after a referral was made,” Dr. Geissler said. More research is needed to examine not only these factors but also the appropriateness of specialty care based on clinical guidelines to ensure high-quality evidence-based care for children with asthma who are insured by Medicaid, she said.
Improve Access and Expand Analysis
Asthma is a chronic and prevalent disease and requires a comprehensive approach that sometimes calls for specialist care, Anne Coates, MD, a pediatric pulmonologist in Portland, Maine, said in an interview.
Dr. Coates said she was surprised by the results of the current study but commended the authors for highlighting the limitations of the study, which illustrate areas for additional research. Notably, “the authors couldn’t observe referrals to specialists from primary care physicians; they used completed visits as a proxy,” Dr. Coates said.
More studies are needed to assess the completion of referral visits regardless of children’s insurance in order to better understand and address the barriers to specialty care, she added.
The current study is important because of the extent of asthma coupled with the significant number of children across the United States who are insured by Medicaid, especially underserved populations, she said.
“The burden of asthma differentially affects people of color who are living in lower resourced areas, and it is important in further research to understanding the barriers to helping people get the care they need,” Dr. Coates told this news organization. Some alternatives might include telehealth visits or even a hybrid visit to a primary care provider (PCP) who has high-speed internet, and the specialist could then conduct a telehealth visit from the PCP’s office, with the PCP acting as on-site eyes and ears, said Dr. Coates, who has used this strategy in her practice in Maine, where many patients live far from specialist care.
The study was supported by the National Heart, Lung, and Blood Institute and the University of Massachusetts Center for Clinical and Translational Science-Biostatistics, Epidemiology & Research Design Component. Dr. Geissler and Dr. Coates had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Primary care clinicians successfully manage many children with asthma, but data on specialist care according to insurance coverage are lacking, wrote Kimberley H. Geissler, PhD, of the University of Massachusetts Chan Medical School–Baystate, Springfield, Massachusetts, and colleagues.
Despite many interventions over time, “low-income children insured by Medicaid, many of whom are from minoritized racial and ethnic groups, continue to have worse outcomes and higher rates of poorly controlled asthma than children who are privately insured,” Dr. Geissler said in an interview.
“Because differences in whether a child sees an asthma specialist could contribute to these disparities, better understanding specialist use among both groups of kids may help inform potential solutions,” she said.
In a study published in JAMA Network Open, the researchers identified children with asthma aged 2-17 years using data from the Massachusetts All-Payer Claims Database for the years 2015-2020. The study population included 198,101 children and 432,455 child-year observations from children with asthma during a year when they met at least one of three criteria with any asthma diagnosis: One or more hospital visits, two or more outpatient visits, or at least one outpatient visit and at least one asthma medication.
Outpatient Visit Outcome
The primary outcome of asthma specialist care was defined as at least one outpatient visit with any asthma diagnosis to a clinician with a code of allergy and immunology, pulmonology, or otolaryngology.
A total of 66.2% of the child-year observations involved Medicaid and 33.8% involved private insurance. Approximately 15% of the children received asthma specialist care. However, nearly twice as many children with private insurance received asthma specialty care compared with those with Medicaid (20.6% vs 11.9%). In a full logistic regression analysis, children with Medicaid insurance were 55% less likely to receive asthma specialist treatment than children with private insurance.
Allergy and immunology was the most common specialty used, and the child-years for this specialty among children with Medicaid were less than half of those among children with private insurance (7.1% vs 15.9%).
Rates of persistent asthma were 20.0% and 16.9% in children with Medicaid and private insurance, respectively. Overall, children with persistent asthma were nearly four times as likely to receive asthma specialist care (adjusted odds ratio, 3.96). However, the difference in odds of receiving specialty care based on insurance type in favor of private insurance was greater among children with persistent asthma than among those without persistent asthma (−24.0 percentage points vs −20.8 percentage points).
The researchers found a similar pattern of difference in asthma specialty care in a sensitivity analysis limiting the results to child-year observations with at least one outpatient visit with any asthma diagnosis in a calendar year, although they also found a slight narrowing of the difference between the groups over time.
“Contrary to expectations, disparities in specialist care by insurance type were even more striking in children with persistent asthma,” the researchers wrote in their discussion. Notably, the growth of specialty drugs such as biologics for moderate to severe asthma are mainly prescribed by specialists, and ensuring access to specialists for children with Medicaid may reduce disparities in asthma control for those with severe or poorly controlled disease, they added.
The study findings were limited by several factors including the use only of data from Massachusetts, which may not generalize to other states, and the use of completed specialist visits without data on referrals, the researchers noted. Other limitations included a lack of data on asthma symptom frequency or control and on the setting in which an asthma diagnosis was made.
However, the results suggest a need for more attention to disparities in asthma care by insurance type, and more research is needed to determine whether these disparities persist in subsets of children with asthma, such as those with allergies or chronic medical conditions, they concluded.
Takeaways and Next Steps
“Perhaps unsurprisingly, children with private insurance were more likely to receive asthma specialist care than children with Medicaid,” Dr. Geissler told this news organization. The researchers expected a smaller gap between insurance types among children with persistent asthma, a marker for asthma severity, she said. However, “we found that the gap between those with Medicaid and those with private insurance is actually larger” for children with persistent asthma, she added.
As improved treatments for hard-to-control asthma become more available, pediatricians and primary care clinicians should follow the latest clinical guidelines for referring children to specialists for asthma care, said Dr. Geissler.
“Additionally, asthma specialists should ensure that their practices are accessible to children with Medicaid, as these families may face higher barriers to care; for example, transportation needs or scheduling challenges,” she said. Other strategies to overcome barriers to care might include electronic consultations with specialists or primary care–oriented interdisciplinary asthma clinics, which may be useful for all children with asthma but may particularly benefit those insured by Medicaid, she noted.
“Based on data limitations, we could not examine why we observed such big differences in specialist use by insurance type; for example, whether pediatricians were referring to specialists less for Medicaid-insured kids, or whether kids with Medicaid were less likely to see a specialist after a referral was made,” Dr. Geissler said. More research is needed to examine not only these factors but also the appropriateness of specialty care based on clinical guidelines to ensure high-quality evidence-based care for children with asthma who are insured by Medicaid, she said.
Improve Access and Expand Analysis
Asthma is a chronic and prevalent disease and requires a comprehensive approach that sometimes calls for specialist care, Anne Coates, MD, a pediatric pulmonologist in Portland, Maine, said in an interview.
Dr. Coates said she was surprised by the results of the current study but commended the authors for highlighting the limitations of the study, which illustrate areas for additional research. Notably, “the authors couldn’t observe referrals to specialists from primary care physicians; they used completed visits as a proxy,” Dr. Coates said.
More studies are needed to assess the completion of referral visits regardless of children’s insurance in order to better understand and address the barriers to specialty care, she added.
The current study is important because of the extent of asthma coupled with the significant number of children across the United States who are insured by Medicaid, especially underserved populations, she said.
“The burden of asthma differentially affects people of color who are living in lower resourced areas, and it is important in further research to understanding the barriers to helping people get the care they need,” Dr. Coates told this news organization. Some alternatives might include telehealth visits or even a hybrid visit to a primary care provider (PCP) who has high-speed internet, and the specialist could then conduct a telehealth visit from the PCP’s office, with the PCP acting as on-site eyes and ears, said Dr. Coates, who has used this strategy in her practice in Maine, where many patients live far from specialist care.
The study was supported by the National Heart, Lung, and Blood Institute and the University of Massachusetts Center for Clinical and Translational Science-Biostatistics, Epidemiology & Research Design Component. Dr. Geissler and Dr. Coates had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.