User login
Walking 10,000 steps a day: Desirable goal or urban myth?
Some myths never die. The idea of taking 10,000 steps a day is one of them. What started as a catchy marketing slogan has become a mantra for anyone promoting physical activity.
It all began in 1965 when the Japanese company Yamasa Tokei began selling a new step-counter which they called manpo-kei (ten-thousand steps meter). They coupled the product launch with an ad campaign – “Let’s walk 10,000 steps a day!” – in a bid to encourage physical activity. The threshold was always somewhat arbitrary, but the idea of 10,000 steps cemented itself in the public consciousness from that point forward.
To be fair, there is nothing wrong with taking 10,000 steps a day, and it does roughly correlate with the generally recommended amount of physical activity. Most people will take somewhere between 5,000 and 7,500 steps a day even if they lead largely sedentary lives. If you add 30 minutes of walking to your daily routine, that will account for an extra 3,000-4,000 steps and bring you close to that 10,000-step threshold. As such, setting a 10,000-step target is a potentially useful shorthand for people aspiring to achieve ideal levels of physical activity.
But walking fewer steps still has a benefit. A study in JAMA Network Open followed a cohort of 2,110 adults from the CARDIA study and found, rather unsurprisingly, that those with more steps per day had lower rates of all-cause mortality. But interestingly, those who averaged 7,000-10,000 steps per day did just as well as those who walked more than 10,000 steps, suggesting that the lower threshold was probably the inflection point.
Other research has shown that improving your step count is probably more important than achieving any specific threshold. In one Canadian study, patients with diabetes were randomized to usual care or to an exercise prescription from their physicians. The intervention group improved their daily step count from around 5,000 steps per day to about 6,200 steps per day. While the increase was less than the researchers had hoped for, it still resulted in improvements in blood sugar control. In another study, a 24-week walking program reduced blood pressure by 11 points in postmenopausal women, even though their increased daily step counts fell shy of the 10,000 goal at about 9,000 steps. Similarly, a small Japanese study found that enrolling postmenopausal women in a weekly exercise program helped improve their lipid profile even though they only increased their daily step count from 6,800 to 8,500 steps per day. And an analysis of U.S. NHANES data showed a mortality benefit when individuals taking more than 8,000 steps were compared with those taking fewer than 4,000 steps per day. The benefits largely plateaued beyond 9,000-10,000 steps.
The reality is that walking 10,000 steps a day is a laudable goal and is almost certainly beneficial. But even lower levels of physical activity have benefits. The trick is not so much to aim for some theoretical ideal but to improve upon your current baseline. Encouraging patients to get into the habit of taking a daily walk (be it in the morning, during lunchtime, or in the evening) is going to pay dividends regardless of their daily step count. The point is that when it comes to physical activity, the greatest benefit seems to be when we go from doing nothing to doing something.
Dr. Labos is a cardiologist at Queen Elizabeth Health Complex, Montreal. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Some myths never die. The idea of taking 10,000 steps a day is one of them. What started as a catchy marketing slogan has become a mantra for anyone promoting physical activity.
It all began in 1965 when the Japanese company Yamasa Tokei began selling a new step-counter which they called manpo-kei (ten-thousand steps meter). They coupled the product launch with an ad campaign – “Let’s walk 10,000 steps a day!” – in a bid to encourage physical activity. The threshold was always somewhat arbitrary, but the idea of 10,000 steps cemented itself in the public consciousness from that point forward.
To be fair, there is nothing wrong with taking 10,000 steps a day, and it does roughly correlate with the generally recommended amount of physical activity. Most people will take somewhere between 5,000 and 7,500 steps a day even if they lead largely sedentary lives. If you add 30 minutes of walking to your daily routine, that will account for an extra 3,000-4,000 steps and bring you close to that 10,000-step threshold. As such, setting a 10,000-step target is a potentially useful shorthand for people aspiring to achieve ideal levels of physical activity.
But walking fewer steps still has a benefit. A study in JAMA Network Open followed a cohort of 2,110 adults from the CARDIA study and found, rather unsurprisingly, that those with more steps per day had lower rates of all-cause mortality. But interestingly, those who averaged 7,000-10,000 steps per day did just as well as those who walked more than 10,000 steps, suggesting that the lower threshold was probably the inflection point.
Other research has shown that improving your step count is probably more important than achieving any specific threshold. In one Canadian study, patients with diabetes were randomized to usual care or to an exercise prescription from their physicians. The intervention group improved their daily step count from around 5,000 steps per day to about 6,200 steps per day. While the increase was less than the researchers had hoped for, it still resulted in improvements in blood sugar control. In another study, a 24-week walking program reduced blood pressure by 11 points in postmenopausal women, even though their increased daily step counts fell shy of the 10,000 goal at about 9,000 steps. Similarly, a small Japanese study found that enrolling postmenopausal women in a weekly exercise program helped improve their lipid profile even though they only increased their daily step count from 6,800 to 8,500 steps per day. And an analysis of U.S. NHANES data showed a mortality benefit when individuals taking more than 8,000 steps were compared with those taking fewer than 4,000 steps per day. The benefits largely plateaued beyond 9,000-10,000 steps.
The reality is that walking 10,000 steps a day is a laudable goal and is almost certainly beneficial. But even lower levels of physical activity have benefits. The trick is not so much to aim for some theoretical ideal but to improve upon your current baseline. Encouraging patients to get into the habit of taking a daily walk (be it in the morning, during lunchtime, or in the evening) is going to pay dividends regardless of their daily step count. The point is that when it comes to physical activity, the greatest benefit seems to be when we go from doing nothing to doing something.
Dr. Labos is a cardiologist at Queen Elizabeth Health Complex, Montreal. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Some myths never die. The idea of taking 10,000 steps a day is one of them. What started as a catchy marketing slogan has become a mantra for anyone promoting physical activity.
It all began in 1965 when the Japanese company Yamasa Tokei began selling a new step-counter which they called manpo-kei (ten-thousand steps meter). They coupled the product launch with an ad campaign – “Let’s walk 10,000 steps a day!” – in a bid to encourage physical activity. The threshold was always somewhat arbitrary, but the idea of 10,000 steps cemented itself in the public consciousness from that point forward.
To be fair, there is nothing wrong with taking 10,000 steps a day, and it does roughly correlate with the generally recommended amount of physical activity. Most people will take somewhere between 5,000 and 7,500 steps a day even if they lead largely sedentary lives. If you add 30 minutes of walking to your daily routine, that will account for an extra 3,000-4,000 steps and bring you close to that 10,000-step threshold. As such, setting a 10,000-step target is a potentially useful shorthand for people aspiring to achieve ideal levels of physical activity.
But walking fewer steps still has a benefit. A study in JAMA Network Open followed a cohort of 2,110 adults from the CARDIA study and found, rather unsurprisingly, that those with more steps per day had lower rates of all-cause mortality. But interestingly, those who averaged 7,000-10,000 steps per day did just as well as those who walked more than 10,000 steps, suggesting that the lower threshold was probably the inflection point.
Other research has shown that improving your step count is probably more important than achieving any specific threshold. In one Canadian study, patients with diabetes were randomized to usual care or to an exercise prescription from their physicians. The intervention group improved their daily step count from around 5,000 steps per day to about 6,200 steps per day. While the increase was less than the researchers had hoped for, it still resulted in improvements in blood sugar control. In another study, a 24-week walking program reduced blood pressure by 11 points in postmenopausal women, even though their increased daily step counts fell shy of the 10,000 goal at about 9,000 steps. Similarly, a small Japanese study found that enrolling postmenopausal women in a weekly exercise program helped improve their lipid profile even though they only increased their daily step count from 6,800 to 8,500 steps per day. And an analysis of U.S. NHANES data showed a mortality benefit when individuals taking more than 8,000 steps were compared with those taking fewer than 4,000 steps per day. The benefits largely plateaued beyond 9,000-10,000 steps.
The reality is that walking 10,000 steps a day is a laudable goal and is almost certainly beneficial. But even lower levels of physical activity have benefits. The trick is not so much to aim for some theoretical ideal but to improve upon your current baseline. Encouraging patients to get into the habit of taking a daily walk (be it in the morning, during lunchtime, or in the evening) is going to pay dividends regardless of their daily step count. The point is that when it comes to physical activity, the greatest benefit seems to be when we go from doing nothing to doing something.
Dr. Labos is a cardiologist at Queen Elizabeth Health Complex, Montreal. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Common eye disorder in children tied to mental illness
Misaligned eyes in children are associated with an increased prevalence of mental illness, results of a large study suggest.
“Psychiatrists who have a patient with depression or anxiety and notice that patient also has strabismus might think about the link between those two conditions and refer that patient,” study investigator Stacy L. Pineles, MD, professor, department of ophthalmology, University of California, Los Angeles, told this news organization.
The study was published online March 10 in JAMA Ophthalmology.
A common condition
Strabismus, a condition in which the eyes don’t line up or are “crossed,” is one of the most common eye diseases in children, with some estimates suggesting it affects more than 1.5 million American youth.
Patients with strabismus have problems making eye contact and are affected socially and functionally, said Dr. Pineles. They’re often met with a negative bias, as shown by children’s responses to pictures of faces with and without strabismus, she said.
There is a signal from previous research suggesting that strabismus is linked to a higher risk of mental illness. However, most of these studies were small and had relatively homogenous populations, said Dr. Pineles.
The new study includes over 12 million children (mean age, 8.0 years) from a private health insurance claims database that represents diverse races and ethnicities as well as geographic regions across the United States.
The sample included 352,636 children with strabismus and 11,652,553 children with no diagnosed eye disease who served as controls. Most participants were White (51.6%), came from a family with an annual household income of $40,000 or more (51.0%), had point-of-service insurance (68.7%), and had at least one comorbid condition (64.5%).
The study evaluated five mental illness diagnoses. These included anxiety disorder, depressive disorder, substance use or addictive disorder, bipolar disorder, and schizophrenia.
Overall, children with strabismus had a higher prevalence of all these illnesses, with the exception of substance use disorder.
After adjusting for age, sex, race and ethnicity, census region, education level of caregiver, family net worth, and presence of at least one comorbid condition, the odds ratios among those with versus without strabismus were: 2.01 (95% confidence interval, 1.99-2.04; P < .001) for anxiety disorder, 1.83 (95% CI, 1.76-1.90; P < .001) for schizophrenia, 1.64 (95% CI, 1.59-1.70; P < .001) for bipolar disorder, and 1.61 (95% CI, 1.59-1.63; P < .001) for depressive disorder.
Substance use disorder had a negative unadjusted association with strabismus, but after adjustment for confounders, the association was not significant (OR, 0.99; 95% CI, 0.97-1.02; P = .48).
Dr. Pineles noted that the study participants, who were all under age 19, may be too young to have substance use disorders.
The results for substance use disorders provided something of an “internal control” and reaffirmed results for the other four conditions, said Dr. Pineles.
“When you do research on such a large database, you’re very likely to find significant associations; the dataset is so large that even very small differences become statistically significant. It was interesting that not everything gave us a positive association.”
Researchers divided the strabismus group into those with esotropia, where the eyes turn inward (52.2%), exotropia, where they turn outward (46.3%), and hypertropia, where one eye wanders upward (12.5%). Investigators found that all three conditions were associated with increased risk of anxiety disorder, depressive disorder, bipolar disorders, and schizophrenia.
Investigators note that rates in the current study were lower than in previous studies, which showed that children with congenital esotropia were 2.6 times more likely to receive a mental health diagnosis, and children with intermittent exotropia were 2.7 times more likely to receive a mental health diagnosis.
“It is probable that our study found a lower risk than these studies, because our study was cross-sectional and claims based, whereas these studies observed the children to early adulthood and were based on medical records,” the investigators note.
It’s impossible to determine from this study how strabismus is connected to mental illness. However, Dr. Pineles believes depression and anxiety might be tied to strabismus via teasing, which affects self-esteem, although genetics could also play a role. For conditions such as schizophrenia, a shared genetic link with strabismus might be more likely, she added.
“Schizophrenia is a pretty severe diagnosis, so just being teased or having poor self-esteem is probably not enough” to develop schizophrenia.
Based on her clinical experience, Dr. Pineles said that realigning the eyes of patients with milder forms of depression or anxiety “definitely anecdotally helps these patients a lot.”
Dr. Pineles and colleagues have another paper in press that examines mental illnesses and other serious eye disorders in children and shows similar findings, she said.
Implications for insurance coverage?
In an accompanying editorial, experts, led by S. Grace Prakalapakorn, MD, department of ophthalmology and pediatrics, Duke University Medical Center, Durham, N.C., noted the exclusion of children covered under government insurance or without insurance is an important study limitation, largely because socioeconomic status is a risk factor for poor mental health.
The editorialists point to studies showing that surgical correction of ocular misalignments may be associated with reduced anxiety and depression. However, health insurance coverage for such surgical correction “may not be available owing to a misconception that these conditions are ‘cosmetic’.”
Evidence of the broader association of strabismus with physical and mental health “may play an important role in shifting policy to promote insurance coverage for timely strabismus care,” they write.
As many mental health disorders begin in childhood or adolescence, “it is paramount to identify, address, and, if possible, prevent mental health disorders at a young age, because failure to intervene in a timely fashion can have lifelong health consequences,” say Dr. Prakalapakorn and colleagues.
With mental health conditions and disorders increasing worldwide, compounded by the stressors of the COVID-19 pandemic, additional studies are needed to explore the causal relationships between ocular and psychiatric phenomena, their treatment, and outcomes, they add.
The study was supported by a grant from the National Eye Institute and an unrestricted grant from Research to Prevent Blindness. Dr. Pineles has reported no relevant conflicts of interest. Commentary author Manpreet K. Singh, MD, has reported receiving research support from Stanford’s Maternal Child Health Research Institute and Stanford’s Department of Psychiatry and Behavioral Sciences, the National Institute of Mental Health, the National Institute on Aging, the Patient-Centered Outcomes Research Institute, Johnson & Johnson, Allergan, and the Brain and Behavior Research Foundation; serving on the advisory board for Sunovion and Skyland Trail; serving as a consultant for Johnson & Johnson; previously serving as a consultant for X, the moonshot factory, Alphabet, and Limbix Health; receiving honoraria from the American Academy of Child and Adolescent Psychiatry; and receiving royalties from American Psychiatric Association Publishing and Thrive Global. Commentary author Nathan Congdon, MD, has reported receiving personal fees from Belkin Vision outside the submitted work.
A version of this article first appeared on Medscape.com.
Misaligned eyes in children are associated with an increased prevalence of mental illness, results of a large study suggest.
“Psychiatrists who have a patient with depression or anxiety and notice that patient also has strabismus might think about the link between those two conditions and refer that patient,” study investigator Stacy L. Pineles, MD, professor, department of ophthalmology, University of California, Los Angeles, told this news organization.
The study was published online March 10 in JAMA Ophthalmology.
A common condition
Strabismus, a condition in which the eyes don’t line up or are “crossed,” is one of the most common eye diseases in children, with some estimates suggesting it affects more than 1.5 million American youth.
Patients with strabismus have problems making eye contact and are affected socially and functionally, said Dr. Pineles. They’re often met with a negative bias, as shown by children’s responses to pictures of faces with and without strabismus, she said.
There is a signal from previous research suggesting that strabismus is linked to a higher risk of mental illness. However, most of these studies were small and had relatively homogenous populations, said Dr. Pineles.
The new study includes over 12 million children (mean age, 8.0 years) from a private health insurance claims database that represents diverse races and ethnicities as well as geographic regions across the United States.
The sample included 352,636 children with strabismus and 11,652,553 children with no diagnosed eye disease who served as controls. Most participants were White (51.6%), came from a family with an annual household income of $40,000 or more (51.0%), had point-of-service insurance (68.7%), and had at least one comorbid condition (64.5%).
The study evaluated five mental illness diagnoses. These included anxiety disorder, depressive disorder, substance use or addictive disorder, bipolar disorder, and schizophrenia.
Overall, children with strabismus had a higher prevalence of all these illnesses, with the exception of substance use disorder.
After adjusting for age, sex, race and ethnicity, census region, education level of caregiver, family net worth, and presence of at least one comorbid condition, the odds ratios among those with versus without strabismus were: 2.01 (95% confidence interval, 1.99-2.04; P < .001) for anxiety disorder, 1.83 (95% CI, 1.76-1.90; P < .001) for schizophrenia, 1.64 (95% CI, 1.59-1.70; P < .001) for bipolar disorder, and 1.61 (95% CI, 1.59-1.63; P < .001) for depressive disorder.
Substance use disorder had a negative unadjusted association with strabismus, but after adjustment for confounders, the association was not significant (OR, 0.99; 95% CI, 0.97-1.02; P = .48).
Dr. Pineles noted that the study participants, who were all under age 19, may be too young to have substance use disorders.
The results for substance use disorders provided something of an “internal control” and reaffirmed results for the other four conditions, said Dr. Pineles.
“When you do research on such a large database, you’re very likely to find significant associations; the dataset is so large that even very small differences become statistically significant. It was interesting that not everything gave us a positive association.”
Researchers divided the strabismus group into those with esotropia, where the eyes turn inward (52.2%), exotropia, where they turn outward (46.3%), and hypertropia, where one eye wanders upward (12.5%). Investigators found that all three conditions were associated with increased risk of anxiety disorder, depressive disorder, bipolar disorders, and schizophrenia.
Investigators note that rates in the current study were lower than in previous studies, which showed that children with congenital esotropia were 2.6 times more likely to receive a mental health diagnosis, and children with intermittent exotropia were 2.7 times more likely to receive a mental health diagnosis.
“It is probable that our study found a lower risk than these studies, because our study was cross-sectional and claims based, whereas these studies observed the children to early adulthood and were based on medical records,” the investigators note.
It’s impossible to determine from this study how strabismus is connected to mental illness. However, Dr. Pineles believes depression and anxiety might be tied to strabismus via teasing, which affects self-esteem, although genetics could also play a role. For conditions such as schizophrenia, a shared genetic link with strabismus might be more likely, she added.
“Schizophrenia is a pretty severe diagnosis, so just being teased or having poor self-esteem is probably not enough” to develop schizophrenia.
Based on her clinical experience, Dr. Pineles said that realigning the eyes of patients with milder forms of depression or anxiety “definitely anecdotally helps these patients a lot.”
Dr. Pineles and colleagues have another paper in press that examines mental illnesses and other serious eye disorders in children and shows similar findings, she said.
Implications for insurance coverage?
In an accompanying editorial, experts, led by S. Grace Prakalapakorn, MD, department of ophthalmology and pediatrics, Duke University Medical Center, Durham, N.C., noted the exclusion of children covered under government insurance or without insurance is an important study limitation, largely because socioeconomic status is a risk factor for poor mental health.
The editorialists point to studies showing that surgical correction of ocular misalignments may be associated with reduced anxiety and depression. However, health insurance coverage for such surgical correction “may not be available owing to a misconception that these conditions are ‘cosmetic’.”
Evidence of the broader association of strabismus with physical and mental health “may play an important role in shifting policy to promote insurance coverage for timely strabismus care,” they write.
As many mental health disorders begin in childhood or adolescence, “it is paramount to identify, address, and, if possible, prevent mental health disorders at a young age, because failure to intervene in a timely fashion can have lifelong health consequences,” say Dr. Prakalapakorn and colleagues.
With mental health conditions and disorders increasing worldwide, compounded by the stressors of the COVID-19 pandemic, additional studies are needed to explore the causal relationships between ocular and psychiatric phenomena, their treatment, and outcomes, they add.
The study was supported by a grant from the National Eye Institute and an unrestricted grant from Research to Prevent Blindness. Dr. Pineles has reported no relevant conflicts of interest. Commentary author Manpreet K. Singh, MD, has reported receiving research support from Stanford’s Maternal Child Health Research Institute and Stanford’s Department of Psychiatry and Behavioral Sciences, the National Institute of Mental Health, the National Institute on Aging, the Patient-Centered Outcomes Research Institute, Johnson & Johnson, Allergan, and the Brain and Behavior Research Foundation; serving on the advisory board for Sunovion and Skyland Trail; serving as a consultant for Johnson & Johnson; previously serving as a consultant for X, the moonshot factory, Alphabet, and Limbix Health; receiving honoraria from the American Academy of Child and Adolescent Psychiatry; and receiving royalties from American Psychiatric Association Publishing and Thrive Global. Commentary author Nathan Congdon, MD, has reported receiving personal fees from Belkin Vision outside the submitted work.
A version of this article first appeared on Medscape.com.
Misaligned eyes in children are associated with an increased prevalence of mental illness, results of a large study suggest.
“Psychiatrists who have a patient with depression or anxiety and notice that patient also has strabismus might think about the link between those two conditions and refer that patient,” study investigator Stacy L. Pineles, MD, professor, department of ophthalmology, University of California, Los Angeles, told this news organization.
The study was published online March 10 in JAMA Ophthalmology.
A common condition
Strabismus, a condition in which the eyes don’t line up or are “crossed,” is one of the most common eye diseases in children, with some estimates suggesting it affects more than 1.5 million American youth.
Patients with strabismus have problems making eye contact and are affected socially and functionally, said Dr. Pineles. They’re often met with a negative bias, as shown by children’s responses to pictures of faces with and without strabismus, she said.
There is a signal from previous research suggesting that strabismus is linked to a higher risk of mental illness. However, most of these studies were small and had relatively homogenous populations, said Dr. Pineles.
The new study includes over 12 million children (mean age, 8.0 years) from a private health insurance claims database that represents diverse races and ethnicities as well as geographic regions across the United States.
The sample included 352,636 children with strabismus and 11,652,553 children with no diagnosed eye disease who served as controls. Most participants were White (51.6%), came from a family with an annual household income of $40,000 or more (51.0%), had point-of-service insurance (68.7%), and had at least one comorbid condition (64.5%).
The study evaluated five mental illness diagnoses. These included anxiety disorder, depressive disorder, substance use or addictive disorder, bipolar disorder, and schizophrenia.
Overall, children with strabismus had a higher prevalence of all these illnesses, with the exception of substance use disorder.
After adjusting for age, sex, race and ethnicity, census region, education level of caregiver, family net worth, and presence of at least one comorbid condition, the odds ratios among those with versus without strabismus were: 2.01 (95% confidence interval, 1.99-2.04; P < .001) for anxiety disorder, 1.83 (95% CI, 1.76-1.90; P < .001) for schizophrenia, 1.64 (95% CI, 1.59-1.70; P < .001) for bipolar disorder, and 1.61 (95% CI, 1.59-1.63; P < .001) for depressive disorder.
Substance use disorder had a negative unadjusted association with strabismus, but after adjustment for confounders, the association was not significant (OR, 0.99; 95% CI, 0.97-1.02; P = .48).
Dr. Pineles noted that the study participants, who were all under age 19, may be too young to have substance use disorders.
The results for substance use disorders provided something of an “internal control” and reaffirmed results for the other four conditions, said Dr. Pineles.
“When you do research on such a large database, you’re very likely to find significant associations; the dataset is so large that even very small differences become statistically significant. It was interesting that not everything gave us a positive association.”
Researchers divided the strabismus group into those with esotropia, where the eyes turn inward (52.2%), exotropia, where they turn outward (46.3%), and hypertropia, where one eye wanders upward (12.5%). Investigators found that all three conditions were associated with increased risk of anxiety disorder, depressive disorder, bipolar disorders, and schizophrenia.
Investigators note that rates in the current study were lower than in previous studies, which showed that children with congenital esotropia were 2.6 times more likely to receive a mental health diagnosis, and children with intermittent exotropia were 2.7 times more likely to receive a mental health diagnosis.
“It is probable that our study found a lower risk than these studies, because our study was cross-sectional and claims based, whereas these studies observed the children to early adulthood and were based on medical records,” the investigators note.
It’s impossible to determine from this study how strabismus is connected to mental illness. However, Dr. Pineles believes depression and anxiety might be tied to strabismus via teasing, which affects self-esteem, although genetics could also play a role. For conditions such as schizophrenia, a shared genetic link with strabismus might be more likely, she added.
“Schizophrenia is a pretty severe diagnosis, so just being teased or having poor self-esteem is probably not enough” to develop schizophrenia.
Based on her clinical experience, Dr. Pineles said that realigning the eyes of patients with milder forms of depression or anxiety “definitely anecdotally helps these patients a lot.”
Dr. Pineles and colleagues have another paper in press that examines mental illnesses and other serious eye disorders in children and shows similar findings, she said.
Implications for insurance coverage?
In an accompanying editorial, experts, led by S. Grace Prakalapakorn, MD, department of ophthalmology and pediatrics, Duke University Medical Center, Durham, N.C., noted the exclusion of children covered under government insurance or without insurance is an important study limitation, largely because socioeconomic status is a risk factor for poor mental health.
The editorialists point to studies showing that surgical correction of ocular misalignments may be associated with reduced anxiety and depression. However, health insurance coverage for such surgical correction “may not be available owing to a misconception that these conditions are ‘cosmetic’.”
Evidence of the broader association of strabismus with physical and mental health “may play an important role in shifting policy to promote insurance coverage for timely strabismus care,” they write.
As many mental health disorders begin in childhood or adolescence, “it is paramount to identify, address, and, if possible, prevent mental health disorders at a young age, because failure to intervene in a timely fashion can have lifelong health consequences,” say Dr. Prakalapakorn and colleagues.
With mental health conditions and disorders increasing worldwide, compounded by the stressors of the COVID-19 pandemic, additional studies are needed to explore the causal relationships between ocular and psychiatric phenomena, their treatment, and outcomes, they add.
The study was supported by a grant from the National Eye Institute and an unrestricted grant from Research to Prevent Blindness. Dr. Pineles has reported no relevant conflicts of interest. Commentary author Manpreet K. Singh, MD, has reported receiving research support from Stanford’s Maternal Child Health Research Institute and Stanford’s Department of Psychiatry and Behavioral Sciences, the National Institute of Mental Health, the National Institute on Aging, the Patient-Centered Outcomes Research Institute, Johnson & Johnson, Allergan, and the Brain and Behavior Research Foundation; serving on the advisory board for Sunovion and Skyland Trail; serving as a consultant for Johnson & Johnson; previously serving as a consultant for X, the moonshot factory, Alphabet, and Limbix Health; receiving honoraria from the American Academy of Child and Adolescent Psychiatry; and receiving royalties from American Psychiatric Association Publishing and Thrive Global. Commentary author Nathan Congdon, MD, has reported receiving personal fees from Belkin Vision outside the submitted work.
A version of this article first appeared on Medscape.com.
Clinical Presentation of Subacute Combined Degeneration in a Patient With Chronic B12 Deficiency
Subacute combined degeneration (SCD) is an acquired neurologic complication of vitamin B12 (cobalamin) or, rarely, vitamin B9 (folate) deficiency. SCD is characterized by progressive demyelination of the dorsal and lateral spinal cord, resulting in peripheral neuropathy; gait ataxia; impaired proprioception, vibration, and fine touch; optic neuropathy; and cognitive impairment.1 In addition to SCD, other neurologic manifestations of B12 deficiency include dementia, depression, visual symptoms due to optic atrophy, and behavioral changes.2 The prevalence of SCD in the US has not been well documented, but B12 deficiency is reported at 6% in those aged < 60 years and 20% in those > 60 years.3
Causes of B12 and B9 deficiency include advanced age, low nutritional intake (eg, vegan diet), impaired absorption (eg, inflammatory bowel disease, autoimmune pernicious anemia, gastrectomy, pancreatic disease), alcohol use, tapeworm infection, medications, and high metabolic states.2,4 Impaired B12 absorption is common in patients taking medications, such as metformin and proton pump inhibitors (PPI), due to suppression of ileal membrane transport and intrinsic factor activity.5-7 B-vitamin deficiency can be exacerbated by states of increased cellular turnover, such as polycythemia vera, due to elevated DNA synthesis.
Patients may experience permanent neurologic damage when the diagnosis and treatment of SCD are missed or delayed. Early diagnosis of SCD can be challenging due to lack of specific hematologic markers. In addition, many other conditions such as diabetic neuropathy, malnutrition, toxic neuropathy, sarcoidosis, HIV, multiple sclerosis, polycythemia vera, and iron deficiency anemia have similar presentations and clinical findings.8 Anemia and/or macrocytosis are not specific to B12 deficiency.4 In addition, patients with B12 deficiency may have a normal complete blood count (CBC); those with concomitant iron deficiency may have minimal or no mean corpuscular volume (MCV) elevation.4 In patients suspected to have B12 deficiency based on clinical presentation or laboratory findings of macrocytosis, serum methylmalonic acid (MMA) can serve as a direct measure of B12 activity, with levels > 0.75 μmol/L almost always indicating cobalamin deficiency. 9 On the other hand, plasma total homocysteine (tHcy) is a sensitive marker for B12 deficiency. The active form of B12, holotranscobalamin, has also emerged as a specific measure of B12 deficiency.9 However, in patients with SCD, measurement of these markers may be unnecessary due to the severity of their clinical symptoms.
The diagnosis of SCD is further complicated because not all individuals who develop B12 or B9 deficiency will develop SCD. It is difficult to determine which patients will develop SCD because the minimum level of serum B12 required for normal function is unknown, and recent studies indicate that SCD may occur even at low-normal B12 and B9 levels.2,4,10 Commonly, a serum B12 level of < 200 pg/mL is considered deficient, while a level between 200 and 300 pg/mL is considered borderline.4 The goal level of serum B12 is > 300 pg/mL, which is considered normal.4 While serologic findings of B-vitamin deficiency are only moderately specific, radiographic findings are highly sensitive and specific for SCD. According to Briani and colleagues, the most consistent finding in SCD on magnetic resonance imaging (MRI) is a “symmetrical, abnormally increased T2 signal intensity, commonly confined to posterior or posterior and lateral columns in the cervical and thoracic spinal cord.”2
We present a case of SCD in a patient with low-normal vitamin B12 levels who presented with progressive sensorimotor deficits and vision loss. The patient was subsequently diagnosed with SCD by radiologic workup. His course was complicated by worsening neurologic deficits despite B12 replacement. The progression of his clinical symptoms demonstrates the need for prompt, aggressive B12 replacement in patients diagnosed with SCD.
Case Presentation
A 63-year-old man presented for neurologic evaluation of progressive gait disturbance, paresthesia, blurred vision, and increasing falls despite use of a walker. Pertinent medical history included polycythemia vera requiring phlebotomy for approximately 9 years, alcohol use disorder (18 servings weekly), type 2 diabetes mellitus, and a remote episode of transient ischemic attack (TIA). The patient reported a 5-year history of burning pain in all extremities. A prior physician diagnosis attributed the symptoms to polyneuropathy secondary to iron deficiency anemia in the setting of chronic phlebotomy for polycythemia vera and high erythrogenesis. He was prescribed gabapentin 600 mg 3 times daily for pain control. B12 deficiency was considered an unlikely etiology due to a low-normal serum level of 305 pg/mL (reference range, 190-950 pg/mL) and normocytosis, with MCV of 88 fL (reference range, 80-100 fL). The patient also reported a 3-year history of blurred vision, which was initially attributed to be secondary to diabetic retinopathy. One week prior to presenting to our clinic, he was evaluated by ophthalmology for new-onset, bilateral central visual field defects, and he was diagnosed with nutritional optic neuropathy.
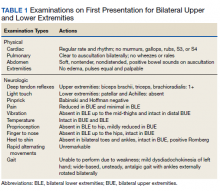
Ophthalmology suspected B12 deficiency. Notable findings included reduced deep tendon reflexes (DTRs) in the upper extremities and absent DTRs in the lower extremities, reduced sensation to light touch in all extremities, absent sensation to pinprick, vibration, and temperature in the lower extremities, positive Romberg sign, and a wide-based antalgic gait with the ankles externally rotated bilaterally (Table 1)
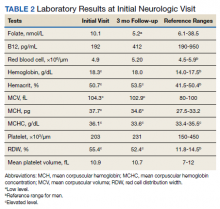
Previous cardiac evaluation failed to provide a diagnosis for syncopal episodes. MRI of the brain revealed nonspecific white matter changes consistent with chronic microvascular ischemic disease. Electromyography was limited due to pain but showed severe peripheral neuropathy. Laboratory results showed megalocytosis, low-normal serum B12 levels, and low serum folate levels (Table 2). The patient was diagnosed with polyneuropathy and was given intramuscular (IM) vitamin B12 1000 mcg once and a daily multivitamin (containing 25 mcg of B12). He was counseled on alcohol abstinence and medication adherence and was scheduled for follow-up in 3 months. He continued outpatient phlebotomy every 6 weeks for polycythemia.
At 3-month follow-up, the patient reported medication adherence, continued alcohol use, and worsening of symptoms. Falls, which now occurred 2 to 3 times weekly despite proper use of a walker, were described as sudden loss of bilateral lower extremity strength without loss of consciousness, palpitations, or other prodrome. Laboratory results showed minimal changes. Physical examination of the patient demonstrated similar deficits as on initial presentation. The patient received one additional B12 1000 mcg IM. Gabapentin was replaced with pregabalin 75 mg twice daily due to persistent uncontrolled pain and paresthesia. The patient was scheduled for a 3-month followup (6 months from initial visit) and repeat serology.
At 6-month follow-up, the patient showed continued progression of disease with significant difficulty using the walker, worsening falls, and wheelchair use required. Physical examination showed decreased sensation bilaterally up to the knees, absent bilateral patellar and Achilles reflexes, and unsteady gait. Laboratory results showed persistent subclinical B12 deficiency. MRI of the brain and spine showed high T2 signaling in a pattern highly specific for SCD. A formal diagnosis of SCD was made. The patient received an additional B12 1000 mcg IM once. Follow-up phone call with the patient 1 month later revealed no progression or improvement of symptoms.
Radiographic Findings
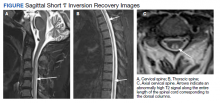
MRI of the cervical and thoracic spine demonstrated abnormal high T2 signal starting from C2 and extending along the course of the cervical and thoracic spinal cord (Figure). MRI in SCD classically shows symmetric, bilateral high T2 signal within the dorsal columns; on axial images, there is typically an inverted “V” sign.2,4 There can also be abnormal cerebral white matter change; however, MRI of the brain in this patient did not show any abnormalities.2 The imaging differential for this appearance includes other metabolic deficiencies/toxicities: copper deficiency; vitamin E deficiency; methotrexateinduced myelopathy, and infectious causes: HIV vacuolar myelopathy; and neurosyphilis (tabes dorsalis).4
Discussion
This case demonstrates the clinical and radiographic findings of SCD and underscores the need for high-intensity dosing of B12 replacement in patients with SCD to prevent progression of the disease and development of morbidities.
Symptoms of SCD may manifest even when the vitamin levels are in low-normal levels. Its presentation is often nonspecific, thus radiologic workup is beneficial to elucidate the clinical picture. We support the use of spinal MRI in patients with clinical suspicion of SCD to help rule out other causes of myelopathy. However, an MRI is not indicated in all patients with B12 deficiency, especially those without myelopathic symptoms. Additionally, follow-up spinal MRIs are useful in monitoring the progression or improvement of SCD after B12 replacement.2 It is important to note that the MRI findings in SCD are not specific to B12 deficiency; other causes may present with similar radiographic findings.4 Therefore, radiologic findings must be correlated with a patient’s clinical presentation.
B12 replacement improves and may resolve clinical symptoms and abnormal radiographic findings of SCD. The treatment duration of B12 deficiency depends on the underlying etiology. Reversible causes, such as metformin use > 4 months, PPI use > 12 months, and dietary deficiency, require treatment until appropriate levels are reached and symptoms are resolved.4,11 The need for chronic metformin and PPI use should also be reassessed regularly. In patients who require long-term metformin use, IM administration of B12 1000 mcg annually should be considered, which will ensure adequate storage for more than 1 year.12,13 In patients who require long-term PPI use, the risk and benefits of continued use should be measured, and if needed, the lowest possible effective PPI dose is recommended.14 Irreversible causes of B12 deficiency, such as advanced age, prior gastrectomy, chronic pancreatitis, or autoimmune pernicious anemia, require lifelong supplementation of B12.4,11
In general, oral vitamin B12 replacement at 1000 to 2000 mcg daily may be as effective as parenteral replacement in patients with mild to moderate deficiency or neurologic symptoms.11 On the other hand, patients with SCD often require parenteral replacement of B12 due to the severity of their deficiency or neurologic symptoms, need for more rapid improvement in symptoms, and prevention of irreversible neurological deficits. 4,11 Appropriate B12 replacement in SCD requires intensive initial therapy which may involve IM B12 1000 mcg every other day for 2 weeks and additional IM supplementation every 2 to 3 months afterward until resolution of deficiency.4,14 IM replacement may also be considered in patients who are nonadherent to oral replacement or have an underlying gastrointestinal condition that impairs enteral absorption.4,11
B12 deficiency is frequently undertreated and can lead to progression of disease with significant morbidity. The need for highintensity dosing of B12 replacement is crucial in patients with SCD. Failure to respond to treatment, as shown from the lack of improvement of serum markers or symptoms, likely suggests undertreatment, treatment nonadherence, iron deficiency anemia, an unidentified malabsorption syndrome, or other diagnoses. In our case, significant undertreatment, compounded by his suspected iron deficiency anemia secondary to his polycythemia vera and chronic phlebotomies, are the most likel etiologies for his lack of clinical improvement.
Multiple factors may affect the prognosis of SCD. Males aged < 50 years with absence of anemia, spinal cord atrophy, Romberg sign, Babinski sign, or sensory deficits on examination have increased likelihood of eventual recovery of signs and symptoms of SCD; those with less spinal cord involvement (< 7 cord segments), contrast enhancement, and spinal cord edema also have improved outcomes.4,15
Conclusion
SCD is a rare but serious complication of chronic vitamin B12 deficiency that presents with a variety of neurological findings and may be easily confused with other illnesses. The condition is easily overlooked or misdiagnosed; thus, it is crucial to differentiate B12 deficiency from other common causes of neurologic symptoms. Specific findings on MRI are useful to support the clinical diagnosis of SCD and guide clinical decisions. Given the prevalence of B12 deficiency in the older adult population, clinicians should remain alert to the possibility of these conditions in patients who present with progressive neuropathy. Once a patient is diagnosed with SCD secondary to a B12 deficiency, appropriate B12 replacement is critical. Appropriate B12 replacement is aggressive and involves IM B12 1000 mcg every other day for 2 to 3 weeks, followed by additional IM administration every 2 months before transitioning to oral therapy. As seen in this case, failure to adequately replenish B12 can lead to progression or lack of resolution of SCD symptoms.
1. Gürsoy AE, Kolukısa M, Babacan-Yıldız G, Celebi A. Subacute Combined Degeneration of the Spinal Cord due to Different Etiologies and Improvement of MRI Findings. Case Rep Neurol Med. 2013;2013:159649. doi:10.1155/2013/159649
2. Briani C, Dalla Torre C, Citton V, et al. Cobalamin deficiency: clinical picture and radiological findings. Nutrients. 2013;5(11):4521-4539. Published 2013 Nov 15. doi:10.3390/nu5114521
3. Hunt A, Harrington D, Robinson S. Vitamin B12 deficiency. BMJ. 2014;349:g5226. Published 2014 Sep 4. doi:10.1136/bmj.g5226
4. Qudsiya Z, De Jesus O. Subacute combined degeneration of the spinal cord. [Updated 2021 Feb 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Updated August 30, 2021. Accessed January 5, 2022. https://www.ncbi.nlm.nih.gov/books /NBK559316/
5. de Jager J, Kooy A, Lehert P, et al. Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: randomised placebo controlled trial. BMJ. 2010;340:c2181. Published 2010 May 20. doi:10.1136/bmj.c2181
6. Aroda VR, Edelstein SL, Goldberg RB, et al. Longterm Metformin Use and Vitamin B12 Deficiency in the Diabetes Prevention Program Outcomes Study. J Clin Endocrinol Metab. 2016;101(4):1754-1761. doi:10.1210/jc.2015-3754
7. Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310(22):2435-2442. doi:10.1001/jama.2013.280490
8. Mihalj M, Titlic´ M, Bonacin D, Dogaš Z. Sensomotor axonal peripheral neuropathy as a first complication of polycythemia rubra vera: A report of 3 cases. Am J Case Rep. 2013;14:385-387. Published 2013 Sep 25. doi:10.12659/AJCR.884016
9. Devalia V, Hamilton MS, Molloy AM; British Committee for Standards in Haematology. Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol. 2014;166(4):496-513. doi:10.1111/bjh.12959
10. Cao J, Xu S, Liu C. Is serum vitamin B12 decrease a necessity for the diagnosis of subacute combined degeneration?: A meta-analysis. Medicine (Baltimore). 2020;99(14):e19700.doi:10.1097/MD.0000000000019700
11. Langan RC, Goodbred AJ. Vitamin B12 Deficiency: Recognition and Management. Am Fam Physician. 2017;96(6):384-389.
12. Mazokopakis EE, Starakis IK. Recommendations for diagnosis and management of metformin-induced vitamin B12 (Cbl) deficiency. Diabetes Res Clin Pract. 2012;97(3):359-367. doi:10.1016/j.diabres.2012.06.001
13. Mahajan R, Gupta K. Revisiting Metformin: Annual Vitamin B12 Supplementation may become Mandatory with Long-Term Metformin Use. J Young Pharm. 2010;2(4):428-429. doi:10.4103/0975-1483.71621
14. Parks NE. Metabolic and Toxic Myelopathies. Continuum (Minneap Minn). 2021;27(1):143-162. doi:10.1212/CON.0000000000000963
15. Vasconcelos OM, Poehm EH, McCarter RJ, Campbell WW, Quezado ZM. Potential outcome factors in subacute combined degeneration: review of observational studies. J Gen Intern Med. 2006;21(10):1063-1068. doi:10.1111/j.1525-1497.2006.00525.x
Subacute combined degeneration (SCD) is an acquired neurologic complication of vitamin B12 (cobalamin) or, rarely, vitamin B9 (folate) deficiency. SCD is characterized by progressive demyelination of the dorsal and lateral spinal cord, resulting in peripheral neuropathy; gait ataxia; impaired proprioception, vibration, and fine touch; optic neuropathy; and cognitive impairment.1 In addition to SCD, other neurologic manifestations of B12 deficiency include dementia, depression, visual symptoms due to optic atrophy, and behavioral changes.2 The prevalence of SCD in the US has not been well documented, but B12 deficiency is reported at 6% in those aged < 60 years and 20% in those > 60 years.3
Causes of B12 and B9 deficiency include advanced age, low nutritional intake (eg, vegan diet), impaired absorption (eg, inflammatory bowel disease, autoimmune pernicious anemia, gastrectomy, pancreatic disease), alcohol use, tapeworm infection, medications, and high metabolic states.2,4 Impaired B12 absorption is common in patients taking medications, such as metformin and proton pump inhibitors (PPI), due to suppression of ileal membrane transport and intrinsic factor activity.5-7 B-vitamin deficiency can be exacerbated by states of increased cellular turnover, such as polycythemia vera, due to elevated DNA synthesis.
Patients may experience permanent neurologic damage when the diagnosis and treatment of SCD are missed or delayed. Early diagnosis of SCD can be challenging due to lack of specific hematologic markers. In addition, many other conditions such as diabetic neuropathy, malnutrition, toxic neuropathy, sarcoidosis, HIV, multiple sclerosis, polycythemia vera, and iron deficiency anemia have similar presentations and clinical findings.8 Anemia and/or macrocytosis are not specific to B12 deficiency.4 In addition, patients with B12 deficiency may have a normal complete blood count (CBC); those with concomitant iron deficiency may have minimal or no mean corpuscular volume (MCV) elevation.4 In patients suspected to have B12 deficiency based on clinical presentation or laboratory findings of macrocytosis, serum methylmalonic acid (MMA) can serve as a direct measure of B12 activity, with levels > 0.75 μmol/L almost always indicating cobalamin deficiency. 9 On the other hand, plasma total homocysteine (tHcy) is a sensitive marker for B12 deficiency. The active form of B12, holotranscobalamin, has also emerged as a specific measure of B12 deficiency.9 However, in patients with SCD, measurement of these markers may be unnecessary due to the severity of their clinical symptoms.
The diagnosis of SCD is further complicated because not all individuals who develop B12 or B9 deficiency will develop SCD. It is difficult to determine which patients will develop SCD because the minimum level of serum B12 required for normal function is unknown, and recent studies indicate that SCD may occur even at low-normal B12 and B9 levels.2,4,10 Commonly, a serum B12 level of < 200 pg/mL is considered deficient, while a level between 200 and 300 pg/mL is considered borderline.4 The goal level of serum B12 is > 300 pg/mL, which is considered normal.4 While serologic findings of B-vitamin deficiency are only moderately specific, radiographic findings are highly sensitive and specific for SCD. According to Briani and colleagues, the most consistent finding in SCD on magnetic resonance imaging (MRI) is a “symmetrical, abnormally increased T2 signal intensity, commonly confined to posterior or posterior and lateral columns in the cervical and thoracic spinal cord.”2
We present a case of SCD in a patient with low-normal vitamin B12 levels who presented with progressive sensorimotor deficits and vision loss. The patient was subsequently diagnosed with SCD by radiologic workup. His course was complicated by worsening neurologic deficits despite B12 replacement. The progression of his clinical symptoms demonstrates the need for prompt, aggressive B12 replacement in patients diagnosed with SCD.
Case Presentation
A 63-year-old man presented for neurologic evaluation of progressive gait disturbance, paresthesia, blurred vision, and increasing falls despite use of a walker. Pertinent medical history included polycythemia vera requiring phlebotomy for approximately 9 years, alcohol use disorder (18 servings weekly), type 2 diabetes mellitus, and a remote episode of transient ischemic attack (TIA). The patient reported a 5-year history of burning pain in all extremities. A prior physician diagnosis attributed the symptoms to polyneuropathy secondary to iron deficiency anemia in the setting of chronic phlebotomy for polycythemia vera and high erythrogenesis. He was prescribed gabapentin 600 mg 3 times daily for pain control. B12 deficiency was considered an unlikely etiology due to a low-normal serum level of 305 pg/mL (reference range, 190-950 pg/mL) and normocytosis, with MCV of 88 fL (reference range, 80-100 fL). The patient also reported a 3-year history of blurred vision, which was initially attributed to be secondary to diabetic retinopathy. One week prior to presenting to our clinic, he was evaluated by ophthalmology for new-onset, bilateral central visual field defects, and he was diagnosed with nutritional optic neuropathy.
Ophthalmology suspected B12 deficiency. Notable findings included reduced deep tendon reflexes (DTRs) in the upper extremities and absent DTRs in the lower extremities, reduced sensation to light touch in all extremities, absent sensation to pinprick, vibration, and temperature in the lower extremities, positive Romberg sign, and a wide-based antalgic gait with the ankles externally rotated bilaterally (Table 1)
Previous cardiac evaluation failed to provide a diagnosis for syncopal episodes. MRI of the brain revealed nonspecific white matter changes consistent with chronic microvascular ischemic disease. Electromyography was limited due to pain but showed severe peripheral neuropathy. Laboratory results showed megalocytosis, low-normal serum B12 levels, and low serum folate levels (Table 2). The patient was diagnosed with polyneuropathy and was given intramuscular (IM) vitamin B12 1000 mcg once and a daily multivitamin (containing 25 mcg of B12). He was counseled on alcohol abstinence and medication adherence and was scheduled for follow-up in 3 months. He continued outpatient phlebotomy every 6 weeks for polycythemia.
At 3-month follow-up, the patient reported medication adherence, continued alcohol use, and worsening of symptoms. Falls, which now occurred 2 to 3 times weekly despite proper use of a walker, were described as sudden loss of bilateral lower extremity strength without loss of consciousness, palpitations, or other prodrome. Laboratory results showed minimal changes. Physical examination of the patient demonstrated similar deficits as on initial presentation. The patient received one additional B12 1000 mcg IM. Gabapentin was replaced with pregabalin 75 mg twice daily due to persistent uncontrolled pain and paresthesia. The patient was scheduled for a 3-month followup (6 months from initial visit) and repeat serology.
At 6-month follow-up, the patient showed continued progression of disease with significant difficulty using the walker, worsening falls, and wheelchair use required. Physical examination showed decreased sensation bilaterally up to the knees, absent bilateral patellar and Achilles reflexes, and unsteady gait. Laboratory results showed persistent subclinical B12 deficiency. MRI of the brain and spine showed high T2 signaling in a pattern highly specific for SCD. A formal diagnosis of SCD was made. The patient received an additional B12 1000 mcg IM once. Follow-up phone call with the patient 1 month later revealed no progression or improvement of symptoms.
Radiographic Findings
MRI of the cervical and thoracic spine demonstrated abnormal high T2 signal starting from C2 and extending along the course of the cervical and thoracic spinal cord (Figure). MRI in SCD classically shows symmetric, bilateral high T2 signal within the dorsal columns; on axial images, there is typically an inverted “V” sign.2,4 There can also be abnormal cerebral white matter change; however, MRI of the brain in this patient did not show any abnormalities.2 The imaging differential for this appearance includes other metabolic deficiencies/toxicities: copper deficiency; vitamin E deficiency; methotrexateinduced myelopathy, and infectious causes: HIV vacuolar myelopathy; and neurosyphilis (tabes dorsalis).4
Discussion
This case demonstrates the clinical and radiographic findings of SCD and underscores the need for high-intensity dosing of B12 replacement in patients with SCD to prevent progression of the disease and development of morbidities.
Symptoms of SCD may manifest even when the vitamin levels are in low-normal levels. Its presentation is often nonspecific, thus radiologic workup is beneficial to elucidate the clinical picture. We support the use of spinal MRI in patients with clinical suspicion of SCD to help rule out other causes of myelopathy. However, an MRI is not indicated in all patients with B12 deficiency, especially those without myelopathic symptoms. Additionally, follow-up spinal MRIs are useful in monitoring the progression or improvement of SCD after B12 replacement.2 It is important to note that the MRI findings in SCD are not specific to B12 deficiency; other causes may present with similar radiographic findings.4 Therefore, radiologic findings must be correlated with a patient’s clinical presentation.
B12 replacement improves and may resolve clinical symptoms and abnormal radiographic findings of SCD. The treatment duration of B12 deficiency depends on the underlying etiology. Reversible causes, such as metformin use > 4 months, PPI use > 12 months, and dietary deficiency, require treatment until appropriate levels are reached and symptoms are resolved.4,11 The need for chronic metformin and PPI use should also be reassessed regularly. In patients who require long-term metformin use, IM administration of B12 1000 mcg annually should be considered, which will ensure adequate storage for more than 1 year.12,13 In patients who require long-term PPI use, the risk and benefits of continued use should be measured, and if needed, the lowest possible effective PPI dose is recommended.14 Irreversible causes of B12 deficiency, such as advanced age, prior gastrectomy, chronic pancreatitis, or autoimmune pernicious anemia, require lifelong supplementation of B12.4,11
In general, oral vitamin B12 replacement at 1000 to 2000 mcg daily may be as effective as parenteral replacement in patients with mild to moderate deficiency or neurologic symptoms.11 On the other hand, patients with SCD often require parenteral replacement of B12 due to the severity of their deficiency or neurologic symptoms, need for more rapid improvement in symptoms, and prevention of irreversible neurological deficits. 4,11 Appropriate B12 replacement in SCD requires intensive initial therapy which may involve IM B12 1000 mcg every other day for 2 weeks and additional IM supplementation every 2 to 3 months afterward until resolution of deficiency.4,14 IM replacement may also be considered in patients who are nonadherent to oral replacement or have an underlying gastrointestinal condition that impairs enteral absorption.4,11
B12 deficiency is frequently undertreated and can lead to progression of disease with significant morbidity. The need for highintensity dosing of B12 replacement is crucial in patients with SCD. Failure to respond to treatment, as shown from the lack of improvement of serum markers or symptoms, likely suggests undertreatment, treatment nonadherence, iron deficiency anemia, an unidentified malabsorption syndrome, or other diagnoses. In our case, significant undertreatment, compounded by his suspected iron deficiency anemia secondary to his polycythemia vera and chronic phlebotomies, are the most likel etiologies for his lack of clinical improvement.
Multiple factors may affect the prognosis of SCD. Males aged < 50 years with absence of anemia, spinal cord atrophy, Romberg sign, Babinski sign, or sensory deficits on examination have increased likelihood of eventual recovery of signs and symptoms of SCD; those with less spinal cord involvement (< 7 cord segments), contrast enhancement, and spinal cord edema also have improved outcomes.4,15
Conclusion
SCD is a rare but serious complication of chronic vitamin B12 deficiency that presents with a variety of neurological findings and may be easily confused with other illnesses. The condition is easily overlooked or misdiagnosed; thus, it is crucial to differentiate B12 deficiency from other common causes of neurologic symptoms. Specific findings on MRI are useful to support the clinical diagnosis of SCD and guide clinical decisions. Given the prevalence of B12 deficiency in the older adult population, clinicians should remain alert to the possibility of these conditions in patients who present with progressive neuropathy. Once a patient is diagnosed with SCD secondary to a B12 deficiency, appropriate B12 replacement is critical. Appropriate B12 replacement is aggressive and involves IM B12 1000 mcg every other day for 2 to 3 weeks, followed by additional IM administration every 2 months before transitioning to oral therapy. As seen in this case, failure to adequately replenish B12 can lead to progression or lack of resolution of SCD symptoms.
Subacute combined degeneration (SCD) is an acquired neurologic complication of vitamin B12 (cobalamin) or, rarely, vitamin B9 (folate) deficiency. SCD is characterized by progressive demyelination of the dorsal and lateral spinal cord, resulting in peripheral neuropathy; gait ataxia; impaired proprioception, vibration, and fine touch; optic neuropathy; and cognitive impairment.1 In addition to SCD, other neurologic manifestations of B12 deficiency include dementia, depression, visual symptoms due to optic atrophy, and behavioral changes.2 The prevalence of SCD in the US has not been well documented, but B12 deficiency is reported at 6% in those aged < 60 years and 20% in those > 60 years.3
Causes of B12 and B9 deficiency include advanced age, low nutritional intake (eg, vegan diet), impaired absorption (eg, inflammatory bowel disease, autoimmune pernicious anemia, gastrectomy, pancreatic disease), alcohol use, tapeworm infection, medications, and high metabolic states.2,4 Impaired B12 absorption is common in patients taking medications, such as metformin and proton pump inhibitors (PPI), due to suppression of ileal membrane transport and intrinsic factor activity.5-7 B-vitamin deficiency can be exacerbated by states of increased cellular turnover, such as polycythemia vera, due to elevated DNA synthesis.
Patients may experience permanent neurologic damage when the diagnosis and treatment of SCD are missed or delayed. Early diagnosis of SCD can be challenging due to lack of specific hematologic markers. In addition, many other conditions such as diabetic neuropathy, malnutrition, toxic neuropathy, sarcoidosis, HIV, multiple sclerosis, polycythemia vera, and iron deficiency anemia have similar presentations and clinical findings.8 Anemia and/or macrocytosis are not specific to B12 deficiency.4 In addition, patients with B12 deficiency may have a normal complete blood count (CBC); those with concomitant iron deficiency may have minimal or no mean corpuscular volume (MCV) elevation.4 In patients suspected to have B12 deficiency based on clinical presentation or laboratory findings of macrocytosis, serum methylmalonic acid (MMA) can serve as a direct measure of B12 activity, with levels > 0.75 μmol/L almost always indicating cobalamin deficiency. 9 On the other hand, plasma total homocysteine (tHcy) is a sensitive marker for B12 deficiency. The active form of B12, holotranscobalamin, has also emerged as a specific measure of B12 deficiency.9 However, in patients with SCD, measurement of these markers may be unnecessary due to the severity of their clinical symptoms.
The diagnosis of SCD is further complicated because not all individuals who develop B12 or B9 deficiency will develop SCD. It is difficult to determine which patients will develop SCD because the minimum level of serum B12 required for normal function is unknown, and recent studies indicate that SCD may occur even at low-normal B12 and B9 levels.2,4,10 Commonly, a serum B12 level of < 200 pg/mL is considered deficient, while a level between 200 and 300 pg/mL is considered borderline.4 The goal level of serum B12 is > 300 pg/mL, which is considered normal.4 While serologic findings of B-vitamin deficiency are only moderately specific, radiographic findings are highly sensitive and specific for SCD. According to Briani and colleagues, the most consistent finding in SCD on magnetic resonance imaging (MRI) is a “symmetrical, abnormally increased T2 signal intensity, commonly confined to posterior or posterior and lateral columns in the cervical and thoracic spinal cord.”2
We present a case of SCD in a patient with low-normal vitamin B12 levels who presented with progressive sensorimotor deficits and vision loss. The patient was subsequently diagnosed with SCD by radiologic workup. His course was complicated by worsening neurologic deficits despite B12 replacement. The progression of his clinical symptoms demonstrates the need for prompt, aggressive B12 replacement in patients diagnosed with SCD.
Case Presentation
A 63-year-old man presented for neurologic evaluation of progressive gait disturbance, paresthesia, blurred vision, and increasing falls despite use of a walker. Pertinent medical history included polycythemia vera requiring phlebotomy for approximately 9 years, alcohol use disorder (18 servings weekly), type 2 diabetes mellitus, and a remote episode of transient ischemic attack (TIA). The patient reported a 5-year history of burning pain in all extremities. A prior physician diagnosis attributed the symptoms to polyneuropathy secondary to iron deficiency anemia in the setting of chronic phlebotomy for polycythemia vera and high erythrogenesis. He was prescribed gabapentin 600 mg 3 times daily for pain control. B12 deficiency was considered an unlikely etiology due to a low-normal serum level of 305 pg/mL (reference range, 190-950 pg/mL) and normocytosis, with MCV of 88 fL (reference range, 80-100 fL). The patient also reported a 3-year history of blurred vision, which was initially attributed to be secondary to diabetic retinopathy. One week prior to presenting to our clinic, he was evaluated by ophthalmology for new-onset, bilateral central visual field defects, and he was diagnosed with nutritional optic neuropathy.
Ophthalmology suspected B12 deficiency. Notable findings included reduced deep tendon reflexes (DTRs) in the upper extremities and absent DTRs in the lower extremities, reduced sensation to light touch in all extremities, absent sensation to pinprick, vibration, and temperature in the lower extremities, positive Romberg sign, and a wide-based antalgic gait with the ankles externally rotated bilaterally (Table 1)
Previous cardiac evaluation failed to provide a diagnosis for syncopal episodes. MRI of the brain revealed nonspecific white matter changes consistent with chronic microvascular ischemic disease. Electromyography was limited due to pain but showed severe peripheral neuropathy. Laboratory results showed megalocytosis, low-normal serum B12 levels, and low serum folate levels (Table 2). The patient was diagnosed with polyneuropathy and was given intramuscular (IM) vitamin B12 1000 mcg once and a daily multivitamin (containing 25 mcg of B12). He was counseled on alcohol abstinence and medication adherence and was scheduled for follow-up in 3 months. He continued outpatient phlebotomy every 6 weeks for polycythemia.
At 3-month follow-up, the patient reported medication adherence, continued alcohol use, and worsening of symptoms. Falls, which now occurred 2 to 3 times weekly despite proper use of a walker, were described as sudden loss of bilateral lower extremity strength without loss of consciousness, palpitations, or other prodrome. Laboratory results showed minimal changes. Physical examination of the patient demonstrated similar deficits as on initial presentation. The patient received one additional B12 1000 mcg IM. Gabapentin was replaced with pregabalin 75 mg twice daily due to persistent uncontrolled pain and paresthesia. The patient was scheduled for a 3-month followup (6 months from initial visit) and repeat serology.
At 6-month follow-up, the patient showed continued progression of disease with significant difficulty using the walker, worsening falls, and wheelchair use required. Physical examination showed decreased sensation bilaterally up to the knees, absent bilateral patellar and Achilles reflexes, and unsteady gait. Laboratory results showed persistent subclinical B12 deficiency. MRI of the brain and spine showed high T2 signaling in a pattern highly specific for SCD. A formal diagnosis of SCD was made. The patient received an additional B12 1000 mcg IM once. Follow-up phone call with the patient 1 month later revealed no progression or improvement of symptoms.
Radiographic Findings
MRI of the cervical and thoracic spine demonstrated abnormal high T2 signal starting from C2 and extending along the course of the cervical and thoracic spinal cord (Figure). MRI in SCD classically shows symmetric, bilateral high T2 signal within the dorsal columns; on axial images, there is typically an inverted “V” sign.2,4 There can also be abnormal cerebral white matter change; however, MRI of the brain in this patient did not show any abnormalities.2 The imaging differential for this appearance includes other metabolic deficiencies/toxicities: copper deficiency; vitamin E deficiency; methotrexateinduced myelopathy, and infectious causes: HIV vacuolar myelopathy; and neurosyphilis (tabes dorsalis).4
Discussion
This case demonstrates the clinical and radiographic findings of SCD and underscores the need for high-intensity dosing of B12 replacement in patients with SCD to prevent progression of the disease and development of morbidities.
Symptoms of SCD may manifest even when the vitamin levels are in low-normal levels. Its presentation is often nonspecific, thus radiologic workup is beneficial to elucidate the clinical picture. We support the use of spinal MRI in patients with clinical suspicion of SCD to help rule out other causes of myelopathy. However, an MRI is not indicated in all patients with B12 deficiency, especially those without myelopathic symptoms. Additionally, follow-up spinal MRIs are useful in monitoring the progression or improvement of SCD after B12 replacement.2 It is important to note that the MRI findings in SCD are not specific to B12 deficiency; other causes may present with similar radiographic findings.4 Therefore, radiologic findings must be correlated with a patient’s clinical presentation.
B12 replacement improves and may resolve clinical symptoms and abnormal radiographic findings of SCD. The treatment duration of B12 deficiency depends on the underlying etiology. Reversible causes, such as metformin use > 4 months, PPI use > 12 months, and dietary deficiency, require treatment until appropriate levels are reached and symptoms are resolved.4,11 The need for chronic metformin and PPI use should also be reassessed regularly. In patients who require long-term metformin use, IM administration of B12 1000 mcg annually should be considered, which will ensure adequate storage for more than 1 year.12,13 In patients who require long-term PPI use, the risk and benefits of continued use should be measured, and if needed, the lowest possible effective PPI dose is recommended.14 Irreversible causes of B12 deficiency, such as advanced age, prior gastrectomy, chronic pancreatitis, or autoimmune pernicious anemia, require lifelong supplementation of B12.4,11
In general, oral vitamin B12 replacement at 1000 to 2000 mcg daily may be as effective as parenteral replacement in patients with mild to moderate deficiency or neurologic symptoms.11 On the other hand, patients with SCD often require parenteral replacement of B12 due to the severity of their deficiency or neurologic symptoms, need for more rapid improvement in symptoms, and prevention of irreversible neurological deficits. 4,11 Appropriate B12 replacement in SCD requires intensive initial therapy which may involve IM B12 1000 mcg every other day for 2 weeks and additional IM supplementation every 2 to 3 months afterward until resolution of deficiency.4,14 IM replacement may also be considered in patients who are nonadherent to oral replacement or have an underlying gastrointestinal condition that impairs enteral absorption.4,11
B12 deficiency is frequently undertreated and can lead to progression of disease with significant morbidity. The need for highintensity dosing of B12 replacement is crucial in patients with SCD. Failure to respond to treatment, as shown from the lack of improvement of serum markers or symptoms, likely suggests undertreatment, treatment nonadherence, iron deficiency anemia, an unidentified malabsorption syndrome, or other diagnoses. In our case, significant undertreatment, compounded by his suspected iron deficiency anemia secondary to his polycythemia vera and chronic phlebotomies, are the most likel etiologies for his lack of clinical improvement.
Multiple factors may affect the prognosis of SCD. Males aged < 50 years with absence of anemia, spinal cord atrophy, Romberg sign, Babinski sign, or sensory deficits on examination have increased likelihood of eventual recovery of signs and symptoms of SCD; those with less spinal cord involvement (< 7 cord segments), contrast enhancement, and spinal cord edema also have improved outcomes.4,15
Conclusion
SCD is a rare but serious complication of chronic vitamin B12 deficiency that presents with a variety of neurological findings and may be easily confused with other illnesses. The condition is easily overlooked or misdiagnosed; thus, it is crucial to differentiate B12 deficiency from other common causes of neurologic symptoms. Specific findings on MRI are useful to support the clinical diagnosis of SCD and guide clinical decisions. Given the prevalence of B12 deficiency in the older adult population, clinicians should remain alert to the possibility of these conditions in patients who present with progressive neuropathy. Once a patient is diagnosed with SCD secondary to a B12 deficiency, appropriate B12 replacement is critical. Appropriate B12 replacement is aggressive and involves IM B12 1000 mcg every other day for 2 to 3 weeks, followed by additional IM administration every 2 months before transitioning to oral therapy. As seen in this case, failure to adequately replenish B12 can lead to progression or lack of resolution of SCD symptoms.
1. Gürsoy AE, Kolukısa M, Babacan-Yıldız G, Celebi A. Subacute Combined Degeneration of the Spinal Cord due to Different Etiologies and Improvement of MRI Findings. Case Rep Neurol Med. 2013;2013:159649. doi:10.1155/2013/159649
2. Briani C, Dalla Torre C, Citton V, et al. Cobalamin deficiency: clinical picture and radiological findings. Nutrients. 2013;5(11):4521-4539. Published 2013 Nov 15. doi:10.3390/nu5114521
3. Hunt A, Harrington D, Robinson S. Vitamin B12 deficiency. BMJ. 2014;349:g5226. Published 2014 Sep 4. doi:10.1136/bmj.g5226
4. Qudsiya Z, De Jesus O. Subacute combined degeneration of the spinal cord. [Updated 2021 Feb 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Updated August 30, 2021. Accessed January 5, 2022. https://www.ncbi.nlm.nih.gov/books /NBK559316/
5. de Jager J, Kooy A, Lehert P, et al. Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: randomised placebo controlled trial. BMJ. 2010;340:c2181. Published 2010 May 20. doi:10.1136/bmj.c2181
6. Aroda VR, Edelstein SL, Goldberg RB, et al. Longterm Metformin Use and Vitamin B12 Deficiency in the Diabetes Prevention Program Outcomes Study. J Clin Endocrinol Metab. 2016;101(4):1754-1761. doi:10.1210/jc.2015-3754
7. Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310(22):2435-2442. doi:10.1001/jama.2013.280490
8. Mihalj M, Titlic´ M, Bonacin D, Dogaš Z. Sensomotor axonal peripheral neuropathy as a first complication of polycythemia rubra vera: A report of 3 cases. Am J Case Rep. 2013;14:385-387. Published 2013 Sep 25. doi:10.12659/AJCR.884016
9. Devalia V, Hamilton MS, Molloy AM; British Committee for Standards in Haematology. Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol. 2014;166(4):496-513. doi:10.1111/bjh.12959
10. Cao J, Xu S, Liu C. Is serum vitamin B12 decrease a necessity for the diagnosis of subacute combined degeneration?: A meta-analysis. Medicine (Baltimore). 2020;99(14):e19700.doi:10.1097/MD.0000000000019700
11. Langan RC, Goodbred AJ. Vitamin B12 Deficiency: Recognition and Management. Am Fam Physician. 2017;96(6):384-389.
12. Mazokopakis EE, Starakis IK. Recommendations for diagnosis and management of metformin-induced vitamin B12 (Cbl) deficiency. Diabetes Res Clin Pract. 2012;97(3):359-367. doi:10.1016/j.diabres.2012.06.001
13. Mahajan R, Gupta K. Revisiting Metformin: Annual Vitamin B12 Supplementation may become Mandatory with Long-Term Metformin Use. J Young Pharm. 2010;2(4):428-429. doi:10.4103/0975-1483.71621
14. Parks NE. Metabolic and Toxic Myelopathies. Continuum (Minneap Minn). 2021;27(1):143-162. doi:10.1212/CON.0000000000000963
15. Vasconcelos OM, Poehm EH, McCarter RJ, Campbell WW, Quezado ZM. Potential outcome factors in subacute combined degeneration: review of observational studies. J Gen Intern Med. 2006;21(10):1063-1068. doi:10.1111/j.1525-1497.2006.00525.x
1. Gürsoy AE, Kolukısa M, Babacan-Yıldız G, Celebi A. Subacute Combined Degeneration of the Spinal Cord due to Different Etiologies and Improvement of MRI Findings. Case Rep Neurol Med. 2013;2013:159649. doi:10.1155/2013/159649
2. Briani C, Dalla Torre C, Citton V, et al. Cobalamin deficiency: clinical picture and radiological findings. Nutrients. 2013;5(11):4521-4539. Published 2013 Nov 15. doi:10.3390/nu5114521
3. Hunt A, Harrington D, Robinson S. Vitamin B12 deficiency. BMJ. 2014;349:g5226. Published 2014 Sep 4. doi:10.1136/bmj.g5226
4. Qudsiya Z, De Jesus O. Subacute combined degeneration of the spinal cord. [Updated 2021 Feb 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Updated August 30, 2021. Accessed January 5, 2022. https://www.ncbi.nlm.nih.gov/books /NBK559316/
5. de Jager J, Kooy A, Lehert P, et al. Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: randomised placebo controlled trial. BMJ. 2010;340:c2181. Published 2010 May 20. doi:10.1136/bmj.c2181
6. Aroda VR, Edelstein SL, Goldberg RB, et al. Longterm Metformin Use and Vitamin B12 Deficiency in the Diabetes Prevention Program Outcomes Study. J Clin Endocrinol Metab. 2016;101(4):1754-1761. doi:10.1210/jc.2015-3754
7. Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310(22):2435-2442. doi:10.1001/jama.2013.280490
8. Mihalj M, Titlic´ M, Bonacin D, Dogaš Z. Sensomotor axonal peripheral neuropathy as a first complication of polycythemia rubra vera: A report of 3 cases. Am J Case Rep. 2013;14:385-387. Published 2013 Sep 25. doi:10.12659/AJCR.884016
9. Devalia V, Hamilton MS, Molloy AM; British Committee for Standards in Haematology. Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol. 2014;166(4):496-513. doi:10.1111/bjh.12959
10. Cao J, Xu S, Liu C. Is serum vitamin B12 decrease a necessity for the diagnosis of subacute combined degeneration?: A meta-analysis. Medicine (Baltimore). 2020;99(14):e19700.doi:10.1097/MD.0000000000019700
11. Langan RC, Goodbred AJ. Vitamin B12 Deficiency: Recognition and Management. Am Fam Physician. 2017;96(6):384-389.
12. Mazokopakis EE, Starakis IK. Recommendations for diagnosis and management of metformin-induced vitamin B12 (Cbl) deficiency. Diabetes Res Clin Pract. 2012;97(3):359-367. doi:10.1016/j.diabres.2012.06.001
13. Mahajan R, Gupta K. Revisiting Metformin: Annual Vitamin B12 Supplementation may become Mandatory with Long-Term Metformin Use. J Young Pharm. 2010;2(4):428-429. doi:10.4103/0975-1483.71621
14. Parks NE. Metabolic and Toxic Myelopathies. Continuum (Minneap Minn). 2021;27(1):143-162. doi:10.1212/CON.0000000000000963
15. Vasconcelos OM, Poehm EH, McCarter RJ, Campbell WW, Quezado ZM. Potential outcome factors in subacute combined degeneration: review of observational studies. J Gen Intern Med. 2006;21(10):1063-1068. doi:10.1111/j.1525-1497.2006.00525.x
Doc sues patient and wins
Urologist Arnaldo Trabucco, MD, was outraged when he learned of a legal claim filed by a patient’s family blaming him for the patient’s death.
Dr. Trabucco had performed a successful laparoscopic left radical nephrectomy on Gerald Scharf, but he died 3 days later from an unrelated condition, said Dr. Trabucco, who now practices in California. The claims alleged not only wrongful death, but also that Dr. Trabucco “committed willful and malicious actions upon” the patient, eventually resulting in his death, and that Dr. Trabucco’s actions constituted “extreme and outrageous behavior.”
The accusations were levied on behalf of the patient’s family by a bankruptcy attorney who admittedly had no experience in medical malpractice litigation.
“It was a vicious allegation that I intentionally tried to kill my own patient,” said Dr. Trabucco, 66. “It’s an allegation that was outlandish. Those were fighting words.”
As the case dragged on, Dr. Trabucco filed a complaint against the Scharfs and bankruptcy attorney Jeffrey Cogan for malicious prosecution and abuse of process. In 2018, Dr. Trabucco prevailed when a jury awarded him $6,232,000 in compensatory damages and $1,768,000 in punitive damages against Mr. Cogan. (No damages were assessed against the patient’s family.)
“This was a principle issue more than anything else,” Dr. Trabucco said. “My name was tarnished and thrown through the mud. I’m very proud of my name and my reputation.”
Mr. Cogan, a bankruptcy attorney licensed in Nevada and California, disputes that his actions were malicious and disagrees with the jury’s verdict.
“I don’t think I did anything wrong with filing the lawsuit,” he said.
Despite the $8 million award, the legal fight between Dr. Trabucco and Mr. Cogan has continued – and is not over yet.
Not your ordinary legal journey
Dr. Trabucco completed his medical training in Rome before moving back to the United States to begin his residency in Brooklyn, New York.
He quietly practiced urology in New York for years before moving to Columbus, Ind., to work at a urology clinic. In 2005, Dr. Trabucco made headlines when the Bartholomew County, Ind., sheriff’s deputies found marijuana plants growing in his home. His medical license was suspended, and he pled guilty to a misdemeanor.
Dr. Trabucco said he was unaware that a family member was growing the plants in the home and that he was not responsible for the operation. The misdemeanor has since been expunged from his record, according to Dr. Trabucco. Bartholomew County records do not list the offense. He later moved to Arizona, where he opened a medical practice.
Before the Scharfs filed their malpractice case in 2013, Dr. Trabucco had been embroiled in a series of unrelated legal disputes, including postdivorce proceedings and a complaint against him by another physician, alleging defamation. In that case, the physician claimed Dr. Trabucco published false and defamatory communications about him, including that the physician committed intentional fraud.
Dr. Trabucco filed his own suit for defamation and infliction of emotional distress against the same physician for alleged threats and harassment and making false reports about him to several Arizona state entities. The defamation suit against Dr. Trabucco ended in a settlement, the terms of which are confidential, according to Mr. Cogan. Dr. Trabucco’s defamation case against the physician was dismissed, according to court records, but they do not specify the reason. Dr. Trabucco said he dropped the defamation case because of the other relentless litigation he was facing.
In November 2012, Dr. Trabucco filed for Chapter 7 bankruptcy protection.
Mr. Cogan was representing Dr. Trabucco’s ex-wife’s interests as a creditor in the bankruptcy proceeding and eventually represented the physician who sued Dr. Trabucco, according to court documents. In all, Mr. Cogan represented six creditors in connection to Dr. Trabucco’s bankruptcy, including the Scharfs. He also took over the Scharfs’ malpractice case in Arizona’s Mohave County Superior Court after their attorney died.
As part of a filing in Nevada bankruptcy court called a “Complaint to Determine Nondischargeabiliy of Debts,” Mr. Cogan alleged that Dr. Trabucco knew he lacked sufficient expertise regarding the laparoscopic nephrectomy, that an intraoperative complication occurred because of his error, and that Dr. Trabucco hid the complication and did not attempt to remedy the situation, among other claims.
In addition to the Mohave County filing and the bankruptcy filing, Mr. Cogan filed a similar medical malpractice case against Dr. Trabucco in Arizona district court.
“I believe, based upon the statements by [a retired medical malpractice attorney], that Dr Trabucco squeezed the abdominal aorta,” Mr. Cogan said. “When he did so, plaque from Mr Scharf blocked blood from going to his remaining his kidney, causing his death. My lawsuit said that Dr Trabucco knew he made a mistake and went home rather than fixing the mistake.”
Dr. Trabucco said the laparoscopic nephrectomy went smoothly. A few hours after the surgery, however, Mr. Scharf was transferred to a Las Vegas hospital with a diagnosis of potential occlusion of the abdominal aorta. Surgery was performed, and while the operative report noted the presence of severe atherosclerosis of the abdominal aorta, there was no indication of an intraoperative injury to the aorta from Dr. Trabucco’s surgery, according to court documents filed in U.S. District Court for the District of Arizona. An autopsy performed on Mr. Scharf reported “severe calcific aortic atherosclerosis, primarily at the aortic arch and abdominal aorta at the level of the branching of the renal arteries with small adherent thrombus.” The autopsy did not include any indication or suggestion of an intraoperative injury to the abdominal aorta.
A federal jury found unanimously in favor of Dr. Trabucco, concluding that he did nothing wrong. The original medical malpractice case in Mohave County court was ultimately dismissed and in 2014, the bankruptcy court entered an order discharging Dr. Trabucco from all prepetition debts, according to court records.
Meanwhile, as the malicious prosecution case against Mr. Cogan continued, Dr. Trabucco’s name again drew media attention. His former girlfriend was arrested for plotting to kidnap and kill an attorney who was representing Dr. Trabucco’s ex-wife in a divorce proceeding. Renee Perillo was sentenced to 27 years in prison for conspiracy to commit kidnapping and murder for hire for trying to kill Noblesville, Ind., attorney Rebecca Eimerman. Ms. Eimerman was pursuing divorce settlement money from Dr. Trabucco on behalf of his ex-wife. Ms. Perillo’s son was also charged in the crime.
No charges related to the crime were filed against Dr. Trabucco, according to the Hamilton County Prosecutor’s Office and federal charging records. Dr. Trabucco said he was not involved in the incident and had no knowledge of Perillo’s plans.
A hard fought – and still continuing – legal battle
After a 3-day trial, jurors in 2018 found that Mr. Cogan owed Dr. Trabucco $8 million for the harm caused by the unfounded claims.
“This has had a tremendous impact on my life,” Dr. Trabucco said. “It’s cost me a lot of time, money, and anguish.”
Mr. Cogan appealed the verdict. An Arizona appeals court in 2020 upheld the finding of liability for malicious prosecution against Mr. Cogan, but it vacated the finding of liability for abuse of process. Because of the partial reversal, the appellate court vacated the $8 million and sent the case back to the Superior Court in Mohave County, Ariz., for a new trial on damages.
But shortly before the December 2021 retrial, both parties agreed to settle for $8 million.
The settlement, however, is not the end of the litigation between the parties.
Late last year, Mr. Cogan filed a new case in the U.S. District Court for the District of Nevada against Dr. Trabucco alleging the Arizona court that tried the malicious prosecution case never had jurisdiction. Mr. Cogan contends that per federal case law, any damages resulting from a bankruptcy court pleading give the federal court exclusive jurisdiction over the matter.
“I believe that I will win the federal court case,” Mr. Cogan said. “If Judge Andrew P. Gordon finds for me, Dr. Trabucco’s judgment in Arizona is void as Arizona did not have subject-matter jurisdiction.”
Dr. Trabucco says the new federal case has no merit.
“The federal courts have no jurisdiction over civil matters, and this should be thrown out,” he said. “However, my attorney is fully prepared to take this to the Supreme Court [if it moves forward].”
At this article’s deadline, the judge had not yet ruled on Dr. Trabucco’s motion to dismiss the federal complaint.
Should a physician sue for malicious prosecution?
Dr. Trabucco’s case raises the question of whether physicians should consider suing a patient after winning their malpractice case. As many doctors know, a successful case outcome doesn’t necessarily undo the time spent, income lost, and reputation harm that often comes with a negligence lawsuit. Is suing for malicious prosecution a reasonable route to recoup some of the damages caused by the claim?
“It’s an uphill struggle to prevail,” said Jeffrey Segal, MD, JD. “Have people done it? Yes, they have. Is it easy? No.”
A physician must hit the marks of a distinct checklist to pursue a malicious prosecution case, said Dr. Segal, CEO and founder of Medical Justice, a company that aids and advises physicians on legal matters. One necessary element is that the malpractice case against the physician must have been adjudicated on the merits, he said. For example, the doctor won the case at trial or the case was dismissed on summary judgment. Summary judgment refers to a court tossing the claim because there was no genuine dispute as to any material fact and because the defendant is entitled to judgment as a matter of law.
If the case was dismissed for another reason, such as a technicality, or dropped by the plaintiff, the case would not meet the threshold for a malicious prosecution claim, Dr. Segal explains.
The physician must also show that the lawsuit was instituted with “malice,” meaning with an intent to hurt the physician, and that the lawsuit was brought without “probable cause,” adds J. Richard Moore, JD, a medical malpractice defense attorney based in Indianapolis.
“The courts are still pretty good at weeding out cases with no merit whatsoever, so even if a physician takes a case to trial and wins, it is exceedingly rare that a case that makes it that far, has no merit whatsoever,” Mr. Moore said.
In addition, a plaintiffs’ attorney may be protected from a malicious prosecution claim because they relied on a medical expert’s opinion that reasonable cause existed to file a lawsuit, notes William Sullivan, DO, JD, an emergency physician and attorney based in Frankfort, Ill.
Dr. Sullivan has personal experience with such a challenge. He filed a malicious prosecution claim against a plaintiffs’ attorney after being dismissed from a medical malpractice case. The claim stemmed from Dr. Sullivan inserting an emergency central line into a trauma patient who was taken to surgery and later died. The suit alleged Dr. Sullivan failed to diagnose internal bleeding and failed to perform surgery, although surgeons at the hospital knew internal bleeding was present and Dr. Sullivan had no privileges to perform surgery, he said.
However, Dr. Sullivan was unable to identify the medical expert involved in the claim. Illinois law allows a malpractice plaintiff to withhold the identity of a medical expert who certifies a malpractice lawsuit. Because Dr. Sullivan couldn’t identify the expert, he could not depose the physician, and the law firm claimed there was no malicious prosecution because it relied on the trauma surgeon’s opinion that Dr. Sullivan was liable.
The trial court agreed with the law firm’s argument and dismissed them from the case, Dr. Sullivan said. The trial court also agreed that the expert trauma surgeon should be allowed to remain anonymous in accordance with Illinois law.
Alternative options for doctors to recover damages
For physicians who want to recoup after a frivolous claim, but don’t want to dive into another lawsuit, there are other options, say legal experts.
One alternative is filing a motion for sanctions, Dr. Sullivan said. If an attorney files a lawsuit that does not have a reasonable basis in fact or law, that attorney could be subject to sanctions, including paying for the physician’s attorney’s fees and costs, he explained. If a motion for sanctions is granted against an opposing attorney, that fact may be reportable to the attorney’s insurance carrier and also reportable to the attorney’s state licensing board. A motion for sanctions does not require filing of a separate lawsuit and filing a motion for sanctions may allow the defendant physician or the defense attorney to depose the plaintiff attorney regarding the reasonable basis that attorney had for filing a lawsuit, Dr. Sullivan said.
Dr. Sullivan recently represented a physician who won a motion for sanctions. During the legal action against him, Dr. Sullivan presented the plaintiff’s attorney with information showing why his lawsuit did not have a reasonable basis, but the attorney repeatedly ignored the information.
“Eventually, I filed a motion to dismiss the physician from the lawsuit and that motion was granted,” he said. “I also filed a motion for sanctions so that the physician could recover the costs involved in defending the claim. The trial court granted our motion for sanctions against the plaintiff and her law firm and awarded my client more than $10,000.”
Physicians who believe a plaintiffs’ attorney is acting unprofessionally can also file a complaint with the attorney’s bar, Dr. Segal said. And expert witnesses acting in bad faith can be reported to professional societies, medical licensing boards, and/or specialty boards.
“There are a number of avenues to address the sense of justice,” he said. “But if you’re looking for a payday, the only way to do that is by going to court.”
A version of this article first appeared on Medscape.com.
Urologist Arnaldo Trabucco, MD, was outraged when he learned of a legal claim filed by a patient’s family blaming him for the patient’s death.
Dr. Trabucco had performed a successful laparoscopic left radical nephrectomy on Gerald Scharf, but he died 3 days later from an unrelated condition, said Dr. Trabucco, who now practices in California. The claims alleged not only wrongful death, but also that Dr. Trabucco “committed willful and malicious actions upon” the patient, eventually resulting in his death, and that Dr. Trabucco’s actions constituted “extreme and outrageous behavior.”
The accusations were levied on behalf of the patient’s family by a bankruptcy attorney who admittedly had no experience in medical malpractice litigation.
“It was a vicious allegation that I intentionally tried to kill my own patient,” said Dr. Trabucco, 66. “It’s an allegation that was outlandish. Those were fighting words.”
As the case dragged on, Dr. Trabucco filed a complaint against the Scharfs and bankruptcy attorney Jeffrey Cogan for malicious prosecution and abuse of process. In 2018, Dr. Trabucco prevailed when a jury awarded him $6,232,000 in compensatory damages and $1,768,000 in punitive damages against Mr. Cogan. (No damages were assessed against the patient’s family.)
“This was a principle issue more than anything else,” Dr. Trabucco said. “My name was tarnished and thrown through the mud. I’m very proud of my name and my reputation.”
Mr. Cogan, a bankruptcy attorney licensed in Nevada and California, disputes that his actions were malicious and disagrees with the jury’s verdict.
“I don’t think I did anything wrong with filing the lawsuit,” he said.
Despite the $8 million award, the legal fight between Dr. Trabucco and Mr. Cogan has continued – and is not over yet.
Not your ordinary legal journey
Dr. Trabucco completed his medical training in Rome before moving back to the United States to begin his residency in Brooklyn, New York.
He quietly practiced urology in New York for years before moving to Columbus, Ind., to work at a urology clinic. In 2005, Dr. Trabucco made headlines when the Bartholomew County, Ind., sheriff’s deputies found marijuana plants growing in his home. His medical license was suspended, and he pled guilty to a misdemeanor.
Dr. Trabucco said he was unaware that a family member was growing the plants in the home and that he was not responsible for the operation. The misdemeanor has since been expunged from his record, according to Dr. Trabucco. Bartholomew County records do not list the offense. He later moved to Arizona, where he opened a medical practice.
Before the Scharfs filed their malpractice case in 2013, Dr. Trabucco had been embroiled in a series of unrelated legal disputes, including postdivorce proceedings and a complaint against him by another physician, alleging defamation. In that case, the physician claimed Dr. Trabucco published false and defamatory communications about him, including that the physician committed intentional fraud.
Dr. Trabucco filed his own suit for defamation and infliction of emotional distress against the same physician for alleged threats and harassment and making false reports about him to several Arizona state entities. The defamation suit against Dr. Trabucco ended in a settlement, the terms of which are confidential, according to Mr. Cogan. Dr. Trabucco’s defamation case against the physician was dismissed, according to court records, but they do not specify the reason. Dr. Trabucco said he dropped the defamation case because of the other relentless litigation he was facing.
In November 2012, Dr. Trabucco filed for Chapter 7 bankruptcy protection.
Mr. Cogan was representing Dr. Trabucco’s ex-wife’s interests as a creditor in the bankruptcy proceeding and eventually represented the physician who sued Dr. Trabucco, according to court documents. In all, Mr. Cogan represented six creditors in connection to Dr. Trabucco’s bankruptcy, including the Scharfs. He also took over the Scharfs’ malpractice case in Arizona’s Mohave County Superior Court after their attorney died.
As part of a filing in Nevada bankruptcy court called a “Complaint to Determine Nondischargeabiliy of Debts,” Mr. Cogan alleged that Dr. Trabucco knew he lacked sufficient expertise regarding the laparoscopic nephrectomy, that an intraoperative complication occurred because of his error, and that Dr. Trabucco hid the complication and did not attempt to remedy the situation, among other claims.
In addition to the Mohave County filing and the bankruptcy filing, Mr. Cogan filed a similar medical malpractice case against Dr. Trabucco in Arizona district court.
“I believe, based upon the statements by [a retired medical malpractice attorney], that Dr Trabucco squeezed the abdominal aorta,” Mr. Cogan said. “When he did so, plaque from Mr Scharf blocked blood from going to his remaining his kidney, causing his death. My lawsuit said that Dr Trabucco knew he made a mistake and went home rather than fixing the mistake.”
Dr. Trabucco said the laparoscopic nephrectomy went smoothly. A few hours after the surgery, however, Mr. Scharf was transferred to a Las Vegas hospital with a diagnosis of potential occlusion of the abdominal aorta. Surgery was performed, and while the operative report noted the presence of severe atherosclerosis of the abdominal aorta, there was no indication of an intraoperative injury to the aorta from Dr. Trabucco’s surgery, according to court documents filed in U.S. District Court for the District of Arizona. An autopsy performed on Mr. Scharf reported “severe calcific aortic atherosclerosis, primarily at the aortic arch and abdominal aorta at the level of the branching of the renal arteries with small adherent thrombus.” The autopsy did not include any indication or suggestion of an intraoperative injury to the abdominal aorta.
A federal jury found unanimously in favor of Dr. Trabucco, concluding that he did nothing wrong. The original medical malpractice case in Mohave County court was ultimately dismissed and in 2014, the bankruptcy court entered an order discharging Dr. Trabucco from all prepetition debts, according to court records.
Meanwhile, as the malicious prosecution case against Mr. Cogan continued, Dr. Trabucco’s name again drew media attention. His former girlfriend was arrested for plotting to kidnap and kill an attorney who was representing Dr. Trabucco’s ex-wife in a divorce proceeding. Renee Perillo was sentenced to 27 years in prison for conspiracy to commit kidnapping and murder for hire for trying to kill Noblesville, Ind., attorney Rebecca Eimerman. Ms. Eimerman was pursuing divorce settlement money from Dr. Trabucco on behalf of his ex-wife. Ms. Perillo’s son was also charged in the crime.
No charges related to the crime were filed against Dr. Trabucco, according to the Hamilton County Prosecutor’s Office and federal charging records. Dr. Trabucco said he was not involved in the incident and had no knowledge of Perillo’s plans.
A hard fought – and still continuing – legal battle
After a 3-day trial, jurors in 2018 found that Mr. Cogan owed Dr. Trabucco $8 million for the harm caused by the unfounded claims.
“This has had a tremendous impact on my life,” Dr. Trabucco said. “It’s cost me a lot of time, money, and anguish.”
Mr. Cogan appealed the verdict. An Arizona appeals court in 2020 upheld the finding of liability for malicious prosecution against Mr. Cogan, but it vacated the finding of liability for abuse of process. Because of the partial reversal, the appellate court vacated the $8 million and sent the case back to the Superior Court in Mohave County, Ariz., for a new trial on damages.
But shortly before the December 2021 retrial, both parties agreed to settle for $8 million.
The settlement, however, is not the end of the litigation between the parties.
Late last year, Mr. Cogan filed a new case in the U.S. District Court for the District of Nevada against Dr. Trabucco alleging the Arizona court that tried the malicious prosecution case never had jurisdiction. Mr. Cogan contends that per federal case law, any damages resulting from a bankruptcy court pleading give the federal court exclusive jurisdiction over the matter.
“I believe that I will win the federal court case,” Mr. Cogan said. “If Judge Andrew P. Gordon finds for me, Dr. Trabucco’s judgment in Arizona is void as Arizona did not have subject-matter jurisdiction.”
Dr. Trabucco says the new federal case has no merit.
“The federal courts have no jurisdiction over civil matters, and this should be thrown out,” he said. “However, my attorney is fully prepared to take this to the Supreme Court [if it moves forward].”
At this article’s deadline, the judge had not yet ruled on Dr. Trabucco’s motion to dismiss the federal complaint.
Should a physician sue for malicious prosecution?
Dr. Trabucco’s case raises the question of whether physicians should consider suing a patient after winning their malpractice case. As many doctors know, a successful case outcome doesn’t necessarily undo the time spent, income lost, and reputation harm that often comes with a negligence lawsuit. Is suing for malicious prosecution a reasonable route to recoup some of the damages caused by the claim?
“It’s an uphill struggle to prevail,” said Jeffrey Segal, MD, JD. “Have people done it? Yes, they have. Is it easy? No.”
A physician must hit the marks of a distinct checklist to pursue a malicious prosecution case, said Dr. Segal, CEO and founder of Medical Justice, a company that aids and advises physicians on legal matters. One necessary element is that the malpractice case against the physician must have been adjudicated on the merits, he said. For example, the doctor won the case at trial or the case was dismissed on summary judgment. Summary judgment refers to a court tossing the claim because there was no genuine dispute as to any material fact and because the defendant is entitled to judgment as a matter of law.
If the case was dismissed for another reason, such as a technicality, or dropped by the plaintiff, the case would not meet the threshold for a malicious prosecution claim, Dr. Segal explains.
The physician must also show that the lawsuit was instituted with “malice,” meaning with an intent to hurt the physician, and that the lawsuit was brought without “probable cause,” adds J. Richard Moore, JD, a medical malpractice defense attorney based in Indianapolis.
“The courts are still pretty good at weeding out cases with no merit whatsoever, so even if a physician takes a case to trial and wins, it is exceedingly rare that a case that makes it that far, has no merit whatsoever,” Mr. Moore said.
In addition, a plaintiffs’ attorney may be protected from a malicious prosecution claim because they relied on a medical expert’s opinion that reasonable cause existed to file a lawsuit, notes William Sullivan, DO, JD, an emergency physician and attorney based in Frankfort, Ill.
Dr. Sullivan has personal experience with such a challenge. He filed a malicious prosecution claim against a plaintiffs’ attorney after being dismissed from a medical malpractice case. The claim stemmed from Dr. Sullivan inserting an emergency central line into a trauma patient who was taken to surgery and later died. The suit alleged Dr. Sullivan failed to diagnose internal bleeding and failed to perform surgery, although surgeons at the hospital knew internal bleeding was present and Dr. Sullivan had no privileges to perform surgery, he said.
However, Dr. Sullivan was unable to identify the medical expert involved in the claim. Illinois law allows a malpractice plaintiff to withhold the identity of a medical expert who certifies a malpractice lawsuit. Because Dr. Sullivan couldn’t identify the expert, he could not depose the physician, and the law firm claimed there was no malicious prosecution because it relied on the trauma surgeon’s opinion that Dr. Sullivan was liable.
The trial court agreed with the law firm’s argument and dismissed them from the case, Dr. Sullivan said. The trial court also agreed that the expert trauma surgeon should be allowed to remain anonymous in accordance with Illinois law.
Alternative options for doctors to recover damages
For physicians who want to recoup after a frivolous claim, but don’t want to dive into another lawsuit, there are other options, say legal experts.
One alternative is filing a motion for sanctions, Dr. Sullivan said. If an attorney files a lawsuit that does not have a reasonable basis in fact or law, that attorney could be subject to sanctions, including paying for the physician’s attorney’s fees and costs, he explained. If a motion for sanctions is granted against an opposing attorney, that fact may be reportable to the attorney’s insurance carrier and also reportable to the attorney’s state licensing board. A motion for sanctions does not require filing of a separate lawsuit and filing a motion for sanctions may allow the defendant physician or the defense attorney to depose the plaintiff attorney regarding the reasonable basis that attorney had for filing a lawsuit, Dr. Sullivan said.
Dr. Sullivan recently represented a physician who won a motion for sanctions. During the legal action against him, Dr. Sullivan presented the plaintiff’s attorney with information showing why his lawsuit did not have a reasonable basis, but the attorney repeatedly ignored the information.
“Eventually, I filed a motion to dismiss the physician from the lawsuit and that motion was granted,” he said. “I also filed a motion for sanctions so that the physician could recover the costs involved in defending the claim. The trial court granted our motion for sanctions against the plaintiff and her law firm and awarded my client more than $10,000.”
Physicians who believe a plaintiffs’ attorney is acting unprofessionally can also file a complaint with the attorney’s bar, Dr. Segal said. And expert witnesses acting in bad faith can be reported to professional societies, medical licensing boards, and/or specialty boards.
“There are a number of avenues to address the sense of justice,” he said. “But if you’re looking for a payday, the only way to do that is by going to court.”
A version of this article first appeared on Medscape.com.
Urologist Arnaldo Trabucco, MD, was outraged when he learned of a legal claim filed by a patient’s family blaming him for the patient’s death.
Dr. Trabucco had performed a successful laparoscopic left radical nephrectomy on Gerald Scharf, but he died 3 days later from an unrelated condition, said Dr. Trabucco, who now practices in California. The claims alleged not only wrongful death, but also that Dr. Trabucco “committed willful and malicious actions upon” the patient, eventually resulting in his death, and that Dr. Trabucco’s actions constituted “extreme and outrageous behavior.”
The accusations were levied on behalf of the patient’s family by a bankruptcy attorney who admittedly had no experience in medical malpractice litigation.
“It was a vicious allegation that I intentionally tried to kill my own patient,” said Dr. Trabucco, 66. “It’s an allegation that was outlandish. Those were fighting words.”
As the case dragged on, Dr. Trabucco filed a complaint against the Scharfs and bankruptcy attorney Jeffrey Cogan for malicious prosecution and abuse of process. In 2018, Dr. Trabucco prevailed when a jury awarded him $6,232,000 in compensatory damages and $1,768,000 in punitive damages against Mr. Cogan. (No damages were assessed against the patient’s family.)
“This was a principle issue more than anything else,” Dr. Trabucco said. “My name was tarnished and thrown through the mud. I’m very proud of my name and my reputation.”
Mr. Cogan, a bankruptcy attorney licensed in Nevada and California, disputes that his actions were malicious and disagrees with the jury’s verdict.
“I don’t think I did anything wrong with filing the lawsuit,” he said.
Despite the $8 million award, the legal fight between Dr. Trabucco and Mr. Cogan has continued – and is not over yet.
Not your ordinary legal journey
Dr. Trabucco completed his medical training in Rome before moving back to the United States to begin his residency in Brooklyn, New York.
He quietly practiced urology in New York for years before moving to Columbus, Ind., to work at a urology clinic. In 2005, Dr. Trabucco made headlines when the Bartholomew County, Ind., sheriff’s deputies found marijuana plants growing in his home. His medical license was suspended, and he pled guilty to a misdemeanor.
Dr. Trabucco said he was unaware that a family member was growing the plants in the home and that he was not responsible for the operation. The misdemeanor has since been expunged from his record, according to Dr. Trabucco. Bartholomew County records do not list the offense. He later moved to Arizona, where he opened a medical practice.
Before the Scharfs filed their malpractice case in 2013, Dr. Trabucco had been embroiled in a series of unrelated legal disputes, including postdivorce proceedings and a complaint against him by another physician, alleging defamation. In that case, the physician claimed Dr. Trabucco published false and defamatory communications about him, including that the physician committed intentional fraud.
Dr. Trabucco filed his own suit for defamation and infliction of emotional distress against the same physician for alleged threats and harassment and making false reports about him to several Arizona state entities. The defamation suit against Dr. Trabucco ended in a settlement, the terms of which are confidential, according to Mr. Cogan. Dr. Trabucco’s defamation case against the physician was dismissed, according to court records, but they do not specify the reason. Dr. Trabucco said he dropped the defamation case because of the other relentless litigation he was facing.
In November 2012, Dr. Trabucco filed for Chapter 7 bankruptcy protection.
Mr. Cogan was representing Dr. Trabucco’s ex-wife’s interests as a creditor in the bankruptcy proceeding and eventually represented the physician who sued Dr. Trabucco, according to court documents. In all, Mr. Cogan represented six creditors in connection to Dr. Trabucco’s bankruptcy, including the Scharfs. He also took over the Scharfs’ malpractice case in Arizona’s Mohave County Superior Court after their attorney died.
As part of a filing in Nevada bankruptcy court called a “Complaint to Determine Nondischargeabiliy of Debts,” Mr. Cogan alleged that Dr. Trabucco knew he lacked sufficient expertise regarding the laparoscopic nephrectomy, that an intraoperative complication occurred because of his error, and that Dr. Trabucco hid the complication and did not attempt to remedy the situation, among other claims.
In addition to the Mohave County filing and the bankruptcy filing, Mr. Cogan filed a similar medical malpractice case against Dr. Trabucco in Arizona district court.
“I believe, based upon the statements by [a retired medical malpractice attorney], that Dr Trabucco squeezed the abdominal aorta,” Mr. Cogan said. “When he did so, plaque from Mr Scharf blocked blood from going to his remaining his kidney, causing his death. My lawsuit said that Dr Trabucco knew he made a mistake and went home rather than fixing the mistake.”
Dr. Trabucco said the laparoscopic nephrectomy went smoothly. A few hours after the surgery, however, Mr. Scharf was transferred to a Las Vegas hospital with a diagnosis of potential occlusion of the abdominal aorta. Surgery was performed, and while the operative report noted the presence of severe atherosclerosis of the abdominal aorta, there was no indication of an intraoperative injury to the aorta from Dr. Trabucco’s surgery, according to court documents filed in U.S. District Court for the District of Arizona. An autopsy performed on Mr. Scharf reported “severe calcific aortic atherosclerosis, primarily at the aortic arch and abdominal aorta at the level of the branching of the renal arteries with small adherent thrombus.” The autopsy did not include any indication or suggestion of an intraoperative injury to the abdominal aorta.
A federal jury found unanimously in favor of Dr. Trabucco, concluding that he did nothing wrong. The original medical malpractice case in Mohave County court was ultimately dismissed and in 2014, the bankruptcy court entered an order discharging Dr. Trabucco from all prepetition debts, according to court records.
Meanwhile, as the malicious prosecution case against Mr. Cogan continued, Dr. Trabucco’s name again drew media attention. His former girlfriend was arrested for plotting to kidnap and kill an attorney who was representing Dr. Trabucco’s ex-wife in a divorce proceeding. Renee Perillo was sentenced to 27 years in prison for conspiracy to commit kidnapping and murder for hire for trying to kill Noblesville, Ind., attorney Rebecca Eimerman. Ms. Eimerman was pursuing divorce settlement money from Dr. Trabucco on behalf of his ex-wife. Ms. Perillo’s son was also charged in the crime.
No charges related to the crime were filed against Dr. Trabucco, according to the Hamilton County Prosecutor’s Office and federal charging records. Dr. Trabucco said he was not involved in the incident and had no knowledge of Perillo’s plans.
A hard fought – and still continuing – legal battle
After a 3-day trial, jurors in 2018 found that Mr. Cogan owed Dr. Trabucco $8 million for the harm caused by the unfounded claims.
“This has had a tremendous impact on my life,” Dr. Trabucco said. “It’s cost me a lot of time, money, and anguish.”
Mr. Cogan appealed the verdict. An Arizona appeals court in 2020 upheld the finding of liability for malicious prosecution against Mr. Cogan, but it vacated the finding of liability for abuse of process. Because of the partial reversal, the appellate court vacated the $8 million and sent the case back to the Superior Court in Mohave County, Ariz., for a new trial on damages.
But shortly before the December 2021 retrial, both parties agreed to settle for $8 million.
The settlement, however, is not the end of the litigation between the parties.
Late last year, Mr. Cogan filed a new case in the U.S. District Court for the District of Nevada against Dr. Trabucco alleging the Arizona court that tried the malicious prosecution case never had jurisdiction. Mr. Cogan contends that per federal case law, any damages resulting from a bankruptcy court pleading give the federal court exclusive jurisdiction over the matter.
“I believe that I will win the federal court case,” Mr. Cogan said. “If Judge Andrew P. Gordon finds for me, Dr. Trabucco’s judgment in Arizona is void as Arizona did not have subject-matter jurisdiction.”
Dr. Trabucco says the new federal case has no merit.
“The federal courts have no jurisdiction over civil matters, and this should be thrown out,” he said. “However, my attorney is fully prepared to take this to the Supreme Court [if it moves forward].”
At this article’s deadline, the judge had not yet ruled on Dr. Trabucco’s motion to dismiss the federal complaint.
Should a physician sue for malicious prosecution?
Dr. Trabucco’s case raises the question of whether physicians should consider suing a patient after winning their malpractice case. As many doctors know, a successful case outcome doesn’t necessarily undo the time spent, income lost, and reputation harm that often comes with a negligence lawsuit. Is suing for malicious prosecution a reasonable route to recoup some of the damages caused by the claim?
“It’s an uphill struggle to prevail,” said Jeffrey Segal, MD, JD. “Have people done it? Yes, they have. Is it easy? No.”
A physician must hit the marks of a distinct checklist to pursue a malicious prosecution case, said Dr. Segal, CEO and founder of Medical Justice, a company that aids and advises physicians on legal matters. One necessary element is that the malpractice case against the physician must have been adjudicated on the merits, he said. For example, the doctor won the case at trial or the case was dismissed on summary judgment. Summary judgment refers to a court tossing the claim because there was no genuine dispute as to any material fact and because the defendant is entitled to judgment as a matter of law.
If the case was dismissed for another reason, such as a technicality, or dropped by the plaintiff, the case would not meet the threshold for a malicious prosecution claim, Dr. Segal explains.
The physician must also show that the lawsuit was instituted with “malice,” meaning with an intent to hurt the physician, and that the lawsuit was brought without “probable cause,” adds J. Richard Moore, JD, a medical malpractice defense attorney based in Indianapolis.
“The courts are still pretty good at weeding out cases with no merit whatsoever, so even if a physician takes a case to trial and wins, it is exceedingly rare that a case that makes it that far, has no merit whatsoever,” Mr. Moore said.
In addition, a plaintiffs’ attorney may be protected from a malicious prosecution claim because they relied on a medical expert’s opinion that reasonable cause existed to file a lawsuit, notes William Sullivan, DO, JD, an emergency physician and attorney based in Frankfort, Ill.
Dr. Sullivan has personal experience with such a challenge. He filed a malicious prosecution claim against a plaintiffs’ attorney after being dismissed from a medical malpractice case. The claim stemmed from Dr. Sullivan inserting an emergency central line into a trauma patient who was taken to surgery and later died. The suit alleged Dr. Sullivan failed to diagnose internal bleeding and failed to perform surgery, although surgeons at the hospital knew internal bleeding was present and Dr. Sullivan had no privileges to perform surgery, he said.
However, Dr. Sullivan was unable to identify the medical expert involved in the claim. Illinois law allows a malpractice plaintiff to withhold the identity of a medical expert who certifies a malpractice lawsuit. Because Dr. Sullivan couldn’t identify the expert, he could not depose the physician, and the law firm claimed there was no malicious prosecution because it relied on the trauma surgeon’s opinion that Dr. Sullivan was liable.
The trial court agreed with the law firm’s argument and dismissed them from the case, Dr. Sullivan said. The trial court also agreed that the expert trauma surgeon should be allowed to remain anonymous in accordance with Illinois law.
Alternative options for doctors to recover damages
For physicians who want to recoup after a frivolous claim, but don’t want to dive into another lawsuit, there are other options, say legal experts.
One alternative is filing a motion for sanctions, Dr. Sullivan said. If an attorney files a lawsuit that does not have a reasonable basis in fact or law, that attorney could be subject to sanctions, including paying for the physician’s attorney’s fees and costs, he explained. If a motion for sanctions is granted against an opposing attorney, that fact may be reportable to the attorney’s insurance carrier and also reportable to the attorney’s state licensing board. A motion for sanctions does not require filing of a separate lawsuit and filing a motion for sanctions may allow the defendant physician or the defense attorney to depose the plaintiff attorney regarding the reasonable basis that attorney had for filing a lawsuit, Dr. Sullivan said.
Dr. Sullivan recently represented a physician who won a motion for sanctions. During the legal action against him, Dr. Sullivan presented the plaintiff’s attorney with information showing why his lawsuit did not have a reasonable basis, but the attorney repeatedly ignored the information.
“Eventually, I filed a motion to dismiss the physician from the lawsuit and that motion was granted,” he said. “I also filed a motion for sanctions so that the physician could recover the costs involved in defending the claim. The trial court granted our motion for sanctions against the plaintiff and her law firm and awarded my client more than $10,000.”
Physicians who believe a plaintiffs’ attorney is acting unprofessionally can also file a complaint with the attorney’s bar, Dr. Segal said. And expert witnesses acting in bad faith can be reported to professional societies, medical licensing boards, and/or specialty boards.
“There are a number of avenues to address the sense of justice,” he said. “But if you’re looking for a payday, the only way to do that is by going to court.”
A version of this article first appeared on Medscape.com.
U.S. primary care seen lagging in key markers
In delivery of primary care, including access and coordination, the U.S. trails well behind 10 other wealthy countries, according to a new report from the Commonwealth Fund.
The document, released March 15, concludes that the shortcomings in the U.S. system – from a lack of a relationship with a primary care physician to unequal access to after-hours care – “disproportionately affect Black and Latinx communities and rural areas, exacerbating disparities that have widened during the COVID-19 pandemic.”
“This report really shows that the U.S. is falling behind. We know that a strong primary care system yields better health outcomes. We have a lot to learn from other high-income countries,” coauthor Munira Z. Gunja, MPH, a senior researcher for the Commonwealth Fund’s International Program in Health Policy and Practice Innovations, told this news organization. “At baseline, we really need to make sure that everyone has health insurance in this country so they can actually use primary care services, and we need to increase the supply of those services.”
The report draws from the Commonwealth Fund’s 2019 and 2020 International Health Policy Surveys and the 2020 International Profiles of Health Care Systems. Among the main points:
- U.S. adults are the least likely to have a regular physician or place of care or a long-standing relationship with a primary care provider: 43% of American adults have a long-term relationship with a primary care doctor, compared with highs of 71% in Germany and the Netherlands.
- Access to home visits or after-hours care – excluding emergency department visits – is lowest in the United States (45%). In the Netherlands, Norway, New Zealand, and Germany, the rate is 90% to 96%.
- Half of primary care providers in the United States report adequate coordination with specialists and hospitals – around the average for the 11 countries studied.
‘Dismal mess’
Experts reacted to the report with a mix of concern and frustration – but not surprise.
“The results in this report are not surprising, and we have known them all for a number of years now,” Timothy Hoff, PhD, a health policy expert at Northeastern University, Boston, said. “Primary care doctors remain the backbone of our primary care system. But there are too few of them in the United States, and there likely will remain too few of them in the future. This opens the door to other and more diverse forms of innovation that will be required to help complement the work they do.”
Dr. Hoff, author of Searching for the Family Doctor: Primary Care on the Brink, added that comparing the United States to smaller countries like Norway or the United Kingdom is “somewhat problematic.”
“Our system has to take care of several hundred million people, trapped in a fragmented and market-based delivery system focused on specialty care, each of whom may have a different insurance plan,” he said. “Doing some of the things very small countries with government-funded insurance and a history of strong primary care delivery do in taking care of far fewer citizens is not realistic.”
Jeffrey Borkan, MD, PhD, chair and professor in the department of family medicine at the Alpert Medical School of Brown University, Providence, R.I., said the most shocking finding in the report is that despite spending far more on health care than any other country, “we cannot manage to provide one of the least expensive and most efficacious services: a relationship with a primary care doctor.”
Arthur Caplan, PhD, director of the Division of Medical Ethics at New York University Langone Medical Center, called primary care in this country “a dismal mess. It has been for many years. This is especially so in mental health. Access in many counties is nonexistent, and many primary care physicians are opting into boutique care.”
R. Shawn Martin, CEO of the 133,000-member American Academy of Family Physicians, said, “None of this surprises me. I think these are trendlines; we have been following this for many, many years here at the Academy.”
Mr. Martin added that he was disappointed that the recent, large investments in sharing and digitizing information have not closed the gaps that hinder the efficient and widespread delivery of primary care.
The findings in the report weren’t all bad. More primary care providers in the United States (30%) screen their patients for social needs such as housing, food security, and transportation – the highest among all 11 nations studied.
Also, Commonwealth Fund said the proportion of patients who said they received information on meeting their social needs and screening for domestic violence or social isolation was low everywhere. However, the percentage in the United States, Canada, and Norway was the highest, at 9%. Sweden had the lowest rate for such screenings, at 1%.
The researchers noted that social determinants of health account for as much as 55% of health outcomes. “In some countries, like the United States, the higher rates of receiving such information may be a response to the higher rates of material hardship, along with a weaker safety net,” the report states.
Ms. Gunja and her colleagues suggested several options for changes in policies, including narrowing the wage gap between primary care providers and higher-paid specialists; subsidizing medical school tuition to give students incentives to enter primary care; investing in telehealth to make primary care more accessible; and rewarding and holding providers accountable for continuity of care.
“The U.S. had the largest wage gap and highest tuition fees among the countries we studied,” Ms. Gunja told this news organization..
Researchers noted that U.S. patients could benefit from the introduction of incentives such as those paid in New Zealand to primary health organizations, which receive additional funding per capita to promote health and coordinate care.
But Dr. Caplan was skeptical that those measures would do much to correct the problems.
“We have no will to fix this ongoing, scandalous situation,” he said. “Specialist care still pays inordinately large salaries. Nurses and physician extenders are underused. Academic prestige does little to reward primary care. Plus, patients are not pressing for better access. Sorry, but I see no solutions pending in the current climate. Obamacare barely survived.”
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In delivery of primary care, including access and coordination, the U.S. trails well behind 10 other wealthy countries, according to a new report from the Commonwealth Fund.
The document, released March 15, concludes that the shortcomings in the U.S. system – from a lack of a relationship with a primary care physician to unequal access to after-hours care – “disproportionately affect Black and Latinx communities and rural areas, exacerbating disparities that have widened during the COVID-19 pandemic.”
“This report really shows that the U.S. is falling behind. We know that a strong primary care system yields better health outcomes. We have a lot to learn from other high-income countries,” coauthor Munira Z. Gunja, MPH, a senior researcher for the Commonwealth Fund’s International Program in Health Policy and Practice Innovations, told this news organization. “At baseline, we really need to make sure that everyone has health insurance in this country so they can actually use primary care services, and we need to increase the supply of those services.”
The report draws from the Commonwealth Fund’s 2019 and 2020 International Health Policy Surveys and the 2020 International Profiles of Health Care Systems. Among the main points:
- U.S. adults are the least likely to have a regular physician or place of care or a long-standing relationship with a primary care provider: 43% of American adults have a long-term relationship with a primary care doctor, compared with highs of 71% in Germany and the Netherlands.
- Access to home visits or after-hours care – excluding emergency department visits – is lowest in the United States (45%). In the Netherlands, Norway, New Zealand, and Germany, the rate is 90% to 96%.
- Half of primary care providers in the United States report adequate coordination with specialists and hospitals – around the average for the 11 countries studied.
‘Dismal mess’
Experts reacted to the report with a mix of concern and frustration – but not surprise.
“The results in this report are not surprising, and we have known them all for a number of years now,” Timothy Hoff, PhD, a health policy expert at Northeastern University, Boston, said. “Primary care doctors remain the backbone of our primary care system. But there are too few of them in the United States, and there likely will remain too few of them in the future. This opens the door to other and more diverse forms of innovation that will be required to help complement the work they do.”
Dr. Hoff, author of Searching for the Family Doctor: Primary Care on the Brink, added that comparing the United States to smaller countries like Norway or the United Kingdom is “somewhat problematic.”
“Our system has to take care of several hundred million people, trapped in a fragmented and market-based delivery system focused on specialty care, each of whom may have a different insurance plan,” he said. “Doing some of the things very small countries with government-funded insurance and a history of strong primary care delivery do in taking care of far fewer citizens is not realistic.”
Jeffrey Borkan, MD, PhD, chair and professor in the department of family medicine at the Alpert Medical School of Brown University, Providence, R.I., said the most shocking finding in the report is that despite spending far more on health care than any other country, “we cannot manage to provide one of the least expensive and most efficacious services: a relationship with a primary care doctor.”
Arthur Caplan, PhD, director of the Division of Medical Ethics at New York University Langone Medical Center, called primary care in this country “a dismal mess. It has been for many years. This is especially so in mental health. Access in many counties is nonexistent, and many primary care physicians are opting into boutique care.”
R. Shawn Martin, CEO of the 133,000-member American Academy of Family Physicians, said, “None of this surprises me. I think these are trendlines; we have been following this for many, many years here at the Academy.”
Mr. Martin added that he was disappointed that the recent, large investments in sharing and digitizing information have not closed the gaps that hinder the efficient and widespread delivery of primary care.
The findings in the report weren’t all bad. More primary care providers in the United States (30%) screen their patients for social needs such as housing, food security, and transportation – the highest among all 11 nations studied.
Also, Commonwealth Fund said the proportion of patients who said they received information on meeting their social needs and screening for domestic violence or social isolation was low everywhere. However, the percentage in the United States, Canada, and Norway was the highest, at 9%. Sweden had the lowest rate for such screenings, at 1%.
The researchers noted that social determinants of health account for as much as 55% of health outcomes. “In some countries, like the United States, the higher rates of receiving such information may be a response to the higher rates of material hardship, along with a weaker safety net,” the report states.
Ms. Gunja and her colleagues suggested several options for changes in policies, including narrowing the wage gap between primary care providers and higher-paid specialists; subsidizing medical school tuition to give students incentives to enter primary care; investing in telehealth to make primary care more accessible; and rewarding and holding providers accountable for continuity of care.
“The U.S. had the largest wage gap and highest tuition fees among the countries we studied,” Ms. Gunja told this news organization..
Researchers noted that U.S. patients could benefit from the introduction of incentives such as those paid in New Zealand to primary health organizations, which receive additional funding per capita to promote health and coordinate care.
But Dr. Caplan was skeptical that those measures would do much to correct the problems.
“We have no will to fix this ongoing, scandalous situation,” he said. “Specialist care still pays inordinately large salaries. Nurses and physician extenders are underused. Academic prestige does little to reward primary care. Plus, patients are not pressing for better access. Sorry, but I see no solutions pending in the current climate. Obamacare barely survived.”
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In delivery of primary care, including access and coordination, the U.S. trails well behind 10 other wealthy countries, according to a new report from the Commonwealth Fund.
The document, released March 15, concludes that the shortcomings in the U.S. system – from a lack of a relationship with a primary care physician to unequal access to after-hours care – “disproportionately affect Black and Latinx communities and rural areas, exacerbating disparities that have widened during the COVID-19 pandemic.”
“This report really shows that the U.S. is falling behind. We know that a strong primary care system yields better health outcomes. We have a lot to learn from other high-income countries,” coauthor Munira Z. Gunja, MPH, a senior researcher for the Commonwealth Fund’s International Program in Health Policy and Practice Innovations, told this news organization. “At baseline, we really need to make sure that everyone has health insurance in this country so they can actually use primary care services, and we need to increase the supply of those services.”
The report draws from the Commonwealth Fund’s 2019 and 2020 International Health Policy Surveys and the 2020 International Profiles of Health Care Systems. Among the main points:
- U.S. adults are the least likely to have a regular physician or place of care or a long-standing relationship with a primary care provider: 43% of American adults have a long-term relationship with a primary care doctor, compared with highs of 71% in Germany and the Netherlands.
- Access to home visits or after-hours care – excluding emergency department visits – is lowest in the United States (45%). In the Netherlands, Norway, New Zealand, and Germany, the rate is 90% to 96%.
- Half of primary care providers in the United States report adequate coordination with specialists and hospitals – around the average for the 11 countries studied.
‘Dismal mess’
Experts reacted to the report with a mix of concern and frustration – but not surprise.
“The results in this report are not surprising, and we have known them all for a number of years now,” Timothy Hoff, PhD, a health policy expert at Northeastern University, Boston, said. “Primary care doctors remain the backbone of our primary care system. But there are too few of them in the United States, and there likely will remain too few of them in the future. This opens the door to other and more diverse forms of innovation that will be required to help complement the work they do.”
Dr. Hoff, author of Searching for the Family Doctor: Primary Care on the Brink, added that comparing the United States to smaller countries like Norway or the United Kingdom is “somewhat problematic.”
“Our system has to take care of several hundred million people, trapped in a fragmented and market-based delivery system focused on specialty care, each of whom may have a different insurance plan,” he said. “Doing some of the things very small countries with government-funded insurance and a history of strong primary care delivery do in taking care of far fewer citizens is not realistic.”
Jeffrey Borkan, MD, PhD, chair and professor in the department of family medicine at the Alpert Medical School of Brown University, Providence, R.I., said the most shocking finding in the report is that despite spending far more on health care than any other country, “we cannot manage to provide one of the least expensive and most efficacious services: a relationship with a primary care doctor.”
Arthur Caplan, PhD, director of the Division of Medical Ethics at New York University Langone Medical Center, called primary care in this country “a dismal mess. It has been for many years. This is especially so in mental health. Access in many counties is nonexistent, and many primary care physicians are opting into boutique care.”
R. Shawn Martin, CEO of the 133,000-member American Academy of Family Physicians, said, “None of this surprises me. I think these are trendlines; we have been following this for many, many years here at the Academy.”
Mr. Martin added that he was disappointed that the recent, large investments in sharing and digitizing information have not closed the gaps that hinder the efficient and widespread delivery of primary care.
The findings in the report weren’t all bad. More primary care providers in the United States (30%) screen their patients for social needs such as housing, food security, and transportation – the highest among all 11 nations studied.
Also, Commonwealth Fund said the proportion of patients who said they received information on meeting their social needs and screening for domestic violence or social isolation was low everywhere. However, the percentage in the United States, Canada, and Norway was the highest, at 9%. Sweden had the lowest rate for such screenings, at 1%.
The researchers noted that social determinants of health account for as much as 55% of health outcomes. “In some countries, like the United States, the higher rates of receiving such information may be a response to the higher rates of material hardship, along with a weaker safety net,” the report states.
Ms. Gunja and her colleagues suggested several options for changes in policies, including narrowing the wage gap between primary care providers and higher-paid specialists; subsidizing medical school tuition to give students incentives to enter primary care; investing in telehealth to make primary care more accessible; and rewarding and holding providers accountable for continuity of care.
“The U.S. had the largest wage gap and highest tuition fees among the countries we studied,” Ms. Gunja told this news organization..
Researchers noted that U.S. patients could benefit from the introduction of incentives such as those paid in New Zealand to primary health organizations, which receive additional funding per capita to promote health and coordinate care.
But Dr. Caplan was skeptical that those measures would do much to correct the problems.
“We have no will to fix this ongoing, scandalous situation,” he said. “Specialist care still pays inordinately large salaries. Nurses and physician extenders are underused. Academic prestige does little to reward primary care. Plus, patients are not pressing for better access. Sorry, but I see no solutions pending in the current climate. Obamacare barely survived.”
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Empagliflozin scores topline win in EMPA-KIDNEY trial
Researchers running the EMPA-KIDNEY trial that’s been testing the safety and efficacy of the SGLT2 inhibitor empagliflozin (Jardiance) in about 6,600 patients with chronic kidney disease (CKD) announced on March 16 that they had stopped the trial early because of positive efficacy that met the study’s prespecified threshold for early termination.
EMPA-KIDNEY is the third major trial of an agent from the sodium-glucose cotransport 2 (SGLT2) inhibitor class tested in patients with CKD to be stopped early because of positive results that met a prespecified termination rule.
In 2020, the DAPA-CKD trial of dapagliflozin (Farxiga) stopped early, after a median follow-up of 2.4 years, because of positive efficacy results. In 2019, the same thing happened in the CREDENCE trial of canagliflozin (Invokana), with the unexpected halt coming after a median follow-up of 2.62 years.
The announcement about EMPA-KIDNEY did not include information on median follow-up, but enrollment into the trial ran from May 2019 to April 2021, which means that the longest that enrolled patients could have been in the study was about 2.85 years.
The primary efficacy endpoint in EMPA-KIDNEY was a composite of a sustained decline in estimated glomerular filtration rate (eGFR) to less than 10 mL/min/1.73 m2, renal death, a sustained decline of at least 40% in eGFR from baseline, or cardiovascular death. The announcement of the trial’s early termination provided no details on the efficacy results.
EMPA-KIDNEY enrolled a wider range of patients
EMPA-KIDNEY expands the scope of types of patients with CKD now shown to benefit from treatment with an SGLT2 inhibitor. CREDENCE tested canagliflozin only in patients with type 2 diabetes and diabetic nephropathy, and in DAPA-CKD, two-thirds of enrolled patients had type 2 diabetes, and all had CKD. In EMPA-KIDNEY, 46% of the 6,609 enrolled patients had diabetes (including a very small number with type 1 diabetes).
Another departure from prior studies of an SGLT2 inhibitor for patients selected primarily for having CKD was that in EMPA-KIDNEY, 20% of patients did not have albuminuria, and for 34%, eGFR at entry was less than 30 mL/min/1.73 m2, with all enrolled patients required to have an eGFR at entry of greater than or equal to 20 mL/min/1.73 m2. Average eGFR in EMPA-KIDNEY was about 38 mL/min/1.73 m2. To be included in the trial, patients were not required to have albuminuria, except those whose eGFR was greater than or equal to 45 mL/min/1.73 m2.
In DAPA-CKD, the minimum eGFR at entry had to be greater than or equal to 25 mL/min/1.73 m2, and roughly 14% of enrolled patients had an eGFR of less than 30 mL/min/1.73 m2. The average eGFR in DAPA-CKD was about 43 mL/min/1.73 m2. In addition, all patients had at least microalbuminuria, with a minimum urinary albumin-to-creatinine ratio of 200. In CREDENCE, the minimum eGFR for enrollment was 30 mL/min/1.73 m2, and the average eGFR was about 56 mL/min/1.73 m2. All patients in CREDENCE had to have macroalbuminuria, with a urinary albumin-to-creatinine ratio of more than 300.
According to the researchers who designed EMPA-KIDNEY, the trial enrollment criteria aimed to include adults with CKD “who are frequently seen in practice but were under-represented in previous SGLT2 inhibitor trials.”
Indications for empagliflozin are expanding
The success of empagliflozin in EMPA-KIDNEY follows its positive results in both the EMPEROR-Reduced and EMPEROR-Preserved trials, which collectively proved the efficacy of the agent for patients with heart failure regardless of their left ventricular ejection fraction and regardless of whether they also had diabetes.
These results led the U.S. Food and Drug Administration to recently expand the labeled indication for empagliflozin to all patients with heart failure. Empagliflozin also has labeled indications for glycemic control in patients with type 2 diabetes and to reduce the risk of cardiovascular death in adults with type 2 diabetes and established cardiovascular disease.
As of today, empagliflozin has no labeled indication for treating patients with CKD. Dapagliflozin received that indication in April 2021, and canagliflozin received an indication for treating patients with type 2 diabetes, diabetic nephropathy, and albuminuria in September 2019.
EMPA-KIDNEY is sponsored by Boehringer Ingelheim and Lilly, the two companies that jointly market empagliflozin (Jardiance).
A version of this article first appeared on Medscape.com.
Researchers running the EMPA-KIDNEY trial that’s been testing the safety and efficacy of the SGLT2 inhibitor empagliflozin (Jardiance) in about 6,600 patients with chronic kidney disease (CKD) announced on March 16 that they had stopped the trial early because of positive efficacy that met the study’s prespecified threshold for early termination.
EMPA-KIDNEY is the third major trial of an agent from the sodium-glucose cotransport 2 (SGLT2) inhibitor class tested in patients with CKD to be stopped early because of positive results that met a prespecified termination rule.
In 2020, the DAPA-CKD trial of dapagliflozin (Farxiga) stopped early, after a median follow-up of 2.4 years, because of positive efficacy results. In 2019, the same thing happened in the CREDENCE trial of canagliflozin (Invokana), with the unexpected halt coming after a median follow-up of 2.62 years.
The announcement about EMPA-KIDNEY did not include information on median follow-up, but enrollment into the trial ran from May 2019 to April 2021, which means that the longest that enrolled patients could have been in the study was about 2.85 years.
The primary efficacy endpoint in EMPA-KIDNEY was a composite of a sustained decline in estimated glomerular filtration rate (eGFR) to less than 10 mL/min/1.73 m2, renal death, a sustained decline of at least 40% in eGFR from baseline, or cardiovascular death. The announcement of the trial’s early termination provided no details on the efficacy results.
EMPA-KIDNEY enrolled a wider range of patients
EMPA-KIDNEY expands the scope of types of patients with CKD now shown to benefit from treatment with an SGLT2 inhibitor. CREDENCE tested canagliflozin only in patients with type 2 diabetes and diabetic nephropathy, and in DAPA-CKD, two-thirds of enrolled patients had type 2 diabetes, and all had CKD. In EMPA-KIDNEY, 46% of the 6,609 enrolled patients had diabetes (including a very small number with type 1 diabetes).
Another departure from prior studies of an SGLT2 inhibitor for patients selected primarily for having CKD was that in EMPA-KIDNEY, 20% of patients did not have albuminuria, and for 34%, eGFR at entry was less than 30 mL/min/1.73 m2, with all enrolled patients required to have an eGFR at entry of greater than or equal to 20 mL/min/1.73 m2. Average eGFR in EMPA-KIDNEY was about 38 mL/min/1.73 m2. To be included in the trial, patients were not required to have albuminuria, except those whose eGFR was greater than or equal to 45 mL/min/1.73 m2.
In DAPA-CKD, the minimum eGFR at entry had to be greater than or equal to 25 mL/min/1.73 m2, and roughly 14% of enrolled patients had an eGFR of less than 30 mL/min/1.73 m2. The average eGFR in DAPA-CKD was about 43 mL/min/1.73 m2. In addition, all patients had at least microalbuminuria, with a minimum urinary albumin-to-creatinine ratio of 200. In CREDENCE, the minimum eGFR for enrollment was 30 mL/min/1.73 m2, and the average eGFR was about 56 mL/min/1.73 m2. All patients in CREDENCE had to have macroalbuminuria, with a urinary albumin-to-creatinine ratio of more than 300.
According to the researchers who designed EMPA-KIDNEY, the trial enrollment criteria aimed to include adults with CKD “who are frequently seen in practice but were under-represented in previous SGLT2 inhibitor trials.”
Indications for empagliflozin are expanding
The success of empagliflozin in EMPA-KIDNEY follows its positive results in both the EMPEROR-Reduced and EMPEROR-Preserved trials, which collectively proved the efficacy of the agent for patients with heart failure regardless of their left ventricular ejection fraction and regardless of whether they also had diabetes.
These results led the U.S. Food and Drug Administration to recently expand the labeled indication for empagliflozin to all patients with heart failure. Empagliflozin also has labeled indications for glycemic control in patients with type 2 diabetes and to reduce the risk of cardiovascular death in adults with type 2 diabetes and established cardiovascular disease.
As of today, empagliflozin has no labeled indication for treating patients with CKD. Dapagliflozin received that indication in April 2021, and canagliflozin received an indication for treating patients with type 2 diabetes, diabetic nephropathy, and albuminuria in September 2019.
EMPA-KIDNEY is sponsored by Boehringer Ingelheim and Lilly, the two companies that jointly market empagliflozin (Jardiance).
A version of this article first appeared on Medscape.com.
Researchers running the EMPA-KIDNEY trial that’s been testing the safety and efficacy of the SGLT2 inhibitor empagliflozin (Jardiance) in about 6,600 patients with chronic kidney disease (CKD) announced on March 16 that they had stopped the trial early because of positive efficacy that met the study’s prespecified threshold for early termination.
EMPA-KIDNEY is the third major trial of an agent from the sodium-glucose cotransport 2 (SGLT2) inhibitor class tested in patients with CKD to be stopped early because of positive results that met a prespecified termination rule.
In 2020, the DAPA-CKD trial of dapagliflozin (Farxiga) stopped early, after a median follow-up of 2.4 years, because of positive efficacy results. In 2019, the same thing happened in the CREDENCE trial of canagliflozin (Invokana), with the unexpected halt coming after a median follow-up of 2.62 years.
The announcement about EMPA-KIDNEY did not include information on median follow-up, but enrollment into the trial ran from May 2019 to April 2021, which means that the longest that enrolled patients could have been in the study was about 2.85 years.
The primary efficacy endpoint in EMPA-KIDNEY was a composite of a sustained decline in estimated glomerular filtration rate (eGFR) to less than 10 mL/min/1.73 m2, renal death, a sustained decline of at least 40% in eGFR from baseline, or cardiovascular death. The announcement of the trial’s early termination provided no details on the efficacy results.
EMPA-KIDNEY enrolled a wider range of patients
EMPA-KIDNEY expands the scope of types of patients with CKD now shown to benefit from treatment with an SGLT2 inhibitor. CREDENCE tested canagliflozin only in patients with type 2 diabetes and diabetic nephropathy, and in DAPA-CKD, two-thirds of enrolled patients had type 2 diabetes, and all had CKD. In EMPA-KIDNEY, 46% of the 6,609 enrolled patients had diabetes (including a very small number with type 1 diabetes).
Another departure from prior studies of an SGLT2 inhibitor for patients selected primarily for having CKD was that in EMPA-KIDNEY, 20% of patients did not have albuminuria, and for 34%, eGFR at entry was less than 30 mL/min/1.73 m2, with all enrolled patients required to have an eGFR at entry of greater than or equal to 20 mL/min/1.73 m2. Average eGFR in EMPA-KIDNEY was about 38 mL/min/1.73 m2. To be included in the trial, patients were not required to have albuminuria, except those whose eGFR was greater than or equal to 45 mL/min/1.73 m2.
In DAPA-CKD, the minimum eGFR at entry had to be greater than or equal to 25 mL/min/1.73 m2, and roughly 14% of enrolled patients had an eGFR of less than 30 mL/min/1.73 m2. The average eGFR in DAPA-CKD was about 43 mL/min/1.73 m2. In addition, all patients had at least microalbuminuria, with a minimum urinary albumin-to-creatinine ratio of 200. In CREDENCE, the minimum eGFR for enrollment was 30 mL/min/1.73 m2, and the average eGFR was about 56 mL/min/1.73 m2. All patients in CREDENCE had to have macroalbuminuria, with a urinary albumin-to-creatinine ratio of more than 300.
According to the researchers who designed EMPA-KIDNEY, the trial enrollment criteria aimed to include adults with CKD “who are frequently seen in practice but were under-represented in previous SGLT2 inhibitor trials.”
Indications for empagliflozin are expanding
The success of empagliflozin in EMPA-KIDNEY follows its positive results in both the EMPEROR-Reduced and EMPEROR-Preserved trials, which collectively proved the efficacy of the agent for patients with heart failure regardless of their left ventricular ejection fraction and regardless of whether they also had diabetes.
These results led the U.S. Food and Drug Administration to recently expand the labeled indication for empagliflozin to all patients with heart failure. Empagliflozin also has labeled indications for glycemic control in patients with type 2 diabetes and to reduce the risk of cardiovascular death in adults with type 2 diabetes and established cardiovascular disease.
As of today, empagliflozin has no labeled indication for treating patients with CKD. Dapagliflozin received that indication in April 2021, and canagliflozin received an indication for treating patients with type 2 diabetes, diabetic nephropathy, and albuminuria in September 2019.
EMPA-KIDNEY is sponsored by Boehringer Ingelheim and Lilly, the two companies that jointly market empagliflozin (Jardiance).
A version of this article first appeared on Medscape.com.
COVID-19–alopecia areata link? Review doesn’t find much evidence
A new
If there is a connection, it’s likely not a strong one, said study author Rachel E. Christensen, a graduate student at Rutgers Robert Wood Johnson Medical School, in an interview. “Based on the reported number of cases following COVID-19, alopecia areata appears to be low on the list of common skin manifestations of COVID-19,” she said. Of 402 articles screened from three databases in the review, only 11 were identified as related to alopecia areata (AA) and COVID-19, and only 9 of those met the study inclusion criteria. “This number alone highlights the very low number of published articles investigating this connection.”
The review was published in JAAD International.
While COVID-19 has been linked to a variety of skin conditions, a 2021 South Korean study of 7,958 cases and 218,779 controls found no connection between infection and AA even after covariates such as age, gender, and income level were taken into account. In a letter to the editor published in 2020, dermatologists in Turkey reported that the percentage of patients with AA at the dermatology outpatient clinic jumped from 0.97% in May 2019 to 1.48% in May 2020. The number of patients in each group wasn’t reported.
Systematic review
The investigators launched the systematic review to gain a wider perspective, although there are still limitations. On the one hand, Ms. Christensen said, “we do know that COVID-19, like other viruses, has been linked to various dermatological disorders.”
However, “it is difficult to tease apart whether any worsening of alopecia areata we see following COVID-19 is due to the virus itself or the increased psychological burden related to the infection or to the pandemic in general,” she said. Indeed, the authors of the report in Turkey attributed the rise in cases to stress.
For the review, the researchers analyzed studies from Italy (four), Turkey (two), Brazil (one), the United States (one), and Poland (one).
Six of the studies reported cases of new-onset AA following COVID-19 infection (seven cases; average age, 37 years; females, three). Another study was a retrospective review of 32 patients with preexisting AA who developed COVID-19; none experienced significant worsening of AA within 6 months.
The review also included a study based on a survey of 389 patients with AA. The investigators found that, at a median 2.14 months after infection, 44% of those who had COVID-19 vs. 12% of those who were COVID negative had a relapse. Finally, a case report noted a patient with preexisting AA whose condition worsened following COVID infection.
The findings suggest that AA “could be a dermatological manifestation of COVID-19, with cases most often appearing 1-2 months following infection,” the authors wrote. “However, the heterogeneity of study designs and high proportion of case reports make it challenging to draw any conclusion.”
In an interview, dermatologist Brett King, MD, PhD, of the department of dermatology, Yale University, New Haven, Conn., said the review findings suggest that “there is little concern of alopecia areata following COVID infection.
Does new-onset AA happen, and are there exacerbations of preexisting disease related to COVID infection? Probably yes, but rarely.”
However, he noted that another form of alopecia, telogen effluvium (TE), is more common after COVID-19 infection. According to Dr. King, who was not involved with the systematic review, TE is typically time-limited, compared with AA’s more common chronic waxing-and-waning course.
“Distinguishing TE and AA is usually straightforward because AA typically presents with well-circumscribed patches of hair loss,” such as circular patches, “while TE manifests as diffuse hair loss,” he explained. “Rarely, however, AA does manifest diffuse hair loss without patches, similar to TE. In those cases, it may be difficult to distinguish them. A biopsy may be helpful if there is a question of the diagnosis.”
No study funding is reported. The review authors and Dr. King report no relevant disclosures.
A new
If there is a connection, it’s likely not a strong one, said study author Rachel E. Christensen, a graduate student at Rutgers Robert Wood Johnson Medical School, in an interview. “Based on the reported number of cases following COVID-19, alopecia areata appears to be low on the list of common skin manifestations of COVID-19,” she said. Of 402 articles screened from three databases in the review, only 11 were identified as related to alopecia areata (AA) and COVID-19, and only 9 of those met the study inclusion criteria. “This number alone highlights the very low number of published articles investigating this connection.”
The review was published in JAAD International.
While COVID-19 has been linked to a variety of skin conditions, a 2021 South Korean study of 7,958 cases and 218,779 controls found no connection between infection and AA even after covariates such as age, gender, and income level were taken into account. In a letter to the editor published in 2020, dermatologists in Turkey reported that the percentage of patients with AA at the dermatology outpatient clinic jumped from 0.97% in May 2019 to 1.48% in May 2020. The number of patients in each group wasn’t reported.
Systematic review
The investigators launched the systematic review to gain a wider perspective, although there are still limitations. On the one hand, Ms. Christensen said, “we do know that COVID-19, like other viruses, has been linked to various dermatological disorders.”
However, “it is difficult to tease apart whether any worsening of alopecia areata we see following COVID-19 is due to the virus itself or the increased psychological burden related to the infection or to the pandemic in general,” she said. Indeed, the authors of the report in Turkey attributed the rise in cases to stress.
For the review, the researchers analyzed studies from Italy (four), Turkey (two), Brazil (one), the United States (one), and Poland (one).
Six of the studies reported cases of new-onset AA following COVID-19 infection (seven cases; average age, 37 years; females, three). Another study was a retrospective review of 32 patients with preexisting AA who developed COVID-19; none experienced significant worsening of AA within 6 months.
The review also included a study based on a survey of 389 patients with AA. The investigators found that, at a median 2.14 months after infection, 44% of those who had COVID-19 vs. 12% of those who were COVID negative had a relapse. Finally, a case report noted a patient with preexisting AA whose condition worsened following COVID infection.
The findings suggest that AA “could be a dermatological manifestation of COVID-19, with cases most often appearing 1-2 months following infection,” the authors wrote. “However, the heterogeneity of study designs and high proportion of case reports make it challenging to draw any conclusion.”
In an interview, dermatologist Brett King, MD, PhD, of the department of dermatology, Yale University, New Haven, Conn., said the review findings suggest that “there is little concern of alopecia areata following COVID infection.
Does new-onset AA happen, and are there exacerbations of preexisting disease related to COVID infection? Probably yes, but rarely.”
However, he noted that another form of alopecia, telogen effluvium (TE), is more common after COVID-19 infection. According to Dr. King, who was not involved with the systematic review, TE is typically time-limited, compared with AA’s more common chronic waxing-and-waning course.
“Distinguishing TE and AA is usually straightforward because AA typically presents with well-circumscribed patches of hair loss,” such as circular patches, “while TE manifests as diffuse hair loss,” he explained. “Rarely, however, AA does manifest diffuse hair loss without patches, similar to TE. In those cases, it may be difficult to distinguish them. A biopsy may be helpful if there is a question of the diagnosis.”
No study funding is reported. The review authors and Dr. King report no relevant disclosures.
A new
If there is a connection, it’s likely not a strong one, said study author Rachel E. Christensen, a graduate student at Rutgers Robert Wood Johnson Medical School, in an interview. “Based on the reported number of cases following COVID-19, alopecia areata appears to be low on the list of common skin manifestations of COVID-19,” she said. Of 402 articles screened from three databases in the review, only 11 were identified as related to alopecia areata (AA) and COVID-19, and only 9 of those met the study inclusion criteria. “This number alone highlights the very low number of published articles investigating this connection.”
The review was published in JAAD International.
While COVID-19 has been linked to a variety of skin conditions, a 2021 South Korean study of 7,958 cases and 218,779 controls found no connection between infection and AA even after covariates such as age, gender, and income level were taken into account. In a letter to the editor published in 2020, dermatologists in Turkey reported that the percentage of patients with AA at the dermatology outpatient clinic jumped from 0.97% in May 2019 to 1.48% in May 2020. The number of patients in each group wasn’t reported.
Systematic review
The investigators launched the systematic review to gain a wider perspective, although there are still limitations. On the one hand, Ms. Christensen said, “we do know that COVID-19, like other viruses, has been linked to various dermatological disorders.”
However, “it is difficult to tease apart whether any worsening of alopecia areata we see following COVID-19 is due to the virus itself or the increased psychological burden related to the infection or to the pandemic in general,” she said. Indeed, the authors of the report in Turkey attributed the rise in cases to stress.
For the review, the researchers analyzed studies from Italy (four), Turkey (two), Brazil (one), the United States (one), and Poland (one).
Six of the studies reported cases of new-onset AA following COVID-19 infection (seven cases; average age, 37 years; females, three). Another study was a retrospective review of 32 patients with preexisting AA who developed COVID-19; none experienced significant worsening of AA within 6 months.
The review also included a study based on a survey of 389 patients with AA. The investigators found that, at a median 2.14 months after infection, 44% of those who had COVID-19 vs. 12% of those who were COVID negative had a relapse. Finally, a case report noted a patient with preexisting AA whose condition worsened following COVID infection.
The findings suggest that AA “could be a dermatological manifestation of COVID-19, with cases most often appearing 1-2 months following infection,” the authors wrote. “However, the heterogeneity of study designs and high proportion of case reports make it challenging to draw any conclusion.”
In an interview, dermatologist Brett King, MD, PhD, of the department of dermatology, Yale University, New Haven, Conn., said the review findings suggest that “there is little concern of alopecia areata following COVID infection.
Does new-onset AA happen, and are there exacerbations of preexisting disease related to COVID infection? Probably yes, but rarely.”
However, he noted that another form of alopecia, telogen effluvium (TE), is more common after COVID-19 infection. According to Dr. King, who was not involved with the systematic review, TE is typically time-limited, compared with AA’s more common chronic waxing-and-waning course.
“Distinguishing TE and AA is usually straightforward because AA typically presents with well-circumscribed patches of hair loss,” such as circular patches, “while TE manifests as diffuse hair loss,” he explained. “Rarely, however, AA does manifest diffuse hair loss without patches, similar to TE. In those cases, it may be difficult to distinguish them. A biopsy may be helpful if there is a question of the diagnosis.”
No study funding is reported. The review authors and Dr. King report no relevant disclosures.
FROM JAAD INTERNATIONAL
‘We don’t want to be an inspiration’
Over 2.5 million people have fled the ghastly war in Ukraine for safety. But, not everyone is trying to leave. Shockingly, hundreds of thousands are actually flocking toward the danger in Ukraine right now. Many of them are women.
I was commuting to work when I first heard this story on a podcast. In astonishing numbers, women have chosen to return to or stay in Ukraine because they’re needed to fight and to protect their families. My reaction, like yours, was to be inspired. What amazing courage! Twitter and Instagram will swell with images of their balaclava masked faces standing in the breach once more. Like the women in medicine who armed themselves with surgical masks and face shields and babies on their backs to join the fight against COVID-19. They will be poster girls, blue sleeves rolled up and red polka dotted bandanas covering their hair.
But that’s not what they want. “We don’t want to be an inspiration,” said one fearless Ukrainian fighter in the story, “we want to be alive.”
At the time of this writing as we celebrate the brilliant accomplishments of women on March 8, International Women’s Day, I wonder if we don’t have it slightly wrong.
Although acknowledgment is appreciated, the women I work alongside don’t need me to be inspired by them. They need me to stand with them, to help them. . The “she-session” it’s been called, refers to the million women who have not rejoined the workforce since COVID-19. This is especially acute for us in medicine where women are significantly more likely than are men to report not working full time, or not working at all.
The truth is that even in 2022, the burdens of family life are still not borne equally. Bias against mothers in particular can be insidious. Take academia, where there is little sympathy for not publishing on schedule. Perhaps there are unexplained gaps, but where exactly on a CV does one put “recurrent pregnancy loss?” Do you know how many clinics or ORs a woman must cancel to attempt maddeningly unpredictable egg retrievals and embryo transfers? A lot. Not to mention the financial burden of doing so.
During the pandemic, female physicians were more likely to manage child care, schooling, and household duties, compared to male physicians.
And yet (perhaps even because of that?) women in medicine make less money. How much? About $80,000 less on average in dermatology. Inspired? Indeed. No thanks. Let’s #BreakTheBias rather.
I’m not a policy expert nor a sociologist. I don’t know what advice might be helpful here. I’d say raising our collective consciousness of the unfairness, highlighting discrepancies, and advocating for equality are good starts. But, International Women’s Day isn’t new. It’s old. Like over a hundred years old (since 1909 to be exact). We don’t just need a better hashtag, we need to do something. Give equity in pay. Offer opportunities for leadership that accommodate the extra duty women might have outside work. Create flexibility in schedules and without the penalty of having to pump at work or leave early to pick up a child. Not to mention all the opportunities we men have to do more of the household work that women currently do.
The gallant women of Ukraine don’t need our approbation. They need our aid and our prayers. Like the women in my department, at my medical center, in my community, they aren’t posing to be made into posters. There’s work to be done and they are flocking toward it right now.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Over 2.5 million people have fled the ghastly war in Ukraine for safety. But, not everyone is trying to leave. Shockingly, hundreds of thousands are actually flocking toward the danger in Ukraine right now. Many of them are women.
I was commuting to work when I first heard this story on a podcast. In astonishing numbers, women have chosen to return to or stay in Ukraine because they’re needed to fight and to protect their families. My reaction, like yours, was to be inspired. What amazing courage! Twitter and Instagram will swell with images of their balaclava masked faces standing in the breach once more. Like the women in medicine who armed themselves with surgical masks and face shields and babies on their backs to join the fight against COVID-19. They will be poster girls, blue sleeves rolled up and red polka dotted bandanas covering their hair.
But that’s not what they want. “We don’t want to be an inspiration,” said one fearless Ukrainian fighter in the story, “we want to be alive.”
At the time of this writing as we celebrate the brilliant accomplishments of women on March 8, International Women’s Day, I wonder if we don’t have it slightly wrong.
Although acknowledgment is appreciated, the women I work alongside don’t need me to be inspired by them. They need me to stand with them, to help them. . The “she-session” it’s been called, refers to the million women who have not rejoined the workforce since COVID-19. This is especially acute for us in medicine where women are significantly more likely than are men to report not working full time, or not working at all.
The truth is that even in 2022, the burdens of family life are still not borne equally. Bias against mothers in particular can be insidious. Take academia, where there is little sympathy for not publishing on schedule. Perhaps there are unexplained gaps, but where exactly on a CV does one put “recurrent pregnancy loss?” Do you know how many clinics or ORs a woman must cancel to attempt maddeningly unpredictable egg retrievals and embryo transfers? A lot. Not to mention the financial burden of doing so.
During the pandemic, female physicians were more likely to manage child care, schooling, and household duties, compared to male physicians.
And yet (perhaps even because of that?) women in medicine make less money. How much? About $80,000 less on average in dermatology. Inspired? Indeed. No thanks. Let’s #BreakTheBias rather.
I’m not a policy expert nor a sociologist. I don’t know what advice might be helpful here. I’d say raising our collective consciousness of the unfairness, highlighting discrepancies, and advocating for equality are good starts. But, International Women’s Day isn’t new. It’s old. Like over a hundred years old (since 1909 to be exact). We don’t just need a better hashtag, we need to do something. Give equity in pay. Offer opportunities for leadership that accommodate the extra duty women might have outside work. Create flexibility in schedules and without the penalty of having to pump at work or leave early to pick up a child. Not to mention all the opportunities we men have to do more of the household work that women currently do.
The gallant women of Ukraine don’t need our approbation. They need our aid and our prayers. Like the women in my department, at my medical center, in my community, they aren’t posing to be made into posters. There’s work to be done and they are flocking toward it right now.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Over 2.5 million people have fled the ghastly war in Ukraine for safety. But, not everyone is trying to leave. Shockingly, hundreds of thousands are actually flocking toward the danger in Ukraine right now. Many of them are women.
I was commuting to work when I first heard this story on a podcast. In astonishing numbers, women have chosen to return to or stay in Ukraine because they’re needed to fight and to protect their families. My reaction, like yours, was to be inspired. What amazing courage! Twitter and Instagram will swell with images of their balaclava masked faces standing in the breach once more. Like the women in medicine who armed themselves with surgical masks and face shields and babies on their backs to join the fight against COVID-19. They will be poster girls, blue sleeves rolled up and red polka dotted bandanas covering their hair.
But that’s not what they want. “We don’t want to be an inspiration,” said one fearless Ukrainian fighter in the story, “we want to be alive.”
At the time of this writing as we celebrate the brilliant accomplishments of women on March 8, International Women’s Day, I wonder if we don’t have it slightly wrong.
Although acknowledgment is appreciated, the women I work alongside don’t need me to be inspired by them. They need me to stand with them, to help them. . The “she-session” it’s been called, refers to the million women who have not rejoined the workforce since COVID-19. This is especially acute for us in medicine where women are significantly more likely than are men to report not working full time, or not working at all.
The truth is that even in 2022, the burdens of family life are still not borne equally. Bias against mothers in particular can be insidious. Take academia, where there is little sympathy for not publishing on schedule. Perhaps there are unexplained gaps, but where exactly on a CV does one put “recurrent pregnancy loss?” Do you know how many clinics or ORs a woman must cancel to attempt maddeningly unpredictable egg retrievals and embryo transfers? A lot. Not to mention the financial burden of doing so.
During the pandemic, female physicians were more likely to manage child care, schooling, and household duties, compared to male physicians.
And yet (perhaps even because of that?) women in medicine make less money. How much? About $80,000 less on average in dermatology. Inspired? Indeed. No thanks. Let’s #BreakTheBias rather.
I’m not a policy expert nor a sociologist. I don’t know what advice might be helpful here. I’d say raising our collective consciousness of the unfairness, highlighting discrepancies, and advocating for equality are good starts. But, International Women’s Day isn’t new. It’s old. Like over a hundred years old (since 1909 to be exact). We don’t just need a better hashtag, we need to do something. Give equity in pay. Offer opportunities for leadership that accommodate the extra duty women might have outside work. Create flexibility in schedules and without the penalty of having to pump at work or leave early to pick up a child. Not to mention all the opportunities we men have to do more of the household work that women currently do.
The gallant women of Ukraine don’t need our approbation. They need our aid and our prayers. Like the women in my department, at my medical center, in my community, they aren’t posing to be made into posters. There’s work to be done and they are flocking toward it right now.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Short DOAC interruption curbs bleeding after cold snare polypectomy
Bleeding risk after cold snare polypectomy is reduced when direct-acting oral anticoagulants (DOACs) are withheld only on the day of the procedure rather than continuing use of these agents, data from a new study suggest.
Findings of the study, led by Atsushi Morita, MD, of the Digestive Disease Center, Showa Inan General Hospital in Komagane, Japan, were published in Gastrointestinal Endoscopy.
This prospective, observational single-center study enrolled two consecutive groups of patients receiving antithrombotic medications who were undergoing cold snare polypectomy of colorectal polyps of 10 mm or less.
All colonoscopies were performed by endoscopists who each perform more than 500 endoscopies a year.
During period 1 of the study (2017 and 2018), DOACs were continued, even on the day of polypectomy (DOAC continued group); during period 2 (2019 and 2020), DOACs were withheld only on the day of the procedure (DOAC withheld group).
The primary outcome was the frequency of delayed bleeding requiring endoscopic treatment within 2 weeks after cold snare polypectomy. Among the secondary outcomes were immediate bleeding and the number of hemostatic clips used.
Clinical features were similar between the two groups. The first group included 204 patients, 34% of whom were female (average age, 75 years); the second group included 264 patients, 34% of whom were female (average age, 74 years). The number of cold snare polypectomies was similar between the groups (47 vs. 66, P = .55), as was the average number of polyps per patient (0.72 vs. 0.70, P = .76).
Delayed bleeding after cold snare polypectomy occurred in 4 out of 47 (8.5%) participants in the continued DOAC group versus 0 out of 66 (0%) participants in the DOAC-withheld group (P < .001). There was similar improvement in immediate postpolypectomy bleeding (secondary outcome) between the two groups.
Immediate bleeding after endoscopy lasting more than 30 seconds occurred about four times as often in continued DOAC group versus the DOAC withheld group (12 out of 47 [25.5%] participants vs. 4 out of 66 [6.1%] participants; P < .008).
Polyps measuring up to 10 mm (excluding tiny hyperplastic polyps in the rectum and distal sigmoid colon), were removed using dedicated cold snares measuring 0.30 mm in diameter.
“This result is consistent with the best practice recommendation of short interruptions of DOACs based on the patient’s creatinine clearance before all polypectomy techniques, including cold snare polypectomy,” the authors wrote.
Countries’ guidelines differ
Guidelines from American Society for Gastrointestinal Endoscopy, the authors noted, currently recommend stopping DOACs before polypectomy, including cold snare procedures, and restarting them only after hemostasis has been achieved. Moreover, since there is no way for a clinician to predict polyp size, the U.S. guidelines further recommend holding warfarin for 5 days and DOACs for 2-3 days before colonoscopy.
In contrast to the U.S. guidelines, the Japanese Gastroenterological Endoscopy Society guidelines suggest clinicians withhold DOACs only on the day of the procedure.
“This policy of withholding DOACs only on the day of colonoscopy should be considered for routine clinical practice,” the authors wrote.
Rajesh N. Keswani, MD, associate professor of medicine in gastroenterology and hepatology at Northwestern University, Chicago, said in an interview it is difficult to draw firm conclusions from this paper because of its study design but added the authors “appear to have delineated a preferred method for managing DOACs prior to colonoscopy.”
He further noted that most polyps encountered during colonoscopy are less than 10 mm and can be safely managed with cold snare polypectomy.
“The management of DOACs prior to colonoscopy is variable,” Dr. Keswani said, “but ranges from cessation of DOACs multiple days prior to colonoscopy versus uninterrupted use of DOACs throughout the colonoscopy period.”
“The authors suggest that holding DOACs on the day of colonoscopy is the optimal balance between minimizing thromboembolic risk and postpolypectomy bleeding. While this data will need to be validated in larger samples, this provides some guidance to colonoscopists tasked with managing DOACs prior to colonoscopy,” Dr. Keswani said.
Limitations of the study included the small number of patients who received DOACs, conduction of the study at a single hospital in Japan, and the definition of immediate bleeding, which differs based on study design.
No commercial funding or conflicts of interest were reported. Dr. Keswani is a consultant for Boston Scientific and Neptune Medical and receives research support from Virgo.
This article was updated March 24, 2022.
Bleeding risk after cold snare polypectomy is reduced when direct-acting oral anticoagulants (DOACs) are withheld only on the day of the procedure rather than continuing use of these agents, data from a new study suggest.
Findings of the study, led by Atsushi Morita, MD, of the Digestive Disease Center, Showa Inan General Hospital in Komagane, Japan, were published in Gastrointestinal Endoscopy.
This prospective, observational single-center study enrolled two consecutive groups of patients receiving antithrombotic medications who were undergoing cold snare polypectomy of colorectal polyps of 10 mm or less.
All colonoscopies were performed by endoscopists who each perform more than 500 endoscopies a year.
During period 1 of the study (2017 and 2018), DOACs were continued, even on the day of polypectomy (DOAC continued group); during period 2 (2019 and 2020), DOACs were withheld only on the day of the procedure (DOAC withheld group).
The primary outcome was the frequency of delayed bleeding requiring endoscopic treatment within 2 weeks after cold snare polypectomy. Among the secondary outcomes were immediate bleeding and the number of hemostatic clips used.
Clinical features were similar between the two groups. The first group included 204 patients, 34% of whom were female (average age, 75 years); the second group included 264 patients, 34% of whom were female (average age, 74 years). The number of cold snare polypectomies was similar between the groups (47 vs. 66, P = .55), as was the average number of polyps per patient (0.72 vs. 0.70, P = .76).
Delayed bleeding after cold snare polypectomy occurred in 4 out of 47 (8.5%) participants in the continued DOAC group versus 0 out of 66 (0%) participants in the DOAC-withheld group (P < .001). There was similar improvement in immediate postpolypectomy bleeding (secondary outcome) between the two groups.
Immediate bleeding after endoscopy lasting more than 30 seconds occurred about four times as often in continued DOAC group versus the DOAC withheld group (12 out of 47 [25.5%] participants vs. 4 out of 66 [6.1%] participants; P < .008).
Polyps measuring up to 10 mm (excluding tiny hyperplastic polyps in the rectum and distal sigmoid colon), were removed using dedicated cold snares measuring 0.30 mm in diameter.
“This result is consistent with the best practice recommendation of short interruptions of DOACs based on the patient’s creatinine clearance before all polypectomy techniques, including cold snare polypectomy,” the authors wrote.
Countries’ guidelines differ
Guidelines from American Society for Gastrointestinal Endoscopy, the authors noted, currently recommend stopping DOACs before polypectomy, including cold snare procedures, and restarting them only after hemostasis has been achieved. Moreover, since there is no way for a clinician to predict polyp size, the U.S. guidelines further recommend holding warfarin for 5 days and DOACs for 2-3 days before colonoscopy.
In contrast to the U.S. guidelines, the Japanese Gastroenterological Endoscopy Society guidelines suggest clinicians withhold DOACs only on the day of the procedure.
“This policy of withholding DOACs only on the day of colonoscopy should be considered for routine clinical practice,” the authors wrote.
Rajesh N. Keswani, MD, associate professor of medicine in gastroenterology and hepatology at Northwestern University, Chicago, said in an interview it is difficult to draw firm conclusions from this paper because of its study design but added the authors “appear to have delineated a preferred method for managing DOACs prior to colonoscopy.”
He further noted that most polyps encountered during colonoscopy are less than 10 mm and can be safely managed with cold snare polypectomy.
“The management of DOACs prior to colonoscopy is variable,” Dr. Keswani said, “but ranges from cessation of DOACs multiple days prior to colonoscopy versus uninterrupted use of DOACs throughout the colonoscopy period.”
“The authors suggest that holding DOACs on the day of colonoscopy is the optimal balance between minimizing thromboembolic risk and postpolypectomy bleeding. While this data will need to be validated in larger samples, this provides some guidance to colonoscopists tasked with managing DOACs prior to colonoscopy,” Dr. Keswani said.
Limitations of the study included the small number of patients who received DOACs, conduction of the study at a single hospital in Japan, and the definition of immediate bleeding, which differs based on study design.
No commercial funding or conflicts of interest were reported. Dr. Keswani is a consultant for Boston Scientific and Neptune Medical and receives research support from Virgo.
This article was updated March 24, 2022.
Bleeding risk after cold snare polypectomy is reduced when direct-acting oral anticoagulants (DOACs) are withheld only on the day of the procedure rather than continuing use of these agents, data from a new study suggest.
Findings of the study, led by Atsushi Morita, MD, of the Digestive Disease Center, Showa Inan General Hospital in Komagane, Japan, were published in Gastrointestinal Endoscopy.
This prospective, observational single-center study enrolled two consecutive groups of patients receiving antithrombotic medications who were undergoing cold snare polypectomy of colorectal polyps of 10 mm or less.
All colonoscopies were performed by endoscopists who each perform more than 500 endoscopies a year.
During period 1 of the study (2017 and 2018), DOACs were continued, even on the day of polypectomy (DOAC continued group); during period 2 (2019 and 2020), DOACs were withheld only on the day of the procedure (DOAC withheld group).
The primary outcome was the frequency of delayed bleeding requiring endoscopic treatment within 2 weeks after cold snare polypectomy. Among the secondary outcomes were immediate bleeding and the number of hemostatic clips used.
Clinical features were similar between the two groups. The first group included 204 patients, 34% of whom were female (average age, 75 years); the second group included 264 patients, 34% of whom were female (average age, 74 years). The number of cold snare polypectomies was similar between the groups (47 vs. 66, P = .55), as was the average number of polyps per patient (0.72 vs. 0.70, P = .76).
Delayed bleeding after cold snare polypectomy occurred in 4 out of 47 (8.5%) participants in the continued DOAC group versus 0 out of 66 (0%) participants in the DOAC-withheld group (P < .001). There was similar improvement in immediate postpolypectomy bleeding (secondary outcome) between the two groups.
Immediate bleeding after endoscopy lasting more than 30 seconds occurred about four times as often in continued DOAC group versus the DOAC withheld group (12 out of 47 [25.5%] participants vs. 4 out of 66 [6.1%] participants; P < .008).
Polyps measuring up to 10 mm (excluding tiny hyperplastic polyps in the rectum and distal sigmoid colon), were removed using dedicated cold snares measuring 0.30 mm in diameter.
“This result is consistent with the best practice recommendation of short interruptions of DOACs based on the patient’s creatinine clearance before all polypectomy techniques, including cold snare polypectomy,” the authors wrote.
Countries’ guidelines differ
Guidelines from American Society for Gastrointestinal Endoscopy, the authors noted, currently recommend stopping DOACs before polypectomy, including cold snare procedures, and restarting them only after hemostasis has been achieved. Moreover, since there is no way for a clinician to predict polyp size, the U.S. guidelines further recommend holding warfarin for 5 days and DOACs for 2-3 days before colonoscopy.
In contrast to the U.S. guidelines, the Japanese Gastroenterological Endoscopy Society guidelines suggest clinicians withhold DOACs only on the day of the procedure.
“This policy of withholding DOACs only on the day of colonoscopy should be considered for routine clinical practice,” the authors wrote.
Rajesh N. Keswani, MD, associate professor of medicine in gastroenterology and hepatology at Northwestern University, Chicago, said in an interview it is difficult to draw firm conclusions from this paper because of its study design but added the authors “appear to have delineated a preferred method for managing DOACs prior to colonoscopy.”
He further noted that most polyps encountered during colonoscopy are less than 10 mm and can be safely managed with cold snare polypectomy.
“The management of DOACs prior to colonoscopy is variable,” Dr. Keswani said, “but ranges from cessation of DOACs multiple days prior to colonoscopy versus uninterrupted use of DOACs throughout the colonoscopy period.”
“The authors suggest that holding DOACs on the day of colonoscopy is the optimal balance between minimizing thromboembolic risk and postpolypectomy bleeding. While this data will need to be validated in larger samples, this provides some guidance to colonoscopists tasked with managing DOACs prior to colonoscopy,” Dr. Keswani said.
Limitations of the study included the small number of patients who received DOACs, conduction of the study at a single hospital in Japan, and the definition of immediate bleeding, which differs based on study design.
No commercial funding or conflicts of interest were reported. Dr. Keswani is a consultant for Boston Scientific and Neptune Medical and receives research support from Virgo.
This article was updated March 24, 2022.
FROM GASTROINTESTINAL ENDOSCOPY
‘Alarming’ worldwide decline in mental health
The Mental Health Million project of Sapien Labs issued its second report, published online March 15, encompassing 34 countries and over 220,000 Internet-enabled adults. It found a continued decline in mental health in all age groups and genders, with English-speaking countries having the lowest mental well-being.
The decline was significantly correlated with the stringency of COVID-19 lockdown measures in each country and was directionally correlated to the cases and deaths per million.
The youngest age group (18-24 years) reported the poorest mental well-being, with better mental health scores rising in every successively older age group.
“Some of our findings, especially regarding mental health in young adults, are alarming,” Tara Thiagarajan, PhD, Sapien Labs founder and chief scientist, told this news organization.
“Our data, which are continually updated in real time, are freely available for nonprofit, noncommercial use and research, and we hope that researchers will get involved in an interdisciplinary way that spans sociology, economics, psychiatry, and other fields,” she said.
Pioneering research
Dr. Thiagarajan and her team pioneered the Mental Health Million project, an ongoing research initiative utilizing a “free and anonymous assessment tool,” the Mental Health Quotient (MHQ), which “encompasses a comprehensive view of our emotional, social, and cognitive function and capability.”
The MHQ consists of 47 “elements of mental well-being,” with scores ranging from –100 to +200. (Negative scores indicate poorer mental well-being.) The MHQ categorizes respondents as “clinical, at-risk, enduring, managing, succeeding, and thriving” and computes scores on the basis of six broad dimensions of mental health: core cognition, complex cognition, mood and outlook, drive and motivation, social self, and mind-body connection.
As reported by this news organization, Sapien Lab’s first Mental Health State of the World report (n = 49,000 adults) was conducted in eight English-speaking countries in 2020. Participants were compared to a smaller sample of people from the same countries polled in 2019.
In this year’s report, “we expanded quite substantially,” Dr. Thiagarajan said. The project added Spanish, French, and Arabic and recruited participants from 34 countries on six continents (n = 223,087) via advertising on Google and Facebook.
Economic prosperity not protective
Across the eight English-speaking countries, there was a decline in mental well-being of 3% from 2020 to 2021, which was smaller than the 8% decline from 2019 to 2020. The percentage of people who were “distressed or struggling” increased from 26% to 30% in 2021.
“Now that a lot of pandemic issue seems to be easing up, I hope we’ll see mental well-being coming back up, but at least it’s a smaller decline than we saw between 2019 and 2020,” said Dr. Thiagarajan.
The decline across countries from 2019 to 2021 was significantly correlated with the stringency of governmental COVID-19-related measures (based on the Oxford COVID-19 Government Response Tracker, 2022; r = .54) and directionally correlated to the cases and deaths per million.
In total, 30% of respondents in English-speaking countries had mental well-being scores in the “distressed” or “struggling” range – higher than the Middle Eastern countries, North Africa, Latin America, and Europe (23%, 23%, 24%, and 18%, respectively).
Only 36% of participants in the English-speaking countries, the Middle East, and North Africa reported “thriving or succeeding,” vs. 45% and 46% in Latin America and Europe, respectively. Venezuela topped the list with an average MHQ of 91, while the United Kingdom and South Africa had the lowest scores, at 46 each.
Mental well-being was slightly higher in males than in females but was dramatically lower in nonbinary/third-gender respondents. In fact, those identifying as nonbinary/third gender had the lowest mental well-being of any group.
Across all countries and languages, higher education was associated with better mental well-being. Employment was also associated with superior mental well-being, compared with being unemployed – particularly in core English-speaking countries.
However, “country indicators of economic prosperity were negatively correlated with mental well-being, particularly for young adults and males, belying the commonly held belief that national economic prosperity translates into greater mental well-being,” said Dr. Thiagarajan.
‘Stark’ contrast
The most dramatic finding was the difference in mental well-being between younger and older adults, which was two- to threefold larger than differences in other dimensions (for example, age, gender, employment). Even the maximum difference between countries overall (15%) was still smaller than the generational gap within any region.
While only 7% (6%- 9%) of participants aged ≥65 years were “distressed and struggling” with their mental well-being to a “clinical” extent, 44% (38%-50%) of those aged 18-24 years reported mental well-being scores in the “distressed or struggling” range – representing a “growing gap between generations that, while present prior to the COVID-19 pandemic, has since been exacerbated,” the authors state.
With every successive decrement in age group, mental well-being “plummeted,” Dr. Thiagarajan said. She noted that research conducted prior to 2010 in several regions of the world showed that young adults typically had the highest well-being. “Our findings stand in stark contrast to these previous patterns.”
The relationship between lockdown stringency and poorer mental health could play a role. “The impact of social isolation may be most strongly felt in younger people,” she said.
Internet a culprit?
“Within almost every region, scores for cognition and drive and motivation were highest while mood and outlook and social self were the lowest,” the authors report.
The aggregate percentage of respondents who reported being “distressed or struggling” in the various MHQ dimensions is shown in the following table.
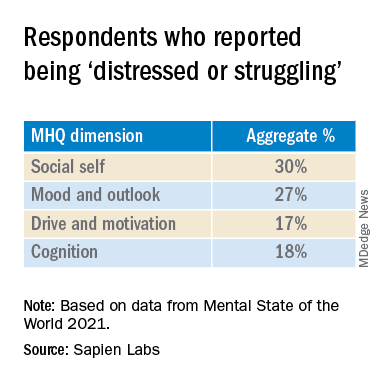
In particular, English-speaking countries scored lowest on the social self scale.
The sense of social self is “how you see yourself with respect to others, how you relate to others and the ability to form strong, stable relationships and maintain them with other people,” said Dr. Thiagarajan.
Internet use might account for the “massive” difference between the youngest and the oldest generations, she suggested. “Following 2010, mobile phone penetration picked up and rose rapidly. ... Mobile phones took over the world.”
Time spent on the Internet – an estimated 7-10 hours per day – “eats into the time people in older generations used in building the social self. Kids who grow up on the Internet are losing thousands of hours in social interactions, which is challenging their ability to form relationships, how they see themselves, and how they fit into the social fabric,” Dr. Thiagarajan added
Sedentary time
Commenting for this news organization, Bernardo Ng, MD, a member of the American Psychiatric Association’s Council on International Psychiatry and Global Health and medical director of Sun Valley Research Center, Imperial, Calif., called the report “interesting, with an impressive sample size” and an “impressive geographic distribution.”
Dr. Ng, who was not involved in the report, said, “I did not think the impact of Internet use on mental health was as dramatic before looking at this report.
“On the other hand, I have personally been interested in the impact of sedentarism in mental health – not only emotionally but also biologically. Sedentarism, which is directly related to screen use time, produces inflammation that worsens brain function.”
Also commenting, Ken Duckworth, MD, chief medical officer of the National Alliance of Mental Illness, called the survey “extremely well timed and creative, although it looked only at Internet-enabled populations, so one cannot make too many overall pronouncements, because a lot of people don’t have access to the Internet.”
The data regarding young people are particularly powerful. “The idea that young people are having a decrease in their experience of mental health across the world is something I haven’t seen before.”
Dr. Duckworth suggested the reason might “have to do with the impact of the COVID lockdown on normal development that young people go through, while older people don’t struggle with these developmental challenges in the same way.”
A version of this article first appeared on Medscape.com.
The Mental Health Million project of Sapien Labs issued its second report, published online March 15, encompassing 34 countries and over 220,000 Internet-enabled adults. It found a continued decline in mental health in all age groups and genders, with English-speaking countries having the lowest mental well-being.
The decline was significantly correlated with the stringency of COVID-19 lockdown measures in each country and was directionally correlated to the cases and deaths per million.
The youngest age group (18-24 years) reported the poorest mental well-being, with better mental health scores rising in every successively older age group.
“Some of our findings, especially regarding mental health in young adults, are alarming,” Tara Thiagarajan, PhD, Sapien Labs founder and chief scientist, told this news organization.
“Our data, which are continually updated in real time, are freely available for nonprofit, noncommercial use and research, and we hope that researchers will get involved in an interdisciplinary way that spans sociology, economics, psychiatry, and other fields,” she said.
Pioneering research
Dr. Thiagarajan and her team pioneered the Mental Health Million project, an ongoing research initiative utilizing a “free and anonymous assessment tool,” the Mental Health Quotient (MHQ), which “encompasses a comprehensive view of our emotional, social, and cognitive function and capability.”
The MHQ consists of 47 “elements of mental well-being,” with scores ranging from –100 to +200. (Negative scores indicate poorer mental well-being.) The MHQ categorizes respondents as “clinical, at-risk, enduring, managing, succeeding, and thriving” and computes scores on the basis of six broad dimensions of mental health: core cognition, complex cognition, mood and outlook, drive and motivation, social self, and mind-body connection.
As reported by this news organization, Sapien Lab’s first Mental Health State of the World report (n = 49,000 adults) was conducted in eight English-speaking countries in 2020. Participants were compared to a smaller sample of people from the same countries polled in 2019.
In this year’s report, “we expanded quite substantially,” Dr. Thiagarajan said. The project added Spanish, French, and Arabic and recruited participants from 34 countries on six continents (n = 223,087) via advertising on Google and Facebook.

Economic prosperity not protective
Across the eight English-speaking countries, there was a decline in mental well-being of 3% from 2020 to 2021, which was smaller than the 8% decline from 2019 to 2020. The percentage of people who were “distressed or struggling” increased from 26% to 30% in 2021.
“Now that a lot of pandemic issue seems to be easing up, I hope we’ll see mental well-being coming back up, but at least it’s a smaller decline than we saw between 2019 and 2020,” said Dr. Thiagarajan.
The decline across countries from 2019 to 2021 was significantly correlated with the stringency of governmental COVID-19-related measures (based on the Oxford COVID-19 Government Response Tracker, 2022; r = .54) and directionally correlated to the cases and deaths per million.
In total, 30% of respondents in English-speaking countries had mental well-being scores in the “distressed” or “struggling” range – higher than the Middle Eastern countries, North Africa, Latin America, and Europe (23%, 23%, 24%, and 18%, respectively).
Only 36% of participants in the English-speaking countries, the Middle East, and North Africa reported “thriving or succeeding,” vs. 45% and 46% in Latin America and Europe, respectively. Venezuela topped the list with an average MHQ of 91, while the United Kingdom and South Africa had the lowest scores, at 46 each.
Mental well-being was slightly higher in males than in females but was dramatically lower in nonbinary/third-gender respondents. In fact, those identifying as nonbinary/third gender had the lowest mental well-being of any group.
Across all countries and languages, higher education was associated with better mental well-being. Employment was also associated with superior mental well-being, compared with being unemployed – particularly in core English-speaking countries.
However, “country indicators of economic prosperity were negatively correlated with mental well-being, particularly for young adults and males, belying the commonly held belief that national economic prosperity translates into greater mental well-being,” said Dr. Thiagarajan.
‘Stark’ contrast
The most dramatic finding was the difference in mental well-being between younger and older adults, which was two- to threefold larger than differences in other dimensions (for example, age, gender, employment). Even the maximum difference between countries overall (15%) was still smaller than the generational gap within any region.
While only 7% (6%- 9%) of participants aged ≥65 years were “distressed and struggling” with their mental well-being to a “clinical” extent, 44% (38%-50%) of those aged 18-24 years reported mental well-being scores in the “distressed or struggling” range – representing a “growing gap between generations that, while present prior to the COVID-19 pandemic, has since been exacerbated,” the authors state.
With every successive decrement in age group, mental well-being “plummeted,” Dr. Thiagarajan said. She noted that research conducted prior to 2010 in several regions of the world showed that young adults typically had the highest well-being. “Our findings stand in stark contrast to these previous patterns.”
The relationship between lockdown stringency and poorer mental health could play a role. “The impact of social isolation may be most strongly felt in younger people,” she said.
Internet a culprit?
“Within almost every region, scores for cognition and drive and motivation were highest while mood and outlook and social self were the lowest,” the authors report.
The aggregate percentage of respondents who reported being “distressed or struggling” in the various MHQ dimensions is shown in the following table.

In particular, English-speaking countries scored lowest on the social self scale.
The sense of social self is “how you see yourself with respect to others, how you relate to others and the ability to form strong, stable relationships and maintain them with other people,” said Dr. Thiagarajan.
Internet use might account for the “massive” difference between the youngest and the oldest generations, she suggested. “Following 2010, mobile phone penetration picked up and rose rapidly. ... Mobile phones took over the world.”
Time spent on the Internet – an estimated 7-10 hours per day – “eats into the time people in older generations used in building the social self. Kids who grow up on the Internet are losing thousands of hours in social interactions, which is challenging their ability to form relationships, how they see themselves, and how they fit into the social fabric,” Dr. Thiagarajan added
Sedentary time
Commenting for this news organization, Bernardo Ng, MD, a member of the American Psychiatric Association’s Council on International Psychiatry and Global Health and medical director of Sun Valley Research Center, Imperial, Calif., called the report “interesting, with an impressive sample size” and an “impressive geographic distribution.”
Dr. Ng, who was not involved in the report, said, “I did not think the impact of Internet use on mental health was as dramatic before looking at this report.
“On the other hand, I have personally been interested in the impact of sedentarism in mental health – not only emotionally but also biologically. Sedentarism, which is directly related to screen use time, produces inflammation that worsens brain function.”
Also commenting, Ken Duckworth, MD, chief medical officer of the National Alliance of Mental Illness, called the survey “extremely well timed and creative, although it looked only at Internet-enabled populations, so one cannot make too many overall pronouncements, because a lot of people don’t have access to the Internet.”
The data regarding young people are particularly powerful. “The idea that young people are having a decrease in their experience of mental health across the world is something I haven’t seen before.”
Dr. Duckworth suggested the reason might “have to do with the impact of the COVID lockdown on normal development that young people go through, while older people don’t struggle with these developmental challenges in the same way.”
A version of this article first appeared on Medscape.com.
The Mental Health Million project of Sapien Labs issued its second report, published online March 15, encompassing 34 countries and over 220,000 Internet-enabled adults. It found a continued decline in mental health in all age groups and genders, with English-speaking countries having the lowest mental well-being.
The decline was significantly correlated with the stringency of COVID-19 lockdown measures in each country and was directionally correlated to the cases and deaths per million.
The youngest age group (18-24 years) reported the poorest mental well-being, with better mental health scores rising in every successively older age group.
“Some of our findings, especially regarding mental health in young adults, are alarming,” Tara Thiagarajan, PhD, Sapien Labs founder and chief scientist, told this news organization.
“Our data, which are continually updated in real time, are freely available for nonprofit, noncommercial use and research, and we hope that researchers will get involved in an interdisciplinary way that spans sociology, economics, psychiatry, and other fields,” she said.
Pioneering research
Dr. Thiagarajan and her team pioneered the Mental Health Million project, an ongoing research initiative utilizing a “free and anonymous assessment tool,” the Mental Health Quotient (MHQ), which “encompasses a comprehensive view of our emotional, social, and cognitive function and capability.”
The MHQ consists of 47 “elements of mental well-being,” with scores ranging from –100 to +200. (Negative scores indicate poorer mental well-being.) The MHQ categorizes respondents as “clinical, at-risk, enduring, managing, succeeding, and thriving” and computes scores on the basis of six broad dimensions of mental health: core cognition, complex cognition, mood and outlook, drive and motivation, social self, and mind-body connection.
As reported by this news organization, Sapien Lab’s first Mental Health State of the World report (n = 49,000 adults) was conducted in eight English-speaking countries in 2020. Participants were compared to a smaller sample of people from the same countries polled in 2019.
In this year’s report, “we expanded quite substantially,” Dr. Thiagarajan said. The project added Spanish, French, and Arabic and recruited participants from 34 countries on six continents (n = 223,087) via advertising on Google and Facebook.

Economic prosperity not protective
Across the eight English-speaking countries, there was a decline in mental well-being of 3% from 2020 to 2021, which was smaller than the 8% decline from 2019 to 2020. The percentage of people who were “distressed or struggling” increased from 26% to 30% in 2021.
“Now that a lot of pandemic issue seems to be easing up, I hope we’ll see mental well-being coming back up, but at least it’s a smaller decline than we saw between 2019 and 2020,” said Dr. Thiagarajan.
The decline across countries from 2019 to 2021 was significantly correlated with the stringency of governmental COVID-19-related measures (based on the Oxford COVID-19 Government Response Tracker, 2022; r = .54) and directionally correlated to the cases and deaths per million.
In total, 30% of respondents in English-speaking countries had mental well-being scores in the “distressed” or “struggling” range – higher than the Middle Eastern countries, North Africa, Latin America, and Europe (23%, 23%, 24%, and 18%, respectively).
Only 36% of participants in the English-speaking countries, the Middle East, and North Africa reported “thriving or succeeding,” vs. 45% and 46% in Latin America and Europe, respectively. Venezuela topped the list with an average MHQ of 91, while the United Kingdom and South Africa had the lowest scores, at 46 each.
Mental well-being was slightly higher in males than in females but was dramatically lower in nonbinary/third-gender respondents. In fact, those identifying as nonbinary/third gender had the lowest mental well-being of any group.
Across all countries and languages, higher education was associated with better mental well-being. Employment was also associated with superior mental well-being, compared with being unemployed – particularly in core English-speaking countries.
However, “country indicators of economic prosperity were negatively correlated with mental well-being, particularly for young adults and males, belying the commonly held belief that national economic prosperity translates into greater mental well-being,” said Dr. Thiagarajan.
‘Stark’ contrast
The most dramatic finding was the difference in mental well-being between younger and older adults, which was two- to threefold larger than differences in other dimensions (for example, age, gender, employment). Even the maximum difference between countries overall (15%) was still smaller than the generational gap within any region.
While only 7% (6%- 9%) of participants aged ≥65 years were “distressed and struggling” with their mental well-being to a “clinical” extent, 44% (38%-50%) of those aged 18-24 years reported mental well-being scores in the “distressed or struggling” range – representing a “growing gap between generations that, while present prior to the COVID-19 pandemic, has since been exacerbated,” the authors state.
With every successive decrement in age group, mental well-being “plummeted,” Dr. Thiagarajan said. She noted that research conducted prior to 2010 in several regions of the world showed that young adults typically had the highest well-being. “Our findings stand in stark contrast to these previous patterns.”
The relationship between lockdown stringency and poorer mental health could play a role. “The impact of social isolation may be most strongly felt in younger people,” she said.
Internet a culprit?
“Within almost every region, scores for cognition and drive and motivation were highest while mood and outlook and social self were the lowest,” the authors report.
The aggregate percentage of respondents who reported being “distressed or struggling” in the various MHQ dimensions is shown in the following table.

In particular, English-speaking countries scored lowest on the social self scale.
The sense of social self is “how you see yourself with respect to others, how you relate to others and the ability to form strong, stable relationships and maintain them with other people,” said Dr. Thiagarajan.
Internet use might account for the “massive” difference between the youngest and the oldest generations, she suggested. “Following 2010, mobile phone penetration picked up and rose rapidly. ... Mobile phones took over the world.”
Time spent on the Internet – an estimated 7-10 hours per day – “eats into the time people in older generations used in building the social self. Kids who grow up on the Internet are losing thousands of hours in social interactions, which is challenging their ability to form relationships, how they see themselves, and how they fit into the social fabric,” Dr. Thiagarajan added
Sedentary time
Commenting for this news organization, Bernardo Ng, MD, a member of the American Psychiatric Association’s Council on International Psychiatry and Global Health and medical director of Sun Valley Research Center, Imperial, Calif., called the report “interesting, with an impressive sample size” and an “impressive geographic distribution.”
Dr. Ng, who was not involved in the report, said, “I did not think the impact of Internet use on mental health was as dramatic before looking at this report.
“On the other hand, I have personally been interested in the impact of sedentarism in mental health – not only emotionally but also biologically. Sedentarism, which is directly related to screen use time, produces inflammation that worsens brain function.”
Also commenting, Ken Duckworth, MD, chief medical officer of the National Alliance of Mental Illness, called the survey “extremely well timed and creative, although it looked only at Internet-enabled populations, so one cannot make too many overall pronouncements, because a lot of people don’t have access to the Internet.”
The data regarding young people are particularly powerful. “The idea that young people are having a decrease in their experience of mental health across the world is something I haven’t seen before.”
Dr. Duckworth suggested the reason might “have to do with the impact of the COVID lockdown on normal development that young people go through, while older people don’t struggle with these developmental challenges in the same way.”
A version of this article first appeared on Medscape.com.