User login
AAD unveils updated guidelines for topical AD treatment in adults
, and topical phosphodiesterase-4 (PDE-4) and Janus kinase (JAK) inhibitors. The guidelines also conditionally recommend the use of bathing and wet wrap therapy but recommend against the use of topical antimicrobials, antiseptics, and antihistamines.
The development updates the AAD’s 2014 recommendations for managing AD with topical therapies, published almost 9 years ago. “At that time, the only U.S. FDA–approved systemic medication for atopic dermatitis was prednisone – universally felt amongst dermatologists to be the least appropriate systemic medication for this condition, at least chronically,” Robert Sidbury, MD, MPH, who cochaired a 14-member multidisciplinary work group that assembled the updated guidelines, told this news organization in an interview.
“Since 2017, there have been two different biologic medications approved for moderate to severe AD (dupilumab and tralokinumab) with certainly a third or more right around the corner. There have been two new oral agents approved for moderate to severe AD – upadacitinib and abrocitinib – with others on the way,” he noted. While these are not topical therapies, the purview of the newly released guidelines, he said, “there have also been new topical medications approved since that time (crisaborole and ruxolitinib). It was high time for an update.”
For the new guidelines, which were published online in the Journal of the American Academy of Dermatology, Dr. Sidbury, chief of the division of dermatology at Seattle Children’s Hospital, guidelines cochair Dawn M. R. Davis, MD, a dermatologist at Mayo Clinic, Rochester, Minn., and colleagues conducted a systematic review of evidence regarding the use of nonprescription topical agents such as moisturizers, bathing practices, and wet wraps, as well as topical pharmacologic modalities such as corticosteroids, calcineurin inhibitors, JAK inhibitors, PDE-4 inhibitors, antimicrobials, and antihistamines.
Next, the work group applied the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach for assessing the certainty of the evidence and formulating and grading clinical recommendations based on relevant randomized trials in the medical literature.
12 recommendations
Of the 12 recommendations made for adults with AD, the work group ranked 7 as “strong” based on the evidence reviewed, and the rest as “conditional.” The “strong” recommendations include the use of moisturizers; the use of tacrolimus 0.03% or 0.1%; the use of pimecrolimus 1% cream for mild to moderate AD; use of topical steroids; intermittent use of medium-potency topical corticosteroids as maintenance therapy to reduce flares and relapse; the use of the topical PDE-4 inhibitor crisaborole, and the use of the topical JAK inhibitor ruxolitinib.
Regarding ruxolitinib cream 1.5%, the work group advised that the treatment area “should not exceed 20% body surface area, and a maximum of 60 grams should be applied per week; these stipulations are aimed at reducing systemic absorption, as black box warnings include serious infections, mortality, malignancies (for example, lymphoma), major adverse cardiovascular events, and thrombosis.”
Conditional recommendations in the guidelines include those for bathing for treatment and maintenance and the use of wet dressings, and those against the use of topical antimicrobials, topical antihistamines, and topical antiseptics.
According to Dr. Sidbury, the topic of bathing generated robust discussion among the work group members. “Though [each group member] has strong opinions and individual practice styles, they were also able to recognize that the evidence is all that matters in a project like this, which led to a ‘conditional’ recommendation regarding bathing frequency backed by ‘low’ evidence,” he said. “While this may seem like ‘guidance’ that doesn’t ‘guide,’ I would argue it informs the guideline consumer exactly where we are in terms of this question and allows them to use their best judgment and experience as their true north here.”
In the realm of topical steroids, Dr. Sidbury said that topical steroid addiction (TSA) and topical steroid withdrawal (TSW) have been a “controversial but persistent concern” from some patients and providers. “Two systematic reviews of this topic were mentioned, and it was made clear that the evidence base [for the concepts] is weak,” he said. “With that important caveat ,the guideline committee delineated both a definition of TSW/TSA and potential risk factors.”
Dr. Sidbury marveled at the potential impact of newer medicines such as crisaborole and ruxolitinib on younger AD patients as well. Crisaborole is now Food and Drug Administration approved down to 3 months of age for mild to moderate AD. “This is extraordinary and expands treatment options for all providers at an age when parents and providers are most conservative in their practice,” he said. “Ruxolitinib, also nonsteroidal, is FDA approved for mild to moderate AD down to 12 years of age. Having spent a good percentage of my practice years either being able to offer only topical steroids, or later topical steroids and topical calcineurin inhibitors like tacrolimus or pimecrolimus, having additional options is wonderful.”
In the guidelines, the work group noted that “significant gaps remain” in current understanding of various topical AD therapies. “Studies are needed which examine quality of life and other patient-important outcomes, changes to the cutaneous microbiome, as well as long term follow-up, and use in special and diverse populations (e.g., pregnancy, lactation, immunosuppression, multiple comorbidities, skin of color, pediatric),” they wrote. “Furthermore, increased use of new systemic AD treatment options (dupilumab, tralokinumab, abrocitinib, upadacitinib) in patients with moderate to severe disease may result in a selection bias toward milder disease in current and future AD topical therapy studies.”
Use of topical therapies to manage AD in pediatric patients will be covered in a forthcoming AAD guideline. The first updated AD guideline, on comorbidities associated with AD in adults, was released in January 2022.
Dr. Sidbury reported that he serves as an advisory board member for Pfizer, a principal investigator for Regeneron, an investigator for Brickell Biotech and Galderma USA, and a consultant for Galderma Global and Microes. Other work group members reported having financial disclosures with many pharmaceutical companies.
, and topical phosphodiesterase-4 (PDE-4) and Janus kinase (JAK) inhibitors. The guidelines also conditionally recommend the use of bathing and wet wrap therapy but recommend against the use of topical antimicrobials, antiseptics, and antihistamines.
The development updates the AAD’s 2014 recommendations for managing AD with topical therapies, published almost 9 years ago. “At that time, the only U.S. FDA–approved systemic medication for atopic dermatitis was prednisone – universally felt amongst dermatologists to be the least appropriate systemic medication for this condition, at least chronically,” Robert Sidbury, MD, MPH, who cochaired a 14-member multidisciplinary work group that assembled the updated guidelines, told this news organization in an interview.
“Since 2017, there have been two different biologic medications approved for moderate to severe AD (dupilumab and tralokinumab) with certainly a third or more right around the corner. There have been two new oral agents approved for moderate to severe AD – upadacitinib and abrocitinib – with others on the way,” he noted. While these are not topical therapies, the purview of the newly released guidelines, he said, “there have also been new topical medications approved since that time (crisaborole and ruxolitinib). It was high time for an update.”
For the new guidelines, which were published online in the Journal of the American Academy of Dermatology, Dr. Sidbury, chief of the division of dermatology at Seattle Children’s Hospital, guidelines cochair Dawn M. R. Davis, MD, a dermatologist at Mayo Clinic, Rochester, Minn., and colleagues conducted a systematic review of evidence regarding the use of nonprescription topical agents such as moisturizers, bathing practices, and wet wraps, as well as topical pharmacologic modalities such as corticosteroids, calcineurin inhibitors, JAK inhibitors, PDE-4 inhibitors, antimicrobials, and antihistamines.
Next, the work group applied the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach for assessing the certainty of the evidence and formulating and grading clinical recommendations based on relevant randomized trials in the medical literature.
12 recommendations
Of the 12 recommendations made for adults with AD, the work group ranked 7 as “strong” based on the evidence reviewed, and the rest as “conditional.” The “strong” recommendations include the use of moisturizers; the use of tacrolimus 0.03% or 0.1%; the use of pimecrolimus 1% cream for mild to moderate AD; use of topical steroids; intermittent use of medium-potency topical corticosteroids as maintenance therapy to reduce flares and relapse; the use of the topical PDE-4 inhibitor crisaborole, and the use of the topical JAK inhibitor ruxolitinib.
Regarding ruxolitinib cream 1.5%, the work group advised that the treatment area “should not exceed 20% body surface area, and a maximum of 60 grams should be applied per week; these stipulations are aimed at reducing systemic absorption, as black box warnings include serious infections, mortality, malignancies (for example, lymphoma), major adverse cardiovascular events, and thrombosis.”
Conditional recommendations in the guidelines include those for bathing for treatment and maintenance and the use of wet dressings, and those against the use of topical antimicrobials, topical antihistamines, and topical antiseptics.
According to Dr. Sidbury, the topic of bathing generated robust discussion among the work group members. “Though [each group member] has strong opinions and individual practice styles, they were also able to recognize that the evidence is all that matters in a project like this, which led to a ‘conditional’ recommendation regarding bathing frequency backed by ‘low’ evidence,” he said. “While this may seem like ‘guidance’ that doesn’t ‘guide,’ I would argue it informs the guideline consumer exactly where we are in terms of this question and allows them to use their best judgment and experience as their true north here.”
In the realm of topical steroids, Dr. Sidbury said that topical steroid addiction (TSA) and topical steroid withdrawal (TSW) have been a “controversial but persistent concern” from some patients and providers. “Two systematic reviews of this topic were mentioned, and it was made clear that the evidence base [for the concepts] is weak,” he said. “With that important caveat ,the guideline committee delineated both a definition of TSW/TSA and potential risk factors.”
Dr. Sidbury marveled at the potential impact of newer medicines such as crisaborole and ruxolitinib on younger AD patients as well. Crisaborole is now Food and Drug Administration approved down to 3 months of age for mild to moderate AD. “This is extraordinary and expands treatment options for all providers at an age when parents and providers are most conservative in their practice,” he said. “Ruxolitinib, also nonsteroidal, is FDA approved for mild to moderate AD down to 12 years of age. Having spent a good percentage of my practice years either being able to offer only topical steroids, or later topical steroids and topical calcineurin inhibitors like tacrolimus or pimecrolimus, having additional options is wonderful.”
In the guidelines, the work group noted that “significant gaps remain” in current understanding of various topical AD therapies. “Studies are needed which examine quality of life and other patient-important outcomes, changes to the cutaneous microbiome, as well as long term follow-up, and use in special and diverse populations (e.g., pregnancy, lactation, immunosuppression, multiple comorbidities, skin of color, pediatric),” they wrote. “Furthermore, increased use of new systemic AD treatment options (dupilumab, tralokinumab, abrocitinib, upadacitinib) in patients with moderate to severe disease may result in a selection bias toward milder disease in current and future AD topical therapy studies.”
Use of topical therapies to manage AD in pediatric patients will be covered in a forthcoming AAD guideline. The first updated AD guideline, on comorbidities associated with AD in adults, was released in January 2022.
Dr. Sidbury reported that he serves as an advisory board member for Pfizer, a principal investigator for Regeneron, an investigator for Brickell Biotech and Galderma USA, and a consultant for Galderma Global and Microes. Other work group members reported having financial disclosures with many pharmaceutical companies.
, and topical phosphodiesterase-4 (PDE-4) and Janus kinase (JAK) inhibitors. The guidelines also conditionally recommend the use of bathing and wet wrap therapy but recommend against the use of topical antimicrobials, antiseptics, and antihistamines.
The development updates the AAD’s 2014 recommendations for managing AD with topical therapies, published almost 9 years ago. “At that time, the only U.S. FDA–approved systemic medication for atopic dermatitis was prednisone – universally felt amongst dermatologists to be the least appropriate systemic medication for this condition, at least chronically,” Robert Sidbury, MD, MPH, who cochaired a 14-member multidisciplinary work group that assembled the updated guidelines, told this news organization in an interview.
“Since 2017, there have been two different biologic medications approved for moderate to severe AD (dupilumab and tralokinumab) with certainly a third or more right around the corner. There have been two new oral agents approved for moderate to severe AD – upadacitinib and abrocitinib – with others on the way,” he noted. While these are not topical therapies, the purview of the newly released guidelines, he said, “there have also been new topical medications approved since that time (crisaborole and ruxolitinib). It was high time for an update.”
For the new guidelines, which were published online in the Journal of the American Academy of Dermatology, Dr. Sidbury, chief of the division of dermatology at Seattle Children’s Hospital, guidelines cochair Dawn M. R. Davis, MD, a dermatologist at Mayo Clinic, Rochester, Minn., and colleagues conducted a systematic review of evidence regarding the use of nonprescription topical agents such as moisturizers, bathing practices, and wet wraps, as well as topical pharmacologic modalities such as corticosteroids, calcineurin inhibitors, JAK inhibitors, PDE-4 inhibitors, antimicrobials, and antihistamines.
Next, the work group applied the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach for assessing the certainty of the evidence and formulating and grading clinical recommendations based on relevant randomized trials in the medical literature.
12 recommendations
Of the 12 recommendations made for adults with AD, the work group ranked 7 as “strong” based on the evidence reviewed, and the rest as “conditional.” The “strong” recommendations include the use of moisturizers; the use of tacrolimus 0.03% or 0.1%; the use of pimecrolimus 1% cream for mild to moderate AD; use of topical steroids; intermittent use of medium-potency topical corticosteroids as maintenance therapy to reduce flares and relapse; the use of the topical PDE-4 inhibitor crisaborole, and the use of the topical JAK inhibitor ruxolitinib.
Regarding ruxolitinib cream 1.5%, the work group advised that the treatment area “should not exceed 20% body surface area, and a maximum of 60 grams should be applied per week; these stipulations are aimed at reducing systemic absorption, as black box warnings include serious infections, mortality, malignancies (for example, lymphoma), major adverse cardiovascular events, and thrombosis.”
Conditional recommendations in the guidelines include those for bathing for treatment and maintenance and the use of wet dressings, and those against the use of topical antimicrobials, topical antihistamines, and topical antiseptics.
According to Dr. Sidbury, the topic of bathing generated robust discussion among the work group members. “Though [each group member] has strong opinions and individual practice styles, they were also able to recognize that the evidence is all that matters in a project like this, which led to a ‘conditional’ recommendation regarding bathing frequency backed by ‘low’ evidence,” he said. “While this may seem like ‘guidance’ that doesn’t ‘guide,’ I would argue it informs the guideline consumer exactly where we are in terms of this question and allows them to use their best judgment and experience as their true north here.”
In the realm of topical steroids, Dr. Sidbury said that topical steroid addiction (TSA) and topical steroid withdrawal (TSW) have been a “controversial but persistent concern” from some patients and providers. “Two systematic reviews of this topic were mentioned, and it was made clear that the evidence base [for the concepts] is weak,” he said. “With that important caveat ,the guideline committee delineated both a definition of TSW/TSA and potential risk factors.”
Dr. Sidbury marveled at the potential impact of newer medicines such as crisaborole and ruxolitinib on younger AD patients as well. Crisaborole is now Food and Drug Administration approved down to 3 months of age for mild to moderate AD. “This is extraordinary and expands treatment options for all providers at an age when parents and providers are most conservative in their practice,” he said. “Ruxolitinib, also nonsteroidal, is FDA approved for mild to moderate AD down to 12 years of age. Having spent a good percentage of my practice years either being able to offer only topical steroids, or later topical steroids and topical calcineurin inhibitors like tacrolimus or pimecrolimus, having additional options is wonderful.”
In the guidelines, the work group noted that “significant gaps remain” in current understanding of various topical AD therapies. “Studies are needed which examine quality of life and other patient-important outcomes, changes to the cutaneous microbiome, as well as long term follow-up, and use in special and diverse populations (e.g., pregnancy, lactation, immunosuppression, multiple comorbidities, skin of color, pediatric),” they wrote. “Furthermore, increased use of new systemic AD treatment options (dupilumab, tralokinumab, abrocitinib, upadacitinib) in patients with moderate to severe disease may result in a selection bias toward milder disease in current and future AD topical therapy studies.”
Use of topical therapies to manage AD in pediatric patients will be covered in a forthcoming AAD guideline. The first updated AD guideline, on comorbidities associated with AD in adults, was released in January 2022.
Dr. Sidbury reported that he serves as an advisory board member for Pfizer, a principal investigator for Regeneron, an investigator for Brickell Biotech and Galderma USA, and a consultant for Galderma Global and Microes. Other work group members reported having financial disclosures with many pharmaceutical companies.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
The Safety and Efficacy of AUC/MIC-Guided vs Trough-Guided Vancomycin Monitoring Among Veterans
Vancomycin is a commonly used glycopeptide antibiotic used to treat infections caused by gram-positive organisms. Vancomycin is most often used as a parenteral agent for empiric or definitive treatment of methicillin-resistant Staphylococcus aureus (MRSA). It can also be used for the treatment of other susceptible Staphylococcus or Enterococcus species. Adverse effects of parenteral vancomycin include infusion-related reactions, ototoxicity, and nephrotoxicity.1 Higher vancomycin trough levels have been associated with an increased risk of nephrotoxicity.1-4 The major safety concern with vancomycin is acute kidney injury (AKI). Even mild AKI can prolong hospitalizations, increase the cost of health care, and increase morbidity.2
In March 2020, the American Society of Health-System Pharmacists, the Infectious Diseases Society of America (IDSA), the Pediatric Infectious Disease Society, and the Society of Infectious Diseases Pharmacists released a consensus statement and guidelines regarding the optimization of vancomycin dosing and monitoring for patients with suspected or definitive serious MRSA infections. Based on these guidelines, it is recommended to target an individualized area under the curve/minimum inhibitory concentration (AUC/MIC) ratio of 400 to 600 mg × h/L to maximize clinical efficacy and minimize the risk of AKI.2
Before March 2020, the vancomycin monitoring recommendation was to target trough levels of 10 to 20 mg/L. A goal trough of 15 to 20 mg/L was recommended for severe infections, including sepsis, endocarditis, hospital-acquired pneumonia, meningitis, and osteomyelitis, caused by MRSA. A goal trough of 10 to 15 mg/L was recommended for noninvasive infections, such as skin and soft tissue infections and urinary tract infections, caused by MRSA. Targeting these trough levels was thought to achieve an AUC/MIC ≥ 400 mg × h/L.5 Evidence has since shown that trough values may not be an optimal marker for AUC/MIC values.2
The updated vancomycin therapeutic drug monitoring (TDM) guidelines recommend that health systems transition to AUC/MIC-guided monitoring for suspected or confirmed infections caused by MRSA. There is not enough evidence to recommend AUC/MIC-guided monitoring in patients with noninvasive infections or infections caused by other microbes.2
AUC/MIC-guided monitoring can be achieved in 2 ways. The first method is collecting Cmax (peak level) and Cmin (trough level) serum concentrations, preferably during the same dosing interval. Ideally, Cmax should be drawn 1 to 2 hours after the vancomycin infusion and Cmin should be drawn at the end of the dosing interval. First-order pharmacokinetic equations are used to estimate the AUC/MIC with this method. Bayesian software pharmacokinetic modeling based on 1 or 2 vancomycin concentrations with 1 trough level also can be used for monitoring. Preferably, 2 levels would be obtained to estimate the AUC/MIC when using Bayesian modeling.2
The bactericidal activity of vancomycin was achieved with AUC/MIC ratios of ≥ 400 mg × h/L. AUC/MIC ratios of < 400 mg × h/L increase the incidence of resistant and intermediate strains of S aureus. AUC/MIC-guided monitoring assumes an MIC of 1 mg/L. When the MIC is > 1 mg/L, it is less likely that an AUC/MIC ≥ 400 mg × h/L is achievable. Regardless of the TDM method used, AUC/MIC ratios ≥ 400 mg × h/L are not achievable with conventional dosing methods if the vancomycin MIC is > 2 mg/L in patients with normal renal function. Alternative therapy is recommended to be used for these patients.2
There are multiple studies investigating the therapeutic dosing of vancomycin and the associated incidence of AKI. Previous studies have correlated vancomycin AUC/MICs of 400 mg to 600 mg × h/L with clinical effectiveness.2,6 In 2017, Neely and colleagues looked at the therapeutic dosing of vancomycin in 252 adults with ≥ 1 vancomycin level.7 During this prospective trial, they evaluated patients for 1 year and targeted trough concentrations of 10 to 20 mg/L with infection-specific goal ranges of 10 to 15 mg/L and 15 to 20 mg/L for noninvasive and invasive infections, respectively. They also targeted AUC/MIC ratios ≥ 400 mg × h/L regardless of trough concentration using Bayesian estimated AUC/MICs for 2 years. They found only 19% of trough concentrations to be therapeutic compared with 70% of AUC/MICs. A secondary outcome assessed by Neely and colleagues was nephrotoxicity, which was identified in 8% of patients with trough targets and 2% of patients with AUC/MIC targets.8
Previous studies evaluating the use of vancomycin in the veteran population have focused on AKI incidence, general nephrotoxicity, and 30-day readmission rates.4,7,9,10 Poston-Blahnik and colleagues investigated the rates of AKI in 200 veterans using AUC/MIC-guided vancomycin TDM.5 They found an AKI incidence of 42% of patients with AUC/MICs ≥ 550 mg × h/L and 2% of patients with AUC/MICs < 550 mg × h/L.5 Gyamlani and colleagues investigated the rates of AKI in 33,527 veterans and found that serum vancomycin trough levels ≥ 20 mg/L were associated with a higher risk of AKI.8 Prabaker and colleagues investigated the association between vancomycin trough levels and nephrotoxicity, defined as 0.5 mg/L or a 50% increase in serum creatinine (sCr) in 348 veterans. They found nephrotoxicity in 8.9% of patients.10 Patel and colleagues investigated the effect of AKI on 30-day readmission rates in 216 veterans.10 AKI occurred in 8.8% of patients and of those 19.4% were readmitted within 30 days.10 Current literature lacks evidence regarding the comparison of the safety and efficacy of vancomycin trough-guided vs AUC/MIC-guided TDM in the veteran population. Therefore, the objective of this study was to investigate the differences in the safety and efficacy of vancomycin TDM in the veteran population based on the different monitoring methods used.
METHODS
This study was a retrospective, single-center, quasi-experimental chart review conducted at the Sioux Falls Veterans Affairs Health Care System (SFVAHCS) in South Dakota. Data were collected from the Computerized Patient Record System (CPRS). The SFVAHCS transitioned from trough-guided to AUC/MIC-guided TDM in November 2020.
Patients included in this study were veterans aged ≥ 18 years with orders for parenteral vancomycin between February 1, 2020, and October 31, 2020, for the trough-guided TDM group and between December 1, 2020, and August 31, 2021, for the AUC/MIC-guided TDM group. Patients with vancomycin courses initiated during November 2020 were excluded as both TDM methods were being used at that time. Patients were excluded if their vancomycin course began before February 1, 2020, for the trough-guided TDM group or began during November 2020 for the AUC/MIC-guided TDM group. Patients were excluded if their vancomycin course extended past October 31, 2020, for the trough group or past August 31, 2021, for the AUC/MIC group. Patients on dialysis or missing Cmax, Cmin, or sCr levels were excluded.
This study evaluated both safety (AKI incidence) and effectiveness (time spent in therapeutic range and time to therapeutic range). The primary endpoint was presence of vancomycin-induced AKI, which was based on the most recent Kidney Disease: Improving Global Outcomes (KDIGO) AKI definition: increased sCr of ≥ 0.3 mg/dL or by 50% from baseline sustained over 48 hours without any other explanation for the change.11 A secondary endpoint was the absence or presence of AKI.
Additional secondary endpoints included the presence of the initial trough or AUC/MIC of each vancomycin course within the therapeutic range and the percentage of all trough levels or AUC/MICs within therapeutic, subtherapeutic, and supratherapeutic ranges. The therapeutic range for AUC/MIC-guided TDM was 400 to 600 mg × h/L and 10 to 20 mg/L depending on indication for trough-guided TDM (15-20 mg/L for severe infections and 10-15 mg/L for less invasive infections). The percentage of trough levels or AUC/MICs within therapeutic, subtherapeutic, and supratherapeutic ranges were calculated as a ratio of levels within each range to total levels taken for each patient.
For AUC/MIC-guided TDM the Cmax levels were ideally drawn 1 to 2 hours after vancomycin infusion and Cmin levels were ideally drawn 30 minutes before the next dose. First-order pharmacokinetic equations were used to estimate the AUC/MIC.12 If the timing of a vancomycin level was inappropriate, actual levels were extrapolated based on the timing of the blood draw compared with the ideal Cmin or Cmax time. Extrapolated levels were used for both trough-guided and AUC/MIC-guided TDM groups when appropriate. Vancomycin levels were excluded if they were drawn during the vancomycin infusion.
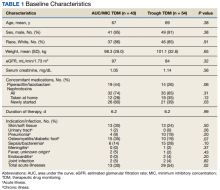
Study participant age, sex, race, weight, baseline estimated glomerular filtration (eGFR) rate, baseline sCr, concomitant nephrotoxic medications, duration of vancomycin course, indication of vancomycin, and acuity of illness based on indication were collected. sCr levels were collected from the initial day vancomycin was ordered through 72 hours following completion of a vancomycin course to evaluate for AKI. Patients’ charts were reviewed for the use of the following nephrotoxic medications: nonsteroidal anti-inflammatories, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, aminoglycosides, piperacillin/tazobactam, loop diuretics, amphotericin B, acyclovir, intravenous contrast, and nephrotoxic chemotherapy (cisplatin). The category of concomitant nephrotoxic medications was also collected including the continuation of a home nephrotoxic medication vs the initiation of a new nephrotoxic medication.
Statistical Analysis
The primary endpoint of the incidence of vancomycin-induced AKI was compared using a Fisher exact test. The secondary endpoint of the percentage of trough levels or AUC/MICs in the therapeutic, subtherapeutic, and supratherapeutic range were compared using a student t test. The secondary endpoint of first level or AUC/MIC within goal range was compared using a χ2 test. Continuous baseline characteristics were reported as a mean and compared using a student t test. Nominal baseline characteristics were reported as a percentage and compared using the χ2 test. P values < .05 were considered statistically significant.
RESULTS
This study included 97 patients, 43 in the AUC/MIC group and 54 in the trough group.
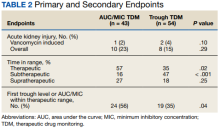
One (2%) patient in the AUC/MIC group and 2 (4%) patients in the trough group experienced vancomycin-induced AKI (P = .10) (Table 2).
DISCUSSION
There was no statistically significant difference between the 2 groups for the vancomycin-induced AKI (P = .10), the primary endpoint, or overall AKI (P = .29), the secondary endpoint. It should be noted that there was more overall AKI in the AUC/MIC group. Veterans in the AUC/MIC group were found to have their first AUC/MIC within the therapeutic range statistically significantly more often than the first trough level in the trough group (P = .04). The percentage of time spent within therapeutic range was statistically significantly higher in the AUC/MIC-guided TDM group (P = .02). The percentage of time spent subtherapeutic of goal range was statistically significantly higher in the trough-guided TDM group (P < .001). There was no statistically significant difference found in the percent of time spent supratherapeutic of goal range (P = .25). However, the observed percentage of time spent supratherapeutic of goal range was higher in the AUC/MIC group. These results indicate that AUC/MIC-guided TDM may be more efficacious with regard to time in therapeutic range and time to therapeutic range.
The finding of increased AKI with AUC/MIC-guided TDM does not align with previous studies.8 The prospective study by Neely and colleagues found that AUC/MIC-guided TDM resulted in more time in the therapeutic range as well as less nephrotoxicity compared with trough-guided TDM, although it was limited by its lack of randomization and did not account for other causes of nephrotoxicity.8 They found that only 19% of trough concentrations were therapeutic compared with 70% of AUC/MICs and found nephrotoxicity in 8% of trough-guided TDM patients compared with 2% of AUC/MIC-guided TDM patients.8
Unlike Nealy and colleagues, our study did not find lower nephrotoxicity associated with AUC/MIC-guided TDM. Multiple factors may have influenced our results. Our AUC/MIC group had significantly more newly started concomitant nephrotoxins and other nephrotoxic medications used during the vancomycin courses compared with the trough-guided group, which may have influenced AKI outcomes. It also should be noted that there was significantly more time spent subtherapeutic of the goal range and significantly less time in the goal range in the trough group compared with the AUC/MIC group. In our study, the trough-guided group had significantly more patients with acute illness compared with the AUC/MIC group (skin, soft tissue, and joint infections were similar between the groups). The group with more acutely ill patients would have been expected to have more nephrotoxicity. However, despite the acute illnesses, patients in the trough-guided group spent more time in the subtherapeutic range. This may explain the increased nephrotoxicity in the AUC/MIC group since those patients spent more time in the therapeutic range.
This study used the most recent KDIGO AKI definition: either an increase in sCr of ≥ 0.3 mg/dL or a 50% increase in sCr from baseline sustained over 48 hours without any other explanation for the change in renal function.11 This AKI definition is stricter than the previous definition, which was used by earlier studies, including Neely and colleagues, to evaluate rates of vancomycin-induced AKI.2,3 Therefore, the rates of overall AKI found in this study may be higher than in previous studies due to the definition of AKI used.
Limitations
This study was limited by its retrospective nature, lack of randomization, and small sample size. To decrease the potential for error in this study, analysis of power and a larger study sample would have been beneficial. During the COVID-19 pandemic, increased pneumonia cases may have hidden bacterial causes and caused an undercount. Nephrotoxicity may also be related to volume depletion, severe systemic illness, dehydration, or hypotension. Screening was completed via chart review for these alternative causes of nephrotoxicity in this study but may not be completely accounted for due to lack of documentation and the retrospective nature of this study.
CONCLUSIONS
This study did not find a significant difference in the rates of vancomycin-induced or overall AKI between AUC/MIC-guided and trough-guided TDM. However, this study may not have been powered to detect a significant difference in the primary endpoint. This study indicated that AUC/MIC-guided TDM of vancomycin resulted in a quicker time to the therapeutic range and a higher percentage of overall time in the therapeutic range as compared with trough-guided TDM. The results of this study indicated that trough-guided monitoring resulted in a higher percentage of time in a subtherapeutic range. This study also found that the first AUC/MIC calculated was within therapeutic range more often than the first trough level collected.
These results indicate that AUC/MIC-guided TDM may be more effective than trough-guided TDM in the veteran population. However, while AUC/MIC-guided TDM may be more effective with regards to time in therapeutic range and time to therapeutic range, this study did not indicate any safety benefit of AUC/MIC-guided over trough-guided TDM with regards to AKI incidence. Our data indicate that AUC/MIC-guided TDM increases the amount of time in the therapeutic range compared with trough-guided TDM and is not more nephrotoxic. The findings of this study support the recommendation to transition to the use of AUC/MIC-guided TDM of vancomycin in the veteran population.
Acknowledgments
This material is the result of work supported with the use of facilities and resources from the Sioux Falls Veterans Affairs Health Care System.
1. Gallagher J, MacDougall C. Glycopeptides and short-acting lipoglycopeptides In: Antibiotics Simplified. Jones & Bartlett Learning; 2018.
2. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835-864. doi:10.1093/ajhp/zxaa036
3. Hermsen ED, Hanson M, Sankaranarayanan J, Stoner JA, Florescu MC, Rupp ME. Clinical outcomes and nephrotoxicity associated with vancomycin trough concentrations during treatment of deep-seated infections. Expert Opin Drug Saf. 2010;9(1):9-14. doi:10.1517/14740330903413514
4. Poston-Blahnik A, Moenster R. Association between vancomycin area under the curve and nephrotoxicity: a single center, retrospective cohort study in a veteran population. Open Forum Infect Dis. 2021;8(5):ofab094. Published 2021 Mar 12. doi:10.1093/ofid/ofab094
5. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82-98. doi:10.2146/ajhp080434
6. Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004;43(13):925-942. doi:10.2165/00003088-200443130-00005
7. Gyamlani G, Potukuchi PK, Thomas F, et al. Vancomycin-Associated Acute Kidney Injury in a Large Veteran Population. Am J Nephrol. 2019;49(2):133-142. doi:10.1159/000496484
8. Neely MN, Kato L, Youn G, et al. Prospective Trial on the Use of Trough Concentration versus Area under the Curve To Determine Therapeutic Vancomycin Dosing. Antimicrob Agents Chemother. 2018;62(2):e02042-17. Published 2018 Jan 25. doi:10.1128/AAC.02042-17
9. Prabaker KK, Tran TP, Pratummas T, Goetz MB, Graber CJ. Elevated vancomycin trough is not associated with nephrotoxicity among inpatient veterans. J Hosp Med. 2012;7(2):91-97. doi:10.1002/jhm.946
10. Patel N, Stornelli N, Sangiovanni RJ, Huang DB, Lodise TP. Effect of vancomycin-associated acute kidney injury on incidence of 30-day readmissions among hospitalized Veterans Affairs patients with skin and skin structure infections. Antimicrob Agents Chemother. 2020;64(10):e01268-20. Published 2020 Sep 21. doi:10.1128/AAC.01268-20
11. Acute Kidney Injury Work Group. Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. 2012;2(suppl 1):1-138.
12. Pai MP, Neely M, Rodvold KA, Lodise TP. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. 2014;77:50-57. doi:10.1016/j.addr.2014.05.016
Vancomycin is a commonly used glycopeptide antibiotic used to treat infections caused by gram-positive organisms. Vancomycin is most often used as a parenteral agent for empiric or definitive treatment of methicillin-resistant Staphylococcus aureus (MRSA). It can also be used for the treatment of other susceptible Staphylococcus or Enterococcus species. Adverse effects of parenteral vancomycin include infusion-related reactions, ototoxicity, and nephrotoxicity.1 Higher vancomycin trough levels have been associated with an increased risk of nephrotoxicity.1-4 The major safety concern with vancomycin is acute kidney injury (AKI). Even mild AKI can prolong hospitalizations, increase the cost of health care, and increase morbidity.2
In March 2020, the American Society of Health-System Pharmacists, the Infectious Diseases Society of America (IDSA), the Pediatric Infectious Disease Society, and the Society of Infectious Diseases Pharmacists released a consensus statement and guidelines regarding the optimization of vancomycin dosing and monitoring for patients with suspected or definitive serious MRSA infections. Based on these guidelines, it is recommended to target an individualized area under the curve/minimum inhibitory concentration (AUC/MIC) ratio of 400 to 600 mg × h/L to maximize clinical efficacy and minimize the risk of AKI.2
Before March 2020, the vancomycin monitoring recommendation was to target trough levels of 10 to 20 mg/L. A goal trough of 15 to 20 mg/L was recommended for severe infections, including sepsis, endocarditis, hospital-acquired pneumonia, meningitis, and osteomyelitis, caused by MRSA. A goal trough of 10 to 15 mg/L was recommended for noninvasive infections, such as skin and soft tissue infections and urinary tract infections, caused by MRSA. Targeting these trough levels was thought to achieve an AUC/MIC ≥ 400 mg × h/L.5 Evidence has since shown that trough values may not be an optimal marker for AUC/MIC values.2
The updated vancomycin therapeutic drug monitoring (TDM) guidelines recommend that health systems transition to AUC/MIC-guided monitoring for suspected or confirmed infections caused by MRSA. There is not enough evidence to recommend AUC/MIC-guided monitoring in patients with noninvasive infections or infections caused by other microbes.2
AUC/MIC-guided monitoring can be achieved in 2 ways. The first method is collecting Cmax (peak level) and Cmin (trough level) serum concentrations, preferably during the same dosing interval. Ideally, Cmax should be drawn 1 to 2 hours after the vancomycin infusion and Cmin should be drawn at the end of the dosing interval. First-order pharmacokinetic equations are used to estimate the AUC/MIC with this method. Bayesian software pharmacokinetic modeling based on 1 or 2 vancomycin concentrations with 1 trough level also can be used for monitoring. Preferably, 2 levels would be obtained to estimate the AUC/MIC when using Bayesian modeling.2
The bactericidal activity of vancomycin was achieved with AUC/MIC ratios of ≥ 400 mg × h/L. AUC/MIC ratios of < 400 mg × h/L increase the incidence of resistant and intermediate strains of S aureus. AUC/MIC-guided monitoring assumes an MIC of 1 mg/L. When the MIC is > 1 mg/L, it is less likely that an AUC/MIC ≥ 400 mg × h/L is achievable. Regardless of the TDM method used, AUC/MIC ratios ≥ 400 mg × h/L are not achievable with conventional dosing methods if the vancomycin MIC is > 2 mg/L in patients with normal renal function. Alternative therapy is recommended to be used for these patients.2
There are multiple studies investigating the therapeutic dosing of vancomycin and the associated incidence of AKI. Previous studies have correlated vancomycin AUC/MICs of 400 mg to 600 mg × h/L with clinical effectiveness.2,6 In 2017, Neely and colleagues looked at the therapeutic dosing of vancomycin in 252 adults with ≥ 1 vancomycin level.7 During this prospective trial, they evaluated patients for 1 year and targeted trough concentrations of 10 to 20 mg/L with infection-specific goal ranges of 10 to 15 mg/L and 15 to 20 mg/L for noninvasive and invasive infections, respectively. They also targeted AUC/MIC ratios ≥ 400 mg × h/L regardless of trough concentration using Bayesian estimated AUC/MICs for 2 years. They found only 19% of trough concentrations to be therapeutic compared with 70% of AUC/MICs. A secondary outcome assessed by Neely and colleagues was nephrotoxicity, which was identified in 8% of patients with trough targets and 2% of patients with AUC/MIC targets.8
Previous studies evaluating the use of vancomycin in the veteran population have focused on AKI incidence, general nephrotoxicity, and 30-day readmission rates.4,7,9,10 Poston-Blahnik and colleagues investigated the rates of AKI in 200 veterans using AUC/MIC-guided vancomycin TDM.5 They found an AKI incidence of 42% of patients with AUC/MICs ≥ 550 mg × h/L and 2% of patients with AUC/MICs < 550 mg × h/L.5 Gyamlani and colleagues investigated the rates of AKI in 33,527 veterans and found that serum vancomycin trough levels ≥ 20 mg/L were associated with a higher risk of AKI.8 Prabaker and colleagues investigated the association between vancomycin trough levels and nephrotoxicity, defined as 0.5 mg/L or a 50% increase in serum creatinine (sCr) in 348 veterans. They found nephrotoxicity in 8.9% of patients.10 Patel and colleagues investigated the effect of AKI on 30-day readmission rates in 216 veterans.10 AKI occurred in 8.8% of patients and of those 19.4% were readmitted within 30 days.10 Current literature lacks evidence regarding the comparison of the safety and efficacy of vancomycin trough-guided vs AUC/MIC-guided TDM in the veteran population. Therefore, the objective of this study was to investigate the differences in the safety and efficacy of vancomycin TDM in the veteran population based on the different monitoring methods used.
METHODS
This study was a retrospective, single-center, quasi-experimental chart review conducted at the Sioux Falls Veterans Affairs Health Care System (SFVAHCS) in South Dakota. Data were collected from the Computerized Patient Record System (CPRS). The SFVAHCS transitioned from trough-guided to AUC/MIC-guided TDM in November 2020.
Patients included in this study were veterans aged ≥ 18 years with orders for parenteral vancomycin between February 1, 2020, and October 31, 2020, for the trough-guided TDM group and between December 1, 2020, and August 31, 2021, for the AUC/MIC-guided TDM group. Patients with vancomycin courses initiated during November 2020 were excluded as both TDM methods were being used at that time. Patients were excluded if their vancomycin course began before February 1, 2020, for the trough-guided TDM group or began during November 2020 for the AUC/MIC-guided TDM group. Patients were excluded if their vancomycin course extended past October 31, 2020, for the trough group or past August 31, 2021, for the AUC/MIC group. Patients on dialysis or missing Cmax, Cmin, or sCr levels were excluded.
This study evaluated both safety (AKI incidence) and effectiveness (time spent in therapeutic range and time to therapeutic range). The primary endpoint was presence of vancomycin-induced AKI, which was based on the most recent Kidney Disease: Improving Global Outcomes (KDIGO) AKI definition: increased sCr of ≥ 0.3 mg/dL or by 50% from baseline sustained over 48 hours without any other explanation for the change.11 A secondary endpoint was the absence or presence of AKI.
Additional secondary endpoints included the presence of the initial trough or AUC/MIC of each vancomycin course within the therapeutic range and the percentage of all trough levels or AUC/MICs within therapeutic, subtherapeutic, and supratherapeutic ranges. The therapeutic range for AUC/MIC-guided TDM was 400 to 600 mg × h/L and 10 to 20 mg/L depending on indication for trough-guided TDM (15-20 mg/L for severe infections and 10-15 mg/L for less invasive infections). The percentage of trough levels or AUC/MICs within therapeutic, subtherapeutic, and supratherapeutic ranges were calculated as a ratio of levels within each range to total levels taken for each patient.
For AUC/MIC-guided TDM the Cmax levels were ideally drawn 1 to 2 hours after vancomycin infusion and Cmin levels were ideally drawn 30 minutes before the next dose. First-order pharmacokinetic equations were used to estimate the AUC/MIC.12 If the timing of a vancomycin level was inappropriate, actual levels were extrapolated based on the timing of the blood draw compared with the ideal Cmin or Cmax time. Extrapolated levels were used for both trough-guided and AUC/MIC-guided TDM groups when appropriate. Vancomycin levels were excluded if they were drawn during the vancomycin infusion.
Study participant age, sex, race, weight, baseline estimated glomerular filtration (eGFR) rate, baseline sCr, concomitant nephrotoxic medications, duration of vancomycin course, indication of vancomycin, and acuity of illness based on indication were collected. sCr levels were collected from the initial day vancomycin was ordered through 72 hours following completion of a vancomycin course to evaluate for AKI. Patients’ charts were reviewed for the use of the following nephrotoxic medications: nonsteroidal anti-inflammatories, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, aminoglycosides, piperacillin/tazobactam, loop diuretics, amphotericin B, acyclovir, intravenous contrast, and nephrotoxic chemotherapy (cisplatin). The category of concomitant nephrotoxic medications was also collected including the continuation of a home nephrotoxic medication vs the initiation of a new nephrotoxic medication.
Statistical Analysis
The primary endpoint of the incidence of vancomycin-induced AKI was compared using a Fisher exact test. The secondary endpoint of the percentage of trough levels or AUC/MICs in the therapeutic, subtherapeutic, and supratherapeutic range were compared using a student t test. The secondary endpoint of first level or AUC/MIC within goal range was compared using a χ2 test. Continuous baseline characteristics were reported as a mean and compared using a student t test. Nominal baseline characteristics were reported as a percentage and compared using the χ2 test. P values < .05 were considered statistically significant.
RESULTS
This study included 97 patients, 43 in the AUC/MIC group and 54 in the trough group.
One (2%) patient in the AUC/MIC group and 2 (4%) patients in the trough group experienced vancomycin-induced AKI (P = .10) (Table 2).
DISCUSSION
There was no statistically significant difference between the 2 groups for the vancomycin-induced AKI (P = .10), the primary endpoint, or overall AKI (P = .29), the secondary endpoint. It should be noted that there was more overall AKI in the AUC/MIC group. Veterans in the AUC/MIC group were found to have their first AUC/MIC within the therapeutic range statistically significantly more often than the first trough level in the trough group (P = .04). The percentage of time spent within therapeutic range was statistically significantly higher in the AUC/MIC-guided TDM group (P = .02). The percentage of time spent subtherapeutic of goal range was statistically significantly higher in the trough-guided TDM group (P < .001). There was no statistically significant difference found in the percent of time spent supratherapeutic of goal range (P = .25). However, the observed percentage of time spent supratherapeutic of goal range was higher in the AUC/MIC group. These results indicate that AUC/MIC-guided TDM may be more efficacious with regard to time in therapeutic range and time to therapeutic range.
The finding of increased AKI with AUC/MIC-guided TDM does not align with previous studies.8 The prospective study by Neely and colleagues found that AUC/MIC-guided TDM resulted in more time in the therapeutic range as well as less nephrotoxicity compared with trough-guided TDM, although it was limited by its lack of randomization and did not account for other causes of nephrotoxicity.8 They found that only 19% of trough concentrations were therapeutic compared with 70% of AUC/MICs and found nephrotoxicity in 8% of trough-guided TDM patients compared with 2% of AUC/MIC-guided TDM patients.8
Unlike Nealy and colleagues, our study did not find lower nephrotoxicity associated with AUC/MIC-guided TDM. Multiple factors may have influenced our results. Our AUC/MIC group had significantly more newly started concomitant nephrotoxins and other nephrotoxic medications used during the vancomycin courses compared with the trough-guided group, which may have influenced AKI outcomes. It also should be noted that there was significantly more time spent subtherapeutic of the goal range and significantly less time in the goal range in the trough group compared with the AUC/MIC group. In our study, the trough-guided group had significantly more patients with acute illness compared with the AUC/MIC group (skin, soft tissue, and joint infections were similar between the groups). The group with more acutely ill patients would have been expected to have more nephrotoxicity. However, despite the acute illnesses, patients in the trough-guided group spent more time in the subtherapeutic range. This may explain the increased nephrotoxicity in the AUC/MIC group since those patients spent more time in the therapeutic range.
This study used the most recent KDIGO AKI definition: either an increase in sCr of ≥ 0.3 mg/dL or a 50% increase in sCr from baseline sustained over 48 hours without any other explanation for the change in renal function.11 This AKI definition is stricter than the previous definition, which was used by earlier studies, including Neely and colleagues, to evaluate rates of vancomycin-induced AKI.2,3 Therefore, the rates of overall AKI found in this study may be higher than in previous studies due to the definition of AKI used.
Limitations
This study was limited by its retrospective nature, lack of randomization, and small sample size. To decrease the potential for error in this study, analysis of power and a larger study sample would have been beneficial. During the COVID-19 pandemic, increased pneumonia cases may have hidden bacterial causes and caused an undercount. Nephrotoxicity may also be related to volume depletion, severe systemic illness, dehydration, or hypotension. Screening was completed via chart review for these alternative causes of nephrotoxicity in this study but may not be completely accounted for due to lack of documentation and the retrospective nature of this study.
CONCLUSIONS
This study did not find a significant difference in the rates of vancomycin-induced or overall AKI between AUC/MIC-guided and trough-guided TDM. However, this study may not have been powered to detect a significant difference in the primary endpoint. This study indicated that AUC/MIC-guided TDM of vancomycin resulted in a quicker time to the therapeutic range and a higher percentage of overall time in the therapeutic range as compared with trough-guided TDM. The results of this study indicated that trough-guided monitoring resulted in a higher percentage of time in a subtherapeutic range. This study also found that the first AUC/MIC calculated was within therapeutic range more often than the first trough level collected.
These results indicate that AUC/MIC-guided TDM may be more effective than trough-guided TDM in the veteran population. However, while AUC/MIC-guided TDM may be more effective with regards to time in therapeutic range and time to therapeutic range, this study did not indicate any safety benefit of AUC/MIC-guided over trough-guided TDM with regards to AKI incidence. Our data indicate that AUC/MIC-guided TDM increases the amount of time in the therapeutic range compared with trough-guided TDM and is not more nephrotoxic. The findings of this study support the recommendation to transition to the use of AUC/MIC-guided TDM of vancomycin in the veteran population.
Acknowledgments
This material is the result of work supported with the use of facilities and resources from the Sioux Falls Veterans Affairs Health Care System.
Vancomycin is a commonly used glycopeptide antibiotic used to treat infections caused by gram-positive organisms. Vancomycin is most often used as a parenteral agent for empiric or definitive treatment of methicillin-resistant Staphylococcus aureus (MRSA). It can also be used for the treatment of other susceptible Staphylococcus or Enterococcus species. Adverse effects of parenteral vancomycin include infusion-related reactions, ototoxicity, and nephrotoxicity.1 Higher vancomycin trough levels have been associated with an increased risk of nephrotoxicity.1-4 The major safety concern with vancomycin is acute kidney injury (AKI). Even mild AKI can prolong hospitalizations, increase the cost of health care, and increase morbidity.2
In March 2020, the American Society of Health-System Pharmacists, the Infectious Diseases Society of America (IDSA), the Pediatric Infectious Disease Society, and the Society of Infectious Diseases Pharmacists released a consensus statement and guidelines regarding the optimization of vancomycin dosing and monitoring for patients with suspected or definitive serious MRSA infections. Based on these guidelines, it is recommended to target an individualized area under the curve/minimum inhibitory concentration (AUC/MIC) ratio of 400 to 600 mg × h/L to maximize clinical efficacy and minimize the risk of AKI.2
Before March 2020, the vancomycin monitoring recommendation was to target trough levels of 10 to 20 mg/L. A goal trough of 15 to 20 mg/L was recommended for severe infections, including sepsis, endocarditis, hospital-acquired pneumonia, meningitis, and osteomyelitis, caused by MRSA. A goal trough of 10 to 15 mg/L was recommended for noninvasive infections, such as skin and soft tissue infections and urinary tract infections, caused by MRSA. Targeting these trough levels was thought to achieve an AUC/MIC ≥ 400 mg × h/L.5 Evidence has since shown that trough values may not be an optimal marker for AUC/MIC values.2
The updated vancomycin therapeutic drug monitoring (TDM) guidelines recommend that health systems transition to AUC/MIC-guided monitoring for suspected or confirmed infections caused by MRSA. There is not enough evidence to recommend AUC/MIC-guided monitoring in patients with noninvasive infections or infections caused by other microbes.2
AUC/MIC-guided monitoring can be achieved in 2 ways. The first method is collecting Cmax (peak level) and Cmin (trough level) serum concentrations, preferably during the same dosing interval. Ideally, Cmax should be drawn 1 to 2 hours after the vancomycin infusion and Cmin should be drawn at the end of the dosing interval. First-order pharmacokinetic equations are used to estimate the AUC/MIC with this method. Bayesian software pharmacokinetic modeling based on 1 or 2 vancomycin concentrations with 1 trough level also can be used for monitoring. Preferably, 2 levels would be obtained to estimate the AUC/MIC when using Bayesian modeling.2
The bactericidal activity of vancomycin was achieved with AUC/MIC ratios of ≥ 400 mg × h/L. AUC/MIC ratios of < 400 mg × h/L increase the incidence of resistant and intermediate strains of S aureus. AUC/MIC-guided monitoring assumes an MIC of 1 mg/L. When the MIC is > 1 mg/L, it is less likely that an AUC/MIC ≥ 400 mg × h/L is achievable. Regardless of the TDM method used, AUC/MIC ratios ≥ 400 mg × h/L are not achievable with conventional dosing methods if the vancomycin MIC is > 2 mg/L in patients with normal renal function. Alternative therapy is recommended to be used for these patients.2
There are multiple studies investigating the therapeutic dosing of vancomycin and the associated incidence of AKI. Previous studies have correlated vancomycin AUC/MICs of 400 mg to 600 mg × h/L with clinical effectiveness.2,6 In 2017, Neely and colleagues looked at the therapeutic dosing of vancomycin in 252 adults with ≥ 1 vancomycin level.7 During this prospective trial, they evaluated patients for 1 year and targeted trough concentrations of 10 to 20 mg/L with infection-specific goal ranges of 10 to 15 mg/L and 15 to 20 mg/L for noninvasive and invasive infections, respectively. They also targeted AUC/MIC ratios ≥ 400 mg × h/L regardless of trough concentration using Bayesian estimated AUC/MICs for 2 years. They found only 19% of trough concentrations to be therapeutic compared with 70% of AUC/MICs. A secondary outcome assessed by Neely and colleagues was nephrotoxicity, which was identified in 8% of patients with trough targets and 2% of patients with AUC/MIC targets.8
Previous studies evaluating the use of vancomycin in the veteran population have focused on AKI incidence, general nephrotoxicity, and 30-day readmission rates.4,7,9,10 Poston-Blahnik and colleagues investigated the rates of AKI in 200 veterans using AUC/MIC-guided vancomycin TDM.5 They found an AKI incidence of 42% of patients with AUC/MICs ≥ 550 mg × h/L and 2% of patients with AUC/MICs < 550 mg × h/L.5 Gyamlani and colleagues investigated the rates of AKI in 33,527 veterans and found that serum vancomycin trough levels ≥ 20 mg/L were associated with a higher risk of AKI.8 Prabaker and colleagues investigated the association between vancomycin trough levels and nephrotoxicity, defined as 0.5 mg/L or a 50% increase in serum creatinine (sCr) in 348 veterans. They found nephrotoxicity in 8.9% of patients.10 Patel and colleagues investigated the effect of AKI on 30-day readmission rates in 216 veterans.10 AKI occurred in 8.8% of patients and of those 19.4% were readmitted within 30 days.10 Current literature lacks evidence regarding the comparison of the safety and efficacy of vancomycin trough-guided vs AUC/MIC-guided TDM in the veteran population. Therefore, the objective of this study was to investigate the differences in the safety and efficacy of vancomycin TDM in the veteran population based on the different monitoring methods used.
METHODS
This study was a retrospective, single-center, quasi-experimental chart review conducted at the Sioux Falls Veterans Affairs Health Care System (SFVAHCS) in South Dakota. Data were collected from the Computerized Patient Record System (CPRS). The SFVAHCS transitioned from trough-guided to AUC/MIC-guided TDM in November 2020.
Patients included in this study were veterans aged ≥ 18 years with orders for parenteral vancomycin between February 1, 2020, and October 31, 2020, for the trough-guided TDM group and between December 1, 2020, and August 31, 2021, for the AUC/MIC-guided TDM group. Patients with vancomycin courses initiated during November 2020 were excluded as both TDM methods were being used at that time. Patients were excluded if their vancomycin course began before February 1, 2020, for the trough-guided TDM group or began during November 2020 for the AUC/MIC-guided TDM group. Patients were excluded if their vancomycin course extended past October 31, 2020, for the trough group or past August 31, 2021, for the AUC/MIC group. Patients on dialysis or missing Cmax, Cmin, or sCr levels were excluded.
This study evaluated both safety (AKI incidence) and effectiveness (time spent in therapeutic range and time to therapeutic range). The primary endpoint was presence of vancomycin-induced AKI, which was based on the most recent Kidney Disease: Improving Global Outcomes (KDIGO) AKI definition: increased sCr of ≥ 0.3 mg/dL or by 50% from baseline sustained over 48 hours without any other explanation for the change.11 A secondary endpoint was the absence or presence of AKI.
Additional secondary endpoints included the presence of the initial trough or AUC/MIC of each vancomycin course within the therapeutic range and the percentage of all trough levels or AUC/MICs within therapeutic, subtherapeutic, and supratherapeutic ranges. The therapeutic range for AUC/MIC-guided TDM was 400 to 600 mg × h/L and 10 to 20 mg/L depending on indication for trough-guided TDM (15-20 mg/L for severe infections and 10-15 mg/L for less invasive infections). The percentage of trough levels or AUC/MICs within therapeutic, subtherapeutic, and supratherapeutic ranges were calculated as a ratio of levels within each range to total levels taken for each patient.
For AUC/MIC-guided TDM the Cmax levels were ideally drawn 1 to 2 hours after vancomycin infusion and Cmin levels were ideally drawn 30 minutes before the next dose. First-order pharmacokinetic equations were used to estimate the AUC/MIC.12 If the timing of a vancomycin level was inappropriate, actual levels were extrapolated based on the timing of the blood draw compared with the ideal Cmin or Cmax time. Extrapolated levels were used for both trough-guided and AUC/MIC-guided TDM groups when appropriate. Vancomycin levels were excluded if they were drawn during the vancomycin infusion.
Study participant age, sex, race, weight, baseline estimated glomerular filtration (eGFR) rate, baseline sCr, concomitant nephrotoxic medications, duration of vancomycin course, indication of vancomycin, and acuity of illness based on indication were collected. sCr levels were collected from the initial day vancomycin was ordered through 72 hours following completion of a vancomycin course to evaluate for AKI. Patients’ charts were reviewed for the use of the following nephrotoxic medications: nonsteroidal anti-inflammatories, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, aminoglycosides, piperacillin/tazobactam, loop diuretics, amphotericin B, acyclovir, intravenous contrast, and nephrotoxic chemotherapy (cisplatin). The category of concomitant nephrotoxic medications was also collected including the continuation of a home nephrotoxic medication vs the initiation of a new nephrotoxic medication.
Statistical Analysis
The primary endpoint of the incidence of vancomycin-induced AKI was compared using a Fisher exact test. The secondary endpoint of the percentage of trough levels or AUC/MICs in the therapeutic, subtherapeutic, and supratherapeutic range were compared using a student t test. The secondary endpoint of first level or AUC/MIC within goal range was compared using a χ2 test. Continuous baseline characteristics were reported as a mean and compared using a student t test. Nominal baseline characteristics were reported as a percentage and compared using the χ2 test. P values < .05 were considered statistically significant.
RESULTS
This study included 97 patients, 43 in the AUC/MIC group and 54 in the trough group.
One (2%) patient in the AUC/MIC group and 2 (4%) patients in the trough group experienced vancomycin-induced AKI (P = .10) (Table 2).
DISCUSSION
There was no statistically significant difference between the 2 groups for the vancomycin-induced AKI (P = .10), the primary endpoint, or overall AKI (P = .29), the secondary endpoint. It should be noted that there was more overall AKI in the AUC/MIC group. Veterans in the AUC/MIC group were found to have their first AUC/MIC within the therapeutic range statistically significantly more often than the first trough level in the trough group (P = .04). The percentage of time spent within therapeutic range was statistically significantly higher in the AUC/MIC-guided TDM group (P = .02). The percentage of time spent subtherapeutic of goal range was statistically significantly higher in the trough-guided TDM group (P < .001). There was no statistically significant difference found in the percent of time spent supratherapeutic of goal range (P = .25). However, the observed percentage of time spent supratherapeutic of goal range was higher in the AUC/MIC group. These results indicate that AUC/MIC-guided TDM may be more efficacious with regard to time in therapeutic range and time to therapeutic range.
The finding of increased AKI with AUC/MIC-guided TDM does not align with previous studies.8 The prospective study by Neely and colleagues found that AUC/MIC-guided TDM resulted in more time in the therapeutic range as well as less nephrotoxicity compared with trough-guided TDM, although it was limited by its lack of randomization and did not account for other causes of nephrotoxicity.8 They found that only 19% of trough concentrations were therapeutic compared with 70% of AUC/MICs and found nephrotoxicity in 8% of trough-guided TDM patients compared with 2% of AUC/MIC-guided TDM patients.8
Unlike Nealy and colleagues, our study did not find lower nephrotoxicity associated with AUC/MIC-guided TDM. Multiple factors may have influenced our results. Our AUC/MIC group had significantly more newly started concomitant nephrotoxins and other nephrotoxic medications used during the vancomycin courses compared with the trough-guided group, which may have influenced AKI outcomes. It also should be noted that there was significantly more time spent subtherapeutic of the goal range and significantly less time in the goal range in the trough group compared with the AUC/MIC group. In our study, the trough-guided group had significantly more patients with acute illness compared with the AUC/MIC group (skin, soft tissue, and joint infections were similar between the groups). The group with more acutely ill patients would have been expected to have more nephrotoxicity. However, despite the acute illnesses, patients in the trough-guided group spent more time in the subtherapeutic range. This may explain the increased nephrotoxicity in the AUC/MIC group since those patients spent more time in the therapeutic range.
This study used the most recent KDIGO AKI definition: either an increase in sCr of ≥ 0.3 mg/dL or a 50% increase in sCr from baseline sustained over 48 hours without any other explanation for the change in renal function.11 This AKI definition is stricter than the previous definition, which was used by earlier studies, including Neely and colleagues, to evaluate rates of vancomycin-induced AKI.2,3 Therefore, the rates of overall AKI found in this study may be higher than in previous studies due to the definition of AKI used.
Limitations
This study was limited by its retrospective nature, lack of randomization, and small sample size. To decrease the potential for error in this study, analysis of power and a larger study sample would have been beneficial. During the COVID-19 pandemic, increased pneumonia cases may have hidden bacterial causes and caused an undercount. Nephrotoxicity may also be related to volume depletion, severe systemic illness, dehydration, or hypotension. Screening was completed via chart review for these alternative causes of nephrotoxicity in this study but may not be completely accounted for due to lack of documentation and the retrospective nature of this study.
CONCLUSIONS
This study did not find a significant difference in the rates of vancomycin-induced or overall AKI between AUC/MIC-guided and trough-guided TDM. However, this study may not have been powered to detect a significant difference in the primary endpoint. This study indicated that AUC/MIC-guided TDM of vancomycin resulted in a quicker time to the therapeutic range and a higher percentage of overall time in the therapeutic range as compared with trough-guided TDM. The results of this study indicated that trough-guided monitoring resulted in a higher percentage of time in a subtherapeutic range. This study also found that the first AUC/MIC calculated was within therapeutic range more often than the first trough level collected.
These results indicate that AUC/MIC-guided TDM may be more effective than trough-guided TDM in the veteran population. However, while AUC/MIC-guided TDM may be more effective with regards to time in therapeutic range and time to therapeutic range, this study did not indicate any safety benefit of AUC/MIC-guided over trough-guided TDM with regards to AKI incidence. Our data indicate that AUC/MIC-guided TDM increases the amount of time in the therapeutic range compared with trough-guided TDM and is not more nephrotoxic. The findings of this study support the recommendation to transition to the use of AUC/MIC-guided TDM of vancomycin in the veteran population.
Acknowledgments
This material is the result of work supported with the use of facilities and resources from the Sioux Falls Veterans Affairs Health Care System.
1. Gallagher J, MacDougall C. Glycopeptides and short-acting lipoglycopeptides In: Antibiotics Simplified. Jones & Bartlett Learning; 2018.
2. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835-864. doi:10.1093/ajhp/zxaa036
3. Hermsen ED, Hanson M, Sankaranarayanan J, Stoner JA, Florescu MC, Rupp ME. Clinical outcomes and nephrotoxicity associated with vancomycin trough concentrations during treatment of deep-seated infections. Expert Opin Drug Saf. 2010;9(1):9-14. doi:10.1517/14740330903413514
4. Poston-Blahnik A, Moenster R. Association between vancomycin area under the curve and nephrotoxicity: a single center, retrospective cohort study in a veteran population. Open Forum Infect Dis. 2021;8(5):ofab094. Published 2021 Mar 12. doi:10.1093/ofid/ofab094
5. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82-98. doi:10.2146/ajhp080434
6. Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004;43(13):925-942. doi:10.2165/00003088-200443130-00005
7. Gyamlani G, Potukuchi PK, Thomas F, et al. Vancomycin-Associated Acute Kidney Injury in a Large Veteran Population. Am J Nephrol. 2019;49(2):133-142. doi:10.1159/000496484
8. Neely MN, Kato L, Youn G, et al. Prospective Trial on the Use of Trough Concentration versus Area under the Curve To Determine Therapeutic Vancomycin Dosing. Antimicrob Agents Chemother. 2018;62(2):e02042-17. Published 2018 Jan 25. doi:10.1128/AAC.02042-17
9. Prabaker KK, Tran TP, Pratummas T, Goetz MB, Graber CJ. Elevated vancomycin trough is not associated with nephrotoxicity among inpatient veterans. J Hosp Med. 2012;7(2):91-97. doi:10.1002/jhm.946
10. Patel N, Stornelli N, Sangiovanni RJ, Huang DB, Lodise TP. Effect of vancomycin-associated acute kidney injury on incidence of 30-day readmissions among hospitalized Veterans Affairs patients with skin and skin structure infections. Antimicrob Agents Chemother. 2020;64(10):e01268-20. Published 2020 Sep 21. doi:10.1128/AAC.01268-20
11. Acute Kidney Injury Work Group. Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. 2012;2(suppl 1):1-138.
12. Pai MP, Neely M, Rodvold KA, Lodise TP. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. 2014;77:50-57. doi:10.1016/j.addr.2014.05.016
1. Gallagher J, MacDougall C. Glycopeptides and short-acting lipoglycopeptides In: Antibiotics Simplified. Jones & Bartlett Learning; 2018.
2. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835-864. doi:10.1093/ajhp/zxaa036
3. Hermsen ED, Hanson M, Sankaranarayanan J, Stoner JA, Florescu MC, Rupp ME. Clinical outcomes and nephrotoxicity associated with vancomycin trough concentrations during treatment of deep-seated infections. Expert Opin Drug Saf. 2010;9(1):9-14. doi:10.1517/14740330903413514
4. Poston-Blahnik A, Moenster R. Association between vancomycin area under the curve and nephrotoxicity: a single center, retrospective cohort study in a veteran population. Open Forum Infect Dis. 2021;8(5):ofab094. Published 2021 Mar 12. doi:10.1093/ofid/ofab094
5. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82-98. doi:10.2146/ajhp080434
6. Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004;43(13):925-942. doi:10.2165/00003088-200443130-00005
7. Gyamlani G, Potukuchi PK, Thomas F, et al. Vancomycin-Associated Acute Kidney Injury in a Large Veteran Population. Am J Nephrol. 2019;49(2):133-142. doi:10.1159/000496484
8. Neely MN, Kato L, Youn G, et al. Prospective Trial on the Use of Trough Concentration versus Area under the Curve To Determine Therapeutic Vancomycin Dosing. Antimicrob Agents Chemother. 2018;62(2):e02042-17. Published 2018 Jan 25. doi:10.1128/AAC.02042-17
9. Prabaker KK, Tran TP, Pratummas T, Goetz MB, Graber CJ. Elevated vancomycin trough is not associated with nephrotoxicity among inpatient veterans. J Hosp Med. 2012;7(2):91-97. doi:10.1002/jhm.946
10. Patel N, Stornelli N, Sangiovanni RJ, Huang DB, Lodise TP. Effect of vancomycin-associated acute kidney injury on incidence of 30-day readmissions among hospitalized Veterans Affairs patients with skin and skin structure infections. Antimicrob Agents Chemother. 2020;64(10):e01268-20. Published 2020 Sep 21. doi:10.1128/AAC.01268-20
11. Acute Kidney Injury Work Group. Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. 2012;2(suppl 1):1-138.
12. Pai MP, Neely M, Rodvold KA, Lodise TP. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. 2014;77:50-57. doi:10.1016/j.addr.2014.05.016
Methacrylate Polymer Powder Dressing for a Lower Leg Surgical Defect
To the Editor:
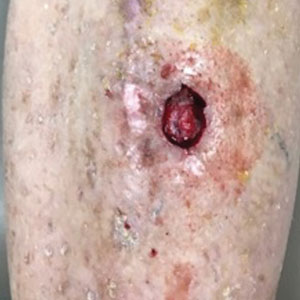
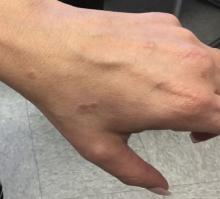
Surgical wounds on the lower leg are challenging to manage because venous stasis, bacterial colonization, and high tension may contribute to protracted healing. Advances in technology led to the development of novel, polymer-based wound-healing modalities that hold promise for the management of these wounds.
A 75-year-old man presented with a well-differentiated squamous cell carcinoma with a 3-mm depth of invasion on the left pretibial region. His comorbidities were notable for hypertension, hypercholesterolemia, varicose veins, myocardial infarction, peripheral vascular disease, and a 32 pack-year cigarette smoking history. Current medications included clopidogrel bisulfate and warfarin sodium to manage a recently placed coronary artery stent.
The tumor was cleared after 2 stages of Mohs micrographic surgery with excision down to tibialis anterior fascia (Figure 1A). The resultant defect measured 43×33 mm in area and 9 mm in depth (wound size, 12,771 mm3). Reconstructive options were discussed, including random-pattern flap repair and skin graft. Given the patient’s risk of bleeding, the decision was made to forego a flap repair. Additionally, the patient was a heavy smoker and could not comply with the wound care and elevation and ambulation restrictions required for optimal skin graft care. Therefore, a decision was made to proceed with secondary intention healing using a methacrylate polymer powder dressing.
After achieving hemostasis, a novel 10-mg sterile, biologically inert methacrylate polymer powder dressing was poured over the wound in a uniform layer to fill and seal the entire wound surface (Figure 1B). Sterile normal saline 0.1 mL was sprayed onto the powder to activate particle aggregation. No secondary dressing was used, and the patient was permitted to get the dressing wet after 48 hours.
The dressing was changed in a similar fashion 4 weeks after application, following gentle debridement with gauze and normal saline. Eight weeks after surgery, the wound exhibited healthy granulation tissue and measured 5×6 mm in area and 2 mm in depth (wound size, 60 mm3), which represented a 99.5% reduction in wound size (Figure 1C). The dressing was not painful, and there were no reported adverse effects. The patient continued to smoke and ambulate fully throughout this period. No antibiotics were used.
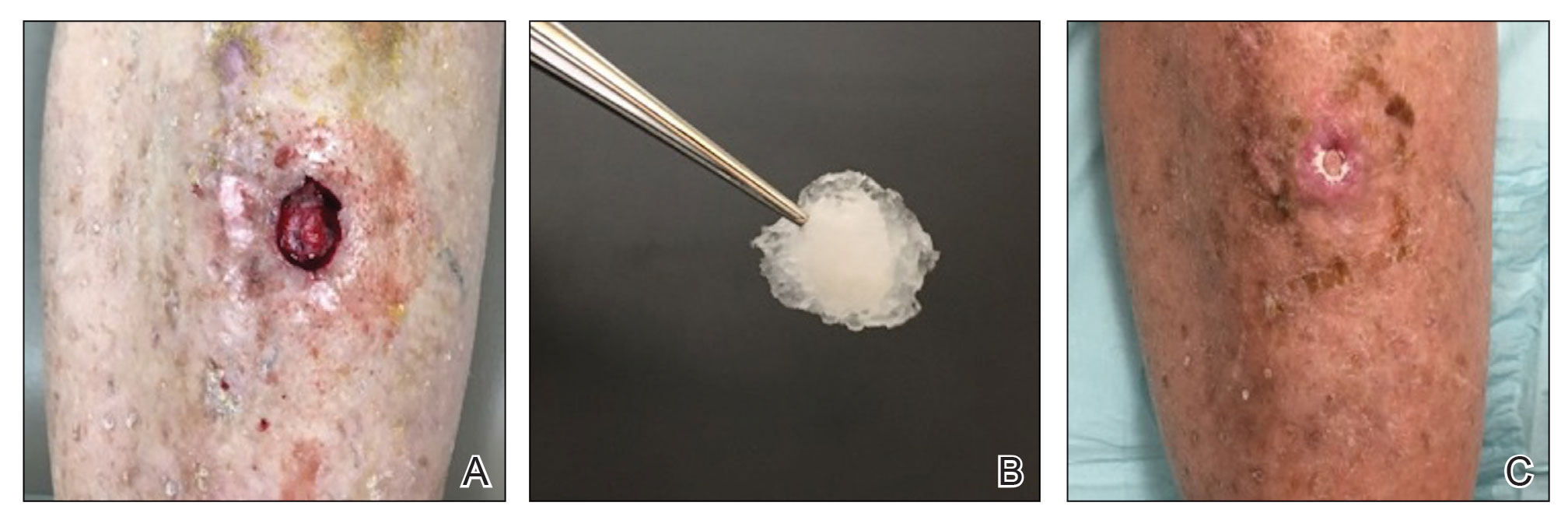
Methacrylate polymer powder dressings are a novel and sophisticated dressing modality with great promise for the management of surgical wounds on the lower limb. The dressing is a sterile powder consisting of 84.8% poly-2-hydroxyethylmethacrylate, 14.9% poly-2-hydroxypropylmethacrylate, and 0.3% sodium deoxycholate. These hydrophilic polymers have a covalent methacrylate backbone with a hydroxyl aliphatic side chain. When saline or wound exudate contacts the powder, the spheres hydrate and nonreversibly aggregate to form a moist, flexible dressing that conforms to the topography of the wound and seals it (Figure 2).1
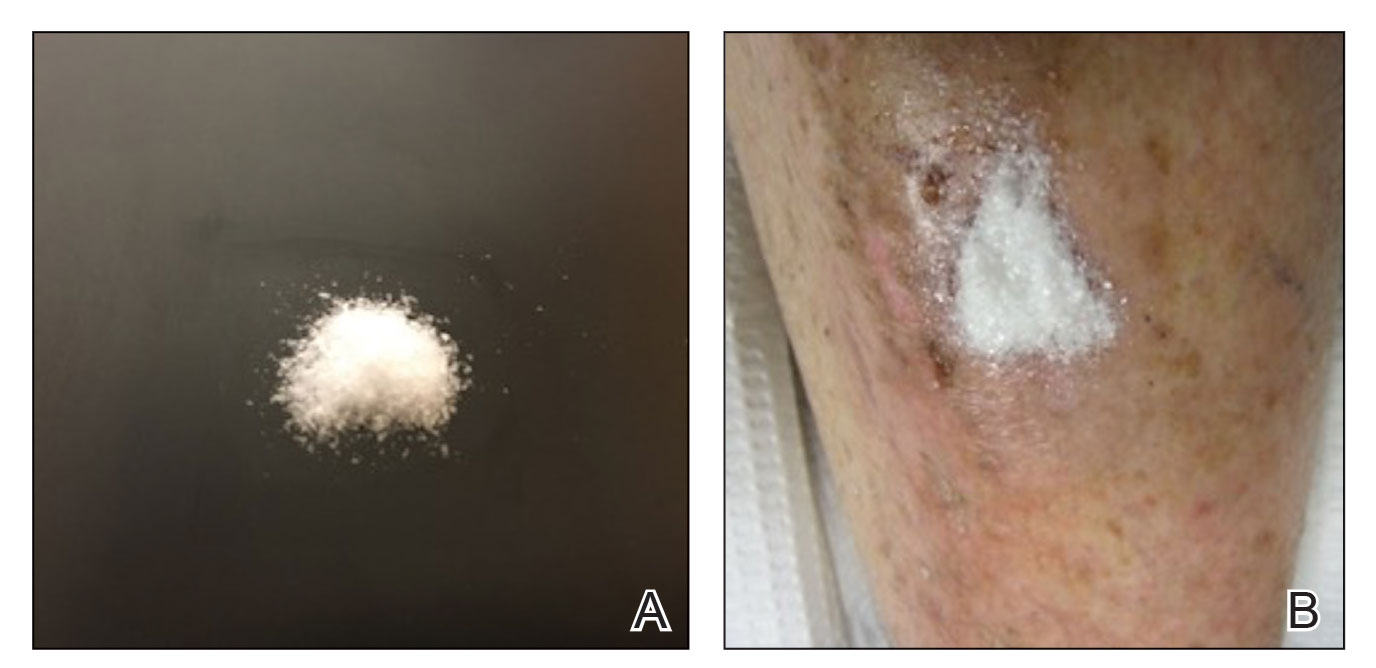
Once the spheres have aggregated, they are designed to orient in a honeycomb formation with 4- to 10-nm openings that serve as capillary channels (Figure 3). This porous architecture of the polymer is essential for adequate moisture management. It allows for vapor transpiration at a rate of 12 L/m2 per day, which ensures the capillary flow from the moist wound surface is evenly distributed through the dressing, contributing to its 68% water content. Notably, this approximately three-fifths water composition is similar to the water makeup of human skin. Optimized moisture management is theorized to enhance epithelial migration, stimulate angiogenesis, retain growth factors, promote autolytic debridement, and maintain ideal voltage and oxygen gradients for wound healing. The risk for infection is not increased by the existence of these pores, as their small size does not allow for bacterial migration.1
This case demonstrates the effectiveness of using a methacrylate polymer powder dressing to promote timely wound healing in a poorly vascularized lower leg surgical wound. The low maintenance, user-friendly dressing was changed at monthly intervals, which spared the patient the inconvenience and pain associated with the repeated application of more conventional primary and secondary dressings. The dressing was well tolerated and resulted in a 99.5% reduction in wound size. Further studies are needed to investigate the utility of this promising technology.
1. Fitzgerald RH, Bharara M, Mills JL, et al. Use of a nanoflex powder dressing for wound management following debridement for necrotising fasciitis in the diabetic foot. Int Wound J. 2009;6:133-139.
To the Editor:
Surgical wounds on the lower leg are challenging to manage because venous stasis, bacterial colonization, and high tension may contribute to protracted healing. Advances in technology led to the development of novel, polymer-based wound-healing modalities that hold promise for the management of these wounds.
A 75-year-old man presented with a well-differentiated squamous cell carcinoma with a 3-mm depth of invasion on the left pretibial region. His comorbidities were notable for hypertension, hypercholesterolemia, varicose veins, myocardial infarction, peripheral vascular disease, and a 32 pack-year cigarette smoking history. Current medications included clopidogrel bisulfate and warfarin sodium to manage a recently placed coronary artery stent.
The tumor was cleared after 2 stages of Mohs micrographic surgery with excision down to tibialis anterior fascia (Figure 1A). The resultant defect measured 43×33 mm in area and 9 mm in depth (wound size, 12,771 mm3). Reconstructive options were discussed, including random-pattern flap repair and skin graft. Given the patient’s risk of bleeding, the decision was made to forego a flap repair. Additionally, the patient was a heavy smoker and could not comply with the wound care and elevation and ambulation restrictions required for optimal skin graft care. Therefore, a decision was made to proceed with secondary intention healing using a methacrylate polymer powder dressing.
After achieving hemostasis, a novel 10-mg sterile, biologically inert methacrylate polymer powder dressing was poured over the wound in a uniform layer to fill and seal the entire wound surface (Figure 1B). Sterile normal saline 0.1 mL was sprayed onto the powder to activate particle aggregation. No secondary dressing was used, and the patient was permitted to get the dressing wet after 48 hours.
The dressing was changed in a similar fashion 4 weeks after application, following gentle debridement with gauze and normal saline. Eight weeks after surgery, the wound exhibited healthy granulation tissue and measured 5×6 mm in area and 2 mm in depth (wound size, 60 mm3), which represented a 99.5% reduction in wound size (Figure 1C). The dressing was not painful, and there were no reported adverse effects. The patient continued to smoke and ambulate fully throughout this period. No antibiotics were used.

Methacrylate polymer powder dressings are a novel and sophisticated dressing modality with great promise for the management of surgical wounds on the lower limb. The dressing is a sterile powder consisting of 84.8% poly-2-hydroxyethylmethacrylate, 14.9% poly-2-hydroxypropylmethacrylate, and 0.3% sodium deoxycholate. These hydrophilic polymers have a covalent methacrylate backbone with a hydroxyl aliphatic side chain. When saline or wound exudate contacts the powder, the spheres hydrate and nonreversibly aggregate to form a moist, flexible dressing that conforms to the topography of the wound and seals it (Figure 2).1

Once the spheres have aggregated, they are designed to orient in a honeycomb formation with 4- to 10-nm openings that serve as capillary channels (Figure 3). This porous architecture of the polymer is essential for adequate moisture management. It allows for vapor transpiration at a rate of 12 L/m2 per day, which ensures the capillary flow from the moist wound surface is evenly distributed through the dressing, contributing to its 68% water content. Notably, this approximately three-fifths water composition is similar to the water makeup of human skin. Optimized moisture management is theorized to enhance epithelial migration, stimulate angiogenesis, retain growth factors, promote autolytic debridement, and maintain ideal voltage and oxygen gradients for wound healing. The risk for infection is not increased by the existence of these pores, as their small size does not allow for bacterial migration.1

This case demonstrates the effectiveness of using a methacrylate polymer powder dressing to promote timely wound healing in a poorly vascularized lower leg surgical wound. The low maintenance, user-friendly dressing was changed at monthly intervals, which spared the patient the inconvenience and pain associated with the repeated application of more conventional primary and secondary dressings. The dressing was well tolerated and resulted in a 99.5% reduction in wound size. Further studies are needed to investigate the utility of this promising technology.
To the Editor:
Surgical wounds on the lower leg are challenging to manage because venous stasis, bacterial colonization, and high tension may contribute to protracted healing. Advances in technology led to the development of novel, polymer-based wound-healing modalities that hold promise for the management of these wounds.
A 75-year-old man presented with a well-differentiated squamous cell carcinoma with a 3-mm depth of invasion on the left pretibial region. His comorbidities were notable for hypertension, hypercholesterolemia, varicose veins, myocardial infarction, peripheral vascular disease, and a 32 pack-year cigarette smoking history. Current medications included clopidogrel bisulfate and warfarin sodium to manage a recently placed coronary artery stent.
The tumor was cleared after 2 stages of Mohs micrographic surgery with excision down to tibialis anterior fascia (Figure 1A). The resultant defect measured 43×33 mm in area and 9 mm in depth (wound size, 12,771 mm3). Reconstructive options were discussed, including random-pattern flap repair and skin graft. Given the patient’s risk of bleeding, the decision was made to forego a flap repair. Additionally, the patient was a heavy smoker and could not comply with the wound care and elevation and ambulation restrictions required for optimal skin graft care. Therefore, a decision was made to proceed with secondary intention healing using a methacrylate polymer powder dressing.
After achieving hemostasis, a novel 10-mg sterile, biologically inert methacrylate polymer powder dressing was poured over the wound in a uniform layer to fill and seal the entire wound surface (Figure 1B). Sterile normal saline 0.1 mL was sprayed onto the powder to activate particle aggregation. No secondary dressing was used, and the patient was permitted to get the dressing wet after 48 hours.
The dressing was changed in a similar fashion 4 weeks after application, following gentle debridement with gauze and normal saline. Eight weeks after surgery, the wound exhibited healthy granulation tissue and measured 5×6 mm in area and 2 mm in depth (wound size, 60 mm3), which represented a 99.5% reduction in wound size (Figure 1C). The dressing was not painful, and there were no reported adverse effects. The patient continued to smoke and ambulate fully throughout this period. No antibiotics were used.

Methacrylate polymer powder dressings are a novel and sophisticated dressing modality with great promise for the management of surgical wounds on the lower limb. The dressing is a sterile powder consisting of 84.8% poly-2-hydroxyethylmethacrylate, 14.9% poly-2-hydroxypropylmethacrylate, and 0.3% sodium deoxycholate. These hydrophilic polymers have a covalent methacrylate backbone with a hydroxyl aliphatic side chain. When saline or wound exudate contacts the powder, the spheres hydrate and nonreversibly aggregate to form a moist, flexible dressing that conforms to the topography of the wound and seals it (Figure 2).1

Once the spheres have aggregated, they are designed to orient in a honeycomb formation with 4- to 10-nm openings that serve as capillary channels (Figure 3). This porous architecture of the polymer is essential for adequate moisture management. It allows for vapor transpiration at a rate of 12 L/m2 per day, which ensures the capillary flow from the moist wound surface is evenly distributed through the dressing, contributing to its 68% water content. Notably, this approximately three-fifths water composition is similar to the water makeup of human skin. Optimized moisture management is theorized to enhance epithelial migration, stimulate angiogenesis, retain growth factors, promote autolytic debridement, and maintain ideal voltage and oxygen gradients for wound healing. The risk for infection is not increased by the existence of these pores, as their small size does not allow for bacterial migration.1

This case demonstrates the effectiveness of using a methacrylate polymer powder dressing to promote timely wound healing in a poorly vascularized lower leg surgical wound. The low maintenance, user-friendly dressing was changed at monthly intervals, which spared the patient the inconvenience and pain associated with the repeated application of more conventional primary and secondary dressings. The dressing was well tolerated and resulted in a 99.5% reduction in wound size. Further studies are needed to investigate the utility of this promising technology.
1. Fitzgerald RH, Bharara M, Mills JL, et al. Use of a nanoflex powder dressing for wound management following debridement for necrotising fasciitis in the diabetic foot. Int Wound J. 2009;6:133-139.
1. Fitzgerald RH, Bharara M, Mills JL, et al. Use of a nanoflex powder dressing for wound management following debridement for necrotising fasciitis in the diabetic foot. Int Wound J. 2009;6:133-139.
PRACTICE POINTS
- Lower leg surgical wounds are difficult to manage, as venous stasis, bacterial colonization, and high tension may contribute to protracted healing.
- A methacrylate polymer powder dressing is user friendly and facilitates granulation and reduction in size of difficult lower leg wounds.
After PCI, 1-month beats 12-month DAPT in high-risk patients
Replacing dual-antiplatelet therapy (DAPT) with clopidogrel alone 1 month after percutaneous intervention (PCI) offers a lower risk of bleeding with comparable protection against cardiovascular events, according to two subgroup analyses of the Japanese STOPDAPT-2 and STOPDAPT-2 ACS trials.
The objective of these two analyses was to evaluate whether there was a benefit-to-risk ratio advantage for those who entered the study with high bleeding risk or who had undergone a complex PCI. Overall, bleeding risk was reduced without a major increase in cardiovascular events regardless of subgroup, according to results published by a multicenter group of Japanese investigators.
In this substudy, like the previously published studies from which the data were drawn, the primary endpoint was a composite of cardiovascular death, myocardial infarction, definite stent thrombosis, stroke, and Thrombolysis In Myocardial Infarction bleeding (major or minor).
The proportion of patients in the 1-month and 12-month DAPT groups reaching this composite endpoint at 1 year was not significantly different among patients stratified by baseline bleeding risk or by PCI complexity, according to a multicenter group of authors led by Takeshi Kimura, MD, department of cardiovascular medicine, Kyoto University.
Shortened DAPT is focus of multiple trials
The new analysis, published in JACC Asia, is a follow-up to the 2019 STOPDAPT-2 trial, published in JAMA, and the 2022 STOPDAPT-2 ACS trial, published in JAMA Cardiology. The first tested 1- versus 12-month DAPT in PCI patients receiving a drug-eluting stent. The second study compared the same strategies in patients undergoing PCI to treat an acute coronary syndrome (ACS).
Both studies were conducted in Japan. DAPT consisted of the P2Y12 receptor inhibitor clopidogrel plus aspirin. The experimental arm received this regimen for 1 month followed by clopidogrel monotherapy. The control arm remained on DAPT for 12 months.
The study is potentially important because it addresses the challenge of finding “the sweet spot of antiplatelet therapy in East Asian patients,” according to the coauthors of an accompanying editorial in the same issue of JACC Asia.
Previous data suggest East Asians have a higher risk of bleeding but lower anti-ischemic benefits from DAPT therapy, explained the coauthors, Antonio Greco, MD and Davide Capodanno, MD, PhD, both from the University of Catania (Italy). They praised the effort to explore this question.
In the STOPDAPT-2 trial, the shortened DAPT regimen was associated with a significantly lower rate of a composite endpoint of cardiovascular and bleeding events than standard DAPT, meeting criteria for superiority as well as noninferiority. In the STOPDAPT-2 ACS trial, shortened DAPT failed to achieve noninferiority to standard DAPT because of an increase in cardiovascular events despite a reduction in bleeding events.
Neither of these studies specifically compared shortened to standard DAPT in patients with high bleeding risk or in patients who underwent complex PCI, which are among the most common patient groups in which to consider a modified DAPT regimen. To do this, two new substudies were performed with the combined data from 5,997 patients in the two STOPDAPT-2 trials.
Two candidate groups for shortened DAPT evaluated
In the first substudy, the 1,893 patients who met criteria for high bleeding risk were compared with the 4,104 who did not. In those with a high risk of bleeding, the proportion reaching a primary endpoint at 1 year was lower, but not significantly different, for those on 1-month versus standard DAPT (5.01% vs. 5.14%). This was also true in those without an elevated bleeding risk (1.90% vs. 2.02%).
In the second substudy, 999 patients who had a complex PCI, defined by such characteristics as implantation of at least three stents or chronic total occlusion in the target lesions, were compared with the 4,998 who did not. Again, the primary endpoint was lower in both those who had a complex PCI (3.15% vs. 4.07%) and those who did not (2.78% vs. 2.82%).
Not surprisingly, patients with a high bleeding risk benefited from a substantially lower risk of bleeding events on the 1-month DAPT regimen (0.66% vs. 2.27%). The cost was a higher risk of cardiovascular events (4.35% vs. 3.52%), but this difference did not reach significance. Those without an elevated bleeding risk also had a lower risk of bleeding events (0.43% vs. 0.85%) but a higher risk of cardiovascular events (1.56% vs. 1.22%). Again, differences were nonsignificant. In the substudy evaluating DAPT duration in relation to complex PCI, the rate of cardiovascular events at 1 year in those treated with short versus 12-month DAPT was nearly identical (2.53% vs. 2.52%). In the non–complex PCI patients, event rates were nonsignificantly greater on the shortened DAPT regimen (2.38% vs. 1.86%), but the bleeding rate was lower on shortened DAPT whether PCI had been complex (0.63% vs. 1.75%) or not (0.48% vs. 1.22%).
In the absence of any major signal that complex PCI benefited from longer duration DAPT, “complex PCI might not be an appropriate determinant for DAPT durations,” according to Dr. Kimura and coinvestigators.
Study data might not be generalizable
Dr. Greco and Dr. Capodanno pointed out that there are differences between patients and PCI practices in Japan relative to other areas of the world, limiting the generalizability of these findings even if the question is relevant.
“This is an approach that might be suggested for patients at high bleeding risk who have the characteristics of the patients enrolled in the STOPDAPT-2 trials,” Dr. Capodanno said in an interview. In his own PCI practice treating ACS patients, “I would not feel safe enough with clopidogrel monotherapy after only 1 month.”
He considers the ACS population to have a particularly “delicate bleeding-ischemia trade-off,” which is why he thinks this question is relevant and needs to be explored further in additional populations. However, he might design trials differently in his own practice setting. For example, he would at the very least be interested in testing a more potent P2Y12 inhibitor such as ticagrelor when considering a single antiplatelet agent after a limited course of DAPT.
One message from this study is that “bleeding risk trumps PCI complexity,” according to Deepak L. Bhatt, MD, who recently assumed the position of director of Mount Sinai Heart in New York. He liked the approach the investigators took to address a complex and relevant clinical issue, but he also expressed reservations about the clinical applicability of this subgroup analysis.
“We really need more data before uniformly shortening DAPT duration in all patients,” Dr. Bhatt said in an interview. He considers this a hot clinical issue that is likely to generate more trials. He hopes these will provide more definitive evidence of when and how DAPT duration can be reduced. Overall, he anticipates progress toward tailoring therapy in specific populations in order to achieve the best risk-to-benefit balance.
Dr. Kimura has financial relationships with Boston Scientific, Daiichi Sankyo, Sanofi, Terumo, and Abbott Medical Japan, which provided funding for the STOPDAPT-2 and STOPDAPT-2 ACS trials. Dr. Capodanno reported financial relationships with Amgen, Arena, Chiesi, Daiichi Sakyo, Sanofi Aventis, and Terumo. Dr. Bhatt reported financial relationships with more than 20 pharmaceutical companies, including Abbott Medical.
Replacing dual-antiplatelet therapy (DAPT) with clopidogrel alone 1 month after percutaneous intervention (PCI) offers a lower risk of bleeding with comparable protection against cardiovascular events, according to two subgroup analyses of the Japanese STOPDAPT-2 and STOPDAPT-2 ACS trials.
The objective of these two analyses was to evaluate whether there was a benefit-to-risk ratio advantage for those who entered the study with high bleeding risk or who had undergone a complex PCI. Overall, bleeding risk was reduced without a major increase in cardiovascular events regardless of subgroup, according to results published by a multicenter group of Japanese investigators.
In this substudy, like the previously published studies from which the data were drawn, the primary endpoint was a composite of cardiovascular death, myocardial infarction, definite stent thrombosis, stroke, and Thrombolysis In Myocardial Infarction bleeding (major or minor).
The proportion of patients in the 1-month and 12-month DAPT groups reaching this composite endpoint at 1 year was not significantly different among patients stratified by baseline bleeding risk or by PCI complexity, according to a multicenter group of authors led by Takeshi Kimura, MD, department of cardiovascular medicine, Kyoto University.
Shortened DAPT is focus of multiple trials
The new analysis, published in JACC Asia, is a follow-up to the 2019 STOPDAPT-2 trial, published in JAMA, and the 2022 STOPDAPT-2 ACS trial, published in JAMA Cardiology. The first tested 1- versus 12-month DAPT in PCI patients receiving a drug-eluting stent. The second study compared the same strategies in patients undergoing PCI to treat an acute coronary syndrome (ACS).
Both studies were conducted in Japan. DAPT consisted of the P2Y12 receptor inhibitor clopidogrel plus aspirin. The experimental arm received this regimen for 1 month followed by clopidogrel monotherapy. The control arm remained on DAPT for 12 months.
The study is potentially important because it addresses the challenge of finding “the sweet spot of antiplatelet therapy in East Asian patients,” according to the coauthors of an accompanying editorial in the same issue of JACC Asia.
Previous data suggest East Asians have a higher risk of bleeding but lower anti-ischemic benefits from DAPT therapy, explained the coauthors, Antonio Greco, MD and Davide Capodanno, MD, PhD, both from the University of Catania (Italy). They praised the effort to explore this question.
In the STOPDAPT-2 trial, the shortened DAPT regimen was associated with a significantly lower rate of a composite endpoint of cardiovascular and bleeding events than standard DAPT, meeting criteria for superiority as well as noninferiority. In the STOPDAPT-2 ACS trial, shortened DAPT failed to achieve noninferiority to standard DAPT because of an increase in cardiovascular events despite a reduction in bleeding events.
Neither of these studies specifically compared shortened to standard DAPT in patients with high bleeding risk or in patients who underwent complex PCI, which are among the most common patient groups in which to consider a modified DAPT regimen. To do this, two new substudies were performed with the combined data from 5,997 patients in the two STOPDAPT-2 trials.
Two candidate groups for shortened DAPT evaluated
In the first substudy, the 1,893 patients who met criteria for high bleeding risk were compared with the 4,104 who did not. In those with a high risk of bleeding, the proportion reaching a primary endpoint at 1 year was lower, but not significantly different, for those on 1-month versus standard DAPT (5.01% vs. 5.14%). This was also true in those without an elevated bleeding risk (1.90% vs. 2.02%).
In the second substudy, 999 patients who had a complex PCI, defined by such characteristics as implantation of at least three stents or chronic total occlusion in the target lesions, were compared with the 4,998 who did not. Again, the primary endpoint was lower in both those who had a complex PCI (3.15% vs. 4.07%) and those who did not (2.78% vs. 2.82%).
Not surprisingly, patients with a high bleeding risk benefited from a substantially lower risk of bleeding events on the 1-month DAPT regimen (0.66% vs. 2.27%). The cost was a higher risk of cardiovascular events (4.35% vs. 3.52%), but this difference did not reach significance. Those without an elevated bleeding risk also had a lower risk of bleeding events (0.43% vs. 0.85%) but a higher risk of cardiovascular events (1.56% vs. 1.22%). Again, differences were nonsignificant. In the substudy evaluating DAPT duration in relation to complex PCI, the rate of cardiovascular events at 1 year in those treated with short versus 12-month DAPT was nearly identical (2.53% vs. 2.52%). In the non–complex PCI patients, event rates were nonsignificantly greater on the shortened DAPT regimen (2.38% vs. 1.86%), but the bleeding rate was lower on shortened DAPT whether PCI had been complex (0.63% vs. 1.75%) or not (0.48% vs. 1.22%).
In the absence of any major signal that complex PCI benefited from longer duration DAPT, “complex PCI might not be an appropriate determinant for DAPT durations,” according to Dr. Kimura and coinvestigators.
Study data might not be generalizable
Dr. Greco and Dr. Capodanno pointed out that there are differences between patients and PCI practices in Japan relative to other areas of the world, limiting the generalizability of these findings even if the question is relevant.
“This is an approach that might be suggested for patients at high bleeding risk who have the characteristics of the patients enrolled in the STOPDAPT-2 trials,” Dr. Capodanno said in an interview. In his own PCI practice treating ACS patients, “I would not feel safe enough with clopidogrel monotherapy after only 1 month.”
He considers the ACS population to have a particularly “delicate bleeding-ischemia trade-off,” which is why he thinks this question is relevant and needs to be explored further in additional populations. However, he might design trials differently in his own practice setting. For example, he would at the very least be interested in testing a more potent P2Y12 inhibitor such as ticagrelor when considering a single antiplatelet agent after a limited course of DAPT.
One message from this study is that “bleeding risk trumps PCI complexity,” according to Deepak L. Bhatt, MD, who recently assumed the position of director of Mount Sinai Heart in New York. He liked the approach the investigators took to address a complex and relevant clinical issue, but he also expressed reservations about the clinical applicability of this subgroup analysis.
“We really need more data before uniformly shortening DAPT duration in all patients,” Dr. Bhatt said in an interview. He considers this a hot clinical issue that is likely to generate more trials. He hopes these will provide more definitive evidence of when and how DAPT duration can be reduced. Overall, he anticipates progress toward tailoring therapy in specific populations in order to achieve the best risk-to-benefit balance.
Dr. Kimura has financial relationships with Boston Scientific, Daiichi Sankyo, Sanofi, Terumo, and Abbott Medical Japan, which provided funding for the STOPDAPT-2 and STOPDAPT-2 ACS trials. Dr. Capodanno reported financial relationships with Amgen, Arena, Chiesi, Daiichi Sakyo, Sanofi Aventis, and Terumo. Dr. Bhatt reported financial relationships with more than 20 pharmaceutical companies, including Abbott Medical.
Replacing dual-antiplatelet therapy (DAPT) with clopidogrel alone 1 month after percutaneous intervention (PCI) offers a lower risk of bleeding with comparable protection against cardiovascular events, according to two subgroup analyses of the Japanese STOPDAPT-2 and STOPDAPT-2 ACS trials.
The objective of these two analyses was to evaluate whether there was a benefit-to-risk ratio advantage for those who entered the study with high bleeding risk or who had undergone a complex PCI. Overall, bleeding risk was reduced without a major increase in cardiovascular events regardless of subgroup, according to results published by a multicenter group of Japanese investigators.
In this substudy, like the previously published studies from which the data were drawn, the primary endpoint was a composite of cardiovascular death, myocardial infarction, definite stent thrombosis, stroke, and Thrombolysis In Myocardial Infarction bleeding (major or minor).
The proportion of patients in the 1-month and 12-month DAPT groups reaching this composite endpoint at 1 year was not significantly different among patients stratified by baseline bleeding risk or by PCI complexity, according to a multicenter group of authors led by Takeshi Kimura, MD, department of cardiovascular medicine, Kyoto University.
Shortened DAPT is focus of multiple trials
The new analysis, published in JACC Asia, is a follow-up to the 2019 STOPDAPT-2 trial, published in JAMA, and the 2022 STOPDAPT-2 ACS trial, published in JAMA Cardiology. The first tested 1- versus 12-month DAPT in PCI patients receiving a drug-eluting stent. The second study compared the same strategies in patients undergoing PCI to treat an acute coronary syndrome (ACS).
Both studies were conducted in Japan. DAPT consisted of the P2Y12 receptor inhibitor clopidogrel plus aspirin. The experimental arm received this regimen for 1 month followed by clopidogrel monotherapy. The control arm remained on DAPT for 12 months.
The study is potentially important because it addresses the challenge of finding “the sweet spot of antiplatelet therapy in East Asian patients,” according to the coauthors of an accompanying editorial in the same issue of JACC Asia.
Previous data suggest East Asians have a higher risk of bleeding but lower anti-ischemic benefits from DAPT therapy, explained the coauthors, Antonio Greco, MD and Davide Capodanno, MD, PhD, both from the University of Catania (Italy). They praised the effort to explore this question.
In the STOPDAPT-2 trial, the shortened DAPT regimen was associated with a significantly lower rate of a composite endpoint of cardiovascular and bleeding events than standard DAPT, meeting criteria for superiority as well as noninferiority. In the STOPDAPT-2 ACS trial, shortened DAPT failed to achieve noninferiority to standard DAPT because of an increase in cardiovascular events despite a reduction in bleeding events.
Neither of these studies specifically compared shortened to standard DAPT in patients with high bleeding risk or in patients who underwent complex PCI, which are among the most common patient groups in which to consider a modified DAPT regimen. To do this, two new substudies were performed with the combined data from 5,997 patients in the two STOPDAPT-2 trials.
Two candidate groups for shortened DAPT evaluated
In the first substudy, the 1,893 patients who met criteria for high bleeding risk were compared with the 4,104 who did not. In those with a high risk of bleeding, the proportion reaching a primary endpoint at 1 year was lower, but not significantly different, for those on 1-month versus standard DAPT (5.01% vs. 5.14%). This was also true in those without an elevated bleeding risk (1.90% vs. 2.02%).
In the second substudy, 999 patients who had a complex PCI, defined by such characteristics as implantation of at least three stents or chronic total occlusion in the target lesions, were compared with the 4,998 who did not. Again, the primary endpoint was lower in both those who had a complex PCI (3.15% vs. 4.07%) and those who did not (2.78% vs. 2.82%).
Not surprisingly, patients with a high bleeding risk benefited from a substantially lower risk of bleeding events on the 1-month DAPT regimen (0.66% vs. 2.27%). The cost was a higher risk of cardiovascular events (4.35% vs. 3.52%), but this difference did not reach significance. Those without an elevated bleeding risk also had a lower risk of bleeding events (0.43% vs. 0.85%) but a higher risk of cardiovascular events (1.56% vs. 1.22%). Again, differences were nonsignificant. In the substudy evaluating DAPT duration in relation to complex PCI, the rate of cardiovascular events at 1 year in those treated with short versus 12-month DAPT was nearly identical (2.53% vs. 2.52%). In the non–complex PCI patients, event rates were nonsignificantly greater on the shortened DAPT regimen (2.38% vs. 1.86%), but the bleeding rate was lower on shortened DAPT whether PCI had been complex (0.63% vs. 1.75%) or not (0.48% vs. 1.22%).
In the absence of any major signal that complex PCI benefited from longer duration DAPT, “complex PCI might not be an appropriate determinant for DAPT durations,” according to Dr. Kimura and coinvestigators.
Study data might not be generalizable
Dr. Greco and Dr. Capodanno pointed out that there are differences between patients and PCI practices in Japan relative to other areas of the world, limiting the generalizability of these findings even if the question is relevant.
“This is an approach that might be suggested for patients at high bleeding risk who have the characteristics of the patients enrolled in the STOPDAPT-2 trials,” Dr. Capodanno said in an interview. In his own PCI practice treating ACS patients, “I would not feel safe enough with clopidogrel monotherapy after only 1 month.”
He considers the ACS population to have a particularly “delicate bleeding-ischemia trade-off,” which is why he thinks this question is relevant and needs to be explored further in additional populations. However, he might design trials differently in his own practice setting. For example, he would at the very least be interested in testing a more potent P2Y12 inhibitor such as ticagrelor when considering a single antiplatelet agent after a limited course of DAPT.
One message from this study is that “bleeding risk trumps PCI complexity,” according to Deepak L. Bhatt, MD, who recently assumed the position of director of Mount Sinai Heart in New York. He liked the approach the investigators took to address a complex and relevant clinical issue, but he also expressed reservations about the clinical applicability of this subgroup analysis.
“We really need more data before uniformly shortening DAPT duration in all patients,” Dr. Bhatt said in an interview. He considers this a hot clinical issue that is likely to generate more trials. He hopes these will provide more definitive evidence of when and how DAPT duration can be reduced. Overall, he anticipates progress toward tailoring therapy in specific populations in order to achieve the best risk-to-benefit balance.
Dr. Kimura has financial relationships with Boston Scientific, Daiichi Sankyo, Sanofi, Terumo, and Abbott Medical Japan, which provided funding for the STOPDAPT-2 and STOPDAPT-2 ACS trials. Dr. Capodanno reported financial relationships with Amgen, Arena, Chiesi, Daiichi Sakyo, Sanofi Aventis, and Terumo. Dr. Bhatt reported financial relationships with more than 20 pharmaceutical companies, including Abbott Medical.
FROM JACC ASIA
Can 6 minutes of intense cycling put the brakes on Alzheimer’s?
new research suggests.
In a small study of healthy adults, 6 minutes of high-intensity cycling increased circulating levels of brain-derived neurotrophic factor (BDNF) to a significantly greater extent than prolonged light cycling or fasting.
However, the data do not suggest that 6 minutes of high-intensity exercise “wards off dementia,” cautioned lead investigator Travis Gibbons, MSc, PhD candidate in environmental physiology at the University of Otago (New Zealand), Dunedin, and now postdoctoral fellow at the University of British Columbia – Okanagan, Kelowna.
“Like all science, this is just a small piece that supports a potential mechanistic role for how exercise might improve brain health,” Dr. Gibbons told this news organization.
The findings were published online in the Journal of Physiology.
Targeting BDNF
Both intermittent fasting and exercise have previously been shown to have potent neuroprotective effects; and an acute upregulation of BDNF appears to be a common mechanistic link.
To tease apart the influence of fasting and exercise on BDNF production, Dr. Gibbons and colleagues studied 12 aerobically fit, healthy men (n = 6) and women (n = 6) aged 20-40 years.
In a study that employed a repeated-measures crossover design, they assessed circulating BDNF levels after a 20-hour fast, prolonged (90-min) light cycling, short (6-min) high-intensity cycling, and combined fasting and exercise.
Six minutes of high-intensity exercise appeared to be the most efficient way to increase BDNF.
Fasting for 20 hours led to a ninefold increase in ketone body delivery to the brain but had no effect on any metric of BDNF in peripheral circulation at rest or during exercise.
Six minutes of high-intensity exercise increased every metric of circulating BDNF four to five times more than prolonged low-intensity exercise.
In addition, the increase in plasma-derived BDNF correlated with a sixfold increase in circulating lactate irrespective of feeding or fasting state.
Lactate delivery?
“My leading theory is that, during and following intense exercise, lactate produced by muscles is delivered and consumed by the brain,” Dr. Gibbons noted.
“It takes high-intensity exercise to provoke this ‘cerebral substrate switch’ from glucose to lactate. Critically, this cerebral substrate switch has been shown to contribute to the early processes that upregulate BDNF production in the brain,” he said.
However, “Whether this translates to ‘warding off dementia’ is not clear,” Dr. Gibbons added.
The study also suggests that increases in plasma volume and platelet concentration appear to play a role in concentrating BDNF in the circulation during exercise.
The investigators note that BDNF and other neurotrophic-based pharmaceutical therapies have shown “great promise” in slowing and even arresting neurodegenerative processes in animals, but attempts to harness the protective power of BDNF in human neurodegeneration have thus far failed.
“Whether episodically upregulating BDNF production with intense exercise is an effective strategy to curb age-related cognitive decline in humans is unknown, but animal models indicate that it is and that BDNF plays a primary role,” the researchers write.
Funding for the study was provided by the Healthcare Otago Charitable Trust. The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
In a small study of healthy adults, 6 minutes of high-intensity cycling increased circulating levels of brain-derived neurotrophic factor (BDNF) to a significantly greater extent than prolonged light cycling or fasting.
However, the data do not suggest that 6 minutes of high-intensity exercise “wards off dementia,” cautioned lead investigator Travis Gibbons, MSc, PhD candidate in environmental physiology at the University of Otago (New Zealand), Dunedin, and now postdoctoral fellow at the University of British Columbia – Okanagan, Kelowna.
“Like all science, this is just a small piece that supports a potential mechanistic role for how exercise might improve brain health,” Dr. Gibbons told this news organization.
The findings were published online in the Journal of Physiology.
Targeting BDNF
Both intermittent fasting and exercise have previously been shown to have potent neuroprotective effects; and an acute upregulation of BDNF appears to be a common mechanistic link.
To tease apart the influence of fasting and exercise on BDNF production, Dr. Gibbons and colleagues studied 12 aerobically fit, healthy men (n = 6) and women (n = 6) aged 20-40 years.
In a study that employed a repeated-measures crossover design, they assessed circulating BDNF levels after a 20-hour fast, prolonged (90-min) light cycling, short (6-min) high-intensity cycling, and combined fasting and exercise.
Six minutes of high-intensity exercise appeared to be the most efficient way to increase BDNF.
Fasting for 20 hours led to a ninefold increase in ketone body delivery to the brain but had no effect on any metric of BDNF in peripheral circulation at rest or during exercise.
Six minutes of high-intensity exercise increased every metric of circulating BDNF four to five times more than prolonged low-intensity exercise.
In addition, the increase in plasma-derived BDNF correlated with a sixfold increase in circulating lactate irrespective of feeding or fasting state.
Lactate delivery?
“My leading theory is that, during and following intense exercise, lactate produced by muscles is delivered and consumed by the brain,” Dr. Gibbons noted.
“It takes high-intensity exercise to provoke this ‘cerebral substrate switch’ from glucose to lactate. Critically, this cerebral substrate switch has been shown to contribute to the early processes that upregulate BDNF production in the brain,” he said.
However, “Whether this translates to ‘warding off dementia’ is not clear,” Dr. Gibbons added.
The study also suggests that increases in plasma volume and platelet concentration appear to play a role in concentrating BDNF in the circulation during exercise.
The investigators note that BDNF and other neurotrophic-based pharmaceutical therapies have shown “great promise” in slowing and even arresting neurodegenerative processes in animals, but attempts to harness the protective power of BDNF in human neurodegeneration have thus far failed.
“Whether episodically upregulating BDNF production with intense exercise is an effective strategy to curb age-related cognitive decline in humans is unknown, but animal models indicate that it is and that BDNF plays a primary role,” the researchers write.
Funding for the study was provided by the Healthcare Otago Charitable Trust. The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
In a small study of healthy adults, 6 minutes of high-intensity cycling increased circulating levels of brain-derived neurotrophic factor (BDNF) to a significantly greater extent than prolonged light cycling or fasting.
However, the data do not suggest that 6 minutes of high-intensity exercise “wards off dementia,” cautioned lead investigator Travis Gibbons, MSc, PhD candidate in environmental physiology at the University of Otago (New Zealand), Dunedin, and now postdoctoral fellow at the University of British Columbia – Okanagan, Kelowna.
“Like all science, this is just a small piece that supports a potential mechanistic role for how exercise might improve brain health,” Dr. Gibbons told this news organization.
The findings were published online in the Journal of Physiology.
Targeting BDNF
Both intermittent fasting and exercise have previously been shown to have potent neuroprotective effects; and an acute upregulation of BDNF appears to be a common mechanistic link.
To tease apart the influence of fasting and exercise on BDNF production, Dr. Gibbons and colleagues studied 12 aerobically fit, healthy men (n = 6) and women (n = 6) aged 20-40 years.
In a study that employed a repeated-measures crossover design, they assessed circulating BDNF levels after a 20-hour fast, prolonged (90-min) light cycling, short (6-min) high-intensity cycling, and combined fasting and exercise.
Six minutes of high-intensity exercise appeared to be the most efficient way to increase BDNF.
Fasting for 20 hours led to a ninefold increase in ketone body delivery to the brain but had no effect on any metric of BDNF in peripheral circulation at rest or during exercise.
Six minutes of high-intensity exercise increased every metric of circulating BDNF four to five times more than prolonged low-intensity exercise.
In addition, the increase in plasma-derived BDNF correlated with a sixfold increase in circulating lactate irrespective of feeding or fasting state.
Lactate delivery?
“My leading theory is that, during and following intense exercise, lactate produced by muscles is delivered and consumed by the brain,” Dr. Gibbons noted.
“It takes high-intensity exercise to provoke this ‘cerebral substrate switch’ from glucose to lactate. Critically, this cerebral substrate switch has been shown to contribute to the early processes that upregulate BDNF production in the brain,” he said.
However, “Whether this translates to ‘warding off dementia’ is not clear,” Dr. Gibbons added.
The study also suggests that increases in plasma volume and platelet concentration appear to play a role in concentrating BDNF in the circulation during exercise.
The investigators note that BDNF and other neurotrophic-based pharmaceutical therapies have shown “great promise” in slowing and even arresting neurodegenerative processes in animals, but attempts to harness the protective power of BDNF in human neurodegeneration have thus far failed.
“Whether episodically upregulating BDNF production with intense exercise is an effective strategy to curb age-related cognitive decline in humans is unknown, but animal models indicate that it is and that BDNF plays a primary role,” the researchers write.
Funding for the study was provided by the Healthcare Otago Charitable Trust. The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF PHYSIOLOGY
Singer is paralyzed after delay in care; hospital must pay
Delay in treatment will cost hospital millions
according to a report on WFAA.com, among other news sites.
On March 21, 2019, Judy “Jessie” Adams, then part of a singing-songwriting duo with her husband, Richard, went to Premier Interventional Pain Management, in Flower Mound, Tex., prior to the couple’s drive to Ohio for a funeral. At Premier, Jesse received an epidural steroid injection (ESI) that she hoped would ease her back pain during the long drive.
Instead, the injection ended up increasing her pain.
“He [the pain physician] gave me the shot, but I couldn’t feel my legs. They were tingling, but I couldn’t feel them,” Mrs. Adams explained. “The pain was so bad in my back.” In their suit, Adams and her husband alleged that the doctor had probably “nicked a blood vessel during the ESI procedure, causing Jessie to hemorrhage.” (The couple’s suit against the doctor was settled prior to trial.)
Mrs. Adams remained under observation at the pain facility for about 1½ hours, at which point she was taken by ambulance to nearby Texas Health Presbyterian Hospital. There, in the emergency department, staff ordered a “STAT MRI” in preparation for an emergency laminectomy.
For reasons that remain murky, the MRI wasn’t performed for 1 hour and 37 minutes. The emergency laminectomy itself wasn’t started until more than 5 hours after Adams had been admitted to the ED. This was a direct violation of hospital protocol, which required that emergency surgeries be performed within 1 hour of admittance in the first available surgical suite. (At trial, Mrs. Adams’s attorneys from Lyons & Simmons offered evidence that a suite became available 49 minutes after Adams had arrived at the ED.)
During the wait, Mrs. Adams continued to experience excruciating pain. “I kept screaming: ‘Help me,’ ” she recalled. At trial, her attorneys argued that the hospital’s delay in addressing her spinal emergency led directly to her current paralysis, which keeps her confined to a wheelchair and renders her incontinent.
The hospital disagreed. In court, it maintained that Mrs. Adams was already paralyzed when she arrived at the ED and that there was no delay in care.
The jury saw things differently, however. Siding with the plaintiffs, it awarded Mrs. Adams and her husband $10.1 million, including $500,000 for Mr. Adams’s loss of future earnings and $1 million for his “loss of consortium” with his wife.
Their music career now effectively over, Mr. Adams spends most of his time taking care of Mrs. Adams.
“Music was our lifeblood for so many years, and he can’t do it anymore,” Mrs. Adams said. “He goes upstairs to play his guitar and write, and suddenly I need him to come and cath me. I just feel like I’m going to wake up from this bad dream, but it’s the same routine.”
Two doctors are absolved in woman’s sudden death
In a 3-2 decision in December 2022, the Pennsylvania Supreme Court ruled that the state’s 2-year statute of limitations in wrongful-death cases applies even in cases in which plaintiffs fail to identify the cause of death in a timely manner, as a report in the Claims Journal indicates.
The decision stems from a lawsuit filed by Linda Reibenstein on behalf of her mother, Mary Ann Whitman, who died in late April 2010 from a ruptured aortic aneurysm.
On April 12, 2010, Ms. Whitman visited Patrick D. Conaboy, MD, a Scranton family physician, complaining of a persistent cough, fever, and lower-back pain. Following an initial examination, Dr. Conaboy ordered an aortic duplex ultrasound scan and a CT scan of the patient’s abdomen.
The ultrasound was performed by radiologist Charles Barax, MD, who reviewed both scans. He identified a “poorly visualized aortic aneurysm.” At this point, Dr. Conaboy referred Ms. Whitman to a vascular surgeon. But before this visit could take place, Whitman’s aneurysm ruptured, killing her. This was listed as the medical cause of death on the patient’s death certificate.
In April 2011, Ms. Reibenstein filed a claim against Dr. Barax, alleging that he had failed to gauge the severity of her mother’s condition. Ms. Reibenstein’s attorney wasn’t able to question Dr. Barax on the record until well after the state’s 2-year statute of limitations had elapsed. When he did testify, Dr. Barax explained that the scans’ image quality prevented him from determining whether Whitman’s aneurysm was rupturing or simply bleeding. Despite this, he insisted that he had warned Dr. Conaboy of the potential for Ms. Whitman’s aneurysm to rupture.
In March 2016, nearly 6 years after her mother’s death, Ms. Reibenstein filed a new lawsuit, this one against Dr. Conaboy, whom she alleged had failed to properly treat her mother’s condition. Dr. Conaboy, in turn, asked the court for summary judgment – that is, a judgment in his favor without a full trial – arguing that the state’s window for filing a wrongful-death claim had long since closed. For their part, Ms. Reibenstein and her attorney argued that the state’s 2-year statute of limitations didn’t start until the plaintiff had discovered the cause of her mother’s death.
Initially refusing to dismiss the case, a lower court reconsidered Dr. Conaboy’s motion for summary judgment and ruled that Ms. Reibenstein had failed to present any evidence of “affirmative misrepresentation or fraudulent concealment.” In other words, in the absence of any willful attempt on the part of the defendant to hide the legal cause of death, which includes “acts, omissions, or events having some causative connection with the death,” the statute of limitations remained in effect, and the defendant’s motion was thereby granted.
Continuing the legal seesaw, a state appeals court reversed the lower-court ruling. Noting that the Pennsylvania malpractice statute was ambiguous, the court argued that it should be interpreted in a way that protects plaintiffs who seek “fair compensation” but encounter willfully erected obstacles in pursuit of their claim.
Dr. Conaboy then took his case to the state’s highest court. In its majority decision, the Pennsylvania Supreme Court staked out a narrow definition of cause of death – one based on the death certificate – and ruled that only willful fraud in that document would constitute the necessary condition for halting the claim’s clock. Furthermore, the high court said, when lawmakers adopted the Medical Care Availability and Reduction of Error Act in 2002, they did so with no guarantee “that all of the information necessary to sustain a claim will be gathered in the limitations period.”
Similarly, the court ruled, “at some point the clock must run out, lest health care providers remain subject to liability exposure indefinitely, with the prospect of a trial marred by the death or diminished memory of material witnesses or the loss of critical evidence.”
A version of this article first appeared on Medscape.com.
Delay in treatment will cost hospital millions
according to a report on WFAA.com, among other news sites.
On March 21, 2019, Judy “Jessie” Adams, then part of a singing-songwriting duo with her husband, Richard, went to Premier Interventional Pain Management, in Flower Mound, Tex., prior to the couple’s drive to Ohio for a funeral. At Premier, Jesse received an epidural steroid injection (ESI) that she hoped would ease her back pain during the long drive.
Instead, the injection ended up increasing her pain.
“He [the pain physician] gave me the shot, but I couldn’t feel my legs. They were tingling, but I couldn’t feel them,” Mrs. Adams explained. “The pain was so bad in my back.” In their suit, Adams and her husband alleged that the doctor had probably “nicked a blood vessel during the ESI procedure, causing Jessie to hemorrhage.” (The couple’s suit against the doctor was settled prior to trial.)
Mrs. Adams remained under observation at the pain facility for about 1½ hours, at which point she was taken by ambulance to nearby Texas Health Presbyterian Hospital. There, in the emergency department, staff ordered a “STAT MRI” in preparation for an emergency laminectomy.
For reasons that remain murky, the MRI wasn’t performed for 1 hour and 37 minutes. The emergency laminectomy itself wasn’t started until more than 5 hours after Adams had been admitted to the ED. This was a direct violation of hospital protocol, which required that emergency surgeries be performed within 1 hour of admittance in the first available surgical suite. (At trial, Mrs. Adams’s attorneys from Lyons & Simmons offered evidence that a suite became available 49 minutes after Adams had arrived at the ED.)
During the wait, Mrs. Adams continued to experience excruciating pain. “I kept screaming: ‘Help me,’ ” she recalled. At trial, her attorneys argued that the hospital’s delay in addressing her spinal emergency led directly to her current paralysis, which keeps her confined to a wheelchair and renders her incontinent.
The hospital disagreed. In court, it maintained that Mrs. Adams was already paralyzed when she arrived at the ED and that there was no delay in care.
The jury saw things differently, however. Siding with the plaintiffs, it awarded Mrs. Adams and her husband $10.1 million, including $500,000 for Mr. Adams’s loss of future earnings and $1 million for his “loss of consortium” with his wife.
Their music career now effectively over, Mr. Adams spends most of his time taking care of Mrs. Adams.
“Music was our lifeblood for so many years, and he can’t do it anymore,” Mrs. Adams said. “He goes upstairs to play his guitar and write, and suddenly I need him to come and cath me. I just feel like I’m going to wake up from this bad dream, but it’s the same routine.”
Two doctors are absolved in woman’s sudden death
In a 3-2 decision in December 2022, the Pennsylvania Supreme Court ruled that the state’s 2-year statute of limitations in wrongful-death cases applies even in cases in which plaintiffs fail to identify the cause of death in a timely manner, as a report in the Claims Journal indicates.
The decision stems from a lawsuit filed by Linda Reibenstein on behalf of her mother, Mary Ann Whitman, who died in late April 2010 from a ruptured aortic aneurysm.
On April 12, 2010, Ms. Whitman visited Patrick D. Conaboy, MD, a Scranton family physician, complaining of a persistent cough, fever, and lower-back pain. Following an initial examination, Dr. Conaboy ordered an aortic duplex ultrasound scan and a CT scan of the patient’s abdomen.
The ultrasound was performed by radiologist Charles Barax, MD, who reviewed both scans. He identified a “poorly visualized aortic aneurysm.” At this point, Dr. Conaboy referred Ms. Whitman to a vascular surgeon. But before this visit could take place, Whitman’s aneurysm ruptured, killing her. This was listed as the medical cause of death on the patient’s death certificate.
In April 2011, Ms. Reibenstein filed a claim against Dr. Barax, alleging that he had failed to gauge the severity of her mother’s condition. Ms. Reibenstein’s attorney wasn’t able to question Dr. Barax on the record until well after the state’s 2-year statute of limitations had elapsed. When he did testify, Dr. Barax explained that the scans’ image quality prevented him from determining whether Whitman’s aneurysm was rupturing or simply bleeding. Despite this, he insisted that he had warned Dr. Conaboy of the potential for Ms. Whitman’s aneurysm to rupture.
In March 2016, nearly 6 years after her mother’s death, Ms. Reibenstein filed a new lawsuit, this one against Dr. Conaboy, whom she alleged had failed to properly treat her mother’s condition. Dr. Conaboy, in turn, asked the court for summary judgment – that is, a judgment in his favor without a full trial – arguing that the state’s window for filing a wrongful-death claim had long since closed. For their part, Ms. Reibenstein and her attorney argued that the state’s 2-year statute of limitations didn’t start until the plaintiff had discovered the cause of her mother’s death.
Initially refusing to dismiss the case, a lower court reconsidered Dr. Conaboy’s motion for summary judgment and ruled that Ms. Reibenstein had failed to present any evidence of “affirmative misrepresentation or fraudulent concealment.” In other words, in the absence of any willful attempt on the part of the defendant to hide the legal cause of death, which includes “acts, omissions, or events having some causative connection with the death,” the statute of limitations remained in effect, and the defendant’s motion was thereby granted.
Continuing the legal seesaw, a state appeals court reversed the lower-court ruling. Noting that the Pennsylvania malpractice statute was ambiguous, the court argued that it should be interpreted in a way that protects plaintiffs who seek “fair compensation” but encounter willfully erected obstacles in pursuit of their claim.
Dr. Conaboy then took his case to the state’s highest court. In its majority decision, the Pennsylvania Supreme Court staked out a narrow definition of cause of death – one based on the death certificate – and ruled that only willful fraud in that document would constitute the necessary condition for halting the claim’s clock. Furthermore, the high court said, when lawmakers adopted the Medical Care Availability and Reduction of Error Act in 2002, they did so with no guarantee “that all of the information necessary to sustain a claim will be gathered in the limitations period.”
Similarly, the court ruled, “at some point the clock must run out, lest health care providers remain subject to liability exposure indefinitely, with the prospect of a trial marred by the death or diminished memory of material witnesses or the loss of critical evidence.”
A version of this article first appeared on Medscape.com.
Delay in treatment will cost hospital millions
according to a report on WFAA.com, among other news sites.
On March 21, 2019, Judy “Jessie” Adams, then part of a singing-songwriting duo with her husband, Richard, went to Premier Interventional Pain Management, in Flower Mound, Tex., prior to the couple’s drive to Ohio for a funeral. At Premier, Jesse received an epidural steroid injection (ESI) that she hoped would ease her back pain during the long drive.
Instead, the injection ended up increasing her pain.
“He [the pain physician] gave me the shot, but I couldn’t feel my legs. They were tingling, but I couldn’t feel them,” Mrs. Adams explained. “The pain was so bad in my back.” In their suit, Adams and her husband alleged that the doctor had probably “nicked a blood vessel during the ESI procedure, causing Jessie to hemorrhage.” (The couple’s suit against the doctor was settled prior to trial.)
Mrs. Adams remained under observation at the pain facility for about 1½ hours, at which point she was taken by ambulance to nearby Texas Health Presbyterian Hospital. There, in the emergency department, staff ordered a “STAT MRI” in preparation for an emergency laminectomy.
For reasons that remain murky, the MRI wasn’t performed for 1 hour and 37 minutes. The emergency laminectomy itself wasn’t started until more than 5 hours after Adams had been admitted to the ED. This was a direct violation of hospital protocol, which required that emergency surgeries be performed within 1 hour of admittance in the first available surgical suite. (At trial, Mrs. Adams’s attorneys from Lyons & Simmons offered evidence that a suite became available 49 minutes after Adams had arrived at the ED.)
During the wait, Mrs. Adams continued to experience excruciating pain. “I kept screaming: ‘Help me,’ ” she recalled. At trial, her attorneys argued that the hospital’s delay in addressing her spinal emergency led directly to her current paralysis, which keeps her confined to a wheelchair and renders her incontinent.
The hospital disagreed. In court, it maintained that Mrs. Adams was already paralyzed when she arrived at the ED and that there was no delay in care.
The jury saw things differently, however. Siding with the plaintiffs, it awarded Mrs. Adams and her husband $10.1 million, including $500,000 for Mr. Adams’s loss of future earnings and $1 million for his “loss of consortium” with his wife.
Their music career now effectively over, Mr. Adams spends most of his time taking care of Mrs. Adams.
“Music was our lifeblood for so many years, and he can’t do it anymore,” Mrs. Adams said. “He goes upstairs to play his guitar and write, and suddenly I need him to come and cath me. I just feel like I’m going to wake up from this bad dream, but it’s the same routine.”
Two doctors are absolved in woman’s sudden death
In a 3-2 decision in December 2022, the Pennsylvania Supreme Court ruled that the state’s 2-year statute of limitations in wrongful-death cases applies even in cases in which plaintiffs fail to identify the cause of death in a timely manner, as a report in the Claims Journal indicates.
The decision stems from a lawsuit filed by Linda Reibenstein on behalf of her mother, Mary Ann Whitman, who died in late April 2010 from a ruptured aortic aneurysm.
On April 12, 2010, Ms. Whitman visited Patrick D. Conaboy, MD, a Scranton family physician, complaining of a persistent cough, fever, and lower-back pain. Following an initial examination, Dr. Conaboy ordered an aortic duplex ultrasound scan and a CT scan of the patient’s abdomen.
The ultrasound was performed by radiologist Charles Barax, MD, who reviewed both scans. He identified a “poorly visualized aortic aneurysm.” At this point, Dr. Conaboy referred Ms. Whitman to a vascular surgeon. But before this visit could take place, Whitman’s aneurysm ruptured, killing her. This was listed as the medical cause of death on the patient’s death certificate.
In April 2011, Ms. Reibenstein filed a claim against Dr. Barax, alleging that he had failed to gauge the severity of her mother’s condition. Ms. Reibenstein’s attorney wasn’t able to question Dr. Barax on the record until well after the state’s 2-year statute of limitations had elapsed. When he did testify, Dr. Barax explained that the scans’ image quality prevented him from determining whether Whitman’s aneurysm was rupturing or simply bleeding. Despite this, he insisted that he had warned Dr. Conaboy of the potential for Ms. Whitman’s aneurysm to rupture.
In March 2016, nearly 6 years after her mother’s death, Ms. Reibenstein filed a new lawsuit, this one against Dr. Conaboy, whom she alleged had failed to properly treat her mother’s condition. Dr. Conaboy, in turn, asked the court for summary judgment – that is, a judgment in his favor without a full trial – arguing that the state’s window for filing a wrongful-death claim had long since closed. For their part, Ms. Reibenstein and her attorney argued that the state’s 2-year statute of limitations didn’t start until the plaintiff had discovered the cause of her mother’s death.
Initially refusing to dismiss the case, a lower court reconsidered Dr. Conaboy’s motion for summary judgment and ruled that Ms. Reibenstein had failed to present any evidence of “affirmative misrepresentation or fraudulent concealment.” In other words, in the absence of any willful attempt on the part of the defendant to hide the legal cause of death, which includes “acts, omissions, or events having some causative connection with the death,” the statute of limitations remained in effect, and the defendant’s motion was thereby granted.
Continuing the legal seesaw, a state appeals court reversed the lower-court ruling. Noting that the Pennsylvania malpractice statute was ambiguous, the court argued that it should be interpreted in a way that protects plaintiffs who seek “fair compensation” but encounter willfully erected obstacles in pursuit of their claim.
Dr. Conaboy then took his case to the state’s highest court. In its majority decision, the Pennsylvania Supreme Court staked out a narrow definition of cause of death – one based on the death certificate – and ruled that only willful fraud in that document would constitute the necessary condition for halting the claim’s clock. Furthermore, the high court said, when lawmakers adopted the Medical Care Availability and Reduction of Error Act in 2002, they did so with no guarantee “that all of the information necessary to sustain a claim will be gathered in the limitations period.”
Similarly, the court ruled, “at some point the clock must run out, lest health care providers remain subject to liability exposure indefinitely, with the prospect of a trial marred by the death or diminished memory of material witnesses or the loss of critical evidence.”
A version of this article first appeared on Medscape.com.
Scant evidence for proton pump inhibitor role in gastric cancer
The available evidence suggests that proton pump inhibitors (PPIs) do not cause gastric cancer, researchers say.
A new study could help resolve a controversy over one of the most serious side effects attributed to the widely used medications.
“Our findings are reassuring, especially to all those patients who have an indication for long-term PPI use and need persistent and effective gastric acid suppression to prevent serious health consequences,” said Daniele Piovani, MSc, PhD, an assistant professor of medical statistics at Humanitas University, Milan, in an email to this news organization.
Previous studies did not take into account the probability that the diseases for which the medications were prescribed might have caused the cancer, Dr. Piovani and colleagues write in Alimentary Pharmacology and Therapeutics.
Researchers have worried about the potential of PPIs to cause cancer after finding that they are associated with enterochromaffin-like cells, gastric atrophy, and changes in gut microbiota and gastric mucosal immunology.
Observational studies and meta-analyses showed a link between PPIs and an increased risk for gastric cancer.
“However, the underlying conditions for which PPIs are prescribed are associated with gastric cancer,” said Dr. Piovani. “This may result in an apparent association between PPIs and gastric cancer.”
Another potential confounding factor is that as-yet undiagnosed cancer might also cause symptoms that are treated with PPIs. Patient behavior also may play a role, she noted.
“Let’s imagine a patient with peptic ulcer who takes PPIs,” said Dr. Piovani. “He may not only have peptic ulcer but also be a heavy smoker. He may drink much more alcohol, have a different dietary pattern, be more likely to be exposed to high levels of stress, etc. in respect to a control [patient] who does not have peptic ulcer and does not take PPIs.”
Comparing two drug classes
More recent studies have compared people taking PPIs to people taking histamine-2 receptor antagonists (H2RAs). H2RAs are often used to treat the same conditions as PPIs, but they are not as strongly linked to hypergastrinemia and are not associated with gastric atrophy, so they might serve as good comparators.
Since results of these studies have been conflicting, Dr. Piovani and colleagues attempted to weigh them together in a systematic review and meta-analysis. They identified two randomized clinical trials and 12 observational studies with a total of over 6 million patients.
One randomized controlled trial involved Helicobacter pylori–negative patients with bleeding ulcers. Researchers assigned 138 to 20 mg daily rabeprazole (a PPI) and 132 to 40 mg famotidine (an H2RA). After a year, no cancer occurred.
The other randomized controlled trial involved H. pylori–negative patients with idiopathic peptic ulcers. Investigators assigned 114 to 30 mg lansoprazole (another PPI) and 114 to 40 mg famotidine. In 2 years, one patient receiving famotidine developed cancer.
The researchers found several methodological problems with these trials. One flaw is that the study periods were not long enough to accurately measure what effects the medications might have on gastric cancers, which are a rare outcome, they note. The evidence from these studies was so weak they could not draw conclusions from the results, the investigators conclude.
Pooling data from the 11 observational trials they were able to combine, the researchers found that PPI users had a one-third higher random relative risk of cancer than H2RA users (95% confidence interval, 1.11-1.59). However, these studies were heterogenous, and five of them did not adjust for age and sex, as well as other potentially confounding covariates.
The remaining six observational studies adjusted for age, sex, and at least two other covariates that could affect the risk for gastric cancer. These studies had a total of 2.5 million patients and 7,372 gastric cancers. Combined, these studies showed an RR of gastric cancer in PPI users, compared with H2RA users of 1.07, which was not statistically significant (95% CI, 0.97-1.19).
The researchers found no clear evidence of a dose-response or of an increased risk with longer-term use of PPIs.
Findings support practice guidance
“I found this relatively reassuring,” Mark Lewis, MD, director of gastrointestinal oncology at Intermountain Healthcare, Murray, Utah, told this news organization.
PPIs do dramatically increase the pH of the stomach, stimulating the stomach to try to compensate in a process that can sometimes give rise to tumors, Dr. Lewis said. But these tumors appear to be benign.
Other concerns about PPI use, such as reduction in bone density, remain under investigation, he said.
Some H2RA blockers might actually pose a greater cancer risk than PPIs, said Dr. Lewis, and many clinicians seem to favor PPIs. “I have seen a huge sea change where most patients are on PPIs. And I would say that H2RA blockers are older and increasingly the exception in terms of usage, not the rule.”
The investigators note that observational studies by their nature cannot prove cause and effect, but because gastric cancer is so rare, a randomized controlled trial of PPIs versus H2RAs that is large enough to be definitive may not be feasible.
They conclude that their findings support the American Gastroenterological Association recommendation that “the decision to discontinue PPIs should be based solely on the lack of an indication for use and not because of concern for PPI-associated adverse effects.”
Dr. Piovani and Dr. Lewis report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The available evidence suggests that proton pump inhibitors (PPIs) do not cause gastric cancer, researchers say.
A new study could help resolve a controversy over one of the most serious side effects attributed to the widely used medications.
“Our findings are reassuring, especially to all those patients who have an indication for long-term PPI use and need persistent and effective gastric acid suppression to prevent serious health consequences,” said Daniele Piovani, MSc, PhD, an assistant professor of medical statistics at Humanitas University, Milan, in an email to this news organization.
Previous studies did not take into account the probability that the diseases for which the medications were prescribed might have caused the cancer, Dr. Piovani and colleagues write in Alimentary Pharmacology and Therapeutics.
Researchers have worried about the potential of PPIs to cause cancer after finding that they are associated with enterochromaffin-like cells, gastric atrophy, and changes in gut microbiota and gastric mucosal immunology.
Observational studies and meta-analyses showed a link between PPIs and an increased risk for gastric cancer.
“However, the underlying conditions for which PPIs are prescribed are associated with gastric cancer,” said Dr. Piovani. “This may result in an apparent association between PPIs and gastric cancer.”
Another potential confounding factor is that as-yet undiagnosed cancer might also cause symptoms that are treated with PPIs. Patient behavior also may play a role, she noted.
“Let’s imagine a patient with peptic ulcer who takes PPIs,” said Dr. Piovani. “He may not only have peptic ulcer but also be a heavy smoker. He may drink much more alcohol, have a different dietary pattern, be more likely to be exposed to high levels of stress, etc. in respect to a control [patient] who does not have peptic ulcer and does not take PPIs.”
Comparing two drug classes
More recent studies have compared people taking PPIs to people taking histamine-2 receptor antagonists (H2RAs). H2RAs are often used to treat the same conditions as PPIs, but they are not as strongly linked to hypergastrinemia and are not associated with gastric atrophy, so they might serve as good comparators.
Since results of these studies have been conflicting, Dr. Piovani and colleagues attempted to weigh them together in a systematic review and meta-analysis. They identified two randomized clinical trials and 12 observational studies with a total of over 6 million patients.
One randomized controlled trial involved Helicobacter pylori–negative patients with bleeding ulcers. Researchers assigned 138 to 20 mg daily rabeprazole (a PPI) and 132 to 40 mg famotidine (an H2RA). After a year, no cancer occurred.
The other randomized controlled trial involved H. pylori–negative patients with idiopathic peptic ulcers. Investigators assigned 114 to 30 mg lansoprazole (another PPI) and 114 to 40 mg famotidine. In 2 years, one patient receiving famotidine developed cancer.
The researchers found several methodological problems with these trials. One flaw is that the study periods were not long enough to accurately measure what effects the medications might have on gastric cancers, which are a rare outcome, they note. The evidence from these studies was so weak they could not draw conclusions from the results, the investigators conclude.
Pooling data from the 11 observational trials they were able to combine, the researchers found that PPI users had a one-third higher random relative risk of cancer than H2RA users (95% confidence interval, 1.11-1.59). However, these studies were heterogenous, and five of them did not adjust for age and sex, as well as other potentially confounding covariates.
The remaining six observational studies adjusted for age, sex, and at least two other covariates that could affect the risk for gastric cancer. These studies had a total of 2.5 million patients and 7,372 gastric cancers. Combined, these studies showed an RR of gastric cancer in PPI users, compared with H2RA users of 1.07, which was not statistically significant (95% CI, 0.97-1.19).
The researchers found no clear evidence of a dose-response or of an increased risk with longer-term use of PPIs.
Findings support practice guidance
“I found this relatively reassuring,” Mark Lewis, MD, director of gastrointestinal oncology at Intermountain Healthcare, Murray, Utah, told this news organization.
PPIs do dramatically increase the pH of the stomach, stimulating the stomach to try to compensate in a process that can sometimes give rise to tumors, Dr. Lewis said. But these tumors appear to be benign.
Other concerns about PPI use, such as reduction in bone density, remain under investigation, he said.
Some H2RA blockers might actually pose a greater cancer risk than PPIs, said Dr. Lewis, and many clinicians seem to favor PPIs. “I have seen a huge sea change where most patients are on PPIs. And I would say that H2RA blockers are older and increasingly the exception in terms of usage, not the rule.”
The investigators note that observational studies by their nature cannot prove cause and effect, but because gastric cancer is so rare, a randomized controlled trial of PPIs versus H2RAs that is large enough to be definitive may not be feasible.
They conclude that their findings support the American Gastroenterological Association recommendation that “the decision to discontinue PPIs should be based solely on the lack of an indication for use and not because of concern for PPI-associated adverse effects.”
Dr. Piovani and Dr. Lewis report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The available evidence suggests that proton pump inhibitors (PPIs) do not cause gastric cancer, researchers say.
A new study could help resolve a controversy over one of the most serious side effects attributed to the widely used medications.
“Our findings are reassuring, especially to all those patients who have an indication for long-term PPI use and need persistent and effective gastric acid suppression to prevent serious health consequences,” said Daniele Piovani, MSc, PhD, an assistant professor of medical statistics at Humanitas University, Milan, in an email to this news organization.
Previous studies did not take into account the probability that the diseases for which the medications were prescribed might have caused the cancer, Dr. Piovani and colleagues write in Alimentary Pharmacology and Therapeutics.
Researchers have worried about the potential of PPIs to cause cancer after finding that they are associated with enterochromaffin-like cells, gastric atrophy, and changes in gut microbiota and gastric mucosal immunology.
Observational studies and meta-analyses showed a link between PPIs and an increased risk for gastric cancer.
“However, the underlying conditions for which PPIs are prescribed are associated with gastric cancer,” said Dr. Piovani. “This may result in an apparent association between PPIs and gastric cancer.”
Another potential confounding factor is that as-yet undiagnosed cancer might also cause symptoms that are treated with PPIs. Patient behavior also may play a role, she noted.
“Let’s imagine a patient with peptic ulcer who takes PPIs,” said Dr. Piovani. “He may not only have peptic ulcer but also be a heavy smoker. He may drink much more alcohol, have a different dietary pattern, be more likely to be exposed to high levels of stress, etc. in respect to a control [patient] who does not have peptic ulcer and does not take PPIs.”
Comparing two drug classes
More recent studies have compared people taking PPIs to people taking histamine-2 receptor antagonists (H2RAs). H2RAs are often used to treat the same conditions as PPIs, but they are not as strongly linked to hypergastrinemia and are not associated with gastric atrophy, so they might serve as good comparators.
Since results of these studies have been conflicting, Dr. Piovani and colleagues attempted to weigh them together in a systematic review and meta-analysis. They identified two randomized clinical trials and 12 observational studies with a total of over 6 million patients.
One randomized controlled trial involved Helicobacter pylori–negative patients with bleeding ulcers. Researchers assigned 138 to 20 mg daily rabeprazole (a PPI) and 132 to 40 mg famotidine (an H2RA). After a year, no cancer occurred.
The other randomized controlled trial involved H. pylori–negative patients with idiopathic peptic ulcers. Investigators assigned 114 to 30 mg lansoprazole (another PPI) and 114 to 40 mg famotidine. In 2 years, one patient receiving famotidine developed cancer.
The researchers found several methodological problems with these trials. One flaw is that the study periods were not long enough to accurately measure what effects the medications might have on gastric cancers, which are a rare outcome, they note. The evidence from these studies was so weak they could not draw conclusions from the results, the investigators conclude.
Pooling data from the 11 observational trials they were able to combine, the researchers found that PPI users had a one-third higher random relative risk of cancer than H2RA users (95% confidence interval, 1.11-1.59). However, these studies were heterogenous, and five of them did not adjust for age and sex, as well as other potentially confounding covariates.
The remaining six observational studies adjusted for age, sex, and at least two other covariates that could affect the risk for gastric cancer. These studies had a total of 2.5 million patients and 7,372 gastric cancers. Combined, these studies showed an RR of gastric cancer in PPI users, compared with H2RA users of 1.07, which was not statistically significant (95% CI, 0.97-1.19).
The researchers found no clear evidence of a dose-response or of an increased risk with longer-term use of PPIs.
Findings support practice guidance
“I found this relatively reassuring,” Mark Lewis, MD, director of gastrointestinal oncology at Intermountain Healthcare, Murray, Utah, told this news organization.
PPIs do dramatically increase the pH of the stomach, stimulating the stomach to try to compensate in a process that can sometimes give rise to tumors, Dr. Lewis said. But these tumors appear to be benign.
Other concerns about PPI use, such as reduction in bone density, remain under investigation, he said.
Some H2RA blockers might actually pose a greater cancer risk than PPIs, said Dr. Lewis, and many clinicians seem to favor PPIs. “I have seen a huge sea change where most patients are on PPIs. And I would say that H2RA blockers are older and increasingly the exception in terms of usage, not the rule.”
The investigators note that observational studies by their nature cannot prove cause and effect, but because gastric cancer is so rare, a randomized controlled trial of PPIs versus H2RAs that is large enough to be definitive may not be feasible.
They conclude that their findings support the American Gastroenterological Association recommendation that “the decision to discontinue PPIs should be based solely on the lack of an indication for use and not because of concern for PPI-associated adverse effects.”
Dr. Piovani and Dr. Lewis report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ALIMENTARY PHARMACOLOGY AND THERAPEUTICS
A 50-year-old woman with no significant history presented with erythematous, annular plaques, and papules on the dorsal hands and arms
. The prevalence and incidence is approximately 0.1%-0.4%. Although the condition is benign, it may be associated with more serious conditions such as HIV and malignancy. GA affects women more frequently than men but can affect any age group, although it most commonly presents in those ages 30 years and younger. While the exact etiology is unknown, GA has been most strongly associated with diabetes mellitus, hyperlipidemia, and autoimmune diseases.
The disease presents as localized, annular erythematous plaques and papules on the dorsal hands and feet in approximately 75% of cases. However, eruptions may appear on the trunk and extremities and can be categorized into patchy, generalized, interstitial, subcutaneous, or perforating subtypes. The lesions are often asymptomatic and typically not associated with any other symptoms.
The pathogenesis of GA is still under investigation, but recent studies suggest that a Th1-mediated dysregulation of the JAK-STAT pathway may contribute to the disease. Other hypotheses include a delayed hypersensitivity reaction or cell mediated immune response. The mechanism may be multifaceted, and epidemiologic research suggests a genetic predisposition in White individuals, but these findings may be associated with socioeconomic factors and disparities in health care.
GA presents on histology with palisading histiocytes surrounding focal collagen necrobiosis with mucin deposition. Tissue samples also display leukocytic infiltration of the dermis featuring multinucleated giant cells. There are defining features of the different subtypes, but focal collagen necrosis, the presence of histiocytes, and mucin deposition are consistent findings across all presentations.
GA lesions commonly regress on their own, but they tend to recur and can be functionally and visually unappealing to patients. The most common treatments for GA include topical corticosteroids, intralesional corticosteroid injections, and other anti-inflammatory drugs. These interventions can be administered in a variety of ways as the inflammation caused by GA exists on a spectrum, and less severe cases can be managed with topical or intralesional treatment. Systemic therapy may be necessary for severe and recalcitrant cases. Other interventions that have shown promise in smaller studies include phototherapy, hydroxychloroquine, and TNF-alpha inhibitors.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Tampa Bay Regional Campus, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Joshi TP and Duvic M. Am J Clin Dermatol. 2022 Jan;23(1):37-50. doi: 10.1007/s40257-021-00636-1.
Muse M et al. Dermatol Online J. 2021 Apr 15;27(4):13030/qt0m50398n.
Schmieder SJ et al. Granuloma Annulare. NIH National Center for Biotechnology Information [Updated 2022 Nov 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan. 7.
. The prevalence and incidence is approximately 0.1%-0.4%. Although the condition is benign, it may be associated with more serious conditions such as HIV and malignancy. GA affects women more frequently than men but can affect any age group, although it most commonly presents in those ages 30 years and younger. While the exact etiology is unknown, GA has been most strongly associated with diabetes mellitus, hyperlipidemia, and autoimmune diseases.
The disease presents as localized, annular erythematous plaques and papules on the dorsal hands and feet in approximately 75% of cases. However, eruptions may appear on the trunk and extremities and can be categorized into patchy, generalized, interstitial, subcutaneous, or perforating subtypes. The lesions are often asymptomatic and typically not associated with any other symptoms.
The pathogenesis of GA is still under investigation, but recent studies suggest that a Th1-mediated dysregulation of the JAK-STAT pathway may contribute to the disease. Other hypotheses include a delayed hypersensitivity reaction or cell mediated immune response. The mechanism may be multifaceted, and epidemiologic research suggests a genetic predisposition in White individuals, but these findings may be associated with socioeconomic factors and disparities in health care.
GA presents on histology with palisading histiocytes surrounding focal collagen necrobiosis with mucin deposition. Tissue samples also display leukocytic infiltration of the dermis featuring multinucleated giant cells. There are defining features of the different subtypes, but focal collagen necrosis, the presence of histiocytes, and mucin deposition are consistent findings across all presentations.
GA lesions commonly regress on their own, but they tend to recur and can be functionally and visually unappealing to patients. The most common treatments for GA include topical corticosteroids, intralesional corticosteroid injections, and other anti-inflammatory drugs. These interventions can be administered in a variety of ways as the inflammation caused by GA exists on a spectrum, and less severe cases can be managed with topical or intralesional treatment. Systemic therapy may be necessary for severe and recalcitrant cases. Other interventions that have shown promise in smaller studies include phototherapy, hydroxychloroquine, and TNF-alpha inhibitors.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Tampa Bay Regional Campus, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Joshi TP and Duvic M. Am J Clin Dermatol. 2022 Jan;23(1):37-50. doi: 10.1007/s40257-021-00636-1.
Muse M et al. Dermatol Online J. 2021 Apr 15;27(4):13030/qt0m50398n.
Schmieder SJ et al. Granuloma Annulare. NIH National Center for Biotechnology Information [Updated 2022 Nov 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan. 7.
. The prevalence and incidence is approximately 0.1%-0.4%. Although the condition is benign, it may be associated with more serious conditions such as HIV and malignancy. GA affects women more frequently than men but can affect any age group, although it most commonly presents in those ages 30 years and younger. While the exact etiology is unknown, GA has been most strongly associated with diabetes mellitus, hyperlipidemia, and autoimmune diseases.
The disease presents as localized, annular erythematous plaques and papules on the dorsal hands and feet in approximately 75% of cases. However, eruptions may appear on the trunk and extremities and can be categorized into patchy, generalized, interstitial, subcutaneous, or perforating subtypes. The lesions are often asymptomatic and typically not associated with any other symptoms.
The pathogenesis of GA is still under investigation, but recent studies suggest that a Th1-mediated dysregulation of the JAK-STAT pathway may contribute to the disease. Other hypotheses include a delayed hypersensitivity reaction or cell mediated immune response. The mechanism may be multifaceted, and epidemiologic research suggests a genetic predisposition in White individuals, but these findings may be associated with socioeconomic factors and disparities in health care.
GA presents on histology with palisading histiocytes surrounding focal collagen necrobiosis with mucin deposition. Tissue samples also display leukocytic infiltration of the dermis featuring multinucleated giant cells. There are defining features of the different subtypes, but focal collagen necrosis, the presence of histiocytes, and mucin deposition are consistent findings across all presentations.
GA lesions commonly regress on their own, but they tend to recur and can be functionally and visually unappealing to patients. The most common treatments for GA include topical corticosteroids, intralesional corticosteroid injections, and other anti-inflammatory drugs. These interventions can be administered in a variety of ways as the inflammation caused by GA exists on a spectrum, and less severe cases can be managed with topical or intralesional treatment. Systemic therapy may be necessary for severe and recalcitrant cases. Other interventions that have shown promise in smaller studies include phototherapy, hydroxychloroquine, and TNF-alpha inhibitors.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Tampa Bay Regional Campus, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Joshi TP and Duvic M. Am J Clin Dermatol. 2022 Jan;23(1):37-50. doi: 10.1007/s40257-021-00636-1.
Muse M et al. Dermatol Online J. 2021 Apr 15;27(4):13030/qt0m50398n.
Schmieder SJ et al. Granuloma Annulare. NIH National Center for Biotechnology Information [Updated 2022 Nov 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan. 7.
COVID leading cause of death among law enforcement for third year
A new report says 70 officers died of COVID-related causes after getting the virus while on the job. The number is down dramatically from 2021, when 405 officer deaths were attributed to COVID.
The annual count was published Wednesday by the National Law Enforcement Officers Memorial Fund.
In total, 226 officers died in the line of duty in 2022, which is a decrease of 61% from 2021.
The decrease “is almost entirely related to the significant reduction in COVID-19 deaths,” the report stated. The authors said the decline was likely due to “reduced infection rates and the broad availability and use of vaccinations.”
Reported deaths included federal, state, tribal, and local law enforcement officers.
Firearms-related fatalities were the second-leading cause of death among officers, with 64 in 2022. That count sustains a 21% increase seen in 2021, up from the decade-long average of 53 firearms-related deaths annually from 2010 to 2020.
Traffic-related causes ranked third for cause of death in 2022, accounting for 56 deaths.
“While overall line-of-duty deaths are trending down, the continuing trend of greater-than-average firearms-related deaths continues to be a serious concern,” Marcia Ferranto, the organization’s chief executive officer, said in a news release. “Using and reporting on this data allows us to highlight the continuing cost of maintaining our democracy, regrettably measured in the lives of the many law enforcement professionals who sacrifice everything fulfilling their promise to serve and protect.”
A version of this article first appeared on WebMD.com.
A new report says 70 officers died of COVID-related causes after getting the virus while on the job. The number is down dramatically from 2021, when 405 officer deaths were attributed to COVID.
The annual count was published Wednesday by the National Law Enforcement Officers Memorial Fund.
In total, 226 officers died in the line of duty in 2022, which is a decrease of 61% from 2021.
The decrease “is almost entirely related to the significant reduction in COVID-19 deaths,” the report stated. The authors said the decline was likely due to “reduced infection rates and the broad availability and use of vaccinations.”
Reported deaths included federal, state, tribal, and local law enforcement officers.
Firearms-related fatalities were the second-leading cause of death among officers, with 64 in 2022. That count sustains a 21% increase seen in 2021, up from the decade-long average of 53 firearms-related deaths annually from 2010 to 2020.
Traffic-related causes ranked third for cause of death in 2022, accounting for 56 deaths.
“While overall line-of-duty deaths are trending down, the continuing trend of greater-than-average firearms-related deaths continues to be a serious concern,” Marcia Ferranto, the organization’s chief executive officer, said in a news release. “Using and reporting on this data allows us to highlight the continuing cost of maintaining our democracy, regrettably measured in the lives of the many law enforcement professionals who sacrifice everything fulfilling their promise to serve and protect.”
A version of this article first appeared on WebMD.com.
A new report says 70 officers died of COVID-related causes after getting the virus while on the job. The number is down dramatically from 2021, when 405 officer deaths were attributed to COVID.
The annual count was published Wednesday by the National Law Enforcement Officers Memorial Fund.
In total, 226 officers died in the line of duty in 2022, which is a decrease of 61% from 2021.
The decrease “is almost entirely related to the significant reduction in COVID-19 deaths,” the report stated. The authors said the decline was likely due to “reduced infection rates and the broad availability and use of vaccinations.”
Reported deaths included federal, state, tribal, and local law enforcement officers.
Firearms-related fatalities were the second-leading cause of death among officers, with 64 in 2022. That count sustains a 21% increase seen in 2021, up from the decade-long average of 53 firearms-related deaths annually from 2010 to 2020.
Traffic-related causes ranked third for cause of death in 2022, accounting for 56 deaths.
“While overall line-of-duty deaths are trending down, the continuing trend of greater-than-average firearms-related deaths continues to be a serious concern,” Marcia Ferranto, the organization’s chief executive officer, said in a news release. “Using and reporting on this data allows us to highlight the continuing cost of maintaining our democracy, regrettably measured in the lives of the many law enforcement professionals who sacrifice everything fulfilling their promise to serve and protect.”
A version of this article first appeared on WebMD.com.
Oramed oral insulin fails to meet goal in type 2 diabetes
Oramed Pharmaceuticals’ investigational oral insulin failed to achieve its primary endpoint in a phase 3 trial, according to top-line results announced by the company.
“Therefore, Oramed expects to discontinue its oral insulin clinical activities for [type 2 diabetes],” according to a company statement.
, ORA-D-013-1, comparing the efficacy of the insulin product ORMD-0801 to placebo in 710 people with type 2 diabetes with inadequate glycemic control on two or three oral glucose-lowering agents.
The participants were randomized 2:2:1:1 into ORMD-0801 dosed at 8 mg once or twice daily, or placebo dosed once or twice daily. They completed a 21-day screening period, followed by a 26-week double-blind treatment period.
The product didn’t achieve the primary endpoint comparing reduction in hemoglobin A1c from baseline to 26 weeks, or the secondary endpoint of mean change in fasting plasma glucose at 26 weeks. There were no serious adverse events.
Oramed Pharmaceuticals specializes in developing oral delivery formulations of drugs currently delivered via injection. The company has offices in the United States and Israel.
Oramed CEO Nadav Kidron commented in the statement, “Today’s outcome is very disappointing, given the positive results from prior trials. Once full data from the studies are available, we expect to share relevant learnings and future plans. We thank all the patients, families, and health care professionals who participated in the trial.”
Insulin manufacturer Novo Nordisk had also been developing an oral insulin product. Successful phase 2a results were presented at the American Diabetes Association’s 2017 Scientific Sessions and full phase 2 feasibility results were published in Lancet Diabetes & Endocrinology in 2019.
However, Novo Nordisk, which manufactures the oral glucagon-like peptide-1 receptor agonist semaglutide (Rybelsus), subsequently discontinued development of their oral insulin product. According to a statement, “Initial results raised questions about truly addressing patients’ unmet needs with insulin therapy. Therefore, we discontinued this work to focus on projects that could in fact improve cardiometabolic outcomes for people living with diabetes.”
A version of this article first appeared on Medscape.com.
Oramed Pharmaceuticals’ investigational oral insulin failed to achieve its primary endpoint in a phase 3 trial, according to top-line results announced by the company.
“Therefore, Oramed expects to discontinue its oral insulin clinical activities for [type 2 diabetes],” according to a company statement.
, ORA-D-013-1, comparing the efficacy of the insulin product ORMD-0801 to placebo in 710 people with type 2 diabetes with inadequate glycemic control on two or three oral glucose-lowering agents.
The participants were randomized 2:2:1:1 into ORMD-0801 dosed at 8 mg once or twice daily, or placebo dosed once or twice daily. They completed a 21-day screening period, followed by a 26-week double-blind treatment period.
The product didn’t achieve the primary endpoint comparing reduction in hemoglobin A1c from baseline to 26 weeks, or the secondary endpoint of mean change in fasting plasma glucose at 26 weeks. There were no serious adverse events.
Oramed Pharmaceuticals specializes in developing oral delivery formulations of drugs currently delivered via injection. The company has offices in the United States and Israel.
Oramed CEO Nadav Kidron commented in the statement, “Today’s outcome is very disappointing, given the positive results from prior trials. Once full data from the studies are available, we expect to share relevant learnings and future plans. We thank all the patients, families, and health care professionals who participated in the trial.”
Insulin manufacturer Novo Nordisk had also been developing an oral insulin product. Successful phase 2a results were presented at the American Diabetes Association’s 2017 Scientific Sessions and full phase 2 feasibility results were published in Lancet Diabetes & Endocrinology in 2019.
However, Novo Nordisk, which manufactures the oral glucagon-like peptide-1 receptor agonist semaglutide (Rybelsus), subsequently discontinued development of their oral insulin product. According to a statement, “Initial results raised questions about truly addressing patients’ unmet needs with insulin therapy. Therefore, we discontinued this work to focus on projects that could in fact improve cardiometabolic outcomes for people living with diabetes.”
A version of this article first appeared on Medscape.com.
Oramed Pharmaceuticals’ investigational oral insulin failed to achieve its primary endpoint in a phase 3 trial, according to top-line results announced by the company.
“Therefore, Oramed expects to discontinue its oral insulin clinical activities for [type 2 diabetes],” according to a company statement.
, ORA-D-013-1, comparing the efficacy of the insulin product ORMD-0801 to placebo in 710 people with type 2 diabetes with inadequate glycemic control on two or three oral glucose-lowering agents.
The participants were randomized 2:2:1:1 into ORMD-0801 dosed at 8 mg once or twice daily, or placebo dosed once or twice daily. They completed a 21-day screening period, followed by a 26-week double-blind treatment period.
The product didn’t achieve the primary endpoint comparing reduction in hemoglobin A1c from baseline to 26 weeks, or the secondary endpoint of mean change in fasting plasma glucose at 26 weeks. There were no serious adverse events.
Oramed Pharmaceuticals specializes in developing oral delivery formulations of drugs currently delivered via injection. The company has offices in the United States and Israel.
Oramed CEO Nadav Kidron commented in the statement, “Today’s outcome is very disappointing, given the positive results from prior trials. Once full data from the studies are available, we expect to share relevant learnings and future plans. We thank all the patients, families, and health care professionals who participated in the trial.”
Insulin manufacturer Novo Nordisk had also been developing an oral insulin product. Successful phase 2a results were presented at the American Diabetes Association’s 2017 Scientific Sessions and full phase 2 feasibility results were published in Lancet Diabetes & Endocrinology in 2019.
However, Novo Nordisk, which manufactures the oral glucagon-like peptide-1 receptor agonist semaglutide (Rybelsus), subsequently discontinued development of their oral insulin product. According to a statement, “Initial results raised questions about truly addressing patients’ unmet needs with insulin therapy. Therefore, we discontinued this work to focus on projects that could in fact improve cardiometabolic outcomes for people living with diabetes.”
A version of this article first appeared on Medscape.com.