User login
The Respect for Marriage Act: How this law supports the health and well-being of LGBTQ+ youth
Childhood and adolescence are periods of life with rapid growth and development in which the psychosocial factors of one’s environment can have a profound effect on health. There is increasing evidence that adverse childhood experiences (ACEs) can have significant negative effects on long-term health with effects persisting into subsequent generations.1 Youth themselves, however, often do not have the voice, ability, or political power to advocate for safe and more supportive environments that are essential to their well-being. Thus, advocacy has been central to the profession of pediatrics since its inception, where providers can partner with their patients, families, and communities to push for changes in the environments in which youth live and grow.2
LGBTQ+ youth are known to be at increased risk for ACEs because of the stress that comes from being part of a minority group and the discrimination they experience by their families, communities, and society at large. These factors within their environments have been shown to be associated with increased rates of anxiety, depression, substance use, sexually transmitted infections, and homelessness.3 As with other health outcomes that have been linked to the social determinants of health, these disparities are not inevitable and could be greatly improved upon through advocacy and changes in the environments of LGBTQ+ youth.
Marriage equality (the recognition that same-sex couples have the same legal right to marry as opposite-sex couples) has been shown to be not only a political issue, but one that affects health. The debates surrounding marriage equality have contributed to minority stress by questioning the validity of same-sex relationships and assigning them less value relative to opposite-sex relationships.4 In 1996, the U.S. Congress passed the Defense of Marriage Act (DOMA), which federally defined marriage as being legally recognized only between opposite-sex couples.
Individual states then continued the marriage equality debate by passing individual state laws either allowing or prohibiting same-sex marriage. During this time, it was shown that, in states where same-sex marriage was legally prohibited, LGBTQ+ adults reported significantly higher rates of generalized anxiety disorder, alcohol use disorder, any mood disorder, and psychiatric comorbidity when compared with states without a legal ban on same-sex marriage.5
Using data from the Youth Risk Behavior Surveillance System, it was shown that state policies recognizing same-sex marriage were associated with a 7% relative reduction in suicide attempts reported by adolescent sexual minority students compared with before these policies.6 It was also shown that children with same-sex parents were overall less likely to have private health insurance, but this disparity was improved in states that legally recognized same-sex marriage and allowed second-parent adoptions.7
In 2013, the U.S. Supreme Court ruled that DOMA was unconstitutional, requiring the federal government to legally recognize same-sex marriages for the purposes of federal benefits. In 2015, the U.S. Supreme Court further ruled that same-sex couples are guaranteed the fundamental right to marry, requiring that all states issue marriage licenses to same-sex couples. These rulings were associated with a decrease in reported levels of stigma over time and increased reported levels of family support, particularly for those in same-sex relationships.8
The Respect for Marriage Act (RFMA) was passed by the U.S. Congress and signed into law by President Biden on Dec. 13, 2022. This law officially repeals DOMA and requires all states and the federal government to recognize same-sex marriages performed in any U.S. state or territory.9
If the U.S. Supreme Court were to overturn the 2015 marriage equality decision, individual state laws ensuring or banning same-sex marriage would again be in effect. However, the RFMA ensures that all states continue to recognize same-sex marriages performed in any U.S. state or territory (even if that state itself bans same-sex marriage). While we do not yet have any studies or data regarding the effect of the RFMA on public health, we can expect positive effects by drawing on the previous evidence on the effect of marriage equality and its effect on the health and well-being of LGBTQ+ individuals. By establishing marriage equality in the United States, our government institutions are affirming the relationships and identities of those in same-sex relationships, with the potential effect of helping to destigmatize the LGBTQ+ community.
Since 2002, the American Academy of Pediatrics has recommended that pediatricians “support the right of every child and family to the financial, psychological, and legal security that results from having legally recognized parents who are committed to each other and to the welfare of their children,” acknowledging that “legislative initiatives assuring legal status equivalent to marriage for gay and lesbian partners … can also attend to providing security and permanence for the children of those partnerships.”10 While changes in legal marriage equality are likely to have a positive effect on those within the LGBTQ+ community, it should also be understood that this will not solve all of the psychosocial effects and resultant health disparities that these children face.
A recent scoping review highlights that, as the result of marriage equality progress, sexual minority adults have reported increased social acceptance and reduced stigma across individual, community, and societal levels, but that sexual minority stigma continues to persist across all levels.11
As pediatricians, we can continue to support LGBTQ+ patients and parents by providing care in a safe and affirming environment in which families understand and embrace the healthy development of gender identity and sexuality in an open and destigmatized manner. Delivering care using this approach in and of itself can be seen as advocacy to promote health and well-being within minoritized populations. Pediatricians are also encouraged to become engaged in local and national advocacy initiatives to have a broader effect in the fight for health equity in minority populations, including LGBTQ+ families and youth.
Pediatricians should work with their patients, families, and communities to advocate for structural change needed to address the social determinants of health for optimal growth and development.
Dr. Warus is an adolescent medicine physician who specializes in care for transgender and gender-nonconforming youth, and LGBTQ health for youth at Children’s Hospital of Los Angeles. He is an assistant professor of pediatrics at University of Southern California, Los Angeles.
Resources
Bright Futures – Promoting Healthy Development of Sexuality and Gender Identity (Implementation Tip Sheet): https://downloads.aap.org/AAP/PDF/BF_HealthySexualityGenderIdentity_Tipsheet.pdf
Bright Futures – Implementing Social Determinants of Health Into Health Supervision Visits (Implementation Tip Sheet): https://downloads.aap.org/AAP/PDF/Bright%20Futures/BF_IntegrateSDoH_Tipsheet.pdf?_ga=2.214227031.1330574154.1673910248-58875083.1673910248
American Academy of Pediatrics – Advocacy Website: https://www.aap.org/en/advocacy/
References
1. Hughes K et al. Lancet Public Health. 2017;2(8):e356-66.
2. Camero K and Javier JR. Pediatr Clin N Am. 2023;70:43-51.
3. Lund EM and Burgess CM. Prim Care Clin Office Pract. 2021;48:179-89.
4. Buffie WC. Am J Public Health. 2011;101(6):986-90.
5. Hatzenbuehler ML et al. Am J Public Health. 2010;100:452-9.
6. Raifman J et al. JAMA Pediatr. 2017;171(4):350-6.
7. Gonzales G and Blewett LA. Pediatrics. 2013;132(4):703-11.
8. Ogolsky BG et al. J Fam Psychol. 2019;33(4):422-32.
9. Library of Congress. H.R.8404 – 117th Congress (2021-2022): Respect for Marriage Act. 2022 Dec 13. www.congress.gov/bill/117th-congress/house-bill/8404/text.
10. Perrin EC and Committee on Psychosocial Aspects of Child and Family Health. Pediatrics. 2002;109(2):341-4.
11. Drabble LA et al. PLoS ONE. 2021;16(5):e0249125.
Childhood and adolescence are periods of life with rapid growth and development in which the psychosocial factors of one’s environment can have a profound effect on health. There is increasing evidence that adverse childhood experiences (ACEs) can have significant negative effects on long-term health with effects persisting into subsequent generations.1 Youth themselves, however, often do not have the voice, ability, or political power to advocate for safe and more supportive environments that are essential to their well-being. Thus, advocacy has been central to the profession of pediatrics since its inception, where providers can partner with their patients, families, and communities to push for changes in the environments in which youth live and grow.2
LGBTQ+ youth are known to be at increased risk for ACEs because of the stress that comes from being part of a minority group and the discrimination they experience by their families, communities, and society at large. These factors within their environments have been shown to be associated with increased rates of anxiety, depression, substance use, sexually transmitted infections, and homelessness.3 As with other health outcomes that have been linked to the social determinants of health, these disparities are not inevitable and could be greatly improved upon through advocacy and changes in the environments of LGBTQ+ youth.
Marriage equality (the recognition that same-sex couples have the same legal right to marry as opposite-sex couples) has been shown to be not only a political issue, but one that affects health. The debates surrounding marriage equality have contributed to minority stress by questioning the validity of same-sex relationships and assigning them less value relative to opposite-sex relationships.4 In 1996, the U.S. Congress passed the Defense of Marriage Act (DOMA), which federally defined marriage as being legally recognized only between opposite-sex couples.
Individual states then continued the marriage equality debate by passing individual state laws either allowing or prohibiting same-sex marriage. During this time, it was shown that, in states where same-sex marriage was legally prohibited, LGBTQ+ adults reported significantly higher rates of generalized anxiety disorder, alcohol use disorder, any mood disorder, and psychiatric comorbidity when compared with states without a legal ban on same-sex marriage.5
Using data from the Youth Risk Behavior Surveillance System, it was shown that state policies recognizing same-sex marriage were associated with a 7% relative reduction in suicide attempts reported by adolescent sexual minority students compared with before these policies.6 It was also shown that children with same-sex parents were overall less likely to have private health insurance, but this disparity was improved in states that legally recognized same-sex marriage and allowed second-parent adoptions.7
In 2013, the U.S. Supreme Court ruled that DOMA was unconstitutional, requiring the federal government to legally recognize same-sex marriages for the purposes of federal benefits. In 2015, the U.S. Supreme Court further ruled that same-sex couples are guaranteed the fundamental right to marry, requiring that all states issue marriage licenses to same-sex couples. These rulings were associated with a decrease in reported levels of stigma over time and increased reported levels of family support, particularly for those in same-sex relationships.8
The Respect for Marriage Act (RFMA) was passed by the U.S. Congress and signed into law by President Biden on Dec. 13, 2022. This law officially repeals DOMA and requires all states and the federal government to recognize same-sex marriages performed in any U.S. state or territory.9
If the U.S. Supreme Court were to overturn the 2015 marriage equality decision, individual state laws ensuring or banning same-sex marriage would again be in effect. However, the RFMA ensures that all states continue to recognize same-sex marriages performed in any U.S. state or territory (even if that state itself bans same-sex marriage). While we do not yet have any studies or data regarding the effect of the RFMA on public health, we can expect positive effects by drawing on the previous evidence on the effect of marriage equality and its effect on the health and well-being of LGBTQ+ individuals. By establishing marriage equality in the United States, our government institutions are affirming the relationships and identities of those in same-sex relationships, with the potential effect of helping to destigmatize the LGBTQ+ community.
Since 2002, the American Academy of Pediatrics has recommended that pediatricians “support the right of every child and family to the financial, psychological, and legal security that results from having legally recognized parents who are committed to each other and to the welfare of their children,” acknowledging that “legislative initiatives assuring legal status equivalent to marriage for gay and lesbian partners … can also attend to providing security and permanence for the children of those partnerships.”10 While changes in legal marriage equality are likely to have a positive effect on those within the LGBTQ+ community, it should also be understood that this will not solve all of the psychosocial effects and resultant health disparities that these children face.
A recent scoping review highlights that, as the result of marriage equality progress, sexual minority adults have reported increased social acceptance and reduced stigma across individual, community, and societal levels, but that sexual minority stigma continues to persist across all levels.11
As pediatricians, we can continue to support LGBTQ+ patients and parents by providing care in a safe and affirming environment in which families understand and embrace the healthy development of gender identity and sexuality in an open and destigmatized manner. Delivering care using this approach in and of itself can be seen as advocacy to promote health and well-being within minoritized populations. Pediatricians are also encouraged to become engaged in local and national advocacy initiatives to have a broader effect in the fight for health equity in minority populations, including LGBTQ+ families and youth.
Pediatricians should work with their patients, families, and communities to advocate for structural change needed to address the social determinants of health for optimal growth and development.
Dr. Warus is an adolescent medicine physician who specializes in care for transgender and gender-nonconforming youth, and LGBTQ health for youth at Children’s Hospital of Los Angeles. He is an assistant professor of pediatrics at University of Southern California, Los Angeles.
Resources
Bright Futures – Promoting Healthy Development of Sexuality and Gender Identity (Implementation Tip Sheet): https://downloads.aap.org/AAP/PDF/BF_HealthySexualityGenderIdentity_Tipsheet.pdf
Bright Futures – Implementing Social Determinants of Health Into Health Supervision Visits (Implementation Tip Sheet): https://downloads.aap.org/AAP/PDF/Bright%20Futures/BF_IntegrateSDoH_Tipsheet.pdf?_ga=2.214227031.1330574154.1673910248-58875083.1673910248
American Academy of Pediatrics – Advocacy Website: https://www.aap.org/en/advocacy/
References
1. Hughes K et al. Lancet Public Health. 2017;2(8):e356-66.
2. Camero K and Javier JR. Pediatr Clin N Am. 2023;70:43-51.
3. Lund EM and Burgess CM. Prim Care Clin Office Pract. 2021;48:179-89.
4. Buffie WC. Am J Public Health. 2011;101(6):986-90.
5. Hatzenbuehler ML et al. Am J Public Health. 2010;100:452-9.
6. Raifman J et al. JAMA Pediatr. 2017;171(4):350-6.
7. Gonzales G and Blewett LA. Pediatrics. 2013;132(4):703-11.
8. Ogolsky BG et al. J Fam Psychol. 2019;33(4):422-32.
9. Library of Congress. H.R.8404 – 117th Congress (2021-2022): Respect for Marriage Act. 2022 Dec 13. www.congress.gov/bill/117th-congress/house-bill/8404/text.
10. Perrin EC and Committee on Psychosocial Aspects of Child and Family Health. Pediatrics. 2002;109(2):341-4.
11. Drabble LA et al. PLoS ONE. 2021;16(5):e0249125.
Childhood and adolescence are periods of life with rapid growth and development in which the psychosocial factors of one’s environment can have a profound effect on health. There is increasing evidence that adverse childhood experiences (ACEs) can have significant negative effects on long-term health with effects persisting into subsequent generations.1 Youth themselves, however, often do not have the voice, ability, or political power to advocate for safe and more supportive environments that are essential to their well-being. Thus, advocacy has been central to the profession of pediatrics since its inception, where providers can partner with their patients, families, and communities to push for changes in the environments in which youth live and grow.2
LGBTQ+ youth are known to be at increased risk for ACEs because of the stress that comes from being part of a minority group and the discrimination they experience by their families, communities, and society at large. These factors within their environments have been shown to be associated with increased rates of anxiety, depression, substance use, sexually transmitted infections, and homelessness.3 As with other health outcomes that have been linked to the social determinants of health, these disparities are not inevitable and could be greatly improved upon through advocacy and changes in the environments of LGBTQ+ youth.
Marriage equality (the recognition that same-sex couples have the same legal right to marry as opposite-sex couples) has been shown to be not only a political issue, but one that affects health. The debates surrounding marriage equality have contributed to minority stress by questioning the validity of same-sex relationships and assigning them less value relative to opposite-sex relationships.4 In 1996, the U.S. Congress passed the Defense of Marriage Act (DOMA), which federally defined marriage as being legally recognized only between opposite-sex couples.
Individual states then continued the marriage equality debate by passing individual state laws either allowing or prohibiting same-sex marriage. During this time, it was shown that, in states where same-sex marriage was legally prohibited, LGBTQ+ adults reported significantly higher rates of generalized anxiety disorder, alcohol use disorder, any mood disorder, and psychiatric comorbidity when compared with states without a legal ban on same-sex marriage.5
Using data from the Youth Risk Behavior Surveillance System, it was shown that state policies recognizing same-sex marriage were associated with a 7% relative reduction in suicide attempts reported by adolescent sexual minority students compared with before these policies.6 It was also shown that children with same-sex parents were overall less likely to have private health insurance, but this disparity was improved in states that legally recognized same-sex marriage and allowed second-parent adoptions.7
In 2013, the U.S. Supreme Court ruled that DOMA was unconstitutional, requiring the federal government to legally recognize same-sex marriages for the purposes of federal benefits. In 2015, the U.S. Supreme Court further ruled that same-sex couples are guaranteed the fundamental right to marry, requiring that all states issue marriage licenses to same-sex couples. These rulings were associated with a decrease in reported levels of stigma over time and increased reported levels of family support, particularly for those in same-sex relationships.8
The Respect for Marriage Act (RFMA) was passed by the U.S. Congress and signed into law by President Biden on Dec. 13, 2022. This law officially repeals DOMA and requires all states and the federal government to recognize same-sex marriages performed in any U.S. state or territory.9
If the U.S. Supreme Court were to overturn the 2015 marriage equality decision, individual state laws ensuring or banning same-sex marriage would again be in effect. However, the RFMA ensures that all states continue to recognize same-sex marriages performed in any U.S. state or territory (even if that state itself bans same-sex marriage). While we do not yet have any studies or data regarding the effect of the RFMA on public health, we can expect positive effects by drawing on the previous evidence on the effect of marriage equality and its effect on the health and well-being of LGBTQ+ individuals. By establishing marriage equality in the United States, our government institutions are affirming the relationships and identities of those in same-sex relationships, with the potential effect of helping to destigmatize the LGBTQ+ community.
Since 2002, the American Academy of Pediatrics has recommended that pediatricians “support the right of every child and family to the financial, psychological, and legal security that results from having legally recognized parents who are committed to each other and to the welfare of their children,” acknowledging that “legislative initiatives assuring legal status equivalent to marriage for gay and lesbian partners … can also attend to providing security and permanence for the children of those partnerships.”10 While changes in legal marriage equality are likely to have a positive effect on those within the LGBTQ+ community, it should also be understood that this will not solve all of the psychosocial effects and resultant health disparities that these children face.
A recent scoping review highlights that, as the result of marriage equality progress, sexual minority adults have reported increased social acceptance and reduced stigma across individual, community, and societal levels, but that sexual minority stigma continues to persist across all levels.11
As pediatricians, we can continue to support LGBTQ+ patients and parents by providing care in a safe and affirming environment in which families understand and embrace the healthy development of gender identity and sexuality in an open and destigmatized manner. Delivering care using this approach in and of itself can be seen as advocacy to promote health and well-being within minoritized populations. Pediatricians are also encouraged to become engaged in local and national advocacy initiatives to have a broader effect in the fight for health equity in minority populations, including LGBTQ+ families and youth.
Pediatricians should work with their patients, families, and communities to advocate for structural change needed to address the social determinants of health for optimal growth and development.
Dr. Warus is an adolescent medicine physician who specializes in care for transgender and gender-nonconforming youth, and LGBTQ health for youth at Children’s Hospital of Los Angeles. He is an assistant professor of pediatrics at University of Southern California, Los Angeles.
Resources
Bright Futures – Promoting Healthy Development of Sexuality and Gender Identity (Implementation Tip Sheet): https://downloads.aap.org/AAP/PDF/BF_HealthySexualityGenderIdentity_Tipsheet.pdf
Bright Futures – Implementing Social Determinants of Health Into Health Supervision Visits (Implementation Tip Sheet): https://downloads.aap.org/AAP/PDF/Bright%20Futures/BF_IntegrateSDoH_Tipsheet.pdf?_ga=2.214227031.1330574154.1673910248-58875083.1673910248
American Academy of Pediatrics – Advocacy Website: https://www.aap.org/en/advocacy/
References
1. Hughes K et al. Lancet Public Health. 2017;2(8):e356-66.
2. Camero K and Javier JR. Pediatr Clin N Am. 2023;70:43-51.
3. Lund EM and Burgess CM. Prim Care Clin Office Pract. 2021;48:179-89.
4. Buffie WC. Am J Public Health. 2011;101(6):986-90.
5. Hatzenbuehler ML et al. Am J Public Health. 2010;100:452-9.
6. Raifman J et al. JAMA Pediatr. 2017;171(4):350-6.
7. Gonzales G and Blewett LA. Pediatrics. 2013;132(4):703-11.
8. Ogolsky BG et al. J Fam Psychol. 2019;33(4):422-32.
9. Library of Congress. H.R.8404 – 117th Congress (2021-2022): Respect for Marriage Act. 2022 Dec 13. www.congress.gov/bill/117th-congress/house-bill/8404/text.
10. Perrin EC and Committee on Psychosocial Aspects of Child and Family Health. Pediatrics. 2002;109(2):341-4.
11. Drabble LA et al. PLoS ONE. 2021;16(5):e0249125.
CDC frets over further dip in kindergarten vaccination rates
The percentage of kindergarteners in the United States who have received routine vaccines to protect against illnesses such as measles, whooping cough, and polio has declined for 2 straight years, a new study has found.
Drops in vaccine coverage leave communities more susceptible to outbreaks of vaccine-preventable diseases, such as those that occurred in 2022, public health officials said.
Coverage for four vaccines – against measles, mumps, and rubella (MMR); diphtheria, tetanus, and acellular pertussis (DTaP); poliovirus; and varicella – among kindergarten students was about 95% in 2019-2020.
The rate fell to 94% the following year.
For the 2021-2022 school year, coverage dropped another point, to 93%, according to the report, published online in Morbidity and Mortality Weekly Report.
The rate of vaccination overall remains high, but about 250,000 kindergarten students may not be protected against measles, the researchers estimate. Measles, which is highly infectious, can lead to serious illness and even death in children who have not been vaccinated against the virus.
“In 2022, two communities in the United States responded to outbreaks of measles where children have been hospitalized,” Georgina Peacock, MD, MPH, director of the immunization services division of the Centers for Disease Control and Prevention, said in a media briefing about the report. “One community reported a case of paralytic polio in an unvaccinated person. These outbreaks were preventable. The best way to prevent these diseases and their devastating impact on children is through vaccination.”
Exemptions steady
For the new study, Ranee Seither, MPH, with the CDC’s National Center for Immunization and Respiratory Diseases and her colleagues analyzed data reported by states to estimate nationwide coverage for the four routine vaccines.
The number of students with exemptions remained low, at 2.6%, but another 3.9% who were without exemptions were not up to date with the MMR vaccine, the investigators report.
In a separate study, researchers found that vaccination coverage for 2-year-olds has increased. Approximately 70% of children were up to date with a seven-vaccine series by age 24 months. The coverage rate was higher for children born during 2018-2019 than for those born during 2016-2017.
Although the COVID-19 pandemic was not associated with decreased vaccination rates in this younger age group overall, coverage fell by 4-5 percentage points for children living below the poverty level or in rural areas, according to the study.
In addition, uninsured children were eight times more likely than those with private insurance to not be vaccinated by their second birthday, the researchers found.
Strategies to increase vaccination coverage include enforcing school vaccination requirements and holding vaccination clinics at schools, the CDC said.
“Providers should review children’s histories and recommend needed vaccinations during every clinical encounter and address parental hesitancy to help reduce disparities and ensure that all children are protected from vaccine-preventable diseases,” the agency said.
To that end, the agency launched an initiative this week called Let’s RISE (Routine Immunizations on Schedule for Everyone) to provide clinicians with resources to help patients get on track with their immunizations.
Hundreds of thousands unprotected
MMR vaccination coverage for kindergartners is the lowest it has been in over a decade, Dr. Peacock noted. Decreased coverage for kindergarten students might be tied to pandemic-related disruptions in health care systems and schools, she said. School administrators and parents may have been less focused on routine vaccination paperwork amid the return to in-person learning, for instance.
Hesitancy about COVID vaccines could be affecting routine vaccinations. “That’s something that we are watching very closely,” Dr. Peacock said.
The 2-point decrease in vaccination coverage “translates to hundreds of thousands of children starting school without being fully protected” against preventable diseases that can spread easily in classrooms, Sean O’Leary, MD, chair of the American Academy of Pediatrics’ Committee on Infectious Diseases, said.
Despite the drop in coverage, Dr. O’Leary said he saw some encouraging signs in the data: Nonmedical exemptions for kindergarten students have not increased. And the vast majority of parents are still having their children vaccinated. At the same time, the reports highlight a need to address child poverty and improve vaccine access in rural areas, he said.
A version of this article first appeared on Medscape.com.
The percentage of kindergarteners in the United States who have received routine vaccines to protect against illnesses such as measles, whooping cough, and polio has declined for 2 straight years, a new study has found.
Drops in vaccine coverage leave communities more susceptible to outbreaks of vaccine-preventable diseases, such as those that occurred in 2022, public health officials said.
Coverage for four vaccines – against measles, mumps, and rubella (MMR); diphtheria, tetanus, and acellular pertussis (DTaP); poliovirus; and varicella – among kindergarten students was about 95% in 2019-2020.
The rate fell to 94% the following year.
For the 2021-2022 school year, coverage dropped another point, to 93%, according to the report, published online in Morbidity and Mortality Weekly Report.
The rate of vaccination overall remains high, but about 250,000 kindergarten students may not be protected against measles, the researchers estimate. Measles, which is highly infectious, can lead to serious illness and even death in children who have not been vaccinated against the virus.
“In 2022, two communities in the United States responded to outbreaks of measles where children have been hospitalized,” Georgina Peacock, MD, MPH, director of the immunization services division of the Centers for Disease Control and Prevention, said in a media briefing about the report. “One community reported a case of paralytic polio in an unvaccinated person. These outbreaks were preventable. The best way to prevent these diseases and their devastating impact on children is through vaccination.”
Exemptions steady
For the new study, Ranee Seither, MPH, with the CDC’s National Center for Immunization and Respiratory Diseases and her colleagues analyzed data reported by states to estimate nationwide coverage for the four routine vaccines.
The number of students with exemptions remained low, at 2.6%, but another 3.9% who were without exemptions were not up to date with the MMR vaccine, the investigators report.
In a separate study, researchers found that vaccination coverage for 2-year-olds has increased. Approximately 70% of children were up to date with a seven-vaccine series by age 24 months. The coverage rate was higher for children born during 2018-2019 than for those born during 2016-2017.
Although the COVID-19 pandemic was not associated with decreased vaccination rates in this younger age group overall, coverage fell by 4-5 percentage points for children living below the poverty level or in rural areas, according to the study.
In addition, uninsured children were eight times more likely than those with private insurance to not be vaccinated by their second birthday, the researchers found.
Strategies to increase vaccination coverage include enforcing school vaccination requirements and holding vaccination clinics at schools, the CDC said.
“Providers should review children’s histories and recommend needed vaccinations during every clinical encounter and address parental hesitancy to help reduce disparities and ensure that all children are protected from vaccine-preventable diseases,” the agency said.
To that end, the agency launched an initiative this week called Let’s RISE (Routine Immunizations on Schedule for Everyone) to provide clinicians with resources to help patients get on track with their immunizations.
Hundreds of thousands unprotected
MMR vaccination coverage for kindergartners is the lowest it has been in over a decade, Dr. Peacock noted. Decreased coverage for kindergarten students might be tied to pandemic-related disruptions in health care systems and schools, she said. School administrators and parents may have been less focused on routine vaccination paperwork amid the return to in-person learning, for instance.
Hesitancy about COVID vaccines could be affecting routine vaccinations. “That’s something that we are watching very closely,” Dr. Peacock said.
The 2-point decrease in vaccination coverage “translates to hundreds of thousands of children starting school without being fully protected” against preventable diseases that can spread easily in classrooms, Sean O’Leary, MD, chair of the American Academy of Pediatrics’ Committee on Infectious Diseases, said.
Despite the drop in coverage, Dr. O’Leary said he saw some encouraging signs in the data: Nonmedical exemptions for kindergarten students have not increased. And the vast majority of parents are still having their children vaccinated. At the same time, the reports highlight a need to address child poverty and improve vaccine access in rural areas, he said.
A version of this article first appeared on Medscape.com.
The percentage of kindergarteners in the United States who have received routine vaccines to protect against illnesses such as measles, whooping cough, and polio has declined for 2 straight years, a new study has found.
Drops in vaccine coverage leave communities more susceptible to outbreaks of vaccine-preventable diseases, such as those that occurred in 2022, public health officials said.
Coverage for four vaccines – against measles, mumps, and rubella (MMR); diphtheria, tetanus, and acellular pertussis (DTaP); poliovirus; and varicella – among kindergarten students was about 95% in 2019-2020.
The rate fell to 94% the following year.
For the 2021-2022 school year, coverage dropped another point, to 93%, according to the report, published online in Morbidity and Mortality Weekly Report.
The rate of vaccination overall remains high, but about 250,000 kindergarten students may not be protected against measles, the researchers estimate. Measles, which is highly infectious, can lead to serious illness and even death in children who have not been vaccinated against the virus.
“In 2022, two communities in the United States responded to outbreaks of measles where children have been hospitalized,” Georgina Peacock, MD, MPH, director of the immunization services division of the Centers for Disease Control and Prevention, said in a media briefing about the report. “One community reported a case of paralytic polio in an unvaccinated person. These outbreaks were preventable. The best way to prevent these diseases and their devastating impact on children is through vaccination.”
Exemptions steady
For the new study, Ranee Seither, MPH, with the CDC’s National Center for Immunization and Respiratory Diseases and her colleagues analyzed data reported by states to estimate nationwide coverage for the four routine vaccines.
The number of students with exemptions remained low, at 2.6%, but another 3.9% who were without exemptions were not up to date with the MMR vaccine, the investigators report.
In a separate study, researchers found that vaccination coverage for 2-year-olds has increased. Approximately 70% of children were up to date with a seven-vaccine series by age 24 months. The coverage rate was higher for children born during 2018-2019 than for those born during 2016-2017.
Although the COVID-19 pandemic was not associated with decreased vaccination rates in this younger age group overall, coverage fell by 4-5 percentage points for children living below the poverty level or in rural areas, according to the study.
In addition, uninsured children were eight times more likely than those with private insurance to not be vaccinated by their second birthday, the researchers found.
Strategies to increase vaccination coverage include enforcing school vaccination requirements and holding vaccination clinics at schools, the CDC said.
“Providers should review children’s histories and recommend needed vaccinations during every clinical encounter and address parental hesitancy to help reduce disparities and ensure that all children are protected from vaccine-preventable diseases,” the agency said.
To that end, the agency launched an initiative this week called Let’s RISE (Routine Immunizations on Schedule for Everyone) to provide clinicians with resources to help patients get on track with their immunizations.
Hundreds of thousands unprotected
MMR vaccination coverage for kindergartners is the lowest it has been in over a decade, Dr. Peacock noted. Decreased coverage for kindergarten students might be tied to pandemic-related disruptions in health care systems and schools, she said. School administrators and parents may have been less focused on routine vaccination paperwork amid the return to in-person learning, for instance.
Hesitancy about COVID vaccines could be affecting routine vaccinations. “That’s something that we are watching very closely,” Dr. Peacock said.
The 2-point decrease in vaccination coverage “translates to hundreds of thousands of children starting school without being fully protected” against preventable diseases that can spread easily in classrooms, Sean O’Leary, MD, chair of the American Academy of Pediatrics’ Committee on Infectious Diseases, said.
Despite the drop in coverage, Dr. O’Leary said he saw some encouraging signs in the data: Nonmedical exemptions for kindergarten students have not increased. And the vast majority of parents are still having their children vaccinated. At the same time, the reports highlight a need to address child poverty and improve vaccine access in rural areas, he said.
A version of this article first appeared on Medscape.com.
FROM THE MMWR
Nearly 50% of patients with dementia experience falls
, suggests new research that also identifies multiple risk factors for these falls.
In a study of more than 5,500 participants, 45.5% of those with dementia experienced one or more falls, compared with 30.9% of their peers without dementia.
Vision impairment and living with a spouse were among the strongest predictors of future fall risk among participants living with dementia. Interestingly, high neighborhood social deprivation, which is reflected by such things as income and education, was associated with lower odds of falling.
Overall, the results highlight the need for a multidisciplinary approach to preventing falls among elderly individuals with dementia, said lead author Safiyyah M. Okoye, PhD, assistant professor, College of Nursing and Health Professions, Drexel University, Philadelphia.
“We need to consider different dimensions and figure out how we can try to go beyond the clinic in our interactions,” she said.
Dr. Okoye noted that in addition to reviewing medications that may contribute to falls and screening for vision problems, clinicians might also consider resources to improve the home environment and ensure that families have appropriate caregiving.
The findings were published online in Alzheimer’s and Dementia: The Journal of the Alzheimer’s Association.
No ‘silver bullet’
Every year, falls cause millions of injuries in older adults, and those with dementia are especially vulnerable. This population has twice the risk of falling and up to three times the risk of incurring serious fall-related injuries, such as fractures, the researchers noted.
Falls are a leading cause of hospitalization among those with dementia. Previous evidence has shown that persons with dementia are more likely to experience negative health consequences, such as delirium, while in hospital, compared with those without dementia. Even minor fall-related injuries are associated with the patient’s being discharged to a nursing home rather than returning home.
Dr. Okoye stressed that many factors contribute to falls, including health status; function, such as the ability to walk and balance; medications; home environment; and activity level.
“There are multidimensional aspects, and we can’t just find one silver bullet to address falls. It should be addressed comprehensively,” she said.
Existing studies “overwhelmingly” focus on factors related to health and function that could be addressed in the doctor’s office or with a referral, rather than on environmental and social factors, Dr. Okoye noted.
And even though the risk of falling is high among community-dwelling seniors with dementia, very few studies have addressed the risk of falls among these adults, she added.
The new analysis included a nationally representative sample of 5,581 community-dwelling adults who participated in both the 2015 and 2016 National Health and Aging Trends Study (NHATS). The NHATS is a population-based survey of health and disability trends and trajectories among Americans aged 65 years and older.
During interviews, participants were asked, personally or by proxy, about falls during the previous 12 months. Having fallen at baseline was evaluated as a possible predictor of falls in the subsequent 12 months.
To determine probable dementia, researchers asked whether a doctor had ever told the participants that they had dementia or Alzheimer’s disease. They also used a dementia screening questionnaire and neuropsychological tests of memory, orientation, and executive function.
Of the total sample, most (n = 5,093) did not have dementia.
Physical environmental factors that were assessed included conditions at home, such as clutter, tripping hazards, and structural issues, as well as neighborhood social and economic deprivation – such as income, education levels, and employment status.
Fall rates and counterintuitive findings
Results showed that significantly more of those with dementia than without experienced one or more falls (45.5% vs. 30.9%; P < .001).
In addition, a history of falling was significantly associated with subsequent falls among those with dementia (odds ratio, 6.20; 95% confidence interval, 3.81-10.09), as was vision impairment (OR, 2.22; 95% CI, 1.12-4.40) and living with a spouse versus alone (OR, 2.43; 95% CI, 1.09-5.43).
A possible explanation for higher fall risk among those living with a partner is that those living alone usually have better functioning, the investigators noted. Also, live-in partners tend to be of a similar age as the person with dementia and may have challenges of their own.
Interestingly, high neighborhood social deprivation was associated with lower odds of falling (OR, 0.55 for the highest deprivation scores; 95% CI, 0.31-0.98), a finding Dr. Okoye said was “counterintuitive.”
This result could be related to the social environment, she noted. “Maybe there are more people around in the house, more people with eyes on the person, or more people in the community who know the person. Despite the low economic resources, there could be social resources there,” she said.
The new findings underscore the idea that falling is a multidimensional phenomenon among older adults with dementia as well as those without dementia, Dr. Okoye noted.
Doctors can play a role in reducing falls among patients with dementia by asking about falls, possibly eliminating medications that are associated with risk of falling, and screening for and correcting vision and hearing impairments, she suggested.
They may also help determine household hazards for a patient, such as clutter and poor lighting, and ensure that these are addressed, Dr. Okoye added.
No surprise
Commenting on the study, David S. Knopman, MD, a clinical neurologist at Mayo Clinic, Rochester, Minn., said the finding that visual impairment and a prior history of falling are predictive of subsequent falls “comes as no surprise.”
Dr. Knopman, whose research focuses on late-life cognitive disorders, was not involved with the current study.
Risk reduction is “of course” a key management goal, he said. “Vigilance and optimizing the patient’s living space to reduce fall risks are the major strategies,” he added.
Dr. Knopman reiterated that falls among those with dementia are associated with higher mortality and often lead to loss of the capacity to live outside of an institution.
The study was supported by the National Institute on Aging. The investigators and Dr. Knopman report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, suggests new research that also identifies multiple risk factors for these falls.
In a study of more than 5,500 participants, 45.5% of those with dementia experienced one or more falls, compared with 30.9% of their peers without dementia.
Vision impairment and living with a spouse were among the strongest predictors of future fall risk among participants living with dementia. Interestingly, high neighborhood social deprivation, which is reflected by such things as income and education, was associated with lower odds of falling.
Overall, the results highlight the need for a multidisciplinary approach to preventing falls among elderly individuals with dementia, said lead author Safiyyah M. Okoye, PhD, assistant professor, College of Nursing and Health Professions, Drexel University, Philadelphia.
“We need to consider different dimensions and figure out how we can try to go beyond the clinic in our interactions,” she said.
Dr. Okoye noted that in addition to reviewing medications that may contribute to falls and screening for vision problems, clinicians might also consider resources to improve the home environment and ensure that families have appropriate caregiving.
The findings were published online in Alzheimer’s and Dementia: The Journal of the Alzheimer’s Association.
No ‘silver bullet’
Every year, falls cause millions of injuries in older adults, and those with dementia are especially vulnerable. This population has twice the risk of falling and up to three times the risk of incurring serious fall-related injuries, such as fractures, the researchers noted.
Falls are a leading cause of hospitalization among those with dementia. Previous evidence has shown that persons with dementia are more likely to experience negative health consequences, such as delirium, while in hospital, compared with those without dementia. Even minor fall-related injuries are associated with the patient’s being discharged to a nursing home rather than returning home.
Dr. Okoye stressed that many factors contribute to falls, including health status; function, such as the ability to walk and balance; medications; home environment; and activity level.
“There are multidimensional aspects, and we can’t just find one silver bullet to address falls. It should be addressed comprehensively,” she said.
Existing studies “overwhelmingly” focus on factors related to health and function that could be addressed in the doctor’s office or with a referral, rather than on environmental and social factors, Dr. Okoye noted.
And even though the risk of falling is high among community-dwelling seniors with dementia, very few studies have addressed the risk of falls among these adults, she added.
The new analysis included a nationally representative sample of 5,581 community-dwelling adults who participated in both the 2015 and 2016 National Health and Aging Trends Study (NHATS). The NHATS is a population-based survey of health and disability trends and trajectories among Americans aged 65 years and older.
During interviews, participants were asked, personally or by proxy, about falls during the previous 12 months. Having fallen at baseline was evaluated as a possible predictor of falls in the subsequent 12 months.
To determine probable dementia, researchers asked whether a doctor had ever told the participants that they had dementia or Alzheimer’s disease. They also used a dementia screening questionnaire and neuropsychological tests of memory, orientation, and executive function.
Of the total sample, most (n = 5,093) did not have dementia.
Physical environmental factors that were assessed included conditions at home, such as clutter, tripping hazards, and structural issues, as well as neighborhood social and economic deprivation – such as income, education levels, and employment status.
Fall rates and counterintuitive findings
Results showed that significantly more of those with dementia than without experienced one or more falls (45.5% vs. 30.9%; P < .001).
In addition, a history of falling was significantly associated with subsequent falls among those with dementia (odds ratio, 6.20; 95% confidence interval, 3.81-10.09), as was vision impairment (OR, 2.22; 95% CI, 1.12-4.40) and living with a spouse versus alone (OR, 2.43; 95% CI, 1.09-5.43).
A possible explanation for higher fall risk among those living with a partner is that those living alone usually have better functioning, the investigators noted. Also, live-in partners tend to be of a similar age as the person with dementia and may have challenges of their own.
Interestingly, high neighborhood social deprivation was associated with lower odds of falling (OR, 0.55 for the highest deprivation scores; 95% CI, 0.31-0.98), a finding Dr. Okoye said was “counterintuitive.”
This result could be related to the social environment, she noted. “Maybe there are more people around in the house, more people with eyes on the person, or more people in the community who know the person. Despite the low economic resources, there could be social resources there,” she said.
The new findings underscore the idea that falling is a multidimensional phenomenon among older adults with dementia as well as those without dementia, Dr. Okoye noted.
Doctors can play a role in reducing falls among patients with dementia by asking about falls, possibly eliminating medications that are associated with risk of falling, and screening for and correcting vision and hearing impairments, she suggested.
They may also help determine household hazards for a patient, such as clutter and poor lighting, and ensure that these are addressed, Dr. Okoye added.
No surprise
Commenting on the study, David S. Knopman, MD, a clinical neurologist at Mayo Clinic, Rochester, Minn., said the finding that visual impairment and a prior history of falling are predictive of subsequent falls “comes as no surprise.”
Dr. Knopman, whose research focuses on late-life cognitive disorders, was not involved with the current study.
Risk reduction is “of course” a key management goal, he said. “Vigilance and optimizing the patient’s living space to reduce fall risks are the major strategies,” he added.
Dr. Knopman reiterated that falls among those with dementia are associated with higher mortality and often lead to loss of the capacity to live outside of an institution.
The study was supported by the National Institute on Aging. The investigators and Dr. Knopman report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, suggests new research that also identifies multiple risk factors for these falls.
In a study of more than 5,500 participants, 45.5% of those with dementia experienced one or more falls, compared with 30.9% of their peers without dementia.
Vision impairment and living with a spouse were among the strongest predictors of future fall risk among participants living with dementia. Interestingly, high neighborhood social deprivation, which is reflected by such things as income and education, was associated with lower odds of falling.
Overall, the results highlight the need for a multidisciplinary approach to preventing falls among elderly individuals with dementia, said lead author Safiyyah M. Okoye, PhD, assistant professor, College of Nursing and Health Professions, Drexel University, Philadelphia.
“We need to consider different dimensions and figure out how we can try to go beyond the clinic in our interactions,” she said.
Dr. Okoye noted that in addition to reviewing medications that may contribute to falls and screening for vision problems, clinicians might also consider resources to improve the home environment and ensure that families have appropriate caregiving.
The findings were published online in Alzheimer’s and Dementia: The Journal of the Alzheimer’s Association.
No ‘silver bullet’
Every year, falls cause millions of injuries in older adults, and those with dementia are especially vulnerable. This population has twice the risk of falling and up to three times the risk of incurring serious fall-related injuries, such as fractures, the researchers noted.
Falls are a leading cause of hospitalization among those with dementia. Previous evidence has shown that persons with dementia are more likely to experience negative health consequences, such as delirium, while in hospital, compared with those without dementia. Even minor fall-related injuries are associated with the patient’s being discharged to a nursing home rather than returning home.
Dr. Okoye stressed that many factors contribute to falls, including health status; function, such as the ability to walk and balance; medications; home environment; and activity level.
“There are multidimensional aspects, and we can’t just find one silver bullet to address falls. It should be addressed comprehensively,” she said.
Existing studies “overwhelmingly” focus on factors related to health and function that could be addressed in the doctor’s office or with a referral, rather than on environmental and social factors, Dr. Okoye noted.
And even though the risk of falling is high among community-dwelling seniors with dementia, very few studies have addressed the risk of falls among these adults, she added.
The new analysis included a nationally representative sample of 5,581 community-dwelling adults who participated in both the 2015 and 2016 National Health and Aging Trends Study (NHATS). The NHATS is a population-based survey of health and disability trends and trajectories among Americans aged 65 years and older.
During interviews, participants were asked, personally or by proxy, about falls during the previous 12 months. Having fallen at baseline was evaluated as a possible predictor of falls in the subsequent 12 months.
To determine probable dementia, researchers asked whether a doctor had ever told the participants that they had dementia or Alzheimer’s disease. They also used a dementia screening questionnaire and neuropsychological tests of memory, orientation, and executive function.
Of the total sample, most (n = 5,093) did not have dementia.
Physical environmental factors that were assessed included conditions at home, such as clutter, tripping hazards, and structural issues, as well as neighborhood social and economic deprivation – such as income, education levels, and employment status.
Fall rates and counterintuitive findings
Results showed that significantly more of those with dementia than without experienced one or more falls (45.5% vs. 30.9%; P < .001).
In addition, a history of falling was significantly associated with subsequent falls among those with dementia (odds ratio, 6.20; 95% confidence interval, 3.81-10.09), as was vision impairment (OR, 2.22; 95% CI, 1.12-4.40) and living with a spouse versus alone (OR, 2.43; 95% CI, 1.09-5.43).
A possible explanation for higher fall risk among those living with a partner is that those living alone usually have better functioning, the investigators noted. Also, live-in partners tend to be of a similar age as the person with dementia and may have challenges of their own.
Interestingly, high neighborhood social deprivation was associated with lower odds of falling (OR, 0.55 for the highest deprivation scores; 95% CI, 0.31-0.98), a finding Dr. Okoye said was “counterintuitive.”
This result could be related to the social environment, she noted. “Maybe there are more people around in the house, more people with eyes on the person, or more people in the community who know the person. Despite the low economic resources, there could be social resources there,” she said.
The new findings underscore the idea that falling is a multidimensional phenomenon among older adults with dementia as well as those without dementia, Dr. Okoye noted.
Doctors can play a role in reducing falls among patients with dementia by asking about falls, possibly eliminating medications that are associated with risk of falling, and screening for and correcting vision and hearing impairments, she suggested.
They may also help determine household hazards for a patient, such as clutter and poor lighting, and ensure that these are addressed, Dr. Okoye added.
No surprise
Commenting on the study, David S. Knopman, MD, a clinical neurologist at Mayo Clinic, Rochester, Minn., said the finding that visual impairment and a prior history of falling are predictive of subsequent falls “comes as no surprise.”
Dr. Knopman, whose research focuses on late-life cognitive disorders, was not involved with the current study.
Risk reduction is “of course” a key management goal, he said. “Vigilance and optimizing the patient’s living space to reduce fall risks are the major strategies,” he added.
Dr. Knopman reiterated that falls among those with dementia are associated with higher mortality and often lead to loss of the capacity to live outside of an institution.
The study was supported by the National Institute on Aging. The investigators and Dr. Knopman report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ALZHEIMER’S AND DEMENTIA
Unintended consequences of perfectly good programs and policies
Some of our worst decisions seemed like really good ideas at the time. We wouldn’t make them otherwise; but often we fall into the unintended consequence of “the cure being worse than the poison.” We have seen this when government is trying to fix a problem, often an emotionally charged problem, without considering the long-term consequences of the “fix.” We have seen the unintended consequences of certain health care policies and programs lead to abuse and negative downstream effects on the same population that they were intended to protect.
It has been postulated that unintended consequences fall into a framework that’s “based upon level of knowledge and the scope for avoidance.” Essentially, that means these consequences fall into one of four categories: knowable and avoidable, knowable and unavoidable, unknowable and avoidable, and unknowable and unavoidable.
What category do the following policies fall into?
Pharmacy benefit managers’ safe harbor from the Anti-Kickback Statute
Let’s start with the “safe harbor” from the Anti-Kickback Statute (AKS) for payments from drug companies to health insurance companies and pharmacy benefit managers (PBMs). The AKS was created in 1972 and its “main purpose is to protect patients and the federal health care programs from fraud and abuse by curtailing the corrupting influence of money on health care decisions.” During the 1990s, a number of safe harbor provisions under the AKS were instituted for certain payments to health insurance companies, PBMs, and other providers. The thinking was that these payments needed a safe harbor because, although they might meet the statutory definition of “kickbacks,” they were beneficial because they would reduce the cost of care and, more specifically, the prices of drugs.
While well-intentioned, those safe harbors now protect a system of such perverse incentives that patients are whipsawed back and forth onto drugs that are the most profitable for the PBMs, who create the annual list of insurance covered drugs (i.e., the formulary). It is clear now that protected kickbacks ($$), in the form of rebates and fees paid by pharmaceutical manufacturers to PBMs, determine what drugs will be on the formulary. PBMs then use utilization management tools such as step therapy to force patients to take those drugs first. Consequently, safe harbor protection from the AKS allows manufacturers to buy market share at the expense of patient’s health. Because these protected kickbacks are based on a percentage of the list price of the drugs, PBMs profit more from higher priced drugs, which PBMs call the lowest cost medications (for them, that is). These bids from various manufacturers can change over the course of a year, allowing PBMs to change formulary coverage (even mid-year) and nonmedically switch stable patients to the drug that is the most profitable. Much of this happens as a result of the unintended consequence of this particular safe harbor from the AKS. Ironically, the safe harbor has helped to create the very behavior that the law was supposed to prevent and has harmed the patients it was supposed to protect. Health care decisions are being corrupted by the influence of profits allowed by safe harbor from the AKS.
340B drug program lacks oversight
Helping hospitals pay for care of the indigent: What could go wrong with that? The 340B Drug Pricing Program was created in 1992 to help low-income patients have better access to outpatient medications. The program requires drug companies to offer deep discounts to safety-net providers and qualified “disproportionate share hospitals,” which have a minimum percentage threshold of Medicare and Medicaid patients. The idea was that these qualified entities would pass these savings through to their low-income patients who needed the medications. Sounds like a great idea!
Apparently, there is a lot of money to be made under the 340B program because what started in 1992 with 90 covered entities had expanded by 2017 to more than 12,000 covered entities. The program became a profit center in part because reimbursement for 340B-acquired drugs far exceeds the acquisition costs. Over the years, in order to increase profits, qualified entities, such as disproportionate share hospitals, added for-profit contracted outpatient pharmacies, significantly increasing the amount of 340B drugs dispensed to commercial patients. From 2010 to 2020, the number of contract pharmacy arrangements increased from 2,000 to over 100,000, massively increasing profits for the qualified hospitals and their for-profit contracted pharmacies, which included a number of Fortune 25 companies.
Unfortunately, there is no oversight of 340B programs, and there are no requirements that the 340B drug profits be used for charitable care. In fact, nearly 10 years ago, two experts stated in Health Affairs that, “our findings support the criticism that the 340B program is being converted from one that serves vulnerable patient populations to one that enriches hospitals and their affiliated clinics.” In spite of the immense profits generated at 340B hospitals, an analysis by Avalere Health revealed that “65 percent of 340B hospitals provide less charity care than the national average for all short-term acute care hospitals, including for-profit hospitals.”
I have seen this dynamic at work in my own community in south Louisiana. There is a major expanding 340B hospital system that refuses Medicaid patients into its clinics once the hospital has reached its minimum disproportionate share of Medicaid patients. Our community has many young female African American patients with lupus, many of whom are covered by Medicaid. Even though this 340B hospital system has rheumatology fellows, it closes its rheumatology clinic doors to patients with lupus who have Medicaid as soon as it has reached its 11.75% of Medicaid patients. Clearly, this is an abuse of a program instituted specifically to take care of those in need – and here in our community, it creates inequitable access to rheumatologic care for patients with lupus.
The statute that created 340B specifically listed certain nonhospital providers who need – and should continue to receive – access to 340B discounts, such as Federally Qualified Health Centers and others. There are many deserving safety net providers and special disease clinics that are taking care of the truly needy and deserve to get the 340B highly discounted drug pricing. However, many so-called nonprofit hospital systems are spreading into wealthy neighborhoods with contracted pharmacies making large profits without caring for those in need. Five years ago, the U.S. Government Accountability Office stated that more oversight of the 340B program was needed, but that still hasn’t happened. The combination of vague statutory language and a lack of oversight has led to unintended consequences of fraud and abuse of the system, with indigent patients not realizing the benefit of the steep discounts, being sued when they can’t pay their bills, and even turned away from clinics when the qualified hospital reaches it mandated minimum of Medicaid patients.
Knowable and avoidable?
Should it have been known that these abuses would result from these policies and programs? And if so, could guardrails have been put in place from the start to avoid these abuses? Maybe the answers to these questions are irrelevant: All we can do now is fix what is not working, which will require changes and oversight to ensure that the safe harbor policy and 340B drug discount program are achieving the desired ends. At this point, unfortunately, it is clear that they’re not. In fact, it looks like they have enabled “profits over patients” all the way.
As recently stated by Dr. Megan Ranney of Brown University: “In this country, we continually forget that the profit motive is not sufficient for the public’s health.” Yes, hindsight is 20/20. But now we need to take off our blinders, see what is happening, and act to finally put “patients over profits.”
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
Some of our worst decisions seemed like really good ideas at the time. We wouldn’t make them otherwise; but often we fall into the unintended consequence of “the cure being worse than the poison.” We have seen this when government is trying to fix a problem, often an emotionally charged problem, without considering the long-term consequences of the “fix.” We have seen the unintended consequences of certain health care policies and programs lead to abuse and negative downstream effects on the same population that they were intended to protect.
It has been postulated that unintended consequences fall into a framework that’s “based upon level of knowledge and the scope for avoidance.” Essentially, that means these consequences fall into one of four categories: knowable and avoidable, knowable and unavoidable, unknowable and avoidable, and unknowable and unavoidable.
What category do the following policies fall into?
Pharmacy benefit managers’ safe harbor from the Anti-Kickback Statute
Let’s start with the “safe harbor” from the Anti-Kickback Statute (AKS) for payments from drug companies to health insurance companies and pharmacy benefit managers (PBMs). The AKS was created in 1972 and its “main purpose is to protect patients and the federal health care programs from fraud and abuse by curtailing the corrupting influence of money on health care decisions.” During the 1990s, a number of safe harbor provisions under the AKS were instituted for certain payments to health insurance companies, PBMs, and other providers. The thinking was that these payments needed a safe harbor because, although they might meet the statutory definition of “kickbacks,” they were beneficial because they would reduce the cost of care and, more specifically, the prices of drugs.
While well-intentioned, those safe harbors now protect a system of such perverse incentives that patients are whipsawed back and forth onto drugs that are the most profitable for the PBMs, who create the annual list of insurance covered drugs (i.e., the formulary). It is clear now that protected kickbacks ($$), in the form of rebates and fees paid by pharmaceutical manufacturers to PBMs, determine what drugs will be on the formulary. PBMs then use utilization management tools such as step therapy to force patients to take those drugs first. Consequently, safe harbor protection from the AKS allows manufacturers to buy market share at the expense of patient’s health. Because these protected kickbacks are based on a percentage of the list price of the drugs, PBMs profit more from higher priced drugs, which PBMs call the lowest cost medications (for them, that is). These bids from various manufacturers can change over the course of a year, allowing PBMs to change formulary coverage (even mid-year) and nonmedically switch stable patients to the drug that is the most profitable. Much of this happens as a result of the unintended consequence of this particular safe harbor from the AKS. Ironically, the safe harbor has helped to create the very behavior that the law was supposed to prevent and has harmed the patients it was supposed to protect. Health care decisions are being corrupted by the influence of profits allowed by safe harbor from the AKS.
340B drug program lacks oversight
Helping hospitals pay for care of the indigent: What could go wrong with that? The 340B Drug Pricing Program was created in 1992 to help low-income patients have better access to outpatient medications. The program requires drug companies to offer deep discounts to safety-net providers and qualified “disproportionate share hospitals,” which have a minimum percentage threshold of Medicare and Medicaid patients. The idea was that these qualified entities would pass these savings through to their low-income patients who needed the medications. Sounds like a great idea!
Apparently, there is a lot of money to be made under the 340B program because what started in 1992 with 90 covered entities had expanded by 2017 to more than 12,000 covered entities. The program became a profit center in part because reimbursement for 340B-acquired drugs far exceeds the acquisition costs. Over the years, in order to increase profits, qualified entities, such as disproportionate share hospitals, added for-profit contracted outpatient pharmacies, significantly increasing the amount of 340B drugs dispensed to commercial patients. From 2010 to 2020, the number of contract pharmacy arrangements increased from 2,000 to over 100,000, massively increasing profits for the qualified hospitals and their for-profit contracted pharmacies, which included a number of Fortune 25 companies.
Unfortunately, there is no oversight of 340B programs, and there are no requirements that the 340B drug profits be used for charitable care. In fact, nearly 10 years ago, two experts stated in Health Affairs that, “our findings support the criticism that the 340B program is being converted from one that serves vulnerable patient populations to one that enriches hospitals and their affiliated clinics.” In spite of the immense profits generated at 340B hospitals, an analysis by Avalere Health revealed that “65 percent of 340B hospitals provide less charity care than the national average for all short-term acute care hospitals, including for-profit hospitals.”
I have seen this dynamic at work in my own community in south Louisiana. There is a major expanding 340B hospital system that refuses Medicaid patients into its clinics once the hospital has reached its minimum disproportionate share of Medicaid patients. Our community has many young female African American patients with lupus, many of whom are covered by Medicaid. Even though this 340B hospital system has rheumatology fellows, it closes its rheumatology clinic doors to patients with lupus who have Medicaid as soon as it has reached its 11.75% of Medicaid patients. Clearly, this is an abuse of a program instituted specifically to take care of those in need – and here in our community, it creates inequitable access to rheumatologic care for patients with lupus.
The statute that created 340B specifically listed certain nonhospital providers who need – and should continue to receive – access to 340B discounts, such as Federally Qualified Health Centers and others. There are many deserving safety net providers and special disease clinics that are taking care of the truly needy and deserve to get the 340B highly discounted drug pricing. However, many so-called nonprofit hospital systems are spreading into wealthy neighborhoods with contracted pharmacies making large profits without caring for those in need. Five years ago, the U.S. Government Accountability Office stated that more oversight of the 340B program was needed, but that still hasn’t happened. The combination of vague statutory language and a lack of oversight has led to unintended consequences of fraud and abuse of the system, with indigent patients not realizing the benefit of the steep discounts, being sued when they can’t pay their bills, and even turned away from clinics when the qualified hospital reaches it mandated minimum of Medicaid patients.
Knowable and avoidable?
Should it have been known that these abuses would result from these policies and programs? And if so, could guardrails have been put in place from the start to avoid these abuses? Maybe the answers to these questions are irrelevant: All we can do now is fix what is not working, which will require changes and oversight to ensure that the safe harbor policy and 340B drug discount program are achieving the desired ends. At this point, unfortunately, it is clear that they’re not. In fact, it looks like they have enabled “profits over patients” all the way.
As recently stated by Dr. Megan Ranney of Brown University: “In this country, we continually forget that the profit motive is not sufficient for the public’s health.” Yes, hindsight is 20/20. But now we need to take off our blinders, see what is happening, and act to finally put “patients over profits.”
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
Some of our worst decisions seemed like really good ideas at the time. We wouldn’t make them otherwise; but often we fall into the unintended consequence of “the cure being worse than the poison.” We have seen this when government is trying to fix a problem, often an emotionally charged problem, without considering the long-term consequences of the “fix.” We have seen the unintended consequences of certain health care policies and programs lead to abuse and negative downstream effects on the same population that they were intended to protect.
It has been postulated that unintended consequences fall into a framework that’s “based upon level of knowledge and the scope for avoidance.” Essentially, that means these consequences fall into one of four categories: knowable and avoidable, knowable and unavoidable, unknowable and avoidable, and unknowable and unavoidable.
What category do the following policies fall into?
Pharmacy benefit managers’ safe harbor from the Anti-Kickback Statute
Let’s start with the “safe harbor” from the Anti-Kickback Statute (AKS) for payments from drug companies to health insurance companies and pharmacy benefit managers (PBMs). The AKS was created in 1972 and its “main purpose is to protect patients and the federal health care programs from fraud and abuse by curtailing the corrupting influence of money on health care decisions.” During the 1990s, a number of safe harbor provisions under the AKS were instituted for certain payments to health insurance companies, PBMs, and other providers. The thinking was that these payments needed a safe harbor because, although they might meet the statutory definition of “kickbacks,” they were beneficial because they would reduce the cost of care and, more specifically, the prices of drugs.
While well-intentioned, those safe harbors now protect a system of such perverse incentives that patients are whipsawed back and forth onto drugs that are the most profitable for the PBMs, who create the annual list of insurance covered drugs (i.e., the formulary). It is clear now that protected kickbacks ($$), in the form of rebates and fees paid by pharmaceutical manufacturers to PBMs, determine what drugs will be on the formulary. PBMs then use utilization management tools such as step therapy to force patients to take those drugs first. Consequently, safe harbor protection from the AKS allows manufacturers to buy market share at the expense of patient’s health. Because these protected kickbacks are based on a percentage of the list price of the drugs, PBMs profit more from higher priced drugs, which PBMs call the lowest cost medications (for them, that is). These bids from various manufacturers can change over the course of a year, allowing PBMs to change formulary coverage (even mid-year) and nonmedically switch stable patients to the drug that is the most profitable. Much of this happens as a result of the unintended consequence of this particular safe harbor from the AKS. Ironically, the safe harbor has helped to create the very behavior that the law was supposed to prevent and has harmed the patients it was supposed to protect. Health care decisions are being corrupted by the influence of profits allowed by safe harbor from the AKS.
340B drug program lacks oversight
Helping hospitals pay for care of the indigent: What could go wrong with that? The 340B Drug Pricing Program was created in 1992 to help low-income patients have better access to outpatient medications. The program requires drug companies to offer deep discounts to safety-net providers and qualified “disproportionate share hospitals,” which have a minimum percentage threshold of Medicare and Medicaid patients. The idea was that these qualified entities would pass these savings through to their low-income patients who needed the medications. Sounds like a great idea!
Apparently, there is a lot of money to be made under the 340B program because what started in 1992 with 90 covered entities had expanded by 2017 to more than 12,000 covered entities. The program became a profit center in part because reimbursement for 340B-acquired drugs far exceeds the acquisition costs. Over the years, in order to increase profits, qualified entities, such as disproportionate share hospitals, added for-profit contracted outpatient pharmacies, significantly increasing the amount of 340B drugs dispensed to commercial patients. From 2010 to 2020, the number of contract pharmacy arrangements increased from 2,000 to over 100,000, massively increasing profits for the qualified hospitals and their for-profit contracted pharmacies, which included a number of Fortune 25 companies.
Unfortunately, there is no oversight of 340B programs, and there are no requirements that the 340B drug profits be used for charitable care. In fact, nearly 10 years ago, two experts stated in Health Affairs that, “our findings support the criticism that the 340B program is being converted from one that serves vulnerable patient populations to one that enriches hospitals and their affiliated clinics.” In spite of the immense profits generated at 340B hospitals, an analysis by Avalere Health revealed that “65 percent of 340B hospitals provide less charity care than the national average for all short-term acute care hospitals, including for-profit hospitals.”
I have seen this dynamic at work in my own community in south Louisiana. There is a major expanding 340B hospital system that refuses Medicaid patients into its clinics once the hospital has reached its minimum disproportionate share of Medicaid patients. Our community has many young female African American patients with lupus, many of whom are covered by Medicaid. Even though this 340B hospital system has rheumatology fellows, it closes its rheumatology clinic doors to patients with lupus who have Medicaid as soon as it has reached its 11.75% of Medicaid patients. Clearly, this is an abuse of a program instituted specifically to take care of those in need – and here in our community, it creates inequitable access to rheumatologic care for patients with lupus.
The statute that created 340B specifically listed certain nonhospital providers who need – and should continue to receive – access to 340B discounts, such as Federally Qualified Health Centers and others. There are many deserving safety net providers and special disease clinics that are taking care of the truly needy and deserve to get the 340B highly discounted drug pricing. However, many so-called nonprofit hospital systems are spreading into wealthy neighborhoods with contracted pharmacies making large profits without caring for those in need. Five years ago, the U.S. Government Accountability Office stated that more oversight of the 340B program was needed, but that still hasn’t happened. The combination of vague statutory language and a lack of oversight has led to unintended consequences of fraud and abuse of the system, with indigent patients not realizing the benefit of the steep discounts, being sued when they can’t pay their bills, and even turned away from clinics when the qualified hospital reaches it mandated minimum of Medicaid patients.
Knowable and avoidable?
Should it have been known that these abuses would result from these policies and programs? And if so, could guardrails have been put in place from the start to avoid these abuses? Maybe the answers to these questions are irrelevant: All we can do now is fix what is not working, which will require changes and oversight to ensure that the safe harbor policy and 340B drug discount program are achieving the desired ends. At this point, unfortunately, it is clear that they’re not. In fact, it looks like they have enabled “profits over patients” all the way.
As recently stated by Dr. Megan Ranney of Brown University: “In this country, we continually forget that the profit motive is not sufficient for the public’s health.” Yes, hindsight is 20/20. But now we need to take off our blinders, see what is happening, and act to finally put “patients over profits.”
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
Medication Overuse Headache (MOH): Prevention and Treatment
Medication overuse headache, previously known as rebound headache or medication-induced headache, may be caused by the frequent or excessive use of various acute care medications. When these medications are used too frequently, they can cause headaches rather than relieving them. (Some headache specialists feel that MOH is the result of recurring severe headaches, and the patients’ overuse of medications to relieve them.) These medications, some of which are painkillers or analgesics, include over-the-counter products such as acetaminophen, aspirin, and anti-inflammatories, as well as prescription medications such as triptans, ergots opioids, opioids, and barbiturates. The one category of acute care medication that does not seem to cause MOH is the gepants, such as rimegepant and ubrogepant.
MOH is the fourth most common headache disorder. It is defined by the International Classification of Headache Disorders (ICHD-3) as a headache present 15 days per month, evolving from regular use of strong acute medication (10 or more days of triptans, ergotamines, butalbital medications, opioids, or combination medications or 15 or more days per month of simple analgesics such as aspirin, acetaminophen, or nonsteroidal anti-inflammatories) for 3 months.
Patients are usually not aware they have MOH, and this is the most problematic aspect of the condition. Patients do not realize that the medicine they are taking is making their headaches worse. It can be difficult to explain to the patient exactly what is going on with MOH, and why they are doing the wrong thing by taking the very medication that was prescribed by their doctor to stop a migraine attack. Many doctors do not fully understand MOH either, which can make it difficult to treat patients with this type of headache; therefore, it is imperative to educate both doctors and patients on the causes and treatments of MOH.
One of the most important facets of treating MOH traditionally has been the process of detoxifying patients from their overused medication by gradually or precipitously withdrawing the offending medication. There is variability in how detoxification can be accomplished. Some of my patients stopped medications abruptly and experienced very bad headaches. Others tried reducing dosages on their own and reported experiencing the worst headaches of their lives—some of which lasted for a few weeks. I have found that if patients can endure 2 to 3 weeks of detox, they start to feel better. But because the headaches can worsen before they get better, patients understandably try to avoid the detoxification process.
I start patients on preventive medicine, then slowly increase it to an effective dose, and have them come back in a month for an evaluation. I then have them gradually reduce, but not completely stop, the pain medication before they return. Once I feel their preventive medication is at a therapeutic level, I have them begin a slow detox. After a month of preventive medication, there is a reasonable chance that headaches will start to decrease and be less severe. I tell them that if their headache is less severe try to avoid taking the medicine that they were overusing to prevent perpetuating the MOH.
One plausible physiologic mechanism behind MOH is that chronic exposure to acute care migraine treatment leads to suppression of the serotonergic/norepinephrinergic endogenous antinociceptive system in the upper brain stem, with facilitation of the trigeminal nociceptive process via up-regulation of calcitonin gene-related peptide (CGRP).This increase in CGRP at the end of peripheral nerve terminals in the trigeminovascular system may facilitate pain transmission. An increase in cortical CGRP may cause cortical spreading depression: a wave of excitement traveling through the cortex, followed by a wave of electrical depression seems to cause headache.
Good, effective prevention often helps avoid MOH; medications such as topiramate, nortriptyline, gabapentin, onabotulinumtoxinA, and CGRP monoclonal antibodies or some type of local nerve block have improved MOH in patients, but detoxification is usually necessary is some patients.
Monoclonal antibodies targeting CGRP or its receptor (CGRP-R), given by subcutaneous or intravenous injection or small molecule CGRP receptor antagonists given orally (gepants), seem to be able to treat MOH in some patients without a detoxification. This has been best demonstrated in the monoclonal antibody group, but there is some evidence showing that it may also occur with gepants. These treatments seem to work even when patients are overusing acute care medications; this helps some patients to self-detoxify at their own pace, which is easier for both the patient and the doctor.
Currently, there are 4 monoclonal antibodies against CGRP or the CGRP-R. Erenumab is the only completely human one and the only antibody that blocks the CGRP receptor to prevent the CGRP ligand from docking and exerting its effect. The other 3 (fremanezumab, galcanezumab, and eptinezumab) are humanized monoclonal antibodies that selectively bind to the CGRP ligand, preventing it from docking on its receptor. Patients started on the monoclonal antibodies against CGRP or its receptor usually have fewer headaches in the first week or two of therapy, and this helps make the self-detox easier for the patient.
Further, substantial data have shown that onabotulinumtoxinA reduces the number/frequency of headaches and reduces the need for patients to take acute medication. OnabotulinumtoxinA is currently the only medication approved for preventive treatment of chronic migraine; it has long-term safety data available and has reported efficacy lasting for up to 3 years when given in multiple injection sites every 3 months. Interestingly, although topiramate is used as a preventive medication, a recent study comparing erenumab vs topiramate for reducing monthly migraine days (MMD) showed that erenumab outperformed topiramate with a 50% reduction in MMD, and with fewer reported adverse events.
We are just starting to learn about some other potential cellular mechanisms that could be causing MOH in patients; these data could help create new and improved therapies for treating and possibly preventing MOH in the future. Patient outcomes could also be improved by encouraging the inclusion of MOH as part of a continuing education program for physicians who could potentially be treating new patients presenting with MOH.
Medication overuse headache, previously known as rebound headache or medication-induced headache, may be caused by the frequent or excessive use of various acute care medications. When these medications are used too frequently, they can cause headaches rather than relieving them. (Some headache specialists feel that MOH is the result of recurring severe headaches, and the patients’ overuse of medications to relieve them.) These medications, some of which are painkillers or analgesics, include over-the-counter products such as acetaminophen, aspirin, and anti-inflammatories, as well as prescription medications such as triptans, ergots opioids, opioids, and barbiturates. The one category of acute care medication that does not seem to cause MOH is the gepants, such as rimegepant and ubrogepant.
MOH is the fourth most common headache disorder. It is defined by the International Classification of Headache Disorders (ICHD-3) as a headache present 15 days per month, evolving from regular use of strong acute medication (10 or more days of triptans, ergotamines, butalbital medications, opioids, or combination medications or 15 or more days per month of simple analgesics such as aspirin, acetaminophen, or nonsteroidal anti-inflammatories) for 3 months.
Patients are usually not aware they have MOH, and this is the most problematic aspect of the condition. Patients do not realize that the medicine they are taking is making their headaches worse. It can be difficult to explain to the patient exactly what is going on with MOH, and why they are doing the wrong thing by taking the very medication that was prescribed by their doctor to stop a migraine attack. Many doctors do not fully understand MOH either, which can make it difficult to treat patients with this type of headache; therefore, it is imperative to educate both doctors and patients on the causes and treatments of MOH.
One of the most important facets of treating MOH traditionally has been the process of detoxifying patients from their overused medication by gradually or precipitously withdrawing the offending medication. There is variability in how detoxification can be accomplished. Some of my patients stopped medications abruptly and experienced very bad headaches. Others tried reducing dosages on their own and reported experiencing the worst headaches of their lives—some of which lasted for a few weeks. I have found that if patients can endure 2 to 3 weeks of detox, they start to feel better. But because the headaches can worsen before they get better, patients understandably try to avoid the detoxification process.
I start patients on preventive medicine, then slowly increase it to an effective dose, and have them come back in a month for an evaluation. I then have them gradually reduce, but not completely stop, the pain medication before they return. Once I feel their preventive medication is at a therapeutic level, I have them begin a slow detox. After a month of preventive medication, there is a reasonable chance that headaches will start to decrease and be less severe. I tell them that if their headache is less severe try to avoid taking the medicine that they were overusing to prevent perpetuating the MOH.
One plausible physiologic mechanism behind MOH is that chronic exposure to acute care migraine treatment leads to suppression of the serotonergic/norepinephrinergic endogenous antinociceptive system in the upper brain stem, with facilitation of the trigeminal nociceptive process via up-regulation of calcitonin gene-related peptide (CGRP).This increase in CGRP at the end of peripheral nerve terminals in the trigeminovascular system may facilitate pain transmission. An increase in cortical CGRP may cause cortical spreading depression: a wave of excitement traveling through the cortex, followed by a wave of electrical depression seems to cause headache.
Good, effective prevention often helps avoid MOH; medications such as topiramate, nortriptyline, gabapentin, onabotulinumtoxinA, and CGRP monoclonal antibodies or some type of local nerve block have improved MOH in patients, but detoxification is usually necessary is some patients.
Monoclonal antibodies targeting CGRP or its receptor (CGRP-R), given by subcutaneous or intravenous injection or small molecule CGRP receptor antagonists given orally (gepants), seem to be able to treat MOH in some patients without a detoxification. This has been best demonstrated in the monoclonal antibody group, but there is some evidence showing that it may also occur with gepants. These treatments seem to work even when patients are overusing acute care medications; this helps some patients to self-detoxify at their own pace, which is easier for both the patient and the doctor.
Currently, there are 4 monoclonal antibodies against CGRP or the CGRP-R. Erenumab is the only completely human one and the only antibody that blocks the CGRP receptor to prevent the CGRP ligand from docking and exerting its effect. The other 3 (fremanezumab, galcanezumab, and eptinezumab) are humanized monoclonal antibodies that selectively bind to the CGRP ligand, preventing it from docking on its receptor. Patients started on the monoclonal antibodies against CGRP or its receptor usually have fewer headaches in the first week or two of therapy, and this helps make the self-detox easier for the patient.
Further, substantial data have shown that onabotulinumtoxinA reduces the number/frequency of headaches and reduces the need for patients to take acute medication. OnabotulinumtoxinA is currently the only medication approved for preventive treatment of chronic migraine; it has long-term safety data available and has reported efficacy lasting for up to 3 years when given in multiple injection sites every 3 months. Interestingly, although topiramate is used as a preventive medication, a recent study comparing erenumab vs topiramate for reducing monthly migraine days (MMD) showed that erenumab outperformed topiramate with a 50% reduction in MMD, and with fewer reported adverse events.
We are just starting to learn about some other potential cellular mechanisms that could be causing MOH in patients; these data could help create new and improved therapies for treating and possibly preventing MOH in the future. Patient outcomes could also be improved by encouraging the inclusion of MOH as part of a continuing education program for physicians who could potentially be treating new patients presenting with MOH.
Medication overuse headache, previously known as rebound headache or medication-induced headache, may be caused by the frequent or excessive use of various acute care medications. When these medications are used too frequently, they can cause headaches rather than relieving them. (Some headache specialists feel that MOH is the result of recurring severe headaches, and the patients’ overuse of medications to relieve them.) These medications, some of which are painkillers or analgesics, include over-the-counter products such as acetaminophen, aspirin, and anti-inflammatories, as well as prescription medications such as triptans, ergots opioids, opioids, and barbiturates. The one category of acute care medication that does not seem to cause MOH is the gepants, such as rimegepant and ubrogepant.
MOH is the fourth most common headache disorder. It is defined by the International Classification of Headache Disorders (ICHD-3) as a headache present 15 days per month, evolving from regular use of strong acute medication (10 or more days of triptans, ergotamines, butalbital medications, opioids, or combination medications or 15 or more days per month of simple analgesics such as aspirin, acetaminophen, or nonsteroidal anti-inflammatories) for 3 months.
Patients are usually not aware they have MOH, and this is the most problematic aspect of the condition. Patients do not realize that the medicine they are taking is making their headaches worse. It can be difficult to explain to the patient exactly what is going on with MOH, and why they are doing the wrong thing by taking the very medication that was prescribed by their doctor to stop a migraine attack. Many doctors do not fully understand MOH either, which can make it difficult to treat patients with this type of headache; therefore, it is imperative to educate both doctors and patients on the causes and treatments of MOH.
One of the most important facets of treating MOH traditionally has been the process of detoxifying patients from their overused medication by gradually or precipitously withdrawing the offending medication. There is variability in how detoxification can be accomplished. Some of my patients stopped medications abruptly and experienced very bad headaches. Others tried reducing dosages on their own and reported experiencing the worst headaches of their lives—some of which lasted for a few weeks. I have found that if patients can endure 2 to 3 weeks of detox, they start to feel better. But because the headaches can worsen before they get better, patients understandably try to avoid the detoxification process.
I start patients on preventive medicine, then slowly increase it to an effective dose, and have them come back in a month for an evaluation. I then have them gradually reduce, but not completely stop, the pain medication before they return. Once I feel their preventive medication is at a therapeutic level, I have them begin a slow detox. After a month of preventive medication, there is a reasonable chance that headaches will start to decrease and be less severe. I tell them that if their headache is less severe try to avoid taking the medicine that they were overusing to prevent perpetuating the MOH.
One plausible physiologic mechanism behind MOH is that chronic exposure to acute care migraine treatment leads to suppression of the serotonergic/norepinephrinergic endogenous antinociceptive system in the upper brain stem, with facilitation of the trigeminal nociceptive process via up-regulation of calcitonin gene-related peptide (CGRP).This increase in CGRP at the end of peripheral nerve terminals in the trigeminovascular system may facilitate pain transmission. An increase in cortical CGRP may cause cortical spreading depression: a wave of excitement traveling through the cortex, followed by a wave of electrical depression seems to cause headache.
Good, effective prevention often helps avoid MOH; medications such as topiramate, nortriptyline, gabapentin, onabotulinumtoxinA, and CGRP monoclonal antibodies or some type of local nerve block have improved MOH in patients, but detoxification is usually necessary is some patients.
Monoclonal antibodies targeting CGRP or its receptor (CGRP-R), given by subcutaneous or intravenous injection or small molecule CGRP receptor antagonists given orally (gepants), seem to be able to treat MOH in some patients without a detoxification. This has been best demonstrated in the monoclonal antibody group, but there is some evidence showing that it may also occur with gepants. These treatments seem to work even when patients are overusing acute care medications; this helps some patients to self-detoxify at their own pace, which is easier for both the patient and the doctor.
Currently, there are 4 monoclonal antibodies against CGRP or the CGRP-R. Erenumab is the only completely human one and the only antibody that blocks the CGRP receptor to prevent the CGRP ligand from docking and exerting its effect. The other 3 (fremanezumab, galcanezumab, and eptinezumab) are humanized monoclonal antibodies that selectively bind to the CGRP ligand, preventing it from docking on its receptor. Patients started on the monoclonal antibodies against CGRP or its receptor usually have fewer headaches in the first week or two of therapy, and this helps make the self-detox easier for the patient.
Further, substantial data have shown that onabotulinumtoxinA reduces the number/frequency of headaches and reduces the need for patients to take acute medication. OnabotulinumtoxinA is currently the only medication approved for preventive treatment of chronic migraine; it has long-term safety data available and has reported efficacy lasting for up to 3 years when given in multiple injection sites every 3 months. Interestingly, although topiramate is used as a preventive medication, a recent study comparing erenumab vs topiramate for reducing monthly migraine days (MMD) showed that erenumab outperformed topiramate with a 50% reduction in MMD, and with fewer reported adverse events.
We are just starting to learn about some other potential cellular mechanisms that could be causing MOH in patients; these data could help create new and improved therapies for treating and possibly preventing MOH in the future. Patient outcomes could also be improved by encouraging the inclusion of MOH as part of a continuing education program for physicians who could potentially be treating new patients presenting with MOH.
AD outcomes improved with lebrikizumab and topical steroids
, according to results of the 16-week phase 3 ADhere trial.
“Lebrikizumab, a monoclonal antibody inhibiting interleukin-13, combined with TCS was associated with reduced overall disease severity of moderate to severe AD in adolescents and adults, and had a safety profile consistent with previous lebrikizumab AD studies,” noted lead author Eric L. Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland, and coauthors in their article on the study, which was published in JAMA Dermatology.
The double-blind trial, conducted at 54 sites across Germany, Poland, Canada, and the United States, included 211 patients, mean age 37.2 years, of whom 48.8% were female and roughly 22% were adolescents. Almost 15% were Asian, and about 13% were Black.
At baseline, participants had a score of 16 or higher on the Eczema Area and Severity Index (EASI), a score of 3 or higher on the Investigator’s Global Assessment (IGA) scale, AD covering a body surface area of 10% or greater, and a history of inadequate response to treatment with topical medications.
After a minimum 1-week washout period from topical and systemic therapy, participants were randomized in a 2:1 ratio to receive lebrikizumab plus TCS (n = 145) or placebo plus TCS (n = 66) for 16 weeks.
Lebrikizumab or placebo was administered by subcutaneous injection every 2 weeks; the loading and week-2 doses of lebrikizumab were 500 mg, followed by 250 mg thereafter. All patients were instructed to use low- to mid-potency TCS at their own discretion. Study sites provided a mid-potency TCS (triamcinolone acetonide 0.1% cream) and a low-potency TCS (hydrocortisone 1% cream), with topical calcineurin inhibitors permitted for sensitive skin areas.
Primary outcomes at 16 weeks included a 2-point or more reduction in IGA score from baseline and EASI-75 response. Patients in the lebrikizumab arm had superior responses on both of these outcomes, with statistical significance achieved as early as week 8 and week 4, respectively, and maintained through week 16. Specifically, 41.2% of those treated with lebrikizumab had an IGA reduction of 2 points or more, compared with 22.1% of those receiving placebo plus TCS (P = .01), and the proportion of patients achieving EASI-75 responses was 69.5% vs. 42.2%, respectively (P < .001).
Patients treated with lebrikizumab also showed statistically significant improvements, compared with TCS alone in all key secondary endpoints, “including skin clearance, improvement in itch, itch interference on sleep, and enhanced QoL [quality of life],” noted the authors. “This study captured the clinical benefit of lebrikizumab through the combined end point of physician-assessed clinical sign of skin clearance (EASI-75) and patient-reported outcome of improvement in itch (Pruritus NRS).”
The percentage of patients who achieved the combined endpoint was more than double for the lebrikizumab plus TCS group vs. the group on TCS alone, indicating that patients treated with lebrikizumab plus TCS “were more likely to experience improvement in skin symptoms and itch,” the investigators added.
The authors noted that most treatment-emergent adverse events “were nonserious, mild, or moderate in severity, and did not lead to study discontinuation.” These included conjunctivitis (4.8%), headache (4.8%), hypertension (2.8%), injection-site reactions (2.8%), and herpes infection (3.4%) – all of which occurred in 1.5% or less of patients in the placebo group.
“The higher incidence of conjunctivitis has also been reported in other biologics inhibiting IL [interleukin]–13 and/or IL-4 signaling, as well as lebrikizumab monotherapy studies,” they noted. The 4.8% rate of conjunctivitis reported in the combination study, they added, is “compared with 7.5% frequency in 16-week data from the lebrikizumab monotherapy studies. Although the mechanism remains unclear, it has been reported that conjunctival goblet cell scarcity due to IL-13 and IL-4 inhibition, and subsequent effects on the homeostasis of the conjunctival mucosal surface, results in ocular AEs [adverse events].”
“This truly is a time of great hope and promise for our patients with AD,” commented Zelma Chiesa Fuxench, MD, who was not involved in the study. “The advent of newer, targeted therapeutic agents for AD continues to revolutionize the treatment experience for our patients, offering the possibility of greater AD disease control with a favorable risk profile and less need for blood work monitoring compared to traditional systemic agents.”
On the basis of the study results, Dr. Chiesa Fuxench, of the department of dermatology at the University of Pennsylvania, Philadelphia, said in an interview that “lebrikizumab represents an additional option in the treatment armamentarium for providers who care for patients with AD.” She added that, “while head-to-head trials comparing lebrikizumab to dupilumab, the first FDA-approved biologic for AD, would be beneficial, to the best of my knowledge this data is currently lacking. However, based on the results of this study, we would expect lebrikizumab to work at least similarly to dupilumab, based on the reported improvements in IGA and EASI score.”
Additionally, lebrikizumab showed a favorable safety profile, “with most treatment-emergent adverse effects reported as nonserious and not leading to drug discontinuation,” she said. “Of interest to clinicians may be the reported rates of conjunctivitis in this study. Rates of conjunctivitis for lebrikizumab appear to be lower than those reported in the LIBERTY AD CHRONOS study for dupilumab – a finding that merits further scrutiny in my opinion, as this one of the most frequent treatment-emergent adverse events that I encounter in my clinical practice.”
The study was funded by Dermira, a subsidiary of Eli Lilly. Dr. Simpson reported personal fees and grants from multiple sources, including Dermira and Eli Lilly, the companies developing lebrikizumab. Several authors were employees of Eli Lilly. Dr. Fuxench disclosed serving as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, Pfizer, AbbVie, and Incyte, for which she has received honoraria for AD-related work. She is the recipient of research grants through Regeneron, Sanofi, Tioga, Vanda, Menlo Therapeutics, Leo Pharma, and Eli Lilly for work related to AD as well as honoraria for continuing medical education work related to AD sponsored through educational grants from Regeneron/Sanofi and Pfizer.
A version of this article first appeared on Medscape.com.
, according to results of the 16-week phase 3 ADhere trial.
“Lebrikizumab, a monoclonal antibody inhibiting interleukin-13, combined with TCS was associated with reduced overall disease severity of moderate to severe AD in adolescents and adults, and had a safety profile consistent with previous lebrikizumab AD studies,” noted lead author Eric L. Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland, and coauthors in their article on the study, which was published in JAMA Dermatology.
The double-blind trial, conducted at 54 sites across Germany, Poland, Canada, and the United States, included 211 patients, mean age 37.2 years, of whom 48.8% were female and roughly 22% were adolescents. Almost 15% were Asian, and about 13% were Black.
At baseline, participants had a score of 16 or higher on the Eczema Area and Severity Index (EASI), a score of 3 or higher on the Investigator’s Global Assessment (IGA) scale, AD covering a body surface area of 10% or greater, and a history of inadequate response to treatment with topical medications.
After a minimum 1-week washout period from topical and systemic therapy, participants were randomized in a 2:1 ratio to receive lebrikizumab plus TCS (n = 145) or placebo plus TCS (n = 66) for 16 weeks.
Lebrikizumab or placebo was administered by subcutaneous injection every 2 weeks; the loading and week-2 doses of lebrikizumab were 500 mg, followed by 250 mg thereafter. All patients were instructed to use low- to mid-potency TCS at their own discretion. Study sites provided a mid-potency TCS (triamcinolone acetonide 0.1% cream) and a low-potency TCS (hydrocortisone 1% cream), with topical calcineurin inhibitors permitted for sensitive skin areas.
Primary outcomes at 16 weeks included a 2-point or more reduction in IGA score from baseline and EASI-75 response. Patients in the lebrikizumab arm had superior responses on both of these outcomes, with statistical significance achieved as early as week 8 and week 4, respectively, and maintained through week 16. Specifically, 41.2% of those treated with lebrikizumab had an IGA reduction of 2 points or more, compared with 22.1% of those receiving placebo plus TCS (P = .01), and the proportion of patients achieving EASI-75 responses was 69.5% vs. 42.2%, respectively (P < .001).
Patients treated with lebrikizumab also showed statistically significant improvements, compared with TCS alone in all key secondary endpoints, “including skin clearance, improvement in itch, itch interference on sleep, and enhanced QoL [quality of life],” noted the authors. “This study captured the clinical benefit of lebrikizumab through the combined end point of physician-assessed clinical sign of skin clearance (EASI-75) and patient-reported outcome of improvement in itch (Pruritus NRS).”
The percentage of patients who achieved the combined endpoint was more than double for the lebrikizumab plus TCS group vs. the group on TCS alone, indicating that patients treated with lebrikizumab plus TCS “were more likely to experience improvement in skin symptoms and itch,” the investigators added.
The authors noted that most treatment-emergent adverse events “were nonserious, mild, or moderate in severity, and did not lead to study discontinuation.” These included conjunctivitis (4.8%), headache (4.8%), hypertension (2.8%), injection-site reactions (2.8%), and herpes infection (3.4%) – all of which occurred in 1.5% or less of patients in the placebo group.
“The higher incidence of conjunctivitis has also been reported in other biologics inhibiting IL [interleukin]–13 and/or IL-4 signaling, as well as lebrikizumab monotherapy studies,” they noted. The 4.8% rate of conjunctivitis reported in the combination study, they added, is “compared with 7.5% frequency in 16-week data from the lebrikizumab monotherapy studies. Although the mechanism remains unclear, it has been reported that conjunctival goblet cell scarcity due to IL-13 and IL-4 inhibition, and subsequent effects on the homeostasis of the conjunctival mucosal surface, results in ocular AEs [adverse events].”
“This truly is a time of great hope and promise for our patients with AD,” commented Zelma Chiesa Fuxench, MD, who was not involved in the study. “The advent of newer, targeted therapeutic agents for AD continues to revolutionize the treatment experience for our patients, offering the possibility of greater AD disease control with a favorable risk profile and less need for blood work monitoring compared to traditional systemic agents.”
On the basis of the study results, Dr. Chiesa Fuxench, of the department of dermatology at the University of Pennsylvania, Philadelphia, said in an interview that “lebrikizumab represents an additional option in the treatment armamentarium for providers who care for patients with AD.” She added that, “while head-to-head trials comparing lebrikizumab to dupilumab, the first FDA-approved biologic for AD, would be beneficial, to the best of my knowledge this data is currently lacking. However, based on the results of this study, we would expect lebrikizumab to work at least similarly to dupilumab, based on the reported improvements in IGA and EASI score.”
Additionally, lebrikizumab showed a favorable safety profile, “with most treatment-emergent adverse effects reported as nonserious and not leading to drug discontinuation,” she said. “Of interest to clinicians may be the reported rates of conjunctivitis in this study. Rates of conjunctivitis for lebrikizumab appear to be lower than those reported in the LIBERTY AD CHRONOS study for dupilumab – a finding that merits further scrutiny in my opinion, as this one of the most frequent treatment-emergent adverse events that I encounter in my clinical practice.”
The study was funded by Dermira, a subsidiary of Eli Lilly. Dr. Simpson reported personal fees and grants from multiple sources, including Dermira and Eli Lilly, the companies developing lebrikizumab. Several authors were employees of Eli Lilly. Dr. Fuxench disclosed serving as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, Pfizer, AbbVie, and Incyte, for which she has received honoraria for AD-related work. She is the recipient of research grants through Regeneron, Sanofi, Tioga, Vanda, Menlo Therapeutics, Leo Pharma, and Eli Lilly for work related to AD as well as honoraria for continuing medical education work related to AD sponsored through educational grants from Regeneron/Sanofi and Pfizer.
A version of this article first appeared on Medscape.com.
, according to results of the 16-week phase 3 ADhere trial.
“Lebrikizumab, a monoclonal antibody inhibiting interleukin-13, combined with TCS was associated with reduced overall disease severity of moderate to severe AD in adolescents and adults, and had a safety profile consistent with previous lebrikizumab AD studies,” noted lead author Eric L. Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland, and coauthors in their article on the study, which was published in JAMA Dermatology.
The double-blind trial, conducted at 54 sites across Germany, Poland, Canada, and the United States, included 211 patients, mean age 37.2 years, of whom 48.8% were female and roughly 22% were adolescents. Almost 15% were Asian, and about 13% were Black.
At baseline, participants had a score of 16 or higher on the Eczema Area and Severity Index (EASI), a score of 3 or higher on the Investigator’s Global Assessment (IGA) scale, AD covering a body surface area of 10% or greater, and a history of inadequate response to treatment with topical medications.
After a minimum 1-week washout period from topical and systemic therapy, participants were randomized in a 2:1 ratio to receive lebrikizumab plus TCS (n = 145) or placebo plus TCS (n = 66) for 16 weeks.
Lebrikizumab or placebo was administered by subcutaneous injection every 2 weeks; the loading and week-2 doses of lebrikizumab were 500 mg, followed by 250 mg thereafter. All patients were instructed to use low- to mid-potency TCS at their own discretion. Study sites provided a mid-potency TCS (triamcinolone acetonide 0.1% cream) and a low-potency TCS (hydrocortisone 1% cream), with topical calcineurin inhibitors permitted for sensitive skin areas.
Primary outcomes at 16 weeks included a 2-point or more reduction in IGA score from baseline and EASI-75 response. Patients in the lebrikizumab arm had superior responses on both of these outcomes, with statistical significance achieved as early as week 8 and week 4, respectively, and maintained through week 16. Specifically, 41.2% of those treated with lebrikizumab had an IGA reduction of 2 points or more, compared with 22.1% of those receiving placebo plus TCS (P = .01), and the proportion of patients achieving EASI-75 responses was 69.5% vs. 42.2%, respectively (P < .001).
Patients treated with lebrikizumab also showed statistically significant improvements, compared with TCS alone in all key secondary endpoints, “including skin clearance, improvement in itch, itch interference on sleep, and enhanced QoL [quality of life],” noted the authors. “This study captured the clinical benefit of lebrikizumab through the combined end point of physician-assessed clinical sign of skin clearance (EASI-75) and patient-reported outcome of improvement in itch (Pruritus NRS).”
The percentage of patients who achieved the combined endpoint was more than double for the lebrikizumab plus TCS group vs. the group on TCS alone, indicating that patients treated with lebrikizumab plus TCS “were more likely to experience improvement in skin symptoms and itch,” the investigators added.
The authors noted that most treatment-emergent adverse events “were nonserious, mild, or moderate in severity, and did not lead to study discontinuation.” These included conjunctivitis (4.8%), headache (4.8%), hypertension (2.8%), injection-site reactions (2.8%), and herpes infection (3.4%) – all of which occurred in 1.5% or less of patients in the placebo group.
“The higher incidence of conjunctivitis has also been reported in other biologics inhibiting IL [interleukin]–13 and/or IL-4 signaling, as well as lebrikizumab monotherapy studies,” they noted. The 4.8% rate of conjunctivitis reported in the combination study, they added, is “compared with 7.5% frequency in 16-week data from the lebrikizumab monotherapy studies. Although the mechanism remains unclear, it has been reported that conjunctival goblet cell scarcity due to IL-13 and IL-4 inhibition, and subsequent effects on the homeostasis of the conjunctival mucosal surface, results in ocular AEs [adverse events].”
“This truly is a time of great hope and promise for our patients with AD,” commented Zelma Chiesa Fuxench, MD, who was not involved in the study. “The advent of newer, targeted therapeutic agents for AD continues to revolutionize the treatment experience for our patients, offering the possibility of greater AD disease control with a favorable risk profile and less need for blood work monitoring compared to traditional systemic agents.”
On the basis of the study results, Dr. Chiesa Fuxench, of the department of dermatology at the University of Pennsylvania, Philadelphia, said in an interview that “lebrikizumab represents an additional option in the treatment armamentarium for providers who care for patients with AD.” She added that, “while head-to-head trials comparing lebrikizumab to dupilumab, the first FDA-approved biologic for AD, would be beneficial, to the best of my knowledge this data is currently lacking. However, based on the results of this study, we would expect lebrikizumab to work at least similarly to dupilumab, based on the reported improvements in IGA and EASI score.”
Additionally, lebrikizumab showed a favorable safety profile, “with most treatment-emergent adverse effects reported as nonserious and not leading to drug discontinuation,” she said. “Of interest to clinicians may be the reported rates of conjunctivitis in this study. Rates of conjunctivitis for lebrikizumab appear to be lower than those reported in the LIBERTY AD CHRONOS study for dupilumab – a finding that merits further scrutiny in my opinion, as this one of the most frequent treatment-emergent adverse events that I encounter in my clinical practice.”
The study was funded by Dermira, a subsidiary of Eli Lilly. Dr. Simpson reported personal fees and grants from multiple sources, including Dermira and Eli Lilly, the companies developing lebrikizumab. Several authors were employees of Eli Lilly. Dr. Fuxench disclosed serving as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, Pfizer, AbbVie, and Incyte, for which she has received honoraria for AD-related work. She is the recipient of research grants through Regeneron, Sanofi, Tioga, Vanda, Menlo Therapeutics, Leo Pharma, and Eli Lilly for work related to AD as well as honoraria for continuing medical education work related to AD sponsored through educational grants from Regeneron/Sanofi and Pfizer.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Children and COVID: ED visits and hospitalizations start to fall again
Emergency department visits and hospitalizations for COVID-19 in children appear to be following the declining trend set by weekly cases since early December, based on data from the Centers for Disease Control and Prevention.
. New cases took a different path that had the weekly total falling through November before taking a big jump during the week of Nov. 27 to Dec. 3 – the count doubled from 30,000 the previous week to 63,000 – and then decreased again, the CDC reported.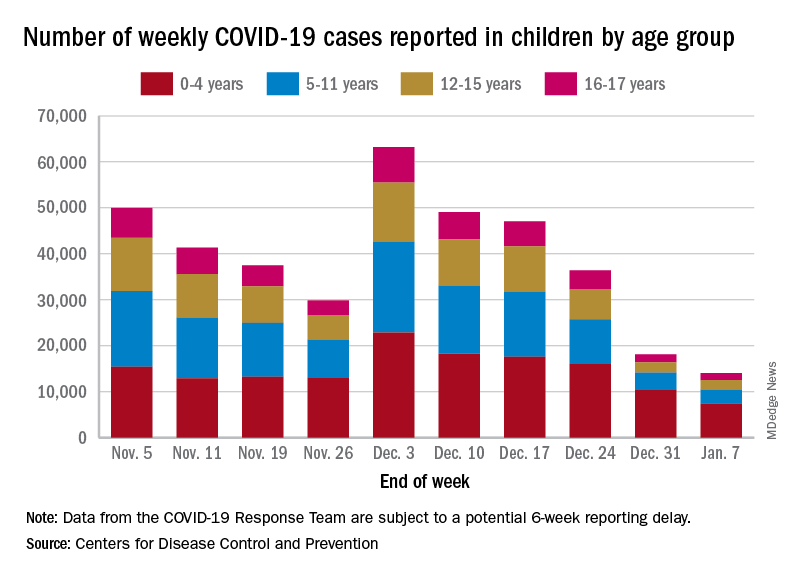
The proportion of ED visits with COVID, which was down to 1.0% of all ED visits (7-day average) for children aged 0-4 years on Nov. 4, was up to 3.2% on Jan. 3 but slipped to 2.5% as of Jan. 10. The patterns for older children are similar, with some differences in timing and lower peaks (1.7% for 12- to 15-year-olds and 1.9% for those aged 16-17), according to the CDC’s COVID Data Tracker.
The trend for new hospital admissions of children with confirmed COVID showed a similar rise through December, and the latest data for the very beginning of January suggest an even faster drop, although there is more of a reporting lag with hospitalization data, compared with ED visits, the CDC noted.
The most current data (Dec. 30 to Jan. 5) available from the American Academy of Pediatrics and the Children’s Hospital Association show less volatility in the number of weekly cases through November and December, with the peak being about 48,000 in mid-December. The AAP/CHA totals for the last 2 weeks, however, were both higher than the CDC’s corresponding counts, which are more preliminary and subject to revision.
The CDC puts the total number of COVID cases in children at 16.7 million – about 17.2% of all cases – as of Jan. 11, with 1,981 deaths reported so far. The AAP and CHA are not tracking deaths, but their case total as of Jan. 5 was 15.2 million, which represents 18.1% of cases in all ages. The AAP/CHA report is based on data reported publicly by an ever-decreasing number of states and territories.
Emergency department visits and hospitalizations for COVID-19 in children appear to be following the declining trend set by weekly cases since early December, based on data from the Centers for Disease Control and Prevention.
. New cases took a different path that had the weekly total falling through November before taking a big jump during the week of Nov. 27 to Dec. 3 – the count doubled from 30,000 the previous week to 63,000 – and then decreased again, the CDC reported.
The proportion of ED visits with COVID, which was down to 1.0% of all ED visits (7-day average) for children aged 0-4 years on Nov. 4, was up to 3.2% on Jan. 3 but slipped to 2.5% as of Jan. 10. The patterns for older children are similar, with some differences in timing and lower peaks (1.7% for 12- to 15-year-olds and 1.9% for those aged 16-17), according to the CDC’s COVID Data Tracker.
The trend for new hospital admissions of children with confirmed COVID showed a similar rise through December, and the latest data for the very beginning of January suggest an even faster drop, although there is more of a reporting lag with hospitalization data, compared with ED visits, the CDC noted.
The most current data (Dec. 30 to Jan. 5) available from the American Academy of Pediatrics and the Children’s Hospital Association show less volatility in the number of weekly cases through November and December, with the peak being about 48,000 in mid-December. The AAP/CHA totals for the last 2 weeks, however, were both higher than the CDC’s corresponding counts, which are more preliminary and subject to revision.
The CDC puts the total number of COVID cases in children at 16.7 million – about 17.2% of all cases – as of Jan. 11, with 1,981 deaths reported so far. The AAP and CHA are not tracking deaths, but their case total as of Jan. 5 was 15.2 million, which represents 18.1% of cases in all ages. The AAP/CHA report is based on data reported publicly by an ever-decreasing number of states and territories.
Emergency department visits and hospitalizations for COVID-19 in children appear to be following the declining trend set by weekly cases since early December, based on data from the Centers for Disease Control and Prevention.
. New cases took a different path that had the weekly total falling through November before taking a big jump during the week of Nov. 27 to Dec. 3 – the count doubled from 30,000 the previous week to 63,000 – and then decreased again, the CDC reported.
The proportion of ED visits with COVID, which was down to 1.0% of all ED visits (7-day average) for children aged 0-4 years on Nov. 4, was up to 3.2% on Jan. 3 but slipped to 2.5% as of Jan. 10. The patterns for older children are similar, with some differences in timing and lower peaks (1.7% for 12- to 15-year-olds and 1.9% for those aged 16-17), according to the CDC’s COVID Data Tracker.
The trend for new hospital admissions of children with confirmed COVID showed a similar rise through December, and the latest data for the very beginning of January suggest an even faster drop, although there is more of a reporting lag with hospitalization data, compared with ED visits, the CDC noted.
The most current data (Dec. 30 to Jan. 5) available from the American Academy of Pediatrics and the Children’s Hospital Association show less volatility in the number of weekly cases through November and December, with the peak being about 48,000 in mid-December. The AAP/CHA totals for the last 2 weeks, however, were both higher than the CDC’s corresponding counts, which are more preliminary and subject to revision.
The CDC puts the total number of COVID cases in children at 16.7 million – about 17.2% of all cases – as of Jan. 11, with 1,981 deaths reported so far. The AAP and CHA are not tracking deaths, but their case total as of Jan. 5 was 15.2 million, which represents 18.1% of cases in all ages. The AAP/CHA report is based on data reported publicly by an ever-decreasing number of states and territories.
Manicure gone wrong leads to cancer diagnosis
. Now, she and her doctor are spreading the word about her ordeal as a lesson that speed and persistence in seeking treatment are the keys that make her type of cancer – squamous cell carcinoma – completely curable.
“She cut me, and the cut wasn’t just a regular cuticle cut. She cut me deep, and that was one of the first times that happened to me,” Grace Garcia, 50, told TODAY.com, recalling the November 2021 incident.
Ms. Garcia had been getting her nails done regularly for 20 years, she said, but happened to go to a different salon than her usual spot because she couldn’t get an appointment during the busy pre-Thanksgiving season. She doesn’t recall whether the technician opened packaging that signals unused tools.
She put antibiotic ointment on the cut, but it didn’t heal after a few days. Eventually, the skin closed and a darkened bump formed. It was painful. She went to her doctor, who said it was a “callus from writing,” she told TODAY.com. But it was on her ring finger, which didn’t seem connected to writing. Her doctor said to keep an eye on it.
Five months after the cut occurred, she mentioned it during a gynecology appointment and was referred to a dermatologist, who also advised keeping an eye on it. A wart developed. She went back to her primary care physician and then to another dermatologist. The spot was biopsied.
Squamous cell carcinoma is a common type of skin cancer, according to the American Academy of Dermatology. It can have many causes, but the cause in Ms. Garcia’s case was both very common and very rare: human papillomavirus, or HPV. HPV is a virus that infects millions of people every year, but it’s not a typical cause of skin cancer.
“It’s pretty rare for several reasons. Generally speaking, the strains that cause cancer from an HPV standpoint tend to be more sexually transmitted,” dermatologist Teo Soleymani told TODAY.com. “In Grace’s case, she had an injury, which became the portal of entry. So that thick skin that we have on our hands and feet that acts as a natural barrier against infections and things like that was no longer the case, and the virus was able to infect her skin.”
Dr. Soleymani said Ms. Garcia’s persistence to get answers likely saved her from losing a finger.
“Your outcomes are entirely dictated by how early you catch them, and very often they’re completely curable,” he said. “Her persistence – not only was she able to have a great outcome, she probably saved herself from having her finger amputated.”
. Now, she and her doctor are spreading the word about her ordeal as a lesson that speed and persistence in seeking treatment are the keys that make her type of cancer – squamous cell carcinoma – completely curable.
“She cut me, and the cut wasn’t just a regular cuticle cut. She cut me deep, and that was one of the first times that happened to me,” Grace Garcia, 50, told TODAY.com, recalling the November 2021 incident.
Ms. Garcia had been getting her nails done regularly for 20 years, she said, but happened to go to a different salon than her usual spot because she couldn’t get an appointment during the busy pre-Thanksgiving season. She doesn’t recall whether the technician opened packaging that signals unused tools.
She put antibiotic ointment on the cut, but it didn’t heal after a few days. Eventually, the skin closed and a darkened bump formed. It was painful. She went to her doctor, who said it was a “callus from writing,” she told TODAY.com. But it was on her ring finger, which didn’t seem connected to writing. Her doctor said to keep an eye on it.
Five months after the cut occurred, she mentioned it during a gynecology appointment and was referred to a dermatologist, who also advised keeping an eye on it. A wart developed. She went back to her primary care physician and then to another dermatologist. The spot was biopsied.
Squamous cell carcinoma is a common type of skin cancer, according to the American Academy of Dermatology. It can have many causes, but the cause in Ms. Garcia’s case was both very common and very rare: human papillomavirus, or HPV. HPV is a virus that infects millions of people every year, but it’s not a typical cause of skin cancer.
“It’s pretty rare for several reasons. Generally speaking, the strains that cause cancer from an HPV standpoint tend to be more sexually transmitted,” dermatologist Teo Soleymani told TODAY.com. “In Grace’s case, she had an injury, which became the portal of entry. So that thick skin that we have on our hands and feet that acts as a natural barrier against infections and things like that was no longer the case, and the virus was able to infect her skin.”
Dr. Soleymani said Ms. Garcia’s persistence to get answers likely saved her from losing a finger.
“Your outcomes are entirely dictated by how early you catch them, and very often they’re completely curable,” he said. “Her persistence – not only was she able to have a great outcome, she probably saved herself from having her finger amputated.”
. Now, she and her doctor are spreading the word about her ordeal as a lesson that speed and persistence in seeking treatment are the keys that make her type of cancer – squamous cell carcinoma – completely curable.
“She cut me, and the cut wasn’t just a regular cuticle cut. She cut me deep, and that was one of the first times that happened to me,” Grace Garcia, 50, told TODAY.com, recalling the November 2021 incident.
Ms. Garcia had been getting her nails done regularly for 20 years, she said, but happened to go to a different salon than her usual spot because she couldn’t get an appointment during the busy pre-Thanksgiving season. She doesn’t recall whether the technician opened packaging that signals unused tools.
She put antibiotic ointment on the cut, but it didn’t heal after a few days. Eventually, the skin closed and a darkened bump formed. It was painful. She went to her doctor, who said it was a “callus from writing,” she told TODAY.com. But it was on her ring finger, which didn’t seem connected to writing. Her doctor said to keep an eye on it.
Five months after the cut occurred, she mentioned it during a gynecology appointment and was referred to a dermatologist, who also advised keeping an eye on it. A wart developed. She went back to her primary care physician and then to another dermatologist. The spot was biopsied.
Squamous cell carcinoma is a common type of skin cancer, according to the American Academy of Dermatology. It can have many causes, but the cause in Ms. Garcia’s case was both very common and very rare: human papillomavirus, or HPV. HPV is a virus that infects millions of people every year, but it’s not a typical cause of skin cancer.
“It’s pretty rare for several reasons. Generally speaking, the strains that cause cancer from an HPV standpoint tend to be more sexually transmitted,” dermatologist Teo Soleymani told TODAY.com. “In Grace’s case, she had an injury, which became the portal of entry. So that thick skin that we have on our hands and feet that acts as a natural barrier against infections and things like that was no longer the case, and the virus was able to infect her skin.”
Dr. Soleymani said Ms. Garcia’s persistence to get answers likely saved her from losing a finger.
“Your outcomes are entirely dictated by how early you catch them, and very often they’re completely curable,” he said. “Her persistence – not only was she able to have a great outcome, she probably saved herself from having her finger amputated.”
Ecopipam reduces Tourette’s tics without common side effects in phase 2 trial
Ecopipam, in development for Tourette syndrome in children and adolescents, has shown in a randomized, controlled trial that, compared with placebo, it reduced tics and reduced the risk for some of the common side effects of other treatments, including weight gain.
Findings of the multicenter, double-blind, trial funded by the drug maker, Emalex Biosciences, were published online in Pediatrics. The trial was conducted at 68 sites in the United States, Canada, Germany, France, and Poland between May 2019 and September 2021.
Donald L. Gilbert, MD, MS, with the division of neurology at Cincinnati Children’s Hospital, and colleagues noted that all Food and Drug Administration–approved medications for Tourette syndrome are antipsychotics. The medications carry a risk of weight gain, electrocardiogram abnormalities, metabolic changes, and drug-induced movement disorders.
First-in-class medication ecopipam, targets the D1 dopamine receptor, while currently approved medications block the D2 receptor. It “may be a safe and effective treatment of Tourette syndrome with advantages over other currently approved therapeutic agents,” the authors wrote.
The study included 153 individuals at least 6 years old up to age 18 with a baseline Yale Global Tic Severity Score Total Tic Score of at least 20.
They were randomly assigned 1:1 to ecopipam or placebo.
Significant reduction in tic severity
Researchers saw a 30% reduction in the tic severity score from baseline to week 12 for the ecopipam group compared with the placebo group.
The data showed a least-squares mean difference of 3.44 (95% confidence interval [CI], 6.09-0.79, P = .01). Researchers also saw improvement in Clinical Global Impression of Tourette Syndrome Severity in the ecopipam group (P = .03).
Sara Pawlowski, MD, division chief for primary care mental health integration at University of Vermont Health Network and assistant professor of psychiatry, University of Vermont, Burlington, said in an interview that several things should be considered with this research.
One is that, though the results show a reduction in tics, the study lasted only 12 weeks and “tics can last a lifetime,” she noted.
“They also can ebb and flow with major life events, stressors, and various other variables. So, I wonder how the effects of improvement can be teased out from the natural ebb and flow of the condition in a 3-month window, which is a snapshot into the course of a known relapsing, remitting, lifetime, and chronically variable condition,” she said.
Headaches, insomnia among side effects
Weight gain was larger in the placebo group than in the ecopipam group: 17.1% in the ecopipam group and 20.3% of those who got a placebo had a weight gain of more than 7% over the study period.
The most common side effects of the study drug were headache (15.8%), insomnia (14.5%), fatigue (7.9%), and somnolence (7.9%).
A limitation of the study was lack of racial and ethnic diversity, as 93.5% of those in the placebo group and 86.8% in the ecopipam group were White.
Guidelines in North America and Europe agree that behavioral treatments should be the first-line therapy.
Dr. Pawlowski said that although effective medications are needed, she urges focusing on better access to nonmedication treatments “that work for children and adolescents” as children who start taking the medications early may take them for the rest of their lives.
Also, while the research didn’t find weight gain in the ecopipam group, the side effects they did find in the group, including headache and insomnia, “do impact a child’s life,” she noted.
“We also can’t be reassured that over the course of chronic treatment there wouldn’t be movement disorders or metabolic disorders that emerge. Those are side effects or disorders that can emerge surreptitiously over time, and more time than 12 weeks,” she said.
The study was funded by Emalex Biosciences. Dr. Gilbert has received consulting fees from Biogen and PTC therapeutics. Study coauthors disclosed ties with Emalex, Alkermes, and Paragon Biosciences. Dr. Pawlowski reports no relevant financial relationships.
Ecopipam, in development for Tourette syndrome in children and adolescents, has shown in a randomized, controlled trial that, compared with placebo, it reduced tics and reduced the risk for some of the common side effects of other treatments, including weight gain.
Findings of the multicenter, double-blind, trial funded by the drug maker, Emalex Biosciences, were published online in Pediatrics. The trial was conducted at 68 sites in the United States, Canada, Germany, France, and Poland between May 2019 and September 2021.
Donald L. Gilbert, MD, MS, with the division of neurology at Cincinnati Children’s Hospital, and colleagues noted that all Food and Drug Administration–approved medications for Tourette syndrome are antipsychotics. The medications carry a risk of weight gain, electrocardiogram abnormalities, metabolic changes, and drug-induced movement disorders.
First-in-class medication ecopipam, targets the D1 dopamine receptor, while currently approved medications block the D2 receptor. It “may be a safe and effective treatment of Tourette syndrome with advantages over other currently approved therapeutic agents,” the authors wrote.
The study included 153 individuals at least 6 years old up to age 18 with a baseline Yale Global Tic Severity Score Total Tic Score of at least 20.
They were randomly assigned 1:1 to ecopipam or placebo.
Significant reduction in tic severity
Researchers saw a 30% reduction in the tic severity score from baseline to week 12 for the ecopipam group compared with the placebo group.
The data showed a least-squares mean difference of 3.44 (95% confidence interval [CI], 6.09-0.79, P = .01). Researchers also saw improvement in Clinical Global Impression of Tourette Syndrome Severity in the ecopipam group (P = .03).
Sara Pawlowski, MD, division chief for primary care mental health integration at University of Vermont Health Network and assistant professor of psychiatry, University of Vermont, Burlington, said in an interview that several things should be considered with this research.
One is that, though the results show a reduction in tics, the study lasted only 12 weeks and “tics can last a lifetime,” she noted.
“They also can ebb and flow with major life events, stressors, and various other variables. So, I wonder how the effects of improvement can be teased out from the natural ebb and flow of the condition in a 3-month window, which is a snapshot into the course of a known relapsing, remitting, lifetime, and chronically variable condition,” she said.
Headaches, insomnia among side effects
Weight gain was larger in the placebo group than in the ecopipam group: 17.1% in the ecopipam group and 20.3% of those who got a placebo had a weight gain of more than 7% over the study period.
The most common side effects of the study drug were headache (15.8%), insomnia (14.5%), fatigue (7.9%), and somnolence (7.9%).
A limitation of the study was lack of racial and ethnic diversity, as 93.5% of those in the placebo group and 86.8% in the ecopipam group were White.
Guidelines in North America and Europe agree that behavioral treatments should be the first-line therapy.
Dr. Pawlowski said that although effective medications are needed, she urges focusing on better access to nonmedication treatments “that work for children and adolescents” as children who start taking the medications early may take them for the rest of their lives.
Also, while the research didn’t find weight gain in the ecopipam group, the side effects they did find in the group, including headache and insomnia, “do impact a child’s life,” she noted.
“We also can’t be reassured that over the course of chronic treatment there wouldn’t be movement disorders or metabolic disorders that emerge. Those are side effects or disorders that can emerge surreptitiously over time, and more time than 12 weeks,” she said.
The study was funded by Emalex Biosciences. Dr. Gilbert has received consulting fees from Biogen and PTC therapeutics. Study coauthors disclosed ties with Emalex, Alkermes, and Paragon Biosciences. Dr. Pawlowski reports no relevant financial relationships.
Ecopipam, in development for Tourette syndrome in children and adolescents, has shown in a randomized, controlled trial that, compared with placebo, it reduced tics and reduced the risk for some of the common side effects of other treatments, including weight gain.
Findings of the multicenter, double-blind, trial funded by the drug maker, Emalex Biosciences, were published online in Pediatrics. The trial was conducted at 68 sites in the United States, Canada, Germany, France, and Poland between May 2019 and September 2021.
Donald L. Gilbert, MD, MS, with the division of neurology at Cincinnati Children’s Hospital, and colleagues noted that all Food and Drug Administration–approved medications for Tourette syndrome are antipsychotics. The medications carry a risk of weight gain, electrocardiogram abnormalities, metabolic changes, and drug-induced movement disorders.
First-in-class medication ecopipam, targets the D1 dopamine receptor, while currently approved medications block the D2 receptor. It “may be a safe and effective treatment of Tourette syndrome with advantages over other currently approved therapeutic agents,” the authors wrote.
The study included 153 individuals at least 6 years old up to age 18 with a baseline Yale Global Tic Severity Score Total Tic Score of at least 20.
They were randomly assigned 1:1 to ecopipam or placebo.
Significant reduction in tic severity
Researchers saw a 30% reduction in the tic severity score from baseline to week 12 for the ecopipam group compared with the placebo group.
The data showed a least-squares mean difference of 3.44 (95% confidence interval [CI], 6.09-0.79, P = .01). Researchers also saw improvement in Clinical Global Impression of Tourette Syndrome Severity in the ecopipam group (P = .03).
Sara Pawlowski, MD, division chief for primary care mental health integration at University of Vermont Health Network and assistant professor of psychiatry, University of Vermont, Burlington, said in an interview that several things should be considered with this research.
One is that, though the results show a reduction in tics, the study lasted only 12 weeks and “tics can last a lifetime,” she noted.
“They also can ebb and flow with major life events, stressors, and various other variables. So, I wonder how the effects of improvement can be teased out from the natural ebb and flow of the condition in a 3-month window, which is a snapshot into the course of a known relapsing, remitting, lifetime, and chronically variable condition,” she said.
Headaches, insomnia among side effects
Weight gain was larger in the placebo group than in the ecopipam group: 17.1% in the ecopipam group and 20.3% of those who got a placebo had a weight gain of more than 7% over the study period.
The most common side effects of the study drug were headache (15.8%), insomnia (14.5%), fatigue (7.9%), and somnolence (7.9%).
A limitation of the study was lack of racial and ethnic diversity, as 93.5% of those in the placebo group and 86.8% in the ecopipam group were White.
Guidelines in North America and Europe agree that behavioral treatments should be the first-line therapy.
Dr. Pawlowski said that although effective medications are needed, she urges focusing on better access to nonmedication treatments “that work for children and adolescents” as children who start taking the medications early may take them for the rest of their lives.
Also, while the research didn’t find weight gain in the ecopipam group, the side effects they did find in the group, including headache and insomnia, “do impact a child’s life,” she noted.
“We also can’t be reassured that over the course of chronic treatment there wouldn’t be movement disorders or metabolic disorders that emerge. Those are side effects or disorders that can emerge surreptitiously over time, and more time than 12 weeks,” she said.
The study was funded by Emalex Biosciences. Dr. Gilbert has received consulting fees from Biogen and PTC therapeutics. Study coauthors disclosed ties with Emalex, Alkermes, and Paragon Biosciences. Dr. Pawlowski reports no relevant financial relationships.
FROM PEDIATRICS
Cardiac Adverse Events Following COVID-19 Vaccination in Patients With Prior Vaccine-Associated Myocarditis
Vaccinations have substantially reduced morbidity and mortality from many infectious diseases. Despite the clear value of vaccinations in public health, efforts to better understand adverse events (AEs) following immunization are important to sustain public trust and vaccine confidence. Noninfectious inflammation of the heart may manifest as myocarditis or pericarditis, or occasionally, with shared signs and symptoms of each, as myopericarditis. This is a rare AE following some immunizations. Vaccine-associated myocarditis, pericarditis, or myopericarditis (VAMP) has been most clearly associated with smallpox vaccines and mRNA COVID-19 vaccines.1-6 Although extremely rare, VAMP also has been associated with other vaccines.7,8 Limited information exists to guide shared clinical decision making on COVID-19 vaccination in persons with a history of VAMP. It is unknown whether individuals with a history of VAMP are at higher risk for developing a recurrence or experiencing a more severe outcome following COVID-19 vaccination.
Methods
As part of the collaborative public health mission with the Centers for Disease Control and Prevention (CDC) for enhanced vaccine AE surveillance, the Defense Health Agency Immunization Healthcare Division (IHD) maintains a clinical database of service members and beneficiaries referred for suspected AEs following immunizations. A review of all AEs following immunization cases in this database from January 1, 2003, through February 28, 2022, identified individuals meeting the following criteria: (a) VAMP prior to receipt of COVID-19 vaccine; (b) receipt of COVID-19 vaccine in 2021; and (c) medical documentation in available electronic health records sufficient to describe health status at least 30 days following COVID-19 vaccination.9 If medical entries suggested cardiac symptoms following a COVID-19 vaccine, additional information was sought to verify VAMP based on current published criteria.10,11 Both the initial VAMP cases and the suspected COVID-19 VAMP cases were adjudicated by a team of vaccine experts and specialists in immunology, cardiology, and preventive medicine.
This retrospective review was approved and conducted in accordance with the Walter Reed National Military Medical Center Institutional Review Board protocol #20664. All individuals with recurrent VAMP consented to share their health records and clinical details.
Results
Among 9260 cases in the IHD database, 431 met the case definition for VAMP.
Among the 179 patients included in this analysis, 171 (96%) were male. Their median age was 39 years at the time of COVID-19 vaccination.
Within 1 month of receipt of any COVID-19 vaccine, 11 individuals had documented symptoms suggesting cardiac involvement, specifically, chest pain, palpitations, or dyspnea. After cardiac evaluation, 4 patients met the criteria for VAMP after COVID-19 vaccination.10,11 Seven patients either did not meet the criteria for VAMP or had alternative causes for their symptoms.
Two men aged 49 and 50 years with a history of vaccine-associated myocarditis following smallpox vaccination (Dryvax and ACAM2000) developed myocarditis 3 days after their second dose of the Moderna vaccine. One of these patients received a Pfizer-BioNTech booster 10 months later with no recurrence of symptoms. A 55-year-old man with a history of vaccine-associated myocarditis following Dryvax vaccination developed myocarditis 2 days after his Pfizer-BioNTech booster. None of the patients who developed post-COVID-19 VAMP reported residual symptoms from their initial VAMP episode, which occurred 12 to 18 years earlier. All were hospitalized briefly for observation and had complete symptom resolution within 6 weeks.
A 25-year-old man developed pericarditis 4 days after his second Pfizer-BioNTech vaccination. His previous ACAM2000 vaccine-associated myocarditis occurred 3 years earlier, with no residual symptoms. Of note, he had a mild COVID-19 infection 78 days before the onset of his pericarditis. After the onset of his COVID-19 vaccine-associated pericarditis, he continued to experience transient bouts of chest pressure and exertional dyspnea that resolved within 7 months of onset.
The median interval between COVID-19 vaccine doses in those who developed post-COVID-19 VAMP was within the recommended mRNA vaccine dosing intervals of 3 to 4 weeks and was consistent with the median mRNA vaccine dosing intervals among the entire cohort.
Due to the small cohort size and other limitations of this study, the suggested rate of cardiac injury in this review (4 cases in 179 persons, or 2.2%) is an imprecise estimate of risk in a small population (95% CI, 0.1%-4.4%). While this rate may seem higher than expected within the general population after COVID-19 vaccination, it is lower than the estimated lifetime risk of recurrent myocarditis from any cause.6,12
Discussion
To our knowledge, this is the first report describing cardiac outcomes after COVID-19 vaccination among a cohort of individuals with prior history of VAMP. Four cases of COVID-19 VAMP were identified among 179 patients with previous VAMP. All cases had experienced VAMP after the smallpox vaccine several years earlier, with complete resolution of symptoms. Three cases presented with recurrent VAMP after their second dose of an mRNA COVID-19 vaccine, and one after an mRNA booster dose. All fully recovered over the course of several months.
Myocarditis is a heterogeneous inflammatory injury with diverse, sometimes idiopathic, etiologies.13 In contrast to infection-related cardiac injury, prior reports of vaccine-associated myocarditis have suggested a hypersensitivity reaction characterized by patchy eosinophilic infiltrates, a benign clinical course, and good prognosis.2,3
There are several common features between VAMP after smallpox and COVID-19 vaccination. Cases occur predominantly in young men. The onset of symptoms after smallpox vaccine (mean, 10 days) and after mRNA COVID-19 vaccine (mean, 3 days) appears to correspond to the timing of peak postvaccination pro-inflammatory cytokine elevation.14 While all VAMP cases are serious events, the majority of patients appear to have a relatively benign clinical course with rapid and full recovery.13
Patients who have experienced an inflammatory cardiac injury may be at higher risk for recurrence, but quantifying risk of this rare phenomenon is challenging. Cases of VAMP after the COVID-19 vaccine have occasionally been reported in patients with previous cardiac injury unrelated to vaccination.15-17 The cases presented here represent the first report of recurrent VAMP following prior non-COVID-19 vaccinations.
Most patients with prior VAMP in this cohort did not experience cardiac-suggestive symptoms following COVID-19 vaccination. Among 11 patients who developed symptoms, 3 had confirmed myocarditis and 1 had confirmed pericarditis. The clinical course for these patients with recurrent VAMP was observed to be no different in severity or duration from those who experience new-onset VAMP.4 All other patients not meeting criteria for VAMP or having alternative explanations for their symptoms also had a benign clinical course. Nonetheless, of the study cohort of 179, recurrent VAMP was diagnosed in 4 of the 11 who developed cardiac-suggestive symptoms following COVID-19 vaccination. The importance of cardiac evaluation should be emphasized for any patient presenting with chest pain, dyspnea, or other cardiac-suggestive symptoms following vaccination.
Strengths and Limitations
The strength of this review of VAMP recurrence associated with COVID-19 vaccination derives from our large and unique longitudinal database of VAMP among current and prior service members. Additionally, the IHD’s ongoing enhanced vaccine AEs surveillance provides the opportunity to contact patients and review their electronic health records over an extended interval of time.
When interpreting this report’s implications, limitations inherent to any retrospective case review should be considered. The cohort of cases of prior VAMP included primarily healthy, fit, young service members; this population is not representative of the general population. The cohort included prior VAMP cases that generally occurred after smallpox vaccination. Experiences after smallpox vaccine may not apply to cardiac injury from other vaccines or etiologies. By the nature of this review, the population studied at the time of COVID-19 vaccination was somewhat older than those most likely to develop an initial bout of VAMP.2 This review was limited by information available in the electronic health records of a small number of patients. Subclinical cases of VAMP and cases without adequate clinical evaluation also could not be included.
Conclusions
Noninfectious inflammation of the heart (myocarditis, pericarditis, or myopericarditis) is a rare AE following certain vaccines, especially live replicating smallpox vaccine and mRNA COVID-19 vaccines. In this observational analysis, the majority of patients with previous VAMP successfully received a COVID-19 vaccine without recurrence. The 4 patients who were identified with recurrent VAMP following COVID-19 vaccination all recovered with supportive care. While the CDC endorses that individuals with a history of infectious myocarditis may receive COVID-19 vaccine after symptoms have resolved, there is currently insufficient safety data regarding COVID-19 vaccination of those with prior non-COVID-19 VAMP or following subsequent COVID-19 vaccination in those with prior VAMP related to COVID-19.10 For these individuals, COVID-19 vaccination is a precaution.10 Although insufficient to determine a precise level of risk, this report does provide data on which to base the CDC-recommended shared decision-making counseling of these patients. More research is needed to better define factors that increase risk for, or protection from, immune-mediated AEs following immunization, including VAMP. While benefits of vaccination have clearly outweighed risks during the COVID-19 pandemic, such research may optimize future vaccine recommendations.18
1. Decker MD, Garman PM, Hughes H, et al. Enhanced safety surveillance study of ACAM2000 smallpox vaccine among US military service members. Vaccine. 2021;39(39):5541-5547. doi:10.1016/j.vaccine.2021.08.041
2. Engler RJ, Nelson MR, Collins LC Jr, et al. A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination. PLoS One. 2015;10(3):e0118283. doi:10.1371/journal.pone.0118283
3. Faix DJ, Gordon DM, Perry LN, et al. Prospective safety surveillance study of ACAM2000 smallpox vaccine in deploying military personnel. Vaccine. 2020;38(46):7323-7330. doi:10.1016/j.vaccine.2020.09.037
4. Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021;6(10):1202-1206. doi:10.1001/jamacardio.2021.2833
5. Witberg G, Barda N, Hoss S, et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med. 2021;385(23):2132-2139. doi:10.1056/NEJMoa2110737
6. Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327(4):331-340. doi:10.1001/jama.2021.24110
7. Su JR, McNeil MM, Welsh KJ, et al. Myopericarditis after vaccination, Vaccine Adverse Event Reporting System (VAERS), 1990-2018. Vaccine. 2021;39(5):839-845. doi:10.1016/j.vaccine.2020.12.046
8. Mei R, Raschi E, Forcesi E, Diemberger I, De Ponti F, Poluzzi E. Myocarditis and pericarditis after immunization: gaining insights through the Vaccine Adverse Event Reporting System. Int J Cardiol. 2018;273:183-186. doi:10.1016/j.ijcard.2018.09.054
9. Centers for Disease Control and Prevention (CDC). Update: cardiac-related events during the civilian smallpox vaccination program—United States, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(21):492-496.
10. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70(27):977-982. doi:10.15585/mmwr.mm7027e2
11. Sexson Tejtel SK, Munoz FM, Al-Ammouri I, et al. Myocarditis and pericarditis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2022;40(10):1499-1511. doi:10.1016/j.vaccine.2021.11.074
12. Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet. 2012;379(9817):738-747. doi:10.1016/S0140-6736(11) 60648-X
13. Heymans S, Cooper LT. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2022;19(2):75-77. doi:10.1038/s41569-021-00662-w
14. Cohen JI, Hohman P, Fulton R, et al. Kinetics of serum cytokines after primary or repeat vaccination with the smallpox vaccine. J Infect Dis. 2010;201(8):1183-1191. doi:10.1086/651453
15. Minocha PK, Better D, Singh RK, Hoque T. Recurrence of acute myocarditis temporally associated with receipt of the mRNA COVID-19 vaccine in an adolescent male. J Pediatr. 2021;238:321-323. doi:10.1016/j.jpeds.2021.06.035
16. Umei TC, Kishino Y, Watanabe K, et al. Recurrence of myopericarditis following mRNA COVID-19 vaccination in a male adolescent. CJC Open. 2022;4(3):350-352. doi:10.1016/j.cjco.2021.12.002
17. Pasha MA, Isaac S, Khan Z. Recurrent myocarditis following COVID-19 infection and the mRNA vaccine. Cureus. 2022;14(7):e26650. doi:10.7759/cureus.26650
18. Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(14):517-523. Published 2022 Apr 8. doi:10.15585/mmwr.mm7114e1
Vaccinations have substantially reduced morbidity and mortality from many infectious diseases. Despite the clear value of vaccinations in public health, efforts to better understand adverse events (AEs) following immunization are important to sustain public trust and vaccine confidence. Noninfectious inflammation of the heart may manifest as myocarditis or pericarditis, or occasionally, with shared signs and symptoms of each, as myopericarditis. This is a rare AE following some immunizations. Vaccine-associated myocarditis, pericarditis, or myopericarditis (VAMP) has been most clearly associated with smallpox vaccines and mRNA COVID-19 vaccines.1-6 Although extremely rare, VAMP also has been associated with other vaccines.7,8 Limited information exists to guide shared clinical decision making on COVID-19 vaccination in persons with a history of VAMP. It is unknown whether individuals with a history of VAMP are at higher risk for developing a recurrence or experiencing a more severe outcome following COVID-19 vaccination.
Methods
As part of the collaborative public health mission with the Centers for Disease Control and Prevention (CDC) for enhanced vaccine AE surveillance, the Defense Health Agency Immunization Healthcare Division (IHD) maintains a clinical database of service members and beneficiaries referred for suspected AEs following immunizations. A review of all AEs following immunization cases in this database from January 1, 2003, through February 28, 2022, identified individuals meeting the following criteria: (a) VAMP prior to receipt of COVID-19 vaccine; (b) receipt of COVID-19 vaccine in 2021; and (c) medical documentation in available electronic health records sufficient to describe health status at least 30 days following COVID-19 vaccination.9 If medical entries suggested cardiac symptoms following a COVID-19 vaccine, additional information was sought to verify VAMP based on current published criteria.10,11 Both the initial VAMP cases and the suspected COVID-19 VAMP cases were adjudicated by a team of vaccine experts and specialists in immunology, cardiology, and preventive medicine.
This retrospective review was approved and conducted in accordance with the Walter Reed National Military Medical Center Institutional Review Board protocol #20664. All individuals with recurrent VAMP consented to share their health records and clinical details.
Results
Among 9260 cases in the IHD database, 431 met the case definition for VAMP.
Among the 179 patients included in this analysis, 171 (96%) were male. Their median age was 39 years at the time of COVID-19 vaccination.
Within 1 month of receipt of any COVID-19 vaccine, 11 individuals had documented symptoms suggesting cardiac involvement, specifically, chest pain, palpitations, or dyspnea. After cardiac evaluation, 4 patients met the criteria for VAMP after COVID-19 vaccination.10,11 Seven patients either did not meet the criteria for VAMP or had alternative causes for their symptoms.
Two men aged 49 and 50 years with a history of vaccine-associated myocarditis following smallpox vaccination (Dryvax and ACAM2000) developed myocarditis 3 days after their second dose of the Moderna vaccine. One of these patients received a Pfizer-BioNTech booster 10 months later with no recurrence of symptoms. A 55-year-old man with a history of vaccine-associated myocarditis following Dryvax vaccination developed myocarditis 2 days after his Pfizer-BioNTech booster. None of the patients who developed post-COVID-19 VAMP reported residual symptoms from their initial VAMP episode, which occurred 12 to 18 years earlier. All were hospitalized briefly for observation and had complete symptom resolution within 6 weeks.
A 25-year-old man developed pericarditis 4 days after his second Pfizer-BioNTech vaccination. His previous ACAM2000 vaccine-associated myocarditis occurred 3 years earlier, with no residual symptoms. Of note, he had a mild COVID-19 infection 78 days before the onset of his pericarditis. After the onset of his COVID-19 vaccine-associated pericarditis, he continued to experience transient bouts of chest pressure and exertional dyspnea that resolved within 7 months of onset.
The median interval between COVID-19 vaccine doses in those who developed post-COVID-19 VAMP was within the recommended mRNA vaccine dosing intervals of 3 to 4 weeks and was consistent with the median mRNA vaccine dosing intervals among the entire cohort.
Due to the small cohort size and other limitations of this study, the suggested rate of cardiac injury in this review (4 cases in 179 persons, or 2.2%) is an imprecise estimate of risk in a small population (95% CI, 0.1%-4.4%). While this rate may seem higher than expected within the general population after COVID-19 vaccination, it is lower than the estimated lifetime risk of recurrent myocarditis from any cause.6,12
Discussion
To our knowledge, this is the first report describing cardiac outcomes after COVID-19 vaccination among a cohort of individuals with prior history of VAMP. Four cases of COVID-19 VAMP were identified among 179 patients with previous VAMP. All cases had experienced VAMP after the smallpox vaccine several years earlier, with complete resolution of symptoms. Three cases presented with recurrent VAMP after their second dose of an mRNA COVID-19 vaccine, and one after an mRNA booster dose. All fully recovered over the course of several months.
Myocarditis is a heterogeneous inflammatory injury with diverse, sometimes idiopathic, etiologies.13 In contrast to infection-related cardiac injury, prior reports of vaccine-associated myocarditis have suggested a hypersensitivity reaction characterized by patchy eosinophilic infiltrates, a benign clinical course, and good prognosis.2,3
There are several common features between VAMP after smallpox and COVID-19 vaccination. Cases occur predominantly in young men. The onset of symptoms after smallpox vaccine (mean, 10 days) and after mRNA COVID-19 vaccine (mean, 3 days) appears to correspond to the timing of peak postvaccination pro-inflammatory cytokine elevation.14 While all VAMP cases are serious events, the majority of patients appear to have a relatively benign clinical course with rapid and full recovery.13
Patients who have experienced an inflammatory cardiac injury may be at higher risk for recurrence, but quantifying risk of this rare phenomenon is challenging. Cases of VAMP after the COVID-19 vaccine have occasionally been reported in patients with previous cardiac injury unrelated to vaccination.15-17 The cases presented here represent the first report of recurrent VAMP following prior non-COVID-19 vaccinations.
Most patients with prior VAMP in this cohort did not experience cardiac-suggestive symptoms following COVID-19 vaccination. Among 11 patients who developed symptoms, 3 had confirmed myocarditis and 1 had confirmed pericarditis. The clinical course for these patients with recurrent VAMP was observed to be no different in severity or duration from those who experience new-onset VAMP.4 All other patients not meeting criteria for VAMP or having alternative explanations for their symptoms also had a benign clinical course. Nonetheless, of the study cohort of 179, recurrent VAMP was diagnosed in 4 of the 11 who developed cardiac-suggestive symptoms following COVID-19 vaccination. The importance of cardiac evaluation should be emphasized for any patient presenting with chest pain, dyspnea, or other cardiac-suggestive symptoms following vaccination.
Strengths and Limitations
The strength of this review of VAMP recurrence associated with COVID-19 vaccination derives from our large and unique longitudinal database of VAMP among current and prior service members. Additionally, the IHD’s ongoing enhanced vaccine AEs surveillance provides the opportunity to contact patients and review their electronic health records over an extended interval of time.
When interpreting this report’s implications, limitations inherent to any retrospective case review should be considered. The cohort of cases of prior VAMP included primarily healthy, fit, young service members; this population is not representative of the general population. The cohort included prior VAMP cases that generally occurred after smallpox vaccination. Experiences after smallpox vaccine may not apply to cardiac injury from other vaccines or etiologies. By the nature of this review, the population studied at the time of COVID-19 vaccination was somewhat older than those most likely to develop an initial bout of VAMP.2 This review was limited by information available in the electronic health records of a small number of patients. Subclinical cases of VAMP and cases without adequate clinical evaluation also could not be included.
Conclusions
Noninfectious inflammation of the heart (myocarditis, pericarditis, or myopericarditis) is a rare AE following certain vaccines, especially live replicating smallpox vaccine and mRNA COVID-19 vaccines. In this observational analysis, the majority of patients with previous VAMP successfully received a COVID-19 vaccine without recurrence. The 4 patients who were identified with recurrent VAMP following COVID-19 vaccination all recovered with supportive care. While the CDC endorses that individuals with a history of infectious myocarditis may receive COVID-19 vaccine after symptoms have resolved, there is currently insufficient safety data regarding COVID-19 vaccination of those with prior non-COVID-19 VAMP or following subsequent COVID-19 vaccination in those with prior VAMP related to COVID-19.10 For these individuals, COVID-19 vaccination is a precaution.10 Although insufficient to determine a precise level of risk, this report does provide data on which to base the CDC-recommended shared decision-making counseling of these patients. More research is needed to better define factors that increase risk for, or protection from, immune-mediated AEs following immunization, including VAMP. While benefits of vaccination have clearly outweighed risks during the COVID-19 pandemic, such research may optimize future vaccine recommendations.18
Vaccinations have substantially reduced morbidity and mortality from many infectious diseases. Despite the clear value of vaccinations in public health, efforts to better understand adverse events (AEs) following immunization are important to sustain public trust and vaccine confidence. Noninfectious inflammation of the heart may manifest as myocarditis or pericarditis, or occasionally, with shared signs and symptoms of each, as myopericarditis. This is a rare AE following some immunizations. Vaccine-associated myocarditis, pericarditis, or myopericarditis (VAMP) has been most clearly associated with smallpox vaccines and mRNA COVID-19 vaccines.1-6 Although extremely rare, VAMP also has been associated with other vaccines.7,8 Limited information exists to guide shared clinical decision making on COVID-19 vaccination in persons with a history of VAMP. It is unknown whether individuals with a history of VAMP are at higher risk for developing a recurrence or experiencing a more severe outcome following COVID-19 vaccination.
Methods
As part of the collaborative public health mission with the Centers for Disease Control and Prevention (CDC) for enhanced vaccine AE surveillance, the Defense Health Agency Immunization Healthcare Division (IHD) maintains a clinical database of service members and beneficiaries referred for suspected AEs following immunizations. A review of all AEs following immunization cases in this database from January 1, 2003, through February 28, 2022, identified individuals meeting the following criteria: (a) VAMP prior to receipt of COVID-19 vaccine; (b) receipt of COVID-19 vaccine in 2021; and (c) medical documentation in available electronic health records sufficient to describe health status at least 30 days following COVID-19 vaccination.9 If medical entries suggested cardiac symptoms following a COVID-19 vaccine, additional information was sought to verify VAMP based on current published criteria.10,11 Both the initial VAMP cases and the suspected COVID-19 VAMP cases were adjudicated by a team of vaccine experts and specialists in immunology, cardiology, and preventive medicine.
This retrospective review was approved and conducted in accordance with the Walter Reed National Military Medical Center Institutional Review Board protocol #20664. All individuals with recurrent VAMP consented to share their health records and clinical details.
Results
Among 9260 cases in the IHD database, 431 met the case definition for VAMP.
Among the 179 patients included in this analysis, 171 (96%) were male. Their median age was 39 years at the time of COVID-19 vaccination.
Within 1 month of receipt of any COVID-19 vaccine, 11 individuals had documented symptoms suggesting cardiac involvement, specifically, chest pain, palpitations, or dyspnea. After cardiac evaluation, 4 patients met the criteria for VAMP after COVID-19 vaccination.10,11 Seven patients either did not meet the criteria for VAMP or had alternative causes for their symptoms.
Two men aged 49 and 50 years with a history of vaccine-associated myocarditis following smallpox vaccination (Dryvax and ACAM2000) developed myocarditis 3 days after their second dose of the Moderna vaccine. One of these patients received a Pfizer-BioNTech booster 10 months later with no recurrence of symptoms. A 55-year-old man with a history of vaccine-associated myocarditis following Dryvax vaccination developed myocarditis 2 days after his Pfizer-BioNTech booster. None of the patients who developed post-COVID-19 VAMP reported residual symptoms from their initial VAMP episode, which occurred 12 to 18 years earlier. All were hospitalized briefly for observation and had complete symptom resolution within 6 weeks.
A 25-year-old man developed pericarditis 4 days after his second Pfizer-BioNTech vaccination. His previous ACAM2000 vaccine-associated myocarditis occurred 3 years earlier, with no residual symptoms. Of note, he had a mild COVID-19 infection 78 days before the onset of his pericarditis. After the onset of his COVID-19 vaccine-associated pericarditis, he continued to experience transient bouts of chest pressure and exertional dyspnea that resolved within 7 months of onset.
The median interval between COVID-19 vaccine doses in those who developed post-COVID-19 VAMP was within the recommended mRNA vaccine dosing intervals of 3 to 4 weeks and was consistent with the median mRNA vaccine dosing intervals among the entire cohort.
Due to the small cohort size and other limitations of this study, the suggested rate of cardiac injury in this review (4 cases in 179 persons, or 2.2%) is an imprecise estimate of risk in a small population (95% CI, 0.1%-4.4%). While this rate may seem higher than expected within the general population after COVID-19 vaccination, it is lower than the estimated lifetime risk of recurrent myocarditis from any cause.6,12
Discussion
To our knowledge, this is the first report describing cardiac outcomes after COVID-19 vaccination among a cohort of individuals with prior history of VAMP. Four cases of COVID-19 VAMP were identified among 179 patients with previous VAMP. All cases had experienced VAMP after the smallpox vaccine several years earlier, with complete resolution of symptoms. Three cases presented with recurrent VAMP after their second dose of an mRNA COVID-19 vaccine, and one after an mRNA booster dose. All fully recovered over the course of several months.
Myocarditis is a heterogeneous inflammatory injury with diverse, sometimes idiopathic, etiologies.13 In contrast to infection-related cardiac injury, prior reports of vaccine-associated myocarditis have suggested a hypersensitivity reaction characterized by patchy eosinophilic infiltrates, a benign clinical course, and good prognosis.2,3
There are several common features between VAMP after smallpox and COVID-19 vaccination. Cases occur predominantly in young men. The onset of symptoms after smallpox vaccine (mean, 10 days) and after mRNA COVID-19 vaccine (mean, 3 days) appears to correspond to the timing of peak postvaccination pro-inflammatory cytokine elevation.14 While all VAMP cases are serious events, the majority of patients appear to have a relatively benign clinical course with rapid and full recovery.13
Patients who have experienced an inflammatory cardiac injury may be at higher risk for recurrence, but quantifying risk of this rare phenomenon is challenging. Cases of VAMP after the COVID-19 vaccine have occasionally been reported in patients with previous cardiac injury unrelated to vaccination.15-17 The cases presented here represent the first report of recurrent VAMP following prior non-COVID-19 vaccinations.
Most patients with prior VAMP in this cohort did not experience cardiac-suggestive symptoms following COVID-19 vaccination. Among 11 patients who developed symptoms, 3 had confirmed myocarditis and 1 had confirmed pericarditis. The clinical course for these patients with recurrent VAMP was observed to be no different in severity or duration from those who experience new-onset VAMP.4 All other patients not meeting criteria for VAMP or having alternative explanations for their symptoms also had a benign clinical course. Nonetheless, of the study cohort of 179, recurrent VAMP was diagnosed in 4 of the 11 who developed cardiac-suggestive symptoms following COVID-19 vaccination. The importance of cardiac evaluation should be emphasized for any patient presenting with chest pain, dyspnea, or other cardiac-suggestive symptoms following vaccination.
Strengths and Limitations
The strength of this review of VAMP recurrence associated with COVID-19 vaccination derives from our large and unique longitudinal database of VAMP among current and prior service members. Additionally, the IHD’s ongoing enhanced vaccine AEs surveillance provides the opportunity to contact patients and review their electronic health records over an extended interval of time.
When interpreting this report’s implications, limitations inherent to any retrospective case review should be considered. The cohort of cases of prior VAMP included primarily healthy, fit, young service members; this population is not representative of the general population. The cohort included prior VAMP cases that generally occurred after smallpox vaccination. Experiences after smallpox vaccine may not apply to cardiac injury from other vaccines or etiologies. By the nature of this review, the population studied at the time of COVID-19 vaccination was somewhat older than those most likely to develop an initial bout of VAMP.2 This review was limited by information available in the electronic health records of a small number of patients. Subclinical cases of VAMP and cases without adequate clinical evaluation also could not be included.
Conclusions
Noninfectious inflammation of the heart (myocarditis, pericarditis, or myopericarditis) is a rare AE following certain vaccines, especially live replicating smallpox vaccine and mRNA COVID-19 vaccines. In this observational analysis, the majority of patients with previous VAMP successfully received a COVID-19 vaccine without recurrence. The 4 patients who were identified with recurrent VAMP following COVID-19 vaccination all recovered with supportive care. While the CDC endorses that individuals with a history of infectious myocarditis may receive COVID-19 vaccine after symptoms have resolved, there is currently insufficient safety data regarding COVID-19 vaccination of those with prior non-COVID-19 VAMP or following subsequent COVID-19 vaccination in those with prior VAMP related to COVID-19.10 For these individuals, COVID-19 vaccination is a precaution.10 Although insufficient to determine a precise level of risk, this report does provide data on which to base the CDC-recommended shared decision-making counseling of these patients. More research is needed to better define factors that increase risk for, or protection from, immune-mediated AEs following immunization, including VAMP. While benefits of vaccination have clearly outweighed risks during the COVID-19 pandemic, such research may optimize future vaccine recommendations.18
1. Decker MD, Garman PM, Hughes H, et al. Enhanced safety surveillance study of ACAM2000 smallpox vaccine among US military service members. Vaccine. 2021;39(39):5541-5547. doi:10.1016/j.vaccine.2021.08.041
2. Engler RJ, Nelson MR, Collins LC Jr, et al. A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination. PLoS One. 2015;10(3):e0118283. doi:10.1371/journal.pone.0118283
3. Faix DJ, Gordon DM, Perry LN, et al. Prospective safety surveillance study of ACAM2000 smallpox vaccine in deploying military personnel. Vaccine. 2020;38(46):7323-7330. doi:10.1016/j.vaccine.2020.09.037
4. Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021;6(10):1202-1206. doi:10.1001/jamacardio.2021.2833
5. Witberg G, Barda N, Hoss S, et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med. 2021;385(23):2132-2139. doi:10.1056/NEJMoa2110737
6. Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327(4):331-340. doi:10.1001/jama.2021.24110
7. Su JR, McNeil MM, Welsh KJ, et al. Myopericarditis after vaccination, Vaccine Adverse Event Reporting System (VAERS), 1990-2018. Vaccine. 2021;39(5):839-845. doi:10.1016/j.vaccine.2020.12.046
8. Mei R, Raschi E, Forcesi E, Diemberger I, De Ponti F, Poluzzi E. Myocarditis and pericarditis after immunization: gaining insights through the Vaccine Adverse Event Reporting System. Int J Cardiol. 2018;273:183-186. doi:10.1016/j.ijcard.2018.09.054
9. Centers for Disease Control and Prevention (CDC). Update: cardiac-related events during the civilian smallpox vaccination program—United States, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(21):492-496.
10. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70(27):977-982. doi:10.15585/mmwr.mm7027e2
11. Sexson Tejtel SK, Munoz FM, Al-Ammouri I, et al. Myocarditis and pericarditis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2022;40(10):1499-1511. doi:10.1016/j.vaccine.2021.11.074
12. Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet. 2012;379(9817):738-747. doi:10.1016/S0140-6736(11) 60648-X
13. Heymans S, Cooper LT. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2022;19(2):75-77. doi:10.1038/s41569-021-00662-w
14. Cohen JI, Hohman P, Fulton R, et al. Kinetics of serum cytokines after primary or repeat vaccination with the smallpox vaccine. J Infect Dis. 2010;201(8):1183-1191. doi:10.1086/651453
15. Minocha PK, Better D, Singh RK, Hoque T. Recurrence of acute myocarditis temporally associated with receipt of the mRNA COVID-19 vaccine in an adolescent male. J Pediatr. 2021;238:321-323. doi:10.1016/j.jpeds.2021.06.035
16. Umei TC, Kishino Y, Watanabe K, et al. Recurrence of myopericarditis following mRNA COVID-19 vaccination in a male adolescent. CJC Open. 2022;4(3):350-352. doi:10.1016/j.cjco.2021.12.002
17. Pasha MA, Isaac S, Khan Z. Recurrent myocarditis following COVID-19 infection and the mRNA vaccine. Cureus. 2022;14(7):e26650. doi:10.7759/cureus.26650
18. Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(14):517-523. Published 2022 Apr 8. doi:10.15585/mmwr.mm7114e1
1. Decker MD, Garman PM, Hughes H, et al. Enhanced safety surveillance study of ACAM2000 smallpox vaccine among US military service members. Vaccine. 2021;39(39):5541-5547. doi:10.1016/j.vaccine.2021.08.041
2. Engler RJ, Nelson MR, Collins LC Jr, et al. A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination. PLoS One. 2015;10(3):e0118283. doi:10.1371/journal.pone.0118283
3. Faix DJ, Gordon DM, Perry LN, et al. Prospective safety surveillance study of ACAM2000 smallpox vaccine in deploying military personnel. Vaccine. 2020;38(46):7323-7330. doi:10.1016/j.vaccine.2020.09.037
4. Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021;6(10):1202-1206. doi:10.1001/jamacardio.2021.2833
5. Witberg G, Barda N, Hoss S, et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med. 2021;385(23):2132-2139. doi:10.1056/NEJMoa2110737
6. Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327(4):331-340. doi:10.1001/jama.2021.24110
7. Su JR, McNeil MM, Welsh KJ, et al. Myopericarditis after vaccination, Vaccine Adverse Event Reporting System (VAERS), 1990-2018. Vaccine. 2021;39(5):839-845. doi:10.1016/j.vaccine.2020.12.046
8. Mei R, Raschi E, Forcesi E, Diemberger I, De Ponti F, Poluzzi E. Myocarditis and pericarditis after immunization: gaining insights through the Vaccine Adverse Event Reporting System. Int J Cardiol. 2018;273:183-186. doi:10.1016/j.ijcard.2018.09.054
9. Centers for Disease Control and Prevention (CDC). Update: cardiac-related events during the civilian smallpox vaccination program—United States, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(21):492-496.
10. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70(27):977-982. doi:10.15585/mmwr.mm7027e2
11. Sexson Tejtel SK, Munoz FM, Al-Ammouri I, et al. Myocarditis and pericarditis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2022;40(10):1499-1511. doi:10.1016/j.vaccine.2021.11.074
12. Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet. 2012;379(9817):738-747. doi:10.1016/S0140-6736(11) 60648-X
13. Heymans S, Cooper LT. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2022;19(2):75-77. doi:10.1038/s41569-021-00662-w
14. Cohen JI, Hohman P, Fulton R, et al. Kinetics of serum cytokines after primary or repeat vaccination with the smallpox vaccine. J Infect Dis. 2010;201(8):1183-1191. doi:10.1086/651453
15. Minocha PK, Better D, Singh RK, Hoque T. Recurrence of acute myocarditis temporally associated with receipt of the mRNA COVID-19 vaccine in an adolescent male. J Pediatr. 2021;238:321-323. doi:10.1016/j.jpeds.2021.06.035
16. Umei TC, Kishino Y, Watanabe K, et al. Recurrence of myopericarditis following mRNA COVID-19 vaccination in a male adolescent. CJC Open. 2022;4(3):350-352. doi:10.1016/j.cjco.2021.12.002
17. Pasha MA, Isaac S, Khan Z. Recurrent myocarditis following COVID-19 infection and the mRNA vaccine. Cureus. 2022;14(7):e26650. doi:10.7759/cureus.26650
18. Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(14):517-523. Published 2022 Apr 8. doi:10.15585/mmwr.mm7114e1