User login
Pay an annual visit to your office
that your patients might see?
We tend not to notice gradual deterioration in the workplace we inhabit every day: Carpets fade and dull with constant traffic and cleaning; wallpaper and paint accumulate dirt, stains, and damage; furniture gets dirty and dented, fabric rips, hardware goes missing; laminate peels off the edges of desks and cabinets.
When did you last take a good look at your waiting room? How clean is it? Patients expect cleanliness in doctor’s offices, and they expect the reception area to be neat. How are the carpeting and upholstery holding up? Sit in your chairs; how do they feel? Patients don’t appreciate a sore back or bottom from any chairs, especially in a medical office. Consider investing in new furniture that will be attractive and comfortable for your patients.
Look at the decor itself; is it dated or just plain “old-looking?” Any interior designer will tell you they can determine quite accurately when a space was last decorated, simply by the color and style of the materials used. If your office is stuck in the ‘90s, it’s probably time for a change. Even if you don’t find anything obvious, it’s wise to check periodically for subtle evidence of age: Find some patches of protected carpeting and flooring under stationary furniture and compare them to exposed floors.
If your color scheme is hopelessly out of date and style, or if you are just tired of it, change it. Wallpaper and carpeting should be long-wearing industrial quality; paint should be high-quality “eggshell” finish to facilitate cleaning, and everything should be professionally applied. (This is neither the time nor place for do-it-yourself experiments.) Consider updating your overhead lighting. The harsh glow of fluorescent lights amid an uninspired decor creates a sterile, uninviting atmosphere.
During renovation, get your building’s maintenance crew to fix any nagging plumbing, electrical, or heating/air conditioning problems while pipes, ducts, and wires are more readily accessible. This is also a good time to clear out old textbooks, journals, and files that you will never open again, in this digital age.
If your wall decorations are dated and unattractive, now would be a good time to replace at least some of them. This need not be an expensive proposition; a few years ago, I redecorated my exam room walls with framed photos from my travel adventures – to very positive responses from patients and staff alike. If you’re not an artist or photographer, invite a family member, or local artists or talented patients, to display some of their creations on your walls. If you get too many contributions, you can rotate them on a periodic basis.
Plants are great aesthetic accents, yet many offices have little or no plant life. Plants naturally aerate an office suite and help make it feel less stuffy. Also, multiple studies have found that plants promote productivity among office staff and create a sense of calm for apprehensive patients. Improvements like this can make a big difference. They show an attention to detail and a willingness to make your practice as inviting as possible for patients and employees alike.
Spruce-up time is also an excellent opportunity to inventory your medical equipment. We’ve all seen “vintage” offices full of gadgets that were state-of-the-art decades ago. Nostalgia is nice; but would you want to be treated by a physician whose office could be a Smithsonian exhibit titled, “Doctor’s Office Circa 1975?” Neither would your patients, for the most part; many – particularly younger ones – assume that doctors who don’t keep up with technological innovations don’t keep up with anything else, either.
If you’re planning a vacation this year (and I hope you are), that would be the perfect time for a re-do. Your patients will be spared the dust and turmoil, tradespeople won’t have to work around your office hours, and you won’t have to cancel any hours that weren’t already canceled. Best of all, you’ll come back to a clean, fresh environment.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
that your patients might see?
We tend not to notice gradual deterioration in the workplace we inhabit every day: Carpets fade and dull with constant traffic and cleaning; wallpaper and paint accumulate dirt, stains, and damage; furniture gets dirty and dented, fabric rips, hardware goes missing; laminate peels off the edges of desks and cabinets.
When did you last take a good look at your waiting room? How clean is it? Patients expect cleanliness in doctor’s offices, and they expect the reception area to be neat. How are the carpeting and upholstery holding up? Sit in your chairs; how do they feel? Patients don’t appreciate a sore back or bottom from any chairs, especially in a medical office. Consider investing in new furniture that will be attractive and comfortable for your patients.
Look at the decor itself; is it dated or just plain “old-looking?” Any interior designer will tell you they can determine quite accurately when a space was last decorated, simply by the color and style of the materials used. If your office is stuck in the ‘90s, it’s probably time for a change. Even if you don’t find anything obvious, it’s wise to check periodically for subtle evidence of age: Find some patches of protected carpeting and flooring under stationary furniture and compare them to exposed floors.
If your color scheme is hopelessly out of date and style, or if you are just tired of it, change it. Wallpaper and carpeting should be long-wearing industrial quality; paint should be high-quality “eggshell” finish to facilitate cleaning, and everything should be professionally applied. (This is neither the time nor place for do-it-yourself experiments.) Consider updating your overhead lighting. The harsh glow of fluorescent lights amid an uninspired decor creates a sterile, uninviting atmosphere.
During renovation, get your building’s maintenance crew to fix any nagging plumbing, electrical, or heating/air conditioning problems while pipes, ducts, and wires are more readily accessible. This is also a good time to clear out old textbooks, journals, and files that you will never open again, in this digital age.
If your wall decorations are dated and unattractive, now would be a good time to replace at least some of them. This need not be an expensive proposition; a few years ago, I redecorated my exam room walls with framed photos from my travel adventures – to very positive responses from patients and staff alike. If you’re not an artist or photographer, invite a family member, or local artists or talented patients, to display some of their creations on your walls. If you get too many contributions, you can rotate them on a periodic basis.
Plants are great aesthetic accents, yet many offices have little or no plant life. Plants naturally aerate an office suite and help make it feel less stuffy. Also, multiple studies have found that plants promote productivity among office staff and create a sense of calm for apprehensive patients. Improvements like this can make a big difference. They show an attention to detail and a willingness to make your practice as inviting as possible for patients and employees alike.
Spruce-up time is also an excellent opportunity to inventory your medical equipment. We’ve all seen “vintage” offices full of gadgets that were state-of-the-art decades ago. Nostalgia is nice; but would you want to be treated by a physician whose office could be a Smithsonian exhibit titled, “Doctor’s Office Circa 1975?” Neither would your patients, for the most part; many – particularly younger ones – assume that doctors who don’t keep up with technological innovations don’t keep up with anything else, either.
If you’re planning a vacation this year (and I hope you are), that would be the perfect time for a re-do. Your patients will be spared the dust and turmoil, tradespeople won’t have to work around your office hours, and you won’t have to cancel any hours that weren’t already canceled. Best of all, you’ll come back to a clean, fresh environment.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
that your patients might see?
We tend not to notice gradual deterioration in the workplace we inhabit every day: Carpets fade and dull with constant traffic and cleaning; wallpaper and paint accumulate dirt, stains, and damage; furniture gets dirty and dented, fabric rips, hardware goes missing; laminate peels off the edges of desks and cabinets.
When did you last take a good look at your waiting room? How clean is it? Patients expect cleanliness in doctor’s offices, and they expect the reception area to be neat. How are the carpeting and upholstery holding up? Sit in your chairs; how do they feel? Patients don’t appreciate a sore back or bottom from any chairs, especially in a medical office. Consider investing in new furniture that will be attractive and comfortable for your patients.
Look at the decor itself; is it dated or just plain “old-looking?” Any interior designer will tell you they can determine quite accurately when a space was last decorated, simply by the color and style of the materials used. If your office is stuck in the ‘90s, it’s probably time for a change. Even if you don’t find anything obvious, it’s wise to check periodically for subtle evidence of age: Find some patches of protected carpeting and flooring under stationary furniture and compare them to exposed floors.
If your color scheme is hopelessly out of date and style, or if you are just tired of it, change it. Wallpaper and carpeting should be long-wearing industrial quality; paint should be high-quality “eggshell” finish to facilitate cleaning, and everything should be professionally applied. (This is neither the time nor place for do-it-yourself experiments.) Consider updating your overhead lighting. The harsh glow of fluorescent lights amid an uninspired decor creates a sterile, uninviting atmosphere.
During renovation, get your building’s maintenance crew to fix any nagging plumbing, electrical, or heating/air conditioning problems while pipes, ducts, and wires are more readily accessible. This is also a good time to clear out old textbooks, journals, and files that you will never open again, in this digital age.
If your wall decorations are dated and unattractive, now would be a good time to replace at least some of them. This need not be an expensive proposition; a few years ago, I redecorated my exam room walls with framed photos from my travel adventures – to very positive responses from patients and staff alike. If you’re not an artist or photographer, invite a family member, or local artists or talented patients, to display some of their creations on your walls. If you get too many contributions, you can rotate them on a periodic basis.
Plants are great aesthetic accents, yet many offices have little or no plant life. Plants naturally aerate an office suite and help make it feel less stuffy. Also, multiple studies have found that plants promote productivity among office staff and create a sense of calm for apprehensive patients. Improvements like this can make a big difference. They show an attention to detail and a willingness to make your practice as inviting as possible for patients and employees alike.
Spruce-up time is also an excellent opportunity to inventory your medical equipment. We’ve all seen “vintage” offices full of gadgets that were state-of-the-art decades ago. Nostalgia is nice; but would you want to be treated by a physician whose office could be a Smithsonian exhibit titled, “Doctor’s Office Circa 1975?” Neither would your patients, for the most part; many – particularly younger ones – assume that doctors who don’t keep up with technological innovations don’t keep up with anything else, either.
If you’re planning a vacation this year (and I hope you are), that would be the perfect time for a re-do. Your patients will be spared the dust and turmoil, tradespeople won’t have to work around your office hours, and you won’t have to cancel any hours that weren’t already canceled. Best of all, you’ll come back to a clean, fresh environment.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Black Veterans Disproportionately Denied VA Benefits
Black veterans are less likely to have their benefits claims processed and paid than are their White peers because of systemic problems within the US Department of Veterans Affairs, according to a lawsuit filed against the agency.
“A Black veteran who served honorably can walk into the VA, file a disability claim, and be at a significantly higher likelihood of having that claim denied,” said Adam Henderson, a student working with the Yale Law School Veterans Legal Services Clinic, one of several groups connected to the lawsuit.
“The VA has denied countless meritorious applications of Black veterans and thus deprived them and their families of the support that they are entitled to.”
The suit, filed in federal court by the clinic on behalf of Vietnam War veteran Conley Monk Jr., asks for “redress for the harms caused by the failure of VA staff and leaders to administer these benefits programs in a manner free from racial discrimination against Black veterans.”
In a press conference announcing the lawsuit, the effort received backing from Sen. Richard Blumenthal (D, Connecticut) who called it an “unacceptable” situation.
“Black veterans are denied benefits at a very significantly disproportionate rate,” he said. “We know the results. We want to know the reason why.”
The suit stems from an analysis of VA claims records released by the department following an earlier legal action. Between 2001 and 2020, the average denial rate for disability claims filed for Black veterans was 29.5%, significantly above the 24.2% for White veterans.
Attorneys allege the problems date back even further and that VA officials should have known about the racial disparities in the system from previous complaints.
“The negligence of VA leadership, and their failure to train, supervise, monitor and instruct agency officials to take steps to identify and correct racial disparities, led to systematic benefits obstruction for Black veterans,” the suit states.
Monk is a Black disabled Marine Corps veteran who previously sued the military to overturn his less-than-honorable military discharge due to complications from undiagnosed posttraumatic stress disorder.
He was subsequently granted access to a host of veterans benefits but not to retroactive payouts for claims he was denied in the 1970s.
“They didn’t fully compensate me or my family,” he said. “I wasn’t able to give my kids my educational benefits. We should have been receiving checks while they were growing up.”
Along with potential past benefits for Monk, individuals involved with the lawsuit said the move could force the VA to reassess thousands of other unfairly dismissed cases. “For decades [the US government] has allowed racially discriminatory practices to obstruct Black veterans from easily accessing veterans housing, education, and health care benefits with wide-reaching economic consequences for Black veterans and their families,” said Richard Brookshire, executive director of the Black Veterans Project.
“This lawsuit reckons with the shameful history of racism by the Department of Veteran Affairs and seeks to redress long-standing improprieties reverberating across generations of Black military service.”
In a statement, VA press secretary Terrence Hayes did not directly respond to the lawsuit but noted that “throughout history, there have been unacceptable disparities in both VA benefits decisions and military discharge status due to racism, which have wrongly left Black veterans without access to VA care and benefits.”
“We are actively working to right these wrongs, and we will stop at nothing to ensure that all Black veterans get the VA services they have earned and deserve,” he said. “We are currently studying racial disparities in benefits claims decisions, and we will publish the results of that study as soon as they are available.”
Hayes said the department has already begun targeted outreach to Black veterans to help them with claims and is “taking steps to ensure that our claims process combats institutional racism, rather than perpetuating it.”
Black veterans are less likely to have their benefits claims processed and paid than are their White peers because of systemic problems within the US Department of Veterans Affairs, according to a lawsuit filed against the agency.
“A Black veteran who served honorably can walk into the VA, file a disability claim, and be at a significantly higher likelihood of having that claim denied,” said Adam Henderson, a student working with the Yale Law School Veterans Legal Services Clinic, one of several groups connected to the lawsuit.
“The VA has denied countless meritorious applications of Black veterans and thus deprived them and their families of the support that they are entitled to.”
The suit, filed in federal court by the clinic on behalf of Vietnam War veteran Conley Monk Jr., asks for “redress for the harms caused by the failure of VA staff and leaders to administer these benefits programs in a manner free from racial discrimination against Black veterans.”
In a press conference announcing the lawsuit, the effort received backing from Sen. Richard Blumenthal (D, Connecticut) who called it an “unacceptable” situation.
“Black veterans are denied benefits at a very significantly disproportionate rate,” he said. “We know the results. We want to know the reason why.”
The suit stems from an analysis of VA claims records released by the department following an earlier legal action. Between 2001 and 2020, the average denial rate for disability claims filed for Black veterans was 29.5%, significantly above the 24.2% for White veterans.
Attorneys allege the problems date back even further and that VA officials should have known about the racial disparities in the system from previous complaints.
“The negligence of VA leadership, and their failure to train, supervise, monitor and instruct agency officials to take steps to identify and correct racial disparities, led to systematic benefits obstruction for Black veterans,” the suit states.
Monk is a Black disabled Marine Corps veteran who previously sued the military to overturn his less-than-honorable military discharge due to complications from undiagnosed posttraumatic stress disorder.
He was subsequently granted access to a host of veterans benefits but not to retroactive payouts for claims he was denied in the 1970s.
“They didn’t fully compensate me or my family,” he said. “I wasn’t able to give my kids my educational benefits. We should have been receiving checks while they were growing up.”
Along with potential past benefits for Monk, individuals involved with the lawsuit said the move could force the VA to reassess thousands of other unfairly dismissed cases. “For decades [the US government] has allowed racially discriminatory practices to obstruct Black veterans from easily accessing veterans housing, education, and health care benefits with wide-reaching economic consequences for Black veterans and their families,” said Richard Brookshire, executive director of the Black Veterans Project.
“This lawsuit reckons with the shameful history of racism by the Department of Veteran Affairs and seeks to redress long-standing improprieties reverberating across generations of Black military service.”
In a statement, VA press secretary Terrence Hayes did not directly respond to the lawsuit but noted that “throughout history, there have been unacceptable disparities in both VA benefits decisions and military discharge status due to racism, which have wrongly left Black veterans without access to VA care and benefits.”
“We are actively working to right these wrongs, and we will stop at nothing to ensure that all Black veterans get the VA services they have earned and deserve,” he said. “We are currently studying racial disparities in benefits claims decisions, and we will publish the results of that study as soon as they are available.”
Hayes said the department has already begun targeted outreach to Black veterans to help them with claims and is “taking steps to ensure that our claims process combats institutional racism, rather than perpetuating it.”
Black veterans are less likely to have their benefits claims processed and paid than are their White peers because of systemic problems within the US Department of Veterans Affairs, according to a lawsuit filed against the agency.
“A Black veteran who served honorably can walk into the VA, file a disability claim, and be at a significantly higher likelihood of having that claim denied,” said Adam Henderson, a student working with the Yale Law School Veterans Legal Services Clinic, one of several groups connected to the lawsuit.
“The VA has denied countless meritorious applications of Black veterans and thus deprived them and their families of the support that they are entitled to.”
The suit, filed in federal court by the clinic on behalf of Vietnam War veteran Conley Monk Jr., asks for “redress for the harms caused by the failure of VA staff and leaders to administer these benefits programs in a manner free from racial discrimination against Black veterans.”
In a press conference announcing the lawsuit, the effort received backing from Sen. Richard Blumenthal (D, Connecticut) who called it an “unacceptable” situation.
“Black veterans are denied benefits at a very significantly disproportionate rate,” he said. “We know the results. We want to know the reason why.”
The suit stems from an analysis of VA claims records released by the department following an earlier legal action. Between 2001 and 2020, the average denial rate for disability claims filed for Black veterans was 29.5%, significantly above the 24.2% for White veterans.
Attorneys allege the problems date back even further and that VA officials should have known about the racial disparities in the system from previous complaints.
“The negligence of VA leadership, and their failure to train, supervise, monitor and instruct agency officials to take steps to identify and correct racial disparities, led to systematic benefits obstruction for Black veterans,” the suit states.
Monk is a Black disabled Marine Corps veteran who previously sued the military to overturn his less-than-honorable military discharge due to complications from undiagnosed posttraumatic stress disorder.
He was subsequently granted access to a host of veterans benefits but not to retroactive payouts for claims he was denied in the 1970s.
“They didn’t fully compensate me or my family,” he said. “I wasn’t able to give my kids my educational benefits. We should have been receiving checks while they were growing up.”
Along with potential past benefits for Monk, individuals involved with the lawsuit said the move could force the VA to reassess thousands of other unfairly dismissed cases. “For decades [the US government] has allowed racially discriminatory practices to obstruct Black veterans from easily accessing veterans housing, education, and health care benefits with wide-reaching economic consequences for Black veterans and their families,” said Richard Brookshire, executive director of the Black Veterans Project.
“This lawsuit reckons with the shameful history of racism by the Department of Veteran Affairs and seeks to redress long-standing improprieties reverberating across generations of Black military service.”
In a statement, VA press secretary Terrence Hayes did not directly respond to the lawsuit but noted that “throughout history, there have been unacceptable disparities in both VA benefits decisions and military discharge status due to racism, which have wrongly left Black veterans without access to VA care and benefits.”
“We are actively working to right these wrongs, and we will stop at nothing to ensure that all Black veterans get the VA services they have earned and deserve,” he said. “We are currently studying racial disparities in benefits claims decisions, and we will publish the results of that study as soon as they are available.”
Hayes said the department has already begun targeted outreach to Black veterans to help them with claims and is “taking steps to ensure that our claims process combats institutional racism, rather than perpetuating it.”
Metformin monotherapy not always best start in type 2 diabetes
Metformin failure in people with type 2 diabetes is very common, particularly among those with high hemoglobin A1c levels at the time of diagnosis, new findings suggest.
An analysis of electronic health record data for more than 22,000 patients starting metformin at three U.S. clinical sites found that over 40% experienced metformin failure.
This was defined as either failure to achieve or maintain A1c less than 7% within 18 months or the use of additional glucose-lowering medications.
Other predictors that metformin use wouldn’t be successful included increasing age, male sex, and race/ethnicity. However, the latter ceased to be linked after adjustment for other clinical risk factors.
“Our study results suggest increased monitoring with potentially earlier treatment intensification to achieve glycemic control may be appropriate in patients with clinical parameters described in this paper,” Suzette J. Bielinski, PhD, and colleagues wrote.
“Further, these results call into question the ubiquitous use of metformin as the first-line therapy and suggest a more individualized approach may be needed to optimize therapy,” they added in their article, published online in the Journal of Clinical Endocrinology and Metabolism.
The study is also noteworthy in that it demonstrated the feasibility of using EHR data with a machine-learning approach to discover risk biomarkers, Dr. Bielinski, professor of epidemiology at the Mayo Clinic, Rochester, Minn., said in an interview.
“We wanted to repurpose clinical data to answer questions ... I think more studies using these types of techniques repurposing data meant for one thing could potentially impact care in other domains. ... If we can get the bang for the buck from all these data that we generate on people I just think it will improve health care and maybe save health care dollars.”
Baseline A1c strongest predictor of metformin failure
The investigators identified a total of 22,047 metformin initiators from three clinical primary care sites: the University of Mississippi’s Jackson centers, which serves a mostly African American population, the Mountain Park Health Center in Arizona, a seven-clinic federally qualified community health center in Phoenix that serves a mostly Latino population, and the Rochester Epidemiology Project, which includes the Mayo Clinic and serves a primarily White population.
Overall, a total of 43% (9,407) of patients met one of two criteria for metformin failure by 18 months. Among those, median time to failure on metformin was 3.9 months.
Unadjusted failure rates were higher among African Americans, Hispanics, and other racial groups, compared with non-Hispanic White patients.
However, the racial groups also differed by baseline characteristics. Mean A1c was 7.7% overall, 8.1% for the African American group, 7.9% for Asians, and 8.2% for Hispanics, compared with 7.6% for non-Hispanic Whites.
Of 150 clinical factors examined, higher A1c was the strongest predictor of metformin failure, with a rapid increase in risk appearing between 7.5% and 8.0%.
“The slope is steep. It gives us some clinical guidance,” Dr. Bielinski said.
Other variables positively correlated with metformin failure included “diabetes with complications,” increased age, and higher levels of potassium, triglycerides, heart rate, and mean cell hemoglobin.
Factors inversely correlated with metformin failure were having received screening for other suspected conditions and medical examination/evaluation, and lower levels of sodium, albumin, and HDL cholesterol.
Three variables – body mass index, LDL cholesterol, and creatinine – had a U-shaped relationship with metformin failure, so that both high and low values were associated with increased risk.
“The racial/ethnic differences disappeared once other clinical factors were considered suggesting that the biological response to metformin is similar regardless of race/ethnicity,” Dr. Bielinski and colleagues wrote.
They also noted that the abnormal lab results which correlated with metformin failure “likely represent biomarkers for chronic illnesses. However, the effect size for lab abnormalities was small compared with that of baseline A1c.”
Dr. Bielinski urged caution in interpreting the findings. “Electronic health records data have limitations. We have evidence that these people were prescribed metformin. We have no idea if they took it. ... I would really be hesitant to be too strong in making clinical recommendations.”
However, she said that the data are “suggestive to say maybe we need to have some kind of threshold where if someone comes in with an A1c of X that they go on dual therapy right away. I think this is opening the door to that.”
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Metformin failure in people with type 2 diabetes is very common, particularly among those with high hemoglobin A1c levels at the time of diagnosis, new findings suggest.
An analysis of electronic health record data for more than 22,000 patients starting metformin at three U.S. clinical sites found that over 40% experienced metformin failure.
This was defined as either failure to achieve or maintain A1c less than 7% within 18 months or the use of additional glucose-lowering medications.
Other predictors that metformin use wouldn’t be successful included increasing age, male sex, and race/ethnicity. However, the latter ceased to be linked after adjustment for other clinical risk factors.
“Our study results suggest increased monitoring with potentially earlier treatment intensification to achieve glycemic control may be appropriate in patients with clinical parameters described in this paper,” Suzette J. Bielinski, PhD, and colleagues wrote.
“Further, these results call into question the ubiquitous use of metformin as the first-line therapy and suggest a more individualized approach may be needed to optimize therapy,” they added in their article, published online in the Journal of Clinical Endocrinology and Metabolism.
The study is also noteworthy in that it demonstrated the feasibility of using EHR data with a machine-learning approach to discover risk biomarkers, Dr. Bielinski, professor of epidemiology at the Mayo Clinic, Rochester, Minn., said in an interview.
“We wanted to repurpose clinical data to answer questions ... I think more studies using these types of techniques repurposing data meant for one thing could potentially impact care in other domains. ... If we can get the bang for the buck from all these data that we generate on people I just think it will improve health care and maybe save health care dollars.”
Baseline A1c strongest predictor of metformin failure
The investigators identified a total of 22,047 metformin initiators from three clinical primary care sites: the University of Mississippi’s Jackson centers, which serves a mostly African American population, the Mountain Park Health Center in Arizona, a seven-clinic federally qualified community health center in Phoenix that serves a mostly Latino population, and the Rochester Epidemiology Project, which includes the Mayo Clinic and serves a primarily White population.
Overall, a total of 43% (9,407) of patients met one of two criteria for metformin failure by 18 months. Among those, median time to failure on metformin was 3.9 months.
Unadjusted failure rates were higher among African Americans, Hispanics, and other racial groups, compared with non-Hispanic White patients.
However, the racial groups also differed by baseline characteristics. Mean A1c was 7.7% overall, 8.1% for the African American group, 7.9% for Asians, and 8.2% for Hispanics, compared with 7.6% for non-Hispanic Whites.
Of 150 clinical factors examined, higher A1c was the strongest predictor of metformin failure, with a rapid increase in risk appearing between 7.5% and 8.0%.
“The slope is steep. It gives us some clinical guidance,” Dr. Bielinski said.
Other variables positively correlated with metformin failure included “diabetes with complications,” increased age, and higher levels of potassium, triglycerides, heart rate, and mean cell hemoglobin.
Factors inversely correlated with metformin failure were having received screening for other suspected conditions and medical examination/evaluation, and lower levels of sodium, albumin, and HDL cholesterol.
Three variables – body mass index, LDL cholesterol, and creatinine – had a U-shaped relationship with metformin failure, so that both high and low values were associated with increased risk.
“The racial/ethnic differences disappeared once other clinical factors were considered suggesting that the biological response to metformin is similar regardless of race/ethnicity,” Dr. Bielinski and colleagues wrote.
They also noted that the abnormal lab results which correlated with metformin failure “likely represent biomarkers for chronic illnesses. However, the effect size for lab abnormalities was small compared with that of baseline A1c.”
Dr. Bielinski urged caution in interpreting the findings. “Electronic health records data have limitations. We have evidence that these people were prescribed metformin. We have no idea if they took it. ... I would really be hesitant to be too strong in making clinical recommendations.”
However, she said that the data are “suggestive to say maybe we need to have some kind of threshold where if someone comes in with an A1c of X that they go on dual therapy right away. I think this is opening the door to that.”
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Metformin failure in people with type 2 diabetes is very common, particularly among those with high hemoglobin A1c levels at the time of diagnosis, new findings suggest.
An analysis of electronic health record data for more than 22,000 patients starting metformin at three U.S. clinical sites found that over 40% experienced metformin failure.
This was defined as either failure to achieve or maintain A1c less than 7% within 18 months or the use of additional glucose-lowering medications.
Other predictors that metformin use wouldn’t be successful included increasing age, male sex, and race/ethnicity. However, the latter ceased to be linked after adjustment for other clinical risk factors.
“Our study results suggest increased monitoring with potentially earlier treatment intensification to achieve glycemic control may be appropriate in patients with clinical parameters described in this paper,” Suzette J. Bielinski, PhD, and colleagues wrote.
“Further, these results call into question the ubiquitous use of metformin as the first-line therapy and suggest a more individualized approach may be needed to optimize therapy,” they added in their article, published online in the Journal of Clinical Endocrinology and Metabolism.
The study is also noteworthy in that it demonstrated the feasibility of using EHR data with a machine-learning approach to discover risk biomarkers, Dr. Bielinski, professor of epidemiology at the Mayo Clinic, Rochester, Minn., said in an interview.
“We wanted to repurpose clinical data to answer questions ... I think more studies using these types of techniques repurposing data meant for one thing could potentially impact care in other domains. ... If we can get the bang for the buck from all these data that we generate on people I just think it will improve health care and maybe save health care dollars.”
Baseline A1c strongest predictor of metformin failure
The investigators identified a total of 22,047 metformin initiators from three clinical primary care sites: the University of Mississippi’s Jackson centers, which serves a mostly African American population, the Mountain Park Health Center in Arizona, a seven-clinic federally qualified community health center in Phoenix that serves a mostly Latino population, and the Rochester Epidemiology Project, which includes the Mayo Clinic and serves a primarily White population.
Overall, a total of 43% (9,407) of patients met one of two criteria for metformin failure by 18 months. Among those, median time to failure on metformin was 3.9 months.
Unadjusted failure rates were higher among African Americans, Hispanics, and other racial groups, compared with non-Hispanic White patients.
However, the racial groups also differed by baseline characteristics. Mean A1c was 7.7% overall, 8.1% for the African American group, 7.9% for Asians, and 8.2% for Hispanics, compared with 7.6% for non-Hispanic Whites.
Of 150 clinical factors examined, higher A1c was the strongest predictor of metformin failure, with a rapid increase in risk appearing between 7.5% and 8.0%.
“The slope is steep. It gives us some clinical guidance,” Dr. Bielinski said.
Other variables positively correlated with metformin failure included “diabetes with complications,” increased age, and higher levels of potassium, triglycerides, heart rate, and mean cell hemoglobin.
Factors inversely correlated with metformin failure were having received screening for other suspected conditions and medical examination/evaluation, and lower levels of sodium, albumin, and HDL cholesterol.
Three variables – body mass index, LDL cholesterol, and creatinine – had a U-shaped relationship with metformin failure, so that both high and low values were associated with increased risk.
“The racial/ethnic differences disappeared once other clinical factors were considered suggesting that the biological response to metformin is similar regardless of race/ethnicity,” Dr. Bielinski and colleagues wrote.
They also noted that the abnormal lab results which correlated with metformin failure “likely represent biomarkers for chronic illnesses. However, the effect size for lab abnormalities was small compared with that of baseline A1c.”
Dr. Bielinski urged caution in interpreting the findings. “Electronic health records data have limitations. We have evidence that these people were prescribed metformin. We have no idea if they took it. ... I would really be hesitant to be too strong in making clinical recommendations.”
However, she said that the data are “suggestive to say maybe we need to have some kind of threshold where if someone comes in with an A1c of X that they go on dual therapy right away. I think this is opening the door to that.”
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Atrial failure or insufficiency: A new syndrome
Atrial dysfunction, widely considered a marker or consequence of other heart diseases, is a relevant clinical entity, which is why it is justified to define atrial failure or insufficiency as “a new syndrome that all cardiologists should be aware of,” said Adrián Baranchuk, MD, PhD, professor of medicine at Queen’s University, Kingston, Ont., during the 2022 48th Argentine Congress of Cardiology in Buenos Aires.
“The atria are like the heart’s silly sisters and can fail just like the ventricle fails. Understanding their function and dysfunction helps us to understand heart failure. And as electrophysiologists and clinical cardiologists, we have to embrace this concept and understand it in depth,” Dr. Baranchuk, president-elect of the Inter-American Society of Cardiology, said in an interview.
The specialist first proposed atrial failure as an entity or syndrome in early 2020 in an article in the Journal of the American College of Cardiology. His four collaborators included the experienced Eugene Braunwald, MD, from Brigham and Women’s Hospital, Boston, and Antoni Bayés de Luna, PhD, from the department of medicine of the autonomous University of Barcelona.
Pathology despite function
“In many patients with heart failure, the pump function is preserved, but what causes the pathology? For the last 5-10 years, attention has been focused on the ventricle: whether it contracts poorly or whether it contracts properly and relaxes poorly. However, we have also seen patients in whom the ventricle contracts properly and relaxes properly. Where else can we look? We started looking at atrial contraction, especially the left atrium,” recalled Dr. Baranchuk.
He and his colleagues proposed the following consensus definition of atrial failure or insufficiency: any atrial dysfunction (anatomical, mechanical, electrical, and rheological, including blood homeostasis) that causes impaired function, heart symptoms, and a worsening of quality of life (or life expectancy) in the absence of significant valvular or ventricular abnormalities.
In his presentation, recorded and projected by video from Canada, Dr. Baranchuk pointed out that there are two large groups of causes of atrial failure: one that has to do with electrical disorders of atrial and interatrial contraction and another related to the progressive development of fibrosis, which gradually leads to dyssynchrony in interatrial contraction, pump failure, and impaired atrial function as a reservoir and as a conduit.
“In turn, these mechanisms trigger neurohormonal alterations that perpetuate atrial failure, so it is not just a matter of progressive fibrosis, which is very difficult to treat, but also of constant neurohormonal activation that guarantees that these phenomena never resolve,” said Dr. Baranchuk. The manifestations or end point of this cascade of events are the known ones: stroke, ischemia, and heart failure.
New entity necessary?
Defining atrial failure or insufficiency as a clinical entity not only restores the hierarchy of the atria in cardiac function, which was already postulated by William Harvey in 1628, but also enables new lines of research that would eventually allow timely preventive interventions.
One key is early recognition of partial or total interatrial block by analyzing the characteristics of the P wave on the electrocardiogram, which could serve to prevent progression to atrial fibrillation. Left atrial enlargement can also be detected by echocardiography.
“When the contractile impairment is severe and you are in atrial fibrillation, all that remains is to apply patches. The strategy is to correct risk factors beforehand, such as high blood pressure, sleep apnea, or high-dose alcohol consumption, as well as tirelessly searching for atrial fibrillation, with Holter electrocardiograms, continuous monitoring devices, such as Apple Watch, KardiaMobile, or an implantable loop recorder,” Dr. Baranchuk said in an interview.
Two ongoing or planned studies, ARCADIA and AMIABLE, will seek to determine whether anticoagulation in patients with elevated cardiovascular risk scores and any of these atrial disorders that have not yet led to atrial fibrillation could reduce the incidence of stroke.
The strategy has a rational basis. In a subanalysis of raw data from the NAVIGATE ESUS study in patients with embolic stroke of unknown cause, Dr. Baranchuk estimated that the presence of interatrial block was a tenfold higher predictor of the risk of experiencing a second stroke. Another 2018 observational study in which he participated found that in outpatients with heart failure, advanced interatrial block approximately tripled the risk of developing atrial fibrillation and ischemic stroke.
For Dr. Baranchuk, other questions that still need to be answered include whether drugs used for heart failure with preserved ejection fraction can be useful in primary atrial failure or whether specific drugs can be repositioned or developed to suppress or slow the process of fibrosis. “From generating the clinical concept, many lines of research are enabled.”
“The concept of atrial failure is very interesting and opens our eyes to treatments,” another speaker at the session, Alejo Tronconi, MD, a cardiologist and electrophysiologist at the Cardiovascular Institute of the South, Cipolletti, Argentina, said in an interview.
“It is necessary to cut circuits that have been extensively studied in heart failure models, and now we are beginning to see their participation in atrial dysfunction,” he said.
Dr. Baranchuk and Dr. Tronconi declared no relevant financial conflict of interest.
A version of this article first appeared on Medscape.com.
Atrial dysfunction, widely considered a marker or consequence of other heart diseases, is a relevant clinical entity, which is why it is justified to define atrial failure or insufficiency as “a new syndrome that all cardiologists should be aware of,” said Adrián Baranchuk, MD, PhD, professor of medicine at Queen’s University, Kingston, Ont., during the 2022 48th Argentine Congress of Cardiology in Buenos Aires.
“The atria are like the heart’s silly sisters and can fail just like the ventricle fails. Understanding their function and dysfunction helps us to understand heart failure. And as electrophysiologists and clinical cardiologists, we have to embrace this concept and understand it in depth,” Dr. Baranchuk, president-elect of the Inter-American Society of Cardiology, said in an interview.
The specialist first proposed atrial failure as an entity or syndrome in early 2020 in an article in the Journal of the American College of Cardiology. His four collaborators included the experienced Eugene Braunwald, MD, from Brigham and Women’s Hospital, Boston, and Antoni Bayés de Luna, PhD, from the department of medicine of the autonomous University of Barcelona.
Pathology despite function
“In many patients with heart failure, the pump function is preserved, but what causes the pathology? For the last 5-10 years, attention has been focused on the ventricle: whether it contracts poorly or whether it contracts properly and relaxes poorly. However, we have also seen patients in whom the ventricle contracts properly and relaxes properly. Where else can we look? We started looking at atrial contraction, especially the left atrium,” recalled Dr. Baranchuk.
He and his colleagues proposed the following consensus definition of atrial failure or insufficiency: any atrial dysfunction (anatomical, mechanical, electrical, and rheological, including blood homeostasis) that causes impaired function, heart symptoms, and a worsening of quality of life (or life expectancy) in the absence of significant valvular or ventricular abnormalities.
In his presentation, recorded and projected by video from Canada, Dr. Baranchuk pointed out that there are two large groups of causes of atrial failure: one that has to do with electrical disorders of atrial and interatrial contraction and another related to the progressive development of fibrosis, which gradually leads to dyssynchrony in interatrial contraction, pump failure, and impaired atrial function as a reservoir and as a conduit.
“In turn, these mechanisms trigger neurohormonal alterations that perpetuate atrial failure, so it is not just a matter of progressive fibrosis, which is very difficult to treat, but also of constant neurohormonal activation that guarantees that these phenomena never resolve,” said Dr. Baranchuk. The manifestations or end point of this cascade of events are the known ones: stroke, ischemia, and heart failure.
New entity necessary?
Defining atrial failure or insufficiency as a clinical entity not only restores the hierarchy of the atria in cardiac function, which was already postulated by William Harvey in 1628, but also enables new lines of research that would eventually allow timely preventive interventions.
One key is early recognition of partial or total interatrial block by analyzing the characteristics of the P wave on the electrocardiogram, which could serve to prevent progression to atrial fibrillation. Left atrial enlargement can also be detected by echocardiography.
“When the contractile impairment is severe and you are in atrial fibrillation, all that remains is to apply patches. The strategy is to correct risk factors beforehand, such as high blood pressure, sleep apnea, or high-dose alcohol consumption, as well as tirelessly searching for atrial fibrillation, with Holter electrocardiograms, continuous monitoring devices, such as Apple Watch, KardiaMobile, or an implantable loop recorder,” Dr. Baranchuk said in an interview.
Two ongoing or planned studies, ARCADIA and AMIABLE, will seek to determine whether anticoagulation in patients with elevated cardiovascular risk scores and any of these atrial disorders that have not yet led to atrial fibrillation could reduce the incidence of stroke.
The strategy has a rational basis. In a subanalysis of raw data from the NAVIGATE ESUS study in patients with embolic stroke of unknown cause, Dr. Baranchuk estimated that the presence of interatrial block was a tenfold higher predictor of the risk of experiencing a second stroke. Another 2018 observational study in which he participated found that in outpatients with heart failure, advanced interatrial block approximately tripled the risk of developing atrial fibrillation and ischemic stroke.
For Dr. Baranchuk, other questions that still need to be answered include whether drugs used for heart failure with preserved ejection fraction can be useful in primary atrial failure or whether specific drugs can be repositioned or developed to suppress or slow the process of fibrosis. “From generating the clinical concept, many lines of research are enabled.”
“The concept of atrial failure is very interesting and opens our eyes to treatments,” another speaker at the session, Alejo Tronconi, MD, a cardiologist and electrophysiologist at the Cardiovascular Institute of the South, Cipolletti, Argentina, said in an interview.
“It is necessary to cut circuits that have been extensively studied in heart failure models, and now we are beginning to see their participation in atrial dysfunction,” he said.
Dr. Baranchuk and Dr. Tronconi declared no relevant financial conflict of interest.
A version of this article first appeared on Medscape.com.
Atrial dysfunction, widely considered a marker or consequence of other heart diseases, is a relevant clinical entity, which is why it is justified to define atrial failure or insufficiency as “a new syndrome that all cardiologists should be aware of,” said Adrián Baranchuk, MD, PhD, professor of medicine at Queen’s University, Kingston, Ont., during the 2022 48th Argentine Congress of Cardiology in Buenos Aires.
“The atria are like the heart’s silly sisters and can fail just like the ventricle fails. Understanding their function and dysfunction helps us to understand heart failure. And as electrophysiologists and clinical cardiologists, we have to embrace this concept and understand it in depth,” Dr. Baranchuk, president-elect of the Inter-American Society of Cardiology, said in an interview.
The specialist first proposed atrial failure as an entity or syndrome in early 2020 in an article in the Journal of the American College of Cardiology. His four collaborators included the experienced Eugene Braunwald, MD, from Brigham and Women’s Hospital, Boston, and Antoni Bayés de Luna, PhD, from the department of medicine of the autonomous University of Barcelona.
Pathology despite function
“In many patients with heart failure, the pump function is preserved, but what causes the pathology? For the last 5-10 years, attention has been focused on the ventricle: whether it contracts poorly or whether it contracts properly and relaxes poorly. However, we have also seen patients in whom the ventricle contracts properly and relaxes properly. Where else can we look? We started looking at atrial contraction, especially the left atrium,” recalled Dr. Baranchuk.
He and his colleagues proposed the following consensus definition of atrial failure or insufficiency: any atrial dysfunction (anatomical, mechanical, electrical, and rheological, including blood homeostasis) that causes impaired function, heart symptoms, and a worsening of quality of life (or life expectancy) in the absence of significant valvular or ventricular abnormalities.
In his presentation, recorded and projected by video from Canada, Dr. Baranchuk pointed out that there are two large groups of causes of atrial failure: one that has to do with electrical disorders of atrial and interatrial contraction and another related to the progressive development of fibrosis, which gradually leads to dyssynchrony in interatrial contraction, pump failure, and impaired atrial function as a reservoir and as a conduit.
“In turn, these mechanisms trigger neurohormonal alterations that perpetuate atrial failure, so it is not just a matter of progressive fibrosis, which is very difficult to treat, but also of constant neurohormonal activation that guarantees that these phenomena never resolve,” said Dr. Baranchuk. The manifestations or end point of this cascade of events are the known ones: stroke, ischemia, and heart failure.
New entity necessary?
Defining atrial failure or insufficiency as a clinical entity not only restores the hierarchy of the atria in cardiac function, which was already postulated by William Harvey in 1628, but also enables new lines of research that would eventually allow timely preventive interventions.
One key is early recognition of partial or total interatrial block by analyzing the characteristics of the P wave on the electrocardiogram, which could serve to prevent progression to atrial fibrillation. Left atrial enlargement can also be detected by echocardiography.
“When the contractile impairment is severe and you are in atrial fibrillation, all that remains is to apply patches. The strategy is to correct risk factors beforehand, such as high blood pressure, sleep apnea, or high-dose alcohol consumption, as well as tirelessly searching for atrial fibrillation, with Holter electrocardiograms, continuous monitoring devices, such as Apple Watch, KardiaMobile, or an implantable loop recorder,” Dr. Baranchuk said in an interview.
Two ongoing or planned studies, ARCADIA and AMIABLE, will seek to determine whether anticoagulation in patients with elevated cardiovascular risk scores and any of these atrial disorders that have not yet led to atrial fibrillation could reduce the incidence of stroke.
The strategy has a rational basis. In a subanalysis of raw data from the NAVIGATE ESUS study in patients with embolic stroke of unknown cause, Dr. Baranchuk estimated that the presence of interatrial block was a tenfold higher predictor of the risk of experiencing a second stroke. Another 2018 observational study in which he participated found that in outpatients with heart failure, advanced interatrial block approximately tripled the risk of developing atrial fibrillation and ischemic stroke.
For Dr. Baranchuk, other questions that still need to be answered include whether drugs used for heart failure with preserved ejection fraction can be useful in primary atrial failure or whether specific drugs can be repositioned or developed to suppress or slow the process of fibrosis. “From generating the clinical concept, many lines of research are enabled.”
“The concept of atrial failure is very interesting and opens our eyes to treatments,” another speaker at the session, Alejo Tronconi, MD, a cardiologist and electrophysiologist at the Cardiovascular Institute of the South, Cipolletti, Argentina, said in an interview.
“It is necessary to cut circuits that have been extensively studied in heart failure models, and now we are beginning to see their participation in atrial dysfunction,” he said.
Dr. Baranchuk and Dr. Tronconi declared no relevant financial conflict of interest.
A version of this article first appeared on Medscape.com.
Exophytic Firm Papulonodule on the Labia in a Patient With Nonspecific Gastrointestinal Symptoms
The Diagnosis: Cutaneous Crohn Disease
Kinyoun and Grocott-Gomori methenamine-silver staining of the labial biopsy were negative for mycobacteria and fungi, respectively. A complete blood cell count, erythrocyte sedimentation rate, C-reactive protein, celiac disease serologies, stool occult blood, and stool calprotectin laboratory test results were within reference range. Magnetic resonance imaging of the pelvis demonstrated an anal fissure extending from the anal verge at the 6 o’clock position, abnormal T2 bright signal in the skin of the buttocks and perineum extending to the labia, and mild mucosal enhancement of the rectal and anal mucosa. Esophagogastroduodenoscopy and magnetic resonance elastography were unremarkable. Colonoscopy demonstrated scattered superficial erythematous patches and erosions in the rectum. Histologically, there was mild to moderately active colitis in the rectum with no evidence of chronicity. Given our patient’s labial edema and exophytic papulonodule (Figure 1) in the setting of nonspecific gastrointestinal symptoms and granulomatous dermatitis seen on pathology (Figure 2), she was diagnosed with cutaneous Crohn disease (CD).
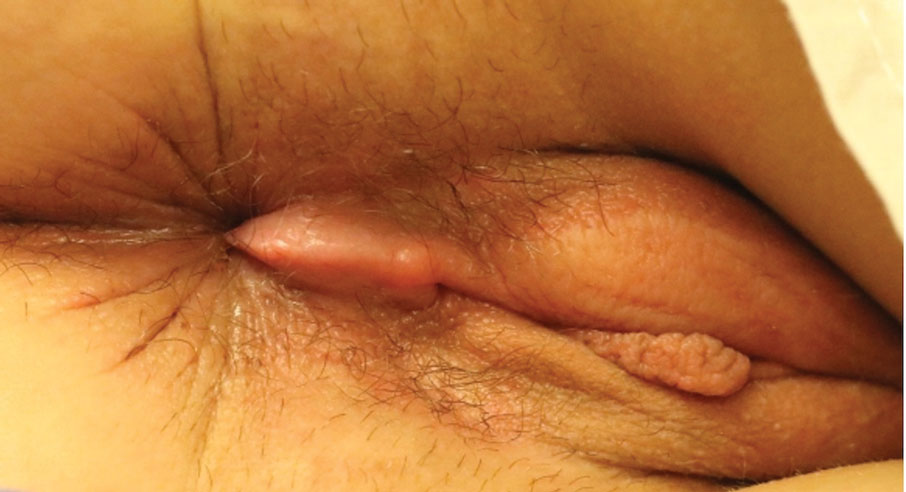
In our patient, labial biopsy was necessary to definitively diagnose CD. Prior to biopsy of the lesion, our patient was diagnosed with irritable bowel syndrome with constipation leading to an anal fissure and skin tag due to lack of laboratory, imaging, and colonoscopy findings commonly associated with CD. Her biopsy results and gastrointestinal symptoms made these diagnoses, as well as condyloma or a large sentinel skin tag, less likely.
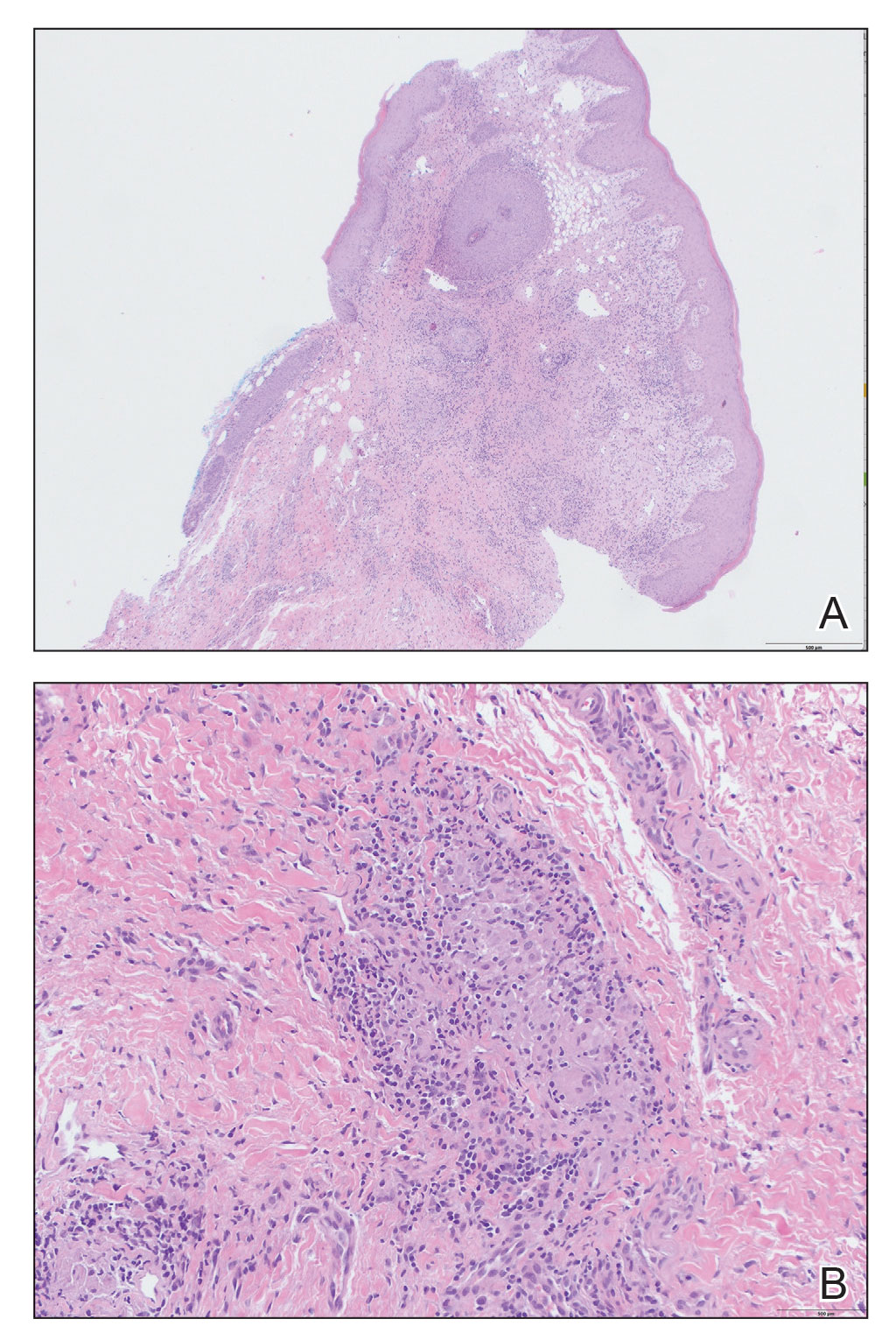
Extraintestinal findings of CD, especially cutaneous manifestations, are relatively frequent and may be present in as many as 44% of patients.1,2 Cutaneous CD often is characterized based on pathogenic mechanisms as either reactive, associated, or CD specific. Reactive cutaneous manifestations include erythema nodosum, pyoderma gangrenosum, and oral aphthae. Associated cutaneous manifestations include vitiligo, palmar erythema, and palmoplantar pustulosis.2 Crohn disease–specific manifestations, including genital or extragenital metastatic CD (MCD), fistulas, and oral involvement, are granulomatous in nature, similar to intestinal CD. Genital manifestations of MCD include edema, erythema, fissures, and/or ulceration of the vulva, penis, or scrotum. Labial swelling is the most common presenting symptom of MCD in females in both pediatric and adult age groups.2 Lymphedema, skin tags, and condylomalike growths also can be seen but are relatively less common.2
Given the labial edema, exophytic papulonodule, and granulomatous dermatitis seen on histopathology, our patient likely fit into the MCD category.2 In adults, most instances of MCD arise in the setting of well-established intestinal CD disease,3 whereas in children 86% of cases occur in patients without concurrent intestinal CD.2
Given the nonspecific and variable presentation of MCD, the differential diagnosis is broad. The differential diagnosis could include infectious etiologies such as condyloma acuminatum (human papillomavirus); syphilitic chancre; or mycobacterial, bacterial, fungal, or parasitic vulvovaginitis. Sexual abuse, sarcoidosis, Behçet disease, or hidradenitis suppurativa, among other diagnoses, also should be considered. Diagnostic workup should include biopsy of the lesion with special stains, polarizing microscopy, and tissue cultures.4 A thorough evaluation for gastrointestinal CD should be completed after diagnosis.3
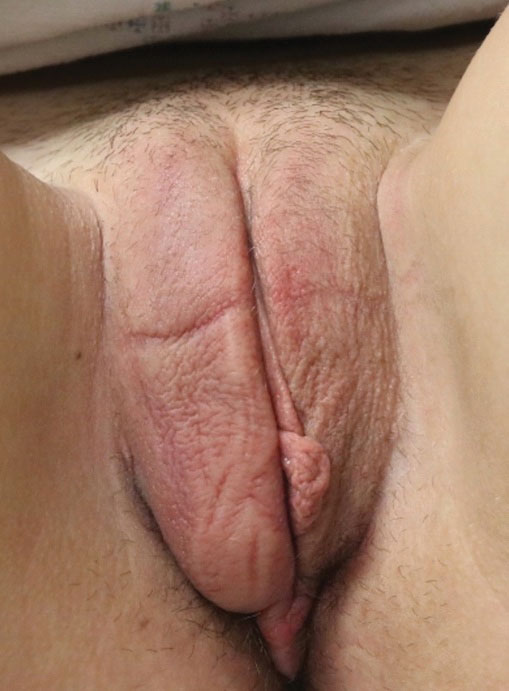
The clinical course of vulvar CD can be unpredictable, with some cases healing spontaneously but most persisting despite treatment and sometimes prompting surgical removal.2,4 Early recognition is crucial, as long-standing MCD lesions can be therapy resistant.5 Due to the rarity of the condition and lack of data, there is a lack of treatment consensus for MCD. In 2014, the American Academy of Dermatology published treatment guidelines recommending superpotent topical steroids or topical tacrolimus as first-line therapy. Next-line therapy includes oral metronidazole, followed by prednisolone if still symptomatic.3 Treatment-resistant disease can warrant treatment with immunomodulators or tumor necrosis factor α inhibitors. Our patient was started on adalimumab; after just 2 months of therapy, the labial swelling decreased and the exophytic nodule was less firm and smaller.
Metastatic CD is a rare manifestation of cutaneous CD and can be present in the absence of gastrointestinal disease.3 This case demonstrates the importance of recognizing the cutaneous signs of CD and the necessity of lesional biopsy for the diagnosis of MCD, as our patient presented with nonspecific gastrointestinal symptoms and a diagnostic workup, including endoscopies, that proved inconclusive for the diagnosis of CD.
- Antonelli E, Bassotti G, Tramontana M, et al. Dermatological manifestations in inflammatory bowel diseases. J Clin Med. 2021;10:1-16. doi:10.3390/JCM10020364
- Schneider SL, Foster K, Patel D, et al. Cutaneous manifestations of metastatic Crohn’s disease. Pediatr Dermatol. 2018;35:566-574. doi:10.1111/PDE.13565
- Kurtzman DJB, Jones T, Lian F, et al. Metastatic Crohn’s disease: a review and approach to therapy. J Am Acad Dermatol. 2014;71:804-813. doi:10.1016/J.JAAD.2014.04.002
- Barret M, De Parades V, Battistella M, et al. Crohn’s disease of the vulva. J Crohns Colitis. 2014;8:563-570. doi:10.1016/J.CROHNS.2013.10.009
- Aberumand B, Howard J, Howard J. Metastatic Crohn’s disease: an approach to an uncommon but important cutaneous disorder [published online January 3, 2017]. Biomed Res Int. 2017;2017:8192150. doi:10.1155/2017/8192150
The Diagnosis: Cutaneous Crohn Disease
Kinyoun and Grocott-Gomori methenamine-silver staining of the labial biopsy were negative for mycobacteria and fungi, respectively. A complete blood cell count, erythrocyte sedimentation rate, C-reactive protein, celiac disease serologies, stool occult blood, and stool calprotectin laboratory test results were within reference range. Magnetic resonance imaging of the pelvis demonstrated an anal fissure extending from the anal verge at the 6 o’clock position, abnormal T2 bright signal in the skin of the buttocks and perineum extending to the labia, and mild mucosal enhancement of the rectal and anal mucosa. Esophagogastroduodenoscopy and magnetic resonance elastography were unremarkable. Colonoscopy demonstrated scattered superficial erythematous patches and erosions in the rectum. Histologically, there was mild to moderately active colitis in the rectum with no evidence of chronicity. Given our patient’s labial edema and exophytic papulonodule (Figure 1) in the setting of nonspecific gastrointestinal symptoms and granulomatous dermatitis seen on pathology (Figure 2), she was diagnosed with cutaneous Crohn disease (CD).

In our patient, labial biopsy was necessary to definitively diagnose CD. Prior to biopsy of the lesion, our patient was diagnosed with irritable bowel syndrome with constipation leading to an anal fissure and skin tag due to lack of laboratory, imaging, and colonoscopy findings commonly associated with CD. Her biopsy results and gastrointestinal symptoms made these diagnoses, as well as condyloma or a large sentinel skin tag, less likely.

Extraintestinal findings of CD, especially cutaneous manifestations, are relatively frequent and may be present in as many as 44% of patients.1,2 Cutaneous CD often is characterized based on pathogenic mechanisms as either reactive, associated, or CD specific. Reactive cutaneous manifestations include erythema nodosum, pyoderma gangrenosum, and oral aphthae. Associated cutaneous manifestations include vitiligo, palmar erythema, and palmoplantar pustulosis.2 Crohn disease–specific manifestations, including genital or extragenital metastatic CD (MCD), fistulas, and oral involvement, are granulomatous in nature, similar to intestinal CD. Genital manifestations of MCD include edema, erythema, fissures, and/or ulceration of the vulva, penis, or scrotum. Labial swelling is the most common presenting symptom of MCD in females in both pediatric and adult age groups.2 Lymphedema, skin tags, and condylomalike growths also can be seen but are relatively less common.2
Given the labial edema, exophytic papulonodule, and granulomatous dermatitis seen on histopathology, our patient likely fit into the MCD category.2 In adults, most instances of MCD arise in the setting of well-established intestinal CD disease,3 whereas in children 86% of cases occur in patients without concurrent intestinal CD.2
Given the nonspecific and variable presentation of MCD, the differential diagnosis is broad. The differential diagnosis could include infectious etiologies such as condyloma acuminatum (human papillomavirus); syphilitic chancre; or mycobacterial, bacterial, fungal, or parasitic vulvovaginitis. Sexual abuse, sarcoidosis, Behçet disease, or hidradenitis suppurativa, among other diagnoses, also should be considered. Diagnostic workup should include biopsy of the lesion with special stains, polarizing microscopy, and tissue cultures.4 A thorough evaluation for gastrointestinal CD should be completed after diagnosis.3
The clinical course of vulvar CD can be unpredictable, with some cases healing spontaneously but most persisting despite treatment and sometimes prompting surgical removal.2,4 Early recognition is crucial, as long-standing MCD lesions can be therapy resistant.5 Due to the rarity of the condition and lack of data, there is a lack of treatment consensus for MCD. In 2014, the American Academy of Dermatology published treatment guidelines recommending superpotent topical steroids or topical tacrolimus as first-line therapy. Next-line therapy includes oral metronidazole, followed by prednisolone if still symptomatic.3 Treatment-resistant disease can warrant treatment with immunomodulators or tumor necrosis factor α inhibitors. Our patient was started on adalimumab; after just 2 months of therapy, the labial swelling decreased and the exophytic nodule was less firm and smaller.
Metastatic CD is a rare manifestation of cutaneous CD and can be present in the absence of gastrointestinal disease.3 This case demonstrates the importance of recognizing the cutaneous signs of CD and the necessity of lesional biopsy for the diagnosis of MCD, as our patient presented with nonspecific gastrointestinal symptoms and a diagnostic workup, including endoscopies, that proved inconclusive for the diagnosis of CD.
The Diagnosis: Cutaneous Crohn Disease
Kinyoun and Grocott-Gomori methenamine-silver staining of the labial biopsy were negative for mycobacteria and fungi, respectively. A complete blood cell count, erythrocyte sedimentation rate, C-reactive protein, celiac disease serologies, stool occult blood, and stool calprotectin laboratory test results were within reference range. Magnetic resonance imaging of the pelvis demonstrated an anal fissure extending from the anal verge at the 6 o’clock position, abnormal T2 bright signal in the skin of the buttocks and perineum extending to the labia, and mild mucosal enhancement of the rectal and anal mucosa. Esophagogastroduodenoscopy and magnetic resonance elastography were unremarkable. Colonoscopy demonstrated scattered superficial erythematous patches and erosions in the rectum. Histologically, there was mild to moderately active colitis in the rectum with no evidence of chronicity. Given our patient’s labial edema and exophytic papulonodule (Figure 1) in the setting of nonspecific gastrointestinal symptoms and granulomatous dermatitis seen on pathology (Figure 2), she was diagnosed with cutaneous Crohn disease (CD).

In our patient, labial biopsy was necessary to definitively diagnose CD. Prior to biopsy of the lesion, our patient was diagnosed with irritable bowel syndrome with constipation leading to an anal fissure and skin tag due to lack of laboratory, imaging, and colonoscopy findings commonly associated with CD. Her biopsy results and gastrointestinal symptoms made these diagnoses, as well as condyloma or a large sentinel skin tag, less likely.

Extraintestinal findings of CD, especially cutaneous manifestations, are relatively frequent and may be present in as many as 44% of patients.1,2 Cutaneous CD often is characterized based on pathogenic mechanisms as either reactive, associated, or CD specific. Reactive cutaneous manifestations include erythema nodosum, pyoderma gangrenosum, and oral aphthae. Associated cutaneous manifestations include vitiligo, palmar erythema, and palmoplantar pustulosis.2 Crohn disease–specific manifestations, including genital or extragenital metastatic CD (MCD), fistulas, and oral involvement, are granulomatous in nature, similar to intestinal CD. Genital manifestations of MCD include edema, erythema, fissures, and/or ulceration of the vulva, penis, or scrotum. Labial swelling is the most common presenting symptom of MCD in females in both pediatric and adult age groups.2 Lymphedema, skin tags, and condylomalike growths also can be seen but are relatively less common.2
Given the labial edema, exophytic papulonodule, and granulomatous dermatitis seen on histopathology, our patient likely fit into the MCD category.2 In adults, most instances of MCD arise in the setting of well-established intestinal CD disease,3 whereas in children 86% of cases occur in patients without concurrent intestinal CD.2
Given the nonspecific and variable presentation of MCD, the differential diagnosis is broad. The differential diagnosis could include infectious etiologies such as condyloma acuminatum (human papillomavirus); syphilitic chancre; or mycobacterial, bacterial, fungal, or parasitic vulvovaginitis. Sexual abuse, sarcoidosis, Behçet disease, or hidradenitis suppurativa, among other diagnoses, also should be considered. Diagnostic workup should include biopsy of the lesion with special stains, polarizing microscopy, and tissue cultures.4 A thorough evaluation for gastrointestinal CD should be completed after diagnosis.3
The clinical course of vulvar CD can be unpredictable, with some cases healing spontaneously but most persisting despite treatment and sometimes prompting surgical removal.2,4 Early recognition is crucial, as long-standing MCD lesions can be therapy resistant.5 Due to the rarity of the condition and lack of data, there is a lack of treatment consensus for MCD. In 2014, the American Academy of Dermatology published treatment guidelines recommending superpotent topical steroids or topical tacrolimus as first-line therapy. Next-line therapy includes oral metronidazole, followed by prednisolone if still symptomatic.3 Treatment-resistant disease can warrant treatment with immunomodulators or tumor necrosis factor α inhibitors. Our patient was started on adalimumab; after just 2 months of therapy, the labial swelling decreased and the exophytic nodule was less firm and smaller.
Metastatic CD is a rare manifestation of cutaneous CD and can be present in the absence of gastrointestinal disease.3 This case demonstrates the importance of recognizing the cutaneous signs of CD and the necessity of lesional biopsy for the diagnosis of MCD, as our patient presented with nonspecific gastrointestinal symptoms and a diagnostic workup, including endoscopies, that proved inconclusive for the diagnosis of CD.
- Antonelli E, Bassotti G, Tramontana M, et al. Dermatological manifestations in inflammatory bowel diseases. J Clin Med. 2021;10:1-16. doi:10.3390/JCM10020364
- Schneider SL, Foster K, Patel D, et al. Cutaneous manifestations of metastatic Crohn’s disease. Pediatr Dermatol. 2018;35:566-574. doi:10.1111/PDE.13565
- Kurtzman DJB, Jones T, Lian F, et al. Metastatic Crohn’s disease: a review and approach to therapy. J Am Acad Dermatol. 2014;71:804-813. doi:10.1016/J.JAAD.2014.04.002
- Barret M, De Parades V, Battistella M, et al. Crohn’s disease of the vulva. J Crohns Colitis. 2014;8:563-570. doi:10.1016/J.CROHNS.2013.10.009
- Aberumand B, Howard J, Howard J. Metastatic Crohn’s disease: an approach to an uncommon but important cutaneous disorder [published online January 3, 2017]. Biomed Res Int. 2017;2017:8192150. doi:10.1155/2017/8192150
- Antonelli E, Bassotti G, Tramontana M, et al. Dermatological manifestations in inflammatory bowel diseases. J Clin Med. 2021;10:1-16. doi:10.3390/JCM10020364
- Schneider SL, Foster K, Patel D, et al. Cutaneous manifestations of metastatic Crohn’s disease. Pediatr Dermatol. 2018;35:566-574. doi:10.1111/PDE.13565
- Kurtzman DJB, Jones T, Lian F, et al. Metastatic Crohn’s disease: a review and approach to therapy. J Am Acad Dermatol. 2014;71:804-813. doi:10.1016/J.JAAD.2014.04.002
- Barret M, De Parades V, Battistella M, et al. Crohn’s disease of the vulva. J Crohns Colitis. 2014;8:563-570. doi:10.1016/J.CROHNS.2013.10.009
- Aberumand B, Howard J, Howard J. Metastatic Crohn’s disease: an approach to an uncommon but important cutaneous disorder [published online January 3, 2017]. Biomed Res Int. 2017;2017:8192150. doi:10.1155/2017/8192150
An 18-year-old woman with chronic constipation presented with an enlarging, painful, and edematous “lump” in the perineum of 1 year’s duration. The lesion became firmer and more painful with bowel movements. Physical examination revealed an enlarged right labia majora, as well as a pink to flesh-colored, exophytic, firm papulonodule in the perineum posterior to the right labia. The patient concomitantly was following with gastroenterology due to abdominal pain that worsened with eating, as well as constipation, nausea, weight loss, and rectal bleeding of 5 years’ duration. The patient denied rash, joint arthralgia, or oral ulcers. A biopsy from the labial lesion was performed.
Dietary zinc seen reducing migraine risk
, according to results from a cross-sectional study of more than 11,000 American adults.
For their research, published online in Headache, Huanxian Liu, MD, and colleagues at Chinese PLA General Hospital in Beijing, analyzed publicly available data from the U.S. National Health and Nutrition Examination Survey to determine whether people self-reporting migraine or severe headache saw lower zinc intake, compared with people without migraine. The data used in the analysis was collected between 1999 and 2004, and contained information on foods and drinks consumed by participants in a 24-hour period, along with additional health information.
An inverse relationship
The investigators divided their study’s 11,088 participants (mean age, 46.5 years; 50% female) into quintiles based on dietary zinc consumption as inferred from foods eaten. They also considered zinc supplementation, for which data was available for 4,324 participants, of whom 2,607 reported use of supplements containing zinc.
Some 20% of the cohort (n = 2,236) reported migraine or severe headache within the previous 3 months. Pregnant women were excluded from analysis, and the investigators adjusted for a range of covariates, including age, sex, ethnicity, education level, body mass, smoking, diabetes, cardiovascular disease, and nutritional factors.
Dr. Liu and colleagues reported an inverse association between dietary zinc consumption and migraine, with the highest-consuming quintile of the cohort (15.8 mg or more zinc per day) seeing lowest risk of migraine (odds ratio, 0.70; 95% confidence interval, 0.52-0.94; P = .029), compared with the low-consuming quintile (5.9 mg or less daily). Among people getting high levels of zinc (19.3-32.5 mg daily) through supplements, risk of migraine was lower still, to between an OR of 0.62 (95% CI: 0.46–0.83, P = 0.019) and an OR of 0.67 (95% CI, 0.49–0.91; P = .045).
While the investigators acknowledged limitations of the study, including its cross-sectional design and use of a broad question to discern prevalence of migraine, the findings suggest that “zinc is an important nutrient that influences migraine,” they wrote, citing evidence for its antioxidant and anti-inflammatory properties.
The importance of nutritional factors
Commenting on the research findings, Deborah I. Friedman, MD, MPH, a headache specialist in Dallas, said that Dr. Liu and colleagues’ findings added to a growing information base about nutritional factors and migraine. For example, “low magnesium levels are common in people with migraine, and magnesium supplementation is a recommended preventive treatment for migraine.”
Dr. Friedman cited a recent study showing that vitamin B12 and magnesium supplementation in women (, combined with high-intensity interval training, “silenced” the inflammation signaling pathway, helped migraine pain and decreased levels of calcitonin gene-related peptide. A 2022 randomized trial found that alpha lipoic acid supplementation reduced migraine severity, frequency and disability in women with episodic migraine.
Vitamin D levels are also lower in people with migraine, compared with controls, Dr. Friedman noted, and a randomized trial of 2,000 IU of vitamin D3 daily saw reduced monthly headache days, attack duration, severe headaches, and analgesic use, compared with placebo. Other nutrients implicated in migraine include coenzyme Q10, calcium, folic acid, vitamin B6, and vitamin B1.
“What should a patient with migraine do with all of this information? First, eat a healthy and balanced diet,” Dr. Friedman said. “Sources of dietary zinc include red meat, nuts, legumes, poultry, shellfish (especially oysters), whole grains, some cereals, and even dark chocolate. The recommended daily dosage of zinc is 9.5 mg in men and 7 mg in women. Most people get enough zinc in their diet; vegetarians, vegans, pregnant or breastfeeding women, and adults over age 65 may need to take supplemental zinc.”
Dr. Liu and colleagues’ work was supported by China’s National Natural Science Foundation. The investigators reported no financial conflicts of interest. Dr. Friedman has received financial support from Alder, Allergan, Amgen, Biohaven, Eli Lilly, Merck, Teva, and other pharmaceutical manufacturers.
, according to results from a cross-sectional study of more than 11,000 American adults.
For their research, published online in Headache, Huanxian Liu, MD, and colleagues at Chinese PLA General Hospital in Beijing, analyzed publicly available data from the U.S. National Health and Nutrition Examination Survey to determine whether people self-reporting migraine or severe headache saw lower zinc intake, compared with people without migraine. The data used in the analysis was collected between 1999 and 2004, and contained information on foods and drinks consumed by participants in a 24-hour period, along with additional health information.
An inverse relationship
The investigators divided their study’s 11,088 participants (mean age, 46.5 years; 50% female) into quintiles based on dietary zinc consumption as inferred from foods eaten. They also considered zinc supplementation, for which data was available for 4,324 participants, of whom 2,607 reported use of supplements containing zinc.
Some 20% of the cohort (n = 2,236) reported migraine or severe headache within the previous 3 months. Pregnant women were excluded from analysis, and the investigators adjusted for a range of covariates, including age, sex, ethnicity, education level, body mass, smoking, diabetes, cardiovascular disease, and nutritional factors.
Dr. Liu and colleagues reported an inverse association between dietary zinc consumption and migraine, with the highest-consuming quintile of the cohort (15.8 mg or more zinc per day) seeing lowest risk of migraine (odds ratio, 0.70; 95% confidence interval, 0.52-0.94; P = .029), compared with the low-consuming quintile (5.9 mg or less daily). Among people getting high levels of zinc (19.3-32.5 mg daily) through supplements, risk of migraine was lower still, to between an OR of 0.62 (95% CI: 0.46–0.83, P = 0.019) and an OR of 0.67 (95% CI, 0.49–0.91; P = .045).
While the investigators acknowledged limitations of the study, including its cross-sectional design and use of a broad question to discern prevalence of migraine, the findings suggest that “zinc is an important nutrient that influences migraine,” they wrote, citing evidence for its antioxidant and anti-inflammatory properties.
The importance of nutritional factors
Commenting on the research findings, Deborah I. Friedman, MD, MPH, a headache specialist in Dallas, said that Dr. Liu and colleagues’ findings added to a growing information base about nutritional factors and migraine. For example, “low magnesium levels are common in people with migraine, and magnesium supplementation is a recommended preventive treatment for migraine.”
Dr. Friedman cited a recent study showing that vitamin B12 and magnesium supplementation in women (, combined with high-intensity interval training, “silenced” the inflammation signaling pathway, helped migraine pain and decreased levels of calcitonin gene-related peptide. A 2022 randomized trial found that alpha lipoic acid supplementation reduced migraine severity, frequency and disability in women with episodic migraine.
Vitamin D levels are also lower in people with migraine, compared with controls, Dr. Friedman noted, and a randomized trial of 2,000 IU of vitamin D3 daily saw reduced monthly headache days, attack duration, severe headaches, and analgesic use, compared with placebo. Other nutrients implicated in migraine include coenzyme Q10, calcium, folic acid, vitamin B6, and vitamin B1.
“What should a patient with migraine do with all of this information? First, eat a healthy and balanced diet,” Dr. Friedman said. “Sources of dietary zinc include red meat, nuts, legumes, poultry, shellfish (especially oysters), whole grains, some cereals, and even dark chocolate. The recommended daily dosage of zinc is 9.5 mg in men and 7 mg in women. Most people get enough zinc in their diet; vegetarians, vegans, pregnant or breastfeeding women, and adults over age 65 may need to take supplemental zinc.”
Dr. Liu and colleagues’ work was supported by China’s National Natural Science Foundation. The investigators reported no financial conflicts of interest. Dr. Friedman has received financial support from Alder, Allergan, Amgen, Biohaven, Eli Lilly, Merck, Teva, and other pharmaceutical manufacturers.
, according to results from a cross-sectional study of more than 11,000 American adults.
For their research, published online in Headache, Huanxian Liu, MD, and colleagues at Chinese PLA General Hospital in Beijing, analyzed publicly available data from the U.S. National Health and Nutrition Examination Survey to determine whether people self-reporting migraine or severe headache saw lower zinc intake, compared with people without migraine. The data used in the analysis was collected between 1999 and 2004, and contained information on foods and drinks consumed by participants in a 24-hour period, along with additional health information.
An inverse relationship
The investigators divided their study’s 11,088 participants (mean age, 46.5 years; 50% female) into quintiles based on dietary zinc consumption as inferred from foods eaten. They also considered zinc supplementation, for which data was available for 4,324 participants, of whom 2,607 reported use of supplements containing zinc.
Some 20% of the cohort (n = 2,236) reported migraine or severe headache within the previous 3 months. Pregnant women were excluded from analysis, and the investigators adjusted for a range of covariates, including age, sex, ethnicity, education level, body mass, smoking, diabetes, cardiovascular disease, and nutritional factors.
Dr. Liu and colleagues reported an inverse association between dietary zinc consumption and migraine, with the highest-consuming quintile of the cohort (15.8 mg or more zinc per day) seeing lowest risk of migraine (odds ratio, 0.70; 95% confidence interval, 0.52-0.94; P = .029), compared with the low-consuming quintile (5.9 mg or less daily). Among people getting high levels of zinc (19.3-32.5 mg daily) through supplements, risk of migraine was lower still, to between an OR of 0.62 (95% CI: 0.46–0.83, P = 0.019) and an OR of 0.67 (95% CI, 0.49–0.91; P = .045).
While the investigators acknowledged limitations of the study, including its cross-sectional design and use of a broad question to discern prevalence of migraine, the findings suggest that “zinc is an important nutrient that influences migraine,” they wrote, citing evidence for its antioxidant and anti-inflammatory properties.
The importance of nutritional factors
Commenting on the research findings, Deborah I. Friedman, MD, MPH, a headache specialist in Dallas, said that Dr. Liu and colleagues’ findings added to a growing information base about nutritional factors and migraine. For example, “low magnesium levels are common in people with migraine, and magnesium supplementation is a recommended preventive treatment for migraine.”
Dr. Friedman cited a recent study showing that vitamin B12 and magnesium supplementation in women (, combined with high-intensity interval training, “silenced” the inflammation signaling pathway, helped migraine pain and decreased levels of calcitonin gene-related peptide. A 2022 randomized trial found that alpha lipoic acid supplementation reduced migraine severity, frequency and disability in women with episodic migraine.
Vitamin D levels are also lower in people with migraine, compared with controls, Dr. Friedman noted, and a randomized trial of 2,000 IU of vitamin D3 daily saw reduced monthly headache days, attack duration, severe headaches, and analgesic use, compared with placebo. Other nutrients implicated in migraine include coenzyme Q10, calcium, folic acid, vitamin B6, and vitamin B1.
“What should a patient with migraine do with all of this information? First, eat a healthy and balanced diet,” Dr. Friedman said. “Sources of dietary zinc include red meat, nuts, legumes, poultry, shellfish (especially oysters), whole grains, some cereals, and even dark chocolate. The recommended daily dosage of zinc is 9.5 mg in men and 7 mg in women. Most people get enough zinc in their diet; vegetarians, vegans, pregnant or breastfeeding women, and adults over age 65 may need to take supplemental zinc.”
Dr. Liu and colleagues’ work was supported by China’s National Natural Science Foundation. The investigators reported no financial conflicts of interest. Dr. Friedman has received financial support from Alder, Allergan, Amgen, Biohaven, Eli Lilly, Merck, Teva, and other pharmaceutical manufacturers.
FROM HEADACHE
Buccal fat pad removal
The buccal fat pads, previously known as Bichat’s fat pads, were first described in 1802. Everyone has them and their size is predominantly related to genetics, but similar to other facial fat pockets, they can shrink or shift over time. Buccal fat pads are often resistant to weight loss and stubbornly persist because of a slower lipolytic rate than subcutaneous fat. Some patients with round facial shapes may seek removal of the midface volume to create a more angular cheek and jawline, which enhances the zygomatic prominence and mandibular angle, creating a more contoured face.
The buccal fat pad is a submuscular fat pad surrounded by a capsule that contains three lobes with four extensions. The anterior lobe rests in front of the anterior border of the masseter muscle. The intermediate lobe extends between the masseter and buccinator muscles. The posterior lobe extends between the temporal masticatory space. These pads range from 7-11 mL in volume and grow from ages 10 to 20 years, declining in size after age 20. Given their location in the central face, they contain a rich vascular supply and are surrounded by the facial nerves, salivary glands, the parotid gland, and muscles of mastication.
The aesthetic contour of the lower face is defined by the mandibular prominence, the masseter muscle, subcutaneous fat, and the buccal fat pad. Surgically, removal is a simple and safe procedure. Complications can include damage to the parotid gland, vessels, salivary duct, or facial nerve. Temporary numbness, swelling, and facial asymmetry are the most common complications.
Increasing popularity, controversy
Removal of the buccal fat pads has become popular because of celebrity media exposure, particularly among young women seeking a slim appearance to their face and jawlines. Although the procedure is relatively simple, there have been no long term studies evaluating the effects of buccal fat pad removal on facial aging.
The shrinking or shifting of fat that occurs with aging makes the removal of these fat pads in young women controversial because when removed, they cannot be effectively replaced. Shrinking of the fat pads with age, loss of midface volume, and solar elastosis can make the cheeks appear gaunt and “sucked in.”
An experienced surgeon will reduce and contour the fat pads – and will not completely remove them – to prevent a complete hollowing of the cheeks over time. Complete removal is not recommended and in men, overzealous removal in men can feminize the face.
In middle-aged men and women, the buccal fat pad can shift to the lower face and often drops below the angle of the mandible giving the appearance of jowls. Complete removal of the shifted buccal fat pad will help align the jawline; however, residual skin laxity is a complication and must be addressed to fully correct the jowls.
In my experience, the best approach to reducing buccal pads as an alternative to surgical removal is “melting” the buccal fat in a systematic, controlled manner over several sessions with either radiofrequency laser or deoxycholic acid injections. This slow, controlled method allows me to contour the cheeks appropriately in concordance with the patient’s anatomy. In younger patients or those with little skin laxity, I choose treatments with deoxycholic acid to remove the pads (which I also use to treat the jowls, as outlined in my 2020 column on treating the jowl overhang with deoxycholic acid).
In patients with more skin laxity, I perform sequential radiofrequency laser treatment over the fat pockets to simultaneously melt the fat pockets and tighten the overlying skin. Both of these methods often require three to six treatments. The controlled, cautious, treatments gradually shrink the fat pockets while preventing the overhollowing of the face.
Dr. Talakoub and Dr. Naissan O. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
References
Dubin B et al. Plast Reconstr Surg. 1989 Feb;83(2):257-64
Jackson IT. Plast Reconstr Surg. 1999 Jun;103(7):2059-60.
Matarasso A. Ann Plast Surg. 1991 May;26(5):413-8.
The buccal fat pads, previously known as Bichat’s fat pads, were first described in 1802. Everyone has them and their size is predominantly related to genetics, but similar to other facial fat pockets, they can shrink or shift over time. Buccal fat pads are often resistant to weight loss and stubbornly persist because of a slower lipolytic rate than subcutaneous fat. Some patients with round facial shapes may seek removal of the midface volume to create a more angular cheek and jawline, which enhances the zygomatic prominence and mandibular angle, creating a more contoured face.
The buccal fat pad is a submuscular fat pad surrounded by a capsule that contains three lobes with four extensions. The anterior lobe rests in front of the anterior border of the masseter muscle. The intermediate lobe extends between the masseter and buccinator muscles. The posterior lobe extends between the temporal masticatory space. These pads range from 7-11 mL in volume and grow from ages 10 to 20 years, declining in size after age 20. Given their location in the central face, they contain a rich vascular supply and are surrounded by the facial nerves, salivary glands, the parotid gland, and muscles of mastication.
The aesthetic contour of the lower face is defined by the mandibular prominence, the masseter muscle, subcutaneous fat, and the buccal fat pad. Surgically, removal is a simple and safe procedure. Complications can include damage to the parotid gland, vessels, salivary duct, or facial nerve. Temporary numbness, swelling, and facial asymmetry are the most common complications.
Increasing popularity, controversy
Removal of the buccal fat pads has become popular because of celebrity media exposure, particularly among young women seeking a slim appearance to their face and jawlines. Although the procedure is relatively simple, there have been no long term studies evaluating the effects of buccal fat pad removal on facial aging.
The shrinking or shifting of fat that occurs with aging makes the removal of these fat pads in young women controversial because when removed, they cannot be effectively replaced. Shrinking of the fat pads with age, loss of midface volume, and solar elastosis can make the cheeks appear gaunt and “sucked in.”
An experienced surgeon will reduce and contour the fat pads – and will not completely remove them – to prevent a complete hollowing of the cheeks over time. Complete removal is not recommended and in men, overzealous removal in men can feminize the face.
In middle-aged men and women, the buccal fat pad can shift to the lower face and often drops below the angle of the mandible giving the appearance of jowls. Complete removal of the shifted buccal fat pad will help align the jawline; however, residual skin laxity is a complication and must be addressed to fully correct the jowls.
In my experience, the best approach to reducing buccal pads as an alternative to surgical removal is “melting” the buccal fat in a systematic, controlled manner over several sessions with either radiofrequency laser or deoxycholic acid injections. This slow, controlled method allows me to contour the cheeks appropriately in concordance with the patient’s anatomy. In younger patients or those with little skin laxity, I choose treatments with deoxycholic acid to remove the pads (which I also use to treat the jowls, as outlined in my 2020 column on treating the jowl overhang with deoxycholic acid).
In patients with more skin laxity, I perform sequential radiofrequency laser treatment over the fat pockets to simultaneously melt the fat pockets and tighten the overlying skin. Both of these methods often require three to six treatments. The controlled, cautious, treatments gradually shrink the fat pockets while preventing the overhollowing of the face.
Dr. Talakoub and Dr. Naissan O. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
References
Dubin B et al. Plast Reconstr Surg. 1989 Feb;83(2):257-64
Jackson IT. Plast Reconstr Surg. 1999 Jun;103(7):2059-60.
Matarasso A. Ann Plast Surg. 1991 May;26(5):413-8.
The buccal fat pads, previously known as Bichat’s fat pads, were first described in 1802. Everyone has them and their size is predominantly related to genetics, but similar to other facial fat pockets, they can shrink or shift over time. Buccal fat pads are often resistant to weight loss and stubbornly persist because of a slower lipolytic rate than subcutaneous fat. Some patients with round facial shapes may seek removal of the midface volume to create a more angular cheek and jawline, which enhances the zygomatic prominence and mandibular angle, creating a more contoured face.
The buccal fat pad is a submuscular fat pad surrounded by a capsule that contains three lobes with four extensions. The anterior lobe rests in front of the anterior border of the masseter muscle. The intermediate lobe extends between the masseter and buccinator muscles. The posterior lobe extends between the temporal masticatory space. These pads range from 7-11 mL in volume and grow from ages 10 to 20 years, declining in size after age 20. Given their location in the central face, they contain a rich vascular supply and are surrounded by the facial nerves, salivary glands, the parotid gland, and muscles of mastication.
The aesthetic contour of the lower face is defined by the mandibular prominence, the masseter muscle, subcutaneous fat, and the buccal fat pad. Surgically, removal is a simple and safe procedure. Complications can include damage to the parotid gland, vessels, salivary duct, or facial nerve. Temporary numbness, swelling, and facial asymmetry are the most common complications.
Increasing popularity, controversy
Removal of the buccal fat pads has become popular because of celebrity media exposure, particularly among young women seeking a slim appearance to their face and jawlines. Although the procedure is relatively simple, there have been no long term studies evaluating the effects of buccal fat pad removal on facial aging.
The shrinking or shifting of fat that occurs with aging makes the removal of these fat pads in young women controversial because when removed, they cannot be effectively replaced. Shrinking of the fat pads with age, loss of midface volume, and solar elastosis can make the cheeks appear gaunt and “sucked in.”
An experienced surgeon will reduce and contour the fat pads – and will not completely remove them – to prevent a complete hollowing of the cheeks over time. Complete removal is not recommended and in men, overzealous removal in men can feminize the face.
In middle-aged men and women, the buccal fat pad can shift to the lower face and often drops below the angle of the mandible giving the appearance of jowls. Complete removal of the shifted buccal fat pad will help align the jawline; however, residual skin laxity is a complication and must be addressed to fully correct the jowls.
In my experience, the best approach to reducing buccal pads as an alternative to surgical removal is “melting” the buccal fat in a systematic, controlled manner over several sessions with either radiofrequency laser or deoxycholic acid injections. This slow, controlled method allows me to contour the cheeks appropriately in concordance with the patient’s anatomy. In younger patients or those with little skin laxity, I choose treatments with deoxycholic acid to remove the pads (which I also use to treat the jowls, as outlined in my 2020 column on treating the jowl overhang with deoxycholic acid).
In patients with more skin laxity, I perform sequential radiofrequency laser treatment over the fat pockets to simultaneously melt the fat pockets and tighten the overlying skin. Both of these methods often require three to six treatments. The controlled, cautious, treatments gradually shrink the fat pockets while preventing the overhollowing of the face.
Dr. Talakoub and Dr. Naissan O. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
References
Dubin B et al. Plast Reconstr Surg. 1989 Feb;83(2):257-64
Jackson IT. Plast Reconstr Surg. 1999 Jun;103(7):2059-60.
Matarasso A. Ann Plast Surg. 1991 May;26(5):413-8.
Simulating psychoanalysis: A review of Freud’s Bones
While psychiatry has been the subject of many films, video games are not a medium commonly known for examining mental illness.1 There have been PC games over the years with psychiatric themes, such as Sanitarium (1998), Depression Quest (2013), Fran Bow (2015), and Night in the Woods (2017). Now for perhaps the first time a game has been developed with the practice of psychiatry as its primary focus.
Freud’s Bones is a 2022 game developed by independent Italian game studio Fortuna Imperatore. The result of a successful Kickstarter crowdfunding campaign, Freud’s Bones is advertised as “the first point & click narrative-drive game to pay homage to the birth of psychoanalysis and its founder, addressing the themes of sexuality and neuroses filled with existential doubts.”
In Freud’s Bones, you take control of Sigmund Freud and guide him through his daily tasks. Gameplay is of the simple point-and-click variety, modeled after classic LucasArts-style adventure games of the 1990s such as The Secret of Monkey Island or Day of the Tentacle. Prior to seeing your first patient, the game provides several documents the player can peruse to become familiar with basic concepts of psychoanalysis. Although the game was originally written in Italian (and translation gaffes occasionally arise), generally the English wording is easy to read. However, some players may feel intimidated or bored by the sheer quantity of text the game provides. All in-game text, including books and spoken words, are written and there is no recorded voice acting. Audio consists largely of unintrusive background music and occasional sound effects. The graphical style is simple and cartoonish but pleasant.
Freud’s personal life is a major focus of the game. His real life dog Jofi is a constant presence in Freud’s office. At various times the player will witness Freud’s dreams, act as a voice inside his head, and attempt to interpret mystical Egyptian messages he receives. Players are also tasked with managing Freud’s reputation in the scientific community. This is apparently intended as a reflection of in-game clinical acumen, but it was sometimes difficult to tell what direct influence my actions had on Freud’s reputation.
Freud’s energy may flag at various points during the game, and the player may choose to give him a cigar or a dose of cocaine to stimulate him. These options sound interesting on the surface, but I found the effect of these substances on the game’s actual outcome to be minimal. Some tasks are presented in a less than user-friendly manner. For example, on my initial playthrough I could not figure out how to complete several optional errands such as shopping for more tobacco or selecting a cover for Freud’s books. The player is also given the opportunity to make choices that affect Freud’s personal life, such as whether to pursue an extramarital affair. The game does have a few narrative surprises, including appearances from some of Freud’s well-known contemporaries. One particularly vivid sequence late in the game involves navigating Freud through a hallucination with some bizarre, but very Freudian, imagery.
By far the most interesting and enjoyable part of the game is the psychoanalysis sessions. The player guides Freud through multiple sessions with four different patients. Each of them has a unique story and associated symptoms, and the player can choose a variety of responses. For example, will you take a comforting, paternalistic approach to the patient uncomfortable with her first appointment? Or will you take the more stoic, quiet approach of the analyst and allow the patient to speak without prompting? Part of the player’s quest in guiding Freud through these sessions is to help patients bring their unconscious thoughts to conscious awareness. This is depicted graphically as the thought moves vertically through images representing the id, superego, and ego. Skillful questioning can bring these thoughts to the surface, but poor choices can leave valuable insights buried in the unconscious.
These therapy sessions were unique and engaging, and I wish they constituted a larger portion of the gameplay in Freud’s Bones. More patients, more sessions with each patient, and longer sessions would all have been welcome additions. These analytic sessions eventually culminate in an opportunity to offer a diagnosis, and the player’s accuracy in treatment can result in divergent outcomes for each patient. The game is not lengthy, as it can be played in its entirety in roughly 5-6 hours. Selecting different options for Freud’s personal life and the analysis sessions provides some replay value for subsequent playthroughs.
Overall, Freud’s Bones is a worthy effort for being uniquely designed as interactive entertainment simulating psychoanalysis. It provides an experience of interest to psychiatrists but is also accessible to the general public. While the game has flaws in that it can be overly text-heavy and goals are not always clear, it shines in the moments where it allows the player to participate directly in the process of psychoanalysis. Freud’s Bones is available for purchase on Steam (currently priced at $13.99) and can be played on Windows PCs.
Dr. Weber is a psychiatrist at Intermountain Logan Regional Hospital in Logan, Utah. He disclosed no relevant financial relationships.
References
1. See, for example, Gabbard GO, Gabbard K. Psychiatry and the Cinema, 2nd ed. American Psychiatric Press, Inc.; 1999.
While psychiatry has been the subject of many films, video games are not a medium commonly known for examining mental illness.1 There have been PC games over the years with psychiatric themes, such as Sanitarium (1998), Depression Quest (2013), Fran Bow (2015), and Night in the Woods (2017). Now for perhaps the first time a game has been developed with the practice of psychiatry as its primary focus.
Freud’s Bones is a 2022 game developed by independent Italian game studio Fortuna Imperatore. The result of a successful Kickstarter crowdfunding campaign, Freud’s Bones is advertised as “the first point & click narrative-drive game to pay homage to the birth of psychoanalysis and its founder, addressing the themes of sexuality and neuroses filled with existential doubts.”
In Freud’s Bones, you take control of Sigmund Freud and guide him through his daily tasks. Gameplay is of the simple point-and-click variety, modeled after classic LucasArts-style adventure games of the 1990s such as The Secret of Monkey Island or Day of the Tentacle. Prior to seeing your first patient, the game provides several documents the player can peruse to become familiar with basic concepts of psychoanalysis. Although the game was originally written in Italian (and translation gaffes occasionally arise), generally the English wording is easy to read. However, some players may feel intimidated or bored by the sheer quantity of text the game provides. All in-game text, including books and spoken words, are written and there is no recorded voice acting. Audio consists largely of unintrusive background music and occasional sound effects. The graphical style is simple and cartoonish but pleasant.
Freud’s personal life is a major focus of the game. His real life dog Jofi is a constant presence in Freud’s office. At various times the player will witness Freud’s dreams, act as a voice inside his head, and attempt to interpret mystical Egyptian messages he receives. Players are also tasked with managing Freud’s reputation in the scientific community. This is apparently intended as a reflection of in-game clinical acumen, but it was sometimes difficult to tell what direct influence my actions had on Freud’s reputation.
Freud’s energy may flag at various points during the game, and the player may choose to give him a cigar or a dose of cocaine to stimulate him. These options sound interesting on the surface, but I found the effect of these substances on the game’s actual outcome to be minimal. Some tasks are presented in a less than user-friendly manner. For example, on my initial playthrough I could not figure out how to complete several optional errands such as shopping for more tobacco or selecting a cover for Freud’s books. The player is also given the opportunity to make choices that affect Freud’s personal life, such as whether to pursue an extramarital affair. The game does have a few narrative surprises, including appearances from some of Freud’s well-known contemporaries. One particularly vivid sequence late in the game involves navigating Freud through a hallucination with some bizarre, but very Freudian, imagery.
By far the most interesting and enjoyable part of the game is the psychoanalysis sessions. The player guides Freud through multiple sessions with four different patients. Each of them has a unique story and associated symptoms, and the player can choose a variety of responses. For example, will you take a comforting, paternalistic approach to the patient uncomfortable with her first appointment? Or will you take the more stoic, quiet approach of the analyst and allow the patient to speak without prompting? Part of the player’s quest in guiding Freud through these sessions is to help patients bring their unconscious thoughts to conscious awareness. This is depicted graphically as the thought moves vertically through images representing the id, superego, and ego. Skillful questioning can bring these thoughts to the surface, but poor choices can leave valuable insights buried in the unconscious.
These therapy sessions were unique and engaging, and I wish they constituted a larger portion of the gameplay in Freud’s Bones. More patients, more sessions with each patient, and longer sessions would all have been welcome additions. These analytic sessions eventually culminate in an opportunity to offer a diagnosis, and the player’s accuracy in treatment can result in divergent outcomes for each patient. The game is not lengthy, as it can be played in its entirety in roughly 5-6 hours. Selecting different options for Freud’s personal life and the analysis sessions provides some replay value for subsequent playthroughs.
Overall, Freud’s Bones is a worthy effort for being uniquely designed as interactive entertainment simulating psychoanalysis. It provides an experience of interest to psychiatrists but is also accessible to the general public. While the game has flaws in that it can be overly text-heavy and goals are not always clear, it shines in the moments where it allows the player to participate directly in the process of psychoanalysis. Freud’s Bones is available for purchase on Steam (currently priced at $13.99) and can be played on Windows PCs.
Dr. Weber is a psychiatrist at Intermountain Logan Regional Hospital in Logan, Utah. He disclosed no relevant financial relationships.
References
1. See, for example, Gabbard GO, Gabbard K. Psychiatry and the Cinema, 2nd ed. American Psychiatric Press, Inc.; 1999.
While psychiatry has been the subject of many films, video games are not a medium commonly known for examining mental illness.1 There have been PC games over the years with psychiatric themes, such as Sanitarium (1998), Depression Quest (2013), Fran Bow (2015), and Night in the Woods (2017). Now for perhaps the first time a game has been developed with the practice of psychiatry as its primary focus.
Freud’s Bones is a 2022 game developed by independent Italian game studio Fortuna Imperatore. The result of a successful Kickstarter crowdfunding campaign, Freud’s Bones is advertised as “the first point & click narrative-drive game to pay homage to the birth of psychoanalysis and its founder, addressing the themes of sexuality and neuroses filled with existential doubts.”
In Freud’s Bones, you take control of Sigmund Freud and guide him through his daily tasks. Gameplay is of the simple point-and-click variety, modeled after classic LucasArts-style adventure games of the 1990s such as The Secret of Monkey Island or Day of the Tentacle. Prior to seeing your first patient, the game provides several documents the player can peruse to become familiar with basic concepts of psychoanalysis. Although the game was originally written in Italian (and translation gaffes occasionally arise), generally the English wording is easy to read. However, some players may feel intimidated or bored by the sheer quantity of text the game provides. All in-game text, including books and spoken words, are written and there is no recorded voice acting. Audio consists largely of unintrusive background music and occasional sound effects. The graphical style is simple and cartoonish but pleasant.
Freud’s personal life is a major focus of the game. His real life dog Jofi is a constant presence in Freud’s office. At various times the player will witness Freud’s dreams, act as a voice inside his head, and attempt to interpret mystical Egyptian messages he receives. Players are also tasked with managing Freud’s reputation in the scientific community. This is apparently intended as a reflection of in-game clinical acumen, but it was sometimes difficult to tell what direct influence my actions had on Freud’s reputation.
Freud’s energy may flag at various points during the game, and the player may choose to give him a cigar or a dose of cocaine to stimulate him. These options sound interesting on the surface, but I found the effect of these substances on the game’s actual outcome to be minimal. Some tasks are presented in a less than user-friendly manner. For example, on my initial playthrough I could not figure out how to complete several optional errands such as shopping for more tobacco or selecting a cover for Freud’s books. The player is also given the opportunity to make choices that affect Freud’s personal life, such as whether to pursue an extramarital affair. The game does have a few narrative surprises, including appearances from some of Freud’s well-known contemporaries. One particularly vivid sequence late in the game involves navigating Freud through a hallucination with some bizarre, but very Freudian, imagery.
By far the most interesting and enjoyable part of the game is the psychoanalysis sessions. The player guides Freud through multiple sessions with four different patients. Each of them has a unique story and associated symptoms, and the player can choose a variety of responses. For example, will you take a comforting, paternalistic approach to the patient uncomfortable with her first appointment? Or will you take the more stoic, quiet approach of the analyst and allow the patient to speak without prompting? Part of the player’s quest in guiding Freud through these sessions is to help patients bring their unconscious thoughts to conscious awareness. This is depicted graphically as the thought moves vertically through images representing the id, superego, and ego. Skillful questioning can bring these thoughts to the surface, but poor choices can leave valuable insights buried in the unconscious.
These therapy sessions were unique and engaging, and I wish they constituted a larger portion of the gameplay in Freud’s Bones. More patients, more sessions with each patient, and longer sessions would all have been welcome additions. These analytic sessions eventually culminate in an opportunity to offer a diagnosis, and the player’s accuracy in treatment can result in divergent outcomes for each patient. The game is not lengthy, as it can be played in its entirety in roughly 5-6 hours. Selecting different options for Freud’s personal life and the analysis sessions provides some replay value for subsequent playthroughs.
Overall, Freud’s Bones is a worthy effort for being uniquely designed as interactive entertainment simulating psychoanalysis. It provides an experience of interest to psychiatrists but is also accessible to the general public. While the game has flaws in that it can be overly text-heavy and goals are not always clear, it shines in the moments where it allows the player to participate directly in the process of psychoanalysis. Freud’s Bones is available for purchase on Steam (currently priced at $13.99) and can be played on Windows PCs.
Dr. Weber is a psychiatrist at Intermountain Logan Regional Hospital in Logan, Utah. He disclosed no relevant financial relationships.
References
1. See, for example, Gabbard GO, Gabbard K. Psychiatry and the Cinema, 2nd ed. American Psychiatric Press, Inc.; 1999.
Borderline patients have longer time to depression remission
Major depressive episodes (MDEs) occur in major depressive disorder (MDD) and bipolar disorder (BD), John J. Söderholm, MD, of the University of Helsinki and colleagues wrote. Borderline personality disorder (BPD) includes an increased risk for depression, but data on the relationship between BPD symptoms and depressive illness are limited. In particular, they noted “a lack of studies prospectively comparing the presence of (hypo)manic symptoms over time during the recovery process from MDE between MDD, MDE/BD, and MDE/BPD patients.”
In a cohort study published in the Journal of Affective Disorders, the researchers collected data from 39 adult MDE patients with MDD, 33 with BD, and 23 with BPD. The patients were diagnosed with MDE using the SCID-I/P and SCID-II interviews, mixed symptoms were identified using the Mix-MDE scale, and borderline symptoms were identified using the Borderline Personality Disorder Severity Index.
Over a 6-month follow-up period, the participants completed biweekly online assessments. The primary outcomes were time to first full remission of symptoms and duration and nature of mood episodes.
Overall, the mean number of distinct mood states was 5.75, and the median duration was 60.9 days. When identified by subcohorts, the median number of mood state periods for MDD, BD, and BPD was 4.49, 8.05, and 4.67, respectively. The median durations were 69.2 days, 40.30 days, and 75.6 days, respectively.
The rates of remission for depressive symptoms were similar for MDD, MDE/BD, and MDE/BPD patients. However, MDE/BD patients had a significantly shorter time to first remission (hazard ratio, 2.44). Patients in the BPD group had a significantly longer time to first remission (HR, 0.95).
“When the cohort was divided into quintiles according to BPD feature severity, there was an approximately 1-month difference in time to first period of remission between the first and third and between the third and fifth quintiles, with longer times seen in patients with more severe BPD symptoms,” the researchers wrote.
The study findings were limited by several factors including the small sample size and short follow-up period that prevented investigation of depressive recurrence, the researchers noted. Other limitations included the lack of diagnostic blinding and variation in patients’ treatment schedules.
However, the results were strengthened by the representative samples of subjects with various disorders, the prospective and multimodal assessment of affective states, and the comparison of three patient groups in a single study.
As BPD was associated with a longer time to remission from depressive symptoms, the results suggest that BPD severity may be an indicator of more severe disease in patients with MDD in the context of depression, the researchers concluded.
The study was supported by the Finska Lakaresallskapet, the City of Helsinki, the Hospital District of Helsinki and Uusimaa, and the Finnish Psychiatric Association. The researchers had no financial conflicts to disclose.
Major depressive episodes (MDEs) occur in major depressive disorder (MDD) and bipolar disorder (BD), John J. Söderholm, MD, of the University of Helsinki and colleagues wrote. Borderline personality disorder (BPD) includes an increased risk for depression, but data on the relationship between BPD symptoms and depressive illness are limited. In particular, they noted “a lack of studies prospectively comparing the presence of (hypo)manic symptoms over time during the recovery process from MDE between MDD, MDE/BD, and MDE/BPD patients.”
In a cohort study published in the Journal of Affective Disorders, the researchers collected data from 39 adult MDE patients with MDD, 33 with BD, and 23 with BPD. The patients were diagnosed with MDE using the SCID-I/P and SCID-II interviews, mixed symptoms were identified using the Mix-MDE scale, and borderline symptoms were identified using the Borderline Personality Disorder Severity Index.
Over a 6-month follow-up period, the participants completed biweekly online assessments. The primary outcomes were time to first full remission of symptoms and duration and nature of mood episodes.
Overall, the mean number of distinct mood states was 5.75, and the median duration was 60.9 days. When identified by subcohorts, the median number of mood state periods for MDD, BD, and BPD was 4.49, 8.05, and 4.67, respectively. The median durations were 69.2 days, 40.30 days, and 75.6 days, respectively.
The rates of remission for depressive symptoms were similar for MDD, MDE/BD, and MDE/BPD patients. However, MDE/BD patients had a significantly shorter time to first remission (hazard ratio, 2.44). Patients in the BPD group had a significantly longer time to first remission (HR, 0.95).
“When the cohort was divided into quintiles according to BPD feature severity, there was an approximately 1-month difference in time to first period of remission between the first and third and between the third and fifth quintiles, with longer times seen in patients with more severe BPD symptoms,” the researchers wrote.
The study findings were limited by several factors including the small sample size and short follow-up period that prevented investigation of depressive recurrence, the researchers noted. Other limitations included the lack of diagnostic blinding and variation in patients’ treatment schedules.
However, the results were strengthened by the representative samples of subjects with various disorders, the prospective and multimodal assessment of affective states, and the comparison of three patient groups in a single study.
As BPD was associated with a longer time to remission from depressive symptoms, the results suggest that BPD severity may be an indicator of more severe disease in patients with MDD in the context of depression, the researchers concluded.
The study was supported by the Finska Lakaresallskapet, the City of Helsinki, the Hospital District of Helsinki and Uusimaa, and the Finnish Psychiatric Association. The researchers had no financial conflicts to disclose.
Major depressive episodes (MDEs) occur in major depressive disorder (MDD) and bipolar disorder (BD), John J. Söderholm, MD, of the University of Helsinki and colleagues wrote. Borderline personality disorder (BPD) includes an increased risk for depression, but data on the relationship between BPD symptoms and depressive illness are limited. In particular, they noted “a lack of studies prospectively comparing the presence of (hypo)manic symptoms over time during the recovery process from MDE between MDD, MDE/BD, and MDE/BPD patients.”
In a cohort study published in the Journal of Affective Disorders, the researchers collected data from 39 adult MDE patients with MDD, 33 with BD, and 23 with BPD. The patients were diagnosed with MDE using the SCID-I/P and SCID-II interviews, mixed symptoms were identified using the Mix-MDE scale, and borderline symptoms were identified using the Borderline Personality Disorder Severity Index.
Over a 6-month follow-up period, the participants completed biweekly online assessments. The primary outcomes were time to first full remission of symptoms and duration and nature of mood episodes.
Overall, the mean number of distinct mood states was 5.75, and the median duration was 60.9 days. When identified by subcohorts, the median number of mood state periods for MDD, BD, and BPD was 4.49, 8.05, and 4.67, respectively. The median durations were 69.2 days, 40.30 days, and 75.6 days, respectively.
The rates of remission for depressive symptoms were similar for MDD, MDE/BD, and MDE/BPD patients. However, MDE/BD patients had a significantly shorter time to first remission (hazard ratio, 2.44). Patients in the BPD group had a significantly longer time to first remission (HR, 0.95).
“When the cohort was divided into quintiles according to BPD feature severity, there was an approximately 1-month difference in time to first period of remission between the first and third and between the third and fifth quintiles, with longer times seen in patients with more severe BPD symptoms,” the researchers wrote.
The study findings were limited by several factors including the small sample size and short follow-up period that prevented investigation of depressive recurrence, the researchers noted. Other limitations included the lack of diagnostic blinding and variation in patients’ treatment schedules.
However, the results were strengthened by the representative samples of subjects with various disorders, the prospective and multimodal assessment of affective states, and the comparison of three patient groups in a single study.
As BPD was associated with a longer time to remission from depressive symptoms, the results suggest that BPD severity may be an indicator of more severe disease in patients with MDD in the context of depression, the researchers concluded.
The study was supported by the Finska Lakaresallskapet, the City of Helsinki, the Hospital District of Helsinki and Uusimaa, and the Finnish Psychiatric Association. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Belimumab for pregnant women with lupus: B-cell concerns remain
The largest combined analysis of birth outcome data for women with systemic lupus erythematosus (SLE) who took belimumab (Benlysta) during pregnancy appears to indicate that the biologic is “unlikely to cause very frequent birth defects,” but the full extent of possible risk remains unknown. The drug’s effect on B cells, immune function, and infections in exposed offspring were not captured in the data, but a separate case report published after the belimumab pregnancy data report indicates that the drug does cross the placenta and builds up in the blood of the newborn, reducing B cells at birth.
Children of women with SLE have increased birth defect risks, and standard SLE therapeutic agents (for example, cyclophosphamide, methotrexate, mycophenolate mofetil) have been implicated in birth defects and pregnancy loss, but birth defect data for biologic drugs such as belimumab are limited. While belimumab animal data revealed no evidence of fetal harm or pregnancy loss rates, there was evidence of immature and mature B-cell count reductions.
Belimumab is approved by the Food and Drug Administration for use in patients aged 5 years and older with active, autoantibody-positive SLE who are taking standard therapy, and also for those with lupus nephritis.
Michelle Petri, MD, of Johns Hopkins University, Baltimore, and coauthors reported in Annals of the Rheumatic Diseases on data they compiled through March 8, 2020, from belimumab clinical trials, the Belimumab Pregnancy Registry (BPR), and postmarketing/spontaneous reports that encompassed 319 pregnancies with known outcomes.
Across 18 clinical trials with 223 live births, birth defects occurred in 4 of 72 (5.6%) belimumab-exposed pregnancies and in 0 of 9 in placebo-exposed pregnancies. Pregnancy loss (excluding elective terminations) occurred in 31.8% (35 of 110) of belimumab-exposed women and 43.8% (7 of 16) of placebo-exposed women in clinical trials. In the BPR retrospective cohort, 4.2% had pregnancy loss. Postmarketing and spontaneous reports had a pregnancy loss rate of 31.4% (43 of 137). Concomitant medications, confounding factors, and/or missing data were noted in all belimumab-exposed women in clinical trials and the BPR cohort. Dr. Petri and colleagues reported no consistent pattern of birth defects across datasets but stated: “Low numbers of exposed pregnancies, presence of confounding factors/other biases, and incomplete information preclude informed recommendations regarding risk of birth defects and pregnancy loss with belimumab use.”
In an interview, coauthor Megan E. B. Clowse, MD, MPH, associate professor of medicine and director of the division of rheumatology and immunology at Duke University, Durham, N.C., said that “the Annals of the Rheumatic Diseases article provides some reassurance that belimumab is unlikely to cause very frequent birth defects. It is clearly not in the risk-range for thalidomide or mycophenolate. However, due to the complexity of collecting these data, this manuscript can’t explore the full extent of possible risks. It also did not provide information about B cells, immune function, or infection risks in exposed offspring.”
A separate case report by Helle Bitter of the department of rheumatology at Sorlandet Hospital Kristiansand (Norway) in Annals of the Rheumatic Diseases is the first to show transplacental passage of belimumab in humans. Other prior reports have shown such transplacental passage for monoclonal IgG antibodies (tumor necrosis factor inhibitors and rituximab). Even though the last infusion was given late in the second trimester, belimumab was present in cord serum at birth, suggesting much higher concentrations before treatment was stopped. While B-cell numbers were reduced at birth, they returned to normal ranges by 4 months post partum when they were undetectable. In the mother, B-cell numbers remained low throughout the study period extending to 7 months after delivery. The authors stated that the child had a normal vaccination response, and except for the reduced B-cell levels at birth, had no adverse effects of prenatal exposure to maternal medication through age 6 years.
“The belimumab transfer in the case report is the level that we would anticipate based on similar studies in infant/mother pairs on other IgG1 antibody biologics like adalimumab – about 60% higher than the maternal level at birth,” Dr. Clowse said. “That the baby has very low B cells at birth is worrisome to me, demonstrating the lasting effect of maternal belimumab on the infant’s immune system, even when the drug was stopped 14 weeks prior to delivery. While this single infant did not have problems with infections, with more widespread use it seems possible that infants would be found to have higher rates of infections after in utero belimumab exposure.”
The field of lupus research greatly needs controlled studies of newer biologics in pregnancy, Dr. Clowse said. “Women with active lupus in pregnancy – particularly with active lupus nephritis – continue to suffer tragic outcomes at an alarming rate. Newer treatments for lupus nephritis provide some hope that we might be able to control lupus nephritis in pregnancy more effectively. The available data suggests the risks of these medications are not so large as to make studies unreasonable. Our current data doesn’t allow us to sufficiently balance the potential risks and benefits in a way that provides clinically useful guidance. Trials of these medications, however, would enable us to identify improved treatment strategies that could result in healthier women, pregnancies, and babies.”
GlaxoSmithKline funded the study. Dr. Clowse reported receiving consulting fees and grants from UCB and GlaxoSmithKline that relate to pregnancy in women with lupus.
The largest combined analysis of birth outcome data for women with systemic lupus erythematosus (SLE) who took belimumab (Benlysta) during pregnancy appears to indicate that the biologic is “unlikely to cause very frequent birth defects,” but the full extent of possible risk remains unknown. The drug’s effect on B cells, immune function, and infections in exposed offspring were not captured in the data, but a separate case report published after the belimumab pregnancy data report indicates that the drug does cross the placenta and builds up in the blood of the newborn, reducing B cells at birth.
Children of women with SLE have increased birth defect risks, and standard SLE therapeutic agents (for example, cyclophosphamide, methotrexate, mycophenolate mofetil) have been implicated in birth defects and pregnancy loss, but birth defect data for biologic drugs such as belimumab are limited. While belimumab animal data revealed no evidence of fetal harm or pregnancy loss rates, there was evidence of immature and mature B-cell count reductions.
Belimumab is approved by the Food and Drug Administration for use in patients aged 5 years and older with active, autoantibody-positive SLE who are taking standard therapy, and also for those with lupus nephritis.
Michelle Petri, MD, of Johns Hopkins University, Baltimore, and coauthors reported in Annals of the Rheumatic Diseases on data they compiled through March 8, 2020, from belimumab clinical trials, the Belimumab Pregnancy Registry (BPR), and postmarketing/spontaneous reports that encompassed 319 pregnancies with known outcomes.
Across 18 clinical trials with 223 live births, birth defects occurred in 4 of 72 (5.6%) belimumab-exposed pregnancies and in 0 of 9 in placebo-exposed pregnancies. Pregnancy loss (excluding elective terminations) occurred in 31.8% (35 of 110) of belimumab-exposed women and 43.8% (7 of 16) of placebo-exposed women in clinical trials. In the BPR retrospective cohort, 4.2% had pregnancy loss. Postmarketing and spontaneous reports had a pregnancy loss rate of 31.4% (43 of 137). Concomitant medications, confounding factors, and/or missing data were noted in all belimumab-exposed women in clinical trials and the BPR cohort. Dr. Petri and colleagues reported no consistent pattern of birth defects across datasets but stated: “Low numbers of exposed pregnancies, presence of confounding factors/other biases, and incomplete information preclude informed recommendations regarding risk of birth defects and pregnancy loss with belimumab use.”
In an interview, coauthor Megan E. B. Clowse, MD, MPH, associate professor of medicine and director of the division of rheumatology and immunology at Duke University, Durham, N.C., said that “the Annals of the Rheumatic Diseases article provides some reassurance that belimumab is unlikely to cause very frequent birth defects. It is clearly not in the risk-range for thalidomide or mycophenolate. However, due to the complexity of collecting these data, this manuscript can’t explore the full extent of possible risks. It also did not provide information about B cells, immune function, or infection risks in exposed offspring.”
A separate case report by Helle Bitter of the department of rheumatology at Sorlandet Hospital Kristiansand (Norway) in Annals of the Rheumatic Diseases is the first to show transplacental passage of belimumab in humans. Other prior reports have shown such transplacental passage for monoclonal IgG antibodies (tumor necrosis factor inhibitors and rituximab). Even though the last infusion was given late in the second trimester, belimumab was present in cord serum at birth, suggesting much higher concentrations before treatment was stopped. While B-cell numbers were reduced at birth, they returned to normal ranges by 4 months post partum when they were undetectable. In the mother, B-cell numbers remained low throughout the study period extending to 7 months after delivery. The authors stated that the child had a normal vaccination response, and except for the reduced B-cell levels at birth, had no adverse effects of prenatal exposure to maternal medication through age 6 years.
“The belimumab transfer in the case report is the level that we would anticipate based on similar studies in infant/mother pairs on other IgG1 antibody biologics like adalimumab – about 60% higher than the maternal level at birth,” Dr. Clowse said. “That the baby has very low B cells at birth is worrisome to me, demonstrating the lasting effect of maternal belimumab on the infant’s immune system, even when the drug was stopped 14 weeks prior to delivery. While this single infant did not have problems with infections, with more widespread use it seems possible that infants would be found to have higher rates of infections after in utero belimumab exposure.”
The field of lupus research greatly needs controlled studies of newer biologics in pregnancy, Dr. Clowse said. “Women with active lupus in pregnancy – particularly with active lupus nephritis – continue to suffer tragic outcomes at an alarming rate. Newer treatments for lupus nephritis provide some hope that we might be able to control lupus nephritis in pregnancy more effectively. The available data suggests the risks of these medications are not so large as to make studies unreasonable. Our current data doesn’t allow us to sufficiently balance the potential risks and benefits in a way that provides clinically useful guidance. Trials of these medications, however, would enable us to identify improved treatment strategies that could result in healthier women, pregnancies, and babies.”
GlaxoSmithKline funded the study. Dr. Clowse reported receiving consulting fees and grants from UCB and GlaxoSmithKline that relate to pregnancy in women with lupus.
The largest combined analysis of birth outcome data for women with systemic lupus erythematosus (SLE) who took belimumab (Benlysta) during pregnancy appears to indicate that the biologic is “unlikely to cause very frequent birth defects,” but the full extent of possible risk remains unknown. The drug’s effect on B cells, immune function, and infections in exposed offspring were not captured in the data, but a separate case report published after the belimumab pregnancy data report indicates that the drug does cross the placenta and builds up in the blood of the newborn, reducing B cells at birth.
Children of women with SLE have increased birth defect risks, and standard SLE therapeutic agents (for example, cyclophosphamide, methotrexate, mycophenolate mofetil) have been implicated in birth defects and pregnancy loss, but birth defect data for biologic drugs such as belimumab are limited. While belimumab animal data revealed no evidence of fetal harm or pregnancy loss rates, there was evidence of immature and mature B-cell count reductions.
Belimumab is approved by the Food and Drug Administration for use in patients aged 5 years and older with active, autoantibody-positive SLE who are taking standard therapy, and also for those with lupus nephritis.
Michelle Petri, MD, of Johns Hopkins University, Baltimore, and coauthors reported in Annals of the Rheumatic Diseases on data they compiled through March 8, 2020, from belimumab clinical trials, the Belimumab Pregnancy Registry (BPR), and postmarketing/spontaneous reports that encompassed 319 pregnancies with known outcomes.
Across 18 clinical trials with 223 live births, birth defects occurred in 4 of 72 (5.6%) belimumab-exposed pregnancies and in 0 of 9 in placebo-exposed pregnancies. Pregnancy loss (excluding elective terminations) occurred in 31.8% (35 of 110) of belimumab-exposed women and 43.8% (7 of 16) of placebo-exposed women in clinical trials. In the BPR retrospective cohort, 4.2% had pregnancy loss. Postmarketing and spontaneous reports had a pregnancy loss rate of 31.4% (43 of 137). Concomitant medications, confounding factors, and/or missing data were noted in all belimumab-exposed women in clinical trials and the BPR cohort. Dr. Petri and colleagues reported no consistent pattern of birth defects across datasets but stated: “Low numbers of exposed pregnancies, presence of confounding factors/other biases, and incomplete information preclude informed recommendations regarding risk of birth defects and pregnancy loss with belimumab use.”
In an interview, coauthor Megan E. B. Clowse, MD, MPH, associate professor of medicine and director of the division of rheumatology and immunology at Duke University, Durham, N.C., said that “the Annals of the Rheumatic Diseases article provides some reassurance that belimumab is unlikely to cause very frequent birth defects. It is clearly not in the risk-range for thalidomide or mycophenolate. However, due to the complexity of collecting these data, this manuscript can’t explore the full extent of possible risks. It also did not provide information about B cells, immune function, or infection risks in exposed offspring.”
A separate case report by Helle Bitter of the department of rheumatology at Sorlandet Hospital Kristiansand (Norway) in Annals of the Rheumatic Diseases is the first to show transplacental passage of belimumab in humans. Other prior reports have shown such transplacental passage for monoclonal IgG antibodies (tumor necrosis factor inhibitors and rituximab). Even though the last infusion was given late in the second trimester, belimumab was present in cord serum at birth, suggesting much higher concentrations before treatment was stopped. While B-cell numbers were reduced at birth, they returned to normal ranges by 4 months post partum when they were undetectable. In the mother, B-cell numbers remained low throughout the study period extending to 7 months after delivery. The authors stated that the child had a normal vaccination response, and except for the reduced B-cell levels at birth, had no adverse effects of prenatal exposure to maternal medication through age 6 years.
“The belimumab transfer in the case report is the level that we would anticipate based on similar studies in infant/mother pairs on other IgG1 antibody biologics like adalimumab – about 60% higher than the maternal level at birth,” Dr. Clowse said. “That the baby has very low B cells at birth is worrisome to me, demonstrating the lasting effect of maternal belimumab on the infant’s immune system, even when the drug was stopped 14 weeks prior to delivery. While this single infant did not have problems with infections, with more widespread use it seems possible that infants would be found to have higher rates of infections after in utero belimumab exposure.”
The field of lupus research greatly needs controlled studies of newer biologics in pregnancy, Dr. Clowse said. “Women with active lupus in pregnancy – particularly with active lupus nephritis – continue to suffer tragic outcomes at an alarming rate. Newer treatments for lupus nephritis provide some hope that we might be able to control lupus nephritis in pregnancy more effectively. The available data suggests the risks of these medications are not so large as to make studies unreasonable. Our current data doesn’t allow us to sufficiently balance the potential risks and benefits in a way that provides clinically useful guidance. Trials of these medications, however, would enable us to identify improved treatment strategies that could result in healthier women, pregnancies, and babies.”
GlaxoSmithKline funded the study. Dr. Clowse reported receiving consulting fees and grants from UCB and GlaxoSmithKline that relate to pregnancy in women with lupus.
FROM ANNALS OF THE RHEUMATIC DISEASES










