User login
Hearing loss strongly tied to increased dementia risk
, new national data show. Investigators also found that even mild hearing loss was associated with increased dementia risk, although it was not statistically significant, and that hearing aid use was tied to a 32% decrease in dementia prevalence.
“Every 10-decibel increase in hearing loss was associated with 16% greater prevalence of dementia, such that prevalence of dementia in older adults with moderate or greater hearing loss was 61% higher than prevalence in those with normal hearing,” said lead investigator Alison Huang, PhD, senior research associate in epidemiology at Johns Hopkins Bloomberg School of Public Health and core faculty in the Cochlear Center for Hearing and Public Health, Baltimore.
The findings were published online in JAMA.
Dose dependent effect
For their study, researchers analyzed data on 2,413 community-dwelling participants in the National Health and Aging Trends Study, a nationally representative, continuous panel study of U.S. Medicare beneficiaries aged 65 and older.
Data from the study was collected during in-home interviews, setting it apart from previous work that relied on data collected in a clinical setting, Dr. Huang said.
“This study was able to capture more vulnerable populations, such as the oldest old and older adults with disabilities, typically excluded from prior epidemiologic studies of the hearing loss–dementia association that use clinic-based data collection, which only captures people who have the ability and means to get to clinics,” Dr. Huang said.
Weighted hearing loss prevalence was 36.7% for mild and 29.8% for moderate to severe hearing loss, and weighted prevalence of dementia was 10.3%.
Those with moderate to severe hearing loss were 61% more likely to have dementia than were those with normal hearing (prevalence ratio, 1.61; 95% confidence interval [CI], 1.09-2.38).
Dementia prevalence increased with increasing severity of hearing loss: Normal hearing: 6.19% (95% CI, 4.31-8.80); mild hearing loss: 8.93% (95% CI, 6.99-11.34); moderate to severe hearing loss: 16.52% (95% CI, 13.81-19.64). But only moderate to severe hearing loss showed a statistically significant association with dementia (P = .02).
Dementia prevalence increased 16% per 10-decibel increase in hearing loss (prevalence ratio 1.16; P < .001).
Among the 853 individuals in the study with moderate to severe hearing loss, those who used hearing aids (n = 414) had a 32% lower risk of dementia compared with those who didn’t use assisted devices (prevalence ratio, 0.68; 95% CI, 0.47-1.00). Similar data were published in JAMA Neurology, suggesting that hearing aids reduce dementia risk.
“With this study, we were able to refine our understanding of the strength of the hearing loss–dementia association in a study more representative of older adults in the United States,” said Dr. Huang.
Robust association
Commenting on the findings, Justin S. Golub, MD, associate professor in the department of otolaryngology–head and neck surgery at Columbia University, New York, said the study supports earlier research and suggests a “robust” association between hearing loss and dementia.
“The particular advantage of this study was that it was high quality and nationally representative,” Dr. Golub said. “It is also among a smaller set of studies that have shown hearing aid use to be associated with lower risk of dementia.”
Although not statistically significant, researchers did find increasing prevalence of dementia among people with only mild hearing loss, and clinicians should take note, said Dr. Golub, who was not involved with this study.
“We would expect the relationship between mild hearing loss and dementia to be weaker than severe hearing loss and dementia and, as a result, it might take more participants to show an association among the mild group,” Dr. Golub said.
“Even though this particular study did not specifically find a relationship between mild hearing loss and dementia, I would still recommend people to start treating their hearing loss when it is early,” Dr. Golub added.
The study was funded by the National Institute on Aging. Dr. Golub reports no relevant financial relationships. Full disclosures for study authors are included in the original article.
A version of this article first appeared on Medscape.com.
, new national data show. Investigators also found that even mild hearing loss was associated with increased dementia risk, although it was not statistically significant, and that hearing aid use was tied to a 32% decrease in dementia prevalence.
“Every 10-decibel increase in hearing loss was associated with 16% greater prevalence of dementia, such that prevalence of dementia in older adults with moderate or greater hearing loss was 61% higher than prevalence in those with normal hearing,” said lead investigator Alison Huang, PhD, senior research associate in epidemiology at Johns Hopkins Bloomberg School of Public Health and core faculty in the Cochlear Center for Hearing and Public Health, Baltimore.
The findings were published online in JAMA.
Dose dependent effect
For their study, researchers analyzed data on 2,413 community-dwelling participants in the National Health and Aging Trends Study, a nationally representative, continuous panel study of U.S. Medicare beneficiaries aged 65 and older.
Data from the study was collected during in-home interviews, setting it apart from previous work that relied on data collected in a clinical setting, Dr. Huang said.
“This study was able to capture more vulnerable populations, such as the oldest old and older adults with disabilities, typically excluded from prior epidemiologic studies of the hearing loss–dementia association that use clinic-based data collection, which only captures people who have the ability and means to get to clinics,” Dr. Huang said.
Weighted hearing loss prevalence was 36.7% for mild and 29.8% for moderate to severe hearing loss, and weighted prevalence of dementia was 10.3%.
Those with moderate to severe hearing loss were 61% more likely to have dementia than were those with normal hearing (prevalence ratio, 1.61; 95% confidence interval [CI], 1.09-2.38).
Dementia prevalence increased with increasing severity of hearing loss: Normal hearing: 6.19% (95% CI, 4.31-8.80); mild hearing loss: 8.93% (95% CI, 6.99-11.34); moderate to severe hearing loss: 16.52% (95% CI, 13.81-19.64). But only moderate to severe hearing loss showed a statistically significant association with dementia (P = .02).
Dementia prevalence increased 16% per 10-decibel increase in hearing loss (prevalence ratio 1.16; P < .001).
Among the 853 individuals in the study with moderate to severe hearing loss, those who used hearing aids (n = 414) had a 32% lower risk of dementia compared with those who didn’t use assisted devices (prevalence ratio, 0.68; 95% CI, 0.47-1.00). Similar data were published in JAMA Neurology, suggesting that hearing aids reduce dementia risk.
“With this study, we were able to refine our understanding of the strength of the hearing loss–dementia association in a study more representative of older adults in the United States,” said Dr. Huang.
Robust association
Commenting on the findings, Justin S. Golub, MD, associate professor in the department of otolaryngology–head and neck surgery at Columbia University, New York, said the study supports earlier research and suggests a “robust” association between hearing loss and dementia.
“The particular advantage of this study was that it was high quality and nationally representative,” Dr. Golub said. “It is also among a smaller set of studies that have shown hearing aid use to be associated with lower risk of dementia.”
Although not statistically significant, researchers did find increasing prevalence of dementia among people with only mild hearing loss, and clinicians should take note, said Dr. Golub, who was not involved with this study.
“We would expect the relationship between mild hearing loss and dementia to be weaker than severe hearing loss and dementia and, as a result, it might take more participants to show an association among the mild group,” Dr. Golub said.
“Even though this particular study did not specifically find a relationship between mild hearing loss and dementia, I would still recommend people to start treating their hearing loss when it is early,” Dr. Golub added.
The study was funded by the National Institute on Aging. Dr. Golub reports no relevant financial relationships. Full disclosures for study authors are included in the original article.
A version of this article first appeared on Medscape.com.
, new national data show. Investigators also found that even mild hearing loss was associated with increased dementia risk, although it was not statistically significant, and that hearing aid use was tied to a 32% decrease in dementia prevalence.
“Every 10-decibel increase in hearing loss was associated with 16% greater prevalence of dementia, such that prevalence of dementia in older adults with moderate or greater hearing loss was 61% higher than prevalence in those with normal hearing,” said lead investigator Alison Huang, PhD, senior research associate in epidemiology at Johns Hopkins Bloomberg School of Public Health and core faculty in the Cochlear Center for Hearing and Public Health, Baltimore.
The findings were published online in JAMA.
Dose dependent effect
For their study, researchers analyzed data on 2,413 community-dwelling participants in the National Health and Aging Trends Study, a nationally representative, continuous panel study of U.S. Medicare beneficiaries aged 65 and older.
Data from the study was collected during in-home interviews, setting it apart from previous work that relied on data collected in a clinical setting, Dr. Huang said.
“This study was able to capture more vulnerable populations, such as the oldest old and older adults with disabilities, typically excluded from prior epidemiologic studies of the hearing loss–dementia association that use clinic-based data collection, which only captures people who have the ability and means to get to clinics,” Dr. Huang said.
Weighted hearing loss prevalence was 36.7% for mild and 29.8% for moderate to severe hearing loss, and weighted prevalence of dementia was 10.3%.
Those with moderate to severe hearing loss were 61% more likely to have dementia than were those with normal hearing (prevalence ratio, 1.61; 95% confidence interval [CI], 1.09-2.38).
Dementia prevalence increased with increasing severity of hearing loss: Normal hearing: 6.19% (95% CI, 4.31-8.80); mild hearing loss: 8.93% (95% CI, 6.99-11.34); moderate to severe hearing loss: 16.52% (95% CI, 13.81-19.64). But only moderate to severe hearing loss showed a statistically significant association with dementia (P = .02).
Dementia prevalence increased 16% per 10-decibel increase in hearing loss (prevalence ratio 1.16; P < .001).
Among the 853 individuals in the study with moderate to severe hearing loss, those who used hearing aids (n = 414) had a 32% lower risk of dementia compared with those who didn’t use assisted devices (prevalence ratio, 0.68; 95% CI, 0.47-1.00). Similar data were published in JAMA Neurology, suggesting that hearing aids reduce dementia risk.
“With this study, we were able to refine our understanding of the strength of the hearing loss–dementia association in a study more representative of older adults in the United States,” said Dr. Huang.
Robust association
Commenting on the findings, Justin S. Golub, MD, associate professor in the department of otolaryngology–head and neck surgery at Columbia University, New York, said the study supports earlier research and suggests a “robust” association between hearing loss and dementia.
“The particular advantage of this study was that it was high quality and nationally representative,” Dr. Golub said. “It is also among a smaller set of studies that have shown hearing aid use to be associated with lower risk of dementia.”
Although not statistically significant, researchers did find increasing prevalence of dementia among people with only mild hearing loss, and clinicians should take note, said Dr. Golub, who was not involved with this study.
“We would expect the relationship between mild hearing loss and dementia to be weaker than severe hearing loss and dementia and, as a result, it might take more participants to show an association among the mild group,” Dr. Golub said.
“Even though this particular study did not specifically find a relationship between mild hearing loss and dementia, I would still recommend people to start treating their hearing loss when it is early,” Dr. Golub added.
The study was funded by the National Institute on Aging. Dr. Golub reports no relevant financial relationships. Full disclosures for study authors are included in the original article.
A version of this article first appeared on Medscape.com.
Scaly facial plaques
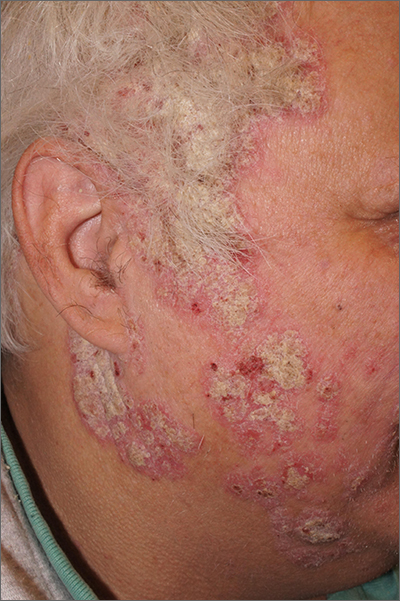
This patient was experiencing a flare of his psoriasis. Three factors contributed to the flare: noncompliance with his treatment regimen, decreased sunlight in the winter, and his lithium therapy. Though carcinogenic, certain wavelengths of UV light are beneficial for psoriasis, and the shorter days of winter can cause flaring of psoriasis (or relative flaring). In addition, lithium—the most effective therapy for this patient’s bipolar disorder—can worsen psoriasis.
Psoriasis is a chronic multisystem inflammatory disorder with characteristic skin findings that include well-demarcated micaceous plaques, nail pitting, and sometimes tendon pain and inflammatory arthritis. Severity can range from small, thin plaques that are intermittently noticeable on the elbows or knees to widespread ash-like plaques covering most of the body.
Good topical choices for facial skin include hydrocortisone 2.5% cream or desonide 0.05%. Nonsteroidal topical therapies that are safe for facial skin include tacrolimus 0.1% ointment or pimecrolimus 1% cream.1 These options may be used twice daily until the disease is controlled.
In many cases (as in this one), the patient’s previous psoriasis outbreaks could not be controlled with topical therapy alone. The patient had not responded to a previous methotrexate regimen, and more recently had been clear for several years on systemic ustekinumab, a monoclonal antibody. Dosed every 12 weeks, or sometimes every 8 weeks, ustekinumab is given by subcutaneous injection, usually in the abdomen, through normal skin. Ustekinumab was recently approved for home use with just 4 injections per year for maintenance therapy. However, the infrequency of the injections sometimes leads to noncompliance, as occurred with this patient. He had missed 2 doses since taking over his own dosing regimen.
Ultimately, the patient’s flare resolved when he was transitioned back to in-office treatment with ustekinumab.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Woo SM, Choi JW, Yoon HS, et al. Classification of facial psoriasis based on the distributions of facial lesions. J Am Acad Dermatol. 2008;58:959-63. doi: 10.1016/j.jaad.2008.02.006

This patient was experiencing a flare of his psoriasis. Three factors contributed to the flare: noncompliance with his treatment regimen, decreased sunlight in the winter, and his lithium therapy. Though carcinogenic, certain wavelengths of UV light are beneficial for psoriasis, and the shorter days of winter can cause flaring of psoriasis (or relative flaring). In addition, lithium—the most effective therapy for this patient’s bipolar disorder—can worsen psoriasis.
Psoriasis is a chronic multisystem inflammatory disorder with characteristic skin findings that include well-demarcated micaceous plaques, nail pitting, and sometimes tendon pain and inflammatory arthritis. Severity can range from small, thin plaques that are intermittently noticeable on the elbows or knees to widespread ash-like plaques covering most of the body.
Good topical choices for facial skin include hydrocortisone 2.5% cream or desonide 0.05%. Nonsteroidal topical therapies that are safe for facial skin include tacrolimus 0.1% ointment or pimecrolimus 1% cream.1 These options may be used twice daily until the disease is controlled.
In many cases (as in this one), the patient’s previous psoriasis outbreaks could not be controlled with topical therapy alone. The patient had not responded to a previous methotrexate regimen, and more recently had been clear for several years on systemic ustekinumab, a monoclonal antibody. Dosed every 12 weeks, or sometimes every 8 weeks, ustekinumab is given by subcutaneous injection, usually in the abdomen, through normal skin. Ustekinumab was recently approved for home use with just 4 injections per year for maintenance therapy. However, the infrequency of the injections sometimes leads to noncompliance, as occurred with this patient. He had missed 2 doses since taking over his own dosing regimen.
Ultimately, the patient’s flare resolved when he was transitioned back to in-office treatment with ustekinumab.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

This patient was experiencing a flare of his psoriasis. Three factors contributed to the flare: noncompliance with his treatment regimen, decreased sunlight in the winter, and his lithium therapy. Though carcinogenic, certain wavelengths of UV light are beneficial for psoriasis, and the shorter days of winter can cause flaring of psoriasis (or relative flaring). In addition, lithium—the most effective therapy for this patient’s bipolar disorder—can worsen psoriasis.
Psoriasis is a chronic multisystem inflammatory disorder with characteristic skin findings that include well-demarcated micaceous plaques, nail pitting, and sometimes tendon pain and inflammatory arthritis. Severity can range from small, thin plaques that are intermittently noticeable on the elbows or knees to widespread ash-like plaques covering most of the body.
Good topical choices for facial skin include hydrocortisone 2.5% cream or desonide 0.05%. Nonsteroidal topical therapies that are safe for facial skin include tacrolimus 0.1% ointment or pimecrolimus 1% cream.1 These options may be used twice daily until the disease is controlled.
In many cases (as in this one), the patient’s previous psoriasis outbreaks could not be controlled with topical therapy alone. The patient had not responded to a previous methotrexate regimen, and more recently had been clear for several years on systemic ustekinumab, a monoclonal antibody. Dosed every 12 weeks, or sometimes every 8 weeks, ustekinumab is given by subcutaneous injection, usually in the abdomen, through normal skin. Ustekinumab was recently approved for home use with just 4 injections per year for maintenance therapy. However, the infrequency of the injections sometimes leads to noncompliance, as occurred with this patient. He had missed 2 doses since taking over his own dosing regimen.
Ultimately, the patient’s flare resolved when he was transitioned back to in-office treatment with ustekinumab.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Woo SM, Choi JW, Yoon HS, et al. Classification of facial psoriasis based on the distributions of facial lesions. J Am Acad Dermatol. 2008;58:959-63. doi: 10.1016/j.jaad.2008.02.006
1. Woo SM, Choi JW, Yoon HS, et al. Classification of facial psoriasis based on the distributions of facial lesions. J Am Acad Dermatol. 2008;58:959-63. doi: 10.1016/j.jaad.2008.02.006
Add this to the list of long COVID symptoms: Stigma
Most people with long COVID find they’re facing stigma due to their condition, according to a new report from researchers in the United Kingdom. In short: Relatives and friends may not believe they’re truly sick.
The U.K. team found that more than three-quarters of people studied had experienced stigma often or always.
In fact, 95% of people with long COVID faced at least one type of stigma at least sometimes, according to the study, published in November in the journal PLOS One.
Those conclusions had surprised the study’s lead researcher, Marija Pantelic, PhD, a public health lecturer at Brighton and Sussex Medical School, England.
“After years of working on HIV-related stigma, I was shocked to see how many people were turning a blind eye to and dismissing the difficulties experienced by people with long COVID,” Dr. Pantelic says. “It has also been clear to me from the start that this stigma is detrimental not just for people’s dignity, but also public health.”
Even some doctors argue that the growing attention paid to long COVID is excessive.
“It’s often normal to experience mild fatigue or weaknesses for weeks after being sick and inactive and not eating well. Calling these cases long COVID is the medicalization of modern life,” Marty Makary, MD, a surgeon and public policy researcher at Johns Hopkins University, Baltimore, wrote in a commentary in the Wall Street Journal.
Other doctors strongly disagree, including Alba Azola, MD, codirector of the Johns Hopkins Post-Acute COVID-19 Team and an expert in the stigma surrounding long COVID.
“Putting that spin on things, it’s just hurting people,” she says.
One example is people who cannot return to work.
“A lot of their family members tell me that they’re being lazy,” Dr. Azola says. “That’s part of the public stigma, that these are people just trying to get out of work.”
Some experts say the U.K. study represents a landmark.
“When you have data like this on long COVID stigma, it becomes more difficult to deny its existence or address it,” says Naomi Torres-Mackie, PhD, a clinical psychologist at Lenox Hill Hospital in New York. She also is head of research at the New York–based Mental Health Coalition, a group of experts working to end the stigma surrounding mental health.
She recalls her first patient with long COVID.
“She experienced the discomfort and pain itself, and then she had this crushing feeling that it wasn’t valid, or real. She felt very alone in it,” Dr. Torres-Mackie says.
Another one of her patients is working at her job from home but facing doubt about her condition from her employers.
“Every month, her medical doctor has to produce a letter confirming her medical condition,” Dr. Torres-Mackie says.
Taking part in the British stigma survey were 1,166 people, including 966 residents of the United Kingdom, with the average age of 48. Nearly 85% were female, and more than three-quarters were educated at the university level or higher.
Half of them said they had a clinical diagnosis of long COVID.
More than 60% of them said that at least some of the time, they were cautious about who they talked to about their condition. And fully 34% of those who did disclose their diagnosis said that they regretted having done so.
That’s a difficult experience for those with long COVID, says Leonard Jason, PhD, a professor of psychology at DePaul University in Chicago.
“It’s like they’re traumatized by the initial experience of being sick, and retraumatized by the response of others to them,” he says.
Unexplained illnesses are not well-regarded by the general public, Dr. Jason says.
He gave the example of multiple sclerosis. Before the 1980s, those with MS were considered to have a psychological illness, he says. “Then, in the 1980s, there were biomarkers that said, ‘Here’s the evidence.’ ”
The British study described three types of stigma stemming from the long COVID diagnosis of those questioned:
- Enacted stigma: People were directly treated unfairly because of their condition.
- Internalized stigma: People felt embarrassed by that condition.
- Anticipated stigma: People expected they would be treated poorly because of their diagnosis.
Dr. Azola calls the medical community a major problem when it comes to dealing with long COVID.
“What I see with my patients is medical trauma,” she says. They may have symptoms that send them to the emergency room, and then the tests come back negative. “Instead of tracking the patients’ symptoms, patients get told, ‘Everything looks good, you can go home, this is a panic attack,’ ” she says.
Some people go online to search for treatments, sometimes launching GoFundMe campaigns to raise money for unreliable treatments.
Long COVID patients may have gone through 5 to 10 doctors before they arrive for treatment with the Johns Hopkins Post-Acute COVID-19 Team. The clinic began in April 2020 remotely and in August of that year in person.
Today, the clinic staff spends an hour with a first-time long COVID patient, hearing their stories and helping relieve anxiety, Dr. Azola says.
The phenomenon of long COVID is similar to what patients have had with chronic fatigue syndrome, lupus, or fibromyalgia, where people have symptoms that are hard to explain, says Jennifer Chevinsky, MD, deputy public health officer for Riverside County, Calif.
“Stigma within medicine or health care is nothing new,” she says.
In Chicago, Dr. Jason notes that the federal government’s decision to invest hundreds of millions of dollars in long COVID research “shows the government is helping destigmatize it.”
Dr. Pantelic says she and her colleagues are continuing their research.
“We are interested in understanding the impacts of this stigma, and how to mitigate any adverse outcomes for patients and services,” she says.
A version of this article first appeared on WebMD.com.
Most people with long COVID find they’re facing stigma due to their condition, according to a new report from researchers in the United Kingdom. In short: Relatives and friends may not believe they’re truly sick.
The U.K. team found that more than three-quarters of people studied had experienced stigma often or always.
In fact, 95% of people with long COVID faced at least one type of stigma at least sometimes, according to the study, published in November in the journal PLOS One.
Those conclusions had surprised the study’s lead researcher, Marija Pantelic, PhD, a public health lecturer at Brighton and Sussex Medical School, England.
“After years of working on HIV-related stigma, I was shocked to see how many people were turning a blind eye to and dismissing the difficulties experienced by people with long COVID,” Dr. Pantelic says. “It has also been clear to me from the start that this stigma is detrimental not just for people’s dignity, but also public health.”
Even some doctors argue that the growing attention paid to long COVID is excessive.
“It’s often normal to experience mild fatigue or weaknesses for weeks after being sick and inactive and not eating well. Calling these cases long COVID is the medicalization of modern life,” Marty Makary, MD, a surgeon and public policy researcher at Johns Hopkins University, Baltimore, wrote in a commentary in the Wall Street Journal.
Other doctors strongly disagree, including Alba Azola, MD, codirector of the Johns Hopkins Post-Acute COVID-19 Team and an expert in the stigma surrounding long COVID.
“Putting that spin on things, it’s just hurting people,” she says.
One example is people who cannot return to work.
“A lot of their family members tell me that they’re being lazy,” Dr. Azola says. “That’s part of the public stigma, that these are people just trying to get out of work.”
Some experts say the U.K. study represents a landmark.
“When you have data like this on long COVID stigma, it becomes more difficult to deny its existence or address it,” says Naomi Torres-Mackie, PhD, a clinical psychologist at Lenox Hill Hospital in New York. She also is head of research at the New York–based Mental Health Coalition, a group of experts working to end the stigma surrounding mental health.
She recalls her first patient with long COVID.
“She experienced the discomfort and pain itself, and then she had this crushing feeling that it wasn’t valid, or real. She felt very alone in it,” Dr. Torres-Mackie says.
Another one of her patients is working at her job from home but facing doubt about her condition from her employers.
“Every month, her medical doctor has to produce a letter confirming her medical condition,” Dr. Torres-Mackie says.
Taking part in the British stigma survey were 1,166 people, including 966 residents of the United Kingdom, with the average age of 48. Nearly 85% were female, and more than three-quarters were educated at the university level or higher.
Half of them said they had a clinical diagnosis of long COVID.
More than 60% of them said that at least some of the time, they were cautious about who they talked to about their condition. And fully 34% of those who did disclose their diagnosis said that they regretted having done so.
That’s a difficult experience for those with long COVID, says Leonard Jason, PhD, a professor of psychology at DePaul University in Chicago.
“It’s like they’re traumatized by the initial experience of being sick, and retraumatized by the response of others to them,” he says.
Unexplained illnesses are not well-regarded by the general public, Dr. Jason says.
He gave the example of multiple sclerosis. Before the 1980s, those with MS were considered to have a psychological illness, he says. “Then, in the 1980s, there were biomarkers that said, ‘Here’s the evidence.’ ”
The British study described three types of stigma stemming from the long COVID diagnosis of those questioned:
- Enacted stigma: People were directly treated unfairly because of their condition.
- Internalized stigma: People felt embarrassed by that condition.
- Anticipated stigma: People expected they would be treated poorly because of their diagnosis.
Dr. Azola calls the medical community a major problem when it comes to dealing with long COVID.
“What I see with my patients is medical trauma,” she says. They may have symptoms that send them to the emergency room, and then the tests come back negative. “Instead of tracking the patients’ symptoms, patients get told, ‘Everything looks good, you can go home, this is a panic attack,’ ” she says.
Some people go online to search for treatments, sometimes launching GoFundMe campaigns to raise money for unreliable treatments.
Long COVID patients may have gone through 5 to 10 doctors before they arrive for treatment with the Johns Hopkins Post-Acute COVID-19 Team. The clinic began in April 2020 remotely and in August of that year in person.
Today, the clinic staff spends an hour with a first-time long COVID patient, hearing their stories and helping relieve anxiety, Dr. Azola says.
The phenomenon of long COVID is similar to what patients have had with chronic fatigue syndrome, lupus, or fibromyalgia, where people have symptoms that are hard to explain, says Jennifer Chevinsky, MD, deputy public health officer for Riverside County, Calif.
“Stigma within medicine or health care is nothing new,” she says.
In Chicago, Dr. Jason notes that the federal government’s decision to invest hundreds of millions of dollars in long COVID research “shows the government is helping destigmatize it.”
Dr. Pantelic says she and her colleagues are continuing their research.
“We are interested in understanding the impacts of this stigma, and how to mitigate any adverse outcomes for patients and services,” she says.
A version of this article first appeared on WebMD.com.
Most people with long COVID find they’re facing stigma due to their condition, according to a new report from researchers in the United Kingdom. In short: Relatives and friends may not believe they’re truly sick.
The U.K. team found that more than three-quarters of people studied had experienced stigma often or always.
In fact, 95% of people with long COVID faced at least one type of stigma at least sometimes, according to the study, published in November in the journal PLOS One.
Those conclusions had surprised the study’s lead researcher, Marija Pantelic, PhD, a public health lecturer at Brighton and Sussex Medical School, England.
“After years of working on HIV-related stigma, I was shocked to see how many people were turning a blind eye to and dismissing the difficulties experienced by people with long COVID,” Dr. Pantelic says. “It has also been clear to me from the start that this stigma is detrimental not just for people’s dignity, but also public health.”
Even some doctors argue that the growing attention paid to long COVID is excessive.
“It’s often normal to experience mild fatigue or weaknesses for weeks after being sick and inactive and not eating well. Calling these cases long COVID is the medicalization of modern life,” Marty Makary, MD, a surgeon and public policy researcher at Johns Hopkins University, Baltimore, wrote in a commentary in the Wall Street Journal.
Other doctors strongly disagree, including Alba Azola, MD, codirector of the Johns Hopkins Post-Acute COVID-19 Team and an expert in the stigma surrounding long COVID.
“Putting that spin on things, it’s just hurting people,” she says.
One example is people who cannot return to work.
“A lot of their family members tell me that they’re being lazy,” Dr. Azola says. “That’s part of the public stigma, that these are people just trying to get out of work.”
Some experts say the U.K. study represents a landmark.
“When you have data like this on long COVID stigma, it becomes more difficult to deny its existence or address it,” says Naomi Torres-Mackie, PhD, a clinical psychologist at Lenox Hill Hospital in New York. She also is head of research at the New York–based Mental Health Coalition, a group of experts working to end the stigma surrounding mental health.
She recalls her first patient with long COVID.
“She experienced the discomfort and pain itself, and then she had this crushing feeling that it wasn’t valid, or real. She felt very alone in it,” Dr. Torres-Mackie says.
Another one of her patients is working at her job from home but facing doubt about her condition from her employers.
“Every month, her medical doctor has to produce a letter confirming her medical condition,” Dr. Torres-Mackie says.
Taking part in the British stigma survey were 1,166 people, including 966 residents of the United Kingdom, with the average age of 48. Nearly 85% were female, and more than three-quarters were educated at the university level or higher.
Half of them said they had a clinical diagnosis of long COVID.
More than 60% of them said that at least some of the time, they were cautious about who they talked to about their condition. And fully 34% of those who did disclose their diagnosis said that they regretted having done so.
That’s a difficult experience for those with long COVID, says Leonard Jason, PhD, a professor of psychology at DePaul University in Chicago.
“It’s like they’re traumatized by the initial experience of being sick, and retraumatized by the response of others to them,” he says.
Unexplained illnesses are not well-regarded by the general public, Dr. Jason says.
He gave the example of multiple sclerosis. Before the 1980s, those with MS were considered to have a psychological illness, he says. “Then, in the 1980s, there were biomarkers that said, ‘Here’s the evidence.’ ”
The British study described three types of stigma stemming from the long COVID diagnosis of those questioned:
- Enacted stigma: People were directly treated unfairly because of their condition.
- Internalized stigma: People felt embarrassed by that condition.
- Anticipated stigma: People expected they would be treated poorly because of their diagnosis.
Dr. Azola calls the medical community a major problem when it comes to dealing with long COVID.
“What I see with my patients is medical trauma,” she says. They may have symptoms that send them to the emergency room, and then the tests come back negative. “Instead of tracking the patients’ symptoms, patients get told, ‘Everything looks good, you can go home, this is a panic attack,’ ” she says.
Some people go online to search for treatments, sometimes launching GoFundMe campaigns to raise money for unreliable treatments.
Long COVID patients may have gone through 5 to 10 doctors before they arrive for treatment with the Johns Hopkins Post-Acute COVID-19 Team. The clinic began in April 2020 remotely and in August of that year in person.
Today, the clinic staff spends an hour with a first-time long COVID patient, hearing their stories and helping relieve anxiety, Dr. Azola says.
The phenomenon of long COVID is similar to what patients have had with chronic fatigue syndrome, lupus, or fibromyalgia, where people have symptoms that are hard to explain, says Jennifer Chevinsky, MD, deputy public health officer for Riverside County, Calif.
“Stigma within medicine or health care is nothing new,” she says.
In Chicago, Dr. Jason notes that the federal government’s decision to invest hundreds of millions of dollars in long COVID research “shows the government is helping destigmatize it.”
Dr. Pantelic says she and her colleagues are continuing their research.
“We are interested in understanding the impacts of this stigma, and how to mitigate any adverse outcomes for patients and services,” she says.
A version of this article first appeared on WebMD.com.
PLOS ONE
Can siRNA improve compliance in patients with hypertension?
Many approaches have been explored in recent years to make life easier for patients living with chronic conditions that require them to take daily medication: subcutaneous implantable devices, nanogels, and, more specifically in the case of hypertension, renal denervation or small interfering RNA (siRNA) with a long half-life.
It’s siRNA that Michel Azizi, MD, PhD, head of the blood pressure clinic at Georges Pompidou European Hospital (HEGP) in Paris, discussed at the International Meeting of the French Society of Hypertension.
These small molecules have already shown their worth in treating rare diseases such as transthyretin amyloidosis. More recently, treating hypercholesterolemia with the PCSK9 inhibitor inclisiran has proven effective. “One subcutaneous injection of inclisiran reduces LDL cholesterol by 50% for a period of 210 days,” said Dr. Azizi.
The benefit of a new therapeutic siRNA – zilebesiran, administered subcutaneously – in treating hypertension is currently the subject of a phase II clinical trial.
This is a double-stranded RNA. One of the strands is linked to a sugar, N-acetylgalactosamine (GalNAc), which protects these highly fragile siRNA and binds with a very strong affinity in the liver. The second strand binds to a specific area of the RNA to prevent synthesis of the precursor peptide of angiotensin, angiotensinogen. The resulting effect is suppression of the production of angiotensin I and II, which leads to a long-lasting lowering of blood pressure.
Lasting efficacy
Phase I studies with zilebesiran have demonstrated a long-term effect, with a reduction of greater than 90% in circulating angiotensinogen over 6 months after a single subcutaneous dose (800 mg). The peak in reduction of circulating angiotensinogen occurs after approximately 3 weeks.
“It’s extremely powerful,” said Dr. Azizi.
Lasting reductions in blood pressure have also been observed, with 24-hour ambulatory blood pressure monitoring showing a reduction in systolic BP of greater than 15 mm Hg 8 weeks after administration of a single dose of zilebesiran (800 mg).
Zilebesiran was also well tolerated, with only mild to moderate reactions at the site of the injection (n = 5/56) and no serious treatment-related adverse events, hypotension, or significant changes in kidney or liver function.
“In terms of benefits, the effect is ongoing. Zilebesiran leads to reduced medication use and causes less variability in blood pressure response. Nevertheless, interfering RNA acts slowly, meaning that zilebesiran would not be suitable for people presenting with a hypertensive crisis. The fact that it blocks the renin-angiotensin system [RAS] for a very long period of time also poses the question of how to reverse its hypotensive effects,” said Dr. Azizi.
Unanswered questions
The lasting RAS antagonist and blood pressure–lowering effects pose a potential safety problem in circumstances involving patients in a state of hypovolemia and hypotension who require rapid blood pressure–raising interventions to prevent morbidity and mortality.
In recent studies, Estrellita Uijl et al. have thus examined strategies to counteract the blood pressure–lowering effect of siRNA in spontaneously hypertensive rats.
Fludrocortisone and a high-salt diet were both successful in gradually increasing blood pressure, which returned to its baseline levels on days 5 and 7, respectively. Yet this rate of response would be wholly inadequate in an urgent clinical situation.
However, midodrine could not reduce blood pressure to normal levels, whether administered subcutaneously or orally.
A rapid and short-lasting increase in blood pressure was observed with bolus doses of vasopressors, but clinically, these would need to be administered intravenously to achieve a lasting effect. Such administration would require hospitalization, close monitoring, and the use of human resources and additional health care provisions.
Encouragingly, the laboratory that created this molecule, Alnylam Pharmaceuticals, has come up with an antidote: Reversir. It is a GalNAc-conjugated, single-stranded, high-affinity oligonucleotide complementary to the zilebesiran strand that achieves effective reversal of siRNA activity in 24 hours.
In the future, after the phase 2 trials have been completed, whether or not zilebesiran reduces the incidence of cardiovascular events and mortality remains to be seen. But as for Dr. Azizi, the director of HEGP’s blood pressure clinic in Paris, he has no doubt that “this approach is about to shake up how we treat patients in the cardiovascular field.”
On the horizon
Zilebesiran is being studied in phase 2 trials in patients with mild to moderate hypertension not taking antihypertensive drugs (KARDIA-1: 375 patients; double-blind, placebo-controlled, five-arm trial; zilebesiran at 150, 300, and 600 mg twice per year and 300 mg once every 3 months) and in patients whose blood pressure is not controlled (KARDIA-2: 800 patients; initial open-label start-up period of 4 weeks with indapamide/amlodipine/olmesartan, followed by a double-blind, placebo-controlled study over 6 months, then an open-label extension study for up to 12 additional months; zilebesiran at 600 mg on the first day of the initial double-blind period, then every 6 months during the open-label extension period).
This article was translated from the Medscape French edition and a version appeared on Medscape.com.
Many approaches have been explored in recent years to make life easier for patients living with chronic conditions that require them to take daily medication: subcutaneous implantable devices, nanogels, and, more specifically in the case of hypertension, renal denervation or small interfering RNA (siRNA) with a long half-life.
It’s siRNA that Michel Azizi, MD, PhD, head of the blood pressure clinic at Georges Pompidou European Hospital (HEGP) in Paris, discussed at the International Meeting of the French Society of Hypertension.
These small molecules have already shown their worth in treating rare diseases such as transthyretin amyloidosis. More recently, treating hypercholesterolemia with the PCSK9 inhibitor inclisiran has proven effective. “One subcutaneous injection of inclisiran reduces LDL cholesterol by 50% for a period of 210 days,” said Dr. Azizi.
The benefit of a new therapeutic siRNA – zilebesiran, administered subcutaneously – in treating hypertension is currently the subject of a phase II clinical trial.
This is a double-stranded RNA. One of the strands is linked to a sugar, N-acetylgalactosamine (GalNAc), which protects these highly fragile siRNA and binds with a very strong affinity in the liver. The second strand binds to a specific area of the RNA to prevent synthesis of the precursor peptide of angiotensin, angiotensinogen. The resulting effect is suppression of the production of angiotensin I and II, which leads to a long-lasting lowering of blood pressure.
Lasting efficacy
Phase I studies with zilebesiran have demonstrated a long-term effect, with a reduction of greater than 90% in circulating angiotensinogen over 6 months after a single subcutaneous dose (800 mg). The peak in reduction of circulating angiotensinogen occurs after approximately 3 weeks.
“It’s extremely powerful,” said Dr. Azizi.
Lasting reductions in blood pressure have also been observed, with 24-hour ambulatory blood pressure monitoring showing a reduction in systolic BP of greater than 15 mm Hg 8 weeks after administration of a single dose of zilebesiran (800 mg).
Zilebesiran was also well tolerated, with only mild to moderate reactions at the site of the injection (n = 5/56) and no serious treatment-related adverse events, hypotension, or significant changes in kidney or liver function.
“In terms of benefits, the effect is ongoing. Zilebesiran leads to reduced medication use and causes less variability in blood pressure response. Nevertheless, interfering RNA acts slowly, meaning that zilebesiran would not be suitable for people presenting with a hypertensive crisis. The fact that it blocks the renin-angiotensin system [RAS] for a very long period of time also poses the question of how to reverse its hypotensive effects,” said Dr. Azizi.
Unanswered questions
The lasting RAS antagonist and blood pressure–lowering effects pose a potential safety problem in circumstances involving patients in a state of hypovolemia and hypotension who require rapid blood pressure–raising interventions to prevent morbidity and mortality.
In recent studies, Estrellita Uijl et al. have thus examined strategies to counteract the blood pressure–lowering effect of siRNA in spontaneously hypertensive rats.
Fludrocortisone and a high-salt diet were both successful in gradually increasing blood pressure, which returned to its baseline levels on days 5 and 7, respectively. Yet this rate of response would be wholly inadequate in an urgent clinical situation.
However, midodrine could not reduce blood pressure to normal levels, whether administered subcutaneously or orally.
A rapid and short-lasting increase in blood pressure was observed with bolus doses of vasopressors, but clinically, these would need to be administered intravenously to achieve a lasting effect. Such administration would require hospitalization, close monitoring, and the use of human resources and additional health care provisions.
Encouragingly, the laboratory that created this molecule, Alnylam Pharmaceuticals, has come up with an antidote: Reversir. It is a GalNAc-conjugated, single-stranded, high-affinity oligonucleotide complementary to the zilebesiran strand that achieves effective reversal of siRNA activity in 24 hours.
In the future, after the phase 2 trials have been completed, whether or not zilebesiran reduces the incidence of cardiovascular events and mortality remains to be seen. But as for Dr. Azizi, the director of HEGP’s blood pressure clinic in Paris, he has no doubt that “this approach is about to shake up how we treat patients in the cardiovascular field.”
On the horizon
Zilebesiran is being studied in phase 2 trials in patients with mild to moderate hypertension not taking antihypertensive drugs (KARDIA-1: 375 patients; double-blind, placebo-controlled, five-arm trial; zilebesiran at 150, 300, and 600 mg twice per year and 300 mg once every 3 months) and in patients whose blood pressure is not controlled (KARDIA-2: 800 patients; initial open-label start-up period of 4 weeks with indapamide/amlodipine/olmesartan, followed by a double-blind, placebo-controlled study over 6 months, then an open-label extension study for up to 12 additional months; zilebesiran at 600 mg on the first day of the initial double-blind period, then every 6 months during the open-label extension period).
This article was translated from the Medscape French edition and a version appeared on Medscape.com.
Many approaches have been explored in recent years to make life easier for patients living with chronic conditions that require them to take daily medication: subcutaneous implantable devices, nanogels, and, more specifically in the case of hypertension, renal denervation or small interfering RNA (siRNA) with a long half-life.
It’s siRNA that Michel Azizi, MD, PhD, head of the blood pressure clinic at Georges Pompidou European Hospital (HEGP) in Paris, discussed at the International Meeting of the French Society of Hypertension.
These small molecules have already shown their worth in treating rare diseases such as transthyretin amyloidosis. More recently, treating hypercholesterolemia with the PCSK9 inhibitor inclisiran has proven effective. “One subcutaneous injection of inclisiran reduces LDL cholesterol by 50% for a period of 210 days,” said Dr. Azizi.
The benefit of a new therapeutic siRNA – zilebesiran, administered subcutaneously – in treating hypertension is currently the subject of a phase II clinical trial.
This is a double-stranded RNA. One of the strands is linked to a sugar, N-acetylgalactosamine (GalNAc), which protects these highly fragile siRNA and binds with a very strong affinity in the liver. The second strand binds to a specific area of the RNA to prevent synthesis of the precursor peptide of angiotensin, angiotensinogen. The resulting effect is suppression of the production of angiotensin I and II, which leads to a long-lasting lowering of blood pressure.
Lasting efficacy
Phase I studies with zilebesiran have demonstrated a long-term effect, with a reduction of greater than 90% in circulating angiotensinogen over 6 months after a single subcutaneous dose (800 mg). The peak in reduction of circulating angiotensinogen occurs after approximately 3 weeks.
“It’s extremely powerful,” said Dr. Azizi.
Lasting reductions in blood pressure have also been observed, with 24-hour ambulatory blood pressure monitoring showing a reduction in systolic BP of greater than 15 mm Hg 8 weeks after administration of a single dose of zilebesiran (800 mg).
Zilebesiran was also well tolerated, with only mild to moderate reactions at the site of the injection (n = 5/56) and no serious treatment-related adverse events, hypotension, or significant changes in kidney or liver function.
“In terms of benefits, the effect is ongoing. Zilebesiran leads to reduced medication use and causes less variability in blood pressure response. Nevertheless, interfering RNA acts slowly, meaning that zilebesiran would not be suitable for people presenting with a hypertensive crisis. The fact that it blocks the renin-angiotensin system [RAS] for a very long period of time also poses the question of how to reverse its hypotensive effects,” said Dr. Azizi.
Unanswered questions
The lasting RAS antagonist and blood pressure–lowering effects pose a potential safety problem in circumstances involving patients in a state of hypovolemia and hypotension who require rapid blood pressure–raising interventions to prevent morbidity and mortality.
In recent studies, Estrellita Uijl et al. have thus examined strategies to counteract the blood pressure–lowering effect of siRNA in spontaneously hypertensive rats.
Fludrocortisone and a high-salt diet were both successful in gradually increasing blood pressure, which returned to its baseline levels on days 5 and 7, respectively. Yet this rate of response would be wholly inadequate in an urgent clinical situation.
However, midodrine could not reduce blood pressure to normal levels, whether administered subcutaneously or orally.
A rapid and short-lasting increase in blood pressure was observed with bolus doses of vasopressors, but clinically, these would need to be administered intravenously to achieve a lasting effect. Such administration would require hospitalization, close monitoring, and the use of human resources and additional health care provisions.
Encouragingly, the laboratory that created this molecule, Alnylam Pharmaceuticals, has come up with an antidote: Reversir. It is a GalNAc-conjugated, single-stranded, high-affinity oligonucleotide complementary to the zilebesiran strand that achieves effective reversal of siRNA activity in 24 hours.
In the future, after the phase 2 trials have been completed, whether or not zilebesiran reduces the incidence of cardiovascular events and mortality remains to be seen. But as for Dr. Azizi, the director of HEGP’s blood pressure clinic in Paris, he has no doubt that “this approach is about to shake up how we treat patients in the cardiovascular field.”
On the horizon
Zilebesiran is being studied in phase 2 trials in patients with mild to moderate hypertension not taking antihypertensive drugs (KARDIA-1: 375 patients; double-blind, placebo-controlled, five-arm trial; zilebesiran at 150, 300, and 600 mg twice per year and 300 mg once every 3 months) and in patients whose blood pressure is not controlled (KARDIA-2: 800 patients; initial open-label start-up period of 4 weeks with indapamide/amlodipine/olmesartan, followed by a double-blind, placebo-controlled study over 6 months, then an open-label extension study for up to 12 additional months; zilebesiran at 600 mg on the first day of the initial double-blind period, then every 6 months during the open-label extension period).
This article was translated from the Medscape French edition and a version appeared on Medscape.com.
AT INTERNATIONAL MEETING OF THE FRENCH SOCIETY OF HYPERTENSION
What the FTC’s proposed ban on noncompete agreements could mean for physicians, other clinicians
The proposed rule seeks to ban companies from enforcing noncompete clauses in employment contracts, a practice that represents an “unfair method of competition” with “exploitative and widespread” impacts, including suppression of wages, innovation, and entrepreneurial spirit, the FTC said. The public has 60 days to submit comments on the proposal before the FTC issues the final rule.
Employers often include noncompete clauses in physician contracts because they want to avoid having patients leave their health care system and follow a doctor to a competitor. A 2018 survey of primary care physicians found that about half of office-based physicians and 37% of physicians employed at hospitals or freestanding care centers were bound by restrictive covenants.
“A federal ban on noncompete agreements will ensure that physicians nationwide can finally change jobs without fear of being sued,” Erik B. Smith, MD, JD, clinical assistant professor of anesthesiology at the University of Southern California, Los Angeles, said in an interview.
Many doctors would like to see noncompete agreements vanish, but some physicians still favor them.
“As a small-practice owner, I am personally against this. The noncompete helps me take a risk and hire a physician. It typically takes 2-3 years for me to break even. I think this will further consolidate employment with large hospital systems unfortunately,” Texas cardiologist Rishin Shah, MD, recently tweeted in response to the FTC announcement.
Dr. Smith, who has advocated for noncompete reform, said about half of states currently allow the controversial clauses.
However, several states have recently passed laws restricting their use. California, North Dakota, and Oklahoma ban noncompetes, although some narrowly defined exceptions, such as the sale of a business, remain.
Other states, like Colorado, Illinois, and Oregon, broadly ban noncompete clauses, except for workers earning above a certain threshold. For example, in Colorado, noncompete agreements are permitted for highly compensated employees earning more than $101,250.
Despite additional restrictions on noncompete agreements for workers in the District of Columbia, the new legislation does not apply to physicians earning total compensation of $250,000 or more. However, their employers must define the geographic parameters of the noncompete and limit postemployment restrictions to 2 years.
Restrictive covenants are “uniquely challenging to family medicine’s emphasis on longitudinal care and the patient-physician relationship,” said Tochi Iroku-Malize, MD, MPH, president of the American Academy of Family Physicians. The limitations imposed by noncompete agreements “potentially reduce patient choice, lower the quality of care for patients, and ultimately harm the foundation of family medicine – our relationships with our patients.”
Although the proposed rule aligns with President Biden’s executive order promoting economic competition, Dr. Smith said a national ban on noncompete agreements may push the limits of FTC authority.
“This new rule will certainly result in a ‘major questions doctrine’ Supreme Court challenge,” said Dr. Smith, and possibly be struck down if the court determines an administrative overstep into areas of “vast economic or political significance.”
A controversial policy
The American Medical Association’s code of ethics discourages covenants that “unreasonably restrict” the ability of physicians to practice following contract termination. And in 2022, the AMA cited “overly broad” noncompete language as a red flag young physicians should watch out for during contract negotiations.
But in 2020, the AMA asked the FTC not to use its rulemaking authority to regulate noncompete clauses in physician employment contracts, and instead, relegate enforcement of such agreements to each state. The American Hospital Association expressed similar views.
Still, the FTC said that eliminating noncompete clauses will increase annual wages by $300 billion, allow 30 million Americans to pursue better job opportunities, and encourage hiring competition among employers. It will also save consumers up to $148 billion in health care costs annually.
“Noncompetes block workers from freely switching jobs, depriving them of higher wages and better working conditions, and depriving businesses of a talent pool that they need to build and expand,” Lina M. Khan, FTC chair, said in a press release about the proposal.
A national ban on noncompetes would keep more physicians in the industry and practicing in their communities, a win for patients and providers, said Dr. Smith. It could also compel employers to offer more competitive employment packages, including fair wages, better work conditions, and a culture of well-being and patient safety.
“Whatever the final rule is, I’m certain it will be legally challenged,” said Dr. Smith, adding that the nation’s most prominent business lobbying group, the Chamber of Commerce, has already issued a statement calling the rule “blatantly unlawful."
A version of this article first appeared on Medscape.com.
The proposed rule seeks to ban companies from enforcing noncompete clauses in employment contracts, a practice that represents an “unfair method of competition” with “exploitative and widespread” impacts, including suppression of wages, innovation, and entrepreneurial spirit, the FTC said. The public has 60 days to submit comments on the proposal before the FTC issues the final rule.
Employers often include noncompete clauses in physician contracts because they want to avoid having patients leave their health care system and follow a doctor to a competitor. A 2018 survey of primary care physicians found that about half of office-based physicians and 37% of physicians employed at hospitals or freestanding care centers were bound by restrictive covenants.
“A federal ban on noncompete agreements will ensure that physicians nationwide can finally change jobs without fear of being sued,” Erik B. Smith, MD, JD, clinical assistant professor of anesthesiology at the University of Southern California, Los Angeles, said in an interview.
Many doctors would like to see noncompete agreements vanish, but some physicians still favor them.
“As a small-practice owner, I am personally against this. The noncompete helps me take a risk and hire a physician. It typically takes 2-3 years for me to break even. I think this will further consolidate employment with large hospital systems unfortunately,” Texas cardiologist Rishin Shah, MD, recently tweeted in response to the FTC announcement.
Dr. Smith, who has advocated for noncompete reform, said about half of states currently allow the controversial clauses.
However, several states have recently passed laws restricting their use. California, North Dakota, and Oklahoma ban noncompetes, although some narrowly defined exceptions, such as the sale of a business, remain.
Other states, like Colorado, Illinois, and Oregon, broadly ban noncompete clauses, except for workers earning above a certain threshold. For example, in Colorado, noncompete agreements are permitted for highly compensated employees earning more than $101,250.
Despite additional restrictions on noncompete agreements for workers in the District of Columbia, the new legislation does not apply to physicians earning total compensation of $250,000 or more. However, their employers must define the geographic parameters of the noncompete and limit postemployment restrictions to 2 years.
Restrictive covenants are “uniquely challenging to family medicine’s emphasis on longitudinal care and the patient-physician relationship,” said Tochi Iroku-Malize, MD, MPH, president of the American Academy of Family Physicians. The limitations imposed by noncompete agreements “potentially reduce patient choice, lower the quality of care for patients, and ultimately harm the foundation of family medicine – our relationships with our patients.”
Although the proposed rule aligns with President Biden’s executive order promoting economic competition, Dr. Smith said a national ban on noncompete agreements may push the limits of FTC authority.
“This new rule will certainly result in a ‘major questions doctrine’ Supreme Court challenge,” said Dr. Smith, and possibly be struck down if the court determines an administrative overstep into areas of “vast economic or political significance.”
A controversial policy
The American Medical Association’s code of ethics discourages covenants that “unreasonably restrict” the ability of physicians to practice following contract termination. And in 2022, the AMA cited “overly broad” noncompete language as a red flag young physicians should watch out for during contract negotiations.
But in 2020, the AMA asked the FTC not to use its rulemaking authority to regulate noncompete clauses in physician employment contracts, and instead, relegate enforcement of such agreements to each state. The American Hospital Association expressed similar views.
Still, the FTC said that eliminating noncompete clauses will increase annual wages by $300 billion, allow 30 million Americans to pursue better job opportunities, and encourage hiring competition among employers. It will also save consumers up to $148 billion in health care costs annually.
“Noncompetes block workers from freely switching jobs, depriving them of higher wages and better working conditions, and depriving businesses of a talent pool that they need to build and expand,” Lina M. Khan, FTC chair, said in a press release about the proposal.
A national ban on noncompetes would keep more physicians in the industry and practicing in their communities, a win for patients and providers, said Dr. Smith. It could also compel employers to offer more competitive employment packages, including fair wages, better work conditions, and a culture of well-being and patient safety.
“Whatever the final rule is, I’m certain it will be legally challenged,” said Dr. Smith, adding that the nation’s most prominent business lobbying group, the Chamber of Commerce, has already issued a statement calling the rule “blatantly unlawful."
A version of this article first appeared on Medscape.com.
The proposed rule seeks to ban companies from enforcing noncompete clauses in employment contracts, a practice that represents an “unfair method of competition” with “exploitative and widespread” impacts, including suppression of wages, innovation, and entrepreneurial spirit, the FTC said. The public has 60 days to submit comments on the proposal before the FTC issues the final rule.
Employers often include noncompete clauses in physician contracts because they want to avoid having patients leave their health care system and follow a doctor to a competitor. A 2018 survey of primary care physicians found that about half of office-based physicians and 37% of physicians employed at hospitals or freestanding care centers were bound by restrictive covenants.
“A federal ban on noncompete agreements will ensure that physicians nationwide can finally change jobs without fear of being sued,” Erik B. Smith, MD, JD, clinical assistant professor of anesthesiology at the University of Southern California, Los Angeles, said in an interview.
Many doctors would like to see noncompete agreements vanish, but some physicians still favor them.
“As a small-practice owner, I am personally against this. The noncompete helps me take a risk and hire a physician. It typically takes 2-3 years for me to break even. I think this will further consolidate employment with large hospital systems unfortunately,” Texas cardiologist Rishin Shah, MD, recently tweeted in response to the FTC announcement.
Dr. Smith, who has advocated for noncompete reform, said about half of states currently allow the controversial clauses.
However, several states have recently passed laws restricting their use. California, North Dakota, and Oklahoma ban noncompetes, although some narrowly defined exceptions, such as the sale of a business, remain.
Other states, like Colorado, Illinois, and Oregon, broadly ban noncompete clauses, except for workers earning above a certain threshold. For example, in Colorado, noncompete agreements are permitted for highly compensated employees earning more than $101,250.
Despite additional restrictions on noncompete agreements for workers in the District of Columbia, the new legislation does not apply to physicians earning total compensation of $250,000 or more. However, their employers must define the geographic parameters of the noncompete and limit postemployment restrictions to 2 years.
Restrictive covenants are “uniquely challenging to family medicine’s emphasis on longitudinal care and the patient-physician relationship,” said Tochi Iroku-Malize, MD, MPH, president of the American Academy of Family Physicians. The limitations imposed by noncompete agreements “potentially reduce patient choice, lower the quality of care for patients, and ultimately harm the foundation of family medicine – our relationships with our patients.”
Although the proposed rule aligns with President Biden’s executive order promoting economic competition, Dr. Smith said a national ban on noncompete agreements may push the limits of FTC authority.
“This new rule will certainly result in a ‘major questions doctrine’ Supreme Court challenge,” said Dr. Smith, and possibly be struck down if the court determines an administrative overstep into areas of “vast economic or political significance.”
A controversial policy
The American Medical Association’s code of ethics discourages covenants that “unreasonably restrict” the ability of physicians to practice following contract termination. And in 2022, the AMA cited “overly broad” noncompete language as a red flag young physicians should watch out for during contract negotiations.
But in 2020, the AMA asked the FTC not to use its rulemaking authority to regulate noncompete clauses in physician employment contracts, and instead, relegate enforcement of such agreements to each state. The American Hospital Association expressed similar views.
Still, the FTC said that eliminating noncompete clauses will increase annual wages by $300 billion, allow 30 million Americans to pursue better job opportunities, and encourage hiring competition among employers. It will also save consumers up to $148 billion in health care costs annually.
“Noncompetes block workers from freely switching jobs, depriving them of higher wages and better working conditions, and depriving businesses of a talent pool that they need to build and expand,” Lina M. Khan, FTC chair, said in a press release about the proposal.
A national ban on noncompetes would keep more physicians in the industry and practicing in their communities, a win for patients and providers, said Dr. Smith. It could also compel employers to offer more competitive employment packages, including fair wages, better work conditions, and a culture of well-being and patient safety.
“Whatever the final rule is, I’m certain it will be legally challenged,” said Dr. Smith, adding that the nation’s most prominent business lobbying group, the Chamber of Commerce, has already issued a statement calling the rule “blatantly unlawful."
A version of this article first appeared on Medscape.com.
Components of coffee other than caffeine linked to reduced NAFLD severity
Increased intake of both regular and decaffeinated coffee was significantly associated with a reduced severity of NAFLD in the study, published in Nutrients. The study participants included 156 overweight adults, most of whom had type 2 diabetes.
A confluence of factors including diet and lifestyle changes and increased obesity have contributed to a rise in type 2 diabetes and of NAFLD, Margarida Coelho, of the Center for Neuroscience and Cell Biology at the University of Coimbra (Portugal), and colleagues wrote.
Previous studies support an association between coffee and protection against NAFLD, but the roles of the caffeine and noncaffeine components of coffee have not been examined, corresponding author John Griffith Jones, PhD, also of the Center for Neuroscience and Cell Biology at the University of Coimbra, said in an interview.
“There have been previous studies indicating a link between coffee intake and NAFLD amelioration, but these were entirely based on self-reporting questionnaire data, but the main limitation of this approach is that it does not provide any information on which components of coffee confer the beneficial effects,” Dr. Jones said. “The development of new analytical techniques allowing reliable profiling of coffee metabolites in urine allowed this limitation to be addressed.”
Dr. Jones and associates examined the relationship between consumption of regular and decaffeinated coffee on the fatty liver index (FLI), a validated predictor of NAFLD. They measured coffee intake of 156 overweight adults, 135 of whom had type 2 diabetes. The study population included 76 women and 80 men with a mean age of 59 years and a mean body mass index of 29 kg/m2.
The participants reported coffee intake via questionnaires, and 98 participants (all with type 2 diabetes) also provided urine samples for measurement of caffeine and noncaffeine metabolites (the products of the body breaking down coffee). NAFLD was assessed using the FLI and a scanning measure of fibrosis.
Overall, no associations appeared between self-reported coffee intake and NAFLD measures. However, urine caffeine metabolite levels were significantly higher among individuals with no liver fibrosis, compared with those with fibrosis, and noncaffeine metabolites showed a significant negative association with FLI measures.
In a multiple regression analysis of 89 individuals with type 2 diabetes, both caffeine and noncaffeine metabolites were negatively associated with FLI, which suggests less severe NAFLD, the researchers noted.
Although the mechanism of action remains unclear, the findings suggest that other noncaffeine coffee components such as polyphenols may reduce the risk of fibrosis by reducing oxidative stress on the liver, they said.
Benefits beyond caffeine
“The main surprise of the study was that both caffeine and noncaffeine metabolites had beneficial effects,” Dr. Jones said. “We had anticipated caffeine, based on its well-known effects on inhibiting liver fibrosis, but the effects of other components were less well described.”
Clinicians can encourage their patients with type 2 diabetes who drink coffee to continue to do so within a normal range (up to three to four cups per day) including decaffeinated coffee; however, “they should be strongly encouraged to drink coffee without added fats and sugars, otherwise the protective benefits [against more severe NAFLD] will not be realized,” Dr. Jones said.
Additional research is needed to extend the analysis to include more coffee compounds, especially those truly unique to coffee, since caffeine can be found in many other foods and beverages, Dr. Jones added.
Limitations include 24-hour time frame
The findings were limited by several factors, including the use of 24-hour urine sample, which may not represent an individual’s habitual coffee consumption, the researchers noted. The urine metabolites measured also may be derived from foods and beverages other than coffee. In addition, the assessment of NAFLD was based on serum markers and ultrasound/elastography, which are less precise than liver biopsy and magnetic resonance spectroscopy.
However, the study is the first known to use urine data to examine coffee’s protective effect against NAFLD and suggests that both caffeine and noncaffeine metabolites are associated with less severe disease, they concluded.
Findings intriguing but not ready for prime time
“The bottom line is that we have a major epidemic of NAFLD in the United States,” Victor L. Roberts, MD, professor of internal medicine at the University of Central Florida, Orlando, said in an interview. NAFLD has become the most common cause of chronic liver disease worldwide, and will become one of the leading causes of cirrhosis – surpassing infections as the main driver of end-stage liver disease.
“In this country, the epidemic of obesity compounds the problem, and risks for NAFLD include obesity and type 2 diabetes,” said Dr. Roberts.
The concept of coffee as beneficial is not new, but data suggest that the effects vary with insulin resistance, he said. If liver disease is advanced, coffee and its components may not have much benefit, but early on, it might have a role.
The likely mechanism of action for the benefits of coffee on the reduction in liver fibrosis is through a complex set of metabolic steps that interrupt the promotion of collagen production and reduce liver stiffness, said Dr. Roberts.
The current study authors were up front about the limitations, mainly the use of self-reports, although including the urine collection provided more scientific data, he said. More studies are needed in other populations, but the findings are interesting enough to merit additional research.
The take-home message for primary care, however, is that drinking coffee – regular or decaf – does not replace standard of care, Dr. Roberts emphasized.
“If a patient is a coffee drinker and they have NAFLD or are at risk, they could be encouraged to continue drinking coffee,” in reasonable amounts, said Dr. Roberts. “Anywhere from 1-3 cups a day is unlikely to be a problem, and there is some hope and interest in this area,” but the findings of the current study “should not be taken as gospel or advocacy as a solution for people with NAFLD.”
Instead, clinicians should focus on the standard of care for management of patients at risk for NAFLD, promoting lifestyle changes such as weight loss, diet, and exercise (challenging as that may be), and prescribing appropriate medications, he said.
The study was supported by the Institute for Scientific Information on Coffee, and the researchers received funding from the ISIC to conduct the study. Dr. Roberts had no financial conflicts to disclose, but he serves on the editorial advisory board of Internal Medicine News.
Increased intake of both regular and decaffeinated coffee was significantly associated with a reduced severity of NAFLD in the study, published in Nutrients. The study participants included 156 overweight adults, most of whom had type 2 diabetes.
A confluence of factors including diet and lifestyle changes and increased obesity have contributed to a rise in type 2 diabetes and of NAFLD, Margarida Coelho, of the Center for Neuroscience and Cell Biology at the University of Coimbra (Portugal), and colleagues wrote.
Previous studies support an association between coffee and protection against NAFLD, but the roles of the caffeine and noncaffeine components of coffee have not been examined, corresponding author John Griffith Jones, PhD, also of the Center for Neuroscience and Cell Biology at the University of Coimbra, said in an interview.
“There have been previous studies indicating a link between coffee intake and NAFLD amelioration, but these were entirely based on self-reporting questionnaire data, but the main limitation of this approach is that it does not provide any information on which components of coffee confer the beneficial effects,” Dr. Jones said. “The development of new analytical techniques allowing reliable profiling of coffee metabolites in urine allowed this limitation to be addressed.”
Dr. Jones and associates examined the relationship between consumption of regular and decaffeinated coffee on the fatty liver index (FLI), a validated predictor of NAFLD. They measured coffee intake of 156 overweight adults, 135 of whom had type 2 diabetes. The study population included 76 women and 80 men with a mean age of 59 years and a mean body mass index of 29 kg/m2.
The participants reported coffee intake via questionnaires, and 98 participants (all with type 2 diabetes) also provided urine samples for measurement of caffeine and noncaffeine metabolites (the products of the body breaking down coffee). NAFLD was assessed using the FLI and a scanning measure of fibrosis.
Overall, no associations appeared between self-reported coffee intake and NAFLD measures. However, urine caffeine metabolite levels were significantly higher among individuals with no liver fibrosis, compared with those with fibrosis, and noncaffeine metabolites showed a significant negative association with FLI measures.
In a multiple regression analysis of 89 individuals with type 2 diabetes, both caffeine and noncaffeine metabolites were negatively associated with FLI, which suggests less severe NAFLD, the researchers noted.
Although the mechanism of action remains unclear, the findings suggest that other noncaffeine coffee components such as polyphenols may reduce the risk of fibrosis by reducing oxidative stress on the liver, they said.
Benefits beyond caffeine
“The main surprise of the study was that both caffeine and noncaffeine metabolites had beneficial effects,” Dr. Jones said. “We had anticipated caffeine, based on its well-known effects on inhibiting liver fibrosis, but the effects of other components were less well described.”
Clinicians can encourage their patients with type 2 diabetes who drink coffee to continue to do so within a normal range (up to three to four cups per day) including decaffeinated coffee; however, “they should be strongly encouraged to drink coffee without added fats and sugars, otherwise the protective benefits [against more severe NAFLD] will not be realized,” Dr. Jones said.
Additional research is needed to extend the analysis to include more coffee compounds, especially those truly unique to coffee, since caffeine can be found in many other foods and beverages, Dr. Jones added.
Limitations include 24-hour time frame
The findings were limited by several factors, including the use of 24-hour urine sample, which may not represent an individual’s habitual coffee consumption, the researchers noted. The urine metabolites measured also may be derived from foods and beverages other than coffee. In addition, the assessment of NAFLD was based on serum markers and ultrasound/elastography, which are less precise than liver biopsy and magnetic resonance spectroscopy.
However, the study is the first known to use urine data to examine coffee’s protective effect against NAFLD and suggests that both caffeine and noncaffeine metabolites are associated with less severe disease, they concluded.
Findings intriguing but not ready for prime time
“The bottom line is that we have a major epidemic of NAFLD in the United States,” Victor L. Roberts, MD, professor of internal medicine at the University of Central Florida, Orlando, said in an interview. NAFLD has become the most common cause of chronic liver disease worldwide, and will become one of the leading causes of cirrhosis – surpassing infections as the main driver of end-stage liver disease.
“In this country, the epidemic of obesity compounds the problem, and risks for NAFLD include obesity and type 2 diabetes,” said Dr. Roberts.
The concept of coffee as beneficial is not new, but data suggest that the effects vary with insulin resistance, he said. If liver disease is advanced, coffee and its components may not have much benefit, but early on, it might have a role.
The likely mechanism of action for the benefits of coffee on the reduction in liver fibrosis is through a complex set of metabolic steps that interrupt the promotion of collagen production and reduce liver stiffness, said Dr. Roberts.
The current study authors were up front about the limitations, mainly the use of self-reports, although including the urine collection provided more scientific data, he said. More studies are needed in other populations, but the findings are interesting enough to merit additional research.
The take-home message for primary care, however, is that drinking coffee – regular or decaf – does not replace standard of care, Dr. Roberts emphasized.
“If a patient is a coffee drinker and they have NAFLD or are at risk, they could be encouraged to continue drinking coffee,” in reasonable amounts, said Dr. Roberts. “Anywhere from 1-3 cups a day is unlikely to be a problem, and there is some hope and interest in this area,” but the findings of the current study “should not be taken as gospel or advocacy as a solution for people with NAFLD.”
Instead, clinicians should focus on the standard of care for management of patients at risk for NAFLD, promoting lifestyle changes such as weight loss, diet, and exercise (challenging as that may be), and prescribing appropriate medications, he said.
The study was supported by the Institute for Scientific Information on Coffee, and the researchers received funding from the ISIC to conduct the study. Dr. Roberts had no financial conflicts to disclose, but he serves on the editorial advisory board of Internal Medicine News.
Increased intake of both regular and decaffeinated coffee was significantly associated with a reduced severity of NAFLD in the study, published in Nutrients. The study participants included 156 overweight adults, most of whom had type 2 diabetes.
A confluence of factors including diet and lifestyle changes and increased obesity have contributed to a rise in type 2 diabetes and of NAFLD, Margarida Coelho, of the Center for Neuroscience and Cell Biology at the University of Coimbra (Portugal), and colleagues wrote.
Previous studies support an association between coffee and protection against NAFLD, but the roles of the caffeine and noncaffeine components of coffee have not been examined, corresponding author John Griffith Jones, PhD, also of the Center for Neuroscience and Cell Biology at the University of Coimbra, said in an interview.
“There have been previous studies indicating a link between coffee intake and NAFLD amelioration, but these were entirely based on self-reporting questionnaire data, but the main limitation of this approach is that it does not provide any information on which components of coffee confer the beneficial effects,” Dr. Jones said. “The development of new analytical techniques allowing reliable profiling of coffee metabolites in urine allowed this limitation to be addressed.”
Dr. Jones and associates examined the relationship between consumption of regular and decaffeinated coffee on the fatty liver index (FLI), a validated predictor of NAFLD. They measured coffee intake of 156 overweight adults, 135 of whom had type 2 diabetes. The study population included 76 women and 80 men with a mean age of 59 years and a mean body mass index of 29 kg/m2.
The participants reported coffee intake via questionnaires, and 98 participants (all with type 2 diabetes) also provided urine samples for measurement of caffeine and noncaffeine metabolites (the products of the body breaking down coffee). NAFLD was assessed using the FLI and a scanning measure of fibrosis.
Overall, no associations appeared between self-reported coffee intake and NAFLD measures. However, urine caffeine metabolite levels were significantly higher among individuals with no liver fibrosis, compared with those with fibrosis, and noncaffeine metabolites showed a significant negative association with FLI measures.
In a multiple regression analysis of 89 individuals with type 2 diabetes, both caffeine and noncaffeine metabolites were negatively associated with FLI, which suggests less severe NAFLD, the researchers noted.
Although the mechanism of action remains unclear, the findings suggest that other noncaffeine coffee components such as polyphenols may reduce the risk of fibrosis by reducing oxidative stress on the liver, they said.
Benefits beyond caffeine
“The main surprise of the study was that both caffeine and noncaffeine metabolites had beneficial effects,” Dr. Jones said. “We had anticipated caffeine, based on its well-known effects on inhibiting liver fibrosis, but the effects of other components were less well described.”
Clinicians can encourage their patients with type 2 diabetes who drink coffee to continue to do so within a normal range (up to three to four cups per day) including decaffeinated coffee; however, “they should be strongly encouraged to drink coffee without added fats and sugars, otherwise the protective benefits [against more severe NAFLD] will not be realized,” Dr. Jones said.
Additional research is needed to extend the analysis to include more coffee compounds, especially those truly unique to coffee, since caffeine can be found in many other foods and beverages, Dr. Jones added.
Limitations include 24-hour time frame
The findings were limited by several factors, including the use of 24-hour urine sample, which may not represent an individual’s habitual coffee consumption, the researchers noted. The urine metabolites measured also may be derived from foods and beverages other than coffee. In addition, the assessment of NAFLD was based on serum markers and ultrasound/elastography, which are less precise than liver biopsy and magnetic resonance spectroscopy.
However, the study is the first known to use urine data to examine coffee’s protective effect against NAFLD and suggests that both caffeine and noncaffeine metabolites are associated with less severe disease, they concluded.
Findings intriguing but not ready for prime time
“The bottom line is that we have a major epidemic of NAFLD in the United States,” Victor L. Roberts, MD, professor of internal medicine at the University of Central Florida, Orlando, said in an interview. NAFLD has become the most common cause of chronic liver disease worldwide, and will become one of the leading causes of cirrhosis – surpassing infections as the main driver of end-stage liver disease.
“In this country, the epidemic of obesity compounds the problem, and risks for NAFLD include obesity and type 2 diabetes,” said Dr. Roberts.
The concept of coffee as beneficial is not new, but data suggest that the effects vary with insulin resistance, he said. If liver disease is advanced, coffee and its components may not have much benefit, but early on, it might have a role.
The likely mechanism of action for the benefits of coffee on the reduction in liver fibrosis is through a complex set of metabolic steps that interrupt the promotion of collagen production and reduce liver stiffness, said Dr. Roberts.
The current study authors were up front about the limitations, mainly the use of self-reports, although including the urine collection provided more scientific data, he said. More studies are needed in other populations, but the findings are interesting enough to merit additional research.
The take-home message for primary care, however, is that drinking coffee – regular or decaf – does not replace standard of care, Dr. Roberts emphasized.
“If a patient is a coffee drinker and they have NAFLD or are at risk, they could be encouraged to continue drinking coffee,” in reasonable amounts, said Dr. Roberts. “Anywhere from 1-3 cups a day is unlikely to be a problem, and there is some hope and interest in this area,” but the findings of the current study “should not be taken as gospel or advocacy as a solution for people with NAFLD.”
Instead, clinicians should focus on the standard of care for management of patients at risk for NAFLD, promoting lifestyle changes such as weight loss, diet, and exercise (challenging as that may be), and prescribing appropriate medications, he said.
The study was supported by the Institute for Scientific Information on Coffee, and the researchers received funding from the ISIC to conduct the study. Dr. Roberts had no financial conflicts to disclose, but he serves on the editorial advisory board of Internal Medicine News.
FROM NUTRIENTS
Diagnosing rare disorders
When I was a resident (back in the Cretaceous era), the idea of autoimmune encephalitis was just beginning to take hold. It was kind of like Bigfoot. A few reports, vague articles, the occasional sighting of what may or may not be a case. …
Unlike Bigfoot, however, the evidence quickly added up until there was no question that such a disorder existed. Then disorder became disorders, and now it seems a few more types are added to the list each year.
This doesn’t change the fact that they’re still, in the grand scheme of general neurology, relatively rare, though no one questions that they exist.
Today people still wishfully take pictures of Bigfoot, but they turn out to be images of bears or other animals, or tricks of light and shadow.
This is an issue with human thought. Many times we see what we want to see, especially if it’s more interesting than a mundane alternative.
An autoimmune encephalitis article in the January 2023 issue of JAMA Neurology looked into this. On reviewing 393 patients diagnosed with the disorder, the researchers found that 27% of them actually didn’t have it at all. Such things as functional disorders, neurodegenerative diseases, and primary psychiatric diagnoses were, instead, the culprits.
I’m not criticizing those who made an incorrect diagnosis. We all do. That’s the nature of medicine.
Which is worse? Missing the diagnosis entirely and not treating, or diagnosing a patient with something else and treating incorrectly? I guess it depends on the disease and nature of treatment.
Certainly, finding a case of autoimmune encephalitis is more interesting than, say toxic-metabolic encephalopathy from a bladder infection, just as getting a picture of Bigfoot is way more cool than one of a bear with mange.
But we need to be careful when faced with equivocal labs and data lest we read too much into them. There are too many gray zones in medicine to lead you astray. Not to say we won’t be.
But it’s not just rare diseases. In the early 1990s two different studies found that 24% of patients diagnosed with Parkinson’s disease were found to have something else on autopsy.
That was 30 years ago. Now we have DaT scans to help. Maybe our abilities as neurologists have also gotten better (though I don’t think the neurological exam has changed much since Charcot).
Our gadgets, labs, and treatments get better every year. We have tools available to us now that were unthinkable a generation ago. For that matter, they were unthinkable when I began my career.
But they don’t change the fact that human error never goes away. All of us are susceptible to it, and all of us make mistakes.
Such is the way of medicine now, and likely always. All we can do is our best and keep moving forward.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
When I was a resident (back in the Cretaceous era), the idea of autoimmune encephalitis was just beginning to take hold. It was kind of like Bigfoot. A few reports, vague articles, the occasional sighting of what may or may not be a case. …
Unlike Bigfoot, however, the evidence quickly added up until there was no question that such a disorder existed. Then disorder became disorders, and now it seems a few more types are added to the list each year.
This doesn’t change the fact that they’re still, in the grand scheme of general neurology, relatively rare, though no one questions that they exist.
Today people still wishfully take pictures of Bigfoot, but they turn out to be images of bears or other animals, or tricks of light and shadow.
This is an issue with human thought. Many times we see what we want to see, especially if it’s more interesting than a mundane alternative.
An autoimmune encephalitis article in the January 2023 issue of JAMA Neurology looked into this. On reviewing 393 patients diagnosed with the disorder, the researchers found that 27% of them actually didn’t have it at all. Such things as functional disorders, neurodegenerative diseases, and primary psychiatric diagnoses were, instead, the culprits.
I’m not criticizing those who made an incorrect diagnosis. We all do. That’s the nature of medicine.
Which is worse? Missing the diagnosis entirely and not treating, or diagnosing a patient with something else and treating incorrectly? I guess it depends on the disease and nature of treatment.
Certainly, finding a case of autoimmune encephalitis is more interesting than, say toxic-metabolic encephalopathy from a bladder infection, just as getting a picture of Bigfoot is way more cool than one of a bear with mange.
But we need to be careful when faced with equivocal labs and data lest we read too much into them. There are too many gray zones in medicine to lead you astray. Not to say we won’t be.
But it’s not just rare diseases. In the early 1990s two different studies found that 24% of patients diagnosed with Parkinson’s disease were found to have something else on autopsy.
That was 30 years ago. Now we have DaT scans to help. Maybe our abilities as neurologists have also gotten better (though I don’t think the neurological exam has changed much since Charcot).
Our gadgets, labs, and treatments get better every year. We have tools available to us now that were unthinkable a generation ago. For that matter, they were unthinkable when I began my career.
But they don’t change the fact that human error never goes away. All of us are susceptible to it, and all of us make mistakes.
Such is the way of medicine now, and likely always. All we can do is our best and keep moving forward.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
When I was a resident (back in the Cretaceous era), the idea of autoimmune encephalitis was just beginning to take hold. It was kind of like Bigfoot. A few reports, vague articles, the occasional sighting of what may or may not be a case. …
Unlike Bigfoot, however, the evidence quickly added up until there was no question that such a disorder existed. Then disorder became disorders, and now it seems a few more types are added to the list each year.
This doesn’t change the fact that they’re still, in the grand scheme of general neurology, relatively rare, though no one questions that they exist.
Today people still wishfully take pictures of Bigfoot, but they turn out to be images of bears or other animals, or tricks of light and shadow.
This is an issue with human thought. Many times we see what we want to see, especially if it’s more interesting than a mundane alternative.
An autoimmune encephalitis article in the January 2023 issue of JAMA Neurology looked into this. On reviewing 393 patients diagnosed with the disorder, the researchers found that 27% of them actually didn’t have it at all. Such things as functional disorders, neurodegenerative diseases, and primary psychiatric diagnoses were, instead, the culprits.
I’m not criticizing those who made an incorrect diagnosis. We all do. That’s the nature of medicine.
Which is worse? Missing the diagnosis entirely and not treating, or diagnosing a patient with something else and treating incorrectly? I guess it depends on the disease and nature of treatment.
Certainly, finding a case of autoimmune encephalitis is more interesting than, say toxic-metabolic encephalopathy from a bladder infection, just as getting a picture of Bigfoot is way more cool than one of a bear with mange.
But we need to be careful when faced with equivocal labs and data lest we read too much into them. There are too many gray zones in medicine to lead you astray. Not to say we won’t be.
But it’s not just rare diseases. In the early 1990s two different studies found that 24% of patients diagnosed with Parkinson’s disease were found to have something else on autopsy.
That was 30 years ago. Now we have DaT scans to help. Maybe our abilities as neurologists have also gotten better (though I don’t think the neurological exam has changed much since Charcot).
Our gadgets, labs, and treatments get better every year. We have tools available to us now that were unthinkable a generation ago. For that matter, they were unthinkable when I began my career.
But they don’t change the fact that human error never goes away. All of us are susceptible to it, and all of us make mistakes.
Such is the way of medicine now, and likely always. All we can do is our best and keep moving forward.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
How Well Does the Third Dose of COVID-19 Vaccine Work?
How effective are the COVID-19 vaccines, third time around? Researchers compared 2 large groups of veterans to find out how well a third dose protected against documented infection, symptomatic COVID-19, and COVID-19–related hospitalization, intensive care unit (ICU) admission, and death.
The research, published in Nature, used electronic health records of 65,196 veterans who received BNT162b2 (Pfizer-BioNTech) and 65,196 who received mRNA-1273 (Moderna). They chose to study the 16 weeks between October 20, 2021 and February 8, 2022, which included both Delta- and Omicron-variant waves.
During the follow-up (median, 77 days), 2994 COVID-19 infections were documented, of which 200 were detected as symptomatic, 194 required hospitalization, and 52 required ICU admission. Twenty-two patients died.
In a previous head-to-head trial comparing breakthrough COVID-19 outcomes after the first doses of the 2 vaccines (given when the Alpha and Delta variants were predominant), the researchers had found a low risk of documented infection and severe outcomes, but lower for the Moderna vaccine. They note that few head-to-head comparisons have been made of third-dose effectiveness.
As expected, in this trial, the researchers found a “nearly identical” pattern for the risk of the 2 vaccine groups. Although the risks for all of the measured outcomes over 16 weeks were low for both vaccines ≤ 4% for documented infection and < 0.03% for death in each group—those veterans who received the Pfizer-BioNTech vaccine had an excess of 45 documented infections and 11 hospitalizations per 10,000 persons, compared with the Moderna group. The Pfizer-BioNTech group also had a higher risk of documented infection over 9 weeks of follow-up, during which an Omicron-variant predominated.
Given the high effectiveness of a third dose of both vaccines, either vaccine is strongly recommended, the researchers conclude. They point to “evidence of clear and comparable benefits” for the most severe outcomes: The difference in estimated 16-week risk of death between the 2 groups was two-thousandths of 1 %.
They add that, while the differences in estimated risk for less severe outcomes between the 2 groups were small on the absolute scale, they may be meaningful when considering the population scale at which these vaccines are deployed.
How effective are the COVID-19 vaccines, third time around? Researchers compared 2 large groups of veterans to find out how well a third dose protected against documented infection, symptomatic COVID-19, and COVID-19–related hospitalization, intensive care unit (ICU) admission, and death.
The research, published in Nature, used electronic health records of 65,196 veterans who received BNT162b2 (Pfizer-BioNTech) and 65,196 who received mRNA-1273 (Moderna). They chose to study the 16 weeks between October 20, 2021 and February 8, 2022, which included both Delta- and Omicron-variant waves.
During the follow-up (median, 77 days), 2994 COVID-19 infections were documented, of which 200 were detected as symptomatic, 194 required hospitalization, and 52 required ICU admission. Twenty-two patients died.
In a previous head-to-head trial comparing breakthrough COVID-19 outcomes after the first doses of the 2 vaccines (given when the Alpha and Delta variants were predominant), the researchers had found a low risk of documented infection and severe outcomes, but lower for the Moderna vaccine. They note that few head-to-head comparisons have been made of third-dose effectiveness.
As expected, in this trial, the researchers found a “nearly identical” pattern for the risk of the 2 vaccine groups. Although the risks for all of the measured outcomes over 16 weeks were low for both vaccines ≤ 4% for documented infection and < 0.03% for death in each group—those veterans who received the Pfizer-BioNTech vaccine had an excess of 45 documented infections and 11 hospitalizations per 10,000 persons, compared with the Moderna group. The Pfizer-BioNTech group also had a higher risk of documented infection over 9 weeks of follow-up, during which an Omicron-variant predominated.
Given the high effectiveness of a third dose of both vaccines, either vaccine is strongly recommended, the researchers conclude. They point to “evidence of clear and comparable benefits” for the most severe outcomes: The difference in estimated 16-week risk of death between the 2 groups was two-thousandths of 1 %.
They add that, while the differences in estimated risk for less severe outcomes between the 2 groups were small on the absolute scale, they may be meaningful when considering the population scale at which these vaccines are deployed.
How effective are the COVID-19 vaccines, third time around? Researchers compared 2 large groups of veterans to find out how well a third dose protected against documented infection, symptomatic COVID-19, and COVID-19–related hospitalization, intensive care unit (ICU) admission, and death.
The research, published in Nature, used electronic health records of 65,196 veterans who received BNT162b2 (Pfizer-BioNTech) and 65,196 who received mRNA-1273 (Moderna). They chose to study the 16 weeks between October 20, 2021 and February 8, 2022, which included both Delta- and Omicron-variant waves.
During the follow-up (median, 77 days), 2994 COVID-19 infections were documented, of which 200 were detected as symptomatic, 194 required hospitalization, and 52 required ICU admission. Twenty-two patients died.
In a previous head-to-head trial comparing breakthrough COVID-19 outcomes after the first doses of the 2 vaccines (given when the Alpha and Delta variants were predominant), the researchers had found a low risk of documented infection and severe outcomes, but lower for the Moderna vaccine. They note that few head-to-head comparisons have been made of third-dose effectiveness.
As expected, in this trial, the researchers found a “nearly identical” pattern for the risk of the 2 vaccine groups. Although the risks for all of the measured outcomes over 16 weeks were low for both vaccines ≤ 4% for documented infection and < 0.03% for death in each group—those veterans who received the Pfizer-BioNTech vaccine had an excess of 45 documented infections and 11 hospitalizations per 10,000 persons, compared with the Moderna group. The Pfizer-BioNTech group also had a higher risk of documented infection over 9 weeks of follow-up, during which an Omicron-variant predominated.
Given the high effectiveness of a third dose of both vaccines, either vaccine is strongly recommended, the researchers conclude. They point to “evidence of clear and comparable benefits” for the most severe outcomes: The difference in estimated 16-week risk of death between the 2 groups was two-thousandths of 1 %.
They add that, while the differences in estimated risk for less severe outcomes between the 2 groups were small on the absolute scale, they may be meaningful when considering the population scale at which these vaccines are deployed.