User login
Characteristics of Matched vs Nonmatched Dermatology Applicants
Dermatology residency continues to be one of the most competitive specialties, with a match rate of 84.7% for US allopathic seniors in the 2019-2020 academic year.1 In the 2019-2020 cycle, dermatology applicants were tied with plastic surgery for the highest median US Medical Licensing Examination (USMLE) Step 1 score compared with other specialties, which suggests that the top medical students are applying, yet only approximately 5 of 6 students are matching.
Factors that have been cited with successful dermatology matching include USMLE Step 1 and Step 2 Clinical Knowledge (CK) scores,2 research accomplishments,3 letters of recommendation,4 medical school performance, personal statement, grades in required clerkships, and volunteer/extracurricular experiences, among others.5
The National Resident Matching Program (NRMP) publishes data each year regarding different academic factors—USMLE scores; number of abstracts, presentations, and papers; work, volunteer, and research experiences—and compares the mean between matched and nonmatched applicants.1 However, the USMLE does not report any demographic information of the applicants and the implication it has for matching. Additionally, the number of couples participating in the couples match continues to increase each year. In the 2019-2020 cycle, 1224 couples participated in the couples match.1 However, NRMP reports only limited data regarding the couples match, and it is not specialty specific.
We aimed to determine the characteristics of matched vs nonmatched dermatology applicants. Secondarily, we aimed to determine any differences among demographics regarding matching rates, academic performance, and research publications. We also aimed to characterize the strategy and outcomes of applicants that couples matched.
Materials and Methods
The Mayo Clinic institutional review board deemed this study exempt. All applicants who applied to Mayo Clinic dermatology residency in Scottsdale, Arizona, during the 2018-2019 cycle were emailed an initial survey (N=475) before Match Day that obtained demographic information, geographic information, gap-year information, USMLE Step 1 score, publications, medical school grades, number of away rotations, and number of interviews. A follow-up survey gathering match data and couples matching data was sent to the applicants who completed the first survey on Match Day. The survey was repeated for the 2019-2020 cycle. In the second survey, Step 2 CK data were obtained. The survey was sent to 629 applicants who applied to Mayo Clinic dermatology residencies in Arizona, Minnesota, and Florida to include a broader group of applicants. For publications, applicants were asked to count only published or accepted manuscripts, not abstracts, posters, conference presentations, or submitted manuscripts. Applicants who did not respond to the second survey (match data) were not included in that part of the analysis. One survey was excluded because of implausible answers (eg, scores outside of range for USMLE Step scores).
Statistical Analysis—For statistical analyses, the applicants from both applications cycles were combined. Descriptive statistics were reported in the form of mean, median, or counts (percentages), as applicable. Means were compared using 2-sided t tests. Group comparisons were examined using χ2 tests for categorical variables. Statistical analyses were performed using the BlueSky Statistics version 6.30. P<.05 was considered significant.
Results
In 2019, a total of 149 applicants completed the initial survey (31.4% response rate), and 112 completed the follow-up survey (75.2% response rate). In 2020, a total of 142 applicants completed the initial survey (22.6% response rate), and 124 completed the follow-up survey (87.3% response rate). Combining the 2 years, after removing 1 survey with implausible answers, there were 290 respondents from the initial survey and 235 from the follow-up survey. The median (SD) age for the total applicants over both years was 27 (3.0) years, and 180 applicants were female (61.9%).
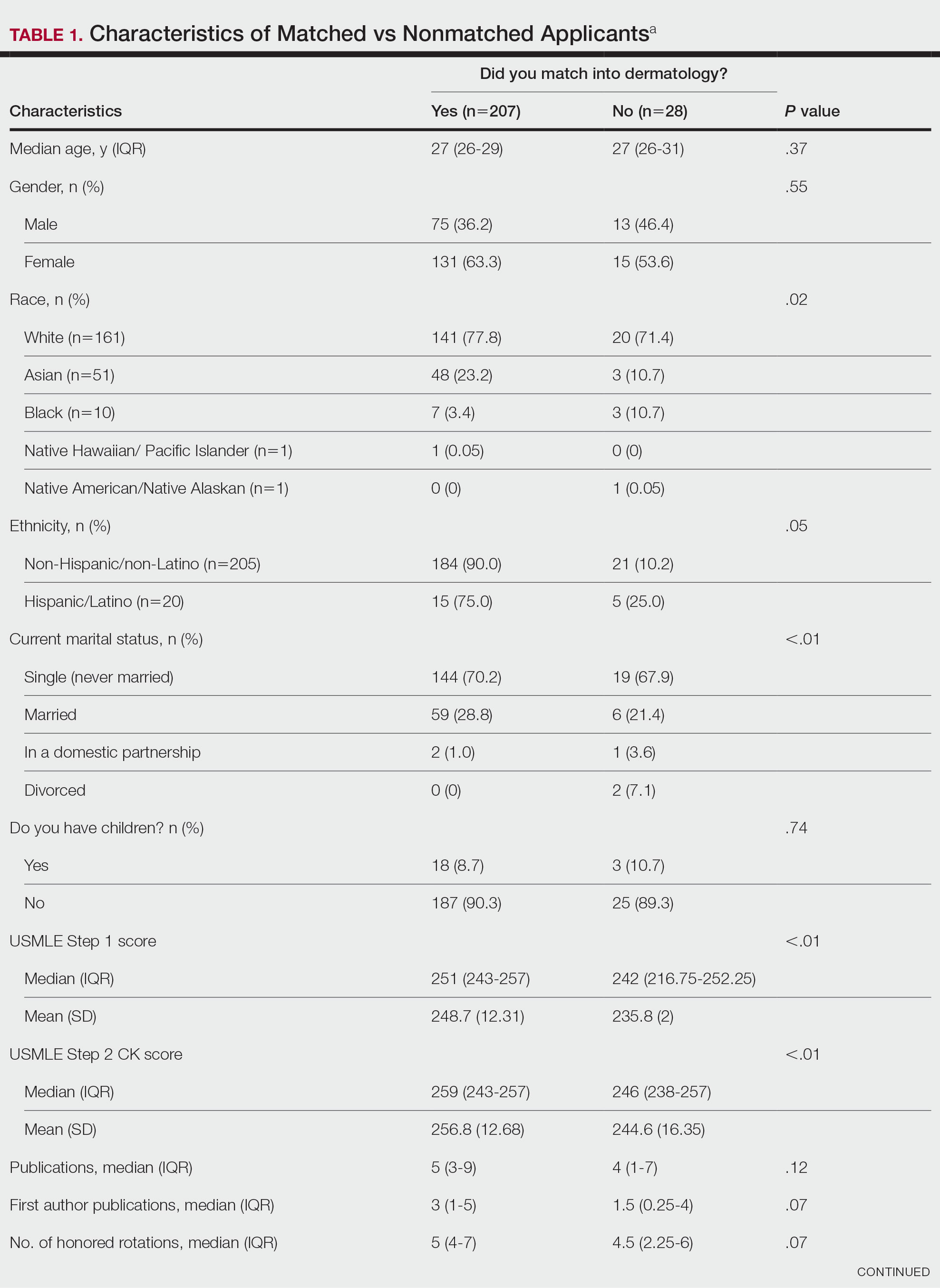
USMLE Scores—The median USMLE Step 1 score was 250, and scores ranged from 196 to 271. The median USMLE Step 2 CK score was 257, and scores ranged from 213 to 281. Higher USMLE Step 1 and Step 2 CK scores and more interviews were associated with higher match rates (Table 1). In addition, students with a dermatology program at their medical school were more likely to match than those without a home dermatology program.
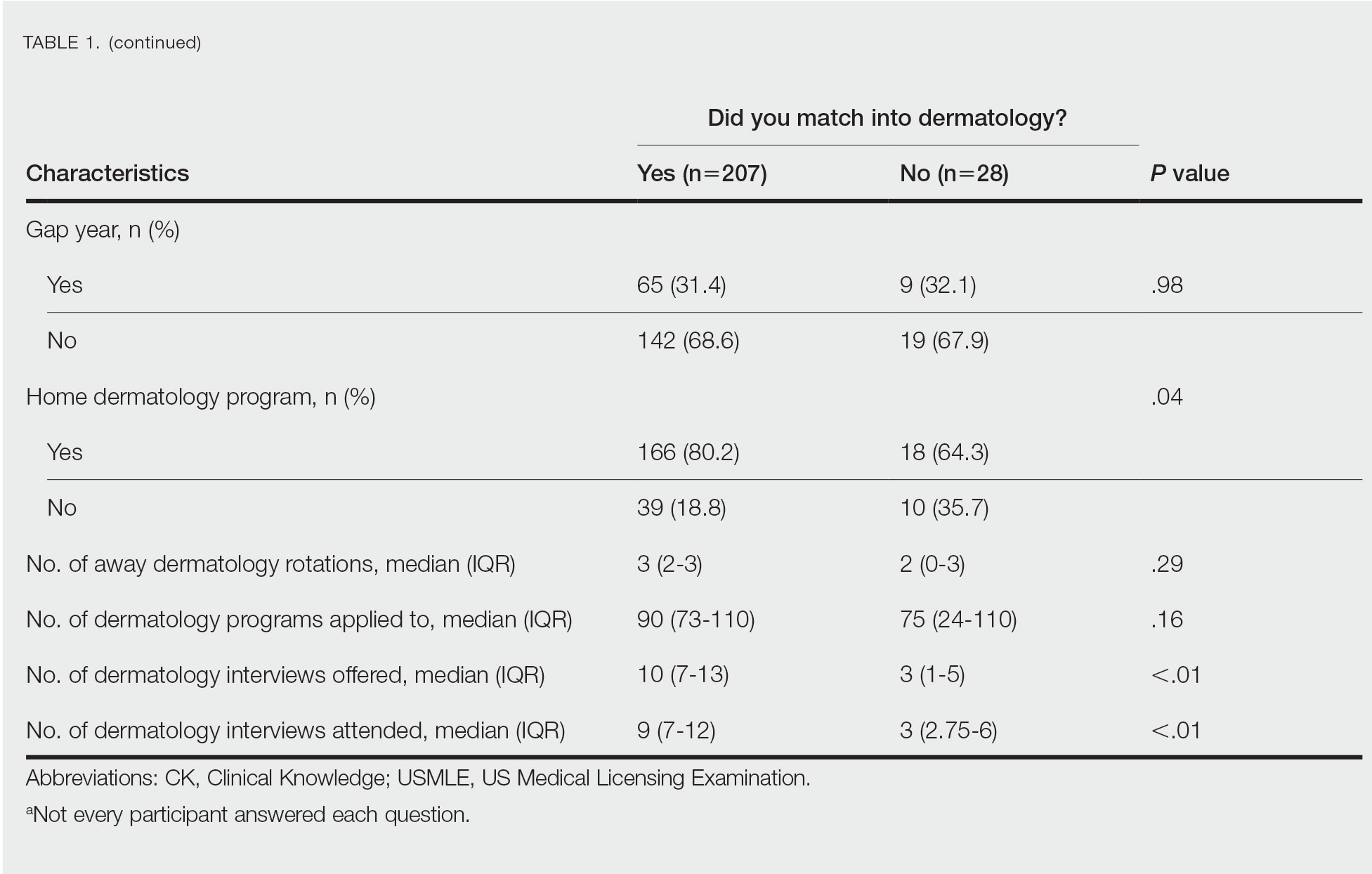
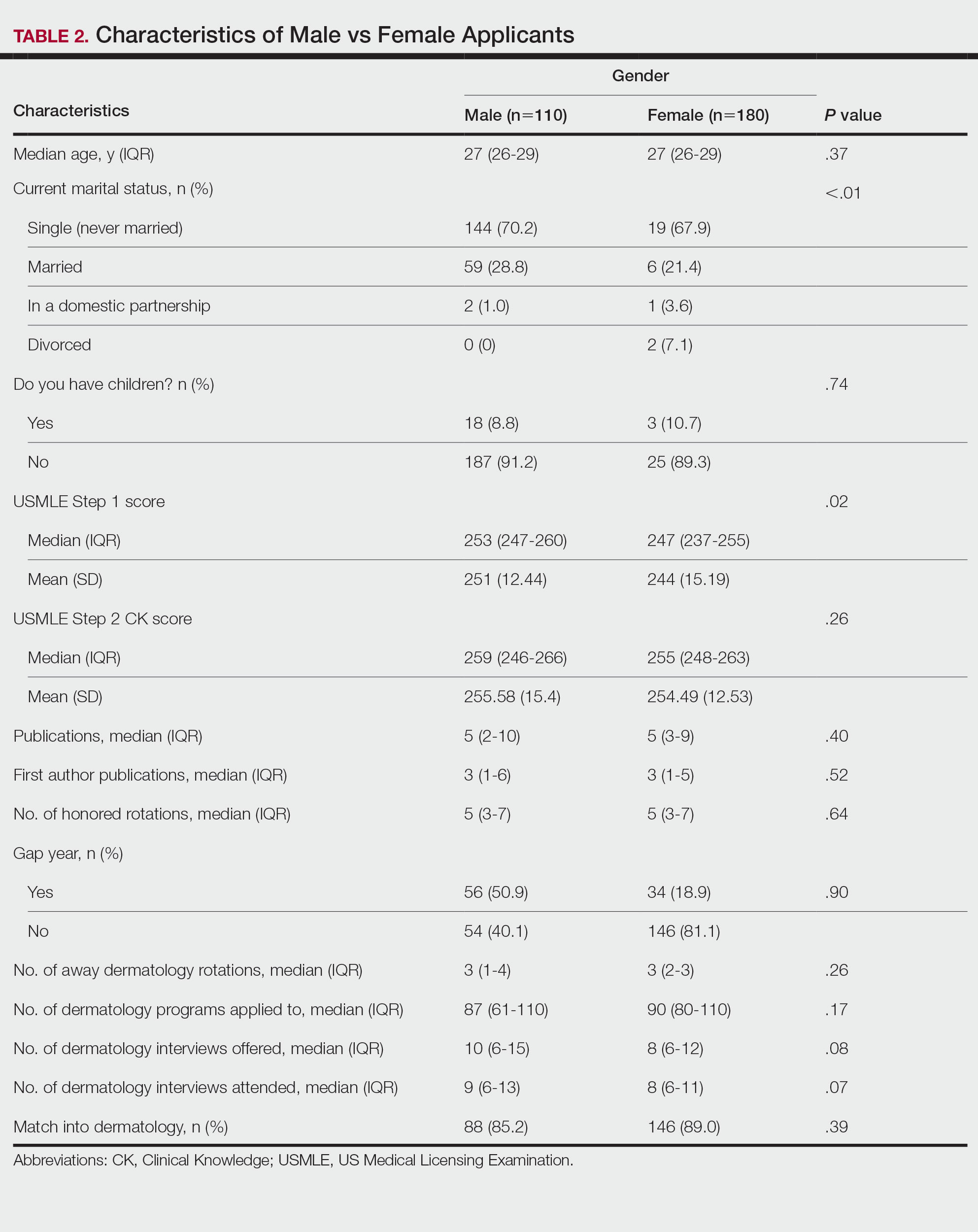
Gender Differences—There were 180 females and 110 males who completed the surveys. Males and females had similar match rates (85.2% vs 89.0%; P=.39)(Table 2).
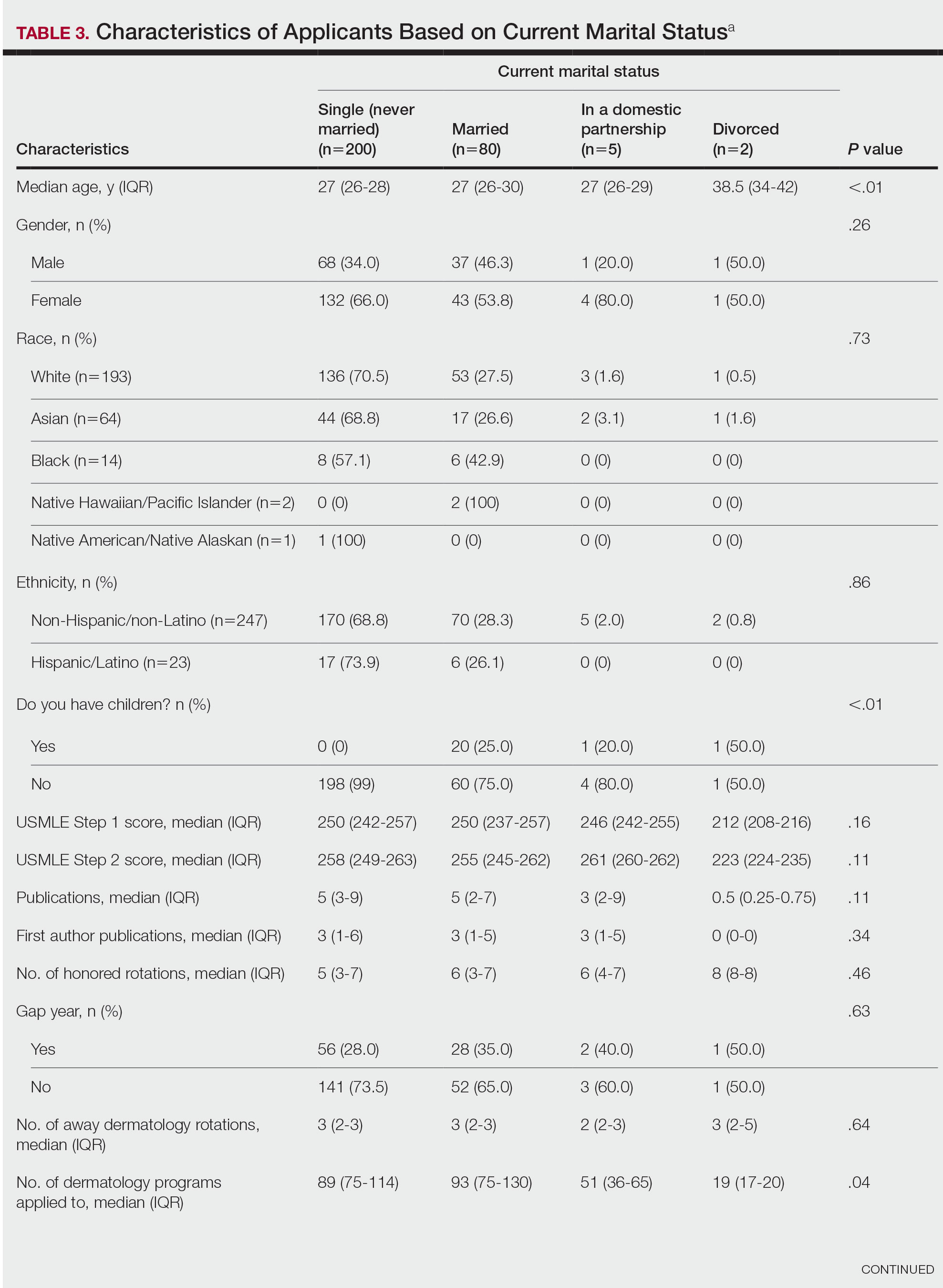
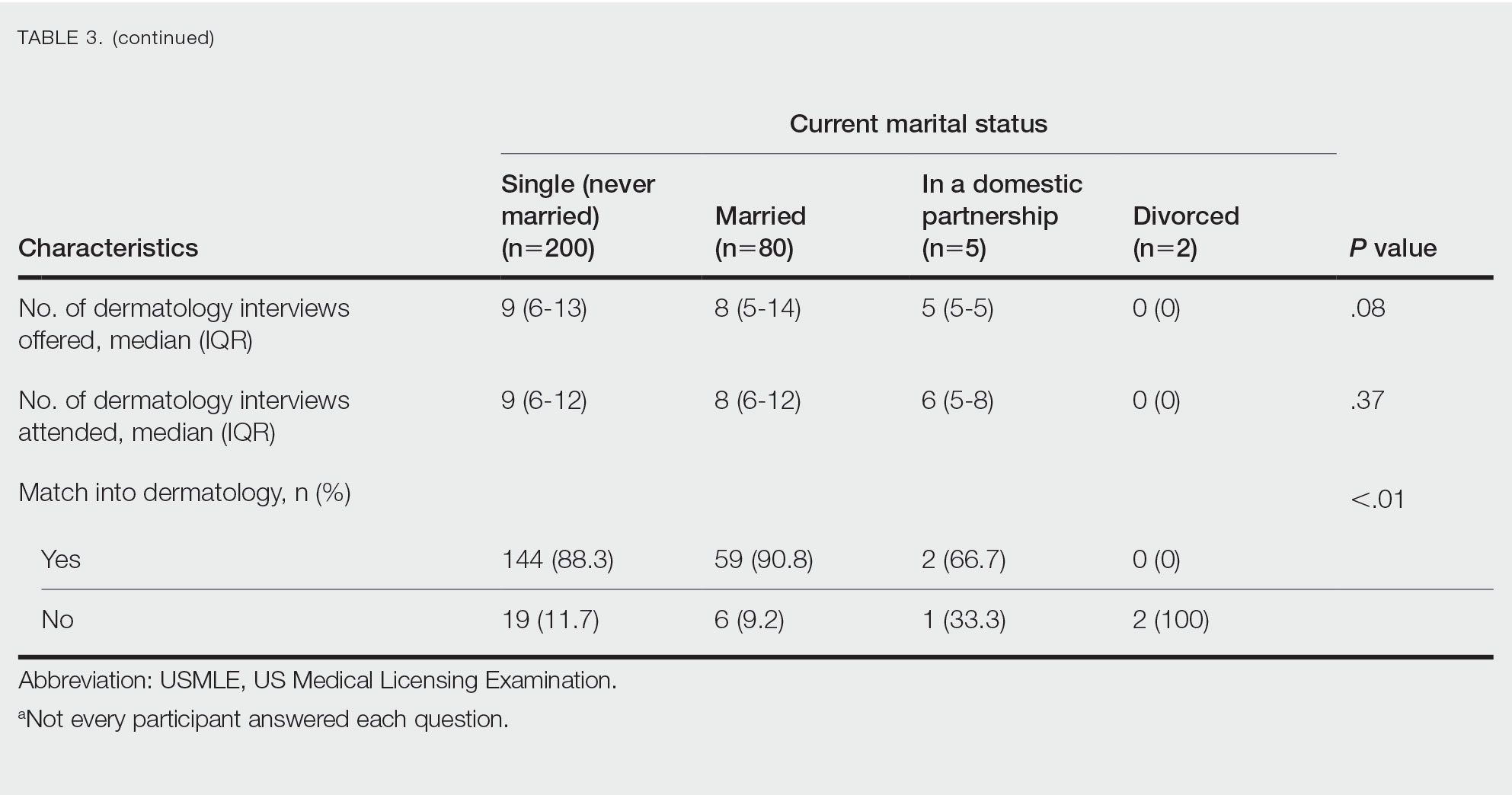
Family Life—In comparing marital status, applicants who were divorced had a higher median age (38.5 years) compared with applicants who were single, married, or in a domestic partnership (all 27 years; P<.01). Differences are outlined in Table 3.
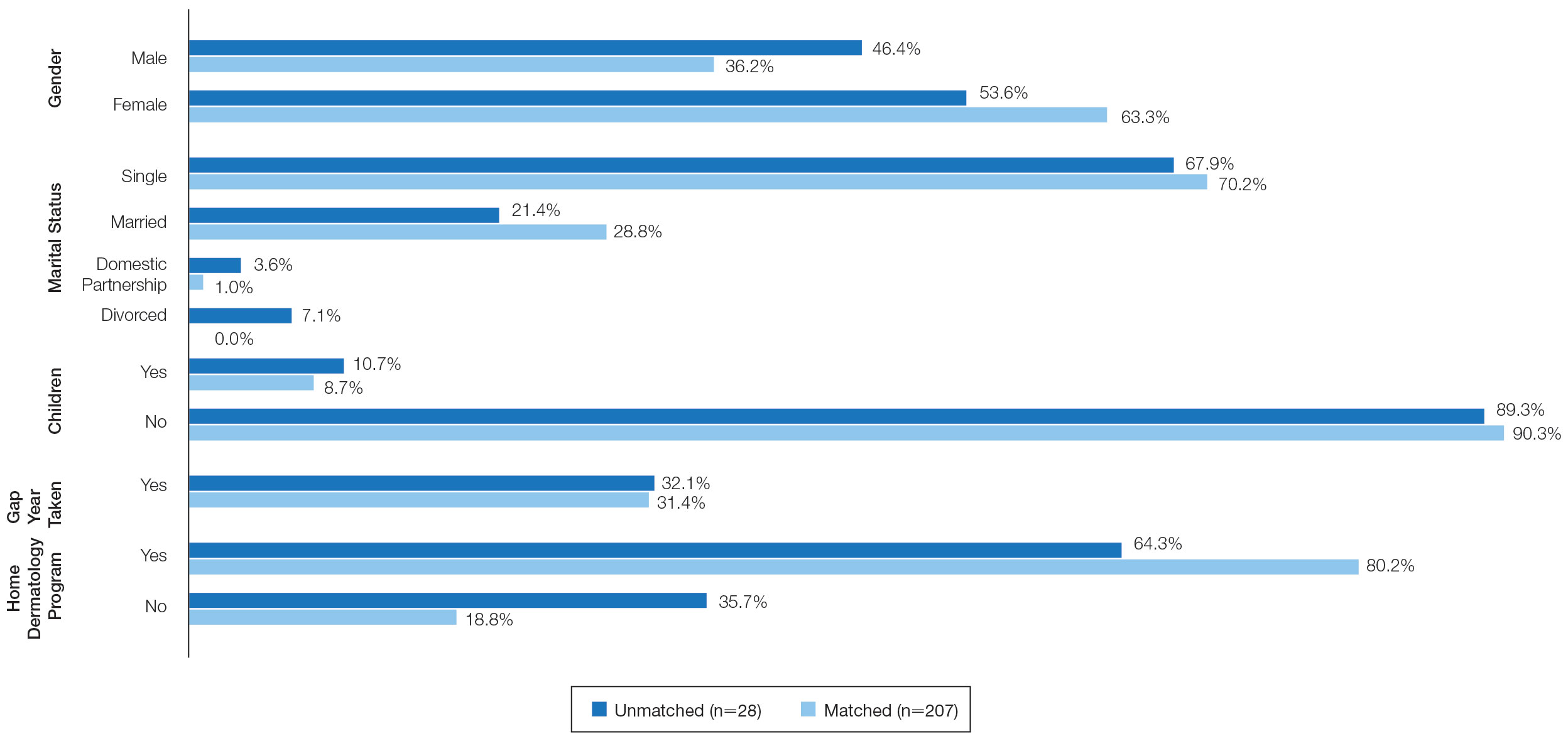
On average, applicants with children (n=27 [15 male, 12 female]; P=.13) were 3 years older than those without (30.5 vs 27; P<.01) and were more likely to be married (88.9% vs 21.5%; P<.01). Applicants with children had a mean USMLE Step 1 score of 241 compared to 251 for those without children (P=.02) and a mean USMLE Step 2 CK score of 246 compared to 258 for those without children (P<.01). Applicants with children had similar debt, number of publications, number of honored rotations, and match rates compared to applicants without children (Figure).
Couples Match—Seventeen individuals in our survey participated in the couples match (7.8%), and all 17 (100%) matched into dermatology. The mean age was 26.7 years, 12 applicants were female, 2 applicants were married, and 1 applicant had children. The mean number of interviews offered was 13.6, and the mean number of interviews attended was 11.3. This was higher than participants who were not couples matching (13.6 vs 9.8 [P=.02] and 11.3 vs 8.9 [P=.04], respectively). Applicants and their partners applied to programs and received interviews in a mean of 10 cities. Sixteen applicants reported that they contacted programs where their partner had interview offers. All participants’ rank lists included programs located in different cities than their partners’ ranked programs, and all but 1 participant ranked programs located in a different state than their partners’ ranked programs. Fifteen participants had options in their rank list for the applicant not to match, even if the partner would match. Similarly, 12 had the option for the applicant to match, even if the partner would not match. Fourteen (82.4%) matched at the same institution as their significant other. Three (17.6%) applicants matched to a program in a different state than the partner’s matched program. Two (11.8%) participants felt their relationship with their partner suffered because of the match, and 1 (5.9%) applicant was undetermined. One applicant described their relationship suffering from “unnecessary tension and anxiety” and noted “difficult conversations” about potentially matching into dermatology in a different location from their partner that could have been “devastating and not something [he or she] should have to choose.”
Comment
Factors for Matching in Dermatology—In our survey, we found the statistically significant factors of matching into dermatology included high USMLE Step 1 and Step 2 CK scores (P<.01), having a home dermatology program (P=.04), and attending a higher number of dermatology interviews (P<.01). These data are similar to NRMP results1; however, the higher likelihood of matching if the medical school has a home dermatology program has not been reported. This finding could be due to multiple factors such as students have less access to academic dermatologists for research projects, letters of recommendations, mentorship, and clinical rotations.
Gender and having children were factors that had no correlation with the match rate. There was a statistical difference of matching based on marital status (P<.01), but this is likely due to the low number of applicants in the divorced category. There were differences among demographics with USMLE Step 1 and Step 2 CK scores, which is a known factor in matching.1,2 Applicants with children had lower USMLE Step 1 and Step 2 CK scores compared to applicants without children. Females also had lower median USMLE Step 1 scores compared to males. This finding may serve as a reminder to programs when comparing USMLE Step examination scores that demographic factors may play a role. The race and ethnicity of applicants likely play a role. It has been reported that underrepresented minorities had lower match rates than White and Asian applicants in dermatology.6 There have been several published articles discussing the lack of diversity in dermatology, with a call to action.7-9
Factors for Couples Matching—The number of applicants participating in the couples match continues to increase yearly. The NMRP does publish data regarding “successful” couples matching but does not specify how many couples match together. There also is little published regarding advice for participation in the couples match. Although we had a limited number of couples that participated in the match, it is interesting to note they had similar strategies, including contacting programs at institutions that had offered interviews to their partners. This strategy may be effective, as dermatology programs offer interviews relatively late compared with other specialties.5 Additionally, this strategy may increase the number of interviews offered and received, as evidenced by the higher number of interviews offered compared with those who were not couples matching. Additionally, this survey highlights the sacrifice often needed by couples in the couples match as revealed by the inclusion of rank-list options in which the couples reside long distance or in which 1 partner does not match. This information may be helpful to applicants who are planning a strategy for the couples match in dermatology. Although this study does not encompass all dermatology applicants in the 2019-2020 cycle, we do believe it may be representative. The USMLE Step 1 scores in this study were similar to the published NRMP data.1,10 According to NRMP data from the 2019-2020 cycle, the mean USMLE Step 1 score was 248 for matched applicants and 239 for unmatched.1 The NRMP reported the mean USMLE Step 2 CK score for matched was 256 and 248 for unmatched, which also is similar to our data. The NRMP reported the mean number of programs ranked was 9.9 for matched and 4.5 for unmatched applicants.1 Again, our data were similar for number of dermatology interviews attended.
Limitations—There are limitations to this study. The main limitation is that the survey is from a single institution and had a limited number of respondents. Given the nature of the study, the accuracy of the data is dependent on the applicants’ honesty in self-reporting academic performance and other variables. There also may be a selection bias given the low response rate. The subanalyses—children and couples matching—were underpowered with the limited number of participants. Further studies that include multiple residency programs and multiple years could be helpful to provide more power and less risk of bias. We did not gather information such as the Medical Student Performance Evaluation letter, letters of recommendation, or personal statements, which do play an important role in the assessment of an applicant. However, because the applicants completed these surveys, and given these are largely blinded to applicants, we did not feel the applicants could accurately respond to those aspects of the application.
Conclusion
Our survey finds that factors associated with matching included a higher USMLE Step 1 score, having a home dermatology program, and a higher number of interviews offered and attended. Some demographics had varying USMLE Step 1 scores but similar match rates.
- National Resident Matching Program. Results and Data: 2020 Main Residency Match. National Resident Matching Program; May 2020. Accessed January 9, 2023. https://www.nrmp.org/wp-content/uploads/2021/12/MM_Results_and-Data_2020-1.pdf
- Gauer JL, Jackson JB. The association of USMLE Step 1 and Step 2 CK scores with residency match specialty and location. Med Educ Online. 2017;22:1358579.
- Wang JV, Keller M. Pressure to publish for residency applicants in dermatology. Dermatol Online J. 2016;22:13030/qt56x1t7ww.
- Wang RF, Zhang M, Kaffenberger JA. Does the dermatology standardized letter of recommendation alter applicants’ chances of matching into residency. J Am Acad Dermatol. 2017;77:e139-e140.
- National Resident Matching Program, Data Release and Research Committee: results of the 2018 NRMP Program Director Survey. Accessed December 19, 2022. https://www.nrmp.org/wp-content/uploads/2021/07/NRMP-2018-Program-Director-Survey-for-WWW.pdf
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role of race and ethnicity in the dermatology applicant match process. J Natl Med Assoc. 2022;113:666-670.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants-turning the tide. JAMA Dermatol. 2017;153:259-260.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49.
- National Resident Matching Program. Charting outcomes in the match: U.S. allopathic seniors. Characteristics of U.S. allopathic seniors who matched to their preferred specialty in the 2018 main residency match. 2nd ed. Accessed December 19, 2022. https://www.nrmp.org/wp-content/uploads/2021/07/Charting-Outcomes-in-the-Match-2018_Seniors-1.pdf
Dermatology residency continues to be one of the most competitive specialties, with a match rate of 84.7% for US allopathic seniors in the 2019-2020 academic year.1 In the 2019-2020 cycle, dermatology applicants were tied with plastic surgery for the highest median US Medical Licensing Examination (USMLE) Step 1 score compared with other specialties, which suggests that the top medical students are applying, yet only approximately 5 of 6 students are matching.
Factors that have been cited with successful dermatology matching include USMLE Step 1 and Step 2 Clinical Knowledge (CK) scores,2 research accomplishments,3 letters of recommendation,4 medical school performance, personal statement, grades in required clerkships, and volunteer/extracurricular experiences, among others.5
The National Resident Matching Program (NRMP) publishes data each year regarding different academic factors—USMLE scores; number of abstracts, presentations, and papers; work, volunteer, and research experiences—and compares the mean between matched and nonmatched applicants.1 However, the USMLE does not report any demographic information of the applicants and the implication it has for matching. Additionally, the number of couples participating in the couples match continues to increase each year. In the 2019-2020 cycle, 1224 couples participated in the couples match.1 However, NRMP reports only limited data regarding the couples match, and it is not specialty specific.
We aimed to determine the characteristics of matched vs nonmatched dermatology applicants. Secondarily, we aimed to determine any differences among demographics regarding matching rates, academic performance, and research publications. We also aimed to characterize the strategy and outcomes of applicants that couples matched.
Materials and Methods
The Mayo Clinic institutional review board deemed this study exempt. All applicants who applied to Mayo Clinic dermatology residency in Scottsdale, Arizona, during the 2018-2019 cycle were emailed an initial survey (N=475) before Match Day that obtained demographic information, geographic information, gap-year information, USMLE Step 1 score, publications, medical school grades, number of away rotations, and number of interviews. A follow-up survey gathering match data and couples matching data was sent to the applicants who completed the first survey on Match Day. The survey was repeated for the 2019-2020 cycle. In the second survey, Step 2 CK data were obtained. The survey was sent to 629 applicants who applied to Mayo Clinic dermatology residencies in Arizona, Minnesota, and Florida to include a broader group of applicants. For publications, applicants were asked to count only published or accepted manuscripts, not abstracts, posters, conference presentations, or submitted manuscripts. Applicants who did not respond to the second survey (match data) were not included in that part of the analysis. One survey was excluded because of implausible answers (eg, scores outside of range for USMLE Step scores).
Statistical Analysis—For statistical analyses, the applicants from both applications cycles were combined. Descriptive statistics were reported in the form of mean, median, or counts (percentages), as applicable. Means were compared using 2-sided t tests. Group comparisons were examined using χ2 tests for categorical variables. Statistical analyses were performed using the BlueSky Statistics version 6.30. P<.05 was considered significant.
Results
In 2019, a total of 149 applicants completed the initial survey (31.4% response rate), and 112 completed the follow-up survey (75.2% response rate). In 2020, a total of 142 applicants completed the initial survey (22.6% response rate), and 124 completed the follow-up survey (87.3% response rate). Combining the 2 years, after removing 1 survey with implausible answers, there were 290 respondents from the initial survey and 235 from the follow-up survey. The median (SD) age for the total applicants over both years was 27 (3.0) years, and 180 applicants were female (61.9%).
USMLE Scores—The median USMLE Step 1 score was 250, and scores ranged from 196 to 271. The median USMLE Step 2 CK score was 257, and scores ranged from 213 to 281. Higher USMLE Step 1 and Step 2 CK scores and more interviews were associated with higher match rates (Table 1). In addition, students with a dermatology program at their medical school were more likely to match than those without a home dermatology program.


Gender Differences—There were 180 females and 110 males who completed the surveys. Males and females had similar match rates (85.2% vs 89.0%; P=.39)(Table 2).

Family Life—In comparing marital status, applicants who were divorced had a higher median age (38.5 years) compared with applicants who were single, married, or in a domestic partnership (all 27 years; P<.01). Differences are outlined in Table 3.


On average, applicants with children (n=27 [15 male, 12 female]; P=.13) were 3 years older than those without (30.5 vs 27; P<.01) and were more likely to be married (88.9% vs 21.5%; P<.01). Applicants with children had a mean USMLE Step 1 score of 241 compared to 251 for those without children (P=.02) and a mean USMLE Step 2 CK score of 246 compared to 258 for those without children (P<.01). Applicants with children had similar debt, number of publications, number of honored rotations, and match rates compared to applicants without children (Figure).

Couples Match—Seventeen individuals in our survey participated in the couples match (7.8%), and all 17 (100%) matched into dermatology. The mean age was 26.7 years, 12 applicants were female, 2 applicants were married, and 1 applicant had children. The mean number of interviews offered was 13.6, and the mean number of interviews attended was 11.3. This was higher than participants who were not couples matching (13.6 vs 9.8 [P=.02] and 11.3 vs 8.9 [P=.04], respectively). Applicants and their partners applied to programs and received interviews in a mean of 10 cities. Sixteen applicants reported that they contacted programs where their partner had interview offers. All participants’ rank lists included programs located in different cities than their partners’ ranked programs, and all but 1 participant ranked programs located in a different state than their partners’ ranked programs. Fifteen participants had options in their rank list for the applicant not to match, even if the partner would match. Similarly, 12 had the option for the applicant to match, even if the partner would not match. Fourteen (82.4%) matched at the same institution as their significant other. Three (17.6%) applicants matched to a program in a different state than the partner’s matched program. Two (11.8%) participants felt their relationship with their partner suffered because of the match, and 1 (5.9%) applicant was undetermined. One applicant described their relationship suffering from “unnecessary tension and anxiety” and noted “difficult conversations” about potentially matching into dermatology in a different location from their partner that could have been “devastating and not something [he or she] should have to choose.”
Comment
Factors for Matching in Dermatology—In our survey, we found the statistically significant factors of matching into dermatology included high USMLE Step 1 and Step 2 CK scores (P<.01), having a home dermatology program (P=.04), and attending a higher number of dermatology interviews (P<.01). These data are similar to NRMP results1; however, the higher likelihood of matching if the medical school has a home dermatology program has not been reported. This finding could be due to multiple factors such as students have less access to academic dermatologists for research projects, letters of recommendations, mentorship, and clinical rotations.
Gender and having children were factors that had no correlation with the match rate. There was a statistical difference of matching based on marital status (P<.01), but this is likely due to the low number of applicants in the divorced category. There were differences among demographics with USMLE Step 1 and Step 2 CK scores, which is a known factor in matching.1,2 Applicants with children had lower USMLE Step 1 and Step 2 CK scores compared to applicants without children. Females also had lower median USMLE Step 1 scores compared to males. This finding may serve as a reminder to programs when comparing USMLE Step examination scores that demographic factors may play a role. The race and ethnicity of applicants likely play a role. It has been reported that underrepresented minorities had lower match rates than White and Asian applicants in dermatology.6 There have been several published articles discussing the lack of diversity in dermatology, with a call to action.7-9
Factors for Couples Matching—The number of applicants participating in the couples match continues to increase yearly. The NMRP does publish data regarding “successful” couples matching but does not specify how many couples match together. There also is little published regarding advice for participation in the couples match. Although we had a limited number of couples that participated in the match, it is interesting to note they had similar strategies, including contacting programs at institutions that had offered interviews to their partners. This strategy may be effective, as dermatology programs offer interviews relatively late compared with other specialties.5 Additionally, this strategy may increase the number of interviews offered and received, as evidenced by the higher number of interviews offered compared with those who were not couples matching. Additionally, this survey highlights the sacrifice often needed by couples in the couples match as revealed by the inclusion of rank-list options in which the couples reside long distance or in which 1 partner does not match. This information may be helpful to applicants who are planning a strategy for the couples match in dermatology. Although this study does not encompass all dermatology applicants in the 2019-2020 cycle, we do believe it may be representative. The USMLE Step 1 scores in this study were similar to the published NRMP data.1,10 According to NRMP data from the 2019-2020 cycle, the mean USMLE Step 1 score was 248 for matched applicants and 239 for unmatched.1 The NRMP reported the mean USMLE Step 2 CK score for matched was 256 and 248 for unmatched, which also is similar to our data. The NRMP reported the mean number of programs ranked was 9.9 for matched and 4.5 for unmatched applicants.1 Again, our data were similar for number of dermatology interviews attended.
Limitations—There are limitations to this study. The main limitation is that the survey is from a single institution and had a limited number of respondents. Given the nature of the study, the accuracy of the data is dependent on the applicants’ honesty in self-reporting academic performance and other variables. There also may be a selection bias given the low response rate. The subanalyses—children and couples matching—were underpowered with the limited number of participants. Further studies that include multiple residency programs and multiple years could be helpful to provide more power and less risk of bias. We did not gather information such as the Medical Student Performance Evaluation letter, letters of recommendation, or personal statements, which do play an important role in the assessment of an applicant. However, because the applicants completed these surveys, and given these are largely blinded to applicants, we did not feel the applicants could accurately respond to those aspects of the application.
Conclusion
Our survey finds that factors associated with matching included a higher USMLE Step 1 score, having a home dermatology program, and a higher number of interviews offered and attended. Some demographics had varying USMLE Step 1 scores but similar match rates.
Dermatology residency continues to be one of the most competitive specialties, with a match rate of 84.7% for US allopathic seniors in the 2019-2020 academic year.1 In the 2019-2020 cycle, dermatology applicants were tied with plastic surgery for the highest median US Medical Licensing Examination (USMLE) Step 1 score compared with other specialties, which suggests that the top medical students are applying, yet only approximately 5 of 6 students are matching.
Factors that have been cited with successful dermatology matching include USMLE Step 1 and Step 2 Clinical Knowledge (CK) scores,2 research accomplishments,3 letters of recommendation,4 medical school performance, personal statement, grades in required clerkships, and volunteer/extracurricular experiences, among others.5
The National Resident Matching Program (NRMP) publishes data each year regarding different academic factors—USMLE scores; number of abstracts, presentations, and papers; work, volunteer, and research experiences—and compares the mean between matched and nonmatched applicants.1 However, the USMLE does not report any demographic information of the applicants and the implication it has for matching. Additionally, the number of couples participating in the couples match continues to increase each year. In the 2019-2020 cycle, 1224 couples participated in the couples match.1 However, NRMP reports only limited data regarding the couples match, and it is not specialty specific.
We aimed to determine the characteristics of matched vs nonmatched dermatology applicants. Secondarily, we aimed to determine any differences among demographics regarding matching rates, academic performance, and research publications. We also aimed to characterize the strategy and outcomes of applicants that couples matched.
Materials and Methods
The Mayo Clinic institutional review board deemed this study exempt. All applicants who applied to Mayo Clinic dermatology residency in Scottsdale, Arizona, during the 2018-2019 cycle were emailed an initial survey (N=475) before Match Day that obtained demographic information, geographic information, gap-year information, USMLE Step 1 score, publications, medical school grades, number of away rotations, and number of interviews. A follow-up survey gathering match data and couples matching data was sent to the applicants who completed the first survey on Match Day. The survey was repeated for the 2019-2020 cycle. In the second survey, Step 2 CK data were obtained. The survey was sent to 629 applicants who applied to Mayo Clinic dermatology residencies in Arizona, Minnesota, and Florida to include a broader group of applicants. For publications, applicants were asked to count only published or accepted manuscripts, not abstracts, posters, conference presentations, or submitted manuscripts. Applicants who did not respond to the second survey (match data) were not included in that part of the analysis. One survey was excluded because of implausible answers (eg, scores outside of range for USMLE Step scores).
Statistical Analysis—For statistical analyses, the applicants from both applications cycles were combined. Descriptive statistics were reported in the form of mean, median, or counts (percentages), as applicable. Means were compared using 2-sided t tests. Group comparisons were examined using χ2 tests for categorical variables. Statistical analyses were performed using the BlueSky Statistics version 6.30. P<.05 was considered significant.
Results
In 2019, a total of 149 applicants completed the initial survey (31.4% response rate), and 112 completed the follow-up survey (75.2% response rate). In 2020, a total of 142 applicants completed the initial survey (22.6% response rate), and 124 completed the follow-up survey (87.3% response rate). Combining the 2 years, after removing 1 survey with implausible answers, there were 290 respondents from the initial survey and 235 from the follow-up survey. The median (SD) age for the total applicants over both years was 27 (3.0) years, and 180 applicants were female (61.9%).
USMLE Scores—The median USMLE Step 1 score was 250, and scores ranged from 196 to 271. The median USMLE Step 2 CK score was 257, and scores ranged from 213 to 281. Higher USMLE Step 1 and Step 2 CK scores and more interviews were associated with higher match rates (Table 1). In addition, students with a dermatology program at their medical school were more likely to match than those without a home dermatology program.


Gender Differences—There were 180 females and 110 males who completed the surveys. Males and females had similar match rates (85.2% vs 89.0%; P=.39)(Table 2).

Family Life—In comparing marital status, applicants who were divorced had a higher median age (38.5 years) compared with applicants who were single, married, or in a domestic partnership (all 27 years; P<.01). Differences are outlined in Table 3.


On average, applicants with children (n=27 [15 male, 12 female]; P=.13) were 3 years older than those without (30.5 vs 27; P<.01) and were more likely to be married (88.9% vs 21.5%; P<.01). Applicants with children had a mean USMLE Step 1 score of 241 compared to 251 for those without children (P=.02) and a mean USMLE Step 2 CK score of 246 compared to 258 for those without children (P<.01). Applicants with children had similar debt, number of publications, number of honored rotations, and match rates compared to applicants without children (Figure).

Couples Match—Seventeen individuals in our survey participated in the couples match (7.8%), and all 17 (100%) matched into dermatology. The mean age was 26.7 years, 12 applicants were female, 2 applicants were married, and 1 applicant had children. The mean number of interviews offered was 13.6, and the mean number of interviews attended was 11.3. This was higher than participants who were not couples matching (13.6 vs 9.8 [P=.02] and 11.3 vs 8.9 [P=.04], respectively). Applicants and their partners applied to programs and received interviews in a mean of 10 cities. Sixteen applicants reported that they contacted programs where their partner had interview offers. All participants’ rank lists included programs located in different cities than their partners’ ranked programs, and all but 1 participant ranked programs located in a different state than their partners’ ranked programs. Fifteen participants had options in their rank list for the applicant not to match, even if the partner would match. Similarly, 12 had the option for the applicant to match, even if the partner would not match. Fourteen (82.4%) matched at the same institution as their significant other. Three (17.6%) applicants matched to a program in a different state than the partner’s matched program. Two (11.8%) participants felt their relationship with their partner suffered because of the match, and 1 (5.9%) applicant was undetermined. One applicant described their relationship suffering from “unnecessary tension and anxiety” and noted “difficult conversations” about potentially matching into dermatology in a different location from their partner that could have been “devastating and not something [he or she] should have to choose.”
Comment
Factors for Matching in Dermatology—In our survey, we found the statistically significant factors of matching into dermatology included high USMLE Step 1 and Step 2 CK scores (P<.01), having a home dermatology program (P=.04), and attending a higher number of dermatology interviews (P<.01). These data are similar to NRMP results1; however, the higher likelihood of matching if the medical school has a home dermatology program has not been reported. This finding could be due to multiple factors such as students have less access to academic dermatologists for research projects, letters of recommendations, mentorship, and clinical rotations.
Gender and having children were factors that had no correlation with the match rate. There was a statistical difference of matching based on marital status (P<.01), but this is likely due to the low number of applicants in the divorced category. There were differences among demographics with USMLE Step 1 and Step 2 CK scores, which is a known factor in matching.1,2 Applicants with children had lower USMLE Step 1 and Step 2 CK scores compared to applicants without children. Females also had lower median USMLE Step 1 scores compared to males. This finding may serve as a reminder to programs when comparing USMLE Step examination scores that demographic factors may play a role. The race and ethnicity of applicants likely play a role. It has been reported that underrepresented minorities had lower match rates than White and Asian applicants in dermatology.6 There have been several published articles discussing the lack of diversity in dermatology, with a call to action.7-9
Factors for Couples Matching—The number of applicants participating in the couples match continues to increase yearly. The NMRP does publish data regarding “successful” couples matching but does not specify how many couples match together. There also is little published regarding advice for participation in the couples match. Although we had a limited number of couples that participated in the match, it is interesting to note they had similar strategies, including contacting programs at institutions that had offered interviews to their partners. This strategy may be effective, as dermatology programs offer interviews relatively late compared with other specialties.5 Additionally, this strategy may increase the number of interviews offered and received, as evidenced by the higher number of interviews offered compared with those who were not couples matching. Additionally, this survey highlights the sacrifice often needed by couples in the couples match as revealed by the inclusion of rank-list options in which the couples reside long distance or in which 1 partner does not match. This information may be helpful to applicants who are planning a strategy for the couples match in dermatology. Although this study does not encompass all dermatology applicants in the 2019-2020 cycle, we do believe it may be representative. The USMLE Step 1 scores in this study were similar to the published NRMP data.1,10 According to NRMP data from the 2019-2020 cycle, the mean USMLE Step 1 score was 248 for matched applicants and 239 for unmatched.1 The NRMP reported the mean USMLE Step 2 CK score for matched was 256 and 248 for unmatched, which also is similar to our data. The NRMP reported the mean number of programs ranked was 9.9 for matched and 4.5 for unmatched applicants.1 Again, our data were similar for number of dermatology interviews attended.
Limitations—There are limitations to this study. The main limitation is that the survey is from a single institution and had a limited number of respondents. Given the nature of the study, the accuracy of the data is dependent on the applicants’ honesty in self-reporting academic performance and other variables. There also may be a selection bias given the low response rate. The subanalyses—children and couples matching—were underpowered with the limited number of participants. Further studies that include multiple residency programs and multiple years could be helpful to provide more power and less risk of bias. We did not gather information such as the Medical Student Performance Evaluation letter, letters of recommendation, or personal statements, which do play an important role in the assessment of an applicant. However, because the applicants completed these surveys, and given these are largely blinded to applicants, we did not feel the applicants could accurately respond to those aspects of the application.
Conclusion
Our survey finds that factors associated with matching included a higher USMLE Step 1 score, having a home dermatology program, and a higher number of interviews offered and attended. Some demographics had varying USMLE Step 1 scores but similar match rates.
- National Resident Matching Program. Results and Data: 2020 Main Residency Match. National Resident Matching Program; May 2020. Accessed January 9, 2023. https://www.nrmp.org/wp-content/uploads/2021/12/MM_Results_and-Data_2020-1.pdf
- Gauer JL, Jackson JB. The association of USMLE Step 1 and Step 2 CK scores with residency match specialty and location. Med Educ Online. 2017;22:1358579.
- Wang JV, Keller M. Pressure to publish for residency applicants in dermatology. Dermatol Online J. 2016;22:13030/qt56x1t7ww.
- Wang RF, Zhang M, Kaffenberger JA. Does the dermatology standardized letter of recommendation alter applicants’ chances of matching into residency. J Am Acad Dermatol. 2017;77:e139-e140.
- National Resident Matching Program, Data Release and Research Committee: results of the 2018 NRMP Program Director Survey. Accessed December 19, 2022. https://www.nrmp.org/wp-content/uploads/2021/07/NRMP-2018-Program-Director-Survey-for-WWW.pdf
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role of race and ethnicity in the dermatology applicant match process. J Natl Med Assoc. 2022;113:666-670.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants-turning the tide. JAMA Dermatol. 2017;153:259-260.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49.
- National Resident Matching Program. Charting outcomes in the match: U.S. allopathic seniors. Characteristics of U.S. allopathic seniors who matched to their preferred specialty in the 2018 main residency match. 2nd ed. Accessed December 19, 2022. https://www.nrmp.org/wp-content/uploads/2021/07/Charting-Outcomes-in-the-Match-2018_Seniors-1.pdf
- National Resident Matching Program. Results and Data: 2020 Main Residency Match. National Resident Matching Program; May 2020. Accessed January 9, 2023. https://www.nrmp.org/wp-content/uploads/2021/12/MM_Results_and-Data_2020-1.pdf
- Gauer JL, Jackson JB. The association of USMLE Step 1 and Step 2 CK scores with residency match specialty and location. Med Educ Online. 2017;22:1358579.
- Wang JV, Keller M. Pressure to publish for residency applicants in dermatology. Dermatol Online J. 2016;22:13030/qt56x1t7ww.
- Wang RF, Zhang M, Kaffenberger JA. Does the dermatology standardized letter of recommendation alter applicants’ chances of matching into residency. J Am Acad Dermatol. 2017;77:e139-e140.
- National Resident Matching Program, Data Release and Research Committee: results of the 2018 NRMP Program Director Survey. Accessed December 19, 2022. https://www.nrmp.org/wp-content/uploads/2021/07/NRMP-2018-Program-Director-Survey-for-WWW.pdf
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role of race and ethnicity in the dermatology applicant match process. J Natl Med Assoc. 2022;113:666-670.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants-turning the tide. JAMA Dermatol. 2017;153:259-260.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49.
- National Resident Matching Program. Charting outcomes in the match: U.S. allopathic seniors. Characteristics of U.S. allopathic seniors who matched to their preferred specialty in the 2018 main residency match. 2nd ed. Accessed December 19, 2022. https://www.nrmp.org/wp-content/uploads/2021/07/Charting-Outcomes-in-the-Match-2018_Seniors-1.pdf
PRACTICE POINTS
- Dermatology residency continues to be one of the most competitive specialties, with a match rate of 84.7% in 2019.
- A high US Medical Licensing Examination (USMLE) Step 1 score and having a home dermatology program and a greater number of interviews may lead to higher likeliness of matching in dermatology.
- Most applicants (82.4%) applied to programs their partner had interviews at, suggesting this may be a helpful strategy.
Geriatrician advises on use of vitamin D supplementation, lecanemab, and texting for her patients
Vitamin D supplementation and incident fractures
Vitamin D supplementation is a commonly recommended intervention for bone health, but data to support its impact on reducing fracture risk has been variable.
A study in the New England Journal of Medicine by LeBoff and colleagues has garnered much attention since its publication in July 2022.1 In the ancillary study of the Vitamin D and Omega-3-Trial (VITAL), the authors examined the impact of vitamin D supplementation versus placebo on incident fractures. The study found that vitamin D supplementation, as compared with placebo, led to no significant difference in the incidence of total, nonvertebral, and hip fractures in midlife and older adults over the 5-year period of follow-up.
The generalizability of these findings has been raised as a concern as the study does not describe adults at higher risk for fracture. The authors of the study specified in their conclusion that vitamin D supplementation does not reduce fracture risk in “generally healthy midlife and older adults who were not selected for vitamin D deficiency, low bone mass or osteoporosis.”
With a mean participant age of 67 and exclusion of participants with a history of cardiovascular disease, stroke, cirrhosis and other serious illnesses, the study does not reflect the multimorbid older adult population that geriatricians typically care for. Furthermore, efficacy of vitamin D supplementation on fracture risk may be the most impactful in those with osteoporosis and with severe vitamin D deficiency (defined by vitamin D 25[OH]D level less than 12 ng/mL).
In post hoc analyses, there was no significant difference in fracture risk in these subgroups, however the authors acknowledged that the findings may be limited by the small percentage of participants with severe vitamin D deficiency (2.4%) and osteoporosis included in the study (5%).
Lecanemab for mild cognitive impairment and early Alzheimer’s dementia
On Jan. 6, 2023, the Food and Drug Administration approved lecanemab, the second-ever disease-modifying treatment for Alzheimer’s dementia following the approval of aducanumab in 2021. Lecanemab is a monoclonal antibody targeting larger amyloid-beta oligomers, which has been shown in vitro to have higher affinity for amyloid-beta, compared with aducanumab. FDA approval followed shortly after the publication of the CLARITY-AD trial, which investigated the effect of lecanemab versus placebo on cognitive decline and burden of amyloid in adults with mild cognitive impairment and mild Alzheimer’s dementia. Over an 18-month period, the study found that participants who received lecanemab, compared with placebo, had a significantly smaller decline in cognition and function, and reduction in amyloid burden on PET CT.2
The clinical significance of these findings, however, is unclear. As noted by an editorial published in the Lancet in 2022, the difference in Clinical Dementia Rating-Sum of Boxes (CDR-SB) scale between the treatment and placebo groups was 0.45. On an 18-point scale, prior research has noted that a minimal clinically significance difference of 0.98 is necessary in those with mild cognitive impairment and 1.63 in mild Alzheimer dementia.3
Additionally, the CLARITY-AD trial reported that lecanemab resulted in infusion reactions in 26.4% of participants and brain edema (an amyloid-related imaging abnormality referred to as ARIA-E) in 12.6% of participants. This finding highlights concerns for safety and the need for close monitoring, as well as ongoing implications of economic feasibility and equitable access for all those who qualify for treatment.2
Social isolation and dementia risk
There is growing awareness of the impact of social isolation on health outcomes, particularly among older adults. Prior research has reported that one in four older adults are considered socially isolated and that social isolation increases risk of premature death, dementia, depression, and cardiovascular disease.4
A study by Huang and colleagues is the first nationally representative cohort study examining the association between social isolation and incident dementia for older adults in community dwelling settings. A cohort of 5,022 older adults participating in the National Health and Aging Trends Study was followed from 2011 to 2020. When adjusting for demographic and health factors, including race, level of education, and number of chronic health conditions, socially isolated adults had a greater risk of developing dementia, compared with adults who were not socially isolated (hazard ratio, 1.27; 95% confidence interval, 1.08-1.49). Potential mechanisms to explain this association include the increased risk of cardiovascular disease and depression in older adults who are socially isolated, thereby increasing dementia risk.
Decreased cognitive activity/engagement and access to resources such as caregiving and health care may also be linked to the increased risk of dementia in socially isolated older adults.5
Another observational cohort study from the National Health and Aging Trends Study investigated whether access and use of technology can lower the risk of social isolation. The study found that older adults who used email or text messaging had a lower risk of social isolation than older adults who did not use technology (incidence rate ratio, 0.64; 95% CI, 0.51-0.80).6 These findings highlight the importance of addressing social isolation as an important modifiable health risk factor, and the need for providing equitable access to technology in vulnerable populations as health intervention.
Dr. Mengru “Ruru” Wang is a geriatrician and internist at the University of Washington, Seattle. She practices full-spectrum medicine, seeing patients in primary care, nursing homes, and acute care. Dr. Wang has no disclosures related to this piece.
References
1. LeBoff MS et al. Supplemental vitamin D and incident fractures in midlife and older adults. N Engl J Med. 2022;387(4):299-30.
2. van Dyck CH et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21.
3. The Lancet. Lecanemab for Alzheimer’s disease: tempering hype and hope. Lancet. 2022; 400:1899.
4. National Academies of Sciences, Engineering, and Medicine. Social Isolation and Loneliness in Older Adults: Opportunities for the Health Care System. Washington, DC: 2020, The National Academies Press.
5. Huang, AR et al. Social isolation and 9-year dementia risk in community dwelling Medicare beneficiaries in the United States. J Am Geriatr Soc. 2023 Jan 11. doi: 10.1111/jgs18140.
6. Umoh ME etal. Impact of technology on social isolation: Longitudinal analysis from the National Health Aging Trends Study. J Am Geriatr Soc. 2022 Dec 15. doi 10.1111/jgs.18179.
Vitamin D supplementation and incident fractures
Vitamin D supplementation is a commonly recommended intervention for bone health, but data to support its impact on reducing fracture risk has been variable.
A study in the New England Journal of Medicine by LeBoff and colleagues has garnered much attention since its publication in July 2022.1 In the ancillary study of the Vitamin D and Omega-3-Trial (VITAL), the authors examined the impact of vitamin D supplementation versus placebo on incident fractures. The study found that vitamin D supplementation, as compared with placebo, led to no significant difference in the incidence of total, nonvertebral, and hip fractures in midlife and older adults over the 5-year period of follow-up.
The generalizability of these findings has been raised as a concern as the study does not describe adults at higher risk for fracture. The authors of the study specified in their conclusion that vitamin D supplementation does not reduce fracture risk in “generally healthy midlife and older adults who were not selected for vitamin D deficiency, low bone mass or osteoporosis.”
With a mean participant age of 67 and exclusion of participants with a history of cardiovascular disease, stroke, cirrhosis and other serious illnesses, the study does not reflect the multimorbid older adult population that geriatricians typically care for. Furthermore, efficacy of vitamin D supplementation on fracture risk may be the most impactful in those with osteoporosis and with severe vitamin D deficiency (defined by vitamin D 25[OH]D level less than 12 ng/mL).
In post hoc analyses, there was no significant difference in fracture risk in these subgroups, however the authors acknowledged that the findings may be limited by the small percentage of participants with severe vitamin D deficiency (2.4%) and osteoporosis included in the study (5%).
Lecanemab for mild cognitive impairment and early Alzheimer’s dementia
On Jan. 6, 2023, the Food and Drug Administration approved lecanemab, the second-ever disease-modifying treatment for Alzheimer’s dementia following the approval of aducanumab in 2021. Lecanemab is a monoclonal antibody targeting larger amyloid-beta oligomers, which has been shown in vitro to have higher affinity for amyloid-beta, compared with aducanumab. FDA approval followed shortly after the publication of the CLARITY-AD trial, which investigated the effect of lecanemab versus placebo on cognitive decline and burden of amyloid in adults with mild cognitive impairment and mild Alzheimer’s dementia. Over an 18-month period, the study found that participants who received lecanemab, compared with placebo, had a significantly smaller decline in cognition and function, and reduction in amyloid burden on PET CT.2
The clinical significance of these findings, however, is unclear. As noted by an editorial published in the Lancet in 2022, the difference in Clinical Dementia Rating-Sum of Boxes (CDR-SB) scale between the treatment and placebo groups was 0.45. On an 18-point scale, prior research has noted that a minimal clinically significance difference of 0.98 is necessary in those with mild cognitive impairment and 1.63 in mild Alzheimer dementia.3
Additionally, the CLARITY-AD trial reported that lecanemab resulted in infusion reactions in 26.4% of participants and brain edema (an amyloid-related imaging abnormality referred to as ARIA-E) in 12.6% of participants. This finding highlights concerns for safety and the need for close monitoring, as well as ongoing implications of economic feasibility and equitable access for all those who qualify for treatment.2
Social isolation and dementia risk
There is growing awareness of the impact of social isolation on health outcomes, particularly among older adults. Prior research has reported that one in four older adults are considered socially isolated and that social isolation increases risk of premature death, dementia, depression, and cardiovascular disease.4
A study by Huang and colleagues is the first nationally representative cohort study examining the association between social isolation and incident dementia for older adults in community dwelling settings. A cohort of 5,022 older adults participating in the National Health and Aging Trends Study was followed from 2011 to 2020. When adjusting for demographic and health factors, including race, level of education, and number of chronic health conditions, socially isolated adults had a greater risk of developing dementia, compared with adults who were not socially isolated (hazard ratio, 1.27; 95% confidence interval, 1.08-1.49). Potential mechanisms to explain this association include the increased risk of cardiovascular disease and depression in older adults who are socially isolated, thereby increasing dementia risk.
Decreased cognitive activity/engagement and access to resources such as caregiving and health care may also be linked to the increased risk of dementia in socially isolated older adults.5
Another observational cohort study from the National Health and Aging Trends Study investigated whether access and use of technology can lower the risk of social isolation. The study found that older adults who used email or text messaging had a lower risk of social isolation than older adults who did not use technology (incidence rate ratio, 0.64; 95% CI, 0.51-0.80).6 These findings highlight the importance of addressing social isolation as an important modifiable health risk factor, and the need for providing equitable access to technology in vulnerable populations as health intervention.
Dr. Mengru “Ruru” Wang is a geriatrician and internist at the University of Washington, Seattle. She practices full-spectrum medicine, seeing patients in primary care, nursing homes, and acute care. Dr. Wang has no disclosures related to this piece.
References
1. LeBoff MS et al. Supplemental vitamin D and incident fractures in midlife and older adults. N Engl J Med. 2022;387(4):299-30.
2. van Dyck CH et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21.
3. The Lancet. Lecanemab for Alzheimer’s disease: tempering hype and hope. Lancet. 2022; 400:1899.
4. National Academies of Sciences, Engineering, and Medicine. Social Isolation and Loneliness in Older Adults: Opportunities for the Health Care System. Washington, DC: 2020, The National Academies Press.
5. Huang, AR et al. Social isolation and 9-year dementia risk in community dwelling Medicare beneficiaries in the United States. J Am Geriatr Soc. 2023 Jan 11. doi: 10.1111/jgs18140.
6. Umoh ME etal. Impact of technology on social isolation: Longitudinal analysis from the National Health Aging Trends Study. J Am Geriatr Soc. 2022 Dec 15. doi 10.1111/jgs.18179.
Vitamin D supplementation and incident fractures
Vitamin D supplementation is a commonly recommended intervention for bone health, but data to support its impact on reducing fracture risk has been variable.
A study in the New England Journal of Medicine by LeBoff and colleagues has garnered much attention since its publication in July 2022.1 In the ancillary study of the Vitamin D and Omega-3-Trial (VITAL), the authors examined the impact of vitamin D supplementation versus placebo on incident fractures. The study found that vitamin D supplementation, as compared with placebo, led to no significant difference in the incidence of total, nonvertebral, and hip fractures in midlife and older adults over the 5-year period of follow-up.
The generalizability of these findings has been raised as a concern as the study does not describe adults at higher risk for fracture. The authors of the study specified in their conclusion that vitamin D supplementation does not reduce fracture risk in “generally healthy midlife and older adults who were not selected for vitamin D deficiency, low bone mass or osteoporosis.”
With a mean participant age of 67 and exclusion of participants with a history of cardiovascular disease, stroke, cirrhosis and other serious illnesses, the study does not reflect the multimorbid older adult population that geriatricians typically care for. Furthermore, efficacy of vitamin D supplementation on fracture risk may be the most impactful in those with osteoporosis and with severe vitamin D deficiency (defined by vitamin D 25[OH]D level less than 12 ng/mL).
In post hoc analyses, there was no significant difference in fracture risk in these subgroups, however the authors acknowledged that the findings may be limited by the small percentage of participants with severe vitamin D deficiency (2.4%) and osteoporosis included in the study (5%).
Lecanemab for mild cognitive impairment and early Alzheimer’s dementia
On Jan. 6, 2023, the Food and Drug Administration approved lecanemab, the second-ever disease-modifying treatment for Alzheimer’s dementia following the approval of aducanumab in 2021. Lecanemab is a monoclonal antibody targeting larger amyloid-beta oligomers, which has been shown in vitro to have higher affinity for amyloid-beta, compared with aducanumab. FDA approval followed shortly after the publication of the CLARITY-AD trial, which investigated the effect of lecanemab versus placebo on cognitive decline and burden of amyloid in adults with mild cognitive impairment and mild Alzheimer’s dementia. Over an 18-month period, the study found that participants who received lecanemab, compared with placebo, had a significantly smaller decline in cognition and function, and reduction in amyloid burden on PET CT.2
The clinical significance of these findings, however, is unclear. As noted by an editorial published in the Lancet in 2022, the difference in Clinical Dementia Rating-Sum of Boxes (CDR-SB) scale between the treatment and placebo groups was 0.45. On an 18-point scale, prior research has noted that a minimal clinically significance difference of 0.98 is necessary in those with mild cognitive impairment and 1.63 in mild Alzheimer dementia.3
Additionally, the CLARITY-AD trial reported that lecanemab resulted in infusion reactions in 26.4% of participants and brain edema (an amyloid-related imaging abnormality referred to as ARIA-E) in 12.6% of participants. This finding highlights concerns for safety and the need for close monitoring, as well as ongoing implications of economic feasibility and equitable access for all those who qualify for treatment.2
Social isolation and dementia risk
There is growing awareness of the impact of social isolation on health outcomes, particularly among older adults. Prior research has reported that one in four older adults are considered socially isolated and that social isolation increases risk of premature death, dementia, depression, and cardiovascular disease.4
A study by Huang and colleagues is the first nationally representative cohort study examining the association between social isolation and incident dementia for older adults in community dwelling settings. A cohort of 5,022 older adults participating in the National Health and Aging Trends Study was followed from 2011 to 2020. When adjusting for demographic and health factors, including race, level of education, and number of chronic health conditions, socially isolated adults had a greater risk of developing dementia, compared with adults who were not socially isolated (hazard ratio, 1.27; 95% confidence interval, 1.08-1.49). Potential mechanisms to explain this association include the increased risk of cardiovascular disease and depression in older adults who are socially isolated, thereby increasing dementia risk.
Decreased cognitive activity/engagement and access to resources such as caregiving and health care may also be linked to the increased risk of dementia in socially isolated older adults.5
Another observational cohort study from the National Health and Aging Trends Study investigated whether access and use of technology can lower the risk of social isolation. The study found that older adults who used email or text messaging had a lower risk of social isolation than older adults who did not use technology (incidence rate ratio, 0.64; 95% CI, 0.51-0.80).6 These findings highlight the importance of addressing social isolation as an important modifiable health risk factor, and the need for providing equitable access to technology in vulnerable populations as health intervention.
Dr. Mengru “Ruru” Wang is a geriatrician and internist at the University of Washington, Seattle. She practices full-spectrum medicine, seeing patients in primary care, nursing homes, and acute care. Dr. Wang has no disclosures related to this piece.
References
1. LeBoff MS et al. Supplemental vitamin D and incident fractures in midlife and older adults. N Engl J Med. 2022;387(4):299-30.
2. van Dyck CH et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21.
3. The Lancet. Lecanemab for Alzheimer’s disease: tempering hype and hope. Lancet. 2022; 400:1899.
4. National Academies of Sciences, Engineering, and Medicine. Social Isolation and Loneliness in Older Adults: Opportunities for the Health Care System. Washington, DC: 2020, The National Academies Press.
5. Huang, AR et al. Social isolation and 9-year dementia risk in community dwelling Medicare beneficiaries in the United States. J Am Geriatr Soc. 2023 Jan 11. doi: 10.1111/jgs18140.
6. Umoh ME etal. Impact of technology on social isolation: Longitudinal analysis from the National Health Aging Trends Study. J Am Geriatr Soc. 2022 Dec 15. doi 10.1111/jgs.18179.
Damar Hamlin’s cardiac arrest: Key lessons
This discussion was recorded on Jan. 9, 2023. This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert D. Glatter, medical adviser for Medscape Emergency Medicine. Today, we have Dr. Paul E. Pepe, an emergency medicine physician based in Florida and a highly recognized expert in emergency medical services (EMS), critical care, sports and event medicine, and resuscitation. Also joining us is Dr. Michael S. (“Mick”) Malloy, an emergency medicine physician based in Ireland, also an expert in prehospital care, resuscitation, and sports and event medicine. Welcome, gentlemen.
Dr. Pepe: Thanks for having us here.
Dr. Glatter: the Buffalo Bills safety who went down suffering a cardiac arrest in front of millions of people. Although we don’t know the exact cause of the events that transpired, the goal of our discussion is to guide our audience through a systematic approach to evaluation and management of an athlete suffering blunt force chest and neck trauma, and then suffering a cardiac arrest. We do know, obviously, that Damar was successfully resuscitated, thanks to the medical staff and trainers.
Almost 50 years ago, Chuck Hughes, a Detroit Lions receiver, went down and died with just a minute to go in the game and, unfortunately, didn’t survive.
Paul, can you tell me your impressions after viewing the replay of the events that evening? What were the most likely causes of this syncopal event and the subsequent cardiac arrest?
Dr. Pepe: We don’t know anything specifically. It’s being kept private about what the events were. It’s a little bit complicated in a sense that he basically had an extended resuscitation in the hospital. My experience has been that most people that have ventricular fibrillation, from whatever cause, will most likely be waking up on the field if you get to them. I’ve had personal experience with that.
More importantly than when it starts, when someone goes down on the field, both Dr. Malloy and I take a broader view. We don’t get tunnel vision and think, “Oh, it was a traumatic event,” or “It was cardiac event,” and we just have our minds open. There are many things that could make you stop breathing on the field. It could be a neck or a severe head injury, and then any kind of other internal injury that occurs.
When I saw in the video that Damar Hamlin stood up, that made it a less likely to be a spinal injury. He seemed to be physically functioning, and then he suddenly collapsed. That went along with something that looks like a ventricular fibrillation or ventricular tachycardia type of event and made me think right away that it was commotio cordis. I’m not a Latin scholar, but commotio is like commotion. A literal translation might be an agitation of the heart. I was thinking that he probably got hit somewhere in the middle of the chest at the right moment where the heart is resetting in that repolarization phase, like an R-on-T phenomenon, and then caused this sudden ventricular dysrhythmia.
Most people associate it to that because we have a couple of dozen cases a year of people getting hockey pucks or a baseball hitting their chest, which is very common with adolescents. On the other hand, you can’t get it from a blunt injury like this, and it was too early for it to be, say, a direct cardiac contusion, unless there was a direct injury there. It just happened so quickly.
In Europe, they’ve had a large amount of experience with this same kind of problem before, even just from a direct shoulder hit, for example. Mick Malloy is the dean of the faculty of sports and exercise medicine at the Royal College of Surgeons in Ireland and has vast experience, and now he is the person overseeing the procedures for this. Mick, have you had those kinds of experiences as well?
Dr. Molloy: Yes. It’s something that has occurred over recent decades and has been more recognized. I note that in professional sports, it’s a very different thing because you’ve got such huge teams and teams trained to respond very quickly. And that’s the most important thing in this scenario – having a team that is well functioning as a high-class emergency response team ready to get out on to that field very quickly after the person collapses, getting the automated external defibrillator (AED) on, and then recognizing whether there needs to be a shock given or not. The machine will tell you all that.
In our scenario, we run courses called CARES (Care of the Athlete Resuscitation and Emergencies in Sport) to make sure that our team physicians and team physiotherapists and trainers are all speaking as one when an emergency arises.
I don’t worry so much about the professional sport. It’s more with the amateur sports and the kids sports that I get a bit more concerned because there isn’t the same level of medical care there. Having everybody trained in basic life support would be very important to reduce unnecessary deaths from these types of conditions.
As Paul mentioned, there is a very specific cardiac cause in some of these circumstances, where you get hit just at the wrong time and that hit occurs at a particular electrical point in time. It causes this ventricular fibrillation, and the only real treatment there is the defibrillator as quickly as possible.
Dr. Glatter: What you’re saying ultimately is an important part about rapid defibrillation, and at first, cardiopulmonary resuscitation (CPR). People are concerned about whether they should begin CPR. We’re talking about out-of-hospital cardiac arrest that is outside of a football stadium, for example. Some people are obsessed with taking a person’s pulse, and that’s been a point of contention. If someone is unconscious and not breathing, we should start CPR. Wouldn›t you agree? They will wake up quickly if you begin chest compressions if they’re not necessary.
Dr. Pepe: I tell people, just do it. You’re right, people will wake up and feel it if they don’t need it.
Getting back to Mick’s point of having things ready to go, for example, 8 years ago, we had a professional player on the bench who suddenly collapsed right there in front of the entire audience. We immediately did CPR, and we got the AED on. We shocked him and he was ready, willing, and able to get back on the bench again. It turns out he had underlying coronary artery disease, but we got him back right away.
I did an initial study where we placed an AED in a public place at the Chicago O’Hare Airport to see if the public would use these. Most cardiac arrests occur at home, of course, but in public places, that was a good place to try it. We had almost 10 cases the first year. What was fascinating was that we had almost no survivors over the previous decade, even though there were paramedics at the airport. When we put these out there, we had nine people go down that first year, and six people who had never operated an AED or seen one before knew to get one and use it. Every one of those people survived neurologically intact, and almost every person was waking up before traditional responders got there. That’s how effective this is, but you need to know where the AED is.
Dr. Glatter: How to turn it on, where it is, and how to operate it.
Dr. Pepe: That was the point: These rescuers saved lives in the first year, and it was tremendous. Two points I make about it are that one, you need to know where it is, and two, just go turn it on. It gives you the instructions to follow through; just be in the Nike mode, because it basically won’t hurt a person. It’s rare that there’s ever been any complication of that. The machine algorithms are so good.
Dr. Glatter: Mick, I want to turn to you about the European experience. Specifically in Denmark, we know that there’s a large public health initiative to have AEDs accessible. There have been studies showing that when the public is engaged, especially with studies looking at an app when access is available, survivability doubled in the past 10 years from having access to AEDs. What’s your experience in Ireland in terms of public access to defibrillators?
Dr. Molloy: We’ve got two different streams here. There was a big push to have more AEDs at all sports venues. That was great, but some of the sporting clubs put them inside the locked door. I said that there’s no point to that because nobody can access it. You need to have an external building and you need to leave it open. If somebody needs to use it, they need to know how to get it, open it, and get away, and not get in through a locked door to get access to a defibrillator. We have AEDs now in most stadiums and even in small rural areas, where you might have only 200 people turn up for a game.
From another public access side, if you dial in – in our scenario, it’s 112, not 911 –we have Community First Responder groups. In the rural areas, you have local people who’ve been trained in basic life support and community first response who have AEDs. They’ll have periods of the day where they come home from work as a teacher, a nurse, a policeman, or a fireman, and they turn on an app on their phone and say, “I’m available for the next 5 hours.” If there’s a cardiac arrest rung in within 5 miles of their community, they will drive directly there with the AED that they have. We’ve had numerous saves from that in the country because it could take 40 minutes to get an EMS vehicle there, and obviously, time is crucial in these scenarios. Our dispatchers will talk people through CPR, and then the community responders arrive with the AED. It has been a fantastic initiative.
Dr. Pepe: In many places, people have apps on their phones where they’re locked into the system, and it will go off and tell them there is something nearby and even GPS them into it, and it’s been fantastic.
The two points I want to make to responding to what we just heard Dean Malloy say is one, we always have a designated spot to have these in various places. If I’m at City Hall, we always have them near the red elevators on every floor and down at security. In all the public high schools, we always have one right below the clock where everybody can see it. We set it up in a very standardized form that anybody and everybody will know where it is at the time an event happens.
The other point he made about having the response teams is fantastic. I live in a large high rise and there are two complexes with many people here, and many are older, so there’s going to be a higher risk for having an event. In fact, we’ve just had one recently. The concept we developed here was a community emergency response team, where we sometimes have doctors, nurses, and paramedics who live here be on call and be responsible, or you could try to find an AED. More importantly, we made sure everybody here knew where they were and where to get them. We’ve got most of the people trained, and we’re doing more training in what actions to take during these periods of time when such events happen.
Dr. Glatter: Yes, it’s critical. I wanted to point out that we’ve looked at the use of drones, especially here in the United States. There have been some pilot studies looking at their utility in the setting of out-of-hospital cardiac arrest. I want to get both of your thoughts on this and the feasibility of this.
Dr. Molloy: In a rural area, it’s a fantastic idea. You’re going to get something there as the crow flies very quickly. You probably have to look at exactly in, say, a rural area like Ireland of 32,000 square kilometers, how many you›ll have to put, what kind of distances they can realistically cover, and make sure the batteries are charged. Certainly, that’s a very good initiative because with the AEDs, you can’t do anything wrong. You can’t give a shock unless a shock needs to be given. The machine directs you what to do, so somebody who has had no training can pick one of these out of the box and start to work with it quickly and confidently that they can’t do anything wrong.
It’s a great idea. It would be a little expensive potentially at the moment in getting the drones and having that volume of drones around. In the U.S., you have completely different air traffic than we have, and in cities, you have more helicopters flying around. We certainly wouldn’t have that in our cities because that could cause a challenge if you’ve got drones flying around as well. It’s about making it safe that nothing else can go wrong from a drone in somebody else’s flight path.
Dr. Pepe: In my experience, the earlier the intervention, the better the results. There is a limit here in terms of the drones if they just can’t get there soon enough. Having said that, we are so fortunate in the city of Seattle to have most citizens knowing CPR, and we’d get that person resuscitated because they were doing such a good job with the CPR up front.
That’s why you’re going to see the Buffalo Bills player survive neurologically intact – because he did get immediate treatment right then and there. In the future, we may even have some better devices that will actually even restore normal blood flow right then and there while you’re still in cardiac arrest. There are limitations in every case. But on the other hand, it’s exciting and it paid off in this case recently.
Dr. Molloy: Just a point of interest coming from this small little country over here. The first portable defibrillator was developed in Belfast, Ireland, in the back of a cardiac response car. Despite us being a tiny little country, we do have some advances ahead of the United States.
Dr. Pepe: That was a breakthrough. Dr. Frank Pantridge and John Geddes did this great work and that caught the imagination of everybody here. At first, they were just going out to give people oxygen and sedate them for their chest pain. It turned out that their defibrillators are what made the difference as they went out there. Absolutely, I have to acknowledge the folks in Ireland for giving us this. Many of the EMS systems got started because of the article they published in The Lancet back in 1967.
Dr. Glatter: I wanted to briefly talk about screening of the athletes at the high school/college level, but also at the professional level. Obviously, there are issues, including the risk for false-positives in terms of low incidence, but there are also false negatives, as the case with Christian Eriksen, who had a cardiac arrest in 2021 and who has been through extensive testing. We can debate the validity of such testing, but I wanted to get both of your takes on the utility of screening in such a population.
Dr. Molloy: That’s a very emotive subject. False-positives are difficult because you’re now saying to somebody that they can’t compete in your sport at a decent level. The difficult part is telling somebody that this is the end of their career.
The false-negative is a little bit more difficult. I don’t know Christian Eriksen and I’m not involved in his team in any way, but that is a one-point examination, and you’re dependent on the scale of the process interpreting the ECG, which is again only a couple of seconds and that particular arrhythmia may not have shown up on that.
Also, athletes, by nature of what they’re doing, are operating at 99% of efficiency on a frequent basis. They are at the peak of their physiologic fitness, and it does make them a little bit more prone to picking up viral illnesses from time to time. They may get a small viral myopericarditis, which causes a new arrhythmia that nobody knew about. They had the screening 2 or 3 years ago, and they now developed a new problem because of what they do, which just may not show up.
I was actually surprised that the gentleman came through it very well, which is fantastic. He wasn’t allowed to play football in the country where he was employed, and he has now moved to another country and is playing football with a defibrillator inserted. I don’t know what the rules are in American football where you can play with implantable defibrillators. I’m not so sure it’s a great idea to do that.
Dr. Pepe: One thing that we should bring up is that there are athletes with underlying cardiomyopathies or hypertrophies and things like that, but that was unlikely in this case. It’s possible, but it’s unlikely, because it would have manifested itself before. In terms of screening, I’ve met some very smart medical doctors who have run those tests, and they have been very encouraged even at the high school levels to have screenings done, whether it’s electrocardiography, echocardiography, and so on. I have to reiterate what Dr Malloy just said in that it may have its downsides as well. If you can pick up real obvious cases, I think that may be of value.
Dr. Glatter: I want to conclude and get some pearls and takeaways from each of you regarding the events that transpired and what our audience can really hold onto.
Dr. Molloy: Look at Formula One in the past 50 years. In Formula One, in the beginning it was a 2-minute job to change a tire. Now, they have this down where they’re measuring in fractions of a second and criticizing each other if one guy is 2.6 seconds and the other guy is 2.9 seconds. For me, that’s phenomenal. It takes me 25 minutes to change a tire.
We’ve looked at that from a resuscitation perspective, and we now do pit crew resuscitation before our events. We’ve planned our team and know who’s going to be occupying what role. After the events at the UEFA championships, we had a new rule brought in by UEFA where they handed me a new document saying, “This is what we would like you to do for resuscitation.” It was a three-man triangle, and I said, “No, we’re not going to do that here.” And they said, “Why, you have to; it’s our rule.”
I said, “No, our rule in Ireland is we have a six-person triangle. We’re not downing our standards because of what you have internationally. You’re covering games in some very low-resource environments, I know that. We have a particular standard here that we’re sticking to. We have a six-person group. We know what we’re all doing; we come very quickly to those downed players and get involved and we’ve had good outcomes, so we’re not going to change the standards.”
That’s the thing: You need to practice these things. The players don’t go out on the weekend and do a move for the very first time without practicing it hundreds of times. We need to look at it the same way as the medical team who are looking after that group of players and the crowd because we also look after the crowd.
A particular challenge in some of our stadiums is that the upper decks are so steep, and it’s very hard to get a patient onto a trolley and do CPR as you’re bringing them down to a zone to get them flat. We’ve had to come up with some innovative techniques to try and do that and accommodate that using some of the mechanical CPR devices. That’s the result you’ll only get from having practiced these events and trying to extricate patients. We want to check response times, so you have to practice your response team activity very frequently.
Dr. Pepe: There are two points made by Mick that I want to react to. One, the pit crew approach is critical in so many ways. We do the same thing in what we call the medical first attack, where we knew who the A, B, and C person would be. When we took it out to the NBA trainers, I recommended for them to have a similar approach so that if an event does happen right in the middle of prime time, they are coordinated.
The second point is that we do mass-gathering medicine. It’s not just the sportspeople on the field or the entertainers that we’re looking after; it is the people in the stands. We will see a cardiac arrest once a month. If you think about it, you might see a cardiac arrest occur in any community on a regular basis. Now you’ve got 100,000 people in one stadium, and something is bound to go wrong over those 3 or 4 hours where they are there and may have a critical emergency. Preparation for all of that is really important as well.
The final point is that on a day-to-day basis, most cardiac arrests do occur in the home. Granted, 80% of them are nonshockable cases, but the people who are more apt to survive are going to be the ones who have an electrical event. In fact, when we looked at our data years ago, we found that, of the cases of people with ventricular fibrillation that we resuscitated, half didn’t even have heart damage. Their enzymes were normal. It was a pure electrical event, and they were more resuscitable. They may have an underlying problem, but we can fix that once we get them back.
Everybody needs to know how to do bystander CPR, and second, we must make sure we have AEDs strategically placed, as I alluded to before. We also go out to other parts of the community and give them advice. All those things must be put in place, but more importantly, just get the training and make the training simple. It’s really a “just do it” philosophy, but make it simple.
For example, when I teach a course, I can do it in 15 minutes, and people retain it because I keep reiterating things like, “Okay, there’s one thing you need to know about choking: Pop the cork.” You give them a physiologic image of what’s happening. Everybody says, “I remember you saying to just do it, pop the cork.”
With AEDs, know where it is – that’s why we should have it in standardized places. Go get it, turn it on, and then follow the instructions. Also, the most important thing is making sure you’re doing quality compressions; and there are videos that can help you with that, as well as classes that you can take that will get you through it.
Dr. Glatter: Absolutely. The public still has the misconception that you need to do mouth-to-mouth resuscitation. The message has not permeated through society that you don’t need to do mouth-to-mouth. Hands-only CPR is the gold standard now.
Dr. Pepe: If people have a reversible cause like ventricular fibrillation, often they’re already gasping, which is better than a delivered breath, by the way. Most important, then, are the compressions to make sure you have oxygen going up to the brain, because you’re still theoretically loaded with oxygen in your bloodstream if you had a sudden cardiac arrest from a ventricular fibrillation.
Your points are well taken, and we found that we had better outcomes when we just gave instructions to do compressions only, and that became the standard. Mick, you’ve had some experiences with that as well.
Dr. Molloy: If we’re going to have a long-term benefit from all this, we have to start doing this in elementary school and teaching kids basic life support and some basic health messaging.
I remember trying to get this across to a teacher one day and the teacher saying, “But why would we teach young kids to resuscitate each other?” I said, “I think you forget that the only 60-year-old person in the room is you. You train them, and we train them. They’re the ones who are going to respond and keep you alive. That’s the way you should be looking at this.” That completely changed the mindset of whether we should be doing this for the kids or not.
Dr. Pepe: In fact, what we find is that that’s exactly who gets saved. I had case after case where the kids at the school had learned CPR and saved the teachers or the administrator at the high school or elementary school. It’s a fantastic point that you bring up, Dr. Malloy.
Dr. Glatter: One other brief thing we can interject here is that the team was excellent on field in that they evaluated Damar Hamlin in a primary survey sense of ABCs (i.e., airway, breathing, and circulation) for things like a tension pneumothorax. In the sense in which he was hit, there are reversible causes. Making sure he didn’t have a tension pneumothorax that caused the arrest, in my mind, was critical.
Dr. Pepe: We do the same thing on a day-to-day basis with a car wreck, because it could be that the person had ventricular fibrillation and then had the wreck. It’s not always trauma. That’s a fantastic point that you’re making. That’s exactly what I think happened, and that’s what we do.
Dr. Glatter: Well, thank you, gentlemen. This was an informative and helpful discussion for our audience. I appreciate your time and expertise.
Dr. Glatter, is an attending physician at Lenox Hill Hospital in New York City and assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is an editorial adviser and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes.
Dr. Pepe is a professor of internal medicine, surgery, pediatrics, public health, and emergency medicine at University of Texas Health Science Center in Houston. He’s also a global coordinator of the U.S. Metropolitan Municipalities EMS Medical Directors (“Eagles”) Coalition.
Dr. Molloy works clinically as a consultant in emergency medicine in Wexford General Hospital, part of the Ireland East Hospital Group (IEHG). Internationally, he is a member of the Disaster Medicine Section of the European Society of Emergency Medicine (EUSEM) and has been appointed by the Irish Medical Organization (IMO) as one of two Irish delegates to serve on the European Board and Section of Emergency Medicine of the European Union of Medical Specialists (UEMS), having served for a number of years on its predecessor, the Multidisciplinary Joint Committee on Emergency Medicine.
A version of this article first appeared on Medscape.com.
This discussion was recorded on Jan. 9, 2023. This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert D. Glatter, medical adviser for Medscape Emergency Medicine. Today, we have Dr. Paul E. Pepe, an emergency medicine physician based in Florida and a highly recognized expert in emergency medical services (EMS), critical care, sports and event medicine, and resuscitation. Also joining us is Dr. Michael S. (“Mick”) Malloy, an emergency medicine physician based in Ireland, also an expert in prehospital care, resuscitation, and sports and event medicine. Welcome, gentlemen.
Dr. Pepe: Thanks for having us here.
Dr. Glatter: the Buffalo Bills safety who went down suffering a cardiac arrest in front of millions of people. Although we don’t know the exact cause of the events that transpired, the goal of our discussion is to guide our audience through a systematic approach to evaluation and management of an athlete suffering blunt force chest and neck trauma, and then suffering a cardiac arrest. We do know, obviously, that Damar was successfully resuscitated, thanks to the medical staff and trainers.
Almost 50 years ago, Chuck Hughes, a Detroit Lions receiver, went down and died with just a minute to go in the game and, unfortunately, didn’t survive.
Paul, can you tell me your impressions after viewing the replay of the events that evening? What were the most likely causes of this syncopal event and the subsequent cardiac arrest?
Dr. Pepe: We don’t know anything specifically. It’s being kept private about what the events were. It’s a little bit complicated in a sense that he basically had an extended resuscitation in the hospital. My experience has been that most people that have ventricular fibrillation, from whatever cause, will most likely be waking up on the field if you get to them. I’ve had personal experience with that.
More importantly than when it starts, when someone goes down on the field, both Dr. Malloy and I take a broader view. We don’t get tunnel vision and think, “Oh, it was a traumatic event,” or “It was cardiac event,” and we just have our minds open. There are many things that could make you stop breathing on the field. It could be a neck or a severe head injury, and then any kind of other internal injury that occurs.
When I saw in the video that Damar Hamlin stood up, that made it a less likely to be a spinal injury. He seemed to be physically functioning, and then he suddenly collapsed. That went along with something that looks like a ventricular fibrillation or ventricular tachycardia type of event and made me think right away that it was commotio cordis. I’m not a Latin scholar, but commotio is like commotion. A literal translation might be an agitation of the heart. I was thinking that he probably got hit somewhere in the middle of the chest at the right moment where the heart is resetting in that repolarization phase, like an R-on-T phenomenon, and then caused this sudden ventricular dysrhythmia.
Most people associate it to that because we have a couple of dozen cases a year of people getting hockey pucks or a baseball hitting their chest, which is very common with adolescents. On the other hand, you can’t get it from a blunt injury like this, and it was too early for it to be, say, a direct cardiac contusion, unless there was a direct injury there. It just happened so quickly.
In Europe, they’ve had a large amount of experience with this same kind of problem before, even just from a direct shoulder hit, for example. Mick Malloy is the dean of the faculty of sports and exercise medicine at the Royal College of Surgeons in Ireland and has vast experience, and now he is the person overseeing the procedures for this. Mick, have you had those kinds of experiences as well?
Dr. Molloy: Yes. It’s something that has occurred over recent decades and has been more recognized. I note that in professional sports, it’s a very different thing because you’ve got such huge teams and teams trained to respond very quickly. And that’s the most important thing in this scenario – having a team that is well functioning as a high-class emergency response team ready to get out on to that field very quickly after the person collapses, getting the automated external defibrillator (AED) on, and then recognizing whether there needs to be a shock given or not. The machine will tell you all that.
In our scenario, we run courses called CARES (Care of the Athlete Resuscitation and Emergencies in Sport) to make sure that our team physicians and team physiotherapists and trainers are all speaking as one when an emergency arises.
I don’t worry so much about the professional sport. It’s more with the amateur sports and the kids sports that I get a bit more concerned because there isn’t the same level of medical care there. Having everybody trained in basic life support would be very important to reduce unnecessary deaths from these types of conditions.
As Paul mentioned, there is a very specific cardiac cause in some of these circumstances, where you get hit just at the wrong time and that hit occurs at a particular electrical point in time. It causes this ventricular fibrillation, and the only real treatment there is the defibrillator as quickly as possible.
Dr. Glatter: What you’re saying ultimately is an important part about rapid defibrillation, and at first, cardiopulmonary resuscitation (CPR). People are concerned about whether they should begin CPR. We’re talking about out-of-hospital cardiac arrest that is outside of a football stadium, for example. Some people are obsessed with taking a person’s pulse, and that’s been a point of contention. If someone is unconscious and not breathing, we should start CPR. Wouldn›t you agree? They will wake up quickly if you begin chest compressions if they’re not necessary.
Dr. Pepe: I tell people, just do it. You’re right, people will wake up and feel it if they don’t need it.
Getting back to Mick’s point of having things ready to go, for example, 8 years ago, we had a professional player on the bench who suddenly collapsed right there in front of the entire audience. We immediately did CPR, and we got the AED on. We shocked him and he was ready, willing, and able to get back on the bench again. It turns out he had underlying coronary artery disease, but we got him back right away.
I did an initial study where we placed an AED in a public place at the Chicago O’Hare Airport to see if the public would use these. Most cardiac arrests occur at home, of course, but in public places, that was a good place to try it. We had almost 10 cases the first year. What was fascinating was that we had almost no survivors over the previous decade, even though there were paramedics at the airport. When we put these out there, we had nine people go down that first year, and six people who had never operated an AED or seen one before knew to get one and use it. Every one of those people survived neurologically intact, and almost every person was waking up before traditional responders got there. That’s how effective this is, but you need to know where the AED is.
Dr. Glatter: How to turn it on, where it is, and how to operate it.
Dr. Pepe: That was the point: These rescuers saved lives in the first year, and it was tremendous. Two points I make about it are that one, you need to know where it is, and two, just go turn it on. It gives you the instructions to follow through; just be in the Nike mode, because it basically won’t hurt a person. It’s rare that there’s ever been any complication of that. The machine algorithms are so good.
Dr. Glatter: Mick, I want to turn to you about the European experience. Specifically in Denmark, we know that there’s a large public health initiative to have AEDs accessible. There have been studies showing that when the public is engaged, especially with studies looking at an app when access is available, survivability doubled in the past 10 years from having access to AEDs. What’s your experience in Ireland in terms of public access to defibrillators?
Dr. Molloy: We’ve got two different streams here. There was a big push to have more AEDs at all sports venues. That was great, but some of the sporting clubs put them inside the locked door. I said that there’s no point to that because nobody can access it. You need to have an external building and you need to leave it open. If somebody needs to use it, they need to know how to get it, open it, and get away, and not get in through a locked door to get access to a defibrillator. We have AEDs now in most stadiums and even in small rural areas, where you might have only 200 people turn up for a game.
From another public access side, if you dial in – in our scenario, it’s 112, not 911 –we have Community First Responder groups. In the rural areas, you have local people who’ve been trained in basic life support and community first response who have AEDs. They’ll have periods of the day where they come home from work as a teacher, a nurse, a policeman, or a fireman, and they turn on an app on their phone and say, “I’m available for the next 5 hours.” If there’s a cardiac arrest rung in within 5 miles of their community, they will drive directly there with the AED that they have. We’ve had numerous saves from that in the country because it could take 40 minutes to get an EMS vehicle there, and obviously, time is crucial in these scenarios. Our dispatchers will talk people through CPR, and then the community responders arrive with the AED. It has been a fantastic initiative.
Dr. Pepe: In many places, people have apps on their phones where they’re locked into the system, and it will go off and tell them there is something nearby and even GPS them into it, and it’s been fantastic.
The two points I want to make to responding to what we just heard Dean Malloy say is one, we always have a designated spot to have these in various places. If I’m at City Hall, we always have them near the red elevators on every floor and down at security. In all the public high schools, we always have one right below the clock where everybody can see it. We set it up in a very standardized form that anybody and everybody will know where it is at the time an event happens.
The other point he made about having the response teams is fantastic. I live in a large high rise and there are two complexes with many people here, and many are older, so there’s going to be a higher risk for having an event. In fact, we’ve just had one recently. The concept we developed here was a community emergency response team, where we sometimes have doctors, nurses, and paramedics who live here be on call and be responsible, or you could try to find an AED. More importantly, we made sure everybody here knew where they were and where to get them. We’ve got most of the people trained, and we’re doing more training in what actions to take during these periods of time when such events happen.
Dr. Glatter: Yes, it’s critical. I wanted to point out that we’ve looked at the use of drones, especially here in the United States. There have been some pilot studies looking at their utility in the setting of out-of-hospital cardiac arrest. I want to get both of your thoughts on this and the feasibility of this.
Dr. Molloy: In a rural area, it’s a fantastic idea. You’re going to get something there as the crow flies very quickly. You probably have to look at exactly in, say, a rural area like Ireland of 32,000 square kilometers, how many you›ll have to put, what kind of distances they can realistically cover, and make sure the batteries are charged. Certainly, that’s a very good initiative because with the AEDs, you can’t do anything wrong. You can’t give a shock unless a shock needs to be given. The machine directs you what to do, so somebody who has had no training can pick one of these out of the box and start to work with it quickly and confidently that they can’t do anything wrong.
It’s a great idea. It would be a little expensive potentially at the moment in getting the drones and having that volume of drones around. In the U.S., you have completely different air traffic than we have, and in cities, you have more helicopters flying around. We certainly wouldn’t have that in our cities because that could cause a challenge if you’ve got drones flying around as well. It’s about making it safe that nothing else can go wrong from a drone in somebody else’s flight path.
Dr. Pepe: In my experience, the earlier the intervention, the better the results. There is a limit here in terms of the drones if they just can’t get there soon enough. Having said that, we are so fortunate in the city of Seattle to have most citizens knowing CPR, and we’d get that person resuscitated because they were doing such a good job with the CPR up front.
That’s why you’re going to see the Buffalo Bills player survive neurologically intact – because he did get immediate treatment right then and there. In the future, we may even have some better devices that will actually even restore normal blood flow right then and there while you’re still in cardiac arrest. There are limitations in every case. But on the other hand, it’s exciting and it paid off in this case recently.
Dr. Molloy: Just a point of interest coming from this small little country over here. The first portable defibrillator was developed in Belfast, Ireland, in the back of a cardiac response car. Despite us being a tiny little country, we do have some advances ahead of the United States.
Dr. Pepe: That was a breakthrough. Dr. Frank Pantridge and John Geddes did this great work and that caught the imagination of everybody here. At first, they were just going out to give people oxygen and sedate them for their chest pain. It turned out that their defibrillators are what made the difference as they went out there. Absolutely, I have to acknowledge the folks in Ireland for giving us this. Many of the EMS systems got started because of the article they published in The Lancet back in 1967.
Dr. Glatter: I wanted to briefly talk about screening of the athletes at the high school/college level, but also at the professional level. Obviously, there are issues, including the risk for false-positives in terms of low incidence, but there are also false negatives, as the case with Christian Eriksen, who had a cardiac arrest in 2021 and who has been through extensive testing. We can debate the validity of such testing, but I wanted to get both of your takes on the utility of screening in such a population.
Dr. Molloy: That’s a very emotive subject. False-positives are difficult because you’re now saying to somebody that they can’t compete in your sport at a decent level. The difficult part is telling somebody that this is the end of their career.
The false-negative is a little bit more difficult. I don’t know Christian Eriksen and I’m not involved in his team in any way, but that is a one-point examination, and you’re dependent on the scale of the process interpreting the ECG, which is again only a couple of seconds and that particular arrhythmia may not have shown up on that.
Also, athletes, by nature of what they’re doing, are operating at 99% of efficiency on a frequent basis. They are at the peak of their physiologic fitness, and it does make them a little bit more prone to picking up viral illnesses from time to time. They may get a small viral myopericarditis, which causes a new arrhythmia that nobody knew about. They had the screening 2 or 3 years ago, and they now developed a new problem because of what they do, which just may not show up.
I was actually surprised that the gentleman came through it very well, which is fantastic. He wasn’t allowed to play football in the country where he was employed, and he has now moved to another country and is playing football with a defibrillator inserted. I don’t know what the rules are in American football where you can play with implantable defibrillators. I’m not so sure it’s a great idea to do that.
Dr. Pepe: One thing that we should bring up is that there are athletes with underlying cardiomyopathies or hypertrophies and things like that, but that was unlikely in this case. It’s possible, but it’s unlikely, because it would have manifested itself before. In terms of screening, I’ve met some very smart medical doctors who have run those tests, and they have been very encouraged even at the high school levels to have screenings done, whether it’s electrocardiography, echocardiography, and so on. I have to reiterate what Dr Malloy just said in that it may have its downsides as well. If you can pick up real obvious cases, I think that may be of value.
Dr. Glatter: I want to conclude and get some pearls and takeaways from each of you regarding the events that transpired and what our audience can really hold onto.
Dr. Molloy: Look at Formula One in the past 50 years. In Formula One, in the beginning it was a 2-minute job to change a tire. Now, they have this down where they’re measuring in fractions of a second and criticizing each other if one guy is 2.6 seconds and the other guy is 2.9 seconds. For me, that’s phenomenal. It takes me 25 minutes to change a tire.
We’ve looked at that from a resuscitation perspective, and we now do pit crew resuscitation before our events. We’ve planned our team and know who’s going to be occupying what role. After the events at the UEFA championships, we had a new rule brought in by UEFA where they handed me a new document saying, “This is what we would like you to do for resuscitation.” It was a three-man triangle, and I said, “No, we’re not going to do that here.” And they said, “Why, you have to; it’s our rule.”
I said, “No, our rule in Ireland is we have a six-person triangle. We’re not downing our standards because of what you have internationally. You’re covering games in some very low-resource environments, I know that. We have a particular standard here that we’re sticking to. We have a six-person group. We know what we’re all doing; we come very quickly to those downed players and get involved and we’ve had good outcomes, so we’re not going to change the standards.”
That’s the thing: You need to practice these things. The players don’t go out on the weekend and do a move for the very first time without practicing it hundreds of times. We need to look at it the same way as the medical team who are looking after that group of players and the crowd because we also look after the crowd.
A particular challenge in some of our stadiums is that the upper decks are so steep, and it’s very hard to get a patient onto a trolley and do CPR as you’re bringing them down to a zone to get them flat. We’ve had to come up with some innovative techniques to try and do that and accommodate that using some of the mechanical CPR devices. That’s the result you’ll only get from having practiced these events and trying to extricate patients. We want to check response times, so you have to practice your response team activity very frequently.
Dr. Pepe: There are two points made by Mick that I want to react to. One, the pit crew approach is critical in so many ways. We do the same thing in what we call the medical first attack, where we knew who the A, B, and C person would be. When we took it out to the NBA trainers, I recommended for them to have a similar approach so that if an event does happen right in the middle of prime time, they are coordinated.
The second point is that we do mass-gathering medicine. It’s not just the sportspeople on the field or the entertainers that we’re looking after; it is the people in the stands. We will see a cardiac arrest once a month. If you think about it, you might see a cardiac arrest occur in any community on a regular basis. Now you’ve got 100,000 people in one stadium, and something is bound to go wrong over those 3 or 4 hours where they are there and may have a critical emergency. Preparation for all of that is really important as well.
The final point is that on a day-to-day basis, most cardiac arrests do occur in the home. Granted, 80% of them are nonshockable cases, but the people who are more apt to survive are going to be the ones who have an electrical event. In fact, when we looked at our data years ago, we found that, of the cases of people with ventricular fibrillation that we resuscitated, half didn’t even have heart damage. Their enzymes were normal. It was a pure electrical event, and they were more resuscitable. They may have an underlying problem, but we can fix that once we get them back.
Everybody needs to know how to do bystander CPR, and second, we must make sure we have AEDs strategically placed, as I alluded to before. We also go out to other parts of the community and give them advice. All those things must be put in place, but more importantly, just get the training and make the training simple. It’s really a “just do it” philosophy, but make it simple.
For example, when I teach a course, I can do it in 15 minutes, and people retain it because I keep reiterating things like, “Okay, there’s one thing you need to know about choking: Pop the cork.” You give them a physiologic image of what’s happening. Everybody says, “I remember you saying to just do it, pop the cork.”
With AEDs, know where it is – that’s why we should have it in standardized places. Go get it, turn it on, and then follow the instructions. Also, the most important thing is making sure you’re doing quality compressions; and there are videos that can help you with that, as well as classes that you can take that will get you through it.
Dr. Glatter: Absolutely. The public still has the misconception that you need to do mouth-to-mouth resuscitation. The message has not permeated through society that you don’t need to do mouth-to-mouth. Hands-only CPR is the gold standard now.
Dr. Pepe: If people have a reversible cause like ventricular fibrillation, often they’re already gasping, which is better than a delivered breath, by the way. Most important, then, are the compressions to make sure you have oxygen going up to the brain, because you’re still theoretically loaded with oxygen in your bloodstream if you had a sudden cardiac arrest from a ventricular fibrillation.
Your points are well taken, and we found that we had better outcomes when we just gave instructions to do compressions only, and that became the standard. Mick, you’ve had some experiences with that as well.
Dr. Molloy: If we’re going to have a long-term benefit from all this, we have to start doing this in elementary school and teaching kids basic life support and some basic health messaging.
I remember trying to get this across to a teacher one day and the teacher saying, “But why would we teach young kids to resuscitate each other?” I said, “I think you forget that the only 60-year-old person in the room is you. You train them, and we train them. They’re the ones who are going to respond and keep you alive. That’s the way you should be looking at this.” That completely changed the mindset of whether we should be doing this for the kids or not.
Dr. Pepe: In fact, what we find is that that’s exactly who gets saved. I had case after case where the kids at the school had learned CPR and saved the teachers or the administrator at the high school or elementary school. It’s a fantastic point that you bring up, Dr. Malloy.
Dr. Glatter: One other brief thing we can interject here is that the team was excellent on field in that they evaluated Damar Hamlin in a primary survey sense of ABCs (i.e., airway, breathing, and circulation) for things like a tension pneumothorax. In the sense in which he was hit, there are reversible causes. Making sure he didn’t have a tension pneumothorax that caused the arrest, in my mind, was critical.
Dr. Pepe: We do the same thing on a day-to-day basis with a car wreck, because it could be that the person had ventricular fibrillation and then had the wreck. It’s not always trauma. That’s a fantastic point that you’re making. That’s exactly what I think happened, and that’s what we do.
Dr. Glatter: Well, thank you, gentlemen. This was an informative and helpful discussion for our audience. I appreciate your time and expertise.
Dr. Glatter, is an attending physician at Lenox Hill Hospital in New York City and assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is an editorial adviser and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes.
Dr. Pepe is a professor of internal medicine, surgery, pediatrics, public health, and emergency medicine at University of Texas Health Science Center in Houston. He’s also a global coordinator of the U.S. Metropolitan Municipalities EMS Medical Directors (“Eagles”) Coalition.
Dr. Molloy works clinically as a consultant in emergency medicine in Wexford General Hospital, part of the Ireland East Hospital Group (IEHG). Internationally, he is a member of the Disaster Medicine Section of the European Society of Emergency Medicine (EUSEM) and has been appointed by the Irish Medical Organization (IMO) as one of two Irish delegates to serve on the European Board and Section of Emergency Medicine of the European Union of Medical Specialists (UEMS), having served for a number of years on its predecessor, the Multidisciplinary Joint Committee on Emergency Medicine.
A version of this article first appeared on Medscape.com.
This discussion was recorded on Jan. 9, 2023. This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert D. Glatter, medical adviser for Medscape Emergency Medicine. Today, we have Dr. Paul E. Pepe, an emergency medicine physician based in Florida and a highly recognized expert in emergency medical services (EMS), critical care, sports and event medicine, and resuscitation. Also joining us is Dr. Michael S. (“Mick”) Malloy, an emergency medicine physician based in Ireland, also an expert in prehospital care, resuscitation, and sports and event medicine. Welcome, gentlemen.
Dr. Pepe: Thanks for having us here.
Dr. Glatter: the Buffalo Bills safety who went down suffering a cardiac arrest in front of millions of people. Although we don’t know the exact cause of the events that transpired, the goal of our discussion is to guide our audience through a systematic approach to evaluation and management of an athlete suffering blunt force chest and neck trauma, and then suffering a cardiac arrest. We do know, obviously, that Damar was successfully resuscitated, thanks to the medical staff and trainers.
Almost 50 years ago, Chuck Hughes, a Detroit Lions receiver, went down and died with just a minute to go in the game and, unfortunately, didn’t survive.
Paul, can you tell me your impressions after viewing the replay of the events that evening? What were the most likely causes of this syncopal event and the subsequent cardiac arrest?
Dr. Pepe: We don’t know anything specifically. It’s being kept private about what the events were. It’s a little bit complicated in a sense that he basically had an extended resuscitation in the hospital. My experience has been that most people that have ventricular fibrillation, from whatever cause, will most likely be waking up on the field if you get to them. I’ve had personal experience with that.
More importantly than when it starts, when someone goes down on the field, both Dr. Malloy and I take a broader view. We don’t get tunnel vision and think, “Oh, it was a traumatic event,” or “It was cardiac event,” and we just have our minds open. There are many things that could make you stop breathing on the field. It could be a neck or a severe head injury, and then any kind of other internal injury that occurs.
When I saw in the video that Damar Hamlin stood up, that made it a less likely to be a spinal injury. He seemed to be physically functioning, and then he suddenly collapsed. That went along with something that looks like a ventricular fibrillation or ventricular tachycardia type of event and made me think right away that it was commotio cordis. I’m not a Latin scholar, but commotio is like commotion. A literal translation might be an agitation of the heart. I was thinking that he probably got hit somewhere in the middle of the chest at the right moment where the heart is resetting in that repolarization phase, like an R-on-T phenomenon, and then caused this sudden ventricular dysrhythmia.
Most people associate it to that because we have a couple of dozen cases a year of people getting hockey pucks or a baseball hitting their chest, which is very common with adolescents. On the other hand, you can’t get it from a blunt injury like this, and it was too early for it to be, say, a direct cardiac contusion, unless there was a direct injury there. It just happened so quickly.
In Europe, they’ve had a large amount of experience with this same kind of problem before, even just from a direct shoulder hit, for example. Mick Malloy is the dean of the faculty of sports and exercise medicine at the Royal College of Surgeons in Ireland and has vast experience, and now he is the person overseeing the procedures for this. Mick, have you had those kinds of experiences as well?
Dr. Molloy: Yes. It’s something that has occurred over recent decades and has been more recognized. I note that in professional sports, it’s a very different thing because you’ve got such huge teams and teams trained to respond very quickly. And that’s the most important thing in this scenario – having a team that is well functioning as a high-class emergency response team ready to get out on to that field very quickly after the person collapses, getting the automated external defibrillator (AED) on, and then recognizing whether there needs to be a shock given or not. The machine will tell you all that.
In our scenario, we run courses called CARES (Care of the Athlete Resuscitation and Emergencies in Sport) to make sure that our team physicians and team physiotherapists and trainers are all speaking as one when an emergency arises.
I don’t worry so much about the professional sport. It’s more with the amateur sports and the kids sports that I get a bit more concerned because there isn’t the same level of medical care there. Having everybody trained in basic life support would be very important to reduce unnecessary deaths from these types of conditions.
As Paul mentioned, there is a very specific cardiac cause in some of these circumstances, where you get hit just at the wrong time and that hit occurs at a particular electrical point in time. It causes this ventricular fibrillation, and the only real treatment there is the defibrillator as quickly as possible.
Dr. Glatter: What you’re saying ultimately is an important part about rapid defibrillation, and at first, cardiopulmonary resuscitation (CPR). People are concerned about whether they should begin CPR. We’re talking about out-of-hospital cardiac arrest that is outside of a football stadium, for example. Some people are obsessed with taking a person’s pulse, and that’s been a point of contention. If someone is unconscious and not breathing, we should start CPR. Wouldn›t you agree? They will wake up quickly if you begin chest compressions if they’re not necessary.
Dr. Pepe: I tell people, just do it. You’re right, people will wake up and feel it if they don’t need it.
Getting back to Mick’s point of having things ready to go, for example, 8 years ago, we had a professional player on the bench who suddenly collapsed right there in front of the entire audience. We immediately did CPR, and we got the AED on. We shocked him and he was ready, willing, and able to get back on the bench again. It turns out he had underlying coronary artery disease, but we got him back right away.
I did an initial study where we placed an AED in a public place at the Chicago O’Hare Airport to see if the public would use these. Most cardiac arrests occur at home, of course, but in public places, that was a good place to try it. We had almost 10 cases the first year. What was fascinating was that we had almost no survivors over the previous decade, even though there were paramedics at the airport. When we put these out there, we had nine people go down that first year, and six people who had never operated an AED or seen one before knew to get one and use it. Every one of those people survived neurologically intact, and almost every person was waking up before traditional responders got there. That’s how effective this is, but you need to know where the AED is.
Dr. Glatter: How to turn it on, where it is, and how to operate it.
Dr. Pepe: That was the point: These rescuers saved lives in the first year, and it was tremendous. Two points I make about it are that one, you need to know where it is, and two, just go turn it on. It gives you the instructions to follow through; just be in the Nike mode, because it basically won’t hurt a person. It’s rare that there’s ever been any complication of that. The machine algorithms are so good.
Dr. Glatter: Mick, I want to turn to you about the European experience. Specifically in Denmark, we know that there’s a large public health initiative to have AEDs accessible. There have been studies showing that when the public is engaged, especially with studies looking at an app when access is available, survivability doubled in the past 10 years from having access to AEDs. What’s your experience in Ireland in terms of public access to defibrillators?
Dr. Molloy: We’ve got two different streams here. There was a big push to have more AEDs at all sports venues. That was great, but some of the sporting clubs put them inside the locked door. I said that there’s no point to that because nobody can access it. You need to have an external building and you need to leave it open. If somebody needs to use it, they need to know how to get it, open it, and get away, and not get in through a locked door to get access to a defibrillator. We have AEDs now in most stadiums and even in small rural areas, where you might have only 200 people turn up for a game.
From another public access side, if you dial in – in our scenario, it’s 112, not 911 –we have Community First Responder groups. In the rural areas, you have local people who’ve been trained in basic life support and community first response who have AEDs. They’ll have periods of the day where they come home from work as a teacher, a nurse, a policeman, or a fireman, and they turn on an app on their phone and say, “I’m available for the next 5 hours.” If there’s a cardiac arrest rung in within 5 miles of their community, they will drive directly there with the AED that they have. We’ve had numerous saves from that in the country because it could take 40 minutes to get an EMS vehicle there, and obviously, time is crucial in these scenarios. Our dispatchers will talk people through CPR, and then the community responders arrive with the AED. It has been a fantastic initiative.
Dr. Pepe: In many places, people have apps on their phones where they’re locked into the system, and it will go off and tell them there is something nearby and even GPS them into it, and it’s been fantastic.
The two points I want to make to responding to what we just heard Dean Malloy say is one, we always have a designated spot to have these in various places. If I’m at City Hall, we always have them near the red elevators on every floor and down at security. In all the public high schools, we always have one right below the clock where everybody can see it. We set it up in a very standardized form that anybody and everybody will know where it is at the time an event happens.
The other point he made about having the response teams is fantastic. I live in a large high rise and there are two complexes with many people here, and many are older, so there’s going to be a higher risk for having an event. In fact, we’ve just had one recently. The concept we developed here was a community emergency response team, where we sometimes have doctors, nurses, and paramedics who live here be on call and be responsible, or you could try to find an AED. More importantly, we made sure everybody here knew where they were and where to get them. We’ve got most of the people trained, and we’re doing more training in what actions to take during these periods of time when such events happen.
Dr. Glatter: Yes, it’s critical. I wanted to point out that we’ve looked at the use of drones, especially here in the United States. There have been some pilot studies looking at their utility in the setting of out-of-hospital cardiac arrest. I want to get both of your thoughts on this and the feasibility of this.
Dr. Molloy: In a rural area, it’s a fantastic idea. You’re going to get something there as the crow flies very quickly. You probably have to look at exactly in, say, a rural area like Ireland of 32,000 square kilometers, how many you›ll have to put, what kind of distances they can realistically cover, and make sure the batteries are charged. Certainly, that’s a very good initiative because with the AEDs, you can’t do anything wrong. You can’t give a shock unless a shock needs to be given. The machine directs you what to do, so somebody who has had no training can pick one of these out of the box and start to work with it quickly and confidently that they can’t do anything wrong.
It’s a great idea. It would be a little expensive potentially at the moment in getting the drones and having that volume of drones around. In the U.S., you have completely different air traffic than we have, and in cities, you have more helicopters flying around. We certainly wouldn’t have that in our cities because that could cause a challenge if you’ve got drones flying around as well. It’s about making it safe that nothing else can go wrong from a drone in somebody else’s flight path.
Dr. Pepe: In my experience, the earlier the intervention, the better the results. There is a limit here in terms of the drones if they just can’t get there soon enough. Having said that, we are so fortunate in the city of Seattle to have most citizens knowing CPR, and we’d get that person resuscitated because they were doing such a good job with the CPR up front.
That’s why you’re going to see the Buffalo Bills player survive neurologically intact – because he did get immediate treatment right then and there. In the future, we may even have some better devices that will actually even restore normal blood flow right then and there while you’re still in cardiac arrest. There are limitations in every case. But on the other hand, it’s exciting and it paid off in this case recently.
Dr. Molloy: Just a point of interest coming from this small little country over here. The first portable defibrillator was developed in Belfast, Ireland, in the back of a cardiac response car. Despite us being a tiny little country, we do have some advances ahead of the United States.
Dr. Pepe: That was a breakthrough. Dr. Frank Pantridge and John Geddes did this great work and that caught the imagination of everybody here. At first, they were just going out to give people oxygen and sedate them for their chest pain. It turned out that their defibrillators are what made the difference as they went out there. Absolutely, I have to acknowledge the folks in Ireland for giving us this. Many of the EMS systems got started because of the article they published in The Lancet back in 1967.
Dr. Glatter: I wanted to briefly talk about screening of the athletes at the high school/college level, but also at the professional level. Obviously, there are issues, including the risk for false-positives in terms of low incidence, but there are also false negatives, as the case with Christian Eriksen, who had a cardiac arrest in 2021 and who has been through extensive testing. We can debate the validity of such testing, but I wanted to get both of your takes on the utility of screening in such a population.
Dr. Molloy: That’s a very emotive subject. False-positives are difficult because you’re now saying to somebody that they can’t compete in your sport at a decent level. The difficult part is telling somebody that this is the end of their career.
The false-negative is a little bit more difficult. I don’t know Christian Eriksen and I’m not involved in his team in any way, but that is a one-point examination, and you’re dependent on the scale of the process interpreting the ECG, which is again only a couple of seconds and that particular arrhythmia may not have shown up on that.
Also, athletes, by nature of what they’re doing, are operating at 99% of efficiency on a frequent basis. They are at the peak of their physiologic fitness, and it does make them a little bit more prone to picking up viral illnesses from time to time. They may get a small viral myopericarditis, which causes a new arrhythmia that nobody knew about. They had the screening 2 or 3 years ago, and they now developed a new problem because of what they do, which just may not show up.
I was actually surprised that the gentleman came through it very well, which is fantastic. He wasn’t allowed to play football in the country where he was employed, and he has now moved to another country and is playing football with a defibrillator inserted. I don’t know what the rules are in American football where you can play with implantable defibrillators. I’m not so sure it’s a great idea to do that.
Dr. Pepe: One thing that we should bring up is that there are athletes with underlying cardiomyopathies or hypertrophies and things like that, but that was unlikely in this case. It’s possible, but it’s unlikely, because it would have manifested itself before. In terms of screening, I’ve met some very smart medical doctors who have run those tests, and they have been very encouraged even at the high school levels to have screenings done, whether it’s electrocardiography, echocardiography, and so on. I have to reiterate what Dr Malloy just said in that it may have its downsides as well. If you can pick up real obvious cases, I think that may be of value.
Dr. Glatter: I want to conclude and get some pearls and takeaways from each of you regarding the events that transpired and what our audience can really hold onto.
Dr. Molloy: Look at Formula One in the past 50 years. In Formula One, in the beginning it was a 2-minute job to change a tire. Now, they have this down where they’re measuring in fractions of a second and criticizing each other if one guy is 2.6 seconds and the other guy is 2.9 seconds. For me, that’s phenomenal. It takes me 25 minutes to change a tire.
We’ve looked at that from a resuscitation perspective, and we now do pit crew resuscitation before our events. We’ve planned our team and know who’s going to be occupying what role. After the events at the UEFA championships, we had a new rule brought in by UEFA where they handed me a new document saying, “This is what we would like you to do for resuscitation.” It was a three-man triangle, and I said, “No, we’re not going to do that here.” And they said, “Why, you have to; it’s our rule.”
I said, “No, our rule in Ireland is we have a six-person triangle. We’re not downing our standards because of what you have internationally. You’re covering games in some very low-resource environments, I know that. We have a particular standard here that we’re sticking to. We have a six-person group. We know what we’re all doing; we come very quickly to those downed players and get involved and we’ve had good outcomes, so we’re not going to change the standards.”
That’s the thing: You need to practice these things. The players don’t go out on the weekend and do a move for the very first time without practicing it hundreds of times. We need to look at it the same way as the medical team who are looking after that group of players and the crowd because we also look after the crowd.
A particular challenge in some of our stadiums is that the upper decks are so steep, and it’s very hard to get a patient onto a trolley and do CPR as you’re bringing them down to a zone to get them flat. We’ve had to come up with some innovative techniques to try and do that and accommodate that using some of the mechanical CPR devices. That’s the result you’ll only get from having practiced these events and trying to extricate patients. We want to check response times, so you have to practice your response team activity very frequently.
Dr. Pepe: There are two points made by Mick that I want to react to. One, the pit crew approach is critical in so many ways. We do the same thing in what we call the medical first attack, where we knew who the A, B, and C person would be. When we took it out to the NBA trainers, I recommended for them to have a similar approach so that if an event does happen right in the middle of prime time, they are coordinated.
The second point is that we do mass-gathering medicine. It’s not just the sportspeople on the field or the entertainers that we’re looking after; it is the people in the stands. We will see a cardiac arrest once a month. If you think about it, you might see a cardiac arrest occur in any community on a regular basis. Now you’ve got 100,000 people in one stadium, and something is bound to go wrong over those 3 or 4 hours where they are there and may have a critical emergency. Preparation for all of that is really important as well.
The final point is that on a day-to-day basis, most cardiac arrests do occur in the home. Granted, 80% of them are nonshockable cases, but the people who are more apt to survive are going to be the ones who have an electrical event. In fact, when we looked at our data years ago, we found that, of the cases of people with ventricular fibrillation that we resuscitated, half didn’t even have heart damage. Their enzymes were normal. It was a pure electrical event, and they were more resuscitable. They may have an underlying problem, but we can fix that once we get them back.
Everybody needs to know how to do bystander CPR, and second, we must make sure we have AEDs strategically placed, as I alluded to before. We also go out to other parts of the community and give them advice. All those things must be put in place, but more importantly, just get the training and make the training simple. It’s really a “just do it” philosophy, but make it simple.
For example, when I teach a course, I can do it in 15 minutes, and people retain it because I keep reiterating things like, “Okay, there’s one thing you need to know about choking: Pop the cork.” You give them a physiologic image of what’s happening. Everybody says, “I remember you saying to just do it, pop the cork.”
With AEDs, know where it is – that’s why we should have it in standardized places. Go get it, turn it on, and then follow the instructions. Also, the most important thing is making sure you’re doing quality compressions; and there are videos that can help you with that, as well as classes that you can take that will get you through it.
Dr. Glatter: Absolutely. The public still has the misconception that you need to do mouth-to-mouth resuscitation. The message has not permeated through society that you don’t need to do mouth-to-mouth. Hands-only CPR is the gold standard now.
Dr. Pepe: If people have a reversible cause like ventricular fibrillation, often they’re already gasping, which is better than a delivered breath, by the way. Most important, then, are the compressions to make sure you have oxygen going up to the brain, because you’re still theoretically loaded with oxygen in your bloodstream if you had a sudden cardiac arrest from a ventricular fibrillation.
Your points are well taken, and we found that we had better outcomes when we just gave instructions to do compressions only, and that became the standard. Mick, you’ve had some experiences with that as well.
Dr. Molloy: If we’re going to have a long-term benefit from all this, we have to start doing this in elementary school and teaching kids basic life support and some basic health messaging.
I remember trying to get this across to a teacher one day and the teacher saying, “But why would we teach young kids to resuscitate each other?” I said, “I think you forget that the only 60-year-old person in the room is you. You train them, and we train them. They’re the ones who are going to respond and keep you alive. That’s the way you should be looking at this.” That completely changed the mindset of whether we should be doing this for the kids or not.
Dr. Pepe: In fact, what we find is that that’s exactly who gets saved. I had case after case where the kids at the school had learned CPR and saved the teachers or the administrator at the high school or elementary school. It’s a fantastic point that you bring up, Dr. Malloy.
Dr. Glatter: One other brief thing we can interject here is that the team was excellent on field in that they evaluated Damar Hamlin in a primary survey sense of ABCs (i.e., airway, breathing, and circulation) for things like a tension pneumothorax. In the sense in which he was hit, there are reversible causes. Making sure he didn’t have a tension pneumothorax that caused the arrest, in my mind, was critical.
Dr. Pepe: We do the same thing on a day-to-day basis with a car wreck, because it could be that the person had ventricular fibrillation and then had the wreck. It’s not always trauma. That’s a fantastic point that you’re making. That’s exactly what I think happened, and that’s what we do.
Dr. Glatter: Well, thank you, gentlemen. This was an informative and helpful discussion for our audience. I appreciate your time and expertise.
Dr. Glatter, is an attending physician at Lenox Hill Hospital in New York City and assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is an editorial adviser and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes.
Dr. Pepe is a professor of internal medicine, surgery, pediatrics, public health, and emergency medicine at University of Texas Health Science Center in Houston. He’s also a global coordinator of the U.S. Metropolitan Municipalities EMS Medical Directors (“Eagles”) Coalition.
Dr. Molloy works clinically as a consultant in emergency medicine in Wexford General Hospital, part of the Ireland East Hospital Group (IEHG). Internationally, he is a member of the Disaster Medicine Section of the European Society of Emergency Medicine (EUSEM) and has been appointed by the Irish Medical Organization (IMO) as one of two Irish delegates to serve on the European Board and Section of Emergency Medicine of the European Union of Medical Specialists (UEMS), having served for a number of years on its predecessor, the Multidisciplinary Joint Committee on Emergency Medicine.
A version of this article first appeared on Medscape.com.
FDA OKs zanubrutinib for CLL or SLL
By giving the nod to these uses of this second-generation Bruton’s tyrosine kinase inhibitor, the FDA expanded on its previous approvals of this drug in mantle cell and marginal zone lymphoma.
“We have seen striking data from the Brukinsa development program demonstrating significant and consistent efficacy across CLL patient subtypes, including the high-risk del17p/TP53-mutated population, and regardless of treatment setting,” Jennifer R. Brown, MD, PhD, of Dana-Farber Cancer Institute in Boston, said in a press release from drug developer BeiGene.
The FDA’s decision was based on two phase 3 trials – SEQUOIA and ALPINE. The SEQUOIA trial assessed 479 patients with treatment-naive CLL/SLL who either received zanubrutinib until disease progression or unacceptable toxicity or bendamustine plus rituximab for six cycles. Median progression-free survival was not reached in the zanubrutinib arm and was 33.7 months in the bendamustine plus rituximab arm (hazard ratio, 0.42).
In a separate, nonrandomized SEQUOIA cohort, investigators assessed zanubrutinib in patients with a 17p deletion and found an overall response rate of 88%. In addition, over the 25-month follow-up, the median duration of response was not reached.
The ALPINE trial included 652 patients with relapsed or refractory CLL/SLL who received either zanubrutinib or ibrutinib. The overall response rate was 80% in the zanubrutinib arm versus 73% in the ibrutinib arm, and the median duration of response was not reached in either arm over the 14-month follow-up period. Median progression-free survival was not reached in the zanubrutinib arm and was 35 months in the ibrutinib group.
Dr. Brown, a lead investigator on both drug trials, suggested that, given the improvements observed in progression-free survival, zanubrutinib could become the standard of care in this setting.
In the ALPINE trial, treatment discontinuation rate was lower among patients receiving zanubrutinib (26%) versus ibrutinib (41.2%), with most discontinuations a result of adverse events or progressive disease.
And across both trials, the most common adverse reactions were decreased neutrophil count (42%), upper respiratory tract infection (39%), decreased platelet count (34%), hemorrhage (30%), and musculoskeletal pain (30%).
A version of this article first appeared on Medscape.com.
By giving the nod to these uses of this second-generation Bruton’s tyrosine kinase inhibitor, the FDA expanded on its previous approvals of this drug in mantle cell and marginal zone lymphoma.
“We have seen striking data from the Brukinsa development program demonstrating significant and consistent efficacy across CLL patient subtypes, including the high-risk del17p/TP53-mutated population, and regardless of treatment setting,” Jennifer R. Brown, MD, PhD, of Dana-Farber Cancer Institute in Boston, said in a press release from drug developer BeiGene.
The FDA’s decision was based on two phase 3 trials – SEQUOIA and ALPINE. The SEQUOIA trial assessed 479 patients with treatment-naive CLL/SLL who either received zanubrutinib until disease progression or unacceptable toxicity or bendamustine plus rituximab for six cycles. Median progression-free survival was not reached in the zanubrutinib arm and was 33.7 months in the bendamustine plus rituximab arm (hazard ratio, 0.42).
In a separate, nonrandomized SEQUOIA cohort, investigators assessed zanubrutinib in patients with a 17p deletion and found an overall response rate of 88%. In addition, over the 25-month follow-up, the median duration of response was not reached.
The ALPINE trial included 652 patients with relapsed or refractory CLL/SLL who received either zanubrutinib or ibrutinib. The overall response rate was 80% in the zanubrutinib arm versus 73% in the ibrutinib arm, and the median duration of response was not reached in either arm over the 14-month follow-up period. Median progression-free survival was not reached in the zanubrutinib arm and was 35 months in the ibrutinib group.
Dr. Brown, a lead investigator on both drug trials, suggested that, given the improvements observed in progression-free survival, zanubrutinib could become the standard of care in this setting.
In the ALPINE trial, treatment discontinuation rate was lower among patients receiving zanubrutinib (26%) versus ibrutinib (41.2%), with most discontinuations a result of adverse events or progressive disease.
And across both trials, the most common adverse reactions were decreased neutrophil count (42%), upper respiratory tract infection (39%), decreased platelet count (34%), hemorrhage (30%), and musculoskeletal pain (30%).
A version of this article first appeared on Medscape.com.
By giving the nod to these uses of this second-generation Bruton’s tyrosine kinase inhibitor, the FDA expanded on its previous approvals of this drug in mantle cell and marginal zone lymphoma.
“We have seen striking data from the Brukinsa development program demonstrating significant and consistent efficacy across CLL patient subtypes, including the high-risk del17p/TP53-mutated population, and regardless of treatment setting,” Jennifer R. Brown, MD, PhD, of Dana-Farber Cancer Institute in Boston, said in a press release from drug developer BeiGene.
The FDA’s decision was based on two phase 3 trials – SEQUOIA and ALPINE. The SEQUOIA trial assessed 479 patients with treatment-naive CLL/SLL who either received zanubrutinib until disease progression or unacceptable toxicity or bendamustine plus rituximab for six cycles. Median progression-free survival was not reached in the zanubrutinib arm and was 33.7 months in the bendamustine plus rituximab arm (hazard ratio, 0.42).
In a separate, nonrandomized SEQUOIA cohort, investigators assessed zanubrutinib in patients with a 17p deletion and found an overall response rate of 88%. In addition, over the 25-month follow-up, the median duration of response was not reached.
The ALPINE trial included 652 patients with relapsed or refractory CLL/SLL who received either zanubrutinib or ibrutinib. The overall response rate was 80% in the zanubrutinib arm versus 73% in the ibrutinib arm, and the median duration of response was not reached in either arm over the 14-month follow-up period. Median progression-free survival was not reached in the zanubrutinib arm and was 35 months in the ibrutinib group.
Dr. Brown, a lead investigator on both drug trials, suggested that, given the improvements observed in progression-free survival, zanubrutinib could become the standard of care in this setting.
In the ALPINE trial, treatment discontinuation rate was lower among patients receiving zanubrutinib (26%) versus ibrutinib (41.2%), with most discontinuations a result of adverse events or progressive disease.
And across both trials, the most common adverse reactions were decreased neutrophil count (42%), upper respiratory tract infection (39%), decreased platelet count (34%), hemorrhage (30%), and musculoskeletal pain (30%).
A version of this article first appeared on Medscape.com.
A freak impalement by a model rocket has this doctor scrambling
North central Washington state is a lot of nothing other than fields. Every year, the Federal Aviation Administration closes the airspace in a remote part of the area for a model rocket competition, the National Association of Rocketry Annual Meet. It’s a 2-day event and a pretty big deal. People come from all over the country to be there.
When you were a kid, you probably saw those rockets that are 3 feet tall. You launch them up in the air, they have a little parachute that comes out and they come back down to the ground. Well, picture that on ultimate steroids. There are anywhere from 3-foot to almost 20-foot-long rockets at this thing. People show up with horse trailers full of rockets and components. I mean, it’s an obsession.
Some of these rockets are super sophisticated. They have different stages where the first stage burns out and the second takes over. They go up thousands of feet to the edge of the stratosphere. Most of them have GoPro cameras, so you get to see when the rocket reaches the top of its trajectory and the last engine burns out. As it starts to descend, a parachute deploys and it can drift back anywhere from pretty close to where you launched it to a couple miles away. Then you use your little GPS to find it.
Why not? I drove out there and parked my Jeep and was walking over to the competition when I noticed something off. A bigger commotion than there should have been.
Here’s what happened 2 minutes before I got there:
A 5-foot-long rocket, 2½ inches in diameter, had reached the top of its several thousand–foot trajectory and was ready to come back to Earth. But its parachute didn’t deploy. It turned itself point-down and literally shot back to earth like a rocket.
It had gone up pretty darn straight and came down just as straight – right into a circle of people sitting in lawn chairs.
It hit a middle-aged man. But you can’t imagine how. First of all, who knows how fast it was going. The point glanced off his forehead and ... how to describe the rest. The man was pretty heavy. So the rocket impaled him through the abdomen and stuck right into the ground. As in, the point entered the top of his belly just below chest level and came out the bottom of his belly. The rocket pinned him to the ground through his belly.
Well, this was not how I planned on spending my day. But my spectator time was over. There were a lot of people running around in circles where he was pinned, not really knowing what to do.
When I said I was an emergency physician, instantly 15 heads looked right at me for direction like, Oh my gosh, please take over! A lot of people were asking: “What can I do? What can I do?” I said: “Well, we don’t need to do CPR. What we really need to do is get this rocket out of the ground. We need to keep him still while we dig out the rocket and get him flat.”
People gently dug around the nose of the rocket. It was in about 6 or 8 inches, enough that we didn’t want to just yank on it (I still marvel at how fast it must have been traveling to both impale the man the way it did and also jam into the ground like that). We wanted to loosen it up and ease it out of the ground.
We managed to dig the nose out and get the guy on his back. Needless to say, he wasn’t particularly comfortable. He looked pretty ashen, like he was in pretty good trouble.
The festival had an EMS kit with some bandages in it, but not a whole lot else. There’s the old joke in emergency medicine: What can you do with duct tape, a Swiss army knife, and a paper clip? It’s like, what has anybody got that might work here?
What we really needed to do was keep both the rocket and the man from moving. We cut off his shirt and got his pants down so that I could better see where it entered and exited. Then we used a couple of clean T-shirts to stabilize the rocket so it didn’t move while he lay flat. It didn’t bleed all that much. And his belly wasn’t massively expanding like he was bleeding internally. I mean, he looked crappy. But so would I!
We were about an hour away from the closest EMS and only a couple people even had cell service out there. But we managed to get hold of EMS. It was also one of those 92-degree days with no shade for 50 miles in any direction.
There was a volunteer firefighter there to man the fire rig. He helped carry the guy into an air-conditioned trailer without moving him very much.
Basically, we stabilized him by keeping him super still and as comfortable as we could until EMS arrived. I rode with him about an hour and a half to the closest trauma center in Central Washington. He was conscious, which was lousy for him but reassuring for me. “You’re still talking to me,” I said. “I think you’re going to be okay.”
One of the take-home points from a medical point of view is never try to remove something sticking out of someone when you’re out in the field. If it’s pushing against something vital, you could do a lot of damage, and if it’s up against a blood vessel, that vessel’s going to bleed uncontrollably.
We got to the trauma center and they took him to the OR. By the grace of friendships, somebody got his wife to the hospital. She was calmer than I think I would have been if my spouse had been hit by a rocket.
The full diagnostic story: The rocket bouncing off his forehead gave him a small skull fracture and slight concussion. That was no big deal. But picture this: The rocket only went through his belly fat. It didn’t hit any of his abdominal organs! I still think this is absolutely amazing. If he had been leaning forward in his lawn chair even a few inches, the rocket would’ve gone through his head and that would’ve been all they wrote.
He stayed in the hospital for a couple of days. I never saw him again, but I received follow-up from the surgeon. And I read the paper the next day. Let me tell you, in Central Washington, this is pretty big news.
It wasn’t the way I’d planned my morning. But you just can’t predict that kind of thing. I don’t know, maybe spiritually or karma wise, I was meant to show up about 90 seconds after he’d been hit. The only emergency physician at the whole event, just by chance. My work blesses me with a certain skill set. I know when to really worry, how to go about keeping somebody safe until you can get them to the ED. It’s something I thank my stars for every single day.
As I said to the guy on the way to the hospital: “Well, it’s not your lucky day, but it sure as heck could have been a whole lot unluckier.”
Stephen Anderson, MD, is an emergency medicine physician in Auburn, Washington and is affiliated with MultiCare Auburn Medical Center.
A version of this article first appeared on Medscape.com.
North central Washington state is a lot of nothing other than fields. Every year, the Federal Aviation Administration closes the airspace in a remote part of the area for a model rocket competition, the National Association of Rocketry Annual Meet. It’s a 2-day event and a pretty big deal. People come from all over the country to be there.
When you were a kid, you probably saw those rockets that are 3 feet tall. You launch them up in the air, they have a little parachute that comes out and they come back down to the ground. Well, picture that on ultimate steroids. There are anywhere from 3-foot to almost 20-foot-long rockets at this thing. People show up with horse trailers full of rockets and components. I mean, it’s an obsession.
Some of these rockets are super sophisticated. They have different stages where the first stage burns out and the second takes over. They go up thousands of feet to the edge of the stratosphere. Most of them have GoPro cameras, so you get to see when the rocket reaches the top of its trajectory and the last engine burns out. As it starts to descend, a parachute deploys and it can drift back anywhere from pretty close to where you launched it to a couple miles away. Then you use your little GPS to find it.
Why not? I drove out there and parked my Jeep and was walking over to the competition when I noticed something off. A bigger commotion than there should have been.
Here’s what happened 2 minutes before I got there:
A 5-foot-long rocket, 2½ inches in diameter, had reached the top of its several thousand–foot trajectory and was ready to come back to Earth. But its parachute didn’t deploy. It turned itself point-down and literally shot back to earth like a rocket.
It had gone up pretty darn straight and came down just as straight – right into a circle of people sitting in lawn chairs.
It hit a middle-aged man. But you can’t imagine how. First of all, who knows how fast it was going. The point glanced off his forehead and ... how to describe the rest. The man was pretty heavy. So the rocket impaled him through the abdomen and stuck right into the ground. As in, the point entered the top of his belly just below chest level and came out the bottom of his belly. The rocket pinned him to the ground through his belly.
Well, this was not how I planned on spending my day. But my spectator time was over. There were a lot of people running around in circles where he was pinned, not really knowing what to do.
When I said I was an emergency physician, instantly 15 heads looked right at me for direction like, Oh my gosh, please take over! A lot of people were asking: “What can I do? What can I do?” I said: “Well, we don’t need to do CPR. What we really need to do is get this rocket out of the ground. We need to keep him still while we dig out the rocket and get him flat.”
People gently dug around the nose of the rocket. It was in about 6 or 8 inches, enough that we didn’t want to just yank on it (I still marvel at how fast it must have been traveling to both impale the man the way it did and also jam into the ground like that). We wanted to loosen it up and ease it out of the ground.
We managed to dig the nose out and get the guy on his back. Needless to say, he wasn’t particularly comfortable. He looked pretty ashen, like he was in pretty good trouble.
The festival had an EMS kit with some bandages in it, but not a whole lot else. There’s the old joke in emergency medicine: What can you do with duct tape, a Swiss army knife, and a paper clip? It’s like, what has anybody got that might work here?
What we really needed to do was keep both the rocket and the man from moving. We cut off his shirt and got his pants down so that I could better see where it entered and exited. Then we used a couple of clean T-shirts to stabilize the rocket so it didn’t move while he lay flat. It didn’t bleed all that much. And his belly wasn’t massively expanding like he was bleeding internally. I mean, he looked crappy. But so would I!
We were about an hour away from the closest EMS and only a couple people even had cell service out there. But we managed to get hold of EMS. It was also one of those 92-degree days with no shade for 50 miles in any direction.
There was a volunteer firefighter there to man the fire rig. He helped carry the guy into an air-conditioned trailer without moving him very much.
Basically, we stabilized him by keeping him super still and as comfortable as we could until EMS arrived. I rode with him about an hour and a half to the closest trauma center in Central Washington. He was conscious, which was lousy for him but reassuring for me. “You’re still talking to me,” I said. “I think you’re going to be okay.”
One of the take-home points from a medical point of view is never try to remove something sticking out of someone when you’re out in the field. If it’s pushing against something vital, you could do a lot of damage, and if it’s up against a blood vessel, that vessel’s going to bleed uncontrollably.
We got to the trauma center and they took him to the OR. By the grace of friendships, somebody got his wife to the hospital. She was calmer than I think I would have been if my spouse had been hit by a rocket.
The full diagnostic story: The rocket bouncing off his forehead gave him a small skull fracture and slight concussion. That was no big deal. But picture this: The rocket only went through his belly fat. It didn’t hit any of his abdominal organs! I still think this is absolutely amazing. If he had been leaning forward in his lawn chair even a few inches, the rocket would’ve gone through his head and that would’ve been all they wrote.
He stayed in the hospital for a couple of days. I never saw him again, but I received follow-up from the surgeon. And I read the paper the next day. Let me tell you, in Central Washington, this is pretty big news.
It wasn’t the way I’d planned my morning. But you just can’t predict that kind of thing. I don’t know, maybe spiritually or karma wise, I was meant to show up about 90 seconds after he’d been hit. The only emergency physician at the whole event, just by chance. My work blesses me with a certain skill set. I know when to really worry, how to go about keeping somebody safe until you can get them to the ED. It’s something I thank my stars for every single day.
As I said to the guy on the way to the hospital: “Well, it’s not your lucky day, but it sure as heck could have been a whole lot unluckier.”
Stephen Anderson, MD, is an emergency medicine physician in Auburn, Washington and is affiliated with MultiCare Auburn Medical Center.
A version of this article first appeared on Medscape.com.
North central Washington state is a lot of nothing other than fields. Every year, the Federal Aviation Administration closes the airspace in a remote part of the area for a model rocket competition, the National Association of Rocketry Annual Meet. It’s a 2-day event and a pretty big deal. People come from all over the country to be there.
When you were a kid, you probably saw those rockets that are 3 feet tall. You launch them up in the air, they have a little parachute that comes out and they come back down to the ground. Well, picture that on ultimate steroids. There are anywhere from 3-foot to almost 20-foot-long rockets at this thing. People show up with horse trailers full of rockets and components. I mean, it’s an obsession.
Some of these rockets are super sophisticated. They have different stages where the first stage burns out and the second takes over. They go up thousands of feet to the edge of the stratosphere. Most of them have GoPro cameras, so you get to see when the rocket reaches the top of its trajectory and the last engine burns out. As it starts to descend, a parachute deploys and it can drift back anywhere from pretty close to where you launched it to a couple miles away. Then you use your little GPS to find it.
Why not? I drove out there and parked my Jeep and was walking over to the competition when I noticed something off. A bigger commotion than there should have been.
Here’s what happened 2 minutes before I got there:
A 5-foot-long rocket, 2½ inches in diameter, had reached the top of its several thousand–foot trajectory and was ready to come back to Earth. But its parachute didn’t deploy. It turned itself point-down and literally shot back to earth like a rocket.
It had gone up pretty darn straight and came down just as straight – right into a circle of people sitting in lawn chairs.
It hit a middle-aged man. But you can’t imagine how. First of all, who knows how fast it was going. The point glanced off his forehead and ... how to describe the rest. The man was pretty heavy. So the rocket impaled him through the abdomen and stuck right into the ground. As in, the point entered the top of his belly just below chest level and came out the bottom of his belly. The rocket pinned him to the ground through his belly.
Well, this was not how I planned on spending my day. But my spectator time was over. There were a lot of people running around in circles where he was pinned, not really knowing what to do.
When I said I was an emergency physician, instantly 15 heads looked right at me for direction like, Oh my gosh, please take over! A lot of people were asking: “What can I do? What can I do?” I said: “Well, we don’t need to do CPR. What we really need to do is get this rocket out of the ground. We need to keep him still while we dig out the rocket and get him flat.”
People gently dug around the nose of the rocket. It was in about 6 or 8 inches, enough that we didn’t want to just yank on it (I still marvel at how fast it must have been traveling to both impale the man the way it did and also jam into the ground like that). We wanted to loosen it up and ease it out of the ground.
We managed to dig the nose out and get the guy on his back. Needless to say, he wasn’t particularly comfortable. He looked pretty ashen, like he was in pretty good trouble.
The festival had an EMS kit with some bandages in it, but not a whole lot else. There’s the old joke in emergency medicine: What can you do with duct tape, a Swiss army knife, and a paper clip? It’s like, what has anybody got that might work here?
What we really needed to do was keep both the rocket and the man from moving. We cut off his shirt and got his pants down so that I could better see where it entered and exited. Then we used a couple of clean T-shirts to stabilize the rocket so it didn’t move while he lay flat. It didn’t bleed all that much. And his belly wasn’t massively expanding like he was bleeding internally. I mean, he looked crappy. But so would I!
We were about an hour away from the closest EMS and only a couple people even had cell service out there. But we managed to get hold of EMS. It was also one of those 92-degree days with no shade for 50 miles in any direction.
There was a volunteer firefighter there to man the fire rig. He helped carry the guy into an air-conditioned trailer without moving him very much.
Basically, we stabilized him by keeping him super still and as comfortable as we could until EMS arrived. I rode with him about an hour and a half to the closest trauma center in Central Washington. He was conscious, which was lousy for him but reassuring for me. “You’re still talking to me,” I said. “I think you’re going to be okay.”
One of the take-home points from a medical point of view is never try to remove something sticking out of someone when you’re out in the field. If it’s pushing against something vital, you could do a lot of damage, and if it’s up against a blood vessel, that vessel’s going to bleed uncontrollably.
We got to the trauma center and they took him to the OR. By the grace of friendships, somebody got his wife to the hospital. She was calmer than I think I would have been if my spouse had been hit by a rocket.
The full diagnostic story: The rocket bouncing off his forehead gave him a small skull fracture and slight concussion. That was no big deal. But picture this: The rocket only went through his belly fat. It didn’t hit any of his abdominal organs! I still think this is absolutely amazing. If he had been leaning forward in his lawn chair even a few inches, the rocket would’ve gone through his head and that would’ve been all they wrote.
He stayed in the hospital for a couple of days. I never saw him again, but I received follow-up from the surgeon. And I read the paper the next day. Let me tell you, in Central Washington, this is pretty big news.
It wasn’t the way I’d planned my morning. But you just can’t predict that kind of thing. I don’t know, maybe spiritually or karma wise, I was meant to show up about 90 seconds after he’d been hit. The only emergency physician at the whole event, just by chance. My work blesses me with a certain skill set. I know when to really worry, how to go about keeping somebody safe until you can get them to the ED. It’s something I thank my stars for every single day.
As I said to the guy on the way to the hospital: “Well, it’s not your lucky day, but it sure as heck could have been a whole lot unluckier.”
Stephen Anderson, MD, is an emergency medicine physician in Auburn, Washington and is affiliated with MultiCare Auburn Medical Center.
A version of this article first appeared on Medscape.com.
Not all white coats are doctors: Why titles are important at the doctor’s office
says Cyndy Flores, a physician assistant (PA) in the emergency department at Vituity, Emeryville, Calif. “Sometimes, I can go through a complete history and physical, explain a treatment plan, and perform a procedure, and [the patient] will say, ‘Thank you, doctor.’ ”
“I always come back and say, ‘You’re very welcome, but my name is Cyndy, and I’m the PA.’ ”
Ms. Flores is used to patients calling her “doctor” when she greets them. She typically offers a quick correction and moves on with the appointment.
With 355,000 nurse practitioners (NPs) and 149,000 certified PAs practicing in the United States, it’s more common than ever for health care providers who don’t go by the title “doctor” to diagnose and treat patients.
A recent report, Evolving Scope of Practice, found that more than 70% of physicians were “somewhat satisfied to very satisfied” with patient treatment by PAs and NPs.
But for patients, having a health care team that includes physicians, NPs, and PAs can be confusing. Additionally, it creates a need for education about their correct titles and roles in patient care.
“It’s really important for patients to understand who is taking care of them,” Ms. Flores says.
Education starts in your practice
Educating patients about the roles of different providers on their health care team starts long before patients enter the exam room, Ms. Flores explains.
Some patients may not understand the difference, some may just forget because they’re used to calling all providers doctors, and others may find it awkward to use a provider’s first name or not know the respectful way to address an NP or a PA.
Practices can help by listing the names and biographies of the health care team on the clinic website. In addition, when patients call for an appointment, Ms. Flores believes front desk staff can reinforce that information. When offering appointments with a physician, NP, or PA, clearly use the practitioner’s title and reiterate it throughout the conversation. For example, “Would you like to see our nurse practitioner, Alice Smith, next week?” or “So, our physician assistant Mrs. Jones will see you Friday at 3 PM.”
The report also found that 76% of patients expressed a preference to see a physician over a PA, and 71% expressed a preference to see a physician over an NP, but offering appointments with nonphysician providers is part of the education process.
“Some families are super savvy and know the differences between nurse practitioners, physician assistants, and doctors, and ... there are families who don’t understand those titles, [and] we need to explain what they do in our practice,” adds Nicole Aaronson, MD, MBA, attending surgeon at Nemours Children’s Health of Delaware. Dr. Aaronson believes there’s an opportunity for educating patients when speaking about all the available providers they may see.
Hanging posters or using brochures in the clinic or hospital is another effective way to reinforce the roles of various providers on the care team. Include biographies and educational information on practice materials and video programs running in the waiting room.
“Patients mean it [calling everyone doctor] as a way to respectfully address the nurse practitioner or physician assistant rather than meaning it as a denigration of the physician,” Dr. Aaronson says. “But everyone appreciates being called by the correct title.”
Helping patients understand the members of their care team and the correct titles to use for those health care professionals could also help patients feel more confident about their health care experience.
“Patients really like knowing that there are specialists in each of the areas taking care of them,” Ms. Flores says. “I think that conveys a feeling of trust in your provider.”
Not everyone is a doctor
Even when PAs and NPs remind patients of their roles and reinforce the use of their preferred names, there will still be patients who continue referring to their nonphysician provider as “doctor.”
“There’s a perception that anyone who walks into a room with a stethoscope is your doctor,” says Graig Straus, DNP, an NP and president and CEO of Rockland Urgent Care Family Health NP, P.C., West Haverstraw, N.Y. “You do get a little bit of burnout correcting people all the time.”
Dr. Straus, who earned his doctorate in nursing practice, notes that patients using the honorific with him aren’t incorrect, but he still educates them on his role within the health care team.
“NPs and PAs have a valuable role to play independently and in concert with the physician,” Dr. Aaronson says. This understanding is essential, as states consider expanding treatment abilities for NPs and PAs.
NPs have expanded treatment abilities or full practice authority in almost half the states, and 31% of the physicians surveyed agreed that NPs should have expanded treatment abilities.
An estimated 1 in 5 states characterizes the physician-PA relationship as collaborative, not supervisory, according to the American Academy of Physician Associates. At the same time, only 39% of physicians surveyed said they favored this trend.
“Patients need great quality care, and there are many different types of providers that can provide that care as part of the team,” Ms. Flores says. “When you have a team taking care of a patient, that patient [gets] the best care possible – and ... that’s why we went into medicine: to deliver high-quality, compassionate care to our patients, and we should all be in this together.”
When practices do their part explaining who is and isn’t a doctor and what each provider’s title and role is and what to call them, and everyone reinforces it, health care becomes not only more manageable for patients to traverse but easier to understand, leading to a better experience.
A version of this article first appeared on Medscape.com.
says Cyndy Flores, a physician assistant (PA) in the emergency department at Vituity, Emeryville, Calif. “Sometimes, I can go through a complete history and physical, explain a treatment plan, and perform a procedure, and [the patient] will say, ‘Thank you, doctor.’ ”
“I always come back and say, ‘You’re very welcome, but my name is Cyndy, and I’m the PA.’ ”
Ms. Flores is used to patients calling her “doctor” when she greets them. She typically offers a quick correction and moves on with the appointment.
With 355,000 nurse practitioners (NPs) and 149,000 certified PAs practicing in the United States, it’s more common than ever for health care providers who don’t go by the title “doctor” to diagnose and treat patients.
A recent report, Evolving Scope of Practice, found that more than 70% of physicians were “somewhat satisfied to very satisfied” with patient treatment by PAs and NPs.
But for patients, having a health care team that includes physicians, NPs, and PAs can be confusing. Additionally, it creates a need for education about their correct titles and roles in patient care.
“It’s really important for patients to understand who is taking care of them,” Ms. Flores says.
Education starts in your practice
Educating patients about the roles of different providers on their health care team starts long before patients enter the exam room, Ms. Flores explains.
Some patients may not understand the difference, some may just forget because they’re used to calling all providers doctors, and others may find it awkward to use a provider’s first name or not know the respectful way to address an NP or a PA.
Practices can help by listing the names and biographies of the health care team on the clinic website. In addition, when patients call for an appointment, Ms. Flores believes front desk staff can reinforce that information. When offering appointments with a physician, NP, or PA, clearly use the practitioner’s title and reiterate it throughout the conversation. For example, “Would you like to see our nurse practitioner, Alice Smith, next week?” or “So, our physician assistant Mrs. Jones will see you Friday at 3 PM.”
The report also found that 76% of patients expressed a preference to see a physician over a PA, and 71% expressed a preference to see a physician over an NP, but offering appointments with nonphysician providers is part of the education process.
“Some families are super savvy and know the differences between nurse practitioners, physician assistants, and doctors, and ... there are families who don’t understand those titles, [and] we need to explain what they do in our practice,” adds Nicole Aaronson, MD, MBA, attending surgeon at Nemours Children’s Health of Delaware. Dr. Aaronson believes there’s an opportunity for educating patients when speaking about all the available providers they may see.
Hanging posters or using brochures in the clinic or hospital is another effective way to reinforce the roles of various providers on the care team. Include biographies and educational information on practice materials and video programs running in the waiting room.
“Patients mean it [calling everyone doctor] as a way to respectfully address the nurse practitioner or physician assistant rather than meaning it as a denigration of the physician,” Dr. Aaronson says. “But everyone appreciates being called by the correct title.”
Helping patients understand the members of their care team and the correct titles to use for those health care professionals could also help patients feel more confident about their health care experience.
“Patients really like knowing that there are specialists in each of the areas taking care of them,” Ms. Flores says. “I think that conveys a feeling of trust in your provider.”
Not everyone is a doctor
Even when PAs and NPs remind patients of their roles and reinforce the use of their preferred names, there will still be patients who continue referring to their nonphysician provider as “doctor.”
“There’s a perception that anyone who walks into a room with a stethoscope is your doctor,” says Graig Straus, DNP, an NP and president and CEO of Rockland Urgent Care Family Health NP, P.C., West Haverstraw, N.Y. “You do get a little bit of burnout correcting people all the time.”
Dr. Straus, who earned his doctorate in nursing practice, notes that patients using the honorific with him aren’t incorrect, but he still educates them on his role within the health care team.
“NPs and PAs have a valuable role to play independently and in concert with the physician,” Dr. Aaronson says. This understanding is essential, as states consider expanding treatment abilities for NPs and PAs.
NPs have expanded treatment abilities or full practice authority in almost half the states, and 31% of the physicians surveyed agreed that NPs should have expanded treatment abilities.
An estimated 1 in 5 states characterizes the physician-PA relationship as collaborative, not supervisory, according to the American Academy of Physician Associates. At the same time, only 39% of physicians surveyed said they favored this trend.
“Patients need great quality care, and there are many different types of providers that can provide that care as part of the team,” Ms. Flores says. “When you have a team taking care of a patient, that patient [gets] the best care possible – and ... that’s why we went into medicine: to deliver high-quality, compassionate care to our patients, and we should all be in this together.”
When practices do their part explaining who is and isn’t a doctor and what each provider’s title and role is and what to call them, and everyone reinforces it, health care becomes not only more manageable for patients to traverse but easier to understand, leading to a better experience.
A version of this article first appeared on Medscape.com.
says Cyndy Flores, a physician assistant (PA) in the emergency department at Vituity, Emeryville, Calif. “Sometimes, I can go through a complete history and physical, explain a treatment plan, and perform a procedure, and [the patient] will say, ‘Thank you, doctor.’ ”
“I always come back and say, ‘You’re very welcome, but my name is Cyndy, and I’m the PA.’ ”
Ms. Flores is used to patients calling her “doctor” when she greets them. She typically offers a quick correction and moves on with the appointment.
With 355,000 nurse practitioners (NPs) and 149,000 certified PAs practicing in the United States, it’s more common than ever for health care providers who don’t go by the title “doctor” to diagnose and treat patients.
A recent report, Evolving Scope of Practice, found that more than 70% of physicians were “somewhat satisfied to very satisfied” with patient treatment by PAs and NPs.
But for patients, having a health care team that includes physicians, NPs, and PAs can be confusing. Additionally, it creates a need for education about their correct titles and roles in patient care.
“It’s really important for patients to understand who is taking care of them,” Ms. Flores says.
Education starts in your practice
Educating patients about the roles of different providers on their health care team starts long before patients enter the exam room, Ms. Flores explains.
Some patients may not understand the difference, some may just forget because they’re used to calling all providers doctors, and others may find it awkward to use a provider’s first name or not know the respectful way to address an NP or a PA.
Practices can help by listing the names and biographies of the health care team on the clinic website. In addition, when patients call for an appointment, Ms. Flores believes front desk staff can reinforce that information. When offering appointments with a physician, NP, or PA, clearly use the practitioner’s title and reiterate it throughout the conversation. For example, “Would you like to see our nurse practitioner, Alice Smith, next week?” or “So, our physician assistant Mrs. Jones will see you Friday at 3 PM.”
The report also found that 76% of patients expressed a preference to see a physician over a PA, and 71% expressed a preference to see a physician over an NP, but offering appointments with nonphysician providers is part of the education process.
“Some families are super savvy and know the differences between nurse practitioners, physician assistants, and doctors, and ... there are families who don’t understand those titles, [and] we need to explain what they do in our practice,” adds Nicole Aaronson, MD, MBA, attending surgeon at Nemours Children’s Health of Delaware. Dr. Aaronson believes there’s an opportunity for educating patients when speaking about all the available providers they may see.
Hanging posters or using brochures in the clinic or hospital is another effective way to reinforce the roles of various providers on the care team. Include biographies and educational information on practice materials and video programs running in the waiting room.
“Patients mean it [calling everyone doctor] as a way to respectfully address the nurse practitioner or physician assistant rather than meaning it as a denigration of the physician,” Dr. Aaronson says. “But everyone appreciates being called by the correct title.”
Helping patients understand the members of their care team and the correct titles to use for those health care professionals could also help patients feel more confident about their health care experience.
“Patients really like knowing that there are specialists in each of the areas taking care of them,” Ms. Flores says. “I think that conveys a feeling of trust in your provider.”
Not everyone is a doctor
Even when PAs and NPs remind patients of their roles and reinforce the use of their preferred names, there will still be patients who continue referring to their nonphysician provider as “doctor.”
“There’s a perception that anyone who walks into a room with a stethoscope is your doctor,” says Graig Straus, DNP, an NP and president and CEO of Rockland Urgent Care Family Health NP, P.C., West Haverstraw, N.Y. “You do get a little bit of burnout correcting people all the time.”
Dr. Straus, who earned his doctorate in nursing practice, notes that patients using the honorific with him aren’t incorrect, but he still educates them on his role within the health care team.
“NPs and PAs have a valuable role to play independently and in concert with the physician,” Dr. Aaronson says. This understanding is essential, as states consider expanding treatment abilities for NPs and PAs.
NPs have expanded treatment abilities or full practice authority in almost half the states, and 31% of the physicians surveyed agreed that NPs should have expanded treatment abilities.
An estimated 1 in 5 states characterizes the physician-PA relationship as collaborative, not supervisory, according to the American Academy of Physician Associates. At the same time, only 39% of physicians surveyed said they favored this trend.
“Patients need great quality care, and there are many different types of providers that can provide that care as part of the team,” Ms. Flores says. “When you have a team taking care of a patient, that patient [gets] the best care possible – and ... that’s why we went into medicine: to deliver high-quality, compassionate care to our patients, and we should all be in this together.”
When practices do their part explaining who is and isn’t a doctor and what each provider’s title and role is and what to call them, and everyone reinforces it, health care becomes not only more manageable for patients to traverse but easier to understand, leading to a better experience.
A version of this article first appeared on Medscape.com.
U.S. ketamine poisonings up 81%
Although the overall ketamine exposures were low, researchers say the findings add to a growing body of research that suggests recreational ketamine use may be on the rise.
“Ketamine is by no means the most dangerous drug, but it could be dangerous if combined with drugs such as alcohol or if used in potentially hazardous situations – physically hazardous or socially hazardous,” lead author Joseph Palamar, PhD, associate professor and epidemiologist at New York University Langone Health, New York, told this news organization.
“People who decide to use ketamine recreationally need to be educated about potential risks,” Dr. Palamar said.
The findings were recently published online in the Journal of Psychopharmacology.
More widespread use
Researchers noted that ketamine use has become more widespread in the United States due in part to increasing availability of ketamine in both clinical and nonclinical settings.
Previous work by Dr. Palamar documented an increase in recreational use of ketamine at dance clubs and an increase in ketamine seizures by the Drug Enforcement Administration.
In the current study, investigators analyzed data from the National Poison Control database and included cases reported by 51 of the 55 poison control centers in the United States.
They identified 758 cases involving ketamine exposure between the first quarter of 2019 and the last quarter of 2021 in individuals aged 13 and older, more than half of whom were men.
The number of ketamine exposures increased 81.1% during the study period, rising from 37 to 67 (P = .018).
Nearly 40% of callers reported intentional misuse or abuse of ketamine, while 19.7% involved a suspected suicide or suicide attempt. The ketamine exposure was unintended in 18.9% of cases, and 10.6% of calls involved an adverse drug reaction.
Onep-third of cases involved co-use of other substances, most commonly benzodiazepines, opioids, or alcohol.
The route of administration was ingestion for 44.3%, injection for 18.8%, and inhalation for 17.6%. Another 19.3% involved another route or a combination of routes.
Nearly 20% of cases reported a major adverse effect or death, 42.8% reported a moderate effect, 25.8% a minor effect, and 11.8% no effect. There were seven deaths reported in ketamine-related calls, although Dr. Palamar noted it is unlikely those deaths were due solely to ketamine use.
Researchers didn’t analyze specific harms reported in the calls, but chronic and heavy ketamine use has been previously associated with cognitive impairment, urinary cystitis and other urinary tract issues, and upper gastrointestinal problems.
In addition, using ketamine with gamma-hydroxybutyrate (GHB) or opioids was associated with a significantly higher risk for major adverse effects (P < .001 for both). Injecting ketamine was also linked to a higher prevalence of major adverse effects, although the association did not quite reach significance (P < .05).
Cause for concern
Commenting on the findings, Timothy Wiegand, MD, director of Addiction Toxicology and Toxicology Consult Service and associate professor of emergency medicine at the University of Rochester Medical Center and Strong Memorial Hospital, New York, noted the data on co-use of ketamine with other drugs were cause for concern.
“I think the co-occurring behaviors are critical here with concomitant use of opioids and GHB, intravenous drug use, or that it is used in an attempt to harm one’s self because it allows for identification of these behaviors or use patterns,” said Dr. Wiegand, who was not involved with the research.
He added that it is important for “addiction providers and others in medicine or in the addiction field to be aware of trends” associated with ketamine.
“At the same time, a focus on general prevention, and access to care and treatment, and understanding how to implement harm reduction strategies remain high priorities,” Dr. Wiegand said.
The study was funded by the National Institute on Drug Abuse. Dr. Palamar has reported consulting for Alkermes. Dr. Wiegand has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although the overall ketamine exposures were low, researchers say the findings add to a growing body of research that suggests recreational ketamine use may be on the rise.
“Ketamine is by no means the most dangerous drug, but it could be dangerous if combined with drugs such as alcohol or if used in potentially hazardous situations – physically hazardous or socially hazardous,” lead author Joseph Palamar, PhD, associate professor and epidemiologist at New York University Langone Health, New York, told this news organization.
“People who decide to use ketamine recreationally need to be educated about potential risks,” Dr. Palamar said.
The findings were recently published online in the Journal of Psychopharmacology.
More widespread use
Researchers noted that ketamine use has become more widespread in the United States due in part to increasing availability of ketamine in both clinical and nonclinical settings.
Previous work by Dr. Palamar documented an increase in recreational use of ketamine at dance clubs and an increase in ketamine seizures by the Drug Enforcement Administration.
In the current study, investigators analyzed data from the National Poison Control database and included cases reported by 51 of the 55 poison control centers in the United States.
They identified 758 cases involving ketamine exposure between the first quarter of 2019 and the last quarter of 2021 in individuals aged 13 and older, more than half of whom were men.
The number of ketamine exposures increased 81.1% during the study period, rising from 37 to 67 (P = .018).
Nearly 40% of callers reported intentional misuse or abuse of ketamine, while 19.7% involved a suspected suicide or suicide attempt. The ketamine exposure was unintended in 18.9% of cases, and 10.6% of calls involved an adverse drug reaction.
Onep-third of cases involved co-use of other substances, most commonly benzodiazepines, opioids, or alcohol.
The route of administration was ingestion for 44.3%, injection for 18.8%, and inhalation for 17.6%. Another 19.3% involved another route or a combination of routes.
Nearly 20% of cases reported a major adverse effect or death, 42.8% reported a moderate effect, 25.8% a minor effect, and 11.8% no effect. There were seven deaths reported in ketamine-related calls, although Dr. Palamar noted it is unlikely those deaths were due solely to ketamine use.
Researchers didn’t analyze specific harms reported in the calls, but chronic and heavy ketamine use has been previously associated with cognitive impairment, urinary cystitis and other urinary tract issues, and upper gastrointestinal problems.
In addition, using ketamine with gamma-hydroxybutyrate (GHB) or opioids was associated with a significantly higher risk for major adverse effects (P < .001 for both). Injecting ketamine was also linked to a higher prevalence of major adverse effects, although the association did not quite reach significance (P < .05).
Cause for concern
Commenting on the findings, Timothy Wiegand, MD, director of Addiction Toxicology and Toxicology Consult Service and associate professor of emergency medicine at the University of Rochester Medical Center and Strong Memorial Hospital, New York, noted the data on co-use of ketamine with other drugs were cause for concern.
“I think the co-occurring behaviors are critical here with concomitant use of opioids and GHB, intravenous drug use, or that it is used in an attempt to harm one’s self because it allows for identification of these behaviors or use patterns,” said Dr. Wiegand, who was not involved with the research.
He added that it is important for “addiction providers and others in medicine or in the addiction field to be aware of trends” associated with ketamine.
“At the same time, a focus on general prevention, and access to care and treatment, and understanding how to implement harm reduction strategies remain high priorities,” Dr. Wiegand said.
The study was funded by the National Institute on Drug Abuse. Dr. Palamar has reported consulting for Alkermes. Dr. Wiegand has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although the overall ketamine exposures were low, researchers say the findings add to a growing body of research that suggests recreational ketamine use may be on the rise.
“Ketamine is by no means the most dangerous drug, but it could be dangerous if combined with drugs such as alcohol or if used in potentially hazardous situations – physically hazardous or socially hazardous,” lead author Joseph Palamar, PhD, associate professor and epidemiologist at New York University Langone Health, New York, told this news organization.
“People who decide to use ketamine recreationally need to be educated about potential risks,” Dr. Palamar said.
The findings were recently published online in the Journal of Psychopharmacology.
More widespread use
Researchers noted that ketamine use has become more widespread in the United States due in part to increasing availability of ketamine in both clinical and nonclinical settings.
Previous work by Dr. Palamar documented an increase in recreational use of ketamine at dance clubs and an increase in ketamine seizures by the Drug Enforcement Administration.
In the current study, investigators analyzed data from the National Poison Control database and included cases reported by 51 of the 55 poison control centers in the United States.
They identified 758 cases involving ketamine exposure between the first quarter of 2019 and the last quarter of 2021 in individuals aged 13 and older, more than half of whom were men.
The number of ketamine exposures increased 81.1% during the study period, rising from 37 to 67 (P = .018).
Nearly 40% of callers reported intentional misuse or abuse of ketamine, while 19.7% involved a suspected suicide or suicide attempt. The ketamine exposure was unintended in 18.9% of cases, and 10.6% of calls involved an adverse drug reaction.
Onep-third of cases involved co-use of other substances, most commonly benzodiazepines, opioids, or alcohol.
The route of administration was ingestion for 44.3%, injection for 18.8%, and inhalation for 17.6%. Another 19.3% involved another route or a combination of routes.
Nearly 20% of cases reported a major adverse effect or death, 42.8% reported a moderate effect, 25.8% a minor effect, and 11.8% no effect. There were seven deaths reported in ketamine-related calls, although Dr. Palamar noted it is unlikely those deaths were due solely to ketamine use.
Researchers didn’t analyze specific harms reported in the calls, but chronic and heavy ketamine use has been previously associated with cognitive impairment, urinary cystitis and other urinary tract issues, and upper gastrointestinal problems.
In addition, using ketamine with gamma-hydroxybutyrate (GHB) or opioids was associated with a significantly higher risk for major adverse effects (P < .001 for both). Injecting ketamine was also linked to a higher prevalence of major adverse effects, although the association did not quite reach significance (P < .05).
Cause for concern
Commenting on the findings, Timothy Wiegand, MD, director of Addiction Toxicology and Toxicology Consult Service and associate professor of emergency medicine at the University of Rochester Medical Center and Strong Memorial Hospital, New York, noted the data on co-use of ketamine with other drugs were cause for concern.
“I think the co-occurring behaviors are critical here with concomitant use of opioids and GHB, intravenous drug use, or that it is used in an attempt to harm one’s self because it allows for identification of these behaviors or use patterns,” said Dr. Wiegand, who was not involved with the research.
He added that it is important for “addiction providers and others in medicine or in the addiction field to be aware of trends” associated with ketamine.
“At the same time, a focus on general prevention, and access to care and treatment, and understanding how to implement harm reduction strategies remain high priorities,” Dr. Wiegand said.
The study was funded by the National Institute on Drug Abuse. Dr. Palamar has reported consulting for Alkermes. Dr. Wiegand has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF PSYCHOPHARMACOLOGY
Canadian Task Force recommendation on screening for postpartum depression misses the mark
Postpartum/perinatal depression (PPD) remains the most common complication in modern obstetrics, with a prevalence of 10%-15% based on multiple studies over the last 2 decades. Over those same 2 decades, there has been growing interest and motivation across the country – from small community hospitals to major academic centers – to promote screening. Such screening is integrated into obstetrical practices, typically using the Edinburgh Postnatal Depression Scale (EPDS), the most widely used validated screen for PPD globally.
As mentioned in previous columns, the U.S. Preventive Services Task Force recommended screening for PPD in 2016, which includes screening women at highest risk, and both acutely treating and preventing PPD.
Since then, screening women for a common clinical problem like PPD has been widely adopted by clinicians representing a broad spectrum of interdisciplinary care. Providers who are engaged in the treatment of postpartum women – obstetricians, psychiatrists, doulas, lactation consultants, facilitators of postpartum support groups, and advocacy groups among others – are included.
An open question and one of great concern recently to our group and others has been what happens after screening. It is clear that identification of PPD per se is not necessarily a challenge, and we have multiple effective treatments from antidepressants to mindfulness-based cognitive therapy to cognitive-behavioral interventions. There is also a growing number of digital applications aimed at mitigation of depressive symptoms in women with postpartum major depressive disorder. One unanswered question is how to engage women after identification of PPD and how to facilitate access to care in a way that maximizes the likelihood that women who actually are suffering from PPD get adequate treatment.
The “perinatal treatment cascade” refers to the majority of women who, on the other side of identification of PPD, fail to receive adequate treatment and continue to have persistent depression. This is perhaps the greatest challenge to the field and to clinicians – how do we, on the other side of screening, see that these women get access to care and get well?
With that backdrop, it is surprising that the Canadian Task Force on Preventive Health Care has recently recommended against screening with systematic questionnaires, noting that benefits were unclear and not a particular advantage relative to standard practice. The recommendation carries an assumption that standard practice involves queries about mental health. While the task force continues to recommend screening for PPD, their recommendation against screening with a standardized questionnaire represents a bold, sweeping, if not myopic view.
While the Canadian Task Force on Preventive Health Care made their recommendation based on a single randomized controlled trial with the assumption that women were getting mental health counseling, and that women liked getting mental health engagement around their depression, that is not a uniform part of practice. Thus, it is puzzling why the task force would make the recommendation based on such sparse data.
The way to optimize access to care and referral systems for women who are suffering from PPD is not to remove a part of the system that’s already working. Well-validated questionnaires such as the EPDS are easy to administer and are routinely integrated into the electronic health systems records of both small and large centers. These questionnaires are an inexpensive way to increase the likelihood that women get identified and referred for a spectrum of potentially helpful interventions.
PPD is also easy to treat with medications and a wide spectrum of nonpharmacologic interventions. Novel interventions are also being explored to maximize access for women with postpartum mood and anxiety disorders such as peer-delivered behavioral activation and cognitive-behavioral therapy, which could be community based and implemented from urban to rural settings across the United States.
What may need the greatest study is the path to accessing effective treatments and resources for these women and this problem has prompted our group to explore these issues in our more recent investigations. Better understanding of those factors that limit access to mental health providers with expertise in perinatal mental health to the logistical issues of navigating the health care system for sleep-deprived new moms and their families demands greater attention and clearer answers.
The whole field has an obligation to postpartum women to figure out the amalgam of practitioners, resources, and platforms that need to be used to engage women so that they get effective treatment – because we have effective treatments. But the solution to improving perinatal mental health outcomes, unlike the approach of our colleagues in Canada, is not to be found in abandoning questionnaire-based screening, but in identifying the best ways to prevent PPD and to maximize access to care.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Postpartum/perinatal depression (PPD) remains the most common complication in modern obstetrics, with a prevalence of 10%-15% based on multiple studies over the last 2 decades. Over those same 2 decades, there has been growing interest and motivation across the country – from small community hospitals to major academic centers – to promote screening. Such screening is integrated into obstetrical practices, typically using the Edinburgh Postnatal Depression Scale (EPDS), the most widely used validated screen for PPD globally.
As mentioned in previous columns, the U.S. Preventive Services Task Force recommended screening for PPD in 2016, which includes screening women at highest risk, and both acutely treating and preventing PPD.
Since then, screening women for a common clinical problem like PPD has been widely adopted by clinicians representing a broad spectrum of interdisciplinary care. Providers who are engaged in the treatment of postpartum women – obstetricians, psychiatrists, doulas, lactation consultants, facilitators of postpartum support groups, and advocacy groups among others – are included.
An open question and one of great concern recently to our group and others has been what happens after screening. It is clear that identification of PPD per se is not necessarily a challenge, and we have multiple effective treatments from antidepressants to mindfulness-based cognitive therapy to cognitive-behavioral interventions. There is also a growing number of digital applications aimed at mitigation of depressive symptoms in women with postpartum major depressive disorder. One unanswered question is how to engage women after identification of PPD and how to facilitate access to care in a way that maximizes the likelihood that women who actually are suffering from PPD get adequate treatment.
The “perinatal treatment cascade” refers to the majority of women who, on the other side of identification of PPD, fail to receive adequate treatment and continue to have persistent depression. This is perhaps the greatest challenge to the field and to clinicians – how do we, on the other side of screening, see that these women get access to care and get well?
With that backdrop, it is surprising that the Canadian Task Force on Preventive Health Care has recently recommended against screening with systematic questionnaires, noting that benefits were unclear and not a particular advantage relative to standard practice. The recommendation carries an assumption that standard practice involves queries about mental health. While the task force continues to recommend screening for PPD, their recommendation against screening with a standardized questionnaire represents a bold, sweeping, if not myopic view.
While the Canadian Task Force on Preventive Health Care made their recommendation based on a single randomized controlled trial with the assumption that women were getting mental health counseling, and that women liked getting mental health engagement around their depression, that is not a uniform part of practice. Thus, it is puzzling why the task force would make the recommendation based on such sparse data.
The way to optimize access to care and referral systems for women who are suffering from PPD is not to remove a part of the system that’s already working. Well-validated questionnaires such as the EPDS are easy to administer and are routinely integrated into the electronic health systems records of both small and large centers. These questionnaires are an inexpensive way to increase the likelihood that women get identified and referred for a spectrum of potentially helpful interventions.
PPD is also easy to treat with medications and a wide spectrum of nonpharmacologic interventions. Novel interventions are also being explored to maximize access for women with postpartum mood and anxiety disorders such as peer-delivered behavioral activation and cognitive-behavioral therapy, which could be community based and implemented from urban to rural settings across the United States.
What may need the greatest study is the path to accessing effective treatments and resources for these women and this problem has prompted our group to explore these issues in our more recent investigations. Better understanding of those factors that limit access to mental health providers with expertise in perinatal mental health to the logistical issues of navigating the health care system for sleep-deprived new moms and their families demands greater attention and clearer answers.
The whole field has an obligation to postpartum women to figure out the amalgam of practitioners, resources, and platforms that need to be used to engage women so that they get effective treatment – because we have effective treatments. But the solution to improving perinatal mental health outcomes, unlike the approach of our colleagues in Canada, is not to be found in abandoning questionnaire-based screening, but in identifying the best ways to prevent PPD and to maximize access to care.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Postpartum/perinatal depression (PPD) remains the most common complication in modern obstetrics, with a prevalence of 10%-15% based on multiple studies over the last 2 decades. Over those same 2 decades, there has been growing interest and motivation across the country – from small community hospitals to major academic centers – to promote screening. Such screening is integrated into obstetrical practices, typically using the Edinburgh Postnatal Depression Scale (EPDS), the most widely used validated screen for PPD globally.
As mentioned in previous columns, the U.S. Preventive Services Task Force recommended screening for PPD in 2016, which includes screening women at highest risk, and both acutely treating and preventing PPD.
Since then, screening women for a common clinical problem like PPD has been widely adopted by clinicians representing a broad spectrum of interdisciplinary care. Providers who are engaged in the treatment of postpartum women – obstetricians, psychiatrists, doulas, lactation consultants, facilitators of postpartum support groups, and advocacy groups among others – are included.
An open question and one of great concern recently to our group and others has been what happens after screening. It is clear that identification of PPD per se is not necessarily a challenge, and we have multiple effective treatments from antidepressants to mindfulness-based cognitive therapy to cognitive-behavioral interventions. There is also a growing number of digital applications aimed at mitigation of depressive symptoms in women with postpartum major depressive disorder. One unanswered question is how to engage women after identification of PPD and how to facilitate access to care in a way that maximizes the likelihood that women who actually are suffering from PPD get adequate treatment.
The “perinatal treatment cascade” refers to the majority of women who, on the other side of identification of PPD, fail to receive adequate treatment and continue to have persistent depression. This is perhaps the greatest challenge to the field and to clinicians – how do we, on the other side of screening, see that these women get access to care and get well?
With that backdrop, it is surprising that the Canadian Task Force on Preventive Health Care has recently recommended against screening with systematic questionnaires, noting that benefits were unclear and not a particular advantage relative to standard practice. The recommendation carries an assumption that standard practice involves queries about mental health. While the task force continues to recommend screening for PPD, their recommendation against screening with a standardized questionnaire represents a bold, sweeping, if not myopic view.
While the Canadian Task Force on Preventive Health Care made their recommendation based on a single randomized controlled trial with the assumption that women were getting mental health counseling, and that women liked getting mental health engagement around their depression, that is not a uniform part of practice. Thus, it is puzzling why the task force would make the recommendation based on such sparse data.
The way to optimize access to care and referral systems for women who are suffering from PPD is not to remove a part of the system that’s already working. Well-validated questionnaires such as the EPDS are easy to administer and are routinely integrated into the electronic health systems records of both small and large centers. These questionnaires are an inexpensive way to increase the likelihood that women get identified and referred for a spectrum of potentially helpful interventions.
PPD is also easy to treat with medications and a wide spectrum of nonpharmacologic interventions. Novel interventions are also being explored to maximize access for women with postpartum mood and anxiety disorders such as peer-delivered behavioral activation and cognitive-behavioral therapy, which could be community based and implemented from urban to rural settings across the United States.
What may need the greatest study is the path to accessing effective treatments and resources for these women and this problem has prompted our group to explore these issues in our more recent investigations. Better understanding of those factors that limit access to mental health providers with expertise in perinatal mental health to the logistical issues of navigating the health care system for sleep-deprived new moms and their families demands greater attention and clearer answers.
The whole field has an obligation to postpartum women to figure out the amalgam of practitioners, resources, and platforms that need to be used to engage women so that they get effective treatment – because we have effective treatments. But the solution to improving perinatal mental health outcomes, unlike the approach of our colleagues in Canada, is not to be found in abandoning questionnaire-based screening, but in identifying the best ways to prevent PPD and to maximize access to care.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Age-related atopic dermatitis phenotypes evaluated in study
, while older adults tend to present with less flexural eczema and the fewest associated signs.
Those are key findings from a study conducted at a single academic medical center, which aimed to identify the age-related clinical phenotypes of AD.
“Previous studies have found differences in the clinical characteristics of AD depending on age of AD onset, ethnic background, and AD severity,” senior author Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research in the department of dermatology at George Washington University, Washington, and his coauthor wrote in the study, which was published online in JAAD International. “However, none have prospectively compared the clinical characteristics and associated signs by age group. Improved understanding of the clinical phenotypes of AD may help guide choice of treatment and improve health outcomes,” they added.
Along with coauthor Sheena Chatrath, a dermatology research fellow in the department, Dr. Silverberg prospectively reviewed self-reported questionnaires that were completed by 380 patients prior to their visit at GWU’s eczema clinic between 2013 and 2019. Questions included age of AD onset, sociodemographics, Visual Analog Scale (VAS) itch and sleep for Scoring AD, and Numeric Rating Scale (NRS) for skin pain and itch. The researchers used the Eczema Area Severity Index to assess AD severity and a dermatologist conducted full body skin exams, noting the distribution of AD involvement as well as associated signs.
Of the 380 patients, 6.1% were younger than aged 18 years, 46.3% were young adults aged 18-39 years, and 47.6% were older adults 40 years of age and older.
Compared with pediatric patients, both young and older adults were less likely to experience AD on the ankles (adjusted odds ratio [aOR], 0.41 and 0.43, respectively), moderate to severe AD lesions on flexures (aOR, 0.47 and 0.30), pityriasis alba (aOR, 0.24 and 0.07), oozing lesions (aOR, 0.44 and 0.35), and moderate to severe excoriations (aOR, 0.49 and 0.44).
In children, severe itch was more common, reported in 47.1%, compared with 43.4% of the young adults and 38.6% of the older adults, and itch was less severe among the young and older adults. “Interestingly, despite increased itch in pediatric patients, we found no difference in the severity of skin pain across all age groups,” the researchers wrote. “Moreover, pediatric patients reported skin pain less often than adult patients. This may be due to age-related differences of pain perception.”
In other findings, compared with pediatric patients, young adults were more likely to experience AD around the eyes (aOR, 2.92), while older adults were less likely to experience AD on elbows (aOR, 0.34), nipples (aOR, 0.40), knees (aOR, 0.27), and less likely to have keratosis pilaris (aOR, 0.38), and lichenification (aOR, 0.47).
Dr. Silverberg and Ms. Chatrath used latent class analysis to identify four classes for distribution of AD lesions. In this model, class 1 had low probabilities of AD involvement at all sites examined and class 2 had low probabilities of scalp, face, and foot involvement, and intermediate probability of all other AD sites. Class 3 had low probabilities of hand and foot involvement, high probability of facial erythema, and intermediate probability of all other AD signs, while class 4 had intermediate probability of postauricular and foot involvement, and high probability of all other AD sites examined.
“Pediatric patients were most commonly in class 4 (33.3%), followed by class 1 and 2 (26.7%), and least commonly in class 3 (13.3%),” the authors wrote. “In young adults, class 4 and 1 were most common (32.4% and 29.4%), followed by class 2 (27.9%), and least commonly class 3 (10.3%). In older adults, class 1 was most common (40.3%), followed by class 4 (23.6%), and least commonly classes 2 and 3 (18.1%).”
The researchers also used latent class analysis to identify four classes for the signs and symptoms of AD. In this model, class 1 had zero-low probability of all AD signs and class 2 had low probability of all AD signs. Class 3 had high probability of oozing lesions and low probability of all other signs, while class 4 had high probability of xerosis, intermediate probability of ichthyosis and palmar hyperlinearity, and low probability of all other AD signs.
In all three groups, the most common class was class 1 (85.6% of older adults, 81.8% of younger adults, and 82.6% of pediatric patients). Among the pediatric patients, they wrote, “class 3 was the second most common (8.7%), followed by class 2 and 4 (4.4%).” Among the young adults, 9.7% were in class 2, 5.7% were in class 4, and 2.8% were in class 3; and among the older adults, 8.3% were in class 4, 4.4% were in class 2, and 1.67% were in class 3.
Zelma Chiesa Fuxench, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, who was asked to comment on the study, said that while AD is traditionally largely thought of as a disease of children primarily involving the flexural areas, “this study provides additional evidence to support that AD is more than just a disease of childhood with a fixed clinical presentation, but is a heterogeneous disease whose clinical presentation varies across different population groups.”
While the study provides insight into the clinical differences that may be observed across AD groups, “care must be taken when interpreting these results as the study was done in a single center with observations collected during one single visit,” she added. “AD is not a ‘static’ disease; its presentation can stay the same in one patient but can vary even in another patient throughout their lifetime. Therefore, studies of a more prospective nature that evaluate the change in clinical presentation using multiple measures throughout time in these individuals would be a step forward to better understand if these phenotypic differences truly exist and, as such, what implications could they have for treatment selection.”
This study was supported by grants from the Agency for Healthcare Research and Quality and the Dermatology Foundation. The researchers reported having no disclosures. Dr. Chiesa Fuxench disclosed serving as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, Pfizer, AbbVie, and Incyte, for which she has received honoraria for AD-related work. She is the recipient of research grants through Regeneron, Sanofi, Tioga, Vanda, Menlo Therapeutics, Leo Pharma, and Eli Lilly for work related to AD as well as honoraria for continuing medical education work related to AD sponsored through educational grants from Regeneron/Sanofi and Pfizer.
, while older adults tend to present with less flexural eczema and the fewest associated signs.
Those are key findings from a study conducted at a single academic medical center, which aimed to identify the age-related clinical phenotypes of AD.
“Previous studies have found differences in the clinical characteristics of AD depending on age of AD onset, ethnic background, and AD severity,” senior author Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research in the department of dermatology at George Washington University, Washington, and his coauthor wrote in the study, which was published online in JAAD International. “However, none have prospectively compared the clinical characteristics and associated signs by age group. Improved understanding of the clinical phenotypes of AD may help guide choice of treatment and improve health outcomes,” they added.
Along with coauthor Sheena Chatrath, a dermatology research fellow in the department, Dr. Silverberg prospectively reviewed self-reported questionnaires that were completed by 380 patients prior to their visit at GWU’s eczema clinic between 2013 and 2019. Questions included age of AD onset, sociodemographics, Visual Analog Scale (VAS) itch and sleep for Scoring AD, and Numeric Rating Scale (NRS) for skin pain and itch. The researchers used the Eczema Area Severity Index to assess AD severity and a dermatologist conducted full body skin exams, noting the distribution of AD involvement as well as associated signs.
Of the 380 patients, 6.1% were younger than aged 18 years, 46.3% were young adults aged 18-39 years, and 47.6% were older adults 40 years of age and older.
Compared with pediatric patients, both young and older adults were less likely to experience AD on the ankles (adjusted odds ratio [aOR], 0.41 and 0.43, respectively), moderate to severe AD lesions on flexures (aOR, 0.47 and 0.30), pityriasis alba (aOR, 0.24 and 0.07), oozing lesions (aOR, 0.44 and 0.35), and moderate to severe excoriations (aOR, 0.49 and 0.44).
In children, severe itch was more common, reported in 47.1%, compared with 43.4% of the young adults and 38.6% of the older adults, and itch was less severe among the young and older adults. “Interestingly, despite increased itch in pediatric patients, we found no difference in the severity of skin pain across all age groups,” the researchers wrote. “Moreover, pediatric patients reported skin pain less often than adult patients. This may be due to age-related differences of pain perception.”
In other findings, compared with pediatric patients, young adults were more likely to experience AD around the eyes (aOR, 2.92), while older adults were less likely to experience AD on elbows (aOR, 0.34), nipples (aOR, 0.40), knees (aOR, 0.27), and less likely to have keratosis pilaris (aOR, 0.38), and lichenification (aOR, 0.47).
Dr. Silverberg and Ms. Chatrath used latent class analysis to identify four classes for distribution of AD lesions. In this model, class 1 had low probabilities of AD involvement at all sites examined and class 2 had low probabilities of scalp, face, and foot involvement, and intermediate probability of all other AD sites. Class 3 had low probabilities of hand and foot involvement, high probability of facial erythema, and intermediate probability of all other AD signs, while class 4 had intermediate probability of postauricular and foot involvement, and high probability of all other AD sites examined.
“Pediatric patients were most commonly in class 4 (33.3%), followed by class 1 and 2 (26.7%), and least commonly in class 3 (13.3%),” the authors wrote. “In young adults, class 4 and 1 were most common (32.4% and 29.4%), followed by class 2 (27.9%), and least commonly class 3 (10.3%). In older adults, class 1 was most common (40.3%), followed by class 4 (23.6%), and least commonly classes 2 and 3 (18.1%).”
The researchers also used latent class analysis to identify four classes for the signs and symptoms of AD. In this model, class 1 had zero-low probability of all AD signs and class 2 had low probability of all AD signs. Class 3 had high probability of oozing lesions and low probability of all other signs, while class 4 had high probability of xerosis, intermediate probability of ichthyosis and palmar hyperlinearity, and low probability of all other AD signs.
In all three groups, the most common class was class 1 (85.6% of older adults, 81.8% of younger adults, and 82.6% of pediatric patients). Among the pediatric patients, they wrote, “class 3 was the second most common (8.7%), followed by class 2 and 4 (4.4%).” Among the young adults, 9.7% were in class 2, 5.7% were in class 4, and 2.8% were in class 3; and among the older adults, 8.3% were in class 4, 4.4% were in class 2, and 1.67% were in class 3.
Zelma Chiesa Fuxench, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, who was asked to comment on the study, said that while AD is traditionally largely thought of as a disease of children primarily involving the flexural areas, “this study provides additional evidence to support that AD is more than just a disease of childhood with a fixed clinical presentation, but is a heterogeneous disease whose clinical presentation varies across different population groups.”
While the study provides insight into the clinical differences that may be observed across AD groups, “care must be taken when interpreting these results as the study was done in a single center with observations collected during one single visit,” she added. “AD is not a ‘static’ disease; its presentation can stay the same in one patient but can vary even in another patient throughout their lifetime. Therefore, studies of a more prospective nature that evaluate the change in clinical presentation using multiple measures throughout time in these individuals would be a step forward to better understand if these phenotypic differences truly exist and, as such, what implications could they have for treatment selection.”
This study was supported by grants from the Agency for Healthcare Research and Quality and the Dermatology Foundation. The researchers reported having no disclosures. Dr. Chiesa Fuxench disclosed serving as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, Pfizer, AbbVie, and Incyte, for which she has received honoraria for AD-related work. She is the recipient of research grants through Regeneron, Sanofi, Tioga, Vanda, Menlo Therapeutics, Leo Pharma, and Eli Lilly for work related to AD as well as honoraria for continuing medical education work related to AD sponsored through educational grants from Regeneron/Sanofi and Pfizer.
, while older adults tend to present with less flexural eczema and the fewest associated signs.
Those are key findings from a study conducted at a single academic medical center, which aimed to identify the age-related clinical phenotypes of AD.
“Previous studies have found differences in the clinical characteristics of AD depending on age of AD onset, ethnic background, and AD severity,” senior author Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research in the department of dermatology at George Washington University, Washington, and his coauthor wrote in the study, which was published online in JAAD International. “However, none have prospectively compared the clinical characteristics and associated signs by age group. Improved understanding of the clinical phenotypes of AD may help guide choice of treatment and improve health outcomes,” they added.
Along with coauthor Sheena Chatrath, a dermatology research fellow in the department, Dr. Silverberg prospectively reviewed self-reported questionnaires that were completed by 380 patients prior to their visit at GWU’s eczema clinic between 2013 and 2019. Questions included age of AD onset, sociodemographics, Visual Analog Scale (VAS) itch and sleep for Scoring AD, and Numeric Rating Scale (NRS) for skin pain and itch. The researchers used the Eczema Area Severity Index to assess AD severity and a dermatologist conducted full body skin exams, noting the distribution of AD involvement as well as associated signs.
Of the 380 patients, 6.1% were younger than aged 18 years, 46.3% were young adults aged 18-39 years, and 47.6% were older adults 40 years of age and older.
Compared with pediatric patients, both young and older adults were less likely to experience AD on the ankles (adjusted odds ratio [aOR], 0.41 and 0.43, respectively), moderate to severe AD lesions on flexures (aOR, 0.47 and 0.30), pityriasis alba (aOR, 0.24 and 0.07), oozing lesions (aOR, 0.44 and 0.35), and moderate to severe excoriations (aOR, 0.49 and 0.44).
In children, severe itch was more common, reported in 47.1%, compared with 43.4% of the young adults and 38.6% of the older adults, and itch was less severe among the young and older adults. “Interestingly, despite increased itch in pediatric patients, we found no difference in the severity of skin pain across all age groups,” the researchers wrote. “Moreover, pediatric patients reported skin pain less often than adult patients. This may be due to age-related differences of pain perception.”
In other findings, compared with pediatric patients, young adults were more likely to experience AD around the eyes (aOR, 2.92), while older adults were less likely to experience AD on elbows (aOR, 0.34), nipples (aOR, 0.40), knees (aOR, 0.27), and less likely to have keratosis pilaris (aOR, 0.38), and lichenification (aOR, 0.47).
Dr. Silverberg and Ms. Chatrath used latent class analysis to identify four classes for distribution of AD lesions. In this model, class 1 had low probabilities of AD involvement at all sites examined and class 2 had low probabilities of scalp, face, and foot involvement, and intermediate probability of all other AD sites. Class 3 had low probabilities of hand and foot involvement, high probability of facial erythema, and intermediate probability of all other AD signs, while class 4 had intermediate probability of postauricular and foot involvement, and high probability of all other AD sites examined.
“Pediatric patients were most commonly in class 4 (33.3%), followed by class 1 and 2 (26.7%), and least commonly in class 3 (13.3%),” the authors wrote. “In young adults, class 4 and 1 were most common (32.4% and 29.4%), followed by class 2 (27.9%), and least commonly class 3 (10.3%). In older adults, class 1 was most common (40.3%), followed by class 4 (23.6%), and least commonly classes 2 and 3 (18.1%).”
The researchers also used latent class analysis to identify four classes for the signs and symptoms of AD. In this model, class 1 had zero-low probability of all AD signs and class 2 had low probability of all AD signs. Class 3 had high probability of oozing lesions and low probability of all other signs, while class 4 had high probability of xerosis, intermediate probability of ichthyosis and palmar hyperlinearity, and low probability of all other AD signs.
In all three groups, the most common class was class 1 (85.6% of older adults, 81.8% of younger adults, and 82.6% of pediatric patients). Among the pediatric patients, they wrote, “class 3 was the second most common (8.7%), followed by class 2 and 4 (4.4%).” Among the young adults, 9.7% were in class 2, 5.7% were in class 4, and 2.8% were in class 3; and among the older adults, 8.3% were in class 4, 4.4% were in class 2, and 1.67% were in class 3.
Zelma Chiesa Fuxench, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, who was asked to comment on the study, said that while AD is traditionally largely thought of as a disease of children primarily involving the flexural areas, “this study provides additional evidence to support that AD is more than just a disease of childhood with a fixed clinical presentation, but is a heterogeneous disease whose clinical presentation varies across different population groups.”
While the study provides insight into the clinical differences that may be observed across AD groups, “care must be taken when interpreting these results as the study was done in a single center with observations collected during one single visit,” she added. “AD is not a ‘static’ disease; its presentation can stay the same in one patient but can vary even in another patient throughout their lifetime. Therefore, studies of a more prospective nature that evaluate the change in clinical presentation using multiple measures throughout time in these individuals would be a step forward to better understand if these phenotypic differences truly exist and, as such, what implications could they have for treatment selection.”
This study was supported by grants from the Agency for Healthcare Research and Quality and the Dermatology Foundation. The researchers reported having no disclosures. Dr. Chiesa Fuxench disclosed serving as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, Pfizer, AbbVie, and Incyte, for which she has received honoraria for AD-related work. She is the recipient of research grants through Regeneron, Sanofi, Tioga, Vanda, Menlo Therapeutics, Leo Pharma, and Eli Lilly for work related to AD as well as honoraria for continuing medical education work related to AD sponsored through educational grants from Regeneron/Sanofi and Pfizer.
FROM JAAD INTERNATIONAL
Two short-term exposure therapies linked to PTSD reductions
Two forms of short-term exposure therapy may help reduce symptoms of posttraumatic stress disorder, new research suggests.
In addition, remission rates of around 50% were sustained in both groups up to the 6-month mark.
“With about two-thirds of study participants reporting clinically meaningful symptom improvement and more than half losing their PTSD diagnosis, this study provides important new evidence that combat-related PTSD can be effectively treated – in as little as 3 weeks,” lead investigator Alan Peterson, PhD, told this news organization.
Dr. Peterson, professor of psychiatry and behavioral sciences at the University of Texas Health Science Center, San Antonio, and director of the Consortium to Alleviate PTSD, noted that while condensed treatments may not be feasible for everyone, “results show that compressed formats adapted to the military context resulted in significant, meaningful, and lasting improvements in PTSD, disability, and functional impairments for most participants.”
The findings were published online in JAMA Network Open.
Breathing, direct exposure, education
The investigators randomly recruited 234 military personnel and veterans from two military treatment facilities and two Veterans Affairs facilities in south and central Texas.
Participants (78% men; mean age, 39 years) were active-duty service members or veterans who had deployed post Sept. 11 and met diagnostic criteria for PTSD. They could receive psychotropic medications at stable doses and were excluded if they had mania, substance abuse, psychosis, or suicidality.
The sample included 44% White participants, 26% Black participants, and 25% Hispanic participants.
The researchers randomly assigned the participants to receive either massed-PE (n = 117) or IOP-PE (n = 117).
PE, the foundation of both protocols, includes psychoeducation about trauma, diaphragmatic breathing, direct and imaginal exposure, and processing of the trauma.
The massed-PE protocol was delivered in 15 daily 90-minute sessions over 3 consecutive weeks, as was the IOP-PE. However, the IOP-PE also included eight additional multiple daily feedback sessions, homework, social support from friends or family, and three booster sessions post treatment.
The investigators conducted baseline assessments and follow-up assessments at 1 month, 3 months, and 6 months. At the 6-month follow-up, there were 57 participants left to analyze in the massed-PE group and 57 in the IOP-PE group.
Significantly decreased symptoms
As measured by the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5), PTSD symptoms decreased significantly from baseline to the 1-month follow-up in both groups (massed-PE mean change, –14.13; P < .001; IOP-PE mean change, –13.85; P < .001).
Both groups also failed to meet PTSD diagnostic criteria at 1-, 3-, and 6-month follow-ups.
At the 1-month follow-up, 62% of participants who received massed-PE and 48% of those who received IOP-PE no longer met diagnostic criteria on the CAPS-5. Diagnostic remission was maintained in more than half of the massed-PE group (52%) and the IOP-PE group (53%) at the 6-month follow-up.
Disability scores as measured by the Sheehan Disability Scale also decreased significantly in both groups (P < .001) from baseline to the 1-month follow-up mark; as did psychosocial functioning scores, as reflected by the Brief Inventory of Psychosocial Functioning (P < .001).
Dr. Peterson noted that the condensed treatment format could be an essential option to consider even in other countries, such as Ukraine, where there are concerns about PTSD in military personnel.
Study limitations included the lack of a placebo or inactive comparison group, and the lack of generalizability of the results to the entire population of U.S. service members and veterans outside of Texas.
Dr. Peterson said he plans to continue his research and that the compressed treatment formats studied “are well-suited for the evaluation of alternative modes of therapy combining cognitive-behavioral treatments with medications and medical devices.”
Generalizability limited?
Commenting on the research, Joshua Morganstein, MD, chair of the American Psychiatric Association’s committee on the psychiatric dimensions of disaster, said he was reassured to see participants achieve and keep improvements throughout the study.
“One of the biggest challenges we have, particularly with trauma and stress disorders, is keeping people in therapy” because of the difficult nature of the exposure therapy, said Dr. Morganstein, who was not involved with the research.
“The number of people assigned to each group and who ultimately completed the last follow-up gives a good idea of the utility of the intervention,” he added.
However, Dr. Morganstein noted that some of the exclusion criteria, particularly suicidality and substance abuse, affected the study’s relevance to real-world populations.
“The people in the study become less representative of those who are actually in clinical care,” he said, noting that these two conditions are often comorbid with PTSD.
The study was funded by the Department of Defense, the Defense Health Program, the Psychological Health and Traumatic Brain Injury Research Program, the Department of Veterans Affairs, the Office of Research and Development, and the Clinical Science Research & Development Service. The investigators and Dr. Morganstein have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Two forms of short-term exposure therapy may help reduce symptoms of posttraumatic stress disorder, new research suggests.
In addition, remission rates of around 50% were sustained in both groups up to the 6-month mark.
“With about two-thirds of study participants reporting clinically meaningful symptom improvement and more than half losing their PTSD diagnosis, this study provides important new evidence that combat-related PTSD can be effectively treated – in as little as 3 weeks,” lead investigator Alan Peterson, PhD, told this news organization.
Dr. Peterson, professor of psychiatry and behavioral sciences at the University of Texas Health Science Center, San Antonio, and director of the Consortium to Alleviate PTSD, noted that while condensed treatments may not be feasible for everyone, “results show that compressed formats adapted to the military context resulted in significant, meaningful, and lasting improvements in PTSD, disability, and functional impairments for most participants.”
The findings were published online in JAMA Network Open.
Breathing, direct exposure, education
The investigators randomly recruited 234 military personnel and veterans from two military treatment facilities and two Veterans Affairs facilities in south and central Texas.
Participants (78% men; mean age, 39 years) were active-duty service members or veterans who had deployed post Sept. 11 and met diagnostic criteria for PTSD. They could receive psychotropic medications at stable doses and were excluded if they had mania, substance abuse, psychosis, or suicidality.
The sample included 44% White participants, 26% Black participants, and 25% Hispanic participants.
The researchers randomly assigned the participants to receive either massed-PE (n = 117) or IOP-PE (n = 117).
PE, the foundation of both protocols, includes psychoeducation about trauma, diaphragmatic breathing, direct and imaginal exposure, and processing of the trauma.
The massed-PE protocol was delivered in 15 daily 90-minute sessions over 3 consecutive weeks, as was the IOP-PE. However, the IOP-PE also included eight additional multiple daily feedback sessions, homework, social support from friends or family, and three booster sessions post treatment.
The investigators conducted baseline assessments and follow-up assessments at 1 month, 3 months, and 6 months. At the 6-month follow-up, there were 57 participants left to analyze in the massed-PE group and 57 in the IOP-PE group.
Significantly decreased symptoms
As measured by the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5), PTSD symptoms decreased significantly from baseline to the 1-month follow-up in both groups (massed-PE mean change, –14.13; P < .001; IOP-PE mean change, –13.85; P < .001).
Both groups also failed to meet PTSD diagnostic criteria at 1-, 3-, and 6-month follow-ups.
At the 1-month follow-up, 62% of participants who received massed-PE and 48% of those who received IOP-PE no longer met diagnostic criteria on the CAPS-5. Diagnostic remission was maintained in more than half of the massed-PE group (52%) and the IOP-PE group (53%) at the 6-month follow-up.
Disability scores as measured by the Sheehan Disability Scale also decreased significantly in both groups (P < .001) from baseline to the 1-month follow-up mark; as did psychosocial functioning scores, as reflected by the Brief Inventory of Psychosocial Functioning (P < .001).
Dr. Peterson noted that the condensed treatment format could be an essential option to consider even in other countries, such as Ukraine, where there are concerns about PTSD in military personnel.
Study limitations included the lack of a placebo or inactive comparison group, and the lack of generalizability of the results to the entire population of U.S. service members and veterans outside of Texas.
Dr. Peterson said he plans to continue his research and that the compressed treatment formats studied “are well-suited for the evaluation of alternative modes of therapy combining cognitive-behavioral treatments with medications and medical devices.”
Generalizability limited?
Commenting on the research, Joshua Morganstein, MD, chair of the American Psychiatric Association’s committee on the psychiatric dimensions of disaster, said he was reassured to see participants achieve and keep improvements throughout the study.
“One of the biggest challenges we have, particularly with trauma and stress disorders, is keeping people in therapy” because of the difficult nature of the exposure therapy, said Dr. Morganstein, who was not involved with the research.
“The number of people assigned to each group and who ultimately completed the last follow-up gives a good idea of the utility of the intervention,” he added.
However, Dr. Morganstein noted that some of the exclusion criteria, particularly suicidality and substance abuse, affected the study’s relevance to real-world populations.
“The people in the study become less representative of those who are actually in clinical care,” he said, noting that these two conditions are often comorbid with PTSD.
The study was funded by the Department of Defense, the Defense Health Program, the Psychological Health and Traumatic Brain Injury Research Program, the Department of Veterans Affairs, the Office of Research and Development, and the Clinical Science Research & Development Service. The investigators and Dr. Morganstein have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Two forms of short-term exposure therapy may help reduce symptoms of posttraumatic stress disorder, new research suggests.
In addition, remission rates of around 50% were sustained in both groups up to the 6-month mark.
“With about two-thirds of study participants reporting clinically meaningful symptom improvement and more than half losing their PTSD diagnosis, this study provides important new evidence that combat-related PTSD can be effectively treated – in as little as 3 weeks,” lead investigator Alan Peterson, PhD, told this news organization.
Dr. Peterson, professor of psychiatry and behavioral sciences at the University of Texas Health Science Center, San Antonio, and director of the Consortium to Alleviate PTSD, noted that while condensed treatments may not be feasible for everyone, “results show that compressed formats adapted to the military context resulted in significant, meaningful, and lasting improvements in PTSD, disability, and functional impairments for most participants.”
The findings were published online in JAMA Network Open.
Breathing, direct exposure, education
The investigators randomly recruited 234 military personnel and veterans from two military treatment facilities and two Veterans Affairs facilities in south and central Texas.
Participants (78% men; mean age, 39 years) were active-duty service members or veterans who had deployed post Sept. 11 and met diagnostic criteria for PTSD. They could receive psychotropic medications at stable doses and were excluded if they had mania, substance abuse, psychosis, or suicidality.
The sample included 44% White participants, 26% Black participants, and 25% Hispanic participants.
The researchers randomly assigned the participants to receive either massed-PE (n = 117) or IOP-PE (n = 117).
PE, the foundation of both protocols, includes psychoeducation about trauma, diaphragmatic breathing, direct and imaginal exposure, and processing of the trauma.
The massed-PE protocol was delivered in 15 daily 90-minute sessions over 3 consecutive weeks, as was the IOP-PE. However, the IOP-PE also included eight additional multiple daily feedback sessions, homework, social support from friends or family, and three booster sessions post treatment.
The investigators conducted baseline assessments and follow-up assessments at 1 month, 3 months, and 6 months. At the 6-month follow-up, there were 57 participants left to analyze in the massed-PE group and 57 in the IOP-PE group.
Significantly decreased symptoms
As measured by the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5), PTSD symptoms decreased significantly from baseline to the 1-month follow-up in both groups (massed-PE mean change, –14.13; P < .001; IOP-PE mean change, –13.85; P < .001).
Both groups also failed to meet PTSD diagnostic criteria at 1-, 3-, and 6-month follow-ups.
At the 1-month follow-up, 62% of participants who received massed-PE and 48% of those who received IOP-PE no longer met diagnostic criteria on the CAPS-5. Diagnostic remission was maintained in more than half of the massed-PE group (52%) and the IOP-PE group (53%) at the 6-month follow-up.
Disability scores as measured by the Sheehan Disability Scale also decreased significantly in both groups (P < .001) from baseline to the 1-month follow-up mark; as did psychosocial functioning scores, as reflected by the Brief Inventory of Psychosocial Functioning (P < .001).
Dr. Peterson noted that the condensed treatment format could be an essential option to consider even in other countries, such as Ukraine, where there are concerns about PTSD in military personnel.
Study limitations included the lack of a placebo or inactive comparison group, and the lack of generalizability of the results to the entire population of U.S. service members and veterans outside of Texas.
Dr. Peterson said he plans to continue his research and that the compressed treatment formats studied “are well-suited for the evaluation of alternative modes of therapy combining cognitive-behavioral treatments with medications and medical devices.”
Generalizability limited?
Commenting on the research, Joshua Morganstein, MD, chair of the American Psychiatric Association’s committee on the psychiatric dimensions of disaster, said he was reassured to see participants achieve and keep improvements throughout the study.
“One of the biggest challenges we have, particularly with trauma and stress disorders, is keeping people in therapy” because of the difficult nature of the exposure therapy, said Dr. Morganstein, who was not involved with the research.
“The number of people assigned to each group and who ultimately completed the last follow-up gives a good idea of the utility of the intervention,” he added.
However, Dr. Morganstein noted that some of the exclusion criteria, particularly suicidality and substance abuse, affected the study’s relevance to real-world populations.
“The people in the study become less representative of those who are actually in clinical care,” he said, noting that these two conditions are often comorbid with PTSD.
The study was funded by the Department of Defense, the Defense Health Program, the Psychological Health and Traumatic Brain Injury Research Program, the Department of Veterans Affairs, the Office of Research and Development, and the Clinical Science Research & Development Service. The investigators and Dr. Morganstein have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN