User login
Asthma affects more than 300 million people worldwide.1 While many of these cases can achieve control with standard therapy, 5% to 10% of these cases are classified as severe asthma, remaining poorly controlled despite treatment with inhaled corticosteroids (ICS) and long-acting β agonists (LABA).2 These patients also account for the majority of morbidity and mortality associated with the disease, with increased hospitalizations, intensive care unit (ICU) stays, detrimental adverse effects of oral corticosteroids (OCS), and lower quality of life.3-6 Additionally, the financial repercussions of severe asthma are notable; in the United States, the estimated cost of asthma management is $82 billion annually, with $3 billion accounting for asthma-related work/school absences.7
In the past several years, the use of anti-immunoglobulin E (IgE), anti-interleukin-4 (IL-4), and anti-IL-5 biologic agents for severe asthma has been shown to decrease asthma exacerbations, improve lung function, reduce corticosteroid use, and decrease hospitalizations, especially for type 2 helper T cell (TH2-high) asthma.8-10 However, clinicians have observed significant barriers to the implementation and widespread use of biologics, including insurance coverage, long wait times, follow-up, and limited access for lower income groups.11,12
This article describes a unique model for a severe asthma clinic located at the Washington DC Veterans Affairs Medical Center (WDCVAMC) that is dually staffed by an allergist and pulmonologist. This clinic uses biologic agents for patients with difficult-to-treat asthma, many of whom require repeated or prolonged steroid use, in addition to prolonged and recurrent hospitalizations for exacerbations. The objective of this clinic is to provide a standardized approach to the management of severe asthma with the perspective of both an allergist and pulmonologist, thereby reducing the need to schedule appointments with multiple specialties and reducing delays in initiating biologics. This article presents the preliminary findings of 30 months of severe asthma management with various biologic agents, examining the impact of these therapies on hospitalizations, asthma exacerbations, ICU stays, and OCS use. The findings of this study support the benefits of biologics and suggest that the use of these agents within a dually staffed clinic may be a particularly effective model through which to manage severe asthma.
Background
Asthma affects approximately 20 million adults in the United States.13 Veterans are a population particularly impacted by asthma. Between 2015 and 2018, 10.9% of all veterans reported being diagnosed with asthma and 5.1% stated that they currently have asthma, compared with 13.4% and 8.0% of nonveterans, respectively.14 Veterans are susceptible to many of the factors that can trigger exacerbations while engaging in military service, such as chemical and environmental exposures both abroad and domestically.15,16 Additionally, medication adherence is often challenging among the veteran population, particularly with more involved therapy, such as inhaler use.17 Such factors contribute to asthma exacerbations, with 2.9% of veterans reporting at least 1 asthma exacerbation in the past 12 months.14
Over the past several years, the development and use of biologic agents have transformed the management of severe asthma.8 Before the development of biologic agents for severe asthma, treatment options for patients were limited. While OCS are frequently used for asthma exacerbations, they are associated with a multiplicity of undesirable adverse effects, including weight gain, mood lability, gastrointestinal upset, hyperglycemia, risk of bone fractures, and hypertension.18-20 The regular use of OCS are particularly problematic among other medical comorbidities commonly affecting the veteran population, such as diabetes and hypertension.21-22
The WDCVAMC severe allergy clinic used 3 biologic agents: omalizumab (anti-IgE), benralizumab (anti-IL-5), and agent dupilumab (anti-IL-4). These medications have shown significant improvements in quality of life, reduction in asthma exacerbations and hospitalizations, and decreased use of OCS.8,9 While research has firmly established the medical benefits of the use of biologic agents in severe asthma, several barriers exist in implementing widespread use.11,12
In Gelhorn and colleagues’ study examining both physician and patient challenges in the use of biologics for severe asthma, scheduling, administrative time, and insurance costs were found to be major barriers to the use of these medications.12 Patients expressed a preference for the administration of these medications in a specialist’s office but cited long wait times and scheduling difficulties as barriers. One of the most notable challenges from the physician perspective was the difficulty in obtaining reimbursement from insurance companies, requiring them to devote significant portions of time to prior authorizations and documentation.12
This article examines a dual specialty clinic that focuses on the treatment of severe asthma with biologic agents. This model is unique for several reasons. First, given the US Department of Veterans Affairs (VA) health care model, the health care practitioners (HCPs) in this clinic can avoid much of the administrative burden of obtaining reimbursement or working with insurance companies. Additionally, by dedicating specific days to the severe asthma clinic, patients do not experience long wait times to see both an allergist and pulmonologist. By seeing both clinicians, concurrent allergic and pulmonary issues can be addressed in the same visit, rather than delaying treatment by waiting on 2 specialist appointments.
Severe Asthma Clinic
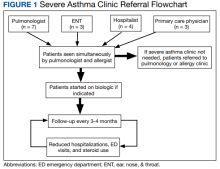
The severe asthma clinic was started in September 2017 by a pulmonologist and an allergist at WDCVAMC. After experiencing substantial delays with the initiation of biologics for their patients and multiple referrals between their clinics, these physicians wanted to start a dually staffed asthma clinic to specifically focus on evaluating and treating severe asthma. A dedicated severe asthma clinic allowed the allergist and pulmonologist to streamline resources and collaborate to advocate for patients with the pharmacy section. Additionally, patients can benefit from the perspective of both specialists, as both the pulmonologist and allergist evaluate each patient and discuss the next steps of management.
This clinic is composed of 4 registered nurses, an allergist, and a pulmonologist. Clinic is held twice monthly through both telemedicine and in-office visits. The VA has strict guidelines for the use of certain biologics, including blood eosinophil count > 150 cells/µL, failure of traditional therapy, and frequent use of OCS. Additionally, to ensure these biologic agents are prescribed to patients that will benefit from them, the patients enrolled in this clinic are already on maximum therapy for their asthma, meaning all other therapeutic options (inhalers and oral medications) are being used. The clinic services all patients with severe asthma, not just patients who are on biologic therapy. Often, patients are referred to the severe allergy clinic late in their disease course given a lack of familiarity with biologic agents from prescribers and both institutional and insurance barriers.
Before the COVID-19 pandemic, spirometry and fractional exhaled nitric oxide (FENO) tests were recorded at each visit. Initially during the pandemic, the clinic transitioned to primarily telemedicine visits due to patients’ hesitance to seek in-person care. More recently, the clinic has transitioned back to primarily office visits; patients are seen in clinic on average every 3 months. At each visit, the patient is seen by both the pulmonologist and allergist. Additionally, the nursing staff reviews inhaler adherence with patients, spacer use, documents, Asthma Control Test (ACT) scores, and schedules follow-up visits.
Every 4 to 8 weeks, patients receive biologics agent at the WDCVAMC infusion center depending on the agent. The infusion center also instructs patients how to handle self-administered medications, like benralizumab, if the patient expresses a preference for taking it at home. Omalizumab has a boxed warning for anaphylaxis, although the other biologics in this study have a low risk of anaphylaxis. All patients receiving omalizumab, benralizumab, and dupilumab were provided with epinephrine injection devices in case of an allergic reaction and were taught how to use them in the clinic.23,24
If patients continued to experience asthma exacerbations after the initiation of a biologic, a change in agent was considered after 4 to 6 months. Additionally, a complete blood count, respiratory allergy panel, and pulmonary function tests (PFTs) were completed.
Clinic Patients
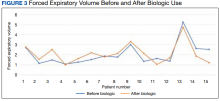
Preliminary data were obtained from a retrospective chart review of 15 patients enrolled in the severe asthma clinic over 30 months. The inclusion criteria for chart review consisted of patients aged > 18 years receiving a biologic agent for > 3 months for the treatment of severe asthma. The outcomes examined included steroid use, emergency department (ED) visits, hospitalizations, FEV1, and ICU stays.
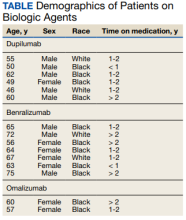
Seven patients used benralizumab, 6 used dupilumab, and 2 used omalizumab (Table).
There was a notable clinical improvement in these patients. Before starting a biologic agent, all the patients in this study were prescribed steroids at least once a year for an asthma exacerbation, with a mean of 4.2 steroid tapers per year.
The initiation of a biologic agent also resulted in fewer ED visits and hospitalizations. Two patients had an ED visit for an asthma exacerbation since starting a biologic agent and 1 patient had a hospital admission for an asthma exacerbation. No patients were hospitalized in the ICU after starting a biologic agent.
Discussion
The 15 patients in this initial data were referred to the severe asthma clinic by pulmonology, ear, nose, and throat (ENT), primary care, and a hospitalist during an in-patient stay. As the enrollment in our clinic grows, an increasing number of patients are referred from the allergy clinic as well. Patients in the severe asthma clinic also are referred by regional centers as news of the clinic is spread by word of mouth to surrounding VA facilities. As our clinic gains the capacity to serve more patients, we hope to contact WDCVAMC primary care, pulmonology, allergy, and ENT departments to raise awareness of the clinic.
Benralizumab and dupilumab were the most used agents in this preliminary data. This finding was largely due to the ability of patients to self-administer benralizumab, which was particularly beneficial during the COVID-19 pandemic. Of note, 5 patients in this study switched from another biologic agent to benralizumab due to the ability to self-administer. Three of 5 patients that required steroids after initiating benralizumab used fewer steroids than they had previously. This finding suggests benralizumab may be the preferred agent when travel time to health care is a challenge, reducing the need for frequent clinic visits and transportation.
This preliminary data supports previous studies that have demonstrated that biologic agents improve clinical outcomes by reducing asthma exacerbations, OCS use, hospitalizations, and ICU stays for patients on all 4 biologic agents. In addition to improving patient health through avoiding complications of prolonged OCS use and hospital stays, the decrease in ED visits and hospitalizations provides a substantial cost reduction to the health care system.
These findings highlight the strength of a unique model of a combined allergy/pulmonary clinic. Before this combined clinic model, both pulmonology and allergy clinics noted delays in the initiation of biologics for patients who were potential candidates. Impediments include referrals between each specialty for evaluation of concurrent pulmonary conditions or allergy testing, overlap in asthma management, and a delay in coordination with the pharmacy department to start biologic agents. A dedicated severe asthma clinic staffed by both an allergist and pulmonologist provides a convenient option for patients to be seen by both specialists, reducing the need for separate appointments with each specialty, transportation to those appointments, and clinical time. This is particularly beneficial in a clinic such as this model, as this clinic serves patients from 4 states and Washington, DC. An additional benefit of this model is trained staff who directly communicate with the pharmacy in the initiation of these agents, allocate time to educating patients in biologic use, and coordinate follow-up.
Limitations
There were several limitations to this report. First, the number of patients examined in this preliminary data set is small. Due to the COVID-19 pandemic, there was a limited ability to see patients in person, and patients were seen exclusively over telemedicine for several months. For this reason, collecting data such as patient surveys and laboratory work following the initiation of a biologic was a challenge. Additionally, during the height of COVID-19, WDCVAMC did not perform aerosolizing procedures, such as PFTs and FENOs; thus, peak flows were obtained instead. Examining metrics, such as FENOs and IgE levels, and expanding PFT data would provide additional insight into the impact of biologic agents on clinical outcomes. Patient survey data in the form of ACTs or satisfaction surveys would also yield important data examining the impact of this clinic design and biologic use on patient experience. As of December 2022, 114 patients are enrolled in the clinic. We are working to collect the above laboratory results and spirometry for these patients so that these results can be published with a more robust data set. Another limitation of the information presented is that it is a retrospective data analysis; the data collected was contingent upon documentation and the assumption that these patients were exclusively receiving care through the VA. For example, steroid use before and after initiation of biologic was taken from asthma clinic notes and the patient’s medication list. Therefore, there is a possibility that not all instances were accounted for if that patient sought care outside the VA or whether it was not documented in a follow-up note.
Conclusions
The model of a combined allergy/pulmonology clinic can be particularly efficacious in the treatment of severe asthma, as it reduces the need for multiple appointments with different specialties, reduces wait time before starting a biologic agent, and offers the perspective of 2 specialists. This kind of model could be an example to many clinics in the VA. With a rapid increase in telemedicine due to the COVID-19 pandemic, multiple physicians consulting simultaneously is becoming a more feasible possibility across multiple specialties. As the use of biologics becomes more widespread, a combined clinic design is an efficient and promising method to improve severe asthma management.
This preliminary data continue to support previous research that shows biologic agents have led to better clinical outcomes through the reduction of asthma exacerbations, hospitalizations, and improved PFTs. While this initial data set highlights the results for 15 patients, there are 86 patients currently enrolled in this clinic. We are collecting additional data to publish more comprehensive results.
1. Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16(1):45-56. doi:10.1038/ni.3049
2. Moore WC, Bleecker ER, Curran-Everett D, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119(2):405-413. doi:10.1016/j.jaci.2006.11.639
3. Barnes PJ, Jonsson B, Klim JB. The costs of asthma. Eur Respir J. 1996;9(4):636-642. doi:10.1183/09031936.96.09040636
4. Bourdin A, Charriot J, Boissin C, et al. Will the asthma revolution fostered by biologics also benefit adult ICU patients?. Allergy. 2021;76(8):2395-2406. doi:10.1111/all.14688
5. Lloyd A, Price D, Brown R. The impact of asthma exacerbations on health-related quality of life in moderate to severe asthma patients in the UK. Prim Care Respir J. 2007;16(1):22-27. doi:10.3132/pcrj.2007.00002
6. Eisner MD, Yelin EH, Katz PP, Lactao G, Iribarren C, Blanc PD. Risk factors for work disability in severe adult asthma. Am J Med. 2006;119(10):884-891. doi:10.1016/j.amjmed.2006.01.016
7. Nurmagambetov T, Kuwahara R, Garbe P. The Economic Burden of Asthma in the United States, 2008-2013. Ann Am Thorac Soc. 2018;15(3):348-356. doi:10.1513/AnnalsATS.201703-259OC
8. McGregor MC, Krings JG, Nair P, Castro M. Role of biologics in asthma. Am J Respir Crit Care Med. 2019;199(4):433-445. doi:10.1164/rccm.201810-1944CI
9. Bice JB, Leechawengwongs E, Montanaro A. Biologic targeted therapy in allergic asthma. Ann Allergy Asthma Immunol. 2014;112(2):108-115. doi:10.1016/j.anai.2013.12.013
10. Darveaux J, Busse WW. Biologics in asthma--the next step toward personalized treatment. J Allergy Clin Immunol Pract. 2015;3(2):152-161. doi:10.1016/j.jaip.2014.09.014
11. Inselman JW, Jeffery MM, Maddux JT, Shah ND, Rank MA. Trends and disparities in asthma biologic use in the United States. J Allergy Clin Immunol Pract. 2020;8(2):549-554.e1. doi:10.1016/j.jaip.2019.08.024
12. Gelhorn HL, Balantac Z, Ambrose CS, Chung YN, Stone B. Patient and physician preferences for attributes of biologic medications for severe asthma. Patient Prefer Adherence. 2019;13:1253-1268. Published 2019 Jul 25. doi:10.2147/PPA.S198953
13. Centers for Disease Control and Prevention, National Center for Environmental Health. 2019 National Health Interview Survey (NHIS) data. Accessed December 6, 2022. https://www.cdc.gov/asthma/nhis/2019/data.htm
14. Zelaya CE BP, Moy E. Crude and age-adjusted percent distribution of respondent-assessed health status among adults aged 20 and over, by veteran status and other selected characteristics: United States, 2015-2018. National Center for Health Statistic. Updated June 19, 2020. Accessed December 12, 2022. https://www.cdc.gov/nchs/nhis/veterans_health_statistics/tables.htm
15. Szema AM, Peters MC, Weissinger KM, Gagliano CA, Chen JJ. New-onset asthma among soldiers serving in Iraq and Afghanistan. Allergy Asthma Proc. 2010;31(5):67-71. doi:10.2500/aap.2010.31.3383
16. Yi SW, Hong JS, Ohrr H, Yi JJ. Agent Orange exposure and disease prevalence in Korean Vietnam veterans: the Korean veterans health study. Environ Res. 2014;133:56-65. doi:10.1016/j.envres.2014.04.027
17. Huetsch JC, Uman JE, Udris EM, Au DH. Predictors of adherence to inhaled medications among veterans with COPD. J Gen Intern Med. 2012;27(11):1506-1512. doi:10.1007/s11606-012-2130-5
18. Mundell L, Lindemann R, Douglas J. Monitoring long-term oral corticosteroids. BMJ Open Qual. 2017;6(2):e000209. Published 2017 Nov 8. doi:10.1136/bmjoq-2017-000209
19. Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2005;20(8):1487-1486. doi:10.1359/jbmr.2005.20.8.1486
20. Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006;81(10):1361-1367. doi:10.4065/81.10.1361
21. Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care. 2004;27(suppl 2):B10-B21. doi:10.2337/diacare.27.suppl_2.b10
22. Acharya T, Tringali S, Singh M, Huang J. Resistant hypertension and associated comorbidities in a Veterans Affairs population. J Clin Hypertens (Greenwich). 2014;16(10):741-745. doi:10.1111/jch.12410
23. Dupixent (dupilumab). Prescribing information. Sanofi and Regeneron Pharmaceuticals; 2022. Accessed December 6, 2022. https://www.dupixenthcp.com/asthma/efficacy/safety-data
24. Li L, Wang Z, Cui L, Xu Y, Guan K, Zhao B. Anaphylactic risk related to omalizumab, benralizumab, reslizumab, mepolizumab, and dupilumab. Clin Transl Allergy. 2021;11(4):e12038. Published 2021 Jun 3. doi:10.1002/clt2.12038
Asthma affects more than 300 million people worldwide.1 While many of these cases can achieve control with standard therapy, 5% to 10% of these cases are classified as severe asthma, remaining poorly controlled despite treatment with inhaled corticosteroids (ICS) and long-acting β agonists (LABA).2 These patients also account for the majority of morbidity and mortality associated with the disease, with increased hospitalizations, intensive care unit (ICU) stays, detrimental adverse effects of oral corticosteroids (OCS), and lower quality of life.3-6 Additionally, the financial repercussions of severe asthma are notable; in the United States, the estimated cost of asthma management is $82 billion annually, with $3 billion accounting for asthma-related work/school absences.7
In the past several years, the use of anti-immunoglobulin E (IgE), anti-interleukin-4 (IL-4), and anti-IL-5 biologic agents for severe asthma has been shown to decrease asthma exacerbations, improve lung function, reduce corticosteroid use, and decrease hospitalizations, especially for type 2 helper T cell (TH2-high) asthma.8-10 However, clinicians have observed significant barriers to the implementation and widespread use of biologics, including insurance coverage, long wait times, follow-up, and limited access for lower income groups.11,12
This article describes a unique model for a severe asthma clinic located at the Washington DC Veterans Affairs Medical Center (WDCVAMC) that is dually staffed by an allergist and pulmonologist. This clinic uses biologic agents for patients with difficult-to-treat asthma, many of whom require repeated or prolonged steroid use, in addition to prolonged and recurrent hospitalizations for exacerbations. The objective of this clinic is to provide a standardized approach to the management of severe asthma with the perspective of both an allergist and pulmonologist, thereby reducing the need to schedule appointments with multiple specialties and reducing delays in initiating biologics. This article presents the preliminary findings of 30 months of severe asthma management with various biologic agents, examining the impact of these therapies on hospitalizations, asthma exacerbations, ICU stays, and OCS use. The findings of this study support the benefits of biologics and suggest that the use of these agents within a dually staffed clinic may be a particularly effective model through which to manage severe asthma.
Background
Asthma affects approximately 20 million adults in the United States.13 Veterans are a population particularly impacted by asthma. Between 2015 and 2018, 10.9% of all veterans reported being diagnosed with asthma and 5.1% stated that they currently have asthma, compared with 13.4% and 8.0% of nonveterans, respectively.14 Veterans are susceptible to many of the factors that can trigger exacerbations while engaging in military service, such as chemical and environmental exposures both abroad and domestically.15,16 Additionally, medication adherence is often challenging among the veteran population, particularly with more involved therapy, such as inhaler use.17 Such factors contribute to asthma exacerbations, with 2.9% of veterans reporting at least 1 asthma exacerbation in the past 12 months.14
Over the past several years, the development and use of biologic agents have transformed the management of severe asthma.8 Before the development of biologic agents for severe asthma, treatment options for patients were limited. While OCS are frequently used for asthma exacerbations, they are associated with a multiplicity of undesirable adverse effects, including weight gain, mood lability, gastrointestinal upset, hyperglycemia, risk of bone fractures, and hypertension.18-20 The regular use of OCS are particularly problematic among other medical comorbidities commonly affecting the veteran population, such as diabetes and hypertension.21-22
The WDCVAMC severe allergy clinic used 3 biologic agents: omalizumab (anti-IgE), benralizumab (anti-IL-5), and agent dupilumab (anti-IL-4). These medications have shown significant improvements in quality of life, reduction in asthma exacerbations and hospitalizations, and decreased use of OCS.8,9 While research has firmly established the medical benefits of the use of biologic agents in severe asthma, several barriers exist in implementing widespread use.11,12
In Gelhorn and colleagues’ study examining both physician and patient challenges in the use of biologics for severe asthma, scheduling, administrative time, and insurance costs were found to be major barriers to the use of these medications.12 Patients expressed a preference for the administration of these medications in a specialist’s office but cited long wait times and scheduling difficulties as barriers. One of the most notable challenges from the physician perspective was the difficulty in obtaining reimbursement from insurance companies, requiring them to devote significant portions of time to prior authorizations and documentation.12
This article examines a dual specialty clinic that focuses on the treatment of severe asthma with biologic agents. This model is unique for several reasons. First, given the US Department of Veterans Affairs (VA) health care model, the health care practitioners (HCPs) in this clinic can avoid much of the administrative burden of obtaining reimbursement or working with insurance companies. Additionally, by dedicating specific days to the severe asthma clinic, patients do not experience long wait times to see both an allergist and pulmonologist. By seeing both clinicians, concurrent allergic and pulmonary issues can be addressed in the same visit, rather than delaying treatment by waiting on 2 specialist appointments.
Severe Asthma Clinic
The severe asthma clinic was started in September 2017 by a pulmonologist and an allergist at WDCVAMC. After experiencing substantial delays with the initiation of biologics for their patients and multiple referrals between their clinics, these physicians wanted to start a dually staffed asthma clinic to specifically focus on evaluating and treating severe asthma. A dedicated severe asthma clinic allowed the allergist and pulmonologist to streamline resources and collaborate to advocate for patients with the pharmacy section. Additionally, patients can benefit from the perspective of both specialists, as both the pulmonologist and allergist evaluate each patient and discuss the next steps of management.
This clinic is composed of 4 registered nurses, an allergist, and a pulmonologist. Clinic is held twice monthly through both telemedicine and in-office visits. The VA has strict guidelines for the use of certain biologics, including blood eosinophil count > 150 cells/µL, failure of traditional therapy, and frequent use of OCS. Additionally, to ensure these biologic agents are prescribed to patients that will benefit from them, the patients enrolled in this clinic are already on maximum therapy for their asthma, meaning all other therapeutic options (inhalers and oral medications) are being used. The clinic services all patients with severe asthma, not just patients who are on biologic therapy. Often, patients are referred to the severe allergy clinic late in their disease course given a lack of familiarity with biologic agents from prescribers and both institutional and insurance barriers.
Before the COVID-19 pandemic, spirometry and fractional exhaled nitric oxide (FENO) tests were recorded at each visit. Initially during the pandemic, the clinic transitioned to primarily telemedicine visits due to patients’ hesitance to seek in-person care. More recently, the clinic has transitioned back to primarily office visits; patients are seen in clinic on average every 3 months. At each visit, the patient is seen by both the pulmonologist and allergist. Additionally, the nursing staff reviews inhaler adherence with patients, spacer use, documents, Asthma Control Test (ACT) scores, and schedules follow-up visits.
Every 4 to 8 weeks, patients receive biologics agent at the WDCVAMC infusion center depending on the agent. The infusion center also instructs patients how to handle self-administered medications, like benralizumab, if the patient expresses a preference for taking it at home. Omalizumab has a boxed warning for anaphylaxis, although the other biologics in this study have a low risk of anaphylaxis. All patients receiving omalizumab, benralizumab, and dupilumab were provided with epinephrine injection devices in case of an allergic reaction and were taught how to use them in the clinic.23,24
If patients continued to experience asthma exacerbations after the initiation of a biologic, a change in agent was considered after 4 to 6 months. Additionally, a complete blood count, respiratory allergy panel, and pulmonary function tests (PFTs) were completed.
Clinic Patients
Preliminary data were obtained from a retrospective chart review of 15 patients enrolled in the severe asthma clinic over 30 months. The inclusion criteria for chart review consisted of patients aged > 18 years receiving a biologic agent for > 3 months for the treatment of severe asthma. The outcomes examined included steroid use, emergency department (ED) visits, hospitalizations, FEV1, and ICU stays.
Seven patients used benralizumab, 6 used dupilumab, and 2 used omalizumab (Table).
There was a notable clinical improvement in these patients. Before starting a biologic agent, all the patients in this study were prescribed steroids at least once a year for an asthma exacerbation, with a mean of 4.2 steroid tapers per year.
The initiation of a biologic agent also resulted in fewer ED visits and hospitalizations. Two patients had an ED visit for an asthma exacerbation since starting a biologic agent and 1 patient had a hospital admission for an asthma exacerbation. No patients were hospitalized in the ICU after starting a biologic agent.
Discussion
The 15 patients in this initial data were referred to the severe asthma clinic by pulmonology, ear, nose, and throat (ENT), primary care, and a hospitalist during an in-patient stay. As the enrollment in our clinic grows, an increasing number of patients are referred from the allergy clinic as well. Patients in the severe asthma clinic also are referred by regional centers as news of the clinic is spread by word of mouth to surrounding VA facilities. As our clinic gains the capacity to serve more patients, we hope to contact WDCVAMC primary care, pulmonology, allergy, and ENT departments to raise awareness of the clinic.
Benralizumab and dupilumab were the most used agents in this preliminary data. This finding was largely due to the ability of patients to self-administer benralizumab, which was particularly beneficial during the COVID-19 pandemic. Of note, 5 patients in this study switched from another biologic agent to benralizumab due to the ability to self-administer. Three of 5 patients that required steroids after initiating benralizumab used fewer steroids than they had previously. This finding suggests benralizumab may be the preferred agent when travel time to health care is a challenge, reducing the need for frequent clinic visits and transportation.
This preliminary data supports previous studies that have demonstrated that biologic agents improve clinical outcomes by reducing asthma exacerbations, OCS use, hospitalizations, and ICU stays for patients on all 4 biologic agents. In addition to improving patient health through avoiding complications of prolonged OCS use and hospital stays, the decrease in ED visits and hospitalizations provides a substantial cost reduction to the health care system.
These findings highlight the strength of a unique model of a combined allergy/pulmonary clinic. Before this combined clinic model, both pulmonology and allergy clinics noted delays in the initiation of biologics for patients who were potential candidates. Impediments include referrals between each specialty for evaluation of concurrent pulmonary conditions or allergy testing, overlap in asthma management, and a delay in coordination with the pharmacy department to start biologic agents. A dedicated severe asthma clinic staffed by both an allergist and pulmonologist provides a convenient option for patients to be seen by both specialists, reducing the need for separate appointments with each specialty, transportation to those appointments, and clinical time. This is particularly beneficial in a clinic such as this model, as this clinic serves patients from 4 states and Washington, DC. An additional benefit of this model is trained staff who directly communicate with the pharmacy in the initiation of these agents, allocate time to educating patients in biologic use, and coordinate follow-up.
Limitations
There were several limitations to this report. First, the number of patients examined in this preliminary data set is small. Due to the COVID-19 pandemic, there was a limited ability to see patients in person, and patients were seen exclusively over telemedicine for several months. For this reason, collecting data such as patient surveys and laboratory work following the initiation of a biologic was a challenge. Additionally, during the height of COVID-19, WDCVAMC did not perform aerosolizing procedures, such as PFTs and FENOs; thus, peak flows were obtained instead. Examining metrics, such as FENOs and IgE levels, and expanding PFT data would provide additional insight into the impact of biologic agents on clinical outcomes. Patient survey data in the form of ACTs or satisfaction surveys would also yield important data examining the impact of this clinic design and biologic use on patient experience. As of December 2022, 114 patients are enrolled in the clinic. We are working to collect the above laboratory results and spirometry for these patients so that these results can be published with a more robust data set. Another limitation of the information presented is that it is a retrospective data analysis; the data collected was contingent upon documentation and the assumption that these patients were exclusively receiving care through the VA. For example, steroid use before and after initiation of biologic was taken from asthma clinic notes and the patient’s medication list. Therefore, there is a possibility that not all instances were accounted for if that patient sought care outside the VA or whether it was not documented in a follow-up note.
Conclusions
The model of a combined allergy/pulmonology clinic can be particularly efficacious in the treatment of severe asthma, as it reduces the need for multiple appointments with different specialties, reduces wait time before starting a biologic agent, and offers the perspective of 2 specialists. This kind of model could be an example to many clinics in the VA. With a rapid increase in telemedicine due to the COVID-19 pandemic, multiple physicians consulting simultaneously is becoming a more feasible possibility across multiple specialties. As the use of biologics becomes more widespread, a combined clinic design is an efficient and promising method to improve severe asthma management.
This preliminary data continue to support previous research that shows biologic agents have led to better clinical outcomes through the reduction of asthma exacerbations, hospitalizations, and improved PFTs. While this initial data set highlights the results for 15 patients, there are 86 patients currently enrolled in this clinic. We are collecting additional data to publish more comprehensive results.
Asthma affects more than 300 million people worldwide.1 While many of these cases can achieve control with standard therapy, 5% to 10% of these cases are classified as severe asthma, remaining poorly controlled despite treatment with inhaled corticosteroids (ICS) and long-acting β agonists (LABA).2 These patients also account for the majority of morbidity and mortality associated with the disease, with increased hospitalizations, intensive care unit (ICU) stays, detrimental adverse effects of oral corticosteroids (OCS), and lower quality of life.3-6 Additionally, the financial repercussions of severe asthma are notable; in the United States, the estimated cost of asthma management is $82 billion annually, with $3 billion accounting for asthma-related work/school absences.7
In the past several years, the use of anti-immunoglobulin E (IgE), anti-interleukin-4 (IL-4), and anti-IL-5 biologic agents for severe asthma has been shown to decrease asthma exacerbations, improve lung function, reduce corticosteroid use, and decrease hospitalizations, especially for type 2 helper T cell (TH2-high) asthma.8-10 However, clinicians have observed significant barriers to the implementation and widespread use of biologics, including insurance coverage, long wait times, follow-up, and limited access for lower income groups.11,12
This article describes a unique model for a severe asthma clinic located at the Washington DC Veterans Affairs Medical Center (WDCVAMC) that is dually staffed by an allergist and pulmonologist. This clinic uses biologic agents for patients with difficult-to-treat asthma, many of whom require repeated or prolonged steroid use, in addition to prolonged and recurrent hospitalizations for exacerbations. The objective of this clinic is to provide a standardized approach to the management of severe asthma with the perspective of both an allergist and pulmonologist, thereby reducing the need to schedule appointments with multiple specialties and reducing delays in initiating biologics. This article presents the preliminary findings of 30 months of severe asthma management with various biologic agents, examining the impact of these therapies on hospitalizations, asthma exacerbations, ICU stays, and OCS use. The findings of this study support the benefits of biologics and suggest that the use of these agents within a dually staffed clinic may be a particularly effective model through which to manage severe asthma.
Background
Asthma affects approximately 20 million adults in the United States.13 Veterans are a population particularly impacted by asthma. Between 2015 and 2018, 10.9% of all veterans reported being diagnosed with asthma and 5.1% stated that they currently have asthma, compared with 13.4% and 8.0% of nonveterans, respectively.14 Veterans are susceptible to many of the factors that can trigger exacerbations while engaging in military service, such as chemical and environmental exposures both abroad and domestically.15,16 Additionally, medication adherence is often challenging among the veteran population, particularly with more involved therapy, such as inhaler use.17 Such factors contribute to asthma exacerbations, with 2.9% of veterans reporting at least 1 asthma exacerbation in the past 12 months.14
Over the past several years, the development and use of biologic agents have transformed the management of severe asthma.8 Before the development of biologic agents for severe asthma, treatment options for patients were limited. While OCS are frequently used for asthma exacerbations, they are associated with a multiplicity of undesirable adverse effects, including weight gain, mood lability, gastrointestinal upset, hyperglycemia, risk of bone fractures, and hypertension.18-20 The regular use of OCS are particularly problematic among other medical comorbidities commonly affecting the veteran population, such as diabetes and hypertension.21-22
The WDCVAMC severe allergy clinic used 3 biologic agents: omalizumab (anti-IgE), benralizumab (anti-IL-5), and agent dupilumab (anti-IL-4). These medications have shown significant improvements in quality of life, reduction in asthma exacerbations and hospitalizations, and decreased use of OCS.8,9 While research has firmly established the medical benefits of the use of biologic agents in severe asthma, several barriers exist in implementing widespread use.11,12
In Gelhorn and colleagues’ study examining both physician and patient challenges in the use of biologics for severe asthma, scheduling, administrative time, and insurance costs were found to be major barriers to the use of these medications.12 Patients expressed a preference for the administration of these medications in a specialist’s office but cited long wait times and scheduling difficulties as barriers. One of the most notable challenges from the physician perspective was the difficulty in obtaining reimbursement from insurance companies, requiring them to devote significant portions of time to prior authorizations and documentation.12
This article examines a dual specialty clinic that focuses on the treatment of severe asthma with biologic agents. This model is unique for several reasons. First, given the US Department of Veterans Affairs (VA) health care model, the health care practitioners (HCPs) in this clinic can avoid much of the administrative burden of obtaining reimbursement or working with insurance companies. Additionally, by dedicating specific days to the severe asthma clinic, patients do not experience long wait times to see both an allergist and pulmonologist. By seeing both clinicians, concurrent allergic and pulmonary issues can be addressed in the same visit, rather than delaying treatment by waiting on 2 specialist appointments.
Severe Asthma Clinic
The severe asthma clinic was started in September 2017 by a pulmonologist and an allergist at WDCVAMC. After experiencing substantial delays with the initiation of biologics for their patients and multiple referrals between their clinics, these physicians wanted to start a dually staffed asthma clinic to specifically focus on evaluating and treating severe asthma. A dedicated severe asthma clinic allowed the allergist and pulmonologist to streamline resources and collaborate to advocate for patients with the pharmacy section. Additionally, patients can benefit from the perspective of both specialists, as both the pulmonologist and allergist evaluate each patient and discuss the next steps of management.
This clinic is composed of 4 registered nurses, an allergist, and a pulmonologist. Clinic is held twice monthly through both telemedicine and in-office visits. The VA has strict guidelines for the use of certain biologics, including blood eosinophil count > 150 cells/µL, failure of traditional therapy, and frequent use of OCS. Additionally, to ensure these biologic agents are prescribed to patients that will benefit from them, the patients enrolled in this clinic are already on maximum therapy for their asthma, meaning all other therapeutic options (inhalers and oral medications) are being used. The clinic services all patients with severe asthma, not just patients who are on biologic therapy. Often, patients are referred to the severe allergy clinic late in their disease course given a lack of familiarity with biologic agents from prescribers and both institutional and insurance barriers.
Before the COVID-19 pandemic, spirometry and fractional exhaled nitric oxide (FENO) tests were recorded at each visit. Initially during the pandemic, the clinic transitioned to primarily telemedicine visits due to patients’ hesitance to seek in-person care. More recently, the clinic has transitioned back to primarily office visits; patients are seen in clinic on average every 3 months. At each visit, the patient is seen by both the pulmonologist and allergist. Additionally, the nursing staff reviews inhaler adherence with patients, spacer use, documents, Asthma Control Test (ACT) scores, and schedules follow-up visits.
Every 4 to 8 weeks, patients receive biologics agent at the WDCVAMC infusion center depending on the agent. The infusion center also instructs patients how to handle self-administered medications, like benralizumab, if the patient expresses a preference for taking it at home. Omalizumab has a boxed warning for anaphylaxis, although the other biologics in this study have a low risk of anaphylaxis. All patients receiving omalizumab, benralizumab, and dupilumab were provided with epinephrine injection devices in case of an allergic reaction and were taught how to use them in the clinic.23,24
If patients continued to experience asthma exacerbations after the initiation of a biologic, a change in agent was considered after 4 to 6 months. Additionally, a complete blood count, respiratory allergy panel, and pulmonary function tests (PFTs) were completed.
Clinic Patients
Preliminary data were obtained from a retrospective chart review of 15 patients enrolled in the severe asthma clinic over 30 months. The inclusion criteria for chart review consisted of patients aged > 18 years receiving a biologic agent for > 3 months for the treatment of severe asthma. The outcomes examined included steroid use, emergency department (ED) visits, hospitalizations, FEV1, and ICU stays.
Seven patients used benralizumab, 6 used dupilumab, and 2 used omalizumab (Table).
There was a notable clinical improvement in these patients. Before starting a biologic agent, all the patients in this study were prescribed steroids at least once a year for an asthma exacerbation, with a mean of 4.2 steroid tapers per year.
The initiation of a biologic agent also resulted in fewer ED visits and hospitalizations. Two patients had an ED visit for an asthma exacerbation since starting a biologic agent and 1 patient had a hospital admission for an asthma exacerbation. No patients were hospitalized in the ICU after starting a biologic agent.
Discussion
The 15 patients in this initial data were referred to the severe asthma clinic by pulmonology, ear, nose, and throat (ENT), primary care, and a hospitalist during an in-patient stay. As the enrollment in our clinic grows, an increasing number of patients are referred from the allergy clinic as well. Patients in the severe asthma clinic also are referred by regional centers as news of the clinic is spread by word of mouth to surrounding VA facilities. As our clinic gains the capacity to serve more patients, we hope to contact WDCVAMC primary care, pulmonology, allergy, and ENT departments to raise awareness of the clinic.
Benralizumab and dupilumab were the most used agents in this preliminary data. This finding was largely due to the ability of patients to self-administer benralizumab, which was particularly beneficial during the COVID-19 pandemic. Of note, 5 patients in this study switched from another biologic agent to benralizumab due to the ability to self-administer. Three of 5 patients that required steroids after initiating benralizumab used fewer steroids than they had previously. This finding suggests benralizumab may be the preferred agent when travel time to health care is a challenge, reducing the need for frequent clinic visits and transportation.
This preliminary data supports previous studies that have demonstrated that biologic agents improve clinical outcomes by reducing asthma exacerbations, OCS use, hospitalizations, and ICU stays for patients on all 4 biologic agents. In addition to improving patient health through avoiding complications of prolonged OCS use and hospital stays, the decrease in ED visits and hospitalizations provides a substantial cost reduction to the health care system.
These findings highlight the strength of a unique model of a combined allergy/pulmonary clinic. Before this combined clinic model, both pulmonology and allergy clinics noted delays in the initiation of biologics for patients who were potential candidates. Impediments include referrals between each specialty for evaluation of concurrent pulmonary conditions or allergy testing, overlap in asthma management, and a delay in coordination with the pharmacy department to start biologic agents. A dedicated severe asthma clinic staffed by both an allergist and pulmonologist provides a convenient option for patients to be seen by both specialists, reducing the need for separate appointments with each specialty, transportation to those appointments, and clinical time. This is particularly beneficial in a clinic such as this model, as this clinic serves patients from 4 states and Washington, DC. An additional benefit of this model is trained staff who directly communicate with the pharmacy in the initiation of these agents, allocate time to educating patients in biologic use, and coordinate follow-up.
Limitations
There were several limitations to this report. First, the number of patients examined in this preliminary data set is small. Due to the COVID-19 pandemic, there was a limited ability to see patients in person, and patients were seen exclusively over telemedicine for several months. For this reason, collecting data such as patient surveys and laboratory work following the initiation of a biologic was a challenge. Additionally, during the height of COVID-19, WDCVAMC did not perform aerosolizing procedures, such as PFTs and FENOs; thus, peak flows were obtained instead. Examining metrics, such as FENOs and IgE levels, and expanding PFT data would provide additional insight into the impact of biologic agents on clinical outcomes. Patient survey data in the form of ACTs or satisfaction surveys would also yield important data examining the impact of this clinic design and biologic use on patient experience. As of December 2022, 114 patients are enrolled in the clinic. We are working to collect the above laboratory results and spirometry for these patients so that these results can be published with a more robust data set. Another limitation of the information presented is that it is a retrospective data analysis; the data collected was contingent upon documentation and the assumption that these patients were exclusively receiving care through the VA. For example, steroid use before and after initiation of biologic was taken from asthma clinic notes and the patient’s medication list. Therefore, there is a possibility that not all instances were accounted for if that patient sought care outside the VA or whether it was not documented in a follow-up note.
Conclusions
The model of a combined allergy/pulmonology clinic can be particularly efficacious in the treatment of severe asthma, as it reduces the need for multiple appointments with different specialties, reduces wait time before starting a biologic agent, and offers the perspective of 2 specialists. This kind of model could be an example to many clinics in the VA. With a rapid increase in telemedicine due to the COVID-19 pandemic, multiple physicians consulting simultaneously is becoming a more feasible possibility across multiple specialties. As the use of biologics becomes more widespread, a combined clinic design is an efficient and promising method to improve severe asthma management.
This preliminary data continue to support previous research that shows biologic agents have led to better clinical outcomes through the reduction of asthma exacerbations, hospitalizations, and improved PFTs. While this initial data set highlights the results for 15 patients, there are 86 patients currently enrolled in this clinic. We are collecting additional data to publish more comprehensive results.
1. Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16(1):45-56. doi:10.1038/ni.3049
2. Moore WC, Bleecker ER, Curran-Everett D, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119(2):405-413. doi:10.1016/j.jaci.2006.11.639
3. Barnes PJ, Jonsson B, Klim JB. The costs of asthma. Eur Respir J. 1996;9(4):636-642. doi:10.1183/09031936.96.09040636
4. Bourdin A, Charriot J, Boissin C, et al. Will the asthma revolution fostered by biologics also benefit adult ICU patients?. Allergy. 2021;76(8):2395-2406. doi:10.1111/all.14688
5. Lloyd A, Price D, Brown R. The impact of asthma exacerbations on health-related quality of life in moderate to severe asthma patients in the UK. Prim Care Respir J. 2007;16(1):22-27. doi:10.3132/pcrj.2007.00002
6. Eisner MD, Yelin EH, Katz PP, Lactao G, Iribarren C, Blanc PD. Risk factors for work disability in severe adult asthma. Am J Med. 2006;119(10):884-891. doi:10.1016/j.amjmed.2006.01.016
7. Nurmagambetov T, Kuwahara R, Garbe P. The Economic Burden of Asthma in the United States, 2008-2013. Ann Am Thorac Soc. 2018;15(3):348-356. doi:10.1513/AnnalsATS.201703-259OC
8. McGregor MC, Krings JG, Nair P, Castro M. Role of biologics in asthma. Am J Respir Crit Care Med. 2019;199(4):433-445. doi:10.1164/rccm.201810-1944CI
9. Bice JB, Leechawengwongs E, Montanaro A. Biologic targeted therapy in allergic asthma. Ann Allergy Asthma Immunol. 2014;112(2):108-115. doi:10.1016/j.anai.2013.12.013
10. Darveaux J, Busse WW. Biologics in asthma--the next step toward personalized treatment. J Allergy Clin Immunol Pract. 2015;3(2):152-161. doi:10.1016/j.jaip.2014.09.014
11. Inselman JW, Jeffery MM, Maddux JT, Shah ND, Rank MA. Trends and disparities in asthma biologic use in the United States. J Allergy Clin Immunol Pract. 2020;8(2):549-554.e1. doi:10.1016/j.jaip.2019.08.024
12. Gelhorn HL, Balantac Z, Ambrose CS, Chung YN, Stone B. Patient and physician preferences for attributes of biologic medications for severe asthma. Patient Prefer Adherence. 2019;13:1253-1268. Published 2019 Jul 25. doi:10.2147/PPA.S198953
13. Centers for Disease Control and Prevention, National Center for Environmental Health. 2019 National Health Interview Survey (NHIS) data. Accessed December 6, 2022. https://www.cdc.gov/asthma/nhis/2019/data.htm
14. Zelaya CE BP, Moy E. Crude and age-adjusted percent distribution of respondent-assessed health status among adults aged 20 and over, by veteran status and other selected characteristics: United States, 2015-2018. National Center for Health Statistic. Updated June 19, 2020. Accessed December 12, 2022. https://www.cdc.gov/nchs/nhis/veterans_health_statistics/tables.htm
15. Szema AM, Peters MC, Weissinger KM, Gagliano CA, Chen JJ. New-onset asthma among soldiers serving in Iraq and Afghanistan. Allergy Asthma Proc. 2010;31(5):67-71. doi:10.2500/aap.2010.31.3383
16. Yi SW, Hong JS, Ohrr H, Yi JJ. Agent Orange exposure and disease prevalence in Korean Vietnam veterans: the Korean veterans health study. Environ Res. 2014;133:56-65. doi:10.1016/j.envres.2014.04.027
17. Huetsch JC, Uman JE, Udris EM, Au DH. Predictors of adherence to inhaled medications among veterans with COPD. J Gen Intern Med. 2012;27(11):1506-1512. doi:10.1007/s11606-012-2130-5
18. Mundell L, Lindemann R, Douglas J. Monitoring long-term oral corticosteroids. BMJ Open Qual. 2017;6(2):e000209. Published 2017 Nov 8. doi:10.1136/bmjoq-2017-000209
19. Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2005;20(8):1487-1486. doi:10.1359/jbmr.2005.20.8.1486
20. Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006;81(10):1361-1367. doi:10.4065/81.10.1361
21. Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care. 2004;27(suppl 2):B10-B21. doi:10.2337/diacare.27.suppl_2.b10
22. Acharya T, Tringali S, Singh M, Huang J. Resistant hypertension and associated comorbidities in a Veterans Affairs population. J Clin Hypertens (Greenwich). 2014;16(10):741-745. doi:10.1111/jch.12410
23. Dupixent (dupilumab). Prescribing information. Sanofi and Regeneron Pharmaceuticals; 2022. Accessed December 6, 2022. https://www.dupixenthcp.com/asthma/efficacy/safety-data
24. Li L, Wang Z, Cui L, Xu Y, Guan K, Zhao B. Anaphylactic risk related to omalizumab, benralizumab, reslizumab, mepolizumab, and dupilumab. Clin Transl Allergy. 2021;11(4):e12038. Published 2021 Jun 3. doi:10.1002/clt2.12038
1. Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16(1):45-56. doi:10.1038/ni.3049
2. Moore WC, Bleecker ER, Curran-Everett D, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119(2):405-413. doi:10.1016/j.jaci.2006.11.639
3. Barnes PJ, Jonsson B, Klim JB. The costs of asthma. Eur Respir J. 1996;9(4):636-642. doi:10.1183/09031936.96.09040636
4. Bourdin A, Charriot J, Boissin C, et al. Will the asthma revolution fostered by biologics also benefit adult ICU patients?. Allergy. 2021;76(8):2395-2406. doi:10.1111/all.14688
5. Lloyd A, Price D, Brown R. The impact of asthma exacerbations on health-related quality of life in moderate to severe asthma patients in the UK. Prim Care Respir J. 2007;16(1):22-27. doi:10.3132/pcrj.2007.00002
6. Eisner MD, Yelin EH, Katz PP, Lactao G, Iribarren C, Blanc PD. Risk factors for work disability in severe adult asthma. Am J Med. 2006;119(10):884-891. doi:10.1016/j.amjmed.2006.01.016
7. Nurmagambetov T, Kuwahara R, Garbe P. The Economic Burden of Asthma in the United States, 2008-2013. Ann Am Thorac Soc. 2018;15(3):348-356. doi:10.1513/AnnalsATS.201703-259OC
8. McGregor MC, Krings JG, Nair P, Castro M. Role of biologics in asthma. Am J Respir Crit Care Med. 2019;199(4):433-445. doi:10.1164/rccm.201810-1944CI
9. Bice JB, Leechawengwongs E, Montanaro A. Biologic targeted therapy in allergic asthma. Ann Allergy Asthma Immunol. 2014;112(2):108-115. doi:10.1016/j.anai.2013.12.013
10. Darveaux J, Busse WW. Biologics in asthma--the next step toward personalized treatment. J Allergy Clin Immunol Pract. 2015;3(2):152-161. doi:10.1016/j.jaip.2014.09.014
11. Inselman JW, Jeffery MM, Maddux JT, Shah ND, Rank MA. Trends and disparities in asthma biologic use in the United States. J Allergy Clin Immunol Pract. 2020;8(2):549-554.e1. doi:10.1016/j.jaip.2019.08.024
12. Gelhorn HL, Balantac Z, Ambrose CS, Chung YN, Stone B. Patient and physician preferences for attributes of biologic medications for severe asthma. Patient Prefer Adherence. 2019;13:1253-1268. Published 2019 Jul 25. doi:10.2147/PPA.S198953
13. Centers for Disease Control and Prevention, National Center for Environmental Health. 2019 National Health Interview Survey (NHIS) data. Accessed December 6, 2022. https://www.cdc.gov/asthma/nhis/2019/data.htm
14. Zelaya CE BP, Moy E. Crude and age-adjusted percent distribution of respondent-assessed health status among adults aged 20 and over, by veteran status and other selected characteristics: United States, 2015-2018. National Center for Health Statistic. Updated June 19, 2020. Accessed December 12, 2022. https://www.cdc.gov/nchs/nhis/veterans_health_statistics/tables.htm
15. Szema AM, Peters MC, Weissinger KM, Gagliano CA, Chen JJ. New-onset asthma among soldiers serving in Iraq and Afghanistan. Allergy Asthma Proc. 2010;31(5):67-71. doi:10.2500/aap.2010.31.3383
16. Yi SW, Hong JS, Ohrr H, Yi JJ. Agent Orange exposure and disease prevalence in Korean Vietnam veterans: the Korean veterans health study. Environ Res. 2014;133:56-65. doi:10.1016/j.envres.2014.04.027
17. Huetsch JC, Uman JE, Udris EM, Au DH. Predictors of adherence to inhaled medications among veterans with COPD. J Gen Intern Med. 2012;27(11):1506-1512. doi:10.1007/s11606-012-2130-5
18. Mundell L, Lindemann R, Douglas J. Monitoring long-term oral corticosteroids. BMJ Open Qual. 2017;6(2):e000209. Published 2017 Nov 8. doi:10.1136/bmjoq-2017-000209
19. Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2005;20(8):1487-1486. doi:10.1359/jbmr.2005.20.8.1486
20. Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006;81(10):1361-1367. doi:10.4065/81.10.1361
21. Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care. 2004;27(suppl 2):B10-B21. doi:10.2337/diacare.27.suppl_2.b10
22. Acharya T, Tringali S, Singh M, Huang J. Resistant hypertension and associated comorbidities in a Veterans Affairs population. J Clin Hypertens (Greenwich). 2014;16(10):741-745. doi:10.1111/jch.12410
23. Dupixent (dupilumab). Prescribing information. Sanofi and Regeneron Pharmaceuticals; 2022. Accessed December 6, 2022. https://www.dupixenthcp.com/asthma/efficacy/safety-data
24. Li L, Wang Z, Cui L, Xu Y, Guan K, Zhao B. Anaphylactic risk related to omalizumab, benralizumab, reslizumab, mepolizumab, and dupilumab. Clin Transl Allergy. 2021;11(4):e12038. Published 2021 Jun 3. doi:10.1002/clt2.12038