User login
Advances in Blood Cancer Care for Veterans
Advances in Blood Cancer Care for Veterans
Click to view more from Cancer Data Trends 2025.
- Li W, ed. The 5th Edition of the World Health Organization Classification of
Hematolymphoid Tumors. In: Leukemia [Internet]. Brisbane (AU): Exon Publications;
October 16, 2022. https://www.ncbi.nlm.nih.gov/books/NBK586208/ - Graf SA, Samples LS, Keating TM, Garcia JM. Clinical research in older adults with
hematologic malignancies: Opportunities for alignment in the Veterans Affairs. Semin
Oncol. 2020;47(1):94-101. doi:10.1053/j.seminoncol.2020.02.010. - Tiu A, McKinnell Z, Liu S, et al. Risk of myeloproliferative neoplasms among
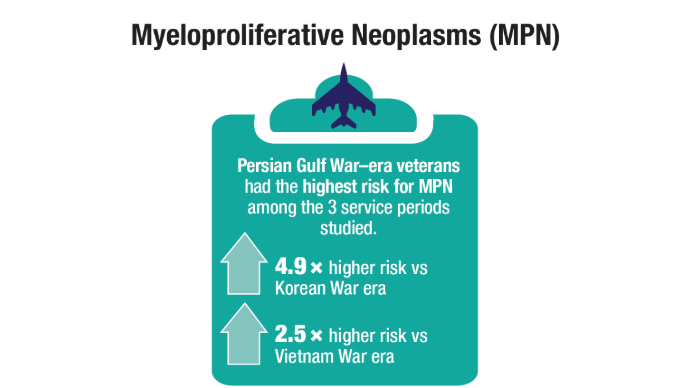
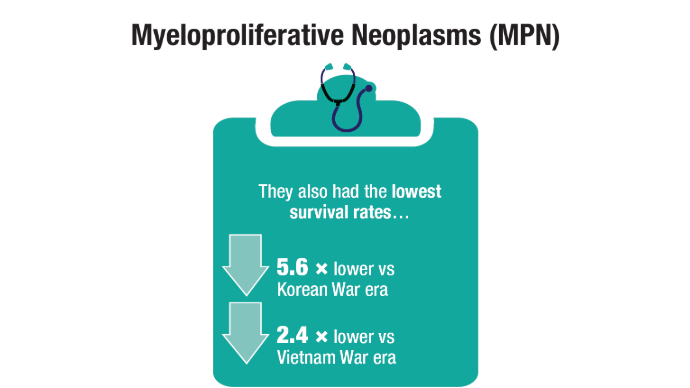
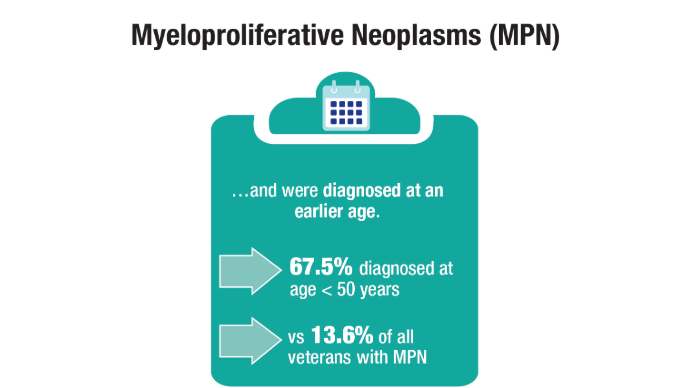
U.S. Veterans from Korean, Vietnam, and Persian Gulf War eras. Am J Hematol.
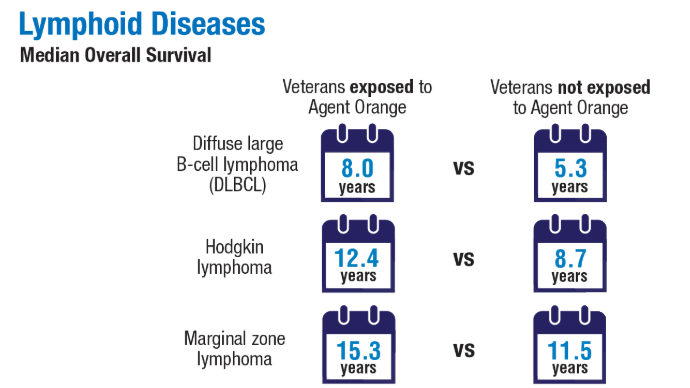
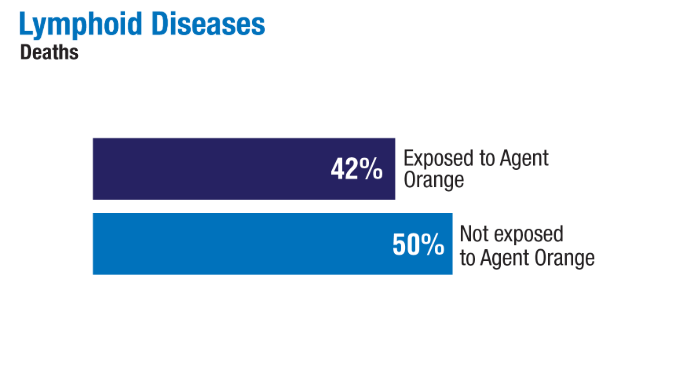
2024;99(10):1969-1978. doi:10.1002/ajh.27438 - Ma H, Wan JY, Cortessis VK, Gupta P, Cozen W. Survival in Agent Orange
exposed and unexposed Vietnam-era veterans who were diagnosed with
lymphoid malignancies. Blood Adv. 2024;8(4):1037-1041. doi:10.1182/
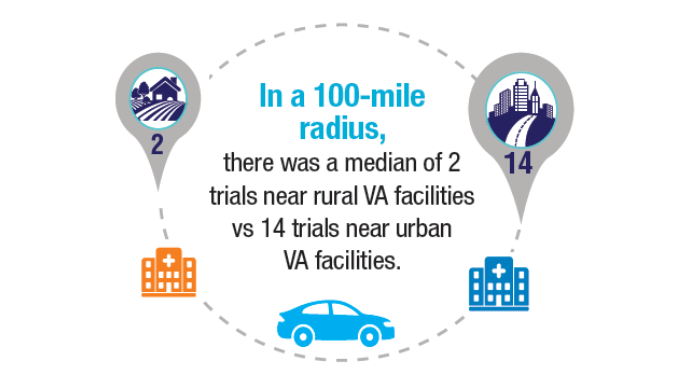
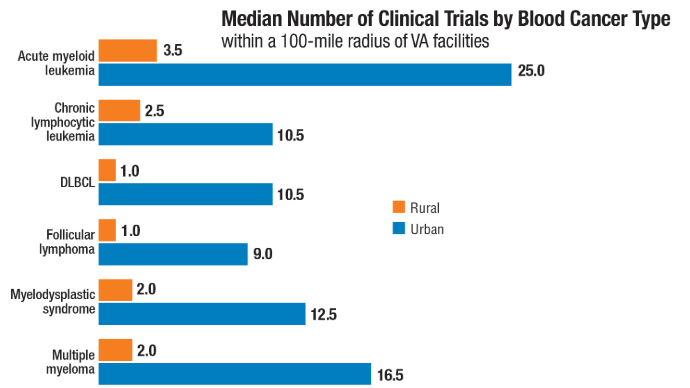
bloodadvances.2023011999 - Friedman DR, Rodgers TD, Kovalick C, Yellapragada S, Szumita L, Weiss ES. Veterans
with blood cancers: Clinical trial navigation and the challenge of rurality. J Rural
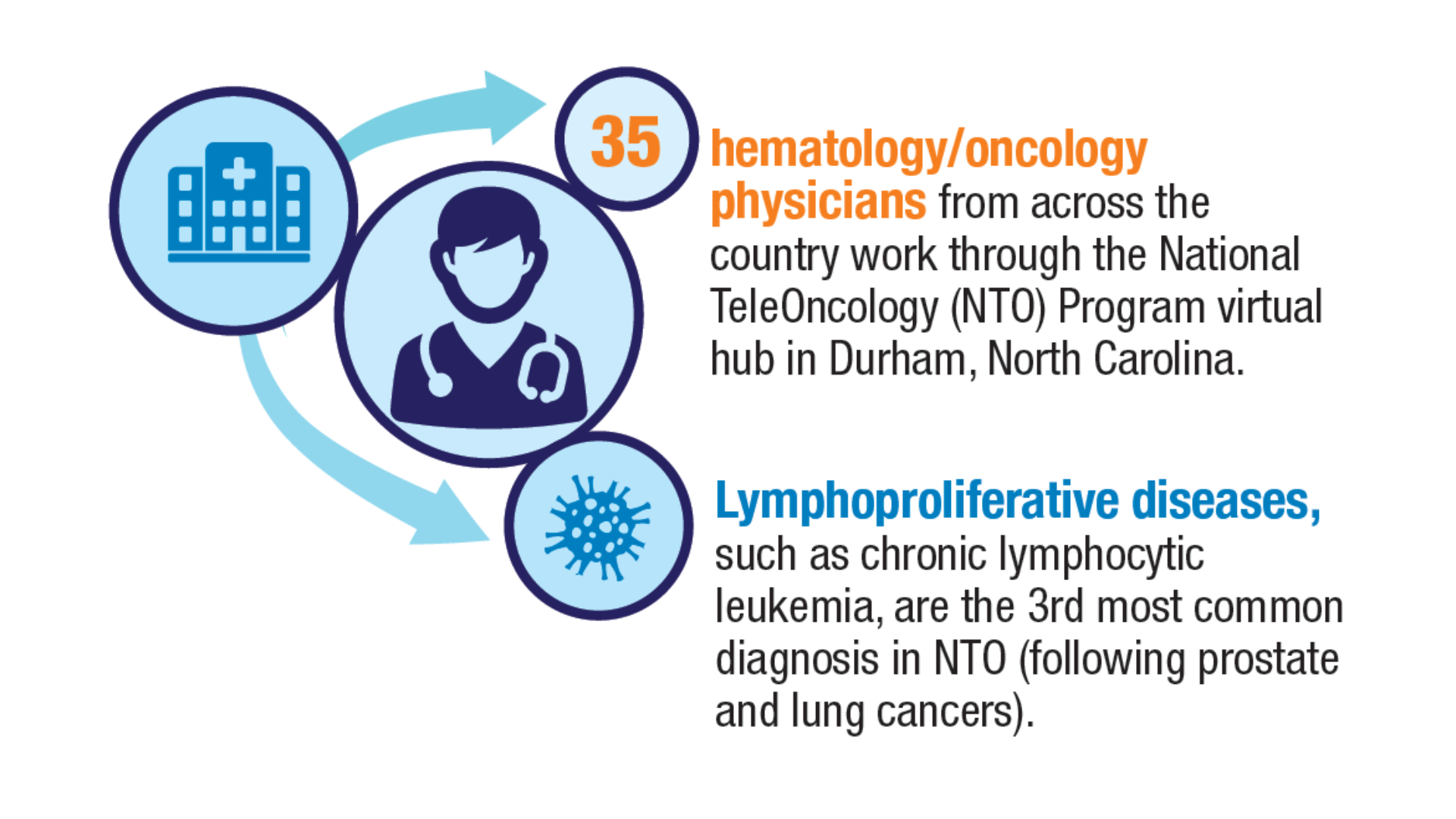
Health. 2024;40(1):114-120. doi:10.1111/jrh.12773 - Parikh DA, Rodgers TD, Passero VA, et al. Teleoncology in the Veterans Health
Administration: Models of Care and the Veteran Experience. Am Soc Clin Oncol Educ
Book. 2024;44(3):e100042. doi:10.1200/EDBK_100042 - Pulumati A, Pulumati A, Dwarakanath BS, Verma A, Papineni RVL. Technological
advancements in cancer diagnostics: Improvements and limitations. Cancer Rep
(Hoboken). 2023;6(2):e1764. doi:10.1002/cnr2.1764
Click to view more from Cancer Data Trends 2025.
Click to view more from Cancer Data Trends 2025.
- Li W, ed. The 5th Edition of the World Health Organization Classification of
Hematolymphoid Tumors. In: Leukemia [Internet]. Brisbane (AU): Exon Publications;
October 16, 2022. https://www.ncbi.nlm.nih.gov/books/NBK586208/ - Graf SA, Samples LS, Keating TM, Garcia JM. Clinical research in older adults with
hematologic malignancies: Opportunities for alignment in the Veterans Affairs. Semin
Oncol. 2020;47(1):94-101. doi:10.1053/j.seminoncol.2020.02.010. - Tiu A, McKinnell Z, Liu S, et al. Risk of myeloproliferative neoplasms among
U.S. Veterans from Korean, Vietnam, and Persian Gulf War eras. Am J Hematol.
2024;99(10):1969-1978. doi:10.1002/ajh.27438 - Ma H, Wan JY, Cortessis VK, Gupta P, Cozen W. Survival in Agent Orange
exposed and unexposed Vietnam-era veterans who were diagnosed with
lymphoid malignancies. Blood Adv. 2024;8(4):1037-1041. doi:10.1182/
bloodadvances.2023011999 - Friedman DR, Rodgers TD, Kovalick C, Yellapragada S, Szumita L, Weiss ES. Veterans
with blood cancers: Clinical trial navigation and the challenge of rurality. J Rural
Health. 2024;40(1):114-120. doi:10.1111/jrh.12773 - Parikh DA, Rodgers TD, Passero VA, et al. Teleoncology in the Veterans Health
Administration: Models of Care and the Veteran Experience. Am Soc Clin Oncol Educ
Book. 2024;44(3):e100042. doi:10.1200/EDBK_100042 - Pulumati A, Pulumati A, Dwarakanath BS, Verma A, Papineni RVL. Technological
advancements in cancer diagnostics: Improvements and limitations. Cancer Rep
(Hoboken). 2023;6(2):e1764. doi:10.1002/cnr2.1764
- Li W, ed. The 5th Edition of the World Health Organization Classification of
Hematolymphoid Tumors. In: Leukemia [Internet]. Brisbane (AU): Exon Publications;
October 16, 2022. https://www.ncbi.nlm.nih.gov/books/NBK586208/ - Graf SA, Samples LS, Keating TM, Garcia JM. Clinical research in older adults with
hematologic malignancies: Opportunities for alignment in the Veterans Affairs. Semin
Oncol. 2020;47(1):94-101. doi:10.1053/j.seminoncol.2020.02.010. - Tiu A, McKinnell Z, Liu S, et al. Risk of myeloproliferative neoplasms among
U.S. Veterans from Korean, Vietnam, and Persian Gulf War eras. Am J Hematol.
2024;99(10):1969-1978. doi:10.1002/ajh.27438 - Ma H, Wan JY, Cortessis VK, Gupta P, Cozen W. Survival in Agent Orange
exposed and unexposed Vietnam-era veterans who were diagnosed with
lymphoid malignancies. Blood Adv. 2024;8(4):1037-1041. doi:10.1182/
bloodadvances.2023011999 - Friedman DR, Rodgers TD, Kovalick C, Yellapragada S, Szumita L, Weiss ES. Veterans
with blood cancers: Clinical trial navigation and the challenge of rurality. J Rural
Health. 2024;40(1):114-120. doi:10.1111/jrh.12773 - Parikh DA, Rodgers TD, Passero VA, et al. Teleoncology in the Veterans Health
Administration: Models of Care and the Veteran Experience. Am Soc Clin Oncol Educ
Book. 2024;44(3):e100042. doi:10.1200/EDBK_100042 - Pulumati A, Pulumati A, Dwarakanath BS, Verma A, Papineni RVL. Technological
advancements in cancer diagnostics: Improvements and limitations. Cancer Rep
(Hoboken). 2023;6(2):e1764. doi:10.1002/cnr2.1764
Advances in Blood Cancer Care for Veterans
Advances in Blood Cancer Care for Veterans
Follicular Lymphoma Highlights From ASH 2022
Highlights in follicular lymphoma from the 2022 American Society of Hematology (ASH) Annual Meeting are discussed by Dr Thomas Rodgers of the Durham VA Medical Center.
Dr Rodgers begins with a prognostic model designed to evaluate the risk for disease progression in high-risk patients within 24 months of starting first-line treatment with the intention of better individualizing management in this group.
Next, he presents long-term phase 3 data comparing first-line rituximab with a watch-and-wait approach. After 12 years of follow-up, results showed no significant difference in overall survival between watch and wait, rituximab induction, and rituximab induction plus maintenance, suggesting to Dr Rodgers that individualized upfront management can lead to similarly excellent outcomes in patients with low tumor burden.
Turning to relapsed/refractory disease, Dr Rodgers cites a study comparing rituximab plus lenalidomide with rituximab plus placebo. The combination yielded superior results and more durable efficacy than did the control group.
He also discusses studies on the use of novel agent tazemetostat in combination with lenalidomide, and the bispecific monoclonal antibody mosunetuzumab as monotherapy. The US Food and Drug Administration approved mosunetuzumab in December, expanding the armamentarium for patients with follicular lymphoma who have undergone multiple lines of therapy.
--
Thomas Rodgers, MD, Assistant Professor, Department of Hematologic Malignancies and Cellular Therapy, Duke University; Staff Physician, Department of Hematology/Oncology, Durham VA Medical Center, Durham, North Carolina
Thomas Rodgers, MD, has disclosed no relevant financial relationships.
Highlights in follicular lymphoma from the 2022 American Society of Hematology (ASH) Annual Meeting are discussed by Dr Thomas Rodgers of the Durham VA Medical Center.
Dr Rodgers begins with a prognostic model designed to evaluate the risk for disease progression in high-risk patients within 24 months of starting first-line treatment with the intention of better individualizing management in this group.
Next, he presents long-term phase 3 data comparing first-line rituximab with a watch-and-wait approach. After 12 years of follow-up, results showed no significant difference in overall survival between watch and wait, rituximab induction, and rituximab induction plus maintenance, suggesting to Dr Rodgers that individualized upfront management can lead to similarly excellent outcomes in patients with low tumor burden.
Turning to relapsed/refractory disease, Dr Rodgers cites a study comparing rituximab plus lenalidomide with rituximab plus placebo. The combination yielded superior results and more durable efficacy than did the control group.
He also discusses studies on the use of novel agent tazemetostat in combination with lenalidomide, and the bispecific monoclonal antibody mosunetuzumab as monotherapy. The US Food and Drug Administration approved mosunetuzumab in December, expanding the armamentarium for patients with follicular lymphoma who have undergone multiple lines of therapy.
--
Thomas Rodgers, MD, Assistant Professor, Department of Hematologic Malignancies and Cellular Therapy, Duke University; Staff Physician, Department of Hematology/Oncology, Durham VA Medical Center, Durham, North Carolina
Thomas Rodgers, MD, has disclosed no relevant financial relationships.
Highlights in follicular lymphoma from the 2022 American Society of Hematology (ASH) Annual Meeting are discussed by Dr Thomas Rodgers of the Durham VA Medical Center.
Dr Rodgers begins with a prognostic model designed to evaluate the risk for disease progression in high-risk patients within 24 months of starting first-line treatment with the intention of better individualizing management in this group.
Next, he presents long-term phase 3 data comparing first-line rituximab with a watch-and-wait approach. After 12 years of follow-up, results showed no significant difference in overall survival between watch and wait, rituximab induction, and rituximab induction plus maintenance, suggesting to Dr Rodgers that individualized upfront management can lead to similarly excellent outcomes in patients with low tumor burden.
Turning to relapsed/refractory disease, Dr Rodgers cites a study comparing rituximab plus lenalidomide with rituximab plus placebo. The combination yielded superior results and more durable efficacy than did the control group.
He also discusses studies on the use of novel agent tazemetostat in combination with lenalidomide, and the bispecific monoclonal antibody mosunetuzumab as monotherapy. The US Food and Drug Administration approved mosunetuzumab in December, expanding the armamentarium for patients with follicular lymphoma who have undergone multiple lines of therapy.
--
Thomas Rodgers, MD, Assistant Professor, Department of Hematologic Malignancies and Cellular Therapy, Duke University; Staff Physician, Department of Hematology/Oncology, Durham VA Medical Center, Durham, North Carolina
Thomas Rodgers, MD, has disclosed no relevant financial relationships.