User login
Gut microbiota and symptoms of psychosis: Is there a link?
The human microbiota refers to the collection of bacteria, archaea, eukarya, and viruses that reside within the human body. The term gut microbiome indicates the composition of these microbes and genetic codes in the intestine.1 Harkening back to the ancient Greek physician Galen, who treated gastrointestinal (GI) symptoms to relieve mental disturbances such as psychosis, the gut has been a therapeutic target in schizophrenia long before antipsychotics and the DSM.2 In recent years, research into the gut microbiome has drastically increased, with genetic sequencing affording a more precise look into the specific bacteria that call the human intestines their home. This has led to the recognition that the gut microbiome may be severely disrupted in schizophrenia, a condition known as dysbiosis. Preliminary research suggests that gut bacteria are more helpful than many human genes in distinguishing individuals with schizophrenia from their healthy counterparts.3,4 In this article, we discuss the potential role of the gut microbiome in schizophrenia, including new research correlating clinical symptoms of psychosis with dysbiosis. We also provide recommendations for promoting a healthy gut microbiome.
The enteric brain across life
The composition of our bodies is far more microbiota than human. Strikingly, microbiota cells in the gut outnumber human cells, and the distal gut alone hosts bacteria with 100 times the genetic content of the entire genome.5 The intricate meshwork of nerves in the gut is often called the enteric brain because the gut consists of 100 million neurons and synthesizes many neuroactive chemicals implicated in mood disorders and psychosis, including serotonin, dopamine, gamma-aminobutyric acid (GABA), and acetylcholine.6 The variety of neuroimmunologic, hormonal, and metabolic paths by which the gut microbiome and the brain interact are collectively known as the gut-microbiota-brain axis.7
How do we acquire our gut microbiome, and how does it come to influence our brain and behavior? On the first day of life, as babies pass through the birth canal, they are bathed in their mother’s vaginal microbiota. In the following weeks, the microbiome expands and colonizes the gut as bacteria are introduced from environmental sources such as skin-to-skin contact and breastmilk.8 The microbiome continues to evolve throughout early life. As children expand their diets and navigate new aspects of the physical world, additional bacteria join the unseen ecosystem growing inside.9 The development of the microbiome coincides with the development of the brain. From preclinical studies, we know the gut microbiome mediates important aspects of neurodevelopment such as the formation of the blood-brain barrier (BBB), synaptic pruning, glial activation, and myelination.10 Interestingly, many of the risk factors for schizophrenia are associated with gut dysbiosis, including obstetric complications, infections treated with antibiotics, and urbanization.11-15
Throughout human life, the gut and brain remain in close communication. The gut microbiota continue to produce monoamines, along with other metabolites that are able to cross the BBB.6 The HPA axis, stimulation of the immune system, and the vagus nerve all provide highways of communication between the gut and the brain.7 The relationship between the enteric brain and cephalic brain continues through life, even up to a person’s final hour. One autopsy study that is often cited (but soberingly, cannot be found online) allegedly revealed that 92% of schizophrenia patients had developed colitis by the time of death.16,17
First-episode psychosis and antipsychotic treatment
For patients with schizophrenia, first-episode psychosis (FEP) represents a cocktail of mounting genetic and environmental factors. Typically, by the time a patient receives psychiatric care, they present with characteristic psychotic symptoms—hallucinations, delusions, bizarre behavior, and unusual thought process—along with a unique gut microbiome profile.
This disrupted microbiome coincides with a marked state of inflammation in the intestines. Inflammation triggers increased endothelial barrier permeability, similar to the way immune signals increase capillary permeability to allow immune cells into the periphery of the blood. Specific gut bacteria play specific roles in maintaining the gut barrier.18,19 Disruptions in the bacteria that maintain the gut barrier, combined with inflammation, contribute to a leaky gut. A leaky gut barrier allows bacterial and immune products to more easily enter the bloodstream and then the brain, which is a potential source of neuroinflammation in schizophrenia.20 This increase in gut permeability (leaky gut syndrome) is likely one of several reasons low-grade inflammation is common in schizophrenia—numerous studies show higher serum levels of proinflammatory cytokines along with antibacterial immunoglobulins in patients with FEP.21,22
Fortunately, antipsychotics, especially the second-generation agents, help restore a healthy gut microbiome and have substantial anti-inflammatory properties.23,24 These medications interact heavily with the gut microbiome: they have been found to have antibiotic properties, even in doses lower than would normally reach the gut microbiome.25 In humans, a randomized controlled trial of probiotic supplementation for schizophrenia patients taking antipsychotics showed a reduction in GI symptoms but no significant improvement in psychotic symptoms.26
Dysbiosis in schizophrenia: cause or effect?
There is no consensus on what constitutes a healthy gut microbiome because the gut microbiome is highly variable, even among healthy individuals, and can change quickly. Those who adopt new diets, for example, see drastic shifts in the gut microbiome within a few days.27 Despite this variation, the main separation between a healthy and dysbiotic gut comes from the diversity of bacteria present in the gut—a healthy gut microbiome is associated with increased diversity. Numerous disease states have been associated with decreased bacterial diversity, including Clostridium difficile infection, Parkinson disease, depression, Crohn disease, and schizophrenia spectrum disorders.28,29
Although there are ethical limitations to studying causality in humans directly, animal models have provided a great deal of insight into the gut microbiome’s role in the development of schizophrenia. A recent study used fecal transplant to provide the gut microbiome from patients with schizophrenia to a group of germ-free mice and compared these animals to a group of mice that received a fecal transplant from individuals with a healthy gut microbiome. The mice receiving the schizophrenia microbiome showed an increased startle response and hyperactivity.3 This was consistent with mouse models of schizophrenia, although with obvious limitations.30 In addition, the brains of these animals showed changes in glutamate, glutamine, and GABA in the hippocampus; these chemicals play a role in the neurophysiology of schizophrenia.3,31 This study has not yet been replicated, and considerable variation remains within the schizophrenia biosignature.
Continue to: Clinical symptoms of psychosis and the gut microbiome
Clinical symptoms of psychosis and the gut microbiome
Previous literature has grouped patients with schizophrenia spectrum disorders as 1 unified study group. But as is the case with many psychiatric conditions, there is a great deal of heterogeneity in neurobiology, genetics, and microbiome composition among individuals with schizophrenia.32
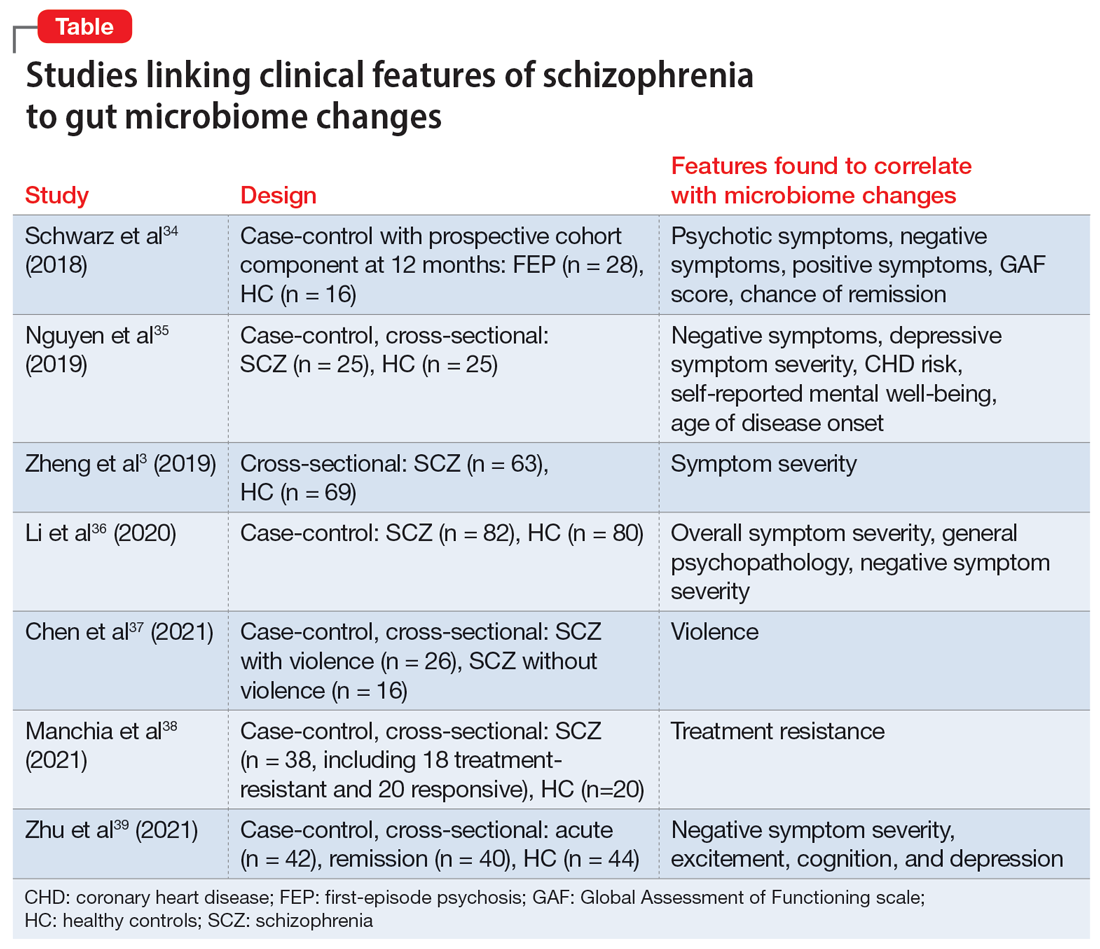
Researchers have begun to investigate ways in which the gut microbiome varies regarding the clinical symptoms of psychosis.33 The Table3,34-39 provides an overview of 7 human studies of gut microbiome changes relating to clinical features of schizophrenia. In these studies, researchers have found correlations between the gut microbiome and a tendency toward violence,37 cognitive deficits,34-36,39 depressive symptoms,35,39 and numerous other clinical features of psychosis. Most of these correlations have not yet been replicated by further studies. But among studies with similar clinical questions, 3 reported changes in gut microbiome correlated with overall symptom severity, and 4 studies correlated changes with negative symptom severity. In 2 studies,3,34 Lachnospiraceae was correlated with worsened symptom severity. However, this may have been the result of poor control for antipsychotic use, as 1 study in bipolar patients found that Lachnospiraceae was increased in those taking antipsychotics compared to those who were not treated with antipsychotics.40 The specific shifts in bacteria seen for overall symptom and negative symptom severity were not consistent across studies. This is not surprising because the gut microbiome varies with diet and geographic region,41 and patients in these studies were from a variety of regions. Multiple studies demonstrated gut microbiome alterations for patients with more severe negative symptoms. This is particularly interesting because negative symptoms are often difficult to treat and do not respond to antipsychotics.42 This research suggests the gut microbiome may be helpful in developing future treatments for patients with negative symptoms that do not respond to existing treatments.
Research of probiotic supplementation for ameliorating symptoms of schizophrenia has yielded mixed results.43 It is possible that studies of probiotic supplementation have failed to consider the variations in the gut microbiome among individuals with schizophrenia. A better understanding of the variations in gut microbiome may allow for the development of more personalized interventions.
Recommendations for a healthy gut microbiome
In addition to antipsychotics, many other evidence-based interventions can be used to help restore a healthy gut microbiome in patients with schizophrenia. To improve the gut microbiome, we suggest discussing the following changes with patients:
- Quitting smoking. Smoking is common among patients with schizophrenia but decreases gut microbiome diversity.44
- Avoiding excessive alcohol use. Excessive alcohol use contributes to dysbiosis and increased intestinal permeability.45 Moderate alcohol consumption does not appear to have the same harmful effects on the microbiome.46
- Avoiding the use of recreational drugs, including marijuana, which impact the gut microbiome.47
- Consuming a diet rich in fiber.48 Presently, there is not enough evidence to recommend probiotic supplementation to reduce symptoms of schizophrenia.41 Similar to probiotics, fermented foods contain Lactobacillus, a bacterial species that produces lactic acid.49 Lactobacillus is enriched in the gut microbiome in some neurodegenerative diseases, and lactic acid can be neurotoxic at high levels.50-52 Therefore, clinicians should not explicitly recommend fermented foods under the assumption of improved brain health. A diet rich in soluble fiber has been consistently shown to promote anti-inflammatory bacteria and is much more likely to be beneficial.53,54 Soluble fiber is found in foods such as fruits, vegetables, beans, and oats.
- Exercising can increase microbiome diversity and provide anti-inflammatory effects in the gut.55,56 A recent review found that steady-state aerobic and high-intensity exercise interventions have positive effects on mood, cognition, and other negative symptoms in patients with schizophrenia.55
- Minimizing stress. Psychological stress and physiological stress from untreated medical conditions are toxic to healthy gut bacteria and weaken the gut barrier.57
- Mitigating exposure to pollution. Environmental pollution, including exposures to air pollution, heavy metals, and pesticides, disrupts the gut microbiome.58
The American Heart Association publishes lifestyle recommendations for individuals with heart disease and the National Institutes of Health publishes lifestyle recommendations for patients with chronic kidney disease. This leads us to question why the American Psychiatric Association has not published lifestyle recommendations for those with severe mental illness. The effects of lifestyle on both the gut microbiome and symptom mitigation is critical. With increasingly shortened appointments, standardized guidelines would benefit psychiatrists and patients alike.
Bottom Line
The gut microbiome is connected to the clinical symptoms of psychosis via a variety of hormonal, neuroimmune, and metabolic mechanisms active across the lifespan. Despite advances in research, there is still much to be understood regarding this relationship. Clinicians should discuss with patients ways to promote a healthy gut microbiome, including consuming a diet rich in fiber, avoiding use of recreational drugs, and exercising regularly.
Related Resources
- Nocera A, Nasrallah HA. The association of the gut microbiota with clinical features in schizophrenia. Behav Sci. 2022;12(4):89.
- Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
1. Bäckhed F, Ley RE, Sonnenburg JL, et al. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915-1920. doi:10.1126/science.1104816
2. Jackson SW. Galen—on mental disorders. J Hist Behav Sci. 1969;5(4):365-384. doi:10.1002/1520-6696(196910)5:4<365::AID-JHBS2300050408>3.0.CO;2-9
3. Zheng P, Zeng B, Liu M, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv. 2019;5(2):eaau8317. doi:10.1126/sciadv.aau8317
4. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421-427. doi:10.1038/nature13595
5. Gill SR, Pop M, DeBoy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312(5778):1355-1359. doi:10.1126/science.1124234
6. Alam R, Abdolmaleky HM, Zhou JR. Microbiome, inflammation, epigenetic alterations, and mental diseases. Am J Med Genet B Neuropsychiatr Genet. 2017;174(6):651-660. doi:10.1002/ajmg.b.32567
7. Cryan JF, O’Riordan KJ, Cowan CSM, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877-2013. doi:10.1152/physrev.00018.2018
8. Mueller NT, Bakacs E, Combellick J, et al. The infant microbiome development: mom matters. Trends Mol Med. 2015;21(2):109-117. doi:10.1016/j.molmed.2014.12.002
9. Fouhy F, Watkins C, Hill CJ, et al. Perinatal factors affect the gut microbiota up to four years after birth. Nat Commun. 2019;10(1):1517. doi:10.1038/s41467-019-09252-4
10. Sharon G, Sampson TR, Geschwind DH, et al. The central nervous system and the gut microbiome. Cell. 2016;167(4):915-932. doi:10.1016/j.cell.2016.10.027
11. Hill CJ, Lynch DB, Murphy K, et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome. 2017;5:4. doi:10.1186/s40168-016-0213-y
12. Gareau MG, Wine E, Rodrigues DM, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307-317. doi:10.1136/gut.2009.202515
13. Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8(343):343ra82. doi:10.1126/scitranslmed.aad7121
14. Mancabelli L, Milani C, Lugli GA, et al. Meta-analysis of the human gut microbiome from urbanized and pre-agricultural populations. Environ Microbiol. 2017;19(4):1379-1390. doi:10.1111/1462-2920.13692
15. Stilo SA, Murray RM. Non-genetic factors in schizophrenia. Curr Psychiatry Rep. 2019;21(10):100. doi:10.1007/s11920-019-1091-3
16. Buscaino VM. Patologia extraneurale della schizofrenia: fegato, tubo digerente, sistema reticolo-endoteliale. Acta Neurologica. 1953;VIII:1-60.
17. Hemmings G. Schizophrenia. Lancet. 2004;364(9442):1312-1313. doi:10.1016/S0140- 6736(04)17181-X
18. Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292(5519):1115-1118. doi:10.1126/science.1058709
19. Ewaschuk JB, Diaz H, Meddings L, et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol-Gastrointest Liver Physiol. 2008;295(5):G1025-G1034. doi:10.1152/ajpgi.90227.2008
20. Alhasson F, Das S, Seth R, et al. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS One. 2017;12(3):e0172914. doi:10.1371/journal.pone.0172914
21. Fillman SG, Cloonan N, Catts VS, et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18(2):206-214. doi:10.1038/mp.2012.110
22. Miller BJ, Buckley P, Seabolt W, et al. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663-671. doi:10.1016/j.biopsych.2011.04.013
23. Al-Amin M, Uddin MMN, Reza HM. Effects of antipsychotics on the inflammatory response system of patients with schizophrenia in peripheral blood mononuclear cell cultures. Clin Psychopharmacol Neurosci. 2013;11(3):144-151. doi:10.9758/cpn.2013.11.3.144
24. Yuan X, Zhang P, Wang Y, et al. Changes in metabolism and microbiota after 24-week risperidone treatment in drug naïve, normal weight patients with first episode schizophrenia. Schizophr Res. 2018;201:299-306. doi:10.1016/j.schres.2018.05.017
25. Maier L, Pruteanu M, Kuhn M, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555(7698):623-628. doi:10.1038/nature25979
26. Dickerson FB, Stallings C, Origoni A, et al. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: a randomized, placebo-controlled trial. Prim Care Companion CNS Disord. 2014;15(1):PCC.13m01579. doi:10.4088/PCC.13m01579
27. David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559-563. doi:10.1038/nature12820
28. Bien J, Palagani V, Bozko P. The intestinal microbiota dysbiosis and Clostridium difficile infection: is there a relationship with inflammatory bowel disease? Ther Adv Gastroenterol. 2013;6(1):53-68. doi:10.1177/1756283X12454590
29. Cryan JF, O’Riordan KJ, Sandhu K, et al. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19(2):179-194. doi:10.1016/S1474-4422(19)30356-4
30. Jones CA, Watson DJG, Fone KCF. Animal models of schizophrenia. Br J Pharmacol. 2011;164(4):1162-1194. doi:10.1111/j.1476-5381.2011.01386.x
31. Schmidt MJ, Mirnics K. Neurodevelopment, GABA system dysfunction, and schizophrenia. Neuropsychopharmacology. 2015;40(1):190-206. doi:10.1038/npp.2014.95
32. Nasrallah, HA. The daunting challenge of schizophrenia: hundreds of biotypes and dozens of theories. Curr. Psychiatry 2018;17(12):4-6,50.
33. Nocera A, Nasrallah HA. The association of the gut microbiota with clinical features in schizophrenia. Behav Sci (Basel). 2022;12(4):89. doi:10.3390/bs12040089
34. Schwarz E, Maukonen J, Hyytiäinen T, et al. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr Res. 2018;192:398-403. doi:10.1016/j.schres.2017.04.017
35. Nguyen TT, Kosciolek T, Maldonado Y, et al. Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophr Res. 2019;204:23-29. doi:10.1016/j.schres.2018.09.014
36. Li S, Zhuo M, Huang X, et al. Altered gut microbiota associated with symptom severity in schizophrenia. PeerJ. 2020;8:e9574. doi:10.7717/peerj.9574
37. Chen X, Xu J, Wang H, et al. Profiling the differences of gut microbial structure between schizophrenia patients with and without violent behaviors based on 16S rRNA gene sequencing. Int J Legal Med. 2021;135(1):131-141. doi:10.1007/s00414-020-02439-1
38. Manchia M, Fontana A, Panebianco C, et al. Involvement of gut microbiota in schizophrenia and treatment resistance to antipsychotics. Biomedicines. 2021;9(8):875. doi:10.3390/biomedicines9080875
39. Zhu C, Zheng M, Ali U, et al. Association between abundance of haemophilus in the gut microbiota and negative symptoms of schizophrenia. Front Psychiatry. 2021;12:685910. doi:10.3389/fpsyt.2021.685910
40. Flowers SA, Evans SJ, Ward KM, et al. Interaction between atypical antipsychotics and the gut microbiome in a bipolar disease cohort. Pharmacotherapy. 2017;37(3):261-267. doi:10.1002/phar.1890
41. Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222-227. doi:10.1038/nature11053
42. Buchanan RW. Persistent negative symptoms in schizophrenia: an overview. Schizophr Bull. 2007;33(4):1013-1022. doi:10.1093/schbul/sb1057
43. Liu JCW, Gorbovskaya I, Hahn MK, et al. The gut microbiome in schizophrenia and the potential benefits of prebiotic and probiotic treatment. Nutrients. 2021;13(4):1152. doi:10.3390/nu13041152
44. Biedermann L, Zeitz J, Mwinyi J, et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PloS One. 2013;8(3):e59260. doi:10.1371/journal.pone.0059260
45. Leclercq S, Matamoros S, Cani PD, et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci. 2014;111(42):e4485-e4493. doi:10.1073/pnas.1415174111
46. Hernández-Quiroz F, Nirmalkar K, Villalobos-Flores LE, et al. Influence of moderate beer consumption on human gut microbiota and its impact on fasting glucose and ß-cell function. Alcohol. 2020;85:77-94. doi:10.1016/j.alcohol.2019.05.006
47. Panee J, Gerschenson M, Chang L. Associations between microbiota, mitochondrial function, and cognition in chronic marijuana users. J Neuroimmune Pharmacol. 2018;13(1):113-122. doi:10.1007/s11481-017-9767-0
48. Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105-108. doi:10.1126/science.1208344
49. Rezac S, Kok CR, Heermann M, et al. Fermented foods as a dietary source of live organisms. Front Microbiol. 2018;9:1785. doi:10.3389/fmicb.2018.01785
50. Chen X, Zhang Y, Wang H, et al. The regulatory effects of lactic acid on neuropsychiatric disorders. Discover Ment Health. 2022;2(1). doi:10.1007/s44192-022-00011-4
51. Karbownik MS, Mokros Ł, Dobielska M, et al. Association between consumption of fermented food and food-derived prebiotics with cognitive performance, depressive, and anxiety symptoms in psychiatrically healthy medical students under psychological stress: a prospective cohort study. Front Nutr. 2022;9:850249. doi:10.3389/fnut.2022.850249
52. Romano S, Savva GM, Bedarf JR, et al. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Parkinsons Dis. 2021;7(1):27. doi:10.1038/s41531-021-00156-z
53. Bourassa MW, Alim I, Bultman SJ, et al. Butyrate, neuroepigenetics and the gut microbiome: can a high fiber diet improve brain health? Neurosci Lett. 2016;625:56-63. doi:10.1016/j.neulet.2016.02.009
54. Matt SM, Allen JM, Lawson MA, et al. Butyrate and dietary soluble fiber improve neuroinflammation associated with aging in mice. Front Immunol. 2018;9:1832. doi:10.3389/fimmu.2018.01832
55. Mittal VA, Vargas T, Osborne KJ, et al. Exercise treatments for psychosis: a review. Curr Treat Options Psychiatry. 2017;4(2):152-166. doi:10.1007/s40501-017-0112-2
56. Estaki M, Pither J, Baumeister P, et al. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. 2016;4(1):42. doi:10.1186/s40168-016-0189-7
57. Karl JP, Margolis LM, Madslien EH, et al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am J Physiol-Gastrointest Liver Physiol. 2017;312(6):G559-G571. doi:10.1152/ajpgi.00066.2017
58. Claus SP, Guillou H, Ellero-Simatos S. The gut microbiota: a major player in the toxicity of environmental pollutants? NPJ Biofilms Microbiomes. 2016;2:16003. doi:10.1038/npjbiofilms.2016.3
The human microbiota refers to the collection of bacteria, archaea, eukarya, and viruses that reside within the human body. The term gut microbiome indicates the composition of these microbes and genetic codes in the intestine.1 Harkening back to the ancient Greek physician Galen, who treated gastrointestinal (GI) symptoms to relieve mental disturbances such as psychosis, the gut has been a therapeutic target in schizophrenia long before antipsychotics and the DSM.2 In recent years, research into the gut microbiome has drastically increased, with genetic sequencing affording a more precise look into the specific bacteria that call the human intestines their home. This has led to the recognition that the gut microbiome may be severely disrupted in schizophrenia, a condition known as dysbiosis. Preliminary research suggests that gut bacteria are more helpful than many human genes in distinguishing individuals with schizophrenia from their healthy counterparts.3,4 In this article, we discuss the potential role of the gut microbiome in schizophrenia, including new research correlating clinical symptoms of psychosis with dysbiosis. We also provide recommendations for promoting a healthy gut microbiome.
The enteric brain across life
The composition of our bodies is far more microbiota than human. Strikingly, microbiota cells in the gut outnumber human cells, and the distal gut alone hosts bacteria with 100 times the genetic content of the entire genome.5 The intricate meshwork of nerves in the gut is often called the enteric brain because the gut consists of 100 million neurons and synthesizes many neuroactive chemicals implicated in mood disorders and psychosis, including serotonin, dopamine, gamma-aminobutyric acid (GABA), and acetylcholine.6 The variety of neuroimmunologic, hormonal, and metabolic paths by which the gut microbiome and the brain interact are collectively known as the gut-microbiota-brain axis.7
How do we acquire our gut microbiome, and how does it come to influence our brain and behavior? On the first day of life, as babies pass through the birth canal, they are bathed in their mother’s vaginal microbiota. In the following weeks, the microbiome expands and colonizes the gut as bacteria are introduced from environmental sources such as skin-to-skin contact and breastmilk.8 The microbiome continues to evolve throughout early life. As children expand their diets and navigate new aspects of the physical world, additional bacteria join the unseen ecosystem growing inside.9 The development of the microbiome coincides with the development of the brain. From preclinical studies, we know the gut microbiome mediates important aspects of neurodevelopment such as the formation of the blood-brain barrier (BBB), synaptic pruning, glial activation, and myelination.10 Interestingly, many of the risk factors for schizophrenia are associated with gut dysbiosis, including obstetric complications, infections treated with antibiotics, and urbanization.11-15
Throughout human life, the gut and brain remain in close communication. The gut microbiota continue to produce monoamines, along with other metabolites that are able to cross the BBB.6 The HPA axis, stimulation of the immune system, and the vagus nerve all provide highways of communication between the gut and the brain.7 The relationship between the enteric brain and cephalic brain continues through life, even up to a person’s final hour. One autopsy study that is often cited (but soberingly, cannot be found online) allegedly revealed that 92% of schizophrenia patients had developed colitis by the time of death.16,17
First-episode psychosis and antipsychotic treatment
For patients with schizophrenia, first-episode psychosis (FEP) represents a cocktail of mounting genetic and environmental factors. Typically, by the time a patient receives psychiatric care, they present with characteristic psychotic symptoms—hallucinations, delusions, bizarre behavior, and unusual thought process—along with a unique gut microbiome profile.
This disrupted microbiome coincides with a marked state of inflammation in the intestines. Inflammation triggers increased endothelial barrier permeability, similar to the way immune signals increase capillary permeability to allow immune cells into the periphery of the blood. Specific gut bacteria play specific roles in maintaining the gut barrier.18,19 Disruptions in the bacteria that maintain the gut barrier, combined with inflammation, contribute to a leaky gut. A leaky gut barrier allows bacterial and immune products to more easily enter the bloodstream and then the brain, which is a potential source of neuroinflammation in schizophrenia.20 This increase in gut permeability (leaky gut syndrome) is likely one of several reasons low-grade inflammation is common in schizophrenia—numerous studies show higher serum levels of proinflammatory cytokines along with antibacterial immunoglobulins in patients with FEP.21,22
Fortunately, antipsychotics, especially the second-generation agents, help restore a healthy gut microbiome and have substantial anti-inflammatory properties.23,24 These medications interact heavily with the gut microbiome: they have been found to have antibiotic properties, even in doses lower than would normally reach the gut microbiome.25 In humans, a randomized controlled trial of probiotic supplementation for schizophrenia patients taking antipsychotics showed a reduction in GI symptoms but no significant improvement in psychotic symptoms.26
Dysbiosis in schizophrenia: cause or effect?
There is no consensus on what constitutes a healthy gut microbiome because the gut microbiome is highly variable, even among healthy individuals, and can change quickly. Those who adopt new diets, for example, see drastic shifts in the gut microbiome within a few days.27 Despite this variation, the main separation between a healthy and dysbiotic gut comes from the diversity of bacteria present in the gut—a healthy gut microbiome is associated with increased diversity. Numerous disease states have been associated with decreased bacterial diversity, including Clostridium difficile infection, Parkinson disease, depression, Crohn disease, and schizophrenia spectrum disorders.28,29
Although there are ethical limitations to studying causality in humans directly, animal models have provided a great deal of insight into the gut microbiome’s role in the development of schizophrenia. A recent study used fecal transplant to provide the gut microbiome from patients with schizophrenia to a group of germ-free mice and compared these animals to a group of mice that received a fecal transplant from individuals with a healthy gut microbiome. The mice receiving the schizophrenia microbiome showed an increased startle response and hyperactivity.3 This was consistent with mouse models of schizophrenia, although with obvious limitations.30 In addition, the brains of these animals showed changes in glutamate, glutamine, and GABA in the hippocampus; these chemicals play a role in the neurophysiology of schizophrenia.3,31 This study has not yet been replicated, and considerable variation remains within the schizophrenia biosignature.
Continue to: Clinical symptoms of psychosis and the gut microbiome
Clinical symptoms of psychosis and the gut microbiome
Previous literature has grouped patients with schizophrenia spectrum disorders as 1 unified study group. But as is the case with many psychiatric conditions, there is a great deal of heterogeneity in neurobiology, genetics, and microbiome composition among individuals with schizophrenia.32
Researchers have begun to investigate ways in which the gut microbiome varies regarding the clinical symptoms of psychosis.33 The Table3,34-39 provides an overview of 7 human studies of gut microbiome changes relating to clinical features of schizophrenia. In these studies, researchers have found correlations between the gut microbiome and a tendency toward violence,37 cognitive deficits,34-36,39 depressive symptoms,35,39 and numerous other clinical features of psychosis. Most of these correlations have not yet been replicated by further studies. But among studies with similar clinical questions, 3 reported changes in gut microbiome correlated with overall symptom severity, and 4 studies correlated changes with negative symptom severity. In 2 studies,3,34 Lachnospiraceae was correlated with worsened symptom severity. However, this may have been the result of poor control for antipsychotic use, as 1 study in bipolar patients found that Lachnospiraceae was increased in those taking antipsychotics compared to those who were not treated with antipsychotics.40 The specific shifts in bacteria seen for overall symptom and negative symptom severity were not consistent across studies. This is not surprising because the gut microbiome varies with diet and geographic region,41 and patients in these studies were from a variety of regions. Multiple studies demonstrated gut microbiome alterations for patients with more severe negative symptoms. This is particularly interesting because negative symptoms are often difficult to treat and do not respond to antipsychotics.42 This research suggests the gut microbiome may be helpful in developing future treatments for patients with negative symptoms that do not respond to existing treatments.
Research of probiotic supplementation for ameliorating symptoms of schizophrenia has yielded mixed results.43 It is possible that studies of probiotic supplementation have failed to consider the variations in the gut microbiome among individuals with schizophrenia. A better understanding of the variations in gut microbiome may allow for the development of more personalized interventions.

Recommendations for a healthy gut microbiome
In addition to antipsychotics, many other evidence-based interventions can be used to help restore a healthy gut microbiome in patients with schizophrenia. To improve the gut microbiome, we suggest discussing the following changes with patients:
- Quitting smoking. Smoking is common among patients with schizophrenia but decreases gut microbiome diversity.44
- Avoiding excessive alcohol use. Excessive alcohol use contributes to dysbiosis and increased intestinal permeability.45 Moderate alcohol consumption does not appear to have the same harmful effects on the microbiome.46
- Avoiding the use of recreational drugs, including marijuana, which impact the gut microbiome.47
- Consuming a diet rich in fiber.48 Presently, there is not enough evidence to recommend probiotic supplementation to reduce symptoms of schizophrenia.41 Similar to probiotics, fermented foods contain Lactobacillus, a bacterial species that produces lactic acid.49 Lactobacillus is enriched in the gut microbiome in some neurodegenerative diseases, and lactic acid can be neurotoxic at high levels.50-52 Therefore, clinicians should not explicitly recommend fermented foods under the assumption of improved brain health. A diet rich in soluble fiber has been consistently shown to promote anti-inflammatory bacteria and is much more likely to be beneficial.53,54 Soluble fiber is found in foods such as fruits, vegetables, beans, and oats.
- Exercising can increase microbiome diversity and provide anti-inflammatory effects in the gut.55,56 A recent review found that steady-state aerobic and high-intensity exercise interventions have positive effects on mood, cognition, and other negative symptoms in patients with schizophrenia.55
- Minimizing stress. Psychological stress and physiological stress from untreated medical conditions are toxic to healthy gut bacteria and weaken the gut barrier.57
- Mitigating exposure to pollution. Environmental pollution, including exposures to air pollution, heavy metals, and pesticides, disrupts the gut microbiome.58
The American Heart Association publishes lifestyle recommendations for individuals with heart disease and the National Institutes of Health publishes lifestyle recommendations for patients with chronic kidney disease. This leads us to question why the American Psychiatric Association has not published lifestyle recommendations for those with severe mental illness. The effects of lifestyle on both the gut microbiome and symptom mitigation is critical. With increasingly shortened appointments, standardized guidelines would benefit psychiatrists and patients alike.
Bottom Line
The gut microbiome is connected to the clinical symptoms of psychosis via a variety of hormonal, neuroimmune, and metabolic mechanisms active across the lifespan. Despite advances in research, there is still much to be understood regarding this relationship. Clinicians should discuss with patients ways to promote a healthy gut microbiome, including consuming a diet rich in fiber, avoiding use of recreational drugs, and exercising regularly.
Related Resources
- Nocera A, Nasrallah HA. The association of the gut microbiota with clinical features in schizophrenia. Behav Sci. 2022;12(4):89.
- Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
The human microbiota refers to the collection of bacteria, archaea, eukarya, and viruses that reside within the human body. The term gut microbiome indicates the composition of these microbes and genetic codes in the intestine.1 Harkening back to the ancient Greek physician Galen, who treated gastrointestinal (GI) symptoms to relieve mental disturbances such as psychosis, the gut has been a therapeutic target in schizophrenia long before antipsychotics and the DSM.2 In recent years, research into the gut microbiome has drastically increased, with genetic sequencing affording a more precise look into the specific bacteria that call the human intestines their home. This has led to the recognition that the gut microbiome may be severely disrupted in schizophrenia, a condition known as dysbiosis. Preliminary research suggests that gut bacteria are more helpful than many human genes in distinguishing individuals with schizophrenia from their healthy counterparts.3,4 In this article, we discuss the potential role of the gut microbiome in schizophrenia, including new research correlating clinical symptoms of psychosis with dysbiosis. We also provide recommendations for promoting a healthy gut microbiome.
The enteric brain across life
The composition of our bodies is far more microbiota than human. Strikingly, microbiota cells in the gut outnumber human cells, and the distal gut alone hosts bacteria with 100 times the genetic content of the entire genome.5 The intricate meshwork of nerves in the gut is often called the enteric brain because the gut consists of 100 million neurons and synthesizes many neuroactive chemicals implicated in mood disorders and psychosis, including serotonin, dopamine, gamma-aminobutyric acid (GABA), and acetylcholine.6 The variety of neuroimmunologic, hormonal, and metabolic paths by which the gut microbiome and the brain interact are collectively known as the gut-microbiota-brain axis.7
How do we acquire our gut microbiome, and how does it come to influence our brain and behavior? On the first day of life, as babies pass through the birth canal, they are bathed in their mother’s vaginal microbiota. In the following weeks, the microbiome expands and colonizes the gut as bacteria are introduced from environmental sources such as skin-to-skin contact and breastmilk.8 The microbiome continues to evolve throughout early life. As children expand their diets and navigate new aspects of the physical world, additional bacteria join the unseen ecosystem growing inside.9 The development of the microbiome coincides with the development of the brain. From preclinical studies, we know the gut microbiome mediates important aspects of neurodevelopment such as the formation of the blood-brain barrier (BBB), synaptic pruning, glial activation, and myelination.10 Interestingly, many of the risk factors for schizophrenia are associated with gut dysbiosis, including obstetric complications, infections treated with antibiotics, and urbanization.11-15
Throughout human life, the gut and brain remain in close communication. The gut microbiota continue to produce monoamines, along with other metabolites that are able to cross the BBB.6 The HPA axis, stimulation of the immune system, and the vagus nerve all provide highways of communication between the gut and the brain.7 The relationship between the enteric brain and cephalic brain continues through life, even up to a person’s final hour. One autopsy study that is often cited (but soberingly, cannot be found online) allegedly revealed that 92% of schizophrenia patients had developed colitis by the time of death.16,17
First-episode psychosis and antipsychotic treatment
For patients with schizophrenia, first-episode psychosis (FEP) represents a cocktail of mounting genetic and environmental factors. Typically, by the time a patient receives psychiatric care, they present with characteristic psychotic symptoms—hallucinations, delusions, bizarre behavior, and unusual thought process—along with a unique gut microbiome profile.
This disrupted microbiome coincides with a marked state of inflammation in the intestines. Inflammation triggers increased endothelial barrier permeability, similar to the way immune signals increase capillary permeability to allow immune cells into the periphery of the blood. Specific gut bacteria play specific roles in maintaining the gut barrier.18,19 Disruptions in the bacteria that maintain the gut barrier, combined with inflammation, contribute to a leaky gut. A leaky gut barrier allows bacterial and immune products to more easily enter the bloodstream and then the brain, which is a potential source of neuroinflammation in schizophrenia.20 This increase in gut permeability (leaky gut syndrome) is likely one of several reasons low-grade inflammation is common in schizophrenia—numerous studies show higher serum levels of proinflammatory cytokines along with antibacterial immunoglobulins in patients with FEP.21,22
Fortunately, antipsychotics, especially the second-generation agents, help restore a healthy gut microbiome and have substantial anti-inflammatory properties.23,24 These medications interact heavily with the gut microbiome: they have been found to have antibiotic properties, even in doses lower than would normally reach the gut microbiome.25 In humans, a randomized controlled trial of probiotic supplementation for schizophrenia patients taking antipsychotics showed a reduction in GI symptoms but no significant improvement in psychotic symptoms.26
Dysbiosis in schizophrenia: cause or effect?
There is no consensus on what constitutes a healthy gut microbiome because the gut microbiome is highly variable, even among healthy individuals, and can change quickly. Those who adopt new diets, for example, see drastic shifts in the gut microbiome within a few days.27 Despite this variation, the main separation between a healthy and dysbiotic gut comes from the diversity of bacteria present in the gut—a healthy gut microbiome is associated with increased diversity. Numerous disease states have been associated with decreased bacterial diversity, including Clostridium difficile infection, Parkinson disease, depression, Crohn disease, and schizophrenia spectrum disorders.28,29
Although there are ethical limitations to studying causality in humans directly, animal models have provided a great deal of insight into the gut microbiome’s role in the development of schizophrenia. A recent study used fecal transplant to provide the gut microbiome from patients with schizophrenia to a group of germ-free mice and compared these animals to a group of mice that received a fecal transplant from individuals with a healthy gut microbiome. The mice receiving the schizophrenia microbiome showed an increased startle response and hyperactivity.3 This was consistent with mouse models of schizophrenia, although with obvious limitations.30 In addition, the brains of these animals showed changes in glutamate, glutamine, and GABA in the hippocampus; these chemicals play a role in the neurophysiology of schizophrenia.3,31 This study has not yet been replicated, and considerable variation remains within the schizophrenia biosignature.
Continue to: Clinical symptoms of psychosis and the gut microbiome
Clinical symptoms of psychosis and the gut microbiome
Previous literature has grouped patients with schizophrenia spectrum disorders as 1 unified study group. But as is the case with many psychiatric conditions, there is a great deal of heterogeneity in neurobiology, genetics, and microbiome composition among individuals with schizophrenia.32
Researchers have begun to investigate ways in which the gut microbiome varies regarding the clinical symptoms of psychosis.33 The Table3,34-39 provides an overview of 7 human studies of gut microbiome changes relating to clinical features of schizophrenia. In these studies, researchers have found correlations between the gut microbiome and a tendency toward violence,37 cognitive deficits,34-36,39 depressive symptoms,35,39 and numerous other clinical features of psychosis. Most of these correlations have not yet been replicated by further studies. But among studies with similar clinical questions, 3 reported changes in gut microbiome correlated with overall symptom severity, and 4 studies correlated changes with negative symptom severity. In 2 studies,3,34 Lachnospiraceae was correlated with worsened symptom severity. However, this may have been the result of poor control for antipsychotic use, as 1 study in bipolar patients found that Lachnospiraceae was increased in those taking antipsychotics compared to those who were not treated with antipsychotics.40 The specific shifts in bacteria seen for overall symptom and negative symptom severity were not consistent across studies. This is not surprising because the gut microbiome varies with diet and geographic region,41 and patients in these studies were from a variety of regions. Multiple studies demonstrated gut microbiome alterations for patients with more severe negative symptoms. This is particularly interesting because negative symptoms are often difficult to treat and do not respond to antipsychotics.42 This research suggests the gut microbiome may be helpful in developing future treatments for patients with negative symptoms that do not respond to existing treatments.
Research of probiotic supplementation for ameliorating symptoms of schizophrenia has yielded mixed results.43 It is possible that studies of probiotic supplementation have failed to consider the variations in the gut microbiome among individuals with schizophrenia. A better understanding of the variations in gut microbiome may allow for the development of more personalized interventions.

Recommendations for a healthy gut microbiome
In addition to antipsychotics, many other evidence-based interventions can be used to help restore a healthy gut microbiome in patients with schizophrenia. To improve the gut microbiome, we suggest discussing the following changes with patients:
- Quitting smoking. Smoking is common among patients with schizophrenia but decreases gut microbiome diversity.44
- Avoiding excessive alcohol use. Excessive alcohol use contributes to dysbiosis and increased intestinal permeability.45 Moderate alcohol consumption does not appear to have the same harmful effects on the microbiome.46
- Avoiding the use of recreational drugs, including marijuana, which impact the gut microbiome.47
- Consuming a diet rich in fiber.48 Presently, there is not enough evidence to recommend probiotic supplementation to reduce symptoms of schizophrenia.41 Similar to probiotics, fermented foods contain Lactobacillus, a bacterial species that produces lactic acid.49 Lactobacillus is enriched in the gut microbiome in some neurodegenerative diseases, and lactic acid can be neurotoxic at high levels.50-52 Therefore, clinicians should not explicitly recommend fermented foods under the assumption of improved brain health. A diet rich in soluble fiber has been consistently shown to promote anti-inflammatory bacteria and is much more likely to be beneficial.53,54 Soluble fiber is found in foods such as fruits, vegetables, beans, and oats.
- Exercising can increase microbiome diversity and provide anti-inflammatory effects in the gut.55,56 A recent review found that steady-state aerobic and high-intensity exercise interventions have positive effects on mood, cognition, and other negative symptoms in patients with schizophrenia.55
- Minimizing stress. Psychological stress and physiological stress from untreated medical conditions are toxic to healthy gut bacteria and weaken the gut barrier.57
- Mitigating exposure to pollution. Environmental pollution, including exposures to air pollution, heavy metals, and pesticides, disrupts the gut microbiome.58
The American Heart Association publishes lifestyle recommendations for individuals with heart disease and the National Institutes of Health publishes lifestyle recommendations for patients with chronic kidney disease. This leads us to question why the American Psychiatric Association has not published lifestyle recommendations for those with severe mental illness. The effects of lifestyle on both the gut microbiome and symptom mitigation is critical. With increasingly shortened appointments, standardized guidelines would benefit psychiatrists and patients alike.
Bottom Line
The gut microbiome is connected to the clinical symptoms of psychosis via a variety of hormonal, neuroimmune, and metabolic mechanisms active across the lifespan. Despite advances in research, there is still much to be understood regarding this relationship. Clinicians should discuss with patients ways to promote a healthy gut microbiome, including consuming a diet rich in fiber, avoiding use of recreational drugs, and exercising regularly.
Related Resources
- Nocera A, Nasrallah HA. The association of the gut microbiota with clinical features in schizophrenia. Behav Sci. 2022;12(4):89.
- Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
1. Bäckhed F, Ley RE, Sonnenburg JL, et al. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915-1920. doi:10.1126/science.1104816
2. Jackson SW. Galen—on mental disorders. J Hist Behav Sci. 1969;5(4):365-384. doi:10.1002/1520-6696(196910)5:4<365::AID-JHBS2300050408>3.0.CO;2-9
3. Zheng P, Zeng B, Liu M, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv. 2019;5(2):eaau8317. doi:10.1126/sciadv.aau8317
4. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421-427. doi:10.1038/nature13595
5. Gill SR, Pop M, DeBoy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312(5778):1355-1359. doi:10.1126/science.1124234
6. Alam R, Abdolmaleky HM, Zhou JR. Microbiome, inflammation, epigenetic alterations, and mental diseases. Am J Med Genet B Neuropsychiatr Genet. 2017;174(6):651-660. doi:10.1002/ajmg.b.32567
7. Cryan JF, O’Riordan KJ, Cowan CSM, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877-2013. doi:10.1152/physrev.00018.2018
8. Mueller NT, Bakacs E, Combellick J, et al. The infant microbiome development: mom matters. Trends Mol Med. 2015;21(2):109-117. doi:10.1016/j.molmed.2014.12.002
9. Fouhy F, Watkins C, Hill CJ, et al. Perinatal factors affect the gut microbiota up to four years after birth. Nat Commun. 2019;10(1):1517. doi:10.1038/s41467-019-09252-4
10. Sharon G, Sampson TR, Geschwind DH, et al. The central nervous system and the gut microbiome. Cell. 2016;167(4):915-932. doi:10.1016/j.cell.2016.10.027
11. Hill CJ, Lynch DB, Murphy K, et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome. 2017;5:4. doi:10.1186/s40168-016-0213-y
12. Gareau MG, Wine E, Rodrigues DM, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307-317. doi:10.1136/gut.2009.202515
13. Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8(343):343ra82. doi:10.1126/scitranslmed.aad7121
14. Mancabelli L, Milani C, Lugli GA, et al. Meta-analysis of the human gut microbiome from urbanized and pre-agricultural populations. Environ Microbiol. 2017;19(4):1379-1390. doi:10.1111/1462-2920.13692
15. Stilo SA, Murray RM. Non-genetic factors in schizophrenia. Curr Psychiatry Rep. 2019;21(10):100. doi:10.1007/s11920-019-1091-3
16. Buscaino VM. Patologia extraneurale della schizofrenia: fegato, tubo digerente, sistema reticolo-endoteliale. Acta Neurologica. 1953;VIII:1-60.
17. Hemmings G. Schizophrenia. Lancet. 2004;364(9442):1312-1313. doi:10.1016/S0140- 6736(04)17181-X
18. Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292(5519):1115-1118. doi:10.1126/science.1058709
19. Ewaschuk JB, Diaz H, Meddings L, et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol-Gastrointest Liver Physiol. 2008;295(5):G1025-G1034. doi:10.1152/ajpgi.90227.2008
20. Alhasson F, Das S, Seth R, et al. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS One. 2017;12(3):e0172914. doi:10.1371/journal.pone.0172914
21. Fillman SG, Cloonan N, Catts VS, et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18(2):206-214. doi:10.1038/mp.2012.110
22. Miller BJ, Buckley P, Seabolt W, et al. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663-671. doi:10.1016/j.biopsych.2011.04.013
23. Al-Amin M, Uddin MMN, Reza HM. Effects of antipsychotics on the inflammatory response system of patients with schizophrenia in peripheral blood mononuclear cell cultures. Clin Psychopharmacol Neurosci. 2013;11(3):144-151. doi:10.9758/cpn.2013.11.3.144
24. Yuan X, Zhang P, Wang Y, et al. Changes in metabolism and microbiota after 24-week risperidone treatment in drug naïve, normal weight patients with first episode schizophrenia. Schizophr Res. 2018;201:299-306. doi:10.1016/j.schres.2018.05.017
25. Maier L, Pruteanu M, Kuhn M, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555(7698):623-628. doi:10.1038/nature25979
26. Dickerson FB, Stallings C, Origoni A, et al. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: a randomized, placebo-controlled trial. Prim Care Companion CNS Disord. 2014;15(1):PCC.13m01579. doi:10.4088/PCC.13m01579
27. David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559-563. doi:10.1038/nature12820
28. Bien J, Palagani V, Bozko P. The intestinal microbiota dysbiosis and Clostridium difficile infection: is there a relationship with inflammatory bowel disease? Ther Adv Gastroenterol. 2013;6(1):53-68. doi:10.1177/1756283X12454590
29. Cryan JF, O’Riordan KJ, Sandhu K, et al. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19(2):179-194. doi:10.1016/S1474-4422(19)30356-4
30. Jones CA, Watson DJG, Fone KCF. Animal models of schizophrenia. Br J Pharmacol. 2011;164(4):1162-1194. doi:10.1111/j.1476-5381.2011.01386.x
31. Schmidt MJ, Mirnics K. Neurodevelopment, GABA system dysfunction, and schizophrenia. Neuropsychopharmacology. 2015;40(1):190-206. doi:10.1038/npp.2014.95
32. Nasrallah, HA. The daunting challenge of schizophrenia: hundreds of biotypes and dozens of theories. Curr. Psychiatry 2018;17(12):4-6,50.
33. Nocera A, Nasrallah HA. The association of the gut microbiota with clinical features in schizophrenia. Behav Sci (Basel). 2022;12(4):89. doi:10.3390/bs12040089
34. Schwarz E, Maukonen J, Hyytiäinen T, et al. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr Res. 2018;192:398-403. doi:10.1016/j.schres.2017.04.017
35. Nguyen TT, Kosciolek T, Maldonado Y, et al. Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophr Res. 2019;204:23-29. doi:10.1016/j.schres.2018.09.014
36. Li S, Zhuo M, Huang X, et al. Altered gut microbiota associated with symptom severity in schizophrenia. PeerJ. 2020;8:e9574. doi:10.7717/peerj.9574
37. Chen X, Xu J, Wang H, et al. Profiling the differences of gut microbial structure between schizophrenia patients with and without violent behaviors based on 16S rRNA gene sequencing. Int J Legal Med. 2021;135(1):131-141. doi:10.1007/s00414-020-02439-1
38. Manchia M, Fontana A, Panebianco C, et al. Involvement of gut microbiota in schizophrenia and treatment resistance to antipsychotics. Biomedicines. 2021;9(8):875. doi:10.3390/biomedicines9080875
39. Zhu C, Zheng M, Ali U, et al. Association between abundance of haemophilus in the gut microbiota and negative symptoms of schizophrenia. Front Psychiatry. 2021;12:685910. doi:10.3389/fpsyt.2021.685910
40. Flowers SA, Evans SJ, Ward KM, et al. Interaction between atypical antipsychotics and the gut microbiome in a bipolar disease cohort. Pharmacotherapy. 2017;37(3):261-267. doi:10.1002/phar.1890
41. Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222-227. doi:10.1038/nature11053
42. Buchanan RW. Persistent negative symptoms in schizophrenia: an overview. Schizophr Bull. 2007;33(4):1013-1022. doi:10.1093/schbul/sb1057
43. Liu JCW, Gorbovskaya I, Hahn MK, et al. The gut microbiome in schizophrenia and the potential benefits of prebiotic and probiotic treatment. Nutrients. 2021;13(4):1152. doi:10.3390/nu13041152
44. Biedermann L, Zeitz J, Mwinyi J, et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PloS One. 2013;8(3):e59260. doi:10.1371/journal.pone.0059260
45. Leclercq S, Matamoros S, Cani PD, et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci. 2014;111(42):e4485-e4493. doi:10.1073/pnas.1415174111
46. Hernández-Quiroz F, Nirmalkar K, Villalobos-Flores LE, et al. Influence of moderate beer consumption on human gut microbiota and its impact on fasting glucose and ß-cell function. Alcohol. 2020;85:77-94. doi:10.1016/j.alcohol.2019.05.006
47. Panee J, Gerschenson M, Chang L. Associations between microbiota, mitochondrial function, and cognition in chronic marijuana users. J Neuroimmune Pharmacol. 2018;13(1):113-122. doi:10.1007/s11481-017-9767-0
48. Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105-108. doi:10.1126/science.1208344
49. Rezac S, Kok CR, Heermann M, et al. Fermented foods as a dietary source of live organisms. Front Microbiol. 2018;9:1785. doi:10.3389/fmicb.2018.01785
50. Chen X, Zhang Y, Wang H, et al. The regulatory effects of lactic acid on neuropsychiatric disorders. Discover Ment Health. 2022;2(1). doi:10.1007/s44192-022-00011-4
51. Karbownik MS, Mokros Ł, Dobielska M, et al. Association between consumption of fermented food and food-derived prebiotics with cognitive performance, depressive, and anxiety symptoms in psychiatrically healthy medical students under psychological stress: a prospective cohort study. Front Nutr. 2022;9:850249. doi:10.3389/fnut.2022.850249
52. Romano S, Savva GM, Bedarf JR, et al. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Parkinsons Dis. 2021;7(1):27. doi:10.1038/s41531-021-00156-z
53. Bourassa MW, Alim I, Bultman SJ, et al. Butyrate, neuroepigenetics and the gut microbiome: can a high fiber diet improve brain health? Neurosci Lett. 2016;625:56-63. doi:10.1016/j.neulet.2016.02.009
54. Matt SM, Allen JM, Lawson MA, et al. Butyrate and dietary soluble fiber improve neuroinflammation associated with aging in mice. Front Immunol. 2018;9:1832. doi:10.3389/fimmu.2018.01832
55. Mittal VA, Vargas T, Osborne KJ, et al. Exercise treatments for psychosis: a review. Curr Treat Options Psychiatry. 2017;4(2):152-166. doi:10.1007/s40501-017-0112-2
56. Estaki M, Pither J, Baumeister P, et al. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. 2016;4(1):42. doi:10.1186/s40168-016-0189-7
57. Karl JP, Margolis LM, Madslien EH, et al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am J Physiol-Gastrointest Liver Physiol. 2017;312(6):G559-G571. doi:10.1152/ajpgi.00066.2017
58. Claus SP, Guillou H, Ellero-Simatos S. The gut microbiota: a major player in the toxicity of environmental pollutants? NPJ Biofilms Microbiomes. 2016;2:16003. doi:10.1038/npjbiofilms.2016.3
1. Bäckhed F, Ley RE, Sonnenburg JL, et al. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915-1920. doi:10.1126/science.1104816
2. Jackson SW. Galen—on mental disorders. J Hist Behav Sci. 1969;5(4):365-384. doi:10.1002/1520-6696(196910)5:4<365::AID-JHBS2300050408>3.0.CO;2-9
3. Zheng P, Zeng B, Liu M, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv. 2019;5(2):eaau8317. doi:10.1126/sciadv.aau8317
4. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421-427. doi:10.1038/nature13595
5. Gill SR, Pop M, DeBoy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312(5778):1355-1359. doi:10.1126/science.1124234
6. Alam R, Abdolmaleky HM, Zhou JR. Microbiome, inflammation, epigenetic alterations, and mental diseases. Am J Med Genet B Neuropsychiatr Genet. 2017;174(6):651-660. doi:10.1002/ajmg.b.32567
7. Cryan JF, O’Riordan KJ, Cowan CSM, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877-2013. doi:10.1152/physrev.00018.2018
8. Mueller NT, Bakacs E, Combellick J, et al. The infant microbiome development: mom matters. Trends Mol Med. 2015;21(2):109-117. doi:10.1016/j.molmed.2014.12.002
9. Fouhy F, Watkins C, Hill CJ, et al. Perinatal factors affect the gut microbiota up to four years after birth. Nat Commun. 2019;10(1):1517. doi:10.1038/s41467-019-09252-4
10. Sharon G, Sampson TR, Geschwind DH, et al. The central nervous system and the gut microbiome. Cell. 2016;167(4):915-932. doi:10.1016/j.cell.2016.10.027
11. Hill CJ, Lynch DB, Murphy K, et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome. 2017;5:4. doi:10.1186/s40168-016-0213-y
12. Gareau MG, Wine E, Rodrigues DM, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307-317. doi:10.1136/gut.2009.202515
13. Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8(343):343ra82. doi:10.1126/scitranslmed.aad7121
14. Mancabelli L, Milani C, Lugli GA, et al. Meta-analysis of the human gut microbiome from urbanized and pre-agricultural populations. Environ Microbiol. 2017;19(4):1379-1390. doi:10.1111/1462-2920.13692
15. Stilo SA, Murray RM. Non-genetic factors in schizophrenia. Curr Psychiatry Rep. 2019;21(10):100. doi:10.1007/s11920-019-1091-3
16. Buscaino VM. Patologia extraneurale della schizofrenia: fegato, tubo digerente, sistema reticolo-endoteliale. Acta Neurologica. 1953;VIII:1-60.
17. Hemmings G. Schizophrenia. Lancet. 2004;364(9442):1312-1313. doi:10.1016/S0140- 6736(04)17181-X
18. Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292(5519):1115-1118. doi:10.1126/science.1058709
19. Ewaschuk JB, Diaz H, Meddings L, et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol-Gastrointest Liver Physiol. 2008;295(5):G1025-G1034. doi:10.1152/ajpgi.90227.2008
20. Alhasson F, Das S, Seth R, et al. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS One. 2017;12(3):e0172914. doi:10.1371/journal.pone.0172914
21. Fillman SG, Cloonan N, Catts VS, et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18(2):206-214. doi:10.1038/mp.2012.110
22. Miller BJ, Buckley P, Seabolt W, et al. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663-671. doi:10.1016/j.biopsych.2011.04.013
23. Al-Amin M, Uddin MMN, Reza HM. Effects of antipsychotics on the inflammatory response system of patients with schizophrenia in peripheral blood mononuclear cell cultures. Clin Psychopharmacol Neurosci. 2013;11(3):144-151. doi:10.9758/cpn.2013.11.3.144
24. Yuan X, Zhang P, Wang Y, et al. Changes in metabolism and microbiota after 24-week risperidone treatment in drug naïve, normal weight patients with first episode schizophrenia. Schizophr Res. 2018;201:299-306. doi:10.1016/j.schres.2018.05.017
25. Maier L, Pruteanu M, Kuhn M, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555(7698):623-628. doi:10.1038/nature25979
26. Dickerson FB, Stallings C, Origoni A, et al. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: a randomized, placebo-controlled trial. Prim Care Companion CNS Disord. 2014;15(1):PCC.13m01579. doi:10.4088/PCC.13m01579
27. David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559-563. doi:10.1038/nature12820
28. Bien J, Palagani V, Bozko P. The intestinal microbiota dysbiosis and Clostridium difficile infection: is there a relationship with inflammatory bowel disease? Ther Adv Gastroenterol. 2013;6(1):53-68. doi:10.1177/1756283X12454590
29. Cryan JF, O’Riordan KJ, Sandhu K, et al. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19(2):179-194. doi:10.1016/S1474-4422(19)30356-4
30. Jones CA, Watson DJG, Fone KCF. Animal models of schizophrenia. Br J Pharmacol. 2011;164(4):1162-1194. doi:10.1111/j.1476-5381.2011.01386.x
31. Schmidt MJ, Mirnics K. Neurodevelopment, GABA system dysfunction, and schizophrenia. Neuropsychopharmacology. 2015;40(1):190-206. doi:10.1038/npp.2014.95
32. Nasrallah, HA. The daunting challenge of schizophrenia: hundreds of biotypes and dozens of theories. Curr. Psychiatry 2018;17(12):4-6,50.
33. Nocera A, Nasrallah HA. The association of the gut microbiota with clinical features in schizophrenia. Behav Sci (Basel). 2022;12(4):89. doi:10.3390/bs12040089
34. Schwarz E, Maukonen J, Hyytiäinen T, et al. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr Res. 2018;192:398-403. doi:10.1016/j.schres.2017.04.017
35. Nguyen TT, Kosciolek T, Maldonado Y, et al. Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophr Res. 2019;204:23-29. doi:10.1016/j.schres.2018.09.014
36. Li S, Zhuo M, Huang X, et al. Altered gut microbiota associated with symptom severity in schizophrenia. PeerJ. 2020;8:e9574. doi:10.7717/peerj.9574
37. Chen X, Xu J, Wang H, et al. Profiling the differences of gut microbial structure between schizophrenia patients with and without violent behaviors based on 16S rRNA gene sequencing. Int J Legal Med. 2021;135(1):131-141. doi:10.1007/s00414-020-02439-1
38. Manchia M, Fontana A, Panebianco C, et al. Involvement of gut microbiota in schizophrenia and treatment resistance to antipsychotics. Biomedicines. 2021;9(8):875. doi:10.3390/biomedicines9080875
39. Zhu C, Zheng M, Ali U, et al. Association between abundance of haemophilus in the gut microbiota and negative symptoms of schizophrenia. Front Psychiatry. 2021;12:685910. doi:10.3389/fpsyt.2021.685910
40. Flowers SA, Evans SJ, Ward KM, et al. Interaction between atypical antipsychotics and the gut microbiome in a bipolar disease cohort. Pharmacotherapy. 2017;37(3):261-267. doi:10.1002/phar.1890
41. Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222-227. doi:10.1038/nature11053
42. Buchanan RW. Persistent negative symptoms in schizophrenia: an overview. Schizophr Bull. 2007;33(4):1013-1022. doi:10.1093/schbul/sb1057
43. Liu JCW, Gorbovskaya I, Hahn MK, et al. The gut microbiome in schizophrenia and the potential benefits of prebiotic and probiotic treatment. Nutrients. 2021;13(4):1152. doi:10.3390/nu13041152
44. Biedermann L, Zeitz J, Mwinyi J, et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PloS One. 2013;8(3):e59260. doi:10.1371/journal.pone.0059260
45. Leclercq S, Matamoros S, Cani PD, et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci. 2014;111(42):e4485-e4493. doi:10.1073/pnas.1415174111
46. Hernández-Quiroz F, Nirmalkar K, Villalobos-Flores LE, et al. Influence of moderate beer consumption on human gut microbiota and its impact on fasting glucose and ß-cell function. Alcohol. 2020;85:77-94. doi:10.1016/j.alcohol.2019.05.006
47. Panee J, Gerschenson M, Chang L. Associations between microbiota, mitochondrial function, and cognition in chronic marijuana users. J Neuroimmune Pharmacol. 2018;13(1):113-122. doi:10.1007/s11481-017-9767-0
48. Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105-108. doi:10.1126/science.1208344
49. Rezac S, Kok CR, Heermann M, et al. Fermented foods as a dietary source of live organisms. Front Microbiol. 2018;9:1785. doi:10.3389/fmicb.2018.01785
50. Chen X, Zhang Y, Wang H, et al. The regulatory effects of lactic acid on neuropsychiatric disorders. Discover Ment Health. 2022;2(1). doi:10.1007/s44192-022-00011-4
51. Karbownik MS, Mokros Ł, Dobielska M, et al. Association between consumption of fermented food and food-derived prebiotics with cognitive performance, depressive, and anxiety symptoms in psychiatrically healthy medical students under psychological stress: a prospective cohort study. Front Nutr. 2022;9:850249. doi:10.3389/fnut.2022.850249
52. Romano S, Savva GM, Bedarf JR, et al. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Parkinsons Dis. 2021;7(1):27. doi:10.1038/s41531-021-00156-z
53. Bourassa MW, Alim I, Bultman SJ, et al. Butyrate, neuroepigenetics and the gut microbiome: can a high fiber diet improve brain health? Neurosci Lett. 2016;625:56-63. doi:10.1016/j.neulet.2016.02.009
54. Matt SM, Allen JM, Lawson MA, et al. Butyrate and dietary soluble fiber improve neuroinflammation associated with aging in mice. Front Immunol. 2018;9:1832. doi:10.3389/fimmu.2018.01832
55. Mittal VA, Vargas T, Osborne KJ, et al. Exercise treatments for psychosis: a review. Curr Treat Options Psychiatry. 2017;4(2):152-166. doi:10.1007/s40501-017-0112-2
56. Estaki M, Pither J, Baumeister P, et al. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. 2016;4(1):42. doi:10.1186/s40168-016-0189-7
57. Karl JP, Margolis LM, Madslien EH, et al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am J Physiol-Gastrointest Liver Physiol. 2017;312(6):G559-G571. doi:10.1152/ajpgi.00066.2017
58. Claus SP, Guillou H, Ellero-Simatos S. The gut microbiota: a major player in the toxicity of environmental pollutants? NPJ Biofilms Microbiomes. 2016;2:16003. doi:10.1038/npjbiofilms.2016.3
Depression guidelines fall short in characterizing withdrawal
Previous research suggests that approximately half of patients who discontinue or decrease dosage of antidepressants experience withdrawal symptoms, wrote Anders Sørensen, MD, of Copenhagen University Hospital, and colleagues. These symptoms are diverse and may include flulike symptoms, fatigue, anxiety, and sensations of electric shock, they noted. Most withdrawal effects last for a few weeks, but some persist for months or years, sometimes described as persistent postwithdrawal disorder, they added.
“Symptoms of withdrawal and depression overlap considerably but constitute two fundamentally different clinical conditions, which makes it important to distinguish between the two,” the researchers emphasized.
In a study published in the Journal of Affective Disorders, the researchers identified 21 clinical practice guidelines (CPGs) for depression published between 1998 and 2022. The guidelines were published in the United Kingdom, the United States, Canada, Australia, Singapore, Ireland, and New Zealand. They compared descriptions of withdrawal from antidepressants and calculated the proportion of CPGs with different information.
Overall, 15 of the 21 studies in the review (71%) noted that antidepressants are associated with withdrawal symptoms, but less than half (43%) used the term “withdrawal symptoms,” or similar. Of the nine guidelines that mentioned withdrawal symptoms, five used the term interchangeably with “discontinuation symptoms” and six used the term “discontinuation symptoms” only when discussing antidepressant withdrawal. In addition, six CPGs specifically stated that patients who stop antidepressants can experience withdrawal symptoms, and five stated that these symptoms also can occur in patients who are reducing or tapering their doses.
The type of withdrawal symptoms was mentioned in 10 CPGs, and the other 11 had no information on potential withdrawal symptoms, the researchers noted. Of the CPGs that mentioned symptoms specifically associated with withdrawal, the number of potential symptoms ranged from 4 to 39.
“None of the CPGs provided an exhaustive list of the potential withdrawal symptoms identified in the research literature,” the researchers wrote in their discussion.
Only four of the guidelines (19%) mentioned the overlap in symptoms between withdrawal from antidepressants and depression relapse, and only one provided guidance on distinguishing between the two conditions. Most of the symptoms of withdrawal, when described, were characterized as mild, brief, or self-limiting, the researchers noted.
“Being in withdrawal is a fundamentally different clinical situation than experiencing relapse, requiring two distinctly different treatment approaches,” the researchers emphasized. “Withdrawal reactions that are more severe and longer lasting than currently defined in the CPGs could risk getting misinterpreted as relapse, potentially leading to resumed unnecessary long-term antidepressant treatment in some patients,” they added.
The findings were limited by several factors including the inclusion only of guidelines from English-speaking countries, which may limit generalizability, the researchers noted. Other potential limitations include the subjective judgments involved in creating different guidelines, they said.
However, the results support the need for improved CPGs that help clinicians distinguish potential withdrawal reactions from depression relapse, and the need for more research on optimal dose reduction strategies for antidepressants, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Previous research suggests that approximately half of patients who discontinue or decrease dosage of antidepressants experience withdrawal symptoms, wrote Anders Sørensen, MD, of Copenhagen University Hospital, and colleagues. These symptoms are diverse and may include flulike symptoms, fatigue, anxiety, and sensations of electric shock, they noted. Most withdrawal effects last for a few weeks, but some persist for months or years, sometimes described as persistent postwithdrawal disorder, they added.
“Symptoms of withdrawal and depression overlap considerably but constitute two fundamentally different clinical conditions, which makes it important to distinguish between the two,” the researchers emphasized.
In a study published in the Journal of Affective Disorders, the researchers identified 21 clinical practice guidelines (CPGs) for depression published between 1998 and 2022. The guidelines were published in the United Kingdom, the United States, Canada, Australia, Singapore, Ireland, and New Zealand. They compared descriptions of withdrawal from antidepressants and calculated the proportion of CPGs with different information.
Overall, 15 of the 21 studies in the review (71%) noted that antidepressants are associated with withdrawal symptoms, but less than half (43%) used the term “withdrawal symptoms,” or similar. Of the nine guidelines that mentioned withdrawal symptoms, five used the term interchangeably with “discontinuation symptoms” and six used the term “discontinuation symptoms” only when discussing antidepressant withdrawal. In addition, six CPGs specifically stated that patients who stop antidepressants can experience withdrawal symptoms, and five stated that these symptoms also can occur in patients who are reducing or tapering their doses.
The type of withdrawal symptoms was mentioned in 10 CPGs, and the other 11 had no information on potential withdrawal symptoms, the researchers noted. Of the CPGs that mentioned symptoms specifically associated with withdrawal, the number of potential symptoms ranged from 4 to 39.
“None of the CPGs provided an exhaustive list of the potential withdrawal symptoms identified in the research literature,” the researchers wrote in their discussion.
Only four of the guidelines (19%) mentioned the overlap in symptoms between withdrawal from antidepressants and depression relapse, and only one provided guidance on distinguishing between the two conditions. Most of the symptoms of withdrawal, when described, were characterized as mild, brief, or self-limiting, the researchers noted.
“Being in withdrawal is a fundamentally different clinical situation than experiencing relapse, requiring two distinctly different treatment approaches,” the researchers emphasized. “Withdrawal reactions that are more severe and longer lasting than currently defined in the CPGs could risk getting misinterpreted as relapse, potentially leading to resumed unnecessary long-term antidepressant treatment in some patients,” they added.
The findings were limited by several factors including the inclusion only of guidelines from English-speaking countries, which may limit generalizability, the researchers noted. Other potential limitations include the subjective judgments involved in creating different guidelines, they said.
However, the results support the need for improved CPGs that help clinicians distinguish potential withdrawal reactions from depression relapse, and the need for more research on optimal dose reduction strategies for antidepressants, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Previous research suggests that approximately half of patients who discontinue or decrease dosage of antidepressants experience withdrawal symptoms, wrote Anders Sørensen, MD, of Copenhagen University Hospital, and colleagues. These symptoms are diverse and may include flulike symptoms, fatigue, anxiety, and sensations of electric shock, they noted. Most withdrawal effects last for a few weeks, but some persist for months or years, sometimes described as persistent postwithdrawal disorder, they added.
“Symptoms of withdrawal and depression overlap considerably but constitute two fundamentally different clinical conditions, which makes it important to distinguish between the two,” the researchers emphasized.
In a study published in the Journal of Affective Disorders, the researchers identified 21 clinical practice guidelines (CPGs) for depression published between 1998 and 2022. The guidelines were published in the United Kingdom, the United States, Canada, Australia, Singapore, Ireland, and New Zealand. They compared descriptions of withdrawal from antidepressants and calculated the proportion of CPGs with different information.
Overall, 15 of the 21 studies in the review (71%) noted that antidepressants are associated with withdrawal symptoms, but less than half (43%) used the term “withdrawal symptoms,” or similar. Of the nine guidelines that mentioned withdrawal symptoms, five used the term interchangeably with “discontinuation symptoms” and six used the term “discontinuation symptoms” only when discussing antidepressant withdrawal. In addition, six CPGs specifically stated that patients who stop antidepressants can experience withdrawal symptoms, and five stated that these symptoms also can occur in patients who are reducing or tapering their doses.
The type of withdrawal symptoms was mentioned in 10 CPGs, and the other 11 had no information on potential withdrawal symptoms, the researchers noted. Of the CPGs that mentioned symptoms specifically associated with withdrawal, the number of potential symptoms ranged from 4 to 39.
“None of the CPGs provided an exhaustive list of the potential withdrawal symptoms identified in the research literature,” the researchers wrote in their discussion.
Only four of the guidelines (19%) mentioned the overlap in symptoms between withdrawal from antidepressants and depression relapse, and only one provided guidance on distinguishing between the two conditions. Most of the symptoms of withdrawal, when described, were characterized as mild, brief, or self-limiting, the researchers noted.
“Being in withdrawal is a fundamentally different clinical situation than experiencing relapse, requiring two distinctly different treatment approaches,” the researchers emphasized. “Withdrawal reactions that are more severe and longer lasting than currently defined in the CPGs could risk getting misinterpreted as relapse, potentially leading to resumed unnecessary long-term antidepressant treatment in some patients,” they added.
The findings were limited by several factors including the inclusion only of guidelines from English-speaking countries, which may limit generalizability, the researchers noted. Other potential limitations include the subjective judgments involved in creating different guidelines, they said.
However, the results support the need for improved CPGs that help clinicians distinguish potential withdrawal reactions from depression relapse, and the need for more research on optimal dose reduction strategies for antidepressants, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Depression and schizophrenia: Many biological and clinical similarities
Clinicians generally regard major depressive disorder (MDD) and schizophrenia as 2 separate and distinct psychiatric brain disorders. However, despite some differences, those 2 psychiatric syndromes have numerous similarities across clinical features and neurobiologic parameters.
Biological similarities
Both disorders share the following variables:
- Highly genetic in etiology but with environmental influences and epigenetics
- Associated with childhood maltreatment, abuse, or neglect
- Disrupted neuroplasticity, especially shrinkage in hippocampal volume
- Significant drop in brain-derived neurotrophic factor resulting in decreased neurogenesis
- Extensive white matter pathology across interhemispheric and intrahemispheric bundles
- Increased levels of serum cortisol, a stress hormone and inflammatory biomarker
- Hypofrontal cerebral blood flow during acute episodes of both MDD and schizophrenia
- Reduced dendritic spines (in number and size) and impaired experiential neuroplasticity
- Neuroinflammation (eg, cytokines, tumor necrosis factor-alpha, C-reactive protein) during acute episodes
- Elevated oxidative stress biomarkers, indicating an increase in free radicals
- Overactive default mode network associated with ruminations in MDD and “daydreaming” in schizophrenia
- Decrease in gamma-aminobutyric acid (GABA) and its inhibitory activity, translating into dysregulation of glutamatergic pathways and other neurotransmitters
- Immune dysregulation and comorbid autoimmune disorders
Clinical similarities
- Psychotic symptoms, especially delusional thinking such as paranoia in schizophrenia and severe self-deprecation in MDD
- Significantly elevated lifetime suicide risk
- Cognitive impairment (more severe in schizophrenia across several cognitive functions)
- Similarity of depressive and negative symptoms (especially anhedonia, apathy, restricted facial expression, social withdrawal)
- Antidepressant medications im-prove depressive and negative symptoms (though not completely in the case of negative symptoms of schizophrenia)
- Both have treatment-resistant subtypes that fail to respond to standard therapies
- Both are associated with comorbid generalized anxiety disorder
- Both are associated with comorbid obsessive-compulsive disorder
- Both are associated with serious alcohol and drug use
- Early mortality from general medical conditions, especially cardiovascular risks due to obesity, diabetes, hypertension, dyslipidemia
- Elevated risk of dementia with aging compared to the unaffected general population
- Opioids improve MDD and psychosis (buprenorphine in MDD and morphine in schizophrenia)
- Several second-generation antipsychotic medications are approved for both MDD and schizophrenia
- Electroconvulsive therapy is effective when pharmacotherapy fails in both MDD and schizophrenia
Biological differences
- Glutamate N-methyl-D-aspartate receptor antagonists (eg, ketamine) improve MDD but worsen schizophrenia
- Muscarinic agonists improve psychosis but worsen depression
- High pain threshold in schizophrenia (pain insensitivity) and low threshold in MDD (in which pain is a common comorbidity)
- Cortical thinning more severe in schizophrenia
- Hippocampal atrophy is reversible with successful treatment in MDD but not in schizophrenia
- Hypofrontality is reversible with remission in MDD but not in schizophrenia
Clinical differences
- Auditory and visual hallucinations are more common in schizophrenia than in MDD
- Anosognosia is common in schizophrenia but not in MDD
- Implausible delusions are more common in schizophrenia than in MDD
- Mood-congruent delusions are more common in MDD than in schizophrenia
- Sadness, crying, pessimism, and self-deprecation are common in MDD but not in schizophrenia
- Achieving full remission is more common in MDD than in schizophrenia
- Long-acting injectable medications are available for schizophrenia but not for MDD
- Evidence-based psychotherapy, without pharmacotherapy, is more likely to be effective in MDD than in schizophrenia
A transdiagnostic model of psychopathology
The significant overlap between MDD and schizophrenia should not be surprising. They are both generated by the same organ, the human brain, with disrupted neurochemical and physiological circuits in the brain.
The overlap is also consistent with the emerging transdiagnostic model of psychopathology.1-9 This model proposes that there is a “core” genetic risk for psychopathology with different iterations. The transdiagnostic model is in stark contrast to the prevailing DSM-5, which categorizes psychiatric disorders in “silos,” as if they are completely independent from each other despite many shared features. This is highly debatable according to the substantial evidence that multiple psychiatric disorders share many genes that influence brain development in utero and predispose individuals to a variety of clinical symptoms in adolescence and young adulthood.
The origin of mental illness is being disentangled by emerging research, which is identifying the common links among the various disorders currently listed in DSM-5.10 However, the evolution of psychiatric diagnosis has come full circle from a single entity before DSM, to multiple entities with DSM, and now back to a unified transdiagnostic model that is rapidly emerging.11 This has implications for the FDA’s persistent dogma that clinical trials for new drugs must be targeted for 1 of the DSM-5 categories, a flawed and narrow assumption. Given the accelerating body of evidence for a unified, transdiagnostic model, it makes much more sense for the FDA to approve medications that target a psychiatric symptom that is shared by multiple psychiatric conditions within a transdiagnostic clinical system. When medications are approved for a symptom regardless of a DSM diagnosis, the term “off-label” and its “stigma” will then fade into history, along with the malignant preauthorization racket that was invented by greedy insurance companies that exploit the off-label use of medications (even when an FDA-approved medication for the patient’s condition does not yet exist) simply to deny coverage, lower their expenses, and fatten their profits.
1. Goodkind M, Eickhoff SB, Oathes DJ, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72(4):305-315.
2. Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175(9):831-844.
3. Krueger RF, Easton NR. Transdiagnostic factors in mental disorders. World Psychiatry. 2015;14(1):27-29.
4. Hyman SE. New evidence for shared risk architecture for mental disorders. JAMA Psychiatry. 2019;76(3):235-236.
5. Selzam S, Coleman JRI, Caspi A, et al. A polygenic p factor for major psychiatric disorders. Translational Psychiatry. 2018;8(1):205.
6. Barch DM. What it means to be transdiagnostic and how do we know? Am J Psychiatry. 2020;177(5):370-372.
7. Nasrallah HA. Is there only 1 neurobiologic psychiatric disorder with different clinical expressions? Current Psychiatry. 2015;14(7):10-12.
8. Nasrallah HA. Pleiotropy of psychiatric disorders will reinvent DSM. Current Psychiatry. 2013;12(4):6-7.
9. Nasrallah HA. Beyond DSM-5: clinical and biological features shared by major psychiatric syndromes. Current Psychiatry. 2017;16(10):4-7.
10. Marshall M. Roots of mental illness: researchers are beginning to untangle the common biology that links supposedly distinct psychiatric conditions. Nature. 2020;581:19-21.
11. Kendler KS. From many to one to many--the search for causes of psychiatric illness. JAMA Psychiatry. 2019;76(10):1085-1091.
Clinicians generally regard major depressive disorder (MDD) and schizophrenia as 2 separate and distinct psychiatric brain disorders. However, despite some differences, those 2 psychiatric syndromes have numerous similarities across clinical features and neurobiologic parameters.
Biological similarities
Both disorders share the following variables:
- Highly genetic in etiology but with environmental influences and epigenetics
- Associated with childhood maltreatment, abuse, or neglect
- Disrupted neuroplasticity, especially shrinkage in hippocampal volume
- Significant drop in brain-derived neurotrophic factor resulting in decreased neurogenesis
- Extensive white matter pathology across interhemispheric and intrahemispheric bundles
- Increased levels of serum cortisol, a stress hormone and inflammatory biomarker
- Hypofrontal cerebral blood flow during acute episodes of both MDD and schizophrenia
- Reduced dendritic spines (in number and size) and impaired experiential neuroplasticity
- Neuroinflammation (eg, cytokines, tumor necrosis factor-alpha, C-reactive protein) during acute episodes
- Elevated oxidative stress biomarkers, indicating an increase in free radicals
- Overactive default mode network associated with ruminations in MDD and “daydreaming” in schizophrenia
- Decrease in gamma-aminobutyric acid (GABA) and its inhibitory activity, translating into dysregulation of glutamatergic pathways and other neurotransmitters
- Immune dysregulation and comorbid autoimmune disorders
Clinical similarities
- Psychotic symptoms, especially delusional thinking such as paranoia in schizophrenia and severe self-deprecation in MDD
- Significantly elevated lifetime suicide risk
- Cognitive impairment (more severe in schizophrenia across several cognitive functions)
- Similarity of depressive and negative symptoms (especially anhedonia, apathy, restricted facial expression, social withdrawal)
- Antidepressant medications im-prove depressive and negative symptoms (though not completely in the case of negative symptoms of schizophrenia)
- Both have treatment-resistant subtypes that fail to respond to standard therapies
- Both are associated with comorbid generalized anxiety disorder
- Both are associated with comorbid obsessive-compulsive disorder
- Both are associated with serious alcohol and drug use
- Early mortality from general medical conditions, especially cardiovascular risks due to obesity, diabetes, hypertension, dyslipidemia
- Elevated risk of dementia with aging compared to the unaffected general population
- Opioids improve MDD and psychosis (buprenorphine in MDD and morphine in schizophrenia)
- Several second-generation antipsychotic medications are approved for both MDD and schizophrenia
- Electroconvulsive therapy is effective when pharmacotherapy fails in both MDD and schizophrenia
Biological differences
- Glutamate N-methyl-D-aspartate receptor antagonists (eg, ketamine) improve MDD but worsen schizophrenia
- Muscarinic agonists improve psychosis but worsen depression
- High pain threshold in schizophrenia (pain insensitivity) and low threshold in MDD (in which pain is a common comorbidity)
- Cortical thinning more severe in schizophrenia
- Hippocampal atrophy is reversible with successful treatment in MDD but not in schizophrenia
- Hypofrontality is reversible with remission in MDD but not in schizophrenia
Clinical differences
- Auditory and visual hallucinations are more common in schizophrenia than in MDD
- Anosognosia is common in schizophrenia but not in MDD
- Implausible delusions are more common in schizophrenia than in MDD
- Mood-congruent delusions are more common in MDD than in schizophrenia
- Sadness, crying, pessimism, and self-deprecation are common in MDD but not in schizophrenia
- Achieving full remission is more common in MDD than in schizophrenia
- Long-acting injectable medications are available for schizophrenia but not for MDD
- Evidence-based psychotherapy, without pharmacotherapy, is more likely to be effective in MDD than in schizophrenia
A transdiagnostic model of psychopathology
The significant overlap between MDD and schizophrenia should not be surprising. They are both generated by the same organ, the human brain, with disrupted neurochemical and physiological circuits in the brain.
The overlap is also consistent with the emerging transdiagnostic model of psychopathology.1-9 This model proposes that there is a “core” genetic risk for psychopathology with different iterations. The transdiagnostic model is in stark contrast to the prevailing DSM-5, which categorizes psychiatric disorders in “silos,” as if they are completely independent from each other despite many shared features. This is highly debatable according to the substantial evidence that multiple psychiatric disorders share many genes that influence brain development in utero and predispose individuals to a variety of clinical symptoms in adolescence and young adulthood.
The origin of mental illness is being disentangled by emerging research, which is identifying the common links among the various disorders currently listed in DSM-5.10 However, the evolution of psychiatric diagnosis has come full circle from a single entity before DSM, to multiple entities with DSM, and now back to a unified transdiagnostic model that is rapidly emerging.11 This has implications for the FDA’s persistent dogma that clinical trials for new drugs must be targeted for 1 of the DSM-5 categories, a flawed and narrow assumption. Given the accelerating body of evidence for a unified, transdiagnostic model, it makes much more sense for the FDA to approve medications that target a psychiatric symptom that is shared by multiple psychiatric conditions within a transdiagnostic clinical system. When medications are approved for a symptom regardless of a DSM diagnosis, the term “off-label” and its “stigma” will then fade into history, along with the malignant preauthorization racket that was invented by greedy insurance companies that exploit the off-label use of medications (even when an FDA-approved medication for the patient’s condition does not yet exist) simply to deny coverage, lower their expenses, and fatten their profits.
Clinicians generally regard major depressive disorder (MDD) and schizophrenia as 2 separate and distinct psychiatric brain disorders. However, despite some differences, those 2 psychiatric syndromes have numerous similarities across clinical features and neurobiologic parameters.
Biological similarities
Both disorders share the following variables:
- Highly genetic in etiology but with environmental influences and epigenetics
- Associated with childhood maltreatment, abuse, or neglect
- Disrupted neuroplasticity, especially shrinkage in hippocampal volume
- Significant drop in brain-derived neurotrophic factor resulting in decreased neurogenesis
- Extensive white matter pathology across interhemispheric and intrahemispheric bundles
- Increased levels of serum cortisol, a stress hormone and inflammatory biomarker
- Hypofrontal cerebral blood flow during acute episodes of both MDD and schizophrenia
- Reduced dendritic spines (in number and size) and impaired experiential neuroplasticity
- Neuroinflammation (eg, cytokines, tumor necrosis factor-alpha, C-reactive protein) during acute episodes
- Elevated oxidative stress biomarkers, indicating an increase in free radicals
- Overactive default mode network associated with ruminations in MDD and “daydreaming” in schizophrenia
- Decrease in gamma-aminobutyric acid (GABA) and its inhibitory activity, translating into dysregulation of glutamatergic pathways and other neurotransmitters
- Immune dysregulation and comorbid autoimmune disorders
Clinical similarities
- Psychotic symptoms, especially delusional thinking such as paranoia in schizophrenia and severe self-deprecation in MDD
- Significantly elevated lifetime suicide risk
- Cognitive impairment (more severe in schizophrenia across several cognitive functions)
- Similarity of depressive and negative symptoms (especially anhedonia, apathy, restricted facial expression, social withdrawal)
- Antidepressant medications im-prove depressive and negative symptoms (though not completely in the case of negative symptoms of schizophrenia)
- Both have treatment-resistant subtypes that fail to respond to standard therapies
- Both are associated with comorbid generalized anxiety disorder
- Both are associated with comorbid obsessive-compulsive disorder
- Both are associated with serious alcohol and drug use
- Early mortality from general medical conditions, especially cardiovascular risks due to obesity, diabetes, hypertension, dyslipidemia
- Elevated risk of dementia with aging compared to the unaffected general population
- Opioids improve MDD and psychosis (buprenorphine in MDD and morphine in schizophrenia)
- Several second-generation antipsychotic medications are approved for both MDD and schizophrenia
- Electroconvulsive therapy is effective when pharmacotherapy fails in both MDD and schizophrenia
Biological differences
- Glutamate N-methyl-D-aspartate receptor antagonists (eg, ketamine) improve MDD but worsen schizophrenia
- Muscarinic agonists improve psychosis but worsen depression
- High pain threshold in schizophrenia (pain insensitivity) and low threshold in MDD (in which pain is a common comorbidity)
- Cortical thinning more severe in schizophrenia
- Hippocampal atrophy is reversible with successful treatment in MDD but not in schizophrenia
- Hypofrontality is reversible with remission in MDD but not in schizophrenia
Clinical differences
- Auditory and visual hallucinations are more common in schizophrenia than in MDD
- Anosognosia is common in schizophrenia but not in MDD
- Implausible delusions are more common in schizophrenia than in MDD
- Mood-congruent delusions are more common in MDD than in schizophrenia
- Sadness, crying, pessimism, and self-deprecation are common in MDD but not in schizophrenia
- Achieving full remission is more common in MDD than in schizophrenia
- Long-acting injectable medications are available for schizophrenia but not for MDD
- Evidence-based psychotherapy, without pharmacotherapy, is more likely to be effective in MDD than in schizophrenia
A transdiagnostic model of psychopathology
The significant overlap between MDD and schizophrenia should not be surprising. They are both generated by the same organ, the human brain, with disrupted neurochemical and physiological circuits in the brain.
The overlap is also consistent with the emerging transdiagnostic model of psychopathology.1-9 This model proposes that there is a “core” genetic risk for psychopathology with different iterations. The transdiagnostic model is in stark contrast to the prevailing DSM-5, which categorizes psychiatric disorders in “silos,” as if they are completely independent from each other despite many shared features. This is highly debatable according to the substantial evidence that multiple psychiatric disorders share many genes that influence brain development in utero and predispose individuals to a variety of clinical symptoms in adolescence and young adulthood.
The origin of mental illness is being disentangled by emerging research, which is identifying the common links among the various disorders currently listed in DSM-5.10 However, the evolution of psychiatric diagnosis has come full circle from a single entity before DSM, to multiple entities with DSM, and now back to a unified transdiagnostic model that is rapidly emerging.11 This has implications for the FDA’s persistent dogma that clinical trials for new drugs must be targeted for 1 of the DSM-5 categories, a flawed and narrow assumption. Given the accelerating body of evidence for a unified, transdiagnostic model, it makes much more sense for the FDA to approve medications that target a psychiatric symptom that is shared by multiple psychiatric conditions within a transdiagnostic clinical system. When medications are approved for a symptom regardless of a DSM diagnosis, the term “off-label” and its “stigma” will then fade into history, along with the malignant preauthorization racket that was invented by greedy insurance companies that exploit the off-label use of medications (even when an FDA-approved medication for the patient’s condition does not yet exist) simply to deny coverage, lower their expenses, and fatten their profits.
1. Goodkind M, Eickhoff SB, Oathes DJ, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72(4):305-315.
2. Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175(9):831-844.
3. Krueger RF, Easton NR. Transdiagnostic factors in mental disorders. World Psychiatry. 2015;14(1):27-29.
4. Hyman SE. New evidence for shared risk architecture for mental disorders. JAMA Psychiatry. 2019;76(3):235-236.
5. Selzam S, Coleman JRI, Caspi A, et al. A polygenic p factor for major psychiatric disorders. Translational Psychiatry. 2018;8(1):205.
6. Barch DM. What it means to be transdiagnostic and how do we know? Am J Psychiatry. 2020;177(5):370-372.
7. Nasrallah HA. Is there only 1 neurobiologic psychiatric disorder with different clinical expressions? Current Psychiatry. 2015;14(7):10-12.
8. Nasrallah HA. Pleiotropy of psychiatric disorders will reinvent DSM. Current Psychiatry. 2013;12(4):6-7.
9. Nasrallah HA. Beyond DSM-5: clinical and biological features shared by major psychiatric syndromes. Current Psychiatry. 2017;16(10):4-7.
10. Marshall M. Roots of mental illness: researchers are beginning to untangle the common biology that links supposedly distinct psychiatric conditions. Nature. 2020;581:19-21.
11. Kendler KS. From many to one to many--the search for causes of psychiatric illness. JAMA Psychiatry. 2019;76(10):1085-1091.
1. Goodkind M, Eickhoff SB, Oathes DJ, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72(4):305-315.
2. Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175(9):831-844.
3. Krueger RF, Easton NR. Transdiagnostic factors in mental disorders. World Psychiatry. 2015;14(1):27-29.
4. Hyman SE. New evidence for shared risk architecture for mental disorders. JAMA Psychiatry. 2019;76(3):235-236.
5. Selzam S, Coleman JRI, Caspi A, et al. A polygenic p factor for major psychiatric disorders. Translational Psychiatry. 2018;8(1):205.
6. Barch DM. What it means to be transdiagnostic and how do we know? Am J Psychiatry. 2020;177(5):370-372.
7. Nasrallah HA. Is there only 1 neurobiologic psychiatric disorder with different clinical expressions? Current Psychiatry. 2015;14(7):10-12.
8. Nasrallah HA. Pleiotropy of psychiatric disorders will reinvent DSM. Current Psychiatry. 2013;12(4):6-7.
9. Nasrallah HA. Beyond DSM-5: clinical and biological features shared by major psychiatric syndromes. Current Psychiatry. 2017;16(10):4-7.
10. Marshall M. Roots of mental illness: researchers are beginning to untangle the common biology that links supposedly distinct psychiatric conditions. Nature. 2020;581:19-21.
11. Kendler KS. From many to one to many--the search for causes of psychiatric illness. JAMA Psychiatry. 2019;76(10):1085-1091.
Disability in medicine: My experience
What does a doctor look like? Throughout history, this concept has shifted due to societal norms and increased access to medical education. Today, the idea of a physician has expanded to incorporate a myriad of people; however, stigma still exists in medicine regarding mental illness and disability. I would like to share my personal journey through high school, college, medical school, and now residency, and how my identity and struggles have shaped me into the physician I am today. There are few conversations around disability—especially disability and mental health—in medicine, and through my own advocacy, I have met many students with disability who feel that medical school is unattainable. Additionally, I have met many medical students, residents, and pre-health advisors who are happy for the experience to learn more about a marginalized group in medicine. My hope in sharing my story is to offer a space for conversation about intersectionality within medical communities and how physicians and physicians in training can facilitate that change, regardless of their position or specialty. Additionally, I hope to shed light on the unique mental health needs of patients with disabilities and how mental health clinicians can address those needs.
Perceived weaknesses turned into strengths
“Why do you walk like that?” “What is that brace on your leg?” The early years of my childhood were marked by these questions and others like them. I was the kid with the limp, the kid with a brace on his leg, and the kid who disappeared multiple times a week for doctor’s appointments or physical therapy. I learned to deflect these questions or give nebulous answers about an accident or injury. The reality is that I was born with cerebral palsy (CP). My CP manifested as hemiparesis on the left side of my body. I was in aggressive physical therapy throughout childhood, received Botox injections for muscle spasticity, and underwent corrective surgery on my left leg to straighten my foot. In childhood, the diagnosis meant nothing more than 2 words that sounded like they belonged to superheroes in comic books. Even with supportive parents and family, I kept my disability a secret, much like the powers and abilities of my favorite superheroes.
However, like all great origin stories, what I once thought were weaknesses turned out to be strengths that pushed me through college, medical school, and now psychiatry residency. Living with a disability has shaped how I see the world and relate to my patients. My experience has helped me connect to my patients in ways others might not. These properties are important in any physician but vital in psychiatry, where many patients feel neglected or stigmatized; this is another reason there should be more doctors with disabilities in medicine. Unfortunately, systemic barriers are still in place that disincentivize those with a disability from pursuing careers in medicine. Stories like mine are important to inspire a reexamination of what a physician should be and how medicine, patients, and communities benefit from this change.
My experience through medical school
My path to psychiatry and residency was shaped by my early experience with the medical field and treatment. From the early days of my diagnosis at age 4, I was told that my brain was “wired differently” and that, because of this disruption in circuitry, I would have difficulty with physical activity. I grew to appreciate the intricacies of the brain and pathology to understand my body. With greater understanding came the existential realization that I would live with a disability for the rest of my life. Rather than dream of a future where I would be “normal,” I focused on adapting my life to my normal. An unfortunate reality of this normal was that no doctor would be able to relate to me, and my health care would focus on limitations rather than possibilities.
I focused on school as a distraction and slowly warmed to the idea of pursuing medicine as a career. The seed was planted years prior by the numerous doctors’ visits and procedures, and was cultivated by a desire to understand pathologies and offer treatment to patients from the perspective of a patient. When I applied to medical school, I did not know how to address my CP. Living as a person with CP was a core reason for my decision to pursue medicine, but I was afraid that a disclosure of disability would preclude any admission to medical school. Research into programs offered little guidance because most institutions only listed vague “physical expectations” of each student. There were times I doubted if I would be accepted anywhere. Many programs I reached out to about my situation seemed unenthusiastic about the prospect of a student with CP, and when I brought up my CP in interviews, the reaction was often of surprise and an admission that they had forgotten about “that part” of my application. Fortunately, I was accepted to medical school, but still struggled with the fear that one day I would be found out and not allowed to continue. No one in my class or school was like me, and a meeting with an Americans with Disabilities Act coordinator who asked me to reexamine the physical competencies of the school before advancing to clinical clerkships only further reinforced this fear. I decided to fly under the radar and not say anything about my disability to my attendings. I slowly worked my way through clerkships by making do with adapted ways to perform procedures and exams with additional practice and maneuvering at home. I found myself drawn to psychiatry because of the similarities I saw in the patients and myself. I empathized with how the patients struggled with chronic conditions that left them feeling separated from society and how they felt that their diagnosis was something they needed to hide. When medical school ended and I decided to pursue psychiatry, I wanted to share my story to inspire others with a disability to consider medicine as a career given their unique experiences. My experience thus far has been uplifting as my journey has echoed so many others.
A need for greater representation
Disability representation in medicine is needed more than ever. According to the CDC, >60 million adults in the United States (1 in 4) live with a disability.1 Although the physical health disparities are often discussed, there is less conversation surrounding mental health for individuals with disabilities. A 2018 study by Cree et al2 found that approximately 17.4 million adults with disabilities experienced frequent mental distress, defined as reporting ≥14 mentally unhealthy days in the past 30 days. Furthermore, compared to individuals without a disability, those with a disability are statistically more likely to have suicidal ideation, suicidal planning, and suicide attempts.3 One way to address this disparity is to recruit medical students with disabilities to become physicians with disabilities. Evidence suggests that physicians who are members of groups that are underrepresented in medicine are more likely to deliver care to underrepresented patients.4 However, medical schools and institutions have been slow to address the disparity. A 2019 survey found an estimated 4.6% of medical students responded “yes” when asked if they had a disability, with most students reporting a psychological or attention/hyperactive disorder.5 Existing barriers include restrictive language surrounding technical standards influenced by long-standing vestiges of what a physician should be.6
An opportunity to connect with patients
I now do not see myself as having a secret identity to hide. Although my CP does not give me any superpowers, it has given me the opportunity to connect with my patients and serve as an example of why medical school recruitment and admissions should expand. Psychiatrists have been on the forefront of change in medicine and can shift the perception of a physician. In doing so, we not only enrich our field but also the lives of our patients who may need it most.
1. Okoro CA, Hollis ND, Cyrus AC, et al. Prevalence of disabilities and health care access by disability status and type among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(32):882-887.
2. Cree RA, Okoro CA, Zack MM, et al. Frequent mental distress among adults, by disability status, disability type, and selected characteristics—United States 2018. MMWR Morb Mortal Wkly Rep. 2020;69(36):1238-1243.
3. Marlow NM, Xie Z, Tanner R, et al. Association between disability and suicide-related outcomes among US adults. Am J Prev Med. 2021;61(6):852-862.
4. Thurmond VB, Kirch DG. Impact of minority physicians on health care. South Med J. 1998;91(11):1009-1013.
5. Meeks LM, Case B, Herzer K, et al. Change in prevalence of disabilities and accommodation practices among US medical schools, 2016 vs 2019. JAMA. 2019;322(20):2022-2024.
6. Stauffer C, Case B, Moreland CJ, et al. Technical standards from newly established medical schools: a review of disability inclusive practices. J Med Educ Curric Dev. 2022;9:23821205211072763.
What does a doctor look like? Throughout history, this concept has shifted due to societal norms and increased access to medical education. Today, the idea of a physician has expanded to incorporate a myriad of people; however, stigma still exists in medicine regarding mental illness and disability. I would like to share my personal journey through high school, college, medical school, and now residency, and how my identity and struggles have shaped me into the physician I am today. There are few conversations around disability—especially disability and mental health—in medicine, and through my own advocacy, I have met many students with disability who feel that medical school is unattainable. Additionally, I have met many medical students, residents, and pre-health advisors who are happy for the experience to learn more about a marginalized group in medicine. My hope in sharing my story is to offer a space for conversation about intersectionality within medical communities and how physicians and physicians in training can facilitate that change, regardless of their position or specialty. Additionally, I hope to shed light on the unique mental health needs of patients with disabilities and how mental health clinicians can address those needs.
Perceived weaknesses turned into strengths
“Why do you walk like that?” “What is that brace on your leg?” The early years of my childhood were marked by these questions and others like them. I was the kid with the limp, the kid with a brace on his leg, and the kid who disappeared multiple times a week for doctor’s appointments or physical therapy. I learned to deflect these questions or give nebulous answers about an accident or injury. The reality is that I was born with cerebral palsy (CP). My CP manifested as hemiparesis on the left side of my body. I was in aggressive physical therapy throughout childhood, received Botox injections for muscle spasticity, and underwent corrective surgery on my left leg to straighten my foot. In childhood, the diagnosis meant nothing more than 2 words that sounded like they belonged to superheroes in comic books. Even with supportive parents and family, I kept my disability a secret, much like the powers and abilities of my favorite superheroes.
However, like all great origin stories, what I once thought were weaknesses turned out to be strengths that pushed me through college, medical school, and now psychiatry residency. Living with a disability has shaped how I see the world and relate to my patients. My experience has helped me connect to my patients in ways others might not. These properties are important in any physician but vital in psychiatry, where many patients feel neglected or stigmatized; this is another reason there should be more doctors with disabilities in medicine. Unfortunately, systemic barriers are still in place that disincentivize those with a disability from pursuing careers in medicine. Stories like mine are important to inspire a reexamination of what a physician should be and how medicine, patients, and communities benefit from this change.
My experience through medical school
My path to psychiatry and residency was shaped by my early experience with the medical field and treatment. From the early days of my diagnosis at age 4, I was told that my brain was “wired differently” and that, because of this disruption in circuitry, I would have difficulty with physical activity. I grew to appreciate the intricacies of the brain and pathology to understand my body. With greater understanding came the existential realization that I would live with a disability for the rest of my life. Rather than dream of a future where I would be “normal,” I focused on adapting my life to my normal. An unfortunate reality of this normal was that no doctor would be able to relate to me, and my health care would focus on limitations rather than possibilities.
I focused on school as a distraction and slowly warmed to the idea of pursuing medicine as a career. The seed was planted years prior by the numerous doctors’ visits and procedures, and was cultivated by a desire to understand pathologies and offer treatment to patients from the perspective of a patient. When I applied to medical school, I did not know how to address my CP. Living as a person with CP was a core reason for my decision to pursue medicine, but I was afraid that a disclosure of disability would preclude any admission to medical school. Research into programs offered little guidance because most institutions only listed vague “physical expectations” of each student. There were times I doubted if I would be accepted anywhere. Many programs I reached out to about my situation seemed unenthusiastic about the prospect of a student with CP, and when I brought up my CP in interviews, the reaction was often of surprise and an admission that they had forgotten about “that part” of my application. Fortunately, I was accepted to medical school, but still struggled with the fear that one day I would be found out and not allowed to continue. No one in my class or school was like me, and a meeting with an Americans with Disabilities Act coordinator who asked me to reexamine the physical competencies of the school before advancing to clinical clerkships only further reinforced this fear. I decided to fly under the radar and not say anything about my disability to my attendings. I slowly worked my way through clerkships by making do with adapted ways to perform procedures and exams with additional practice and maneuvering at home. I found myself drawn to psychiatry because of the similarities I saw in the patients and myself. I empathized with how the patients struggled with chronic conditions that left them feeling separated from society and how they felt that their diagnosis was something they needed to hide. When medical school ended and I decided to pursue psychiatry, I wanted to share my story to inspire others with a disability to consider medicine as a career given their unique experiences. My experience thus far has been uplifting as my journey has echoed so many others.
A need for greater representation
Disability representation in medicine is needed more than ever. According to the CDC, >60 million adults in the United States (1 in 4) live with a disability.1 Although the physical health disparities are often discussed, there is less conversation surrounding mental health for individuals with disabilities. A 2018 study by Cree et al2 found that approximately 17.4 million adults with disabilities experienced frequent mental distress, defined as reporting ≥14 mentally unhealthy days in the past 30 days. Furthermore, compared to individuals without a disability, those with a disability are statistically more likely to have suicidal ideation, suicidal planning, and suicide attempts.3 One way to address this disparity is to recruit medical students with disabilities to become physicians with disabilities. Evidence suggests that physicians who are members of groups that are underrepresented in medicine are more likely to deliver care to underrepresented patients.4 However, medical schools and institutions have been slow to address the disparity. A 2019 survey found an estimated 4.6% of medical students responded “yes” when asked if they had a disability, with most students reporting a psychological or attention/hyperactive disorder.5 Existing barriers include restrictive language surrounding technical standards influenced by long-standing vestiges of what a physician should be.6
An opportunity to connect with patients
I now do not see myself as having a secret identity to hide. Although my CP does not give me any superpowers, it has given me the opportunity to connect with my patients and serve as an example of why medical school recruitment and admissions should expand. Psychiatrists have been on the forefront of change in medicine and can shift the perception of a physician. In doing so, we not only enrich our field but also the lives of our patients who may need it most.
What does a doctor look like? Throughout history, this concept has shifted due to societal norms and increased access to medical education. Today, the idea of a physician has expanded to incorporate a myriad of people; however, stigma still exists in medicine regarding mental illness and disability. I would like to share my personal journey through high school, college, medical school, and now residency, and how my identity and struggles have shaped me into the physician I am today. There are few conversations around disability—especially disability and mental health—in medicine, and through my own advocacy, I have met many students with disability who feel that medical school is unattainable. Additionally, I have met many medical students, residents, and pre-health advisors who are happy for the experience to learn more about a marginalized group in medicine. My hope in sharing my story is to offer a space for conversation about intersectionality within medical communities and how physicians and physicians in training can facilitate that change, regardless of their position or specialty. Additionally, I hope to shed light on the unique mental health needs of patients with disabilities and how mental health clinicians can address those needs.
Perceived weaknesses turned into strengths
“Why do you walk like that?” “What is that brace on your leg?” The early years of my childhood were marked by these questions and others like them. I was the kid with the limp, the kid with a brace on his leg, and the kid who disappeared multiple times a week for doctor’s appointments or physical therapy. I learned to deflect these questions or give nebulous answers about an accident or injury. The reality is that I was born with cerebral palsy (CP). My CP manifested as hemiparesis on the left side of my body. I was in aggressive physical therapy throughout childhood, received Botox injections for muscle spasticity, and underwent corrective surgery on my left leg to straighten my foot. In childhood, the diagnosis meant nothing more than 2 words that sounded like they belonged to superheroes in comic books. Even with supportive parents and family, I kept my disability a secret, much like the powers and abilities of my favorite superheroes.
However, like all great origin stories, what I once thought were weaknesses turned out to be strengths that pushed me through college, medical school, and now psychiatry residency. Living with a disability has shaped how I see the world and relate to my patients. My experience has helped me connect to my patients in ways others might not. These properties are important in any physician but vital in psychiatry, where many patients feel neglected or stigmatized; this is another reason there should be more doctors with disabilities in medicine. Unfortunately, systemic barriers are still in place that disincentivize those with a disability from pursuing careers in medicine. Stories like mine are important to inspire a reexamination of what a physician should be and how medicine, patients, and communities benefit from this change.
My experience through medical school
My path to psychiatry and residency was shaped by my early experience with the medical field and treatment. From the early days of my diagnosis at age 4, I was told that my brain was “wired differently” and that, because of this disruption in circuitry, I would have difficulty with physical activity. I grew to appreciate the intricacies of the brain and pathology to understand my body. With greater understanding came the existential realization that I would live with a disability for the rest of my life. Rather than dream of a future where I would be “normal,” I focused on adapting my life to my normal. An unfortunate reality of this normal was that no doctor would be able to relate to me, and my health care would focus on limitations rather than possibilities.
I focused on school as a distraction and slowly warmed to the idea of pursuing medicine as a career. The seed was planted years prior by the numerous doctors’ visits and procedures, and was cultivated by a desire to understand pathologies and offer treatment to patients from the perspective of a patient. When I applied to medical school, I did not know how to address my CP. Living as a person with CP was a core reason for my decision to pursue medicine, but I was afraid that a disclosure of disability would preclude any admission to medical school. Research into programs offered little guidance because most institutions only listed vague “physical expectations” of each student. There were times I doubted if I would be accepted anywhere. Many programs I reached out to about my situation seemed unenthusiastic about the prospect of a student with CP, and when I brought up my CP in interviews, the reaction was often of surprise and an admission that they had forgotten about “that part” of my application. Fortunately, I was accepted to medical school, but still struggled with the fear that one day I would be found out and not allowed to continue. No one in my class or school was like me, and a meeting with an Americans with Disabilities Act coordinator who asked me to reexamine the physical competencies of the school before advancing to clinical clerkships only further reinforced this fear. I decided to fly under the radar and not say anything about my disability to my attendings. I slowly worked my way through clerkships by making do with adapted ways to perform procedures and exams with additional practice and maneuvering at home. I found myself drawn to psychiatry because of the similarities I saw in the patients and myself. I empathized with how the patients struggled with chronic conditions that left them feeling separated from society and how they felt that their diagnosis was something they needed to hide. When medical school ended and I decided to pursue psychiatry, I wanted to share my story to inspire others with a disability to consider medicine as a career given their unique experiences. My experience thus far has been uplifting as my journey has echoed so many others.
A need for greater representation
Disability representation in medicine is needed more than ever. According to the CDC, >60 million adults in the United States (1 in 4) live with a disability.1 Although the physical health disparities are often discussed, there is less conversation surrounding mental health for individuals with disabilities. A 2018 study by Cree et al2 found that approximately 17.4 million adults with disabilities experienced frequent mental distress, defined as reporting ≥14 mentally unhealthy days in the past 30 days. Furthermore, compared to individuals without a disability, those with a disability are statistically more likely to have suicidal ideation, suicidal planning, and suicide attempts.3 One way to address this disparity is to recruit medical students with disabilities to become physicians with disabilities. Evidence suggests that physicians who are members of groups that are underrepresented in medicine are more likely to deliver care to underrepresented patients.4 However, medical schools and institutions have been slow to address the disparity. A 2019 survey found an estimated 4.6% of medical students responded “yes” when asked if they had a disability, with most students reporting a psychological or attention/hyperactive disorder.5 Existing barriers include restrictive language surrounding technical standards influenced by long-standing vestiges of what a physician should be.6
An opportunity to connect with patients
I now do not see myself as having a secret identity to hide. Although my CP does not give me any superpowers, it has given me the opportunity to connect with my patients and serve as an example of why medical school recruitment and admissions should expand. Psychiatrists have been on the forefront of change in medicine and can shift the perception of a physician. In doing so, we not only enrich our field but also the lives of our patients who may need it most.
1. Okoro CA, Hollis ND, Cyrus AC, et al. Prevalence of disabilities and health care access by disability status and type among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(32):882-887.
2. Cree RA, Okoro CA, Zack MM, et al. Frequent mental distress among adults, by disability status, disability type, and selected characteristics—United States 2018. MMWR Morb Mortal Wkly Rep. 2020;69(36):1238-1243.
3. Marlow NM, Xie Z, Tanner R, et al. Association between disability and suicide-related outcomes among US adults. Am J Prev Med. 2021;61(6):852-862.
4. Thurmond VB, Kirch DG. Impact of minority physicians on health care. South Med J. 1998;91(11):1009-1013.
5. Meeks LM, Case B, Herzer K, et al. Change in prevalence of disabilities and accommodation practices among US medical schools, 2016 vs 2019. JAMA. 2019;322(20):2022-2024.
6. Stauffer C, Case B, Moreland CJ, et al. Technical standards from newly established medical schools: a review of disability inclusive practices. J Med Educ Curric Dev. 2022;9:23821205211072763.
1. Okoro CA, Hollis ND, Cyrus AC, et al. Prevalence of disabilities and health care access by disability status and type among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(32):882-887.
2. Cree RA, Okoro CA, Zack MM, et al. Frequent mental distress among adults, by disability status, disability type, and selected characteristics—United States 2018. MMWR Morb Mortal Wkly Rep. 2020;69(36):1238-1243.
3. Marlow NM, Xie Z, Tanner R, et al. Association between disability and suicide-related outcomes among US adults. Am J Prev Med. 2021;61(6):852-862.
4. Thurmond VB, Kirch DG. Impact of minority physicians on health care. South Med J. 1998;91(11):1009-1013.
5. Meeks LM, Case B, Herzer K, et al. Change in prevalence of disabilities and accommodation practices among US medical schools, 2016 vs 2019. JAMA. 2019;322(20):2022-2024.
6. Stauffer C, Case B, Moreland CJ, et al. Technical standards from newly established medical schools: a review of disability inclusive practices. J Med Educ Curric Dev. 2022;9:23821205211072763.
Discontinuing a long-acting injectable antipsychotic: What to consider
Mr. R, age 29, was diagnosed with schizophrenia 6 years ago. To manage his disorder, he has been receiving paliperidone palmitate long-acting injectable (LAI) 156 mg once a month for 2 years. Prior to maintenance with paliperidone palmitate, Mr. R was stabilized on oral paliperidone 9 mg/d. Though he was originally initiated on paliperidone palmitate due to nonadherence concerns, Mr. R has been adherent with each injection for 1 year.
At a recent visit, Mr. R says he wants to discontinue the injection because he is not interested in receiving an ongoing injectable medication and is not able to continue monthly clinic visits. He wants to take a daily oral antipsychotic again, despite the availability of longer-acting products.
A paucity of evidence exists regarding the discontinuation of LAI antipsychotics and the next steps that follow in treatment. There is neither a consensus nor recognized guidelines advising how and when to discontinue an LAI and restart an oral antipsychotic. A recent systematic review and meta-analysis evaluated different maintenance treatment strategies; however, switching from an LAI antipsychotic to an oral medication was not a focus.1 In this article, we outline a possible approach to discontinuing an LAI antipsychotic and restarting an oral formulation. Before discontinuing an LAI antipsychotic, clinicians should review with the patient the risks and benefits of switching medications, including the risk of decompensation and potential adverse effects.
Switching to an oral antipsychotic
The first step in the discontinuation process is to determine whether the patient will continue the same oral medication as the LAI antipsychotic or if a different oral antipsychotic will be initiated. Next, determining when to initiate the oral medication requires several pieces of information, including the oral dose equivalent of the patient’s current LAI, the half-life of the LAI, and the release mechanism of the LAI (Table 1).2-5 To determine the appropriate time frame for restarting oral treatment, it is also vital to know the date of the last injection.
Based on the date of the next injection, the clinician will utilize the LAI’s half-life and its release mechanism to determine the appropriate time to start a new oral antipsychotic. Research demonstrates that in patients who have achieved steady state with a first-generation antipsychotic, plasma concentrations stay relatively consistent for 6 to 7 weeks after the last injection, which suggests oral medications may not need to be initiated until that time.6-9
For many second-generation LAI antipsychotics, oral medications may be initiated at the date of the next injection. Initiation of an oral antipsychotic may require more time between the last injection dose and the date of administration for oral medication due to the pharmacokinetic profile of risperidone microspheres. Once a patient is at steady state with risperidone microspheres, trough levels are not observed until 3 to 4 weeks after discontinuation.10
Previous pharmacokinetic model–based stimulations of active moiety plasma concentrations of risperidone microspheres demonstrate that 2 weeks after an injection of risperidone microspheres, the concentration of active moiety continued to approximate the steady-state concentration for 3 to 5 weeks.11 This is likely due to the product’s delay in release being 3 weeks from the time of injection to the last release phase. Of note, there was a rapid decline in the active moiety concentration; it reached nearly 0 by Week 5.11 The same pharmacokinetic model–based stimulation demonstrated a steady and slow decline of the concentration of active moiety of paliperidone palmitate after discontinuation of the LAI.11
Continue to: No guidance exists for...
No guidance exists for aripiprazole LAI medications; however, based on the pharmacokinetic data, administration of oral medications should be initiated at the date of next injection. Given the long half-life of aripiprazole, a cross-titration of the LAI with oral medication is reasonable.
Monitoring drug levels
In addition to utilizing the pharmacokinetic data from LAI antipsychotics, therapeutic drug levels can be instrumental in determining the dose of oral medication to use and when to begin titration (Table 2).12-14 Obtaining a drug level on the date of the next injection can provide the clinician with data regarding the release of the medication specific to the patient. Based on the level and the current symptomatology, the clinician could choose to start the oral medication at a lower dose and titrate back to the LAI equivalent oral dose, or initiate the oral dose at the LAI equivalent oral dose. Continued therapeutic drug levels can aid in this determination.
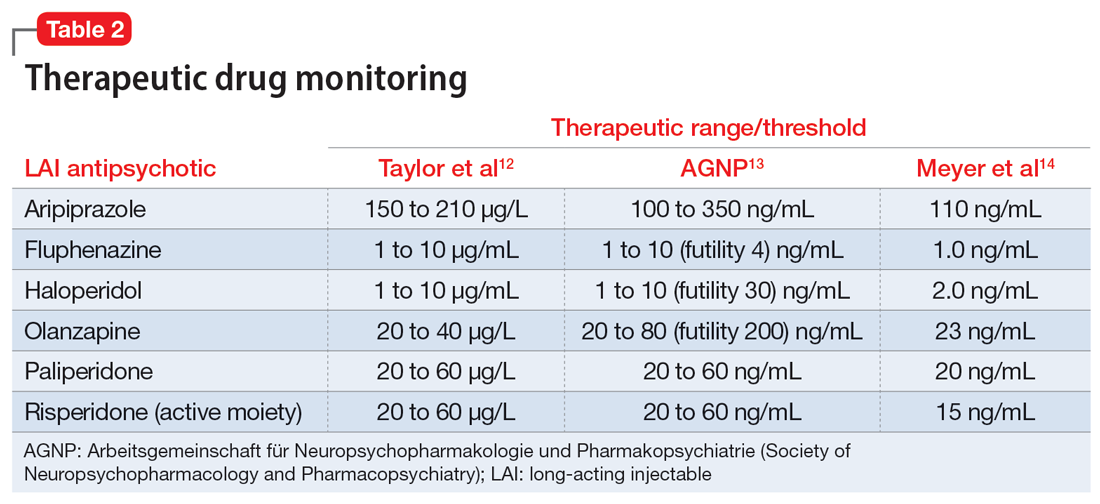
No guidance exists on the appropriate discontinuation of LAI antipsychotics. Utilizing a medication’s half-life and release mechanism, as well as the patient’s previous medication history, date of last injection, and therapeutic drug levels, should be considered when determining the schedule for restarting an oral antipsychotic.
CASE CONTINUED
Based on the current dosing of paliperidone palmitate of 156 mg once a month, Mr. R likely requires 9 mg/d of oral paliperidone upon discontinuation of the LAI. On the date of the next injection, the clinician could decide to initiate a lower dose of paliperidone, such as to 3 mg/d or 6 mg/d, and increase the dose as tolerated over the next 10 to 14 days as the paliperidone palmitate is further metabolized. Additionally, the clinician may consider obtaining a therapeutic drug level to determine the current paliperidone level prior to initiating the oral medication. Each treatment option offers individual risks and benefits. The decision on when and how to initiate the oral medication will be based on the individual patient’s situation and history, as well as the comfort and discretion of the clinician. The clinician should arrange appropriate monitoring for potential increased symptomatology during the transition, and adverse effects should be assessed regularly until steady state is achieved with the targeted oral dose of medication.
Related Resources
- Parmentier BL. Second-generation long-acting injectable antipsychotics: a practical guide. Current Psychiatry. 2020;19(3):24-32.
- Thippaiah SM, Fargason RE, Birur B. Switching antipsychotics: a guide to dose equivalents. Current Psychiatry. 2021;20(4):13-14. doi:10.12788/cp.0103
Drug Brand Names
Aripiprazole lauroxil • Aristada
Aripiprazole monohydrate • Maintena
Haloperidol injection • Haldol decanoate
Olanzapine pamoate • Zyprexa Relprevv
Paliperidone • Invega
Paliperidone palmitate once monthly • Invega Sustenna
Paliperidone palmitate every 3 months • Invega Trinza
Paliperidone palmitate every 6 months • Invega Hafyera
Risperidone microspheres • Risperdal Consta
Risperidone polymer • Perseris
1. Ostuzzi G, Vita G, Bertolini F, et al. Continuing, reducing, switching, or stopping antipsychotics in individuals with schizophrenia-spectrum disorders who are clinically stable: a systematic review and network meta-analysis. Lancet Psychiatry. 2022;9(8):614-624.
2. Correll CU, Kim E, Sliwa JK, et al. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. 2021;35(1):39-59.
3. Spanarello S, La Ferla T. The pharmacokinetics of long-acting antipsychotic medications. Curr Clin Pharmacol. 2014;9(3):310-317.
4. Meyer JM. Understanding depot antipsychotics: an illustrated guide to kinetics. CNS Spectr. 2013;18(Suppl 1):58-68.
5. Invega Hafyera [package insert]. Janssen Pharmaceuticals, Inc; 2021.
6. Gitlin MJ, Midha KK, Fogelson D, et al. Persistence of fluphenazine in plasma after decanoate withdrawal. J Clin Psychopharmacol. 1988;8(1):53-56.
7. Wistedt B, Jørgensen A, Wiles D. A depot neuroleptic withdrawal study. Plasma concentration of fluphenazine and flupenthixol and relapse frequency. Psychopharmacology. 1982;78(4):301-304.
8. Chang WH, Lin SK, Juang DJ, et al. Prolonged haloperidol and reduced haloperidol plasma concentrations after decanoate withdrawal. Schizophr Res. 1993;9(1):35-40.
9. Eklund K, Forsman A. Minimal effective dose and relapse—double-blind trial: haloperidol decanoate vs. placebo. Clin Neuropharmacol. 1991;1(Suppl 2):S7-S15.
10. Wilson WH. A visual guide to expected blood levels of long-acting injectable risperidone in clinical practice. J Psychiatry Pract. 2004;10(6):393-401.
11. Samtani MN, Sheehan JJ, Fu DJ, et al. Management of antipsychotic treatment discontinuation and interruptions using model-based simulations. Clin Pharmacol. 2012;4:25-40.
12. Taylor D, Barnes TRE, Young AH. The Maudsley Prescribing Guidelines in Psychiatry. 13th ed. Wiley-Blackwell; 2018.
13. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-2):9-62.
14. Meyer JM, Stahl SM. The Clinical Use of Antipsychotic Plasma Levels. Cambridge University Press; 2021.
Mr. R, age 29, was diagnosed with schizophrenia 6 years ago. To manage his disorder, he has been receiving paliperidone palmitate long-acting injectable (LAI) 156 mg once a month for 2 years. Prior to maintenance with paliperidone palmitate, Mr. R was stabilized on oral paliperidone 9 mg/d. Though he was originally initiated on paliperidone palmitate due to nonadherence concerns, Mr. R has been adherent with each injection for 1 year.
At a recent visit, Mr. R says he wants to discontinue the injection because he is not interested in receiving an ongoing injectable medication and is not able to continue monthly clinic visits. He wants to take a daily oral antipsychotic again, despite the availability of longer-acting products.
A paucity of evidence exists regarding the discontinuation of LAI antipsychotics and the next steps that follow in treatment. There is neither a consensus nor recognized guidelines advising how and when to discontinue an LAI and restart an oral antipsychotic. A recent systematic review and meta-analysis evaluated different maintenance treatment strategies; however, switching from an LAI antipsychotic to an oral medication was not a focus.1 In this article, we outline a possible approach to discontinuing an LAI antipsychotic and restarting an oral formulation. Before discontinuing an LAI antipsychotic, clinicians should review with the patient the risks and benefits of switching medications, including the risk of decompensation and potential adverse effects.
Switching to an oral antipsychotic
The first step in the discontinuation process is to determine whether the patient will continue the same oral medication as the LAI antipsychotic or if a different oral antipsychotic will be initiated. Next, determining when to initiate the oral medication requires several pieces of information, including the oral dose equivalent of the patient’s current LAI, the half-life of the LAI, and the release mechanism of the LAI (Table 1).2-5 To determine the appropriate time frame for restarting oral treatment, it is also vital to know the date of the last injection.

Based on the date of the next injection, the clinician will utilize the LAI’s half-life and its release mechanism to determine the appropriate time to start a new oral antipsychotic. Research demonstrates that in patients who have achieved steady state with a first-generation antipsychotic, plasma concentrations stay relatively consistent for 6 to 7 weeks after the last injection, which suggests oral medications may not need to be initiated until that time.6-9
For many second-generation LAI antipsychotics, oral medications may be initiated at the date of the next injection. Initiation of an oral antipsychotic may require more time between the last injection dose and the date of administration for oral medication due to the pharmacokinetic profile of risperidone microspheres. Once a patient is at steady state with risperidone microspheres, trough levels are not observed until 3 to 4 weeks after discontinuation.10
Previous pharmacokinetic model–based stimulations of active moiety plasma concentrations of risperidone microspheres demonstrate that 2 weeks after an injection of risperidone microspheres, the concentration of active moiety continued to approximate the steady-state concentration for 3 to 5 weeks.11 This is likely due to the product’s delay in release being 3 weeks from the time of injection to the last release phase. Of note, there was a rapid decline in the active moiety concentration; it reached nearly 0 by Week 5.11 The same pharmacokinetic model–based stimulation demonstrated a steady and slow decline of the concentration of active moiety of paliperidone palmitate after discontinuation of the LAI.11
Continue to: No guidance exists for...
No guidance exists for aripiprazole LAI medications; however, based on the pharmacokinetic data, administration of oral medications should be initiated at the date of next injection. Given the long half-life of aripiprazole, a cross-titration of the LAI with oral medication is reasonable.
Monitoring drug levels
In addition to utilizing the pharmacokinetic data from LAI antipsychotics, therapeutic drug levels can be instrumental in determining the dose of oral medication to use and when to begin titration (Table 2).12-14 Obtaining a drug level on the date of the next injection can provide the clinician with data regarding the release of the medication specific to the patient. Based on the level and the current symptomatology, the clinician could choose to start the oral medication at a lower dose and titrate back to the LAI equivalent oral dose, or initiate the oral dose at the LAI equivalent oral dose. Continued therapeutic drug levels can aid in this determination.

No guidance exists on the appropriate discontinuation of LAI antipsychotics. Utilizing a medication’s half-life and release mechanism, as well as the patient’s previous medication history, date of last injection, and therapeutic drug levels, should be considered when determining the schedule for restarting an oral antipsychotic.
CASE CONTINUED
Based on the current dosing of paliperidone palmitate of 156 mg once a month, Mr. R likely requires 9 mg/d of oral paliperidone upon discontinuation of the LAI. On the date of the next injection, the clinician could decide to initiate a lower dose of paliperidone, such as to 3 mg/d or 6 mg/d, and increase the dose as tolerated over the next 10 to 14 days as the paliperidone palmitate is further metabolized. Additionally, the clinician may consider obtaining a therapeutic drug level to determine the current paliperidone level prior to initiating the oral medication. Each treatment option offers individual risks and benefits. The decision on when and how to initiate the oral medication will be based on the individual patient’s situation and history, as well as the comfort and discretion of the clinician. The clinician should arrange appropriate monitoring for potential increased symptomatology during the transition, and adverse effects should be assessed regularly until steady state is achieved with the targeted oral dose of medication.
Related Resources
- Parmentier BL. Second-generation long-acting injectable antipsychotics: a practical guide. Current Psychiatry. 2020;19(3):24-32.
- Thippaiah SM, Fargason RE, Birur B. Switching antipsychotics: a guide to dose equivalents. Current Psychiatry. 2021;20(4):13-14. doi:10.12788/cp.0103
Drug Brand Names
Aripiprazole lauroxil • Aristada
Aripiprazole monohydrate • Maintena
Haloperidol injection • Haldol decanoate
Olanzapine pamoate • Zyprexa Relprevv
Paliperidone • Invega
Paliperidone palmitate once monthly • Invega Sustenna
Paliperidone palmitate every 3 months • Invega Trinza
Paliperidone palmitate every 6 months • Invega Hafyera
Risperidone microspheres • Risperdal Consta
Risperidone polymer • Perseris
Mr. R, age 29, was diagnosed with schizophrenia 6 years ago. To manage his disorder, he has been receiving paliperidone palmitate long-acting injectable (LAI) 156 mg once a month for 2 years. Prior to maintenance with paliperidone palmitate, Mr. R was stabilized on oral paliperidone 9 mg/d. Though he was originally initiated on paliperidone palmitate due to nonadherence concerns, Mr. R has been adherent with each injection for 1 year.
At a recent visit, Mr. R says he wants to discontinue the injection because he is not interested in receiving an ongoing injectable medication and is not able to continue monthly clinic visits. He wants to take a daily oral antipsychotic again, despite the availability of longer-acting products.
A paucity of evidence exists regarding the discontinuation of LAI antipsychotics and the next steps that follow in treatment. There is neither a consensus nor recognized guidelines advising how and when to discontinue an LAI and restart an oral antipsychotic. A recent systematic review and meta-analysis evaluated different maintenance treatment strategies; however, switching from an LAI antipsychotic to an oral medication was not a focus.1 In this article, we outline a possible approach to discontinuing an LAI antipsychotic and restarting an oral formulation. Before discontinuing an LAI antipsychotic, clinicians should review with the patient the risks and benefits of switching medications, including the risk of decompensation and potential adverse effects.
Switching to an oral antipsychotic
The first step in the discontinuation process is to determine whether the patient will continue the same oral medication as the LAI antipsychotic or if a different oral antipsychotic will be initiated. Next, determining when to initiate the oral medication requires several pieces of information, including the oral dose equivalent of the patient’s current LAI, the half-life of the LAI, and the release mechanism of the LAI (Table 1).2-5 To determine the appropriate time frame for restarting oral treatment, it is also vital to know the date of the last injection.

Based on the date of the next injection, the clinician will utilize the LAI’s half-life and its release mechanism to determine the appropriate time to start a new oral antipsychotic. Research demonstrates that in patients who have achieved steady state with a first-generation antipsychotic, plasma concentrations stay relatively consistent for 6 to 7 weeks after the last injection, which suggests oral medications may not need to be initiated until that time.6-9
For many second-generation LAI antipsychotics, oral medications may be initiated at the date of the next injection. Initiation of an oral antipsychotic may require more time between the last injection dose and the date of administration for oral medication due to the pharmacokinetic profile of risperidone microspheres. Once a patient is at steady state with risperidone microspheres, trough levels are not observed until 3 to 4 weeks after discontinuation.10
Previous pharmacokinetic model–based stimulations of active moiety plasma concentrations of risperidone microspheres demonstrate that 2 weeks after an injection of risperidone microspheres, the concentration of active moiety continued to approximate the steady-state concentration for 3 to 5 weeks.11 This is likely due to the product’s delay in release being 3 weeks from the time of injection to the last release phase. Of note, there was a rapid decline in the active moiety concentration; it reached nearly 0 by Week 5.11 The same pharmacokinetic model–based stimulation demonstrated a steady and slow decline of the concentration of active moiety of paliperidone palmitate after discontinuation of the LAI.11
Continue to: No guidance exists for...
No guidance exists for aripiprazole LAI medications; however, based on the pharmacokinetic data, administration of oral medications should be initiated at the date of next injection. Given the long half-life of aripiprazole, a cross-titration of the LAI with oral medication is reasonable.
Monitoring drug levels
In addition to utilizing the pharmacokinetic data from LAI antipsychotics, therapeutic drug levels can be instrumental in determining the dose of oral medication to use and when to begin titration (Table 2).12-14 Obtaining a drug level on the date of the next injection can provide the clinician with data regarding the release of the medication specific to the patient. Based on the level and the current symptomatology, the clinician could choose to start the oral medication at a lower dose and titrate back to the LAI equivalent oral dose, or initiate the oral dose at the LAI equivalent oral dose. Continued therapeutic drug levels can aid in this determination.

No guidance exists on the appropriate discontinuation of LAI antipsychotics. Utilizing a medication’s half-life and release mechanism, as well as the patient’s previous medication history, date of last injection, and therapeutic drug levels, should be considered when determining the schedule for restarting an oral antipsychotic.
CASE CONTINUED
Based on the current dosing of paliperidone palmitate of 156 mg once a month, Mr. R likely requires 9 mg/d of oral paliperidone upon discontinuation of the LAI. On the date of the next injection, the clinician could decide to initiate a lower dose of paliperidone, such as to 3 mg/d or 6 mg/d, and increase the dose as tolerated over the next 10 to 14 days as the paliperidone palmitate is further metabolized. Additionally, the clinician may consider obtaining a therapeutic drug level to determine the current paliperidone level prior to initiating the oral medication. Each treatment option offers individual risks and benefits. The decision on when and how to initiate the oral medication will be based on the individual patient’s situation and history, as well as the comfort and discretion of the clinician. The clinician should arrange appropriate monitoring for potential increased symptomatology during the transition, and adverse effects should be assessed regularly until steady state is achieved with the targeted oral dose of medication.
Related Resources
- Parmentier BL. Second-generation long-acting injectable antipsychotics: a practical guide. Current Psychiatry. 2020;19(3):24-32.
- Thippaiah SM, Fargason RE, Birur B. Switching antipsychotics: a guide to dose equivalents. Current Psychiatry. 2021;20(4):13-14. doi:10.12788/cp.0103
Drug Brand Names
Aripiprazole lauroxil • Aristada
Aripiprazole monohydrate • Maintena
Haloperidol injection • Haldol decanoate
Olanzapine pamoate • Zyprexa Relprevv
Paliperidone • Invega
Paliperidone palmitate once monthly • Invega Sustenna
Paliperidone palmitate every 3 months • Invega Trinza
Paliperidone palmitate every 6 months • Invega Hafyera
Risperidone microspheres • Risperdal Consta
Risperidone polymer • Perseris
1. Ostuzzi G, Vita G, Bertolini F, et al. Continuing, reducing, switching, or stopping antipsychotics in individuals with schizophrenia-spectrum disorders who are clinically stable: a systematic review and network meta-analysis. Lancet Psychiatry. 2022;9(8):614-624.
2. Correll CU, Kim E, Sliwa JK, et al. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. 2021;35(1):39-59.
3. Spanarello S, La Ferla T. The pharmacokinetics of long-acting antipsychotic medications. Curr Clin Pharmacol. 2014;9(3):310-317.
4. Meyer JM. Understanding depot antipsychotics: an illustrated guide to kinetics. CNS Spectr. 2013;18(Suppl 1):58-68.
5. Invega Hafyera [package insert]. Janssen Pharmaceuticals, Inc; 2021.
6. Gitlin MJ, Midha KK, Fogelson D, et al. Persistence of fluphenazine in plasma after decanoate withdrawal. J Clin Psychopharmacol. 1988;8(1):53-56.
7. Wistedt B, Jørgensen A, Wiles D. A depot neuroleptic withdrawal study. Plasma concentration of fluphenazine and flupenthixol and relapse frequency. Psychopharmacology. 1982;78(4):301-304.
8. Chang WH, Lin SK, Juang DJ, et al. Prolonged haloperidol and reduced haloperidol plasma concentrations after decanoate withdrawal. Schizophr Res. 1993;9(1):35-40.
9. Eklund K, Forsman A. Minimal effective dose and relapse—double-blind trial: haloperidol decanoate vs. placebo. Clin Neuropharmacol. 1991;1(Suppl 2):S7-S15.
10. Wilson WH. A visual guide to expected blood levels of long-acting injectable risperidone in clinical practice. J Psychiatry Pract. 2004;10(6):393-401.
11. Samtani MN, Sheehan JJ, Fu DJ, et al. Management of antipsychotic treatment discontinuation and interruptions using model-based simulations. Clin Pharmacol. 2012;4:25-40.
12. Taylor D, Barnes TRE, Young AH. The Maudsley Prescribing Guidelines in Psychiatry. 13th ed. Wiley-Blackwell; 2018.
13. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-2):9-62.
14. Meyer JM, Stahl SM. The Clinical Use of Antipsychotic Plasma Levels. Cambridge University Press; 2021.
1. Ostuzzi G, Vita G, Bertolini F, et al. Continuing, reducing, switching, or stopping antipsychotics in individuals with schizophrenia-spectrum disorders who are clinically stable: a systematic review and network meta-analysis. Lancet Psychiatry. 2022;9(8):614-624.
2. Correll CU, Kim E, Sliwa JK, et al. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. 2021;35(1):39-59.
3. Spanarello S, La Ferla T. The pharmacokinetics of long-acting antipsychotic medications. Curr Clin Pharmacol. 2014;9(3):310-317.
4. Meyer JM. Understanding depot antipsychotics: an illustrated guide to kinetics. CNS Spectr. 2013;18(Suppl 1):58-68.
5. Invega Hafyera [package insert]. Janssen Pharmaceuticals, Inc; 2021.
6. Gitlin MJ, Midha KK, Fogelson D, et al. Persistence of fluphenazine in plasma after decanoate withdrawal. J Clin Psychopharmacol. 1988;8(1):53-56.
7. Wistedt B, Jørgensen A, Wiles D. A depot neuroleptic withdrawal study. Plasma concentration of fluphenazine and flupenthixol and relapse frequency. Psychopharmacology. 1982;78(4):301-304.
8. Chang WH, Lin SK, Juang DJ, et al. Prolonged haloperidol and reduced haloperidol plasma concentrations after decanoate withdrawal. Schizophr Res. 1993;9(1):35-40.
9. Eklund K, Forsman A. Minimal effective dose and relapse—double-blind trial: haloperidol decanoate vs. placebo. Clin Neuropharmacol. 1991;1(Suppl 2):S7-S15.
10. Wilson WH. A visual guide to expected blood levels of long-acting injectable risperidone in clinical practice. J Psychiatry Pract. 2004;10(6):393-401.
11. Samtani MN, Sheehan JJ, Fu DJ, et al. Management of antipsychotic treatment discontinuation and interruptions using model-based simulations. Clin Pharmacol. 2012;4:25-40.
12. Taylor D, Barnes TRE, Young AH. The Maudsley Prescribing Guidelines in Psychiatry. 13th ed. Wiley-Blackwell; 2018.
13. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-2):9-62.
14. Meyer JM, Stahl SM. The Clinical Use of Antipsychotic Plasma Levels. Cambridge University Press; 2021.
Medication-induced rhabdomyolysis
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Ms. A, age 32, has a history of anxiety, bipolar disorder, and borderline personality disorder. She is undergoing treatment with lamotrigine 200 mg/d at bedtime, aripiprazole 5 mg/d, trazodone 100 mg/d at bedtime, clonazepam 0.5 mg twice a day, and hydroxyzine 25 mg twice a day. She presents to the emergency department with myalgia, left upper and lower extremity numbness, and weakness. These symptoms started at approximately 3
Ms. A’s vital signs are hemodynamically stable, but her pulse is 113 bpm. On examination, she appears anxious and has decreased sensation in her upper and lower extremities, with 3/5 strength on the left side. Her laboratory results indicate mild leukocytosis, hyponatremia (129 mmol/L; reference range 136 to 145 mmol/L), and elevations in serum creatinine (3.7 mg/dL; reference range 0.6 to 1.2 mg/dL), aspartate aminotransferase (654 U/L; reference range 10 to 42 U/L), alanine transaminase (234 U/L; reference range 10 to 60 U/L), and troponin (2.11 ng/mL; reference range 0 to 0.04 ng/mL). A urinalysis reveals darkly colored urine with large red blood cells.
Neurology and Cardiology consultations are requested to rule out stroke and acute coronary syndromes. A computed tomography scan of the head shows no acute intracranial findings. Her creatinine kinase (CK) level is elevated (>42,670 U/L; reference range 22 to 232 U/L), which prompts a search for causes of rhabdomyolysis, a breakdown of muscle tissue that releases muscle fiber contents into the blood. Ms. A reports no history of recent trauma or strenuous exercise. Infectious, endocrine, and other workups are negative. After a consult to Psychiatry, the treating clinicians suspect that the most likely cause for rhabdomyolysis is aripiprazole.
Ms. A is treated with IV isotonic fluids. Aripiprazole is stopped and her CK levels are closely monitored. CK levels continue to trend down, and by Day 6 of hospitalization her CK level is 1,648 U/L. Her transaminase levels also improve; these elevations are considered likely secondary to rhabdomyolysis. Because there is notable improvement in CK and transaminase levels after stopping aripiprazole, Ms. A is discharged and instructed to follow up with a psychiatrist for further management.
Aripiprazole and rhabdomyolysis
According to the National Institute of Mental Health, an estimated 2.8% of the US population has bipolar disorder and 0.24% to 0.64% has schizophrenia.1,2 Antipsychotics are often used to treat these disorders. The prevalence of antipsychotic use in the general adult population is 1.6%.3 The use of second-generation antipsychotics (SGAs) has increased over recent years with the availability of a variety of formulations, such as immediate-release injectable, long-acting injectable, and orally disintegrating tablets in addition to the customary oral tablets. SGAs can cause several adverse effects, including weight gain, hyperlipidemia, diabetes, QTc prolongation, extrapyramidal side effects, myocarditis, agranulocytosis, cataracts, and sexual adverse effects.4
Antipsychotic use is more commonly associated with serotonin syndrome and neuroleptic malignant syndrome than it is with rhabdomyolysis. Rhabdomyolysis as an adverse effect of antipsychotic use has not been well understood or reported. One study found the prevalence of rhabdomyolysis was approximately 10% among patients who received an antipsychotic medication.5 There have been 4 case reports of clozapine use, 6 of olanzapine use, and 3 of aripiprazole use associated with rhabdomyolysis.6-8 Therefore, this would be the fourth case report to describe aripiprazole-associated rhabdomyolysis.
Aripiprazole is FDA-approved for the treatment of schizophrenia. In this case report, we found that aripiprazole could have led to rhabdomyolysis. Aripiprazole is a quinoline derivative that acts by binding to the 5-HT1A and 5-HT2A receptors.9,10 It acts as a partial agonist at 5-HT1A receptors, an antagonist at 5-HT2A receptors, and a partial agonist and stabilizer at the D2 receptor. By binding to the dopamine receptor in its G protein–coupled state, aripiprazole blocks the receptor in the presence of excessive dopamine.11-13 The mechanism of how aripiprazole could cause rhabdomyolysis is unclear. One proposed mechanism is that it can increase the permeability of skeletal muscle by 5-HT2A antagonism. This leads to a decrease in glucose reuptake in the cell and increases the permeability of the cell membrane, leading to elevations in CK levels.14 Another proposed mechanism is that dopamine blockade in the nigrostriatal pathway can result in muscle stiffness, rigidity, parkinsonian-like symptoms, and akathisia, which can result in elevated CK levels.15 There are only 3 other published cases of aripiprazole-induced rhabdomyolysis; we hope this case report will add value to the available literature. More evidence is needed to establish the safety profile of aripiprazole.
1. National Institute of Mental Health. Prevalence of bipolar disorder among adults. Accessed December 21, 2022. https://www.nimh.nih.gov/health/statistics/bipolar-disorder#part_2605
2. National Institute of Mental Health. Schizophrenia. Accessed December 21, 2022. https://www.nimh.nih.gov/health/statistics/schizophrenia#part_2543
3. Dennis JA, Gittner LS, Payne JD, et al. Characteristics of U.S. adults taking prescription antipsychotic medications, National Health and Nutrition Examination Survey 2013-2018. BMC Psychiatry. 2020;20(1):483. doi: 10.1186/s12888-020-02895-4
4. Willner K, Vasan S, Abdijadid S. Atypical antipsychotic agents. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated May 2, 2022. Accessed December 22, 2022. https://www.ncbi.nlm.nih.gov/books/NBK448156/
5. Packard K, Price P, Hanson A. Antipsychotic use and the risk of rhabdomyolysis. J Pharm Pract 2014;27(5):501-512. doi: 10.1177/0897190013516509
6. Wu YF, Chang KY. Aripiprazole-associated rhabdomyolysis in a patient with schizophrenia. J Neuropsychiatry Clin Neurosci. 2011;23(3):E51.
7. Marzetti E, Bocchino L, Teramo S, et al. Rhabdomyolysis in a patient on aripiprazole with traumatic hip prosthesis luxation. J Neuropsychiatry Clin Neurosci. 2012;24(4):E40-E41.
8. Zhu X, Hu J, Deng S, et al. Rhabdomyolysis and elevated liver enzymes after rapid correction of hyponatremia due to pneumonia and concurrent use of aripiprazole: a case report. Aust N Z J Psychiatry. 2018;52(2):206. doi:10.1177/0004867417743342
9. Stahl SM. Essential Psychopharmacology: Neuroscientific Basis and Practical Application. 2nd ed. Cambridge University Press; 2000.
10. Stahl SM. “Hit-and-run” actions at dopamine receptors, part 1: mechanism of action of atypical antipsychotics. J Clin Psychiatry. 2001;62(9):670-671.
11. Leysen JE, Janssen PM, Schotte A, et al. Interaction of antipsychotic drugs with neurotransmitter receptor sites in vitro and in vivo in relation to pharmacological and clinical effects: role of 5HT2 receptors. Psychopharmacology (Berl). 1993;112(1 Suppl):S40-S54.
12. Millan MJ. Improving the treatment of schizophrenia: focus on serotonin (5-HT)(1A) receptors. J Pharmacol Exp Ther. 2000;295(3):853-861.
13. Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70(2):83-244.
14. Meltzer HY, Cola PA, Parsa M. Marked elevations of serum creatine kinase activity associated with antipsychotic drug treatment. Neuropsychopharmacology. 1996;15(4):395-405.
15. Devarajan S, Dursun SM. Antipsychotic drugs, serum creatine kinase (CPK) and possible mechanisms. Psychopharmacology (Berl). 2000;152(1):122.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Ms. A, age 32, has a history of anxiety, bipolar disorder, and borderline personality disorder. She is undergoing treatment with lamotrigine 200 mg/d at bedtime, aripiprazole 5 mg/d, trazodone 100 mg/d at bedtime, clonazepam 0.5 mg twice a day, and hydroxyzine 25 mg twice a day. She presents to the emergency department with myalgia, left upper and lower extremity numbness, and weakness. These symptoms started at approximately 3
Ms. A’s vital signs are hemodynamically stable, but her pulse is 113 bpm. On examination, she appears anxious and has decreased sensation in her upper and lower extremities, with 3/5 strength on the left side. Her laboratory results indicate mild leukocytosis, hyponatremia (129 mmol/L; reference range 136 to 145 mmol/L), and elevations in serum creatinine (3.7 mg/dL; reference range 0.6 to 1.2 mg/dL), aspartate aminotransferase (654 U/L; reference range 10 to 42 U/L), alanine transaminase (234 U/L; reference range 10 to 60 U/L), and troponin (2.11 ng/mL; reference range 0 to 0.04 ng/mL). A urinalysis reveals darkly colored urine with large red blood cells.
Neurology and Cardiology consultations are requested to rule out stroke and acute coronary syndromes. A computed tomography scan of the head shows no acute intracranial findings. Her creatinine kinase (CK) level is elevated (>42,670 U/L; reference range 22 to 232 U/L), which prompts a search for causes of rhabdomyolysis, a breakdown of muscle tissue that releases muscle fiber contents into the blood. Ms. A reports no history of recent trauma or strenuous exercise. Infectious, endocrine, and other workups are negative. After a consult to Psychiatry, the treating clinicians suspect that the most likely cause for rhabdomyolysis is aripiprazole.
Ms. A is treated with IV isotonic fluids. Aripiprazole is stopped and her CK levels are closely monitored. CK levels continue to trend down, and by Day 6 of hospitalization her CK level is 1,648 U/L. Her transaminase levels also improve; these elevations are considered likely secondary to rhabdomyolysis. Because there is notable improvement in CK and transaminase levels after stopping aripiprazole, Ms. A is discharged and instructed to follow up with a psychiatrist for further management.
Aripiprazole and rhabdomyolysis
According to the National Institute of Mental Health, an estimated 2.8% of the US population has bipolar disorder and 0.24% to 0.64% has schizophrenia.1,2 Antipsychotics are often used to treat these disorders. The prevalence of antipsychotic use in the general adult population is 1.6%.3 The use of second-generation antipsychotics (SGAs) has increased over recent years with the availability of a variety of formulations, such as immediate-release injectable, long-acting injectable, and orally disintegrating tablets in addition to the customary oral tablets. SGAs can cause several adverse effects, including weight gain, hyperlipidemia, diabetes, QTc prolongation, extrapyramidal side effects, myocarditis, agranulocytosis, cataracts, and sexual adverse effects.4
Antipsychotic use is more commonly associated with serotonin syndrome and neuroleptic malignant syndrome than it is with rhabdomyolysis. Rhabdomyolysis as an adverse effect of antipsychotic use has not been well understood or reported. One study found the prevalence of rhabdomyolysis was approximately 10% among patients who received an antipsychotic medication.5 There have been 4 case reports of clozapine use, 6 of olanzapine use, and 3 of aripiprazole use associated with rhabdomyolysis.6-8 Therefore, this would be the fourth case report to describe aripiprazole-associated rhabdomyolysis.
Aripiprazole is FDA-approved for the treatment of schizophrenia. In this case report, we found that aripiprazole could have led to rhabdomyolysis. Aripiprazole is a quinoline derivative that acts by binding to the 5-HT1A and 5-HT2A receptors.9,10 It acts as a partial agonist at 5-HT1A receptors, an antagonist at 5-HT2A receptors, and a partial agonist and stabilizer at the D2 receptor. By binding to the dopamine receptor in its G protein–coupled state, aripiprazole blocks the receptor in the presence of excessive dopamine.11-13 The mechanism of how aripiprazole could cause rhabdomyolysis is unclear. One proposed mechanism is that it can increase the permeability of skeletal muscle by 5-HT2A antagonism. This leads to a decrease in glucose reuptake in the cell and increases the permeability of the cell membrane, leading to elevations in CK levels.14 Another proposed mechanism is that dopamine blockade in the nigrostriatal pathway can result in muscle stiffness, rigidity, parkinsonian-like symptoms, and akathisia, which can result in elevated CK levels.15 There are only 3 other published cases of aripiprazole-induced rhabdomyolysis; we hope this case report will add value to the available literature. More evidence is needed to establish the safety profile of aripiprazole.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Ms. A, age 32, has a history of anxiety, bipolar disorder, and borderline personality disorder. She is undergoing treatment with lamotrigine 200 mg/d at bedtime, aripiprazole 5 mg/d, trazodone 100 mg/d at bedtime, clonazepam 0.5 mg twice a day, and hydroxyzine 25 mg twice a day. She presents to the emergency department with myalgia, left upper and lower extremity numbness, and weakness. These symptoms started at approximately 3
Ms. A’s vital signs are hemodynamically stable, but her pulse is 113 bpm. On examination, she appears anxious and has decreased sensation in her upper and lower extremities, with 3/5 strength on the left side. Her laboratory results indicate mild leukocytosis, hyponatremia (129 mmol/L; reference range 136 to 145 mmol/L), and elevations in serum creatinine (3.7 mg/dL; reference range 0.6 to 1.2 mg/dL), aspartate aminotransferase (654 U/L; reference range 10 to 42 U/L), alanine transaminase (234 U/L; reference range 10 to 60 U/L), and troponin (2.11 ng/mL; reference range 0 to 0.04 ng/mL). A urinalysis reveals darkly colored urine with large red blood cells.
Neurology and Cardiology consultations are requested to rule out stroke and acute coronary syndromes. A computed tomography scan of the head shows no acute intracranial findings. Her creatinine kinase (CK) level is elevated (>42,670 U/L; reference range 22 to 232 U/L), which prompts a search for causes of rhabdomyolysis, a breakdown of muscle tissue that releases muscle fiber contents into the blood. Ms. A reports no history of recent trauma or strenuous exercise. Infectious, endocrine, and other workups are negative. After a consult to Psychiatry, the treating clinicians suspect that the most likely cause for rhabdomyolysis is aripiprazole.
Ms. A is treated with IV isotonic fluids. Aripiprazole is stopped and her CK levels are closely monitored. CK levels continue to trend down, and by Day 6 of hospitalization her CK level is 1,648 U/L. Her transaminase levels also improve; these elevations are considered likely secondary to rhabdomyolysis. Because there is notable improvement in CK and transaminase levels after stopping aripiprazole, Ms. A is discharged and instructed to follow up with a psychiatrist for further management.
Aripiprazole and rhabdomyolysis
According to the National Institute of Mental Health, an estimated 2.8% of the US population has bipolar disorder and 0.24% to 0.64% has schizophrenia.1,2 Antipsychotics are often used to treat these disorders. The prevalence of antipsychotic use in the general adult population is 1.6%.3 The use of second-generation antipsychotics (SGAs) has increased over recent years with the availability of a variety of formulations, such as immediate-release injectable, long-acting injectable, and orally disintegrating tablets in addition to the customary oral tablets. SGAs can cause several adverse effects, including weight gain, hyperlipidemia, diabetes, QTc prolongation, extrapyramidal side effects, myocarditis, agranulocytosis, cataracts, and sexual adverse effects.4
Antipsychotic use is more commonly associated with serotonin syndrome and neuroleptic malignant syndrome than it is with rhabdomyolysis. Rhabdomyolysis as an adverse effect of antipsychotic use has not been well understood or reported. One study found the prevalence of rhabdomyolysis was approximately 10% among patients who received an antipsychotic medication.5 There have been 4 case reports of clozapine use, 6 of olanzapine use, and 3 of aripiprazole use associated with rhabdomyolysis.6-8 Therefore, this would be the fourth case report to describe aripiprazole-associated rhabdomyolysis.
Aripiprazole is FDA-approved for the treatment of schizophrenia. In this case report, we found that aripiprazole could have led to rhabdomyolysis. Aripiprazole is a quinoline derivative that acts by binding to the 5-HT1A and 5-HT2A receptors.9,10 It acts as a partial agonist at 5-HT1A receptors, an antagonist at 5-HT2A receptors, and a partial agonist and stabilizer at the D2 receptor. By binding to the dopamine receptor in its G protein–coupled state, aripiprazole blocks the receptor in the presence of excessive dopamine.11-13 The mechanism of how aripiprazole could cause rhabdomyolysis is unclear. One proposed mechanism is that it can increase the permeability of skeletal muscle by 5-HT2A antagonism. This leads to a decrease in glucose reuptake in the cell and increases the permeability of the cell membrane, leading to elevations in CK levels.14 Another proposed mechanism is that dopamine blockade in the nigrostriatal pathway can result in muscle stiffness, rigidity, parkinsonian-like symptoms, and akathisia, which can result in elevated CK levels.15 There are only 3 other published cases of aripiprazole-induced rhabdomyolysis; we hope this case report will add value to the available literature. More evidence is needed to establish the safety profile of aripiprazole.
1. National Institute of Mental Health. Prevalence of bipolar disorder among adults. Accessed December 21, 2022. https://www.nimh.nih.gov/health/statistics/bipolar-disorder#part_2605
2. National Institute of Mental Health. Schizophrenia. Accessed December 21, 2022. https://www.nimh.nih.gov/health/statistics/schizophrenia#part_2543
3. Dennis JA, Gittner LS, Payne JD, et al. Characteristics of U.S. adults taking prescription antipsychotic medications, National Health and Nutrition Examination Survey 2013-2018. BMC Psychiatry. 2020;20(1):483. doi: 10.1186/s12888-020-02895-4
4. Willner K, Vasan S, Abdijadid S. Atypical antipsychotic agents. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated May 2, 2022. Accessed December 22, 2022. https://www.ncbi.nlm.nih.gov/books/NBK448156/
5. Packard K, Price P, Hanson A. Antipsychotic use and the risk of rhabdomyolysis. J Pharm Pract 2014;27(5):501-512. doi: 10.1177/0897190013516509
6. Wu YF, Chang KY. Aripiprazole-associated rhabdomyolysis in a patient with schizophrenia. J Neuropsychiatry Clin Neurosci. 2011;23(3):E51.
7. Marzetti E, Bocchino L, Teramo S, et al. Rhabdomyolysis in a patient on aripiprazole with traumatic hip prosthesis luxation. J Neuropsychiatry Clin Neurosci. 2012;24(4):E40-E41.
8. Zhu X, Hu J, Deng S, et al. Rhabdomyolysis and elevated liver enzymes after rapid correction of hyponatremia due to pneumonia and concurrent use of aripiprazole: a case report. Aust N Z J Psychiatry. 2018;52(2):206. doi:10.1177/0004867417743342
9. Stahl SM. Essential Psychopharmacology: Neuroscientific Basis and Practical Application. 2nd ed. Cambridge University Press; 2000.
10. Stahl SM. “Hit-and-run” actions at dopamine receptors, part 1: mechanism of action of atypical antipsychotics. J Clin Psychiatry. 2001;62(9):670-671.
11. Leysen JE, Janssen PM, Schotte A, et al. Interaction of antipsychotic drugs with neurotransmitter receptor sites in vitro and in vivo in relation to pharmacological and clinical effects: role of 5HT2 receptors. Psychopharmacology (Berl). 1993;112(1 Suppl):S40-S54.
12. Millan MJ. Improving the treatment of schizophrenia: focus on serotonin (5-HT)(1A) receptors. J Pharmacol Exp Ther. 2000;295(3):853-861.
13. Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70(2):83-244.
14. Meltzer HY, Cola PA, Parsa M. Marked elevations of serum creatine kinase activity associated with antipsychotic drug treatment. Neuropsychopharmacology. 1996;15(4):395-405.
15. Devarajan S, Dursun SM. Antipsychotic drugs, serum creatine kinase (CPK) and possible mechanisms. Psychopharmacology (Berl). 2000;152(1):122.
1. National Institute of Mental Health. Prevalence of bipolar disorder among adults. Accessed December 21, 2022. https://www.nimh.nih.gov/health/statistics/bipolar-disorder#part_2605
2. National Institute of Mental Health. Schizophrenia. Accessed December 21, 2022. https://www.nimh.nih.gov/health/statistics/schizophrenia#part_2543
3. Dennis JA, Gittner LS, Payne JD, et al. Characteristics of U.S. adults taking prescription antipsychotic medications, National Health and Nutrition Examination Survey 2013-2018. BMC Psychiatry. 2020;20(1):483. doi: 10.1186/s12888-020-02895-4
4. Willner K, Vasan S, Abdijadid S. Atypical antipsychotic agents. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated May 2, 2022. Accessed December 22, 2022. https://www.ncbi.nlm.nih.gov/books/NBK448156/
5. Packard K, Price P, Hanson A. Antipsychotic use and the risk of rhabdomyolysis. J Pharm Pract 2014;27(5):501-512. doi: 10.1177/0897190013516509
6. Wu YF, Chang KY. Aripiprazole-associated rhabdomyolysis in a patient with schizophrenia. J Neuropsychiatry Clin Neurosci. 2011;23(3):E51.
7. Marzetti E, Bocchino L, Teramo S, et al. Rhabdomyolysis in a patient on aripiprazole with traumatic hip prosthesis luxation. J Neuropsychiatry Clin Neurosci. 2012;24(4):E40-E41.
8. Zhu X, Hu J, Deng S, et al. Rhabdomyolysis and elevated liver enzymes after rapid correction of hyponatremia due to pneumonia and concurrent use of aripiprazole: a case report. Aust N Z J Psychiatry. 2018;52(2):206. doi:10.1177/0004867417743342
9. Stahl SM. Essential Psychopharmacology: Neuroscientific Basis and Practical Application. 2nd ed. Cambridge University Press; 2000.
10. Stahl SM. “Hit-and-run” actions at dopamine receptors, part 1: mechanism of action of atypical antipsychotics. J Clin Psychiatry. 2001;62(9):670-671.
11. Leysen JE, Janssen PM, Schotte A, et al. Interaction of antipsychotic drugs with neurotransmitter receptor sites in vitro and in vivo in relation to pharmacological and clinical effects: role of 5HT2 receptors. Psychopharmacology (Berl). 1993;112(1 Suppl):S40-S54.
12. Millan MJ. Improving the treatment of schizophrenia: focus on serotonin (5-HT)(1A) receptors. J Pharmacol Exp Ther. 2000;295(3):853-861.
13. Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70(2):83-244.
14. Meltzer HY, Cola PA, Parsa M. Marked elevations of serum creatine kinase activity associated with antipsychotic drug treatment. Neuropsychopharmacology. 1996;15(4):395-405.
15. Devarajan S, Dursun SM. Antipsychotic drugs, serum creatine kinase (CPK) and possible mechanisms. Psychopharmacology (Berl). 2000;152(1):122.
Subtle cognitive decline in a patient with depression and anxiety
CASE Anxious and confused
Mr. M, age 53, a surgeon, presents to the emergency department (ED) following a panic attack and concerns from his staff that he appears confused. Specifically, staff members report that in the past 4 months, Mr. M was observed having problems completing some postoperative tasks related to chart documentation. Mr. M has a history of major depressive disorder (MDD), hypertension, hyperlipidemia, and type 2 diabetes.
HISTORY A long-standing diagnosis of depression
Mr. M reports that 30 years ago, he received care from a psychiatrist to address symptoms of MDD. He says that around the time he arrived at the ED, he had noticed subtle but gradual changes in his cognition, which led him to skip words and often struggle to find the correct words. These episodes left him confused. Mr. M started getting anxious about these cognitive issues because they disrupted his work and forced him to reduce his duties. He does not have any known family history of mental illness, is single, and lives alone.
EVALUATION After stroke is ruled out, a psychiatric workup
In the ED, a comprehensive exam rules out an acute cerebrovascular event. A neurologic evaluation notes some delay in processing information and observes Mr. M having difficulty following simple commands. Laboratory investigations, including a comprehensive metabolic panel, are unremarkable. An MRI of Mr. M’s brain, with and without contrast, notes no acute findings. He is discharged from the ED with a diagnosis of MDD.
Before he presented to the ED, Mr. M’s medication regimen included duloxetine 60 mg/d, buspirone 10 mg 3 times a day, and aripiprazole 5 mg/d for MDD and anxiety. After the ED visit, Mr. M’s physician refers him to an outpatient psychiatrist for management of worsening depression and panic attacks. During the psychiatrist’s evaluation, Mr. M reports a decreased interest in activities, decreased motivation, being easily fatigued, and having poor sleep. He denies having a depressed mood, difficulty concentrating, or having problems with his appetite. He also denies suicidal thoughts, both past and present.
Mr. M describes his mood as anxious, primarily surrounding his recent cognitive changes. He does not have a substance use disorder, psychotic illness, mania or hypomania, posttraumatic stress disorder, or obsessive-compulsive disorder. He reports adherence to his psychiatric medications. A mental status exam reveals Mr. M to be anxious. His attention is not well sustained, and he has difficulty describing details of his cognitive struggles, providing vague descriptions such as “skipping thought” and “skipping words.” Mr. M’s affect is congruent to his mood with some restriction and the psychiatrist notes that he is experiencing thought latency, poverty of content of thoughts, word-finding difficulties, and circumlocution. Mr. M denies any perceptual abnormalities, and there is no evidence of delusions.
[polldaddy:11320112]
The authors’ observations
Mr. M’s symptoms are significant for subacute cognitive decline that is subtle but gradual and can be easily missed, especially in the beginning. Though his ED evaluation—including brain imaging—ruled out acute or focal neurologic findings and his primary psychiatric presentation was anxiety, Mr. M’s medical history and mental status exam were suggestive of cognitive deficits.
Collateral information was obtained from his work colleagues, which confirmed both cognitive problems and comorbid anxiety. Additionally, given Mr. M’s high cognitive baseline as a surgeon, the new-onset cognitive changes over 4 months warranted further cognitive and neurologic evaluation. There are many causes of cognitive impairment (vascular, cancer, infection, autoimmune, medications, substances or toxins, neurodegenerative, psychiatric, vitamin deficiencies), all of which need to be considered in a patient with a nonspecific presentation such as Mr. M’s. The psychiatrist confirmed Mr. M’s current medication regimen, and discussed tapering aripiprazole while continuing duloxetine and buspirone.
Continue to: EVALUATION A closer look at cognitive deficits
EVALUATION A closer look at cognitive deficits
Mr. M scores 12/30 on the Montreal Cognitive Assessment (MoCA), indicating moderate cognitive impairment (Table 1). The psychiatrist refers Mr. M to Neurology. During his neurologic evaluation, Mr. M continues to report feeling anxious that “something is wrong” and skips his words. The neurologist confirms Mr. M’s symptoms may have started 2 to 3 months before he presented to the ED. Mr. M reports unusual eating habits, including yogurt and cookies for breakfast, Mexican food for lunch, and more cookies for dinner. He denies having a fever, gaining or losing weight, rashes, headaches, neck stiffness, tingling or weakness or stiffness of limbs, vertigo, visual changes, photophobia, unsteady gait, bowel or bladder incontinence, or tremors.
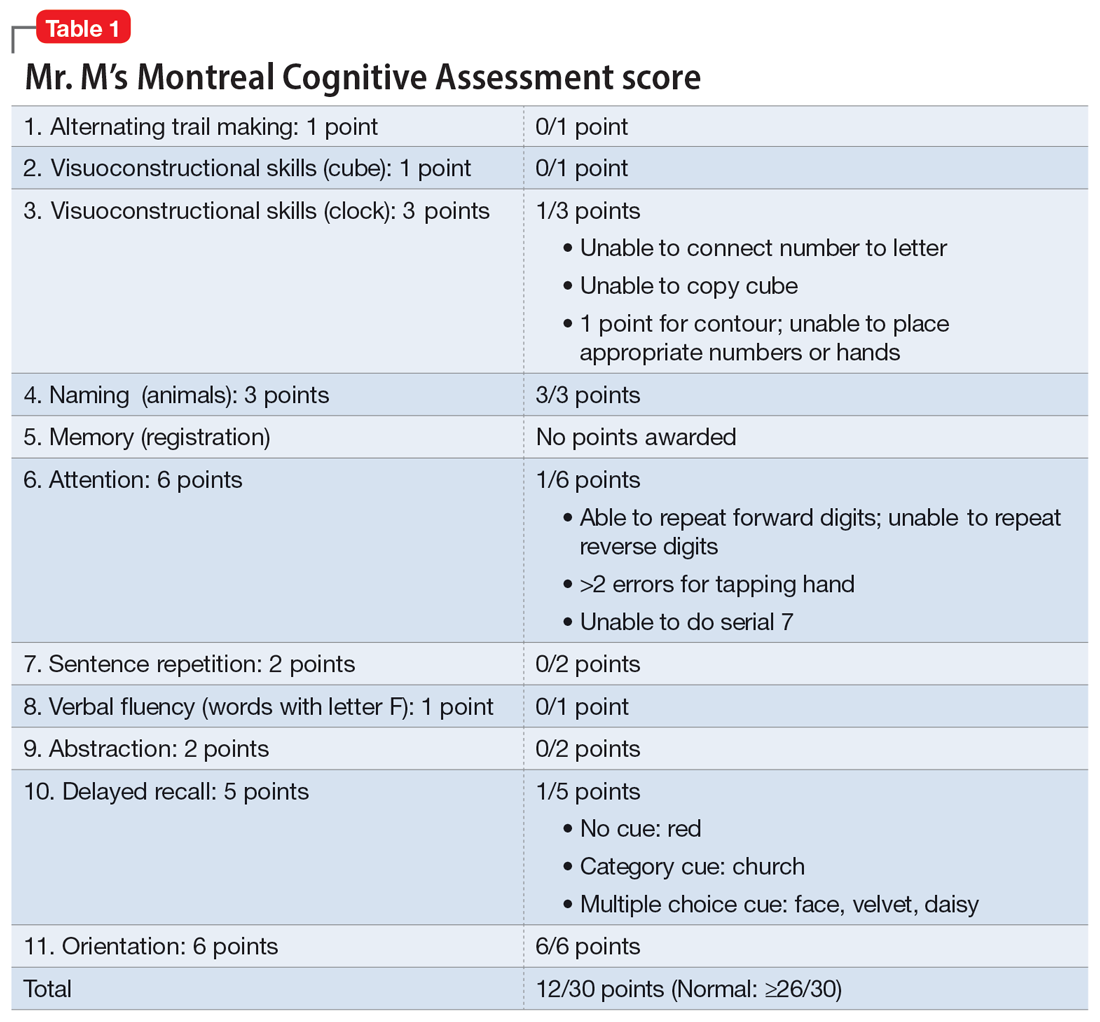
When the neurologist repeats the MoCA, Mr. M again scores 12. The neurologist notes that Mr. M answers questions a little slowly and pauses for thoughts when unable to find an answer. Mr. M has difficulty following some simple commands, such as “touch a finger to your nose.” Other in-office neurologic physical exams (cranial nerves, involuntary movements or tremors, sensation, muscle strength, reflexes, cerebellar signs) are unremarkable except for mildly decreased vibration sense of his toes. The neurologist concludes that Mr. M’s presentation is suggestive of subacute to chronic bradyphrenia and orders additional evaluation, including neuropsychological testing.
[polldaddy:11320114]
The authors’ observations
Physical and neurologic exams were not suggestive of any obvious causes of cognitive decline. Both the mental status exam and 2 serial MoCAs suggested deficits in executive function, language, and memory. Each of the differential diagnoses considered was ruled out with workup or exams (Table 2), which led to a most likely diagnosis of neurodegenerative disorder with PPA. Neuropsychological testing confirmed the diagnosis of nonfluent PPA.
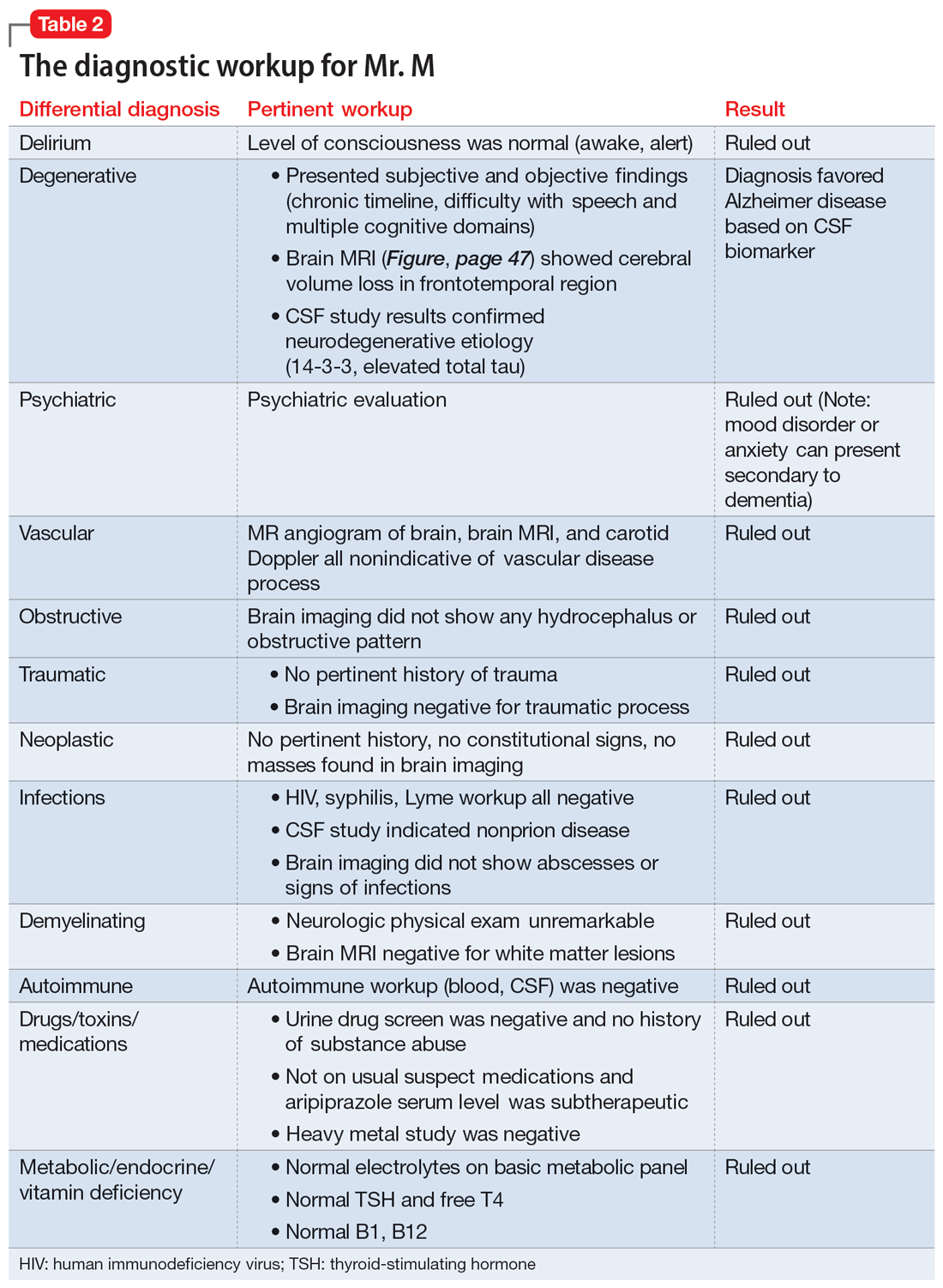
Primary progressive aphasia
PPA is an uncommon, heterogeneous group of disorders stemming from focal degeneration of language-governing centers of the brain.1,2 The estimated prevalence of PPA is 3 in 100,000 cases.2,3 There are 4 major variants of PPA (Table 34), and each presents with distinct language, cognitive, neuroanatomical, and neuropathological characteristics.4 PPA is usually diagnosed in late middle life; however, diagnosis is often delayed due to the relative obscurity of the disorder.4 In Mr. M’s case, it took approximately 4 months of evaluations by various specialists before a diagnosis was confirmed.
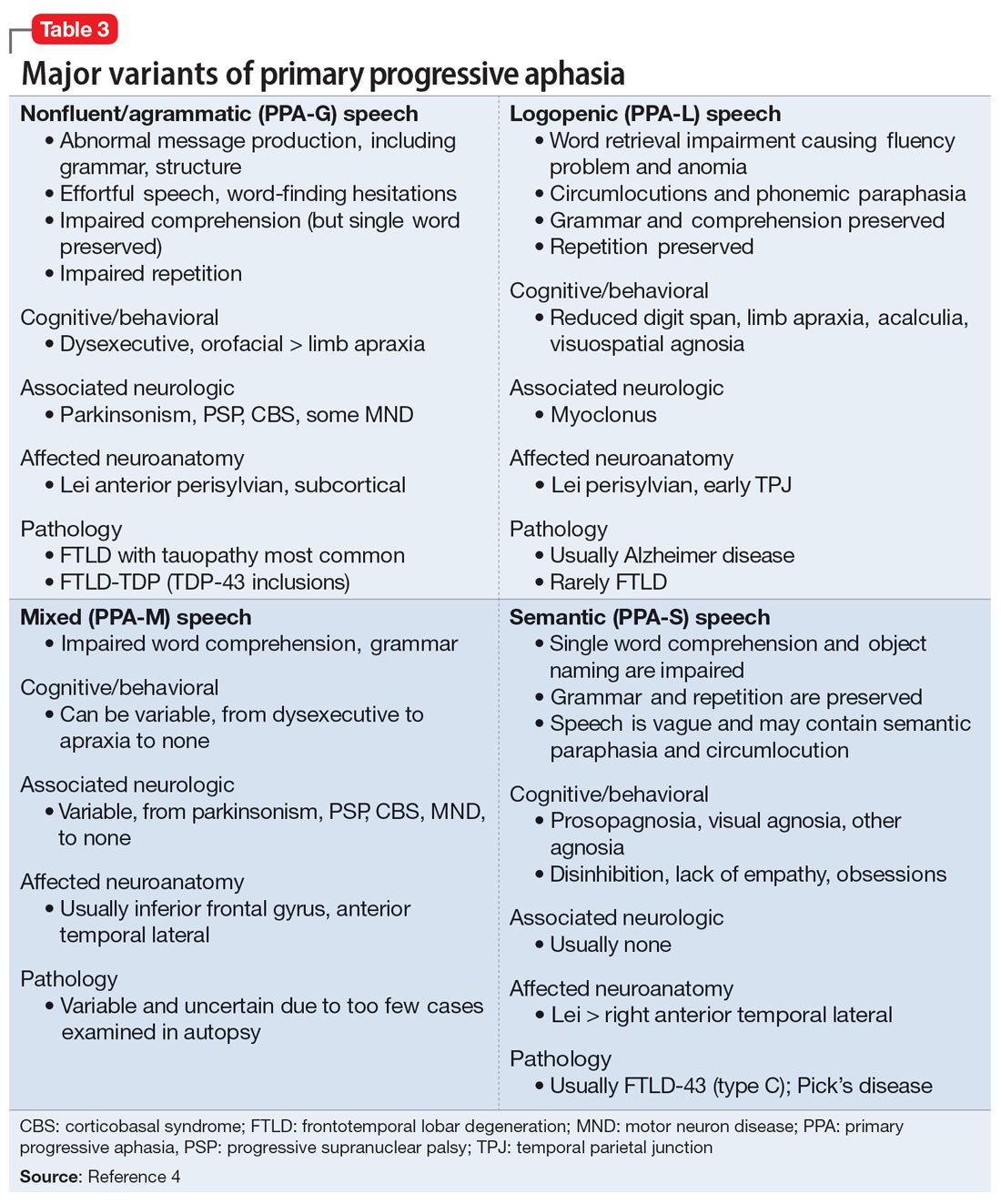
The initial phase of PPA can present as a diagnostic challenge because patients can have difficulty articulating their cognitive and language deficits. PPA can be commonly mistaken for a primary psychiatric disorder such as MDD or anxiety, which can further delay an accurate diagnosis and treatment. Special attention to the mental status exam, close observation of the patient’s language, and assessment of cognitive abilities using standardized screenings such as the MoCA or Mini-Mental State Examination can be helpful in clarifying the diagnosis. It is also important to rule out developmental problems (eg, dyslexia) and hearing difficulties, particularly in older patients.
Continue to: TREATMENT Adjusting the medication regimen
TREATMENT Adjusting the medication regimen
The neurologist completes additional examinations to rule out causes of rare neurodegenerative disorders, including CSF autoimmune disorders, Creutzfeldt-Jakob disease, and Alzheimer disease (AD) (Table 4). Mr. M continues to follow up with his outpatient psychiatrist and his medication regimen is adjusted. Aripiprazole and buspirone are discontinued, and duloxetine is titrated to 60 mg twice a day. During follow-up visits, Mr. M discusses his understanding of his neurologic condition. His concerns shift to his illness and prognosis. During these visits, he continues to deny suicidality.
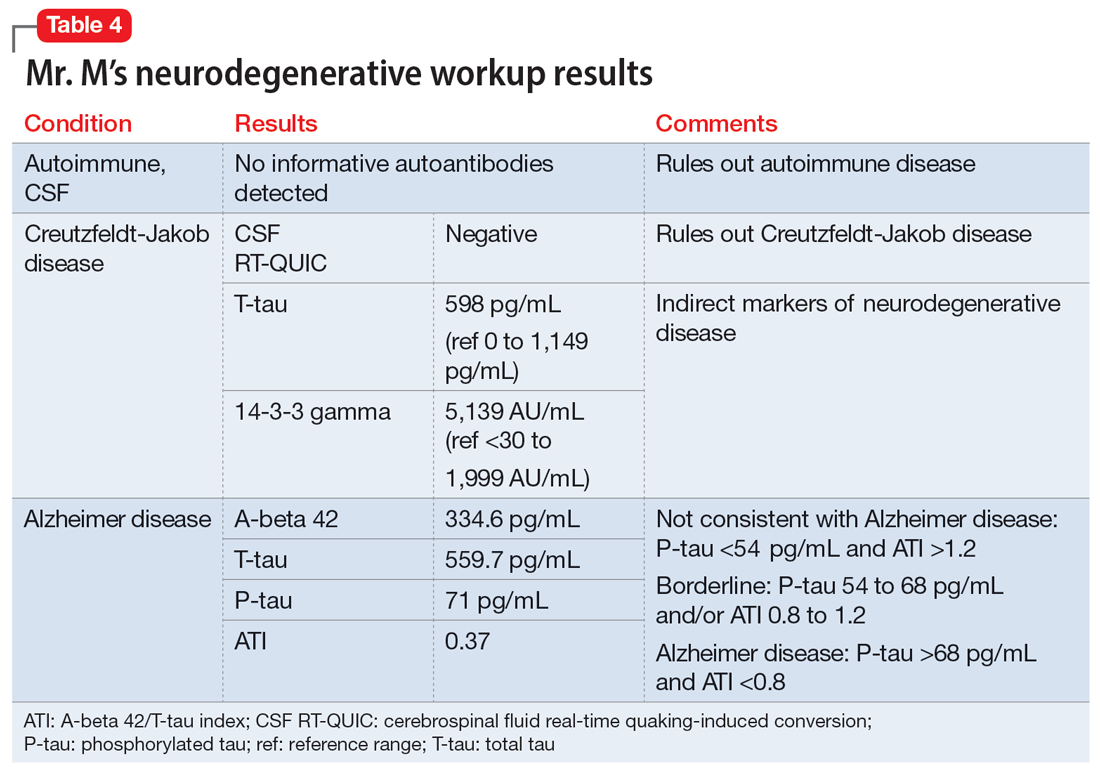
[polldaddy:11320115]
The authors’ observations
Mr. M’s neurodegenerative workup identified an intriguing diagnostic challenge. A repeat brain MRI (Figure) showed atrophy patterns suggestive of frontotemporal lobar degeneration (FTLD). On the other hand, his CSF ATI (A-beta 42/T-tau index, a value used to aid in the diagnosis of AD) was <1, suggesting early-onset AD.5,6 Although significant advances have been made to distinguish AD and FTLD following an autopsy, there are still no reliable or definitive biomarkers to distinguish AD from FTLD (particularly in the early stages of FTLD). This can often leave the confirmatory diagnosis as a question.7
A PPA diagnosis (and other dementias) can have a significant impact on the patient and their family due to the uncertain nature of the progression of the disease and quality-of-life issues related to language and other cognitive deficits. Early identification and accurate diagnosis of PPA and its etiology (ie, AD vs FTLD) is important to avoid unnecessary exposure to medications or the use of polypharmacy to treat an inaccurate diagnosis of a primary psychiatric illness. For example, Mr. M was being treated with 3 psychiatric medications (aripiprazole, buspirone, and duloxetine) for depression and anxiety prior to the diagnosis of PPA.
Nonpharmacologic interventions can play an important role in the management of patients with PPA. These include educating the patient and their family about the diagnosis and discussions about future planning, including appropriate social support, employment, and finances.4 Pharmacologic interventions may be limited, as there are currently no disease-modifying treatments for PPA or FTLD. For patients with nonfluent PPA or AD, cholinesterase inhibitors such as donepezil or N-methyl
Psychiatrists should continue to treat patients with PPA for comorbid anxiety or depression, with appropriate medications and/or supportive therapy to guide the patient through the process of grief. Assessing for suicide risk is also important in patients diagnosed with dementia. A retrospective cohort study of patients age ≥60 with a diagnosis of dementia suggested that the majority of suicides occurred in those with a new dementia diagnosis.9 End-of-life decisions such as advanced directives should be made when the patient still has legal capacity, ideally as soon as possible after diagnosis.10
OUTCOME Remaining engaged in treatment
Mr. M continues to follow-up with the Neurology team. He has also been regularly seeing his psychiatric team for medication management and supportive therapy, and his psychiatric medications have been optimized to reduce polypharmacy. During his sessions, Mr. M discusses his grief and plans for the future. Despite his anxiety about the uncertainty of his prognosis, Mr. M continues to report that he is doing reasonably well and remains engaged in treatment.
Bottom Line
Patients with primary progressive aphasia and rare neurodegenerative disorders may present to an outpatient or emergency setting with symptoms of anxiety and confusion. They are frequently misdiagnosed with a primary psychiatric disorder due to the nature of cognitive and language deficits, particularly in the early stages of the disease. Paying close attention to language and conducting cognitive screening are critical in identifying the true cause of a patient’s symptoms.
Related Resources
- Primary progressive aphasia. National Center for Advancing Translational Sciences. Genetic and Rare Diseases Information Center. https://rarediseases.info.nih.gov/diseases/8541/primary-progressive-aphasia
- Moller MD, Parmenter BA, Lane DW. Neuropsychological testing: A useful but underutilized resource. Current Psychiatry. 2019;18(11):40-46,51.
Drug Brand Names
Aripiprazole • Abilify
Donepezil • Aricept
Duloxetine • Cymbalta
Memantine • Namenda
1. Grossman M. Primary progressive aphasia: clinicopathological correlations. Nat Rev Neurol. 2010;6(2):88-97. doi:10.1038/nrneurol.2009.216
2. Mesulam M-M, Rogalski EJ, Wieneke C, et al. Primary progressive aphasia and the evolving neurology of the language network. Nat Rev Neurol. 2014;10(10):554-569. doi:10.1038/nrneurol.2014.159
3. Coyle-Gilchrist ITS, Dick KM, Patterson K, et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86(18):1736-1743. doi:10.1212/WNL.0000000000002638
4. Marshall CR, Hardy CJD, Volkmer A, et al. Primary progressive aphasia: a clinical approach. J Neurol. 2018;265(6):1474-1490. doi:10.1007/s00415-018-8762-6
5. Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer’s disease. NeuroRx. 2004;1(2):213-225. doi:10.1602/neurorx.1.2.213
6. Hulstaert F, Blennow K, Ivanoiu A, et al. Improved discrimination of AD patients using beta-amyloid(1-42) and tau levels in CSF. Neurology. 1999;52(8):1555-1562. doi:10.1212/wnl.52.8.1555
7. Thijssen EH, La Joie R, Wolf A, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat Med. 2020;26(3):387-397. doi:10.1038/s41591-020-0762-2
8. Newhart M, Davis C, Kannan V, et al. Therapy for naming deficits in two variants of primary progressive aphasia. Aphasiology. 2009;23(7-8):823-834. doi:10.1080/02687030802661762
9. Seyfried LS, Kales HC, Ignacio RV, et al. Predictors of suicide in patients with dementia. Alzheimers Dement. 2011;7(6):567-573. doi:10.1016/j.jalz.2011.01.006
10. Porteri C. Advance directives as a tool to respect patients’ values and preferences: discussion on the case of Alzheimer’s disease. BMC Med Ethics. 2018;19(1):9. doi:10.1186/s12910-018-0249-6
CASE Anxious and confused
Mr. M, age 53, a surgeon, presents to the emergency department (ED) following a panic attack and concerns from his staff that he appears confused. Specifically, staff members report that in the past 4 months, Mr. M was observed having problems completing some postoperative tasks related to chart documentation. Mr. M has a history of major depressive disorder (MDD), hypertension, hyperlipidemia, and type 2 diabetes.
HISTORY A long-standing diagnosis of depression
Mr. M reports that 30 years ago, he received care from a psychiatrist to address symptoms of MDD. He says that around the time he arrived at the ED, he had noticed subtle but gradual changes in his cognition, which led him to skip words and often struggle to find the correct words. These episodes left him confused. Mr. M started getting anxious about these cognitive issues because they disrupted his work and forced him to reduce his duties. He does not have any known family history of mental illness, is single, and lives alone.
EVALUATION After stroke is ruled out, a psychiatric workup
In the ED, a comprehensive exam rules out an acute cerebrovascular event. A neurologic evaluation notes some delay in processing information and observes Mr. M having difficulty following simple commands. Laboratory investigations, including a comprehensive metabolic panel, are unremarkable. An MRI of Mr. M’s brain, with and without contrast, notes no acute findings. He is discharged from the ED with a diagnosis of MDD.
Before he presented to the ED, Mr. M’s medication regimen included duloxetine 60 mg/d, buspirone 10 mg 3 times a day, and aripiprazole 5 mg/d for MDD and anxiety. After the ED visit, Mr. M’s physician refers him to an outpatient psychiatrist for management of worsening depression and panic attacks. During the psychiatrist’s evaluation, Mr. M reports a decreased interest in activities, decreased motivation, being easily fatigued, and having poor sleep. He denies having a depressed mood, difficulty concentrating, or having problems with his appetite. He also denies suicidal thoughts, both past and present.
Mr. M describes his mood as anxious, primarily surrounding his recent cognitive changes. He does not have a substance use disorder, psychotic illness, mania or hypomania, posttraumatic stress disorder, or obsessive-compulsive disorder. He reports adherence to his psychiatric medications. A mental status exam reveals Mr. M to be anxious. His attention is not well sustained, and he has difficulty describing details of his cognitive struggles, providing vague descriptions such as “skipping thought” and “skipping words.” Mr. M’s affect is congruent to his mood with some restriction and the psychiatrist notes that he is experiencing thought latency, poverty of content of thoughts, word-finding difficulties, and circumlocution. Mr. M denies any perceptual abnormalities, and there is no evidence of delusions.
[polldaddy:11320112]
The authors’ observations
Mr. M’s symptoms are significant for subacute cognitive decline that is subtle but gradual and can be easily missed, especially in the beginning. Though his ED evaluation—including brain imaging—ruled out acute or focal neurologic findings and his primary psychiatric presentation was anxiety, Mr. M’s medical history and mental status exam were suggestive of cognitive deficits.
Collateral information was obtained from his work colleagues, which confirmed both cognitive problems and comorbid anxiety. Additionally, given Mr. M’s high cognitive baseline as a surgeon, the new-onset cognitive changes over 4 months warranted further cognitive and neurologic evaluation. There are many causes of cognitive impairment (vascular, cancer, infection, autoimmune, medications, substances or toxins, neurodegenerative, psychiatric, vitamin deficiencies), all of which need to be considered in a patient with a nonspecific presentation such as Mr. M’s. The psychiatrist confirmed Mr. M’s current medication regimen, and discussed tapering aripiprazole while continuing duloxetine and buspirone.
Continue to: EVALUATION A closer look at cognitive deficits
EVALUATION A closer look at cognitive deficits
Mr. M scores 12/30 on the Montreal Cognitive Assessment (MoCA), indicating moderate cognitive impairment (Table 1). The psychiatrist refers Mr. M to Neurology. During his neurologic evaluation, Mr. M continues to report feeling anxious that “something is wrong” and skips his words. The neurologist confirms Mr. M’s symptoms may have started 2 to 3 months before he presented to the ED. Mr. M reports unusual eating habits, including yogurt and cookies for breakfast, Mexican food for lunch, and more cookies for dinner. He denies having a fever, gaining or losing weight, rashes, headaches, neck stiffness, tingling or weakness or stiffness of limbs, vertigo, visual changes, photophobia, unsteady gait, bowel or bladder incontinence, or tremors.

When the neurologist repeats the MoCA, Mr. M again scores 12. The neurologist notes that Mr. M answers questions a little slowly and pauses for thoughts when unable to find an answer. Mr. M has difficulty following some simple commands, such as “touch a finger to your nose.” Other in-office neurologic physical exams (cranial nerves, involuntary movements or tremors, sensation, muscle strength, reflexes, cerebellar signs) are unremarkable except for mildly decreased vibration sense of his toes. The neurologist concludes that Mr. M’s presentation is suggestive of subacute to chronic bradyphrenia and orders additional evaluation, including neuropsychological testing.
[polldaddy:11320114]
The authors’ observations
Physical and neurologic exams were not suggestive of any obvious causes of cognitive decline. Both the mental status exam and 2 serial MoCAs suggested deficits in executive function, language, and memory. Each of the differential diagnoses considered was ruled out with workup or exams (Table 2), which led to a most likely diagnosis of neurodegenerative disorder with PPA. Neuropsychological testing confirmed the diagnosis of nonfluent PPA.

Primary progressive aphasia
PPA is an uncommon, heterogeneous group of disorders stemming from focal degeneration of language-governing centers of the brain.1,2 The estimated prevalence of PPA is 3 in 100,000 cases.2,3 There are 4 major variants of PPA (Table 34), and each presents with distinct language, cognitive, neuroanatomical, and neuropathological characteristics.4 PPA is usually diagnosed in late middle life; however, diagnosis is often delayed due to the relative obscurity of the disorder.4 In Mr. M’s case, it took approximately 4 months of evaluations by various specialists before a diagnosis was confirmed.

The initial phase of PPA can present as a diagnostic challenge because patients can have difficulty articulating their cognitive and language deficits. PPA can be commonly mistaken for a primary psychiatric disorder such as MDD or anxiety, which can further delay an accurate diagnosis and treatment. Special attention to the mental status exam, close observation of the patient’s language, and assessment of cognitive abilities using standardized screenings such as the MoCA or Mini-Mental State Examination can be helpful in clarifying the diagnosis. It is also important to rule out developmental problems (eg, dyslexia) and hearing difficulties, particularly in older patients.
Continue to: TREATMENT Adjusting the medication regimen
TREATMENT Adjusting the medication regimen
The neurologist completes additional examinations to rule out causes of rare neurodegenerative disorders, including CSF autoimmune disorders, Creutzfeldt-Jakob disease, and Alzheimer disease (AD) (Table 4). Mr. M continues to follow up with his outpatient psychiatrist and his medication regimen is adjusted. Aripiprazole and buspirone are discontinued, and duloxetine is titrated to 60 mg twice a day. During follow-up visits, Mr. M discusses his understanding of his neurologic condition. His concerns shift to his illness and prognosis. During these visits, he continues to deny suicidality.

[polldaddy:11320115]
The authors’ observations
Mr. M’s neurodegenerative workup identified an intriguing diagnostic challenge. A repeat brain MRI (Figure) showed atrophy patterns suggestive of frontotemporal lobar degeneration (FTLD). On the other hand, his CSF ATI (A-beta 42/T-tau index, a value used to aid in the diagnosis of AD) was <1, suggesting early-onset AD.5,6 Although significant advances have been made to distinguish AD and FTLD following an autopsy, there are still no reliable or definitive biomarkers to distinguish AD from FTLD (particularly in the early stages of FTLD). This can often leave the confirmatory diagnosis as a question.7

A PPA diagnosis (and other dementias) can have a significant impact on the patient and their family due to the uncertain nature of the progression of the disease and quality-of-life issues related to language and other cognitive deficits. Early identification and accurate diagnosis of PPA and its etiology (ie, AD vs FTLD) is important to avoid unnecessary exposure to medications or the use of polypharmacy to treat an inaccurate diagnosis of a primary psychiatric illness. For example, Mr. M was being treated with 3 psychiatric medications (aripiprazole, buspirone, and duloxetine) for depression and anxiety prior to the diagnosis of PPA.
Nonpharmacologic interventions can play an important role in the management of patients with PPA. These include educating the patient and their family about the diagnosis and discussions about future planning, including appropriate social support, employment, and finances.4 Pharmacologic interventions may be limited, as there are currently no disease-modifying treatments for PPA or FTLD. For patients with nonfluent PPA or AD, cholinesterase inhibitors such as donepezil or N-methyl
Psychiatrists should continue to treat patients with PPA for comorbid anxiety or depression, with appropriate medications and/or supportive therapy to guide the patient through the process of grief. Assessing for suicide risk is also important in patients diagnosed with dementia. A retrospective cohort study of patients age ≥60 with a diagnosis of dementia suggested that the majority of suicides occurred in those with a new dementia diagnosis.9 End-of-life decisions such as advanced directives should be made when the patient still has legal capacity, ideally as soon as possible after diagnosis.10
OUTCOME Remaining engaged in treatment
Mr. M continues to follow-up with the Neurology team. He has also been regularly seeing his psychiatric team for medication management and supportive therapy, and his psychiatric medications have been optimized to reduce polypharmacy. During his sessions, Mr. M discusses his grief and plans for the future. Despite his anxiety about the uncertainty of his prognosis, Mr. M continues to report that he is doing reasonably well and remains engaged in treatment.
Bottom Line
Patients with primary progressive aphasia and rare neurodegenerative disorders may present to an outpatient or emergency setting with symptoms of anxiety and confusion. They are frequently misdiagnosed with a primary psychiatric disorder due to the nature of cognitive and language deficits, particularly in the early stages of the disease. Paying close attention to language and conducting cognitive screening are critical in identifying the true cause of a patient’s symptoms.
Related Resources
- Primary progressive aphasia. National Center for Advancing Translational Sciences. Genetic and Rare Diseases Information Center. https://rarediseases.info.nih.gov/diseases/8541/primary-progressive-aphasia
- Moller MD, Parmenter BA, Lane DW. Neuropsychological testing: A useful but underutilized resource. Current Psychiatry. 2019;18(11):40-46,51.
Drug Brand Names
Aripiprazole • Abilify
Donepezil • Aricept
Duloxetine • Cymbalta
Memantine • Namenda
CASE Anxious and confused
Mr. M, age 53, a surgeon, presents to the emergency department (ED) following a panic attack and concerns from his staff that he appears confused. Specifically, staff members report that in the past 4 months, Mr. M was observed having problems completing some postoperative tasks related to chart documentation. Mr. M has a history of major depressive disorder (MDD), hypertension, hyperlipidemia, and type 2 diabetes.
HISTORY A long-standing diagnosis of depression
Mr. M reports that 30 years ago, he received care from a psychiatrist to address symptoms of MDD. He says that around the time he arrived at the ED, he had noticed subtle but gradual changes in his cognition, which led him to skip words and often struggle to find the correct words. These episodes left him confused. Mr. M started getting anxious about these cognitive issues because they disrupted his work and forced him to reduce his duties. He does not have any known family history of mental illness, is single, and lives alone.
EVALUATION After stroke is ruled out, a psychiatric workup
In the ED, a comprehensive exam rules out an acute cerebrovascular event. A neurologic evaluation notes some delay in processing information and observes Mr. M having difficulty following simple commands. Laboratory investigations, including a comprehensive metabolic panel, are unremarkable. An MRI of Mr. M’s brain, with and without contrast, notes no acute findings. He is discharged from the ED with a diagnosis of MDD.
Before he presented to the ED, Mr. M’s medication regimen included duloxetine 60 mg/d, buspirone 10 mg 3 times a day, and aripiprazole 5 mg/d for MDD and anxiety. After the ED visit, Mr. M’s physician refers him to an outpatient psychiatrist for management of worsening depression and panic attacks. During the psychiatrist’s evaluation, Mr. M reports a decreased interest in activities, decreased motivation, being easily fatigued, and having poor sleep. He denies having a depressed mood, difficulty concentrating, or having problems with his appetite. He also denies suicidal thoughts, both past and present.
Mr. M describes his mood as anxious, primarily surrounding his recent cognitive changes. He does not have a substance use disorder, psychotic illness, mania or hypomania, posttraumatic stress disorder, or obsessive-compulsive disorder. He reports adherence to his psychiatric medications. A mental status exam reveals Mr. M to be anxious. His attention is not well sustained, and he has difficulty describing details of his cognitive struggles, providing vague descriptions such as “skipping thought” and “skipping words.” Mr. M’s affect is congruent to his mood with some restriction and the psychiatrist notes that he is experiencing thought latency, poverty of content of thoughts, word-finding difficulties, and circumlocution. Mr. M denies any perceptual abnormalities, and there is no evidence of delusions.
[polldaddy:11320112]
The authors’ observations
Mr. M’s symptoms are significant for subacute cognitive decline that is subtle but gradual and can be easily missed, especially in the beginning. Though his ED evaluation—including brain imaging—ruled out acute or focal neurologic findings and his primary psychiatric presentation was anxiety, Mr. M’s medical history and mental status exam were suggestive of cognitive deficits.
Collateral information was obtained from his work colleagues, which confirmed both cognitive problems and comorbid anxiety. Additionally, given Mr. M’s high cognitive baseline as a surgeon, the new-onset cognitive changes over 4 months warranted further cognitive and neurologic evaluation. There are many causes of cognitive impairment (vascular, cancer, infection, autoimmune, medications, substances or toxins, neurodegenerative, psychiatric, vitamin deficiencies), all of which need to be considered in a patient with a nonspecific presentation such as Mr. M’s. The psychiatrist confirmed Mr. M’s current medication regimen, and discussed tapering aripiprazole while continuing duloxetine and buspirone.
Continue to: EVALUATION A closer look at cognitive deficits
EVALUATION A closer look at cognitive deficits
Mr. M scores 12/30 on the Montreal Cognitive Assessment (MoCA), indicating moderate cognitive impairment (Table 1). The psychiatrist refers Mr. M to Neurology. During his neurologic evaluation, Mr. M continues to report feeling anxious that “something is wrong” and skips his words. The neurologist confirms Mr. M’s symptoms may have started 2 to 3 months before he presented to the ED. Mr. M reports unusual eating habits, including yogurt and cookies for breakfast, Mexican food for lunch, and more cookies for dinner. He denies having a fever, gaining or losing weight, rashes, headaches, neck stiffness, tingling or weakness or stiffness of limbs, vertigo, visual changes, photophobia, unsteady gait, bowel or bladder incontinence, or tremors.

When the neurologist repeats the MoCA, Mr. M again scores 12. The neurologist notes that Mr. M answers questions a little slowly and pauses for thoughts when unable to find an answer. Mr. M has difficulty following some simple commands, such as “touch a finger to your nose.” Other in-office neurologic physical exams (cranial nerves, involuntary movements or tremors, sensation, muscle strength, reflexes, cerebellar signs) are unremarkable except for mildly decreased vibration sense of his toes. The neurologist concludes that Mr. M’s presentation is suggestive of subacute to chronic bradyphrenia and orders additional evaluation, including neuropsychological testing.
[polldaddy:11320114]
The authors’ observations
Physical and neurologic exams were not suggestive of any obvious causes of cognitive decline. Both the mental status exam and 2 serial MoCAs suggested deficits in executive function, language, and memory. Each of the differential diagnoses considered was ruled out with workup or exams (Table 2), which led to a most likely diagnosis of neurodegenerative disorder with PPA. Neuropsychological testing confirmed the diagnosis of nonfluent PPA.

Primary progressive aphasia
PPA is an uncommon, heterogeneous group of disorders stemming from focal degeneration of language-governing centers of the brain.1,2 The estimated prevalence of PPA is 3 in 100,000 cases.2,3 There are 4 major variants of PPA (Table 34), and each presents with distinct language, cognitive, neuroanatomical, and neuropathological characteristics.4 PPA is usually diagnosed in late middle life; however, diagnosis is often delayed due to the relative obscurity of the disorder.4 In Mr. M’s case, it took approximately 4 months of evaluations by various specialists before a diagnosis was confirmed.

The initial phase of PPA can present as a diagnostic challenge because patients can have difficulty articulating their cognitive and language deficits. PPA can be commonly mistaken for a primary psychiatric disorder such as MDD or anxiety, which can further delay an accurate diagnosis and treatment. Special attention to the mental status exam, close observation of the patient’s language, and assessment of cognitive abilities using standardized screenings such as the MoCA or Mini-Mental State Examination can be helpful in clarifying the diagnosis. It is also important to rule out developmental problems (eg, dyslexia) and hearing difficulties, particularly in older patients.
Continue to: TREATMENT Adjusting the medication regimen
TREATMENT Adjusting the medication regimen
The neurologist completes additional examinations to rule out causes of rare neurodegenerative disorders, including CSF autoimmune disorders, Creutzfeldt-Jakob disease, and Alzheimer disease (AD) (Table 4). Mr. M continues to follow up with his outpatient psychiatrist and his medication regimen is adjusted. Aripiprazole and buspirone are discontinued, and duloxetine is titrated to 60 mg twice a day. During follow-up visits, Mr. M discusses his understanding of his neurologic condition. His concerns shift to his illness and prognosis. During these visits, he continues to deny suicidality.

[polldaddy:11320115]
The authors’ observations
Mr. M’s neurodegenerative workup identified an intriguing diagnostic challenge. A repeat brain MRI (Figure) showed atrophy patterns suggestive of frontotemporal lobar degeneration (FTLD). On the other hand, his CSF ATI (A-beta 42/T-tau index, a value used to aid in the diagnosis of AD) was <1, suggesting early-onset AD.5,6 Although significant advances have been made to distinguish AD and FTLD following an autopsy, there are still no reliable or definitive biomarkers to distinguish AD from FTLD (particularly in the early stages of FTLD). This can often leave the confirmatory diagnosis as a question.7

A PPA diagnosis (and other dementias) can have a significant impact on the patient and their family due to the uncertain nature of the progression of the disease and quality-of-life issues related to language and other cognitive deficits. Early identification and accurate diagnosis of PPA and its etiology (ie, AD vs FTLD) is important to avoid unnecessary exposure to medications or the use of polypharmacy to treat an inaccurate diagnosis of a primary psychiatric illness. For example, Mr. M was being treated with 3 psychiatric medications (aripiprazole, buspirone, and duloxetine) for depression and anxiety prior to the diagnosis of PPA.
Nonpharmacologic interventions can play an important role in the management of patients with PPA. These include educating the patient and their family about the diagnosis and discussions about future planning, including appropriate social support, employment, and finances.4 Pharmacologic interventions may be limited, as there are currently no disease-modifying treatments for PPA or FTLD. For patients with nonfluent PPA or AD, cholinesterase inhibitors such as donepezil or N-methyl
Psychiatrists should continue to treat patients with PPA for comorbid anxiety or depression, with appropriate medications and/or supportive therapy to guide the patient through the process of grief. Assessing for suicide risk is also important in patients diagnosed with dementia. A retrospective cohort study of patients age ≥60 with a diagnosis of dementia suggested that the majority of suicides occurred in those with a new dementia diagnosis.9 End-of-life decisions such as advanced directives should be made when the patient still has legal capacity, ideally as soon as possible after diagnosis.10
OUTCOME Remaining engaged in treatment
Mr. M continues to follow-up with the Neurology team. He has also been regularly seeing his psychiatric team for medication management and supportive therapy, and his psychiatric medications have been optimized to reduce polypharmacy. During his sessions, Mr. M discusses his grief and plans for the future. Despite his anxiety about the uncertainty of his prognosis, Mr. M continues to report that he is doing reasonably well and remains engaged in treatment.
Bottom Line
Patients with primary progressive aphasia and rare neurodegenerative disorders may present to an outpatient or emergency setting with symptoms of anxiety and confusion. They are frequently misdiagnosed with a primary psychiatric disorder due to the nature of cognitive and language deficits, particularly in the early stages of the disease. Paying close attention to language and conducting cognitive screening are critical in identifying the true cause of a patient’s symptoms.
Related Resources
- Primary progressive aphasia. National Center for Advancing Translational Sciences. Genetic and Rare Diseases Information Center. https://rarediseases.info.nih.gov/diseases/8541/primary-progressive-aphasia
- Moller MD, Parmenter BA, Lane DW. Neuropsychological testing: A useful but underutilized resource. Current Psychiatry. 2019;18(11):40-46,51.
Drug Brand Names
Aripiprazole • Abilify
Donepezil • Aricept
Duloxetine • Cymbalta
Memantine • Namenda
1. Grossman M. Primary progressive aphasia: clinicopathological correlations. Nat Rev Neurol. 2010;6(2):88-97. doi:10.1038/nrneurol.2009.216
2. Mesulam M-M, Rogalski EJ, Wieneke C, et al. Primary progressive aphasia and the evolving neurology of the language network. Nat Rev Neurol. 2014;10(10):554-569. doi:10.1038/nrneurol.2014.159
3. Coyle-Gilchrist ITS, Dick KM, Patterson K, et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86(18):1736-1743. doi:10.1212/WNL.0000000000002638
4. Marshall CR, Hardy CJD, Volkmer A, et al. Primary progressive aphasia: a clinical approach. J Neurol. 2018;265(6):1474-1490. doi:10.1007/s00415-018-8762-6
5. Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer’s disease. NeuroRx. 2004;1(2):213-225. doi:10.1602/neurorx.1.2.213
6. Hulstaert F, Blennow K, Ivanoiu A, et al. Improved discrimination of AD patients using beta-amyloid(1-42) and tau levels in CSF. Neurology. 1999;52(8):1555-1562. doi:10.1212/wnl.52.8.1555
7. Thijssen EH, La Joie R, Wolf A, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat Med. 2020;26(3):387-397. doi:10.1038/s41591-020-0762-2
8. Newhart M, Davis C, Kannan V, et al. Therapy for naming deficits in two variants of primary progressive aphasia. Aphasiology. 2009;23(7-8):823-834. doi:10.1080/02687030802661762
9. Seyfried LS, Kales HC, Ignacio RV, et al. Predictors of suicide in patients with dementia. Alzheimers Dement. 2011;7(6):567-573. doi:10.1016/j.jalz.2011.01.006
10. Porteri C. Advance directives as a tool to respect patients’ values and preferences: discussion on the case of Alzheimer’s disease. BMC Med Ethics. 2018;19(1):9. doi:10.1186/s12910-018-0249-6
1. Grossman M. Primary progressive aphasia: clinicopathological correlations. Nat Rev Neurol. 2010;6(2):88-97. doi:10.1038/nrneurol.2009.216
2. Mesulam M-M, Rogalski EJ, Wieneke C, et al. Primary progressive aphasia and the evolving neurology of the language network. Nat Rev Neurol. 2014;10(10):554-569. doi:10.1038/nrneurol.2014.159
3. Coyle-Gilchrist ITS, Dick KM, Patterson K, et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86(18):1736-1743. doi:10.1212/WNL.0000000000002638
4. Marshall CR, Hardy CJD, Volkmer A, et al. Primary progressive aphasia: a clinical approach. J Neurol. 2018;265(6):1474-1490. doi:10.1007/s00415-018-8762-6
5. Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer’s disease. NeuroRx. 2004;1(2):213-225. doi:10.1602/neurorx.1.2.213
6. Hulstaert F, Blennow K, Ivanoiu A, et al. Improved discrimination of AD patients using beta-amyloid(1-42) and tau levels in CSF. Neurology. 1999;52(8):1555-1562. doi:10.1212/wnl.52.8.1555
7. Thijssen EH, La Joie R, Wolf A, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat Med. 2020;26(3):387-397. doi:10.1038/s41591-020-0762-2
8. Newhart M, Davis C, Kannan V, et al. Therapy for naming deficits in two variants of primary progressive aphasia. Aphasiology. 2009;23(7-8):823-834. doi:10.1080/02687030802661762
9. Seyfried LS, Kales HC, Ignacio RV, et al. Predictors of suicide in patients with dementia. Alzheimers Dement. 2011;7(6):567-573. doi:10.1016/j.jalz.2011.01.006
10. Porteri C. Advance directives as a tool to respect patients’ values and preferences: discussion on the case of Alzheimer’s disease. BMC Med Ethics. 2018;19(1):9. doi:10.1186/s12910-018-0249-6
More on psilocybin
I would like to remark on “Psychedelics for treating psychiatric disorders: Are they safe?” (
The Oregon Psilocybin Services that will begin in 2023 are not specific to therapeutic use; this is a common misconception. These are specifically referred to as “psilocybin services” in the Oregon Administrative Rules (OAR), and psilocybin facilitators are required to limit their scope such that they are not practicing psychotherapy or other interventions, even if they do have a medical or psychotherapy background. The intention of the Oregon Psilocybin Services rollout was that these services would not be of the medical model. In the spirit of this, services do not require a medical diagnosis or referral, and services are not a medical or clinical treatment (OAR 333-333-5040). Additionally, services cannot be provided in a health care facility (OAR 441). Facilitators receive robust training as defined by Oregon law, and licensed facilitators provide this information during preparation for services. When discussing this model on a large public scale, I have noticed substantial misconceptions; it is imperative that we refer to these services as they are defined so that individuals with mental health conditions who seek them are aware that such services are different from psilocybin-assisted psychotherapy. Instead, Oregon Psilocybin Services might be better categorized as supported psilocybin use.
I would like to remark on “Psychedelics for treating psychiatric disorders: Are they safe?” (
The Oregon Psilocybin Services that will begin in 2023 are not specific to therapeutic use; this is a common misconception. These are specifically referred to as “psilocybin services” in the Oregon Administrative Rules (OAR), and psilocybin facilitators are required to limit their scope such that they are not practicing psychotherapy or other interventions, even if they do have a medical or psychotherapy background. The intention of the Oregon Psilocybin Services rollout was that these services would not be of the medical model. In the spirit of this, services do not require a medical diagnosis or referral, and services are not a medical or clinical treatment (OAR 333-333-5040). Additionally, services cannot be provided in a health care facility (OAR 441). Facilitators receive robust training as defined by Oregon law, and licensed facilitators provide this information during preparation for services. When discussing this model on a large public scale, I have noticed substantial misconceptions; it is imperative that we refer to these services as they are defined so that individuals with mental health conditions who seek them are aware that such services are different from psilocybin-assisted psychotherapy. Instead, Oregon Psilocybin Services might be better categorized as supported psilocybin use.
I would like to remark on “Psychedelics for treating psychiatric disorders: Are they safe?” (
The Oregon Psilocybin Services that will begin in 2023 are not specific to therapeutic use; this is a common misconception. These are specifically referred to as “psilocybin services” in the Oregon Administrative Rules (OAR), and psilocybin facilitators are required to limit their scope such that they are not practicing psychotherapy or other interventions, even if they do have a medical or psychotherapy background. The intention of the Oregon Psilocybin Services rollout was that these services would not be of the medical model. In the spirit of this, services do not require a medical diagnosis or referral, and services are not a medical or clinical treatment (OAR 333-333-5040). Additionally, services cannot be provided in a health care facility (OAR 441). Facilitators receive robust training as defined by Oregon law, and licensed facilitators provide this information during preparation for services. When discussing this model on a large public scale, I have noticed substantial misconceptions; it is imperative that we refer to these services as they are defined so that individuals with mental health conditions who seek them are aware that such services are different from psilocybin-assisted psychotherapy. Instead, Oregon Psilocybin Services might be better categorized as supported psilocybin use.
Advances in fertility preservation: Q & A
From the first obscure reference until the 19th century, the maternal mortality rate from an ectopic pregnancy was nearly 100%. In the past 140 years, because of early detection and prompt surgical management, the mortality rate from an ectopic pregnancy declined from 72%-90% in 1880 to 0.48% from 2004 to 2008.1 Given this remarkable reduction in mortality, the 20th-century approach to ectopic pregnancy evolved from preserving the life of the mother to preserving fertility by utilizing conservative treatment with methotrexate and/or tubal surgery.
Why the reference to ectopic pregnancy? Advances in oncology have comparably affected our approach to cancer patients. The increase in survival rates following a cancer diagnosis has fostered revolutionary developments in fertility preservation to obviate the effect of gonadotoxic therapy. We have evolved from shielding and transposing ovaries to ovarian tissue cryopreservation2,3 with rapid implementation.
One of the leaders in the field of female fertility preservation is Kutluk Oktay, MD, of Yale University, New Haven, Conn. I posed the following salient questions to him on the state of fertility preservation as well as expectations for the future.
Q1. What medication/treatment is gonadotoxic that warrants a consultation for fertility preservation?
A: While new drugs for cancer treatment continue to be approved and require testing for gonadotoxicity, evidence is clear on the damaging effects of alkylating agents such as cyclophosphamide, ifosfamide, chlorambucil, and melphalan on primordial follicle reserve.4 A useful tool to determine the risk of alkylating agents affecting fertility is the Cyclophosphamide Equivalent Dose (CED) Calculator. Likewise, topoisomerase inhibitors, such as doxorubicin4 induce ovarian reserve damage by causing double-strand DNA breaks (DSBs) in oocytes.5-7 Contrary to common belief, chemotherapy exposure suppresses the mechanisms that can initiate follicle growth.6 When DSBs occur, some oocytes may be able to repair such damage, otherwise apoptosis is triggered, which results in irreversible ovarian reserve loss.7 Younger individuals have much higher repair capacity, the magnitude of damage can be hard to predict, and it is variable.8,9 So, prior exposure to gonadotoxic drugs does not preclude consideration of fertility preservation.10
In addition, pelvic radiation, in a dose-dependent manner, causes severe DSBs and triggers the same cell suicide mechanisms while also potentially damaging uterine function. Additional information can be found in the American Society of Clinical Oncology Fertility Preservation Guidelines.4
Q2. What are the current options for fertility preservation in patients who will be exposed to gonadotoxic medication/treatment?
A: The current fertility preservation options for female patients faced with gonadotoxic treatments are embryo, oocyte, and ovarian tissue cryopreservation (OTC). Selection of fertility preservation is typically contingent upon the timetable of treatment. Oocyte and embryo cryopreservation have been the standard of care. Recently, OTC had its experimental designation removed by American Society for Reproductive Medicine11 with the advantage of not requiring ovarian stimulation or sexual maturity; and it may to be performed while patients are receiving chemotherapy. If successful, OTC followed by orthotopic transplantation has the potential to restore natural ovarian function, thereby allowing spontaneous conception.10 Especially in young adults, ovarian reserve loss is fractional and can remain at reasonable levels after a few courses of chemotherapy. Ovarian stimulation is risky after the initiation of chemotherapy because of the severe DNA damage to oocytes of developing follicles and the associated poor response.7 Hence, ovarian stimulation should be initiated and completed before the initiation of chemotherapy.
Q3. How successful are the approved fertility preservation options in obtaining oocytes for future utilization by ART?
A: We have decades of experience with embryo cryopreservation and proven success rates that patients can check on the SART.org website for individual clinics. For oocyte cryopreservation, models are used to provide calculation estimates because the technique is less established.12 Although success rates are approaching those with fresh oocytes, they are still not equal.13 OTC followed by orthotopic tissue transplantation has the least outcomes data (approximately 200 reported livebirths to date with a 25% live birth rate per recipient worldwide10 since the first success was reported in 2000.2,14
With our robotic surgical approach to orthotopic and heterotopic ovarian tissue transplantation and the utility of neovascularizing agents, we have found that ovarian graft longevity is extended. Oocytes/embryos can be obtained and has resulted in one to two livebirths in all our recipients to date.10 Unfortunately, if any of the critical steps are not up to standards (freezing, thawing, or transplantation), success rates can dramatically decline. Therefore, providers and patients should seek centers with experience in all three stages of this procedure to maximize outcomes.
Q4. Are there concerns of increasing recurrence/mortality with fertility preservation given hormonal exposure?
A: Yes, this concern exists, at least in theory for estrogen-sensitive cancers, most commonly breast cancer. We developed ovarian stimulation protocols supplemented with anti-estrogen treatments (tamoxifen, an estrogen-receptor antagonist, and letrozole, an aromatase inhibitor) that appear equally effective and reduce estrogen exposure in any susceptible cancer.15,16 Even in estrogen receptor–negative tumors, high estrogen exposure may activate non–estrogen receptor–dependent pathways. In addition, even those tumors that are practically deemed estrogen receptor negative may still contain a small percentage of estrogen receptors, which may become active at high estrogen levels.
Therefore, when we approach women with estrogen-sensitive cancers, e.g., breast and endometrial, we do not alter our approach based on receptor status. One exception occurs in women with BRCA mutations, especially the BRCA1, as they have 25% lower serum anti-müllerian hormone (AMH) levels,8,17 yield fewer oocytes in response to ovarian stimulation,18,19 and have lower fertilization rates and embryo numbers20 compared with those without the mutations.
Q5. Are all reproductive centers capable of offering fertility preservation? If not, how does a patient find a center?
A: All IVF clinics offer embryo and, presumably, oocyte cryopreservation. Pregnancy outcomes vary based on the center’s experience. Globally, major differences exist in the availability and competency of OTC along with the subsequent transplantation approach. A limited number of centers have competency in all aspects of OTC, i.e., cryopreservation, thawing, and transplantation. In general, fertility preservation patients have a multitude of medical issues that necessitate management expertise and the bandwidth to coordinate with cancer health professionals. The reproductive centers offering fertility preservation should be prepared to respond immediately and accommodate patients about to undergo gonadotoxic treatment.
Q6. How should a patient be counseled before proceeding with fertility preservation?
A: The candidate should be counseled on the likelihood of damage from gonadotoxic therapy and all fertility preservation options, on the basis of the urgency of treatment and the woman’s long-term goals. For example, the desire for a large family may compel a patient to undergo multiple cycles of ovarian stimulation or a combination of oocyte/embryo cryopreservation with OTC. In patients who are undergoing embryo cryopreservation, I recommend preimplantation genetic testing for aneuploidies, although there are limitations to its application. Other novel pieces of information we are using in counseling are baseline AMH levels and BRCA mutation status for women with breast cancer. In an 8-year-long NIH-funded prospective longitudinal study we found that women with both baseline AMH < 2 ng/mL and BRCA mutations are at significantly higher risk of losing their ovarian reserve and developing amenorrhea.21 Because the oocytes of women with BRCA mutations are deficient in DNA repair as we have previously shown,19 they are more liable to death upon exposure to DNA-damaging cancer drugs such as cyclophosphamide and doxorubicin.22
Q7. What is the time limit for use of cryopreserved oocytes/tissue?
A: Under optimal storage conditions, cryopreserved oocytes/tissue can be utilized indefinitely without a negative effect on pregnancy outcomes.
Q8. What does the future hold for fertility preservation?
A: The future holds promise for both the medical and nonmedical (planned) utility of fertility preservation. With the former, we will see that the utility of OTC and orthotopic and heterotopic tissue transplantation increase as success rates improve. Improved neovascularizing agents will make the transplants last longer and enhance pregnancy outcomes.23,24 I see planned fertility preservation increasing, based on the experience gained from cancer patients and some preliminary experience with planned OTC, especially for healthy women who wish to consider delaying menopause.25,26
Because of attrition from apoptosis, approximately 2,000 oocytes are wasted per ovulation. Through calculation models, we predict that if an equivalent of one-third of a woman’s ovarian cortex can be cryopreserved (which may not significantly affect the age at natural menopause) before age 40 years, transplantation at perimenopause may provide sufficient primordial follicles to delay menopause for 5 years or longer.26 Because ovarian tissue can also be transplanted subcutaneously under local anesthesia, as we have shown,27,28 repeated heterotopic transplants can be performed in an office setting at reduced cost, invasiveness, and with enhanced effectiveness. We can expect increasing reports and progress on this planned use of OTC and transplantation in the future.
Dr. Oktay is professor of obstetrics & gynecology and reproductive sciences and director of the Laboratory of Molecular Reproduction and Fertility Preservation at Yale University, New Haven, Conn. Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Lurie S. Eur J Obstet Gynecol Reprod Biol. 1992 Jan 9;43(1):1-7.
2. Oktay K and Karlikaya G. N Engl J Med. 2000 Jun 22;342(25):1919.
3. Sonmezer and Oktay K. Hum Reprod Update. 2004;10(3):251-66.
4. Oktay K et al. J Clin Oncol. 2018 Jul 1;36(19):1994-2001.
5. Goldfarb SB et al. Breast Cancer Res Treat. 2021;185:165-73.
6. Titus S et al. Sci Rep. 2021 Jan 11;11(1):407.
7. Soleimani R et al. Aging (Albany NY). 2011 Aug;3(8):782-93.
8. Titus S et al. Sci Transl Med. 2013 Feb 13;5(172):172ra21.
9. Oktay KH et al. Fertil Steril. 2022 Jan 5:S0015-0282(21)02293-7.
10. Oktay K et al. Fertil Steril. 2022;117(1):181-92.
11. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2019;112(6):1022–33.
12. Cil A et al. Fertil Steril. 2013 Aug;100(2):492-9.e3.
13. Goldman KN et al. Fertil Steril. 2013 Sep;100(3):712-7.
14. Marin L and Oktay K. Scientific history of ovarian tissue cryopreservation and transplantation. In: Oktay K (ed.), Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier;2022:1-10.
15. Oktay K et al. J Clin Oncol. 2005 Jul 1;23(19):4347-53.
16. Kim JY et al. J Clin Endocrinol Metab. 2016 Apr;101(4):1364-71.
17. Turan V et al. J Clin Oncol. 2021;39:18.
18. Oktay K et al. J Clin Oncol. 2010 Jan 10;28(2):240-4.
19. Lin W et al. J Clin Endocrinol Metab. 2017;102(10):3839-47.
20. Turan V et al. Reprod Sci. 2018;(25):26-32.
21. Oktay K et al. Presence of BRCA mutations and a pre-chemotherapy AMH level of < 2ng/mL strongly predict risk of amenorrhea in women with breast cancer P-291. Presented at the American Society for Reproductive Medicine 78th annual meeting, Anaheim, Calif. Oct. 22-26, 2022.
22. Oktay KH et al. Fertil Steril. 2020;113(6):1251‐60.e1.
23. Soleimani R et al. PLoS One. 2011 Apr 29;6(4):e19475.
24. Marin L et al. Future aspects of ovarian cryopreservation and transplantation. In: Oktay K (ed.). Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier; 2022;223-30.
25. Oktay KH et al. Trends Mol Med. 2021;27(8):753-61.
26. Oktay K and Marin L. Ovarian tissue cryopreservation for delaying childbearing and menopause. In: Oktay, K. (Ed.), Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier;2022:195-204.
27. Oktay K et al. JAMA. 2001 Sep 26;286(12):1490-3.
28. Oktay K et al. Lancet. 2004 Mar 13;363(9412):837-40.
From the first obscure reference until the 19th century, the maternal mortality rate from an ectopic pregnancy was nearly 100%. In the past 140 years, because of early detection and prompt surgical management, the mortality rate from an ectopic pregnancy declined from 72%-90% in 1880 to 0.48% from 2004 to 2008.1 Given this remarkable reduction in mortality, the 20th-century approach to ectopic pregnancy evolved from preserving the life of the mother to preserving fertility by utilizing conservative treatment with methotrexate and/or tubal surgery.
Why the reference to ectopic pregnancy? Advances in oncology have comparably affected our approach to cancer patients. The increase in survival rates following a cancer diagnosis has fostered revolutionary developments in fertility preservation to obviate the effect of gonadotoxic therapy. We have evolved from shielding and transposing ovaries to ovarian tissue cryopreservation2,3 with rapid implementation.
One of the leaders in the field of female fertility preservation is Kutluk Oktay, MD, of Yale University, New Haven, Conn. I posed the following salient questions to him on the state of fertility preservation as well as expectations for the future.
Q1. What medication/treatment is gonadotoxic that warrants a consultation for fertility preservation?
A: While new drugs for cancer treatment continue to be approved and require testing for gonadotoxicity, evidence is clear on the damaging effects of alkylating agents such as cyclophosphamide, ifosfamide, chlorambucil, and melphalan on primordial follicle reserve.4 A useful tool to determine the risk of alkylating agents affecting fertility is the Cyclophosphamide Equivalent Dose (CED) Calculator. Likewise, topoisomerase inhibitors, such as doxorubicin4 induce ovarian reserve damage by causing double-strand DNA breaks (DSBs) in oocytes.5-7 Contrary to common belief, chemotherapy exposure suppresses the mechanisms that can initiate follicle growth.6 When DSBs occur, some oocytes may be able to repair such damage, otherwise apoptosis is triggered, which results in irreversible ovarian reserve loss.7 Younger individuals have much higher repair capacity, the magnitude of damage can be hard to predict, and it is variable.8,9 So, prior exposure to gonadotoxic drugs does not preclude consideration of fertility preservation.10
In addition, pelvic radiation, in a dose-dependent manner, causes severe DSBs and triggers the same cell suicide mechanisms while also potentially damaging uterine function. Additional information can be found in the American Society of Clinical Oncology Fertility Preservation Guidelines.4
Q2. What are the current options for fertility preservation in patients who will be exposed to gonadotoxic medication/treatment?
A: The current fertility preservation options for female patients faced with gonadotoxic treatments are embryo, oocyte, and ovarian tissue cryopreservation (OTC). Selection of fertility preservation is typically contingent upon the timetable of treatment. Oocyte and embryo cryopreservation have been the standard of care. Recently, OTC had its experimental designation removed by American Society for Reproductive Medicine11 with the advantage of not requiring ovarian stimulation or sexual maturity; and it may to be performed while patients are receiving chemotherapy. If successful, OTC followed by orthotopic transplantation has the potential to restore natural ovarian function, thereby allowing spontaneous conception.10 Especially in young adults, ovarian reserve loss is fractional and can remain at reasonable levels after a few courses of chemotherapy. Ovarian stimulation is risky after the initiation of chemotherapy because of the severe DNA damage to oocytes of developing follicles and the associated poor response.7 Hence, ovarian stimulation should be initiated and completed before the initiation of chemotherapy.
Q3. How successful are the approved fertility preservation options in obtaining oocytes for future utilization by ART?
A: We have decades of experience with embryo cryopreservation and proven success rates that patients can check on the SART.org website for individual clinics. For oocyte cryopreservation, models are used to provide calculation estimates because the technique is less established.12 Although success rates are approaching those with fresh oocytes, they are still not equal.13 OTC followed by orthotopic tissue transplantation has the least outcomes data (approximately 200 reported livebirths to date with a 25% live birth rate per recipient worldwide10 since the first success was reported in 2000.2,14
With our robotic surgical approach to orthotopic and heterotopic ovarian tissue transplantation and the utility of neovascularizing agents, we have found that ovarian graft longevity is extended. Oocytes/embryos can be obtained and has resulted in one to two livebirths in all our recipients to date.10 Unfortunately, if any of the critical steps are not up to standards (freezing, thawing, or transplantation), success rates can dramatically decline. Therefore, providers and patients should seek centers with experience in all three stages of this procedure to maximize outcomes.
Q4. Are there concerns of increasing recurrence/mortality with fertility preservation given hormonal exposure?
A: Yes, this concern exists, at least in theory for estrogen-sensitive cancers, most commonly breast cancer. We developed ovarian stimulation protocols supplemented with anti-estrogen treatments (tamoxifen, an estrogen-receptor antagonist, and letrozole, an aromatase inhibitor) that appear equally effective and reduce estrogen exposure in any susceptible cancer.15,16 Even in estrogen receptor–negative tumors, high estrogen exposure may activate non–estrogen receptor–dependent pathways. In addition, even those tumors that are practically deemed estrogen receptor negative may still contain a small percentage of estrogen receptors, which may become active at high estrogen levels.
Therefore, when we approach women with estrogen-sensitive cancers, e.g., breast and endometrial, we do not alter our approach based on receptor status. One exception occurs in women with BRCA mutations, especially the BRCA1, as they have 25% lower serum anti-müllerian hormone (AMH) levels,8,17 yield fewer oocytes in response to ovarian stimulation,18,19 and have lower fertilization rates and embryo numbers20 compared with those without the mutations.
Q5. Are all reproductive centers capable of offering fertility preservation? If not, how does a patient find a center?
A: All IVF clinics offer embryo and, presumably, oocyte cryopreservation. Pregnancy outcomes vary based on the center’s experience. Globally, major differences exist in the availability and competency of OTC along with the subsequent transplantation approach. A limited number of centers have competency in all aspects of OTC, i.e., cryopreservation, thawing, and transplantation. In general, fertility preservation patients have a multitude of medical issues that necessitate management expertise and the bandwidth to coordinate with cancer health professionals. The reproductive centers offering fertility preservation should be prepared to respond immediately and accommodate patients about to undergo gonadotoxic treatment.
Q6. How should a patient be counseled before proceeding with fertility preservation?
A: The candidate should be counseled on the likelihood of damage from gonadotoxic therapy and all fertility preservation options, on the basis of the urgency of treatment and the woman’s long-term goals. For example, the desire for a large family may compel a patient to undergo multiple cycles of ovarian stimulation or a combination of oocyte/embryo cryopreservation with OTC. In patients who are undergoing embryo cryopreservation, I recommend preimplantation genetic testing for aneuploidies, although there are limitations to its application. Other novel pieces of information we are using in counseling are baseline AMH levels and BRCA mutation status for women with breast cancer. In an 8-year-long NIH-funded prospective longitudinal study we found that women with both baseline AMH < 2 ng/mL and BRCA mutations are at significantly higher risk of losing their ovarian reserve and developing amenorrhea.21 Because the oocytes of women with BRCA mutations are deficient in DNA repair as we have previously shown,19 they are more liable to death upon exposure to DNA-damaging cancer drugs such as cyclophosphamide and doxorubicin.22
Q7. What is the time limit for use of cryopreserved oocytes/tissue?
A: Under optimal storage conditions, cryopreserved oocytes/tissue can be utilized indefinitely without a negative effect on pregnancy outcomes.
Q8. What does the future hold for fertility preservation?
A: The future holds promise for both the medical and nonmedical (planned) utility of fertility preservation. With the former, we will see that the utility of OTC and orthotopic and heterotopic tissue transplantation increase as success rates improve. Improved neovascularizing agents will make the transplants last longer and enhance pregnancy outcomes.23,24 I see planned fertility preservation increasing, based on the experience gained from cancer patients and some preliminary experience with planned OTC, especially for healthy women who wish to consider delaying menopause.25,26
Because of attrition from apoptosis, approximately 2,000 oocytes are wasted per ovulation. Through calculation models, we predict that if an equivalent of one-third of a woman’s ovarian cortex can be cryopreserved (which may not significantly affect the age at natural menopause) before age 40 years, transplantation at perimenopause may provide sufficient primordial follicles to delay menopause for 5 years or longer.26 Because ovarian tissue can also be transplanted subcutaneously under local anesthesia, as we have shown,27,28 repeated heterotopic transplants can be performed in an office setting at reduced cost, invasiveness, and with enhanced effectiveness. We can expect increasing reports and progress on this planned use of OTC and transplantation in the future.
Dr. Oktay is professor of obstetrics & gynecology and reproductive sciences and director of the Laboratory of Molecular Reproduction and Fertility Preservation at Yale University, New Haven, Conn. Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Lurie S. Eur J Obstet Gynecol Reprod Biol. 1992 Jan 9;43(1):1-7.
2. Oktay K and Karlikaya G. N Engl J Med. 2000 Jun 22;342(25):1919.
3. Sonmezer and Oktay K. Hum Reprod Update. 2004;10(3):251-66.
4. Oktay K et al. J Clin Oncol. 2018 Jul 1;36(19):1994-2001.
5. Goldfarb SB et al. Breast Cancer Res Treat. 2021;185:165-73.
6. Titus S et al. Sci Rep. 2021 Jan 11;11(1):407.
7. Soleimani R et al. Aging (Albany NY). 2011 Aug;3(8):782-93.
8. Titus S et al. Sci Transl Med. 2013 Feb 13;5(172):172ra21.
9. Oktay KH et al. Fertil Steril. 2022 Jan 5:S0015-0282(21)02293-7.
10. Oktay K et al. Fertil Steril. 2022;117(1):181-92.
11. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2019;112(6):1022–33.
12. Cil A et al. Fertil Steril. 2013 Aug;100(2):492-9.e3.
13. Goldman KN et al. Fertil Steril. 2013 Sep;100(3):712-7.
14. Marin L and Oktay K. Scientific history of ovarian tissue cryopreservation and transplantation. In: Oktay K (ed.), Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier;2022:1-10.
15. Oktay K et al. J Clin Oncol. 2005 Jul 1;23(19):4347-53.
16. Kim JY et al. J Clin Endocrinol Metab. 2016 Apr;101(4):1364-71.
17. Turan V et al. J Clin Oncol. 2021;39:18.
18. Oktay K et al. J Clin Oncol. 2010 Jan 10;28(2):240-4.
19. Lin W et al. J Clin Endocrinol Metab. 2017;102(10):3839-47.
20. Turan V et al. Reprod Sci. 2018;(25):26-32.
21. Oktay K et al. Presence of BRCA mutations and a pre-chemotherapy AMH level of < 2ng/mL strongly predict risk of amenorrhea in women with breast cancer P-291. Presented at the American Society for Reproductive Medicine 78th annual meeting, Anaheim, Calif. Oct. 22-26, 2022.
22. Oktay KH et al. Fertil Steril. 2020;113(6):1251‐60.e1.
23. Soleimani R et al. PLoS One. 2011 Apr 29;6(4):e19475.
24. Marin L et al. Future aspects of ovarian cryopreservation and transplantation. In: Oktay K (ed.). Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier; 2022;223-30.
25. Oktay KH et al. Trends Mol Med. 2021;27(8):753-61.
26. Oktay K and Marin L. Ovarian tissue cryopreservation for delaying childbearing and menopause. In: Oktay, K. (Ed.), Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier;2022:195-204.
27. Oktay K et al. JAMA. 2001 Sep 26;286(12):1490-3.
28. Oktay K et al. Lancet. 2004 Mar 13;363(9412):837-40.
From the first obscure reference until the 19th century, the maternal mortality rate from an ectopic pregnancy was nearly 100%. In the past 140 years, because of early detection and prompt surgical management, the mortality rate from an ectopic pregnancy declined from 72%-90% in 1880 to 0.48% from 2004 to 2008.1 Given this remarkable reduction in mortality, the 20th-century approach to ectopic pregnancy evolved from preserving the life of the mother to preserving fertility by utilizing conservative treatment with methotrexate and/or tubal surgery.
Why the reference to ectopic pregnancy? Advances in oncology have comparably affected our approach to cancer patients. The increase in survival rates following a cancer diagnosis has fostered revolutionary developments in fertility preservation to obviate the effect of gonadotoxic therapy. We have evolved from shielding and transposing ovaries to ovarian tissue cryopreservation2,3 with rapid implementation.
One of the leaders in the field of female fertility preservation is Kutluk Oktay, MD, of Yale University, New Haven, Conn. I posed the following salient questions to him on the state of fertility preservation as well as expectations for the future.
Q1. What medication/treatment is gonadotoxic that warrants a consultation for fertility preservation?
A: While new drugs for cancer treatment continue to be approved and require testing for gonadotoxicity, evidence is clear on the damaging effects of alkylating agents such as cyclophosphamide, ifosfamide, chlorambucil, and melphalan on primordial follicle reserve.4 A useful tool to determine the risk of alkylating agents affecting fertility is the Cyclophosphamide Equivalent Dose (CED) Calculator. Likewise, topoisomerase inhibitors, such as doxorubicin4 induce ovarian reserve damage by causing double-strand DNA breaks (DSBs) in oocytes.5-7 Contrary to common belief, chemotherapy exposure suppresses the mechanisms that can initiate follicle growth.6 When DSBs occur, some oocytes may be able to repair such damage, otherwise apoptosis is triggered, which results in irreversible ovarian reserve loss.7 Younger individuals have much higher repair capacity, the magnitude of damage can be hard to predict, and it is variable.8,9 So, prior exposure to gonadotoxic drugs does not preclude consideration of fertility preservation.10
In addition, pelvic radiation, in a dose-dependent manner, causes severe DSBs and triggers the same cell suicide mechanisms while also potentially damaging uterine function. Additional information can be found in the American Society of Clinical Oncology Fertility Preservation Guidelines.4
Q2. What are the current options for fertility preservation in patients who will be exposed to gonadotoxic medication/treatment?
A: The current fertility preservation options for female patients faced with gonadotoxic treatments are embryo, oocyte, and ovarian tissue cryopreservation (OTC). Selection of fertility preservation is typically contingent upon the timetable of treatment. Oocyte and embryo cryopreservation have been the standard of care. Recently, OTC had its experimental designation removed by American Society for Reproductive Medicine11 with the advantage of not requiring ovarian stimulation or sexual maturity; and it may to be performed while patients are receiving chemotherapy. If successful, OTC followed by orthotopic transplantation has the potential to restore natural ovarian function, thereby allowing spontaneous conception.10 Especially in young adults, ovarian reserve loss is fractional and can remain at reasonable levels after a few courses of chemotherapy. Ovarian stimulation is risky after the initiation of chemotherapy because of the severe DNA damage to oocytes of developing follicles and the associated poor response.7 Hence, ovarian stimulation should be initiated and completed before the initiation of chemotherapy.
Q3. How successful are the approved fertility preservation options in obtaining oocytes for future utilization by ART?
A: We have decades of experience with embryo cryopreservation and proven success rates that patients can check on the SART.org website for individual clinics. For oocyte cryopreservation, models are used to provide calculation estimates because the technique is less established.12 Although success rates are approaching those with fresh oocytes, they are still not equal.13 OTC followed by orthotopic tissue transplantation has the least outcomes data (approximately 200 reported livebirths to date with a 25% live birth rate per recipient worldwide10 since the first success was reported in 2000.2,14
With our robotic surgical approach to orthotopic and heterotopic ovarian tissue transplantation and the utility of neovascularizing agents, we have found that ovarian graft longevity is extended. Oocytes/embryos can be obtained and has resulted in one to two livebirths in all our recipients to date.10 Unfortunately, if any of the critical steps are not up to standards (freezing, thawing, or transplantation), success rates can dramatically decline. Therefore, providers and patients should seek centers with experience in all three stages of this procedure to maximize outcomes.
Q4. Are there concerns of increasing recurrence/mortality with fertility preservation given hormonal exposure?
A: Yes, this concern exists, at least in theory for estrogen-sensitive cancers, most commonly breast cancer. We developed ovarian stimulation protocols supplemented with anti-estrogen treatments (tamoxifen, an estrogen-receptor antagonist, and letrozole, an aromatase inhibitor) that appear equally effective and reduce estrogen exposure in any susceptible cancer.15,16 Even in estrogen receptor–negative tumors, high estrogen exposure may activate non–estrogen receptor–dependent pathways. In addition, even those tumors that are practically deemed estrogen receptor negative may still contain a small percentage of estrogen receptors, which may become active at high estrogen levels.
Therefore, when we approach women with estrogen-sensitive cancers, e.g., breast and endometrial, we do not alter our approach based on receptor status. One exception occurs in women with BRCA mutations, especially the BRCA1, as they have 25% lower serum anti-müllerian hormone (AMH) levels,8,17 yield fewer oocytes in response to ovarian stimulation,18,19 and have lower fertilization rates and embryo numbers20 compared with those without the mutations.
Q5. Are all reproductive centers capable of offering fertility preservation? If not, how does a patient find a center?
A: All IVF clinics offer embryo and, presumably, oocyte cryopreservation. Pregnancy outcomes vary based on the center’s experience. Globally, major differences exist in the availability and competency of OTC along with the subsequent transplantation approach. A limited number of centers have competency in all aspects of OTC, i.e., cryopreservation, thawing, and transplantation. In general, fertility preservation patients have a multitude of medical issues that necessitate management expertise and the bandwidth to coordinate with cancer health professionals. The reproductive centers offering fertility preservation should be prepared to respond immediately and accommodate patients about to undergo gonadotoxic treatment.
Q6. How should a patient be counseled before proceeding with fertility preservation?
A: The candidate should be counseled on the likelihood of damage from gonadotoxic therapy and all fertility preservation options, on the basis of the urgency of treatment and the woman’s long-term goals. For example, the desire for a large family may compel a patient to undergo multiple cycles of ovarian stimulation or a combination of oocyte/embryo cryopreservation with OTC. In patients who are undergoing embryo cryopreservation, I recommend preimplantation genetic testing for aneuploidies, although there are limitations to its application. Other novel pieces of information we are using in counseling are baseline AMH levels and BRCA mutation status for women with breast cancer. In an 8-year-long NIH-funded prospective longitudinal study we found that women with both baseline AMH < 2 ng/mL and BRCA mutations are at significantly higher risk of losing their ovarian reserve and developing amenorrhea.21 Because the oocytes of women with BRCA mutations are deficient in DNA repair as we have previously shown,19 they are more liable to death upon exposure to DNA-damaging cancer drugs such as cyclophosphamide and doxorubicin.22
Q7. What is the time limit for use of cryopreserved oocytes/tissue?
A: Under optimal storage conditions, cryopreserved oocytes/tissue can be utilized indefinitely without a negative effect on pregnancy outcomes.
Q8. What does the future hold for fertility preservation?
A: The future holds promise for both the medical and nonmedical (planned) utility of fertility preservation. With the former, we will see that the utility of OTC and orthotopic and heterotopic tissue transplantation increase as success rates improve. Improved neovascularizing agents will make the transplants last longer and enhance pregnancy outcomes.23,24 I see planned fertility preservation increasing, based on the experience gained from cancer patients and some preliminary experience with planned OTC, especially for healthy women who wish to consider delaying menopause.25,26
Because of attrition from apoptosis, approximately 2,000 oocytes are wasted per ovulation. Through calculation models, we predict that if an equivalent of one-third of a woman’s ovarian cortex can be cryopreserved (which may not significantly affect the age at natural menopause) before age 40 years, transplantation at perimenopause may provide sufficient primordial follicles to delay menopause for 5 years or longer.26 Because ovarian tissue can also be transplanted subcutaneously under local anesthesia, as we have shown,27,28 repeated heterotopic transplants can be performed in an office setting at reduced cost, invasiveness, and with enhanced effectiveness. We can expect increasing reports and progress on this planned use of OTC and transplantation in the future.
Dr. Oktay is professor of obstetrics & gynecology and reproductive sciences and director of the Laboratory of Molecular Reproduction and Fertility Preservation at Yale University, New Haven, Conn. Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Lurie S. Eur J Obstet Gynecol Reprod Biol. 1992 Jan 9;43(1):1-7.
2. Oktay K and Karlikaya G. N Engl J Med. 2000 Jun 22;342(25):1919.
3. Sonmezer and Oktay K. Hum Reprod Update. 2004;10(3):251-66.
4. Oktay K et al. J Clin Oncol. 2018 Jul 1;36(19):1994-2001.
5. Goldfarb SB et al. Breast Cancer Res Treat. 2021;185:165-73.
6. Titus S et al. Sci Rep. 2021 Jan 11;11(1):407.
7. Soleimani R et al. Aging (Albany NY). 2011 Aug;3(8):782-93.
8. Titus S et al. Sci Transl Med. 2013 Feb 13;5(172):172ra21.
9. Oktay KH et al. Fertil Steril. 2022 Jan 5:S0015-0282(21)02293-7.
10. Oktay K et al. Fertil Steril. 2022;117(1):181-92.
11. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2019;112(6):1022–33.
12. Cil A et al. Fertil Steril. 2013 Aug;100(2):492-9.e3.
13. Goldman KN et al. Fertil Steril. 2013 Sep;100(3):712-7.
14. Marin L and Oktay K. Scientific history of ovarian tissue cryopreservation and transplantation. In: Oktay K (ed.), Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier;2022:1-10.
15. Oktay K et al. J Clin Oncol. 2005 Jul 1;23(19):4347-53.
16. Kim JY et al. J Clin Endocrinol Metab. 2016 Apr;101(4):1364-71.
17. Turan V et al. J Clin Oncol. 2021;39:18.
18. Oktay K et al. J Clin Oncol. 2010 Jan 10;28(2):240-4.
19. Lin W et al. J Clin Endocrinol Metab. 2017;102(10):3839-47.
20. Turan V et al. Reprod Sci. 2018;(25):26-32.
21. Oktay K et al. Presence of BRCA mutations and a pre-chemotherapy AMH level of < 2ng/mL strongly predict risk of amenorrhea in women with breast cancer P-291. Presented at the American Society for Reproductive Medicine 78th annual meeting, Anaheim, Calif. Oct. 22-26, 2022.
22. Oktay KH et al. Fertil Steril. 2020;113(6):1251‐60.e1.
23. Soleimani R et al. PLoS One. 2011 Apr 29;6(4):e19475.
24. Marin L et al. Future aspects of ovarian cryopreservation and transplantation. In: Oktay K (ed.). Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier; 2022;223-30.
25. Oktay KH et al. Trends Mol Med. 2021;27(8):753-61.
26. Oktay K and Marin L. Ovarian tissue cryopreservation for delaying childbearing and menopause. In: Oktay, K. (Ed.), Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier;2022:195-204.
27. Oktay K et al. JAMA. 2001 Sep 26;286(12):1490-3.
28. Oktay K et al. Lancet. 2004 Mar 13;363(9412):837-40.
Mastocytosis: Rare, underdiagnosed, potentially fatal
Nationwide, approximately 1,000 adults are diagnosed with systemic mastocytosis annually. This rare disease is a myeloid neoplasm with a highly variable phenotypic expression, in which abnormal mast cells proliferate and infiltrate organs and tissues. It swings widely from a nonadvanced form, composed of indolent or smoldering disease, to advanced disease that progresses to leukemia in 6% of cases.
More than 80% of systemic mastocytosis is driven by the KIT D816V mutation. Along with a host of other rare KIT mutations, KIT D816V activates KIT-receptor tyrosine kinase to trigger mast cell proliferation.
Dr. Gotlib could not be contacted for an interview. However, there are many good reasons to identify patients with systemic mastocytosis, according to Attilio Orazi, MD, professor and chair of the department of pathology at Texas Tech University, El Paso. The chief reason is that the patient may be in grave peril.
“The degree of heterogeneity is amazing. ... There’s very indolent [disease], which is really not a big deal. And then you have a disease in which you’re dead in 3 months,” Dr. Orazi said. “So you run the gamut between an indolent, no-problem cutaneous disease to a very nasty systemic, aggressive leukemia-like neoplasm.”
Since 2001, the diagnosis of mastocytosis has been guided by the World Health Organization Classification of Tumours, or “Blue Book.” In 2022, Dr. Orazi along with 137 other senior experts, most of whom were involved in past editions of the Blue Book, published their own version: The International Consensus Classification of Myeloid Neoplasms and Acute Leukemias (the ICC 2022).
In September 2021, this group of specialists held a virtual/in-person advisory committee meeting at the University of Chicago to create the document. One factor in their decision to go it alone, Dr. Orazi said, was that WHO decided to proceed with the fifth edition of the Blue Book using its own internal editorial group without convening an advisory committee, despite repeated requests to do so.
ICC 2022 divides advanced systemic mastocytosis into three subtypes: aggressive systemic mastocytosis (ASM), systemic mastocytosis with an associated hematologic neoplasm (SM-AHN), and mast cell leukemia (MCL). Median survival is 3.5 years for patients with ASM, 2 years for those with SM-AHN and as low as 2 months for MCL.
The second key reason to increase awareness of mastocytosis among physicians, said Dr. Orazi, is that patients falling through the net are likely to be ambulatory, and their presentation can be “a little confusing.”
Patients with indolent disease are relatively straightforward to recognize, explained Dr. Orazi. Similarly, very sick patients with SM-AHN or MCL are easily recognized by hem-oncs.
“But if you see a patient in an ambulatory setting, in your clinic or whatever, and you’re suspicious, then you need to decide [how] you’re going to investigate that patient further,” he said, Dr. Orazi noted the next step is not always obvious, especially for primary-practice or internal medicine physicians likely to be unfamiliar with such a rare disease.
A practice survey published in 2022 by other researchers backed up Dr. Orazi’s remarks. The study found that community/solo-practice physicians were less likely to have tested systemic mastocytosis patients for KIT816V mutation than academic/specialty physicians (58% vs. 80%; P = .004; n = 111). Clinicians treating these patients estimated that it took an average of 8.5 months for a “typical” patient to receive the diagnosis from the time of symptom onset.
The research was headed by Ruben Mesa, MD, director of University of Texas Health, San Antonio, and funded by Blueprint Medicines, the manufacturer of avapritinib (Ayvakit), a new drug for the disease.
Dr. Orazi urged clinicians to have a high degree of suspicion for mastocytosis in a patient who walks into the clinic with any combination of the following: urticarial-type skin manifestations, especially if persistent into adulthood; history of undue reaction to an insect sting; a big spleen in a patient with a history of cutaneous flushing or rash; chronic diarrhea, especially if a biopsy has shown “too many mast cells” in the lamina propria of the small bowel; and positivity for KIT816V mutation.
Dr. Orazi stressed that the majority of patients will have indolent disease, but for the few patients for whom immediate treatment is essential, “the distinction between indolent and aggressive [disease] is really very, very important.”
Patients with advanced systemic mastocytosis can now be effectively treated, following the arrival of midostaurin (Rydapt, Tauritmo) and avapritinib.
Midostaurin, a multikinase/KIT inhibitor, was approved by the Food and Drug Administration in 2017 for the treatment of advanced systemic mastocytosis (ASM, SM-AHN, and MCL). Avapritinib, a selective kinase inhibitor of KIT816V and platelet-derived growth factor receptor alpha as well as multiple KIT exon 11, 11/17 and 17 mutants, gained the same indication in June 2021.
As with all rare diseases, it is challenging to obtain accurate numbers on how many patients are affected by systemic mastocytosis. The first population-based study of the disorder, presented at the 2018 annual meeting of the American Society of Hematology, used the Surveillance, Epidemiology, and End Results database from 2000 to 2014 to estimate incidence at 0.046 per 10,000, which translates to 1,050 new adult cases per year. The study data have never been published in full.
How many of these cases are advanced disease? There are no U.S. data but extrapolating from a Danish registry study that found 82% of systemic mastocytosis cases to be indolent disease, the incidence of advanced systemic mastocytosis in the United States could be as low as 200 adults a year.
This information, in turn, suggests that identifying more patients with advanced disease would not only benefit those patients but would also benefit clinical trial investigators who are seeking the proverbial needle in the haystack.
Nationwide, five clinical trials are recruiting individuals with advanced systemic mastocytosis, collectively looking for 352 patients in the United States. Two of the studies focus on mast-cell activation (NCT0544944) and cutaneous mastocytoses (NCT04846348). Two trials in a range of hematological malignancies are testing bispecific antibodies flotetuzumab and MGD024 (both from Macrogenics; NCT04681105, NCT05362773).
Apex, a phase 2 study of tyrosine-kinase inhibitor bezuclastinib (a Cogent hopeful), is specifically focusing on advanced disease. Dr. Gotlib and coinvestigators are aiming for 140 participants.
As a pathologist, Dr. Orazi said he find mastocytosis fascinating because he believes he has “a truly useful role,” contrasting with some other hematological diseases in which the molecular profile rules.
“Pathology plays a major role here,” he explained, “because you have to correlate what you see at the microscope with the full clinical picture, selected laboratory tests such as CBC and serum tryptase, and molecular results. You often need integration through a pathologist to put all the pieces together.
“It’s easier to treat once you know exactly what disease you’re dealing with and whether it is an aggressive or indolent subtype,” Dr. Orazi concluded.
Dr. Orazi disclosed no conflicts of interest. Dr. Gotlib has disclosed ties with Blueprint Medicines, Deciphera, Incyte, and Kartos Therapeutics, and has led committees for Blueprint Medicine’s EXPLORER and PATHFINDER studies, Deciphera’s Study Steering Committee for ripretinib in AdvSM, and the Central Response Review Committee for the phase 2 study of bezuclastinib in AdvSM.
Nationwide, approximately 1,000 adults are diagnosed with systemic mastocytosis annually. This rare disease is a myeloid neoplasm with a highly variable phenotypic expression, in which abnormal mast cells proliferate and infiltrate organs and tissues. It swings widely from a nonadvanced form, composed of indolent or smoldering disease, to advanced disease that progresses to leukemia in 6% of cases.
More than 80% of systemic mastocytosis is driven by the KIT D816V mutation. Along with a host of other rare KIT mutations, KIT D816V activates KIT-receptor tyrosine kinase to trigger mast cell proliferation.
Dr. Gotlib could not be contacted for an interview. However, there are many good reasons to identify patients with systemic mastocytosis, according to Attilio Orazi, MD, professor and chair of the department of pathology at Texas Tech University, El Paso. The chief reason is that the patient may be in grave peril.
“The degree of heterogeneity is amazing. ... There’s very indolent [disease], which is really not a big deal. And then you have a disease in which you’re dead in 3 months,” Dr. Orazi said. “So you run the gamut between an indolent, no-problem cutaneous disease to a very nasty systemic, aggressive leukemia-like neoplasm.”
Since 2001, the diagnosis of mastocytosis has been guided by the World Health Organization Classification of Tumours, or “Blue Book.” In 2022, Dr. Orazi along with 137 other senior experts, most of whom were involved in past editions of the Blue Book, published their own version: The International Consensus Classification of Myeloid Neoplasms and Acute Leukemias (the ICC 2022).
In September 2021, this group of specialists held a virtual/in-person advisory committee meeting at the University of Chicago to create the document. One factor in their decision to go it alone, Dr. Orazi said, was that WHO decided to proceed with the fifth edition of the Blue Book using its own internal editorial group without convening an advisory committee, despite repeated requests to do so.
ICC 2022 divides advanced systemic mastocytosis into three subtypes: aggressive systemic mastocytosis (ASM), systemic mastocytosis with an associated hematologic neoplasm (SM-AHN), and mast cell leukemia (MCL). Median survival is 3.5 years for patients with ASM, 2 years for those with SM-AHN and as low as 2 months for MCL.
The second key reason to increase awareness of mastocytosis among physicians, said Dr. Orazi, is that patients falling through the net are likely to be ambulatory, and their presentation can be “a little confusing.”
Patients with indolent disease are relatively straightforward to recognize, explained Dr. Orazi. Similarly, very sick patients with SM-AHN or MCL are easily recognized by hem-oncs.
“But if you see a patient in an ambulatory setting, in your clinic or whatever, and you’re suspicious, then you need to decide [how] you’re going to investigate that patient further,” he said, Dr. Orazi noted the next step is not always obvious, especially for primary-practice or internal medicine physicians likely to be unfamiliar with such a rare disease.
A practice survey published in 2022 by other researchers backed up Dr. Orazi’s remarks. The study found that community/solo-practice physicians were less likely to have tested systemic mastocytosis patients for KIT816V mutation than academic/specialty physicians (58% vs. 80%; P = .004; n = 111). Clinicians treating these patients estimated that it took an average of 8.5 months for a “typical” patient to receive the diagnosis from the time of symptom onset.
The research was headed by Ruben Mesa, MD, director of University of Texas Health, San Antonio, and funded by Blueprint Medicines, the manufacturer of avapritinib (Ayvakit), a new drug for the disease.
Dr. Orazi urged clinicians to have a high degree of suspicion for mastocytosis in a patient who walks into the clinic with any combination of the following: urticarial-type skin manifestations, especially if persistent into adulthood; history of undue reaction to an insect sting; a big spleen in a patient with a history of cutaneous flushing or rash; chronic diarrhea, especially if a biopsy has shown “too many mast cells” in the lamina propria of the small bowel; and positivity for KIT816V mutation.
Dr. Orazi stressed that the majority of patients will have indolent disease, but for the few patients for whom immediate treatment is essential, “the distinction between indolent and aggressive [disease] is really very, very important.”
Patients with advanced systemic mastocytosis can now be effectively treated, following the arrival of midostaurin (Rydapt, Tauritmo) and avapritinib.
Midostaurin, a multikinase/KIT inhibitor, was approved by the Food and Drug Administration in 2017 for the treatment of advanced systemic mastocytosis (ASM, SM-AHN, and MCL). Avapritinib, a selective kinase inhibitor of KIT816V and platelet-derived growth factor receptor alpha as well as multiple KIT exon 11, 11/17 and 17 mutants, gained the same indication in June 2021.
As with all rare diseases, it is challenging to obtain accurate numbers on how many patients are affected by systemic mastocytosis. The first population-based study of the disorder, presented at the 2018 annual meeting of the American Society of Hematology, used the Surveillance, Epidemiology, and End Results database from 2000 to 2014 to estimate incidence at 0.046 per 10,000, which translates to 1,050 new adult cases per year. The study data have never been published in full.
How many of these cases are advanced disease? There are no U.S. data but extrapolating from a Danish registry study that found 82% of systemic mastocytosis cases to be indolent disease, the incidence of advanced systemic mastocytosis in the United States could be as low as 200 adults a year.
This information, in turn, suggests that identifying more patients with advanced disease would not only benefit those patients but would also benefit clinical trial investigators who are seeking the proverbial needle in the haystack.
Nationwide, five clinical trials are recruiting individuals with advanced systemic mastocytosis, collectively looking for 352 patients in the United States. Two of the studies focus on mast-cell activation (NCT0544944) and cutaneous mastocytoses (NCT04846348). Two trials in a range of hematological malignancies are testing bispecific antibodies flotetuzumab and MGD024 (both from Macrogenics; NCT04681105, NCT05362773).
Apex, a phase 2 study of tyrosine-kinase inhibitor bezuclastinib (a Cogent hopeful), is specifically focusing on advanced disease. Dr. Gotlib and coinvestigators are aiming for 140 participants.
As a pathologist, Dr. Orazi said he find mastocytosis fascinating because he believes he has “a truly useful role,” contrasting with some other hematological diseases in which the molecular profile rules.
“Pathology plays a major role here,” he explained, “because you have to correlate what you see at the microscope with the full clinical picture, selected laboratory tests such as CBC and serum tryptase, and molecular results. You often need integration through a pathologist to put all the pieces together.
“It’s easier to treat once you know exactly what disease you’re dealing with and whether it is an aggressive or indolent subtype,” Dr. Orazi concluded.
Dr. Orazi disclosed no conflicts of interest. Dr. Gotlib has disclosed ties with Blueprint Medicines, Deciphera, Incyte, and Kartos Therapeutics, and has led committees for Blueprint Medicine’s EXPLORER and PATHFINDER studies, Deciphera’s Study Steering Committee for ripretinib in AdvSM, and the Central Response Review Committee for the phase 2 study of bezuclastinib in AdvSM.
Nationwide, approximately 1,000 adults are diagnosed with systemic mastocytosis annually. This rare disease is a myeloid neoplasm with a highly variable phenotypic expression, in which abnormal mast cells proliferate and infiltrate organs and tissues. It swings widely from a nonadvanced form, composed of indolent or smoldering disease, to advanced disease that progresses to leukemia in 6% of cases.
More than 80% of systemic mastocytosis is driven by the KIT D816V mutation. Along with a host of other rare KIT mutations, KIT D816V activates KIT-receptor tyrosine kinase to trigger mast cell proliferation.
Dr. Gotlib could not be contacted for an interview. However, there are many good reasons to identify patients with systemic mastocytosis, according to Attilio Orazi, MD, professor and chair of the department of pathology at Texas Tech University, El Paso. The chief reason is that the patient may be in grave peril.
“The degree of heterogeneity is amazing. ... There’s very indolent [disease], which is really not a big deal. And then you have a disease in which you’re dead in 3 months,” Dr. Orazi said. “So you run the gamut between an indolent, no-problem cutaneous disease to a very nasty systemic, aggressive leukemia-like neoplasm.”
Since 2001, the diagnosis of mastocytosis has been guided by the World Health Organization Classification of Tumours, or “Blue Book.” In 2022, Dr. Orazi along with 137 other senior experts, most of whom were involved in past editions of the Blue Book, published their own version: The International Consensus Classification of Myeloid Neoplasms and Acute Leukemias (the ICC 2022).
In September 2021, this group of specialists held a virtual/in-person advisory committee meeting at the University of Chicago to create the document. One factor in their decision to go it alone, Dr. Orazi said, was that WHO decided to proceed with the fifth edition of the Blue Book using its own internal editorial group without convening an advisory committee, despite repeated requests to do so.
ICC 2022 divides advanced systemic mastocytosis into three subtypes: aggressive systemic mastocytosis (ASM), systemic mastocytosis with an associated hematologic neoplasm (SM-AHN), and mast cell leukemia (MCL). Median survival is 3.5 years for patients with ASM, 2 years for those with SM-AHN and as low as 2 months for MCL.
The second key reason to increase awareness of mastocytosis among physicians, said Dr. Orazi, is that patients falling through the net are likely to be ambulatory, and their presentation can be “a little confusing.”
Patients with indolent disease are relatively straightforward to recognize, explained Dr. Orazi. Similarly, very sick patients with SM-AHN or MCL are easily recognized by hem-oncs.
“But if you see a patient in an ambulatory setting, in your clinic or whatever, and you’re suspicious, then you need to decide [how] you’re going to investigate that patient further,” he said, Dr. Orazi noted the next step is not always obvious, especially for primary-practice or internal medicine physicians likely to be unfamiliar with such a rare disease.
A practice survey published in 2022 by other researchers backed up Dr. Orazi’s remarks. The study found that community/solo-practice physicians were less likely to have tested systemic mastocytosis patients for KIT816V mutation than academic/specialty physicians (58% vs. 80%; P = .004; n = 111). Clinicians treating these patients estimated that it took an average of 8.5 months for a “typical” patient to receive the diagnosis from the time of symptom onset.
The research was headed by Ruben Mesa, MD, director of University of Texas Health, San Antonio, and funded by Blueprint Medicines, the manufacturer of avapritinib (Ayvakit), a new drug for the disease.
Dr. Orazi urged clinicians to have a high degree of suspicion for mastocytosis in a patient who walks into the clinic with any combination of the following: urticarial-type skin manifestations, especially if persistent into adulthood; history of undue reaction to an insect sting; a big spleen in a patient with a history of cutaneous flushing or rash; chronic diarrhea, especially if a biopsy has shown “too many mast cells” in the lamina propria of the small bowel; and positivity for KIT816V mutation.
Dr. Orazi stressed that the majority of patients will have indolent disease, but for the few patients for whom immediate treatment is essential, “the distinction between indolent and aggressive [disease] is really very, very important.”
Patients with advanced systemic mastocytosis can now be effectively treated, following the arrival of midostaurin (Rydapt, Tauritmo) and avapritinib.
Midostaurin, a multikinase/KIT inhibitor, was approved by the Food and Drug Administration in 2017 for the treatment of advanced systemic mastocytosis (ASM, SM-AHN, and MCL). Avapritinib, a selective kinase inhibitor of KIT816V and platelet-derived growth factor receptor alpha as well as multiple KIT exon 11, 11/17 and 17 mutants, gained the same indication in June 2021.
As with all rare diseases, it is challenging to obtain accurate numbers on how many patients are affected by systemic mastocytosis. The first population-based study of the disorder, presented at the 2018 annual meeting of the American Society of Hematology, used the Surveillance, Epidemiology, and End Results database from 2000 to 2014 to estimate incidence at 0.046 per 10,000, which translates to 1,050 new adult cases per year. The study data have never been published in full.
How many of these cases are advanced disease? There are no U.S. data but extrapolating from a Danish registry study that found 82% of systemic mastocytosis cases to be indolent disease, the incidence of advanced systemic mastocytosis in the United States could be as low as 200 adults a year.
This information, in turn, suggests that identifying more patients with advanced disease would not only benefit those patients but would also benefit clinical trial investigators who are seeking the proverbial needle in the haystack.
Nationwide, five clinical trials are recruiting individuals with advanced systemic mastocytosis, collectively looking for 352 patients in the United States. Two of the studies focus on mast-cell activation (NCT0544944) and cutaneous mastocytoses (NCT04846348). Two trials in a range of hematological malignancies are testing bispecific antibodies flotetuzumab and MGD024 (both from Macrogenics; NCT04681105, NCT05362773).
Apex, a phase 2 study of tyrosine-kinase inhibitor bezuclastinib (a Cogent hopeful), is specifically focusing on advanced disease. Dr. Gotlib and coinvestigators are aiming for 140 participants.
As a pathologist, Dr. Orazi said he find mastocytosis fascinating because he believes he has “a truly useful role,” contrasting with some other hematological diseases in which the molecular profile rules.
“Pathology plays a major role here,” he explained, “because you have to correlate what you see at the microscope with the full clinical picture, selected laboratory tests such as CBC and serum tryptase, and molecular results. You often need integration through a pathologist to put all the pieces together.
“It’s easier to treat once you know exactly what disease you’re dealing with and whether it is an aggressive or indolent subtype,” Dr. Orazi concluded.
Dr. Orazi disclosed no conflicts of interest. Dr. Gotlib has disclosed ties with Blueprint Medicines, Deciphera, Incyte, and Kartos Therapeutics, and has led committees for Blueprint Medicine’s EXPLORER and PATHFINDER studies, Deciphera’s Study Steering Committee for ripretinib in AdvSM, and the Central Response Review Committee for the phase 2 study of bezuclastinib in AdvSM.