User login
PCSK9 inhibitors for severe COVID? Pilot trial signals of benefit
PCSK9 inhibitors may best be known for their powerful LDL-lowering effects but are less appreciated as anti-inflammatory agents with potential beyond cardiovascular health.
In a small pilot trial, for example, patients hospitalized with severe COVID-19 who received a single injection of PCSK9 inhibitor became less sick and more likely to survive than those given a placebo. Their 30-day risk of death or intubation fell significantly, as did their levels of the inflammatory cytokine interleukin 6 (IL-6).
Indeed, survival gains in the PCSK9-inhibitor group were greatest among patients with higher baseline concentrations of IL-6. Although the trial wasn’t powered for clinical outcomes, it suggests the drugs’ efficacy in COVID-19 tracks with intensity of inflammation, proposes a report published in the Journal of the American College of Cardiology.
Therefore, “PCSK9 inhibition may represent a novel therapeutic pathway in addition to currently recommended therapeutic approaches for severe COVID-19,” conclude the authors, led by Eliano P. Navarese, MD, PhD, Nicolaus Copernicus University, Bydgoszcz, Poland.
PCSK9 inhibitors as anti-inflammatories
Although the study was small and only hypothesis-generating, the fact that outcomes for actively treated patients were proportional to baseline IL-6 levels “strongly suggests that PCSK9 inhibition can directly modulate inflammation in COVID-19,” argues an editorial accompanying the report.
and likely sheds light on “mechanisms through which PCSK9 inhibition dually modulates lipoprotein metabolism and inflammation,” write Sascha N. Goonewardena, MD, University of Michigan, Ann Arbor, and Robert S. Rosenson, MD, Icahn School of Medicine at Mount Sinai, New York.
The results are consistent with prior evidence that the drugs are anti-inflammatory at least partly because of their interference with inflammatory pathways triggered by PCSK9 and mediated by IL-6, as described by Dr. Navarese and colleagues.
Indeed, they write, PCSK9 inhibitors may improve COVID outcomes mostly through mechanisms unrelated to LDL-receptor expression, “including direct inhibition of PCSK9-triggered inflammation.”
If true, the authors observe, it might explain “why the positive findings of the present study have not been consistently observed in trials involving other lipid-lowering agents, such as statins.” Those drugs are well-known to decrease levels of the inflammatory biomarker C-reactive protein.
In patients with stable coronary disease, in whom inflammation is typically tracked by measuring CRP, “the PCSK9 inhibitors have not been shown to have an anti-inflammatory effect,” Dr. Rosenson further explained.
But the current study’s patients with acute, severe COVID-19, a “profound inflammatory insult” with upregulation of IL-6, were “a good population” for evaluating the drugs’ potential anti-inflammatory effects, Dr. Rosenson said in an interview. The results “are quite enticing but require corroboration in a larger trial.”
A single injection
The IMPACT-SIRIO 5 trial entered 60 adults hospitalized with severe COVID-19 and elevated IL-6 at four centers in Poland. Patients with other known active infections were excluded.
They were randomly assigned double-blind to receive a 140 mg injection of evolocumab (Repatha) or placebo. The 2 groups were similar with respect to demographics, body-mass index, time since symptom onset, and treatments for managing COVID-19 and its complications.
Rates of death or need for intubation at 30 days, the primary endpoint, were 23.3% in the PCSK9-inhibitor group and 53.3% for controls, a risk difference of 30% (95% confidence interval –53.4% to –6.6%). The median durations of oxygen therapy were significantly different at 13 days and 20 days, respectively, the report states.
Serum IL-6 levels fell further over 30 days in the PCSK9-inhibitor group (–56% vs. –21% among controls). A drop by more than 90% was seen in 60% of patients in the PCSK9-inhibitor group and in 27% of controls.
The average hospital stay was shorter for those getting the PCSK9 inhibitor, compared with placebo, 16 days versus 22 days, and their 30-day mortality was numerically lower, 16% versus 33.3%.
Patients’ baseline IL-6 levels above the median, the report states, had a lower mortality on the PCSK9 inhibitor versus placebo (risk difference –37.5%; 95% CI –68.2% to –6.70%).
A larger trial to corroborate these results would potentially enter similar patients hospitalized with COVID-19 with reproducible evidence of an ongoing cytokine storm, such as elevated levels of IL-6, who would be assigned to either a PCSK9 inhibitor or placebo, Dr. Rosenson proposed.
Although the current primary endpoint that combines mortality and intubation was “reasonable” for a small pilot trial, he said, if the researchers embark on a larger study, “they’ll want to look at those events separately.”
Dr. Navarese discloses receiving speaker and consultancy fees from Amgen, Sanofi-Regeneron, Bayer; and grants from Abbott. Disclosures for the other authors are in the report. Rosenson discloses receiving research funding to his institution from Amgen, Arrowhead, Eli Lilly, Novartis, and Regeneron; consulting fees from Amgen, Arrowhead, CRISPR Therapeutics, Eli Lilly, Lipigon, Novartis, Precision Biosciences, Regeneron, Ultragenyx, and Verve; speaking fees from Amgen, Kowa, and Regeneron; and royalties from Wolters Kluwer; and owning stock in MediMergent. Dr. Goonewardena reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
PCSK9 inhibitors may best be known for their powerful LDL-lowering effects but are less appreciated as anti-inflammatory agents with potential beyond cardiovascular health.
In a small pilot trial, for example, patients hospitalized with severe COVID-19 who received a single injection of PCSK9 inhibitor became less sick and more likely to survive than those given a placebo. Their 30-day risk of death or intubation fell significantly, as did their levels of the inflammatory cytokine interleukin 6 (IL-6).
Indeed, survival gains in the PCSK9-inhibitor group were greatest among patients with higher baseline concentrations of IL-6. Although the trial wasn’t powered for clinical outcomes, it suggests the drugs’ efficacy in COVID-19 tracks with intensity of inflammation, proposes a report published in the Journal of the American College of Cardiology.
Therefore, “PCSK9 inhibition may represent a novel therapeutic pathway in addition to currently recommended therapeutic approaches for severe COVID-19,” conclude the authors, led by Eliano P. Navarese, MD, PhD, Nicolaus Copernicus University, Bydgoszcz, Poland.
PCSK9 inhibitors as anti-inflammatories
Although the study was small and only hypothesis-generating, the fact that outcomes for actively treated patients were proportional to baseline IL-6 levels “strongly suggests that PCSK9 inhibition can directly modulate inflammation in COVID-19,” argues an editorial accompanying the report.
and likely sheds light on “mechanisms through which PCSK9 inhibition dually modulates lipoprotein metabolism and inflammation,” write Sascha N. Goonewardena, MD, University of Michigan, Ann Arbor, and Robert S. Rosenson, MD, Icahn School of Medicine at Mount Sinai, New York.
The results are consistent with prior evidence that the drugs are anti-inflammatory at least partly because of their interference with inflammatory pathways triggered by PCSK9 and mediated by IL-6, as described by Dr. Navarese and colleagues.
Indeed, they write, PCSK9 inhibitors may improve COVID outcomes mostly through mechanisms unrelated to LDL-receptor expression, “including direct inhibition of PCSK9-triggered inflammation.”
If true, the authors observe, it might explain “why the positive findings of the present study have not been consistently observed in trials involving other lipid-lowering agents, such as statins.” Those drugs are well-known to decrease levels of the inflammatory biomarker C-reactive protein.
In patients with stable coronary disease, in whom inflammation is typically tracked by measuring CRP, “the PCSK9 inhibitors have not been shown to have an anti-inflammatory effect,” Dr. Rosenson further explained.
But the current study’s patients with acute, severe COVID-19, a “profound inflammatory insult” with upregulation of IL-6, were “a good population” for evaluating the drugs’ potential anti-inflammatory effects, Dr. Rosenson said in an interview. The results “are quite enticing but require corroboration in a larger trial.”
A single injection
The IMPACT-SIRIO 5 trial entered 60 adults hospitalized with severe COVID-19 and elevated IL-6 at four centers in Poland. Patients with other known active infections were excluded.
They were randomly assigned double-blind to receive a 140 mg injection of evolocumab (Repatha) or placebo. The 2 groups were similar with respect to demographics, body-mass index, time since symptom onset, and treatments for managing COVID-19 and its complications.
Rates of death or need for intubation at 30 days, the primary endpoint, were 23.3% in the PCSK9-inhibitor group and 53.3% for controls, a risk difference of 30% (95% confidence interval –53.4% to –6.6%). The median durations of oxygen therapy were significantly different at 13 days and 20 days, respectively, the report states.
Serum IL-6 levels fell further over 30 days in the PCSK9-inhibitor group (–56% vs. –21% among controls). A drop by more than 90% was seen in 60% of patients in the PCSK9-inhibitor group and in 27% of controls.
The average hospital stay was shorter for those getting the PCSK9 inhibitor, compared with placebo, 16 days versus 22 days, and their 30-day mortality was numerically lower, 16% versus 33.3%.
Patients’ baseline IL-6 levels above the median, the report states, had a lower mortality on the PCSK9 inhibitor versus placebo (risk difference –37.5%; 95% CI –68.2% to –6.70%).
A larger trial to corroborate these results would potentially enter similar patients hospitalized with COVID-19 with reproducible evidence of an ongoing cytokine storm, such as elevated levels of IL-6, who would be assigned to either a PCSK9 inhibitor or placebo, Dr. Rosenson proposed.
Although the current primary endpoint that combines mortality and intubation was “reasonable” for a small pilot trial, he said, if the researchers embark on a larger study, “they’ll want to look at those events separately.”
Dr. Navarese discloses receiving speaker and consultancy fees from Amgen, Sanofi-Regeneron, Bayer; and grants from Abbott. Disclosures for the other authors are in the report. Rosenson discloses receiving research funding to his institution from Amgen, Arrowhead, Eli Lilly, Novartis, and Regeneron; consulting fees from Amgen, Arrowhead, CRISPR Therapeutics, Eli Lilly, Lipigon, Novartis, Precision Biosciences, Regeneron, Ultragenyx, and Verve; speaking fees from Amgen, Kowa, and Regeneron; and royalties from Wolters Kluwer; and owning stock in MediMergent. Dr. Goonewardena reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
PCSK9 inhibitors may best be known for their powerful LDL-lowering effects but are less appreciated as anti-inflammatory agents with potential beyond cardiovascular health.
In a small pilot trial, for example, patients hospitalized with severe COVID-19 who received a single injection of PCSK9 inhibitor became less sick and more likely to survive than those given a placebo. Their 30-day risk of death or intubation fell significantly, as did their levels of the inflammatory cytokine interleukin 6 (IL-6).
Indeed, survival gains in the PCSK9-inhibitor group were greatest among patients with higher baseline concentrations of IL-6. Although the trial wasn’t powered for clinical outcomes, it suggests the drugs’ efficacy in COVID-19 tracks with intensity of inflammation, proposes a report published in the Journal of the American College of Cardiology.
Therefore, “PCSK9 inhibition may represent a novel therapeutic pathway in addition to currently recommended therapeutic approaches for severe COVID-19,” conclude the authors, led by Eliano P. Navarese, MD, PhD, Nicolaus Copernicus University, Bydgoszcz, Poland.
PCSK9 inhibitors as anti-inflammatories
Although the study was small and only hypothesis-generating, the fact that outcomes for actively treated patients were proportional to baseline IL-6 levels “strongly suggests that PCSK9 inhibition can directly modulate inflammation in COVID-19,” argues an editorial accompanying the report.
and likely sheds light on “mechanisms through which PCSK9 inhibition dually modulates lipoprotein metabolism and inflammation,” write Sascha N. Goonewardena, MD, University of Michigan, Ann Arbor, and Robert S. Rosenson, MD, Icahn School of Medicine at Mount Sinai, New York.
The results are consistent with prior evidence that the drugs are anti-inflammatory at least partly because of their interference with inflammatory pathways triggered by PCSK9 and mediated by IL-6, as described by Dr. Navarese and colleagues.
Indeed, they write, PCSK9 inhibitors may improve COVID outcomes mostly through mechanisms unrelated to LDL-receptor expression, “including direct inhibition of PCSK9-triggered inflammation.”
If true, the authors observe, it might explain “why the positive findings of the present study have not been consistently observed in trials involving other lipid-lowering agents, such as statins.” Those drugs are well-known to decrease levels of the inflammatory biomarker C-reactive protein.
In patients with stable coronary disease, in whom inflammation is typically tracked by measuring CRP, “the PCSK9 inhibitors have not been shown to have an anti-inflammatory effect,” Dr. Rosenson further explained.
But the current study’s patients with acute, severe COVID-19, a “profound inflammatory insult” with upregulation of IL-6, were “a good population” for evaluating the drugs’ potential anti-inflammatory effects, Dr. Rosenson said in an interview. The results “are quite enticing but require corroboration in a larger trial.”
A single injection
The IMPACT-SIRIO 5 trial entered 60 adults hospitalized with severe COVID-19 and elevated IL-6 at four centers in Poland. Patients with other known active infections were excluded.
They were randomly assigned double-blind to receive a 140 mg injection of evolocumab (Repatha) or placebo. The 2 groups were similar with respect to demographics, body-mass index, time since symptom onset, and treatments for managing COVID-19 and its complications.
Rates of death or need for intubation at 30 days, the primary endpoint, were 23.3% in the PCSK9-inhibitor group and 53.3% for controls, a risk difference of 30% (95% confidence interval –53.4% to –6.6%). The median durations of oxygen therapy were significantly different at 13 days and 20 days, respectively, the report states.
Serum IL-6 levels fell further over 30 days in the PCSK9-inhibitor group (–56% vs. –21% among controls). A drop by more than 90% was seen in 60% of patients in the PCSK9-inhibitor group and in 27% of controls.
The average hospital stay was shorter for those getting the PCSK9 inhibitor, compared with placebo, 16 days versus 22 days, and their 30-day mortality was numerically lower, 16% versus 33.3%.
Patients’ baseline IL-6 levels above the median, the report states, had a lower mortality on the PCSK9 inhibitor versus placebo (risk difference –37.5%; 95% CI –68.2% to –6.70%).
A larger trial to corroborate these results would potentially enter similar patients hospitalized with COVID-19 with reproducible evidence of an ongoing cytokine storm, such as elevated levels of IL-6, who would be assigned to either a PCSK9 inhibitor or placebo, Dr. Rosenson proposed.
Although the current primary endpoint that combines mortality and intubation was “reasonable” for a small pilot trial, he said, if the researchers embark on a larger study, “they’ll want to look at those events separately.”
Dr. Navarese discloses receiving speaker and consultancy fees from Amgen, Sanofi-Regeneron, Bayer; and grants from Abbott. Disclosures for the other authors are in the report. Rosenson discloses receiving research funding to his institution from Amgen, Arrowhead, Eli Lilly, Novartis, and Regeneron; consulting fees from Amgen, Arrowhead, CRISPR Therapeutics, Eli Lilly, Lipigon, Novartis, Precision Biosciences, Regeneron, Ultragenyx, and Verve; speaking fees from Amgen, Kowa, and Regeneron; and royalties from Wolters Kluwer; and owning stock in MediMergent. Dr. Goonewardena reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Microneedling With Bimatoprost to Treat Hypopigmented Skin Caused by Burn Scars
To the Editor:
Microneedling is a percutaneous collagen induction therapy frequently used in cosmetic dermatology to promote skin rejuvenation and hair growth and to treat scars by taking advantage of the body’s natural wound-healing cascade.1 The procedure works by generating thousands of microscopic wounds in the dermis with minimal damage to the epidermis, thus initiating the wound-healing cascade and subsequently promoting collagen production in a manner safe for all Fitzpatrick classification skin types.1-3 This therapy effectively treats scars by breaking down scarred collagen and replacing it with new healthy collagen. Microneedling also has application in drug delivery by increasing the permeability of the skin; the microwounds generated can serve as a portal for drug delivery.4
Bimatoprost is a prostaglandin analogue typically used to treat hypotrichosis and open-angle glaucoma.5-7 A known side effect of bimatoprost is hyperpigmentation of surrounding skin; the drug increases melanogenesis, melanocyte proliferation, and melanocyte dendricity, resulting in activation of the inflammatory response and subsequent prostaglandin release, which stimulates melanogenesis. This effect is similar to UV radiation–induced inflammation and hyperpigmentation.6,8
Capitalizing on this effect, a novel application of bimatoprost has been proposed—treating vitiligo, in which hypopigmentation results from destruction of melanocytes in certain areas of the skin. Bimatoprost ophthalmic solution 0.3% utilized as an off-label treatment for vitiligo has been shown to notably increase melanogenesis and return pigmentation to hypopigmented areas.8-10
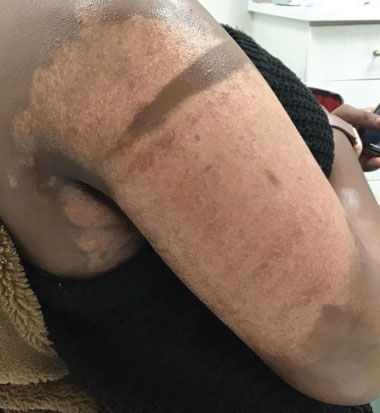
A 32-year-old Black woman presented to our clinic with a 40×15-cm scar that was marked by postinflammatory hypopigmentation from a second-degree burn on the right proximal arm. The patient had been burned 5 months prior by boiling water that was spilled on the arm while cooking. She had immediately sought treatment at an emergency department and subsequently in a burn unit, where the burn was debrided twice; medication was not prescribed to continue treatment. The patient reported that the scarring and hypopigmentation had taken a psychologic toll; her hope was to have pigmentation restored to the affected area to boost her confidence.
Physical examination revealed that the burn wound had healed but visible scarring and severe hypopigmentation due to destroyed melanocytes remained (Figure 1). To inhibit inflammation and stimulate repigmentation, we prescribed the calcineurin inhibitor tacrolimus ointment 0.1% to be applied daily to the affected area. The patient returned to the clinic 1 month later. Perifollicular hyperpigmentation was noted at the site of the scar.
Monthly microneedling sessions with bimatoprost ophthalmic solution 0.3% were started. To avoid damaging any potentially remaining unhealed hypodermis and vasculature, the first microneedling session was performed with 9 needles set at minimal needle depth and frequency. The number of needles and their depth and frequency gradually were increased with each subsequent treatment. The patient continued tacrolimus ointment 0.1% throughout the course of treatment.
For each microneedling procedure, a handheld motorized microneedling device was applied to the skin at a depth of 0.25 mm, which was gradually increased until pinpoint petechiae were achieved. Bimatoprost ophthalmic solution 0.3% was then painted on the skin and allowed to absorb. Microneedling was performed again, ensuring that bimatoprost entered the skin in the area of the burn scar.
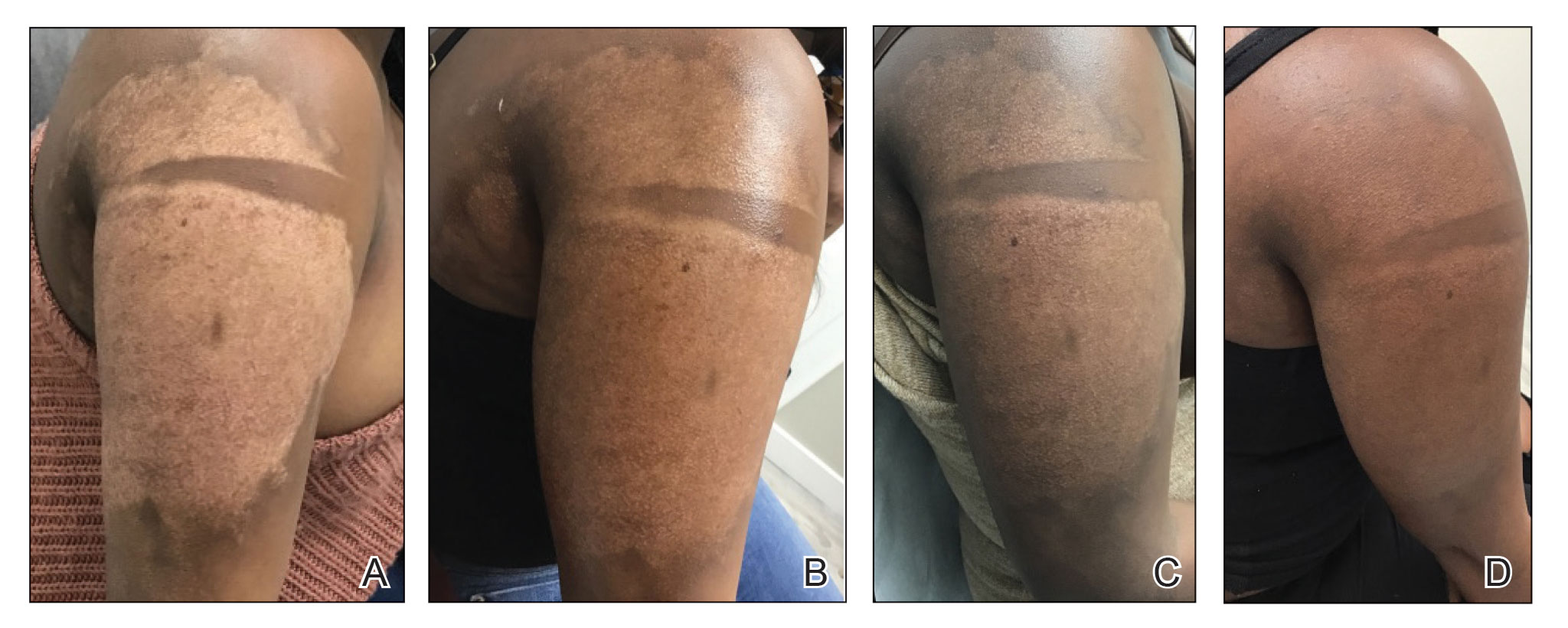
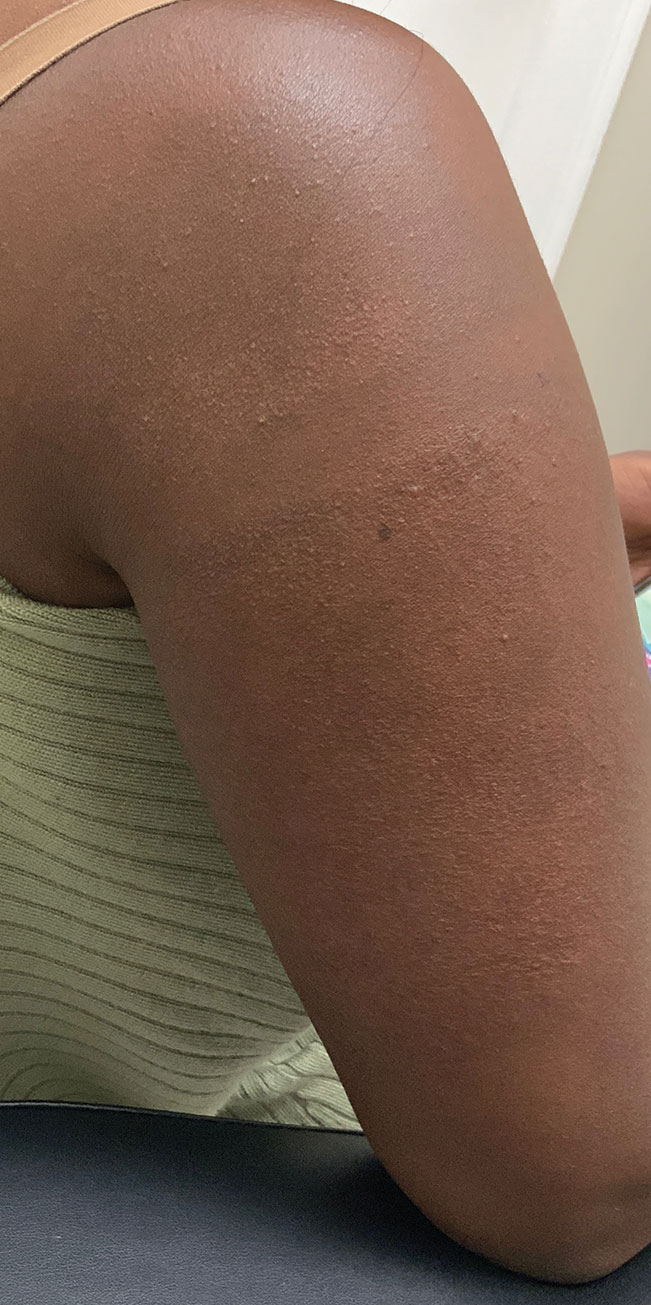
Microneedling procedures were performed monthly for 6 months, then once 3 months later, and once more 3 months later—8 treatments in total over the course of 1 year. Improvement in skin pigmentation was noted at each visit (Figure 2). Repigmentation was first noticed surrounding hair follicles; after later visits, it was observed that pigmentation began to spread from hair follicles to fill in remaining skin. The darkest areas of pigmentation were first noted around hair follicles; over time, melanocytes appeared to spontaneously regenerate and fill in surrounding areas as the scar continued to heal. The patient continued use of tacrolimus during the entire course of microneedling treatments and for the following 4 months. Sixteen months after initiation of treatment, the appearance of the skin was texturally smooth and returned to almost its original pigmentation (Figure 3).
We report a successful outcome in a patient with a hypopigmented burn scar who was treated with bimatoprost administered with traditional microneedling and alongside a tacrolimus regimen. Tacrolimus ointment inhibited the inflammatory response to allow melanocytes to heal and regenerate; bimatoprost and microneedling promoted hyperpigmentation of hair follicles in the affected area, eventually restoring pigmentation to the entire area. Our patient was extremely satisfied with the results of this combination treatment. She has reported feeling more confident going out and wearing short-sleeved clothing. Percutaneous drug delivery of bimatoprost ophthalmic solution 0.3% combined with topical tacrolimus may be an effective treatment for skin repigmentation. Further investigation of this regimen is needed to develop standardized treatment protocols.
- Juhasz MLW, Cohen JL. Micro-needling for the treatment of scars: an update for clinicians. Clin Cosmet Investig Dermatol. 2020;13:997-1003. doi:10.2147/CCID.S267192
- Alster TS, Li MKY. Micro-needling of scars: a large prospective study with long-term follow-up. Plast Reconstr Surg. 2020;145:358-364. doi:10.1097/PRS.0000000000006462
- Aust MC, Knobloch K, Reimers K, et al. Percutaneous collagen induction therapy: an alternative treatment for burn scars. Burns. 2010;36:836-843. doi:10.1016/j.burns.2009.11.014
- Kim Y-C, Park J-H, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64:1547-1568. doi:10.1016/j.addr.2012.04.005
- Doshi M, Edward DP, Osmanovic S. Clinical course of bimatoprost-induced periocular skin changes in Caucasians. Ophthalmology. 2006;113:1961-1967. doi:10.1016/j.ophtha.2006.05.041
- Kapur R, Osmanovic S, Toyran S, et al. Bimatoprost-induced periocular skin hyperpigmentation: histopathological study. Arch Ophthalmol. 2005;123:1541-1546. doi:10.1001/archopht.123.11.1541
- Priluck JC, Fu S. Latisse-induced periocular skin hyperpigmentation. Arch Ophthalmol. 2010;128:792-793. doi:10.1001/archophthalmol.2010.89
- Grimes PE. Bimatoprost 0.03% solution for the treatment of nonfacial vitiligo. J Drugs Dermatol. 2016;15:703-710.
- Barbulescu C, Goldstein N, Roop D, et al. Harnessing the power of regenerative therapy for vitiligo and alopecia areata. J Invest Dermatol. 2020;140: 29-37. doi:10.1016/j.jid.2019.03.1142
- Kanokrungsee S, Pruettivorawongse D, Rajatanavin N. Clinicaloutcomes of topical bimatoprost for nonsegmental facial vitiligo: a preliminary study. J Cosmet Dermatol. 2021;20:812-818. doi.org/10.1111/jocd.13648
To the Editor:
Microneedling is a percutaneous collagen induction therapy frequently used in cosmetic dermatology to promote skin rejuvenation and hair growth and to treat scars by taking advantage of the body’s natural wound-healing cascade.1 The procedure works by generating thousands of microscopic wounds in the dermis with minimal damage to the epidermis, thus initiating the wound-healing cascade and subsequently promoting collagen production in a manner safe for all Fitzpatrick classification skin types.1-3 This therapy effectively treats scars by breaking down scarred collagen and replacing it with new healthy collagen. Microneedling also has application in drug delivery by increasing the permeability of the skin; the microwounds generated can serve as a portal for drug delivery.4
Bimatoprost is a prostaglandin analogue typically used to treat hypotrichosis and open-angle glaucoma.5-7 A known side effect of bimatoprost is hyperpigmentation of surrounding skin; the drug increases melanogenesis, melanocyte proliferation, and melanocyte dendricity, resulting in activation of the inflammatory response and subsequent prostaglandin release, which stimulates melanogenesis. This effect is similar to UV radiation–induced inflammation and hyperpigmentation.6,8
Capitalizing on this effect, a novel application of bimatoprost has been proposed—treating vitiligo, in which hypopigmentation results from destruction of melanocytes in certain areas of the skin. Bimatoprost ophthalmic solution 0.3% utilized as an off-label treatment for vitiligo has been shown to notably increase melanogenesis and return pigmentation to hypopigmented areas.8-10
A 32-year-old Black woman presented to our clinic with a 40×15-cm scar that was marked by postinflammatory hypopigmentation from a second-degree burn on the right proximal arm. The patient had been burned 5 months prior by boiling water that was spilled on the arm while cooking. She had immediately sought treatment at an emergency department and subsequently in a burn unit, where the burn was debrided twice; medication was not prescribed to continue treatment. The patient reported that the scarring and hypopigmentation had taken a psychologic toll; her hope was to have pigmentation restored to the affected area to boost her confidence.
Physical examination revealed that the burn wound had healed but visible scarring and severe hypopigmentation due to destroyed melanocytes remained (Figure 1). To inhibit inflammation and stimulate repigmentation, we prescribed the calcineurin inhibitor tacrolimus ointment 0.1% to be applied daily to the affected area. The patient returned to the clinic 1 month later. Perifollicular hyperpigmentation was noted at the site of the scar.

Monthly microneedling sessions with bimatoprost ophthalmic solution 0.3% were started. To avoid damaging any potentially remaining unhealed hypodermis and vasculature, the first microneedling session was performed with 9 needles set at minimal needle depth and frequency. The number of needles and their depth and frequency gradually were increased with each subsequent treatment. The patient continued tacrolimus ointment 0.1% throughout the course of treatment.
For each microneedling procedure, a handheld motorized microneedling device was applied to the skin at a depth of 0.25 mm, which was gradually increased until pinpoint petechiae were achieved. Bimatoprost ophthalmic solution 0.3% was then painted on the skin and allowed to absorb. Microneedling was performed again, ensuring that bimatoprost entered the skin in the area of the burn scar.
Microneedling procedures were performed monthly for 6 months, then once 3 months later, and once more 3 months later—8 treatments in total over the course of 1 year. Improvement in skin pigmentation was noted at each visit (Figure 2). Repigmentation was first noticed surrounding hair follicles; after later visits, it was observed that pigmentation began to spread from hair follicles to fill in remaining skin. The darkest areas of pigmentation were first noted around hair follicles; over time, melanocytes appeared to spontaneously regenerate and fill in surrounding areas as the scar continued to heal. The patient continued use of tacrolimus during the entire course of microneedling treatments and for the following 4 months. Sixteen months after initiation of treatment, the appearance of the skin was texturally smooth and returned to almost its original pigmentation (Figure 3).

We report a successful outcome in a patient with a hypopigmented burn scar who was treated with bimatoprost administered with traditional microneedling and alongside a tacrolimus regimen. Tacrolimus ointment inhibited the inflammatory response to allow melanocytes to heal and regenerate; bimatoprost and microneedling promoted hyperpigmentation of hair follicles in the affected area, eventually restoring pigmentation to the entire area. Our patient was extremely satisfied with the results of this combination treatment. She has reported feeling more confident going out and wearing short-sleeved clothing. Percutaneous drug delivery of bimatoprost ophthalmic solution 0.3% combined with topical tacrolimus may be an effective treatment for skin repigmentation. Further investigation of this regimen is needed to develop standardized treatment protocols.

To the Editor:
Microneedling is a percutaneous collagen induction therapy frequently used in cosmetic dermatology to promote skin rejuvenation and hair growth and to treat scars by taking advantage of the body’s natural wound-healing cascade.1 The procedure works by generating thousands of microscopic wounds in the dermis with minimal damage to the epidermis, thus initiating the wound-healing cascade and subsequently promoting collagen production in a manner safe for all Fitzpatrick classification skin types.1-3 This therapy effectively treats scars by breaking down scarred collagen and replacing it with new healthy collagen. Microneedling also has application in drug delivery by increasing the permeability of the skin; the microwounds generated can serve as a portal for drug delivery.4
Bimatoprost is a prostaglandin analogue typically used to treat hypotrichosis and open-angle glaucoma.5-7 A known side effect of bimatoprost is hyperpigmentation of surrounding skin; the drug increases melanogenesis, melanocyte proliferation, and melanocyte dendricity, resulting in activation of the inflammatory response and subsequent prostaglandin release, which stimulates melanogenesis. This effect is similar to UV radiation–induced inflammation and hyperpigmentation.6,8
Capitalizing on this effect, a novel application of bimatoprost has been proposed—treating vitiligo, in which hypopigmentation results from destruction of melanocytes in certain areas of the skin. Bimatoprost ophthalmic solution 0.3% utilized as an off-label treatment for vitiligo has been shown to notably increase melanogenesis and return pigmentation to hypopigmented areas.8-10
A 32-year-old Black woman presented to our clinic with a 40×15-cm scar that was marked by postinflammatory hypopigmentation from a second-degree burn on the right proximal arm. The patient had been burned 5 months prior by boiling water that was spilled on the arm while cooking. She had immediately sought treatment at an emergency department and subsequently in a burn unit, where the burn was debrided twice; medication was not prescribed to continue treatment. The patient reported that the scarring and hypopigmentation had taken a psychologic toll; her hope was to have pigmentation restored to the affected area to boost her confidence.
Physical examination revealed that the burn wound had healed but visible scarring and severe hypopigmentation due to destroyed melanocytes remained (Figure 1). To inhibit inflammation and stimulate repigmentation, we prescribed the calcineurin inhibitor tacrolimus ointment 0.1% to be applied daily to the affected area. The patient returned to the clinic 1 month later. Perifollicular hyperpigmentation was noted at the site of the scar.

Monthly microneedling sessions with bimatoprost ophthalmic solution 0.3% were started. To avoid damaging any potentially remaining unhealed hypodermis and vasculature, the first microneedling session was performed with 9 needles set at minimal needle depth and frequency. The number of needles and their depth and frequency gradually were increased with each subsequent treatment. The patient continued tacrolimus ointment 0.1% throughout the course of treatment.
For each microneedling procedure, a handheld motorized microneedling device was applied to the skin at a depth of 0.25 mm, which was gradually increased until pinpoint petechiae were achieved. Bimatoprost ophthalmic solution 0.3% was then painted on the skin and allowed to absorb. Microneedling was performed again, ensuring that bimatoprost entered the skin in the area of the burn scar.
Microneedling procedures were performed monthly for 6 months, then once 3 months later, and once more 3 months later—8 treatments in total over the course of 1 year. Improvement in skin pigmentation was noted at each visit (Figure 2). Repigmentation was first noticed surrounding hair follicles; after later visits, it was observed that pigmentation began to spread from hair follicles to fill in remaining skin. The darkest areas of pigmentation were first noted around hair follicles; over time, melanocytes appeared to spontaneously regenerate and fill in surrounding areas as the scar continued to heal. The patient continued use of tacrolimus during the entire course of microneedling treatments and for the following 4 months. Sixteen months after initiation of treatment, the appearance of the skin was texturally smooth and returned to almost its original pigmentation (Figure 3).

We report a successful outcome in a patient with a hypopigmented burn scar who was treated with bimatoprost administered with traditional microneedling and alongside a tacrolimus regimen. Tacrolimus ointment inhibited the inflammatory response to allow melanocytes to heal and regenerate; bimatoprost and microneedling promoted hyperpigmentation of hair follicles in the affected area, eventually restoring pigmentation to the entire area. Our patient was extremely satisfied with the results of this combination treatment. She has reported feeling more confident going out and wearing short-sleeved clothing. Percutaneous drug delivery of bimatoprost ophthalmic solution 0.3% combined with topical tacrolimus may be an effective treatment for skin repigmentation. Further investigation of this regimen is needed to develop standardized treatment protocols.

- Juhasz MLW, Cohen JL. Micro-needling for the treatment of scars: an update for clinicians. Clin Cosmet Investig Dermatol. 2020;13:997-1003. doi:10.2147/CCID.S267192
- Alster TS, Li MKY. Micro-needling of scars: a large prospective study with long-term follow-up. Plast Reconstr Surg. 2020;145:358-364. doi:10.1097/PRS.0000000000006462
- Aust MC, Knobloch K, Reimers K, et al. Percutaneous collagen induction therapy: an alternative treatment for burn scars. Burns. 2010;36:836-843. doi:10.1016/j.burns.2009.11.014
- Kim Y-C, Park J-H, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64:1547-1568. doi:10.1016/j.addr.2012.04.005
- Doshi M, Edward DP, Osmanovic S. Clinical course of bimatoprost-induced periocular skin changes in Caucasians. Ophthalmology. 2006;113:1961-1967. doi:10.1016/j.ophtha.2006.05.041
- Kapur R, Osmanovic S, Toyran S, et al. Bimatoprost-induced periocular skin hyperpigmentation: histopathological study. Arch Ophthalmol. 2005;123:1541-1546. doi:10.1001/archopht.123.11.1541
- Priluck JC, Fu S. Latisse-induced periocular skin hyperpigmentation. Arch Ophthalmol. 2010;128:792-793. doi:10.1001/archophthalmol.2010.89
- Grimes PE. Bimatoprost 0.03% solution for the treatment of nonfacial vitiligo. J Drugs Dermatol. 2016;15:703-710.
- Barbulescu C, Goldstein N, Roop D, et al. Harnessing the power of regenerative therapy for vitiligo and alopecia areata. J Invest Dermatol. 2020;140: 29-37. doi:10.1016/j.jid.2019.03.1142
- Kanokrungsee S, Pruettivorawongse D, Rajatanavin N. Clinicaloutcomes of topical bimatoprost for nonsegmental facial vitiligo: a preliminary study. J Cosmet Dermatol. 2021;20:812-818. doi.org/10.1111/jocd.13648
- Juhasz MLW, Cohen JL. Micro-needling for the treatment of scars: an update for clinicians. Clin Cosmet Investig Dermatol. 2020;13:997-1003. doi:10.2147/CCID.S267192
- Alster TS, Li MKY. Micro-needling of scars: a large prospective study with long-term follow-up. Plast Reconstr Surg. 2020;145:358-364. doi:10.1097/PRS.0000000000006462
- Aust MC, Knobloch K, Reimers K, et al. Percutaneous collagen induction therapy: an alternative treatment for burn scars. Burns. 2010;36:836-843. doi:10.1016/j.burns.2009.11.014
- Kim Y-C, Park J-H, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64:1547-1568. doi:10.1016/j.addr.2012.04.005
- Doshi M, Edward DP, Osmanovic S. Clinical course of bimatoprost-induced periocular skin changes in Caucasians. Ophthalmology. 2006;113:1961-1967. doi:10.1016/j.ophtha.2006.05.041
- Kapur R, Osmanovic S, Toyran S, et al. Bimatoprost-induced periocular skin hyperpigmentation: histopathological study. Arch Ophthalmol. 2005;123:1541-1546. doi:10.1001/archopht.123.11.1541
- Priluck JC, Fu S. Latisse-induced periocular skin hyperpigmentation. Arch Ophthalmol. 2010;128:792-793. doi:10.1001/archophthalmol.2010.89
- Grimes PE. Bimatoprost 0.03% solution for the treatment of nonfacial vitiligo. J Drugs Dermatol. 2016;15:703-710.
- Barbulescu C, Goldstein N, Roop D, et al. Harnessing the power of regenerative therapy for vitiligo and alopecia areata. J Invest Dermatol. 2020;140: 29-37. doi:10.1016/j.jid.2019.03.1142
- Kanokrungsee S, Pruettivorawongse D, Rajatanavin N. Clinicaloutcomes of topical bimatoprost for nonsegmental facial vitiligo: a preliminary study. J Cosmet Dermatol. 2021;20:812-818. doi.org/10.1111/jocd.13648
PRACTICE POINTS
- Microneedling is a percutaneous collagen induction therapy that also may be used in drug delivery.
- Hypopigmentation can cause considerable distress for patients with skin of color.
- Percutaneous drug delivery of bimatoprost may be helpful in skin repigmentation.
Guidelines recommend CBT alone for mild acute depression, more options for more severe cases
The guidelines also state that patients with mild depression should start with CBT alone, and if a patient with moderate to severe depression prefers, they can use a combination of both CBT and an SGA.
These nuanced recommendations contrast sharply with the 2016 ACP guidelines for depression, which lumped all stages and severity levels together, and came with just one recommendation: Clinicians should choose between CBT and an SGA.
More data have come to light over the years, requiring the present update, reported lead author Amir Qaseem, MD, PhD, vice president of Clinical Policy and the Center for Evidence Reviews at the ACP, and adjunct faculty at Thomas Jefferson University, Philadelphia, and colleagues.
In addition to the focus on acute depression, Dr. Qaseem and colleagues highlighted the new guidelines' “consideration of patient values and preferences, and costs,” as well as responses to therapy.
Recommendations were derived from a network meta-analysis that included studies evaluating nonpharmacologic and pharmacologic therapies, the authors wrote in Annals of Internal Medicine. They compared effectiveness across a range of SGAs, “including selective serotonin reuptake inhibitors; serotonin-norepinephrine reuptake inhibitors; and others such as bupropion, mirtazapine, nefazodone, trazodone, vilazodone, and vortioxetine.”
This analysis yielded three pieces of clinical advice.
First, patients in the acute phase of mild depression should receive CBT alone as their initial treatment.
Dr. Qaseem and colleagues noted that many depression studies for pharmacologic therapies excluded these patients in favor of those with moderate to severe depression, leaving an evidence gap.
“Furthermore, the Clinical Guidelines Committee had concerns about adverse effects of SGAs in these patients and suggests that the use of SGAs as initial treatment of these patients should be based on additional considerations, such as limited access to or cost of CBT, history of moderate or severe major depressive disorder, or patient preferences,” they added.
The committee’s next recommendation, based on moderate-certainty evidence, suggested that CBT alone or an SGA alone should be considered for patients in the acute phase of moderate to severe depression. This call for monotherapy is balanced by a conditional recommendation based on low-certainty evidence that the same group may benefit from initial combination therapy with both CBT and an SGA.
“The informed decision on the options of monotherapy with CBT versus SGAs, or combination therapy, should be personalized and based on discussion of potential treatment benefits, harms, adverse effect profiles, cost, feasibility, patients’ specific symptoms (such as insomnia, hypersomnia, or fluctuation in appetite), comorbidities, concomitant medication use, and patient preferences,” the guidelines state.
The third and final recommendation offers an algorithm for patients who do not respond to initial therapy with an SGA. Multiple pathways are provided: Switch to CBT or augment with CBT; or switch to a different SGA or augment with a second pharmacologic therapy, such as mirtazapine, bupropion, or buspirone.
“These second-line treatment strategies show similar efficacy when compared with each other,” the guidelines committee noted.
Again, the guidelines suggest that second-line choices should be personalized based on the various factors previously discussed.
A timely update
“The new guideline is very different from the last guideline,” said Ryan Mire, MD, president of the ACP and practicing internal medicine physician in Nashville, Tenn. in a written comment. “ACP decided to update the depression guidelines with a focus on acute depression because approximately 70% of patients with major depressive disorder do not achieve remission and remain in the acute phase after the initial pharmacologic treatment attempt. In addition, there is new evidence on second-line treatments since the 2016 ACP guideline was published.”
Neil S. Skolnik, MD, of Thomas Jefferson University, Philadelphia, agreed that the guidelines offer a necessary and fresh perspective on caring for patients with depression.
“These guidelines are a helpful update, assuring us that we are using the latest, evidence-based therapies, and [they] are written in a practical, easy-to-implement manner,” Dr. Skolnik said in a written comment.
“First, the guidelines reaffirm that CBT is an effective first-line option, with or without the concurrent use of an SGA,” Dr. Skolnik said, noting that CBT alone may reduce likelihood of recurrence, compared with an SGA alone. “Many patients do not like the idea of medication, or the potential side effects of medications, and CBT is an evidenced-based approach that can be very helpful for patients.”
Dr. Skolnik also applauded the guidelines authors for offering a clear path forward for patients who do not have full remission after treatment – a common clinical scenario.
He went on to offer some more detailed steps forward.
“If someone chooses to be treated with an SGA alone and has not had much response at all to an initial SGA, usually a selective serotonin reuptake inhibitor, I’ll usually switch to a different SSRI or serotonin and norepinephrine reuptake inhibitor (SNRI) and/or add CBT,” Dr. Skolnik said. “If they have had a partial response, I’ll often encourage CBT and consider the addition of augmentation with an additional medication as discussed in the guidelines.”
Valuable despite the gaps
Other experts expressed mixed impressions of the update, noting both highs and lows.
“Although [this guideline] has some gaps, it is more valuable in several ways than other widely consulted practice guidelines for depression,” wrote Miriam Shuchman, MD and Elia Abi-Jaoude, MSc, MD, PhD, of the University of Toronto, in an accompanying editorial.
Specifically, they praised the publication’s focus on shared decision-making in the treatment planning process.
“This effort to respond to patient preferences is crucial and may even increase the chance that patients will improve with treatment,” they wrote.
They also applauded the ACP’s efforts to recuse any committee members who may have had conflicts of interest “that could affect their judgment about treatments for depression.”
After highlighting these attributes, Dr. Shuchman and Dr. Abi-Jaoude noted that the guidelines still contain “significant gaps.”
Foremost, they pointed out the guidelines' emphasis on CBT to the exclusion of other nonpharmacologic options.
“The guideline does patients a disservice by leaving out several nonmedication treatment options that clinicians can offer as first- or second-line therapies,” they wrote.
This oversight may increase risk that patients simply hop from one SGA to another, which is a common, and often ineffective, strategy, according to Dr. Shuchman and Dr. Abi-Jaoude.
“Patients often go from one drug to the next in the hopes of landing on one that ‘works,’ ” the editorialists wrote. “This narrow clinical approach of pursuing medication-based treatments ignores the ways difficulties in a person’s work or relationships may contribute to their struggles with depression. At a time when the COVID-19 pandemic has underscored the importance of the social context of mental health, clinicians may need to consider other forms of support and tailor prescribing to what is most relevant and accessible for a particular patient.”
Dr. Shuchman and Dr. Abi-Jaoude went on to suggest several nonpharmacologic options beyond CBT, including interpersonal therapy, psychodynamic therapy, problem solving, behavioral activation, and guided self-help.
The other key gap they pointed out relates to withdrawal.
Although the guideline does advise physicians to taper antidepressants to reduce risk of withdrawal, the editorialists suggested that this recommendation lacked sufficient emphasis, as it can be a particularly difficult period in the treatment process.
“Tapering of an antidepressant may need to be done over months or years, not weeks, and a patient may need to visit a compounding pharmacy to obtain doses of a second-generation antidepressant not marketed by drug manufacturers so that prescriptions can be tapered even more slowly,” they suggested.
Financial costs remain unclear
Beyond the above medical considerations, one other piece of the depression puzzle remains unsolved: cost.
In a simultaneously published rapid review, Andreea Dobrescu, MD, PhD, of Cochrane Austria, and colleagues evaluated the relative cost-effectiveness of first- and second-step treatment strategies.
For most comparisons, evidence was insufficient to reach a conclusion, although they suggested that CBT may be more cost effective at the 5-year mark.
“For most pharmacologic and nonpharmacologic interventions for major depressive disorder, evidence was missing or was insufficient to draw conclusions about the cost-effectiveness of first- or second-step treatments for MDD,” Dr. Dobrescu and colleagues wrote. “The strongest evidence (albeit still low certainty of evidence) was for the cost-effectiveness of CBT compared with SGA as a first-step treatment over a 5-year time horizon from the societal and health care sector perspectives. However, this evidence should also be interpreted cautiously considering it is based on a single study.”
When asked about the financial findings, Dr. Mire agreed that more data are needed, especially because CBT and SGA costs range widely. He suggested that cost, for each patient, should be considered in the personalized approach now highlighted by the new guidelines.
The guidelines and the Cochrane cost-effectiveness study were supported by the ACP. The guidelines' authors and other individuals quoted in this article reported no conflicts of interest.
The guidelines also state that patients with mild depression should start with CBT alone, and if a patient with moderate to severe depression prefers, they can use a combination of both CBT and an SGA.
These nuanced recommendations contrast sharply with the 2016 ACP guidelines for depression, which lumped all stages and severity levels together, and came with just one recommendation: Clinicians should choose between CBT and an SGA.
More data have come to light over the years, requiring the present update, reported lead author Amir Qaseem, MD, PhD, vice president of Clinical Policy and the Center for Evidence Reviews at the ACP, and adjunct faculty at Thomas Jefferson University, Philadelphia, and colleagues.
In addition to the focus on acute depression, Dr. Qaseem and colleagues highlighted the new guidelines' “consideration of patient values and preferences, and costs,” as well as responses to therapy.
Recommendations were derived from a network meta-analysis that included studies evaluating nonpharmacologic and pharmacologic therapies, the authors wrote in Annals of Internal Medicine. They compared effectiveness across a range of SGAs, “including selective serotonin reuptake inhibitors; serotonin-norepinephrine reuptake inhibitors; and others such as bupropion, mirtazapine, nefazodone, trazodone, vilazodone, and vortioxetine.”
This analysis yielded three pieces of clinical advice.
First, patients in the acute phase of mild depression should receive CBT alone as their initial treatment.
Dr. Qaseem and colleagues noted that many depression studies for pharmacologic therapies excluded these patients in favor of those with moderate to severe depression, leaving an evidence gap.
“Furthermore, the Clinical Guidelines Committee had concerns about adverse effects of SGAs in these patients and suggests that the use of SGAs as initial treatment of these patients should be based on additional considerations, such as limited access to or cost of CBT, history of moderate or severe major depressive disorder, or patient preferences,” they added.
The committee’s next recommendation, based on moderate-certainty evidence, suggested that CBT alone or an SGA alone should be considered for patients in the acute phase of moderate to severe depression. This call for monotherapy is balanced by a conditional recommendation based on low-certainty evidence that the same group may benefit from initial combination therapy with both CBT and an SGA.
“The informed decision on the options of monotherapy with CBT versus SGAs, or combination therapy, should be personalized and based on discussion of potential treatment benefits, harms, adverse effect profiles, cost, feasibility, patients’ specific symptoms (such as insomnia, hypersomnia, or fluctuation in appetite), comorbidities, concomitant medication use, and patient preferences,” the guidelines state.
The third and final recommendation offers an algorithm for patients who do not respond to initial therapy with an SGA. Multiple pathways are provided: Switch to CBT or augment with CBT; or switch to a different SGA or augment with a second pharmacologic therapy, such as mirtazapine, bupropion, or buspirone.
“These second-line treatment strategies show similar efficacy when compared with each other,” the guidelines committee noted.
Again, the guidelines suggest that second-line choices should be personalized based on the various factors previously discussed.
A timely update
“The new guideline is very different from the last guideline,” said Ryan Mire, MD, president of the ACP and practicing internal medicine physician in Nashville, Tenn. in a written comment. “ACP decided to update the depression guidelines with a focus on acute depression because approximately 70% of patients with major depressive disorder do not achieve remission and remain in the acute phase after the initial pharmacologic treatment attempt. In addition, there is new evidence on second-line treatments since the 2016 ACP guideline was published.”
Neil S. Skolnik, MD, of Thomas Jefferson University, Philadelphia, agreed that the guidelines offer a necessary and fresh perspective on caring for patients with depression.
“These guidelines are a helpful update, assuring us that we are using the latest, evidence-based therapies, and [they] are written in a practical, easy-to-implement manner,” Dr. Skolnik said in a written comment.
“First, the guidelines reaffirm that CBT is an effective first-line option, with or without the concurrent use of an SGA,” Dr. Skolnik said, noting that CBT alone may reduce likelihood of recurrence, compared with an SGA alone. “Many patients do not like the idea of medication, or the potential side effects of medications, and CBT is an evidenced-based approach that can be very helpful for patients.”
Dr. Skolnik also applauded the guidelines authors for offering a clear path forward for patients who do not have full remission after treatment – a common clinical scenario.
He went on to offer some more detailed steps forward.
“If someone chooses to be treated with an SGA alone and has not had much response at all to an initial SGA, usually a selective serotonin reuptake inhibitor, I’ll usually switch to a different SSRI or serotonin and norepinephrine reuptake inhibitor (SNRI) and/or add CBT,” Dr. Skolnik said. “If they have had a partial response, I’ll often encourage CBT and consider the addition of augmentation with an additional medication as discussed in the guidelines.”
Valuable despite the gaps
Other experts expressed mixed impressions of the update, noting both highs and lows.
“Although [this guideline] has some gaps, it is more valuable in several ways than other widely consulted practice guidelines for depression,” wrote Miriam Shuchman, MD and Elia Abi-Jaoude, MSc, MD, PhD, of the University of Toronto, in an accompanying editorial.
Specifically, they praised the publication’s focus on shared decision-making in the treatment planning process.
“This effort to respond to patient preferences is crucial and may even increase the chance that patients will improve with treatment,” they wrote.
They also applauded the ACP’s efforts to recuse any committee members who may have had conflicts of interest “that could affect their judgment about treatments for depression.”
After highlighting these attributes, Dr. Shuchman and Dr. Abi-Jaoude noted that the guidelines still contain “significant gaps.”
Foremost, they pointed out the guidelines' emphasis on CBT to the exclusion of other nonpharmacologic options.
“The guideline does patients a disservice by leaving out several nonmedication treatment options that clinicians can offer as first- or second-line therapies,” they wrote.
This oversight may increase risk that patients simply hop from one SGA to another, which is a common, and often ineffective, strategy, according to Dr. Shuchman and Dr. Abi-Jaoude.
“Patients often go from one drug to the next in the hopes of landing on one that ‘works,’ ” the editorialists wrote. “This narrow clinical approach of pursuing medication-based treatments ignores the ways difficulties in a person’s work or relationships may contribute to their struggles with depression. At a time when the COVID-19 pandemic has underscored the importance of the social context of mental health, clinicians may need to consider other forms of support and tailor prescribing to what is most relevant and accessible for a particular patient.”
Dr. Shuchman and Dr. Abi-Jaoude went on to suggest several nonpharmacologic options beyond CBT, including interpersonal therapy, psychodynamic therapy, problem solving, behavioral activation, and guided self-help.
The other key gap they pointed out relates to withdrawal.
Although the guideline does advise physicians to taper antidepressants to reduce risk of withdrawal, the editorialists suggested that this recommendation lacked sufficient emphasis, as it can be a particularly difficult period in the treatment process.
“Tapering of an antidepressant may need to be done over months or years, not weeks, and a patient may need to visit a compounding pharmacy to obtain doses of a second-generation antidepressant not marketed by drug manufacturers so that prescriptions can be tapered even more slowly,” they suggested.
Financial costs remain unclear
Beyond the above medical considerations, one other piece of the depression puzzle remains unsolved: cost.
In a simultaneously published rapid review, Andreea Dobrescu, MD, PhD, of Cochrane Austria, and colleagues evaluated the relative cost-effectiveness of first- and second-step treatment strategies.
For most comparisons, evidence was insufficient to reach a conclusion, although they suggested that CBT may be more cost effective at the 5-year mark.
“For most pharmacologic and nonpharmacologic interventions for major depressive disorder, evidence was missing or was insufficient to draw conclusions about the cost-effectiveness of first- or second-step treatments for MDD,” Dr. Dobrescu and colleagues wrote. “The strongest evidence (albeit still low certainty of evidence) was for the cost-effectiveness of CBT compared with SGA as a first-step treatment over a 5-year time horizon from the societal and health care sector perspectives. However, this evidence should also be interpreted cautiously considering it is based on a single study.”
When asked about the financial findings, Dr. Mire agreed that more data are needed, especially because CBT and SGA costs range widely. He suggested that cost, for each patient, should be considered in the personalized approach now highlighted by the new guidelines.
The guidelines and the Cochrane cost-effectiveness study were supported by the ACP. The guidelines' authors and other individuals quoted in this article reported no conflicts of interest.
The guidelines also state that patients with mild depression should start with CBT alone, and if a patient with moderate to severe depression prefers, they can use a combination of both CBT and an SGA.
These nuanced recommendations contrast sharply with the 2016 ACP guidelines for depression, which lumped all stages and severity levels together, and came with just one recommendation: Clinicians should choose between CBT and an SGA.
More data have come to light over the years, requiring the present update, reported lead author Amir Qaseem, MD, PhD, vice president of Clinical Policy and the Center for Evidence Reviews at the ACP, and adjunct faculty at Thomas Jefferson University, Philadelphia, and colleagues.
In addition to the focus on acute depression, Dr. Qaseem and colleagues highlighted the new guidelines' “consideration of patient values and preferences, and costs,” as well as responses to therapy.
Recommendations were derived from a network meta-analysis that included studies evaluating nonpharmacologic and pharmacologic therapies, the authors wrote in Annals of Internal Medicine. They compared effectiveness across a range of SGAs, “including selective serotonin reuptake inhibitors; serotonin-norepinephrine reuptake inhibitors; and others such as bupropion, mirtazapine, nefazodone, trazodone, vilazodone, and vortioxetine.”
This analysis yielded three pieces of clinical advice.
First, patients in the acute phase of mild depression should receive CBT alone as their initial treatment.
Dr. Qaseem and colleagues noted that many depression studies for pharmacologic therapies excluded these patients in favor of those with moderate to severe depression, leaving an evidence gap.
“Furthermore, the Clinical Guidelines Committee had concerns about adverse effects of SGAs in these patients and suggests that the use of SGAs as initial treatment of these patients should be based on additional considerations, such as limited access to or cost of CBT, history of moderate or severe major depressive disorder, or patient preferences,” they added.
The committee’s next recommendation, based on moderate-certainty evidence, suggested that CBT alone or an SGA alone should be considered for patients in the acute phase of moderate to severe depression. This call for monotherapy is balanced by a conditional recommendation based on low-certainty evidence that the same group may benefit from initial combination therapy with both CBT and an SGA.
“The informed decision on the options of monotherapy with CBT versus SGAs, or combination therapy, should be personalized and based on discussion of potential treatment benefits, harms, adverse effect profiles, cost, feasibility, patients’ specific symptoms (such as insomnia, hypersomnia, or fluctuation in appetite), comorbidities, concomitant medication use, and patient preferences,” the guidelines state.
The third and final recommendation offers an algorithm for patients who do not respond to initial therapy with an SGA. Multiple pathways are provided: Switch to CBT or augment with CBT; or switch to a different SGA or augment with a second pharmacologic therapy, such as mirtazapine, bupropion, or buspirone.
“These second-line treatment strategies show similar efficacy when compared with each other,” the guidelines committee noted.
Again, the guidelines suggest that second-line choices should be personalized based on the various factors previously discussed.
A timely update
“The new guideline is very different from the last guideline,” said Ryan Mire, MD, president of the ACP and practicing internal medicine physician in Nashville, Tenn. in a written comment. “ACP decided to update the depression guidelines with a focus on acute depression because approximately 70% of patients with major depressive disorder do not achieve remission and remain in the acute phase after the initial pharmacologic treatment attempt. In addition, there is new evidence on second-line treatments since the 2016 ACP guideline was published.”
Neil S. Skolnik, MD, of Thomas Jefferson University, Philadelphia, agreed that the guidelines offer a necessary and fresh perspective on caring for patients with depression.
“These guidelines are a helpful update, assuring us that we are using the latest, evidence-based therapies, and [they] are written in a practical, easy-to-implement manner,” Dr. Skolnik said in a written comment.
“First, the guidelines reaffirm that CBT is an effective first-line option, with or without the concurrent use of an SGA,” Dr. Skolnik said, noting that CBT alone may reduce likelihood of recurrence, compared with an SGA alone. “Many patients do not like the idea of medication, or the potential side effects of medications, and CBT is an evidenced-based approach that can be very helpful for patients.”
Dr. Skolnik also applauded the guidelines authors for offering a clear path forward for patients who do not have full remission after treatment – a common clinical scenario.
He went on to offer some more detailed steps forward.
“If someone chooses to be treated with an SGA alone and has not had much response at all to an initial SGA, usually a selective serotonin reuptake inhibitor, I’ll usually switch to a different SSRI or serotonin and norepinephrine reuptake inhibitor (SNRI) and/or add CBT,” Dr. Skolnik said. “If they have had a partial response, I’ll often encourage CBT and consider the addition of augmentation with an additional medication as discussed in the guidelines.”
Valuable despite the gaps
Other experts expressed mixed impressions of the update, noting both highs and lows.
“Although [this guideline] has some gaps, it is more valuable in several ways than other widely consulted practice guidelines for depression,” wrote Miriam Shuchman, MD and Elia Abi-Jaoude, MSc, MD, PhD, of the University of Toronto, in an accompanying editorial.
Specifically, they praised the publication’s focus on shared decision-making in the treatment planning process.
“This effort to respond to patient preferences is crucial and may even increase the chance that patients will improve with treatment,” they wrote.
They also applauded the ACP’s efforts to recuse any committee members who may have had conflicts of interest “that could affect their judgment about treatments for depression.”
After highlighting these attributes, Dr. Shuchman and Dr. Abi-Jaoude noted that the guidelines still contain “significant gaps.”
Foremost, they pointed out the guidelines' emphasis on CBT to the exclusion of other nonpharmacologic options.
“The guideline does patients a disservice by leaving out several nonmedication treatment options that clinicians can offer as first- or second-line therapies,” they wrote.
This oversight may increase risk that patients simply hop from one SGA to another, which is a common, and often ineffective, strategy, according to Dr. Shuchman and Dr. Abi-Jaoude.
“Patients often go from one drug to the next in the hopes of landing on one that ‘works,’ ” the editorialists wrote. “This narrow clinical approach of pursuing medication-based treatments ignores the ways difficulties in a person’s work or relationships may contribute to their struggles with depression. At a time when the COVID-19 pandemic has underscored the importance of the social context of mental health, clinicians may need to consider other forms of support and tailor prescribing to what is most relevant and accessible for a particular patient.”
Dr. Shuchman and Dr. Abi-Jaoude went on to suggest several nonpharmacologic options beyond CBT, including interpersonal therapy, psychodynamic therapy, problem solving, behavioral activation, and guided self-help.
The other key gap they pointed out relates to withdrawal.
Although the guideline does advise physicians to taper antidepressants to reduce risk of withdrawal, the editorialists suggested that this recommendation lacked sufficient emphasis, as it can be a particularly difficult period in the treatment process.
“Tapering of an antidepressant may need to be done over months or years, not weeks, and a patient may need to visit a compounding pharmacy to obtain doses of a second-generation antidepressant not marketed by drug manufacturers so that prescriptions can be tapered even more slowly,” they suggested.
Financial costs remain unclear
Beyond the above medical considerations, one other piece of the depression puzzle remains unsolved: cost.
In a simultaneously published rapid review, Andreea Dobrescu, MD, PhD, of Cochrane Austria, and colleagues evaluated the relative cost-effectiveness of first- and second-step treatment strategies.
For most comparisons, evidence was insufficient to reach a conclusion, although they suggested that CBT may be more cost effective at the 5-year mark.
“For most pharmacologic and nonpharmacologic interventions for major depressive disorder, evidence was missing or was insufficient to draw conclusions about the cost-effectiveness of first- or second-step treatments for MDD,” Dr. Dobrescu and colleagues wrote. “The strongest evidence (albeit still low certainty of evidence) was for the cost-effectiveness of CBT compared with SGA as a first-step treatment over a 5-year time horizon from the societal and health care sector perspectives. However, this evidence should also be interpreted cautiously considering it is based on a single study.”
When asked about the financial findings, Dr. Mire agreed that more data are needed, especially because CBT and SGA costs range widely. He suggested that cost, for each patient, should be considered in the personalized approach now highlighted by the new guidelines.
The guidelines and the Cochrane cost-effectiveness study were supported by the ACP. The guidelines' authors and other individuals quoted in this article reported no conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE
FDA approves new type 2 diabetes drug bexagliflozin
The U.S. Food and Drug Administration has approved bexagliflozin (Brenzavvy, TheracosBio) for the treatment of adults with type 2 diabetes.
The once-daily 20-mg oral sodium-glucose cotransporter 2 (SGLT2) inhibitor is indicated as an adjunct to diet and exercise to improve glycemic control for those with type 2 diabetes, but not type 1 diabetes. It can be used in adults with an estimated glomerular filtration rate (eGFR) > 30 mL/min per 1.73 m2.
Approval was based on results from 23 clinical trials with more than 5,000 participants, including more than 300 patients with stage 3 kidney disease (eGFR < 60 and > 30 mL/min per 1.73 m2).
In the phase 3 studies, bexagliflozin significantly reduced hemoglobin A1c and fasting blood glucose at 24 weeks as monotherapy or as add-on to metformin and other glucose-lowering drugs and combinations. It also produced modest reductions in body weight and systolic blood pressure.
In the phase 3 Bexagliflozin Efficacy and Safety Trial (BEST) cardiovascular outcomes trial, the drug met its efficacy and safety objectives in patients at high cardiovascular risk. Noninferiority was demonstrated for the composite outcome of cardiovascular death, myocardial infarction, stroke, or unstable angina.
“As a class of drugs, SGLT2 inhibitors have shown tremendous benefit in treating adults with type 2 diabetes,” said Mason Freeman, MD, director of the Translational Research Center at Massachusetts General Hospital, Boston, in a press release from TheracosBio.
“Being involved in all of the clinical trials for Brenzavvy, I am greatly impressed with the efficacy of the drug in reducing blood glucose levels and I believe it is an important addition to the SGLT2 inhibitor class of drugs.”
As with other SGLT2 inhibitors, adverse events seen in the trials include ketoacidosis, lower limb amputation, volume depletion, urosepsis, pyelonephritis, Fournier’s gangrene, genital mycotic infections, and hypoglycemia when used with insulin or insulin secretagogues.
Bexagliflozin joins an already crowded field of SGLT2 inhibitors, some of which have been approved for additional cardiovascular and kidney indications.
Of interest, bexagliflozin was approved by the FDA for diabetes in cats in December 2022, as the first oral new animal drug to improve glycemic control in otherwise healthy cats with diabetes not previously treated with insulin.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved bexagliflozin (Brenzavvy, TheracosBio) for the treatment of adults with type 2 diabetes.
The once-daily 20-mg oral sodium-glucose cotransporter 2 (SGLT2) inhibitor is indicated as an adjunct to diet and exercise to improve glycemic control for those with type 2 diabetes, but not type 1 diabetes. It can be used in adults with an estimated glomerular filtration rate (eGFR) > 30 mL/min per 1.73 m2.
Approval was based on results from 23 clinical trials with more than 5,000 participants, including more than 300 patients with stage 3 kidney disease (eGFR < 60 and > 30 mL/min per 1.73 m2).
In the phase 3 studies, bexagliflozin significantly reduced hemoglobin A1c and fasting blood glucose at 24 weeks as monotherapy or as add-on to metformin and other glucose-lowering drugs and combinations. It also produced modest reductions in body weight and systolic blood pressure.
In the phase 3 Bexagliflozin Efficacy and Safety Trial (BEST) cardiovascular outcomes trial, the drug met its efficacy and safety objectives in patients at high cardiovascular risk. Noninferiority was demonstrated for the composite outcome of cardiovascular death, myocardial infarction, stroke, or unstable angina.
“As a class of drugs, SGLT2 inhibitors have shown tremendous benefit in treating adults with type 2 diabetes,” said Mason Freeman, MD, director of the Translational Research Center at Massachusetts General Hospital, Boston, in a press release from TheracosBio.
“Being involved in all of the clinical trials for Brenzavvy, I am greatly impressed with the efficacy of the drug in reducing blood glucose levels and I believe it is an important addition to the SGLT2 inhibitor class of drugs.”
As with other SGLT2 inhibitors, adverse events seen in the trials include ketoacidosis, lower limb amputation, volume depletion, urosepsis, pyelonephritis, Fournier’s gangrene, genital mycotic infections, and hypoglycemia when used with insulin or insulin secretagogues.
Bexagliflozin joins an already crowded field of SGLT2 inhibitors, some of which have been approved for additional cardiovascular and kidney indications.
Of interest, bexagliflozin was approved by the FDA for diabetes in cats in December 2022, as the first oral new animal drug to improve glycemic control in otherwise healthy cats with diabetes not previously treated with insulin.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved bexagliflozin (Brenzavvy, TheracosBio) for the treatment of adults with type 2 diabetes.
The once-daily 20-mg oral sodium-glucose cotransporter 2 (SGLT2) inhibitor is indicated as an adjunct to diet and exercise to improve glycemic control for those with type 2 diabetes, but not type 1 diabetes. It can be used in adults with an estimated glomerular filtration rate (eGFR) > 30 mL/min per 1.73 m2.
Approval was based on results from 23 clinical trials with more than 5,000 participants, including more than 300 patients with stage 3 kidney disease (eGFR < 60 and > 30 mL/min per 1.73 m2).
In the phase 3 studies, bexagliflozin significantly reduced hemoglobin A1c and fasting blood glucose at 24 weeks as monotherapy or as add-on to metformin and other glucose-lowering drugs and combinations. It also produced modest reductions in body weight and systolic blood pressure.
In the phase 3 Bexagliflozin Efficacy and Safety Trial (BEST) cardiovascular outcomes trial, the drug met its efficacy and safety objectives in patients at high cardiovascular risk. Noninferiority was demonstrated for the composite outcome of cardiovascular death, myocardial infarction, stroke, or unstable angina.
“As a class of drugs, SGLT2 inhibitors have shown tremendous benefit in treating adults with type 2 diabetes,” said Mason Freeman, MD, director of the Translational Research Center at Massachusetts General Hospital, Boston, in a press release from TheracosBio.
“Being involved in all of the clinical trials for Brenzavvy, I am greatly impressed with the efficacy of the drug in reducing blood glucose levels and I believe it is an important addition to the SGLT2 inhibitor class of drugs.”
As with other SGLT2 inhibitors, adverse events seen in the trials include ketoacidosis, lower limb amputation, volume depletion, urosepsis, pyelonephritis, Fournier’s gangrene, genital mycotic infections, and hypoglycemia when used with insulin or insulin secretagogues.
Bexagliflozin joins an already crowded field of SGLT2 inhibitors, some of which have been approved for additional cardiovascular and kidney indications.
Of interest, bexagliflozin was approved by the FDA for diabetes in cats in December 2022, as the first oral new animal drug to improve glycemic control in otherwise healthy cats with diabetes not previously treated with insulin.
A version of this article first appeared on Medscape.com.
Adding venetoclax improves ibrutinib outcomes in CLL
Investigators led by Philip Thompson, MD, a hematologist/oncologist at the center, explained that CLL patients receiving ibrutinib, a Bruton’s kinase inhibitor, “rarely achieve complete remission with undetectable measurable residual disease,” so they stay on the costly treatment indefinitely or until disease progression or accumulating adverse events force a switch to venetoclax.
Using the two agents together, instead of consecutively, may allow strong responders to stop treatment altogether and suboptimal responders to have longer remissions, they said.
“We would not advocate prolonged Bruton’s kinase inhibitor use prior to starting venetoclax in treatment-naive patients, as the safety and efficacy of commencing venetoclax after a 3-month ibrutinib monotherapy phase has been repeatedly demonstrated,” the team said.
However, the investigators noted that their “study was not intended to directly answer the question of whether combination therapy is superior to the current paradigm of sequential monotherapy.” Randomized trials are looking into the matter. The study was published recently as a preprint on ResearchSquare.com and has not been peer reviewed.
Complete remission in over half
The 45 adult subjects had one or more high-risk features for CLL progression and had received at least 1 year of ibrutinib at 140-420 mg once daily, depending on tolerance. They had bone marrow detectable disease at study entry but did not meet criteria for progression. Median duration of ibrutinib at baseline was 32 months, and about half the subjects were on it as their initial therapy.
Venetoclax, a BCL2 inhibitor with a completely different mechanisms of action, was added to ibrutinib for up to 2 years, escalated up to a target dose of 400 mg once daily.
On intention-to-treat analysis, venetoclax add-on improved ibrutinib response to complete remission in 55% of patients; complete remission was defined as less than 1 CLL cell per 10,000 leukocytes in bone marrow on two consecutive occasions 6 months apart.
The rate of undetectable bone marrow disease was 57% after 1 year of combined treatment and 71% after venetoclax completion, at which point 23 patients with undetectable disease stopped ibrutinib along with venetoclax.
Five patients had disease progression at a median of 41 months after venetoclax initiation, one during combined therapy, three during ibrutinib maintenance afterward, and one with Richter transformation after complete remission and discontinuation of all treatment. No patient had died from CLL.
“There has so far been no significant difference noted in” time to residual disease re-emergence, the team said, based on whether or not patients continued ibrutinib after venetoclax add-on.
There was no significant difference in the rate of bone marrow clearance according to the presence or absence of TP53 abnormalities, complex karyotypes, or prior treatment status.
The most common grade 3/4 adverse event was neutropenia in 20% of patients. Nine patients developed nonmelanoma skin cancer during the trial; six were diagnosed with other solid tumors; three came down with grade 3 infections, and two developed myelodysplastic syndrome, both with a prior history of chemotherapy.
No one stopped venetoclax because of toxicity, but about a third of subjects required dose reductions, most often because of neutropenia.
The study was funded by AbbVie, which is commercializing venetoclax along with Genentech. Investigators disclosed ties to both companies and many others. Dr. Thompson disclosed ties to AbbVie, Pharmacyclics, Lilly, Adaptive Biotechnologies, Janssen, Beigene, and Genentech.
Investigators led by Philip Thompson, MD, a hematologist/oncologist at the center, explained that CLL patients receiving ibrutinib, a Bruton’s kinase inhibitor, “rarely achieve complete remission with undetectable measurable residual disease,” so they stay on the costly treatment indefinitely or until disease progression or accumulating adverse events force a switch to venetoclax.
Using the two agents together, instead of consecutively, may allow strong responders to stop treatment altogether and suboptimal responders to have longer remissions, they said.
“We would not advocate prolonged Bruton’s kinase inhibitor use prior to starting venetoclax in treatment-naive patients, as the safety and efficacy of commencing venetoclax after a 3-month ibrutinib monotherapy phase has been repeatedly demonstrated,” the team said.
However, the investigators noted that their “study was not intended to directly answer the question of whether combination therapy is superior to the current paradigm of sequential monotherapy.” Randomized trials are looking into the matter. The study was published recently as a preprint on ResearchSquare.com and has not been peer reviewed.
Complete remission in over half
The 45 adult subjects had one or more high-risk features for CLL progression and had received at least 1 year of ibrutinib at 140-420 mg once daily, depending on tolerance. They had bone marrow detectable disease at study entry but did not meet criteria for progression. Median duration of ibrutinib at baseline was 32 months, and about half the subjects were on it as their initial therapy.
Venetoclax, a BCL2 inhibitor with a completely different mechanisms of action, was added to ibrutinib for up to 2 years, escalated up to a target dose of 400 mg once daily.
On intention-to-treat analysis, venetoclax add-on improved ibrutinib response to complete remission in 55% of patients; complete remission was defined as less than 1 CLL cell per 10,000 leukocytes in bone marrow on two consecutive occasions 6 months apart.
The rate of undetectable bone marrow disease was 57% after 1 year of combined treatment and 71% after venetoclax completion, at which point 23 patients with undetectable disease stopped ibrutinib along with venetoclax.
Five patients had disease progression at a median of 41 months after venetoclax initiation, one during combined therapy, three during ibrutinib maintenance afterward, and one with Richter transformation after complete remission and discontinuation of all treatment. No patient had died from CLL.
“There has so far been no significant difference noted in” time to residual disease re-emergence, the team said, based on whether or not patients continued ibrutinib after venetoclax add-on.
There was no significant difference in the rate of bone marrow clearance according to the presence or absence of TP53 abnormalities, complex karyotypes, or prior treatment status.
The most common grade 3/4 adverse event was neutropenia in 20% of patients. Nine patients developed nonmelanoma skin cancer during the trial; six were diagnosed with other solid tumors; three came down with grade 3 infections, and two developed myelodysplastic syndrome, both with a prior history of chemotherapy.
No one stopped venetoclax because of toxicity, but about a third of subjects required dose reductions, most often because of neutropenia.
The study was funded by AbbVie, which is commercializing venetoclax along with Genentech. Investigators disclosed ties to both companies and many others. Dr. Thompson disclosed ties to AbbVie, Pharmacyclics, Lilly, Adaptive Biotechnologies, Janssen, Beigene, and Genentech.
Investigators led by Philip Thompson, MD, a hematologist/oncologist at the center, explained that CLL patients receiving ibrutinib, a Bruton’s kinase inhibitor, “rarely achieve complete remission with undetectable measurable residual disease,” so they stay on the costly treatment indefinitely or until disease progression or accumulating adverse events force a switch to venetoclax.
Using the two agents together, instead of consecutively, may allow strong responders to stop treatment altogether and suboptimal responders to have longer remissions, they said.
“We would not advocate prolonged Bruton’s kinase inhibitor use prior to starting venetoclax in treatment-naive patients, as the safety and efficacy of commencing venetoclax after a 3-month ibrutinib monotherapy phase has been repeatedly demonstrated,” the team said.
However, the investigators noted that their “study was not intended to directly answer the question of whether combination therapy is superior to the current paradigm of sequential monotherapy.” Randomized trials are looking into the matter. The study was published recently as a preprint on ResearchSquare.com and has not been peer reviewed.
Complete remission in over half
The 45 adult subjects had one or more high-risk features for CLL progression and had received at least 1 year of ibrutinib at 140-420 mg once daily, depending on tolerance. They had bone marrow detectable disease at study entry but did not meet criteria for progression. Median duration of ibrutinib at baseline was 32 months, and about half the subjects were on it as their initial therapy.
Venetoclax, a BCL2 inhibitor with a completely different mechanisms of action, was added to ibrutinib for up to 2 years, escalated up to a target dose of 400 mg once daily.
On intention-to-treat analysis, venetoclax add-on improved ibrutinib response to complete remission in 55% of patients; complete remission was defined as less than 1 CLL cell per 10,000 leukocytes in bone marrow on two consecutive occasions 6 months apart.
The rate of undetectable bone marrow disease was 57% after 1 year of combined treatment and 71% after venetoclax completion, at which point 23 patients with undetectable disease stopped ibrutinib along with venetoclax.
Five patients had disease progression at a median of 41 months after venetoclax initiation, one during combined therapy, three during ibrutinib maintenance afterward, and one with Richter transformation after complete remission and discontinuation of all treatment. No patient had died from CLL.
“There has so far been no significant difference noted in” time to residual disease re-emergence, the team said, based on whether or not patients continued ibrutinib after venetoclax add-on.
There was no significant difference in the rate of bone marrow clearance according to the presence or absence of TP53 abnormalities, complex karyotypes, or prior treatment status.
The most common grade 3/4 adverse event was neutropenia in 20% of patients. Nine patients developed nonmelanoma skin cancer during the trial; six were diagnosed with other solid tumors; three came down with grade 3 infections, and two developed myelodysplastic syndrome, both with a prior history of chemotherapy.
No one stopped venetoclax because of toxicity, but about a third of subjects required dose reductions, most often because of neutropenia.
The study was funded by AbbVie, which is commercializing venetoclax along with Genentech. Investigators disclosed ties to both companies and many others. Dr. Thompson disclosed ties to AbbVie, Pharmacyclics, Lilly, Adaptive Biotechnologies, Janssen, Beigene, and Genentech.
FROM RESEARCHSQUARE
AHA scientific statement on rapid evaluation for suspected TIA
TIAs are “warning shots” of a future stroke and require emergency evaluation, Hardik Amin, MD, chair of the writing committee and medical stroke director, Yale New Haven (Conn.) Hospital, said in an AHA podcast.
A key aim of the scientific statement is to help clinicians properly risk-stratify patients with suspected TIA and determine which patients need to be admitted to the hospital and which patients might be safely discharged as long as proper and prompt follow-up has been arranged, Dr. Amin explained.
The statement, published online in the journal Stroke, addresses “how we can identify and be confident in diagnosing a TIA patient and what might suggest an alternative diagnosis,” he added.
Diagnostic challenge
It’s estimated that nearly one in five people who suffer a TIA will have a full-blown stroke within 3 months; close to half of these strokes will happen within 2 days.
The challenge with TIAs is that they can be tough to diagnose because many patients no longer have symptoms when they arrive at the emergency department. There is also no confirmatory test. Limited resources and access to stroke specialists in rural centers may exacerbate these challenges, the authors noted.
The statement pointed out that the F.A.S.T. acronym for stroke symptoms (Face drooping, Arm weakness, Speech difficulty, Time to call 911) can also be used to identify a TIA – even if the symptoms resolve.
The statement also provided guidance on how to tell the difference between a TIA and a TIA mimic.
If available, a noncontrast head CT (NCCT) scan should be done initially in the emergency department to evaluate for subacute ischemia, hemorrhage, or mass lesion. Although the sensitivity of NCCT to detect an acute infarct is low, NCCT is useful for ruling out TIA mimics, the writing group said.
Multimodal brain MRI is the “preferred” method to evaluate for acute ischemic infarct and ideally should be obtained within 24 hours of symptom onset, and in most centers will follow an NCCT.
“When MRI cannot be obtained acutely to definitively distinguish TIA from stroke, it remains reasonable to make a clinical diagnosis of TIA in the ED on the basis of a negative NCCT and symptom resolution within 24 hours,” the authors said.
“A potential next step would be hospital admission for MRI, comprehensive workup, and neurology consultation. Other options might include transferring patients to a facility with advanced imaging and vascular neurology expertise or arranging a timely (ideally < 24 hours) outpatient MRI,” they advised.
The statement also provides guidance on the advantages, limitations, and considerations of Doppler ultrasonography, CT angiography, and magnetic resonance angiography for TIA assessment.
Once TIA is diagnosed, a cardiac work-up is advised because of the potential for heart-related factors to cause a TIA.
An individual’s risk of future stroke after TIA can be rapidly assessed using the ABCD2 score, which stratifies patients into low, medium, and high risk based on age, blood pressure, clinical features, duration of symptoms, and diabetes.
“It is up to each center to use the resources available and create a pathway to ensure successful management and disposition of patients with TIA, with the ultimate goal of reducing the risk of future stroke,” the authors concluded.
This scientific statement was prepared by the volunteer writing group on behalf of the American Heart Association’s Emergency Neurovascular Care Committee of the Stroke Council and the Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists, and it is endorsed by the American Association of Neurological Surgeons/Congress of Neurological Surgeons.
A version of this article first appeared on Medscape.com.
TIAs are “warning shots” of a future stroke and require emergency evaluation, Hardik Amin, MD, chair of the writing committee and medical stroke director, Yale New Haven (Conn.) Hospital, said in an AHA podcast.
A key aim of the scientific statement is to help clinicians properly risk-stratify patients with suspected TIA and determine which patients need to be admitted to the hospital and which patients might be safely discharged as long as proper and prompt follow-up has been arranged, Dr. Amin explained.
The statement, published online in the journal Stroke, addresses “how we can identify and be confident in diagnosing a TIA patient and what might suggest an alternative diagnosis,” he added.
Diagnostic challenge
It’s estimated that nearly one in five people who suffer a TIA will have a full-blown stroke within 3 months; close to half of these strokes will happen within 2 days.
The challenge with TIAs is that they can be tough to diagnose because many patients no longer have symptoms when they arrive at the emergency department. There is also no confirmatory test. Limited resources and access to stroke specialists in rural centers may exacerbate these challenges, the authors noted.
The statement pointed out that the F.A.S.T. acronym for stroke symptoms (Face drooping, Arm weakness, Speech difficulty, Time to call 911) can also be used to identify a TIA – even if the symptoms resolve.
The statement also provided guidance on how to tell the difference between a TIA and a TIA mimic.
If available, a noncontrast head CT (NCCT) scan should be done initially in the emergency department to evaluate for subacute ischemia, hemorrhage, or mass lesion. Although the sensitivity of NCCT to detect an acute infarct is low, NCCT is useful for ruling out TIA mimics, the writing group said.
Multimodal brain MRI is the “preferred” method to evaluate for acute ischemic infarct and ideally should be obtained within 24 hours of symptom onset, and in most centers will follow an NCCT.
“When MRI cannot be obtained acutely to definitively distinguish TIA from stroke, it remains reasonable to make a clinical diagnosis of TIA in the ED on the basis of a negative NCCT and symptom resolution within 24 hours,” the authors said.
“A potential next step would be hospital admission for MRI, comprehensive workup, and neurology consultation. Other options might include transferring patients to a facility with advanced imaging and vascular neurology expertise or arranging a timely (ideally < 24 hours) outpatient MRI,” they advised.
The statement also provides guidance on the advantages, limitations, and considerations of Doppler ultrasonography, CT angiography, and magnetic resonance angiography for TIA assessment.
Once TIA is diagnosed, a cardiac work-up is advised because of the potential for heart-related factors to cause a TIA.
An individual’s risk of future stroke after TIA can be rapidly assessed using the ABCD2 score, which stratifies patients into low, medium, and high risk based on age, blood pressure, clinical features, duration of symptoms, and diabetes.
“It is up to each center to use the resources available and create a pathway to ensure successful management and disposition of patients with TIA, with the ultimate goal of reducing the risk of future stroke,” the authors concluded.
This scientific statement was prepared by the volunteer writing group on behalf of the American Heart Association’s Emergency Neurovascular Care Committee of the Stroke Council and the Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists, and it is endorsed by the American Association of Neurological Surgeons/Congress of Neurological Surgeons.
A version of this article first appeared on Medscape.com.
TIAs are “warning shots” of a future stroke and require emergency evaluation, Hardik Amin, MD, chair of the writing committee and medical stroke director, Yale New Haven (Conn.) Hospital, said in an AHA podcast.
A key aim of the scientific statement is to help clinicians properly risk-stratify patients with suspected TIA and determine which patients need to be admitted to the hospital and which patients might be safely discharged as long as proper and prompt follow-up has been arranged, Dr. Amin explained.
The statement, published online in the journal Stroke, addresses “how we can identify and be confident in diagnosing a TIA patient and what might suggest an alternative diagnosis,” he added.
Diagnostic challenge
It’s estimated that nearly one in five people who suffer a TIA will have a full-blown stroke within 3 months; close to half of these strokes will happen within 2 days.
The challenge with TIAs is that they can be tough to diagnose because many patients no longer have symptoms when they arrive at the emergency department. There is also no confirmatory test. Limited resources and access to stroke specialists in rural centers may exacerbate these challenges, the authors noted.
The statement pointed out that the F.A.S.T. acronym for stroke symptoms (Face drooping, Arm weakness, Speech difficulty, Time to call 911) can also be used to identify a TIA – even if the symptoms resolve.
The statement also provided guidance on how to tell the difference between a TIA and a TIA mimic.
If available, a noncontrast head CT (NCCT) scan should be done initially in the emergency department to evaluate for subacute ischemia, hemorrhage, or mass lesion. Although the sensitivity of NCCT to detect an acute infarct is low, NCCT is useful for ruling out TIA mimics, the writing group said.
Multimodal brain MRI is the “preferred” method to evaluate for acute ischemic infarct and ideally should be obtained within 24 hours of symptom onset, and in most centers will follow an NCCT.
“When MRI cannot be obtained acutely to definitively distinguish TIA from stroke, it remains reasonable to make a clinical diagnosis of TIA in the ED on the basis of a negative NCCT and symptom resolution within 24 hours,” the authors said.
“A potential next step would be hospital admission for MRI, comprehensive workup, and neurology consultation. Other options might include transferring patients to a facility with advanced imaging and vascular neurology expertise or arranging a timely (ideally < 24 hours) outpatient MRI,” they advised.
The statement also provides guidance on the advantages, limitations, and considerations of Doppler ultrasonography, CT angiography, and magnetic resonance angiography for TIA assessment.
Once TIA is diagnosed, a cardiac work-up is advised because of the potential for heart-related factors to cause a TIA.
An individual’s risk of future stroke after TIA can be rapidly assessed using the ABCD2 score, which stratifies patients into low, medium, and high risk based on age, blood pressure, clinical features, duration of symptoms, and diabetes.
“It is up to each center to use the resources available and create a pathway to ensure successful management and disposition of patients with TIA, with the ultimate goal of reducing the risk of future stroke,” the authors concluded.
This scientific statement was prepared by the volunteer writing group on behalf of the American Heart Association’s Emergency Neurovascular Care Committee of the Stroke Council and the Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists, and it is endorsed by the American Association of Neurological Surgeons/Congress of Neurological Surgeons.
A version of this article first appeared on Medscape.com.
FROM STROKE
3D-printed tumor models could advance new cancer therapies
Scientists have made big strides in the fight against cancer. A person’s risk of dying of cancer in the U.S. fell by 27% in the past 2 decades, thanks in large part to researchers who continue to uncover the complex details of how cancer works and to make advances in treatment.
Now the by enabling scientists to develop 3D tumor models that better represent samples from patients.
The impact could be “huge,” says Y. Shrike Zhang, PhD, an assistant professor of medicine at Harvard Medical School and associate bioengineer at Brigham and Women’s Hospital, both in Boston, who studies 3D bioprinting. “It is not the only technology that may allow modeling of tumors in vitro, but it certainly is one of the most capable.”
Why does that matter? Because the 2D cell cultures that scientists often use now may not capture all the complexities of how cancer grows, spreads, and responds to treatment. It’s one reason why so few potential new cancer drugs – 3.4%, according to one estimate – can pass all clinical trials. Results may not carry over from the culture dish to the patient.
Researchers say these 3D-printed blood vessels may treat certain dangerous health problems that affect your veins, arteries, or capillaries.
A 3D-bioprinted model, on the other hand, may be better at copying a tumor’s “microenvironment” – all the parts (cells, molecules, blood vessels) that surround a tumor.
“The tumor microenvironment plays an integral role in defining how cancer progresses,” says Madhuri Dey, a PhD candidate and researcher at Penn State University. “In vitro 3D models are an attempt at reconstituting a [cancer] microenvironment, which sheds light on how tumors respond to chemo or immunotherapeutic treatments when they are present in a native-like microenvironment.”
Ms. Dey is the lead author of a study funded by the National Science Foundation in which breast cancer tumors were 3D-bioprinted and successfully treated. Unlike some previous 3D models of cancer cells, this model did a better job of imitating that microenvironment, she explains.
So far, “3D bioprinting of cancer models has been limited to bioprinting of individual cancer cells laden in hydrogels,” she says. But she and her colleagues developed a technique called aspiration-assisted bioprinting that lets them control where blood vessels are located relative to the tumor. “This model lays the foundation for studying these nuances of cancer,” Ms. Dey says.
“This is a quite cool work,” Dr. Zhang says of the Penn State study (which he was not involved in). “Vascularization is always a key component in [a] majority of the tumor types.” A model that incorporates blood vessels provides a “critical niche” to help tumor models reach their full potential in cancer research.
A 3D printer for your body
Chances are you’ve heard of 3D printing and may even own (or know someone who owns) a 3D printer. The concept is like regular printing, but instead of spewing ink onto paper, a 3D printer releases layers of plastic or other materials, hundreds or thousands of times, to build an object from the ground up.
Three-dimensional bioprinting works much the same way, except those layers are made of living cells to create biological structures like skin, vessels, organs, or bone.
Bioprinting has been around since 1988. So far, it’s mainly used in research settings, such as in the field of regenerative medicine. Research is underway for ear reconstruction, nerve regeneration, and skin regeneration. The technology was also recently used to create eye tissue to help researchers study eye diseases.
The technology’s potential for use in cancer research has yet to be fully realized, Ms. Dey says. But that may be changing.
“The use of 3D-bioprinted tumor models is getting close to translations in cancer research,” says Dr. Zhang. “They are being increasingly adopted by the research field, and [the technology] has started to be explored by the pharma industry for use towards cancer drug development.”
Because bioprinting can be automated, it could allow researchers to create high-quality, complex tumor models at scale, Dr. Zhang says.
Such 3D models also have the potential to replace or reduce the use of animals in tumor drug testing, Ms. Dey notes. They “are expected to provide a more accurate drug response, compared [with] animal models, as animal physiology does not match humans’.”
The FDA Modernization Act 2.0, a new U.S. law eliminating the requirement that drugs be tested in animals before humans, has “further paved the way for such technologies in the drug development pipeline,” Dr. Zhang says.
What if we could build a custom tumor model for each patient?
Possible uses for bioprinting go beyond the lab, Ms. Dey says. Imagine if we could customize 3D tumor models based on biopsies from individual patients. Doctors could test many treatments on these patient-specific models, letting them more accurately predict how each patient would respond to different therapies. This would help doctors decide which course of treatment is best.
In Ms. Dey’s study, the 3D model was treated with chemotherapy and with immunotherapy, and it responded to both. This highlights the potential for such 3D models to reveal the body’s immune response and be used to screen therapies, she says. “We hope is that in the future, this technique can be adapted in the hospital, which would speed up the course of cancer treatment.”
To that end, she and her colleagues are now working with real breast cancer tumors removed from patients, re-creating them in the lab in 3D to use for chemo and immunotherapy screening.
A version of this article first appeared on WebMD.com.
Scientists have made big strides in the fight against cancer. A person’s risk of dying of cancer in the U.S. fell by 27% in the past 2 decades, thanks in large part to researchers who continue to uncover the complex details of how cancer works and to make advances in treatment.
Now the by enabling scientists to develop 3D tumor models that better represent samples from patients.
The impact could be “huge,” says Y. Shrike Zhang, PhD, an assistant professor of medicine at Harvard Medical School and associate bioengineer at Brigham and Women’s Hospital, both in Boston, who studies 3D bioprinting. “It is not the only technology that may allow modeling of tumors in vitro, but it certainly is one of the most capable.”
Why does that matter? Because the 2D cell cultures that scientists often use now may not capture all the complexities of how cancer grows, spreads, and responds to treatment. It’s one reason why so few potential new cancer drugs – 3.4%, according to one estimate – can pass all clinical trials. Results may not carry over from the culture dish to the patient.
Researchers say these 3D-printed blood vessels may treat certain dangerous health problems that affect your veins, arteries, or capillaries.
A 3D-bioprinted model, on the other hand, may be better at copying a tumor’s “microenvironment” – all the parts (cells, molecules, blood vessels) that surround a tumor.
“The tumor microenvironment plays an integral role in defining how cancer progresses,” says Madhuri Dey, a PhD candidate and researcher at Penn State University. “In vitro 3D models are an attempt at reconstituting a [cancer] microenvironment, which sheds light on how tumors respond to chemo or immunotherapeutic treatments when they are present in a native-like microenvironment.”
Ms. Dey is the lead author of a study funded by the National Science Foundation in which breast cancer tumors were 3D-bioprinted and successfully treated. Unlike some previous 3D models of cancer cells, this model did a better job of imitating that microenvironment, she explains.
So far, “3D bioprinting of cancer models has been limited to bioprinting of individual cancer cells laden in hydrogels,” she says. But she and her colleagues developed a technique called aspiration-assisted bioprinting that lets them control where blood vessels are located relative to the tumor. “This model lays the foundation for studying these nuances of cancer,” Ms. Dey says.
“This is a quite cool work,” Dr. Zhang says of the Penn State study (which he was not involved in). “Vascularization is always a key component in [a] majority of the tumor types.” A model that incorporates blood vessels provides a “critical niche” to help tumor models reach their full potential in cancer research.
A 3D printer for your body
Chances are you’ve heard of 3D printing and may even own (or know someone who owns) a 3D printer. The concept is like regular printing, but instead of spewing ink onto paper, a 3D printer releases layers of plastic or other materials, hundreds or thousands of times, to build an object from the ground up.
Three-dimensional bioprinting works much the same way, except those layers are made of living cells to create biological structures like skin, vessels, organs, or bone.
Bioprinting has been around since 1988. So far, it’s mainly used in research settings, such as in the field of regenerative medicine. Research is underway for ear reconstruction, nerve regeneration, and skin regeneration. The technology was also recently used to create eye tissue to help researchers study eye diseases.
The technology’s potential for use in cancer research has yet to be fully realized, Ms. Dey says. But that may be changing.
“The use of 3D-bioprinted tumor models is getting close to translations in cancer research,” says Dr. Zhang. “They are being increasingly adopted by the research field, and [the technology] has started to be explored by the pharma industry for use towards cancer drug development.”
Because bioprinting can be automated, it could allow researchers to create high-quality, complex tumor models at scale, Dr. Zhang says.
Such 3D models also have the potential to replace or reduce the use of animals in tumor drug testing, Ms. Dey notes. They “are expected to provide a more accurate drug response, compared [with] animal models, as animal physiology does not match humans’.”
The FDA Modernization Act 2.0, a new U.S. law eliminating the requirement that drugs be tested in animals before humans, has “further paved the way for such technologies in the drug development pipeline,” Dr. Zhang says.
What if we could build a custom tumor model for each patient?
Possible uses for bioprinting go beyond the lab, Ms. Dey says. Imagine if we could customize 3D tumor models based on biopsies from individual patients. Doctors could test many treatments on these patient-specific models, letting them more accurately predict how each patient would respond to different therapies. This would help doctors decide which course of treatment is best.
In Ms. Dey’s study, the 3D model was treated with chemotherapy and with immunotherapy, and it responded to both. This highlights the potential for such 3D models to reveal the body’s immune response and be used to screen therapies, she says. “We hope is that in the future, this technique can be adapted in the hospital, which would speed up the course of cancer treatment.”
To that end, she and her colleagues are now working with real breast cancer tumors removed from patients, re-creating them in the lab in 3D to use for chemo and immunotherapy screening.
A version of this article first appeared on WebMD.com.
Scientists have made big strides in the fight against cancer. A person’s risk of dying of cancer in the U.S. fell by 27% in the past 2 decades, thanks in large part to researchers who continue to uncover the complex details of how cancer works and to make advances in treatment.
Now the by enabling scientists to develop 3D tumor models that better represent samples from patients.
The impact could be “huge,” says Y. Shrike Zhang, PhD, an assistant professor of medicine at Harvard Medical School and associate bioengineer at Brigham and Women’s Hospital, both in Boston, who studies 3D bioprinting. “It is not the only technology that may allow modeling of tumors in vitro, but it certainly is one of the most capable.”
Why does that matter? Because the 2D cell cultures that scientists often use now may not capture all the complexities of how cancer grows, spreads, and responds to treatment. It’s one reason why so few potential new cancer drugs – 3.4%, according to one estimate – can pass all clinical trials. Results may not carry over from the culture dish to the patient.
Researchers say these 3D-printed blood vessels may treat certain dangerous health problems that affect your veins, arteries, or capillaries.
A 3D-bioprinted model, on the other hand, may be better at copying a tumor’s “microenvironment” – all the parts (cells, molecules, blood vessels) that surround a tumor.
“The tumor microenvironment plays an integral role in defining how cancer progresses,” says Madhuri Dey, a PhD candidate and researcher at Penn State University. “In vitro 3D models are an attempt at reconstituting a [cancer] microenvironment, which sheds light on how tumors respond to chemo or immunotherapeutic treatments when they are present in a native-like microenvironment.”
Ms. Dey is the lead author of a study funded by the National Science Foundation in which breast cancer tumors were 3D-bioprinted and successfully treated. Unlike some previous 3D models of cancer cells, this model did a better job of imitating that microenvironment, she explains.
So far, “3D bioprinting of cancer models has been limited to bioprinting of individual cancer cells laden in hydrogels,” she says. But she and her colleagues developed a technique called aspiration-assisted bioprinting that lets them control where blood vessels are located relative to the tumor. “This model lays the foundation for studying these nuances of cancer,” Ms. Dey says.
“This is a quite cool work,” Dr. Zhang says of the Penn State study (which he was not involved in). “Vascularization is always a key component in [a] majority of the tumor types.” A model that incorporates blood vessels provides a “critical niche” to help tumor models reach their full potential in cancer research.
A 3D printer for your body
Chances are you’ve heard of 3D printing and may even own (or know someone who owns) a 3D printer. The concept is like regular printing, but instead of spewing ink onto paper, a 3D printer releases layers of plastic or other materials, hundreds or thousands of times, to build an object from the ground up.
Three-dimensional bioprinting works much the same way, except those layers are made of living cells to create biological structures like skin, vessels, organs, or bone.
Bioprinting has been around since 1988. So far, it’s mainly used in research settings, such as in the field of regenerative medicine. Research is underway for ear reconstruction, nerve regeneration, and skin regeneration. The technology was also recently used to create eye tissue to help researchers study eye diseases.
The technology’s potential for use in cancer research has yet to be fully realized, Ms. Dey says. But that may be changing.
“The use of 3D-bioprinted tumor models is getting close to translations in cancer research,” says Dr. Zhang. “They are being increasingly adopted by the research field, and [the technology] has started to be explored by the pharma industry for use towards cancer drug development.”
Because bioprinting can be automated, it could allow researchers to create high-quality, complex tumor models at scale, Dr. Zhang says.
Such 3D models also have the potential to replace or reduce the use of animals in tumor drug testing, Ms. Dey notes. They “are expected to provide a more accurate drug response, compared [with] animal models, as animal physiology does not match humans’.”
The FDA Modernization Act 2.0, a new U.S. law eliminating the requirement that drugs be tested in animals before humans, has “further paved the way for such technologies in the drug development pipeline,” Dr. Zhang says.
What if we could build a custom tumor model for each patient?
Possible uses for bioprinting go beyond the lab, Ms. Dey says. Imagine if we could customize 3D tumor models based on biopsies from individual patients. Doctors could test many treatments on these patient-specific models, letting them more accurately predict how each patient would respond to different therapies. This would help doctors decide which course of treatment is best.
In Ms. Dey’s study, the 3D model was treated with chemotherapy and with immunotherapy, and it responded to both. This highlights the potential for such 3D models to reveal the body’s immune response and be used to screen therapies, she says. “We hope is that in the future, this technique can be adapted in the hospital, which would speed up the course of cancer treatment.”
To that end, she and her colleagues are now working with real breast cancer tumors removed from patients, re-creating them in the lab in 3D to use for chemo and immunotherapy screening.
A version of this article first appeared on WebMD.com.
People with cancer should be wary of taking dietary supplements
Cancer dietitian Lisa Cianciotta often finds herself sitting across from a patient who suddenly fishes a bottle of antioxidant supplements from their bag and says, “My friend told me this works really well,” or “I read on the Internet that this is supposed to be really good for cancer.”
Although taking an antioxidant pill sounds harmless, Ms. Cianciotta, a clinical dietitian who works with cancer patients at New York–Presbyterian Hospital, knows well that this popular dietary supplement can interfere with a patient’s radiation or chemotherapy.
But many patients with cancer believe these over-the-counter vitamins, minerals, or herbal remedies will help them, and most use at least one dietary supplement alongside their cancer treatment.
And that leaves Ms. Cianciotta with a delicate conversation ahead of her.
. Popular dietary supplements may, for instance, cancel the effects of a cancer treatment, making it less effective, or increase serious side effects, such as liver toxicity. But in other cases, supplementation, such as vitamin D for patients who lack the vitamin, may be beneficial, Ms. Cianciotta said.
These drug-supplement interactions can be hard to pinpoint, given that more than two-thirds of doctors don’t know their patients are using supplements.
Here’s what patients need to know about the potential risks of supplement use during treatment, and how oncologists can address this thorny, often poorly understood topic with patients.
The complex drug-supplement landscape
The list of dietary supplements and how they can interact with different treatments and cancer types is long and nuanced.
But certain supplements appear to affect cancer treatments regardless of other things and should be avoided. Any supplement that strongly alters the body’s levels of the protein cytochromes P450 is one example. This group of enzymes plays a key role in metabolizing drugs, including chemotherapy and immunotherapy agents.
Certain supplements – most notably St. John’s wort extract – may decrease or increase the activity of cytochrome P450, which can then affect the concentrations of anticancer drugs in the blood, said William Figg, PharmD, an associate director of the Center for Cancer Research at the National Cancer Institute in Bethesda, Md. Studies show, for instance, that this common herbal supplement can increase the activity of cytochrome P450, resulting in lower levels of cancer drugs.
Outside of drug metabolism, patients with hormone-related cancers, such as breast and prostate cancers, should steer clear of dietary supplements that can alter levels of testosterone or estrogen, Dr. Figg said. The evergreen shrub ashwagandha, for example, is marketed to reduce stress and fatigue, but can also increase testosterone levels – a potential problem for those with prostate cancer receiving androgen deprivation therapy, which lowers testosterone levels.
Many oncologists counsel patients against using antioxidant-based dietary supplements – particularly turmeric and green tea extract – while they have radiation therapy and certain chemotherapies. These therapies work by creating an abundance of highly reactive molecules called free radicals in tumor cells, which increase stress within these cells, ultimately killing them off. Antioxidants, in theory, can neutralize this effect, said Skyler Johnson, MD, a radiation oncologist at Huntsman Cancer Institute at the University of Utah, Salt Lake City. Some studies suggest that antioxidant supplements may lessen the effects of radiation and chemotherapy, although the evidence is mixed.
Some dietary supplements, including high-dose green tea extract and vitamin A, can cause kidney or liver toxicity, and “many cancer patients already have compromised kidney or liver function,” said Jun J. Mao, MD, chief of integrative medicine at Memorial Sloan Kettering Cancer Center in New York. Even herbs that don’t interfere with how well a cancer drug works, such as stevia, can increase treatment-related side effects, such as nausea and vomiting.
Another potential problem with dietary supplements: It’s nearly impossible to know exactly what’s in them. For instance, just last year, the Food and Drug Administration sent nearly 50 warning letters to companies marketing dietary supplements. The issue is that federal regulations governing production are less strict for supplements than for medications. As a result, some supplements contain ingredients not listed on the label.
One historical example was the supplement PC-SPES, a mix of eight herbs, marketed to men with prostate cancer. The supplement was recalled in 2002 after certain batches were found to contain traces of prescription drugs, including diethylstilbestrol, ethinyl estradiol, warfarin, and alprazolam.
To further complicate matters, some dietary supplements can be helpful. Most patients with cancer “are malnourished and missing out on nutrients they could be getting from food,” said Ms. Cianciotta.
Patients are tested routinely for vitamin deficiencies and receive supplements as needed, she said. Vitamin D and folic acid are two of the most common deficiencies in this patient population. Vitamin D supplementation can improve outcomes in patients who have received a stem cell transplant by aiding engraftment and rebuilding the immune system, while folic acid supplementation can help to raise low red blood cell counts and hemoglobin levels.
Although she rarely sees vitamin toxicity, Ms. Cianciotta stressed that more is not always better and supplement use, even when it seems safe or warranted due to a deficiency, should be taken under supervision, and monitored carefully by the patient’s care team.
Bringing supplement use into the light
Too often, providers are unaware of a patient’s supplement use.
A core reason: Dietary supplements are often touted as natural, which many patients equate with safety, said Samantha Heller, a senior clinical nutritionist at New York (N.Y.) University Langone Health.
That means patients may not know a supplement can act like a drug and interfere with their cancer treatment, and thus may not see the importance of telling their doctors.
Still, the promise of herbs, vitamins, and minerals can be alluring, and there are many reasons patients decide to partake. One major appeal: Dietary supplements can help some patients feel empowered.
“Cancer is a disease that takes away a lot of control from the individual. Taking supplements or herbs is a way to regain some sense of control,” said Dr. Mao.
The phenomenon can also be cultural, he said. Some people grow up taking herbs and supplements to stay healthy or combat health woes.
Pressure or advice from family or friends who may think they are helping a loved one with their dietary recommendations may play a role as well. Friends and family “cannot prescribe chemo, but they can buy herbs and supplements,” Dr. Mao said.
Patients seeking greater control over their health or who feel high levels of anxiety may be more likely to take suggestions from friends and family or more likely to believe false or misleading claims about the efficacy or safety of supplements, explained medical oncologist William Dahut, MD, chief scientific officer for the American Cancer Society.
Plus, social media often amplifies and normalizes this misinformation, noted Dr. Johnson. In a 2021 study published in the Journal of the National Cancer Institute, he and colleagues found that one-third of the most popular articles on cancer treatment posted to social media in 2018 and 2019 contained false, inaccurate, or misleading information that was often harmful.
Some of the false claims centered on unproven, potentially unsafe herbal remedies, according to Dr. Johnson. These included “lung cancer can be cured with cannabis oil” and “golden berries cure and prevent cancer.”
Given exaggerated claims of “cures,” some patients may keep their supplement use under the radar out of fear they will be judged or criticized.
“Clinicians should avoid making patients feel judged or telling people not to go online to do their own research,” Dr. Johnson said.
Guiding patients, instead, to accurate sources of online information may be one way to help patients feel empowered, he said. Cancer.gov and the Memorial Sloan Kettering Cancer Center’s About Herbs database provide accessible and accurate information on dietary supplements and cancer treatment for both health care professionals and patients, he noted.
If a particular supplement is not safe during treatment, providers should be able to explain why, said Ms. Cianciotta. In a recent study, 80% of health care providers surveyed believed that interactions between herbals and medications could be problematic, but only 15% could explain why.
“Being able to explain why we are discouraging a particular supplement right now tends to be much better received than just telling a patient not to take something, because it is bad,” she said.
Another key is listening closely to patients to understand why they may be taking a particular supplement. Does the patient feel out of control? Is nausea a problem?
“Allowing patients to tell you why they are using a particular supplement will often reveal unmet needs or psychosocial challenges,” Dr. Mao said. This information can allow providers to suggest an evidence-based alternative, such as mindfulness meditation or acupuncture, to manage stress.
And if a patient has received a dietary supplement from well-meaning family and friends?
“Simply telling a patient that a given supplement is useless or harmful could create family tension,” said Dr. Mao.
Instead, he recommends reframing the issue.
“We want to have a better understanding of how patients are tolerating chemo or immunotherapy before throwing other things on top of it. Let them know that now may just not be the right time to add a supplement to the mix,” Dr. Mao said.
The bottom line: “Patients want to play an active role in their own care, and we want to help them do that in a safe way,” he said.
A version of this article first appeared on WebMD.com.
Cancer dietitian Lisa Cianciotta often finds herself sitting across from a patient who suddenly fishes a bottle of antioxidant supplements from their bag and says, “My friend told me this works really well,” or “I read on the Internet that this is supposed to be really good for cancer.”
Although taking an antioxidant pill sounds harmless, Ms. Cianciotta, a clinical dietitian who works with cancer patients at New York–Presbyterian Hospital, knows well that this popular dietary supplement can interfere with a patient’s radiation or chemotherapy.
But many patients with cancer believe these over-the-counter vitamins, minerals, or herbal remedies will help them, and most use at least one dietary supplement alongside their cancer treatment.
And that leaves Ms. Cianciotta with a delicate conversation ahead of her.
. Popular dietary supplements may, for instance, cancel the effects of a cancer treatment, making it less effective, or increase serious side effects, such as liver toxicity. But in other cases, supplementation, such as vitamin D for patients who lack the vitamin, may be beneficial, Ms. Cianciotta said.
These drug-supplement interactions can be hard to pinpoint, given that more than two-thirds of doctors don’t know their patients are using supplements.
Here’s what patients need to know about the potential risks of supplement use during treatment, and how oncologists can address this thorny, often poorly understood topic with patients.
The complex drug-supplement landscape
The list of dietary supplements and how they can interact with different treatments and cancer types is long and nuanced.
But certain supplements appear to affect cancer treatments regardless of other things and should be avoided. Any supplement that strongly alters the body’s levels of the protein cytochromes P450 is one example. This group of enzymes plays a key role in metabolizing drugs, including chemotherapy and immunotherapy agents.
Certain supplements – most notably St. John’s wort extract – may decrease or increase the activity of cytochrome P450, which can then affect the concentrations of anticancer drugs in the blood, said William Figg, PharmD, an associate director of the Center for Cancer Research at the National Cancer Institute in Bethesda, Md. Studies show, for instance, that this common herbal supplement can increase the activity of cytochrome P450, resulting in lower levels of cancer drugs.
Outside of drug metabolism, patients with hormone-related cancers, such as breast and prostate cancers, should steer clear of dietary supplements that can alter levels of testosterone or estrogen, Dr. Figg said. The evergreen shrub ashwagandha, for example, is marketed to reduce stress and fatigue, but can also increase testosterone levels – a potential problem for those with prostate cancer receiving androgen deprivation therapy, which lowers testosterone levels.
Many oncologists counsel patients against using antioxidant-based dietary supplements – particularly turmeric and green tea extract – while they have radiation therapy and certain chemotherapies. These therapies work by creating an abundance of highly reactive molecules called free radicals in tumor cells, which increase stress within these cells, ultimately killing them off. Antioxidants, in theory, can neutralize this effect, said Skyler Johnson, MD, a radiation oncologist at Huntsman Cancer Institute at the University of Utah, Salt Lake City. Some studies suggest that antioxidant supplements may lessen the effects of radiation and chemotherapy, although the evidence is mixed.
Some dietary supplements, including high-dose green tea extract and vitamin A, can cause kidney or liver toxicity, and “many cancer patients already have compromised kidney or liver function,” said Jun J. Mao, MD, chief of integrative medicine at Memorial Sloan Kettering Cancer Center in New York. Even herbs that don’t interfere with how well a cancer drug works, such as stevia, can increase treatment-related side effects, such as nausea and vomiting.
Another potential problem with dietary supplements: It’s nearly impossible to know exactly what’s in them. For instance, just last year, the Food and Drug Administration sent nearly 50 warning letters to companies marketing dietary supplements. The issue is that federal regulations governing production are less strict for supplements than for medications. As a result, some supplements contain ingredients not listed on the label.
One historical example was the supplement PC-SPES, a mix of eight herbs, marketed to men with prostate cancer. The supplement was recalled in 2002 after certain batches were found to contain traces of prescription drugs, including diethylstilbestrol, ethinyl estradiol, warfarin, and alprazolam.
To further complicate matters, some dietary supplements can be helpful. Most patients with cancer “are malnourished and missing out on nutrients they could be getting from food,” said Ms. Cianciotta.
Patients are tested routinely for vitamin deficiencies and receive supplements as needed, she said. Vitamin D and folic acid are two of the most common deficiencies in this patient population. Vitamin D supplementation can improve outcomes in patients who have received a stem cell transplant by aiding engraftment and rebuilding the immune system, while folic acid supplementation can help to raise low red blood cell counts and hemoglobin levels.
Although she rarely sees vitamin toxicity, Ms. Cianciotta stressed that more is not always better and supplement use, even when it seems safe or warranted due to a deficiency, should be taken under supervision, and monitored carefully by the patient’s care team.
Bringing supplement use into the light
Too often, providers are unaware of a patient’s supplement use.
A core reason: Dietary supplements are often touted as natural, which many patients equate with safety, said Samantha Heller, a senior clinical nutritionist at New York (N.Y.) University Langone Health.
That means patients may not know a supplement can act like a drug and interfere with their cancer treatment, and thus may not see the importance of telling their doctors.
Still, the promise of herbs, vitamins, and minerals can be alluring, and there are many reasons patients decide to partake. One major appeal: Dietary supplements can help some patients feel empowered.
“Cancer is a disease that takes away a lot of control from the individual. Taking supplements or herbs is a way to regain some sense of control,” said Dr. Mao.
The phenomenon can also be cultural, he said. Some people grow up taking herbs and supplements to stay healthy or combat health woes.
Pressure or advice from family or friends who may think they are helping a loved one with their dietary recommendations may play a role as well. Friends and family “cannot prescribe chemo, but they can buy herbs and supplements,” Dr. Mao said.
Patients seeking greater control over their health or who feel high levels of anxiety may be more likely to take suggestions from friends and family or more likely to believe false or misleading claims about the efficacy or safety of supplements, explained medical oncologist William Dahut, MD, chief scientific officer for the American Cancer Society.
Plus, social media often amplifies and normalizes this misinformation, noted Dr. Johnson. In a 2021 study published in the Journal of the National Cancer Institute, he and colleagues found that one-third of the most popular articles on cancer treatment posted to social media in 2018 and 2019 contained false, inaccurate, or misleading information that was often harmful.
Some of the false claims centered on unproven, potentially unsafe herbal remedies, according to Dr. Johnson. These included “lung cancer can be cured with cannabis oil” and “golden berries cure and prevent cancer.”
Given exaggerated claims of “cures,” some patients may keep their supplement use under the radar out of fear they will be judged or criticized.
“Clinicians should avoid making patients feel judged or telling people not to go online to do their own research,” Dr. Johnson said.
Guiding patients, instead, to accurate sources of online information may be one way to help patients feel empowered, he said. Cancer.gov and the Memorial Sloan Kettering Cancer Center’s About Herbs database provide accessible and accurate information on dietary supplements and cancer treatment for both health care professionals and patients, he noted.
If a particular supplement is not safe during treatment, providers should be able to explain why, said Ms. Cianciotta. In a recent study, 80% of health care providers surveyed believed that interactions between herbals and medications could be problematic, but only 15% could explain why.
“Being able to explain why we are discouraging a particular supplement right now tends to be much better received than just telling a patient not to take something, because it is bad,” she said.
Another key is listening closely to patients to understand why they may be taking a particular supplement. Does the patient feel out of control? Is nausea a problem?
“Allowing patients to tell you why they are using a particular supplement will often reveal unmet needs or psychosocial challenges,” Dr. Mao said. This information can allow providers to suggest an evidence-based alternative, such as mindfulness meditation or acupuncture, to manage stress.
And if a patient has received a dietary supplement from well-meaning family and friends?
“Simply telling a patient that a given supplement is useless or harmful could create family tension,” said Dr. Mao.
Instead, he recommends reframing the issue.
“We want to have a better understanding of how patients are tolerating chemo or immunotherapy before throwing other things on top of it. Let them know that now may just not be the right time to add a supplement to the mix,” Dr. Mao said.
The bottom line: “Patients want to play an active role in their own care, and we want to help them do that in a safe way,” he said.
A version of this article first appeared on WebMD.com.
Cancer dietitian Lisa Cianciotta often finds herself sitting across from a patient who suddenly fishes a bottle of antioxidant supplements from their bag and says, “My friend told me this works really well,” or “I read on the Internet that this is supposed to be really good for cancer.”
Although taking an antioxidant pill sounds harmless, Ms. Cianciotta, a clinical dietitian who works with cancer patients at New York–Presbyterian Hospital, knows well that this popular dietary supplement can interfere with a patient’s radiation or chemotherapy.
But many patients with cancer believe these over-the-counter vitamins, minerals, or herbal remedies will help them, and most use at least one dietary supplement alongside their cancer treatment.
And that leaves Ms. Cianciotta with a delicate conversation ahead of her.
. Popular dietary supplements may, for instance, cancel the effects of a cancer treatment, making it less effective, or increase serious side effects, such as liver toxicity. But in other cases, supplementation, such as vitamin D for patients who lack the vitamin, may be beneficial, Ms. Cianciotta said.
These drug-supplement interactions can be hard to pinpoint, given that more than two-thirds of doctors don’t know their patients are using supplements.
Here’s what patients need to know about the potential risks of supplement use during treatment, and how oncologists can address this thorny, often poorly understood topic with patients.
The complex drug-supplement landscape
The list of dietary supplements and how they can interact with different treatments and cancer types is long and nuanced.
But certain supplements appear to affect cancer treatments regardless of other things and should be avoided. Any supplement that strongly alters the body’s levels of the protein cytochromes P450 is one example. This group of enzymes plays a key role in metabolizing drugs, including chemotherapy and immunotherapy agents.
Certain supplements – most notably St. John’s wort extract – may decrease or increase the activity of cytochrome P450, which can then affect the concentrations of anticancer drugs in the blood, said William Figg, PharmD, an associate director of the Center for Cancer Research at the National Cancer Institute in Bethesda, Md. Studies show, for instance, that this common herbal supplement can increase the activity of cytochrome P450, resulting in lower levels of cancer drugs.
Outside of drug metabolism, patients with hormone-related cancers, such as breast and prostate cancers, should steer clear of dietary supplements that can alter levels of testosterone or estrogen, Dr. Figg said. The evergreen shrub ashwagandha, for example, is marketed to reduce stress and fatigue, but can also increase testosterone levels – a potential problem for those with prostate cancer receiving androgen deprivation therapy, which lowers testosterone levels.
Many oncologists counsel patients against using antioxidant-based dietary supplements – particularly turmeric and green tea extract – while they have radiation therapy and certain chemotherapies. These therapies work by creating an abundance of highly reactive molecules called free radicals in tumor cells, which increase stress within these cells, ultimately killing them off. Antioxidants, in theory, can neutralize this effect, said Skyler Johnson, MD, a radiation oncologist at Huntsman Cancer Institute at the University of Utah, Salt Lake City. Some studies suggest that antioxidant supplements may lessen the effects of radiation and chemotherapy, although the evidence is mixed.
Some dietary supplements, including high-dose green tea extract and vitamin A, can cause kidney or liver toxicity, and “many cancer patients already have compromised kidney or liver function,” said Jun J. Mao, MD, chief of integrative medicine at Memorial Sloan Kettering Cancer Center in New York. Even herbs that don’t interfere with how well a cancer drug works, such as stevia, can increase treatment-related side effects, such as nausea and vomiting.
Another potential problem with dietary supplements: It’s nearly impossible to know exactly what’s in them. For instance, just last year, the Food and Drug Administration sent nearly 50 warning letters to companies marketing dietary supplements. The issue is that federal regulations governing production are less strict for supplements than for medications. As a result, some supplements contain ingredients not listed on the label.
One historical example was the supplement PC-SPES, a mix of eight herbs, marketed to men with prostate cancer. The supplement was recalled in 2002 after certain batches were found to contain traces of prescription drugs, including diethylstilbestrol, ethinyl estradiol, warfarin, and alprazolam.
To further complicate matters, some dietary supplements can be helpful. Most patients with cancer “are malnourished and missing out on nutrients they could be getting from food,” said Ms. Cianciotta.
Patients are tested routinely for vitamin deficiencies and receive supplements as needed, she said. Vitamin D and folic acid are two of the most common deficiencies in this patient population. Vitamin D supplementation can improve outcomes in patients who have received a stem cell transplant by aiding engraftment and rebuilding the immune system, while folic acid supplementation can help to raise low red blood cell counts and hemoglobin levels.
Although she rarely sees vitamin toxicity, Ms. Cianciotta stressed that more is not always better and supplement use, even when it seems safe or warranted due to a deficiency, should be taken under supervision, and monitored carefully by the patient’s care team.
Bringing supplement use into the light
Too often, providers are unaware of a patient’s supplement use.
A core reason: Dietary supplements are often touted as natural, which many patients equate with safety, said Samantha Heller, a senior clinical nutritionist at New York (N.Y.) University Langone Health.
That means patients may not know a supplement can act like a drug and interfere with their cancer treatment, and thus may not see the importance of telling their doctors.
Still, the promise of herbs, vitamins, and minerals can be alluring, and there are many reasons patients decide to partake. One major appeal: Dietary supplements can help some patients feel empowered.
“Cancer is a disease that takes away a lot of control from the individual. Taking supplements or herbs is a way to regain some sense of control,” said Dr. Mao.
The phenomenon can also be cultural, he said. Some people grow up taking herbs and supplements to stay healthy or combat health woes.
Pressure or advice from family or friends who may think they are helping a loved one with their dietary recommendations may play a role as well. Friends and family “cannot prescribe chemo, but they can buy herbs and supplements,” Dr. Mao said.
Patients seeking greater control over their health or who feel high levels of anxiety may be more likely to take suggestions from friends and family or more likely to believe false or misleading claims about the efficacy or safety of supplements, explained medical oncologist William Dahut, MD, chief scientific officer for the American Cancer Society.
Plus, social media often amplifies and normalizes this misinformation, noted Dr. Johnson. In a 2021 study published in the Journal of the National Cancer Institute, he and colleagues found that one-third of the most popular articles on cancer treatment posted to social media in 2018 and 2019 contained false, inaccurate, or misleading information that was often harmful.
Some of the false claims centered on unproven, potentially unsafe herbal remedies, according to Dr. Johnson. These included “lung cancer can be cured with cannabis oil” and “golden berries cure and prevent cancer.”
Given exaggerated claims of “cures,” some patients may keep their supplement use under the radar out of fear they will be judged or criticized.
“Clinicians should avoid making patients feel judged or telling people not to go online to do their own research,” Dr. Johnson said.
Guiding patients, instead, to accurate sources of online information may be one way to help patients feel empowered, he said. Cancer.gov and the Memorial Sloan Kettering Cancer Center’s About Herbs database provide accessible and accurate information on dietary supplements and cancer treatment for both health care professionals and patients, he noted.
If a particular supplement is not safe during treatment, providers should be able to explain why, said Ms. Cianciotta. In a recent study, 80% of health care providers surveyed believed that interactions between herbals and medications could be problematic, but only 15% could explain why.
“Being able to explain why we are discouraging a particular supplement right now tends to be much better received than just telling a patient not to take something, because it is bad,” she said.
Another key is listening closely to patients to understand why they may be taking a particular supplement. Does the patient feel out of control? Is nausea a problem?
“Allowing patients to tell you why they are using a particular supplement will often reveal unmet needs or psychosocial challenges,” Dr. Mao said. This information can allow providers to suggest an evidence-based alternative, such as mindfulness meditation or acupuncture, to manage stress.
And if a patient has received a dietary supplement from well-meaning family and friends?
“Simply telling a patient that a given supplement is useless or harmful could create family tension,” said Dr. Mao.
Instead, he recommends reframing the issue.
“We want to have a better understanding of how patients are tolerating chemo or immunotherapy before throwing other things on top of it. Let them know that now may just not be the right time to add a supplement to the mix,” Dr. Mao said.
The bottom line: “Patients want to play an active role in their own care, and we want to help them do that in a safe way,” he said.
A version of this article first appeared on WebMD.com.
Development of a Safety Awards Program at a Veterans Affairs Health Care System: A Quality Improvement Initiative
ABSTRACT
Objective: Promoting a culture of safety is a critical component of improving health care quality. Recognizing staff who stop the line for safety can positively impact the growth of a culture of safety. The purpose of this initiative was to demonstrate to staff the importance of speaking up for safety and being acknowledged for doing so.
Methods: Following a review of the literature on safety awards programs and their role in promoting a culture of safety in health care covering the period 2017 to 2020, a formal process was developed and implemented to disseminate safety awards to employees.
Results: During the initial 18 months of the initiative, a total of 59 awards were presented. The awards were well received by the recipients and other staff members. Within this period, adjustments were made to enhance the scope and reach of the program.
Conclusion: Recognizing staff behaviors that support a culture of safety is important for improving health care quality and employee engagement. Future research should focus on a formal evaluation of the impact of safety awards programs on patient safety outcomes.
Keywords: patient safety, culture of safety, incident reporting, near miss.
A key aspect of improving health care quality is promoting and sustaining a culture of safety in the workplace. Improving the quality of health care services and systems involves making informed choices regarding the types of strategies to implement.1 An essential aspect of supporting a safety culture is safety-event reporting. To approach the goal of zero harm, all safety events, whether they result in actual harm or are considered near misses, need to be reported. Near-miss events are errors that occur while care is being provided but are detected and corrected before harm reaches the patient.1-3 Near-miss reporting plays a critical role in helping to identify and correct weaknesses in health care delivery systems and processes.4 However, evidence shows that there are a multitude of barriers to the reporting of near-miss events, such as fear of punitive actions, additional workload, unsupportive work environments, a culture with poor psychological safety, knowledge deficit, and lack of recognition of staff who do report near misses.4-11
According to The Joint Commission (TJC), acknowledging health care team members who recognize and report unsafe conditions that provide insight for improving patient safety is a key method for promoting the reporting of near-miss events.6 As a result, some health care organizations and patient safety agencies have started to institute some form of recognition for their employees in the realm of safety.8-10 The Pennsylvania Patient Safety Authority offers exceptional guidance for creating a safety awards program to promote a culture of safety.12 Furthermore, TJC supports recognizing individuals and health care teams who identify and report near misses, or who have suggestions for initiatives to promote patient safety, with “good catch” awards. Individuals or teams working to promote and sustain a culture of safety should be recognized for their efforts. Acknowledging “good catches” to reward the identification, communication, and resolution of safety issues is an effective strategy for improving patient safety and health care quality.6,8
This quality improvement (QI) initiative was undertaken to demonstrate to staff that, in building an organizational culture of safety, it is important that staff be encouraged to speak up for safety and be acknowledged for doing so. If health care organizations want staff to be motivated to report near misses and improve safety and health care quality, the culture needs to shift from focusing on blame to incentivizing individuals and teams to speak up when they have concerns.8-10 Although deciding which safety actions are worthy of recognition can be challenging, recognizing all safe acts, regardless of how big or small they are perceived to be, is important. This QI initiative aimed to establish a tiered approach to recognize staff members for various categories of safety acts.
METHODS
A review of the literature from January 2017 to May 2020 for peer-reviewed publications regarding how other organizations implemented safety award programs to promote a culture of safety resulted in a dearth of evidence. This prompted us at the Veterans Affairs Connecticut Healthcare System to develop and implement a formal program to disseminate safety awards to employees.
Program Launch and Promotion
In 2020, our institution embarked on a journey to high reliability with the goal of approaching zero harm. As part of efforts to promote a culture of safety, the hospital’s High Reliability Organization (HRO) team worked to develop a safety awards recognition program. Prior to the launch, the hospital’s patient safety committee recognized staff members through the medical center safety event reporting system (the Joint Patient Safety Reporting system [JPSR]) or through direct communication with staff members on safety actions they were engaged in. JPSR is the Veterans Health Administration National Center for Patient Safety incident reporting system for reporting, tracking, and trending of patient incidents in a national database. The award consisted of a certificate presented by the patient safety committee chairpersons to the employee in front of their peers in their respective work area. Hospital leadership was not involved in the safety awards recognition program at that time. No nomination process existed prior to our QI launch.
Once the QI initiative was launched and marketed heavily at staff meetings, we started to receive nominations for actions that were truly exceptional, while many others were submitted for behaviors that were within the day-to-day scope of practice of the staff member. For those early nominations that did not meet criteria for an award, we thanked staff for their submissions with a gentle statement that their nomination did not meet the criteria for an award. After following this practice for a few weeks, we became concerned that if we did not acknowledge the staff who came forward to request recognition for their routine work that supported safety, we could risk losing their engagement in a culture of safety. As such, we decided to create 3 levels of awards to recognize behaviors that went above and beyond while also acknowledging staff for actions within their scope of practice. Additionally, hospital leadership wanted to ensure that all staff recognize that their safety efforts are valued by leadership and that that sense of value will hopefully contribute to a culture of safety over time.
Initially, the single award system was called the “Good Catch Award” to acknowledge staff who go above and beyond to speak up and take action when they have safety concerns. This particular recognition includes a certificate, an encased baseball card that has been personalized by including the staff member’s picture and safety event identified, a stress-release baseball, and a stick of Bazooka gum (similar to what used to come in baseball cards packs). The award is presented to employees in their work area by the HRO and patient safety teams and includes representatives from the executive leadership team (ELT). The safety event identified is described by an ELT member, and all items are presented to the employee. Participation by the leadership team communicates how much the work being done to promote a culture of safety and advance quality health care is appreciated. This action also encourages others in the organization to identify and report safety concerns.13
With the rollout of the QI initiative, the volume of nominations submitted quickly increased (eg, approximately 1 every 2 months before to 3 per month following implementation). Frequently, nominations were for actions believed to be within the scope of the employee’s responsibilities. Our institution’s leadership team quickly recognized that, as an organization, not diminishing the importance of the “Good Catch Award” was important. However, the
The original Good Catch Award was labelled as a Level 1 award. The Level 2 safety recognition award, named the HRO Safety Champion Award, is given to employees who stop the line for a safety concern within their scope of practice and also participate as part of a team to investigate and improve processes to avoid recurring safety concerns in the future. For the Level Two award, a certificate is presented to an employee by the hospital’s HRO lead, HRO physician champion, patient safety manager, immediate supervisor, and peers. With the Level 3 award, the Culture of Safety Appreciation Award, individuals are recognized for addressing safety concerns within their assigned scope of responsibilities. Recognition is bestowed by an email of appreciation sent to the employee, acknowledging their commitment to promoting a culture of safety and quality health care. The recipient’s direct supervisor and other hospital leaders are copied on the message.14 See Table 1 for a

Our institution’s HRO and patient safety teams utilized many additional venues to disseminate information regarding awardees and their actions. These included our monthly HRO newsletter, monthly safety forums, and biweekly Team Connecticut Healthcare system-wide huddles.
Nomination Process
Awards nominations are submitted via the hospital intranet homepage, where there is an “HRO Safety Award Nomination” icon. Once a staff member clicks the icon, a template opens asking for information, such as the reason for the nomination submission, and then walks them through the template using the CAR (C-context, A-actions, and R-results)15 format for describing the situation, identifying actions taken, and specifying the outcome of the action. Emails with award nominations can also be sent to the HRO lead, HRO champion, or Patient Safety Committee co-chairs. Calls for nominations are made at several venues attended by employees as well as supervisors. These include monthly safety forums, biweekly Team Connecticut Healthcare system-wide huddles, supervisory staff meetings, department and unit-based staff meetings, and many other formal and informal settings. This QI initiative has allowed us to capture potential awardees through several avenues, including self-nominations. All nominations are reviewed by a safety awards committee. Each committee member ranks the nomination as a Level 1, 2, or 3 award. For nominations where conflicting scores are obtained, the committee discusses the nomination together to resolve discrepancies.
Needed Resources
Material resources required for this QI initiative include certificate paper, plastic baseball card sleeves, stress-release baseballs, and Bazooka gum. The largest resource investment was the time needed to support the initiative. This included the time spent scheduling the Level 1 and 2 award presentations with staff and leadership. Time was also required to put the individual award packages together, which included printing the paper certificates, obtaining awardee pictures, placing them with their safety stories in a plastic baseball card sleeve, and arranging for the hospital photographer to take pictures of the awardees with their peers and leaders.
RESULTS
Prior to this QI initiative launch, 14 awards were given out over the preceding 2-year period. During the initial 18 months of the initiative (December 2020 to June 2022), 59 awards were presented (Level 1, n = 26; Level 2, n = 22; and Level 3, n = 11). Looking further into the Level 1 awards presented, 25 awardees worked in clinical roles and 1 in a nonclinical position (Table 2). The awardees represented multidisciplinary areas, including medical/surgical (med/surg) inpatient units, anesthesia, operating room, pharmacy, mental health clinics, surgical intensive care, specialty care clinics, and nutrition and food services. With the Level 2 awards, 18 clinical staff and 4 nonclinical staff received awards from the areas of med/surg inpatient, outpatient surgical suites, the medical center director’s office, radiology, pharmacy, primary care, facilities management, environmental management, infection prevention, and emergency services. All Level 3 awardees were from clinical areas, including primary care, hospital education, sterile processing, pharmacies, operating rooms, and med/surg inpatient units.
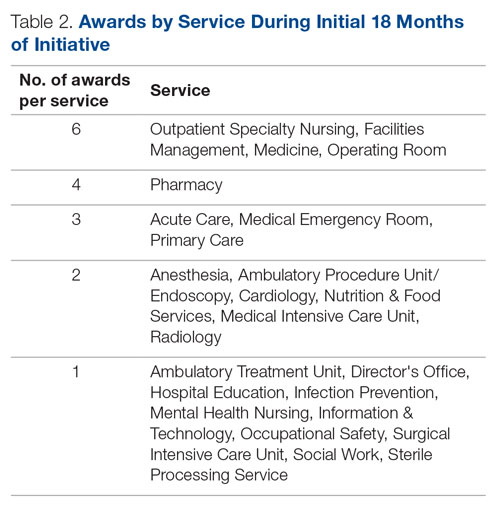
With the inception of this QI initiative, our organization has begun to see trends reflecting increased reporting of both actual and close-call events in JPSR (Figure 1). 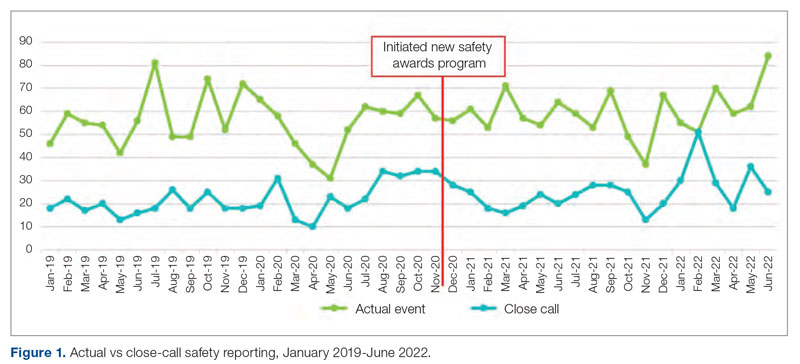
With the inclusion of information regarding awardees and their actions in monthly safety forums, attendance at these forums has increased from an average of 64 attendees per month in 2021 to an average of 131 attendees per month in 2022 (Figure 2).
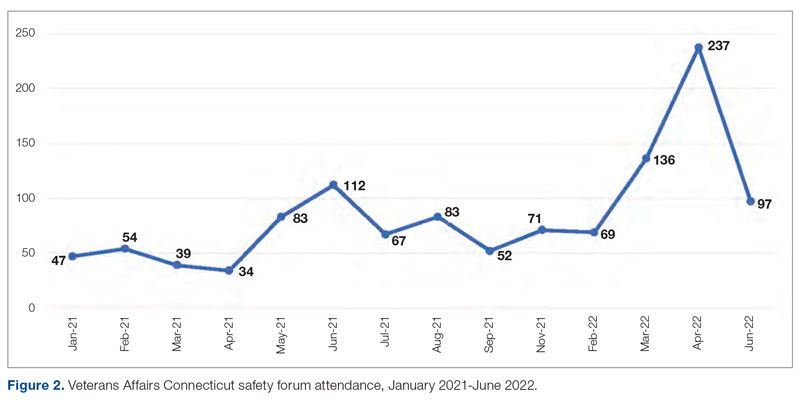
Finally, our organization’s annual All-Employee Survey results have shown incremental increases in staff reporting feeling psychologically safe and not fearing reprisal (Figure 3). It is important to note that there may be other contributing factors to these incremental changes.
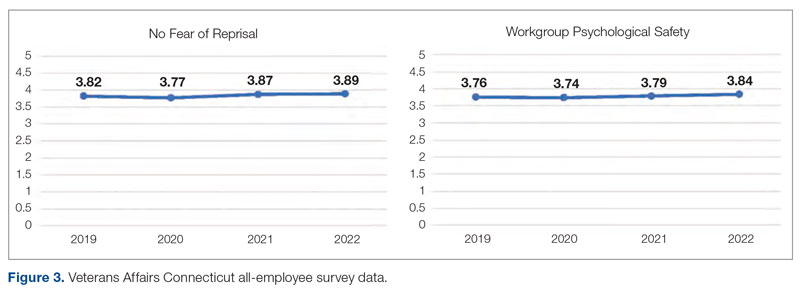
Level 1 – Good Catch Award. M.S. was assigned as a continuous safety monitor, or “sitter,” on one of the med/surg inpatient units. M.S. arrived at the bedside and asked for a report on the patient at a change in shift. The report stated that the patient was sleeping and had not moved in a while. M.S. set about to perform the functions of a sitter and did her usual tasks in cleaning and tidying the room for the patient for breakfast and taking care of all items in the room, in general. M.S. introduced herself to the patient, who she thought might wake up because of her speaking to him. She thought the patient was in an odd position, and knowing that a patient should be a little further up in the bed, she tried with touch to awaken him to adjust his position. M.S. found that the patient was rather chilly to the touch and immediately became concerned. She continued to attempt to rouse the patient. M.S. called for the nurse and began to adjust the patient’s position. M.S. insisted that the patient was cold and “something was wrong.” A set of vitals was taken and a rapid response team code was called. The patient was immediately transferred to the intensive care unit to receive a higher level of care. If not for the diligence and caring attitude of M.S., this patient may have had a very poor outcome.
Reason for criteria being met: The scope of practice of a sitter is to be present in a patient’s room to monitor for falls and overall safety. This employee noticed that the patient was not responsive to verbal or tactile stimuli. Her immediate reporting of her concern to the nurse resulted in prompt intervention. If she had let the patient be, the patient could have died. The staff member went above and beyond by speaking up and taking action when she had a patient safety concern.
Level 2 – HRO Safety Champion Award. A patient presented to an outpatient clinic for monoclonal antibody (mAb) therapy for a COVID-19 infection; the treatment has been scheduled by the patient’s primary care provider. At that time, outpatient mAb therapy was the recommended care option for patients stable enough to receive treatment in this setting, but it is contraindicated in patients who are too unstable to receive mAb therapy in an outpatient setting, such as those with increased oxygen demands. R.L., a staff nurse, assessed the patient on arrival and found that his vital signs were stable, except for a slightly elevated respiratory rate. Upon questioning, the patient reported that he had increased his oxygen use at home from 2 to 4 L via a nasal cannula. R.L. assessed that the patient was too high-risk for outpatient mAb therapy and had the patient checked into the emergency department (ED) to receive a full diagnostic workup and evaluation by Dr. W., an ED provider. The patient required admission to the hospital for a higher level of care in an inpatient unit because of severe COVID-19 infection. Within 48 hours of admission, the patient’s condition further declined, requiring an upgrade to the medical intensive care unit with progressive interventions. Owing to the clinical assessment skills and prompt action of R.L., the patient was admitted to the hospital instead of receiving treatment in a suboptimal care setting and returning home. Had the patient gone home, his rapid decline could have had serious consequences.
Reason for criteria being met: On a cursory look, the patient may have passed as someone sufficiently stable to undergo outpatient treatment. However, the nurse stopped the line, paid close attention, and picked up on an abnormal vital sign and the projected consequences. The nurse brought the patient to a higher level of care in the ED so that he could get the attention he needed. If this patient was given mAb therapy in the outpatient setting, he would have been discharged and become sicker with the COVID-19 illness. As a result of this incident, R.L. is working with the outpatient clinic and ED staff to enahance triage and evaluation of patients referred for outpatient therapy for COVID-19 infections to prevent a similar event from recurring.
Level 3 – Culture of Safety Appreciation Award. While C.C. was reviewing the hazardous item
Reason for criteria being met: The employee works in the hospital education department. It is within her scope of responsibilities to provide ongoing education to staff in order to address potential safety concerns.
DISCUSSION
This QI initiative was undertaken to demonstrate to staff that, in building an organizational culture of safety and advancing quality health care, it is important that staff be encouraged to speak up for safety and be acknowledged for doing so. As part of efforts to continuously build on a safety-first culture, transparency and celebration of successes were demonstrated. This QI initiative demonstrated that a diverse and wide range of employees were reached, from clinical to nonclinical staff, and frontline to supervisory staff, as all were included in the recognition process. While many award nominations were received through the submission of safety concerns to the high-reliability team and patient safety office, several came directly from staff who wanted to recognize their peers for their work, supporting a culture of safety. This showed that staff felt that taking the time to submit a write-up to recognize a peer was an important task. Achieving zero harm for patients and staff alike is a top priority for our institution and guides all decisions, which reinforces that everyone has a responsibility to ensure that safety is always the first consideration. A culture of safety is enhanced by staff recognition. This QI initiative also showed that staff felt valued when they were acknowledged, regardless of the level of recognition they received. The theme of feeling valued came from unsolicited feedback. For example, some direct comments from awardees are presented in the Box.

In addition to endorsing the importance of safe practices to staff, safety award programs can identify gaps in existing standard procedures that can be updated quickly and shared broadly across a health care organization. The authors observed that the existence of the award program gives staff permission to use their voice to speak up when they have questions or concerns related to safety and to proactively engage in safety practices; a cultural shift of this kind informs safety practices and procedures and contributes to a more inspiring workplace. Staff at our organization who have received any of the safety awards, and those who are aware of these awards, have embraced the program readily. At the time of submission of this manuscript, there was a relative paucity of published literature on the details, performance, and impact of such programs. This initiative aims to share a road map highlighting the various dimensions of staff recognition and how the program supports our health care system in fostering a strong, sustainable culture of safety and health care quality. A next step is to formally assess the impact of the awards program on our culture of safety and quality using a psychometrically sound measurement tool, as recommended by TJC,16 such as the
CONCLUSION
A health care organization safety awards program is a strategy for building and sustaining a culture of safety. This QI initiative may be valuable to other organizations in the process of establishing a safety awards program of their own. Future research should focus on a formal evaluation of the impact of safety awards programs on patient safety outcomes.
Corresponding author: John S. Murray, PhD, MPH, MSGH, RN, FAAN, 20 Chapel Street, Unit A502, Brookline, MA 02446; [email protected]
Disclosures: None reported.
1. National Center for Biotechnology Information. Improving healthcare quality in Europe: Characteristics, effectiveness and implementation of different strategies. National Library of Medicine; 2019.
2.
3. Agency for Healthcare Research and Quality. Implementing near-miss reporting and improvement tracking in primary care practices: lessons learned. Agency for Healthcare Research and Quality; 2017.
4. Hamed M, Konstantinidis S. Barriers to incident reporting among nurses: a qualitative systematic review. West J Nurs Res. 2022;44(5):506-523. doi:10.1177/0193945921999449
5. Mohamed M, Abubeker IY, Al-Mohanadi D, et al. Perceived barriers of incident reporting among internists: results from Hamad medical corporation in Qatar. Avicenna J Med. 2021;11(3):139-144. doi:10.1055/s-0041-1734386
6. The Joint Commission. The essential role of leadership in developing a safety culture. The Joint Commission; 2017.
7. Yali G, Nzala S. Healthcare providers’ perspective on barriers to patient safety incident reporting in Lusaka District. J Prev Rehabil Med. 2022;4:44-52. doi:10.21617/jprm2022.417
8. Herzer KR, Mirrer M, Xie Y, et al. Patient safety reporting systems: sustained quality improvement using a multidisciplinary team and “good catch” awards. Jt Comm J Qual Patient Saf. 2012;38(8):339-347. doi:10.1016/s1553-7250(12)38044-6
9. Rogers E, Griffin E, Carnie W, et al. A just culture approach to managing medication errors. Hosp Pharm. 2017;52(4):308-315. doi:10.1310/hpj5204-308
10. Murray JS, Clifford J, Larson S, et al. Implementing just culture to improve patient safety. Mil Med. 2022;0: 1. doi:10.1093/milmed/usac115
11. Paradiso L, Sweeney N. Just culture: it’s more than policy. Nurs Manag. 2019;50(6):38–45. doi:10.1097/01.NUMA.0000558482.07815.ae
12. Wallace S, Mamrol M, Finley E; Pennsylvania Patient Safety Authority. Promote a culture of safety with good catch reports. PA Patient Saf Advis. 2017;14(3).
13. Tan KH, Pang NL, Siau C, et al: Building an organizational culture of patient safety. J Patient Saf Risk Manag. 2019;24:253-261. doi.10.1177/251604351987897
14. Merchant N, O’Neal J, Dealino-Perez C, et al: A high reliability mindset. Am J Med Qual. 2022;37(6):504-510. doi:10.1097/JMQ.0000000000000086
15. Behavioral interview questions and answers. Hudson. Accessed December 23, 2022. https://au.hudson.com/insights/career-advice/job-interviews/behavioural-interview-questions-and-answers/
16. The Joint Commission. Safety culture assessment: Improving the survey process. Accessed December 26, 2022. https://www.jointcommission.org/-/media/tjc/documents/accred-and-cert/safety_culture_assessment_improving_the_survey_process.pdf
17. Reis CT, Paiva SG, Sousa P. The patient safety culture: a systematic review by characteristics of hospital survey on patient safety culture dimensions. Int J Qual Heal Care. 2018;30(9):660-677. doi:10.1093/intqhc/mzy080
18. Fourar YO, Benhassine W, Boughaba A, et al. Contribution to the assessment of patient safety culture in Algerian healthcare settings: the ASCO project. Int J Healthc Manag. 2022;15:52-61. doi.org/10.1080/20479700.2020.1836736
ABSTRACT
Objective: Promoting a culture of safety is a critical component of improving health care quality. Recognizing staff who stop the line for safety can positively impact the growth of a culture of safety. The purpose of this initiative was to demonstrate to staff the importance of speaking up for safety and being acknowledged for doing so.
Methods: Following a review of the literature on safety awards programs and their role in promoting a culture of safety in health care covering the period 2017 to 2020, a formal process was developed and implemented to disseminate safety awards to employees.
Results: During the initial 18 months of the initiative, a total of 59 awards were presented. The awards were well received by the recipients and other staff members. Within this period, adjustments were made to enhance the scope and reach of the program.
Conclusion: Recognizing staff behaviors that support a culture of safety is important for improving health care quality and employee engagement. Future research should focus on a formal evaluation of the impact of safety awards programs on patient safety outcomes.
Keywords: patient safety, culture of safety, incident reporting, near miss.
A key aspect of improving health care quality is promoting and sustaining a culture of safety in the workplace. Improving the quality of health care services and systems involves making informed choices regarding the types of strategies to implement.1 An essential aspect of supporting a safety culture is safety-event reporting. To approach the goal of zero harm, all safety events, whether they result in actual harm or are considered near misses, need to be reported. Near-miss events are errors that occur while care is being provided but are detected and corrected before harm reaches the patient.1-3 Near-miss reporting plays a critical role in helping to identify and correct weaknesses in health care delivery systems and processes.4 However, evidence shows that there are a multitude of barriers to the reporting of near-miss events, such as fear of punitive actions, additional workload, unsupportive work environments, a culture with poor psychological safety, knowledge deficit, and lack of recognition of staff who do report near misses.4-11
According to The Joint Commission (TJC), acknowledging health care team members who recognize and report unsafe conditions that provide insight for improving patient safety is a key method for promoting the reporting of near-miss events.6 As a result, some health care organizations and patient safety agencies have started to institute some form of recognition for their employees in the realm of safety.8-10 The Pennsylvania Patient Safety Authority offers exceptional guidance for creating a safety awards program to promote a culture of safety.12 Furthermore, TJC supports recognizing individuals and health care teams who identify and report near misses, or who have suggestions for initiatives to promote patient safety, with “good catch” awards. Individuals or teams working to promote and sustain a culture of safety should be recognized for their efforts. Acknowledging “good catches” to reward the identification, communication, and resolution of safety issues is an effective strategy for improving patient safety and health care quality.6,8
This quality improvement (QI) initiative was undertaken to demonstrate to staff that, in building an organizational culture of safety, it is important that staff be encouraged to speak up for safety and be acknowledged for doing so. If health care organizations want staff to be motivated to report near misses and improve safety and health care quality, the culture needs to shift from focusing on blame to incentivizing individuals and teams to speak up when they have concerns.8-10 Although deciding which safety actions are worthy of recognition can be challenging, recognizing all safe acts, regardless of how big or small they are perceived to be, is important. This QI initiative aimed to establish a tiered approach to recognize staff members for various categories of safety acts.
METHODS
A review of the literature from January 2017 to May 2020 for peer-reviewed publications regarding how other organizations implemented safety award programs to promote a culture of safety resulted in a dearth of evidence. This prompted us at the Veterans Affairs Connecticut Healthcare System to develop and implement a formal program to disseminate safety awards to employees.
Program Launch and Promotion
In 2020, our institution embarked on a journey to high reliability with the goal of approaching zero harm. As part of efforts to promote a culture of safety, the hospital’s High Reliability Organization (HRO) team worked to develop a safety awards recognition program. Prior to the launch, the hospital’s patient safety committee recognized staff members through the medical center safety event reporting system (the Joint Patient Safety Reporting system [JPSR]) or through direct communication with staff members on safety actions they were engaged in. JPSR is the Veterans Health Administration National Center for Patient Safety incident reporting system for reporting, tracking, and trending of patient incidents in a national database. The award consisted of a certificate presented by the patient safety committee chairpersons to the employee in front of their peers in their respective work area. Hospital leadership was not involved in the safety awards recognition program at that time. No nomination process existed prior to our QI launch.
Once the QI initiative was launched and marketed heavily at staff meetings, we started to receive nominations for actions that were truly exceptional, while many others were submitted for behaviors that were within the day-to-day scope of practice of the staff member. For those early nominations that did not meet criteria for an award, we thanked staff for their submissions with a gentle statement that their nomination did not meet the criteria for an award. After following this practice for a few weeks, we became concerned that if we did not acknowledge the staff who came forward to request recognition for their routine work that supported safety, we could risk losing their engagement in a culture of safety. As such, we decided to create 3 levels of awards to recognize behaviors that went above and beyond while also acknowledging staff for actions within their scope of practice. Additionally, hospital leadership wanted to ensure that all staff recognize that their safety efforts are valued by leadership and that that sense of value will hopefully contribute to a culture of safety over time.
Initially, the single award system was called the “Good Catch Award” to acknowledge staff who go above and beyond to speak up and take action when they have safety concerns. This particular recognition includes a certificate, an encased baseball card that has been personalized by including the staff member’s picture and safety event identified, a stress-release baseball, and a stick of Bazooka gum (similar to what used to come in baseball cards packs). The award is presented to employees in their work area by the HRO and patient safety teams and includes representatives from the executive leadership team (ELT). The safety event identified is described by an ELT member, and all items are presented to the employee. Participation by the leadership team communicates how much the work being done to promote a culture of safety and advance quality health care is appreciated. This action also encourages others in the organization to identify and report safety concerns.13
With the rollout of the QI initiative, the volume of nominations submitted quickly increased (eg, approximately 1 every 2 months before to 3 per month following implementation). Frequently, nominations were for actions believed to be within the scope of the employee’s responsibilities. Our institution’s leadership team quickly recognized that, as an organization, not diminishing the importance of the “Good Catch Award” was important. However, the
The original Good Catch Award was labelled as a Level 1 award. The Level 2 safety recognition award, named the HRO Safety Champion Award, is given to employees who stop the line for a safety concern within their scope of practice and also participate as part of a team to investigate and improve processes to avoid recurring safety concerns in the future. For the Level Two award, a certificate is presented to an employee by the hospital’s HRO lead, HRO physician champion, patient safety manager, immediate supervisor, and peers. With the Level 3 award, the Culture of Safety Appreciation Award, individuals are recognized for addressing safety concerns within their assigned scope of responsibilities. Recognition is bestowed by an email of appreciation sent to the employee, acknowledging their commitment to promoting a culture of safety and quality health care. The recipient’s direct supervisor and other hospital leaders are copied on the message.14 See Table 1 for a

Our institution’s HRO and patient safety teams utilized many additional venues to disseminate information regarding awardees and their actions. These included our monthly HRO newsletter, monthly safety forums, and biweekly Team Connecticut Healthcare system-wide huddles.
Nomination Process
Awards nominations are submitted via the hospital intranet homepage, where there is an “HRO Safety Award Nomination” icon. Once a staff member clicks the icon, a template opens asking for information, such as the reason for the nomination submission, and then walks them through the template using the CAR (C-context, A-actions, and R-results)15 format for describing the situation, identifying actions taken, and specifying the outcome of the action. Emails with award nominations can also be sent to the HRO lead, HRO champion, or Patient Safety Committee co-chairs. Calls for nominations are made at several venues attended by employees as well as supervisors. These include monthly safety forums, biweekly Team Connecticut Healthcare system-wide huddles, supervisory staff meetings, department and unit-based staff meetings, and many other formal and informal settings. This QI initiative has allowed us to capture potential awardees through several avenues, including self-nominations. All nominations are reviewed by a safety awards committee. Each committee member ranks the nomination as a Level 1, 2, or 3 award. For nominations where conflicting scores are obtained, the committee discusses the nomination together to resolve discrepancies.
Needed Resources
Material resources required for this QI initiative include certificate paper, plastic baseball card sleeves, stress-release baseballs, and Bazooka gum. The largest resource investment was the time needed to support the initiative. This included the time spent scheduling the Level 1 and 2 award presentations with staff and leadership. Time was also required to put the individual award packages together, which included printing the paper certificates, obtaining awardee pictures, placing them with their safety stories in a plastic baseball card sleeve, and arranging for the hospital photographer to take pictures of the awardees with their peers and leaders.
RESULTS
Prior to this QI initiative launch, 14 awards were given out over the preceding 2-year period. During the initial 18 months of the initiative (December 2020 to June 2022), 59 awards were presented (Level 1, n = 26; Level 2, n = 22; and Level 3, n = 11). Looking further into the Level 1 awards presented, 25 awardees worked in clinical roles and 1 in a nonclinical position (Table 2). The awardees represented multidisciplinary areas, including medical/surgical (med/surg) inpatient units, anesthesia, operating room, pharmacy, mental health clinics, surgical intensive care, specialty care clinics, and nutrition and food services. With the Level 2 awards, 18 clinical staff and 4 nonclinical staff received awards from the areas of med/surg inpatient, outpatient surgical suites, the medical center director’s office, radiology, pharmacy, primary care, facilities management, environmental management, infection prevention, and emergency services. All Level 3 awardees were from clinical areas, including primary care, hospital education, sterile processing, pharmacies, operating rooms, and med/surg inpatient units.

With the inception of this QI initiative, our organization has begun to see trends reflecting increased reporting of both actual and close-call events in JPSR (Figure 1). 
With the inclusion of information regarding awardees and their actions in monthly safety forums, attendance at these forums has increased from an average of 64 attendees per month in 2021 to an average of 131 attendees per month in 2022 (Figure 2).

Finally, our organization’s annual All-Employee Survey results have shown incremental increases in staff reporting feeling psychologically safe and not fearing reprisal (Figure 3). It is important to note that there may be other contributing factors to these incremental changes.

Level 1 – Good Catch Award. M.S. was assigned as a continuous safety monitor, or “sitter,” on one of the med/surg inpatient units. M.S. arrived at the bedside and asked for a report on the patient at a change in shift. The report stated that the patient was sleeping and had not moved in a while. M.S. set about to perform the functions of a sitter and did her usual tasks in cleaning and tidying the room for the patient for breakfast and taking care of all items in the room, in general. M.S. introduced herself to the patient, who she thought might wake up because of her speaking to him. She thought the patient was in an odd position, and knowing that a patient should be a little further up in the bed, she tried with touch to awaken him to adjust his position. M.S. found that the patient was rather chilly to the touch and immediately became concerned. She continued to attempt to rouse the patient. M.S. called for the nurse and began to adjust the patient’s position. M.S. insisted that the patient was cold and “something was wrong.” A set of vitals was taken and a rapid response team code was called. The patient was immediately transferred to the intensive care unit to receive a higher level of care. If not for the diligence and caring attitude of M.S., this patient may have had a very poor outcome.
Reason for criteria being met: The scope of practice of a sitter is to be present in a patient’s room to monitor for falls and overall safety. This employee noticed that the patient was not responsive to verbal or tactile stimuli. Her immediate reporting of her concern to the nurse resulted in prompt intervention. If she had let the patient be, the patient could have died. The staff member went above and beyond by speaking up and taking action when she had a patient safety concern.
Level 2 – HRO Safety Champion Award. A patient presented to an outpatient clinic for monoclonal antibody (mAb) therapy for a COVID-19 infection; the treatment has been scheduled by the patient’s primary care provider. At that time, outpatient mAb therapy was the recommended care option for patients stable enough to receive treatment in this setting, but it is contraindicated in patients who are too unstable to receive mAb therapy in an outpatient setting, such as those with increased oxygen demands. R.L., a staff nurse, assessed the patient on arrival and found that his vital signs were stable, except for a slightly elevated respiratory rate. Upon questioning, the patient reported that he had increased his oxygen use at home from 2 to 4 L via a nasal cannula. R.L. assessed that the patient was too high-risk for outpatient mAb therapy and had the patient checked into the emergency department (ED) to receive a full diagnostic workup and evaluation by Dr. W., an ED provider. The patient required admission to the hospital for a higher level of care in an inpatient unit because of severe COVID-19 infection. Within 48 hours of admission, the patient’s condition further declined, requiring an upgrade to the medical intensive care unit with progressive interventions. Owing to the clinical assessment skills and prompt action of R.L., the patient was admitted to the hospital instead of receiving treatment in a suboptimal care setting and returning home. Had the patient gone home, his rapid decline could have had serious consequences.
Reason for criteria being met: On a cursory look, the patient may have passed as someone sufficiently stable to undergo outpatient treatment. However, the nurse stopped the line, paid close attention, and picked up on an abnormal vital sign and the projected consequences. The nurse brought the patient to a higher level of care in the ED so that he could get the attention he needed. If this patient was given mAb therapy in the outpatient setting, he would have been discharged and become sicker with the COVID-19 illness. As a result of this incident, R.L. is working with the outpatient clinic and ED staff to enahance triage and evaluation of patients referred for outpatient therapy for COVID-19 infections to prevent a similar event from recurring.
Level 3 – Culture of Safety Appreciation Award. While C.C. was reviewing the hazardous item
Reason for criteria being met: The employee works in the hospital education department. It is within her scope of responsibilities to provide ongoing education to staff in order to address potential safety concerns.
DISCUSSION
This QI initiative was undertaken to demonstrate to staff that, in building an organizational culture of safety and advancing quality health care, it is important that staff be encouraged to speak up for safety and be acknowledged for doing so. As part of efforts to continuously build on a safety-first culture, transparency and celebration of successes were demonstrated. This QI initiative demonstrated that a diverse and wide range of employees were reached, from clinical to nonclinical staff, and frontline to supervisory staff, as all were included in the recognition process. While many award nominations were received through the submission of safety concerns to the high-reliability team and patient safety office, several came directly from staff who wanted to recognize their peers for their work, supporting a culture of safety. This showed that staff felt that taking the time to submit a write-up to recognize a peer was an important task. Achieving zero harm for patients and staff alike is a top priority for our institution and guides all decisions, which reinforces that everyone has a responsibility to ensure that safety is always the first consideration. A culture of safety is enhanced by staff recognition. This QI initiative also showed that staff felt valued when they were acknowledged, regardless of the level of recognition they received. The theme of feeling valued came from unsolicited feedback. For example, some direct comments from awardees are presented in the Box.

In addition to endorsing the importance of safe practices to staff, safety award programs can identify gaps in existing standard procedures that can be updated quickly and shared broadly across a health care organization. The authors observed that the existence of the award program gives staff permission to use their voice to speak up when they have questions or concerns related to safety and to proactively engage in safety practices; a cultural shift of this kind informs safety practices and procedures and contributes to a more inspiring workplace. Staff at our organization who have received any of the safety awards, and those who are aware of these awards, have embraced the program readily. At the time of submission of this manuscript, there was a relative paucity of published literature on the details, performance, and impact of such programs. This initiative aims to share a road map highlighting the various dimensions of staff recognition and how the program supports our health care system in fostering a strong, sustainable culture of safety and health care quality. A next step is to formally assess the impact of the awards program on our culture of safety and quality using a psychometrically sound measurement tool, as recommended by TJC,16 such as the
CONCLUSION
A health care organization safety awards program is a strategy for building and sustaining a culture of safety. This QI initiative may be valuable to other organizations in the process of establishing a safety awards program of their own. Future research should focus on a formal evaluation of the impact of safety awards programs on patient safety outcomes.
Corresponding author: John S. Murray, PhD, MPH, MSGH, RN, FAAN, 20 Chapel Street, Unit A502, Brookline, MA 02446; [email protected]
Disclosures: None reported.
ABSTRACT
Objective: Promoting a culture of safety is a critical component of improving health care quality. Recognizing staff who stop the line for safety can positively impact the growth of a culture of safety. The purpose of this initiative was to demonstrate to staff the importance of speaking up for safety and being acknowledged for doing so.
Methods: Following a review of the literature on safety awards programs and their role in promoting a culture of safety in health care covering the period 2017 to 2020, a formal process was developed and implemented to disseminate safety awards to employees.
Results: During the initial 18 months of the initiative, a total of 59 awards were presented. The awards were well received by the recipients and other staff members. Within this period, adjustments were made to enhance the scope and reach of the program.
Conclusion: Recognizing staff behaviors that support a culture of safety is important for improving health care quality and employee engagement. Future research should focus on a formal evaluation of the impact of safety awards programs on patient safety outcomes.
Keywords: patient safety, culture of safety, incident reporting, near miss.
A key aspect of improving health care quality is promoting and sustaining a culture of safety in the workplace. Improving the quality of health care services and systems involves making informed choices regarding the types of strategies to implement.1 An essential aspect of supporting a safety culture is safety-event reporting. To approach the goal of zero harm, all safety events, whether they result in actual harm or are considered near misses, need to be reported. Near-miss events are errors that occur while care is being provided but are detected and corrected before harm reaches the patient.1-3 Near-miss reporting plays a critical role in helping to identify and correct weaknesses in health care delivery systems and processes.4 However, evidence shows that there are a multitude of barriers to the reporting of near-miss events, such as fear of punitive actions, additional workload, unsupportive work environments, a culture with poor psychological safety, knowledge deficit, and lack of recognition of staff who do report near misses.4-11
According to The Joint Commission (TJC), acknowledging health care team members who recognize and report unsafe conditions that provide insight for improving patient safety is a key method for promoting the reporting of near-miss events.6 As a result, some health care organizations and patient safety agencies have started to institute some form of recognition for their employees in the realm of safety.8-10 The Pennsylvania Patient Safety Authority offers exceptional guidance for creating a safety awards program to promote a culture of safety.12 Furthermore, TJC supports recognizing individuals and health care teams who identify and report near misses, or who have suggestions for initiatives to promote patient safety, with “good catch” awards. Individuals or teams working to promote and sustain a culture of safety should be recognized for their efforts. Acknowledging “good catches” to reward the identification, communication, and resolution of safety issues is an effective strategy for improving patient safety and health care quality.6,8
This quality improvement (QI) initiative was undertaken to demonstrate to staff that, in building an organizational culture of safety, it is important that staff be encouraged to speak up for safety and be acknowledged for doing so. If health care organizations want staff to be motivated to report near misses and improve safety and health care quality, the culture needs to shift from focusing on blame to incentivizing individuals and teams to speak up when they have concerns.8-10 Although deciding which safety actions are worthy of recognition can be challenging, recognizing all safe acts, regardless of how big or small they are perceived to be, is important. This QI initiative aimed to establish a tiered approach to recognize staff members for various categories of safety acts.
METHODS
A review of the literature from January 2017 to May 2020 for peer-reviewed publications regarding how other organizations implemented safety award programs to promote a culture of safety resulted in a dearth of evidence. This prompted us at the Veterans Affairs Connecticut Healthcare System to develop and implement a formal program to disseminate safety awards to employees.
Program Launch and Promotion
In 2020, our institution embarked on a journey to high reliability with the goal of approaching zero harm. As part of efforts to promote a culture of safety, the hospital’s High Reliability Organization (HRO) team worked to develop a safety awards recognition program. Prior to the launch, the hospital’s patient safety committee recognized staff members through the medical center safety event reporting system (the Joint Patient Safety Reporting system [JPSR]) or through direct communication with staff members on safety actions they were engaged in. JPSR is the Veterans Health Administration National Center for Patient Safety incident reporting system for reporting, tracking, and trending of patient incidents in a national database. The award consisted of a certificate presented by the patient safety committee chairpersons to the employee in front of their peers in their respective work area. Hospital leadership was not involved in the safety awards recognition program at that time. No nomination process existed prior to our QI launch.
Once the QI initiative was launched and marketed heavily at staff meetings, we started to receive nominations for actions that were truly exceptional, while many others were submitted for behaviors that were within the day-to-day scope of practice of the staff member. For those early nominations that did not meet criteria for an award, we thanked staff for their submissions with a gentle statement that their nomination did not meet the criteria for an award. After following this practice for a few weeks, we became concerned that if we did not acknowledge the staff who came forward to request recognition for their routine work that supported safety, we could risk losing their engagement in a culture of safety. As such, we decided to create 3 levels of awards to recognize behaviors that went above and beyond while also acknowledging staff for actions within their scope of practice. Additionally, hospital leadership wanted to ensure that all staff recognize that their safety efforts are valued by leadership and that that sense of value will hopefully contribute to a culture of safety over time.
Initially, the single award system was called the “Good Catch Award” to acknowledge staff who go above and beyond to speak up and take action when they have safety concerns. This particular recognition includes a certificate, an encased baseball card that has been personalized by including the staff member’s picture and safety event identified, a stress-release baseball, and a stick of Bazooka gum (similar to what used to come in baseball cards packs). The award is presented to employees in their work area by the HRO and patient safety teams and includes representatives from the executive leadership team (ELT). The safety event identified is described by an ELT member, and all items are presented to the employee. Participation by the leadership team communicates how much the work being done to promote a culture of safety and advance quality health care is appreciated. This action also encourages others in the organization to identify and report safety concerns.13
With the rollout of the QI initiative, the volume of nominations submitted quickly increased (eg, approximately 1 every 2 months before to 3 per month following implementation). Frequently, nominations were for actions believed to be within the scope of the employee’s responsibilities. Our institution’s leadership team quickly recognized that, as an organization, not diminishing the importance of the “Good Catch Award” was important. However, the
The original Good Catch Award was labelled as a Level 1 award. The Level 2 safety recognition award, named the HRO Safety Champion Award, is given to employees who stop the line for a safety concern within their scope of practice and also participate as part of a team to investigate and improve processes to avoid recurring safety concerns in the future. For the Level Two award, a certificate is presented to an employee by the hospital’s HRO lead, HRO physician champion, patient safety manager, immediate supervisor, and peers. With the Level 3 award, the Culture of Safety Appreciation Award, individuals are recognized for addressing safety concerns within their assigned scope of responsibilities. Recognition is bestowed by an email of appreciation sent to the employee, acknowledging their commitment to promoting a culture of safety and quality health care. The recipient’s direct supervisor and other hospital leaders are copied on the message.14 See Table 1 for a

Our institution’s HRO and patient safety teams utilized many additional venues to disseminate information regarding awardees and their actions. These included our monthly HRO newsletter, monthly safety forums, and biweekly Team Connecticut Healthcare system-wide huddles.
Nomination Process
Awards nominations are submitted via the hospital intranet homepage, where there is an “HRO Safety Award Nomination” icon. Once a staff member clicks the icon, a template opens asking for information, such as the reason for the nomination submission, and then walks them through the template using the CAR (C-context, A-actions, and R-results)15 format for describing the situation, identifying actions taken, and specifying the outcome of the action. Emails with award nominations can also be sent to the HRO lead, HRO champion, or Patient Safety Committee co-chairs. Calls for nominations are made at several venues attended by employees as well as supervisors. These include monthly safety forums, biweekly Team Connecticut Healthcare system-wide huddles, supervisory staff meetings, department and unit-based staff meetings, and many other formal and informal settings. This QI initiative has allowed us to capture potential awardees through several avenues, including self-nominations. All nominations are reviewed by a safety awards committee. Each committee member ranks the nomination as a Level 1, 2, or 3 award. For nominations where conflicting scores are obtained, the committee discusses the nomination together to resolve discrepancies.
Needed Resources
Material resources required for this QI initiative include certificate paper, plastic baseball card sleeves, stress-release baseballs, and Bazooka gum. The largest resource investment was the time needed to support the initiative. This included the time spent scheduling the Level 1 and 2 award presentations with staff and leadership. Time was also required to put the individual award packages together, which included printing the paper certificates, obtaining awardee pictures, placing them with their safety stories in a plastic baseball card sleeve, and arranging for the hospital photographer to take pictures of the awardees with their peers and leaders.
RESULTS
Prior to this QI initiative launch, 14 awards were given out over the preceding 2-year period. During the initial 18 months of the initiative (December 2020 to June 2022), 59 awards were presented (Level 1, n = 26; Level 2, n = 22; and Level 3, n = 11). Looking further into the Level 1 awards presented, 25 awardees worked in clinical roles and 1 in a nonclinical position (Table 2). The awardees represented multidisciplinary areas, including medical/surgical (med/surg) inpatient units, anesthesia, operating room, pharmacy, mental health clinics, surgical intensive care, specialty care clinics, and nutrition and food services. With the Level 2 awards, 18 clinical staff and 4 nonclinical staff received awards from the areas of med/surg inpatient, outpatient surgical suites, the medical center director’s office, radiology, pharmacy, primary care, facilities management, environmental management, infection prevention, and emergency services. All Level 3 awardees were from clinical areas, including primary care, hospital education, sterile processing, pharmacies, operating rooms, and med/surg inpatient units.

With the inception of this QI initiative, our organization has begun to see trends reflecting increased reporting of both actual and close-call events in JPSR (Figure 1). 
With the inclusion of information regarding awardees and their actions in monthly safety forums, attendance at these forums has increased from an average of 64 attendees per month in 2021 to an average of 131 attendees per month in 2022 (Figure 2).

Finally, our organization’s annual All-Employee Survey results have shown incremental increases in staff reporting feeling psychologically safe and not fearing reprisal (Figure 3). It is important to note that there may be other contributing factors to these incremental changes.

Level 1 – Good Catch Award. M.S. was assigned as a continuous safety monitor, or “sitter,” on one of the med/surg inpatient units. M.S. arrived at the bedside and asked for a report on the patient at a change in shift. The report stated that the patient was sleeping and had not moved in a while. M.S. set about to perform the functions of a sitter and did her usual tasks in cleaning and tidying the room for the patient for breakfast and taking care of all items in the room, in general. M.S. introduced herself to the patient, who she thought might wake up because of her speaking to him. She thought the patient was in an odd position, and knowing that a patient should be a little further up in the bed, she tried with touch to awaken him to adjust his position. M.S. found that the patient was rather chilly to the touch and immediately became concerned. She continued to attempt to rouse the patient. M.S. called for the nurse and began to adjust the patient’s position. M.S. insisted that the patient was cold and “something was wrong.” A set of vitals was taken and a rapid response team code was called. The patient was immediately transferred to the intensive care unit to receive a higher level of care. If not for the diligence and caring attitude of M.S., this patient may have had a very poor outcome.
Reason for criteria being met: The scope of practice of a sitter is to be present in a patient’s room to monitor for falls and overall safety. This employee noticed that the patient was not responsive to verbal or tactile stimuli. Her immediate reporting of her concern to the nurse resulted in prompt intervention. If she had let the patient be, the patient could have died. The staff member went above and beyond by speaking up and taking action when she had a patient safety concern.
Level 2 – HRO Safety Champion Award. A patient presented to an outpatient clinic for monoclonal antibody (mAb) therapy for a COVID-19 infection; the treatment has been scheduled by the patient’s primary care provider. At that time, outpatient mAb therapy was the recommended care option for patients stable enough to receive treatment in this setting, but it is contraindicated in patients who are too unstable to receive mAb therapy in an outpatient setting, such as those with increased oxygen demands. R.L., a staff nurse, assessed the patient on arrival and found that his vital signs were stable, except for a slightly elevated respiratory rate. Upon questioning, the patient reported that he had increased his oxygen use at home from 2 to 4 L via a nasal cannula. R.L. assessed that the patient was too high-risk for outpatient mAb therapy and had the patient checked into the emergency department (ED) to receive a full diagnostic workup and evaluation by Dr. W., an ED provider. The patient required admission to the hospital for a higher level of care in an inpatient unit because of severe COVID-19 infection. Within 48 hours of admission, the patient’s condition further declined, requiring an upgrade to the medical intensive care unit with progressive interventions. Owing to the clinical assessment skills and prompt action of R.L., the patient was admitted to the hospital instead of receiving treatment in a suboptimal care setting and returning home. Had the patient gone home, his rapid decline could have had serious consequences.
Reason for criteria being met: On a cursory look, the patient may have passed as someone sufficiently stable to undergo outpatient treatment. However, the nurse stopped the line, paid close attention, and picked up on an abnormal vital sign and the projected consequences. The nurse brought the patient to a higher level of care in the ED so that he could get the attention he needed. If this patient was given mAb therapy in the outpatient setting, he would have been discharged and become sicker with the COVID-19 illness. As a result of this incident, R.L. is working with the outpatient clinic and ED staff to enahance triage and evaluation of patients referred for outpatient therapy for COVID-19 infections to prevent a similar event from recurring.
Level 3 – Culture of Safety Appreciation Award. While C.C. was reviewing the hazardous item
Reason for criteria being met: The employee works in the hospital education department. It is within her scope of responsibilities to provide ongoing education to staff in order to address potential safety concerns.
DISCUSSION
This QI initiative was undertaken to demonstrate to staff that, in building an organizational culture of safety and advancing quality health care, it is important that staff be encouraged to speak up for safety and be acknowledged for doing so. As part of efforts to continuously build on a safety-first culture, transparency and celebration of successes were demonstrated. This QI initiative demonstrated that a diverse and wide range of employees were reached, from clinical to nonclinical staff, and frontline to supervisory staff, as all were included in the recognition process. While many award nominations were received through the submission of safety concerns to the high-reliability team and patient safety office, several came directly from staff who wanted to recognize their peers for their work, supporting a culture of safety. This showed that staff felt that taking the time to submit a write-up to recognize a peer was an important task. Achieving zero harm for patients and staff alike is a top priority for our institution and guides all decisions, which reinforces that everyone has a responsibility to ensure that safety is always the first consideration. A culture of safety is enhanced by staff recognition. This QI initiative also showed that staff felt valued when they were acknowledged, regardless of the level of recognition they received. The theme of feeling valued came from unsolicited feedback. For example, some direct comments from awardees are presented in the Box.

In addition to endorsing the importance of safe practices to staff, safety award programs can identify gaps in existing standard procedures that can be updated quickly and shared broadly across a health care organization. The authors observed that the existence of the award program gives staff permission to use their voice to speak up when they have questions or concerns related to safety and to proactively engage in safety practices; a cultural shift of this kind informs safety practices and procedures and contributes to a more inspiring workplace. Staff at our organization who have received any of the safety awards, and those who are aware of these awards, have embraced the program readily. At the time of submission of this manuscript, there was a relative paucity of published literature on the details, performance, and impact of such programs. This initiative aims to share a road map highlighting the various dimensions of staff recognition and how the program supports our health care system in fostering a strong, sustainable culture of safety and health care quality. A next step is to formally assess the impact of the awards program on our culture of safety and quality using a psychometrically sound measurement tool, as recommended by TJC,16 such as the
CONCLUSION
A health care organization safety awards program is a strategy for building and sustaining a culture of safety. This QI initiative may be valuable to other organizations in the process of establishing a safety awards program of their own. Future research should focus on a formal evaluation of the impact of safety awards programs on patient safety outcomes.
Corresponding author: John S. Murray, PhD, MPH, MSGH, RN, FAAN, 20 Chapel Street, Unit A502, Brookline, MA 02446; [email protected]
Disclosures: None reported.
1. National Center for Biotechnology Information. Improving healthcare quality in Europe: Characteristics, effectiveness and implementation of different strategies. National Library of Medicine; 2019.
2.
3. Agency for Healthcare Research and Quality. Implementing near-miss reporting and improvement tracking in primary care practices: lessons learned. Agency for Healthcare Research and Quality; 2017.
4. Hamed M, Konstantinidis S. Barriers to incident reporting among nurses: a qualitative systematic review. West J Nurs Res. 2022;44(5):506-523. doi:10.1177/0193945921999449
5. Mohamed M, Abubeker IY, Al-Mohanadi D, et al. Perceived barriers of incident reporting among internists: results from Hamad medical corporation in Qatar. Avicenna J Med. 2021;11(3):139-144. doi:10.1055/s-0041-1734386
6. The Joint Commission. The essential role of leadership in developing a safety culture. The Joint Commission; 2017.
7. Yali G, Nzala S. Healthcare providers’ perspective on barriers to patient safety incident reporting in Lusaka District. J Prev Rehabil Med. 2022;4:44-52. doi:10.21617/jprm2022.417
8. Herzer KR, Mirrer M, Xie Y, et al. Patient safety reporting systems: sustained quality improvement using a multidisciplinary team and “good catch” awards. Jt Comm J Qual Patient Saf. 2012;38(8):339-347. doi:10.1016/s1553-7250(12)38044-6
9. Rogers E, Griffin E, Carnie W, et al. A just culture approach to managing medication errors. Hosp Pharm. 2017;52(4):308-315. doi:10.1310/hpj5204-308
10. Murray JS, Clifford J, Larson S, et al. Implementing just culture to improve patient safety. Mil Med. 2022;0: 1. doi:10.1093/milmed/usac115
11. Paradiso L, Sweeney N. Just culture: it’s more than policy. Nurs Manag. 2019;50(6):38–45. doi:10.1097/01.NUMA.0000558482.07815.ae
12. Wallace S, Mamrol M, Finley E; Pennsylvania Patient Safety Authority. Promote a culture of safety with good catch reports. PA Patient Saf Advis. 2017;14(3).
13. Tan KH, Pang NL, Siau C, et al: Building an organizational culture of patient safety. J Patient Saf Risk Manag. 2019;24:253-261. doi.10.1177/251604351987897
14. Merchant N, O’Neal J, Dealino-Perez C, et al: A high reliability mindset. Am J Med Qual. 2022;37(6):504-510. doi:10.1097/JMQ.0000000000000086
15. Behavioral interview questions and answers. Hudson. Accessed December 23, 2022. https://au.hudson.com/insights/career-advice/job-interviews/behavioural-interview-questions-and-answers/
16. The Joint Commission. Safety culture assessment: Improving the survey process. Accessed December 26, 2022. https://www.jointcommission.org/-/media/tjc/documents/accred-and-cert/safety_culture_assessment_improving_the_survey_process.pdf
17. Reis CT, Paiva SG, Sousa P. The patient safety culture: a systematic review by characteristics of hospital survey on patient safety culture dimensions. Int J Qual Heal Care. 2018;30(9):660-677. doi:10.1093/intqhc/mzy080
18. Fourar YO, Benhassine W, Boughaba A, et al. Contribution to the assessment of patient safety culture in Algerian healthcare settings: the ASCO project. Int J Healthc Manag. 2022;15:52-61. doi.org/10.1080/20479700.2020.1836736
1. National Center for Biotechnology Information. Improving healthcare quality in Europe: Characteristics, effectiveness and implementation of different strategies. National Library of Medicine; 2019.
2.
3. Agency for Healthcare Research and Quality. Implementing near-miss reporting and improvement tracking in primary care practices: lessons learned. Agency for Healthcare Research and Quality; 2017.
4. Hamed M, Konstantinidis S. Barriers to incident reporting among nurses: a qualitative systematic review. West J Nurs Res. 2022;44(5):506-523. doi:10.1177/0193945921999449
5. Mohamed M, Abubeker IY, Al-Mohanadi D, et al. Perceived barriers of incident reporting among internists: results from Hamad medical corporation in Qatar. Avicenna J Med. 2021;11(3):139-144. doi:10.1055/s-0041-1734386
6. The Joint Commission. The essential role of leadership in developing a safety culture. The Joint Commission; 2017.
7. Yali G, Nzala S. Healthcare providers’ perspective on barriers to patient safety incident reporting in Lusaka District. J Prev Rehabil Med. 2022;4:44-52. doi:10.21617/jprm2022.417
8. Herzer KR, Mirrer M, Xie Y, et al. Patient safety reporting systems: sustained quality improvement using a multidisciplinary team and “good catch” awards. Jt Comm J Qual Patient Saf. 2012;38(8):339-347. doi:10.1016/s1553-7250(12)38044-6
9. Rogers E, Griffin E, Carnie W, et al. A just culture approach to managing medication errors. Hosp Pharm. 2017;52(4):308-315. doi:10.1310/hpj5204-308
10. Murray JS, Clifford J, Larson S, et al. Implementing just culture to improve patient safety. Mil Med. 2022;0: 1. doi:10.1093/milmed/usac115
11. Paradiso L, Sweeney N. Just culture: it’s more than policy. Nurs Manag. 2019;50(6):38–45. doi:10.1097/01.NUMA.0000558482.07815.ae
12. Wallace S, Mamrol M, Finley E; Pennsylvania Patient Safety Authority. Promote a culture of safety with good catch reports. PA Patient Saf Advis. 2017;14(3).
13. Tan KH, Pang NL, Siau C, et al: Building an organizational culture of patient safety. J Patient Saf Risk Manag. 2019;24:253-261. doi.10.1177/251604351987897
14. Merchant N, O’Neal J, Dealino-Perez C, et al: A high reliability mindset. Am J Med Qual. 2022;37(6):504-510. doi:10.1097/JMQ.0000000000000086
15. Behavioral interview questions and answers. Hudson. Accessed December 23, 2022. https://au.hudson.com/insights/career-advice/job-interviews/behavioural-interview-questions-and-answers/
16. The Joint Commission. Safety culture assessment: Improving the survey process. Accessed December 26, 2022. https://www.jointcommission.org/-/media/tjc/documents/accred-and-cert/safety_culture_assessment_improving_the_survey_process.pdf
17. Reis CT, Paiva SG, Sousa P. The patient safety culture: a systematic review by characteristics of hospital survey on patient safety culture dimensions. Int J Qual Heal Care. 2018;30(9):660-677. doi:10.1093/intqhc/mzy080
18. Fourar YO, Benhassine W, Boughaba A, et al. Contribution to the assessment of patient safety culture in Algerian healthcare settings: the ASCO project. Int J Healthc Manag. 2022;15:52-61. doi.org/10.1080/20479700.2020.1836736
Teaching Quality Improvement to Internal Medicine Residents to Address Patient Care Gaps in Ambulatory Quality Metrics
ABSTRACT
Objective: To teach internal medicine residents quality improvement (QI) principles in an effort to improve resident knowledge and comfort with QI, as well as address quality care gaps in resident clinic primary care patient panels.
Design: A QI curriculum was implemented for all residents rotating through a primary care block over a 6-month period. Residents completed Institute for Healthcare Improvement (IHI) modules, participated in a QI workshop, and received panel data reports, ultimately completing a plan-do-study-act (PDSA) cycle to improve colorectal cancer screening and hypertension control.
Setting and participants: This project was undertaken at Tufts Medical Center Primary Care, Boston, Massachusetts, the primary care teaching practice for all 75 internal medicine residents at Tufts Medical Center. All internal medicine residents were included, with 55 (73%) of the 75 residents completing the presurvey, and 39 (52%) completing the postsurvey.
Measurements: We administered a 10-question pre- and postsurvey looking at resident attitudes toward and comfort with QI and familiarity with their panel data as well as measured rates of colorectal cancer screening and hypertension control in resident panels.
Results: There was an increase in the numbers of residents who performed a PDSA cycle (P = .002), completed outreach based on their panel data (P = .02), and felt comfortable in both creating aim statements and designing and implementing PDSA cycles (P < .0001). The residents’ knowledge of their panel data significantly increased. There was no significant improvement in hypertension control, but there was an increase in colorectal cancer screening rates (P < .0001).
Conclusion: Providing panel data and performing targeted QI interventions can improve resident comfort with QI, translating to improvement in patient outcomes.
Keywords: quality improvement, resident education, medical education, care gaps, quality metrics.
As quality improvement (QI) has become an integral part of clinical practice, residency training programs have continued to evolve in how best to teach QI. The Accreditation Council for Graduate Medical Education (ACGME) Common Program requirements mandate that core competencies in residency programs include practice-based learning and improvement and systems-based practice.1 Residents should receive education in QI, receive data on quality metrics and benchmarks related to their patient population, and participate in QI activities. The Clinical Learning Environment Review (CLER) program was established to provide feedback to institutions on 6 focused areas, including patient safety and health care quality. In visits to institutions across the United States, the CLER committees found that many residents had limited knowledge of QI concepts and limited access to data on quality metrics and benchmarks.2
There are many barriers to implementing a QI curriculum in residency programs, and creating and maintaining successful strategies has proven challenging.3 Many QI curricula for internal medicine residents have been described in the literature, but the results of many of these studies focus on resident self-assessment of QI knowledge and numbers of projects rather than on patient outcomes.4-13 As there is some evidence suggesting that patients treated by residents have worse outcomes on ambulatory quality measures when compared with patients treated by staff physicians,14,15 it is important to also look at patient outcomes when evaluating a QI curriculum. Experts in education recommend the following to optimize learning: exposure to both didactic and experiential opportunities, connection to health system improvement efforts, and assessment of patient outcomes in addition to learner feedback.16,17 A study also found that providing panel data to residents could improve quality metrics.18
In this study, we sought to investigate the effects of a resident QI intervention during an ambulatory block on both residents’ self-assessments of QI knowledge and attitudes as well as on patient quality metrics.
Methods
Curriculum
We implemented this educational initiative at Tufts Medical Center Primary Care, Boston, Massachusetts, the primary care teaching practice for all 75 internal medicine residents at Tufts Medical Center. Co-located with the 415-bed academic medical center in downtown Boston, the practice serves more than 40,000 patients, approximately 7000 of whom are cared for by resident primary care physicians (PCPs). The internal medicine residents rotate through the primary care clinic as part of continuity clinic during ambulatory or elective blocks. In addition to continuity clinic, the residents have 2 dedicated 3-week primary care rotations during the course of an academic year. Primary care rotations consist of 5 clinic sessions a week as well as structured teaching sessions. Each resident inherits a panel of patients from an outgoing senior resident, with an average panel size of 96 patients per resident.
Prior to this study intervention, we did not do any formal QI teaching to our residents as part of their primary care curriculum, and previous panel management had focused more on chart reviews of patients whom residents perceived to be higher risk. Residents from all 3 years were included in the intervention. We taught a QI curriculum to our residents from January 2018 to June 2018 during the 3-week primary care rotation, which consisted of the following components:
- Institute for Healthcare Improvement (IHI) module QI 102 completed independently online.
- A 2-hour QI workshop led by 1 of 2 primary care faculty with backgrounds in QI, during which residents were taught basic principles of QI, including how to craft aim statements and design plan-do-study-act (PDSA) cycles, and participated in a hands-on QI activity designed to model rapid cycle improvement (the Paper Airplane Factory19).
- Distribution of individualized reports of residents’ patient panel data by email at the start of the primary care block that detailed patients’ overall rates of colorectal cancer screening and hypertension (HTN) control, along with the average resident panel rates and the average attending panel rates. The reports also included a list of all residents’ patients who were overdue for colorectal cancer screening or whose last blood pressure (BP) was uncontrolled (systolic BP ≥ 140 mm Hg or diastolic BP ≥ 90 mm Hg). These reports were originally designed by our practice’s QI team and run and exported in Microsoft Excel format monthly by our information technology (IT) administrator.
- Instruction on aim statements as a group, followed by the expectation that each resident create an individualized aim statement tailored to each resident’s patient panel rates, with the PDSA cycle to be implemented during the remainder of the primary care rotation, focusing on improvement of colorectal cancer screening and HTN control (see supplementary eFigure 1 online for the worksheet used for the workshop).
- Residents were held accountable for their interventions by various check-ins. At the end of the primary care block, residents were required to submit their completed worksheets showing the intervention they had undertaken and when it was performed. The 2 primary care attendings primarily responsible for QI education would review the resident’s work approximately 1 to 2 months after they submitted their worksheets describing their intervention. These attendings sent the residents personalized feedback based on whether the intervention had been completed or successful as evidenced by documentation in the chart, including direct patient outreach by phone, letter, or portal; outreach to the resident coordinator; scheduled follow-up appointment; or booking or completion of colorectal cancer screening. Along with this feedback, residents were also sent suggestions for next steps. Resident preceptors were copied on the email to facilitate reinforcement of the goals and plans. Finally, the resident preceptors also helped with accountability by going through the residents’ worksheets and patient panel metrics with the residents during biannual evaluations.
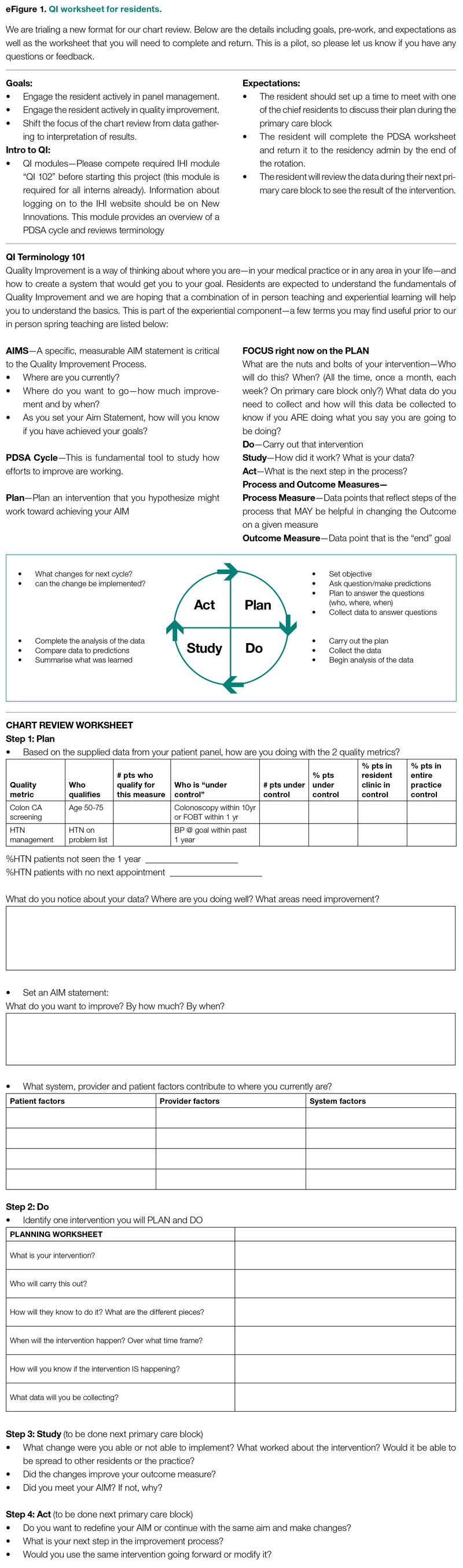
Evaluation
Residents were surveyed with a 10-item questionnaire pre and post intervention regarding their attitudes toward QI, understanding of QI principles, and familiarity with their patient panel data. Surveys were anonymous and distributed via the SurveyMonkey platform (see supplementary eFigure 2 online). Residents were asked if they had ever performed a PDSA cycle, performed patient outreach, or performed an intervention and whether they knew the rates of diabetes, HTN, and colorectal cancer screening in their patient panels. Questions rated on a 5-point Likert scale were used to assess comfort with panel management, developing an aim statement, designing and implementing a PDSA cycle, as well as interest in pursuing QI as a career. For the purposes of analysis, these questions were dichotomized into “somewhat comfortable” and “very comfortable” vs “neutral,” “somewhat uncomfortable,” and “very uncomfortable.” Similarly, we dichotomized the question about interest in QI as a career into “somewhat interested” and “very interested” vs “neutral,” “somewhat disinterested,” and “very disinterested.” As the surveys were anonymous, we were unable to pair the pre- and postintervention surveys and used a chi-square test to evaluate whether there was an association between survey assessments pre intervention vs post intervention and a positive or negative response to the question.
We also examined rates of HTN control and colorectal cancer screening in all 75 resident panels pre and post intervention. The paired t-test was used to determine whether the mean change from pre to post intervention was significant. SAS 9.4 (SAS Institute Inc.) was used for all analyses. Institutional Review Board exemption was obtained from the Tufts Medical Center IRB. There was no funding received for this study.
Results
Respondents
Of the 75 residents, 55 (73%) completed the survey prior to the intervention, and 39 (52%) completed the survey after the intervention.
Panel Knowledge and Intervention
Prior to the intervention, 45% of residents had performed a PDSA cycle, compared with 77% post intervention, which was a significant increase (P = .002) (Table 1). Sixty-two percent of residents had performed outreach or an intervention based on their patient panel reports prior to the intervention, compared with 85% of residents post intervention, which was also a significant increase (P = .02). The increase post intervention was not 100%, as there were residents who either missed the initial workshop or who did not follow through with their planned intervention. Common interventions included the residents giving their coordinators a list of patients to call to schedule appointments, utilizing fellow team members (eg, pharmacists, social workers) for targeted patient outreach, or calling patients themselves to reestablish a connection.
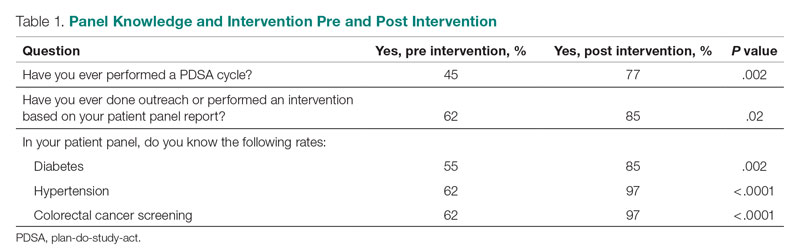
In terms of knowledge of their patient panels, prior to the intervention, 55%, 62%, and 62% of residents knew the rates of patients in their panel with diabetes, HTN, and colorectal cancer screening, respectively. After the intervention, the residents’ knowledge of these rates increased significantly, to 85% for diabetes (P = .002), 97% for HTN (P < .0001), and 97% for colorectal cancer screening (P < .0001).
Comfort With QI Approaches
Prior to the intervention, 82% of residents were comfortable managing their primary care panel, which did not change significantly post intervention (Table 2). The residents’ comfort with designing an aim statement did significantly increase, from 55% to 95% (P < .0001). The residents also had a significant increase in comfort with both designing and implementing a PDSA cycle. Prior to the intervention, 22% felt comfortable designing a PDSA cycle, which increased to 79% (P < .0001) post intervention, and 24% felt comfortable implementing a PDSA cycle, which increased to 77% (P < .0001) post intervention.
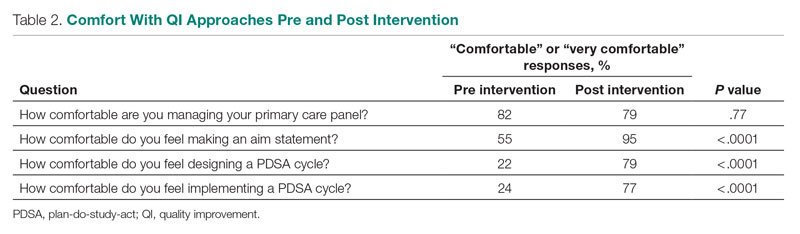
Patient Outcome Measures
The rate of HTN control in the residents' patient panels did not change significantly pre and post intervention (Table 3). The rate of resident patients who were up to date with colorectal cancer screening increased by 6.5% post intervention (P < .0001).
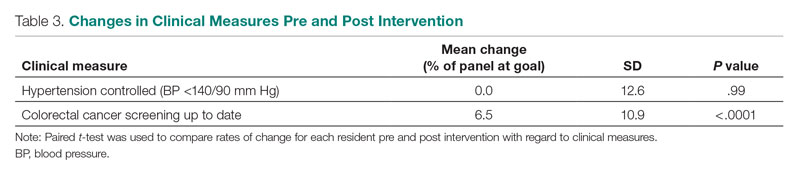
Interest in QI as a Career
As part of the survey, residents were asked how interested they were in making QI a part of their career. Fifty percent of residents indicated an interest in QI pre intervention, and 54% indicated an interest post intervention, which was not a significant difference (P = .72).
Discussion
In this study, we found that integration of a QI curriculum into a primary care rotation improved both residents’ knowledge of their patient panels and comfort with QI approaches, which translated to improvement in patient outcomes. Several previous studies have found improvements in resident self-assessment or knowledge after implementation of a QI curriculum.4-13 Liao et al implemented a longitudinal curriculum including both didactic and experiential components and found an improvement in both QI confidence and knowledge.3 Similarly, Duello et al8 found that a curriculum including both didactic lectures and QI projects improved subjective QI knowledge and comfort. Interestingly, Fok and Wong9 found that resident knowledge could be sustained post curriculum after completion of a QI project, suggesting that experiential learning may be helpful in maintaining knowledge.
Studies also have looked at providing performance data to residents. Hwang et al18 found that providing audit and feedback in the form of individual panel performance data to residents compared with practice targets led to statistically significant improvement in cancer screening rates and composite quality score, indicating that there is tremendous potential in providing residents with their data. While the ACGME mandates that residents should receive data on their quality metrics, on CLER visits, many residents interviewed noted limited access to data on their metrics and benchmarks.1,2
Though previous studies have individually looked at teaching QI concepts, providing panel data, or targeting select metrics, our study was unique in that it reviewed both self-reported resident outcomes data as well as actual patient outcomes. In addition to finding increased knowledge of patient panels and comfort with QI approaches, we found a significant increase in colorectal cancer screening rates post intervention. We thought this finding was particularly important given some data that residents' patients have been found to have worse outcomes on quality metrics compared with patients cared for by staff physicians.14,15 Given that having a resident physician as a PCP has been associated with failing to meet quality measures, it is especially important to focus targeted quality improvement initiatives in this patient population to reduce disparities in care.
We found that residents had improved knowledge on their patient panels as a result of this initiative. The residents were noted to have a higher knowledge of their HTN and colorectal cancer screening rates in comparison to their diabetes metrics. We suspect this is because residents are provided with multiple metrics related to diabetes, including process measures such as A1c testing, as well as outcome measures such as A1c control, so it may be harder for them to elucidate exactly how they are doing with their diabetes patients, whereas in HTN control and colorectal cancer screening, there is only 1 associated metric. Interestingly, even though HTN and colorectal cancer screening were the 2 measures focused on in the study, the residents had a significant improvement in knowledge of the rates of diabetes in their panel as well. This suggests that even just receiving data alone is valuable, hopefully translating to better outcomes with better baseline understanding of panels. We believe that our intervention was successful because it included both a didactic and an experiential component, as well as the use of individual panel performance data.
There were several limitations to our study. It was performed at a single institution, translating to a small sample size. Our data analysis was limited because we were unable to pair our pre- and postintervention survey responses because we used an anonymous survey. We also did not have full participation in postintervention surveys from all residents, which may have biased the study in favor of high performers. Another limitation was that our survey relied on self-reported outcomes for the questions about the residents knowing their patient panels.
This study required a 2-hour workshop every 3 weeks led by a faculty member trained in QI. Given the amount of time needed for the curriculum, this study may be difficult to replicate at other institutions, especially if faculty with an interest or training in QI are not available. Given our finding that residents had increased knowledge of their patient panels after receiving panel metrics, simply providing data with the goal of smaller, focused interventions may be easier to implement. At our institution, we discontinued the longer 2-hour QI workshops designed to teach QI approaches more broadly. We continue to provide individualized panel data to all residents during their primary care rotations and conduct half-hour, small group workshops with the interns that focus on drafting aim statements and planning interventions. All residents are required to submit worksheets to us at the end of their primary care blocks listing their current rates of each predetermined metric and laying out their aim statements and planned interventions. Residents also continue to receive feedback from our faculty with expertise in QI afterward on their plans and evidence of follow-through in the chart, with their preceptors included on the feedback emails. Even without the larger QI workshop, this approach has continued to be successful and appreciated. In fact, it does appear as though improvement in colorectal cancer screening has been sustained over several years. At the end of our study period, the resident patient colorectal cancer screening rate rose from 34% to 43%, and for the 2021-2022 academic year, the rate rose further, from 46% to 50%.
Given that the resident clinic patient population is at higher risk overall, targeted outreach and approaches to improve quality must be continued. Future areas of research include looking at which interventions, whether QI curriculum, provision of panel data, or required panel management interventions, translate to the greatest improvements in patient outcomes in this vulnerable population.
Conclusion
Our study showed that a dedicated QI curriculum for the residents and access to quality metric data improved both resident knowledge and comfort with QI approaches. Beyond resident-centered outcomes, there was also translation to improved patient outcomes, with a significant increase in colon cancer screening rates post intervention.
Corresponding author: Kinjalika Sathi, MD, 800 Washington St., Boston, MA 02111; [email protected]
Disclosures: None reported.
1. Accreditation Council for Graduate Medical Education. ACGME Common Program Requirements (Residency). Approved June 13, 2021. Updated July 1, 2022. Accessed December 29, 2022. https://www.acgme.org/globalassets/pfassets/programrequirements/cprresidency_2022v3.pdf
2. Koh NJ, Wagner R, Newton RC, et al; on behalf of the CLER Evaluation Committee and the CLER Program. CLER National Report of Findings 2021. Accreditation Council for Graduate Medical Education; 2021. Accessed December 29, 2022. https://www.acgme.org/globalassets/pdfs/cler/2021clernationalreportoffindings.pdf
3. Liao JM, Co JP, Kachalia A. Providing educational content and context for training the next generation of physicians in quality improvement. Acad Med. 2015;90(9):1241-1245. doi:10.1097/ACM.0000000000000799
4. Johnson KM, Fiordellisi W, Kuperman E, et al. X + Y = time for QI: meaningful engagement of residents in quality improvement during the ambulatory block. J Grad Med Educ. 2018;10(3):316-324. doi:10.4300/JGME-D-17-00761.1
5. Kesari K, Ali S, Smith S. Integrating residents with institutional quality improvement teams. Med Educ. 2017;51(11):1173. doi:10.1111/medu.13431
6. Ogrinc G, Cohen ES, van Aalst R, et al. Clinical and educational outcomes of an integrated inpatient quality improvement curriculum for internal medicine residents. J Grad Med Educ. 2016;8(4):563-568. doi:10.4300/JGME-D-15-00412.1
7. Malayala SV, Qazi KJ, Samdani AJ, et al. A multidisciplinary performance improvement rotation in an internal medicine training program. Int J Med Educ. 2016;7:212-213. doi:10.5116/ijme.5765.0bda
8. Duello K, Louh I, Greig H, et al. Residents’ knowledge of quality improvement: the impact of using a group project curriculum. Postgrad Med J. 2015;91(1078):431-435. doi:10.1136/postgradmedj-2014-132886
9. Fok MC, Wong RY. Impact of a competency based curriculum on quality improvement among internal medicine residents. BMC Med Educ. 2014;14:252. doi:10.1186/s12909-014-0252-7
10. Wilper AP, Smith CS, Weppner W. Instituting systems-based practice and practice-based learning and improvement: a curriculum of inquiry. Med Educ Online. 2013;18:21612. doi:10.3402/meo.v18i0.21612
11. Weigel C, Suen W, Gupte G. Using lean methodology to teach quality improvement to internal medicine residents at a safety net hospital. Am J Med Qual. 2013;28(5):392-399. doi:10.1177/1062860612474062
12. Tomolo AM, Lawrence RH, Watts B, et al. Pilot study evaluating a practice-based learning and improvement curriculum focusing on the development of system-level quality improvement skills. J Grad Med Educ. 2011;3(1):49-58. doi:10.4300/JGME-D-10-00104.1
13. Djuricich AM, Ciccarelli M, Swigonski NL. A continuous quality improvement curriculum for residents: addressing core competency, improving systems. Acad Med. 2004;79(10 Suppl):S65-S67. doi:10.1097/00001888-200410001-00020
14. Essien UR, He W, Ray A, et al. Disparities in quality of primary care by resident and staff physicians: is there a conflict between training and equity? J Gen Intern Med. 2019;34(7):1184-1191. doi:10.1007/s11606-019-04960-5
15. Amat M, Norian E, Graham KL. Unmasking a vulnerable patient care process: a qualitative study describing the current state of resident continuity clinic in a nationwide cohort of internal medicine residency programs. Am J Med. 2022;135(6):783-786. doi:10.1016/j.amjmed.2022.02.007
16. Wong BM, Etchells EE, Kuper A, et al. Teaching quality improvement and patient safety to trainees: a systematic review. Acad Med. 2010;85(9):1425-1439. doi:10.1097/ACM.0b013e3181e2d0c6
17. Armstrong G, Headrick L, Madigosky W, et al. Designing education to improve care. Jt Comm J Qual Patient Saf. 2012;38:5-14. doi:10.1016/s1553-7250(12)38002-1
18. Hwang AS, Harding AS, Chang Y, et al. An audit and feedback intervention to improve internal medicine residents’ performance on ambulatory quality measures: a randomized controlled trial. Popul Health Manag. 2019;22(6):529-535. doi:10.1089/pop.2018.0217
19. Institute for Healthcare Improvement. Open school. The paper airplane factory. Accessed December 29, 2022. https://www.ihi.org/education/IHIOpenSchool/resources/Pages/Activities/PaperAirplaneFactory.aspx
ABSTRACT
Objective: To teach internal medicine residents quality improvement (QI) principles in an effort to improve resident knowledge and comfort with QI, as well as address quality care gaps in resident clinic primary care patient panels.
Design: A QI curriculum was implemented for all residents rotating through a primary care block over a 6-month period. Residents completed Institute for Healthcare Improvement (IHI) modules, participated in a QI workshop, and received panel data reports, ultimately completing a plan-do-study-act (PDSA) cycle to improve colorectal cancer screening and hypertension control.
Setting and participants: This project was undertaken at Tufts Medical Center Primary Care, Boston, Massachusetts, the primary care teaching practice for all 75 internal medicine residents at Tufts Medical Center. All internal medicine residents were included, with 55 (73%) of the 75 residents completing the presurvey, and 39 (52%) completing the postsurvey.
Measurements: We administered a 10-question pre- and postsurvey looking at resident attitudes toward and comfort with QI and familiarity with their panel data as well as measured rates of colorectal cancer screening and hypertension control in resident panels.
Results: There was an increase in the numbers of residents who performed a PDSA cycle (P = .002), completed outreach based on their panel data (P = .02), and felt comfortable in both creating aim statements and designing and implementing PDSA cycles (P < .0001). The residents’ knowledge of their panel data significantly increased. There was no significant improvement in hypertension control, but there was an increase in colorectal cancer screening rates (P < .0001).
Conclusion: Providing panel data and performing targeted QI interventions can improve resident comfort with QI, translating to improvement in patient outcomes.
Keywords: quality improvement, resident education, medical education, care gaps, quality metrics.
As quality improvement (QI) has become an integral part of clinical practice, residency training programs have continued to evolve in how best to teach QI. The Accreditation Council for Graduate Medical Education (ACGME) Common Program requirements mandate that core competencies in residency programs include practice-based learning and improvement and systems-based practice.1 Residents should receive education in QI, receive data on quality metrics and benchmarks related to their patient population, and participate in QI activities. The Clinical Learning Environment Review (CLER) program was established to provide feedback to institutions on 6 focused areas, including patient safety and health care quality. In visits to institutions across the United States, the CLER committees found that many residents had limited knowledge of QI concepts and limited access to data on quality metrics and benchmarks.2
There are many barriers to implementing a QI curriculum in residency programs, and creating and maintaining successful strategies has proven challenging.3 Many QI curricula for internal medicine residents have been described in the literature, but the results of many of these studies focus on resident self-assessment of QI knowledge and numbers of projects rather than on patient outcomes.4-13 As there is some evidence suggesting that patients treated by residents have worse outcomes on ambulatory quality measures when compared with patients treated by staff physicians,14,15 it is important to also look at patient outcomes when evaluating a QI curriculum. Experts in education recommend the following to optimize learning: exposure to both didactic and experiential opportunities, connection to health system improvement efforts, and assessment of patient outcomes in addition to learner feedback.16,17 A study also found that providing panel data to residents could improve quality metrics.18
In this study, we sought to investigate the effects of a resident QI intervention during an ambulatory block on both residents’ self-assessments of QI knowledge and attitudes as well as on patient quality metrics.
Methods
Curriculum
We implemented this educational initiative at Tufts Medical Center Primary Care, Boston, Massachusetts, the primary care teaching practice for all 75 internal medicine residents at Tufts Medical Center. Co-located with the 415-bed academic medical center in downtown Boston, the practice serves more than 40,000 patients, approximately 7000 of whom are cared for by resident primary care physicians (PCPs). The internal medicine residents rotate through the primary care clinic as part of continuity clinic during ambulatory or elective blocks. In addition to continuity clinic, the residents have 2 dedicated 3-week primary care rotations during the course of an academic year. Primary care rotations consist of 5 clinic sessions a week as well as structured teaching sessions. Each resident inherits a panel of patients from an outgoing senior resident, with an average panel size of 96 patients per resident.
Prior to this study intervention, we did not do any formal QI teaching to our residents as part of their primary care curriculum, and previous panel management had focused more on chart reviews of patients whom residents perceived to be higher risk. Residents from all 3 years were included in the intervention. We taught a QI curriculum to our residents from January 2018 to June 2018 during the 3-week primary care rotation, which consisted of the following components:
- Institute for Healthcare Improvement (IHI) module QI 102 completed independently online.
- A 2-hour QI workshop led by 1 of 2 primary care faculty with backgrounds in QI, during which residents were taught basic principles of QI, including how to craft aim statements and design plan-do-study-act (PDSA) cycles, and participated in a hands-on QI activity designed to model rapid cycle improvement (the Paper Airplane Factory19).
- Distribution of individualized reports of residents’ patient panel data by email at the start of the primary care block that detailed patients’ overall rates of colorectal cancer screening and hypertension (HTN) control, along with the average resident panel rates and the average attending panel rates. The reports also included a list of all residents’ patients who were overdue for colorectal cancer screening or whose last blood pressure (BP) was uncontrolled (systolic BP ≥ 140 mm Hg or diastolic BP ≥ 90 mm Hg). These reports were originally designed by our practice’s QI team and run and exported in Microsoft Excel format monthly by our information technology (IT) administrator.
- Instruction on aim statements as a group, followed by the expectation that each resident create an individualized aim statement tailored to each resident’s patient panel rates, with the PDSA cycle to be implemented during the remainder of the primary care rotation, focusing on improvement of colorectal cancer screening and HTN control (see supplementary eFigure 1 online for the worksheet used for the workshop).
- Residents were held accountable for their interventions by various check-ins. At the end of the primary care block, residents were required to submit their completed worksheets showing the intervention they had undertaken and when it was performed. The 2 primary care attendings primarily responsible for QI education would review the resident’s work approximately 1 to 2 months after they submitted their worksheets describing their intervention. These attendings sent the residents personalized feedback based on whether the intervention had been completed or successful as evidenced by documentation in the chart, including direct patient outreach by phone, letter, or portal; outreach to the resident coordinator; scheduled follow-up appointment; or booking or completion of colorectal cancer screening. Along with this feedback, residents were also sent suggestions for next steps. Resident preceptors were copied on the email to facilitate reinforcement of the goals and plans. Finally, the resident preceptors also helped with accountability by going through the residents’ worksheets and patient panel metrics with the residents during biannual evaluations.

Evaluation
Residents were surveyed with a 10-item questionnaire pre and post intervention regarding their attitudes toward QI, understanding of QI principles, and familiarity with their patient panel data. Surveys were anonymous and distributed via the SurveyMonkey platform (see supplementary eFigure 2 online). Residents were asked if they had ever performed a PDSA cycle, performed patient outreach, or performed an intervention and whether they knew the rates of diabetes, HTN, and colorectal cancer screening in their patient panels. Questions rated on a 5-point Likert scale were used to assess comfort with panel management, developing an aim statement, designing and implementing a PDSA cycle, as well as interest in pursuing QI as a career. For the purposes of analysis, these questions were dichotomized into “somewhat comfortable” and “very comfortable” vs “neutral,” “somewhat uncomfortable,” and “very uncomfortable.” Similarly, we dichotomized the question about interest in QI as a career into “somewhat interested” and “very interested” vs “neutral,” “somewhat disinterested,” and “very disinterested.” As the surveys were anonymous, we were unable to pair the pre- and postintervention surveys and used a chi-square test to evaluate whether there was an association between survey assessments pre intervention vs post intervention and a positive or negative response to the question.

We also examined rates of HTN control and colorectal cancer screening in all 75 resident panels pre and post intervention. The paired t-test was used to determine whether the mean change from pre to post intervention was significant. SAS 9.4 (SAS Institute Inc.) was used for all analyses. Institutional Review Board exemption was obtained from the Tufts Medical Center IRB. There was no funding received for this study.
Results
Respondents
Of the 75 residents, 55 (73%) completed the survey prior to the intervention, and 39 (52%) completed the survey after the intervention.
Panel Knowledge and Intervention
Prior to the intervention, 45% of residents had performed a PDSA cycle, compared with 77% post intervention, which was a significant increase (P = .002) (Table 1). Sixty-two percent of residents had performed outreach or an intervention based on their patient panel reports prior to the intervention, compared with 85% of residents post intervention, which was also a significant increase (P = .02). The increase post intervention was not 100%, as there were residents who either missed the initial workshop or who did not follow through with their planned intervention. Common interventions included the residents giving their coordinators a list of patients to call to schedule appointments, utilizing fellow team members (eg, pharmacists, social workers) for targeted patient outreach, or calling patients themselves to reestablish a connection.

In terms of knowledge of their patient panels, prior to the intervention, 55%, 62%, and 62% of residents knew the rates of patients in their panel with diabetes, HTN, and colorectal cancer screening, respectively. After the intervention, the residents’ knowledge of these rates increased significantly, to 85% for diabetes (P = .002), 97% for HTN (P < .0001), and 97% for colorectal cancer screening (P < .0001).
Comfort With QI Approaches
Prior to the intervention, 82% of residents were comfortable managing their primary care panel, which did not change significantly post intervention (Table 2). The residents’ comfort with designing an aim statement did significantly increase, from 55% to 95% (P < .0001). The residents also had a significant increase in comfort with both designing and implementing a PDSA cycle. Prior to the intervention, 22% felt comfortable designing a PDSA cycle, which increased to 79% (P < .0001) post intervention, and 24% felt comfortable implementing a PDSA cycle, which increased to 77% (P < .0001) post intervention.

Patient Outcome Measures
The rate of HTN control in the residents' patient panels did not change significantly pre and post intervention (Table 3). The rate of resident patients who were up to date with colorectal cancer screening increased by 6.5% post intervention (P < .0001).

Interest in QI as a Career
As part of the survey, residents were asked how interested they were in making QI a part of their career. Fifty percent of residents indicated an interest in QI pre intervention, and 54% indicated an interest post intervention, which was not a significant difference (P = .72).
Discussion
In this study, we found that integration of a QI curriculum into a primary care rotation improved both residents’ knowledge of their patient panels and comfort with QI approaches, which translated to improvement in patient outcomes. Several previous studies have found improvements in resident self-assessment or knowledge after implementation of a QI curriculum.4-13 Liao et al implemented a longitudinal curriculum including both didactic and experiential components and found an improvement in both QI confidence and knowledge.3 Similarly, Duello et al8 found that a curriculum including both didactic lectures and QI projects improved subjective QI knowledge and comfort. Interestingly, Fok and Wong9 found that resident knowledge could be sustained post curriculum after completion of a QI project, suggesting that experiential learning may be helpful in maintaining knowledge.
Studies also have looked at providing performance data to residents. Hwang et al18 found that providing audit and feedback in the form of individual panel performance data to residents compared with practice targets led to statistically significant improvement in cancer screening rates and composite quality score, indicating that there is tremendous potential in providing residents with their data. While the ACGME mandates that residents should receive data on their quality metrics, on CLER visits, many residents interviewed noted limited access to data on their metrics and benchmarks.1,2
Though previous studies have individually looked at teaching QI concepts, providing panel data, or targeting select metrics, our study was unique in that it reviewed both self-reported resident outcomes data as well as actual patient outcomes. In addition to finding increased knowledge of patient panels and comfort with QI approaches, we found a significant increase in colorectal cancer screening rates post intervention. We thought this finding was particularly important given some data that residents' patients have been found to have worse outcomes on quality metrics compared with patients cared for by staff physicians.14,15 Given that having a resident physician as a PCP has been associated with failing to meet quality measures, it is especially important to focus targeted quality improvement initiatives in this patient population to reduce disparities in care.
We found that residents had improved knowledge on their patient panels as a result of this initiative. The residents were noted to have a higher knowledge of their HTN and colorectal cancer screening rates in comparison to their diabetes metrics. We suspect this is because residents are provided with multiple metrics related to diabetes, including process measures such as A1c testing, as well as outcome measures such as A1c control, so it may be harder for them to elucidate exactly how they are doing with their diabetes patients, whereas in HTN control and colorectal cancer screening, there is only 1 associated metric. Interestingly, even though HTN and colorectal cancer screening were the 2 measures focused on in the study, the residents had a significant improvement in knowledge of the rates of diabetes in their panel as well. This suggests that even just receiving data alone is valuable, hopefully translating to better outcomes with better baseline understanding of panels. We believe that our intervention was successful because it included both a didactic and an experiential component, as well as the use of individual panel performance data.
There were several limitations to our study. It was performed at a single institution, translating to a small sample size. Our data analysis was limited because we were unable to pair our pre- and postintervention survey responses because we used an anonymous survey. We also did not have full participation in postintervention surveys from all residents, which may have biased the study in favor of high performers. Another limitation was that our survey relied on self-reported outcomes for the questions about the residents knowing their patient panels.
This study required a 2-hour workshop every 3 weeks led by a faculty member trained in QI. Given the amount of time needed for the curriculum, this study may be difficult to replicate at other institutions, especially if faculty with an interest or training in QI are not available. Given our finding that residents had increased knowledge of their patient panels after receiving panel metrics, simply providing data with the goal of smaller, focused interventions may be easier to implement. At our institution, we discontinued the longer 2-hour QI workshops designed to teach QI approaches more broadly. We continue to provide individualized panel data to all residents during their primary care rotations and conduct half-hour, small group workshops with the interns that focus on drafting aim statements and planning interventions. All residents are required to submit worksheets to us at the end of their primary care blocks listing their current rates of each predetermined metric and laying out their aim statements and planned interventions. Residents also continue to receive feedback from our faculty with expertise in QI afterward on their plans and evidence of follow-through in the chart, with their preceptors included on the feedback emails. Even without the larger QI workshop, this approach has continued to be successful and appreciated. In fact, it does appear as though improvement in colorectal cancer screening has been sustained over several years. At the end of our study period, the resident patient colorectal cancer screening rate rose from 34% to 43%, and for the 2021-2022 academic year, the rate rose further, from 46% to 50%.
Given that the resident clinic patient population is at higher risk overall, targeted outreach and approaches to improve quality must be continued. Future areas of research include looking at which interventions, whether QI curriculum, provision of panel data, or required panel management interventions, translate to the greatest improvements in patient outcomes in this vulnerable population.
Conclusion
Our study showed that a dedicated QI curriculum for the residents and access to quality metric data improved both resident knowledge and comfort with QI approaches. Beyond resident-centered outcomes, there was also translation to improved patient outcomes, with a significant increase in colon cancer screening rates post intervention.
Corresponding author: Kinjalika Sathi, MD, 800 Washington St., Boston, MA 02111; [email protected]
Disclosures: None reported.
ABSTRACT
Objective: To teach internal medicine residents quality improvement (QI) principles in an effort to improve resident knowledge and comfort with QI, as well as address quality care gaps in resident clinic primary care patient panels.
Design: A QI curriculum was implemented for all residents rotating through a primary care block over a 6-month period. Residents completed Institute for Healthcare Improvement (IHI) modules, participated in a QI workshop, and received panel data reports, ultimately completing a plan-do-study-act (PDSA) cycle to improve colorectal cancer screening and hypertension control.
Setting and participants: This project was undertaken at Tufts Medical Center Primary Care, Boston, Massachusetts, the primary care teaching practice for all 75 internal medicine residents at Tufts Medical Center. All internal medicine residents were included, with 55 (73%) of the 75 residents completing the presurvey, and 39 (52%) completing the postsurvey.
Measurements: We administered a 10-question pre- and postsurvey looking at resident attitudes toward and comfort with QI and familiarity with their panel data as well as measured rates of colorectal cancer screening and hypertension control in resident panels.
Results: There was an increase in the numbers of residents who performed a PDSA cycle (P = .002), completed outreach based on their panel data (P = .02), and felt comfortable in both creating aim statements and designing and implementing PDSA cycles (P < .0001). The residents’ knowledge of their panel data significantly increased. There was no significant improvement in hypertension control, but there was an increase in colorectal cancer screening rates (P < .0001).
Conclusion: Providing panel data and performing targeted QI interventions can improve resident comfort with QI, translating to improvement in patient outcomes.
Keywords: quality improvement, resident education, medical education, care gaps, quality metrics.
As quality improvement (QI) has become an integral part of clinical practice, residency training programs have continued to evolve in how best to teach QI. The Accreditation Council for Graduate Medical Education (ACGME) Common Program requirements mandate that core competencies in residency programs include practice-based learning and improvement and systems-based practice.1 Residents should receive education in QI, receive data on quality metrics and benchmarks related to their patient population, and participate in QI activities. The Clinical Learning Environment Review (CLER) program was established to provide feedback to institutions on 6 focused areas, including patient safety and health care quality. In visits to institutions across the United States, the CLER committees found that many residents had limited knowledge of QI concepts and limited access to data on quality metrics and benchmarks.2
There are many barriers to implementing a QI curriculum in residency programs, and creating and maintaining successful strategies has proven challenging.3 Many QI curricula for internal medicine residents have been described in the literature, but the results of many of these studies focus on resident self-assessment of QI knowledge and numbers of projects rather than on patient outcomes.4-13 As there is some evidence suggesting that patients treated by residents have worse outcomes on ambulatory quality measures when compared with patients treated by staff physicians,14,15 it is important to also look at patient outcomes when evaluating a QI curriculum. Experts in education recommend the following to optimize learning: exposure to both didactic and experiential opportunities, connection to health system improvement efforts, and assessment of patient outcomes in addition to learner feedback.16,17 A study also found that providing panel data to residents could improve quality metrics.18
In this study, we sought to investigate the effects of a resident QI intervention during an ambulatory block on both residents’ self-assessments of QI knowledge and attitudes as well as on patient quality metrics.
Methods
Curriculum
We implemented this educational initiative at Tufts Medical Center Primary Care, Boston, Massachusetts, the primary care teaching practice for all 75 internal medicine residents at Tufts Medical Center. Co-located with the 415-bed academic medical center in downtown Boston, the practice serves more than 40,000 patients, approximately 7000 of whom are cared for by resident primary care physicians (PCPs). The internal medicine residents rotate through the primary care clinic as part of continuity clinic during ambulatory or elective blocks. In addition to continuity clinic, the residents have 2 dedicated 3-week primary care rotations during the course of an academic year. Primary care rotations consist of 5 clinic sessions a week as well as structured teaching sessions. Each resident inherits a panel of patients from an outgoing senior resident, with an average panel size of 96 patients per resident.
Prior to this study intervention, we did not do any formal QI teaching to our residents as part of their primary care curriculum, and previous panel management had focused more on chart reviews of patients whom residents perceived to be higher risk. Residents from all 3 years were included in the intervention. We taught a QI curriculum to our residents from January 2018 to June 2018 during the 3-week primary care rotation, which consisted of the following components:
- Institute for Healthcare Improvement (IHI) module QI 102 completed independently online.
- A 2-hour QI workshop led by 1 of 2 primary care faculty with backgrounds in QI, during which residents were taught basic principles of QI, including how to craft aim statements and design plan-do-study-act (PDSA) cycles, and participated in a hands-on QI activity designed to model rapid cycle improvement (the Paper Airplane Factory19).
- Distribution of individualized reports of residents’ patient panel data by email at the start of the primary care block that detailed patients’ overall rates of colorectal cancer screening and hypertension (HTN) control, along with the average resident panel rates and the average attending panel rates. The reports also included a list of all residents’ patients who were overdue for colorectal cancer screening or whose last blood pressure (BP) was uncontrolled (systolic BP ≥ 140 mm Hg or diastolic BP ≥ 90 mm Hg). These reports were originally designed by our practice’s QI team and run and exported in Microsoft Excel format monthly by our information technology (IT) administrator.
- Instruction on aim statements as a group, followed by the expectation that each resident create an individualized aim statement tailored to each resident’s patient panel rates, with the PDSA cycle to be implemented during the remainder of the primary care rotation, focusing on improvement of colorectal cancer screening and HTN control (see supplementary eFigure 1 online for the worksheet used for the workshop).
- Residents were held accountable for their interventions by various check-ins. At the end of the primary care block, residents were required to submit their completed worksheets showing the intervention they had undertaken and when it was performed. The 2 primary care attendings primarily responsible for QI education would review the resident’s work approximately 1 to 2 months after they submitted their worksheets describing their intervention. These attendings sent the residents personalized feedback based on whether the intervention had been completed or successful as evidenced by documentation in the chart, including direct patient outreach by phone, letter, or portal; outreach to the resident coordinator; scheduled follow-up appointment; or booking or completion of colorectal cancer screening. Along with this feedback, residents were also sent suggestions for next steps. Resident preceptors were copied on the email to facilitate reinforcement of the goals and plans. Finally, the resident preceptors also helped with accountability by going through the residents’ worksheets and patient panel metrics with the residents during biannual evaluations.

Evaluation
Residents were surveyed with a 10-item questionnaire pre and post intervention regarding their attitudes toward QI, understanding of QI principles, and familiarity with their patient panel data. Surveys were anonymous and distributed via the SurveyMonkey platform (see supplementary eFigure 2 online). Residents were asked if they had ever performed a PDSA cycle, performed patient outreach, or performed an intervention and whether they knew the rates of diabetes, HTN, and colorectal cancer screening in their patient panels. Questions rated on a 5-point Likert scale were used to assess comfort with panel management, developing an aim statement, designing and implementing a PDSA cycle, as well as interest in pursuing QI as a career. For the purposes of analysis, these questions were dichotomized into “somewhat comfortable” and “very comfortable” vs “neutral,” “somewhat uncomfortable,” and “very uncomfortable.” Similarly, we dichotomized the question about interest in QI as a career into “somewhat interested” and “very interested” vs “neutral,” “somewhat disinterested,” and “very disinterested.” As the surveys were anonymous, we were unable to pair the pre- and postintervention surveys and used a chi-square test to evaluate whether there was an association between survey assessments pre intervention vs post intervention and a positive or negative response to the question.

We also examined rates of HTN control and colorectal cancer screening in all 75 resident panels pre and post intervention. The paired t-test was used to determine whether the mean change from pre to post intervention was significant. SAS 9.4 (SAS Institute Inc.) was used for all analyses. Institutional Review Board exemption was obtained from the Tufts Medical Center IRB. There was no funding received for this study.
Results
Respondents
Of the 75 residents, 55 (73%) completed the survey prior to the intervention, and 39 (52%) completed the survey after the intervention.
Panel Knowledge and Intervention
Prior to the intervention, 45% of residents had performed a PDSA cycle, compared with 77% post intervention, which was a significant increase (P = .002) (Table 1). Sixty-two percent of residents had performed outreach or an intervention based on their patient panel reports prior to the intervention, compared with 85% of residents post intervention, which was also a significant increase (P = .02). The increase post intervention was not 100%, as there were residents who either missed the initial workshop or who did not follow through with their planned intervention. Common interventions included the residents giving their coordinators a list of patients to call to schedule appointments, utilizing fellow team members (eg, pharmacists, social workers) for targeted patient outreach, or calling patients themselves to reestablish a connection.

In terms of knowledge of their patient panels, prior to the intervention, 55%, 62%, and 62% of residents knew the rates of patients in their panel with diabetes, HTN, and colorectal cancer screening, respectively. After the intervention, the residents’ knowledge of these rates increased significantly, to 85% for diabetes (P = .002), 97% for HTN (P < .0001), and 97% for colorectal cancer screening (P < .0001).
Comfort With QI Approaches
Prior to the intervention, 82% of residents were comfortable managing their primary care panel, which did not change significantly post intervention (Table 2). The residents’ comfort with designing an aim statement did significantly increase, from 55% to 95% (P < .0001). The residents also had a significant increase in comfort with both designing and implementing a PDSA cycle. Prior to the intervention, 22% felt comfortable designing a PDSA cycle, which increased to 79% (P < .0001) post intervention, and 24% felt comfortable implementing a PDSA cycle, which increased to 77% (P < .0001) post intervention.

Patient Outcome Measures
The rate of HTN control in the residents' patient panels did not change significantly pre and post intervention (Table 3). The rate of resident patients who were up to date with colorectal cancer screening increased by 6.5% post intervention (P < .0001).

Interest in QI as a Career
As part of the survey, residents were asked how interested they were in making QI a part of their career. Fifty percent of residents indicated an interest in QI pre intervention, and 54% indicated an interest post intervention, which was not a significant difference (P = .72).
Discussion
In this study, we found that integration of a QI curriculum into a primary care rotation improved both residents’ knowledge of their patient panels and comfort with QI approaches, which translated to improvement in patient outcomes. Several previous studies have found improvements in resident self-assessment or knowledge after implementation of a QI curriculum.4-13 Liao et al implemented a longitudinal curriculum including both didactic and experiential components and found an improvement in both QI confidence and knowledge.3 Similarly, Duello et al8 found that a curriculum including both didactic lectures and QI projects improved subjective QI knowledge and comfort. Interestingly, Fok and Wong9 found that resident knowledge could be sustained post curriculum after completion of a QI project, suggesting that experiential learning may be helpful in maintaining knowledge.
Studies also have looked at providing performance data to residents. Hwang et al18 found that providing audit and feedback in the form of individual panel performance data to residents compared with practice targets led to statistically significant improvement in cancer screening rates and composite quality score, indicating that there is tremendous potential in providing residents with their data. While the ACGME mandates that residents should receive data on their quality metrics, on CLER visits, many residents interviewed noted limited access to data on their metrics and benchmarks.1,2
Though previous studies have individually looked at teaching QI concepts, providing panel data, or targeting select metrics, our study was unique in that it reviewed both self-reported resident outcomes data as well as actual patient outcomes. In addition to finding increased knowledge of patient panels and comfort with QI approaches, we found a significant increase in colorectal cancer screening rates post intervention. We thought this finding was particularly important given some data that residents' patients have been found to have worse outcomes on quality metrics compared with patients cared for by staff physicians.14,15 Given that having a resident physician as a PCP has been associated with failing to meet quality measures, it is especially important to focus targeted quality improvement initiatives in this patient population to reduce disparities in care.
We found that residents had improved knowledge on their patient panels as a result of this initiative. The residents were noted to have a higher knowledge of their HTN and colorectal cancer screening rates in comparison to their diabetes metrics. We suspect this is because residents are provided with multiple metrics related to diabetes, including process measures such as A1c testing, as well as outcome measures such as A1c control, so it may be harder for them to elucidate exactly how they are doing with their diabetes patients, whereas in HTN control and colorectal cancer screening, there is only 1 associated metric. Interestingly, even though HTN and colorectal cancer screening were the 2 measures focused on in the study, the residents had a significant improvement in knowledge of the rates of diabetes in their panel as well. This suggests that even just receiving data alone is valuable, hopefully translating to better outcomes with better baseline understanding of panels. We believe that our intervention was successful because it included both a didactic and an experiential component, as well as the use of individual panel performance data.
There were several limitations to our study. It was performed at a single institution, translating to a small sample size. Our data analysis was limited because we were unable to pair our pre- and postintervention survey responses because we used an anonymous survey. We also did not have full participation in postintervention surveys from all residents, which may have biased the study in favor of high performers. Another limitation was that our survey relied on self-reported outcomes for the questions about the residents knowing their patient panels.
This study required a 2-hour workshop every 3 weeks led by a faculty member trained in QI. Given the amount of time needed for the curriculum, this study may be difficult to replicate at other institutions, especially if faculty with an interest or training in QI are not available. Given our finding that residents had increased knowledge of their patient panels after receiving panel metrics, simply providing data with the goal of smaller, focused interventions may be easier to implement. At our institution, we discontinued the longer 2-hour QI workshops designed to teach QI approaches more broadly. We continue to provide individualized panel data to all residents during their primary care rotations and conduct half-hour, small group workshops with the interns that focus on drafting aim statements and planning interventions. All residents are required to submit worksheets to us at the end of their primary care blocks listing their current rates of each predetermined metric and laying out their aim statements and planned interventions. Residents also continue to receive feedback from our faculty with expertise in QI afterward on their plans and evidence of follow-through in the chart, with their preceptors included on the feedback emails. Even without the larger QI workshop, this approach has continued to be successful and appreciated. In fact, it does appear as though improvement in colorectal cancer screening has been sustained over several years. At the end of our study period, the resident patient colorectal cancer screening rate rose from 34% to 43%, and for the 2021-2022 academic year, the rate rose further, from 46% to 50%.
Given that the resident clinic patient population is at higher risk overall, targeted outreach and approaches to improve quality must be continued. Future areas of research include looking at which interventions, whether QI curriculum, provision of panel data, or required panel management interventions, translate to the greatest improvements in patient outcomes in this vulnerable population.
Conclusion
Our study showed that a dedicated QI curriculum for the residents and access to quality metric data improved both resident knowledge and comfort with QI approaches. Beyond resident-centered outcomes, there was also translation to improved patient outcomes, with a significant increase in colon cancer screening rates post intervention.
Corresponding author: Kinjalika Sathi, MD, 800 Washington St., Boston, MA 02111; [email protected]
Disclosures: None reported.
1. Accreditation Council for Graduate Medical Education. ACGME Common Program Requirements (Residency). Approved June 13, 2021. Updated July 1, 2022. Accessed December 29, 2022. https://www.acgme.org/globalassets/pfassets/programrequirements/cprresidency_2022v3.pdf
2. Koh NJ, Wagner R, Newton RC, et al; on behalf of the CLER Evaluation Committee and the CLER Program. CLER National Report of Findings 2021. Accreditation Council for Graduate Medical Education; 2021. Accessed December 29, 2022. https://www.acgme.org/globalassets/pdfs/cler/2021clernationalreportoffindings.pdf
3. Liao JM, Co JP, Kachalia A. Providing educational content and context for training the next generation of physicians in quality improvement. Acad Med. 2015;90(9):1241-1245. doi:10.1097/ACM.0000000000000799
4. Johnson KM, Fiordellisi W, Kuperman E, et al. X + Y = time for QI: meaningful engagement of residents in quality improvement during the ambulatory block. J Grad Med Educ. 2018;10(3):316-324. doi:10.4300/JGME-D-17-00761.1
5. Kesari K, Ali S, Smith S. Integrating residents with institutional quality improvement teams. Med Educ. 2017;51(11):1173. doi:10.1111/medu.13431
6. Ogrinc G, Cohen ES, van Aalst R, et al. Clinical and educational outcomes of an integrated inpatient quality improvement curriculum for internal medicine residents. J Grad Med Educ. 2016;8(4):563-568. doi:10.4300/JGME-D-15-00412.1
7. Malayala SV, Qazi KJ, Samdani AJ, et al. A multidisciplinary performance improvement rotation in an internal medicine training program. Int J Med Educ. 2016;7:212-213. doi:10.5116/ijme.5765.0bda
8. Duello K, Louh I, Greig H, et al. Residents’ knowledge of quality improvement: the impact of using a group project curriculum. Postgrad Med J. 2015;91(1078):431-435. doi:10.1136/postgradmedj-2014-132886
9. Fok MC, Wong RY. Impact of a competency based curriculum on quality improvement among internal medicine residents. BMC Med Educ. 2014;14:252. doi:10.1186/s12909-014-0252-7
10. Wilper AP, Smith CS, Weppner W. Instituting systems-based practice and practice-based learning and improvement: a curriculum of inquiry. Med Educ Online. 2013;18:21612. doi:10.3402/meo.v18i0.21612
11. Weigel C, Suen W, Gupte G. Using lean methodology to teach quality improvement to internal medicine residents at a safety net hospital. Am J Med Qual. 2013;28(5):392-399. doi:10.1177/1062860612474062
12. Tomolo AM, Lawrence RH, Watts B, et al. Pilot study evaluating a practice-based learning and improvement curriculum focusing on the development of system-level quality improvement skills. J Grad Med Educ. 2011;3(1):49-58. doi:10.4300/JGME-D-10-00104.1
13. Djuricich AM, Ciccarelli M, Swigonski NL. A continuous quality improvement curriculum for residents: addressing core competency, improving systems. Acad Med. 2004;79(10 Suppl):S65-S67. doi:10.1097/00001888-200410001-00020
14. Essien UR, He W, Ray A, et al. Disparities in quality of primary care by resident and staff physicians: is there a conflict between training and equity? J Gen Intern Med. 2019;34(7):1184-1191. doi:10.1007/s11606-019-04960-5
15. Amat M, Norian E, Graham KL. Unmasking a vulnerable patient care process: a qualitative study describing the current state of resident continuity clinic in a nationwide cohort of internal medicine residency programs. Am J Med. 2022;135(6):783-786. doi:10.1016/j.amjmed.2022.02.007
16. Wong BM, Etchells EE, Kuper A, et al. Teaching quality improvement and patient safety to trainees: a systematic review. Acad Med. 2010;85(9):1425-1439. doi:10.1097/ACM.0b013e3181e2d0c6
17. Armstrong G, Headrick L, Madigosky W, et al. Designing education to improve care. Jt Comm J Qual Patient Saf. 2012;38:5-14. doi:10.1016/s1553-7250(12)38002-1
18. Hwang AS, Harding AS, Chang Y, et al. An audit and feedback intervention to improve internal medicine residents’ performance on ambulatory quality measures: a randomized controlled trial. Popul Health Manag. 2019;22(6):529-535. doi:10.1089/pop.2018.0217
19. Institute for Healthcare Improvement. Open school. The paper airplane factory. Accessed December 29, 2022. https://www.ihi.org/education/IHIOpenSchool/resources/Pages/Activities/PaperAirplaneFactory.aspx
1. Accreditation Council for Graduate Medical Education. ACGME Common Program Requirements (Residency). Approved June 13, 2021. Updated July 1, 2022. Accessed December 29, 2022. https://www.acgme.org/globalassets/pfassets/programrequirements/cprresidency_2022v3.pdf
2. Koh NJ, Wagner R, Newton RC, et al; on behalf of the CLER Evaluation Committee and the CLER Program. CLER National Report of Findings 2021. Accreditation Council for Graduate Medical Education; 2021. Accessed December 29, 2022. https://www.acgme.org/globalassets/pdfs/cler/2021clernationalreportoffindings.pdf
3. Liao JM, Co JP, Kachalia A. Providing educational content and context for training the next generation of physicians in quality improvement. Acad Med. 2015;90(9):1241-1245. doi:10.1097/ACM.0000000000000799
4. Johnson KM, Fiordellisi W, Kuperman E, et al. X + Y = time for QI: meaningful engagement of residents in quality improvement during the ambulatory block. J Grad Med Educ. 2018;10(3):316-324. doi:10.4300/JGME-D-17-00761.1
5. Kesari K, Ali S, Smith S. Integrating residents with institutional quality improvement teams. Med Educ. 2017;51(11):1173. doi:10.1111/medu.13431
6. Ogrinc G, Cohen ES, van Aalst R, et al. Clinical and educational outcomes of an integrated inpatient quality improvement curriculum for internal medicine residents. J Grad Med Educ. 2016;8(4):563-568. doi:10.4300/JGME-D-15-00412.1
7. Malayala SV, Qazi KJ, Samdani AJ, et al. A multidisciplinary performance improvement rotation in an internal medicine training program. Int J Med Educ. 2016;7:212-213. doi:10.5116/ijme.5765.0bda
8. Duello K, Louh I, Greig H, et al. Residents’ knowledge of quality improvement: the impact of using a group project curriculum. Postgrad Med J. 2015;91(1078):431-435. doi:10.1136/postgradmedj-2014-132886
9. Fok MC, Wong RY. Impact of a competency based curriculum on quality improvement among internal medicine residents. BMC Med Educ. 2014;14:252. doi:10.1186/s12909-014-0252-7
10. Wilper AP, Smith CS, Weppner W. Instituting systems-based practice and practice-based learning and improvement: a curriculum of inquiry. Med Educ Online. 2013;18:21612. doi:10.3402/meo.v18i0.21612
11. Weigel C, Suen W, Gupte G. Using lean methodology to teach quality improvement to internal medicine residents at a safety net hospital. Am J Med Qual. 2013;28(5):392-399. doi:10.1177/1062860612474062
12. Tomolo AM, Lawrence RH, Watts B, et al. Pilot study evaluating a practice-based learning and improvement curriculum focusing on the development of system-level quality improvement skills. J Grad Med Educ. 2011;3(1):49-58. doi:10.4300/JGME-D-10-00104.1
13. Djuricich AM, Ciccarelli M, Swigonski NL. A continuous quality improvement curriculum for residents: addressing core competency, improving systems. Acad Med. 2004;79(10 Suppl):S65-S67. doi:10.1097/00001888-200410001-00020
14. Essien UR, He W, Ray A, et al. Disparities in quality of primary care by resident and staff physicians: is there a conflict between training and equity? J Gen Intern Med. 2019;34(7):1184-1191. doi:10.1007/s11606-019-04960-5
15. Amat M, Norian E, Graham KL. Unmasking a vulnerable patient care process: a qualitative study describing the current state of resident continuity clinic in a nationwide cohort of internal medicine residency programs. Am J Med. 2022;135(6):783-786. doi:10.1016/j.amjmed.2022.02.007
16. Wong BM, Etchells EE, Kuper A, et al. Teaching quality improvement and patient safety to trainees: a systematic review. Acad Med. 2010;85(9):1425-1439. doi:10.1097/ACM.0b013e3181e2d0c6
17. Armstrong G, Headrick L, Madigosky W, et al. Designing education to improve care. Jt Comm J Qual Patient Saf. 2012;38:5-14. doi:10.1016/s1553-7250(12)38002-1
18. Hwang AS, Harding AS, Chang Y, et al. An audit and feedback intervention to improve internal medicine residents’ performance on ambulatory quality measures: a randomized controlled trial. Popul Health Manag. 2019;22(6):529-535. doi:10.1089/pop.2018.0217
19. Institute for Healthcare Improvement. Open school. The paper airplane factory. Accessed December 29, 2022. https://www.ihi.org/education/IHIOpenSchool/resources/Pages/Activities/PaperAirplaneFactory.aspx





