User login
Doctors’ happiness has not rebounded as pandemic drags on
Physicians reported similar levels of unhappiness in 2022 too.
Fewer than half of physicians said they were currently somewhat or very happy at work, compared with 75% of physicians who said they were somewhat or very happy at work in a previous survey conducted before the pandemic, the new Medscape Physician Lifestyle & Happiness Report 2023 shows.*
“I am not surprised that we’re less happy now,” said Amaryllis Sánchez, MD, a board-certified family medicine physician and a certified physician coach.
“I speak to physicians around the country and I hear that their workplaces are understaffed, they’re overworked and they don’t feel safe. Although we’re in a different phase of the pandemic, doctors feel that the ground beneath them is still shaky,” said Dr. Sánchez, the author of “Recapturing Joy in Medicine.”
Most doctors are seeing more patients than they can handle and are expected to do that consistently. “When you no longer have the capacity to give of yourself, that becomes a nearly impossible task,” said Dr. Sánchez.
Also, physicians in understaffed workplaces often must take on additional work such as administrative or nursing duties, said Katie Cole, DO, a board-certified psychiatrist and a physician coach.
While health systems are aware that physicians need time to rest and recharge, staffing shortages prevent doctors from taking time off because they can’t find coverage, said Dr. Cole.
“While we know that it’s important for physicians to take vacations, more than one-third of doctors still take 2 weeks or less of vacation annually,” said Dr. Cole.
Physicians also tend to have less compassion for themselves and sacrifice self-care compared to other health care workers. “When a patient dies, nurses get together, debrief, and hug each other, whereas doctors have another patient to see. The culture of medicine doesn’t support self-compassion for physicians,” said Dr. Cole.
Physicians also felt less safe at work during the pandemic because of to shortages of personal protective equipment, said Dr. Sánchez. They have also witnessed or experienced an increase in abusive behavior, violence and threats of violence.
Physicians’ personal life suffers
Doctors maintain their mental health primarily by spending time with family members and friends, according to 2022’s Medscape Physician Lifestyle & Happiness Report. Yet half of doctors reported in a survey by the Physicians Foundation that they withdrew from family, friends or coworkers in 2022, said Dr. Sánchez.
“When you exceed your mental, emotional, and physical capacity at work, you have no reserve left for your personal life,” said Dr. Cole.
That may explain why only 58% of doctors reported feeling somewhat or very happy outside of work, compared with 84% who felt that way before the pandemic.
More women doctors said they deal with stronger feelings of conflict in trying to balance parenting responsibilities with a highly demanding job. Nearly one in two women physician-parents reported feeling very conflicted at work, compared with about one in four male physician-parents.
When physicians go home, they may be emotionally drained and tired mentally from making a lot of decisions at work, said Dr. Cole.
“As a woman, if you have children and a husband and you’re responsible for dinner, picking up the kids at daycare or helping them with homework, and making all these decisions when you get home, it’s overwhelming,” said Dr. Cole.
Prioritize your well-being
Doctors need to prioritize their own well-being, said Dr. Sánchez. “That’s not being selfish, that’s doing what’s necessary to stay well and be able to take care of patients. If doctors don’t take care of themselves, no one else will.”
Dr. Sánchez recommended that doctors regularly interact with relatives, friends, trusted colleagues, or clergy to help maintain their well-being, rather than waiting until a crisis to reach out.
A good coach, mentor, or counselor can help physicians gain enough self-awareness to handle their emotions and gain more clarity about what changes need to be made, she said.
Dr. Cole suggested that doctors figure out what makes them happy and fulfilled at work and try to spend more time on that activity. “Knowing what makes you happy and your strengths are foundational for creating a life you love.”
She urged doctors to “start thinking now about what you love about medicine and what is going right at home, and what areas you want to change. Then, start advocating for your needs.”
A version of this article originally appeared on Medscape.com.
Correction, 1/26/23: An earlier version of this article misstated the findings of the survey.
Physicians reported similar levels of unhappiness in 2022 too.
Fewer than half of physicians said they were currently somewhat or very happy at work, compared with 75% of physicians who said they were somewhat or very happy at work in a previous survey conducted before the pandemic, the new Medscape Physician Lifestyle & Happiness Report 2023 shows.*
“I am not surprised that we’re less happy now,” said Amaryllis Sánchez, MD, a board-certified family medicine physician and a certified physician coach.
“I speak to physicians around the country and I hear that their workplaces are understaffed, they’re overworked and they don’t feel safe. Although we’re in a different phase of the pandemic, doctors feel that the ground beneath them is still shaky,” said Dr. Sánchez, the author of “Recapturing Joy in Medicine.”
Most doctors are seeing more patients than they can handle and are expected to do that consistently. “When you no longer have the capacity to give of yourself, that becomes a nearly impossible task,” said Dr. Sánchez.
Also, physicians in understaffed workplaces often must take on additional work such as administrative or nursing duties, said Katie Cole, DO, a board-certified psychiatrist and a physician coach.
While health systems are aware that physicians need time to rest and recharge, staffing shortages prevent doctors from taking time off because they can’t find coverage, said Dr. Cole.
“While we know that it’s important for physicians to take vacations, more than one-third of doctors still take 2 weeks or less of vacation annually,” said Dr. Cole.
Physicians also tend to have less compassion for themselves and sacrifice self-care compared to other health care workers. “When a patient dies, nurses get together, debrief, and hug each other, whereas doctors have another patient to see. The culture of medicine doesn’t support self-compassion for physicians,” said Dr. Cole.
Physicians also felt less safe at work during the pandemic because of to shortages of personal protective equipment, said Dr. Sánchez. They have also witnessed or experienced an increase in abusive behavior, violence and threats of violence.
Physicians’ personal life suffers
Doctors maintain their mental health primarily by spending time with family members and friends, according to 2022’s Medscape Physician Lifestyle & Happiness Report. Yet half of doctors reported in a survey by the Physicians Foundation that they withdrew from family, friends or coworkers in 2022, said Dr. Sánchez.
“When you exceed your mental, emotional, and physical capacity at work, you have no reserve left for your personal life,” said Dr. Cole.
That may explain why only 58% of doctors reported feeling somewhat or very happy outside of work, compared with 84% who felt that way before the pandemic.
More women doctors said they deal with stronger feelings of conflict in trying to balance parenting responsibilities with a highly demanding job. Nearly one in two women physician-parents reported feeling very conflicted at work, compared with about one in four male physician-parents.
When physicians go home, they may be emotionally drained and tired mentally from making a lot of decisions at work, said Dr. Cole.
“As a woman, if you have children and a husband and you’re responsible for dinner, picking up the kids at daycare or helping them with homework, and making all these decisions when you get home, it’s overwhelming,” said Dr. Cole.
Prioritize your well-being
Doctors need to prioritize their own well-being, said Dr. Sánchez. “That’s not being selfish, that’s doing what’s necessary to stay well and be able to take care of patients. If doctors don’t take care of themselves, no one else will.”
Dr. Sánchez recommended that doctors regularly interact with relatives, friends, trusted colleagues, or clergy to help maintain their well-being, rather than waiting until a crisis to reach out.
A good coach, mentor, or counselor can help physicians gain enough self-awareness to handle their emotions and gain more clarity about what changes need to be made, she said.
Dr. Cole suggested that doctors figure out what makes them happy and fulfilled at work and try to spend more time on that activity. “Knowing what makes you happy and your strengths are foundational for creating a life you love.”
She urged doctors to “start thinking now about what you love about medicine and what is going right at home, and what areas you want to change. Then, start advocating for your needs.”
A version of this article originally appeared on Medscape.com.
Correction, 1/26/23: An earlier version of this article misstated the findings of the survey.
Physicians reported similar levels of unhappiness in 2022 too.
Fewer than half of physicians said they were currently somewhat or very happy at work, compared with 75% of physicians who said they were somewhat or very happy at work in a previous survey conducted before the pandemic, the new Medscape Physician Lifestyle & Happiness Report 2023 shows.*
“I am not surprised that we’re less happy now,” said Amaryllis Sánchez, MD, a board-certified family medicine physician and a certified physician coach.
“I speak to physicians around the country and I hear that their workplaces are understaffed, they’re overworked and they don’t feel safe. Although we’re in a different phase of the pandemic, doctors feel that the ground beneath them is still shaky,” said Dr. Sánchez, the author of “Recapturing Joy in Medicine.”
Most doctors are seeing more patients than they can handle and are expected to do that consistently. “When you no longer have the capacity to give of yourself, that becomes a nearly impossible task,” said Dr. Sánchez.
Also, physicians in understaffed workplaces often must take on additional work such as administrative or nursing duties, said Katie Cole, DO, a board-certified psychiatrist and a physician coach.
While health systems are aware that physicians need time to rest and recharge, staffing shortages prevent doctors from taking time off because they can’t find coverage, said Dr. Cole.
“While we know that it’s important for physicians to take vacations, more than one-third of doctors still take 2 weeks or less of vacation annually,” said Dr. Cole.
Physicians also tend to have less compassion for themselves and sacrifice self-care compared to other health care workers. “When a patient dies, nurses get together, debrief, and hug each other, whereas doctors have another patient to see. The culture of medicine doesn’t support self-compassion for physicians,” said Dr. Cole.
Physicians also felt less safe at work during the pandemic because of to shortages of personal protective equipment, said Dr. Sánchez. They have also witnessed or experienced an increase in abusive behavior, violence and threats of violence.
Physicians’ personal life suffers
Doctors maintain their mental health primarily by spending time with family members and friends, according to 2022’s Medscape Physician Lifestyle & Happiness Report. Yet half of doctors reported in a survey by the Physicians Foundation that they withdrew from family, friends or coworkers in 2022, said Dr. Sánchez.
“When you exceed your mental, emotional, and physical capacity at work, you have no reserve left for your personal life,” said Dr. Cole.
That may explain why only 58% of doctors reported feeling somewhat or very happy outside of work, compared with 84% who felt that way before the pandemic.
More women doctors said they deal with stronger feelings of conflict in trying to balance parenting responsibilities with a highly demanding job. Nearly one in two women physician-parents reported feeling very conflicted at work, compared with about one in four male physician-parents.
When physicians go home, they may be emotionally drained and tired mentally from making a lot of decisions at work, said Dr. Cole.
“As a woman, if you have children and a husband and you’re responsible for dinner, picking up the kids at daycare or helping them with homework, and making all these decisions when you get home, it’s overwhelming,” said Dr. Cole.
Prioritize your well-being
Doctors need to prioritize their own well-being, said Dr. Sánchez. “That’s not being selfish, that’s doing what’s necessary to stay well and be able to take care of patients. If doctors don’t take care of themselves, no one else will.”
Dr. Sánchez recommended that doctors regularly interact with relatives, friends, trusted colleagues, or clergy to help maintain their well-being, rather than waiting until a crisis to reach out.
A good coach, mentor, or counselor can help physicians gain enough self-awareness to handle their emotions and gain more clarity about what changes need to be made, she said.
Dr. Cole suggested that doctors figure out what makes them happy and fulfilled at work and try to spend more time on that activity. “Knowing what makes you happy and your strengths are foundational for creating a life you love.”
She urged doctors to “start thinking now about what you love about medicine and what is going right at home, and what areas you want to change. Then, start advocating for your needs.”
A version of this article originally appeared on Medscape.com.
Correction, 1/26/23: An earlier version of this article misstated the findings of the survey.
Diagnostic Errors in Hospitalized Patients
Abstract
Diagnostic errors in hospitalized patients are a leading cause of preventable morbidity and mortality. Significant challenges in defining and measuring diagnostic errors and underlying process failure points have led to considerable variability in reported rates of diagnostic errors and adverse outcomes. In this article, we explore the diagnostic process and its discrete components, emphasizing the centrality of the patient in decision-making as well as the continuous nature of the process. We review the incidence of diagnostic errors in hospitalized patients and different methodological approaches that have been used to arrive at these estimates. We discuss different but interdependent provider- and system-related process-failure points that lead to diagnostic errors. We examine specific challenges related to measurement of diagnostic errors and describe traditional and novel approaches that are being used to obtain the most precise estimates. Finally, we examine various patient-, provider-, and organizational-level interventions that have been proposed to improve diagnostic safety in hospitalized patients.
Keywords: diagnostic error, hospital medicine, patient safety.
Diagnosis is defined as a “pre-existing set of categories agreed upon by the medical profession to designate a specific condition.”1 The diagnostic process involves obtaining a clinical history, performing a physical examination, conducting diagnostic testing, and consulting with other clinical providers to gather data that are relevant to understanding the underlying disease processes. This exercise involves generating hypotheses and updating prior probabilities as more information and evidence become available. Throughout this process of information gathering, integration, and interpretation, there is an ongoing assessment of whether sufficient and necessary knowledge has been obtained to make an accurate diagnosis and provide appropriate treatment.2
Diagnostic error is defined as a missed opportunity to make a timely diagnosis as part of this iterative process, including the failure of communicating the diagnosis to the patient in a timely manner.3 It can be categorized as a missed, delayed, or incorrect diagnosis based on available evidence at the time. Establishing the correct diagnosis has important implications. A timely and precise diagnosis ensures the patient the highest probability of having a positive health outcome that reflects an appropriate understanding of underlying disease processes and is consistent with their overall goals of care.3 When diagnostic errors occur, they can cause patient harm. Adverse events due to medical errors, including diagnostic errors, are estimated to be the third leading cause of death in the United States.4 Most people will experience at least 1 diagnostic error in their lifetime. In the 2015 National Academy of Medicine report Improving Diagnosis in Healthcare, diagnostic errors were identified as a major hazard as well as an opportunity to improve patient outcomes.2
Diagnostic errors during hospitalizations are especially concerning, as they are more likely to be implicated in a wider spectrum of harm, including permanent disability and death. This has become even more relevant for hospital medicine physicians and other clinical providers as they encounter increasing cognitive and administrative workloads, rising dissatisfaction and burnout, and unique obstacles such as night-time scheduling.5
Incidence of Diagnostic Errors in Hospitalized Patients
Several methodological approaches have been used to estimate the incidence of diagnostic errors in hospitalized patients. These include retrospective reviews of a sample of all hospital admissions, evaluations of selected adverse outcomes including autopsy studies, patient and provider surveys, and malpractice claims. Laboratory testing audits and secondary reviews in other diagnostic subspecialities (eg, radiology, pathology, and microbiology) are also essential to improving diagnostic performance in these specialized fields, which in turn affects overall hospital diagnostic error rates.6-8 These diverse approaches provide unique insights regarding our ability to assess the degree to which potential harms, ranging from temporary impairment to permanent disability, to death, are attributable to different failure points in the diagnostic process.
Large retrospective chart reviews of random hospital admissions remain the most accurate way to determine the overall incidence of diagnostic errors in hospitalized patients.9 The Harvard Medical Practice Study, published in 1991, laid the groundwork for measuring the incidence of adverse events in hospitalized patients and assessing their relation to medical error, negligence, and disability. Reviewing 30,121 randomly selected records from 51 randomly selected acute care hospitals in New York State, the study found that adverse events occurred in 3.7% of hospitalizations, diagnostic errors accounted for 13.8% of these events, and these errors were likely attributable to negligence in 74.7% of cases. The study not only outlined individual-level process failures, but also focused attention on some of the systemic causes, setting the agenda for quality improvement research in hospital-based care for years to come.10-12 A recent systematic review and meta-analysis of 22 hospital admission studies found a pooled rate of 0.7% (95% CI, 0.5%-1.1%) for harmful diagnostic errors.9 It found significant variations in the rates of adverse events, diagnostic errors, and range of diagnoses that were missed. This was primarily because of variabilities in pre-test probabilities in detecting diagnostic errors in these specific cohorts, as well as due to heterogeneity in study definitions and methodologies, especially regarding how they defined and measured “diagnostic error.” The analysis, however, did not account for diagnostic errors that were not related to patient harm (missed opportunities); therefore, it likely significantly underestimated the true incidence of diagnostic errors in these study populations. Table 1 summarizes some of key studies that have examined the incidence of harmful diagnostic errors in hospitalized patients.9-21
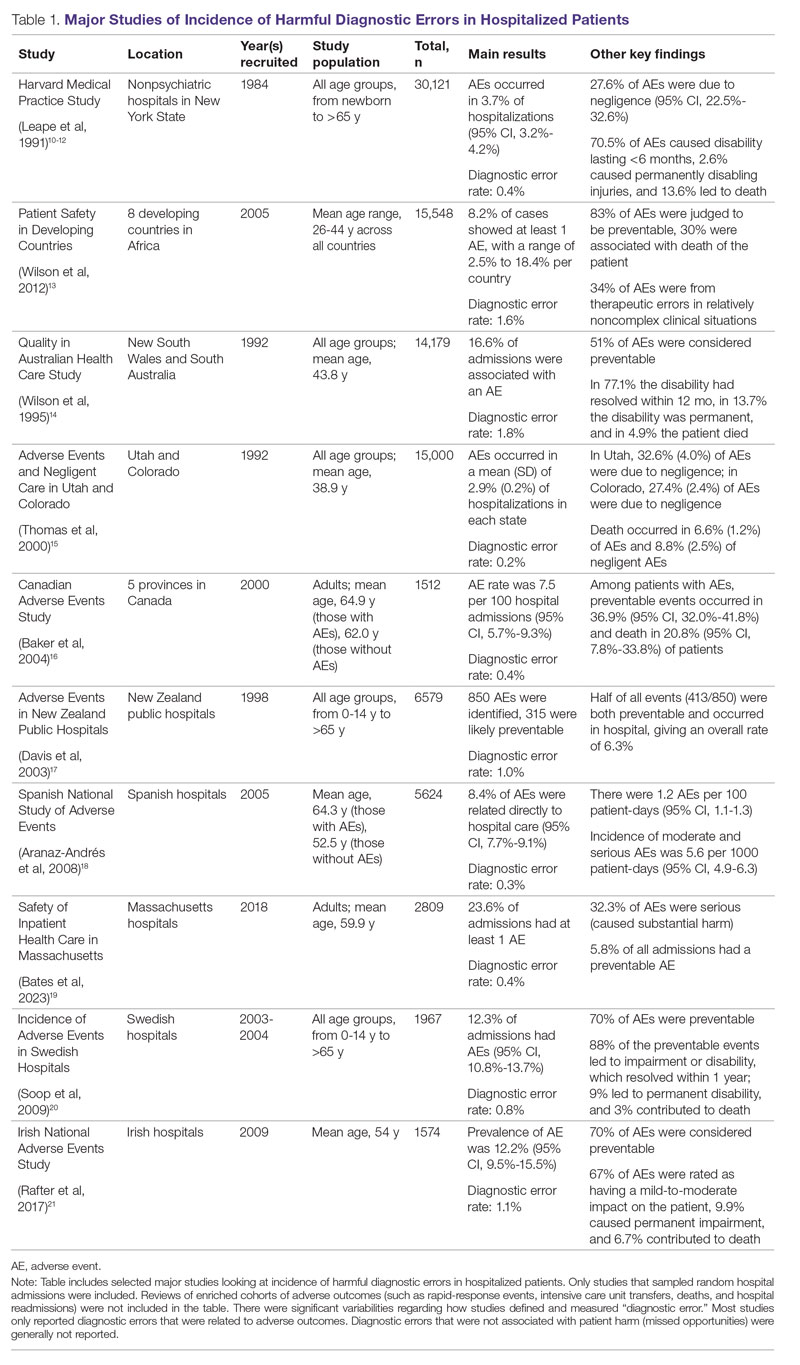
The chief limitation of reviewing random hospital admissions is that, since overall rates of diagnostic errors are still relatively low, a large number of case reviews are required to identify a sufficient sample of adverse outcomes to gain a meaningful understanding of the underlying process failure points and develop tools for remediation. Patient and provider surveys or data from malpractice claims can be high-yield starting points for research on process errors.22,23 Reviews of enriched cohorts of adverse outcomes, such as rapid-response events, intensive care unit (ICU) transfers, deaths, and hospital readmissions, can be an efficient way to identify process failures that lead to greatest harm. Depending on the research approach and the types of underlying patient populations sampled, rates of diagnostic errors in these high-risk groups have been estimated to be approximately 5% to 20%, or even higher.6,24-31 For example, a retrospective study of 391 cases of unplanned 7-day readmissions found that 5.6% of cases contained at least 1 diagnostic error during the index admission.32 In a study conducted at 6 Belgian acute-care hospitals, 56% of patients requiring an unplanned transfer to a higher level of care were determined to have had an adverse event, and of these adverse events, 12.4% of cases were associated with errors in diagnosis.29 A systematic review of 16 hospital-based studies estimated that 3.1% of all inpatient deaths were likely preventable, which corresponded to 22,165 deaths annually in the United States.30 Another such review of 31 autopsy studies reported that 28% of autopsied ICU patients had at least 1 misdiagnosis; of these diagnostic errors, 8% were classified as potentially lethal, and 15% were considered major but not lethal.31 Significant drawbacks of such enriched cohort studies, however, are their poor generalizability and inability to detect failure points that do not lead to patient harm (near-miss events).33
Causes of Diagnostic Errors in Hospitalized Patients
All aspects of the diagnostic process are susceptible to errors. These errors stem from a variety of faulty processes, including failure of the patient to engage with the health care system (eg, due to lack of insurance or transportation, or delay in seeking care); failure in information gathering (eg, missed history or exam findings, ordering wrong tests, laboratory errors); failure in information interpretation (eg, exam finding or test result misinterpretation); inaccurate hypothesis generation (eg, due to suboptimal prioritization or weighing of supporting evidence); and failure in communication (eg, with other team members or with the patient).2,34 Reasons for diagnostic process failures vary widely across different health care settings. While clinician assessment errors (eg, failure to consider or alternatively overweigh competing diagnoses) and errors in testing and the monitoring phase (eg, failure to order or follow up diagnostic tests) can lead to a majority of diagnostic errors in some patient populations, in other settings, social (eg, poor health literacy, punitive cultural practices) and economic factors (eg, lack of access to appropriate diagnostic tests or to specialty expertise) play a more prominent role.34,35
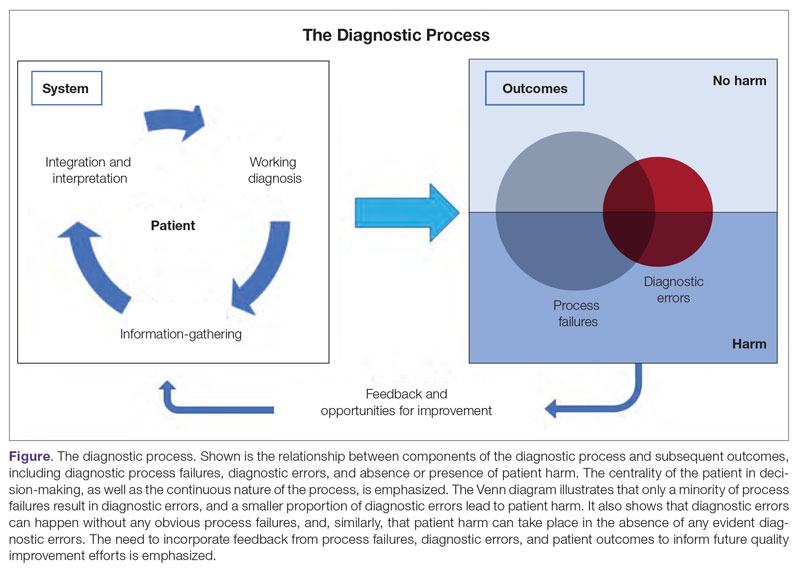
The Figure describes the relationship between components of the diagnostic process and subsequent outcomes, including diagnostic process failures, diagnostic errors, and absence or presence of patient harm.2,36,37 It reemphasizes the centrality of the patient in decision-making and the continuous nature of the process. The Figure also illustrates that only a minority of process failures result in diagnostic errors, and a smaller proportion of diagnostic errors actually lead to patient harm. Conversely, it also shows that diagnostic errors can happen without any obvious process-failure points, and, similarly, patient harm can take place in the absence of any evident diagnostic errors.36-38 Finally, it highlights the need to incorporate feedback from process failures, diagnostic errors, and favorable and unfavorable patient outcomes in order to inform future quality improvement efforts and research.
A significant proportion of diagnostic errors are due to system-related vulnerabilities, such as limitations in availability, adoption or quality of work force training, health informatics resources, and diagnostic capabilities. Lack of institutional culture that promotes safety and transparency also predisposes to diagnostic errors.39,40 The other major domain of process failures is related to cognitive errors in clinician decision-making. Anchoring, confirmation bias, availability bias, and base-rate neglect are some of the common cognitive biases that, along with personality traits (aversion to risk or ambiguity, overconfidence) and affective biases (influence of emotion on decision-making), often determine the degree of utilization of resources and the possibility of suboptimal diagnostic performance.41,42 Further, implicit biases related to age, race, gender, and sexual orientation contribute to disparities in access to health care and outcomes.43 In a large number of cases of preventable adverse outcomes, however, there are multiple interdependent individual and system-related failure points that lead to diagnostic error and patient harm.6,32
Challenges in Defining and Measuring Diagnostic Errors
In order to develop effective, evidence-based interventions to reduce diagnostic errors in hospitalized patients, it is essential to be able to first operationally define, and then accurately measure, diagnostic errors and the process failures that contribute to these errors in a standardized way that is reproducible across different settings.6,44 There are a number of obstacles in this endeavor.
A fundamental problem is that establishing a diagnosis is not a single act but a process. Patterns of symptoms and clinical presentations often differ for the same disease. Information required to make a diagnosis is usually gathered in stages, where the clinician obtains additional data, while considering many possibilities, of which 1 may be ultimately correct. Diagnoses evolve over time and in different care settings. “The most likely diagnosis” is not always the same as “the final correct diagnosis.” Moreover, the diagnostic process is influenced by patients’ individual clinical courses and preferences over time. This makes determination of missed, delayed, or incorrect diagnoses challenging.45,46
For hospitalized patients, generally the goal is to first rule out more serious and acute conditions (eg, pulmonary embolism or stroke), even if their probability is rather low. Conversely, a diagnosis that appears less consequential if delayed (eg, chronic anemia of unclear etiology) might not be pursued on an urgent basis, and is often left to outpatient providers to examine, but still may manifest in downstream harm (eg, delayed diagnosis of gastrointestinal malignancy or recurrent admissions for heart failure due to missed iron-deficiency anemia). Therefore, coming up with disease diagnosis likelihoods in hindsight may turn out to be highly subjective and not always accurate. This can be particularly difficult when clinician and other team deliberations are not recorded in their entirety.47
Another hurdle in the practice of diagnostic medicine is to preserve the balance between underdiagnosing versus pursuing overly aggressive diagnostic approaches. Conducting laboratory, imaging, or other diagnostic studies without a clear shared understanding of how they would affect clinical decision-making (eg, use of prostate-specific antigen to detect prostate cancer) not only leads to increased costs but can also delay appropriate care. Worse, subsequent unnecessary diagnostic tests and treatments can sometimes lead to serious harm.48,49
Finally, retrospective reviews by clinicians are subject to multiple potential limitations that include failure to create well-defined research questions, poorly developed inclusion and exclusion criteria, and issues related to inter- and intra-rater reliability.50 These methodological deficiencies can occur despite following "best practice" guidelines during the study planning, execution, and analysis phases. They further add to the challenge of defining and measuring diagnostic errors.47
Strategies to Improve Measurement of Diagnostic Errors
Development of new methodologies to reliably measure diagnostic errors is an area of active research. The advancement of uniform and universally agreed-upon frameworks to define and identify process failure points and diagnostic errors would help reduce measurement error and support development and testing of interventions that could be generalizable across different health care settings. To more accurately define and measure diagnostic errors, several novel approaches have been proposed (Table 2).
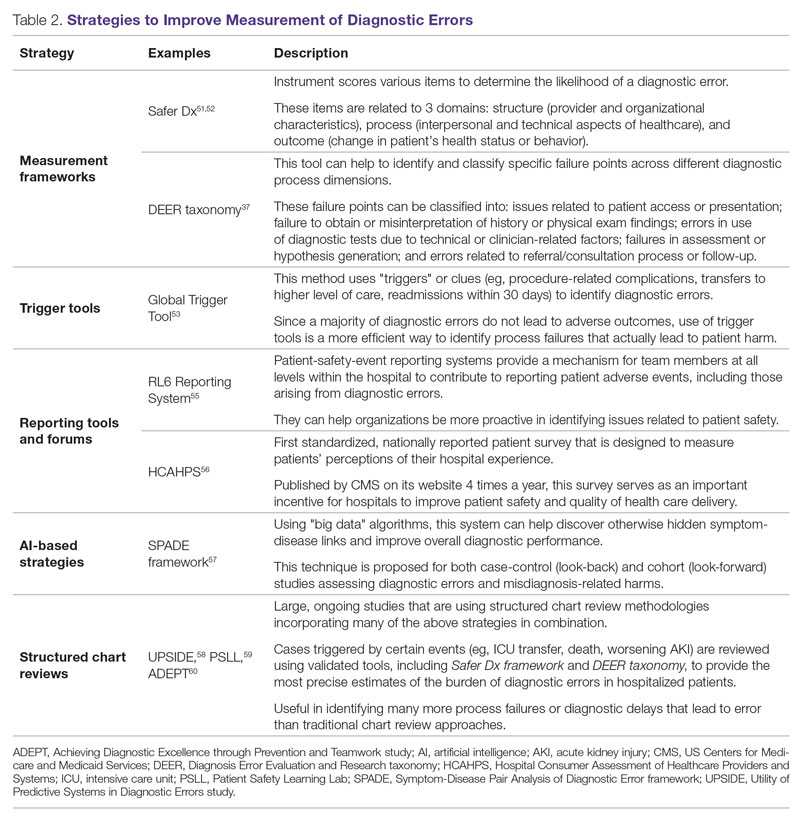
The Safer Dx framework is an all-round tool developed to advance the discipline of measuring diagnostic errors. For an episode of care under review, the instrument scores various items to determine the likelihood of a diagnostic error. These items evaluate multiple dimensions affecting diagnostic performance and measurements across 3 broad domains: structure (provider and organizational characteristics—from everyone involved with patient care, to computing infrastructure, to policies and regulations), process (elements of the patient-provider encounter, diagnostic test performance and follow-up, and subspecialty- and referral-specific factors), and outcome (establishing accurate and timely diagnosis as opposed to missed, delayed, or incorrect diagnosis). This instrument has been revised and can be further modified by a variety of stakeholders, including clinicians, health care organizations, and policymakers, to identify potential diagnostic errors in a standardized way for patient safety and quality improvement research.51,52
Use of standardized tools, such as the Diagnosis Error Evaluation and Research (DEER) taxonomy, can help to identify and classify specific failure points across different diagnostic process dimensions.37 These failure points can be classified into: issues related to patient presentation or access to health care; failure to obtain or misinterpretation of history or physical exam findings; errors in use of diagnostics tests due to technical or clinician-related factors; failures in appropriate weighing of evidence and hypothesis generation; errors associated with referral or consultation process; and failure to monitor the patient or obtain timely follow-up.34 The DEER taxonomy can also be modified based on specific research questions and study populations. Further, it can be recategorized to correspond to Safer Dx framework diagnostic process dimensions to provide insights into reasons for specific process failures and to develop new interventions to mitigate errors and patient harm.6
Since a majority of diagnostic errors do not lead to actual harm, use of “triggers” or clues (eg, procedure-related complications, patient falls, transfers to a higher level of care, readmissions within 30 days) can be a more efficient method to identify diagnostic errors and adverse events that do cause harm. The Global Trigger Tool, developed by the Institute for Healthcare Improvement, uses this strategy. This tool has been shown to identify a significantly higher number of serious adverse events than comparable methods.53 This facilitates selection and development of strategies at the institutional level that are most likely to improve patient outcomes.24
Encouraging and facilitating voluntary or prompted reporting from patients and clinicians can also play an important role in capturing diagnostic errors. Patients and clinicians are not only the key stakeholders but are also uniquely placed within the diagnostic process to detect and report potential errors.25,54 Patient-safety-event reporting systems, such as RL6, play a vital role in reporting near-misses and adverse events. These systems provide a mechanism for team members at all levels within the hospital to contribute toward reporting patient adverse events, including those arising from diagnostic errors.55 The Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey is the first standardized, nationally reported patient survey designed to measure patients’ perceptions of their hospital experience. The US Centers for Medicare and Medicaid Services (CMS) publishes HCAHPS results on its website 4 times a year, which serves as an important incentive for hospitals to improve patient safety and quality of health care delivery.56
Another novel approach links multiple symptoms to a range of target diseases using the Symptom-Disease Pair Analysis of Diagnostic Error (SPADE) framework. Using “big data” technologies, this technique can help discover otherwise hidden symptom-disease links and improve overall diagnostic performance. This approach is proposed for both case-control (look-back) and cohort (look-forward) studies assessing diagnostic errors and misdiagnosis-related harms. For example, starting with a known diagnosis with high potential for harm (eg, stroke), the “look-back” approach can be used to identify high-risk symptoms (eg, dizziness, vertigo). In the “look-forward” approach, a single symptom or exposure risk factor known to be frequently misdiagnosed (eg, dizziness) can be analyzed to identify potential adverse disease outcomes (eg, stroke, migraine).57
Many large ongoing studies looking at diagnostic errors among hospitalized patients, such as Utility of Predictive Systems to identify Inpatient Diagnostic Errors (UPSIDE),58 Patient Safety Learning Lab (PSLL),59 and Achieving Diagnostic Excellence through Prevention and Teamwork (ADEPT),60 are using structured chart review methodologies incorporating many of the above strategies in combination. Cases triggered by certain events (eg, ICU transfer, death, rapid response event, new or worsening acute kidney injury) are reviewed using validated tools, including Safer Dx framework and DEER taxonomy, to provide the most precise estimates of the burden of diagnostic errors in hospitalized patients. These estimates may be much higher than previously predicted using traditional chart review approaches.6,24 For example, a recently published study of 2809 random admissions in 11 Massachusetts hospitals identified 978 adverse events but only 10 diagnostic errors (diagnostic error rate, 0.4%).19 This was likely because the trigger method used in the study did not specifically examine the diagnostic process as critically as done by the Safer Dx framework and DEER taxonomy tools, thereby underestimating the total number of diagnostic errors. Further, these ongoing studies (eg, UPSIDE, ADEPT) aim to employ new and upcoming advanced machine-learning methods to create models that can improve overall diagnostic performance. This would pave the way to test and build novel, efficient, and scalable interventions to reduce diagnostic errors and improve patient outcomes.
Strategies to Improve Diagnostic Safety in Hospitalized Patients
Disease-specific biomedical research, as well as advances in laboratory, imaging, and other technologies, play a critical role in improving diagnostic accuracy. However, these technical approaches do not address many of the broader clinician- and system-level failure points and opportunities for improvement. Various patient-, provider-, and organizational-level interventions that could make diagnostic processes more resilient and reduce the risk of error and patient harm have been proposed.61
Among these strategies are approaches to empower patients and their families. Fostering therapeutic relationships between patients and members of the care team is essential to reducing diagnostic errors.62 Facilitating timely access to health records, ensuring transparency in decision making, and tailoring communication strategies to patients’ cultural and educational backgrounds can reduce harm.63 Similarly, at the system level, enhancing communication among different providers by use of tools such as structured handoffs can prevent communication breakdowns and facilitate positive outcomes.64
Interventions targeted at individual health care providers, such as educational programs to improve content-specific knowledge, can enhance diagnostic performance. Regular feedback, strategies to enhance equity, and fostering an environment where all providers are actively encouraged to think critically and participate in the diagnostic process (training programs to use “diagnostic time-outs” and making it a “team sport”) can improve clinical reasoning.65,66 Use of standardized patients can help identify individual-level cognitive failure points and facilitate creation of new interventions to improve clinical decision-making processes.67
Novel health information technologies can further augment these efforts. These include effective documentation by maintaining dynamic and accurate patient histories, problem lists, and medication lists68-70; use of electronic health record–based algorithms to identify potential diagnostic delays for serious conditions71,72; use of telemedicine technologies to improve accessibility and coordination73; application of mobile health and wearable technologies to facilitate data-gathering and care delivery74,75; and use of computerized decision-support tools, including applications to interpret electrocardiograms, imaging studies, and other diagnostic tests.76
Use of precision medicine, powered by new artificial intelligence (AI) tools, is becoming more widespread. Algorithms powered by AI can augment and sometimes even outperform clinician decision-making in areas such as oncology, radiology, and primary care.77 Creation of large biobanks like the All of Us research program can be used to study thousands of environmental and genetic risk factors and health conditions simultaneously, and help identify specific treatments that work best for people of different backgrounds.78 Active research in these areas holds great promise in terms of how and when we diagnose diseases and make appropriate preventative and treatment decisions. Significant scientific, ethical, and regulatory challenges will need to be overcome before these technologies can address some of the most complex problems in health care.79
Finally, diagnostic performance is affected by the external environment, including the functioning of the medical liability system. Diagnostic errors that lead to patient harm are a leading cause of malpractice claims.80 Developing a legal environment, in collaboration with patient advocacy groups and health care organizations, that promotes and facilitates timely disclosure of diagnostic errors could decrease the incentive to hide errors, advance care processes, and improve outcomes.81,82
Conclusion
The burden of diagnostic errors in hospitalized patients is unacceptably high and remains an underemphasized cause of preventable morbidity and mortality. Diagnostic errors often result from a breakdown in multiple interdependent processes that involve patient-, provider-, and system-level factors. Significant challenges remain in defining and identifying diagnostic errors as well as underlying process-failure points. The most effective interventions to reduce diagnostic errors will require greater patient participation in the diagnostic process and a mix of evidence-based interventions that promote individual-provider excellence as well as system-level changes. Further research and collaboration among various stakeholders should help improve diagnostic safety for hospitalized patients.
Corresponding author: Abhishek Goyal, MD, MPH; [email protected]
Disclosures: Dr. Dalal disclosed receiving income ≥ $250 from MayaMD.
1. Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165(13):1493-1499. doi:10.1001/archinte.165.13.1493
2. National Academies of Sciences, Engineering, and Medicine. 2015. Improving Diagnosis in Health Care. The National Academies Press. doi:10.17226/21794
3. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373(26):2493-2495. doi:10.1056/NEJMp1512241
4. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139. doi:10.1136/bmj.i2139
5. Flanders SA, Centor B, Weber V, McGinn T, Desalvo K, Auerbach A. Challenges and opportunities in academic hospital medicine: report from the academic hospital medicine summit. J Gen Intern Med. 2009;24(5):636-641. doi:10.1007/s11606-009-0944-6
6. Griffin JA, Carr K, Bersani K, et al. Analyzing diagnostic errors in the acute setting: a process-driven approach. Diagnosis (Berl). 2021;9(1):77-88. doi:10.1515/dx-2021-0033
7. Itri JN, Tappouni RR, McEachern RO, Pesch AJ, Patel SH. Fundamentals of diagnostic error in imaging. RadioGraphics. 2018;38(6):1845-1865. doi:10.1148/rg.2018180021
8. Hammerling JA. A Review of medical errors in laboratory diagnostics and where we are today. Lab Med. 2012;43(2):41-44. doi:10.1309/LM6ER9WJR1IHQAUY
9. Gunderson CG, Bilan VP, Holleck JL, et al. Prevalence of harmful diagnostic errors in hospitalised adults: a systematic review and meta-analysis. BMJ Qual Saf. 2020;29(12):1008-1018. doi:10.1136/bmjqs-2019-010822
10. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. doi:10.1056/NEJM199102073240604
11. Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324(6):377-384. doi:10.1056/NEJM199102073240605
12. Localio AR, Lawthers AG, Brennan TA, et al. Relation between malpractice claims and adverse events due to negligence. Results of the Harvard Medical Practice Study III. N Engl J Med. 1991;325(4):245-251. doi:10.1056/NEJM199107253250405
13. Wilson RM, Michel P, Olsen S, et al. Patient safety in developing countries: retrospective estimation of scale and nature of harm to patients in hospital. BMJ. 2012;344:e832. doi:10.1136/bmj.e832
14. Wilson RM, Runciman WB, Gibberd RW, Harrison BT, Newby L, Hamilton JD. The Quality in Australian Health Care Study. Med J Aust. 1995;163(9):458-471. doi:10.5694/j.1326-5377.1995.tb124691.x
15. Thomas EJ, Studdert DM, Burstin HR, et al. Incidence and types of adverse events and negligent care in Utah and Colorado. Med Care. 2000;38(3):261-271. doi:10.1097/00005650-200003000-00003
16. Baker GR, Norton PG, Flintoft V, et al. The Canadian Adverse Events Study: the incidence of adverse events among hospital patients in Canada. CMAJ. 2004;170(11):1678-1686. doi:10.1503/cmaj.1040498
17. Davis P, Lay-Yee R, Briant R, Ali W, Scott A, Schug S. Adverse events in New Zealand public hospitals II: preventability and clinical context. N Z Med J. 2003;116(1183):U624.
18. Aranaz-Andrés JM, Aibar-Remón C, Vitaller-Murillo J, et al. Incidence of adverse events related to health care in Spain: results of the Spanish National Study of Adverse Events. J Epidemiol Community Health. 2008;62(12):1022-1029. doi:10.1136/jech.2007.065227
19. Bates DW, Levine DM, Salmasian H, et al. The safety of inpatient health care. N Engl J Med. 2023;388(2):142-153. doi:10.1056/NEJMsa2206117
20. Soop M, Fryksmark U, Köster M, Haglund B. The incidence of adverse events in Swedish hospitals: a retrospective medical record review study. Int J Qual Health Care. 2009;21(4):285-291. doi:10.1093/intqhc/mzp025
21. Rafter N, Hickey A, Conroy RM, et al. The Irish National Adverse Events Study (INAES): the frequency and nature of adverse events in Irish hospitals—a retrospective record review study. BMJ Qual Saf. 2017;26(2):111-119. doi:10.1136/bmjqs-2015-004828
22. Blendon RJ, DesRoches CM, Brodie M, et al. Views of practicing physicians and the public on medical errors. N Engl J Med. 2002;347(24):1933-1940. doi:10.1056/NEJMsa022151
23. Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986-2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672-680. doi:10.1136/bmjqs-2012-001550
24. Malik MA, Motta-Calderon D, Piniella N, et al. A structured approach to EHR surveillance of diagnostic error in acute care: an exploratory analysis of two institutionally-defined case cohorts. Diagnosis (Berl). 2022;9(4):446-457. doi:10.1515/dx-2022-0032
25. Graber ML. The incidence of diagnostic error in medicine. BMJ Qual Saf. 2013;22(suppl 2):ii21-ii27. doi:10.1136/bmjqs-2012-001615
26. Bergl PA, Taneja A, El-Kareh R, Singh H, Nanchal RS. Frequency, risk factors, causes, and consequences of diagnostic errors in critically ill medical patients: a retrospective cohort study. Crit Care Med. 2019;47(11):e902-e910. doi:10.1097/CCM.0000000000003976
27. Hogan H, Healey F, Neale G, Thomson R, Vincent C, Black N. Preventable deaths due to problems in care in English acute hospitals: a retrospective case record review study. BMJ Qual Saf. 2012;21(9):737-745. doi:10.1136/bmjqs-2011-001159
28. Bergl PA, Nanchal RS, Singh H. Diagnostic error in the critically ill: defining the problem and exploring next steps to advance intensive care unit safety. Ann Am Thorac Soc. 2018;15(8):903-907. doi:10.1513/AnnalsATS.201801-068PS
29. Marquet K, Claes N, De Troy E, et al. One fourth of unplanned transfers to a higher level of care are associated with a highly preventable adverse event: a patient record review in six Belgian hospitals. Crit Care Med. 2015;43(5):1053-1061. doi:10.1097/CCM.0000000000000932
30. Rodwin BA, Bilan VP, Merchant NB, et al. Rate of preventable mortality in hospitalized patients: a systematic review and meta-analysis. J Gen Intern Med. 2020;35(7):2099-2106. doi:10.1007/s11606-019-05592-5
31. Winters B, Custer J, Galvagno SM, et al. Diagnostic errors in the intensive care unit: a systematic review of autopsy studies. BMJ Qual Saf. 2012;21(11):894-902. doi:10.1136/bmjqs-2012-000803
32. Raffel KE, Kantor MA, Barish P, et al. Prevalence and characterisation of diagnostic error among 7-day all-cause hospital medicine readmissions: a retrospective cohort study. BMJ Qual Saf. 2020;29(12):971-979. doi:10.1136/bmjqs-2020-010896
33. Weingart SN, Pagovich O, Sands DZ, et al. What can hospitalized patients tell us about adverse events? learning from patient-reported incidents. J Gen Intern Med. 2005;20(9):830-836. doi:10.1111/j.1525-1497.2005.0180.x
34. Schiff GD, Hasan O, Kim S, et al. Diagnostic error in medicine: analysis of 583 physician-reported errors. Arch Intern Med. 2009;169(20):1881-1887. doi:10.1001/archinternmed.2009.333
35. Singh H, Schiff GD, Graber ML, Onakpoya I, Thompson MJ. The global burden of diagnostic errors in primary care. BMJ Qual Saf. 2017;26(6):484-494. doi:10.1136/bmjqs-2016-005401
36. Schiff GD, Leape LL. Commentary: how can we make diagnosis safer? Acad Med J Assoc Am Med Coll. 2012;87(2):135-138. doi:10.1097/ACM.0b013e31823f711c
37. Schiff GD, Kim S, Abrams R, et al. Diagnosing diagnosis errors: lessons from a multi-institutional collaborative project. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety: From Research to Implementation. Volume 2: Concepts and Methodology. AHRQ Publication No. 05-0021-2. Agency for Healthcare Research and Quality (US); 2005. Accessed January 16, 2023. http://www.ncbi.nlm.nih.gov/books/NBK20492/
38. Newman-Toker DE. A unified conceptual model for diagnostic errors: underdiagnosis, overdiagnosis, and misdiagnosis. Diagnosis (Berl). 2014;1(1):43-48. doi:10.1515/dx-2013-0027
39. Abimanyi-Ochom J, Bohingamu Mudiyanselage S, Catchpool M, Firipis M, Wanni Arachchige Dona S, Watts JJ. Strategies to reduce diagnostic errors: a systematic review. BMC Med Inform Decis Mak. 2019;19(1):174. doi:10.1186/s12911-019-0901-1
40. Gupta A, Harrod M, Quinn M, et al. Mind the overlap: how system problems contribute to cognitive failure and diagnostic errors. Diagnosis (Berl). 2018;5(3):151-156. doi:10.1515/dx-2018-0014
41. Saposnik G, Redelmeier D, Ruff CC, Tobler PN. Cognitive biases associated with medical decisions: a systematic review. BMC Med Inform Decis Mak. 2016;16:138. doi:10.1186/s12911-016-0377-1
42. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78(8):775-780. doi: 10.1097/00001888-200308000-00003
43. Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med. 2013;28(11):1504-1510. doi:10.1007/s11606-013-2441-1
44. Zwaan L, Singh H. The challenges in defining and measuring diagnostic error. Diagnosis (Ber). 2015;2(2):97-103. doi:10.1515/dx-2014-0069
45. Arkes HR, Wortmann RL, Saville PD, Harkness AR. Hindsight bias among physicians weighing the likelihood of diagnoses. J Appl Psychol. 1981;66(2):252-254.
46. Singh H. Editorial: Helping health care organizations to define diagnostic errors as missed opportunities in diagnosis. Jt Comm J Qual Patient Saf. 2014;40(3):99-101. doi:10.1016/s1553-7250(14)40012-6
47. Vassar M, Holzmann M. The retrospective chart review: important methodological considerations. J Educ Eval Health Prof. 2013;10:12. doi:10.3352/jeehp.2013.10.12
48. Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605-613. doi:10.1093/jnci/djq099
49. Moynihan R, Doust J, Henry D. Preventing overdiagnosis: how to stop harming the healthy. BMJ. 2012;344:e3502. doi:10.1136/bmj.e3502
50. Hayward RA, Hofer TP. Estimating hospital deaths due to medical errors: preventability is in the eye of the reviewer. JAMA. 2001;286(4):415-420. doi:10.1001/jama.286.4.415
51. Singh H, Sittig DF. Advancing the science of measurement of diagnostic errors in healthcare: the Safer Dx framework. BMJ Qual Saf. 2015;24(2):103-110. doi:10.1136/bmjqs-2014-003675
52. Singh H, Khanna A, Spitzmueller C, Meyer AND. Recommendations for using the Revised Safer Dx Instrument to help measure and improve diagnostic safety. Diagnosis (Berl). 2019;6(4):315-323. doi:10.1515/dx-2019-0012
53. Classen DC, Resar R, Griffin F, et al. “Global trigger tool” shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff (Millwood). 2011;30(4):581-589. doi:10.1377/hlthaff.2011.0190
54. Schiff GD. Minimizing diagnostic error: the importance of follow-up and feedback. Am J Med. 2008;121(5 suppl):S38-S42. doi:10.1016/j.amjmed.2008.02.004
55. Mitchell I, Schuster A, Smith K, Pronovost P, Wu A. Patient safety incident reporting: a qualitative study of thoughts and perceptions of experts 15 years after “To Err is Human.” BMJ Qual Saf. 2016;25(2):92-99. doi:10.1136/bmjqs-2015-004405
56. Mazurenko O, Collum T, Ferdinand A, Menachemi N. Predictors of hospital patient satisfaction as measured by HCAHPS: a systematic review. J Healthc Manag. 2017;62(4):272-283. doi:10.1097/JHM-D-15-00050
57. Liberman AL, Newman-Toker DE. Symptom-Disease Pair Analysis of Diagnostic Error (SPADE): a conceptual framework and methodological approach for unearthing misdiagnosis-related harms using big data. BMJ Qual Saf. 2018;27(7):557-566. doi:10.1136/bmjqs-2017-007032
58. Utility of Predictive Systems to Identify Inpatient Diagnostic Errors: the UPSIDE study. NIH RePort/RePORTER. Accessed January 14, 2023. https://reporter.nih.gov/search/rpoHXlEAcEudQV3B9ld8iw/project-details/10020962
59. Overview of Patient Safety Learning Laboratory (PSLL) Projects. Agency for Healthcare Research and Quality. Accessed January 14, 2023. https://www.ahrq.gov/patient-safety/resources/learning-lab/index.html
60. Achieving Diagnostic Excellence through Prevention and Teamwork (ADEPT). NIH RePort/RePORTER. Accessed January 14, 2023. https://reporter.nih.gov/project-details/10642576
61. Zwaan L, Singh H. Diagnostic error in hospitals: finding forests not just the big trees. BMJ Qual Saf. 2020;29(12):961-964. doi:10.1136/bmjqs-2020-011099
62. Longtin Y, Sax H, Leape LL, Sheridan SE, Donaldson L, Pittet D. Patient participation: current knowledge and applicability to patient safety. Mayo Clin Proc. 2010;85(1):53-62. doi:10.4065/mcp.2009.0248
63. Murphy DR, Singh H, Berlin L. Communication breakdowns and diagnostic errors: a radiology perspective. Diagnosis (Berl). 2014;1(4):253-261. doi:10.1515/dx-2014-0035
64. Singh H, Naik AD, Rao R, Petersen LA. Reducing diagnostic errors through effective communication: harnessing the power of information technology. J Gen Intern Med. 2008;23(4):489-494. doi:10.1007/s11606-007-0393-z
65. Singh H, Connor DM, Dhaliwal G. Five strategies for clinicians to advance diagnostic excellence. BMJ. 2022;376:e068044. doi:10.1136/bmj-2021-068044
66. Yale S, Cohen S, Bordini BJ. Diagnostic time-outs to improve diagnosis. Crit Care Clin. 2022;38(2):185-194. doi:10.1016/j.ccc.2021.11.008
67. Schwartz A, Peskin S, Spiro A, Weiner SJ. Impact of unannounced standardized patient audit and feedback on care, documentation, and costs: an experiment and claims analysis. J Gen Intern Med. 2021;36(1):27-34. doi:10.1007/s11606-020-05965-1
68. Carpenter JD, Gorman PN. Using medication list—problem list mismatches as markers of potential error. Proc AMIA Symp. 2002:106-110.
69. Hron JD, Manzi S, Dionne R, et al. Electronic medication reconciliation and medication errors. Int J Qual Health Care. 2015;27(4):314-319. doi:10.1093/intqhc/mzv046
70. Graber ML, Siegal D, Riah H, Johnston D, Kenyon K. Electronic health record–related events in medical malpractice claims. J Patient Saf. 2019;15(2):77-85. doi:10.1097/PTS.0000000000000240
71. Murphy DR, Wu L, Thomas EJ, Forjuoh SN, Meyer AND, Singh H. Electronic trigger-based intervention to reduce delays in diagnostic evaluation for cancer: a cluster randomized controlled trial. J Clin Oncol. 2015;33(31):3560-3567. doi:10.1200/JCO.2015.61.1301
72. Singh H, Giardina TD, Forjuoh SN, et al. Electronic health record-based surveillance of diagnostic errors in primary care. BMJ Qual Saf. 2012;21(2):93-100. doi:10.1136/bmjqs-2011-000304
73. Armaignac DL, Saxena A, Rubens M, et al. Impact of telemedicine on mortality, length of stay, and cost among patients in progressive care units: experience from a large healthcare system. Crit Care Med. 2018;46(5):728-735. doi:10.1097/CCM.0000000000002994
74. MacKinnon GE, Brittain EL. Mobile health technologies in cardiopulmonary disease. Chest. 2020;157(3):654-664. doi:10.1016/j.chest.2019.10.015
75. DeVore AD, Wosik J, Hernandez AF. The future of wearables in heart failure patients. JACC Heart Fail. 2019;7(11):922-932. doi:10.1016/j.jchf.2019.08.008
76. Tsai TL, Fridsma DB, Gatti G. Computer decision support as a source of interpretation error: the case of electrocardiograms. J Am Med Inform Assoc. 2003;10(5):478-483. doi:10.1197/jamia.M1279
77. Lin SY, Mahoney MR, Sinsky CA. Ten ways artificial intelligence will transform primary care. J Gen Intern Med. 2019;34(8):1626-1630. doi:10.1007/s11606-019-05035-1
78. Ramirez AH, Gebo KA, Harris PA. Progress with the All Of Us research program: opening access for researchers. JAMA. 2021;325(24):2441-2442. doi:10.1001/jama.2021.7702
79. Johnson KB, Wei W, Weeraratne D, et al. Precision medicine, AI, and the future of personalized health care. Clin Transl Sci. 2021;14(1):86-93. doi:10.1111/cts.12884
80. Gupta A, Snyder A, Kachalia A, Flanders S, Saint S, Chopra V. Malpractice claims related to diagnostic errors in the hospital. BMJ Qual Saf. 2017;27(1):bmjqs-2017-006774. doi:10.1136/bmjqs-2017-006774
81. Renkema E, Broekhuis M, Ahaus K. Conditions that influence the impact of malpractice litigation risk on physicians’ behavior regarding patient safety. BMC Health Serv Res. 2014;14(1):38. doi:10.1186/1472-6963-14-38
82. Kachalia A, Mello MM, Nallamothu BK, Studdert DM. Legal and policy interventions to improve patient safety. Circulation. 2016;133(7):661-671. doi:10.1161/CIRCULATIONAHA.115.015880
Abstract
Diagnostic errors in hospitalized patients are a leading cause of preventable morbidity and mortality. Significant challenges in defining and measuring diagnostic errors and underlying process failure points have led to considerable variability in reported rates of diagnostic errors and adverse outcomes. In this article, we explore the diagnostic process and its discrete components, emphasizing the centrality of the patient in decision-making as well as the continuous nature of the process. We review the incidence of diagnostic errors in hospitalized patients and different methodological approaches that have been used to arrive at these estimates. We discuss different but interdependent provider- and system-related process-failure points that lead to diagnostic errors. We examine specific challenges related to measurement of diagnostic errors and describe traditional and novel approaches that are being used to obtain the most precise estimates. Finally, we examine various patient-, provider-, and organizational-level interventions that have been proposed to improve diagnostic safety in hospitalized patients.
Keywords: diagnostic error, hospital medicine, patient safety.
Diagnosis is defined as a “pre-existing set of categories agreed upon by the medical profession to designate a specific condition.”1 The diagnostic process involves obtaining a clinical history, performing a physical examination, conducting diagnostic testing, and consulting with other clinical providers to gather data that are relevant to understanding the underlying disease processes. This exercise involves generating hypotheses and updating prior probabilities as more information and evidence become available. Throughout this process of information gathering, integration, and interpretation, there is an ongoing assessment of whether sufficient and necessary knowledge has been obtained to make an accurate diagnosis and provide appropriate treatment.2
Diagnostic error is defined as a missed opportunity to make a timely diagnosis as part of this iterative process, including the failure of communicating the diagnosis to the patient in a timely manner.3 It can be categorized as a missed, delayed, or incorrect diagnosis based on available evidence at the time. Establishing the correct diagnosis has important implications. A timely and precise diagnosis ensures the patient the highest probability of having a positive health outcome that reflects an appropriate understanding of underlying disease processes and is consistent with their overall goals of care.3 When diagnostic errors occur, they can cause patient harm. Adverse events due to medical errors, including diagnostic errors, are estimated to be the third leading cause of death in the United States.4 Most people will experience at least 1 diagnostic error in their lifetime. In the 2015 National Academy of Medicine report Improving Diagnosis in Healthcare, diagnostic errors were identified as a major hazard as well as an opportunity to improve patient outcomes.2
Diagnostic errors during hospitalizations are especially concerning, as they are more likely to be implicated in a wider spectrum of harm, including permanent disability and death. This has become even more relevant for hospital medicine physicians and other clinical providers as they encounter increasing cognitive and administrative workloads, rising dissatisfaction and burnout, and unique obstacles such as night-time scheduling.5
Incidence of Diagnostic Errors in Hospitalized Patients
Several methodological approaches have been used to estimate the incidence of diagnostic errors in hospitalized patients. These include retrospective reviews of a sample of all hospital admissions, evaluations of selected adverse outcomes including autopsy studies, patient and provider surveys, and malpractice claims. Laboratory testing audits and secondary reviews in other diagnostic subspecialities (eg, radiology, pathology, and microbiology) are also essential to improving diagnostic performance in these specialized fields, which in turn affects overall hospital diagnostic error rates.6-8 These diverse approaches provide unique insights regarding our ability to assess the degree to which potential harms, ranging from temporary impairment to permanent disability, to death, are attributable to different failure points in the diagnostic process.
Large retrospective chart reviews of random hospital admissions remain the most accurate way to determine the overall incidence of diagnostic errors in hospitalized patients.9 The Harvard Medical Practice Study, published in 1991, laid the groundwork for measuring the incidence of adverse events in hospitalized patients and assessing their relation to medical error, negligence, and disability. Reviewing 30,121 randomly selected records from 51 randomly selected acute care hospitals in New York State, the study found that adverse events occurred in 3.7% of hospitalizations, diagnostic errors accounted for 13.8% of these events, and these errors were likely attributable to negligence in 74.7% of cases. The study not only outlined individual-level process failures, but also focused attention on some of the systemic causes, setting the agenda for quality improvement research in hospital-based care for years to come.10-12 A recent systematic review and meta-analysis of 22 hospital admission studies found a pooled rate of 0.7% (95% CI, 0.5%-1.1%) for harmful diagnostic errors.9 It found significant variations in the rates of adverse events, diagnostic errors, and range of diagnoses that were missed. This was primarily because of variabilities in pre-test probabilities in detecting diagnostic errors in these specific cohorts, as well as due to heterogeneity in study definitions and methodologies, especially regarding how they defined and measured “diagnostic error.” The analysis, however, did not account for diagnostic errors that were not related to patient harm (missed opportunities); therefore, it likely significantly underestimated the true incidence of diagnostic errors in these study populations. Table 1 summarizes some of key studies that have examined the incidence of harmful diagnostic errors in hospitalized patients.9-21

The chief limitation of reviewing random hospital admissions is that, since overall rates of diagnostic errors are still relatively low, a large number of case reviews are required to identify a sufficient sample of adverse outcomes to gain a meaningful understanding of the underlying process failure points and develop tools for remediation. Patient and provider surveys or data from malpractice claims can be high-yield starting points for research on process errors.22,23 Reviews of enriched cohorts of adverse outcomes, such as rapid-response events, intensive care unit (ICU) transfers, deaths, and hospital readmissions, can be an efficient way to identify process failures that lead to greatest harm. Depending on the research approach and the types of underlying patient populations sampled, rates of diagnostic errors in these high-risk groups have been estimated to be approximately 5% to 20%, or even higher.6,24-31 For example, a retrospective study of 391 cases of unplanned 7-day readmissions found that 5.6% of cases contained at least 1 diagnostic error during the index admission.32 In a study conducted at 6 Belgian acute-care hospitals, 56% of patients requiring an unplanned transfer to a higher level of care were determined to have had an adverse event, and of these adverse events, 12.4% of cases were associated with errors in diagnosis.29 A systematic review of 16 hospital-based studies estimated that 3.1% of all inpatient deaths were likely preventable, which corresponded to 22,165 deaths annually in the United States.30 Another such review of 31 autopsy studies reported that 28% of autopsied ICU patients had at least 1 misdiagnosis; of these diagnostic errors, 8% were classified as potentially lethal, and 15% were considered major but not lethal.31 Significant drawbacks of such enriched cohort studies, however, are their poor generalizability and inability to detect failure points that do not lead to patient harm (near-miss events).33
Causes of Diagnostic Errors in Hospitalized Patients
All aspects of the diagnostic process are susceptible to errors. These errors stem from a variety of faulty processes, including failure of the patient to engage with the health care system (eg, due to lack of insurance or transportation, or delay in seeking care); failure in information gathering (eg, missed history or exam findings, ordering wrong tests, laboratory errors); failure in information interpretation (eg, exam finding or test result misinterpretation); inaccurate hypothesis generation (eg, due to suboptimal prioritization or weighing of supporting evidence); and failure in communication (eg, with other team members or with the patient).2,34 Reasons for diagnostic process failures vary widely across different health care settings. While clinician assessment errors (eg, failure to consider or alternatively overweigh competing diagnoses) and errors in testing and the monitoring phase (eg, failure to order or follow up diagnostic tests) can lead to a majority of diagnostic errors in some patient populations, in other settings, social (eg, poor health literacy, punitive cultural practices) and economic factors (eg, lack of access to appropriate diagnostic tests or to specialty expertise) play a more prominent role.34,35
The Figure describes the relationship between components of the diagnostic process and subsequent outcomes, including diagnostic process failures, diagnostic errors, and absence or presence of patient harm.2,36,37 It reemphasizes the centrality of the patient in decision-making and the continuous nature of the process. The Figure also illustrates that only a minority of process failures result in diagnostic errors, and a smaller proportion of diagnostic errors actually lead to patient harm. Conversely, it also shows that diagnostic errors can happen without any obvious process-failure points, and, similarly, patient harm can take place in the absence of any evident diagnostic errors.36-38 Finally, it highlights the need to incorporate feedback from process failures, diagnostic errors, and favorable and unfavorable patient outcomes in order to inform future quality improvement efforts and research.

A significant proportion of diagnostic errors are due to system-related vulnerabilities, such as limitations in availability, adoption or quality of work force training, health informatics resources, and diagnostic capabilities. Lack of institutional culture that promotes safety and transparency also predisposes to diagnostic errors.39,40 The other major domain of process failures is related to cognitive errors in clinician decision-making. Anchoring, confirmation bias, availability bias, and base-rate neglect are some of the common cognitive biases that, along with personality traits (aversion to risk or ambiguity, overconfidence) and affective biases (influence of emotion on decision-making), often determine the degree of utilization of resources and the possibility of suboptimal diagnostic performance.41,42 Further, implicit biases related to age, race, gender, and sexual orientation contribute to disparities in access to health care and outcomes.43 In a large number of cases of preventable adverse outcomes, however, there are multiple interdependent individual and system-related failure points that lead to diagnostic error and patient harm.6,32
Challenges in Defining and Measuring Diagnostic Errors
In order to develop effective, evidence-based interventions to reduce diagnostic errors in hospitalized patients, it is essential to be able to first operationally define, and then accurately measure, diagnostic errors and the process failures that contribute to these errors in a standardized way that is reproducible across different settings.6,44 There are a number of obstacles in this endeavor.
A fundamental problem is that establishing a diagnosis is not a single act but a process. Patterns of symptoms and clinical presentations often differ for the same disease. Information required to make a diagnosis is usually gathered in stages, where the clinician obtains additional data, while considering many possibilities, of which 1 may be ultimately correct. Diagnoses evolve over time and in different care settings. “The most likely diagnosis” is not always the same as “the final correct diagnosis.” Moreover, the diagnostic process is influenced by patients’ individual clinical courses and preferences over time. This makes determination of missed, delayed, or incorrect diagnoses challenging.45,46
For hospitalized patients, generally the goal is to first rule out more serious and acute conditions (eg, pulmonary embolism or stroke), even if their probability is rather low. Conversely, a diagnosis that appears less consequential if delayed (eg, chronic anemia of unclear etiology) might not be pursued on an urgent basis, and is often left to outpatient providers to examine, but still may manifest in downstream harm (eg, delayed diagnosis of gastrointestinal malignancy or recurrent admissions for heart failure due to missed iron-deficiency anemia). Therefore, coming up with disease diagnosis likelihoods in hindsight may turn out to be highly subjective and not always accurate. This can be particularly difficult when clinician and other team deliberations are not recorded in their entirety.47
Another hurdle in the practice of diagnostic medicine is to preserve the balance between underdiagnosing versus pursuing overly aggressive diagnostic approaches. Conducting laboratory, imaging, or other diagnostic studies without a clear shared understanding of how they would affect clinical decision-making (eg, use of prostate-specific antigen to detect prostate cancer) not only leads to increased costs but can also delay appropriate care. Worse, subsequent unnecessary diagnostic tests and treatments can sometimes lead to serious harm.48,49
Finally, retrospective reviews by clinicians are subject to multiple potential limitations that include failure to create well-defined research questions, poorly developed inclusion and exclusion criteria, and issues related to inter- and intra-rater reliability.50 These methodological deficiencies can occur despite following "best practice" guidelines during the study planning, execution, and analysis phases. They further add to the challenge of defining and measuring diagnostic errors.47
Strategies to Improve Measurement of Diagnostic Errors
Development of new methodologies to reliably measure diagnostic errors is an area of active research. The advancement of uniform and universally agreed-upon frameworks to define and identify process failure points and diagnostic errors would help reduce measurement error and support development and testing of interventions that could be generalizable across different health care settings. To more accurately define and measure diagnostic errors, several novel approaches have been proposed (Table 2).

The Safer Dx framework is an all-round tool developed to advance the discipline of measuring diagnostic errors. For an episode of care under review, the instrument scores various items to determine the likelihood of a diagnostic error. These items evaluate multiple dimensions affecting diagnostic performance and measurements across 3 broad domains: structure (provider and organizational characteristics—from everyone involved with patient care, to computing infrastructure, to policies and regulations), process (elements of the patient-provider encounter, diagnostic test performance and follow-up, and subspecialty- and referral-specific factors), and outcome (establishing accurate and timely diagnosis as opposed to missed, delayed, or incorrect diagnosis). This instrument has been revised and can be further modified by a variety of stakeholders, including clinicians, health care organizations, and policymakers, to identify potential diagnostic errors in a standardized way for patient safety and quality improvement research.51,52
Use of standardized tools, such as the Diagnosis Error Evaluation and Research (DEER) taxonomy, can help to identify and classify specific failure points across different diagnostic process dimensions.37 These failure points can be classified into: issues related to patient presentation or access to health care; failure to obtain or misinterpretation of history or physical exam findings; errors in use of diagnostics tests due to technical or clinician-related factors; failures in appropriate weighing of evidence and hypothesis generation; errors associated with referral or consultation process; and failure to monitor the patient or obtain timely follow-up.34 The DEER taxonomy can also be modified based on specific research questions and study populations. Further, it can be recategorized to correspond to Safer Dx framework diagnostic process dimensions to provide insights into reasons for specific process failures and to develop new interventions to mitigate errors and patient harm.6
Since a majority of diagnostic errors do not lead to actual harm, use of “triggers” or clues (eg, procedure-related complications, patient falls, transfers to a higher level of care, readmissions within 30 days) can be a more efficient method to identify diagnostic errors and adverse events that do cause harm. The Global Trigger Tool, developed by the Institute for Healthcare Improvement, uses this strategy. This tool has been shown to identify a significantly higher number of serious adverse events than comparable methods.53 This facilitates selection and development of strategies at the institutional level that are most likely to improve patient outcomes.24
Encouraging and facilitating voluntary or prompted reporting from patients and clinicians can also play an important role in capturing diagnostic errors. Patients and clinicians are not only the key stakeholders but are also uniquely placed within the diagnostic process to detect and report potential errors.25,54 Patient-safety-event reporting systems, such as RL6, play a vital role in reporting near-misses and adverse events. These systems provide a mechanism for team members at all levels within the hospital to contribute toward reporting patient adverse events, including those arising from diagnostic errors.55 The Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey is the first standardized, nationally reported patient survey designed to measure patients’ perceptions of their hospital experience. The US Centers for Medicare and Medicaid Services (CMS) publishes HCAHPS results on its website 4 times a year, which serves as an important incentive for hospitals to improve patient safety and quality of health care delivery.56
Another novel approach links multiple symptoms to a range of target diseases using the Symptom-Disease Pair Analysis of Diagnostic Error (SPADE) framework. Using “big data” technologies, this technique can help discover otherwise hidden symptom-disease links and improve overall diagnostic performance. This approach is proposed for both case-control (look-back) and cohort (look-forward) studies assessing diagnostic errors and misdiagnosis-related harms. For example, starting with a known diagnosis with high potential for harm (eg, stroke), the “look-back” approach can be used to identify high-risk symptoms (eg, dizziness, vertigo). In the “look-forward” approach, a single symptom or exposure risk factor known to be frequently misdiagnosed (eg, dizziness) can be analyzed to identify potential adverse disease outcomes (eg, stroke, migraine).57
Many large ongoing studies looking at diagnostic errors among hospitalized patients, such as Utility of Predictive Systems to identify Inpatient Diagnostic Errors (UPSIDE),58 Patient Safety Learning Lab (PSLL),59 and Achieving Diagnostic Excellence through Prevention and Teamwork (ADEPT),60 are using structured chart review methodologies incorporating many of the above strategies in combination. Cases triggered by certain events (eg, ICU transfer, death, rapid response event, new or worsening acute kidney injury) are reviewed using validated tools, including Safer Dx framework and DEER taxonomy, to provide the most precise estimates of the burden of diagnostic errors in hospitalized patients. These estimates may be much higher than previously predicted using traditional chart review approaches.6,24 For example, a recently published study of 2809 random admissions in 11 Massachusetts hospitals identified 978 adverse events but only 10 diagnostic errors (diagnostic error rate, 0.4%).19 This was likely because the trigger method used in the study did not specifically examine the diagnostic process as critically as done by the Safer Dx framework and DEER taxonomy tools, thereby underestimating the total number of diagnostic errors. Further, these ongoing studies (eg, UPSIDE, ADEPT) aim to employ new and upcoming advanced machine-learning methods to create models that can improve overall diagnostic performance. This would pave the way to test and build novel, efficient, and scalable interventions to reduce diagnostic errors and improve patient outcomes.
Strategies to Improve Diagnostic Safety in Hospitalized Patients
Disease-specific biomedical research, as well as advances in laboratory, imaging, and other technologies, play a critical role in improving diagnostic accuracy. However, these technical approaches do not address many of the broader clinician- and system-level failure points and opportunities for improvement. Various patient-, provider-, and organizational-level interventions that could make diagnostic processes more resilient and reduce the risk of error and patient harm have been proposed.61
Among these strategies are approaches to empower patients and their families. Fostering therapeutic relationships between patients and members of the care team is essential to reducing diagnostic errors.62 Facilitating timely access to health records, ensuring transparency in decision making, and tailoring communication strategies to patients’ cultural and educational backgrounds can reduce harm.63 Similarly, at the system level, enhancing communication among different providers by use of tools such as structured handoffs can prevent communication breakdowns and facilitate positive outcomes.64
Interventions targeted at individual health care providers, such as educational programs to improve content-specific knowledge, can enhance diagnostic performance. Regular feedback, strategies to enhance equity, and fostering an environment where all providers are actively encouraged to think critically and participate in the diagnostic process (training programs to use “diagnostic time-outs” and making it a “team sport”) can improve clinical reasoning.65,66 Use of standardized patients can help identify individual-level cognitive failure points and facilitate creation of new interventions to improve clinical decision-making processes.67
Novel health information technologies can further augment these efforts. These include effective documentation by maintaining dynamic and accurate patient histories, problem lists, and medication lists68-70; use of electronic health record–based algorithms to identify potential diagnostic delays for serious conditions71,72; use of telemedicine technologies to improve accessibility and coordination73; application of mobile health and wearable technologies to facilitate data-gathering and care delivery74,75; and use of computerized decision-support tools, including applications to interpret electrocardiograms, imaging studies, and other diagnostic tests.76
Use of precision medicine, powered by new artificial intelligence (AI) tools, is becoming more widespread. Algorithms powered by AI can augment and sometimes even outperform clinician decision-making in areas such as oncology, radiology, and primary care.77 Creation of large biobanks like the All of Us research program can be used to study thousands of environmental and genetic risk factors and health conditions simultaneously, and help identify specific treatments that work best for people of different backgrounds.78 Active research in these areas holds great promise in terms of how and when we diagnose diseases and make appropriate preventative and treatment decisions. Significant scientific, ethical, and regulatory challenges will need to be overcome before these technologies can address some of the most complex problems in health care.79
Finally, diagnostic performance is affected by the external environment, including the functioning of the medical liability system. Diagnostic errors that lead to patient harm are a leading cause of malpractice claims.80 Developing a legal environment, in collaboration with patient advocacy groups and health care organizations, that promotes and facilitates timely disclosure of diagnostic errors could decrease the incentive to hide errors, advance care processes, and improve outcomes.81,82
Conclusion
The burden of diagnostic errors in hospitalized patients is unacceptably high and remains an underemphasized cause of preventable morbidity and mortality. Diagnostic errors often result from a breakdown in multiple interdependent processes that involve patient-, provider-, and system-level factors. Significant challenges remain in defining and identifying diagnostic errors as well as underlying process-failure points. The most effective interventions to reduce diagnostic errors will require greater patient participation in the diagnostic process and a mix of evidence-based interventions that promote individual-provider excellence as well as system-level changes. Further research and collaboration among various stakeholders should help improve diagnostic safety for hospitalized patients.
Corresponding author: Abhishek Goyal, MD, MPH; [email protected]
Disclosures: Dr. Dalal disclosed receiving income ≥ $250 from MayaMD.
Abstract
Diagnostic errors in hospitalized patients are a leading cause of preventable morbidity and mortality. Significant challenges in defining and measuring diagnostic errors and underlying process failure points have led to considerable variability in reported rates of diagnostic errors and adverse outcomes. In this article, we explore the diagnostic process and its discrete components, emphasizing the centrality of the patient in decision-making as well as the continuous nature of the process. We review the incidence of diagnostic errors in hospitalized patients and different methodological approaches that have been used to arrive at these estimates. We discuss different but interdependent provider- and system-related process-failure points that lead to diagnostic errors. We examine specific challenges related to measurement of diagnostic errors and describe traditional and novel approaches that are being used to obtain the most precise estimates. Finally, we examine various patient-, provider-, and organizational-level interventions that have been proposed to improve diagnostic safety in hospitalized patients.
Keywords: diagnostic error, hospital medicine, patient safety.
Diagnosis is defined as a “pre-existing set of categories agreed upon by the medical profession to designate a specific condition.”1 The diagnostic process involves obtaining a clinical history, performing a physical examination, conducting diagnostic testing, and consulting with other clinical providers to gather data that are relevant to understanding the underlying disease processes. This exercise involves generating hypotheses and updating prior probabilities as more information and evidence become available. Throughout this process of information gathering, integration, and interpretation, there is an ongoing assessment of whether sufficient and necessary knowledge has been obtained to make an accurate diagnosis and provide appropriate treatment.2
Diagnostic error is defined as a missed opportunity to make a timely diagnosis as part of this iterative process, including the failure of communicating the diagnosis to the patient in a timely manner.3 It can be categorized as a missed, delayed, or incorrect diagnosis based on available evidence at the time. Establishing the correct diagnosis has important implications. A timely and precise diagnosis ensures the patient the highest probability of having a positive health outcome that reflects an appropriate understanding of underlying disease processes and is consistent with their overall goals of care.3 When diagnostic errors occur, they can cause patient harm. Adverse events due to medical errors, including diagnostic errors, are estimated to be the third leading cause of death in the United States.4 Most people will experience at least 1 diagnostic error in their lifetime. In the 2015 National Academy of Medicine report Improving Diagnosis in Healthcare, diagnostic errors were identified as a major hazard as well as an opportunity to improve patient outcomes.2
Diagnostic errors during hospitalizations are especially concerning, as they are more likely to be implicated in a wider spectrum of harm, including permanent disability and death. This has become even more relevant for hospital medicine physicians and other clinical providers as they encounter increasing cognitive and administrative workloads, rising dissatisfaction and burnout, and unique obstacles such as night-time scheduling.5
Incidence of Diagnostic Errors in Hospitalized Patients
Several methodological approaches have been used to estimate the incidence of diagnostic errors in hospitalized patients. These include retrospective reviews of a sample of all hospital admissions, evaluations of selected adverse outcomes including autopsy studies, patient and provider surveys, and malpractice claims. Laboratory testing audits and secondary reviews in other diagnostic subspecialities (eg, radiology, pathology, and microbiology) are also essential to improving diagnostic performance in these specialized fields, which in turn affects overall hospital diagnostic error rates.6-8 These diverse approaches provide unique insights regarding our ability to assess the degree to which potential harms, ranging from temporary impairment to permanent disability, to death, are attributable to different failure points in the diagnostic process.
Large retrospective chart reviews of random hospital admissions remain the most accurate way to determine the overall incidence of diagnostic errors in hospitalized patients.9 The Harvard Medical Practice Study, published in 1991, laid the groundwork for measuring the incidence of adverse events in hospitalized patients and assessing their relation to medical error, negligence, and disability. Reviewing 30,121 randomly selected records from 51 randomly selected acute care hospitals in New York State, the study found that adverse events occurred in 3.7% of hospitalizations, diagnostic errors accounted for 13.8% of these events, and these errors were likely attributable to negligence in 74.7% of cases. The study not only outlined individual-level process failures, but also focused attention on some of the systemic causes, setting the agenda for quality improvement research in hospital-based care for years to come.10-12 A recent systematic review and meta-analysis of 22 hospital admission studies found a pooled rate of 0.7% (95% CI, 0.5%-1.1%) for harmful diagnostic errors.9 It found significant variations in the rates of adverse events, diagnostic errors, and range of diagnoses that were missed. This was primarily because of variabilities in pre-test probabilities in detecting diagnostic errors in these specific cohorts, as well as due to heterogeneity in study definitions and methodologies, especially regarding how they defined and measured “diagnostic error.” The analysis, however, did not account for diagnostic errors that were not related to patient harm (missed opportunities); therefore, it likely significantly underestimated the true incidence of diagnostic errors in these study populations. Table 1 summarizes some of key studies that have examined the incidence of harmful diagnostic errors in hospitalized patients.9-21

The chief limitation of reviewing random hospital admissions is that, since overall rates of diagnostic errors are still relatively low, a large number of case reviews are required to identify a sufficient sample of adverse outcomes to gain a meaningful understanding of the underlying process failure points and develop tools for remediation. Patient and provider surveys or data from malpractice claims can be high-yield starting points for research on process errors.22,23 Reviews of enriched cohorts of adverse outcomes, such as rapid-response events, intensive care unit (ICU) transfers, deaths, and hospital readmissions, can be an efficient way to identify process failures that lead to greatest harm. Depending on the research approach and the types of underlying patient populations sampled, rates of diagnostic errors in these high-risk groups have been estimated to be approximately 5% to 20%, or even higher.6,24-31 For example, a retrospective study of 391 cases of unplanned 7-day readmissions found that 5.6% of cases contained at least 1 diagnostic error during the index admission.32 In a study conducted at 6 Belgian acute-care hospitals, 56% of patients requiring an unplanned transfer to a higher level of care were determined to have had an adverse event, and of these adverse events, 12.4% of cases were associated with errors in diagnosis.29 A systematic review of 16 hospital-based studies estimated that 3.1% of all inpatient deaths were likely preventable, which corresponded to 22,165 deaths annually in the United States.30 Another such review of 31 autopsy studies reported that 28% of autopsied ICU patients had at least 1 misdiagnosis; of these diagnostic errors, 8% were classified as potentially lethal, and 15% were considered major but not lethal.31 Significant drawbacks of such enriched cohort studies, however, are their poor generalizability and inability to detect failure points that do not lead to patient harm (near-miss events).33
Causes of Diagnostic Errors in Hospitalized Patients
All aspects of the diagnostic process are susceptible to errors. These errors stem from a variety of faulty processes, including failure of the patient to engage with the health care system (eg, due to lack of insurance or transportation, or delay in seeking care); failure in information gathering (eg, missed history or exam findings, ordering wrong tests, laboratory errors); failure in information interpretation (eg, exam finding or test result misinterpretation); inaccurate hypothesis generation (eg, due to suboptimal prioritization or weighing of supporting evidence); and failure in communication (eg, with other team members or with the patient).2,34 Reasons for diagnostic process failures vary widely across different health care settings. While clinician assessment errors (eg, failure to consider or alternatively overweigh competing diagnoses) and errors in testing and the monitoring phase (eg, failure to order or follow up diagnostic tests) can lead to a majority of diagnostic errors in some patient populations, in other settings, social (eg, poor health literacy, punitive cultural practices) and economic factors (eg, lack of access to appropriate diagnostic tests or to specialty expertise) play a more prominent role.34,35
The Figure describes the relationship between components of the diagnostic process and subsequent outcomes, including diagnostic process failures, diagnostic errors, and absence or presence of patient harm.2,36,37 It reemphasizes the centrality of the patient in decision-making and the continuous nature of the process. The Figure also illustrates that only a minority of process failures result in diagnostic errors, and a smaller proportion of diagnostic errors actually lead to patient harm. Conversely, it also shows that diagnostic errors can happen without any obvious process-failure points, and, similarly, patient harm can take place in the absence of any evident diagnostic errors.36-38 Finally, it highlights the need to incorporate feedback from process failures, diagnostic errors, and favorable and unfavorable patient outcomes in order to inform future quality improvement efforts and research.

A significant proportion of diagnostic errors are due to system-related vulnerabilities, such as limitations in availability, adoption or quality of work force training, health informatics resources, and diagnostic capabilities. Lack of institutional culture that promotes safety and transparency also predisposes to diagnostic errors.39,40 The other major domain of process failures is related to cognitive errors in clinician decision-making. Anchoring, confirmation bias, availability bias, and base-rate neglect are some of the common cognitive biases that, along with personality traits (aversion to risk or ambiguity, overconfidence) and affective biases (influence of emotion on decision-making), often determine the degree of utilization of resources and the possibility of suboptimal diagnostic performance.41,42 Further, implicit biases related to age, race, gender, and sexual orientation contribute to disparities in access to health care and outcomes.43 In a large number of cases of preventable adverse outcomes, however, there are multiple interdependent individual and system-related failure points that lead to diagnostic error and patient harm.6,32
Challenges in Defining and Measuring Diagnostic Errors
In order to develop effective, evidence-based interventions to reduce diagnostic errors in hospitalized patients, it is essential to be able to first operationally define, and then accurately measure, diagnostic errors and the process failures that contribute to these errors in a standardized way that is reproducible across different settings.6,44 There are a number of obstacles in this endeavor.
A fundamental problem is that establishing a diagnosis is not a single act but a process. Patterns of symptoms and clinical presentations often differ for the same disease. Information required to make a diagnosis is usually gathered in stages, where the clinician obtains additional data, while considering many possibilities, of which 1 may be ultimately correct. Diagnoses evolve over time and in different care settings. “The most likely diagnosis” is not always the same as “the final correct diagnosis.” Moreover, the diagnostic process is influenced by patients’ individual clinical courses and preferences over time. This makes determination of missed, delayed, or incorrect diagnoses challenging.45,46
For hospitalized patients, generally the goal is to first rule out more serious and acute conditions (eg, pulmonary embolism or stroke), even if their probability is rather low. Conversely, a diagnosis that appears less consequential if delayed (eg, chronic anemia of unclear etiology) might not be pursued on an urgent basis, and is often left to outpatient providers to examine, but still may manifest in downstream harm (eg, delayed diagnosis of gastrointestinal malignancy or recurrent admissions for heart failure due to missed iron-deficiency anemia). Therefore, coming up with disease diagnosis likelihoods in hindsight may turn out to be highly subjective and not always accurate. This can be particularly difficult when clinician and other team deliberations are not recorded in their entirety.47
Another hurdle in the practice of diagnostic medicine is to preserve the balance between underdiagnosing versus pursuing overly aggressive diagnostic approaches. Conducting laboratory, imaging, or other diagnostic studies without a clear shared understanding of how they would affect clinical decision-making (eg, use of prostate-specific antigen to detect prostate cancer) not only leads to increased costs but can also delay appropriate care. Worse, subsequent unnecessary diagnostic tests and treatments can sometimes lead to serious harm.48,49
Finally, retrospective reviews by clinicians are subject to multiple potential limitations that include failure to create well-defined research questions, poorly developed inclusion and exclusion criteria, and issues related to inter- and intra-rater reliability.50 These methodological deficiencies can occur despite following "best practice" guidelines during the study planning, execution, and analysis phases. They further add to the challenge of defining and measuring diagnostic errors.47
Strategies to Improve Measurement of Diagnostic Errors
Development of new methodologies to reliably measure diagnostic errors is an area of active research. The advancement of uniform and universally agreed-upon frameworks to define and identify process failure points and diagnostic errors would help reduce measurement error and support development and testing of interventions that could be generalizable across different health care settings. To more accurately define and measure diagnostic errors, several novel approaches have been proposed (Table 2).

The Safer Dx framework is an all-round tool developed to advance the discipline of measuring diagnostic errors. For an episode of care under review, the instrument scores various items to determine the likelihood of a diagnostic error. These items evaluate multiple dimensions affecting diagnostic performance and measurements across 3 broad domains: structure (provider and organizational characteristics—from everyone involved with patient care, to computing infrastructure, to policies and regulations), process (elements of the patient-provider encounter, diagnostic test performance and follow-up, and subspecialty- and referral-specific factors), and outcome (establishing accurate and timely diagnosis as opposed to missed, delayed, or incorrect diagnosis). This instrument has been revised and can be further modified by a variety of stakeholders, including clinicians, health care organizations, and policymakers, to identify potential diagnostic errors in a standardized way for patient safety and quality improvement research.51,52
Use of standardized tools, such as the Diagnosis Error Evaluation and Research (DEER) taxonomy, can help to identify and classify specific failure points across different diagnostic process dimensions.37 These failure points can be classified into: issues related to patient presentation or access to health care; failure to obtain or misinterpretation of history or physical exam findings; errors in use of diagnostics tests due to technical or clinician-related factors; failures in appropriate weighing of evidence and hypothesis generation; errors associated with referral or consultation process; and failure to monitor the patient or obtain timely follow-up.34 The DEER taxonomy can also be modified based on specific research questions and study populations. Further, it can be recategorized to correspond to Safer Dx framework diagnostic process dimensions to provide insights into reasons for specific process failures and to develop new interventions to mitigate errors and patient harm.6
Since a majority of diagnostic errors do not lead to actual harm, use of “triggers” or clues (eg, procedure-related complications, patient falls, transfers to a higher level of care, readmissions within 30 days) can be a more efficient method to identify diagnostic errors and adverse events that do cause harm. The Global Trigger Tool, developed by the Institute for Healthcare Improvement, uses this strategy. This tool has been shown to identify a significantly higher number of serious adverse events than comparable methods.53 This facilitates selection and development of strategies at the institutional level that are most likely to improve patient outcomes.24
Encouraging and facilitating voluntary or prompted reporting from patients and clinicians can also play an important role in capturing diagnostic errors. Patients and clinicians are not only the key stakeholders but are also uniquely placed within the diagnostic process to detect and report potential errors.25,54 Patient-safety-event reporting systems, such as RL6, play a vital role in reporting near-misses and adverse events. These systems provide a mechanism for team members at all levels within the hospital to contribute toward reporting patient adverse events, including those arising from diagnostic errors.55 The Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey is the first standardized, nationally reported patient survey designed to measure patients’ perceptions of their hospital experience. The US Centers for Medicare and Medicaid Services (CMS) publishes HCAHPS results on its website 4 times a year, which serves as an important incentive for hospitals to improve patient safety and quality of health care delivery.56
Another novel approach links multiple symptoms to a range of target diseases using the Symptom-Disease Pair Analysis of Diagnostic Error (SPADE) framework. Using “big data” technologies, this technique can help discover otherwise hidden symptom-disease links and improve overall diagnostic performance. This approach is proposed for both case-control (look-back) and cohort (look-forward) studies assessing diagnostic errors and misdiagnosis-related harms. For example, starting with a known diagnosis with high potential for harm (eg, stroke), the “look-back” approach can be used to identify high-risk symptoms (eg, dizziness, vertigo). In the “look-forward” approach, a single symptom or exposure risk factor known to be frequently misdiagnosed (eg, dizziness) can be analyzed to identify potential adverse disease outcomes (eg, stroke, migraine).57
Many large ongoing studies looking at diagnostic errors among hospitalized patients, such as Utility of Predictive Systems to identify Inpatient Diagnostic Errors (UPSIDE),58 Patient Safety Learning Lab (PSLL),59 and Achieving Diagnostic Excellence through Prevention and Teamwork (ADEPT),60 are using structured chart review methodologies incorporating many of the above strategies in combination. Cases triggered by certain events (eg, ICU transfer, death, rapid response event, new or worsening acute kidney injury) are reviewed using validated tools, including Safer Dx framework and DEER taxonomy, to provide the most precise estimates of the burden of diagnostic errors in hospitalized patients. These estimates may be much higher than previously predicted using traditional chart review approaches.6,24 For example, a recently published study of 2809 random admissions in 11 Massachusetts hospitals identified 978 adverse events but only 10 diagnostic errors (diagnostic error rate, 0.4%).19 This was likely because the trigger method used in the study did not specifically examine the diagnostic process as critically as done by the Safer Dx framework and DEER taxonomy tools, thereby underestimating the total number of diagnostic errors. Further, these ongoing studies (eg, UPSIDE, ADEPT) aim to employ new and upcoming advanced machine-learning methods to create models that can improve overall diagnostic performance. This would pave the way to test and build novel, efficient, and scalable interventions to reduce diagnostic errors and improve patient outcomes.
Strategies to Improve Diagnostic Safety in Hospitalized Patients
Disease-specific biomedical research, as well as advances in laboratory, imaging, and other technologies, play a critical role in improving diagnostic accuracy. However, these technical approaches do not address many of the broader clinician- and system-level failure points and opportunities for improvement. Various patient-, provider-, and organizational-level interventions that could make diagnostic processes more resilient and reduce the risk of error and patient harm have been proposed.61
Among these strategies are approaches to empower patients and their families. Fostering therapeutic relationships between patients and members of the care team is essential to reducing diagnostic errors.62 Facilitating timely access to health records, ensuring transparency in decision making, and tailoring communication strategies to patients’ cultural and educational backgrounds can reduce harm.63 Similarly, at the system level, enhancing communication among different providers by use of tools such as structured handoffs can prevent communication breakdowns and facilitate positive outcomes.64
Interventions targeted at individual health care providers, such as educational programs to improve content-specific knowledge, can enhance diagnostic performance. Regular feedback, strategies to enhance equity, and fostering an environment where all providers are actively encouraged to think critically and participate in the diagnostic process (training programs to use “diagnostic time-outs” and making it a “team sport”) can improve clinical reasoning.65,66 Use of standardized patients can help identify individual-level cognitive failure points and facilitate creation of new interventions to improve clinical decision-making processes.67
Novel health information technologies can further augment these efforts. These include effective documentation by maintaining dynamic and accurate patient histories, problem lists, and medication lists68-70; use of electronic health record–based algorithms to identify potential diagnostic delays for serious conditions71,72; use of telemedicine technologies to improve accessibility and coordination73; application of mobile health and wearable technologies to facilitate data-gathering and care delivery74,75; and use of computerized decision-support tools, including applications to interpret electrocardiograms, imaging studies, and other diagnostic tests.76
Use of precision medicine, powered by new artificial intelligence (AI) tools, is becoming more widespread. Algorithms powered by AI can augment and sometimes even outperform clinician decision-making in areas such as oncology, radiology, and primary care.77 Creation of large biobanks like the All of Us research program can be used to study thousands of environmental and genetic risk factors and health conditions simultaneously, and help identify specific treatments that work best for people of different backgrounds.78 Active research in these areas holds great promise in terms of how and when we diagnose diseases and make appropriate preventative and treatment decisions. Significant scientific, ethical, and regulatory challenges will need to be overcome before these technologies can address some of the most complex problems in health care.79
Finally, diagnostic performance is affected by the external environment, including the functioning of the medical liability system. Diagnostic errors that lead to patient harm are a leading cause of malpractice claims.80 Developing a legal environment, in collaboration with patient advocacy groups and health care organizations, that promotes and facilitates timely disclosure of diagnostic errors could decrease the incentive to hide errors, advance care processes, and improve outcomes.81,82
Conclusion
The burden of diagnostic errors in hospitalized patients is unacceptably high and remains an underemphasized cause of preventable morbidity and mortality. Diagnostic errors often result from a breakdown in multiple interdependent processes that involve patient-, provider-, and system-level factors. Significant challenges remain in defining and identifying diagnostic errors as well as underlying process-failure points. The most effective interventions to reduce diagnostic errors will require greater patient participation in the diagnostic process and a mix of evidence-based interventions that promote individual-provider excellence as well as system-level changes. Further research and collaboration among various stakeholders should help improve diagnostic safety for hospitalized patients.
Corresponding author: Abhishek Goyal, MD, MPH; [email protected]
Disclosures: Dr. Dalal disclosed receiving income ≥ $250 from MayaMD.
1. Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165(13):1493-1499. doi:10.1001/archinte.165.13.1493
2. National Academies of Sciences, Engineering, and Medicine. 2015. Improving Diagnosis in Health Care. The National Academies Press. doi:10.17226/21794
3. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373(26):2493-2495. doi:10.1056/NEJMp1512241
4. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139. doi:10.1136/bmj.i2139
5. Flanders SA, Centor B, Weber V, McGinn T, Desalvo K, Auerbach A. Challenges and opportunities in academic hospital medicine: report from the academic hospital medicine summit. J Gen Intern Med. 2009;24(5):636-641. doi:10.1007/s11606-009-0944-6
6. Griffin JA, Carr K, Bersani K, et al. Analyzing diagnostic errors in the acute setting: a process-driven approach. Diagnosis (Berl). 2021;9(1):77-88. doi:10.1515/dx-2021-0033
7. Itri JN, Tappouni RR, McEachern RO, Pesch AJ, Patel SH. Fundamentals of diagnostic error in imaging. RadioGraphics. 2018;38(6):1845-1865. doi:10.1148/rg.2018180021
8. Hammerling JA. A Review of medical errors in laboratory diagnostics and where we are today. Lab Med. 2012;43(2):41-44. doi:10.1309/LM6ER9WJR1IHQAUY
9. Gunderson CG, Bilan VP, Holleck JL, et al. Prevalence of harmful diagnostic errors in hospitalised adults: a systematic review and meta-analysis. BMJ Qual Saf. 2020;29(12):1008-1018. doi:10.1136/bmjqs-2019-010822
10. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. doi:10.1056/NEJM199102073240604
11. Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324(6):377-384. doi:10.1056/NEJM199102073240605
12. Localio AR, Lawthers AG, Brennan TA, et al. Relation between malpractice claims and adverse events due to negligence. Results of the Harvard Medical Practice Study III. N Engl J Med. 1991;325(4):245-251. doi:10.1056/NEJM199107253250405
13. Wilson RM, Michel P, Olsen S, et al. Patient safety in developing countries: retrospective estimation of scale and nature of harm to patients in hospital. BMJ. 2012;344:e832. doi:10.1136/bmj.e832
14. Wilson RM, Runciman WB, Gibberd RW, Harrison BT, Newby L, Hamilton JD. The Quality in Australian Health Care Study. Med J Aust. 1995;163(9):458-471. doi:10.5694/j.1326-5377.1995.tb124691.x
15. Thomas EJ, Studdert DM, Burstin HR, et al. Incidence and types of adverse events and negligent care in Utah and Colorado. Med Care. 2000;38(3):261-271. doi:10.1097/00005650-200003000-00003
16. Baker GR, Norton PG, Flintoft V, et al. The Canadian Adverse Events Study: the incidence of adverse events among hospital patients in Canada. CMAJ. 2004;170(11):1678-1686. doi:10.1503/cmaj.1040498
17. Davis P, Lay-Yee R, Briant R, Ali W, Scott A, Schug S. Adverse events in New Zealand public hospitals II: preventability and clinical context. N Z Med J. 2003;116(1183):U624.
18. Aranaz-Andrés JM, Aibar-Remón C, Vitaller-Murillo J, et al. Incidence of adverse events related to health care in Spain: results of the Spanish National Study of Adverse Events. J Epidemiol Community Health. 2008;62(12):1022-1029. doi:10.1136/jech.2007.065227
19. Bates DW, Levine DM, Salmasian H, et al. The safety of inpatient health care. N Engl J Med. 2023;388(2):142-153. doi:10.1056/NEJMsa2206117
20. Soop M, Fryksmark U, Köster M, Haglund B. The incidence of adverse events in Swedish hospitals: a retrospective medical record review study. Int J Qual Health Care. 2009;21(4):285-291. doi:10.1093/intqhc/mzp025
21. Rafter N, Hickey A, Conroy RM, et al. The Irish National Adverse Events Study (INAES): the frequency and nature of adverse events in Irish hospitals—a retrospective record review study. BMJ Qual Saf. 2017;26(2):111-119. doi:10.1136/bmjqs-2015-004828
22. Blendon RJ, DesRoches CM, Brodie M, et al. Views of practicing physicians and the public on medical errors. N Engl J Med. 2002;347(24):1933-1940. doi:10.1056/NEJMsa022151
23. Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986-2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672-680. doi:10.1136/bmjqs-2012-001550
24. Malik MA, Motta-Calderon D, Piniella N, et al. A structured approach to EHR surveillance of diagnostic error in acute care: an exploratory analysis of two institutionally-defined case cohorts. Diagnosis (Berl). 2022;9(4):446-457. doi:10.1515/dx-2022-0032
25. Graber ML. The incidence of diagnostic error in medicine. BMJ Qual Saf. 2013;22(suppl 2):ii21-ii27. doi:10.1136/bmjqs-2012-001615
26. Bergl PA, Taneja A, El-Kareh R, Singh H, Nanchal RS. Frequency, risk factors, causes, and consequences of diagnostic errors in critically ill medical patients: a retrospective cohort study. Crit Care Med. 2019;47(11):e902-e910. doi:10.1097/CCM.0000000000003976
27. Hogan H, Healey F, Neale G, Thomson R, Vincent C, Black N. Preventable deaths due to problems in care in English acute hospitals: a retrospective case record review study. BMJ Qual Saf. 2012;21(9):737-745. doi:10.1136/bmjqs-2011-001159
28. Bergl PA, Nanchal RS, Singh H. Diagnostic error in the critically ill: defining the problem and exploring next steps to advance intensive care unit safety. Ann Am Thorac Soc. 2018;15(8):903-907. doi:10.1513/AnnalsATS.201801-068PS
29. Marquet K, Claes N, De Troy E, et al. One fourth of unplanned transfers to a higher level of care are associated with a highly preventable adverse event: a patient record review in six Belgian hospitals. Crit Care Med. 2015;43(5):1053-1061. doi:10.1097/CCM.0000000000000932
30. Rodwin BA, Bilan VP, Merchant NB, et al. Rate of preventable mortality in hospitalized patients: a systematic review and meta-analysis. J Gen Intern Med. 2020;35(7):2099-2106. doi:10.1007/s11606-019-05592-5
31. Winters B, Custer J, Galvagno SM, et al. Diagnostic errors in the intensive care unit: a systematic review of autopsy studies. BMJ Qual Saf. 2012;21(11):894-902. doi:10.1136/bmjqs-2012-000803
32. Raffel KE, Kantor MA, Barish P, et al. Prevalence and characterisation of diagnostic error among 7-day all-cause hospital medicine readmissions: a retrospective cohort study. BMJ Qual Saf. 2020;29(12):971-979. doi:10.1136/bmjqs-2020-010896
33. Weingart SN, Pagovich O, Sands DZ, et al. What can hospitalized patients tell us about adverse events? learning from patient-reported incidents. J Gen Intern Med. 2005;20(9):830-836. doi:10.1111/j.1525-1497.2005.0180.x
34. Schiff GD, Hasan O, Kim S, et al. Diagnostic error in medicine: analysis of 583 physician-reported errors. Arch Intern Med. 2009;169(20):1881-1887. doi:10.1001/archinternmed.2009.333
35. Singh H, Schiff GD, Graber ML, Onakpoya I, Thompson MJ. The global burden of diagnostic errors in primary care. BMJ Qual Saf. 2017;26(6):484-494. doi:10.1136/bmjqs-2016-005401
36. Schiff GD, Leape LL. Commentary: how can we make diagnosis safer? Acad Med J Assoc Am Med Coll. 2012;87(2):135-138. doi:10.1097/ACM.0b013e31823f711c
37. Schiff GD, Kim S, Abrams R, et al. Diagnosing diagnosis errors: lessons from a multi-institutional collaborative project. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety: From Research to Implementation. Volume 2: Concepts and Methodology. AHRQ Publication No. 05-0021-2. Agency for Healthcare Research and Quality (US); 2005. Accessed January 16, 2023. http://www.ncbi.nlm.nih.gov/books/NBK20492/
38. Newman-Toker DE. A unified conceptual model for diagnostic errors: underdiagnosis, overdiagnosis, and misdiagnosis. Diagnosis (Berl). 2014;1(1):43-48. doi:10.1515/dx-2013-0027
39. Abimanyi-Ochom J, Bohingamu Mudiyanselage S, Catchpool M, Firipis M, Wanni Arachchige Dona S, Watts JJ. Strategies to reduce diagnostic errors: a systematic review. BMC Med Inform Decis Mak. 2019;19(1):174. doi:10.1186/s12911-019-0901-1
40. Gupta A, Harrod M, Quinn M, et al. Mind the overlap: how system problems contribute to cognitive failure and diagnostic errors. Diagnosis (Berl). 2018;5(3):151-156. doi:10.1515/dx-2018-0014
41. Saposnik G, Redelmeier D, Ruff CC, Tobler PN. Cognitive biases associated with medical decisions: a systematic review. BMC Med Inform Decis Mak. 2016;16:138. doi:10.1186/s12911-016-0377-1
42. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78(8):775-780. doi: 10.1097/00001888-200308000-00003
43. Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med. 2013;28(11):1504-1510. doi:10.1007/s11606-013-2441-1
44. Zwaan L, Singh H. The challenges in defining and measuring diagnostic error. Diagnosis (Ber). 2015;2(2):97-103. doi:10.1515/dx-2014-0069
45. Arkes HR, Wortmann RL, Saville PD, Harkness AR. Hindsight bias among physicians weighing the likelihood of diagnoses. J Appl Psychol. 1981;66(2):252-254.
46. Singh H. Editorial: Helping health care organizations to define diagnostic errors as missed opportunities in diagnosis. Jt Comm J Qual Patient Saf. 2014;40(3):99-101. doi:10.1016/s1553-7250(14)40012-6
47. Vassar M, Holzmann M. The retrospective chart review: important methodological considerations. J Educ Eval Health Prof. 2013;10:12. doi:10.3352/jeehp.2013.10.12
48. Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605-613. doi:10.1093/jnci/djq099
49. Moynihan R, Doust J, Henry D. Preventing overdiagnosis: how to stop harming the healthy. BMJ. 2012;344:e3502. doi:10.1136/bmj.e3502
50. Hayward RA, Hofer TP. Estimating hospital deaths due to medical errors: preventability is in the eye of the reviewer. JAMA. 2001;286(4):415-420. doi:10.1001/jama.286.4.415
51. Singh H, Sittig DF. Advancing the science of measurement of diagnostic errors in healthcare: the Safer Dx framework. BMJ Qual Saf. 2015;24(2):103-110. doi:10.1136/bmjqs-2014-003675
52. Singh H, Khanna A, Spitzmueller C, Meyer AND. Recommendations for using the Revised Safer Dx Instrument to help measure and improve diagnostic safety. Diagnosis (Berl). 2019;6(4):315-323. doi:10.1515/dx-2019-0012
53. Classen DC, Resar R, Griffin F, et al. “Global trigger tool” shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff (Millwood). 2011;30(4):581-589. doi:10.1377/hlthaff.2011.0190
54. Schiff GD. Minimizing diagnostic error: the importance of follow-up and feedback. Am J Med. 2008;121(5 suppl):S38-S42. doi:10.1016/j.amjmed.2008.02.004
55. Mitchell I, Schuster A, Smith K, Pronovost P, Wu A. Patient safety incident reporting: a qualitative study of thoughts and perceptions of experts 15 years after “To Err is Human.” BMJ Qual Saf. 2016;25(2):92-99. doi:10.1136/bmjqs-2015-004405
56. Mazurenko O, Collum T, Ferdinand A, Menachemi N. Predictors of hospital patient satisfaction as measured by HCAHPS: a systematic review. J Healthc Manag. 2017;62(4):272-283. doi:10.1097/JHM-D-15-00050
57. Liberman AL, Newman-Toker DE. Symptom-Disease Pair Analysis of Diagnostic Error (SPADE): a conceptual framework and methodological approach for unearthing misdiagnosis-related harms using big data. BMJ Qual Saf. 2018;27(7):557-566. doi:10.1136/bmjqs-2017-007032
58. Utility of Predictive Systems to Identify Inpatient Diagnostic Errors: the UPSIDE study. NIH RePort/RePORTER. Accessed January 14, 2023. https://reporter.nih.gov/search/rpoHXlEAcEudQV3B9ld8iw/project-details/10020962
59. Overview of Patient Safety Learning Laboratory (PSLL) Projects. Agency for Healthcare Research and Quality. Accessed January 14, 2023. https://www.ahrq.gov/patient-safety/resources/learning-lab/index.html
60. Achieving Diagnostic Excellence through Prevention and Teamwork (ADEPT). NIH RePort/RePORTER. Accessed January 14, 2023. https://reporter.nih.gov/project-details/10642576
61. Zwaan L, Singh H. Diagnostic error in hospitals: finding forests not just the big trees. BMJ Qual Saf. 2020;29(12):961-964. doi:10.1136/bmjqs-2020-011099
62. Longtin Y, Sax H, Leape LL, Sheridan SE, Donaldson L, Pittet D. Patient participation: current knowledge and applicability to patient safety. Mayo Clin Proc. 2010;85(1):53-62. doi:10.4065/mcp.2009.0248
63. Murphy DR, Singh H, Berlin L. Communication breakdowns and diagnostic errors: a radiology perspective. Diagnosis (Berl). 2014;1(4):253-261. doi:10.1515/dx-2014-0035
64. Singh H, Naik AD, Rao R, Petersen LA. Reducing diagnostic errors through effective communication: harnessing the power of information technology. J Gen Intern Med. 2008;23(4):489-494. doi:10.1007/s11606-007-0393-z
65. Singh H, Connor DM, Dhaliwal G. Five strategies for clinicians to advance diagnostic excellence. BMJ. 2022;376:e068044. doi:10.1136/bmj-2021-068044
66. Yale S, Cohen S, Bordini BJ. Diagnostic time-outs to improve diagnosis. Crit Care Clin. 2022;38(2):185-194. doi:10.1016/j.ccc.2021.11.008
67. Schwartz A, Peskin S, Spiro A, Weiner SJ. Impact of unannounced standardized patient audit and feedback on care, documentation, and costs: an experiment and claims analysis. J Gen Intern Med. 2021;36(1):27-34. doi:10.1007/s11606-020-05965-1
68. Carpenter JD, Gorman PN. Using medication list—problem list mismatches as markers of potential error. Proc AMIA Symp. 2002:106-110.
69. Hron JD, Manzi S, Dionne R, et al. Electronic medication reconciliation and medication errors. Int J Qual Health Care. 2015;27(4):314-319. doi:10.1093/intqhc/mzv046
70. Graber ML, Siegal D, Riah H, Johnston D, Kenyon K. Electronic health record–related events in medical malpractice claims. J Patient Saf. 2019;15(2):77-85. doi:10.1097/PTS.0000000000000240
71. Murphy DR, Wu L, Thomas EJ, Forjuoh SN, Meyer AND, Singh H. Electronic trigger-based intervention to reduce delays in diagnostic evaluation for cancer: a cluster randomized controlled trial. J Clin Oncol. 2015;33(31):3560-3567. doi:10.1200/JCO.2015.61.1301
72. Singh H, Giardina TD, Forjuoh SN, et al. Electronic health record-based surveillance of diagnostic errors in primary care. BMJ Qual Saf. 2012;21(2):93-100. doi:10.1136/bmjqs-2011-000304
73. Armaignac DL, Saxena A, Rubens M, et al. Impact of telemedicine on mortality, length of stay, and cost among patients in progressive care units: experience from a large healthcare system. Crit Care Med. 2018;46(5):728-735. doi:10.1097/CCM.0000000000002994
74. MacKinnon GE, Brittain EL. Mobile health technologies in cardiopulmonary disease. Chest. 2020;157(3):654-664. doi:10.1016/j.chest.2019.10.015
75. DeVore AD, Wosik J, Hernandez AF. The future of wearables in heart failure patients. JACC Heart Fail. 2019;7(11):922-932. doi:10.1016/j.jchf.2019.08.008
76. Tsai TL, Fridsma DB, Gatti G. Computer decision support as a source of interpretation error: the case of electrocardiograms. J Am Med Inform Assoc. 2003;10(5):478-483. doi:10.1197/jamia.M1279
77. Lin SY, Mahoney MR, Sinsky CA. Ten ways artificial intelligence will transform primary care. J Gen Intern Med. 2019;34(8):1626-1630. doi:10.1007/s11606-019-05035-1
78. Ramirez AH, Gebo KA, Harris PA. Progress with the All Of Us research program: opening access for researchers. JAMA. 2021;325(24):2441-2442. doi:10.1001/jama.2021.7702
79. Johnson KB, Wei W, Weeraratne D, et al. Precision medicine, AI, and the future of personalized health care. Clin Transl Sci. 2021;14(1):86-93. doi:10.1111/cts.12884
80. Gupta A, Snyder A, Kachalia A, Flanders S, Saint S, Chopra V. Malpractice claims related to diagnostic errors in the hospital. BMJ Qual Saf. 2017;27(1):bmjqs-2017-006774. doi:10.1136/bmjqs-2017-006774
81. Renkema E, Broekhuis M, Ahaus K. Conditions that influence the impact of malpractice litigation risk on physicians’ behavior regarding patient safety. BMC Health Serv Res. 2014;14(1):38. doi:10.1186/1472-6963-14-38
82. Kachalia A, Mello MM, Nallamothu BK, Studdert DM. Legal and policy interventions to improve patient safety. Circulation. 2016;133(7):661-671. doi:10.1161/CIRCULATIONAHA.115.015880
1. Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165(13):1493-1499. doi:10.1001/archinte.165.13.1493
2. National Academies of Sciences, Engineering, and Medicine. 2015. Improving Diagnosis in Health Care. The National Academies Press. doi:10.17226/21794
3. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373(26):2493-2495. doi:10.1056/NEJMp1512241
4. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139. doi:10.1136/bmj.i2139
5. Flanders SA, Centor B, Weber V, McGinn T, Desalvo K, Auerbach A. Challenges and opportunities in academic hospital medicine: report from the academic hospital medicine summit. J Gen Intern Med. 2009;24(5):636-641. doi:10.1007/s11606-009-0944-6
6. Griffin JA, Carr K, Bersani K, et al. Analyzing diagnostic errors in the acute setting: a process-driven approach. Diagnosis (Berl). 2021;9(1):77-88. doi:10.1515/dx-2021-0033
7. Itri JN, Tappouni RR, McEachern RO, Pesch AJ, Patel SH. Fundamentals of diagnostic error in imaging. RadioGraphics. 2018;38(6):1845-1865. doi:10.1148/rg.2018180021
8. Hammerling JA. A Review of medical errors in laboratory diagnostics and where we are today. Lab Med. 2012;43(2):41-44. doi:10.1309/LM6ER9WJR1IHQAUY
9. Gunderson CG, Bilan VP, Holleck JL, et al. Prevalence of harmful diagnostic errors in hospitalised adults: a systematic review and meta-analysis. BMJ Qual Saf. 2020;29(12):1008-1018. doi:10.1136/bmjqs-2019-010822
10. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. doi:10.1056/NEJM199102073240604
11. Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324(6):377-384. doi:10.1056/NEJM199102073240605
12. Localio AR, Lawthers AG, Brennan TA, et al. Relation between malpractice claims and adverse events due to negligence. Results of the Harvard Medical Practice Study III. N Engl J Med. 1991;325(4):245-251. doi:10.1056/NEJM199107253250405
13. Wilson RM, Michel P, Olsen S, et al. Patient safety in developing countries: retrospective estimation of scale and nature of harm to patients in hospital. BMJ. 2012;344:e832. doi:10.1136/bmj.e832
14. Wilson RM, Runciman WB, Gibberd RW, Harrison BT, Newby L, Hamilton JD. The Quality in Australian Health Care Study. Med J Aust. 1995;163(9):458-471. doi:10.5694/j.1326-5377.1995.tb124691.x
15. Thomas EJ, Studdert DM, Burstin HR, et al. Incidence and types of adverse events and negligent care in Utah and Colorado. Med Care. 2000;38(3):261-271. doi:10.1097/00005650-200003000-00003
16. Baker GR, Norton PG, Flintoft V, et al. The Canadian Adverse Events Study: the incidence of adverse events among hospital patients in Canada. CMAJ. 2004;170(11):1678-1686. doi:10.1503/cmaj.1040498
17. Davis P, Lay-Yee R, Briant R, Ali W, Scott A, Schug S. Adverse events in New Zealand public hospitals II: preventability and clinical context. N Z Med J. 2003;116(1183):U624.
18. Aranaz-Andrés JM, Aibar-Remón C, Vitaller-Murillo J, et al. Incidence of adverse events related to health care in Spain: results of the Spanish National Study of Adverse Events. J Epidemiol Community Health. 2008;62(12):1022-1029. doi:10.1136/jech.2007.065227
19. Bates DW, Levine DM, Salmasian H, et al. The safety of inpatient health care. N Engl J Med. 2023;388(2):142-153. doi:10.1056/NEJMsa2206117
20. Soop M, Fryksmark U, Köster M, Haglund B. The incidence of adverse events in Swedish hospitals: a retrospective medical record review study. Int J Qual Health Care. 2009;21(4):285-291. doi:10.1093/intqhc/mzp025
21. Rafter N, Hickey A, Conroy RM, et al. The Irish National Adverse Events Study (INAES): the frequency and nature of adverse events in Irish hospitals—a retrospective record review study. BMJ Qual Saf. 2017;26(2):111-119. doi:10.1136/bmjqs-2015-004828
22. Blendon RJ, DesRoches CM, Brodie M, et al. Views of practicing physicians and the public on medical errors. N Engl J Med. 2002;347(24):1933-1940. doi:10.1056/NEJMsa022151
23. Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986-2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672-680. doi:10.1136/bmjqs-2012-001550
24. Malik MA, Motta-Calderon D, Piniella N, et al. A structured approach to EHR surveillance of diagnostic error in acute care: an exploratory analysis of two institutionally-defined case cohorts. Diagnosis (Berl). 2022;9(4):446-457. doi:10.1515/dx-2022-0032
25. Graber ML. The incidence of diagnostic error in medicine. BMJ Qual Saf. 2013;22(suppl 2):ii21-ii27. doi:10.1136/bmjqs-2012-001615
26. Bergl PA, Taneja A, El-Kareh R, Singh H, Nanchal RS. Frequency, risk factors, causes, and consequences of diagnostic errors in critically ill medical patients: a retrospective cohort study. Crit Care Med. 2019;47(11):e902-e910. doi:10.1097/CCM.0000000000003976
27. Hogan H, Healey F, Neale G, Thomson R, Vincent C, Black N. Preventable deaths due to problems in care in English acute hospitals: a retrospective case record review study. BMJ Qual Saf. 2012;21(9):737-745. doi:10.1136/bmjqs-2011-001159
28. Bergl PA, Nanchal RS, Singh H. Diagnostic error in the critically ill: defining the problem and exploring next steps to advance intensive care unit safety. Ann Am Thorac Soc. 2018;15(8):903-907. doi:10.1513/AnnalsATS.201801-068PS
29. Marquet K, Claes N, De Troy E, et al. One fourth of unplanned transfers to a higher level of care are associated with a highly preventable adverse event: a patient record review in six Belgian hospitals. Crit Care Med. 2015;43(5):1053-1061. doi:10.1097/CCM.0000000000000932
30. Rodwin BA, Bilan VP, Merchant NB, et al. Rate of preventable mortality in hospitalized patients: a systematic review and meta-analysis. J Gen Intern Med. 2020;35(7):2099-2106. doi:10.1007/s11606-019-05592-5
31. Winters B, Custer J, Galvagno SM, et al. Diagnostic errors in the intensive care unit: a systematic review of autopsy studies. BMJ Qual Saf. 2012;21(11):894-902. doi:10.1136/bmjqs-2012-000803
32. Raffel KE, Kantor MA, Barish P, et al. Prevalence and characterisation of diagnostic error among 7-day all-cause hospital medicine readmissions: a retrospective cohort study. BMJ Qual Saf. 2020;29(12):971-979. doi:10.1136/bmjqs-2020-010896
33. Weingart SN, Pagovich O, Sands DZ, et al. What can hospitalized patients tell us about adverse events? learning from patient-reported incidents. J Gen Intern Med. 2005;20(9):830-836. doi:10.1111/j.1525-1497.2005.0180.x
34. Schiff GD, Hasan O, Kim S, et al. Diagnostic error in medicine: analysis of 583 physician-reported errors. Arch Intern Med. 2009;169(20):1881-1887. doi:10.1001/archinternmed.2009.333
35. Singh H, Schiff GD, Graber ML, Onakpoya I, Thompson MJ. The global burden of diagnostic errors in primary care. BMJ Qual Saf. 2017;26(6):484-494. doi:10.1136/bmjqs-2016-005401
36. Schiff GD, Leape LL. Commentary: how can we make diagnosis safer? Acad Med J Assoc Am Med Coll. 2012;87(2):135-138. doi:10.1097/ACM.0b013e31823f711c
37. Schiff GD, Kim S, Abrams R, et al. Diagnosing diagnosis errors: lessons from a multi-institutional collaborative project. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety: From Research to Implementation. Volume 2: Concepts and Methodology. AHRQ Publication No. 05-0021-2. Agency for Healthcare Research and Quality (US); 2005. Accessed January 16, 2023. http://www.ncbi.nlm.nih.gov/books/NBK20492/
38. Newman-Toker DE. A unified conceptual model for diagnostic errors: underdiagnosis, overdiagnosis, and misdiagnosis. Diagnosis (Berl). 2014;1(1):43-48. doi:10.1515/dx-2013-0027
39. Abimanyi-Ochom J, Bohingamu Mudiyanselage S, Catchpool M, Firipis M, Wanni Arachchige Dona S, Watts JJ. Strategies to reduce diagnostic errors: a systematic review. BMC Med Inform Decis Mak. 2019;19(1):174. doi:10.1186/s12911-019-0901-1
40. Gupta A, Harrod M, Quinn M, et al. Mind the overlap: how system problems contribute to cognitive failure and diagnostic errors. Diagnosis (Berl). 2018;5(3):151-156. doi:10.1515/dx-2018-0014
41. Saposnik G, Redelmeier D, Ruff CC, Tobler PN. Cognitive biases associated with medical decisions: a systematic review. BMC Med Inform Decis Mak. 2016;16:138. doi:10.1186/s12911-016-0377-1
42. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78(8):775-780. doi: 10.1097/00001888-200308000-00003
43. Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med. 2013;28(11):1504-1510. doi:10.1007/s11606-013-2441-1
44. Zwaan L, Singh H. The challenges in defining and measuring diagnostic error. Diagnosis (Ber). 2015;2(2):97-103. doi:10.1515/dx-2014-0069
45. Arkes HR, Wortmann RL, Saville PD, Harkness AR. Hindsight bias among physicians weighing the likelihood of diagnoses. J Appl Psychol. 1981;66(2):252-254.
46. Singh H. Editorial: Helping health care organizations to define diagnostic errors as missed opportunities in diagnosis. Jt Comm J Qual Patient Saf. 2014;40(3):99-101. doi:10.1016/s1553-7250(14)40012-6
47. Vassar M, Holzmann M. The retrospective chart review: important methodological considerations. J Educ Eval Health Prof. 2013;10:12. doi:10.3352/jeehp.2013.10.12
48. Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605-613. doi:10.1093/jnci/djq099
49. Moynihan R, Doust J, Henry D. Preventing overdiagnosis: how to stop harming the healthy. BMJ. 2012;344:e3502. doi:10.1136/bmj.e3502
50. Hayward RA, Hofer TP. Estimating hospital deaths due to medical errors: preventability is in the eye of the reviewer. JAMA. 2001;286(4):415-420. doi:10.1001/jama.286.4.415
51. Singh H, Sittig DF. Advancing the science of measurement of diagnostic errors in healthcare: the Safer Dx framework. BMJ Qual Saf. 2015;24(2):103-110. doi:10.1136/bmjqs-2014-003675
52. Singh H, Khanna A, Spitzmueller C, Meyer AND. Recommendations for using the Revised Safer Dx Instrument to help measure and improve diagnostic safety. Diagnosis (Berl). 2019;6(4):315-323. doi:10.1515/dx-2019-0012
53. Classen DC, Resar R, Griffin F, et al. “Global trigger tool” shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff (Millwood). 2011;30(4):581-589. doi:10.1377/hlthaff.2011.0190
54. Schiff GD. Minimizing diagnostic error: the importance of follow-up and feedback. Am J Med. 2008;121(5 suppl):S38-S42. doi:10.1016/j.amjmed.2008.02.004
55. Mitchell I, Schuster A, Smith K, Pronovost P, Wu A. Patient safety incident reporting: a qualitative study of thoughts and perceptions of experts 15 years after “To Err is Human.” BMJ Qual Saf. 2016;25(2):92-99. doi:10.1136/bmjqs-2015-004405
56. Mazurenko O, Collum T, Ferdinand A, Menachemi N. Predictors of hospital patient satisfaction as measured by HCAHPS: a systematic review. J Healthc Manag. 2017;62(4):272-283. doi:10.1097/JHM-D-15-00050
57. Liberman AL, Newman-Toker DE. Symptom-Disease Pair Analysis of Diagnostic Error (SPADE): a conceptual framework and methodological approach for unearthing misdiagnosis-related harms using big data. BMJ Qual Saf. 2018;27(7):557-566. doi:10.1136/bmjqs-2017-007032
58. Utility of Predictive Systems to Identify Inpatient Diagnostic Errors: the UPSIDE study. NIH RePort/RePORTER. Accessed January 14, 2023. https://reporter.nih.gov/search/rpoHXlEAcEudQV3B9ld8iw/project-details/10020962
59. Overview of Patient Safety Learning Laboratory (PSLL) Projects. Agency for Healthcare Research and Quality. Accessed January 14, 2023. https://www.ahrq.gov/patient-safety/resources/learning-lab/index.html
60. Achieving Diagnostic Excellence through Prevention and Teamwork (ADEPT). NIH RePort/RePORTER. Accessed January 14, 2023. https://reporter.nih.gov/project-details/10642576
61. Zwaan L, Singh H. Diagnostic error in hospitals: finding forests not just the big trees. BMJ Qual Saf. 2020;29(12):961-964. doi:10.1136/bmjqs-2020-011099
62. Longtin Y, Sax H, Leape LL, Sheridan SE, Donaldson L, Pittet D. Patient participation: current knowledge and applicability to patient safety. Mayo Clin Proc. 2010;85(1):53-62. doi:10.4065/mcp.2009.0248
63. Murphy DR, Singh H, Berlin L. Communication breakdowns and diagnostic errors: a radiology perspective. Diagnosis (Berl). 2014;1(4):253-261. doi:10.1515/dx-2014-0035
64. Singh H, Naik AD, Rao R, Petersen LA. Reducing diagnostic errors through effective communication: harnessing the power of information technology. J Gen Intern Med. 2008;23(4):489-494. doi:10.1007/s11606-007-0393-z
65. Singh H, Connor DM, Dhaliwal G. Five strategies for clinicians to advance diagnostic excellence. BMJ. 2022;376:e068044. doi:10.1136/bmj-2021-068044
66. Yale S, Cohen S, Bordini BJ. Diagnostic time-outs to improve diagnosis. Crit Care Clin. 2022;38(2):185-194. doi:10.1016/j.ccc.2021.11.008
67. Schwartz A, Peskin S, Spiro A, Weiner SJ. Impact of unannounced standardized patient audit and feedback on care, documentation, and costs: an experiment and claims analysis. J Gen Intern Med. 2021;36(1):27-34. doi:10.1007/s11606-020-05965-1
68. Carpenter JD, Gorman PN. Using medication list—problem list mismatches as markers of potential error. Proc AMIA Symp. 2002:106-110.
69. Hron JD, Manzi S, Dionne R, et al. Electronic medication reconciliation and medication errors. Int J Qual Health Care. 2015;27(4):314-319. doi:10.1093/intqhc/mzv046
70. Graber ML, Siegal D, Riah H, Johnston D, Kenyon K. Electronic health record–related events in medical malpractice claims. J Patient Saf. 2019;15(2):77-85. doi:10.1097/PTS.0000000000000240
71. Murphy DR, Wu L, Thomas EJ, Forjuoh SN, Meyer AND, Singh H. Electronic trigger-based intervention to reduce delays in diagnostic evaluation for cancer: a cluster randomized controlled trial. J Clin Oncol. 2015;33(31):3560-3567. doi:10.1200/JCO.2015.61.1301
72. Singh H, Giardina TD, Forjuoh SN, et al. Electronic health record-based surveillance of diagnostic errors in primary care. BMJ Qual Saf. 2012;21(2):93-100. doi:10.1136/bmjqs-2011-000304
73. Armaignac DL, Saxena A, Rubens M, et al. Impact of telemedicine on mortality, length of stay, and cost among patients in progressive care units: experience from a large healthcare system. Crit Care Med. 2018;46(5):728-735. doi:10.1097/CCM.0000000000002994
74. MacKinnon GE, Brittain EL. Mobile health technologies in cardiopulmonary disease. Chest. 2020;157(3):654-664. doi:10.1016/j.chest.2019.10.015
75. DeVore AD, Wosik J, Hernandez AF. The future of wearables in heart failure patients. JACC Heart Fail. 2019;7(11):922-932. doi:10.1016/j.jchf.2019.08.008
76. Tsai TL, Fridsma DB, Gatti G. Computer decision support as a source of interpretation error: the case of electrocardiograms. J Am Med Inform Assoc. 2003;10(5):478-483. doi:10.1197/jamia.M1279
77. Lin SY, Mahoney MR, Sinsky CA. Ten ways artificial intelligence will transform primary care. J Gen Intern Med. 2019;34(8):1626-1630. doi:10.1007/s11606-019-05035-1
78. Ramirez AH, Gebo KA, Harris PA. Progress with the All Of Us research program: opening access for researchers. JAMA. 2021;325(24):2441-2442. doi:10.1001/jama.2021.7702
79. Johnson KB, Wei W, Weeraratne D, et al. Precision medicine, AI, and the future of personalized health care. Clin Transl Sci. 2021;14(1):86-93. doi:10.1111/cts.12884
80. Gupta A, Snyder A, Kachalia A, Flanders S, Saint S, Chopra V. Malpractice claims related to diagnostic errors in the hospital. BMJ Qual Saf. 2017;27(1):bmjqs-2017-006774. doi:10.1136/bmjqs-2017-006774
81. Renkema E, Broekhuis M, Ahaus K. Conditions that influence the impact of malpractice litigation risk on physicians’ behavior regarding patient safety. BMC Health Serv Res. 2014;14(1):38. doi:10.1186/1472-6963-14-38
82. Kachalia A, Mello MM, Nallamothu BK, Studdert DM. Legal and policy interventions to improve patient safety. Circulation. 2016;133(7):661-671. doi:10.1161/CIRCULATIONAHA.115.015880
Safety in Health Care: An Essential Pillar of Quality
Each year, 40,000 to 98,000 deaths occur due to medical errors.1 The Harvard Medical Practice Study (HMPS), published in 1991, found that 3.7% of hospitalized patients were harmed by adverse events and 1% were harmed by adverse events due to negligence.2 The latest HMPS showed that, despite significant improvements in patient safety over the past 3 decades, patient safety challenges persist. This study found that inpatient care leads to harm in nearly a quarter of patients, and that 1 in 4 of these adverse events are preventable.3
Since the first HMPS study was published, efforts to improve patient safety have focused on identifying causes of medical error and the design and implementation of interventions to mitigate errors. Factors contributing to medical errors have been well documented: the complexity of care delivery from inpatient to outpatient settings, with transitions of care and extensive use of medications; multiple comorbidities; and the fragmentation of care across multiple systems and specialties. Although most errors are related to process or system failure, accountability of each practitioner and clinician is essential to promoting a culture of safety. Many medical errors are preventable through multifaceted approaches employed throughout the phases of the care,4 with medication errors, both prescribing and administration, and diagnostic and treatment errors encompassing most risk prevention areas. Broadly, safety efforts should emphasize building a culture of safety where all safety events are reported, including near-miss events.
Two articles in this issue of JCOM address key elements of patient safety: building a safety culture and diagnostic error. Merchant et al5 report on an initiative designed to promote a safety culture by recognizing and rewarding staff who identify and report near misses. The tiered awards program they designed led to significantly increased staff participation in the safety awards nomination process and was associated with increased reporting of actual and close-call events and greater attendance at monthly safety forums. Goyal et al,6 noting that diagnostic error rates in hospitalized patients remain unacceptably high, provide a concise update on diagnostic error among inpatients, focusing on issues related to defining and measuring diagnostic errors and current strategies to improve diagnostic safety in hospitalized patients. In a third article, Sathi et al report on efforts to teach quality improvement (QI) methods to internal medicine trainees; their project increased residents’ knowledge of their patient panels and comfort with QI approaches and led to improved patient outcomes.
Major progress has been made to improve health care safety since the first HMPS was published. However, the latest HMPS shows that patient safety efforts must continue, given the persistent risk for patient harm in the current health care delivery system. Safety, along with clear accountability for identifying, reporting, and addressing errors, should be a top priority for health care systems throughout the preventive, diagnostic, and therapeutic phases of care.
Corresponding author: Ebrahim Barkoudah, MD, MPH; [email protected]
1. Clancy C, Munier W, Brady J. National healthcare quality report. Agency for Healthcare Research and Quality; 2013.
2. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. doi:10.1056/NEJM199102073240604
3. Bates DW, Levine DM, Salmasian H, et al. The safety of inpatient health care. N Engl J Med. 2023;388(2):142-153. doi:10.1056/NEJMsa2206117
4. Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events: implications for prevention. JAMA. 1995;274(1):29-34.
5. Merchant NB, O’Neal J, Murray JS. Development of a safety awards program at a Veterans Affairs health care system: a quality improvement initiative. J Clin Outcome Manag. 2023;30(1):9-16. doi:10.12788/jcom.0120
6. Goyal A, Martin-Doyle W, Dalal AK. Diagnostic errors in hospitalized patients. J Clin Outcome Manag. 2023;30(1):17-27. doi:10.12788/jcom.0121
7. Sathi K, Huang KTL, Chandler DM, et al. Teaching quality improvement to internal medicine residents to address patient care gaps in ambulatory quality metrics. J Clin Outcome Manag. 2023;30(1):1-6.doi:10.12788/jcom.0119
Each year, 40,000 to 98,000 deaths occur due to medical errors.1 The Harvard Medical Practice Study (HMPS), published in 1991, found that 3.7% of hospitalized patients were harmed by adverse events and 1% were harmed by adverse events due to negligence.2 The latest HMPS showed that, despite significant improvements in patient safety over the past 3 decades, patient safety challenges persist. This study found that inpatient care leads to harm in nearly a quarter of patients, and that 1 in 4 of these adverse events are preventable.3
Since the first HMPS study was published, efforts to improve patient safety have focused on identifying causes of medical error and the design and implementation of interventions to mitigate errors. Factors contributing to medical errors have been well documented: the complexity of care delivery from inpatient to outpatient settings, with transitions of care and extensive use of medications; multiple comorbidities; and the fragmentation of care across multiple systems and specialties. Although most errors are related to process or system failure, accountability of each practitioner and clinician is essential to promoting a culture of safety. Many medical errors are preventable through multifaceted approaches employed throughout the phases of the care,4 with medication errors, both prescribing and administration, and diagnostic and treatment errors encompassing most risk prevention areas. Broadly, safety efforts should emphasize building a culture of safety where all safety events are reported, including near-miss events.
Two articles in this issue of JCOM address key elements of patient safety: building a safety culture and diagnostic error. Merchant et al5 report on an initiative designed to promote a safety culture by recognizing and rewarding staff who identify and report near misses. The tiered awards program they designed led to significantly increased staff participation in the safety awards nomination process and was associated with increased reporting of actual and close-call events and greater attendance at monthly safety forums. Goyal et al,6 noting that diagnostic error rates in hospitalized patients remain unacceptably high, provide a concise update on diagnostic error among inpatients, focusing on issues related to defining and measuring diagnostic errors and current strategies to improve diagnostic safety in hospitalized patients. In a third article, Sathi et al report on efforts to teach quality improvement (QI) methods to internal medicine trainees; their project increased residents’ knowledge of their patient panels and comfort with QI approaches and led to improved patient outcomes.
Major progress has been made to improve health care safety since the first HMPS was published. However, the latest HMPS shows that patient safety efforts must continue, given the persistent risk for patient harm in the current health care delivery system. Safety, along with clear accountability for identifying, reporting, and addressing errors, should be a top priority for health care systems throughout the preventive, diagnostic, and therapeutic phases of care.
Corresponding author: Ebrahim Barkoudah, MD, MPH; [email protected]
Each year, 40,000 to 98,000 deaths occur due to medical errors.1 The Harvard Medical Practice Study (HMPS), published in 1991, found that 3.7% of hospitalized patients were harmed by adverse events and 1% were harmed by adverse events due to negligence.2 The latest HMPS showed that, despite significant improvements in patient safety over the past 3 decades, patient safety challenges persist. This study found that inpatient care leads to harm in nearly a quarter of patients, and that 1 in 4 of these adverse events are preventable.3
Since the first HMPS study was published, efforts to improve patient safety have focused on identifying causes of medical error and the design and implementation of interventions to mitigate errors. Factors contributing to medical errors have been well documented: the complexity of care delivery from inpatient to outpatient settings, with transitions of care and extensive use of medications; multiple comorbidities; and the fragmentation of care across multiple systems and specialties. Although most errors are related to process or system failure, accountability of each practitioner and clinician is essential to promoting a culture of safety. Many medical errors are preventable through multifaceted approaches employed throughout the phases of the care,4 with medication errors, both prescribing and administration, and diagnostic and treatment errors encompassing most risk prevention areas. Broadly, safety efforts should emphasize building a culture of safety where all safety events are reported, including near-miss events.
Two articles in this issue of JCOM address key elements of patient safety: building a safety culture and diagnostic error. Merchant et al5 report on an initiative designed to promote a safety culture by recognizing and rewarding staff who identify and report near misses. The tiered awards program they designed led to significantly increased staff participation in the safety awards nomination process and was associated with increased reporting of actual and close-call events and greater attendance at monthly safety forums. Goyal et al,6 noting that diagnostic error rates in hospitalized patients remain unacceptably high, provide a concise update on diagnostic error among inpatients, focusing on issues related to defining and measuring diagnostic errors and current strategies to improve diagnostic safety in hospitalized patients. In a third article, Sathi et al report on efforts to teach quality improvement (QI) methods to internal medicine trainees; their project increased residents’ knowledge of their patient panels and comfort with QI approaches and led to improved patient outcomes.
Major progress has been made to improve health care safety since the first HMPS was published. However, the latest HMPS shows that patient safety efforts must continue, given the persistent risk for patient harm in the current health care delivery system. Safety, along with clear accountability for identifying, reporting, and addressing errors, should be a top priority for health care systems throughout the preventive, diagnostic, and therapeutic phases of care.
Corresponding author: Ebrahim Barkoudah, MD, MPH; [email protected]
1. Clancy C, Munier W, Brady J. National healthcare quality report. Agency for Healthcare Research and Quality; 2013.
2. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. doi:10.1056/NEJM199102073240604
3. Bates DW, Levine DM, Salmasian H, et al. The safety of inpatient health care. N Engl J Med. 2023;388(2):142-153. doi:10.1056/NEJMsa2206117
4. Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events: implications for prevention. JAMA. 1995;274(1):29-34.
5. Merchant NB, O’Neal J, Murray JS. Development of a safety awards program at a Veterans Affairs health care system: a quality improvement initiative. J Clin Outcome Manag. 2023;30(1):9-16. doi:10.12788/jcom.0120
6. Goyal A, Martin-Doyle W, Dalal AK. Diagnostic errors in hospitalized patients. J Clin Outcome Manag. 2023;30(1):17-27. doi:10.12788/jcom.0121
7. Sathi K, Huang KTL, Chandler DM, et al. Teaching quality improvement to internal medicine residents to address patient care gaps in ambulatory quality metrics. J Clin Outcome Manag. 2023;30(1):1-6.doi:10.12788/jcom.0119
1. Clancy C, Munier W, Brady J. National healthcare quality report. Agency for Healthcare Research and Quality; 2013.
2. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. doi:10.1056/NEJM199102073240604
3. Bates DW, Levine DM, Salmasian H, et al. The safety of inpatient health care. N Engl J Med. 2023;388(2):142-153. doi:10.1056/NEJMsa2206117
4. Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events: implications for prevention. JAMA. 1995;274(1):29-34.
5. Merchant NB, O’Neal J, Murray JS. Development of a safety awards program at a Veterans Affairs health care system: a quality improvement initiative. J Clin Outcome Manag. 2023;30(1):9-16. doi:10.12788/jcom.0120
6. Goyal A, Martin-Doyle W, Dalal AK. Diagnostic errors in hospitalized patients. J Clin Outcome Manag. 2023;30(1):17-27. doi:10.12788/jcom.0121
7. Sathi K, Huang KTL, Chandler DM, et al. Teaching quality improvement to internal medicine residents to address patient care gaps in ambulatory quality metrics. J Clin Outcome Manag. 2023;30(1):1-6.doi:10.12788/jcom.0119
A patient named ‘Settle’ decides to sue instead
On Nov. 1, 2020, Dallas Settle went to Plateau Medical Center, Oak Hill, W.Va., complaining of pain that was later described in court documents as being “in his right mid-abdomen migrating to his right lower abdomen.” Following a CT scan, Mr. Settle was diagnosed with diverticulitis resulting in pneumoperitoneum, which is the presence of air or other gas in the abdominal cavity. The patient, it was decided, required surgery to correct the problem, but Plateau Medical Center didn’t have the staff to perform the procedure.
Mr. Settle was then transferred to another West Virginia hospital, Charleston Area Medical Center (CAMC). Here, he was evaluated by doctors in the facility’s General Division, who initiated treatment with IV fluids and opiate analgesics. He was then placed under the care of a trauma surgeon, who initially decided to treat the patient nonoperatively. If that approach failed, the surgeon believed, Mr. Settle would probably require a laparotomy, bowel resection, and ostomy.
Another surgical team performed an exploratory laparotomy the following day. The team determined that Mr. Settle was suffering from a ruptured appendicitis and allegedly performed an appendectomy. But Mr. Settle’s condition continued to deteriorate the following day.
Another CT scan followed. It revealed various problems – multiple fluid collections, an ileus, distended loops of the patient’s small bowel, a left renal cyst, subcentimeter mesenteric, and retroperitoneal adenopathy. Additional CT scans conducted 4 days later indicated other problems, including fluid collections in the patient’s right- and left-lower quadrants.
Over the next few days, doctors performed further exploratory laparotomies. Finally, on Nov. 22, Mr. Settle was transferred out of the intensive care unit in preparation for his discharge the following day.
His pain continued to worsen, however, and he was readmitted to CAMC a day later. At this point, an examination revealed that his surgical incisions had become infected.
Worse news was on the horizon. On Nov. 28, the trauma surgeon who had first agreed to treat Mr. Settle informed him that, despite claims to the contrary, his appendix hadn’t been removed.
Eventually, Mr. Settle was referred to the Cleveland Clinic, where at press time he was still being treated.
Mr. Settle has hired the firm Calwell Luce diTrapano to sue CAMC, accusing it of medical malpractice, medical negligence, and other lapses in the standard of care. In his complaint, he accused the hospital and its staff of breaching their duty of care “by negligently and improperly treating him” and by failing “to exercise the degree of care, skill, and learning required and expected of reasonable health care providers.”
His suit seeks not only compensatory damages and other relief but also punitive damages.
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article originally appeared on Medscape.com.
On Nov. 1, 2020, Dallas Settle went to Plateau Medical Center, Oak Hill, W.Va., complaining of pain that was later described in court documents as being “in his right mid-abdomen migrating to his right lower abdomen.” Following a CT scan, Mr. Settle was diagnosed with diverticulitis resulting in pneumoperitoneum, which is the presence of air or other gas in the abdominal cavity. The patient, it was decided, required surgery to correct the problem, but Plateau Medical Center didn’t have the staff to perform the procedure.
Mr. Settle was then transferred to another West Virginia hospital, Charleston Area Medical Center (CAMC). Here, he was evaluated by doctors in the facility’s General Division, who initiated treatment with IV fluids and opiate analgesics. He was then placed under the care of a trauma surgeon, who initially decided to treat the patient nonoperatively. If that approach failed, the surgeon believed, Mr. Settle would probably require a laparotomy, bowel resection, and ostomy.
Another surgical team performed an exploratory laparotomy the following day. The team determined that Mr. Settle was suffering from a ruptured appendicitis and allegedly performed an appendectomy. But Mr. Settle’s condition continued to deteriorate the following day.
Another CT scan followed. It revealed various problems – multiple fluid collections, an ileus, distended loops of the patient’s small bowel, a left renal cyst, subcentimeter mesenteric, and retroperitoneal adenopathy. Additional CT scans conducted 4 days later indicated other problems, including fluid collections in the patient’s right- and left-lower quadrants.
Over the next few days, doctors performed further exploratory laparotomies. Finally, on Nov. 22, Mr. Settle was transferred out of the intensive care unit in preparation for his discharge the following day.
His pain continued to worsen, however, and he was readmitted to CAMC a day later. At this point, an examination revealed that his surgical incisions had become infected.
Worse news was on the horizon. On Nov. 28, the trauma surgeon who had first agreed to treat Mr. Settle informed him that, despite claims to the contrary, his appendix hadn’t been removed.
Eventually, Mr. Settle was referred to the Cleveland Clinic, where at press time he was still being treated.
Mr. Settle has hired the firm Calwell Luce diTrapano to sue CAMC, accusing it of medical malpractice, medical negligence, and other lapses in the standard of care. In his complaint, he accused the hospital and its staff of breaching their duty of care “by negligently and improperly treating him” and by failing “to exercise the degree of care, skill, and learning required and expected of reasonable health care providers.”
His suit seeks not only compensatory damages and other relief but also punitive damages.
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article originally appeared on Medscape.com.
On Nov. 1, 2020, Dallas Settle went to Plateau Medical Center, Oak Hill, W.Va., complaining of pain that was later described in court documents as being “in his right mid-abdomen migrating to his right lower abdomen.” Following a CT scan, Mr. Settle was diagnosed with diverticulitis resulting in pneumoperitoneum, which is the presence of air or other gas in the abdominal cavity. The patient, it was decided, required surgery to correct the problem, but Plateau Medical Center didn’t have the staff to perform the procedure.
Mr. Settle was then transferred to another West Virginia hospital, Charleston Area Medical Center (CAMC). Here, he was evaluated by doctors in the facility’s General Division, who initiated treatment with IV fluids and opiate analgesics. He was then placed under the care of a trauma surgeon, who initially decided to treat the patient nonoperatively. If that approach failed, the surgeon believed, Mr. Settle would probably require a laparotomy, bowel resection, and ostomy.
Another surgical team performed an exploratory laparotomy the following day. The team determined that Mr. Settle was suffering from a ruptured appendicitis and allegedly performed an appendectomy. But Mr. Settle’s condition continued to deteriorate the following day.
Another CT scan followed. It revealed various problems – multiple fluid collections, an ileus, distended loops of the patient’s small bowel, a left renal cyst, subcentimeter mesenteric, and retroperitoneal adenopathy. Additional CT scans conducted 4 days later indicated other problems, including fluid collections in the patient’s right- and left-lower quadrants.
Over the next few days, doctors performed further exploratory laparotomies. Finally, on Nov. 22, Mr. Settle was transferred out of the intensive care unit in preparation for his discharge the following day.
His pain continued to worsen, however, and he was readmitted to CAMC a day later. At this point, an examination revealed that his surgical incisions had become infected.
Worse news was on the horizon. On Nov. 28, the trauma surgeon who had first agreed to treat Mr. Settle informed him that, despite claims to the contrary, his appendix hadn’t been removed.
Eventually, Mr. Settle was referred to the Cleveland Clinic, where at press time he was still being treated.
Mr. Settle has hired the firm Calwell Luce diTrapano to sue CAMC, accusing it of medical malpractice, medical negligence, and other lapses in the standard of care. In his complaint, he accused the hospital and its staff of breaching their duty of care “by negligently and improperly treating him” and by failing “to exercise the degree of care, skill, and learning required and expected of reasonable health care providers.”
His suit seeks not only compensatory damages and other relief but also punitive damages.
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article originally appeared on Medscape.com.
Oncologist to insurer: ‘This denial will not stand’
“Is this really the hill you want to die on?” asked Rebecca Shatsky, MD, a medical oncologist at the University of California, San Diego.
It was Nov. 18 and Dr. Shatsky was on the phone with a retired oncologist working for the health insurance company Premera Blue Cross.
Dr. Shatsky was appealing a prior authorization denial for pembrolizumab (Keytruda) to treat her patient with stage IIIc triple-negative breast cancer (TNBC). She hoped the peer-to-peer would reverse the denial. The Food and Drug Administration had approved the immunotherapy for people with high-risk TNBC both in the neoadjuvant setting alongside chemotherapy and, in her patient’s case, as a single-agent adjuvant treatment based on data from the KEYNOTE 522 trial.
In the peer-to-peer, Dr. Shatsky laid out the evidence, but she could tell the physician wasn’t going to budge.
When she pressed him further, asking why he was denying potentially lifesaving care for her patient, he said the data on whether patients really need adjuvant pembrolizumab were not clear yet.
“The man – who was not a breast oncologist – was essentially mansplaining breast oncology to me,” she said in an interview. “I don’t need a nonexpert giving me their misinterpretation of the data.”
Dr. Shatsky informed him that this decision would not stand. She would be escalating the claim.
“I’m not going to let you get in way of my patient’s survival,” Dr. Shatsky told the physician during the peer-to-peer. “We have one shot to cure this, and if we don’t do it now, patients’ average lifespan is 17 months.”
The conversation turned a few heads in her office.
“My whole office stopped and stared. But then they clapped after they realized why I was yelling,” she tweeted later that night.
She continued: “@premera picked the wrong oncologist to mess with today. I will not be letting this go. This denial. Will. Not. Stand. An insurance company should not get to tell me how to practice medicine when Phase III RCT data and @NCCN + @ASCO guideline support my decision!”
A spokesperson for Premera said in a statement that, “while we did see many of the details about the case were posted to Twitter, we cannot comment on the specifics you noted due to privacy policies.”
The spokesperson explained that Premera has “the same goal as our provider partners: ensure our members have access to quality health care,” noting that prior authorization helps health plans evaluate the medical necessity and safety of health care services given that “15%-30% of care is unnecessary.”
“We also understand that providers may not agree with our decisions, which is why we have a robust appeals process,” the spokesperson said, suggesting Dr. Shatsky could have appealed the decision a second time.
And “if the member or provider still disagrees with Premera’s coverage decision after the initial appeal, providers can request review by a medical expert outside Premera who works for an independent review organization,” and the company “will pay for” and “abide by” that decision, the spokesperson added.
The Twitter storm
After Dr. Shatsky tweeted about her experience with Premera, she received a flood of support from the Twitterverse. The thread garnered tens of thousands of likes and hundreds of comments offering support and advice.
Several people suggested asking Merck for help accessing the drug. But Dr. Shatsky said no, “I’m tired of laying down and letting [insurance companies] win. It IS worth fighting for.”
The next morning, Dr. Shatsky got a call. It was the vice president of medical management at Premera.
“We’ve talked again, and we’ll give you the drug,” Dr. Shatsky recalled the Premera vice president saying.
The next day, Monday morning, Dr. Shatsky’s patient received her first infusion of pembrolizumab.
Although relieved, Dr. Shatsky noted that it wasn’t until she posted her experience to Twitter that Premera seemed to take notice.
Plus, “an oncologist without a strong social media following may not have gotten care approved and that’s not how medicine should work,” said Dr. Anderson, assistant professor in the department of clinical pharmacy, University of Colorado at Denver, Aurora.
Tatiana Prowell, MD, expressed similar concerns in a Nov. 20 tweet: “And sadly, the patients with cancer & an even busier, more exhausted doctor who doesn’t have a big [reach] on social media will be denied appropriate care. And that’s bank for insurers.”
But, Dr. Prowell noted sarcastically: “At least a patient with cancer had her care delayed & a dedicated OncTwitter colleague’s Physician Burnout was exacerbated.”
In this case, the prior authorization process took about a week – requiring an initial prior authorization request, an appeal after the request was denied, a peer-to-peer resulting in a second denial, and finally a tweet and a phone call from a top executive at the company.
In fact, these delays have become so common that Dr. Shatsky needs to anticipate and incorporate likely delays into her workflow.
“I learn which drugs will take a long time to get prior authorization for and then plan enough time so that my patient’s care is hopefully not delayed,” Dr. Shatsky said. “It should not be so hard to get appropriate and time-sensitive care for our patients.”
A version of this article first appeared on Medscape.com.
“Is this really the hill you want to die on?” asked Rebecca Shatsky, MD, a medical oncologist at the University of California, San Diego.
It was Nov. 18 and Dr. Shatsky was on the phone with a retired oncologist working for the health insurance company Premera Blue Cross.
Dr. Shatsky was appealing a prior authorization denial for pembrolizumab (Keytruda) to treat her patient with stage IIIc triple-negative breast cancer (TNBC). She hoped the peer-to-peer would reverse the denial. The Food and Drug Administration had approved the immunotherapy for people with high-risk TNBC both in the neoadjuvant setting alongside chemotherapy and, in her patient’s case, as a single-agent adjuvant treatment based on data from the KEYNOTE 522 trial.
In the peer-to-peer, Dr. Shatsky laid out the evidence, but she could tell the physician wasn’t going to budge.
When she pressed him further, asking why he was denying potentially lifesaving care for her patient, he said the data on whether patients really need adjuvant pembrolizumab were not clear yet.
“The man – who was not a breast oncologist – was essentially mansplaining breast oncology to me,” she said in an interview. “I don’t need a nonexpert giving me their misinterpretation of the data.”
Dr. Shatsky informed him that this decision would not stand. She would be escalating the claim.
“I’m not going to let you get in way of my patient’s survival,” Dr. Shatsky told the physician during the peer-to-peer. “We have one shot to cure this, and if we don’t do it now, patients’ average lifespan is 17 months.”
The conversation turned a few heads in her office.
“My whole office stopped and stared. But then they clapped after they realized why I was yelling,” she tweeted later that night.
She continued: “@premera picked the wrong oncologist to mess with today. I will not be letting this go. This denial. Will. Not. Stand. An insurance company should not get to tell me how to practice medicine when Phase III RCT data and @NCCN + @ASCO guideline support my decision!”
A spokesperson for Premera said in a statement that, “while we did see many of the details about the case were posted to Twitter, we cannot comment on the specifics you noted due to privacy policies.”
The spokesperson explained that Premera has “the same goal as our provider partners: ensure our members have access to quality health care,” noting that prior authorization helps health plans evaluate the medical necessity and safety of health care services given that “15%-30% of care is unnecessary.”
“We also understand that providers may not agree with our decisions, which is why we have a robust appeals process,” the spokesperson said, suggesting Dr. Shatsky could have appealed the decision a second time.
And “if the member or provider still disagrees with Premera’s coverage decision after the initial appeal, providers can request review by a medical expert outside Premera who works for an independent review organization,” and the company “will pay for” and “abide by” that decision, the spokesperson added.
The Twitter storm
After Dr. Shatsky tweeted about her experience with Premera, she received a flood of support from the Twitterverse. The thread garnered tens of thousands of likes and hundreds of comments offering support and advice.
Several people suggested asking Merck for help accessing the drug. But Dr. Shatsky said no, “I’m tired of laying down and letting [insurance companies] win. It IS worth fighting for.”
The next morning, Dr. Shatsky got a call. It was the vice president of medical management at Premera.
“We’ve talked again, and we’ll give you the drug,” Dr. Shatsky recalled the Premera vice president saying.
The next day, Monday morning, Dr. Shatsky’s patient received her first infusion of pembrolizumab.
Although relieved, Dr. Shatsky noted that it wasn’t until she posted her experience to Twitter that Premera seemed to take notice.
Plus, “an oncologist without a strong social media following may not have gotten care approved and that’s not how medicine should work,” said Dr. Anderson, assistant professor in the department of clinical pharmacy, University of Colorado at Denver, Aurora.
Tatiana Prowell, MD, expressed similar concerns in a Nov. 20 tweet: “And sadly, the patients with cancer & an even busier, more exhausted doctor who doesn’t have a big [reach] on social media will be denied appropriate care. And that’s bank for insurers.”
But, Dr. Prowell noted sarcastically: “At least a patient with cancer had her care delayed & a dedicated OncTwitter colleague’s Physician Burnout was exacerbated.”
In this case, the prior authorization process took about a week – requiring an initial prior authorization request, an appeal after the request was denied, a peer-to-peer resulting in a second denial, and finally a tweet and a phone call from a top executive at the company.
In fact, these delays have become so common that Dr. Shatsky needs to anticipate and incorporate likely delays into her workflow.
“I learn which drugs will take a long time to get prior authorization for and then plan enough time so that my patient’s care is hopefully not delayed,” Dr. Shatsky said. “It should not be so hard to get appropriate and time-sensitive care for our patients.”
A version of this article first appeared on Medscape.com.
“Is this really the hill you want to die on?” asked Rebecca Shatsky, MD, a medical oncologist at the University of California, San Diego.
It was Nov. 18 and Dr. Shatsky was on the phone with a retired oncologist working for the health insurance company Premera Blue Cross.
Dr. Shatsky was appealing a prior authorization denial for pembrolizumab (Keytruda) to treat her patient with stage IIIc triple-negative breast cancer (TNBC). She hoped the peer-to-peer would reverse the denial. The Food and Drug Administration had approved the immunotherapy for people with high-risk TNBC both in the neoadjuvant setting alongside chemotherapy and, in her patient’s case, as a single-agent adjuvant treatment based on data from the KEYNOTE 522 trial.
In the peer-to-peer, Dr. Shatsky laid out the evidence, but she could tell the physician wasn’t going to budge.
When she pressed him further, asking why he was denying potentially lifesaving care for her patient, he said the data on whether patients really need adjuvant pembrolizumab were not clear yet.
“The man – who was not a breast oncologist – was essentially mansplaining breast oncology to me,” she said in an interview. “I don’t need a nonexpert giving me their misinterpretation of the data.”
Dr. Shatsky informed him that this decision would not stand. She would be escalating the claim.
“I’m not going to let you get in way of my patient’s survival,” Dr. Shatsky told the physician during the peer-to-peer. “We have one shot to cure this, and if we don’t do it now, patients’ average lifespan is 17 months.”
The conversation turned a few heads in her office.
“My whole office stopped and stared. But then they clapped after they realized why I was yelling,” she tweeted later that night.
She continued: “@premera picked the wrong oncologist to mess with today. I will not be letting this go. This denial. Will. Not. Stand. An insurance company should not get to tell me how to practice medicine when Phase III RCT data and @NCCN + @ASCO guideline support my decision!”
A spokesperson for Premera said in a statement that, “while we did see many of the details about the case were posted to Twitter, we cannot comment on the specifics you noted due to privacy policies.”
The spokesperson explained that Premera has “the same goal as our provider partners: ensure our members have access to quality health care,” noting that prior authorization helps health plans evaluate the medical necessity and safety of health care services given that “15%-30% of care is unnecessary.”
“We also understand that providers may not agree with our decisions, which is why we have a robust appeals process,” the spokesperson said, suggesting Dr. Shatsky could have appealed the decision a second time.
And “if the member or provider still disagrees with Premera’s coverage decision after the initial appeal, providers can request review by a medical expert outside Premera who works for an independent review organization,” and the company “will pay for” and “abide by” that decision, the spokesperson added.
The Twitter storm
After Dr. Shatsky tweeted about her experience with Premera, she received a flood of support from the Twitterverse. The thread garnered tens of thousands of likes and hundreds of comments offering support and advice.
Several people suggested asking Merck for help accessing the drug. But Dr. Shatsky said no, “I’m tired of laying down and letting [insurance companies] win. It IS worth fighting for.”
The next morning, Dr. Shatsky got a call. It was the vice president of medical management at Premera.
“We’ve talked again, and we’ll give you the drug,” Dr. Shatsky recalled the Premera vice president saying.
The next day, Monday morning, Dr. Shatsky’s patient received her first infusion of pembrolizumab.
Although relieved, Dr. Shatsky noted that it wasn’t until she posted her experience to Twitter that Premera seemed to take notice.
Plus, “an oncologist without a strong social media following may not have gotten care approved and that’s not how medicine should work,” said Dr. Anderson, assistant professor in the department of clinical pharmacy, University of Colorado at Denver, Aurora.
Tatiana Prowell, MD, expressed similar concerns in a Nov. 20 tweet: “And sadly, the patients with cancer & an even busier, more exhausted doctor who doesn’t have a big [reach] on social media will be denied appropriate care. And that’s bank for insurers.”
But, Dr. Prowell noted sarcastically: “At least a patient with cancer had her care delayed & a dedicated OncTwitter colleague’s Physician Burnout was exacerbated.”
In this case, the prior authorization process took about a week – requiring an initial prior authorization request, an appeal after the request was denied, a peer-to-peer resulting in a second denial, and finally a tweet and a phone call from a top executive at the company.
In fact, these delays have become so common that Dr. Shatsky needs to anticipate and incorporate likely delays into her workflow.
“I learn which drugs will take a long time to get prior authorization for and then plan enough time so that my patient’s care is hopefully not delayed,” Dr. Shatsky said. “It should not be so hard to get appropriate and time-sensitive care for our patients.”
A version of this article first appeared on Medscape.com.
Tucatinib plus trastuzumab approved for HER2+ colorectal cancer
The U.S. Food and Drug Administration has granted accelerated approval to tucatinib (Tukysa) in combination with trastuzumab for use in RAS wild-type, HER2-positive unresectable or metastatic colorectal cancer that has progressed after fluoropyrimidine, oxaliplatin, and irinotecan-based chemotherapy.
This is the first FDA-approved treatment for HER2-positive metastatic colorectal cancer, maker Seagen said in a Jan. 19 press release.
“Historically, patients with HER2-positive metastatic colorectal cancer who have progressed following frontline therapy have had poor outcomes. The FDA approval of a chemotherapy-free combination regimen that specifically targets HER2 is great news for these patients,” John Strickler, MD, associate professor of medicine at Duke University Medical Center, Durham, N.C., said in the press release.
Dr. Strickler was the lead investigator on the approval trial, dubbed MOUNTAINEER, which involved 84 patients who met the treatment criteria and who had also been treated with an anti-VEGF antibody. Participants whose tumors were deficient in mismatch repair proteins or were microsatellite instability–high must also have received a PD-1 inhibitor. Patients who received prior anti-HER2 therapy were excluded, the FDA explained in its own press release.
Participants were treated with tucatinib 300 mg orally twice daily– the recommended dose in product labeling – with trastuzumab administered at a loading dose of 8 mg/kg intravenously on day 1 of cycle 1 followed by a maintenance dose of trastuzumab 6 mg/kg on day 1 of each subsequent 21-day cycle.
Overall response rate was 38%, and median duration of response was 12.4 months.
The most common adverse events, occurring in at least 20% of study participants, were diarrhea, fatigue, rash, nausea, abdominal pain, infusion related reactions, and pyrexia. The most common laboratory abnormalities were increased creatinine, decreased lymphocytes, increased alanine aminotransferase, and decreased hemoglobin, among others.
Serious adverse reactions occurred in 22% of patients. The most common (occurring in ≥ 2% of patients) were intestinal obstruction (7%); urinary tract infection (3.5%); and pneumonia, abdominal pain, and rectal perforation (2.3% each). Adverse reactions leading to permanent discontinuation occurred in 6% of patients, including increased alanine aminotransferase in 2.3%.
Continued approval for the indication may be contingent upon verification and description of clinical benefit in confirmatory trials, the company said.
A global, randomized phase 3 clinical trial (MOUNTAINEER-03) is ongoing and is comparing tucatinib in combination with trastuzumab and mFOLFOX6 with standard of care and is intended to serve as a confirmatory trial, the company said.
Tucatinib is already approved in combination with trastuzumab and capecitabine for use in the treatment of advanced unresectable or metastatic HER2-positive breast cancer.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has granted accelerated approval to tucatinib (Tukysa) in combination with trastuzumab for use in RAS wild-type, HER2-positive unresectable or metastatic colorectal cancer that has progressed after fluoropyrimidine, oxaliplatin, and irinotecan-based chemotherapy.
This is the first FDA-approved treatment for HER2-positive metastatic colorectal cancer, maker Seagen said in a Jan. 19 press release.
“Historically, patients with HER2-positive metastatic colorectal cancer who have progressed following frontline therapy have had poor outcomes. The FDA approval of a chemotherapy-free combination regimen that specifically targets HER2 is great news for these patients,” John Strickler, MD, associate professor of medicine at Duke University Medical Center, Durham, N.C., said in the press release.
Dr. Strickler was the lead investigator on the approval trial, dubbed MOUNTAINEER, which involved 84 patients who met the treatment criteria and who had also been treated with an anti-VEGF antibody. Participants whose tumors were deficient in mismatch repair proteins or were microsatellite instability–high must also have received a PD-1 inhibitor. Patients who received prior anti-HER2 therapy were excluded, the FDA explained in its own press release.
Participants were treated with tucatinib 300 mg orally twice daily– the recommended dose in product labeling – with trastuzumab administered at a loading dose of 8 mg/kg intravenously on day 1 of cycle 1 followed by a maintenance dose of trastuzumab 6 mg/kg on day 1 of each subsequent 21-day cycle.
Overall response rate was 38%, and median duration of response was 12.4 months.
The most common adverse events, occurring in at least 20% of study participants, were diarrhea, fatigue, rash, nausea, abdominal pain, infusion related reactions, and pyrexia. The most common laboratory abnormalities were increased creatinine, decreased lymphocytes, increased alanine aminotransferase, and decreased hemoglobin, among others.
Serious adverse reactions occurred in 22% of patients. The most common (occurring in ≥ 2% of patients) were intestinal obstruction (7%); urinary tract infection (3.5%); and pneumonia, abdominal pain, and rectal perforation (2.3% each). Adverse reactions leading to permanent discontinuation occurred in 6% of patients, including increased alanine aminotransferase in 2.3%.
Continued approval for the indication may be contingent upon verification and description of clinical benefit in confirmatory trials, the company said.
A global, randomized phase 3 clinical trial (MOUNTAINEER-03) is ongoing and is comparing tucatinib in combination with trastuzumab and mFOLFOX6 with standard of care and is intended to serve as a confirmatory trial, the company said.
Tucatinib is already approved in combination with trastuzumab and capecitabine for use in the treatment of advanced unresectable or metastatic HER2-positive breast cancer.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has granted accelerated approval to tucatinib (Tukysa) in combination with trastuzumab for use in RAS wild-type, HER2-positive unresectable or metastatic colorectal cancer that has progressed after fluoropyrimidine, oxaliplatin, and irinotecan-based chemotherapy.
This is the first FDA-approved treatment for HER2-positive metastatic colorectal cancer, maker Seagen said in a Jan. 19 press release.
“Historically, patients with HER2-positive metastatic colorectal cancer who have progressed following frontline therapy have had poor outcomes. The FDA approval of a chemotherapy-free combination regimen that specifically targets HER2 is great news for these patients,” John Strickler, MD, associate professor of medicine at Duke University Medical Center, Durham, N.C., said in the press release.
Dr. Strickler was the lead investigator on the approval trial, dubbed MOUNTAINEER, which involved 84 patients who met the treatment criteria and who had also been treated with an anti-VEGF antibody. Participants whose tumors were deficient in mismatch repair proteins or were microsatellite instability–high must also have received a PD-1 inhibitor. Patients who received prior anti-HER2 therapy were excluded, the FDA explained in its own press release.
Participants were treated with tucatinib 300 mg orally twice daily– the recommended dose in product labeling – with trastuzumab administered at a loading dose of 8 mg/kg intravenously on day 1 of cycle 1 followed by a maintenance dose of trastuzumab 6 mg/kg on day 1 of each subsequent 21-day cycle.
Overall response rate was 38%, and median duration of response was 12.4 months.
The most common adverse events, occurring in at least 20% of study participants, were diarrhea, fatigue, rash, nausea, abdominal pain, infusion related reactions, and pyrexia. The most common laboratory abnormalities were increased creatinine, decreased lymphocytes, increased alanine aminotransferase, and decreased hemoglobin, among others.
Serious adverse reactions occurred in 22% of patients. The most common (occurring in ≥ 2% of patients) were intestinal obstruction (7%); urinary tract infection (3.5%); and pneumonia, abdominal pain, and rectal perforation (2.3% each). Adverse reactions leading to permanent discontinuation occurred in 6% of patients, including increased alanine aminotransferase in 2.3%.
Continued approval for the indication may be contingent upon verification and description of clinical benefit in confirmatory trials, the company said.
A global, randomized phase 3 clinical trial (MOUNTAINEER-03) is ongoing and is comparing tucatinib in combination with trastuzumab and mFOLFOX6 with standard of care and is intended to serve as a confirmatory trial, the company said.
Tucatinib is already approved in combination with trastuzumab and capecitabine for use in the treatment of advanced unresectable or metastatic HER2-positive breast cancer.
A version of this article first appeared on Medscape.com.
Reversing abortion drug’s approval would harm public interest, FDA says
(Reuters) – President Joe Biden’s administration is urging a judge to reject a request by abortion opponents for a court order withdrawing federal approval for the drug used in medication abortions – which account for more than half of U.S. abortions – citing potential dangers to women seeking to end their pregnancies.
The U.S. Food and Drug Administration’s filing to U.S. District Judge Matthew Kacsmaryk, made available online on Tuesday, came in a lawsuit in Texas by antiabortion groups challenging the agency’s approval of the drug mifepristone in 2000 for medication abortion.
“The public interest would be dramatically harmed by effectively withdrawing from the marketplace a safe and effective drug that has lawfully been on the market for 22 years,” lawyers for the FDA said in the filing to Mr. Kacsmaryk, who is based in Amarillo.
Mifepristone is available under the brand name Mifeprex and as a generic. Used in conjunction with another drug, it is approved to terminate a pregnancy within the first 10 weeks of a pregnancy. The FDA on Jan. 3 said the government for the first time will allow mifepristone to be dispensed at retail pharmacies.
Medication abortion has drawn increasing attention since the U.S. Supreme Court last June overturned its landmark 1973 Roe v. Wade decision that had legalized abortion nationwide. Nearly all abortions, including medication abortions, are now banned in 12 states, and 16 states that permit some abortions also had laws restricting medication abortion as of November, according to the Guttmacher Institute, a research group that supports abortion rights.
“No abortion is safe, and chemical abortions are particularly dangerous,” said Julie Blake, senior counsel at the conservative legal group Alliance Defending Freedom, which represents the plaintiffs in the lawsuit. “The FDA, by approving chemical abortion drugs for home use, puts a woman or girl’s life at risk.”
The American College of Obstetricians and Gynecologists and the American Medical Association said in a joint letter to the Biden administration last June that “robust evidence exists regarding the safety of mifepristone for medication-induced abortion.”
Antiabortion groups including the Alliance for Hippocratic Medicine and the American Association of Pro-Life Obstetricians and Gynecologists sued the FDA in November, saying the agency improperly used an accelerated process to approve mifepristone and failed to study its risks for minors adequately.
In its court filing, the FDA said there was no basis for second-guessing the FDA’s judgment. The FDA said that pulling the drug would force patients seeking abortions in many cases to undergo unnecessary and more invasive surgical abortion. That would result in longer wait times and would carry risks for some patients including those intolerant to anesthesia, the FDA added.
In support of its position, the agency submitted declarations from abortion providers. For example, nonprofit Maine Family Planning said it would have to eliminate abortion services at 17 of its 18 clinics if mifepristone were no longer available.
Mifeprex maker Danco Laboratories on Friday also asked to intervene in the lawsuit to protect its ability to sell the drug.
A version of this article first appeared on Medscape.com.
(Reuters) – President Joe Biden’s administration is urging a judge to reject a request by abortion opponents for a court order withdrawing federal approval for the drug used in medication abortions – which account for more than half of U.S. abortions – citing potential dangers to women seeking to end their pregnancies.
The U.S. Food and Drug Administration’s filing to U.S. District Judge Matthew Kacsmaryk, made available online on Tuesday, came in a lawsuit in Texas by antiabortion groups challenging the agency’s approval of the drug mifepristone in 2000 for medication abortion.
“The public interest would be dramatically harmed by effectively withdrawing from the marketplace a safe and effective drug that has lawfully been on the market for 22 years,” lawyers for the FDA said in the filing to Mr. Kacsmaryk, who is based in Amarillo.
Mifepristone is available under the brand name Mifeprex and as a generic. Used in conjunction with another drug, it is approved to terminate a pregnancy within the first 10 weeks of a pregnancy. The FDA on Jan. 3 said the government for the first time will allow mifepristone to be dispensed at retail pharmacies.
Medication abortion has drawn increasing attention since the U.S. Supreme Court last June overturned its landmark 1973 Roe v. Wade decision that had legalized abortion nationwide. Nearly all abortions, including medication abortions, are now banned in 12 states, and 16 states that permit some abortions also had laws restricting medication abortion as of November, according to the Guttmacher Institute, a research group that supports abortion rights.
“No abortion is safe, and chemical abortions are particularly dangerous,” said Julie Blake, senior counsel at the conservative legal group Alliance Defending Freedom, which represents the plaintiffs in the lawsuit. “The FDA, by approving chemical abortion drugs for home use, puts a woman or girl’s life at risk.”
The American College of Obstetricians and Gynecologists and the American Medical Association said in a joint letter to the Biden administration last June that “robust evidence exists regarding the safety of mifepristone for medication-induced abortion.”
Antiabortion groups including the Alliance for Hippocratic Medicine and the American Association of Pro-Life Obstetricians and Gynecologists sued the FDA in November, saying the agency improperly used an accelerated process to approve mifepristone and failed to study its risks for minors adequately.
In its court filing, the FDA said there was no basis for second-guessing the FDA’s judgment. The FDA said that pulling the drug would force patients seeking abortions in many cases to undergo unnecessary and more invasive surgical abortion. That would result in longer wait times and would carry risks for some patients including those intolerant to anesthesia, the FDA added.
In support of its position, the agency submitted declarations from abortion providers. For example, nonprofit Maine Family Planning said it would have to eliminate abortion services at 17 of its 18 clinics if mifepristone were no longer available.
Mifeprex maker Danco Laboratories on Friday also asked to intervene in the lawsuit to protect its ability to sell the drug.
A version of this article first appeared on Medscape.com.
(Reuters) – President Joe Biden’s administration is urging a judge to reject a request by abortion opponents for a court order withdrawing federal approval for the drug used in medication abortions – which account for more than half of U.S. abortions – citing potential dangers to women seeking to end their pregnancies.
The U.S. Food and Drug Administration’s filing to U.S. District Judge Matthew Kacsmaryk, made available online on Tuesday, came in a lawsuit in Texas by antiabortion groups challenging the agency’s approval of the drug mifepristone in 2000 for medication abortion.
“The public interest would be dramatically harmed by effectively withdrawing from the marketplace a safe and effective drug that has lawfully been on the market for 22 years,” lawyers for the FDA said in the filing to Mr. Kacsmaryk, who is based in Amarillo.
Mifepristone is available under the brand name Mifeprex and as a generic. Used in conjunction with another drug, it is approved to terminate a pregnancy within the first 10 weeks of a pregnancy. The FDA on Jan. 3 said the government for the first time will allow mifepristone to be dispensed at retail pharmacies.
Medication abortion has drawn increasing attention since the U.S. Supreme Court last June overturned its landmark 1973 Roe v. Wade decision that had legalized abortion nationwide. Nearly all abortions, including medication abortions, are now banned in 12 states, and 16 states that permit some abortions also had laws restricting medication abortion as of November, according to the Guttmacher Institute, a research group that supports abortion rights.
“No abortion is safe, and chemical abortions are particularly dangerous,” said Julie Blake, senior counsel at the conservative legal group Alliance Defending Freedom, which represents the plaintiffs in the lawsuit. “The FDA, by approving chemical abortion drugs for home use, puts a woman or girl’s life at risk.”
The American College of Obstetricians and Gynecologists and the American Medical Association said in a joint letter to the Biden administration last June that “robust evidence exists regarding the safety of mifepristone for medication-induced abortion.”
Antiabortion groups including the Alliance for Hippocratic Medicine and the American Association of Pro-Life Obstetricians and Gynecologists sued the FDA in November, saying the agency improperly used an accelerated process to approve mifepristone and failed to study its risks for minors adequately.
In its court filing, the FDA said there was no basis for second-guessing the FDA’s judgment. The FDA said that pulling the drug would force patients seeking abortions in many cases to undergo unnecessary and more invasive surgical abortion. That would result in longer wait times and would carry risks for some patients including those intolerant to anesthesia, the FDA added.
In support of its position, the agency submitted declarations from abortion providers. For example, nonprofit Maine Family Planning said it would have to eliminate abortion services at 17 of its 18 clinics if mifepristone were no longer available.
Mifeprex maker Danco Laboratories on Friday also asked to intervene in the lawsuit to protect its ability to sell the drug.
A version of this article first appeared on Medscape.com.
Hope for catching infants with CP early
A new prognostic tool may help identify infants with cerebral palsy (CP) earlier, allowing them to receive therapies to improve later outcomes.
Researchers from Canada used 12 clinical variables to predict the condition. The tool accurately predicted 75% of CP cases. The study was published in JAMA Pediatrics.
The prevalence of CP in the United States is 2-3 children per 1,000, a rate that has been relatively unchanged for decades. Although recent innovations in diagnosis using motor scores and MRI scans have aided in diagnosis, these techniques have historically been reserved only for infants who were cared for in neonatal intensive care units, were born prematurely, or who had other neurologic risk factors, such as birth defects.
The tool identified 2.4 times more children with CP than would have been detected using current diagnostic methods, according to the researchers.
“We developed the prediction tool to try to make these findings accessible to any health care provider, which will hopefully help break down the long-held perception that CP is usually related to prematurity or a difficult delivery,” said Mary Dunbar, MD, an author of the study. “We know that about half of children with CP aren’t premature and didn’t have a particularly difficult birth.”
The bedside tool weighs factors such as the use by mothers of illicit drugs and tobacco; the presence of diabetes and preeclampsia during pregnancy; whether the infant is male; birth weight; and the number of miscarriages the mother had prior to the birth. The tool also factors in results from a test that measures how well the infant is adjusting to life outside the womb.
Dr. Dunbar and colleagues compared 1,265 infants with CP from the Canadian Cerebral Palsy Registry from 2003 to 2019 with a control group of 1,985 children without CP from the Alberta Pregnancy Outcomes and Nutrition longitudinal study.
The study authors hope that the prognostic tool can be integrated into existing newborn screenings and completed by nurses or physicians as part of routine care.
“Its cost is low especially in comparison to MRI and specialized neurological assessments,” said Sarah Taylor, MD, section chief of neonatal-perinatal medicine at Yale New Haven Children’s Hospital in New Haven, Conn. Health systems and doctors may be more apt to adopt the tool, since it does not require specialized equipment or training.
Surprising findings
Several clinical variables independently increased the risk of CP, including independent 5-minute Apgar test scores of <6, chorioamnionitis, and illicit drug use during the pregnancy. Dr. Dunbar and colleagues recommend that primary care clinicians provide enhanced surveillance for these infants.
“I think there are also really important public health implications to address maternal and reproductive health to support pregnant people, since this study shows that common pregnancy conditions that are potentially treatable may additively contribute to cerebral palsy risk,” said Dr. Dunbar, a pediatric neurologist and assistant professor at the University of Calgary (Alta.)
For infants identified as being at risk, the study authors also suggest that doctors conduct focused examinations for CP at 3-, 6- and 12-month well-baby visits. If results of an examination are abnormal, doctors can advise the caregiver to conduct an early expert evaluation for a general movements assessment. Interventions for children with CP usually start in the first few years of life and can include occupational therapy, use of orthotic devices, and medication.
Dr. Dunbar and colleagues acknowledge that the test is not perfect and that additional work is needed.
“As helpful as the prediction tool may be to identify cases of CP early, we know there are still a minority of CP cases that it won’t catch because they don’t have any of the known risk factors,” Dr. Dunbar said. “We’re currently working on further research about this unique group.”
The researchers cited several limitations to the dataset used in the study, including a control group that was skewed toward older patients and persons of higher socioeconomic status. In addition, the data included a greater proportion of White women than the average Canadian population.
The Canadian Cerebral Palsy Registry was supported by the NeuroDevNet, KidsBrainHealth, the Harvey Guyda Chair of McGill University, Montreal Children’s Hospital, and the Public Health Agency of Canada. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new prognostic tool may help identify infants with cerebral palsy (CP) earlier, allowing them to receive therapies to improve later outcomes.
Researchers from Canada used 12 clinical variables to predict the condition. The tool accurately predicted 75% of CP cases. The study was published in JAMA Pediatrics.
The prevalence of CP in the United States is 2-3 children per 1,000, a rate that has been relatively unchanged for decades. Although recent innovations in diagnosis using motor scores and MRI scans have aided in diagnosis, these techniques have historically been reserved only for infants who were cared for in neonatal intensive care units, were born prematurely, or who had other neurologic risk factors, such as birth defects.
The tool identified 2.4 times more children with CP than would have been detected using current diagnostic methods, according to the researchers.
“We developed the prediction tool to try to make these findings accessible to any health care provider, which will hopefully help break down the long-held perception that CP is usually related to prematurity or a difficult delivery,” said Mary Dunbar, MD, an author of the study. “We know that about half of children with CP aren’t premature and didn’t have a particularly difficult birth.”
The bedside tool weighs factors such as the use by mothers of illicit drugs and tobacco; the presence of diabetes and preeclampsia during pregnancy; whether the infant is male; birth weight; and the number of miscarriages the mother had prior to the birth. The tool also factors in results from a test that measures how well the infant is adjusting to life outside the womb.
Dr. Dunbar and colleagues compared 1,265 infants with CP from the Canadian Cerebral Palsy Registry from 2003 to 2019 with a control group of 1,985 children without CP from the Alberta Pregnancy Outcomes and Nutrition longitudinal study.
The study authors hope that the prognostic tool can be integrated into existing newborn screenings and completed by nurses or physicians as part of routine care.
“Its cost is low especially in comparison to MRI and specialized neurological assessments,” said Sarah Taylor, MD, section chief of neonatal-perinatal medicine at Yale New Haven Children’s Hospital in New Haven, Conn. Health systems and doctors may be more apt to adopt the tool, since it does not require specialized equipment or training.
Surprising findings
Several clinical variables independently increased the risk of CP, including independent 5-minute Apgar test scores of <6, chorioamnionitis, and illicit drug use during the pregnancy. Dr. Dunbar and colleagues recommend that primary care clinicians provide enhanced surveillance for these infants.
“I think there are also really important public health implications to address maternal and reproductive health to support pregnant people, since this study shows that common pregnancy conditions that are potentially treatable may additively contribute to cerebral palsy risk,” said Dr. Dunbar, a pediatric neurologist and assistant professor at the University of Calgary (Alta.)
For infants identified as being at risk, the study authors also suggest that doctors conduct focused examinations for CP at 3-, 6- and 12-month well-baby visits. If results of an examination are abnormal, doctors can advise the caregiver to conduct an early expert evaluation for a general movements assessment. Interventions for children with CP usually start in the first few years of life and can include occupational therapy, use of orthotic devices, and medication.
Dr. Dunbar and colleagues acknowledge that the test is not perfect and that additional work is needed.
“As helpful as the prediction tool may be to identify cases of CP early, we know there are still a minority of CP cases that it won’t catch because they don’t have any of the known risk factors,” Dr. Dunbar said. “We’re currently working on further research about this unique group.”
The researchers cited several limitations to the dataset used in the study, including a control group that was skewed toward older patients and persons of higher socioeconomic status. In addition, the data included a greater proportion of White women than the average Canadian population.
The Canadian Cerebral Palsy Registry was supported by the NeuroDevNet, KidsBrainHealth, the Harvey Guyda Chair of McGill University, Montreal Children’s Hospital, and the Public Health Agency of Canada. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new prognostic tool may help identify infants with cerebral palsy (CP) earlier, allowing them to receive therapies to improve later outcomes.
Researchers from Canada used 12 clinical variables to predict the condition. The tool accurately predicted 75% of CP cases. The study was published in JAMA Pediatrics.
The prevalence of CP in the United States is 2-3 children per 1,000, a rate that has been relatively unchanged for decades. Although recent innovations in diagnosis using motor scores and MRI scans have aided in diagnosis, these techniques have historically been reserved only for infants who were cared for in neonatal intensive care units, were born prematurely, or who had other neurologic risk factors, such as birth defects.
The tool identified 2.4 times more children with CP than would have been detected using current diagnostic methods, according to the researchers.
“We developed the prediction tool to try to make these findings accessible to any health care provider, which will hopefully help break down the long-held perception that CP is usually related to prematurity or a difficult delivery,” said Mary Dunbar, MD, an author of the study. “We know that about half of children with CP aren’t premature and didn’t have a particularly difficult birth.”
The bedside tool weighs factors such as the use by mothers of illicit drugs and tobacco; the presence of diabetes and preeclampsia during pregnancy; whether the infant is male; birth weight; and the number of miscarriages the mother had prior to the birth. The tool also factors in results from a test that measures how well the infant is adjusting to life outside the womb.
Dr. Dunbar and colleagues compared 1,265 infants with CP from the Canadian Cerebral Palsy Registry from 2003 to 2019 with a control group of 1,985 children without CP from the Alberta Pregnancy Outcomes and Nutrition longitudinal study.
The study authors hope that the prognostic tool can be integrated into existing newborn screenings and completed by nurses or physicians as part of routine care.
“Its cost is low especially in comparison to MRI and specialized neurological assessments,” said Sarah Taylor, MD, section chief of neonatal-perinatal medicine at Yale New Haven Children’s Hospital in New Haven, Conn. Health systems and doctors may be more apt to adopt the tool, since it does not require specialized equipment or training.
Surprising findings
Several clinical variables independently increased the risk of CP, including independent 5-minute Apgar test scores of <6, chorioamnionitis, and illicit drug use during the pregnancy. Dr. Dunbar and colleagues recommend that primary care clinicians provide enhanced surveillance for these infants.
“I think there are also really important public health implications to address maternal and reproductive health to support pregnant people, since this study shows that common pregnancy conditions that are potentially treatable may additively contribute to cerebral palsy risk,” said Dr. Dunbar, a pediatric neurologist and assistant professor at the University of Calgary (Alta.)
For infants identified as being at risk, the study authors also suggest that doctors conduct focused examinations for CP at 3-, 6- and 12-month well-baby visits. If results of an examination are abnormal, doctors can advise the caregiver to conduct an early expert evaluation for a general movements assessment. Interventions for children with CP usually start in the first few years of life and can include occupational therapy, use of orthotic devices, and medication.
Dr. Dunbar and colleagues acknowledge that the test is not perfect and that additional work is needed.
“As helpful as the prediction tool may be to identify cases of CP early, we know there are still a minority of CP cases that it won’t catch because they don’t have any of the known risk factors,” Dr. Dunbar said. “We’re currently working on further research about this unique group.”
The researchers cited several limitations to the dataset used in the study, including a control group that was skewed toward older patients and persons of higher socioeconomic status. In addition, the data included a greater proportion of White women than the average Canadian population.
The Canadian Cerebral Palsy Registry was supported by the NeuroDevNet, KidsBrainHealth, the Harvey Guyda Chair of McGill University, Montreal Children’s Hospital, and the Public Health Agency of Canada. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA PEDIATRICS
Holding out hope for ambroxol
How many of you hadn’t heard of ambroxol until the last few weeks?
How many of you have gotten at least one call asking for a prescription for it in that time?
I’ll raise my hand on both accounts.
Ambroxol seems relatively innocuous – an over-the-counter cold medication commonly used on planet Earth (though not approved in the U.S. for whatever reason). But in the last few years some interesting data have cropped up that it may help with Parkinson’s disease.
“May” being the key word here.
Now, I’m not saying it will or won’t do something. The trials that are being started will show that. It would be totally awesome if it did.
But we’ve been here before: The hope that some old, inexpensive, and widely available medication would turn out to have an amazing benefit we didn’t anticipate. We saw this with hydroxychloroquine and ivermectin during the pandemic. Before that we saw all kinds of speculative ideas that statins would be effective for diseases from multiple sclerosis to Alzheimer’s disease.
And, as with many incurable diseases, patients and their families are hoping for a breakthrough. We have plenty of treatments for Parkinson’s disease, but no cures yet. So any potentially effective drug news makes the rounds quickly on news sites, patient advocacy sites, Facebook, and others.
Like the childrens’ telephone game, each time the story is repeated it changes a bit. We’ve gone from an article saying the drug is starting clinical trials to see if it works, to it being a cure now on the marketplace.
Which is when people start calling my office. Most are disappointed to learn that its benefit (if any) is unknown and that it’s not even available. A few get confrontational, accusing me of withholding treatment, when “everyone knows” the drug works.
Believe me, if I had a cure I’d be thrilled to be able to offer it.
I understand that patients and families want a cure.
I understand hope.
I want ambroxol to work for Parkinson’s disease and make a huge difference in the lives of those affected by it. Maybe it will. Or maybe it won’t.
But these things take time to figure out. None of the amazing medications and hi-tech toys we have came about overnight. They were all years in the making.
That’s how science works, and medicine is as much a science as an art.
The art is being able to explain this to patients, and still allow them to hope.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
How many of you hadn’t heard of ambroxol until the last few weeks?
How many of you have gotten at least one call asking for a prescription for it in that time?
I’ll raise my hand on both accounts.
Ambroxol seems relatively innocuous – an over-the-counter cold medication commonly used on planet Earth (though not approved in the U.S. for whatever reason). But in the last few years some interesting data have cropped up that it may help with Parkinson’s disease.
“May” being the key word here.
Now, I’m not saying it will or won’t do something. The trials that are being started will show that. It would be totally awesome if it did.
But we’ve been here before: The hope that some old, inexpensive, and widely available medication would turn out to have an amazing benefit we didn’t anticipate. We saw this with hydroxychloroquine and ivermectin during the pandemic. Before that we saw all kinds of speculative ideas that statins would be effective for diseases from multiple sclerosis to Alzheimer’s disease.
And, as with many incurable diseases, patients and their families are hoping for a breakthrough. We have plenty of treatments for Parkinson’s disease, but no cures yet. So any potentially effective drug news makes the rounds quickly on news sites, patient advocacy sites, Facebook, and others.
Like the childrens’ telephone game, each time the story is repeated it changes a bit. We’ve gone from an article saying the drug is starting clinical trials to see if it works, to it being a cure now on the marketplace.
Which is when people start calling my office. Most are disappointed to learn that its benefit (if any) is unknown and that it’s not even available. A few get confrontational, accusing me of withholding treatment, when “everyone knows” the drug works.
Believe me, if I had a cure I’d be thrilled to be able to offer it.
I understand that patients and families want a cure.
I understand hope.
I want ambroxol to work for Parkinson’s disease and make a huge difference in the lives of those affected by it. Maybe it will. Or maybe it won’t.
But these things take time to figure out. None of the amazing medications and hi-tech toys we have came about overnight. They were all years in the making.
That’s how science works, and medicine is as much a science as an art.
The art is being able to explain this to patients, and still allow them to hope.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
How many of you hadn’t heard of ambroxol until the last few weeks?
How many of you have gotten at least one call asking for a prescription for it in that time?
I’ll raise my hand on both accounts.
Ambroxol seems relatively innocuous – an over-the-counter cold medication commonly used on planet Earth (though not approved in the U.S. for whatever reason). But in the last few years some interesting data have cropped up that it may help with Parkinson’s disease.
“May” being the key word here.
Now, I’m not saying it will or won’t do something. The trials that are being started will show that. It would be totally awesome if it did.
But we’ve been here before: The hope that some old, inexpensive, and widely available medication would turn out to have an amazing benefit we didn’t anticipate. We saw this with hydroxychloroquine and ivermectin during the pandemic. Before that we saw all kinds of speculative ideas that statins would be effective for diseases from multiple sclerosis to Alzheimer’s disease.
And, as with many incurable diseases, patients and their families are hoping for a breakthrough. We have plenty of treatments for Parkinson’s disease, but no cures yet. So any potentially effective drug news makes the rounds quickly on news sites, patient advocacy sites, Facebook, and others.
Like the childrens’ telephone game, each time the story is repeated it changes a bit. We’ve gone from an article saying the drug is starting clinical trials to see if it works, to it being a cure now on the marketplace.
Which is when people start calling my office. Most are disappointed to learn that its benefit (if any) is unknown and that it’s not even available. A few get confrontational, accusing me of withholding treatment, when “everyone knows” the drug works.
Believe me, if I had a cure I’d be thrilled to be able to offer it.
I understand that patients and families want a cure.
I understand hope.
I want ambroxol to work for Parkinson’s disease and make a huge difference in the lives of those affected by it. Maybe it will. Or maybe it won’t.
But these things take time to figure out. None of the amazing medications and hi-tech toys we have came about overnight. They were all years in the making.
That’s how science works, and medicine is as much a science as an art.
The art is being able to explain this to patients, and still allow them to hope.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Should pediatricians fret over their falling board scores?
Few pediatricians have warm, fuzzy memories about taking their initial board exam.
But many reacted strongly when they read a recent post on Twitter by Bryan Carmody, MD, who noted that the passing rate for first-time test takers had dipped to its lowest level in 5 years – hitting 81% in 2021, down from 91% 3 years earlier.
“It’s literally an awfully written exam,” replied one person who posted. Another asked: “At what point is the exam just not reflective of clinical practice?” And, inevitably, the question of the effect of COVID-19 surfaced: “Is any of this attributable to pulling early career physicians into the pandemic?”
But Dr. Carmody, an associate professor of pediatrics at the University of Eastern Virginia Medical School, Norfolk, isn’t buying that explanation. He researched board scores for internal medicine, general surgery, and family medicine for 2021. All were stable during the same period, he said, leading him to dismiss the idea that the pandemic drove the decline. “It’s not really clear to me why other specialties wouldn’t have seen similar drops,” Dr. Carmody said.
The slip has caught the attention of the American Board of Pediatrics, according to Judy Schaechter, MD, MBA, who was chair of the department of pediatrics at the University of Miami before taking her post as president and CEO of the American Board of Pediatrics in 2021.
“So, our first question was, was this within the range of what one might expect?” Dr. Schaechter said. “Were there other factors that might have come into play?”
The board performs an extensive analysis every year before releasing scores, and it didn’t uncover any changes in the difficulty or content of the test in 2021, nor did the score that was needed to pass increase. Dr. Schaechter pointed out that the passing rate that year was not unprecedented – in 2016, it also dipped to 81%.
Dr. Schaechter said COVID-19 might have affected test takers. “Remember, pediatrics was different from any other specialty during the pandemic,” she said. The census in pediatric wards around the country dropped dramatically in the first two winters of the pandemic, leaving residents with less hands-on experience with patients and mentorship from attendings – both of which can help test-takers pass the exam.
Eyal Ben-Isaac, MD, an associate professor of the department of pediatrics at the Keck School of Medicine at the University of Southern California, Los Angeles, said residents likely suffered during the pandemic, when noon lectures and grand rounds became virtual events.
“I’m sure that clearly affected a person’s ability to sit and listen and really learn the material, as opposed to either doing it hands on or learning the material from a faculty member face to face,” Dr. Ben-Isaac said.
But how much do the didactic experiences of residency programs contribute to residents’ readiness to take the boards? Thomas Welch, MD, professor and chair emeritus of the department of pediatrics at SUNY Upstate Medical University, Syracuse, credits his own success in advancing through college, medical school, pediatric residency, and nephrology fellowship to his skill as a test taker.
He confirmed his suspicions by conducting a study that evaluated correlations between residents’ performance on the United States Medical Licensing Exam (USMLE) taken during medical school and their board scores after completing residency.
Dr. Welch said he wasn’t surprised to find that “the best predictor of one’s passing the pediatric boards was not the training program in which one worked. It was their performance on Step 2 [taken during the fourth year of medical school] of the USMLE.”
Although Dr. Ben-Isaac felt that changes in residency training opportunities might have partially explained the drop in passing rates, he agreed that other factors contribute to success on boards. As director of the pediatric residency program at Children’s Hospital of Los Angeles from 1994 to 2019, one of his first goals was to increase the pass rate of graduates. He developed a board review course for residents, revising it over time on the basis of resident feedback and adding individual coaching for residents who wanted more help.
“Without a question, it raised our board pass rate to being one of the highest in the country,” he said.
Dr. Welch said that while “being up all night with a sick child teaches you a lot about medicine and certainly makes you a better doctor, it doesn’t do anything to improve your board scores.”
None of the pediatricians was too worried about a 1-year drop in scores, and the consensus was that supporting residents with review courses and coaching on how to take multiple choice tests would raise passing rates.
“There are definitely people who are amazing clinicians who did not pass the boards on their first attempt,” Dr. Ben-Isaac said.
But Dr. Schaechter defended the importance of the examination. “Our first obligation is really to the public,” she said. The ABP’s role is to ensure that pediatricians “provide the care that parents want their kids to have.”
As Dr. Welch put it, “Would I trust someone who didn’t pass the board exam to take care of my own kid? Probably not.”
A version of this article first appeared on Medscape.com.
Few pediatricians have warm, fuzzy memories about taking their initial board exam.
But many reacted strongly when they read a recent post on Twitter by Bryan Carmody, MD, who noted that the passing rate for first-time test takers had dipped to its lowest level in 5 years – hitting 81% in 2021, down from 91% 3 years earlier.
“It’s literally an awfully written exam,” replied one person who posted. Another asked: “At what point is the exam just not reflective of clinical practice?” And, inevitably, the question of the effect of COVID-19 surfaced: “Is any of this attributable to pulling early career physicians into the pandemic?”
But Dr. Carmody, an associate professor of pediatrics at the University of Eastern Virginia Medical School, Norfolk, isn’t buying that explanation. He researched board scores for internal medicine, general surgery, and family medicine for 2021. All were stable during the same period, he said, leading him to dismiss the idea that the pandemic drove the decline. “It’s not really clear to me why other specialties wouldn’t have seen similar drops,” Dr. Carmody said.
The slip has caught the attention of the American Board of Pediatrics, according to Judy Schaechter, MD, MBA, who was chair of the department of pediatrics at the University of Miami before taking her post as president and CEO of the American Board of Pediatrics in 2021.
“So, our first question was, was this within the range of what one might expect?” Dr. Schaechter said. “Were there other factors that might have come into play?”
The board performs an extensive analysis every year before releasing scores, and it didn’t uncover any changes in the difficulty or content of the test in 2021, nor did the score that was needed to pass increase. Dr. Schaechter pointed out that the passing rate that year was not unprecedented – in 2016, it also dipped to 81%.
Dr. Schaechter said COVID-19 might have affected test takers. “Remember, pediatrics was different from any other specialty during the pandemic,” she said. The census in pediatric wards around the country dropped dramatically in the first two winters of the pandemic, leaving residents with less hands-on experience with patients and mentorship from attendings – both of which can help test-takers pass the exam.
Eyal Ben-Isaac, MD, an associate professor of the department of pediatrics at the Keck School of Medicine at the University of Southern California, Los Angeles, said residents likely suffered during the pandemic, when noon lectures and grand rounds became virtual events.
“I’m sure that clearly affected a person’s ability to sit and listen and really learn the material, as opposed to either doing it hands on or learning the material from a faculty member face to face,” Dr. Ben-Isaac said.
But how much do the didactic experiences of residency programs contribute to residents’ readiness to take the boards? Thomas Welch, MD, professor and chair emeritus of the department of pediatrics at SUNY Upstate Medical University, Syracuse, credits his own success in advancing through college, medical school, pediatric residency, and nephrology fellowship to his skill as a test taker.
He confirmed his suspicions by conducting a study that evaluated correlations between residents’ performance on the United States Medical Licensing Exam (USMLE) taken during medical school and their board scores after completing residency.
Dr. Welch said he wasn’t surprised to find that “the best predictor of one’s passing the pediatric boards was not the training program in which one worked. It was their performance on Step 2 [taken during the fourth year of medical school] of the USMLE.”
Although Dr. Ben-Isaac felt that changes in residency training opportunities might have partially explained the drop in passing rates, he agreed that other factors contribute to success on boards. As director of the pediatric residency program at Children’s Hospital of Los Angeles from 1994 to 2019, one of his first goals was to increase the pass rate of graduates. He developed a board review course for residents, revising it over time on the basis of resident feedback and adding individual coaching for residents who wanted more help.
“Without a question, it raised our board pass rate to being one of the highest in the country,” he said.
Dr. Welch said that while “being up all night with a sick child teaches you a lot about medicine and certainly makes you a better doctor, it doesn’t do anything to improve your board scores.”
None of the pediatricians was too worried about a 1-year drop in scores, and the consensus was that supporting residents with review courses and coaching on how to take multiple choice tests would raise passing rates.
“There are definitely people who are amazing clinicians who did not pass the boards on their first attempt,” Dr. Ben-Isaac said.
But Dr. Schaechter defended the importance of the examination. “Our first obligation is really to the public,” she said. The ABP’s role is to ensure that pediatricians “provide the care that parents want their kids to have.”
As Dr. Welch put it, “Would I trust someone who didn’t pass the board exam to take care of my own kid? Probably not.”
A version of this article first appeared on Medscape.com.
Few pediatricians have warm, fuzzy memories about taking their initial board exam.
But many reacted strongly when they read a recent post on Twitter by Bryan Carmody, MD, who noted that the passing rate for first-time test takers had dipped to its lowest level in 5 years – hitting 81% in 2021, down from 91% 3 years earlier.
“It’s literally an awfully written exam,” replied one person who posted. Another asked: “At what point is the exam just not reflective of clinical practice?” And, inevitably, the question of the effect of COVID-19 surfaced: “Is any of this attributable to pulling early career physicians into the pandemic?”
But Dr. Carmody, an associate professor of pediatrics at the University of Eastern Virginia Medical School, Norfolk, isn’t buying that explanation. He researched board scores for internal medicine, general surgery, and family medicine for 2021. All were stable during the same period, he said, leading him to dismiss the idea that the pandemic drove the decline. “It’s not really clear to me why other specialties wouldn’t have seen similar drops,” Dr. Carmody said.
The slip has caught the attention of the American Board of Pediatrics, according to Judy Schaechter, MD, MBA, who was chair of the department of pediatrics at the University of Miami before taking her post as president and CEO of the American Board of Pediatrics in 2021.
“So, our first question was, was this within the range of what one might expect?” Dr. Schaechter said. “Were there other factors that might have come into play?”
The board performs an extensive analysis every year before releasing scores, and it didn’t uncover any changes in the difficulty or content of the test in 2021, nor did the score that was needed to pass increase. Dr. Schaechter pointed out that the passing rate that year was not unprecedented – in 2016, it also dipped to 81%.
Dr. Schaechter said COVID-19 might have affected test takers. “Remember, pediatrics was different from any other specialty during the pandemic,” she said. The census in pediatric wards around the country dropped dramatically in the first two winters of the pandemic, leaving residents with less hands-on experience with patients and mentorship from attendings – both of which can help test-takers pass the exam.
Eyal Ben-Isaac, MD, an associate professor of the department of pediatrics at the Keck School of Medicine at the University of Southern California, Los Angeles, said residents likely suffered during the pandemic, when noon lectures and grand rounds became virtual events.
“I’m sure that clearly affected a person’s ability to sit and listen and really learn the material, as opposed to either doing it hands on or learning the material from a faculty member face to face,” Dr. Ben-Isaac said.
But how much do the didactic experiences of residency programs contribute to residents’ readiness to take the boards? Thomas Welch, MD, professor and chair emeritus of the department of pediatrics at SUNY Upstate Medical University, Syracuse, credits his own success in advancing through college, medical school, pediatric residency, and nephrology fellowship to his skill as a test taker.
He confirmed his suspicions by conducting a study that evaluated correlations between residents’ performance on the United States Medical Licensing Exam (USMLE) taken during medical school and their board scores after completing residency.
Dr. Welch said he wasn’t surprised to find that “the best predictor of one’s passing the pediatric boards was not the training program in which one worked. It was their performance on Step 2 [taken during the fourth year of medical school] of the USMLE.”
Although Dr. Ben-Isaac felt that changes in residency training opportunities might have partially explained the drop in passing rates, he agreed that other factors contribute to success on boards. As director of the pediatric residency program at Children’s Hospital of Los Angeles from 1994 to 2019, one of his first goals was to increase the pass rate of graduates. He developed a board review course for residents, revising it over time on the basis of resident feedback and adding individual coaching for residents who wanted more help.
“Without a question, it raised our board pass rate to being one of the highest in the country,” he said.
Dr. Welch said that while “being up all night with a sick child teaches you a lot about medicine and certainly makes you a better doctor, it doesn’t do anything to improve your board scores.”
None of the pediatricians was too worried about a 1-year drop in scores, and the consensus was that supporting residents with review courses and coaching on how to take multiple choice tests would raise passing rates.
“There are definitely people who are amazing clinicians who did not pass the boards on their first attempt,” Dr. Ben-Isaac said.
But Dr. Schaechter defended the importance of the examination. “Our first obligation is really to the public,” she said. The ABP’s role is to ensure that pediatricians “provide the care that parents want their kids to have.”
As Dr. Welch put it, “Would I trust someone who didn’t pass the board exam to take care of my own kid? Probably not.”
A version of this article first appeared on Medscape.com.

