User login
Hearing voices, time traveling, and being hit with a high-heeled shoe
CASE Grief and confusion
Mr. P, age 47, is arrested for entering the apartment of a woman he does not know and tossing her belongings out the window. When he is assessed to determine if he can participate in his legal defense, examiners find an attentive, courteous man who is baffled by his own behavior.
Mr. P says that he had been “stressed out” after the recent death of his grandmother, with whom he was close. He says he entered the apartment because voices told him to do so. He has no recent history of substance abuse or psychiatric hospitalizations, but he had a similar episode of “confusion” years before, when another close family member died.
Mr. P is found not fit to stand trial and the charges are dropped. He accepts haloperidol, 10 mg/d, and benztropine, 2 mg/d, and is transferred to a hospital for psychiatric treatment.
On interview, Mr. P is well groomed, soft-spoken, and shy, without formal thought disorder. Physical exam and routine lab tests are within normal limits. He says that 18 months before his arrest, he and his frail grandmother moved to a large city in hopes that he would find a wife. Both depended on the grandmother’s Social Security benefits while he cared for her.
In the 2 months after she died, he reports that he felt sad and alone and slept poorly, but made efforts to find a job and keep his apartment. When his efforts failed and he lost the apartment, he stayed with various friends for a few days at a time, then spent several days in the subway before ending up on the streets.
His arrest on the current charge occurred 4 days after he began walking the streets.
a) continue haloperidol to treat psychotic symptoms
b) discontinue haloperidol and observe him
c) add an antidepressant to haloperidol
HISTORY Imagining nonsense
Mr. P cannot explain why he started “trashing” the woman’s apartment, but says he entered it because he thought it was his apartment. With embarrassment and regret, he admits he has been depressed and confused, “imagining things”—“foolish things,” he admits—such as being in a different “time zone.”
Contradicting his earlier statements, Mr. P now admits that he had “a few beers” and denies that he experienced auditory hallucinations, saying he only talks to himself. He now says that within 2 days after his arrest, he was “all over it.” Mr. P denies current symptoms, including hallucinations, but, when pressed, waffles, then admits to a strange belief: that some people, including him, can move from one “time zone” to another.
Mr. P says he was treated for psychiatric problems 4 years earlier when his parents were killed in a car crash. By his recollection, his reaction to their death was similar to his reaction to his grandmother’s death: He became upset and wandered the streets for a few days, “moving between time zones” and talking to himself but not experiencing hallucinations. After he was taken to a hospital and “given an injection,” he calmed down and was released. Within a few days he recovered and returned to supporting himself and caring for his grandmother. Mr. P says the idea of travelling between “time zones” is embarrassing and nonsensical but adds that he was affected in this way because he “bickered” with his mother.
Mr. P’s grandmother raised him until he was age 15, although he frequently visited his parents, who lived nearby and worked during the day. Mr. P initially denies substance abuse, then admits to smoking marijuana every day for about a year before admission. He also admits to cocaine abuse in his 20s. He denies a history of suicide attempts.
The author’s observations
Mr. P reported only 2 episodes of “confusion” (or psychosis) and strange behavior in his life, both precipitated by the loss of a loved one, and at least 1 while under the influence of alcohol and Cannabis. He gave an inconsistent and ambiguous history of auditory hallucinations associated with episodes of confusion. He believes that time travel is possible, an idea that he acknowledged is nonsense. This alone was not enough to warrant long-term antipsychotic treatment. The most likely diagnosis seemed to be brief psychotic episode induced by Cannabis and the stressors of homelessness and his grandmother’s death.
EVALUATION Changing stories
No longer taking haloperidol, Mr. P continues to deny hallucinations and depressed mood, but keeps to himself. Nine days after admission he becomes tearful after he informs his aunt of his grandmother’s death in a telephone call, then approaches a nurse and complains of sadness and auditory hallucinations.
Mr. P confesses that he denied hallucinations on admission because he feared he would remain in the hospital for years if he revealed the truth that he had been experiencing auditory hallucinations almost continuously from age 10. He reports that the voices distracted him when he worked; seem to be male; often spoke gibberish; and alternate between deprecating and positive and supportive. Mr. P is reluctant to disclose more about what the voices actually say, although he acknowledges that they are not commenting or conversing with him, and that he has never believed the voices were his own thoughts but did believe that they came from inside his brain.
With haloperidol, the voices stopped. They resumed, however, when haloperidol was discontinued.
When we ask what happened to him at age 10, Mr. P shrugs.
a) childhood onset schizophrenia
b) substance abuse
c) posttraumatic stress disorder (PTSD)
d) none
The author’s observations
In community samples of children and adolescents, auditory hallucinations are not rare and usually do not cause distress or dysfunction. In a study of 3,870 children age 7 and 8,1 9% endorsed auditory hallucinations. Most heard 1 voice, once a week or less, at low volume. In 85% of children who experienced hallucinations, they caused minimal or no suffering; 97% reported minimal or no interference with daily functioning. Among children who experienced auditory hallucinations at age 7 or 8, 24% continued to hallucinate 5 years later.2 Persistent hallucinations were associated with more problematic behaviors at baseline and follow up.
In a group of 12-year-old twins, 4.2% reported auditory hallucinations.3 In that study, hallucinations were not related to Cannabis use; rather, they were heritable and related to risk factors such as cognitive impairment; behavioral, emotional, and educational problems at age 5; and a history of physical abuse and self-harm at age 12. The authors noted that these are risk factors and correlates of schizophrenia, but are not specific to schizophrenia.
Hallucinations and delusions have been found in 4% to 8% of children and adolescents referred for psychiatric treatment,4 far more than the prevalence of childhood-onset schizophrenia (0.01% of children).5 Psychotic symptoms in children have been associated with bipolar disorder, but also with anxiety disorders, obsessive-compulsive disorder, PTSD, pervasive developmental disorder, conduct disorder, and substance abuse.4
Childhood-onset schizophrenia is rare and would require that Mr. P have a diagnosis of schizophrenia as an adult. It is possible that Mr. P’s childhood symptoms were related to substance abuse but he was not asked for this history because it seemed unlikely in a 10-year-old boy. A PTSD diagnosis requires a traumatic event, which Mr. P did not reveal. It is possible that at age 10 he did not have a psychiatric disorder.
a) PTSD
b) dissociative disorder
c) borderline personality disorder
d) chronic schizophrenia
e) no psychiatric diagnosis
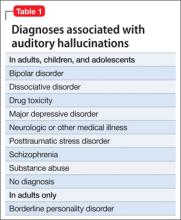
Among adults in the general population, 10% to 15% report auditory hallucinations.6 Hallucinations could be caused by substance abuse or psychiatric conditions other than schizophrenia; however, in adults—as in children—auditory hallucinations can occur in the absence of these conditions (Table 1) and rarely cause distress or dysfunction.6 In Sommer and colleagues’6 study of 103 healthy persons, none who heard voices had disorganization or negative symptoms. Those who heard voices had significantly more schizotypal symptoms and more childhood trauma, including emotional, physical, and sexual abuse, than those who did not hear voices.6
Conditions associated with hallucinations
PTSD is associated with auditory hallucinations and other psychotic symptoms.7 Most studies are of combat veterans with PTSD, in whom auditory hallucinations and delusions were associated with major depressive disorder, not a thought disorder or inappropriate affect.8 In a community sample,9 psychotic symptoms—particularly auditory hallucinations—were associated with PTSD. Subjects with PTSD and psychotic symptoms were more likely to have other psychiatric disorders, including major depressive disorder and substance use disorder, than patients with PTSD but no psychotic symptoms; however, the relationship between PTSD and psychosis remained after controlling for other psychiatric disorders.
Hallucinations can occur in persons with dissociative disorders in the absence of distinct personality states.10 Hallucinations have been seen transiently and chronically in persons with borderline personality disorder and can be associated with comorbid conditions such as substance abuse disorders, mood disorders, and PTSD.11
Mr. P lacked the reduced capacity for interpersonal relationships required for a schizotypal personality disorder diagnosis. A diagnosis of PTSD or dissociative disorder requires a history of trauma, which Mr. P did not report.
“Time travelling” with incomprehensible behavior could be interpreted as dissociation, but dissociative fugue or dissociative disorder not otherwise specified (NOS) cannot be diagnosed if symptoms might be the direct effect of a substance, such as Cannabis. Mr. P admitted to substance abuse. We can rule out borderline personality disorder because he did not display or admit to tempestuous interpersonal relationships.
A schizophrenia diagnosis requires the presence of auditory hallucinations that commented on his behavior or conversed among themselves, a second psychotic symptom for ≥1 month, or negative symptoms, which Mr. P lacked (unless belief in time travel is considered delusional).
Last, a physician might have considered malingering or a factitious disorder when Mr. P was found not able to participate in his own defense, but this seemed less likely after he revealed that he experienced auditory hallucinations since age 10.
HISTORY Bad beatings
With a few days of beginning risperidone, 4 mg/d, Mr. P reports that his hallucinations have stopped and he feels less sad. He reveals that, at age 10, when the hallucinations began, his mother hit him over the head with a high-heeled shoe, causing a scalp laceration that required a visit to the emergency room for suturing. His mother beat Mr. P for as long as he could remember. She beat him “bad” at least twice weekly, and he was taken to the hospital 7 or 8 times for injury, but she also beat him “constantly” with a belt buckle, sometimes striking his head. She instructed him to tell nobody.
The author’s observations
Auditory hallucinations in adults have been associated with childhood abuse, particularly childhood sexual abuse,12 in clinical and non-clinical samples.13 Some argue13 that child abuse itself causes hallucinations and other psychotic symptoms.
OUTCOME Depressed and sleepless
Mr. P admits that he had been smoking marijuana 2 to 3 times daily for a year. He also reports insomnia, sleeping approximately 4 hours a night and spending hours awake in bed thinking of his grandmother, with depressed mood and tearfulness. He denies suicidal ideas and hallucinations. He is treated for depressive disorder NOS first with amitriptyline, 50 mg at bedtime, for sleep, then paroxetine, 20 mg/d, for depressive symptoms, in addition to risperidone, 4 mg/d. Although Mr. P does not describe re-experiencing his childhood trauma, avoidance of stimuli associated with the trauma, or symptoms of increased arousal (except for insomnia), the treatment team did not ask, so it remains uncertain if he has PTSD (Table 2).
When Mr. P is discharged to a clinic, he smiles easily and is positive and supportive with other patients. He spruces up his appearance by wearing jewelry and works in the hospital kitchen.
Bottom Line
Chronic auditory hallucinations are associated with psychiatric illnesses other than chronic schizophrenia, particularly those resulting from trauma such as posttraumatic stress disorder. They can also occur in the absence of diagnosable psychiatric illness and rarely cause distress or functional impairment. Auditory hallucinations in adults have been associated with childhood abuse.
Related Resources
- Moskowitz A, Schafer I, Dorahy MJ. Psychosis, trauma and dissociation: emerging perspectives on severe psychopathology. West Sussex, UK: John Wiley and Sons, Ltd.; 2008.
- The International Hearing Voices Network. www.intervoiceonline.org.
Drug Brand Names
Amitriptyline • Elavil Paroxetine • Paxil
Benztropine • Cogentin Risperidone • Risperdal
Haloperidol • Haldol
Disclosure
Dr. Crowner reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Barthel-Velthuis AA, Jenner JA, van de Willige G, et al Prevalence and correlates of auditory vocal hallucinations in middle childhood. Br J Psychiatry. 2010;196(1):41-46.
2. Bartels-Velthuis AA, van de Willige G, Jenner JA, et al. Course of auditory vocal hallucinations in childhood: 5-year follow-up study. Br J Psychiatry. 2011;199(4):296-302.
3. Polanczyk G, Moffitt TE, Arsensault L, et al. Etiological and clinical features of childhood psychotic symptoms: results from a birth cohort. Arch Gen Psychiatry. 2010;67(4):328-338.
4. Biederman J, Pety C, Faracone SV, et al. Phenomenology of childhood psychosis: Findings from a large sample of psychiatrically referred youth. J Nerv Ment Dis 2004;192(9):607-614.
5. American Academy of Child and Adolescent Psychiatry. Practice parameters for the assessment and treatment of children and adolescents with schizophrenia. J Am Acad Child Adolesc Psychiatry. 2001;40(suppl 7):4SS-23S.
6. Sommer IEC, Daalman K, Rietkerk T, et al. Healthy individuals with auditory verbal hallucinations; Who are they? Psychiatric assessments of a selected sample of 103 subjects. Schizophr Bull. 2010;36(3):633-641.
7. Butler RW, Mueser KT, Sprock J, et al. Positive symptoms of psychosis in posttraumatic stress disorder. Biol Psychiatry. 1996;39:839-844.
8. David D, Kutcher GS, Jackson EI, et al Psychotic symptoms in combat-related posttraumatic stress disorder. J Clin Psychiatry. 1999;60(1):29-32.
9. Sareen J, Cox BJ, Goodwin RD, et al. Co-occurrence of posttraumatic stress disorder with positive psychotic symptoms in a nationally representative sample. J Trauma Stress. 2005;18(4):313-322.
10. Sar V, Akyuv G, Dogan O. Prevalence of dissociative disorders among women in the general population. Psychiatry Res. 2007;149:169-176.
11. Barnow S, Arens EA, Sieswerda S, et al. Borderline personality disorder and psychosis: a review. Curr Psychiatry Rep. 2010;12(3):186-195.
12. Bebbington P, Jonas S, Kuipers E, et al. Childhood sexual abuse and psychosis: data from a cross-sectional national psychiatric survey in England. Br J Psychiatry. 2011;199(1):29-37.
13. Read J, van Os J, Morrison AP, et al. Childhood trauma, psychosis and schizophrenia: a literature review with theoretical and clinical implications. Acta Psychiatr Scand. 2005;112(5):330-350.
CASE Grief and confusion
Mr. P, age 47, is arrested for entering the apartment of a woman he does not know and tossing her belongings out the window. When he is assessed to determine if he can participate in his legal defense, examiners find an attentive, courteous man who is baffled by his own behavior.
Mr. P says that he had been “stressed out” after the recent death of his grandmother, with whom he was close. He says he entered the apartment because voices told him to do so. He has no recent history of substance abuse or psychiatric hospitalizations, but he had a similar episode of “confusion” years before, when another close family member died.
Mr. P is found not fit to stand trial and the charges are dropped. He accepts haloperidol, 10 mg/d, and benztropine, 2 mg/d, and is transferred to a hospital for psychiatric treatment.
On interview, Mr. P is well groomed, soft-spoken, and shy, without formal thought disorder. Physical exam and routine lab tests are within normal limits. He says that 18 months before his arrest, he and his frail grandmother moved to a large city in hopes that he would find a wife. Both depended on the grandmother’s Social Security benefits while he cared for her.
In the 2 months after she died, he reports that he felt sad and alone and slept poorly, but made efforts to find a job and keep his apartment. When his efforts failed and he lost the apartment, he stayed with various friends for a few days at a time, then spent several days in the subway before ending up on the streets.
His arrest on the current charge occurred 4 days after he began walking the streets.
a) continue haloperidol to treat psychotic symptoms
b) discontinue haloperidol and observe him
c) add an antidepressant to haloperidol
HISTORY Imagining nonsense
Mr. P cannot explain why he started “trashing” the woman’s apartment, but says he entered it because he thought it was his apartment. With embarrassment and regret, he admits he has been depressed and confused, “imagining things”—“foolish things,” he admits—such as being in a different “time zone.”
Contradicting his earlier statements, Mr. P now admits that he had “a few beers” and denies that he experienced auditory hallucinations, saying he only talks to himself. He now says that within 2 days after his arrest, he was “all over it.” Mr. P denies current symptoms, including hallucinations, but, when pressed, waffles, then admits to a strange belief: that some people, including him, can move from one “time zone” to another.
Mr. P says he was treated for psychiatric problems 4 years earlier when his parents were killed in a car crash. By his recollection, his reaction to their death was similar to his reaction to his grandmother’s death: He became upset and wandered the streets for a few days, “moving between time zones” and talking to himself but not experiencing hallucinations. After he was taken to a hospital and “given an injection,” he calmed down and was released. Within a few days he recovered and returned to supporting himself and caring for his grandmother. Mr. P says the idea of travelling between “time zones” is embarrassing and nonsensical but adds that he was affected in this way because he “bickered” with his mother.
Mr. P’s grandmother raised him until he was age 15, although he frequently visited his parents, who lived nearby and worked during the day. Mr. P initially denies substance abuse, then admits to smoking marijuana every day for about a year before admission. He also admits to cocaine abuse in his 20s. He denies a history of suicide attempts.
The author’s observations
Mr. P reported only 2 episodes of “confusion” (or psychosis) and strange behavior in his life, both precipitated by the loss of a loved one, and at least 1 while under the influence of alcohol and Cannabis. He gave an inconsistent and ambiguous history of auditory hallucinations associated with episodes of confusion. He believes that time travel is possible, an idea that he acknowledged is nonsense. This alone was not enough to warrant long-term antipsychotic treatment. The most likely diagnosis seemed to be brief psychotic episode induced by Cannabis and the stressors of homelessness and his grandmother’s death.
EVALUATION Changing stories
No longer taking haloperidol, Mr. P continues to deny hallucinations and depressed mood, but keeps to himself. Nine days after admission he becomes tearful after he informs his aunt of his grandmother’s death in a telephone call, then approaches a nurse and complains of sadness and auditory hallucinations.
Mr. P confesses that he denied hallucinations on admission because he feared he would remain in the hospital for years if he revealed the truth that he had been experiencing auditory hallucinations almost continuously from age 10. He reports that the voices distracted him when he worked; seem to be male; often spoke gibberish; and alternate between deprecating and positive and supportive. Mr. P is reluctant to disclose more about what the voices actually say, although he acknowledges that they are not commenting or conversing with him, and that he has never believed the voices were his own thoughts but did believe that they came from inside his brain.
With haloperidol, the voices stopped. They resumed, however, when haloperidol was discontinued.
When we ask what happened to him at age 10, Mr. P shrugs.
a) childhood onset schizophrenia
b) substance abuse
c) posttraumatic stress disorder (PTSD)
d) none
The author’s observations
In community samples of children and adolescents, auditory hallucinations are not rare and usually do not cause distress or dysfunction. In a study of 3,870 children age 7 and 8,1 9% endorsed auditory hallucinations. Most heard 1 voice, once a week or less, at low volume. In 85% of children who experienced hallucinations, they caused minimal or no suffering; 97% reported minimal or no interference with daily functioning. Among children who experienced auditory hallucinations at age 7 or 8, 24% continued to hallucinate 5 years later.2 Persistent hallucinations were associated with more problematic behaviors at baseline and follow up.
In a group of 12-year-old twins, 4.2% reported auditory hallucinations.3 In that study, hallucinations were not related to Cannabis use; rather, they were heritable and related to risk factors such as cognitive impairment; behavioral, emotional, and educational problems at age 5; and a history of physical abuse and self-harm at age 12. The authors noted that these are risk factors and correlates of schizophrenia, but are not specific to schizophrenia.
Hallucinations and delusions have been found in 4% to 8% of children and adolescents referred for psychiatric treatment,4 far more than the prevalence of childhood-onset schizophrenia (0.01% of children).5 Psychotic symptoms in children have been associated with bipolar disorder, but also with anxiety disorders, obsessive-compulsive disorder, PTSD, pervasive developmental disorder, conduct disorder, and substance abuse.4
Childhood-onset schizophrenia is rare and would require that Mr. P have a diagnosis of schizophrenia as an adult. It is possible that Mr. P’s childhood symptoms were related to substance abuse but he was not asked for this history because it seemed unlikely in a 10-year-old boy. A PTSD diagnosis requires a traumatic event, which Mr. P did not reveal. It is possible that at age 10 he did not have a psychiatric disorder.
a) PTSD
b) dissociative disorder
c) borderline personality disorder
d) chronic schizophrenia
e) no psychiatric diagnosis
Among adults in the general population, 10% to 15% report auditory hallucinations.6 Hallucinations could be caused by substance abuse or psychiatric conditions other than schizophrenia; however, in adults—as in children—auditory hallucinations can occur in the absence of these conditions (Table 1) and rarely cause distress or dysfunction.6 In Sommer and colleagues’6 study of 103 healthy persons, none who heard voices had disorganization or negative symptoms. Those who heard voices had significantly more schizotypal symptoms and more childhood trauma, including emotional, physical, and sexual abuse, than those who did not hear voices.6
Conditions associated with hallucinations
PTSD is associated with auditory hallucinations and other psychotic symptoms.7 Most studies are of combat veterans with PTSD, in whom auditory hallucinations and delusions were associated with major depressive disorder, not a thought disorder or inappropriate affect.8 In a community sample,9 psychotic symptoms—particularly auditory hallucinations—were associated with PTSD. Subjects with PTSD and psychotic symptoms were more likely to have other psychiatric disorders, including major depressive disorder and substance use disorder, than patients with PTSD but no psychotic symptoms; however, the relationship between PTSD and psychosis remained after controlling for other psychiatric disorders.
Hallucinations can occur in persons with dissociative disorders in the absence of distinct personality states.10 Hallucinations have been seen transiently and chronically in persons with borderline personality disorder and can be associated with comorbid conditions such as substance abuse disorders, mood disorders, and PTSD.11
Mr. P lacked the reduced capacity for interpersonal relationships required for a schizotypal personality disorder diagnosis. A diagnosis of PTSD or dissociative disorder requires a history of trauma, which Mr. P did not report.
“Time travelling” with incomprehensible behavior could be interpreted as dissociation, but dissociative fugue or dissociative disorder not otherwise specified (NOS) cannot be diagnosed if symptoms might be the direct effect of a substance, such as Cannabis. Mr. P admitted to substance abuse. We can rule out borderline personality disorder because he did not display or admit to tempestuous interpersonal relationships.
A schizophrenia diagnosis requires the presence of auditory hallucinations that commented on his behavior or conversed among themselves, a second psychotic symptom for ≥1 month, or negative symptoms, which Mr. P lacked (unless belief in time travel is considered delusional).
Last, a physician might have considered malingering or a factitious disorder when Mr. P was found not able to participate in his own defense, but this seemed less likely after he revealed that he experienced auditory hallucinations since age 10.
HISTORY Bad beatings
With a few days of beginning risperidone, 4 mg/d, Mr. P reports that his hallucinations have stopped and he feels less sad. He reveals that, at age 10, when the hallucinations began, his mother hit him over the head with a high-heeled shoe, causing a scalp laceration that required a visit to the emergency room for suturing. His mother beat Mr. P for as long as he could remember. She beat him “bad” at least twice weekly, and he was taken to the hospital 7 or 8 times for injury, but she also beat him “constantly” with a belt buckle, sometimes striking his head. She instructed him to tell nobody.
The author’s observations
Auditory hallucinations in adults have been associated with childhood abuse, particularly childhood sexual abuse,12 in clinical and non-clinical samples.13 Some argue13 that child abuse itself causes hallucinations and other psychotic symptoms.
OUTCOME Depressed and sleepless
Mr. P admits that he had been smoking marijuana 2 to 3 times daily for a year. He also reports insomnia, sleeping approximately 4 hours a night and spending hours awake in bed thinking of his grandmother, with depressed mood and tearfulness. He denies suicidal ideas and hallucinations. He is treated for depressive disorder NOS first with amitriptyline, 50 mg at bedtime, for sleep, then paroxetine, 20 mg/d, for depressive symptoms, in addition to risperidone, 4 mg/d. Although Mr. P does not describe re-experiencing his childhood trauma, avoidance of stimuli associated with the trauma, or symptoms of increased arousal (except for insomnia), the treatment team did not ask, so it remains uncertain if he has PTSD (Table 2).
When Mr. P is discharged to a clinic, he smiles easily and is positive and supportive with other patients. He spruces up his appearance by wearing jewelry and works in the hospital kitchen.
Bottom Line
Chronic auditory hallucinations are associated with psychiatric illnesses other than chronic schizophrenia, particularly those resulting from trauma such as posttraumatic stress disorder. They can also occur in the absence of diagnosable psychiatric illness and rarely cause distress or functional impairment. Auditory hallucinations in adults have been associated with childhood abuse.
Related Resources
- Moskowitz A, Schafer I, Dorahy MJ. Psychosis, trauma and dissociation: emerging perspectives on severe psychopathology. West Sussex, UK: John Wiley and Sons, Ltd.; 2008.
- The International Hearing Voices Network. www.intervoiceonline.org.
Drug Brand Names
Amitriptyline • Elavil Paroxetine • Paxil
Benztropine • Cogentin Risperidone • Risperdal
Haloperidol • Haldol
Disclosure
Dr. Crowner reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Grief and confusion
Mr. P, age 47, is arrested for entering the apartment of a woman he does not know and tossing her belongings out the window. When he is assessed to determine if he can participate in his legal defense, examiners find an attentive, courteous man who is baffled by his own behavior.
Mr. P says that he had been “stressed out” after the recent death of his grandmother, with whom he was close. He says he entered the apartment because voices told him to do so. He has no recent history of substance abuse or psychiatric hospitalizations, but he had a similar episode of “confusion” years before, when another close family member died.
Mr. P is found not fit to stand trial and the charges are dropped. He accepts haloperidol, 10 mg/d, and benztropine, 2 mg/d, and is transferred to a hospital for psychiatric treatment.
On interview, Mr. P is well groomed, soft-spoken, and shy, without formal thought disorder. Physical exam and routine lab tests are within normal limits. He says that 18 months before his arrest, he and his frail grandmother moved to a large city in hopes that he would find a wife. Both depended on the grandmother’s Social Security benefits while he cared for her.
In the 2 months after she died, he reports that he felt sad and alone and slept poorly, but made efforts to find a job and keep his apartment. When his efforts failed and he lost the apartment, he stayed with various friends for a few days at a time, then spent several days in the subway before ending up on the streets.
His arrest on the current charge occurred 4 days after he began walking the streets.
a) continue haloperidol to treat psychotic symptoms
b) discontinue haloperidol and observe him
c) add an antidepressant to haloperidol
HISTORY Imagining nonsense
Mr. P cannot explain why he started “trashing” the woman’s apartment, but says he entered it because he thought it was his apartment. With embarrassment and regret, he admits he has been depressed and confused, “imagining things”—“foolish things,” he admits—such as being in a different “time zone.”
Contradicting his earlier statements, Mr. P now admits that he had “a few beers” and denies that he experienced auditory hallucinations, saying he only talks to himself. He now says that within 2 days after his arrest, he was “all over it.” Mr. P denies current symptoms, including hallucinations, but, when pressed, waffles, then admits to a strange belief: that some people, including him, can move from one “time zone” to another.
Mr. P says he was treated for psychiatric problems 4 years earlier when his parents were killed in a car crash. By his recollection, his reaction to their death was similar to his reaction to his grandmother’s death: He became upset and wandered the streets for a few days, “moving between time zones” and talking to himself but not experiencing hallucinations. After he was taken to a hospital and “given an injection,” he calmed down and was released. Within a few days he recovered and returned to supporting himself and caring for his grandmother. Mr. P says the idea of travelling between “time zones” is embarrassing and nonsensical but adds that he was affected in this way because he “bickered” with his mother.
Mr. P’s grandmother raised him until he was age 15, although he frequently visited his parents, who lived nearby and worked during the day. Mr. P initially denies substance abuse, then admits to smoking marijuana every day for about a year before admission. He also admits to cocaine abuse in his 20s. He denies a history of suicide attempts.
The author’s observations
Mr. P reported only 2 episodes of “confusion” (or psychosis) and strange behavior in his life, both precipitated by the loss of a loved one, and at least 1 while under the influence of alcohol and Cannabis. He gave an inconsistent and ambiguous history of auditory hallucinations associated with episodes of confusion. He believes that time travel is possible, an idea that he acknowledged is nonsense. This alone was not enough to warrant long-term antipsychotic treatment. The most likely diagnosis seemed to be brief psychotic episode induced by Cannabis and the stressors of homelessness and his grandmother’s death.
EVALUATION Changing stories
No longer taking haloperidol, Mr. P continues to deny hallucinations and depressed mood, but keeps to himself. Nine days after admission he becomes tearful after he informs his aunt of his grandmother’s death in a telephone call, then approaches a nurse and complains of sadness and auditory hallucinations.
Mr. P confesses that he denied hallucinations on admission because he feared he would remain in the hospital for years if he revealed the truth that he had been experiencing auditory hallucinations almost continuously from age 10. He reports that the voices distracted him when he worked; seem to be male; often spoke gibberish; and alternate between deprecating and positive and supportive. Mr. P is reluctant to disclose more about what the voices actually say, although he acknowledges that they are not commenting or conversing with him, and that he has never believed the voices were his own thoughts but did believe that they came from inside his brain.
With haloperidol, the voices stopped. They resumed, however, when haloperidol was discontinued.
When we ask what happened to him at age 10, Mr. P shrugs.
a) childhood onset schizophrenia
b) substance abuse
c) posttraumatic stress disorder (PTSD)
d) none
The author’s observations
In community samples of children and adolescents, auditory hallucinations are not rare and usually do not cause distress or dysfunction. In a study of 3,870 children age 7 and 8,1 9% endorsed auditory hallucinations. Most heard 1 voice, once a week or less, at low volume. In 85% of children who experienced hallucinations, they caused minimal or no suffering; 97% reported minimal or no interference with daily functioning. Among children who experienced auditory hallucinations at age 7 or 8, 24% continued to hallucinate 5 years later.2 Persistent hallucinations were associated with more problematic behaviors at baseline and follow up.
In a group of 12-year-old twins, 4.2% reported auditory hallucinations.3 In that study, hallucinations were not related to Cannabis use; rather, they were heritable and related to risk factors such as cognitive impairment; behavioral, emotional, and educational problems at age 5; and a history of physical abuse and self-harm at age 12. The authors noted that these are risk factors and correlates of schizophrenia, but are not specific to schizophrenia.
Hallucinations and delusions have been found in 4% to 8% of children and adolescents referred for psychiatric treatment,4 far more than the prevalence of childhood-onset schizophrenia (0.01% of children).5 Psychotic symptoms in children have been associated with bipolar disorder, but also with anxiety disorders, obsessive-compulsive disorder, PTSD, pervasive developmental disorder, conduct disorder, and substance abuse.4
Childhood-onset schizophrenia is rare and would require that Mr. P have a diagnosis of schizophrenia as an adult. It is possible that Mr. P’s childhood symptoms were related to substance abuse but he was not asked for this history because it seemed unlikely in a 10-year-old boy. A PTSD diagnosis requires a traumatic event, which Mr. P did not reveal. It is possible that at age 10 he did not have a psychiatric disorder.
a) PTSD
b) dissociative disorder
c) borderline personality disorder
d) chronic schizophrenia
e) no psychiatric diagnosis
Among adults in the general population, 10% to 15% report auditory hallucinations.6 Hallucinations could be caused by substance abuse or psychiatric conditions other than schizophrenia; however, in adults—as in children—auditory hallucinations can occur in the absence of these conditions (Table 1) and rarely cause distress or dysfunction.6 In Sommer and colleagues’6 study of 103 healthy persons, none who heard voices had disorganization or negative symptoms. Those who heard voices had significantly more schizotypal symptoms and more childhood trauma, including emotional, physical, and sexual abuse, than those who did not hear voices.6
Conditions associated with hallucinations
PTSD is associated with auditory hallucinations and other psychotic symptoms.7 Most studies are of combat veterans with PTSD, in whom auditory hallucinations and delusions were associated with major depressive disorder, not a thought disorder or inappropriate affect.8 In a community sample,9 psychotic symptoms—particularly auditory hallucinations—were associated with PTSD. Subjects with PTSD and psychotic symptoms were more likely to have other psychiatric disorders, including major depressive disorder and substance use disorder, than patients with PTSD but no psychotic symptoms; however, the relationship between PTSD and psychosis remained after controlling for other psychiatric disorders.
Hallucinations can occur in persons with dissociative disorders in the absence of distinct personality states.10 Hallucinations have been seen transiently and chronically in persons with borderline personality disorder and can be associated with comorbid conditions such as substance abuse disorders, mood disorders, and PTSD.11
Mr. P lacked the reduced capacity for interpersonal relationships required for a schizotypal personality disorder diagnosis. A diagnosis of PTSD or dissociative disorder requires a history of trauma, which Mr. P did not report.
“Time travelling” with incomprehensible behavior could be interpreted as dissociation, but dissociative fugue or dissociative disorder not otherwise specified (NOS) cannot be diagnosed if symptoms might be the direct effect of a substance, such as Cannabis. Mr. P admitted to substance abuse. We can rule out borderline personality disorder because he did not display or admit to tempestuous interpersonal relationships.
A schizophrenia diagnosis requires the presence of auditory hallucinations that commented on his behavior or conversed among themselves, a second psychotic symptom for ≥1 month, or negative symptoms, which Mr. P lacked (unless belief in time travel is considered delusional).
Last, a physician might have considered malingering or a factitious disorder when Mr. P was found not able to participate in his own defense, but this seemed less likely after he revealed that he experienced auditory hallucinations since age 10.
HISTORY Bad beatings
With a few days of beginning risperidone, 4 mg/d, Mr. P reports that his hallucinations have stopped and he feels less sad. He reveals that, at age 10, when the hallucinations began, his mother hit him over the head with a high-heeled shoe, causing a scalp laceration that required a visit to the emergency room for suturing. His mother beat Mr. P for as long as he could remember. She beat him “bad” at least twice weekly, and he was taken to the hospital 7 or 8 times for injury, but she also beat him “constantly” with a belt buckle, sometimes striking his head. She instructed him to tell nobody.
The author’s observations
Auditory hallucinations in adults have been associated with childhood abuse, particularly childhood sexual abuse,12 in clinical and non-clinical samples.13 Some argue13 that child abuse itself causes hallucinations and other psychotic symptoms.
OUTCOME Depressed and sleepless
Mr. P admits that he had been smoking marijuana 2 to 3 times daily for a year. He also reports insomnia, sleeping approximately 4 hours a night and spending hours awake in bed thinking of his grandmother, with depressed mood and tearfulness. He denies suicidal ideas and hallucinations. He is treated for depressive disorder NOS first with amitriptyline, 50 mg at bedtime, for sleep, then paroxetine, 20 mg/d, for depressive symptoms, in addition to risperidone, 4 mg/d. Although Mr. P does not describe re-experiencing his childhood trauma, avoidance of stimuli associated with the trauma, or symptoms of increased arousal (except for insomnia), the treatment team did not ask, so it remains uncertain if he has PTSD (Table 2).
When Mr. P is discharged to a clinic, he smiles easily and is positive and supportive with other patients. He spruces up his appearance by wearing jewelry and works in the hospital kitchen.
Bottom Line
Chronic auditory hallucinations are associated with psychiatric illnesses other than chronic schizophrenia, particularly those resulting from trauma such as posttraumatic stress disorder. They can also occur in the absence of diagnosable psychiatric illness and rarely cause distress or functional impairment. Auditory hallucinations in adults have been associated with childhood abuse.
Related Resources
- Moskowitz A, Schafer I, Dorahy MJ. Psychosis, trauma and dissociation: emerging perspectives on severe psychopathology. West Sussex, UK: John Wiley and Sons, Ltd.; 2008.
- The International Hearing Voices Network. www.intervoiceonline.org.
Drug Brand Names
Amitriptyline • Elavil Paroxetine • Paxil
Benztropine • Cogentin Risperidone • Risperdal
Haloperidol • Haldol
Disclosure
Dr. Crowner reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Barthel-Velthuis AA, Jenner JA, van de Willige G, et al Prevalence and correlates of auditory vocal hallucinations in middle childhood. Br J Psychiatry. 2010;196(1):41-46.
2. Bartels-Velthuis AA, van de Willige G, Jenner JA, et al. Course of auditory vocal hallucinations in childhood: 5-year follow-up study. Br J Psychiatry. 2011;199(4):296-302.
3. Polanczyk G, Moffitt TE, Arsensault L, et al. Etiological and clinical features of childhood psychotic symptoms: results from a birth cohort. Arch Gen Psychiatry. 2010;67(4):328-338.
4. Biederman J, Pety C, Faracone SV, et al. Phenomenology of childhood psychosis: Findings from a large sample of psychiatrically referred youth. J Nerv Ment Dis 2004;192(9):607-614.
5. American Academy of Child and Adolescent Psychiatry. Practice parameters for the assessment and treatment of children and adolescents with schizophrenia. J Am Acad Child Adolesc Psychiatry. 2001;40(suppl 7):4SS-23S.
6. Sommer IEC, Daalman K, Rietkerk T, et al. Healthy individuals with auditory verbal hallucinations; Who are they? Psychiatric assessments of a selected sample of 103 subjects. Schizophr Bull. 2010;36(3):633-641.
7. Butler RW, Mueser KT, Sprock J, et al. Positive symptoms of psychosis in posttraumatic stress disorder. Biol Psychiatry. 1996;39:839-844.
8. David D, Kutcher GS, Jackson EI, et al Psychotic symptoms in combat-related posttraumatic stress disorder. J Clin Psychiatry. 1999;60(1):29-32.
9. Sareen J, Cox BJ, Goodwin RD, et al. Co-occurrence of posttraumatic stress disorder with positive psychotic symptoms in a nationally representative sample. J Trauma Stress. 2005;18(4):313-322.
10. Sar V, Akyuv G, Dogan O. Prevalence of dissociative disorders among women in the general population. Psychiatry Res. 2007;149:169-176.
11. Barnow S, Arens EA, Sieswerda S, et al. Borderline personality disorder and psychosis: a review. Curr Psychiatry Rep. 2010;12(3):186-195.
12. Bebbington P, Jonas S, Kuipers E, et al. Childhood sexual abuse and psychosis: data from a cross-sectional national psychiatric survey in England. Br J Psychiatry. 2011;199(1):29-37.
13. Read J, van Os J, Morrison AP, et al. Childhood trauma, psychosis and schizophrenia: a literature review with theoretical and clinical implications. Acta Psychiatr Scand. 2005;112(5):330-350.
1. Barthel-Velthuis AA, Jenner JA, van de Willige G, et al Prevalence and correlates of auditory vocal hallucinations in middle childhood. Br J Psychiatry. 2010;196(1):41-46.
2. Bartels-Velthuis AA, van de Willige G, Jenner JA, et al. Course of auditory vocal hallucinations in childhood: 5-year follow-up study. Br J Psychiatry. 2011;199(4):296-302.
3. Polanczyk G, Moffitt TE, Arsensault L, et al. Etiological and clinical features of childhood psychotic symptoms: results from a birth cohort. Arch Gen Psychiatry. 2010;67(4):328-338.
4. Biederman J, Pety C, Faracone SV, et al. Phenomenology of childhood psychosis: Findings from a large sample of psychiatrically referred youth. J Nerv Ment Dis 2004;192(9):607-614.
5. American Academy of Child and Adolescent Psychiatry. Practice parameters for the assessment and treatment of children and adolescents with schizophrenia. J Am Acad Child Adolesc Psychiatry. 2001;40(suppl 7):4SS-23S.
6. Sommer IEC, Daalman K, Rietkerk T, et al. Healthy individuals with auditory verbal hallucinations; Who are they? Psychiatric assessments of a selected sample of 103 subjects. Schizophr Bull. 2010;36(3):633-641.
7. Butler RW, Mueser KT, Sprock J, et al. Positive symptoms of psychosis in posttraumatic stress disorder. Biol Psychiatry. 1996;39:839-844.
8. David D, Kutcher GS, Jackson EI, et al Psychotic symptoms in combat-related posttraumatic stress disorder. J Clin Psychiatry. 1999;60(1):29-32.
9. Sareen J, Cox BJ, Goodwin RD, et al. Co-occurrence of posttraumatic stress disorder with positive psychotic symptoms in a nationally representative sample. J Trauma Stress. 2005;18(4):313-322.
10. Sar V, Akyuv G, Dogan O. Prevalence of dissociative disorders among women in the general population. Psychiatry Res. 2007;149:169-176.
11. Barnow S, Arens EA, Sieswerda S, et al. Borderline personality disorder and psychosis: a review. Curr Psychiatry Rep. 2010;12(3):186-195.
12. Bebbington P, Jonas S, Kuipers E, et al. Childhood sexual abuse and psychosis: data from a cross-sectional national psychiatric survey in England. Br J Psychiatry. 2011;199(1):29-37.
13. Read J, van Os J, Morrison AP, et al. Childhood trauma, psychosis and schizophrenia: a literature review with theoretical and clinical implications. Acta Psychiatr Scand. 2005;112(5):330-350.
Dementia, bizarre creatures, and a white knight to the rescue
CASE Strange creatures
Ms. L, age 78, is admitted to the inpatient unit for treatment of psychosis and behavioral changes. In the months before this admission, she had visited the emergency room several times for recurrent falls. CT scans of the head show no acute changes; brain and spinal MRI reveal evidence of chronic white matter disease and degenerative changes of the spine. Medical workup is unremarkable and includes evaluation for syncope and ambulation impairments related to degenerative disease of the hip joints.
Ms. L and her family are instructed to follow-up with her primary care physician and a neurologist for neuromuscular workup.
She next presents to her primary care physician, describing hallucinations of strangers walking around her house. Over a few weeks, hallucinations expand to include a fixed hallucination of creatures that she describes as having qualities of insects and plants, “piling up” around her. She describes tactile hallucinations of these creatures crawling on her skin, and she tracks their movements around her. She complains of vivid visual hallucinations of these creatures spinning webs across the room and she says she keeps the lights on at night. Ms. L becomes anxious and depressed, and her insomnia becomes worse.
She is referred for outpatient psychiatric evaluation and treatment.
Ms. L’s family notes lapses of short-term memory, disorganization, and difficulty with tasks such as cooking because she has trouble following steps. These deficits come and go, with periods when she is functional and others during which she experiences considerable confusion. The family is uncertain when these signs and symptoms first appeared, but are clear that these deficits are having an impact on her day-to-day life. She can conduct activities of daily living, but with increasing difficulty—and only with help from her husband for tasks that require complex order and movement.
Over several months, Ms. L’s gait stability decreases and she begins to rely on a walker to keep from falling. On the Montreal Cognitive Assessment screening for cognitive dysfunction, she scores 19 out of 30 (normal range >25). This suggests cognitive impairment greater than expected for her age, compared with normal controls, and, when coupled with her functional impairment, raises the possibility of a diagnosis of dementia with Lewy bodies (DLB).
a) donepezil
b) memantine
c) quetiapine
d) low-dose clozapine
The authors’ observations
Limited literature exists of placebo-controlled, large-scale studies on DLB treatment. Cholinesterase inhibitors have shown some symptomatic benefit, including for hallucinations.1-3 Memantine, an N-methyl-d-aspartate receptor blocker, shows mixed results.4 Many studies explore the use of neuroleptics for treating hallucinations in psychosis in Parkinson’s disease and Parkinson’s disease dementia (PDD) but, in DLB, the literature primarily consists of case reports.2 Much of DLB treatment is inferred and intermixed with studies on PDD.5,6
Low-dose clozapine has become a standard treatment for psychosis in Parkinson’s disease based on the findings of several trials.6 Despite its side-effect profile, clozapine has been shown to ameliorate hallucinations in PDD without exacerbating parkinsonian symptoms,7,8 and is the only medication with proven efficacy in PDD.2 The French Clozapine Parkinson Study Group demonstrated relief of psychotic symptoms of Parkinson’s disease with clozapine, 6.25 mg/d.9 The Clozapine Study Group found complete resolution of hallucinations in some patients within 1 day of initiating clozapine. Among patients in this study who did not see immediate benefit, most showed significant improvement of psychotic symptoms in 1 or 2 weeks.10
TREATMENT Few options
Ms. L’s psychiatrist and primary care physician start her on a series of medications. Donepezil is initiated for suspected dementia. We begin a trial of quetiapine to address the hallucinations, but the drug makes her movement symptoms worse. Risperidone also is tried but, again, the drugs make movement symptoms, particularly gait instability, tremor, and rigidity worse without alleviating the hallucinations. Neuroleptics seem to exacerbate confusion. Because of worsening depressive symptoms and our concern over possible pseudodementia, we try several selective serotonin reuptake inhibitors (SSRIs) and mirtazapine. Antidepressants have little effect on her depressive symptoms and do not improve hallucinations or insomnia.
Ms. L’s signs and symptoms become worse over the next few months, with more severe hallucinations, agitation, insomnia, and gait instability. Her agitation over the hallucinations increases and she begins pouring bleach around herself in bed and spraying her house with toxic bug spray. Ms. L’s family brings her to the hospital after they observe her scratching the hallucinatory creatures off of her skin with a razor blade and trying to pry them out of her mouth with a piece of metal.
In the hospital, medical and neurologic workups rule out organic causes for her symptoms and signs. MRI is consistent with imaging from 6 months earlier. Focal neurologic signs are absent. Blood work is within normal limits, failing to reveal any pathology that would suggest a cause for her symptoms and signs, such as syphilis, vitamin deficiency, and Lyme disease.
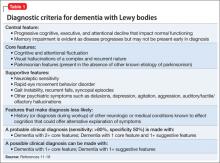
Ms. L’s symptoms were consistent with consensus guideline criteria for a clinical diagnosis of DLB (Table 1).11-18
She is started on low-dose quetiapine, which she tolerates poorly with worsening confusion, rigidity, tremor, and gait instability. Because other agents failed, Ms. L’s providers and family decide on a trial of clozapine.
Within 24 hours after the first dose of clozapine, 25 mg, sleep improves, the tactile component of hallucinations diminish, and she begins to spend increasing periods of time “observing the creatures” rather than fighting with them.
Over the next few days, Ms. L’s attitude towards the creatures changes. Now, as she sits observing them intently, the hallucinations evolve: rather than tormenting her and causing distress, the plant-creatures burst apart and a miniature knight on horseback charges out. The rest of the creatures then gather into a rank and file and the knight leads them to the nearest exit.
Clozapine is titrated to 50 mg/d, which she tolerates well without exacerbation of cognitive symptoms or movement disorder. The only notable adverse effect at the time of her discharge is sialorrhea.
with an antipsychotic?
a) start low and go slow
b) monitor her heart rate and blood pressure
c) readminister the Montreal Cognitive Assessment
d) all of the above
The authors’ observations
Ideally, in psychosis, antipsychotics eliminate positive symptoms such as hallucinations and delusions. In DLB, the aim is to alleviate the agitation and suffering brought on by the psychotic symptoms without exacerbating other motor and cognitive symptoms. The hallucinations are obstinate, and it is a well-known quality of this disorder that patients are exceptionally susceptible to a range of antipsychotic side effects including cognitive impairment, fatigue, neuroleptic malignant syndrome, and parkinsonism.19
Treatment in DLB requires trial and error, and medications with fewer associated risks should be administered first. Patients with DLB treated with neuroleptics have an increased risk of death compared with those who are not treated.19 Moreover, prescribing information for clozapine includes a black-box warning that the drug:
- is not approved for dementia-related psychosis and
- is associated with an increased risk of death in elderly patients with these conditions, similar to what is seen with other neuroleptics.20
Despite these well-known concerns, it remains difficult for clinicians not to try to treat the distress caused by these symptoms.
We chose clozapine for Ms. L because:
- other neuroleptics failed
- acetylcholinesterase inhibitors did not alleviate Ms. L’s psychosis and associated behavioral disturbance
- there is substantial evidence that the drug can be effective in Parkinson’s disease with psychosis.
There is controversy regarding use of clozapine in DLB. In one case series, clozapine trigger extreme neuroleptic reactions in some patients, similar to what occurs with other second-generation antipsychotics.21 Another case series provides examples of the drug’s efficacy in treating hallucinations and delusions with minimal adverse effects.22
It is important to emphasize that Ms. L’s hallucinations did not go away; rather, they changed to a more benign presentation that she could manage and, occasionally, found pleasant. Ultimately, her agitation—the primary target of treatment—improved markedly with the arrival of the knight in shining armor.
Treatment recommendations
If neuropsychiatric symptoms in DLB are the primary concern of the patient and family, we recommend the following:
- Begin treatment with a cholinesterase inhibitor. The best evidence exists for rivastigmine and donepezil. These drugs have a low risk of side effects, which are primarily gastrointestinal effects with some reports of worsening extrapyramidal symptoms.23-25
- If the patient obtains minimal benefit or develops a significant adverse effect from cholinesterase inhibitors, consider memantine. Its efficacy is under examination and results are mixed; it can be used in combination with cholinesterase inhibitors.26-28
- If psychotic symptoms are upsetting and refractory to other therapies, consider antipsychotics. Avoid first-generation antipsychotics. The American Psychiatric Association recommends aripiprazole or quetiapine initially, although there is little evidence comparing neuroleptics in DLB.29 Because of its risks, reserve clozapine for refractory cases. An exception might be made for patients sensitive to extrapyramidal effects, in whom clozapine could be considered earlier.
There are no formal neuroleptic dosing guidelines beyond a general urging towards minimalism. Mosimann and McKeith30 recommend clozapine, 12.5 mg/d; olanzapine, 2.5 mg/d; risperidone, 0.25 mg/d; or quetiapine, 12.5 mg/d. Such dosages might be effective while producing only minimal side effects.9,31
SSRIs and other antidepressants have not been shown to improve neuropsychiatric symptoms, and often are poorly tolerated.32
One study found efficacy with electroconvulsive therapy and transcranial magnetic stimulation in treatment-resistant patients.33
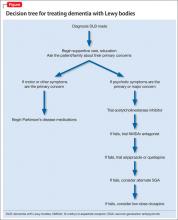
In addition to these treatments, nonpharmaceutical interventions should be employed from the earliest stages of diagnosis and treatment (Table 2). See the Figure for an algorithm for treating DLB. These include educational and behavioral interventions, social support, psychological interventions, and environmental therapies and modifications.
OUTCOME New friends
The creatures return from time to time, Ms. L reports, but are no longer upsetting because the white knight (a sort of mental deus ex machina) leads the once-terrifying things away. She describes the hallucination as a kind of zoological observation, refers to the creatures that once horrified her as “her friends,” and chuckles as she observes their natural history. This new, far more benign hallucination becomes a mainstay of her symptoms, and she is discharged to the care of her husband and family.
Soon after her discharge, her hallucinations resolved completely, but returned briefly when Ms. L resumed smoking cigarettes because smoking is known to lower clozapine serum levels.34
Bottom Line
Consider a low dosage of a neuroleptic when a patient suffers significant distress and behavioral disturbance related to psychotic symptoms in dementia with Lewy bodies and those problems are not relieved by other agents. Low-dose clozapine is an option for refractory psychotic symptoms or in patients with severe extrapyramidal sensitivity. Start low, and go slow.
Related Resources
- Bishnoi RJ, Grossberg GT, Manepalli J. Differentiating Alzheimer’s disease from dementia with Lewy bodies. Current Psychiatry. 2012;11(11):22-27.
- McKeith I, Emre M. Management of Parkinson’s disease dementia and dementia with Lewy bodies. In: Emre M, ed. Cognitive impairment and dementia in Parkinson’s disease. Oxford, United Kingdom: Oxford University Press; 2010:245-256.
Drug Brand Names
Aripiprazole • Abilify Mirtazapine • Remeron
Clozapine • Clozaril Olanzapine • Zyprexa
Donepezil • Aricept Quetiapine • Seroquel
Haloperidol • Haldol Risperidone • Risperdal
Memantine • Namenda Rivastigmine • Exelon
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Wesnes KA, McKeith IG, Ferrara R, et al. Effects of rivastigmine on cognitive function in dementia with Lewy bodies: a randomised placebo-controlled international study using the Cognitive Drug Research computerized assessment system. Dement Geriatr Cogn Disord. 2002; 13(3):183-192.
2. Weintraub D, Hurtig HI. Presentation and management of psychosis in Parkinson’s disease and dementia with Lewy bodies. Am J Psychiatry. 2007;164(10):1491-1498.
3. McKeith IG, Wesnes KA, Perry E, et al. Hallucinations predict attentional improvements with rivastigmine in dementia with Lewy bodies. Dement Geriatr Cogn Disord. 2004;18(1):94-100.
4. Emre M, Tsolaki , Bonuccelli U, et al. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9(10):969-977.
5. Aarsland D, Ballard C, Walker Z, et al. Clinical trials of dementia with Lewy bodies and Parkinson’s disease dementia. Curr Neurol Neurosci Rep. 2012;12(5):492-501.
6. Drach LM. Drug treatment of dementia with Lewy bodies and Parkinson’s disease dementia--common features and differences [in German]. Med Monatsschr Pharm. 2011; 34(2):47-52.
7. Frieling H, Hillemacher T, Ziegenbein M, et al. Treating dopamimetic psychosis in Parkinson’s disease: Structured review and meta-analysis. Eur Neuropsychopharmacol. 2007;17(3):165-171.
8. Marti MJ, Tolosa E, de la Cerda A. Dementia in Parkinson’s disease. J Neurol. 2007;254(suppl 5):41-48.
9. French Clozapine Parkinson Study Group. Clozapine in drug-induced psychosis in Parkinson’s disease. Lancet. 1999;353(9169):2041-2042.
10. Friedman JH, Factor SA. Atypical antipsychotics in the treatment of drug-induced psychosis in Parkinson’s disease. Mov Disord. 2000;15(2):201-211.
11. McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47(5):1113-1124.
12. McKeith IG, Ballard CG, Perry RH et al. Prospective validation of consensus criteria for the diagnosis of dementia with Lewy bodies. Neurology. 2000;54(5):1050-1058.
13. McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863-1872.
14. McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis. 2006;9(suppl 3):417-423.
15. Geser F, Wenning GK, Poewe W, et al. How to diagnose dementia with Lewy bodies: state of the art. Mov Disord. 2005;20(suppl 12):S11-20.
16. Latoo J, Jan F. Dementia with Lewy bodies: clinical review. British Journal of Medical Practioners. 2008;1(1):10-14.
17. McKeith I. Dementia with Lewy bodies. Dialogues Clin Neurosci. 2004;6(3):333-341.
18. Litvan I, Bhatia KP, Burn DJ, et al; Movement Disorders Society Scientific Issues Committee. SIC Task Force Appraisal of clinical diagnostic criteria for parkinsonian disorders. Mov Disord. 2003;18(5):467-486.
19. McKeith I, Fairbairn A, Perry R, et al. Neuroleptic sensitivity in patients with senile dementia of Lewy body type. BMJ. 1992;305(6855):673-678.
20. Clozapine Monitoring Guidelines. 2008. http://www.clozapineregistry.com/resuming_treatment_after_interruption.pdf.ashx. Accessed October 31, 2013.
21. Burke WJ, Pfeiffer RF, McComb RD. Neuroleptic sensitivity to clozapine in dementia with Lewy bodies. J Neuropsychiatry Clin Neurosci. 1998;10(2):227-229.
22. Chacko RC, Hurley RA, Jankovic J. Clozapine use in diffuse Lewy body disease. J Neuropsychiatry Clin Neurosci. 1993;5(2):206-208.
23. McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356(9247):2031-2036
24. Mori E, Ikeda M, Kosaka K; Donepezil-DLB Study Investiagors. Donepezil for dementia with Lewy bodies: a randomized, placebo‐controlled trial. Ann Neurol. 2012; 72(1):41-52.
25. Ukai K, Aleksic B, Ishihara R, et al. Efficacy of donepezil for the treatment of visual and multiple sensory hallucinations in dementia with Lewy bodies. Clinical Neuropsychopharmacology and Therapeutics. 2011;2:56-58.
26. Aarsland D, Ballard C, Walker Z, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009;8(7):613-618.
27. Boeve BF, Silber MH, Ferman TJ. Melatonin for treatment of REM sleep behavior disorder in neurologic disorders: results in 14 patients. Sleep Med. 2003;4(4):281-284.
28. Mathys ML, McCarrell J, Sleeper RB, et al. Visual hallucinations treated with the reinitiation of memantine in a patient with Lewy body dementia. Ann Pharmacother. 2013;47(2):e10.
29. American Psychiatric Association. Practice guideline for the treatment of patients with Alzheimer’s disease and other dementias. 2nd ed. http://psychiatryonline.org/pdfaccess.ashx?ResourceID=243205&PDFSource=6. Accessed November 1, 2013.
30. Mosimann U, McKeith IG. Dementia with lewy bodies—diagnosis and treatment. Swiss Med Wkly. 2003;133:131-142.
31. Baskys A, Davis P Atypical antipsychotic quetiapine in the treatment of the psychosis associated with Lewy body dementia. Neurobiol Aging. 2002;23:S63.
32. Culo S, Mulsant BH, Rosen J, et al. Treating neuropsychiatric symptoms in dementia with Lewy bodies: a randomized controlled-trial. Alzheimer Dis Assoc Disord. 2010;24(4):306-364.
33. Takahashi S, Mizukami K, Yasuno F, et al. Depression associated with dementia with Lewy bodies (DLB) and the effect of somatotherapy. Psychogeriatrics. 2009;9(2):56-61.
34. van der Weide J, Steijns LS, van Weelden MJ. The effect of smoking and cytochrome P450 CYP1A2 genetic polymorphism on clozapine clearance and dose requirement. Pharmacogenetics. 2003;13(3):169-172.
CASE Strange creatures
Ms. L, age 78, is admitted to the inpatient unit for treatment of psychosis and behavioral changes. In the months before this admission, she had visited the emergency room several times for recurrent falls. CT scans of the head show no acute changes; brain and spinal MRI reveal evidence of chronic white matter disease and degenerative changes of the spine. Medical workup is unremarkable and includes evaluation for syncope and ambulation impairments related to degenerative disease of the hip joints.
Ms. L and her family are instructed to follow-up with her primary care physician and a neurologist for neuromuscular workup.
She next presents to her primary care physician, describing hallucinations of strangers walking around her house. Over a few weeks, hallucinations expand to include a fixed hallucination of creatures that she describes as having qualities of insects and plants, “piling up” around her. She describes tactile hallucinations of these creatures crawling on her skin, and she tracks their movements around her. She complains of vivid visual hallucinations of these creatures spinning webs across the room and she says she keeps the lights on at night. Ms. L becomes anxious and depressed, and her insomnia becomes worse.
She is referred for outpatient psychiatric evaluation and treatment.
Ms. L’s family notes lapses of short-term memory, disorganization, and difficulty with tasks such as cooking because she has trouble following steps. These deficits come and go, with periods when she is functional and others during which she experiences considerable confusion. The family is uncertain when these signs and symptoms first appeared, but are clear that these deficits are having an impact on her day-to-day life. She can conduct activities of daily living, but with increasing difficulty—and only with help from her husband for tasks that require complex order and movement.
Over several months, Ms. L’s gait stability decreases and she begins to rely on a walker to keep from falling. On the Montreal Cognitive Assessment screening for cognitive dysfunction, she scores 19 out of 30 (normal range >25). This suggests cognitive impairment greater than expected for her age, compared with normal controls, and, when coupled with her functional impairment, raises the possibility of a diagnosis of dementia with Lewy bodies (DLB).
a) donepezil
b) memantine
c) quetiapine
d) low-dose clozapine
The authors’ observations
Limited literature exists of placebo-controlled, large-scale studies on DLB treatment. Cholinesterase inhibitors have shown some symptomatic benefit, including for hallucinations.1-3 Memantine, an N-methyl-d-aspartate receptor blocker, shows mixed results.4 Many studies explore the use of neuroleptics for treating hallucinations in psychosis in Parkinson’s disease and Parkinson’s disease dementia (PDD) but, in DLB, the literature primarily consists of case reports.2 Much of DLB treatment is inferred and intermixed with studies on PDD.5,6
Low-dose clozapine has become a standard treatment for psychosis in Parkinson’s disease based on the findings of several trials.6 Despite its side-effect profile, clozapine has been shown to ameliorate hallucinations in PDD without exacerbating parkinsonian symptoms,7,8 and is the only medication with proven efficacy in PDD.2 The French Clozapine Parkinson Study Group demonstrated relief of psychotic symptoms of Parkinson’s disease with clozapine, 6.25 mg/d.9 The Clozapine Study Group found complete resolution of hallucinations in some patients within 1 day of initiating clozapine. Among patients in this study who did not see immediate benefit, most showed significant improvement of psychotic symptoms in 1 or 2 weeks.10
TREATMENT Few options
Ms. L’s psychiatrist and primary care physician start her on a series of medications. Donepezil is initiated for suspected dementia. We begin a trial of quetiapine to address the hallucinations, but the drug makes her movement symptoms worse. Risperidone also is tried but, again, the drugs make movement symptoms, particularly gait instability, tremor, and rigidity worse without alleviating the hallucinations. Neuroleptics seem to exacerbate confusion. Because of worsening depressive symptoms and our concern over possible pseudodementia, we try several selective serotonin reuptake inhibitors (SSRIs) and mirtazapine. Antidepressants have little effect on her depressive symptoms and do not improve hallucinations or insomnia.
Ms. L’s signs and symptoms become worse over the next few months, with more severe hallucinations, agitation, insomnia, and gait instability. Her agitation over the hallucinations increases and she begins pouring bleach around herself in bed and spraying her house with toxic bug spray. Ms. L’s family brings her to the hospital after they observe her scratching the hallucinatory creatures off of her skin with a razor blade and trying to pry them out of her mouth with a piece of metal.
In the hospital, medical and neurologic workups rule out organic causes for her symptoms and signs. MRI is consistent with imaging from 6 months earlier. Focal neurologic signs are absent. Blood work is within normal limits, failing to reveal any pathology that would suggest a cause for her symptoms and signs, such as syphilis, vitamin deficiency, and Lyme disease.
Ms. L’s symptoms were consistent with consensus guideline criteria for a clinical diagnosis of DLB (Table 1).11-18
She is started on low-dose quetiapine, which she tolerates poorly with worsening confusion, rigidity, tremor, and gait instability. Because other agents failed, Ms. L’s providers and family decide on a trial of clozapine.
Within 24 hours after the first dose of clozapine, 25 mg, sleep improves, the tactile component of hallucinations diminish, and she begins to spend increasing periods of time “observing the creatures” rather than fighting with them.
Over the next few days, Ms. L’s attitude towards the creatures changes. Now, as she sits observing them intently, the hallucinations evolve: rather than tormenting her and causing distress, the plant-creatures burst apart and a miniature knight on horseback charges out. The rest of the creatures then gather into a rank and file and the knight leads them to the nearest exit.
Clozapine is titrated to 50 mg/d, which she tolerates well without exacerbation of cognitive symptoms or movement disorder. The only notable adverse effect at the time of her discharge is sialorrhea.
with an antipsychotic?
a) start low and go slow
b) monitor her heart rate and blood pressure
c) readminister the Montreal Cognitive Assessment
d) all of the above
The authors’ observations
Ideally, in psychosis, antipsychotics eliminate positive symptoms such as hallucinations and delusions. In DLB, the aim is to alleviate the agitation and suffering brought on by the psychotic symptoms without exacerbating other motor and cognitive symptoms. The hallucinations are obstinate, and it is a well-known quality of this disorder that patients are exceptionally susceptible to a range of antipsychotic side effects including cognitive impairment, fatigue, neuroleptic malignant syndrome, and parkinsonism.19
Treatment in DLB requires trial and error, and medications with fewer associated risks should be administered first. Patients with DLB treated with neuroleptics have an increased risk of death compared with those who are not treated.19 Moreover, prescribing information for clozapine includes a black-box warning that the drug:
- is not approved for dementia-related psychosis and
- is associated with an increased risk of death in elderly patients with these conditions, similar to what is seen with other neuroleptics.20
Despite these well-known concerns, it remains difficult for clinicians not to try to treat the distress caused by these symptoms.
We chose clozapine for Ms. L because:
- other neuroleptics failed
- acetylcholinesterase inhibitors did not alleviate Ms. L’s psychosis and associated behavioral disturbance
- there is substantial evidence that the drug can be effective in Parkinson’s disease with psychosis.
There is controversy regarding use of clozapine in DLB. In one case series, clozapine trigger extreme neuroleptic reactions in some patients, similar to what occurs with other second-generation antipsychotics.21 Another case series provides examples of the drug’s efficacy in treating hallucinations and delusions with minimal adverse effects.22
It is important to emphasize that Ms. L’s hallucinations did not go away; rather, they changed to a more benign presentation that she could manage and, occasionally, found pleasant. Ultimately, her agitation—the primary target of treatment—improved markedly with the arrival of the knight in shining armor.
Treatment recommendations
If neuropsychiatric symptoms in DLB are the primary concern of the patient and family, we recommend the following:
- Begin treatment with a cholinesterase inhibitor. The best evidence exists for rivastigmine and donepezil. These drugs have a low risk of side effects, which are primarily gastrointestinal effects with some reports of worsening extrapyramidal symptoms.23-25
- If the patient obtains minimal benefit or develops a significant adverse effect from cholinesterase inhibitors, consider memantine. Its efficacy is under examination and results are mixed; it can be used in combination with cholinesterase inhibitors.26-28
- If psychotic symptoms are upsetting and refractory to other therapies, consider antipsychotics. Avoid first-generation antipsychotics. The American Psychiatric Association recommends aripiprazole or quetiapine initially, although there is little evidence comparing neuroleptics in DLB.29 Because of its risks, reserve clozapine for refractory cases. An exception might be made for patients sensitive to extrapyramidal effects, in whom clozapine could be considered earlier.
There are no formal neuroleptic dosing guidelines beyond a general urging towards minimalism. Mosimann and McKeith30 recommend clozapine, 12.5 mg/d; olanzapine, 2.5 mg/d; risperidone, 0.25 mg/d; or quetiapine, 12.5 mg/d. Such dosages might be effective while producing only minimal side effects.9,31
SSRIs and other antidepressants have not been shown to improve neuropsychiatric symptoms, and often are poorly tolerated.32
One study found efficacy with electroconvulsive therapy and transcranial magnetic stimulation in treatment-resistant patients.33
In addition to these treatments, nonpharmaceutical interventions should be employed from the earliest stages of diagnosis and treatment (Table 2). See the Figure for an algorithm for treating DLB. These include educational and behavioral interventions, social support, psychological interventions, and environmental therapies and modifications.
OUTCOME New friends
The creatures return from time to time, Ms. L reports, but are no longer upsetting because the white knight (a sort of mental deus ex machina) leads the once-terrifying things away. She describes the hallucination as a kind of zoological observation, refers to the creatures that once horrified her as “her friends,” and chuckles as she observes their natural history. This new, far more benign hallucination becomes a mainstay of her symptoms, and she is discharged to the care of her husband and family.
Soon after her discharge, her hallucinations resolved completely, but returned briefly when Ms. L resumed smoking cigarettes because smoking is known to lower clozapine serum levels.34
Bottom Line
Consider a low dosage of a neuroleptic when a patient suffers significant distress and behavioral disturbance related to psychotic symptoms in dementia with Lewy bodies and those problems are not relieved by other agents. Low-dose clozapine is an option for refractory psychotic symptoms or in patients with severe extrapyramidal sensitivity. Start low, and go slow.
Related Resources
- Bishnoi RJ, Grossberg GT, Manepalli J. Differentiating Alzheimer’s disease from dementia with Lewy bodies. Current Psychiatry. 2012;11(11):22-27.
- McKeith I, Emre M. Management of Parkinson’s disease dementia and dementia with Lewy bodies. In: Emre M, ed. Cognitive impairment and dementia in Parkinson’s disease. Oxford, United Kingdom: Oxford University Press; 2010:245-256.
Drug Brand Names
Aripiprazole • Abilify Mirtazapine • Remeron
Clozapine • Clozaril Olanzapine • Zyprexa
Donepezil • Aricept Quetiapine • Seroquel
Haloperidol • Haldol Risperidone • Risperdal
Memantine • Namenda Rivastigmine • Exelon
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Strange creatures
Ms. L, age 78, is admitted to the inpatient unit for treatment of psychosis and behavioral changes. In the months before this admission, she had visited the emergency room several times for recurrent falls. CT scans of the head show no acute changes; brain and spinal MRI reveal evidence of chronic white matter disease and degenerative changes of the spine. Medical workup is unremarkable and includes evaluation for syncope and ambulation impairments related to degenerative disease of the hip joints.
Ms. L and her family are instructed to follow-up with her primary care physician and a neurologist for neuromuscular workup.
She next presents to her primary care physician, describing hallucinations of strangers walking around her house. Over a few weeks, hallucinations expand to include a fixed hallucination of creatures that she describes as having qualities of insects and plants, “piling up” around her. She describes tactile hallucinations of these creatures crawling on her skin, and she tracks their movements around her. She complains of vivid visual hallucinations of these creatures spinning webs across the room and she says she keeps the lights on at night. Ms. L becomes anxious and depressed, and her insomnia becomes worse.
She is referred for outpatient psychiatric evaluation and treatment.
Ms. L’s family notes lapses of short-term memory, disorganization, and difficulty with tasks such as cooking because she has trouble following steps. These deficits come and go, with periods when she is functional and others during which she experiences considerable confusion. The family is uncertain when these signs and symptoms first appeared, but are clear that these deficits are having an impact on her day-to-day life. She can conduct activities of daily living, but with increasing difficulty—and only with help from her husband for tasks that require complex order and movement.
Over several months, Ms. L’s gait stability decreases and she begins to rely on a walker to keep from falling. On the Montreal Cognitive Assessment screening for cognitive dysfunction, she scores 19 out of 30 (normal range >25). This suggests cognitive impairment greater than expected for her age, compared with normal controls, and, when coupled with her functional impairment, raises the possibility of a diagnosis of dementia with Lewy bodies (DLB).
a) donepezil
b) memantine
c) quetiapine
d) low-dose clozapine
The authors’ observations
Limited literature exists of placebo-controlled, large-scale studies on DLB treatment. Cholinesterase inhibitors have shown some symptomatic benefit, including for hallucinations.1-3 Memantine, an N-methyl-d-aspartate receptor blocker, shows mixed results.4 Many studies explore the use of neuroleptics for treating hallucinations in psychosis in Parkinson’s disease and Parkinson’s disease dementia (PDD) but, in DLB, the literature primarily consists of case reports.2 Much of DLB treatment is inferred and intermixed with studies on PDD.5,6
Low-dose clozapine has become a standard treatment for psychosis in Parkinson’s disease based on the findings of several trials.6 Despite its side-effect profile, clozapine has been shown to ameliorate hallucinations in PDD without exacerbating parkinsonian symptoms,7,8 and is the only medication with proven efficacy in PDD.2 The French Clozapine Parkinson Study Group demonstrated relief of psychotic symptoms of Parkinson’s disease with clozapine, 6.25 mg/d.9 The Clozapine Study Group found complete resolution of hallucinations in some patients within 1 day of initiating clozapine. Among patients in this study who did not see immediate benefit, most showed significant improvement of psychotic symptoms in 1 or 2 weeks.10
TREATMENT Few options
Ms. L’s psychiatrist and primary care physician start her on a series of medications. Donepezil is initiated for suspected dementia. We begin a trial of quetiapine to address the hallucinations, but the drug makes her movement symptoms worse. Risperidone also is tried but, again, the drugs make movement symptoms, particularly gait instability, tremor, and rigidity worse without alleviating the hallucinations. Neuroleptics seem to exacerbate confusion. Because of worsening depressive symptoms and our concern over possible pseudodementia, we try several selective serotonin reuptake inhibitors (SSRIs) and mirtazapine. Antidepressants have little effect on her depressive symptoms and do not improve hallucinations or insomnia.
Ms. L’s signs and symptoms become worse over the next few months, with more severe hallucinations, agitation, insomnia, and gait instability. Her agitation over the hallucinations increases and she begins pouring bleach around herself in bed and spraying her house with toxic bug spray. Ms. L’s family brings her to the hospital after they observe her scratching the hallucinatory creatures off of her skin with a razor blade and trying to pry them out of her mouth with a piece of metal.
In the hospital, medical and neurologic workups rule out organic causes for her symptoms and signs. MRI is consistent with imaging from 6 months earlier. Focal neurologic signs are absent. Blood work is within normal limits, failing to reveal any pathology that would suggest a cause for her symptoms and signs, such as syphilis, vitamin deficiency, and Lyme disease.
Ms. L’s symptoms were consistent with consensus guideline criteria for a clinical diagnosis of DLB (Table 1).11-18
She is started on low-dose quetiapine, which she tolerates poorly with worsening confusion, rigidity, tremor, and gait instability. Because other agents failed, Ms. L’s providers and family decide on a trial of clozapine.
Within 24 hours after the first dose of clozapine, 25 mg, sleep improves, the tactile component of hallucinations diminish, and she begins to spend increasing periods of time “observing the creatures” rather than fighting with them.
Over the next few days, Ms. L’s attitude towards the creatures changes. Now, as she sits observing them intently, the hallucinations evolve: rather than tormenting her and causing distress, the plant-creatures burst apart and a miniature knight on horseback charges out. The rest of the creatures then gather into a rank and file and the knight leads them to the nearest exit.
Clozapine is titrated to 50 mg/d, which she tolerates well without exacerbation of cognitive symptoms or movement disorder. The only notable adverse effect at the time of her discharge is sialorrhea.
with an antipsychotic?
a) start low and go slow
b) monitor her heart rate and blood pressure
c) readminister the Montreal Cognitive Assessment
d) all of the above
The authors’ observations
Ideally, in psychosis, antipsychotics eliminate positive symptoms such as hallucinations and delusions. In DLB, the aim is to alleviate the agitation and suffering brought on by the psychotic symptoms without exacerbating other motor and cognitive symptoms. The hallucinations are obstinate, and it is a well-known quality of this disorder that patients are exceptionally susceptible to a range of antipsychotic side effects including cognitive impairment, fatigue, neuroleptic malignant syndrome, and parkinsonism.19
Treatment in DLB requires trial and error, and medications with fewer associated risks should be administered first. Patients with DLB treated with neuroleptics have an increased risk of death compared with those who are not treated.19 Moreover, prescribing information for clozapine includes a black-box warning that the drug:
- is not approved for dementia-related psychosis and
- is associated with an increased risk of death in elderly patients with these conditions, similar to what is seen with other neuroleptics.20
Despite these well-known concerns, it remains difficult for clinicians not to try to treat the distress caused by these symptoms.
We chose clozapine for Ms. L because:
- other neuroleptics failed
- acetylcholinesterase inhibitors did not alleviate Ms. L’s psychosis and associated behavioral disturbance
- there is substantial evidence that the drug can be effective in Parkinson’s disease with psychosis.
There is controversy regarding use of clozapine in DLB. In one case series, clozapine trigger extreme neuroleptic reactions in some patients, similar to what occurs with other second-generation antipsychotics.21 Another case series provides examples of the drug’s efficacy in treating hallucinations and delusions with minimal adverse effects.22
It is important to emphasize that Ms. L’s hallucinations did not go away; rather, they changed to a more benign presentation that she could manage and, occasionally, found pleasant. Ultimately, her agitation—the primary target of treatment—improved markedly with the arrival of the knight in shining armor.
Treatment recommendations
If neuropsychiatric symptoms in DLB are the primary concern of the patient and family, we recommend the following:
- Begin treatment with a cholinesterase inhibitor. The best evidence exists for rivastigmine and donepezil. These drugs have a low risk of side effects, which are primarily gastrointestinal effects with some reports of worsening extrapyramidal symptoms.23-25
- If the patient obtains minimal benefit or develops a significant adverse effect from cholinesterase inhibitors, consider memantine. Its efficacy is under examination and results are mixed; it can be used in combination with cholinesterase inhibitors.26-28
- If psychotic symptoms are upsetting and refractory to other therapies, consider antipsychotics. Avoid first-generation antipsychotics. The American Psychiatric Association recommends aripiprazole or quetiapine initially, although there is little evidence comparing neuroleptics in DLB.29 Because of its risks, reserve clozapine for refractory cases. An exception might be made for patients sensitive to extrapyramidal effects, in whom clozapine could be considered earlier.
There are no formal neuroleptic dosing guidelines beyond a general urging towards minimalism. Mosimann and McKeith30 recommend clozapine, 12.5 mg/d; olanzapine, 2.5 mg/d; risperidone, 0.25 mg/d; or quetiapine, 12.5 mg/d. Such dosages might be effective while producing only minimal side effects.9,31
SSRIs and other antidepressants have not been shown to improve neuropsychiatric symptoms, and often are poorly tolerated.32
One study found efficacy with electroconvulsive therapy and transcranial magnetic stimulation in treatment-resistant patients.33
In addition to these treatments, nonpharmaceutical interventions should be employed from the earliest stages of diagnosis and treatment (Table 2). See the Figure for an algorithm for treating DLB. These include educational and behavioral interventions, social support, psychological interventions, and environmental therapies and modifications.
OUTCOME New friends
The creatures return from time to time, Ms. L reports, but are no longer upsetting because the white knight (a sort of mental deus ex machina) leads the once-terrifying things away. She describes the hallucination as a kind of zoological observation, refers to the creatures that once horrified her as “her friends,” and chuckles as she observes their natural history. This new, far more benign hallucination becomes a mainstay of her symptoms, and she is discharged to the care of her husband and family.
Soon after her discharge, her hallucinations resolved completely, but returned briefly when Ms. L resumed smoking cigarettes because smoking is known to lower clozapine serum levels.34
Bottom Line
Consider a low dosage of a neuroleptic when a patient suffers significant distress and behavioral disturbance related to psychotic symptoms in dementia with Lewy bodies and those problems are not relieved by other agents. Low-dose clozapine is an option for refractory psychotic symptoms or in patients with severe extrapyramidal sensitivity. Start low, and go slow.
Related Resources
- Bishnoi RJ, Grossberg GT, Manepalli J. Differentiating Alzheimer’s disease from dementia with Lewy bodies. Current Psychiatry. 2012;11(11):22-27.
- McKeith I, Emre M. Management of Parkinson’s disease dementia and dementia with Lewy bodies. In: Emre M, ed. Cognitive impairment and dementia in Parkinson’s disease. Oxford, United Kingdom: Oxford University Press; 2010:245-256.
Drug Brand Names
Aripiprazole • Abilify Mirtazapine • Remeron
Clozapine • Clozaril Olanzapine • Zyprexa
Donepezil • Aricept Quetiapine • Seroquel
Haloperidol • Haldol Risperidone • Risperdal
Memantine • Namenda Rivastigmine • Exelon
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Wesnes KA, McKeith IG, Ferrara R, et al. Effects of rivastigmine on cognitive function in dementia with Lewy bodies: a randomised placebo-controlled international study using the Cognitive Drug Research computerized assessment system. Dement Geriatr Cogn Disord. 2002; 13(3):183-192.
2. Weintraub D, Hurtig HI. Presentation and management of psychosis in Parkinson’s disease and dementia with Lewy bodies. Am J Psychiatry. 2007;164(10):1491-1498.
3. McKeith IG, Wesnes KA, Perry E, et al. Hallucinations predict attentional improvements with rivastigmine in dementia with Lewy bodies. Dement Geriatr Cogn Disord. 2004;18(1):94-100.
4. Emre M, Tsolaki , Bonuccelli U, et al. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9(10):969-977.
5. Aarsland D, Ballard C, Walker Z, et al. Clinical trials of dementia with Lewy bodies and Parkinson’s disease dementia. Curr Neurol Neurosci Rep. 2012;12(5):492-501.
6. Drach LM. Drug treatment of dementia with Lewy bodies and Parkinson’s disease dementia--common features and differences [in German]. Med Monatsschr Pharm. 2011; 34(2):47-52.
7. Frieling H, Hillemacher T, Ziegenbein M, et al. Treating dopamimetic psychosis in Parkinson’s disease: Structured review and meta-analysis. Eur Neuropsychopharmacol. 2007;17(3):165-171.
8. Marti MJ, Tolosa E, de la Cerda A. Dementia in Parkinson’s disease. J Neurol. 2007;254(suppl 5):41-48.
9. French Clozapine Parkinson Study Group. Clozapine in drug-induced psychosis in Parkinson’s disease. Lancet. 1999;353(9169):2041-2042.
10. Friedman JH, Factor SA. Atypical antipsychotics in the treatment of drug-induced psychosis in Parkinson’s disease. Mov Disord. 2000;15(2):201-211.
11. McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47(5):1113-1124.
12. McKeith IG, Ballard CG, Perry RH et al. Prospective validation of consensus criteria for the diagnosis of dementia with Lewy bodies. Neurology. 2000;54(5):1050-1058.
13. McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863-1872.
14. McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis. 2006;9(suppl 3):417-423.
15. Geser F, Wenning GK, Poewe W, et al. How to diagnose dementia with Lewy bodies: state of the art. Mov Disord. 2005;20(suppl 12):S11-20.
16. Latoo J, Jan F. Dementia with Lewy bodies: clinical review. British Journal of Medical Practioners. 2008;1(1):10-14.
17. McKeith I. Dementia with Lewy bodies. Dialogues Clin Neurosci. 2004;6(3):333-341.
18. Litvan I, Bhatia KP, Burn DJ, et al; Movement Disorders Society Scientific Issues Committee. SIC Task Force Appraisal of clinical diagnostic criteria for parkinsonian disorders. Mov Disord. 2003;18(5):467-486.
19. McKeith I, Fairbairn A, Perry R, et al. Neuroleptic sensitivity in patients with senile dementia of Lewy body type. BMJ. 1992;305(6855):673-678.
20. Clozapine Monitoring Guidelines. 2008. http://www.clozapineregistry.com/resuming_treatment_after_interruption.pdf.ashx. Accessed October 31, 2013.
21. Burke WJ, Pfeiffer RF, McComb RD. Neuroleptic sensitivity to clozapine in dementia with Lewy bodies. J Neuropsychiatry Clin Neurosci. 1998;10(2):227-229.
22. Chacko RC, Hurley RA, Jankovic J. Clozapine use in diffuse Lewy body disease. J Neuropsychiatry Clin Neurosci. 1993;5(2):206-208.
23. McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356(9247):2031-2036
24. Mori E, Ikeda M, Kosaka K; Donepezil-DLB Study Investiagors. Donepezil for dementia with Lewy bodies: a randomized, placebo‐controlled trial. Ann Neurol. 2012; 72(1):41-52.
25. Ukai K, Aleksic B, Ishihara R, et al. Efficacy of donepezil for the treatment of visual and multiple sensory hallucinations in dementia with Lewy bodies. Clinical Neuropsychopharmacology and Therapeutics. 2011;2:56-58.
26. Aarsland D, Ballard C, Walker Z, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009;8(7):613-618.
27. Boeve BF, Silber MH, Ferman TJ. Melatonin for treatment of REM sleep behavior disorder in neurologic disorders: results in 14 patients. Sleep Med. 2003;4(4):281-284.
28. Mathys ML, McCarrell J, Sleeper RB, et al. Visual hallucinations treated with the reinitiation of memantine in a patient with Lewy body dementia. Ann Pharmacother. 2013;47(2):e10.
29. American Psychiatric Association. Practice guideline for the treatment of patients with Alzheimer’s disease and other dementias. 2nd ed. http://psychiatryonline.org/pdfaccess.ashx?ResourceID=243205&PDFSource=6. Accessed November 1, 2013.
30. Mosimann U, McKeith IG. Dementia with lewy bodies—diagnosis and treatment. Swiss Med Wkly. 2003;133:131-142.
31. Baskys A, Davis P Atypical antipsychotic quetiapine in the treatment of the psychosis associated with Lewy body dementia. Neurobiol Aging. 2002;23:S63.
32. Culo S, Mulsant BH, Rosen J, et al. Treating neuropsychiatric symptoms in dementia with Lewy bodies: a randomized controlled-trial. Alzheimer Dis Assoc Disord. 2010;24(4):306-364.
33. Takahashi S, Mizukami K, Yasuno F, et al. Depression associated with dementia with Lewy bodies (DLB) and the effect of somatotherapy. Psychogeriatrics. 2009;9(2):56-61.
34. van der Weide J, Steijns LS, van Weelden MJ. The effect of smoking and cytochrome P450 CYP1A2 genetic polymorphism on clozapine clearance and dose requirement. Pharmacogenetics. 2003;13(3):169-172.
1. Wesnes KA, McKeith IG, Ferrara R, et al. Effects of rivastigmine on cognitive function in dementia with Lewy bodies: a randomised placebo-controlled international study using the Cognitive Drug Research computerized assessment system. Dement Geriatr Cogn Disord. 2002; 13(3):183-192.
2. Weintraub D, Hurtig HI. Presentation and management of psychosis in Parkinson’s disease and dementia with Lewy bodies. Am J Psychiatry. 2007;164(10):1491-1498.
3. McKeith IG, Wesnes KA, Perry E, et al. Hallucinations predict attentional improvements with rivastigmine in dementia with Lewy bodies. Dement Geriatr Cogn Disord. 2004;18(1):94-100.
4. Emre M, Tsolaki , Bonuccelli U, et al. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9(10):969-977.
5. Aarsland D, Ballard C, Walker Z, et al. Clinical trials of dementia with Lewy bodies and Parkinson’s disease dementia. Curr Neurol Neurosci Rep. 2012;12(5):492-501.
6. Drach LM. Drug treatment of dementia with Lewy bodies and Parkinson’s disease dementia--common features and differences [in German]. Med Monatsschr Pharm. 2011; 34(2):47-52.
7. Frieling H, Hillemacher T, Ziegenbein M, et al. Treating dopamimetic psychosis in Parkinson’s disease: Structured review and meta-analysis. Eur Neuropsychopharmacol. 2007;17(3):165-171.
8. Marti MJ, Tolosa E, de la Cerda A. Dementia in Parkinson’s disease. J Neurol. 2007;254(suppl 5):41-48.
9. French Clozapine Parkinson Study Group. Clozapine in drug-induced psychosis in Parkinson’s disease. Lancet. 1999;353(9169):2041-2042.
10. Friedman JH, Factor SA. Atypical antipsychotics in the treatment of drug-induced psychosis in Parkinson’s disease. Mov Disord. 2000;15(2):201-211.
11. McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47(5):1113-1124.
12. McKeith IG, Ballard CG, Perry RH et al. Prospective validation of consensus criteria for the diagnosis of dementia with Lewy bodies. Neurology. 2000;54(5):1050-1058.
13. McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863-1872.
14. McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis. 2006;9(suppl 3):417-423.
15. Geser F, Wenning GK, Poewe W, et al. How to diagnose dementia with Lewy bodies: state of the art. Mov Disord. 2005;20(suppl 12):S11-20.
16. Latoo J, Jan F. Dementia with Lewy bodies: clinical review. British Journal of Medical Practioners. 2008;1(1):10-14.
17. McKeith I. Dementia with Lewy bodies. Dialogues Clin Neurosci. 2004;6(3):333-341.
18. Litvan I, Bhatia KP, Burn DJ, et al; Movement Disorders Society Scientific Issues Committee. SIC Task Force Appraisal of clinical diagnostic criteria for parkinsonian disorders. Mov Disord. 2003;18(5):467-486.
19. McKeith I, Fairbairn A, Perry R, et al. Neuroleptic sensitivity in patients with senile dementia of Lewy body type. BMJ. 1992;305(6855):673-678.
20. Clozapine Monitoring Guidelines. 2008. http://www.clozapineregistry.com/resuming_treatment_after_interruption.pdf.ashx. Accessed October 31, 2013.
21. Burke WJ, Pfeiffer RF, McComb RD. Neuroleptic sensitivity to clozapine in dementia with Lewy bodies. J Neuropsychiatry Clin Neurosci. 1998;10(2):227-229.
22. Chacko RC, Hurley RA, Jankovic J. Clozapine use in diffuse Lewy body disease. J Neuropsychiatry Clin Neurosci. 1993;5(2):206-208.
23. McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356(9247):2031-2036
24. Mori E, Ikeda M, Kosaka K; Donepezil-DLB Study Investiagors. Donepezil for dementia with Lewy bodies: a randomized, placebo‐controlled trial. Ann Neurol. 2012; 72(1):41-52.
25. Ukai K, Aleksic B, Ishihara R, et al. Efficacy of donepezil for the treatment of visual and multiple sensory hallucinations in dementia with Lewy bodies. Clinical Neuropsychopharmacology and Therapeutics. 2011;2:56-58.
26. Aarsland D, Ballard C, Walker Z, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009;8(7):613-618.
27. Boeve BF, Silber MH, Ferman TJ. Melatonin for treatment of REM sleep behavior disorder in neurologic disorders: results in 14 patients. Sleep Med. 2003;4(4):281-284.
28. Mathys ML, McCarrell J, Sleeper RB, et al. Visual hallucinations treated with the reinitiation of memantine in a patient with Lewy body dementia. Ann Pharmacother. 2013;47(2):e10.
29. American Psychiatric Association. Practice guideline for the treatment of patients with Alzheimer’s disease and other dementias. 2nd ed. http://psychiatryonline.org/pdfaccess.ashx?ResourceID=243205&PDFSource=6. Accessed November 1, 2013.
30. Mosimann U, McKeith IG. Dementia with lewy bodies—diagnosis and treatment. Swiss Med Wkly. 2003;133:131-142.
31. Baskys A, Davis P Atypical antipsychotic quetiapine in the treatment of the psychosis associated with Lewy body dementia. Neurobiol Aging. 2002;23:S63.
32. Culo S, Mulsant BH, Rosen J, et al. Treating neuropsychiatric symptoms in dementia with Lewy bodies: a randomized controlled-trial. Alzheimer Dis Assoc Disord. 2010;24(4):306-364.
33. Takahashi S, Mizukami K, Yasuno F, et al. Depression associated with dementia with Lewy bodies (DLB) and the effect of somatotherapy. Psychogeriatrics. 2009;9(2):56-61.
34. van der Weide J, Steijns LS, van Weelden MJ. The effect of smoking and cytochrome P450 CYP1A2 genetic polymorphism on clozapine clearance and dose requirement. Pharmacogenetics. 2003;13(3):169-172.
Angry, inattentive, and sidelined
CASE Angry and depressed
Y is a 16-year-old male who presents with his mother to our clinic for medication evaluation because of anger issues and problems learning in school. He says he has been feeling depressed for several months and noticed significant irritability. Y sleeps excessively, sometimes for more than 12 hours a day, and eats more than he usually does. He reports feeling hopeless, helpless, and guilty for letting his family down. Y, who is in the 10th grade, acknowledges trouble focusing and concentrating but attributed this to a previous diagnosis of attention-deficit/hyperactivity disorder (ADHD). He stopped taking his stimulant medication several months ago because he did not like taking it. He denies thoughts of self-harm or thinking about death.
Y’s mother reports that her son had been athletic but had to stop playing football because he has had 5 concussions. Y’s inability to play sports appears to be a precipitating factor in his decline in mood (Box). He had his first concussion at age 13; the last one was several months before his presenting to the clinic. Y experienced loss of consciousness and unsteady gait after his concussions and was hospitalized for some of them. Y says his life goals are “playing sports and being a marine,” which may be compromised because of his head injuries.
His mother reports Y is having more anger outbursts and says his personality is changing. Y viewed this change as just being more assertive and fails to see that others may be scared by his behavior. He is getting into more fights at school and is more impulsive and unpredictable, according to his mother. Y is struggling in school with cognitive deficits and memory problems; his grade point average (GPA) drops from 3.5 to 0.3 over several months. He had been homeschooled initially because of uncontrolled impulsivity and aggression, but was reintegrated to public school. Y has a history of a mathematics disorder but had done well without school accommodations before the head injuries. Lack of access to his peers and poor self-esteem because of his declining grades are making his mood worse. He denies a history of substance use and his urine drug screen is negative.
Recently, Y’s grandfather, with whom he had been close, died and 2 friends were killed in car accidents in the last few years. Y has no history of psychiatric hospitalization. He had seen a psychotherapist for depression. He had been on lisdexamfetamine, 30 mg/d, citalopram, 10 mg/d, and an unknown dose of dextroamphetamine. He had no major medical comorbidities. He lives with his mother. His parents are separated but he has frequent contact with his father. His developmental history is unremarkable. There was a questionable family history of schizophrenia, “nervous breakdowns,” depression, and bipolar disorder. There was no family history of suicide.
On his initial mental status examination, Y appears to be his stated age and is dressed appropriately. He is well dressed, suggesting that he puts a lot of care into his personal appearance. He is alert and oriented. He is cooperative and has fair eye contact. His gait is normal and no motor abnormalities are evident. His speech is normal in rate, rhythm, and volume. He can remember events with great accuracy. He reports that his mood is depressed and “down.” His affect appears irritable and he has low frustration tolerance, especially towards his mother. He is easy to anger but is re-directable. He does not endorse thoughts of suicidality or harm to others. He denies auditory or visual hallucinations, and paranoia. He does not appear to be responding to internal stimuli. His judgment and insight are fair.
a) major depressive disorder
b) oppositional defiant disorder
c) bipolar disorder, most recent episode depressed
d) ADHD, untreated
e) post-concussion syndrome
The authors' observations
Traumatic brain injury (TBI) affects 1.7 to 3.8 million people in the United States. More than 473,000 children present to the emergency room annually with TBI, approximately 75% of whom are given a label of mild TBI in the United States.1-3 TBI patients present with varying degrees of problems ranging from headaches to cognitive deficits and death. Symptoms may be transient or permanent.4 Prepubescent children are at higher risk and are more likely to sustain permanent damage post-TBI, with problems in attention, executive functioning, memory, and cognition.5-7
Prognosis depends on severity of injury and environmental factors, including socioeconomic status, family dysfunction, and access to resources.8 Patients may present during the acute concussion phase with physical symptoms, such as headaches, nausea, vomiting, sensitivity to light and sounds, and memory deficits, and psychiatric complaints such as anger, irritability, and mood swings. Symptoms may persist post-concussion, leading to problems in personal relationships and social and occupational functioning, and neuropsychiatric manifestations, including changes in personality, depression, suicidal thoughts, and substance dependence. As seen in this case, Y had neuropsychiatric manifestations after his TBI but other factors, such as his ADHD diagnosis and the death of his grandfather and friends, may have contributed to his presentation.
Up to one-half of children with brain injuries may be at increased risk for unfavorable behavioral outcomes, which include internalizing and externalizing presentations.9 These behavioral problems may emerge several years after the injury and often persist or get worse with time. Behavioral functioning before injury usually dictates long-term outcomes post injury. The American Academy of Neurology recently released guidelines for the assessment and treatment of athletes with concussions (see Related Resources).
TREATMENT Restart medication
We restart Y on citalopram, 10 mg/d, which he tolerated in the past, and increase it to 20 mg/d after 4 days to address his depression and irritability. He also is restarted on lisdexamfetamine, 30 mg/d, for his ADHD. We give his mother the Child Behavior Checklist and Teacher’s Report Forms to gather additional collateral information. We ask Y to follow up in 1 month and we encourage him to continue seeing his psychotherapist.
a) neuropsychological testing
b) neurology referral
c) imaging studies
d) no testing
EVALUATION Testing
Although Y denies feeling depressed to the neuropsychologist, the examiner notes her concerns about his depression based on his mental status examination during testing.
Neuropsychological testing reveals a discrepancy noted between normal verbal skills and perceptual intellectual skills that were in the borderline range (Table). Testing revealed results supporting executive dysfunction and distractibility, which are consistent with his history of ADHD. Y’s broad reading scores are in the 20th percentile and math scores in the 30th percentile. Although he has a history of a mathematics disorder, his reading deficits are considered a decline compared with his previous performance.
The authors' observations
Y is a 16-year-old male who presented with anger, depression, and academic problems. He had genetic loading with a questionable family history of schizophrenia, “nervous breakdowns,” depression, and bipolar disorder. Other than his concussions, Y was healthy, however, he had pre-morbid, untreated ADHD. He was doing well academically until his concussions, after which he started to see a steep decline in his grades. He was struggling with low self-esteem, which affected his mood. Multiple contributors perpetuated his difficulties, including, his inability to play sports; being home-schooled; removal from his friends; deaths of close friends and family; and a concern that his medical limitation to refrain from physical activities was affecting his career ambitions, contributing to his sense of hopelessness.
Y responded well to the stimulant and antidepressant, but it is important to note the increased risk of non-compliance in teenagers, even when they report seemingly minor side effects, despite doing well clinically. Y required frequent psychiatric follow up and repeat neuropsychological evaluation to monitor his progress.
OUTCOME Back on the playing field
At Y’s 1 month follow up, he reports feeling less depressed but citalopram, 20 mg/d, makes him feel “plain.” His GPA increases to 2.5 and he completes 10th grade. Lisdexamfetamine is titrated to 60 mg/d, he is focusing at school, and his anger is better controlled. Y’s mother is hesitant to change any medications because of her son’s overall improvement.
A few weeks before his next follow up appointment, Y’s mother calls stating that his depression is worse as he has not been taking citalopram because he doesn’t like how it makes him feel. He is started on fluoxetine, 10 mg/d. At his next appointment, Y says that he tolerates fluoxetine. His mood improves substantially and he is doing much better. Y’s mother says she feel that her son is more social, smiling more, and sleeping and eating better.
Several months after Y’s school performance, mood, and behaviors improve, his physicians give him permission to play non-contact sports. He is excited to play baseball. Because of his symptoms, we recommend continuing treating his ADHD and depressive symptoms and monitoring the need for medication. We discussed with Y nonpharmacotherapeutic options, including access to an individualized education plan at school, individual therapy, and formalized cognitive training.
Bottom Line
Traumatic brain injury (TBI) affects children and adults with long-term sequelae, which affects outcomes. Outcome is dependent on several risk factors. Many patients with TBI also suffer from neuropsychiatric symptoms that affect their functioning at home and in social and occupational settings. Those with premorbid psychiatric conditions need to be closely monitored because they may be at greater risk for problems with mood and executive function. Treatment should be targeted to individual complaints.
Related Resources
- Giza CC, Kutcher JS, Ashwal S, et al. Summary of evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80(24): 2250-2257.
- Reardon CL, Factor RM. Sport psychiatry: a systematic review of diagnosis and medical treatment of mental illness in athletes. Sports Med. 2010;40(11):961-980.
Drug Brand Names
Citalopram • Celexa Dextroamphetamine • Adderall
Fluoxetine • Prozac Lisdexamfetamine dimesylate • Vyvanse
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jager TE, Weiss HB, Coben JH, et al. Traumatic brain injuries evaluated in US emergency departments, 1992-1994. Acad Emerg Med. 2000;7(2):134-140.
2. Committee on Quality Improvement American Academy of Pediatrics; Commission on Clinical Policies and Research American Academy of Family Physicians. The management of minor closed head injury in children. Pediatrics. 1999;104(6):1407-1415.
3. Koepsell TD, Rivara FP, Vavilala MS, et al. Incidence and descriptive epidemiologic features of traumatic brain injury in King County, Washington. Pediatrics. 2011;128(5):946-954.
4. Sahler CS, Greenwald BD. Traumatic brain injury in sports: a review [published online July 9, 2012]. Rehabil Res Pract. 2012;2012:659652. doi: 10.1155/2012/659652.
5. Crowe L, Babl F, Anderson V, et al. The epidemiology of paediatric head injuries: data from a referral centre in Victoria, Australia. J Paediatr Child Health. 2009;45(6):346-350.
6. Anderson V, Catroppa C, Morse S, et al. Intellectual outcome from preschool traumatic brain injury: a 5-year prospective, longitudinal study. Pediatrics. 2009;124(6):e1064-1071.
7. Jaffe KM, Fay GC, Polissar NL, et al. Severity of pediatric traumatic brain injury and neurobehavioral recovery at one year—a cohort study. Arch Phys Med Rehabil. 1993; 74(6):587-595.
8. Anderson VA, Catroppa C, Dudgeon P, et al. Understanding predictors of functional recovery and outcome 30 months following early childhood head injury. Neuropsychology. 2006;20(1):42-57.
9. Li L, Liu J. The effect of pediatric traumatic brain injury on behavioral outcomes: a systematic review. Dev Med Child Neurol. 2013;55(1):37-45.
CASE Angry and depressed
Y is a 16-year-old male who presents with his mother to our clinic for medication evaluation because of anger issues and problems learning in school. He says he has been feeling depressed for several months and noticed significant irritability. Y sleeps excessively, sometimes for more than 12 hours a day, and eats more than he usually does. He reports feeling hopeless, helpless, and guilty for letting his family down. Y, who is in the 10th grade, acknowledges trouble focusing and concentrating but attributed this to a previous diagnosis of attention-deficit/hyperactivity disorder (ADHD). He stopped taking his stimulant medication several months ago because he did not like taking it. He denies thoughts of self-harm or thinking about death.
Y’s mother reports that her son had been athletic but had to stop playing football because he has had 5 concussions. Y’s inability to play sports appears to be a precipitating factor in his decline in mood (Box). He had his first concussion at age 13; the last one was several months before his presenting to the clinic. Y experienced loss of consciousness and unsteady gait after his concussions and was hospitalized for some of them. Y says his life goals are “playing sports and being a marine,” which may be compromised because of his head injuries.
His mother reports Y is having more anger outbursts and says his personality is changing. Y viewed this change as just being more assertive and fails to see that others may be scared by his behavior. He is getting into more fights at school and is more impulsive and unpredictable, according to his mother. Y is struggling in school with cognitive deficits and memory problems; his grade point average (GPA) drops from 3.5 to 0.3 over several months. He had been homeschooled initially because of uncontrolled impulsivity and aggression, but was reintegrated to public school. Y has a history of a mathematics disorder but had done well without school accommodations before the head injuries. Lack of access to his peers and poor self-esteem because of his declining grades are making his mood worse. He denies a history of substance use and his urine drug screen is negative.
Recently, Y’s grandfather, with whom he had been close, died and 2 friends were killed in car accidents in the last few years. Y has no history of psychiatric hospitalization. He had seen a psychotherapist for depression. He had been on lisdexamfetamine, 30 mg/d, citalopram, 10 mg/d, and an unknown dose of dextroamphetamine. He had no major medical comorbidities. He lives with his mother. His parents are separated but he has frequent contact with his father. His developmental history is unremarkable. There was a questionable family history of schizophrenia, “nervous breakdowns,” depression, and bipolar disorder. There was no family history of suicide.
On his initial mental status examination, Y appears to be his stated age and is dressed appropriately. He is well dressed, suggesting that he puts a lot of care into his personal appearance. He is alert and oriented. He is cooperative and has fair eye contact. His gait is normal and no motor abnormalities are evident. His speech is normal in rate, rhythm, and volume. He can remember events with great accuracy. He reports that his mood is depressed and “down.” His affect appears irritable and he has low frustration tolerance, especially towards his mother. He is easy to anger but is re-directable. He does not endorse thoughts of suicidality or harm to others. He denies auditory or visual hallucinations, and paranoia. He does not appear to be responding to internal stimuli. His judgment and insight are fair.
a) major depressive disorder
b) oppositional defiant disorder
c) bipolar disorder, most recent episode depressed
d) ADHD, untreated
e) post-concussion syndrome
The authors' observations
Traumatic brain injury (TBI) affects 1.7 to 3.8 million people in the United States. More than 473,000 children present to the emergency room annually with TBI, approximately 75% of whom are given a label of mild TBI in the United States.1-3 TBI patients present with varying degrees of problems ranging from headaches to cognitive deficits and death. Symptoms may be transient or permanent.4 Prepubescent children are at higher risk and are more likely to sustain permanent damage post-TBI, with problems in attention, executive functioning, memory, and cognition.5-7
Prognosis depends on severity of injury and environmental factors, including socioeconomic status, family dysfunction, and access to resources.8 Patients may present during the acute concussion phase with physical symptoms, such as headaches, nausea, vomiting, sensitivity to light and sounds, and memory deficits, and psychiatric complaints such as anger, irritability, and mood swings. Symptoms may persist post-concussion, leading to problems in personal relationships and social and occupational functioning, and neuropsychiatric manifestations, including changes in personality, depression, suicidal thoughts, and substance dependence. As seen in this case, Y had neuropsychiatric manifestations after his TBI but other factors, such as his ADHD diagnosis and the death of his grandfather and friends, may have contributed to his presentation.
Up to one-half of children with brain injuries may be at increased risk for unfavorable behavioral outcomes, which include internalizing and externalizing presentations.9 These behavioral problems may emerge several years after the injury and often persist or get worse with time. Behavioral functioning before injury usually dictates long-term outcomes post injury. The American Academy of Neurology recently released guidelines for the assessment and treatment of athletes with concussions (see Related Resources).
TREATMENT Restart medication
We restart Y on citalopram, 10 mg/d, which he tolerated in the past, and increase it to 20 mg/d after 4 days to address his depression and irritability. He also is restarted on lisdexamfetamine, 30 mg/d, for his ADHD. We give his mother the Child Behavior Checklist and Teacher’s Report Forms to gather additional collateral information. We ask Y to follow up in 1 month and we encourage him to continue seeing his psychotherapist.
a) neuropsychological testing
b) neurology referral
c) imaging studies
d) no testing
EVALUATION Testing
Although Y denies feeling depressed to the neuropsychologist, the examiner notes her concerns about his depression based on his mental status examination during testing.
Neuropsychological testing reveals a discrepancy noted between normal verbal skills and perceptual intellectual skills that were in the borderline range (Table). Testing revealed results supporting executive dysfunction and distractibility, which are consistent with his history of ADHD. Y’s broad reading scores are in the 20th percentile and math scores in the 30th percentile. Although he has a history of a mathematics disorder, his reading deficits are considered a decline compared with his previous performance.
The authors' observations
Y is a 16-year-old male who presented with anger, depression, and academic problems. He had genetic loading with a questionable family history of schizophrenia, “nervous breakdowns,” depression, and bipolar disorder. Other than his concussions, Y was healthy, however, he had pre-morbid, untreated ADHD. He was doing well academically until his concussions, after which he started to see a steep decline in his grades. He was struggling with low self-esteem, which affected his mood. Multiple contributors perpetuated his difficulties, including, his inability to play sports; being home-schooled; removal from his friends; deaths of close friends and family; and a concern that his medical limitation to refrain from physical activities was affecting his career ambitions, contributing to his sense of hopelessness.
Y responded well to the stimulant and antidepressant, but it is important to note the increased risk of non-compliance in teenagers, even when they report seemingly minor side effects, despite doing well clinically. Y required frequent psychiatric follow up and repeat neuropsychological evaluation to monitor his progress.
OUTCOME Back on the playing field
At Y’s 1 month follow up, he reports feeling less depressed but citalopram, 20 mg/d, makes him feel “plain.” His GPA increases to 2.5 and he completes 10th grade. Lisdexamfetamine is titrated to 60 mg/d, he is focusing at school, and his anger is better controlled. Y’s mother is hesitant to change any medications because of her son’s overall improvement.
A few weeks before his next follow up appointment, Y’s mother calls stating that his depression is worse as he has not been taking citalopram because he doesn’t like how it makes him feel. He is started on fluoxetine, 10 mg/d. At his next appointment, Y says that he tolerates fluoxetine. His mood improves substantially and he is doing much better. Y’s mother says she feel that her son is more social, smiling more, and sleeping and eating better.
Several months after Y’s school performance, mood, and behaviors improve, his physicians give him permission to play non-contact sports. He is excited to play baseball. Because of his symptoms, we recommend continuing treating his ADHD and depressive symptoms and monitoring the need for medication. We discussed with Y nonpharmacotherapeutic options, including access to an individualized education plan at school, individual therapy, and formalized cognitive training.
Bottom Line
Traumatic brain injury (TBI) affects children and adults with long-term sequelae, which affects outcomes. Outcome is dependent on several risk factors. Many patients with TBI also suffer from neuropsychiatric symptoms that affect their functioning at home and in social and occupational settings. Those with premorbid psychiatric conditions need to be closely monitored because they may be at greater risk for problems with mood and executive function. Treatment should be targeted to individual complaints.
Related Resources
- Giza CC, Kutcher JS, Ashwal S, et al. Summary of evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80(24): 2250-2257.
- Reardon CL, Factor RM. Sport psychiatry: a systematic review of diagnosis and medical treatment of mental illness in athletes. Sports Med. 2010;40(11):961-980.
Drug Brand Names
Citalopram • Celexa Dextroamphetamine • Adderall
Fluoxetine • Prozac Lisdexamfetamine dimesylate • Vyvanse
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Angry and depressed
Y is a 16-year-old male who presents with his mother to our clinic for medication evaluation because of anger issues and problems learning in school. He says he has been feeling depressed for several months and noticed significant irritability. Y sleeps excessively, sometimes for more than 12 hours a day, and eats more than he usually does. He reports feeling hopeless, helpless, and guilty for letting his family down. Y, who is in the 10th grade, acknowledges trouble focusing and concentrating but attributed this to a previous diagnosis of attention-deficit/hyperactivity disorder (ADHD). He stopped taking his stimulant medication several months ago because he did not like taking it. He denies thoughts of self-harm or thinking about death.
Y’s mother reports that her son had been athletic but had to stop playing football because he has had 5 concussions. Y’s inability to play sports appears to be a precipitating factor in his decline in mood (Box). He had his first concussion at age 13; the last one was several months before his presenting to the clinic. Y experienced loss of consciousness and unsteady gait after his concussions and was hospitalized for some of them. Y says his life goals are “playing sports and being a marine,” which may be compromised because of his head injuries.
His mother reports Y is having more anger outbursts and says his personality is changing. Y viewed this change as just being more assertive and fails to see that others may be scared by his behavior. He is getting into more fights at school and is more impulsive and unpredictable, according to his mother. Y is struggling in school with cognitive deficits and memory problems; his grade point average (GPA) drops from 3.5 to 0.3 over several months. He had been homeschooled initially because of uncontrolled impulsivity and aggression, but was reintegrated to public school. Y has a history of a mathematics disorder but had done well without school accommodations before the head injuries. Lack of access to his peers and poor self-esteem because of his declining grades are making his mood worse. He denies a history of substance use and his urine drug screen is negative.
Recently, Y’s grandfather, with whom he had been close, died and 2 friends were killed in car accidents in the last few years. Y has no history of psychiatric hospitalization. He had seen a psychotherapist for depression. He had been on lisdexamfetamine, 30 mg/d, citalopram, 10 mg/d, and an unknown dose of dextroamphetamine. He had no major medical comorbidities. He lives with his mother. His parents are separated but he has frequent contact with his father. His developmental history is unremarkable. There was a questionable family history of schizophrenia, “nervous breakdowns,” depression, and bipolar disorder. There was no family history of suicide.
On his initial mental status examination, Y appears to be his stated age and is dressed appropriately. He is well dressed, suggesting that he puts a lot of care into his personal appearance. He is alert and oriented. He is cooperative and has fair eye contact. His gait is normal and no motor abnormalities are evident. His speech is normal in rate, rhythm, and volume. He can remember events with great accuracy. He reports that his mood is depressed and “down.” His affect appears irritable and he has low frustration tolerance, especially towards his mother. He is easy to anger but is re-directable. He does not endorse thoughts of suicidality or harm to others. He denies auditory or visual hallucinations, and paranoia. He does not appear to be responding to internal stimuli. His judgment and insight are fair.
a) major depressive disorder
b) oppositional defiant disorder
c) bipolar disorder, most recent episode depressed
d) ADHD, untreated
e) post-concussion syndrome
The authors' observations
Traumatic brain injury (TBI) affects 1.7 to 3.8 million people in the United States. More than 473,000 children present to the emergency room annually with TBI, approximately 75% of whom are given a label of mild TBI in the United States.1-3 TBI patients present with varying degrees of problems ranging from headaches to cognitive deficits and death. Symptoms may be transient or permanent.4 Prepubescent children are at higher risk and are more likely to sustain permanent damage post-TBI, with problems in attention, executive functioning, memory, and cognition.5-7
Prognosis depends on severity of injury and environmental factors, including socioeconomic status, family dysfunction, and access to resources.8 Patients may present during the acute concussion phase with physical symptoms, such as headaches, nausea, vomiting, sensitivity to light and sounds, and memory deficits, and psychiatric complaints such as anger, irritability, and mood swings. Symptoms may persist post-concussion, leading to problems in personal relationships and social and occupational functioning, and neuropsychiatric manifestations, including changes in personality, depression, suicidal thoughts, and substance dependence. As seen in this case, Y had neuropsychiatric manifestations after his TBI but other factors, such as his ADHD diagnosis and the death of his grandfather and friends, may have contributed to his presentation.
Up to one-half of children with brain injuries may be at increased risk for unfavorable behavioral outcomes, which include internalizing and externalizing presentations.9 These behavioral problems may emerge several years after the injury and often persist or get worse with time. Behavioral functioning before injury usually dictates long-term outcomes post injury. The American Academy of Neurology recently released guidelines for the assessment and treatment of athletes with concussions (see Related Resources).
TREATMENT Restart medication
We restart Y on citalopram, 10 mg/d, which he tolerated in the past, and increase it to 20 mg/d after 4 days to address his depression and irritability. He also is restarted on lisdexamfetamine, 30 mg/d, for his ADHD. We give his mother the Child Behavior Checklist and Teacher’s Report Forms to gather additional collateral information. We ask Y to follow up in 1 month and we encourage him to continue seeing his psychotherapist.
a) neuropsychological testing
b) neurology referral
c) imaging studies
d) no testing
EVALUATION Testing
Although Y denies feeling depressed to the neuropsychologist, the examiner notes her concerns about his depression based on his mental status examination during testing.
Neuropsychological testing reveals a discrepancy noted between normal verbal skills and perceptual intellectual skills that were in the borderline range (Table). Testing revealed results supporting executive dysfunction and distractibility, which are consistent with his history of ADHD. Y’s broad reading scores are in the 20th percentile and math scores in the 30th percentile. Although he has a history of a mathematics disorder, his reading deficits are considered a decline compared with his previous performance.
The authors' observations
Y is a 16-year-old male who presented with anger, depression, and academic problems. He had genetic loading with a questionable family history of schizophrenia, “nervous breakdowns,” depression, and bipolar disorder. Other than his concussions, Y was healthy, however, he had pre-morbid, untreated ADHD. He was doing well academically until his concussions, after which he started to see a steep decline in his grades. He was struggling with low self-esteem, which affected his mood. Multiple contributors perpetuated his difficulties, including, his inability to play sports; being home-schooled; removal from his friends; deaths of close friends and family; and a concern that his medical limitation to refrain from physical activities was affecting his career ambitions, contributing to his sense of hopelessness.
Y responded well to the stimulant and antidepressant, but it is important to note the increased risk of non-compliance in teenagers, even when they report seemingly minor side effects, despite doing well clinically. Y required frequent psychiatric follow up and repeat neuropsychological evaluation to monitor his progress.
OUTCOME Back on the playing field
At Y’s 1 month follow up, he reports feeling less depressed but citalopram, 20 mg/d, makes him feel “plain.” His GPA increases to 2.5 and he completes 10th grade. Lisdexamfetamine is titrated to 60 mg/d, he is focusing at school, and his anger is better controlled. Y’s mother is hesitant to change any medications because of her son’s overall improvement.
A few weeks before his next follow up appointment, Y’s mother calls stating that his depression is worse as he has not been taking citalopram because he doesn’t like how it makes him feel. He is started on fluoxetine, 10 mg/d. At his next appointment, Y says that he tolerates fluoxetine. His mood improves substantially and he is doing much better. Y’s mother says she feel that her son is more social, smiling more, and sleeping and eating better.
Several months after Y’s school performance, mood, and behaviors improve, his physicians give him permission to play non-contact sports. He is excited to play baseball. Because of his symptoms, we recommend continuing treating his ADHD and depressive symptoms and monitoring the need for medication. We discussed with Y nonpharmacotherapeutic options, including access to an individualized education plan at school, individual therapy, and formalized cognitive training.
Bottom Line
Traumatic brain injury (TBI) affects children and adults with long-term sequelae, which affects outcomes. Outcome is dependent on several risk factors. Many patients with TBI also suffer from neuropsychiatric symptoms that affect their functioning at home and in social and occupational settings. Those with premorbid psychiatric conditions need to be closely monitored because they may be at greater risk for problems with mood and executive function. Treatment should be targeted to individual complaints.
Related Resources
- Giza CC, Kutcher JS, Ashwal S, et al. Summary of evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80(24): 2250-2257.
- Reardon CL, Factor RM. Sport psychiatry: a systematic review of diagnosis and medical treatment of mental illness in athletes. Sports Med. 2010;40(11):961-980.
Drug Brand Names
Citalopram • Celexa Dextroamphetamine • Adderall
Fluoxetine • Prozac Lisdexamfetamine dimesylate • Vyvanse
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jager TE, Weiss HB, Coben JH, et al. Traumatic brain injuries evaluated in US emergency departments, 1992-1994. Acad Emerg Med. 2000;7(2):134-140.
2. Committee on Quality Improvement American Academy of Pediatrics; Commission on Clinical Policies and Research American Academy of Family Physicians. The management of minor closed head injury in children. Pediatrics. 1999;104(6):1407-1415.
3. Koepsell TD, Rivara FP, Vavilala MS, et al. Incidence and descriptive epidemiologic features of traumatic brain injury in King County, Washington. Pediatrics. 2011;128(5):946-954.
4. Sahler CS, Greenwald BD. Traumatic brain injury in sports: a review [published online July 9, 2012]. Rehabil Res Pract. 2012;2012:659652. doi: 10.1155/2012/659652.
5. Crowe L, Babl F, Anderson V, et al. The epidemiology of paediatric head injuries: data from a referral centre in Victoria, Australia. J Paediatr Child Health. 2009;45(6):346-350.
6. Anderson V, Catroppa C, Morse S, et al. Intellectual outcome from preschool traumatic brain injury: a 5-year prospective, longitudinal study. Pediatrics. 2009;124(6):e1064-1071.
7. Jaffe KM, Fay GC, Polissar NL, et al. Severity of pediatric traumatic brain injury and neurobehavioral recovery at one year—a cohort study. Arch Phys Med Rehabil. 1993; 74(6):587-595.
8. Anderson VA, Catroppa C, Dudgeon P, et al. Understanding predictors of functional recovery and outcome 30 months following early childhood head injury. Neuropsychology. 2006;20(1):42-57.
9. Li L, Liu J. The effect of pediatric traumatic brain injury on behavioral outcomes: a systematic review. Dev Med Child Neurol. 2013;55(1):37-45.
1. Jager TE, Weiss HB, Coben JH, et al. Traumatic brain injuries evaluated in US emergency departments, 1992-1994. Acad Emerg Med. 2000;7(2):134-140.
2. Committee on Quality Improvement American Academy of Pediatrics; Commission on Clinical Policies and Research American Academy of Family Physicians. The management of minor closed head injury in children. Pediatrics. 1999;104(6):1407-1415.
3. Koepsell TD, Rivara FP, Vavilala MS, et al. Incidence and descriptive epidemiologic features of traumatic brain injury in King County, Washington. Pediatrics. 2011;128(5):946-954.
4. Sahler CS, Greenwald BD. Traumatic brain injury in sports: a review [published online July 9, 2012]. Rehabil Res Pract. 2012;2012:659652. doi: 10.1155/2012/659652.
5. Crowe L, Babl F, Anderson V, et al. The epidemiology of paediatric head injuries: data from a referral centre in Victoria, Australia. J Paediatr Child Health. 2009;45(6):346-350.
6. Anderson V, Catroppa C, Morse S, et al. Intellectual outcome from preschool traumatic brain injury: a 5-year prospective, longitudinal study. Pediatrics. 2009;124(6):e1064-1071.
7. Jaffe KM, Fay GC, Polissar NL, et al. Severity of pediatric traumatic brain injury and neurobehavioral recovery at one year—a cohort study. Arch Phys Med Rehabil. 1993; 74(6):587-595.
8. Anderson VA, Catroppa C, Dudgeon P, et al. Understanding predictors of functional recovery and outcome 30 months following early childhood head injury. Neuropsychology. 2006;20(1):42-57.
9. Li L, Liu J. The effect of pediatric traumatic brain injury on behavioral outcomes: a systematic review. Dev Med Child Neurol. 2013;55(1):37-45.
Weakness and facial droop: Is it a stroke?
CASE Sudden weakness
Ms. G, age 59, presents to a local critical access (rural) hospital after an episode of sudden-onset left-sided weakness followed by unconsciousness. She regained consciousness quickly and is awake when she arrives at the hospital. This event was not witnessed, although family members were nearby to call emergency personnel.
a) CT scan
b) MRI
c) EEG
d) head and neck magnetic resonance angiogram (MRA)
EXAMINATION Unremarkable
In the emergency department, Ms. G demonstrates left facial droop, left-sided weakness of her arm and leg, and aphasia. She says she has a severe headache that began after she regained consciousness. She is unable to see out of her left eye.
Ms. G’s NIH Stroke Scale score is 13, indicating a moderate stroke; an emergent head CT does not demonstrate any acute hemorrhagic process. Tissue plasminogen activator (tPA) is administered for a suspected stroke approximately 2 hours after her symptoms began. She is transferred to a larger, tertiary care hospital for further workup and observation.
Upon admission to the ICU, Ms. G’s laboratory values are: sodium, 137 mEq/L; potassium, 5.1 mEq/L; creatinine, 1.26 mg/dL; lipase, 126 U/L; and lactic acid, 9 mg/dL. The glucose level is within normal limits and her urinalysis is unremarkable.
Vital signs are stable and Ms. G is not in acute distress. A physical exam demonstrates 4/5 strength in the left-upper and -lower extremities. Additionally, there are 2+ deep tendon reflexes bilaterally in the biceps, triceps, and brachioradialis. She has left-sided facial droop while in the ICU, and continues to demonstrate some aphasia—although she is alert and oriented to person, time, and place.
The medical history is significant for depression, restless leg syndrome, tonic-clonic seizures, and previous stroke-like events. Medications include amitriptyline, 25 mg/d; citalopram, 20 mg/d; valproate, 1,200 mg/d; and ropinirole, 0.5 mg/d. Her mother has a history of stroke-like events, but her family history and social history are otherwise unremarkable.
The authors' observations
Conversion disorder requires the exclusion of medical causes that could explain the patient’s neurologic symptoms. It is prudent to rule out the most serious of the potential contributors to Ms. G’s condition—namely, an acute cerebrovascular accident. A CT scan did not find any significant pathology, however. In the ICU, an MRI showed no evidence of acute infarction based on diffusion-weighted imaging. A head and neck MRA demonstrated no hemodynamically significant stenosis of the internal carotid arteries. An EEG revealed generalized, polymorphic slow activity without evidence of seizures or epilepsy. An electrocardiogram showed normal ventricular size with an appropriate ejection fraction.
The ICU staff consulted psychiatry to evaluate a psychiatric cause of Ms. G’s symptoms.
An exhaustive and comprehensive workup was performed; there were no significant findings. Although laboratory tests were performed, it was the physical exam that suggested the diagnosis of conversion disorder. In that sense, the diagnostic tests were more of a supportive adjunct to the findings of the physical examination, which consistently failed to indicate a neurologic insult.
Hoover’s sign is a well-established test of functional weakness, in which the patient extends his (her) hip when the contralateral hip is flexed. However, there are other tests of functional weakness that can be useful when considering a conversion disorder diagnosis, including co-contraction, the so-called arm-drop sign, and the sternocleidomastoid test. Diukova and colleagues reported that 80% of patients with functional weakness demonstrated ipsilateral sternocleidomastoid weakness, compared with 11% with vascular hemiparesis.1
a) stroke
b) transient ischemic attack
c) conversion disorder
d) seizure disorder
Ms. G appeared to have suffered an acute ischemic event that caused her neurologic symptoms; her rather extensive psychiatric history was overlooked before the psychiatric service was consulted. When Ms. G was admitted to the ICU, the working differential was postictal seizure state rather than cerebrovascular accident. Ms. G had a poorly defined seizure history, and her history of stroke-like events was murky, at best. She had not been treated previously with tPA, and in all past instances her symptoms resolved spontaneously.
Ms. G’s case illustrates why conversion disorder is difficult to diagnose and why, perhaps, it is even a dangerous diagnostic consideration. Booij and colleagues described two patients with neurologic sequelae thought to be the result of conversion disorder; subsequent imaging demonstrated a posterior stroke.2 Over a 6-year period in an emergency department, Glick and coworkers identified six patients with neurologic pathology who were misdiagnosed with conversion disorder.3 In a study of 4,220 patients presenting to a psychiatric emergency service, three patients complained of extremity paralysis or pain, which was attributed to conversion disorder but later attributed to an organic disease.4
These studies emphasize the precarious nature of diagnosing conversion disorder. For that reason, an extensive medical workup is necessary prior to considering a diagnosis of conversion disorder. In Ms. G’s case, a reasonably thorough workup failed to reveal any obvious pathology. Only then was conversion disorder included as a diagnostic possibility.
EVALUATION Childhood abuse
When performing a mental status exam, Ms. G has poor eye contact, but is cooperative with our interview. She is disheveled and overweight, and denies suicidal or homicidal ideation. She displays constricted affect.
During the interview, we note a left facial droop, although Ms. G is able to smile fully. As the interview progresses, her facial droop seems to become more apparent as we discuss her past, including a history of childhood physical and sexual abuse. She has a history of depression and has been seeing an outpatient psychiatrist for the past year. Ms. G describes being hospitalized in a psychiatric unit, but she is unable to provide any details about when and where this occurred.
Ms. G admits to occasional auditory and visual hallucinations, mostly relating to the abuse she experienced as a child by her parents. She exhibits no other signs or symptoms of psychosis; the hallucinations she describes are consistent with flashbacks and vivid memories relating to the abuse. Ms. G also recently lost her job and is experiencing numerous financial stressors.
The authors' observations
There are many examples in the literature of patients with conversion disorder (Table 1),4 ranging from pseudoseizures, which are relatively common, to intriguing cases, such as cochlear implant failure.5
Some studies estimate that the prevalence of conversion disorder symptoms ranges from 16.1% to 21.9% in the general population.6 Somatoform disorders, including conversion disorder, often are comorbid with anxiety and depression. In one study, 26% of somatoform disorder patients also had depression or anxiety, or both.7 Patients with conversion disorder often report a history of childhood physical or sexual abuse.6 In many patients with conversion disorder, there also appears to be a significant association between the disorder and a recent and distant history of psychosocial stressors.8
Ms. G had an extensive history of abuse by her parents. Conversion disorder presenting as a stroke with realistic and convincing physical manifestations is an unusual presentation. There are case reports that detail this presentation, particularly in the emergency department setting.6
Clinical considerations
The relative uncertainty that accompanies a diagnosis of conversion disorder can be discomforting for clinicians. As demonstrated by Ms. G, as well as other case reports of conversion disorder, it takes time for the patient to find a clinician who will consider a diagnosis of conversion disorder.9 Largely, this is because DSM-5 requires that other medical causes be ruled out (Table 2).10 This often proves to be problematic because feigning, or the lack thereof, is difficult to prove.9
Further complicating the diagnosis is the lack of a diagnostic test. Neurologists can use video EEG or physical exam maneuvers such as the Hoover’s sign to help make a diagnosis of conversion disorder.11 In this sense, the physical exam maneuvers form the basis of making a diagnosis, while imaging and lab work support the diagnosis. Hoover’s sign, for example, has not been well studied in a controlled manner, but is recognized as a test that may aid a conversion disorder diagnosis. Clinicians should not solely rely upon these physical exam maneuvers; interpreting them in the context of the patient’s overall presentation is critical. This demonstrates the importance of using the physical exam as a way to guide the diagnosis in association with other tests.12
Despite the lack of pathology, studies demonstrate that patients with conversion disorder may have abnormal brain activity that causes them to perceive motor symptoms as involuntary.11 Therefore, there is a clear need for an increased understanding of psychiatric and neurologic components of diagnosing conversion disorder.8
With Ms. G, it was prudent to make a conversion disorder diagnosis to prevent harm to the patient should future stroke-like events occur. Without considering a conversion disorder diagnosis, a patient may continue to receive unnecessary interventions. Basic physical exam maneuvers, such as Hoover’s sign, can be performed quickly in the ED setting before proceeding with other potentially harmful interventions, such as administering tPA.
Treatment. There are few therapies for conversion disorder. This is, in part, because of lack of understanding about the disorder’s neurologic and biologic etiologies. Although there are some studies that support the use of cognitive-behavioral therapy (CBT), there is little evidence advocating the use of a single mechanism to treat conversion disorder.13 There is evidence that CBT is an effective treatment for several somatoform disorders, including conversion disorder. Research suggests that patients with somatoform disorder have better outcomes when CBT is added to a traditional follow-up.14,15
In Ms. G’s case, we provided information about the diagnosis and scheduled visits to continue her outpatient therapy.
Bottom Line
Conversion disorder is difficult to diagnose, and can mimic potentially life- threatening medical conditions. Conduct a thorough medical workup of these patients, even when it is tempting to jump to a diagnosis of conversion disorder. The use of physical exam maneuvers such as Hoover’s sign may help guide the diagnosis when used in conjunction with other testing.
Related Resources
- Conversion disorder. www.nlm.nih.gov/medlineplus/ency/ article/000954.htm.
- Couprie W, Wijdicks EF, Rooijmans HG, et al. Outcome in conver- sion disorder: a follow up study. J Neurol Neurosurg Psychiatry. 1995;58(6):750-752.
Drug Brand Names
Amitriptyline • Elavil Citalopram • Celexa
Ropinirole • Requip Valproate • Depakote
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diukova GM, Stolajrova AV, Vein AM. Sternocleidomastoid (SCM) muscle test in patients with hysterical and organic paresis. J Neurol Sci. 2001;187(suppl 1):S108.
2. Booij HA, Hamburger HL, Jöbsis GJ, et al. Stroke mimicking conversion disorder: two young women who put our feet back on the ground. Pract Neurol. 2012;12(3):179-181.
3. Glick TH, Workman TP, Gaufberg SV. Suspected conversion disorder: foreseeable risks and avoidable errors. Acad Emerg Med. 2000;7(11):1272-1277.
4. Fishbain DA, Goldberg M. The misdiagnosis of conversion disorder in a psychiatric emergency service. Gen Hosp Psychiatry. 1991;13(3):177-181.
5. Carlson ML, Archibald DJ, Gifford RH, et al. Conversion disorder: a missed diagnosis leading to cochlear reimplantation. Otol Neurotol. 2011;32(1):36-38.
6. Sar V, Akyüz G, Kundakçi T, et al. Childhood trauma, dissociation, and psychiatric comorbidity in patients with conversion disorder. Am J Psychiatry. 2004;161(12):2271-2276.
7. de Waal MW, Arnold IA, Eekhof JA, et al. Somatoform disorders in general practice: prevalence, functional impairment and comorbidity with anxiety and depressive disorders. Br J Psychiatry. 2004;184:470-476.
8. Nicholson TR, Stone J, Kanaan RA. Conversion disorder: a problematic diagnosis. J Neurol Neurosurg Psychiatry. 2011;82(11):1267-1273.
9. Stone J, LaFrance WC, Jr, Levenson JL, et al. Issues for
DSM-5: conversion disorder. Am J Psychiatry. 2010;167(6): 626-627.
10. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
11. Voon V, Gallea C, Hattori N, et al. The involuntary nature of conversion disorder. Neurology. 2010;74(3):223-228.
12. Stone J, Zeman A, Sharpe M. Functional weakness and sensory disturbance. J Neurol Neurosurg Psychiatry. 2002; 73:241-245.
13. Aybek S, Kanaan RA, David AS. The neuropsychiatry of conversion disorder. Curr Opin Psychiatry. 2008;21(3):275-280.
14. Kroenke K. Efficacy of treatment for somatoform disorders: a review of randomized controlled trials. Psychosom Med. 2007;69(9):881-888.
15. Sharpe M, Walker J, Williams C, et al. Guided self-help for functional (psychogenic) symptoms: a randomized controlled efficacy trial. Neurology. 2011;77(6):564-572.
CASE Sudden weakness
Ms. G, age 59, presents to a local critical access (rural) hospital after an episode of sudden-onset left-sided weakness followed by unconsciousness. She regained consciousness quickly and is awake when she arrives at the hospital. This event was not witnessed, although family members were nearby to call emergency personnel.
a) CT scan
b) MRI
c) EEG
d) head and neck magnetic resonance angiogram (MRA)
EXAMINATION Unremarkable
In the emergency department, Ms. G demonstrates left facial droop, left-sided weakness of her arm and leg, and aphasia. She says she has a severe headache that began after she regained consciousness. She is unable to see out of her left eye.
Ms. G’s NIH Stroke Scale score is 13, indicating a moderate stroke; an emergent head CT does not demonstrate any acute hemorrhagic process. Tissue plasminogen activator (tPA) is administered for a suspected stroke approximately 2 hours after her symptoms began. She is transferred to a larger, tertiary care hospital for further workup and observation.
Upon admission to the ICU, Ms. G’s laboratory values are: sodium, 137 mEq/L; potassium, 5.1 mEq/L; creatinine, 1.26 mg/dL; lipase, 126 U/L; and lactic acid, 9 mg/dL. The glucose level is within normal limits and her urinalysis is unremarkable.
Vital signs are stable and Ms. G is not in acute distress. A physical exam demonstrates 4/5 strength in the left-upper and -lower extremities. Additionally, there are 2+ deep tendon reflexes bilaterally in the biceps, triceps, and brachioradialis. She has left-sided facial droop while in the ICU, and continues to demonstrate some aphasia—although she is alert and oriented to person, time, and place.
The medical history is significant for depression, restless leg syndrome, tonic-clonic seizures, and previous stroke-like events. Medications include amitriptyline, 25 mg/d; citalopram, 20 mg/d; valproate, 1,200 mg/d; and ropinirole, 0.5 mg/d. Her mother has a history of stroke-like events, but her family history and social history are otherwise unremarkable.
The authors' observations
Conversion disorder requires the exclusion of medical causes that could explain the patient’s neurologic symptoms. It is prudent to rule out the most serious of the potential contributors to Ms. G’s condition—namely, an acute cerebrovascular accident. A CT scan did not find any significant pathology, however. In the ICU, an MRI showed no evidence of acute infarction based on diffusion-weighted imaging. A head and neck MRA demonstrated no hemodynamically significant stenosis of the internal carotid arteries. An EEG revealed generalized, polymorphic slow activity without evidence of seizures or epilepsy. An electrocardiogram showed normal ventricular size with an appropriate ejection fraction.
The ICU staff consulted psychiatry to evaluate a psychiatric cause of Ms. G’s symptoms.
An exhaustive and comprehensive workup was performed; there were no significant findings. Although laboratory tests were performed, it was the physical exam that suggested the diagnosis of conversion disorder. In that sense, the diagnostic tests were more of a supportive adjunct to the findings of the physical examination, which consistently failed to indicate a neurologic insult.
Hoover’s sign is a well-established test of functional weakness, in which the patient extends his (her) hip when the contralateral hip is flexed. However, there are other tests of functional weakness that can be useful when considering a conversion disorder diagnosis, including co-contraction, the so-called arm-drop sign, and the sternocleidomastoid test. Diukova and colleagues reported that 80% of patients with functional weakness demonstrated ipsilateral sternocleidomastoid weakness, compared with 11% with vascular hemiparesis.1
a) stroke
b) transient ischemic attack
c) conversion disorder
d) seizure disorder
Ms. G appeared to have suffered an acute ischemic event that caused her neurologic symptoms; her rather extensive psychiatric history was overlooked before the psychiatric service was consulted. When Ms. G was admitted to the ICU, the working differential was postictal seizure state rather than cerebrovascular accident. Ms. G had a poorly defined seizure history, and her history of stroke-like events was murky, at best. She had not been treated previously with tPA, and in all past instances her symptoms resolved spontaneously.
Ms. G’s case illustrates why conversion disorder is difficult to diagnose and why, perhaps, it is even a dangerous diagnostic consideration. Booij and colleagues described two patients with neurologic sequelae thought to be the result of conversion disorder; subsequent imaging demonstrated a posterior stroke.2 Over a 6-year period in an emergency department, Glick and coworkers identified six patients with neurologic pathology who were misdiagnosed with conversion disorder.3 In a study of 4,220 patients presenting to a psychiatric emergency service, three patients complained of extremity paralysis or pain, which was attributed to conversion disorder but later attributed to an organic disease.4
These studies emphasize the precarious nature of diagnosing conversion disorder. For that reason, an extensive medical workup is necessary prior to considering a diagnosis of conversion disorder. In Ms. G’s case, a reasonably thorough workup failed to reveal any obvious pathology. Only then was conversion disorder included as a diagnostic possibility.
EVALUATION Childhood abuse
When performing a mental status exam, Ms. G has poor eye contact, but is cooperative with our interview. She is disheveled and overweight, and denies suicidal or homicidal ideation. She displays constricted affect.
During the interview, we note a left facial droop, although Ms. G is able to smile fully. As the interview progresses, her facial droop seems to become more apparent as we discuss her past, including a history of childhood physical and sexual abuse. She has a history of depression and has been seeing an outpatient psychiatrist for the past year. Ms. G describes being hospitalized in a psychiatric unit, but she is unable to provide any details about when and where this occurred.
Ms. G admits to occasional auditory and visual hallucinations, mostly relating to the abuse she experienced as a child by her parents. She exhibits no other signs or symptoms of psychosis; the hallucinations she describes are consistent with flashbacks and vivid memories relating to the abuse. Ms. G also recently lost her job and is experiencing numerous financial stressors.
The authors' observations
There are many examples in the literature of patients with conversion disorder (Table 1),4 ranging from pseudoseizures, which are relatively common, to intriguing cases, such as cochlear implant failure.5
Some studies estimate that the prevalence of conversion disorder symptoms ranges from 16.1% to 21.9% in the general population.6 Somatoform disorders, including conversion disorder, often are comorbid with anxiety and depression. In one study, 26% of somatoform disorder patients also had depression or anxiety, or both.7 Patients with conversion disorder often report a history of childhood physical or sexual abuse.6 In many patients with conversion disorder, there also appears to be a significant association between the disorder and a recent and distant history of psychosocial stressors.8
Ms. G had an extensive history of abuse by her parents. Conversion disorder presenting as a stroke with realistic and convincing physical manifestations is an unusual presentation. There are case reports that detail this presentation, particularly in the emergency department setting.6
Clinical considerations
The relative uncertainty that accompanies a diagnosis of conversion disorder can be discomforting for clinicians. As demonstrated by Ms. G, as well as other case reports of conversion disorder, it takes time for the patient to find a clinician who will consider a diagnosis of conversion disorder.9 Largely, this is because DSM-5 requires that other medical causes be ruled out (Table 2).10 This often proves to be problematic because feigning, or the lack thereof, is difficult to prove.9
Further complicating the diagnosis is the lack of a diagnostic test. Neurologists can use video EEG or physical exam maneuvers such as the Hoover’s sign to help make a diagnosis of conversion disorder.11 In this sense, the physical exam maneuvers form the basis of making a diagnosis, while imaging and lab work support the diagnosis. Hoover’s sign, for example, has not been well studied in a controlled manner, but is recognized as a test that may aid a conversion disorder diagnosis. Clinicians should not solely rely upon these physical exam maneuvers; interpreting them in the context of the patient’s overall presentation is critical. This demonstrates the importance of using the physical exam as a way to guide the diagnosis in association with other tests.12
Despite the lack of pathology, studies demonstrate that patients with conversion disorder may have abnormal brain activity that causes them to perceive motor symptoms as involuntary.11 Therefore, there is a clear need for an increased understanding of psychiatric and neurologic components of diagnosing conversion disorder.8
With Ms. G, it was prudent to make a conversion disorder diagnosis to prevent harm to the patient should future stroke-like events occur. Without considering a conversion disorder diagnosis, a patient may continue to receive unnecessary interventions. Basic physical exam maneuvers, such as Hoover’s sign, can be performed quickly in the ED setting before proceeding with other potentially harmful interventions, such as administering tPA.
Treatment. There are few therapies for conversion disorder. This is, in part, because of lack of understanding about the disorder’s neurologic and biologic etiologies. Although there are some studies that support the use of cognitive-behavioral therapy (CBT), there is little evidence advocating the use of a single mechanism to treat conversion disorder.13 There is evidence that CBT is an effective treatment for several somatoform disorders, including conversion disorder. Research suggests that patients with somatoform disorder have better outcomes when CBT is added to a traditional follow-up.14,15
In Ms. G’s case, we provided information about the diagnosis and scheduled visits to continue her outpatient therapy.
Bottom Line
Conversion disorder is difficult to diagnose, and can mimic potentially life- threatening medical conditions. Conduct a thorough medical workup of these patients, even when it is tempting to jump to a diagnosis of conversion disorder. The use of physical exam maneuvers such as Hoover’s sign may help guide the diagnosis when used in conjunction with other testing.
Related Resources
- Conversion disorder. www.nlm.nih.gov/medlineplus/ency/ article/000954.htm.
- Couprie W, Wijdicks EF, Rooijmans HG, et al. Outcome in conver- sion disorder: a follow up study. J Neurol Neurosurg Psychiatry. 1995;58(6):750-752.
Drug Brand Names
Amitriptyline • Elavil Citalopram • Celexa
Ropinirole • Requip Valproate • Depakote
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Sudden weakness
Ms. G, age 59, presents to a local critical access (rural) hospital after an episode of sudden-onset left-sided weakness followed by unconsciousness. She regained consciousness quickly and is awake when she arrives at the hospital. This event was not witnessed, although family members were nearby to call emergency personnel.
a) CT scan
b) MRI
c) EEG
d) head and neck magnetic resonance angiogram (MRA)
EXAMINATION Unremarkable
In the emergency department, Ms. G demonstrates left facial droop, left-sided weakness of her arm and leg, and aphasia. She says she has a severe headache that began after she regained consciousness. She is unable to see out of her left eye.
Ms. G’s NIH Stroke Scale score is 13, indicating a moderate stroke; an emergent head CT does not demonstrate any acute hemorrhagic process. Tissue plasminogen activator (tPA) is administered for a suspected stroke approximately 2 hours after her symptoms began. She is transferred to a larger, tertiary care hospital for further workup and observation.
Upon admission to the ICU, Ms. G’s laboratory values are: sodium, 137 mEq/L; potassium, 5.1 mEq/L; creatinine, 1.26 mg/dL; lipase, 126 U/L; and lactic acid, 9 mg/dL. The glucose level is within normal limits and her urinalysis is unremarkable.
Vital signs are stable and Ms. G is not in acute distress. A physical exam demonstrates 4/5 strength in the left-upper and -lower extremities. Additionally, there are 2+ deep tendon reflexes bilaterally in the biceps, triceps, and brachioradialis. She has left-sided facial droop while in the ICU, and continues to demonstrate some aphasia—although she is alert and oriented to person, time, and place.
The medical history is significant for depression, restless leg syndrome, tonic-clonic seizures, and previous stroke-like events. Medications include amitriptyline, 25 mg/d; citalopram, 20 mg/d; valproate, 1,200 mg/d; and ropinirole, 0.5 mg/d. Her mother has a history of stroke-like events, but her family history and social history are otherwise unremarkable.
The authors' observations
Conversion disorder requires the exclusion of medical causes that could explain the patient’s neurologic symptoms. It is prudent to rule out the most serious of the potential contributors to Ms. G’s condition—namely, an acute cerebrovascular accident. A CT scan did not find any significant pathology, however. In the ICU, an MRI showed no evidence of acute infarction based on diffusion-weighted imaging. A head and neck MRA demonstrated no hemodynamically significant stenosis of the internal carotid arteries. An EEG revealed generalized, polymorphic slow activity without evidence of seizures or epilepsy. An electrocardiogram showed normal ventricular size with an appropriate ejection fraction.
The ICU staff consulted psychiatry to evaluate a psychiatric cause of Ms. G’s symptoms.
An exhaustive and comprehensive workup was performed; there were no significant findings. Although laboratory tests were performed, it was the physical exam that suggested the diagnosis of conversion disorder. In that sense, the diagnostic tests were more of a supportive adjunct to the findings of the physical examination, which consistently failed to indicate a neurologic insult.
Hoover’s sign is a well-established test of functional weakness, in which the patient extends his (her) hip when the contralateral hip is flexed. However, there are other tests of functional weakness that can be useful when considering a conversion disorder diagnosis, including co-contraction, the so-called arm-drop sign, and the sternocleidomastoid test. Diukova and colleagues reported that 80% of patients with functional weakness demonstrated ipsilateral sternocleidomastoid weakness, compared with 11% with vascular hemiparesis.1
a) stroke
b) transient ischemic attack
c) conversion disorder
d) seizure disorder
Ms. G appeared to have suffered an acute ischemic event that caused her neurologic symptoms; her rather extensive psychiatric history was overlooked before the psychiatric service was consulted. When Ms. G was admitted to the ICU, the working differential was postictal seizure state rather than cerebrovascular accident. Ms. G had a poorly defined seizure history, and her history of stroke-like events was murky, at best. She had not been treated previously with tPA, and in all past instances her symptoms resolved spontaneously.
Ms. G’s case illustrates why conversion disorder is difficult to diagnose and why, perhaps, it is even a dangerous diagnostic consideration. Booij and colleagues described two patients with neurologic sequelae thought to be the result of conversion disorder; subsequent imaging demonstrated a posterior stroke.2 Over a 6-year period in an emergency department, Glick and coworkers identified six patients with neurologic pathology who were misdiagnosed with conversion disorder.3 In a study of 4,220 patients presenting to a psychiatric emergency service, three patients complained of extremity paralysis or pain, which was attributed to conversion disorder but later attributed to an organic disease.4
These studies emphasize the precarious nature of diagnosing conversion disorder. For that reason, an extensive medical workup is necessary prior to considering a diagnosis of conversion disorder. In Ms. G’s case, a reasonably thorough workup failed to reveal any obvious pathology. Only then was conversion disorder included as a diagnostic possibility.
EVALUATION Childhood abuse
When performing a mental status exam, Ms. G has poor eye contact, but is cooperative with our interview. She is disheveled and overweight, and denies suicidal or homicidal ideation. She displays constricted affect.
During the interview, we note a left facial droop, although Ms. G is able to smile fully. As the interview progresses, her facial droop seems to become more apparent as we discuss her past, including a history of childhood physical and sexual abuse. She has a history of depression and has been seeing an outpatient psychiatrist for the past year. Ms. G describes being hospitalized in a psychiatric unit, but she is unable to provide any details about when and where this occurred.
Ms. G admits to occasional auditory and visual hallucinations, mostly relating to the abuse she experienced as a child by her parents. She exhibits no other signs or symptoms of psychosis; the hallucinations she describes are consistent with flashbacks and vivid memories relating to the abuse. Ms. G also recently lost her job and is experiencing numerous financial stressors.
The authors' observations
There are many examples in the literature of patients with conversion disorder (Table 1),4 ranging from pseudoseizures, which are relatively common, to intriguing cases, such as cochlear implant failure.5
Some studies estimate that the prevalence of conversion disorder symptoms ranges from 16.1% to 21.9% in the general population.6 Somatoform disorders, including conversion disorder, often are comorbid with anxiety and depression. In one study, 26% of somatoform disorder patients also had depression or anxiety, or both.7 Patients with conversion disorder often report a history of childhood physical or sexual abuse.6 In many patients with conversion disorder, there also appears to be a significant association between the disorder and a recent and distant history of psychosocial stressors.8
Ms. G had an extensive history of abuse by her parents. Conversion disorder presenting as a stroke with realistic and convincing physical manifestations is an unusual presentation. There are case reports that detail this presentation, particularly in the emergency department setting.6
Clinical considerations
The relative uncertainty that accompanies a diagnosis of conversion disorder can be discomforting for clinicians. As demonstrated by Ms. G, as well as other case reports of conversion disorder, it takes time for the patient to find a clinician who will consider a diagnosis of conversion disorder.9 Largely, this is because DSM-5 requires that other medical causes be ruled out (Table 2).10 This often proves to be problematic because feigning, or the lack thereof, is difficult to prove.9
Further complicating the diagnosis is the lack of a diagnostic test. Neurologists can use video EEG or physical exam maneuvers such as the Hoover’s sign to help make a diagnosis of conversion disorder.11 In this sense, the physical exam maneuvers form the basis of making a diagnosis, while imaging and lab work support the diagnosis. Hoover’s sign, for example, has not been well studied in a controlled manner, but is recognized as a test that may aid a conversion disorder diagnosis. Clinicians should not solely rely upon these physical exam maneuvers; interpreting them in the context of the patient’s overall presentation is critical. This demonstrates the importance of using the physical exam as a way to guide the diagnosis in association with other tests.12
Despite the lack of pathology, studies demonstrate that patients with conversion disorder may have abnormal brain activity that causes them to perceive motor symptoms as involuntary.11 Therefore, there is a clear need for an increased understanding of psychiatric and neurologic components of diagnosing conversion disorder.8
With Ms. G, it was prudent to make a conversion disorder diagnosis to prevent harm to the patient should future stroke-like events occur. Without considering a conversion disorder diagnosis, a patient may continue to receive unnecessary interventions. Basic physical exam maneuvers, such as Hoover’s sign, can be performed quickly in the ED setting before proceeding with other potentially harmful interventions, such as administering tPA.
Treatment. There are few therapies for conversion disorder. This is, in part, because of lack of understanding about the disorder’s neurologic and biologic etiologies. Although there are some studies that support the use of cognitive-behavioral therapy (CBT), there is little evidence advocating the use of a single mechanism to treat conversion disorder.13 There is evidence that CBT is an effective treatment for several somatoform disorders, including conversion disorder. Research suggests that patients with somatoform disorder have better outcomes when CBT is added to a traditional follow-up.14,15
In Ms. G’s case, we provided information about the diagnosis and scheduled visits to continue her outpatient therapy.
Bottom Line
Conversion disorder is difficult to diagnose, and can mimic potentially life- threatening medical conditions. Conduct a thorough medical workup of these patients, even when it is tempting to jump to a diagnosis of conversion disorder. The use of physical exam maneuvers such as Hoover’s sign may help guide the diagnosis when used in conjunction with other testing.
Related Resources
- Conversion disorder. www.nlm.nih.gov/medlineplus/ency/ article/000954.htm.
- Couprie W, Wijdicks EF, Rooijmans HG, et al. Outcome in conver- sion disorder: a follow up study. J Neurol Neurosurg Psychiatry. 1995;58(6):750-752.
Drug Brand Names
Amitriptyline • Elavil Citalopram • Celexa
Ropinirole • Requip Valproate • Depakote
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diukova GM, Stolajrova AV, Vein AM. Sternocleidomastoid (SCM) muscle test in patients with hysterical and organic paresis. J Neurol Sci. 2001;187(suppl 1):S108.
2. Booij HA, Hamburger HL, Jöbsis GJ, et al. Stroke mimicking conversion disorder: two young women who put our feet back on the ground. Pract Neurol. 2012;12(3):179-181.
3. Glick TH, Workman TP, Gaufberg SV. Suspected conversion disorder: foreseeable risks and avoidable errors. Acad Emerg Med. 2000;7(11):1272-1277.
4. Fishbain DA, Goldberg M. The misdiagnosis of conversion disorder in a psychiatric emergency service. Gen Hosp Psychiatry. 1991;13(3):177-181.
5. Carlson ML, Archibald DJ, Gifford RH, et al. Conversion disorder: a missed diagnosis leading to cochlear reimplantation. Otol Neurotol. 2011;32(1):36-38.
6. Sar V, Akyüz G, Kundakçi T, et al. Childhood trauma, dissociation, and psychiatric comorbidity in patients with conversion disorder. Am J Psychiatry. 2004;161(12):2271-2276.
7. de Waal MW, Arnold IA, Eekhof JA, et al. Somatoform disorders in general practice: prevalence, functional impairment and comorbidity with anxiety and depressive disorders. Br J Psychiatry. 2004;184:470-476.
8. Nicholson TR, Stone J, Kanaan RA. Conversion disorder: a problematic diagnosis. J Neurol Neurosurg Psychiatry. 2011;82(11):1267-1273.
9. Stone J, LaFrance WC, Jr, Levenson JL, et al. Issues for
DSM-5: conversion disorder. Am J Psychiatry. 2010;167(6): 626-627.
10. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
11. Voon V, Gallea C, Hattori N, et al. The involuntary nature of conversion disorder. Neurology. 2010;74(3):223-228.
12. Stone J, Zeman A, Sharpe M. Functional weakness and sensory disturbance. J Neurol Neurosurg Psychiatry. 2002; 73:241-245.
13. Aybek S, Kanaan RA, David AS. The neuropsychiatry of conversion disorder. Curr Opin Psychiatry. 2008;21(3):275-280.
14. Kroenke K. Efficacy of treatment for somatoform disorders: a review of randomized controlled trials. Psychosom Med. 2007;69(9):881-888.
15. Sharpe M, Walker J, Williams C, et al. Guided self-help for functional (psychogenic) symptoms: a randomized controlled efficacy trial. Neurology. 2011;77(6):564-572.
1. Diukova GM, Stolajrova AV, Vein AM. Sternocleidomastoid (SCM) muscle test in patients with hysterical and organic paresis. J Neurol Sci. 2001;187(suppl 1):S108.
2. Booij HA, Hamburger HL, Jöbsis GJ, et al. Stroke mimicking conversion disorder: two young women who put our feet back on the ground. Pract Neurol. 2012;12(3):179-181.
3. Glick TH, Workman TP, Gaufberg SV. Suspected conversion disorder: foreseeable risks and avoidable errors. Acad Emerg Med. 2000;7(11):1272-1277.
4. Fishbain DA, Goldberg M. The misdiagnosis of conversion disorder in a psychiatric emergency service. Gen Hosp Psychiatry. 1991;13(3):177-181.
5. Carlson ML, Archibald DJ, Gifford RH, et al. Conversion disorder: a missed diagnosis leading to cochlear reimplantation. Otol Neurotol. 2011;32(1):36-38.
6. Sar V, Akyüz G, Kundakçi T, et al. Childhood trauma, dissociation, and psychiatric comorbidity in patients with conversion disorder. Am J Psychiatry. 2004;161(12):2271-2276.
7. de Waal MW, Arnold IA, Eekhof JA, et al. Somatoform disorders in general practice: prevalence, functional impairment and comorbidity with anxiety and depressive disorders. Br J Psychiatry. 2004;184:470-476.
8. Nicholson TR, Stone J, Kanaan RA. Conversion disorder: a problematic diagnosis. J Neurol Neurosurg Psychiatry. 2011;82(11):1267-1273.
9. Stone J, LaFrance WC, Jr, Levenson JL, et al. Issues for
DSM-5: conversion disorder. Am J Psychiatry. 2010;167(6): 626-627.
10. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
11. Voon V, Gallea C, Hattori N, et al. The involuntary nature of conversion disorder. Neurology. 2010;74(3):223-228.
12. Stone J, Zeman A, Sharpe M. Functional weakness and sensory disturbance. J Neurol Neurosurg Psychiatry. 2002; 73:241-245.
13. Aybek S, Kanaan RA, David AS. The neuropsychiatry of conversion disorder. Curr Opin Psychiatry. 2008;21(3):275-280.
14. Kroenke K. Efficacy of treatment for somatoform disorders: a review of randomized controlled trials. Psychosom Med. 2007;69(9):881-888.
15. Sharpe M, Walker J, Williams C, et al. Guided self-help for functional (psychogenic) symptoms: a randomized controlled efficacy trial. Neurology. 2011;77(6):564-572.
Obsessed with Facebook
CASE: Paranoid and online
Mr. M, age 22, is brought to the emergency department by family because they are concerned about his paranoia and increasing agitation related to Facebook posts by friends and siblings. At age 8, Mr. M was diagnosed with depression, attention-deficit/hyperactivity disorder (ADHD), and anger management problems, which were well controlled with fluoxetine until last year, when he discontinued psychiatric follow-up. Mr. M’s girlfriend ended their relationship 1 month ago, although it is unclear whether the break-up was caused by his depressive symptoms or exacerbated them. In the last 2 days, his parents have noticed an increase in his delusional thoughts and aggressive behavior.
Family psychiatric history is not significant. Five years ago, Mr. M suffered a head injury in a motor vehicle collision, but completed high school without evidence of cognitive impairment or behavioral changes.
Mr. M appears disheveled and irritable. He reports his mood as “depressed,” but denies suicidal or homicidal ideations. He has no history of violence or antisocial behavior.
Mr. M is alert and oriented with clear speech, intact language, and grossly intact memory and concentration—although, he admits, “I just obsess over certain thoughts.” He endorses feelings of anxiety, insomnia, low energy, lack of sleep secondary to his paranoia, and claims that “something was said on Facebook about a girl and everyone is in on it.” He explains that his Facebook friends talk in “analogies” about him, and reports that, “I can just tell that’s what they are talking about even if they don’t say it directly.”
a) impulse control disorder
b) brief psychotic episode
c) psychotic depression
d) bipolar disorder
The authors’ observations
The last decade has seen a rise in the creation and use of social networking sites such as Facebook, Myspace, and Twitter. Facebook has 1.15 billion monthly active users.1 Seventy-five percent of teenagers own cell phones, and 25% report using their phones to access social media outlets.2 More than 50% of teenagers visit a social networking site daily, with 22% logging in to their favorite social media network more than 10 times a day.3 The easy accessibility of social media outlets has prompted study of the association of that accessibility with anxiety, depression, and self-esteem.3-7
Although not a DSM-5 or ICD-10 diagnosis, internet addiction has been correlated with depression.8 Similarly, O’Keefe and colleagues describe Facebook depression in teens who spend a large amount of time on social networking sites.4 The recently developed Bergen Facebook Addiction Scale (BFAS)9 evaluates the six core elements of addiction (salience, mood modification, tolerance, withdrawal, conflict, and relapse) in Facebook users.
Facebook certainly provides a valuable mechanism for friends to stay connected in an increasingly global society, and has acknowledged the potential it has to address mental illness. In 2011, Facebook partnered with the National Suicide Prevention Lifeline to allow users to report observed suicidal content, thereby utilizing the online community to facilitate delivery of mental health resources.10,11
HISTORY: Sibling rivalry
Mr. M had a romantic relationship with “Ms. B” in high school that he describes as “on and off,” beginning during his sophomore year. He describes himself as a “quick learner” who is task-oriented. He says he was outgoing in high school but became more introverted during his last year there. After high school, Mr. M worked as an electrician and discontinued psychiatric follow-up because he “felt fine.” He lives at home with his parents, two older sisters, and twin brother, who he identifies as being a lifelong “rival.”
After Ms. B ended her relationship with Mr. M, he began to suspect that she had become romantically involved with his twin brother. After Mr. M observed his brother leaving the house one night, he confronted his twin, who denied any involvement with Ms. B. After his brother left, Mr. M became enraged and punched a wall, fracturing his hand.
Two weeks before admission, Mr. M became increasingly preoccupied with suspicions of his brother’s involvement with Ms. B and looked for evidence on Facebook. Mr. M intensely monitored his Facebook news feed, which constantly updates to show public posts made by a user’s Facebook friends. He interpreted his friends’ posts as either directly relating to him or to a new relationship between Ms. B and his twin brother, stating that his friends were “talking in analogies” rather than directly using names.
Mr. M’s Facebook use rapidly increased to 3 or more hours a day. He can access Facebook from his laptop or cell phone, and reports logging in more than 10 times throughout the day. He says that, on Facebook, “it’s easier to talk trash” because people can say things they would not normally say face to face. He also states that Facebook is “ruining personal relationships,” and that it is “so easy to be in touch with everyone without really being in touch.”
The authors’ observations
In Mr. M’s case, Facebook served as a vehicle through which he could pursue a non-bizarre delusion. Mr. M openly admitted to viewing his twin brother as a rival; it is not surprising, therefore, that his delusions targeted his brother and ex-girlfriend.
Before social networks, the perseveration of this delusion might have been limited to internal thinking, or gathering corroborative information by means of stalking. Social media outlets have provided a means to perseverate and implicate others remotely, however, and Mr. M soon expanded his delusions to include more peers.
After beginning to suspect that friends and family are commenting on or criticizing him through Facebook, Mr. M experienced an irresistible impulse to repeatedly check the social network, which may have provided short-term relief of anticipatory anxiety, but that perpetuated the cycle. Constant access to the internet facilitated and intensified Mr. M’s cycle of paranoia, anxiety, and dysphoria. He called this process an “addiction.” A conceptual framework of the development of Mr. M’s maladaptive use of Facebook is illustrated in Figure 1.
Risk factors
Insecurity with one’s self-worth also may be a warning sign. Online social networking circumvents the need for physical interaction. A Facebook profile allows a person to selectively portray himself (herself) to the world, which may not be congruous with how his peers see him in everyday life. Patients who fear criticism or judgment may be more prone to maladaptive Facebook use, because they might feel empowered by the control they have over how others see them—online, at least.
Limited or, in Mr. M’s case, singular romantic experience may have influenced the course of his illness. Mr. M described his romantic involvement as a single, tumultuous relationship that lasted several years. Young patients with limited romantic experience may struggle to develop healthy protective mechanisms and may become preoccupied with the details of the situation, such that it interferes with functioning.
Mr. M’s history of ADHD might be a risk factor for abnormal patterns of internet use. Patients with ADHD have increased attentiveness with visually stimulating tasks—specifically, computers and video games.12
Last, it is unclear how, or if, Mr. M’s history of head injury contributed to his symptoms. There were no clear, temporal changes in cognition or emotion associated with the head injury, and he did not receive regular follow-up. Significant cognitive impairment does not appear to be a factor.
a) restart fluoxetine
b) begin an atypical antipsychotic
c) begin a mood stabilizer and atypical antipsychotic
d) encourage Mr. M to deactivate his Facebook account
TREATMENT: Observed use
Quetiapine is selected to target psychosis, agitation, and insomnia characterized by difficulty with sleep initiation. Risperidone is added as a short-term agent to boost antipsychotic effect during the day when Mr. M is not fully responsive to quetiapine alone. Valproic acid is added on admission as a mood stabilizer to target emotional lability, impulsiveness, and possible mania.
After several days of treatment, and without access to a computer, Mr. M is calmer. We begin to assess the challenges of self-limiting time spent on Facebook; Mr. M explains that, before hospitalization, he had deactivated his Facebook account several times to try to rid himself of what he describes as an “addiction to social media”; soon afterward, however, he experienced overwhelming anxiety that led him to reactivate his account.
We sit with Mr. M as he logs in to Facebook and discuss the range of alternative explanations that specific public messages on his news feed could have. Explicitly listing alternative explanations is a technique used in cognitive-behavioral therapy. Mr. M begins to demonstrate increased insight regarding his paranoia and possible misinterpretation of information gleaned via Facebook; however, he still believes that masked references to him had existed. During his hospital stay he begins to acknowledge the problems that online interactions pose compared with face-to-face interactions, stating that, “There’s no emotion in [Facebook], so you can easily misinterpret what someone says.”
The authors’ observations
Mr. M was discharged after 7 days of treatment and has been seen weekly as an outpatient for 3 months without need for further hospitalization.
Bottom Line
Pervasive access to social media represents a vehicle for relapse of many psychiatric conditions. Younger patients may be especially at risk because they are more likely to use social media and are in the age range for onset of psychiatric illness. Although some degree of dependence on online networks can be considered normal, patients suffering from mental illness represent a vulnerable population for maladaptive online interactions.
Related Resources
• Sandler EP. If you’re in crisis, go online. Psychology Today. www.psychologytoday.com/blog/promoting-hope-preventing-suicide/201110/if-you-re-in-crisis-go-online. Published October 26, 2011.
• Nitzan U, Shoshan E, Lev-Ran S, et al. Internet-related psychosis−a sign of the times. Isr J Psychiatry Relat Sci. 2011;48(3):207-211.
• Martin EA, Bailey DH, Cicero DC, et al. Social networking profile correlates of schizotypy. Psychiatry Res. 2012;200(2-3):641-646.
Drug Brand Names
Fluoxetine • Prozac Quetiapine • Seroquel
Risperidone • Risperdal Valproic acid • Depakote
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Facebook. Facebook reports second quarter 2013 results. http://investor.fb.com/releasedetail.cfm?ReleaseID= 780093. Updated July 24, 2013. Accessed July 29, 2013.
2. Hinduja S, Patchin JW. Offline consequences of online victimization: school violence and delinquency. Journal of School Violence. 2007;6(3):89-112.
3. Pantic I, Damjanovic A, Todorovic J, et al. Associations between online social networking and depression in high school students: behavioral physiology viewpoint. Psychiatr Danub. 2012;24(1):90-93.
4. O’Keeffe GS, Clarke-Pearson K; Council on Communications and Media. The impact of social media on children, adolescents, and families. Pediatrics. 2011;127(4):800-804.
5. Gonzales AL, Hancock JT. Mirror, mirror on my Facebook wall: effects of exposure to Facebook on self-esteem. Cyberpsychol Behav Soc Netw. 2011;14(1-2):79-83.
6. Hinduja S, Patchin JW. Bullying, cyberbullying, and suicide. Arch Suicide Res. 2010;14(3):206-221.
7. Selfhout MH, Branje SJ, Delsing M, et al. Different types of Internet use, depression, and social anxiety: the role of perceived friendship quality. J Adolesc. 2009;32(4):819-833.
8. Morrison CM, Gore H. The relationship between excessive internet use and depression: a questionnaire-based study of 1,319 young people and adults. Psychopathology. 2010; 43:121-126.
9. Andreassen CS, Torsheim T, Brunborg GS, et al. Development of a Facebook Addiction Scale. Psychol Rep. 2012;110(2):501-517.
10. SAMHSA News. Suicide prevention: a national priority. vol 20, no 3. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012.
11. Facebook. New partnership between Facebook and the National Suicide Prevention Lifeline http://www.facebook.com/note.php?note_id=310287485658707. Accessed July 25, 2013.
12. Weinstein A, Weizman A. Emerging association between addictive gaming and attention-deficit/hyperactivity disorder. Curr Psychiatry Rep. 2012;14(5):590-597.
CASE: Paranoid and online
Mr. M, age 22, is brought to the emergency department by family because they are concerned about his paranoia and increasing agitation related to Facebook posts by friends and siblings. At age 8, Mr. M was diagnosed with depression, attention-deficit/hyperactivity disorder (ADHD), and anger management problems, which were well controlled with fluoxetine until last year, when he discontinued psychiatric follow-up. Mr. M’s girlfriend ended their relationship 1 month ago, although it is unclear whether the break-up was caused by his depressive symptoms or exacerbated them. In the last 2 days, his parents have noticed an increase in his delusional thoughts and aggressive behavior.
Family psychiatric history is not significant. Five years ago, Mr. M suffered a head injury in a motor vehicle collision, but completed high school without evidence of cognitive impairment or behavioral changes.
Mr. M appears disheveled and irritable. He reports his mood as “depressed,” but denies suicidal or homicidal ideations. He has no history of violence or antisocial behavior.
Mr. M is alert and oriented with clear speech, intact language, and grossly intact memory and concentration—although, he admits, “I just obsess over certain thoughts.” He endorses feelings of anxiety, insomnia, low energy, lack of sleep secondary to his paranoia, and claims that “something was said on Facebook about a girl and everyone is in on it.” He explains that his Facebook friends talk in “analogies” about him, and reports that, “I can just tell that’s what they are talking about even if they don’t say it directly.”
a) impulse control disorder
b) brief psychotic episode
c) psychotic depression
d) bipolar disorder
The authors’ observations
The last decade has seen a rise in the creation and use of social networking sites such as Facebook, Myspace, and Twitter. Facebook has 1.15 billion monthly active users.1 Seventy-five percent of teenagers own cell phones, and 25% report using their phones to access social media outlets.2 More than 50% of teenagers visit a social networking site daily, with 22% logging in to their favorite social media network more than 10 times a day.3 The easy accessibility of social media outlets has prompted study of the association of that accessibility with anxiety, depression, and self-esteem.3-7
Although not a DSM-5 or ICD-10 diagnosis, internet addiction has been correlated with depression.8 Similarly, O’Keefe and colleagues describe Facebook depression in teens who spend a large amount of time on social networking sites.4 The recently developed Bergen Facebook Addiction Scale (BFAS)9 evaluates the six core elements of addiction (salience, mood modification, tolerance, withdrawal, conflict, and relapse) in Facebook users.
Facebook certainly provides a valuable mechanism for friends to stay connected in an increasingly global society, and has acknowledged the potential it has to address mental illness. In 2011, Facebook partnered with the National Suicide Prevention Lifeline to allow users to report observed suicidal content, thereby utilizing the online community to facilitate delivery of mental health resources.10,11
HISTORY: Sibling rivalry
Mr. M had a romantic relationship with “Ms. B” in high school that he describes as “on and off,” beginning during his sophomore year. He describes himself as a “quick learner” who is task-oriented. He says he was outgoing in high school but became more introverted during his last year there. After high school, Mr. M worked as an electrician and discontinued psychiatric follow-up because he “felt fine.” He lives at home with his parents, two older sisters, and twin brother, who he identifies as being a lifelong “rival.”
After Ms. B ended her relationship with Mr. M, he began to suspect that she had become romantically involved with his twin brother. After Mr. M observed his brother leaving the house one night, he confronted his twin, who denied any involvement with Ms. B. After his brother left, Mr. M became enraged and punched a wall, fracturing his hand.
Two weeks before admission, Mr. M became increasingly preoccupied with suspicions of his brother’s involvement with Ms. B and looked for evidence on Facebook. Mr. M intensely monitored his Facebook news feed, which constantly updates to show public posts made by a user’s Facebook friends. He interpreted his friends’ posts as either directly relating to him or to a new relationship between Ms. B and his twin brother, stating that his friends were “talking in analogies” rather than directly using names.
Mr. M’s Facebook use rapidly increased to 3 or more hours a day. He can access Facebook from his laptop or cell phone, and reports logging in more than 10 times throughout the day. He says that, on Facebook, “it’s easier to talk trash” because people can say things they would not normally say face to face. He also states that Facebook is “ruining personal relationships,” and that it is “so easy to be in touch with everyone without really being in touch.”
The authors’ observations
In Mr. M’s case, Facebook served as a vehicle through which he could pursue a non-bizarre delusion. Mr. M openly admitted to viewing his twin brother as a rival; it is not surprising, therefore, that his delusions targeted his brother and ex-girlfriend.
Before social networks, the perseveration of this delusion might have been limited to internal thinking, or gathering corroborative information by means of stalking. Social media outlets have provided a means to perseverate and implicate others remotely, however, and Mr. M soon expanded his delusions to include more peers.
After beginning to suspect that friends and family are commenting on or criticizing him through Facebook, Mr. M experienced an irresistible impulse to repeatedly check the social network, which may have provided short-term relief of anticipatory anxiety, but that perpetuated the cycle. Constant access to the internet facilitated and intensified Mr. M’s cycle of paranoia, anxiety, and dysphoria. He called this process an “addiction.” A conceptual framework of the development of Mr. M’s maladaptive use of Facebook is illustrated in Figure 1.
Risk factors
Insecurity with one’s self-worth also may be a warning sign. Online social networking circumvents the need for physical interaction. A Facebook profile allows a person to selectively portray himself (herself) to the world, which may not be congruous with how his peers see him in everyday life. Patients who fear criticism or judgment may be more prone to maladaptive Facebook use, because they might feel empowered by the control they have over how others see them—online, at least.
Limited or, in Mr. M’s case, singular romantic experience may have influenced the course of his illness. Mr. M described his romantic involvement as a single, tumultuous relationship that lasted several years. Young patients with limited romantic experience may struggle to develop healthy protective mechanisms and may become preoccupied with the details of the situation, such that it interferes with functioning.
Mr. M’s history of ADHD might be a risk factor for abnormal patterns of internet use. Patients with ADHD have increased attentiveness with visually stimulating tasks—specifically, computers and video games.12
Last, it is unclear how, or if, Mr. M’s history of head injury contributed to his symptoms. There were no clear, temporal changes in cognition or emotion associated with the head injury, and he did not receive regular follow-up. Significant cognitive impairment does not appear to be a factor.
a) restart fluoxetine
b) begin an atypical antipsychotic
c) begin a mood stabilizer and atypical antipsychotic
d) encourage Mr. M to deactivate his Facebook account
TREATMENT: Observed use
Quetiapine is selected to target psychosis, agitation, and insomnia characterized by difficulty with sleep initiation. Risperidone is added as a short-term agent to boost antipsychotic effect during the day when Mr. M is not fully responsive to quetiapine alone. Valproic acid is added on admission as a mood stabilizer to target emotional lability, impulsiveness, and possible mania.
After several days of treatment, and without access to a computer, Mr. M is calmer. We begin to assess the challenges of self-limiting time spent on Facebook; Mr. M explains that, before hospitalization, he had deactivated his Facebook account several times to try to rid himself of what he describes as an “addiction to social media”; soon afterward, however, he experienced overwhelming anxiety that led him to reactivate his account.
We sit with Mr. M as he logs in to Facebook and discuss the range of alternative explanations that specific public messages on his news feed could have. Explicitly listing alternative explanations is a technique used in cognitive-behavioral therapy. Mr. M begins to demonstrate increased insight regarding his paranoia and possible misinterpretation of information gleaned via Facebook; however, he still believes that masked references to him had existed. During his hospital stay he begins to acknowledge the problems that online interactions pose compared with face-to-face interactions, stating that, “There’s no emotion in [Facebook], so you can easily misinterpret what someone says.”
The authors’ observations
Mr. M was discharged after 7 days of treatment and has been seen weekly as an outpatient for 3 months without need for further hospitalization.
Bottom Line
Pervasive access to social media represents a vehicle for relapse of many psychiatric conditions. Younger patients may be especially at risk because they are more likely to use social media and are in the age range for onset of psychiatric illness. Although some degree of dependence on online networks can be considered normal, patients suffering from mental illness represent a vulnerable population for maladaptive online interactions.
Related Resources
• Sandler EP. If you’re in crisis, go online. Psychology Today. www.psychologytoday.com/blog/promoting-hope-preventing-suicide/201110/if-you-re-in-crisis-go-online. Published October 26, 2011.
• Nitzan U, Shoshan E, Lev-Ran S, et al. Internet-related psychosis−a sign of the times. Isr J Psychiatry Relat Sci. 2011;48(3):207-211.
• Martin EA, Bailey DH, Cicero DC, et al. Social networking profile correlates of schizotypy. Psychiatry Res. 2012;200(2-3):641-646.
Drug Brand Names
Fluoxetine • Prozac Quetiapine • Seroquel
Risperidone • Risperdal Valproic acid • Depakote
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Paranoid and online
Mr. M, age 22, is brought to the emergency department by family because they are concerned about his paranoia and increasing agitation related to Facebook posts by friends and siblings. At age 8, Mr. M was diagnosed with depression, attention-deficit/hyperactivity disorder (ADHD), and anger management problems, which were well controlled with fluoxetine until last year, when he discontinued psychiatric follow-up. Mr. M’s girlfriend ended their relationship 1 month ago, although it is unclear whether the break-up was caused by his depressive symptoms or exacerbated them. In the last 2 days, his parents have noticed an increase in his delusional thoughts and aggressive behavior.
Family psychiatric history is not significant. Five years ago, Mr. M suffered a head injury in a motor vehicle collision, but completed high school without evidence of cognitive impairment or behavioral changes.
Mr. M appears disheveled and irritable. He reports his mood as “depressed,” but denies suicidal or homicidal ideations. He has no history of violence or antisocial behavior.
Mr. M is alert and oriented with clear speech, intact language, and grossly intact memory and concentration—although, he admits, “I just obsess over certain thoughts.” He endorses feelings of anxiety, insomnia, low energy, lack of sleep secondary to his paranoia, and claims that “something was said on Facebook about a girl and everyone is in on it.” He explains that his Facebook friends talk in “analogies” about him, and reports that, “I can just tell that’s what they are talking about even if they don’t say it directly.”
a) impulse control disorder
b) brief psychotic episode
c) psychotic depression
d) bipolar disorder
The authors’ observations
The last decade has seen a rise in the creation and use of social networking sites such as Facebook, Myspace, and Twitter. Facebook has 1.15 billion monthly active users.1 Seventy-five percent of teenagers own cell phones, and 25% report using their phones to access social media outlets.2 More than 50% of teenagers visit a social networking site daily, with 22% logging in to their favorite social media network more than 10 times a day.3 The easy accessibility of social media outlets has prompted study of the association of that accessibility with anxiety, depression, and self-esteem.3-7
Although not a DSM-5 or ICD-10 diagnosis, internet addiction has been correlated with depression.8 Similarly, O’Keefe and colleagues describe Facebook depression in teens who spend a large amount of time on social networking sites.4 The recently developed Bergen Facebook Addiction Scale (BFAS)9 evaluates the six core elements of addiction (salience, mood modification, tolerance, withdrawal, conflict, and relapse) in Facebook users.
Facebook certainly provides a valuable mechanism for friends to stay connected in an increasingly global society, and has acknowledged the potential it has to address mental illness. In 2011, Facebook partnered with the National Suicide Prevention Lifeline to allow users to report observed suicidal content, thereby utilizing the online community to facilitate delivery of mental health resources.10,11
HISTORY: Sibling rivalry
Mr. M had a romantic relationship with “Ms. B” in high school that he describes as “on and off,” beginning during his sophomore year. He describes himself as a “quick learner” who is task-oriented. He says he was outgoing in high school but became more introverted during his last year there. After high school, Mr. M worked as an electrician and discontinued psychiatric follow-up because he “felt fine.” He lives at home with his parents, two older sisters, and twin brother, who he identifies as being a lifelong “rival.”
After Ms. B ended her relationship with Mr. M, he began to suspect that she had become romantically involved with his twin brother. After Mr. M observed his brother leaving the house one night, he confronted his twin, who denied any involvement with Ms. B. After his brother left, Mr. M became enraged and punched a wall, fracturing his hand.
Two weeks before admission, Mr. M became increasingly preoccupied with suspicions of his brother’s involvement with Ms. B and looked for evidence on Facebook. Mr. M intensely monitored his Facebook news feed, which constantly updates to show public posts made by a user’s Facebook friends. He interpreted his friends’ posts as either directly relating to him or to a new relationship between Ms. B and his twin brother, stating that his friends were “talking in analogies” rather than directly using names.
Mr. M’s Facebook use rapidly increased to 3 or more hours a day. He can access Facebook from his laptop or cell phone, and reports logging in more than 10 times throughout the day. He says that, on Facebook, “it’s easier to talk trash” because people can say things they would not normally say face to face. He also states that Facebook is “ruining personal relationships,” and that it is “so easy to be in touch with everyone without really being in touch.”
The authors’ observations
In Mr. M’s case, Facebook served as a vehicle through which he could pursue a non-bizarre delusion. Mr. M openly admitted to viewing his twin brother as a rival; it is not surprising, therefore, that his delusions targeted his brother and ex-girlfriend.
Before social networks, the perseveration of this delusion might have been limited to internal thinking, or gathering corroborative information by means of stalking. Social media outlets have provided a means to perseverate and implicate others remotely, however, and Mr. M soon expanded his delusions to include more peers.
After beginning to suspect that friends and family are commenting on or criticizing him through Facebook, Mr. M experienced an irresistible impulse to repeatedly check the social network, which may have provided short-term relief of anticipatory anxiety, but that perpetuated the cycle. Constant access to the internet facilitated and intensified Mr. M’s cycle of paranoia, anxiety, and dysphoria. He called this process an “addiction.” A conceptual framework of the development of Mr. M’s maladaptive use of Facebook is illustrated in Figure 1.
Risk factors
Insecurity with one’s self-worth also may be a warning sign. Online social networking circumvents the need for physical interaction. A Facebook profile allows a person to selectively portray himself (herself) to the world, which may not be congruous with how his peers see him in everyday life. Patients who fear criticism or judgment may be more prone to maladaptive Facebook use, because they might feel empowered by the control they have over how others see them—online, at least.
Limited or, in Mr. M’s case, singular romantic experience may have influenced the course of his illness. Mr. M described his romantic involvement as a single, tumultuous relationship that lasted several years. Young patients with limited romantic experience may struggle to develop healthy protective mechanisms and may become preoccupied with the details of the situation, such that it interferes with functioning.
Mr. M’s history of ADHD might be a risk factor for abnormal patterns of internet use. Patients with ADHD have increased attentiveness with visually stimulating tasks—specifically, computers and video games.12
Last, it is unclear how, or if, Mr. M’s history of head injury contributed to his symptoms. There were no clear, temporal changes in cognition or emotion associated with the head injury, and he did not receive regular follow-up. Significant cognitive impairment does not appear to be a factor.
a) restart fluoxetine
b) begin an atypical antipsychotic
c) begin a mood stabilizer and atypical antipsychotic
d) encourage Mr. M to deactivate his Facebook account
TREATMENT: Observed use
Quetiapine is selected to target psychosis, agitation, and insomnia characterized by difficulty with sleep initiation. Risperidone is added as a short-term agent to boost antipsychotic effect during the day when Mr. M is not fully responsive to quetiapine alone. Valproic acid is added on admission as a mood stabilizer to target emotional lability, impulsiveness, and possible mania.
After several days of treatment, and without access to a computer, Mr. M is calmer. We begin to assess the challenges of self-limiting time spent on Facebook; Mr. M explains that, before hospitalization, he had deactivated his Facebook account several times to try to rid himself of what he describes as an “addiction to social media”; soon afterward, however, he experienced overwhelming anxiety that led him to reactivate his account.
We sit with Mr. M as he logs in to Facebook and discuss the range of alternative explanations that specific public messages on his news feed could have. Explicitly listing alternative explanations is a technique used in cognitive-behavioral therapy. Mr. M begins to demonstrate increased insight regarding his paranoia and possible misinterpretation of information gleaned via Facebook; however, he still believes that masked references to him had existed. During his hospital stay he begins to acknowledge the problems that online interactions pose compared with face-to-face interactions, stating that, “There’s no emotion in [Facebook], so you can easily misinterpret what someone says.”
The authors’ observations
Mr. M was discharged after 7 days of treatment and has been seen weekly as an outpatient for 3 months without need for further hospitalization.
Bottom Line
Pervasive access to social media represents a vehicle for relapse of many psychiatric conditions. Younger patients may be especially at risk because they are more likely to use social media and are in the age range for onset of psychiatric illness. Although some degree of dependence on online networks can be considered normal, patients suffering from mental illness represent a vulnerable population for maladaptive online interactions.
Related Resources
• Sandler EP. If you’re in crisis, go online. Psychology Today. www.psychologytoday.com/blog/promoting-hope-preventing-suicide/201110/if-you-re-in-crisis-go-online. Published October 26, 2011.
• Nitzan U, Shoshan E, Lev-Ran S, et al. Internet-related psychosis−a sign of the times. Isr J Psychiatry Relat Sci. 2011;48(3):207-211.
• Martin EA, Bailey DH, Cicero DC, et al. Social networking profile correlates of schizotypy. Psychiatry Res. 2012;200(2-3):641-646.
Drug Brand Names
Fluoxetine • Prozac Quetiapine • Seroquel
Risperidone • Risperdal Valproic acid • Depakote
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Facebook. Facebook reports second quarter 2013 results. http://investor.fb.com/releasedetail.cfm?ReleaseID= 780093. Updated July 24, 2013. Accessed July 29, 2013.
2. Hinduja S, Patchin JW. Offline consequences of online victimization: school violence and delinquency. Journal of School Violence. 2007;6(3):89-112.
3. Pantic I, Damjanovic A, Todorovic J, et al. Associations between online social networking and depression in high school students: behavioral physiology viewpoint. Psychiatr Danub. 2012;24(1):90-93.
4. O’Keeffe GS, Clarke-Pearson K; Council on Communications and Media. The impact of social media on children, adolescents, and families. Pediatrics. 2011;127(4):800-804.
5. Gonzales AL, Hancock JT. Mirror, mirror on my Facebook wall: effects of exposure to Facebook on self-esteem. Cyberpsychol Behav Soc Netw. 2011;14(1-2):79-83.
6. Hinduja S, Patchin JW. Bullying, cyberbullying, and suicide. Arch Suicide Res. 2010;14(3):206-221.
7. Selfhout MH, Branje SJ, Delsing M, et al. Different types of Internet use, depression, and social anxiety: the role of perceived friendship quality. J Adolesc. 2009;32(4):819-833.
8. Morrison CM, Gore H. The relationship between excessive internet use and depression: a questionnaire-based study of 1,319 young people and adults. Psychopathology. 2010; 43:121-126.
9. Andreassen CS, Torsheim T, Brunborg GS, et al. Development of a Facebook Addiction Scale. Psychol Rep. 2012;110(2):501-517.
10. SAMHSA News. Suicide prevention: a national priority. vol 20, no 3. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012.
11. Facebook. New partnership between Facebook and the National Suicide Prevention Lifeline http://www.facebook.com/note.php?note_id=310287485658707. Accessed July 25, 2013.
12. Weinstein A, Weizman A. Emerging association between addictive gaming and attention-deficit/hyperactivity disorder. Curr Psychiatry Rep. 2012;14(5):590-597.
1. Facebook. Facebook reports second quarter 2013 results. http://investor.fb.com/releasedetail.cfm?ReleaseID= 780093. Updated July 24, 2013. Accessed July 29, 2013.
2. Hinduja S, Patchin JW. Offline consequences of online victimization: school violence and delinquency. Journal of School Violence. 2007;6(3):89-112.
3. Pantic I, Damjanovic A, Todorovic J, et al. Associations between online social networking and depression in high school students: behavioral physiology viewpoint. Psychiatr Danub. 2012;24(1):90-93.
4. O’Keeffe GS, Clarke-Pearson K; Council on Communications and Media. The impact of social media on children, adolescents, and families. Pediatrics. 2011;127(4):800-804.
5. Gonzales AL, Hancock JT. Mirror, mirror on my Facebook wall: effects of exposure to Facebook on self-esteem. Cyberpsychol Behav Soc Netw. 2011;14(1-2):79-83.
6. Hinduja S, Patchin JW. Bullying, cyberbullying, and suicide. Arch Suicide Res. 2010;14(3):206-221.
7. Selfhout MH, Branje SJ, Delsing M, et al. Different types of Internet use, depression, and social anxiety: the role of perceived friendship quality. J Adolesc. 2009;32(4):819-833.
8. Morrison CM, Gore H. The relationship between excessive internet use and depression: a questionnaire-based study of 1,319 young people and adults. Psychopathology. 2010; 43:121-126.
9. Andreassen CS, Torsheim T, Brunborg GS, et al. Development of a Facebook Addiction Scale. Psychol Rep. 2012;110(2):501-517.
10. SAMHSA News. Suicide prevention: a national priority. vol 20, no 3. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012.
11. Facebook. New partnership between Facebook and the National Suicide Prevention Lifeline http://www.facebook.com/note.php?note_id=310287485658707. Accessed July 25, 2013.
12. Weinstein A, Weizman A. Emerging association between addictive gaming and attention-deficit/hyperactivity disorder. Curr Psychiatry Rep. 2012;14(5):590-597.
Pregnant and catatonic
CASE: Mute and unresponsive
Mrs. K, a 42-year-old Haitian who is 31 weeks pregnant, presents with a 4-week history of progressive mutism and psychomotor retardation. At inpatient admission, she is awake and alert but does not speak and resists the treatment team’s attempts to engage her. Mrs. K’s eyes are open, but she has a vacant stare and avoids eye contact. Her affect is flat and nonreactive and she appears internally preoccupied. Mrs. K exhibits motoric immobility, displays a rigid posture, and resists attempts to get her to move. Features of catatonic excitement, echo phenomena, posturing, stereotypies, and mannerisms are absent during the initial evaluation.
Mrs. K’s husband reports that they had been on vacation for 6 days before he brought her for psychiatric evaluation. He denies any recent evidence of psychosis or mood disturbance, stating that his wife was excited when she learned of the pregnancy, and attended all prenatal appointments. He reports that when this episode began, Mrs. K stopped talking to her 3-year-old daughter, did not respond to her name, and did not pay attention to those around her.
According to her husband, a similar episode occurred 2 years earlier, during which Mrs. K was selectively mute for approximately 1 month. She did not eat for 5 days and neglected the care of her daughter. There were 2 additional brief periods of prominent psychomotor retardation for which she was hospitalized in Haiti. According to the patient’s aunt, Mrs. K complained that her husband had cast a “voodoo spell” on her because he wanted sole custody of their daughter. Her husband recounted an episode, when they lived in Haiti, during which his wife became paranoid, left the house, and wandered the streets for 2 days.
The medical history is significant for a cervical polyp that was removed 2 years ago. Mrs. K has no history of substance abuse. She was born and raised in Haiti where she studied medicine. Her family reports that Mrs. K’s husband is “controlling,” which causes her distress.
a) major depressive disorder, severe with psychosis, with catatonia
b) schizophrenia, with catatonia
c) conversion disorder, with catatonia
d) bipolar I disorder, with psychosis and catatonia
The authors' observations
Catatonia is a neuropsychiatric syndrome that can occur in schizophrenia, mood disorders, mental retardation, neurologic disease, metabolic conditions, and drug intoxication.1 Catatonia can present in several ways, from catatonic stupor to extreme purposeless agitation; more than 60 catatonic signs and symptoms have been described.1 According to DSM-52 catatonia is characterized by 3 or more of the following symptoms:
• stupor
• catalepsy
• waxy flexibility
• mutism
• negativism
• posturing
• mannerism
• stereotypy
• agitation, not influenced by external stimuli
• grimacing
• echolalia
• echopraxia.
Mrs. K exhibited stupor, mutism, posturing, and grimacing (Table 1).2 We thought that her catatonic features were secondary to schizophrenia because she had paranoid delusions and displayed disorganized behavior while in Haiti. There was no evidence of past or current mood disorder, metabolic condition, neurologic illness, or substance abuse.
Catatonia and pregnancy
There is little available information to guide clinicians who are treating a pregnant woman who has a catatonic syndrome. Espinola-Nadurille and co-workers described a 22-year-old pregnant (21 weeks) woman from rural Mexico who was hospitalized with agitation, disorganized speech, restlessness, and hallucinations after several weeks of alternating agitation and withdrawal with mutism and refusal to eat or drink.3 This patient developed malignant catatonia with creatine phosphokinase elevation and leukocytosis and eventually responded to treatment with lorazepam and electroconvulsive therapy (ECT). She was given a diagnosis of schizophreniform disorder. Treating her catatonic symptoms did not result in any adverse effects on the pregnancy or the fetus.
Exacerbation of schizophrenia during pregnancy can lead to neglect of pregnancy and prenatal care,4 imminent harm to the fetus because of malnutrition and dehydration, and risk of preterm delivery and low weight at birth. Prolonged catatonia can cause medical complications such as decubitus ulcers, incontinence, recurrent urinary tract infections, aspiration pneumonia, increased risk of deep venous thrombosis, malnutrition, and ocular complications because of prolonged staring and reduced blinking (Table 25-10). For these reasons, it is important to treat this condition early and aggressively to improve pregnancy outcome and infant well-being.
of Mrs. K’s symptoms?
a) Positive and Negative Symptom Scale
b) The Northoff Catatonia Rating Scale
c) The Bush-Francis Catatonia Rating Scale (BFCRS)
d) The Rogers Catatonia Scale
EVALUATION: Flat affect
The mental status examination on admission describes a tall, black, Haitian woman with unkempt hair and fair hygiene. Mrs. K has a prominent abdomen, consistent with a 31-week pregnancy. She exhibits a blank stare without direct eye contact; she is mute, and exhibits flat affect. We cannot evaluate her thought processes and content because Mrs. K is mute, although she does appear internally preoccupied.
Physical examination on admission is unremarkable; vital signs are stable and within normal limits. Laboratory work-up reveals a urinary tract infection, which is treated with ceftriaxone. Mrs. K also has macrocytic anemia (hemoglobin, 11.7 g/dL; hematocrit, 34.7%; mean corpuscular volume, 99.2 μm3). Albumin is low at 2.6 g/dL. Urine drug toxicology screen is negative. Fingerstick glucose reading is 139 mg/dL. Mrs. K is given a presumptive diagnosis of schizophrenia with catatonia.
Mrs. K’s BFCRS11 score is 22 at admission. She is mute, holds postures for longer than a minute, and is resistant to repositioning. She also has extreme hypoactivity and does not interact with others. She has a fixed, blank stare, and exhibits mild grimacing.
The authors' observations
BFCRS defines each catatonic sign, rates its severity, and provides a standardized schema for clinical examination.11 The BFCRS is preferred for routine use because of its validity, reliability, and ease of administration.12 The treatment team rated Mrs. K at admission, during the course of treatment, and at discharge, showing a substantial improvement at the end of the hospitalization (Figure).
a) ECT
b) lorazepam
c) haloperidol
d) olanzapine
The authors' observations
Benzodiazepines (particularly lorazepam) and ECT are considered the treatment of choice for catatonic symptoms.11 More than 72% of patients with catatonic symptoms remit after a trial of a benzodiazepine.1 ECT is considered when patients do not respond to a benzodiazepine after 48 to 72 hours.3 Several cases of complete resolution of catatonic symptoms have been linked to ECT (Table 3).13-18
A recent retrospective review revealed that patients who do not respond to lorazepam are more likely to come from a rural setting, have a longer duration of illness, exhibit mutism, and exhibit third-person auditory hallucinations and made phenomena (the feeling that some aspect of the individual is under the external control of others).19 Case reports of treatment of catatonic patients with ECT vs lorazepam are listed in Table 3.13-18
In pregnancy, ECT can be considered early in the course of illness. A review of the literature on ECT during pregnancy reported at least partial remission in 78% of studies reporting efficacy data.20 Among these 339 patients, there were 25 fetal or neonatal complications—only 11 of these were related to ECT—and 20 maternal complications, of which 18 were related to ECT. The authors of this review concluded that 1) ECT is an effective treatment for severe mental illness during pregnancy and 2) the risks to fetus and mother are low.
A 2007 study identified 1,979 infants whose mothers reported use of benzodiazepines or hypnotic benzodiazepine-receptor agonists during early pregnancy.21 An additional 401 infants born to mothers who were prescribed these medications during late pregnancy also were included in this study. Women who took these medications were at an increased risk for preterm birth and low birth weight. The rate of congenital malformations in this study was moderately increased among infants exposed in early pregnancy (adjusted odds ratio = 1.24 [95% confidence interval, 1.00 to 1.55]).
Because catatonic symptoms can appear during the course of schizophrenia, several antipsychotics have been used to treat this condition. The efficacy and safety of antipsychotics for treating catatonia remains largely unknown, however.1
OUTCOME: Recovery, baby girl
We begin oral haloperidol, 10 mg/d, for Mrs. K, which we then increase to 20 mg/d. Because she shows little response to haloperidol, we suggest a trial of ECT, but her husband refuses to consent. She is started on IM lorazepam, 6 mg/d.
Mrs. K gradually improves and increases her intake of food and liquids. After 10 days of lorazepam treatment, her BFCRS score decreases to 13. Mrs. K begins to speak and her gaze is less fixed. Negativistic behaviors are nearly absent.
Because we are concerned about Mrs. K’s pregnancy, lab tests are repeated. A complete metabolic panel shows an elevated glucose level (122 mg/dL); urinalysis reveals glycosuria (glucose, 1,000 mg/dL), proteinuria (protein, 10 mg/dL), and ketonuria, (ketones, 20 mg/dL). She is transferred to the obstetrics service for evaluation of gestational diabetes.
Psychotropics are continued while Mrs. K is on the obstetrics service; she returns to the inpatient psychiatric unit on an insulin regimen. IM lorazepam is increased to 8 mg/d, and haloperidol is decreased from 20 mg/d, to 10 mg/d, to prevent worsening of catatonia, which can occur when catatonic patients receive a psychotropic.11 Three days later, Mrs. K’s BFCRS score is 12 and she shows only mild rigidity. Mrs. K briefly interacts with staff, particularly when she wants something.
Lorazepam is decreased to 1 mg/d in anticipation of cesarean delivery. Mrs. K becomes more adherent with her medications; often, she takes the oral dose of haloperidol, rather than the IM formulation. On mental status examination she exhibits poor eye contact, rather than a fixed gaze, and her BFCRS score decreases to 7 by day 25.
By the end of lorazepam treatment, Mrs. K has fully recovered from her catatonic state. She interacts with staff, engages with the treatment team, and is excited to go home. At discharge, she is given a diagnosis of schizophrenia with catatonia, and is taking haloperidol, 5 mg, twice a day. She gives birth to a healthy girl.
The authors' observations
Mrs. K was treated initially with haloperidol for several reasons. Haloperidol is relatively safe during pregnancy (FDA pregnancy category C) as shown by a recent multicenter, prospective, cohort study in which babies exposed in utero to haloperidol showed a congenital malformation (limb defect) rate within the expected baseline risk for the general population.22 Lorazepam is FDA category D for use in pregnancy and can cause preterm delivery,23 floppy infant syndrome, and withdrawal syndromes.24 We did not use a second-generation antipsychotic (SGA) because it could have made Mrs. K’s hyperglycemia worse. SGAs can induce gestational diabetes and increase the incidence of large-for-gestational-age newborns, compared with first-generation antipsychotics.24 Last, Mrs. K’s family rejected ECT.
Because of Mrs. K’s poor response to haloperidol, the treatment team decided to start IM lorazepam, which eventually was increased to 8 mg/d. The haloperidol dose was reduced by half to avoid worsening of catatonia and reduce the risk of neuroleptic malignant syndrome.1,25 When clinical response was achieved, lorazepam was tapered and Mrs. K was discharged with only haloperidol.
In the absence of well-designed prospective follow-up studies, information on the potential impact of prenatal exposure to antipsychotics and benzodiazepines on a child’s cognitive development is limited.26 This case adds to the scant literature on the treatment of catatonia during pregnancy and illustrates how the BFCRS can be utilized during serial patient evaluations to monitor clinical improvement.
Bottom Line
Psychosis and catatonia during pregnancy are associated with complications to mother and child. The Bush-Francis Catatonia Rating Scale can be used to identify and track catatonic symptoms. Lorazepam and electroconvulsive therapy have been used safely and with good outcomes in mentally ill pregnant women when used appropriately.
Related Resources
- Fink M. Catatonia: a syndrome appears, disappears, and is rediscovered. Can J Psychiatry. 2009;54(7):437-445.
- Seethalakshmi R, Dhavale S, Suggu K, et al. Catatonic syndrome: importance of detection and treatment with lorazepam. Ann Clin Psychiatry. 2008;20(1):5-8.
- Salam S, Kilzieh N. Lorazepam treatment of psychogenic catatonia: an update. J Clin Psychiatry, 1988;49(suppl):16-21.
Drug Brand Names
Ceftriaxone • Rocephin Olanzapine • Zyprexa
Haloperidol • Haldol Short-acting Insulin • Novolin, Humulin
Lorazepam • Ativan
Disclosures
Dr. Runyan receives grant support from Lippincott, Williams, & Wilkins. Drs. Durant, Prudent, and Sotelo report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Weder N, Muralee S, Penland H, et al. Catatonia: a review. Ann Clin Psychiatry. 2008;20(2):97-107.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Arlington, VA: American Psychiatric Association; 2013.
3. Espinola-Nadurille M, Ramirez-Bermudez J, Fricchione GL. Pregnancy and malignant catatonia. Gen Hosp Psychiatry. 2007;29(1):69-71.
4. Solari H, Dickson KE, Miller L. Understanding and treating women with schizophrenia during pregnancy and postpartum. Can J Clin Pharmacol. 2009;16(1):e23-e32.
5. Gross AF, Smith FA, Stern TA. Dread complications of catatonia: a case discussion and review of the literature. Prim Care Companion J Clin Psychiatry. 2008;10(12):
153-155.
6. Larsen HH, Ritchie JC, McNutt MD, et al. Pulmonary embolism in a patient with catatonia: an old disease, changing times. Psychosomatics. 2011;52(4):387-391.
7. Lachner C, Sandson NB. Medical complications of catatonia: a case of catatonia-induced deep venous thrombosis. Psychosomatics. 2003;44(6):512-4.
8. Morioka H, Nagatomo I, Yamada K, et al. Deep venous thrombosis of the leg due to psychiatric stupor. Psychiatry Clin Neurosci, 1997;51(5):323-326.
9. Nomoto H, Hatta K, Usui C, et al. Vitamin K deficiency due to prolongation of antibiotic treatment and decrease in food intake in a catatonia patient. Psychosomatics. 2011;52(5):
486-487.
10. Srivastava A, Gupta A, Murthy P, et al. Catatonia and multiple pressure ulcers: a rare complication in psychiatric setting. Indian J Psychiatry. 2009;51(3):206-208.
11. Francis A. Catatonia: diagnosis, classification, and treatment. Curr Psychiatry Rep. 2010;12(3):180-185.
12. Sienaert P, Rooseleer J, De Fruyt J. Measuring catatonia: a systematic review of rating scales. J Affect Disord. 2011;135(1):1-9.
13. Zisselman MH, Jaffe RL. ECT in the treatment of a patient with catatonia: consent and complications. Am J Psychiatry. 2010;167(2):127-132.
14. Rohland BM, Carroll BT, Jacoby RG. ECT in the treatment of the catatonic syndrome. J Affect Disord. 1993;29(4):255-261.
15. Raveendranathan D, Narayanaswamy JC, Reddi SV. Response rate of catatonia to electroconvulsive therapy and its clinical correlates. Eur Arch Psychiatry Clin Neurosci. 2012;262(5):425-430.
16. Payee H, Chandrasekaran R, Raju GV. Catatonic syndrome: treatment response to lorazepam. Indian J Psychiatry. 1999; 41(1):49-53.
17. Bush G, Fink M, Petrides G, et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137-143.
18. Huang TL. Lorazepam and diazepam rapidly relieve catatonic signs in patients with schizophrenia. Psychiatry Clin Neurosci. 2005;59(1):52-55.
19. Narayanaswamy JC, Tibrewal P, Zutshi A, et al. Clinical predictors of response to treatment in catatonia. Gen Hosp Psychiatry. 2012;34:312-316.
20. Anderson EL, Reti IM. ECT in pregnancy: a review of the literature from 1941 to 2007. Psychosom Med. 2009;71:
235-242.
21. Wikner BN, Stiller CO, Bergman U, et al. Use of benzodiazepines and benzodiazepine receptor agonists during pregnancy: neonatal outcome and congenital malformations. Pharmacoepidemiol Drug Saf. 2007;16:
1203-1210.
22. Gentile S. Antipsychotic therapy during early and late pregnancy. A systematic review. Schizophr Bull. 2010;36:
518-544.
23. Calderon-Margalit R, Qiu C, Ornoy A, et al. Risk of preterm delivery and other adverse perinatal outcomes in relation to maternal use of psychotropic medications during pregnancy. Am J Obstet Gynecol. 2009;201:579e1-8.
24. Howland RH. Prescribing psychotropic medications during pregnancy and lactation: principles and guidelines. J Psychosoc Nurs Ment Health Serv. 2009;47(5):19-23.
25. White DA, Robins AH. Catatonia: harbinger of the neuroleptic malignant syndrome. Br J Psychiatry. 1991; 158:419-421.
26. Gentile S. Neurodevelopmental effects of prenatal exposure to psychotropic medications. Depress Anxiety. 2010; 27(7):675-686.
CASE: Mute and unresponsive
Mrs. K, a 42-year-old Haitian who is 31 weeks pregnant, presents with a 4-week history of progressive mutism and psychomotor retardation. At inpatient admission, she is awake and alert but does not speak and resists the treatment team’s attempts to engage her. Mrs. K’s eyes are open, but she has a vacant stare and avoids eye contact. Her affect is flat and nonreactive and she appears internally preoccupied. Mrs. K exhibits motoric immobility, displays a rigid posture, and resists attempts to get her to move. Features of catatonic excitement, echo phenomena, posturing, stereotypies, and mannerisms are absent during the initial evaluation.
Mrs. K’s husband reports that they had been on vacation for 6 days before he brought her for psychiatric evaluation. He denies any recent evidence of psychosis or mood disturbance, stating that his wife was excited when she learned of the pregnancy, and attended all prenatal appointments. He reports that when this episode began, Mrs. K stopped talking to her 3-year-old daughter, did not respond to her name, and did not pay attention to those around her.
According to her husband, a similar episode occurred 2 years earlier, during which Mrs. K was selectively mute for approximately 1 month. She did not eat for 5 days and neglected the care of her daughter. There were 2 additional brief periods of prominent psychomotor retardation for which she was hospitalized in Haiti. According to the patient’s aunt, Mrs. K complained that her husband had cast a “voodoo spell” on her because he wanted sole custody of their daughter. Her husband recounted an episode, when they lived in Haiti, during which his wife became paranoid, left the house, and wandered the streets for 2 days.
The medical history is significant for a cervical polyp that was removed 2 years ago. Mrs. K has no history of substance abuse. She was born and raised in Haiti where she studied medicine. Her family reports that Mrs. K’s husband is “controlling,” which causes her distress.
a) major depressive disorder, severe with psychosis, with catatonia
b) schizophrenia, with catatonia
c) conversion disorder, with catatonia
d) bipolar I disorder, with psychosis and catatonia
The authors' observations
Catatonia is a neuropsychiatric syndrome that can occur in schizophrenia, mood disorders, mental retardation, neurologic disease, metabolic conditions, and drug intoxication.1 Catatonia can present in several ways, from catatonic stupor to extreme purposeless agitation; more than 60 catatonic signs and symptoms have been described.1 According to DSM-52 catatonia is characterized by 3 or more of the following symptoms:
• stupor
• catalepsy
• waxy flexibility
• mutism
• negativism
• posturing
• mannerism
• stereotypy
• agitation, not influenced by external stimuli
• grimacing
• echolalia
• echopraxia.
Mrs. K exhibited stupor, mutism, posturing, and grimacing (Table 1).2 We thought that her catatonic features were secondary to schizophrenia because she had paranoid delusions and displayed disorganized behavior while in Haiti. There was no evidence of past or current mood disorder, metabolic condition, neurologic illness, or substance abuse.
Catatonia and pregnancy
There is little available information to guide clinicians who are treating a pregnant woman who has a catatonic syndrome. Espinola-Nadurille and co-workers described a 22-year-old pregnant (21 weeks) woman from rural Mexico who was hospitalized with agitation, disorganized speech, restlessness, and hallucinations after several weeks of alternating agitation and withdrawal with mutism and refusal to eat or drink.3 This patient developed malignant catatonia with creatine phosphokinase elevation and leukocytosis and eventually responded to treatment with lorazepam and electroconvulsive therapy (ECT). She was given a diagnosis of schizophreniform disorder. Treating her catatonic symptoms did not result in any adverse effects on the pregnancy or the fetus.
Exacerbation of schizophrenia during pregnancy can lead to neglect of pregnancy and prenatal care,4 imminent harm to the fetus because of malnutrition and dehydration, and risk of preterm delivery and low weight at birth. Prolonged catatonia can cause medical complications such as decubitus ulcers, incontinence, recurrent urinary tract infections, aspiration pneumonia, increased risk of deep venous thrombosis, malnutrition, and ocular complications because of prolonged staring and reduced blinking (Table 25-10). For these reasons, it is important to treat this condition early and aggressively to improve pregnancy outcome and infant well-being.
of Mrs. K’s symptoms?
a) Positive and Negative Symptom Scale
b) The Northoff Catatonia Rating Scale
c) The Bush-Francis Catatonia Rating Scale (BFCRS)
d) The Rogers Catatonia Scale
EVALUATION: Flat affect
The mental status examination on admission describes a tall, black, Haitian woman with unkempt hair and fair hygiene. Mrs. K has a prominent abdomen, consistent with a 31-week pregnancy. She exhibits a blank stare without direct eye contact; she is mute, and exhibits flat affect. We cannot evaluate her thought processes and content because Mrs. K is mute, although she does appear internally preoccupied.
Physical examination on admission is unremarkable; vital signs are stable and within normal limits. Laboratory work-up reveals a urinary tract infection, which is treated with ceftriaxone. Mrs. K also has macrocytic anemia (hemoglobin, 11.7 g/dL; hematocrit, 34.7%; mean corpuscular volume, 99.2 μm3). Albumin is low at 2.6 g/dL. Urine drug toxicology screen is negative. Fingerstick glucose reading is 139 mg/dL. Mrs. K is given a presumptive diagnosis of schizophrenia with catatonia.
Mrs. K’s BFCRS11 score is 22 at admission. She is mute, holds postures for longer than a minute, and is resistant to repositioning. She also has extreme hypoactivity and does not interact with others. She has a fixed, blank stare, and exhibits mild grimacing.
The authors' observations
BFCRS defines each catatonic sign, rates its severity, and provides a standardized schema for clinical examination.11 The BFCRS is preferred for routine use because of its validity, reliability, and ease of administration.12 The treatment team rated Mrs. K at admission, during the course of treatment, and at discharge, showing a substantial improvement at the end of the hospitalization (Figure).
a) ECT
b) lorazepam
c) haloperidol
d) olanzapine
The authors' observations
Benzodiazepines (particularly lorazepam) and ECT are considered the treatment of choice for catatonic symptoms.11 More than 72% of patients with catatonic symptoms remit after a trial of a benzodiazepine.1 ECT is considered when patients do not respond to a benzodiazepine after 48 to 72 hours.3 Several cases of complete resolution of catatonic symptoms have been linked to ECT (Table 3).13-18
A recent retrospective review revealed that patients who do not respond to lorazepam are more likely to come from a rural setting, have a longer duration of illness, exhibit mutism, and exhibit third-person auditory hallucinations and made phenomena (the feeling that some aspect of the individual is under the external control of others).19 Case reports of treatment of catatonic patients with ECT vs lorazepam are listed in Table 3.13-18
In pregnancy, ECT can be considered early in the course of illness. A review of the literature on ECT during pregnancy reported at least partial remission in 78% of studies reporting efficacy data.20 Among these 339 patients, there were 25 fetal or neonatal complications—only 11 of these were related to ECT—and 20 maternal complications, of which 18 were related to ECT. The authors of this review concluded that 1) ECT is an effective treatment for severe mental illness during pregnancy and 2) the risks to fetus and mother are low.
A 2007 study identified 1,979 infants whose mothers reported use of benzodiazepines or hypnotic benzodiazepine-receptor agonists during early pregnancy.21 An additional 401 infants born to mothers who were prescribed these medications during late pregnancy also were included in this study. Women who took these medications were at an increased risk for preterm birth and low birth weight. The rate of congenital malformations in this study was moderately increased among infants exposed in early pregnancy (adjusted odds ratio = 1.24 [95% confidence interval, 1.00 to 1.55]).
Because catatonic symptoms can appear during the course of schizophrenia, several antipsychotics have been used to treat this condition. The efficacy and safety of antipsychotics for treating catatonia remains largely unknown, however.1
OUTCOME: Recovery, baby girl
We begin oral haloperidol, 10 mg/d, for Mrs. K, which we then increase to 20 mg/d. Because she shows little response to haloperidol, we suggest a trial of ECT, but her husband refuses to consent. She is started on IM lorazepam, 6 mg/d.
Mrs. K gradually improves and increases her intake of food and liquids. After 10 days of lorazepam treatment, her BFCRS score decreases to 13. Mrs. K begins to speak and her gaze is less fixed. Negativistic behaviors are nearly absent.
Because we are concerned about Mrs. K’s pregnancy, lab tests are repeated. A complete metabolic panel shows an elevated glucose level (122 mg/dL); urinalysis reveals glycosuria (glucose, 1,000 mg/dL), proteinuria (protein, 10 mg/dL), and ketonuria, (ketones, 20 mg/dL). She is transferred to the obstetrics service for evaluation of gestational diabetes.
Psychotropics are continued while Mrs. K is on the obstetrics service; she returns to the inpatient psychiatric unit on an insulin regimen. IM lorazepam is increased to 8 mg/d, and haloperidol is decreased from 20 mg/d, to 10 mg/d, to prevent worsening of catatonia, which can occur when catatonic patients receive a psychotropic.11 Three days later, Mrs. K’s BFCRS score is 12 and she shows only mild rigidity. Mrs. K briefly interacts with staff, particularly when she wants something.
Lorazepam is decreased to 1 mg/d in anticipation of cesarean delivery. Mrs. K becomes more adherent with her medications; often, she takes the oral dose of haloperidol, rather than the IM formulation. On mental status examination she exhibits poor eye contact, rather than a fixed gaze, and her BFCRS score decreases to 7 by day 25.
By the end of lorazepam treatment, Mrs. K has fully recovered from her catatonic state. She interacts with staff, engages with the treatment team, and is excited to go home. At discharge, she is given a diagnosis of schizophrenia with catatonia, and is taking haloperidol, 5 mg, twice a day. She gives birth to a healthy girl.
The authors' observations
Mrs. K was treated initially with haloperidol for several reasons. Haloperidol is relatively safe during pregnancy (FDA pregnancy category C) as shown by a recent multicenter, prospective, cohort study in which babies exposed in utero to haloperidol showed a congenital malformation (limb defect) rate within the expected baseline risk for the general population.22 Lorazepam is FDA category D for use in pregnancy and can cause preterm delivery,23 floppy infant syndrome, and withdrawal syndromes.24 We did not use a second-generation antipsychotic (SGA) because it could have made Mrs. K’s hyperglycemia worse. SGAs can induce gestational diabetes and increase the incidence of large-for-gestational-age newborns, compared with first-generation antipsychotics.24 Last, Mrs. K’s family rejected ECT.
Because of Mrs. K’s poor response to haloperidol, the treatment team decided to start IM lorazepam, which eventually was increased to 8 mg/d. The haloperidol dose was reduced by half to avoid worsening of catatonia and reduce the risk of neuroleptic malignant syndrome.1,25 When clinical response was achieved, lorazepam was tapered and Mrs. K was discharged with only haloperidol.
In the absence of well-designed prospective follow-up studies, information on the potential impact of prenatal exposure to antipsychotics and benzodiazepines on a child’s cognitive development is limited.26 This case adds to the scant literature on the treatment of catatonia during pregnancy and illustrates how the BFCRS can be utilized during serial patient evaluations to monitor clinical improvement.
Bottom Line
Psychosis and catatonia during pregnancy are associated with complications to mother and child. The Bush-Francis Catatonia Rating Scale can be used to identify and track catatonic symptoms. Lorazepam and electroconvulsive therapy have been used safely and with good outcomes in mentally ill pregnant women when used appropriately.
Related Resources
- Fink M. Catatonia: a syndrome appears, disappears, and is rediscovered. Can J Psychiatry. 2009;54(7):437-445.
- Seethalakshmi R, Dhavale S, Suggu K, et al. Catatonic syndrome: importance of detection and treatment with lorazepam. Ann Clin Psychiatry. 2008;20(1):5-8.
- Salam S, Kilzieh N. Lorazepam treatment of psychogenic catatonia: an update. J Clin Psychiatry, 1988;49(suppl):16-21.
Drug Brand Names
Ceftriaxone • Rocephin Olanzapine • Zyprexa
Haloperidol • Haldol Short-acting Insulin • Novolin, Humulin
Lorazepam • Ativan
Disclosures
Dr. Runyan receives grant support from Lippincott, Williams, & Wilkins. Drs. Durant, Prudent, and Sotelo report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Mute and unresponsive
Mrs. K, a 42-year-old Haitian who is 31 weeks pregnant, presents with a 4-week history of progressive mutism and psychomotor retardation. At inpatient admission, she is awake and alert but does not speak and resists the treatment team’s attempts to engage her. Mrs. K’s eyes are open, but she has a vacant stare and avoids eye contact. Her affect is flat and nonreactive and she appears internally preoccupied. Mrs. K exhibits motoric immobility, displays a rigid posture, and resists attempts to get her to move. Features of catatonic excitement, echo phenomena, posturing, stereotypies, and mannerisms are absent during the initial evaluation.
Mrs. K’s husband reports that they had been on vacation for 6 days before he brought her for psychiatric evaluation. He denies any recent evidence of psychosis or mood disturbance, stating that his wife was excited when she learned of the pregnancy, and attended all prenatal appointments. He reports that when this episode began, Mrs. K stopped talking to her 3-year-old daughter, did not respond to her name, and did not pay attention to those around her.
According to her husband, a similar episode occurred 2 years earlier, during which Mrs. K was selectively mute for approximately 1 month. She did not eat for 5 days and neglected the care of her daughter. There were 2 additional brief periods of prominent psychomotor retardation for which she was hospitalized in Haiti. According to the patient’s aunt, Mrs. K complained that her husband had cast a “voodoo spell” on her because he wanted sole custody of their daughter. Her husband recounted an episode, when they lived in Haiti, during which his wife became paranoid, left the house, and wandered the streets for 2 days.
The medical history is significant for a cervical polyp that was removed 2 years ago. Mrs. K has no history of substance abuse. She was born and raised in Haiti where she studied medicine. Her family reports that Mrs. K’s husband is “controlling,” which causes her distress.
a) major depressive disorder, severe with psychosis, with catatonia
b) schizophrenia, with catatonia
c) conversion disorder, with catatonia
d) bipolar I disorder, with psychosis and catatonia
The authors' observations
Catatonia is a neuropsychiatric syndrome that can occur in schizophrenia, mood disorders, mental retardation, neurologic disease, metabolic conditions, and drug intoxication.1 Catatonia can present in several ways, from catatonic stupor to extreme purposeless agitation; more than 60 catatonic signs and symptoms have been described.1 According to DSM-52 catatonia is characterized by 3 or more of the following symptoms:
• stupor
• catalepsy
• waxy flexibility
• mutism
• negativism
• posturing
• mannerism
• stereotypy
• agitation, not influenced by external stimuli
• grimacing
• echolalia
• echopraxia.
Mrs. K exhibited stupor, mutism, posturing, and grimacing (Table 1).2 We thought that her catatonic features were secondary to schizophrenia because she had paranoid delusions and displayed disorganized behavior while in Haiti. There was no evidence of past or current mood disorder, metabolic condition, neurologic illness, or substance abuse.
Catatonia and pregnancy
There is little available information to guide clinicians who are treating a pregnant woman who has a catatonic syndrome. Espinola-Nadurille and co-workers described a 22-year-old pregnant (21 weeks) woman from rural Mexico who was hospitalized with agitation, disorganized speech, restlessness, and hallucinations after several weeks of alternating agitation and withdrawal with mutism and refusal to eat or drink.3 This patient developed malignant catatonia with creatine phosphokinase elevation and leukocytosis and eventually responded to treatment with lorazepam and electroconvulsive therapy (ECT). She was given a diagnosis of schizophreniform disorder. Treating her catatonic symptoms did not result in any adverse effects on the pregnancy or the fetus.
Exacerbation of schizophrenia during pregnancy can lead to neglect of pregnancy and prenatal care,4 imminent harm to the fetus because of malnutrition and dehydration, and risk of preterm delivery and low weight at birth. Prolonged catatonia can cause medical complications such as decubitus ulcers, incontinence, recurrent urinary tract infections, aspiration pneumonia, increased risk of deep venous thrombosis, malnutrition, and ocular complications because of prolonged staring and reduced blinking (Table 25-10). For these reasons, it is important to treat this condition early and aggressively to improve pregnancy outcome and infant well-being.
of Mrs. K’s symptoms?
a) Positive and Negative Symptom Scale
b) The Northoff Catatonia Rating Scale
c) The Bush-Francis Catatonia Rating Scale (BFCRS)
d) The Rogers Catatonia Scale
EVALUATION: Flat affect
The mental status examination on admission describes a tall, black, Haitian woman with unkempt hair and fair hygiene. Mrs. K has a prominent abdomen, consistent with a 31-week pregnancy. She exhibits a blank stare without direct eye contact; she is mute, and exhibits flat affect. We cannot evaluate her thought processes and content because Mrs. K is mute, although she does appear internally preoccupied.
Physical examination on admission is unremarkable; vital signs are stable and within normal limits. Laboratory work-up reveals a urinary tract infection, which is treated with ceftriaxone. Mrs. K also has macrocytic anemia (hemoglobin, 11.7 g/dL; hematocrit, 34.7%; mean corpuscular volume, 99.2 μm3). Albumin is low at 2.6 g/dL. Urine drug toxicology screen is negative. Fingerstick glucose reading is 139 mg/dL. Mrs. K is given a presumptive diagnosis of schizophrenia with catatonia.
Mrs. K’s BFCRS11 score is 22 at admission. She is mute, holds postures for longer than a minute, and is resistant to repositioning. She also has extreme hypoactivity and does not interact with others. She has a fixed, blank stare, and exhibits mild grimacing.
The authors' observations
BFCRS defines each catatonic sign, rates its severity, and provides a standardized schema for clinical examination.11 The BFCRS is preferred for routine use because of its validity, reliability, and ease of administration.12 The treatment team rated Mrs. K at admission, during the course of treatment, and at discharge, showing a substantial improvement at the end of the hospitalization (Figure).
a) ECT
b) lorazepam
c) haloperidol
d) olanzapine
The authors' observations
Benzodiazepines (particularly lorazepam) and ECT are considered the treatment of choice for catatonic symptoms.11 More than 72% of patients with catatonic symptoms remit after a trial of a benzodiazepine.1 ECT is considered when patients do not respond to a benzodiazepine after 48 to 72 hours.3 Several cases of complete resolution of catatonic symptoms have been linked to ECT (Table 3).13-18
A recent retrospective review revealed that patients who do not respond to lorazepam are more likely to come from a rural setting, have a longer duration of illness, exhibit mutism, and exhibit third-person auditory hallucinations and made phenomena (the feeling that some aspect of the individual is under the external control of others).19 Case reports of treatment of catatonic patients with ECT vs lorazepam are listed in Table 3.13-18
In pregnancy, ECT can be considered early in the course of illness. A review of the literature on ECT during pregnancy reported at least partial remission in 78% of studies reporting efficacy data.20 Among these 339 patients, there were 25 fetal or neonatal complications—only 11 of these were related to ECT—and 20 maternal complications, of which 18 were related to ECT. The authors of this review concluded that 1) ECT is an effective treatment for severe mental illness during pregnancy and 2) the risks to fetus and mother are low.
A 2007 study identified 1,979 infants whose mothers reported use of benzodiazepines or hypnotic benzodiazepine-receptor agonists during early pregnancy.21 An additional 401 infants born to mothers who were prescribed these medications during late pregnancy also were included in this study. Women who took these medications were at an increased risk for preterm birth and low birth weight. The rate of congenital malformations in this study was moderately increased among infants exposed in early pregnancy (adjusted odds ratio = 1.24 [95% confidence interval, 1.00 to 1.55]).
Because catatonic symptoms can appear during the course of schizophrenia, several antipsychotics have been used to treat this condition. The efficacy and safety of antipsychotics for treating catatonia remains largely unknown, however.1
OUTCOME: Recovery, baby girl
We begin oral haloperidol, 10 mg/d, for Mrs. K, which we then increase to 20 mg/d. Because she shows little response to haloperidol, we suggest a trial of ECT, but her husband refuses to consent. She is started on IM lorazepam, 6 mg/d.
Mrs. K gradually improves and increases her intake of food and liquids. After 10 days of lorazepam treatment, her BFCRS score decreases to 13. Mrs. K begins to speak and her gaze is less fixed. Negativistic behaviors are nearly absent.
Because we are concerned about Mrs. K’s pregnancy, lab tests are repeated. A complete metabolic panel shows an elevated glucose level (122 mg/dL); urinalysis reveals glycosuria (glucose, 1,000 mg/dL), proteinuria (protein, 10 mg/dL), and ketonuria, (ketones, 20 mg/dL). She is transferred to the obstetrics service for evaluation of gestational diabetes.
Psychotropics are continued while Mrs. K is on the obstetrics service; she returns to the inpatient psychiatric unit on an insulin regimen. IM lorazepam is increased to 8 mg/d, and haloperidol is decreased from 20 mg/d, to 10 mg/d, to prevent worsening of catatonia, which can occur when catatonic patients receive a psychotropic.11 Three days later, Mrs. K’s BFCRS score is 12 and she shows only mild rigidity. Mrs. K briefly interacts with staff, particularly when she wants something.
Lorazepam is decreased to 1 mg/d in anticipation of cesarean delivery. Mrs. K becomes more adherent with her medications; often, she takes the oral dose of haloperidol, rather than the IM formulation. On mental status examination she exhibits poor eye contact, rather than a fixed gaze, and her BFCRS score decreases to 7 by day 25.
By the end of lorazepam treatment, Mrs. K has fully recovered from her catatonic state. She interacts with staff, engages with the treatment team, and is excited to go home. At discharge, she is given a diagnosis of schizophrenia with catatonia, and is taking haloperidol, 5 mg, twice a day. She gives birth to a healthy girl.
The authors' observations
Mrs. K was treated initially with haloperidol for several reasons. Haloperidol is relatively safe during pregnancy (FDA pregnancy category C) as shown by a recent multicenter, prospective, cohort study in which babies exposed in utero to haloperidol showed a congenital malformation (limb defect) rate within the expected baseline risk for the general population.22 Lorazepam is FDA category D for use in pregnancy and can cause preterm delivery,23 floppy infant syndrome, and withdrawal syndromes.24 We did not use a second-generation antipsychotic (SGA) because it could have made Mrs. K’s hyperglycemia worse. SGAs can induce gestational diabetes and increase the incidence of large-for-gestational-age newborns, compared with first-generation antipsychotics.24 Last, Mrs. K’s family rejected ECT.
Because of Mrs. K’s poor response to haloperidol, the treatment team decided to start IM lorazepam, which eventually was increased to 8 mg/d. The haloperidol dose was reduced by half to avoid worsening of catatonia and reduce the risk of neuroleptic malignant syndrome.1,25 When clinical response was achieved, lorazepam was tapered and Mrs. K was discharged with only haloperidol.
In the absence of well-designed prospective follow-up studies, information on the potential impact of prenatal exposure to antipsychotics and benzodiazepines on a child’s cognitive development is limited.26 This case adds to the scant literature on the treatment of catatonia during pregnancy and illustrates how the BFCRS can be utilized during serial patient evaluations to monitor clinical improvement.
Bottom Line
Psychosis and catatonia during pregnancy are associated with complications to mother and child. The Bush-Francis Catatonia Rating Scale can be used to identify and track catatonic symptoms. Lorazepam and electroconvulsive therapy have been used safely and with good outcomes in mentally ill pregnant women when used appropriately.
Related Resources
- Fink M. Catatonia: a syndrome appears, disappears, and is rediscovered. Can J Psychiatry. 2009;54(7):437-445.
- Seethalakshmi R, Dhavale S, Suggu K, et al. Catatonic syndrome: importance of detection and treatment with lorazepam. Ann Clin Psychiatry. 2008;20(1):5-8.
- Salam S, Kilzieh N. Lorazepam treatment of psychogenic catatonia: an update. J Clin Psychiatry, 1988;49(suppl):16-21.
Drug Brand Names
Ceftriaxone • Rocephin Olanzapine • Zyprexa
Haloperidol • Haldol Short-acting Insulin • Novolin, Humulin
Lorazepam • Ativan
Disclosures
Dr. Runyan receives grant support from Lippincott, Williams, & Wilkins. Drs. Durant, Prudent, and Sotelo report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Weder N, Muralee S, Penland H, et al. Catatonia: a review. Ann Clin Psychiatry. 2008;20(2):97-107.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Arlington, VA: American Psychiatric Association; 2013.
3. Espinola-Nadurille M, Ramirez-Bermudez J, Fricchione GL. Pregnancy and malignant catatonia. Gen Hosp Psychiatry. 2007;29(1):69-71.
4. Solari H, Dickson KE, Miller L. Understanding and treating women with schizophrenia during pregnancy and postpartum. Can J Clin Pharmacol. 2009;16(1):e23-e32.
5. Gross AF, Smith FA, Stern TA. Dread complications of catatonia: a case discussion and review of the literature. Prim Care Companion J Clin Psychiatry. 2008;10(12):
153-155.
6. Larsen HH, Ritchie JC, McNutt MD, et al. Pulmonary embolism in a patient with catatonia: an old disease, changing times. Psychosomatics. 2011;52(4):387-391.
7. Lachner C, Sandson NB. Medical complications of catatonia: a case of catatonia-induced deep venous thrombosis. Psychosomatics. 2003;44(6):512-4.
8. Morioka H, Nagatomo I, Yamada K, et al. Deep venous thrombosis of the leg due to psychiatric stupor. Psychiatry Clin Neurosci, 1997;51(5):323-326.
9. Nomoto H, Hatta K, Usui C, et al. Vitamin K deficiency due to prolongation of antibiotic treatment and decrease in food intake in a catatonia patient. Psychosomatics. 2011;52(5):
486-487.
10. Srivastava A, Gupta A, Murthy P, et al. Catatonia and multiple pressure ulcers: a rare complication in psychiatric setting. Indian J Psychiatry. 2009;51(3):206-208.
11. Francis A. Catatonia: diagnosis, classification, and treatment. Curr Psychiatry Rep. 2010;12(3):180-185.
12. Sienaert P, Rooseleer J, De Fruyt J. Measuring catatonia: a systematic review of rating scales. J Affect Disord. 2011;135(1):1-9.
13. Zisselman MH, Jaffe RL. ECT in the treatment of a patient with catatonia: consent and complications. Am J Psychiatry. 2010;167(2):127-132.
14. Rohland BM, Carroll BT, Jacoby RG. ECT in the treatment of the catatonic syndrome. J Affect Disord. 1993;29(4):255-261.
15. Raveendranathan D, Narayanaswamy JC, Reddi SV. Response rate of catatonia to electroconvulsive therapy and its clinical correlates. Eur Arch Psychiatry Clin Neurosci. 2012;262(5):425-430.
16. Payee H, Chandrasekaran R, Raju GV. Catatonic syndrome: treatment response to lorazepam. Indian J Psychiatry. 1999; 41(1):49-53.
17. Bush G, Fink M, Petrides G, et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137-143.
18. Huang TL. Lorazepam and diazepam rapidly relieve catatonic signs in patients with schizophrenia. Psychiatry Clin Neurosci. 2005;59(1):52-55.
19. Narayanaswamy JC, Tibrewal P, Zutshi A, et al. Clinical predictors of response to treatment in catatonia. Gen Hosp Psychiatry. 2012;34:312-316.
20. Anderson EL, Reti IM. ECT in pregnancy: a review of the literature from 1941 to 2007. Psychosom Med. 2009;71:
235-242.
21. Wikner BN, Stiller CO, Bergman U, et al. Use of benzodiazepines and benzodiazepine receptor agonists during pregnancy: neonatal outcome and congenital malformations. Pharmacoepidemiol Drug Saf. 2007;16:
1203-1210.
22. Gentile S. Antipsychotic therapy during early and late pregnancy. A systematic review. Schizophr Bull. 2010;36:
518-544.
23. Calderon-Margalit R, Qiu C, Ornoy A, et al. Risk of preterm delivery and other adverse perinatal outcomes in relation to maternal use of psychotropic medications during pregnancy. Am J Obstet Gynecol. 2009;201:579e1-8.
24. Howland RH. Prescribing psychotropic medications during pregnancy and lactation: principles and guidelines. J Psychosoc Nurs Ment Health Serv. 2009;47(5):19-23.
25. White DA, Robins AH. Catatonia: harbinger of the neuroleptic malignant syndrome. Br J Psychiatry. 1991; 158:419-421.
26. Gentile S. Neurodevelopmental effects of prenatal exposure to psychotropic medications. Depress Anxiety. 2010; 27(7):675-686.
1. Weder N, Muralee S, Penland H, et al. Catatonia: a review. Ann Clin Psychiatry. 2008;20(2):97-107.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Arlington, VA: American Psychiatric Association; 2013.
3. Espinola-Nadurille M, Ramirez-Bermudez J, Fricchione GL. Pregnancy and malignant catatonia. Gen Hosp Psychiatry. 2007;29(1):69-71.
4. Solari H, Dickson KE, Miller L. Understanding and treating women with schizophrenia during pregnancy and postpartum. Can J Clin Pharmacol. 2009;16(1):e23-e32.
5. Gross AF, Smith FA, Stern TA. Dread complications of catatonia: a case discussion and review of the literature. Prim Care Companion J Clin Psychiatry. 2008;10(12):
153-155.
6. Larsen HH, Ritchie JC, McNutt MD, et al. Pulmonary embolism in a patient with catatonia: an old disease, changing times. Psychosomatics. 2011;52(4):387-391.
7. Lachner C, Sandson NB. Medical complications of catatonia: a case of catatonia-induced deep venous thrombosis. Psychosomatics. 2003;44(6):512-4.
8. Morioka H, Nagatomo I, Yamada K, et al. Deep venous thrombosis of the leg due to psychiatric stupor. Psychiatry Clin Neurosci, 1997;51(5):323-326.
9. Nomoto H, Hatta K, Usui C, et al. Vitamin K deficiency due to prolongation of antibiotic treatment and decrease in food intake in a catatonia patient. Psychosomatics. 2011;52(5):
486-487.
10. Srivastava A, Gupta A, Murthy P, et al. Catatonia and multiple pressure ulcers: a rare complication in psychiatric setting. Indian J Psychiatry. 2009;51(3):206-208.
11. Francis A. Catatonia: diagnosis, classification, and treatment. Curr Psychiatry Rep. 2010;12(3):180-185.
12. Sienaert P, Rooseleer J, De Fruyt J. Measuring catatonia: a systematic review of rating scales. J Affect Disord. 2011;135(1):1-9.
13. Zisselman MH, Jaffe RL. ECT in the treatment of a patient with catatonia: consent and complications. Am J Psychiatry. 2010;167(2):127-132.
14. Rohland BM, Carroll BT, Jacoby RG. ECT in the treatment of the catatonic syndrome. J Affect Disord. 1993;29(4):255-261.
15. Raveendranathan D, Narayanaswamy JC, Reddi SV. Response rate of catatonia to electroconvulsive therapy and its clinical correlates. Eur Arch Psychiatry Clin Neurosci. 2012;262(5):425-430.
16. Payee H, Chandrasekaran R, Raju GV. Catatonic syndrome: treatment response to lorazepam. Indian J Psychiatry. 1999; 41(1):49-53.
17. Bush G, Fink M, Petrides G, et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137-143.
18. Huang TL. Lorazepam and diazepam rapidly relieve catatonic signs in patients with schizophrenia. Psychiatry Clin Neurosci. 2005;59(1):52-55.
19. Narayanaswamy JC, Tibrewal P, Zutshi A, et al. Clinical predictors of response to treatment in catatonia. Gen Hosp Psychiatry. 2012;34:312-316.
20. Anderson EL, Reti IM. ECT in pregnancy: a review of the literature from 1941 to 2007. Psychosom Med. 2009;71:
235-242.
21. Wikner BN, Stiller CO, Bergman U, et al. Use of benzodiazepines and benzodiazepine receptor agonists during pregnancy: neonatal outcome and congenital malformations. Pharmacoepidemiol Drug Saf. 2007;16:
1203-1210.
22. Gentile S. Antipsychotic therapy during early and late pregnancy. A systematic review. Schizophr Bull. 2010;36:
518-544.
23. Calderon-Margalit R, Qiu C, Ornoy A, et al. Risk of preterm delivery and other adverse perinatal outcomes in relation to maternal use of psychotropic medications during pregnancy. Am J Obstet Gynecol. 2009;201:579e1-8.
24. Howland RH. Prescribing psychotropic medications during pregnancy and lactation: principles and guidelines. J Psychosoc Nurs Ment Health Serv. 2009;47(5):19-23.
25. White DA, Robins AH. Catatonia: harbinger of the neuroleptic malignant syndrome. Br J Psychiatry. 1991; 158:419-421.
26. Gentile S. Neurodevelopmental effects of prenatal exposure to psychotropic medications. Depress Anxiety. 2010; 27(7):675-686.
Psychotic and needing prayer
CASE: Psychotic and assaultive
Mr. A, age 34, is involuntarily admitted to a psychiatric hospital after assaulting a family member and a police officer. He is charged with 2 counts of first-degree assault. He describes auditory hallucinations and believes God is telling him to refuse medication. One year earlier he was diagnosed with schizophrenia. Mr. A informs hospital staff that he is a Christian Scientist, and his religion precludes him from taking any medications. The local parish of the First Church of Christ, Scientist confirms that he is an active member. One day after admission, Mr. A is threatening and belligerent, and he continues to refuse any treatment.
How would you initially treat Mr. A?a) seek emergency guardianship
b) seek help from the Christian Science community
c) order the appropriate medication to effectively treat his symptoms
TREATMENT: Involuntary treatment
While in the hospital, Mr. A’s psychotic symptoms and aggressive behavior toward the staff and other patients lead to several psychiatric emergencies being declared and involuntary administration of antipsychotic medication. Because IM haloperidol, 5 mg/d, rapidly alleviates his symptoms, there is no need to pursue guardianship. Mr. A asks to meet with a member of the Christian Science community before his discharge, which is arranged. Upon being discharged, Mr. A schedules outpatient treatment at the community mental health center.
The author's observations
Mr. A’s case challenged staff to balance his clinical needs with his religious philosophy. Although psychotic, Mr. A provided a reason for refusing treatment—his belief in Christian Science—which would be considered a valid spiritual choice based on his values. However, his psychiatric symptoms created a dangerous situation for himself and others, which lead to emergency administration of antipsychotics against his will. Resolution of his symptoms did not warrant a petition for guardianship or a long-term involuntary hospitalization (Table 1). Allowing Mr. A to meet with a member of his church was crucial because it validated Mr. A’s religious practices and showed the staff’s willingness to respect his Christian Science beliefs.1,2
Honoring religious beliefs
Christian Science is based on the writings of Mary Baker Eddy and the Bible. Adherents believe that any form of evil, such as sin, disease, or death, is the opposite of God and is an illusion. Health care and treatment within the Christian Science community do not focus on what is wrong with the physical body, but rather what is wrong with the mind. Christian Scientists attempt healing through specific forms of prayer, not conventional methods such as medications or surgery.3 Christian Scientists believe there are no limits to the type of medical conditions that can be healed through prayer. Community members go to Christian Science practitioners for healing via prayer, focusing on the Bible and Mary Baker Eddy’s writings to alleviate their suffering.
The Christian Science church does not forbid its members from receiving conventional medical treatments, although prayer clearly is the preferred method of healing.4 Members can make their own choice about obtaining medical treatment. If they choose medical care, they cannot receive simultaneous treatment from Christian Science practitioners, but they can participate in other church activities. However, members compelled to get medical or psychiatric treatment via a guardianship or a court order can receive concurrent treatment from a Christian Science practitioner.
Other faith traditions generally do not draw such a clear line between medical treatment and religious healing. For example, Jehovah’s Witnesses have no prohibition against obtaining medical care, but they refuse blood transfusions, although they do accept medical alternatives to blood.5
ASSESSMENT: Remorse, reluctance
Mr. A stops taking his medication a few days after discharge, becomes psychotic, assaults his landlord, and is involuntarily readmitted to the hospital. His symptoms again are alleviated with IM haloperidol, 5 mg/d, and Mr. A is remorseful about his behavior while psychotic. He repeats his belief that his illness can be cured with prayer. The staff is reluctant to discharge Mr. A because of his history of nonadherence to treatment and assaultive episodes.
What are the next steps to consider in Mr. A’s treatment?a) seek guardianship because Mr. A does not appreciate the need for treatment
b) obtain a long-term commitment to the hospital with plans to conditionally release Mr. A when he is clinically stable
c) begin treatment with a long-acting injectable antipsychotic
EVALUATION: Next steps
The psychiatrist requests and receives a 3-year commitment for Mr. A from the probate court. The psychiatrist works with Mr. A and the community mental health center clinician to develop a conditional discharge plan in which Mr. A agrees to take medications as prescribed as a condition of his release. Mr. A initially is resistant to this plan. He is allowed to meet frequently with his Christian Science practitioner to discuss ways to continue treatment. Hospital staff supports these meetings, while explaining the importance of adhering to medication and how this will effectively treat his psychotic symptoms. Hospital staff does not negate or minimize Mr. A’s religious beliefs. The Christian Science practitioner allows Mr. A to continue his religious healing while receiving psychiatric care because he is a under court-ordered involuntary commitment. This leads Mr. A to find common ground between his religious beliefs and need for psychiatric treatment. Mr. A maintains his belief that he can be healed by prayer, but agrees to accept medications under the law of the probate commitment. To maximize adherence, he agrees to haloperidol decanoate, a long-acting injectable antipsychotic. He is conditionally discharged to continuing outpatient treatment at the community mental health center.
Mr. A adheres to treatment but begins to develop early signs of tardive dyskinesia (mild lip smacking and some tongue protrusion). Therefore, haloperidol decanoate is discontinued and replaced with oral olanzapine, 20 mg/d. Mr. A is no longer psychotic, and his psychotic symptoms are in remission. He continues to hold fast to his Christian Science beliefs.
One month before the end of his 3-year commitment, Mr. A informs his psychiatrist that he plans to stop his antipsychotic when the commitment ends and to pursue treatment with his Christian Science practitioner via prayer. He wants to prove to everyone that medications are no longer necessary.
What should Mr. A’s treating psychiatrist do?a) immediately readmit Mr. A involuntarily because of his potential dangerousness and impending treatment nonadherence
b) pursue guardianship because Mr. A is incapable of understanding that he has a serious mental illness
c) not pursue legal action but continue to treat Mr. A with antipsychotics and encourage compliance
d) readmit Mr. A to the hospital, request an extension of the commitment order, and consider a medication holiday in a safe setting to address Mr. A’s religious beliefs
OUTCOME: Court-ordered treatment
Mr. A agrees to hospitalization and at a court hearing is committed to the hospital for a period not to exceed 5 years. The judge also orders that Mr. A undergo a period of reducing or stopping his antipsychotic to see if he decompensates. The judicial order states that if it is determined that Mr. A no longer needs medication, the judge may reconsider the terms of the long-term commitment.
Mr. A, his inpatient and outpatient psychiatrists, and a Christian Science practitioner work together to develop a plan to taper his medication. Over 2 weeks, olanzapine is tapered from 20 mg/d to 10 mg/d. Two weeks into the taper, Mr. A becomes increasingly irritable, paranoid, and vigilant. The staff gives him prompt feedback about his apparent decompensation. Mr. A accepts this. He resumes taking olanzapine, 20 mg/d, and his symptoms resolve. He feels discouraged because taking medication is against his religious values. Nevertheless, he accepts the 5-year commitment as a court-mandated treatment that he must abide by. He is conditionally discharged from the hospital. For a summary of Mr. A’s clinical course, see Table 2.
Mr. A continues to do well in the community. New Hampshire’s law allowing up to a 5-year commitment to the hospital has been effective in maximizing Mr. A’s treatment adherence (Table 3).6 He has not been rehospitalized and his psychotic symptoms are in remission. Mr. A still believes his symptoms can be best treated with Christian Science prayer, but sees the state-imposed conditional discharge as a necessary “evil” that he must adhere to. He continues to be an active member of his church.
The author's observations
With the support of his outpatient and inpatient psychiatrists, treatment teams, and Christian Science practitioner, Mr. A has successfully integrated 2 seemingly opposing views regarding treatment, allowing him to live successfully in the community.
From this case, we learned that clinicians:
- need to understand patients’ religious beliefs and how these beliefs can impact their care
- must be aware that caring for patients from different religious traditions may present unique treatment challenges
- need to put their personal views regarding a patient’s religious beliefs aside and work with the patient to alleviate suffering
- must give patients ample opportunity to meet with their faith community, allowing adequate time for discussion and problem solving
Bottom Line
Balancing a patient’s clinical and spiritual needs can be challenging when those needs seem mutually exclusive. Clear communication, legal guidance, careful planning, and a strong therapeutic alliance can create opportunities for the patient to make both needs work to his advantage.
Related Resources
- Christian Science. www.christianscience.com.
- de Nesnera A, Vidaver RM. New Hampshire’s commitment law: treatment implications. New Hampshire Bar Journal. 2007;48(2):68-73.
- Ehman J. Religious diversity: practical points for health care
providers: www.uphs.upenn.edu/pastoral/resed/diversity_points.html.
Drug Brand Names
Haloperidol • Haldol
Olanzapine • Zyprexa
Disclosure
Dr. de Nesnera reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Pavlo AM, Bursztajn H, Gutheil T. Christian Science and competence to make treatment choices: clinical challenges in assessing values. Int J Law Psych. 1987;10(4):395-401.
2. Pavlo AM, Bursztajn H, Gutheil T, et al. Weighing religious beliefs in determining competence. Hosp Comm Psych. 1987;38(4):350-352.
3. Eddy MB. Science and health with key to the scriptures. Boston, MA: Christian Science Publishing Company; 1875:1-17.
4. Eddy M. Science and health with key to the scriptures. Boston, MA: Christian Science Publishing Company; 1875: 167.
5. The growing demand for bloodless medicine and surgery. Awake! January 8, 2000:4-6. http://wol.jw.org/en/wol/d/r1/lp-e/102000003. Accessed June 4, 2013.
6. NH Rev Stat Ann § 135-C:27-46.
CASE: Psychotic and assaultive
Mr. A, age 34, is involuntarily admitted to a psychiatric hospital after assaulting a family member and a police officer. He is charged with 2 counts of first-degree assault. He describes auditory hallucinations and believes God is telling him to refuse medication. One year earlier he was diagnosed with schizophrenia. Mr. A informs hospital staff that he is a Christian Scientist, and his religion precludes him from taking any medications. The local parish of the First Church of Christ, Scientist confirms that he is an active member. One day after admission, Mr. A is threatening and belligerent, and he continues to refuse any treatment.
How would you initially treat Mr. A?a) seek emergency guardianship
b) seek help from the Christian Science community
c) order the appropriate medication to effectively treat his symptoms
TREATMENT: Involuntary treatment
While in the hospital, Mr. A’s psychotic symptoms and aggressive behavior toward the staff and other patients lead to several psychiatric emergencies being declared and involuntary administration of antipsychotic medication. Because IM haloperidol, 5 mg/d, rapidly alleviates his symptoms, there is no need to pursue guardianship. Mr. A asks to meet with a member of the Christian Science community before his discharge, which is arranged. Upon being discharged, Mr. A schedules outpatient treatment at the community mental health center.
The author's observations
Mr. A’s case challenged staff to balance his clinical needs with his religious philosophy. Although psychotic, Mr. A provided a reason for refusing treatment—his belief in Christian Science—which would be considered a valid spiritual choice based on his values. However, his psychiatric symptoms created a dangerous situation for himself and others, which lead to emergency administration of antipsychotics against his will. Resolution of his symptoms did not warrant a petition for guardianship or a long-term involuntary hospitalization (Table 1). Allowing Mr. A to meet with a member of his church was crucial because it validated Mr. A’s religious practices and showed the staff’s willingness to respect his Christian Science beliefs.1,2
Honoring religious beliefs
Christian Science is based on the writings of Mary Baker Eddy and the Bible. Adherents believe that any form of evil, such as sin, disease, or death, is the opposite of God and is an illusion. Health care and treatment within the Christian Science community do not focus on what is wrong with the physical body, but rather what is wrong with the mind. Christian Scientists attempt healing through specific forms of prayer, not conventional methods such as medications or surgery.3 Christian Scientists believe there are no limits to the type of medical conditions that can be healed through prayer. Community members go to Christian Science practitioners for healing via prayer, focusing on the Bible and Mary Baker Eddy’s writings to alleviate their suffering.
The Christian Science church does not forbid its members from receiving conventional medical treatments, although prayer clearly is the preferred method of healing.4 Members can make their own choice about obtaining medical treatment. If they choose medical care, they cannot receive simultaneous treatment from Christian Science practitioners, but they can participate in other church activities. However, members compelled to get medical or psychiatric treatment via a guardianship or a court order can receive concurrent treatment from a Christian Science practitioner.
Other faith traditions generally do not draw such a clear line between medical treatment and religious healing. For example, Jehovah’s Witnesses have no prohibition against obtaining medical care, but they refuse blood transfusions, although they do accept medical alternatives to blood.5
ASSESSMENT: Remorse, reluctance
Mr. A stops taking his medication a few days after discharge, becomes psychotic, assaults his landlord, and is involuntarily readmitted to the hospital. His symptoms again are alleviated with IM haloperidol, 5 mg/d, and Mr. A is remorseful about his behavior while psychotic. He repeats his belief that his illness can be cured with prayer. The staff is reluctant to discharge Mr. A because of his history of nonadherence to treatment and assaultive episodes.
What are the next steps to consider in Mr. A’s treatment?a) seek guardianship because Mr. A does not appreciate the need for treatment
b) obtain a long-term commitment to the hospital with plans to conditionally release Mr. A when he is clinically stable
c) begin treatment with a long-acting injectable antipsychotic
EVALUATION: Next steps
The psychiatrist requests and receives a 3-year commitment for Mr. A from the probate court. The psychiatrist works with Mr. A and the community mental health center clinician to develop a conditional discharge plan in which Mr. A agrees to take medications as prescribed as a condition of his release. Mr. A initially is resistant to this plan. He is allowed to meet frequently with his Christian Science practitioner to discuss ways to continue treatment. Hospital staff supports these meetings, while explaining the importance of adhering to medication and how this will effectively treat his psychotic symptoms. Hospital staff does not negate or minimize Mr. A’s religious beliefs. The Christian Science practitioner allows Mr. A to continue his religious healing while receiving psychiatric care because he is a under court-ordered involuntary commitment. This leads Mr. A to find common ground between his religious beliefs and need for psychiatric treatment. Mr. A maintains his belief that he can be healed by prayer, but agrees to accept medications under the law of the probate commitment. To maximize adherence, he agrees to haloperidol decanoate, a long-acting injectable antipsychotic. He is conditionally discharged to continuing outpatient treatment at the community mental health center.
Mr. A adheres to treatment but begins to develop early signs of tardive dyskinesia (mild lip smacking and some tongue protrusion). Therefore, haloperidol decanoate is discontinued and replaced with oral olanzapine, 20 mg/d. Mr. A is no longer psychotic, and his psychotic symptoms are in remission. He continues to hold fast to his Christian Science beliefs.
One month before the end of his 3-year commitment, Mr. A informs his psychiatrist that he plans to stop his antipsychotic when the commitment ends and to pursue treatment with his Christian Science practitioner via prayer. He wants to prove to everyone that medications are no longer necessary.
What should Mr. A’s treating psychiatrist do?a) immediately readmit Mr. A involuntarily because of his potential dangerousness and impending treatment nonadherence
b) pursue guardianship because Mr. A is incapable of understanding that he has a serious mental illness
c) not pursue legal action but continue to treat Mr. A with antipsychotics and encourage compliance
d) readmit Mr. A to the hospital, request an extension of the commitment order, and consider a medication holiday in a safe setting to address Mr. A’s religious beliefs
OUTCOME: Court-ordered treatment
Mr. A agrees to hospitalization and at a court hearing is committed to the hospital for a period not to exceed 5 years. The judge also orders that Mr. A undergo a period of reducing or stopping his antipsychotic to see if he decompensates. The judicial order states that if it is determined that Mr. A no longer needs medication, the judge may reconsider the terms of the long-term commitment.
Mr. A, his inpatient and outpatient psychiatrists, and a Christian Science practitioner work together to develop a plan to taper his medication. Over 2 weeks, olanzapine is tapered from 20 mg/d to 10 mg/d. Two weeks into the taper, Mr. A becomes increasingly irritable, paranoid, and vigilant. The staff gives him prompt feedback about his apparent decompensation. Mr. A accepts this. He resumes taking olanzapine, 20 mg/d, and his symptoms resolve. He feels discouraged because taking medication is against his religious values. Nevertheless, he accepts the 5-year commitment as a court-mandated treatment that he must abide by. He is conditionally discharged from the hospital. For a summary of Mr. A’s clinical course, see Table 2.
Mr. A continues to do well in the community. New Hampshire’s law allowing up to a 5-year commitment to the hospital has been effective in maximizing Mr. A’s treatment adherence (Table 3).6 He has not been rehospitalized and his psychotic symptoms are in remission. Mr. A still believes his symptoms can be best treated with Christian Science prayer, but sees the state-imposed conditional discharge as a necessary “evil” that he must adhere to. He continues to be an active member of his church.
The author's observations
With the support of his outpatient and inpatient psychiatrists, treatment teams, and Christian Science practitioner, Mr. A has successfully integrated 2 seemingly opposing views regarding treatment, allowing him to live successfully in the community.
From this case, we learned that clinicians:
- need to understand patients’ religious beliefs and how these beliefs can impact their care
- must be aware that caring for patients from different religious traditions may present unique treatment challenges
- need to put their personal views regarding a patient’s religious beliefs aside and work with the patient to alleviate suffering
- must give patients ample opportunity to meet with their faith community, allowing adequate time for discussion and problem solving
Bottom Line
Balancing a patient’s clinical and spiritual needs can be challenging when those needs seem mutually exclusive. Clear communication, legal guidance, careful planning, and a strong therapeutic alliance can create opportunities for the patient to make both needs work to his advantage.
Related Resources
- Christian Science. www.christianscience.com.
- de Nesnera A, Vidaver RM. New Hampshire’s commitment law: treatment implications. New Hampshire Bar Journal. 2007;48(2):68-73.
- Ehman J. Religious diversity: practical points for health care
providers: www.uphs.upenn.edu/pastoral/resed/diversity_points.html.
Drug Brand Names
Haloperidol • Haldol
Olanzapine • Zyprexa
Disclosure
Dr. de Nesnera reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Psychotic and assaultive
Mr. A, age 34, is involuntarily admitted to a psychiatric hospital after assaulting a family member and a police officer. He is charged with 2 counts of first-degree assault. He describes auditory hallucinations and believes God is telling him to refuse medication. One year earlier he was diagnosed with schizophrenia. Mr. A informs hospital staff that he is a Christian Scientist, and his religion precludes him from taking any medications. The local parish of the First Church of Christ, Scientist confirms that he is an active member. One day after admission, Mr. A is threatening and belligerent, and he continues to refuse any treatment.
How would you initially treat Mr. A?a) seek emergency guardianship
b) seek help from the Christian Science community
c) order the appropriate medication to effectively treat his symptoms
TREATMENT: Involuntary treatment
While in the hospital, Mr. A’s psychotic symptoms and aggressive behavior toward the staff and other patients lead to several psychiatric emergencies being declared and involuntary administration of antipsychotic medication. Because IM haloperidol, 5 mg/d, rapidly alleviates his symptoms, there is no need to pursue guardianship. Mr. A asks to meet with a member of the Christian Science community before his discharge, which is arranged. Upon being discharged, Mr. A schedules outpatient treatment at the community mental health center.
The author's observations
Mr. A’s case challenged staff to balance his clinical needs with his religious philosophy. Although psychotic, Mr. A provided a reason for refusing treatment—his belief in Christian Science—which would be considered a valid spiritual choice based on his values. However, his psychiatric symptoms created a dangerous situation for himself and others, which lead to emergency administration of antipsychotics against his will. Resolution of his symptoms did not warrant a petition for guardianship or a long-term involuntary hospitalization (Table 1). Allowing Mr. A to meet with a member of his church was crucial because it validated Mr. A’s religious practices and showed the staff’s willingness to respect his Christian Science beliefs.1,2
Honoring religious beliefs
Christian Science is based on the writings of Mary Baker Eddy and the Bible. Adherents believe that any form of evil, such as sin, disease, or death, is the opposite of God and is an illusion. Health care and treatment within the Christian Science community do not focus on what is wrong with the physical body, but rather what is wrong with the mind. Christian Scientists attempt healing through specific forms of prayer, not conventional methods such as medications or surgery.3 Christian Scientists believe there are no limits to the type of medical conditions that can be healed through prayer. Community members go to Christian Science practitioners for healing via prayer, focusing on the Bible and Mary Baker Eddy’s writings to alleviate their suffering.
The Christian Science church does not forbid its members from receiving conventional medical treatments, although prayer clearly is the preferred method of healing.4 Members can make their own choice about obtaining medical treatment. If they choose medical care, they cannot receive simultaneous treatment from Christian Science practitioners, but they can participate in other church activities. However, members compelled to get medical or psychiatric treatment via a guardianship or a court order can receive concurrent treatment from a Christian Science practitioner.
Other faith traditions generally do not draw such a clear line between medical treatment and religious healing. For example, Jehovah’s Witnesses have no prohibition against obtaining medical care, but they refuse blood transfusions, although they do accept medical alternatives to blood.5
ASSESSMENT: Remorse, reluctance
Mr. A stops taking his medication a few days after discharge, becomes psychotic, assaults his landlord, and is involuntarily readmitted to the hospital. His symptoms again are alleviated with IM haloperidol, 5 mg/d, and Mr. A is remorseful about his behavior while psychotic. He repeats his belief that his illness can be cured with prayer. The staff is reluctant to discharge Mr. A because of his history of nonadherence to treatment and assaultive episodes.
What are the next steps to consider in Mr. A’s treatment?a) seek guardianship because Mr. A does not appreciate the need for treatment
b) obtain a long-term commitment to the hospital with plans to conditionally release Mr. A when he is clinically stable
c) begin treatment with a long-acting injectable antipsychotic
EVALUATION: Next steps
The psychiatrist requests and receives a 3-year commitment for Mr. A from the probate court. The psychiatrist works with Mr. A and the community mental health center clinician to develop a conditional discharge plan in which Mr. A agrees to take medications as prescribed as a condition of his release. Mr. A initially is resistant to this plan. He is allowed to meet frequently with his Christian Science practitioner to discuss ways to continue treatment. Hospital staff supports these meetings, while explaining the importance of adhering to medication and how this will effectively treat his psychotic symptoms. Hospital staff does not negate or minimize Mr. A’s religious beliefs. The Christian Science practitioner allows Mr. A to continue his religious healing while receiving psychiatric care because he is a under court-ordered involuntary commitment. This leads Mr. A to find common ground between his religious beliefs and need for psychiatric treatment. Mr. A maintains his belief that he can be healed by prayer, but agrees to accept medications under the law of the probate commitment. To maximize adherence, he agrees to haloperidol decanoate, a long-acting injectable antipsychotic. He is conditionally discharged to continuing outpatient treatment at the community mental health center.
Mr. A adheres to treatment but begins to develop early signs of tardive dyskinesia (mild lip smacking and some tongue protrusion). Therefore, haloperidol decanoate is discontinued and replaced with oral olanzapine, 20 mg/d. Mr. A is no longer psychotic, and his psychotic symptoms are in remission. He continues to hold fast to his Christian Science beliefs.
One month before the end of his 3-year commitment, Mr. A informs his psychiatrist that he plans to stop his antipsychotic when the commitment ends and to pursue treatment with his Christian Science practitioner via prayer. He wants to prove to everyone that medications are no longer necessary.
What should Mr. A’s treating psychiatrist do?a) immediately readmit Mr. A involuntarily because of his potential dangerousness and impending treatment nonadherence
b) pursue guardianship because Mr. A is incapable of understanding that he has a serious mental illness
c) not pursue legal action but continue to treat Mr. A with antipsychotics and encourage compliance
d) readmit Mr. A to the hospital, request an extension of the commitment order, and consider a medication holiday in a safe setting to address Mr. A’s religious beliefs
OUTCOME: Court-ordered treatment
Mr. A agrees to hospitalization and at a court hearing is committed to the hospital for a period not to exceed 5 years. The judge also orders that Mr. A undergo a period of reducing or stopping his antipsychotic to see if he decompensates. The judicial order states that if it is determined that Mr. A no longer needs medication, the judge may reconsider the terms of the long-term commitment.
Mr. A, his inpatient and outpatient psychiatrists, and a Christian Science practitioner work together to develop a plan to taper his medication. Over 2 weeks, olanzapine is tapered from 20 mg/d to 10 mg/d. Two weeks into the taper, Mr. A becomes increasingly irritable, paranoid, and vigilant. The staff gives him prompt feedback about his apparent decompensation. Mr. A accepts this. He resumes taking olanzapine, 20 mg/d, and his symptoms resolve. He feels discouraged because taking medication is against his religious values. Nevertheless, he accepts the 5-year commitment as a court-mandated treatment that he must abide by. He is conditionally discharged from the hospital. For a summary of Mr. A’s clinical course, see Table 2.
Mr. A continues to do well in the community. New Hampshire’s law allowing up to a 5-year commitment to the hospital has been effective in maximizing Mr. A’s treatment adherence (Table 3).6 He has not been rehospitalized and his psychotic symptoms are in remission. Mr. A still believes his symptoms can be best treated with Christian Science prayer, but sees the state-imposed conditional discharge as a necessary “evil” that he must adhere to. He continues to be an active member of his church.
The author's observations
With the support of his outpatient and inpatient psychiatrists, treatment teams, and Christian Science practitioner, Mr. A has successfully integrated 2 seemingly opposing views regarding treatment, allowing him to live successfully in the community.
From this case, we learned that clinicians:
- need to understand patients’ religious beliefs and how these beliefs can impact their care
- must be aware that caring for patients from different religious traditions may present unique treatment challenges
- need to put their personal views regarding a patient’s religious beliefs aside and work with the patient to alleviate suffering
- must give patients ample opportunity to meet with their faith community, allowing adequate time for discussion and problem solving
Bottom Line
Balancing a patient’s clinical and spiritual needs can be challenging when those needs seem mutually exclusive. Clear communication, legal guidance, careful planning, and a strong therapeutic alliance can create opportunities for the patient to make both needs work to his advantage.
Related Resources
- Christian Science. www.christianscience.com.
- de Nesnera A, Vidaver RM. New Hampshire’s commitment law: treatment implications. New Hampshire Bar Journal. 2007;48(2):68-73.
- Ehman J. Religious diversity: practical points for health care
providers: www.uphs.upenn.edu/pastoral/resed/diversity_points.html.
Drug Brand Names
Haloperidol • Haldol
Olanzapine • Zyprexa
Disclosure
Dr. de Nesnera reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Pavlo AM, Bursztajn H, Gutheil T. Christian Science and competence to make treatment choices: clinical challenges in assessing values. Int J Law Psych. 1987;10(4):395-401.
2. Pavlo AM, Bursztajn H, Gutheil T, et al. Weighing religious beliefs in determining competence. Hosp Comm Psych. 1987;38(4):350-352.
3. Eddy MB. Science and health with key to the scriptures. Boston, MA: Christian Science Publishing Company; 1875:1-17.
4. Eddy M. Science and health with key to the scriptures. Boston, MA: Christian Science Publishing Company; 1875: 167.
5. The growing demand for bloodless medicine and surgery. Awake! January 8, 2000:4-6. http://wol.jw.org/en/wol/d/r1/lp-e/102000003. Accessed June 4, 2013.
6. NH Rev Stat Ann § 135-C:27-46.
1. Pavlo AM, Bursztajn H, Gutheil T. Christian Science and competence to make treatment choices: clinical challenges in assessing values. Int J Law Psych. 1987;10(4):395-401.
2. Pavlo AM, Bursztajn H, Gutheil T, et al. Weighing religious beliefs in determining competence. Hosp Comm Psych. 1987;38(4):350-352.
3. Eddy MB. Science and health with key to the scriptures. Boston, MA: Christian Science Publishing Company; 1875:1-17.
4. Eddy M. Science and health with key to the scriptures. Boston, MA: Christian Science Publishing Company; 1875: 167.
5. The growing demand for bloodless medicine and surgery. Awake! January 8, 2000:4-6. http://wol.jw.org/en/wol/d/r1/lp-e/102000003. Accessed June 4, 2013.
6. NH Rev Stat Ann § 135-C:27-46.
A teen who is wasting away
CASE: Weak and passive
Cassandra, age 17, recently was discharged from a medical rehabilitation facility with a diagnosis of conversion disorder. Her school performance and attendance had been steadily declining for the last 6 months as she lost strength and motivation to take care of herself. Cassandra lives with her father, who is her primary caretaker. Her parents are separated and her mother has fibromyalgia and chronic fatigue syndrome, which leaves her unable to care for her daughter or participate in appointments.
Now lethargic and wasting away physically, Cassandra is pushed in a wheelchair by her weary father into a child psychiatrist’s office. She does not look up or make eye contact. Her father says “the doctors didn’t know what they were doing. That needle test, a nerve conduction study they did, is what made her worse.” Although Cassandra moves her arms to adjust herself in the wheelchair, she does not move her legs or try to move the wheelchair.
Cassandra’s father states that she has “congenital neuromyopathy. Her mother gave it to her in utero. But nobody listens to me or orders the tests that will prove I am right.” He insists on obscure and specialized blood tests and immune function panels to prove that a congenital condition is causing his daughter’s deterioration and physical debility. He is unwilling to accept that there is any other cause of her condition.
Cassandra’s father is unemployed and has no social contacts or supports. He asserts that “the medical system” is against him, and he believes medical interventions are harming his daughter. He keeps Cassandra isolated from friends and other family members.
How would you proceed?
a) separate Cassandra from her father during the interview
b) contact Cassandra’s mother for collateral information
c) assure Cassandra that there is no medical cause for her physical condition
d) order the testing her father requests
EVALUATION: Demoralized, hopeless
Cassandra is uncooperative with the interview and answers questions with one-word answers. Her affect is irritable and her demeanor is frustrated. She does not seem concerned that she needs assistance with eating and toileting.
When outpatient treatment with her primary care physician did not stop her physical deterioration, she was referred to a tertiary care academic medical center for a complete medical and neurologic workup. The workup, including an MRI, electroencephalogram, nerve conduction studies, and full immunologic panels, was negative for any physical illness, including neuromuscular degenerative disease. A muscle biopsy was considered, but not ordered because Cassandra and her father resisted.
During this hospitalization, she was diagnosed with conversion disorder by the psychiatry consultation service, and transferred to a physical rehabilitation facility for further care. At the rehab facility, Cassandra’s father interfered with her care, arguing constantly with the medical team. Cassandra demonstrated no effort to work with physical or occupational therapy and was discharged after 2 weeks because of noncompliance with treatment. Cassandra and her father are resentful that no physical cause was found and feel that the medical workup and time at the rehabilitation facility made her condition worse. The rehabilitation hospital referred Cassandra to an outpatient child psychiatrist for follow-up.
During the intake evaluation and follow-up appointments with the child psychiatrist, her affect is negativistic and restrictive. She is resistant to talking about her condition and accepting psychotherapeutic interventions. She is quick to blame others for her lack of progress and unable to take responsibility for working on her treatment plan. Cassandra feels demoralized, depressed, and hopeless about her situation and prospects for recovery. She feels that no one is listening to her father and if “they did just the tests he wants, we will know what is wrong with me and that he is right.”
The author’s observations
Table 1 lists conditions to consider in the differential diagnosis of conversion disorder. Although Cassandra’s conversion disorder diagnosis appears to be appropriate, it is important to consider 2 other possibilities: delusional disorder, somatic type with familial features, and Munchausen syndrome by proxy. An underlying depressive or anxiety disorder also should be considered and treated appropriately.
Conversion disorder has a challenging and often complex presentation in children and adolescents. Conversion disorders in children commonly are associated with stressful family situations including divorce, marital conflict, or loss of a close family member.1 An overbearing and conflict-prone parenting style also is associated with childhood conversion disorders.2 Common physical symptoms in conversion disorder are functional abdominal pain, partial paralysis, numbness, or seizures. Individuals such as Cassandra who are unable to express or verbalize their emotional distress are vulnerable to expressing their distress in somatic symptoms. Cassandra demonstrates La belle indifference, the characteristic attitude of not being overly concerned about what others would consider an alarming functional impairment.
Delusional disorder. A diagnosis of delusional disorder, somatic type with familial features was considered because Cassandra and her father shared persecutory and paranoid beliefs that her condition was brought on by some hidden, unrecognized medical condition. A delusional disorder with shared or “familial” features develops when a parent has strongly held delusional beliefs that are transferred to the child. Typically, it develops within the context of a close relationship with the parent, is similar in content to the parent’s belief, and is not preceded by psychosis or prodromal to schizophrenia.3
Because Cassandra’s father transferred his delusional system to his daughter, she clung to the belief that her physical symptoms and immobility were caused by medical misdiagnosis and failure to recognize her illness. Cassandra’s father strongly resisted and defended against accepting his role in her medical condition.
Munchausen by proxy. Because Cassandra and her father share a delusional system that prevented her from accepting and following treatment recommendations, it is possible that her father created her condition. Munchausen syndrome by proxy is a condition whereby illness-producing behavior in a child is exaggerated, fabricated, or induced by a parent or guardian.4 Separating Cassandra from her father and initiating antipsychotic treatment for him are critical considerations for her recovery.
How would you treat Cassandra?
a) call Child Protective Services (CPS) to remove Cassandra from her father’s custody
b) hospitalize Cassandra for intensive treatment of conversion disorder
c) start Cassandra on an atypical antipsychotic
d) begin cognitive-behavioral therapy (CBT) and an antidepressant
Treatment approach
Treating a patient with a conversion disorder, somatic type starts with validating that the patient’s and parent’s distress is real to them (Table 2).5 The clinician acknowledges that no physical evidence of physiological dysfunction has been found, which can be reassuring to the patient and family. The clinician then states that the patient’s condition and the physical manifestation of the symptoms are real. A patient’s or parent’s resistance to this reassurance may indicate that they have a large investment in the symptoms and perpetuating the dysfunction.
Taking a mind-body approach—explaining that the child’s condition is created and perpetuated by a mind-body connection and is not under their voluntary control—often is well received by patients and parents. The treating clinician emphasizes that the condition is physically disabling and that careful, appropriate, and intensive treatment is necessary.
A rehabilitation model has power for patients with conversion disorder because it acknowledges the patient’s discomfort and loss of function while shifting the focus away from finding what is wrong. The goal is to actively engage patients in their own care to help them return to normal functioning.6
Cassandra was encouraged to participate in physical therapy, go to school, and take care of herself. Actively participating in her care and recovery meant that Cassandra had to leave the sick role behind, which was impossible for her father, who saw her as passive, helpless, and fragile.
TREATMENT: Pharmacotherapy, CBT
During psychiatric evaluation, it becomes clear that in addition to her physical debility, Cassandra has major depressive disorder, moderate without psychotic features. Her depression contributes to her hopelessness and lack of participation in treatment. After discussion with her family about how her depressive symptoms are preventing her recovery, Cassandra is started on escitalopram, 10 mg/d. CBT helps her manage her depressive symptoms, prevent further somatization, and correct misperceptions about her body function and disabilities.
For conversion disorder patients, physical therapy can be combined with incentives tied to improvements in functioning. Cassandra has overwhelming anxiety while attempting physical therapy, which interferes with her participation in the therapy. Lorazepam, 0.5 mg/d, is prescribed for her intense anxiety and panic attacks, which led her to avoid physical therapy.
Staff at the rehabilitation hospital calls CPS because Cassandra’s father interferes with her care and treatment plan. CPS continues to monitor Cassandra’s progress through outpatient care. An individualized education plan and psychoeducational testing help determine a school placement to meet Cassandra’s educational needs.
CPS directs Cassandra to stay with her mother for alternating weeks. While at her mother’s, Cassandra is more interested in taking care of herself. She helps with getting herself into bed and to the toilet. Upon returning to her father’s home, these gains are lost.
The author’s observations
Psychodynamic and unconscious motivators for conversion disorder operate on a deeper, hidden level. The underlying primary conflict in pseudoseizures—a more common conversion disorder—has been described as an inability to express negative emotions such as anger. Social problems, conflict with parents, learning disorders,7 or sexual abuse8 produce the negative emotions caused by the primary conflict. Cassandra yearned for a closer relationship with her mother, yet she remained enmeshed with poor intrapsychic boundaries with her father. The fact that he assisted his 17-year-old daughter with toileting raised the possibility of sexual abuse. Sexual abuse could have led to her depression and physical decline. Cassandra’s physical debility also may have been her way to foster dependency on her father and protect him from perceived persecution.
Conversion disorder may have been a result of Cassandra’s defense mechanisms against admitting abuse and protecting against abandonment. Establishing a therapeutic alliance with Cassandra is essential to allow a graceful exit from the conversion disorder symptoms and her father’s hold on her thinking about her illness. However, this alliance may seem to threaten the child’s special connection with the parent. A therapeutic alliance was elusive in Cassandra’s case and likely nearly impossible.
Both parents underwent court-ordered psychological testing as part of the CPS evaluation. Testing on Cassandra’s father indicated a rigid personality structure with long-standing paranoia and mistrust of authority. Because Cassandra endorsed his delusional system completely, it is likely that her father inculcated her into believing his beliefs and transmitted his delusions to her by their close proximity and time together. Based upon this delusional belief system, Cassandra gave up trying to move her legs and her muscles atrophied. Her legs were so weak that she stopped trying to walk or move, illustrating the power of the mind-body connection to produce functional and physiological changes.
Children who live with a mother with chronic illness are at risk of developing psychosomatic disorders.9 Cassandra’s mother had fibromyalgia and chronic pain with symptoms of headache, weakness, and muscle pain and frequent medical office visits and tests without definitive results or symptom relief. Although Cassandra did not live with her mother, Cassandra’s somatization symptoms may be a result of modeling or observational learning within her family.9 Cassandra may have unconsciously adopted her mother’s symptoms and behaviors as a way to cope with stress and gain attention to her needs.
Cassandra’s negative affect, sensitivity to change, and lack of resiliency were further risk factors for developing a somatoform illness.10 She resisted and would not follow through with physical therapy. Krisnakumar10 also reported that an inability to persist in completing tasks is a risk factor for somatoform disorder. Family dynamics of problematic parental interactions also played a role in her somatoform disorder (Table 3).11
OUTCOME: Foster care, improvement
Cassandra receives weekly CBT and biweekly medication monitoring and demonstrates a moderate improvement in mood with less negativity and irritability. Her anxiety symptoms gradually respond to treatment. However, her emotional gains are not matched with improvement in her physical functioning or participation in physical therapy. Cassandra does not recover her muscular strength or control and shows little improvement in her physical capacity and independence.
After 3 months of treatment, Cassandra does not make sufficient progress or actively participate in treatment. Because her father continues to interfere with the treatment plan and does not receive treatment himself, CPS obtains a court order to prevent her father from directing her medical care and telling her treating physicians which tests to order.
Because these interventions do not improve her treatment response, Cassandra is removed from her parents’ care and placed in a therapeutic foster care home, thereby improving her independence and chances for recovery. After 3 months in foster care, she more actively participates in her physical rehabilitation. Water therapy, with the buoyancy and support in water, helps her regain muscle strength and control of her lower extremities.
Bottom Line
Patients with conversion disorder present with functional impairment and physical symptoms without clear physiological causes. Parents have a strong influence on the presentation and course of conversion disorder in children and adolescents. Parents’ mental and physical illnesses are independent risk factors for childhood somatoform disorders. Evaluation of parents’ psychological and psychiatric state is essential to determine intervention.
Related Resource
- Seltzer WJ. Conversion disorder in childhood and adolescence: a familial/cultural approach. Family Systems Medicine. 1985;3(3):261-280.
Drug Brand Names
Escitalopram • Lexapro
Lorazepam • Ativan
Disclosure
Dr. Leipsic reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Wyllie E, Glazer JP, Benbadis S, et al. Psychiatric features of children and adolescents with pseudoseizures. Arch Pediatr Adolesc Med. 1999;153(3):244-248.
2. Salmon P, Al-Marzooqi SM, Baker G, et al. Childhood family dysfunction and associated abuse in patients with nonepileptic seizures: towards a casual model. Psychosom Med. 2003;65(4):695-700.
3. Manschreck T. Delusional disorder and shared psychotic disorder. In: Sadock BJ, Sadock VA, eds. Kaplan & Sadock’s comprehensive textbook of psychiatry. 7th ed.
Philadelphia, PA: Lippincott Williams & Wilkins; 2000: 1243-1264.
4. Meadow R. Munchausen syndrome by proxy. The hinterland of child abuse. Lancet. 1977;2(8033):343-345.
5. Campo JV, Fritsch SL. Somatization in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1994; 33(9):1223-1235.
6. Campo JV, Fritz G. A management model for pediatric somatization. Psychosomatics. 2001;42(6):467-476.
7. Silver LB. Conversion disorder with pseudoseizures in adolescence: a stress reaction to unrecognized and untreated learning disabilities. J Am Accad Child Psychiatry. 1982; 21(5):508-512.
8. AlperK,DevinskyO,PerrineK,etal.Nonepilepticseizures and childhood sexual and physical abuse. Neurology. 1993; 43(10):1950-1953.
9. Jamison RN, Walker LS. Illness behavior in children of chronic pain patients. Int J Psychiatry Med. 1992;22(4): 329-342.
10. Krisnakumar P, Sumesh P, Mathews L. Tempermental traits associated with conversion disorder. Indian Pediatr. 2006;43(10):895-899.
11. Minuchin S, Rosman BL, Baker L. Psychosomatic families: anorexia nervosa in context. Cambridge, MA: Harvard University Press; 1978.
CASE: Weak and passive
Cassandra, age 17, recently was discharged from a medical rehabilitation facility with a diagnosis of conversion disorder. Her school performance and attendance had been steadily declining for the last 6 months as she lost strength and motivation to take care of herself. Cassandra lives with her father, who is her primary caretaker. Her parents are separated and her mother has fibromyalgia and chronic fatigue syndrome, which leaves her unable to care for her daughter or participate in appointments.
Now lethargic and wasting away physically, Cassandra is pushed in a wheelchair by her weary father into a child psychiatrist’s office. She does not look up or make eye contact. Her father says “the doctors didn’t know what they were doing. That needle test, a nerve conduction study they did, is what made her worse.” Although Cassandra moves her arms to adjust herself in the wheelchair, she does not move her legs or try to move the wheelchair.
Cassandra’s father states that she has “congenital neuromyopathy. Her mother gave it to her in utero. But nobody listens to me or orders the tests that will prove I am right.” He insists on obscure and specialized blood tests and immune function panels to prove that a congenital condition is causing his daughter’s deterioration and physical debility. He is unwilling to accept that there is any other cause of her condition.
Cassandra’s father is unemployed and has no social contacts or supports. He asserts that “the medical system” is against him, and he believes medical interventions are harming his daughter. He keeps Cassandra isolated from friends and other family members.
How would you proceed?
a) separate Cassandra from her father during the interview
b) contact Cassandra’s mother for collateral information
c) assure Cassandra that there is no medical cause for her physical condition
d) order the testing her father requests
EVALUATION: Demoralized, hopeless
Cassandra is uncooperative with the interview and answers questions with one-word answers. Her affect is irritable and her demeanor is frustrated. She does not seem concerned that she needs assistance with eating and toileting.
When outpatient treatment with her primary care physician did not stop her physical deterioration, she was referred to a tertiary care academic medical center for a complete medical and neurologic workup. The workup, including an MRI, electroencephalogram, nerve conduction studies, and full immunologic panels, was negative for any physical illness, including neuromuscular degenerative disease. A muscle biopsy was considered, but not ordered because Cassandra and her father resisted.
During this hospitalization, she was diagnosed with conversion disorder by the psychiatry consultation service, and transferred to a physical rehabilitation facility for further care. At the rehab facility, Cassandra’s father interfered with her care, arguing constantly with the medical team. Cassandra demonstrated no effort to work with physical or occupational therapy and was discharged after 2 weeks because of noncompliance with treatment. Cassandra and her father are resentful that no physical cause was found and feel that the medical workup and time at the rehabilitation facility made her condition worse. The rehabilitation hospital referred Cassandra to an outpatient child psychiatrist for follow-up.
During the intake evaluation and follow-up appointments with the child psychiatrist, her affect is negativistic and restrictive. She is resistant to talking about her condition and accepting psychotherapeutic interventions. She is quick to blame others for her lack of progress and unable to take responsibility for working on her treatment plan. Cassandra feels demoralized, depressed, and hopeless about her situation and prospects for recovery. She feels that no one is listening to her father and if “they did just the tests he wants, we will know what is wrong with me and that he is right.”
The author’s observations
Table 1 lists conditions to consider in the differential diagnosis of conversion disorder. Although Cassandra’s conversion disorder diagnosis appears to be appropriate, it is important to consider 2 other possibilities: delusional disorder, somatic type with familial features, and Munchausen syndrome by proxy. An underlying depressive or anxiety disorder also should be considered and treated appropriately.
Conversion disorder has a challenging and often complex presentation in children and adolescents. Conversion disorders in children commonly are associated with stressful family situations including divorce, marital conflict, or loss of a close family member.1 An overbearing and conflict-prone parenting style also is associated with childhood conversion disorders.2 Common physical symptoms in conversion disorder are functional abdominal pain, partial paralysis, numbness, or seizures. Individuals such as Cassandra who are unable to express or verbalize their emotional distress are vulnerable to expressing their distress in somatic symptoms. Cassandra demonstrates La belle indifference, the characteristic attitude of not being overly concerned about what others would consider an alarming functional impairment.
Delusional disorder. A diagnosis of delusional disorder, somatic type with familial features was considered because Cassandra and her father shared persecutory and paranoid beliefs that her condition was brought on by some hidden, unrecognized medical condition. A delusional disorder with shared or “familial” features develops when a parent has strongly held delusional beliefs that are transferred to the child. Typically, it develops within the context of a close relationship with the parent, is similar in content to the parent’s belief, and is not preceded by psychosis or prodromal to schizophrenia.3
Because Cassandra’s father transferred his delusional system to his daughter, she clung to the belief that her physical symptoms and immobility were caused by medical misdiagnosis and failure to recognize her illness. Cassandra’s father strongly resisted and defended against accepting his role in her medical condition.
Munchausen by proxy. Because Cassandra and her father share a delusional system that prevented her from accepting and following treatment recommendations, it is possible that her father created her condition. Munchausen syndrome by proxy is a condition whereby illness-producing behavior in a child is exaggerated, fabricated, or induced by a parent or guardian.4 Separating Cassandra from her father and initiating antipsychotic treatment for him are critical considerations for her recovery.
How would you treat Cassandra?
a) call Child Protective Services (CPS) to remove Cassandra from her father’s custody
b) hospitalize Cassandra for intensive treatment of conversion disorder
c) start Cassandra on an atypical antipsychotic
d) begin cognitive-behavioral therapy (CBT) and an antidepressant
Treatment approach
Treating a patient with a conversion disorder, somatic type starts with validating that the patient’s and parent’s distress is real to them (Table 2).5 The clinician acknowledges that no physical evidence of physiological dysfunction has been found, which can be reassuring to the patient and family. The clinician then states that the patient’s condition and the physical manifestation of the symptoms are real. A patient’s or parent’s resistance to this reassurance may indicate that they have a large investment in the symptoms and perpetuating the dysfunction.
Taking a mind-body approach—explaining that the child’s condition is created and perpetuated by a mind-body connection and is not under their voluntary control—often is well received by patients and parents. The treating clinician emphasizes that the condition is physically disabling and that careful, appropriate, and intensive treatment is necessary.
A rehabilitation model has power for patients with conversion disorder because it acknowledges the patient’s discomfort and loss of function while shifting the focus away from finding what is wrong. The goal is to actively engage patients in their own care to help them return to normal functioning.6
Cassandra was encouraged to participate in physical therapy, go to school, and take care of herself. Actively participating in her care and recovery meant that Cassandra had to leave the sick role behind, which was impossible for her father, who saw her as passive, helpless, and fragile.
TREATMENT: Pharmacotherapy, CBT
During psychiatric evaluation, it becomes clear that in addition to her physical debility, Cassandra has major depressive disorder, moderate without psychotic features. Her depression contributes to her hopelessness and lack of participation in treatment. After discussion with her family about how her depressive symptoms are preventing her recovery, Cassandra is started on escitalopram, 10 mg/d. CBT helps her manage her depressive symptoms, prevent further somatization, and correct misperceptions about her body function and disabilities.
For conversion disorder patients, physical therapy can be combined with incentives tied to improvements in functioning. Cassandra has overwhelming anxiety while attempting physical therapy, which interferes with her participation in the therapy. Lorazepam, 0.5 mg/d, is prescribed for her intense anxiety and panic attacks, which led her to avoid physical therapy.
Staff at the rehabilitation hospital calls CPS because Cassandra’s father interferes with her care and treatment plan. CPS continues to monitor Cassandra’s progress through outpatient care. An individualized education plan and psychoeducational testing help determine a school placement to meet Cassandra’s educational needs.
CPS directs Cassandra to stay with her mother for alternating weeks. While at her mother’s, Cassandra is more interested in taking care of herself. She helps with getting herself into bed and to the toilet. Upon returning to her father’s home, these gains are lost.
The author’s observations
Psychodynamic and unconscious motivators for conversion disorder operate on a deeper, hidden level. The underlying primary conflict in pseudoseizures—a more common conversion disorder—has been described as an inability to express negative emotions such as anger. Social problems, conflict with parents, learning disorders,7 or sexual abuse8 produce the negative emotions caused by the primary conflict. Cassandra yearned for a closer relationship with her mother, yet she remained enmeshed with poor intrapsychic boundaries with her father. The fact that he assisted his 17-year-old daughter with toileting raised the possibility of sexual abuse. Sexual abuse could have led to her depression and physical decline. Cassandra’s physical debility also may have been her way to foster dependency on her father and protect him from perceived persecution.
Conversion disorder may have been a result of Cassandra’s defense mechanisms against admitting abuse and protecting against abandonment. Establishing a therapeutic alliance with Cassandra is essential to allow a graceful exit from the conversion disorder symptoms and her father’s hold on her thinking about her illness. However, this alliance may seem to threaten the child’s special connection with the parent. A therapeutic alliance was elusive in Cassandra’s case and likely nearly impossible.
Both parents underwent court-ordered psychological testing as part of the CPS evaluation. Testing on Cassandra’s father indicated a rigid personality structure with long-standing paranoia and mistrust of authority. Because Cassandra endorsed his delusional system completely, it is likely that her father inculcated her into believing his beliefs and transmitted his delusions to her by their close proximity and time together. Based upon this delusional belief system, Cassandra gave up trying to move her legs and her muscles atrophied. Her legs were so weak that she stopped trying to walk or move, illustrating the power of the mind-body connection to produce functional and physiological changes.
Children who live with a mother with chronic illness are at risk of developing psychosomatic disorders.9 Cassandra’s mother had fibromyalgia and chronic pain with symptoms of headache, weakness, and muscle pain and frequent medical office visits and tests without definitive results or symptom relief. Although Cassandra did not live with her mother, Cassandra’s somatization symptoms may be a result of modeling or observational learning within her family.9 Cassandra may have unconsciously adopted her mother’s symptoms and behaviors as a way to cope with stress and gain attention to her needs.
Cassandra’s negative affect, sensitivity to change, and lack of resiliency were further risk factors for developing a somatoform illness.10 She resisted and would not follow through with physical therapy. Krisnakumar10 also reported that an inability to persist in completing tasks is a risk factor for somatoform disorder. Family dynamics of problematic parental interactions also played a role in her somatoform disorder (Table 3).11
OUTCOME: Foster care, improvement
Cassandra receives weekly CBT and biweekly medication monitoring and demonstrates a moderate improvement in mood with less negativity and irritability. Her anxiety symptoms gradually respond to treatment. However, her emotional gains are not matched with improvement in her physical functioning or participation in physical therapy. Cassandra does not recover her muscular strength or control and shows little improvement in her physical capacity and independence.
After 3 months of treatment, Cassandra does not make sufficient progress or actively participate in treatment. Because her father continues to interfere with the treatment plan and does not receive treatment himself, CPS obtains a court order to prevent her father from directing her medical care and telling her treating physicians which tests to order.
Because these interventions do not improve her treatment response, Cassandra is removed from her parents’ care and placed in a therapeutic foster care home, thereby improving her independence and chances for recovery. After 3 months in foster care, she more actively participates in her physical rehabilitation. Water therapy, with the buoyancy and support in water, helps her regain muscle strength and control of her lower extremities.
Bottom Line
Patients with conversion disorder present with functional impairment and physical symptoms without clear physiological causes. Parents have a strong influence on the presentation and course of conversion disorder in children and adolescents. Parents’ mental and physical illnesses are independent risk factors for childhood somatoform disorders. Evaluation of parents’ psychological and psychiatric state is essential to determine intervention.
Related Resource
- Seltzer WJ. Conversion disorder in childhood and adolescence: a familial/cultural approach. Family Systems Medicine. 1985;3(3):261-280.
Drug Brand Names
Escitalopram • Lexapro
Lorazepam • Ativan
Disclosure
Dr. Leipsic reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Weak and passive
Cassandra, age 17, recently was discharged from a medical rehabilitation facility with a diagnosis of conversion disorder. Her school performance and attendance had been steadily declining for the last 6 months as she lost strength and motivation to take care of herself. Cassandra lives with her father, who is her primary caretaker. Her parents are separated and her mother has fibromyalgia and chronic fatigue syndrome, which leaves her unable to care for her daughter or participate in appointments.
Now lethargic and wasting away physically, Cassandra is pushed in a wheelchair by her weary father into a child psychiatrist’s office. She does not look up or make eye contact. Her father says “the doctors didn’t know what they were doing. That needle test, a nerve conduction study they did, is what made her worse.” Although Cassandra moves her arms to adjust herself in the wheelchair, she does not move her legs or try to move the wheelchair.
Cassandra’s father states that she has “congenital neuromyopathy. Her mother gave it to her in utero. But nobody listens to me or orders the tests that will prove I am right.” He insists on obscure and specialized blood tests and immune function panels to prove that a congenital condition is causing his daughter’s deterioration and physical debility. He is unwilling to accept that there is any other cause of her condition.
Cassandra’s father is unemployed and has no social contacts or supports. He asserts that “the medical system” is against him, and he believes medical interventions are harming his daughter. He keeps Cassandra isolated from friends and other family members.
How would you proceed?
a) separate Cassandra from her father during the interview
b) contact Cassandra’s mother for collateral information
c) assure Cassandra that there is no medical cause for her physical condition
d) order the testing her father requests
EVALUATION: Demoralized, hopeless
Cassandra is uncooperative with the interview and answers questions with one-word answers. Her affect is irritable and her demeanor is frustrated. She does not seem concerned that she needs assistance with eating and toileting.
When outpatient treatment with her primary care physician did not stop her physical deterioration, she was referred to a tertiary care academic medical center for a complete medical and neurologic workup. The workup, including an MRI, electroencephalogram, nerve conduction studies, and full immunologic panels, was negative for any physical illness, including neuromuscular degenerative disease. A muscle biopsy was considered, but not ordered because Cassandra and her father resisted.
During this hospitalization, she was diagnosed with conversion disorder by the psychiatry consultation service, and transferred to a physical rehabilitation facility for further care. At the rehab facility, Cassandra’s father interfered with her care, arguing constantly with the medical team. Cassandra demonstrated no effort to work with physical or occupational therapy and was discharged after 2 weeks because of noncompliance with treatment. Cassandra and her father are resentful that no physical cause was found and feel that the medical workup and time at the rehabilitation facility made her condition worse. The rehabilitation hospital referred Cassandra to an outpatient child psychiatrist for follow-up.
During the intake evaluation and follow-up appointments with the child psychiatrist, her affect is negativistic and restrictive. She is resistant to talking about her condition and accepting psychotherapeutic interventions. She is quick to blame others for her lack of progress and unable to take responsibility for working on her treatment plan. Cassandra feels demoralized, depressed, and hopeless about her situation and prospects for recovery. She feels that no one is listening to her father and if “they did just the tests he wants, we will know what is wrong with me and that he is right.”
The author’s observations
Table 1 lists conditions to consider in the differential diagnosis of conversion disorder. Although Cassandra’s conversion disorder diagnosis appears to be appropriate, it is important to consider 2 other possibilities: delusional disorder, somatic type with familial features, and Munchausen syndrome by proxy. An underlying depressive or anxiety disorder also should be considered and treated appropriately.
Conversion disorder has a challenging and often complex presentation in children and adolescents. Conversion disorders in children commonly are associated with stressful family situations including divorce, marital conflict, or loss of a close family member.1 An overbearing and conflict-prone parenting style also is associated with childhood conversion disorders.2 Common physical symptoms in conversion disorder are functional abdominal pain, partial paralysis, numbness, or seizures. Individuals such as Cassandra who are unable to express or verbalize their emotional distress are vulnerable to expressing their distress in somatic symptoms. Cassandra demonstrates La belle indifference, the characteristic attitude of not being overly concerned about what others would consider an alarming functional impairment.
Delusional disorder. A diagnosis of delusional disorder, somatic type with familial features was considered because Cassandra and her father shared persecutory and paranoid beliefs that her condition was brought on by some hidden, unrecognized medical condition. A delusional disorder with shared or “familial” features develops when a parent has strongly held delusional beliefs that are transferred to the child. Typically, it develops within the context of a close relationship with the parent, is similar in content to the parent’s belief, and is not preceded by psychosis or prodromal to schizophrenia.3
Because Cassandra’s father transferred his delusional system to his daughter, she clung to the belief that her physical symptoms and immobility were caused by medical misdiagnosis and failure to recognize her illness. Cassandra’s father strongly resisted and defended against accepting his role in her medical condition.
Munchausen by proxy. Because Cassandra and her father share a delusional system that prevented her from accepting and following treatment recommendations, it is possible that her father created her condition. Munchausen syndrome by proxy is a condition whereby illness-producing behavior in a child is exaggerated, fabricated, or induced by a parent or guardian.4 Separating Cassandra from her father and initiating antipsychotic treatment for him are critical considerations for her recovery.
How would you treat Cassandra?
a) call Child Protective Services (CPS) to remove Cassandra from her father’s custody
b) hospitalize Cassandra for intensive treatment of conversion disorder
c) start Cassandra on an atypical antipsychotic
d) begin cognitive-behavioral therapy (CBT) and an antidepressant
Treatment approach
Treating a patient with a conversion disorder, somatic type starts with validating that the patient’s and parent’s distress is real to them (Table 2).5 The clinician acknowledges that no physical evidence of physiological dysfunction has been found, which can be reassuring to the patient and family. The clinician then states that the patient’s condition and the physical manifestation of the symptoms are real. A patient’s or parent’s resistance to this reassurance may indicate that they have a large investment in the symptoms and perpetuating the dysfunction.
Taking a mind-body approach—explaining that the child’s condition is created and perpetuated by a mind-body connection and is not under their voluntary control—often is well received by patients and parents. The treating clinician emphasizes that the condition is physically disabling and that careful, appropriate, and intensive treatment is necessary.
A rehabilitation model has power for patients with conversion disorder because it acknowledges the patient’s discomfort and loss of function while shifting the focus away from finding what is wrong. The goal is to actively engage patients in their own care to help them return to normal functioning.6
Cassandra was encouraged to participate in physical therapy, go to school, and take care of herself. Actively participating in her care and recovery meant that Cassandra had to leave the sick role behind, which was impossible for her father, who saw her as passive, helpless, and fragile.
TREATMENT: Pharmacotherapy, CBT
During psychiatric evaluation, it becomes clear that in addition to her physical debility, Cassandra has major depressive disorder, moderate without psychotic features. Her depression contributes to her hopelessness and lack of participation in treatment. After discussion with her family about how her depressive symptoms are preventing her recovery, Cassandra is started on escitalopram, 10 mg/d. CBT helps her manage her depressive symptoms, prevent further somatization, and correct misperceptions about her body function and disabilities.
For conversion disorder patients, physical therapy can be combined with incentives tied to improvements in functioning. Cassandra has overwhelming anxiety while attempting physical therapy, which interferes with her participation in the therapy. Lorazepam, 0.5 mg/d, is prescribed for her intense anxiety and panic attacks, which led her to avoid physical therapy.
Staff at the rehabilitation hospital calls CPS because Cassandra’s father interferes with her care and treatment plan. CPS continues to monitor Cassandra’s progress through outpatient care. An individualized education plan and psychoeducational testing help determine a school placement to meet Cassandra’s educational needs.
CPS directs Cassandra to stay with her mother for alternating weeks. While at her mother’s, Cassandra is more interested in taking care of herself. She helps with getting herself into bed and to the toilet. Upon returning to her father’s home, these gains are lost.
The author’s observations
Psychodynamic and unconscious motivators for conversion disorder operate on a deeper, hidden level. The underlying primary conflict in pseudoseizures—a more common conversion disorder—has been described as an inability to express negative emotions such as anger. Social problems, conflict with parents, learning disorders,7 or sexual abuse8 produce the negative emotions caused by the primary conflict. Cassandra yearned for a closer relationship with her mother, yet she remained enmeshed with poor intrapsychic boundaries with her father. The fact that he assisted his 17-year-old daughter with toileting raised the possibility of sexual abuse. Sexual abuse could have led to her depression and physical decline. Cassandra’s physical debility also may have been her way to foster dependency on her father and protect him from perceived persecution.
Conversion disorder may have been a result of Cassandra’s defense mechanisms against admitting abuse and protecting against abandonment. Establishing a therapeutic alliance with Cassandra is essential to allow a graceful exit from the conversion disorder symptoms and her father’s hold on her thinking about her illness. However, this alliance may seem to threaten the child’s special connection with the parent. A therapeutic alliance was elusive in Cassandra’s case and likely nearly impossible.
Both parents underwent court-ordered psychological testing as part of the CPS evaluation. Testing on Cassandra’s father indicated a rigid personality structure with long-standing paranoia and mistrust of authority. Because Cassandra endorsed his delusional system completely, it is likely that her father inculcated her into believing his beliefs and transmitted his delusions to her by their close proximity and time together. Based upon this delusional belief system, Cassandra gave up trying to move her legs and her muscles atrophied. Her legs were so weak that she stopped trying to walk or move, illustrating the power of the mind-body connection to produce functional and physiological changes.
Children who live with a mother with chronic illness are at risk of developing psychosomatic disorders.9 Cassandra’s mother had fibromyalgia and chronic pain with symptoms of headache, weakness, and muscle pain and frequent medical office visits and tests without definitive results or symptom relief. Although Cassandra did not live with her mother, Cassandra’s somatization symptoms may be a result of modeling or observational learning within her family.9 Cassandra may have unconsciously adopted her mother’s symptoms and behaviors as a way to cope with stress and gain attention to her needs.
Cassandra’s negative affect, sensitivity to change, and lack of resiliency were further risk factors for developing a somatoform illness.10 She resisted and would not follow through with physical therapy. Krisnakumar10 also reported that an inability to persist in completing tasks is a risk factor for somatoform disorder. Family dynamics of problematic parental interactions also played a role in her somatoform disorder (Table 3).11
OUTCOME: Foster care, improvement
Cassandra receives weekly CBT and biweekly medication monitoring and demonstrates a moderate improvement in mood with less negativity and irritability. Her anxiety symptoms gradually respond to treatment. However, her emotional gains are not matched with improvement in her physical functioning or participation in physical therapy. Cassandra does not recover her muscular strength or control and shows little improvement in her physical capacity and independence.
After 3 months of treatment, Cassandra does not make sufficient progress or actively participate in treatment. Because her father continues to interfere with the treatment plan and does not receive treatment himself, CPS obtains a court order to prevent her father from directing her medical care and telling her treating physicians which tests to order.
Because these interventions do not improve her treatment response, Cassandra is removed from her parents’ care and placed in a therapeutic foster care home, thereby improving her independence and chances for recovery. After 3 months in foster care, she more actively participates in her physical rehabilitation. Water therapy, with the buoyancy and support in water, helps her regain muscle strength and control of her lower extremities.
Bottom Line
Patients with conversion disorder present with functional impairment and physical symptoms without clear physiological causes. Parents have a strong influence on the presentation and course of conversion disorder in children and adolescents. Parents’ mental and physical illnesses are independent risk factors for childhood somatoform disorders. Evaluation of parents’ psychological and psychiatric state is essential to determine intervention.
Related Resource
- Seltzer WJ. Conversion disorder in childhood and adolescence: a familial/cultural approach. Family Systems Medicine. 1985;3(3):261-280.
Drug Brand Names
Escitalopram • Lexapro
Lorazepam • Ativan
Disclosure
Dr. Leipsic reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Wyllie E, Glazer JP, Benbadis S, et al. Psychiatric features of children and adolescents with pseudoseizures. Arch Pediatr Adolesc Med. 1999;153(3):244-248.
2. Salmon P, Al-Marzooqi SM, Baker G, et al. Childhood family dysfunction and associated abuse in patients with nonepileptic seizures: towards a casual model. Psychosom Med. 2003;65(4):695-700.
3. Manschreck T. Delusional disorder and shared psychotic disorder. In: Sadock BJ, Sadock VA, eds. Kaplan & Sadock’s comprehensive textbook of psychiatry. 7th ed.
Philadelphia, PA: Lippincott Williams & Wilkins; 2000: 1243-1264.
4. Meadow R. Munchausen syndrome by proxy. The hinterland of child abuse. Lancet. 1977;2(8033):343-345.
5. Campo JV, Fritsch SL. Somatization in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1994; 33(9):1223-1235.
6. Campo JV, Fritz G. A management model for pediatric somatization. Psychosomatics. 2001;42(6):467-476.
7. Silver LB. Conversion disorder with pseudoseizures in adolescence: a stress reaction to unrecognized and untreated learning disabilities. J Am Accad Child Psychiatry. 1982; 21(5):508-512.
8. AlperK,DevinskyO,PerrineK,etal.Nonepilepticseizures and childhood sexual and physical abuse. Neurology. 1993; 43(10):1950-1953.
9. Jamison RN, Walker LS. Illness behavior in children of chronic pain patients. Int J Psychiatry Med. 1992;22(4): 329-342.
10. Krisnakumar P, Sumesh P, Mathews L. Tempermental traits associated with conversion disorder. Indian Pediatr. 2006;43(10):895-899.
11. Minuchin S, Rosman BL, Baker L. Psychosomatic families: anorexia nervosa in context. Cambridge, MA: Harvard University Press; 1978.
1. Wyllie E, Glazer JP, Benbadis S, et al. Psychiatric features of children and adolescents with pseudoseizures. Arch Pediatr Adolesc Med. 1999;153(3):244-248.
2. Salmon P, Al-Marzooqi SM, Baker G, et al. Childhood family dysfunction and associated abuse in patients with nonepileptic seizures: towards a casual model. Psychosom Med. 2003;65(4):695-700.
3. Manschreck T. Delusional disorder and shared psychotic disorder. In: Sadock BJ, Sadock VA, eds. Kaplan & Sadock’s comprehensive textbook of psychiatry. 7th ed.
Philadelphia, PA: Lippincott Williams & Wilkins; 2000: 1243-1264.
4. Meadow R. Munchausen syndrome by proxy. The hinterland of child abuse. Lancet. 1977;2(8033):343-345.
5. Campo JV, Fritsch SL. Somatization in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1994; 33(9):1223-1235.
6. Campo JV, Fritz G. A management model for pediatric somatization. Psychosomatics. 2001;42(6):467-476.
7. Silver LB. Conversion disorder with pseudoseizures in adolescence: a stress reaction to unrecognized and untreated learning disabilities. J Am Accad Child Psychiatry. 1982; 21(5):508-512.
8. AlperK,DevinskyO,PerrineK,etal.Nonepilepticseizures and childhood sexual and physical abuse. Neurology. 1993; 43(10):1950-1953.
9. Jamison RN, Walker LS. Illness behavior in children of chronic pain patients. Int J Psychiatry Med. 1992;22(4): 329-342.
10. Krisnakumar P, Sumesh P, Mathews L. Tempermental traits associated with conversion disorder. Indian Pediatr. 2006;43(10):895-899.
11. Minuchin S, Rosman BL, Baker L. Psychosomatic families: anorexia nervosa in context. Cambridge, MA: Harvard University Press; 1978.
Psychotic and sexually deviant
CASE: Paranoid and distressed
Mr. P, age 21, is a single, white college student who presents to a psychiatric emergency room with his father at his psychotherapist’s recommendation. The psychotherapist, who has been treating Mr. P for anxiety and depression, recommended he be evaluated because of increased erratic behavior and paranoia. Mr. P reports that he has been feeling increasingly “anxious” and “paranoid” and thinks the security cameras at his college have been following him. He also describes an increased connection with God and hearing God’s voice as a commentary on his behaviors. Mr. P denies euphoria, depression, increased goal-directed activities, distractibility, increased impulsivity, or rapid speech. He is admitted voluntarily to the psychiatric unit for further evaluation.
During the hospitalization, Mr. P discloses that he has been viewing child pornography for 2 years, and during the past 6 months he has been distressed by the intensity of his sexual fantasies involving sexual contact with prepubescent girls. He also continues to experience paranoia and increased religiosity.
Mr. P says he began looking at pornography on the internet at age 14. He says he was watching “regular straight porn” and he would use it to masturbate and achieve orgasm. Mr. P began looking at child pornography at age 19. He stated that “regular porn” was no longer sufficiently arousing for him. Mr. P explains, “First, I started looking for 15- or 16-year-olds. They would work for a while [referring to sexual gratification], but then I would look for younger girls.” He says the images of younger girls are sexually arousing, typically “young girls, 8 to 10 years old” who are nude or involved in sex acts.
Mr. P denies sexual contact with prepubescent individuals and says his thoughts about such contact are “distressing.” He reports that he has viewed child pornography even when he wasn’t experiencing psychotic or mood symptoms. Mr. P’s outpatient psychotherapist reports that Mr. P first disclosed viewing child pornography and his attraction to prepubescent girls 2 years before this admission.
The authors’ observations
DSM-IV-TR diagnostic criteria for pedophilia (Table 1)1 are based on a history of sexual arousal to prepubescent individuals. A subset of sex offenders meet criteria for a paraphilia (Table 2),1 an axis I disorder, and a subset of sex offenders with paraphilia meet diagnostic criteria for pedophilia. Dunsieth et al2 found that among a sample of 113 male sex offenders, 74% had a diagnosable paraphilia, and 50% of individuals with paraphilia met criteria for pedophilia.
Table 1
DSM-IV-TR diagnostic criteria for pedophilia
| A) | Over a period of ≥6 months, recurrent, intense sexually arousing fantasies, sexual urges, or behaviors involving sexual activity with a prepubescent child or children (generally age ≤13) |
| B) | The person has acted on these sexual urges, or the sexual urges or fantasies cause marked distress or interpersonal difficulty |
| C) | The person is age ≥16 and ≥5 years older than the child or children in criterion A |
| Note: Do not include an individual in late adolescence involved in an ongoing sexual relationship with a 12- or 13-year-old | |
| Source: Reference 1 | |
DSM-IV-TR diagnostic criteria for a paraphilia
| The essential features of a paraphilia are recurrent, intense sexually arousing fantasies, sexual urges, or behaviors generally involving: | |
| A) | nonhuman objects, the suffering or humiliation of oneself or one’s partner, or children or other nonconsenting persons that occur over a period of ≥6 months |
| B) | The behavior, sexual urges, or fantasies cause clinically significant distress or impairment in social, occupational, or other important areas of functioning |
| Source: Reference 1 | |
Although most schizophrenia patients without a history of sexual offenses do not exhibit sexual deviancy, sexual content in hallucinations and delusions is common.6 Confusion about sexual identity and the boundaries of one’s body are common and may contribute to sexual deviancy.6 Psychiatric inpatients without a history of sexual offenses—including but not limited to psychotic patients—have higher rates of sexually deviant fantasies and behaviors compared with those without psychiatric illness.6 In one survey, 15% of men with schizophrenia displayed paraphilic behaviors and 20% had atypical sexual thoughts.7
Alish et al4 found that pedophilia was not necessarily linked to psychotic behavior or antisocial personality features when comparing pedophilia rates in individuals with or without schizophrenia. In a sample of 22 adolescent males who sexually molested a child at least once, axis I morbidity was common, and 55% met criteria for bipolar disorder.8
Few experts in paraphilias
A patient who endorses deviant sexual fantasies should be evaluated by a mental health professional with specialized training in paraphilias. Although paraphilias are not recognized as a subspecialty in psychiatry, diagnosing and treating patients with a paraphilia requires additional training. There is a scarcity of psychiatrists trained to evaluate and treat patients with paraphilias.
Sexual evaluation. Evaluating a patient who presents with problematic sexual behaviors includes performing a comprehensive psychiatric history with a focus on sexual history. A psychosexual history is distinct from general psychiatric evaluations because of the level of detail regarding a sexual history (Table 3). In addition to the clinical interview, objective testing to determine sexual interests may be useful in some patients (Table 4).9
Actuarial tools—risk assessment instruments based on statistically significant risk factors—are valid tools for determining the risk of sexual reoffending. There are several validated actuarial tools in the assessment of sex offender recidivism, such as the Static-99R,10 Stable-2007,11 and the Sex Offender Risk Appraisal Guide.12 However, these tools are used for sex offenders, and would not be used for individuals who have not committed a sex offense, such as Mr. P.
Table 3
Psychosexual evaluation
| Aspect of evaluation | Measures |
| Sexual behavior history | History of sexual abuse Childhood exposure to sex Masturbation history Preferred sexual partners Kinsey Scale |
| Sexual addiction or compulsion | Total Sexual Outlet measure Amount of time in sexual fantasy Financial, legal, or social cost of sexual behavior Prior treatment of sexual behavior |
| Sexual interests | Sex, age, and number of partner(s) Review of criteria for all paraphilias (exposing, voyeurism, cross-dressing, sadistic or masochistic interests) |
Objective testing to determine sexual interests
| Test | Results |
|---|---|
| Penile plethysmograph | Measures penis circumference with a mercury-in-rubber strain gauge. Used clinically by measuring circumferential changes in the penis while the patient is listening to audio or video stimuli of various sexual vignettes |
| Abel Assessment for Sexual Interests-3 | An objective method for evaluating deviant sexual interest uses noninvasive means to achieve objective measures of sexual interest. The subject’s visual response time is measured while viewing images of males and females of varying age. Visual reaction time is correlated with sexual interests |
| Source: Reference 9 | |
Medicolegal aspects of a psychosexual evaluation may include mandated reporting, confidentiality, and documentation. Mental health professionals are mandated to report to law enforcement or child welfare agencies when they observe or suspect physical, sexual, or other types of abuse in vulnerable populations such as children. In psychosexual evaluations, the evaluator is legally required to report if a patient discloses current sexual behavior with a child with a plan to continue to engage in the behavior. In Mr. P’s case, there was no duty to report because although he described viewing child pornography and had a sexual interest in prepubescent individuals, he did not report a history of engaging in handson sexual behaviors with children or impulses to do so. When an individual has engaged in sexual contact with a prepubescent individual, reporting is not mandated unless the individual continues to engage in sexual behavior with a minor. Mental health professionals are not responsible for calling the police or alerting authorities after a crime has been committed.
The relationship between viewing child pornography and pedophilia is unclear. Some child pornography viewers are pedophilic, others are sexually compulsive, and others are viewing out of curiosity and have no sexual deviance. Seto et al13 suggested that child pornography offenders show greater sexual arousal to children than to adults. Persistent child pornography use is a stronger diagnostic indicator of pedophilia than sexually offending against child victims.13 A clinician who learns that a patient is viewing child pornography should take a detailed sexual history, including a review of criteria for paraphilias. In addition, when appropriate, the clinician should perform a risk assessment to determine the patient’s risk of engaging in sexual offenses with children.
OUTCOME: Expert consultation
We start Mr. P on risperidone, 1 mg/d, to treat his paranoia and request a consultation with an expert in paraphilias to determine if Mr. P has a paraphilia and to discuss treatment options.
Mr. P’s initial diagnosis is psychotic disorder not otherwise specified. His viewing of child pornography and sexual interest in prepubescent individuals is not limited to his current mental status, and these interests persist in the absence of mood and psychotic states. Mr. P’s viewing of child pornography and sexual attraction to prepubescent girls meet the diagnostic criteria for pedophilia. During hospitalization, we educate Mr. P about his diagnoses and need for continued treatment. We refer him to a sexual disorders outpatient clinic, which continues to address his deviant sexual interests.
The authors’ observations
A meta-analysis indicates that a combination of pharmacologic and behavioral treatments coupled with close legal supervision seems to reduce the risk of repeated sexual offenses.14 Legal supervision is a general term to describe oversight of offenders in the community by supervisory boards, such as probation or parole, and tracking devices such as GPS. Currently, pedophilia treatment focuses on minimizing deviant sexual arousal through behavioral modification, cognitive-behavioral therapies, and testosterone-lowering medications, such as medroxyprogesterone or leuprolide. The decision to prescribe testosterone-lowering medication should be based on informed consent and the patient’s risk of dangerous sexual behaviors.
- Reijnen L, Bulten E, Nijman H. Demographic and personality characteristics of internet child pornography downloaders in comparison to other offenders. J Child Sex Abus. 2009;18(6):611-622.
- Hall RC, Hall RC. A profile of pedophilia: definition, characteristics of offenders, recidivism, treatment outcomes, and forensic issues. Mayo Clin Proc. 2007;82(4):457-471.
- Leuprolide • Eligard, Lupron
- Medroxyprogesterone • Cycrin, Provera
- Risperidone • Risperdal
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
2. Dunsieth NW, Jr, Nelson EB, Brusman-Lovins LA, et al. Psychiatric and legal features of 113 men convicted of sexual offenses. J Clin Psychiatry. 2004;65(3):293-300.
3. Wallace C, Mullen P, Burgess P, et al. Serious criminal offending and mental disorder. Case linkage study. Br J Psychiatry. 1998;172:477-484.
4. Alish Y, Birger M, Manor N, et al. Schizophrenia sex offenders: a clinical and epidemiological comparison study. Int J Law Psychiatry. 2007;30(6):459-466.
5. Smith AD, Taylor PJ. Serious sex offending against women by men with schizophrenia. Relationship of illness and psychiatric symptoms to offending. Br J Psychiatry. 1999;174:233-237.
6. Drake CR, Pathé M. Understanding sexual offending in schizophrenia. Crim Behav Ment Health. 2004;14(2):108-120.
7. Harley EW, Boardman J, Craig T. Sexual problems in schizophrenia prevalence and characteristics: a cross sectional survey. Soc Psychiatry Psychiatr Epidemiol. 2010;45(7):759-766.
8. Galli V, McElroy SL, Soutullo CA, et al. The psychiatric diagnoses of twenty-two adolescents who have sexually molested other children. Compr Psychiatry. 1999;40(2):85-88.
9. Abel GG, Jordan A, Hand CG, et al. Classification models of child molesters utilizing the Abel Assessment for sexual interest. Child Abuse Negl. 2001;25(5):703-718.
10. Hanson RK, Thornton D. Improving risk assessments for sex offenders: a comparison of three actuarial scales. Law Hum Behav. 2000;24(1):119-136.
11. Hanson RK, Harris AJ, Scott TL, et al. Assessing the risk of sexual offenders on community supervision: The Dynamic Supervision Project. Vol 5. Ottawa, Canada: Public Safety Canada; 2007.
12. Quinsey VL, Harris AJ, Rice ME, et al. Violent offenders: appraising and managing risk. 2nd ed. Washington DC: American Psychological Association; 2006.
13. Seto M, Cantor JM, Blanchard R. Child pornography offenses are a valid diagnostic indicator of pedophilia. J Abnorm Psychol. 2006;115(3):610-615.
14. Thibaut F, De La Barra F, Gordon H, et al. WFSBP Task Force on Sexual Disorders. The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of paraphilias. World J Biol Psychiatry. 2010;11(4):604-655.
CASE: Paranoid and distressed
Mr. P, age 21, is a single, white college student who presents to a psychiatric emergency room with his father at his psychotherapist’s recommendation. The psychotherapist, who has been treating Mr. P for anxiety and depression, recommended he be evaluated because of increased erratic behavior and paranoia. Mr. P reports that he has been feeling increasingly “anxious” and “paranoid” and thinks the security cameras at his college have been following him. He also describes an increased connection with God and hearing God’s voice as a commentary on his behaviors. Mr. P denies euphoria, depression, increased goal-directed activities, distractibility, increased impulsivity, or rapid speech. He is admitted voluntarily to the psychiatric unit for further evaluation.
During the hospitalization, Mr. P discloses that he has been viewing child pornography for 2 years, and during the past 6 months he has been distressed by the intensity of his sexual fantasies involving sexual contact with prepubescent girls. He also continues to experience paranoia and increased religiosity.
Mr. P says he began looking at pornography on the internet at age 14. He says he was watching “regular straight porn” and he would use it to masturbate and achieve orgasm. Mr. P began looking at child pornography at age 19. He stated that “regular porn” was no longer sufficiently arousing for him. Mr. P explains, “First, I started looking for 15- or 16-year-olds. They would work for a while [referring to sexual gratification], but then I would look for younger girls.” He says the images of younger girls are sexually arousing, typically “young girls, 8 to 10 years old” who are nude or involved in sex acts.
Mr. P denies sexual contact with prepubescent individuals and says his thoughts about such contact are “distressing.” He reports that he has viewed child pornography even when he wasn’t experiencing psychotic or mood symptoms. Mr. P’s outpatient psychotherapist reports that Mr. P first disclosed viewing child pornography and his attraction to prepubescent girls 2 years before this admission.
The authors’ observations
DSM-IV-TR diagnostic criteria for pedophilia (Table 1)1 are based on a history of sexual arousal to prepubescent individuals. A subset of sex offenders meet criteria for a paraphilia (Table 2),1 an axis I disorder, and a subset of sex offenders with paraphilia meet diagnostic criteria for pedophilia. Dunsieth et al2 found that among a sample of 113 male sex offenders, 74% had a diagnosable paraphilia, and 50% of individuals with paraphilia met criteria for pedophilia.
Table 1
DSM-IV-TR diagnostic criteria for pedophilia
| A) | Over a period of ≥6 months, recurrent, intense sexually arousing fantasies, sexual urges, or behaviors involving sexual activity with a prepubescent child or children (generally age ≤13) |
| B) | The person has acted on these sexual urges, or the sexual urges or fantasies cause marked distress or interpersonal difficulty |
| C) | The person is age ≥16 and ≥5 years older than the child or children in criterion A |
| Note: Do not include an individual in late adolescence involved in an ongoing sexual relationship with a 12- or 13-year-old | |
| Source: Reference 1 | |
DSM-IV-TR diagnostic criteria for a paraphilia
| The essential features of a paraphilia are recurrent, intense sexually arousing fantasies, sexual urges, or behaviors generally involving: | |
| A) | nonhuman objects, the suffering or humiliation of oneself or one’s partner, or children or other nonconsenting persons that occur over a period of ≥6 months |
| B) | The behavior, sexual urges, or fantasies cause clinically significant distress or impairment in social, occupational, or other important areas of functioning |
| Source: Reference 1 | |
Although most schizophrenia patients without a history of sexual offenses do not exhibit sexual deviancy, sexual content in hallucinations and delusions is common.6 Confusion about sexual identity and the boundaries of one’s body are common and may contribute to sexual deviancy.6 Psychiatric inpatients without a history of sexual offenses—including but not limited to psychotic patients—have higher rates of sexually deviant fantasies and behaviors compared with those without psychiatric illness.6 In one survey, 15% of men with schizophrenia displayed paraphilic behaviors and 20% had atypical sexual thoughts.7
Alish et al4 found that pedophilia was not necessarily linked to psychotic behavior or antisocial personality features when comparing pedophilia rates in individuals with or without schizophrenia. In a sample of 22 adolescent males who sexually molested a child at least once, axis I morbidity was common, and 55% met criteria for bipolar disorder.8
Few experts in paraphilias
A patient who endorses deviant sexual fantasies should be evaluated by a mental health professional with specialized training in paraphilias. Although paraphilias are not recognized as a subspecialty in psychiatry, diagnosing and treating patients with a paraphilia requires additional training. There is a scarcity of psychiatrists trained to evaluate and treat patients with paraphilias.
Sexual evaluation. Evaluating a patient who presents with problematic sexual behaviors includes performing a comprehensive psychiatric history with a focus on sexual history. A psychosexual history is distinct from general psychiatric evaluations because of the level of detail regarding a sexual history (Table 3). In addition to the clinical interview, objective testing to determine sexual interests may be useful in some patients (Table 4).9
Actuarial tools—risk assessment instruments based on statistically significant risk factors—are valid tools for determining the risk of sexual reoffending. There are several validated actuarial tools in the assessment of sex offender recidivism, such as the Static-99R,10 Stable-2007,11 and the Sex Offender Risk Appraisal Guide.12 However, these tools are used for sex offenders, and would not be used for individuals who have not committed a sex offense, such as Mr. P.
Table 3
Psychosexual evaluation
| Aspect of evaluation | Measures |
| Sexual behavior history | History of sexual abuse Childhood exposure to sex Masturbation history Preferred sexual partners Kinsey Scale |
| Sexual addiction or compulsion | Total Sexual Outlet measure Amount of time in sexual fantasy Financial, legal, or social cost of sexual behavior Prior treatment of sexual behavior |
| Sexual interests | Sex, age, and number of partner(s) Review of criteria for all paraphilias (exposing, voyeurism, cross-dressing, sadistic or masochistic interests) |
Objective testing to determine sexual interests
| Test | Results |
|---|---|
| Penile plethysmograph | Measures penis circumference with a mercury-in-rubber strain gauge. Used clinically by measuring circumferential changes in the penis while the patient is listening to audio or video stimuli of various sexual vignettes |
| Abel Assessment for Sexual Interests-3 | An objective method for evaluating deviant sexual interest uses noninvasive means to achieve objective measures of sexual interest. The subject’s visual response time is measured while viewing images of males and females of varying age. Visual reaction time is correlated with sexual interests |
| Source: Reference 9 | |
Medicolegal aspects of a psychosexual evaluation may include mandated reporting, confidentiality, and documentation. Mental health professionals are mandated to report to law enforcement or child welfare agencies when they observe or suspect physical, sexual, or other types of abuse in vulnerable populations such as children. In psychosexual evaluations, the evaluator is legally required to report if a patient discloses current sexual behavior with a child with a plan to continue to engage in the behavior. In Mr. P’s case, there was no duty to report because although he described viewing child pornography and had a sexual interest in prepubescent individuals, he did not report a history of engaging in handson sexual behaviors with children or impulses to do so. When an individual has engaged in sexual contact with a prepubescent individual, reporting is not mandated unless the individual continues to engage in sexual behavior with a minor. Mental health professionals are not responsible for calling the police or alerting authorities after a crime has been committed.
The relationship between viewing child pornography and pedophilia is unclear. Some child pornography viewers are pedophilic, others are sexually compulsive, and others are viewing out of curiosity and have no sexual deviance. Seto et al13 suggested that child pornography offenders show greater sexual arousal to children than to adults. Persistent child pornography use is a stronger diagnostic indicator of pedophilia than sexually offending against child victims.13 A clinician who learns that a patient is viewing child pornography should take a detailed sexual history, including a review of criteria for paraphilias. In addition, when appropriate, the clinician should perform a risk assessment to determine the patient’s risk of engaging in sexual offenses with children.
OUTCOME: Expert consultation
We start Mr. P on risperidone, 1 mg/d, to treat his paranoia and request a consultation with an expert in paraphilias to determine if Mr. P has a paraphilia and to discuss treatment options.
Mr. P’s initial diagnosis is psychotic disorder not otherwise specified. His viewing of child pornography and sexual interest in prepubescent individuals is not limited to his current mental status, and these interests persist in the absence of mood and psychotic states. Mr. P’s viewing of child pornography and sexual attraction to prepubescent girls meet the diagnostic criteria for pedophilia. During hospitalization, we educate Mr. P about his diagnoses and need for continued treatment. We refer him to a sexual disorders outpatient clinic, which continues to address his deviant sexual interests.
The authors’ observations
A meta-analysis indicates that a combination of pharmacologic and behavioral treatments coupled with close legal supervision seems to reduce the risk of repeated sexual offenses.14 Legal supervision is a general term to describe oversight of offenders in the community by supervisory boards, such as probation or parole, and tracking devices such as GPS. Currently, pedophilia treatment focuses on minimizing deviant sexual arousal through behavioral modification, cognitive-behavioral therapies, and testosterone-lowering medications, such as medroxyprogesterone or leuprolide. The decision to prescribe testosterone-lowering medication should be based on informed consent and the patient’s risk of dangerous sexual behaviors.
- Reijnen L, Bulten E, Nijman H. Demographic and personality characteristics of internet child pornography downloaders in comparison to other offenders. J Child Sex Abus. 2009;18(6):611-622.
- Hall RC, Hall RC. A profile of pedophilia: definition, characteristics of offenders, recidivism, treatment outcomes, and forensic issues. Mayo Clin Proc. 2007;82(4):457-471.
- Leuprolide • Eligard, Lupron
- Medroxyprogesterone • Cycrin, Provera
- Risperidone • Risperdal
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Paranoid and distressed
Mr. P, age 21, is a single, white college student who presents to a psychiatric emergency room with his father at his psychotherapist’s recommendation. The psychotherapist, who has been treating Mr. P for anxiety and depression, recommended he be evaluated because of increased erratic behavior and paranoia. Mr. P reports that he has been feeling increasingly “anxious” and “paranoid” and thinks the security cameras at his college have been following him. He also describes an increased connection with God and hearing God’s voice as a commentary on his behaviors. Mr. P denies euphoria, depression, increased goal-directed activities, distractibility, increased impulsivity, or rapid speech. He is admitted voluntarily to the psychiatric unit for further evaluation.
During the hospitalization, Mr. P discloses that he has been viewing child pornography for 2 years, and during the past 6 months he has been distressed by the intensity of his sexual fantasies involving sexual contact with prepubescent girls. He also continues to experience paranoia and increased religiosity.
Mr. P says he began looking at pornography on the internet at age 14. He says he was watching “regular straight porn” and he would use it to masturbate and achieve orgasm. Mr. P began looking at child pornography at age 19. He stated that “regular porn” was no longer sufficiently arousing for him. Mr. P explains, “First, I started looking for 15- or 16-year-olds. They would work for a while [referring to sexual gratification], but then I would look for younger girls.” He says the images of younger girls are sexually arousing, typically “young girls, 8 to 10 years old” who are nude or involved in sex acts.
Mr. P denies sexual contact with prepubescent individuals and says his thoughts about such contact are “distressing.” He reports that he has viewed child pornography even when he wasn’t experiencing psychotic or mood symptoms. Mr. P’s outpatient psychotherapist reports that Mr. P first disclosed viewing child pornography and his attraction to prepubescent girls 2 years before this admission.
The authors’ observations
DSM-IV-TR diagnostic criteria for pedophilia (Table 1)1 are based on a history of sexual arousal to prepubescent individuals. A subset of sex offenders meet criteria for a paraphilia (Table 2),1 an axis I disorder, and a subset of sex offenders with paraphilia meet diagnostic criteria for pedophilia. Dunsieth et al2 found that among a sample of 113 male sex offenders, 74% had a diagnosable paraphilia, and 50% of individuals with paraphilia met criteria for pedophilia.
Table 1
DSM-IV-TR diagnostic criteria for pedophilia
| A) | Over a period of ≥6 months, recurrent, intense sexually arousing fantasies, sexual urges, or behaviors involving sexual activity with a prepubescent child or children (generally age ≤13) |
| B) | The person has acted on these sexual urges, or the sexual urges or fantasies cause marked distress or interpersonal difficulty |
| C) | The person is age ≥16 and ≥5 years older than the child or children in criterion A |
| Note: Do not include an individual in late adolescence involved in an ongoing sexual relationship with a 12- or 13-year-old | |
| Source: Reference 1 | |
DSM-IV-TR diagnostic criteria for a paraphilia
| The essential features of a paraphilia are recurrent, intense sexually arousing fantasies, sexual urges, or behaviors generally involving: | |
| A) | nonhuman objects, the suffering or humiliation of oneself or one’s partner, or children or other nonconsenting persons that occur over a period of ≥6 months |
| B) | The behavior, sexual urges, or fantasies cause clinically significant distress or impairment in social, occupational, or other important areas of functioning |
| Source: Reference 1 | |
Although most schizophrenia patients without a history of sexual offenses do not exhibit sexual deviancy, sexual content in hallucinations and delusions is common.6 Confusion about sexual identity and the boundaries of one’s body are common and may contribute to sexual deviancy.6 Psychiatric inpatients without a history of sexual offenses—including but not limited to psychotic patients—have higher rates of sexually deviant fantasies and behaviors compared with those without psychiatric illness.6 In one survey, 15% of men with schizophrenia displayed paraphilic behaviors and 20% had atypical sexual thoughts.7
Alish et al4 found that pedophilia was not necessarily linked to psychotic behavior or antisocial personality features when comparing pedophilia rates in individuals with or without schizophrenia. In a sample of 22 adolescent males who sexually molested a child at least once, axis I morbidity was common, and 55% met criteria for bipolar disorder.8
Few experts in paraphilias
A patient who endorses deviant sexual fantasies should be evaluated by a mental health professional with specialized training in paraphilias. Although paraphilias are not recognized as a subspecialty in psychiatry, diagnosing and treating patients with a paraphilia requires additional training. There is a scarcity of psychiatrists trained to evaluate and treat patients with paraphilias.
Sexual evaluation. Evaluating a patient who presents with problematic sexual behaviors includes performing a comprehensive psychiatric history with a focus on sexual history. A psychosexual history is distinct from general psychiatric evaluations because of the level of detail regarding a sexual history (Table 3). In addition to the clinical interview, objective testing to determine sexual interests may be useful in some patients (Table 4).9
Actuarial tools—risk assessment instruments based on statistically significant risk factors—are valid tools for determining the risk of sexual reoffending. There are several validated actuarial tools in the assessment of sex offender recidivism, such as the Static-99R,10 Stable-2007,11 and the Sex Offender Risk Appraisal Guide.12 However, these tools are used for sex offenders, and would not be used for individuals who have not committed a sex offense, such as Mr. P.
Table 3
Psychosexual evaluation
| Aspect of evaluation | Measures |
| Sexual behavior history | History of sexual abuse Childhood exposure to sex Masturbation history Preferred sexual partners Kinsey Scale |
| Sexual addiction or compulsion | Total Sexual Outlet measure Amount of time in sexual fantasy Financial, legal, or social cost of sexual behavior Prior treatment of sexual behavior |
| Sexual interests | Sex, age, and number of partner(s) Review of criteria for all paraphilias (exposing, voyeurism, cross-dressing, sadistic or masochistic interests) |
Objective testing to determine sexual interests
| Test | Results |
|---|---|
| Penile plethysmograph | Measures penis circumference with a mercury-in-rubber strain gauge. Used clinically by measuring circumferential changes in the penis while the patient is listening to audio or video stimuli of various sexual vignettes |
| Abel Assessment for Sexual Interests-3 | An objective method for evaluating deviant sexual interest uses noninvasive means to achieve objective measures of sexual interest. The subject’s visual response time is measured while viewing images of males and females of varying age. Visual reaction time is correlated with sexual interests |
| Source: Reference 9 | |
Medicolegal aspects of a psychosexual evaluation may include mandated reporting, confidentiality, and documentation. Mental health professionals are mandated to report to law enforcement or child welfare agencies when they observe or suspect physical, sexual, or other types of abuse in vulnerable populations such as children. In psychosexual evaluations, the evaluator is legally required to report if a patient discloses current sexual behavior with a child with a plan to continue to engage in the behavior. In Mr. P’s case, there was no duty to report because although he described viewing child pornography and had a sexual interest in prepubescent individuals, he did not report a history of engaging in handson sexual behaviors with children or impulses to do so. When an individual has engaged in sexual contact with a prepubescent individual, reporting is not mandated unless the individual continues to engage in sexual behavior with a minor. Mental health professionals are not responsible for calling the police or alerting authorities after a crime has been committed.
The relationship between viewing child pornography and pedophilia is unclear. Some child pornography viewers are pedophilic, others are sexually compulsive, and others are viewing out of curiosity and have no sexual deviance. Seto et al13 suggested that child pornography offenders show greater sexual arousal to children than to adults. Persistent child pornography use is a stronger diagnostic indicator of pedophilia than sexually offending against child victims.13 A clinician who learns that a patient is viewing child pornography should take a detailed sexual history, including a review of criteria for paraphilias. In addition, when appropriate, the clinician should perform a risk assessment to determine the patient’s risk of engaging in sexual offenses with children.
OUTCOME: Expert consultation
We start Mr. P on risperidone, 1 mg/d, to treat his paranoia and request a consultation with an expert in paraphilias to determine if Mr. P has a paraphilia and to discuss treatment options.
Mr. P’s initial diagnosis is psychotic disorder not otherwise specified. His viewing of child pornography and sexual interest in prepubescent individuals is not limited to his current mental status, and these interests persist in the absence of mood and psychotic states. Mr. P’s viewing of child pornography and sexual attraction to prepubescent girls meet the diagnostic criteria for pedophilia. During hospitalization, we educate Mr. P about his diagnoses and need for continued treatment. We refer him to a sexual disorders outpatient clinic, which continues to address his deviant sexual interests.
The authors’ observations
A meta-analysis indicates that a combination of pharmacologic and behavioral treatments coupled with close legal supervision seems to reduce the risk of repeated sexual offenses.14 Legal supervision is a general term to describe oversight of offenders in the community by supervisory boards, such as probation or parole, and tracking devices such as GPS. Currently, pedophilia treatment focuses on minimizing deviant sexual arousal through behavioral modification, cognitive-behavioral therapies, and testosterone-lowering medications, such as medroxyprogesterone or leuprolide. The decision to prescribe testosterone-lowering medication should be based on informed consent and the patient’s risk of dangerous sexual behaviors.
- Reijnen L, Bulten E, Nijman H. Demographic and personality characteristics of internet child pornography downloaders in comparison to other offenders. J Child Sex Abus. 2009;18(6):611-622.
- Hall RC, Hall RC. A profile of pedophilia: definition, characteristics of offenders, recidivism, treatment outcomes, and forensic issues. Mayo Clin Proc. 2007;82(4):457-471.
- Leuprolide • Eligard, Lupron
- Medroxyprogesterone • Cycrin, Provera
- Risperidone • Risperdal
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
2. Dunsieth NW, Jr, Nelson EB, Brusman-Lovins LA, et al. Psychiatric and legal features of 113 men convicted of sexual offenses. J Clin Psychiatry. 2004;65(3):293-300.
3. Wallace C, Mullen P, Burgess P, et al. Serious criminal offending and mental disorder. Case linkage study. Br J Psychiatry. 1998;172:477-484.
4. Alish Y, Birger M, Manor N, et al. Schizophrenia sex offenders: a clinical and epidemiological comparison study. Int J Law Psychiatry. 2007;30(6):459-466.
5. Smith AD, Taylor PJ. Serious sex offending against women by men with schizophrenia. Relationship of illness and psychiatric symptoms to offending. Br J Psychiatry. 1999;174:233-237.
6. Drake CR, Pathé M. Understanding sexual offending in schizophrenia. Crim Behav Ment Health. 2004;14(2):108-120.
7. Harley EW, Boardman J, Craig T. Sexual problems in schizophrenia prevalence and characteristics: a cross sectional survey. Soc Psychiatry Psychiatr Epidemiol. 2010;45(7):759-766.
8. Galli V, McElroy SL, Soutullo CA, et al. The psychiatric diagnoses of twenty-two adolescents who have sexually molested other children. Compr Psychiatry. 1999;40(2):85-88.
9. Abel GG, Jordan A, Hand CG, et al. Classification models of child molesters utilizing the Abel Assessment for sexual interest. Child Abuse Negl. 2001;25(5):703-718.
10. Hanson RK, Thornton D. Improving risk assessments for sex offenders: a comparison of three actuarial scales. Law Hum Behav. 2000;24(1):119-136.
11. Hanson RK, Harris AJ, Scott TL, et al. Assessing the risk of sexual offenders on community supervision: The Dynamic Supervision Project. Vol 5. Ottawa, Canada: Public Safety Canada; 2007.
12. Quinsey VL, Harris AJ, Rice ME, et al. Violent offenders: appraising and managing risk. 2nd ed. Washington DC: American Psychological Association; 2006.
13. Seto M, Cantor JM, Blanchard R. Child pornography offenses are a valid diagnostic indicator of pedophilia. J Abnorm Psychol. 2006;115(3):610-615.
14. Thibaut F, De La Barra F, Gordon H, et al. WFSBP Task Force on Sexual Disorders. The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of paraphilias. World J Biol Psychiatry. 2010;11(4):604-655.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
2. Dunsieth NW, Jr, Nelson EB, Brusman-Lovins LA, et al. Psychiatric and legal features of 113 men convicted of sexual offenses. J Clin Psychiatry. 2004;65(3):293-300.
3. Wallace C, Mullen P, Burgess P, et al. Serious criminal offending and mental disorder. Case linkage study. Br J Psychiatry. 1998;172:477-484.
4. Alish Y, Birger M, Manor N, et al. Schizophrenia sex offenders: a clinical and epidemiological comparison study. Int J Law Psychiatry. 2007;30(6):459-466.
5. Smith AD, Taylor PJ. Serious sex offending against women by men with schizophrenia. Relationship of illness and psychiatric symptoms to offending. Br J Psychiatry. 1999;174:233-237.
6. Drake CR, Pathé M. Understanding sexual offending in schizophrenia. Crim Behav Ment Health. 2004;14(2):108-120.
7. Harley EW, Boardman J, Craig T. Sexual problems in schizophrenia prevalence and characteristics: a cross sectional survey. Soc Psychiatry Psychiatr Epidemiol. 2010;45(7):759-766.
8. Galli V, McElroy SL, Soutullo CA, et al. The psychiatric diagnoses of twenty-two adolescents who have sexually molested other children. Compr Psychiatry. 1999;40(2):85-88.
9. Abel GG, Jordan A, Hand CG, et al. Classification models of child molesters utilizing the Abel Assessment for sexual interest. Child Abuse Negl. 2001;25(5):703-718.
10. Hanson RK, Thornton D. Improving risk assessments for sex offenders: a comparison of three actuarial scales. Law Hum Behav. 2000;24(1):119-136.
11. Hanson RK, Harris AJ, Scott TL, et al. Assessing the risk of sexual offenders on community supervision: The Dynamic Supervision Project. Vol 5. Ottawa, Canada: Public Safety Canada; 2007.
12. Quinsey VL, Harris AJ, Rice ME, et al. Violent offenders: appraising and managing risk. 2nd ed. Washington DC: American Psychological Association; 2006.
13. Seto M, Cantor JM, Blanchard R. Child pornography offenses are a valid diagnostic indicator of pedophilia. J Abnorm Psychol. 2006;115(3):610-615.
14. Thibaut F, De La Barra F, Gordon H, et al. WFSBP Task Force on Sexual Disorders. The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of paraphilias. World J Biol Psychiatry. 2010;11(4):604-655.
Sleepless and paranoid
CASE: Worsening insomnia
Mr. Q, age 44, presents for evaluation of altered mental status characterized by disorientation, impaired attention and concentration, paranoid delusions, and prominent auditory and visual hallucinations. His initial Folstein Mini-Mental State Examination (MMSE) score is 7 of 30, indicating severe impairment. He further describes a recent history of nausea, intermittent vomiting, and anorexia. He takes hydrocodone/acetaminophen, 5/500 mg, 4 times daily for lower back and joint pain. Additionally, he has a pacemaker, which was placed when Mr. Q was in his late 30s to treat sinus bradycardia.
Mr. Q’s fiancée describes his 6-month history of worsening sleep disturbance, noting insomnia, fractured sleep, dream enactment, and daytime fatigue. During this time, Mr. Q averaged 3 to 4 hours of sleep nightly without day-time naps. Ten days ago, he stopped sleeping completely and his cognitive function decompensated rapidly. He became increasingly paranoid, believing government agents had been dispatched to kill him. Several days before admission, Mr. Q developed auditory and visual hallucinations. He reports that he hears voices warning him of Armageddon and sees reincarnated spirits of deceased relatives. He describes his mood as “fine” and “okay” and lacks insight into his psychiatric symptoms other than his sleeplessness.
Mr. Q’s family says he has a history of transient mild depression after his older brother died from an unknown neurologic disease 3 years ago. Mr. Q did not receive pharmacotherapy or psychotherapy but his symptoms resolved. His family says that Mr. Q has been using marijuana daily for several years, but they are unaware of other substance use. They deny a family history of psychiatric illness.
On physical examination, Mr. Q appears thin, agitated, and in mild distress. He has a fever of 99.2°F. His blood pressure drops intermittently from a baseline of 120/70 mm Hg to 100/60 mm Hg, at which point he experiences transient normal sinus tachycardia. Neurologic examination reveals psychomotor agitation and diffuse myoclonic tremor.
The authors’ observations
Table 1
Differential diagnosis of insomnia
| Type of disorder | Examples |
|---|---|
| Sleep disorders | Narcolepsy, REM sleep disorder, periodic limb movement disorder, restless leg syndrome, parasomniac conditions |
| Psychiatric disorders | Mania or hypomania, psychosis, substance intoxication or withdrawal, dementia, delirium |
| Neurologic disorders | Stroke, malignancy, infection or abscess, metabolic or viral encephalopathy, seizure disorder, prion disease |
| Somatic conditions | Cardiorespiratory disease, central or obstructive sleep apnea, congestive heart failure (Cheyne-Stokes respiration), pain, nocturnal movement disorder, gastroesophageal reflux disease, nocturia |
| Other causes | Jet lag, shift work, environment, lifestyle, medication |
| REM: rapid eye movement Source: Reference 1 | |
Medications that can cause or exacerbate insomnia
| Class/category | Medication(s) |
|---|---|
| Stimulants | Bupropion, dextroamphetamine, methylphenidate |
| Decongestants | Pseudoephedrine, phenylephrine |
| Antihypertensives or antiarrythmics | α- and β-antagonists |
| Respiratory medications | Albuterol, theophylline |
| Hormones | Corticosteroids, thyroid medications |
| Anticonvulsants | Lamotrigine |
| Medications that induce rebound insomnia | Benzodiazepines, sedative-hypnotics, opioids |
| Nonprescription medications | Caffeine, alcohol, nicotine, illicit psychostimulants |
EVALUATION: Inconclusive results
Routine laboratory studies reveal mild normocytic anemia and mild hypokalemia. Liver panel, renal function, cardiac profile, brain natriuretic peptide level, folate and vitamin B12 levels, thyroid studies, and human immunodeficiency virus serology are negative or within normal limits. Urinalysis reveals the presence of ketones, indicative of Mr. Q’s recent anorexia. Chest radiography and CT imaging of the head, abdomen, and pelvis also are unremarkable. MRI is contraindicated because of Mr. Q’s implanted pacemaker. Pulse oximetry does not suggest apneic events. Mr. Q and his family refuse a lumbar puncture, which precludes cerebrospinal fluid (CSF) analysis. Electroencephalography (EEG) records normal patterns of wakefulness oscillating with transient periods of stage 1 sleep. A detailed family interview reveals that Mr. Q’s older brother had a history of epilepsy and died at age 49 following a prolonged hospitalization for recurrent seizures and similar insomnia symptoms. History from the patient’s paternal lineage is not available.
The authors’ observations
American Psychiatric Association practice guidelines3 do not support first-line use of benzodiazepines for non-alcohol withdrawal-related delirium. Benzodiazepines are ineffective for treating delirium and may exacerbate symptoms.4 Laboratory evidence confirmed Mr. Q has no history of alcohol or benzodiazepine use. Although treating the underlying cause of delirium is essential, prescribing a sedative-hypnotic medication such as zolpidem for Mr. Q’s insomnia may worsen his condition. These agents are known to impair cognition and may induce or intensify psychosis.5 Melatonin and melatonin receptor agonists, such as ramelteon, promote sleep by regulating the sleep-wake rhythm through their action on melatonin receptors in the hypothalamus.6 Recently, a randomized control trial (RCT)7 found melatonin protected against delirium in hospitalized patients age ≥65. However, no RCT has examined use of exogenous melatonin or melatonin receptor agonists to treat delirium. In Mr. Q’s case, we chose to administer haloperidol. First- and second-generation antipsychotics have shown efficacy in treating acute delirium. Although more clinical experience has accumulated using first-generation agents such as haloperidol, a 2007 Cochrane meta-analysis8 demonstrated equal benefit with second-generation antipsychotics, while noting a decreased incidence of adverse effects.
TREATMENT: Adverse effects
Mr. Q receives an IM injection of haloperidol, 5 mg, for severe agitation, followed 15 hours later by IM aripiprazole, 9.75 mg. Within hours of receiving aripiprazole, Mr. Q develops hyperkinetic perioral and tongue movements. He initially is diagnosed with acute reactionary dystonia, although closer examination reveals myoclonus consistent with his overall presentation. Additionally, his QTc interval increases by 120 ms. Subsequently, all antipsychotics are stopped. We prescribe lorazepam, 1 mg IM every 4 hours as needed, for agitation. Mr. Q receives 2 consecutive doses of lorazepam, although neither effectively reduces his agitation or promotes sleep. Mr. Q is not assessed with positron-emission tomography (PET) or polysomnography.
The authors’ observations
There was no evidence of neurologic disease on Mr. Q’s CT scan and EEG was within normal limits. Other imaging and laboratory studies did not reveal possible infection, malignancy, or cardiovascular disease. Despite its rarity, we considered the possibility of a prion disease, given Mr. Q’s unique presentation and family history. Familial fatal insomnia (FFI) is an autosomal dominant disease caused by a point mutation in the prion protein gene. Prion proteins are theorized to play a role in myelin stability. The aberrant isoform produced in FFI is structurally misfolded so that it resists degradation by proteolytic enzymes. The accumulation of irregular prion proteins in the medial thalamic nucleus results in progressive neurodegeneration. Patients with FFI present with increasingly severe insomnia, mild fever, dysautonomia, spontaneous myoclonus, cognitive dysfunction, and hallucinations.9 Generally, patients die from sudden cardiorespiratory failure or ensuing infections 9 to 24 months after symptom onset. In vivo, FFI diagnosis is suggested by a loss of sleep spindles on polysomnogram and by decreased thalamic metabolism on PET scan. Other imaging modalities and testing, including EEG and CSF analysis, lack sensitivity and/or specificity.10
OUTCOME: Improvement, discharge
On his fourth hospital day, Mr. Q’s symptoms begin to remit spontaneously. His gastrointestinal (GI) upset improves and the following night he sleeps for approximately 4 hours. As his sleep improves, his delusional thinking and hallucinations resolve. Orientation, memory, and concentration gradually improve. Before discharge, his MMSE score is 24 out of 30, indicating improved cognition. His heart rate, blood pressure, and body temperature normalize and his myoclonus improves. Mr. Q is discharged after 6 days in the hospital and returns home. He follows up with his primary care physician, denies any recurrence of sleep disturbance, and reports that his cognition and perception have returned to his baseline.
The authors’ observations
12
Table 3
DSM-IV-TR diagnostic criteria for opioid withdrawal
| A. | Either of the following:
|
| B. | ≥3 of the following, developing within minutes to several days after criterion A:
|
| C. | The symptoms of criterion B cause clinically significant distress or impairment in social, occupational, or other important areas of functioning |
| D. | The symptoms are not due to a general medical condition and are not better accounted for by another mental disorder |
| Source: Reference 11 | |
- Morin CM, Benca R. Chronic insomnia. Lancet. 2012; 379(9821):1129-1141.
- Pressman MR, Orr WC, eds. Understanding sleep: the evolution and treatment of sleep disorders. Washington, DC: American Psychological Association; 1997.
- NIH State-of-the-Science Conference Statement on manifestations and management of chronic insomnia in adults. NIH Consens State Sci Statements. 2005;22(2):1-30.
- Albuterol • Proventil, Ventolin
- Aripiprazole • Abilify
- Bupropion • Wellbutrin, Zyban
- Dextroamphetamine • Dexadrine
- Haloperidol • Haldol
- Hydrocodone/Acetaminophen • Vicodin
- Lamotrigine • Lamictal
- Lorazepam • Ativan
- Methylphenidate • Methylin, Ritalin
- Phenylephrine • Neo-Synephrine
- Pseudoephedrine • Sudafed
- Ramelteon • Rozerem
- Theophylline • Elixophyllin, Slo-Phyllin
- Zolpidem • Ambien
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Mai E, Buysse DJ. Insomnia: prevalence impact, pathogenesis, differential diagnosis, and evaluation. Sleep Med Clin. 2008;3(2):167-174.
2. Kennedy N, Boydell J, Kalidindi S, et al. Gender differences in incidence and age at onset of mania and bipolar disorder over a 35-year period in Camberwell, England. Am J Psychiatry. 2005;162(2):257-262.
3. Cook IA. American Psychiatric Association. Guideline watch: practice guidelines for the treatment of patients with delirium. http://psychiatryonline.org/content.aspx?bookid=28§ionid=1681952. Accessed June 20 2012.
4. Lonergan E, Luxenberg J, Areosa Sastre A, et al. Benzodiazepines for delirium. Cochrane Database Syst Rev. 2009;21(1):CD006379.-
5. Toner LC, Tsambiras BM, Catalano G, et al. Central nervous system side effects associated with zolpidem treatment. Clin Neuropharmacol. 2000;23(1):54-58.
6. Srinivasan V, Pandi-Perumal SR, Trahkt I, et al. Melatonin and melatonergic drugs on sleep: possible mechanisms of action. Int J Neurosci. 2009;119(6):821-846.
7. Al-Aama T, Brymer C, Gutmanis I, et al. Melatonin decreases delirium in elderly patients: a randomized, placebo-controlled trial. Int J Geriatr Psychiatry. 2011;26(7):687-694.
8. Lonergan E, Britton AM, Luxenberg J, et al. Antipsychotics for delirium. Cochrane Database Syst Rev. 2007;18(2):CD005594.-
9. Medori R, Tritschler HJ, LeBlanc A, et al. Fatal familial insomnia, a prion disease with a mutation codon 178 of the prion protein gene. N Engl J Med. 1992;326(7):444-449.
10. Lugaresi E, Provini F, Cortelli P. Agrypnia excitata. Sleep Med. 2011;12(suppl 2):S3-S10.
11. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
12. Jaffe JH, Strain EC. Opioid-related disorders. In: Sadock BJ Sadock VA, eds. Kaplan and Sadock’s comprehensive textbook of psychiatry. 8th ed. Baltimore, MD: Lippincott Williams & Wilkins, 2005:1164, 1272-1274.
CASE: Worsening insomnia
Mr. Q, age 44, presents for evaluation of altered mental status characterized by disorientation, impaired attention and concentration, paranoid delusions, and prominent auditory and visual hallucinations. His initial Folstein Mini-Mental State Examination (MMSE) score is 7 of 30, indicating severe impairment. He further describes a recent history of nausea, intermittent vomiting, and anorexia. He takes hydrocodone/acetaminophen, 5/500 mg, 4 times daily for lower back and joint pain. Additionally, he has a pacemaker, which was placed when Mr. Q was in his late 30s to treat sinus bradycardia.
Mr. Q’s fiancée describes his 6-month history of worsening sleep disturbance, noting insomnia, fractured sleep, dream enactment, and daytime fatigue. During this time, Mr. Q averaged 3 to 4 hours of sleep nightly without day-time naps. Ten days ago, he stopped sleeping completely and his cognitive function decompensated rapidly. He became increasingly paranoid, believing government agents had been dispatched to kill him. Several days before admission, Mr. Q developed auditory and visual hallucinations. He reports that he hears voices warning him of Armageddon and sees reincarnated spirits of deceased relatives. He describes his mood as “fine” and “okay” and lacks insight into his psychiatric symptoms other than his sleeplessness.
Mr. Q’s family says he has a history of transient mild depression after his older brother died from an unknown neurologic disease 3 years ago. Mr. Q did not receive pharmacotherapy or psychotherapy but his symptoms resolved. His family says that Mr. Q has been using marijuana daily for several years, but they are unaware of other substance use. They deny a family history of psychiatric illness.
On physical examination, Mr. Q appears thin, agitated, and in mild distress. He has a fever of 99.2°F. His blood pressure drops intermittently from a baseline of 120/70 mm Hg to 100/60 mm Hg, at which point he experiences transient normal sinus tachycardia. Neurologic examination reveals psychomotor agitation and diffuse myoclonic tremor.
The authors’ observations
Table 1
Differential diagnosis of insomnia
| Type of disorder | Examples |
|---|---|
| Sleep disorders | Narcolepsy, REM sleep disorder, periodic limb movement disorder, restless leg syndrome, parasomniac conditions |
| Psychiatric disorders | Mania or hypomania, psychosis, substance intoxication or withdrawal, dementia, delirium |
| Neurologic disorders | Stroke, malignancy, infection or abscess, metabolic or viral encephalopathy, seizure disorder, prion disease |
| Somatic conditions | Cardiorespiratory disease, central or obstructive sleep apnea, congestive heart failure (Cheyne-Stokes respiration), pain, nocturnal movement disorder, gastroesophageal reflux disease, nocturia |
| Other causes | Jet lag, shift work, environment, lifestyle, medication |
| REM: rapid eye movement Source: Reference 1 | |
Medications that can cause or exacerbate insomnia
| Class/category | Medication(s) |
|---|---|
| Stimulants | Bupropion, dextroamphetamine, methylphenidate |
| Decongestants | Pseudoephedrine, phenylephrine |
| Antihypertensives or antiarrythmics | α- and β-antagonists |
| Respiratory medications | Albuterol, theophylline |
| Hormones | Corticosteroids, thyroid medications |
| Anticonvulsants | Lamotrigine |
| Medications that induce rebound insomnia | Benzodiazepines, sedative-hypnotics, opioids |
| Nonprescription medications | Caffeine, alcohol, nicotine, illicit psychostimulants |
EVALUATION: Inconclusive results
Routine laboratory studies reveal mild normocytic anemia and mild hypokalemia. Liver panel, renal function, cardiac profile, brain natriuretic peptide level, folate and vitamin B12 levels, thyroid studies, and human immunodeficiency virus serology are negative or within normal limits. Urinalysis reveals the presence of ketones, indicative of Mr. Q’s recent anorexia. Chest radiography and CT imaging of the head, abdomen, and pelvis also are unremarkable. MRI is contraindicated because of Mr. Q’s implanted pacemaker. Pulse oximetry does not suggest apneic events. Mr. Q and his family refuse a lumbar puncture, which precludes cerebrospinal fluid (CSF) analysis. Electroencephalography (EEG) records normal patterns of wakefulness oscillating with transient periods of stage 1 sleep. A detailed family interview reveals that Mr. Q’s older brother had a history of epilepsy and died at age 49 following a prolonged hospitalization for recurrent seizures and similar insomnia symptoms. History from the patient’s paternal lineage is not available.
The authors’ observations
American Psychiatric Association practice guidelines3 do not support first-line use of benzodiazepines for non-alcohol withdrawal-related delirium. Benzodiazepines are ineffective for treating delirium and may exacerbate symptoms.4 Laboratory evidence confirmed Mr. Q has no history of alcohol or benzodiazepine use. Although treating the underlying cause of delirium is essential, prescribing a sedative-hypnotic medication such as zolpidem for Mr. Q’s insomnia may worsen his condition. These agents are known to impair cognition and may induce or intensify psychosis.5 Melatonin and melatonin receptor agonists, such as ramelteon, promote sleep by regulating the sleep-wake rhythm through their action on melatonin receptors in the hypothalamus.6 Recently, a randomized control trial (RCT)7 found melatonin protected against delirium in hospitalized patients age ≥65. However, no RCT has examined use of exogenous melatonin or melatonin receptor agonists to treat delirium. In Mr. Q’s case, we chose to administer haloperidol. First- and second-generation antipsychotics have shown efficacy in treating acute delirium. Although more clinical experience has accumulated using first-generation agents such as haloperidol, a 2007 Cochrane meta-analysis8 demonstrated equal benefit with second-generation antipsychotics, while noting a decreased incidence of adverse effects.
TREATMENT: Adverse effects
Mr. Q receives an IM injection of haloperidol, 5 mg, for severe agitation, followed 15 hours later by IM aripiprazole, 9.75 mg. Within hours of receiving aripiprazole, Mr. Q develops hyperkinetic perioral and tongue movements. He initially is diagnosed with acute reactionary dystonia, although closer examination reveals myoclonus consistent with his overall presentation. Additionally, his QTc interval increases by 120 ms. Subsequently, all antipsychotics are stopped. We prescribe lorazepam, 1 mg IM every 4 hours as needed, for agitation. Mr. Q receives 2 consecutive doses of lorazepam, although neither effectively reduces his agitation or promotes sleep. Mr. Q is not assessed with positron-emission tomography (PET) or polysomnography.
The authors’ observations
There was no evidence of neurologic disease on Mr. Q’s CT scan and EEG was within normal limits. Other imaging and laboratory studies did not reveal possible infection, malignancy, or cardiovascular disease. Despite its rarity, we considered the possibility of a prion disease, given Mr. Q’s unique presentation and family history. Familial fatal insomnia (FFI) is an autosomal dominant disease caused by a point mutation in the prion protein gene. Prion proteins are theorized to play a role in myelin stability. The aberrant isoform produced in FFI is structurally misfolded so that it resists degradation by proteolytic enzymes. The accumulation of irregular prion proteins in the medial thalamic nucleus results in progressive neurodegeneration. Patients with FFI present with increasingly severe insomnia, mild fever, dysautonomia, spontaneous myoclonus, cognitive dysfunction, and hallucinations.9 Generally, patients die from sudden cardiorespiratory failure or ensuing infections 9 to 24 months after symptom onset. In vivo, FFI diagnosis is suggested by a loss of sleep spindles on polysomnogram and by decreased thalamic metabolism on PET scan. Other imaging modalities and testing, including EEG and CSF analysis, lack sensitivity and/or specificity.10
OUTCOME: Improvement, discharge
On his fourth hospital day, Mr. Q’s symptoms begin to remit spontaneously. His gastrointestinal (GI) upset improves and the following night he sleeps for approximately 4 hours. As his sleep improves, his delusional thinking and hallucinations resolve. Orientation, memory, and concentration gradually improve. Before discharge, his MMSE score is 24 out of 30, indicating improved cognition. His heart rate, blood pressure, and body temperature normalize and his myoclonus improves. Mr. Q is discharged after 6 days in the hospital and returns home. He follows up with his primary care physician, denies any recurrence of sleep disturbance, and reports that his cognition and perception have returned to his baseline.
The authors’ observations
12
Table 3
DSM-IV-TR diagnostic criteria for opioid withdrawal
| A. | Either of the following:
|
| B. | ≥3 of the following, developing within minutes to several days after criterion A:
|
| C. | The symptoms of criterion B cause clinically significant distress or impairment in social, occupational, or other important areas of functioning |
| D. | The symptoms are not due to a general medical condition and are not better accounted for by another mental disorder |
| Source: Reference 11 | |
- Morin CM, Benca R. Chronic insomnia. Lancet. 2012; 379(9821):1129-1141.
- Pressman MR, Orr WC, eds. Understanding sleep: the evolution and treatment of sleep disorders. Washington, DC: American Psychological Association; 1997.
- NIH State-of-the-Science Conference Statement on manifestations and management of chronic insomnia in adults. NIH Consens State Sci Statements. 2005;22(2):1-30.
- Albuterol • Proventil, Ventolin
- Aripiprazole • Abilify
- Bupropion • Wellbutrin, Zyban
- Dextroamphetamine • Dexadrine
- Haloperidol • Haldol
- Hydrocodone/Acetaminophen • Vicodin
- Lamotrigine • Lamictal
- Lorazepam • Ativan
- Methylphenidate • Methylin, Ritalin
- Phenylephrine • Neo-Synephrine
- Pseudoephedrine • Sudafed
- Ramelteon • Rozerem
- Theophylline • Elixophyllin, Slo-Phyllin
- Zolpidem • Ambien
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Worsening insomnia
Mr. Q, age 44, presents for evaluation of altered mental status characterized by disorientation, impaired attention and concentration, paranoid delusions, and prominent auditory and visual hallucinations. His initial Folstein Mini-Mental State Examination (MMSE) score is 7 of 30, indicating severe impairment. He further describes a recent history of nausea, intermittent vomiting, and anorexia. He takes hydrocodone/acetaminophen, 5/500 mg, 4 times daily for lower back and joint pain. Additionally, he has a pacemaker, which was placed when Mr. Q was in his late 30s to treat sinus bradycardia.
Mr. Q’s fiancée describes his 6-month history of worsening sleep disturbance, noting insomnia, fractured sleep, dream enactment, and daytime fatigue. During this time, Mr. Q averaged 3 to 4 hours of sleep nightly without day-time naps. Ten days ago, he stopped sleeping completely and his cognitive function decompensated rapidly. He became increasingly paranoid, believing government agents had been dispatched to kill him. Several days before admission, Mr. Q developed auditory and visual hallucinations. He reports that he hears voices warning him of Armageddon and sees reincarnated spirits of deceased relatives. He describes his mood as “fine” and “okay” and lacks insight into his psychiatric symptoms other than his sleeplessness.
Mr. Q’s family says he has a history of transient mild depression after his older brother died from an unknown neurologic disease 3 years ago. Mr. Q did not receive pharmacotherapy or psychotherapy but his symptoms resolved. His family says that Mr. Q has been using marijuana daily for several years, but they are unaware of other substance use. They deny a family history of psychiatric illness.
On physical examination, Mr. Q appears thin, agitated, and in mild distress. He has a fever of 99.2°F. His blood pressure drops intermittently from a baseline of 120/70 mm Hg to 100/60 mm Hg, at which point he experiences transient normal sinus tachycardia. Neurologic examination reveals psychomotor agitation and diffuse myoclonic tremor.
The authors’ observations
Table 1
Differential diagnosis of insomnia
| Type of disorder | Examples |
|---|---|
| Sleep disorders | Narcolepsy, REM sleep disorder, periodic limb movement disorder, restless leg syndrome, parasomniac conditions |
| Psychiatric disorders | Mania or hypomania, psychosis, substance intoxication or withdrawal, dementia, delirium |
| Neurologic disorders | Stroke, malignancy, infection or abscess, metabolic or viral encephalopathy, seizure disorder, prion disease |
| Somatic conditions | Cardiorespiratory disease, central or obstructive sleep apnea, congestive heart failure (Cheyne-Stokes respiration), pain, nocturnal movement disorder, gastroesophageal reflux disease, nocturia |
| Other causes | Jet lag, shift work, environment, lifestyle, medication |
| REM: rapid eye movement Source: Reference 1 | |
Medications that can cause or exacerbate insomnia
| Class/category | Medication(s) |
|---|---|
| Stimulants | Bupropion, dextroamphetamine, methylphenidate |
| Decongestants | Pseudoephedrine, phenylephrine |
| Antihypertensives or antiarrythmics | α- and β-antagonists |
| Respiratory medications | Albuterol, theophylline |
| Hormones | Corticosteroids, thyroid medications |
| Anticonvulsants | Lamotrigine |
| Medications that induce rebound insomnia | Benzodiazepines, sedative-hypnotics, opioids |
| Nonprescription medications | Caffeine, alcohol, nicotine, illicit psychostimulants |
EVALUATION: Inconclusive results
Routine laboratory studies reveal mild normocytic anemia and mild hypokalemia. Liver panel, renal function, cardiac profile, brain natriuretic peptide level, folate and vitamin B12 levels, thyroid studies, and human immunodeficiency virus serology are negative or within normal limits. Urinalysis reveals the presence of ketones, indicative of Mr. Q’s recent anorexia. Chest radiography and CT imaging of the head, abdomen, and pelvis also are unremarkable. MRI is contraindicated because of Mr. Q’s implanted pacemaker. Pulse oximetry does not suggest apneic events. Mr. Q and his family refuse a lumbar puncture, which precludes cerebrospinal fluid (CSF) analysis. Electroencephalography (EEG) records normal patterns of wakefulness oscillating with transient periods of stage 1 sleep. A detailed family interview reveals that Mr. Q’s older brother had a history of epilepsy and died at age 49 following a prolonged hospitalization for recurrent seizures and similar insomnia symptoms. History from the patient’s paternal lineage is not available.
The authors’ observations
American Psychiatric Association practice guidelines3 do not support first-line use of benzodiazepines for non-alcohol withdrawal-related delirium. Benzodiazepines are ineffective for treating delirium and may exacerbate symptoms.4 Laboratory evidence confirmed Mr. Q has no history of alcohol or benzodiazepine use. Although treating the underlying cause of delirium is essential, prescribing a sedative-hypnotic medication such as zolpidem for Mr. Q’s insomnia may worsen his condition. These agents are known to impair cognition and may induce or intensify psychosis.5 Melatonin and melatonin receptor agonists, such as ramelteon, promote sleep by regulating the sleep-wake rhythm through their action on melatonin receptors in the hypothalamus.6 Recently, a randomized control trial (RCT)7 found melatonin protected against delirium in hospitalized patients age ≥65. However, no RCT has examined use of exogenous melatonin or melatonin receptor agonists to treat delirium. In Mr. Q’s case, we chose to administer haloperidol. First- and second-generation antipsychotics have shown efficacy in treating acute delirium. Although more clinical experience has accumulated using first-generation agents such as haloperidol, a 2007 Cochrane meta-analysis8 demonstrated equal benefit with second-generation antipsychotics, while noting a decreased incidence of adverse effects.
TREATMENT: Adverse effects
Mr. Q receives an IM injection of haloperidol, 5 mg, for severe agitation, followed 15 hours later by IM aripiprazole, 9.75 mg. Within hours of receiving aripiprazole, Mr. Q develops hyperkinetic perioral and tongue movements. He initially is diagnosed with acute reactionary dystonia, although closer examination reveals myoclonus consistent with his overall presentation. Additionally, his QTc interval increases by 120 ms. Subsequently, all antipsychotics are stopped. We prescribe lorazepam, 1 mg IM every 4 hours as needed, for agitation. Mr. Q receives 2 consecutive doses of lorazepam, although neither effectively reduces his agitation or promotes sleep. Mr. Q is not assessed with positron-emission tomography (PET) or polysomnography.
The authors’ observations
There was no evidence of neurologic disease on Mr. Q’s CT scan and EEG was within normal limits. Other imaging and laboratory studies did not reveal possible infection, malignancy, or cardiovascular disease. Despite its rarity, we considered the possibility of a prion disease, given Mr. Q’s unique presentation and family history. Familial fatal insomnia (FFI) is an autosomal dominant disease caused by a point mutation in the prion protein gene. Prion proteins are theorized to play a role in myelin stability. The aberrant isoform produced in FFI is structurally misfolded so that it resists degradation by proteolytic enzymes. The accumulation of irregular prion proteins in the medial thalamic nucleus results in progressive neurodegeneration. Patients with FFI present with increasingly severe insomnia, mild fever, dysautonomia, spontaneous myoclonus, cognitive dysfunction, and hallucinations.9 Generally, patients die from sudden cardiorespiratory failure or ensuing infections 9 to 24 months after symptom onset. In vivo, FFI diagnosis is suggested by a loss of sleep spindles on polysomnogram and by decreased thalamic metabolism on PET scan. Other imaging modalities and testing, including EEG and CSF analysis, lack sensitivity and/or specificity.10
OUTCOME: Improvement, discharge
On his fourth hospital day, Mr. Q’s symptoms begin to remit spontaneously. His gastrointestinal (GI) upset improves and the following night he sleeps for approximately 4 hours. As his sleep improves, his delusional thinking and hallucinations resolve. Orientation, memory, and concentration gradually improve. Before discharge, his MMSE score is 24 out of 30, indicating improved cognition. His heart rate, blood pressure, and body temperature normalize and his myoclonus improves. Mr. Q is discharged after 6 days in the hospital and returns home. He follows up with his primary care physician, denies any recurrence of sleep disturbance, and reports that his cognition and perception have returned to his baseline.
The authors’ observations
12
Table 3
DSM-IV-TR diagnostic criteria for opioid withdrawal
| A. | Either of the following:
|
| B. | ≥3 of the following, developing within minutes to several days after criterion A:
|
| C. | The symptoms of criterion B cause clinically significant distress or impairment in social, occupational, or other important areas of functioning |
| D. | The symptoms are not due to a general medical condition and are not better accounted for by another mental disorder |
| Source: Reference 11 | |
- Morin CM, Benca R. Chronic insomnia. Lancet. 2012; 379(9821):1129-1141.
- Pressman MR, Orr WC, eds. Understanding sleep: the evolution and treatment of sleep disorders. Washington, DC: American Psychological Association; 1997.
- NIH State-of-the-Science Conference Statement on manifestations and management of chronic insomnia in adults. NIH Consens State Sci Statements. 2005;22(2):1-30.
- Albuterol • Proventil, Ventolin
- Aripiprazole • Abilify
- Bupropion • Wellbutrin, Zyban
- Dextroamphetamine • Dexadrine
- Haloperidol • Haldol
- Hydrocodone/Acetaminophen • Vicodin
- Lamotrigine • Lamictal
- Lorazepam • Ativan
- Methylphenidate • Methylin, Ritalin
- Phenylephrine • Neo-Synephrine
- Pseudoephedrine • Sudafed
- Ramelteon • Rozerem
- Theophylline • Elixophyllin, Slo-Phyllin
- Zolpidem • Ambien
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Mai E, Buysse DJ. Insomnia: prevalence impact, pathogenesis, differential diagnosis, and evaluation. Sleep Med Clin. 2008;3(2):167-174.
2. Kennedy N, Boydell J, Kalidindi S, et al. Gender differences in incidence and age at onset of mania and bipolar disorder over a 35-year period in Camberwell, England. Am J Psychiatry. 2005;162(2):257-262.
3. Cook IA. American Psychiatric Association. Guideline watch: practice guidelines for the treatment of patients with delirium. http://psychiatryonline.org/content.aspx?bookid=28§ionid=1681952. Accessed June 20 2012.
4. Lonergan E, Luxenberg J, Areosa Sastre A, et al. Benzodiazepines for delirium. Cochrane Database Syst Rev. 2009;21(1):CD006379.-
5. Toner LC, Tsambiras BM, Catalano G, et al. Central nervous system side effects associated with zolpidem treatment. Clin Neuropharmacol. 2000;23(1):54-58.
6. Srinivasan V, Pandi-Perumal SR, Trahkt I, et al. Melatonin and melatonergic drugs on sleep: possible mechanisms of action. Int J Neurosci. 2009;119(6):821-846.
7. Al-Aama T, Brymer C, Gutmanis I, et al. Melatonin decreases delirium in elderly patients: a randomized, placebo-controlled trial. Int J Geriatr Psychiatry. 2011;26(7):687-694.
8. Lonergan E, Britton AM, Luxenberg J, et al. Antipsychotics for delirium. Cochrane Database Syst Rev. 2007;18(2):CD005594.-
9. Medori R, Tritschler HJ, LeBlanc A, et al. Fatal familial insomnia, a prion disease with a mutation codon 178 of the prion protein gene. N Engl J Med. 1992;326(7):444-449.
10. Lugaresi E, Provini F, Cortelli P. Agrypnia excitata. Sleep Med. 2011;12(suppl 2):S3-S10.
11. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
12. Jaffe JH, Strain EC. Opioid-related disorders. In: Sadock BJ Sadock VA, eds. Kaplan and Sadock’s comprehensive textbook of psychiatry. 8th ed. Baltimore, MD: Lippincott Williams & Wilkins, 2005:1164, 1272-1274.
1. Mai E, Buysse DJ. Insomnia: prevalence impact, pathogenesis, differential diagnosis, and evaluation. Sleep Med Clin. 2008;3(2):167-174.
2. Kennedy N, Boydell J, Kalidindi S, et al. Gender differences in incidence and age at onset of mania and bipolar disorder over a 35-year period in Camberwell, England. Am J Psychiatry. 2005;162(2):257-262.
3. Cook IA. American Psychiatric Association. Guideline watch: practice guidelines for the treatment of patients with delirium. http://psychiatryonline.org/content.aspx?bookid=28§ionid=1681952. Accessed June 20 2012.
4. Lonergan E, Luxenberg J, Areosa Sastre A, et al. Benzodiazepines for delirium. Cochrane Database Syst Rev. 2009;21(1):CD006379.-
5. Toner LC, Tsambiras BM, Catalano G, et al. Central nervous system side effects associated with zolpidem treatment. Clin Neuropharmacol. 2000;23(1):54-58.
6. Srinivasan V, Pandi-Perumal SR, Trahkt I, et al. Melatonin and melatonergic drugs on sleep: possible mechanisms of action. Int J Neurosci. 2009;119(6):821-846.
7. Al-Aama T, Brymer C, Gutmanis I, et al. Melatonin decreases delirium in elderly patients: a randomized, placebo-controlled trial. Int J Geriatr Psychiatry. 2011;26(7):687-694.
8. Lonergan E, Britton AM, Luxenberg J, et al. Antipsychotics for delirium. Cochrane Database Syst Rev. 2007;18(2):CD005594.-
9. Medori R, Tritschler HJ, LeBlanc A, et al. Fatal familial insomnia, a prion disease with a mutation codon 178 of the prion protein gene. N Engl J Med. 1992;326(7):444-449.
10. Lugaresi E, Provini F, Cortelli P. Agrypnia excitata. Sleep Med. 2011;12(suppl 2):S3-S10.
11. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
12. Jaffe JH, Strain EC. Opioid-related disorders. In: Sadock BJ Sadock VA, eds. Kaplan and Sadock’s comprehensive textbook of psychiatry. 8th ed. Baltimore, MD: Lippincott Williams & Wilkins, 2005:1164, 1272-1274.