User login
A taste for the unusual
Discuss this article at www.facebook.com/CurrentPsychiatry
CASE: Nauseous and full
Ms. O, age 48, presents to the emergency department reporting a 3-day history of vomiting approximately 5 minutes after consuming solids or liquids. She’s had 10 vomiting episodes, which were associated with “fullness” and an “aching” sensation she rates as 6 on a 10-point scale pain scale that is diffuse over the upper epigastric area, with no palliative factors. Ms. O has not had a bowel movement for 3 days and her last menstrual period was 8 days ago. She is taking lorazepam, 1 mg/d. Her medical and psychiatric history includes anxiety, depression, personality disorder symptoms of affective dysregulation, obesity (270 lbs; medium height), and pica. She was 352 lbs when she underwent a Roux-en-Y gastric bypass 2 years ago. One year earlier, she had a laparoscopic gastric bezoar removal and an incisional hernia repair. Ms. O had no pica-related surgeries before undergoing gastric bypass surgery.
Ms. O denies shortness of breath, chest pain, allergies, smoking, or alcohol abuse, but reports uncontrollable cravings for paper products, specifically cardboard, which she describes as “just so delicious.” This craving led her to consume large amounts of cardboard and newspaper in the days before she began vomiting.
What may be causing Ms. O’s pica symptoms?
- iron deficiency anemia
- complications from gastric bypass surgery
- personality disorder
- generalized anxiety disorder (GAD)
The authors’ observations
DSM-IV-TR diagnostic criteria for pica include the persistent eating of non-nutritive substances for ≥1 month that is inappropriate for the level of a person’s development and not an acceptable part of one’s culture.1 If pica occurs with other mental disorders, it must be severe enough to indicate further clinical assessment to receive a separate diagnosis. Often associated with pregnancy, iron deficiency anemia, early development, and mental retardation, pica has been observed in post-gastric bypass surgery patients, all of whom presented with pagophagia (compulsive ice eating), and in one case was associated with a bezoar causing obstruction of the GI tract.1,2 With the dramatic increase in gastric bypass surgery and the required presurgical mental health evaluation, the consequences of failing to screen patients for pica behaviors can be devastating.
EVALUATION: Low iron
Ms. O’s vital signs on admission are stable, and physical exam is notable for mild abdominal distention with no guarding, tenderness, rigidity, or masses. No rebound tenderness is elicited. CT scan shows evidence of post-surgical changes involving the small bowel consistent with gastric bypass surgery and a hiatal hernia, but no obstruction, focal inflammation, free fluids, or gas. Lab values for amylase, lipase, urinalysis, coagulation studies, cardiac enzymes, and complete metabolic profile are within normal limits. Although not anemic, Ms. O is iron deficient, with ferritin, 10 ng/mL (normal 10 to 120 ng/mL); B12, 299 pg/mL (normal 100 to 700 pg/mL); and iron, 25 μg/dL (normal 50 to 170 μg/dL).
A foreign body is removed endoscopically and the specimen is sent to pathology. It is determined to be a gastric bezoar, yellowish-green in color, measuring 2.5 cm × 1 cm × 0.8 cm. After bezoar removal, Ms. O tolerates food and is discharged home on vitamin B12, 1,000 mcg/d for 2 weeks; folate, 1 mg/d for 1 month; calcium with vitamin D, 1 g/d; and esomeprazole, 40 mg/d for frequent heartburn. She is referred to psychiatry for behavioral modification therapy and medication management.
How would you treat Ms. O?
- start a selective serotonin reuptake inhibitor (SSRI)
- prescribe an atypical antipsychotic
- continue lorazepam
- begin behavioral therapy
HISTORY: Pica during pregnancy
During psychiatric workup, Ms. O admits to having pica urges most of her life, but experienced an uncontrollable exacerbation after gastric bypass surgery. This led to intense, chaotic periods of pica, resulting in a previous bezoar removal. She is particularly attracted to cardboard and newspaper cartoons, but notes she also has felt the urge to eat charcoal, moist soil, clay, chalk, pencils, and new shoes, which she chews on. In the past, her extreme anxiety and preoccupation with these urges had lead to diagnoses of personality disorder not otherwise specified, GAD, and obsessive-compulsive disorder.
Her first experience with pica was during her first pregnancy at age 15, when she had an impulse to eat soil. The urges briefly stopped until she became pregnant again. During each of her 5 pregnancies her pica symptoms returned. At one point during her last pregnancy she reports having felt out of control, eating 2 to 3 pencils with the eraser per day, after which she would feel intense relaxation. Her mother also exhibited symptoms of pica toward charcoal and soil. Ms. O had been taking unknown dosages of lorazepam for anxiety and fluoxetine for depression, both of which she stopped because she feared side effects during her last pregnancy. However, she never experienced any side effects.
The authors’ observations
Although pica is most commonly observed in young children, it sometimes is seen in pregnant women.1 Pica frequently is associated with other mental disorders, such as pervasive developmental disorder and mental retardation,1 and can be associated with premorbid psychosis and anxiety disorders. Occasional vitamin and mineral deficiencies, such as iron or zinc, have been reported, but usually patients’ lab values are normal. Treatment usually is initiated in the context of medical complications, such as iron deficiency anemia. In Ms. O’s case, the precipitating event was mechanical bowel obstruction due to a bezoar.
Several theories about the origins of pica have been proposed, but none truly are explanatory or satisfactory. The nutritional theory—that patients eat non-nutritive substances to compensate for mineral deficiencies—is popular because of pica’s frequent association with mineral deficiencies, but it is unknown whether pica is the cause or the result of the deficiency. An example of this is anemia due to eating clay instead of foods that contain iron. Another theory is that because pica is normal in early childhood development, it may be a manifestation of delayed development or mental retardation. The cultural theory is attractive because pregnant women in several cultures eat starch or clay as a part of their native rituals, and the incidence of pica is relatively high among pregnant African American women who live in rural areas.3 In the Roux-en-Y procedure, bypass of the duodenum and proximal jejunum can significantly decrease a patient’s iron uptake, leading to iron deficiency anemia, and could trigger pica in a susceptible patient.4
Exacerbation after gastric bypass
Kushner et al4 describes re-emergent pica after bariatric surgery in 2 patients with pagophagia associated with concomitant iron deficiency anemia. A 41-year-old white woman presented with pagophagia and a history of childhood consumption of dirt, chalk, and clay. Another patient, a 34-year-old African American woman, suffered from a lifelong desire to eat dirt, which she was able to resist, but experienced pagophagia during pregnancy and later when she developed iron deficiency anemia.4 In another case series, Kushner et al5 describes a 35-year-old woman with iron deficiency anemia with pagophagia presenting 2 years after Roux-en-Y. Her history was significant for eating clay as a child, but this new-onset pagophagia was so intense she purchased 2 snow cone machines, one for home and one for work, to feed her urges. Another patient, a 45-year-old African American woman, had an irresistible craving for calcium carbonate antacids, eating 40 to 50 a day, as well as several 30-ounce cups of ice.5 A third case report details a 33-year-old woman with iron deficiency anemia who presented with nocturnal pagophagia after Roux-en-Y anastomosis. She repeatedly rose during the night to eat the frost off the ice maker in her refrigerator.6 Another case described a female patient who ate cardboard after having a Roux-en-Y.2
Common themes in these case reports are female sex, Roux-en-Y, and dramatic resurgence of previously noted pica behaviors after gastric bypass surgery. Several studies have shown that pagophagia and pica in patients who are iron deficient or have iron deficiency anemia can be rapidly curbed with iron supplements.5 Ms. O, who has low iron, is taking iron supplementation, yet continues to experience pica cravings, albeit less severely. Her pica could be psychiatric in origin, perhaps related to her history of anxiety.
OUTCOME: Combination therapy
We start Ms. O on ziprasidone, 80 mg twice a day, restart lorazepam, 1 mg/d, and schedule monthly follow-up appointments to monitor her pica symptoms. We prescribe ziprasidone because it could treat paranoia and preoccupations and is considered to be weight-neutral. She continues her supplements, including ferrous sulfate, 325 mg 3 times daily. Ms. O attends weekly behavioral therapy sessions, during which the therapist monitors her mood and cravings with response prevention, which entails purposely avoiding behaviors after initiating a distressing stimulus. Ms. O responds well to medication and psychotherapy 1 month after the gastric bezoar removal, and she reports a decreased urge to eat cardboard. She is able to increase the amount of time she can go without eating non-nutritive substances—once daily, rather than repeatedly throughout the day.
The authors’ observations
Each patient with pica likely needs customized care. Children need to be supervised to prevent ingestion of lead-containing substances such as paint chips. Iron supplements are recommended for iron deficiency anemia and prophylaxis for iron deficiency anemia in Roux-en-Y patients.3,4 Pica in pregnant patients should be addressed to maintain adequate nutrition and prevent accidental poisonings.7 Behavioral intervention strategies are based on positive reinforcement and punishment (Table).8 A report of 3 young children with pica noted successful treatment of one with automatic reinforcement, and the other 2 with a combination of social and automatic reinforcement.9 There are no FDA-approved medications for pica. Positive effects have been seen with SSRIs, bupropion, atypical antipsychotics, buprenorphine, and chlorimipramine.10 Olanzapine has shown positive results as a treatment for pica.11 Most pica patients need concurrent psychotherapy.10
Table
Behavioral interventions for pica
| Intervention | Comments |
|---|---|
| Environmental enrichment | Providing additional stimulus to increase neuronal activity and focus behaviors |
| Noncontingent reinforcement | Presenting reinforcers according to a fixed schedule |
| Differential reinforcement | Desired behaviors are reinforced and inappropriate behaviors are ignored |
| Response blocking | Physically block a patient’s attempts to eat nonedible items |
| Source: Reference 8 | |
Related Resources
- Blinder BJ, Salama C. An update on pica: prevalence, contributing causes, and treatment. Psychiatric Times. www.psychiatrictimes.com/display/article/10168/1159376?pageNumber=1. Published May 1, 2008.
- Nurcombe B. Developmental disorders of attachment, feeding, elimination, & sleeping. In: Ebert MH, Loosen PT, Nurcombe B, et al, eds. CURRENT diagnosis & treatment: psychiatry. 2nd ed. New York, NY: McGraw Hill; 2008.
Drug Brand Names
- Buprenorphine • Subutex
- Bupropion • Wellbutrin, Zyban
- Chlorimipramine • Anafranil
- Esomeprazole • Nexium
- Fluoxetine • Prozac
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
2. Patton W, Gibbs K. Cardboard bezoar complicating laparoscopic gastric bypass. Surg Obes Relat Dis. 2010;6(3):313-315.
3. Nurcombe B. Developmental disorders of attachment feeding, elimination, & sleeping. In: Ebert MH, Loosen PT, Nurcombe B, et al, eds. CURRENT diagnosis & treatment: psychiatry. 2nd ed. New York, NY: McGraw Hill; 2008.
4. Kushner F, Gleason B, Shanta-Retelny V. Reemergence of pica following gastric bypass surgery for obesity: a new presentation of an old problem. J Am Diet Assoc. 2004;104(9):1393-1397.
5. Kushner F, Shanta Retelny V. Emergence of pica (ingestion of non-food substances) accompanying iron deficiency anemia after gastric bypass surgery. Obes Surg. 2005;15(10):1491-1495.
6. Marinella MA. Nocturnal pagophagia complicating gastric bypass. Mayo Clin Proc. 2008;83(8):961.-
7. Bernstein B, Weinstein M. Normal pregnancy & prenatal care. In: DeCherney AH Nathan L, Goodwin TM, et al, eds. CURRENT diagnosis & treatment obstetrics & gynecology. 10th ed. New York, NY: McGraw Hill; 2007.
8. Piazza C, Fisher W, Hanley P, et al. Treatment of pica through multiple analyses of its reinforcing functions. J Appl Behav Anal. 1998;31(2):165-189.
9. Williams DE, McAdam D. Assessment behavioral treatment, and prevention of pica: clinical guidelines and recommendations for practitioners. Res Dev Disabil. 2012;33(6):2050-2057.
10. Blinder BJ, Salama C. An update on pica: prevalence contributing causes, and treatment. Psychiatric Times. http://www.psychiatrictimes.com/display/article/10168/1159376?pageNumber=1. Published May 1, 2008. Accessed January 23, 2013.
11. Lerner AJ. Treatment of pica behavior with olanzapine. CNS Spectr. 2008;13(1):19.-
Discuss this article at www.facebook.com/CurrentPsychiatry
CASE: Nauseous and full
Ms. O, age 48, presents to the emergency department reporting a 3-day history of vomiting approximately 5 minutes after consuming solids or liquids. She’s had 10 vomiting episodes, which were associated with “fullness” and an “aching” sensation she rates as 6 on a 10-point scale pain scale that is diffuse over the upper epigastric area, with no palliative factors. Ms. O has not had a bowel movement for 3 days and her last menstrual period was 8 days ago. She is taking lorazepam, 1 mg/d. Her medical and psychiatric history includes anxiety, depression, personality disorder symptoms of affective dysregulation, obesity (270 lbs; medium height), and pica. She was 352 lbs when she underwent a Roux-en-Y gastric bypass 2 years ago. One year earlier, she had a laparoscopic gastric bezoar removal and an incisional hernia repair. Ms. O had no pica-related surgeries before undergoing gastric bypass surgery.
Ms. O denies shortness of breath, chest pain, allergies, smoking, or alcohol abuse, but reports uncontrollable cravings for paper products, specifically cardboard, which she describes as “just so delicious.” This craving led her to consume large amounts of cardboard and newspaper in the days before she began vomiting.
What may be causing Ms. O’s pica symptoms?
- iron deficiency anemia
- complications from gastric bypass surgery
- personality disorder
- generalized anxiety disorder (GAD)
The authors’ observations
DSM-IV-TR diagnostic criteria for pica include the persistent eating of non-nutritive substances for ≥1 month that is inappropriate for the level of a person’s development and not an acceptable part of one’s culture.1 If pica occurs with other mental disorders, it must be severe enough to indicate further clinical assessment to receive a separate diagnosis. Often associated with pregnancy, iron deficiency anemia, early development, and mental retardation, pica has been observed in post-gastric bypass surgery patients, all of whom presented with pagophagia (compulsive ice eating), and in one case was associated with a bezoar causing obstruction of the GI tract.1,2 With the dramatic increase in gastric bypass surgery and the required presurgical mental health evaluation, the consequences of failing to screen patients for pica behaviors can be devastating.
EVALUATION: Low iron
Ms. O’s vital signs on admission are stable, and physical exam is notable for mild abdominal distention with no guarding, tenderness, rigidity, or masses. No rebound tenderness is elicited. CT scan shows evidence of post-surgical changes involving the small bowel consistent with gastric bypass surgery and a hiatal hernia, but no obstruction, focal inflammation, free fluids, or gas. Lab values for amylase, lipase, urinalysis, coagulation studies, cardiac enzymes, and complete metabolic profile are within normal limits. Although not anemic, Ms. O is iron deficient, with ferritin, 10 ng/mL (normal 10 to 120 ng/mL); B12, 299 pg/mL (normal 100 to 700 pg/mL); and iron, 25 μg/dL (normal 50 to 170 μg/dL).
A foreign body is removed endoscopically and the specimen is sent to pathology. It is determined to be a gastric bezoar, yellowish-green in color, measuring 2.5 cm × 1 cm × 0.8 cm. After bezoar removal, Ms. O tolerates food and is discharged home on vitamin B12, 1,000 mcg/d for 2 weeks; folate, 1 mg/d for 1 month; calcium with vitamin D, 1 g/d; and esomeprazole, 40 mg/d for frequent heartburn. She is referred to psychiatry for behavioral modification therapy and medication management.
How would you treat Ms. O?
- start a selective serotonin reuptake inhibitor (SSRI)
- prescribe an atypical antipsychotic
- continue lorazepam
- begin behavioral therapy
HISTORY: Pica during pregnancy
During psychiatric workup, Ms. O admits to having pica urges most of her life, but experienced an uncontrollable exacerbation after gastric bypass surgery. This led to intense, chaotic periods of pica, resulting in a previous bezoar removal. She is particularly attracted to cardboard and newspaper cartoons, but notes she also has felt the urge to eat charcoal, moist soil, clay, chalk, pencils, and new shoes, which she chews on. In the past, her extreme anxiety and preoccupation with these urges had lead to diagnoses of personality disorder not otherwise specified, GAD, and obsessive-compulsive disorder.
Her first experience with pica was during her first pregnancy at age 15, when she had an impulse to eat soil. The urges briefly stopped until she became pregnant again. During each of her 5 pregnancies her pica symptoms returned. At one point during her last pregnancy she reports having felt out of control, eating 2 to 3 pencils with the eraser per day, after which she would feel intense relaxation. Her mother also exhibited symptoms of pica toward charcoal and soil. Ms. O had been taking unknown dosages of lorazepam for anxiety and fluoxetine for depression, both of which she stopped because she feared side effects during her last pregnancy. However, she never experienced any side effects.
The authors’ observations
Although pica is most commonly observed in young children, it sometimes is seen in pregnant women.1 Pica frequently is associated with other mental disorders, such as pervasive developmental disorder and mental retardation,1 and can be associated with premorbid psychosis and anxiety disorders. Occasional vitamin and mineral deficiencies, such as iron or zinc, have been reported, but usually patients’ lab values are normal. Treatment usually is initiated in the context of medical complications, such as iron deficiency anemia. In Ms. O’s case, the precipitating event was mechanical bowel obstruction due to a bezoar.
Several theories about the origins of pica have been proposed, but none truly are explanatory or satisfactory. The nutritional theory—that patients eat non-nutritive substances to compensate for mineral deficiencies—is popular because of pica’s frequent association with mineral deficiencies, but it is unknown whether pica is the cause or the result of the deficiency. An example of this is anemia due to eating clay instead of foods that contain iron. Another theory is that because pica is normal in early childhood development, it may be a manifestation of delayed development or mental retardation. The cultural theory is attractive because pregnant women in several cultures eat starch or clay as a part of their native rituals, and the incidence of pica is relatively high among pregnant African American women who live in rural areas.3 In the Roux-en-Y procedure, bypass of the duodenum and proximal jejunum can significantly decrease a patient’s iron uptake, leading to iron deficiency anemia, and could trigger pica in a susceptible patient.4
Exacerbation after gastric bypass
Kushner et al4 describes re-emergent pica after bariatric surgery in 2 patients with pagophagia associated with concomitant iron deficiency anemia. A 41-year-old white woman presented with pagophagia and a history of childhood consumption of dirt, chalk, and clay. Another patient, a 34-year-old African American woman, suffered from a lifelong desire to eat dirt, which she was able to resist, but experienced pagophagia during pregnancy and later when she developed iron deficiency anemia.4 In another case series, Kushner et al5 describes a 35-year-old woman with iron deficiency anemia with pagophagia presenting 2 years after Roux-en-Y. Her history was significant for eating clay as a child, but this new-onset pagophagia was so intense she purchased 2 snow cone machines, one for home and one for work, to feed her urges. Another patient, a 45-year-old African American woman, had an irresistible craving for calcium carbonate antacids, eating 40 to 50 a day, as well as several 30-ounce cups of ice.5 A third case report details a 33-year-old woman with iron deficiency anemia who presented with nocturnal pagophagia after Roux-en-Y anastomosis. She repeatedly rose during the night to eat the frost off the ice maker in her refrigerator.6 Another case described a female patient who ate cardboard after having a Roux-en-Y.2
Common themes in these case reports are female sex, Roux-en-Y, and dramatic resurgence of previously noted pica behaviors after gastric bypass surgery. Several studies have shown that pagophagia and pica in patients who are iron deficient or have iron deficiency anemia can be rapidly curbed with iron supplements.5 Ms. O, who has low iron, is taking iron supplementation, yet continues to experience pica cravings, albeit less severely. Her pica could be psychiatric in origin, perhaps related to her history of anxiety.
OUTCOME: Combination therapy
We start Ms. O on ziprasidone, 80 mg twice a day, restart lorazepam, 1 mg/d, and schedule monthly follow-up appointments to monitor her pica symptoms. We prescribe ziprasidone because it could treat paranoia and preoccupations and is considered to be weight-neutral. She continues her supplements, including ferrous sulfate, 325 mg 3 times daily. Ms. O attends weekly behavioral therapy sessions, during which the therapist monitors her mood and cravings with response prevention, which entails purposely avoiding behaviors after initiating a distressing stimulus. Ms. O responds well to medication and psychotherapy 1 month after the gastric bezoar removal, and she reports a decreased urge to eat cardboard. She is able to increase the amount of time she can go without eating non-nutritive substances—once daily, rather than repeatedly throughout the day.
The authors’ observations
Each patient with pica likely needs customized care. Children need to be supervised to prevent ingestion of lead-containing substances such as paint chips. Iron supplements are recommended for iron deficiency anemia and prophylaxis for iron deficiency anemia in Roux-en-Y patients.3,4 Pica in pregnant patients should be addressed to maintain adequate nutrition and prevent accidental poisonings.7 Behavioral intervention strategies are based on positive reinforcement and punishment (Table).8 A report of 3 young children with pica noted successful treatment of one with automatic reinforcement, and the other 2 with a combination of social and automatic reinforcement.9 There are no FDA-approved medications for pica. Positive effects have been seen with SSRIs, bupropion, atypical antipsychotics, buprenorphine, and chlorimipramine.10 Olanzapine has shown positive results as a treatment for pica.11 Most pica patients need concurrent psychotherapy.10
Table
Behavioral interventions for pica
| Intervention | Comments |
|---|---|
| Environmental enrichment | Providing additional stimulus to increase neuronal activity and focus behaviors |
| Noncontingent reinforcement | Presenting reinforcers according to a fixed schedule |
| Differential reinforcement | Desired behaviors are reinforced and inappropriate behaviors are ignored |
| Response blocking | Physically block a patient’s attempts to eat nonedible items |
| Source: Reference 8 | |
Related Resources
- Blinder BJ, Salama C. An update on pica: prevalence, contributing causes, and treatment. Psychiatric Times. www.psychiatrictimes.com/display/article/10168/1159376?pageNumber=1. Published May 1, 2008.
- Nurcombe B. Developmental disorders of attachment, feeding, elimination, & sleeping. In: Ebert MH, Loosen PT, Nurcombe B, et al, eds. CURRENT diagnosis & treatment: psychiatry. 2nd ed. New York, NY: McGraw Hill; 2008.
Drug Brand Names
- Buprenorphine • Subutex
- Bupropion • Wellbutrin, Zyban
- Chlorimipramine • Anafranil
- Esomeprazole • Nexium
- Fluoxetine • Prozac
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
CASE: Nauseous and full
Ms. O, age 48, presents to the emergency department reporting a 3-day history of vomiting approximately 5 minutes after consuming solids or liquids. She’s had 10 vomiting episodes, which were associated with “fullness” and an “aching” sensation she rates as 6 on a 10-point scale pain scale that is diffuse over the upper epigastric area, with no palliative factors. Ms. O has not had a bowel movement for 3 days and her last menstrual period was 8 days ago. She is taking lorazepam, 1 mg/d. Her medical and psychiatric history includes anxiety, depression, personality disorder symptoms of affective dysregulation, obesity (270 lbs; medium height), and pica. She was 352 lbs when she underwent a Roux-en-Y gastric bypass 2 years ago. One year earlier, she had a laparoscopic gastric bezoar removal and an incisional hernia repair. Ms. O had no pica-related surgeries before undergoing gastric bypass surgery.
Ms. O denies shortness of breath, chest pain, allergies, smoking, or alcohol abuse, but reports uncontrollable cravings for paper products, specifically cardboard, which she describes as “just so delicious.” This craving led her to consume large amounts of cardboard and newspaper in the days before she began vomiting.
What may be causing Ms. O’s pica symptoms?
- iron deficiency anemia
- complications from gastric bypass surgery
- personality disorder
- generalized anxiety disorder (GAD)
The authors’ observations
DSM-IV-TR diagnostic criteria for pica include the persistent eating of non-nutritive substances for ≥1 month that is inappropriate for the level of a person’s development and not an acceptable part of one’s culture.1 If pica occurs with other mental disorders, it must be severe enough to indicate further clinical assessment to receive a separate diagnosis. Often associated with pregnancy, iron deficiency anemia, early development, and mental retardation, pica has been observed in post-gastric bypass surgery patients, all of whom presented with pagophagia (compulsive ice eating), and in one case was associated with a bezoar causing obstruction of the GI tract.1,2 With the dramatic increase in gastric bypass surgery and the required presurgical mental health evaluation, the consequences of failing to screen patients for pica behaviors can be devastating.
EVALUATION: Low iron
Ms. O’s vital signs on admission are stable, and physical exam is notable for mild abdominal distention with no guarding, tenderness, rigidity, or masses. No rebound tenderness is elicited. CT scan shows evidence of post-surgical changes involving the small bowel consistent with gastric bypass surgery and a hiatal hernia, but no obstruction, focal inflammation, free fluids, or gas. Lab values for amylase, lipase, urinalysis, coagulation studies, cardiac enzymes, and complete metabolic profile are within normal limits. Although not anemic, Ms. O is iron deficient, with ferritin, 10 ng/mL (normal 10 to 120 ng/mL); B12, 299 pg/mL (normal 100 to 700 pg/mL); and iron, 25 μg/dL (normal 50 to 170 μg/dL).
A foreign body is removed endoscopically and the specimen is sent to pathology. It is determined to be a gastric bezoar, yellowish-green in color, measuring 2.5 cm × 1 cm × 0.8 cm. After bezoar removal, Ms. O tolerates food and is discharged home on vitamin B12, 1,000 mcg/d for 2 weeks; folate, 1 mg/d for 1 month; calcium with vitamin D, 1 g/d; and esomeprazole, 40 mg/d for frequent heartburn. She is referred to psychiatry for behavioral modification therapy and medication management.
How would you treat Ms. O?
- start a selective serotonin reuptake inhibitor (SSRI)
- prescribe an atypical antipsychotic
- continue lorazepam
- begin behavioral therapy
HISTORY: Pica during pregnancy
During psychiatric workup, Ms. O admits to having pica urges most of her life, but experienced an uncontrollable exacerbation after gastric bypass surgery. This led to intense, chaotic periods of pica, resulting in a previous bezoar removal. She is particularly attracted to cardboard and newspaper cartoons, but notes she also has felt the urge to eat charcoal, moist soil, clay, chalk, pencils, and new shoes, which she chews on. In the past, her extreme anxiety and preoccupation with these urges had lead to diagnoses of personality disorder not otherwise specified, GAD, and obsessive-compulsive disorder.
Her first experience with pica was during her first pregnancy at age 15, when she had an impulse to eat soil. The urges briefly stopped until she became pregnant again. During each of her 5 pregnancies her pica symptoms returned. At one point during her last pregnancy she reports having felt out of control, eating 2 to 3 pencils with the eraser per day, after which she would feel intense relaxation. Her mother also exhibited symptoms of pica toward charcoal and soil. Ms. O had been taking unknown dosages of lorazepam for anxiety and fluoxetine for depression, both of which she stopped because she feared side effects during her last pregnancy. However, she never experienced any side effects.
The authors’ observations
Although pica is most commonly observed in young children, it sometimes is seen in pregnant women.1 Pica frequently is associated with other mental disorders, such as pervasive developmental disorder and mental retardation,1 and can be associated with premorbid psychosis and anxiety disorders. Occasional vitamin and mineral deficiencies, such as iron or zinc, have been reported, but usually patients’ lab values are normal. Treatment usually is initiated in the context of medical complications, such as iron deficiency anemia. In Ms. O’s case, the precipitating event was mechanical bowel obstruction due to a bezoar.
Several theories about the origins of pica have been proposed, but none truly are explanatory or satisfactory. The nutritional theory—that patients eat non-nutritive substances to compensate for mineral deficiencies—is popular because of pica’s frequent association with mineral deficiencies, but it is unknown whether pica is the cause or the result of the deficiency. An example of this is anemia due to eating clay instead of foods that contain iron. Another theory is that because pica is normal in early childhood development, it may be a manifestation of delayed development or mental retardation. The cultural theory is attractive because pregnant women in several cultures eat starch or clay as a part of their native rituals, and the incidence of pica is relatively high among pregnant African American women who live in rural areas.3 In the Roux-en-Y procedure, bypass of the duodenum and proximal jejunum can significantly decrease a patient’s iron uptake, leading to iron deficiency anemia, and could trigger pica in a susceptible patient.4
Exacerbation after gastric bypass
Kushner et al4 describes re-emergent pica after bariatric surgery in 2 patients with pagophagia associated with concomitant iron deficiency anemia. A 41-year-old white woman presented with pagophagia and a history of childhood consumption of dirt, chalk, and clay. Another patient, a 34-year-old African American woman, suffered from a lifelong desire to eat dirt, which she was able to resist, but experienced pagophagia during pregnancy and later when she developed iron deficiency anemia.4 In another case series, Kushner et al5 describes a 35-year-old woman with iron deficiency anemia with pagophagia presenting 2 years after Roux-en-Y. Her history was significant for eating clay as a child, but this new-onset pagophagia was so intense she purchased 2 snow cone machines, one for home and one for work, to feed her urges. Another patient, a 45-year-old African American woman, had an irresistible craving for calcium carbonate antacids, eating 40 to 50 a day, as well as several 30-ounce cups of ice.5 A third case report details a 33-year-old woman with iron deficiency anemia who presented with nocturnal pagophagia after Roux-en-Y anastomosis. She repeatedly rose during the night to eat the frost off the ice maker in her refrigerator.6 Another case described a female patient who ate cardboard after having a Roux-en-Y.2
Common themes in these case reports are female sex, Roux-en-Y, and dramatic resurgence of previously noted pica behaviors after gastric bypass surgery. Several studies have shown that pagophagia and pica in patients who are iron deficient or have iron deficiency anemia can be rapidly curbed with iron supplements.5 Ms. O, who has low iron, is taking iron supplementation, yet continues to experience pica cravings, albeit less severely. Her pica could be psychiatric in origin, perhaps related to her history of anxiety.
OUTCOME: Combination therapy
We start Ms. O on ziprasidone, 80 mg twice a day, restart lorazepam, 1 mg/d, and schedule monthly follow-up appointments to monitor her pica symptoms. We prescribe ziprasidone because it could treat paranoia and preoccupations and is considered to be weight-neutral. She continues her supplements, including ferrous sulfate, 325 mg 3 times daily. Ms. O attends weekly behavioral therapy sessions, during which the therapist monitors her mood and cravings with response prevention, which entails purposely avoiding behaviors after initiating a distressing stimulus. Ms. O responds well to medication and psychotherapy 1 month after the gastric bezoar removal, and she reports a decreased urge to eat cardboard. She is able to increase the amount of time she can go without eating non-nutritive substances—once daily, rather than repeatedly throughout the day.
The authors’ observations
Each patient with pica likely needs customized care. Children need to be supervised to prevent ingestion of lead-containing substances such as paint chips. Iron supplements are recommended for iron deficiency anemia and prophylaxis for iron deficiency anemia in Roux-en-Y patients.3,4 Pica in pregnant patients should be addressed to maintain adequate nutrition and prevent accidental poisonings.7 Behavioral intervention strategies are based on positive reinforcement and punishment (Table).8 A report of 3 young children with pica noted successful treatment of one with automatic reinforcement, and the other 2 with a combination of social and automatic reinforcement.9 There are no FDA-approved medications for pica. Positive effects have been seen with SSRIs, bupropion, atypical antipsychotics, buprenorphine, and chlorimipramine.10 Olanzapine has shown positive results as a treatment for pica.11 Most pica patients need concurrent psychotherapy.10
Table
Behavioral interventions for pica
| Intervention | Comments |
|---|---|
| Environmental enrichment | Providing additional stimulus to increase neuronal activity and focus behaviors |
| Noncontingent reinforcement | Presenting reinforcers according to a fixed schedule |
| Differential reinforcement | Desired behaviors are reinforced and inappropriate behaviors are ignored |
| Response blocking | Physically block a patient’s attempts to eat nonedible items |
| Source: Reference 8 | |
Related Resources
- Blinder BJ, Salama C. An update on pica: prevalence, contributing causes, and treatment. Psychiatric Times. www.psychiatrictimes.com/display/article/10168/1159376?pageNumber=1. Published May 1, 2008.
- Nurcombe B. Developmental disorders of attachment, feeding, elimination, & sleeping. In: Ebert MH, Loosen PT, Nurcombe B, et al, eds. CURRENT diagnosis & treatment: psychiatry. 2nd ed. New York, NY: McGraw Hill; 2008.
Drug Brand Names
- Buprenorphine • Subutex
- Bupropion • Wellbutrin, Zyban
- Chlorimipramine • Anafranil
- Esomeprazole • Nexium
- Fluoxetine • Prozac
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
2. Patton W, Gibbs K. Cardboard bezoar complicating laparoscopic gastric bypass. Surg Obes Relat Dis. 2010;6(3):313-315.
3. Nurcombe B. Developmental disorders of attachment feeding, elimination, & sleeping. In: Ebert MH, Loosen PT, Nurcombe B, et al, eds. CURRENT diagnosis & treatment: psychiatry. 2nd ed. New York, NY: McGraw Hill; 2008.
4. Kushner F, Gleason B, Shanta-Retelny V. Reemergence of pica following gastric bypass surgery for obesity: a new presentation of an old problem. J Am Diet Assoc. 2004;104(9):1393-1397.
5. Kushner F, Shanta Retelny V. Emergence of pica (ingestion of non-food substances) accompanying iron deficiency anemia after gastric bypass surgery. Obes Surg. 2005;15(10):1491-1495.
6. Marinella MA. Nocturnal pagophagia complicating gastric bypass. Mayo Clin Proc. 2008;83(8):961.-
7. Bernstein B, Weinstein M. Normal pregnancy & prenatal care. In: DeCherney AH Nathan L, Goodwin TM, et al, eds. CURRENT diagnosis & treatment obstetrics & gynecology. 10th ed. New York, NY: McGraw Hill; 2007.
8. Piazza C, Fisher W, Hanley P, et al. Treatment of pica through multiple analyses of its reinforcing functions. J Appl Behav Anal. 1998;31(2):165-189.
9. Williams DE, McAdam D. Assessment behavioral treatment, and prevention of pica: clinical guidelines and recommendations for practitioners. Res Dev Disabil. 2012;33(6):2050-2057.
10. Blinder BJ, Salama C. An update on pica: prevalence contributing causes, and treatment. Psychiatric Times. http://www.psychiatrictimes.com/display/article/10168/1159376?pageNumber=1. Published May 1, 2008. Accessed January 23, 2013.
11. Lerner AJ. Treatment of pica behavior with olanzapine. CNS Spectr. 2008;13(1):19.-
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
2. Patton W, Gibbs K. Cardboard bezoar complicating laparoscopic gastric bypass. Surg Obes Relat Dis. 2010;6(3):313-315.
3. Nurcombe B. Developmental disorders of attachment feeding, elimination, & sleeping. In: Ebert MH, Loosen PT, Nurcombe B, et al, eds. CURRENT diagnosis & treatment: psychiatry. 2nd ed. New York, NY: McGraw Hill; 2008.
4. Kushner F, Gleason B, Shanta-Retelny V. Reemergence of pica following gastric bypass surgery for obesity: a new presentation of an old problem. J Am Diet Assoc. 2004;104(9):1393-1397.
5. Kushner F, Shanta Retelny V. Emergence of pica (ingestion of non-food substances) accompanying iron deficiency anemia after gastric bypass surgery. Obes Surg. 2005;15(10):1491-1495.
6. Marinella MA. Nocturnal pagophagia complicating gastric bypass. Mayo Clin Proc. 2008;83(8):961.-
7. Bernstein B, Weinstein M. Normal pregnancy & prenatal care. In: DeCherney AH Nathan L, Goodwin TM, et al, eds. CURRENT diagnosis & treatment obstetrics & gynecology. 10th ed. New York, NY: McGraw Hill; 2007.
8. Piazza C, Fisher W, Hanley P, et al. Treatment of pica through multiple analyses of its reinforcing functions. J Appl Behav Anal. 1998;31(2):165-189.
9. Williams DE, McAdam D. Assessment behavioral treatment, and prevention of pica: clinical guidelines and recommendations for practitioners. Res Dev Disabil. 2012;33(6):2050-2057.
10. Blinder BJ, Salama C. An update on pica: prevalence contributing causes, and treatment. Psychiatric Times. http://www.psychiatrictimes.com/display/article/10168/1159376?pageNumber=1. Published May 1, 2008. Accessed January 23, 2013.
11. Lerner AJ. Treatment of pica behavior with olanzapine. CNS Spectr. 2008;13(1):19.-
Bipolar disorder or something else?
CASE: Unclear diagnosis
Police find Ms. S, age 31, extremely intoxicated and drinking alcohol in her car in a city park parking lot. In the emergency room, she becomes increasingly somnolent and clinicians intubate her trachea to protect her airway. Lab testing shows she has elevated acetaminophen and lithium serum levels, and she is transferred to our hospital for further management after being started on N-acetylcysteine to treat acetaminophen toxicity. Her “ex-fiancé,” the father of her 2 children, saw her earlier the day of the episode and says she was distraught, intoxicated, and had several empty pill bottles in her purse.
In our hospital, Ms. S’ lithium level increases from 2.3 mEq/L to a peak of 5.32 mEq/L, and she undergoes hemodialysis. On hospital day 2, her serum lithium level is trending downward. After Ms. S is able to breathe spontaneously, her trachea is extubated and her hemodialysis line is removed. A psychiatric consultation is obtained, but she is unable to provide a coherent history and the treating clinicians believe she has delirium caused by multiple factors.
On hospital day 3, Ms. S’ delirium clears enough for her to engage in an interview, and she is transferred to our inpatient psychiatry ward for further monitoring and stabilization.
She reports that she was diagnosed with bipolar disorder (BD) at age 12, when she faced multiple psychosocial stressors, including physical abuse by her mother’s boyfriend. She took several psychotropics—although she cannot remember which ones—until age 14, when she stopped all medications until the year before her current hospitalization. Although throughout adolescence and adulthood Ms. S experienced chronic irritability, anxiety, impulsive behavior, poor self-esteem, abusive relationships, self-cutting, and depressed mood, she maintains that she felt worse when she was taking psychotropics and doubts the BD diagnosis. She attributes her longstanding mood issues to low self-worth, a “codependent nature,” and a tendency to gravitate toward abusive relationships. Although she admits to experimenting with several illicit drugs during adolescence, she denies more recent substance use and states she drinks alcohol only once every few months.
The authors’ observations
BD is underdiagnosed in several patient populations, such as individuals previously diagnosed with MDD.1-3 Misdiagnosis can have severe implications, including delay in receiving treatment with effective medications (eg, mood stabilizers) or use of agents that can induce mania or rapid-cycling, such as antidepressants. Perhaps in response to this concern, in recent years clinicians increasingly have diagnosed BD in adolescents and adults. An analysis of a national database of physician practices found a 40-fold increase in office visits for BD among youth and a near doubling among adults from 1994 to 2003.4
Although underdiagnosis of BD remains important, some researchers have suggested that overdiagnosis may be more prevalent and equally harmful. In a study of 180 patients being treated for depression in a family care clinic, there was a 21.6% initial underdiagnosis rate among those eventually found to have BD.1 However, among 43 patients with a prior BD diagnosis, the diagnosis was not confirmed in 33%.1 In a study of 700 psychiatric outpatients in Rhode Island, only 43% of 145 patients who reported a prior BD diagnosis had that diagnosis confirmed.5 Three times as many patients were overdiagnosed with BD as underdiagnosed.
Are there characteristics common to individuals incorrectly diagnosed with BD? In a study that compared patients who had been mistakenly diagnosed with BD with those who had not been diagnosed with BD, the overdiagnosis group was significantly more likely to be diagnosed with a personality disorder, in particular borderline or antisocial personality disorder.6 Only lifetime and current BPD, current posttraumatic stress disorder (PTSD), and lifetime impulse control disorders were independently associated with BD overdiagnosis. The odds ratio for overdiagnosis of BD in patients found to have BPD was 3.7.
EVALUATION: Rethink the diagnosis
In the last few months, Ms. S had complained to her primary care provider (PCP) of worsening anxiety and depressed mood. She was the victim of ongoing physical and emotional abuse by her ex-fiancé and was concerned that she may lose custody of her 2 sons. Approximately 8 months before admission, Ms. S’ PCP prescribed lithium, 450 mg, 3 times a day, for “mood stabilization” and depression because she’d already been diagnosed with BD. This was the first mood stabilizer she’d taken since she was 14. She also was taking unknown doses of hydrocodone/acetaminophen, cyclobenzaprine, and tramadol for pain and temazepam for insomnia. Ms. S continued to suffer from labile and depressed mood, and fought with her ex-fiancé and legal authorities to maintain custody of her 2 children until she was found in the park.
Throughout her hospitalization she denies that she attempted suicide that day, and maintains that this incident was caused by unintentional mismanagement of her medications. Although she continues to have a sense of low self-worth, she denies feeling depressed; in contrast, she says she feels like she has a “new lease on life.” During several interviews she cannot provide a history of any prolonged (ie, several days) episodes of elevated mood, increased goal-directed behavior, decreased need for sleep, tangential thought, pressured speech, or other symptoms that suggest hypomania or mania. She does not endorse prolonged periods of neurovegetative symptoms that would indicate a major depressive episode.
We feel that Ms. S’ symptoms of affective dysregulation, impulsivity, and interpersonal dysfunction are consistent with BPD, and we determine that she meets 6 of the 9 DSM-IV-TR diagnostic features of BPD (≥5 are required for a BPD diagnosis) (Table 1).7 Ms. S describes efforts to avoid abandonment, unstable and intense interpersonal relationships, marked and persistent unstable self-image, recurrent suicidal and self-mutilating behavior, affective instability, and chronic feelings of emptiness. She is discharged to follow up with a psychotherapist and family practitioner. She is not continued on any psychotropic medications.
The authors’ observations
Although it can be difficult to accurately diagnose psychiatric illness during a brief inpatient hospitalization, several clinicians who cared for Ms. S felt that her presentation was more consistent with BPD than BD. Her case is an example of the potential harm of incorrectly diagnosing personality-disordered patients with BD. Ms. S is impulsive and used lithium—a medication that is the standard of care for BD—in an overdose, which lead to a costly and dangerous hospitalization marked by a difficult tracheal intubation and hemodialysis.
Table 1
DSM-IV-TR diagnostic criteria for borderline personality disorder
| A pervasive pattern of instability of interpersonal relationships, self-image, and affects, and marked impulsivity, as indicated by ≥5 of the following: |
|
| Source: Reference 7 |
Distinguishing BD and BPD
There is considerable overlap in symptoms of BD and BPD. Although the episodic nature of BD is well differentiated from the more chronic course of BPD, many hypomania and mania symptoms are similar to those of BPD (Table 2).7 For example, patients with BD or BPD may exhibit impulsive behavior and labile moods. Substance use, risky and self-destructive behaviors, and inflammatory interpersonal relationships can occur in both disorders. Some researchers have suggested that pathophysiologically, BPD may fall on a spectrum of bipolar illness, and have proposed a clinical entity they call bipolar type IV or ultra-rapid cycling BD.2,8,9 There may be more co-occurrence of BD with BPD than would be expected by chance10; 1 review of BPD studies found the rate of comorbid BD ranged from 5.6% to 19%.11 However, because of differences in several factors—including phenomenology, family prevalence, longitudinal course, and medication response—some researchers have concluded that evidence does not support categorizing BPD as part of a bipolar spectrum.10-14 Nonetheless, BPD and other personality disorders often co-occur with axis I disorders, including MDD, BD, or PTSD.
Some research has suggested that the increasing availability and marketing campaigns of medications to treat BD may promote diagnosis of the disorder.15 Zimmerman15 hypothesizes that physicians may be more likely to diagnose a condition that responds to medication (ie, BD) than one that is less responsive (ie, BPD). Financial compensation for treating axis I disorders is significantly better than for treating personality disorders.16 The inpatient setting confers barriers to accurately diagnosing personality disorders, including limits on the amount of time that clinicians can spend with patients or ability to communicate with sources of collateral information. A patient’s observed personality and behaviors while hospitalized may not accurately reflect his or her personality and behaviors in that patient’s “natural” environment.
Several diagnostic strategies can help distinguish BPD from BD. For BD to be the primary diagnosis, a patient must have had a hypomanic or manic episode. Sustained episodes of elation or extreme irritability without evident stressors suggest BD rather than BPD.10 According to Gunderson et al,10 “repeated angry outbursts, suicide attempts, or acts of deliberate self harm that are reactive to interpersonal stress and reflect extreme rejection sensitivity are axiomatic of borderline personality disorder.” In a review of clinical practice, Gunderson17 found that hypersensitivity to rejection and fearful preoccupation with expected abandonment are the most distinctive characteristics of BPD patients. He suggested that clinicians can establish the diagnosis by asking patients directly if they believe the criteria for BPD characterize them, which also can help a patient to accept the diagnosis.
Finally, during a short hospitalization, it can be helpful to obtain collateral information from the patient’s friends and family or further characterize the time course of symptoms and diagnostic features in the patient’s natural environment. Clinicians who are reluctant to diagnose BPD in an inpatient setting could suggest the presence of borderline traits or discuss the possibility of the BPD diagnosis in documentation (eg, in the assessment or formulation). Doing so would avoid a premature BPD diagnosis and allow outpatient providers to confirm or rule out personality disorder diagnoses over time. It is important to screen patients with BPD for co-occurring axis I disorders, including BD, MDD, PTSD, and substance abuse.
A false-positive BD diagnosis in patients with BPD has serious treatment implications. Antipsychotics, antidepressants, and anticonvulsants have been used to target BPD symptoms such as affective dysregulation, impulsivity, and cognitive/perceptual abnormalities, but no medications are FDA-approved for treating BPD. American Psychiatric Association guidelines recommend symptom-based pharmacologic strategies for BPD,18 although some researchers believe that these recommendations are out-of-date and not evidence-based.17,19 Some evidence suggests pharmacotherapy can have modest short-term benefits on specific BPD symptoms, but no data suggest that medication can reduce the severity of BPD or lead to remission.19-23 Just 1 randomized controlled trial (N = 17) has examined lithium for BPD and found no effect on mood.11,24
Misdiagnosis of BD in the context of BPD may create unrealistic expectations regarding the potential efficacy of medications for relieving symptoms. Patients may be diverted from potentially helpful psychotherapeutic treatments—such as DBT or mentalization therapy—which evidence suggests can effectively reduce symptoms, the need for additional treatments, and self-harm or suicidal behaviors.10,17,19 Evidence from long-term longitudinal studies suggests that psychosocial or psychotherapeutic treatment may protect against suicide in BPD patients.25
Table 2
DSM-IV-TR diagnostic criteria for a manic episode
|
The DSM-IV-TR diagnostic criteria for a hypomanic episode are similar to criteria for a manic episode, except:
|
| Source: Reference 7 |
Related Resources
- National Education Alliance Borderline Personality Disorder. www.borderlinepersonalitydisorder.com.
- Hoffman PD, Steiner-Grossman P. Borderline personality disorder: meeting the challenges to successful treatment. Philadelphia, PA: Haworth Press; 2008.
Drug Brand Names
- Cyclobenzaprine • Flexeril
- Hydrocodone/acetaminophen • Lorcet, Vicodin, others
- Lithium • Eskalith, Lithobid
- Temazepam • Restoril
- Tramadol • Ultram
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Hirschfeld RM, Cass AR, Holt DC, et al. Screening for bipolar disorder in patients treated for depression in a family medicine clinic. J Am Board Fam Pract. 2005;18(4):233-239.
2. Ghaemi SN, Ko JY, Goodwin FK. “Cade’s disease” and beyond: Misdiagnosis antidepressant use, and a proposed definition for bipolar spectrum disorder. Can J Psychiatry. 2002;47(2):125-134.
3. Bowden CL. Strategies to reduce misdiagnosis of bipolar depression. Psychiatr Serv. 2001;52(1):51-55.
4. Moreno C, Laje G, Blanco C, et al. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry. 2007;64(9):1032-1039.
5. Zimmerman M, Ruggero CJ, Chelminski I, et al. Is bipolar disorder overdiagnosed? J Clin Psychiatry. 2008;69(6):935-940.
6. Zimmerman M, Ruggero CJ, Chelminski I, et al. Psychiatric diagnoses in patients previously overdiagnosed with bipolar disorder. J Clin Psychiatry. 2010;71(1):26-31.
7. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
8. Akiskal HS. The bipolar spectrum-the shaping of a new paradigm in psychiatry. Curr Psychiatry Rep. 2002;4(1):1-3.
9. Akiskal HS, Pinto O. The evolving bipolar spectrum. Prototypes I II, III, and IV. Psychiatr Clin North Am. 1999;22(3):517-534, vii.
10. Gunderson JG, Weinberg I, Daversa MT, et al. Descriptive and longitudinal observations on the relationship of borderline personality disorder and bipolar disorder. Am J Psychiatry. 2006;163(7):1173-1178.
11. Paris J, Gunderson J, Weinberg I. The interface between borderline personality disorder and bipolar spectrum disorders. Compr Psychiatry. 2007;48(2):145-154.
12. Paris J. Why psychiatrists are reluctant to diagnose: borderline personality disorder. Psychiatry (Edgmont). 2007;4(1):35-39.
13. Paris J. Borderline or bipolar? Distinguishing borderline personality disorder from bipolar spectrum disorders. Harv Rev Psychiatry. 2004;12(3):140-145.
14. Ruggero CJ, Zimmerman M, Chelminski I, et al. Borderline personality disorder and the misdiagnosis of bipolar disorder. J Psychiatr Res. 2010;44(6):405-408.
15. Zimmerman M. Problems diagnosing bipolar disorder in clinical practice. Expert Rev Neurother. 2010;10(7):1019-1021.
16. Stone MH. Relationship of borderline personality disorder and bipolar disorder. Am J Psychiatry. 2006;163(7):1126-1128.
17. Gunderson JG. Clinical practice. Borderline personality disorder. N Engl J Med. 2011;364(21):2037-2042.
18. American Psychiatric Association. Practice guideline for the treatment of patients with borderline personality disorder. Washington D.C.: American Psychiatric Association; 2001.
19. Paris J. The treatment of borderline personality disorder: implications of research on diagnosis etiology, and outcome. Annu Rev Clin Psychol. 2009;5:277-290.
20. Stoffers J, Völlm BA, Rücker G, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2010;(6):CD005653.-
21. Ripoll LH, Triebwasser J, Siever LJ. Evidence-based pharmacotherapy for personality disorders. Int J Neuropsychopharmacol. 2011;14(9):1257-1288.
22. Mercer D, Douglass AB, Links PS. Meta-analyses of mood stabilizers antidepressants and antipsychotics in the treatment of borderline personality disorder: effectiveness for depression and anger symptoms. J Pers Disord. 2009;23(2):156-174.
23. Lieb K, Völlm B, Rücker G, et al. Pharmacotherapy for borderline personality disorder: Cochrane systematic review of randomised trials. Br J Psychiatry. 2010;196(1):4-12.
24. Links PS, Steiner M, Boiago I, et al. Lithium therapy for borderline patients: preliminary findings. J Pers Disord. 1990;4(2):173-181.
25. Goodman M, Roiff T, Oakes AH, et al. Suicidal risk and management in borderline personality disorder. Curr Psychiatry Rep. 2012;14(1):79-85.
CASE: Unclear diagnosis
Police find Ms. S, age 31, extremely intoxicated and drinking alcohol in her car in a city park parking lot. In the emergency room, she becomes increasingly somnolent and clinicians intubate her trachea to protect her airway. Lab testing shows she has elevated acetaminophen and lithium serum levels, and she is transferred to our hospital for further management after being started on N-acetylcysteine to treat acetaminophen toxicity. Her “ex-fiancé,” the father of her 2 children, saw her earlier the day of the episode and says she was distraught, intoxicated, and had several empty pill bottles in her purse.
In our hospital, Ms. S’ lithium level increases from 2.3 mEq/L to a peak of 5.32 mEq/L, and she undergoes hemodialysis. On hospital day 2, her serum lithium level is trending downward. After Ms. S is able to breathe spontaneously, her trachea is extubated and her hemodialysis line is removed. A psychiatric consultation is obtained, but she is unable to provide a coherent history and the treating clinicians believe she has delirium caused by multiple factors.
On hospital day 3, Ms. S’ delirium clears enough for her to engage in an interview, and she is transferred to our inpatient psychiatry ward for further monitoring and stabilization.
She reports that she was diagnosed with bipolar disorder (BD) at age 12, when she faced multiple psychosocial stressors, including physical abuse by her mother’s boyfriend. She took several psychotropics—although she cannot remember which ones—until age 14, when she stopped all medications until the year before her current hospitalization. Although throughout adolescence and adulthood Ms. S experienced chronic irritability, anxiety, impulsive behavior, poor self-esteem, abusive relationships, self-cutting, and depressed mood, she maintains that she felt worse when she was taking psychotropics and doubts the BD diagnosis. She attributes her longstanding mood issues to low self-worth, a “codependent nature,” and a tendency to gravitate toward abusive relationships. Although she admits to experimenting with several illicit drugs during adolescence, she denies more recent substance use and states she drinks alcohol only once every few months.
The authors’ observations
BD is underdiagnosed in several patient populations, such as individuals previously diagnosed with MDD.1-3 Misdiagnosis can have severe implications, including delay in receiving treatment with effective medications (eg, mood stabilizers) or use of agents that can induce mania or rapid-cycling, such as antidepressants. Perhaps in response to this concern, in recent years clinicians increasingly have diagnosed BD in adolescents and adults. An analysis of a national database of physician practices found a 40-fold increase in office visits for BD among youth and a near doubling among adults from 1994 to 2003.4
Although underdiagnosis of BD remains important, some researchers have suggested that overdiagnosis may be more prevalent and equally harmful. In a study of 180 patients being treated for depression in a family care clinic, there was a 21.6% initial underdiagnosis rate among those eventually found to have BD.1 However, among 43 patients with a prior BD diagnosis, the diagnosis was not confirmed in 33%.1 In a study of 700 psychiatric outpatients in Rhode Island, only 43% of 145 patients who reported a prior BD diagnosis had that diagnosis confirmed.5 Three times as many patients were overdiagnosed with BD as underdiagnosed.
Are there characteristics common to individuals incorrectly diagnosed with BD? In a study that compared patients who had been mistakenly diagnosed with BD with those who had not been diagnosed with BD, the overdiagnosis group was significantly more likely to be diagnosed with a personality disorder, in particular borderline or antisocial personality disorder.6 Only lifetime and current BPD, current posttraumatic stress disorder (PTSD), and lifetime impulse control disorders were independently associated with BD overdiagnosis. The odds ratio for overdiagnosis of BD in patients found to have BPD was 3.7.
EVALUATION: Rethink the diagnosis
In the last few months, Ms. S had complained to her primary care provider (PCP) of worsening anxiety and depressed mood. She was the victim of ongoing physical and emotional abuse by her ex-fiancé and was concerned that she may lose custody of her 2 sons. Approximately 8 months before admission, Ms. S’ PCP prescribed lithium, 450 mg, 3 times a day, for “mood stabilization” and depression because she’d already been diagnosed with BD. This was the first mood stabilizer she’d taken since she was 14. She also was taking unknown doses of hydrocodone/acetaminophen, cyclobenzaprine, and tramadol for pain and temazepam for insomnia. Ms. S continued to suffer from labile and depressed mood, and fought with her ex-fiancé and legal authorities to maintain custody of her 2 children until she was found in the park.
Throughout her hospitalization she denies that she attempted suicide that day, and maintains that this incident was caused by unintentional mismanagement of her medications. Although she continues to have a sense of low self-worth, she denies feeling depressed; in contrast, she says she feels like she has a “new lease on life.” During several interviews she cannot provide a history of any prolonged (ie, several days) episodes of elevated mood, increased goal-directed behavior, decreased need for sleep, tangential thought, pressured speech, or other symptoms that suggest hypomania or mania. She does not endorse prolonged periods of neurovegetative symptoms that would indicate a major depressive episode.
We feel that Ms. S’ symptoms of affective dysregulation, impulsivity, and interpersonal dysfunction are consistent with BPD, and we determine that she meets 6 of the 9 DSM-IV-TR diagnostic features of BPD (≥5 are required for a BPD diagnosis) (Table 1).7 Ms. S describes efforts to avoid abandonment, unstable and intense interpersonal relationships, marked and persistent unstable self-image, recurrent suicidal and self-mutilating behavior, affective instability, and chronic feelings of emptiness. She is discharged to follow up with a psychotherapist and family practitioner. She is not continued on any psychotropic medications.
The authors’ observations
Although it can be difficult to accurately diagnose psychiatric illness during a brief inpatient hospitalization, several clinicians who cared for Ms. S felt that her presentation was more consistent with BPD than BD. Her case is an example of the potential harm of incorrectly diagnosing personality-disordered patients with BD. Ms. S is impulsive and used lithium—a medication that is the standard of care for BD—in an overdose, which lead to a costly and dangerous hospitalization marked by a difficult tracheal intubation and hemodialysis.
Table 1
DSM-IV-TR diagnostic criteria for borderline personality disorder
| A pervasive pattern of instability of interpersonal relationships, self-image, and affects, and marked impulsivity, as indicated by ≥5 of the following: |
|
| Source: Reference 7 |
Distinguishing BD and BPD
There is considerable overlap in symptoms of BD and BPD. Although the episodic nature of BD is well differentiated from the more chronic course of BPD, many hypomania and mania symptoms are similar to those of BPD (Table 2).7 For example, patients with BD or BPD may exhibit impulsive behavior and labile moods. Substance use, risky and self-destructive behaviors, and inflammatory interpersonal relationships can occur in both disorders. Some researchers have suggested that pathophysiologically, BPD may fall on a spectrum of bipolar illness, and have proposed a clinical entity they call bipolar type IV or ultra-rapid cycling BD.2,8,9 There may be more co-occurrence of BD with BPD than would be expected by chance10; 1 review of BPD studies found the rate of comorbid BD ranged from 5.6% to 19%.11 However, because of differences in several factors—including phenomenology, family prevalence, longitudinal course, and medication response—some researchers have concluded that evidence does not support categorizing BPD as part of a bipolar spectrum.10-14 Nonetheless, BPD and other personality disorders often co-occur with axis I disorders, including MDD, BD, or PTSD.
Some research has suggested that the increasing availability and marketing campaigns of medications to treat BD may promote diagnosis of the disorder.15 Zimmerman15 hypothesizes that physicians may be more likely to diagnose a condition that responds to medication (ie, BD) than one that is less responsive (ie, BPD). Financial compensation for treating axis I disorders is significantly better than for treating personality disorders.16 The inpatient setting confers barriers to accurately diagnosing personality disorders, including limits on the amount of time that clinicians can spend with patients or ability to communicate with sources of collateral information. A patient’s observed personality and behaviors while hospitalized may not accurately reflect his or her personality and behaviors in that patient’s “natural” environment.
Several diagnostic strategies can help distinguish BPD from BD. For BD to be the primary diagnosis, a patient must have had a hypomanic or manic episode. Sustained episodes of elation or extreme irritability without evident stressors suggest BD rather than BPD.10 According to Gunderson et al,10 “repeated angry outbursts, suicide attempts, or acts of deliberate self harm that are reactive to interpersonal stress and reflect extreme rejection sensitivity are axiomatic of borderline personality disorder.” In a review of clinical practice, Gunderson17 found that hypersensitivity to rejection and fearful preoccupation with expected abandonment are the most distinctive characteristics of BPD patients. He suggested that clinicians can establish the diagnosis by asking patients directly if they believe the criteria for BPD characterize them, which also can help a patient to accept the diagnosis.
Finally, during a short hospitalization, it can be helpful to obtain collateral information from the patient’s friends and family or further characterize the time course of symptoms and diagnostic features in the patient’s natural environment. Clinicians who are reluctant to diagnose BPD in an inpatient setting could suggest the presence of borderline traits or discuss the possibility of the BPD diagnosis in documentation (eg, in the assessment or formulation). Doing so would avoid a premature BPD diagnosis and allow outpatient providers to confirm or rule out personality disorder diagnoses over time. It is important to screen patients with BPD for co-occurring axis I disorders, including BD, MDD, PTSD, and substance abuse.
A false-positive BD diagnosis in patients with BPD has serious treatment implications. Antipsychotics, antidepressants, and anticonvulsants have been used to target BPD symptoms such as affective dysregulation, impulsivity, and cognitive/perceptual abnormalities, but no medications are FDA-approved for treating BPD. American Psychiatric Association guidelines recommend symptom-based pharmacologic strategies for BPD,18 although some researchers believe that these recommendations are out-of-date and not evidence-based.17,19 Some evidence suggests pharmacotherapy can have modest short-term benefits on specific BPD symptoms, but no data suggest that medication can reduce the severity of BPD or lead to remission.19-23 Just 1 randomized controlled trial (N = 17) has examined lithium for BPD and found no effect on mood.11,24
Misdiagnosis of BD in the context of BPD may create unrealistic expectations regarding the potential efficacy of medications for relieving symptoms. Patients may be diverted from potentially helpful psychotherapeutic treatments—such as DBT or mentalization therapy—which evidence suggests can effectively reduce symptoms, the need for additional treatments, and self-harm or suicidal behaviors.10,17,19 Evidence from long-term longitudinal studies suggests that psychosocial or psychotherapeutic treatment may protect against suicide in BPD patients.25
Table 2
DSM-IV-TR diagnostic criteria for a manic episode
|
The DSM-IV-TR diagnostic criteria for a hypomanic episode are similar to criteria for a manic episode, except:
|
| Source: Reference 7 |
Related Resources
- National Education Alliance Borderline Personality Disorder. www.borderlinepersonalitydisorder.com.
- Hoffman PD, Steiner-Grossman P. Borderline personality disorder: meeting the challenges to successful treatment. Philadelphia, PA: Haworth Press; 2008.
Drug Brand Names
- Cyclobenzaprine • Flexeril
- Hydrocodone/acetaminophen • Lorcet, Vicodin, others
- Lithium • Eskalith, Lithobid
- Temazepam • Restoril
- Tramadol • Ultram
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Unclear diagnosis
Police find Ms. S, age 31, extremely intoxicated and drinking alcohol in her car in a city park parking lot. In the emergency room, she becomes increasingly somnolent and clinicians intubate her trachea to protect her airway. Lab testing shows she has elevated acetaminophen and lithium serum levels, and she is transferred to our hospital for further management after being started on N-acetylcysteine to treat acetaminophen toxicity. Her “ex-fiancé,” the father of her 2 children, saw her earlier the day of the episode and says she was distraught, intoxicated, and had several empty pill bottles in her purse.
In our hospital, Ms. S’ lithium level increases from 2.3 mEq/L to a peak of 5.32 mEq/L, and she undergoes hemodialysis. On hospital day 2, her serum lithium level is trending downward. After Ms. S is able to breathe spontaneously, her trachea is extubated and her hemodialysis line is removed. A psychiatric consultation is obtained, but she is unable to provide a coherent history and the treating clinicians believe she has delirium caused by multiple factors.
On hospital day 3, Ms. S’ delirium clears enough for her to engage in an interview, and she is transferred to our inpatient psychiatry ward for further monitoring and stabilization.
She reports that she was diagnosed with bipolar disorder (BD) at age 12, when she faced multiple psychosocial stressors, including physical abuse by her mother’s boyfriend. She took several psychotropics—although she cannot remember which ones—until age 14, when she stopped all medications until the year before her current hospitalization. Although throughout adolescence and adulthood Ms. S experienced chronic irritability, anxiety, impulsive behavior, poor self-esteem, abusive relationships, self-cutting, and depressed mood, she maintains that she felt worse when she was taking psychotropics and doubts the BD diagnosis. She attributes her longstanding mood issues to low self-worth, a “codependent nature,” and a tendency to gravitate toward abusive relationships. Although she admits to experimenting with several illicit drugs during adolescence, she denies more recent substance use and states she drinks alcohol only once every few months.
The authors’ observations
BD is underdiagnosed in several patient populations, such as individuals previously diagnosed with MDD.1-3 Misdiagnosis can have severe implications, including delay in receiving treatment with effective medications (eg, mood stabilizers) or use of agents that can induce mania or rapid-cycling, such as antidepressants. Perhaps in response to this concern, in recent years clinicians increasingly have diagnosed BD in adolescents and adults. An analysis of a national database of physician practices found a 40-fold increase in office visits for BD among youth and a near doubling among adults from 1994 to 2003.4
Although underdiagnosis of BD remains important, some researchers have suggested that overdiagnosis may be more prevalent and equally harmful. In a study of 180 patients being treated for depression in a family care clinic, there was a 21.6% initial underdiagnosis rate among those eventually found to have BD.1 However, among 43 patients with a prior BD diagnosis, the diagnosis was not confirmed in 33%.1 In a study of 700 psychiatric outpatients in Rhode Island, only 43% of 145 patients who reported a prior BD diagnosis had that diagnosis confirmed.5 Three times as many patients were overdiagnosed with BD as underdiagnosed.
Are there characteristics common to individuals incorrectly diagnosed with BD? In a study that compared patients who had been mistakenly diagnosed with BD with those who had not been diagnosed with BD, the overdiagnosis group was significantly more likely to be diagnosed with a personality disorder, in particular borderline or antisocial personality disorder.6 Only lifetime and current BPD, current posttraumatic stress disorder (PTSD), and lifetime impulse control disorders were independently associated with BD overdiagnosis. The odds ratio for overdiagnosis of BD in patients found to have BPD was 3.7.
EVALUATION: Rethink the diagnosis
In the last few months, Ms. S had complained to her primary care provider (PCP) of worsening anxiety and depressed mood. She was the victim of ongoing physical and emotional abuse by her ex-fiancé and was concerned that she may lose custody of her 2 sons. Approximately 8 months before admission, Ms. S’ PCP prescribed lithium, 450 mg, 3 times a day, for “mood stabilization” and depression because she’d already been diagnosed with BD. This was the first mood stabilizer she’d taken since she was 14. She also was taking unknown doses of hydrocodone/acetaminophen, cyclobenzaprine, and tramadol for pain and temazepam for insomnia. Ms. S continued to suffer from labile and depressed mood, and fought with her ex-fiancé and legal authorities to maintain custody of her 2 children until she was found in the park.
Throughout her hospitalization she denies that she attempted suicide that day, and maintains that this incident was caused by unintentional mismanagement of her medications. Although she continues to have a sense of low self-worth, she denies feeling depressed; in contrast, she says she feels like she has a “new lease on life.” During several interviews she cannot provide a history of any prolonged (ie, several days) episodes of elevated mood, increased goal-directed behavior, decreased need for sleep, tangential thought, pressured speech, or other symptoms that suggest hypomania or mania. She does not endorse prolonged periods of neurovegetative symptoms that would indicate a major depressive episode.
We feel that Ms. S’ symptoms of affective dysregulation, impulsivity, and interpersonal dysfunction are consistent with BPD, and we determine that she meets 6 of the 9 DSM-IV-TR diagnostic features of BPD (≥5 are required for a BPD diagnosis) (Table 1).7 Ms. S describes efforts to avoid abandonment, unstable and intense interpersonal relationships, marked and persistent unstable self-image, recurrent suicidal and self-mutilating behavior, affective instability, and chronic feelings of emptiness. She is discharged to follow up with a psychotherapist and family practitioner. She is not continued on any psychotropic medications.
The authors’ observations
Although it can be difficult to accurately diagnose psychiatric illness during a brief inpatient hospitalization, several clinicians who cared for Ms. S felt that her presentation was more consistent with BPD than BD. Her case is an example of the potential harm of incorrectly diagnosing personality-disordered patients with BD. Ms. S is impulsive and used lithium—a medication that is the standard of care for BD—in an overdose, which lead to a costly and dangerous hospitalization marked by a difficult tracheal intubation and hemodialysis.
Table 1
DSM-IV-TR diagnostic criteria for borderline personality disorder
| A pervasive pattern of instability of interpersonal relationships, self-image, and affects, and marked impulsivity, as indicated by ≥5 of the following: |
|
| Source: Reference 7 |
Distinguishing BD and BPD
There is considerable overlap in symptoms of BD and BPD. Although the episodic nature of BD is well differentiated from the more chronic course of BPD, many hypomania and mania symptoms are similar to those of BPD (Table 2).7 For example, patients with BD or BPD may exhibit impulsive behavior and labile moods. Substance use, risky and self-destructive behaviors, and inflammatory interpersonal relationships can occur in both disorders. Some researchers have suggested that pathophysiologically, BPD may fall on a spectrum of bipolar illness, and have proposed a clinical entity they call bipolar type IV or ultra-rapid cycling BD.2,8,9 There may be more co-occurrence of BD with BPD than would be expected by chance10; 1 review of BPD studies found the rate of comorbid BD ranged from 5.6% to 19%.11 However, because of differences in several factors—including phenomenology, family prevalence, longitudinal course, and medication response—some researchers have concluded that evidence does not support categorizing BPD as part of a bipolar spectrum.10-14 Nonetheless, BPD and other personality disorders often co-occur with axis I disorders, including MDD, BD, or PTSD.
Some research has suggested that the increasing availability and marketing campaigns of medications to treat BD may promote diagnosis of the disorder.15 Zimmerman15 hypothesizes that physicians may be more likely to diagnose a condition that responds to medication (ie, BD) than one that is less responsive (ie, BPD). Financial compensation for treating axis I disorders is significantly better than for treating personality disorders.16 The inpatient setting confers barriers to accurately diagnosing personality disorders, including limits on the amount of time that clinicians can spend with patients or ability to communicate with sources of collateral information. A patient’s observed personality and behaviors while hospitalized may not accurately reflect his or her personality and behaviors in that patient’s “natural” environment.
Several diagnostic strategies can help distinguish BPD from BD. For BD to be the primary diagnosis, a patient must have had a hypomanic or manic episode. Sustained episodes of elation or extreme irritability without evident stressors suggest BD rather than BPD.10 According to Gunderson et al,10 “repeated angry outbursts, suicide attempts, or acts of deliberate self harm that are reactive to interpersonal stress and reflect extreme rejection sensitivity are axiomatic of borderline personality disorder.” In a review of clinical practice, Gunderson17 found that hypersensitivity to rejection and fearful preoccupation with expected abandonment are the most distinctive characteristics of BPD patients. He suggested that clinicians can establish the diagnosis by asking patients directly if they believe the criteria for BPD characterize them, which also can help a patient to accept the diagnosis.
Finally, during a short hospitalization, it can be helpful to obtain collateral information from the patient’s friends and family or further characterize the time course of symptoms and diagnostic features in the patient’s natural environment. Clinicians who are reluctant to diagnose BPD in an inpatient setting could suggest the presence of borderline traits or discuss the possibility of the BPD diagnosis in documentation (eg, in the assessment or formulation). Doing so would avoid a premature BPD diagnosis and allow outpatient providers to confirm or rule out personality disorder diagnoses over time. It is important to screen patients with BPD for co-occurring axis I disorders, including BD, MDD, PTSD, and substance abuse.
A false-positive BD diagnosis in patients with BPD has serious treatment implications. Antipsychotics, antidepressants, and anticonvulsants have been used to target BPD symptoms such as affective dysregulation, impulsivity, and cognitive/perceptual abnormalities, but no medications are FDA-approved for treating BPD. American Psychiatric Association guidelines recommend symptom-based pharmacologic strategies for BPD,18 although some researchers believe that these recommendations are out-of-date and not evidence-based.17,19 Some evidence suggests pharmacotherapy can have modest short-term benefits on specific BPD symptoms, but no data suggest that medication can reduce the severity of BPD or lead to remission.19-23 Just 1 randomized controlled trial (N = 17) has examined lithium for BPD and found no effect on mood.11,24
Misdiagnosis of BD in the context of BPD may create unrealistic expectations regarding the potential efficacy of medications for relieving symptoms. Patients may be diverted from potentially helpful psychotherapeutic treatments—such as DBT or mentalization therapy—which evidence suggests can effectively reduce symptoms, the need for additional treatments, and self-harm or suicidal behaviors.10,17,19 Evidence from long-term longitudinal studies suggests that psychosocial or psychotherapeutic treatment may protect against suicide in BPD patients.25
Table 2
DSM-IV-TR diagnostic criteria for a manic episode
|
The DSM-IV-TR diagnostic criteria for a hypomanic episode are similar to criteria for a manic episode, except:
|
| Source: Reference 7 |
Related Resources
- National Education Alliance Borderline Personality Disorder. www.borderlinepersonalitydisorder.com.
- Hoffman PD, Steiner-Grossman P. Borderline personality disorder: meeting the challenges to successful treatment. Philadelphia, PA: Haworth Press; 2008.
Drug Brand Names
- Cyclobenzaprine • Flexeril
- Hydrocodone/acetaminophen • Lorcet, Vicodin, others
- Lithium • Eskalith, Lithobid
- Temazepam • Restoril
- Tramadol • Ultram
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Hirschfeld RM, Cass AR, Holt DC, et al. Screening for bipolar disorder in patients treated for depression in a family medicine clinic. J Am Board Fam Pract. 2005;18(4):233-239.
2. Ghaemi SN, Ko JY, Goodwin FK. “Cade’s disease” and beyond: Misdiagnosis antidepressant use, and a proposed definition for bipolar spectrum disorder. Can J Psychiatry. 2002;47(2):125-134.
3. Bowden CL. Strategies to reduce misdiagnosis of bipolar depression. Psychiatr Serv. 2001;52(1):51-55.
4. Moreno C, Laje G, Blanco C, et al. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry. 2007;64(9):1032-1039.
5. Zimmerman M, Ruggero CJ, Chelminski I, et al. Is bipolar disorder overdiagnosed? J Clin Psychiatry. 2008;69(6):935-940.
6. Zimmerman M, Ruggero CJ, Chelminski I, et al. Psychiatric diagnoses in patients previously overdiagnosed with bipolar disorder. J Clin Psychiatry. 2010;71(1):26-31.
7. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
8. Akiskal HS. The bipolar spectrum-the shaping of a new paradigm in psychiatry. Curr Psychiatry Rep. 2002;4(1):1-3.
9. Akiskal HS, Pinto O. The evolving bipolar spectrum. Prototypes I II, III, and IV. Psychiatr Clin North Am. 1999;22(3):517-534, vii.
10. Gunderson JG, Weinberg I, Daversa MT, et al. Descriptive and longitudinal observations on the relationship of borderline personality disorder and bipolar disorder. Am J Psychiatry. 2006;163(7):1173-1178.
11. Paris J, Gunderson J, Weinberg I. The interface between borderline personality disorder and bipolar spectrum disorders. Compr Psychiatry. 2007;48(2):145-154.
12. Paris J. Why psychiatrists are reluctant to diagnose: borderline personality disorder. Psychiatry (Edgmont). 2007;4(1):35-39.
13. Paris J. Borderline or bipolar? Distinguishing borderline personality disorder from bipolar spectrum disorders. Harv Rev Psychiatry. 2004;12(3):140-145.
14. Ruggero CJ, Zimmerman M, Chelminski I, et al. Borderline personality disorder and the misdiagnosis of bipolar disorder. J Psychiatr Res. 2010;44(6):405-408.
15. Zimmerman M. Problems diagnosing bipolar disorder in clinical practice. Expert Rev Neurother. 2010;10(7):1019-1021.
16. Stone MH. Relationship of borderline personality disorder and bipolar disorder. Am J Psychiatry. 2006;163(7):1126-1128.
17. Gunderson JG. Clinical practice. Borderline personality disorder. N Engl J Med. 2011;364(21):2037-2042.
18. American Psychiatric Association. Practice guideline for the treatment of patients with borderline personality disorder. Washington D.C.: American Psychiatric Association; 2001.
19. Paris J. The treatment of borderline personality disorder: implications of research on diagnosis etiology, and outcome. Annu Rev Clin Psychol. 2009;5:277-290.
20. Stoffers J, Völlm BA, Rücker G, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2010;(6):CD005653.-
21. Ripoll LH, Triebwasser J, Siever LJ. Evidence-based pharmacotherapy for personality disorders. Int J Neuropsychopharmacol. 2011;14(9):1257-1288.
22. Mercer D, Douglass AB, Links PS. Meta-analyses of mood stabilizers antidepressants and antipsychotics in the treatment of borderline personality disorder: effectiveness for depression and anger symptoms. J Pers Disord. 2009;23(2):156-174.
23. Lieb K, Völlm B, Rücker G, et al. Pharmacotherapy for borderline personality disorder: Cochrane systematic review of randomised trials. Br J Psychiatry. 2010;196(1):4-12.
24. Links PS, Steiner M, Boiago I, et al. Lithium therapy for borderline patients: preliminary findings. J Pers Disord. 1990;4(2):173-181.
25. Goodman M, Roiff T, Oakes AH, et al. Suicidal risk and management in borderline personality disorder. Curr Psychiatry Rep. 2012;14(1):79-85.
1. Hirschfeld RM, Cass AR, Holt DC, et al. Screening for bipolar disorder in patients treated for depression in a family medicine clinic. J Am Board Fam Pract. 2005;18(4):233-239.
2. Ghaemi SN, Ko JY, Goodwin FK. “Cade’s disease” and beyond: Misdiagnosis antidepressant use, and a proposed definition for bipolar spectrum disorder. Can J Psychiatry. 2002;47(2):125-134.
3. Bowden CL. Strategies to reduce misdiagnosis of bipolar depression. Psychiatr Serv. 2001;52(1):51-55.
4. Moreno C, Laje G, Blanco C, et al. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry. 2007;64(9):1032-1039.
5. Zimmerman M, Ruggero CJ, Chelminski I, et al. Is bipolar disorder overdiagnosed? J Clin Psychiatry. 2008;69(6):935-940.
6. Zimmerman M, Ruggero CJ, Chelminski I, et al. Psychiatric diagnoses in patients previously overdiagnosed with bipolar disorder. J Clin Psychiatry. 2010;71(1):26-31.
7. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
8. Akiskal HS. The bipolar spectrum-the shaping of a new paradigm in psychiatry. Curr Psychiatry Rep. 2002;4(1):1-3.
9. Akiskal HS, Pinto O. The evolving bipolar spectrum. Prototypes I II, III, and IV. Psychiatr Clin North Am. 1999;22(3):517-534, vii.
10. Gunderson JG, Weinberg I, Daversa MT, et al. Descriptive and longitudinal observations on the relationship of borderline personality disorder and bipolar disorder. Am J Psychiatry. 2006;163(7):1173-1178.
11. Paris J, Gunderson J, Weinberg I. The interface between borderline personality disorder and bipolar spectrum disorders. Compr Psychiatry. 2007;48(2):145-154.
12. Paris J. Why psychiatrists are reluctant to diagnose: borderline personality disorder. Psychiatry (Edgmont). 2007;4(1):35-39.
13. Paris J. Borderline or bipolar? Distinguishing borderline personality disorder from bipolar spectrum disorders. Harv Rev Psychiatry. 2004;12(3):140-145.
14. Ruggero CJ, Zimmerman M, Chelminski I, et al. Borderline personality disorder and the misdiagnosis of bipolar disorder. J Psychiatr Res. 2010;44(6):405-408.
15. Zimmerman M. Problems diagnosing bipolar disorder in clinical practice. Expert Rev Neurother. 2010;10(7):1019-1021.
16. Stone MH. Relationship of borderline personality disorder and bipolar disorder. Am J Psychiatry. 2006;163(7):1126-1128.
17. Gunderson JG. Clinical practice. Borderline personality disorder. N Engl J Med. 2011;364(21):2037-2042.
18. American Psychiatric Association. Practice guideline for the treatment of patients with borderline personality disorder. Washington D.C.: American Psychiatric Association; 2001.
19. Paris J. The treatment of borderline personality disorder: implications of research on diagnosis etiology, and outcome. Annu Rev Clin Psychol. 2009;5:277-290.
20. Stoffers J, Völlm BA, Rücker G, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2010;(6):CD005653.-
21. Ripoll LH, Triebwasser J, Siever LJ. Evidence-based pharmacotherapy for personality disorders. Int J Neuropsychopharmacol. 2011;14(9):1257-1288.
22. Mercer D, Douglass AB, Links PS. Meta-analyses of mood stabilizers antidepressants and antipsychotics in the treatment of borderline personality disorder: effectiveness for depression and anger symptoms. J Pers Disord. 2009;23(2):156-174.
23. Lieb K, Völlm B, Rücker G, et al. Pharmacotherapy for borderline personality disorder: Cochrane systematic review of randomised trials. Br J Psychiatry. 2010;196(1):4-12.
24. Links PS, Steiner M, Boiago I, et al. Lithium therapy for borderline patients: preliminary findings. J Pers Disord. 1990;4(2):173-181.
25. Goodman M, Roiff T, Oakes AH, et al. Suicidal risk and management in borderline personality disorder. Curr Psychiatry Rep. 2012;14(1):79-85.
Paranoid, agitated, and manipulative
CASE: Agitation
Mrs. M, age 39, presents to the emergency department (ED) with altered mental status. She is escorted by her husband and the police. She has a history of severe alcohol dependence, bipolar disorder (BD), anxiety, borderline personality disorder (BPD), hypothyroidism, and bulimia, and had gastric bypass surgery 4 years ago. Her husband called 911 when he could no longer manage Mrs. M’s agitated state. The police found her to be extremely paranoid, restless, and disoriented. Her husband reports that she shouted “the world is going to end” before she escaped naked into her neighborhood streets.
On several occasions Mrs. M had been admitted to the same hospital for alcohol withdrawal and dependence with subsequent liver failure, leading to jaundice, coagulopathy, and ascites. During these hospitalizations, she exhibited poor behavioral tendencies, unhealthy psychological defenses, and chronic maladaptive coping and defense mechanisms congruent with her BPD diagnosis. Specifically, she engaged in splitting of hospital staff, ranging from extreme flattery to overt devaluation and hostility. Other defense mechanisms included denial, distortion, acting out, and passive-aggressive behavior. During these admissions, Mrs. M often displayed deficits in recall and attention on Mini-Mental State Examination (MMSE), but these deficits were associated with concurrent alcohol use and improved rapidly during her stay.
In her current presentation, Mrs. M’s mental status change is more pronounced and atypical compared with earlier admissions. Her outpatient medication regimen includes lamotrigine, 100 mg/d, levothyroxine, 88 mcg/d, venlafaxine extended release (XR), 75 mg/d, clonazepam, 3 mg/d, docusate as needed for constipation, and a daily multivitamin.
The authors’ observations
Delirium is a disturbance of consciousness manifested by a reduced clarity of awareness (impairment in attention) and change in cognition (impairment in orientation, memory, and language).1,2 The disturbance develops over a short time and tends to fluctuate during the day. Delirium is a direct physiological consequence of a general medical condition, substance use (intoxication or withdrawal), or both (Table).3
Delirium generally is a reversible mental disorder but can progress to irreversible brain damage. Prompt and accurate diagnosis of delirium is essential,4 although the condition often is underdiagnosed or misdiagnosed because of lack of recognition.
Table
DSM-IV-TR diagnostic criteria for delirium
|
| Source: Reference 3 |
Patients who have convoluted histories, such as Mrs. M, are common and difficult to manage and treat. These patients become substantially more complex when they are admitted to inpatient medical or surgical services. The need to clarify between delirium (primarily medical) and depression (primarily psychiatric) becomes paramount when administering treatment and evaluating decision-making capacity.5 In Mrs. M’s case, internal medicine, neurology, and psychiatry teams each had a different approach to altered mental status. Each team’s different terminology, assessment, and objectives further complicated an already challenging case.6
EVALUATION: Confounding results
The ED physicians offer a working diagnosis of acute mental status change, administer IV lorazepam, 4 mg, and order restraints for Mrs. M’s severe agitation. Her initial vital signs reveal slightly elevated blood pressure (140/90 mm Hg) and tachycardia (115 beats per minute). Internal medicine clinicians note that Mrs. M is not in acute distress, although she refuses to speak and has a small amount of dried blood on her lips, presumably from a struggle with the police before coming to the hospital, but this is not certain. Her abdomen is not tender; she has normal bowel sounds, and no asterixis is noted on neurologic exam. Physical exam is otherwise normal. A noncontrast head CT scan shows no acute process. Initial lab values show elevations in ammonia (277 μg/dL) and γ-glutamyl transpeptidase (68 U/L). Thyroid-stimulating hormone is 1.45 mlU/L, prothrombin time is 19.5 s, partial thromboplastin time is 40.3 s, and international normalized ratio is 1.67. The internal medicine team admits Mrs. M to the intensive care unit (ICU) for further management of her mental status change with alcohol withdrawal or hepatic encephalopathy as the most likely etiologies.
Mrs. M’s husband says that his wife has not consumed alcohol in the last 4 months in preparation for a possible liver transplant; however, past interactions with Mrs. M’s family suggest they are unreliable. The Clinical Institute Withdrawal Assessment (CIWA) protocol is implemented in case her symptoms are caused by alcohol withdrawal. Her vital signs are stable and IV lorazepam, 4 mg, is administered once for agitation. Mrs. M’s husband also reports that 1 month ago his wife underwent a transjugular intrahepatic portosystemic shunt (TIPS) procedure for portal hypertension. Outpatient psychotropics (lamotrigine, 100 mg/d, and venlafaxine XR, 75 mg/d) are restarted because withdrawal from these drugs may exacerbate her symptoms. In the ICU Mrs. M experiences a tonic-clonic seizure with fecal incontinence and bitten tongue, which results in a consultation from neurology and the psychiatry consultation-liaison service.
Psychiatry recommends withholding psychotropics, stopping CIWA, and using vital sign parameters along with objective signs of diaphoresis and tremors as indicators of alcohol withdrawal for lorazepam administration. Mrs. M receives IV haloperidol, 1 mg, once during her second day in the hospital for severe agitation, but this medication is discontinued because of concern about lowering her seizure threshold.7 After treatment with lactulose, her ammonia levels trend down to 33 μg/dL, but her altered mental state persists with significant deficits in attention and orientation.
The neurology service performs an EEG that shows no slow-wave, triphasic waves, or epileptiform activity, which likely would be present in delirium or seizures. See Figure 1 for an example of triphasic waves on an EEG and Figure 2 for Mrs. M's EEG results. Subsequent lumbar puncture, MRI, and a second EEG are unremarkable. By the fifth hospital day, Mrs. M is calm and her paranoia has subsided, but she still is confused and disoriented. Psychiatry orders a third EEG while she is in this confused state; it shows no pathologic process. Based on these examinations, neurology posits that Mrs. M is not encephalopathic.
Figure 1: Representative sample of triphasic waves
This EEG tracing is from a 54-year-old woman who underwent prolonged abdominal surgery for lysis of adhesions during which she suffered an intraoperative left subinsular stroke followed by nonconvulsive status epilepticus. The tracing demonstrates typical morphology with the positive sharp transient preceded and followed by smaller amplitude negative deflections. Symmetric, frontal predominance of findings seen is this tracing is common
Figure 2: Mrs. M’s EEG results
This is a representative tracing of Mrs. M’s 3 EEGs revealing an 8.5 to 9 Hz dominant alpha rhythm. There is superimposed frontally dominant beta fast activity, which is consistent with known administration of benzodiazepines
The authors’ observations
Mrs. M had repeated admissions for alcohol dependence and subsequent liver failure. Her recent hospitalization was complicated by a TIPS procedure done 1 month ago. The incidence of hepatic encephalopathy in patients undergoing TIPS is >30%, especially in the first month post-procedure, which raised suspicion that hepatic encephalopathy played a significant role in Mrs. M’s delirium.8
Because of frequent hospitalization, Mrs. M was well known to the internal medicine, neurology, and psychiatry teams, and each used different terms to describe her mental state. Internal medicine used the phrase “acute mental status change,” which covers a broad differential. Neurology used “encephalopathy,” which also is a general term. Psychiatry used “delirium,” which has narrower and more specific diagnostic criteria. Engel et al9 described the delirious patient as having “cerebral insufficiency” with universally abnormal EEG. Regardless of terminology, based on Mrs. M’s acute confusion, one would expect an abnormal EEG, but repeat EEGs were unremarkable.
Interpreting EEG
EEG is one of the few tools available for measuring acute changes in cerebral function, and an EEG slowing remains a hallmark in encephalopathic processes.10,11 Initially, the 3 specialties agreed that Mrs. M’s presentation likely was caused by underlying medical issues or substances (alcohol or others). EEG can help recognize delirium, and, in some cases, elucidate the underlying cause.10,12 It was surprising that Mrs. M’s EEGs were normal despite a clinical presentation of delirium. Because of the normal EEG findings, neurology leaned toward a primary psychiatric (“functional”) etiology as the cause of her delirium vs a general medical condition or alcohol withdrawal (“organic”).
A literature search in regards to sensitivity of EEG in delirium revealed conflicting statements and data. A standard textbook in neurology and psychiatry states that “a normal EEG virtually excludes a toxic-metabolic encephalopathy.”13 The American Psychiatric Association’s (APA) practice guidelines for delirium states: “The presence of EEG abnormalities has fairly good sensitivities for delirium (in one study, the sensitivity was found to be 75%), but the absence does not rule out the diagnosis; thus the EEG is no substitute for careful clinical observation.”6
At the beginning of Mrs. M’s care, in discussion with the neurology and internal medicine teams, we argued that Mrs. M was experiencing delirium despite her initial normal EEG. We did not expect that 2 subsequent EEGs would be normal, especially because the teams witnessed the final EEG being performed while Mrs. M was clinically evaluated and observed to be in a state of delirium.
OUTCOME: Cause still unknown
By the 6th day of hospitalization, Mrs. M’s vitals are normal and she remains hemodynamically stable. Differential diagnosis remains wide and unclear. The psychiatry team feels she could have atypical catatonia due to an underlying mood disorder. One hour after a trial of IV lorazepam, 1 mg, Mrs. M is more lucid and fully oriented, with MMSE of 28/30 (recall was 1/3), indicating normal cognition. During the exam, a psychiatry resident notes Mrs. M winks and feigns a yawn at the medical students and nurses in the room, displaying her boredom with the interview and simplicity of the mental status exam questions. Later that evening, Mrs. M exhibits bizarre sexual gestures toward male hospital staff, including licking a male nursing staff member’s hand.
Although Mrs. M’s initial confusion resolved, the severity of her comorbid psychiatric history warrants inpatient psychiatric hospitalization. She agrees to transfer to the psychiatric ward after she confesses anxiety regarding death, intense demoralization, and guilt related to her condition and her relationship with her 12-year-old daughter. She tearfully reports that she discontinued her psychotropic medications shortly after stopping alcohol 4 months ago. Shortly before her transfer, psychiatry is called back to the medicine floor because of Mrs. M’s disruptive behavior.
The team finds Mrs. M in her hospital gown, pursuing her husband in the hallway as he is leaving, yelling profanities and blaming him for her horrible experience in the hospital. Based on her demeanor, the team determines that she is back to her baseline mental state despite her mood disorder, and that her upcoming inpatient psychiatric stay likely would be too short to address her comorbid personality disorder. The next day she signs out of the hospital against medical advice.
The authors’ observations
We never clearly identified the specific etiology responsible for Mrs. M’s delirium. We assume at the initial presentation she had toxic-metabolic encephalopathy that rapidly resolved with lactulose treatment and lowering her ammonia. She then had a single tonic-clonic seizure, perhaps related to stopping and then restarting her psychotropics. Her subsequent confusion, bizarre sexual behavior, and demeanor on her final hospital days were more indicative of her psychiatric diagnoses. We now suspect that Mrs. M’s delirium was briefer than presumed and she returned to her baseline borderline personality, resulting in some factitious staging of delirium to confuse her 3 treating teams (a psychoanalyst may say this was a form of projective identification).
We felt that if Mrs. M truly was delirious due to metabolic or hepatic dysfunction or alcohol withdrawal, she would have had abnormal EEG findings. We discovered that the notion of “75% sensitivity” of EEG abnormalities cited in the APA guidelines comes from studies that include patients with “psychogenic” and “organic” delirium. Acute manias and agitated psychoses were termed “psychogenic delirium” and acute confusion due to medical conditions or substance issues was termed “organic delirium.”9,12,14-16
This poses a circular reasoning in the diagnostic criteria and clinical approach to delirium. The fallacy is that, according to DSM-IV-TR, delirium is supposed to be the result of a direct physiological consequence of a general medical condition or substance use (criterion D), and cannot be due to psychosis (eg, schizophrenia) or mania (eg, BD). We question the presumptive 75% sensitivity of EEG abnormalities in patients with delirium because it is possible that when some of these studies were conducted the definition of delirium was not solidified or fully understood. We suspect the sensitivity would be much higher if the correct definition of delirium according to DSM-IV-TR is used in future studies. To improve interdisciplinary communication and future research, it would be constructive if all disciplines could agree on a single term, with the same diagnostic criteria, when evaluating a patient with acute confusion.
Related Resources
- Meagher D. Delirium: the role of psychiatry. Advances in Psychiatric Treatment. 2001;7:433-442.
- Casey DA, DeFazio JV Jr, Vansickle K, et al. Delirium. Quick recognition, careful evaluation, and appropriate treatment. Postgrad Med. 1996;100(1):121-4, 128, 133-134.
Drug Brand Names
- Clonazepam • Klonopin
- Docusate • Surfak
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Lorazepam • Ativan
- Levothyroxine • Levoxyl, Synthtoid
- Venlafaxine XR • Effexor XR
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the U.S. Government. The authors are employees of the U.S. Government. This work was prepared as part of their official duties. Title 17 U.S.C. 105 provides that “Copyright protection under this title is not available for any work of the U.S. Government.” Title 17 U.S.C. 101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
1. Katz IR, Mossey J, Sussman N, et al. Bedside clinical and electrophysiological assessment: assessment of change in vulnerable patients. Int Psychogeriatr. 1991;3(2):289-300.
2. Inouye SK. Delirium in older persons. N Engl J Med. 2006;354(11):1157-1165.
3. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
4. McPhee SJ, Papadakis M, Rabow MW. CURRENT medical diagnosis and treatment. New York NY: McGraw Hill Medical; 2012.
5. Brody B. Who has capacity? N Engl J Med. 2009;361(3):232-233.
6. Practice guideline for the treatment of patients with delirium. American Psychiatric Association. Am J Psychiatry. 1999;156(5 suppl):1-20.
7. Fricchione GL, Nejad SH, Esses JA, et al. Postoperative delirium. Am J Psychiatry. 2008;165(7):803-812.
8. Sanyal AJ, Freedman AM, Shiffman ML, et al. Portosystemic encephalopathy after transjugular intrahepatic portosystemic shunt: results of a prospective controlled study. Hepatology. 1994;20(1 pt 1):46-55.
9. Engel GL, Romano J. Delirium a syndrome of cerebral insufficiency. 1959. J Neuropsychiatry Clin Neurosci. 2004;16(4):526-538.
10. Pro JD, Wells CE. The use of the electroencephalogram in the diagnosis of delirium. Dis Nerv Syst. 1977;38(10):804-808.
11. Sidhu KS, Balon R, Ajluni V, et al. Standard EEG and the difficult-to-assess mental status. Ann Clin Psychiatry. 2009;21(2):103-108.
12. Brenner RP. Utility of EEG in delirium: past views and current practice. Int Psychogeriatr. 1991;3(2):211-229.
13. Kaufman DM. Clinical neurology for psychiatrists. 5th ed. Philadelphia PA: Saunders; 2001: 230-232.
14. Bond TC. Recognition of acute delirious mania. Arch Gen Psychiatry. 1980;37(5):553-554.
15. Krauthammer C, Klerman GL. Secondary mania: manic syndromes associated with antecedent physical illness or drugs. Arch Gen Psychiatry. 1978;35(11):1333-1339.
16. Larson EW, Richelson E. Organic causes of mania. Mayo Clin Proc. 1988;63(9):906-912.
CASE: Agitation
Mrs. M, age 39, presents to the emergency department (ED) with altered mental status. She is escorted by her husband and the police. She has a history of severe alcohol dependence, bipolar disorder (BD), anxiety, borderline personality disorder (BPD), hypothyroidism, and bulimia, and had gastric bypass surgery 4 years ago. Her husband called 911 when he could no longer manage Mrs. M’s agitated state. The police found her to be extremely paranoid, restless, and disoriented. Her husband reports that she shouted “the world is going to end” before she escaped naked into her neighborhood streets.
On several occasions Mrs. M had been admitted to the same hospital for alcohol withdrawal and dependence with subsequent liver failure, leading to jaundice, coagulopathy, and ascites. During these hospitalizations, she exhibited poor behavioral tendencies, unhealthy psychological defenses, and chronic maladaptive coping and defense mechanisms congruent with her BPD diagnosis. Specifically, she engaged in splitting of hospital staff, ranging from extreme flattery to overt devaluation and hostility. Other defense mechanisms included denial, distortion, acting out, and passive-aggressive behavior. During these admissions, Mrs. M often displayed deficits in recall and attention on Mini-Mental State Examination (MMSE), but these deficits were associated with concurrent alcohol use and improved rapidly during her stay.
In her current presentation, Mrs. M’s mental status change is more pronounced and atypical compared with earlier admissions. Her outpatient medication regimen includes lamotrigine, 100 mg/d, levothyroxine, 88 mcg/d, venlafaxine extended release (XR), 75 mg/d, clonazepam, 3 mg/d, docusate as needed for constipation, and a daily multivitamin.
The authors’ observations
Delirium is a disturbance of consciousness manifested by a reduced clarity of awareness (impairment in attention) and change in cognition (impairment in orientation, memory, and language).1,2 The disturbance develops over a short time and tends to fluctuate during the day. Delirium is a direct physiological consequence of a general medical condition, substance use (intoxication or withdrawal), or both (Table).3
Delirium generally is a reversible mental disorder but can progress to irreversible brain damage. Prompt and accurate diagnosis of delirium is essential,4 although the condition often is underdiagnosed or misdiagnosed because of lack of recognition.
Table
DSM-IV-TR diagnostic criteria for delirium
|
| Source: Reference 3 |
Patients who have convoluted histories, such as Mrs. M, are common and difficult to manage and treat. These patients become substantially more complex when they are admitted to inpatient medical or surgical services. The need to clarify between delirium (primarily medical) and depression (primarily psychiatric) becomes paramount when administering treatment and evaluating decision-making capacity.5 In Mrs. M’s case, internal medicine, neurology, and psychiatry teams each had a different approach to altered mental status. Each team’s different terminology, assessment, and objectives further complicated an already challenging case.6
EVALUATION: Confounding results
The ED physicians offer a working diagnosis of acute mental status change, administer IV lorazepam, 4 mg, and order restraints for Mrs. M’s severe agitation. Her initial vital signs reveal slightly elevated blood pressure (140/90 mm Hg) and tachycardia (115 beats per minute). Internal medicine clinicians note that Mrs. M is not in acute distress, although she refuses to speak and has a small amount of dried blood on her lips, presumably from a struggle with the police before coming to the hospital, but this is not certain. Her abdomen is not tender; she has normal bowel sounds, and no asterixis is noted on neurologic exam. Physical exam is otherwise normal. A noncontrast head CT scan shows no acute process. Initial lab values show elevations in ammonia (277 μg/dL) and γ-glutamyl transpeptidase (68 U/L). Thyroid-stimulating hormone is 1.45 mlU/L, prothrombin time is 19.5 s, partial thromboplastin time is 40.3 s, and international normalized ratio is 1.67. The internal medicine team admits Mrs. M to the intensive care unit (ICU) for further management of her mental status change with alcohol withdrawal or hepatic encephalopathy as the most likely etiologies.
Mrs. M’s husband says that his wife has not consumed alcohol in the last 4 months in preparation for a possible liver transplant; however, past interactions with Mrs. M’s family suggest they are unreliable. The Clinical Institute Withdrawal Assessment (CIWA) protocol is implemented in case her symptoms are caused by alcohol withdrawal. Her vital signs are stable and IV lorazepam, 4 mg, is administered once for agitation. Mrs. M’s husband also reports that 1 month ago his wife underwent a transjugular intrahepatic portosystemic shunt (TIPS) procedure for portal hypertension. Outpatient psychotropics (lamotrigine, 100 mg/d, and venlafaxine XR, 75 mg/d) are restarted because withdrawal from these drugs may exacerbate her symptoms. In the ICU Mrs. M experiences a tonic-clonic seizure with fecal incontinence and bitten tongue, which results in a consultation from neurology and the psychiatry consultation-liaison service.
Psychiatry recommends withholding psychotropics, stopping CIWA, and using vital sign parameters along with objective signs of diaphoresis and tremors as indicators of alcohol withdrawal for lorazepam administration. Mrs. M receives IV haloperidol, 1 mg, once during her second day in the hospital for severe agitation, but this medication is discontinued because of concern about lowering her seizure threshold.7 After treatment with lactulose, her ammonia levels trend down to 33 μg/dL, but her altered mental state persists with significant deficits in attention and orientation.
The neurology service performs an EEG that shows no slow-wave, triphasic waves, or epileptiform activity, which likely would be present in delirium or seizures. See Figure 1 for an example of triphasic waves on an EEG and Figure 2 for Mrs. M's EEG results. Subsequent lumbar puncture, MRI, and a second EEG are unremarkable. By the fifth hospital day, Mrs. M is calm and her paranoia has subsided, but she still is confused and disoriented. Psychiatry orders a third EEG while she is in this confused state; it shows no pathologic process. Based on these examinations, neurology posits that Mrs. M is not encephalopathic.
Figure 1: Representative sample of triphasic waves
This EEG tracing is from a 54-year-old woman who underwent prolonged abdominal surgery for lysis of adhesions during which she suffered an intraoperative left subinsular stroke followed by nonconvulsive status epilepticus. The tracing demonstrates typical morphology with the positive sharp transient preceded and followed by smaller amplitude negative deflections. Symmetric, frontal predominance of findings seen is this tracing is common
Figure 2: Mrs. M’s EEG results
This is a representative tracing of Mrs. M’s 3 EEGs revealing an 8.5 to 9 Hz dominant alpha rhythm. There is superimposed frontally dominant beta fast activity, which is consistent with known administration of benzodiazepines
The authors’ observations
Mrs. M had repeated admissions for alcohol dependence and subsequent liver failure. Her recent hospitalization was complicated by a TIPS procedure done 1 month ago. The incidence of hepatic encephalopathy in patients undergoing TIPS is >30%, especially in the first month post-procedure, which raised suspicion that hepatic encephalopathy played a significant role in Mrs. M’s delirium.8
Because of frequent hospitalization, Mrs. M was well known to the internal medicine, neurology, and psychiatry teams, and each used different terms to describe her mental state. Internal medicine used the phrase “acute mental status change,” which covers a broad differential. Neurology used “encephalopathy,” which also is a general term. Psychiatry used “delirium,” which has narrower and more specific diagnostic criteria. Engel et al9 described the delirious patient as having “cerebral insufficiency” with universally abnormal EEG. Regardless of terminology, based on Mrs. M’s acute confusion, one would expect an abnormal EEG, but repeat EEGs were unremarkable.
Interpreting EEG
EEG is one of the few tools available for measuring acute changes in cerebral function, and an EEG slowing remains a hallmark in encephalopathic processes.10,11 Initially, the 3 specialties agreed that Mrs. M’s presentation likely was caused by underlying medical issues or substances (alcohol or others). EEG can help recognize delirium, and, in some cases, elucidate the underlying cause.10,12 It was surprising that Mrs. M’s EEGs were normal despite a clinical presentation of delirium. Because of the normal EEG findings, neurology leaned toward a primary psychiatric (“functional”) etiology as the cause of her delirium vs a general medical condition or alcohol withdrawal (“organic”).
A literature search in regards to sensitivity of EEG in delirium revealed conflicting statements and data. A standard textbook in neurology and psychiatry states that “a normal EEG virtually excludes a toxic-metabolic encephalopathy.”13 The American Psychiatric Association’s (APA) practice guidelines for delirium states: “The presence of EEG abnormalities has fairly good sensitivities for delirium (in one study, the sensitivity was found to be 75%), but the absence does not rule out the diagnosis; thus the EEG is no substitute for careful clinical observation.”6
At the beginning of Mrs. M’s care, in discussion with the neurology and internal medicine teams, we argued that Mrs. M was experiencing delirium despite her initial normal EEG. We did not expect that 2 subsequent EEGs would be normal, especially because the teams witnessed the final EEG being performed while Mrs. M was clinically evaluated and observed to be in a state of delirium.
OUTCOME: Cause still unknown
By the 6th day of hospitalization, Mrs. M’s vitals are normal and she remains hemodynamically stable. Differential diagnosis remains wide and unclear. The psychiatry team feels she could have atypical catatonia due to an underlying mood disorder. One hour after a trial of IV lorazepam, 1 mg, Mrs. M is more lucid and fully oriented, with MMSE of 28/30 (recall was 1/3), indicating normal cognition. During the exam, a psychiatry resident notes Mrs. M winks and feigns a yawn at the medical students and nurses in the room, displaying her boredom with the interview and simplicity of the mental status exam questions. Later that evening, Mrs. M exhibits bizarre sexual gestures toward male hospital staff, including licking a male nursing staff member’s hand.
Although Mrs. M’s initial confusion resolved, the severity of her comorbid psychiatric history warrants inpatient psychiatric hospitalization. She agrees to transfer to the psychiatric ward after she confesses anxiety regarding death, intense demoralization, and guilt related to her condition and her relationship with her 12-year-old daughter. She tearfully reports that she discontinued her psychotropic medications shortly after stopping alcohol 4 months ago. Shortly before her transfer, psychiatry is called back to the medicine floor because of Mrs. M’s disruptive behavior.
The team finds Mrs. M in her hospital gown, pursuing her husband in the hallway as he is leaving, yelling profanities and blaming him for her horrible experience in the hospital. Based on her demeanor, the team determines that she is back to her baseline mental state despite her mood disorder, and that her upcoming inpatient psychiatric stay likely would be too short to address her comorbid personality disorder. The next day she signs out of the hospital against medical advice.
The authors’ observations
We never clearly identified the specific etiology responsible for Mrs. M’s delirium. We assume at the initial presentation she had toxic-metabolic encephalopathy that rapidly resolved with lactulose treatment and lowering her ammonia. She then had a single tonic-clonic seizure, perhaps related to stopping and then restarting her psychotropics. Her subsequent confusion, bizarre sexual behavior, and demeanor on her final hospital days were more indicative of her psychiatric diagnoses. We now suspect that Mrs. M’s delirium was briefer than presumed and she returned to her baseline borderline personality, resulting in some factitious staging of delirium to confuse her 3 treating teams (a psychoanalyst may say this was a form of projective identification).
We felt that if Mrs. M truly was delirious due to metabolic or hepatic dysfunction or alcohol withdrawal, she would have had abnormal EEG findings. We discovered that the notion of “75% sensitivity” of EEG abnormalities cited in the APA guidelines comes from studies that include patients with “psychogenic” and “organic” delirium. Acute manias and agitated psychoses were termed “psychogenic delirium” and acute confusion due to medical conditions or substance issues was termed “organic delirium.”9,12,14-16
This poses a circular reasoning in the diagnostic criteria and clinical approach to delirium. The fallacy is that, according to DSM-IV-TR, delirium is supposed to be the result of a direct physiological consequence of a general medical condition or substance use (criterion D), and cannot be due to psychosis (eg, schizophrenia) or mania (eg, BD). We question the presumptive 75% sensitivity of EEG abnormalities in patients with delirium because it is possible that when some of these studies were conducted the definition of delirium was not solidified or fully understood. We suspect the sensitivity would be much higher if the correct definition of delirium according to DSM-IV-TR is used in future studies. To improve interdisciplinary communication and future research, it would be constructive if all disciplines could agree on a single term, with the same diagnostic criteria, when evaluating a patient with acute confusion.
Related Resources
- Meagher D. Delirium: the role of psychiatry. Advances in Psychiatric Treatment. 2001;7:433-442.
- Casey DA, DeFazio JV Jr, Vansickle K, et al. Delirium. Quick recognition, careful evaluation, and appropriate treatment. Postgrad Med. 1996;100(1):121-4, 128, 133-134.
Drug Brand Names
- Clonazepam • Klonopin
- Docusate • Surfak
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Lorazepam • Ativan
- Levothyroxine • Levoxyl, Synthtoid
- Venlafaxine XR • Effexor XR
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the U.S. Government. The authors are employees of the U.S. Government. This work was prepared as part of their official duties. Title 17 U.S.C. 105 provides that “Copyright protection under this title is not available for any work of the U.S. Government.” Title 17 U.S.C. 101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
CASE: Agitation
Mrs. M, age 39, presents to the emergency department (ED) with altered mental status. She is escorted by her husband and the police. She has a history of severe alcohol dependence, bipolar disorder (BD), anxiety, borderline personality disorder (BPD), hypothyroidism, and bulimia, and had gastric bypass surgery 4 years ago. Her husband called 911 when he could no longer manage Mrs. M’s agitated state. The police found her to be extremely paranoid, restless, and disoriented. Her husband reports that she shouted “the world is going to end” before she escaped naked into her neighborhood streets.
On several occasions Mrs. M had been admitted to the same hospital for alcohol withdrawal and dependence with subsequent liver failure, leading to jaundice, coagulopathy, and ascites. During these hospitalizations, she exhibited poor behavioral tendencies, unhealthy psychological defenses, and chronic maladaptive coping and defense mechanisms congruent with her BPD diagnosis. Specifically, she engaged in splitting of hospital staff, ranging from extreme flattery to overt devaluation and hostility. Other defense mechanisms included denial, distortion, acting out, and passive-aggressive behavior. During these admissions, Mrs. M often displayed deficits in recall and attention on Mini-Mental State Examination (MMSE), but these deficits were associated with concurrent alcohol use and improved rapidly during her stay.
In her current presentation, Mrs. M’s mental status change is more pronounced and atypical compared with earlier admissions. Her outpatient medication regimen includes lamotrigine, 100 mg/d, levothyroxine, 88 mcg/d, venlafaxine extended release (XR), 75 mg/d, clonazepam, 3 mg/d, docusate as needed for constipation, and a daily multivitamin.
The authors’ observations
Delirium is a disturbance of consciousness manifested by a reduced clarity of awareness (impairment in attention) and change in cognition (impairment in orientation, memory, and language).1,2 The disturbance develops over a short time and tends to fluctuate during the day. Delirium is a direct physiological consequence of a general medical condition, substance use (intoxication or withdrawal), or both (Table).3
Delirium generally is a reversible mental disorder but can progress to irreversible brain damage. Prompt and accurate diagnosis of delirium is essential,4 although the condition often is underdiagnosed or misdiagnosed because of lack of recognition.
Table
DSM-IV-TR diagnostic criteria for delirium
|
| Source: Reference 3 |
Patients who have convoluted histories, such as Mrs. M, are common and difficult to manage and treat. These patients become substantially more complex when they are admitted to inpatient medical or surgical services. The need to clarify between delirium (primarily medical) and depression (primarily psychiatric) becomes paramount when administering treatment and evaluating decision-making capacity.5 In Mrs. M’s case, internal medicine, neurology, and psychiatry teams each had a different approach to altered mental status. Each team’s different terminology, assessment, and objectives further complicated an already challenging case.6
EVALUATION: Confounding results
The ED physicians offer a working diagnosis of acute mental status change, administer IV lorazepam, 4 mg, and order restraints for Mrs. M’s severe agitation. Her initial vital signs reveal slightly elevated blood pressure (140/90 mm Hg) and tachycardia (115 beats per minute). Internal medicine clinicians note that Mrs. M is not in acute distress, although she refuses to speak and has a small amount of dried blood on her lips, presumably from a struggle with the police before coming to the hospital, but this is not certain. Her abdomen is not tender; she has normal bowel sounds, and no asterixis is noted on neurologic exam. Physical exam is otherwise normal. A noncontrast head CT scan shows no acute process. Initial lab values show elevations in ammonia (277 μg/dL) and γ-glutamyl transpeptidase (68 U/L). Thyroid-stimulating hormone is 1.45 mlU/L, prothrombin time is 19.5 s, partial thromboplastin time is 40.3 s, and international normalized ratio is 1.67. The internal medicine team admits Mrs. M to the intensive care unit (ICU) for further management of her mental status change with alcohol withdrawal or hepatic encephalopathy as the most likely etiologies.
Mrs. M’s husband says that his wife has not consumed alcohol in the last 4 months in preparation for a possible liver transplant; however, past interactions with Mrs. M’s family suggest they are unreliable. The Clinical Institute Withdrawal Assessment (CIWA) protocol is implemented in case her symptoms are caused by alcohol withdrawal. Her vital signs are stable and IV lorazepam, 4 mg, is administered once for agitation. Mrs. M’s husband also reports that 1 month ago his wife underwent a transjugular intrahepatic portosystemic shunt (TIPS) procedure for portal hypertension. Outpatient psychotropics (lamotrigine, 100 mg/d, and venlafaxine XR, 75 mg/d) are restarted because withdrawal from these drugs may exacerbate her symptoms. In the ICU Mrs. M experiences a tonic-clonic seizure with fecal incontinence and bitten tongue, which results in a consultation from neurology and the psychiatry consultation-liaison service.
Psychiatry recommends withholding psychotropics, stopping CIWA, and using vital sign parameters along with objective signs of diaphoresis and tremors as indicators of alcohol withdrawal for lorazepam administration. Mrs. M receives IV haloperidol, 1 mg, once during her second day in the hospital for severe agitation, but this medication is discontinued because of concern about lowering her seizure threshold.7 After treatment with lactulose, her ammonia levels trend down to 33 μg/dL, but her altered mental state persists with significant deficits in attention and orientation.
The neurology service performs an EEG that shows no slow-wave, triphasic waves, or epileptiform activity, which likely would be present in delirium or seizures. See Figure 1 for an example of triphasic waves on an EEG and Figure 2 for Mrs. M's EEG results. Subsequent lumbar puncture, MRI, and a second EEG are unremarkable. By the fifth hospital day, Mrs. M is calm and her paranoia has subsided, but she still is confused and disoriented. Psychiatry orders a third EEG while she is in this confused state; it shows no pathologic process. Based on these examinations, neurology posits that Mrs. M is not encephalopathic.
Figure 1: Representative sample of triphasic waves
This EEG tracing is from a 54-year-old woman who underwent prolonged abdominal surgery for lysis of adhesions during which she suffered an intraoperative left subinsular stroke followed by nonconvulsive status epilepticus. The tracing demonstrates typical morphology with the positive sharp transient preceded and followed by smaller amplitude negative deflections. Symmetric, frontal predominance of findings seen is this tracing is common
Figure 2: Mrs. M’s EEG results
This is a representative tracing of Mrs. M’s 3 EEGs revealing an 8.5 to 9 Hz dominant alpha rhythm. There is superimposed frontally dominant beta fast activity, which is consistent with known administration of benzodiazepines
The authors’ observations
Mrs. M had repeated admissions for alcohol dependence and subsequent liver failure. Her recent hospitalization was complicated by a TIPS procedure done 1 month ago. The incidence of hepatic encephalopathy in patients undergoing TIPS is >30%, especially in the first month post-procedure, which raised suspicion that hepatic encephalopathy played a significant role in Mrs. M’s delirium.8
Because of frequent hospitalization, Mrs. M was well known to the internal medicine, neurology, and psychiatry teams, and each used different terms to describe her mental state. Internal medicine used the phrase “acute mental status change,” which covers a broad differential. Neurology used “encephalopathy,” which also is a general term. Psychiatry used “delirium,” which has narrower and more specific diagnostic criteria. Engel et al9 described the delirious patient as having “cerebral insufficiency” with universally abnormal EEG. Regardless of terminology, based on Mrs. M’s acute confusion, one would expect an abnormal EEG, but repeat EEGs were unremarkable.
Interpreting EEG
EEG is one of the few tools available for measuring acute changes in cerebral function, and an EEG slowing remains a hallmark in encephalopathic processes.10,11 Initially, the 3 specialties agreed that Mrs. M’s presentation likely was caused by underlying medical issues or substances (alcohol or others). EEG can help recognize delirium, and, in some cases, elucidate the underlying cause.10,12 It was surprising that Mrs. M’s EEGs were normal despite a clinical presentation of delirium. Because of the normal EEG findings, neurology leaned toward a primary psychiatric (“functional”) etiology as the cause of her delirium vs a general medical condition or alcohol withdrawal (“organic”).
A literature search in regards to sensitivity of EEG in delirium revealed conflicting statements and data. A standard textbook in neurology and psychiatry states that “a normal EEG virtually excludes a toxic-metabolic encephalopathy.”13 The American Psychiatric Association’s (APA) practice guidelines for delirium states: “The presence of EEG abnormalities has fairly good sensitivities for delirium (in one study, the sensitivity was found to be 75%), but the absence does not rule out the diagnosis; thus the EEG is no substitute for careful clinical observation.”6
At the beginning of Mrs. M’s care, in discussion with the neurology and internal medicine teams, we argued that Mrs. M was experiencing delirium despite her initial normal EEG. We did not expect that 2 subsequent EEGs would be normal, especially because the teams witnessed the final EEG being performed while Mrs. M was clinically evaluated and observed to be in a state of delirium.
OUTCOME: Cause still unknown
By the 6th day of hospitalization, Mrs. M’s vitals are normal and she remains hemodynamically stable. Differential diagnosis remains wide and unclear. The psychiatry team feels she could have atypical catatonia due to an underlying mood disorder. One hour after a trial of IV lorazepam, 1 mg, Mrs. M is more lucid and fully oriented, with MMSE of 28/30 (recall was 1/3), indicating normal cognition. During the exam, a psychiatry resident notes Mrs. M winks and feigns a yawn at the medical students and nurses in the room, displaying her boredom with the interview and simplicity of the mental status exam questions. Later that evening, Mrs. M exhibits bizarre sexual gestures toward male hospital staff, including licking a male nursing staff member’s hand.
Although Mrs. M’s initial confusion resolved, the severity of her comorbid psychiatric history warrants inpatient psychiatric hospitalization. She agrees to transfer to the psychiatric ward after she confesses anxiety regarding death, intense demoralization, and guilt related to her condition and her relationship with her 12-year-old daughter. She tearfully reports that she discontinued her psychotropic medications shortly after stopping alcohol 4 months ago. Shortly before her transfer, psychiatry is called back to the medicine floor because of Mrs. M’s disruptive behavior.
The team finds Mrs. M in her hospital gown, pursuing her husband in the hallway as he is leaving, yelling profanities and blaming him for her horrible experience in the hospital. Based on her demeanor, the team determines that she is back to her baseline mental state despite her mood disorder, and that her upcoming inpatient psychiatric stay likely would be too short to address her comorbid personality disorder. The next day she signs out of the hospital against medical advice.
The authors’ observations
We never clearly identified the specific etiology responsible for Mrs. M’s delirium. We assume at the initial presentation she had toxic-metabolic encephalopathy that rapidly resolved with lactulose treatment and lowering her ammonia. She then had a single tonic-clonic seizure, perhaps related to stopping and then restarting her psychotropics. Her subsequent confusion, bizarre sexual behavior, and demeanor on her final hospital days were more indicative of her psychiatric diagnoses. We now suspect that Mrs. M’s delirium was briefer than presumed and she returned to her baseline borderline personality, resulting in some factitious staging of delirium to confuse her 3 treating teams (a psychoanalyst may say this was a form of projective identification).
We felt that if Mrs. M truly was delirious due to metabolic or hepatic dysfunction or alcohol withdrawal, she would have had abnormal EEG findings. We discovered that the notion of “75% sensitivity” of EEG abnormalities cited in the APA guidelines comes from studies that include patients with “psychogenic” and “organic” delirium. Acute manias and agitated psychoses were termed “psychogenic delirium” and acute confusion due to medical conditions or substance issues was termed “organic delirium.”9,12,14-16
This poses a circular reasoning in the diagnostic criteria and clinical approach to delirium. The fallacy is that, according to DSM-IV-TR, delirium is supposed to be the result of a direct physiological consequence of a general medical condition or substance use (criterion D), and cannot be due to psychosis (eg, schizophrenia) or mania (eg, BD). We question the presumptive 75% sensitivity of EEG abnormalities in patients with delirium because it is possible that when some of these studies were conducted the definition of delirium was not solidified or fully understood. We suspect the sensitivity would be much higher if the correct definition of delirium according to DSM-IV-TR is used in future studies. To improve interdisciplinary communication and future research, it would be constructive if all disciplines could agree on a single term, with the same diagnostic criteria, when evaluating a patient with acute confusion.
Related Resources
- Meagher D. Delirium: the role of psychiatry. Advances in Psychiatric Treatment. 2001;7:433-442.
- Casey DA, DeFazio JV Jr, Vansickle K, et al. Delirium. Quick recognition, careful evaluation, and appropriate treatment. Postgrad Med. 1996;100(1):121-4, 128, 133-134.
Drug Brand Names
- Clonazepam • Klonopin
- Docusate • Surfak
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Lorazepam • Ativan
- Levothyroxine • Levoxyl, Synthtoid
- Venlafaxine XR • Effexor XR
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the U.S. Government. The authors are employees of the U.S. Government. This work was prepared as part of their official duties. Title 17 U.S.C. 105 provides that “Copyright protection under this title is not available for any work of the U.S. Government.” Title 17 U.S.C. 101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
1. Katz IR, Mossey J, Sussman N, et al. Bedside clinical and electrophysiological assessment: assessment of change in vulnerable patients. Int Psychogeriatr. 1991;3(2):289-300.
2. Inouye SK. Delirium in older persons. N Engl J Med. 2006;354(11):1157-1165.
3. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
4. McPhee SJ, Papadakis M, Rabow MW. CURRENT medical diagnosis and treatment. New York NY: McGraw Hill Medical; 2012.
5. Brody B. Who has capacity? N Engl J Med. 2009;361(3):232-233.
6. Practice guideline for the treatment of patients with delirium. American Psychiatric Association. Am J Psychiatry. 1999;156(5 suppl):1-20.
7. Fricchione GL, Nejad SH, Esses JA, et al. Postoperative delirium. Am J Psychiatry. 2008;165(7):803-812.
8. Sanyal AJ, Freedman AM, Shiffman ML, et al. Portosystemic encephalopathy after transjugular intrahepatic portosystemic shunt: results of a prospective controlled study. Hepatology. 1994;20(1 pt 1):46-55.
9. Engel GL, Romano J. Delirium a syndrome of cerebral insufficiency. 1959. J Neuropsychiatry Clin Neurosci. 2004;16(4):526-538.
10. Pro JD, Wells CE. The use of the electroencephalogram in the diagnosis of delirium. Dis Nerv Syst. 1977;38(10):804-808.
11. Sidhu KS, Balon R, Ajluni V, et al. Standard EEG and the difficult-to-assess mental status. Ann Clin Psychiatry. 2009;21(2):103-108.
12. Brenner RP. Utility of EEG in delirium: past views and current practice. Int Psychogeriatr. 1991;3(2):211-229.
13. Kaufman DM. Clinical neurology for psychiatrists. 5th ed. Philadelphia PA: Saunders; 2001: 230-232.
14. Bond TC. Recognition of acute delirious mania. Arch Gen Psychiatry. 1980;37(5):553-554.
15. Krauthammer C, Klerman GL. Secondary mania: manic syndromes associated with antecedent physical illness or drugs. Arch Gen Psychiatry. 1978;35(11):1333-1339.
16. Larson EW, Richelson E. Organic causes of mania. Mayo Clin Proc. 1988;63(9):906-912.
1. Katz IR, Mossey J, Sussman N, et al. Bedside clinical and electrophysiological assessment: assessment of change in vulnerable patients. Int Psychogeriatr. 1991;3(2):289-300.
2. Inouye SK. Delirium in older persons. N Engl J Med. 2006;354(11):1157-1165.
3. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
4. McPhee SJ, Papadakis M, Rabow MW. CURRENT medical diagnosis and treatment. New York NY: McGraw Hill Medical; 2012.
5. Brody B. Who has capacity? N Engl J Med. 2009;361(3):232-233.
6. Practice guideline for the treatment of patients with delirium. American Psychiatric Association. Am J Psychiatry. 1999;156(5 suppl):1-20.
7. Fricchione GL, Nejad SH, Esses JA, et al. Postoperative delirium. Am J Psychiatry. 2008;165(7):803-812.
8. Sanyal AJ, Freedman AM, Shiffman ML, et al. Portosystemic encephalopathy after transjugular intrahepatic portosystemic shunt: results of a prospective controlled study. Hepatology. 1994;20(1 pt 1):46-55.
9. Engel GL, Romano J. Delirium a syndrome of cerebral insufficiency. 1959. J Neuropsychiatry Clin Neurosci. 2004;16(4):526-538.
10. Pro JD, Wells CE. The use of the electroencephalogram in the diagnosis of delirium. Dis Nerv Syst. 1977;38(10):804-808.
11. Sidhu KS, Balon R, Ajluni V, et al. Standard EEG and the difficult-to-assess mental status. Ann Clin Psychiatry. 2009;21(2):103-108.
12. Brenner RP. Utility of EEG in delirium: past views and current practice. Int Psychogeriatr. 1991;3(2):211-229.
13. Kaufman DM. Clinical neurology for psychiatrists. 5th ed. Philadelphia PA: Saunders; 2001: 230-232.
14. Bond TC. Recognition of acute delirious mania. Arch Gen Psychiatry. 1980;37(5):553-554.
15. Krauthammer C, Klerman GL. Secondary mania: manic syndromes associated with antecedent physical illness or drugs. Arch Gen Psychiatry. 1978;35(11):1333-1339.
16. Larson EW, Richelson E. Organic causes of mania. Mayo Clin Proc. 1988;63(9):906-912.
Paranoia and slowed cognition
CASE: Behavioral changes
Mr. K, age 45, is brought to the emergency department (ED) by his wife for severe paranoia, combative behavior, confusion, and slowed cognition. Mr. K tells the ED staff that a chemical abrasion he sustained a few weeks earlier has spread to his penis, and insists that his penis is retracting into his body. He has tied a string around his penis to keep it from disappearing into his body. According to Mr. K’s wife, he went to an urgent care clinic 2 weeks ago after he sustained chemical abrasions from exposure to cleaning solution at home. The provider at the urgent care clinic started Mr. K on an unknown dose of oral prednisone.
Mr. K’s wife reports that her husband had a dysphoric episode approximately 6 months ago when his business was struggling but his mood improved without psychiatric care. Mr. K’s medical history includes episodic sarcoidosis of the eyes, skin, and lungs. In the past these symptoms remitted after he received oral prednisone.
ED clinicians consider neurosarcoidosis and substance-induced delirium in the differential diagnosis (Table).1 A CT scan of the head fails to show lesions suggestive of neurosarcoidosis. Chest radiography does not reveal lesions suggestive of lung sarcoids and Mr. K has no skin lesions.
Table
DSM-IV-TR criteria for substance-induced delirium
|
| Source: Reference 1 |
Mr. K is admitted to the psychiatric inpatient unit for acute stabilization, where he remains aggressive and combative. He throws chairs at his peers and staff on the unit and is placed in physical restraints. He requires several doses of IM haloperidol, 5 mg, lorazepam, 2 mg, and diphenhydramine, 50 mg, for severe agitation. Mr. K is guarded, perseverative, and selectively mute. He avoids eye contact and has poor grooming. He has slow thought processing and displays concrete thought process. Prednisone is discontinued and olanzapine, titrated to 30 mg/d, and mirtazapine, titrated to 30 mg/d, are started for psychosis and depression.
Mr. K’s mood and behavior eventually return to baseline but slowed cognition persists. He is discharged from our facility.
The authors’ observations
Cortisone was first used to treat rheumatoid arthritis in 1948 and corticosteroids have been linked to multiple neuropsychiatric complications that have been broadly defined as steroid psychosis. This syndrome includes reversible behavioral manifestations such as hypomania, irritability, mood reactivity, anxiety, and insomnia in addition to more severe symptoms such as depression, mania, and psychosis.2 Although mild cognitive deficits have been noted in patients taking corticosteroids, most published cases have focused on steroid-induced psychosis.
In 1984, Varney et al3 noted a phenomenon they called “steroid dementia” in 6 patients treated with corticosteroids. On first evaluation, these patients presented with symptoms similar to early Alzheimer’s dementia—impaired memory, attention, and concentration. Three patients initially were diagnosed first with Alzheimer’s dementia until their symptoms spontaneously improved when steroids were reduced or discontinued. Although their presentation resembled Alzheimer’s dementia, patients with steroid dementia had a specific cognitive presentation associated with corticosteroid use. Symptoms included impaired verbal memory and spatial thinking but normal procedural memory. These patients showed intact immediate recall but impaired delayed recall with difficulty tracking conversations and word finding. Overall, patients with steroid dementia showed a predominance of verbal declarative memory deficits out of proportion to other cognitive symptoms. These symptoms and recent corticosteroid exposure differentiated steroid dementia from other forms of dementia.
In a later article, Varney reviewed electroencephalography (EEG) and CT findings associated with steroid dementia, noting bilateral EEG abnormalities and acute cortical atrophy on CT.4 Steroid dementia largely was reversible, resolving 3 to 11 months after corticosteroid discontinuation. Additionally, Varney noted that patients who had psychosis and dementia had more severe and longer-lasting dementia.
TREATMENT: Progressive decline
Mr. K is college educated, has been married for 15 years, has 2 children, age 9 and 11, and owns a successful basketball coaching business. He has no history of substance abuse, legal issues, or violence. He reports a good childhood with normal developmental milestones and no history of trauma.
In the 6 months after his initial psychiatric admission, Mr. K sees various outpatient providers, who change his psychotropics multiple times. He also receives 4 courses of prednisone for ocular sarcoidosis. He is admitted twice to other psychiatric facilities. After he has paranoid interactions with colleagues and families of the youth he coaches, his business fails.
After his third psychiatric inpatient hospitalization, Mr. K becomes severely paranoid, believing his wife is having an affair. He becomes physically abusive to his wife, who obtains a restraining order and leaves with their children. Mr. K barely leaves his house and stops grooming. A friend notes that Mr. K’s home has become uninhabitable, and it goes into foreclosure. After Mr. K’s neighbors report combative behavior and paranoia, police bring him in on an involuntary hold for a fourth psychiatric hospitalization (the second in our facility).
During this hospitalization—6 months after the initial ED presentation—the neurology team conducts a repeat medical workup. EEG shows generalized slowing. Head CT and MRI show diffuse cortical atrophy that was not seen in previous imaging. Mr. K has ocular lesions characteristic of ocular sarcoidosis. His mental status examination is similar to his first presentation except that the psychosis and thought disorganization are considerably worse. His cognitive functioning also shows significant decline. Cognitive screening reveals intact remote memory with impaired recent memory. His thinking is concrete and his verbal memory is markedly impaired. His Mini-Mental State Examination score is 27/30, indicating functional capacity that is better than his clinical presentation. Because of difficulty with concentration and verbal processing, Mr. K is unable to complete the Minnesota Multiphasic Personality Inventory despite substantial assistance. On most days he cannot recall recent conversations with his wife, staff, or physicians. He is taking no medications at this time.
Mr. K is restarted on olanzapine, titrated to 30 mg/d, to control his psychosis; this medication was effective during his last stay in our facility. Oral prednisone is discontinued and methotrexate, 10 mg/week, is initiated for ocular sarcoidosis. Based on recommendations from a case series report,5 we start Mr. K on lithium, titrated to 600 mg twice a day, for steroid-induced mood symptoms, Mr. K’s psychosis and mood improve dramatically once he reaches a therapeutic lithium level; however, his cognition remains slowed and he is unable to care for his basic needs.
The authors’ observations
Steroid dementia may be the result of effects in the medial temporal lobe, specifically dorsolateral prefrontal cortex, which impairs working memory, and the parahippocampal gyrus.6,7 The cognitive presentation of steroid dementia Varney et al3 described has been replicated in healthy volunteers who received corticosteroids.3 Patients with Cushing’s syndrome also have been noted to have diminished hippocampal volume and similar cognitive deficits. Cognitive impairment experienced by patients treated with corticosteroids may be caused by neuronal death in the hippocampus and dorsolateral prefrontal cortex. The etiology of cell death is multifactorial and includes glutamate-mediated excitotoxicity, activation of proinflammatory pathways, inhibited utilization of glucose in the hippocampus, telomere shortening, and diminished cell repair by brain-derived neurotrophic factor. The net result is significant, widespread damage that in some cases is irreversible.8
Because of the severity of Mr. K’s psychosis and personality change from baseline, his cognitive symptoms were largely overlooked during his first psychiatric hospitalization. The affective flattening, delayed verbal response, and markedly concrete thought process were considered within the spectrum of resolving psychosis. After further hospitalizations and abnormal results on cognitive testing, Mr. K’s cognitive impairment was fully noted. His symptoms match those of previously documented cases of steroid dementia, including verbal deficits out of proportion to other impairment, acute cerebral atrophy on CT after corticosteroid treatment, and gradual improvement of symptoms when corticosteroids were discontinued.
Management recommendations
Educate patients taking steroids about possible side effects of mood changes, psychosis, and cognitive deficits. Close monitoring of patients on corticosteroids is paramount. If psychiatric or cognitive symptoms develop, gradually discontinue the corticosteroid and seek other treatments.
Randomized, placebo-controlled trials of lamotrigine and memantine have shown these medications are cognitively protective for patients taking prednisone.9
OUTCOME: Long-term deficits
After a 33-day stay in our adult inpatient psychiatric facility, the county places Mr. K in a permanent conservatorship for severe grave disability. He is discharged to a long-term psychiatric care locked facility for ongoing management. Mr. K spends 20 months in the long-term care facility while his family remains hopeful for his recovery and return home. He is admitted to our facility for acute stabilization of psychotic symptoms after he is released from the locked facility. Although no imaging studies are conducted, he remains significantly forgetful. Additionally, his paranoia persists.
Mr. K is poorly compliant with his psychotropics, which include divalproex, 1,000 mg/d, and olanzapine, 30 mg/d. Although he is discharged home with his family, his functional capacity is less than expected and he requires continuous support. Insisting that Mr. K abstain from steroids after the first psychiatric hospitalization might have prevented this seemingly irreversible dementia.
Related Resources
- Sacks O, Shulman M. Steroid dementia: an overlooked diagnosis? Neurology. 2005;64(4):707-709.
- Cipriani G, Picchi L, Vedovello M, et al. Reversible dementia from corticosteroid therapy. Clinical Geriatrics. 2012;20(7):38-41.
Drug Brand Names
- Diphenhydramine • Benadryl
- Divalproex • Depakote
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid
- Lorazepam • Ativan
- Memantine • Namenda
- Methotrexate • Rheumatrex, Trexall
- Mirtazapine • Remeron
- Olanzapine • Zyprexa
- Prednisone • Deltasone, Meticorten, others
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders 4th ed, text rev. Arlington VA: American Psychiatric Association; 2000.
2. Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006;81(10):1361-1367.
3. Varney NR, Alexander B, MacIndoe JH. Reversible steroid dementia in patients without steroid psychosis. Am J Psychiatry. 1984;141(3):369-372.
4. Varney NR. A case of reversible steroid dementia. Arch Clin Neuropsychol. 1997;12(2):167-171.
5. Sirois F. Steroid psychosis: a review. Gen Hosp Psychiatry. 2003;25(1):27-33.
6. Wolkowitz OM, Burke H, Epel ES, et al. Glucocorticoids: mood, memory, and mechanisms. Ann N Y Acad Sci. 2009;1179:19-40.
7. Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognition: integration of animal and human model studies. Brain Res Brain Res Rev. 1997;24(1):1-27.
8. Sapolsky RM. The physiological relevance of glucocorticoid endangerment of the hippocampus. Ann NY Acad Sci. 1994;746:294-304.
9. Brown ES. Effects of glucocorticoids on mood memory and the hippocampus. Treatment and preventative therapy. Ann N Y Acad Sci. 2009;1179:41-55.
CASE: Behavioral changes
Mr. K, age 45, is brought to the emergency department (ED) by his wife for severe paranoia, combative behavior, confusion, and slowed cognition. Mr. K tells the ED staff that a chemical abrasion he sustained a few weeks earlier has spread to his penis, and insists that his penis is retracting into his body. He has tied a string around his penis to keep it from disappearing into his body. According to Mr. K’s wife, he went to an urgent care clinic 2 weeks ago after he sustained chemical abrasions from exposure to cleaning solution at home. The provider at the urgent care clinic started Mr. K on an unknown dose of oral prednisone.
Mr. K’s wife reports that her husband had a dysphoric episode approximately 6 months ago when his business was struggling but his mood improved without psychiatric care. Mr. K’s medical history includes episodic sarcoidosis of the eyes, skin, and lungs. In the past these symptoms remitted after he received oral prednisone.
ED clinicians consider neurosarcoidosis and substance-induced delirium in the differential diagnosis (Table).1 A CT scan of the head fails to show lesions suggestive of neurosarcoidosis. Chest radiography does not reveal lesions suggestive of lung sarcoids and Mr. K has no skin lesions.
Table
DSM-IV-TR criteria for substance-induced delirium
|
| Source: Reference 1 |
Mr. K is admitted to the psychiatric inpatient unit for acute stabilization, where he remains aggressive and combative. He throws chairs at his peers and staff on the unit and is placed in physical restraints. He requires several doses of IM haloperidol, 5 mg, lorazepam, 2 mg, and diphenhydramine, 50 mg, for severe agitation. Mr. K is guarded, perseverative, and selectively mute. He avoids eye contact and has poor grooming. He has slow thought processing and displays concrete thought process. Prednisone is discontinued and olanzapine, titrated to 30 mg/d, and mirtazapine, titrated to 30 mg/d, are started for psychosis and depression.
Mr. K’s mood and behavior eventually return to baseline but slowed cognition persists. He is discharged from our facility.
The authors’ observations
Cortisone was first used to treat rheumatoid arthritis in 1948 and corticosteroids have been linked to multiple neuropsychiatric complications that have been broadly defined as steroid psychosis. This syndrome includes reversible behavioral manifestations such as hypomania, irritability, mood reactivity, anxiety, and insomnia in addition to more severe symptoms such as depression, mania, and psychosis.2 Although mild cognitive deficits have been noted in patients taking corticosteroids, most published cases have focused on steroid-induced psychosis.
In 1984, Varney et al3 noted a phenomenon they called “steroid dementia” in 6 patients treated with corticosteroids. On first evaluation, these patients presented with symptoms similar to early Alzheimer’s dementia—impaired memory, attention, and concentration. Three patients initially were diagnosed first with Alzheimer’s dementia until their symptoms spontaneously improved when steroids were reduced or discontinued. Although their presentation resembled Alzheimer’s dementia, patients with steroid dementia had a specific cognitive presentation associated with corticosteroid use. Symptoms included impaired verbal memory and spatial thinking but normal procedural memory. These patients showed intact immediate recall but impaired delayed recall with difficulty tracking conversations and word finding. Overall, patients with steroid dementia showed a predominance of verbal declarative memory deficits out of proportion to other cognitive symptoms. These symptoms and recent corticosteroid exposure differentiated steroid dementia from other forms of dementia.
In a later article, Varney reviewed electroencephalography (EEG) and CT findings associated with steroid dementia, noting bilateral EEG abnormalities and acute cortical atrophy on CT.4 Steroid dementia largely was reversible, resolving 3 to 11 months after corticosteroid discontinuation. Additionally, Varney noted that patients who had psychosis and dementia had more severe and longer-lasting dementia.
TREATMENT: Progressive decline
Mr. K is college educated, has been married for 15 years, has 2 children, age 9 and 11, and owns a successful basketball coaching business. He has no history of substance abuse, legal issues, or violence. He reports a good childhood with normal developmental milestones and no history of trauma.
In the 6 months after his initial psychiatric admission, Mr. K sees various outpatient providers, who change his psychotropics multiple times. He also receives 4 courses of prednisone for ocular sarcoidosis. He is admitted twice to other psychiatric facilities. After he has paranoid interactions with colleagues and families of the youth he coaches, his business fails.
After his third psychiatric inpatient hospitalization, Mr. K becomes severely paranoid, believing his wife is having an affair. He becomes physically abusive to his wife, who obtains a restraining order and leaves with their children. Mr. K barely leaves his house and stops grooming. A friend notes that Mr. K’s home has become uninhabitable, and it goes into foreclosure. After Mr. K’s neighbors report combative behavior and paranoia, police bring him in on an involuntary hold for a fourth psychiatric hospitalization (the second in our facility).
During this hospitalization—6 months after the initial ED presentation—the neurology team conducts a repeat medical workup. EEG shows generalized slowing. Head CT and MRI show diffuse cortical atrophy that was not seen in previous imaging. Mr. K has ocular lesions characteristic of ocular sarcoidosis. His mental status examination is similar to his first presentation except that the psychosis and thought disorganization are considerably worse. His cognitive functioning also shows significant decline. Cognitive screening reveals intact remote memory with impaired recent memory. His thinking is concrete and his verbal memory is markedly impaired. His Mini-Mental State Examination score is 27/30, indicating functional capacity that is better than his clinical presentation. Because of difficulty with concentration and verbal processing, Mr. K is unable to complete the Minnesota Multiphasic Personality Inventory despite substantial assistance. On most days he cannot recall recent conversations with his wife, staff, or physicians. He is taking no medications at this time.
Mr. K is restarted on olanzapine, titrated to 30 mg/d, to control his psychosis; this medication was effective during his last stay in our facility. Oral prednisone is discontinued and methotrexate, 10 mg/week, is initiated for ocular sarcoidosis. Based on recommendations from a case series report,5 we start Mr. K on lithium, titrated to 600 mg twice a day, for steroid-induced mood symptoms, Mr. K’s psychosis and mood improve dramatically once he reaches a therapeutic lithium level; however, his cognition remains slowed and he is unable to care for his basic needs.
The authors’ observations
Steroid dementia may be the result of effects in the medial temporal lobe, specifically dorsolateral prefrontal cortex, which impairs working memory, and the parahippocampal gyrus.6,7 The cognitive presentation of steroid dementia Varney et al3 described has been replicated in healthy volunteers who received corticosteroids.3 Patients with Cushing’s syndrome also have been noted to have diminished hippocampal volume and similar cognitive deficits. Cognitive impairment experienced by patients treated with corticosteroids may be caused by neuronal death in the hippocampus and dorsolateral prefrontal cortex. The etiology of cell death is multifactorial and includes glutamate-mediated excitotoxicity, activation of proinflammatory pathways, inhibited utilization of glucose in the hippocampus, telomere shortening, and diminished cell repair by brain-derived neurotrophic factor. The net result is significant, widespread damage that in some cases is irreversible.8
Because of the severity of Mr. K’s psychosis and personality change from baseline, his cognitive symptoms were largely overlooked during his first psychiatric hospitalization. The affective flattening, delayed verbal response, and markedly concrete thought process were considered within the spectrum of resolving psychosis. After further hospitalizations and abnormal results on cognitive testing, Mr. K’s cognitive impairment was fully noted. His symptoms match those of previously documented cases of steroid dementia, including verbal deficits out of proportion to other impairment, acute cerebral atrophy on CT after corticosteroid treatment, and gradual improvement of symptoms when corticosteroids were discontinued.
Management recommendations
Educate patients taking steroids about possible side effects of mood changes, psychosis, and cognitive deficits. Close monitoring of patients on corticosteroids is paramount. If psychiatric or cognitive symptoms develop, gradually discontinue the corticosteroid and seek other treatments.
Randomized, placebo-controlled trials of lamotrigine and memantine have shown these medications are cognitively protective for patients taking prednisone.9
OUTCOME: Long-term deficits
After a 33-day stay in our adult inpatient psychiatric facility, the county places Mr. K in a permanent conservatorship for severe grave disability. He is discharged to a long-term psychiatric care locked facility for ongoing management. Mr. K spends 20 months in the long-term care facility while his family remains hopeful for his recovery and return home. He is admitted to our facility for acute stabilization of psychotic symptoms after he is released from the locked facility. Although no imaging studies are conducted, he remains significantly forgetful. Additionally, his paranoia persists.
Mr. K is poorly compliant with his psychotropics, which include divalproex, 1,000 mg/d, and olanzapine, 30 mg/d. Although he is discharged home with his family, his functional capacity is less than expected and he requires continuous support. Insisting that Mr. K abstain from steroids after the first psychiatric hospitalization might have prevented this seemingly irreversible dementia.
Related Resources
- Sacks O, Shulman M. Steroid dementia: an overlooked diagnosis? Neurology. 2005;64(4):707-709.
- Cipriani G, Picchi L, Vedovello M, et al. Reversible dementia from corticosteroid therapy. Clinical Geriatrics. 2012;20(7):38-41.
Drug Brand Names
- Diphenhydramine • Benadryl
- Divalproex • Depakote
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid
- Lorazepam • Ativan
- Memantine • Namenda
- Methotrexate • Rheumatrex, Trexall
- Mirtazapine • Remeron
- Olanzapine • Zyprexa
- Prednisone • Deltasone, Meticorten, others
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Behavioral changes
Mr. K, age 45, is brought to the emergency department (ED) by his wife for severe paranoia, combative behavior, confusion, and slowed cognition. Mr. K tells the ED staff that a chemical abrasion he sustained a few weeks earlier has spread to his penis, and insists that his penis is retracting into his body. He has tied a string around his penis to keep it from disappearing into his body. According to Mr. K’s wife, he went to an urgent care clinic 2 weeks ago after he sustained chemical abrasions from exposure to cleaning solution at home. The provider at the urgent care clinic started Mr. K on an unknown dose of oral prednisone.
Mr. K’s wife reports that her husband had a dysphoric episode approximately 6 months ago when his business was struggling but his mood improved without psychiatric care. Mr. K’s medical history includes episodic sarcoidosis of the eyes, skin, and lungs. In the past these symptoms remitted after he received oral prednisone.
ED clinicians consider neurosarcoidosis and substance-induced delirium in the differential diagnosis (Table).1 A CT scan of the head fails to show lesions suggestive of neurosarcoidosis. Chest radiography does not reveal lesions suggestive of lung sarcoids and Mr. K has no skin lesions.
Table
DSM-IV-TR criteria for substance-induced delirium
|
| Source: Reference 1 |
Mr. K is admitted to the psychiatric inpatient unit for acute stabilization, where he remains aggressive and combative. He throws chairs at his peers and staff on the unit and is placed in physical restraints. He requires several doses of IM haloperidol, 5 mg, lorazepam, 2 mg, and diphenhydramine, 50 mg, for severe agitation. Mr. K is guarded, perseverative, and selectively mute. He avoids eye contact and has poor grooming. He has slow thought processing and displays concrete thought process. Prednisone is discontinued and olanzapine, titrated to 30 mg/d, and mirtazapine, titrated to 30 mg/d, are started for psychosis and depression.
Mr. K’s mood and behavior eventually return to baseline but slowed cognition persists. He is discharged from our facility.
The authors’ observations
Cortisone was first used to treat rheumatoid arthritis in 1948 and corticosteroids have been linked to multiple neuropsychiatric complications that have been broadly defined as steroid psychosis. This syndrome includes reversible behavioral manifestations such as hypomania, irritability, mood reactivity, anxiety, and insomnia in addition to more severe symptoms such as depression, mania, and psychosis.2 Although mild cognitive deficits have been noted in patients taking corticosteroids, most published cases have focused on steroid-induced psychosis.
In 1984, Varney et al3 noted a phenomenon they called “steroid dementia” in 6 patients treated with corticosteroids. On first evaluation, these patients presented with symptoms similar to early Alzheimer’s dementia—impaired memory, attention, and concentration. Three patients initially were diagnosed first with Alzheimer’s dementia until their symptoms spontaneously improved when steroids were reduced or discontinued. Although their presentation resembled Alzheimer’s dementia, patients with steroid dementia had a specific cognitive presentation associated with corticosteroid use. Symptoms included impaired verbal memory and spatial thinking but normal procedural memory. These patients showed intact immediate recall but impaired delayed recall with difficulty tracking conversations and word finding. Overall, patients with steroid dementia showed a predominance of verbal declarative memory deficits out of proportion to other cognitive symptoms. These symptoms and recent corticosteroid exposure differentiated steroid dementia from other forms of dementia.
In a later article, Varney reviewed electroencephalography (EEG) and CT findings associated with steroid dementia, noting bilateral EEG abnormalities and acute cortical atrophy on CT.4 Steroid dementia largely was reversible, resolving 3 to 11 months after corticosteroid discontinuation. Additionally, Varney noted that patients who had psychosis and dementia had more severe and longer-lasting dementia.
TREATMENT: Progressive decline
Mr. K is college educated, has been married for 15 years, has 2 children, age 9 and 11, and owns a successful basketball coaching business. He has no history of substance abuse, legal issues, or violence. He reports a good childhood with normal developmental milestones and no history of trauma.
In the 6 months after his initial psychiatric admission, Mr. K sees various outpatient providers, who change his psychotropics multiple times. He also receives 4 courses of prednisone for ocular sarcoidosis. He is admitted twice to other psychiatric facilities. After he has paranoid interactions with colleagues and families of the youth he coaches, his business fails.
After his third psychiatric inpatient hospitalization, Mr. K becomes severely paranoid, believing his wife is having an affair. He becomes physically abusive to his wife, who obtains a restraining order and leaves with their children. Mr. K barely leaves his house and stops grooming. A friend notes that Mr. K’s home has become uninhabitable, and it goes into foreclosure. After Mr. K’s neighbors report combative behavior and paranoia, police bring him in on an involuntary hold for a fourth psychiatric hospitalization (the second in our facility).
During this hospitalization—6 months after the initial ED presentation—the neurology team conducts a repeat medical workup. EEG shows generalized slowing. Head CT and MRI show diffuse cortical atrophy that was not seen in previous imaging. Mr. K has ocular lesions characteristic of ocular sarcoidosis. His mental status examination is similar to his first presentation except that the psychosis and thought disorganization are considerably worse. His cognitive functioning also shows significant decline. Cognitive screening reveals intact remote memory with impaired recent memory. His thinking is concrete and his verbal memory is markedly impaired. His Mini-Mental State Examination score is 27/30, indicating functional capacity that is better than his clinical presentation. Because of difficulty with concentration and verbal processing, Mr. K is unable to complete the Minnesota Multiphasic Personality Inventory despite substantial assistance. On most days he cannot recall recent conversations with his wife, staff, or physicians. He is taking no medications at this time.
Mr. K is restarted on olanzapine, titrated to 30 mg/d, to control his psychosis; this medication was effective during his last stay in our facility. Oral prednisone is discontinued and methotrexate, 10 mg/week, is initiated for ocular sarcoidosis. Based on recommendations from a case series report,5 we start Mr. K on lithium, titrated to 600 mg twice a day, for steroid-induced mood symptoms, Mr. K’s psychosis and mood improve dramatically once he reaches a therapeutic lithium level; however, his cognition remains slowed and he is unable to care for his basic needs.
The authors’ observations
Steroid dementia may be the result of effects in the medial temporal lobe, specifically dorsolateral prefrontal cortex, which impairs working memory, and the parahippocampal gyrus.6,7 The cognitive presentation of steroid dementia Varney et al3 described has been replicated in healthy volunteers who received corticosteroids.3 Patients with Cushing’s syndrome also have been noted to have diminished hippocampal volume and similar cognitive deficits. Cognitive impairment experienced by patients treated with corticosteroids may be caused by neuronal death in the hippocampus and dorsolateral prefrontal cortex. The etiology of cell death is multifactorial and includes glutamate-mediated excitotoxicity, activation of proinflammatory pathways, inhibited utilization of glucose in the hippocampus, telomere shortening, and diminished cell repair by brain-derived neurotrophic factor. The net result is significant, widespread damage that in some cases is irreversible.8
Because of the severity of Mr. K’s psychosis and personality change from baseline, his cognitive symptoms were largely overlooked during his first psychiatric hospitalization. The affective flattening, delayed verbal response, and markedly concrete thought process were considered within the spectrum of resolving psychosis. After further hospitalizations and abnormal results on cognitive testing, Mr. K’s cognitive impairment was fully noted. His symptoms match those of previously documented cases of steroid dementia, including verbal deficits out of proportion to other impairment, acute cerebral atrophy on CT after corticosteroid treatment, and gradual improvement of symptoms when corticosteroids were discontinued.
Management recommendations
Educate patients taking steroids about possible side effects of mood changes, psychosis, and cognitive deficits. Close monitoring of patients on corticosteroids is paramount. If psychiatric or cognitive symptoms develop, gradually discontinue the corticosteroid and seek other treatments.
Randomized, placebo-controlled trials of lamotrigine and memantine have shown these medications are cognitively protective for patients taking prednisone.9
OUTCOME: Long-term deficits
After a 33-day stay in our adult inpatient psychiatric facility, the county places Mr. K in a permanent conservatorship for severe grave disability. He is discharged to a long-term psychiatric care locked facility for ongoing management. Mr. K spends 20 months in the long-term care facility while his family remains hopeful for his recovery and return home. He is admitted to our facility for acute stabilization of psychotic symptoms after he is released from the locked facility. Although no imaging studies are conducted, he remains significantly forgetful. Additionally, his paranoia persists.
Mr. K is poorly compliant with his psychotropics, which include divalproex, 1,000 mg/d, and olanzapine, 30 mg/d. Although he is discharged home with his family, his functional capacity is less than expected and he requires continuous support. Insisting that Mr. K abstain from steroids after the first psychiatric hospitalization might have prevented this seemingly irreversible dementia.
Related Resources
- Sacks O, Shulman M. Steroid dementia: an overlooked diagnosis? Neurology. 2005;64(4):707-709.
- Cipriani G, Picchi L, Vedovello M, et al. Reversible dementia from corticosteroid therapy. Clinical Geriatrics. 2012;20(7):38-41.
Drug Brand Names
- Diphenhydramine • Benadryl
- Divalproex • Depakote
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid
- Lorazepam • Ativan
- Memantine • Namenda
- Methotrexate • Rheumatrex, Trexall
- Mirtazapine • Remeron
- Olanzapine • Zyprexa
- Prednisone • Deltasone, Meticorten, others
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders 4th ed, text rev. Arlington VA: American Psychiatric Association; 2000.
2. Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006;81(10):1361-1367.
3. Varney NR, Alexander B, MacIndoe JH. Reversible steroid dementia in patients without steroid psychosis. Am J Psychiatry. 1984;141(3):369-372.
4. Varney NR. A case of reversible steroid dementia. Arch Clin Neuropsychol. 1997;12(2):167-171.
5. Sirois F. Steroid psychosis: a review. Gen Hosp Psychiatry. 2003;25(1):27-33.
6. Wolkowitz OM, Burke H, Epel ES, et al. Glucocorticoids: mood, memory, and mechanisms. Ann N Y Acad Sci. 2009;1179:19-40.
7. Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognition: integration of animal and human model studies. Brain Res Brain Res Rev. 1997;24(1):1-27.
8. Sapolsky RM. The physiological relevance of glucocorticoid endangerment of the hippocampus. Ann NY Acad Sci. 1994;746:294-304.
9. Brown ES. Effects of glucocorticoids on mood memory and the hippocampus. Treatment and preventative therapy. Ann N Y Acad Sci. 2009;1179:41-55.
1. Diagnostic and statistical manual of mental disorders 4th ed, text rev. Arlington VA: American Psychiatric Association; 2000.
2. Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006;81(10):1361-1367.
3. Varney NR, Alexander B, MacIndoe JH. Reversible steroid dementia in patients without steroid psychosis. Am J Psychiatry. 1984;141(3):369-372.
4. Varney NR. A case of reversible steroid dementia. Arch Clin Neuropsychol. 1997;12(2):167-171.
5. Sirois F. Steroid psychosis: a review. Gen Hosp Psychiatry. 2003;25(1):27-33.
6. Wolkowitz OM, Burke H, Epel ES, et al. Glucocorticoids: mood, memory, and mechanisms. Ann N Y Acad Sci. 2009;1179:19-40.
7. Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognition: integration of animal and human model studies. Brain Res Brain Res Rev. 1997;24(1):1-27.
8. Sapolsky RM. The physiological relevance of glucocorticoid endangerment of the hippocampus. Ann NY Acad Sci. 1994;746:294-304.
9. Brown ES. Effects of glucocorticoids on mood memory and the hippocampus. Treatment and preventative therapy. Ann N Y Acad Sci. 2009;1179:41-55.
Something smells different
CASE: Depressed and hopeless
Ms. D, age 69, has a 20-year history of bipolar II disorder, for which she is taking citalopram, 30 mg/d. She presents to her outpatient psychotherapist with a chief complaint of depressed mood. The therapist refers her for psychiatric hospitalization and electroconvulsive therapy consultation. Upon admission, Ms. D reports that her depressed mood has worsened over the past 5 weeks after a trip to the Dominican Republic. Ms. D had a negative encounter with airport security that she attributed to her 2 artificial knees and caused her to miss her flight. She endorses poor appetite, loss of energy, anhedonia, difficulty concentrating, poor memory, and feelings of hopelessness.
Ms. D reports increasingly frequent panic attacks as well as intermittent right-sided discomfort, unusual noxious smells, and increased falls. She says the falls likely are a result of new bilateral lower extremity weakness coupled with long-standing imbalance. Ms. D says she has experienced brief occasions of foul-smelling odors while showering without evidence of an offending substance. She also reports a mild, occipitally located headache.
Four years ago, Ms. D was hospitalized for a depressive episode without psychotic features and diagnosed with generalized anxiety disorder, for which she is taking clonazepam, 1.5 mg/d. Her last hypomanic episode was several years ago, and was characterized by increased energy with decreased need for sleep, flight of ideas, increased productivity, and impulsivity. Her medical history includes non-insulin dependent diabetes mellitus, chronic low back pain, hyperlipidemia, arthritis, and gastroesophageal reflux disease; her medications include pioglitazone, 30 mg/d, oxybutynin, 15 mg/d, rosuvastatin, 20 mg/d, losartan, 50 mg/d, and omeprazole, 20 mg/d. She also had bilateral knee replacements 9 years ago and an L4-S1 spinal fusion 11 years ago. She has no history of head injuries or seizures. Ms. D’s father had major depressive disorder, her mother died of a cerebrovascular accident at an unknown age, and her brother died of a myocardial infarction at age 52.
The authors’ observations
A striking aspect of Ms. D’s presenting complaints was her intermittent experience of foul smells. Although olfactory hallucinations can occur with psychotic and affective states, they also may be harbingers of an organic etiology involving the temporal lobe.1 Olfactory hallucinations associated with a psychiatric disorder often have an accompanying delusional belief regarding the cause of the smell.2
Olfactory hallucinations have been associated with migraines, epilepsy, and Parkinson’s disease.1-3 Neoplasms, cerebrovascular events, or traumatic brain injuries that result in focal mesial temporal lobe lesions can present as a partial complex seizure with olfactory or gustatory hallucinations and progress to automatisms.4 Characteristic odors in these hallucinations are unpleasant; patients with temporal lobe epilepsy describe the smells as “bad,” “rotten,” “sickening,” and “like burning food.”2 Ms. D’s report of unusual smells warranted consideration of an organic etiology for her mood change and a thorough neurologic examination.
EVALUATION: Neurologic signs
At the time of admission, Ms. D has a blood pressure of 127/68 mm Hg, heart rate of 74 beats per minute, respiratory rate of 16 breaths per minute, and temperature of 36.5°C. Neurologic examination reveals a left facial droop of unknown duration. Motor strength is weak throughout with left-sided focal weakness. Ms. D’s daughter notes that her mother’s smile appears “funny” in her admission photograph but is unsure when the asymmetry in her facial appearance began. Ms. D had been ambulatory before admission. Nursing staff observes Ms. D leans toward her left side and exhibits possible left-sided neglect during the first 12 hours of hospitalization.
When asked about her facial droop, Ms. D replies that she had not noticed any change in her appearance lately. She does not appear to be concerned about her worsening ambulation. On hospital day 2, Ms. D seems to have difficulty using utensils to eat breakfast. Ms. D is dismissive of her worsening motor function and asks to be left alone to finish her meal.
The authors’ observations
Ms. D’s focal neurologic deficits and complaint of a headache on admission were concerning because they could be caused by a cerebrovascular event or space-occupying brain lesion with potential for increased intracranial pressure. Neurologic examination with evaluation for papilledema is indicated, followed by medical transport to the closest medical center for emergent brain imaging. Neither Ms. D nor her daughter could pinpoint the onset of Ms. D’s left-sided facial droop, which precluded administering tissue plasminogen activator for a potential acute ischemic stroke.5
Ms. D’s case prompted us to consider what constitutes timely brain imaging in a patient who presents with psychiatric symptoms. Several neurologic conditions may present first with neurobehavioral symptoms before findings on physical exam. Two series of autopsies conducted >70 years ago at psychiatric hospitals found incidences of brain tumors of 3.45%6 and 13.5%.7 In a 5-year retrospective study, 21% of meningioma cases presented with psychiatric symptoms alone.8 These historical cases suggest that affective, behavioral, and psychotic symptoms may be the only clinical indicators of brain lesions that merit surgery.9-11
Imaging and radiation exposure
With the advent of CT scans in the 1970s, psychiatrists gained a new method of investigating potential structural CNS pathology in patients presenting with psychiatric symptoms. The dramatic increase in CT scan use in recent years and resulting radiation exposure is responsible for 1.5% to 2% of all cancers in the United States.12,13 Certainly, physicians must balance the advantage of early detection of brain lesions with cost-effectiveness and exposure to radiation.14
There is no consensus regarding use of brain imaging in a patient who presents with new-onset psychiatric symptoms. Certainly, patients with localizing neurologic deficits or symptoms of increased intracranial pressure should undergo brain imaging. As for psychiatric patients without neurologic findings, Filley and Kleinschmidt-DeMasters15 provide recommendations based on their 1995 case series, and other authors have recommended imaging for patients age ≥4016 vs ≥5017,18 who present with atypical mental status changes.
OUTCOME: Scan, then surgery
Ms. D’s head CT reveals a large right-sided temporoparietal low-density lesion with 8-mm left lower midline shift (Figure). She undergoes a right temporal craniotomy with resection of the mass, which is confirmed by surgical pathology to be a glioblastoma multiforme World Health Organization grade 4 tumor. Postoperative MRI shows evidence of infarction in the right posterior cerebral artery distribution and residual tumor is identified on follow-up imaging. Ms. D is referred to radiation oncology, where she receives a prognostic median life expectancy of 14 months with radiation and temozolomide treatment.19

Figure: Ms. D’s MRI results
MRI with contrast shows a large right temporal heterogeneous mass consistent with glioblastoma multiforme
The authors’ observations
Glioblastoma is a rare cancer that comprises 25% of all malignant nervous system tumors.20 It is associated with a poor prognosis, with a <30% relative survival rate for adults at 1 year and 3% at 5 years.20 Headaches, seizures, motor weakness, and progressive neurologic deficits are common symptoms of glioblastoma at diagnosis.20 Ms. D was offered the standard of care treatment for a high-grade glioma, including surgical resection followed by concomitant external-beam radiotherapy and chemotherapy.21
Consider structural brain lesions in patients who present with neurobehavioral symptoms, although most of these patients will be diagnosed with a primary psychiatric disorder. Ms. D had a known psychiatric disorder that predated the onset of neurologic symptoms and diagnosis of a rare brain cancer. Before she developed neurologic signs, Ms. D experienced symptoms uncharacteristic of her previous depressive episodes, including olfactory hallucinations, that provided an early indicator of a CNS lesion. Consider brain imaging in patients of any age who do not respond to medications targeting the presumed psychiatric diagnosis to ensure that insidious brain tumors are not missed (Table 1).15
Table 1
When to order neuroimaging for psychiatric patients
| Patient’s age | Most common types of brain tumor | MRI vs CT | Indications to image |
|---|---|---|---|
| ≥40 years | Metastases High-grade gliomas Meningiomas | Roughly equivalent for imaging common tumor types. Base on cost, availability, and relative patient contraindications | New-onset cognitive or emotional dysfunction. Patient is not responding to appropriate pharmacotherapy for psychiatric diagnosis |
| <40 years | Low-grade astrocytomas Oligodendrogliomas | MRI preferred | New-onset cognitive or emotional dysfunction with associated somatic symptoms (headache, nausea, vomiting, papilledema, seizures, or focal deficits). Patient is not responding to appropriate pharmacotherapy for the psychiatric diagnosis |
| Source: Reference 15 | |||
Compared with cerebrovascular lesions, neoplasms are more difficult to clinically correlate with their anatomic location. Neurobehavioral symptoms are more frequently associated with tumors originating in the frontal lobe or temporolimbic regions of the brain. The 3 types of frontal lobe syndromes are dorsolateral, orbitofrontal, and medial-frontal (Table 2).15 Temporolimbic tumors may present with hallucinations, mania, panic attacks, or amnesia. A meta-analysis found a statistically significant association between anorexia and hypothalamic tumors.22 Reports of neuropsychiatric symptoms that respond to pharmacologic treatment further confound the clinical picture.16
Table 2
Frontal lobe syndromes
| Syndrome | Characteristics |
|---|---|
| Dorsolateral | Deficits in executive functioning, including organization and behavior planning |
| Orbitofrontal | Prominent disinhibition |
| Medial-frontal | Apathy, abulia |
| Source: Reference 15 | |
It is uncommon for a patient with a long-standing mood disorder to develop a primary brain cancer. However, Ms. D’s case serves as an important reminder to consider medical comorbidities in our aging psychiatric population. In particular, a patient who develops unusual symptoms or does not respond to previously effective treatments should be more closely examined and the differential diagnosis broadened.
Related Resources
- MD Anderson Cancer Center. Brain tumor videos and podcasts. www.mdanderson.org/patient-and-cancer-information/cancer-information/cancer-types/brain-tumor/videos-and-podcasts/index.html.
- Braun CM, Dumont M, Duval J, et al. Brain modules of hallucination: an analysis of multiple patients with brain lesions. J Psychiatry Neurosci. 2003;28(6):432-449.
Drug Brand Names
- Citalopram • Celexa
- Clonazepam • Klonopin
- Losartan • Cozaar
- Omeprazole • Prilosec
- Oxybutynin • Ditropan
- Pioglitazone • Actos
- Rosuvastatin • Crestor
- Temozolomide • Temodar
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Assad G, Shapiro B. Hallucinations: theoretical and clinical overview. Am J Psychiatry. 1986;143(9):1088-1097.
2. Carter JL. Visual somatosensory, olfactory, and gustatory hallucinations. Psychiatr Clin North Am. 1992;15(2):347-358.
3. Fuller GN, Guiloff RJ. Migrainous olfactory hallucinations. J Neurol Neurosurg Psychiatry. 1987;50(12):1688-1690.
4. Chang BS, Lowenstein DH. Mechanisms of disease: epilepsy. N Engl J Med. 2003;349(13):1257-1266.
5. Lansberg MG, Bluhmki E, Thijs VN. Efficacy and safety of tissue plasminogen activator 3 to 4.5 hours after acute ischemic stroke: a metaanalysis. Stroke. 2009;40(7):2438-2441.
6. Hoffman JL. Intracranial neoplasms: their incidence and mental manifestations. Psychiatr Q. 1937;11(4):561-575.
7. Larson CP. Intracranial tumors in mental hospital patients. Am J Psychiatry. 1940;97(1):49-58.
8. Gupta RK, Kumar R. Benign brain tumours and psychiatric morbidity: a 5-years retrospective data analysis. Aust N Z J Psychiatry. 2004;38(5):316-319.
9. Chambers WR. Neurosurgical conditions masquerading as psychiatric diseases. Am J Psychiatry. 1955;112(5):387-389.
10. Trimble MR, Mendez MF, Cummings JL. Neuropsychiatric symptoms from the temporolimbic lobes. J Neuropsychiatry Clin Neurosci. 1997;9(3):429-438.
11. Uribe VM. Psychiatric symptoms and brain tumor. Am Fam Physician. 1986;34(2):95-98.
12. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(2):2277-2284.
13. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077.
14. Weinberger DR. Brain disease and psychiatric illness: when should a psychiatrist order a CAT scan? Am J Psychiatry. 1984;141(12):1521-1526.
15. Filley CM, Kleinschmidt-DeMasters BK. Neurobehavioral presentations of brain neoplasms. West J Med. 1995;163(1):19-25.
16. Moise D, Madhusoodanan S. Psychiatric symptoms associated with brain tumors: a clinical engima. CNS Spectr. 2006;11(1):28-31.
17. Bunevicius A, Deltuva VP, Deltuviene D, et al. Brain lesions manifesting as psychiatric disorders: eight cases. CNS Spectr. 2008;13(11):950-958.
18. Hollister LE, Boutros N. Clinical use of CT and MR scans in psychiatric patients. J Psychiatr Neurosci. 1991;16(4):194-198.
19. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996.
20. Brandes AA, Tosoni A, Franceschi E, et al. Glioblastoma in adults. Crit Rev Oncol Hematol. 2008;67(2):139-152.
21. Chandana SR, Movva S, Arora M, et al. Primary brain tumors in adults. Am Fam Physician. 2008;77(10):1423-1430.
22. Madhusoodanan S, Opler MG, Moise D, et al. Brain tumor location and psychiatric symptoms: is there any association? A meta-analysis of published case studies. Expert Rev Neurother. 2010;10(10):1529-1536.
CASE: Depressed and hopeless
Ms. D, age 69, has a 20-year history of bipolar II disorder, for which she is taking citalopram, 30 mg/d. She presents to her outpatient psychotherapist with a chief complaint of depressed mood. The therapist refers her for psychiatric hospitalization and electroconvulsive therapy consultation. Upon admission, Ms. D reports that her depressed mood has worsened over the past 5 weeks after a trip to the Dominican Republic. Ms. D had a negative encounter with airport security that she attributed to her 2 artificial knees and caused her to miss her flight. She endorses poor appetite, loss of energy, anhedonia, difficulty concentrating, poor memory, and feelings of hopelessness.
Ms. D reports increasingly frequent panic attacks as well as intermittent right-sided discomfort, unusual noxious smells, and increased falls. She says the falls likely are a result of new bilateral lower extremity weakness coupled with long-standing imbalance. Ms. D says she has experienced brief occasions of foul-smelling odors while showering without evidence of an offending substance. She also reports a mild, occipitally located headache.
Four years ago, Ms. D was hospitalized for a depressive episode without psychotic features and diagnosed with generalized anxiety disorder, for which she is taking clonazepam, 1.5 mg/d. Her last hypomanic episode was several years ago, and was characterized by increased energy with decreased need for sleep, flight of ideas, increased productivity, and impulsivity. Her medical history includes non-insulin dependent diabetes mellitus, chronic low back pain, hyperlipidemia, arthritis, and gastroesophageal reflux disease; her medications include pioglitazone, 30 mg/d, oxybutynin, 15 mg/d, rosuvastatin, 20 mg/d, losartan, 50 mg/d, and omeprazole, 20 mg/d. She also had bilateral knee replacements 9 years ago and an L4-S1 spinal fusion 11 years ago. She has no history of head injuries or seizures. Ms. D’s father had major depressive disorder, her mother died of a cerebrovascular accident at an unknown age, and her brother died of a myocardial infarction at age 52.
The authors’ observations
A striking aspect of Ms. D’s presenting complaints was her intermittent experience of foul smells. Although olfactory hallucinations can occur with psychotic and affective states, they also may be harbingers of an organic etiology involving the temporal lobe.1 Olfactory hallucinations associated with a psychiatric disorder often have an accompanying delusional belief regarding the cause of the smell.2
Olfactory hallucinations have been associated with migraines, epilepsy, and Parkinson’s disease.1-3 Neoplasms, cerebrovascular events, or traumatic brain injuries that result in focal mesial temporal lobe lesions can present as a partial complex seizure with olfactory or gustatory hallucinations and progress to automatisms.4 Characteristic odors in these hallucinations are unpleasant; patients with temporal lobe epilepsy describe the smells as “bad,” “rotten,” “sickening,” and “like burning food.”2 Ms. D’s report of unusual smells warranted consideration of an organic etiology for her mood change and a thorough neurologic examination.
EVALUATION: Neurologic signs
At the time of admission, Ms. D has a blood pressure of 127/68 mm Hg, heart rate of 74 beats per minute, respiratory rate of 16 breaths per minute, and temperature of 36.5°C. Neurologic examination reveals a left facial droop of unknown duration. Motor strength is weak throughout with left-sided focal weakness. Ms. D’s daughter notes that her mother’s smile appears “funny” in her admission photograph but is unsure when the asymmetry in her facial appearance began. Ms. D had been ambulatory before admission. Nursing staff observes Ms. D leans toward her left side and exhibits possible left-sided neglect during the first 12 hours of hospitalization.
When asked about her facial droop, Ms. D replies that she had not noticed any change in her appearance lately. She does not appear to be concerned about her worsening ambulation. On hospital day 2, Ms. D seems to have difficulty using utensils to eat breakfast. Ms. D is dismissive of her worsening motor function and asks to be left alone to finish her meal.
The authors’ observations
Ms. D’s focal neurologic deficits and complaint of a headache on admission were concerning because they could be caused by a cerebrovascular event or space-occupying brain lesion with potential for increased intracranial pressure. Neurologic examination with evaluation for papilledema is indicated, followed by medical transport to the closest medical center for emergent brain imaging. Neither Ms. D nor her daughter could pinpoint the onset of Ms. D’s left-sided facial droop, which precluded administering tissue plasminogen activator for a potential acute ischemic stroke.5
Ms. D’s case prompted us to consider what constitutes timely brain imaging in a patient who presents with psychiatric symptoms. Several neurologic conditions may present first with neurobehavioral symptoms before findings on physical exam. Two series of autopsies conducted >70 years ago at psychiatric hospitals found incidences of brain tumors of 3.45%6 and 13.5%.7 In a 5-year retrospective study, 21% of meningioma cases presented with psychiatric symptoms alone.8 These historical cases suggest that affective, behavioral, and psychotic symptoms may be the only clinical indicators of brain lesions that merit surgery.9-11
Imaging and radiation exposure
With the advent of CT scans in the 1970s, psychiatrists gained a new method of investigating potential structural CNS pathology in patients presenting with psychiatric symptoms. The dramatic increase in CT scan use in recent years and resulting radiation exposure is responsible for 1.5% to 2% of all cancers in the United States.12,13 Certainly, physicians must balance the advantage of early detection of brain lesions with cost-effectiveness and exposure to radiation.14
There is no consensus regarding use of brain imaging in a patient who presents with new-onset psychiatric symptoms. Certainly, patients with localizing neurologic deficits or symptoms of increased intracranial pressure should undergo brain imaging. As for psychiatric patients without neurologic findings, Filley and Kleinschmidt-DeMasters15 provide recommendations based on their 1995 case series, and other authors have recommended imaging for patients age ≥4016 vs ≥5017,18 who present with atypical mental status changes.
OUTCOME: Scan, then surgery
Ms. D’s head CT reveals a large right-sided temporoparietal low-density lesion with 8-mm left lower midline shift (Figure). She undergoes a right temporal craniotomy with resection of the mass, which is confirmed by surgical pathology to be a glioblastoma multiforme World Health Organization grade 4 tumor. Postoperative MRI shows evidence of infarction in the right posterior cerebral artery distribution and residual tumor is identified on follow-up imaging. Ms. D is referred to radiation oncology, where she receives a prognostic median life expectancy of 14 months with radiation and temozolomide treatment.19

Figure: Ms. D’s MRI results
MRI with contrast shows a large right temporal heterogeneous mass consistent with glioblastoma multiforme
The authors’ observations
Glioblastoma is a rare cancer that comprises 25% of all malignant nervous system tumors.20 It is associated with a poor prognosis, with a <30% relative survival rate for adults at 1 year and 3% at 5 years.20 Headaches, seizures, motor weakness, and progressive neurologic deficits are common symptoms of glioblastoma at diagnosis.20 Ms. D was offered the standard of care treatment for a high-grade glioma, including surgical resection followed by concomitant external-beam radiotherapy and chemotherapy.21
Consider structural brain lesions in patients who present with neurobehavioral symptoms, although most of these patients will be diagnosed with a primary psychiatric disorder. Ms. D had a known psychiatric disorder that predated the onset of neurologic symptoms and diagnosis of a rare brain cancer. Before she developed neurologic signs, Ms. D experienced symptoms uncharacteristic of her previous depressive episodes, including olfactory hallucinations, that provided an early indicator of a CNS lesion. Consider brain imaging in patients of any age who do not respond to medications targeting the presumed psychiatric diagnosis to ensure that insidious brain tumors are not missed (Table 1).15
Table 1
When to order neuroimaging for psychiatric patients
| Patient’s age | Most common types of brain tumor | MRI vs CT | Indications to image |
|---|---|---|---|
| ≥40 years | Metastases High-grade gliomas Meningiomas | Roughly equivalent for imaging common tumor types. Base on cost, availability, and relative patient contraindications | New-onset cognitive or emotional dysfunction. Patient is not responding to appropriate pharmacotherapy for psychiatric diagnosis |
| <40 years | Low-grade astrocytomas Oligodendrogliomas | MRI preferred | New-onset cognitive or emotional dysfunction with associated somatic symptoms (headache, nausea, vomiting, papilledema, seizures, or focal deficits). Patient is not responding to appropriate pharmacotherapy for the psychiatric diagnosis |
| Source: Reference 15 | |||
Compared with cerebrovascular lesions, neoplasms are more difficult to clinically correlate with their anatomic location. Neurobehavioral symptoms are more frequently associated with tumors originating in the frontal lobe or temporolimbic regions of the brain. The 3 types of frontal lobe syndromes are dorsolateral, orbitofrontal, and medial-frontal (Table 2).15 Temporolimbic tumors may present with hallucinations, mania, panic attacks, or amnesia. A meta-analysis found a statistically significant association between anorexia and hypothalamic tumors.22 Reports of neuropsychiatric symptoms that respond to pharmacologic treatment further confound the clinical picture.16
Table 2
Frontal lobe syndromes
| Syndrome | Characteristics |
|---|---|
| Dorsolateral | Deficits in executive functioning, including organization and behavior planning |
| Orbitofrontal | Prominent disinhibition |
| Medial-frontal | Apathy, abulia |
| Source: Reference 15 | |
It is uncommon for a patient with a long-standing mood disorder to develop a primary brain cancer. However, Ms. D’s case serves as an important reminder to consider medical comorbidities in our aging psychiatric population. In particular, a patient who develops unusual symptoms or does not respond to previously effective treatments should be more closely examined and the differential diagnosis broadened.
Related Resources
- MD Anderson Cancer Center. Brain tumor videos and podcasts. www.mdanderson.org/patient-and-cancer-information/cancer-information/cancer-types/brain-tumor/videos-and-podcasts/index.html.
- Braun CM, Dumont M, Duval J, et al. Brain modules of hallucination: an analysis of multiple patients with brain lesions. J Psychiatry Neurosci. 2003;28(6):432-449.
Drug Brand Names
- Citalopram • Celexa
- Clonazepam • Klonopin
- Losartan • Cozaar
- Omeprazole • Prilosec
- Oxybutynin • Ditropan
- Pioglitazone • Actos
- Rosuvastatin • Crestor
- Temozolomide • Temodar
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Depressed and hopeless
Ms. D, age 69, has a 20-year history of bipolar II disorder, for which she is taking citalopram, 30 mg/d. She presents to her outpatient psychotherapist with a chief complaint of depressed mood. The therapist refers her for psychiatric hospitalization and electroconvulsive therapy consultation. Upon admission, Ms. D reports that her depressed mood has worsened over the past 5 weeks after a trip to the Dominican Republic. Ms. D had a negative encounter with airport security that she attributed to her 2 artificial knees and caused her to miss her flight. She endorses poor appetite, loss of energy, anhedonia, difficulty concentrating, poor memory, and feelings of hopelessness.
Ms. D reports increasingly frequent panic attacks as well as intermittent right-sided discomfort, unusual noxious smells, and increased falls. She says the falls likely are a result of new bilateral lower extremity weakness coupled with long-standing imbalance. Ms. D says she has experienced brief occasions of foul-smelling odors while showering without evidence of an offending substance. She also reports a mild, occipitally located headache.
Four years ago, Ms. D was hospitalized for a depressive episode without psychotic features and diagnosed with generalized anxiety disorder, for which she is taking clonazepam, 1.5 mg/d. Her last hypomanic episode was several years ago, and was characterized by increased energy with decreased need for sleep, flight of ideas, increased productivity, and impulsivity. Her medical history includes non-insulin dependent diabetes mellitus, chronic low back pain, hyperlipidemia, arthritis, and gastroesophageal reflux disease; her medications include pioglitazone, 30 mg/d, oxybutynin, 15 mg/d, rosuvastatin, 20 mg/d, losartan, 50 mg/d, and omeprazole, 20 mg/d. She also had bilateral knee replacements 9 years ago and an L4-S1 spinal fusion 11 years ago. She has no history of head injuries or seizures. Ms. D’s father had major depressive disorder, her mother died of a cerebrovascular accident at an unknown age, and her brother died of a myocardial infarction at age 52.
The authors’ observations
A striking aspect of Ms. D’s presenting complaints was her intermittent experience of foul smells. Although olfactory hallucinations can occur with psychotic and affective states, they also may be harbingers of an organic etiology involving the temporal lobe.1 Olfactory hallucinations associated with a psychiatric disorder often have an accompanying delusional belief regarding the cause of the smell.2
Olfactory hallucinations have been associated with migraines, epilepsy, and Parkinson’s disease.1-3 Neoplasms, cerebrovascular events, or traumatic brain injuries that result in focal mesial temporal lobe lesions can present as a partial complex seizure with olfactory or gustatory hallucinations and progress to automatisms.4 Characteristic odors in these hallucinations are unpleasant; patients with temporal lobe epilepsy describe the smells as “bad,” “rotten,” “sickening,” and “like burning food.”2 Ms. D’s report of unusual smells warranted consideration of an organic etiology for her mood change and a thorough neurologic examination.
EVALUATION: Neurologic signs
At the time of admission, Ms. D has a blood pressure of 127/68 mm Hg, heart rate of 74 beats per minute, respiratory rate of 16 breaths per minute, and temperature of 36.5°C. Neurologic examination reveals a left facial droop of unknown duration. Motor strength is weak throughout with left-sided focal weakness. Ms. D’s daughter notes that her mother’s smile appears “funny” in her admission photograph but is unsure when the asymmetry in her facial appearance began. Ms. D had been ambulatory before admission. Nursing staff observes Ms. D leans toward her left side and exhibits possible left-sided neglect during the first 12 hours of hospitalization.
When asked about her facial droop, Ms. D replies that she had not noticed any change in her appearance lately. She does not appear to be concerned about her worsening ambulation. On hospital day 2, Ms. D seems to have difficulty using utensils to eat breakfast. Ms. D is dismissive of her worsening motor function and asks to be left alone to finish her meal.
The authors’ observations
Ms. D’s focal neurologic deficits and complaint of a headache on admission were concerning because they could be caused by a cerebrovascular event or space-occupying brain lesion with potential for increased intracranial pressure. Neurologic examination with evaluation for papilledema is indicated, followed by medical transport to the closest medical center for emergent brain imaging. Neither Ms. D nor her daughter could pinpoint the onset of Ms. D’s left-sided facial droop, which precluded administering tissue plasminogen activator for a potential acute ischemic stroke.5
Ms. D’s case prompted us to consider what constitutes timely brain imaging in a patient who presents with psychiatric symptoms. Several neurologic conditions may present first with neurobehavioral symptoms before findings on physical exam. Two series of autopsies conducted >70 years ago at psychiatric hospitals found incidences of brain tumors of 3.45%6 and 13.5%.7 In a 5-year retrospective study, 21% of meningioma cases presented with psychiatric symptoms alone.8 These historical cases suggest that affective, behavioral, and psychotic symptoms may be the only clinical indicators of brain lesions that merit surgery.9-11
Imaging and radiation exposure
With the advent of CT scans in the 1970s, psychiatrists gained a new method of investigating potential structural CNS pathology in patients presenting with psychiatric symptoms. The dramatic increase in CT scan use in recent years and resulting radiation exposure is responsible for 1.5% to 2% of all cancers in the United States.12,13 Certainly, physicians must balance the advantage of early detection of brain lesions with cost-effectiveness and exposure to radiation.14
There is no consensus regarding use of brain imaging in a patient who presents with new-onset psychiatric symptoms. Certainly, patients with localizing neurologic deficits or symptoms of increased intracranial pressure should undergo brain imaging. As for psychiatric patients without neurologic findings, Filley and Kleinschmidt-DeMasters15 provide recommendations based on their 1995 case series, and other authors have recommended imaging for patients age ≥4016 vs ≥5017,18 who present with atypical mental status changes.
OUTCOME: Scan, then surgery
Ms. D’s head CT reveals a large right-sided temporoparietal low-density lesion with 8-mm left lower midline shift (Figure). She undergoes a right temporal craniotomy with resection of the mass, which is confirmed by surgical pathology to be a glioblastoma multiforme World Health Organization grade 4 tumor. Postoperative MRI shows evidence of infarction in the right posterior cerebral artery distribution and residual tumor is identified on follow-up imaging. Ms. D is referred to radiation oncology, where she receives a prognostic median life expectancy of 14 months with radiation and temozolomide treatment.19

Figure: Ms. D’s MRI results
MRI with contrast shows a large right temporal heterogeneous mass consistent with glioblastoma multiforme
The authors’ observations
Glioblastoma is a rare cancer that comprises 25% of all malignant nervous system tumors.20 It is associated with a poor prognosis, with a <30% relative survival rate for adults at 1 year and 3% at 5 years.20 Headaches, seizures, motor weakness, and progressive neurologic deficits are common symptoms of glioblastoma at diagnosis.20 Ms. D was offered the standard of care treatment for a high-grade glioma, including surgical resection followed by concomitant external-beam radiotherapy and chemotherapy.21
Consider structural brain lesions in patients who present with neurobehavioral symptoms, although most of these patients will be diagnosed with a primary psychiatric disorder. Ms. D had a known psychiatric disorder that predated the onset of neurologic symptoms and diagnosis of a rare brain cancer. Before she developed neurologic signs, Ms. D experienced symptoms uncharacteristic of her previous depressive episodes, including olfactory hallucinations, that provided an early indicator of a CNS lesion. Consider brain imaging in patients of any age who do not respond to medications targeting the presumed psychiatric diagnosis to ensure that insidious brain tumors are not missed (Table 1).15
Table 1
When to order neuroimaging for psychiatric patients
| Patient’s age | Most common types of brain tumor | MRI vs CT | Indications to image |
|---|---|---|---|
| ≥40 years | Metastases High-grade gliomas Meningiomas | Roughly equivalent for imaging common tumor types. Base on cost, availability, and relative patient contraindications | New-onset cognitive or emotional dysfunction. Patient is not responding to appropriate pharmacotherapy for psychiatric diagnosis |
| <40 years | Low-grade astrocytomas Oligodendrogliomas | MRI preferred | New-onset cognitive or emotional dysfunction with associated somatic symptoms (headache, nausea, vomiting, papilledema, seizures, or focal deficits). Patient is not responding to appropriate pharmacotherapy for the psychiatric diagnosis |
| Source: Reference 15 | |||
Compared with cerebrovascular lesions, neoplasms are more difficult to clinically correlate with their anatomic location. Neurobehavioral symptoms are more frequently associated with tumors originating in the frontal lobe or temporolimbic regions of the brain. The 3 types of frontal lobe syndromes are dorsolateral, orbitofrontal, and medial-frontal (Table 2).15 Temporolimbic tumors may present with hallucinations, mania, panic attacks, or amnesia. A meta-analysis found a statistically significant association between anorexia and hypothalamic tumors.22 Reports of neuropsychiatric symptoms that respond to pharmacologic treatment further confound the clinical picture.16
Table 2
Frontal lobe syndromes
| Syndrome | Characteristics |
|---|---|
| Dorsolateral | Deficits in executive functioning, including organization and behavior planning |
| Orbitofrontal | Prominent disinhibition |
| Medial-frontal | Apathy, abulia |
| Source: Reference 15 | |
It is uncommon for a patient with a long-standing mood disorder to develop a primary brain cancer. However, Ms. D’s case serves as an important reminder to consider medical comorbidities in our aging psychiatric population. In particular, a patient who develops unusual symptoms or does not respond to previously effective treatments should be more closely examined and the differential diagnosis broadened.
Related Resources
- MD Anderson Cancer Center. Brain tumor videos and podcasts. www.mdanderson.org/patient-and-cancer-information/cancer-information/cancer-types/brain-tumor/videos-and-podcasts/index.html.
- Braun CM, Dumont M, Duval J, et al. Brain modules of hallucination: an analysis of multiple patients with brain lesions. J Psychiatry Neurosci. 2003;28(6):432-449.
Drug Brand Names
- Citalopram • Celexa
- Clonazepam • Klonopin
- Losartan • Cozaar
- Omeprazole • Prilosec
- Oxybutynin • Ditropan
- Pioglitazone • Actos
- Rosuvastatin • Crestor
- Temozolomide • Temodar
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Assad G, Shapiro B. Hallucinations: theoretical and clinical overview. Am J Psychiatry. 1986;143(9):1088-1097.
2. Carter JL. Visual somatosensory, olfactory, and gustatory hallucinations. Psychiatr Clin North Am. 1992;15(2):347-358.
3. Fuller GN, Guiloff RJ. Migrainous olfactory hallucinations. J Neurol Neurosurg Psychiatry. 1987;50(12):1688-1690.
4. Chang BS, Lowenstein DH. Mechanisms of disease: epilepsy. N Engl J Med. 2003;349(13):1257-1266.
5. Lansberg MG, Bluhmki E, Thijs VN. Efficacy and safety of tissue plasminogen activator 3 to 4.5 hours after acute ischemic stroke: a metaanalysis. Stroke. 2009;40(7):2438-2441.
6. Hoffman JL. Intracranial neoplasms: their incidence and mental manifestations. Psychiatr Q. 1937;11(4):561-575.
7. Larson CP. Intracranial tumors in mental hospital patients. Am J Psychiatry. 1940;97(1):49-58.
8. Gupta RK, Kumar R. Benign brain tumours and psychiatric morbidity: a 5-years retrospective data analysis. Aust N Z J Psychiatry. 2004;38(5):316-319.
9. Chambers WR. Neurosurgical conditions masquerading as psychiatric diseases. Am J Psychiatry. 1955;112(5):387-389.
10. Trimble MR, Mendez MF, Cummings JL. Neuropsychiatric symptoms from the temporolimbic lobes. J Neuropsychiatry Clin Neurosci. 1997;9(3):429-438.
11. Uribe VM. Psychiatric symptoms and brain tumor. Am Fam Physician. 1986;34(2):95-98.
12. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(2):2277-2284.
13. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077.
14. Weinberger DR. Brain disease and psychiatric illness: when should a psychiatrist order a CAT scan? Am J Psychiatry. 1984;141(12):1521-1526.
15. Filley CM, Kleinschmidt-DeMasters BK. Neurobehavioral presentations of brain neoplasms. West J Med. 1995;163(1):19-25.
16. Moise D, Madhusoodanan S. Psychiatric symptoms associated with brain tumors: a clinical engima. CNS Spectr. 2006;11(1):28-31.
17. Bunevicius A, Deltuva VP, Deltuviene D, et al. Brain lesions manifesting as psychiatric disorders: eight cases. CNS Spectr. 2008;13(11):950-958.
18. Hollister LE, Boutros N. Clinical use of CT and MR scans in psychiatric patients. J Psychiatr Neurosci. 1991;16(4):194-198.
19. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996.
20. Brandes AA, Tosoni A, Franceschi E, et al. Glioblastoma in adults. Crit Rev Oncol Hematol. 2008;67(2):139-152.
21. Chandana SR, Movva S, Arora M, et al. Primary brain tumors in adults. Am Fam Physician. 2008;77(10):1423-1430.
22. Madhusoodanan S, Opler MG, Moise D, et al. Brain tumor location and psychiatric symptoms: is there any association? A meta-analysis of published case studies. Expert Rev Neurother. 2010;10(10):1529-1536.
1. Assad G, Shapiro B. Hallucinations: theoretical and clinical overview. Am J Psychiatry. 1986;143(9):1088-1097.
2. Carter JL. Visual somatosensory, olfactory, and gustatory hallucinations. Psychiatr Clin North Am. 1992;15(2):347-358.
3. Fuller GN, Guiloff RJ. Migrainous olfactory hallucinations. J Neurol Neurosurg Psychiatry. 1987;50(12):1688-1690.
4. Chang BS, Lowenstein DH. Mechanisms of disease: epilepsy. N Engl J Med. 2003;349(13):1257-1266.
5. Lansberg MG, Bluhmki E, Thijs VN. Efficacy and safety of tissue plasminogen activator 3 to 4.5 hours after acute ischemic stroke: a metaanalysis. Stroke. 2009;40(7):2438-2441.
6. Hoffman JL. Intracranial neoplasms: their incidence and mental manifestations. Psychiatr Q. 1937;11(4):561-575.
7. Larson CP. Intracranial tumors in mental hospital patients. Am J Psychiatry. 1940;97(1):49-58.
8. Gupta RK, Kumar R. Benign brain tumours and psychiatric morbidity: a 5-years retrospective data analysis. Aust N Z J Psychiatry. 2004;38(5):316-319.
9. Chambers WR. Neurosurgical conditions masquerading as psychiatric diseases. Am J Psychiatry. 1955;112(5):387-389.
10. Trimble MR, Mendez MF, Cummings JL. Neuropsychiatric symptoms from the temporolimbic lobes. J Neuropsychiatry Clin Neurosci. 1997;9(3):429-438.
11. Uribe VM. Psychiatric symptoms and brain tumor. Am Fam Physician. 1986;34(2):95-98.
12. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(2):2277-2284.
13. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077.
14. Weinberger DR. Brain disease and psychiatric illness: when should a psychiatrist order a CAT scan? Am J Psychiatry. 1984;141(12):1521-1526.
15. Filley CM, Kleinschmidt-DeMasters BK. Neurobehavioral presentations of brain neoplasms. West J Med. 1995;163(1):19-25.
16. Moise D, Madhusoodanan S. Psychiatric symptoms associated with brain tumors: a clinical engima. CNS Spectr. 2006;11(1):28-31.
17. Bunevicius A, Deltuva VP, Deltuviene D, et al. Brain lesions manifesting as psychiatric disorders: eight cases. CNS Spectr. 2008;13(11):950-958.
18. Hollister LE, Boutros N. Clinical use of CT and MR scans in psychiatric patients. J Psychiatr Neurosci. 1991;16(4):194-198.
19. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996.
20. Brandes AA, Tosoni A, Franceschi E, et al. Glioblastoma in adults. Crit Rev Oncol Hematol. 2008;67(2):139-152.
21. Chandana SR, Movva S, Arora M, et al. Primary brain tumors in adults. Am Fam Physician. 2008;77(10):1423-1430.
22. Madhusoodanan S, Opler MG, Moise D, et al. Brain tumor location and psychiatric symptoms: is there any association? A meta-analysis of published case studies. Expert Rev Neurother. 2010;10(10):1529-1536.
Stalked by a ‘patient’
CASE: Delusions and threats
For over 20 months, Ms. I, age 48, sends a psychiatric resident letters and postcards that total approximately 3,000 pages and come from dozens of return addresses. Ms. I expresses romantic feelings toward the resident and believes that he was her physician and prescribed medications, including “mood stabilizers.” The resident never treated Ms. I; to his knowledge, he has never interacted with her.
Ms. I describes the resident’s refusal to continue treating her as “abandonment” and states that she is contemplating self-harm because of this rejection. In her letters, Ms. I admits that she was a long-term patient in a state psychiatric hospital in her home state and suffers from persistent auditory hallucinations. She also wants a romantic relationship with the resident and repeatedly threatens the resident’s female acquaintances and former romantic partners whose relationships she had surmised from news articles available on the Internet. Ms. I also threatens to strangle the resident. The resident sends her multiple written requests that she cease contact, but they are not acknowledged.
The authors’ observations
Stalking—repeated, unwanted attention or communication that would cause a reasonable person fear—is a serious threat for many psychiatric clinicians.1 Prevalence rates among mental health care providers range from 3% to 21%.2,3 Most stalkers have engaged in previous stalking behavior.3
Being stalked is highly distressing,4 and mental health professionals often do not reveal such experiences to colleagues.5 Irrational feelings of guilt or embarrassment, such as being thought to have poorly managed interactions with the stalker, often motivate a self-imposed silence (Table 1).6 This isolation may foster anxiety, interfere with receiving problem-solving advice, and increase physical vulnerability. In the case involving Ms. I, the psychiatric resident’s primary responsibility is safeguarding his own physical and psychological welfare.
Clinicians who work in a hospital or other institutional setting who are being stalked should inform their supervisors and the facility’s security personnel. Security personnel may be able to gather data about the stalker, decrease the stalker’s ability to communicate with the victim, and reduce unwanted physical access to the victim by distributing a photo of the stalker or installing a camera or receptionist-controlled door lock in patient entryways. Security personnel also may collaborate with local law enforcement. Having a third party respond to a stalker’s aggressive behavior—rather than the victim responding directly—avoids rewarding the stalker, which may generate further unwanted contact.7 Any intervention by the victim may increase the risk of violence, creating an “intervention dilemma.” Resnick8 argues that before deciding how best to address the stalker’s behavior, a stalking victim must “first separate the risk of continued stalking from the risk that the stalker will commit a violent act.”
Mental health professionals in private practice who are being stalked should consider retaining an attorney. An attorney often can maintain privacy of communications regarding the stalker via the attorney-client and attorney-work product privileges, which may help during legal proceedings.
Table 1
Factors that can impede psychiatrists from reporting stalking
| Fear of being perceived as a failure |
| Embarrassment |
| High professional tolerance for antisocial and threatening behavior |
| Misplaced sense of duty |
| Source: Reference 6 |
RESPONSE: Involving police
Over 2 months, Ms. I phones the resident’s home 105 times (the resident screens the calls). During 1 call, she states that she is hidden in a closet in her home and will hurt herself unless the resident “resumes” her psychiatric care. The resident contacts police in his city and Ms. I’s community, but authorities are reluctant to act when he acknowledges that he is not Ms. I’s psychiatrist and does not know her. Police officers in Ms. I’s hometown tell the resident no one answered the door when they visited her home. They state that they would enter the residence forcibly only if Ms. I’s physician or a family member asked them to do so, and because the resident admits that he is not her psychiatrist, they cannot take further action. Ms. I leaves the resident a phone message several hours later to inform him she is safe.
The authors’ observations
Stalking-induced countertransference responses may lead a psychiatrist to unwittingly place himself in harm’s way. For example, intense rage at a stalker’s request for treatment may generate guilt that motivates the psychiatrist to agree to treat the stalker. Feelings of helplessness may produce a frantic desire to do something even when such activity is ill-advised. Psychiatrists may develop a tolerance for antisocial or threatening behavior—which is common in mental health settings—and could accept unnecessary risks.
A psychiatrist who is being stalked may be able to assist a mentally ill stalker in a way that does not create a duty to treat and does not expose the psychiatrist to harm, such as contacting a mobile crisis intervention team, a mental health professional who recently treated the stalker, a family member of the stalker, or law enforcement personnel. A psychiatrist who is thrust from the role of helper to victim and must protect his or her own well-being instead of attending to a patient’s welfare is prone to suffer substantial countertransference distress.
The situation with Ms. I was particularly challenging because the resident did not know her complete history and therefore had little information to gauge how likely she was to act on her aggressive threats. Factors that predict future violence include:
- a history of violence
- significant prior criminality
- young age at first arrest
- concomitant substance abuse
- male sex.9
Unfortunately, other than sex, this data regarding Ms. I could not be readily obtained.
A psychiatrist’s duty
Although sympathetic to his stalker’s distress, the resident did not want to treat this woman, nor was he ethically or legally obligated to do so. An individual’s wish to be treated by a particular psychiatrist does not create a duty for the psychiatrist to satisfy this wish.10 State-based “Good Samaritan” laws encourage physicians to assist those in acute need by shielding them from liability, as long as they reasonably act within the scope of their expertise.11 However, they do not require a physician to care for an individual in acute need. A delusional wish for treatment or a false belief of already being in treatment does not create a duty to care for a person.
OUTCOME: Seeking help
Ms. I’s phone calls and letters continue. The resident discusses the situation with his associate residency director, who refers him to the hospital’s legal and investigative staffs. Based on advice from the hospital’s private investigator, the resident sends Ms. I a formal “cease and desist” letter that threatens her with legal action and possible jail time. The staff at the front desk of the clinic where the resident works and the hospital’s security department are instructed to watch for a visitor with Ms. I’s name and description, although the hospital’s investigator is unable to obtain a photograph of her. Shortly after the resident sends the letter, Ms. I ceases communication.
The authors’ observations
This case is unusual because most stalking victims know their stalkers. Identifying a stalker’s motivation can be helpful in formulating a risk assessment. One classification system recognizes 5 categories of stalkers: rejected, intimacy seeking, incompetent, resentful, and predatory (Table 2).1 Rejected stalkers appear to pose the greatest risk of violence and homicide.8 However, all stalkers may pose a risk of violence and therefore all stalking behavior should be treated seriously.
Table 2
Classification of stalkers
| Category | Common features |
|---|---|
| Rejected | Most have a personality disorder; often seeking reconciliation and revenge; most frequent victims are ex-romantic partners, but also target estranged relatives, former friends |
| Intimacy seeking | Erotomania; “morbid infatuation” |
| Incompetent | Lacking social skills; often have stalked others |
| Resentful | Pursuing a vendetta; generally feeling aggrieved |
| Predatory | Often comorbid with paraphilias; may have past convictions for sex offenses |
| Source: Adapted from reference 1 | |
Responding to a stalker
The approach should be tailored to the stalker’s characteristics.12 Silence—ie, lack of acknowledgement of a stalker’s intrusions—is one tactic.13 Consistent and persistent lack of engagement may bore the stalker, but also may provoke frustration or narcissistic or paranoia-fueled rage, and increased efforts to interact with the mental health professional. Other responses include:
- obtaining a protection or restraining order
- promoting the stalker’s participation in adversarial civil litigation, such as a lawsuit
- issuing verbal counterthreats.
Restraining orders are controversial and assessments of their effectiveness vary.14 How well a restraining order works may depend on the stalker’s:
- ability to appreciate reality, and how likely he or she is to experience anxiety when confronted with adverse consequences of his or her actions
- how consistently, rapidly, and harshly the criminal justice system responds to violations of restraining orders.
Restraining orders also may provide the victim a false sense of security.15 One of her letters revealed that Ms. I violated a criminal plea arrangement years earlier, which suggests she was capable of violating a restraining order.
Litigation. A stalker may initiate civil litigation against the victim to feel that he or she has an impact on the victim, which may reduce the stalker’s risk of violence if he or she is emotionally engaged in the litigation. Based on the authors’ experience, as long as the stalker is talking, he or she generally is less likely to act out violently and terminate a satisfying process. Adversarial civil litigation could give a stalker the opportunity to be “close” to the victim and a means of expressing aggressive wishes. The benefit of litigation lasts only as long as the case persists and the stalker believes he or she may prevail. In one of her letters, Ms. I bragged that she had represented herself as a pro se litigant in a complex civil matter, suggesting that she might be constructively channeled into litigation.
Promoting litigation carries significant risk.16 Being a defendant in pro se litigation may be emotionally and financially stressful. This approach may be desirable if the psychiatrist’s institution is willing to offer substantial support. For example, an institution may provide legal assistance—including helping to defray the cost of litigation—and litigation-related scheduling flexibility. An attorney may serve as a boundary between the victim and the pro se litigant’s sometimes ceaseless, time-devouring, anxiety-inducing legal maneuvers.
Counterthreats. Warning a stalker that he or she will face severe civil and criminal consequences if his or her behavior continues can make clear that his or her conduct is unacceptable.17 Such warnings may be delivered verbally or in writing by a legal representative, law enforcement personnel, a private security agent, or the victim.
Issuing a counterthreat can be risky. Stalkers with antisocial or narcissistic personality features may perceive a counterthreat as narcissistically diminishing, and to save face will escalate their stalking in retaliation. Avoid counterthreats if you believe the stalker might be psychotic because destabilizing such an individual—such as by precipitating a short psychotic episode—may increase unpredictability and diminish their responsive to interventions.
Ms. I’s contact with the resident lasted approximately 20 months, slightly less than the average 26 months reported in a survey of mental health professionals.3 Because stalkers are unpredictable, the psychiatric resident remains cautious.
Related Resources
- National Center for Victims of Crime. Stalking resource center. www.victimsofcrime.org/our-programs/stalking-resource-center.
- Mullen PE, Pathé M, Purcell R. Stalkers and their victims. New York, NY: Cambridge University Press; 2009.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Mullen PE, Pathé M, Purcell R, et al. Study of stalkers. Am J Psychiatry. 1999;156(8):1244-1249.
2. Sandberg DA, McNiel DE, Binder RL. Stalking threatening, and harassing behavior by psychiatric patients toward clinicians. J Am Acad Psychiatry Law. 2002;30(2):221-229.
3. McIvor R, Potter L, Davies L. Stalking behavior by patients towards psychiatrists in a large mental health organization. Int J Soc Psychiatry. 2008;54(4):350-357.
4. Mullen PE, Pathé M. Stalking. Crime and Justice. 2002;29:273-318.
5. Bird S. Strategies for managing and minimizing the impact of harassment and stalking by patients. ANZ J Surg. 2009;79(7-8):537-538.
6. Sinwelski SA, Vinton L. Stalking: the constant threat of violence. Affilia. 2001;16(1):46-65.
7. Meloy JR. Commentary: stalking threatening, and harassing behavior by patients—the risk-management response. J Am Acad Psychiatry Law. 2002;30(2):230-231.
8. Resnick PJ. Stalking risk assessment. In: Pinals DA, ed. Stalking: psychiatric perspectives and practical approaches. New York, NY: Oxford University Press; 2007:61–84.
9. Dietz PE. Defenses against dangerous people when arrest and commitment fail. In: Simon RI, ed. American Psychiatric Press review of clinical psychiatry and the law. 1st ed. Washington, DC: American Psychiatric Press; 1989:205–219.
10. Hilliard J. Termination of treatment with troublesome patients. In: Lifson LE, Simon RI, eds. The mental health practitioner and the law: a comprehensive handbook. Cambridge, MA: Harvard University Press; 1998:216–224.
11. Paterick TJ, Paterick BB, Paterick TE. Implications of Good Samaritan laws for physicians. J Med Pract Manage. 2008;23(6):372-375.
12. MacKenzie RD, James DV. Management and treatment of stalkers: problems options, and solutions. Behav Sci Law. 2011;29(2):220-239.
13. Fremouw WJ, Westrup D, Pennypacker J. Stalking on campus: the prevalence and strategies for coping with stalking. J Forensic Sci. 1997;42(4):666-669.
14. Nicastro AM, Cousins AV, Spitzberg BH. The tactical face of stalking. Journal of Criminal Justice. 2000;28(1):69-82.
15. Spitzberg BH. The tactical topography of stalking victimization and management. Trauma Violence Abuse. 2002;3(4):261-288.
16. Pathé M, MacKenzie R, Mullen PE. Stalking by law: damaging victims and rewarding offenders. J Law Med. 2004;12(1):103-111.
17. Lion JR, Herschler JA. The stalking of physicians by their patients. In: Meloy JR. The psychology of stalking: clinical and forensic perspectives. San Diego, CA: Academic Press; 1998:163–173.
CASE: Delusions and threats
For over 20 months, Ms. I, age 48, sends a psychiatric resident letters and postcards that total approximately 3,000 pages and come from dozens of return addresses. Ms. I expresses romantic feelings toward the resident and believes that he was her physician and prescribed medications, including “mood stabilizers.” The resident never treated Ms. I; to his knowledge, he has never interacted with her.
Ms. I describes the resident’s refusal to continue treating her as “abandonment” and states that she is contemplating self-harm because of this rejection. In her letters, Ms. I admits that she was a long-term patient in a state psychiatric hospital in her home state and suffers from persistent auditory hallucinations. She also wants a romantic relationship with the resident and repeatedly threatens the resident’s female acquaintances and former romantic partners whose relationships she had surmised from news articles available on the Internet. Ms. I also threatens to strangle the resident. The resident sends her multiple written requests that she cease contact, but they are not acknowledged.
The authors’ observations
Stalking—repeated, unwanted attention or communication that would cause a reasonable person fear—is a serious threat for many psychiatric clinicians.1 Prevalence rates among mental health care providers range from 3% to 21%.2,3 Most stalkers have engaged in previous stalking behavior.3
Being stalked is highly distressing,4 and mental health professionals often do not reveal such experiences to colleagues.5 Irrational feelings of guilt or embarrassment, such as being thought to have poorly managed interactions with the stalker, often motivate a self-imposed silence (Table 1).6 This isolation may foster anxiety, interfere with receiving problem-solving advice, and increase physical vulnerability. In the case involving Ms. I, the psychiatric resident’s primary responsibility is safeguarding his own physical and psychological welfare.
Clinicians who work in a hospital or other institutional setting who are being stalked should inform their supervisors and the facility’s security personnel. Security personnel may be able to gather data about the stalker, decrease the stalker’s ability to communicate with the victim, and reduce unwanted physical access to the victim by distributing a photo of the stalker or installing a camera or receptionist-controlled door lock in patient entryways. Security personnel also may collaborate with local law enforcement. Having a third party respond to a stalker’s aggressive behavior—rather than the victim responding directly—avoids rewarding the stalker, which may generate further unwanted contact.7 Any intervention by the victim may increase the risk of violence, creating an “intervention dilemma.” Resnick8 argues that before deciding how best to address the stalker’s behavior, a stalking victim must “first separate the risk of continued stalking from the risk that the stalker will commit a violent act.”
Mental health professionals in private practice who are being stalked should consider retaining an attorney. An attorney often can maintain privacy of communications regarding the stalker via the attorney-client and attorney-work product privileges, which may help during legal proceedings.
Table 1
Factors that can impede psychiatrists from reporting stalking
| Fear of being perceived as a failure |
| Embarrassment |
| High professional tolerance for antisocial and threatening behavior |
| Misplaced sense of duty |
| Source: Reference 6 |
RESPONSE: Involving police
Over 2 months, Ms. I phones the resident’s home 105 times (the resident screens the calls). During 1 call, she states that she is hidden in a closet in her home and will hurt herself unless the resident “resumes” her psychiatric care. The resident contacts police in his city and Ms. I’s community, but authorities are reluctant to act when he acknowledges that he is not Ms. I’s psychiatrist and does not know her. Police officers in Ms. I’s hometown tell the resident no one answered the door when they visited her home. They state that they would enter the residence forcibly only if Ms. I’s physician or a family member asked them to do so, and because the resident admits that he is not her psychiatrist, they cannot take further action. Ms. I leaves the resident a phone message several hours later to inform him she is safe.
The authors’ observations
Stalking-induced countertransference responses may lead a psychiatrist to unwittingly place himself in harm’s way. For example, intense rage at a stalker’s request for treatment may generate guilt that motivates the psychiatrist to agree to treat the stalker. Feelings of helplessness may produce a frantic desire to do something even when such activity is ill-advised. Psychiatrists may develop a tolerance for antisocial or threatening behavior—which is common in mental health settings—and could accept unnecessary risks.
A psychiatrist who is being stalked may be able to assist a mentally ill stalker in a way that does not create a duty to treat and does not expose the psychiatrist to harm, such as contacting a mobile crisis intervention team, a mental health professional who recently treated the stalker, a family member of the stalker, or law enforcement personnel. A psychiatrist who is thrust from the role of helper to victim and must protect his or her own well-being instead of attending to a patient’s welfare is prone to suffer substantial countertransference distress.
The situation with Ms. I was particularly challenging because the resident did not know her complete history and therefore had little information to gauge how likely she was to act on her aggressive threats. Factors that predict future violence include:
- a history of violence
- significant prior criminality
- young age at first arrest
- concomitant substance abuse
- male sex.9
Unfortunately, other than sex, this data regarding Ms. I could not be readily obtained.
A psychiatrist’s duty
Although sympathetic to his stalker’s distress, the resident did not want to treat this woman, nor was he ethically or legally obligated to do so. An individual’s wish to be treated by a particular psychiatrist does not create a duty for the psychiatrist to satisfy this wish.10 State-based “Good Samaritan” laws encourage physicians to assist those in acute need by shielding them from liability, as long as they reasonably act within the scope of their expertise.11 However, they do not require a physician to care for an individual in acute need. A delusional wish for treatment or a false belief of already being in treatment does not create a duty to care for a person.
OUTCOME: Seeking help
Ms. I’s phone calls and letters continue. The resident discusses the situation with his associate residency director, who refers him to the hospital’s legal and investigative staffs. Based on advice from the hospital’s private investigator, the resident sends Ms. I a formal “cease and desist” letter that threatens her with legal action and possible jail time. The staff at the front desk of the clinic where the resident works and the hospital’s security department are instructed to watch for a visitor with Ms. I’s name and description, although the hospital’s investigator is unable to obtain a photograph of her. Shortly after the resident sends the letter, Ms. I ceases communication.
The authors’ observations
This case is unusual because most stalking victims know their stalkers. Identifying a stalker’s motivation can be helpful in formulating a risk assessment. One classification system recognizes 5 categories of stalkers: rejected, intimacy seeking, incompetent, resentful, and predatory (Table 2).1 Rejected stalkers appear to pose the greatest risk of violence and homicide.8 However, all stalkers may pose a risk of violence and therefore all stalking behavior should be treated seriously.
Table 2
Classification of stalkers
| Category | Common features |
|---|---|
| Rejected | Most have a personality disorder; often seeking reconciliation and revenge; most frequent victims are ex-romantic partners, but also target estranged relatives, former friends |
| Intimacy seeking | Erotomania; “morbid infatuation” |
| Incompetent | Lacking social skills; often have stalked others |
| Resentful | Pursuing a vendetta; generally feeling aggrieved |
| Predatory | Often comorbid with paraphilias; may have past convictions for sex offenses |
| Source: Adapted from reference 1 | |
Responding to a stalker
The approach should be tailored to the stalker’s characteristics.12 Silence—ie, lack of acknowledgement of a stalker’s intrusions—is one tactic.13 Consistent and persistent lack of engagement may bore the stalker, but also may provoke frustration or narcissistic or paranoia-fueled rage, and increased efforts to interact with the mental health professional. Other responses include:
- obtaining a protection or restraining order
- promoting the stalker’s participation in adversarial civil litigation, such as a lawsuit
- issuing verbal counterthreats.
Restraining orders are controversial and assessments of their effectiveness vary.14 How well a restraining order works may depend on the stalker’s:
- ability to appreciate reality, and how likely he or she is to experience anxiety when confronted with adverse consequences of his or her actions
- how consistently, rapidly, and harshly the criminal justice system responds to violations of restraining orders.
Restraining orders also may provide the victim a false sense of security.15 One of her letters revealed that Ms. I violated a criminal plea arrangement years earlier, which suggests she was capable of violating a restraining order.
Litigation. A stalker may initiate civil litigation against the victim to feel that he or she has an impact on the victim, which may reduce the stalker’s risk of violence if he or she is emotionally engaged in the litigation. Based on the authors’ experience, as long as the stalker is talking, he or she generally is less likely to act out violently and terminate a satisfying process. Adversarial civil litigation could give a stalker the opportunity to be “close” to the victim and a means of expressing aggressive wishes. The benefit of litigation lasts only as long as the case persists and the stalker believes he or she may prevail. In one of her letters, Ms. I bragged that she had represented herself as a pro se litigant in a complex civil matter, suggesting that she might be constructively channeled into litigation.
Promoting litigation carries significant risk.16 Being a defendant in pro se litigation may be emotionally and financially stressful. This approach may be desirable if the psychiatrist’s institution is willing to offer substantial support. For example, an institution may provide legal assistance—including helping to defray the cost of litigation—and litigation-related scheduling flexibility. An attorney may serve as a boundary between the victim and the pro se litigant’s sometimes ceaseless, time-devouring, anxiety-inducing legal maneuvers.
Counterthreats. Warning a stalker that he or she will face severe civil and criminal consequences if his or her behavior continues can make clear that his or her conduct is unacceptable.17 Such warnings may be delivered verbally or in writing by a legal representative, law enforcement personnel, a private security agent, or the victim.
Issuing a counterthreat can be risky. Stalkers with antisocial or narcissistic personality features may perceive a counterthreat as narcissistically diminishing, and to save face will escalate their stalking in retaliation. Avoid counterthreats if you believe the stalker might be psychotic because destabilizing such an individual—such as by precipitating a short psychotic episode—may increase unpredictability and diminish their responsive to interventions.
Ms. I’s contact with the resident lasted approximately 20 months, slightly less than the average 26 months reported in a survey of mental health professionals.3 Because stalkers are unpredictable, the psychiatric resident remains cautious.
Related Resources
- National Center for Victims of Crime. Stalking resource center. www.victimsofcrime.org/our-programs/stalking-resource-center.
- Mullen PE, Pathé M, Purcell R. Stalkers and their victims. New York, NY: Cambridge University Press; 2009.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Delusions and threats
For over 20 months, Ms. I, age 48, sends a psychiatric resident letters and postcards that total approximately 3,000 pages and come from dozens of return addresses. Ms. I expresses romantic feelings toward the resident and believes that he was her physician and prescribed medications, including “mood stabilizers.” The resident never treated Ms. I; to his knowledge, he has never interacted with her.
Ms. I describes the resident’s refusal to continue treating her as “abandonment” and states that she is contemplating self-harm because of this rejection. In her letters, Ms. I admits that she was a long-term patient in a state psychiatric hospital in her home state and suffers from persistent auditory hallucinations. She also wants a romantic relationship with the resident and repeatedly threatens the resident’s female acquaintances and former romantic partners whose relationships she had surmised from news articles available on the Internet. Ms. I also threatens to strangle the resident. The resident sends her multiple written requests that she cease contact, but they are not acknowledged.
The authors’ observations
Stalking—repeated, unwanted attention or communication that would cause a reasonable person fear—is a serious threat for many psychiatric clinicians.1 Prevalence rates among mental health care providers range from 3% to 21%.2,3 Most stalkers have engaged in previous stalking behavior.3
Being stalked is highly distressing,4 and mental health professionals often do not reveal such experiences to colleagues.5 Irrational feelings of guilt or embarrassment, such as being thought to have poorly managed interactions with the stalker, often motivate a self-imposed silence (Table 1).6 This isolation may foster anxiety, interfere with receiving problem-solving advice, and increase physical vulnerability. In the case involving Ms. I, the psychiatric resident’s primary responsibility is safeguarding his own physical and psychological welfare.
Clinicians who work in a hospital or other institutional setting who are being stalked should inform their supervisors and the facility’s security personnel. Security personnel may be able to gather data about the stalker, decrease the stalker’s ability to communicate with the victim, and reduce unwanted physical access to the victim by distributing a photo of the stalker or installing a camera or receptionist-controlled door lock in patient entryways. Security personnel also may collaborate with local law enforcement. Having a third party respond to a stalker’s aggressive behavior—rather than the victim responding directly—avoids rewarding the stalker, which may generate further unwanted contact.7 Any intervention by the victim may increase the risk of violence, creating an “intervention dilemma.” Resnick8 argues that before deciding how best to address the stalker’s behavior, a stalking victim must “first separate the risk of continued stalking from the risk that the stalker will commit a violent act.”
Mental health professionals in private practice who are being stalked should consider retaining an attorney. An attorney often can maintain privacy of communications regarding the stalker via the attorney-client and attorney-work product privileges, which may help during legal proceedings.
Table 1
Factors that can impede psychiatrists from reporting stalking
| Fear of being perceived as a failure |
| Embarrassment |
| High professional tolerance for antisocial and threatening behavior |
| Misplaced sense of duty |
| Source: Reference 6 |
RESPONSE: Involving police
Over 2 months, Ms. I phones the resident’s home 105 times (the resident screens the calls). During 1 call, she states that she is hidden in a closet in her home and will hurt herself unless the resident “resumes” her psychiatric care. The resident contacts police in his city and Ms. I’s community, but authorities are reluctant to act when he acknowledges that he is not Ms. I’s psychiatrist and does not know her. Police officers in Ms. I’s hometown tell the resident no one answered the door when they visited her home. They state that they would enter the residence forcibly only if Ms. I’s physician or a family member asked them to do so, and because the resident admits that he is not her psychiatrist, they cannot take further action. Ms. I leaves the resident a phone message several hours later to inform him she is safe.
The authors’ observations
Stalking-induced countertransference responses may lead a psychiatrist to unwittingly place himself in harm’s way. For example, intense rage at a stalker’s request for treatment may generate guilt that motivates the psychiatrist to agree to treat the stalker. Feelings of helplessness may produce a frantic desire to do something even when such activity is ill-advised. Psychiatrists may develop a tolerance for antisocial or threatening behavior—which is common in mental health settings—and could accept unnecessary risks.
A psychiatrist who is being stalked may be able to assist a mentally ill stalker in a way that does not create a duty to treat and does not expose the psychiatrist to harm, such as contacting a mobile crisis intervention team, a mental health professional who recently treated the stalker, a family member of the stalker, or law enforcement personnel. A psychiatrist who is thrust from the role of helper to victim and must protect his or her own well-being instead of attending to a patient’s welfare is prone to suffer substantial countertransference distress.
The situation with Ms. I was particularly challenging because the resident did not know her complete history and therefore had little information to gauge how likely she was to act on her aggressive threats. Factors that predict future violence include:
- a history of violence
- significant prior criminality
- young age at first arrest
- concomitant substance abuse
- male sex.9
Unfortunately, other than sex, this data regarding Ms. I could not be readily obtained.
A psychiatrist’s duty
Although sympathetic to his stalker’s distress, the resident did not want to treat this woman, nor was he ethically or legally obligated to do so. An individual’s wish to be treated by a particular psychiatrist does not create a duty for the psychiatrist to satisfy this wish.10 State-based “Good Samaritan” laws encourage physicians to assist those in acute need by shielding them from liability, as long as they reasonably act within the scope of their expertise.11 However, they do not require a physician to care for an individual in acute need. A delusional wish for treatment or a false belief of already being in treatment does not create a duty to care for a person.
OUTCOME: Seeking help
Ms. I’s phone calls and letters continue. The resident discusses the situation with his associate residency director, who refers him to the hospital’s legal and investigative staffs. Based on advice from the hospital’s private investigator, the resident sends Ms. I a formal “cease and desist” letter that threatens her with legal action and possible jail time. The staff at the front desk of the clinic where the resident works and the hospital’s security department are instructed to watch for a visitor with Ms. I’s name and description, although the hospital’s investigator is unable to obtain a photograph of her. Shortly after the resident sends the letter, Ms. I ceases communication.
The authors’ observations
This case is unusual because most stalking victims know their stalkers. Identifying a stalker’s motivation can be helpful in formulating a risk assessment. One classification system recognizes 5 categories of stalkers: rejected, intimacy seeking, incompetent, resentful, and predatory (Table 2).1 Rejected stalkers appear to pose the greatest risk of violence and homicide.8 However, all stalkers may pose a risk of violence and therefore all stalking behavior should be treated seriously.
Table 2
Classification of stalkers
| Category | Common features |
|---|---|
| Rejected | Most have a personality disorder; often seeking reconciliation and revenge; most frequent victims are ex-romantic partners, but also target estranged relatives, former friends |
| Intimacy seeking | Erotomania; “morbid infatuation” |
| Incompetent | Lacking social skills; often have stalked others |
| Resentful | Pursuing a vendetta; generally feeling aggrieved |
| Predatory | Often comorbid with paraphilias; may have past convictions for sex offenses |
| Source: Adapted from reference 1 | |
Responding to a stalker
The approach should be tailored to the stalker’s characteristics.12 Silence—ie, lack of acknowledgement of a stalker’s intrusions—is one tactic.13 Consistent and persistent lack of engagement may bore the stalker, but also may provoke frustration or narcissistic or paranoia-fueled rage, and increased efforts to interact with the mental health professional. Other responses include:
- obtaining a protection or restraining order
- promoting the stalker’s participation in adversarial civil litigation, such as a lawsuit
- issuing verbal counterthreats.
Restraining orders are controversial and assessments of their effectiveness vary.14 How well a restraining order works may depend on the stalker’s:
- ability to appreciate reality, and how likely he or she is to experience anxiety when confronted with adverse consequences of his or her actions
- how consistently, rapidly, and harshly the criminal justice system responds to violations of restraining orders.
Restraining orders also may provide the victim a false sense of security.15 One of her letters revealed that Ms. I violated a criminal plea arrangement years earlier, which suggests she was capable of violating a restraining order.
Litigation. A stalker may initiate civil litigation against the victim to feel that he or she has an impact on the victim, which may reduce the stalker’s risk of violence if he or she is emotionally engaged in the litigation. Based on the authors’ experience, as long as the stalker is talking, he or she generally is less likely to act out violently and terminate a satisfying process. Adversarial civil litigation could give a stalker the opportunity to be “close” to the victim and a means of expressing aggressive wishes. The benefit of litigation lasts only as long as the case persists and the stalker believes he or she may prevail. In one of her letters, Ms. I bragged that she had represented herself as a pro se litigant in a complex civil matter, suggesting that she might be constructively channeled into litigation.
Promoting litigation carries significant risk.16 Being a defendant in pro se litigation may be emotionally and financially stressful. This approach may be desirable if the psychiatrist’s institution is willing to offer substantial support. For example, an institution may provide legal assistance—including helping to defray the cost of litigation—and litigation-related scheduling flexibility. An attorney may serve as a boundary between the victim and the pro se litigant’s sometimes ceaseless, time-devouring, anxiety-inducing legal maneuvers.
Counterthreats. Warning a stalker that he or she will face severe civil and criminal consequences if his or her behavior continues can make clear that his or her conduct is unacceptable.17 Such warnings may be delivered verbally or in writing by a legal representative, law enforcement personnel, a private security agent, or the victim.
Issuing a counterthreat can be risky. Stalkers with antisocial or narcissistic personality features may perceive a counterthreat as narcissistically diminishing, and to save face will escalate their stalking in retaliation. Avoid counterthreats if you believe the stalker might be psychotic because destabilizing such an individual—such as by precipitating a short psychotic episode—may increase unpredictability and diminish their responsive to interventions.
Ms. I’s contact with the resident lasted approximately 20 months, slightly less than the average 26 months reported in a survey of mental health professionals.3 Because stalkers are unpredictable, the psychiatric resident remains cautious.
Related Resources
- National Center for Victims of Crime. Stalking resource center. www.victimsofcrime.org/our-programs/stalking-resource-center.
- Mullen PE, Pathé M, Purcell R. Stalkers and their victims. New York, NY: Cambridge University Press; 2009.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Mullen PE, Pathé M, Purcell R, et al. Study of stalkers. Am J Psychiatry. 1999;156(8):1244-1249.
2. Sandberg DA, McNiel DE, Binder RL. Stalking threatening, and harassing behavior by psychiatric patients toward clinicians. J Am Acad Psychiatry Law. 2002;30(2):221-229.
3. McIvor R, Potter L, Davies L. Stalking behavior by patients towards psychiatrists in a large mental health organization. Int J Soc Psychiatry. 2008;54(4):350-357.
4. Mullen PE, Pathé M. Stalking. Crime and Justice. 2002;29:273-318.
5. Bird S. Strategies for managing and minimizing the impact of harassment and stalking by patients. ANZ J Surg. 2009;79(7-8):537-538.
6. Sinwelski SA, Vinton L. Stalking: the constant threat of violence. Affilia. 2001;16(1):46-65.
7. Meloy JR. Commentary: stalking threatening, and harassing behavior by patients—the risk-management response. J Am Acad Psychiatry Law. 2002;30(2):230-231.
8. Resnick PJ. Stalking risk assessment. In: Pinals DA, ed. Stalking: psychiatric perspectives and practical approaches. New York, NY: Oxford University Press; 2007:61–84.
9. Dietz PE. Defenses against dangerous people when arrest and commitment fail. In: Simon RI, ed. American Psychiatric Press review of clinical psychiatry and the law. 1st ed. Washington, DC: American Psychiatric Press; 1989:205–219.
10. Hilliard J. Termination of treatment with troublesome patients. In: Lifson LE, Simon RI, eds. The mental health practitioner and the law: a comprehensive handbook. Cambridge, MA: Harvard University Press; 1998:216–224.
11. Paterick TJ, Paterick BB, Paterick TE. Implications of Good Samaritan laws for physicians. J Med Pract Manage. 2008;23(6):372-375.
12. MacKenzie RD, James DV. Management and treatment of stalkers: problems options, and solutions. Behav Sci Law. 2011;29(2):220-239.
13. Fremouw WJ, Westrup D, Pennypacker J. Stalking on campus: the prevalence and strategies for coping with stalking. J Forensic Sci. 1997;42(4):666-669.
14. Nicastro AM, Cousins AV, Spitzberg BH. The tactical face of stalking. Journal of Criminal Justice. 2000;28(1):69-82.
15. Spitzberg BH. The tactical topography of stalking victimization and management. Trauma Violence Abuse. 2002;3(4):261-288.
16. Pathé M, MacKenzie R, Mullen PE. Stalking by law: damaging victims and rewarding offenders. J Law Med. 2004;12(1):103-111.
17. Lion JR, Herschler JA. The stalking of physicians by their patients. In: Meloy JR. The psychology of stalking: clinical and forensic perspectives. San Diego, CA: Academic Press; 1998:163–173.
1. Mullen PE, Pathé M, Purcell R, et al. Study of stalkers. Am J Psychiatry. 1999;156(8):1244-1249.
2. Sandberg DA, McNiel DE, Binder RL. Stalking threatening, and harassing behavior by psychiatric patients toward clinicians. J Am Acad Psychiatry Law. 2002;30(2):221-229.
3. McIvor R, Potter L, Davies L. Stalking behavior by patients towards psychiatrists in a large mental health organization. Int J Soc Psychiatry. 2008;54(4):350-357.
4. Mullen PE, Pathé M. Stalking. Crime and Justice. 2002;29:273-318.
5. Bird S. Strategies for managing and minimizing the impact of harassment and stalking by patients. ANZ J Surg. 2009;79(7-8):537-538.
6. Sinwelski SA, Vinton L. Stalking: the constant threat of violence. Affilia. 2001;16(1):46-65.
7. Meloy JR. Commentary: stalking threatening, and harassing behavior by patients—the risk-management response. J Am Acad Psychiatry Law. 2002;30(2):230-231.
8. Resnick PJ. Stalking risk assessment. In: Pinals DA, ed. Stalking: psychiatric perspectives and practical approaches. New York, NY: Oxford University Press; 2007:61–84.
9. Dietz PE. Defenses against dangerous people when arrest and commitment fail. In: Simon RI, ed. American Psychiatric Press review of clinical psychiatry and the law. 1st ed. Washington, DC: American Psychiatric Press; 1989:205–219.
10. Hilliard J. Termination of treatment with troublesome patients. In: Lifson LE, Simon RI, eds. The mental health practitioner and the law: a comprehensive handbook. Cambridge, MA: Harvard University Press; 1998:216–224.
11. Paterick TJ, Paterick BB, Paterick TE. Implications of Good Samaritan laws for physicians. J Med Pract Manage. 2008;23(6):372-375.
12. MacKenzie RD, James DV. Management and treatment of stalkers: problems options, and solutions. Behav Sci Law. 2011;29(2):220-239.
13. Fremouw WJ, Westrup D, Pennypacker J. Stalking on campus: the prevalence and strategies for coping with stalking. J Forensic Sci. 1997;42(4):666-669.
14. Nicastro AM, Cousins AV, Spitzberg BH. The tactical face of stalking. Journal of Criminal Justice. 2000;28(1):69-82.
15. Spitzberg BH. The tactical topography of stalking victimization and management. Trauma Violence Abuse. 2002;3(4):261-288.
16. Pathé M, MacKenzie R, Mullen PE. Stalking by law: damaging victims and rewarding offenders. J Law Med. 2004;12(1):103-111.
17. Lion JR, Herschler JA. The stalking of physicians by their patients. In: Meloy JR. The psychology of stalking: clinical and forensic perspectives. San Diego, CA: Academic Press; 1998:163–173.
Epilepsy or something else?
CASE: Seizure-like symptoms
Ms. T, age 20, is brought to the emergency room (ER) by her father because she refuses to eat and drink, is unable to function at home, lies in bed all day, and does not attend to her activities of daily living (ADLs). Ms. T lives with her family, is not enrolled in school, and is unemployed. In the ER she initially is uncooperative and mute and then suddenly becomes agitated and has a seizure-like episode characterized by jerking of her trunk followed by random, asymmetrical movements of her legs and arms, closing both eyes, weeping, foaming at the mouth, moaning, and marked unresponsiveness. The episode lasts for >5 minutes.
The authors’ observations
Based on Ms. T’s presentation, the medical team considered acute epileptic seizures. Asymmetrical jerking of the body may be seen in frontal lobe epilepsy or seizures of the supplementary sensorimotor area. Frontal lobe epilepsy can present with bilateral asynchronous motor activity with consciousness during the event and a lack of postictal confusion.1 Seizures of the supplementary sensorimotor area—also known as the secondary motor area—are particularly problematic because typically they present with bilateral asymmetric tonic posturing followed by a few clonic movements, intact consciousness, and rarely postictal confusion. Adding to the diagnostic uncertainty, some “soft signs” thought to indicate PNES (eg, pelvic thrusting, crying) are common with frontal lobe epilepsy.1,2
PNES are episodes of altered movement, sensation, or experience that may be mistaken for epileptic seizures but are not a consequence of abnormal cortical discharges. Instead they are caused by physiological or psychological factors.3 Behaviors or signs that strongly suggest PNES include:
- gradual onset or termination
- pseudosleep, when the patient appears to be asleep but electroencephalography (EEG) findings indicate he or she is awake
- discontinuous (stop-and-go), irregular, or asynchronous (out-of-phase) activity—including side-to-side head movement, pelvic thrusting, and opisthotonic posturing—stuttering, and weeping4
- eye closure.5
Ms. T’s father said his daughter had been hospitalized several times for episodes characterized by pelvic thrusting, stuttering, and pseudosleep, which raised the possibility of PNES. Definitive diagnosis of PNES comes from video EEG when a patient is observed having typical seizures without accompanying EEG abnormalities.6
EVALUATION: Inconclusive data
Ms. T is admitted to the medical unit to rule out a seizure disorder. Physical examination is unremarkable and laboratory tests are within normal limits. The neurology service requests a head MRI, which is inconclusive. Inpatient video EEG with 24-hour monitoring does not indicate acute epileptic seizures. Ms. T’s father says that she has experienced many paroxysmal motor episodes and all neurologic tests, exams, and labs have failed to find a cause for these episodes. She did not receive any antiepileptic medications. A psychiatric consult is requested to clarify the diagnosis. Ms. T is transferred to an inpatient psychiatric unit for further evaluation and management.
The authors’ observations
Fleisher et al7 suggested that traumatic events may lead to presentations similar to PNES. Because Ms. T was molested by a family friend as a child, we considered posttraumatic stress disorder (PTSD) in the differential diagnosis, although she has not reported symptoms of intrusive recollections, avoidance, numbing, or hyperarousal.
We also considered conversion disorder and dissociative disorder. Patients with conversion disorder have ≥1 symptoms or signs that affect voluntary motor or sensory function that cannot be explained by a neurologic or general medical condition.8 Dissociative disorder is a disruption in usually integrated functions of consciousness, memory, identity, or perception of the environment.8 The presentation of patients with PNES may resemble that of patients with dissociative disorder.8 In a study of 45 adult PNES patients, Bowman et al8 found that PNES often are comorbid with other psychiatric disorders, including somatoform disorders (89%), dissociative disorders (91%), affective disorders (64%), personality disorders (62%), PTSD (49%), and other anxiety disorders (47%).
TREATMENT: Managing aggression
In the psychiatric unit, Ms. T initially is irritable and disorganized with poor oral intake and regressed behavior; she often is found in the fetal position, crying and talking in a childish manner. Throughout her admission, she receives several anxiolytics and antipsychotics—including lorazepam, up to 6 mg/d, clonazepam, up to 3 mg/d, haloperidol, up to 10 mg/d, and quetiapine, up to 200 mg/d—to help manage her aggressive behaviors after her seizure-like episodes. Further evaluation reveals that Ms. T has no psychotic symptoms, overt delusions, or perceptual disturbances and her thought process is coherent and clear. She has no history of substance abuse. Her ability to perform ADLs improves within a few days. She complains of depressed mood and engages in head banging, which requires close observation.
Ms. T has a history of mood and behavioral problems since early childhood characterized by episodic dysphoric mood, anxiety, and agitation. She has had trials of several antidepressants, including sertraline, fluoxetine, venlafaxine, and escitalopram, and anxiolytics, including lorazepam, clonazepam, and alprazolam. Her outpatient psychiatrist describes a history of physical and sexual abuse starting at age 7. At age 9, after her mother died from breast cancer, Ms. T and her siblings were moved to foster care, where she was physically abused by the staff. She remained in foster care until age 18.
The authors’ observations
PNES pose a diagnostic and therapeutic challenge. Many PNES patients seek medical attention for their seizures. PNES patients misdiagnosed as having epilepsy have a worse prognosis because they do not receive appropriate treatment9 and may experience side effects if antiepileptics are prescribed.10 Finally, the financial burden of medical care can be significant. Ms. T had several hospitalizations, including extensive neurologic workup, intensive care unit admissions for intubation, and use of antiepileptics with almost no benefit.
Psychosocial assessments of PNES patients have revealed that sexual abuse, family conflicts, and death of a family member often play an important role.11 It is possible that as a result of childhood trauma, Ms. T exhibited a regressed and primitive defense mechanism to deal with the trauma. PNES usually are considered when a patient presents with:
- absence of therapeutic response to antiepileptics
- loss of response (therapeutic failure) to antiepileptics
- paradoxical response to antiepileptics (worsening or unexpected responses)
- atypical, multiple, or inconsistent seizures
- seizures that occur soon after emotional stress.12
We concluded Ms. T had PNES because of the unusual presentations of her seizures, negative video EEG findings, failure to respond to antiepileptics, lack of risk factors for epilepsy, and aggressive behaviors before or after the seizures ( Table ).4,10,11,13 Diagnosing PNES early allows clinicians to focus on appropriate treatment modalities (eg, psychotherapy, antidepressants), prevents costly neurologic workups and treatments (eg, routine EEGs, trials of several antiepileptics), and provides patients with diagnostic assurance.10
Table
Characteristics of psychogenic nonepileptic seizures
| Characteristic | Comment |
|---|---|
| Duration | May be prolonged |
| Timing | Usually occur only during the day |
| Physical harm | Rare |
| Tongue biting | Rare |
| Urinary incontinence | Rare |
| Motor activity | Prolonged |
| Cyanosis | No |
| Postictal confusion | Rare |
| Related to medication changes | No |
| Interictal EEG | Normal |
| Ictal EEG | Normal |
| Presence of secondary gain | Common |
| EEG: electroencephalography Source: References 4,10,11,13 | |
3 components of treatment
Presenting the PNES diagnosis to the patient. The neurologist and the psychiatrist should convey to the patient that they see the symptoms as “real” and not “all in your head.”14
Withdrawing antiepileptic medications. Antiepileptic medication withdrawal is recommended when a thorough diagnostic workup shows no evidence of epileptic seizures.15 Oto et al16 reported 49% of PNES patients became seizure-free 12 months after discontinuing antiepileptics.
Psychotherapy and pharmacotherapy. Open-label studies of psychological treatments for PNES have demonstrated that a cognitive-behavioral therapy-based approach and brief augmented psychodynamic interpersonal therapy could reduce seizures.17 In a pilot, randomized, placebo-controlled trial, PNES patients who received flexibly dosed sertraline reported a 45% reduction in seizures compared with an 8% increase in the placebo group.18 Similar improvements in seizure frequency have been reported in PNES patients with anxiety or depression treated with venlafaxine.19
OUTCOME: Support, improvement
During the next several days, Ms. T has random episodes of seizures with foaming of the mouth and unresponsiveness. These episodes last from 5 to 30 minutes and require transfer to the ER. After each episode, Ms. T is medically cleared and sent back to the psychiatric unit. The neurologist recommends avoiding antiepileptics. Ms. T responds well to the structured inpatient setting and supportive psychotherapy. Her episodes decrease and her mood becomes more stable. She refrains from self-injurious behaviors and is discharged home with outpatient follow-up.
Related Resource
- Marsh P, Benbadis S, Fernandez F. Psychogenic nonepileptic seizures: ways to win over skeptical patients. Current Psychiatry. 2008;7(1):21-35.
Drug Brand Names
- Alprazolam • Xanax
- Clonazepam • Klonopin
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Haloperidol • Haldol
- Lorazepam • Ativan
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kellinghaus C, Lüders HO. Frontal lobe epilepsy. Epileptic Disord. 2004;6(4):223-239.
2. Kanner AM, Morris HH, Lüders H, et al. Supplementary motor seizures mimicking pseudoseizures: some clinical differences. Neurology. 1990;40(9):1404-1407.
3. Hall-Patch L, Brown R, House A, et al. Acceptability and effectiveness of a strategy for the communication of the diagnosis of psychogenic nonepileptic seizures. Epilepsia. 2010;51(1):70-78.
4. Reuber M, Elger CE. Psychogenic nonepileptic seizures: review and update. Epilepsy Behav. 2003;4(3):205-216.
5. Chung SS, Gerber P, Kirlin KA. Ictal eye closure is a reliable indicator for psychogenic nonepileptic seizures. Neurology. 2006;66(11):1730-1731.
6. Mostacci B, Bisulli F, Alvisi L, et al. Ictal characteristics of psychogenic nonepileptic seizures: what we have learned from video/EEG recordings—a literature review. Epilepsy Behav. 2011;22(2):144-153.
7. Fleisher W, Staley D, Krawetz P, et al. Comparative study of trauma-related phenomena in subjects with pseudoseizures and subjects with epilepsy. Am J Psychiatry. 2002;159(4):660-663.
8. Bowman ES, Markand ON. Psychodynamics and psychiatric diagnoses of pseudoseizure subjects. Am J Psychiatry. 1996;153(1):57-63.
9. Benbadis SR. The EEG in nonepileptic seizures. J Clin Neurophysiol. 2006;23(4):340-352.
10. Brown RJ, Syed TU, Benbadis S, et al. Psychogenic nonepileptic seizures. Epilepsy Behav. 2011;22(1):85-93.
11. Bodde NM, Brooks JL, Baker GA, et al. Psychogenic non-epileptic seizures—definition, etiology, treatment and prognostic issues: a critical review. Seizure. 2009;18(8):543-553.
12. Alsaadi TM, Marquez AV. Psychogenic nonepileptic seizures. Am Fam Physician. 2005;72(5):849-856.
13. Bradley WG, Daroff RB, Fenichel GM, et al. eds. Neurology in clinical practice: principles of diagnosis and management. 4th ed. Philadelphia, PA: Butterworth Heinemann; 2004:19-20, 1971–1972.
14. Harden CL, Ferrando SJ. Delivering the diagnosis of psychogenic pseudoseizures: should the neurologist or the psychiatrist be responsible? Epilepsy Behav. 2001;2(6):519-523.
15. Oto M, Espie CA, Duncan R. An exploratory randomized controlled trial of immediate versus delayed withdrawal of antiepileptic drugs in patients with psychogenic nonepileptic attacks (PNEAs). Epilepsia. 2010;51(10):1994-1999.
16. Oto M, Espie C, Pelosi A, et al. The safety of antiepileptic drug withdrawal in patients with non-epileptic seizures. J Neurol Neurosurg Psychiatry. 2005;76(12):1682-1685.
17. Goldstein LH, Mellers JD. Recent developments in our understanding of the semiology and treatment of psychogenic nonepileptic seizures. Curr Neurol Neurosci Rep. 2012;12(4):436-444.
18. LaFrance WC, Jr, Keitner GI, Papandonatos GD, et al. Pilot pharmacologic randomized controlled trial for psychogenic nonepileptic seizures. Neurology. 2010;75(13):1166-1173.
19. Pintor L, Baillés E, Matrai S, et al. Efficiency of venlafaxine in patients with psychogenic nonepileptic seizures and anxiety and/or depressive disorders. J Neuropsychiatry Clin Neurosci. 2010;22(4):401-408.
CASE: Seizure-like symptoms
Ms. T, age 20, is brought to the emergency room (ER) by her father because she refuses to eat and drink, is unable to function at home, lies in bed all day, and does not attend to her activities of daily living (ADLs). Ms. T lives with her family, is not enrolled in school, and is unemployed. In the ER she initially is uncooperative and mute and then suddenly becomes agitated and has a seizure-like episode characterized by jerking of her trunk followed by random, asymmetrical movements of her legs and arms, closing both eyes, weeping, foaming at the mouth, moaning, and marked unresponsiveness. The episode lasts for >5 minutes.
The authors’ observations
Based on Ms. T’s presentation, the medical team considered acute epileptic seizures. Asymmetrical jerking of the body may be seen in frontal lobe epilepsy or seizures of the supplementary sensorimotor area. Frontal lobe epilepsy can present with bilateral asynchronous motor activity with consciousness during the event and a lack of postictal confusion.1 Seizures of the supplementary sensorimotor area—also known as the secondary motor area—are particularly problematic because typically they present with bilateral asymmetric tonic posturing followed by a few clonic movements, intact consciousness, and rarely postictal confusion. Adding to the diagnostic uncertainty, some “soft signs” thought to indicate PNES (eg, pelvic thrusting, crying) are common with frontal lobe epilepsy.1,2
PNES are episodes of altered movement, sensation, or experience that may be mistaken for epileptic seizures but are not a consequence of abnormal cortical discharges. Instead they are caused by physiological or psychological factors.3 Behaviors or signs that strongly suggest PNES include:
- gradual onset or termination
- pseudosleep, when the patient appears to be asleep but electroencephalography (EEG) findings indicate he or she is awake
- discontinuous (stop-and-go), irregular, or asynchronous (out-of-phase) activity—including side-to-side head movement, pelvic thrusting, and opisthotonic posturing—stuttering, and weeping4
- eye closure.5
Ms. T’s father said his daughter had been hospitalized several times for episodes characterized by pelvic thrusting, stuttering, and pseudosleep, which raised the possibility of PNES. Definitive diagnosis of PNES comes from video EEG when a patient is observed having typical seizures without accompanying EEG abnormalities.6
EVALUATION: Inconclusive data
Ms. T is admitted to the medical unit to rule out a seizure disorder. Physical examination is unremarkable and laboratory tests are within normal limits. The neurology service requests a head MRI, which is inconclusive. Inpatient video EEG with 24-hour monitoring does not indicate acute epileptic seizures. Ms. T’s father says that she has experienced many paroxysmal motor episodes and all neurologic tests, exams, and labs have failed to find a cause for these episodes. She did not receive any antiepileptic medications. A psychiatric consult is requested to clarify the diagnosis. Ms. T is transferred to an inpatient psychiatric unit for further evaluation and management.
The authors’ observations
Fleisher et al7 suggested that traumatic events may lead to presentations similar to PNES. Because Ms. T was molested by a family friend as a child, we considered posttraumatic stress disorder (PTSD) in the differential diagnosis, although she has not reported symptoms of intrusive recollections, avoidance, numbing, or hyperarousal.
We also considered conversion disorder and dissociative disorder. Patients with conversion disorder have ≥1 symptoms or signs that affect voluntary motor or sensory function that cannot be explained by a neurologic or general medical condition.8 Dissociative disorder is a disruption in usually integrated functions of consciousness, memory, identity, or perception of the environment.8 The presentation of patients with PNES may resemble that of patients with dissociative disorder.8 In a study of 45 adult PNES patients, Bowman et al8 found that PNES often are comorbid with other psychiatric disorders, including somatoform disorders (89%), dissociative disorders (91%), affective disorders (64%), personality disorders (62%), PTSD (49%), and other anxiety disorders (47%).
TREATMENT: Managing aggression
In the psychiatric unit, Ms. T initially is irritable and disorganized with poor oral intake and regressed behavior; she often is found in the fetal position, crying and talking in a childish manner. Throughout her admission, she receives several anxiolytics and antipsychotics—including lorazepam, up to 6 mg/d, clonazepam, up to 3 mg/d, haloperidol, up to 10 mg/d, and quetiapine, up to 200 mg/d—to help manage her aggressive behaviors after her seizure-like episodes. Further evaluation reveals that Ms. T has no psychotic symptoms, overt delusions, or perceptual disturbances and her thought process is coherent and clear. She has no history of substance abuse. Her ability to perform ADLs improves within a few days. She complains of depressed mood and engages in head banging, which requires close observation.
Ms. T has a history of mood and behavioral problems since early childhood characterized by episodic dysphoric mood, anxiety, and agitation. She has had trials of several antidepressants, including sertraline, fluoxetine, venlafaxine, and escitalopram, and anxiolytics, including lorazepam, clonazepam, and alprazolam. Her outpatient psychiatrist describes a history of physical and sexual abuse starting at age 7. At age 9, after her mother died from breast cancer, Ms. T and her siblings were moved to foster care, where she was physically abused by the staff. She remained in foster care until age 18.
The authors’ observations
PNES pose a diagnostic and therapeutic challenge. Many PNES patients seek medical attention for their seizures. PNES patients misdiagnosed as having epilepsy have a worse prognosis because they do not receive appropriate treatment9 and may experience side effects if antiepileptics are prescribed.10 Finally, the financial burden of medical care can be significant. Ms. T had several hospitalizations, including extensive neurologic workup, intensive care unit admissions for intubation, and use of antiepileptics with almost no benefit.
Psychosocial assessments of PNES patients have revealed that sexual abuse, family conflicts, and death of a family member often play an important role.11 It is possible that as a result of childhood trauma, Ms. T exhibited a regressed and primitive defense mechanism to deal with the trauma. PNES usually are considered when a patient presents with:
- absence of therapeutic response to antiepileptics
- loss of response (therapeutic failure) to antiepileptics
- paradoxical response to antiepileptics (worsening or unexpected responses)
- atypical, multiple, or inconsistent seizures
- seizures that occur soon after emotional stress.12
We concluded Ms. T had PNES because of the unusual presentations of her seizures, negative video EEG findings, failure to respond to antiepileptics, lack of risk factors for epilepsy, and aggressive behaviors before or after the seizures ( Table ).4,10,11,13 Diagnosing PNES early allows clinicians to focus on appropriate treatment modalities (eg, psychotherapy, antidepressants), prevents costly neurologic workups and treatments (eg, routine EEGs, trials of several antiepileptics), and provides patients with diagnostic assurance.10
Table
Characteristics of psychogenic nonepileptic seizures
| Characteristic | Comment |
|---|---|
| Duration | May be prolonged |
| Timing | Usually occur only during the day |
| Physical harm | Rare |
| Tongue biting | Rare |
| Urinary incontinence | Rare |
| Motor activity | Prolonged |
| Cyanosis | No |
| Postictal confusion | Rare |
| Related to medication changes | No |
| Interictal EEG | Normal |
| Ictal EEG | Normal |
| Presence of secondary gain | Common |
| EEG: electroencephalography Source: References 4,10,11,13 | |
3 components of treatment
Presenting the PNES diagnosis to the patient. The neurologist and the psychiatrist should convey to the patient that they see the symptoms as “real” and not “all in your head.”14
Withdrawing antiepileptic medications. Antiepileptic medication withdrawal is recommended when a thorough diagnostic workup shows no evidence of epileptic seizures.15 Oto et al16 reported 49% of PNES patients became seizure-free 12 months after discontinuing antiepileptics.
Psychotherapy and pharmacotherapy. Open-label studies of psychological treatments for PNES have demonstrated that a cognitive-behavioral therapy-based approach and brief augmented psychodynamic interpersonal therapy could reduce seizures.17 In a pilot, randomized, placebo-controlled trial, PNES patients who received flexibly dosed sertraline reported a 45% reduction in seizures compared with an 8% increase in the placebo group.18 Similar improvements in seizure frequency have been reported in PNES patients with anxiety or depression treated with venlafaxine.19
OUTCOME: Support, improvement
During the next several days, Ms. T has random episodes of seizures with foaming of the mouth and unresponsiveness. These episodes last from 5 to 30 minutes and require transfer to the ER. After each episode, Ms. T is medically cleared and sent back to the psychiatric unit. The neurologist recommends avoiding antiepileptics. Ms. T responds well to the structured inpatient setting and supportive psychotherapy. Her episodes decrease and her mood becomes more stable. She refrains from self-injurious behaviors and is discharged home with outpatient follow-up.
Related Resource
- Marsh P, Benbadis S, Fernandez F. Psychogenic nonepileptic seizures: ways to win over skeptical patients. Current Psychiatry. 2008;7(1):21-35.
Drug Brand Names
- Alprazolam • Xanax
- Clonazepam • Klonopin
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Haloperidol • Haldol
- Lorazepam • Ativan
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Seizure-like symptoms
Ms. T, age 20, is brought to the emergency room (ER) by her father because she refuses to eat and drink, is unable to function at home, lies in bed all day, and does not attend to her activities of daily living (ADLs). Ms. T lives with her family, is not enrolled in school, and is unemployed. In the ER she initially is uncooperative and mute and then suddenly becomes agitated and has a seizure-like episode characterized by jerking of her trunk followed by random, asymmetrical movements of her legs and arms, closing both eyes, weeping, foaming at the mouth, moaning, and marked unresponsiveness. The episode lasts for >5 minutes.
The authors’ observations
Based on Ms. T’s presentation, the medical team considered acute epileptic seizures. Asymmetrical jerking of the body may be seen in frontal lobe epilepsy or seizures of the supplementary sensorimotor area. Frontal lobe epilepsy can present with bilateral asynchronous motor activity with consciousness during the event and a lack of postictal confusion.1 Seizures of the supplementary sensorimotor area—also known as the secondary motor area—are particularly problematic because typically they present with bilateral asymmetric tonic posturing followed by a few clonic movements, intact consciousness, and rarely postictal confusion. Adding to the diagnostic uncertainty, some “soft signs” thought to indicate PNES (eg, pelvic thrusting, crying) are common with frontal lobe epilepsy.1,2
PNES are episodes of altered movement, sensation, or experience that may be mistaken for epileptic seizures but are not a consequence of abnormal cortical discharges. Instead they are caused by physiological or psychological factors.3 Behaviors or signs that strongly suggest PNES include:
- gradual onset or termination
- pseudosleep, when the patient appears to be asleep but electroencephalography (EEG) findings indicate he or she is awake
- discontinuous (stop-and-go), irregular, or asynchronous (out-of-phase) activity—including side-to-side head movement, pelvic thrusting, and opisthotonic posturing—stuttering, and weeping4
- eye closure.5
Ms. T’s father said his daughter had been hospitalized several times for episodes characterized by pelvic thrusting, stuttering, and pseudosleep, which raised the possibility of PNES. Definitive diagnosis of PNES comes from video EEG when a patient is observed having typical seizures without accompanying EEG abnormalities.6
EVALUATION: Inconclusive data
Ms. T is admitted to the medical unit to rule out a seizure disorder. Physical examination is unremarkable and laboratory tests are within normal limits. The neurology service requests a head MRI, which is inconclusive. Inpatient video EEG with 24-hour monitoring does not indicate acute epileptic seizures. Ms. T’s father says that she has experienced many paroxysmal motor episodes and all neurologic tests, exams, and labs have failed to find a cause for these episodes. She did not receive any antiepileptic medications. A psychiatric consult is requested to clarify the diagnosis. Ms. T is transferred to an inpatient psychiatric unit for further evaluation and management.
The authors’ observations
Fleisher et al7 suggested that traumatic events may lead to presentations similar to PNES. Because Ms. T was molested by a family friend as a child, we considered posttraumatic stress disorder (PTSD) in the differential diagnosis, although she has not reported symptoms of intrusive recollections, avoidance, numbing, or hyperarousal.
We also considered conversion disorder and dissociative disorder. Patients with conversion disorder have ≥1 symptoms or signs that affect voluntary motor or sensory function that cannot be explained by a neurologic or general medical condition.8 Dissociative disorder is a disruption in usually integrated functions of consciousness, memory, identity, or perception of the environment.8 The presentation of patients with PNES may resemble that of patients with dissociative disorder.8 In a study of 45 adult PNES patients, Bowman et al8 found that PNES often are comorbid with other psychiatric disorders, including somatoform disorders (89%), dissociative disorders (91%), affective disorders (64%), personality disorders (62%), PTSD (49%), and other anxiety disorders (47%).
TREATMENT: Managing aggression
In the psychiatric unit, Ms. T initially is irritable and disorganized with poor oral intake and regressed behavior; she often is found in the fetal position, crying and talking in a childish manner. Throughout her admission, she receives several anxiolytics and antipsychotics—including lorazepam, up to 6 mg/d, clonazepam, up to 3 mg/d, haloperidol, up to 10 mg/d, and quetiapine, up to 200 mg/d—to help manage her aggressive behaviors after her seizure-like episodes. Further evaluation reveals that Ms. T has no psychotic symptoms, overt delusions, or perceptual disturbances and her thought process is coherent and clear. She has no history of substance abuse. Her ability to perform ADLs improves within a few days. She complains of depressed mood and engages in head banging, which requires close observation.
Ms. T has a history of mood and behavioral problems since early childhood characterized by episodic dysphoric mood, anxiety, and agitation. She has had trials of several antidepressants, including sertraline, fluoxetine, venlafaxine, and escitalopram, and anxiolytics, including lorazepam, clonazepam, and alprazolam. Her outpatient psychiatrist describes a history of physical and sexual abuse starting at age 7. At age 9, after her mother died from breast cancer, Ms. T and her siblings were moved to foster care, where she was physically abused by the staff. She remained in foster care until age 18.
The authors’ observations
PNES pose a diagnostic and therapeutic challenge. Many PNES patients seek medical attention for their seizures. PNES patients misdiagnosed as having epilepsy have a worse prognosis because they do not receive appropriate treatment9 and may experience side effects if antiepileptics are prescribed.10 Finally, the financial burden of medical care can be significant. Ms. T had several hospitalizations, including extensive neurologic workup, intensive care unit admissions for intubation, and use of antiepileptics with almost no benefit.
Psychosocial assessments of PNES patients have revealed that sexual abuse, family conflicts, and death of a family member often play an important role.11 It is possible that as a result of childhood trauma, Ms. T exhibited a regressed and primitive defense mechanism to deal with the trauma. PNES usually are considered when a patient presents with:
- absence of therapeutic response to antiepileptics
- loss of response (therapeutic failure) to antiepileptics
- paradoxical response to antiepileptics (worsening or unexpected responses)
- atypical, multiple, or inconsistent seizures
- seizures that occur soon after emotional stress.12
We concluded Ms. T had PNES because of the unusual presentations of her seizures, negative video EEG findings, failure to respond to antiepileptics, lack of risk factors for epilepsy, and aggressive behaviors before or after the seizures ( Table ).4,10,11,13 Diagnosing PNES early allows clinicians to focus on appropriate treatment modalities (eg, psychotherapy, antidepressants), prevents costly neurologic workups and treatments (eg, routine EEGs, trials of several antiepileptics), and provides patients with diagnostic assurance.10
Table
Characteristics of psychogenic nonepileptic seizures
| Characteristic | Comment |
|---|---|
| Duration | May be prolonged |
| Timing | Usually occur only during the day |
| Physical harm | Rare |
| Tongue biting | Rare |
| Urinary incontinence | Rare |
| Motor activity | Prolonged |
| Cyanosis | No |
| Postictal confusion | Rare |
| Related to medication changes | No |
| Interictal EEG | Normal |
| Ictal EEG | Normal |
| Presence of secondary gain | Common |
| EEG: electroencephalography Source: References 4,10,11,13 | |
3 components of treatment
Presenting the PNES diagnosis to the patient. The neurologist and the psychiatrist should convey to the patient that they see the symptoms as “real” and not “all in your head.”14
Withdrawing antiepileptic medications. Antiepileptic medication withdrawal is recommended when a thorough diagnostic workup shows no evidence of epileptic seizures.15 Oto et al16 reported 49% of PNES patients became seizure-free 12 months after discontinuing antiepileptics.
Psychotherapy and pharmacotherapy. Open-label studies of psychological treatments for PNES have demonstrated that a cognitive-behavioral therapy-based approach and brief augmented psychodynamic interpersonal therapy could reduce seizures.17 In a pilot, randomized, placebo-controlled trial, PNES patients who received flexibly dosed sertraline reported a 45% reduction in seizures compared with an 8% increase in the placebo group.18 Similar improvements in seizure frequency have been reported in PNES patients with anxiety or depression treated with venlafaxine.19
OUTCOME: Support, improvement
During the next several days, Ms. T has random episodes of seizures with foaming of the mouth and unresponsiveness. These episodes last from 5 to 30 minutes and require transfer to the ER. After each episode, Ms. T is medically cleared and sent back to the psychiatric unit. The neurologist recommends avoiding antiepileptics. Ms. T responds well to the structured inpatient setting and supportive psychotherapy. Her episodes decrease and her mood becomes more stable. She refrains from self-injurious behaviors and is discharged home with outpatient follow-up.
Related Resource
- Marsh P, Benbadis S, Fernandez F. Psychogenic nonepileptic seizures: ways to win over skeptical patients. Current Psychiatry. 2008;7(1):21-35.
Drug Brand Names
- Alprazolam • Xanax
- Clonazepam • Klonopin
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Haloperidol • Haldol
- Lorazepam • Ativan
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kellinghaus C, Lüders HO. Frontal lobe epilepsy. Epileptic Disord. 2004;6(4):223-239.
2. Kanner AM, Morris HH, Lüders H, et al. Supplementary motor seizures mimicking pseudoseizures: some clinical differences. Neurology. 1990;40(9):1404-1407.
3. Hall-Patch L, Brown R, House A, et al. Acceptability and effectiveness of a strategy for the communication of the diagnosis of psychogenic nonepileptic seizures. Epilepsia. 2010;51(1):70-78.
4. Reuber M, Elger CE. Psychogenic nonepileptic seizures: review and update. Epilepsy Behav. 2003;4(3):205-216.
5. Chung SS, Gerber P, Kirlin KA. Ictal eye closure is a reliable indicator for psychogenic nonepileptic seizures. Neurology. 2006;66(11):1730-1731.
6. Mostacci B, Bisulli F, Alvisi L, et al. Ictal characteristics of psychogenic nonepileptic seizures: what we have learned from video/EEG recordings—a literature review. Epilepsy Behav. 2011;22(2):144-153.
7. Fleisher W, Staley D, Krawetz P, et al. Comparative study of trauma-related phenomena in subjects with pseudoseizures and subjects with epilepsy. Am J Psychiatry. 2002;159(4):660-663.
8. Bowman ES, Markand ON. Psychodynamics and psychiatric diagnoses of pseudoseizure subjects. Am J Psychiatry. 1996;153(1):57-63.
9. Benbadis SR. The EEG in nonepileptic seizures. J Clin Neurophysiol. 2006;23(4):340-352.
10. Brown RJ, Syed TU, Benbadis S, et al. Psychogenic nonepileptic seizures. Epilepsy Behav. 2011;22(1):85-93.
11. Bodde NM, Brooks JL, Baker GA, et al. Psychogenic non-epileptic seizures—definition, etiology, treatment and prognostic issues: a critical review. Seizure. 2009;18(8):543-553.
12. Alsaadi TM, Marquez AV. Psychogenic nonepileptic seizures. Am Fam Physician. 2005;72(5):849-856.
13. Bradley WG, Daroff RB, Fenichel GM, et al. eds. Neurology in clinical practice: principles of diagnosis and management. 4th ed. Philadelphia, PA: Butterworth Heinemann; 2004:19-20, 1971–1972.
14. Harden CL, Ferrando SJ. Delivering the diagnosis of psychogenic pseudoseizures: should the neurologist or the psychiatrist be responsible? Epilepsy Behav. 2001;2(6):519-523.
15. Oto M, Espie CA, Duncan R. An exploratory randomized controlled trial of immediate versus delayed withdrawal of antiepileptic drugs in patients with psychogenic nonepileptic attacks (PNEAs). Epilepsia. 2010;51(10):1994-1999.
16. Oto M, Espie C, Pelosi A, et al. The safety of antiepileptic drug withdrawal in patients with non-epileptic seizures. J Neurol Neurosurg Psychiatry. 2005;76(12):1682-1685.
17. Goldstein LH, Mellers JD. Recent developments in our understanding of the semiology and treatment of psychogenic nonepileptic seizures. Curr Neurol Neurosci Rep. 2012;12(4):436-444.
18. LaFrance WC, Jr, Keitner GI, Papandonatos GD, et al. Pilot pharmacologic randomized controlled trial for psychogenic nonepileptic seizures. Neurology. 2010;75(13):1166-1173.
19. Pintor L, Baillés E, Matrai S, et al. Efficiency of venlafaxine in patients with psychogenic nonepileptic seizures and anxiety and/or depressive disorders. J Neuropsychiatry Clin Neurosci. 2010;22(4):401-408.
1. Kellinghaus C, Lüders HO. Frontal lobe epilepsy. Epileptic Disord. 2004;6(4):223-239.
2. Kanner AM, Morris HH, Lüders H, et al. Supplementary motor seizures mimicking pseudoseizures: some clinical differences. Neurology. 1990;40(9):1404-1407.
3. Hall-Patch L, Brown R, House A, et al. Acceptability and effectiveness of a strategy for the communication of the diagnosis of psychogenic nonepileptic seizures. Epilepsia. 2010;51(1):70-78.
4. Reuber M, Elger CE. Psychogenic nonepileptic seizures: review and update. Epilepsy Behav. 2003;4(3):205-216.
5. Chung SS, Gerber P, Kirlin KA. Ictal eye closure is a reliable indicator for psychogenic nonepileptic seizures. Neurology. 2006;66(11):1730-1731.
6. Mostacci B, Bisulli F, Alvisi L, et al. Ictal characteristics of psychogenic nonepileptic seizures: what we have learned from video/EEG recordings—a literature review. Epilepsy Behav. 2011;22(2):144-153.
7. Fleisher W, Staley D, Krawetz P, et al. Comparative study of trauma-related phenomena in subjects with pseudoseizures and subjects with epilepsy. Am J Psychiatry. 2002;159(4):660-663.
8. Bowman ES, Markand ON. Psychodynamics and psychiatric diagnoses of pseudoseizure subjects. Am J Psychiatry. 1996;153(1):57-63.
9. Benbadis SR. The EEG in nonepileptic seizures. J Clin Neurophysiol. 2006;23(4):340-352.
10. Brown RJ, Syed TU, Benbadis S, et al. Psychogenic nonepileptic seizures. Epilepsy Behav. 2011;22(1):85-93.
11. Bodde NM, Brooks JL, Baker GA, et al. Psychogenic non-epileptic seizures—definition, etiology, treatment and prognostic issues: a critical review. Seizure. 2009;18(8):543-553.
12. Alsaadi TM, Marquez AV. Psychogenic nonepileptic seizures. Am Fam Physician. 2005;72(5):849-856.
13. Bradley WG, Daroff RB, Fenichel GM, et al. eds. Neurology in clinical practice: principles of diagnosis and management. 4th ed. Philadelphia, PA: Butterworth Heinemann; 2004:19-20, 1971–1972.
14. Harden CL, Ferrando SJ. Delivering the diagnosis of psychogenic pseudoseizures: should the neurologist or the psychiatrist be responsible? Epilepsy Behav. 2001;2(6):519-523.
15. Oto M, Espie CA, Duncan R. An exploratory randomized controlled trial of immediate versus delayed withdrawal of antiepileptic drugs in patients with psychogenic nonepileptic attacks (PNEAs). Epilepsia. 2010;51(10):1994-1999.
16. Oto M, Espie C, Pelosi A, et al. The safety of antiepileptic drug withdrawal in patients with non-epileptic seizures. J Neurol Neurosurg Psychiatry. 2005;76(12):1682-1685.
17. Goldstein LH, Mellers JD. Recent developments in our understanding of the semiology and treatment of psychogenic nonepileptic seizures. Curr Neurol Neurosci Rep. 2012;12(4):436-444.
18. LaFrance WC, Jr, Keitner GI, Papandonatos GD, et al. Pilot pharmacologic randomized controlled trial for psychogenic nonepileptic seizures. Neurology. 2010;75(13):1166-1173.
19. Pintor L, Baillés E, Matrai S, et al. Efficiency of venlafaxine in patients with psychogenic nonepileptic seizures and anxiety and/or depressive disorders. J Neuropsychiatry Clin Neurosci. 2010;22(4):401-408.
The avoidant psychotherapy patient
CASE: Unexplained panic
Mr. J, age 35, is a married, unemployed musician who presents for outpatient treatment for panic attacks. He experienced his first panic attack at his oldest son’s baptism 12 years ago, but does not know why it occurred at that moment. He rarely has panic attacks now, but wants to continue medication management. He denies depressive symptoms, saying, “I’m the most optimistic person in the world.” Mr. J tried several medications for his panic attacks before clonazepam, 2 mg/d, proved effective, but always has been vehemently opposed to antidepressants. Despite his insistence that he needs only medication management, Mr. J chooses to enroll in a resident-run psychotherapy clinic.
In sessions, Mr. J describes his father, who also has panic disorder, as a powerful figure who is physically and emotionally abusive, but also charismatic, charming, and “impossible not to love.” However, Mr. J felt his father was impossible to live with, and moved out at age 18 to marry his high school sweetheart. They have 3 children, ages 12, 10, and 8. Mr. J worked for his father at his construction company, but was not able to satisfy him or live up to his standards so he quit because he was tired of being cut down and emasculated.
Mr. J’s parents divorced 15 years ago after his mother had an affair with her husband’s friend. His father learned of the affair and threatened his wife with a handgun. Although Mr. J and his mother were close before her affair, he has been unable to forgive or empathize with her, and rarely speaks to her. Mr. J’s mother could not protect him from his father’s abuse, and later compounded her failure by abandoning her husband and son through her sexual affair. Growing up with a father he did not respect or get comfort from and sharing a common fear and alliance with his mother likely made it difficult for Mr. J to navigate his Oedipal phase,1 and made her abandonment even more painful.
When Mr. J was 6 years old, he was molested by one of his father’s friends. His father stabbed the man in the shoulder when he found out about the molestation and received probation. Although Mr. J knows he was molested, he does not remember it and has repressed most of his childhood.
The authors’ observations
I (JF) wanted to discuss with Mr. J why his first panic attack occurred during such a symbolic occasion. His panic could be the result of a struggle between a murderous wish toward his father and paternal protective instinct toward his son. The baptism placed his son in a highly vulnerable position, which reminded Mr. J of his own vulnerability and impotent rage toward his father. Anxiety often results when an individual has 2 opposing wishes,2 and a murderous wish often is involved when anxiety progresses to panic. Getting to the root of this with Mr. J could allow for further psychological growth.3 His murderous wishes and fantasies are ego-dystonic, and panic could be a way of punishing himself for these thoughts. When Mr. J identified himself as his son during the baptism, he likely was flooded with thoughts that his defenses were no longer able to repress. Seeing his son submerged in the baptismal font brought back an aspect of his own life that he had completely split off from consciousness, and likely will take time to process. Considering the current therapeutic dynamic, I decided that it was not the best time to address this potential conflict; however, I could have chosen a manualized form of psychodynamic psychotherapy for panic disorder.4 See Table 1 for an outline of the phases of psychodynamic psychotherapy for panic disorder.
Although Mr. J’s initial willingness to discuss his past was encouraging, he refused to schedule more than 1 session every 4 weeks. He also began to keep the content of our sessions superficial, which caused me angst because he seemed to be withholding information and would not come more frequently. Although I was not proud of my feelings, I had to be honest with myself that I had started to dislike Mr. J.
Table 1
Psychodynamic psychotherapy for panic disorder
| Phase | Comments |
|---|---|
| Treatment of acute panic | Therapy focuses on discovering the conscious and unconscious meaning of panic symptoms |
| Treatment of panic vulnerability | Core dynamic conflicts related to panic are understood and altered. Tasks include addressing the nature of the transference and working through them |
| Termination | The therapist directly addresses patients’ difficulties with separation and independence as they emerge in treatment. After treatment, patients may be better able to manage separations, anger, and independence |
| Source: Adapted from reference 4 | |
Countertransference reactions
Countertransference is a therapist's emotional reaction to a patient. Just as patients form reactions based on past relationships brought to present, therapists develop similar reactions.5 Noting one’s countertransference provides a window into how the patient’s thoughts and actions evoke feelings in others. It also can shed light on an aspect of the doctor-patient relationship that may have gone unnoticed.2
Countertransference hatred can occur when a therapist begins to dislike a patient. Typically, patients with borderline personality disorder, masochistic tendencies, or suicidality arouse strong countertransference reactions6; however, any patient can evoke these emotions. This type of hateful patient can precipitate antitherapeutic feelings such as aversion or malice that can be a major obstacle to treatment.7 Aversion leads the therapist to withdraw from the patient, and malice can trigger cruel impulses.
Maltsberger and Buie7 identified 5 defenses therapists may use to combat countertransference hatred (Table 2). When treating Mr. J, I used several of these defenses, including projection and turning against the self to protect myself from this challenging patient. In turning against the self, I became doubtful and critical of my skills and increasingly submissive to Mr. J. Additionally, I projected this countertransference hatred onto Mr. J, focusing on the negative transference that he brought to our therapeutic encounters. On an unconscious level, I may have feared retribution from Mr. J.
I became so frustrated with Mr. J that I reduced the frequency of our sessions to once every 6 weeks, which I realized could be evidence of my feelings regarding Mr. J’s minimization and avoidant style.
Table 2
Defenses against countertransference hate
| Defense mechanism | Description |
|---|---|
| Repression | Remaining unconscious of feelings of hate; may manifest as difficulty paying attention to what the patient is saying or feeling bored or tired |
| Turning against oneself | Doubting one’s capacity to help the patient; may feel inadequate, helpless, and hopeless. May lead to giving up on the patient because the therapist feels incompetent |
| Reaction formation | Turning hatred into the opposite emotion. The therapist may be too preoccupied with being helpful or overly concerned about the patient’s welfare and comfort |
| Projection | Feeling that the patient hates the therapist, leading to feelings of dread and fear |
| Distortion of reality | Devaluing the patient and seeing the patient as a hopeless case or a dangerous person. The therapist may feel indifference, pity, or anger toward the patient |
| Source: Reference 7 | |
TREATMENT: A breakthrough
Mr. J presents with obvious unease at the first visit after we had decreased the frequency of our sessions. At this point, Mr. J opens up to me. He says he has not been truthful with me, and has had worsening depression, anhedonia, and agoraphobia over the past year. He also reveals that he has homosexual fantasies that he cannot stop, which disturb him because he says he is heterosexual. He agrees to come once a week, and reluctantly admits that he desperately needs help.
Although Mr. J only takes clonazepam and citalopram, 20 mg/d, which I prescribed after he admitted to depression and anxiety, he has hyperlipidemia and a family history of heart disease. In addition to being a musician and working at his father’s construction company, he has worked as a security guard, bounty hunter, and computer technician. His careers have been solitary in nature, and, with the exception of computer work, permitted an outlet for aggression. However, he recently started taking online college classes and wants to become a music teacher because he feels he has a lot to offer children as a result of his life experiences. His fantasy of being a teacher shows considerably less aggression, and could be a sign of psychological growth.
Mr. J is struggling financially and his home is on the verge of foreclosure. Early in treatment he told me that he stopped paying his mortgage, but demonstrated blind optimism that things would “work out.” I asked if this was a wise decision, but he seemed confident and dismissive of my concerns. Although he now struggles with this situation, I consider this healthier than his constant pseudo-happy state, and a sign of psychological development.8 Despite his financial stressors, he wants to pursue his dream of being a famous musician, and says he “could never work a 9-to-5 job in a cubicle.”
The authors’ observations
I do not think it’s a coincidence that Mr. J stopped minimizing his symptoms when we decreased the frequency of his sessions. I had viewed our sessions as unproductive and blamed Mr. J for wasting both of our time with his resistance and minimization and had begun to dislike him. I felt impotent because he had been controlling each session with long, elaborate stories that had little relevance to his panic attacks, and I could not redirect him or get him to focus on pertinent issues. It was as if I was an audience for him, and provided nothing useful. However, I was interested in these superficial stories because Mr. J was charming and engaging. He likely reenacted his relationship with his father with me. Mr. J’s superficial relationship with me caused me to dislike him, and, similar to his father, reject him. This rejection likely was damaging because I was unable to anticipate his needs, which would have been to increase—rather than decrease—the frequency of our sessions. Just like his father, I was not able to take care of him.
Mr. J is deeply conflicted about his father. He states that his father “is a monster who instills fear and intimidation into everyone around him, but he’s charismatic, and I’ll always love him.” His view of his domineering father likely developed into a castration anxiety because he was afraid of competing for his mother’s love, contributing to a muddled sexual identity. This was intensified when Mr. J was sexually abused; he may have been stimulated by the molestation, adding to his confusion. Although Mr. J has repressed the abuse and split off most of his childhood, he suffers from shame, guilt, and depression because of his ego-dystonic homosexual fantasies. Homosexuality is at odds with his self-image and contributes to his anxiety and panic attacks. He cannot adequately discharge this dangerous libidinal energy, and as he becomes more conscious of it, his anxiety intensifies.
OUTCOME: Overcoming fear
As Mr. J sits crying in my office, he says he hasn’t cried in front of another man in years. I wonder aloud what his father would think of this situation. His states that his father does not respect any type of weakness and probably would “knock his teeth in.” Overcoming this fear of opening up will be a goal of Mr. J’s treatment. His unbridled optimism borders on pathologic, and is a defense against reality.8 Additionally, his reluctance to accept that he is suffering from depression, which he perceives as a weakness, will be a struggle throughout therapy. He likely will continue to minimize his symptoms when possible, making the true depths of his illness difficult to grasp.
Related Resources
- Waska R. Using countertransference: analytic contact, projective identification, and transference phantasy states. Am J Psychother. 2008;62(4):333-351.
- Gabbard GO, Litowitz BE, Williams P. Textbook of psychoanalysis. Arlington, VA: American Psychiatric Publishing, Inc; 2011.
Drug Brand Names
- Citalopram • Celexa
- Clonazepam • Klonopin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Blos P. Son and father: before and beyond the Oedipus complex. New York NY: Free Press/Macmillan; 1985.
2. Ursano RJ, Sonnenberg SM, Lazar SG. Concise guide to psychodynamic psychotherapy. Arlington VA: American Psychiatric Publishing; 2004.
3. Akhtar S. Comprehensive dictionary of psychoanalysis. London United Kingdom: Kamac Books; 2009.
4. Busch FN, Milrod BL, Singer MB. Theory and technique in psychodynamic treatment of panic disorder. J Psychother Pract Res. 1999;8(3):234-242.
5. Freud S. The future prospects of psycho-analytic therapy. The standard edition of the complete psychological works of Sigmund Freud volume XI (1910): five lectures on psycho-analysis, Leonardo da Vinci and other works. London, United Kingdom: Hogarth Press; 1957:139–152.
6. Winnicott DW. Hate in the counter-transference. J Psychother Pract Res. 1994;3(4):348-356.
7. Maltsberger JT, Buie DH. Countertransference hate in the treatment of suicidal patients. Arch Gen Psychiatry. 1974;30(5):625-633.
8. Akhtar S. “Someday…” and “if only…” fantasies: pathological optimism and inordinate nostalgia as related forms of idealization. J Am Psychoanal Assoc. 1996;44(3):723-753.
CASE: Unexplained panic
Mr. J, age 35, is a married, unemployed musician who presents for outpatient treatment for panic attacks. He experienced his first panic attack at his oldest son’s baptism 12 years ago, but does not know why it occurred at that moment. He rarely has panic attacks now, but wants to continue medication management. He denies depressive symptoms, saying, “I’m the most optimistic person in the world.” Mr. J tried several medications for his panic attacks before clonazepam, 2 mg/d, proved effective, but always has been vehemently opposed to antidepressants. Despite his insistence that he needs only medication management, Mr. J chooses to enroll in a resident-run psychotherapy clinic.
In sessions, Mr. J describes his father, who also has panic disorder, as a powerful figure who is physically and emotionally abusive, but also charismatic, charming, and “impossible not to love.” However, Mr. J felt his father was impossible to live with, and moved out at age 18 to marry his high school sweetheart. They have 3 children, ages 12, 10, and 8. Mr. J worked for his father at his construction company, but was not able to satisfy him or live up to his standards so he quit because he was tired of being cut down and emasculated.
Mr. J’s parents divorced 15 years ago after his mother had an affair with her husband’s friend. His father learned of the affair and threatened his wife with a handgun. Although Mr. J and his mother were close before her affair, he has been unable to forgive or empathize with her, and rarely speaks to her. Mr. J’s mother could not protect him from his father’s abuse, and later compounded her failure by abandoning her husband and son through her sexual affair. Growing up with a father he did not respect or get comfort from and sharing a common fear and alliance with his mother likely made it difficult for Mr. J to navigate his Oedipal phase,1 and made her abandonment even more painful.
When Mr. J was 6 years old, he was molested by one of his father’s friends. His father stabbed the man in the shoulder when he found out about the molestation and received probation. Although Mr. J knows he was molested, he does not remember it and has repressed most of his childhood.
The authors’ observations
I (JF) wanted to discuss with Mr. J why his first panic attack occurred during such a symbolic occasion. His panic could be the result of a struggle between a murderous wish toward his father and paternal protective instinct toward his son. The baptism placed his son in a highly vulnerable position, which reminded Mr. J of his own vulnerability and impotent rage toward his father. Anxiety often results when an individual has 2 opposing wishes,2 and a murderous wish often is involved when anxiety progresses to panic. Getting to the root of this with Mr. J could allow for further psychological growth.3 His murderous wishes and fantasies are ego-dystonic, and panic could be a way of punishing himself for these thoughts. When Mr. J identified himself as his son during the baptism, he likely was flooded with thoughts that his defenses were no longer able to repress. Seeing his son submerged in the baptismal font brought back an aspect of his own life that he had completely split off from consciousness, and likely will take time to process. Considering the current therapeutic dynamic, I decided that it was not the best time to address this potential conflict; however, I could have chosen a manualized form of psychodynamic psychotherapy for panic disorder.4 See Table 1 for an outline of the phases of psychodynamic psychotherapy for panic disorder.
Although Mr. J’s initial willingness to discuss his past was encouraging, he refused to schedule more than 1 session every 4 weeks. He also began to keep the content of our sessions superficial, which caused me angst because he seemed to be withholding information and would not come more frequently. Although I was not proud of my feelings, I had to be honest with myself that I had started to dislike Mr. J.
Table 1
Psychodynamic psychotherapy for panic disorder
| Phase | Comments |
|---|---|
| Treatment of acute panic | Therapy focuses on discovering the conscious and unconscious meaning of panic symptoms |
| Treatment of panic vulnerability | Core dynamic conflicts related to panic are understood and altered. Tasks include addressing the nature of the transference and working through them |
| Termination | The therapist directly addresses patients’ difficulties with separation and independence as they emerge in treatment. After treatment, patients may be better able to manage separations, anger, and independence |
| Source: Adapted from reference 4 | |
Countertransference reactions
Countertransference is a therapist's emotional reaction to a patient. Just as patients form reactions based on past relationships brought to present, therapists develop similar reactions.5 Noting one’s countertransference provides a window into how the patient’s thoughts and actions evoke feelings in others. It also can shed light on an aspect of the doctor-patient relationship that may have gone unnoticed.2
Countertransference hatred can occur when a therapist begins to dislike a patient. Typically, patients with borderline personality disorder, masochistic tendencies, or suicidality arouse strong countertransference reactions6; however, any patient can evoke these emotions. This type of hateful patient can precipitate antitherapeutic feelings such as aversion or malice that can be a major obstacle to treatment.7 Aversion leads the therapist to withdraw from the patient, and malice can trigger cruel impulses.
Maltsberger and Buie7 identified 5 defenses therapists may use to combat countertransference hatred (Table 2). When treating Mr. J, I used several of these defenses, including projection and turning against the self to protect myself from this challenging patient. In turning against the self, I became doubtful and critical of my skills and increasingly submissive to Mr. J. Additionally, I projected this countertransference hatred onto Mr. J, focusing on the negative transference that he brought to our therapeutic encounters. On an unconscious level, I may have feared retribution from Mr. J.
I became so frustrated with Mr. J that I reduced the frequency of our sessions to once every 6 weeks, which I realized could be evidence of my feelings regarding Mr. J’s minimization and avoidant style.
Table 2
Defenses against countertransference hate
| Defense mechanism | Description |
|---|---|
| Repression | Remaining unconscious of feelings of hate; may manifest as difficulty paying attention to what the patient is saying or feeling bored or tired |
| Turning against oneself | Doubting one’s capacity to help the patient; may feel inadequate, helpless, and hopeless. May lead to giving up on the patient because the therapist feels incompetent |
| Reaction formation | Turning hatred into the opposite emotion. The therapist may be too preoccupied with being helpful or overly concerned about the patient’s welfare and comfort |
| Projection | Feeling that the patient hates the therapist, leading to feelings of dread and fear |
| Distortion of reality | Devaluing the patient and seeing the patient as a hopeless case or a dangerous person. The therapist may feel indifference, pity, or anger toward the patient |
| Source: Reference 7 | |
TREATMENT: A breakthrough
Mr. J presents with obvious unease at the first visit after we had decreased the frequency of our sessions. At this point, Mr. J opens up to me. He says he has not been truthful with me, and has had worsening depression, anhedonia, and agoraphobia over the past year. He also reveals that he has homosexual fantasies that he cannot stop, which disturb him because he says he is heterosexual. He agrees to come once a week, and reluctantly admits that he desperately needs help.
Although Mr. J only takes clonazepam and citalopram, 20 mg/d, which I prescribed after he admitted to depression and anxiety, he has hyperlipidemia and a family history of heart disease. In addition to being a musician and working at his father’s construction company, he has worked as a security guard, bounty hunter, and computer technician. His careers have been solitary in nature, and, with the exception of computer work, permitted an outlet for aggression. However, he recently started taking online college classes and wants to become a music teacher because he feels he has a lot to offer children as a result of his life experiences. His fantasy of being a teacher shows considerably less aggression, and could be a sign of psychological growth.
Mr. J is struggling financially and his home is on the verge of foreclosure. Early in treatment he told me that he stopped paying his mortgage, but demonstrated blind optimism that things would “work out.” I asked if this was a wise decision, but he seemed confident and dismissive of my concerns. Although he now struggles with this situation, I consider this healthier than his constant pseudo-happy state, and a sign of psychological development.8 Despite his financial stressors, he wants to pursue his dream of being a famous musician, and says he “could never work a 9-to-5 job in a cubicle.”
The authors’ observations
I do not think it’s a coincidence that Mr. J stopped minimizing his symptoms when we decreased the frequency of his sessions. I had viewed our sessions as unproductive and blamed Mr. J for wasting both of our time with his resistance and minimization and had begun to dislike him. I felt impotent because he had been controlling each session with long, elaborate stories that had little relevance to his panic attacks, and I could not redirect him or get him to focus on pertinent issues. It was as if I was an audience for him, and provided nothing useful. However, I was interested in these superficial stories because Mr. J was charming and engaging. He likely reenacted his relationship with his father with me. Mr. J’s superficial relationship with me caused me to dislike him, and, similar to his father, reject him. This rejection likely was damaging because I was unable to anticipate his needs, which would have been to increase—rather than decrease—the frequency of our sessions. Just like his father, I was not able to take care of him.
Mr. J is deeply conflicted about his father. He states that his father “is a monster who instills fear and intimidation into everyone around him, but he’s charismatic, and I’ll always love him.” His view of his domineering father likely developed into a castration anxiety because he was afraid of competing for his mother’s love, contributing to a muddled sexual identity. This was intensified when Mr. J was sexually abused; he may have been stimulated by the molestation, adding to his confusion. Although Mr. J has repressed the abuse and split off most of his childhood, he suffers from shame, guilt, and depression because of his ego-dystonic homosexual fantasies. Homosexuality is at odds with his self-image and contributes to his anxiety and panic attacks. He cannot adequately discharge this dangerous libidinal energy, and as he becomes more conscious of it, his anxiety intensifies.
OUTCOME: Overcoming fear
As Mr. J sits crying in my office, he says he hasn’t cried in front of another man in years. I wonder aloud what his father would think of this situation. His states that his father does not respect any type of weakness and probably would “knock his teeth in.” Overcoming this fear of opening up will be a goal of Mr. J’s treatment. His unbridled optimism borders on pathologic, and is a defense against reality.8 Additionally, his reluctance to accept that he is suffering from depression, which he perceives as a weakness, will be a struggle throughout therapy. He likely will continue to minimize his symptoms when possible, making the true depths of his illness difficult to grasp.
Related Resources
- Waska R. Using countertransference: analytic contact, projective identification, and transference phantasy states. Am J Psychother. 2008;62(4):333-351.
- Gabbard GO, Litowitz BE, Williams P. Textbook of psychoanalysis. Arlington, VA: American Psychiatric Publishing, Inc; 2011.
Drug Brand Names
- Citalopram • Celexa
- Clonazepam • Klonopin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Unexplained panic
Mr. J, age 35, is a married, unemployed musician who presents for outpatient treatment for panic attacks. He experienced his first panic attack at his oldest son’s baptism 12 years ago, but does not know why it occurred at that moment. He rarely has panic attacks now, but wants to continue medication management. He denies depressive symptoms, saying, “I’m the most optimistic person in the world.” Mr. J tried several medications for his panic attacks before clonazepam, 2 mg/d, proved effective, but always has been vehemently opposed to antidepressants. Despite his insistence that he needs only medication management, Mr. J chooses to enroll in a resident-run psychotherapy clinic.
In sessions, Mr. J describes his father, who also has panic disorder, as a powerful figure who is physically and emotionally abusive, but also charismatic, charming, and “impossible not to love.” However, Mr. J felt his father was impossible to live with, and moved out at age 18 to marry his high school sweetheart. They have 3 children, ages 12, 10, and 8. Mr. J worked for his father at his construction company, but was not able to satisfy him or live up to his standards so he quit because he was tired of being cut down and emasculated.
Mr. J’s parents divorced 15 years ago after his mother had an affair with her husband’s friend. His father learned of the affair and threatened his wife with a handgun. Although Mr. J and his mother were close before her affair, he has been unable to forgive or empathize with her, and rarely speaks to her. Mr. J’s mother could not protect him from his father’s abuse, and later compounded her failure by abandoning her husband and son through her sexual affair. Growing up with a father he did not respect or get comfort from and sharing a common fear and alliance with his mother likely made it difficult for Mr. J to navigate his Oedipal phase,1 and made her abandonment even more painful.
When Mr. J was 6 years old, he was molested by one of his father’s friends. His father stabbed the man in the shoulder when he found out about the molestation and received probation. Although Mr. J knows he was molested, he does not remember it and has repressed most of his childhood.
The authors’ observations
I (JF) wanted to discuss with Mr. J why his first panic attack occurred during such a symbolic occasion. His panic could be the result of a struggle between a murderous wish toward his father and paternal protective instinct toward his son. The baptism placed his son in a highly vulnerable position, which reminded Mr. J of his own vulnerability and impotent rage toward his father. Anxiety often results when an individual has 2 opposing wishes,2 and a murderous wish often is involved when anxiety progresses to panic. Getting to the root of this with Mr. J could allow for further psychological growth.3 His murderous wishes and fantasies are ego-dystonic, and panic could be a way of punishing himself for these thoughts. When Mr. J identified himself as his son during the baptism, he likely was flooded with thoughts that his defenses were no longer able to repress. Seeing his son submerged in the baptismal font brought back an aspect of his own life that he had completely split off from consciousness, and likely will take time to process. Considering the current therapeutic dynamic, I decided that it was not the best time to address this potential conflict; however, I could have chosen a manualized form of psychodynamic psychotherapy for panic disorder.4 See Table 1 for an outline of the phases of psychodynamic psychotherapy for panic disorder.
Although Mr. J’s initial willingness to discuss his past was encouraging, he refused to schedule more than 1 session every 4 weeks. He also began to keep the content of our sessions superficial, which caused me angst because he seemed to be withholding information and would not come more frequently. Although I was not proud of my feelings, I had to be honest with myself that I had started to dislike Mr. J.
Table 1
Psychodynamic psychotherapy for panic disorder
| Phase | Comments |
|---|---|
| Treatment of acute panic | Therapy focuses on discovering the conscious and unconscious meaning of panic symptoms |
| Treatment of panic vulnerability | Core dynamic conflicts related to panic are understood and altered. Tasks include addressing the nature of the transference and working through them |
| Termination | The therapist directly addresses patients’ difficulties with separation and independence as they emerge in treatment. After treatment, patients may be better able to manage separations, anger, and independence |
| Source: Adapted from reference 4 | |
Countertransference reactions
Countertransference is a therapist's emotional reaction to a patient. Just as patients form reactions based on past relationships brought to present, therapists develop similar reactions.5 Noting one’s countertransference provides a window into how the patient’s thoughts and actions evoke feelings in others. It also can shed light on an aspect of the doctor-patient relationship that may have gone unnoticed.2
Countertransference hatred can occur when a therapist begins to dislike a patient. Typically, patients with borderline personality disorder, masochistic tendencies, or suicidality arouse strong countertransference reactions6; however, any patient can evoke these emotions. This type of hateful patient can precipitate antitherapeutic feelings such as aversion or malice that can be a major obstacle to treatment.7 Aversion leads the therapist to withdraw from the patient, and malice can trigger cruel impulses.
Maltsberger and Buie7 identified 5 defenses therapists may use to combat countertransference hatred (Table 2). When treating Mr. J, I used several of these defenses, including projection and turning against the self to protect myself from this challenging patient. In turning against the self, I became doubtful and critical of my skills and increasingly submissive to Mr. J. Additionally, I projected this countertransference hatred onto Mr. J, focusing on the negative transference that he brought to our therapeutic encounters. On an unconscious level, I may have feared retribution from Mr. J.
I became so frustrated with Mr. J that I reduced the frequency of our sessions to once every 6 weeks, which I realized could be evidence of my feelings regarding Mr. J’s minimization and avoidant style.
Table 2
Defenses against countertransference hate
| Defense mechanism | Description |
|---|---|
| Repression | Remaining unconscious of feelings of hate; may manifest as difficulty paying attention to what the patient is saying or feeling bored or tired |
| Turning against oneself | Doubting one’s capacity to help the patient; may feel inadequate, helpless, and hopeless. May lead to giving up on the patient because the therapist feels incompetent |
| Reaction formation | Turning hatred into the opposite emotion. The therapist may be too preoccupied with being helpful or overly concerned about the patient’s welfare and comfort |
| Projection | Feeling that the patient hates the therapist, leading to feelings of dread and fear |
| Distortion of reality | Devaluing the patient and seeing the patient as a hopeless case or a dangerous person. The therapist may feel indifference, pity, or anger toward the patient |
| Source: Reference 7 | |
TREATMENT: A breakthrough
Mr. J presents with obvious unease at the first visit after we had decreased the frequency of our sessions. At this point, Mr. J opens up to me. He says he has not been truthful with me, and has had worsening depression, anhedonia, and agoraphobia over the past year. He also reveals that he has homosexual fantasies that he cannot stop, which disturb him because he says he is heterosexual. He agrees to come once a week, and reluctantly admits that he desperately needs help.
Although Mr. J only takes clonazepam and citalopram, 20 mg/d, which I prescribed after he admitted to depression and anxiety, he has hyperlipidemia and a family history of heart disease. In addition to being a musician and working at his father’s construction company, he has worked as a security guard, bounty hunter, and computer technician. His careers have been solitary in nature, and, with the exception of computer work, permitted an outlet for aggression. However, he recently started taking online college classes and wants to become a music teacher because he feels he has a lot to offer children as a result of his life experiences. His fantasy of being a teacher shows considerably less aggression, and could be a sign of psychological growth.
Mr. J is struggling financially and his home is on the verge of foreclosure. Early in treatment he told me that he stopped paying his mortgage, but demonstrated blind optimism that things would “work out.” I asked if this was a wise decision, but he seemed confident and dismissive of my concerns. Although he now struggles with this situation, I consider this healthier than his constant pseudo-happy state, and a sign of psychological development.8 Despite his financial stressors, he wants to pursue his dream of being a famous musician, and says he “could never work a 9-to-5 job in a cubicle.”
The authors’ observations
I do not think it’s a coincidence that Mr. J stopped minimizing his symptoms when we decreased the frequency of his sessions. I had viewed our sessions as unproductive and blamed Mr. J for wasting both of our time with his resistance and minimization and had begun to dislike him. I felt impotent because he had been controlling each session with long, elaborate stories that had little relevance to his panic attacks, and I could not redirect him or get him to focus on pertinent issues. It was as if I was an audience for him, and provided nothing useful. However, I was interested in these superficial stories because Mr. J was charming and engaging. He likely reenacted his relationship with his father with me. Mr. J’s superficial relationship with me caused me to dislike him, and, similar to his father, reject him. This rejection likely was damaging because I was unable to anticipate his needs, which would have been to increase—rather than decrease—the frequency of our sessions. Just like his father, I was not able to take care of him.
Mr. J is deeply conflicted about his father. He states that his father “is a monster who instills fear and intimidation into everyone around him, but he’s charismatic, and I’ll always love him.” His view of his domineering father likely developed into a castration anxiety because he was afraid of competing for his mother’s love, contributing to a muddled sexual identity. This was intensified when Mr. J was sexually abused; he may have been stimulated by the molestation, adding to his confusion. Although Mr. J has repressed the abuse and split off most of his childhood, he suffers from shame, guilt, and depression because of his ego-dystonic homosexual fantasies. Homosexuality is at odds with his self-image and contributes to his anxiety and panic attacks. He cannot adequately discharge this dangerous libidinal energy, and as he becomes more conscious of it, his anxiety intensifies.
OUTCOME: Overcoming fear
As Mr. J sits crying in my office, he says he hasn’t cried in front of another man in years. I wonder aloud what his father would think of this situation. His states that his father does not respect any type of weakness and probably would “knock his teeth in.” Overcoming this fear of opening up will be a goal of Mr. J’s treatment. His unbridled optimism borders on pathologic, and is a defense against reality.8 Additionally, his reluctance to accept that he is suffering from depression, which he perceives as a weakness, will be a struggle throughout therapy. He likely will continue to minimize his symptoms when possible, making the true depths of his illness difficult to grasp.
Related Resources
- Waska R. Using countertransference: analytic contact, projective identification, and transference phantasy states. Am J Psychother. 2008;62(4):333-351.
- Gabbard GO, Litowitz BE, Williams P. Textbook of psychoanalysis. Arlington, VA: American Psychiatric Publishing, Inc; 2011.
Drug Brand Names
- Citalopram • Celexa
- Clonazepam • Klonopin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Blos P. Son and father: before and beyond the Oedipus complex. New York NY: Free Press/Macmillan; 1985.
2. Ursano RJ, Sonnenberg SM, Lazar SG. Concise guide to psychodynamic psychotherapy. Arlington VA: American Psychiatric Publishing; 2004.
3. Akhtar S. Comprehensive dictionary of psychoanalysis. London United Kingdom: Kamac Books; 2009.
4. Busch FN, Milrod BL, Singer MB. Theory and technique in psychodynamic treatment of panic disorder. J Psychother Pract Res. 1999;8(3):234-242.
5. Freud S. The future prospects of psycho-analytic therapy. The standard edition of the complete psychological works of Sigmund Freud volume XI (1910): five lectures on psycho-analysis, Leonardo da Vinci and other works. London, United Kingdom: Hogarth Press; 1957:139–152.
6. Winnicott DW. Hate in the counter-transference. J Psychother Pract Res. 1994;3(4):348-356.
7. Maltsberger JT, Buie DH. Countertransference hate in the treatment of suicidal patients. Arch Gen Psychiatry. 1974;30(5):625-633.
8. Akhtar S. “Someday…” and “if only…” fantasies: pathological optimism and inordinate nostalgia as related forms of idealization. J Am Psychoanal Assoc. 1996;44(3):723-753.
1. Blos P. Son and father: before and beyond the Oedipus complex. New York NY: Free Press/Macmillan; 1985.
2. Ursano RJ, Sonnenberg SM, Lazar SG. Concise guide to psychodynamic psychotherapy. Arlington VA: American Psychiatric Publishing; 2004.
3. Akhtar S. Comprehensive dictionary of psychoanalysis. London United Kingdom: Kamac Books; 2009.
4. Busch FN, Milrod BL, Singer MB. Theory and technique in psychodynamic treatment of panic disorder. J Psychother Pract Res. 1999;8(3):234-242.
5. Freud S. The future prospects of psycho-analytic therapy. The standard edition of the complete psychological works of Sigmund Freud volume XI (1910): five lectures on psycho-analysis, Leonardo da Vinci and other works. London, United Kingdom: Hogarth Press; 1957:139–152.
6. Winnicott DW. Hate in the counter-transference. J Psychother Pract Res. 1994;3(4):348-356.
7. Maltsberger JT, Buie DH. Countertransference hate in the treatment of suicidal patients. Arch Gen Psychiatry. 1974;30(5):625-633.
8. Akhtar S. “Someday…” and “if only…” fantasies: pathological optimism and inordinate nostalgia as related forms of idealization. J Am Psychoanal Assoc. 1996;44(3):723-753.
A perplexing case of altered mental status
Discuss this article at www.facebook.com/CurrentPsychiatry
CASE: Agitated and paranoid
Mr. E, age 55, presents to the emergency department (ED) with a 2-week history of altered mental status (AMS). His wife reports, “He was normal one day and the next day he was not.” Mr. E also presents with sleep disturbance, decreased appetite and speech, and a 20-lb weight loss. His family reports no recent stressors or head trauma. Mr. E is agitated in the ED and receives a single dose of IV haloperidol, 5 mg. He exhibits paranoia and is afraid to get a CT scan. The medical team attempts a lumbar puncture (LP), but Mr. E does not cooperate.
His laboratory values are: potassium, 3.0 mEq/L; creatinine, 1.60 mg/dL; calcium, 10.6 mg/dL; thyroid-stimulating hormone, 0.177 IU/L; vitamin B12, >1500 pg/mL; folate, >20 ng/mL; and creatine kinase, 281 U/L. Urine drug screen is positive for benzodiazepines and opiates, neither of which was prescribed, and blood alcohol is negative.
Mr. E is admitted for further workup of AMS. His daughter-in-law states that Mr. E is an alcoholic and she is concerned that he may have mixed drugs and alcohol. The medical service starts Mr. E on empiric antimicrobials—vancomycin, ceftriaxone, and acyclovir—because of his AMS, and performs an LP to rule out intracranial pathology. His LP results are unremarkable.
Mr. E appears to be confused during psychiatric evaluation. He requests to be “hypnotized on a helicopter to find out what is wrong with me.” His wife states that Mr. E drank vodka daily but decreased his alcohol consumption after surgery 5 months ago. Before his current admission, he was drinking approximately half a glass of vodka every few days, according to his wife. Mr. E says he has no prior psychiatric admissions. During the mental status exam, his eye contact is poor, with latency of response to questions, thought blocking, and psychomotor retardation. He is alert, oriented to time, place, and person, and cooperative. He cannot concentrate or focus during the interview. He denies suicidal or homicidal ideation.
The authors’ observations
Mr. E appeared to be delirious, as evidenced by the sudden onset of waxing and waning changes in consciousness, attention deficits, and cognition. He also had a history of daily alcohol use and decreased his alcohol intake after a surgery 5 months ago, which puts him at risk for Wernicke’s encephalopathy.1-3 The type of surgery and whether he received adequate thiamine supplementation at that time was unclear. Because Mr. E is older, he has a higher risk of mortality and morbidity from delirium.4,5 We started Mr. E on quetiapine, 50 mg/d, for delirium and an IV lorazepam taper, starting at 2 mg every 8 hours, because the extent of his alcohol and benzodiazepine use was unclear—we weren’t sure how forthcoming he was about his alcohol use. He received IV thiamine supplementation followed by oral thiamine, 100 mg/d.
The authors’ recommendations
We requested a neurology consult, EEG, CSF cultures, and brain MRI (Table 1).6 EEG, chest radiography, thyroid scan, and CT scan were normal and MRI showed no acute intracranial process. However, there was a redemonstration of increased T1 signal seen within the bilateral basal ganglia and relative diminutive appearance to the bilateral mamillary bodies, which suggests possible liver disease and/or alcohol abuse. These findings were unchanged from an MRI Mr. E received 10 years ago, were consistent with his history of alcohol abuse, and may indicate an underlying predisposition to delirium. A CT scan of the abdomen showed hepatic cirrhosis with prominent, tortuous vessels of the upper abdomen, subtle ill-defined hypodensity of the anterior aspect of the liver, and an enlarged spleen.
Mr. E’s mental functioning did not improve with quetiapine and lorazepam. Further evaluation revealed a negative human immunodeficiency virus test and normal heavy metals, ammonia, ceruloplasmin, and thiamine. We suspected limbic encephalitis because of Mr. E’s memory problems and behavioral and psychiatric manifestations,7 but CSF was unremarkable and limbic encephalitis workup of CSF and paraneoplastic antibody panel were negative.
Mr. E’s primary care physician stated that at an appointment 1 month ago, Mr. E was alert, oriented, and conversational with normal thought processing. At that time he had presented with rectal bleeding, occult blood in his stool, and an unintentional 25-lb weight loss over 2 months. It was not clear if his weight loss was caused by poor nutrition—which is common among chronic alcoholics—or an occult disease process.
After 10 days, Mr. E was discharged home from the medicine service with no clear cause of his AMS.
Table 1
Suggested workup for altered mental status
| Complete blood count, basic metabolic profile, creatine kinase |
| Thyroid-stimulating hormone, thyroid scan |
| Vitamin B12, folate, thiamine |
| Blood alcohol, urine drug screen |
| Urine analysis and cultures |
| Lumbar puncture—CSF staining and cultures |
| Chest radiography |
| CT and MRI scan of brain |
| Electroencephalography |
| Neuropsychiatric testing |
| CSF: cerebrospinal fluid Source: Reference 6 |
EVALUATION: Worsening behavior
One week later, Mr. E presents to the ED with continued AMS and worsening behavior at home. Two days ago, he attempted to strangle his dog and cut himself with a knife. His paranoia was worsening and his oral intake continued to decrease. In the ED, Mr. E does not want a chest radiograph because, “I don’t like radiation contaminating my body”; his family stated that he had been suspicious of radiography all of his life. He receives empiric ceftriaxone because of a possible urinary tract infection. Urine culture is positive for Pseudomonas aeruginosa and he is switched to ciprofloxacin. Mr. E is admitted for further workup.
Mr. E’s mother states, “I think this change in behavior is related to my son drinking alcohol for 20 years. This is exactly how he acted when he was on drugs. I think he is having a flashback.” She also reports her son purchased multiple chemicals—the details of which are unclear—that he left lying around the house.
His wife says that after discharge a week ago, Mr. E was stable for 1 or 2 days and then “he started going downhill.” He became more paranoid and he started talking about cameras watching him in his house. Mr. E took quetiapine, 50 mg/d, for a few days, then refused because he thought there was something in the medication. Mr. E’s family feels that at times he is responding to internal stimuli. He makes statements about his DNA being changed and reports that he has 2 wives and the wife in the room was not the real one, which suggests Capgras syndrome. His wife provides a home medication list that includes vitamin B complex, vitamins B12, E, and C, a multivitamin, zinc, magnesium, fish oil, garlic, calcium, glucosamine, chondroitin, herbal supplements, and gingko. The psychiatry team recommends switching from quetiapine to olanzapine, 15 mg/d, because Mr. E was paranoid about taking quetiapine.
We determine that Mr. E does not have medical decision-making capacity.
Because his symptoms do not improve, Mr. E is transferred to the psychiatric intensive care unit. His mental status shows little change while there. Neuropsychiatric testing shows only “cognitive deficits.” He shows signs consistent with neurologic dysfunction in terms of stimulus-bound responding and perseveration, which is compatible with the bilateral basal ganglia lesion seen on MRI. However, some of his behaviors suggest psychiatric and motivationally driven or manipulative etiology. During this testing he was difficult to evaluate and needed to be convinced to engage. At times he was illogical and at other times he showed good focus and recall. It is difficult to draw more definitive conclusions and Mr. E is discharged home with minor improvement in his symptoms. He didn’t attend follow-up appointments. During a courtesy call a few months after his admission, his wife revealed that Mr. E had died after shooting himself. It is unclear if it was an accident or suicide.
The authors’ observations
Mr. E’s diagnosis remains unclear (for a summary of his clinical course, see Table 2). Although his initial presentation was consistent with delirium, the lack of an identifiable medical cause, prolonged time course, and lack of improvement with dopamine blocking agents suggest additional diagnoses such as Wernicke-Korsakoff syndrome, rapidly progressive dementia, or a substance-induced disorder. He displayed paranoia and bizarre delusions, which would suggest a thought disorder. However, he also had a history of substance use. A few months after we saw Mr. E, “bath salt” (methylenedioxypyrovalerone) abuse gained national attention. Patients with bath salt intoxication present with confusion, paranoia, and behavioral disturbances as well as a prolonged course.8
Mr. E’s CT and MRI scans, history of alcohol use, and cirrhosis also point to Wernicke-Korsakoff syndrome as an underlying diagnosis. It is unclear whether Mr. E experienced alcohol withdrawal and IV glucose without adequate thiamine replacement during a prior surgery. However, MRI findings were unchanged from a previous study 10 years ago.
It is puzzling whether Mr. E’s AMS was a first psychotic break, a result of drug and alcohol use, rapidly progressing dementia, or another neurologic problem that we have not identified. Our tentative diagnosis was Wernicke-Korsakoff syndrome because of his history of alcohol use and imaging findings.
Although we used a multidisciplinary team approach that included psychiatry, internal medicine, neurology, neuropsychology, and an aggressive and thorough workup, we could not establish a definitive diagnosis. Unsolved cases such as this can leave patients and clinicians frustrated and may lead to unfavorable outcomes. Additional resources such as a telephone call after the first missed appointment may have been warranted.
Table 2
Mr. E’s clinical course
| Symptoms | Treatment | |
|---|---|---|
| First ED visit | Agitation Confusion Sleep disturbance Decreased appetite and speech 20-lb weight loss | Empiric antimicrobials for possible meningitis Haloperidol for agitation Quetiapine for delirium Lorazepam taper Thiamine supplementation |
| Second ED visit | Violent behavior Worsening paranoia Responding to internal stimuli Mr. E believes he has 2 wives, but the wife in the room is not the real one, which suggests possible Capgras syndrome Cognitive deficits on mental status exam | Switch from ceftriaxone to ciprofloxacin for Pseudomonas aeruginosa Switch from quetiapine to olanzapine |
| ED: emergency department | ||
Related Resources
- Kaufman DM. Clinical neurology for psychiatrists. 6th ed. Philadelphia, PA: Saunders Elsevier; 2007.
- Sidhu KS, Balon R, Ajluni V, et al. Standard EEG and the difficult-to-assess mental status. Ann Clin Psychiatry. 2009;21(2):103-108.
Drug Brand Names
- Acyclovir • Zovirax
- Ceftriaxone • Rocephin
- Ciprofloxacin • Cipro
- Haloperidol • Haldol
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Thiamine • Betaxin
- Vancomycin • Vancocin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jiang W, Gagliardi JP, Raj YP, et al. Acute psychotic disorder after gastric bypass surgery: differential diagnosis and treatment. Am J Psychiatry. 2006;163(1):15-19.
2. Harrison RA, Vu T, Hunter AJ. Wernicke’s encephalopathy in a patient with schizophrenia. J Gen Intern Med. 2006;21(12):C8-C11.
3. Sechi GP, Serra A. Wernicke’s encephalopathy: new clinical settings and recent advances in diagnosis and treatment. Lancet Neurol. 2007;6(5):442-455.
4. American Psychiatric Association. Practice guidelines for the treatment of patients with delirium. Am J Psychiatry. 1999;156(5 suppl):1-20.
5. Sharon KI, Fearing MA, Marcantonio RA. Delirium. In: Halter JB Ouslander JG, Tinetti ME, et al, eds. Hazzard’s geriatric medicine and gerontology. 6th ed. New York, NY: McGraw-Hill Medical; 2009:647–658.
6. Sadock BJ, Sadock VA. Delirium dementia, and amnestic and other cognitive disorders. In: Sadock BJ, Kaplan HI, Sadock VA. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry. Philadelphia, PA: Lippincott Williams and Wilkins; 2007:319–372.
7. Ahmad SA, Archer HA, Rice CM, et al. Seronegative limbic encephalitis: case report, literature review and proposed treatment algorithm. Pract Neurol. 2011;11(6):355-361.
8. Ross EA, Watson M, Goldberger B. “Bath salts” intoxication. N Engl J Med. 2011;365(10):967-968.
Discuss this article at www.facebook.com/CurrentPsychiatry
CASE: Agitated and paranoid
Mr. E, age 55, presents to the emergency department (ED) with a 2-week history of altered mental status (AMS). His wife reports, “He was normal one day and the next day he was not.” Mr. E also presents with sleep disturbance, decreased appetite and speech, and a 20-lb weight loss. His family reports no recent stressors or head trauma. Mr. E is agitated in the ED and receives a single dose of IV haloperidol, 5 mg. He exhibits paranoia and is afraid to get a CT scan. The medical team attempts a lumbar puncture (LP), but Mr. E does not cooperate.
His laboratory values are: potassium, 3.0 mEq/L; creatinine, 1.60 mg/dL; calcium, 10.6 mg/dL; thyroid-stimulating hormone, 0.177 IU/L; vitamin B12, >1500 pg/mL; folate, >20 ng/mL; and creatine kinase, 281 U/L. Urine drug screen is positive for benzodiazepines and opiates, neither of which was prescribed, and blood alcohol is negative.
Mr. E is admitted for further workup of AMS. His daughter-in-law states that Mr. E is an alcoholic and she is concerned that he may have mixed drugs and alcohol. The medical service starts Mr. E on empiric antimicrobials—vancomycin, ceftriaxone, and acyclovir—because of his AMS, and performs an LP to rule out intracranial pathology. His LP results are unremarkable.
Mr. E appears to be confused during psychiatric evaluation. He requests to be “hypnotized on a helicopter to find out what is wrong with me.” His wife states that Mr. E drank vodka daily but decreased his alcohol consumption after surgery 5 months ago. Before his current admission, he was drinking approximately half a glass of vodka every few days, according to his wife. Mr. E says he has no prior psychiatric admissions. During the mental status exam, his eye contact is poor, with latency of response to questions, thought blocking, and psychomotor retardation. He is alert, oriented to time, place, and person, and cooperative. He cannot concentrate or focus during the interview. He denies suicidal or homicidal ideation.
The authors’ observations
Mr. E appeared to be delirious, as evidenced by the sudden onset of waxing and waning changes in consciousness, attention deficits, and cognition. He also had a history of daily alcohol use and decreased his alcohol intake after a surgery 5 months ago, which puts him at risk for Wernicke’s encephalopathy.1-3 The type of surgery and whether he received adequate thiamine supplementation at that time was unclear. Because Mr. E is older, he has a higher risk of mortality and morbidity from delirium.4,5 We started Mr. E on quetiapine, 50 mg/d, for delirium and an IV lorazepam taper, starting at 2 mg every 8 hours, because the extent of his alcohol and benzodiazepine use was unclear—we weren’t sure how forthcoming he was about his alcohol use. He received IV thiamine supplementation followed by oral thiamine, 100 mg/d.
The authors’ recommendations
We requested a neurology consult, EEG, CSF cultures, and brain MRI (Table 1).6 EEG, chest radiography, thyroid scan, and CT scan were normal and MRI showed no acute intracranial process. However, there was a redemonstration of increased T1 signal seen within the bilateral basal ganglia and relative diminutive appearance to the bilateral mamillary bodies, which suggests possible liver disease and/or alcohol abuse. These findings were unchanged from an MRI Mr. E received 10 years ago, were consistent with his history of alcohol abuse, and may indicate an underlying predisposition to delirium. A CT scan of the abdomen showed hepatic cirrhosis with prominent, tortuous vessels of the upper abdomen, subtle ill-defined hypodensity of the anterior aspect of the liver, and an enlarged spleen.
Mr. E’s mental functioning did not improve with quetiapine and lorazepam. Further evaluation revealed a negative human immunodeficiency virus test and normal heavy metals, ammonia, ceruloplasmin, and thiamine. We suspected limbic encephalitis because of Mr. E’s memory problems and behavioral and psychiatric manifestations,7 but CSF was unremarkable and limbic encephalitis workup of CSF and paraneoplastic antibody panel were negative.
Mr. E’s primary care physician stated that at an appointment 1 month ago, Mr. E was alert, oriented, and conversational with normal thought processing. At that time he had presented with rectal bleeding, occult blood in his stool, and an unintentional 25-lb weight loss over 2 months. It was not clear if his weight loss was caused by poor nutrition—which is common among chronic alcoholics—or an occult disease process.
After 10 days, Mr. E was discharged home from the medicine service with no clear cause of his AMS.
Table 1
Suggested workup for altered mental status
| Complete blood count, basic metabolic profile, creatine kinase |
| Thyroid-stimulating hormone, thyroid scan |
| Vitamin B12, folate, thiamine |
| Blood alcohol, urine drug screen |
| Urine analysis and cultures |
| Lumbar puncture—CSF staining and cultures |
| Chest radiography |
| CT and MRI scan of brain |
| Electroencephalography |
| Neuropsychiatric testing |
| CSF: cerebrospinal fluid Source: Reference 6 |
EVALUATION: Worsening behavior
One week later, Mr. E presents to the ED with continued AMS and worsening behavior at home. Two days ago, he attempted to strangle his dog and cut himself with a knife. His paranoia was worsening and his oral intake continued to decrease. In the ED, Mr. E does not want a chest radiograph because, “I don’t like radiation contaminating my body”; his family stated that he had been suspicious of radiography all of his life. He receives empiric ceftriaxone because of a possible urinary tract infection. Urine culture is positive for Pseudomonas aeruginosa and he is switched to ciprofloxacin. Mr. E is admitted for further workup.
Mr. E’s mother states, “I think this change in behavior is related to my son drinking alcohol for 20 years. This is exactly how he acted when he was on drugs. I think he is having a flashback.” She also reports her son purchased multiple chemicals—the details of which are unclear—that he left lying around the house.
His wife says that after discharge a week ago, Mr. E was stable for 1 or 2 days and then “he started going downhill.” He became more paranoid and he started talking about cameras watching him in his house. Mr. E took quetiapine, 50 mg/d, for a few days, then refused because he thought there was something in the medication. Mr. E’s family feels that at times he is responding to internal stimuli. He makes statements about his DNA being changed and reports that he has 2 wives and the wife in the room was not the real one, which suggests Capgras syndrome. His wife provides a home medication list that includes vitamin B complex, vitamins B12, E, and C, a multivitamin, zinc, magnesium, fish oil, garlic, calcium, glucosamine, chondroitin, herbal supplements, and gingko. The psychiatry team recommends switching from quetiapine to olanzapine, 15 mg/d, because Mr. E was paranoid about taking quetiapine.
We determine that Mr. E does not have medical decision-making capacity.
Because his symptoms do not improve, Mr. E is transferred to the psychiatric intensive care unit. His mental status shows little change while there. Neuropsychiatric testing shows only “cognitive deficits.” He shows signs consistent with neurologic dysfunction in terms of stimulus-bound responding and perseveration, which is compatible with the bilateral basal ganglia lesion seen on MRI. However, some of his behaviors suggest psychiatric and motivationally driven or manipulative etiology. During this testing he was difficult to evaluate and needed to be convinced to engage. At times he was illogical and at other times he showed good focus and recall. It is difficult to draw more definitive conclusions and Mr. E is discharged home with minor improvement in his symptoms. He didn’t attend follow-up appointments. During a courtesy call a few months after his admission, his wife revealed that Mr. E had died after shooting himself. It is unclear if it was an accident or suicide.
The authors’ observations
Mr. E’s diagnosis remains unclear (for a summary of his clinical course, see Table 2). Although his initial presentation was consistent with delirium, the lack of an identifiable medical cause, prolonged time course, and lack of improvement with dopamine blocking agents suggest additional diagnoses such as Wernicke-Korsakoff syndrome, rapidly progressive dementia, or a substance-induced disorder. He displayed paranoia and bizarre delusions, which would suggest a thought disorder. However, he also had a history of substance use. A few months after we saw Mr. E, “bath salt” (methylenedioxypyrovalerone) abuse gained national attention. Patients with bath salt intoxication present with confusion, paranoia, and behavioral disturbances as well as a prolonged course.8
Mr. E’s CT and MRI scans, history of alcohol use, and cirrhosis also point to Wernicke-Korsakoff syndrome as an underlying diagnosis. It is unclear whether Mr. E experienced alcohol withdrawal and IV glucose without adequate thiamine replacement during a prior surgery. However, MRI findings were unchanged from a previous study 10 years ago.
It is puzzling whether Mr. E’s AMS was a first psychotic break, a result of drug and alcohol use, rapidly progressing dementia, or another neurologic problem that we have not identified. Our tentative diagnosis was Wernicke-Korsakoff syndrome because of his history of alcohol use and imaging findings.
Although we used a multidisciplinary team approach that included psychiatry, internal medicine, neurology, neuropsychology, and an aggressive and thorough workup, we could not establish a definitive diagnosis. Unsolved cases such as this can leave patients and clinicians frustrated and may lead to unfavorable outcomes. Additional resources such as a telephone call after the first missed appointment may have been warranted.
Table 2
Mr. E’s clinical course
| Symptoms | Treatment | |
|---|---|---|
| First ED visit | Agitation Confusion Sleep disturbance Decreased appetite and speech 20-lb weight loss | Empiric antimicrobials for possible meningitis Haloperidol for agitation Quetiapine for delirium Lorazepam taper Thiamine supplementation |
| Second ED visit | Violent behavior Worsening paranoia Responding to internal stimuli Mr. E believes he has 2 wives, but the wife in the room is not the real one, which suggests possible Capgras syndrome Cognitive deficits on mental status exam | Switch from ceftriaxone to ciprofloxacin for Pseudomonas aeruginosa Switch from quetiapine to olanzapine |
| ED: emergency department | ||
Related Resources
- Kaufman DM. Clinical neurology for psychiatrists. 6th ed. Philadelphia, PA: Saunders Elsevier; 2007.
- Sidhu KS, Balon R, Ajluni V, et al. Standard EEG and the difficult-to-assess mental status. Ann Clin Psychiatry. 2009;21(2):103-108.
Drug Brand Names
- Acyclovir • Zovirax
- Ceftriaxone • Rocephin
- Ciprofloxacin • Cipro
- Haloperidol • Haldol
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Thiamine • Betaxin
- Vancomycin • Vancocin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
CASE: Agitated and paranoid
Mr. E, age 55, presents to the emergency department (ED) with a 2-week history of altered mental status (AMS). His wife reports, “He was normal one day and the next day he was not.” Mr. E also presents with sleep disturbance, decreased appetite and speech, and a 20-lb weight loss. His family reports no recent stressors or head trauma. Mr. E is agitated in the ED and receives a single dose of IV haloperidol, 5 mg. He exhibits paranoia and is afraid to get a CT scan. The medical team attempts a lumbar puncture (LP), but Mr. E does not cooperate.
His laboratory values are: potassium, 3.0 mEq/L; creatinine, 1.60 mg/dL; calcium, 10.6 mg/dL; thyroid-stimulating hormone, 0.177 IU/L; vitamin B12, >1500 pg/mL; folate, >20 ng/mL; and creatine kinase, 281 U/L. Urine drug screen is positive for benzodiazepines and opiates, neither of which was prescribed, and blood alcohol is negative.
Mr. E is admitted for further workup of AMS. His daughter-in-law states that Mr. E is an alcoholic and she is concerned that he may have mixed drugs and alcohol. The medical service starts Mr. E on empiric antimicrobials—vancomycin, ceftriaxone, and acyclovir—because of his AMS, and performs an LP to rule out intracranial pathology. His LP results are unremarkable.
Mr. E appears to be confused during psychiatric evaluation. He requests to be “hypnotized on a helicopter to find out what is wrong with me.” His wife states that Mr. E drank vodka daily but decreased his alcohol consumption after surgery 5 months ago. Before his current admission, he was drinking approximately half a glass of vodka every few days, according to his wife. Mr. E says he has no prior psychiatric admissions. During the mental status exam, his eye contact is poor, with latency of response to questions, thought blocking, and psychomotor retardation. He is alert, oriented to time, place, and person, and cooperative. He cannot concentrate or focus during the interview. He denies suicidal or homicidal ideation.
The authors’ observations
Mr. E appeared to be delirious, as evidenced by the sudden onset of waxing and waning changes in consciousness, attention deficits, and cognition. He also had a history of daily alcohol use and decreased his alcohol intake after a surgery 5 months ago, which puts him at risk for Wernicke’s encephalopathy.1-3 The type of surgery and whether he received adequate thiamine supplementation at that time was unclear. Because Mr. E is older, he has a higher risk of mortality and morbidity from delirium.4,5 We started Mr. E on quetiapine, 50 mg/d, for delirium and an IV lorazepam taper, starting at 2 mg every 8 hours, because the extent of his alcohol and benzodiazepine use was unclear—we weren’t sure how forthcoming he was about his alcohol use. He received IV thiamine supplementation followed by oral thiamine, 100 mg/d.
The authors’ recommendations
We requested a neurology consult, EEG, CSF cultures, and brain MRI (Table 1).6 EEG, chest radiography, thyroid scan, and CT scan were normal and MRI showed no acute intracranial process. However, there was a redemonstration of increased T1 signal seen within the bilateral basal ganglia and relative diminutive appearance to the bilateral mamillary bodies, which suggests possible liver disease and/or alcohol abuse. These findings were unchanged from an MRI Mr. E received 10 years ago, were consistent with his history of alcohol abuse, and may indicate an underlying predisposition to delirium. A CT scan of the abdomen showed hepatic cirrhosis with prominent, tortuous vessels of the upper abdomen, subtle ill-defined hypodensity of the anterior aspect of the liver, and an enlarged spleen.
Mr. E’s mental functioning did not improve with quetiapine and lorazepam. Further evaluation revealed a negative human immunodeficiency virus test and normal heavy metals, ammonia, ceruloplasmin, and thiamine. We suspected limbic encephalitis because of Mr. E’s memory problems and behavioral and psychiatric manifestations,7 but CSF was unremarkable and limbic encephalitis workup of CSF and paraneoplastic antibody panel were negative.
Mr. E’s primary care physician stated that at an appointment 1 month ago, Mr. E was alert, oriented, and conversational with normal thought processing. At that time he had presented with rectal bleeding, occult blood in his stool, and an unintentional 25-lb weight loss over 2 months. It was not clear if his weight loss was caused by poor nutrition—which is common among chronic alcoholics—or an occult disease process.
After 10 days, Mr. E was discharged home from the medicine service with no clear cause of his AMS.
Table 1
Suggested workup for altered mental status
| Complete blood count, basic metabolic profile, creatine kinase |
| Thyroid-stimulating hormone, thyroid scan |
| Vitamin B12, folate, thiamine |
| Blood alcohol, urine drug screen |
| Urine analysis and cultures |
| Lumbar puncture—CSF staining and cultures |
| Chest radiography |
| CT and MRI scan of brain |
| Electroencephalography |
| Neuropsychiatric testing |
| CSF: cerebrospinal fluid Source: Reference 6 |
EVALUATION: Worsening behavior
One week later, Mr. E presents to the ED with continued AMS and worsening behavior at home. Two days ago, he attempted to strangle his dog and cut himself with a knife. His paranoia was worsening and his oral intake continued to decrease. In the ED, Mr. E does not want a chest radiograph because, “I don’t like radiation contaminating my body”; his family stated that he had been suspicious of radiography all of his life. He receives empiric ceftriaxone because of a possible urinary tract infection. Urine culture is positive for Pseudomonas aeruginosa and he is switched to ciprofloxacin. Mr. E is admitted for further workup.
Mr. E’s mother states, “I think this change in behavior is related to my son drinking alcohol for 20 years. This is exactly how he acted when he was on drugs. I think he is having a flashback.” She also reports her son purchased multiple chemicals—the details of which are unclear—that he left lying around the house.
His wife says that after discharge a week ago, Mr. E was stable for 1 or 2 days and then “he started going downhill.” He became more paranoid and he started talking about cameras watching him in his house. Mr. E took quetiapine, 50 mg/d, for a few days, then refused because he thought there was something in the medication. Mr. E’s family feels that at times he is responding to internal stimuli. He makes statements about his DNA being changed and reports that he has 2 wives and the wife in the room was not the real one, which suggests Capgras syndrome. His wife provides a home medication list that includes vitamin B complex, vitamins B12, E, and C, a multivitamin, zinc, magnesium, fish oil, garlic, calcium, glucosamine, chondroitin, herbal supplements, and gingko. The psychiatry team recommends switching from quetiapine to olanzapine, 15 mg/d, because Mr. E was paranoid about taking quetiapine.
We determine that Mr. E does not have medical decision-making capacity.
Because his symptoms do not improve, Mr. E is transferred to the psychiatric intensive care unit. His mental status shows little change while there. Neuropsychiatric testing shows only “cognitive deficits.” He shows signs consistent with neurologic dysfunction in terms of stimulus-bound responding and perseveration, which is compatible with the bilateral basal ganglia lesion seen on MRI. However, some of his behaviors suggest psychiatric and motivationally driven or manipulative etiology. During this testing he was difficult to evaluate and needed to be convinced to engage. At times he was illogical and at other times he showed good focus and recall. It is difficult to draw more definitive conclusions and Mr. E is discharged home with minor improvement in his symptoms. He didn’t attend follow-up appointments. During a courtesy call a few months after his admission, his wife revealed that Mr. E had died after shooting himself. It is unclear if it was an accident or suicide.
The authors’ observations
Mr. E’s diagnosis remains unclear (for a summary of his clinical course, see Table 2). Although his initial presentation was consistent with delirium, the lack of an identifiable medical cause, prolonged time course, and lack of improvement with dopamine blocking agents suggest additional diagnoses such as Wernicke-Korsakoff syndrome, rapidly progressive dementia, or a substance-induced disorder. He displayed paranoia and bizarre delusions, which would suggest a thought disorder. However, he also had a history of substance use. A few months after we saw Mr. E, “bath salt” (methylenedioxypyrovalerone) abuse gained national attention. Patients with bath salt intoxication present with confusion, paranoia, and behavioral disturbances as well as a prolonged course.8
Mr. E’s CT and MRI scans, history of alcohol use, and cirrhosis also point to Wernicke-Korsakoff syndrome as an underlying diagnosis. It is unclear whether Mr. E experienced alcohol withdrawal and IV glucose without adequate thiamine replacement during a prior surgery. However, MRI findings were unchanged from a previous study 10 years ago.
It is puzzling whether Mr. E’s AMS was a first psychotic break, a result of drug and alcohol use, rapidly progressing dementia, or another neurologic problem that we have not identified. Our tentative diagnosis was Wernicke-Korsakoff syndrome because of his history of alcohol use and imaging findings.
Although we used a multidisciplinary team approach that included psychiatry, internal medicine, neurology, neuropsychology, and an aggressive and thorough workup, we could not establish a definitive diagnosis. Unsolved cases such as this can leave patients and clinicians frustrated and may lead to unfavorable outcomes. Additional resources such as a telephone call after the first missed appointment may have been warranted.
Table 2
Mr. E’s clinical course
| Symptoms | Treatment | |
|---|---|---|
| First ED visit | Agitation Confusion Sleep disturbance Decreased appetite and speech 20-lb weight loss | Empiric antimicrobials for possible meningitis Haloperidol for agitation Quetiapine for delirium Lorazepam taper Thiamine supplementation |
| Second ED visit | Violent behavior Worsening paranoia Responding to internal stimuli Mr. E believes he has 2 wives, but the wife in the room is not the real one, which suggests possible Capgras syndrome Cognitive deficits on mental status exam | Switch from ceftriaxone to ciprofloxacin for Pseudomonas aeruginosa Switch from quetiapine to olanzapine |
| ED: emergency department | ||
Related Resources
- Kaufman DM. Clinical neurology for psychiatrists. 6th ed. Philadelphia, PA: Saunders Elsevier; 2007.
- Sidhu KS, Balon R, Ajluni V, et al. Standard EEG and the difficult-to-assess mental status. Ann Clin Psychiatry. 2009;21(2):103-108.
Drug Brand Names
- Acyclovir • Zovirax
- Ceftriaxone • Rocephin
- Ciprofloxacin • Cipro
- Haloperidol • Haldol
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Thiamine • Betaxin
- Vancomycin • Vancocin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jiang W, Gagliardi JP, Raj YP, et al. Acute psychotic disorder after gastric bypass surgery: differential diagnosis and treatment. Am J Psychiatry. 2006;163(1):15-19.
2. Harrison RA, Vu T, Hunter AJ. Wernicke’s encephalopathy in a patient with schizophrenia. J Gen Intern Med. 2006;21(12):C8-C11.
3. Sechi GP, Serra A. Wernicke’s encephalopathy: new clinical settings and recent advances in diagnosis and treatment. Lancet Neurol. 2007;6(5):442-455.
4. American Psychiatric Association. Practice guidelines for the treatment of patients with delirium. Am J Psychiatry. 1999;156(5 suppl):1-20.
5. Sharon KI, Fearing MA, Marcantonio RA. Delirium. In: Halter JB Ouslander JG, Tinetti ME, et al, eds. Hazzard’s geriatric medicine and gerontology. 6th ed. New York, NY: McGraw-Hill Medical; 2009:647–658.
6. Sadock BJ, Sadock VA. Delirium dementia, and amnestic and other cognitive disorders. In: Sadock BJ, Kaplan HI, Sadock VA. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry. Philadelphia, PA: Lippincott Williams and Wilkins; 2007:319–372.
7. Ahmad SA, Archer HA, Rice CM, et al. Seronegative limbic encephalitis: case report, literature review and proposed treatment algorithm. Pract Neurol. 2011;11(6):355-361.
8. Ross EA, Watson M, Goldberger B. “Bath salts” intoxication. N Engl J Med. 2011;365(10):967-968.
1. Jiang W, Gagliardi JP, Raj YP, et al. Acute psychotic disorder after gastric bypass surgery: differential diagnosis and treatment. Am J Psychiatry. 2006;163(1):15-19.
2. Harrison RA, Vu T, Hunter AJ. Wernicke’s encephalopathy in a patient with schizophrenia. J Gen Intern Med. 2006;21(12):C8-C11.
3. Sechi GP, Serra A. Wernicke’s encephalopathy: new clinical settings and recent advances in diagnosis and treatment. Lancet Neurol. 2007;6(5):442-455.
4. American Psychiatric Association. Practice guidelines for the treatment of patients with delirium. Am J Psychiatry. 1999;156(5 suppl):1-20.
5. Sharon KI, Fearing MA, Marcantonio RA. Delirium. In: Halter JB Ouslander JG, Tinetti ME, et al, eds. Hazzard’s geriatric medicine and gerontology. 6th ed. New York, NY: McGraw-Hill Medical; 2009:647–658.
6. Sadock BJ, Sadock VA. Delirium dementia, and amnestic and other cognitive disorders. In: Sadock BJ, Kaplan HI, Sadock VA. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry. Philadelphia, PA: Lippincott Williams and Wilkins; 2007:319–372.
7. Ahmad SA, Archer HA, Rice CM, et al. Seronegative limbic encephalitis: case report, literature review and proposed treatment algorithm. Pract Neurol. 2011;11(6):355-361.
8. Ross EA, Watson M, Goldberger B. “Bath salts” intoxication. N Engl J Med. 2011;365(10):967-968.
Overwhelmed by side effects
CASE: Medication sensitivity
Mrs. C, age 48, is admitted to a tertiary care inpatient mood disorder unit for evaluation of severe depression characterized by depressed mood, anhedonia, and insomnia. Her initial Hamilton Rating Scale for Depression 17-Item (HRSD-17) score is 30, indicating severe depression. Her medications are fluoxetine, 10 mg/d, and diazepam, 0.5 mg/d.
Mrs. C describes a 10-month history of depression and extreme anxiety in the context of several psychosocial stressors. Her father recently died and she is having difficulty with the demands of administering her father’s estate. She is intensely obsessive and focused on nihilistic themes, her diagnosis, somatic themes, and medications side effects. Her husband confirms our observations. No history or current symptoms of typical compulsions (eg, washing hands or checking doors) are elicited. She has limited insight into her obsessive tendencies.
Mrs. C had no psychiatric history before her depressive and obsessive symptoms developed 10 months ago. However, in the past 10 months, she has been hospitalized in a psychiatric facility twice. She also received a series of 8 electroconvulsive therapy treatments, but reported minimal improvement of her depressive symptoms. Mrs. C had a few cognitive-behavioral therapy (CBT) sessions with a psychotherapist, but she said they didn’t help much.
Mrs. C has substantial difficulty adhering to medications, even at subtherapeutic doses. She states she is “extremely sensitive” to all medications. Mrs. C says she develops dizziness, increased anxiety, insomnia, nausea, and other vague reactions whenever she attempts to increase her psychotropics to therapeutic doses. She took sertraline, 10 mg/d, for 4 days, but discontinued it because of unspecified side effects. She then received escitalopram, 2.5 mg/d, for 10 days, but again stopped it because of vague side effects. She was taking paroxetine, 10 mg/d, for 2 days, but experienced vomiting and discontinued the drug. She tried venlafaxine at a low dose and also discontinued it because of vomiting. Mrs. C stayed on mirtazapine, 22.5 mg/d, for 3 months, but stopped it because of lack of efficacy and she was unwilling to increase the dose. Other unsuccessful trials include citalopram and doxepin. Mrs. C is hesitant to try another medication or increase to therapeutic doses any of the previous medications.
The authors’ observations
Before initiating another treatment, the treatment team considered Mrs. C’s pervasive medication intolerance. Her enzymatic activity may be genetically compromised, which could lead to high blood levels of medications and significant side effects when she takes very low doses. Individual variations in response to psychotropics are influenced by genetic factors.1 Variants in the cytochrome P450 (CYP450) genes produce enzymes with increased activity, normal activity, reduced activity, or no activity, creating phenotypes of ultrarapid metabolizers, extensive metabolizers, intermediate metabolizers, and poor metabolizers, respectively. These genetic variations can affect blood levels of medications that employ these enzymes in their metabolic pathways.2 Mrs. C could be a poor metabolizer of common CYP450 variant enzymes, which led to her exquisite sensitivity to psychotropics. We felt this was a reasonable hypothesis given her tumultuous 10-month course of psychiatric treatment and multiple failed medication trials.
An alternative hypothesis is that Mrs. C’s somatic obsessions about drug side effects were the primary clinical issue that led to her severe medication intolerance. Mrs. C spends hours questioning the inpatient staff about her diagnosis (eg, “Are you sure I don’t have bipolar disorder?”), medications (eg, “Are you sure this medication won’t make me sick?”), somatic themes (eg, “Are you sure I don’t have Meniere’s disease with all my dizziness?”), and nihilistic themes (eg, “What if I never get better?”). Mrs. C’s husband attested that she has spent hours researching her new medications on the Internet and reading the medication handouts from the pharmacy. She admits to mentally cycling through the DSM-IV-TR criteria for hours at a time to “figure out” if she has bipolar disorder (BD).
We initiated pharmacogenomic testing to help distinguish between these hypotheses. Mrs. C’s results are presented in Table 1. Genotype results were applied using an interpretive algorithm (Figure) in which 26 psychiatric medications were placed in categories of “use as directed” (green column), “use with caution” (yellow column), and “use with caution or more frequent monitoring” (red column). The algorithm incorporates the genetic information with the known pharmacologic profile for each of the medications in the panel. Highlights of Mrs. C’s interpretive report are shown in Table 2.
Table 1
Mrs. C’s genotype results
| Gene | Allele | Predicted phenotype |
|---|---|---|
| CYP2D6 | *1/*4 | Intermediate metabolizer |
| CYP2C19 | *1/*1 | Extensive metabolizer |
| SLC6A4 | S/S | Low activity |
| 5HTR2A | G/G | Reduced activity |
Figure
Genotype-phenotype integration into Mrs. C’s interpretive report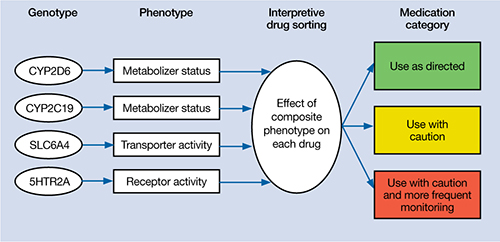
Table 2
Mrs. C’s pharmacogenomic-based interpretive report
| Use as directed | Use with caution | Use with caution and more frequent monitoring |
|---|---|---|
| Antidepressants: Duloxetine, mirtazapine Antipsychotics: Clozapine, olanzapine, quetiapine, ziprasidone | Antidepressants: Amitriptyline,a,b bupropion,a citalopram,c clomipramine,a,b desipramine,a,b escitalopram,c fluoxetine,a fluvoxamine,c imipramine,a,b nortriptyline,a,b sertraline,c paroxetine,c trazodone,a venlafaxinea Antipsychotics: Aripiprazole,a haloperidol,a perphenazine,a risperidonea | None |
| aSerum level may be too high, lower doses may be required bSerum levels may be outside of optimal range cGenotype suggests less than optimal response | ||
The authors’ observations
Mrs. C’s genotype might explain some sensitivity to medications metabolized by CYP2D6 (eg, venlafaxine, paroxetine, fluoxetine), but does not explain her acute sensitivity to all of the medications she has taken. For example, she is an extensive metabolizer for CYP2C19, which metabolizes escitalopram; therefore, it is unlikely escitalopram, 2.5 mg/d, would result in high blood levels and side effects.3 Regardless of the next step in treatment, we deemed her somatic obsessions to be the most important clinical issue. It seems unlikely that Mrs. C would adhere to any medication regimen until this underlying problem was addressed.
The focus of treatment shifted to Mrs. C’s obsessions about her medications and their side effects. Mrs. C was fixated on the content of her obsessions (eg, medications, side effects) rather than the process of her obsessional thinking. The goal was to help Mrs. C identify, label, and ultimately create distance from her obsessive thoughts associated with side effects. The treatment team employed an acceptance and commitment therapy (ACT) model of observing and defusing thoughts in the inpatient setting (Table 3).4 ACT is based on mindfulness and committed, values-based action.5 When patients are “fused” with their thoughts, they believe these thoughts are important and representative of reality. In Mrs. C’s case, she fused with the concept that her medications were making her sick and the idea that she may have BD. The treatment team thought these fused thoughts were the major problem that resulted in 10 months of protracted illness.
Conversely, in a “defused” state, patients can separate from their thoughts and observe them as disparate sounds, words, stories, or bits of language. The goal is to observe and allow the patient’s thoughts to simply be thoughts rather than trying to determine if they are “true.” Mrs. C was fused with the idea that her medications were making her ill, so this belief became the story underlying her obsessional thinking. Helping her disengage from this story became the focus of her treatment.
Table 3
6 core principles of acceptance and commitment therapy
| Defusion | Learning to step back and observe thoughts as separate from the self |
| Acceptance | Allowing unpleasant thoughts to come and go without trying to control them |
| Contact with the present moment | Full awareness and engagement with present experiences |
| Observing the present self | Accessing a transcendent sense of self |
| Values | Clarifying what is most important to the patient |
| Committed action | Setting goals and taking action to achieve them |
| Source: Reference 4 | |
Results guide pharmacotherapy
In addition to helping change the focus of Mrs. C’s psychotherapy, we used the pharmacogenomic results to guide medication treatment. We initially prescribed fluvoxamine, 50 mg/d, because her partially compromised CYP2D6 pathway probably would play only a minor role in metabolizing the drug.1 Smoking induces CYP1A2, which is fluvoxamine’s primary metabolic pathway; however, Mrs. C does not smoke.6 When we saw Mrs. C in January 2009, the author (JGW) was unaware of any available genetic testing for CYP1A2, although now such testing is clinically available.
Mirtazapine is in the “use as directed” category for Mrs. C’s genotype (Table 2) and was the only medication she had adhered to at a therapeutic dose for more than a few days. However, she indicated that she would not adhere to this medication if we prescribed it again. Duloxetine also is in the “use as directed” category; however, given the entire clinical picture, we chose fluvoxamine because of Mrs. C’s obsessive symptomatology and because she had never reached a therapeutic dose of a selective serotonin reuptake inhibitor.
OUTCOME: Obsessions abate
Given Mrs. C’s lack of insight, we initiate a family approach to help broach the topic of obsessions as the focus of treatment. With her husband’s help, she participates in defusion techniques as an inpatient and follows up with an acceptance-based psychotherapist after discharge. After we share the pharmacogenomic information with Mrs. C, she agrees to try fluvoxamine, which is titrated to 100 mg/d. She maintains this dose at her 4-week follow-up visit. Notably, this was only the second time Mrs. C adhered to a medication trial since illness onset. Upon admission, Mrs. C had an HRSD-17 score of 30, indicating severe depression; at 4 weeks, her HRSD-17 score is 8, indicating mild depression.
The authors’ observations
In a complementary case, the author (JGW) consulted on a patient who was taking paroxetine and experiencing anorgasmia, weight gain, and loss of libido. Pharmacogenomic testing revealed that the patient was a poor metabolizer of CYP2D6. Paroxetine is substantially metabolized by CYP2D6; therefore, it was likely that high blood levels were contributing to the side effects.3,7 The key clinical distinction is that although this patient was bothered by intrusive side effects, he was not fixated on them like Mrs. C. His pharmacogenomic test results were used to identify a metabolic issue that was causing the side effects. This is in contrast with Mrs. C, for whom the pharmacogenomic information ruled out a metabolic issue as the primary problem and helped guide the next step in treatment.
Mrs. C’s case illustrates how pharmacogenomics and ACT complemented each other to create a desirable outcome. Pharmacogenomic testing originally was developed as a safety mechanism for medication choice and dosing, but clinical applications have grown as astute clinicians utilize it to help care for their patients.8 ACT can be a powerful tool for patients who have difficulties creating distance from their thoughts. Both pharmacogenomic testing and ACT are noninvasive interventions that can be implemented as part of a multi-faceted treatment approach.
Related Resources
- Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: The process and practice of mindful change. 2nd ed. New York, NY: The Guilford Press; 2011.
- Mrazek DA. Psychiatric pharmacogenomics. New York, NY: Oxford University Press; 2010.
Drug Brand Names
- Amitriptyline • Elavil
- Aripiprazole • Abilify
- Bupropion • Wellbutrin, Zyban
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clozapine • Clozaril
- Desipramine • Norpramin
- Diazepam • Valium
- Doxepin • Adapin, Silenor
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Haloperidol • Haldol
- Imipramine • Tofranil
- Lithium • Eskalith, Lithobid
- Mirtazapine • Remeron
- Olanzapine • Zyprexa
- Nortriptyline • Pamelor
- Paroxetine • Paxil
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Trazodone • Desyrel, Oleptro
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosure
The authors are employed by AssureRx Health, Inc., the provider of the pharmacogenomic testing used in this article.
1. Mrazek DA. Psychiatric pharmacogenomics. New York, NY: Oxford University Press; 2010.
2. Kirchheiner J, Nickchen K, Bauer M, et al. Pharmacogenetics of antidepressants and antipsychotics: the contribution of allelic variations to the phenotype of drug response. Mol Psychiatry. 2004;9(5):442-473.
3. Kircheiner J, Brøsen K, Dahl ML, et al. CYP2D6 and CYP2C19 genotype-based dose recommendations for antidepressants: a first step towards subpopulation-specific dosages. Acta Psychiatr Scand. 2001;104(3):173-192.
4. Harris R. Embracing your demons: an overview of acceptance and commitment therapy. Psychotherapy in Australia. 2006;12(4):2-8.
5. Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: an experiential approach to behavior change. New York, NY: Guilford Press; 2003.
6. Luvox CR [package insert] Palo Alto CA: Jazz Pharmaceuticals, Inc.; 2011.
7. Kaneda Y, Kawamura I, Fujii A, et al. Serotonin syndrome– ‘potential’ role of the CYP2D6 genetic polymorphism in Asians. Int J Neuropsychopharmacol. 2002;5(1):105-106.
8. Kung S, Li X. The clinical use of pharmacogenomic testing in treatment-resistant depression. Primary Psychiatry. 2010;17(5):46-51.
CASE: Medication sensitivity
Mrs. C, age 48, is admitted to a tertiary care inpatient mood disorder unit for evaluation of severe depression characterized by depressed mood, anhedonia, and insomnia. Her initial Hamilton Rating Scale for Depression 17-Item (HRSD-17) score is 30, indicating severe depression. Her medications are fluoxetine, 10 mg/d, and diazepam, 0.5 mg/d.
Mrs. C describes a 10-month history of depression and extreme anxiety in the context of several psychosocial stressors. Her father recently died and she is having difficulty with the demands of administering her father’s estate. She is intensely obsessive and focused on nihilistic themes, her diagnosis, somatic themes, and medications side effects. Her husband confirms our observations. No history or current symptoms of typical compulsions (eg, washing hands or checking doors) are elicited. She has limited insight into her obsessive tendencies.
Mrs. C had no psychiatric history before her depressive and obsessive symptoms developed 10 months ago. However, in the past 10 months, she has been hospitalized in a psychiatric facility twice. She also received a series of 8 electroconvulsive therapy treatments, but reported minimal improvement of her depressive symptoms. Mrs. C had a few cognitive-behavioral therapy (CBT) sessions with a psychotherapist, but she said they didn’t help much.
Mrs. C has substantial difficulty adhering to medications, even at subtherapeutic doses. She states she is “extremely sensitive” to all medications. Mrs. C says she develops dizziness, increased anxiety, insomnia, nausea, and other vague reactions whenever she attempts to increase her psychotropics to therapeutic doses. She took sertraline, 10 mg/d, for 4 days, but discontinued it because of unspecified side effects. She then received escitalopram, 2.5 mg/d, for 10 days, but again stopped it because of vague side effects. She was taking paroxetine, 10 mg/d, for 2 days, but experienced vomiting and discontinued the drug. She tried venlafaxine at a low dose and also discontinued it because of vomiting. Mrs. C stayed on mirtazapine, 22.5 mg/d, for 3 months, but stopped it because of lack of efficacy and she was unwilling to increase the dose. Other unsuccessful trials include citalopram and doxepin. Mrs. C is hesitant to try another medication or increase to therapeutic doses any of the previous medications.
The authors’ observations
Before initiating another treatment, the treatment team considered Mrs. C’s pervasive medication intolerance. Her enzymatic activity may be genetically compromised, which could lead to high blood levels of medications and significant side effects when she takes very low doses. Individual variations in response to psychotropics are influenced by genetic factors.1 Variants in the cytochrome P450 (CYP450) genes produce enzymes with increased activity, normal activity, reduced activity, or no activity, creating phenotypes of ultrarapid metabolizers, extensive metabolizers, intermediate metabolizers, and poor metabolizers, respectively. These genetic variations can affect blood levels of medications that employ these enzymes in their metabolic pathways.2 Mrs. C could be a poor metabolizer of common CYP450 variant enzymes, which led to her exquisite sensitivity to psychotropics. We felt this was a reasonable hypothesis given her tumultuous 10-month course of psychiatric treatment and multiple failed medication trials.
An alternative hypothesis is that Mrs. C’s somatic obsessions about drug side effects were the primary clinical issue that led to her severe medication intolerance. Mrs. C spends hours questioning the inpatient staff about her diagnosis (eg, “Are you sure I don’t have bipolar disorder?”), medications (eg, “Are you sure this medication won’t make me sick?”), somatic themes (eg, “Are you sure I don’t have Meniere’s disease with all my dizziness?”), and nihilistic themes (eg, “What if I never get better?”). Mrs. C’s husband attested that she has spent hours researching her new medications on the Internet and reading the medication handouts from the pharmacy. She admits to mentally cycling through the DSM-IV-TR criteria for hours at a time to “figure out” if she has bipolar disorder (BD).
We initiated pharmacogenomic testing to help distinguish between these hypotheses. Mrs. C’s results are presented in Table 1. Genotype results were applied using an interpretive algorithm (Figure) in which 26 psychiatric medications were placed in categories of “use as directed” (green column), “use with caution” (yellow column), and “use with caution or more frequent monitoring” (red column). The algorithm incorporates the genetic information with the known pharmacologic profile for each of the medications in the panel. Highlights of Mrs. C’s interpretive report are shown in Table 2.
Table 1
Mrs. C’s genotype results
| Gene | Allele | Predicted phenotype |
|---|---|---|
| CYP2D6 | *1/*4 | Intermediate metabolizer |
| CYP2C19 | *1/*1 | Extensive metabolizer |
| SLC6A4 | S/S | Low activity |
| 5HTR2A | G/G | Reduced activity |
Figure
Genotype-phenotype integration into Mrs. C’s interpretive report
Table 2
Mrs. C’s pharmacogenomic-based interpretive report
| Use as directed | Use with caution | Use with caution and more frequent monitoring |
|---|---|---|
| Antidepressants: Duloxetine, mirtazapine Antipsychotics: Clozapine, olanzapine, quetiapine, ziprasidone | Antidepressants: Amitriptyline,a,b bupropion,a citalopram,c clomipramine,a,b desipramine,a,b escitalopram,c fluoxetine,a fluvoxamine,c imipramine,a,b nortriptyline,a,b sertraline,c paroxetine,c trazodone,a venlafaxinea Antipsychotics: Aripiprazole,a haloperidol,a perphenazine,a risperidonea | None |
| aSerum level may be too high, lower doses may be required bSerum levels may be outside of optimal range cGenotype suggests less than optimal response | ||
The authors’ observations
Mrs. C’s genotype might explain some sensitivity to medications metabolized by CYP2D6 (eg, venlafaxine, paroxetine, fluoxetine), but does not explain her acute sensitivity to all of the medications she has taken. For example, she is an extensive metabolizer for CYP2C19, which metabolizes escitalopram; therefore, it is unlikely escitalopram, 2.5 mg/d, would result in high blood levels and side effects.3 Regardless of the next step in treatment, we deemed her somatic obsessions to be the most important clinical issue. It seems unlikely that Mrs. C would adhere to any medication regimen until this underlying problem was addressed.
The focus of treatment shifted to Mrs. C’s obsessions about her medications and their side effects. Mrs. C was fixated on the content of her obsessions (eg, medications, side effects) rather than the process of her obsessional thinking. The goal was to help Mrs. C identify, label, and ultimately create distance from her obsessive thoughts associated with side effects. The treatment team employed an acceptance and commitment therapy (ACT) model of observing and defusing thoughts in the inpatient setting (Table 3).4 ACT is based on mindfulness and committed, values-based action.5 When patients are “fused” with their thoughts, they believe these thoughts are important and representative of reality. In Mrs. C’s case, she fused with the concept that her medications were making her sick and the idea that she may have BD. The treatment team thought these fused thoughts were the major problem that resulted in 10 months of protracted illness.
Conversely, in a “defused” state, patients can separate from their thoughts and observe them as disparate sounds, words, stories, or bits of language. The goal is to observe and allow the patient’s thoughts to simply be thoughts rather than trying to determine if they are “true.” Mrs. C was fused with the idea that her medications were making her ill, so this belief became the story underlying her obsessional thinking. Helping her disengage from this story became the focus of her treatment.
Table 3
6 core principles of acceptance and commitment therapy
| Defusion | Learning to step back and observe thoughts as separate from the self |
| Acceptance | Allowing unpleasant thoughts to come and go without trying to control them |
| Contact with the present moment | Full awareness and engagement with present experiences |
| Observing the present self | Accessing a transcendent sense of self |
| Values | Clarifying what is most important to the patient |
| Committed action | Setting goals and taking action to achieve them |
| Source: Reference 4 | |
Results guide pharmacotherapy
In addition to helping change the focus of Mrs. C’s psychotherapy, we used the pharmacogenomic results to guide medication treatment. We initially prescribed fluvoxamine, 50 mg/d, because her partially compromised CYP2D6 pathway probably would play only a minor role in metabolizing the drug.1 Smoking induces CYP1A2, which is fluvoxamine’s primary metabolic pathway; however, Mrs. C does not smoke.6 When we saw Mrs. C in January 2009, the author (JGW) was unaware of any available genetic testing for CYP1A2, although now such testing is clinically available.
Mirtazapine is in the “use as directed” category for Mrs. C’s genotype (Table 2) and was the only medication she had adhered to at a therapeutic dose for more than a few days. However, she indicated that she would not adhere to this medication if we prescribed it again. Duloxetine also is in the “use as directed” category; however, given the entire clinical picture, we chose fluvoxamine because of Mrs. C’s obsessive symptomatology and because she had never reached a therapeutic dose of a selective serotonin reuptake inhibitor.
OUTCOME: Obsessions abate
Given Mrs. C’s lack of insight, we initiate a family approach to help broach the topic of obsessions as the focus of treatment. With her husband’s help, she participates in defusion techniques as an inpatient and follows up with an acceptance-based psychotherapist after discharge. After we share the pharmacogenomic information with Mrs. C, she agrees to try fluvoxamine, which is titrated to 100 mg/d. She maintains this dose at her 4-week follow-up visit. Notably, this was only the second time Mrs. C adhered to a medication trial since illness onset. Upon admission, Mrs. C had an HRSD-17 score of 30, indicating severe depression; at 4 weeks, her HRSD-17 score is 8, indicating mild depression.
The authors’ observations
In a complementary case, the author (JGW) consulted on a patient who was taking paroxetine and experiencing anorgasmia, weight gain, and loss of libido. Pharmacogenomic testing revealed that the patient was a poor metabolizer of CYP2D6. Paroxetine is substantially metabolized by CYP2D6; therefore, it was likely that high blood levels were contributing to the side effects.3,7 The key clinical distinction is that although this patient was bothered by intrusive side effects, he was not fixated on them like Mrs. C. His pharmacogenomic test results were used to identify a metabolic issue that was causing the side effects. This is in contrast with Mrs. C, for whom the pharmacogenomic information ruled out a metabolic issue as the primary problem and helped guide the next step in treatment.
Mrs. C’s case illustrates how pharmacogenomics and ACT complemented each other to create a desirable outcome. Pharmacogenomic testing originally was developed as a safety mechanism for medication choice and dosing, but clinical applications have grown as astute clinicians utilize it to help care for their patients.8 ACT can be a powerful tool for patients who have difficulties creating distance from their thoughts. Both pharmacogenomic testing and ACT are noninvasive interventions that can be implemented as part of a multi-faceted treatment approach.
Related Resources
- Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: The process and practice of mindful change. 2nd ed. New York, NY: The Guilford Press; 2011.
- Mrazek DA. Psychiatric pharmacogenomics. New York, NY: Oxford University Press; 2010.
Drug Brand Names
- Amitriptyline • Elavil
- Aripiprazole • Abilify
- Bupropion • Wellbutrin, Zyban
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clozapine • Clozaril
- Desipramine • Norpramin
- Diazepam • Valium
- Doxepin • Adapin, Silenor
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Haloperidol • Haldol
- Imipramine • Tofranil
- Lithium • Eskalith, Lithobid
- Mirtazapine • Remeron
- Olanzapine • Zyprexa
- Nortriptyline • Pamelor
- Paroxetine • Paxil
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Trazodone • Desyrel, Oleptro
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosure
The authors are employed by AssureRx Health, Inc., the provider of the pharmacogenomic testing used in this article.
CASE: Medication sensitivity
Mrs. C, age 48, is admitted to a tertiary care inpatient mood disorder unit for evaluation of severe depression characterized by depressed mood, anhedonia, and insomnia. Her initial Hamilton Rating Scale for Depression 17-Item (HRSD-17) score is 30, indicating severe depression. Her medications are fluoxetine, 10 mg/d, and diazepam, 0.5 mg/d.
Mrs. C describes a 10-month history of depression and extreme anxiety in the context of several psychosocial stressors. Her father recently died and she is having difficulty with the demands of administering her father’s estate. She is intensely obsessive and focused on nihilistic themes, her diagnosis, somatic themes, and medications side effects. Her husband confirms our observations. No history or current symptoms of typical compulsions (eg, washing hands or checking doors) are elicited. She has limited insight into her obsessive tendencies.
Mrs. C had no psychiatric history before her depressive and obsessive symptoms developed 10 months ago. However, in the past 10 months, she has been hospitalized in a psychiatric facility twice. She also received a series of 8 electroconvulsive therapy treatments, but reported minimal improvement of her depressive symptoms. Mrs. C had a few cognitive-behavioral therapy (CBT) sessions with a psychotherapist, but she said they didn’t help much.
Mrs. C has substantial difficulty adhering to medications, even at subtherapeutic doses. She states she is “extremely sensitive” to all medications. Mrs. C says she develops dizziness, increased anxiety, insomnia, nausea, and other vague reactions whenever she attempts to increase her psychotropics to therapeutic doses. She took sertraline, 10 mg/d, for 4 days, but discontinued it because of unspecified side effects. She then received escitalopram, 2.5 mg/d, for 10 days, but again stopped it because of vague side effects. She was taking paroxetine, 10 mg/d, for 2 days, but experienced vomiting and discontinued the drug. She tried venlafaxine at a low dose and also discontinued it because of vomiting. Mrs. C stayed on mirtazapine, 22.5 mg/d, for 3 months, but stopped it because of lack of efficacy and she was unwilling to increase the dose. Other unsuccessful trials include citalopram and doxepin. Mrs. C is hesitant to try another medication or increase to therapeutic doses any of the previous medications.
The authors’ observations
Before initiating another treatment, the treatment team considered Mrs. C’s pervasive medication intolerance. Her enzymatic activity may be genetically compromised, which could lead to high blood levels of medications and significant side effects when she takes very low doses. Individual variations in response to psychotropics are influenced by genetic factors.1 Variants in the cytochrome P450 (CYP450) genes produce enzymes with increased activity, normal activity, reduced activity, or no activity, creating phenotypes of ultrarapid metabolizers, extensive metabolizers, intermediate metabolizers, and poor metabolizers, respectively. These genetic variations can affect blood levels of medications that employ these enzymes in their metabolic pathways.2 Mrs. C could be a poor metabolizer of common CYP450 variant enzymes, which led to her exquisite sensitivity to psychotropics. We felt this was a reasonable hypothesis given her tumultuous 10-month course of psychiatric treatment and multiple failed medication trials.
An alternative hypothesis is that Mrs. C’s somatic obsessions about drug side effects were the primary clinical issue that led to her severe medication intolerance. Mrs. C spends hours questioning the inpatient staff about her diagnosis (eg, “Are you sure I don’t have bipolar disorder?”), medications (eg, “Are you sure this medication won’t make me sick?”), somatic themes (eg, “Are you sure I don’t have Meniere’s disease with all my dizziness?”), and nihilistic themes (eg, “What if I never get better?”). Mrs. C’s husband attested that she has spent hours researching her new medications on the Internet and reading the medication handouts from the pharmacy. She admits to mentally cycling through the DSM-IV-TR criteria for hours at a time to “figure out” if she has bipolar disorder (BD).
We initiated pharmacogenomic testing to help distinguish between these hypotheses. Mrs. C’s results are presented in Table 1. Genotype results were applied using an interpretive algorithm (Figure) in which 26 psychiatric medications were placed in categories of “use as directed” (green column), “use with caution” (yellow column), and “use with caution or more frequent monitoring” (red column). The algorithm incorporates the genetic information with the known pharmacologic profile for each of the medications in the panel. Highlights of Mrs. C’s interpretive report are shown in Table 2.
Table 1
Mrs. C’s genotype results
| Gene | Allele | Predicted phenotype |
|---|---|---|
| CYP2D6 | *1/*4 | Intermediate metabolizer |
| CYP2C19 | *1/*1 | Extensive metabolizer |
| SLC6A4 | S/S | Low activity |
| 5HTR2A | G/G | Reduced activity |
Figure
Genotype-phenotype integration into Mrs. C’s interpretive report
Table 2
Mrs. C’s pharmacogenomic-based interpretive report
| Use as directed | Use with caution | Use with caution and more frequent monitoring |
|---|---|---|
| Antidepressants: Duloxetine, mirtazapine Antipsychotics: Clozapine, olanzapine, quetiapine, ziprasidone | Antidepressants: Amitriptyline,a,b bupropion,a citalopram,c clomipramine,a,b desipramine,a,b escitalopram,c fluoxetine,a fluvoxamine,c imipramine,a,b nortriptyline,a,b sertraline,c paroxetine,c trazodone,a venlafaxinea Antipsychotics: Aripiprazole,a haloperidol,a perphenazine,a risperidonea | None |
| aSerum level may be too high, lower doses may be required bSerum levels may be outside of optimal range cGenotype suggests less than optimal response | ||
The authors’ observations
Mrs. C’s genotype might explain some sensitivity to medications metabolized by CYP2D6 (eg, venlafaxine, paroxetine, fluoxetine), but does not explain her acute sensitivity to all of the medications she has taken. For example, she is an extensive metabolizer for CYP2C19, which metabolizes escitalopram; therefore, it is unlikely escitalopram, 2.5 mg/d, would result in high blood levels and side effects.3 Regardless of the next step in treatment, we deemed her somatic obsessions to be the most important clinical issue. It seems unlikely that Mrs. C would adhere to any medication regimen until this underlying problem was addressed.
The focus of treatment shifted to Mrs. C’s obsessions about her medications and their side effects. Mrs. C was fixated on the content of her obsessions (eg, medications, side effects) rather than the process of her obsessional thinking. The goal was to help Mrs. C identify, label, and ultimately create distance from her obsessive thoughts associated with side effects. The treatment team employed an acceptance and commitment therapy (ACT) model of observing and defusing thoughts in the inpatient setting (Table 3).4 ACT is based on mindfulness and committed, values-based action.5 When patients are “fused” with their thoughts, they believe these thoughts are important and representative of reality. In Mrs. C’s case, she fused with the concept that her medications were making her sick and the idea that she may have BD. The treatment team thought these fused thoughts were the major problem that resulted in 10 months of protracted illness.
Conversely, in a “defused” state, patients can separate from their thoughts and observe them as disparate sounds, words, stories, or bits of language. The goal is to observe and allow the patient’s thoughts to simply be thoughts rather than trying to determine if they are “true.” Mrs. C was fused with the idea that her medications were making her ill, so this belief became the story underlying her obsessional thinking. Helping her disengage from this story became the focus of her treatment.
Table 3
6 core principles of acceptance and commitment therapy
| Defusion | Learning to step back and observe thoughts as separate from the self |
| Acceptance | Allowing unpleasant thoughts to come and go without trying to control them |
| Contact with the present moment | Full awareness and engagement with present experiences |
| Observing the present self | Accessing a transcendent sense of self |
| Values | Clarifying what is most important to the patient |
| Committed action | Setting goals and taking action to achieve them |
| Source: Reference 4 | |
Results guide pharmacotherapy
In addition to helping change the focus of Mrs. C’s psychotherapy, we used the pharmacogenomic results to guide medication treatment. We initially prescribed fluvoxamine, 50 mg/d, because her partially compromised CYP2D6 pathway probably would play only a minor role in metabolizing the drug.1 Smoking induces CYP1A2, which is fluvoxamine’s primary metabolic pathway; however, Mrs. C does not smoke.6 When we saw Mrs. C in January 2009, the author (JGW) was unaware of any available genetic testing for CYP1A2, although now such testing is clinically available.
Mirtazapine is in the “use as directed” category for Mrs. C’s genotype (Table 2) and was the only medication she had adhered to at a therapeutic dose for more than a few days. However, she indicated that she would not adhere to this medication if we prescribed it again. Duloxetine also is in the “use as directed” category; however, given the entire clinical picture, we chose fluvoxamine because of Mrs. C’s obsessive symptomatology and because she had never reached a therapeutic dose of a selective serotonin reuptake inhibitor.
OUTCOME: Obsessions abate
Given Mrs. C’s lack of insight, we initiate a family approach to help broach the topic of obsessions as the focus of treatment. With her husband’s help, she participates in defusion techniques as an inpatient and follows up with an acceptance-based psychotherapist after discharge. After we share the pharmacogenomic information with Mrs. C, she agrees to try fluvoxamine, which is titrated to 100 mg/d. She maintains this dose at her 4-week follow-up visit. Notably, this was only the second time Mrs. C adhered to a medication trial since illness onset. Upon admission, Mrs. C had an HRSD-17 score of 30, indicating severe depression; at 4 weeks, her HRSD-17 score is 8, indicating mild depression.
The authors’ observations
In a complementary case, the author (JGW) consulted on a patient who was taking paroxetine and experiencing anorgasmia, weight gain, and loss of libido. Pharmacogenomic testing revealed that the patient was a poor metabolizer of CYP2D6. Paroxetine is substantially metabolized by CYP2D6; therefore, it was likely that high blood levels were contributing to the side effects.3,7 The key clinical distinction is that although this patient was bothered by intrusive side effects, he was not fixated on them like Mrs. C. His pharmacogenomic test results were used to identify a metabolic issue that was causing the side effects. This is in contrast with Mrs. C, for whom the pharmacogenomic information ruled out a metabolic issue as the primary problem and helped guide the next step in treatment.
Mrs. C’s case illustrates how pharmacogenomics and ACT complemented each other to create a desirable outcome. Pharmacogenomic testing originally was developed as a safety mechanism for medication choice and dosing, but clinical applications have grown as astute clinicians utilize it to help care for their patients.8 ACT can be a powerful tool for patients who have difficulties creating distance from their thoughts. Both pharmacogenomic testing and ACT are noninvasive interventions that can be implemented as part of a multi-faceted treatment approach.
Related Resources
- Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: The process and practice of mindful change. 2nd ed. New York, NY: The Guilford Press; 2011.
- Mrazek DA. Psychiatric pharmacogenomics. New York, NY: Oxford University Press; 2010.
Drug Brand Names
- Amitriptyline • Elavil
- Aripiprazole • Abilify
- Bupropion • Wellbutrin, Zyban
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clozapine • Clozaril
- Desipramine • Norpramin
- Diazepam • Valium
- Doxepin • Adapin, Silenor
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Haloperidol • Haldol
- Imipramine • Tofranil
- Lithium • Eskalith, Lithobid
- Mirtazapine • Remeron
- Olanzapine • Zyprexa
- Nortriptyline • Pamelor
- Paroxetine • Paxil
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Trazodone • Desyrel, Oleptro
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosure
The authors are employed by AssureRx Health, Inc., the provider of the pharmacogenomic testing used in this article.
1. Mrazek DA. Psychiatric pharmacogenomics. New York, NY: Oxford University Press; 2010.
2. Kirchheiner J, Nickchen K, Bauer M, et al. Pharmacogenetics of antidepressants and antipsychotics: the contribution of allelic variations to the phenotype of drug response. Mol Psychiatry. 2004;9(5):442-473.
3. Kircheiner J, Brøsen K, Dahl ML, et al. CYP2D6 and CYP2C19 genotype-based dose recommendations for antidepressants: a first step towards subpopulation-specific dosages. Acta Psychiatr Scand. 2001;104(3):173-192.
4. Harris R. Embracing your demons: an overview of acceptance and commitment therapy. Psychotherapy in Australia. 2006;12(4):2-8.
5. Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: an experiential approach to behavior change. New York, NY: Guilford Press; 2003.
6. Luvox CR [package insert] Palo Alto CA: Jazz Pharmaceuticals, Inc.; 2011.
7. Kaneda Y, Kawamura I, Fujii A, et al. Serotonin syndrome– ‘potential’ role of the CYP2D6 genetic polymorphism in Asians. Int J Neuropsychopharmacol. 2002;5(1):105-106.
8. Kung S, Li X. The clinical use of pharmacogenomic testing in treatment-resistant depression. Primary Psychiatry. 2010;17(5):46-51.
1. Mrazek DA. Psychiatric pharmacogenomics. New York, NY: Oxford University Press; 2010.
2. Kirchheiner J, Nickchen K, Bauer M, et al. Pharmacogenetics of antidepressants and antipsychotics: the contribution of allelic variations to the phenotype of drug response. Mol Psychiatry. 2004;9(5):442-473.
3. Kircheiner J, Brøsen K, Dahl ML, et al. CYP2D6 and CYP2C19 genotype-based dose recommendations for antidepressants: a first step towards subpopulation-specific dosages. Acta Psychiatr Scand. 2001;104(3):173-192.
4. Harris R. Embracing your demons: an overview of acceptance and commitment therapy. Psychotherapy in Australia. 2006;12(4):2-8.
5. Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: an experiential approach to behavior change. New York, NY: Guilford Press; 2003.
6. Luvox CR [package insert] Palo Alto CA: Jazz Pharmaceuticals, Inc.; 2011.
7. Kaneda Y, Kawamura I, Fujii A, et al. Serotonin syndrome– ‘potential’ role of the CYP2D6 genetic polymorphism in Asians. Int J Neuropsychopharmacol. 2002;5(1):105-106.
8. Kung S, Li X. The clinical use of pharmacogenomic testing in treatment-resistant depression. Primary Psychiatry. 2010;17(5):46-51.