User login
Overwhelmed by side effects
CASE: Medication sensitivity
Mrs. C, age 48, is admitted to a tertiary care inpatient mood disorder unit for evaluation of severe depression characterized by depressed mood, anhedonia, and insomnia. Her initial Hamilton Rating Scale for Depression 17-Item (HRSD-17) score is 30, indicating severe depression. Her medications are fluoxetine, 10 mg/d, and diazepam, 0.5 mg/d.
Mrs. C describes a 10-month history of depression and extreme anxiety in the context of several psychosocial stressors. Her father recently died and she is having difficulty with the demands of administering her father’s estate. She is intensely obsessive and focused on nihilistic themes, her diagnosis, somatic themes, and medications side effects. Her husband confirms our observations. No history or current symptoms of typical compulsions (eg, washing hands or checking doors) are elicited. She has limited insight into her obsessive tendencies.
Mrs. C had no psychiatric history before her depressive and obsessive symptoms developed 10 months ago. However, in the past 10 months, she has been hospitalized in a psychiatric facility twice. She also received a series of 8 electroconvulsive therapy treatments, but reported minimal improvement of her depressive symptoms. Mrs. C had a few cognitive-behavioral therapy (CBT) sessions with a psychotherapist, but she said they didn’t help much.
Mrs. C has substantial difficulty adhering to medications, even at subtherapeutic doses. She states she is “extremely sensitive” to all medications. Mrs. C says she develops dizziness, increased anxiety, insomnia, nausea, and other vague reactions whenever she attempts to increase her psychotropics to therapeutic doses. She took sertraline, 10 mg/d, for 4 days, but discontinued it because of unspecified side effects. She then received escitalopram, 2.5 mg/d, for 10 days, but again stopped it because of vague side effects. She was taking paroxetine, 10 mg/d, for 2 days, but experienced vomiting and discontinued the drug. She tried venlafaxine at a low dose and also discontinued it because of vomiting. Mrs. C stayed on mirtazapine, 22.5 mg/d, for 3 months, but stopped it because of lack of efficacy and she was unwilling to increase the dose. Other unsuccessful trials include citalopram and doxepin. Mrs. C is hesitant to try another medication or increase to therapeutic doses any of the previous medications.
The authors’ observations
Before initiating another treatment, the treatment team considered Mrs. C’s pervasive medication intolerance. Her enzymatic activity may be genetically compromised, which could lead to high blood levels of medications and significant side effects when she takes very low doses. Individual variations in response to psychotropics are influenced by genetic factors.1 Variants in the cytochrome P450 (CYP450) genes produce enzymes with increased activity, normal activity, reduced activity, or no activity, creating phenotypes of ultrarapid metabolizers, extensive metabolizers, intermediate metabolizers, and poor metabolizers, respectively. These genetic variations can affect blood levels of medications that employ these enzymes in their metabolic pathways.2 Mrs. C could be a poor metabolizer of common CYP450 variant enzymes, which led to her exquisite sensitivity to psychotropics. We felt this was a reasonable hypothesis given her tumultuous 10-month course of psychiatric treatment and multiple failed medication trials.
An alternative hypothesis is that Mrs. C’s somatic obsessions about drug side effects were the primary clinical issue that led to her severe medication intolerance. Mrs. C spends hours questioning the inpatient staff about her diagnosis (eg, “Are you sure I don’t have bipolar disorder?”), medications (eg, “Are you sure this medication won’t make me sick?”), somatic themes (eg, “Are you sure I don’t have Meniere’s disease with all my dizziness?”), and nihilistic themes (eg, “What if I never get better?”). Mrs. C’s husband attested that she has spent hours researching her new medications on the Internet and reading the medication handouts from the pharmacy. She admits to mentally cycling through the DSM-IV-TR criteria for hours at a time to “figure out” if she has bipolar disorder (BD).
We initiated pharmacogenomic testing to help distinguish between these hypotheses. Mrs. C’s results are presented in Table 1. Genotype results were applied using an interpretive algorithm (Figure) in which 26 psychiatric medications were placed in categories of “use as directed” (green column), “use with caution” (yellow column), and “use with caution or more frequent monitoring” (red column). The algorithm incorporates the genetic information with the known pharmacologic profile for each of the medications in the panel. Highlights of Mrs. C’s interpretive report are shown in Table 2.
Table 1
Mrs. C’s genotype results
| Gene | Allele | Predicted phenotype |
|---|---|---|
| CYP2D6 | *1/*4 | Intermediate metabolizer |
| CYP2C19 | *1/*1 | Extensive metabolizer |
| SLC6A4 | S/S | Low activity |
| 5HTR2A | G/G | Reduced activity |
Figure
Genotype-phenotype integration into Mrs. C’s interpretive report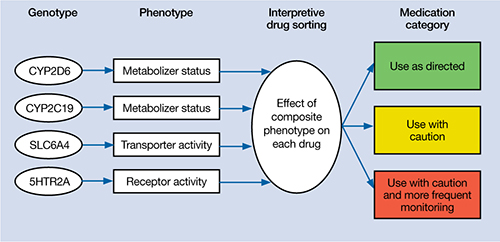
Table 2
Mrs. C’s pharmacogenomic-based interpretive report
| Use as directed | Use with caution | Use with caution and more frequent monitoring |
|---|---|---|
| Antidepressants: Duloxetine, mirtazapine Antipsychotics: Clozapine, olanzapine, quetiapine, ziprasidone | Antidepressants: Amitriptyline,a,b bupropion,a citalopram,c clomipramine,a,b desipramine,a,b escitalopram,c fluoxetine,a fluvoxamine,c imipramine,a,b nortriptyline,a,b sertraline,c paroxetine,c trazodone,a venlafaxinea Antipsychotics: Aripiprazole,a haloperidol,a perphenazine,a risperidonea | None |
| aSerum level may be too high, lower doses may be required bSerum levels may be outside of optimal range cGenotype suggests less than optimal response | ||
The authors’ observations
Mrs. C’s genotype might explain some sensitivity to medications metabolized by CYP2D6 (eg, venlafaxine, paroxetine, fluoxetine), but does not explain her acute sensitivity to all of the medications she has taken. For example, she is an extensive metabolizer for CYP2C19, which metabolizes escitalopram; therefore, it is unlikely escitalopram, 2.5 mg/d, would result in high blood levels and side effects.3 Regardless of the next step in treatment, we deemed her somatic obsessions to be the most important clinical issue. It seems unlikely that Mrs. C would adhere to any medication regimen until this underlying problem was addressed.
The focus of treatment shifted to Mrs. C’s obsessions about her medications and their side effects. Mrs. C was fixated on the content of her obsessions (eg, medications, side effects) rather than the process of her obsessional thinking. The goal was to help Mrs. C identify, label, and ultimately create distance from her obsessive thoughts associated with side effects. The treatment team employed an acceptance and commitment therapy (ACT) model of observing and defusing thoughts in the inpatient setting (Table 3).4 ACT is based on mindfulness and committed, values-based action.5 When patients are “fused” with their thoughts, they believe these thoughts are important and representative of reality. In Mrs. C’s case, she fused with the concept that her medications were making her sick and the idea that she may have BD. The treatment team thought these fused thoughts were the major problem that resulted in 10 months of protracted illness.
Conversely, in a “defused” state, patients can separate from their thoughts and observe them as disparate sounds, words, stories, or bits of language. The goal is to observe and allow the patient’s thoughts to simply be thoughts rather than trying to determine if they are “true.” Mrs. C was fused with the idea that her medications were making her ill, so this belief became the story underlying her obsessional thinking. Helping her disengage from this story became the focus of her treatment.
Table 3
6 core principles of acceptance and commitment therapy
| Defusion | Learning to step back and observe thoughts as separate from the self |
| Acceptance | Allowing unpleasant thoughts to come and go without trying to control them |
| Contact with the present moment | Full awareness and engagement with present experiences |
| Observing the present self | Accessing a transcendent sense of self |
| Values | Clarifying what is most important to the patient |
| Committed action | Setting goals and taking action to achieve them |
| Source: Reference 4 | |
Results guide pharmacotherapy
In addition to helping change the focus of Mrs. C’s psychotherapy, we used the pharmacogenomic results to guide medication treatment. We initially prescribed fluvoxamine, 50 mg/d, because her partially compromised CYP2D6 pathway probably would play only a minor role in metabolizing the drug.1 Smoking induces CYP1A2, which is fluvoxamine’s primary metabolic pathway; however, Mrs. C does not smoke.6 When we saw Mrs. C in January 2009, the author (JGW) was unaware of any available genetic testing for CYP1A2, although now such testing is clinically available.
Mirtazapine is in the “use as directed” category for Mrs. C’s genotype (Table 2) and was the only medication she had adhered to at a therapeutic dose for more than a few days. However, she indicated that she would not adhere to this medication if we prescribed it again. Duloxetine also is in the “use as directed” category; however, given the entire clinical picture, we chose fluvoxamine because of Mrs. C’s obsessive symptomatology and because she had never reached a therapeutic dose of a selective serotonin reuptake inhibitor.
OUTCOME: Obsessions abate
Given Mrs. C’s lack of insight, we initiate a family approach to help broach the topic of obsessions as the focus of treatment. With her husband’s help, she participates in defusion techniques as an inpatient and follows up with an acceptance-based psychotherapist after discharge. After we share the pharmacogenomic information with Mrs. C, she agrees to try fluvoxamine, which is titrated to 100 mg/d. She maintains this dose at her 4-week follow-up visit. Notably, this was only the second time Mrs. C adhered to a medication trial since illness onset. Upon admission, Mrs. C had an HRSD-17 score of 30, indicating severe depression; at 4 weeks, her HRSD-17 score is 8, indicating mild depression.
The authors’ observations
In a complementary case, the author (JGW) consulted on a patient who was taking paroxetine and experiencing anorgasmia, weight gain, and loss of libido. Pharmacogenomic testing revealed that the patient was a poor metabolizer of CYP2D6. Paroxetine is substantially metabolized by CYP2D6; therefore, it was likely that high blood levels were contributing to the side effects.3,7 The key clinical distinction is that although this patient was bothered by intrusive side effects, he was not fixated on them like Mrs. C. His pharmacogenomic test results were used to identify a metabolic issue that was causing the side effects. This is in contrast with Mrs. C, for whom the pharmacogenomic information ruled out a metabolic issue as the primary problem and helped guide the next step in treatment.
Mrs. C’s case illustrates how pharmacogenomics and ACT complemented each other to create a desirable outcome. Pharmacogenomic testing originally was developed as a safety mechanism for medication choice and dosing, but clinical applications have grown as astute clinicians utilize it to help care for their patients.8 ACT can be a powerful tool for patients who have difficulties creating distance from their thoughts. Both pharmacogenomic testing and ACT are noninvasive interventions that can be implemented as part of a multi-faceted treatment approach.
Related Resources
- Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: The process and practice of mindful change. 2nd ed. New York, NY: The Guilford Press; 2011.
- Mrazek DA. Psychiatric pharmacogenomics. New York, NY: Oxford University Press; 2010.
Drug Brand Names
- Amitriptyline • Elavil
- Aripiprazole • Abilify
- Bupropion • Wellbutrin, Zyban
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clozapine • Clozaril
- Desipramine • Norpramin
- Diazepam • Valium
- Doxepin • Adapin, Silenor
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Haloperidol • Haldol
- Imipramine • Tofranil
- Lithium • Eskalith, Lithobid
- Mirtazapine • Remeron
- Olanzapine • Zyprexa
- Nortriptyline • Pamelor
- Paroxetine • Paxil
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Trazodone • Desyrel, Oleptro
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosure
The authors are employed by AssureRx Health, Inc., the provider of the pharmacogenomic testing used in this article.
1. Mrazek DA. Psychiatric pharmacogenomics. New York, NY: Oxford University Press; 2010.
2. Kirchheiner J, Nickchen K, Bauer M, et al. Pharmacogenetics of antidepressants and antipsychotics: the contribution of allelic variations to the phenotype of drug response. Mol Psychiatry. 2004;9(5):442-473.
3. Kircheiner J, Brøsen K, Dahl ML, et al. CYP2D6 and CYP2C19 genotype-based dose recommendations for antidepressants: a first step towards subpopulation-specific dosages. Acta Psychiatr Scand. 2001;104(3):173-192.
4. Harris R. Embracing your demons: an overview of acceptance and commitment therapy. Psychotherapy in Australia. 2006;12(4):2-8.
5. Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: an experiential approach to behavior change. New York, NY: Guilford Press; 2003.
6. Luvox CR [package insert] Palo Alto CA: Jazz Pharmaceuticals, Inc.; 2011.
7. Kaneda Y, Kawamura I, Fujii A, et al. Serotonin syndrome– ‘potential’ role of the CYP2D6 genetic polymorphism in Asians. Int J Neuropsychopharmacol. 2002;5(1):105-106.
8. Kung S, Li X. The clinical use of pharmacogenomic testing in treatment-resistant depression. Primary Psychiatry. 2010;17(5):46-51.
CASE: Medication sensitivity
Mrs. C, age 48, is admitted to a tertiary care inpatient mood disorder unit for evaluation of severe depression characterized by depressed mood, anhedonia, and insomnia. Her initial Hamilton Rating Scale for Depression 17-Item (HRSD-17) score is 30, indicating severe depression. Her medications are fluoxetine, 10 mg/d, and diazepam, 0.5 mg/d.
Mrs. C describes a 10-month history of depression and extreme anxiety in the context of several psychosocial stressors. Her father recently died and she is having difficulty with the demands of administering her father’s estate. She is intensely obsessive and focused on nihilistic themes, her diagnosis, somatic themes, and medications side effects. Her husband confirms our observations. No history or current symptoms of typical compulsions (eg, washing hands or checking doors) are elicited. She has limited insight into her obsessive tendencies.
Mrs. C had no psychiatric history before her depressive and obsessive symptoms developed 10 months ago. However, in the past 10 months, she has been hospitalized in a psychiatric facility twice. She also received a series of 8 electroconvulsive therapy treatments, but reported minimal improvement of her depressive symptoms. Mrs. C had a few cognitive-behavioral therapy (CBT) sessions with a psychotherapist, but she said they didn’t help much.
Mrs. C has substantial difficulty adhering to medications, even at subtherapeutic doses. She states she is “extremely sensitive” to all medications. Mrs. C says she develops dizziness, increased anxiety, insomnia, nausea, and other vague reactions whenever she attempts to increase her psychotropics to therapeutic doses. She took sertraline, 10 mg/d, for 4 days, but discontinued it because of unspecified side effects. She then received escitalopram, 2.5 mg/d, for 10 days, but again stopped it because of vague side effects. She was taking paroxetine, 10 mg/d, for 2 days, but experienced vomiting and discontinued the drug. She tried venlafaxine at a low dose and also discontinued it because of vomiting. Mrs. C stayed on mirtazapine, 22.5 mg/d, for 3 months, but stopped it because of lack of efficacy and she was unwilling to increase the dose. Other unsuccessful trials include citalopram and doxepin. Mrs. C is hesitant to try another medication or increase to therapeutic doses any of the previous medications.
The authors’ observations
Before initiating another treatment, the treatment team considered Mrs. C’s pervasive medication intolerance. Her enzymatic activity may be genetically compromised, which could lead to high blood levels of medications and significant side effects when she takes very low doses. Individual variations in response to psychotropics are influenced by genetic factors.1 Variants in the cytochrome P450 (CYP450) genes produce enzymes with increased activity, normal activity, reduced activity, or no activity, creating phenotypes of ultrarapid metabolizers, extensive metabolizers, intermediate metabolizers, and poor metabolizers, respectively. These genetic variations can affect blood levels of medications that employ these enzymes in their metabolic pathways.2 Mrs. C could be a poor metabolizer of common CYP450 variant enzymes, which led to her exquisite sensitivity to psychotropics. We felt this was a reasonable hypothesis given her tumultuous 10-month course of psychiatric treatment and multiple failed medication trials.
An alternative hypothesis is that Mrs. C’s somatic obsessions about drug side effects were the primary clinical issue that led to her severe medication intolerance. Mrs. C spends hours questioning the inpatient staff about her diagnosis (eg, “Are you sure I don’t have bipolar disorder?”), medications (eg, “Are you sure this medication won’t make me sick?”), somatic themes (eg, “Are you sure I don’t have Meniere’s disease with all my dizziness?”), and nihilistic themes (eg, “What if I never get better?”). Mrs. C’s husband attested that she has spent hours researching her new medications on the Internet and reading the medication handouts from the pharmacy. She admits to mentally cycling through the DSM-IV-TR criteria for hours at a time to “figure out” if she has bipolar disorder (BD).
We initiated pharmacogenomic testing to help distinguish between these hypotheses. Mrs. C’s results are presented in Table 1. Genotype results were applied using an interpretive algorithm (Figure) in which 26 psychiatric medications were placed in categories of “use as directed” (green column), “use with caution” (yellow column), and “use with caution or more frequent monitoring” (red column). The algorithm incorporates the genetic information with the known pharmacologic profile for each of the medications in the panel. Highlights of Mrs. C’s interpretive report are shown in Table 2.
Table 1
Mrs. C’s genotype results
| Gene | Allele | Predicted phenotype |
|---|---|---|
| CYP2D6 | *1/*4 | Intermediate metabolizer |
| CYP2C19 | *1/*1 | Extensive metabolizer |
| SLC6A4 | S/S | Low activity |
| 5HTR2A | G/G | Reduced activity |
Figure
Genotype-phenotype integration into Mrs. C’s interpretive report
Table 2
Mrs. C’s pharmacogenomic-based interpretive report
| Use as directed | Use with caution | Use with caution and more frequent monitoring |
|---|---|---|
| Antidepressants: Duloxetine, mirtazapine Antipsychotics: Clozapine, olanzapine, quetiapine, ziprasidone | Antidepressants: Amitriptyline,a,b bupropion,a citalopram,c clomipramine,a,b desipramine,a,b escitalopram,c fluoxetine,a fluvoxamine,c imipramine,a,b nortriptyline,a,b sertraline,c paroxetine,c trazodone,a venlafaxinea Antipsychotics: Aripiprazole,a haloperidol,a perphenazine,a risperidonea | None |
| aSerum level may be too high, lower doses may be required bSerum levels may be outside of optimal range cGenotype suggests less than optimal response | ||
The authors’ observations
Mrs. C’s genotype might explain some sensitivity to medications metabolized by CYP2D6 (eg, venlafaxine, paroxetine, fluoxetine), but does not explain her acute sensitivity to all of the medications she has taken. For example, she is an extensive metabolizer for CYP2C19, which metabolizes escitalopram; therefore, it is unlikely escitalopram, 2.5 mg/d, would result in high blood levels and side effects.3 Regardless of the next step in treatment, we deemed her somatic obsessions to be the most important clinical issue. It seems unlikely that Mrs. C would adhere to any medication regimen until this underlying problem was addressed.
The focus of treatment shifted to Mrs. C’s obsessions about her medications and their side effects. Mrs. C was fixated on the content of her obsessions (eg, medications, side effects) rather than the process of her obsessional thinking. The goal was to help Mrs. C identify, label, and ultimately create distance from her obsessive thoughts associated with side effects. The treatment team employed an acceptance and commitment therapy (ACT) model of observing and defusing thoughts in the inpatient setting (Table 3).4 ACT is based on mindfulness and committed, values-based action.5 When patients are “fused” with their thoughts, they believe these thoughts are important and representative of reality. In Mrs. C’s case, she fused with the concept that her medications were making her sick and the idea that she may have BD. The treatment team thought these fused thoughts were the major problem that resulted in 10 months of protracted illness.
Conversely, in a “defused” state, patients can separate from their thoughts and observe them as disparate sounds, words, stories, or bits of language. The goal is to observe and allow the patient’s thoughts to simply be thoughts rather than trying to determine if they are “true.” Mrs. C was fused with the idea that her medications were making her ill, so this belief became the story underlying her obsessional thinking. Helping her disengage from this story became the focus of her treatment.
Table 3
6 core principles of acceptance and commitment therapy
| Defusion | Learning to step back and observe thoughts as separate from the self |
| Acceptance | Allowing unpleasant thoughts to come and go without trying to control them |
| Contact with the present moment | Full awareness and engagement with present experiences |
| Observing the present self | Accessing a transcendent sense of self |
| Values | Clarifying what is most important to the patient |
| Committed action | Setting goals and taking action to achieve them |
| Source: Reference 4 | |
Results guide pharmacotherapy
In addition to helping change the focus of Mrs. C’s psychotherapy, we used the pharmacogenomic results to guide medication treatment. We initially prescribed fluvoxamine, 50 mg/d, because her partially compromised CYP2D6 pathway probably would play only a minor role in metabolizing the drug.1 Smoking induces CYP1A2, which is fluvoxamine’s primary metabolic pathway; however, Mrs. C does not smoke.6 When we saw Mrs. C in January 2009, the author (JGW) was unaware of any available genetic testing for CYP1A2, although now such testing is clinically available.
Mirtazapine is in the “use as directed” category for Mrs. C’s genotype (Table 2) and was the only medication she had adhered to at a therapeutic dose for more than a few days. However, she indicated that she would not adhere to this medication if we prescribed it again. Duloxetine also is in the “use as directed” category; however, given the entire clinical picture, we chose fluvoxamine because of Mrs. C’s obsessive symptomatology and because she had never reached a therapeutic dose of a selective serotonin reuptake inhibitor.
OUTCOME: Obsessions abate
Given Mrs. C’s lack of insight, we initiate a family approach to help broach the topic of obsessions as the focus of treatment. With her husband’s help, she participates in defusion techniques as an inpatient and follows up with an acceptance-based psychotherapist after discharge. After we share the pharmacogenomic information with Mrs. C, she agrees to try fluvoxamine, which is titrated to 100 mg/d. She maintains this dose at her 4-week follow-up visit. Notably, this was only the second time Mrs. C adhered to a medication trial since illness onset. Upon admission, Mrs. C had an HRSD-17 score of 30, indicating severe depression; at 4 weeks, her HRSD-17 score is 8, indicating mild depression.
The authors’ observations
In a complementary case, the author (JGW) consulted on a patient who was taking paroxetine and experiencing anorgasmia, weight gain, and loss of libido. Pharmacogenomic testing revealed that the patient was a poor metabolizer of CYP2D6. Paroxetine is substantially metabolized by CYP2D6; therefore, it was likely that high blood levels were contributing to the side effects.3,7 The key clinical distinction is that although this patient was bothered by intrusive side effects, he was not fixated on them like Mrs. C. His pharmacogenomic test results were used to identify a metabolic issue that was causing the side effects. This is in contrast with Mrs. C, for whom the pharmacogenomic information ruled out a metabolic issue as the primary problem and helped guide the next step in treatment.
Mrs. C’s case illustrates how pharmacogenomics and ACT complemented each other to create a desirable outcome. Pharmacogenomic testing originally was developed as a safety mechanism for medication choice and dosing, but clinical applications have grown as astute clinicians utilize it to help care for their patients.8 ACT can be a powerful tool for patients who have difficulties creating distance from their thoughts. Both pharmacogenomic testing and ACT are noninvasive interventions that can be implemented as part of a multi-faceted treatment approach.
Related Resources
- Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: The process and practice of mindful change. 2nd ed. New York, NY: The Guilford Press; 2011.
- Mrazek DA. Psychiatric pharmacogenomics. New York, NY: Oxford University Press; 2010.
Drug Brand Names
- Amitriptyline • Elavil
- Aripiprazole • Abilify
- Bupropion • Wellbutrin, Zyban
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clozapine • Clozaril
- Desipramine • Norpramin
- Diazepam • Valium
- Doxepin • Adapin, Silenor
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Haloperidol • Haldol
- Imipramine • Tofranil
- Lithium • Eskalith, Lithobid
- Mirtazapine • Remeron
- Olanzapine • Zyprexa
- Nortriptyline • Pamelor
- Paroxetine • Paxil
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Trazodone • Desyrel, Oleptro
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosure
The authors are employed by AssureRx Health, Inc., the provider of the pharmacogenomic testing used in this article.
CASE: Medication sensitivity
Mrs. C, age 48, is admitted to a tertiary care inpatient mood disorder unit for evaluation of severe depression characterized by depressed mood, anhedonia, and insomnia. Her initial Hamilton Rating Scale for Depression 17-Item (HRSD-17) score is 30, indicating severe depression. Her medications are fluoxetine, 10 mg/d, and diazepam, 0.5 mg/d.
Mrs. C describes a 10-month history of depression and extreme anxiety in the context of several psychosocial stressors. Her father recently died and she is having difficulty with the demands of administering her father’s estate. She is intensely obsessive and focused on nihilistic themes, her diagnosis, somatic themes, and medications side effects. Her husband confirms our observations. No history or current symptoms of typical compulsions (eg, washing hands or checking doors) are elicited. She has limited insight into her obsessive tendencies.
Mrs. C had no psychiatric history before her depressive and obsessive symptoms developed 10 months ago. However, in the past 10 months, she has been hospitalized in a psychiatric facility twice. She also received a series of 8 electroconvulsive therapy treatments, but reported minimal improvement of her depressive symptoms. Mrs. C had a few cognitive-behavioral therapy (CBT) sessions with a psychotherapist, but she said they didn’t help much.
Mrs. C has substantial difficulty adhering to medications, even at subtherapeutic doses. She states she is “extremely sensitive” to all medications. Mrs. C says she develops dizziness, increased anxiety, insomnia, nausea, and other vague reactions whenever she attempts to increase her psychotropics to therapeutic doses. She took sertraline, 10 mg/d, for 4 days, but discontinued it because of unspecified side effects. She then received escitalopram, 2.5 mg/d, for 10 days, but again stopped it because of vague side effects. She was taking paroxetine, 10 mg/d, for 2 days, but experienced vomiting and discontinued the drug. She tried venlafaxine at a low dose and also discontinued it because of vomiting. Mrs. C stayed on mirtazapine, 22.5 mg/d, for 3 months, but stopped it because of lack of efficacy and she was unwilling to increase the dose. Other unsuccessful trials include citalopram and doxepin. Mrs. C is hesitant to try another medication or increase to therapeutic doses any of the previous medications.
The authors’ observations
Before initiating another treatment, the treatment team considered Mrs. C’s pervasive medication intolerance. Her enzymatic activity may be genetically compromised, which could lead to high blood levels of medications and significant side effects when she takes very low doses. Individual variations in response to psychotropics are influenced by genetic factors.1 Variants in the cytochrome P450 (CYP450) genes produce enzymes with increased activity, normal activity, reduced activity, or no activity, creating phenotypes of ultrarapid metabolizers, extensive metabolizers, intermediate metabolizers, and poor metabolizers, respectively. These genetic variations can affect blood levels of medications that employ these enzymes in their metabolic pathways.2 Mrs. C could be a poor metabolizer of common CYP450 variant enzymes, which led to her exquisite sensitivity to psychotropics. We felt this was a reasonable hypothesis given her tumultuous 10-month course of psychiatric treatment and multiple failed medication trials.
An alternative hypothesis is that Mrs. C’s somatic obsessions about drug side effects were the primary clinical issue that led to her severe medication intolerance. Mrs. C spends hours questioning the inpatient staff about her diagnosis (eg, “Are you sure I don’t have bipolar disorder?”), medications (eg, “Are you sure this medication won’t make me sick?”), somatic themes (eg, “Are you sure I don’t have Meniere’s disease with all my dizziness?”), and nihilistic themes (eg, “What if I never get better?”). Mrs. C’s husband attested that she has spent hours researching her new medications on the Internet and reading the medication handouts from the pharmacy. She admits to mentally cycling through the DSM-IV-TR criteria for hours at a time to “figure out” if she has bipolar disorder (BD).
We initiated pharmacogenomic testing to help distinguish between these hypotheses. Mrs. C’s results are presented in Table 1. Genotype results were applied using an interpretive algorithm (Figure) in which 26 psychiatric medications were placed in categories of “use as directed” (green column), “use with caution” (yellow column), and “use with caution or more frequent monitoring” (red column). The algorithm incorporates the genetic information with the known pharmacologic profile for each of the medications in the panel. Highlights of Mrs. C’s interpretive report are shown in Table 2.
Table 1
Mrs. C’s genotype results
| Gene | Allele | Predicted phenotype |
|---|---|---|
| CYP2D6 | *1/*4 | Intermediate metabolizer |
| CYP2C19 | *1/*1 | Extensive metabolizer |
| SLC6A4 | S/S | Low activity |
| 5HTR2A | G/G | Reduced activity |
Figure
Genotype-phenotype integration into Mrs. C’s interpretive report
Table 2
Mrs. C’s pharmacogenomic-based interpretive report
| Use as directed | Use with caution | Use with caution and more frequent monitoring |
|---|---|---|
| Antidepressants: Duloxetine, mirtazapine Antipsychotics: Clozapine, olanzapine, quetiapine, ziprasidone | Antidepressants: Amitriptyline,a,b bupropion,a citalopram,c clomipramine,a,b desipramine,a,b escitalopram,c fluoxetine,a fluvoxamine,c imipramine,a,b nortriptyline,a,b sertraline,c paroxetine,c trazodone,a venlafaxinea Antipsychotics: Aripiprazole,a haloperidol,a perphenazine,a risperidonea | None |
| aSerum level may be too high, lower doses may be required bSerum levels may be outside of optimal range cGenotype suggests less than optimal response | ||
The authors’ observations
Mrs. C’s genotype might explain some sensitivity to medications metabolized by CYP2D6 (eg, venlafaxine, paroxetine, fluoxetine), but does not explain her acute sensitivity to all of the medications she has taken. For example, she is an extensive metabolizer for CYP2C19, which metabolizes escitalopram; therefore, it is unlikely escitalopram, 2.5 mg/d, would result in high blood levels and side effects.3 Regardless of the next step in treatment, we deemed her somatic obsessions to be the most important clinical issue. It seems unlikely that Mrs. C would adhere to any medication regimen until this underlying problem was addressed.
The focus of treatment shifted to Mrs. C’s obsessions about her medications and their side effects. Mrs. C was fixated on the content of her obsessions (eg, medications, side effects) rather than the process of her obsessional thinking. The goal was to help Mrs. C identify, label, and ultimately create distance from her obsessive thoughts associated with side effects. The treatment team employed an acceptance and commitment therapy (ACT) model of observing and defusing thoughts in the inpatient setting (Table 3).4 ACT is based on mindfulness and committed, values-based action.5 When patients are “fused” with their thoughts, they believe these thoughts are important and representative of reality. In Mrs. C’s case, she fused with the concept that her medications were making her sick and the idea that she may have BD. The treatment team thought these fused thoughts were the major problem that resulted in 10 months of protracted illness.
Conversely, in a “defused” state, patients can separate from their thoughts and observe them as disparate sounds, words, stories, or bits of language. The goal is to observe and allow the patient’s thoughts to simply be thoughts rather than trying to determine if they are “true.” Mrs. C was fused with the idea that her medications were making her ill, so this belief became the story underlying her obsessional thinking. Helping her disengage from this story became the focus of her treatment.
Table 3
6 core principles of acceptance and commitment therapy
| Defusion | Learning to step back and observe thoughts as separate from the self |
| Acceptance | Allowing unpleasant thoughts to come and go without trying to control them |
| Contact with the present moment | Full awareness and engagement with present experiences |
| Observing the present self | Accessing a transcendent sense of self |
| Values | Clarifying what is most important to the patient |
| Committed action | Setting goals and taking action to achieve them |
| Source: Reference 4 | |
Results guide pharmacotherapy
In addition to helping change the focus of Mrs. C’s psychotherapy, we used the pharmacogenomic results to guide medication treatment. We initially prescribed fluvoxamine, 50 mg/d, because her partially compromised CYP2D6 pathway probably would play only a minor role in metabolizing the drug.1 Smoking induces CYP1A2, which is fluvoxamine’s primary metabolic pathway; however, Mrs. C does not smoke.6 When we saw Mrs. C in January 2009, the author (JGW) was unaware of any available genetic testing for CYP1A2, although now such testing is clinically available.
Mirtazapine is in the “use as directed” category for Mrs. C’s genotype (Table 2) and was the only medication she had adhered to at a therapeutic dose for more than a few days. However, she indicated that she would not adhere to this medication if we prescribed it again. Duloxetine also is in the “use as directed” category; however, given the entire clinical picture, we chose fluvoxamine because of Mrs. C’s obsessive symptomatology and because she had never reached a therapeutic dose of a selective serotonin reuptake inhibitor.
OUTCOME: Obsessions abate
Given Mrs. C’s lack of insight, we initiate a family approach to help broach the topic of obsessions as the focus of treatment. With her husband’s help, she participates in defusion techniques as an inpatient and follows up with an acceptance-based psychotherapist after discharge. After we share the pharmacogenomic information with Mrs. C, she agrees to try fluvoxamine, which is titrated to 100 mg/d. She maintains this dose at her 4-week follow-up visit. Notably, this was only the second time Mrs. C adhered to a medication trial since illness onset. Upon admission, Mrs. C had an HRSD-17 score of 30, indicating severe depression; at 4 weeks, her HRSD-17 score is 8, indicating mild depression.
The authors’ observations
In a complementary case, the author (JGW) consulted on a patient who was taking paroxetine and experiencing anorgasmia, weight gain, and loss of libido. Pharmacogenomic testing revealed that the patient was a poor metabolizer of CYP2D6. Paroxetine is substantially metabolized by CYP2D6; therefore, it was likely that high blood levels were contributing to the side effects.3,7 The key clinical distinction is that although this patient was bothered by intrusive side effects, he was not fixated on them like Mrs. C. His pharmacogenomic test results were used to identify a metabolic issue that was causing the side effects. This is in contrast with Mrs. C, for whom the pharmacogenomic information ruled out a metabolic issue as the primary problem and helped guide the next step in treatment.
Mrs. C’s case illustrates how pharmacogenomics and ACT complemented each other to create a desirable outcome. Pharmacogenomic testing originally was developed as a safety mechanism for medication choice and dosing, but clinical applications have grown as astute clinicians utilize it to help care for their patients.8 ACT can be a powerful tool for patients who have difficulties creating distance from their thoughts. Both pharmacogenomic testing and ACT are noninvasive interventions that can be implemented as part of a multi-faceted treatment approach.
Related Resources
- Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: The process and practice of mindful change. 2nd ed. New York, NY: The Guilford Press; 2011.
- Mrazek DA. Psychiatric pharmacogenomics. New York, NY: Oxford University Press; 2010.
Drug Brand Names
- Amitriptyline • Elavil
- Aripiprazole • Abilify
- Bupropion • Wellbutrin, Zyban
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clozapine • Clozaril
- Desipramine • Norpramin
- Diazepam • Valium
- Doxepin • Adapin, Silenor
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Haloperidol • Haldol
- Imipramine • Tofranil
- Lithium • Eskalith, Lithobid
- Mirtazapine • Remeron
- Olanzapine • Zyprexa
- Nortriptyline • Pamelor
- Paroxetine • Paxil
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Trazodone • Desyrel, Oleptro
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosure
The authors are employed by AssureRx Health, Inc., the provider of the pharmacogenomic testing used in this article.
1. Mrazek DA. Psychiatric pharmacogenomics. New York, NY: Oxford University Press; 2010.
2. Kirchheiner J, Nickchen K, Bauer M, et al. Pharmacogenetics of antidepressants and antipsychotics: the contribution of allelic variations to the phenotype of drug response. Mol Psychiatry. 2004;9(5):442-473.
3. Kircheiner J, Brøsen K, Dahl ML, et al. CYP2D6 and CYP2C19 genotype-based dose recommendations for antidepressants: a first step towards subpopulation-specific dosages. Acta Psychiatr Scand. 2001;104(3):173-192.
4. Harris R. Embracing your demons: an overview of acceptance and commitment therapy. Psychotherapy in Australia. 2006;12(4):2-8.
5. Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: an experiential approach to behavior change. New York, NY: Guilford Press; 2003.
6. Luvox CR [package insert] Palo Alto CA: Jazz Pharmaceuticals, Inc.; 2011.
7. Kaneda Y, Kawamura I, Fujii A, et al. Serotonin syndrome– ‘potential’ role of the CYP2D6 genetic polymorphism in Asians. Int J Neuropsychopharmacol. 2002;5(1):105-106.
8. Kung S, Li X. The clinical use of pharmacogenomic testing in treatment-resistant depression. Primary Psychiatry. 2010;17(5):46-51.
1. Mrazek DA. Psychiatric pharmacogenomics. New York, NY: Oxford University Press; 2010.
2. Kirchheiner J, Nickchen K, Bauer M, et al. Pharmacogenetics of antidepressants and antipsychotics: the contribution of allelic variations to the phenotype of drug response. Mol Psychiatry. 2004;9(5):442-473.
3. Kircheiner J, Brøsen K, Dahl ML, et al. CYP2D6 and CYP2C19 genotype-based dose recommendations for antidepressants: a first step towards subpopulation-specific dosages. Acta Psychiatr Scand. 2001;104(3):173-192.
4. Harris R. Embracing your demons: an overview of acceptance and commitment therapy. Psychotherapy in Australia. 2006;12(4):2-8.
5. Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: an experiential approach to behavior change. New York, NY: Guilford Press; 2003.
6. Luvox CR [package insert] Palo Alto CA: Jazz Pharmaceuticals, Inc.; 2011.
7. Kaneda Y, Kawamura I, Fujii A, et al. Serotonin syndrome– ‘potential’ role of the CYP2D6 genetic polymorphism in Asians. Int J Neuropsychopharmacol. 2002;5(1):105-106.
8. Kung S, Li X. The clinical use of pharmacogenomic testing in treatment-resistant depression. Primary Psychiatry. 2010;17(5):46-51.